- Online Degree Explore Bachelor’s & Master’s degrees
- MasterTrack™ Earn credit towards a Master’s degree
- University Certificates Advance your career with graduate-level learning
- Top Courses
- Join for Free

How to Become a Clinical Research Associate
A clinical research associate acts as a liaison between research sponsors and the clinics conducting research. Here’s how you can become one.
![clinical research associate degree [Featured image] A nurse in a blue uniform with a stethoscope around her neck is standing in a hospital office.](https://d3njjcbhbojbot.cloudfront.net/api/utilities/v1/imageproxy/https://images.ctfassets.net/wp1lcwdav1p1/2EZApeHxRLWMt56zIFvoQS/814604c92b6086423df286bcc3e97f68/GettyImages-1292777549__4_.jpg?w=1500&h=680&q=60&fit=fill&f=faces&fm=jpg&fl=progressive&auto=format%2Ccompress&dpr=1&w=1000)
Every pill, vaccine, procedure, therapy, or medical device that might be prescribed or used to improve physical or mental health undergoes clinical research trials. During these trials, a drug or a medical device might get approved for consumer or hospital use.
Clinical research associates (CRA) play a critical role in the health care industry and in improving public health. They liaise between those who sponsor research and those who facilitate clinical research. Even before the COVID-19 pandemic, rising population density and international travel have increased the spread of new and existing diseases. Clinical research is necessary to gain evidence-based insights on how well a drug or vaccine does.
A career as a clinical research associate can be rewarding for individuals who are excited by the prospect of a dynamic role overseeing many different kinds of clinical trials. Here’s how to get started.
What is a clinical research associate?
Clinical research associates act as liaisons between the institutions that sponsor and fund the clinical research trials and the clinics that conduct the research. They're in charge of ensuring the clinical trials run smoothly, monitoring all the procedures, processes, and results, and ensuring the researchers follow established guidelines and protocols at every step.
A clinical research associate works on behalf of the sponsor (pharmaceutical company, university, or health organization) or for a contract research organization (CRO) that funds the research. Clinical trials are the long, scientific process of ensuring that certain drugs, therapies, and devices are safe and effective for public consumption and use. CRAs guide the trials forward following ethics and safety regulations.
Clinical research associate job description
As a clinical research associate, these are the typical tasks and responsibilities:
Monitor the clinical research process, including managing supplies and coordination
Oversee data collection and documentation and input data into systems databases
Outline the trial objectives and present the trial protocols to a committee
Coordinate with an ethics committee that protects trial subject confidentiality
Prepare post-trial reports and manage the creation of publications
Where you’ll work
Field-based CRAs travel to different locations to deal with medical professionals in clinics or hospitals. Some CROs hire in-house CRAs to focus only on document review and management, making only occasional site visits.
Skills needed
Clinical research associates need certain skills to get hired and be successful in their roles. Whether or not you currently possess these skills, you can learn and acquire them through online courses or on the job. Some important skills you’ll need include:
Administrative skills, including the ability to document important information accurately
IT and computer skills, such as databases and systems management
Written and oral communication skills
Keen attention to detail and organization
Ability to manage and coordinate with several stakeholders
Strong understanding of the clinical research trials and health care space, along with medical terminology
Clinical research associate salary and job outlook
The salary for a clinical research associate can vary depending on the organization you work for and your experience level. According to Glassdoor, clinical research associates in Canada earn an average salary of $ 73,209 [ 1 ]. The job outlook for clinical research associates is projected to be moderate to good in nearly all provinces and territories in Canada through 2026, according to the Job Bank [ 2 ].
How to become a clinical research associate.
A CRA career can be fulfilling because you are essentially part translator, part project manager, and part administrator for trials that have the potential to save lives. Here’s how to get started as a clinical research associate.
1. Qualify for certification.
You can take several paths to becoming a certified CRA in Canada. One path is to earn a high school diploma and clock 3,000 to 3,500 part-time hours of work experience in the field. At least one of those years of part-time work must be in Canada and must have been within the past five years. This work is typically in a supporting role, helping experienced CRAs. Your tasks will likely start mundane, but you will get more advanced assignments as you gain experience.
Another path is to graduate high school and gain two years of qualifying experience—again, typically in a support role—in the last five years.
The third path is to earn a post-graduate certificate in clinical research and then earn at least one year of Canadian clinical research working experience in the last two years.
To enrol in a post-graduate certificate program, you will need a bachelor’s degree in health sciences or to be a registered nurse or registered in another regulated health profession. You can also qualify with a certification with a recognized body in health technology or if you’re currently employed in clinical research.
2. Get certified.
After qualifying, you are eligible to sit for the Certified Clinical Research Professionals Society (CCRPS) CRA certification exam. The CRA course and exam costs USD$450.
3. Apply for jobs.
You can start applying for jobs when you have the necessary qualifications to become a CRA. Visit job sites such as Indeed or LinkedIn and type in “clinical research associate” to search for entry- or junior-level positions.
Enhance your resume with any health-care-related experiences you may have, including volunteer activities and internships. You’ll want to quantify your accomplishments with statements such as “I managed clinical trials in seven different states in 2020.”
Prepare for interviews by researching the company and preparing your best answers. Don’t forget to write a list of questions to ask your interviewer.
4. Continue learning.
Earning a master’s degree can help you land a managerial position or salary boost as a CRA. Many types of organizations need clinical research associates, so pursuing higher education can lead to more interesting and dynamic job opportunities.
Start your health care career with Coursera.
Launch your career in the health care industry by honing your skills in medical terminology. You’ll be able to identify parts of words commonly used in medicine, understand health records, and more with the Medical Terminology specialization from Rice University.
Article sources
Glassdoor. " Clinical Research Associate Salaries in Canada , https://www.glassdoor.ca/Salaries/canada-clinical-research-associate-salary-SRCH_IL.0,6_IN3_KO7,34.htm" Accessed April 23, 2024.
Government of Canada Job Bank. " Clinical Research Associate in Canada , https://www.jobbank.gc.ca/marketreport/outlook-occupation/23070/ca." Accessed April 23, 2024.
Keep reading
Coursera staff.
Editorial Team
Coursera’s editorial team is comprised of highly experienced professional editors, writers, and fact...
This content has been made available for informational purposes only. Learners are advised to conduct additional research to ensure that courses and other credentials pursued meet their personal, professional, and financial goals.
- Programs and Courses
- Explore Programs
- Find Courses
- Careers and Relevant NC State Programs
- How to Apply
- Graduate Students
- Non-Degree Studies
- International Students
- Military and Veterans
- Affordability
- Tuition and Fees
- Financial Aid
- Student Resources
- State Authorization
- Professional Licensure
- Student Complaint and Grievance Process
- Faculty and Staff
- Frequently Asked Questions

Science and Research
- Microbiology Undergraduate Certificates
- Textile Chemistry Master's Degrees
Clinical Research Associate
What does a professional in this career do.
A Clinical Research Associate is responsible for running clinical trials, usually for an organization undertaking pharmacological drug testing. Will ensure that trials adhere to good clinical practice guidelines for monitoring clinical trials.
Job Outlook
There were 380 Clinical Research Associate job postings in North Carolina in the past year and 8966 in the United States.
In combination with other careers in the Clinical Research Coordinator / Manager industry, which includes the Clinical Research Associate career, the following graph shows the number of people employed for each year since 2015:
Many new Clinical Research Associate jobs have salaries estimated to be in the following ranges, based on the requirements and responsibilities listed in job postings from the past year.
The average estimated salary in the United States for this career, based on job postings in the past year, is $88,182.
The average estimated salary in North Carolina for this career, based on job postings in the past year, is $93,770.
Percentiles represent the percentage that is lower than the value. For example, 25% of estimated salaries for Clinical Research Associate postings in the United States in the past year were lower than $58,056.
Education and Experience
Posted Clinical Research Associate jobs typically require the following level of education. The numbers below are based on job postings in the United States from the past year. Not all job postings list education requirements.
Posted Clinical Research Associate jobs typically require the following number of years of experience. The numbers below are based on job postings in the United States from the past year. Not all job postings list experience requirements.
Below are listings of the most common general and specialized skills Clinical Research Associate positions expect applicants to have as well as the most common skills that distinguish individuals from their peers. The percentage of job postings that specifically mention each skill is also listed.
Baseline Skills
A skill that is required across a broad range of occupations, including this one.
- Research (53.22%)
- Management (42.95%)
- Communication (38.51%)
- Writing (20.08%)
- Operations (19.82%)
- Detail Oriented (19.74%)
- Microsoft Office (19.16%)
- Microsoft Excel (19.14%)
- Accountability (17.79%)
- Coordinating (17.56%)
Defining Skills
A core skill for this occupation, it occurs frequently in job postings.
- Clinical Trials (63.24%)
- Institutional Review Board (IRB) (22.45%)
- Clinical Research (92.92%)
- Case Report Forms (20.13%)
- Electronic Data Capture (EDC) (20.98%)
- ICH Guidelines (12.01%)
- Clinical Monitoring (16.11%)
- Good Clinical Practices (GCP) (39.01%)
- Trial Master File (8.76%)
Necessary Skills
A skill that is requested frequently in this occupation but isn’t specific to it.
- Medical Devices (11.5%)
- Regulatory Requirements (8.81%)
- Auditing (20.32%)
- Regulatory Documents (19.96%)
- Medical Terminology (15.13%)
- Life Sciences (9.15%)
- Clinical Research Coordination (6.61%)
- Biology (10.98%)
- Biotechnology (8.01%)
- Drug Development (3.51%)
- Oncology (18.4%)
- Pharmaceuticals (12.07%)
- Standard Operating Procedure (10.79%)
- Data Integrity (8.14%)
- Data Collection (19.27%)
- Phlebotomy (8.23%)
- Project Management (12.16%)
- Nursing (11.06%)
- Clinical Trial Management Systems (18.25%)
- Informed Consent (23.38%)
- Reconciliation (7.87%)
- Process Improvement (8.98%)
- Medical Records (12.22%)
- Data Entry (13.32%)
- Data Management (12.87%)
Distinguishing Skills
A skill that may distinguish a subset of the occupation.
- Coherent Remote File System (CRFS) (6.56%)
- Source Document (3.22%)
- Clinical Investigations (4.49%)
Salary Boosting Skills
A professional who wishes to excel in this career path may consider developing the following highly valued skills. The percentage of job postings that specifically mention each skill is listed.
- Clinical Trials (93.78%)
- Case Report Forms (29.86%)
Alternative Job Titles
Sometimes employers post jobs with Clinical Research Associate skills but a different job title. Some common alternative job titles include:
- Clinical Research Assistant
- Oncology Clinical Research Associate
- Clinical Trial Associate
- Oncology Registered Nurse
- CRA Manager
- Clinical Research Study Assistant
- Regional Clinical Research Associate
- Clinical Research Fellow
- Clinical Trials Management Associate
Similar Occupations
If you are interested in exploring occupations with similar skills, you may want to research the following job titles. Note that we only list occupations that have at least one corresponding NC State Online and Distance Education program.
- Clinical Research Manager
- Clinical Trial Manager
- Clinical Research Director
- Clinical Research Coordinator
- Clinical Project Manager
- Natural Science Research Manager (General)
- Clinical Quality Manager
Common Employers
Here are the employers that have posted the most Clinical Research Associate jobs in the past year along with how many they have posted.
United States
- IQVIA (456)
- Actalent (305)
- Johnson & Johnson (290)
- Labcorp Drug Development (223)
- Boston Children's Hospital (182)
- Grifols (167)
- University of Pennsylvania (123)
- Merck (120)
- Children's Hospital of Philadelphia (114)
North Carolina
- Labcorp Drug Development (27)
- Actalent (23)
- East Carolina University (19)
- University of North Carolina (14)
- Grifols (13)
- North Carolina State University (11)
- Thermo Fisher Scientific (10)
- Novasyte (10)
- The Henry M. Jackson Foundation For The Advancement Of Military Medicine (9)
NC State Programs Relevant to this Career
If you are interested in preparing for a career in this field, the following NC State Online and Distance Education programs offer a great place to start!
All wages, job posting statistics, employment trend projections, and information about skill desirability on this page represents historical data and does not guarantee future conditions. Data is provided by and downloaded regularly from Lightcast. For more information about how Lightcast gathers data and what it represents, see Lightcast Data: Basic Overview on Lightcast's Knowledge Base website.

How to Become a
Clinical research associate (cra), quick degree finder, why we love it.
- $136,570 Potential Avg. Salary
- 3.3% Job Growth Rate
- Growing Demand Job Outlook
- Don't Take Work Home Career Attribute
As a clinical research associate (CRA), your chief responsibility is to collect and organize data from research and field-studies. In this role, you would be running clinical trials to ensure the performance and safety of drugs before they are accessible in the market.
Recommended Schools
What is a clinical research associate (cra).
There will be several tasks allotted to a CRA depending on the company, but some of them are presented here:
- Collaborates with investigators and research staff to successfully implement clinical trials in accordance with regulatory requirements and protocols.
- Evaluates that quality of materials and collected data, including supporting the on-site personnel with internal audits and inspection.
- Identifying and assessing sites as potential facilities for conducting clinical trials.
- Verifying the integrity of data entered on case report forms or (CRFs) in comparison with clinical notes of a patient.
- Preparing reports, research manuscripts for publication and other documents for record filing and collation.
Day In The Life
A day in the life of a CRA can differ greatly. Clinical trials are carried out at various stages and are conducted on healthy humans or patients suffering from a particular disease. Generally, your work schedule revolves around establishing secure trial sites, monitoring each and every trial and maintaining records of the procedure for study documentation.
Once trials are completed, you are also part of the team that closes down the site in compliance with industry standards and ensures that all unused trial supplies are flagged. Further, recruitment of efficient investigators or consultants for conducting clinical trials might be part of your work day.
If you are in a field-based role, you are likely to travel often – 3 to 4 days a week – and be on the road. Your day involves working alongside doctors and research nurses at trial centres or hospitals to supervise and evaluate duties regarding clinical trials. On the other hand, your role can be in-house at an office environment, where you will mostly verify trial correspondence such as reports and manage projects.
Work Schedule And Typical Hours
Work locations for these roles can be few and far between – often located in select areas of the country. This can require extensive commuting and longer hours at work. Working hours can vary, with overtime, weekend and holiday work days being fairly common. There will be many tight deadlines which will result in a fast-paced work environment among teams.
Positions in the field are also an option whereby you are based in a specific area and working at a study centre to compile relevant research. Short-term contracts of 6-12 months with a private company is another flexible work option, rather than becoming a full-time employee. A position with international companies will lead to arranging for clinical trials in other countries, such that overseas travel is required.
Growth Of The Job
The US Bureau of Labour Statistics (BLS) projects faster than average growth of around 18% for clinical and medical lab technicians. Working as a clinical research associate leads to career opportunities in a growing sector – pharmaceutical research and development. There is a rise in employment potential of this sector due to companies looking for accurate ways to verify medical products prior to distribution, in order to safeguard against legal proceedings or irregularities.
Typical Employers
Clinical Research Associate positions are frequently offered by research-based medical drug and device companies like Phoenix Medical Systems, Medpace, etc. Clinical research institutes are also interested in recruiting for CRA positions, such as the Sarah Cannon Research Institute, focusing on providing advanced therapies for cancer patients, and the Walter Reed Army Institute of Research, which conducts basic and applied research in biomedical science.
How To Become a Clinical Research Associate (CRA)
A relevant college degree is required to find job opportunities in this field. For instance, you could get a college nursing diploma or complete a bachelor’s degree in biomedical science, health science, pharmacology or pharmacy. Some employers also prefer a master’s degree, demonstrating a technical specialty. Having a grasp over multiple languages gives aspiring candidates a competitive edge as they would be comfortable in international positions.
Further, clinical research associates are expected to be very organized and possess superior analytical and statistical skills. They should be great at solving complicated problems with an evidence-based approach and collect accurate data. Part of the job involves coordinating with professionals from various walks of life, including doctors, research nurses, clinical trial sponsors and participants. Thus, you must have solid communication skills to succeed in this demanding field.
Clinical Research Associate (CRA) Salary Data
We’ve provided you the following to learn more about this career. The salary and growth data on this page comes from recently published Bureau of Labor Statistics data while the recommendations and editorial content are based on our research.
National Anual Salary
National hourly wage.
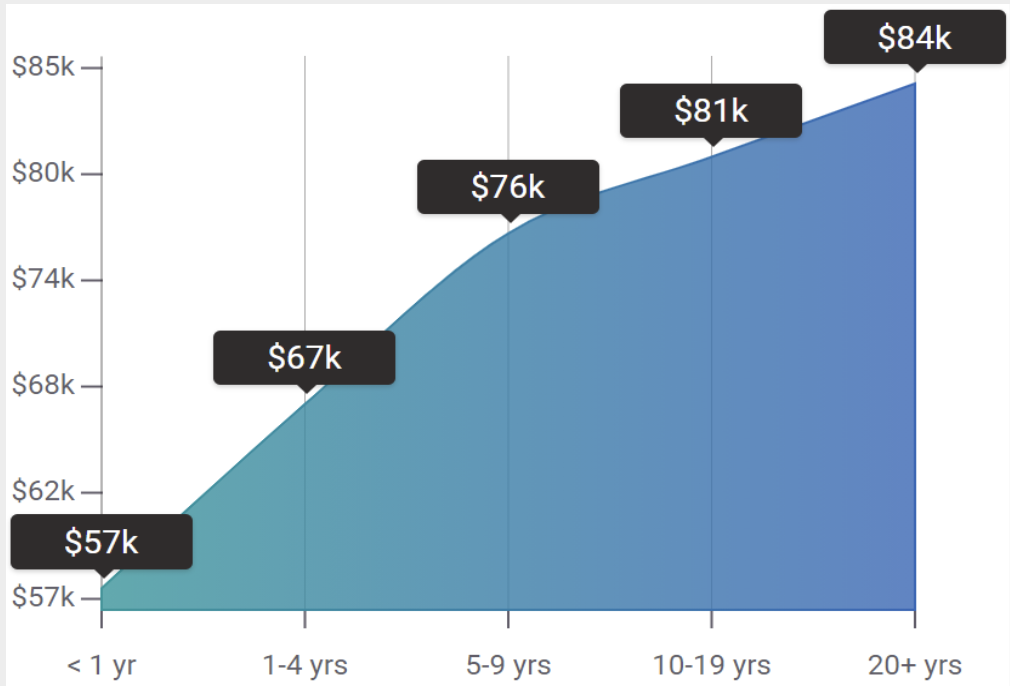
How do Clinical Research Associate (CRA) salaries stack up to other jobs across the country? Based on the latest jobs data nationwide, Clinical Research Associate (CRA)'s can make an average annual salary of $136,570, or $66 per hour. On the lower end, they can make $91,990 or $44 per hour, perhaps when just starting out or based on the state you live in.
Salary Rankings And Facts
#20 nationally for all careers, above average salary nationally, highest education among clinical research associate (cra)s.
- 27.2 % Doctorate
- 31.4 % Masters
- 33.2 % Bachelors
- 2.3 % Associates
- 4 % College
- 1.4 % High School
- 0.6 % Less than High School
Job Growth Projections and Forecast
2014 total jobs, 2024 est. jobs, job growth rate, est. new jobs.
How does Clinical Research Associate (CRA) job growth stack up to other jobs across the country? By 2024, there will be a change of 1,800 jobs for a total of 56,900 people employed in the career nationwide. This is a 3.3% change in growth over the next ten years, giving the career a growth rate nationwide of Above Average.
Growth Rankings And Facts
#510 nationally for all careers, above avg. growth nationally, what companies employ the most clinical research associate (cra)s, similar careers, welfare caseworker, correctional counselor.

Human Resources Specialist (HR)

Conservation Officer

District Attorney or Prosecutor

Fraud Investigator

Human Resources (HR) Manager or Director

Courtroom Clerk

Marine Corps Recruiter

Want To Be a Clinical Research Associate (CRA)? Get Started!
Generate your free SmartPlan™ to identify colleges you like, and potential ways to save on a degree or certification program toward your career with courses, offers, and much more!
Enroll Now and Get Started
or Learn More →

Clinical Research Associate: A Full Guide on Becoming A CRA

Clinical Research Associate
A complete guide on how to become a clinical research associate.
Over 1.9 million students receive a bachelors of science every year. While a few go on to PhD, Masters, and Medical programs; many are ready to start clinical research certification online to start a career in the frontiers of medical research and patient care.
As a new student applying to the science job market, you may only find internships or recognize that even entry-level science jobs requires 1-2 years of experience. More so, you may realize many of these jobs require intense labor in the lab or just did not meet your expectations for your science degree.
This is why a career as a CRA should be considered with clinical research coordinator training. We train over 500 students each month in clinical research coordinator training and clinical research associate training (depending on prior background).
For those who have always wanted a career in medicine or have a gap year before medical school; Clinical Research Training is the next step to getting a head start in your career.
Because the position is unlike actually working in the lab and more of a management role; you get 1-on-1 connections with physicians and medical staff that can lead to a better application for medical school and other medical careers later on.
Best of all; many of these positions accept remote staff (and some allow you to travel 45-75% with full expenses including travel, accommodation, meals, and other per-dime expenses covered).
Clinical Research Training can help you save money while also increasing your salary. CRA’s with our level of training can expect to make between $6,500-$12,000 a month with an estimated promotion rate of 33% a year: an amount that is uncommon in other science-degree careers.
CCRPS is one of the only major US-based ACCRE, ACCME, ANCC, ACPE, and Transcelerate Biopharma accredited CRA certification courses that accepts students with no prior background for certification. T
his is because our course is thorough and created by Senior CRAs who have been in the field for long enough to understand what you need to know to begin working and applying. The course can be completed in as little as 7 days with dedicated full-day study time.
Clinical Research Associate Certification Qualifications
A Clinical Research Associate or Coordinator directs and supervises clinical trials that are run by physicians, nurses, and other science-degree holders.
Many CRA students are actually matriculated foreign doctors who opted not to take the USMLE or repeat their residency training. In fact, some of our Clinical Research Training Students come to us immediately after moving to the U.S. wondering what to do with an MBBS degree in US.
Unlike what you’ve learned during your 3-8 years in university or graduate school, Clinical Research Training after your degree is rarely a repetition of any course you’ve taken before.
Thus, we have 110 Clinical Research Training modules (more than any other course available) to make sure you get the position you want as a CRA.
Unlike the jobs you currently can apply to on the market, a position as a CRA is actually much more difficult to obtain.
While many generic courses exist on the market; we have seen that many of these students cannot find a job afterwords because of the lack of content depth. This is why our course offers a Senior Clinical Research Associate level of training with 110 intense modules.
This science-based medical position is now a high-demand job which can be done privately for pharmaceutical companies such as Pfizer, or academically in medical schools. In addition, we have the largest number of clinical research courses online.
Why Take A CRA Certification Course
The role of the clinical research associate is to ensure that medical devices, new treatments and new drugs are approved for patients' use.
This field is taken as a certificate program course in many schools. For example, you may find associate degree programs. These programs can be completed in two years and can be offered through both the online and the hybrid formats. Hybrid formats combine both online and on-campus courses together.
If you opt for a fully online program, you can still get an immersive education. Different platforms like emails and discussion boards are used to ensure and promote interaction between the students as well as the lecturers.
Online learning platforms are used to upload the syllabus, course materials, lectures and assignments. Some online programs include field work as part of their requirements, in order for students to gain first hand experience working with clinical trials and patients. Depending on the school, they may have a list of approved clinical research institutes and other facilities. Otherwise, you will have to find a facility for yourself and get the school's approval.
These certificate programs are generally designed for professionals that are already in the medical fields (like medical assistants or nurses) and are interested in moving to the field of clinical research.
They may therefore ask for a copy of your CV or resumé or they may ask for a letter from your employers to verify that you have the needed medical experience. Some programs may require just an undergraduate degree in a medical science or life science related field.
Clinical research associates are trained to assist clinical researchers and investigators in the coordination, administration and management of clinical trials.
During this training, different courses will be taught revolving around subjects like safety procedures, subject recruitment, regulatory requirements, drug development, accountability, trial management, medical terminology etc.
The importance of the role of the clinical research associate means that companies that conduct clinical trials are usually very selective. The need to comply with strict regulations often inform their decision when making a choice of their clinical research associate. It is therefore very difficult to get a job as a clinical research associate without previous experience in clinical trials.
Many companies require around at least two years experience in clinical monitoring as a clinical project assistant or clinical trial administrator before considering applicants for this important role.
In applying for the post of a clinical research associate , ensure that you read the job description and indicate or highlights the relevant experience on your curriculum vitae. Your cover letter should be specific to the company you're applying to.
Do not use a one-for-all cover letter. Personalize your cover letter to each company and highlight the skills that fit the specific requirements of the role. Not all companies advertise their vacancies, so you can try to find out about other unadvertised vacancies, you might increase your chances.
Further certification can enhance your resume such as the ACCRE accredited CRA program which contains 110 learning modules for Clinical Research Associate Training and Placement
The Best CRA Certification Course For Entry-Levels
There is a huge shortage of well-trained CRAs, but many companies are reluctant to hire untrained entry-level clinical monitors because of patient and trial safety. Because of this, even the beginner entry-level jobs require certification or training.
Our program is considered one of the top clinical research graduate programs online. Most courses provide very light training that may look good because of the company names, but alone is not sufficient to pass the interview rounds a company conducts.
Because our modules are prepared help even Senior Clinical Research Associates, we find more of our students with no background quickly passing their interview rounds.
CCRPS Course covers double to triple the amount of course content than other courses. While many courses are simply 5-20 simple interactive modules, our course covers 140 dense modules in thorough detail.
After each session, students can ask their questions privately with the course instructor, all of whom have 15+ years of CRA experience.
Currently, 82% of our students are hired within the first month of taking the course. Students with limited background or those looking to gain extra experience are offered a remote internship of up to 6 months during the time they are interviewing.
This advantage allows many students with limited experience to get hired with a higher paying job than previously offered.
While a majority of our students are physicians, a majority of the CRA workforce are Science Grads and Nurses. nonetheless, we train all students at a Senior CRA level regardless of background because clinical research monitoring is vastly different from any lab or science course you may have taken.
Clinical research associates are given the protocol of a study including all medical protocol that must be followed but because they do not diagnose or treat. Medical knowledge is supplemental but not sufficient in this career path.
This is the main reason why our Clinical Research Training includes all possible scenarios you may face at the protocol and guideline level in your future company.
How To Get Experience For Clinical Research Associate Jobs
CCRPS, like other educational institutes, is only associated with educating and certifying clinical research professionals so we do not provide job placement. We want to make sure you apply with your best foot forward. Below are links we readily refer to graduates who are looking for job support. Having a great CV and cover letter are essential to applying for jobs. Recruiters are paid by the company which hires you and thus are free for searching employees. Be realistic but also be driven. Make sure you get continue reaching out until you get a true rejection from any job you apply to as they may never have seen your application if you received no response.
Clinical Research Job Advising: Kunal at ClinicalTrialPodcast
Free Resume Review: TopCV TopCV provides a free review and feedback for your current resume.
Resume Distribution: ResumeRabbit Resume rabbit distributes your resume to 60 job posting sites.
Clinical Research Recruiters: I-Recruit I-Recruit distributes your resume to clinical research recruiters.
Clinical Research Job Bulletin: Indeed Indeed usually provides the most uptodate job bulletin for clinical research jobs
Always use a cover letter specific for the company and job when applying if you are not using a recruiter.
The ICH-GCP in Clinical Research
Regardless of the type of clinical research or function of an IP being tested, it is important that clinical research should meet two critical criteria:
The clinical research process should respect the rights, freedom and dignity of tested patients (human participants).
Data from the clinical research process should be accurately collected, safely stored, rigorously scrutinized and correctly interpreted.
One way to ensure that these requirements are met is to follow a set of internationally recognized and accepted standards for clinical research.
Most countries across the world today follow ICH-GCP, that is, International Committee for Harmonization of Good Clinical Practice guidelines in conducting clinical research on human participants7.
The ICH-GCP outlines procedures and precautions that are essential in order to protect the safety and wellbeing of human research participants during clinical research, and to ensure the integrity of data from clinical research studies.
In the USA, clinical studies are required to comply with the FDA Guidance for Good Clinical Practice, outlined in a document titled ‘E6(R2) Good Clinical Practice: Integrated Addendum to E6(R1)’8.
In the USA, clinical studies are required to comply with the FDA Guidance for Good Clinical Practice, outlined in a document titled ‘E6(R2) Good Clinical Practice: Integrated Addendum to E6(R1)’8.z
Qualifications and Qualities of a CRA
According to the International Accrediting Organization for Clinical Research (IAOCR), candidates for CRA positions usually hold either a biological science degree, or one in medicine or nursing10.
The New Scientist recommends that aspiring CRAs should possess a good working knowledge of one or more of the following subjects – anatomy, biology, biochemistry, chemistry, immunology, microbiology, pharmacology, physiology or toxicology11.
In addition to a background in medical or life sciences, a CRA is required to have a good grasp of data management, including Electronic Data Capture (EDC), data analytics and reporting12.
Sketching the CRA work profile, the authors Diane St. Germain and Marjorie Good state that CRAs are the ones who scrutinize clinical study data most closely from start to finish—as a result, they are often the first to notice critical patterns and interesting trends, and to report these to the research team as well as to the CRO13.
Equally if not more importantly, a CRA must possess a high level of emotional and interpersonal savvy. This is a crucial area, since a CRA’s success hin ges upon his/her ability to elicit the best from team members, in terms of both performance and probity.
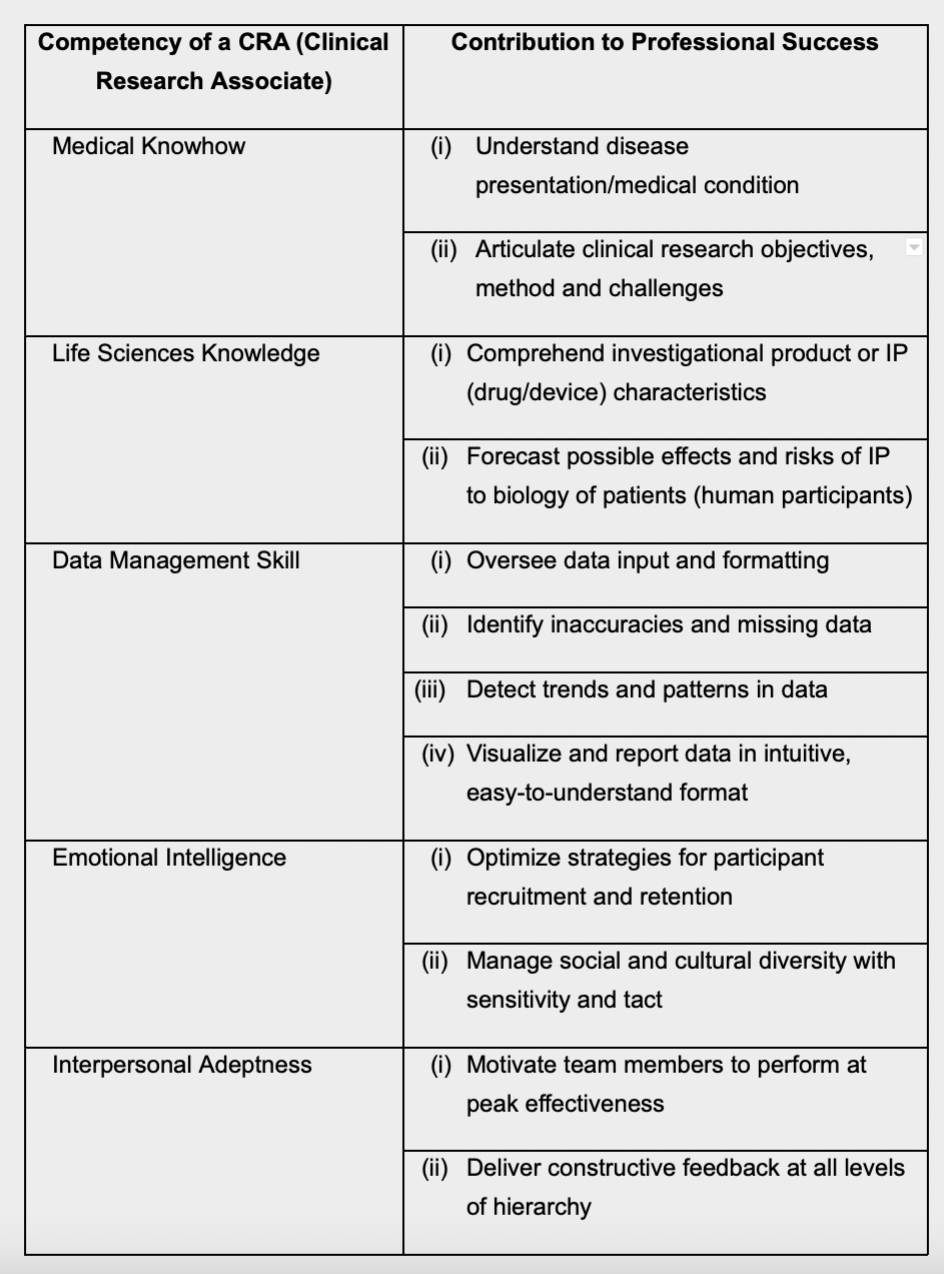
Core Competency Framework for CRAs
To illustrate, the ACRP’s ‘Core Competency
Framework for Clinical Study Monitoring’
requires that a CRA should be able to identify
and correct compliance violations at a study
site. The CRA must not only bring such
violations to the attention of site staff, s/he
must induce them to take corrective action,
as well as reporting the matter and even
escalating it, where necessary14.
The table below summarizes the ideal
competencies of a CRA, and provides
insights on how each ability contributes to
the CRA’s performance.

CRA Career Path
In the past, CRA positions were often filled by individuals with medical or nursing backgrounds, with little thought given to their lack of research training15. As awareness grew about the importance of research experience for a CRA, employers began preferring those with years of experience in clinical research settings, such as Clinical Trials Assistants (CTAs) and Clinical Research Coordinators (CRCs)16.
However, in recent years, the focus has shifted once again from a tenure-based mindset to a skills-based evaluation17. In part, this change has been brought about by the growth in professional courses and training programs in the field.
For instance, many leading US Universities today offer master’s programs in clinical research18. In addition, there are some widely recognized certification programs for clinical research associates, such as those offered by the ACRP19 and the Society of Clinical Research Associates (SOCRA) 20.
Note: You must already be working as a CRA to qualify for the ACRP and SOCRA certification programs.
A Toe in the Door: CRA Certification for a Non-CRA
By this point, you might be wondering, “I have no research experience… I’ve never worked as a Clinical Trials Assistant (CTA) or a s a Clinical Research Coordinator (CRC). Nor do I have a degree in Clinical Research. Can I still become a CRA?”
The simple answer is, yes, you can.
You might be a life sciences graduate looking for a lucrative career in the pharmaceutical or biotechnology sectors. Or, you’re excited by a career in research, but unsure whether the drudgery of a Ph.D. is your thing.
Maybe you’re just looking for a job that represents a great option for someone with your combo of science background plus detail-orientedness.
Whichever of these descriptions best applies to you, a career as a Clinical Research Associate could be exactly right for you.
With the right training, you can be recruited directly to a Clinical Research Associate position, even without a background in clinical research.
So, what kind of training will help me break through the ‘experience’ barrier and land a job as a CRA?
As you’ve already gathered from the table, the skill-set required to be a successful CRA is pretty extensive.
Aside from an in-depth knowledge of scientific and medical concepts and principles, a CRA must have a sound grasp of medical research regulatory requirements, a penchant for being thorough and systematic, as well as a knack for coordinating and managing people with diverse skills, roles and backgrounds.
To our knowledge, CCRPC’s ‘Advanced Clinical Research Associate Certification’ (ACRAC) is one of a kind: The ACRAC is the only multi-accredited* certification program in the US that offers the kind of exhaustive as well as intensive training that equips candidates from a non-clinical background with the abilities and competencies that make a good CRA.
Best of all? The ACRAC is open to fresh graduates holding a B.S. degree in any of the life sciences, with no requirement for prior exposure or experience in clinical research.
*The ACRAC program offered by CCRPC is accredited to ACCRE (Accreditation Council for Clinical Research & Education), ACCME (Accreditation Council for Continuing Medical Education), ACPE (Accreditation Council for Pharmacy Education), ANCC (A merican Nurses Credentialing Center), as well as Transcelerate Biopharma.

Training to be a CRA through CCRPS ACRAC
The ACRAC program includes over 100 course modules that cover all the important knowledge domains and skill-sets required by a CRA.
Designed for a total study time of approximately 250 hours, this training program can be completed at your own pace, or, for those able to dedicate the whole day to study, in as little as two to three weeks.
Starting with a broad overview of clinical research jargon and terminology, the course walks students through the principles of Good Clinical Practice, familiarizing you with the relevant sections of the ICH-GCP and the FDA’s E6(R2).
The program places particular emphasis on ethical practices in research with vulnerable populations.
Students going through the ACRAC are trained in all major aspects of designing a Clinical Trial Protocol in keeping with the Code of Federal Regulations (CFR).
They additionally learn the steps involved in the IRB/IEC approvals process and how to prepare required documents.
Finally, students become aware of the importance of pharmacovigilance and the regulatory process for new drug testing.
A major chunk of the ACRAC certification centers around equipping the CRA for day-to-day responsibilities, such as different types of site visits – preliminary (Site Qualification), preparatory (Site Initiation) and progress monitoring visits (Routine Monitoring).
Crucially, the ACRAC covers essential documentation such as the Case Report Form and Trial Master File, as well as electronic data capture (EDC) and remote monitoring systems.
A vital component of the training program involves empowering students to tackle challenging situations.
For a CRA, these include identifying protocol deviations and violations, and recognizing as well as reporting research fraud and ethical misconduct.
In addition to its comprehensive coverage, the ACRAC certification offers the great advantage of including 17.5 CME credits – that is, course credits that count towards ‘Continuing Medical Education’.
These credits can be used by individuals desiring to further their education and/or careers in healthcare-related fields, including medicine, nursing, pharmacy and research.

Clinical Research Associate Training
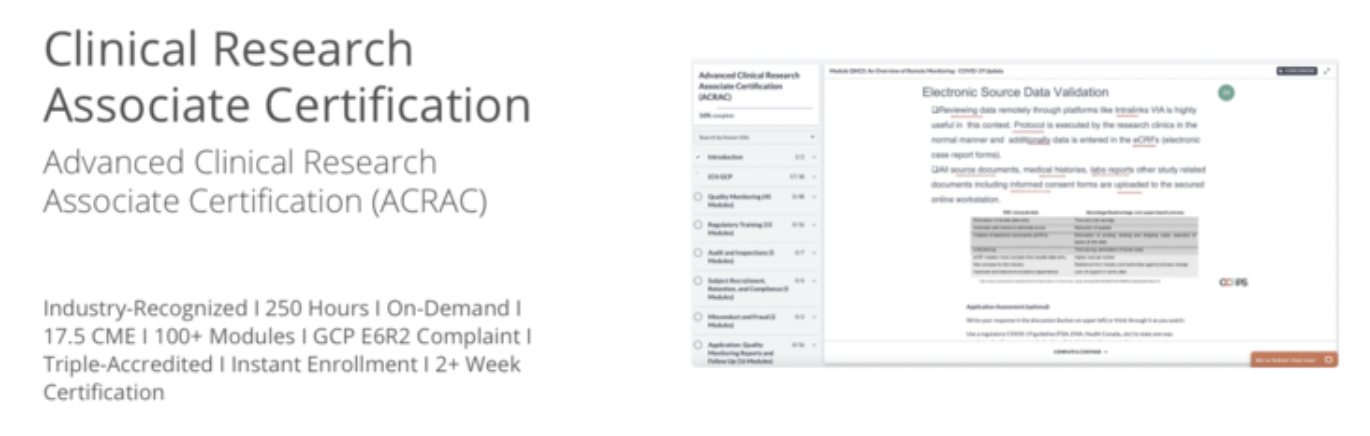
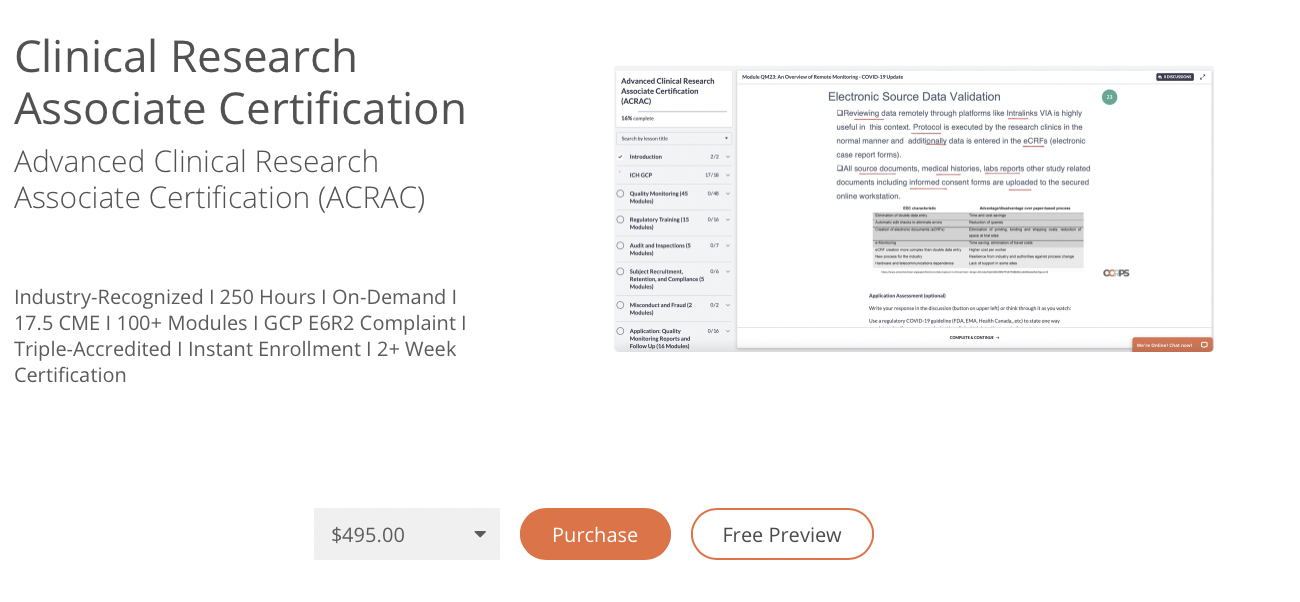
Get ahead in clinical research with advanced accredited online CRA certification for $450. Demo our on-demand course below.
Clinical Research Associate Certification
Advanced clinical research associate certification (acrac).
Chapter 1: Introduction
This chapter orients you to the concept of Continuing Medical Education (CME) and outlines how the CCRPS CRA program contents meets AMA requirements for CME. Given that, across the US, physician practitioners are required to complete between 20 and 50 hours of CME credits yearly, the ACCME-accredited CCRPS CRA course can be used not only to build knowledge and skills in the field of clinical trial management, but also to further a successful medical career. Additionally, the introductory chapter introduces you to the clinical terminology and abbreviations commonly encountered in clinical research, for example, Investigational Product (IP), Good Clinical Practice (GCP), Institutional Review Board (IRB) and so on.
Chapter 2: Roles and Relationships in Clinical Trials
The unit presents the foundational background to beginning and building a career as a clinical research associate (CRA). As you know, a CRA plays a critical role in setting up as well as monitoring the clinical trials process for an investigational product or IP – a medical drug or device under development. In this unit, you will learn how a CRA interacts with other stakeholders, including the Clinical Research Organization (CRO) or Sponsor of the clinical trials, the Principal Investigator (PI) as well as other research site staff, the trials monitoring team including the Clinical Research Coordinator (CRC),other CRAs and the Data Safety Monitoring Board (DSMB), as well as the research ethics committee (Institutional Review Board or IRB).
Chapter 3: Sponsor and Investigator Roles
In this unit, you will gain insight into the ICH-GCP guidelines, particularly addendum E6, sections 2 through 5, which outline procedures and precautions essential for protecting the safety and wellbeing of human research participants during clinical research. These include guidelines for obtaining informed consent from human subjects, maintenance of trial records, reporting of compliance, safety and research progress, as well as procedures for suspension or termination of the trials process. The chapter familiarizes you with the critical importance of monitoring for Adverse Events (AEs), including types of AEs and regulations for documentation and reporting.
Chapter 4: Clinical Trial Design
In this chapter, you will acquire insight into the different phases of the clinical trials process, from the pre-clinical phase through Phases 0 to 4 of clinical testing. The unit will familiarize you with important concepts of clinical trials, such as the structure and goals of each phase of clinical trials, approaches to dosing, toxicology of pharmaceutical products, in vitro and in vivo testing, dose escalation and so on. Finally, the chapter reviews the FDA’s drug approval process.
Chapter 5: ICH-GCP – Overview
The chapter dives deep into GCP, including a review of the history of medical research leading up to the ICH-GCP. The unit covers all four QSEM categories of the guidelines for ensuring Quality, Safety and Efficacy of the IP, as well as Multidisciplinary guidelines (mainly pertaining to documentation and electronic data safety standards). In addition, the chapter includes an overview of MedDRA software that provides a standardized system of terminology and notation for documenting clinical research, as well as principles of budgeting for clinical trials.
Chapter 6: Ethical Research in Vulnerable Populations
The unit provides a detailed walk-through of the regulations and compliance requirements for conducting clinical trials with human subjects who meet the definition of a ‘vulnerable population’, including pregnant women and fetuses, children, mentally incapacitated individuals (those with cognitive functioning impaired by neurolopsychological conditions or chronic substance abuse), as well as prisoners. You will acquire familiarity with the challenges of research in such populations, including the requirement for parental consent, fair but not excessive incentive, justifiable deception or incomplete disclosure, coercive practices and so forth.
Chapter 7: Adverse Events
Through this module, you will gain a bird’s eye view of the protocol for documenting, reporting and responding to AEs or adverse events during the clinical trials process. The unit covers concepts such as expectedness, severity and seriousness of AEs, Adverse Drug Reactions (ADRs) as a sub-category of AEs, Investigational New Drug or IND reports, causality analysis for AEs and so on. In addition, the chapter reviews the responsibilities of both research sponsors as well as IRBs in sharing AE information with subjects.
Chapter 8: Clinical Trial Protocol
The chapter provides an in-depth tutorial on the structure and elements of a CTP or clinical trial protocol, as well as guidelines on writing a CTP. Important concepts reviewed include study Risk Benefit Analysis (RBA), study sample statistics (sample size, statistical power, plan for data analysis), risk management and study administration. Additionally, the module covers concepts central to study sample selection, addressing inclusion and exclusion criteria, especially safety and ethics considerations in sampling.
Chapter 9: Protocol Deviations and Violations
Through this unit, you will gain familiarity with the many potential causes of protocol deviations and violations, learning to distinguish between minor (deviations) and major departures or violations of protocol. Content provides understanding of the most commonly occurring violations, including both minor (off-schedule subject assessments, subjects’ use of prohibited drugs, and so on) as well as major violations (failure to obtain informed consent, failure to report AEs and so forth). Further, the chapter reviews principles for reporting protocol deviations, IRB approval for planned deviations and related concepts.
Chapter 10: IRB and DSMB
This chapter briefly reviews the history of IRBs and examines the principles guiding IRB decision-making. In addition, the unit discusses recent developments in compliance, including sIRB (single IRB) and SmartIRB for institutions that are part of the CTSA (Clinical and Translational Science Awards). The bulk of this module dives into the categories of IRB review, including full board and expedited review, examining criteria for review exemption such as educational or purely behavioral research, as well as studies collecting identifiable data, surveys and interviews.
Chapter 11: Review Questions
The module provides a self-assessment tool by including questions that review the content covered in previous chapters. The set of 71 questions examines all aspects of ICH-GCP previously discussed.
Chapter 12: Site Monitoring Visits
In this module, an overview is provided of the different types of site monitoring visits, including site selection or qualification visit, study initiation visit, routine or progress monitoring visit, as well as study termination or close-out visit. Important concepts discussed include pre-qualification preparations and site feasibility assessment as well as study monitoring criteria (data omission, incorrect entries, inaccurate calculations, documentation of corrections and so on). For each type of site monitoring visit, the chapter reviews relevant documentation.
Chapter 13: Site Qualification Visit (SQV)
The chapter gives an in-depth understanding of the stages and steps involved in selecting a study site. Elements reviewed within the module include the process of investigator selection and criteria for site evaluation (the four P’s: Patient, Protocol, Performance, Profit). Importantly, the module reviews the most common errors in feasibility assessment, including overestimation of sample availability at site, selection of site staff with low motivation, poor-performing sites owing to high competition for personnel and resources (for example, owing to multiple studies running on a single site), and so on.
Chapter 14: Site Initiation Visit (SIV)
The module dives into the details of an SIV or site initiation visit. You will review the procedure for pre-SIV preparation, including filing for IRB and other necessary approvals, permits and licenses. Additionally, the chapter examines elements of the SIV agenda, mainly orientation and training of site staff, creation of important study-related documents such as the Trial Master File (TMF) and post-SIV filing of compliance documents such as FDA form 1572 and Financial Disclosure Form (FDF) for relevant site personnel.
Chapter 15: Routine Monitoring Visit (RMV)
In this unit, the elements of a routine or periodic monitoring visit are discussed in detail. You will become familiar with the agenda of an RMV, which prioritizes receiving updates on AEs from site staff (incidence, documentation, seriousness and so on), as well as oversight of the overall progress of trials. The chapter covers different approaches to site monitoring, contrasting traditional (full-scale) monitoring with risk-based monitoring (RBM), as well as comparing on-site monitoring with remote monitoring. A crucial concept addressed by the unit is Source Data Verification (SDV), which is central to obtaining meaningful, high-quality data from clinical trials.
Chapter 16: Site Close-Out Visit (SCOV)
The module gives you a comprehensive overview of the protocol and procedures involved in terminating or closing out a trial site. Aspects covered in the chapter include pre-SCOV preparations such as IRB notification and schedule coordination among site staff (PI, other investigators, medical staff) and monitoring team (CRC, CRAs and so on), agenda for an SCOV – drug inventory management, database verification and lockdown, subject intimation and completion of all subject-related documents, staff-related documentation as well as other administrative tasks including close-out report compilation.
Chapter 17: Tools for Monitoring Visits
This unit outlines a host of tips and tools that can help a CRA in successfully tackling the complex process of monitoring clinical trials. The chapter lists numerous physical accessories you can use for effective monitoring, including scheduling and calculation aids, ready reckoners for drug information and medical terminology, as well as document templates to speed up the process of obtaining trial updates while also serving as checklists for the site visit agenda. Additionally, the unit highlights helpful strategies that a CRA can use to ensure that site visits go smoothly, from travel advice to team-building suggestions.
Chapter 18: Audit and Inspections
The module deals with one of the most crucial and often most feared aspects of a CRA’s career – audits and inspections by the CRO (sponsor), FDA or other regulatory authority. Starting from the basic distinction between an audit and an inspection, the chapter covers in detail the protocols for both audits and inspections. Crucially, the chapter will enable you to grasp the difference between a routine audit/ inspection and a ‘for-cause’ audit/ inspection. Further, it lays out the sequence of an FDA inspection in full (including a detailed walk-through of the FDA BIMO or Biomedical Research Monitoring Program inspection), and provides important guidelines on the do’s and dont’s for CRAs during an audit/ inspection, such as the critical ‘3 to 5 minute rule’. You will acquire familiarity with important audit and inspection-related documents such as FDA Form 482 (Notice of Inspection) and Form 483 (Notice of Observation) as well as the Establishment Inspection Report (EIR) prepared by the auditor/ inspector. Finally, you will gain insight into the classes of observations provided in an EIR, including NAI (no action indicated), VAI (voluntary action indicated) and OAI (official action indicated)—the last is commonly termed an ‘FDA warning letter’.
Chapter 19: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, as well as a 15-item quiz. Questions and quiz examine all aspects of clinical trial quality monitoring, including monitoring visits, tools as well as audits and inspections.
Chapter 20: SDV and Informed Consent
In this chapter, the ICH-GCP section 4.8 guidelines on obtaining informed consent from subjects are discussed in detail, highlighting the need for using non-technical language, transparent delineation of risks, consent without undue influence, obtaining consent (and assent) from minors and their Legally Acceptable Representatives (LARs), as well as consent from non-English speakers and sedated subjects. The chapter additionally covers important aspects of Source Data Verification (SDV) with respect to electronic as well as paper-based medical records, and highlights the central goal of SDV, which is to conform to ICH-GCP requirements that subject trial data (as recorded in Case Report Forms or CRFs) must correspond to source data (previous medical records).
Chapter 21: Case Report Form
The module provides an in-depth tutorial on the structure and elements of a Case Report Form or CRF, including the different forms for PI verification, subject enrollment, eligibility and randomization, medical history, physical examination and laboratory data, compliance, adverse events and so on. In addition, the chapter outlines important data notation rules, such as the use of accepted acronyms (‘ND’ for missing data and ‘UNK’ for unknown information, MM-DD-YY format, time-stamp data and so forth), as well as guidelines for the design of CRFs (such as consistency of notation, avoidance of data fields that can be computed and of duplicate data fields and so on).
Chapter 22: Quality Control and Safety
Within this unit, you will learn the central concepts of Quality Control (QC) in the context of clinical trials, including definitions of QC and its relationship with the complementary process of Quality Assurance (QA), the use of Key Performance Indicators (KPIs) in QC, need for a Corrective and Preventive Action (CAPA) plan and so on. Additionally, the module examines the QA process, focusing on the central role of RBM or risk-based monitoring in present-day QA as well as providing guidelines on Quality Metrics (QMs) for evaluating the trials process. The chapter also reviews ICH-GCP guidelines on subject safety, underlining risk-benefit assessment, stoppage rules (for instance, in case of SAEs) and reporting responsibilities. Finally, it introduces the FDA’s Human Research Protection Program (HRPP) as a platform that provides training and support for personnel involved in clinical trials.
Chapter 23: Technology in Trials
In this chapter, an in-depth tutorial is provided of the systems used in modern clinical trials for Electronic Data Capture (EDC) and database management. Systems such as Interactive Response Technologies (IRTs) including IVRS and IWRS (Interactive Voice and Web Response Systems, respectively) as well as RTSM systems for Randomization and Trial Supply Management are examined. The unit reviews the benefits of standardized data management and data sharing, approaches to database management and the concept of an Independent Data Monitoring Committee (IDMC). Critical elements of data integrity, such as proper anonymisation and coding, completeness of data, data safety precautions and logging of site visits and other progress reports are highlighted. The unit further examines the essential features of a good Clinical Data Management (CDM) system that complies with FDA CFR Title 21 and HIPAA regulations, such as setting access privileges, tracking changes and updates, data security and locking, flagging and reconciliation of AEs and so forth. Finally, the chapter looks at CTMSs (Clinical Trial Management Systems) in depth, covering the aspects that allow management of day-to-day trials in multi-site studies.
Chapter 24: Modernized Monitoring (Remote, Risk-based, Centralized)
This chapter offers a detailed walk-through of modern, remote monitoring of clinical trials, which evolved into a full-fledged system in response to the COVID-19 pandemic. Important concepts discussed include the critical site initiation process, Electronic Source Data Verification (ESDV) and FDA regulatory guidance for remote monitoring of clinical trials. In this module, you will learn how FDA’s ALCOA (Attributable, Legible, Contemporaneous, Original and Accurate) criteria for data quality have been adapted to remote monitoring. Further, the unit discusses how HIPAA compliance in remote monitoring is achieved by using limited data sets (wherein sensitive individual information is concealed through anonymous subject codes) regulated by data use agreements. Finally, the unit examines how risk-based monitoring approaches have allowed centralized monitoring to evolve into a cost-effective and safe method for clinical trial monitoring.
Chapter 25: Pharmacovigilance and Regulatory Affairs
Through this unit, you will gain insight into the process and rationale behind pharmacovigilance (PV) and its central role in the clinical trials process. The chapter reviews the statistics on AEs, distinguishes between Type A and Type B AEs, and profiles seriousness of ADRs or Adverse Drug Reactions as well as the iGuard Drug Risk Rating System. Importantly, the unit covers ADR causality assessment in detail, including both severity and probability assessment. An important element of PV addressed in this module is the Individual Case Safety Report (ICSR), its structure, content and role in trial monitoring. Other concepts discussed include types of PV inspections (routine vs. ‘for cause’), PSURs or Periodic Safety Update Reports and study criteria for instituting DSMBs (Data Safety Management Boards). Finally, the module also reviews the domain of Regulatory Affairs (RA) as a function of PV, outlining roles and responsibilities of RA personnel as well as the importance of RA in streamlining the process of drug development by ensuring compliance throughout manufacturing, clinical trials, marketing and advertising.
Chapter 26: Investigational Product
In this chapter, an in-depth review is provided of the protocol for receiving, storing and dispensing the IP or investigational product. At every stage, guidelines lay down strategies for ensuring verifiability, accountability and safety of both study subjects and staff. Thus, IP handling precautions include the need for logging date of manufacture, temperature throughout transit, as well as batch number and individual unit numbers (such as bottle or tube identifiers) carefully and accurately, as well as recording shipping details and filing shipping receipts. Additionally, the unit addresses the need for IP dispensing precautions, such as limiting dispensation to authorized personnel only, as well as maintaining individual subject IP logs.
Chapter 27: Local and Central Labs
The module profiles the evolution of lab testing in clinical trials, from error-prone localized laboratory testing to centralized testing that allows homogeneity of testing procedures and measurements, thus minimizing errors and improving outcomes. The chapter reviews standards for clinical trial laboratories as per the GLCP (Good Clinical Laboratory Practice) and CLIA norms (Clinical Laboratory Improvement Amendments), as well as providing guidelines for lab audits, including fire safety, protective gear, staff training and so forth.
Chapter 28: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, as well as a 15-item quiz. Questions and quiz examine all aspects of trial documentation (SDV, CRF, ICSR), quality control, pharmacovigilance, as well as IP and lab guidelines.
Chapter 29: Regulatory Documents in Clinical Trials
The chapter reviews essential documentation to be created and maintained throughout the course of the clinical trials, including the Trial Master File (TMF), FDA forms 1571, 1572, 3674, 3454/3455 and CFR Title 21 Form 312, besides ethics approval documents such as the IRB-approved protocol, informed consent form, subject education and study advertising materials. You will acquire in-depth familiarity with each of these forms, and learn the importance of maintaining and updating records, for example by incorporating IRB revisions and amendments, periodic renewals of permissions and licenses and copies of submitted reports. In addition, the unit summarizes the need for filing documents outlining study- and site-specific procedures, including SOP (Standard Operating Procedure), MOP (Manual of Procedures), Investigator Brochure (IB), Delegation of Authority Log (DOAL), site staff CVs, SAE notifications, logs of subject screening and enrollment, IP storage (temperature, humidity, etc.) and all relevant study parameters.
Chapter 30: CFR Title 21 Part 11 – Electronic Signatures
This unit gives you an overview of Title 21 of the FDA Code of Federal Regulations (CFR), including Chapter 1 sections on informed consent (Section 50), IRB approval (Section 56) and so on, Series on food (100), pharmaceuticals (200 and 300) and so on, as well as FDA Drug Schedules. The major part of the module focuses on Part 11 which deals with Electronic Records and Electronic Signatures (ERES), laying down the criteria for determining safety and reliability (trustworthiness) of electronic data and signatures.
Chapter 31: New Drug Application
Through this module, you will gain knowledge of the FDA process for evaluating a drug under development, and the role of a CRA in streamlining this process. An important distinction covered here is the difference between an IND (Investigational New Drug) and an NDA (New Drug Application). The chapter discusses in-depth the criteria used in evaluating an IND, including toxicology and pharmacokinetics data, as well as requirements for different drug classes (oncology vs. non-oncology). Additionally, the unit covers FDA requirements for AE reporting, including assessment of seriousness, expectedness and format for expedited reporting of life-threatening SARs, as well as safety reporting requirements for investigators.
Chapter 32: Trial Master File
The unit provides a detailed breakdown of the organization of a TMF or Trial Master File, listing the various binders that should be included within the TMF, as well as their contents. Thus, the TMF should contain binders pertaining to the study protocol and IRB, investigator qualifications, FDA forms and correspondence, FDFs or Financial Disclosure Forms, communications with the CRO, and other relevant trial aspects. A helpful templatic guide to creating a TMF is also provided in this chapter, as well as a self-assessment quiz of 10 items on important sections of a TMF.
Chapter 33: Disclosures and Payments for PI, Site, Patients
In this chapter, FDA guidelines regulating financial disclosure are discussed in-depth, covering the definition of ‘conflict of interest’ and the stipulations of Title 21 Section 54 on disclosure requirements. The unit helpfully contrasts FDA requirements with Canadian and UK/EU policies. You will study real life case examples of conflict of interest, as well as lawsuits pertaining to financial disclosure disputes to help gain a better understanding of the potential problems arising from failure to disclose financial interests in clinical trials. Another important dimension covered in the module is the regulation of payments to PIs and other investigators as well as patient payments, which must comply with CMS (Center for Medicare and Medicaid Services) policy on ‘fair market value’ as well as the Federal ‘Anti-Kickback Statute’. The unit contains guidelines on clinical trial budgeting and subject payments. Finally, the chapter reviews IRB guidelines on advertising to recruit human participants for clinical trials, including stipulations against misleading and coercive language, as well as excessive incentives.
Chapter 34: Patient Recruitment, Retention and Compliance
The unit provides an overview of the process of patient (subject) recruitment in clinical trials, from population research to identify motives for participation, to media support for building up public awareness and interest, to community and physician outreach for referrals and enrollment. Additionally, the chapter identifies common barriers to meeting recruitment goals and outlines strategies for maximizing recruitment, such as relaxing overly stringent criteria, offering reasonable incentives such as travel reimbursement and highlighting benefits of participation. Similarly, the unit covers common causes of patient drop-out as well as strategies for minimizing drop-outs, such as improving patient experience (increased attention and listening to patients, flexible scheduling of visits to suit patients’ convenience and so on). Finally, the unit discusses novel strategies to increase patient retention and improve compliance in clinical trials; these techniques harness technology to yield better outcomes, for example, simplifying form completion through digitized forms with auto-fill features, gamifying elements of compliance reporting, and so forth.
Chapter 35: Misconduct and Fraud
This module discusses the various motives for committing scientific fraud and the fallout of fraudulent practices in clinical trials. A scale for classifying errors in clinical trial data is presented, with ‘honest, isolated mistake’ at one end of the spectrum and ‘deliberate data falsification with malicious intent’ at the other. Types of clinical data that may be falsified, methods used in falsification (fabrication, substitution, omission), as well as scenarios in clinical trials where falsification may be occurring are presented. Through this chapter, you will gain familiarity with the signs to watch out for during the actual clinical trials process.
Chapter 36: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, including questions on all aspects of regulatory documents, site documents (TMF and contents), trial budgeting and payments, patient recruitment and scientific fraud.
Chapter 37: Site Visit Templates
This module contains a set of templates that you can use for documenting the details of site monitoring as a CRA, either in their current form, or in a form adapted to the needs of your own study. The templates included in this unit include:
Site Qualification Visit (SQV) – checklist for preparations, questionnaire for assessing the site prior to the actual visit, assessment form and follow-up letter
Site Initiation Visit (SIV) – agenda for visit, confirmation letter to request PI attendance during SIV, report following SIV
Routine Monitoring Visit (RMV) – confirmation letter to request PI attendance, report following RMV, follow-up letter
Site Close-Out Visit (SCOV) – confirmation letter to request PI attendance, agenda for SCOV, report following SCOV, follow-up letter
CRA transition letter – document notifying site PI of appointment of new monitor (yourself as CRA)
Chapter 38: Interviewing and Career
In this unit, you will find suggestions and recommendations for making a positive impact in interviews for CRA positions, as well as tips and strategies for making rapid progress in a clinical research career.
Chapter 39: Final Examination
This module comprises a comprehensive 51-item, self-paced quiz to assess your competency in the skills and knowledge required for a Clinical Research Associate position.
https://www.beroeinc.com/category-intelligence/clinical-research-organizations-market/
https://www.linkedin.com/jobs/search?keywords=Clinical%20Research%20Associate&location=United%20States&geoId=103644278&trk=public_jobs_jobs-search-bar_search-submit&position=1&pageNum=0
https://www.centerwatch.com/articles/24791-demand-for-experienced-clinical-trial-professionals-outpacing-supply-acrp-says
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3317309/
https://www.niaid.nih.gov/research/dmid-investigational-product
https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
Dixon JR. 1999. The international conference on harmonization good clinical practice guideline. Quality Assurance. 6(2): 65-74. DOI: 10.1080/105294199277860
https://www.fda.gov/files/drugs/published/E6%28R2%29-Good-Clinical-Practice--Integrated-Addendum-to-ICH-E6%28R1%29.pdf
https://www.who.int/groups/research-ethics-review-committee/recommended-format-for-a-research-protocol/
https://iaocr.com/finding-first-clinical-research-job/
https://jobs.newscientist.com/en-au/article/a-career-in-clinical-research/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326906/
St. Germain DC, Good MJ. 2017. Data management in clinical trials. In: Gallin JI, Ognibene FP, Lee Johnson L, editors. Principles and practice of clinical research. San Diego: Academic Press. p. 531-545. ISBN 978-0-12-849905-4
https://acrpnet.org/wp-content/uploads/dlm_uploads/2017/04/clinical-study-monitoring-competencies.pdf
https://www.clinicalleader.com/doc/starting-a-career-in-clinical-research-things-we-wish-we-knew-0001
https://www.proclinical.com/blogs/2021-9/how-to-get-a-job-as-a-clinical-research-associate-cra
https://acrpnet.org/2018/06/11/5-clinical-research-trends-emerge-at-acrp-2018/
https://www.collegechoice.net/sciences/clinical-research/best-masters-degrees/
https://acrpnet.org/certifications/cra-certification/
https://www.socra.org/certification/program-overview/
Pharmacovigilance: A Complete Guide to Pharmacovigilance and Drug Safety Training
The ultimate guide to clinical research monitoring.
- Program Overview
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning, and advancing global health.
The Society of Clinical Research Associates (SOCRA) established the Certification Program for Clinical Research Professionals in order to create an internationally accepted standard of knowledge, education, and experience by which clinical research professionals will be recognized by the clinical research community. Those individuals so recognized may use the "Certified Clinical Research Professional" or "CCRP ® " designation.
Path to Certification
CCRP certification is awarded upon meeting two criteria: a successful written application and a passing CCRP examination score. The benefits of obtaining certification are numerous. It not only validates knowledge, skills, and abilities but also enhances credibility and peer recognition. Career advancement and increased earning potential become tangible outcomes, reflecting a commitment to standards, compliance, and integrity.
Scope and Standards of Practice
The standards upon which this certification program is based have been set forth by SOCRA to promote recognition and continuing excellence in the ethical conduct of clinical trials. It is the goal of SOCRA to encourage members, and assure the competency of certified members, in their knowledge, understanding, and application of the conduct of clinical investigations involving humans in accordance with the ICH Guidelines, the U.S. Code of Federal Regulations, and the ethical principles that guide clinical research. Members are expected to adhere to national, state, local and provincial regulations and to international guidelines published by the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and all applicable federal, state and local laws and policies.
Standards of Practice include an understanding of and application of basic concepts of Good Clinical (Research) Practice, including:
- The Nuremberg Code
- The Belmont Report
- The Declaration of Helsinki
- 21 U.S. Code of Federal Regulations – Parts 11, 50, 56, 312, 812
- 45 U.S. Code of Federal Regulations - Part 46
- ICH Harmonised Guideline for Good Clinical Practice E6(R2), and
- ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A)
- 42 CFR Part 11 (ClinicalTrials.gov)
Certification Exam
The SOCRA Certification Examination is offered in two formats: paper and pencil (at SOCRA sponsored sites), and computer based (at Prometric testing centers or through Home Proctoring).
SOCRA Sponsored Sites: Paper and Pencil
- Hosted exams offered in various location throughout the US and Canada.
- Visit the paper and pencil exam schedule for dates and locations.
- A complete application must be received by the deadline date as stated on the examination schedule.
- Score reports mailed to you in 4-6 weeks after exam.
Computer Based Testing: Testing Centers and Remote Proctoring
- Offered at Prometric testing centers throughout the world or through Home Proctoring
- Click here for a list of test centers.
- Allow 2-4 weeks for application processing.
- Once application is approved, schedule exam at a testing center. Exam sessions are available at least 6 weeks in advance.
- Score reports received immediately upon completion of exam.
Candidate Handbook
For more information, please view the Candidate Handbook.
Certification
- CCRP Certification Quick Facts
- Definition of a Clinical Research Professional
- Certification Program Policies
- Removal of CCRP® Credential
- Verify Certification
- Exam Overview
- Candidate Eligibility
- Application and Fee
- Computer Based Testing Exams
- Paper and Pencil Exams
- Refunds, Rescheduling and Retesting
- SOCRA Sponsored Exam Schedule
- Preparing for the Exam
- Preparation Resources
- Examination Results
- Host an Exam at Your Site
- Apply Online
- Exam Schedule SOCRA Sponsored Sites
- Requirements for Maintaining Certification
- Continuing Education Requirements
- Descriptions of Acceptable CE
- CE Recordkeeping Requirements
- Request for SOCRA CE for Courses / Workshops
- Installment Plan Payment
- Renewal of Certification
- Recertification Audit
- Recertification Learning Module
- Accreditation
Summary of Certification Activities
11,145 CCRPs (as of 12/31/2022)
- 1,391 candidates took CCRP exam
- 73% passed CCRP exam
- 2,649 CCRPs recertified
- 946 candidates took CCRP exam
- 65% passed CCRP exam
- 2,783 CCRPs recertified
- 2,060 candidates took CCRP exam
- 70% passed CCRP exam
- 3,801 CCRPs recertified
- 1,980 candidates took CCRP exam
- 71% passed CCRP exam
- 3,188 CCRPs recertified
- 104 exam sites hosted
- 2,175 candidates took CCRP exam
- 2,491 CCRPs recertified
- 91 exam sites hosted
- 2,141 candidates took CCRP exam
- 2,421CCRPs recertified
Map | myFSCJ | Search
- I'M LOOKING FOR...
- Current Student
- Future Student
- Military/Veteran
- Transient Student
- News & Events
- Faculty/Staff
- Explore Programs
- Short Term Programs
- Class Schedules
- Associate in Arts
- Associate in Science Degrees
- Bachelor's Degrees
- Certificate Programs
- Continuing Education
- Adult/ESOL Education
- Credit for Prior Learning
- FSCJ Course Syllabus Tool
- FSCJ Online
- Dual Enrollment
- Honors Program
- Future Students
- Steps to Enroll
- Orientation
- Admissions Events Calendar
- Transcripts
- Academic Advising
- First Year Experience
- International Students
- Student Records
- Request for Information
FUND YOUR FUTURE
- Tuition and Fees
- Financial Aid Services
- Scholarships
- Student Financial Services
- Student Employment
- Veterans Benefits
- Veteran Tuition and Waivers
STUDENT RESOURCES
- Career Development
- Student Life
- Personal Support Services
- Student Support Services
- Food Pantry
- Library and Learning Commons
- Student Computing Resources
- Public Safety
- Discover FSCJ
- Mission & Vision
- Human Resources
- Governance & Administration
- Employee Directory
- Office of the President
- District Board of Trustees
- Policies & Procedures
- Consumer Information
COMMUNITY ENGAGEMENT
- Center on Economic and Financial Education (CEFE)
- Center for Civic Engagement
- Vision Education & Rehabilitation Center
- FSCJ Foundation
- FSCJ Artist Series
- Academic Calendar
- Adult & ESOL Advising
- Assessment/Testing
- Bluewave Athletics
- Board Rules & APMs
- Calendar of Events
- 2024-25 Term & Session Calendar
- Campus Tours
- Can't Find A Class
- Center for Cultures, Languages and Societies
- Commencement
- Educator Preparation Institute
- F1 International Advising
- First Time Student Advising
- FSCJ Syllabus Search Tool
- FSCJ Fitness Centers
- FSCJ Annual Security Report
- Student Housing
- Military Advising
- Student Handbook
- TRIO Student Support Services
- Downtown Campus
- Kent Campus
- North Campus
- South Campus
- Cecil Center
- Deerwood Center
- Nassau Center
- Urban Resource Center
- Fire Academy of the South
- Northeast Florida Criminal Justice Center
- Nathan H. Wilson Center for the Arts

Clinical Research Professional (A.S.)

The Clinical Research Professional Associate in Science (A.S.) program gives students the skills necessary to maintain and elevate the health and wellbeing of the population worldwide. Through clinical research trials and historical studies, these critical research professionals work to identify better ways to detect, diagnose, treat and prevent the spread of disease.
There has never been a time where the need for clinical research professionals has been greater. Florida ranks within the top five states in the country for clinical studies and is home to many of the nation’s largest research facilities and organizations.
This program prepares students for employment as entry-level clinical research professionals. Program graduates will possess the knowledge and skills required to initiate, implement and follow-up on clinical trials while adhering to all applicable regulations.
The Clinical Research Coordinator Technical Certificate (T.C.) is embedded within this program. Students may pursue the A.S. degree and earn the certificate while completing the requirements for the degree, or pursue the certificate to develop or upgrade their skills.
Degree: Associate in Science (A.S.) Credit Hours: 60 credits Online Program : Yes Program Code: 2408 Financial Aid Eligible : Yes
[email protected] (904) 713-4545
Certifications/Licensing
Graduates will be encouraged to take the Association of Clinical Research Professionals (ACRP) Clinical Research Coordinator (CRC) certification exam or the Society of Clinical Research Association (SOCRA) Certified Clinical Research Professionals (CRPS) exam once work experience eligibility requirements have been fulfilled.
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
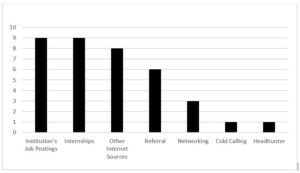
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
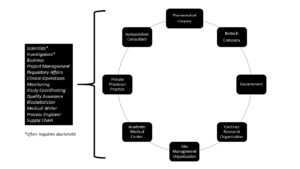
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework
Home » Blog » CCRP Certification: ACRP vs. SOCRA

CCRP Certification: ACRP vs. SOCRA
Table of Contents
The Value of Certification & Certifying Organizations
In addition to experience and career accomplishments, CCRP certification can provide added credentials for CRAs to progress further. It is common to see job advertisements that list certification as one of the preferred quality a candidate should possess. CCRP certification shows that a CRA is willing to go the extra step to pursue professional certification.
Below, I’ll talk about the 2 main clinical research organizations (ACRP and SOCRA) that are well recognized among those in the industry and the type of certifications that they offer.
The Association of Clinical Research Professionals (ACRP)
The Association of Clinical Research Professionals (ACRP) is one of the two clinical research professional organizations that are well recognized. ACRP is the older (established in 1976) and the larger of the two organizations, with more members worldwide.
ACRP offers membership, certifications, classes, conferences, career resources, and job postings. ACRP also offers an online forum and local chapter meeting around the world for networking opportunities and learning events.
For certification, ACRP offers a certification for clinical research coordinators (CRC), which is called Certified Clinical Research Coordinator (CCRC). I had a CCRC certification when I was working at a hospital site as a CRC. ACRP also offers a certification for clinical research associates (CRA), which is called Certified Clinical Research Associate (CCRA). Below are some steps for a CRA to become a certified CCRA through ACRP:
- Become an ACRP member ($150 annual membership fee in United States, $60 annual membership fee for emerging market – electronic membership)
- Register for CRA certification exam ($460 – $600 fee, must meet one of the eligibility options below and provide detailed CV/resume and job description.
- Take and pass the CCRA examination
- Renew CCRA your certification every 2 years ($215 – $325 renewal fee)
- Report twenty-four (24) contact hours / points every two (2) years -or-
- Take the current Certification Exam
The Society of Clinical Research Associates (SOCRA)
The Society of Clinical Research Associates (SOCRA) is the newer of the two clinical research professional organizations. It was established in 1991. Similar to ACRP, SOCRA is well recognized among those in the industry. SOCRA offers membership, certifications, classes, conferences, career resources, and job posting. SOCRA does not offer an online forum, but SOCRA does have local chapter meetings around the world for networking opportunities and learning events.
As for certification, SOCRA is different than ACRP in its approach. Instead of offering separate certification for Clinical Research Coordinators (CRC) and Clinical Research Associates (CRA), SOCRA offers one certification for both. This is called the Certified Clinical Research Professional CCRP certification. I got my CCRP certification through SOCRA. Below are some steps for a CRA to become a certified CCRP through SOCRA:
- Become a SOCRA member ($75 annual membership fee)
- Register for the CCRP certification exam ($395 fee, must meet one of the eligibility options below and provide detailed CV/resume, verification of employment, and job description. Click here for more details.
- Take and pass the CCRP certification exam
- Renew your CCRP certification every 3 years (maintain membership during the 3 year period, $75 fee per year plus a $100 processing fee)
- Report 45 continuing education hours every three (3) years AND
- Take the recertification knowledge quiz (open-book quiz)
ACRP vs. SOCRA CCRP Certification
Both ACRP and SOCRA are well recognized among those in the industry. The decision to choose between ACRP and SOCRA for a CRA may come down to the consideration below:
Which organization do you want to be a member of?
- ACRP is older and has more members
- ACRP is has an online forum
Which eligibility requirements do you meet?
- SOCRA’s CCRP certification is available to anyone who has worked full time in the field of clinical research for at least 2 years as a Clinical Research Professional.
- ACRP’s CCRA certification is available to only those meeting specific CRA/monitoring job functions
How much studying are you willing to put into to passing the exam?
- SOCRA’s CCRP certification exam is easier (unofficial opinion from my own experience and from what I heard from friends and colleagues over the years).
How often do you need to renew the certification and the number of continuing education credit needed?
- ACRP requires 24 credits every 2 years (12 credits/year)
- SOCRA requires 45 credits every 3 years (15 credits/year)
How much does it cost for membership and maintaining your certification?
Below are some estimates of total cost (assuming membership is maintained during renewal period)
Why I Chose SOCRA CCRP Certification
I had ACRP’s CCRC certification when I was a CRC in 2010. I chose not to renew the CCRC certification after becoming a CRA soon after. I have been SOCRA CCRP certified since September 2013. The reason I chose SOCRA’s CCRP certification was due to the lower cost and the longer period of renewal.
For those considering SOCRA’s CCRP certification, I have a study guide that helped me pass the exam with 96% out of 100% grade ( click here to see my score). This study guide contains the notes that I compiled for my own study.
Even though materials on the notes are from publicly available sources (ICH GCP, FDA’s CFR, resources from internet, etc.), I find the vast amount of information to be overwhelming, especially with limited study time in between my daily workload.
This study guide includes summaries of information in an easy-to-read format, as well as my own knowledge and experience in clinical research. I bring both clinical research coordinator (CRC) and clinical research associate (CRA) perspectives, as I had worked in both roles in my career. Visit the homepage to learn more!

Ernie Sakchalathorn
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *

Clinical Research

Lea Bingham Assistant Dean, University Transfer Phillips (Building 3), room 126 919-536-7200, ext. 8004 [email protected]
University Transfer Options
Graduates of clinical research degree programs find careers in pharmaceutical companies, universities, contract research organizations (CROs), and government agencies.
Students have two paths to a bachelor's degree in Clinical Research:
Associate in Applied Science (AAS) Option
Campbell university.
For graduates of Durham Tech’s Clinical Trials Research Associate or Pharmacy Technology AAS degree programs who wish to advance their career, Durham Tech has partnered with Campbell University’s Bachelor of Applied Science in Clinical Research program. Campbell will accept up to 64 credits from the Clinical Research Trials Associate and Pharmacy Technology Associate of applied Science degrees. The program requires a minimum of 124 credits to earn the bachelor’s degree and core courses will strengthen students’ skills in managing clinical research trials, biostatistics, scientific writing, compliance, and ethics. Students must earn a C or higher in each course and complete the AAS degree with a minimum 2.0 GPA.
University Transfer Option
The Associate in Science (AS) degree will transfer to all UNC System schools, most North Carolina independent colleges and universities, and most out of state institutions.
Course Selection Guide
This course selection guide shown below has been developed by referencing the Baccalaureate Degree plans or the student plans for the Bachelor of Science degrees at the universities listed below.
- North Carolina Central University (NCCU) - BS in Clinical Research Sciences
- UNC-Wilmington (UNCW) - BS in Clinical Research
Students should print out and save the baccalaureate degree plan (BDP) for their major and intended transfer university.
Printable Clinical Research Course Selection Guide (pdf)
General Education – 45 SHC (“Semester Hours of Credit”)
Universal general education transfer component (ugetc) courses (34 shc).
UGETC courses are courses which are guaranteed to fulfill a general education component at every public university in North Carolina and the private colleges that are signatories to the 2015 Independent Comprehensive Articulation Agreement.
Select two courses from separate disciplines
Select two courses from from MAT 171, 172, 263, 271, 272.
MAT 172 is required for completion of the AS degree, but is not required by UNCW.
Two courses taken in a sequence selected from BIO 111 and 112; CHM 151 and 152; PHY 151 and 152 or PHY 251 and PHY 252
Additional General Education Transfer Hours (11 SHC)
Note: UNCW requires two semesters of a new language, or a third semester of a language begun in high school.
Other Required Hours (15 SHC)
Guaranteed admissions pathways.
Durham Tech has partnered with several universities to develop guaranteed admissions pathways for a seamless transition into their baccalaureate programs for eligible students who complete a Durham Tech university transfer degree (AA, AS, AFA, and AE). Students must apply for acceptance into these programs.
View more information about Guaranteed Admissions Pathways and the list of universities.
- Programs and Pathways
- Programs by Name
- About Guided Career Pathways
- Pathway Contacts
- Career Services
- Honors Program
- Online Learning
- Service-Learning
- Transfer Programs
Suggested Paths
Clinical trials research associate, university transfer, career explorer, clinical trial research coordinator, starting salary, median annual wage, job outlook, annual job openings, clinical trial research assistant, visit campus.


Clinical Research Associate 1
Coordination of clinical research studies. performance of regulatory tasks including irb and sponsor/cro regulatory correspondence..
- Science and Medical Research
- Opening on: Apr 22 2024
- Research Foundation
- Clinical Research Associate I, E99
Job Summary:
Coordination of clinical research studies. Performance of regulatory tasks including IRB and sponsor/CRO regulatory correspondence. Communication with IRBs, sponsors, and protocol-related Upstate Departments. Pre-screen, Screen, and enroll research participants in outpatient and inpatient clinical trials. Process and ship lab samples. Schedule and conduct follow up research appointments for clinical trial participants. Data collection and entry into paper and electronic databases. Maintenance of clinical research supply inventory. Processing clinical trial billing and payments. Administration of study questionnaires and assessments. Occasional travel.
Minimum Qualifications:
Bachelor's degree and 2-year's related experience or equivalent combination of education and experience.
Preferred Qualifications:
Prior experience with clinical research protocols and/or experience with coordinating clinical trials. ACRP and/or SOCRA certification.
Mon- Fri 8-4:30 with occasional after hours
Message to Applicants:
Salary Range-$58,000-$60,000
Recruitment Office: Human Resources
We are an Equal Opportunity Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, age, protected veteran status or disability or other protected classes under State and Federal law.
Share this job
Thank you for sharing!
Recently Posted Jobs
Hospital attendant 1 (ust), student assistant - imt - autonomous machines, administrative assistant, before you go would you like to receive updates and information from suny upstate medical university on available jobs and career opportunitites.

Cookies are small pieces of information that are stored by the user's browser on the hard drive of a personal computer. The use of cookies is a standard practice among Internet Web sites. Most of Upstate's Web sites do not use cookies, however occasional "session cookies" may be used to enhance or customize a visit to Upstate's Web sites. Session cookies can be created automatically on the device used to access Upstate's Web sites, and do not contain personal information and do not compromise privacy or security. We may use the cookie feature to store a randomly generated identifying tag on the device used to access Upstate's Web sites. A session cookie is erased when a browser is closed.

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
The Division of Cardiovascular Medicine , within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care. Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease.
We are seeking a Clinical Research Coordinator Associate (CRCA) who is passionate about clinical research and wants to deliver results. The CRCA will work with a robust clinical research team, hand in hand with Principal Investigators, Clinical Research Managers, Associates and other stakeholders in support of patients with lymphedema and other lymphatic disorders.
The Clinical Research Coordinator Associate will be responsible for the overall implementation of an assigned set of research protocols assuring efficiency and regulatory compliance. Other responsibilities will include recruiting, screening, assisting in the informed consent process and enrolling subjects in accordance with good clinical practice guidelines as well as collecting, recording and maintaining complete data files using good clinical practice in accordance with HIPAA regulations. The CRCA will participate in data retrieval, reporting, and preparation of files and Case Report Forms for the various studies. Other duties may include processing of blood samples, reporting serious adverse events and maintenance of drug accountability, supplies and equipment.
CV Med Clinical Research is a growing, dynamic team who is dedicated to supporting translational medicine and contributing to Stanford Medicine’s mission. If you are eager to quickly achieve lasting results, we invite you to join our team!
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from start-up through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure Institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
DESIRED QUALIFICATIONS:
- Bachelor's degree in a related field or an equivalent combination of related education and relevant experience; additional 1-2 years clinical trial experience preferred.
EDUCATION & EXPERIENCE (REQUIRED):
- Two year college degree and two years related work experience or a Bachelor’s degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
- Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
PHYSICAL REQUIREMENTS:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
- Will be working at three different locations; Stanford Boswell Clinics, Portola Valley, and Santa Clara sites
WORK STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University's Administrative Guide, http://adminguide.stanford.edu .
The expected pay range for this position is $31.73 to $36.54 per hour.
Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow . We offer career development programs, tuition reimbursement, or audit a course. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture . We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you . Climb our rock wall, or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun . Stroll through historic sculptures, trails, and museums.
- Enviable resources . Enjoy free commuter programs, ridesharing incentives, discounts and more.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources at [email protected]. For all other inquiries, please submit a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Regular
- Requisition ID: 102959
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: Jan 29, 2024
Post Date: Feb 15, 2023
Post Date: Aug 05, 2022
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs

IMAGES
VIDEO
COMMENTS
A clinical research associate (CRA) is a liaison between the sponsor and the clinics conducting research. To become one, you need a bachelor's degree in a health-related field and certification from ACRP or SOCRA. Learn more about the job description, skills, salary, and career outlook of a CRA.
Here's how to get started as a clinical research associate. 1. Qualify for certification. You can take several paths to becoming a certified CRA in Canada. One path is to earn a high school diploma and clock 3,000 to 3,500 part-time hours of work experience in the field.
Learn about the duties, salary, education and certification of clinical research associates who conduct medical research and run clinical trials. Find out how to pursue a career in this field with FAQs and tips.
Learn about the role, salary, skills, and education requirements of a Clinical Research Associate, who runs clinical trials for pharmacological drug testing. Find out the job outlook, common employers, and NC State programs related to this career.
Learn about the education and credentials required to become a clinical research associate (CRA) in the US. Find out the steps to become a CRA with a high school diploma, an associate degree, or a bachelor's degree in health sciences or related fields. Compare the certification options for SOCRA and ACRP.
Now that you've chosen to pursue your degree, most high-level clinical research associate positions require a bachelor's or a master's in a healthcare-related field. However, if you're interested in becoming a management-level clinical research associate, attend a four-year college in an area of study such as: Nursing. Health sciences.
CCRA (Certified Clinical Research Associate) is a credential for clinical research professionals who monitor and supervise clinical trials. Learn about the eligibility, exam content, fees, and preparation for the CCRA certification exam.
Clinical Research Associate Skills and Qualifications. As you set your sights on becoming a CRA, it's essential to equip yourself with the following skills and qualifications: Excellent Communication: Effective communication is paramount in this role, both in writing and verbally. Presentation Skills: The ability to articulate ideas clearly ...
Learn clinical research skills and prepare for certification with Drexel's online program. Choose from various courses and electives to customize your study plan and career goals.
Learn how to become a clinical research associate (CRA), who collects and organizes data from clinical trials. Find out the average salary, job growth, employers, and education requirements for this field.
Learn about the role, qualifications, and benefits of a clinical research associate (CRA) who directs and supervises clinical trials. Find out how to get certified online with CCRPS, a US-based accredited course provider with 110 modules and remote opportunities.
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
With a 30-year legacy, ACRP Certification is the most reputable credentialing program in clinical research. Since 1992, more than 40,000 professionals and their employers have come to trust ACRP Certification as the mark of excellence in clinical research. "Joining ACRP and becoming certified was the best thing I ever did to jumpstart my ...
The Clinical Research Professional Associate in Science (A.S.) program gives students the skills necessary to maintain and elevate the health and wellbeing of the population worldwide. ... (T.C.) is embedded within this program. Students may pursue the A.S. degree and earn the certificate while completing the requirements for the degree, or ...
Clinical research degrees are available at different degree levels, for example, the Associate of Applied Science in Clinical Research Coordination, Bachelor of Science in Clinical Research and Master of Science in Clinical Research. You can find training through courses that place an emphasis on mathematics, reading comprehension, biology and ...
After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate). The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry.
Become a Certified Clinical Research Associate (CCRA) Certifying Agency: The Association of Clinical Research Professionals (ACRP) Eligibility Requirements: Applicants must attest to having at least 3,000 hours of work experience performing the tasks and knowledge of the content areas of the CCRA exam. Experience older than ten years will not be considered.
ACRP also offers a certification for clinical research associates (CRA), which is called Certified Clinical Research Associate (CCRA). Below are some steps for a CRA to become a certified CCRA through ACRP: ... Associate's degree: 4,500 hours: 3: Other, such as LPN, LVN, Medical Assistant, Lab Technician or High School diploma: 6,000 hours:
Learn how to assist in clinical research studies for new drugs, products, and treatments. Durham Tech offers an accredited program with online and on-campus courses, and career opportunities in various settings.
Campbell will accept up to 64 credits from the Clinical Research Trials Associate and Pharmacy Technology Associate of applied Science degrees. The program requires a minimum of 124 credits to earn the bachelor's degree and core courses will strengthen students' skills in managing clinical research trials, biostatistics, scientific writing ...
Pima offers the associate degree in Clinical Research Coordinator, with many required courses offered online to accommodate the scheduling needs of today's busy student. The program does include a professional internship that may lead to a job as CRC graduates are in high demand nationally. Title IV Financial Aid eligible: Yes.
Clinical Research Associate. Abbott Laboratories. South Portland, ME. $48,000 - $96,000 a year. Full-time. Working knowledge of clinical trial databases. Working knowledge of clinical trial practices and regulations. At Abbott, you can do work that matters, grow, and…. Posted.
The MSCR MD/MS Program is a one-year articulated degree program that allows interested UCLA Medical Students to complete the Master of Science in Clinical Research Program (MSCR) during their Discovery Year (Year 3). The MSCR MD/MS Program leads to a Master of Science in Clinical Research graduate degree and is designed to develop physician ...
Best online Doctor of Nursing Practice: University of Central Florida. Best online Doctor of Business Administration: Walsh College. Best online doctorate in physical therapy: Texas Tech ...
Novartis is committed to building an outstanding, inclusive work environment and diverse team's representative of the patients and communities we serve. Role Requirements : Minimum Requirements: • Degree in scientific or healthcare discipline. • 2 years pharmaceutical industry experience or other relevant experience.
Performance of regulatory tasks including IRB and sponsor/CRO regulatory correspondence. Syracuse. Science and Medical Research. Full-time. Opening on: Apr 22 2024. Neurology. Research Foundation. Clinical Research Associate I, E99. 80279.
Bachelor's degree in a related field or an equivalent combination of related education and relevant experience; additional 1-2 years clinical trial experience preferred. ... Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred. PHYSICAL REQUIREMENTS: Frequently stand, walk, twist ...