Home — Essay Samples — Environment — Water — The Importance of Water: The Vital Essence

The Importance of Water: The Vital Essence
- Categories: Nutrition Water
About this sample

Words: 652 |
Published: Sep 7, 2023
Words: 652 | Page: 1 | 4 min read
Table of contents
Water and human health, environmental balance, agriculture and food security, industrial and economic significance, challenges of water scarcity and pollution, responsible water management.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr. Karlyna PhD
Verified writer
- Expert in: Nursing & Health Environment

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
3 pages / 1169 words
1 pages / 559 words
2 pages / 859 words
5 pages / 2919 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Water
Water is a ubiquitous substance that plays a crucial role in shaping our world in numerous ways, including its impact on time. From the rhythmic ebb and flow of tides to the soothing sound of raindrops on a windowpane, water has [...]
A Long Walk to Water is a compelling novel by Linda Sue Park that tells the story of two young individuals, Nya and Salva, who are living in Sudan and struggling to access clean water. The novel sheds light on the harsh [...]
Water, the elixir of life, is a finite resource essential for all living organisms on Earth. Yet, despite its undeniable importance, water shortage has become a critical global issue. This essay delves into the causes, [...]
Water is a fundamental substance for all living organisms on Earth. It plays a crucial role in various biological processes, including metabolism, digestion, and temperature regulation. One of the unique properties of water is [...]
In conclusion, the origin of water remains an intriguing and complex puzzle. While the cometary delivery hypothesis, proto-Earth hypothesis, and planetary embryo hypothesis offer compelling explanations, they also face [...]
Transpiration, or the loss of water vapor from the leaves and stems of plants, is a process that assists in evaporative cooling, gas exchange, and the absorption and distribution of minerals and water throughout the plant. The [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 22 March 2021
Sustainable implementation of innovative technologies for water purification
- Bart Van der Bruggen ORCID: orcid.org/0000-0002-3921-7472 1 , 2
Nature Reviews Chemistry volume 5 , pages 217–218 ( 2021 ) Cite this article
9875 Accesses
72 Citations
3 Altmetric
Metrics details
- Environmental sciences
- Scientific community
One of the sustainable development goals set by the United Nations General Assembly is to ensure the availability and sustainable management of water and sanitation for all. This requires investment in water purification technologies. World Water Day offers an opportunity to discuss whether such investment will help achieve this laudable goal.
Wastewater and seawater have long been considered as potential sources from which to produce freshwater. Several technologies have been developed over the past few decades aimed at their reuse and recycle, but unfortunately the treatment of both sources may have perfidious effects.
Of the approaches presently available, desalination seems to have the greatest potential, given that seawater is a nearly unlimited resource. However, desalination is an energy-intensive process. The state-of-the-art technology, seawater reverse osmosis (SWRO), has undergone huge improvements over the past five decades: the specific energy consumption of SWRO was reduced from 20 kWh m −3 in 1970 to only 2.5 kWh m −3 in 2010. It has been estimated that a further 0.69–0.79 kWh m −3 might be saved by a smart process integration with intrinsic heat recovery 1 , but desalination of typical seawater (with an average salt concentration of 35 g l −1 ) requires a minimum of 1.07 kWh m −3 , offering only a little room for improvement. This limit is the foundation of the water–energy nexus and prompts further research on renewable energy sources for desalination, which remain scarce. In a case study, Delgado-Torres and co-workers 2 used tidal and solar energy for desalination at a semi-arid location in Broome, Australia. Similar studies focus on desalination driven by wind energy, photovoltaics or solar thermal energy. Although such approaches to water desalination may be viable to supply clean water in small or spatially confined communities — as was demonstrated in the island of Aruba 3 — they offer very little for the water challenges of large cities such as Beijing, Cairo or Cape Town.

In a cost–benefit analysis, wastewater recycling is more favourable than seawater desalination, because the former does not require the expensive separation of salts from water. This may seem surprising given that reverse osmosis is the key technology in both cases. The difference is that wastewater recycling would operate at much lower pressure. Such recycling has been practised for more than half a century in Windhoek, Namibia, and is accepted practice in water-scarce places such as Singapore 4 . Southern California is presently implementing a large-scale scheme to use recycled water as a potable source 5 and other countries and locations will surely follow. This trend pushes researchers to develop fouling-resistant, high-flux membranes for reverse osmosis and related membrane processes such as nano- or ultrafiltration. However, new challenges also arise. The production of (polymer) membranes for purification typically requires the use of polar aprotic solvents such as N,N -dimethylformamide (DMF), N,N -dimethylacetamide (DMA), 1,4-dioxane and tetrahydrofuran (THF). These solvents have a considerable environmental impact and significant effort is invested in their replacement with ‘greener’ solvents such as organic carbonates 6 or dimethyl sulfoxide (DMSO) 7 . Another limitation for present membrane technologies lies in the availability, processing and scale-up of materials for their manufacture. For example, two 2006 reports describe how incorporating carbon nanotubes into membranes affords permeabilities one to two orders of magnitude larger than those of conventional membranes. However, scaling up the synthesis of such membranes was not expected to be easy 8 — and, indeed, it has, so far, not happened. Since these reports emerged, there have been numerous studies on mixed-matrix membranes combining other nanostructures with polymeric matrices but, thus far, none has yet been applied on a large scale. Typically, good results are obtained in the laboratory, but the cost of producing the required nanostructures or issues associated with toxicity or leaching of nanoparticles from membranes have proven prohibitive for industrial use. Researchers need to place greater focus on the development of realistic membranes rather than just better membranes.
Closing the water cycle by either desalination or wastewater purification promises to provide virtually unlimited volumes of freshwater: in principle, it would enable an increase in water consumption by a factor equal to the inverse of the recycled fraction. However, we must be cognizant of unintended consequences. Water availability is one of the limiting factors for population growth and greater availability would certainly stimulate population growth. History has shown that humankind naturally makes use of available resources, sometimes with dramatic consequences, as exemplified by the agricultural and industrial revolutions 9 . A historical, sociological and demographic analysis by Harari shows that if water recycling is practised on a large scale, water consumption per capita may remain the same but our population will grow by the inverse of the recycled fraction 9 . This would then automatically lead to new challenges. A disenchanting example is the present SARS-CoV-2 virus: the scale of the outbreak would have been much more contained in a modest, local society without overpopulation. Water technologies may catalyse global growth more than any other technology because water is one of very few commodities that humankind cannot do without. This is of course not the case for industrialized countries, where water is not a limiting factor, but in most parts of the world it is. Harari was criticized for being unfamiliar with technologies, and, while this may be a fair criticism, warnings from other disciplines should not be summarily dismissed by technology developers.
In conclusion, the scope of water technologies may need to be reconsidered. There is no need for a major technological breakthrough in water recycling or desalination. What is really needed is for present technologies to be available to children growing up without access to clean water sources, as stated in the United Nations sustainable development goals . This will require dedicated, embedded actions towards maintaining the demographic status quo while respecting the basic human rights of all. The goals then are a useful tool to monitor progress but must be considered in context because the indicators that are used can result in tunnel vision 10 . Furthermore, lifestyle choices in terms of water — reduce, reuse and recycle — need to be thoroughly considered and be more than just a hollow slogan.
Park, K., Kim, J. B., Yang, D. R. & Hong, S. K. Towards a low-energy seawater reverse osmosis desalination plant: a review and theoretical analysis for future directions. J. Membr. Sci. 595 , 117607 (2020).
Article CAS Google Scholar
Delgado-Torres, A. M., García-Rodríguez, L. & Jiménez del Moral, M. Preliminary assessment of innovative seawater reverse osmosis (SWRO) desalination powered by a hybrid solar photovoltaic (PV) - tidal range energy system. Desalination 477 , 114247 (2020).
Brendel, L. P. M., Shah, V. M., Groll, E. A. & Braun, J. E. A methodology for analyzing renewable energy opportunities for desalination and its application to Aruba. Desalination 493 , 114613 (2020).
Lafforgue, M. & Lenouvel, V. Closing the urban water loop: lessons from Singapore and Windhoek. Environ. Sci. Water Res. Technol. 1 , 622–631 (2015).
Article Google Scholar
Chalmers, R. B., Tremblay, M. & Soni, R. A new water source for Southern California: the regional recycled water program. J. AWWA 112 , 6–19 (2020).
Rasool, M. A., Pescarmona, P. P. & Vankelecom, I. F. J. Applicability of organic carbonates as green solvents for membrane preparation. ACS Sustain. Chem. Eng. 7 , 13774–13785 (2019).
Evenepoel, N., Wen, S., Tsehaye, M. T. & Van der Bruggen, B. Potential of DMSO as greener solvent for PES ultra- and nanofiltration membrane preparation. J. Appl. Polym. Sci. 135 , 46494 (2018).
Sholl, D. S. & Johnson, J. K. Making high-flux membranes with carbon nanotubes. Science 312 , 1003–1004 (2006).
Harari, Y. N. Sapiens: A Brief History of Humankind (Harper Collins, 2015).
Weststrate, J., Dijkstra, G., Eshuis, J., Gianoli, A. & Rusca, M. The sustainable development goal on water and sanitation: learning from the millennium development goals. Soc. Indic. Res. 143 , 795–810 (2019).
Download references
Author information
Authors and affiliations.
Department of Chemical Engineering, KU Leuven, Leuven, Belgium
Bart Van der Bruggen
Faculty of Engineering and the Built Environment, Tshwane University of Technology, Pretoria, South Africa
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Bart Van der Bruggen .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Related links.
United Nations sustainable development goals: https://sdgs.un.org/goals
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Van der Bruggen, B. Sustainable implementation of innovative technologies for water purification. Nat Rev Chem 5 , 217–218 (2021). https://doi.org/10.1038/s41570-021-00264-7
Download citation
Published : 22 March 2021
Issue Date : April 2021
DOI : https://doi.org/10.1038/s41570-021-00264-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Benefits and limitations of recycled water systems in the building sector: a review.
- Zhonghao Chen
- Pow-Seng Yap
Environmental Chemistry Letters (2024)
Nature-inspired wood-based solar evaporation system for efficient desalination and water purification
Journal of Materials Science (2023)
Superhydrophobicity-improved Ethanol-Water Separation
- Linfeng Chen
Chemical Research in Chinese Universities (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS)
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Redlands East Valley High School, Redlands, California, United States of America
- Bill B. Wang

- Published: September 28, 2021
- https://doi.org/10.1371/journal.pone.0257865
- Reader Comments
This study, based in San Bernardino County, Southern California, collected and examined tap water samples within the area to explore the feasibility of adopting non-industrial equipment and methods to reduce water hardness and total dissolved solids(TDS). We investigated how water quality could be improved by utilizing water boiling, activated carbon and sodium bicarbonate additives, as well as electrolysis methods. The results show that heating is effective at lower temperatures rather than long boils, as none of the boiling tests were lower than the original value. Activated carbon is unable to lower TDS, because it is unable to bind to any impurities present in the water. This resulted in an overall TDS increase of 3.5%. However, adding small amounts of sodium bicarbonate(NaHCO 3 ) will further eliminate water hardness by reacting with magnesium ions and improve taste, while increasing the pH. When added to room temperature tap water, there is a continuous increase in TDS of 24.8% at the 30 mg/L mark. The new findings presented in this study showed that electrolysis was the most successful method in eliminating TDS, showing an inverse proportion where an increasing electrical current and duration of electrical lowers more amounts of solids. This method created a maximum decrease in TDS by a maximum of 22.7%, with 3 tests resulting in 15.3–16.6% decreases. Furthermore, when water is heated to a temperature around 50°C (122°F), a decrease in TDS of around 16% was also shown. The reduction of these solids will help lower water hardness and improve the taste of tap water. These results will help direct residents to drink more tap water rather than bottled water with similar taste and health benefits for a cheaper price as well as a reduction on plastic usage.
Citation: Wang BB (2021) Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS). PLoS ONE 16(9): e0257865. https://doi.org/10.1371/journal.pone.0257865
Editor: Mahendra Singh Dhaka, Mohanlal Sukhadia University, INDIA
Received: June 22, 2021; Accepted: September 14, 2021; Published: September 28, 2021
Copyright: © 2021 Bill B. Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The author received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The concentration of total dissolved solids(TDS) present in water is one of the most significant factors in giving water taste and also provides important ions such as calcium, magnesium, potassium, and sodium [ 1 – 3 ]. However, water with high TDS measurements usually indicates contamination by human activities, such as soil and agricultural runoff caused by irrigation, unregulated animal grazing and wildlife impacts, environmentally damaging farming methods such as slash and burn agriculture, and the overuse of nitrate-based fertilizer [ 4 , 5 ], etc. Around tourist areas as well as state parks, these factors will slowly add up over time and influence the water sources nearby [ 5 ]. Water that flows through natural springs and waterways with high concentrations of organic salts within minerals and rocks, or groundwater that originates from wells with high salt concentration will also result in higher particle measurements [ 6 ].
Water sources can be contaminated by substances and ions such as nitrate, lead, arsenic, and copper [ 7 , 8 ] and may cause many health problems related to heavy metal consumption and poisoning. Water reservoirs and treatments plants that do not consider water contamination by motor vehicles, as well as locations that struggle to provide the necessary components required for water treatment will be more prone to indirect contamination [ 9 – 11 ]. Many plants are effective in ensuring the quality and reduction of these contaminants, but often leave out the secondary considerations, The United States Environmental Protection Agency(US EPA)’s secondary regulations recommend that TDS should be below 500 mg/L [ 2 ], which is also supported by the World Health Organization(WHO) recommendation of below 600 mg/L and an absolute maximum of less than 1,000 mg/L [ 3 ]. These substances also form calcium or magnesium scales within water boilers, heaters, and pipes, causing excess buildup and drain problems, and nitrate ions may pose a risk to human health by risking the formation of N -nitroso compounds(NOC) and less public knowledge about such substances [ 12 – 15 ]. Nitrates can pose a non-carcinogenic threat to different communities, but continue to slip past water treatment standards [ 15 ]. Furthermore, most people do not tolerate or prefer water with high hardness or chlorine additives [ 16 ], as the taste changes tremendously and becomes unpreferable. Even so, TDS levels are not accounted for in mandatory water regulations, because the essential removal of harmful toxins and heavy metals is what matters the most in water safety. Some companies indicate risks in certain ions and alkali metals, showing how water hardness is mostly disregarded and is not as well treated as commercial water bottling companies [ 17 , 18 ].
In Southern California, water quality is not as well maintained than the northern counties as most treatment plants in violation of a regulation or standard are located in Central-Southern California [ 19 ], with southern counties having the largest number of people affected [ 20 ]. This study is focused on the Redlands area, which has had no state code violations within the last decade [ 21 ]. A previous study has analyzed TDS concentrations throughout the Santa Ana Basin, and found concentrations ranging from 190–600 ppm as treated wastewater and samples obtained from mountain sites, taking into account the urban runoff and untreated groundwater as reasons for elevated levels of TDS but providing no solution in helping reduce TDS [ 22 ]. Also, samples have not been taken directly through home water supplies, where the consumer is most affected. Other water quality studies in this region have been focused on the elimination of perchlorates in soil and groundwater and distribution of nitrates, but such research on chemicals have ceased for the last decade, demonstrated by safe levels of perchlorates and nitrates in water reports [ 23 , 24 ]. In addition to these studies, despite the improving quality of the local water treatment process, people prefer bottled water instead of tap water because of the taste and hardness of tap water [ 25 ]. Although water quality tests are taken and documented regularly, the taste of the water is not a factor to be accounted for in city water supplies, and neither is the residue left behind after boiling water. The residue can build up over time and cause appliance damage or clogs in drainage pipes.
This study will build upon previous analyses of TDS studies and attempt to raise new solutions to help develop a more efficient method in reducing local TDS levels, as well as compare current measurements to previous analyses to determine the magnitude to which local treatment plants have improved and regulated its treatment processes.
Several methods that lower TDS are reviewed: boiling and heating tap water with and without NaHCO₃, absorption by food-grade activated carbon [ 26 , 27 ], and battery-powered electrolysis [ 28 – 30 ]. By obtaining water samples and determining the difference in TDS before and after the listed experiments, we can determine the effectiveness of lowering TDS. The results of this study will provide options for residents and water treatment plants to find ways to maintain the general taste of the tap water, but also preserve the lifespan of accessories and pipelines. By determining a better way to lower TDS and treat water hardness, water standards can be updated to include TDS levels as a mandatory measurement.
Materials and methods
All experiments utilized tap water sourced from Redlands homes. This water is partially supplied from the Mill Creek (Henry Tate) and Santa Ana (Hinckley) Water Sheds/Treatment Plants, as well as local groundwater pumps. Water sampling and sourcing were done at relatively stable temperatures of 26.9°C (80.42°F) through tap water supplies. The average TDS was measured at 159 ppm, which is slightly lower than the reported 175 ppm by the City of Redlands. Permission is obtained by the author from the San Bernardino Municipal Water Department website to permit the testing procedures and the usage of private water treatment devices for the purpose of lowering water hardness and improving taste and odor. The turbidity was reported as 0.03 Nephelometric Turbidity Units (NTU) post-treatment. Residual nitrate measured at 2.3mg/L in groundwater before treatment and 0.2 mg/L after treatment and perchlorate measured at 0.9 μg/L before treatment, barely staying below the standard of 1 μg/L; it was not detected within post-treatment water. Lead content was not detected at all, while copper was detected at 0.15 mg/L.
For each test, all procedures were done indoors under controlled temperatures, and 20 L of fresh water was retrieved before each test. Water samples were taken before each experimental set and measured for TDS and temperature, and all equipment were cleaned thoroughly with purified water before and after each measurement. TDS consists of inorganic salts and organic material present in solution, and consists mostly of calcium, magnesium, sodium, potassium, carbonate, chloride, nitrate, and sulfate ions. These ions can be drawn out by leaving the water to settle, or binding to added ions and purified by directly separating the water and ions. Equipment include a 50 L container, 1 L beakers for water, a graduated cylinder, a stir rod, a measuring spoon, tweezers, a scale, purified water, and a TDS meter. A standard TDS meter is used, operated by measuring the conductivity of the total amount of ionized solids in the water, and is also cleaned in the same manner as aforementioned equipment. The instrument is also calibrated by 3 pH solutions prior to testing.All results were recorded for and then compiled for graphing and analysis.
Heating/Boiling water for various lengths of time
The heating method was selected because heat is able to break down calcium bicarbonate into calcium carbonate ions that are able to settle to the bottom of the sample. Four flasks of 1 L of tap water were each heated to 40°C, 50°C, 60°C, and 80°C (104–176°F) and observed using a laser thermometer. The heated water was then left to cool and measurements were made using a TDS meter at the 5, 10, 20, 30, and 60-minute marks.
For the boiling experiments, five flasks of 1 L of tap water were heated to boil at 100°C (212°F). Each flask, which was labeled corresponding to its boiling duration, was marked with 2, 4, 6, 10, and 20 minutes. Each flask was boiled for its designated time, left to cool under open air, and measurements were made using a TDS meter at the 5, 10, 20, 30, 60, and 120-minute marks. The reason that the boiling experiment was extended to 120 minutes was to allow the water to cool down to room temperature.
Activated carbon as a water purification additive
This test was performed to see if food-grade, powdered activated carbon had any possibility of binding with and settling out residual particles. Activated carbon was measured using a milligram scale and separated into batches of 1, 2, 4, 5, 10, 30, and 50 mg. Each batch of the activated carbon were added to a separate flask of water and stirred for five minutes, and finally left to settle for another five minutes. TDS measurements were recorded after the water settled.
Baking soda as a water purification additive
To lower scale error and increase experimental accuracy, a concentration of 200 mg/L NaHCO₃ solution was made with purified water and pure NaHCO₃. For each part, an initial TDS measurement was taken before each experiment.
In separate flasks of 1 L tap water, each labeled 1, 2, 4, 5, 10, and 30 mg of NaHCO 3 , a batch was added to each flask appropriately and stirred for 5 minutes to ensure that everything dissolved. Measurements were taken after the water was left to settle for another 5 minutes for any TDS to settle.
Next, 6 flasks of 1 L tap water were labeled, with 5 mg (25 mL solution) of NaHCO₃ added to three flasks and 10 mg (50 mL solution) of NaHCO₃ added to the remaining three. One flask from each concentration of NaHCO₃ was boiled for 2 mins., 4 mins., or 6 mins., and then left to cool. A TDS measurement was taken at the 5, 10, 20, 30, 60, and 120-minute marks after removal from heat.
Electrolysis under low voltages
This test was performed because the ionization of the TDS could be manipulated with electricity to isolate an area of water with lower TDS. For this test, two 10cm long graphite pieces were connected via copper wiring to a group of batteries, with each end of the graphite pieces submerged in a beaker of tap water, ~3 cm apart.
Using groups of 1.5 V double-A batteries, 4 beakers with 40mL of tap water were each treated with either 7.5, 9.0, 10.5, and 12.0 V of current. Electrolysis was observed to be present by the bubbling of the water each test, and measurements were taken at the 3, 5, 7, and 10 minute marks.
Results/Discussion
Heating water to various temperatures until the boiling point.
The goal for this test was to use heat to reduce the amount of dissolved oxygen and carbon dioxide within the water, as shown by this chemical equation: Heat: Ca(HCO 3 ) 2 → CaCO 3 ↓ + H 2 O + CO 2 ↑.
This would decompose ions of calcium bicarbonate down into calcium carbonate and water and carbon dioxide byproducts.
Patterns and trends in decreasing temperatures.
The following trend lines are based on a dataset of changes in temperature obtained from the test results and graphed as Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0257865.g001
To predict the precise temperature measurements of the tap water at 26.9°C, calculations were made based on Fig 1 . The fitting equations are in the format, y = a.e bx . The values for the fitting coefficients a and b, and correlation coefficient R 2 are listed in Table 1 as column a, b and R 2 . The calculated values and the target temperature are listed in Table 1 .
https://doi.org/10.1371/journal.pone.0257865.t001
Fig 2 was obtained by compiling TDS results with different temperatures and times.
https://doi.org/10.1371/journal.pone.0257865.g002
The fitting equations for Fig 2 are also in the format, y = a.e bx . The fitting coefficients a and b, and correlation coefficient R 2 values are listed in Table 2 . Based on the fitting curves in Fig 2 and the duration to the target temperature in Table 1 , We calculated the TDS at 26.9°C as listed in column calculated TDS in Table 2 based on the values we reported on Fig 2 .
https://doi.org/10.1371/journal.pone.0257865.t002
Based on the heating temperature and the calculated TDS with the same target water temperature, we obtained the following heating temperature vs TDS removal trend line and its corresponding fitting curve in Table 2 .
In Fig 1 , a trend in the rate of cooling is seen, where a higher heating temperature creates a steeper curve. During the first five minutes of cooling, the water cools quicker as the absorbed heat is quickly released into the surrounding environment. By the 10-minute mark, the water begins to cool in a linear rate of change. One detail to note is that the 100°C water cools quicker than the 80°C and eventually cools even faster than the 60°C graph. Table 1 supports this observation as the duration to target temperature begins to decrease from a maximum point of 94.8 mins to 80.95 mins after the 80°C mark.
As shown in Fig 2 , all TDS values decrease as the temperature starts to cool to room temperature, demonstrating a proportional relationship where a lower temperature shows lower TDS. This can partially be explained by the ions settling in the flasks. Visible particles can also be observed during experimentation as small white masses on the bottom, as well as a thin ring that forms where the edge of the water contacts the flask. When the water is heated to 40°C and cooled, a 3.8% decrease in TDS is observed. When 50°C is reached, the TDS drops at its fastest rate from an initial value of 202 ppm to 160 ppm after 60 minutes of settling and cooling. The TDS measurements in these experiements reach a maximum of 204 ppm at the 60°C mark. However, an interesting phenomenon to point out is that the water does not hit a new maximum at 100°C. meaning that TDS reaches a plateau at 60°C. Also, the rate of decrease begins to slow down after 20 minutes, showing that an unknown factor is affecting the rate of decrease. It is also hypothesized that the slight increase in TDS between the 5–20 minute range is caused by a disturbance in the settling of the water, where the temperature starts to decrease at a more gradual and constant rate. The unstable and easy formation of CaCO 3 scaling has also been the subject of a study of antiscaling methods, which also supports the result that temperature is a significant influence for scale formation [ 12 ].
In Table 2 , calculations for TDS and the time it takes for each test to cool were made. Using the data, it is determined that the test with 50°C water decreased the most by 16% from the initial measurement of 159ppm. This means that it is most effective when water is heated between temperatures of 40–60°C when it comes to lowering TDS, with a difference of ~7–16%. When water is heated to temperatures greater than 80°C, the water begins to evaporate, increasing the concentration of the ions, causing the TDS to increase substantially when cooled to room temperature.
Finally, in Fig 3 , a line of best fit of function f(x) = -0.0007x 3 + 0.1641x 2 –10.962x + 369.36 is used with R 2 = 0.9341. Using this function, the local minimum of the graph would be reached at 48.4°C.
https://doi.org/10.1371/journal.pone.0257865.g003
This data shows that heating water at low temperatures (i.e. 40–50°C) may be more beneficial than heating water to higher temperatures. This study segment has not been presented in any section within the United States EPA Report on water management for different residual particles/substances. However, warmer water temperatures are more prone to microorganism growth and algal blooms, requiring more intensive treatment in other areas such as chlorine, ozone, and ultraviolet disinfection.
Using the specific heat capacity equation, we can also determine the amount of energy and voltage needed to heat 1 L of water up to 50°C: Q = mcΔT, where c, the specific heat capacity of water, is 4.186 J/g°C, ΔT, the change in temperature from the experimental maximum to room temperature, is 30°C, and m, the mass of the water, is 1000 g. This means that the amount of energy required will be 125580 J, which is 0.035 kWh or 2.1 kW.
After taking all of the different measurements obtained during TDS testing, and compiling the data onto this plot, Fig 4 is created with a corresponding line of best fit:
https://doi.org/10.1371/journal.pone.0257865.g004
In Fig 4 , it can be observed that the relationship between the temperature of the water and its relative TDS value is a downwards facing parabolic graph. As the temperature increases, the TDS begins to decrease after the steep incline at 50–60°C. The line of best fit is represented by the function f(x) = -0.0142x 2 + 2.258x + 105.84. R 2 = 0.6781. Because the R 2 value is less than expected, factors such as the time spent settling and the reaction rate of the ions should be considered. To determine the specifics within this experiment, deeper research and prolonged studies with more highly accurate analyses must be utilized to solve this problem.
Boiling water for various amounts of time
Trend of boiling duration and rate of cooling..
Using the same methods to create the figures and tables for the previous section, Fig 5 depicts how the duration of time spent boiling water affects how fast the water cools.
https://doi.org/10.1371/journal.pone.0257865.g005
As seen in Fig 5 , within the first 10 minutes of the cooling time, the five different graphs are entwined with each other, with all lines following a similar pattern. However, the graph showing 20 minutes of boiling is much steeper than the other graphs, showing a faster rate of cooling. This data continues to support a previous claim in Fig 2 , as this is most likely represented by a relationship a longer the boil creates a faster cooling curve. This also shows that the first 5 minutes of cooling have the largest deviance compared to any other time frame.
The cooling pattern is hypothesized by possible changes in the orderly structure of the hydrogen bonds in the water molecules, or the decreased heat capacity of water due to the increasing concentration of TDS.
Effect on TDS as boiling duration increases.
In Fig 6 , all lines except for the 20-minute line are clustered in the bottom area of the graph. By excluding the last measurement temporarily due to it being an outlier, we have observed that the difference between the initial and final TDS value of each test decreases.
https://doi.org/10.1371/journal.pone.0257865.g006
Despite following a similar trend of an increase in TDS at the start of the tests and a slow decrease overtime, this experiment had an interesting result, with the final test measuring nearly twice the amount of particles compared to any previous tests at 310 ppm, as shown in Fig 6 . It is confirmed that the long boiling time caused a significant amount of water to evaporate, causing the minerals to be more concentrated, thus resulting in a 300 ppm reading. Fig 6 follows the same trend as Fig 2 , except the TDS reading veers away when the boiling duration reaches 20 minutes. Also, with the long duration of heating, the water has developed an unfavorable taste from intense concentrations of CaCO₃. This also causes a buildup of a thin crust of CaCO₃ and other impurities around the container that is difficult to remove entirely. This finding is in accordance with the introductory statement of hot boiling water causing mineral buildups within pipes and appliances [ 9 ]. A TDS reading of 300ppm is still well below federal secondary standards of TDS, and can still even be compared to bottled water, in which companies may fluctuate and contain 335ppm within their water [ 1 , 2 ].
This experiment continues to stupport that the cooling rate of the water increases as the time spent boiling increases. Based on this test, a prediction can be made in which an increased concentration of dissolved solids lowers the total specific heat capacity of the sample, as the total volume of water decreases. This means that a method can be derived to measure TDS using the heat capacity of a tap water mixture and volume, in addition to current methods of using the electrical conductivity of aqueous ions.
Adding food-grade activated carbon to untreated tap water
Fig 7 presents a line graph with little to no change in TDS, with an initial spike from 157 to 163 ppm. The insoluble carbon remains in the water and shows no benefit.
https://doi.org/10.1371/journal.pone.0257865.g007
The food-grade activated carbon proved no benefit to removing TDS from tap water, and instead added around 5–7 ppm extra, which settled down to around +4 ppm at 120 minutes. The carbon, which is not 100% pure from inorganic compounds and materials present in the carbon, can dissolve into the water, adding to the existing concentration of TDS. Furthermore, household tap water has already been treated in processing facilities using a variety of filters, including carbon, so household charcoal filters are not effective in further reducing dissolved solids [ 18 ].
Adding sodium bicarbonate solution to boiled tap water
As seen in Fig 8 , after adding 1 mg of NaHCO 3 in, the TDS rises to 161 ppm, showing a minuscule increase. When 4 mg was added, the TDS drops down to 158 ppm. Then, when 5 mg was added, a sudden spike to 172 ppm was observed. This means that NaHCO 3 is able to ionize some Ca 2+ and Mg 2+ ions, but also adds Na + back into the water. This also means that adding NaHCO 3 has little to no effect on TDS, with 4mg being the upper limit of effectiveness.
https://doi.org/10.1371/journal.pone.0257865.g008
To examine whether or not the temperature plays a role in the effectiveness in adding NaHCO 3 , a boiling experiment was performed, and the data is graphed in Fig 9 .
https://doi.org/10.1371/journal.pone.0257865.g009
Fig 9 presents the relationship between the amount of common baking soda(NaHCO₃) added, the boiling time involved, and the resulting TDS measurements. After boiling each flask for designated amounts of time, the results showed a downward trend line from a spike but does not reach a TDS value significantly lower than the initial sample. It is apparent that the NaHCO₃ has not lowered the TDS of the boiling water, but instead adds smaller quantities of ions, raising the final value. This additive does not contribute to the lowering of the hardness of the tap water. However, tests boiled with 5 mg/L of baking soda maintained a downward pattern as the water was boiled for an increasing amount of time, compared to the seemingly random graphs of boiling with 10 mg/L.
In some households, however, people often add NaHCO₃ to increase the pH for taste and health benefits. However, as shown in the test results, it is not an effective way of reducing TDS levels in the water [ 10 , 16 ], but instead raises the pH, determined by the concentration added. Even under boiling conditions, the water continues to follow the trend of high growth in TDS, of +25–43 ppm right after boiling and the slow drop in TDS (but maintaining a high concentration) as the particles settle to the bottom.
Utilizing the experimental results, we can summarize that after adding small batches of NaHCO3 and waiting up to 5 minutes will reduce water hardness making it less prone to crystallizing within household appliances such as water brewers. Also, this process raises the pH, which is used more within commercial water companies. However, the cost comes at increasing TDS.
Using electrolysis to treat TDS in tap water
Different voltages were passed through the water to observe the change in TDS overtime, with the data being compiled as Fig 10 .
https://doi.org/10.1371/journal.pone.0257865.g010
The process of electrolysis in this experiment was not to and directly remove the existing TDS, but to separate the water sample into three different areas: the anode, cathode, and an area of clean water between the two nodes [ 19 ]. The anions in the water such as OH - , SO 4 2- , HCO 3 - move to the anode, while the cations such as H + , Ca 2+ 、Mg 2+ 、Na + move to the cathode. The middle area would then be left as an area that is more deprived of such ions, with Fig 10 proving this.
As shown in Fig 10 , electrolysis is effective in lower the TDS within tap water. Despite the lines being extremely tangled and unpredictable, the general trend was a larger decrease with a longer duration of time. At 10 minutes, all lines except 10.5 V are approaching the same value, meaning that the deviation was most likely caused by disturbances to the water during measurement from the low volume of water. With each different voltage test, a decrease of 12.7% for 6.0 V, 14.9% for 9.0 V, 22.7% for 10.5 V. and 19.5% for 12.0 V respectfully were observed. In the treatment of wastewate leachate, a study has shown that with 90 minutes of electrical treatment, 34.58% of TDS content were removed, supporting the effectiveness of electricity and its usage in wastewater treatment [ 29 ].
This experiment concludes that electrolysis is effective in lowering TDS, with the possibility to improve this process by further experimentation, development of a water cleaning system utilizing this cathode-anode setup to process water. This system would be a more specific and limited version of a reverse osmosis system by taking away ions through attraction, rather than a filter.
The Southern Californian tap water supply maintains TDS values below the federal regulations. However, crystalline scale buildup in household appliances is a major issue as it is hard to clean and eliminate. To easily improve the taste and quality of tap water at home as well as eliminating the formation of scales, the following methods were demonstrated as viable:
- By heating water to around 50°C (122°F), TDS and water hardness will decrease the most. Also, the boiling process is effective in killing microorganisms and removing contaminants. This process cannot surpass 10 minutes, as the concentration of the ions in the water is too high, which poses human health risks if consumed. These, along with activated carbon and NaHCO₃ additives, are inefficient methods that have minimal effects for lowering TDS.
- Electrolysis is one of the most effective methods of eliminating TDS. Experiments have proven that increased current and duration of time helps lower TDS. However, this method has yet to be implemented into conventional commercial water filtration systems.
Also, some observations made in these experiments could not be explained, and require further research and experimentation to resolve these problems. The first observation is that TDS and increasing water temperature maintain a parabolic relationship, with a maximum being reached at 80°C, followed by a gradual decrease. The second observation is that when water is boiled for an increased duration of time, the rate of cooling also increases.
This experiment utilized non-professional scientific equipment which are prone to mistakes and less precise. These results may deviate from professionally derived data, and will require further study using more advanced equipment to support these findings.
Acknowledgments
The author thanks Tsinghua University Professor and PLOS ONE editor Dr. Huan Li for assisting in experimental setups as well as data processing and treatment. The author also thanks Redlands East Valley High School’s Dr. Melissa Cartagena for her experimental guidance, and Tsinghua University Professor Dr. Cheng Yang for proofreading the manuscript.
- View Article
- Google Scholar
- 2. United States Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories Tables (EPA 822-F-18-001). US EPA, Washington D.C., USA, 2018; pp. 9–19.
- 3. World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. WHO, Geneva, Switzerland, 2017; pp. 7, 219–230, 423.
- PubMed/NCBI
- 6. Chloride, Salinity, and Dissolved Solids. 2019 Mar 1, [cited on 20 September 2020] Available from: https://www.usgs.gov/mission-areas/water-resources/science/chloride-salinity-and-dissolved-solids?qt-science_center_objects=0#qt-science_center_objects .
- 13. Shoukat, Ammara, Hussain, M., Shoukat, Asra. Effects of Temperature on Total dissolved Solid in water. Water Quality Study Conference, Mehran University Sindh, Pakistan, February 2020.
- 17. United States Environmental Protection Agency. Drinking Water Treatment Plant Residuals Management—Technical Report: Summary of Residuals Generation, Treatment, and Disposal at Large Community Water Systems. US EPA, Washington D.C., USA, 2011; pp. 177–182.
- 19. Exceedance and Compliance Status of Public Water Systems. 2012 Feb 1, [cited 22 September 2020] Available from: https://www.arcgis.com/apps/MapJournal/index.html?appid=143794cd74e344a29eb8b96190f4658b# .
- 20. 2019 Water Quality Status Report California. California Water Boards, 2019 July 1, [cited 22 September 2020] Available from: https://gispublic.waterboards.ca.gov/portal/apps/MapJournal/index.html?appid=6cde29ac0afc4d55b0fdaaae6bfc1aa4 .
- 21. City of Redlands—Water Quality Consumer Confidence Reports. City of Redlands, 2010–2020, [cited 23 September 2020] Available from: https://www.cityofredlands.org/post/water-quality .
- 25. Consumers’ Preference For Bottled Water Is Growing And They Want It Available Wherever Drinks Are Sold. 2019 Jan 8, [cited 23 September 2020] Available from: https://www.bottledwater.org/consumers’-preference-bottled-water-growing-and-they-want-it-available-wherever-drinks-are-sold .
Essay On Water Management
500 words essay on water management.
Water management refers to activities that plan, develop, distribute and manage the optimum use of water resources. Everyone can do this from local authorities to individuals at home. Good water management allows access to safe water for everyone. Through an essay on water management, we will go through it in detail.

Importance of Water Management
Water management impacts various aspects of our lives. As water is common, we do not think much of its management. But, if we ask the deprived people, they will know the importance of water management very well.
As we require drinking water, clean drinking water is a necessity. No human can survive without water. Further, we also need water management for cleaning and washing. For instance, we bathe, wash our clothes and utensils to maintain hygiene .
Further, agriculture requires water for growing the food that we eat every day. Thus, a good water supply becomes essential. Moreover, we also enjoy swimming, boating and other leisure activities in the water.
For instance, swimming pools and more. Thus, water needs to be managed so people can enjoy all this. Most importantly, water management ensures that our rivers and lakes do not contaminate. Thus, it helps maintain biodiversity.
Ways of Water Management
There are various ways available through which we can manage water. The major ways of water management include recycling and treating wastewater. When we treat wastewater , it becomes safe to be piped back to our homes.
Thus, we use it for drinking, washing and more. In addition, an irrigation system is a very good way of water management. It involves a good quality irrigation system which we can deploy for nourishing crops in drought-hit areas.
By managing these systems, we can ensure water does not go to waste and avoid unnecessarily depleting water supplies. Most importantly, conserving water is essential at every level.
Whether it is a big company or a small house, we all must practise water management. The big industries use gallons of water on a daily basis. At homes, we can conserve water by using it less.
Further, it also applies to our way of consumption of products. A large amount of water goes into the production of cars or a simple item like a shirt. Thus, we must not buy things unnecessarily but consciously.
It is also essential to care for natural supplies like lakes, rivers , seas and more. As you know, these ecosystems are home to a variety of organisms. Without its support, they will go extinct. Thus, water management becomes essential to ensure we are not polluting these resources.
It is also crucial to ensure that everyone gets access to enough water. Some parts of the world are completely deprived of clean water while some have it in abundance. This is unfair to those who do not get it which also causes many deaths. Thus, we need water management to avoid all this.
Get the huge list of more than 500 Essay Topics and Ideas
Conclusion of the Essay on Water Management
If we look at the current situation of water depletion, it is evident that we are in dire need of water management. We must come together to do our best to ensure that everyone is getting access to safe water daily so that we can lead happy lives.

FAQ of Essay on Water Management
Question 1: What is meant by water management?
Answer 1: Water management refers to the control and movement of water resources for minimizing damage to life and property. Moreover, it is to maximize effective beneficial use.
Question 2: What are the ways of water management?
Answer 2: There are numerous ways of water management. Some of them are the treatment of wastewater, deploying good irrigation methods, conserving water whenever possible. Further, we must also care for natural sources of water like rivers, seas, lakes and more.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App


25,000+ students realised their study abroad dream with us. Take the first step today
Meet top uk universities from the comfort of your home, here’s your new year gift, one app for all your, study abroad needs, start your journey, track your progress, grow with the community and so much more.

Verification Code
An OTP has been sent to your registered mobile no. Please verify

Thanks for your comment !
Our team will review it before it's shown to our readers.

- School Education /
Essay on Water Conservation: Samples in 150, 200, 250 Words
- Updated on
- Oct 17, 2023

What makes you curious to write an essay on water conservation? This life-saving resource is essential for all forms of life on Earth. Water is the essential natural resource present on Earth. Out of the total water present on Earth, 97.5% is salt water and 2.5% is fresh water. 70% of the human body is made of water. But, with the growing population , and climatic crisis , we are facing the urgent need to conserve water.
Water conservation is a hot topic, if you need a sample essay on water conservation then, you are at the right place. In this blog post, we have covered essays on water conservation in 100, 200, and 250 words. So, stay tuned and read further to get some ideas about water conservation!
This Blog Includes:
Essay on water conservation in 100 words, essay on water conservation in 200 words, water scarcity, ways to conserve water.
Also Read: World Water Day
Water is crucial for all components of life which makes it a necessary resource for day-to-day activities. We use water for domestic activities like cooking, bathing, drinking, washing, etc. So, ultimately the consumption of water is very high. This makes it necessary to conserve water. Just as air, water is also important for life. Besides, water consumption, water pollution, and water scarcity are also some of the major water-related issues that need attention so that we can conserve water.
Every year we celebrate World Water Day on 22 March. This day is celebrated to spread awareness about the importance of water and run campaigns to conserve water on Earth. There are several ways to conserve water such as switching to showers, turning off taps when not in use, don’t pollute water bodies, storing rainwater, etc.
Also Read: Essay on Water Pollution
Water is one of the Earth’s most precious resources. But the world is facing water scarcity. As per the SDA report 2022, around 2 billion people worldwide are lacking safe drinking water. This means they are more vulnerable to diseases and unhealthy life.
Apart from the increasing population, climatic change is also hampering the quality of water. Floods and Droughts are more frequent due to the vulnerability of climate, thereby increasing the need to conserve water.
Water conservation is vital to meet the growing global demand for fresh water. Water consumption is very high for agriculture, industry, and households. By conserving water, we can ensure that there is a surplus amount of water to use and avoid conflicts over this limited resource.
Water conservation helps to maintain a balance in the ecosystem because every living thing on this planet is directly associated with the use of water. Reducing water consumption reduces the energy footprint associated with water supply.
The best ways of water conservation are rainwater harvesting , installing water plants, reusing water for gardening purposes, turning off taps when not in use, proper irrigation, installing automatic tap shut-off devices, not polluting water sources, and many more.
If we don’t want to witness the world die due to water scarcity then, it’s high time to conserve water and save the planet and future generations.
Also Read: Essay on Save Water
Also Read: Speech on Save Water
Water Conservation Essay 250 Words
Water conservation is a crucial step in protecting the environment. It is an important compound that supports life on Earth. The world has been facing water-related disasters due to scarcity of freshwater. 70% of the earth as well as the human body is composed of water, but there is a limited amount of freshwater to use. Owing to the ever-increasing population, climatic changes, global warming, and pollution, the need for the conservation of water is increasing. To do so, it is our fundamental duty to conserve water by planting more trees, managing water plants, storing rainwater, and making smart use of water.
Water scarcity is a critical global issue that needs strict attention when the demand for freshwater exceeds the available supply of water. It can manifest in various ways, including a lack of access to clean drinking water, inadequate water for agriculture and industrial processes, and stressed or depleted natural water sources.
Here are some factors that contribute to water scarcity:
- Climate change
- Growing population
- Global warming
- Inefficient water management
- Water pollution
- Increasing demand
- Poor irrigation techniques
- Wastage of water, and much more.
Conserving water is crucial to help address water scarcity and ensure a sustainable water supply for both present and future generations. You can contribute individually by taking small measures to conserve water like turning off the tap. Likewise, here are some ways to conserve water:
- Drip irrigation technique
- Soil management
- Plantation of drought-tolerant crops
- Apply Mulching
- Recycle and reuse water
- Rainwater harvesting
- Desalination
- Spread awareness to conserve water
- Donate to the water cleaning campaign
- Implement proper water management techniques.
Also Read: Types of Water Pollution
Related Articles:
Water conservation is the individual or collective practice of efficient use of water. This helps in protecting the earth from the situation of water scarcity. We can individually contribute to water conservation by not wasting water, reducing the over-consumption of water, rainwater harvesting, etc. Water conservation is an important call because there is a limited amount of fresh water available on earth.
Here are 10 ways to save water. 1. Rainwater harvesting 2 Install water plants 3. Reuse water 4. Maintain proper water management plans 5. Fix the irrigation system 6. Use a bucket 7. Turn off the tap when not in use 8. Keep a regular check on pipe leakage 9. Do not pollute water bodies 10. Participate in water cleaning campaigns
Here are 5 points on the importance of water conservation: It helps the ecosystem; Water conservation is necessary for drought-prone areas; It helps reduce costs; Water conservation improves the quality of water; and Maintains the health of the aquatic ecosystem.
For more information on such interesting topics, visit our essay writing page and follow Leverage Edu .
Kajal Thareja
Hi, I am Kajal, a pharmacy graduate, currently pursuing management and is an experienced content writer. I have 2-years of writing experience in Ed-tech (digital marketing) company. I am passionate towards writing blogs and am on the path of discovering true potential professionally in the field of content marketing. I am engaged in writing creative content for students which is simple yet creative and engaging and leaves an impact on the reader's mind.
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Contact no. *

Connect With Us

25,000+ students realised their study abroad dream with us. Take the first step today.

Resend OTP in

Need help with?
Study abroad.
UK, Canada, US & More
IELTS, GRE, GMAT & More
Scholarship, Loans & Forex
Country Preference
New Zealand
Which English test are you planning to take?
Which academic test are you planning to take.
Not Sure yet
When are you planning to take the exam?
Already booked my exam slot
Within 2 Months
Want to learn about the test
Which Degree do you wish to pursue?
When do you want to start studying abroad.
January 2024
September 2024
What is your budget to study abroad?

How would you describe this article ?
Please rate this article
We would like to hear more.
Have something on your mind?

Make your study abroad dream a reality in January 2022 with
India's Biggest Virtual University Fair

Essex Direct Admission Day
Why attend .

Don't Miss Out

Essay on Clean Water and Sanitation
Students are often asked to write an essay on Clean Water and Sanitation in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Clean Water and Sanitation
Importance of clean water.
Clean water is vital for life. Every living organism needs it for survival. It helps in digestion, removes toxins, and keeps us hydrated. Without clean water, we risk diseases.
Role of Sanitation
Sanitation is as important as clean water. It prevents the spread of germs, ensuring we stay healthy. Good sanitation practices include proper waste disposal and maintaining cleanliness.
Link Between Clean Water and Sanitation
Clean water and sanitation are interconnected. Contaminated water can lead to poor sanitation, and vice versa. Hence, both are essential for a healthy life.
250 Words Essay on Clean Water and Sanitation
Introduction.
Clean water and sanitation are fundamental components of human health and wellbeing. They are deeply intertwined with socioeconomic development, environmental sustainability, and human dignity.
The Importance of Clean Water
Water is the lifeblood of our planet. It is essential for maintaining biodiversity, facilitating agricultural processes, and supporting human life. However, the quality of this precious resource is threatened by pollution, overexploitation, and climate change. Access to clean water is not just about quenching thirst; it’s about ensuring the health of individuals and communities. Waterborne diseases, often a result of poor water quality, account for substantial morbidity and mortality worldwide.
Sanitation: More than Hygiene
Sanitation extends beyond personal hygiene. It involves the management of human waste, solid waste, and wastewater. Proper sanitation practices reduce the incidence of diseases, enhance the quality of life, and contribute to social and economic development. Inadequate sanitation is a pressing issue in many parts of the world, leading to serious public health crises.
Linking Clean Water and Sanitation
The connection between clean water and sanitation is undeniable. Contaminated water sources due to poor sanitation practices can lead to the spread of diseases like cholera, dysentery, and typhoid. Therefore, efforts to improve water quality must go hand in hand with improving sanitation facilities.
The challenges surrounding clean water and sanitation are formidable, but not insurmountable. Through concerted efforts from governments, communities, and individuals, we can ensure access to these fundamental human rights for everyone, thereby paving the way for a healthier, more sustainable world.
500 Words Essay on Clean Water and Sanitation
Clean water and sanitation are fundamental to human health and well-being. Despite being recognized as a human right by the United Nations, millions of people worldwide still lack access to these basic necessities. The importance of clean water and sanitation cannot be overstated, as they play a crucial role in preventing disease, promoting health, and improving overall quality of life.
Water is a vital resource for all forms of life. However, clean and safe drinking water is not universally available. Contaminated water can transmit diseases such as diarrhea, cholera, dysentery, typhoid, and polio, leading to significant morbidity and mortality, particularly in developing countries. Furthermore, the lack of clean water can impede social and economic development, as individuals may spend significant time and effort obtaining water, rather than engaging in productive activities or education.
The Necessity of Sanitation
Sanitation, the provision of facilities and services for the safe disposal of human waste, is equally important. Poor sanitation can lead to the contamination of drinking water sources and the environment, resulting in a range of health problems. Moreover, inadequate sanitation facilities can compromise personal safety and dignity, particularly for women and girls. Improved sanitation contributes to social development by enhancing people’s living conditions and dignity, and to economic development by reducing healthcare costs and improving productivity.
Challenges and Solutions
Despite the critical importance of clean water and sanitation, numerous challenges hinder universal access. These include inadequate infrastructure, lack of funding, and insufficient awareness about the importance of hygiene. Addressing these challenges requires concerted efforts from governments, non-governmental organizations, and communities.
Infrastructure development is crucial for providing clean water and sanitation facilities, particularly in rural and marginalized areas. This includes building water purification systems, sewage treatment plants, and toilets. However, such initiatives require significant financial resources. Therefore, increased investment from both public and private sectors is necessary.
Education and awareness programs can also play a vital role in improving water and sanitation conditions. By educating communities about the importance of hygiene and the risks associated with contaminated water and poor sanitation, we can encourage behavior change and promote the utilization of water and sanitation facilities.
In conclusion, clean water and sanitation are not just basic human needs, but they are also fundamental human rights. Despite the challenges, achieving universal access to clean water and sanitation is possible through infrastructure development, increased funding, and education. By ensuring everyone has access to these basic services, we can significantly improve global health, foster social and economic development, and ultimately, create a more equitable world.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Challenges of Clean Water and Their Solutions
- Essay on Bottled Water
- Essay on Blood is Thicker Than Water
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Water Quality & Drinking Water Treatment Exploratory Essay
The new york city water report, water treatment plant configuration, steps involved in the water treatment.
This reflective report analyses New York City’s drinking water quality and the treatment process. The paper identifies the contaminants in New York City water and analyzes these contaminants through a broad spectrum approach.
The fluoride contaminant violated the maximum requirement level in the New York drinking water. The fluoride content of 2.2 mg/L in this water is almost double the concentration level of 1.0mg/L as situated by the New York City Health Code.
The calcium contaminant was very close to violating the maximum contaminant levels in the New York City drinking water. The concentration of calcium contaminant was recorded at 5.3 mg/L against an average of between 4.4 and 6.7 mg/L.
Contaminant detected: Nitrate
Water in its natural source is often subject to fecal contamination, primarily derived from processes of decomposition of organic nitrogenous material present in water.
While ammonia (ammonium) and nitrites indicate an organic contamination, there are several harmless contaminants present in water such iron and calcium among others. The diagram below represents a typical water treatment plant summarizing the processes involved in water treatment.
Schematic water treatment diagram
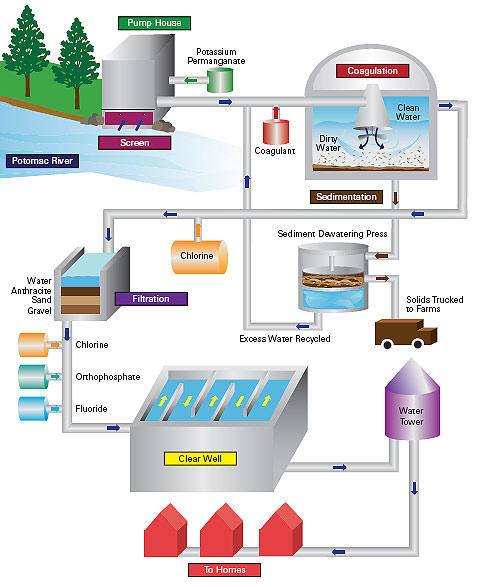
Step 1: Screening
The raw water is passed through a sieving screen to eliminate relatively large pieces of foreign material such as rocks, leaves, and sticks. At this stage, Potassium permanganate chemical may be used when the raw water has traces of algal bloom.
Step 2: Coagulation
This involves passing the raw water into a coagulation tank where visible particles that remained after the screening stage are separated and channeled to an ejection tank for farm use.
Step 3: Sedimentation
The water is then moved to a special tank that is designed to allow for any remaining particle in the water to patch up at the base of the sediment tank.
Step 4: Filtration
The dual media in the filtration tank consisting of anthracite and sand ensures that all the visible pollutants are eliminated.
Step 5: Disinfection
Regulated amount of chlorine is passed into the water to inactivate any pathogens that might have passed through the previous steps. At this stage, controlled quantity of fluoride ingredient is added to the disinfected water to reduce incidences of tooth discoloration and decay upon use of this water.
Step 6: Storage and distribution
The fully treated water is then channel to storage tanks for a while before being distributed to the final user.
Water treatment is carried out by using special purification equipments that measured the 4 parameters of contamination (nitrates, phosphates, chlorides and sulfur). However, the concentration of each parameter detected varies due to the conditions and flow of water from its source. There are significant differences between the parameters, pH, temperature and dissolved oxygen in different water sources.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, December 19). Water Quality & Drinking Water Treatment. https://ivypanda.com/essays/water-quality-drinking-water-treatment/
"Water Quality & Drinking Water Treatment." IvyPanda , 19 Dec. 2023, ivypanda.com/essays/water-quality-drinking-water-treatment/.
IvyPanda . (2023) 'Water Quality & Drinking Water Treatment'. 19 December.
IvyPanda . 2023. "Water Quality & Drinking Water Treatment." December 19, 2023. https://ivypanda.com/essays/water-quality-drinking-water-treatment/.
1. IvyPanda . "Water Quality & Drinking Water Treatment." December 19, 2023. https://ivypanda.com/essays/water-quality-drinking-water-treatment/.
Bibliography
IvyPanda . "Water Quality & Drinking Water Treatment." December 19, 2023. https://ivypanda.com/essays/water-quality-drinking-water-treatment/.
- Nitrite-Indole Reaction: Spectrophotometric Study
- Disseminated Intravascular Coagulation
- Dye Wastewater Purification Using Coagulation
- Fluoride Varnish and Foams and Gels Preventing Cavities
- Reclamation of Grey Water & Refinery Oily Wastewater Using Bioprocesses Treatment
- Fluoride in Drinking Water, Its Costs and Benefits to Oral Health
- Chemicals Used for Microbial Preservation of Food
- Vitamin D, Round up Glyphosate, Nicotine and Sodium Nitrate Toxicity
- Acid Rain Investigation: Problem and Hypothesis
- Fluoride-Containing Varnishes Development and Application in Dentistry
- The Renewable and Non-renewable Electricity Sources
- Electricity Production and Consumption in the US
- Demand for Energy. Energy Sources
- Vulnerability of World Countries to Climate Change
- Aquatic Life in Indiana
To gate or not to gate: Revisiting drinking water microbial assessment through flow cytometry fingerprinting
- Claveau, L.
- Jeffrey, P.
- Hassard, F.
Flow cytometry has been utilized for over a decade as a rapid and reproducible approach to assessing microbial quality of drinking water. However, the need for specialized expertise in gating-a fundamental strategy for distinguishing cell populations-introduces the potential for human error and obstructs the standardization of methods. This work conducts a comprehensive analysis of various gating approaches applied to flow cytometric scatter plots, using a dataset spanning a year. A sensitivity analysis is carried out to examine the impact of different gating strategies on final cell count results. The findings show that dynamic gating, which requires user intervention, is essential for the analysis of highly variable raw waters and distributed water. In contrast, static gating proved suitable for more stable water sources, interstage sample locations, and water presenting a particularly low cell count. Our conclusions suggest that cell count analysis should be supplemented with fluorescence fingerprinting to gain a more complete understanding of the variability in microbial populations within drinking water supplies. Establishing dynamic baselines for each water type in FCM monitoring studies is essential for choosing the correct gating strategy. FCM fingerprinting offers a dynamic approach to quantify treatment processes, enabling options for much better monitoring and control. This study offers new insights into the vagaries of various flow cytometry gating strategies, thereby substantially contributing to best practices in the water industry. The findings foster more efficient and reliable water analysis, improving of standardizing methods in microbial water quality assessment using FCM.
- Flow cytometry;
- Drinking water;
- Cell gating;
- Fluorescence fingerprinting;
- Public health

IMAGES
VIDEO
COMMENTS
Water Purification Conclusion. 1. Reason for the process. Water plays such an important role in our daily lives. 70% of our body is composed of water. 70% of the earth surface is also made up of water, but out of the 70%, only 1/3 of water is consumable. In fact, this amount has been continuously to decrease as more and more industries began to ...
water purification, process by which undesired chemical compounds, organic and inorganic materials, and biological contaminants are removed from water.That process also includes distillation (the conversion of a liquid into vapour to condense it back to liquid form) and deionization (ion removal through the extraction of dissolved salts). One major purpose of water purification is to provide ...
Water purification involves physical and chemical processes, which are carried out stepwise to ensure the water is safe and free from any harm. This directional process essay synthesizes the steps, which have to be followed to achieve this task. In essence, water purification denotes the process used to free water from impurities like bacteria ...
Download. Essay, Pages 9 (2030 words) Views. 10327. Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids and gases from contaminated water. The goal is to produce water fit for a specific purpose. Most water is purified for human consumption (drinking water), but water purification may ...
Several treatment technologies, as well as water treatment materials, have now been developed. Nevertheless, the practical application value in water treatment is often ignored. This is not consistent with the sustainable develop-ment of water resources. Many studies focus on developing something new, like "to write new versus with a false ...
Water purification. Control room and schematics of the water purification plant of Lac de Bret, Switzerland. Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected ...
Ozcan Konur, in Water Purification, 2017. 3 Conclusions. The area of water purification has been one of the most dynamic research fields in recent years with strong public policy implications with over 39,000 papers. Similarly, the areas of nanomaterials and nanoprocesses have been one of the most dynamic research fields in recent years with ...
Infrastructure Development: Investment in water infrastructure, including water treatment plants, distribution systems, and wastewater treatment, is vital for providing clean and safe water to communities. ... Origin Of Water Essay. In conclusion, the origin of water remains an intriguing and complex puzzle. While the cometary delivery ...
This requires investment in water purification technologies. World Water Day offers an opportunity to discuss whether such investment will help achieve this laudable goal. ... In conclusion, the ...
These are the initial procedures during treatment of water. Chemical substances possessing a positive charge are added to water in this compartment. The positive charge neutralizes the negative charge from dirt leading to the formation of huge fragments known as floc. We will write a custom essay on your topic. 809 writers online.
Essay About Water Purification. This essay sample was donated by a student to help the academic community. Papers provided by EduBirdie writers usually outdo students' samples. Purifying seawater has many benefits. Among those include providing potable water for third world countries, adequate amounts of agricultural water supply, and the ...
In your essay about water, you might want to focus on water as one of the most valuable natural resources. Consider exploring the issues of water pollution, purification, conservation, or management. Whether you need to prepare an essay, a research paper, or a presentation, our article will be helpful. Here we've collected water essay topics ...
United States FDA approved it in the top 4 techniques to purify water. Ultra Violet Water Purification proved to be quick, cost effective and reliable method of purifying water. UV is safe, clean and easy method. It uses Ultra Violet light to kill microorganisms. This technology is already proven with no drawbacks.
6210 Words. 25 Pages. Open Document. Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids and gases from contaminated water. The goal is to produce water fit for a specific purpose. Most water is purified for human consumption (drinking water), but water purification may also be designed ...
This study, based in San Bernardino County, Southern California, collected and examined tap water samples within the area to explore the feasibility of adopting non-industrial equipment and methods to reduce water hardness and total dissolved solids(TDS). We investigated how water quality could be improved by utilizing water boiling, activated carbon and sodium bicarbonate additives, as well ...
Answer 2: There are numerous ways of water management. Some of them are the treatment of wastewater, deploying good irrigation methods, conserving water whenever possible. Further, we must also care for natural sources of water like rivers, seas, lakes and more. Share with friends. Previous.
In conclusion, a treatment can purify drinking water largely than city treatment plants and distillation, this option, made a successful at removing some contaminants, it is expensive and wasteful, but it is safe. The perfect technology for treating water and removing unwanted contaminants is water filtration.
River/Lake Water Purification Process Distillation. This technology involves heating water to boiling point. The water vapor which is given out is directed to a condenser where cooling water acts on it to bring down the temperature and cause it to condense, whereupon it is gathered and stored.
Water Conservation Essay 250 Words. Water conservation is a crucial step in protecting the environment. It is an important compound that supports life on Earth. The world has been facing water-related disasters due to scarcity of freshwater. 70% of the earth as well as the human body is composed of water, but there is a limited amount of ...
Linking Clean Water and Sanitation. The connection between clean water and sanitation is undeniable. Contaminated water sources due to poor sanitation practices can lead to the spread of diseases like cholera, dysentery, and typhoid. Therefore, efforts to improve water quality must go hand in hand with improving sanitation facilities.
Water Purification Conclusion. 2018 Words9 Pages. Introduction. For this research task I have chosen compare various methods of water purification in a household as researching the health and environmental factors. I will look at the following sources of water: Oasis purified water, bottled water and the municipal tap water (Municipal- muzunduzi).
This review paper focuses on the various types of Filtration techniques. available and whic h tec hnique is most suitable for which t ype of region. W e. will study the t ypes of natural ...
Water treatment is carried out by using special purification equipments that measured the 4 parameters of contamination (nitrates, phosphates, chlorides and sulfur). However, the concentration of each parameter detected varies due to the conditions and flow of water from its source. There are significant differences between the parameters, pH ...
Our conclusions suggest that cell count analysis should be supplemented with fluorescence fingerprinting to gain a more complete understanding of the variability in microbial populations within drinking water supplies. Establishing dynamic baselines for each water type in FCM monitoring studies is essential for choosing the correct gating strategy.