Green synthesis of nanoparticles using plant extracts: a review
- Published: 13 August 2020
- Volume 19 , pages 355–374, ( 2021 )

Cite this article

- Sapana Jadoun ORCID: orcid.org/0000-0002-3572-7934 1 ,
- Rizwan Arif 1 ,
- Nirmala Kumari Jangid 2 &
- Rajesh Kumar Meena 3
19k Accesses
513 Citations
4 Altmetric
Explore all metrics
Green synthesis of nanoparticles has many potential applications in environmental and biomedical fields. Green synthesis aims in particular at decreasing the usage of toxic chemicals. For instance, the use of biological materials such as plants is usually safe. Plants also contain reducing and capping agents. Here we present the principles of green chemistry, and we review plant-mediated synthesis of nanoparticles and their recent applications. Nanoparticles include gold, silver, copper, palladium, platinum, zinc oxide, and titanium dioxide.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
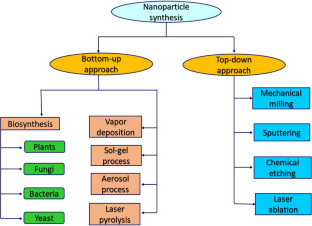
Similar content being viewed by others

Applications of Green Synthesized Metal Nanoparticles — a Review

Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review

Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties
Abbreviations.
4-Amino phenol
Brunauer–Emmett–Teller
Dynamic light scattering
2,4-Dinitrophenilhydrazine
Energy-dispersive spectroscopy
Field emission scanning electron microscopy
Fourier transform infrared
Graphene oxide
Methylene blue
Methyl orange
4-Nitrophenol
Rhodamine B
Reduced graphene oxide
Surface-enhanced Raman scattering
Tannery wastewater
Abisharani JM, Devikala S, Dinesh Kumar R, et al (2019) Green synthesis of TiO 2 nanoparticles using Cucurbita pepo seeds extract. In: Materials today: proceedings
Agarwal H, Venkat Kumar S, Rajeshkumar S (2017) A review on green synthesis of zinc oxide nanoparticles—an eco-friendly approach. Resource-Effic Technol 3:406–413. https://doi.org/10.1016/j.reffit.2017.03.002
Article Google Scholar
Ahmad W, Jaiswal KK, Soni S (2020) Green synthesis of titanium dioxide (TiO 2 ) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg Nano-Met Chem. https://doi.org/10.1080/24701556.2020.1732419
Ahmed S, Ahmad M, Swami BL, Ikram S (2016a) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28. https://doi.org/10.1016/j.jare.2015.02.007
Article CAS Google Scholar
Ahmed S, Annu, Ikram S, Yudha S (2016b) Biosynthesis of gold nanoparticles: a green approach. J Photochem Photobiol B Biol 161:141–153
CAS Google Scholar
Ahmed S, Saifullah Ahmad M et al (2016c) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9:1–7
Google Scholar
Akhter G, Khan A, Ali SG et al (2020) Antibacterial and nematicidal properties of biosynthesized Cu nanoparticles using extract of holoparasitic plant. SN Appl Sci. https://doi.org/10.1007/s42452-020-3068-6
Al Ansari MS (2012) A review of optimal designs in relation to supply chains and sustainable chemical processes. Modern Appl Sci 6:74
Ali S, Perveen S, Ali M et al (2020) Bioinspired morphology-controlled silver nanoparticles for antimicrobial application. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2019.110421
Al-Radadi NS (2019) Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab J Chem 12:330–349. https://doi.org/10.1016/j.arabjc.2018.05.008
Al-Radadi NS, Adam SIY (2020) Green biosynthesis of Pt-nanoparticles from Anbara fruits: toxic and protective effects on CCl4 induced hepatotoxicity in Wister rats. Arab J Chem 13:4386–4403. https://doi.org/10.1016/j.arabjc.2019.08.008
Ambika S, Sundrarajan M (2015) Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J Photochem Photobiol B 146:52–57
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312
Ankamwar B (2010) Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia Catappa. E-J Chem. https://doi.org/10.1155/2010/745120
Arabi N, Kianvash A, Hajalilou A et al (2020) A facile and green synthetic approach toward fabrication of Alcea- and Thyme-stabilized TiO 2 nanoparticles for photocatalytic applications. Arab J Chem 13:2132–2141. https://doi.org/10.1016/j.arabjc.2018.03.014
Arockiya Aarthi Rajathi F, Arumugam R, Saravanan S, Anantharaman P (2014) Phytofabrication of gold nanoparticles assisted by leaves of Suaeda monoica and its free radical scavenging property. J Photochem Photobiol B Biol. https://doi.org/10.1016/j.jphotobiol.2014.03.016
Arsiya F, Sayadi MH, Sobhani S (2017) Green synthesis of palladium nanoparticles using Chlorella vulgaris . Mater Lett 186:113–115
Aswini R, Murugesan S, Kannan K (2020) Bio-engineered TiO 2 nanoparticles using Ledebouria revoluta extract: larvicidal, histopathological, antibacterial and anticancer activity. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1718668
Auld DS (2001) Zinc coordination sphere in biochemical zinc sites. In: Maret W (ed) Zinc biochemistry, physiology, and homeostasis. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-3728-9_6
Chapter Google Scholar
Aygun A, Gülbagca F, Ozer LY et al (2020) Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2019.112961
Aygün A, Özdemir S, Gülcan M et al (2020) Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J Pharm Biomed Anal 178:112970. https://doi.org/10.1016/j.jpba.2019.112970
Bakand S, Hayes A, Dechsakulthorn F (2012) Nanoparticles: a review of particle toxicology following inhalation exposure. Inhalation Toxicol 24:125–135
Balantrapu K, Goia DV (2009) Silver nanoparticles for printable electronics and biological applications. J Mater Res 24:2828–2836
Banasiuk R, Krychowiak M, Swigon D et al (2020) Carnivorous plants used for green synthesis of silver nanoparticles with broad-spectrum antimicrobial activity. Arab J Chem. https://doi.org/10.1016/j.arabjc.2017.11.013
Bar H, Bhui DK, Sahoo GP et al (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surfaces A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2009.07.021
Bayrami A, Haghgooie S, Rahim Pouran S et al (2020) Synergistic antidiabetic activity of ZnO nanoparticles encompassed by Urtica dioica extract. Adv Powder Technol 31:2110–2118. https://doi.org/10.1016/j.apt.2020.03.004
Bergmann CP, de Andrade MJ (2011) Nanostructured materials for engineering applications. Springer, Berlin
Bhagat DS, Gurnule WB, Pande SG, et al (2020) Biosynthesis of gold nanoparticles for detection of dichlorvos residue from different samples. In: Materials today: proceedings
Bhuyan T, Mishra K, Khanuja M et al (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Boomi P, Poorani GP, Selvam S et al (2020) Green biosynthesis of gold nanoparticles using Croton sparsiflorus leaves extract and evaluation of UV protection, antibacterial and anticancer applications. Appl Organomet Chem. https://doi.org/10.1002/aoc.5574
Carolin CF, Kumar PS, Saravanan A et al (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5:2782–2799
Centi G, Perathonar S (2009) From green to sustainable industrial chemistry. In: Cavani F, Centi G, Perathoner S, Trifiró F (eds) Sustainable industrial chemistry. https://doi.org/10.1002/9783527629114.ch1
Chandra C, Khan F (2020) Nano-scale zerovalent copper: green synthesis, characterization and efficient removal of uranium. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-020-07080-1
Chandran SP, Chaudhary M, Pasricha R et al (2006a) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. https://doi.org/10.1021/bp0501423
Chandran SP, Chaudhary M, Pasricha R et al (2006b) Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol Prog 22:577–583
Chaudhari PR, Masurkar SA, Shidore VB, Kamble SP (2012) Biosynthesis of silver nanoparticles using Saccharum officinarum and its antimicrobial activity. Micro Nano Lett 7:646–650
Cheirmadurai K, Biswas S, Murali R, Thanikaivelan P (2014) Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. https://doi.org/10.1039/c4ra01414f
Clark JH, Macquarrie DJ (2008) Handbook of green chemistry and technology. Wiley, Hoboken
Crowl DA, Louvar JF (2001) Chemical process safety: fundamentals with applications. Pearson Education, London
Dar P, Waqas U, Hina A, et al (2016) Biogenic synthesis, characterization of silver nanoparticles using Multani mitti (Fullers Earth), Tomato (Solanum lycopersicum) seeds, Rice Husk (Oryza sativa) and evaluation of their potential antimicrobial activity. J Chem Soc Pak 38:665
Das J, Velusamy P (2014) Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora L. J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2014.04.005
Doan V-D, Thieu AT, Nguyen T-D et al (2020) Biosynthesis of gold nanoparticles using Litsea cubeba fruit extract for catalytic reduction of 4-Nitrophenol. J Nanomater 2020:4548790. https://doi.org/10.1155/2020/4548790
Du L, Xian L, Feng J-X (2011) Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. J Nanopart Res 13:921–930
Duan H, Wang D, Li Y (2015) Green chemistry for nanoparticle synthesis. Chem Soc Rev 44:5778–5792
Dubchak S, Ogar A, Mietelski JW, Turnau K (2010) Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus . Span J Agric Res 1:103–108
Dubey M, Bhadauria S, Kushwah BS (2009) Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (safeda) leaf. Dig J Nanomater Biostruct 4:537–543
Dvir T, Timko BP, Kohane DS, Langer R (2011) Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6:13
El-Sayed IH, Huang X, El-Sayed MA (2005) Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett 5:829–834
Fernando SID, Judan Cruz KG (2020) Ethnobotanical biosynthesis of gold nanoparticles and its downregulation of Quorum sensing-linked AhyR gene in Aeromonas hydrophila . SN Appl Sci. https://doi.org/10.1007/s42452-020-2368-1
Frey NA, Peng S, Cheng K, Sun S (2009) Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem Soc Rev 38:2532–2542
Ghosh P, Han G, De M et al (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60:1307–1315
Ghosh NS, Pandey E, Giilhotra RM, Singh R (2020) Biosynthesis of gold nanoparticles using leaf extract of Desmodium gangeticum and their antioxidant activity. Res J Pharm Technol 13:2685–2689
Goutam SP, Saxena G, Singh V et al (2018) Green synthesis of TiO 2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem Eng J. https://doi.org/10.1016/j.cej.2017.12.029
Guo M, Li W, Yang F, Liu H (2015) Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2015.01.109
Huang X, El-Sayed MA (2010) Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res 1:13–28
Huang J, Lin L, Li Q et al (2008) Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried Cinnamomum camphora leaf in tubular microreactors. Ind Eng Chem Res 47:6081–6090
Hulkoti NI, Taranath TC (2014) Biosynthesis of nanoparticles using microbes—a review. Colloids Surfaces B Biointerfaces 121:474–483. https://doi.org/10.1016/j.colsurfb.2014.05.027
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. https://doi.org/10.1039/C1GC15386B
Islam NU, Jalil K, Shahid M et al (2019) Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba . Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.06.025
Jain PK, Lee KS, El-Sayed IH, El-Sayed MA (2006) Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. https://doi.org/10.1021/jp057170o
Jameel MS, Aziz AA, Dheyab MA (2020) Comparative analysis of platinum nanoparticles synthesized using sonochemical-assisted and conventional green methods. Nano-Struct Nano-Objects 23:100484. https://doi.org/10.1016/j.nanoso.2020.100484
Jeong S, Choi SY, Park J et al (2011) Low-toxicity chitosan gold nanoparticles for small hairpin RNA delivery in human lung adenocarcinoma cells. J Mater Chem 21:13853–13859
Jeyarani S, Vinita NM, Puja P et al (2020) Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J Photochem Photobiol B Biol. https://doi.org/10.1016/j.jphotobiol.2019.111715
Kalaiselvi A, Roopan SM, Madhumitha G et al (2015) Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2014.07.010
Karimi J, Mohsenzadeh S (2015) Rapid, green, and eco-friendly biosynthesis of copper nanoparticles using flower extract of Aloe vera. Synth React Inorg Met-Org Nano-Met Chem. https://doi.org/10.1080/15533174.2013.862644
Kazlagić A, Abud OA, Ćibo M et al (2020) Green synthesis of silver nanoparticles using apple extract and its antimicrobial properties. Health Technol 10:147–150. https://doi.org/10.1007/s12553-019-00378-5
Keijok WJ, Pereira RHA, Alvarez LAC et al (2019) Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci Rep. https://doi.org/10.1038/s41598-019-52496-9
Keshari AK, Srivastava R, Singh P et al (2020) Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum . J Ayurveda Integr Med 1:37–44. https://doi.org/10.1016/j.jaim.2017.11.003
Kharey P, Dutta SB, Gorey A et al (2020) Pimenta dioica mediated biosynthesis of gold nanoparticles and evaluation of its potential for theranostic applications. ChemistrySelect 5:7901–7908. https://doi.org/10.1002/slct.202001230
Kora AJ, Rastogi L (2018) Green synthesis of palladium nanoparticles using gum ghatti ( Anogeissus latifolia ) and its application as an antioxidant and catalyst. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.06.024
Kumar KM, Mandal BK, Tammina SK (2013) Green synthesis of nano platinum using naturally occurring polyphenols. RSC Adv. https://doi.org/10.1039/c3ra22959a
Kumar PSM, Francis AP, Devasena T (2014) Biosynthesized and chemically synthesized titania nanoparticles comparative analysis of antibacterial activity. J Environ Nanotechnol. https://doi.org/10.13074/jent.2014.09.143098
Kumar PV, Kala SMJ, Prakash KS (2019) Green synthesis of gold nanoparticles using Croton Caudatus Geisel leaf extract and their biological studies. Mater Lett 236:19–22
Kumar CRR, Betageri VS, Nagaraju G et al (2020) One-pot synthesis of ZnO nanoparticles for nitrite sensing, photocatalytic and antibacterial studies. J Inorg Organometall Polym Mater. https://doi.org/10.1007/s10904-020-01544-3
Lateef A, Ojo SA, Elegbede JA (2016) The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol Rev 5:601–622
Lebaschi S, Hekmati M, Veisi H (2017) Green synthesis of palladium nanoparticles mediated by black tea leaves ( Camellia sinensis ) extract: catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2016.09.027
Lee SH, Salunke BK, Kim BS (2014) Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using Magnolia kobus plant leaf extracts. Biotechnol Bioprocess Eng 19:169–174
Li S, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem 9:852–858
Louis C, Pluchery O (2012) Gold nanoparticles for physics, chemistry and biology. World Scientific, Singapore
Lukman AI, Gong B, Marjo CE et al (2011) Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci 353:433–444
Manjare SB, Chaudhari RA (2020) Palladium nanoparticle-bentonite hybrid using leaves of syzygium aqueum plant from India: design and assessment in the catalysis of –C–C– coupling reaction. Chemistry Africa. https://doi.org/10.1007/s42250-020-00139-2
Mansoori GA (2005) Principles of nanotechnology: molecular-based study of condensed matter in small systems. World Scientific, Singapore
Marimuthu S, Rahuman AA, Jayaseelan C et al (2013) Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa . Asian Pac J Trop Med. https://doi.org/10.1016/S1995-7645(13)60118-2
Mehdizadeh T, Zamani A, Abtahi Froushani SM (2020) Preparation of Cu nanoparticles fixed on cellulosic walnut shell material and investigation of its antibacterial, antioxidant and anticancer effects. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e03528
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507–517
Murthy HCA, Desalegn T, Kassa M et al (2020) Synthesis of green copper nanoparticles using medicinal plant Hagenia abyssinica (Brace) JF. Gmel. Leaf extract: antimicrobial properties. J Nanomater. https://doi.org/10.1155/2020/3924081
Nabi G, Ain Q-U, Tahir MB et al (2020) Green synthesis of TiO 2 nanoparticles using lemon peel extract: their optical and photocatalytic properties. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1722816
Nadaf NY, Kanase SS (2019) Biosynthesis of gold nanoparticles by Bacillus marisflavi and its potential in catalytic dye degradation. Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.09.020
Nadeem M, Tungmunnithum D, Hano C et al (2018) The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem Lett and Rev. https://doi.org/10.1080/17518253.2018.1538118430
Naghdi S, Sajjadi M, Nasrollahzadeh M et al (2018) Cuscuta reflexa leaf extract mediated green synthesis of the Cu nanoparticles on graphene oxide/manganese dioxide nanocomposite and its catalytic activity toward reduction of nitroarenes and organic dyes. J Taiwan Inst Chem Eng 86:158–173. https://doi.org/10.1016/j.jtice.2017.12.017
Narasaiah BP, Mandal BK (2020) Remediation of azo-dyes based toxicity by agro-waste cotton boll peels mediated palladium nanoparticles. J Saudi Chem Soc 24:267–281. https://doi.org/10.1016/j.jscs.2019.11.003
Narayanan KB, Sakthivel N (2008) Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett. https://doi.org/10.1016/j.matlet.2008.08.044
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci 169:59–79. https://doi.org/10.1016/j.cis.2011.08.004
Nasrollahzadeh M, Maham M, Rostami-Vartooni A et al (2015a) Barberry fruit extract assisted in situ green synthesis of Cu nanoparticles supported on a reduced graphene oxide-Fe 3 O 4 nanocomposite as a magnetically separable and reusable catalyst for the O-arylation of phenols with aryl halides under ligand-free cond. RSC Adv. https://doi.org/10.1039/c5ra10037b
Nasrollahzadeh M, Sajadi SM, Maham M (2015b) Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki-Miyaura coupling in water. J Mol Catal A Chem. https://doi.org/10.1016/j.molcata.2014.10.019
Nath D, Banerjee P (2013) Green nanotechnology—a new hope for medical biology. Environ Toxicol Pharmacol 36:997–1014. https://doi.org/10.1016/j.etap.2013.09.002
Nelson D, Priscyla DM, Oswaldo LA et al (2005) Mechanical aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3:8
Odeniyi MA, Okumah VC, Adebayo-Tayo BC, Odeniyi OA (2020) Green synthesis and cream formulations of silver nanoparticles of Nauclea latifolia (African peach) fruit extracts and evaluation of antimicrobial and antioxidant activities. Sustain Chem Pharm. https://doi.org/10.1016/j.scp.2019.100197
Omer AM (2008) Energy, environment and sustainable development. Renew Sustain Energy Rev 12:2265–2300
Ovais M, Khalil AT, Islam NU et al (2018) Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechnol 102:6799–6814
Pal G, Rai P, Pandey A (2019) Chapter 1—Green synthesis of nanoparticles: a greener approach for a cleaner future. In: Shukla AK, Iravani (eds) Characterization and applications of nanoparticles SBT-GS micro and nano technologies. Elsevier, Amsterdam, pp 1–26
Paul B, Bhuyan B, Dhar Purkayastha D et al (2015) Green synthesis of gold nanoparticles using Pogestemon benghalensis (B) O. Ktz. leaf extract and studies of their photocatalytic activity in degradation of methylene blue. Mater Lett. https://doi.org/10.1016/j.matlet.2015.02.054
Philip D (2009) Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2009.02.037
Philip D (2010) Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys E Low-Dimens Syst Nanostruct. https://doi.org/10.1016/j.physe.2009.11.081
Rabiee N, Bagherzadeh M, Kiani M, Ghadiri AM (2020) Rosmarinus officinalis directed palladium nanoparticle synthesis: investigation of potential anti-bacterial, anti-fungal and Mizoroki–Heck catalytic activities. Adv Powder Technol. https://doi.org/10.1016/j.apt.2020.01.024
Rahmatullah M, Sultan S, Toma TT et al (2010) Effect of Cuscuta reflexa stem and Calotropis procera leaf extracts on glucose tolerance in glucose-induced hyperglycemic rats and mice. Afr J Tradit Complement Altern Med. https://doi.org/10.4314/ajtcam.v7i2.50864
Rajkumar T, Sapi A, Das G et al (2019) Biosynthesis of silver nanoparticle using extract of Zea mays (corn flour) and investigation of its cytotoxicity effect and radical scavenging potential. J Photochem Photobiol B Biol 193:1–7
Ray PC (2010) Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem Rev 110:5332–5365
Razavi M, Salahinejad E, Fahmy M et al (2015) Green chemical and biological synthesis of nanoparticles and their biomedical applications. In: Basiuk V, Basiuk E (eds) Green processes for nanotechnology. Springer, Cham. https://doi.org/10.1007/978-3-319-15461-9_7
Robert KW, Parris TM, Leiserowitz AA (2005) What is sustainable development? Goals, indicators, values, and practice. Environ Sci Policy Sustain Dev 47:8–21
Roopan SM, Bharathi A, Prabhakarn A et al (2012) Efficient phyto-synthesis and structural characterization of rutile TiO 2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2012.08.055
Santhoshkumar T, Rahuman AA, Rajakumar G et al (2011) Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. https://doi.org/10.1007/s00436-010-2115-4
Sastry ABS, Karthik Aamanchi RB, Sree Rama Linga Prasad C, Murty BS (2013) Large-scale green synthesis of Cu nanoparticles. Environm Chem Lett. https://doi.org/10.1007/s10311-012-0395-x
Sathishkumar M, Sneha K, Won SW et al (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surfaces B Biointerfaces. https://doi.org/10.1016/j.colsurfb.2009.06.005
Selvarajan E, Mohanasrinivasan V (2013) Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater Lett 112:180–182. https://doi.org/10.1016/j.matlet.2013.09.020
Selvi AM, Palanisamy S, Jeyanthi S et al (2020) Synthesis of Tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: antioxidant, antibacterial and mitochondria-associated apoptosis in HeLa cells. Process Biochem. https://doi.org/10.1016/j.procbio.2020.07.008
Sethy NK, Arif Z, Mishra PK, Kumar P (2020) Green synthesis of TiO 2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process Synth. https://doi.org/10.1515/gps-2020-0018
Shahid M, Dumat C, Khalid S et al (2017) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazardous Mater 325:36–58. https://doi.org/10.1016/j.jhazmat.2016.11.063
Shankar SS, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. https://doi.org/10.1039/b303808b
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem ( Azadirachta indica ) leaf broth. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2004.03.003
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83–96
Sheik Mydeen S, Raj Kumar R, Kottaisamy M, Vasantha VS (2020) Biosynthesis of ZnO nanoparticles through extract from Prosopis juliflora plant leaf: antibacterial activities and a new approach by rust-induced photocatalysis. J Saudi Chem Soc 24:393–406. https://doi.org/10.1016/j.jscs.2020.03.003
Sheny DS, Philip D, Mathew J (2012) Rapid green synthesis of palladium nanoparticles using the dried leaf of Anacardium occidentale . Spectrochimica Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2012.01.063
Siddiqi KS, Husen A (2016) Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res Lett 11:482. https://doi.org/10.1186/s11671-016-1695-z
Singh J, Dutta T, Kim K-H et al (2018) ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:84
Singh T, Singh A, Wang W, et al (2019) Biosynthesized nanoparticles and its implications in agriculture. In: Biological synthesis of nanoparticles and their applications. CRC Press, pp 257–274. ISBN-13:978-0-367-21069-4
Sivaranjani V, Philominathan P (2016) Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. https://doi.org/10.1016/j.wndm.2015.11.002
Song JY, Jang HK, Kim BS (2009) Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. https://doi.org/10.1016/j.procbio.2009.06.005
Song JY, Kwon EY, Kim BS (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-009-0373-2
Soundarrajan C, Sankari A, Dhandapani P et al (2012) Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-011-0666-0
Sperling RA, Gil PR, Zhang F et al (2008) Biological applications of gold nanoparticles. Chem Soc Rev. https://doi.org/10.1039/b712170a
Stark WJ, Stoessel PR, Wohlleben W, Hafner A (2015) Industrial applications of nanoparticles. Chem Soc Rev 44:5793–5805
Subbiah R, Veerapandian M, Yun SK (2010) Nanoparticles: functionalization and multifunctional applications in biomedical sciences. Curr Med Chem 17:4559–4577
Sundaram PA, Augustine R, Kannan M (2012) Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol Bioprocess Eng 17:835–840
Tahir K, Nazir S, Li B et al (2015) Nerium oleander leaves extract mediated synthesis of gold nanoparticles and its antioxidant activity. Mater Lett. https://doi.org/10.1016/j.matlet.2015.05.062
Tamuly C, Hazarika M, Bordoloi M (2013) Biosynthesis of Au nanoparticles by Gymnocladus assamicus and its catalytic activity. Mater Lett. https://doi.org/10.1016/j.matlet.2013.07.020
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med 6:257–262
Tolaymat TM, El Badawy AM, Genaidy A et al (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ 408:999–1006
Velayutham K, Rahuman AA, Rajakumar G et al (2012) Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis . Parasitol Res. https://doi.org/10.1007/s00436-011-2676-x
Velmurugan P, Shim J, Kim K, Oh BT (2016) Prunus × yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater Lett. https://doi.org/10.1016/j.matlet.2016.03.069
Vijayakumar S, Arulmozhi P, Kumar N et al (2020) Acalypha fruticosa L. leaf extract mediated synthesis of ZnO nanoparticles: characterization and antimicrobialactivities. Mater Today Proc 23:73–80. https://doi.org/10.1016/j.matpr.2019.06.660
Vijikumar S, Ramanathan K, Devi BP (2011) Cuscuta reflexa ROXB. A wonderful miracle plant in ethnomedicine. Indian J Nat Sci 976:997
Wang Y, O’Connor D, Shen Z et al (2019) Green synthesis of nanoparticles for the remediation of contaminated waters and soils: constituents, synthesizing methods, and influencing factors. J Clean Prod 226:540–549
Wilson MP, Schwarzman MR (2009) Toward a new US chemicals policy: rebuilding the foundation to advance new science, green chemistry, and environmental health. Environ Health Perspect 117:1202–1209
Zangeneh MM, Zangeneh A (2020) Novel green synthesis of Hibiscus sabdariffa flower extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia effect in comparison to daunorubicin in a leukemic rodent model. Appl Organomet Chem. https://doi.org/10.1002/aoc.5271
Zhang YX, Zheng J, Gao G et al (2011) Biosynthesis of gold nanoparticles using chloroplasts. Int J Nanomed. https://doi.org/10.2147/ijn.s24785
Download references
Acknowledgements
The authors are thankful to the Elsevier, Springer, and Taylor & Francis for copyright permission.
Author information
Authors and affiliations.
Department of Chemistry, Lingayas Vidyapeeth, Faridabad, Haryana, 121002, India
Sapana Jadoun & Rizwan Arif
Department of Chemistry, Banasthali Vidyapith, Banasthali, Jaipur, 304022, India
Nirmala Kumari Jangid
Department of Chemistry, Kalindi College, Delhi, 110008, India
Rajesh Kumar Meena
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sapana Jadoun .
Ethics declarations
Conflict of interest.
There is no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Jadoun, S., Arif, R., Jangid, N.K. et al. Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett 19 , 355–374 (2021). https://doi.org/10.1007/s10311-020-01074-x
Download citation
Received : 19 July 2020
Accepted : 06 August 2020
Published : 13 August 2020
Issue Date : February 2021
DOI : https://doi.org/10.1007/s10311-020-01074-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Green synthesis
- Nanoparticles
- Sustainability
- Waste treatment
- Dye degradation
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 October 2020
Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan
- Ebtihal Alsadig Ahmed Mohamed 1 , 2 ,
- Ali Mahmoud Muddathir 1 &
- Magda Abker Osman 3
Scientific Reports volume 10 , Article number: 17148 ( 2020 ) Cite this article
17k Accesses
42 Citations
1 Altmetric
Metrics details
- Microbiology
- Plant sciences
The search for plant extracts with highly antimicrobial activity has been increased nowadays. This study evaluated the antimicrobial activity of Pulicaria crispa (Forsk.) Oliv., and Pulicaria undulata (L.) C.A.Mey., which have been used traditionally in Sudan as insect replants. The antimicrobial activity was evaluated against six pathogenic microorganisms, four bacteria (two Gram-positive; Bacillus subtilis and Staphylococcus aureus , two Gram-negative; Escherichia coli , and Pseudomonas aeruginosa ), and two fungi ( Aspergillus niger and Candida albicans ) using disc diffusion method. The extraction of the crude extracts was done by maceration. The essential oils were extracted by hydro-distillation. Phytochemical screening was done using reference method. Essential oils were analyzed using Gas Chromatography Mass Spectrometry. The results indicated that all used the microorganisms were sensitive to the plants extracts. Results of the preliminary phytochemical screening showed the presence of saponins, comarins, tannins, sterols, and triterpenes, and absence of alkaloids, anthraquinones, and flavonoids. Twenty-eight and forty-five constituents were identified in P. crispa and P. undulata, essential oils, respectively. The main constituents in the essential oil of P. crispa were 1,4-ditert butylbenzene (22.81%), caryophyllene (13.19%), carvone (11.80%), and neryl(s)-2-methylbutanoate (10.33%), and for P. undulata were camphor (44.48%), and thymyl acetate (10.31%). Data from this study could be used for developing of natural bioactive agents to improve human health.
Similar content being viewed by others
Phytochemical screening, HPLC analysis, antimicrobial and antioxidant effect of Euphorbia parviflora L. (Euphorbiaceae Juss.)

Essential oil composition and antimicrobial potential of aromatic plants grown in the mid-hill conditions of the Western Himalayas
Antimicrobial activity of the methanolic leaf extract of Prosopis laevigata
Introduction.
The search for substances with highly antimicrobial activity has been one of the most intensive field of research to minimize the risk of infectious diseases that caused by bacteria, fungi, viruses, and parasites, which are pathogenic to humans. Plants extracts are still the major sources of many therapeutic agents including antimicrobial agents for the treatment of infectious diseases 1 , 2 .
The family Asteraceae includes about 100 genera, and 2300 species. The genus Pulicaria is one of these genera, and it includes 100 species distributed worldwide 3 . Seven species of the genus Pulicaria have been reported in Sudan, namely; P. attenuata, P. crispa, P. dysenterica var. stenophylla, P. grantii , P. petiolaris, P. undulata, and P. vulgaris 4 . Only three species of the genus Pulicaria have been found in Khartoum State, which are P. crispa , P. grantii, and P. undulata 5 .
Pulicaria crispa (Forsk.) Oliv. (synonym Francoeuria (Forsk.)) and Pulicaria undulata (L.) C.A.Mey., are two wild aromatic plants growing in Sudan. Their local names are "alrabul", and "altager", and these plants contain plenty of compounds with medicinal importance 6 . P. crispa and P. undulata are annual herbs or sometimes perennial sub-shrubs, with small yellow flowers containing essential oil characterized by a strong aromatic odor. These plants are one of the most widespread desert plants growing wild in Sudan, Saudi Arabia, Kuwait, Iran, Iraq, Southern Egypt, Afghanistan, Pakistan, India, and parts of north & west tropical Africa 7 , 8 , 9 .
Different Pulicaria species have been traditionally used in several countries to repel insects, to treat back pain, to treat intestinal disorders, to treat inflammation, and to reduce influenza, and common cold symptoms. Pulicaria species contain many bioactive compounds such as monoterpenes, sesquiterpene acetylenes, flavonoids, isocomene, alkaloids, glycosides, comarins, and tannins 1 , 6 .
Many studies have reported that, P. undulata have been used traditionally in Sudan against alopecia, as a tea substitute, as an antispasmoic, as an ingredient of local perfumes. In addition, the plant has been used in folk medicine in many countries as an antiepileptic, as galactagogue, and as insect repellent (farmers are used to but it inside the vegetables packing containers). Also it has been reported that the essential oil of P. undulata has been used in the preparation of tonics, as sedative, and as an antibacterial agent 9 , 10 , 11 , 12 , 13 .
A group of researcher reported that P. crispa was found to have many folkloric medicinal uses in many countries. It has been used for many years in conventional medicine for the cure of heart diseases due to its antioxidative nature, also it has been used by the people of Sudan, Southern Egypt and Saudi Arabia to treat inflammation, as an antimicrobial agent, as an insect repellent, for the treatment of colds, coughs, colic, excessive sweating, and as carminative 9 , 14 , 15 .
The antimicrobial activity of P. crispa and P. undulata, methanolic crude extracts and essential oils were studied against pathogenic bacteria, and fungi. A preliminary phytochemical screening of the crude extracts, and the constituents of the essential oils have also been investigated in the present study.
Results and discussion
The yield percentages of the crude methanolic extracts and the essential oils.
Data in Table 1 show the yield percentages of the crude methanolic extracts, and the essential oils. The crude methanolic extraction yield of P. crispa and P. undulata was 22.6% and 23%, respectively, (w/w) on dry weights bases. The essential oils of both species obtained by hydro-distillation from whole plants of P. crispa and P. undulata was 0.1% and 0.4%, respectively, (v/w) on dry weights bases. The odor of the aerial parts (stems, leaves, and flowers) of P. undulata was sharper than that of P. crispa, due to the percentages, and essential oils constituents of the two species 14 . Reported that the methanolic crude extract of P. crispa yielded 27% crude extract 16 . Stated that the essential oil extracted from P. crispa grown wild in Egypt reach to 0.23%. These differences in yield percentages of crude extract and essential oils could by refer to the differences in environmental condition.
Antimicrobial activity of the crude methanolic extracts and the essential oils of P. crispa and P. undulata
The extracts of the studied plants exhibited varying degrees of inhibition activity against the tested bacteria (Table 2 ); and the results were expressed in terms of the diameter of the growth-inhibition zone (clear zones). The results clearly showed that tested bacteria were susceptible to the four extracts. There were significant differences ( p < 0.05) in mean diameter inhibition zone between the four extracts. P. crispa methanolic crude extract showed high activity against S. aureus (19 mm), and moderate activity against B. subtilis (16 mm), E. coli (15 mm), and P. aeruginosa (15 mm). P. undulata methanolic crude extract showed high activity against B. subtilis (18 mm), and moderate activity against E. coli (16 mm), P. aeruginosa (16 mm), and S. aureus (15 mm), which is also similar in activity to Tetracycline. Regarding the essential oil of P. crispa, it shows high activity against S. aureus (18 mm), which is higher than positive controls; also it showed moderate activity against B. subtilis (17 mm), P. aeruginosa (17 mm), and E. coli (16 mm). P. crispa essential oil showed high activity against S. aureus (18 mm). Regarding the essential oil of P. undulata , it showed high activity against B. subtilis (25 mm), P. aeruginosa (24 mm), and was found to be moderately active against E. coli (17 mm) and S. aureus (17 mm), even though it is higher in activity than tetracycline (16 mm). Generally the essential oil of P. undulata showed high activity against B. subtilis , and P. aeruginosa compared to positive controls.
Antifungal activity of the methanolic crude extracts, and the essential oils of P. crispa and P. undulata was presented in Table 3 . There were significant differences ( p < 0.05) in mean diameter inhibition zone between the four extracts. All the extracts showed activity against the two fungal microorganisms. However, P. undulata essential oil showed the highest activity against the two fungi compared to the other extracts. Generally the extracts showed high activity against C. albicans; P. undulata essential oil 23 mm, P. undulata methanolic crude extract 21 mm, P. crispa methanolic crude extract 20 mm, and P. crispa essential oil 19 mm, While the extracts were found to be relatively less active against A. niger ; P. undulata essential oil 22 mm, P. crispa essential oil 21 mm, P. undulata methanolic crude extract 20 mm, and P. crispa methanolic crude extract 19 mm, which was lower than positive controls (Nystatin (26 mm) and Clotrimazole (34 mm).
Minimum inhibitory concentrations (MIC) of P. crispa and P. undulata methanolic crude extracts, and essential oils
The results of MIC presented in Table 4 showed that all microorganisms were very susceptible to the minimum inhibitory concentration of P. crispa essential oil (6.25 mg/ml) except for B. subtilis the MIC value was 25 mg/ml, similarly all microorganisms were susceptible to minimum inhibitory concentration of P. undulata essential oil (6.25 mg/ml), except for E. coli and B. subtilis with MIC value (50 mg/ml). Regarding P. crispa methanolic crude extract the minimum inhibitory concentration was 6.25 mg/ml for P. aeruginosa and A. niger, 25 mg/ml for E. coli and S. aureus, 50 mg/ml for C. albicans and 100 mg/ml for B. subtilis. While, minimum inhibitory concentration of P. undulata methanolic crude extract was 6.25 mg/ml for A. niger and C. albicans, 12.5 mg/ml for P. aeruginosa and S. aureus, the MIC was 50 mg/ml for E. coli and 100 mg/ml for B. subtilis.
Preliminary phytochemical screening of the crude extracts of P. crispa and P. undulata
Data presented in Table 5 show the preliminary phytochemical examination of the methanolic crude extracts of P. crispa and P. undulata , which were rich in sterols, and terpenes, tannins, comarins, saponins. At the same time data confirm the absence of alkaloids, flavonoids, and anthraquinones. These results are in line with the findings of previous researches 16 , 17 , 18 , 19 , 20 . These groups of phytochemicals might be responsible for the observed antimicrobial activity P. crispa and P. undulata .
The chemical constituents of P. crispa and P. undulata essential oils
The hydro-distillation of the dry aerial parts of P. crispa, and P. undulata grown in Sudan gave yellow- colored essential oils. The percentage composition, and identification of each Pulicaria species essential oil are listed in Tables 6 and 7 .
GC–MS analysis of the essential oils resulted in identification of twenty-eight constituents in P. crispa essential oil, and forty-five constituents in P. undulata essential oil. The main constituents of the essential oil of P. crispa were 1,4-ditert-butylbenzene (22.81%), caryophyllene (13.19%), carvone (11.80%), neryl (s)- 2-methylbutanoate (10.33%). In addition, the main constituents of the essential oil of P. undulata were camphor (44.48%), thymyl acetate (10.31%), bicycle (3.46%), and azulenol (3.40%), other minor constituents have been identified in the essential oils of P. crispa and P. undulata . Both linalool, and camphor are presented in the essential oil of P. crispa and P. undulata . Result of P. undulata essential oil constituents agrees with those obtained by 21 of P. undulata collected from Yemen in linalool, camphor, and thymol.
The bactericidal properties of P. undulata essential oil were due to the presence of thymol, and thymol derivatives, which were found to have a significant antimicrobial activity 22 .
Conclusions
This study showed that the essential oils and the methanolic crude extracts of P. crispa and P. undulata, inhibited the growth of various tested species of Gram-positive, Gram-negative bacteria, and fungi. Generally, we can conclude that P. crispa and P. undulata methanolic crude extracts, and essential oils have antimicrobial activity. 3- caryophyllene oxide and carvomenthenone were the major compounds in P. crispa and P. undulata essential oil, respectivily. The above-mentioned results may provide a promising topic for further in vitro and in vivo studies to develop curative plant extracts from P. crispa and P. undulata.
This study was carried out at the Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Center for Research, Khartoum, Sudan.
Plant material
Plants were selected randomly followed by antimicrobial assays. Aerial parts of P. crispa and P. undulata were collected during the flowering stage in July 2015 from different locations in Khartoum State, Sudan. The plants materials were taxonomically authenticated at the herbarium of Medicinal and Aromatic Plants and Traditional Medicine Research Institute, P. crispa voucher specimen number is W-1995-41-MAPTRI-H, and P. undulata voucher specimen number is A-1995-126-MAPTRI-H.
Preparation of the plant materials
P. crispa and P. undulata, plants parts were freed from dust, and foreign material, then dried indoors at room temperature for three days, powdered, then kept in plastic containers at room temperature until used.
Preparation of the crude extracts
P. crispa and P. undulata, methanolic crude extracts were prepared by maceration of the dried powdered plants materials in organic solvent (methanol). Twenty grams of each plant sample were extracted using 50 ml of absolute methanol as solvent. The mixture was allowed to stand for 72 h at room temperature with daily filtration using a standard filter paper (Whatman No. 2, England). The solvent was evaporated under reduced pressure to dryness using rotary evaporator, then the crude extracts have left to dry at room temperature for three days. The yield percentages were determined by dividing the weight of extract by the weight of the sample multiplied by 100. The extracts were stored at 4 °C until used 23 .
Preparation of the essential oils
P. crispa and P. undulata essential oils were prepared by hydro-distillation of the dried powdered plants materials in water. 100 grams of each sample were submitted to hydro-distillation for four hours using Clevenger- type apparatus (Duran West Germany). The obtained essential oils were calculated as a relative’s percentage (v/w), and dried over anhydrous sodium sulfate, filtered, and stored at 4 °C until used 16 .
Antimicrobial activity screening of the methanolic crude extracts, and the essential oils of P. crispa and P. undulata, microorganisms
The antimicrobial activity of P. crispa and P. undulata methanolic crude extracts, and the essential oils were evaluated by disc diffuison method using ATCC (American Type Culture Collection), and NCTC (National Collection of Type Cultures) strains. The strains were four bacterial strains; two Gram-positive ( Bacillus subtilis (NCTC 8236) and Staphylococcus aureus (ATCC 25923)), two Gram-negative ( Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853)), and two fungi ( Aspergillus niger (ATCC9763) and Candida albicans (ATCC7596)).
Preparation of the microorganism culture
All the test microorganisms were inoculated on blood agar, and nutrient agar plates. The bacterial strains were incubated at 37 ºC for 24 h, and the fungal strains were incubated at 37 °C for 48 h in the inverted position, incubated aerobically, and the obtained growth were then stored in the refrigerator at 4 °C till used.
Determination of antimicrobial activity of the methanolic crude extracts, and the essential oils of P. crispa , and P. undulata, by disc diffuison method
The paper disc diffusion method was used to screen the antimicrobial activity of the plants extracts, and performed by using Mueller Hinton agar (MHA). The experiment was carried out according to the National Committee for Clinical Laboratory Standards 24 . The bacterial suspension was diluted with a sterile physiological solution to 108 CFU/ml. 100 µl of the bacterial suspension were swabbed uniformly on the surface of MHA and the inoculum was allowed to dry for five minutes. The sterilized filter paper discs (Whatman No. 2, England) were placed individually on the surface of the MHA and impregnated with 20 µl of samples solution. The inoculated plates with bacteria were incubated at 37 °C for 24 h in the inverted position and 48 h for the fungal strains. The diameters (mm) of the inhibition zones were measured; the values of the antimicrobial activity were expressed as the mean of inhibition zones (mm) with three replicates for each treatment. Gentamicin, Tetracycline, Clotrimazole and Nystatin served as positive controls. The results of the diameters of the zones of inhibitions of the extracts were interpreted as sensitive; (> 18 mm), intermediate (14–17 mm), and resistant (< 14 mm) 17 , 18 .
Determination of the minimum inhibitory concentration (MIC)
The principle of the agar plate dilution is the inhibition of growth on the surface of the agar by the plant extracts incorporated into the medium was used to determine the minimum inhibitory concentration (MIC) according to 25 . The MICs of the extracts were used in concentrations (6.25–100 mg/ml). Agars were prepared in the series of increasing concentrations of the plant extract. The bottom of each plate was marked off into four segments. The organisms tested were growing in broth overnight to contain 108 CFU/ml. Loop-full of diluted culture is spotted with a standard loop that delivers 0.001 ml on the surface of the segment and incubation for 24 h or 48 h at 37 °C. The MIC were determined visually in the agar as the lowest concentrations of the extracts in which no bacterial/fungal growth was visible. The MICs values were express as mg/ml.
Phytochemical screening of the methanolic crude extracts
The methanolic crude extracts were subjected to qualitative examination for the detection of various phytochemical constituents (saponins, comarins, alkaloids, anthraquinones, tannins, flavonoids, sterols, and triterpenes) using standard procedures 26 , 27 , 28 .
Essential oils analysis by the gas chromatography-mass spectrometry (GC–MS)
P. crispa and P. undulata, essential oils were analyzed using Shimadzu Gas Chromatography Mass Spectrometry Apparatus (Japan) (GC.MS- QP2010 Ultra). Analysis was carried out on a Varian 3400 system equipped with a DB-5 fused silica column (30 m length × 0.25 mm diameter, 0.25 μm film thickness). Helium was used as the carrier gas (1.2 ml/min), and the program used was four minutes isothermal at 35 °C , following by 40–240 °C at the rate of 4 °C/min, then held at 260 °C, for three minutes, the injection temperature was 250 °C. The components of the essential oils were identified by library searches 29 , based on comparing their retention indices, and mass spectra with those obtained from authentic samples, and/or the NIST/NBS, Wiley libraries, and the literature.
Statistical analysis
The collected data were subjected to the analysis of variance (ANOVA), and the means were separated using the least significant difference (LSD) at p ≤ 0.05 and at p ≤ 0.01.
Touati, N. et al. Antibacterial activity of phenolic compounds of Pulicaria odora , wild plant in northern Algeria. Int. Food Res. J. 25 (5), 2121–2130 (2018).
CAS Google Scholar
Cos, P., Vlietinck, A. J., Berghe, D. V. & Maes, L. Anti-infective potential of natural products: how to develop a stronger in vitro proof-of-concept’. J. Ethnopharmacol. 106 , 290–302. https://doi.org/10.1016/j.jep.2006.04.003 (2006).
Article CAS PubMed Google Scholar
Hutchinson, J. The Families of Flowering Plants 2nd edn. (Oxford University Press, Oxford, 1959).
Google Scholar
Andrews, F. W. The Flowering Plants of the Sudan Vol. 3 (T. Buncle Ltd, Arbroath, 1956).
. El Ghazali, G. E. B Khaild, H. E. El-Tohami, M. S. Abdalla, W. E. & Yagi, S. M. A. Medicinal Plants of the Sudan. Medicinal Plants of Khartoum State. NCR, Khartoum (1998).
Al-Hajj, N. Q. M. et al. Antimicrobial and antioxidant activities of the essential oils of some aromatic medicinal plants ( Pulicaria inuloides - Asteraceae and Ocimum forskolei -Lamiaceae). Trop. J. Pharma. Res. 13 (8), 1287–1293. https://doi.org/10.4314/tjpr.v13i8.13 (2014).
Article CAS Google Scholar
Abdelmageed, E., Bushara, H. O., Babiker M. Y. A. & Abdelgadir, M. Pulicaria crispa (Asteraceae) extract affects survival and fecundity of Bulinus truncatus vector snails of Schistosomes. Adv. Biores. 8 (6), 135–139. https://doi.org/10.15515/abr.0976-4585.8.6.135139 (2017).
Eliebaa, E. M., Lebdaa, M. A., Tahaa, N. M., Mandora, A. A. & El-Magdb, M. A. Consumption of Pulicaria undulata and Salvadora persica extracts is safe and has a growth promoter effect on broilers. Arab. J. Med. Sci. 1 (1), 31–34 (2018).
El Ghazali, G.E.B., El Tohami, M.S. & El Egami, A.A.B. Medicinal Plants of the Sudan. Part 3. Medicinal Plants of the White Nile State. NCR, MAPRI, Khartoum (1994).
Ghazanfar, S. A. Handbook of Arabian Medicinal Plants. Vol. 98 (CRC Press, Inc., Boca Raton, 1994).
Ali, A. A., Makboul, M. A., Assaf, M. H. & Anton, R. Essential oil of Pulicaria undulata L. growing in Egypt and its effect on animal behaviour. Bull. Pharm. Sci . Assiut Univ. 10 , 37–49 (1987).
Karryev, M. O. Biol. Nauk. (6):46, via CA. 70, 99568 (1968).
Bishay, D. W., Gomaa, C. S. & Saleh, M. A. The second Inter African Sympiosum on Traditional Pharmacopeia and African Medicinal Plants, Cairo, 7–12 July (1975).
Elshiekh, Y. H. & AbdElMoniem, M. A. Phytochemical, antibacterial screening and antioxidantactivity of Pulicaria crispa extracts. Pharma. Innov. 3 (12), 12–15 (2015).
Foudah, A. I. et al. Pharmacognostical, antioxidant and antimicrobial studies of aerial part of Pulicaria crispa (Family: Asteraceae). Bull. Environ. Pharmacol. Life Sci. 4 (12), 19–27 (2015).
Ahmed, S.S., & Ibrahim, M.E. Chemical investigation and antimicrobial activity of Francoeuria crispa ( Forssk ) grown wild. Int. J. Mater. Environ. Sci. 9 (1):266–271. https://doi.org/10.26872/jmes.2018.9.1.30 (2018).
Barry, A. J., Garcia, F. & Thrupp, L. D. Interpretation of sensitivity test results. Am. J. Clin. Pathol. 53 , 140 (1970).
Article Google Scholar
Cruikshank, R., Duguid, J. P., Marmion, B. P., &. Swain, R. H. Medical Microbiology: Medical Microbiology, Part II 12th edn (Churchill, Livingston (Pub.), Edinburgh, 1975).
Jaffer, H. J., Mahmoud, M. J., Jawad, A. M., Naji, A. & Al-Naib, A. Phytochemical and biological screening of some Iraqi plants. Fitoterapia 59 (3), 29–233 (1988).
Al-yahya, M. A. et al. Potential cancer chemopreventative and cytotoxic agents from Pulicaria crispa . J. Nat. Prod. 51 (3), 621–624. https://doi.org/10.1021/np50057a038 (1988).
Ali, N. A. A. et al. Chemical composition and biological activity of essential oil from Pulicaria undulata from Yemen. Nat. Prod. Commun. 7 (2), 257–260 (2012).
CAS PubMed Google Scholar
Hanbali, F. E. L. et al. Chemical composition and antibacterial activity of essential oil of Pulicaria odora L. J. Ethnopharma. 99 , 399–401 (2005).
Sukhdev, S. H., Suman, P. S. K. Gennaro, L. & Dev, D. R. Extraction Technologies for Medicinal and Aromatic Plants. United Nation Industrial Development Organization and the International Center for Science and High Technology, pp 116 (2008).
NCCLS–National Committee for Clinical Laboratory Standards., Performance standards for anti-microbial susceptibility testing: eleventh informational supplement. Document M100-S11. National Committee for Clinical Laboratory Standard, Wayne, PA, USA (2001).
Wiegand, I., Hilpert, K. & Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentrations (MIC) of antimicrobial substances. Nat. Proto. 3 , 163–175. https://doi.org/10.1038/nprot.2007.521 (2008).
Sofowora, A. Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa 2 edn (Spectrum Books Ltd, Nigeria, 1993).
Martinez, A., Valencia, G., & fitoquimica, M. In: Manual de prácticas de Farmacognosia y Fitoquímica. 1 st edn. Medellin:Universidad de Antioquia. Phytochemical Screening Methods, pp 59–65 (1999).
Wall, M., Eddy, C. R., McClenna, M. L. & Klump, M. E. Detection and estimation of steroid and sapogenins in plant tissue. Anal. Chem. 24 , 1337–1342. https://doi.org/10.1021/ac60068a018 (1952).
Adams, R. P. Allured, Carol Stream, Illinois, and USA . ISBN: 0–931710–42–1 (1995).
Download references
Acknowledgements
Authors would like to thank the researcher of the herbarium of Medicinal and Aromatic Plants and Traditional Medicine Research Institute for taxonomically authenticating the plants.
Author information
Authors and affiliations.
Department of Horticulture, Faculty of Agriculture, University of Khartoum, Shambat, 13314, Khartoum, Sudan
Ebtihal Alsadig Ahmed Mohamed & Ali Mahmoud Muddathir
Horticultural Crops Research Center, Agricultural Research Corporation, Shambat 30, Khartoum, Sudan
Ebtihal Alsadig Ahmed Mohamed
Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Centre for Research, 2404, Khartoum, Sudan
Magda Abker Osman
You can also search for this author in PubMed Google Scholar
Contributions
E.A.A.M. was responsible for the conceptualization, and the designing of the research work, responsible for the elaboration of the research project, performed the research work, technical work (laboratory), responsible for the interpretation of the data, wrote, and revised the manuscript. A.M.M. supervised the research project, and revised the manuscript. M.A.O. contributed in the conceptualization, and the designing of the research work, contributed in the supervision of the research project, performed the statistical analysis, contributed in the data interpretation, and revised the manuscript. All authors reviewed the manuscript.

Corresponding author
Correspondence to Ebtihal Alsadig Ahmed Mohamed .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mohamed, E.A.A., Muddathir, A.M. & Osman, M.A. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci Rep 10 , 17148 (2020). https://doi.org/10.1038/s41598-020-74262-y
Download citation
Received : 16 June 2020
Accepted : 29 September 2020
Published : 13 October 2020
DOI : https://doi.org/10.1038/s41598-020-74262-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A critical review on pulicaria species occurring in qatar: traditional uses, phytochemistry and biological activities.
- Deepak M. Kasote
- Malik Adil Nawaz
- Mohammed Alsafran
Phytochemistry Reviews (2024)
Solution combustion method for synthesis of ZnO NPs from Syzygium hemisphericum bark extract and a comparative analysis of the same with the crude bark extract for biomedical applications
- C. H. Sushmitha
- G. Krishnakumar
- K. Meghana Navada
Chemical Papers (2024)
Eco-Friendly Fabrication of Silver Nanoparticles for Sustainable Water Purification and Antibacterial Synergy
- Parvathalu Kalakonda
- Mahesh Thodeti
- Bala Bhaskar Podila
Plasmonics (2024)
In vitro antibacterial, non-cytotoxic and antioxidant activities of Boscia Senegalensis and Tapinanthus dodoneifolius, plants used by pastoralists in Cameroon
- Ronald Romuald Bebey Vougat Ngom
- Harquin Simplice Foyet
Pastoralism (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- Open access
- Published: 04 January 2021
HPTLC analysis of Fumaria parviflora (Lam.) methanolic extract of whole plant
- Anjali Bhargava 1 ,
- Pragya Shrivastava 1 &
- Anita Tilwari 2
Future Journal of Pharmaceutical Sciences volume 7 , Article number: 1 ( 2021 ) Cite this article
7648 Accesses
124 Citations
Metrics details
Fumaria parviflora (Lam.), commonly known as “fine-leaved fumitory,” is well known for its therapeutic properties in the Indian traditional medicinal system. The presence of important bioactive compounds in plants makes them pharmacologically valuable. Therefore, in the present study, the high-performance thin layer chromatography (HPTLC) analysis of Fumaria parviflora (whole plant) methanolic extract was performed for its phytochemical profiling.
The HPTLC densitometric analysis of the methanolic extract of Fumaria parviflora (whole plant) was carried out using CAMAG HPTLC system, and the results were obtained in the form of chromatograms (scanned at the wavelength of 254 nm and 366 nm) representing several peaks. The phytochemical profile of the plant was determined and presented in the tables showing the total number of peaks, peak heights, peak area, percent area, and Rf values.
The study concluded that F. parviflora methanolic extract of the whole plant contains a rich variety of phytochemicals which might be accountable for its therapeutic value and thus justifies its traditional use in India.
Medicinal plants, due to the presence of bioactive phytochemicals, play a very important role in human life for maintaining good health. The use of medicinal herbs in the treatment of infection is an age-old practice, and several natural products are used as phytotherapic for the treatment of many diseases [ 1 ]. The search for a newer source of antibiotics is a global challenge, since many infectious agents are becoming resistant to synthetic drugs [ 2 ]. There are thousands of medicinal plants known to have a long history of usage for their curative properties against various diseases and ailments [ 3 ]. The use of herbal drugs is once more escalating in the form of Complementary and Alternative Medicine (CAM) [ 4 ].
Fumaria parviflora Lam., commonly known as fine-leaved fumitory (in English), Shahatra, Pittapapara, or Pittapapada (in Hindi), belongs to the family Fumariaceae. Fumaria parviflora (Fumariaceae) is a pale green, diffuse, much branched annual herb widely used in Ayurvedic medicine as well as in traditional Yunani system of medicine throughout India [ 5 ]. The entire herb is traditionally used in leprosy, fever [ 6 ], and detoxification and as laxative, diuretic, and diaphoretic [ 7 ].
The World Health Organization (WHO) has stressed on the need for scientific validity of herbal drugs and ensuring, devising, and implementing sound science [ 8 ]. Several techniques are available for the qualitative and quantitative estimation of phytochemicals present in plants. Nowadays, new technology has made it possible to identify, screen, and isolate these active compounds [ 9 ]. The HPTLC (high-performance thin layer chromatography) is an advanced form of TLC as it provides high resolution and much accurate data. It is accepted all over the world as one of the most powerful analytical techniques used for phytochemical and biomedical analysis. It is an inexpensive, simple, and rapid method for the estimation of chemical components present in test sample and therefore most widely used by pharmaceutical industries for new drug discovery. The present study was performed for the phytochemical profiling of Fumaria parviflora (whole plant) methanolic extract by the HPTLC technique.
The plant material was washed and then kept for shade drying for 7 days. The dried plant sample was powdered by mechanical grinder into a fine powder. The air-dried powdered material of the whole plant of Fumaria parviflora (100 g) was extracted with hydroalcoholic solvent [methanol and water solvent (1:1 v/v)] using the Soxhletion process with the help of a Soxhlet apparatus. Excess solvent was then evaporated in a water bath at 50–100 °C to obtain the crude and stored in airtight containers.
Instrumentation
A CAMAG HPTLC system equipped with LINOMAT 5 applicator fitted with 100 μl syringe, CAMAG TLC scanner, and winCATS software was used.
Chemicals and solvents
All the solvents used were of chromatography grade, and all the chemicals used were of analytical reagent grade.
Preparation of samples
Dried extract (10 g) of F. parviflora was dissolved in 100 ml HPTLC grade methanol and filtered. This solution was used as a test solution for the HPTLC study.
Chromatographic conditions
The HPTLC was performed on 7.0 × 10.0 cm precoated silica gel 60 F 254 HPTLC plate (E. MERCK KGaA). No pre-washing and modification of the plate were done. The sample solution was applied as bands to the plate by CAMAG Linomat applicator fitted with 100 μl syringe (Table 1 ). The stable application rate was 150 nl/s. The sample loaded plate was kept in automatic development chamber with mobile phase—chloroform:ethyl acetate:formic acid (5:4:1 v/v/v). Densitometric scanning was performed with CAMAG TLC scanner-4 equipped with winCATS software. The bands were visualized using CAMAG visualizer, and the images were captured in white light and 254 nm (short UV) and 366 nm (long UV) wavelengths (Table 2 ). When exposed to short-wave UV light of 254 nm, UV-active compounds will undergo fluorescence quenching and appear as dark spots on a bright background. Conversely, compounds that absorb 366 nm UV light will appear as bright spots on a dark background [ 10 ].
The HPTLC analysis of F. parviflora Lam. revealed the presence of various phytochemicals as illustrated in the figures and tables below. The chromatograms (Figs. 1 , 2 , 3 , and 4 ) were obtained upon scanning at UV 254 nm and 366 nm, and peak tables were generated. The Rf values, peak height, peak area, and percent area of the unknown substances are depicted in the tables (Tables 3 , 4 , 5 , and 6 ).
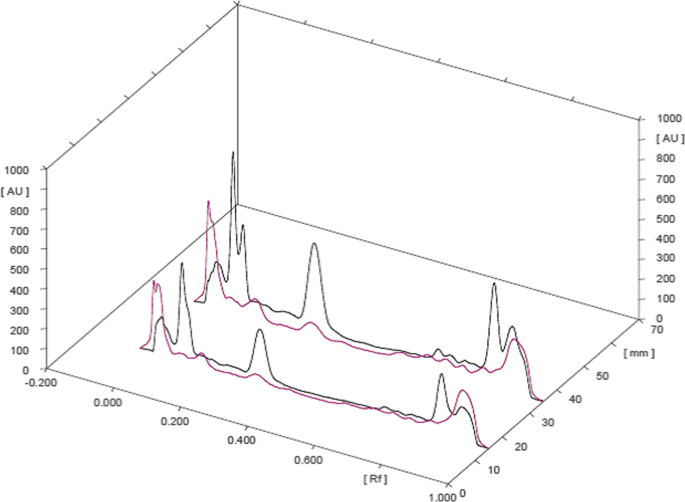
3D overlay of HPTLC chromatogram of all tracks, at all wavelengths
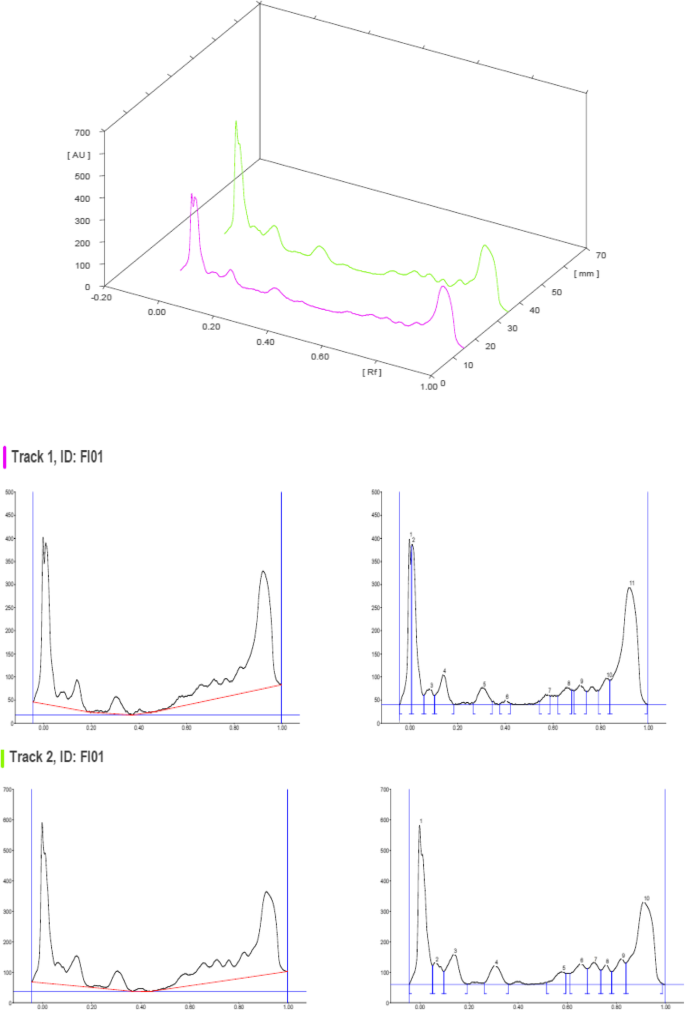
HPTLC chromatograms scanned at 254 nm
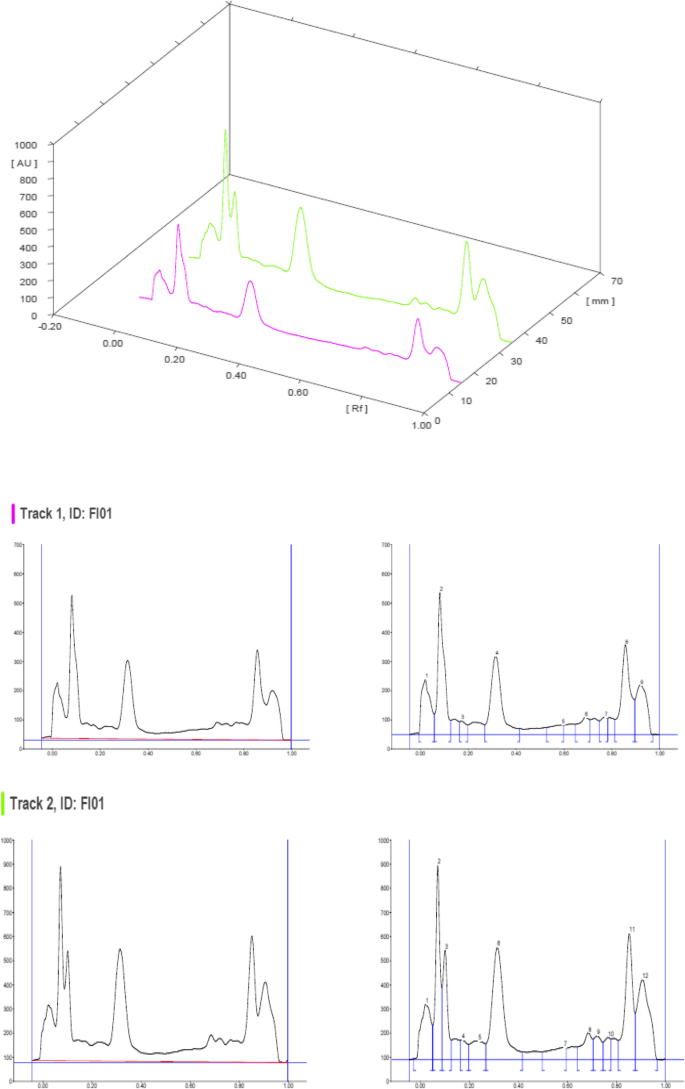
HPTLC chromatograms scanned at 366 nm
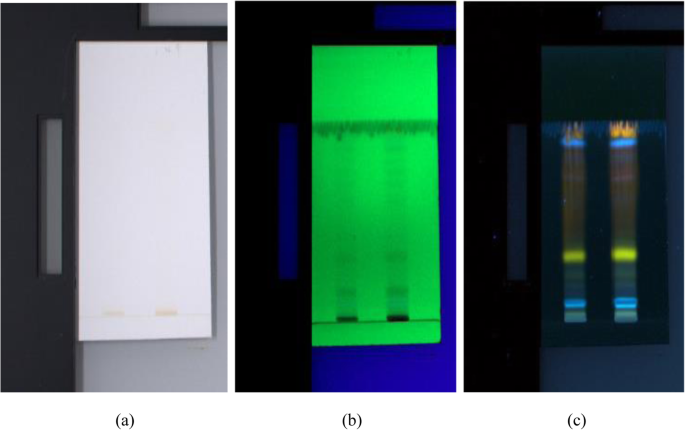
HPTLC chromatograms visualized under a white light, b UV 254 nm, and c UV 366 nm
The HPTLC performed on the methanolic extract of Fumaria parviflora (Lam.) showed the presence of various phytoconstituents in different concentrations as illustrated in figures and tables. Figure 1 represents the 3-dimensional overlay of the chromatogram of all tracks, at all measured wavelengths. The chromatogram scanned at 254 nm (Fig. 2 ) represents 11 and 10 peaks for track 1 and track 2, respectively, whereas the chromatogram scanned at 366 nm (Fig. 3 ) indicates 9 and 12 peaks for track 1 and track 2, respectively. The number of peaks indicates the presence of different phytoconstituents present in the sample. The Rf values (Tables 3 , 4 , 5 , and 6 ) calculated for the phytoconstituents present in the tested sample would be helpful in the identification of the unknown compounds by comparing them with the reference standards, and from the values of peak area, the concentration of the compounds can be determined. The bands of separated compounds can be seen (Fig. 4 ) on the TLC plates visualized under white light and UV of wavelengths 254 nm and 366 nm.
It has been reported from the previous studies that a wide range of bioactive compounds of medicinal significance are present in various species of Fumaria . The HPTLC study conducted on Fumaria vaillantii showed the presence of protopine and rutin in methanol extract of the whole plant at Rf 0.51 and 0.26, respectively [ 11 ]. Some of the Fumaria species are known to exhibit antifungal [ 12 ], antibacterial [ 13 ], and anti-inflammatory [ 14 ] activities due to the presence of bioactive phytochemicals such as alkaloids, polyphenols, and flavonoids. Thus, from the earlier researches, it is evident that various species of Fumaria contain some bioactive compounds important for pharmaceutical industries.
The findings of the present study are limited to the HPTLC analysis of Fumaria parviflora methanolic extract to estimate the presence of different phytochemicals from the chromatogram peaks and obtain the peak tables; however, the identification of the unknown phytochemicals is not done.
The present study revealed the presence of several phytochemicals in F. parviflora which might be the cause for its healing properties and thus justifies its usage as a remedy in various ailments. New drug formulations require the isolation and identification of important phyto-compounds possessing pharmacological properties. The HPTLC study carried out for F. parviflora chemical profiling will be helpful in the identification of bioactive compounds and markers, by comparing the Rf values of the compounds with the reference standards.
Availability of data and materials
All data and material are available upon request.
Abbreviations
Retention factor
High-performance thin layer chromatography
Ultraviolet
Hydrargyrum (mercury)
Sisodiya D, Shrivastava P (2018) Antimicrobial activity of Euphorbia thymifolia (L.) and Manilkara hexandra (Roxb.). Int J Curr Adv Res 7(2):9660–9663
Google Scholar
Latha SP, Kannabiran K (2006) Antimicrobial activity and phytochemicals of Solanum trilobatum Linn. Afr J Biotechnol 5:2402–2404
Sisodiya D, Shrivastava P (2018) Phytochemical screening, thin layer chromatography and quantitative estimation of bioactive constituents in aqueous extract of Manilkara hexandra (Roxb.) dubard. Int J Recent Sci Res 9(1):23083–23086
Cooper LN, Blais BS (2004) Theory of cortical plasticity. World scientific publishing, Singapore
Book Google Scholar
Kumar S, Sharma AK, Kamboj A (2017) Fumaria parviflora Lam. (Fumitory): a traditional herbal medicine with modern evidence. Asian J Pharm Pharmacol 3(6):200–207
CAS Google Scholar
Anonymous (2004) The ayurvedic pharmacopoeia of India, 1 Ministry of Health and Family Welfare, New Delhi, Government of India, 84–86.
Anonymous (2007) Fumaria parviflora Lam. In: Khare CP (ed) Indian medicinal plants. Springer, Heidelberg, p 275
Tilburt JC, Kaptchuk TJ (2008) Herbal medicine research and global health: an ethical analysis. Bull World Health Organ 86:594–599
Article Google Scholar
Sisodiya D, Shrivastava P (2017) Qualitative and quantitative estimation of bioactive compounds of Euphorbia thymifolia L. Asian J Pharm Edu Res 6(3):34–43
Thin-layer chromatography evaluation. https://www.merckmillipore.com . Accessed 6 Nov 2020.
Upadhye AS, Rajopadhye AA (2011) Botanical and phytochemical standardization of Fumaria vaillantii Loisel. Indian J Nat Prod Resour 2(3):369–374
Moghtader M (2013) In vitro antifungal effects of Fumaria vaillantii Loisel. essential oil on Aspergillus flavus . J Yeast Fungal Res 4:21–25
Khamtache-Abderrahima S, Lequart-Pillonb M, Gontierb E, Gaillardb I, Pilardb S et al (2016) Isoquinoline alkaloid fractions of Fumaria officinalis : characterization and evaluation of their antioxidant and antibacterial activities. Ind Crops Prod 94:1001–1008
Bribi N, Algieri F, Rodriguez-Nogales A, Vezza T, Garrido-Mesa J et al (2016) Intestinal anti-inflammatory effects of total alkaloid extract from Fumaria capreolata in the DNBS model of mice colitis and intestinal epithelial CMT93 cells. Phytomedicine 23:901–913
Article CAS Google Scholar
Download references
Acknowledgements
The authors are thankful to Vindhya Herbal Testing & Research Laboratory, Bhopal, Madhya Pradesh, for providing technical facilities and assistance required for this work.
Collection, identification, and authentication of plant
The plant material was collected in the month of July 2020 and identified taxonomically by Dr. Suman Mishra, Consultant Taxonomist, Xcellventure Institute of Fundamental Research Pvt. Ltd., Bhopal (MP). She is also a botany scientist in MFP-PARC, Barkheda Pathani, Bhopal. The plant was identified and authenticated as Fumaria parviflora Lam. belonging to the family Fumariaceae by its macroscopic, microscopic, and powder microscopic examination.
No funding was received for this research.
Author information
Authors and affiliations.
Department of Life Science, Rabindranath Tagore University, Village-Mendua, Post-Bhojpur, Distt. Raisen, Bhopal, Madhya Pradesh, 464993, India
Anjali Bhargava & Pragya Shrivastava
Madhya Pradesh Council of Science and Technology, Bhopal, Madhya Pradesh, India
Anita Tilwari
You can also search for this author in PubMed Google Scholar
Contributions
AB executed the work and prepared the manuscript. PS planned the work and provided proper guidance for the research. AT contributed to the research design and edited the manuscript. All the authors have read and approved the manuscript.
Corresponding author
Correspondence to Anjali Bhargava .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bhargava, A., Shrivastava, P. & Tilwari, A. HPTLC analysis of Fumaria parviflora (Lam.) methanolic extract of whole plant. Futur J Pharm Sci 7 , 1 (2021). https://doi.org/10.1186/s43094-020-00150-x
Download citation
Received : 23 September 2020
Accepted : 30 November 2020
Published : 04 January 2021
DOI : https://doi.org/10.1186/s43094-020-00150-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fumaria parviflora
- Methanolic extract
- Densitometry
- Chromatogram
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Afr J Tradit Complement Altern Med
- v.8(1); 2011

Extraction, Isolation and Characterization of Bioactive Compounds from Plants' Extracts
S sasidharan.
1 Institute for Research in Molecular Medicine (INFORM), Universiti Sains Malaysia, Minden 11800, Malaysia
D Saravanan
2 Centre for Drug Research, University Science of Malaysia, 11800 Minden, Pulau Pinang. Malaysia
K M Sundram
3 School of Pharmacy, AIMST University, Jalan Bedong-Semeling, Batu 3 ½, Bukit Air Nasi, Bedong 08100, Kedah, Malaysia
L Yoga Latha
Natural products from medicinal plants, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug leads because of the unmatched availability of chemical diversity. Due to an increasing demand for chemical diversity in screening programs, seeking therapeutic drugs from natural products, interest particularly in edible plants has grown throughout the world. Botanicals and herbal preparations for medicinal usage contain various types of bioactive compounds. The focus of this paper is on the analytical methodologies, which include the extraction, isolation and characterization of active ingredients in botanicals and herbal preparations. The common problems and key challenges in the extraction, isolation and characterization of active ingredients in botanicals and herbal preparations are discussed. As extraction is the most important step in the analysis of constituents present in botanicals and herbal preparations, the strengths and weaknesses of different extraction techniques are discussed. The analysis of bioactive compounds present in the plant extracts involving the applications of common phytochemical screening assays, chromatographic techniques such as HPLC and, TLC as well as non-chromatographic techniques such as immunoassay and Fourier Transform Infra Red (FTIR) are discussed.
Introduction
Natural products, such as plants extract, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug discoveries because of the unmatched availability of chemical diversity (Cos et al., 2006). According to the World Health Organization (WHO), more than 80% of the world's population relies on traditional medicine for their primary healthcare needs. The use of herbal medicines in Asia represents a long history of human interactions with the environment. Plants used for traditional medicine contain a wide range of substances that can be used to treat chronic as well as infectious diseases ( Duraipandiyan et al., 2006 ). Due to the development of adverse effects and microbial resistance to the chemically synthesized drugs, men turned to ethnopharmacognosy. They found literally thousands of phytochemicals from plants as safe and broadly effective alternatives with less adverse effect. Many beneficial biological activity such as anticancer, antimicrobial, antioxidant, antidiarrheal, analgesic and wound healing activity were reported. In many cases the people claim the good benefit of certain natural or herbal products. However, clinical trials are necessary to demonstrate the effectiveness of a bioactive compound to verify this traditional claim. Clinical trials directed towards understanding the pharmacokinetics, bioavailability, efficacy, safety and drug interactions of newly developed bioactive compounds and their formulations (extracts) require a careful evaluation. Clinical trials are carefully planned to safeguard the health of the participants as well as answer specific research questions by evaluating for both immediate and long-term side effects and their outcomes are measured before the drug is widely applied to patients.
According to the World Health Organization (WHO), nearly 20,000 medicinal plants exist in 91 countries including 12 mega biodiversity countries. The premier steps to utilize the biologically active compound from plant resources are extraction, pharmacological screening, isolation and characterization of bioactive compound, toxicological evaluation and clinical evaluation. A brief summary of the general approaches in extraction, isolation and characterization of bioactive compound from plants extract can be found in Figure 1 . This paper provides details in extraction, isolation and characterization of bioactive compound from plants extract with common phytochemical screening assay, chromatographic techniques, such as HPLC, and HPLC/MS and Fourier Transform Mass Spectrometry (FTMS).

A brief summary of the general approaches in extraction, isolation and characterization of bioactive compound from plants extract
Extraction is the crucial first step in the analysis of medicinal plants, because it is necessary to extract the desired chemical components from the plant materials for further separation and characterization. The basic operation included steps, such as pre-washing, drying of plant materials or freeze drying, grinding to obtain a homogenous sample and often improving the kinetics of analytic extraction and also increasing the contact of sample surface with the solvent system. Proper actions must be taken to assure that potential active constituents are not lost, distorted or destroyed during the preparation of the extract from plant samples. If the plant was selected on the basis of traditional uses ( Fabricant and Farnsworth, 2001 ), then it is needed to prepare the extract as described by the traditional healer in order to mimic as closely as possible the traditional ‘herbal’ drug. The selection of solvent system largely depends on the specific nature of the bioactive compound being targeted. Different solvent systems are available to extract the bioactive compound from natural products. The extraction of hydrophilic compounds uses polar solvents such as methanol, ethanol or ethyl-acetate. For extraction of more lipophilic compounds, dichloromethane or a mixture of dichloromethane/methanol in ratio of 1:1 are used. In some instances, extraction with hexane is used to remove chlorophyll (Cos et al., 2006).
As the target compounds may be non-polar to polar and thermally labile, the suitability of the methods of extraction must be considered. Various methods, such as sonification, heating under reflux, soxhlet extraction and others are commonly used (United States Pharmacopeia and National Formulary, 2002; Pharmacopoeia of the People's Republic of China, 2000; The Japanese Pharmacopeia, 2001) for the plant samples extraction. In addition, plant extracts are also prepared by maceration or percolation of fresh green plants or dried powdered plant material in water and/or organic solvent systems. A brief summary of the experimental conditions for the various methods of extraction is shown in Table 1 .
A brief summary of the experimental conditions for various methods of extraction for plants material
The other modern extraction techniques include solid-phase micro-extraction, supercritical-fluid extraction, pressurized-liquid extraction, microwave-assisted extraction, solid-phase extraction, and surfactant-mediated techniques, which possess certain advantages. These are the reduction in organic solvent consumption and in sample degradation, elimination of additional sample clean-up and concentration steps before chromatographic analysis, improvement in extraction efficiency, selectivity, and/ kinetics of extraction. The ease of automation for these techniques also favors their usage for the extraction of plants materials ( Huie, 2002 ).
Identification and characterization
Due to the fact that plant extracts usually occur as a combination of various type of bioactive compounds or phytochemicals with different polarities, their separation still remains a big challenge for the process of identification and characterization of bioactive compounds. It is a common practice in isolation of these bioactive compounds that a number of different separation techniques such as TLC, column chromatography, flash chromatography, Sephadex chromatography and HPLC, should be used to obtain pure compounds. The pure compounds are then used for the determination of structure and biological activity. Beside that, non-chromatographic techniques such as immunoassay, which use monoclonal antibodies (MAbs), phytochemical screening assay, Fourier-transform infrared spectroscopy (FTIR), can also be used to obtain and facilitate the identification of the bioactive compounds.
Chromatographic techniques
Thin-layer chromatography (tlc) and bio-autographic methods.
TLC is a simple, quick, and inexpensive procedure that gives the researcher a quick answer as to how many components are in a mixture. TLC is also used to support the identity of a compound in a mixture when the R f of a compound is compared with the R f of a known compound. Additional tests involve the spraying of phytochemical screening reagents, which cause color changes according to the phytochemicals existing in a plants extract; or by viewing the plate under the UV light. This has also been used for confirmation of purity and identity of isolated compounds.
Bio-autography is a useful technique to determine bioactive compound with antimicrobial activity from plant extract. TLC bioautographic methods combine chromatographic separation and in situ activity determination facilitating the localization and target-directed isolation of active constituents in a mixture. Traditionally, bioautographic technique has used the growth inhibition of microorganisms to detect anti-microbial components of extracts chromatographed on a TLC layer. This methodology has been considered as the most efficacious assay for the detection of anti-microbial compounds ( Shahverdi, 2007 ). Bio-autography localizes antimicrobial activity on a chromatogram using three approaches: (i) direct bio-autography, where the micro-organism grows directly on the thin-layer chromatographic (TLC) plate, (ii) contact bio-autography, where the antimicrobial compounds are transferred from the TLC plate to an inoculated agar plate through direct contact and (iii) agar overlay bio-autography, where a seeded agar medium is applied directly onto the TLC plate ( Hamburger and Cordell, 1987 ; Rahalison et al., 1991 ). The inhibition zones produced on TLC plates by one of the above bioautographic technique will be use to visualize the position of the bioactive compound with antimicrobial activity in the TLC fingerprint with reference to Rf values ( Homans and Fuchs, 1970 ). Preparative TLC plates with a thickness of 1mm were prepared using the same stationary and mobile phases as above, with the objective of isolating the bioactive components that exhibited the antimicrobial activity against the test strain. These areas were scraped from the plates, and the substance eluted from the silica with ethanol or methanol. Eluted samples were further purified using the above preparative chromatography method. Finally, the components were identified by HPLC, LCMS and GCMS. Although it has high sensitivity, its applicability is limited to micro-organisms that easily grow on TLC plates. Other problems are the need for complete removal of residual low volatile solvents, such as n -BuOH, trifluoroacetic acid and ammonia and the transfer of the active compounds from the stationary phase into the agar layer by diffusion (Cos et al., 2006). Because bio-autography allows localizing antimicrobial activities of an extract on the chromatogram, it supports a quick search for new antimicrobial agents through bioassay-guided isolation (Cos et al., 2006). The bioautography agar overlay method is advantageous in that, firstly it uses very little amount of sample when compared to the normal disc diffusion method and hence, it can be used for bioassay-guided isolation of compounds. Secondly, since the crude extract is resolved into its different components, this technique simplifies the process of identification and isolation of the bioactive compounds ( Rahalison et al., 1991 ).
High performance liquid chromatography
High performance liquid chromatography (HPLC) is a versatile, robust, and widely used technique for the isolation of natural products ( Cannell, 1998 ). Currently, this technique is gaining popularity among various analytical techniques as the main choice for fingerprinting study for the quality control of herbal plants ( Fan et al., 2006 ). Natural products are frequently isolated following the evaluation of a relatively crude extract in a biological assay in order to fully characterize the active entity. The biologically active entity is often present only as minor component in the extract and the resolving power of HPLC is ideally suited to the rapid processing of such multicomponent samples on both an analytical and preparative scale. Many bench top HPLC instruments now are modular in design and comprise a solvent delivery pump, a sample introduction device such as an auto-sampler or manual injection valve, an analytical column, a guard column, detector and a recorder or a printer.
Chemical separations can be accomplished using HPLC by utilizing the fact that certain compounds have different migration rates given a particular column and mobile phase. The extent or degree of separation is mostly determined by the choice of stationary phase and mobile phase. Generally the identification and separation of phytochemicals can be accomplished using isocratic system (using single unchanging mobile phase system). Gradient elution in which the proportion of organic solvent to water is altered with time may be desirable if more than one sample component is being studied and differ from each other significantly in retention under the conditions employed.
Purification of the compound of interest using HPLC is the process of separating or extracting the target compound from other (possibly structurally related) compounds or contaminants. Each compound should have a characteristic peak under certain chromatographic conditions. Depending on what needs to be separated and how closely related the samples are, the chromatographer may choose the conditions, such as the proper mobile phase, flow rate, suitable detectors and columns to get an optimum separation.
Identification of compounds by HPLC is a crucial part of any HPLC assay. In order to identify any compound by HPLC, a detector must first be selected. Once the detector is selected and is set to optimal detection settings, a separation assay must be developed. The parameters of this assay should be such that a clean peak of the known sample is observed from the chromatograph. The identifying peak should have a reasonable retention time and should be well separated from extraneous peaks at the detection levels which the assay will be performed. UV detectors are popular among all the detectors because they offer high sensitivity (Lia et al., 2004) and also because majority of naturally occurring compounds encountered have some UV absorbance at low wavelengths (190–210 nm) ( Cannell, 1998 ). The high sensitivity of UV detection is bonus if a compound of interest is only present in small amounts within the sample. Besides UV, other detection methods are also being employed to detect phytochemicals among which is the diode array detector (DAD) coupled with mass spectrometer (MS) ( Tsao and Deng, 2004 ). Liquid chromatography coupled with mass spectrometry (LC/MS) is also a powerful technique for the analysis of complex botanical extracts ( Cai et al., 2002 ; He, 2000 ). It provides abundant information for structural elucidation of the compounds when tandem mass spectrometry (MS n ) is applied. Therefore, the combination of HPLC and MS facilitates rapid and accurate identification of chemical compounds in medicinal herbs, especially when a pure standard is unavailable ( Ye et al., 2007 ).
The processing of a crude source material to provide a sample suitable for HPLC analysis as well as the choice of solvent for sample reconstitution can have a significant bearing on the overall success of natural product isolation. The source material, e.g., dried powdered plant, will initially need to be treated in such a way as to ensure that the compound of interest is efficiently liberated into solution. In the case of dried plant material, an organic solvent (e.g., methanol, chloroform) may be used as the initial extractant and following a period of maceration, solid material is then removed by decanting off the extract by filteration. The filtrate is then concentrated and injected into HPLC for separation. The usage of guard columns is necessary in the analysis of crude extract. Many natural product materials contain significant level of strongly binding components, such as chlorophyll and other endogenous materials that may in the long term compromise the performance of analytical columns. Therefore, the guard columns will significantly protect the lifespan of the analytical columns.
Non-chromatographic techniques
Immunoassay.
Immunoassays, which use monoclonal antibodies against drugs and low molecular weight natural bioactive compounds, are becoming important tools in bioactive compound analyses. They show high specificity and sensitivity for receptor binding analyses, enzyme assays and qualitative as well as quantitative analytical techniques. Enzyme-linked immunosorbent essay (ELISA) based on MAbs are in many cases more sensitive than conventional HPLC methods. Monoclonal antibodies can be produced in specialized cells through a technique known as hybridoma technology ( Shoyama et al., 2006 ). The following steps are involved in the production of monoclonal antibodies via hybridoma technology against plant drugs:
- A rabbit is immunized through repeated injection of specific plant drugs for the production of specific antibody, facilitated due to proliferation of the desired B cells.
- Tumors are produced in a mouse or a rabbit.
- From the above two types of animals, spleen cell (these cells are rich in B cells and T cells) are cultured separately. The separately cultured spleen cells produce specific antibodies against the plants drug, and against myeloma cells that produce tumors.
- The production of hybridoma by fusion of spleen cells to myeloma cells is induced using polyethylene glycol (PEG). The hybrid cells are grown in selective hypoxanthine aminopterin thymidine (HAT) medium.
- The desired hybridoma is selected for cloning and antibody production against a plant drug. This process is facilitated by preparing single cell colonies that will grow and can be used for screening of antibody producing hybridomas.
- The selected hybridoma cells are cultured for the production of monoclonal antibodies in large quantity against the specific plants drugs.
- The monoclonal antibodies are used to determine similar drugs in the plants extract mixture through enzyme-linked immunosorbent essay (ELISA).
Phytochemical screening assay
Phytochemicals are chemicals derived from plants and the term is often used to describe the large number of secondary metabolic compounds found in plants. Phytochemical screening assay is a simple, quick, and inexpensive procedure that gives the researcher a quick answer to the various types of phytochemicals in a mixture and an important tool in bioactive compound analyses. A brief summary of the experimental procedures for the various phytochemical screening methods for the secondary metabolites is shown in Table 2 . After obtaining the crude extract or active fraction from plant material, phytochemical screening can be performed with the appropriate tests as shown in the Table 2 to get an idea regarding the type of phytochemicals existing in the extract mixture or fraction.
A brief summary of phytochemical screening of secondary metabolites
Fourier-transform infrared spectroscopy (FTIR )
FTIR has proven to be a valuable tool for the characterization and identification of compounds or functional groups (chemical bonds) present in an unknown mixture of plants extract ( Eberhardt et al., 2007 ; Hazra et al., 2007 ). In addition, FTIR spectra of pure compounds are usually so unique that they are like a molecular “fingerprint”. For most common plant compounds, the spectrum of an unknown compound can be identified by comparison to a library of known compounds. Samples for FTIR can be prepared in a number of ways. For liquid samples, the easiest is to place one drop of sample between two plates of sodium chloride. The drop forms a thin film between the plates. Solid samples can be milled with potassium bromide (KBr) to and then compressed into a thin pellet which can be analyzed. Otherwise, solid samples can be dissolved in a solvent such as methylene chloride, and the solution then placed onto a single salt plate. The solvent is then evaporated off, leaving a thin film of the original material on the plate.
Since bioactive compounds occurring in plant material consist of multi-component mixtures, their separation and determination still creates problems. Practically most of them have to be purified by the combination of several chromatographic techniques and various other purification methods to isolate bioactive compound(s).

IMAGES
VIDEO
COMMENTS
This Special Issue of Antioxidants is dedicated to current research on the aspects related to the anti-inflammatory and antioxidant properties of plant extracts, as well as the modulators and pathways involved in these therapeutic actions. In this issue, 16 original research papers and 6 reviews have been published.
The antioxidant activity of the plant extracts against DPPH was determined using the method proposed by . A methanolic dilution of DPPH 1 × 10 −4 M was prepared. Aliquots of 1 mL of each sample in the methanolic extract were collected (at 4 different concentrations: 0.1, 0.5, 1, and 2 mg/mL; two replicates per sample and concentration) and ...
Nowadays, plant extracts are increasingly becoming important additives in the food industry due to their content in bioactive compounds such as polyphenols [ 1] and carotenoids [ 2 ], which have antimicrobial and antioxidant activity, especially against low-density lipoprotein (LDL) and deoxyribonucleic acid (DNA) oxidative changes [ 3 ].
Research on finding novel drugs in medicinal plants involves screening the plant extracts for new compounds and then conducting biological activity tests. Suspected new molecules or bioactive compounds are then isolated and purified for molecular structure elucidation and further pharmacological or toxicological tests [5]. The research ...
Different phenolic contents of different plant samples have been reported in the literature 12,18,19,20,21,22,23,24,25.For instance, the total phenol content of sage and peppermint was 27.94 and ...
For centuries, plants have been part of human civilisation, serving as food, healing substances and treatments for various diseases. In recent years, advances in biological research have revealed the remarkable potential of plant compounds in the food and medicine sectors [].In the food industry, plant extracts are used to improve the safety, nutritional value and shelf life of food products.
You are welcome to participate in this Special Issue with original research papers and reviews on the biologic activity of plant extracts, with the following topics: anti-inflammatory, antioxidant, antidiabetic, anti-aging, antitumor, antiviral, antiallergic, or antimicrobial properties of plant extracts, as well as the mechanisms involved in ...
The current study was done in 2022 seasons to study the influence of foliar application by moringa leaf extract at (2, 4, and 6 ml.l-1), garlic cloves extract (5, 10, and 15 ml.l-1), and turmeric ...
Plant-based medicines are often prepared from crude plant extracts comprising of complex ... pharmaceutical and technological research, ... using Whatman No. 1 filter paper, and the filtrates were ...
Extraction is the separation of medicinally mixture of many plant metabolit es, such as alkaloids, glycosides, p henolics, te rpenoids, and fla vonoids using selective. solvent s throug h standard ...
This paper is aimed at giving an insight into the extraction, isolation, and characterization of the rich medicinal plant biodiversity of potential pharmaceutical importance and the major ...
To 50 µl of 10-100 µg/mL plant extract, 2 ml DPPH was added and kept in dark at room temperature for 30 min. Then, 1 ml methanol and 2 ml DPPH was used as negative control while methanol ...
A solution of plant extract was placed into test tube followed by 2mL of 1% gelatin solution and shaken. Appearance of white precipitate indicates the presence of phenols.[3,19,20,21] (d) Mayer's reagent test (potassium mercuric iodide test). To a solution of plant extract, 1mL of Mayer's reagent was added in an acidic solution.
The dried sample goes to the process of weighing and crushing. Afterward, plants extract is mixed with Milli-Q H 2 O as per desired concentration and boiled with continuous stirring. The obtained solution is then filtered with Whatman filter paper, and the part in which there is a clear solution was useful for sample (plant extract) (Wang et al ...
Background This study was focused on the measurement of anticancer properties of six medicinal plants from western Nepal in three cell lines; HeLa, Hep3B, and HCT116, and anti-inflammatory properties in RAW 264.7 cell line through NO, PGE2, and TNF-α production. In addition, the phytochemical screening, total phenolic, flavonoid content, and antioxidant properties were evaluated. Results The ...
Plant extracts containing bioactive alkaloids, phenolic acids, polyphenols, proteins, sugars, and terpenoids are believed to have an important role in first reducing the metallic ions and then stabilizing them [99, 100]. The biological method also reduces time consumption when compared with the chemical and physical methods.
The objective of this paper is to provide a review of phytochemical studies that have addressed extracting, measuring and identifying bioactive compounds of plants. ... Extensive research has been performed in order to increase stearic acid content oil production in the most widely consumed legume crop in the world, soybeans. ... plant extracts ...
Antimicrobial activity of the crude methanolic extracts and the essential oils of P.crispa and P. undulata. The extracts of the studied plants exhibited varying degrees of inhibition activity ...
The modern methods includes microwave assisted extraction (MAE), ultrasonication assisted. extraction (UAE), supercritical fluid extraction (SFE), solid phase mic ro extraction ( S PME), Soxhwave ...
A multidimensional therapeutic approach to inflammation using phytoextracts is described, however, further research is needed to provide more information about the effectiveness of herbal extracts, as well as their combinations, in people with inflammatory joint diseases. Medicinal plants and their secondary metabolites are increasingly used in the treatment of diseases in complex therapy.
The HPTLC performed on the methanolic extract of Fumaria parviflora (Lam.) showed the presence of various phytoconstituents in different concentrations as illustrated in figures and tables. Figure 1 represents the 3-dimensional overlay of the chromatogram of all tracks, at all measured wavelengths. The chromatogram scanned at 254 nm (Fig. 2) represents 11 and 10 peaks for track 1 and track 2 ...
The application of plant extracts in cell therapy has gradually increased with the rapid development of cell therapy. For example, the increased therapeutic effects of stem cells induced by plant extracts have been reported in studies of Alzheimer's disease [ 10 ], chronic kidney disease [ 11 ], and stroke [ 12 ], and the therapeutic effects ...
Fresh leaves of plants were collected, and the aqueous crude extract was prepared. Phytochemicals were extracted using refluxing technique. A concentration series of crude extract of leaves were prepared separately from 20 to 100 mg/L. Batches of each containing 100 third instar larvae of Ae. aegypti were used for larval bioassays.
In recent years, the use of deep neural network in effective network feature extraction and the design of efficient and high-precision hyperspectral image classification algorithms has gradually become a research hotspot for scholars. However, due to the difficulty of obtaining hyperspectral images and the high cost of annotation, the training samples are very limited. In order to cope with ...
Plants extracts, fractions, and molecules recognized in the pharmaceutical industry due to their broad spectrum of structural diversity and hold promising scope to formulate appropriate drugs.
The focus of this paper is on the analytical methodologies, which include the extraction, isolation and characterization of active ingredients in botanicals and herbal preparations. ... such as plants extract, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug discoveries because of the unmatched ...