Basics of HSV (Herpes Simplex Virus) Keratitis
What is hsv (herpes simplex virus) keratitis.
HSV (Herpes Simplex Virus) keratitis is an infection of the cornea—the clear dome that covers the colored part of the eye—that is caused by HSV. The infection usually heals without damaging the eye, but more severe infections can lead to scarring of the cornea or blindness. HSV keratitis is a major cause of blindness worldwide 1 . HSV-1, which is the type of HSV that also causes cold sores on the mouth, is the most common cause of corneal infections.

What are the symptoms of HSV keratitis?
Symptoms of HSV keratitis include 2 :
- Eye redness
- Blurred vision
- Sensitivity to light
- Watery discharge
If you experience any of these symptoms, remove your contact lenses (if you wear them) and call your eye doctor right away. If left untreated, HSV keratitis can result in vision loss or blindness.
Where is HSV found?
HSV is only found in humans and is spread through direct contact with someone who is infected with the virus 3 . Most HSV keratitis infections happen after another part of the body—most commonly the mouth 4 —has already been infected by HSV 5 . HSV keratitis is often the result of a “flare up” (reactivation) of the earlier infection.
What puts people at risk for HSV keratitis?
People who have had HSV keratitis are at risk for recurrences of the same infection 2 . For these people, wearing contact lenses may further increase the risk 6 .
People most at risk for HSV-1 (but not necessarily HSV keratitis) are 7 :
- Non-Hispanic black or Mexican American
- Born outside the United States
- Sexually active, or have had 3 or more lifetime sex partners
How is HSV keratitis diagnosed?
HSV keratitis is usually diagnosed based on a patient’s health history and findings from an eye exam. Lab testing is not usually necessary, but certain lab tests may further help to confirm HSV-1.
How is HSV keratitis treated?
The treatment of HSV keratitis usually involves medicine, including eye drops or antiviral medications taken by mouth 4 . Surgery is rarely necessary but may be considered if scarring on the eye from HSV keratitis causes vision problems. Each case of HSV keratitis is unique, and an eye doctor should determine the best treatment for each patient. While some treatments can greatly lower the severity and recurrence of symptoms, there is no cure for HSV.
How do you prevent HSV keratitis?
Currently there are no proven methods for preventing HSV keratitis, but some steps available from the Mayo Clinic may help to control HSV keratitis recurrences:
- Avoid touching your eyes or the area around your eyes unless you have washed your hands properly—especially if you have a cold sore or herpes blister.
- Only use eye drops that have been prescribed or recommended by an eye doctor or health care provider 8 .
Follow these tips to keep your eyes healthy while wearing contact lenses. Your daily habits, your contact lenses and supplies, and your eye doctor are all important.
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance . Cornea. 2001;20(1):1-13.
- Welder JD, Kitzmann AS, Wagoner MD. Herpes Simplex Keratitis. EyeRounds.org.
- Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy . Am J Ophthalmol. 2006;141(3):547-57.
- Kaye S, Choudhary A. Herpes simplex keratitis . Prog Retin Eye Res. 2006;25(4):355-80.
- Kennedy DP, Clement C, Arceneaux RL, Bhattacharjee PS, Huq TS, Hill JM. Ocular herpes simplex virus type 1: is the cornea a reservoir for viral latency or a fast pit stop? Cornea. 2011;30(3):251-9.
- Mucci JJ, Utz VM, Galor A, Feuer W, Jeng BH. Recurrence rates of herpes simplex virus keratitis in contact lens and non-contact lens wearers . Eye Contact Lens. 2009;35(4):185-7.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of Herpes Simplex Virus Types 1 and 2–United States, 1999-2010 . J Infect Dis. 2013.
- Mayo Clinic. Keratitis. Diseases and conditions 2012.
To receive email updates about this topic, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Patient Care & Health Information
- Diseases & Conditions
Diagnosing keratitis typically involves the following:
- Eye exam. Although it may be uncomfortable to open your eyes for the exam, it's important to have your eye care provider examine your eyes.
- Penlight exam. Your eye doctor may examine your eye using a penlight, to check your pupil's reaction, size and other factors. A stain may be applied to the surface of your eye. Used with the light, this stain makes it easier to see damage to the surface of the cornea.
- Slit-lamp exam. Your eye care provider will examine your eyes with a special instrument called a slit lamp. It provides a bright source of light and magnification to detect the character and extent of keratitis, as well as the effect it may have on other structures of the eye.
- Laboratory analysis. Your eye care provider may take a sample of tears or some cells from your cornea for laboratory analysis to determine the cause of keratitis and to help develop a treatment plan for you.
Noninfectious keratitis
Treatment of noninfectious keratitis varies depending on the severity. For example, with mild discomfort from a corneal scratch, artificial tear drops may be the only treatment. However, if keratitis is causing significant tearing and pain, topical eye medications may be necessary.
Infectious keratitis
Treatment of infectious keratitis varies, depending on the cause of the infection.
- Bacterial keratitis. Antibiotic eye drops are the primary treatment for bacterial keratitis. Depending on the severity of the infection, drop frequency can range from around four times a day to every 30 minutes, even during the night. Sometimes oral antibiotics are used as a supplement.
- Fungal keratitis. Keratitis caused by fungi typically requires antifungal eye drops and oral antifungal medication.
- Viral keratitis. If a virus is causing the infection, antiviral eye drops and oral antiviral medications may be effective. Other viruses need only supportive care such as artificial tear drops.
- Acanthamoeba keratitis. Keratitis caused by the parasite acanthamoeba can be difficult to treat. Antiparasitic eye drops are used, but some acanthamoeba infections are resistant to medication and can require treatment for several months. Severe cases of acanthamoeba keratitis may require a cornea transplant.
If keratitis doesn't respond to medication, or if it causes permanent damage to the cornea that significantly impairs your vision, your eye care provider may recommend a cornea transplant.
Preparing for your appointment
You may start by seeing or calling your health care provider if you have eye-related symptoms that worry you. Depending on the type and severity of your symptoms, your provider may refer you to an eye specialist, called an ophthalmologist.
What you can do
- Be aware of any pre-appointment restrictions when you make the appointment. Ask if there's anything you need to do in advance, such as stop wearing contact lenses or stop using eye drops.
- Write down any symptoms you're experiencing, including any that may seem unrelated to the reason for which you scheduled the appointment.
- Make a list of all medications, including vitamins and supplements that you're taking.
- Write down questions to ask during your appointment.
Your time is limited, so preparing a list of questions can help you make the most of your appointment. For keratitis, some basic questions to ask include:
- What is likely causing my symptoms?
- What are other possible causes for my symptoms?
- What kinds of tests do I need?
- What is the best course of action?
- What are the alternatives to the approach you're suggesting?
- I have other health conditions. How can I best manage them together?
- Are there any restrictions that I need to follow?
- Should I see a specialist? Is there a generic alternative to the medicine you're prescribing?
- Are there any brochures or other printed material that I can take with me? What websites do you recommend?
- What will determine whether I need to be seen for a follow-up visit?
In addition to the questions you've prepared, don't hesitate to ask other questions anytime you don't understand something.
What to expect from your doctor
Your provider is likely to ask you a number of questions, including:
- When did you begin experiencing symptoms?
- Have your symptoms been continuous or occasional?
- How severe are your symptoms?
- What, if anything, seems to improve your symptoms?
- What, if anything, appears to worsen your symptoms?
- Has your eye been injured recently?
- Have you been swimming or been in a hot tub recently?
- Do your symptoms affect one eye or both eyes?
- Do you use contact lenses?
- Do you sleep in your contact lenses?
- How do you clean your contact lenses?
- How often do you replace your contact lens storage case?
- Have you had a similar problem in the past?
- Are you using eye drops now or have you used any recently?
- How is your general health?
- Have you ever had a sexually transmitted infection?
- Are you taking prescription medications or supplements?
- Have you recently changed the type of cosmetics that you are using?
- What is a corneal ulcer (keratitis)? American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/corneal-ulcer. Accessed Aug. 25, 2022.
- Yanoff M, et al., eds. Bacterial keratitis. In: Ophthalmology. 5th ed. Elsevier; 2019. https://www.clinicalkey.com. Accessed Aug. 25, 2022.
- Basics of bacterial keratitis. Centers for Disease Control and Prevention. https://www.cdc.gov/contactlenses/bacterial-keratitis.html. Accessed Aug. 25, 2022.
- Cherry JD, et al., eds. Ocular infections. In: Feigin and Cherry's Textbook of Pediatric Infectious Diseases. 8th ed. Philadelphia, Pa.: Elsevier; 2019. https://www.clinicalkey.com. Accessed Aug. 25, 2022.
- Jacobs DS. The red eye: Evaluation and management. https://www.uptodate.com/contents/search. Accessed Aug. 25, 2022.
- Austin A, et al. Update on the management of infectious keratitis. Ophthalmology. 2017; doi:10.1016/j.ophtha.2017.05.012.
- What is bacterial keratitis? American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/what-is-bacterial-keratitis. Accessed Aug. 25, 2022.
- Basics of HSV (herpes simplex virus) keratitis. Centers for Disease Control and Prevention. https://www.cdc.gov/contactlenses/viral-keratitis.html. Accessed Aug. 25, 2022.
- Chodnicki KD (expert opinion). Mayo Clinic. Aug. 26, 2022.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
- Symptoms & causes
- Diagnosis & treatment
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 07 January 2021
Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance
- Darren Shu Jeng Ting ORCID: orcid.org/0000-0003-1081-1141 1 , 2 na1 ,
- Charlotte Shan Ho ORCID: orcid.org/0000-0003-1131-1448 2 na1 ,
- Rashmi Deshmukh 2 ,
- Dalia G. Said 1 , 2 &
- Harminder S. Dua ORCID: orcid.org/0000-0002-4683-6917 1 , 2
Eye volume 35 , pages 1084–1101 ( 2021 ) Cite this article
20k Accesses
175 Citations
22 Altmetric
Metrics details
- Corneal diseases
Epidemiology
- Risk factors
A Correction to this article was published on 11 May 2021
This article has been updated
Corneal opacity is the 5th leading cause of blindness and visual impairment globally, affecting ~6 million of the world population. In addition, it is responsible for 1.5–2.0 million new cases of monocular blindness per year, highlighting an ongoing uncurbed burden on human health. Among all aetiologies such as infection, trauma, inflammation, degeneration and nutritional deficiency, infectious keratitis (IK) represents the leading cause of corneal blindness in both developed and developing countries, with an estimated incidence ranging from 2.5 to 799 per 100,000 population-year. IK can be caused by a wide range of microorganisms, including bacteria, fungi, virus, parasites and polymicrobial infection. Subject to the geographical and temporal variations, bacteria and fungi have been shown to be the most common causative microorganisms for corneal infection. Although viral and Acanthamoeba keratitis are less common, they represent important causes for corneal blindness in the developed countries. Contact lens wear, trauma, ocular surface diseases, lid diseases, and post-ocular surgery have been shown to be the major risk factors for IK. Broad-spectrum topical antimicrobial treatment is the current mainstay of treatment for IK, though its effectiveness is being challenged by the emergence of antimicrobial resistance, including multidrug resistance, in some parts of the world. In this review, we aim to provide an updated review on IK, encompassing the epidemiology, causative microorganisms, major risk factors and the impact of antimicrobial resistance.
角膜混浊是全球致盲和视力障碍的第五大原因, 世界范围内大约600万人受其影响。此外, 角膜混浊每年导致的单眼失明约150-200万例, 突显其对人类健康造成的持久性负担。在感染、创伤、炎症、变性和营养缺乏等所有的致病因素中, 感染性角膜炎 (IK) 是发达国家和发展中国家角膜病致盲的主要原因, 大概每100000人口中2.5-799人罹患此病。IK可由多种微生物引起, 包括细菌、真菌、病毒、寄生虫和多重感染。受地理和时间变化的影响, 细菌和真菌已被证明是角膜感染最常见的病原微生物。虽然病毒性角膜炎和棘阿米巴角膜炎并不常见, 但在发达国家, 它们是角膜病致盲的重要原因。接触镜的佩戴、外伤、眼表疾病、眼睑疾病以及眼部手术已证实是IK的主要危险因素。广谱抗生素是目前IK治疗的主要选择, 但是在世界某些地区, 其有效性正在受到抗菌药物耐药性的挑战, 其中包含多重耐药性等。本篇综述旨在提供有关IK的最新进展, 包括流行病学、病原微生物、主要危险因素以及抗生素耐药性对于疗效的影响。
Similar content being viewed by others

Molecular mechanisms of antibiotic resistance revisited

Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics

Introduction
Corneal opacity represents the 5th leading cause of blindness globally, accounting for ~3.2% of all cases [ 1 ]. The recent World Health Organisation (WHO) report highlighted that ~6 million of the world population are affected by cornea-related blindness or moderate/severe visual impairment, including 2 million of those who are affected by trachoma [ 1 , 2 ]. In addition, corneal opacity is estimated to be responsible for 1.5–2.0 million cases of unilateral blindness annually, highlighting an ongoing unchecked burden on human health [ 3 , 4 ].
Any significant insult to the cornea such as infection, trauma, inflammation, degeneration, or nutritional deficiency can result in corneal opacity with visual impairment. Among all, infectious keratitis (IK) has been shown to be the most common cause for corneal blindness in both developed and developing countries [ 5 ]. According to a nationwide study, IK was shown to be the most common cause of all corneal blindness in China, primarily attributed to increased risk of trauma, low socioeconomic status and illiteracy [ 6 ]. IK is a common yet potentially vision-threatening ophthalmic condition, characterised by acute ocular pain, decreased vision, corneal ulceration, and/or stromal infiltrates [ 5 ]. Previously, it has been recognised as a “silent epidemic” in the developing world [ 3 ], and recently, a consortium-led proposal has suggested the designation of IK as a “neglected tropical disease (NTD)” [ 7 ], adding on to the list of NTDs in ophthalmology (i.e. trachoma, onchocerciasis and leprosy). The proposal to attain status of an NTD aims to draw concerted global effort to tackle IK in under-resourced tropical countries, to ameliorate the societal and humanistic burden of IK.
IK can be caused by a wide variety of pathogens including bacteria, fungi, protozoa and viruses. In addition, polymicrobial infection has shown to be accountable for ~2–15% of all IK cases [ 8 , 9 , 10 , 11 ]. As the ocular surface is equipped with highly regulated innate and adaptive defense mechanisms [ 12 ], IK rarely occurs in the absence of predisposing factors such as contact lens (CL) wear, trauma, ocular surface diseases (OSDs), and post-corneal surgery, which are some of the common risk factors implicated in IK [ 13 ].
IK not only causes visual impairment, but also negatively impacts on the quality of life (QOL) of the affected individuals. A study from Uganda reported that IK affected both vision-related QOL (attributed to vision loss) and health-related QOL (attributed to pain in the acute phase) [ 14 ]. The psychological impact on these patients was related to the fear of losing the eye and the social stigma attached. Even when the visual recovery was complete, the individuals affected by IK displayed a lower QOL score than the unaffected controls [ 14 ]. Apart from the impact on the individuals which can affect their economic productivity, IK is also responsible for a huge economic burden on society. According to a report in 2010, the US spent an estimated 175 million dollars on the treatment of IK [ 15 ]. Furthermore, complications of IK such as corneal perforations and scarring form the major indications of corneal transplants in developing countries such as India, Thailand and China [ 13 ], placing additional burden on the limited pool of donor corneas.
Considering that most parts of the world affected by IK are under-resourced, it is highly likely that the actual burden of IK is underestimated due to the lack of surveillance and under-reporting. In view of the global burden of IK, this review aims to provide an updated and comprehensive overview of the epidemiology, causative microorganisms, risk factors and the impact of antimicrobial resistance in relation to IK.
B.1. Incidence
To date, there are limited studies available in the literature that examined the incidence of IK and the majority of studies were conducted more than a decade ago [ 5 ]. Depending on the geographical location and study design, the incidence of IK has been estimated to be in the range of 2.5–799 cases per 100,000 population/year [ 16 , 17 ], particularly more prevalent in the low-income countries. Previous IK studies reported an estimated incidence of 2.5–27.6 per 100,000 population-year in the US [ 16 , 18 ] and 2.6–40.3 per 100,000 population-year in the UK [ 19 , 20 ]. Our recent Nottingham IK Study concurred with the findings of these older studies. We observed a relatively stable incidence of 34.7 per 100,000 population-year in Nottingham, UK, between 2007 and 2019 [ 8 ], highlighting a persistent burden of IK in the developed countries. Another recent study conducted in Australia similarly demonstrated a low IK incidence of 6.6 per 100,000 population-year during the period of 2005–2015 [ 21 ]. However, it is noteworthy that the incidence reported in these two studies is likely to be underestimated as the numbers were based on IK patients who underwent corneal scraping.
In contrast, a substantially higher rate of IK has been reported in under-resourced countries such as South India (113 per 100,000 population-year) [ 22 ] and Nepal (799 per 100,000 population-year) [ 17 ]. The higher incidence observed in these regions was primarily attributable to the poorer environmental and personal hygiene, lower level of education, agricultural industry, increased risk to work-related corneal trauma and poorer access to sanitation and healthcare facility.
The epidemiological patterns and risk factors have been found to vary with demographic factors such as age, gender and socioeconomic status. A tabulated summary of the demographic factors and microbiological profiles of IK is provided in Table 1 [ 8 , 9 , 10 , 13 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ].
IK has been shown to affect individuals across all age groups. Based on large-scale studies (>500 patients), IK most commonly affected people aged between 30 and 55 years (Table 1 ) [ 8 , 9 , 10 , 13 , 21 , 24 , 25 , 29 , 31 , 35 , 37 , 39 , 42 , 43 ], primarily attributed to the underlying risk factors such as CL wear and ocular trauma associated with the working age group. Patients affected by trauma-related IK secondary to agricultural products and foreign bodies are usually around 45–55 years old [ 18 , 44 ]. The employed workforce of some developing countries is mainly composed of farmers and manual labourers, rendering them more susceptible to IK of traumatic aetiology [ 13 , 45 ]. On the other hand, patients affected by CL-related IK are usually between 25 and 40 years old [ 18 , 44 , 46 , 47 ].
Although prevalence of IK is generally low in the extremes of age [ 18 , 48 , 49 , 50 , 51 ], IK may serve as a major contributor to childhood blindness in some countries. For instance, IK was shown to be the second most common cause of visual impairment in children aged <15 years in Uganda [ 52 ]. Ophthalmia neonatorum, defined as conjunctivitis occurring in newborns within 28 days of life, is another important cause of childhood corneal blindness in developing countries, particularly when it is affected by Neisseria gonorrhoea where bilateral ocular involvement is common [ 4 ].
In addition, some studies have demonstrated that elderly patients affected by IK were associated with poor visual outcome (around 40–75% with visual acuity of <6/60) and higher rate of complications such as corneal melting, perforation and loss of eye (i.e. evisceration or enucleation) [ 11 , 53 , 54 ]. This might be related to the higher rate of ocular co-morbidities and the delay in presentation and/or diagnosis of IK as elderly patients are usually dependent on spouse or family when seeking medical care and they may relate their condition to “normal” age-related changes [ 55 , 56 ].
B.3. Gender
The majority of studies did not observe any gender predilection in IK (Table 1 ). However, when gender difference or predominance exists, it is usually attributed to the underlying risk factors in different regions. For instance, CL-related IK has been shown to exhibit a female predominance of 57–69% [ 18 , 44 , 46 , 57 ], whereas trauma-related IK is associated with a male predominance of 74–78% [ 18 , 44 , 46 ], correlating with a high male prevalence (58–75%) of IK in the under-resourced regions such as South America [ 29 , 32 ], Asia [ 13 , 45 , 49 , 58 ], and Africa [ 51 , 59 , 60 ]. Interestingly, a study in Nepal [ 49 ] found that there are significantly more male than female patients across all the age groups. This might be due to a combination of higher rate of trauma, lower number of CL wear, and reduced opportunities among the females to access medical services due to cultural customs.
B.4. Socioeconomic status and level of education
Low socioeconomic status has been shown to increase the risk of developing IK, primarily attributed to poor education, lack of ocular protection and personal hygiene, and limited access to eye care in rural communities [ 6 , 13 , 45 , 51 , 61 ]. In Asia and Africa, amongst those who were diagnosed with IK, ~45–71% of the patients were illiterate and 62–79% of them resided in rural areas with a poorer access to healthcare facilities [ 51 , 60 , 62 ]. In addition, it was found that farmers, rural residents and illiterates were at a higher risk of refractory IK with poorer outcomes [ 51 ].
In some countries such as Nigeria and Malawi, residents in rural communities were shown to be more likely to self-medicate or approach village healers for traditional eye medicine [ 59 , 63 ]. Although it would be unfair to conclude that all therapies performed by traditional healers are inimical, common beliefs or practises of applying breast milk or plant products directly to the eye may actually worsen their keratitis [ 63 ]. In addition, patients who had prior use of traditional eye medicine tended to present later to the eye care professionals, resulting in delayed treatment and poorer visual outcome [ 63 ]. Another study conducted in Nepal reported almost half of the patients with keratitis did not use any medication, self-medicated or treated with undocumented medicine [ 61 ].
Causative microorganisms
A wide range of microorganisms, including bacteria, fungi, protozoa (particularly Acanthamoeba), and viruses, are capable of causing IK. Recently, Ung et al [ 5 ]. have provided a comprehensive summary of the literature concerning the causative microorganisms of IK (up to June 2018). In view of the recent growing literature, this section aimed to summarise the evidence based on large IK studies (>500 sample size) published during 2010–2020 (Table 1 ) [ 8 , 9 , 10 , 13 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ].
C.1. Bacteria
Bacteria are commonly categorised into Gram-positive and Gram-negative bacteria based on the difference in the compositions of bacterial cell envelope. In addition to the universal structure of inner/cytoplasmic membrane, Gram-positive bacteria possess a thick outer cell wall, which is composed of layers of peptidoglycan interspersed with teichoic acids and lipotechoic acids, whereas Gram-negative bacteria consist of a thin middle-layer peptidoglycan and an additional outer membrane primarily made of lipopolysaccharide, which has been shown to play an important role in the pathogenesis of infection (including IK) and the contribution to host inflammatory responses [ 64 , 65 ].
Bacterial keratitis represents the most common type of IK in most regions, including the UK (91–93%) [ 8 , 9 , 10 , 24 ], North America (86–92%) [ 25 ], South America (79–88%) [ 29 , 30 , 31 ], Middle East (91.8%) [ 42 ], and Australasia (93–100%) [ 21 , 43 ]. In terms of specific bacterial strains, coagulase negative staphylococci (CoNS), which are a group of common ocular commensal [ 66 ], were shown to be the most commonly isolated organisms (24–46%) in about half of the included studies [ 9 , 10 , 21 , 23 , 24 , 25 , 29 , 31 , 32 , 35 , 39 , 40 , 41 , 42 , 43 ]. Other common bacteria implicated in IK included S. aureus (5–36%) , Streptococci spp. (7–16%), Pseudomonas aeruginosa (5–24%), Enterobacteriaceae spp . (15%), Corynebacterium spp . (14%), and Propionibacterium spp . (9%; see Table 1 ). Over the past decade, there were several studies in the UK documenting a significant increase in Moraxella keratitis, which are often associated with longer corneal healing time [ 8 , 9 , 10 ]. Interestingly, Nocardia keratitis, a rare cause of IK, was identified as the third most common microorganism (11% of all cases) in the Steroids for Corneal Ulcers Trial (SCUT), and the outcome was found to be negatively influenced by the use of topical steroids [ 67 , 68 ]. Acid-fast bacilli such as non-tuberculous mycobacteria (NTM) serve as another important group of pathogens that are capable of causing IK [ 69 ]. NTM keratitis is commonly associated with refractive surgery and trauma, and it often requires prolonged and aggressive treatment for complete eradication, largely attributed to their propensity to form biofilms [ 69 , 70 ].
Fungi can be broadly divided into two categories, namely filamentous and yeast or yeast-like fungi. Filamentous fungi such as Fusarium spp . and Aspergillus spp . normally thrives in tropical climates whereas yeast-like fungi such as Candida spp. were more commonly observed in temperate regions [ 71 ]. Several studies have demonstrated that Fusarium spp . (13–24%) and Aspergillus spp . (8–30%) were the main causes of IK in Asia, particularly India and China (Table 1 ) [ 13 , 33 , 34 , 36 , 37 , 41 ]. In 2018, the Asian Cornea Society Infectious Keratitis Study (ACSIKS) included more than 6000 patients from eight Asian countries and re-confirmed the dominance of Fusarium spp . keratitis within China (26%) and India (31%) established two decades ago [ 72 , 73 , 74 ]. Although the prevalence of fungal keratitis in temperate regions such as the UK, Europe and North America was reportedly lower, the growth of yeast-like fungi such as Candida spp . is relatively common in patients with history of corneal transplantation or OSDs [ 44 ]. In view of the recent improvement in the diagnostic techniques, rare pathogens such as Cryptococcus curvatus , Arthrographis kalrae , Pythium spp ., and many others are increasingly being identified and reported as rare causes of fungal keratitis [ 75 , 76 , 77 ].
C.3. Protozoa
Acanthamoeba is a free-living protozoan that is found ubiquitously in the environment such as water, soil, air and dust [ 78 ]. Although not as common as bacterial or fungal keratitis, Acanthamoeba keratitis serves as another important cause of IK as it is often associated with prolonged treatment course and poor visual outcome [ 78 ]. It was estimated that Acanthamoeba keratitis affects 1–33 per million CL wearers per year [ 78 ]. In the UK, Carnt et al [ 79 ]. recently confirmed an outbreak of Acanthamoeba keratitis in the South East England during 2010–2016, with an approximately threefold increase compared to the preceding decade.
Based on recent large studies, Acanthamoeba keratitis accounts for ~0–5% of all IK (Table 1 ). Most of the Acanthamoeba keratitis were observed in CL wearer (71–91%) [ 32 , 60 , 80 ]. However, non-CL wearers can also develop this infection if their eyes are exposed to contaminated water, soil or dust, [ 81 , 82 ]. One of the Indian studies reported that only 4% of Acanthamoeba keratitis cases were associated with CL wear and the remainder were associated with trauma and/or exposure to contaminated water [ 82 ]. In addition, the clinical features of non-CL related Acanthamoeba keratitis may differ from CL-related cases [ 82 ]. Moreover, Acanthamoeba sclerokeratitis may manifest as a rare but difficult-to-treat clinical entity that is usually associated with poor clinical outcomes [ 83 ].
Microsporidial keratitis represents another type of parasitic IK that accounts for ~0.4% cases of all IK [ 84 ]. It is mainly observed in Asian countries and may manifest as superficial keratoconjunctivitis or stromal keratitis. It is commonly associated with ocular trauma, exposure to contaminated water/soil, and potentially acquired immunodeficiency syndrome [ 84 , 85 ].
C.4. Viruses
Viral keratitis, most commonly in the form of herpes simplex keratitis (HSK) and herpes zoster keratitis (HZK), represents a common cause of IK [ 86 , 87 ]. However, as viral keratitis cases are commonly treated based on their typical clinical appearance (e.g. dendritic corneal ulcer in HSK) and/or previous ocular history, the majority of cases did not require any microbiological investigation and hence were not captured in many IK studies. Nonetheless, the ACSIKS study demonstrated that viral keratitis represented the most common cause (46%) of IK in China, primarily attributed to HSK (24%) and HZK (17%) [ 13 ]. Another two studies, conducted in Egypt and China, respectively, observed that 15–21% of IK were caused by herpetic keratitis [ 51 , 58 ]. Based on these results, it is likely that viral keratitis represents an important and common cause of IK in many other regions, though further studies are required to elucidate this. Herpetic keratitis is often associated with neurotrophic keratopathy, which can result in poor corneal healing, increased risk of further IK and other corneal complications such as melting and perforation [ 86 , 88 ].
C.5. Polymicrobial infection
Polymicrobial keratitis (IK caused by two or more causative microorganisms) has been reported in around 2–15% of all IK cases [ 8 , 9 , 10 , 11 , 21 ]. Depending on the study design and the definition used, polymicrobial keratitis may include two or more types of organisms from the same category (e.g. bacteria-bacteria, fungus-fungus) or different categories (bacteria-fungus, fungus-protozoan). Polymicrobial keratitis often poses significant diagnostic and therapeutic challenges, and usually fares worse than monomicrobial keratitis [ 11 , 75 , 89 ]. Khoo et al [ 11 ]. observed that patients affected by polymicrobial keratitis (median of 6/60 vision) had a significantly worse visual outcome as compared to those affected by bacterial keratitis (median of 6/18 vision) or culture negative IK (median of 6/9 vision). In another retrospective comparative study, Lim et al [ 89 ]. demonstrated that medical therapy was sufficient to resolve all monomicrobial IK cases but only 81% of polymicrobial IK. In view of the relatively common occurrence of polymicrobial keratitis and variably low culture yield of current microbiological investigation, clinicians should always maintain a low threshold of repeating corneal scraping if patients are not responding to either antibacterial or antifungal therapy, even in the presence of positive culture results.
C.6. Seasonal variations
Pathogens are tremendously adaptive to climate and seasonality. Many studies have shown that IK was most prevalent during the summer season, with P. aeruginosa being one of the most frequently isolated microbes [ 34 , 90 , 91 ]. P. aeruginosa is a well-recognised organisms associated with environmental water as in swimming pools [ 92 ] and CL [ 44 , 46 , 48 , 93 , 94 ]. The seasonal predilection of IK during summer is attributed to the likely increased use of CL wear and engagement in water activities. On the other hand, several studies have shown that the incidence of fungal keratitis in India peaked during the windy and harvest seasons, primarily related to a higher risk of trauma secondary to agricultural activities and agricultural debris being blown in the eyes by the wind [ 34 , 62 ].
Seasonal variation was similarly observed in Acanthamoeba keratitis, though with conflicting results. Lin et al [ 34 ]. observed that Acanthamoeba keratitis occurred more commonly during summer in South India, potentially related to the higher temperature and increased risk of corneal trauma during windy seasons, whereas Walkden et al [ 91 ]. reported an increase in Acanthamoeba keratitis during the winter in the UK.
Major risk factors
In the majority of IK cases, local and/or systemic risk factors are usually present. The most common risk factors include CL wear, ocular trauma, OSDs (e.g. dry eye diseases (DEDs), neurotrophic keratopathy, rosacea, etc.), lid diseases, post-corneal surgery (e.g. keratoplasty, corneal cross-linking (CXL)), and systemic diseases (e.g. diabetes, immunosuppression), amongst others. A tabulated summary of large IK studies reporting the risk factors of IK is provided in Table 2 [ 11 , 13 , 18 , 29 , 32 , 35 , 44 , 45 , 46 , 48 , 49 , 50 , 51 , 58 , 59 , 60 , 61 , 62 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 ].
D.1. Contact lens (CL) wear
CL wear has been recognised as one of the most common risk factors of IK, particularly in developed countries. A study conducted in Northern California reported that the incidence of IK among CL wearers was ~9.3 times higher than the non-CL wearers (130.4 vs. 14.0 per 100,000 person-years) [ 18 ]. Based on the large studies (>200 patients) published in the recent literature, CL wear was shown to be the main predisposing factor (29–64%) of IK in developed countries like Portugal [ 48 ], France [ 93 ], Sweden [ 95 ], the US [ 18 , 44 , 97 ], Singapore[ 46 ] and Australia [ 11 ]. On the contrary, CL-related IK was considerably less common (0–18%) in developing countries due to less number of CL wearers [ 13 , 35 , 50 , 59 , 60 ], highlighting the geographical disparity in the risk factors as well as the causative microorganisms of IK between high income and low-income countries (Table 2 ).
The pathogenesis of CL-related IK is complex and multifactorial. Although it is commonly believed that CL-related IK is triggered by superficial injury secondary to CL wear, several studies had refuted this hypothesis as it was shown that the presence or absence of epithelial injury did not influence the risk or severity of IK [ 65 ]. Plausible mechanisms of CL-related IK include reduction of tear exchange during blinking (which leads to potential degradation of protective components at ocular surface), tear stagnation under CL (particularly soft CL) resulting in accumulation and adherence of microbes to the cornea, reduced corneal epithelial cell desquamation, and alteration of tear fluid biochemistry [ 65 ]. In addition, multiple predisposing factors of CL-related IK have been identified, including the types of CL used (higher risk in soft CL than rigid gas permeable CL), poor CL and CL case hygiene, overnight wear, use of expired CL, types of CL solution used, and CL being prescribed/dispensed by non-ophthalmologists or non-opticians [ 93 , 102 , 103 , 104 , 105 , 106 ]. Reports of IK secondary to the use of cosmetic lens and orthokeratology lens have also been highlighted [ 107 , 108 ].
In terms of underlying aetiologies, CL-related keratitis is most commonly associated with P. aeruginosa and Acanthamoeba spp ., which are both free-living microorganisms that are ubiquitously present in the environment, including water and CL solutions [ 47 ]. As noted above, Pseudomonas keratitis is one of the most common causes of IK, especially in the developed countries where there is increased prevalence of CL wear. Yildiz et al [ 102 ]. and Tong et al [ 46 ]. observed that P. aeruginosa was responsible for 63% and 70% of the CL-related IK, respectively. While Acanthamoeba keratitis is uncommon, most of these cases (71–91%) were observed in CL wearers [ 32 , 60 , 80 ]. Yu et al [ 32 ]. observed that more than 90% of the Acanthamoeba keratitis were associated with CL use. In a 32-year Brazilian study of over 6000 IK cases, Cariello et al [ 29 ]. reported that CL wearers had a 1.7 times higher risk of developing Acanthamoeba-positive culture than non-CL wearers. Interestingly, CL wear was also shown to be a major risk factor for fungal keratitis in a US study [ 44 ].
D.2. Trauma
Trauma serves as another common risk factor for IK in both developed and developing countries. Based on the IK studies reported in the literature, farmers (54–70%) and manual labour workers (11–17%) constituted the main occupations in Asia [ 13 , 45 , 49 , 51 , 58 , 59 , 109 ]. These groups of workers were at a high risk of developing IK due to the increased occupational exposure to plant materials and foreign bodies, which was frequently compounded by the lack of eye protection [ 45 , 51 , 58 , 98 , 109 ].
Fungal keratitis is by far the most common cause (47–83%) of trauma-related IK, especially in regions such as Asia and Africa which are dominated by agricultural communities [ 45 , 51 , 58 , 60 , 94 ]. Occupational exposures to vegetative matter, organic materials and animal products, predominantly in males in the working age group, are the main causes in these regions. The risk of fungal keratitis is further magnified by tropical climates, which are conducive to fungal growth [ 51 , 60 ]. Cariello et al [ 29 ]. observed that the risk of developing culture-proven fungal keratitis was increased by four times if the patients suffered from plant-related trauma. In addition, some studies demonstrated that trauma-related IK fared worse than non-traumatic cases [ 46 , 58 ]. Pan et al [ 58 ]. conducted a 10-year study in China and revealed that patients who presented with trauma-related IK were at a high risk of developing fungal keratitis and requiring surgical interventions (89%), including therapeutic keratoplasty and evisceration/enucleation.
On the other hand, the majority of trauma-related IK reported in European countries were caused by Gram-positive bacteria, including CoNS, S . aureus, Streptococci , and Corynebacterium [ 48 , 95 ]. These are common ocular surface commensals, which have the ability to tolerate hot and dry climates in temperate and sub-tropical zones [ 51 , 110 , 111 ]. Corneal trauma resulting from non-vegetative matter with consequent secondary opportunistic infection with ocular surface commensals could explain the high rate of Gram-positive infection in trauma-related IK in this region.
D.3. Ocular surface and eyelid diseases
Ocular surface diseases (OSDs), encompassing DEDs, blepharitis, neurotrophic keratopathy, Steven–Johnson syndrome, ocular cicatricial pemphigoid and bullous keratopathy, have been identified as one of the main risk factors for IK in both developed and developing countries [ 18 , 44 , 49 , 60 , 97 , 112 ]. OSD-related IK is most commonly caused by Gram-positive bacteria (around 60–80%) [ 11 , 60 , 95 , 112 ], which constitute the main group of ocular surface commensals. In particular, CoNS and S. aureus were shown to be the main culprits in OSD-related IK [ 95 , 112 ].
DED is the most common OSD that is characterised by “ a loss of tear film homeostasis with ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles ” [ 113 ]. The dysregulated ocular surface health can lead to breakdown of the corneal epithelium, a vital ocular surface defence, and ocular surface inflammation, consequently increasing the risk of IK [ 60 , 114 ].
Posterior blepharitis or meibomian gland disease (MGD) is a common eyelid disease, which is difficult to cure. It can lead to an array of ocular surface complications, including evaporative DED, marginal keratitis and IK, amongst others [ 115 ]. Meibomian gland abnormalities (e.g. gland dropout and hyperkeratinisation), alteration of the secreted lipid products, and the dysregulation of bacterial populations and their corresponding lipase or esterase activity are believed to contribute to the ocular surface inflammation and infection. In a 5-year Australian study, MGD was shown to be the most common cause (79%) of OSD implicated in IK [ 112 ]. In addition, nasolacrimal duct obstruction (NLDO) can also increase the risk of IK, primarily attributed to tear stagnation and reduction of tear exchange, resulting in the accumulation of microbes and debris on the ocular surface with increased risk of IK. Chidambaram et al [ 45 ]. showed that NLDO could increase the risk of fungal and bacterial IK, particularly S. pneumonia keratitis.
D.5. Post-ocular surgery
IK may occur following various ocular surgeries, including corneal transplant, refractive surgery, CXL, pterygium surgery, cataract surgery, and others [ 29 , 51 , 116 , 117 ]. Corneal transplant serves as the main sight-restoring surgery for a wide range of corneal diseases, though postoperative complications such as graft failure and IK may develop. In a retrospective study of over 2000 corneal transplants, Dohse et al [ 116 ]. reported an incidence of post-keratoplasty IK of 4%, with loose and broken sutures being reported as one of the most common risk factors (24%) [ 116 ]. Cariello et al [ 29 ]. demonstrated that 22% of the IK cases were associated with prior ocular surgery, particularly corneal graft (56%). In addition, the paradigm shift of penetrating keratoplasty to lamellar keratoplasty has created a new array of host-graft interface complications such as interface infectious keratitis (IIK), which often causes diagnostic and therapeutic challenges due to the deep-seated location of the infection [ 118 , 119 ]. We have recently highlighted a clinically challenging case of post-endothelial keratoplasty interface fungal keratitis, which required in vivo confocal microscopy for confirmatory diagnosis in the absence of positive culture results [ 118 ]. Fortunately the interface infection resolved quickly after the discontinuation of topical steroids and initiation of appropriate antifungal treatment.
Although IK rarely develops after refractive surgery, the significant amount of refractive surgeries performed globally render this an important clinical entity [ 120 ]. This was supported by a Brazilian study where refractive surgery was shown to be the second commonest surgery associated with IK [ 29 ]. Post-refractive surgery IK is most commonly caused by Gram-positive bacteria and NTM, though fungal and Acanthamoeba infection may also occur [ 120 ]. The high rate of Gram-positive bacterial IK following other types of ocular surgeries (e.g. cataract surgery, pterygium surgery) were also observed, most likely as a result of opportunistic infection secondary to ocular surface commensals [ 51 , 93 , 95 ].
In the recent years, CXL has emerged as a therapeutic modality for managing corneal ectactic conditions [ 121 , 122 ] and moderate-to-severe IK [ 123 , 124 , 125 ]. However, the intraoperative removal of corneal epithelium and postoperative insertion of bandage CL (which is the current standard practice in most institutes) can increase the risk of IK following CXL, particularly in patients with OSD such as vernal or atopic keratoconjunctivitis [ 117 , 126 , 127 ]. Post-CXL IK may be further complicated by the reactivation of herpetic keratitis [ 126 ] and manifestation of acute hydrops [ 127 ] and corneal melt/perforation [ 117 ].
D.6. Use of topical steroids
Steroids are commonly used in ophthalmology as a topical immunosuppressive/immunomodulatory agent to manage a wide range of intraocular and ocular surface inflammatory diseases, including DED, allergic eye disease, non-IK, chemical eye injury, cicatricial conjunctivitis and many others [ 128 , 129 ]. The recent SCUT study also demonstrated the benefit of adjuvant topical steroids in improving the visual outcome in patients with severe and central bacterial keratitis [ 67 ]. In addition to managing OSDs, topical steroids are also frequently used as postoperative topical treatment following intraocular and ocular surface surgeries, including corneal transplantation [ 130 ].
However, topical steroids can sometimes act as a double-edge sword. Studies have shown that topical steroids can increase the risk of IK, particularly fungal keratitis and/or polymicrobial keratitis [ 11 , 44 , 118 ]. In a study of 733 fungal keratitis, Keay et al [ 44 ]. reported that 13% of the cases were associated with chronic use of topical steroids. In addition, a study has shown that previous use of topical steroid could negatively impact on the clinical outcome of IK, with 73% ending with poor outcome (defined as worse than 6/60 vision, decreased vision during treatment, or perforation) [ 11 ]. While topical steroids serve as an effective treatment for stromal HSK, which is primarily an immune-related keratitis [ 131 ], its use can potentially exacerbate epithelial HSK and culminate in geographic ulcer [ 132 ]. Interestingly, an Indian study showed that 41% of the Acanthamoeba keratitis cases were associated with the use of topical steroid [ 45 ]. The high rate of prior steroid use might be related to the fact that Acanthamoeba keratitis often presents with non-specific corneal epithelial changes and is mismanaged as viral keratitis [ 104 ].
D.7. Systemic immunosuppression
Systemic immunosuppression, either secondary to diseases or immunosuppressive agents, has been shown to increase the risk of IK. Diabetes mellitus serves as one of the most important systemic risk factors for IK. Hyperglycaemia has been shown to facilitate microbial growth and alter the microbiota of ocular surface, including an upregulation of Pseudomonas spp . and Acinetobacter spp . [ 133 ], as well as affect the homeostasis, corneal sensation and wound healing of the corneal epithelium, thereby increasing the risk of IK [ 134 ]. Sub-basal corneal nerve plexus of patients with diabetic neuropathy is often affected and can lead to neuropathic keratopathy with complications such as corneal melt and IK [ 135 ].
Several large studies have highlighted the association between diabetes and IK (around 8–16%), particularly fungal and bacterial keratitis [ 45 , 58 , 60 , 136 , 137 ]. Zbiba et al [ 60 ]. observed that diabetes was relatively common in patients with bacterial keratitis (15%) and fungal keratitis (16%) as well as mixed bacterial and fungal keratitis (29%). In addition, viral keratitis was also reported to have a high prevalence amongst patients with diabetes [ 138 ]. Viruses, particularly HSV, are omnipresent in the general population, with an estimated prevalence of 1.5 per 1000 population [ 139 ]. Kaiserman et al [ 140 ]. demonstrated that patients with diabetes had a significantly higher incidence and recurrence rate of ocular surface herpetic eye diseases when compared to non-diabetic patients. Pan et al [ 58 ]. observed that 17% patients with diabetes had a substantially higher rate of HSK as compared to bacterial or fungal keratitis. Another study described that all patients with diabetes presented with IK were of viral origin, though the sample size was small [ 51 ]. The heterogeneity in the subtypes of microorganisms associated with diabetes observed in different studies was likely related to the disparity in the ocular predisposing factors of the studied cohort since more than one risk factor is often present in patients with IK [ 11 ].
Apart from diabetes, Jeng et al [ 18 ]. observed an approximately tenfold increased risk of IK in individuals affected by human immunodeficiency viruses compared to healthy individuals (238.1 vs. 27.6 per 100,000 population-year), highlighting the importance of host immunity in ocular surface defence. Intriguingly, a study demonstrated that 55% of the patients with HSK had a history of upper respiratory tract infection prior to the infection or recurrence [ 58 ]. This could be potentially explained by the mechanism linked to a host cell enzyme called heparanase [ 141 ], which is a known contributing factor to the pathogenesis of several viruses, including HSV, respiratory syncytial virus, human papilloma virus, and others. End-stage renal disease, particularly associated with diabetes, was also shown to be a risk factor for IK [ 142 ].
Antimicrobial resistance (AMR)
E.1. overview.
AMR has been recognised as a major public health crisis in the past two decades, with many infectious organisms developing resistance against previously effective antimicrobial agents [ 143 ]. The development of AMR is largely driven by a multitude of factors, including the overuse/abuse of antimicrobial agents in agricultural sectors due to commercial pressure, uncertainty in diagnosis (e.g. bacterial infection vs. viral infection) leading to inappropriate use of antibiotics, financial incentives for prescribing antibiotic, and use of non-prescription antibiotics among the general public, particularly in low- and middle-income countries [ 143 , 144 ]. From the genetic point of view, bacteria primarily develop AMR through two strategies, namely genetic mutational resistance and horizontal gene transfer. The genetic and mechanistic basis of AMR can be referred to a recent excellent review provided by Munita and Arias [ 144 ].
E.2. AMR in the context of IK
Broad-spectrum topical antibiotic therapy is the gold standard treatment for IK. Depending on the disease severity and clinicians’ preference, antibiotic therapy is commonly administered in the form of dual therapy using cephalosporin and aminoglycoside or monotherapy using fluoroquinolone [ 145 ]. As intensive topical antibiotics are applied directly and frequently during the treatment of IK, high concentration of antibiotics can be effectively achieved at the target site (i.e. the infected cornea), which could potentially reduce the risk of AMR in ocular infections. However, a few recent IK studies have highlighted the emergence of AMR in ocular infections, particularly in the US [ 28 ], China [ 41 ] and India [ 40 ]. The driving force is likely to be multifactorial, including the injudicious widespread use of antibiotics in both ocular and systemic infections [ 146 ], incorrect dosing regimen [ 147 ], and representations of the community prevalence of drug resistance, with consequent colonisation of ocular surface by drug resistant pathogens [ 148 ]. For instance, in the SCUT trial, there was a 3.5-fold higher MIC for bacteria isolated from patients who had previous treatment with fluoroquinolones compared to treatment naive patients [ 149 ].
A tabulated summary of the literature concerning the in vitro antibiotic susceptibility and resistance of IK-related bacteria is provided in Table 3 [ 8 , 9 , 21 , 24 , 25 , 26 , 28 , 31 , 35 , 38 , 40 , 41 , 42 , 43 , 150 ]. Overall, fluoroquinolone-resistant, methicillin-resistant and multidrug resistant (MDR; i.e. resistant to 3 or more antibiotics) infections are being increasingly reported in IK [ 28 , 31 , 35 , 40 , 41 , 150 ]. Geographical and temporal factors play a role in the variation of AMR pattern in ocular infections. Reports from Southern India demonstrated that MDR was commonly observed among S. pneumoniae (44%), S. epidermidis (14.8%), S. aureus (14%), and P. aeruginosa (6%). However, gatifloxacin—a fourth-generation fluoroquinolone—was effective against the majority of Gram-negative bacteria (~90%), including P. aeruginosa and Acinetobacter spp ., thus its use as a monotherapy in Gram-negative IK was recommended in that region [ 35 ]. Another study from Southern China similarly reported an increase in MDR among Gram-positive cocci from 2010 to 2018, while susceptibility to fluoroquinolone and aminoglycoside among Gram-negative bacilli remained stable [ 41 ]. In contrast, a Northern India study reported a high rate of resistance of P. aeruginosa against ciprofloxacin (57%), moxifloxacin (47%), and aminoglycoside (52–60%) [ 40 ], highlighting the geographical disparity in the AMR pattern and the importance of region-specific interrogation of the AMR profile in ocular infections.
An increasing trend of MRSA-related ocular infection has also been reported in several studies in the past decade [ 28 , 31 , 41 ]. The Antibiotic Resistance Among Ocular Microorganisms study in the US observed that a high rate of AMR, specifically methicillin resistance, was observed among Staphylococci spp . and Streptococci spp . and the risk increased with age [ 28 ]. More worryingly, ~75% of the MRSA and MR-CoNS were MDR. Another US study demonstrated an increased rate of MRSA-related IK as well as resistance against fluoroquinolones, which questioned their ongoing use as primary monotherapy [ 26 ]. Similarly, a 10-year Mexico study showed that 21–79% of the S. aureus and 48–71% of the CoNS were resistant to oxacillin (or methicillin). P. aeruginosa and other Gram-negative infections displayed resistance against oxacillin (86% and 90%, respectively) and vancomycin (97% and 70%, respectively), with an increasing trend of resistance to ceftazidime observed over time [ 31 ]. Another study conducted in Taiwan also highlighted the emerging issue of methicillin resistance, with MRSA accounting for 43% of all Gram-positive IK [ 38 ]. On the other hand, an increase in voriconazole resistance was observed in the Mycotic Ulcer Treatment Trial (MUTT)-I for fungal keratitis, with a 2.1-fold increase in the mean MIC per year after adjustment for causative organism [ 151 ].
Reassuringly, reports from the UK showed that Gram-positive bacteria exhibited a high susceptibility to cephalosporin (87–100%), but a moderate susceptibility to fluoroquinolone (61–81%). However, Gram-negative bacteria were highly susceptible to both aminoglycoside (97–100%) and fluoroquinolone (91–100%) [ 8 , 9 , 24 ], suggesting that current antibiotic regimen (fluoroquinolone monotherapy or cephalosporin-aminoglycoside dual therapy) could safely remain as the first-line treatment in the UK. In our recent 12-year Nottingham IK Study, we observed an increasing trend of resistance against penicillin over time in both Gram-positive and Gram-negative isolates but a generally good susceptibility to aminoglycosides and fluoroquinolones was maintained; therefore, no change of antibiotic regimen was required [ 8 ].
E.3. Clinical impact
AMR represents a global challenge with a huge impact on morbidity and mortality. It was estimated that 2 million people/year in USA are infected with antimicrobial resistant organisms, with a $20 billion cost incurred on the healthcare system. A recent UK report also predicted a global loss of $100 trillion by 2050 related to AMR [ 152 ].
Within the context of IK, AMR was found to negatively affect the clinical outcome of IK. Kaye et al [ 153 ]. observed that the corneal healing time of IK was prolonged with the increase of minimum inhibitory concentration (MIC; i.e. antibiotic resistance) of the causative organisms, including P. aeruginosa , S. aureus and Enterobacteriaceae spp ., against fluoroquinolone monotherapy. In addition, Lalitha et al [ 154 ]. demonstrated that higher level of MIC was associated with a significantly increase risk of corneal perforation in fungal keratitis.
AMR is continuing to increase in an alarming way. There is a pressing need to increase the awareness amongst prescribers on judicious use of antimicrobials, to tighten the control of ‘over the counter (OTC)” antimicrobials in many countries, and to develop novel therapeutic modalities and strategies for IK, including therapeutic CXL and host defence peptides (or previously known as antimicrobial peptides), which hold great promises as a new class of antimicrobials in the future [ 123 , 155 , 156 , 157 ].
Conclusions
IK represents a persistent burden on human health in both developed and developing countries. As the incidence of IK is likely to be underestimated in the recent studies, well-designed prospective studies including all types of microorganisms (i.e. bacteria, fungi, protozoa and viruses) are required to truly ascertain the incidence and impact of IK. Understanding of the major risk factors for IK in different regions, particularly CL wear, trauma, OSD, and post-ocular surgery, will facilitate a more effective public health intervention to modify and reduce the risk of IK. The increase rate of AMR in ocular infection in several countries, including the US, China, and India, over the past decade highlights the need for judicious use of antimicrobials, tighter control of OTC antimicrobials and development of new antimicrobials and strategies for therapy. Improvement in the diagnostic yield of microbiological investigations of IK with emerging technologies such as next-generation sequencing and artificial intelligence-assisted platforms could also provide a better guidance on the appropriate use of antimicrobial therapy in the future, ultimately reducing the risk of AMR [ 158 , 159 ].
Methods of literature review
Two authors (DSJT and CSH) searched the PubMed (January 1980–May 2020) for relevant articles related to IK. Keywords such as “corneal infection”, “corneal ulcer”, “IK”, “microbial keratitis”, “incidence”, “prevalence”, “epidemiology”, “risk factors”, “antibiotic resistance” and “antimicrobial resistance” were used. There was no restriction to the language used. Bibliographies of included articles were manually screened to identify further relevant studies. The final search was updated on 15 June 2020.
A web application designed for systematic reviews, Rayyan (Qatar), was used to help collate the potential studies and expedite the initial screening of abstracts and titles [ 160 ]. The titles and abstracts obtained from the searches were independently screened by two authors (DSJT and CSH) to include studies that fulfilled the eligibility criteria. The authors then independently assessed the full-text version of all selected articles and extracted data onto a standardised data collection form for data synthesis. The extracted data included the authors, year of publication, country, sample size, demographic factors, culture results, risk factors and in vitro antibiotic susceptibility. Discrepancies were resolved by group consensus and independent adjudication (HSD) if consensus could not be reached. The summary of literature search is detailed in the PRISMA flow chart (Fig. 1 ).
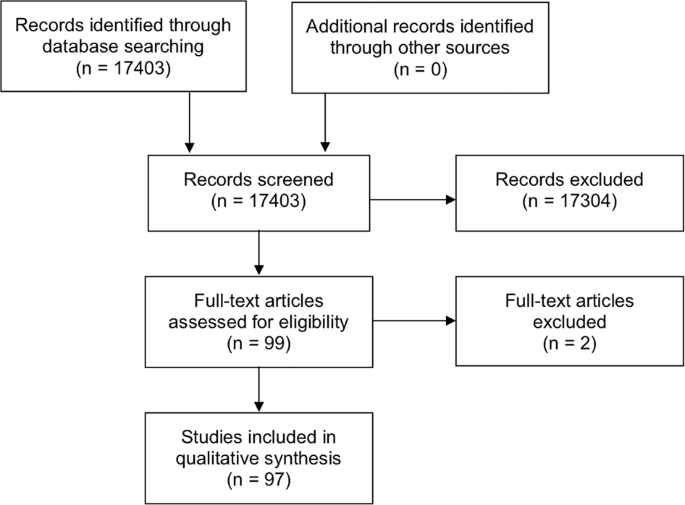
The PRISMA flow chart detailing the process and results of literature search for articles related to infectious keratitis.
Corneal opacity represents the 5th leading cause of blindness globally, with infectious keratitis (IK) being the main culprit.
IK can be caused by a wide variety of pathogens, including bacteria, fungi, viruses, parasites and polymicrobial infection.
Contact lens wear, trauma and ocular surface diseases are the three most common risk factors of IK.
Several studies have highlighted the emerging trends in antimicrobial resistance in ocular infections, particularly in the US, China and India.
Change history
11 may 2021.
A Correction to this paper has been published: https://doi.org/10.1038/s41433-021-01568-0
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e34.
Article PubMed Google Scholar
https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment . [accessed on 1st May 2020]
Whitcher JP, Srinivasan M. Corneal ulceration in the developing world-a silent epidemic. Br J Ophthalmol. 1997;81:622–3.
Article CAS PubMed PubMed Central Google Scholar
Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–21.
CAS PubMed PubMed Central Google Scholar
Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64:255–71.
Song X, Xie L, Tan X, Wang Z, Yang Y, Yuan Y, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS ONE. 2014;9:e113843.
Article PubMed PubMed Central Google Scholar
Ung L, Acharya NR, Agarwal T, Alfonso EC, Bagga B, Bispo PJ, et al. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull World Health Organ. 2019;97:854–6.
Ting DSJ, Ho CS, Cairns J, Elsahn A, Al-Aqaba M, Boswell T, et al. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham Infectious Keratitis Study. Br J Ophthalmol. 2020; https://doi.org/10.1136/bjophthalmol-2020-316128 .
Tan SZ, Walkden A, Au L, Fullwood C, Hamilton A, Qamruddin A, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye (Lond). 2017;31:1229–36.
Article CAS Google Scholar
Ting DSJ, Settle C, Morgan SJ, Baylis O, Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. Eye (Lond). 2018;32:1416–7.
Article Google Scholar
Khoo P, Cabrera-Aguas MP, Nguyen V, Lahra MM, Watson SL. Microbial keratitis in Sydney, Australia: risk factors, patient outcomes, and seasonal variation. Graefes Arch Clin Exp Ophthalmol. 2020; https://doi.org/10.1007/s00417-020-04681-0 .
Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When clarity is crucial: regulating ocular surface immunity. Trends Immunol. 2018;39:288–301.
Article CAS PubMed Google Scholar
Khor WB, Prajna VN, Garg P, Mehta JS, Xie L, Liu Z, et al. The Asia Cornea Society Infectious Keratitis Study: a Prospective Multicenter Study of Infectious Keratitis in Asia. Am J Ophthalmol. 2018;195:161–70.
Arunga S, Wiafe G, Habtamu E, Onyango J, Gichuhi S, Leck A, et al. The impact of microbial keratitis on quality of life in Uganda. BMJ Open Ophthalmol. 2019;4:e000351.
Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, et al. Estimated burden of keratitis-United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63:1027–30.
PubMed PubMed Central Google Scholar
Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111:1665–71.
Upadhyay MP, Karmacharya PC, Koirala S, Shah DN, Shakya S, Shrestha JK, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85:388–92.
Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–8.
Seal DV, Kirkness CM, Bennett HG, Peterson M. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye. 1999;22:49–57.
Ibrahim YW, Boase DL, Cree IA. Incidence of Infectious Corneal Ulcers, Portsmouth Study, UK. J Clin Exp Ophthalmol. 2012;S6:001.
Google Scholar
Green M, Carnt N, Apel A, Stapleton F. Queensland Microbial Keratitis Database: 2005-2015. Br J Ophthalmol. 2019;103:1481–6.
Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3:159–66.
Kaye R, Kaye A, Sueke H, Neal T, Winstanley C, Horsburgh M, et al. Recurrent bacterial keratitis. Investig Ophthalmol Vis Sci. 2013;54:4136–9.
Tavassoli S, Nayar G, Darcy K, Grzeda M, Luck J, Williams OM, et al. An 11-year analysis of microbial keratitis in the South West of England using brain-heart infusion broth. Eye (Lond). 2019;33:1619–25.
Tam ALC, Côté E, Saldanha M, Lichtinger A, Slomovic AR. Bacterial Keratitis in Toronto: a 16-Year Review of the Microorganisms Isolated and the Resistance Patterns Observed. Cornea. 2017;36:1528–34.
Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37:84–7.
Kowalski RP, Nayyar SV, Romanowski EG, Shanks RMQ, Mammen A, Dhaliwal DK, et al. The Prevalence of Bacteria, Fungi, Viruses, and Acanthamoeba From 3,004 Cases of Keratitis, Endophthalmitis, and Conjunctivitis. Eye Contact Lens. 2019; https://doi.org/10.1097/ICL.0000000000000642 .
Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH. Trends in Antibiotic Resistance Among Ocular Microorganisms in the United States From 2009 to 2018. JAMA Ophthalmol. 2020;138:1–12.
Article PubMed Central Google Scholar
Cariello AJ, Passos RM, Yu MC, Hofling-Lima AL. Microbial keratitis at a referral center in Brazil. Int Ophthalmol. 2011;31:197–204.
Marujo FI, Hirai FE, Yu MC, Hofling-Lima AL, Freitas D, Sato EH. [Distribution of infectious keratitis in a tertiary hospital in Brazil]. Arq Bras Oftalmol. 2013;76:370–3.
Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, Ramirez-Miranda A, Navas A, Pedro-Aguilar L, et al. Trends in Microbiological and Antibiotic Sensitivity Patterns in Infectious Keratitis: 10-Year Experience in Mexico City. Cornea 2015;34:778–85.
Yu MC, Höfling-Lima AL, Furtado GH. Microbiological and epidemiological study of infectious keratitis in children and adolescents. Arq Bras Oftalmol. 2016;79:289–93.
Rautaraya B, Sharma S, Kar S, Das S, Sahu SK. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol. 2011;11:39.
Lin CC, Lalitha P, Srinivasan M, Prajna NV, McLeod SD, Acharya NR, et al. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31:1123–7.
Kaliamurthy J, Kalavathy CM, Parmar P, Nelson Jesudasan CA, Thomas PA. Spectrum of bacterial keratitis at a tertiary eye care centre in India. Biomed Res Int. 2013;2013:181564.
Lalitha P, Prajna NV, Manoharan G, Srinivasan M, Mascarenhas J, Das M, et al. Trends in bacterial and fungal keratitis in South India, 2002-2012. Br J Ophthalmol. 2015;99:192–4.
Wang L, Han L, Yin W. Study of Pathogens of Fungal Keratitis and the Sensitivity of Pathogenic Fungi to Therapeutic Agents with the Disk Diffusion Method. Curr Eye Res. 2015;40:1095–101.
Hsiao CH, Sun CC, Yeh LK, Ma DH, Chen PY, Lin HC, et al. Shifting Trends in Bacterial Keratitis in Taiwan: a 10-Year Review in a Tertiary-Care Hospital. Cornea. 2016;35:313–7.
Zhang Y, Wang ZQ, Sun XG. [Etiological analysis and in vitro drug sensitivity of bacterial keratitis in northern China in the period of 2006-2015]. Zhonghua Yan Ke Za Zhi. 2017;53:662–7.
CAS PubMed Google Scholar
Acharya M, Farooqui JH, Singh A, Gandhi A, Mathur U. Bacterial isolates in microbial keratitis: three-year trend analysis from North India. Indian J Ophthalmol. 2019;67:1508–9.
Lin L, Duan F, Yang Y, Lou B, Liang L, Lin X. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of microbial keratitis from a large referral eye center in southern China. Infect Drug Resist. 2019;12:1295–302.
Politis M, Wajnsztajn D, Rosin B, Block C, Solomon A. Trends of Bacterial Keratitis Culture Isolates in Jerusalem; a 13- Years Analysis. PLoS ONE. 2016;11:e0165223.
Cabrera-Aguas M, Khoo P, George CRR, Lahra MM, Watson S. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin Exp Ophthalmol. 2019;48:183–91.
Keay LJ, Gower EW, Iovieno A, Oechsler RA, Alfonso EC, Matoba A, et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001-2007: a multicenter study. Ophthalmology. 2011;118:920–6.
Chidambaram JD, Venkatesh Prajna N, Srikanthi P, Lanjewar S, Shah M, Elakkiya S, et al. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis in South India. Ophthalmic Epidemiol. 2018;25:297–305.
Tong W, Chen D, Chai C, Tan AM, Manotosh R. Disease patterns of microbial keratitis in Singapore: a retrospective case series. Cont Lens Anterior Eye. 2019;42:455–61.
Stapleton F. Contact lens-related corneal infection in Australia. Clin Exp Optom. 2020;103:408–17.
Ferreira CS, Figueira L, Moreira-Gonçalves N, Moreira R, Torrão L, Falcão-Reis F. Clinical and Microbiological Profile of Bacterial Microbial Keratitis in a Portuguese Tertiary Referral Center-Where Are We in 2015? Eye Contact Lens. 2018;44:15–20.
Ganguly S, Salma KC, Kansakar I, Sharma M, Bastola P, Pradhan R. Pattern of fungal isolates in cases of corneal ulcer in the western periphery of Nepal. Nepal J Ophthalmol. 2011;3:118–22.
Al-Ghafri A, Al-Raisi A. The epidemiology of nonviral microbial keratitis in a tertiary care center in Muscat, Oman. Oman J Ophthalmol. 2018;11:213–9.
Mandour SS, Marey HM, Farahat HG. Resistant Microbial Keratitis in South Nile Delta, Egypt: influence of Regional Risk Factors. Semin Ophthalmol. 2016;31:473–8.
PubMed Google Scholar
Waddell KM. Childhood blindness and low vision in Uganda. Eye (Lond). 1998;12:184–92.
Butler TK, Spencer NA, Chan CC, Singh Gilhotra J, McClellan K. Infective keratitis in older patients: a 4 year review, 1998-2002. Br J Ophthalmol. 2005;89:591–6.
Kunimoto DY, Sharma S, Garg P, Gopinathan U, Miller D, Rao GN. Corneal ulceration in the elderly in Hyderabad, south India. Br J Ophthalmol. 2000;84:54–9.
Barua K, Borah M, Deka C, Kakati R. Morbidity pattern and health-seeking behavior of elderly in urban slums: a cross-sectional study in Assam, India. J Fam Med Prim Care. 2017;6:345–50.
Srivastava S, Gill A. Untreated morbidity and treatment-seeking behaviour among the elderly in India: analysis based on National Sample Survey 2004 and 2014. SSM Popul Health. 2020;10:100557.
Green M, Sara S, Hughes I, Apel A, Stapleton F. Trends in contact lens microbial keratitis 1999 to 2015: a retrospective clinical review. Clin Exp Ophthalmol. 2019;47:726–32.
Pan XJ, Jiang T, Zhu H, Liu PP, Zhou ZY, Mao AJ. Corneal infection in Shandong peninsula of China: a 10-year retrospective study on 578 cases. Int J Ophthalmol. 2016;9:53–7.
Oladigbolu K, Rafindadi A, Abah E, Samaila E. Corneal ulcers in a tertiary hospital in Northern Nigeria. Ann Afr Med. 2013;12:165–70.
Zbiba W, Abdesslem NB. Acanthamoeba keratitis: an emerging disease among microbial keratitis in the Cap Bon region of Tunisia. Exp Parasitol. 2018;192:42–5.
Gautam V, Chaudhary A, Singh SK, Rai PG. Profile of Corneal Ulcer in a Month of harvesting Season in a Tertiary Level Eye Hospital of Eastern Nepal. Nepal J Ophthalmol. 2018;10:32–8.
Kumar A, Pandya S, Kavathia G, Antala S, Madan M, Javdekar T. Microbial keratitis in Gujarat, Western India: findings from 200 cases. Pan Afr Med J. 2011;10:48.
Courtright P, Lewallen S, Kanjaloti S, Divala DJ. Traditional eye medicine use among patients with corneal disease in rural Malawi. Br J Ophthalmol. 1994;78:810–2.
Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414.
Fleiszig SMJ, Kroken AR, Nieto V, Grosser MR, Wan SJ, Metruccio MME, et al. Contact lens-related corneal infection: intrinsic resistance and its compromise. Prog Retin Eye Res. 2020;76:100804.
Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27:870–926.
Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, et al. The steroids for corneal ulcers trial: study design and baseline characteristics. Arch Ophthalmol. 2012;130:151–7.
Lalitha P, Srinivasan M, Rajaraman R, Ravindran M, Mascarenhas J, Priya JL, et al. Nocardia keratitis: clinical course and effect of corticosteroids. Am J Ophthalmol. 2012;154:934–9.e1.
Chu HS, Hu FR. Non-tuberculous mycobacterial keratitis. Clin Microbiol Infect. 2013;19:221–6.
Faria S, Joao I, Jordao L. General Overview on Nontuberculous Mycobacteria, Biofilms, and Human Infection. J Pathog. 2015;2015:809014.
Castano G, Elnahry AG, Mada PK. Fungal Keratitis. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2020.
Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–5.
Sharma S, Kunimoto DY, Gopinathan U, Athmanathan S, Garg P, Rao GN. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea. 2002;21:643–7.
Sun XG, Zhang Y, Li R, Wang ZQ, Luo SY, Jin XY, et al. Etiological analysis on ocular fungal infection in the period of 1989 - 2000. Chin Med J (Engl). 2004;117:598–600.
Ting DSJ, Bignardi G, Koerner R, Irion LD, Johnson E, Morgan SJ, et al. Polymicrobial Keratitis With Cryptococcus curvatus, Candida parapsilosis, and Stenotrophomonas maltophilia After Penetrating Keratoplasty: a Rare Case Report With Literature Review. Eye Contact Lens. 2019;45:e5–e10.
Ting DSJ, McKenna M, Sadiq SN, Martin J, Mudhar HS, Meeney A, et al. Arthrographis kalrae Keratitis Complicated by Endophthalmitis: a Case Report With Literature Review. Eye Contact Lens. 2020; https://doi.org/10.1097/ICL.0000000000000713 .
Sahay P, Goel S, Nagpal R, Maharana PK, Sinha R, Agarwal T, et al. Infectious Keratitis Caused by Rare and Emerging Micro-Organisms. Curr Eye Res. 2020;45:761–73.
Somani SN, Ronquillo Y, Moshirfar M. Acanthamoeba Keratitis. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2020.
Carnt N, Hoffman JJ, Verma S, Hau S, Radford CF, Minassian DC, et al. Acanthamoeba keratitis: confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol. 2018;102:1621–8.
Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–99.e2.
Brown AC, Ross J, Jones DB, Collier SA, Ayers TL, Hoekstra RM, et al. Risk Factors for Acanthamoeba Keratitis-A Multistate Case-Control Study, 2008-2011. Eye Contact Lens. 2018;44:S173–S8.
Garg P, Kalra P, Joseph J. Non-contact lens related Acanthamoeba keratitis. Indian J Ophthalmol. 2017;65:1079–86.
Iovieno A, Gore DM, Carnt N, Dart JK. Acanthamoeba sclerokeratitis: epidemiology, clinical features, and treatment outcomes. Ophthalmology. 2014;121:2340–7.
Moshirfar M, Somani SN, Shmunes KM, Espandar L, Gokhale NS, Ronquillo YC, et al. A Narrative Review of Microsporidial Infections of the Cornea. Ophthalmol Ther. 2020;9:265–78.
Friedberg DN, Stenson SM, Orenstein JM, Tierno PM, Charles NC. Microsporidial keratoconjunctivitis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1990;108:504–8.
Ting DSJ, Ghosh N, Ghosh S. Herpes zoster ophthalmicus. BMJ. 2019;364:k5234.
Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013;32:88–101.
Tuli S, Gray M, Shah A. Surgical management of herpetic keratitis. Curr Opin Ophthalmol. 2018;29:347–54.
Lim NC, Lim DK, Ray M. Polymicrobial versus monomicrobial keratitis: a retrospective comparative study. Eye Contact Lens. 2013;39:348–54.
Gorski M, Genis A, Yushvayev S, Awwad A, Lazzaro DR. Seasonal Variation in the Presentation of Infectious Keratitis. Eye Contact Lens. 2016;42:295–7.
Walkden A, Fullwood C, Tan SZ, Au L, Armstrong M, Brahma AK, et al. Association Between Season, Temperature and Causative Organism in Microbial Keratitis in the UK. Cornea. 2018;37:1555–60.
Shankar J, Sueke H, Wiehlmann L, Horsburgh MJ, Tuft S, Neal TJ, et al. Genotypic analysis of UK keratitis-associated Pseudomonas aeruginosa suggests adaptation to environmental water as a key component in the development of eye infections. FEMS Microbiol Lett. 2012;334:79–86.
Dethorey G, Daruich A, Hay A, Renard G, Bourges JL. [Severe bacterial keratitis referred to ophthalmology emergency departments: a retrospective study of 268 cases]. J Fr Ophtalmol. 2013;36:129–37.
Khor HG, Cho I, Lee K, Chieng LL. Spectrum of Microbial Keratitis Encountered in the Tropics. Eye Contact Lens. 2020;46:17–23.
Sagerfors S, Ejdervik-Lindblad B, Söderquist B. Infectious keratitis: isolated microbes and their antibiotic susceptibility pattern during 2004-2014 in Region Örebro County, Sweden. Acta Ophthalmol. 2020;98:255–60.
French DD, Margo CE. Demographic patterns of ED patients diagnosed as having corneal ulcer. Am J Emerg Med. 2013;31:1082–5.
Truong DT, Bui MT, Memon P, Cavanagh HD. Microbial Keratitis at an Urban Public Hospital: a 10-Year Update. J Clin Exp Ophthalmol. 2015;6:498.
Dhakhwa K, Sharma MK, Bajimaya S, Dwivedi AK, Rai S. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepal. J Ophthalmol. 2012;4:119–27.
CAS Google Scholar
Hussain I, Khan BS, Soni M, Iqbal M. Habibullah. Non-viral microbial keratitis: etiology, clinical features and visual outcome. J Coll Physicians Surg Pak. 2012;22:151–4.
Deorukhkar S, Katiyar R, Saini S. Epidemiological features and laboratory results of bacterial and fungal keratitis: a five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singap Med J. 2012;53:264–7.
Sitoula RP, Singh SK, Mahaseth V, Sharma A, Labh RK. Epidemiology and etiological diagnosis of infective keratitis in eastern region of Nepal. Nepal J Ophthalmol. 2015;7:10–5.
Yildiz EH, Airiani S, Hammersmith KM, Rapuano CJ, Laibson PR, Virdi AS, et al. Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea. 2012;31:1097–102.
Sauer A, Greth M, Letsch J, Becmeur PH, Borderie V, Daien V, et al. Contact Lenses and Infectious Keratitis: from a Case-Control Study to a Computation of the Risk for Wearers. Cornea. 2020;39:769–74.
Carnt N, Robaei D, Minassian DC, Dart JKG. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol. 2018;102:1431–5.
Stapleton F, Naduvilath T, Keay L, Radford C, Dart J, Edwards K, et al. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS ONE. 2017;12:e0181343.
Hoddenbach JG, Boekhoorn SS, Wubbels R, Vreugdenhil W, Van Rooij J, Geerards AJ. Clinical presentation and morbidity of contact lens-associated microbial keratitis: a retrospective study. Graefes Arch Clin Exp Ophthalmol. 2014;252:299–306.
Sauer A, Meyer N, Bourcier T, Keratitis FSGfCLRM. Risk Factors for Contact Lens-Related Microbial Keratitis: a Case-Control Multicenter Study. Eye Contact Lens. 2016;42:158–62.
Scanzera AC, Tu EY, Joslin CE. Acanthamoeba Keratitis in Minors With Orthokeratology (OK) Lens Use: a Case Series. Eye Contact Lens. 2020; https://doi.org/10.1097/ICL.0000000000000728 .
Kumar A, Khurana A, Sharma M, Chauhan L. Causative fungi and treatment outcome of dematiaceous fungal keratitis in North India. Indian J Ophthalmol. 2019;67:1048–53.
Suzuki T, Sutani T, Nakai H, Shirahige K, Kinoshita S. The Microbiome of the Meibum and Ocular Surface in Healthy Subjects. Investig Ophthalmol Vis Sci. 2020;61:18.
Graham JE, Moore JE, Jiru X, Goodall EA, Dooley JS, Hayes VE, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Investig Ophthalmol Vis Sci. 2007;48:5616–23.
Khoo P, Cabrera-Aguas M, Robaei D, Lahra MM, Watson S. Microbial Keratitis and Ocular Surface Disease: a 5-Year Study of the Microbiology, Risk Factors and Clinical Outcomes in Sydney, Australia. Curr Eye Res. 2019;44:1195–202.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83.
Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510.
McCulley JP, Shine WE. Changing concepts in the diagnosis and management of blepharitis. Cornea. 2000;19:650–8.
Dohse N, Wibbelsman TD, Rapuano SB, Hammersmith KM, Nagra PK, Rapuano CJ, et al. Microbial keratitis and clinical outcomes following penetrating and endothelial keratoplasty. Acta Ophthalmol. 2020; https://doi.org/10.1111/aos.14404 .
Maharana PK, Sahay P, Sujeeth M, Singhal D, Rathi A, Titiyal JS, et al. Microbial Keratitis After Accelerated Corneal Collagen Cross-Linking in Keratoconus. Cornea. 2018;37:162–7.
Ting DSJ, Said DG, Dua HS. Interface Haze After Descemet Stripping Automated Endothelial Keratoplasty. JAMA Ophthalmol. 2019;137:1201–2.
Fontana L, Moramarco A, Mandarà E, Russello G, Iovieno A. Interface infectious keratitis after anterior and posterior lamellar keratoplasty. Clinical features and treatment strategies. A review. Br J Ophthalmol. 2019;103:307–14.
Randleman JB, Shah RD. LASIK interface complications: etiology, management, and outcomes. J Refract Surg. 2012;28:575–86.
Elmassry A, Said Ahmed OI, Abdalla MF, Gaballah K. Ten years experience of corneal collagen cross-linking: an observational study of 6120 cases. Eur J Ophthalmol. 2020:1120672120928921.
Ting DSJ, Rana-Rahman R, Chen Y, Bell D, Danjoux JP, Morgan SJ, et al. Effectiveness and safety of accelerated (9 mW/cm(2)) corneal collagen cross-linking for progressive keratoconus: a 24-month follow-up. Eye (Lond). 2019;33:812–8.
Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis - Corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17:624–34.
Prajna NV, Radhakrishnan N, Lalitha P, Austin A, Ray KJ, Keenan JD, et al. Cross-Linking-Assisted Infection Reduction: a Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Outcomes in Fungal Keratitis. Ophthalmology. 2020;127:159–66.
Said DG, Elalfy MS, Gatzioufas Z, El-Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121:1377–82.
Dhawan S, Rao K, Natrajan S. Complications of corneal collagen cross-linking. J Ophthalmol. 2011;2011:869015.
Ting DSJ, Bandyopadhyay J, Patel T. Microbial keratitis complicated by acute hydrops following corneal collagen cross-linking for keratoconus. Clin Exp Optom. 2019;102:434–6.
Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15:575–628.
Tempest-Roe S, Joshi L, Dick AD, Taylor SR. Local therapies for inflammatory eye disease in translation: past, present and future. BMC Ophthalmol. 2013;13:39.
Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R. Management of high-risk corneal transplantation. Surv Ophthalmol. 2017;62:816–27.
Wilhelmus KR, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. Herpetic Eye Disease Study: a Controlled Trial of Topical Corticosteroids for Herpes Simplex Stromal Keratitis. Ophthalmology. 2020;127:S5–s18.
Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13.
Li S, Yi G, Peng H, Li Z, Chen S, Zhong H, et al. How Ocular Surface Microbiota Debuts in Type 2 Diabetes Mellitus. Front Cell Infect Microbiol. 2019;9:202.
Zhu L, Titone R, Robertson DM. The impact of hyperglycemia on the corneal epithelium: molecular mechanisms and insight. Ocul Surf. 2019;17:644–54.
Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762.
Dan J, Zhou Q, Zhai H, Cheng J, Wan L, Ge C, et al. Clinical analysis of fungal keratitis in patients with and without diabetes. PLoS ONE. 2018;13:e0196741.
Wang B, Yang S, Zhai HL, Zhang YY, Cui CX, Wang JY, et al. A comparative study of risk factors for corneal infection in diabetic and non-diabetic patients. Int J Ophthalmol. 2018;11:43–7.
Kaiserman I, Kaiserman N, Nakar S, Vinker S. Herpetic eye disease in diabetic patients. Ophthalmology. 2005;112:2184–8.
Liesegang TJ. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1160–5.
Vadoothker S, Andrews L, Jeng BH, Levin MR. Management of Herpes Simplex Virus Keratitis in the Pediatric Population. Pediatr Infect Dis J. 2018;37:949–51.
Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019;17:40–9.
Jan RL, Tai MC, Weng SF, Chang C, Wang JJ, Chang YS. Risk of corneal ulcer in patients with end-stage renal disease: a retrospective large-scale cohort study. Br J Ophthalmol. 2018;102:868–72.
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98.
Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4. https://doi.org/10.1128/microbiolspec.VMBF-0016-2015 .
McDonald EM, Ram FS, Patel DV, McGhee CN. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br J Ophthalmol. 2014;98:1470–7.
Brown L. Resistance to ocular antibiotics: an overview. Clin Exp Optom. 2007;90:258–62.
Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56:2795–805.
Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:322–45.
Ray KJ, Prajna L, Srinivasan M, Geetha M, Karpagam R, Glidden D, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131:310–3.
Vola ME, Moriyama AS, Lisboa R, Vola MM, Hirai FE, Bispo PJ, et al. Prevalence and antibiotic susceptibility of methicillin-resistant Staphylococcus aureus in ocular infections. Arq Bras Oftalmol. 2013;76:350–3.
Prajna NV, Lalitha P, Rajaraman R, Krishnan T, Raghavan A, Srinivasan M, et al. Changing Azole Resistance: a Secondary Analysis of the MUTT I Randomized Clinical Trial. JAMA Ophthalmol. 2016;134:693–6.
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Rev Antimicrobial Resist. 2016:1–81.
Kaye S, Tuft S, Neal T, Tole D, Leeming J, Figueiredo F, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Investig Ophthalmol Vis Sci. 2010;51:362–8.
Lalitha P, Prajna NV, Oldenburg CE, Srinivasan M, Krishnan T, Mascarenhas J, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31:662–7.
Ting DSJ, Beuerman RW, Dua HS, Lakshminarayanan R, Mohammed I. Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front Immunol. 2020;11:983.
Mayandi V, Xi Q, Leng Goh ET, Koh SK, Jie Toh TY, Barathi VA, et al. Rational Substitution of ε-Lysine for α-Lysine Enhances the Cell and Membrane Selectivity of Pore-Forming Melittin. J Med Chem. 2020;63:3522–37.
Mohammed I, Said DG, Nubile M, Mastropasqua L, Dua HS. Cathelicidin-Derived Synthetic Peptide Improves Therapeutic Potential of Vancomycin Against Pseudomonas aeruginosa. Front Microbiol. 2019;10:2190.
Ung L, Bispo PJM, Doan T, Van Gelder RN, Gilmore MS, Lietman T, et al. Clinical metagenomics for infectious corneal ulcers: Rags to riches? Ocul Surf. 2020;18:1–12.
Ting DSJ, Foo VH, Yang LWY, Sia JT, Ang M, Lin H, et al. Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. Br J Ophthalmol. 2020; https://doi.org/10.1136/bjophthalmol-2019-315651 .
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Download references
DSJT acknowledges support from the Medical Research Council/Fight for Sight (FFS) Clinical Research Fellowship (MR/T001674/1) and the FFS/John Lee, Royal College of Ophthalmologists Primer Fellowship (24CO4).
Author information
These authors contributed equally: Darren Shu Jeng Ting, Charlotte Shan Ho
Authors and Affiliations
Academic Ophthalmology, Division of Clinical Neuroscience, School of Medicine, University of Nottingham, Nottingham, UK
Darren Shu Jeng Ting, Dalia G. Said & Harminder S. Dua
Department of Ophthalmology, Queen’s Medical Centre, Nottingham, UK
Darren Shu Jeng Ting, Charlotte Shan Ho, Rashmi Deshmukh, Dalia G. Said & Harminder S. Dua
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Harminder S. Dua .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ting, D.S.J., Ho, C.S., Deshmukh, R. et al. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 35 , 1084–1101 (2021). https://doi.org/10.1038/s41433-020-01339-3
Download citation
Received : 20 July 2020
Revised : 22 October 2020
Accepted : 24 November 2020
Published : 07 January 2021
Issue Date : April 2021
DOI : https://doi.org/10.1038/s41433-020-01339-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Potential applications of artificial intelligence in image analysis in cornea diseases: a review.
- Kai Yuan Tey
- Ezekiel Ze Ken Cheong
Eye and Vision (2024)
Prognostic indicators of corneal ulcer clinical outcomes at a tertiary care center in the Bronx, New York
- Sruthi Kodali
- Behram Khan
- Richard P. Gibralter
Journal of Ophthalmic Inflammation and Infection (2024)
A comparison of antimicrobial regimen outcomes and antibiogram development in microbial keratitis: a prospective cohort study in Alexandria, Egypt
- Amira A. Nayel
- Noha A. Hamdy
- Nelly M. Mohamed
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
Monitoring the transition from corneal ulceration to healed scar using a Scheimpflug tomography–based densitometry
- Yen-Cheng Chen
- Yu-Ting Hsiao
- Ming-Tse Kuo
Green synthesis of broad spectrum microbicidal silver nanoparticles and griseofulvin loaded casein/hydroxypropyl methylcellulose nanocomposite
- Mohamed H. El-Newehy
- Badr M. Thamer
- Meera Moydeen Abdul Hameed
Biomass Conversion and Biorefinery (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Create account
Herpes Simplex Virus Stromal Keratitis and Endotheliitis
All content on Eyewiki is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- 1.1 Disease
- 1.2 Etiology
- 1.3 Epidemiology
- 1.4 Clinical manifestations
- 1.5 Pathophysiology
- 2.1 Differential diagnosis
- 3.1 Medical therapy
- 3.2 Surgical therapy
- 3.3 Prophylactic therapy
- 3.4 Current research
- 4 References
Disease Entity
Herpes Simplex Virus (HSV) Stromal Keratitis and Endotheliitis
HSV infection can cause inflammation in nearly every ocular tissue. In cases of corneal involvement, the epithelium, stroma, or endothelium may be affected. Both herpes stromal keratitis (HSK) and HSV endotheliitis can present clinically with stromal opacity and, therefore, may be difficult to distinguish. In HSK, stromal opacity is due to immunopathology within the stroma, is often associated with corneal neovascularization , and recurrent episodes can lead to irreversible stromal scarring and vision loss.
In HSV endotheliitis, either secondary inflammation caused by the virus and/or direct infection of endothelial cells is thought to cause endothelial dysfunction and subsequent stromal edema and opacity. Stromal scarring from true endotheliitis is rare. While presentation may appear similar between HSK and HSV endotheliitis, they represent two distinct pathologic mechanisms that are both induced by HSV infection.
The majority of ocular HSV infections are caused by HSV type 1 (HSV-1), except in cases of neonatal ocular infections, which are largely caused by HSV-2 contracted during decent through an infected birth canal. [1] The herpes viruses are unique in their ability to cause productive infections as well as establish latency, a relatively quiescent state in which the viral genome is retained in the absence of productive virus particle production. Therefore, the virus is never completely eradicated from the host and may reactivate causing recurrent disease. In response to certain stressors, including exposure to ultraviolet light, [2] psychological stress [3] , and hormonal fluctuation, [4] HSV may reactivate from latency to produce infectious virions with recurrent disease at the initial sight of infection. HSV infection of the mucosal and epithelial surfaces of the orofacial region, including the cornea, precedes retrograde axonal transport of the virus within the respective division of the trigeminal nerve to the trigeminal ganglia, where additional viral replication occurs and latency is established within neuronal nuclei. In times of stress, the virus may reactivate, travel back down the ophthalmic division of the trigeminal nerve and cause recurrent disease in the cornea. It has also been suggested that the cornea itself may be a reservoir for viral latency, although this theory has yet to be definitively proven. [5]
Epidemiology
HSV-1 infects the majority of the world’s adult population with recent seroprevalence studies from the United States and Germany detecting 57.7% and ~75% HSV-1 positivity, respectively. [6] [7] The annual incidence of ocular HSV infections has recently been estimated at 11.8 per 100000 people in the United States [8] and 13.2 per 100000 in France, where the incidence of recurrence was 18.3 per 100000 people. [9] In the Herpetic Eye Disease Study, 18% of patients diagnosed with HSV-1 ocular disease experienced a recurrence involving the stroma, [10] and stromal keratitis represented 44% of all recurrences. [11] Furthermore, previous bouts of HSK significantly increased the risk of future recurrences of stromal disease. Therefore, HSK represents a significant burden of ocular disease caused by HSV-1 infection.
The epidemiology of HSV endotheliitis is less clear at least in part because many publications include disciform HSV endotheliitis as a form of HSK.
Clinical manifestations
While both HSK and HSV endotheliitis can present with stromal opacity, they are separate pathological processes. The stromal opacity in HSK is driven by inflammation within the stroma that is often accompanied by neovascularization (Figure 1). The opacity in HSV endotheliitis is the result of stromal edema due to HSV-mediated endothelial dysfunction in the absence of stromal inflammation or neovascularization (Figure 2).

HSK can be classified as either necrotizing or non-necrotizing. [12] [13] In necrotizing HSK, an overlying epithelial defect is often present, and the risk of stromal melting and perforation is high. Both viral and immune-mediated destruction of the cornea is implicated in necrotizing HSK. Conversely, in non-necrotizing HSK, also known as immune or interstitial HSK, the epithelium is intact, and the pathology is thought to driven primarily by the host immune response.

In HSV endotheliitis, stromal edema is often accompanied by underlying keratic precipitates (KP) and an anterior chamber inflammatory reaction. HSV endotheliitis is categorized into three main forms, namely disciform, which is the most common, diffuse, and linear, based on the pattern of endothelial dysfunction and stromal edema. Intraocular pressure may be elevated secondary to trabeculitis. [14]
- Stages of severity

Mild herpes simplex stromal keratitis (Courtesy of Christopher J. Rapuano, MD)

Moderate herpes simplex stromal keratitis (Courtesy of Christopher J. Rapuano, MD)

Severe herpes simplex stromal keratitis (Courtesy of Christopher J. Rapuano, MD)

Pathophysiology
The pathophysiology of HSK is complex and remains incompletely understood. Current data suggests that CD4 T cells of the adaptive immune system are required for the development and maintenance of HSK. [15]
Based on extensive studies in animal models, several theories exist as to the stimulus for the immunopathological CD4 T cell response:
- CD4 T cells are HSV-specific and driven by the presence of HSV antigen within the stroma; [16]
- The inflammatory milieu created by HSV corneal infection drives a non-specific bystander CD4 T cell response; [17]
- Autoreactive CD4 T cells are stimulated by corneal proteins that mimic HSV proteins, [18] although this observation has not been reproduced and likely represents a phenomenon specific to the animal model used in the study
- Or a combination of these mechanisms.
HSV DNA, protein, or live virus is inconsistently found in cases of HSK, in comparison to HSV epithelial keratitis where HSV is often identified. Other leukocytes, such as neutrophils, also infiltrate the stroma during HSK, and stromal neovascularization is a key component of the inflammatory cascade that leads to stromal opacity. [19] [20]
Deturgesence of the corneal stroma is accomplished in part by the pump mechanism of the endothelium. Endothelial cell dysfunction can lead to inadequate stromal dehydration with subsequent edema and opacity. In HSV endotheliitis, endothelial cells are dysfunctional due to either direct infection with HSV or as a consequence of the anterior chamber inflammation induced by HSV infection. Inflammation with the stoma can be a component of HSV endotheliitis.
The diagnosis of HSK and HSV endotheliitis is predominantly clinical, based on a history of recurrent herpetic ocular disease and slit lamp examination of the eye revealing characteristic herpetic lesions as described above. Additionally, PCR analysis, enzyme-linked assays, and viral culture of the tear film may reveal HSV-1 DNA, protein, or live virus, respectively. [21] However, sensitivity of these tests is greatly reduced in the absence of active corneal ulceration, such as in non-necrotizing HSK and HSV endotheliitis. [22] While virus has been isolated from the anterior chamber of patients with HSV endotheliitis, it is rarely detected in the tear film. [23]
Differential diagnosis
The differential diagnosis for HSK includes other etiologies of infectious interstitial keratitis , such as other viruses (Varicella zoster virus, Epstein-Barr virus), bacteria (syphilis, lyme ), fungus, and Acanthamoeba, as well as immune-mediated etiologies, including sarcoidosis and Cogan syndrome .
The differential diagnosis for HSV endotheliitis includes endothelial dysfunction caused by other herpes viruses (Varicella zoster virus, cytomegalovirus) as well as the inflammatory glaucomatous conditions Fuchs’ heterochromic iridocyclitis and Posner-Schlossman syndrome , which have been postulated to have viral etiologies, including herpes viruses. [24] [25] [26]
Current management of acute HSK and HSV endotheliitis is aimed at inhibiting viral replication with oral or topical antiviral medications and reducing inflammatory damage with topical corticosteroids.
Medical therapy
As discussed above, the immune response to corneal HSV-1 infection is a major contributor to the stromal damage and subsequent scarring responsible for the blinding complications of HSK. Thus, the standard of care for treatment of HSK involves topical steroids, which broadly inhibit the damaging immune response, often in combination with oral antiviral medication to block potential viral replication [27]
Surgical therapy
In cases of chronic or recurrent HSK in which stromal scarring has caused severe visual impairment, corneal transplantation in the form of either full-thickness penetrating keratoplasty or anterior lamellar keratoplasty may be attempted to restore corneal clarity. It is well established that full-thickness grafts placed in patients with a history of HSK have a higher rate and incidence of rejection compared to grafts placed for non-inflammatory conditions. [28]
The exact mechanisms responsible for accelerated graft rejection in patients with a history of herpetic eye disease remains incompletely understood but viral reactivation induced by the grafting procedure with severing of trigeminal nerve termini is thought to contribute to the rejection process. Thus, it is common practice to place patients undergoing corneal transplantation for HSV corneal infection on long-term systemic antiviral therapy to inhibit potential viral replication.
More recently, deep anterior lamellar keratoplasty (DALK) has been used as an alternative surgical approach in patients with visually significant stromal scars from HSK in which the endothelium remains healthy. DALK offers the option of replacing only the corneal stroma with preservation of the host endothelium, which is often the focus of transplant rejection. Initial results using DALK appear promising with several small studies demonstrating high rates of graft survival. [29] [30] [31]
Prophylactic therapy
The potentially blinding complications of HSK stem from the recurrent nature of the disease. Repeated bouts of stromal inflammation may eventually lead to irreversible scarring and loss of sight. Recurrences are likely driven by viral reactivation in the trigeminal ganglion with transport of the virus to the cornea, leading to a local immune response within the stroma. A potentially useful mechanism of therapy, therefore, involves inhibiting viral reactivation to prevent recurrent corneal inflammation. A landmark trial that was part of the Herpetic Eye Disease Study demonstrated that systemic antiviral therapy with oral acyclovir significantly reduced the frequency of recurrent ocular HSV-1 disease. [32] In patients with a history of stromal disease specifically, oral acyclovir treatment reduced the probability of HSK recurrence by 50%, from 28% to 14%. These data suggest that blocking viral replication systemically, likely within the trigeminal ganglia where reactivation begins, inhibits the impetus for recurrent stromal inflammation. Moreover, acyclovir’s mechanism of action, phosphorylation by viral thymidine kinase with subsequent incorporation into viral DNA by viral DNA polymerase resulting in strand termination, requires active viral DNA replication, suggesting that HSV-1 must be at least in the process of reactivating for acyclovir to function.
Current research
Current research has investigated therapeutic HSV vaccination strategies with the goal of inhibiting viral reactivation within the trigeminal ganglia and preventing reactivation events. CD8 T cells of the adaptive immune response function to inhibit viral reactivation within the trigeminal ganglion using non-lytic mechanisms. [33] Therefore, a therapeutic vaccine designed to boost the CD8 T cell response within latently infected ganglia may achieve inhibition of viral reactivation and subsequent corneal inflammation without the need for daily oral medication. Consistent with this hypothesis, a small controlled trial in humans demonstrated a significant reduction in HSV-1 ocular recurrences following subcutaneous administration of heat-inactivated HSV-1. [34] Although there was a trend toward decreased HSK recurrences in the vaccinated group, the study was not powered to achieve statistical significance for this subgroup of HSV ocular disease. Larger clinical trials are required to validate this finding.
- ↑ Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol 2006;141(3):547-57.
- ↑ Spurney RV, Rosenthal MS. Ultraviolet-induced recurrent herpes simplex virus keratitis. Am J Ophthalmol 1972;73(4):609-10.
- ↑ Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol 2007;179(1):322-8.
- ↑ Cherpes TL, Busch JL, Sheridan BS, et al. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol 2008;181(2):969-75.
- ↑ Kennedy DP, Clement C, Arceneaux RL, et al. Ocular herpes simplex virus type 1: Is the cornea a reservoir for viral latency or a fast pit stop? Cornea 2011;30(3):251-9.
- ↑ Rabenau HF, Buxbaum S, Preiser W, et al. Seroprevalence of herpes simplex virus types 1 and type 2 in the Frankfurt am Main area, Germany. Med Microbiol Immunol 2002;190(4):153- 60.
- ↑ Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006;296(8):964-73.
- ↑ Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: The effect of oral antiviral prophylaxis. Arch Ophthalmol 2010;128(9):1178-83.
- ↑ Labetoulle M, Auquier P, Conrad H, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmol 2005;112(5):888-95.
- ↑ Predictors of recurrent herpes simplex virus keratitis. Herpetic Eye Disease Study Group. Cornea 2001;20(2):123-8.
- ↑ Oral acyclovir for herpes simplex virus eye disease: Effect on prevention of epithelial keratitis and stromal keratitis. Herpetic Eye Disease Study Group. Arch Ophthalmol 2000;118(8):1030-6.
- ↑ Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea 1999;18(2):144-54.
- ↑ Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea 1999;18(2):127-43.
- ↑ Amano S, Oshika T, Kaji Y, et al. Herpes simplex virus in the trabeculum of an eye with corneal endotheliitis. Am J Ophthalmol 1999;127(6):721-2.
- ↑ Knickelbein JE, Beula KA, Hendricks RL. Herpes stromal keratitis: Erosion of ocular immune privilege by herpes simplex virus. Future Virol 2010;5(6):699-708.
- ↑ Verjans GM, Remeijer L, van Binnendijk RS, et al. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis 1998;177(2):484-8.
- ↑ Gangappa S, Deshpande SP, Rouse BT. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest Ophthalmol Vis Sci 2000;41(2):453-9.
- ↑ Zhao ZS, Granucci F, Yeh L, et al. Molecular mimicry by herpes simplex virus-type 1: Autoimmune disease after viral infection. Science 1998;279(5355):1344-7.
- ↑ Rowe AM, St Leger AJ, Jeon S, et al. Herpes keratitis. Prog Retin Eye Res 2012; Epub ahead of print.
- ↑ Gimenez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis - A focus on corneal neovascularization. Prog Retin Eye Res 2012; Epub ahead of print.
- ↑ Kowalski RP, Gordon YJ, Romanowski EG, et al. A comparison of enzyme immunoassay and polymerase chain reaction with the clinical examination for diagnosing ocular herpetic disease. Ophthalmol 1993;100(4):530-3.
- ↑ Fukuda M, Deai T, Higaki S, et al. Presence of a large amount of herpes simplex virus genome in tear fluid of herpetic stromal keratitis and persistent epithelial defect patients. Semin Ophthalmol 2008;23(4):217-20.
- ↑ Fukuda M, Deai T, Hibino T, et al. Quantitative analysis of herpes simplex virus genome in tears from patients with herpetic keratitis. Cornea 2003;22(7 Suppl):60.
- ↑ de Groot-Mijnes JD, de Visser L, Rothova A, et al. Rubella virus is associated with fuchs heterochromic iridocyclitis. Am J Ophthalmol 2006;141(1):212-4.
- ↑ Barequet IS, Li Q, Wang Y, et al. Herpes simplex virus DNA identification from aqueous fluid in Fuchs heterochromic iridocyclitis. Am J Ophthalmol 2000;129(5):672-3.
- ↑ Yamamoto S, Pavan-Langston D, Tada R, et al. Possible role of herpes simplex virus in the origin of Posner-Schlossman syndrome. Am J Ophthalmol 1995;119(6):796-8.
- ↑ Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: An evidence-based review. Surv Ophthalmol 2009;54(2):226-34.
- ↑ Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol 2005;140(6):1112-22.
- ↑ Sarnicola V, Toro P. Deep anterior lamellar keratoplasty in herpes simplex corneal opacities. Cornea 2010;29(1):60-4.
- ↑ Wang J, Zhao G, Xie L, et al. Therapeutic effect of deep anterior lamellar keratoplasty for active or quiescent herpetic stromal keratitis. Graefes Arch Clin Exp Ophthalmol 2012;250(8):1187-94.
- ↑ Wu SQ, Zhou P, Zhang B, et al. Long-term comparison of full-bed deep lamellar keratoplasty with penetrating keratoplasty in treating corneal leucoma caused by herpes simplex keratitis. Am J Ophthalmol 2012;153(2):291-9 e2.
- ↑ Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. NEJM 1998;339(5):300-6.
- ↑ Knickelbein JE, Khanna KM, Yee MB, et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 2008;322(5899):268-71.
- ↑ Pivetti-Pezzi P, Accorinti M, Colabelli-Gisoldi RA, et al. Herpes simplex virus vaccine in recurrent herpetic ocular infection. Cornea 1999;18(1):47-51.
- Cornea/External Disease

- For Ophthalmologists
- For Practice Management
- For Clinical Teams
- For Public & Patients
Museum of the Eye
- Browse All Education
- Learning Plans
- Interactive
- Focal Points
- Wills Eye Manual
- Disease Reviews
- Clinical Webinars
- Diagnose This
- Self-Assessments
- Glaucoma Education Center
- Pediatric Ophthalmology Education Center
- Global Ophthalmology Guide
- Laser Surgery Education Center
- Redmond Ethics Center
- Ocular Trauma Resources
- Myopia Resources
- Thyroid Eye Disease Resources
- Practice Guidelines
- Drug-Resistant Pseudomonas Outbreak
- Preferred Practice Patterns
- Clinical Statements
- Ophthalmic Technology Assessments
- Patient Safety Statements
- Complementary Therapy Assessments
- Medical Information Technology
- Diagnostic Excellence
- Choosing Wisely
- Eye Care for Older Adults
- Eye Disease Statistics
- About the Hoskins Center
- Artificial Intelligence
- Premium IOLs
- Patient-Reported Outcomes with LASIK Symptoms and Satisfaction
- Multimedia Library
- 1-Minute Videos
- Presentations and Lectures
- Master Class Videos
- Basic Skills Videos
- Clinical and Surgical Videos
- Resident Lectures
- Submit a Video
- YO Video Contest
- Browse Podcast Library
- Experts InSight Podcast
- Ophthalmology Journal Podcast
- Submit an Image
- Browse Clinical News
- Editors' Choice
- Current Insight
- CME Central
- About Continuing Certification
- Claim CME Credit and View Transcript
- CME Planning Resources
- Complete Your Financial Disclosure
- LEO Continuing Education Recognition Award
- Safe ER/LA Opioid Prescribing
- Check Your Industry Payment Records
- Resident Education Home
- Flashcards and Study Presentations
- Interactive Cases and Simulations
- Diversity and Inclusion Education
- News and Advice from YO Info
- Board Prep Resources
- OKAP and Board Review Presentations
- Study Flashcards
- PGY-1 and PGY-2 Resources
- Physician Wellness
- Resident Knowledge Exchange
- Simulation in Resident Education
- Ophthalmology Job Center
- Clinical Education /
- Multimedia /
Log in to view this page

Viral keratitis
- Mark Complete
Primary herpes simplex ocular infection usually presents as a unilateral foreignbody sensation with watery discharge. There may be skin vesicles on the lids or enlarged preauricular lymph nodes. The herpes simplex virus resides in the trigeminal ganglia, and recurrent outbreaks of herpetic lesions result from periodic reactivation of the virus.

All content on the Academy’s website is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- About the Academy
- Jobs at the Academy
- Financial Relationships with Industry
- Medical Disclaimer
- Privacy Policy
- Terms of Service
- Statement on Artificial Intelligence
- For Advertisers
FOLLOW THE ACADEMY
Medical Professionals
Public & Patients
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest news
- Stock market
- Premium news
- Biden economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance transfer cards
- Cash-back cards
- Rewards cards
- Travel cards
- Personal loans
- Student loans
- Car insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Excision biotherapeutics announces oral presentation of preclinical hsv-1 keratitis data at crisprmed24 conference on april 24, 2024.
Treatment with EBT-104 resulted in over 90% reduction in viral shedding in HSV-1 Keratitis model
SAN FRANCISCO, April 22, 2024 (GLOBE NEWSWIRE) -- Excision BioTherapeutics, Inc. (“Excision”, the “Company”), a clinical-stage biotechnology company developing CRISPR-based therapies to cure serious latent viral infectious diseases, today announced that it will deliver an oral presentation highlighting new data from its herpes simplex virus-1 keratitis (HSV-1 Keratitis) program, EBT-104, at CRISPRMED24, the CRISPR Medicine Conference, which is being held from April 23-25 in Copenhagen, Denmark.
Excision’s EBT-104 is a CRISPR-based gene therapy that is being developed as a potential cure for HSV-1 Keratitis. EBT-104 utilizes a CRISPR/Cas gene editing system to inactivate the latent HSV-1 virus.
“This research marks a significant advancement in our understanding and treatment of HSV-1 keratitis and further demonstrates the broad potential of our unique gene editing platform to treat latent viral infections,” said Daniel Dornbusch, Chief Executive Officer of Excision. “The exceptional in vivo efficacy demonstrated by our gene editing approach offers new hope for patients suffering from this debilitating condition. We look forward to sharing these new data from our EBT-104 program at the first CRISPRMED24 Conference.”
To assess the efficacy of CRISPR/Cas9-mediated gene editing on HSV-1 in vivo , a single all-in-one AAV8(Y733F) and AAV9 vectors delivery of SaCas9 and paired gRNAs were employed in a latent rabbit model of HSV-1 keratitis via corneal scarification. This approach led to a remarkable reduction of over 60% in viral shedding from the treated rabbit eyes. Building upon this success, the intravenous administration of all-in-one AAV8(Y733F) and AAV9 vectors expressing SaCas9 and paired gRNAs was explored in the same rabbit model. Impressively, 91.7% (11/12) of treated eyes exhibited no viral shedding. Even at low AAV dose (6E+12 VG/kg), we observed significant levels of AAV vector genomes in the trigeminal ganglia (TG) where the latent HSV-1 resides. Additionally, we detected reduced copies of HSV-1 viral DNA and latency-associated transcript (LAT) RNA in the trigeminal ganglia (TG) of rabbits treated with the AAV9-SaCas9 vector compared to the control group. These results demonstrate that the delivery of all-in-one AAV9-SaCas9 vectors can serve as an effective and safe one-time therapeutic strategy for treating HSV-1 keratitis.
About Herpes Simplex Keratitis
Herpes Simplex Keratitis (HSK) caused by the infection of herpes simplex virus type 1 (HSV-1) in the cornea is a major cause of blindness worldwide. Although current anti-HSV-1 therapies interfere with viral DNA replication, they do not eliminate HSV-1 reservoirs or prevent recurrence. CRISPR/Cas-mediated gene editing can potentially address the underlying causes of the disease by directly eliminating the latent HSV-1 reservoirs.
About Excision BioTherapeutics, Inc. Excision BioTherapeutics, Inc. develops CRISPR-based medicines as potential cures for serious viral latent infectious diseases. The Company’s proprietary, multiplexed gene editing platform unites CRISPR technologies with a novel gene editing approach which demonstrated the ability to stop viral replication. Excision’s pipeline targets large, underserved markets including herpes simplex virus (HSV-1 keratitis), hepatitis B virus (HBV), and human immunodeficiency virus-1 (HIV-1). Excision’s foundational technologies were developed in the laboratories of Dr. Kamel Khalili at Temple University and Dr. Jennifer Doudna at the University of California, Berkeley. For more information, please visit www.excision.bio .
Contact: John Fraunces LifeSci Advisors 917-355-2395 [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley-Blackwell Online Open

Infectious keratitis: A review
Maria cabrera‐aguas.
1 Save Sight Institute, Discipline of Ophthalmology, Faculty of Medicine and Health, The University of Sydney, Sydney New South Wales, Australia
2 Corneal Unit, Sydney Eye Hospital, Sydney New South Wales, Australia
Pauline Khoo
Stephanie l. watson.
Globally, infectious keratitis is the fifth leading cause of blindness. The main predisposing factors include contact lens wear, ocular injury and ocular surface disease. Staphylococcus species, Pseudomonas aeruginosa, Fusarium species, Candida species and Acanthamoeba species are the most common causal organisms. Culture of corneal scrapes is the preferred initial test to identify the culprit organism. Polymerase chain reaction (PCR) tests and in vivo confocal microscopy can complement the diagnosis. Empiric therapy is typically commenced with fluoroquinolones, or fortified antibiotics for bacterial keratitis; topical natamycin for fungal keratitis; and polyhexamethylene biguanide or chlorhexidine for acanthamoeba keratitis. Herpes simplex keratitis is mainly diagnosed clinically; however, PCR can also be used to confirm the initial diagnosis and in atypical cases. Antivirals and topical corticosteroids are indicated depending on the corneal layer infected. Vision impairment, blindness and even loss of the eye can occur with a delay in diagnosis and inappropriate antimicrobial therapy.
1. INTRODUCTION
Infectious keratitis is an infection of the cornea also known as infectious corneal ulcer or corneal opacity. Infectious keratitis can be classified as microbial keratitis (bacteria, fungi or parasites), or viral keratitis (herpes viruses). 1 , 2 The number of cases of corneal blindness due to infectious keratitis has decreased from about 1.6 million in 1990 to 1.3 million in 2015, 3 , 4 , 5 , 6 and of vision impairment from 3.3 million to 2.9 million cases during the same period, 4 despite these data being underreported. Infectious keratitis is the most common cause of non‐trachomatous corneal opacification and the fifth leading cause of blindness overall causing 3.5% (36 million) of all blind individuals up to 2015. 5 Epidemiological data for infectious keratitis is difficult to capture as most data are reported under ‘corneal blindness’ comprising traumatic, infectious, inflammatory and inherited conditions. 2
Microbial keratitis incidence differs worldwide. In developed countries, the incidence has been reported at 27.6 per 100 000 years in the United States (US) in 1999, 40.3 per 100 000 in England in 2006, and 6.6 per 100 000 in Australia in 2015. 2 , 7 A contrasting situation is found in developing countries in Asia where infectious keratitis is a public health threat. These countries face difficulties in accessing health care, poor health indices and higher proportion of workers in farming and agriculture with incidences as high as 113 per 100 000 in Madurai, Tamil Nadu, India; 339 per 100 000 in Bhutan; 710 in Burma; and 799 in Nepal. 2 , 3 , 6
In the United States, infectious keratitis is responsible for about 1 million visits to health professionals and 58 000 to emergency departments annually costing the US health system 175 million dollars in direct health expenditure. 1 , 2 The real burden of the disease worldwide is difficult to ascertain; however, poor rural and agricultural populations are likely to be disproportionately affected. 2
2. BACTERIAL KERATITIS
Bacterial keratitis (BK) is the most common cause of microbial keratitis. 1 It is an ophthalmic emergency requiring immediate attention as it can progress rapidly. 8 , 9 BK is one of the most common causes of visual impairment in working age adults. 3
2.1. Predisposing factors
Predisposing risk factors for BK include contact lens wear (CLW, Figure 1 )), previous topical steroid use, ocular surface disease (OSD), ocular trauma, previous keratitis and prior surgery (Figure 2 ) and corneal disease (Figure 3 ). 1 , 10 , 11 , 12 Studies have shown the risk of contact lens (CL)‐related keratitis decreases with age. 10 , 13 While the risk of keratitis related to a history of corneal transplant, previous ocular surgery within the last 3 months prior to infection, OSD and diabetes mellitus significantly increases with age. 10 , 13

Slit lamp image of a case of bacterial keratitis in a contact lens wearer with typical features; there is a central corneal infiltrate with an overlying epithelial defect and conjunctival hyperaemia

Bacterial keratitis in a failed corneal graft with a broken suture. The graft is oedematous and inferiorly a white infiltrate and larger epithelial defect can be seen within the graft. There is peripheral host vascularisation and conjunctival hyperaemia

Slit lamp image of a protruding cornea with bacterial keratitis. The patient has keratoconus complicated by corneal hydrops and then bacterial infection. Scattered infiltrates can be seen across most of the protuberant cornea and the conjunctiva is hyperaemic
The main risk factor for BK in developed countries is CLW 10 , 14 whereas trauma is the main risk factor in developing countries. 14 In the United States, there are about 45 million contact lens wearers. In 2010, the estimated incidence of microbial keratitis cases per 100 000 person‐year was 130 among CLW versus 14 non‐wearers in the United States. 1
2.2. Clinical features
Clinical features and symptoms of BK are described in Table 1 . BK can occur in a range of clinical scenarios and present with variable clinical findings though most cases will have a corneal infiltrate, epithelial defect and conjunctival hyperemia as shown in Figures 1 , ,2, 2 , ,3. 3 . Figure 1 illustrates a case of BK in a contact lens wearer with a central infiltrate and defect. In Figure 2 , a failed corneal graft with a broken suture predisposed the cornea to infection and the signs of graft failure and BK are both present. Keratoconus can be complicated by corneal hydrops in which a break in Descemet's membrane produces corneal oedema, in Figure 3 the diffuse corneal oedema and BK have resulted in widespread corneal opacity and infiltrates.
Summary of causal organism(s), clinical features, diagnostic tests and treatment of four types of infectious keratitis
Abbreviations: ACV, aciclovir; BD, twice daily; CL, contact lenses; CoNS, coagulase‐negative staphylococci; FBS, foreign body sensation; HSK, herpes simplex keratitis; IVCM, in vivo confocal microscopy; Occ, ointment; PCR, polymerase chain reaction; PHMB, polyhexamethylene biguanide; spp ., species; TDS, three times daily; VLC, valaciclovir.
The severity of BK can be divided into mild, moderate and severe. Mild corneal ulcers are those <2 mm in size with the depth of the ulcer <20% or 100 μm corneal thickness. Superficial infiltrates near the ulcer may also be seen. Moderate corneal ulcers range between 2 and 5 mm in size, depth of 20%–50% (100–275 μm) of the cornea, with dense infiltrates extending to the mid stroma. Severe ulcers are ≥5 mm, with a depth of more than 50% (>275 μm) and dense infiltrates reaching the deep layers of the corneal stroma. 17 Poor patient outcomes have been associated with increased severity. 10 , 17
2.3. Diagnostic tests
A diagnosis of BK is made from the patient's history as well as microbiology tests. Preferably, all corneal ulcers should be cultured for the identification of the causal organism and the antibiotic susceptibility before commencing antimicrobial therapy. 11 The American Academy of Ophthalmology (AAO), BK preferred practice pattern, recommends smears and/or cultures in the following situations 12 :
- Central and large corneal infiltrate and/or associated with significant stromal involvement or melting
- Chronic or unresponsive infection to broad‐spectrum antibiotic therapy
- History of corneal surgeries
- Atypical clinical features suggesting fungal, amoebic or mycobacterial keratitis
- Multiple infiltrates on the cornea
2.3.1. Microbiology evaluation
Microbiology evaluation includes smear examination and culture of corneal scrapings into several media to grow organisms for identification. 18 The culture media (two blood agars, chocolate agar, Sabouraud's agar slope and cooked meat medium) should be taken from the fridge and left for 1 h to reach room temperature. The corneal ulcer samples are then collected from the area of corneal infiltration using blades or typically 25‐gauge needles after instilling an anaesthetic eye drop (i.e., lignocaine 1%), with the first samples placed on glass slides for staining and then onto the media for culture. 14 , 19 Superficial corneal samples can be processed to 10% KOH‐calcoflour white wet mount, Gram or Giemsa staining onto glass slides for microscopy. 18 Gram staining is beneficial providing prompt results in 5 min, can identify aerobic and anaerobic bacteria, fungi, amoeba and microsporidia, documents morphology of rods and cocci and distinguishes Gram‐positive and Gram‐negative organisms. 11 Gram staining detects the type of organism in 60%–75% of bacterial cases. 14
The positive culture rate from corneal scrapes ranges from 38% to 66% 6 , 7 , 9 , 20 , 21 , 22 , 23 , 24 from different studies worldwide. 25 , 26 In cases of progressive BK or where a negative result has been obtained from corneal scrapes or the organism identified does not match the clinical picture, a corneal biopsy can be performed. A lamellar corneal biopsy can be taken using a dermal trephine or freehand dissection, the specimen is divided into two halves to allow histopathological and microbiological analysis. 12 , 27
2.3.2. Polymerase chain reaction test
There is a need for more sensitive and fast‐processing diagnostic methods due to the delay in identifying the causal organism(s) from corneal scrape cultures. Another test used in the diagnosis of microbial keratitis is the polymerase chain reaction (PCR) test. 28 This is a molecular technique for the detection and analysis of specific DNA sequencies consisting of repeated cycles of denaturation, amplification and replication in which segments of DNA are continuously multiplied to enable their detection. 25 , 28 , 29 All bacteria have the 16S ribosomal DNA (16S RNA) gene which consists in highly conserved regions of nucleotide sequences, interspersed with nine variable regions that are genus or species specific. The broad PCR primers target the conserved regions amplifying the variable regions. The genus or species of bacteria is identified after following sequencing and comparison to stored sequences in a database. 26 , 30 The advantages of PCR include its speed, sensitivity and cost‐effectiveness relative to culture and staining, ability to quickly differentiate bacterial and fungal ulcers, and the detection of slow‐growing bacteria and organisms that are traditionally difficult to cultivate or identify with traditional microbiological methods. 25 , 26 , 29 On the other hand, the disadvantages include the high rate of false positive errors from commensal contaminants or dead bacteria, lower specificity compared with culture and staining, need to narrow the list of causative agents to use specific primers, difficulty for treating clinician to interpret which of the identified organisms is the causal one, less cost‐effective when performed with a multi‐organisms PCR approach, supply costs, equipment fees and training expenses. 25 , 26 , 29
Different studies have compared culture versus PCR results in BK. Eleinen et al, reported the sensitivity to culture of 57.58% versus PCR sensitivity of 87.88%, 29 , 31 while Kim et al. reported a similar result for sensitivity to culture (56%) but lower PCR sensitivity (76%). 29 , 32 A study from Liverpool, United Kingdom (UK) reported that the overall BK detection rate was 36%, using culture and PCR analysis. Of these, 72.2% of isolates were detected by 16S rRNA gene PCR and 63.9% by culture. A combination of both PCR and culture detection methods significantly increased the overall isolation rate by 13% compared with using culture alone. Nevertheless, in negative cultures, 16S PCR yielded more results suggestive of potential organisms than cultures in 16S PCR negative samples, hence there were more 16S PCR positive samples with inconclusive results compared to cultures. 26 Surprisingly, another study from the UK, reported bacterial PCR sensitivity of 25% versus culture of 95.6%. The authors discussed that the higher rates of PCR sensitivity in other studies may have been due to the detection of non‐pathogenic bacteria or better culture in combination with less effective PCR in their laboratories. 28
The PCR related technologies also have an important role in diagnosing rare organisms such as atypical mycobacteria and Nocardia species . 33 Atypical mycobacteria can be identified by a rapid and sensitive test such as the LightCycle system which combines real‐time PCR with fluorescence resonance energy transfer to obtain fast PCR results to identify different organisms. 23 , 34 , 35 This system performs a melting curve analysis to differentiate closely related organisms including polyomaviruses, Bordetella species and Bartonella species . 34 , 36 Molecular tests such as PCR and gene sequencing with restriction endonuclease analysis of 16S rRNA gene and restriction fragment length polymorphism analysis of heat shock protein gene, DNA sequencing and pyrosequencing can be used for the identification of Nocardia species . 35 , 37 , 38 Gene sequencing have identified several Nocardia species with a sensitivity of 88% and specificity of 76%. The PCR based hsp65 gene sequencing can isolate species causing ocular Nocardiosis. 38 PCR has the advantage of detecting even fastidious microorganisms from a small specimen and can be rapidly performed compared with prolonged culture times for such organisms. However, PCR is expensive and not readily available at all sites. 33 , 37 , 38 Despite these drawbacks, the evidence suggests that having multiple diagnostic tests available is needed to optimise the yield of positive cases to assist in an adequate diagnosis and antibiotic therapy. 28
2.4. Microbiological patterns
The type of causative organism varies according to the patient's predisposing risk factors and geographical regions. However, despite local and regional variations in BK, the most commonly reported causative organisms appear consistent worldwide, with a higher proportion of infections caused by Gram‐positive (48%–89%) than Gram‐negative isolates (11%–50%). 2 Caution is needed when interpreting results as most eyelid and ocular surface commensal organisms are Gram‐positive and likely to contaminate the sample. 39 Nonetheless, the most common Gram‐positive organisms include Staphylococcus aureus , Coagulase‐negative staphylococci (CoNS), and Streptococcus pneumoniae . 1 , 2 , 9 , 39 Among Gram‐negative organisms, Pseudomonas aeruginosa has been reported to be the most common causative organism and has been implicated in BK among CLWs. 1 , 10 While, CoNS have been implicated in OSD patients. 40
2.5. Treatment
2.5.1. antibiotic therapy.
Adequate treatment for BK is key to avoid serious complications such as vision impairment or even the loss of the eye. 9 , 12 The initial treatment is generally empiric as culture results can take over 48 h, and the infection can progress rapidly without treatment. The mainstay of treatment is broad‐spectrum topical antibiotics which should be used until culture results are available (Table 1 ). Ocular ointment may be useful at bedtime in less severe cases or as adjunctive therapy. Subconjunctival antibiotics may be useful in scleral or intraocular infections. 12 For central or severe keratitis, an initial frequent dosage every 5–15 min is recommended followed by hourly applications. Cycloplegic agents may be also used to decreased synechiae formation and reduce eye pain. They are indicated in cases with significant anterior chamber inflammation. 12
The AAO BK Preferred Practice Pattern, the Royal College of Ophthalmologists Focus, UK and the Australian Therapeutics Guidelines initially recommend monotherapy with fluoroquinolones (ciprofloxacin 3 mg/ml, ofloxacin 3 mg/ml, moxifloxacin 5 mg/ml, levofloxacin 15 mg/ml, gatifloxacin 3 mg/ml or besifloxacin 6 mg/ml). An alternative includes a combination of cephalosporin or vancomycin plus and an aminoglycoside. Vancomycin should be used in case of multi‐drug resistant Gram‐positive isolates 11 , 12 , 15 , 41 The current guidelines in Australia recommend empiric therapy with fluoroquinolones; 0.3% ciprofloxacin or 0.3% ofloxacin, or fortified combination therapy with 5% cephazolin plus 0.9% gentamicin; either treatment with one drop every hour including overnight. 15
Treatment should be modified based on the results of culture and susceptibility testing. 12 In patients with a history of OSD, care should be taken when prescribing fortified antibiotics to these patients, as fortified antibiotics have been reported to have drug toxicity five times greater than ofloxacin alone. Furthermore, poorer patient outcomes have been reported in OSD patients who were prescribed combination fortified antibiotics when compared with ofloxacin alone. 40
2.5.2. Antimicrobial resistance
Generally, BK cases respond to either of the above therapies; however, increasing resistance to fluoroquinolones has been reported in the US since the 1990s. 9 , 14 , 42 Goldstein et al. reported an increasing trend in resistance for ciprofloxacin in S. aureus (5.8% to 35%) and CoNS (15% to 39%) cases and a significant resistance among Streptococcus species (50%) during 1993 and 1997 in Pittsburg. 42 The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOUR) cumulative report from 2009 to 2018 reported that 34.9% of S. aureus were methicillin‐resistant S. aureus (MRSA). Resistance to ciprofloxacin was 32.2% among all S. aureus (10.4% for Methicillin‐sensitive S. aureus and 72.7% for MRSA) and 32.2% for CoNS. 43 In addition, the ARMOUR cumulative report from 2009 to 2020 reported a decreasing trend in resistance noted to ciprofloxacin among S. aureus (39% to 33%) and CoNS (46% to 26%). 44 On the other hand, in Australia, the Bacterial Ocular Surveillance System reported lower rates of resistance to ciprofloxacin with 16% among all isolates of S. aureus and 6% of CoNS. 8 , 9 , 14 , 42
2.5.3. Topical corticosteroid therapy
The use of adjuvant topical corticosteroid therapy remains controversial. 14 , 45 , 46 The aim of this therapy is the suppression of inflammation to reduce corneal scarring, neovascularisation and vision loss. However, the disadvantages include worsening of the infection, local immunosuppression, corneal melting and increased intraocular pressure. 12 , 46 The SCUT trial evaluated the effect of adjunctive corticosteroids (topical prednisolone phosphate, 1.0%) on clinical outcomes in patients with BK. At 12 months, the trial concluded that the adjunctive therapy may be associated with improved clinical outcomes in culture proven non‐ Nocardia BK after at least 48 h of improvement with antibiotic therapy. 12 , 41 , 45 If the corneal infiltrate compromises the visual axis, topical corticosteroid may be added to the management after at least 2–3 days of improvement with topical antibiotics, when the causal organism has been identified and it is not a fungus for which corticosteroids are contraindicated. 12
2.6. Complications
Surgical interventions are indicated in severe lesions that present progressive stromal thinning, descemetocele formation and local perforation. 41 The application of cyanoacrylate tissue adhesive is the first line intervention for corneal perforation providing a successful tectonic support for a short time, although requiring reapplication with a month after first application. 41 , 47 , 48 , 49 , 50 The success of this adhesive ranges between 29% and 86% depending on the cause of the perforation, indications for applications and definition of success. 47 Complications associated with its application include increased ocular inflammation, corneal neovascularisation and giant papillary conjunctivitis as well as long‐term adhesion. 41 , 47 , 48 , 49 , 50 Another alternative is amniotic membrane transplantation which has anti‐inflammatory effects to accelerate corneal healing. 41 However, a therapeutic penetrating keratoplasty (PK) remains the major intervention for the management of rapidly progressing infections and in large corneal perforations. 41 Although it is usually a successful intervention, the probability of graft survival is reduced in about a half, at 4 years post‐intervention, in eyes with inflammation or with corticosteroid use at the time of graft. 41 , 51
2.7. Future direction in diagnosis of BK
2.7.1. metagenomics next‐generation sequencing.
A promising diagnostic test is the metagenomics next‐generation sequencing (NGS). Ideally, NGS can detect all the microorganisms from a sample, producing sequencing data to be decoded potentially improving diagnostic yield, as it is inherently unbiased and hypothesis‐free. 30 Targeted amplicon sequencing and metagenomics (mNGS) are two approaches to NGS. The first technique consists in primer‐mediated amplification of specific suspected genomic targets (16S rRNA for bacteria). Selective amplification and sequencing can also be used for probing genomic regions of special interest (loci that confer AMR). This approach is less expensive, provides more depth in complex microbial communities and has successfully studied genomes in molecular epidemiological studies of Zika and Ebola. In contrast, with a single primer set, the search for organisms across multiple microbial kingdoms is not feasible. For example, by sequencing only conserved genes such as 16S rRNA, low taxonomic resolution is provided with restrictions to the identification of organisms at genus level generating false‐positive results. 52
Metagenomics NGS amplifies all nucleic acids within specimens without a target providing a considerable number of reads. Nevertheless, offering quantifiable phylogenetic identification of both known and unknown organisms within a specimen. This approach has been used as the last alternative to identify organisms in patients with severe systemic diseases when conventional tests have failed in identifying the causing organism. This approach also assists in molecular epidemiology studies investigating biogeographical and spatial distributions of pathogens in the context of their metagenome, and in high resolution evolutionary and outbreak tracing. 52 Challenges include that the turnaround time is about 5–7 days similar to a standard culture, but with higher costs. Currently, if this approach yields a result not obtained in the culture, independent confirmation of this result with another assay in a certified laboratory is needed. With a culture sensitivity between 30% and 60%, this will occur frequently. 53 Another challenge is how to determine whether a potentially contaminant organism is the actual causal organism of the infection. Perhaps other comparative sequence analysis algorithms may be needed to be explored. 11 , 53
Although NGS and dot matrix hybridization can simultaneously detect target pathogens or specific gene loci, NGS is not ideal for clinical use. NGS requires amplification of the target sequence or enrichment of desirable DNA sequences along with post‐sequencing analysis.
2.7.2. Deep learning
Deep learning algorithms are increasingly being recognised as having potential for screening and making management recommendations for patients with painful red eyes 54 ; distinguishing active corneal infection from scarring 55 ; and differentiating between causal organisms in keratitis 56 —for example between fungal and BK. 57 Convolutional neural networks apply very effectively deep learning for image classification. Algorithms such as ResNet, DenseNet, ResNeXt, SENet, VGG and EfcientNet can potentially develop models for image diagnosis of BK. 54 , 57 A study from Thailand used three algorithms, DenseNet121, REstNet50, VGG19 to classify images of patients with infectious keratitis. The test accuracy (F1 score) was higher for VGG19 (78%) followed by DenseNet121 (71%) and REstNet50 (68%). The authors created their own model called Deepkeratitis combining these algorithms with a F1 score of 83% which showed the best performance in differentiating BK from fungal keratitis (FK) compared with single models. 57 Investigators using external eye photographs to assess deep learning frameworks in BK have reported that the diagnostic accuracy of different models ranged from 69% to 72%; comparable to ophthalmologists (66% to 74%). 54 In areas or circumstances where patients are unable to access ophthalmic care, the ability to diagnose and assess microbial keratitis through artificial intelligence using external eye photos, such as could be taken with a mobile phone, may allow for appropriate therapy to be commenced without delay. 2 , 5 , 54 , 55 , 56
3. HERPES SIMPLEX KERATITIS
3.1. epidemiology.
Herpes simplex virus keratitis (HSK) is a leading cause of monocular infectious blindness in developed countries due to stromal opacification. 58 , 59 , 60 Herpes simplex virus (HSV) is an enveloped double‐stranded DNA virus belonging to the Herpesviridae family responsible for this corneal infection. This virus has two forms: HSV‐1, more related to ocular and perioral disease and HSV‐2 with anogenital infections. 58 , 61 , 62 In the United States, an estimated 500 000 people have ocular HSV infection which treatment of new and recurrent cases costs the country US$ 17.7 million annually. 61 , 63 One of five people with ocular HSV infection can develop stromal HSK with the attendant risk of blindness. 58 In 2012, Farooq and Shukla, estimated the incidence of HSK at about 1.5 million, with 40 000 new cases of severe monocular visual impairment or blindness each year across the world. 61 Herpes simplex keratitis needs frequent visits to the ophthalmologist and is responsible to loss of work and productivity, and income. 64 In the US in 2003, it was estimated that the HSK treatment cost in excess of 17.7 million dollars annually representing an important burden to the healthcare system. 65 HSK is a leading cause of monocular infectious blindness in developed countries due to stromal opacification. 58 , 59 , 60
3.1.1. Predisposing factors
The susceptibility of the host to the virus and the local susceptibility of the host target tissue determine the severity and frequency of recurrent HSK episodes. The susceptibility of the host to the virus is driven by their immune status; therefore, any inherited or acquired immunosuppressive conditions, age and atopy increase the frequency of HSK recurrences or severe disease. 65 Some immunosuppressive conditions include organ transplant recipients, diabetes mellitus, measles infections and human immunodeficiency virus (HIV). 65 , 66 , 67 In terms of age, as children generally have a more robust immune response, they tend to present with severe ocular HSV inflammatory disease, more recurrences, and complications compared to adults. 61 , 65 , 68 Complications include stromal scarring, corneal opacification, irregular astigmatism and amblyopia. 65 Children present more commonly with bilateral ocular HSV disease in primary infections than in recurrent infections, with rates ranging from 3.4% to 26% and a recurrence rate within the first year of 45 to 50%, when compared to adults (1.3% to 12% and 18%, respectively). 65 In a study from California, the United States, patients with severe atopic disease had between 2 and 4.8‐fold higher odds to have ocular HSV disease than people without atopy. 61 , 65 , 67 , 68
The local susceptibility of the cornea may be affected in cases such as application of medications, trauma and inflammation. 65 Medications such as prostaglandin agonists (latanoprost) for the management of elevated intraocular pressure and corticosteroids may increase the risk of recurrent ocular HSV disease. 65 Any surgery on an eye with previous ocular HSV disease increases the risk of recurrence of the infection. 65 , 69 The trauma caused by the surgery and the local immunosuppression of the perioperative corticosteroids may contribute this recurrence. Hence, the recommendation of an antiviral prophylactic therapy in the immediate perioperative period especially while the patient is also on corticosteroid therapy. 65 , 67 The Australian Corneal Graft Registry reported that penetrating grafts with active HSV have a probability of survival of 0.58 versus grafts with history of HSV with survival of 0.83 at year 4 post‐graft. 51 , 67
3.1.2. Clinical diagnosis
Primary HSV infection can be transmitted by direct contact with infected lesion or their secretions. It generally occurs upon exposure to virus shed asymptomatically by mucosal tissues with an incubation period from 1 to 28 days. 59 , 64 After primary infection, the HSV spreads via retrograde axonal transport to establish a latent infection in sensory nerve ganglia including the trigeminal ganglion. Recurrent infections occur when there is a viral reactivation transporting the virus down to the eye. 59 , 64 , 67
A diagnosis of HSK is made under clinical examination and after evaluating the patient's medical history. A history of labial cold sores or history of HSK could be the first clues to the diagnosis. 60 The clinical features and signs vary with the type of HSK and chronicity of the disease as summarised in Table 1 and illustrated in Figure 4 . 58 , 59 , 60 , 64 , 65 A classification system based on the type of corneal layer infected was introduced in the ‘Herpes Simplex Virus Keratitis: a treatment guideline’ by the AAO in 2014. 65 Epithelial HSK typically presents with a characteristic epithelial dendritic ulcer (Figure 4B ). Whereas in stromal HSK, lipid keratopathy and vascularisation are classic features of chronic disease (Figure 4B ) and ulceration may occur acutely (Figure 4C ). In keratouveitis (Figure 4D ) anterior chamber inflammation is associated with signs of HSK (Figure 4D ).

(A) Dendritic ulcer in epithelial herpes simplex keratitis stained with fluorescein. (B) Stromal herpes simplex keratitis with lipid keratopathy and vascularisation. (C) Stromal herpes simplex keratitis with ulceration. (D) Herpes simplex keratouveitis with anterior chamber cells
3.1.3. Diagnostic tests
Diagnostic tests maybe requested in the following cases: to confirm the initial diagnosis, atypical or complicated cases, uncertain diagnosis and suspected neonatal HSV infection. Viral culture is considered as the gold standard for epithelial HSK. It has a high specificity; yet with a limited use in clinical settings due to its low sensitivity, need of a skilled technician and slow turnaround (up to 10 days). 65 , 66
The PCR test detects viral DNA and quantifies the number of viral copies differentiating viral shedding from replication. 64 In HSK, the specimen for PCR is typically obtained by swabbing an active herpetic lesion such as an epithelial keratitis or stromal keratitis with ulceration. Advantages of the PCR test include its high sensitivity and fast results. Disadvantages include the need for a skilled technician, special equipment and appropriate facilities with parameters for ocular samples, and inability to differentiate HSV shedding from infection. 64 , 65 Diverse studies have determined the sensitivity of PCR testing to be between 70% and 100% and specificity of 67.9% to 98%. 69 , 70 , 71 However, in a retrospective case series from Sydney, Australia, the overall PCR positivity rate was 27%. It should be noted that 34% of epithelial HSK cases and 39% of stromal HSK with ulceration cases had a positive PCR contrasting to zero stromal HSK without ulceration cases and zero cases of endothelial HSK. 72 This confirms that the interpretation of the PCR test is more likely to diagnose patients with typical lesions or patients who have not used antiviral medications. 63 , 64 , 72 Nonetheless it can be a useful test when it is able to confirm for the clinician and patient that keratitis is due to HSV. As in cases of recurrent keratitis a diagnosis of HSK can then be readily made.
3.1.4. Treatment
The appropriate therapy for each type of HSK generally depends on the correct diagnosis under clinical examination. The current treatment recommendations for HSK treatment were based on the results of Herpetic Eye Disease Study (HEDS) group clinical trials in the 1990s. 58 , 63 , 65 , 67 However, there are currently newer antivirals and the availability of them varies according to the country. 58 , 65 , 67 The AAO released a treatment guideline in 2014 which recommended ganciclovir as the first line topical therapy with alternatives such as oral aciclovir, famciclovir and trifluridine for epithelial HSK. 65 , 69 However, ganciclovir and trifluridine are not easily accessible in Australia as they are in the United States, where topical aciclovir is not Food and Drug Administration (FDA) approved for HSK. 58 A study from Sydney, Australia, found diverse prescribing patterns for HSK therapeutic and prophylactic treatments. These were not aligned to the HEDS treatment recommendations. 58 As a result, an evidence‐based HSK treatment guideline was developed, implemented and evaluated to standardise the initial treatment for this condition (Table 1 ). 16 , 58 , 60 , 61 , 65 The Royal Australian and New Zealand College of Ophthalmologists (RANZCO) endorsed the treatment guideline in April 2020.
3.1.5. Complications
Recurrent HSK episodes can damage the corneal nerves causing neurotrophic keratopathy. Patients present with a decreased corneal sensation from irregular epithelial surface to an oval‐shaped neurotrophic ulcer with a heaped‐up border, blink reflex and tear production due to the damage to the sensory fibres innervating the cornea. 63 , 69 The infection causes a significant regression of the sensory afferents innervating the cornea, particularly substance P and calcitonin gene‐related protein nociceptive fibres with the loss of corneal sensitivity. 63 Substance P and calcitonin gene‐related protein are neuropeptides involved in the epithelial renewal and wound repair. Following the infection, the cornea reinnervates but with a different organisation of its fibres and reduced concentrations of the substance P. If the breakdown of the epithelium is not appropriately treated early, it may lead to corneal scarring, thinning, vascularisation, perforation or secondary corneal infection. 63
There are diverse treatments to stimulate epithelial growth and prevent further disruption of the ocular surface depending on the severity of the condition. For early and moderate cases, ocular lubricants, bandage contact lens, tarsorrhaphy, botulinum toxin‐induced ptosis, growth factors and autologous plasma maybe indicated. For more severe and complicated cases, collagenase inhibitors, tissue adhesives, conjunctival flap, amniotic membrane use and PK or lamellar keratoplasty can be used considering that poorer outcomes occur more in severely anaesthetic corneas. 63 , 69
3.2. Herpes zoster keratitis
Herpes zoster keratitis (HZK) usually manifests within 1 month of the onset of Herpes zoster ophthalmicus (HZO) and can affect any layer of the cornea. About 6% to 10% of cases of HZO can present with vision loss mainly due to corneal scarring or haze following acute epithelial and/or stromal HZK. 61 , 74 , 75
3.2.1. Epidemiology
It has been estimated that 200 000 new cases of HZO occur each year in the US. 76 , 77 , 78 Varicella zoster virus (VZV) is highly prevalent in the general population, with rates between 97.5% and 100% for 5 to 9 and 75‐ to 79‐year‐olds. 79 A trend towards younger age at presentation for HZO has been reported and maybe associated with childhood varicella vaccination. 77 , 78 Further, vaccination of older adults is increasing due to its effectiveness in reducing disease burden of HZO. 61 , 80 , 81
3.2.2. Predisposing factors
HZO occurs due to the reactivation of latent VZV from the ophthalmic division of the trigeminal nerve. Similar to HSK, primary infection follows latent and recurrent infection and is frequently associated with chronic and/or recurrent disease. 80 Predisposing factors for HZO and HZK include immunosuppression, advancing age, overexposure to the sun, a family history, trauma and ocular surgery such as cataract surgery. 60 , 81 , 82 , 83 , 84 Recently, COVID‐19 vaccination may predispose to HZO. 85
3.2.3. Clinical diagnosis
The clinical appearance of HZK depends on the layer of the cornea affected. Epithelial HZK is common and occurs in about half of patients with ocular involvement in HZO. In epithelial HZK, punctuate epithelial lesions appear 2 days after the onset of the vesicular skin rash. At around day 6, the epithelial lesions form pseudodendrites, which are small and fine lesions in a branching pattern, formed by swollen and heaped up corneal epithelial cells. 74 , 82 , 87 In contrast to the dendritic ulcers in HSK, the pseudodendrites lack of terminal bulbs and are usually located more in the peripheral cornea. They generally resolve spontaneously, however, in around half of cases, there is a progression to stromal HZK. 74
Stromal HZK presents in 6%–16% of patients with ocular involvement of HZO. Stromal HZK usually manifests after the epithelial disease and at around day 10 after the onset of HZO. Signs include stromal opacity, vascularisation, nummular corneal opacity, scarring and lipid keratopathy. 74 , 82 Keratouveitis/endotheliitis occurs rarely in up to 7% of patients within a week of the onset of HZO. Signs include localised corneal oedema, cell and flare and a complement‐mediated immune Wessely ring, elevated intraocular pressure, anterior chamber involvement, and hypopyon or hyphema from the vasculitis in severe cases. 74 , 82
3.2.4. Diagnostic tests
The diagnosis of HZK is usually made clinically on examination. In the acute phase, vesicular lesions maybe seen on the forehead and chronically there maybe scarring in the ophthalmic division of the trigeminal nerve. A swab may be taken from a vesicular skin lesion, a corneal lesion or AC tap for a PCR test to detect VZV DNA with rapid and sensitive results. 88 Higher VZV DNA copy numbers have been associated with more recurrent disease. 88
3.2.5. Treatment
Oral antiviral agents should be commenced within 72 h of onset of HZO; Aciclovir, valaciclovir and famciclovir can be used. 61 , 74 , 89 , 90 , 91 , 92 Despite treatment recommendations for patients with HZO, there is little consensus on the management of keratitis. 87 Diverse antiviral agents alone or in combination with topical corticosteroids can be effective for pseudodendritic keratitis despite current or recent oral antiviral therapy. 93 , 94 , 95 For instance, topical ganciclovir 0.15% gel was successful in these cases. 74 , 87 Topical corticosteroid use aims to control the inflammation in stromal and keratouveitis/endotheliitis cases; but may be challenging to taper off. Clinicians should monitor for side effects such as glaucoma and cataracts when topical corticosteroids are used. 74
3.2.6. Complications
In the long‐term stromal inflammation from HZV can result in stromal keratitis with corneal vascularization and lipid keratopathy, scarring and possible perforation. Nerve damage may lead to neurotrophic keratopathy with loss of corneal sensation and of corneal epithelial integrity and tear dysfunction. 82 Neurotrophic keratopathy may manifest months after HZO with diffuse epitheliopathy and chronic surface dysfunction and followed by band keratopathy. Corneal oedema can occur as the chronic end stage of corneal endothelial destruction caused by the virus or the related inflammation in keratouveitis/endotheliitis cases. 82 Corneal mucous plaques or delayed pseudodendrites may also occur months or year later typically in a quiescent eye. 61
3.3. Fungal keratitis
FK is a devastating condition and one of the main causes of blindness in Asia. 97 , 98 FK accounts for 6% to 53% of all cases of infectious keratitis depending on the country. 97 , 99 , 100 Predisposing factors, causal organisms and clinical outcomes depend on the geographic location, occupation, available medications, and gross national income. 98
3.3.1. Predisposing factors and microbiology
Corneal injury, microtrauma with CL wear, medical history of systemic conditions, topical corticosteroid use and history of OSD such as dry eye, blepharitis, Steven‐Johnson syndrome, bullous keratopathy and exposure keratitis and are the main predisposing factors. 98 , 100 , 101 In a corneal injury with vegetative matter or objects contaminated with soil, the fungus is introduced directly into the epithelial defect or the defect is infected during the trauma. This type of trauma occurs mainly in individuals working in farms, agriculture or outdoor settings. 102 Filamentary saprophytic fungi are more commonly associated with corneal injuries and are more prevalent in tropical and sub‐tropical climates. 103 , 104 A study from India reported that 90% of FK cases were caused by injury while 11%–44% of FK in the United States were injury related.
Hard and soft‐extended CLW are related to P. aeruginosa keratitis; but filamentous and yeasts have been also associated with CLW. For example, Candida albicans can adhere to CL secreting exopolymers almost impenetrable to antibiotics and difficult to remove. In addition, this type of contact lens causes relative hypoxia of the corneal epithelium which may modify the cell surface glycoproteins. Microtrauma due to CLW can increase the organism adherence to the non‐adherent epithelium. Fungi and bacteria adherent to CLs come from poor CL handling including cleaning and lenses storage. 102 Candida species is more commonly found in temperate climates, in diverse environmental settings and is part of the normal human microbiome. It is commonly found as a commensal organism in human gut, respiratory and mucous membranes. Candida related FK is more common in patients with prior OSD, recent ocular surgery and topical immunosuppression. 98 , 102 , 103 , 104 , 105 The most common filamentary fungi include Fusarium species , Aspergillus species and Curvularia species and the most common yeasts, C. albicans and Candida parapsilosis . 98 , 99 , 103 , 105 , 106
3.3.2. Clinical presentation
Signs and symptoms of FK and are summarised in Table 1 . The signs vary with whether the fungi are filamentous or yeast ( Candida species ). 100 , 101 , 104 , 105 Figure 5 illustrates the classic signs of Candida keratitis with a stromal infiltrate, overlying epithelial defect, and conjunctival hyperaemia; similar to BK (Figures 1 and and2). 2 ). Whereas in filamentous FK the stromal infiltrate may have feathery margins and there maybe satellite lesions with a thick endothelial exudate.

Corneal ulcer and infiltrates in a case Candida keratitis; the signs are similar to those found in bacterial keratitis
3.3.3. Diagnostic methods
Microbiology.
Staining and corneal scrape culture are the preferred diagnostic methods for FK. 102 , 104 Direct microscopy is a very valuable and fast method to detect fungal filaments from corneal scrapes. About 65% to 75% of Gram or Giemsa are positive to fungal hyphae. 14 , 101 The 10% potassium hydroxide (KOH) staining is another common procedure with sensitivity between 61% and 99.23% and specificity between 91% and 97%. 100 , 101
Culture media such as blood and chocolate agar and Sabouraud dextrose agar have been used to isolate and identify fungi. 99 Sabouraud agar has a lower pH and sometimes with addition of antibiotics, this agar can be tailored to selectively grow fungi instead of bacteria. 104 Fungal culture media should be maintained at 22 to 25 degrees in a cooling incubator if available. Fungi can be confirmed in blood agar and Sabouraud agar at a minimum of 48 to 72 hours. 99 Brain‐heart infusion and thioglycolate broth liquid media can also be used; but there are not selective for fungi. 104 C. albicans appear as smooth, glossy, raised, cream‐coloured colonies clustered together on Sabouraud dextrose agar; while Fusarium species grow as flat and spreading colonies with feathery borders. Despite being the gold standard for diagnosis, the culture sensitivity from corneal scrapings is limited with low rates of 25%, as fungi can take days or weeks to grow. 99 In vitro susceptibility tests for fungi are not performed routinely due to poor correlation to the clinical response. 106 Corneal biopsies may be needed to isolate the causal organism, as filamentous fungi grow slowly in culture, and for progressive infections despite an adequate antimicrobial therapy. 98
In vivo confocal microscopy
Another non‐invasive imaging tool is in vivo confocal microscopy (IVCM) which provides in vivo images of the cornea with a resolution of 1 μm, from the epithelium to endothelium, nerves and cells, sufficient to yield images larger than a few micrometres of filamentous fungi or Acanthamoeaba cysts. 97 , 99 , 103 Sensitivity of IVCM has been reported as between 80% and 94%, and specificity between 78% and 91.1%. 98 , 99 It has been reported to be of variable value in the diagnosis and monitoring of fungal and acanthamoeba keratitis (AK) 106 and is highly dependent on the experience of the observer. 107 , 108
Advantages of IVCM include ‘non‐invasiveness’, real‐time and early identification of the organism, for monitoring and guidance of the therapy, and determination of the depth of the infection. Limitations of IVCM include the need for an experienced operator, patient co‐operation, unsuitability for smaller organisms, motion artefacts and dense corneal infiltrates and/or scarring can affect the proper tissue penetration and visualisation. 99 , 100 , 104 Typically, IVCM is performed in cases of progressive keratitis and/or when acanthamoeba or FK are suspected. Anterior segment OCT has also been used to image the cornea, it is emerging as a diagnostic tool in microbial keratitis. 110
Polymerase chain reaction test
The sensitivity of the PCR ranges from 75% to 100%, and specificity from 50% to 100% for the diagnosis of FK compared to the corneal scrape culture. 29 , 97 , 99 , 103 In culture or staining negative results, the PCR has the highest positive detection rate. PCR advantages include that a small sample is required for diagnosis, yielding a fast result within 4 to 8 h when available, compared to cultures results which are available between 2 to 7 days. PCR also appears to be useful in earlier infections with low fungal load. 99 , 103 Major disadvantages are its high cost and lack of wide availability. Nevertheless, PCR is a supplementary diagnostic tool to guide early antifungal therapy while awaiting for other diagnostic test results. 99
3.3.4. Management
Management of FK includes antifungal agents, cycloplegics to relieve anterior uveitis, antibiotics for secondary bacterial infection if present and surgical intervention if required. 98 FK generally has poor clinical outcomes due to the reduced ocular penetration and efficacy of antifungal medications and the difficult diagnosis of this condition to commence an adequate initial therapy. 98 Management of FK includes antifungal agents, cycloplegics to relieve anterior uveitis, antibiotics for secondary bacterial infection if present and surgical intervention if required. 98
The selection of antifungal medications may depend on their availability, clinician preference and consultation with infectious diseases specialists. 98 Topical natamycin 5% is FDA approved and commercially available in the United States and has been associated with better outcomes in Fusarium keratitis, despite its poor penetration. Topical voriconazole and amphotericin B 0.15% can also be considered as alternatives. Topical voriconazole's limitations include its cost and being less effective than topical natamycin. Topical amphotericin can be prescribed as first choice to yeasts and as alternative to filamentous fungi; its limitations include its preparation and stability. 97 , 98 , 100 , 104
Oral medications such as voriconazole, ketoconazole, itraconazole or oral fluconazole may be added; although the Mycotic Ulcer Treatment Trial 2 (MUTT 2) concluded that oral voriconazole made no difference in the treatment of severe filamentous keratitis and the incidence of corneal perforation. 104 , 111 Posaconazole is a new medication; its mechanism of action is blocking fungal cell wall ergosterol synthesis. It has a broad‐spectrum activity against Candida species, Aspergillus species and Cryptococcus neoformans . It is also effective in cases of Fusarium species resistant to other antifungals without toxicity. 100 Intracameral or intrastromal antifungals maybe considered when the infection involves the deep stromal layers, significant anterior chamber reaction and ulcers not responding topical and oral medication as well as during corneal transplant surgery for FK. 100 , 104 , 112 The risk of corneal scarring from intrastromal injection must be weighed against that of progressive infection. A PK is indicated when the medical therapy has failed and maybe considered earlier in progressive keratitis, severe corneal thinning, impending perforation and keratitis involving the limbus. Unfortunately, PK has a high rate of recurrent infections ranging from 5% to 14%, usually in cases which involve the limbus and with preoperative hypopyon and corneal perforation. In addition, a study from India reported the media graft survival of 5.9 months with two risk factors: size of corneal infiltrate and size of corneal graft. 100 , 104
3.4. Microsporidial keratitis
Microsporidia are unicellular organisms from the phylum Microspora and kingdom Protista. They have been reclassified as fungi. The intracellular spore is the infectious form of the organism. 113 , 114 The infection can be transmitted via faeco‐oral, contaminated water or food for intestinal microsporidosis; however, the source for ocular infections is unknown. 33 , 114 Risk factors for this infection include CLW, rainy season and exposure to muddy water. 33
3.4.1. Clinical features
This organism can cause keratoconjunctivitis; usually in immunocompromised patients; endophthalmitis and stromal keratitis, in immunocompetent patients. 33 , 113 The infection is typically insidious, difficult to diagnose, and often mistaken for viral keratitis. 33 , 113 It can present as epithelial keratopathy or stromal keratitis, which is less common than keratoconjunctivitis. Stromal keratitis presents with diffuse congestion, greyish white stromal infiltration, oedema without suppuration, or deep stromal infiltrate with or without an overlying epithelial defect. 33 , 114
3.4.2. Diagnostic tests
This organism can be identified as bright turquoise to white intracellular oval bodies clustered in groups against a dark background in 0.1% calcofluor white or 10% potassium hydroxide (KOH) stains. 33 , 113 , 114 Bright purple, ovoid, refractile spores similar to Gram‐positive organisms can be seen in Gram stains. Calcoflour white and modified Ziehl‐Neelsen stains are the most sensitives stains for identifying this organism. 33 , 114 Madin‐Darby canine kidney (MDCK), Vero, HeLa and SIRC cell lines culture media can be used to grow Microsporidia. Other tests such as PCR and transmission electron microscopy (TEM) can be used to identify the species. 33 TEM is the gold standard for diagnosis of microsporidial spores but it is not easily accessible to most laboratories and further tests are needed to determine the species. 114 Pan microsporidian 16S rRNA has been used to identify the microsporidial species with a sensitivity of 83% and specificity of 98%. 114 A microsporidial infection should be considered as a differential diagnosis in culture‐negative stromal keratitis not responding to standard antimicrobial therapy. 33 , 114
3.4.3. Treatment
There is no standard therapy for microsporidal infection. Therapies with albendazole, itraconazole propamidine isethionate 0.1%, PHMB 0.02%, chlorhexidine 0.02%, voriconazole 1%, fluconazole 0.3% and fumagillin 0.3% have had some success requiring a long‐term therapy for several weeks. 33 , 114 Fluoroquinolones have also been used in combination with albendazole and topical fumagillin. 33 Therapeutic penetrating keratoplasty may be needed in a non‐responding infection to medical therapy and for definitive therapy. 33 Microsporidia stromal keratitis has poor clinical outcomes and surgery is needed in most of the cases. 33 , 113 , 114
3.5. Acanthamoeba keratitis
Acanthamoeba species are ubiquitous free‐living amoebae. At least 24 amoebic protozoa species exist worldwide, and they exist in both soil and nearly all water sources. Human ocular involvement with Acanthamoeba presents in the form of keratitis. AK is a rare, sight‐threatening infection. The incidence of AK differs between developed and developing countries, as well as between geographical areas. 115
3.5.1. Predisposing factors
The incidence of AK is lower in developing countries compared with developed countries. 116 In the latter, the majority of cases are linked with CLW, specifically soft CLs. 116 , 117 Diagnosis is often late due to its low incidence of around 3%–15% in the United Kingdom and United States 115 and 3.6 cases per year in Australia. 118 In developing countries like India, CLW is less prevalent and most AK cases are associated with trauma. 119 In non‐CLW, AK cases are associated with contaminated soil, water and surgical trauma. Younger age is associated with increased incidence of AK, this may be related to the increased prevalence of CLW worldwide. 120 , 121 The infection is often caused by contamination during cleaning procedures. 115 Furthermore, warmer periods of the year (i.e., summer) are associated with higher incidence. This is because during the warmer months there is an increased number of amoebae in surface water and prolonged water activities occur. 122 , 123
3.5.2. Clinical features
Symptoms and signs are described in Table 1 and in Figures 6 and and7. 7 . During the early stages, patients may also present with eyelid ptosis, conjunctival hyphemia and pseudodendrites. Keratoneuritis or radial nerve enlargement with perineural infiltrates maybe present but are not pathognomonic, as they may also occur in pseudomonas keratitis and be absent late in the disease. 124 , 125 Deep stromal infiltrates, corneal perforation, satellite lesions, scleritis and anterior uveitis with hypopyon may occur as the disease progresses. 118

Ring infiltrate in acanthamoeba keratitis

Advanced acanthamoeba keratitis, scattered stromal infiltrates with corneal vascularisation and conjunctival hyperaemia are noted
3.5.3. Diagnostic tests
A provisional diagnosis of AK can be made from the patient's history, clinical features and IVCM (Figure 8 ). During IVCM, acanthamoeba cysts appear as hyperreflective, spherical and well‐defined double‐wall structures, while trophozoites are difficult to discriminate from leukocytes and keratocyte nuclei. 126 Identification of Acanthamoeba species via corneal scrape or PCR should also be performed to confirm diagnosis. Epithelial debridement as part of the scrape procedure can also assist management by reducing the acanthamoeba load. The culture specimen should then be inoculated onto Escherichia coli plated over non‐nutrient agar. Cultures for bacterial, fungal and viral infections should also be performed as early clinical signs are nonspecific and indistinguishable from other types of keratitis. 127 While, culture on E. coli agar plates remains the gold standard for diagnosing A canthamoeba species , PCR testing has become well established and demonstrated to have higher sensitivity than corneal culture (67% to 75% vs. 31% to 33%). 128 , 129 Furthermore, E. coli plates may not be available in all centres. 128 , 130 In the case of deep corneal involvement, a corneal biopsy may be needed for diagnosis. 27

In vivo confocal microscopy of acanthamoeba keratitis
3.5.4. Treatment
AK is a complicated infection, however, early diagnosis and aggressive medical therapies have improved the management of this disease. A combination of various topical acanthamoeba agents is usually utilised as no single drug can eliminate both cystic and trophozoite forms. The cyst form tends to be highly resistant to therapy, therefore, a combination of agents is generally used. Polyhexamethylene biguanide (PHMB) and chlorhexidine are topical agents effective against acanthamoeba trophozoites, with variable efficacy against cysts. 121 , 130 Chlorhexidine is often used in combination with propamidine or hexamidine and has shown good results if the treatment is commenced early during the course of infection. 131 However, propamidine and hexamidine are not available in all countries.
Post corneal scrape procedure, topical anti‐acanthamoeba drugs should be administered every hour for the first several days, the frequency then reduced depending on clinical response. Treatment is recommended for 6 to 12 months with close observation to prevent recurrent infection. 132 Therapeutic penetrating keratoplasty is reserved as a measure of last resort in cases of impending corneal perforation. Robaei et al. suggest delaying corneal transplantation where possible until the eye is no longer inflamed and after completion of anti‐acanthamoeba treatment. 133 Penetrating keratoplasty should be considered when the infection spreads to the paracentral corneal stroma, as performing this procedure on a more localised infection may allow for the total removal of the organism. 134 To control inflammation, topical steroids may be used but only after anti‐acanthamoeba therapy has been commenced. 134 , 135 To control inflammation, topical steroids may be used but only after anti‐acanthamoeba therapy has been commenced. 135
Complications such as scleritis and treatment toxicity can occur. Clinicians should instruct patients on proper cleaning of CLs and remind patients to avoid wearing CLs while swimming or showering 136 as this can prevent the occurrence of the disease.
4. CONCLUSION
Infectious keratitis is the fifth leading cause of blindness overall worldwide. Early diagnosis and adequate therapy are key to avoid complications such as vision impairment and blindness. For bacterial, fungal and AK, culture of corneal scrapes is the initial diagnostic test to grow and identify the causing organism. Alternative diagnostic tools such as PCR and IVCM can be also used to aid determination of the causal organism(s). In HSK, the diagnosis is mainly based on clinical examination. PCR testing can also be used; however, it is not useful in stromal and endothelial HSK due to their immune‐related pathogenesis. Newer diagnostic tests such as NGS and deep learning models are being used in selective health settings with the hope that they maybe widely utilised in the near future. Challenges remain in infectious keratitis. First, educating patients with predisposing factors such CLW, OSD or agricultural workers about the risks of infection is crucial to avoid acquiring the infection and encouraging early presentation. Second, developing new diagnostic tests to determine the causal organism in a timely manner, with good sensitivity and specificity while being cost‐effective. Finally, a judicious use of antimicrobials is needed to avoid increasing AMR rates which may lead to sight‐threating complications.
FUNDING INFORMATION
This study was funded by the Sydney Eye Hospital Foundation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Cabrera‐Aguas M, Khoo P, Watson SL. Infectious keratitis: A review . Clin Experiment Ophthalmol . 2022; 50 ( 5 ):543‐562. doi: 10.1111/ceo.14113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Funding information Sydney Eye Hospital Foundation

IMAGES
VIDEO
COMMENTS
Neurotrophic keratitis is the fourth subtype of HSK, wherein HSV damages the nerves which innervate the cornea, resulting in reduced sensitivity. Herpes infections make up 27% of all neurotrophic keratitis cases ( Bonini et al. 2000 ). The clinical presentation of HSK can be divided into three stages.
Herpes simplex virus (HSV) keratitis is an infectious disease of the cornea. ENTITY. Herpes simplex virus keratitis, includes entities with the following ICD-9 and ICD-10 classifications: ... For example, atopy may result in an atypical course or presentation of HSV keratitis. The immune system may also be stressed by infection and fever ...
HSV (Herpes Simplex Virus) keratitis is an infection of the cornea—the clear dome that covers the colored part of the eye—that is caused by HSV. The infection usually heals without damaging the eye, but more severe infections can lead to scarring of the cornea or blindness. HSV keratitis is a major cause of blindness worldwide 1.
Herpes simplex keratitis is a common and potentially blinding condition caused by recurrent corneal infections with the herpes simplex (HSV) virus. Two herpes simplex viruses, HSV-1 and HSV-2, affect humans, who are their only natural hosts. Herpes simplex virus-1 is usually the cause of most infections in the oral, labial, and ocular areas ...
Patients with herpes simplex virus (HSV) keratitis may report the following: Pain. Photophobia. Blurred vision. Tearing. Redness. A history of prior episodes in patients with recurrent disease may exist. Patients with ocular HSV who have previous stromal involvement have a significantly higher risk for subsequent stromal keratitis; in contrast ...
Noninfectious keratitis can be caused by a relatively minor injury, such as from wearing your contact lenses too long or getting a foreign body in the eye. Infectious keratitis can be caused by bacteria, viruses, fungi and parasites. If you have eye redness or other symptoms of keratitis, make an appointment to see an eye specialist.
Herpes simplex virus (HSV) keratitis is one of the leading causes of blindness worldwide. Additionally, up to 90% of the population in some countries is seropositive for HSV. HSV can cause a wide spectrum of ocular disease ranging from blepharitis to retinitis. Although the initial clinical expressions of HSV-1 and HSV-2 are similar, HSV-2 has ...
Practice Essentials. Herpes simplex virus (HSV) keratitis is the most frequent cause of blindness due to corneal disease in the United States and the most common source of infectious blindness in the Western world. [ 1, 2] The prognosis in HSV keratitis, however, generally is favorable with aggressive treatment.
Viral keratitis. If a virus is causing the infection, antiviral eye drops and oral antiviral medications may be effective. Other viruses need only supportive care such as artificial tear drops. Acanthamoeba keratitis. Keratitis caused by the parasite acanthamoeba can be difficult to treat. Antiparasitic eye drops are used, but some acanthamoeba ...
In addition, viral keratitis was also reported to have a high prevalence amongst patients with diabetes . Viruses, particularly HSV, are omnipresent in the general population, with an estimated ...
Disease. HSV infection can cause inflammation in nearly every ocular tissue. In cases of corneal involvement, the epithelium, stroma, or endothelium may be affected. Both herpes stromal keratitis (HSK) and HSV endotheliitis can present clinically with stromal opacity and, therefore, may be difficult to distinguish.
Infectious keratitis is a major global cause of visual impairment and blindness, often affecting marginalized populations. Proper diagnosis of the causative organism is critical, and although culture remains the prevailing diagnostic tool, newer techniques such as in vivo confocal microscopy are helpful for diagnosing fungus and Acanthamoeba.Next-generation sequencing holds the potential for ...
Viral keratitis. reprinted, with permission, from Trobe JD, The Physician's Guide to Eye Care, 2nd Edition, San Francisco: American Academy of Ophthalmology; 2001. Primary herpes simplex ocular infection usually presents as a unilateral foreignbody sensation with watery discharge. There may be skin vesicles on the lids or enlarged ...
69 likes • 11,058 views. AI-enhanced title. P. pragati jain. seminar on viral keratits. Health & Medicine. 1 of 71. Download now. Viral Keratitis: Causes, Symptoms and Treatment - Download as a PDF or view online for free.
7 likes • 606 views. Obaidur Rehman. Types of viral keratitis, diagnosis of different types and management of each. Clinical evaluation with images described. Standard treatment protocols given. Health & Medicine. 1 of 73. Download now. Viral keratitis: Diagnosis and management - Download as a PDF or view online for free.
Introduction. Keratitis or corneal ulcer refers to sight-threatening infection and inflammation of the cornea.. Bacterial and viral keratitis represent the most common forms of microbial keratitis, but rarely the cause may be fungal or protozoan (acanthamoeba).. The following article aims to provide an overview of microbial keratitis at the level expected of final-year medical students and ...
Viral Keratitis. Adenoviral keratitis: Adenoviral keratitis often has associated conjunctivitis, so the exact terminology would be Epidemic adenoviral keratoconjunctivitis (Human adenovirus types 8,19,37 and 54). Presentation is usually unilateral to start with; however, it becomes bilateral later. A predominantly follicular reaction is noted.
2. INTRODUCTION • Infective keratitis is suppurative infection of cornea which may be associated with epithelial defects and or signs of inflammation. • Worldwide 45 million blind • 1.5-2.0 million blind due to corneal diseases added every year. • Prevalence of corneal blindness (<6/60 in worse eye) in Indian population is 0.66% i.e (one out of every 150 people in India) • In India ...
Keratitis is a pathological condition involving inflammation of the cornea. It can be an infectious or non-infectious disease. The causative organisms of keratitis are categorized as bacteria, viruses, fungi, or parasites. The viruses responsible for causing keratitis are herpes simplex virus (HSV), varicella-zoster virus, and adenoviruses. The clinical features of this infection may range ...
Treatment with EBT-104 resulted in over 90% reduction in viral shedding in HSV-1 Keratitis modelSAN FRANCISCO, April 22, 2024 (GLOBE NEWSWIRE) -- Excision BioTherapeutics, Inc. ("Excision ...
Oral presentation to discuss CRISPR-associated gene editing inactivating herpes virus; Two poster presentations highlight the potential of EBT-104 for the treatment of HSV-1 Keratitis ...
1. INTRODUCTION. Infectious keratitis is an infection of the cornea also known as infectious corneal ulcer or corneal opacity. Infectious keratitis can be classified as microbial keratitis (bacteria, fungi or parasites), or viral keratitis (herpes viruses). 1 , 2 The number of cases of corneal blindness due to infectious keratitis has decreased from about 1.6 million in 1990 to 1.3 million in ...
Dr. Gaurav Shukla. Viral keratitis. Healthcare. 1 of 100. Download now. 1. DR.GAURAV SHUKLA. 2. Viruses- Viruses are small (10 - 400 nm in diameter) infectious units with a single- or double-stranded nucleic acid genome A protein capsid shell, with or without an external lipid envelope.
About Herpes Simplex Keratitis. Herpes Simplex Keratitis (HSK) caused by the infection of herpes simplex virus type 1 (HSV-1) in the cornea is a major cause of blindness worldwide. Although current anti-HSV-1 therapies interfere with viral DNA replication, they do not eliminate HSV-1 reservoirs or prevent recurrence.
To assess the efficacy of CRISPR/Cas9-mediated gene editing on HSV-1 in vivo, a single all-in-one AAV8(Y733F) and AAV9 vectors delivery of SaCas9 and paired gRNAs were employed in a latent rabbit model of HSV-1 keratitis via corneal scarification.This approach led to a remarkable reduction of over 60% in viral shedding from the treated rabbit eyes.
Viral Keratitis dr. Frenky DJ • Caused by reactivation of live virus. • C/F; pain, photopbobia, thin watery discharge, decrease vision if lesion is central. • Earliest epithelial lesion is characterized by corneal epithelial vesicles. 1. DENDRITIC ULCER • Most common presentation of HSV keratitis.
Oral presentation to discuss CRISPR-associated gene editing inactivating herpes virus; Two poster presentations highlight the potential of EBT-104 for the treatment of HSV-1 Keratitis ...