Vitiligo Research


Vitiligo Advancements and Discoveries
There has been an increase in the amount of research being undertaken in vitiligo over recent years and dermatologists have an improved understanding of the natural history and different types of the condition. Here you will find a brief summary of research into several areas, with references to the original research articles, for those of you who wish to follow these up.
Researchers are looking at:
- The effectiveness of existing treatments;
- Possible causes of vitiligo;
- How the condition develops;
- Segmental vitiligo;
- The association of vitiligo with other conditions;
- The psychological effects of vitiligo.
It is hoped that the improvements in scientific understanding will in future lead to more effective treatments for vitiligo.
Are psychological interventions important for vitiligo patients?
Yes, a survey of vitiligo patients and healthcare professionals found that psychological interventions are important for managing the impact of vitiligo on patients’ lives.
A survey was conducted to identify psychological interventions for vitiligo. The survey was funded by the UK Dermatology Clinical Trials Network and involved patients and health professionals. The survey recorded personal data and focused on the effect of vitiligo on normal life, as well as the most difficult problems faced by patients and which approaches would be helpful.
- Patients with vitiligo reported key issues such as acceptance of their disease, the duration of the disease and managing embarrassment.
- Other concerns were participating in sporting activities and exposure to sunlight.
- Interventions considered useful by professionals to address these issues included cognitive behavioural therapy (CBT), acceptance and commitment therapy (ACT), and mindfulness therapy.
Psychological interventions for vitiligo are a research priority, but there is little published on appropriate therapy from both patient and clinician perspectives. The unique survey referenced here is therefore of value to the future treatment of vitiligo patients.
Will piperine treat vitiligo?
Although promising results have been seen in cell and animal studies, and early work toward clinical trials in humans is underway, the effectiveness and safety of piperine as a treatment for vitiligo in humans has yet to be fully established.
Ongoing research is being conducted, but funding is needed to support further studies. Therefore, it is unclear at this time whether piperine will ultimately prove to be an effective treatment for vitiligo.
Amala Soumyanath led the research that discovered piperine as a potential treatment for vitiligo. In her own words, she shares the story of her research journey and provides an update on the latest developments. Become a member today and access more resources and stories like this.
How was piperine discovered as a potential treatment for vitiligo?
Piperine was discovered as a potential treatment for vitiligo through research and testing of herbal extracts , where a water extract of black pepper was found to stimulate melanocyte growth and dendrite formation. The compound responsible for this effect was identified as piperine, which could be developed for use in treating vitiligo.
How was piperine validated as a “lead” molecule for the treatment of vitiligo?
Piperine was validated as a “lead” molecule for the treatment of vitiligo through studies conducted at King’s College London. They tested extracts from various herbs and found that piperine from black pepper was the most effective at stimulating the growth of pigment cells. Further studies were conducted to make chemical variations (analogs) of piperine and two of these analogs showed good activity.
All three compounds, piperine, THP, and RCHP, were found to stimulate the growth of pigment cells in mice, causing their skin to visibly darken. These studies allowed the researchers to secure international patents for the use of piperine and its analogs to treat vitiligo.
How was piperine’s effectiveness and safety in treating vitiligo validated?
Piperine’s effectiveness and safety in treating vitiligo were validated through a detailed plan for a clinical trial of piperine in patients with vitiligo. Prior to the clinical study, experiments were conducted to investigate the effects of piperine on human pigment cells, including melanocytes from the uninvolved skin of a vitiligo patient.
Piperine was found to stimulate the replication of human melanocytes in culture and when grown within a reconstructed skin model. Colleagues in OHSU’s Biomedical Engineering and Dermatology departments used innovative optical methods to image pigmentation and melanocytes in the skin models.
What were its effects on human pigment cells and melanoma?
Experiments funded by AdPharma, Inc. showed that piperine has an inhibitory effect on cultured melanoma cells and prevents melanoma cell growth in a reconstructed full-skin model. To further study this aspect, the HGF mouse model of melanoma was introduced to OHSU.
The effects of piperine in this model are currently being studied with pilot funding from the Department of Dermatology’s Jesse Ettelson Fund for the Advancement of Dermatology Research. These ongoing studies are essential to establish the safety of piperine.
What is the status of piperine for treating vitiligo in humans?
In 2013, the appointment of Professor Sancy Leachman, a dermatologist and expert in pigment cell biology, gave a significant boost to the project of developing piperine as a new treatment for vitiligo. Dr. Pamela Cassidy and Eric Smith also joined the team, and a core group is working to bring this discovery to the clinic. The current status of piperine as a treatment for vitiligo in humans remains unclear.
Amala Soumyanath’s Personal and Professional Journey to Develop a Treatment for Vitiligo
Amala Soumyanath’s journey began when she received a phone call from Maxine Whitton, an MBE-awarded vitiligo service provider, sparking an idea to develop piperine as a potential treatment for vitiligo. With dedication and persistence, Amala’s knowledge of drug development processes led her to develop piperine to the point of being tested in humans.
Her personal experience with vitiligo, developing noticeable patches in 2006, fueled her drive to find a treatment for this difficult condition. Alongside a team of talented researchers at OHSU, they continue to evaluate piperine’s efficacy and understand its effects on melanocytes, with Dr. Sancy Leachman leading the project and Amala as the ongoing champion.
Is piperine the new treatment for vitiligo?
Amala Soumyanath and her team at OHSU are developing piperine as a potential treatment for vitiligo. A “proof of concept” human study demonstrating piperine’s safety and efficacy could attract large pharmaceutical companies to move forward with the project, but funding is needed. Donations of any size can make a real difference to the project’s progress. While piperine shows promise as a treatment for vitiligo, further research is required before it can be established as a new treatment.
How can you help?
The team at OHSU is reaching out to the general community for funding to support their ongoing studies on piperine for vitiligo at both the clinical and basic science levels. Donations of any size from those affected by vitiligo or anyone interested in supporting the research can be made online to the Vitiligo Research Fund .
Read Amala Soumyanath’s full story here .
What impact does vitiligo have on a person’s quality of life?
Vitiligo can have a moderate to severe impact on a person’s quality of life, including depression, stigmatization, and impaired sex lives. The location of the lesions and cultural values related to appearance and status may also play a role. Research has found that:
- Quality of life is closely related to the patients’ apprehensions about their disease, psychosocial adjustment, and psychiatric morbidity.
- British Asian women with vitiligo often feel visibly different and have experienced stigmatization due to cultural values related to appearance, status, and myths linked to the cause of the condition.
- Quality of life impairment in women affected with vitiligo assessed using the DLQI was equal to the impairment caused by psoriasis.
- Vitiligo had a negative impact on the sex lives of women with vitiligo.
To learn more about the impact vitiligo has on an individual and their quality of life you can find the full articles below:
- Quality of life of patients with vitiligo attending the Regional Dermatology Training Center in Northern Tanzania
- Depression, anxiety and health‐related quality of life in children and adolescents with vitiligo
- Quality of life and psychological adaptation of Korean adolescents with vitiligo
- Vitiligo linked to stigmatization in British South Asian women: a qualitative study of the experiences of living with vitiligo
- Effect of vitiligo on self‐reported health‐related quality of life
- The Problems in Sexual Functions of Vitiligo and Chronic Urticaria Patients
Can thyroid issues cause vitiligo?
There is evidence to suggest that thyroid issues can be associated with vitiligo. The frequency of thyroid disease in vitiligo patients is higher compared to the general population, and it is recommended that all patients with vitiligo have their thyroid function checked.
In the course of their clinical work, dermatologists discovered:
- the frequency of thyroid disease in vitiligo patients was 15.1%,
- autoimmune thyroid disease was 14.3%
- and the presence of thyroid-specific autoantibodies was 20.8%.
To learn more about the association between thyroid issues and vitiligo you can find the full article here .
Does vitiligo increase your risk of skin cancer?
Although patients with vitiligo have a tendency to burn in the sun, a survey conducted by a team from The Netherlands found that patients with vitiligo have a threefold lower probability of developing malignant melanoma and non-melanoma skin cancer. The reasons for this are not yet fully understood.
Read the entire survey here and learn more about this on BBC iPlayer .
What is segmental vitiligo?
Segmental vitiligo is a form of vitiligo that presents with patches distributed unilaterally and locally . It has been compared with a possible mosaic or neurogenic background, but its distribution pattern is not entirely similar to any other skin condition. Cutaneous mosaicism may be involved in segmental vitiligo. However, the underlying mechanism of segmental vitiligo is still unknown.
Learn more about the distribution pattern of segmental vitiligo here .
How is vitiligo classified?
Segmental vitiligo is classified separately from all other forms of vitiligo, with the term ‘vitiligo’ being used as an umbrella term for all non-segmental forms, including mixed vitiligo in which segmental and non-segmental vitiligo are combined and which is considered a subgroup of vitiligo.
Experts recommend that disease stability is best assessed based on the stability of individual lesions rather than the overall stability of the condition.
Read the entire article about the classification of vitiligo here .
What is the Koebner phenomenon in relation to vitiligo and how can it be assessed?
The Koebner phenomenon (KP) refers to the development of vitiligo within an area of skin that has been damaged by localised, often mild trauma (e.g. an injury). Dr. N van Geel and colleagues of Ghent have looked at this phenomenon. They developed a new assessment method for KP, taking into account both the history and clinical examination of people with vitiligo; this seems to be a useful and valuable tool for assessing KP in daily practice.
The results support the hypothesis that KP may be used to assess and predict the course of vitiligo (access the entire article here ).
What is the relationship between Halo Nevi and vitiligo?
Halo nevi are common moles with a white ring around them, showing the sort of pigment loss that is seen in vitiligo. They may represent a distinct condition, but in some cases, they may be an initiating factor in the development of vitiligo, according to research by Dr. van Geel and researchers (access the entire article here ).
What are the mechanisms of pathogenesis of vitiligo?
The pathogenesis of vitiligo is believed to involve oxidative stress, which leads to an imbalance between reactive oxygen species (ROS) and the body’s ability to detoxify them. (Access the entire article here ).
According to research (access the entire article here ):
- Mitochondria within melanocytes and blood cells generate reactive oxygen species (ROS) that may be relevant in vitiligo development.
- Modification of membrane lipid components in vitiligo cells may cause mitochondrial impairment and the production of intracellular ROS after exposure to mild stress.
- Autoimmunity plays a role in the pathogenesis of vitiligo, with tyrosine hydroxylase identified as an autoantigen target.
- Tyrosine hydroxylase antibodies are more frequent in people with active non-segmental vitiligo (23%) but not in the segmental type.
How does vitiligo affect the layers of skin?
Genetic studies show that susceptibility to vitiligo is related to proteins or parts of the pigment cell involved in the immune system (access the entire article here ). Research from Dalian, China, reveals that alterations in skin biophysical properties, such as stratum corneum (SC) hydration, melanin and erythema index, are lower in vitiligo-affected skin (access the entire article here ).
However, no difference in skin surface acidity was observed, and the SC integrity was similar in involved and uninvolved areas. Barrier recovery in vitiligo-involved areas was significantly delayed compared to uninvolved areas.
What are the systemic treatment options for vitiligo?
It is difficult to find a systemic treatment for vitiligo at the moment (one that affects the whole body). Some of the commonly used systemic treatments for vitiligo include:
- Ginkgo biloba – taking 60 mg of Ginkgo biloba BID was associated with a significant improvement in total Vitiligo Area Scoring Index (VASI) and Vitiligo European Task Force (VETF) scores, but more clinical trials are needed (access the entire article here ).
- Piperine – has been suggested as a potential treatment for vitiligo, yet only a few studies have been conducted and most have been on animals (access the entire article here ).
- Cosmetic camouflage – not only conceals the depigmented patches but has been shown to improve the quality of life in patients (access the entire article here )
What are the surgical treatment options for vitiligo?
Surgical treatment options for vitiligo involve transplanting melanocytes from normally pigmented skin to the depigmented areas and are only suitable for patients with stable vitiligo. It has been proven that suspending melanocytes in the patient’s own serum (plasma in the blood) can improve the effectiveness of the transplant (access the entire article here ).
Another new procedure called ReCell involves taking a sample of normal skin, separating out the skin cells, and spraying them onto the vitiligo patches (access the entire article here). Studies comparing Recell with conventional transplantation have shown varying degrees of repigmentation, but it is not widely available in the UK (access the entire article here ).
What are effective topical treatments and light therapies for vitiligo?
Creams or ointments, known as topical immunomodulators, are usually the first line of treatment for vitiligo. Topical tacrolimus and pimecrolimus have been found to be effective for localised vitiligo. Targeted narrow-band ultraviolet B (UVB) light treatment using the Excimer laser is also known to be effective, but not widely available. Other lasers such as the Q-switched ruby laser have been shown to induce depigmentation more quickly, but with more discomfort.
To learn more about effective topical treatments and light therapies for vitiligo you can find the full articles below:
- Comparative Therapeutic Evaluation of Different Topicals and Narrow Band Ultraviolet B Therapy
- Pimecrolimus: a new choice in the treatment of vitiligo?
- Laser for treating vitiligo: a randomized study
- Treatment of vitiligo: advantages and disadvantages, indications for use and outcomes
Table of contents

FDA Approves New Vitiligo Treatment, Ruxolitinib (Opzelura)
The JAK inhibitor cream is the first medication that can restore pigment in people with this autoimmune disease.
On July 18, the U.S. Food and Drug Administration (FDA) approved ruxolitinib ( Opzelura ) cream 1.5 percent as a treatment for the most common form of vitiligo, according to a statement by Incyte, the manufacturer of the drug.
Vitiligo is a chronic autoimmune condition that causes patches of skin to lose pigment and turn milky white. The most prevalent form is nonsegmental (also known as generalized) vitiligo, in which white patches appear symmetrically on both sides of the body, such as on both hands or both knees, often covering large areas.
Ruxolitinib is the first medication that can restore pigment in patients with nonsegmental vitiligo. The FDA approved Incyte’s ruxolitinib cream for adults and children ages 12 and up.
“This approval is monumental,” says Daniel Gutierrez, MD , assistant professor of dermatology at NYU Grossman School of Medicine and dermatologist at NYU Langone Health in New York City, who was not involved in the drug development. “With Opzelura, we will have an FDA-approved pharmaceutical treatment option that can actually bring back color in patients who have vitiligo,” says Dr. Gutierrez.
He adds that prior to ruxolitinib, the only FDA-approved medication for vitiligo was monobenzyl ether of hydroquinone, a topical drug that removes pigment from skin to even out tones.
What Is Vitiligo?
Researchers estimate that between 1.9 and 2.8 million adults in the United States have vitiligo, with perhaps 40 percent of adults with vitiligo going undiagnosed.
Vitiligo causes immune cells to destroy melanocytes, the skin cells that produce pigment, according to the National Institute of Arthritis and Musculoskeletal and Skin Diseases . “This makes vitiligo much more noticeable in patients of color — people whose skin is much more richly pigmented — because there is going to be much more of a contrast between the unaffected skin and the skin affected by the vitiligo,” says Gutierrez.
Vitiligo can occur at any age, but most people experience the initial symptoms before age 30.
About 50 Percent of People Using Ruxolitinib Had Significant Repigmentation After One Year
Ruxolitinib belongs to a class of drugs called Janus kinase (JAK) inhibitors. While doctors prescribe oral JAK inhibitors for diseases such as rheumatoid arthritis, ruxolitinib is the only topical JAK inhibitor approved in the United States.
The FDA previously approved ruxolitinib for mild to moderate atopic dermatitis (eczema) , in the fall of 2021.
JAK inhibitors work by decreasing the activity of the immune system, blocking certain enzymes that cause inflammation.
Patients using ruxolitinib apply the cream twice daily to the affected areas, covering up to 10 percent of their body’s surface area. It may take 24 weeks or more for people with vitiligo to see satisfactory results, according to Incyte.
The FDA based its approval on data from a clinical trial program that compared ruxolitinib to a placebo cream in more than 600 people (age 12 and older) with nonsegmental vitiligo. Investigators used the Vitiligo Area Scoring Index (VASI), a tool used to gauge disease severity and to measure improvements in face and body repigmentation.
In the two trials, by week 24 approximately 30 percent of people treated with ruxolitinib experienced significant improvements (at least 75 percent) as measured by VASI, which was the goal of the study. At one year, about 50 percent of those using the medication achieved that level of repigmentation.
“People using Opzelura had much more improvement in their vitiligo — very meaningful — compared to the placebo,” says Gutierrez.
The most common side effects seen in the trials were application-site acne, redness and itchiness, pharynx and nasal cavity inflammation, headache, urinary tract infection, and fever.
Ruxolitinib Comes With a Black Box Warning
The FDA added a black box warning to ruxolitinib, based on data showing that people taking oral JAK inhibitors faced a small increased risk of serious infections, major heart issues, clotting (thrombosis), cancer, and even death.
“However, in the clinical trials for people using ruxolitinib as a topical cream, the concentrations of the drug found in the blood were observed to be much lower compared to people who take ruxolitinib orally,” says Gutierrez. The same risks were not observed in the ruxolitinib trials, but the FDA is taking a “better safe than sorry” approach by including a warning on the box, he adds.
A conversation with your healthcare provider is the best way to determine whether the benefits of ruxolitinib outweigh the potential risks, as well as the need for any baseline and/or ongoing monitoring.
Patients Can Use Ruxolitinib on Their Face
Although dermatologists sometimes prescribe topical steroids off-label for vitiligo, there are risks when applying these medications to the face — the area where loss of pigment can impact appearance the most, says Gutierrez.
When used on the face, topical steroids can cause an acne-like rash that can persist for many months, called perioral dermatitis . Plus, “they can cause atrophy or dispigmentation, meaning you can have skin color changes. They can also thin the skin, cause stretch marks, and cause the growth of small blood vessels in the area,” Gutierrez says.
Ruxolitinib does not pose these risks, notes Gutierrez.
FDA Approval Means Better Access to Vitiligo Treatment
The FDA’s approval of ruxolitinib will definitely improve access to the drug by validating it as medically necessary. “Because vitiligo just creates a color change in the skin — there’s no itching or dermatitis under normal circumstances — sometimes it’s considered a cosmetic condition, meaning it’s not medically necessary to treat,” Gutierrez says. As a result, some insurers have declined to cover vitiligo treatments , according to the Vitiligo Research Foundation .
“However, this condition can dramatically impact how a patient sees themselves and how they present to the world. Vitiligo can cause significant psychological distress and negatively impact quality of life,” says Gutierrez.
“Vitiligo disproportionately impacts patients of color,” he adds. “This approval is an important step in improving a health disparity that does exist, and hopefully there will be more treatment options for vitiligo in the pipeline.”
How Much Will Ruxolitinib Cost?
The current Wholesale Acquisition Cost pricing is $1,950 for a 60 gram tube of Opzelura, according to Gabriella Greig, a spokesperson for Incyte. The actual cost to the consumer will vary depending on insurance coverage and how much of the cream is required for treatment.
“Incyte is committed to working with insurance providers in the U.S. to ensure eligible patients who can benefit from Incyte’s products have access to them,” says Greig. The company offers a copay savings card on its website for people with commercial insurance.
A new treatment is restoring skin coloration to some with vitiligo. It's giving patients hope.

- Vitiligo is an autoimmune disorder that leads to the loss of skin pigmentation.
- A recent study shows a medicated cream called ruxolitinib is extremely effective in about one-third of patients.
- The cream is giving patients hope that even if they don't benefit from the treatment there will soon be others.
Sarah Hayden owns a lot of turtlenecks. She also has chunky necklaces and tons of makeup – anything to cover up the blotchy white skin on her face, neck, hands and knees.
When she was about 23, Hayden was diagnosed with vitiligo, a noncontagious skin condition that leads to the loss of pigmentation. It struck first on the back of her neck and then, as with many people, slowly progressed under her eyes and across her face, before jumping down to her knees, elbows and fingertips.
Once, when she stepped into a hot tub, a woman made a comment about how people with skin conditions shouldn't be in hot tubs and paraded herself and her daughter out. Hayden pretended it didn't matter. That she still felt beautiful on the inside.
It was only when she joined a clinical trial for an experimental drug and her vitiligo began to recede that she "realized that having the skin condition affected me more than I thought it did," said Hayden, now 41, of Hood River, Oregon.
The medicated cream she used as part of that trial, ruxolitinib, was approved by the Food and Drug Administration this summer. Late Wednesday, The New England Journal of Medicine published a study showing ruxolitinib cream is extremely effective in about one-third of patients who use it for at least six months.
What is vitiligo and its treatment?
Somewhere between half a percent and 2% of people worldwide have vitiligo, which is now understood to be an autoimmune disorder, where the immune system attacks the cells in the skin that provide pigment.
Famous people with vitiligo include Michael Jackson, model Winnie Harlow, actor and director Jon Hamm, comedian Steve Martin, commentator and comedian Joe Rogan, and NFL player-turned coach Karl Dunbar.
The condition can be particularly distressing for people with naturally dark skin because the light blotches stand out even more.
Only one treatment has previously been approved by the FDA and it removes more coloration, to avoid blotchiness, rather than restoring the skin's natural color as ruxolitinib cream can sometimes do.
Right now, many patients are treated with steroids, which don't work well, or given controlled phototherapy sessions, which can be hard to access for people who live far from a center that offers it, said Dr. David Rosmarin, a lead author on the new study.
MORE: For patients with earliest stage of breast cancer, how much treatment is enough?
How ruxolitinib works
The use of ruxolitinib cream reflects a new understanding of vitiligo, Rosmarin said. It works by tamping down an overactive immune response. "We are now better at modulating, rebalancing" that arm of the immune system, Rosmarin said.
About 30% of the 450 people who received active treatment as part of two studies saw a dramatic improvement in facial pigmentation after six months. Up to half did after a year of treatment, indicating that the cream became more potent over time. More than 80% of people in both trials were white and only 3% to 5% were Black or Asian.
Formulated as a cream ruxolitinib does not affect the whole body, so side effects are relatively minor, usually just some acne where the cream is used, Rosmarin said.
Ruxolitinib cream seems to work best on the head and neck, with hands and feet the hardest to repigment, Rosmarin said. It's not yet known whether someone can take ruxolitinib for a period of time and then stop, or whether vitiligo will return without constant dosing.
Commercial insurers and Medicare have been covering ruxolitinib cream, Rosmarin said, now that there's "broad agreement in the medical community that it is a medical not cosmetic condition and treatment should be covered." One tube of the cream costs about $2,000 and can last anywhere from a few weeks to a few months, depending on how much skin needs to be covered.
The amount of time a patient had vitiligo didn't affect their likelihood of success.
People who had poliosis vitiligo, or a total loss of pigmentation leading to pure white hairs, did not improve on the drug, said Dr. Brett King, who was not directly involved in the study but consults for Incyte, the Delaware-based company that makes ruxolitinib cream and sells it under the brand name Opzelura.
Researchers are also developing other approaches to treating vitiligo for those who don't see much improvement with ruxolitinib cream, Rosmarin said.
The study and recent approval of ruxolitinib cream "sets a pathway for other treatments to hopefully move forward," he said. "This is just the start."
RESEARCH: These rats have human cells in their brains. They may help scientists understand autism and schizophrenia.
The origins of the new approach date to 2017 when King, an associate professor of dermatology at Yale University, decided to test a rheumatoid arthritis drug in a mouse that works in the same way as ruxolitinib. It seemed to help, so he gave it to a patient who had the condition "from nose to toes," he said. Her dramatic improvement "was our first clue" that his approach might work.
"When you have an observation, but in particular one anchored in science, not just in chance observation, it leads to paradigm shifts in how we think about disease and treat disease," King said.
The success with ruxolitinib will encourage other drug companies to develop vitiligo treatments, he said.
Because it takes so long to see a benefit from ruxolitinib cream, it would be useful to find a way of distinguishing patients who will respond well from those who won't, Dr. Liv Eidsmo wrote in an editorial accompanying the new study.
Eidsmo, a dermatologist and researcher at Karolinska University Hospital in Sweden, also pointed out that it's not clear what will happen if patients stop using the cream and raised concerns about the lack of diversity among trial participants, who were largely white.
Still, she wrote, thanks to ruxolitinib, "patients with vitiligo finally have the hope of efficient treatments."
WHAT DOCS WANT YOU TO KNOW: Study raises questions about colonoscopies
'It gave me back something I lost'
Hayden is one of those lucky patients.
The cream has returned about 90% of the pigment to her face. Even her hands, considered the hardest to reach for the drug, have mostly cleared up.
"It gave me back something I lost," said Hayden, who was wearing a V-neck shirt and a thin gold necklace on a recent video call. On weekends, she no longer insists on wearing makeup before leaving the house.
An instructional coach in her town's school district, Hayden had almost no side effects from the cream and didn't find it a burden to spread on her face, chest and hands twice a day during the two-year trial. "It was just part of my morning routine," she said, "and it was part of my bedtime routine after I brushed my teeth."
The treatment changed her relationship with the sun. Before, she would wear high SPF sunblock, a hat and long sleeves if she was in the sun for long periods. With vitiligo, she said "you are either normal or burnt," there was no such thing as a tan.
During the trial, she said, she was encouraged to get limited amounts of sun exposure to promote skin regeneration. "Now, I feel like when I'm in the sunlight, it's regenerative instead of harmful," she said. "I have to be careful, but it's not the same burden as it was."
Hayden said she's grateful to have been able to participate in the research to help others avoid some of the same challenges she's faced.
"That really kind of fueled my 'why' for engaging in the trial," she said. "Not just for my own hope and excitement – which it did 100% and I would do it again in a heartbeat – but also potentially having this available to other people living with vitiligo."
Contact Karen Weintraub at [email protected].
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 30 June 2020
- Correction 06 July 2020
Temprian Therapeutics: developing a gene-based treatment for vitiligo
- Charles Schmidt 0
Charles Schmidt is a freelance writer in Portland, Maine.
You can also search for this author in PubMed Google Scholar
Temprian Therapeutics is a spin-off from Northwestern University in Chicago, Illinois.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-020-01808-5
This article is part of Nature Outlook: The Spinoff Prize 2020 , an editorially independent supplement produced with the financial support of third parties. About this content .
Updates & Corrections
Correction 06 July 2020 : An earlier version of this profile gave the wrong specialty for Caroline Le Poole and the wrong campus for Northwestern University.
Mosenson, J. A. et al. Sci. Transl. Med. 5 , 174ra28 (2013).
Article PubMed Google Scholar
Henning, S. W. et al. J. Invest. Dermatol. 138 , 2531–2539 (2018).
Download references
Related Articles

- Biotechnology
- Drug discovery
- Therapeutics
Vaccine-enhancing plant extract could be mass produced in yeast
News & Views 08 MAY 24

Computationally restoring the potency of a clinical antibody against Omicron
Article 08 MAY 24
Complete biosynthesis of QS-21 in engineered yeast
Accurate structure prediction of biomolecular interactions with AlphaFold 3

Major AlphaFold upgrade offers boost for drug discovery
News 08 MAY 24

Discovery of potent small-molecule inhibitors of lipoprotein(a) formation
Airway hillocks are injury-resistant reservoirs of unique plastic stem cells
Article 01 MAY 24
Cells destroy donated mitochondria to build blood vessels
News & Views 01 MAY 24
Rat neurons repair mouse brains — and restore sense of smell
News 25 APR 24
Recruitment of Principal Investigators by the School of Life Sciences, Peking University
The School of Life Sciences at Peking University is actively seeking talents, with an emphasis on bioinformatics/computational biology/AI/RNA biology.
Beijing (CN)
School of Life Sciences, Peking University
2024 Recruitment notice Shenzhen Institute of Synthetic Biology: Shenzhen, China
The wide-ranging expertise drawing from technical, engineering or science professions...
Shenzhen,China
Shenzhen Institute of Synthetic Biology
Head of Operational Excellence
In this key position, you’ll be responsible for ensuring efficiency and quality in journal workflows through continuous improvement and innovation.
United States (US) - Remote
American Physical Society
Rowland Fellowship
The Rowland Institute at Harvard seeks outstanding early-career experimentalists in all fields of science and engineering.
Cambridge, Massachusetts
Rowland Institute at Harvard
Postdoctoral Fellowship: Chemical and Cell Biology
The 2-year fellowship within a project that will combine biochemical, cell biological and chemical genetic approaches to elucidate migrasome biology
Umeå, Sweden
Umeå University
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Worldwide expert recommendations for the diagnosis and management of vitiligo: Position statement from the international Vitiligo Task Force-Part 2: Specific treatment recommendations
Affiliations.
- 1 Department of Dermatology and Pediatric Dermatology, National Reference Center for Rare Skin Disorders, Hospital Saint-André, ImmunoConcept, CNRS UMR 5164, Bordeaux University, Bordeaux, France.
- 2 Department of Dermatology, Ghent University Hospital, Ghent, Belgium.
- 3 Department of Dermatology and Pediatric Dermatology, National Reference Center for Rare Skin Disorders, Hospital Saint-André, BRIC, UMR 1312, Inserm, University Bordeaux, Bordeaux, France.
- 4 Department of Dermatology, Netherlands Institute for Pigment Disorders, Amsterdam University Medical Centers, Amsterdam Institute for Infection and Immunity, University of Amsterdam, Amsterdam, The Netherlands.
- 5 Department of Dermatology, University Hospital of Nice, Nice, France.
- 6 Department of Dermatology, The University of Texas Southwestern Medical Center, Dallas, Texas, USA.
- 7 Department of Dermatology, Henry Ford Health, Detroit, Michigan, USA.
- 8 Department of Dermatology, University Hospital Henri Mondor, EpiDermE EA 7379, Université Paris-Est Créteil Val de Marne, Créteil, France.
- 9 Department of Dermatology and Skin Science, University of British Columbia, Vancouver, British Columbia, Canada.
- 10 Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China.
- 11 Skin Research Institute of Singapore, ASTAR, Singapore, Singapore.
- 12 Department of Dermatology Integrated Medicine, Osaka University Graduate School of Medicine, Osaka, Japan.
- 13 Department of Dermatology, Yamagata University Faculty of Medicine, Yamagata, Japan.
- 14 Department of Dermatology, Tufts University School of Medicine, Boston, Massachusetts, USA.
- 15 Chroma Dermatology, Pigment and Skin of Colour Centre, Wheelers Hill, Victoria, Australia.
- 16 Department of Dermatology, The Royal Children's Hospital, Parkville, Victoria, Australia.
- 17 Department of Dermatology, Westville Hospital, Durban, South Africa.
- 18 Deutscher Vitiligo-Bund e.V., Adelsdorf, Germany.
- 19 Department of Dermatology, Venereology & Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- 20 Department of Dermatology, Kindai University Nara Hospital, Ikoma, Japan.
- 21 Vitiligo.nl, The Hague, The Netherlands.
- 22 Vitiligo International Patient Organisations, Paris, France.
- 23 Association Française du Vitiligo, Paris, France.
- 24 Department of Dermatology, Microbiology and Immunology, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, Illinois, USA.
- 25 Photodermatology and Vitiligo Treatment Unit, Israelite Hospital, Roma.
- 26 Department of Dermatology, College of Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 27 Department of Dermatology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
- 28 Pigmentation Research and Therapeutics, Osaka Metropolitan University, Osaka, Japan.
- 29 Department of Dermatology, Severance Hospital, Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, South Korea.
- 30 Department of Dermatology, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
- 31 Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India.
- 32 Vitiligo & Pigmentation Institute of Southern California, Los Angeles, California, USA.
- 33 Skin Physicians Pte Ltd, Singapore, Singapore.
- 34 Department of Dermatology, Jaslok Hospital and Research Hospital and South Mumbai Dermatology Clinic, Mumbai, India.
- 35 Department of Dermatology, Faculty of Medicine Cairo University, Cairo, Egypt.
- 36 New Cross Hospital, The Royal Wolverhampton NHS Trust, Wolverhampton, UK.
- 37 Department of Dermatology, University Hospital Münster, Münster, Germany.
- 38 Department of Dermatology, Mohammed V University, Ibn Sina University Hospital, Rabat, Morocco.
- 39 Department of Dermatology, Netherlands Institute for Pigment Disorders, Amsterdam University Medical Centers, Amsterdam Institute for Infection and Immunity, VU University, Amsterdam, The Netherlands.
- 40 Department of Dermatology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea.
- 41 Department of Dermatology, Clinica Dermatologica Moragas, Barcelona, Spain.
- 42 Dermatology, Andrology, and Venereology Department, Ain Shams University, Cairo, Egypt.
- 43 Unicamillus International University, Rome, Italy.
- PMID: 37715487
- DOI: 10.1111/jdv.19450
Background: The treatment of vitiligo can be challenging. Up-to-date agreed consensus recommendations on the use of topical and systemic therapies to facilitate the clinical management of vitiligo are currently lacking.
Objectives: To develop internationally agreed-upon expert-based recommendations for the treatment of vitiligo.
Methods: In this consensus statement, a consortium of 42 international vitiligo experts and four patient representatives participated in different online and live meetings to develop a consensus management strategy for vitiligo. At least two vitiligo experts summarized the evidence for different topics included in the algorithms. A survey was then given to a core group of eight experts to resolve the remaining issues. Subsequently, the recommendations were finalized and validated based on further input from the entire group during two live meetings.
Results: The recommendations provided summarize the latest evidence regarding the use of topical therapies (steroids, calcineurin inhibitors and Jak-inhibitors) and systemic therapies, including steroids and other systemic immunomodulating or antioxidant agents. The different modalities of phototherapies (NB-UVB, photochemotherapy, excimer devices and home phototherapy), which are often combined with other therapies, are also summarized. Interventional approaches as well as depigmentation strategies are presented for specific indications. Finally, the status of innovative and targeted therapies under development is discussed.
Conclusions: This international consensus statement culminated in expert-based clinical practice recommendations for the treatment of vitiligo. The development of new therapies is ongoing in vitiligo, and this will likely improve the future management of vitiligo, a disease that still has many unmet needs.
© 2023 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
- Combined Modality Therapy
- Photochemotherapy*
- Phototherapy
- Steroids / therapeutic use
- Treatment Outcome
- Ultraviolet Therapy*
- Vitiligo* / drug therapy
- Vitiligo* / therapy
- 2024-05-07 Understanding the Timeline for Vitiligo Treatment Treatment times can vary widely due to factors like health, genetic makeup, and where the spots appear. Here's what you need to know: Early Treatment is Key: Catching new spots within the first 6-8 weeks can dramatically improve outcomes. Loca...
- 2024-05-07 Update: Vitiligo Insurance Coverage Navigating the insurance maze for vitiligo treatments can be as unpredictable as the condition itself. Vitiligo, often misunderstood as merely a cosmetic issue, is in fact a serious autoimmune disease with significant emotional and physical implic...
- 2024-04-18 Beyond the Surface: New Frontiers in Dermatology Explored in Iași The Iași Dermatological Spring (PDI) conference is a cornerstone event for Europe's dermatology community. This year, it is set against the elegant backdrop of Hotel Internațional from April 15-19. Under the skilled leadership of Prof. Dr. Dacian...
- 2024-04-14 Impact of Diet and Nutrition on Vitiligo Recent research underscores the potential of diet and nutrition in managing vitiligo, particularly as some traditional therapies focusing solely on its autoimmune aspects may fall short. Dietary supplements and herbal adjuncts are emerging as prom...
- 2024-04-05 Vitiligo Lifeline: Free Opzelura VRF is proud to highlight a new patient support initiative launched by Incyte, named Opzelura On Trac. This program is designed for U.S. patients diagnosed with vitiligo who are prescribed Opzelura, providing essential support for those navigating...
- 2024-04-04 MORE THAN OUR SKIN Wins LA Film Fest We're ecstatic to announce that More Than Our Skin, crafted by Tonia Magras—a three-time Emmy-award winner with over three decades of film and production experience—has been honored as the Best Documentary Feature at the 2024 Los Angeles Film Awar...
- 2024-04-02 Vitiligo: A Silent Struggle A recent study "Burden of Disease and Treatment Patterns Amongst Patients With Vitiligo: Findings From a National, Longitudinal Retrospective Study in the United Kingdom" has shone a light on vitiligo. It found that in 2021, about 0.38% of people...
- 2024-03-23 France Approves Compassionate Vitiligo Treatment with Protopic On March 21, 2024, French health authorities granted special permission for doctors to prescribe Protopic 0.1% (Tacrolimus) for treating vitiligo in adults and children over 2 years old. This kind of permission is given when there's already a diff...
- 2024-03-18 🇨🇴 World Vitiligo Day 2024 Lands in Cali, Colombia The World Vitiligo Day (WVD) Committee proudly announces the Colombia as the host for WVD 2024. This momentous event will be held under the esteemed honorary presidency of Dr. Rafael Falabella, a distinguished figure in dermatology and skincare re...
- 2024-03-15 New Expert Recommendations on Pediatric Vitiligo Care The latest consensus statement released in JAMA Dermatology on March 13, 2024, sets a new standard in the USA for vitiligo treatment among children, teenagers, and young adults. This pioneering guideline, led by Drs. Yael Renert-Yuval and Nanette ...
- 2024-03-13 Lessons In Marketing: How To Sell A Disease To Big Pharma We've actually done it! 🤘Seeing Incyte's "See What's Possible" campaign featuring huge posters with vitiligo models at AAD congress in San Diego was a revelation. Vitiligo, once flying under the radar, has grabbed the healthcare industry by storm...
- 2024-03-12 Join Our Vitiligo Study: Limited Spots, Earn Up to $130! The SurveyEngine, in collaboration with VR Foundation, is conducting a comprehensive research study. Our objective is to understand the varied experiences and treatment expectations of individuals living with vitiligo across different regions, sta...
- 2024-03-08 Vitiligo City in San Diego After the enlightening IMCAS congress in Paris, it's time to dive into the vitiligo ventures on this side of the Atlantic. Landed in San Diego for the AAD 2024 Congress, and it's Vitiligo City! Everywhere you look, "See What’s Possible" banners fe...
- 2024-02-28 Economic Burden Among Patients With Vitiligo in the United States This study, conducted in the United States, evaluates the financial and healthcare resource utilization (HCRU) impact of vitiligo by comparing patients with the condition to those without it. Utilizing data from the Merative MarketScan Commercial ...
- 2024-02-28 AI-News On Vitiligo. All Episodes
- 2024-02-24 Recoloring Vitiligo With AVITA's Spray-On AVITA Medical took a moment during their latest earnings call to update us on their vitiligo treatment efforts. They've wrapped up enrolling patients for their TONE study, which looks into how their treatments are making life better for people wit...
- 2024-02-13 Alys Pharmaceuticals: A New Kid on the Vitiligo Block Alys Pharmaceuticals emerges as a new player in dermatology, pooling the strengths of six biotech startups with a $100M boost from Medicxi. Targeting conditions from vitiligo to psoriasis, Alys' launch heralds cautious optimism for skin treatment ...
- 2024-02-13 📌 Vitiligo Drug Pipeline Analysis and Market Insights UPDATED with recent developments from Alys Pharmeceuticals on February 13, 2024. Our exclusive analysis will help you understand a higly competitive environment for vitiligo therapies, the key biotech and pharma companies involved in vitiligo dr...
- 2024-02-09 AI-News On Vitiligo In the dynamic realm of vitiligo awareness and research, the Vitiligo Research Foundation (VRF) has been subtly yet significantly influencing the field. Eschewing grandiose declarations typical of innovation announcements, VRF has embarked on a jo...
- 2024-02-04 Vitiligo Treatment News at IMCAS 2024 The IMCAS Congress in Paris recently emerged as a pivotal gathering for nearly 20,000 skin specialists worldwide. Thanks to an educational grant from IMCAS and a sponsored booth (K203), Prof. Torello Lotti and I engaged directly with attendees in...
- 2024-01-28 Vitiligo Patient Journey Map Imagine the journey of someone with vitiligo: beginning with a small, innocuous white spot and evolving into a complex, sometimes years-long journey of diagnosis and treatment. This winding path is marked by numerous crossroads and pitstops, yet i...
- 2024-01-25 Association Between Vitiligo Onset and COVID Vaccination A recent study, published in Cureus, investigates the potential association between COVID-19 vaccination and the onset of vitiligo, including a case study and a systematic literature review. Vitiligo, typically a sporadic condition influenced by ...
- 2023-12-10 Use of AI in Patient Education In this enlightening presentation from the Revolutionizing Vitiligo (ReV) Virtual Conference 2023, Yan Valle, VRF CEO, delves into the transformative role of Artificial Intelligence in patient education and its profound impact on doctor-patient re...
- 2023-12-07 Revolutionizing Vitiligo (ReV) Virtual Conference We are thrilled to announce the second Revolutionizing Vitiligo (ReV) Conference, scheduled for Sunday, December 10, 2023. This virtual, CME-accredited conference is dedicated to vitiligo, focusing on diagnosis, treatment, and management. AGENDA ...
- 2023-12-01 UPDATED: World Vitiligo Day 2024 UPDATE: Dear Community, We would like to inform you that the headquarters for World Vitiligo Day 2024 have been relocated from Bayahibe, Dominican Republic, to Cali, Colombia. This decision was made with the safety and security of all participa...
FAQ Other Questions
Contrary to popular belief, vitiligo is not a cosmetic disorder but a systemic disease affecting the largest body organ and other vital systems, with multiple comorbidities. Fo...
Traditional medicines may be helpful in chronic, metabolic, and stress-related conditions early in the disease manifestation, before extensive tissue and organ damage has occurr...
Extracts of the tropical fern Polypodium leucotomos appear to have beneficial properties for the vitiligious skin. Polypodium leucotomos (also classified as Polypodium aureum) a...
Donate Today
Our work is entirely funded by private donations – we receive no money from government. Your money will help us continue funding research into vitiligo and supporting people affected by the condition.
Though it is not always easy to treat vitiligo, there is much to be gained by clearly understanding the diagnosis, the future implications, treatment options and their outcomes.
Many people deal with vitiligo while remaining in the public eye, maintaining a positive outlook, and having a successful career.
By taking a little time to fill in the anonymous questionnaire, you can help researchers better understand and fight vitiligo.
Project Background
The study of multifactorial diseases, such as vitiligo, requires analysis of complex interplay of symptoms, treatments and outcomes across a large number of people. Population surveys and biobanks are indispensable research tools, required for downstream therapy development. Even small collections of biosamples may be extremely precious for researcher in academic institution or biopharma company.
Until recently, vitiligo researchers were generally limited to conducting studies on patient samples they could acquire themselves. When the Foundation started there were no centralized biological database along with the pre-existing body of the clinical management or the historical study data, which is required in order to proceed with the development of specific therapies. We have run a special investigation study to determine whether VRF shall establish its own biobank.
Then the project's leadership crafted a careful strategy for vitiligo biobank development, with special attention paid to the security and confidentiality of the donor's information. 'Future proofing' involves collecting and processing samples to permit the widest possible range of scientific uses, while avoiding approaches that would impede possible future uses.
We have started the first Vitiligo Biobank with a 100+ sample collection from the completed research project in genetics in late January 2013. Three months later, it held approximately 1,000 biosamples and detailed clinical profiles. Our target number is 10,000 samples and we encourage patients to donate samples . The primary biorepository is located in Moscow (Russia) with networked locations in 11 countries.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
January 31, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study reveals the economic burden for patients with vitiligo in the US is significant
by Elsevier

A novel study in the Journal of Investigative Dermatology , shows that patients with vitiligo incur significantly higher health care costs than people without this skin condition. The findings reveal an unmet need for cost-effective treatments and highlight the importance of fully identifying the drivers of economic burden for patients with vitiligo.
Lead investigator Khaled Ezzedine, MD, Ph.D., Department of Dermatology, AP-HP, Henri Mondor University Hospital, and Epidemiology in Dermatology and Evaluation of Therapeutics (EpiDermE)—EA 7379, Université Paris-Est Créteil (UPEC), explains, "Data regarding the economic burden of vitiligo are scarce and outdated. Our study quantifies the health care costs and health care resource utilization (HCRU) among patients with vitiligo. Determining medical costs will help identify the main expenditure predictors and spending patterns."
Vitiligo, a chronic autoimmune disorder that affects 0.5-2.0% of the United States population, is characterized by skin depigmentation caused by the loss of melanocytes. Patients with vitiligo incur direct costs associated with their condition through medical fees, pharmacy expenses, and out-of-pocket costs (e.g., sunscreens, protective clothing, cosmetic concealers, and camouflage products). They may also experience indirect costs owing to psychosocial effects, loss of work productivity, and lost opportunities (e.g., marriage, career choice, promotions, salary increases, or education).
Dr. Ezzedine continues, "Patients with vitiligo are often reported to have psychological problems , such as depression, anxiety, and shame, leading to low self-esteem and social isolation. Higher costs for patients with vitiligo than for persons without it may partly be explained by a higher risk of mental health conditions as well as other comorbidities among patients with vitiligo, including thyroid disease, diabetes, and alopecia areata that impact the cost of the disease."
For this retrospective cohort analysis, the Merative MarketScan Commercial Database, health care costs, and HCRU were evaluated for 49,512 patients with vitiligo compared to 99,024 people without vitiligo in the US between January 2007 and December 2021.
Outcomes included all-cause and vitiligo-related costs (2021 dollars) and all-cause HCRU, including mental health-related HCRU. Patients with vitiligo incurred significantly higher all-cause costs ($15,551 vs. $7,735) and vitiligo-related costs ($3,490 vs. $54) costs than controls. Mental health-related HCRU was also significantly higher among patients with vitiligo. Taken together, health care costs and HCRU were significantly higher among patients with vitiligo than among controls.
In this analysis, the increased costs were associated with significantly higher inpatient costs, ER visits, ambulatory visits, number of prescriptions and prescriptions costs, and other costs (e.g., medical equipment and home health care ), illustrating the importance of independently evaluating the economic burden of different skin conditions. The results from this study show that the economic burden of vitiligo was comparable with those of other well-studied dermatologic conditions, such as atopic dermatitis and psoriasis.
This study shows that the increased health care costs for patients with vitiligo versus those of non-vitiligo controls were driven by medical costs rather than pharmacy costs, and the increased HCRU was primarily the result of outpatient visits compared with inpatient or ER visits, which aligns with the main cost drivers identified in studies of the economic burden of atopic dermatitis and psoriasis.
Dr. Ezzedine concludes, "The health care costs and HCRU for patients in the US with vitiligo in this study were significantly higher than for patients without a vitiligo diagnosis. The economic burden was markedly higher for patients receiving treatment with systemic effects or with new mental health diagnoses than for the total vitiligo population. These findings reveal an unmet need for cost-effective treatments and highlight the importance of fully identifying the drivers of economic burden for patients with vitiligo."
Explore further
Feedback to editors

Artificial intelligence tool detects sex-related differences in brain structure

Over 20,000 people join UK search for new dementia treatments

Clinical trial: New drug makes exercise, everyday tasks easier for people with common heart condition
9 hours ago

Semaglutide can yield weight loss, lower heart issues for at least four years in non-diabetic adults with overweight
12 hours ago

Study reveals key role of glutamate tRNA fragments in brain aging and Alzheimer's disease
13 hours ago

New molecule mimics the anti-clotting action of blood-sucking organisms

Axons in female mammal brains may be more prone to concussions

Herpes cure with gene editing makes progress in laboratory studies

Breast cancer risk variants identified for women of African ancestry
14 hours ago

Variations in telomere lengthening genes may predispose some people to papillary thyroid cancer
Related stories.
Risk for autoimmunity up with concomitant vitiligo, psoriasis
May 26, 2021

Reduced mortality risk seen for patients with vitiligo
Oct 1, 2023
Risk for vitiligo increased for transplant recipients
Dec 21, 2023

Half of patients with newly diagnosed vitiligo do not receive treatment
Oct 17, 2023

New evidence indicates vitiligo-associated autoimmunity may contribute to reduced morbidity and mortality risk
Sep 19, 2023
Limited economic evidence for vitiligo treatments
Aug 17, 2017
Recommended for you

New synthetic biomarker technology differentiates between prior Zika and dengue infections
15 hours ago

Probability of developing Lyme disease is genetically influenced, research suggests
19 hours ago

Commonly used antibiotic brings more complications, death in the sickest patients

Team studies factors related to a sense of economic insecurity in older adults
18 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

- General Dermatology
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Psoriatic Arthritis
- Atopic Dermatitis
- Skin Cancer
- Hidradenitis Suppurativa
- Pigmentary Disorders
- Pediatric Dermatology
- Practice Management
Vitiligo’s Emerging Drug Pipeline
Ruxolitinib cream 1.5% may soon be the first FDA-approved treatment for repigmentation of vitiligo. Experts share what dermatologists need to know regarding efficacy, safety, and cost of this topical drug.
Vitiligo’s cause is unknown, but its detrimental effect on patients’ quality of life is clear. 1 Medications for this skin disease may work slower than patients anticipate, causing them to feel hopeless, according to Pearl E. Grimes, MD, FAAD, a clinical professor of dermatology at the David Geffen School of Medicine at UCLA and founder and director of the Vitiligo and Pigmentation Institute of Southern California in Los Angeles.
Discouraged patients may give up and quit treatment, creating tension in their relationship with their physician, Grimes continued. “So many patients that I see come in with the mindset that there is going to be a quick fix for vitiligo. When they don’t see changes in perhaps 2 weeks or 4 weeks, they abandon treatment,” she said.
But the future—possibly the very near future—holds promise. Treatments in the pipeline offer quicker results and a relatively safe profile; there’s even hope of one day finding a cure for this disease, said Seemal Desai, MD, FAAD, an assistant professor in the Department of Dermatology at UT Southwestern and founder and director of Innovative Dermatology in Dallas, Texas. But meanwhile, unmet needs remain, Grimes and Desai agreed.
The drugs’ different mechanism of action will tackle inflammation, perhaps by targeting inflammatory cytokine pathways and increasing production of melanocytes, Desai said. “I think the mechanism [of action] helps to explain and [helps us] better understand the pathophysiology of the disease,” he said. Approval of the first such treatment for vitiligo may be imminent: Ruxolitinib cream 1.5% (Opzelura; Incyte), a topical Janise kinase (JAK) inhibitor, initially had a Prescription Drug User Fee Act (PDUFA) date of April 18, 2022, but has since been extended 3 months to July 18, 2022. 2,3
Patients who have active unstable disease or chronic and refractory disease or have head and neck involvement are ideal candidates for the new medications. Desai emphasized that these treatments, especially JAK inhibitors, are meant for specific disease states. “These aren’t going to be the type of product in...a 1-pound jar that patients can slather [on from] head to toe,” he said. Instead, they are meant to be used in conjunction with other therapies such as oral antioxidants, systemic steroids, and narrow-band UV-B for targeted phototherapy. Grimes agreed with Desai’s comments, adding that she does not think ruxolitinib 1.5% cream will replace current treatments, which, when used in combination—particularly for lesions on the face and neck—do work.
The issue, she continued, is how the role of existing treatments might change. “My guess is that we will use these drugs as a primary and as a secondary approach,” Grimes said. Other treatments on the horizon are IL-15 and CXCL10 blockades and other more selective JAK inhibitors, such as TYK and TEC inhibitors, Desai said. 4,5
The broadening treatment horizon raises a burning question: Will these treatments be affordable and accessible? Grimes said the drugs have the potential to be expensive and, if not covered by insurance, cost prohibitive. Many physicians will have to work with insurance carriers to help patients get ruxolitinib covered, she said.
Grimes is hopeful that vitiligo will soon have a robust armamentarium, mirroring that of psoriasis, and shares Desai’s hope of a cure.
For more details, visit dermatologytimes. com, where Grimes and Desai discuss onboarding patients, the risks and rewards of treatments, safety signals to heed when beginning a new regimen, and what the latest in research.
References:
- Vitiligo. American Osteopathic College of Dermatology. Accessed April 7, 2022. https://www.aocd.org/page/Vitiligo
- Incyte announces acceptance and priority review of sNDA for ruxolitinib cream (Opzelura) as a treatment for patients with vitiligo. Incyte. Accessed Decem- ber 15, 2021. https://investor.incyte.com/press-releases/press-releases/2021/ Incyte-Announces-Acceptance-and-Priority-Review-of-sNDA-for-Ruxolitinib- Cream-Opzelura-as-a-Treatment-for-Patients-with-Vitiligo/default.aspx
- FDA extends review of Opzelura for skin condition. Formulary Watch®. March 14, 2022. Accessed April 19, 2022. https://www.formularywatch.com/view/ fda-extends-review-of-opzelura-for-skin-condition
- Evaluation of Amg 714 for Vitiligo (REVEAL). ClinicalTrials.gov. Updated November 1, 2021. Accessed April 6, 2022. https://clinicaltrials.gov/ct2/show/ NCT04338581
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6(223):223ra23. doi:10.1126/scitranslmed.3007811

VYNE Therapeutics Provides Updates on VYN201 for Nonsegmental Vitiligo and VYN202 for Inflammatory Diseases

ReV Up Your Vitiligo Treatment Strategies
Patients With Vitiligo Have Decreased Risk of Subsequent Pulmonary Embolism, Peripheral Vascular Disease

The Cutaneous Connection: Deep Dive Into the World of Vitiligo
Study Becomes First to Demonstrate Efficacy of Topical Isoniazid in Melasma

Fire Needle Combined With Excimer Laser Safe, Effective Therapy for Vitiligo, Review Finds
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

MINI REVIEW article
Advances in vitiligo: update on therapeutic targets.

- Department of Dermatology, Jiangsu Province People’s Hospital and Nanjing Medical University First Affiliated Hospital, Nanjing, China
Vitiligo, whose treatment remains a serious concern and challenge, is an autoimmune skin disease characterized by patches of depigmentation. The increasing application of molecular-targeted therapy in skin diseases, such as psoriasis and systemic lupus erythematosus, has dramatically improved their condition. Besides, there is a favorable effect of repigmentation in the treatment of the above diseases combined with vitiligo, implying that molecular-targeted therapy may also have utility in vitiligo treatment. Recently, the role of cytokine and signaling pathways in vitiligo pathogenesis are increasingly recognized. Thus, investigations are underway targeting the molecules described above. In this paper, we present a synopsis of current practices in vitiligo treatment and introduce the improvement in identifying new molecular targets and applying molecular-targeted therapies, including those under development in vitiligo treatment, providing valuable insight into establishing further precision medicine for vitiligo patients.
1 Introduction
Vitiligo is a primary, circumscribed, or generalized depigmentation of the skin and mucosa, related to genetic factors, self-destruction of melanocytes, cytokines, autoimmunity, and oxidative stress ( 1 ). While the detailed molecular mechanisms still require further investigation. In recent years, various studies have showed that the IFN-γ-CXCL9/10-CXCR3 axis appears to be important in vitiligo, via inhibiting melanogenesis, inducing apoptosis of melanocytes, and further recruiting T cells to the skin. These are all involved in the JAK/STAT pathway. In addition, cytokine, including HSP70i, IL-15, IL-17/23, TNF as well as wnt signaling pathway, Tregs, miRNAs have also been proved to be involved in the pathogenesis of vitiligo.
Vitiligo can be treated by different modalities of phototherapy, surgical procedures, and topical therapies, such as glucocorticosteroids, immunosuppressive agents, calcineurin inhibitors, and vitamin D. However, current treatments for vitiligo remain suboptimal, which may not be equally effective in all vitiligo patients, and it would be inconvenient for patients to visit clinics for phototherapy. Targeted therapies, such as biologics targeting cytokines and small-molecule inhibitors targeting intracellular signaling molecules, are recently emerging as promising therapeutics for autoimmune diseases. Their applications also promote our understanding of the detailed molecular mechanism of vitiligo and are essential for guiding a more precise vitiligo treatment. In this article, details of the roles that related cytokines and pathways play as well as the efficacy of targeted therapy have been described.
2 Current treatment
Topical, systemic treatment, and phototherapy are useful for stabilization and repigmentation of vitiligo. Treatment modalities are chosen in the individual patient, based on disease severity, disease activity (stable versus progressive disease), patient preference (including cost and accessibility), and response evaluation. For rapidly progressive disease, low-dose oral glucocorticoids and phototherapy are useful in stabilizing the disease. Therapeutic options for stable, segmental vitiligo include topical therapies (eg, topical corticosteroids, topical calcineurin inhibitors), targeted phototherapy, and surgical therapy (tissue grafts and cellular grafts) ( Table 1 ) ( 14 ). In recent years, attempts have been made to improve the repigmentation of vitiligo phototherapy by combination therapies, including NB-UVB with glucocorticoids ( 15 ), and topical calcineurin inhibitors ( 16 ). While their positive results were not confirmed in all studies. However, the method of treatment described, which were nonspecific, general, off-label, non-targeted with modest efficacy led to the problem of recurrence after stopping treatment. Therefore, efforts should be made to achieve a more comprehensive understanding of vitiligo pathogenesis to develop novel effective therapies ( Table 2 ).
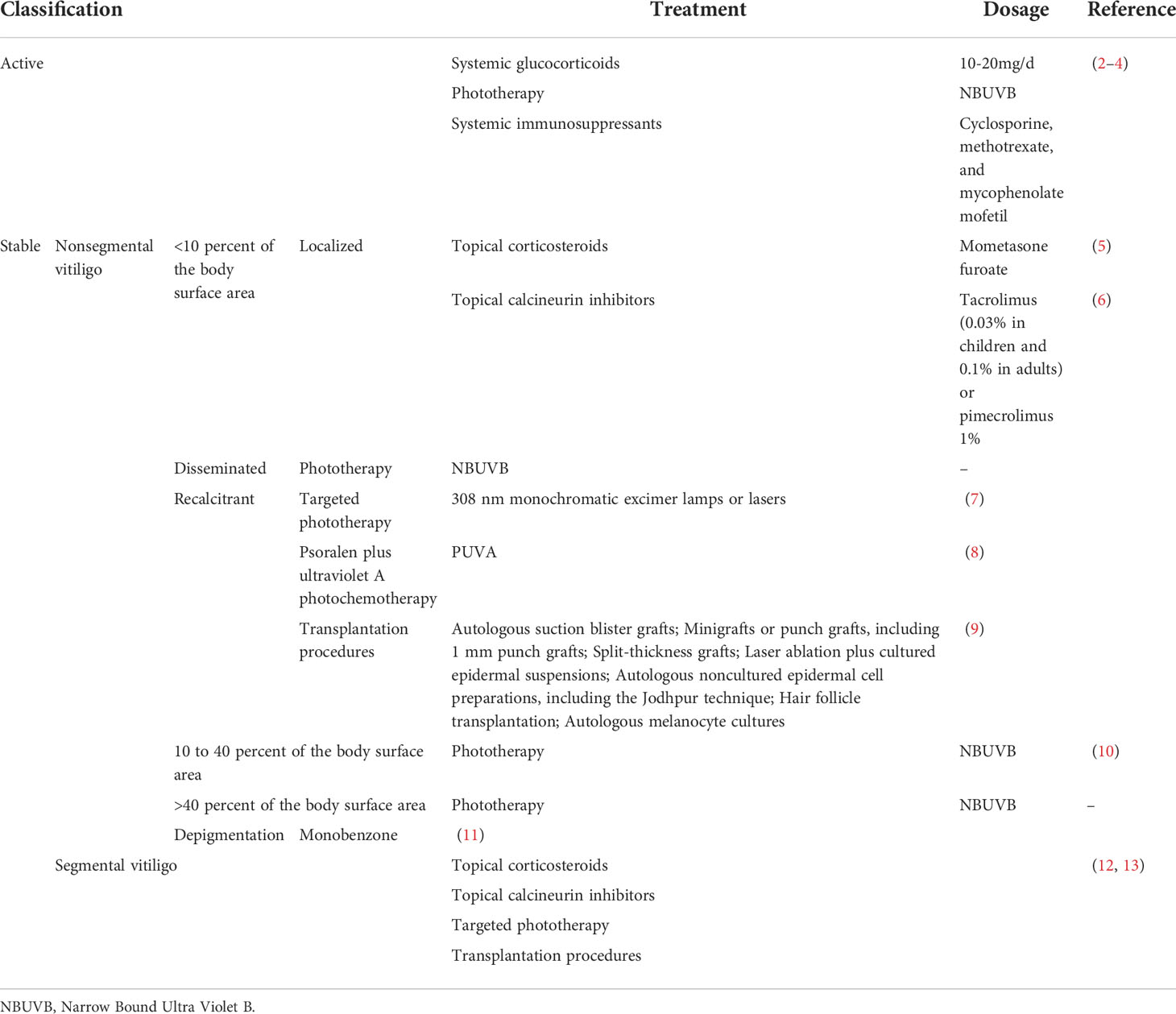
Table 1 Current treatment modalities for vitiligo.
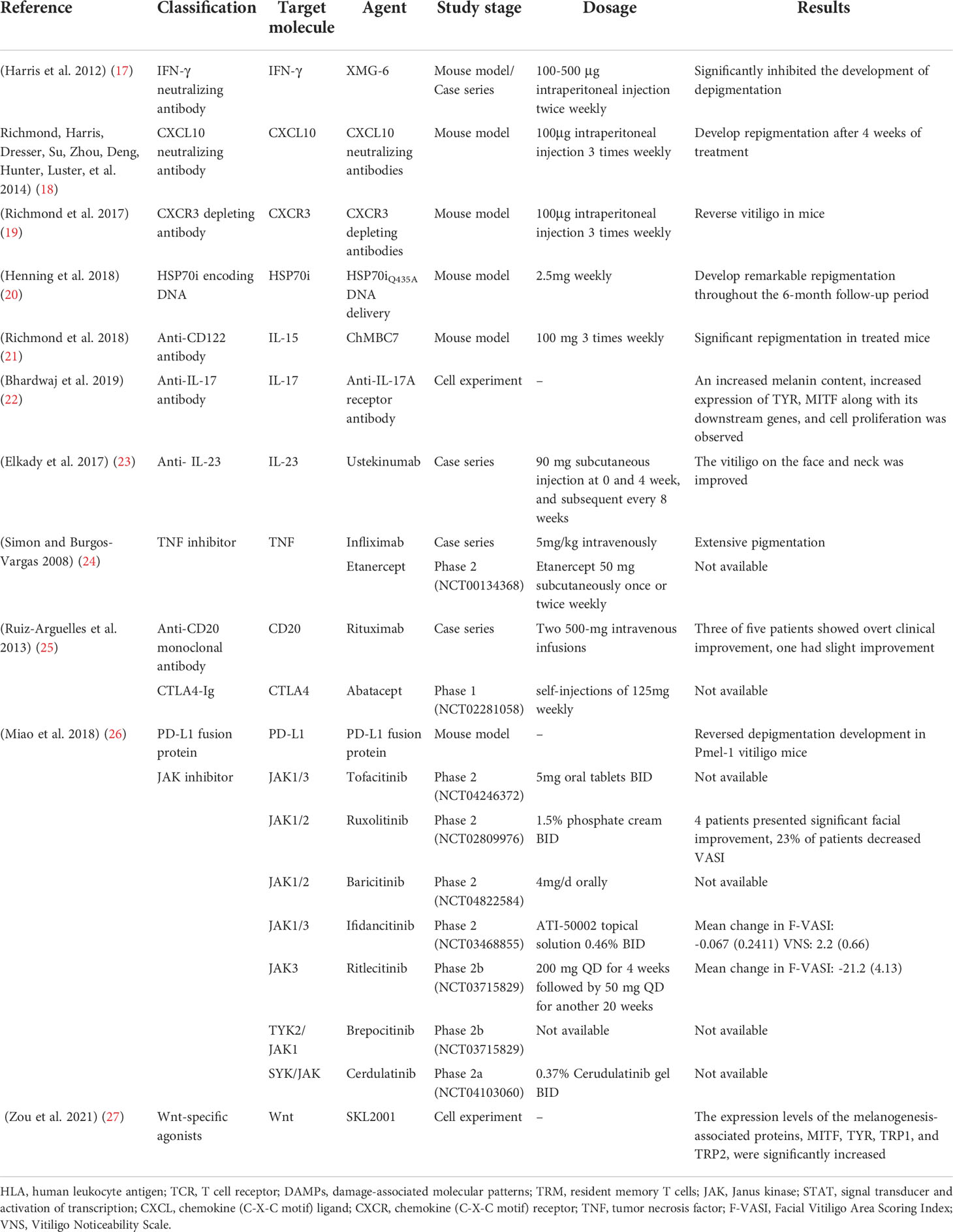
Table 2 Molecular-targeted therapies for the treatment of vitiligo.
3 Small molecules
3.1 emerging therapeutics targeting janus-activated kinase (jak) signaling.
The Janus kinases family consists of JAK1, JAK2, JAK3, and TYK2, which is engaged in the important JAK/STAT pathway, exhibiting pleiotropic effects on transducing multiple extracellular signals involved in regulating proliferative signaling, differentiation, migration, and apoptotic properties ( 28 ).
There are no licensed JAK/STAT inhibitors available against dermatological problems, however, some of them (ruxolitinib and tofacitinib) are used to treat other conditions such as myelofibrosis and RA. However, off-label usage of these medications in the treatment of vitiligo has shown promising outcomes.
JAK-STAT inhibitors promote Sonic Hedgehog and Wnt signaling in epidermal pigmentation, with the former inducing the migration, proliferation, and differentiation of melanocyte ( 29 ). Expanding our knowledge of these medications’ efficacy and safety profiles, as well as their use in dermatological conditions, is critical for establishing their risk-benefit ratio.
3.1.1 Tofacitinib
Tofacitinib is an FDA-cleared JAK1/3 inhibitor for treating RA, PsA, and active ulcerative colitis.
Tofacitinib 5-10 mg QD/BID has demonstrated superior efficacy against vitiligo, with improvement ratios of 5.4% in 5/10 patients with sun-exposed areas or areas treated only with phototherapy ( 30 ), and a reduced rate in vitiligo area scoring index (VASI) score of 4.68 at baseline to 3.95 at 5 months in another trial ( 31 ). In addition, a decline in the number of CD8 + T cells and chemokines, such as CXCL9 and CXCL10 has been observed after tofacitinib treatment, but no variations were observed for the percentage of melanocyte-specific T cells ( 30 ).
Unfortunately, this oral medication is associated with a host of systemic side effects, including infections, malignancies, and cytopenia. Thus, topical JAK inhibitors may be more preferred. 11 vitiligo patients treated with 2% tofacitinib cream twice a day in conjunction with NB-UVB therapy thrice-weekly demonstrated a mean improvement of 70% in facial VASI. There was also a significant difference between facial and non-facial lesions (P=0.022) ( 32 ).
3.1.2 Ruxolitinib
Ruxolitinib, the first Jakinib to get FDA approval, is a JAK1/2 inhibitor designed to deal with polycythemia vera and intermediate- and high-risk primary myelofibrosis ( 33 ).
Studies have shown that except for JAK inhibition, ruxolitinib also inhibited the differentiation and migration of DCs in vitiligo, increasing CD8 + cytotoxic T cell responses ( 34 ). In a double-blind phase 2 trial, 157 recruited vitiligo patients were randomized, in a 1:1:1:1:1 ratio, to receive topical ruxolitinib cream 1.5% BID, 1.5% QD, 0.5% QD, 0.15% QD, or a vehicle for 24 weeks, with the result showing considerably decreased CXCL9 and CXCL10 expression in 1.5% BID and 1.5% QD groups, and more individuals in groups receiving ruxolitinib cream 1.5% BID, 1.5% QD and 0.5% QD achieving F-VASI50, during which 1.5% BID group produced the highest responses in F-VASI50 (58%), F-VASI75 (52%), and F-VASI90 (33%). Besides, three positive responsive groups demonstrated significant repigmentation of vitiligo lesions and acceptable tolerability with a follow-up period of 52 weeks ( 35 ). Vitiligo on the face appears to respond more vigorously to therapy than non-facial lesions, reinforced by a 20-week, open-label trial in which patients with significant facial involvement experienced a 76% improvement in facial VASI scores ( 36 ). Furthermore, better repigmentation rates could be achieved both in oral and topical ruxolitinib treatment combined with phototherapy ( 37 ).
3.1.3 Baricitinib
Baricitinib is a selective JAK1/2 inhibitor that inhibits signal transduction of numerous proinflammatory cytokines ( 38 ), approved for the treatment of RA. To our knowledge, there was only one case report describing repigmentation in vitiligo patients with baricitinib 4 mg daily for the treatment of RA. Besides, an ongoing phase 2 trial (NCT04822584) in which patients received a combination therapy of baricitinib 4mg/d and phototherapy is being performed.
3.1.4 Ifidancitinib (ATI-50002)
Ifidancitinib is another dual JAK1/3 inhibitor for alopecia areata treatment, which is now undergoing phase II clinical trials for its application in vitiligo treatment. Patients with facial NSV(NCT03468855) receiving topical ATI-50002 BID for 24 weeks presented with an improved F-VASI and the Vitiligo Noticeability Scale (VNS) ( 39 ).
3.1.5 Ritlecitinib (PF-06651600) and Brepocitinib (PF-06700841)
Ritlecitinib, an irreversible inhibitor of JAK3 and tyrosine kinase applicable to the treatment of moderate-to-severe RA ( 40 ) and Brepocitinib, a TYK2/JAK1 inhibitor, are currently undergoing evaluation of their efficacy and safety profile in active NSV in combination with phototherapy (NCT03715829) ( 41 ).
3.1.6 Cerdulatinib (PRT062070)
Cerdulatinib, an SYK/JAK dual kinase inhibitor ( 42 ), has been assessed (NCT04103060) for its safety and tolerability for vitiligo treatment in topical formation (0.37% cerudulatinib gel BID).
However, additional studies are needed to determine the best-suited drug regimen and recommended dosage forms and doses to attain the optimum curative effect and minimal toxicity. As the occurrence of depigmentation after the withdrawal of JAK inhibitors, the mechanisms underlying need further exploration, and more work need to be done to corroborate the effectiveness in combination with other therapies.
3.2 Wnt signaling and its agonists
It has been shown that Wnt/β-catenin signaling plays a pivotal role in the proliferation, migration, and differentiation of melanocytes in vitiligo patients ( 29 ), which could be inhibited by oxidative stress ( 43 ). In addition, the Wnt/β-catenin pathway participates in the activation of MITF and its downstream enzymes ( 44 ). Intradermal injection of IWR-1 (inhibitor of Wnt response 1), a chemical inhibitor of β-catenin activation, and small interfering RNA (siRNA) against Wnt7α suppressed the number of epidermal melanocytes ( 45 ). This evidence suggested that stimulation of Wnt signaling may be an adjuvant therapy for vitiligo treatment. Micro-injury ( 46 ) as well as some phenanthridine-derived Wnt-specific agonists binding with the Axin protein have been proved to promote melanogenesis ( 47 ) and induce repigmentation.
3.3 Emerging therapeutics targeting microRNAs (miRNAs)
MiRNAs, which are a highly conservative small class of non-coding RNA molecules, participate in mRNA expression regulation via degradation or repression of mRNA translation ( 48 ). Previous studies have demonstrated that miRNAs were associated with genetic polymorphisms (e.g., miR-196a-2 rs11614913), immune response (e.g., miR-133b, miR-224-3p, miR-4712-3p, miR-3940-5p, miR-21−5p), oxidative stress (e.g., miR-135a, miR-9, miR-34a, miR-183, miR-184, miR-1, miR-25, miR-211, miR-383, miR-577, miR-421) and melanocyte functions (e.g., miR-434-5p, miR-330-5p, miR-137, miR-148, miR-145, miR-155, miR-203, miR-125, miR-377, miR-2909, miR-200c, hsa-miR-149-5p) ( 49 – 54 ), participating in pathological mechanism of vitiligo. These findings suggest that miRNAs may be involved in vitiligo pathogenesis via the modulation of vital genes expression in melanocytes and serve as novel therapeutic targets for vitiligo therapy.
There are two strategies for the therapeutic application of miRNAs: 1) anti-miRNAs, locked-nucleic acids (LNA), or antagomiRs ( 55 ) can be used to counteract the over-activation of miRNA. Short tandem target mimic (STTM)- miR-508-3p has been validated to upregulate SOX6 expression, leading to increased expression of key melanogenic genes CREB, MITF, TYR, and TYRP1/2 with increased melanogenesis ( 56 ). Besides, STTM-miR-143-5p also upregulates the expression of MYO5A, leading to an increase in the level of MITF, TYR, TYRP1, melanin, and Rab27a ( 57 ). 2) miRNA replacement, involving the reintroduction of a gene-suppressor miRNA mimic or AAV (adeno-associated virus)-mediated miRNA gain-of-function to modulate gene expression ( 55 ). A study demonstrated that the migratory capacity of melanocytes was altered by the application of miR-211 mimic through the p53-TRPM1/miR-211-MMP9 axis ( 58 ).
3.4 Emerging therapeutics targeting regulatory T-cells (Tregs)
Tregs are a suppressive CD4 + T cell subset that possesses a capacity to suppress self-reactive T cell activation and expansion ( 59 ). A clear decrease in Treg cells was observed in vitiligo skin within lesional, non-lesional, and perilesional sections ( 60 ), indicating that increasing the number of Tregs with normal function might be an important therapeutic intervention for vitiligo treatment.
Infusing purified populations of Tregs is the most direct way for the supply of Tregs. The current methods mainly include polyclonally-expanded Tregs, antigen-specific Tregs, and engineered Treg cells. In a mouse model of vitiligo, adoptive transfer of polyclonal Tregs may be effective in the short-term ( 61 ), which might however impart systemic immunosuppression ( 62 ). Besides, a TCR transgenic mouse with spontaneous vitiligo, receiving CAR Tregs treatment, developed a significant delay in depigmentation ( 63 ).
However, a limitation of infusing purified populations of Tregs might be the technical difficulty for therapeutic agent delivery to specific cells. A topical application of Tregs or the combination with CCR4 Treg homing receptor ligand CCL22 ( 64 ) by local needle-free jet injection of DNA ( 20 ) or CCL22-encoding plasmid DNA ( 64 ) may help resolve that issue. Besides, various strategies have been applied towards the modulation of Tregs function by targeting Treg-intrinsic pathways and functional modulators for Tregs. HO-1, a functional modulator of Tregs, was decreased in vitiligo Tregs. Treatment with Hemin, an agonist of HO-1, was found to enhance HO-1-induced restoration of Tregs function by up-regulating IL-10 expression ( 65 ). In addition, therapeutic method for microbiota modulation, such as neomycin treatment can significantly delay depigmentation in vitiligo mice and promote the infiltration of Tregs to the skin ( 66 ). Rapamycin, an inhibitor of PI3Kakt-mTORC1 signaling ( 67 ), efficiently halts the depigmentation process by increasing the abundance of Treg in h3TA2 mice, which effect lasted till 6 weeks after treatment ( 61 ). At present, a phase 2 clinical trial(NCT05342519) is underway for assessing the efficacy of the application of 0.1% topical rapamycin ( 68 ) (2022). In addition, nanoparticles containing rapamycin and autoantigen HEL46-61(NPHEL46-61/Rapa) were synthesized, the administration of which halted the disease progression ( 69 ). Also, the calcium-NFATc1-signaling pathway may be involved in defective Tregs function, indicating a potential therapeutic target for vitiligo treatment ( 70 ).
4 Cytokine-targeted therapies
Multiple monoclonal antibodies are available for vitiligo treatment, targeting IFN-γ, CXCL10, CXCR3, HSP70i, IL-15, IL-17/23, and TNF. In addition to full-size immunoglobulin, affibodies and nanobodies, composed of considerably smaller proteins, are currently being developed, which have higher bioavailability as well as affinity and specificity to the targeted molecules.
4.1 IFN-γ and the inhibitors
The IFN-γ-CXCL9/10-CXCR3 axis may be crucial for vitiligo pathogenesis, contributing to disease progression by inhibiting melanogenesis, inducing apoptosis of melanocytes, and further recruiting T cells to the skin ( Figure 1 ) ( 71 ). A study showed a higher expression of IFN-γ mRNA in non-lesional and perilesional skin, especially in active vitiligo ( 72 ), which is associated with disease activity ( 73 ).
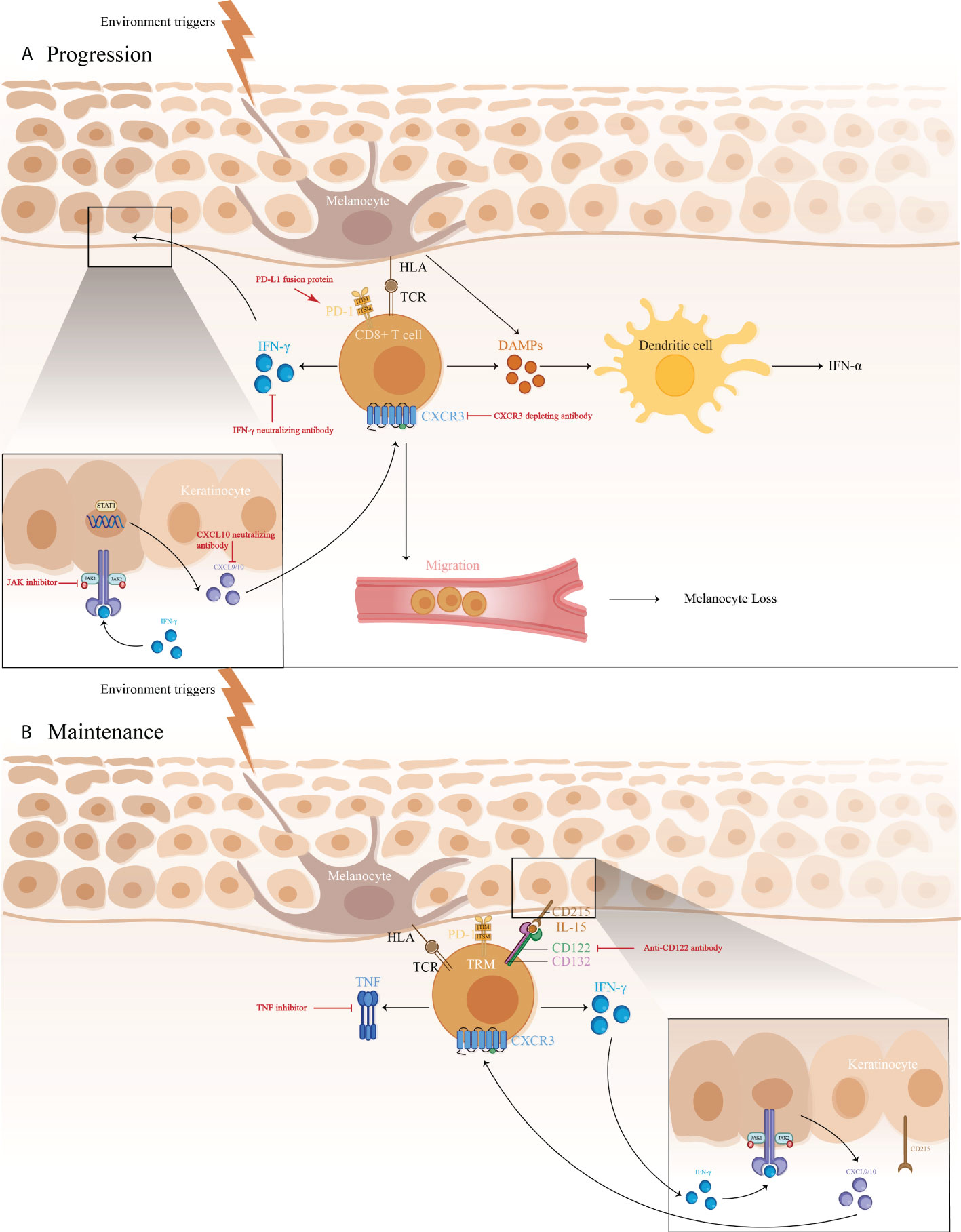
Figure 1 1) The immune pathogenesis of vitiligo: (A) CD8 + T cell expression of IFN-γ in vitiligo lesions activated the JAK/STAT pathway after binding to IFN-γ receptor, thus facilitating the release of CXCL9/10. The binding of CXCL9/10 to CXCR3 increased CXCR3+ T cells recruitment; (B) Maintenance of vitiligo lesions was influenced by the function of IL-15-dependent TRM cells, which produce IFN-γ and TNF-α. 2)Targeted therapeutic interventions in vitiligo mainly include therapies targeting IFN-γ-CXCL9/10-CXCR3 axis (IFN-γ neutralizing antibody, CXCL10 neutralizing antibody, and CXCR3 depleting antibody, as well as JAK inhibitors), anti-CD122 antibody (IL-15 receptor subunit) to decrease IFN-γ production and deplete autoreactive CD8 + TRM cells, TNF inhibitor to inhibit autoantibody production, and PD-L1 fusion protein to reduce the numbers of melanocyte-reactive T cells.
Anti-IFN-γ can have been proved to be effective in rheumatoid arthritis (RA), multiple sclerosis (MS), prevention of corneal rejection, autoimmune skin diseases, and others. In a recent study, vitiligo induction mice, treated with intraperitoneal injection with IFN-γ neutralizing antibody (XMG-6) at a dose of 100-500 μg twice a week, presented with significant improvement of depigmentation ( 17 ), with the same trend observed in vitiligo patients. Four patients who received intradermal perilesional injections presented with repigmentation of the treated area and boundary retreat ( 74 ). More research is warranted to be initiated for further definition of the role that IFN-γ plays in vitiligo and to examine whether IFN-γ neutralization would be more viable in reversing skin depigmentation.
4.2 CXCL10 and the inhibitors
Recent studies report a Th1/IFN-γ immune response in both human and a mouse model of vitiligo that involves elevated CXCL9, 10, and 11 productions, among which CXCL10 participated in the targeted migration of T cells ( 18 ), triggering an immune cell infiltration at the early stage ( 72 ), and involved in the downregulation of keratinocyte glycoprotein non-metastatic melanoma protein B (GPNMB) ( 75 ). A study showed that mice receiving CXCL10 neutralizing antibodies developed more repigmentation after 4 weeks’ treatment, which continued for an additional 4 weeks ( 18 ), thereby supporting CXCL10 suppression as a great therapeutic strategy.
4.3 CXCR3 antibodies
CXCR3 has been proved to be expressed in skin lesions, autoreactive T cells ( 18 ), and the vast majority of skin infiltrating CD8 + resident memory T cells (TRM), which stimulate the secretion of IFN-γ and TNF-α ( 76 ).
In a study, vitiligo mice with >75% depigmentation on their tails are treated with CXCR3 depleting antibodies for 7-8 weeks, which significantly reversed the clinical disease in a perifollicular pattern and a diminution of PMEL in the epidermis, with slightly reduced host CD8 + T cell numbers ( 19 ) compared to neutralizing antibody treatment ( 18 ). Although these results are preliminary, they may provide justification for further studies in targeting CXCR3 in vitiligo ( 19 ), which proposes the use of a depleting Ab to create a greater clinical efficacy by removing autoreactive cells rather than modulating their migration phenotype.
4.4 Inducible HSP70 (HSP70i) DNA
Indeed, HSP70i is the core participant in vitiligo predominantly through HSP70i-plasmacytoid dendritic cells (pDCs)-IFN-α-CXCL9 and CXCL10-cytotoxic T lymphocyte (CTL) axis. Pmel-1 mice vaccinated with HSP70i encoding DNA exhibited significant depigmentation, rarely seen in models knockout for HSP70i, indicating that elevated HSP70i expression alone would be enough to induce depigmentation in vitiligo prone animals ( 77 ). A study revealed that the expression of HSP-70 mRNA in skin lesions of active vitiligo patients was much higher ( 78 ), correlated with the disease activity.
Blocking HSP70i activity might have the potential to reverse vitiligo development. A recent study showed that a Sinclair swine, receiving HSP70iQ435A-encoding DNA treatment, showed remarkable repigmentation with an initial influx of T cells and increased CD4/CD8 ratios ( 20 ), which was also detected in mice with HSP70i Q435A -encoding DNA treatment, resulting in 76% restoration of skin pigmentation. Furthermore, the treatment halted T cells accumulation and transition to T cell phenotype in mice and human skin, engaging HSP70i Q435A DNA delivery as a potent effective therapeutic intervention for vitiligo ( 79 ).
4.5 IL-15 and the inhibitors
It has been established that IL-15 seems to participate in IL-17 regulation and maintenance of TRM signals ( 80 ), with the latter responsible for long-term maintenance and potential relapse of vitiligo ( 81 ). The study has demonstrated a higher serum level of IL-15 in vitiligo patients than in controls, highly associated with epidermal H 2 O 2 content and the disease activity ( 82 , 83 ).
In vitiligo mice, an anti-CD122 antibody that targets IL-15 signaling was reported to effectively reverse depigmentation. Anti-CD122 therapy, either systemically or locally, decreases TRM-induced IFN-γ production and results in long-term repigmentation. These findings consider CD122-targeted drugs as a valid therapy method, which results in effective and long-lasting responses in vitiligo and other tissue-specific autoimmune disorders involving TRM ( 21 ).
4.6 PD-1/PD-L1 pathway
Involvement of the PD-1/PD-L1 pathway has been shown in many autoimmune diseases, including RA, MS, and vitiligo. PD-L1 expression was found limited in normal skin, and only expressed on dermal T cells, and increased in primary melanocytes and fibroblasts after exposure to IFN-γ. No such effect was seen in vitiligo patients, indicating the absence of self-protection ability for melanocytes against T-cell attack during vitiligo pathogenesis. In agreement with this, treatment with PD-L1 fusion protein reduced the numbers of melanocyte-reactive T cells, inhibited the activation of Vβ12-expressing T cells, and increased Tregs numbers, reversing depigmentation in a Pmel-1 T-cell receptor transgenic vitiligo mouse model ( 26 ). However, PD-L1 treatment may still call for extended phototherapy treatment, especially NB-UVB therapy, which likely upregulates PD-L1 expression in an NF-κB-dependent manner ( 84 ), indicating a combination use of local PD-1/PD-L1 agonistic treatment and NB-UVB therapy as a promising option.
4.7 Other cytokine-targeted therapies under investigation
4.7.1 il-17/23 and the inhibitors.
Studies on the effect of IL-17/23 in vitiligo resulted in contradictory findings. On one hand, Th17 cells and IL-17 in vitiligo patients may inhibit function-related factors, repress melanogenesis, and dramatically induct other Th17 type cytokines as well as IL-1β production from dermal fibroblasts and keratinocytes ( 85 ). Elevated Th17 cells and IL-17/23 levels in skin lesions and serum of vitiligo patients, were positively correlated with disease activity ( 86 , 87 ), and decreased after narrowband ultraviolet B (NBUVB) treatment ( 88 ). Primary melanocyte culture showed an increased expression of MITF and its downstream genes, increased melanin pigment, and cell proliferation after blockade with anti-IL-17RA ( 22 ). Besides, incidences of repigmentation have been documented in ustekinumab treatment of vitiligo ( 23 ). However, secukinumab treatment in patients with active non‐segmental vitiligo (NSV) contributed to disease progression in 7/8 patients with no general reduction in CXCL9/10, sCD25/27, Th1 cells, or cytotoxic cells, resulting in early termination of study ( 89 ). There are also reports of ustekinumab-induced new-onset vitiligo and alopecia areata. The above studies showed IL-17/23 signal may not play a direct role in vitiligo pathogenesis, which needs further investigation to confirm this conjecture.
4.7.2 TNF and the inhibitors
As an anti-inflammatory mediator, TNF-α is considered to play a role in vitiligo, which may promote apoptosis in melanocytes, induce B-cell activation, increase autoantibody production, and inhibit melanogenesis ( 90 ). Recent data has shown a significantly higher expression of TNF-α in vitiligo skin. TNF inhibitors are beneficial in the treatment of plaque-type psoriasis, psoriatic arthritis (PsA), RA, and inflammatory bowel disease (IBD), arousing growing interest in their use in vitiligo.
Infliximab is a chimeric anti-TNF-α monoclonal antibody specifically binding to both soluble and membrane-bound TNF ( 91 , 92 ). Intravenous infliximab is widely licensed in the treatment of RA, psoriasis, ankylosing spondylitis (AS), IBD, uveitis, and Behcet’s disease. A 24-year-old patient with ankylosing spondylitis and refractory vitiligo improved significantly following six months of infliximab therapy at a dose of 5mg/kg intravenously in weeks 0, 2, and 6, and then every eight weeks for ten months ( 24 ). Besides, Etanercept is a monoclonal antibody targeted against TNF-α ( 93 ), which has been approved for the treatment of RA, juvenile RA, AS, psoriasis, and PsA. Treatment with etanercept 50 mg subcutaneously once or twice weekly for at least 2 months has shown a great curative effect on established vitiligo ( 94 ).
However, it has been shown that anti‐TNF‐α agents, especially adalimumab and infliximab ( 95 ), may exacerbate established vitiligo and induce new-onset vitiligo during treatment of other autoimmune diseases, including AS ( 96 ), Crohn’s disease ( 97 ), ulcerative colitis ( 98 ), psoriasis ( 99 ), and RA ( 100 ). The mechanism responsible for the TNF-α inhibitors-induced vitiligo is not fully understood. On the one hand, TNF-α inhibitors may increase the nucleosome-mediated autoantibody formation, interfere with the cytotoxic T-cell suppression of autoreactive B cells, and decrease Treg synthesis and activation. Additionally, infliximab increases pDC-produced IFN-γ, participating in further T cells recruiting. Although very rare, new-onset or exacerbations of vitiligo can occur in the anti‐TNF‐α treatment of other autoimmune diseases, the risk of which must not be ignored.
4.7.3 Rituximab
Rituximab has specific affinity for the B-lymphocyte transmembrane protein, CD20, which is expressed on B cells ( 101 ), participating in the activation of the CD8 + T cells and the ensuing autoreactive reaction ( 102 ). Rituximab is licensed for the treatment of lymphomas, leukemias, transplant rejection crisis, and a series of autoimmune diseases ( 103 , 104 ). An intravenous infusion of Rituximab was administered to five active disseminated vitiligo patients, the three of whom exhibited a considerable improvement in both the disease’s symptoms and histology ( 25 ).
4.7.4 Abatacept
Abatacept, a fusion protein consisting of IgG1 coupled to the extracellular domain of CTLA-4 via the immunoglobulin’s Fc region, was licensed for treating moderate to severe RA. Ten eligible patients with active vitiligo have been included to receive self-injections of 125mg abatacept weekly from week 0 to week 24. Secondary endpoints will be evaluated during a 32-week follow-up visit ( 105 ).
5 Future therapeutic prospects
As a future direction, new therapeutic approaches should be developed to reduce vitiligo progression. Among the new approaches being developed, the strategy of targeting the IFN-γ-CXCL9/10-CXCR3 axis has been clinically tested. OPZELURA has been indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. MiRNA-based therapeutics are also in development. However, the absence of organ or tissue selectivity may also lead to off-target side effects, which must be considered and excluded in the process of miRNA-based therapeutics development. Besides, a suitable vector system, as well as the assurance of chemical and biological stability should also be taken into account. Adoptive Treg cell therapy has also been the research hotspot in recent years. However, it has always been a difficult point for reassurance for safety and the development of the delivery system.
Treating vitiligo remains a challenge. As is presented in this paper, a greater variety of precision treatments is currently being studied. With a better understanding and further validation of these therapeutic targets, patients can be stratified to achieve individualized treatment.
6 Conclusion
Current models of treatment for vitiligo are often nonspecific and general. Various therapy options are available for active vitiligo patients, including systemic glucocorticoids, phototherapy, and systemic immunosuppressants. While stable vitiligo patients may benefit from topical corticosteroids, topical calcineurin inhibitors, phototherapy, as well as transplantation procedures. Recently, a better understanding of the pathophysiological processes of vitiligo led to the advent of novel targeted therapies. To date, JAK inhibitors are the only category that has been proved to have a good tolerability profile and functional outcomes in vitiligo treatment, even though the risk of activation of latent infection and systemic side effects still existed, like other immunosuppressive agents. Research is in progress to investigate the important cytokines involved in the pathogenesis of vitiligo, including IFN-γ, CXCL10, CXCR3, HSP70i, IL-15, IL-17/23, and TNF, the blockade of which has undergone preliminary attempts in animal models and some patients. In addition, studies on miRNA-based therapeutics as well as adoptive Treg cell therapy are still primary, and more studies are necessary.
Author contributions
YFF and YL contributed to the conceptual design, writing, editing, and generation of figures for this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet (London England) (20159988) 386:74–84. doi: 10.1016/S0140-6736(14)60763-7
CrossRef Full Text | Google Scholar
2. Bishnoi A, Vinay K, Kumaran MS, Parsad D. “Oral mycophenolate mofetil as a stabilizing treatment for progressive non-segmental vitiligo: results from a prospective, randomized, investigator-blinded pilot study”. Arch Dermatol Res (2021) 313(5):357–65. doi: 10.1007/s00403-020-02108-8
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Garza-Mayers AC, Kroshinsky D. “Low-dose methotrexate for vitiligo”. Drugs Dermatol (2017) 16(7):705–6.
Google Scholar
4. Singh H, Kumaran MS, Bains A, Parsad D. A randomized comparative study of oral corticosteroid minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatology (2015) 231(3):286–90. doi: 10.1159/000433424
5. Taieb A, Alomar A, Bohm M, Dell'anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: The European dermatology forum consensus. Br J Dermatol (2013) 168(1):5–19. doi: 10.1111/j.1365-2133.2012.11197.x
6. Lee JiH, Kwon HS, Jung HMi, Lee H, Kim GM, Yim HW, et al. Treatment outcomes of topical calcineurin inhibitor therapy for patients with vitiligo: A systematic review and meta-analysis. JAMA Dermatol (2019) 155(8):929–38. doi: 10.1001/jamadermatol.2019.0696
7. Lopes C, Trevisani VF, Melnik T. Efficacy and safety of 308-nm monochromatic excimer lamp versus other phototherapy devices for vitiligo: A systematic review with meta-analysis. Am J Clin Dermatol (2016) 17(1):23–32. doi: 10.1007/s40257-015-0164-2
8. Zubair R, Hamzavi IH. Phototherapy for vitiligo. Dermatol Clinics (2020) 38(1):55–62. doi: 10.1016/j.det.2019.08.005
9. Mohammad TF, Hamzavi IH. Surgical therapies for vitiligo. Dermatol Clinics (2017) 35(2):193–203. doi: 10.1016/j.det.2016.11.009
10. Mohammad TF, Al-Jamal M, Hamzavi IH, Harris JE, Leone G, Cabrera R, et al. The vitiligo working group recommendations for narrowband ultraviolet b light phototherapy treatment of vitiligo. J Am Acad Dermatol (2017) 76(5):879–88. doi: 10.1016/j.jaad.2016.12.041
11. Grimes PE, Nashawati R. Depigmentation therapies for vitiligo. Dermatol Clinics (2017) 35(2):219.
12. Mulekar SV, Al Eisa A, Delvi MB, Al Issa A, Al Saeed AH. Childhood vitiligo: A long-term study of localized vitiligo treated by noncultured cellular grafting. Pediatr Dermatol (2010) 27(2):132–6. doi: 10.1111/j.1525-1470.2009.00978.x
13. Mulekar SV. Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol (2004) 140(10):1211–5. doi: 10.1001/archderm.140.10.1211
14. Bohm M, Schunter JA, Fritz K, Salavastru C, Dargatz S, Augustin M, et al. S1 guideline: Diagnosis and therapy of vitiligo. J Dtsch Dermatol Ges (2022) 20(3):365–78. doi: 10.1111/ddg.14713
15. Batchelor JM, Thomas KS, Akram P, Azad J, Bewley A, Chalmers JR, et al. Home-based narrowband UVB, topical corticosteroid or combination for children and adults with vitiligo: HI-light vitiligo three-arm RCT. Health Technol Assess (2020) 24(64):1–128. doi: 10.3310/hta24640
16. Li R, Qiao M, Wang X, Zhao X, Sun Q. Effect of narrow band ultraviolet b phototherapy as monotherapy or combination therapy for vitiligo: A meta-analysis. Photodermatol Photoimmunol Photomed (2017) 33(1):22–31. doi: 10.1111/phpp.12277
17. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol (2012) 132(7):1869–76. doi: 10.1038/jid.2011.463
18. Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su M-W, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Trans Med (2014) 6(223):223ra23. doi: 10.1126/scitranslmed.3007811
19. Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, Harris JE. CXCR3 depleting antibodies prevent and reverse vitiligo in mice. J Invest Dermatol (2017) 137(4):982–5. doi: 10.1016/j.jid.2016.10.048
20. Henning SW, Fernandez MF, Mahon JP, Duff R, Azarafrooz F, Guevara-Patino JA, et al. HSP70iQ435A-encoding DNA repigments vitiligo lesions in Sinclair swine. J Invest Dermatol (2018) 138(12):2531–9. doi: 10.1016/j.jid.2018.06.186
21. Richmond JM, Strassner JP, Zapata L, Garg M, Riding RL, Refat MA, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Trans Med (2018) 10(450):eaam7710. doi: 10.1126/scitranslmed.aam7710
22. Bhardwaj S, Bhatia A, Kumaran MS, Parsad D. Role of IL-17A receptor blocking in melanocyte survival: A strategic intervention against vitiligo. Exp Dermatol (2019) 28(6):682–9. doi: 10.1111/exd.13773
23. Elkady A, Bonomo L, Amir Y, Vekaria AS, Guttman-Yassky E. Effective use of ustekinumab in a patient with concomitant psoriasis, vitiligo, and alopecia areata. JAAD Case Rep (2017) 3(6):477–9. doi: 10.1016/j.jdcr.2017.07.009
24. Simon JA, Burgos-Vargas R. Vitiligo improvement in a patient with ankylosing spondylitis treated with infliximab. Dermatology (2008) 216(3):234–5. doi: 10.1159/000112932
25. Ruiz-Arguelles A, Garcia-Carrasco M, Jimenez-Brito G, Sanchez-Sosa S, Perez-Romano B, Garces-Eisele J, et al. Treatment of vitiligo with a chimeric monoclonal antibody to CD20: A pilot study. Clin Exp Immunol (2013) 174(2):229–36. doi: 10.1111/cei.12168
26. Miao X, Xu R, Fan B, Chen J, Li X, Mao W, et al. PD-L1 reverses depigmentation in pmel-1 vitiligo mice by increasing the abundance of tregs in the skin. Sci Rep (2018) 8(1):1605. doi: 10.1038/s41598-018-19407-w
27. Zou DP, Chen YM, Zhang LZ, Yuan XH, Zhang YJ, Inggawati A, et al. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the wnt/beta-catenin signaling. Genes Dis (2021) 8(5):677–88. doi: 10.1016/j.gendis.2020.06.003
28. Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci (2004) 117(Pt 8):1281–3. doi: 10.1242/jcs.00963
29. Birlea SA, Costin GE, Roop DR, Norris DA. Trends in regenerative medicine: Repigmentation in vitiligo through melanocyte stem cell mobilization. Med Res Rev (2017) 37(4):907–35. doi: 10.1002/med.21426
30. Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol (2017) 77(4):675–82.e1. doi: 10.1016/j.jaad.2017.05.043
31. Vu M, Heyes C, Robertson SJ, Varigos GA, Ross G. Oral tofacitinib: A promising treatment in atopic dermatitis, alopecia areata and vitiligo. Clin Exp Dermatol (2017) 42(8):942–4. doi: 10.1111/ced.13290
32. Mobasher P, Guerra R, Li SJ, Frangos J, Ganesan AK, Huang V. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br J Dermatol (2020) 182(4):1047–9. doi: 10.1111/bjd.18606
33. Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. food and drug administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res (2012) 18(12):3212–7. doi: 10.1158/1078-0432.CCR-12-0653
34. Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo . Blood (2013) 122(7):1192–202. doi: 10.1182/blood-2013-03-484642
35. Rosmarin D, Pandya AG, Lebwohl M, Grimes P, Hamzavi I, Gottlieb AB, et al. Ruxolitinib cream for treatment of vitiligo: A randomised, controlled, phase 2 trial. Lancet (2020) 396(10244):110–20. doi: 10.1016/S0140-6736(20)30609-7
36. Rothstein B, Joshipura D, Saraiya A, Abdat R, Ashkar H, Turkowski Y, et al. Treatment of vitiligo with the topical janus kinase inhibitor ruxolitinib. J Am Acad Dermatol (2017) 76(6):1054–60.e1. doi: 10.1016/j.jaad.2017.02.049
37. Gianfaldoni S, Tchernev G, Wollina U, Roccia MG, Fioranelli M, Lotti J, et al. Micro - focused phototherapy associated to janus kinase inhibitor: A promising valid therapeutic option for patients with localized vitiligo. Open Access Maced J Med Sci (2018) 6(1):46–8. doi: 10.3889/oamjms.2018.042
38. Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs (2014) 23(8):1067–77. doi: 10.1517/13543784.2014.918604
39. A study of ATI-50002 topical solution for the treatment of vitiligo (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03468855 .
40. Robinson MF, Damjanov N, Stamenkovic B, Radunovic G, Kivitz A, Cox L, et al. Efficacy and safety of PF-06651600 (Ritlecitinib), a novel JAK3/TEC inhibitor, in patients with moderate-to-Severe rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol (2020) 72(10):1621–31. doi: 10.1002/art.41316
41. A phase 2b study to evaluate the efficacy and safety profile of PF-06651600 and PF-06700841 in active non-segmental vitiligo subjects (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03715829 .
42. Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker DC, et al. The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and b-cell cancer. J Pharmacol Exp Ther (2014) 351(3):538–48. doi: 10.1124/jpet.114.218164
43. Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: A promising target for repigmenting vitiligo patients. J Invest Dermatol (2015) 135(12):3105–14. doi: 10.1038/jid.2015.335
44. Harris JE. Melanocyte regeneration in vitiligo requires WNT beneath their wings. J Invest Dermatol (2015) 135(12):2921–3. doi: 10.1038/jid.2015.372
45. Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, et al. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol (2013) 133(12):2753–62. doi: 10.1038/jid.2013.235
46. Han X, Chang Li, Qiu Z, Lin M, Wang Y, Liu D, et al. Micro-injury induces hair regeneration and vitiligo repigmentation through wnt/β-catenin pathway. Stem Cells Dev (2022) 31(5-6):111–8. doi: 10.1089/scd.2021.0276
47. Yang BJ, Fan SR, Zhang XF, Cai JY, Ruan T, Xiang ZR, et al. Design, synthesis and structure-activity relationship optimization of phenanthridine derivatives as new anti-vitiligo compounds. Bioorg Chem (2022) 119:105582. doi: 10.1016/j.bioorg.2021.105582
48. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature (2008) 455(7209):64–71. doi: 10.1038/nature07242
49. Li L. The role of MicroRNAs in vitiligo: Regulators and therapeutic targets. Ann Dermatol (2020) 32(6):441–51. doi: 10.5021/ad.2020.32.6.441
50. Huo J, Liu T, Li F, Song X, Hou X. MicroRNA215p protects melanocytes via targeting STAT3 and modulating Treg/Teff balance to alleviate vitiligo. Mol Med Rep (2021) 23(1):51. doi: 10.3892/mmr.2020.11689
51. Vaish U, Kumar AA, Varshney S, Ghosh S, Sengupta S, Sood C, et al. Micro RNAs upregulated in vitiligo skin play an important role in its aetiopathogenesis by altering TRP1 expression and keratinocyte-melanocytes cross-talk. Sci Rep (2019) 9(1):10079. doi: 10.1038/s41598-019-46529-6
52. Zhao C, Wang D, Wang X, Mao Y, Xu Z, Sun Y, et al. Down-regulation of exosomal miR-200c derived from keratinocytes in vitiligo lesions suppresses melanogenesis. J Cell Mol Med (2020) 24(20):12164–75. doi: 10.1111/jcmm.15864
53. Li L, Xie Z, Qian X, Wang T, Jiang M, Qin J, et al. Identification of a potentially functional circRNA-miRNA-mRNA regulatory network in melanocytes for investigating pathogenesis of vitiligo. Front Genet (2021) 12:663091. doi: 10.3389/fgene.2021.663091
54. Shang Z, Li H. Altered expression of four miRNA (miR-1238-3p, miR-202-3p, miR-630 and miR-766-3p) and their potential targets in peripheral blood from vitiligo patients. J Dermatol (2017) 44(10):1138–44. doi: 10.1111/1346-8138.13886
55. Kwekkeboom RF, Lei Z, Doevendans PA, Musters RJ, Sluijter JP. Targeted delivery of miRNA therapeutics for cardiovascular diseases: opportunities and challenges. Clin Sci (Lond) (2014) 127(6):351–65. doi: 10.1042/CS20140005
56. Liu B, Zhang J, Yang S, Ji K, Liu X, Du B, et al. Effect of silencing microRNA-508 by STTM on melanogenesis in alpaca (Vicugna pacos). Gene (2018) 678:343–8. doi: 10.1016/j.gene.2018.08.011
57. Qi S, Liu B, Zhang J, Liu X, Dong C, Fan R. Knockdown of microRNA1435p by STTM technology affects eumelanin and pheomelanin production in melanocytes. Mol Med Rep (2019) 20(3):2649–56. doi: 10.3892/mmr.2019.10492
58. Su M, Miao F, Jiang S, Shi Y, Luo L, He X, et al. Role of the p53−TRPM1/miR−211−MMP9 axis in UVB−induced human melanocyte migration and its potential in repigmentation. Int J Mol Med (2020) 45(4):1017–26. doi: 10.3892/ijmm.2020.4478
59. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med (2001) 193(11):1295–302.
PubMed Abstract | Google Scholar
60. Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, et al. Reduced skin homing by functional treg in vitiligo. Pigment Cell Melanoma Res (2010) 23(2):276–86. doi: 10.1111/j.1755-148X.2010.00688.x
61. Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang HK, Kaur N, et al. A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol (2014) 134(5):1285–94. doi: 10.1038/jid.2013.540
62. Alzhrani A, Bottomley M, Wood K, Hester J, Issa F. Identification, selection, and expansion of non-gene modified alloantigen-reactive tregs for clinical therapeutic use. Cell Immunol (2020) 357:104214. doi: 10.1016/j.cellimm.2020.104214
63. Mukhatayev Z, Dellacecca ER, Cosgrove C, Shivde R, Jaishankar D, Pontarolo-Maag K, et al. Antigen specificity enhances disease control by tregs in vitiligo. Front Immunol (2020) 11:581433. doi: 10.3389/fimmu.2020.581433
64. Eby JM, Kang HK, Tully ST, Bindeman WE, Peiffer DS, Chatterjee S, et al. CCL22 to activate treg migration and suppress depigmentation in vitiligo. J Invest Dermatol (2015) 135(6):1574–80. doi: 10.1038/jid.2015.26
65. Zhang Q, Cui T, Chang Y, Zhang W, Li S, He Y, et al. HO-1 regulates the function of treg: Association with the immune intolerance in vitiligo. J Cell Mol Med (2018) 22(9):4335–43. doi: 10.1111/jcmm.13723
66. Dellacecca ER, Cosgrove C, Mukhatayev Z, Akhtar S, Engelhard VH, Rademaker AW, et al. Antibiotics drive microbial imbalance and vitiligo development in mice. J Invest Dermatol (2020) 140(3):676–687 e6. doi: 10.1016/j.jid.2019.08.435
67. Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity (2010) 33(3):301–11. doi: 10.1016/j.immuni.2010.09.002
68. Daily topical rapamycin for vitiligo (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05342519 .
69. Zhang X, Liu D, He M, Lin M, Tu C, Zhang B. Polymeric nanoparticles containing rapamycin and autoantigen induce antigen-specific immunological tolerance for preventing vitiligo in mice. Hum Vaccin Immunother (2021) 17(7):1923–9. doi: 10.1080/21645515.2021.1872342
70. Giri PS, Bharti AH, Begum R, Dwivedi M. Calcium controlled NFATc1 activation enhances suppressive capacity of regulatory T cells isolated from generalized vitiligo patients. Immunology (2022). doi: 10.1111/imm.13538
71. Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, et al. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol (2015) 95(6):664–70. doi: 10.2340/00015555-2080
72. Maouia A, Sormani L, Youssef M, Helal AN, Kassab A, Passeron T. Differential expression of CXCL9, CXCL10, and IFN-gamma in vitiligo and alopecia areata patients. Pigment Cell Melanoma Res (2017) 30(2):259–61. doi: 10.1111/pcmr.12559
73. Shi F, Erf GF. IFN-gamma, IL-21, and IL-10 co-expression in evolving autoimmune vitiligo lesions of smyth line chickens. J Invest Dermatol (2012) 132(3 Pt 1):642–9. doi: 10.1038/jid.2011.377
74. Skurkovich S, Skurkovich B, Kelly J. Anticytokine therapy, particularly anti-IFN-gamma, in Th1-mediated autoimmune diseases. Expert Rev Clin Immunol (2005) 1(1):11–25. doi: 10.1586/1744666X.1.1.11
75. Biswas KB, Takahashi A, Mizutani Y, Takayama S, Ishitsuka A, Yang L, et al. GPNMB is expressed in human epidermal keratinocytes but disappears in the vitiligo lesional skin. Sci Rep (2020) 10(1):4930. doi: 10.1038/s41598-020-61931-1
76. Boniface K, Jacquemin Clément, Darrigade A-S, Dessarthe Benoît, Martins C, Boukhedouni N, et al. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Invest Dermatol (2018) 138(2):355–64. doi: 10.1016/j.jid.2017.08.038
77. Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole ICLe. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell melanoma Res (2012) 25(1):88–98. doi: 10.1111/j.1755-148X.2011.00916.x
78. Doss RW, El-Rifaie AA, Abdel-Wahab AM, Gohary YM, Rashed LA. Heat shock protein-70 expression in vitiligo and its relation to the disease activity. Indian J Dermatol (2016) 61(4):408–12. doi: 10.4103/0019-5154.185704
79. Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med (2013) 5(174):174ra28. doi: 10.1126/scitranslmed.3005127
80. Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Pathophysiology of skin resident memory T cells. Front Immunol (2020) 11:618897. doi: 10.3389/fimmu.2020.618897
81. Richmond JM, Strassner JP, Rashighi M, Agarwal P, Garg M, Essien KI, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol (2019) 139(4):769–78. doi: 10.1016/j.jid.2018.10.032
82. Chen X, Guo W, Chang Y, Chen J, Kang P, Yi X, et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8(+) T cells activation via JAK-STAT pathway in vitiligo. Free Radic Biol Med (2019) 139:80–91. doi: 10.1016/j.freeradbiomed.2019.05.011
83. Atwa MA, Ali SMM, Youssef N, Mahmoud Marie RE. Elevated serum level of interleukin-15 in vitiligo patients and its correlation with disease severity but not activity. J Cosmet Dermatol (2021) 20(8):2640–4. doi: 10.1111/jocd.13908
84. Wang W, Chapman NM, Zhang B, Li M, Fan M, Laribee RN, et al. Upregulation of PD-L1 via HMGB1-activated IRF3 and NF-kappaB contributes to UV radiation-induced immune suppression. Cancer Res (2019) 79(11):2909–22. doi: 10.1158/0008-5472.CAN-18-3134
85. Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res (2012) 25(2):219–30. doi: 10.1111/j.1755-148X.2011.00945.x
86. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol (2011) 36(3):292–7. doi: 10.1111/j.1365-2230.2010.03972.x
87. Vaccaro M, Cannavo SP, Imbesi S, Cristani M, Barbuzza O, Tigano V, et al. Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int J Dermatol (2015) 54(6):672–4. doi: 10.1111/ijd.12392
88. Hegazy RA, Fawzy MM, Gawdat HI, Samir N, Rashed LA. T helper 17 and tregs: A novel proposed mechanism for NB-UVB in vitiligo. Exp Dermatol (2014) 23(4):283–6. doi: 10.1111/exd.12369
89. Speeckaert R, Mylle S, van Geel N. IL-17A is not a treatment target in progressive vitiligo. Pigment Cell melanoma Res (2019) 32(6):842–7. doi: 10.1111/pcmr.12789
90. Birol A, Kisa U, Kurtipek GS, Kara F, Kocak M, Erkek E, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol (2006) 45(8):992–3. doi: 10.1111/j.1365-4632.2006.02744.x
91. Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol (2010) 10(5):301–16.
92. Horiuchi T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (2010) 49(7):1215–28.
93. Spencer-Green and G. Etanercept (Enbrel): update on therapeutic use. Ann Rheum Dis (2000) 59 Suppl 1(90001):i46–9.
94. Webb KC, Tung R, Winterfield LS, Gottlieb AB, Eby JM, Henning SW, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol (2015) 173(3):641–50. doi: 10.1111/bjd.14016
95. Bae JM, Kim M, Lee HH, Kim KJ, Shin H, Ju HJ, et al. Increased risk of vitiligo following anti-tumor necrosis factor therapy: A 10-year population-based cohort study. J Invest Dermatol (2018) 138(4):768–74. doi: 10.1016/j.jid.2017.11.012
96. Toussirot E, Salard D, Algros MP, Aubin F. [Occurrence of vitiligo in a patient with ankylosing spondylitis receiving adalimumab]. Ann Dermatol Venereol (2013) 140(12):801–2. doi: 10.1016/j.annder.2013.09.158
97. Jung JM, Lee YJ, Won CH, Chang SE, Lee MW, Choi JH, et al. Development of vitiligo during treatment with adalimumab: A plausible or paradoxical response?". Ann Dermatol (2015) 27(5):620–1. doi: 10.5021/ad.2015.27.5.620
98. Ryu TH, Lee DW, Choi JE, Ahn HH, Kye YC, Seo SH. A type II segmental vitiligo developed under infliximab treatment for ulcerative colitis. Ann Dermatol (2017) 29(6):826–7. doi: 10.5021/ad.2017.29.6.826
99. Smith DI, Heffernan MP. Vitiligo after the resolution of psoriatic plaques during treatment with adalimumab. J Am Acad Dermatol (2008) 58(2 Suppl):S50–2. doi: 10.1016/j.jaad.2006.05.035
100. Carvalho CLDB, Ortigosa LCM. Segmental vitiligo after infliximab use for rheumatoid arthritis–a case report. Anais bras dermatol (2014) 89(1):154–6. doi: 10.1590/abd1806-4841.20142887
101. Banchereau J, Rousset F. Human b lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol (1992) 52:125–262. doi: 10.1016/s0065-2776(08)60876-7
102. Lin X, Tian H, Xianmin M. Possible roles of b lymphocyte activating factor of the tumour necrosis factor family in vitiligo autoimmunity. Med Hypotheses (2011) 76(3):339–42. doi: 10.1016/j.mehy.2010.10.034
103. Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol (2006) 2(1):20–7. doi: 10.1038/ncprheum0042
104. Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: A therapeutic challenge. Postgrad Med J (2002) 78(924):599–606. doi: 10.1136/pmj.78.924.599
105. Open-label pilot study of abatacept for the treatment of vitiligo . Available at: https://clinicaltrials.gov/ct2/show/NCT02281058 .
Keywords: vitiligo, targeted therapy, JAK inhibitors, biological, treatment, miRNA - microRNA, Treg
Citation: Feng Y and Lu Y (2022) Advances in vitiligo: Update on therapeutic targets. Front. Immunol. 13:986918. doi: 10.3389/fimmu.2022.986918
Received: 05 July 2022; Accepted: 04 August 2022; Published: 31 August 2022.
Reviewed by:
Copyright © 2022 Feng and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Lu, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New treatment could reverse hair loss caused by an autoimmune skin disease
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Researchers at MIT, Brigham and Women’s Hospital, and Harvard Medical School have developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss and affects people of all ages, including children.
For most patients with this type of hair loss, there is no effective treatment. The team developed a microneedle patch that can be painlessly applied to the scalp and releases drugs that help to rebalance the immune response at the site, halting the autoimmune attack.
In a study of mice, the researchers found that this treatment allowed hair to regrow and dramatically reduced inflammation at the treatment site, while avoiding systemic immune effects elsewhere in the body. This strategy could also be adapted to treat other autoimmune skin diseases such as vitiligo, atopic dermatitis, and psoriasis, the researchers say.
“This innovative approach marks a paradigm shift. Rather than suppressing the immune system, we’re now focusing on regulating it precisely at the site of antigen encounter to generate immune tolerance,” says Natalie Artzi, a principal research scientist in MIT’s Institute for Medical Engineering and Science, an associate professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, and an associate faculty member at the Wyss Institute of Harvard University.
Artzi and Jamil R. Azzi, an associate professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, are the senior authors of the new study , which appears in the journal Advanced Materials . Nour Younis, a Brigham and Women’s postdoc, and Nuria Puigmal, a Brigham and Women’s postdoc and former MIT research affiliate, are the lead authors of the paper.
The researchers are now working on launching a company to further develop the technology, led by Puigmal, who was recently awarded a Harvard Business School Blavatnik Fellowship.
Direct delivery
Alopecia areata, which affects more than 6 million Americans, occurs when the body’s own T cells attack hair follicles, leading the hair to fall out. The only treatment available to most patients — injections of immunosuppressant steroids into the scalp — is painful and patients often can’t tolerate it.
Some patients with alopecia areata and other autoimmune skin diseases can also be treated with immunosuppressant drugs that are given orally, but these drugs lead to widespread suppression of the immune system, which can have adverse side effects.
“This approach silences the entire immune system, offering relief from inflammation symptoms but leading to frequent recurrences. Moreover, it increases susceptibility to infections, cardiovascular diseases, and cancer,” Artzi says.
A few years ago, at a working group meeting in Washington, Artzi happened to be seated next to Azzi (the seating was alphabetical), an immunologist and transplant physican who was seeking new ways to deliver drugs directly to the skin to treat skin-related diseases.
Their conversation led to a new collaboration, and the two labs joined forces to work on a microneedle patch to deliver drugs to the skin. In 2021, they reported that such a patch can be used to prevent rejection following skin transplant. In the new study, they began applying this approach to autoimmune skin disorders.
“The skin is the only organ in our body that we can see and touch, and yet when it comes to drug delivery to the skin, we revert to systemic administration. We saw great potential in utilizing the microneedle patch to reprogram the immune system locally,” Azzi says.
The microneedle patches used in this study are made from hyaluronic acid crosslinked with polyethylene glycol (PEG), both of which are biocompatible and commonly used in medical applications. With this delivery method, drugs can pass through the tough outer layer of the epidermis, which can’t be penetrated by creams applied to the skin.
“This polymer formulation allows us to create highly durable needles capable of effectively penetrating the skin. Additionally, it gives us the flexibility to incorporate any desired drug,” Artzi says. For this study, the researchers loaded the patches with a combination of the cytokines IL-2 and CCL-22. Together, these immune molecules help to recruit regulatory T cells, which proliferate and help to tamp down inflammation. These cells also help the immune system learn to recognize that hair follicles are not foreign antigens, so that it will stop attacking them.
Hair regrowth
The researchers found that mice treated with this patch every other day for three weeks had many more regulatory T cells present at the site, along with a reduction in inflammation. Hair was able to regrow at those sites, and this growth was maintained for several weeks after the treatment ended. In these mice, there were no changes in the levels of regulatory T cells in the spleen or lymph nodes, suggesting that the treatment affected only the site where the patch was applied.
In another set of experiments, the researchers grafted human skin onto mice with a humanized immune system. In these mice, the microneedle treatment also induced proliferation of regulatory T cells and a reduction in inflammation.
The researchers designed the microneedle patches so that after releasing their drug payload, they can also collect samples that could be used to monitor the progress of the treatment. Hyaluronic acid causes the needles to swell about tenfold after entering the skin, which allows them to absorb interstitial fluid containing biomolecules and immune cells from the skin.
Following patch removal, researchers can analyze samples to measure levels of regulatory T cells and inflammation markers. This could prove valuable for monitoring future patients who may undergo this treatment.
The researchers now plan to further develop this approach for treating alopecia, and to expand into other autoimmune skin diseases.
The research was funded by the Ignite Fund and Shark Tank Fund awards from the Department of Medicine at Brigham and Women’s Hospital.
Share this news article on:
Related links.
- Natalie Artzi
- Institute for Medical Engineering and Science
Related Topics
- Drug delivery
- Health sciences and technology
- Institute for Medical Engineering and Science (IMES)
Related Articles

A sprayable gel could make minimally invasive surgeries simpler and safer
Patch that delivers drug, gene, and light-based therapy to tumor sites shows promising results

MIT researchers design tailored tissue adhesives
Previous item Next item
More MIT News

MIT researchers discover the universe’s oldest stars in our own galactic backyard
Read full story →

Four from MIT named 2024 Knight-Hennessy Scholars
Taking RNAi from interesting science to impactful new treatments

The power of App Inventor: Democratizing possibilities for mobile applications
Using MRI, engineers have found a way to detect light deep in the brain

From steel engineering to ovarian tumor research
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
New treatment could reverse hair loss caused by an autoimmune skin disease
A microneedle patch that delivers immune-regulating molecules can teach t cells not to attack hair follicles, helping hair to regrow.
Researchers at MIT, Brigham and Women's Hospital, and Harvard Medical School have developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss and affects people of all ages, including children.
For most patients with this type of hair loss, there is no effective treatment. The team developed a microneedle patch that can be painlessly applied to the scalp and releases drugs that help to rebalance the immune response at the site, halting the autoimmune attack.
In a study of mice, the researchers found that this treatment allowed hair to regrow and dramatically reduced inflammation at the treatment site, while avoiding systemic immune effects elsewhere in the body. This strategy could also be adapted to treat other autoimmune skin diseases such as vitiligo, atopic dermatitis, and psoriasis, the researchers say.
"This innovative approach marks a paradigm shift. Rather than suppressing the immune system, we're now focusing on regulating it precisely at the site of antigen encounter to generate immune tolerance," says Natalie Artzi, a principal research scientist in MIT's Institute for Medical Engineering and Science, an associate professor of medicine at Harvard Medical School and Brigham and Women's Hospital, and an associate faculty member at the Wyss Institute of Harvard University.
Artzi and Jamil R. Azzi, an associate professor of medicine at Harvard Medical School and Brigham and Women's Hospital, are the senior authors of the new study, which appears in the journal Advanced Materials . Nour Younis, a Brigham and Women's postdoc, and Nuria Puigmal, a Brigham and Women's postdoc and former MIT research affiliate, are the lead authors of the paper.
The researchers are now working on launching a company to further develop the technology, led by Puigmal, who was recently awarded a Harvard Business School Blavatnik Fellowship.
Direct delivery
Alopecia areata, which affects more than 6 million Americans, occurs when the body's own T cells attack hair follicles, leading the hair to fall out. The only treatment available to most patients -- injections of immunosuppressant steroids into the scalp -- is painful and patients often can't tolerate it.
Some patients with alopecia areata and other autoimmune skin diseases can also be treated with immunosuppressant drugs that are given orally, but these drugs lead to widespread suppression of the immune system, which can have adverse side effects.
"This approach silences the entire immune system, offering relief from inflammation symptoms but leading to frequent recurrences. Moreover, it increases susceptibility to infections, cardiovascular diseases, and cancer," Artzi says.
A few years ago, at a working group meeting in Washington, Artzi happened to be seated next to Azzi (the seating was alphabetical), an immunologist and transplant physican who was seeking new ways to deliver drugs directly to the skin to treat skin-related diseases.
Their conversation led to a new collaboration, and the two labs joined forces to work on a microneedle patch to deliver drugs to the skin. In 2021, they reported that such a patch can be used to prevent rejection following skin transplant. In the new study, they began applying this approach to autoimmune skin disorders.
"The skin is the only organ in our body that we can see and touch, and yet when it comes to drug delivery to the skin, we revert to systemic administration. We saw great potential in utilizing the microneedle patch to reprogram the immune system locally," Azzi says.
The microneedle patches used in this study are made from hyaluronic acid crosslinked with polyethylene glycol (PEG), both of which are biocompatible and commonly used in medical applications. With this delivery method, drugs can pass through the tough outer layer of the epidermis, which can't be penetrated by creams applied to the skin.
"This polymer formulation allows us to create highly durable needles capable of effectively penetrating the skin. Additionally, it gives us the flexibility to incorporate any desired drug," Artzi says. For this study, the researchers loaded the patches with a combination of the cytokines IL-2 and CCL-22. Together, these immune molecules help to recruit regulatory T cells, which proliferate and help to tamp down inflammation. These cells also help the immune system learn to recognize that hair follicles are not foreign antigens, so that it will stop attacking them.
Hair regrowth
The researchers found that mice treated with this patch every other day for three weeks had many more regulatory T cells present at the site, along with a reduction in inflammation. Hair was able to regrow at those sites, and this growth was maintained for several weeks after the treatment ended. In these mice, there were no changes in the levels of regulatory T cells in the spleen or lymph nodes, suggesting that the treatment affected only the site where the patch was applied.
In another set of experiments, the researchers grafted human skin onto mice with a humanized immune system. In these mice, the microneedle treatment also induced proliferation of regulatory T cells and a reduction in inflammation.
The researchers designed the microneedle patches so that after releasing their drug payload, they can also collect samples that could be used to monitor the progress of the treatment. Hyaluronic acid causes the needles to swell about tenfold after entering the skin, which allows them to absorb interstitial fluid containing biomolecules and immune cells from the skin.
Following patch removal, researchers can analyze samples to measure levels of regulatory T cells and inflammation markers. This could prove valuable for monitoring future patients who may undergo this treatment.
The researchers now plan to further develop this approach for treating alopecia, and to expand into other autoimmune skin diseases.
The research was funded by the Ignite Fund and Shark Tank Fund awards from the Department of Medicine at Brigham and Women's Hospital.
- Immune System
- Diseases and Conditions
- Skin Cancer
- Human Biology
- Hair follicle
- Baldness treatments
- Rheumatoid arthritis
- Domestic goat
Story Source:
Materials provided by Massachusetts Institute of Technology . Original written by Anne Trafton. Note: Content may be edited for style and length.
Journal Reference :
- Nour Younis, Núria Puigmal, Abdallah El Kurdi, Andrew Badaoui, Dongliang Zhang, Claudia Morales, Anis Saad, Diane Cruz, Nadim Al Rahy, Andrea Daccache, Triana Huerta, Christa Deban, Ahmad Halawi, John Choi, Pere Dosta, Christine Lian, Natalie Artzi, Jamil R. Azzi. Microneedle‐mediated Delivery of Immunomodulators Restores Immune Privilege in Hair Follicles and Reverses Immune‐Mediated Alopecia . Advanced Materials , 2024; DOI: 10.1002/adma.202312088
Cite This Page :
Explore More
- Fastest Rate of CO2 Rise Over Last 50,000 Years
- Like Dad and Like Mum...all in One Plant
- What Makes a Memory? Did Your Brain Work Hard?
- Plant Virus Treatment for Metastatic Cancers
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
Vitiligo Advancements and Discoveries. There has been an increase in the amount of research being undertaken in vitiligo over recent years and dermatologists have an improved understanding of the natural history and different types of the condition. Here you will find a brief summary of research into several areas, with references to the ...
1. Introduction. Vitiligo is the most common disorder of depigmentation, and in 2012 its worldwide prevalence ranged from 0.06-2.28% (1,2).It is characterized by the absence of pigment in the skin, secondary to the loss of melanocytes (1,3).Melanocytes are found in several tissues in the skin, hair follicles, eyes, inner ear, bones, heart and brain ().
On July 18, the U.S. Food and Drug Administration (FDA) approved ruxolitinib ( Opzelura) cream 1.5 percent as a treatment for the most common form of vitiligo, according to a statement by Incyte ...
More research is warranted to be initiated for further definition of the role that IFN-γ plays in vitiligo and to examine whether IFN-γ neutralization would be more viable in reversing skin depigmentation. ... There are also reports of ustekinumab-induced new-onset vitiligo and alopecia areata. The above studies showed IL-17/23 signal may not ...
Latest Research and Reviews. ... Vitiligo is an autoimmune condition that results in skin depigmentation due to melanocyte loss, but the root causes are not well understood. Here they identify ...
Vitiligo is an autoimmune disorder that leads to the loss of skin pigmentation. A recent study shows a medicated cream called ruxolitinib is extremely effective in about one-third of patients. The ...
Vitiligo is a chronic autoimmune disease that causes skin depigmentation. ... Research Summary ... Harris JE. Interfering with the IFN-γ/CXCL10 pathway to develop new targeted treatments for ...
Vitiligo affects 0.5 to 2% of the population worldwide. This chronic autoimmune disease manifests as pale skin patches, caused by local death of pigment-producing melanocytes. The extent and percei...
Vitiligo is a chronic pigmentary disease that affects approximately 1% of the world's population. This dermatological condition affects skin and hair and manifests itself by characteristic white macules and patches. The disease can be segmental, localized in one area of the body, or generalized, affecting a broader area.
Temprian Therapeutics is a spin-off from Northwestern University in Chicago, Illinois. Vitiligo is an autoimmune disease that affects an estimated 50 million people worldwide. It isn't life ...
The U.S. Food and Drug Administration has approved Opzelura (ruxolitinib) as the first topical treatment for vitiligo. The 1.5 percent cream is approved for continuous topical use twice daily to ...
Discovery may lead to new treatments to reduce inflammation in vitiligo disease. Download PDF Copy. Reviewed. Reviewed by Emily Henderson, B.Sc. Jun 7 2022. A new study, led by researchers from ...
Vitiligo is a common autoimmune-related depigmenting disease resulting from epidermal melanocyte loss. It is characterized by white patches in the skin or mucous, affecting around 0.1-2% of the ... Treatment update for vitiligo based on autoimmune inhibition and melanocyte protection: Expert Opinion on Therapeutic Targets: Vol 27 , No 3 - Get ...
Lego Launches New Vitiligo Minifigure; Maybe it's NOT vitiligo! New NIH Grant Awarded to Create the Vitiligo Center of Research Translation; Opzelura, the first FDA-approved drug to treat vitiligo! Our new research study in Science Translational Medicine describes a new treatment that may have long-term benefit in vitiligo patients; Patterns ...
This international consensus statement culminated in expert-based clinical practice recommendations for the treatment of vitiligo. The development of new therapies is ongoing in vitiligo, and this will likely improve the future management of vitiligo, a disease that still has many unmet needs.
We address every phase in the vitiligo drug discovery process, from basic research all the way to the clinic, in order for new treatments to reach patients. At the Vitiligo Research Foundation, we have only one incentive: to bring a vitiligo treatment to market for nearly a 100 million people worldwide who are affected by this devastating ...
Please use one of the following formats to cite this article in your essay, paper or report: APA. Francisco de Souza, Hugo. (2024, March 13). Diet's role in fighting vitiligo highlighted in new ...
Vitiligo is a chronic immune-mediated skin disorder that can affect a patient's psychological well-being, quality of life, and social acceptance. It is characterized by depigmented white patches and macules due to the targeted destruction of melanocytes - which are responsible for giving color to the skin. The exact cause is unknown, but it is ...
When Dr. Anand K. Ganesan started a vitiligo specialty practice in 2018 at the UCI Health Beckman Laser Institute & Medical Clinic, it was to find new therapies to reverse the disfiguring skin disorder.. Vitiligo — pronounced vit-ill-EYE-go — causes white patches, often on the face and hands, the result of the immune system destroying the skin's pigment-producing cells.
Outcomes included all-cause and vitiligo-related costs (2021 dollars) and all-cause HCRU, including mental health-related HCRU. Patients with vitiligo incurred significantly higher all-cause costs ...
Vitiligo's cause is unknown, but its detrimental effect on patients' quality of life is clear. 1 Medications for this skin disease may work slower than patients anticipate, causing them to feel hopeless, according to Pearl E. Grimes, MD, FAAD, a clinical professor of dermatology at the David Geffen School of Medicine at UCLA and founder and director of the Vitiligo and Pigmentation ...
V is a multifaceted skin condition lacking a conclusive remedy, many types of vitiligo, but the most common one is Non-segmental Vitiligo (NSV), honmental and genetic are the most important causes of vitiligo. Vitiligo is a chronic autoimmune disorder characterized by patches of skin losing pigment due to the destruction of melanocytes, the cells responsible for producing pigment.
Among the new approaches being developed, the strategy of targeting the IFN-γ-CXCL9/10-CXCR3 axis has been clinically tested. OPZELURA has been indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. MiRNA-based therapeutics are also in development.
A New Era of Vitiligo Research and Treatment. This is a very exciting phase of vitiligo research in which vitiligo is being tackled by multipronged attacks in the form of advancement in basic research, genetics and treatment including surgical management. In order to achieve the ultimate goal of total stability and complete repigmentation ...
Notably, the stigma scores significantly differed based on the physician-assessed and child- or caregiver-assessed disease visibility and severity. Nearly three quarters of children with skin ...
Caption: Researchers developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss. The new microneedle patch delivers immune-regulating molecules that can teach T cells not to attack hair follicles, helping hair regrow. Pictured is an up-close view of the microneedles.
Date: May 9, 2024. Source: Massachusetts Institute of Technology. Summary: Researchers developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss. The ...