
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

What Is a Synthesis Reaction? Definition and Examples

A synthesis reaction is one of the four main types of chemical reactions , along with decomposition, single replacement , and double replacement reactions. Here is the synthesis reaction definition, examples of the reaction using elements and compounds, a look at how many reactants are involved, and how to recognize a synthesis reaction.
Synthesis Reaction Definition
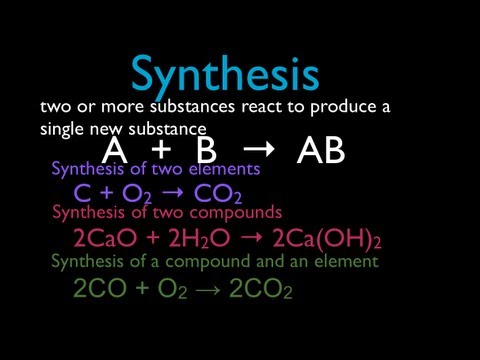
A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product . A + B → AB This type of reaction is also called a direct combination reaction or simply a combination reaction. It’s the type of reaction that forms compounds from their elements. Synthesis reactions also make large molecules from smaller ones. A synthesis reaction is the opposite of a decomposition reaction , which breaks complex molecules into simpler ones.
Synthesis Reaction Examples
There are many examples of synthesis reactions. Some involve elements. In others, an element reacts with a compound. In still other cases, compounds react with other compounds to form larger molecules.
Synthesis Reactions Between Elements
- Iron and sulfur react to form iron sulfide. 8 Fe + S 8 → 8 FeS
- Potassium and chlorine react to form potassium chloride. 2K (s) + Cl 2(g) → 2KCl (s)
- Iron and oxygen react to form rust. 4 Fe (s) + 3 O 2 (g) → 2 Fe 2 O 3 (s)
- Hydrogen reacts with oxygen to form water. 2 H 2 (g) + O 2 (g) → 2 H 2 O(g)
Synthesis Reactions Between an Element and a Compound
- Carbon monoxide reacts with oxygen to form carbon dioxide. 2 CO(g) + O 2 (g) → 2CO 2 (g)
- Nitric oxide reacts with oxygen to form nitrogen dioxide. 2NO + O 2 → 2NO 2
- CH 2 CH 2 (g) + Br 2 (ℓ) → CH 2 BrCH 2 Br
Synthesis Reactions Between Compounds
- Sulfur oxide reacts with water to form sulfuric acid. SO 3 (g) + H 2 O (l) → H 2 SO 4 (aq)
- Calcium oxide reacts with water to form calcium hydroxide. 2CaO (s) + 2H 2 O (l) → 2Ca(OH) 2 (aq)
- Iron oxide and sulfur oxide react to form iron sulfate. Fe 2 O 3 + 3SO 3 → Fe 2 (SO 4 ) 3
How Many Reactants Are There?
Usually, there are two reactants in a synthesis reaction. They could be two elements, an element and a compound, or two compounds. However, sometimes more reactants combine to form a product. Here are examples of synthesis reactions involving three reactants:
- Sodium carbonate reacts with water and carbon dioxide to form sodium bicarbonate. Na 2 CO 3 + H 2 O + CO 2 → 2NaHCO 3
- Nitrogen reacts with water and oxygen to form ammonium nitrate. 2N 2 (g) + 4H 2 O(g) + O 2 (g) → 2NH 4 NO 3 (s)
How to Recognize a Synthesis Reaction
The easiest way to recognize a synthesis reaction is to look for a reaction where multiple reactants produce a single product. However, sometimes a synthesis reaction equation includes multiple products and reactants. A good example is the overall reaction for photosynthesis, in which carbon dioxide and water combine to form glucose and oxygen. CO 2 + H 2 O → C 6 H 12 O 6 + O 2 But, even in this case, two simpler molecules react to form a more complex one. So, this is the key in synthesis reaction identification.
Some synthesis reactions form predictable products. If you recognize them, it’s easy to recognize the reaction type:
- Reacting two elements forms a binary compound. For example, hydrogen and oxygen react to form water.
- When two nonmetals react, more than one product is possible. For example, sulfur and oxygen react to form sulfur dioxide or sulfur trioxide.
- Alkali metals react with nonmetals to form ionic compounds. For example, sodium and chlorine form sodium chloride.
- Transition metals react with nonmetals to form more than one possible product. To predict the product, you need to know the oxidation state (charge) or the metallic cation.
- Nonmetal oxides react with water to form acids. For example sulfur dioxide reacts with water to make sulfurous acid.
- Metallic oxides react with water to form bases.
- Nonmetal oxides react with one another to form salts.
Related Posts
Synthesis Reaction Description Plus Examples
Two or more simple substances combine to form more complex products
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
While there are many types of chemical reactions , they all fall into at least one of four broad categories: synthesis reactions, decomposition reactions , single displacement reactions, and double displacement reactions.
A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound.
General Form of Synthesis Reactions
The general form of a synthesis reaction is:
A + B → AB
Examples of Synthesis Reactions
Here are some examples of synthesis reactions:
- Water: 2 H 2 (g) + O 2 (g) → 2 H 2 O(g)
- Carbon dioxide: 2 CO(g) + O 2 (g) → 2CO 2 (g)
- Ammonia: 3 H 2 (g) + N 2 (g) → 2 NH 3 (g)
- Aluminum oxide: 4 Al(s) + 3 O 2 (g) → 2 Al 2 O 3 (s)
- Iron sulfide: 8 Fe + S 8 → 8 FeS
- Potassium chloride: 2 K(s) + Cl 2 (g) → 2 KCl(s)
Recognizing Synthesis Reactions
The hallmark of a synthesis reaction is that a more complex product is formed from the reactants. One easy-to-recognize type of synthesis reaction occurs when two or more elements combine to form a compound. The other type of synthesis reaction happens when an element and a compound combine to form a new compound.
Basically, to identify this reaction, look for a product that contains all the reactant atoms. Be sure to count the number of atoms in both the reactants and the products. Sometimes when a chemical equation is written, "extra" information is given that might make it hard to recognize what is going on in a reaction. Counting numbers and types of atoms makes it easier to identify reaction types.
- Chemical Reaction Definition and Examples
- Synthesis Reaction Definition and Examples
- Types of Chemical Reactions
- How Many Types of Chemical Reactions Are There?
- Single-Displacement Reaction Definition and Examples
- What Is a Chemical Reaction?
- Chemical Reaction Classification Practice Test
- Chemical Reaction vs. Chemical Equation
- Reactant Definition and Examples
- Decomposition Reaction Definition
- 4 Types of Inorganic Chemical Reactions
- Combustion Reactions in Chemistry
- How to Calculate Theoretical Yield of a Reaction
- What Is a Chemical Equation?
- Equilibrium Constants Practice Test
- Double Displacement Reaction Definition and Examples
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Synthesis Reaction (Combination Reaction)
What is a synthesis reaction, how to identify a synthesis reaction, example of synthesis reaction, different types of synthesis reaction, examples of synthesis reactions in everyday life.
A synthesis reaction is a reaction in which two or more reactants chemically bond and combine to form a product. A synthesis reaction is also known as a combination reaction. Most synthesis reactions are exothermic reactions, i.e., heat is released during the reaction.
General Equation
The general chemical equation for a synthesis reaction is given by the following equation.
When writing an actual reaction, the reaction must be balanced.

A synthesis reaction combines all the reactants of the reaction to form a product. To recognize a synthesis reaction, look for a product that contains all the reactant atoms.
An example of a combination reaction is the combination of aluminum (Al) and oxygen (O 2 ) to aluminum oxide (Al 2 O 3 ).
4 Al (s) + 3 O 2 (g) → 2 Al 2 O 3 (s)
There are three types of synthesis reaction.
1. Reaction between two elements
- The reaction between hydrogen (H 2 ) and nitrogen (N 2 ) to form ammonia (NH 3 )
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
- The reaction between carbon (C) and oxygen (O 2 ) to form carbon dioxide (CO 2 )
C (s) + O 2 (g) → CO 2 (g)
- When sodium (Na) metal reacts with chlorine (Cl 2 ) gas, the reaction results in sodium chloride (NaCl), also known as common salt
2 Na (s) + Cl 2 (g) → 2 NaCl (s)
- The reaction between iron (Fe) and oxygen (O 2 ) results in iron (III) oxide (Fe 2 O 3 ), commonly known as rust. Rusting is a naturally occurring phenomenon.
4 Fe (s) + 3 O 2 (g) → 2 Fe 2 O 3 (s)
2. Reaction between two compounds
- When magnesium oxide (MgO) and carbon dioxide (CO 2 ) combine, the resulting product is magnesium carbonate (MgCO 3 )
MgO (s) + CO 2 (s) → MgCO 3 (s)
3. Reaction between an element and a compound
- The reaction between carbon monoxide (CO) and oxygen (O 2 ) yields carbon dioxide (CO 2 ).
2 CO (g) + O 2 (g) → 2 CO 2 (g)

There are a few examples of the synthesis reaction in real and daily life. Almost all real-life examples are seen in the industry. During industrial production, the synthesis reaction plays a significant part in synthesizing new compounds.
- Synthesis of ammonia
- Commercial production of slaked lime (calcium hydroxide)
- Production of sodium chloride or common salt
- Preparation of hydrochloric acid and ammonium chloride
Other examples include
- Photosynthesis
Ans. A decomposition reaction is one when a substance decomposes into two or more products. This reaction is the opposite of what a combination does.
Ans. Yes. In fact, most common oxidation-reduction (redox) reactions are combination reactions.
Ans. Yes, a combination reaction can be an oxidation reaction if one of the reactants is oxygen.
Ans. While all combination reactions are exothermic, there can be an exception. The production of nitric oxide (NO) from nitrogen and oxygen is an endothermic reaction.
Ans. Dehydration synthesis is the formation of larger molecules from smaller reactants, followed by the loss of a water molecule.
Ans. Dehydration synthesis reactions build up the molecules and generally require energy, while hydrolysis reactions break down the molecules and generally release energy. The two are opposite to one another.
- Opentextbc.ca
- Chem.wisc.edu
- Cpanhd.sitehost.iu.edu
- Amrita.olabs.edu.in
- Chem.libretexts.org
Trending Topics
© 2024 ( Chemistry Learner )
9.9 An Introduction to Organic Synthesis
9.9 • An Introduction to Organic Synthesis
As mentioned in the introduction, one of the purposes of this chapter is to use alkyne chemistry as a vehicle to begin looking at some of the general strategies used in organic synthesis—the construction of complex molecules in the laboratory. There are many reasons for carrying out the laboratory synthesis of an organic compound. In the pharmaceutical industry, new molecules are designed and synthesized in the hope that some might be useful new drugs. In the chemical industry, syntheses are done to devise more economical routes to known compounds. In academic laboratories, the synthesis of extremely complex molecules is sometimes done just for the intellectual challenge involved in mastering so difficult a subject. The successful synthesis route is a highly creative work that is sometimes described by such subjective terms as elegant or beautiful .
In this book, too, we will often devise syntheses of molecules from simpler precursors, but the purpose here is to learn. The ability to plan a successful multistep synthetic sequence requires a working knowledge of the uses and limitations of many different organic reactions. Furthermore, it requires the practical ability to piece together the steps in a sequence such that each reaction does only what is desired without causing changes elsewhere in the molecule. Planning a synthesis makes you approach a chemical problem in a logical way, draw on your knowledge of chemical reactions, and organize that knowledge into a workable plan—it helps you learn organic chemistry.
There’s no secret to planning an organic synthesis: all it takes is a knowledge of the different reactions and some practice. The only real trick is to work backward in what is often called a retrosynthetic direction. Don’t look at a potential starting material and ask yourself what reactions it might undergo. Instead, look at the final product and ask, “What was the immediate precursor of that product?” For example, if the final product is an alkyl halide, the immediate precursor might be an alkene, to which you could add HX. If the final product is a cis alkene, the immediate precursor might be an alkyne, which you could hydrogenate using the Lindlar catalyst. Having found an immediate precursor, work backward again, one step at a time, until you get back to the starting material. You have to keep the starting material in mind, of course, so that you can work back to it, but you don’t want that starting material to be your main focus.
Let’s work several examples of increasing complexity.
Worked Example 9.1
Devising a synthesis route.
How would you synthesize cis -2-hexene from 1-pentyne and an alkyl halide? More than one step is needed.
The product in this case is a cis-disubstituted alkene, so the first question is, “What is an immediate precursor of a cis-disubstituted alkene?” We know that an alkene can be prepared from an alkyne by reduction and that the right choice of experimental conditions will allow us to prepare either a trans-disubstituted alkene (using lithium in liquid ammonia) or a cis-disubstituted alkene (using catalytic hydrogenation over the Lindlar catalyst). Thus, reduction of 2-hexyne by catalytic hydrogenation using the Lindlar catalyst should yield cis -2-hexene.
Next ask, “What is an immediate precursor of 2-hexyne?” We’ve seen that an internal alkyne can be prepared by alkylation of a terminal alkyne anion. In the present instance, we’re told to start with 1-pentyne and an alkyl halide. Thus, alkylation of the anion of 1-pentyne with iodomethane should yield 2-hexyne.
Worked Example 9.2
How would you synthesize 2-bromopentane from acetylene and an alkyl halide? More than one step is needed.
What is an immediate precursor of an alkene? Perhaps an alkyne, which could be reduced.
What is an immediate precursor of a terminal alkyne? Perhaps sodium acetylide and an alkyl halide.
The desired product can be synthesized in four steps from acetylene and 1-bromopropane.
Worked Example 9.3
How would you synthesize 5-methyl-1-hexanol (5-methyl-1-hydroxyhexane) from acetylene and an alkyl halide?
What is an immediate precursor of a terminal alkene? Perhaps a terminal alkyne, which could be reduced.
What is an immediate precursor of 5-methyl-1-hexyne? Perhaps acetylene and 1-bromo-3-methylbutane.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution-NonCommercial-ShareAlike License and you must attribute OpenStax.
Access for free at https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Authors: John McMurry, Professor Emeritus
- Publisher/website: OpenStax
- Book title: Organic Chemistry
- Publication date: Sep 20, 2023
- Location: Houston, Texas
- Book URL: https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Section URL: https://openstax.org/books/organic-chemistry/pages/9-9-an-introduction-to-organic-synthesis
© Jan 9, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Chapter 2: Atoms and Elements
Chapter 3: molecules, compounds, and chemical equations, chapter 4: chemical quantities and aqueous reactions, chapter 5: gases, chapter 6: thermochemistry, chapter 7: electronic structure of atoms, chapter 8: periodic properties of the elements, chapter 9: chemical bonding: basic concepts, chapter 10: chemical bonding: molecular geometry and bonding theories, chapter 11: liquids, solids, and intermolecular forces, chapter 12: solutions and colloids, chapter 13: chemical kinetics, chapter 14: chemical equilibrium, chapter 15: acids and bases, chapter 16: acid-base and solubility equilibria, chapter 17: thermodynamics, chapter 18: electrochemistry, chapter 19: radioactivity and nuclear chemistry, chapter 20: transition metals and coordination complexes, chapter 21: biochemistry.
The JoVE video player is compatible with HTML5 and Adobe Flash. Older browsers that do not support HTML5 and the H.264 video codec will still use a Flash-based video player. We recommend downloading the newest version of Flash here, but we support all versions 10 and above.
Generally, in a chemical reaction, molecules interact by breaking one set of bonds and forming a new set of bonds.
A redox, or oxidation–reduction, reaction is a type of chemical reaction involving the partial or complete transfer of electrons. In such reactions, one reactant is oxidized and the other is reduced, with an observable change in their oxidation states.
The oxidized element, which has lost electrons, undergoes an increase in oxidation state. The reduced element, which has gained electrons, undergoes a decrease in oxidation state.
Among the most common redox reactions are synthesis and decomposition reactions. The synthesis of proteins from different amino acids and the digestion of proteins into amino acids are important examples.
Synthesis, or combination, reactions involve the formation of bonds between reactants to create a single product. The reactants may include only elements, elements and compounds, or only compounds.
Examples are the combination of elemental hydrogen and oxygen to create water, the addition of carbon monoxide to elemental oxygen to form carbon dioxide, and the combination of calcium oxide and water to form calcium hydroxide.
Notice that in all cases, multiple simpler reactants combined into a single complex product.
A decomposition reaction is the opposite of a synthesis reaction. In decomposition reactions, a single complex reactant breaks down into simpler products like elements, elements and compounds, or just compounds.
Decomposition reactions require an input of some form of energy. For example, under the influence of an electric field, water breaks down to give hydrogen and oxygen.
In the presence of sunlight, hydrogen peroxide decomposes into oxygen and water. Similarly, calcium hydroxide, upon being heated, decomposes into calcium oxide and water.
4.13: Synthesis and Decomposition Reactions
Synthesis and decomposition are two types of redox reactions. Synthesis means to make something, whereas decomposition means to break something. The reactions are accompanied by chemical and energy changes.
Synthesis Reactions
Synthesis reactions are also called combination reactions. It is a reaction in which two or more substances combine to form a complex substance. Synthesis reactions are generally represented as: A + B → AB or A + B → C. The formation of nitrogen dioxide is a synthesis reaction: 2 NO ( g ) + O 2 ( g ) → 2 NO 2 ( g ).
In synthesis reactions, the reactants could be all elements (1), or a combination of an element and a compound (2), or all compounds (3).
1) C ( s ) + O 2 ( g ) → CO 2 ( g ) 2) 2 CO ( g ) + O 2 ( g ) → 2 CO 2 ( g ) 3) 2 CaO ( s ) + 2 H 2 O ( l ) → 2 Ca(OH) 2 ( s )
A combination reaction between a metal and a nonmetal always produces an ionic solid. For example, the formation of sodium chloride or table salt from sodium and chlorine is a combination reaction: 2 Na (s) + Cl 2 ( g ) → 2 NaCl ( s ).
A synthesis reaction is generally accompanied by the release of energy. In the above example of sodium chloride, 787 kJ of heat energy is released.
Decomposition Reactions
Oxygen was first discovered by the scientist Joseph Priestley, in 1774, by heating mercury oxide with a burning glass. The reaction was a result of decomposition. Priestley had broken down mercury(II) oxide with heat into its elements. The reaction is represented as: 2 HgO ( s ) → 2 Hg ( l ) + O 2 ( g )
Decomposition reactions involve breaking down a more complex substance into two or more smaller substances. This reaction is often represented as: AB → A + B or C → A + B. Decomposition reactions occur everywhere. For instance, the digestion of proteins, fats, and carbohydrates in our food is an important decomposition reaction. Another example is the decomposition of sodium azide into nitrogen gas.
The reaction is represented as: 2 NaN 3 ( s ) → 2 Na ( s ) + 3 N 2 ( g )
In the above reaction, although the coefficient 2 indicates two molecules of sodium azide being decomposed, there is only one reactant. It is, therefore, a decomposition reaction. Similar to the synthesis reaction, in a decomposition reaction, the products formed could be all elements (1), or a combination of elements and compounds (2), or all compounds (3).
1) 2 Al 2 O 3 ( s ) → 4 Al ( s ) + 3 O 2 ( g ) 2) 2 KClO 3 ( s ) → 2 KCl ( s ) + 3 O 2 ( g ) 3) NH 4 Cl ( s ) → NH 3 ( g ) + HCl ( g )
Get cutting-edge science videos from J o VE sent straight to your inbox every month.
mktb-description
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.
A + B ---> AB
Mg + O 2 ---> MgO H 2 + O 2 ---> H 2 O K + Cl 2 ---> KCl Fe + O 2 ---> Fe 2 O 3
CaO + CO 2 ---> CaCO 3 Na 2 O + CO 2 ---> Na 2 CO 3 KCl + O 2 ---> KClO 3 Ba(ClO 3 ) 2 ---> BaCl 2 + O 2
CO 2 + H 2 O ---> C 6 H 12 O 6 + O 2
H 2 + O 2 ---> H 2 O 2
1) Direct union of two elements will produce a binary compound. 2) Metallic oxides and carbon dioxide react to produce carbonates. 3. Binary salts and oxygen react to produce a chlorate.
CaO + H 2 O ---> Ca(OH) 2 Na 2 O + H 2 O ---> NaOH N 2 O 5 + H 2 O ---> HNO 3 P 2 O 5 + H 2 O ---> H 3 PO 4
1) LiCl + O 2 ---> 2) Na 2 O + CO 2 ---> 3) SO 3 + H 2 O ---> 4) N 2 + H 2 --->
LiCl + O 2 are the products of a chlorate decomposing.
Chlorate is always ClO 3 ¯ Li is plus one
LiCl + O 2 ---> LiClO 3
Na 2 O + CO 2 are the products of a carbonate decomposing.
Carbonate is always CO 3 2 ¯ Na is plus one
Na 2 O + CO 2 ---> Na 2 CO 3
SO 3 + H 2 O are the products of an acid decomposing.
In SO 3 the S has an oxidation number of +6 H has its usual value of +1 and O has its usual value of -2
SO 3 + H 2 O ---> H 2 SO 4
N 2 + H 2 are the products of a binary compound decomposing.
N has a charge of -3 H has its usual value of +1
N 2 + H 2 ---> NH 3
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science ⋅
- Chemistry ⋅
What is a Synthesis Reaction?

Reduction of Benzophenone by Sodium Borohydride
Did you eat a synthesis reaction for breakfast? It's highly likely if you consumed taurine, which is the result of an organic synthesis reaction and commonly found in milk and eggs. In chemistry, a synthesis reaction is when two or more chemicals combine and form a more complex product. You will also have more reactants than products since two or more chemical species combine to form one new larger compound.
What Happens in a Synthesis Reaction?
In a synthesis reaction, two or more chemical species combine, forming a more complex product in the reaction. It is also called a direct reaction and is one of the most common chemical reactions. When the two or more reactants combine they make a larger compound. A synthesis reaction is the opposite of a decomposition reaction, which is when the bonds are broken in a complex product, and it splits the product into its respective components or elements.
What Is the General Form of a Synthesis Reaction?
The word synthesis means to put together. When two or more products are put together it produces a new single product. The basic form of the chemical equation is written as:
What are Some Synthesis Reaction Examples?
Some synthesis reactions occur when burning various metals by adding oxygen to them. Here are some examples:
Magnesium + oxygen → magnesium oxide
Alternatively, in the chemical equation:
2Mg + O 2 → 2MgO
This synthesis reaction gives off a very bright light, so if you perform it, wear safety goggles and don't look directly at the light, or you can harm your eyes.
Aluminum + bromine → aluminum bromide
Or in the chemical equation:
2Al + 3Br 2 → 2AlBr 3
What Is a Synthesis Reaction in Organic Chemistry?
Organic synthesis reactions involve organic compounds. Organic molecules are more complex than their inorganic counterparts are. In many cases, because of the complexity, synthesis reactions of organic compounds require several steps one after the other to create a single product. This makes intermediate compounds for each step before the final single product.
For example, when water combines with ethyl leads it forms ethanol or:
CH 2 = CH 2 + HCl → CH 3 -CH 2 Cl
Other Considerations of a Synthesis Reaction
A synthesis reaction can occur when combining elements and producing a new compound, combining compounds to produce a new compound, or combining both elements and compounds to result in a new compound.
When a metal and non-metal are combined, they produce an ionic compound.
When two non-metals combine, they produce a covalent compound.
When combining metal oxide and water (both compounds), it produces a new compound of a metal hydroxide.
Non-metal and water combinations result in an oxy acid compound.
Metal oxides and carbon dioxide combined produce metal carbonates.
The combination of an element and a compound to produce a new compound can be seen in carbon dioxide. This is the product of carbon monoxide and oxygen, written in a chemical equation as:
2CO (g) + O 2 (g) → 2CO 2 (g)
Related Articles
Three types of aqueous reactions, the differences between anionic and cationic single..., what are the reactants & products in a combustion reaction, how to identify the 6 types of chemical reactions, what is a double replacement reaction, what is a dehydration reaction, easy and fun chemical reaction experiments, why does vinegar affect limestone, type of reactions with copper & nitric acid, what are the reactants & products in the equation..., how are oxidation-reduction reactions used in everyday..., how to make sodium nitrate, physical and chemical properties for the element aluminum, how to prepare potassium chloride, which liquids will tarnish a penny faster, activation energy in an endergonic reaction, what is the difference between reactants & products....
- ThoughtCo.: Synthesis Reaction Definition and Examples
- Chemical Formula: Synthesis Reaction
- What Health: Taurine Overview
About the Author
Mary Lougee has been writing about chemistry, biology, algebra, geometry, trigonometry and calculus for more than 12 years. She gained the knowledge in these fields by taking accelerated classes throughout college while gaining her degree.
Find Your Next Great Science Fair Project! GO
We Have More Great Sciencing Articles!
Examples of Chemical Synthesis
The differences between anionic and cationic single replacement.
Synthesis Reactions — Definition & Examples - Expii
Synthesis reactions — definition & examples.
In a synthesis reaction, or combination reaction, simpler reactants combine to form more complex products. The general equation is A + B → AB.
Explanations (4)
Synthesis reactions.
What are Synthesis Reactions?
- Synthesis reactions are reactions that involve multiple reactants reacting to form one single product.
Example: A + B → AB
- Synthesis reactions are exothermic reactions. So, they release energy as heat or light .
They involve the formation of either ionic or covalent bonds. The formation of a bond releases energy and increases stability. In contrast, breaking bonds requires energy.

Image source: Caroline Monahan
Synthesis Reaction Practice Problem
Which of the following are synthesis reactions?
2S + 3O2 → 2SO3
HCl + NaOH → NaCl + H2O
MgO + CO2 → MgCO3
CO2 + H2O → H2CO3
2Ni2O3 → 4Ni + 3O2
Related Lessons
(Video) Synthesis Reactions: Chemical Reaction (5 of 11) Synthesis Reactions, an Explanation
By Step by Step Science
In this video, you will learn that a synthesis reaction occurs when two or more compounds or elements react with each other to form a singular compound. You will also learn different examples of synthesis reactions in this video.
Synthesis Reactions: What are synthesis reactions?
Image Source: Leo Dong
What is Chemical Synthesis like for Professional Chemists?
In general chemistry, synthesis reactions are the least complicated chemical reaction. You take two reactants and make one product. A generic balanced chemical equation would be A+B→AB. Unfortunately, in the real world, chemical synthesis is rarely so straightforward.
I remember a nightmare scenario from my organic chemistry class. I had one question left on our online homework. I was so close to finishing! I clicked the next button and saw the final problem. It was a fifteen-step synthesis! When you're learning organic chemistry, that's not a short puzzle. Often in chemical synthesis, there are many steps. Why? There are a couple of possible reasons. Sometimes, the reactants that produce an easy synthesis reaction are expensive. So, you might have to start with cheap reactants and build up your molecules. Other times, the reaction intermediates may be unstable. They only form during a series of chemical reactions. So, again we have to build up our reactants.
Planning a Chemical Synthesis
Chemical synthesis is the heart of applied chemistry . The chemist works to develop a procedure to produce their desired compound. What's the result? They construct the reaction mechanism , piece by piece. Along the way, they often have to manipulate specific steps to achieve their goals. They use their knowledge of chemistry principles to influence the reaction.
How would a chemist go about planning a synthesis? Most often, they start at the final product and work backward. Each step focuses on changing one specific bond or group of atoms . In organic chemistry, a compound's reactivity comes from functional groups. They are groupings of atoms with set chemical properties . For example, a carbon - oxygen - hydrogen set is the alcohol group. Because oxygen is bound to hydrogen, it is always a polar group . The oxygen-hydrogen bond is also a weak acid . So, we might manipulate it with a strong base . Other chemists, like inorganic chemists, often focus on individual atoms. But they also have to consider the reactivity of the element .
Performing the Lab Work
In college, I had a friend that was doing a research project. One of the steps in his synthesis formed a hydrate . But, before he could proceed to the next step, he needed to remove the water. So, his compound had to sit in a chemical oven for twelve hours. He didn't get it in until after his afternoon classes. So, he had to return to the lab at 3:00 am to take it out! In chemical synthesis, the lab work is often the most challenging part. Why? A chemical reaction gets influenced by so many factors! We must consider temperature , pressure , pH , time, and other factors. Often we have to manipulate many factors along the way.
Sometimes, we use chemical manipulation . For example, maybe step seven of a fifteen-step reaction is the rate-limiting step . What could we do? We might try to develop a catalyst . Remember, they lower a reaction's activation energy and improve its rate . Sometimes, we can research the published work of other chemists for possible catalysts. Other times, we may need to use theoretical modeling to develop a catalyst. Sometimes, we even have to synthesize the catalyst!
Other times, we need to use physical manipulations. For example, one of our steps could be an equilibrium reaction. But we want it to go to completion. How do we manipulate the equilibrium? We take advantage of Le' Chatlier's principle . If the reaction is endothermic, we could apply heat. What if it's exothermic? We could cool it. Sometimes we can physically separate our products. By removing the products, we alter the equilibrium.
What are Your Results?
Chemistry is complex. For example, sometimes, a reaction can produce multiple products. So, even if we successfully implement our plan, we may not get our desired result. Why? We also have to worry about the percent yield . Part of planning the mechanism is calculating the theoretical yield . But often, one of the steps will act as a limiting reagent . So, very rarely are the theoretical and actual yields equivalent. Sometimes, experimental errors mean we don't produce our desired amount.
Search suggestions
- conductivity
- counting platform scale
- All Categories
- Events & Webinars
Synthesis Reactions
Providing automated tools to deliver life changing products, applications, publications, related products.
- More Information

What Is a Synthesis Reaction?
A synthesis reaction, also known as a direct combination or combination reaction, is a chemical process in which two or more simple elements or compounds combine to form a more complex product. It is represented by the equation: A + B → AB.
Synthesis reactions play a crucial role in creating compounds from their constituent elements and generating larger molecules from smaller ones. They are the opposite of decomposition reactions, which break down complex substances into simpler components. Synthesis reactions are one of the major classes of chemical reactions, which include single displacement, double displacement, and combustion reactions.
Complex Synthesis Reactions
Many synthesis reactions are far more complex than the above reaction: A + B → AB. For example, organic synthesis reactions may involve more than two different molecules, and mixtures of products can occur along with unreacted starting materials. Intermediate molecules may form that can lead to the formation of byproducts. In addition, depending on how the two colliding reactant molecules orient, both the desired product and byproducts may form - which may effect product purity.
There are various types of synthesis reactions. For example, nucleophilic and electrophilic addition and substitution reactions are broad reaction types that yield innumerable examples of synthesis reactions.
When two or more reactants combine to form a more complex molecule, the composition of the final reaction mixture is dependent on the conditions under which the reaction is carried out.
Factors Influencing Successful Synthesis Reactions
A successful synthesis reaction maximizes the creation of desired molecules and minimizes byproduct molecules. A thorough understanding of reaction kinetics, mechanism, and effect of reaction variables are keys to successful synthesis reactions.
- Quality of Reactants, Reagents, and Catalysts - The quality and purity of starting materials and stable sources/vendors of those materials is key to successful, reproducible synthesis reactions and processes.
- Reaction Conditions - Since synthesis reactions are sensitive to reaction parameters, such as temperature, pressure, agitation rate, and dosing rate, precise and accurate control of these variables is crucial to the successful outcome. EasyMax chemical synthesis reactor provides automated parameter control, accuracy, and precision of reaction parameters.
- Reaction Equipment - In the pharmaceutical industry, most synthesis reactions run in batch mode. The physical configuration of EasyMax reactors are an improvement over the classic round bottom flask due to surface area and agitation efficiency considerations. Continuous flow processes are rapidly becoming more frequently used, and ReactIR technology accommodates the real-time analysis of continuous flow and batch syntheses.
- Reaction Kinetics - A thorough understanding of reaction rates is crucial to ensure optimized product yield and minimum byproducts. Through data-rich experiments, ReactIR simplifies and speeds the measurement of kinetic factors in synthesis reactions.
- Product Isolation/Purity - Though separation techniques are a mainstay for product isolation and purity, an understanding of reaction variables to reduce the presence of impurities that may be difficult to separate from the product is important. By optimizing reaction variables, ReactIR with EasyMax aids in impurity reduction. As important, a thorough understanding and control of crystallization via ParticleTrack and ParticleView technology is critical to ensuring purity and ease of isolating desired products.
- Safety - Commercially important chemistry requires lab-to-plant protocols that provide optimized yield, acceptable impurity profiles, and safe operation. ReactIR advances reaction scale-up by elucidating the effects of reaction variables on overall synthesis performance. Reaction calorimetry ensures safe reactions from screening through scale-up to process by measuring heat of reaction. ReactIR in-situ analytics minimizes the exposure of scientists and technicians to toxic chemicals and potentially hazardous reactions by eliminating grab sampling for offline analysis. When offline analysis is required, EasySampler provides automated, in-situ sampling and dilution of samples for HPLC, eliminating worker exposure.

Workstations for Automated and Unattended Synthesis Reactions
Individually, or as an integrated chemical workstation, these tools provide critical support for better synthesis reactions:
- Chemical Synthesis Reactors (EasyMax and OptiMax) Unattended, precise control and data collection of reaction conditions
- FTIR and Raman Spectrometers Real-time tracking and profiling of key reaction species as a function of reaction time to aid kinetics and mechanistic investigations
- Automated, Inline Sampling (EasySampler) Unattended, representative sampling of reactions when offline analysis is required
- EasyViewer In-situ video microscopy of particle/droplet size and shape to quickly increase purity and yield during work-up
- Powerful Analytical Software (iC) Integrates data streams for comprehensive understanding and data management

Replace Manual Synthesis Reaction Steps
With automated synthesis workstations.
Smart chemical synthesis reactors, combined with unattended dosing and automated sampling, provide a simple and safe way to precisely control reaction parameters and obtain reaction information unattended and around the clock.
- Automatically record recipe steps, experimental conditions and analytical data making it easy to repeat experiments and share findings with colleagues
- Run reactions at any temperature from -90°C to 180°C without an ice bath, oil bath, or heating mantle
- Configure parameter controls (such as temperature, dosing, sampling, and stirring) separately for each vessel
- For multi-parameter analysis, including Design of Experiments (DoE) studies , precise and reproducible control help to yield accurate results
- Interchangeable sleeves, glass reactors, and tubes provide flexibility to synthesize at volumes from 0.5 mL to 1000 mL

Enhance Understanding of Synthesis Reactions with FTIR & Raman
Gain in-depth information about reaction kinetics, mechanisms, and pathways. Support safe and optimized scale-up of chemistry. ReactIR and ReactRaman spectrometers provide in-situ, real-time monitoring of chemical reactions for batch and continuous flow syntheses.
- Develop real-time trending profiles for key reaction species: reactants, intermediates, products, and byproducts
- Obtain data-rich information for traditional kinetic analysis or Reaction Progress Kinetics Analysis (RPKA) methods
- Monitor reactions where removing a sample for offline analysis is difficult, impossible, or undesirable – low temperature, elevated temperature/pressure, viscous, toxic reagents, highly energetic reactions, air/moisture sensitive, transient intermediates
- Investigate key stages of a reaction or process, such as reaction start, induction period, accumulation, conversion, and endpoint. Detect reaction stalling or upsets
- Rapidly determine the effect of variables on reactions
- Investigate the broadest range of chemical reactions with ReactIR and ReactRaman. Choose the best technique to match specific chemistries and reaction variables.
- Enhance understanding of solution crystallization processes with ReactRaman for monitoring crystallographic form and polymorphism, and ReactIR for investigating solvent effects and supersaturation.
View a Live eDemo from your work or home office on your schedule.

Automated Sampling For Synthesis Reactions
EasySampler is an automated, unattended technology delivering representative and reproducible samples. The probe-based technology has a micro-pocket, which takes samples at any given time, quenches in situ, and dilutes for ready-to-analyze offline samples.
EasySampler supports reaction understanding by providing samples on demand. Sampling is performed under reaction conditions, making it truly representative. The samples, once collected and time-stamped, can be analyzed via offline analytical methods and the result integrated back into the data stream. An additional value lies in the increased data quality through automatic and seamless data collection. The increased accuracy and precision of automated sampling provide higher quality than manual sampling.

Improve Synthesis Reactions with Fewer Experiments
Combine pat data with advanced modeling.
Reaction Lab uses process analytical technology (PAT) data to accurately model the effect of a range of variables simultaneously, thereby revealing the best set of operating conditions for synthesis reactions. The response of the reaction to the effect of varying specific parameters and conditions is determined, and response surfaces generated give insight into product yield/impurity tradeoffs.
Furthermore, the information from PAT and Reaction Lab facilitates a greater understanding and support for proposed reaction mechanisms and permits processes to be more effectively designed based on this insight.
Featured Application: Understanding α,β-Unsaturated Imine Formation
Determine relative reaction rates and further mechanistic understanding.
Calow, A. D. J., Carbó, J. J., Cid, J., Fernández, E., & Whiting, A. (2014). Understanding α,β-Unsaturated Imine Formation from Amine Additions to α,β-Unsaturated Aldehydes and Ketones: An Analytical and Theoretical Investigation. The Journal of Organic Chemistry , 79 (11), 5163–5172. In previous work, the researchers had reported a catalytic method to synthesize chiral γ-amino alcohols via in-situ generation of α,β-unsaturated imines. They stated that there was a lack of kinetic or mechanistic studies regarding the relative 1,2- versus 1,4- addition of primary amines to α,β-unsaturated aldehydes and ketones. To further this understanding, the researchers used in-situ ReactIR spectroscopy along with NMR studies and DFT calculations, to better characterize the addition of primary amines to α,β-unsaturated aldehydes and ketones (1,2- vs 1,4-addition) and examine the relative rates of these reactions.
ReactIR data showed that when benzylamine was added to crotonaldehyde, 1,2- addition resulted exclusively whereas when benzylamine was added to methyl vinyl ketone, 1,4- addition resulted exclusively.

Synthesis Reaction Examples
- Polymerization Reactions
- Organometallic Chemistry
- Metal-Catalyzed Reactions
- Flow Chemistry
- Design of Experiments (DoE)
- Low Temperature Chemistry
- Elevated Pressure Chemistry ( Hydrogenation Reactions )
- Enzyme Catalyzed Reactions Biocatalysis
- Oligonucleotide Synthesis

Automated Chemistry Solutions for Synthesis Reactions in Industry-Related Publications
Below is a selection of publications where automated solutions are used for synthesis reactions.
- Wang, C., Wang, H., Huang, C., Wu, C., & Sun, T. (2023). Precise Control of the Oxidation Reaction in a High-Purity Dexlansoprazole Synthesis Process Using In Situ Infrared. Organic Process Research & Development . https://doi.org/10.1021/acs.oprd.3c00098
- Yang, H. S., Macha, L., Ha, H. J., & Yang, J. W. (2021). Functionalisation of esters via 1,3-chelation using NaOtBu: mechanistic investigations and synthetic applications. Organic Chemistry Frontiers , 8 (1), 53–60. https://doi.org/10.1039/d0qo01135e
- Millward, M. J., Ellis, E., Ward, J. W., & Clayden, J. (2021). Hydantoin-bridged medium ring scaffolds by migratory insertion of urea-tethered nitrile anions into aromatic C–N bonds. Chemical Science , 12 (6), 2091–2096. https://doi.org/10.1039/d0sc06188c
- Jurica, J. A., & McMullen, J. P. (2021). Automation Technologies to Enable Data-Rich Experimentation: Beyond Design of Experiments for Process Modeling in Late-Stage Process Development. Organic Process Research & Development , 25 (2), 282–291. https://doi.org/10.1021/acs.oprd.0c00496
- Sato, Y., Liu, J., Kukor, A. J., Culhane, J. C., Tucker, J. V., Kucera, D. J., Cochran, B. M., & Hein, J. E. (2021). Real-Time Monitoring of Solid–Liquid Slurries: Optimized Synthesis of Tetrabenazine. Journal of Organic Chemistry , 86 (20), 14069–14078. https://doi.org/10.1021/acs.joc.1c01098
- Shi, Y., Prieto, P. L., Zepel, T., Grunert, S., & Hein, J. E. (2021). Automated Experimentation Powers Data Science in Chemistry. Accounts of Chemical Research , 54 (3), 546–555. https://doi.org/10.1021/acs.accounts.0c00736
- Connor, C. G., DeForest, J. C., Dietrich, P., Do, N. M., Doyle, K. M., Eisenbeis, S., Greenberg, E., Griffin, S. H., Jones, B. P., Jones, K. N., Karmilowicz, M., Kumar, R., Lewis, C. A., McInturff, E. L., McWilliams, J. C., Mehta, R., Nguyen, B. D., Rane, A. M., Samas, B., . . . Webster, M. E. (2020). Development of a Nitrene-Type Rearrangement for the Commercial Route of the JAK1 Inhibitor Abrocitinib. Organic Process Research & Development , 25 (3), 608–615. https://doi.org/10.1021/acs.oprd.0c00366
- Glace, A. W., Cohen, B. M., Dixon, D. D., Beutner, G. L., Vanyo, D., Akpinar, F., Rosso, V., Fraunhoffer, K. J., DelMonte, A. J., Santana, E., Wilbert, C., Gallo, F., & Bartels, W. (2020). Safe Scale-up of an Oxygen-Releasing Cleavage of Evans Oxazolidinone with Hydrogen Peroxide. Organic Process Research & Development , 24 (2), 172–182. https://doi.org/10.1021/acs.oprd.9b00462
- Benkovics, T., McIntosh, J., Silverman, S., Kong, J., Maligres, P., Itoh, T., Yang, H., Huffman, M., Verma, D., Pan, W., Ho, H. I., Vroom, J., Knight, A., Hurtak, J., Morris, W., Strotman, N., Murphy, G., Maloney, K., & Fier, P. (2020). Evolving to an Ideal Synthesis of Molnupiravir, an Investigational Treatment for COVID-19. ChemRxiv . Published. https://doi.org/10.26434/chemrxiv.13472373.v1
- Mennen, S. M., Alhambra, C., Allen, C. L., Barberis, M., Berritt, S., Brandt, T. A., Campbell, A. D., Castañón, J., Cherney, A. H., Christensen, M., Damon, D. B., Eugenio de Diego, J., García-Cerrada, S., García-Losada, P., Haro, R., Janey, J., Leitch, D. C., Li, L., Liu, F., . . . Zajac, M. A. (2019). The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Organic Process Research & Development , 23 (6), 1213–1242. https://doi.org/10.1021/acs.oprd.9b00140
- Wang, K., Han, L., Mustakis, J., Li, B., Magano, J., Damon, D. B., Dion, A., Maloney, M. T., Post, R., & Li, R. (2019). Kinetic and Data-Driven Reaction Analysis for Pharmaceutical Process Development. Industrial & Engineering Chemistry Research , 59 (6), 2409–2421. https://doi.org/10.1021/acs.iecr.9b03578

Chemical Synthesis Reactors

Automated Sampling Systems

FTIR and Raman Spectrometers

Particle Size Analyzers

iC Software Suite

Scale‑up Suite
Request quote or info get a quote instant quote solution information request a demo.

19 Synthesis Reaction Example: Detailed Explanations
In this article, “synthesis reaction example”, different types of synthesis (Williamson synthesis, balanced synthesis and peptide synthesis) example with detailed explanations are discusses briefly.
The examples are-
Synthesis of ethyl methyl ether
Synthesis of anisole, synthesis of 2-ethoxynaphthalene, synthesis of phenyl propyl ether.
- Synthesis of Benzyl-tert butyl ether
Synthesis of tert-butyl methyl ether
Synthesis of ethoxy benzene, synthesis of cyclopentyl methyl ether, synthesis of water, synthesis of carbon-dioxide, synthesis of ammonia.
- Synthesis of Aluminium Oxide

Synthesis of Iron Sulfide
Synthesis of potassium chloride, formation of rust, synthesis of calcium carbonate, synthesis of zinc oxide.
- Synthesis of dipeptide (Gly-Ala)
Solid Phase Peptide Synthesis
What is synthesis reaction.
Synthesis reaction is one type of chemical reaction in which two different atoms involve in the reaction, react with each other to form a totally different molecular compound. In most of the synthesis reaction, energy is released from the reaction medium and known as exothermic reaction.

Example of Williamson Synthesis
Williamson synthesis process is the best method to synthesis ethyl methyl ether (CH 3 -O- CH 2 CH 3 ). This reaction proceeds through SN 2 pathway. To obtain ethyl methyl ether as the synthesized product, sodium methoxide (CH 3 ONa) and ethyl chloride (C 2 H 5 Cl) reacts with each other. Sodium methoxide acts as nucleophile and attacks the electrophilic centre of ethyl chloride to eliminate the leaving group (Cl – ). Ethyl methyl ether is obtained as the Williamson synthesized product.

This ether can also be synthesized by Williamson ether synthesis . To obtain anisole, sodium phenoxide (C 5 H 5 ONa) will react with methyl iodide (CH 3 I) and sodium phenoxide (nucleophile) attacks the electrophilic centre of methyl iodide. Iodide (I – ) will be eliminated as it is a good leaving group and anisole is formed.

To know more please check: 12+ Exothermic Reaction Examples: Detailed Explanations
To proceed this reaction, hydroxyl group should be inserted at the 2 position of naphthalene group and reacts with bromoethane. The reaction medium should be basis. Thus, sodium hydride (NaH) is used. Nucleophilic oxygen atom of OH group in naphthalene attacks the CH 2 centre of CH 3 CH 2 Br and Br – is eliminated as the leaving group.

To synthesis phenyl propyl ether the reactants that are chosen are phenol, sodium metal and n-propyl bromide. Solvent that is used in this synthesis reaction is a polar aprotic solvent. The first step is to react phenol with sodium to form sodium phenoxide (active nucleophile). This nucleophile reacts with n-propyl bromide (electrophile) to synthesize phenyl propyl ether after elimination of bromide ion.

To know more please follow: 11+ First Order Reaction Example: Detailed Explanations
Synthesis of Benzyl-tertbutyl ether
William synthesis pathway is followed for the formation of benzyl-tertbutyl ether. Sodium tert-butoxide and benzyl bromide is taken as the reactants. O – ion from sodium tert-butoxide attacks the electron deficient centre of benzyl bromide Br – is eliminated as the leaving group to form the desired product.

This synthesis process almost similar to the synthesis of benzyl tert-butyl ether. One of the reactants is also same, sodium tert-butoxide and the another reactant is methyl bromide (CH 3 Br). Tertiary sodium tert butoxide reacts as nucleophile and attacks the methyl carbon center to eliminate bromide ion.

To know more please go through: 10+ Covalent bond types of elements: Detailed Insights And Facts
In this process of synthesis of ethoxy benzene, Williamson synthesis process is followed. Sodium ethoxide reacts with phenyl bromide to form ethoxy benzene. O – attacks the electrophilic centre of phenyl bromide and ethoxy benzene is obtained.

In this Williamson ether synthesis, cyclopentanol and methyl bromide is reacted with each other in a basic medium. In presence of base, hydrogen in O-H bond is eliminated and O – attacks the methyl bromide to form the cyclopentyl methyl ether.

Example of Balanced Synthesis
Hydrogen and oxygen-these two gases are the two main reactants of this synthesis. Water molecule that is formed is also in gaseous state. Two molecules of hydrogen react with one molecule of oxygen to form water molecule. Dissociation of water is taken place by passing electric through water.
2H 2 +O 2 = 2H 2 O
Electrolysis of water results-
- Reduction at cathode: 2H + (aq) +2e – = H 2 (g)
- Oxidation at anode: 2H 2 O = O 2 (g) + 4H + (aq) + 4e –
- Net balanced equation: 2H 2 O= 2H 2 + O 2
Carbon dioxide is synthesized during the different decay process of various different material and fermentation of sugars. It can be produced by combustion process of wood or other organic materials. Another procedure is to react metal carbonates with dilute acid for the formation of water. For example, carbon dioxide can be synthesized by the reaction between sodium carbonate with dilute HCl .
Haber-Bosch process is the most well known process to synthesize ammonia. High pressure and high temperature is the two most important driving force of ammonia production. It is an exothermic process (del H= -91.8 KJ/mol). Ammonia is widely used as fertilizer.
N 2 (g) + 3H 2 (g) = 2NH 3
To know more please check: Disulfide reduction: How, What, Methods and Several Facts
Synthesis of Aluminium oxide
Aluminium hydroxide is the main reactant for the formation of aluminium oxide. Solid Al(OH) 3 is decomposed over 1100 0 C and form aluminium oxide (Al 2 O 3 ). Besides that aluminium is oxidized in presence of oxygen to form aluminium oxide.
2Al(OH) 3 = (Al 2 O 3 ) + 3H 2 O
4al (s) + 3o 2 (g)= 2al 2 o 3.
Iron after reaction with sulfur forms iron sulfide (pyrrhotite) in presence of heat energy. Iron sulphide (FeS) has totally different physical and chemical properties with respect to two reactants, iron and sulphur. The ratio of iron with sulphur is 1:1. Equal amount of iron is reacted with equal amount of sulphur to form iron sulphide.
Fe + S = FeS
Potassium chloride is basically an ionic salt. It can be synthesized by the reaction bases of potassium like potassium hydroxide (KOH) with strong acid, hydrochloric acid . In this synthesis reaction, strong acid (HCl) is completely neutralized by strong base (KOH). Water is also produced along with the KCl.
KOH (aq) + HCl (aq) = KCl (s) + H 2 O (liq)
Rust is reddish brown iron oxide formed by reacting iron with oxygen. Water or air takes part in this synthesis reaction as catalyst. Chemical formula of rust is Fe 2 O 3 .Nh 2 O and iron oxide hydroxide (FeO (OH),Fe(OH) 3 ).
- Fe(OH) 2 = FeO + H 2 O
- Fe(OH) 3 = FeO(OH) + H 2 O
- 2FeO(OH) = Fe 2 O 3 +H 2 O
In this synthesis reaction, calcium oxide (CaO) and carbon dioxide is reacted to form calcium carbonate. At first step, calcium hydroxide is prepared by the reaction between calcium oxide with water. After that Ca(OH) 2 is reacted with carbon dioxide and as a product calcium carbonate is obtained.
- CaO +H 2 O = Ca(OH) 2
- Ca(OH) 2 +CO 2 = CaCO 3 +H 2 O
High temperature is one of the most important driving forces. Zinc vapour is reacted with air (oxygen) at 910 0 C. It is mainly an oxidation process and ZnO is produced.
Example of Peptide Synthesis
Synthesis of dipeptide ( gly – ala).
To synthesis of a dipeptide the following steps should be followed-
- At first alpha amino group of glycine should be blocked by tert-butyloxycarbonylchloride.
- After giving the protection to alpha amino acid of glycine, alanine will react with the previously formed compound.
- Then the tert-butyloxycarbonylchloride group is eliminated by reacting with dilute acid and dipeptide (ala-gly) is obtained as the final product.

This synthesis procedure is known as Merrifield synthesis discovered by scientist R.Bruce Merrifield. In this peptide synthesis procedure, homogenous solution is not used for deprotection. This deprotection is carried out at the surface of an insoluble polymer or any solid support.
The carboxyl terminal amino acid is covalently linked with the Merrifield Resin and the length of the peptide chain is increased. Reagents are used to remove the resin with the soluble by products from the peptide chain and at the end desired peptide chain is obtained.

- Maillard reaction
- First order reaction example
- Is oxidation a redox reaction
- Light independent reaction in photosynthesis
- Nuclear fission reaction
- Addition reaction example
- Oxidation reaction example
- Decomposition reaction example
- Redox reactions
- Nuclear fusion reaction

Hello, I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way. Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS AND VIEWS
- 03 April 2024
Trio of radicals choreographed for versatile chemical reaction
- Kenneth F. Clark 0 &
- John A. Murphy 1
Kenneth F. Clark is in the Department of Early Chemical Development, Pharmaceutical Sciences, R&D, AstraZeneca, Macclesfield, SK10 2NA, UK.
You can also search for this author in PubMed Google Scholar
John A. Murphy is in the Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow G1 1XL, UK.
The formation of carbon–carbon bonds is at the heart of the chemistry used to synthesize pharmaceuticals, agrochemicals and advanced materials. Chemists’ skills in harnessing highly reactive free radicals for this purpose have grown steadily. Writing in Nature , Wang et al . 1 describe the ordered construction of molecules using reactions in which three types of radical are present simultaneously, but have distinct roles — a remarkable level of control for such highly reactive chemical species. The secret to success lies in an emerging strategy for organic chemistry, known as radical sorting.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 628 , 42-43 (2024)
doi: https://doi.org/10.1038/d41586-024-00735-z
Wang, J. Z., Lyon, W. L. & MacMillan, D. W. C. Nature 628 , 104–109 (2024).
Article Google Scholar
Chatgilialoglu, C. & Studer, A. Encyclopedia of Radicals in Chemistry, Biology and Materials (Wiley, 2012).
Google Scholar
Dong, Z. & MacMillan, D. W. C. Nature 598 , 451–456 (2021).
Article PubMed Google Scholar
Liu, W., Lavagnino, M. N., Gould, C. A., Alcázar, J. & MacMillan, D. W. C. Science 374 , 1258–1263 (2021).
Sakai, H. & MacMillan, D. W. C. J. Am. Chem. Soc. 144 , 6185–6192 (2022).
Tsymbal, A. V., Bizzoni, L. D. & MacMillan, D. W. C. J. Am. Chem. Soc. 144 , 21278–21286 (2022).
Mao, E. & MacMillan, D. W. C. J. Am. Chem. Soc. 145 , 2787–2793 (2023).
Gould, C. A., Pace, A. L. & MacMillan, D. W. C. J. Am. Chem. Soc. 145 , 16330–16336 (2023).
Bour, J. R., Ferguson, D. M., McClain, E. J., Kampf, J. W. & Sanford, M. S. J. Am. Chem. Soc. 141 , 8914–8920 (2019).
Wang, Y., Wen, X., Cui, X. & Zhang, X. P. J. Am. Chem. Soc. 140 , 4792–4796 (2018).
Hu, Y. et al. Angew. Chem. Int. Edn 58 , 2670–2674 (2019).
Zhou, M., Lankelma, M., van der Vlugt, J. I. & de Bruin, B. Angew. Chem. Int. Edn 59 , 11073–11079 (2020).
Wang, X. et al. J. Am. Chem. Soc. 143 , 11121–11129 (2021).
Wang, S. et al. Nature Commun. 13 , 2432 (2022).
Gan, X.-c. et al. J. Am. Chem. Soc. 145 , 15714–15720 (2023).
Download references
Reprints and permissions
Competing Interests
The authors declare no competing interests.
Related Articles

See all News & Views
- Organic chemistry
Copper-catalyzed dehydrogenation or lactonization of C(sp3)−H bonds
Article 28 MAR 24
Couple-close construction of polycyclic rings from diradicals
Article 13 MAR 24

Symmetry breaking and chiral amplification in prebiotic ligation reactions
Article 28 FEB 24

Identifying general reaction conditions by bandit optimization
Stereodivergent 1,3-difunctionalization of alkenes by charge relocation
Article 31 JAN 24
Catalytic asymmetric cationic shifts of aliphatic hydrocarbons
Article 10 JAN 24
Heat flows enrich prebiotic building blocks and enhance their reactivity
Article 03 APR 24

How synthetic biologists are building better biofactories
Technology Feature 01 APR 24
Laboratory Director
Houston, Texas (US)
Baylor College of Medicine (BCM)
PhD, Postdoc and Technician positions in the Cluster of Excellence "MicroPlanet"
PhD, Postdoc and Technician positions in interdisciplinary microbiome project
Austria (AT) - Vienna, and Lower Austria
University of Vienna - Centre for Microbiology and Environmental Systems Science
Assistant Professor in Integrated Photonics
We offer you the chance to design a unique and autonomous research program, networking with specialists, students and entrepreneurs.
Gothenburg (Stad), Västra Götaland (SE)
Chalmers University of Technology
Senior Group Leader
The Gregor Mendel Institute (GMI) is recruiting a Senior Group Leader. The GMI is a research institute of the Austrian Academy of Sciences, devoted...
Vienna (Landbezirke) (AT)
Gregor Mendel Institut of Molecular Plant Biology
Postdoctoral Fellow (Aging, Metabolic stress, Lipid sensing, Brain Injury)
Seeking a Postdoctoral Fellow to apply advanced knowledge & skills to generate insights into aging, metabolic stress, lipid sensing, & brain Injury.
Dallas, Texas (US)
UT Southwestern Medical Center - Douglas Laboratory
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.10: Classifying Chemical Reactions
- Last updated
- Save as PDF
- Page ID 47512
Learning Objectives
- Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction.
- Predict the products of simple reactions.
The chemical reactions we have described are only a tiny sampling of the infinite number of chemical reactions possible. How do chemists cope with this overwhelming diversity? How do they predict which compounds will react with one another and what products will be formed? The key to success is to find useful ways to categorize reactions. Familiarity with a few basic types of reactions will help you to predict the products that form when certain kinds of compounds or elements come in contact.
Most chemical reactions can be classified into one or more of five basic types: acid–base reactions, exchange reactions, condensation reactions (and the reverse, cleavage reactions), and oxidation–reduction reactions. The general forms of these five kinds of reactions are summarized in Table \(\PageIndex{1}\), along with examples of each. It is important to note, however, that many reactions can be assigned to more than one classification, as you will see in our discussion.
The classification scheme is only for convenience; the same reaction can be classified in different ways, depending on which of its characteristics is most important. Oxidation–reduction reactions, in which there is a net transfer of electrons from one atom to another, and condensation reactions are discussed in this section. Acid–base reactions are one kind of exchange reaction—the formation of an insoluble salt, such as barium sulfate, when solutions of two soluble salts are mixed together.
Combination Reactions
A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is:
\[\ce{A} + \ce{B} \rightarrow \ce{AB} \nonumber \]
One combination reaction is two elements combining to form a compound. Solid sodium metal reacts with chlorine gas to produce solid sodium chloride.
\[2 \ce{Na} \left( s \right) + \ce{Cl_2} \left( g \right) \rightarrow 2 \ce{NaCl} \left( s \right) \nonumber \]
Notice that in order to write and balance the equation correctly, it is important to remember the seven elements that exist in nature as diatomic molecules (\(\ce{H_2}\), \(\ce{N_2}\), \(\ce{O_2}\), \(\ce{F_2}\), \(\ce{Cl_2}\), \(\ce{Br_2}\), and \(\ce{I_2}\)).
One type of combination reaction that occurs frequently is the reaction of an element with oxygen to form an oxide. Metals and nonmetals both react readily with oxygen under most conditions. Magnesium reacts rapidly and dramatically when ignited, combining with oxygen from the air to produce a fine powder of magnesium oxide:
\[2 \ce{Mg} \left( s \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{MgO} \left( s \right) \nonumber \]
Sulfur reacts with oxygen to form sulfur dioxide:
\[\ce{S} \left( s \right) + \ce{O_2} \left( g \right) \rightarrow \ce{SO_2} \left( g \right) \nonumber \]
When nonmetals react with one another, the product is a molecular compound. Often, the nonmetal reactants can combine in different ratios and produce different products. Sulfur can also combine with oxygen to form sulfur trioxide:
\[2 \ce{S} \left( s \right) + 3 \ce{O_2} \left( g \right) \rightarrow 2 \ce{SO_3} \left( g \right) \nonumber \]
Transition metals are capable of adopting multiple positive charges within their ionic compounds. Therefore, most transition metals are capable of forming different products in a combination reaction. Iron reacts with oxygen to form both iron (II) oxide and iron (III) oxide:
\[2 \ce{Fe} \left( s \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{FeO} \left( s \right) \nonumber \]
\[4 \ce{Fe} \left( s \right) + 3 \ce{O_2} \left( g \right) \rightarrow 2 \ce{Fe_2O_3} \left( s \right) \nonumber \]
Example \(\PageIndex{1}\): Combustion of Solid Potassium
Potassium is a very reactive alkali metal that must be stored under oil in order to prevent it from reacting with air. Write the balanced chemical equation for the combination reaction of potassium with oxygen.
Combination reactions can also take place when an element reacts with a compound to form a new compound composed of a larger number of atoms. Carbon monoxide reacts with oxygen to form carbon dioxide according to the equation:
\[2 \ce{CO} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{CO_2} \left( g \right) \nonumber \]
Two compounds may also react to form a more complex compound. A very common example is the reactions of oxides with water. Calcium oxide reacts readily with water to produce an aqueous solution of calcium hydroxide:
\[\ce{CaO} \left( s \right) + \ce{H_2O} \left( l \right) \rightarrow \ce{Ca(OH)_2} \left( aq \right) \nonumber \]
Sulfur trioxide gas reacts with water to form sulfuric acid. This is an unfortunately common reaction that occurs in the atmosphere in some places where oxides of sulfur are present as pollutants. The acid formed in the reaction falls to the ground as acid rain.
\[\ce{SO_3} \left( g \right) + \ce{H_2O} \left( l \right) \rightarrow \ce{H_2SO_4} \left( aq \right) \nonumber \]

Exercise \(\PageIndex{1}\)
- Write the chemical equation for the synthesis of silver bromide, \(\ce{AgBr}\).
- Predict the products for the following reaction: \(\ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right)\)
Decomposition Reactions
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a decomposition reaction is:
\[\ce{AB} \rightarrow \ce{A} + \ce{B} \nonumber \]
Most decomposition reactions require an input of energy in the form of heat, light, or electricity.
Binary compounds are compounds composed of just two elements. The simplest kind of decomposition reaction is when a binary compound decomposes into its elements. Mercury (II) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas:
\[2 \ce{HgO} \left( s \right) \rightarrow 2 \ce{Hg} \left( l \right) + \ce{O_2} \left( g \right) \nonumber \]
Video \(\PageIndex{2}\): Mercury (II) oxide is a red solid. When it is heated, it decomposes into mercury metal and oxygen gas.
A reaction is also considered to be a decomposition reaction even when one or more of the products are still compounds. A metal carbonate decomposes into a metal oxide and carbon dioxide gas. For example, calcium carbonate decomposes into calcium oxide and carbon dioxide:
\[\ce{CaCO_3} \left( s \right) \rightarrow \ce{CaO} \left( s \right) + \ce{CO_2} \left( g \right) \nonumber \]
Metal hydroxides decompose on heating to yield metal oxides and water. Sodium hydroxide decomposes to produce sodium oxide and water:
\[2 \ce{NaOH} \left( s \right) \rightarrow \ce{Na_2O} \left( s \right) + \ce{H_2O} \left( g \right) \nonumber \]
Some unstable acids decompose to produce nonmetal oxides and water. Carbonic acid decomposes easily at room temperature into carbon dioxide and water:
\[\ce{H_2CO_3} \left( aq \right) \rightarrow \ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right) \nonumber \]
Example \(\PageIndex{2}\): Electrolysis of Water
When an electric current is passed through pure water, it decomposes into its elements. Write a balanced equation for the decomposition of water.
Exercise \(\PageIndex{2}\)
Write the chemical equation for the decomposition of:
- \(\ce{Al_2O_3}\)
- \(\ce{Ag_2S}\)
Single Replacement Reactions
A third type of reaction is the single replacement reaction, in which one element replaces a similar element in a compound. The general form of a single-replacement (also called single-displacement) reaction is:
\[\ce{A} + \ce{BC} \rightarrow \ce{AC} + \ce{B} \nonumber \]
In this general reaction, element \(\ce{A}\) is a metal and replaces element \(\ce{B}\), also a metal, in the compound. When the element that is doing the replacing is a nonmetal, it must replace another nonmetal in a compound, and the general equation becomes:
\[\ce{Y} + \ce{XZ} \rightarrow \ce{XY} + \ce{Z} \nonumber \]
where \(\ce{Y}\) is a nonmetal and replaces the nonmetal \(\ce{Z}\) in the compound with \(\ce{X}\).
Metal Replacement
Magnesium is a more reactive metal than copper. When a strip of magnesium metal is placed in an aqueous solution of copper (II) nitrate, it replaces the copper. The products of the reaction are aqueous magnesium nitrate and solid copper metal.
\[\ce{Mg} \left( s \right) + \ce{Cu(NO_3)_2} \left( aq \right) \rightarrow \ce{Mg(NO_3)_2} \left( aq \right) + \ce{Cu} \left( s \right) \nonumber \]
This subcategory of single-replacement reactions is called a metal replacement reaction because it is a metal that is being replaced (copper).
Hydrogen Replacement
Many metals react easily with acids and when they do so, one of the products of the reaction is hydrogen gas. Zinc reacts with hydrochloric acid to produce aqueous zinc chloride and hydrogen (figure below).
\[\ce{Zn} \left( s \right) + 2 \ce{HCl} \left( aq \right) \rightarrow \ce{ZnCl_2} \left( aq \right) + \ce{H_2} \left( g \right) \nonumber \]
In a hydrogen replacement reaction, the hydrogen in the acid is replaced by an active metal. Some metals are so reactive that they are capable of replacing the hydrogen in water. The products of such a reaction are the metal hydroxide and hydrogen gas. All Group 1 metals undergo this type of reaction. Sodium reacts vigorously with water to produce aqueous sodium hydroxide and hydrogen (see figure below).
\[2 \ce{Na} \left( s \right) + 2 \ce{H_2O} \left( l \right) \rightarrow 2 \ce{NaOH} \left( aq \right) + \ce{H_2} \left( g \right) \nonumber \]

Halogen Replacement
The element chlorine reacts with an aqueous solution of sodium bromide to produce aqueous sodium chloride and elemental bromine:
\[\ce{Cl_2} \left( g \right) + 2 \ce{NaBr} \left( aq \right) \rightarrow 2 \ce{NaCl} \left( aq \right) + \ce{Br_2} \left( l \right) \nonumber \]
The reactivity of the halogen group (group 17) decreases from top to bottom within the group. Fluorine is the most reactive halogen, while iodine is the least. Since chlorine is above bromine, it is more reactive than bromine and can replace it in a halogen replacement reaction.
Example \(\PageIndex{3}\)
What are the products of the reaction between solid aluminum (\(\ce{Al}\)) and iron (III) oxide (\(\ce{Fe_2O_3}\))?
Exercise \(\PageIndex{3}\)
- Write the chemical equation for the single replacement reaction between zinc solid and lead (II) nitrate solution to produce zinc nitrate solution and solid lead. (Note that zinc forms ions with a \(+2\) charge.)
- Predict the products for the following reaction: \(\ce{Fe} + \ce{CuSO_4}\). (In this reaction, assume iron forms ions with a \(+2\) charge.)
Double Replacement Reactions
A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds. The general form of a double-replacement (also called double-displacement) reaction is:
\[\ce{AB} + \ce{CD} \rightarrow \ce{AD} + \ce{BC} \nonumber \]
In this reaction, \(\ce{A}\) and \(\ce{C}\) are positively-charged cations, while \(\ce{B}\) and \(\ce{D}\) are negatively-charged anions. Double-replacement reactions generally occur between substances in aqueous solution. In order for a reaction to occur, one of the products is usually a solid precipitate, a gas, or a molecular compound such as water.
Formation of a Precipitate
A precipitate forms in a double-replacement reaction when the cations from one of the reactants combine with the anions from the other reactant to form an insoluble ionic compound. When aqueous solutions of potassium iodide and lead (II) nitrate are mixed, the following reaction occurs:
\[2 \ce{KI} \left( aq \right) + \ce{Pb(NO_3)_2} \left( aq \right) \rightarrow 2 \ce{KNO_3} \left( aq \right) + \ce{PbI_2} \left( s \right) \label{eq10} \]
There are very strong attractive forces that occur between \(\ce{Pb^{2+}}\) and \(\ce{I^-}\) ions and the result is a brilliant yellow precipitate (Figure \(\PageIndex{3}\)). The other product of the reaction, potassium nitrate, remains soluble.
_iodide_precipitating_out_of_solution.jpg?revision=1&size=bestfit&width=194&height=240)
Formation of a Gas
Some double-replacement reactions produce a gaseous product which then bubbles out of the solution and escapes into the air. When solutions of sodium sulfide and hydrochloric acid are mixed, the products of the reaction are aqueous sodium chloride and hydrogen sulfide gas:
\[\ce{Na_2S} \left( aq \right) + 2 \ce{HCl} \left( aq \right) \rightarrow 2 \ce{NaCl} \left( aq \right) + \ce{H_2S} \left( g \right) \nonumber \]
Formation of a Molecular Compound
Another kind of double-replacement reaction is one that produces a molecular compound as one of its products. Many examples in this category are reactions that produce water. When aqueous hydrochloric acid is reacted with aqueous sodium hydroxide, the products are aqueous sodium chloride and water:
\[\ce{HCl} \left( aq \right) + \ce{NaOH} \left( aq \right) \rightarrow \ce{NaCl} \left( aq \right) + \ce{H_2O} \left( l \right) \nonumber \]
Example \(\PageIndex{4}\)
Write a complete and balanced chemical equation for the double-replacement reaction \(\ce{NaCN} \left( aq \right) + \ce{HBr} \left( aq \right) \rightarrow\) (hydrogen cyanide gas is formed).
Exercise \(\PageIndex{4}\)
Write a complete and balanced chemical equation for the double-replacement reaction \(\ce{(NH_4)_2SO_4} \left( aq \right) + \ce{Ba(NO_3)_2} \left( aq \right) \rightarrow\) (a precipitate of barium sulfate forms).
Occasionally, a reaction will produce both a gas and a molecular compound. The reaction of a sodium carbonate solution with hydrochloric acid produces aqueous sodium chloride, carbon dioxide gas, and water:
\[\ce{Na_2CO_3} \left( aq \right) + 2 \ce{HCl} \left( aq \right) \rightarrow 2 \ce{NaCl} \left( aq \right) + \ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right) \nonumber \]
Synthesis and characterization of metal–organic framework (MOF): importance in electro-catalysts for oxygen reduction reaction
- Published: 08 April 2024
- Volume 56 , article number 884 , ( 2024 )
Cite this article
- Ehab A. Abdelrahman 1 , 2 &
- Gharieb S. El-Sayyad ORCID: orcid.org/0000-0001-5410-7936 3 , 4
The reaction of oxygen reduction is essential for media of energy storage and transformation, such as fuel cells. The development of effective and highly stable electro-catalysts, such as single-atom catalysts, poses a definite limitation in clean power technologies. Currently, metal–organic frameworks (MOFs) with a flexible structure and regularly-separated active sites have been developed as unusual and promising precursors for the development of composite particles based on carbon, exhibiting outstanding properties for various applications, particularly in electrochemistry.
In this mini-review, the synthesis, characterization, and the role of different MOF-based electro-catalysts, including MOF-based single atom electro-catalysts have been summarized. The impact of active sites (like hetero-atoms and metal central ions), chemical structure, electronic composition, and porosity of MOF-based nano-composites on the behavior of oxygen reduction reaction were explained by combining both laboratory results and analytical estimates. Taking into account ways to improve mass density of sufficient active sites and preserving them from destruction and corrosion.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
(Adapted from Ref. Cavka et al. ( 2008 )) American Chemical Society Copyright (2008), e UiO-67 structure illustrating a single octahedral cage was shared with eight tetrahedral cages; Adapted from Ref. Katz et al. ( 2013 )) Royal Society of chemistry Copyright (2013), and f MOF-808 is comprised from Zr 6 O 4 (OH) 4 (-CO 2 ) 6 (HCOO) which combined with linkers (BTC)(Adapted from Ref. Jiang et al. ( 2014 )), American Chemical Society Copyright (2014)
(Adapted from Ref. Li et al. ( 2019a , b )); Elsevier Copyright (2018), b the synthesis of nano-frames-like ZIF-8 using microwave irradiation. (Adapted from Ref. Zhang et al. ( 2018a , b )); American Chemical Society Copyright (2018), c the growth of the ZIF-8 membrane via LPE technique. (Adapted from Ref. Shekhah et al. ( 2014 )); Royal Society of chemistry Copyright (1996), and d the seeded samples via layer-by-layer technique. (Adapted from Ref. Lee et al. ( 2012 )); Copyright Elsevier (2012)
© 2012 American Chemical Society
(Adapted from Ref. Wang et al. ( 2019 )); Copyright Elsevier (2019), f E 1/2 ~ [G-N]; g E 1/2 ~ [Zn-N]; h E 1/2 ~ [Py-N]; and i E 1/2 ~ [P-N], and j ORR free energy diagram on Zn-N 4 substrate at various electrode potential U (V) at alkaline medium. Adapted from Ref. Song et al. ( 2017 )); Copyright WILEY–VCH Verlag GmbH & Co (2017). KGaA, Weinheim
Data availability
The corresponding author is willing to provide the datasets used and analyzed during the current work upon reasonable request.
AbdulHalim, R.: Metal organic frameworks: explorations and design strategies for MOF synthesis. KAUST Res. Repos. (2016). https://doi.org/10.25781/KAUST-9KEZ7
Article Google Scholar
Cao, X., Tan, C., Sindoro, M., Zhang, H.: Hybrid micro-/nano-structures derived from metal–organic frameworks: preparation and applications in energy storage and conversion. Chem. Soc. Rev. 46 , 2660–2677 (2017)
Cao, J., Feng, Y., Liu, B., Li, H.: Carbon skeleton doped with Co, N, S and P as efficient electrocatalyst for oxygen evolution reaction. Sci. China Mater. 61 , 686–696 (2018)
Cavka, J.H., Jakobsen, S., Olsbye, U., Guillou, N., Lamberti, C., Bordiga, S., Lillerud, K.P.: A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130 (42), 13850–13851 (2008)
Chacón, A.M., Fuentes, A.B., Partida, A.Z.: Synthesis and Characterization of a Gallic Acid Metal Organic Framework for Antitumoral Therapy. Biosaia: Revista de los másteres de Biotecnología Sanitaria y Biotecnología Ambiental, Industrial y Alimentaria 5 , 2254–3821 (2016)
Google Scholar
Dang, S., Zhu, Q.-L., Xu, Q.: Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater. 3 , 1–14 (2017)
Díaz, U., Corma, A.: Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coord. Chem. Rev. 311 , 85–124 (2016)
Eddaoudi, M., Karim, A., Belmabkhout, Y., Shekhah, O., Bhatt, P.M., Cadiau, A.: Highly stable [mambf6-n (o/h2o) n (ligand) 2 (solvent) x] n metal organic frameworks. Google Patents (2019). https://patents.google.com/patent/US10328414B2/en
Gupta, S., Zhao, S., Wang, X.X., Hwang, S., Karakalos, S., Devaguptapu, S.V., Mukherjee, S., Su, D., Xu, H., Wu, G.: Quaternary FeCoNiMn-based nanocarbon electrocatalysts for bifunctional oxygen reduction and evolution: promotional role of Mn doping in stabilizing carbon. ACS Catal. 7 , 8386–8393 (2017)
He, Y., Zhou, W., Qian, G., Chen, B.: Methane storage in metal–organic frameworks. Chem. Soc. Rev. 43 , 5657–5678 (2014)
Jia, N., Weng, Q., Shi, Y., Shi, X., Chen, X., Chen, P., An, Z., Chen, Y.: N-doped carbon nanocages: bifunctional electrocatalysts for the oxygen reduction and evolution reactions. Nano Res. 11 , 1905–1916 (2018)
Jiang, J., Gándara, F., Zhang, Y.-B., Na, K., Yaghi, O.M., Klemperer, W.G.: Superacidity in sulfated metal–organic framework-808. J. Am. Chem. Soc. 136 (37), 12844–12847 (2014)
Jiao, M., Song, W., Li, K., Wang, Y., Wu, Z.: First-principles study on nitrobenzene-doped graphene as a metal-free electrocatalyst for oxygen reduction reaction. The Journal of Physical Chemistry C 120 , 8804–8812 (2016)
Katz, M.J., Brown, Z.J., Colón, Y.J., Siu, P.W., Scheidt, K.A., Snurr, R.Q., Hupp, J.T., Farha, O.K.: A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 49 (82), 9449–9451 (2013)
Kramm, U.I., Herranz, J., Larouche, N., Arruda, T.M., Lefevre, M., Jaouen, F., Bogdanoff, P., Fiechter, S., Abs-Wurmbach, I., Mukerjee, S.: Structure of the catalytic sites in Fe/N/C-catalysts for O 2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 14 , 11673–11688 (2012)
Lee, D.-J., Li, Q., Kim, H., Lee, K.: Preparation of Ni-MOF-74 membrane for CO2 separation by layer-by-layer seeding technique. Microporous Mesoporous Mater. 163 , 169–177 (2012)
Li, Y., Xie, M., Zhang, X., Liu, Q., Lin, D., Xu, C., Xie, F., Sun, X.: Co-MOF nanosheet array: a high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators, B Chem. 278 , 126–132 (2019a)
Li, D., Xu, H.-Q., Jiao, L., Jiang, H.-L.: Metal-organic frameworks for catalysis: state-of-the-art, challenges, and opportunities. EnergyChem 100005 (2019b). https://doi.org/10.1016/j.enchem.2019.100005
Liang, Z., Qu, C., Xia, D., Zou, R., Xu, Q.: Atomically dispersed metal sites in MOF-based materials for electrocatalytic and photocatalytic energy conversion. Angew. Chem. Int. Ed. 57 (31), 9604–9633 (2018)
Liang, Z., Zhao, R., Qiu, T., Zou, R., Xu, Q.: Metal-organic framework-derived materials for electrochemical energy applications. EnergyChem 100001 (2019). https://doi.org/10.1016/j.enchem.2019.100001
Lin, Q., Bu, X., Kong, A., Mao, C., Zhao, X., Bu, F., Feng, P.: New heterometallic zirconium metalloporphyrin frameworks and their heteroatom-activated high-surface-area carbon derivatives. J. Am. Chem. Soc. 137 , 2235–2238 (2015)
Lin, R., Albani, D., Fako, E., Kaiser, S.K., Safonova, O.V., López, N., Pérez-Ramírez, J.: Design of single gold atoms on nitrogen-doped carbon for molecular recognition in alkyne semi-hydrogenation. Angew. Chem. Int. Ed. 58 , 504–509 (2019)
Liu, J., Jiao, M., Lu, L., Barkholtz, H.M., Li, Y., Wang, Y., Jiang, L., Wu, Z., Liu, D.-j, Zhuang, L.: High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 8 , 1–10 (2017)
ADS Google Scholar
Orellana-Tavra, C., Baxter, E.F., Tian, T., Bennett, T.D., Slater, N.K., Cheetham, A.K., Fairen-Jimenez, D.: Amorphous metal–organic frameworks for drug delivery. Chem. Commun. 51 , 13878–13881 (2015)
Rodenas, T., Luz, I., Prieto, G., Seoane, B., Miro, H., Corma, A., Kapteijn, F., i Xamena, F.X.L., Gascon, J.: Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 14 , 48–55 (2015)
Article ADS Google Scholar
Schuler, T., Ciccone, J.M., Krentscher, B., Marone, F., Peter, C., Schmidt, T.J., Büchi, F.N.: Hierarchically structured porous transport layers for polymer electrolyte water electrolysis. Adv. Energy Mater. 10 , 1903216 (2020). https://doi.org/10.1002/aenm.201903216
Shekhah, O., Swaidan, R., Belmabkhout, Y., Du Plessis, M., Jacobs, T., Barbour, L.J., Pinnau, I., Eddaoudi, M.: The liquid phase epitaxy approach for the successful construction of ultra-thin and defect-free ZIF-8 membranes: pure and mixed gas transport study. Chem. Commun. 50 (17), 2089–2092 (2014)
Song, P., Luo, M., Liu, X., Xing, W., Xu, W., Jiang, Z., Gu, L.: Zn single atom catalyst for highly efficient oxygen reduction reaction. Adv. Func. Mater. 27 (28), 1700802 (2017). https://doi.org/10.1002/adfm.201700802
Su, J., Ge, R., Dong, Y., Hao, F., Chen, L.: Recent progress in single-atom electrocatalysts: concept, synthesis, and applications in clean energy conversion. Journal of Materials Chemistry A 6 , 14025–14042 (2018)
Sun, T., Tian, B., Lu, J., Su, C.: Recent advances in Fe (or Co)/N/C electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells. Journal of Materials Chemistry A 5 , 18933–18950 (2017)
Tonigold, M.S.: Novel copper-and cobalt-based metal-organic polyhedra and frameworks: synthesis, structure, properties and applications. Verl. nicht ermittelbar (2011). https://doi.org/10.18725/OPARU-1944
Wang, Z., Huang, J., Guo, Z., Dong, X., Liu, Y., Wang, Y., Xia, Y.: A metal-organic framework host for highly reversible dendrite-free zinc metal anodes. Joule 3 (5), 1289–1300 (2019)
Wen, X., Zhang, Q., Guan, J.: Applications of metal–organic framework-derived materials in fuel cells and metal-air batteries. Coord. Chem. Rev. 409 , 213214 (2020). https://doi.org/10.1016/j.ccr.2020.213214
Wu, H.B., Lou, X.W.D.: Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion promises and challenges. Sci. Adv. 3 , eaap9252 (2017). https://doi.org/10.1126/sciadv.aap9252
Xia, W., Mahmood, A., Zou, R., Xu, Q.: Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 8 , 1837–1866 (2015)
Yang, J., Zhang, F., Lu, H., Hong, X., Jiang, H., Wu, Y., Li, Y.: Hollow Zn/Co ZIF particles derived from Core-Shell ZIF-67@ ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Ed. 54 , 10889–10893 (2015)
Zhang, T., Tang, X., Zhang, J., Zhou, T., Wang, H., Wu, C., Xia, X., Xie, C., Zeng, D.: Metal-organic framework-assisted construction of TiO2/Co3O4 highly ordered necklace-like heterostructures for enhanced ethanol vapor sensing performance. Langmuir 34 , 14577–14585 (2018a)
Zhang, P., Xiao, Y., Sun, H., Dai, X., Zhang, X., Su, H., Qin, Y., Gao, D., Jin, A., Wang, H.: Microwave-assisted, Ni-induced fabrication of hollow ZIF-8 nanoframes for the knoevenagel reaction. Cryst. Growth Des. 18 (7), 3841–3850 (2018b)
Zhao, D., Timmons, D.J., Yuan, D., Zhou, H.-C.: Tuning the topology and functionality of metal—organic frameworks by ligand design. Acc. Chem. Res. 44 , 123–133 (2011)
Zou, L., Hou, C.-C., Liu, Z., Pang, H., Xu, Q.: Superlong single-crystal metal-organic framework nanotubes. J. Am. Chem. Soc. 140 , 15393–15401 (2018)
Download references
Acknowledgements
The authors would like to thank the Chemical Engineering Department, Military Technical College (MTC), Egyptian Armed Forces, Cairo, Egypt and the Zeiss microscope team in Cairo for their invaluable advice during this mini-review. The authors would like to express their special thanks to Dr. Dounia Elfadil (Hassan II University of Casablanca, Mohammedia, Morocco) for her participation in language editing of this manuscript.
Not applicable.
Author information
Authors and affiliations.
Department of Chemistry, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), 11623, Riyadh, Saudi Arabia
Ehab A. Abdelrahman
Chemistry Department, Faculty of Science, Benha University, Benha, 13518, Egypt
Chemical Engineering Department, Military Technical College (MTC), Egyptian Armed Forces, Cairo, Egypt
Gharieb S. El-Sayyad
Department of Microbiology and Immunology, Faculty of Pharmacy, Ahram Canadian University (ACU), Giza, Egypt
You can also search for this author in PubMed Google Scholar
Contributions
EAA: suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. GSE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing.
Corresponding author
Correspondence to Gharieb S. El-Sayyad .
Ethics declarations
Competing interest.
The authors affirm that they have no financial or other conflicts of interest.
Ethics approval
Consent to participate, consent to publish, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Abdelrahman, E.A., El-Sayyad, G.S. Synthesis and characterization of metal–organic framework (MOF): importance in electro-catalysts for oxygen reduction reaction. Opt Quant Electron 56 , 884 (2024). https://doi.org/10.1007/s11082-024-06768-y
Download citation
Received : 11 May 2023
Accepted : 27 February 2024
Published : 08 April 2024
DOI : https://doi.org/10.1007/s11082-024-06768-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Oxygen reduction reaction
- Single-atom catalysts
- Solvothermal method
- Find a journal
- Publish with us
- Track your research
New Journal of Chemistry
Selenol-ene chemistry-based one pot reaction for asymmetric selenides synthesis and rate regulation of selenide oxidative elimination.
Stimulus-responsive materials containing selenide under oxidizing conditions have wide application prospects, especially in biomedicine. Due to the intricate preparation process, the scarcity of studies on the structure-activity relationship of regulatory factors in the oxidative stimulus response poses challenges to material design. Herein, based on selenol-ene chemistry, a diverse library of asymmetric selenides is synthesized, enabling systematic investigation into the regulation of selenide oxidative elimination with variable substituent groups and positions. The α/β-positions of β-carbonyl asymmetric selenides are efficiently modified with electron-donating or electron-withdrawing groups through a one-pot, two-step reaction involving ammonolysis of γ-selenobutyrolactone (SBL), followed by nucleophilic addition with unsaturated acrylate. In situ NMR tracking shows that the oxidative elimination of selenide had a distinct substituent effect. Both the electron and steric effects of the substituent influence the induction period of the selenide oxidative and elimination reactions. With the optimal substituent and position, the selenides would exhibit a timebomb-type response upon oxidation stimulus. Finally, a novel amphiphilic polymer comprising selenide-linked polyethylene glycol (PEG) and cholesterol moieties is synthesized as a representative ROS-sensitive material and exhibits a time-dependent reduction in micelle size.
Supplementary files
- Supplementary information PDF (6777K)
Article information
Download citation, permissions.
Y. Xiang, J. Zhang, Y. Xu, D. Wang, Z. Zhang, J. Zhu, W. Lu, H. He and X. Pan, New J. Chem. , 2024, Accepted Manuscript , DOI: 10.1039/D4NJ00194J
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
Help | Advanced Search
Physics > Applied Physics
Title: entropy engineered middle-in synthesis of dual single-atom compounds for nitrate reduction reaction.
Abstract: Despite the immense potential of Dual Single-Atom Compounds (DSACs), the challenges in their synthesis process, including complexity, stability, purity, and scalability, remain primary concerns in current research. Here, we present a general strategy, termed "Entropy-Engineered Middle-In Synthesis of Dual Single-Atom Compounds" (EEMIS-DSAC), which is meticulously crafted to produce a diverse range of DSACs, effectively addressing the aforementioned issues. Our strategy integrates the advantages of both bottom-up and top-down paradigms, proposing a new insight to optimize the catalyst structure. The as-fabricated DSACs exhibited excellent activity and stability in the nitrate reduction reaction (NO3RR). In a significant advancement, our prototypical CuNi DSACs demonstrated outstanding performance under conditions reminiscent of industrial wastewater. Specifically, under a NO3- concentration of 2000 ppm, it yielded a Faradaic efficiency (FE) for NH3 of 96.97 %, coupled with a mass productivity of 131.47 mg h-1 mg-1 and an area productivity of 10.06 mg h-1 cm-2. Impressively, even under a heightened NO3- concentration of 0.5 M, the FE for NH3 peaked at 90.61 %, with mass productivity reaching 1024.50 mg h-1 mg-1 and an area productivity of 78.41 mg h-1 cm-2. This work underpins the potential of the EEMIS-DSAC approach, signaling a promising frontier for high-performing DSACs.
Submission history
Access paper:.
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .

IMAGES
VIDEO
COMMENTS
A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. Learn how to recognize a synthesis reaction, its types, and its examples with elements and compounds.
Learn what a synthesis reaction is and how to identify it from the reverse of a decomposition reaction. See examples of simple and complex synthesis reactions with one or more products, and how they are predictable or not.
A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Learn the general form, examples and how to recognize synthesis reactions with this article from ThoughtCo.
Synthesis Reactions. A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound. The general equation for a synthesis reaction is: A + B → AB.
A synthesis reaction is a reaction in which two or more reactants chemically bond and combine to form a product. Learn about the types, general equation, and examples of synthesis reactions in everyday life and industry.
Combination Reactions. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is: A + B → AB A + B → AB. One combination reaction is two elements combining to form a compound.
Learn how to plan and execute organic syntheses of complex molecules from simpler precursors using different reactions. Work through worked examples and practice problems involving alkynes, alkenes, and alcohols.
A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions .The general form of a combination reaction is: \[\ce{A} + \ce{B} \rightarrow \ce{AB}\]
Chemical synthesis is the construction of complex compounds from simpler ones by breaking and forming chemical bonds. Learn about the types, methods, and applications of chemical synthesis, as well as some examples of organic and inorganic reactions.
Chemical synthesis. In chemistry, chemical synthesis ( chemical combination) is the artificial execution of chemical reactions to obtain one or several products. [1] This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable.
This organic chemistry video tutorial focuses on multistep synthesis reactions and retrosynthesis problems. It contains plenty of tips, techniques, examples...
Learn how synthesis and decomposition reactions are types of redox reactions that involve combining or breaking apart substances. See examples of synthesis reactions with elements and compounds, and decomposition reactions with complex substances.
Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, more complex compound. These pieces can be elements or simpler compounds. Complex simply means that the product compound has more atoms than the reactant molecules.
Describes the basics of synthesis reactions, how to identify them, predict the product and balance the chemical equation. Two examples are also shown, synth...
A synthesis reaction is when two or more chemical species combine to form a more complex product, such as a new compound or a larger compound. Learn the general form, examples, and types of synthesis reactions in chemistry, and how they differ from decomposition reactions.
Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. In a synthesis reaction two or more substances undergo a chemical reaction producing a new substance. Click Create Assignment to assign this modality to your LMS.
Synthesis reactions are exothermic reactions. So, they release energy as heat or light. They involve the formation of either ionic or covalent bonds. The formation of a bond releases energy and increases stability. In contrast, breaking bonds requires energy. Image source: Caroline Monahan.
The overall yield drops down considerably for the synthesis of the right isomer. Reactions that yield single isomers (Diastereospecific reactions) in good yields are therefore preferred. Some reactions like the Diels Alder Reaction generate several stereopoints (points at which stereoisomers are generated) simultaneously in one step in a highly ...
Synthesis reactions are one of the major classes of chemical reactions, which include single displacement, double displacement, and combustion reactions. Complex Synthesis Reactions. Many synthesis reactions are far more complex than the above reaction: A + B → AB. For example, organic synthesis reactions may involve more than two different ...
Example of Williamson Synthesis Synthesis of ethyl methyl ether. Williamson synthesis process is the best method to synthesis ethyl methyl ether (CH 3-O- CH 2 CH 3).This reaction proceeds through SN 2 pathway. To obtain ethyl methyl ether as the synthesized product, sodium methoxide (CH 3 ONa) and ethyl chloride (C 2 H 5 Cl) reacts with each other. Sodium methoxide acts as nucleophile and ...
What happens during a synthesis reaction? Types of synthesis reactions explained quickly! #chemistry #tutoring #science #recommended #reaction #synthesis #co...
A 'radical sorting' strategy for organic synthesis. The idea that three different free radicals could be used together to carry out specific steps in a chemical reaction has long been implausible.
Hydrothermal methods are widely used to synthesize functional inorganic materials. The interplay between the reactive species, solution chemistry, and the nanoscale product makes it challenging to control the reaction pathway to achieve a uniform product. Here, we resolve the heterogeneity that arises during hydrothermal synthesis across different length scales. We combine spatially resolved ...
A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is: \[\ce{A} + \ce{B} \rightarrow \ce{AB} \nonumber \] One combination reaction is two elements combining to form a compound.
The reaction of oxygen reduction is essential for media of energy storage and transformation, such as fuel cells. The development of effective and highly stable electro-catalysts, such as single-atom catalysts, poses a definite limitation in clean power technologies. Currently, metal-organic frameworks (MOFs) with a flexible structure and regularly-separated active sites have been developed ...
English . Term. Definition. synthesis reaction. chemical reaction in which two or more reactants combine to form a single product.
Selenol-Ene Chemistry-based One Pot Reaction for Asymmetric Selenides Synthesis and Rate Regulation of Selenide Oxidative Elimination ... two-step reaction involving ammonolysis of γ-selenobutyrolactone (SBL), followed by nucleophilic addition with unsaturated acrylate. In situ NMR tracking shows that the oxidative elimination of selenide had ...
Despite the immense potential of Dual Single-Atom Compounds (DSACs), the challenges in their synthesis process, including complexity, stability, purity, and scalability, remain primary concerns in current research. Here, we present a general strategy, termed "Entropy-Engineered Middle-In Synthesis of Dual Single-Atom Compounds" (EEMIS-DSAC), which is meticulously crafted to produce a diverse ...