
Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download

Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
- Last modified on: 1 week ago
- Reading Time: 11 Minutes
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Here, we have provided case based/passage based questions for Class 10 Science Chapter 1 Chemical Reactions and Equations.

Case Study/Passage Based Questions on Chemical Reactions and Equations
Case Study/Passage Based Questions
Question 1:
Corrosion is the phenomenon of deterioration of surface of metal in presence of air and moisture. It is a natural process and in the presence of a moist atmosphere, chemically active metals get corroded. This is an oxidation reaction. Rusting is the process where iron corrodes due to exposure to the atmosphere. The main circumstance of corrosion occurs with iron because it is a structural material in construction, bridges, buildings, rail transport, ships, etc. Aluminium is also an important structural metal, but even aluminium undergoes oxidation reactions. However, aluminium doesn’t corrode or oxidize as rapidly as its reactivity suggests. Copper (Cu) corrodes and forms a basic green carbonate.
(i) What is rusting?
(ii) Which two metals do not corrode easily?
(iii) Write the chemical name of the compound formed on corrosion of silver.
(iv) Corrosion is (a) a redox reaction (b) a reduction reaction (c) a displacement reaction (d) an oxidation reaction
Also read: Assertion Reason Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question 2:
Oxidation is the process of gaining of oxygen, or losing of hydrogen. Reduction is the process of losing of oxygen or gaining of hydrogen. The substance which undergoes oxidation is the reducing agent while the substance which undergoes reduction is known as the oxidising agent. Oxidation and reduction always take place together and these type of reactions are known as redox reactions. Some of the examples of redox reactions are given below:

(i) Give two examples of oxidation reaction from your everyday life.
(ii) Write the oxidising agent in the reaction III and VI.
(iii) Which of the following is an oxidising agent? (a) LiAlH 4 (b) Alkaline KMnO 4 (c) Acidified K 2 Cr 2 O 7 (d) Both (b) and (c)
(iv) Out of oxidation and reduction, which reaction takes place at anode?
Also read: Extra Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question 3:
A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The chemical reaction in which a single substance breaks down into two or more simpler substances upon heating is known as (a) thermal decomposition reaction (b) photo decomposition reaction (c) electric decomposition reaction (d) both (a) and (c)
(ii) The massive force that pushes the rocket forward through space is generated due to the (a) combination reaction (b) decomposition reaction (c) displacement reaction (d) double displacement reaction
(iii) A white salt on heating decomposes to give brown fumes and yellow residue is left behind. The yellow residue left is of (a) lead nitrate (b) nitrogen oxide (c) lead oxide (d) oxygen gas
(iv) Which of the following reactions represents a combination reaction? (a) CaO (s) + H 2 O (l) → Ca(OH) 2 (aq) (b) CaCO 3 (s) → CaO (s) + CO 2 (g) (c) Zn(s) + CuSO 4 (aq) → ZnSO 4 (aq) + Cu(s) (d) 2FeSO 4 (s) → Fe 2 O 3 (s) +SO 2 (g) + SO 3 (g)
(v) Complete the following statements by choosing correct type of reaction for X and Y. Statement 1: The heating of lead nitrate is an example of ‘X’ reaction. Statement 2: The burning of magnesium is an example of ‘Y’ reaction. (a) X- Combination, Y- Decomposition (b) X- Decomposition, Y-Combination (c) X- Combination, Y-Displacement (d) X- Displacement, Y-Decomposition
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
3 thoughts on “ Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations ”
Good examples! But can you please available practical types and equations type of case based questions which we can read and learn an then they help us to solve the Boards examm. Pleaseeww🙂🙂🙂
would love to see more equation based questions. nevertheless, it proved quite useful in my revision!
after going through the above content child should develops ideas to answer based on knowledge acquired.
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

Case Study Questions Class 10 Science Chapter 1 Chemical Reactions and Equations
- Post author: studyrate
- Post published:
- Post category: class 10th
- Post comments: 0 Comments
CBSE Board Exam is on the way, so you must practice some good Case Studies and Passage Based Questions of Class 10 Science to boost your preparation to score 95+% on Boards. In this post, you will get Case Study and Passage Based Questions that will come in CBSE Class 10 Science Board Exams. These Case Study Questions Class 10 Science are written by experts.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Chemical Reactions and Equations Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Case Study/Passage-Based Questions
Question 1:
A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation, and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The chemical reaction in which a single substance breaks down into two or simpler substances upon heating is known as (a) thermal decomposition reaction (b) photodecomposition reaction (c) electric decomposition reaction (d) both (a) and (c)
Answer: (a) The chemical reaction in which a single substance breaks down into two or more simpler substances upon heating is known as thermal decomposition reaction.
(ii) The massive force that pushes the rocket forward through space is generated due to the (a) combination reaction (b) decomposition reaction (c) displacement reaction (d) double displacement reaction
Answer: (b) The massive force that pushes the rocket forward through space is generated due to the decomposition reaction. Hydrogen peroxide decomposes and provides it with a considerable reaction force thrust.
(iii) A white salt on heating decomposes to give brown fumes and the yellow residue is left behind. The yellow residue left is of (a) lead nitrate (b) nitrogen oxide (c) lead oxide (d) oxygen gas
Answer: (c) Lead nitrate decomposes to give brown fumes of nitrogen dioxide gas and yellow residue of lead oxide is left behind.
(iv) Which of the following reactions represents a combination reaction? (a) CaO (s) + H 2 O (l) → Ca(OH) 2 (aq) (b) CaCO 3 (s) → CaO (s) + CO 2 (g) (c) Zn(s) + CuSO 4 (aq) → ZnSO 4 (aq) + Cu(s) (d) 2FeSO 4 (s) → Fe 2 O 3 (s) +SO 2 (g) + SO 3 (g)
Answer: (a) A reaction in which two or more reactants combine to form a single product is known as a combination reaction.
(v) Complete the following statements by choosing correct type of reaction for X and Y. Statement 1: The heating of lead nitrate is an example of ‘X’ reaction. Statement 2: The burning of magnesium is an example of ‘Y’ reaction. (a) X- Combination, Y- Decomposition (b) X- Decomposition, Y-Combination (c) X- Combination, Y-Displacement (d) X- Displacement, Y-Decomposition
Answer: (b) Heating of lead nitrate to form nitrogen dioxide and lead oxide is an example of thermal decomposition reaction and the burning of magnesium ribbon in the air to form magnesium oxide is an example of combination reaction.
Question 2:
In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be observed easily. These easily observed features which take place as a result of chemical reactions are known as characteristics of chemical reactions. Some important characteristics of chemical reactions are: (I) Evolution of heat (II) Formation of a precipitate (III) Change in color (IV) Change in temperature (V) Change in state
Anyone of these general characteristics can tell us whether a chemical reaction has taken place or not.
(i) Reaction of magnesium with air is a/an
Answer: (a) exothermic reaction
(ii) In the following reaction Ca 2+ (aq)+2OH−(aq)⟶Ca(OH) 2 (s)Ca(aq) 2 ++2OH(aq)−⟶Ca(OH) 2 (s) precipitate of calcium hydroxide will be of
Answer: (d) white colour
(iii) In the given reaction, S(s)+O 2 (g)⟶SO 2 S(s)+O 2 (g)⟶SO 2 the physical state of SO 2 is
Answer: (c) gaseous
(iv) Which one of the following processes involves chemical reactions?
Answer: (d) Heating copper wire in the presence of air at high temperature.
(v) In which of the following reactions, high amount of heat energy will be evolved?
Answer: (c) Burning of L.P.G.
Case Study 3: Chemical reactions and equations are fundamental concepts in chemistry that help us understand the transformation of substances. A chemical reaction involves the rearrangement of atoms to form new substances with different properties. In a chemical equation, the reactants are written on the left side, and the products are written on the right side, separated by an arrow. The number of atoms of each element must be balanced on both sides of the equation. This is achieved by using coefficients to adjust the number of molecules involved in the reaction. Chemical reactions can be classified into various types, such as combination reactions, decomposition reactions, displacement reactions, and redox reactions. Understanding and balancing chemical equations is crucial for studying chemical reactions, predicting the products formed, and analyzing the stoichiometry of reactions.
What do chemical reactions involve? a) Formation of new substances with different properties b) Rearrangement of atoms c) Balancing of equations d) All of the above Answer: d) All of the above
How are reactants and products represented in a chemical equation? a) Reactants on the left side, products on the right side b) Reactants on the right side, products on the left side c) Reactants and products mixed together d) Reactants and products in different equations Answer: a) Reactants on the left side, products on the right side
What must be balanced in a chemical equation? a) Number of molecules b) Number of atoms of each element c) Physical properties of substances d) Coefficients Answer: b) Number of atoms of each element
Which type of chemical reaction involves the breakdown of a compound into simpler substances? a) Combination reaction b) Decomposition reaction c) Displacement reaction d) Redox reaction Answer: b) Decomposition reaction
Why is balancing chemical equations important? a) To predict the products formed in a reaction b) To analyze the stoichiometry of reactions c) To study chemical reactions d) All of the above Answer: d) All of the above
Hope the information shed above regarding Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 10 Science Chemical Reactions and Equations Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible. By Team Study Rate
You Might Also Like
Mcq class 10 english fire and ice questions with answers english poem 2, cbse class 10 maths term 1 mcq questions with answers pdf download, class 10 maths case study questions chapter 8 introduction to trigonometry, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

- Book Solutions
- State Boards
Case Study Questions Class 10 Science Chemical Reactions and Equations
Case study questions class 10 science chapter 1 chemical reactions and equations.

At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks.
CBSE Case Study Questions Class 10 Science Chemical Reactions and Equations
Case study 1.
A solution of slaked lime produced by the reaction is used for white washing walls. Calcium hydroxide reacts slowly with the carbon dioxide in air to form a thin layer of calcium carbonate on the walls. Calcium carbonate is formed after two to three days of white washing and gives a shiny finish to the walls. It is interesting to note that the chemical formula for marble is also CaCO3.
On the basis of above paragraph answer the following questions:
1.) Give the reaction for the formation of calcium carbonate with physical states.
Ca(OH) 2 (s) + CO 2 (g) → CaCO 3 (s)↓ + H 2 O(l)
2.) Write the formulas of slaked lime, quick lime.
Slaked lime: Calcium hydroxide is called as slaked lime with a formula of : Ca(OH)2 ,whereas calcium oxide is called as quick lime with a formula of : Ca0.
3.) Explain why calcium carbonate is used for white washing and not any other substance.
Calcium carbonate is used for whitewashing as it produces a shiny film whilst the production of carbon dioxide and act as hard coating for the walls.
4.) Explain the importance of writing the physical states in a chemical equation.
In any chemical reaction, physical states mention the nature of the reaction and their practical aspects which are necessary for lab uses.Physical states also explains whether reaction is exothermic or endothermic.
5.) Write any one application of calcium carbonate other than white washing.
Calcium carbonate is also used in the production of antacids and can also be used to increase the levels of calcium in body.
CASE STUDY : 2
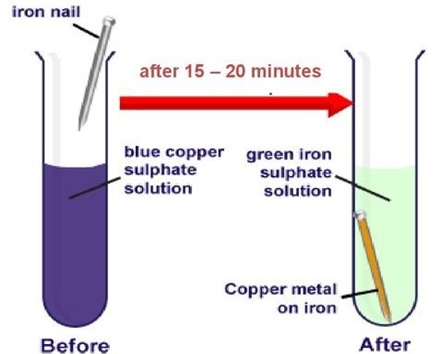
You must have observed that iron articles are shiny when new, but get coated with a reddish brown powder when left for some time. This process is commonly known as rusting of iron. Some other metals also get tarnished in this manner. Have you noticed the colour of the coating formed on copper and silver? When a metal is attacked by substances around it such as moisture, acids, etc., it is said to corrode and this process is called corrosion. The black coating on silver and the green coating on copper are other examples of corrosion.
1.) Explain one benefit of corrosion.
Ans. Corrosion occurring in red blood cells of iron makes the colour of cells red which is highly useful in transportation of oxygen.
2.) Write the formula of corrosion of iron.
Ans. The general formula of corrosion of iron is Fe2 O3.
3.) If corrosion occurs in the case of iron articles, why is the iron pillar at Qutub Minar not effected?
Ans. The iron pillar at Qutub Minar is an ancient piece of metal knowledge in which the metal is very very pure( wrought iron) with low sulphur content and high phosphorus making it resistive to the outer environment.
4.) Write any two ways to prevent rusting.
Ans. The two ways to prevent rusting are:
A) Galvanization.
B) Painting of iron articles.
5.) What doe you mean by galvanization?
Ans. Galvanization is the process of applying a protective layer of Zn coating on materials which are prone to rust making it resistive and long lasting.
CASE STUDY : 3
When fats and oils are oxidized, they become rancid and their smell and taste change. Usually substances which prevent oxidation (antioxidants) are added to foods containing fats and oil. Keeping food in air tight containers helps to slow down oxidation.
1.) What do you mean by the word RANCIDITY?
Ans. The spoiling of food due to oxidation of fats and oils present in the food material
2.) Write any three methods to prevent rancidity.
Ans. Three methods to prevent rancidity of food includes:
B) Adding preservatives like vinegar
C) Storing in air tight containers.
3.) What is the meaning of antioxidants. Give an example.
Ans. The type of substances which can prevent the oxidation process of certain food materials are called as antioxidants. Some natural antioxidants are vitamin c, selenium etc.
4.) Which gas is filled in the chips packets to keep them crunchy?
Ans. The gas filled in chips packets is nitrogen gas which can keep the chips crunchy.
5.) Write any traditional method used by our ancestors to prevent rancidity.
Ans. There are a few methods used by our ancestors to prevent rancidity:
B) Sugaring
C) Pickling
CASE STUDY : 4
We have seen that the decomposition reactions require energy either in the form of heat, light or electricity for breaking down the reactants. Reactions in which energy is absorbed are known as endothermic reactions.
1.) Write the definition of exothermic reaction.
Ans. Reactions in which heat is evolved during the process are called as exothermic reactions, such as mixing of calcium oxide with water.
2.) What do you mean endothermic reactions?
Ans. Reactions in which heat is trapped or absorbed are called as endothermic reactions, such as glucose mixed with water.
3.) Write a reaction which falls under endothermic reaction.
Ans. Melting of ice, evaporation are considered under endothermic reactions. A chemical equation for these types of reaction is:
N2 + O2 + heat → 2 NO
4.) What is decomposition reaction?
Ans. Reactions in which a substance or reactant fragments to give one or many products.
5.) Explain photolysis.
Ans. Reactions in which reactant id decomposed with the help os sunlight are called as photolytic decomposition.
Ex: 2AgCl + sunlight → 2Ag + Cl2
For more update follow net explanations page
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Rs aggarwal class 8 math first chapter rational numbers exercise 1c solution, new learning composite mathematics class 5 s.k. gupta anubhuti gangal volume chapter 18a solution, new learning composite mathematics class 5 s.k. gupta anubhuti gangal volume chapter 18b solution, madam rides the bus extra questions and answers english first flight chapter 9.
Sign in to your account
Username or Email Address
Remember Me
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Case Study Chapter 1 Chemical Reactions and Equations
Please refer to Chapter 1 Chemical Reactions and Equations Case Study Questions with answers provided below. We have provided Case Study Questions for Class 10 Science for all chapters as per CBSE, NCERT and KVS examination guidelines. These case based questions are expected to come in your exams this year. Please practise these case study based Class 10 Science Questions and answers to get more marks in examinations.
Case Study Questions Chapter 1 Chemical Reactions and Equations
Case/Passage – 1 The reaction between MnO 2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released .

Question: Identify the correct statement from the following: (a) MnO2 is getting reduced whereas HCl is getting oxidized (b) MnO2 is getting oxidized whereas HCl is getting reduced. (c) MnO2 and HCl both are getting reduced. (d) MnO2 and HCl both are getting oxidized.
Question: Chlorine gas reacts with _____ to form bleaching powder. (a) dry Ca(OH) 2 (b) dil. solution of Ca(OH) 2 (c) conc. solution of Ca(OH) 2 (d) dry CaO
Question: In the above discussed reaction, what is the nature of MnO2? (a) Acidic oxide (b) Basic oxide (c) Neutral oxide (d) Amphoteric oxide
Question: The chemical reaction between MnO2 and HCl is an example of: (a) displacement reaction (b) combination reaction (c) redox reaction (d) decomposition reaction.
Question: What will happen if we take dry HCl gas instead of aqueous solution of HCl? (a) Reaction will occur faster. (b) Reaction will not occur. (c) Reaction rate will be slow. (d) Reaction rate will remain the same.
Case/Passage – 2
Chemistry in Automobiles: For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car, the distributor and battery provide this starting energy by creating an electrical “spark”, which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustionof gasoline in full supply of air: 2C 8 H 18 (I) + 25O 2 (g) → 16 ‘X’ + Y Question: Which of the following are the products obtained from the reaction mentioned in the above case? Product ‘ X’ Product ‘Y’ (a) CO 2 H 2 O 2 (b) H 2 O CO (c) CH 3 OH H 2 O (d) CO 2 H 2 O
Question: On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel? (i) Photosynthesis in plants (ii) Respiration in the human body (iii) Decomposition of vegetable matter (iv) Decomposition of ferrous sulphate. (a) (ii) & (iii) (b) (i) & (ii) (c) (iii) & (iv) (d) (ii) & (i)
Question: ‘Although nitrogen is the most abundant gas in the atmosphere, it does not take part in combustion’. Identify the correct reason for this statement. (a) Nitrogen is a reactive gas (b) Nitrogen is an inert gas (c) Nitrogen is an explosive gas (d) Only hydrocarbons can take part in combustion
Question: Identify the types of chemical reaction occurring during the combustion of fuel: (a) Oxidation & Endothermic reaction (b) Decomposition & Exothermic reaction (c) Oxidation & Exothermic reaction (d) Combination & Endothermic reaction
Question: ‘A student while walking on the road observed that a cloud of black smoke belched out from the exhaust stack of moving trucks on the road.’ Choose the correct reason for the production of black smoke: (a) Limited supply of air leads to incomplete combustion of fuel. (b) Rich supply of air leads to complete combustion of fuel. (c) Rich supply of air leads to a combination reaction. (d) Limited supply of air leads to complete combustion of fuel.
Related Posts

CBSE Class 10 English The Hack Driver Summary

Periodic Classification Of Elements Class 10 Science Notes And Questions

A Horse and Two Goats Summary by RK Narayan
CBSE Expert
Class 10 Science: Case Study Chapter 1 Chemical Reactions and Equations PDF Download
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given.

Here we are providing you with Class 10 Science Chapter 1 Chemical Reactions and Equations Case Study Questions, by practicing this Case Study and Passage Based Questions will help you in your Class 10th Board Exam.
Case Study Chapter 1 Chemical Reactions and Equations
Question 1:
A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation, and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The chemical reaction in which a single substance breaks down into two or simpler substances upon heating is known as (a) thermal decomposition reaction (b) photodecomposition reaction (c) electric decomposition reaction (d) both (a) and (c)
Answer: (a) The chemical reaction in which a single substance breaks down into two or more simpler substances upon heating is known as thermal decomposition reaction.
(ii) The massive force that pushes the rocket forward through space is generated due to the (a) combination reaction (b) decomposition reaction (c) displacement reaction (d) double displacement reaction
Answer: (b) The massive force that pushes the rocket forward through space is generated due to the decomposition reaction. Hydrogen peroxide decomposes and provides it with a considerable reaction force thrust.
(iii) A white salt on heating decomposes to give brown fumes and the yellow residue is left behind. The yellow residue left is of (a) lead nitrate (b) nitrogen oxide (c) lead oxide (d) oxygen gas
Answer: (c) Lead nitrate decomposes to give brown fumes of nitrogen dioxide gas and yellow residue of lead oxide is left behind.
(iv) Which of the following reactions represents a combination reaction? (a) CaO (s) + H 2 O (l) → Ca(OH) 2 (aq) (b) CaCO 3 (s) → CaO (s) + CO 2 (g) (c) Zn(s) + CuSO 4 (aq) → ZnSO 4 (aq) + Cu(s) (d) 2FeSO 4 (s) → Fe 2 O 3 (s) +SO 2 (g) + SO 3 (g)
Answer: (a) A reaction in which two or more reactants combine to form a single product is known as a combination reaction.
(v) Complete the following statements by choosing correct type of reaction for X and Y. Statement 1: The heating of lead nitrate is an example of ‘X’ reaction. Statement 2: The burning of magnesium is an example of ‘Y’ reaction. (a) X- Combination, Y- Decomposition (b) X- Decomposition, Y-Combination (c) X- Combination, Y-Displacement (d) X- Displacement, Y-Decomposition
Answer: (b) Heating of lead nitrate to form nitrogen dioxide and lead oxide is an example of thermal decomposition reaction and the burning of magnesium ribbon in the air to form magnesium oxide is an example of combination reaction.
Question 2:
In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be observed easily. These easily observed features which take place as a result of chemical reactions are known as characteristics of chemical reactions. Some important characteristics of chemical reactions are: (I) Evolution of heat (II) Formation of a precipitate (III) Change in color (IV) Change in temperature (V) Change in state
Anyone of these general characteristics can tell us whether a chemical reaction has taken place or not.
(i) Reaction of magnesium with air is a/an
Answer: (a) exothermic reaction
(ii) In the following reaction Ca 2+ (aq)+2OH−(aq)⟶Ca(OH) 2 (s)Ca(aq) 2 ++2OH(aq)−⟶Ca(OH) 2 (s) precipitate of calcium hydroxide will be of
Answer: (d) white colour
(iii) In the given reaction, S(s)+O 2 (g)⟶SO 2 S(s)+O 2 (g)⟶SO 2 the physical state of SO 2 is
Answer: (c) gaseous
(iv) Which one of the following processes involves chemical reactions?
Answer: (d) Heating copper wire in the presence of air at high temperature.
(v) In which of the following reactions, a high amount of heat energy will be evolved?
Answer: (c) Burning of L.P.G.
You can also practice Class 10 Science MCQ Questions for Board Exams.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Unit 4: Chemical reactions
About this unit.
This unit introduces chemical reactions, the processes that create and transform matter. Learn about net ionic equations, reaction stoichiometry, titration, common reaction types, and more. Practice what you’ve learned and study for the AP Chemistry exam with 80 AP-aligned questions.
Net ionic equations
- Molecular, complete ionic, and net ionic equations (Opens a modal)
- Net ionic equations Get 3 of 4 questions to level up!

Representations of reactions
- Visualizing chemical equations using particulate models (Opens a modal)
- Drawing particulate models of reaction mixtures (Opens a modal)
- Representations of reactions Get 3 of 4 questions to level up!
Physical and chemical changes
- Physical and chemical changes (Opens a modal)
- Physical and chemical changes Get 3 of 4 questions to level up!
Stoichiometry
- Stoichiometry (Opens a modal)
- Worked example: Calculating amounts of reactants and products (Opens a modal)
- Limiting reactant and reaction yields (Opens a modal)
- Worked example: Calculating the amount of product formed from a limiting reactant (Opens a modal)
- Worked example: Relating reaction stoichiometry and the ideal gas law (Opens a modal)
- Stoichiometry Get 3 of 4 questions to level up!
Oxidation–reduction (redox) reactions
- Oxidation–reduction (redox) reactions (Opens a modal)
- Worked example: Using oxidation numbers to identify oxidation and reduction (Opens a modal)
- Balancing redox equations (Opens a modal)
- Worked example: Balancing a simple redox equation (Opens a modal)
- Worked example: Balancing a redox equation in acidic solution (Opens a modal)
- Worked example: Balancing a redox equation in basic solution (Opens a modal)
- Oxidation–reduction (redox) reactions Get 3 of 4 questions to level up!
Introduction to acid–base reactions
- Brønsted–Lowry acids and bases (Opens a modal)
- Conjugate acid–base pairs (Opens a modal)
- Introduction to acid–base reactions Get 3 of 4 questions to level up!
Introduction to titration
- Acid–base titrations (Opens a modal)
- Worked example: Determining solute concentration by acid–base titration (Opens a modal)
- Redox titrations (Opens a modal)
- Introduction to titration Get 3 of 4 questions to level up!

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- ML Aggarwal Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam

SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences
- Chemical Reactions and Equations 14 April, 2021, 6:02 pm
- Acids, Bases, and Salts 14 April, 2021, 6:02 pm
- Metals and Non-Metals 14 April, 2021, 6:02 pm
- Carbon and its Compounds 14 April, 2021, 6:02 pm
- Periodic Classification of Elements 14 April, 2021, 6:02 pm
- Life Processes 14 April, 2021, 6:02 pm
- How Do Organisms Reproduce 14 April, 2021, 6:02 pm
- Heredity and Evolution 14 April, 2021, 6:02 pm
- Light Reflection and Refraction 14 April, 2021, 6:02 pm

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.

Class 10 Science Chapter 1 Case Based Questions - Chemical Reactions and Equations
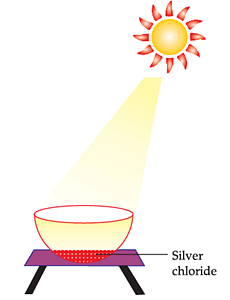
Question 1: The type of chemical reaction that will take place is (a) Photochemical decomposition (b) Displacement reaction (c) Reduction reaction (d) Combination reaction
Correct Answer is Option (a) Let’s look at all the options one-by-one Photochemical decomposition: Photo means light, therefore the reactions in which a compound is broken down/decomposed in the presence of light are known as photochemical reactions. Displacement reaction: Reaction in which more reactive element takes the place of less reactive element. Reduction: The loss of oxygen is known as reduction. Combination Reaction: When two or more substances react to form a single product, it is known as combination reaction. Since the above reaction is taking place in the presence of light, it is a photochemical decomposition reaction . So, the correct answer is (a)
Question 2: When decomposition is carried out by heating, it is called as: (a) Heat decomposition (b) Photolytic decomposition (c) Electrolytic decomposition (d) Thermal decomposition
Correct Answer is Option (d) Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition.
Question 3: The other silver salt which behaves like silver chloride in sunlight is: (a) silver hydride (b) silver bromide (c) silver iodide (d) silver nitrite
Correct Answer is Option (b) Silver Bromide behaves like Silver Chloride So, the correct answer is (b)

(a) 15-20 min (b) 10-15 min (c) 5-10 min (d) 0-5 min
Correct Answer is Option (d) This is the Pressure Time Graph Maximum decomposition is when the pressure is maximum We see that from 0 to 5 minutes, the pressure increases from 0 to 0.625 atm, This indicates maximum increase in pressure due to maximum CO 2 formation
Chemistry in Automobiles: For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car, the distributor and battery provide this starting energy by creating an electrical "spark", which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustion of gasoline in full supply of air: 2C 8 H 18 (l) + 25O 2 (g) → 16 'X' + Y Question 5: On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel? (i) Photosynthesis in plants (ii) Respiration in the human body (iii) Decomposition of vegetable matter (iv) Decomposition of ferrous sulphate. (a) (ii) & (iii) (b) (i) & (ii) (c) (iii) & (iv) (d) (ii) & (i)
Correct Answer is Option (a) In a Combustion reaction, heat is released (evolved) Let’s check all the options: In photosynthesis, the plants make food by absorbing the energy of the sun. So, this is not similar to Combustion The breakdown of food to release energy is known as respiration . Hence, energy is released. So, this is similar to Combustion Decomposition of vegetable matter involves release of energy. So, this is similar to Combustion In order to carry out the decomposition of ferrous sulphate, we need to supply heat. So, this is not similar to Combustion So, the correct answer is (a)
The physical states of the reactants and products can be represented by using the symbols (s) for solids, (l) for liquids, (g) for gases and (aq) for aqueous solution along with their respective formulae. The word aqueous is written if the reactant or product is present as a solution in water. Precipitate can also be represented by using an arrow pointing downwards (↓) instead of using symbol (s). In the same way, the gaseous state of an evolved gas can be represented by using an arrow pointing upward direction (↑) instead of using symbol (g). The specific condition of the reaction like temperature, pressure, catalyst etc. is written above or below the arrow in the chemical equation. Question 6: The correct way to represent the evolution of gas, is to use which of the following symbol: (a) ↓ (b) → (c) ↑ (d) (g)
Correct Answer is Option (c) Reaction of Magnesium with Hydrochloric Acid So, the correct answer is (c) ↑
Question 7: Which of the following reaction is balanced? (a) NaCl + 2H 2 O → 2NaOH + 2Cl 2 + H 2 (b) 2NaCl + H 2 O → 2NaOH + 2Cl 2 + H 2 (c) 2NaCl + 2H 2 O → 2NaOH + Cl 2 + H 2 (d) 2NaCl + 2H 2 O → NaOH + Cl 2 + H 2
Correct Answer is Option (c) A balanced chemical equation is one in which the number of atoms of both reactants and products are equal. In reaction I: Since the number of Na and Cl atoms in reactant and product is not equal, it is not a balanced equation. In reaction II: Since the number of H, O and Cl atoms in reactant and product is not equal. It is not a balanced equation. In reaction III: Since the number of all atoms in reactants and products is equal, it is a balanced equation. In Reaction IV: Since the number of Na, H, and O atoms is not equal in reactant and product, it is not a balanced equation. So, the correct answer is (c) 2NaCl + 2H 2 O → 2NaOH + Cl 2 + H 2
In the following chemical reaction ‘‘zinc oxide reacts with carbon to produce zinc metal and carbon monoxide.’’ Answer any four question from I to V. ZnO + C → Zn + CO Question 8: Name the type of reaction: (a) oxidation reaction (b) reduction reaction (c) redox reaction (d) decomposition reaction
Correct Answer is Option (c) Oxidation and Reduction Reaction Example Since both oxidation and reduction is taking place in this reaction, it is a redox reaction. So, the correct answer is (c) redox reaction
Question 9: Which of the following is the effect of oxidation reaction in everyday life: (a) Precipitation (b) Fermentation (c) Corrosion (d) Hydrogenation of oil
Correct Answer is Option (c) Let’s look at each of the reaction, one by one Precipitation reaction reaction is not an oxidation reaction because this simply involves the formation of an insoluble substance (precipitate). Fermentation breaking down of food in the absence of oxygen Corrosion involves the oxidation of iron to form rust Hydrogenation of oil is not an oxidation reaction because in this, hydrogen atoms are added over the double bonds of hydrocarbons. So, the correct answer is (c) Corrosion
P, Q and R are 3 elements which undergo chemical reactions according to the following equations Answer any four question from I to V: (i) P 2 O 3 + 2Q → Q 2 O 3 + 2P (ii) 3RSO 4 + 2Q → Q 2 (SO 4 ) 3 + 3R (iii) 3RO + 2P → P 2 O 3 + 3R Question 10: Choose the correct statement: (a) Zinc and lead are more reactive elements than copper. (b) Zinc and lead are less reactive elements than copper. (c) Zinc and copper are more reactive elements than lead. (d) Copper and lead are more reactive elements than zinc.
Correct Answer is Option (a) According to the reactivity series, Zinc and lead are more reactive than copper because they both are able to replace copper from its salt solution.
Question 11: Na 2 SO 4 (aq) + BaCl 2 (aq) → BaSO 4 (s) + 2NaCl(aq) The above reaction is an example of: (a) Double displacement reaction. (b) Displacement reaction. (c) Can be both. (d) None of the above.
Correct Answer is Option (a) Since in this reaction the two reacting compounds are switching their positive and negative ions, it is a double displacement reaction. So, the correct answer is (a)
Question 12: The type of reaction is: (a) Displacement reaction (b) Combination reaction (c) Neutralisation reaction (d) Substitution reaction
Correct Answer is Option (a) Let’s look at all the options Displacement Reaction: Reaction in which more reactive element takes the place of less reactive element. Combination Reaction: When two or more substances react to form a single product, it is known as combination reaction. Neutralisation reaction: A reaction in which acid and base react to form salt and water Substitution Reaction: In this reaction, one functional group in a chemical compound is replaced by another. Since in the above mentioned reactions, the more reactive elements (Q, P) are replacing the less reactive element (R), this is a displacement reaction . So, the correct answer is (a) Displacement reaction
Top Courses for Class 9
Faqs on class 10 science chapter 1 case based questions - chemical reactions and equations, mock tests for examination, study material, practice quizzes, objective type questions, semester notes, shortcuts and tricks, video lectures, past year papers, important questions, viva questions, extra questions, previous year questions with solutions, sample paper.

Visual Case Based Type Questions: Chemical Reactions & Equations - 2 Free PDF Download
Importance of visual case based type questions: chemical reactions & equations - 2, visual case based type questions: chemical reactions & equations - 2 notes, visual case based type questions: chemical reactions & equations - 2 class 9, study visual case based type questions: chemical reactions & equations - 2 on the app, welcome back, create your account for free.

Forgot Password
Unattempted tests, change country.
- Bihar Board
SRM University
Up board result 2024, assam hslc result 2024.
- UP Board 10th Result 2024
- UP Board 12th Result 2024
- Punjab Board Result 2024
- JAC Board Result 2024
- Assam Board Result 2024
- Karnataka Board Result
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- नए भारत का नया उत्तर प्रदेश
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
- CBSE Class 10
CBSE Class 10 Chemistry Chapter 1 Important Questions and Answers for 2023
Cbse class 10 chemistry chapter 1 important questions: in this article, we are going to discuss the important questions of cbse class 10 chemistry chapter 1 chemical reactions and equations for 2023 class 10th board examination. the answers are also given towards the end. download solutions in pdf. .

CBSE Class 10 Chemistry Chapter 1 Important Questions: Central Board of Secondary Education is likely to release the 2023 class 10th board examination date sheet soon. The board has not yet announced a fixed date for the release of the date sheet.
The board exams are likely to commence in February, 2023.
With about two months left for CBSE Class 10 Science board examinations, students should begin their preparations if they haven’t already done so.
CBSE Class 10 Chemistry Chapter 1 Important Questions:
Chapter 1 chemical reactions and equations, multiple choice questions.
- When Ag is exposed to air it gets a black coating of
(a) AgNO3
(b) Ag2S
(c) Ag2O
- Which of the reactions is used in black and white photography?
(a) Combination Reaction
(b) Decomposition Reaction
(c) Displacement reaction
(d) Oxidation reaction
- Identify the substance oxidized in the above equation.
(a) MnCl2
(b) HCl
- Zinc reacts with silver nitrate to form which compounds?
- In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
- a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
- The brown gas evolved on heating of copper nitrate is
(a) O2
(b) NO2
(c) N2
- Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
(a) 1: 1
(b) 2:1
(c) 4:1
- A substance ‘X’ is used in white-washing and is obtained by heating limestone in the absence of air. Identify ‘X’.
(a) CaOCl2
(b) Ca (OH)2
(c) CaO
- 2HNO3 + Ca (OH)2 → Ca (NO3)2 + 2H2O; is an example of
(i) displacement reaction
(ii) double displacement reaction
(iii) neutralisation reaction
(iv) combination reaction.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
- A substance X which is a group 2 element is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution which turns red litmus blue. Element X is
(a) Cu
(b) Ca
(c)Na
ASSERTION- REASON TYPE QUESTIONS
DIRECTION : Each of these questions contains an assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
(a) If both Assertion and Reason are correct and reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are correct, but reason is not the correct explanation of Assertion.
(c) If Assertion is correct but Reason is incorrect.
(d) If Assertion is incorrect but Reason is correct.
Q1. Assertion (A): Photosynthesis is considered as an endothermic reaction.
Reason (R): Energy gets released in the process of photosynthesis
Q2. Assertion (A): MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O is redox reaction.
Reason (R): MnO2 oxidises HCl to Cl2 and gets reduced to MnCl2.
Q3. Assertion (A): When HCl is added to zinc granules, a chemical reaction occurs.
Reason (R): Evolution of a gas indicates that the chemical reaction is taking place.
Q4. Assertion (A): White silver chloride turns grey in sunlight.
Reason (R): Decomposition of silver chloride in presence of sunlight takes place to form silver metal and chlorine gas.
Q5. Assertion (A): Chemical reaction changes the physical and chemical properties of a substance
These practise papers will not just boost your Chemistry preparation but also help you in analysing what your weak points are.
CASE STUDY QUESTIONS
- A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The massive force that pushes the rocket forward through space is generated due to the
(a) combination reaction
(b) decomposition reaction
(c) displacement reaction
(d) double displacement reaction
(ii) A white salt on heating decomposes to give brown fumes and yellow residue is left behind. The yellow residue left is of
(a) lead nitrate
(b) nitrogen oxide
(c) lead oxide
(d) oxygen gas
(iii) Which of the following reactions represents a combination reaction?
(a) CaO (s) + H2O (l) → Ca (OH)2 (aq)
(b) CaCO3 (s) → CaO (s) + CO2(g)
(c) Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu(s)
(d) 2FeSO4(s) → Fe2O3 (s) +SO2(g) + SO3(g)
(iv) Complete the following statements by choosing correct type of reaction for X and Y.
Statement 1: The heating of lead nitrate is an example of ‘X’ reaction.
Statement 2: The burning of magnesium is an example of ‘Y’ reaction.
(a)X-Combination,Y-Decomposition
(b)X-Decomposition,Y-Combination
(c)X-Combination,Y-Displacement
- Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. A double displacement reaction usually occurs in solution and one of the products, being insoluble, precipitate out (separates as a solid). Any reaction in which an insoluble solid (called precipitate) is formed that separates from the solution is called a precipitation reaction. The reaction in which acid or acidic oxide reacts with base or basic oxide to form salt and water is called neutralisation reaction.
For example, 2NaOH+H2SO4⟶Na2SO4 + 2 H2O
(i) When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained, and the sulphuric acid so formed remains in the solution. The reaction is an example of a
(a) combination reaction
(b) displacement reaction
(c) decomposition reaction
(ii) Barium chloride on reaction with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(I) Displacement reaction
(II) Precipitation reaction
(III) Combination reaction
(IV) Double displacement reaction
(a) (I) only
(b) (II) only
(c) (III) and (IV) only
(d) (II) and ( V) only
(iii) Identify A in the following reaction.
AlCl3(aq) + 3NH4OH (aq)⟶A + 3NH4Cl(aq)
(a) Al (OH)3
(b) Al2 O3
(c) AlH3
(iv) Consider the following reaction, BaCl2 + Na2SO4⟶ BaSO4 + 2NaCl. Identify the precipitate in the reaction,
(a) BaCl2
(b) BaSO4
(c) Na2SO4
Short Answer Type Questions-I (2 Marks)
- You might have noted that when copper powder is heated in a China dish, the reddish brown surface of copper powder becomes coated with a black substance.
(a) Why is this black substance formed?
(b) What is the black substance?
(c) Write the chemical equation of the reaction that takes place.
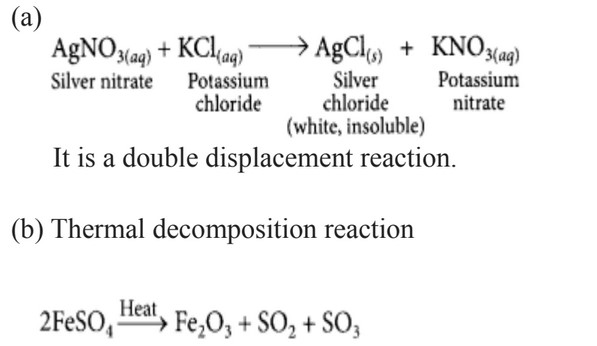
- Define a combination reaction. Give one example of a combination reaction which is also exothermic.
(a) A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction.
(b) Ferrous sulphate when heated, decomposes with the evolution of a gas having a characteristic odour of burning sulphur. Write the chemical reaction involved and identify the type of reaction.
- What is observed when carbon dioxide gas is passed through lime water.
(i) For a short duration
(ii) For long duration?
Also, write the chemical equations for the reaction involved.
- Lead nitrate solution is added to a test tube containing potassium iodide solution.
(a) Write the name and colour of the compound precipitated.
(b) Write the balanced chemical equation for the reaction involved.
(c) Name the type of this reaction justifying your answer.
- Name the type of chemical reaction represented by the following equation:
(Board Term I, 2016)
(i) CaO + H2O → Ca(OH)2
(ii) 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
- 2 g of silver chloride is taken in a China dish and the China dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
- List four observations that help us to determine whether a chemical reaction has taken place.
- Identify the type of reactions taking place in each of the following cases and write the balanced chemical equation for the reactions.
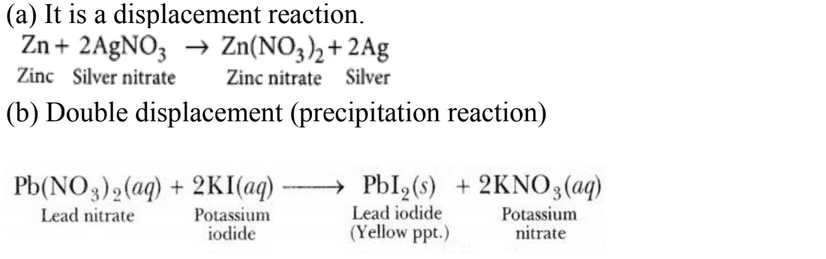
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(b) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
- i) Can a displacement reaction be a redox reaction? Explain with the help of an
- ii) Identify the substances that are oxidised and the substances that are reduced in the
following reactions.
(Board Term I, 2015)
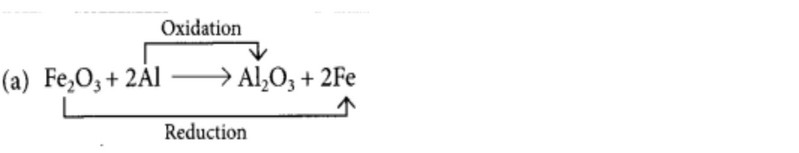
(a) Fe2O3 + 2Al → Al2O3 + 2Fe
(b) 2PbO + C → 2Pb + CO2
Also check:
CBSE Class 10 Science Syllabus 2022-23
Answers to CBSE Class 10 Chemistry Chapter 1 Important Questions:
DOWNLOAD: Answers to CBSE Class 10 Chemistry Chapter 1 Important Questions
The massive force that pushes the rocket forward through space is generated due to the decomposition reaction. Hydrogen peroxide decomposes and provides it with a considerable reaction force thrust.
ii) (c)
Lead nitrate decomposes to give brown fumes of nitrogen dioxide gas and yellow residue of lead oxide is left behind.
iii) (a)

2. A combination reaction is said to have occurred when two or more than two substances combine to form a single substance. CaO + H 2 O → Ca(OH) 2
4. (i) For short duration: Limewater turns milky due to the formation of CaCO3, Which is insoluble in water. CaOH 2 + Co 2 → CaCo 3 + H 2 O
(ii) For Long duration: A clear solution is obtained due to the formation of calcium bicarbonate. Ca (HCO3)2 which is soluble in water. CaCO 3 + H 2 O → Ca(HCO 3 ) 2
5. (a) When lead nitrate is added to potassium iodide then yellow precipitate of lead iodide is formed along with potassium nitrate.
(b) Balanced chemical reaction is as follows : Pb(NO 3 ) 2 + 2KI → PbI 2 + 2KNO 3
6. (i) Combination reaction.
(ii) Precipitation reaction or double displacement reaction.
(iii) Thermal decomposition reaction.
7. When 2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time, the white sodium chloride turns grey due to the decomposition reaction through the sunlight that decomposes silver chloride into silver and chlorine by light. 2 AgCl (s) → 2 Ag (s) + Cl 2 (g)
8. (i) Evolution of gas
(ii) Change in temperature
(iii) Change in state
(iv) Change in colour
(i) Consider the following displacement reaction: Zn(s)+ CuSO4(aq) → ZnSO4(aq) + Cu(s) Here, Zn has changed into ZnSO4 (i.e., Zn2+ ions) by loss of electrons. Hence, Zn has been oxidised. CuSO4 (i.e., Cu2+) has changed into Cu by gain of electrons. Hence, CuSO4 has been reduced. Thus, the above reaction is a displacement reaction as well as a redox reaction.
CBSE Class 10 Chemistry Chapter 2 Important Questions and Answers for 2023
CBSE Class 10 Chemistry Chapter 3 Important Questions and Answers for 2023
Important Resources for Class 10 CBSE Board Exam 2023
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- Jharkhand Board Result 2024
- Jharkhand Board 10th Result 2024
- JAC 10th Result 2024
- JAC Board 10th Result 2024
- jacresults.com 2024 Class 10th Result
- jacresults.com Result 2024
- jac.jharkhand.gov.in 2024 Class 10th Result
- jac.jharkhand.gov.in Result 2024
- JAC 10th Topper List 2024
- Jharkhand Board 10th Toppers List 2024
- CBSE Class 10 Subjects
- CBSE Class 10 Chemistry
- CBSE Class 10 QnA
Latest Education News
AP 10th Results 2024 Manabadi LIVE: BSEAP SSC(Class10) Result on April 22 (Monday) at 11 AM, Check Official Website Link
CDS Answer key 2024:यहाँ देखें सीडीएस परीक्षा की अनऑफिसियल उत्तर कुंजी, ये रहा डायरेक्ट डाउनलोड लिंक
NDA Question Paper 2024 Download UPSC Paper 1 and Paper 2 Papers Here
[Official] AP SSC Results 2024 Manabadi Date and Time Announced: Check Notice for BSEAP 10th Results Here
NDA Answer Key 2024: Download for Maths and GAT (Unofficial) Paper PDF
UPSC CDS Question Paper 2024: Download English, Maths, GK PDF Here
CSK Highest And Lowest Score in IPL Match And Innings
CDS Answer Key 2024: Download Maths, GK, English, Paper PDF (Unofficial)
Today’s IPL Match (21 April) - KKR vs RCB: Team Squad, Match Time, Where to Watch Live and Stadium
[Today] IPL 2024 Points Table: Team Rankings and Net Run Rate
Who Won Yesterday IPL Match: DC vs SRH, Match 35, Check All Details and Latest Points Table
[Current] Orange Cap and Purple Cap Holders in IPL 2024
Purple Cap in IPL 2024: Top Players List with Most Wickets in TATA IPL
Orange Cap in IPL 2024: Top Players List with Most Runs in TATA IPL
Odd One Out Brain Teaser: Can You Find What Is Not Fit In This Grid Of 590 Within 13 Seconds?
(Updated) RCB vs KKR Head to Head in IPL: Check Stats, Records and Results
UP Board Class 12th Result 2024 OUT: रिजल्ट जारी, 82.6 फीसदी बच्चे पास, शुभम ने किया टॉप, अमरोहा जिला रहा टॉप पर
UP Board Class 10 Toppers List 2024 (आउट): मिलिए प्रदेश की टॉपर Sitapur की प्राची Nigam से जिन्हें मिले 591 मार्क्स
UP Board 2024 10th District Topper Names: यूपी बोर्ड जिलेवार 10वीं टॉपर्स लिस्ट यहां देखें
Highest Team Score In IPL: SRH का दबदबा, आईपीएल इतिहास के 11 सबसे बड़े टीम स्कोर कौन-से है?

Class 10th Science - Chemical Reactions and Equations Case Study Questions and Answers 2022 - 2023
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 10 Science Subject - Chemical Reactions and Equations, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Chemical reactions and equations case study questions with answer key.
10th Standard CBSE
Final Semester - June 2015
Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it. In a chemical equation, the substances which combine or react are called reactants and new substances produced are called products. A chemical equation is a short hand method of representing a chemical reaction. A balanced chemical equation has equal number of atoms of different elements in the reactants and products side. An unbalanced chemical equation has unequal number of atoms of one or more elements in reactants and products. Formulae of elements and compounds are not changed to balance an equation. (i) Consider the following reaction: pMg 3 N 2 + qH 2 O ⇾ rMg(OH) 2 + sNH 3 When the equation is balanced, the coefficients p, q, r, s respectively are
(ii) Which of the following information is not conveyed by a balanced chemical equation?
(iii) The balancing of chemical equations is in accordance with
(iv) Which of the following chemical equations is an unbalanced one?
(v) Which of the following statements is/are correct?
In decomposition reactions, a single reactant breaks down to form two or more products. A decomposition reaction is opposite to combination reaction. Thermal decomposition reactions use the energy in form of heat for the decomposition of reactants. Electrolytic decomposition reactions involve the use of electrical energy for the decomposition of reactant molecules. Photolysis or photochemical decomposition involves the use of light energy for the purpose of decomposition. (i) Which of the following reactions is a decomposition reaction?
\({ (ii) \ } 2 \mathrm{~Pb}\left(\mathrm{NO}_{3}\right)_{2} \longrightarrow 2 \mathrm{PbO}+n A+\mathrm{O}_{2}\) What is nA in the given reaction?
(iii) Amino acid is formed by the decomposition of which component of our diet?
(iv) Silver chloride on exposure to sunlight for a long duration turns grey due to (I) the formation of silver by decomposition of silver chloride (II) sublimation of silver chloride (III) decomposition of chlorine gas from silver chloride (IV) oxidation of silver chloride The correct statement(s) is/are
(v) What type of chemical reaction takes place when electricity is passed through water?
Redox reactions are those reactions in which oxidation and reduction occur Simultaneously. A redox reaction is made up of two half reactions. In the first half reaction, oxidation takes place and in second half reaction, reduction occurs. Oxidation is a process in which a substance loses electrons and in reduction, a substance gains electrons. The substance which gains electrons is reduced and acts as an oxidising agent. On the other hand, a substance which loses electrons is oxidised and acts as a reducing agent. (i) Which of the following is a redox reaction?
(ii) Identify the reaction in which H2 02 is acting as a reducing agent.
(iii) For the following reactions, identify the one in which H 2 S acts as a reducing agent.
(iv) For the following reaction, identify the correct statement. \(\mathrm{ZnO}+\mathrm{CO} \longrightarrow \mathrm{Zn}+\mathrm{CO}_{2}\)
(v) In the following reaction, which substance is reduced? \(\mathrm{PbS}+4 \mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow \mathrm{PbSO}_{4}+4 \mathrm{H}_{2} \mathrm{O}\)
In a balanced chemical reaction, equal number of atoms are present on both sides of reaction. A balanced chemical reaction is based on law of conservation of mass which means that total mass of reactants and products participating in a reaction must be equal. For example, a balanced chemical equation of burning of magnesium in oxygen to form magnesium oxide is written as : \(2 \mathrm{Mg}+\mathrm{O}_{2} \longrightarrow 2 \mathrm{MgO}\) The mass of reactants (2 x 24 + 32 = 80) is equal to the mass of products [2 x (24 + 16) = 80] (i) In a reaction, 35 g of reactant, PQ breaks down into 20 g of product, P and an unknown amount of product, Q. Using the law of conservation of mass, weight of products, Q will be
(ii) When solid mercury (II) oxide is heated, liquid mercury and oxygen gas are produced. Which of the following statements is true regarding the balanced chemical equation for this process? (a) 1 mole of mercury (II) oxide produces two moles of mercury and one mole of oxygen gas (b) 2 moles of mercury (II) oxide produce one mole of mercury and one mole of oxygen gas (c) 1 mole of mercury (II) oxide produces half mole of mercury and half mole of oxygen gas (d) 2 moles of mercury (II) oxide produce 2 moles of mercury and one mole of oxygen gas (iii) Which of the following laws is satisfied by a balanced chemical equation?
(iv) In the given chemical reaction \(\mathrm{C}_{6} \mathrm{H}_{6(l)}+15 \mathrm{O}_{2(g)} \longrightarrow m \mathrm{CO}_{2(g)}+n \mathrm{H}_{2} \mathrm{O}_{(l)}\) The values of m and n are respectively
(v) Sulphur dioxide reacts with oxygen to form sulphur trioxide. What would be the molar ratio of sulphur dioxide to sulphur trioxide?
In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be observed easily. These easily observed features which take place as a result of chemical reaction are known as characteristics of chemicals reactions. Some important characteristics of chemical reactions are: (I) Evolution of heat (II) Formation of precipitate (III) Change in colour (IV) Change in temperature (V) Change in state Anyone of these general characteristics can tell us whether a chemical reaction has taken place or not. (i) Reaction of magnesium with air is a/an
(ii) In the following reaction \(\mathrm{Ca}_{(a q)}^{2+}+2 \mathrm{OH}_{(a q)}^{-} \longrightarrow \mathrm{Ca}(\mathrm{OH})_{2(s)}\) precipitate of calcium hydroxide will be of
(iii) In the given reaction, \(\mathrm{S}_{(s)}+\mathrm{O}_{2(g)} \longrightarrow \mathrm{SO}_{2}\) the physical state of SO 2 is
(iv) Which one of the following processes involve chemical reactions?
(v) In which of the following reactions, high amount of heat energy will be evolved?
A reaction in which two or more reactants combine to form a single product is called a combination reaction. For example, calcium oxide reacts vigorously with water to form calcium hydroxide. The reaction is highly exothermic in nature, as lots of heat is produced during the reaction. \(\mathrm{CaO}_{(s)}+\mathrm{H}_{2} \mathrm{O}_{(l)} \longrightarrow \mathrm{Ca}(\mathrm{OH})_{2(a q)}+\text { Heat }\) Calcium oxide Water Calcium hydroxide Solution of Ca(OH) 2 is used for white wash the walls. Calcium hydroxide reacts slowly with carbon dioxide in air to form a thin layer of calcium carbonate on the wall which gives a shiny appearance to wall. Calcium carbonate will form after two or three days of white wash. (i) What is the chemical name of quick lime?
(ii) When carbon dioxide is passed through lime water,
(iv) Quick lime combines Vigorously with water to form (A) which reacts slowly with the carbon dioxide in air to form (B) Identify the compounds(A) and (B)
(v) Among the following, the endothermic reaction is
Reactions in which one element takes place of another element in a compound, are known as displacement reactions. In general, more reactive elements displaces a less reactive element from its compound. In all single displacement reactions, only one element displaces another element from its compound. The single displacement reactions are, however, written as just displacement reactions. The displacement reaction between iron (III) oxide and powdered aluminium produces so much heat that iron metal obtained is in molten form. (i) Copper displaces which of the following metals from its salt solution?
(ii) When zinc reacts with dilute sulphuric acid, the gas evolved is
(iv) Which of the following reactions is a displacement reaction?
(v) When dilute hydrochloric acid is added to granulated zinc placed in a test tube, the observation made is
Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. A double displacement reaction usually occurs in solution and one of the products, being insoluble, precipitate out (separates as a solid). Any reaction in which an insoluble solid (called precipitate) is formed that separates from the solution is called a precipitation reaction. The reaction in which acid or acidic oxide reacts with base or basic oxide to form salt and water is called neutralisation reaction. For example, \(2 \mathrm{NaOH}+\mathrm{H}_{2} \mathrm{SO}_{4} \longrightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{H}_{2} \mathrm{O}\) (i) When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a
(ii) Which of the following is not a double displacement reaction?
(iii) Barium chloride on reaction with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved? (I) Displacement reaction (II) Precipitation reaction (III) Combination reaction (IV) Double displacement reaction
(iv) Identify A in the following reaction. \(\mathrm{AlCl}_{3(a q)}+3 \mathrm{NH}_{4} \mathrm{OH}_{(a q)} \longrightarrow A+3 \mathrm{NH}_{4} \mathrm{Cl}_{(a q)}\)
(v) Consider the following reaction, \(\mathrm{BaCl}_{2}+\mathrm{Na}_{2} \mathrm{SO}_{4} \longrightarrow \mathrm{BaSO}_{4}+2 \mathrm{NaCl}\) identify the precipitate in the reaction,
(ii) In the reaction, \(\mathrm{H}_{2} \mathrm{~S}+\mathrm{Cl}_{2} \longrightarrow \mathrm{S}+2 \mathrm{HCl}\)
(iii) Which ofthe following processes does not involve either oxidation or reduction?
\(\text { (iv) } \mathrm{Mg}+\mathrm{CuO} \longrightarrow \mathrm{MgO}+\mathrm{Cu}\)
Which of the following is wrong relating to the above reaction?
(v) Identify the correct oxidising agent and reducing agent in the following reaction. \(\mathrm{Fe}_{2} \mathrm{O}_{3}+2 \mathrm{Al} \longrightarrow 2 \mathrm{Fe}+\mathrm{Al}_{2} \mathrm{O}_{3}\)
Oxidation has damaging effect on metals as well as on food. The damaging effect of oxidation on metal is studied as corrosion and that on food is studied as rancidity. The phenomenon due to which metals are slowly eaten away by the reaction of air, water and chemicals present in atmosphere, is called corrosion. For example, iron articles are shiny when new, but get coated with a reddish brown powder when left for sometime. This process is known as rusting of iron. Rancidity is the process of slow oxidation of oil and fat (which are volatile in nature) present in the food materials resulting in the change of smell and taste in them. (i) Rancidity can be prevented by
(ii) Combination of phosphorus and oxygen is an example of
(iii) A science teacher wrote the following statements about rancidity : (I) When fats and oils are reduced, they become rancid. (II) In chips packet, rancidity is prevented by oxygen. (III) Rancidity is prevented by adding antioxidants. Select the correct option.
(iv) Two statements are given below regarding rusting of iron. (I) The rusting of iron is a redox reaction and reaction occurs as, \(4 \mathrm{Fe}+3 \mathrm{O}_{2} \longrightarrow 4 \mathrm{Fe}^{3+}+6 \mathrm{O}^{2-}\) (II) The metallic iron is oxidised to \(\mathrm{Fe}^{2+} \text { and } \mathrm{O}_{2} \text { is reduced to } \mathrm{O}^{2-}\) Select the correct statement(s).
(v) Which of the following measures can be adopted to prevent or slow down rancidity? (I) Food materials should be packed in air tight container. (II) Food should be refrigerated. (III) Food materials and cooked food should be kept away from direct sunlight
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. Study this table related to the different types of reactions / processes and answer the questions that follow.
(i) The reaction in which two or more substances combine to form a single substance under suitable conditions is (a) combination reaction (b) combustion (c) decomposition reaction (d) photosynthesis (ii) Which of the following is essential for photosynthesis? (a) Sunlight (b) Chlorophyll (c) Glucose (d) Both 'a' and 'b’ Photosynthesis is the process by which green plants and some other organisms use sunlight to synthesis nutrients from carbon dioxide and water. (iii) When a chemical compound decomposes on absorbing light and energy, then the reaction which takes place is known as (a) photosynthesis (b) photodecomposition (c) combination (d) thermal decomposition. A photodecomposition is a chemical reaction in which an inorganic chemical (or an organic chemical) is broken down by photons and is the interaction of one or more photons with one target molecule. (iv) Which of the following reactions is an example of combustion reaction ? \((a) \mathrm{C}_{(s)}+\mathrm{O}_{2(g)} \longrightarrow \mathrm{CO}_{2(g)}\\ (b) \mathrm{Zn}_{(s)}+\mathrm{H}_{2} \mathrm{SO}_{4(a q)} \longrightarrow \mathrm{ZnSO}_{4(a q)}+\mathrm{H}_{2(g)}\\ (c) \mathrm{Zn}_{(s)}+2 \mathrm{HCl}_{(a q)} \longrightarrow \mathrm{ZnCl}_{2(a q)}+\mathrm{H}_{2(g)}\\ (d) 3 \mathrm{Mg}_{(s)}+\mathrm{N}_{2(g)} \longrightarrow \mathrm{Mg}_{3} \mathrm{~N}_{2(s)}\) A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. (v) Which of the following is an example of combination reaction? \((a) \mathrm{H}_{2(g)}+\mathrm{Cl}_{2(g)} \stackrel{\text { light }}{\longrightarrow} 2 \mathrm{HCl}_{(g)}\\ (b) \mathrm{Fe}_{(s)}+S_{(s)} \longrightarrow \mathrm{FeS}_{(g)}\\ (c) 2 \mathrm{H}_{2(g)}+\mathrm{O}_{2(g)} \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}_{(l)}\\ (d) All \ of \ them\) A combination reaction (also known as a synthesis reaction) is a reaction where two or more elements or compounds (reactants) combine to form a single compound (product).
*****************************************
Related 10th standard cbse science materials, other 10th standard cbse materials.

CBSE 10th Maths Probability Chapter Case Study Question with Answers
Cbse 10th maths statistics chapter case study question with answers, cbse 10th maths surface areas and volumes chapter case study question with answers, cbse 10th maths areas related to circles chapter case study question with answers, cbse 10th maths circles chapter case study question with answers, cbse 10th maths some applications of trigonometry chapter case study question with answers, cbse 10th maths introduction to trigonometry chapter case study question with answers, cbse 10th maths coordinate geometry chapter case study question with answers, cbse 10th maths triangles chapter case study question with answers, cbse 10th maths arithmetic progressions chapter case study questions with answers, cbse 10th maths quadratic equations chapter case study questions with answers.

CBSE 10th Social Science The Making Of A Global World Chapter Case Study Question with Answers
Cbse 10th social science nationalism in india chapter case study question with answers, cbse 10th social science the rise of nationalism in europe chapter case study question with answers, cbse 10th maths pair of linear equation in two variables chapter case study question with answers, tamilnadu stateboard 10th standard cbse study materials.

Tamilnadu Stateboard 10th Standard CBSE Subjects


NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations

Chapter 1 Chemical Reactions and Equations Class 10 NCERT Solutions
Ncert solutions for class 10 science chapters:, state the law which is kept in mind while we balance a chemical equation., what is basic difference between a physical change and a chemical change, what is meant by a chemical reaction, is burning of a candle, a physical change or a chemical change, why are decomposing reactions called the opposite of combination reactions, contact form.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.5: Case Study- Balancing Acts in Pharmacological Synthesis
- Last updated
- Save as PDF
- Page ID 465657
Introduction: Chemical equations are the language through which chemists communicate the subtleties of chemical reactions. In pharmacological research, where the synthesis of new drug molecules is both an art and a science, balancing these equations is crucial. This case study explores the importance of balancing chemical equations in the synthesis of new drugs and how this relates to enzyme kinetics in the body.
Scenario: In the high-tech research laboratory of Apex Pharmaceuticals, a team of researchers, including Dr. Sophie Lee, is on the verge of a breakthrough. They have developed a promising new drug molecule that could potentially treat a rare genetic disorder. However, before they can move forward with production and trials, they must first balance the chemical equation for the synthesis reaction of the drug to ensure the process is efficient, cost-effective, and yields the correct product in the desired quantities.
The Art of Balancing Equations: Dr. Lee and her team meticulously account for each atom of the reactants and products, adhering to the law of conservation of mass. They adjust coefficients to balance the number of atoms on both sides of the equation, a step that is critical for the synthesis process to proceed correctly and safely.
Case Study Questions:
- Why is balancing chemical equations important in drug synthesis?
- How do reaction rates relate to enzyme kinetics?
Hands-On Classroom Activities:
- Synthesis Lab: In the "Synthesis Lab" activity, students will engage in the synthesis of aspirin and are required to balance the chemical equation for this reaction. This exercise is designed to connect their understanding of chemical reactions and stoichiometry with the practical aspects of drug synthesis and pharmaceutical chemistry.
- Enzyme Kinetics Experiment: During the "Enzyme Kinetics Experiment," students will conduct investigations into reaction rates by observing enzyme-catalyzed reactions. They will then be tasked with relating their observations to the principles of balancing chemical equations, thereby enhancing their understanding of the role of enzymes in biological reactions and the importance of precise chemical equations in biochemistry.
Conclusion: For Dr. Lee and her team at Apex Pharmaceuticals, the balancing of chemical equations is not just a necessary step in the synthesis of new drug molecules—it is a critical process that affects every stage from design to delivery. It ensures that each synthesis step is predictable and controllable, which is vital for the safe and effective production of pharmaceuticals. Moreover, by understanding the principles of enzyme kinetics and reaction rates, researchers can better anticipate how a drug will perform in vivo, ultimately leading to more effective treatments with fewer side effects. The meticulous balancing of chemical equations thus stands as the cornerstone of successful drug development and patient care.

IMAGES
VIDEO
COMMENTS
Case Study/Passage Based Questions on Chemical Reactions and Equations. Case Study/Passage Based Questions. Question 1: Corrosion is the phenomenon of deterioration of surface of metal in presence of air and moisture. It is a natural process and in the presence of a moist atmosphere, chemically active metals get corroded. This is an oxidation ...
Case Study 3: Chemical reactions and equations are fundamental concepts in chemistry that help us understand the transformation of substances. A chemical reaction involves the rearrangement of atoms to form new substances with different properties. In a chemical equation, the reactants are written on the left side, and the products are written on the right side, separated by an arrow.
CBSE 10th Standard Science Subject Chemical Reactions and Equations Case Study Questions With Solution 2021. 10th Standard CBSE. Reg.No. : Science. Time : 00:30:00 Hrs. Total Marks : 20. Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it.
It is interesting to note that the chemical formula for marble is also CaCO3. On the basis of above paragraph answer the following questions: 1.) Give the reaction for the formation of calcium carbonate with physical states. Ca (OH) 2 (s) + CO 2 (g) → CaCO 3 (s)↓ + H 2 O (l) 2.) Write the formulas of slaked lime, quick lime.
Case Study Questions Chapter 1 Chemical Reactions and Equations. The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released . (b) MnO2 is getting oxidized whereas HCl is getting reduced. (c) MnO2 and HCl both are getting reduced.
These questions are asked in the Multiple Choice Questions (MCQ). These case based questions are a type of open book test. These case based questions can help students to score well in the particular subject. These Chemical Reactions and Equations Case Study Based Questions can also be asked in the form of CBSE Assertion and Reason.
Case Study Chapter 1 Chemical Reactions and Equations. Question 1: A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation, and reduction reactions.
Chemical Reactions and Equations Case Study Questions (CSQ's) Select the number of questions for the test: TopperLearning provides a complete collection of case studies for CBSE Class 10 Chemistry Chemical Reactions and Equations chapter. Improve your understanding of biological concepts and develop problem-solving skills with expert advice.
5 Passages. 25 questions. Download case study question pdfs for CBSE Class 10th Maths, CBSE Class 10th English, CBSE Class 10th Sciece, CBSE Class 10th SST. As the CBSE 10th Term-1 Board Exams are approaching fast, you can use these worksheets for FREE for practice by students for the new case study formats for CBSE introduced this year.
Learn Class 10 chemistry chapter 1 | Chemical Reactions and Equations | case study Questions | case study based question | case study questions and answers ...
This unit introduces chemical reactions, the processes that create and transform matter. Learn about net ionic equations, reaction stoichiometry, titration, common reaction types, and more. Practice what you've learned and study for the AP Chemistry exam with 80 AP-aligned questions.
Chemical Reactions and Equations Case Study Questions With Answer Key Answer Keys. (iii) (c): In a balanced chemical equation, total mass of reactants must be equal to the total mass of products. This is the statement of law of conservation of mass. (iii) (c) : Proteins in our diet get broken down into amino acids.
Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). 7.5: Case Study- Balancing Acts in Pharmacological Synthesis; 7.E: Exercises
Chemical Reactions and Equations Case Study Based Questions Class 10 SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks.
Document Description: Visual Case Based Type Questions: Chemical Reactions & Equations - 2 for Class 9 2024 is part of Advance Learner Course: Science Class 9 preparation. The notes and questions for Visual Case Based Type Questions: Chemical Reactions & Equations - 2 have been prepared according to the Class 9 exam syllabus. Information about Visual Case Based Type Questions: Chemical ...
🟢Chemical Reaction and Equation | Case Based Questions - CBSE 10 | Anubha Ma'am @VedantuClass910 _____👉🏻 A...
CASE STUDY QUESTIONS. A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like ...
General, Organic, and Biochemistry with Problems, Case Studies, and Activities 7: Chemical Equations and Reactions Expand/collapse global location 7: Chemical Equations and Reactions Last updated; Save as PDF ... Chemical Equations and Reactions is shared under a CC BY-SA license and was authored, remixed, and/or curated by LibreTexts. Back to top;
A balanced chemical equation has equal number of atoms of different elements in the reactants and products side. An unbalanced chemical equation has unequal number of atoms of one or more elements in reactants and products. Formulae of elements and compounds are not changed to balance an equation. (i) Consider the following reaction:
1. A solution of a substance 'X' is used for white washing. (i) Name the substance 'X' and write its formula. (ii) Write the reaction of the substance 'X' named in (i) above with water. Answer. (i) The substance 'X' is calcium oxide. Its chemical formula is CaO. (ii) Calcium oxide reacts vigorously with water to form calcium ...
This case study explores the importance of balancing chemical equations in the synthesis of new drugs and how this relates to enzyme kinetics in the body. Scenario: In the high-tech research laboratory of Apex Pharmaceuticals, a team of researchers, including Dr. Sophie Lee, is on the verge of a breakthrough. They have developed a promising new ...
Take a quick interactive quiz on the concepts in Creating Particle Models for Balanced Chemical Equations or print the worksheet to practice offline. These practice questions will help you master ...