Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2023

Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks
- Golla Madhu ORCID: orcid.org/0000-0002-4170-3146 1 ,
- Ali Wagdy Mohamed ORCID: orcid.org/0000-0002-5895-2632 2 , 3 ,
- Sandeep Kautish ORCID: orcid.org/0000-0001-5120-5741 4 ,
- Mohd Asif Shah ORCID: orcid.org/0000-0002-0351-9559 5 , 6 , 7 &
- Irfan Ali ORCID: orcid.org/0000-0002-1790-5450 8
Scientific Reports volume 13 , Article number: 13377 ( 2023 ) Cite this article
3384 Accesses
3 Citations
1 Altmetric
Metrics details
- Epidemiology
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period, and delaying exact treatment might result in the development of further complications. The most prevalent method now available for detecting malaria is the microscope. Under a microscope, blood smears are typically examined for malaria diagnosis. Despite its advantages, this method is time-consuming, subjective, and requires highly skilled personnel. Therefore, an automated malaria diagnosis system is imperative for ensuring accurate and efficient treatment. This research develops an innovative approach utilizing an urgent, inception-based capsule network to distinguish parasitized and uninfected cells from microscopic images. This diagnostic model incorporates neural networks based on Inception and Imperative Capsule networks. The inception block extracts rich characteristics from images of malaria cells using a pre-trained model, such as Inception V3, which facilitates efficient representation learning. Subsequently, the dynamic imperative capsule neural network detects malaria parasites in microscopic images by classifying them into parasitized and healthy cells, enabling the detection of malaria parasites. The experiment results demonstrate a significant improvement in malaria parasite recognition. Compared to traditional manual microscopy, the proposed system is more accurate and faster. Finally, this study demonstrates the need to provide robust and efficient diagnostic solutions by leveraging state-of-the-art technologies to combat malaria.
Similar content being viewed by others

Reducing data dimension boosts neural network-based stage-specific malaria detection
Enhancing parasitic organism detection in microscopy images through deep learning and fine-tuned optimizer
Classification for avian malaria parasite Plasmodium gallinaceum blood stages by using deep convolutional neural networks
Introduction.
Malaria is a life-threatening disease that involves the Plasmodium parasite, which poses a high death rate. It is transmitted to humans by biting an infected female mosquito with the parasite. Malaria is predominantly a tropical disease since mosquitoes thrive in tropical areas, and it is both preventable and treated. According to the latest Global Malaria Report, there are projected to be around 241 million malaria cases and 627 thousand fatalities worldwide by 2022 1 . Moreover, research by the World Health Organization (WHO) suggests that concerns related to COVID-19 could triple the number of malaria cases 2 , 3 . In response to this global epidemic, the WHO has enacted policies to prevent, treat, eradicate, and monitor malaria 4 . Malaria, a preventable disease, can be controlled and prevented if adequate processes and protocols are used, including early diagnosis of the malarial parasite 4 . Several laboratory techniques, including polymerase chain reaction (PCR), microscopy, and rapid diagnostic test (RDT) are commonly used for investigating malaria using thick or thin blood smears 5 , 6 , 7 , 8 . However, conventional methods tend to rely heavily on manually examining blood smears under a microscope. These methods are time-consuming, subjective, and require highly trained personnel. Additionally, the reliance on clinical experts raises concerns about the consistency and accuracy of the diagnosis. To address these deficiencies, computer-aided diagnostic (CAD) methods for malaria evaluation are being developed to reduce mortality rate 9 . Therefore, automated and accurate diagnostic systems are needed to improve malaria detection. Artificial intelligence has gained more and more attention in the scientific community. It has contributed to improving detection through various diagnostic processes. Most medical imaging analyses now incorporate CAD procedures that leverage deep learning techniques for effective model learning.
However, despite advancements, malaria remains endemic in some areas where the disease is common. Early screening plays a crucial role in detecting malaria and saving lives. Consequently, this motivates us to create faster and more accurate malaria diagnosis procedures. Recently, deep learning architectures have received much attention in terms of research and are the most important method to detect disease automatically and more accurately. These generic deep networks have played a vital role in image classification, detection, and recognition 10 , 11 . In a similar vein, data-driven deep learning (DL) algorithms have surpassed manually constructed feature extraction techniques 12 . A convolutional neural network (CNN) is a type of deep learning model that employs different mechanisms, such as local receptive fields, shared weights, and clustering layers, to leverage information. Its purpose is not limited to extracting features but also extends to generating predictive targets and furnishing actionable predictive models that can effectively aid physicians 10 , 13 . Deep neural networks have shown outstanding performance in computer vision tasks in recent years. This is done using methods like the ResNet-32 network model to identify ductal carcinomas 14 precisely. Despite their effectiveness, CNN suffers from limitations in the modeling of spatial relationships and the lack of an internal representation of the geometrical restrictions on the image data. When these flaws are applied to microscopic cell images, the diagnostic model may be misclassified. The need for a more precise and efficient model arises to improve the performance of detecting and classifying malaria parasites. These challenges have prompted us to develop a rapid and more accurate diagnosis procedure for malaria. The specific hypotheses tested in this study include:
Hypothesis 1
Using the inception neural network will enable the extraction of rich and discriminative features from microscopic images of malaria cells, improving parasite detection and classification accuracy.
Hypothesis 2
The incorporation of the imperative capsule neural network will enhance the modeling of spatial relationships within the images, allowing for a more precise classification of malaria parasites.
By testing these hypotheses, the study aims to demonstrate the superiority of the proposed approach over traditional manual microscopy and other existing methods for malaria diagnosis.
This paper is organized as follows: The relevant research is presented in Section “ Related works ”, and the proposed inception-based imperative capsule neural network is discussed in Section “ Materials and methods ”. Part “ Experimental results ” summarizes and describes the outcomes of this network. Part “ Conclusions ” concludes with the article's conclusions and suggested recommendations for further study.
Related works
Several researchers have demonstrated promising results in medical applications by using data-driven machine learning (ML) and deep learning (DL) models. This study examines contemporary deep-learning applications that elicit key decision-making factors in the diagnosis process. Liang et al. 15 presented a 16-layer CNN to classify the parasitized and uninfected cells in thin blood smears. Features are extracted using a pre-trained AlexNet 16 , and a support vector machine (SVM) is trained on these features, and the model has an average accuracy of 97.37%. However, the transfer learning method achieves only 91.99% accuracy. Bibin et al. 17 proposed and tested a six-layer deep belief network to detect malaria parasites in cell images. Based on their findings, the study achieved 96.4% classification accuracy on a custom dataset using training or test randomization. Dong et al. 18 presented SVM and CNN-based approaches for classifying malaria parasites from cell images. This study attained an accuracy of more than 95% using pre-trained deep learning models such as those used in LeNet 19 , AlexNet 16 , and GoogLeNet 20 . Rajaraman et al. 21 proposed a deep-learning model for malaria parasite detection and classification. The method visualizes the activation maps of each layer and understands the probabilities of the different layers to understand the modeling process. As a result, it obtains an accuracy of 98.61%. Mahdi Postchi et al. 22 surveyed the latest advancements in image analysis and machine-learning techniques for diagnosing malaria through microscopy. Although many machine learning models using traditional features have been developed for image classification and decision-making, these models may lack generalization ability. Sivaramakrishnan et al. 23 suggested a customized CNN model and evaluated the effectiveness of pre-trained and deep-learning CNN models as feature extractors for microscopic images to differentiate between healthy and parasitic blood cells. The model uses surface features to achieve more outstanding results than deep features and applies a level-set-based algorithm to detect and segment red blood cells. This model achieved 98.6% (cell-level) accuracy. Yang et al. 24 presented a fivefold cross-validation for two-step CNN models. In the first step, the model uses an intensity-based iterative Global Mini-mum Screening method to recognize parasites, and then a CNN uses a custom CNN to classify the presence of parasites. The success rate of this method is 93.46%. Vijayalakshmi et al. 25 presented a transfer learning method with a classification accuracy of 93.13% to discriminate between illustrations of malaria-diseased cells and healthy using the VGG16 model and a support vector machine. Madhu et al. 26 proposed an improved dynamic routing process to classify malaria-infected cells from healthy cells using a fully trained capsule network, and the model achieved an accuracy of 98.82%. Loddo et al. 27 used the DenseNet-201 neural network to categorize Plasmodium falciparum life stages into four groups and used two different datasets to assess the robustness of the model. The binary classification accuracy rate was 97.68%, and the multi-classification accuracy rate was 99.40%. Meng et al. 28 proposed a neighborhood correlation graph convolutional network to identify multistage malaria parasites. The model has excellent recognition ability for multistage malaria parasites, outperforming the comparison method by at least 8.67%. Madhu et al. 29 proposed an automated diagnostic model based on deep Siamese capsule arrays for uniquely detecting and classifying malaria parasites. When simplified on the largest test sample (test = 40%), the model achieved an accuracy of 96.61% and 98%, respectively. Ha et al. 30 presented a semi-supervised graph learning framework to solve the problem of identifying apicomplexan parasites. Hybrid graph learning is also used in this approach to explore the relationships between different parasites with and without labels.
In malaria, the Plasmodium parasite causes an acute fever that is carried by female Anopheles mosquitoes. It produces life-threatening sickness if left untreated for a long time, and delaying exact treatment might lead to the development of additional comorbidities. A microscope is currently the most prevalent method for detecting malaria. Consequently, an automated approach to diagnosing malaria is required. This study proposes the development of an urgent, inception-based capsule network for classifying parasitized and uninfected cells from micrographs. These diagnostic models contain neural networks based on the Inception and Imperative Capsule architectures. Using a trained model, such as Inception V3, the first block collects rich characteristics from images of malaria cells. In the second block, a dynamic imperative capsule neural network classifies malaria cells into infected and uninfected red blood cells. The experiment's findings indicate a considerable improvement in recognizing malaria parasites, which contributes to better illness diagnosis and prevention.
By observing the existing challenges, this study aims to develop an automatic diagnostic prototype for classifying malaria parasites from microscopic cell images using the Inception neural network with the Imperative Capsule neural network. The preliminary results of this study are presented as follows:
To develop an innovative approach employing an urgent, inception-based capsule network to recognize parasitized and uninfected cells from microscopic images.
The Inception block extracts rich features from malaria cell images using a pre-trained model, such as Inception V3, which facilitates efficient representation learning to recognize the parasites.
The dynamic imperative capsule neural network is utilized to classify microscopic images into parasitized and healthy cells, enabling the detection of malaria parasites.
To compute routing by agreement among low-level and higher-level capsules that can be used to predict malaria cells and classify them into parasitized and uninfected cells using L2-Norm.
This study underscores the importance of leveraging state-of-the-art technologies to combat malaria by providing a robust and efficient diagnostic solution.
Materials and methods
Dataset collection.
Images of thin blood smears containing two distinct strains of malaria—one infected and the other not—were used in the study. These samples were gathered from patients and healthy controls who had Plasmodium falciparum infections, and they were stored at the National Institutes of Health (NIH) repository, which is open to the public for study 23 . The collection includes 13,779 images of parasites and 13,779 images of uninfected cells, totaling 27,558 images of labeled and segmented cells from thin Giemsa-stained blood smear slides. Figure 1 offers some parasitic and uninfected cell images to visualize their physical traits.
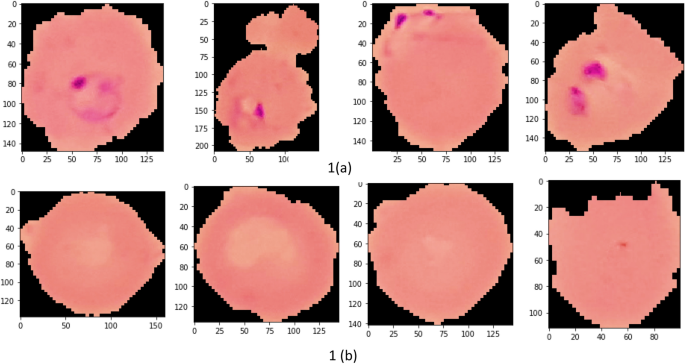
Illustration of sample malaria cell images: ( a ) Infected images; ( b ) Uninfected images (without parasites).
k-fold cross-validation (CV) test
The dataset contains 27,558 blood cell images with malaria-positive and negative samples, which were evaluated in our study for data sample training and testing, and used k-folds (k = 10, 20, 30, 40, 50) Cross-validation to evaluate the proposed model. As shown in Table 1 , the dataset is split into training and testing subsets.
Inception neural network and the imperative capsule neural network
Geoffrey Hinton et al. 31 motivated this research by addressing the limitations of traditional CNNs by proposing inception-based capsule neural networks, which require small data but have higher computational complexity.
This research develops an inception-based imperative capsule neural network for malaria detection, and its basic architecture is shown in Fig. 2 , which is similar to the architecture advocated for image classification problems by Sabour et al. 31 . According to Fig. 2 , input is first routed through fully connected inception blocks, which receive the parasitized and uninfected portions of the cell images as input and extract features on the parasitized and uninfected portions of the cell images. The inception block's output is used as the primary capsule layer's input. The primary and higher capsule layers utilize an imperative routing mechanism to learn the captured features by discerning the spatial orientation of the parasites on the extracted features. After multiple iterations, the resulting output is a feature vector with a length equivalent to the probability of the interval [0, 1], which preserves the object's pose information, minimizing the information loss caused by the feature vector extraction. This feature vector is then used to classify a test sample as infected or healthy cells, aiding in its classification.
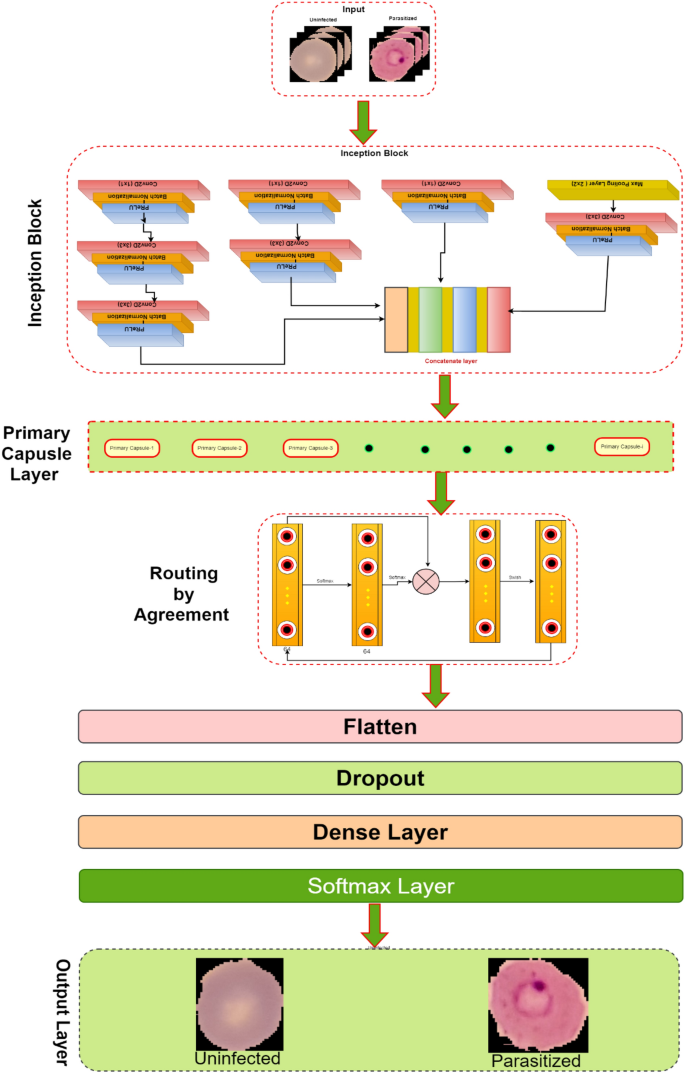
The proposed architecture of Inception-based capsule neural network.
Inception neural network block
In 2015, Google introduced a module for GoogleNet 32 , also known as Inception V3, a convolutional neural network that helps us with image analysis and object detection.
Convolutional layers are frequently employed in convolutional neural networks (CNNs) to extract information from images of malaria blood cells. The CNN's initialization block, which is made up of parallel convolutional layers with filters and kernels of various sizes, extracts feature from various scales to obtain multi-view information on parasites and healthy cells. The structure of the inception block, which is used to extract characteristics at various scales, is shown in Fig. 3 . To extract features at various sizes, this block has four parallel convolutional layers with various kernels (1 × 1, 3 × 3, and 3 × 3). A max-pooling layer with a kernel size of 2 × 2, a convolution layer with a kernel size of 1 × 1, and a batch normalizing layer make up the final parallel convolutional layer. Each parallel layer's computational cost and channel count can be decreased by using a 1 × 1 convolutional layer, and the model's computational cost can be decreased by employing a 3 × 3 max-pooling layer. The output feature maps of each of the four simultaneous convolutional layers are combined after computation to produce new feature maps that are used as the input for the capsule network.
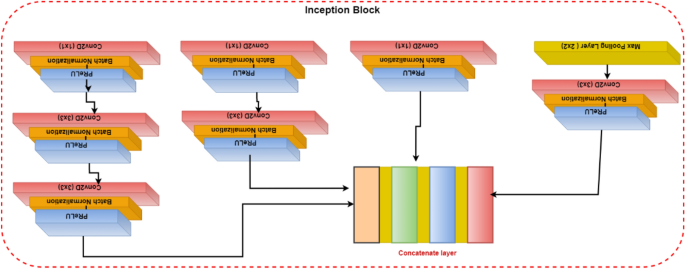
Illustration of the inception block.
Capsule networks block
To classify the items in the MNIST dataset, Sabour et al. 31 presented a capsule network (CapsNet). It uses a neural network to produce an output vector that includes both a scalar and a vector encoding the features of the objects in the image. In our experiment, these capsule networks are trained by carefully adjusting the number of rounds in the dynamic routing algorithm. Using Parametric ReLU (PReLU), it is possible to investigate the behavior of nonlinear activations during dynamic routing 33 . The presence of features in the form of vectors containing low-level entity instantiation parameters is estimated using the principal capsule layer. CapsNet transforms the scalar output using feature detectors in this layer, then passes the vector output of the capsules to the following layer using a modified routing method 31 . Because parameter tuning is critical for better network learning and faster convergence, proper initialization is used to start the routing procedure with kernel initializer before the primary capsule layer; the dynamic routing algorithm is activated with Glorot-normalization 34 . Each capsule, \(i\) has an activity vector \({u}_{i}\in R\) in the layer of \(l,\) which captures information about the features extracted from an entity (i.e., blood cell image). The output of the activity vector \({u}_{i}\) of the \(i\) th level capsule is fed as data into the next level layer, i.e., \(l+1\) layer. The \({j}{\text{th}}\) layer capsules of layer \(l+1\) will get data from \({u}_{i}\) and compute the product weight matrix \({W}_{ij}^{T}\) . The results are stored in the form of \({\widehat{u}}_{(j|i)}.\) This vector is the layer of capsules \(i\) at level \(l\) layer, which is the transformation of the entity represented by capsule \(j\) at the level of \(l+1\) . Then apply the transformation matrix \({W}_{ij}^{T}\) to capsule output \({u}_{i}\) of the previous layer, as shown in Eq. ( 1 ).
In Eq. ( 1 ), capsule \(i\) is the primary capsule layer, \(j\) is the higher-level capsule layer, and \({u}_{i}\) is the output of the capsule network of the upper layer and \({W}_{ij}^{T}\) is the learnable weighted matrix between the \({i}{\text{th}}\) capsule to \({j}{\text{th}}\) capsule. Which is multiplied by each output vector and the coupling coefficient \({C}_{ij}\) is added to the linear sum stage. Then the capsules are in the higher level, which is filled with the sum of the output vector in the lower-level layer, and we add it with a coupling coefficient \({C}_{ij}\) which is computed during the routing method shown in Eq. ( 2 ).
In dynamic routing, the coupling coefficient is determined by Eq. ( 2 ). In the process of calculating \({S}_{j}\) in forward propagation, \({W}_{ij}^{T}\) is set to a random value, \({a}_{ij}\) is initialized to zero, \({u}_{i}\) is the output of the previous layer, and then compute a weighted sum \({S}_{j}\) with weights \({C}_{ij}\) (the sum of these coefficients is equal to one) and it is denoted as follows:
The squashing function map of \({S}_{j}\) yields the output vector \({v}_{j},\) which is obtained is defined as follows:
The squashing function, defined by Eq. ( 4 ), ensures that short vectors are reduced to fewer dimensions near zero while long vectors are scaled to unit length, thus introducing nonlinearity to the capsule network. The total input Sj processed by the jth dimensional capsule array contributes to the coupling coefficient Cij. An activation function PReLU is applied to update the coupling coefficients, instead of the squashing function, by operating on Sj. During the iterative learning phase, these coupling coefficients are updated using Eq. ( 5 ), which proceeds as follows:
In Eq. ( 5 ), \({a}_{ij}\) is a parameter used as a weighted proxy, which means that it gives higher weights to appropriate predictions, and it starts at zero and is modified as the training progress.
However, it is initialized with the current input weights to improve the learning method by reducing the computational cost and improving the predictive ability. The number of routing iterations (n = 3) is used as a hyperparameter allowing one to choose a specific number of iterations during the training (here, epochs = 100) period, and the details of this network parameters are shown in Table 2 . The learning period is evaluated by evaluating the convergence, and our model is repeated for only three iterations. Figure 4 depicts the comprehensive learning curves for iterations over 100 epochs.
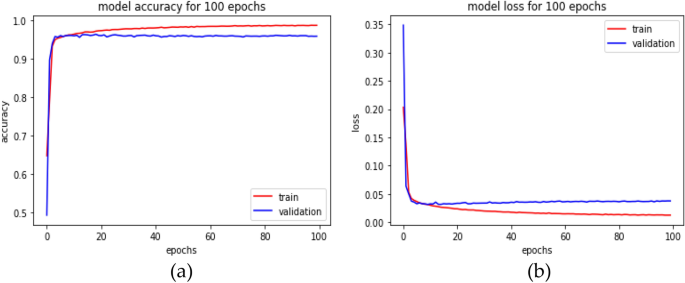
An inception-based capsule network with a router in 3 iterations, depicted as ( a ) accuracy curves and ( b ) loss decay curves.
PReLU activations are utilized during the routing by agreement process to improve the understanding of feature invariance in the captured images of malaria cells. In a conventional capsule network, the squash activation function is typically used as a non-linearity. However, using PReLU as a non-linearity is believed to lead to better generalization and convergence over time. The last layer of the network comprises two capsules (parasitized and uninfected cells) reflecting the probability of the interval [0, 1] and the position information of the object, preserving the pose information to reduce information loss caused by the extracted feature vector. This enables the classification of test samples into either parasitized or uninfected cells, thus aiding in cell feeding.
Loss function
Our current loss function 31 also includes the mean squared error rate (MSE) alongside the marginal loss. Change the settings for faster convergence and add proper model regularization and noise addition when training the classification model with a value set to 0.45.
In Eq. ( 6 ), \({m}^{+}\) and \({m}^{-}\) are the category prediction values, \(\sigma \) is the balance coefficient, \({T}_{x} \mathrm{is \, the \, label \, of \, category}, \) and classification probability vector \(\Vert {v}_{x}\Vert \) is the size. For this study, the default values are set as \({m}^{+}=0.85 \& {m}^{-}=0.15\) , \(\sigma =0.45\) . The total loss function, in this case, refers to the loss of capsules representing both malaria-parasitized and uninfected classes.
Experimental results
This section describes the proposed model's implementation in-depth and thoroughly analyses how well it performs under various restrictions. The proposed network was evaluated against front-line classification models created by several authors, which were pre-trained using NIH malaria datasets 23 and other private datasets to assess whether red blood cells are parasitized or not. According to Table 3 , the proposed model for malaria parasite identification and classification performed well on the NIH malaria dataset, along with the comparison findings. It is important to note that most models typically exhibit low performance on this dataset. Although their weights can handle common classification datasets, they frequently fall short because of ineffective feature extraction brought on by too much depth. Instead, the Inception-based capsule network model classifies parasitized and uninfected cells accurately during the diagnostic process by utilizing external knowledge to produce rich characteristics. On international benchmarks, the suggested model performs noticeably better.
As stated in the Table 4 , our model is assessed for layer-wise testing cell images, varying from training to 80% and testing to 20%.
In this analysis, experiments are conducted on various distributions, and the suggested network's implementation, as shown in Table 4 , achieves an accuracy of 99.35% and an AUC score of at least 99.73% at a test ratio of 20%. Table 4 shows the models' overall generality as measured by various standard classification metrics, including accuracy score, AUC–ROC, sensitivity, and specificity. Limiting diagnostic power does not assess the likelihood that a certain patient will acquire a disease, but it does affect diagnostic accuracy, even though they choose sensitivity and specificity. Table 5 displays the effectiveness of the suggested capsule array at various nonlinearity levels. Compared to the performance of cutting-edge pre-trained models, the generalization distribution for the training and test samples is 80% to 20%.
The performance metrics for every deep learning architecture are compiled in Table 5 . The proposed malaria detection algorithm outperforms the compared deep learning models in terms of performance. The results showed an accuracy of more than 99.35%, an AUC score of 99.73%, and an F1 score of 99.36%. The accuracy score is a well-known metric with a domain that is invariant to general utility; hence it is imperative to note. As a result, the effectiveness of the suggested model is assessed using various measuring techniques. The model was created to be assessed by segregating partition samples that vary from 10 to 50%, ensuring that the model is adequately generalized. Figure 5 displays the predicted results of the suggested model on images of malarial cells. The true value is shown on the x-axis, and the model forecast is shown on the y-axis.
Illustration of some prediction results of the proposed model.
Time complexity analysis
According to our study, the learning model was trained for 100 epochs to assess the time complexity of the model. The results show that our model takes around 33.8667 min for training and 3 s for complete testing, which is less than all the compared models. This study addresses the urgent need for automated malaria detection and classification. It proposes a novel approach based on integrating inception and imperative capsule neural networks. This research has the potential to significantly improve malaria diagnosis, contributing to more effective disease management and prevention. Additionally, the study contributes to the growing field of deep learning in medical image analysis. It showcases the applicability of advanced neural network architectures to address critical healthcare challenges.
Conclusions
This research develops a deep-learning approach by combining the imperative capsule neural network with the inception neural network to distinguish between malaria-parasitized and uninfected cells. This enhances the classification accuracy of identifying malaria parasites from photographs of blood cells. With well-chosen parameters, the capsule model can efficiently finish the procedure for classifying uninfected cells or parasites into different categories. Models with different loss parameters are compared to the proposed model, and the results show that the model's performance can be increased by adjusting the loss parameters. The proposed network achieves higher classification accuracy while analyzing blood cell images for malaria than competing deep learning methods. Under the worst-case scenario (50/50 split), the model obtains an accuracy of 98.10% on the test, while on the 20% split, it achieves an accuracy of 99.355%. These experimental results are helpful since the developed model is robust and flexible and has outperformed competing models. In the work's future scope, the model may be utilized to recognize parasite species and stages in thin blood smears. This research opens opportunities for future advancements in malaria diagnosis and surveillance, including using mobile and portable imaging devices for point-of-care testing.
Data availability
The data that support the findings of this study are openly available in the National Library of Medicine (NLM)—Malaria Data: https://lhncbc.nlm.nih.gov/LHC-research/LHC-projects/image-processing/malaria-datasheet.html and reference number Ref. 23 .
https://www.who.int/news-room/fact-sheets/detail/malaria .
Alnussairi, M. H. D. & İbrahim, A. A. Malaria parasite detection using deep learning algorithms based on (CNNs) technique. Comput. Electr. Eng. 103 , 108316 (2022).
Article Google Scholar
Chakradeo, K., Delves, M. & Titarenko, S. Malaria parasite detection using deep learning methods. Int. J. Comput. Inf. Eng. 15 (2), 175–182 (2021).
Google Scholar
Fact Sheet about MALARIA. https://www.who.int/news-room/fact-sheets/detail/malaria . Accessed 26 Nov 2022.
Devi, S. S., Roy, A., Singha, J., Sheikh, S. A. & Laskar, R. H. Malaria infected erythrocyte classification based on a hybrid classifier using microscopic images of thin blood smear. Multimed. Tools Appl. 77 (1), 631–660 (2018).
Mfuh, K. O. et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar. J. 18 (1), 1–8 (2019).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 , 36–55 (2018).
Article PubMed PubMed Central Google Scholar
Hanscheid, T. & Valadas, E. Malaria diagnosis. Am. J. Trop. Med. Hyg. 61 , 179. https://doi.org/10.4269/ajtmh.1999.61.179 (1999).
Article CAS PubMed Google Scholar
Alonso-Ramírez, A. A. et al. Classifying parasitized and uninfected malaria red blood cells using convolutional-recurrent neural networks. IEEE Access 10 , 97348–97359 (2022).
Krizhevsky, A., Ilya Sutskever, S. & Geoffrey, E. H. ImageNet classification with deep convolutional neural networks. Commun. ACM 60 (6), 84–90 (2017).
Redmon, J., Divvala, S., Girshick, R. & Farhadi, A. You only look once: Unified, real-time object detection. in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition , 779–788 (2016).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521 (7553), 436–444 (2015).
Article ADS CAS PubMed Google Scholar
Razzak, M. I., Naz, S. & Zaib, A. Deep Learning for Medical Image Processing: Overview, Challenges, and the Future 323–350 (Springer, 2018).
Praveen, S. P., Srinivasu, P. N., Shafi, J., Wozniak, M. & Ijaz, M. F. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci. Rep. 12 (1), 20804 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Liang, Z. et al . CNN-based image analysis for malaria diagnosis. in IEEE International Conference on Bioinformatics and Biomedicine, IEEE , 493–496 (2016).
Krizhevsky, A., Sutskever, I. & Hinton, G. E. ImageNet classification with deep convolutional neural networks. in Proceedings of the 25th International Conference on Neural Information Processing Systems, Volume 1 (NIPS'12) , 1097–1105 (2012).
Bibin, D., Nair, M. S. & Punitha, P. Malaria parasite detection from peripheral blood smear images using deep belief networks. IEEE Access 5 , 9099–9108 (2017).
Dong, Y. et al . Evaluations of deep convolutional neural networks for automatic identification of malaria-infected cells. in EMBS International Conference on Biomedical & Health Informatics, IEEE , 101–104 (2017).
Lecun, Y., Bottou, L., Bengio, Y. & Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 86 (11), 2278–2324 (1998).
Szegedy, C. et al . Going deeper with convolutions. in Proceedings of the 2015 (CVPR) , 1–9 (2015).
Sivaramakrishnan, R., Antani, S. & Jaeger, S. Visualizing deep learning activations for improved malaria cell classification. Med. Inf. Healthc. 1 , 40–47 (2017).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 (6), 36–55 (2018).
Sivaramakrishnan, R. et al. Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. PeerJ 6 , e4568 (2018).
Yang, F. et al. Deep learning for smartphone-based malaria parasite detection in thick blood smears. IEEE J. Biomed. Health Inform. 24 (5), 1427–1438 (2019).
Article PubMed Google Scholar
Vijayalakshmi, A. & Rajesh Kanna, B. Deep learning approach to detect malaria from microscopic images. Multimed. Tools Appl. 79 (21), 1–21 (2020).
Madhu, G. et al. Imperative dynamic routing between capsules network for malaria classification. Comput. Mater. Contin. 68 (1), 903–919 (2021).
Loddo, A., Fadda, C. & Di Ruberto, C. An empirical evaluation of convolutional networks for malaria diagnosis. J. Imaging 8 , 3. https://doi.org/10.3390/jimaging8030066 (2022).
Meng, X., Ha, Y. & Tian, J. Neighbor correlated graph convolutional network for multi-stage malaria parasite recognition. Multimed. Tools Appl. 81 , 11393–11414. https://doi.org/10.1007/s11042-022-12098-6 (2022).
Madhu, G. et al. DSCN-net: A deep Siamese capsule neural network model for automatic diagnosis of malaria parasites detection. Multimed. Tools Appl. 81 , 34105–34127. https://doi.org/10.1007/s11042-022-13008-6 (2022).
Ha, Y., Meng, X., Du, Z., Tian, J. & Yuan, Y. Semi-supervised graph learning framework for apicomplexan parasite classification. Biomed. Signal Process. Control 81 , 104502. https://doi.org/10.1016/j.bspc.2022.104502 (2022).
Sabour, S., Frosst, N. & Hinton, G. E. Dynamic routing between capsules. Adv. Neural Inf. Process. Syst. 1 , 3856–3866 (2017).
Szegedy, C. et al . Going deeper with convolutions. in Proc. CVPR 2015 , 1–9 (2015).
He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. in Proceedings of the IEEE International Conference on Computer Vision , 1026–1034 (2015).
Glorot, X. & Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. in Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics , 249–256 (2010).
Das, D. K., Maiti, A. K. & Chakraborty, C. Automated system for characterization and classification of malaria-infected stages using light microscopic images of thin blood smears. J. Microsc. 257 (3), 238–252 (2015).
Díaz, G., González, F. A. & Romero, E. A semi-automatic method for quantification and classification of erythrocytes infected with malaria parasites in microscopic images. J. Biomed. Inform. 42 (2), 296–307 (2009).
Gopakumar, G. P. et al. Convolutional neural network-based malaria diagnosis from focus stack of blood smear images acquired using custom-built slide scanner. J. Biophoton. 11 (3), e201700003 (2018).
Rahman, A. et al . Improving malaria parasite detection from red blood cell using deep convolutional neural networks. (2019). arXiv:1907.10418 .
Download references
Author information
Authors and affiliations.
Department of Information Technology, VNR Vignana Jyothi Institute of Engineering and Technology, Hyderabad, Telangana, 500090, India
Golla Madhu
Operations Research Department, Faculty of Graduate Studies for Statistical Research, Cairo University, Giza, 12613, Egypt
Ali Wagdy Mohamed
Applied Science Research Center, Applied Science Private University, Amman, Jordan
LBEF Campus (Asia Pacific University of Technology & Innovation, Malaysia), Kathmandu, 44600, Nepal
Sandeep Kautish
College of Business and Economics, Kabridahar University, Po Box 250, Kabridahar, Ethiopia
Mohd Asif Shah
School of Business, Woxsen University, Kamkole, Sadasivpet, Hyderabad, 502345, Telangana, India
Division of Research and Development, Lovely Professional University, Phagwara, 144001, Punjab, India
Department of Statistics & Operations Research, Aligarh Muslim University, Aligarh, 202002, India
You can also search for this author in PubMed Google Scholar
Contributions
G.M. conceived and designed the experiments, performed the experiments, and prepared figures and/or tables. A.W.M., S.K., M.A.S. and I.A. supervised the study, analyzed the results, and provided insightful suggestions for the manuscript. All authors have read and authored or reviewed drafts of the paper and approved the final draft.
Corresponding author
Correspondence to Mohd Asif Shah .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Madhu, G., Mohamed, A.W., Kautish, S. et al. Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks. Sci Rep 13 , 13377 (2023). https://doi.org/10.1038/s41598-023-40317-z
Download citation
Received : 13 April 2023
Accepted : 08 August 2023
Published : 17 August 2023
DOI : https://doi.org/10.1038/s41598-023-40317-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- UNC Chapel Hill
MALARIA RESEARCH
- 1 Within Host Diversity of Malaria Infections
- 2 Spatial Epidemiology of Malaria
- 3 Diagnosis Resistant Malaria
- 4.1 Molecular Epidemiology of Drug Resistance
- 4.2 Chloroquine Resistance in Plasmodium vivax
- 4.3 Impacts of ACT Partner Drugs on Population Structure
- 5 Population Genetics of Plasmodium vivax
- 6 Malaria Vaccine Antigen Diversity
- 7 Transmission of Malaria
- 8 Malaria Relapse
Within Host Diversity of Malaria Infections
A major focus of IDEEL@Carolina’s research has been studying the diversity of malaria parasites both within populations as well as within people. Early on, our work focused on studying drug resistance and outcomes of clinical trials and the impact that with-in host diversity might have on interpreting studies. We were the first investigators to report on the presence of low level variants containing drug resistance in polyclonal malaria infections. These “minority variant” parasite strains in these infections have the potential to act as a reservoir of rug resistance in a population. This could limit the re-introduction of drugs to areas where sensitive parasites have repopulated after withdrawal of an antimalarial therapy. These minority variants also have the potential to lead to misclassification of results from clinical trials . We showed that WHO recommended genotyping methods can miss variants within an infection potentially leading to misclassification of trial results and impacting drug efficacy estimates.
After these initial advances, Dr. Juliano began to leverage second generation sequencing technologies to understand with-in host diversity of malaria infections. He was the first person to publish using these techniques , showing a much higher with-in host diversity of infections than previously thought. Since that time, he and other IDEEL@Carolina investigators have continued to use these methods for not only describing with-in host diversity, but to try to understand basic biology processes that occur within the host, such as strain selection by antimalarials or relapse patterns in Plasmodium vivax .
Key to this work has been the collaboration with Dr. Jeffrey Bailey at UMass (see Collaborators). Dr. Bailey’s group has been critical in developing the bioinformatic tools necessary for conducting these studies. His group has launched a free analysis tool, called SeekDeep , for analysis of deep sequencing data from polyclonal infection. This tool is not only applicable to malaria, but can be used to analyze microbiome data or other systems in which more than one strain infects the same host.
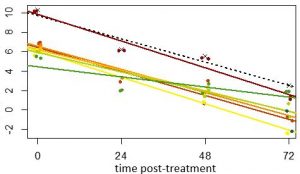
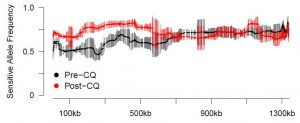
The advances in bioinformatics has allowed us to now use these tools to track the relative frequency of parasite strains with-in individuals over time. Using these tools, IDEEL@Carolina investigators have found parasite strains that clear more slowly in Africa to artemisinin combination therapies. The clearance half-lives are similar to those of artemisinin resistant parasites currently found in SE Asia. We are currently following up these initial results with a clinical study with our collaborators in Tanzania and Kenya.
Spatial Epidemiology of Malaria
As has been historically seen in India and Sri Lanka, when malaria control efforts are reduced, malaria resurges. However, it is unclear how malaria is being reintroduced to areas where control has waned. This reintroduction could occur due to immigration of infected people or mosquitoes. We do not know how quickly this occurs, what geographic factors are most important, nor the relative contributions of infected mosquitoes and people.
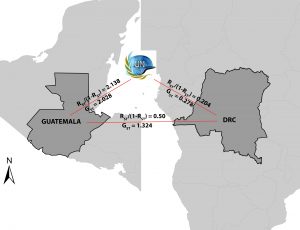
Ecologists use landscape genetics to measure geographic factors which promote as well as prevent dispersal of infectious diseases, as measured by genetic intermingling. For example, grey wolf populations are less genetically related the farther apart they are and if there are bodies of water intervening. Landscape genetics is just starting to be used to measure rate of disease spread, using the rate of gene flow as a proxy. While the rates of gene flow in malaria have never been measured, determinants of genetic distance (Fst/[1-Fst]) have been studied for P. falciparum isolates from different islands on the Comoros archipelago. Genetic distance tended to increase with increasing Euclidian distance, and to decrease as the numbers of travelers between islands increased. Our group has been keenly interested in studying how malaria moves through the environment across landscape, as knowledge of the factors involved are critical for helping with malaria eradication and elimination. As an example, we have used gene flow to study the i mportation of malaria to Guatemala from the Congo as part of UN Peacekeeping efforts .
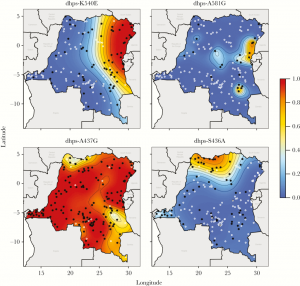
Recently, in collaboration with our colleagues at the University of Massachussetts and Imperial College, we constructed maps of P. falciparum genotypes using molecular inversion probes. We found little structure among neutral markers (not under selection) but marked geographic structure for drug-resistant parasites.
Diagnosis Resistant Malaria
We recently completed the first national population-based study of P. falciparum parasites with deletions of the pfhrp2 gene, which produces the antigen (HRP2) detected by commonly deployed malaria rapid diagnostic tests (RDTs). RDTs currently account for 70% of malaria diagnoses in Africa and represent a major investment by multilateral donors. Because the vast majority of rapid diagnostic tests deployed in Africa are HRP2-based, pfhrp2 -deleted parasites can escape detection.
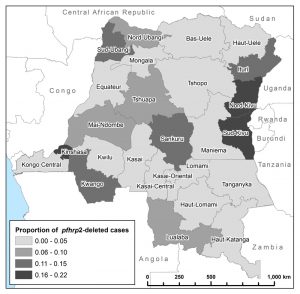
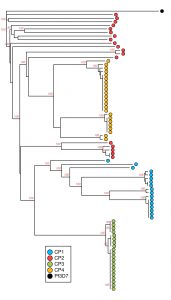
Dr. Parr and Dr. Meshnick led a multi-disciplinary effort to describe the prevalence and evolution of these mutant parasites in the Democratic Republic of Congo (DRC), resulting in the discovery of two clusters of pfhrp2 -deleted parasites. Initial population genetic analyses indicate that mutant parasites from these clusters are genetically distinct from wild-type parasites. Additional studies are underway to characterize their evolution using novel next-generation sequencing approaches and to understand their clinical impact. Through collaboration with the WHO and other partners, we are working to develop strategies for surveillance and improved policies for malaria control programs.
Malaria Drug Resistance
Molecular epidemiology of drug resistance.

The emergence of drug resistance to antimalarial has been a major impediment to global control of malaria. Resistance emerges rapidly to every antimalarial that has been used globally. Molecular epidemiology studies can help with understanding how resistance emerges and spreads.

Molecular markers of resistance have now been described for many antimalarials and have been useful for helping to monitor resistance. IDEEL@Carolina investigators have been involved with studies of these molecular markers in multiple countries around the globe. Recent work has included studying how resistance to sulfadoxine-pyramethamine (SP) developed and segregated between Eastern and Western Africa in the DRC , the global distribution of SP resistance mutations and their implications for SP use in IPTp as part of the Malaria in Pregnancy Consortium , and the emergence of mefloquine resistance in SE Asia . They have also been involved in developing new ways to monitor these molecular markers. They were the first group to use a pooling approach to assay for drug resistance mutations using next generation sequencing . Using these techniques, they were the first group to publish on K13 mutations , the gene associated with artemisinin resistance in SE Asia, in a large scale survey of African malaria infections (>1,000 individuals).
Chloroquine Resistance in Plasmodium vivax
Dr. Juliano has been working in a collaboration led by Thomas Wellems at the NIH to discover the genetic mechanisms behind chloroquine resistance in Plasmodium vivax . Chloroquine resistance has been spreading slowly since the 1980’s, starting in Indonessia and Papau and spreading globally. The molecular mechanism of resistance in P. vivax is different than that in Plasmodium falciparum , as coding mutations in the ortholog ( pvcrt ) of the gene associated with resistance in falciparum ( pfcrt) are not associated with resistance. Using a genetic cross generated in a chimpanzee, this team has been searching for genetic loci associated with resistance. Dr. Juliano’s lab has been responsible for the genome sequencing in this project and have help to identify a critical loci associated with resistance to chloroquine in the cross progeny.
The work on the NIH cross has led to a potential molecular marker of chloroquine resistance for P. vivax . Drs. Juliano and Lin are currently working with AFRIMS in Thailand and the Eijkman Institute of Molecular Biology in Indonesia to study these polymorphisms in natural parasite populations and to evaluate their relationship to chloroquine failure using well characterized clinical samples.
Impacts of ACT Partner Drugs on Population Structure
and Juliano have been using whole genome sequencing to study the potential impacts of artemisinin combination therapy (ACT) partner drug resistance on the population structure of Plasmodium falciparum in Cambodia. Their work suggests that partner drug resistance is promoting clonal expansion of artemisinin resistant parasites in the region. These findings have implications for understanding the evolution of partner drug resistance in the face of artemisinin resistance, and have implications for the use of triple ACTs in the region.
Population Genetics of Plasmodium vivax
Drs. Lin and Juliano have been studying the population structure, demographic history and genomic signatures of selection on the P. vivax populations in Cambodia using whole genome sequencing. Their work suggests that modification of transcription regulation might underly vivax malaria’s resilience to control measures in the region. The work highlights the needs for a better understanding of transcriptional regulation in malaria in order to better inform elimination and control efforts.
Malaria Vaccine Antigen Diversity
Although tremendous gains have been made against malaria, global control and elimination are unlikely to occur without the development of an effective vaccine. Many of the candidate vaccine antigens for malaria are highly polymorphic in natural parsite populations, leading to concerns about strain specific immunity decreasing vaccine efficacy. IDEEL@Carolina investigators have been involved in characterizing vaccine antigen diversity for many antigen using our deep sequencing approaches including: 1) pfs 25 and pfs 48/45 in P. falciparum , 2) circumsporozoite protein (the antigen in RTS,S) in P. falciparum and P. vivax , 3) apical membrane antigen 1 in P. falciparum and 4) merozoite surface protein 1 in P. vivax .
These types of studies can help elucidate critical aspects of how the antigen interacts with the human immune systems . Currently, we have a large effort going into understanding the diversity of var2csa from P. falciparum in a project led by Dr. Meshnick. This antigen is the ligand responsible for malaria binding to the placenta. An effective vaccine targeting this antigen could prevent placental malaria, a leading preventable cause of low birth weight infants in Africa. This work is being done in conjunction with the Institute Pasteur.
Transmission of Malaria

Efforts to eliminate malaria altogether hinge on the ability to prevent transmission. Dr. Lin is working with investigators at AFRIMS to assess the effect of transmission-blocking drugs and other interventions on human to mosquito transmission of malaria in SE Asia. Since much malaria is asymptomatic and microscopic, we are interested in learning the contribution of the asymptomatic reservoir to ongoing transmission and which field deployable diagnostics are most suited to elimination efforts. We are also studying the genetics of the parasite in the mosquito stages to learn how mosquito-borne transmission affects the spread of drug resistance.
Malaria Relapse
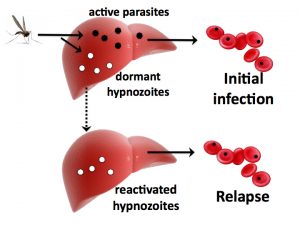
Plasmodium vivax is the second most prevalent malaria species in the world and causes much morbidity through its ability to reactivate from the liver and cause relapse. Interestingly, relapses of vivax malaria commonly occur after treatment of falciparum malaria . Safe and effective treatments are lacking, further complicated by the difficulty of distinguishing re-infections from relapse. IDEEL investigators have teamed with investigators in Thailand, Cambodia, and Indonesia to characterize genotypic signatures of relapse and are applying next generation sequencing techniques to samples from patients with multiple relapses as well as soldiers returning to non-endemic regions with known relapse. The long term goal of this work is to identify genetic determinants of relapse that can guide the development of new therapies.
- Open access
- Published: 12 May 2024
Prevalence of malaria and associated risk factors among household members in South Ethiopia: a multi-site cross-sectional study
- Girma Yutura 1 ,
- Fekadu Massebo 2 ,
- Nigatu Eligo 2 ,
- Abena Kochora 3 &
- Teklu Wegayehu 2
Malaria Journal volume 23 , Article number: 143 ( 2024 ) Cite this article
Metrics details
Despite continuous prevention and control strategies in place, malaria remains a major public health problem in sub-Saharan Africa including Ethiopia. Moreover, prevalence of malaria differs in different geographical settings and epidemiological data were inadequate to assure disease status in the study area. This study was aimed to determine the prevalence of malaria and associated risk factors in selected rural kebeles in South Ethiopia.
A community-based cross-sectional study was conducted between February to June 2019 in eight malaria-endemic kebeles situated in four zones in South Ethiopia. Mult-stage sampling techniques were employed to select the study zones, districts, kebeles and households . Blood sample were collected from 1674 participants in 345 households by finger prick and smears were examined by microscopy. Sociodemographic data as well as risk factors for Plasmodium infection were collected using questionnaires. Bivariate and multivariate logistic regressions were used to analyse the data.
The overall prevalence of malaria in the study localities was 4.5% (76/1674). The prevalence was varied among the study localities with high prevalence in Bashilo (14.6%; 33/226) followed by Mehal Korga (12.1%; 26/214). Plasmodium falciparum was the dominant parasite accounted for 65.8% (50/76), while Plasmodium vivax accounted 18.4% (14/76). Co-infection of P. falciparum and P. vivax was 15.8% (12/76). Among the three age groups prevalence was 7.8% (27/346) in age less than 5 years and 7.5% (40/531) in 5–14 years. The age groups > 14years were less likely infected with Plasmodium parasite (AOR = 0.14, 95% CI 0.02–0.82) than under five children. Non-febrile individuals 1638 (97.8%) were more likely to had Plasmodium infection (AOR = 28.4, 95% CI 011.4–70.6) than febrile 36 (2.2%). Individuals living proximity to mosquito breeding sites have higher Plasmodium infection (AOR = 6.17, 95% CI 2.66–14.3) than those at distant of breeding sites.
Conclusions
Malaria remains a public health problem in the study localities. Thus, malaria prevention and control strategies targeting children, non-febrile cases and individuals living proximity to breeding sites are crucial to reduce malaria related morbidity and mortality.
Malaria continues to remain a global burden and a public health threat despite increasing efforts aimed at improving vector control, therapeutics and diagnostics approaches worldwide [ 1 ]. According to World Health Organization (WHO), there were 249 million estimated malaria cases in 85 malaria endemic countries in 2022, an increase of 5 million cases compared with 2021 [ 1 ]. Most of the increase in case numbers and deaths over the past 5 years occurred in countries in the WHO African Region. Ethiopia is one of the main countries contributing to the increase in cases and death between 2021 and 2022 [ 1 ].
In Ethiopia, malaria transmission is seasonal with two peak transmissions seasons following the bimodal rainfall pattern. Like in most parts of Ethiopia, the peak season for the transmission of malaria in the current study area is from September to December, following the major rainy season [ 2 ]. It affects two-thirds of landmass with 60% of the population living in low to high malaria risk areas, making malaria a leading public health problem in the country [ 3 ]. Plasmodium falciparum and Plasmodium vivax accounting to 60% and 40% of the disease in the country [ 2 , 4 ]. Plasmodium falciparum is highly virulent species which causes severe malaria and death in the country [ 5 , 6 ]. In the country, there were 2.78 million cases and 8041 deaths were reported in 2021 [ 7 ].
Ethiopia is currently working on a malaria elimination programme that aims to eradicate the disease by 2030 [ 8 , 9 ]. In the fight against the disease, the distribution of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are critical. Additionally, increased healthcare utilization, early diagnosis, prompt treatment, prevention, and rapid management of malaria epidemics, were among the interventions used. However, malaria control programmes need to target active case detection for capturing asymptomatic infections as it challenges the ongoing malaria control and elimination efforts worldwide [ 10 , 11 ]. Most P. falciparum and P. vivax infections are likely to be asymptomatic [ 12 ]. Such infections are missed by passive surveillance, but remain infectious to mosquitoes. Treatment of asymptomatic carriers could help reduce disease transmission by depleting the reservoir of parasites available for infection of mosquitoes [ 13 ]. Without identification and targeting of asymptomatic infectious pool, transmission interruption might not be possible [ 12 ].
Several studies have been conducted to describe parasitological and entomological data of malaria in various malaria-endemic areas in Ethiopia. A recent study conducted in South Ethiopia has indicated Anopheles arabiensis to be the primary vector of P. falciparum after decades of malaria control [ 14 ]. On the other hand, studies consider malaria prevalence and risk in remote Ethiopian communities like the current study setting are limited. Therefore, a community-based study on malaria will provide data that is critical for making evidence-based decisions. The aim of the present study was to assess the prevalence of malaria and the associated risk factors among communities in various geographical settings in selected sites of South Ethiopia.
Study areas description
This study was conducted in four zones namely South Omo, Gamo, Wolaita, and Hadiya Zones of the former South Nations Nationalities Peoples Regional State (SNNPRs) (Fig. 1 ). The SNNPR was one of the regional states in Ethiopia, which include 17 administrative zones and 7 special woredas . The region has an elevation of 376 to 4207 m above sea level. Average elevation of the study kebeles ranged from 553 m a.s.l at Duma to 1720 m a.s.l. at Mehal Korga. The mean annual rainfall ranges from 500 – 2200 mm and temperature ranges between 15 °C and 30 °C. Malaria continues to be a significant health problem in the region, but the transmission intensity varies across different local settings [ 15 ].
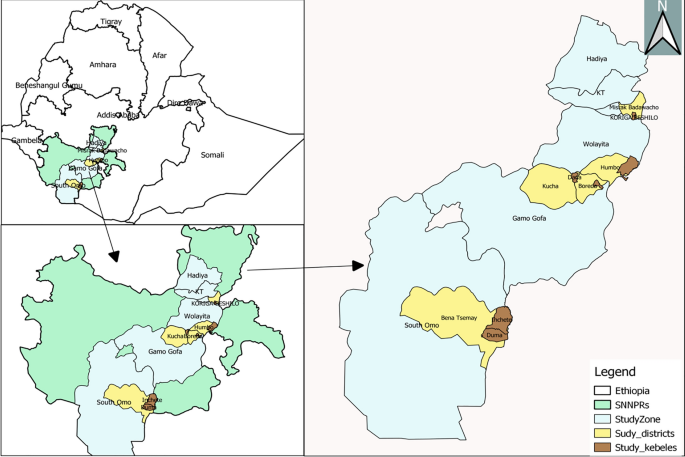
Map of study areas (Arc GIS version 10.1)
Study design and period
Community based cross-sectional study was conducted between February to June, 2019 to determine prevalence of Plasmodium infection and associated risk factors among household members in South Ethiopia.
Study participants
People residing in all the study kebeles could be taken as source population and individuals in selected households were included as study participants based on the following inclusion and exclusion criteria.
Inclusion and exclusion criteria
All household members who lived in the kebele for at least 6 months were included in the study regardless of the age and sex. Individuals, who receiving malaria treatment during survey and non-consenting respondents were excluded.
Sample size determination and sampling techniques
The sample size was determined using single population proportion formula of Fink and Kosecoff [ 16 ] assuming, 16% expected prevalence [ 17 ], 2.5% margin error, design effect 2, α = 5% (95% confidence level), and 15% non-response rate. Accordingly, the sample size was calculated as follows:
where n = the sample size, Z 1 -α/2 = the Z-value at a given confidence level, P = estimated prevalence of malaria in the study population, d = margin of error or sample error. Therefore, sample size was calculated as
Multistage sampling was used to select districts, kebeles , and households. According to the zonal health department report, one high-malaria-prevalent district in each zone were included, except the Gamo zone, where two districts were included. The Gamo zone included two districts as it had wider geographical coverage during conception of the study as Gamo-Gofa Zone. However, the Gamo-Gofa Zone became two independent zones during the study period and two of the districts located in Gamo Zone. Finally, two malarious kebeles were purposefully selected in each district based on the malaria incidence (Fig. 2 ).
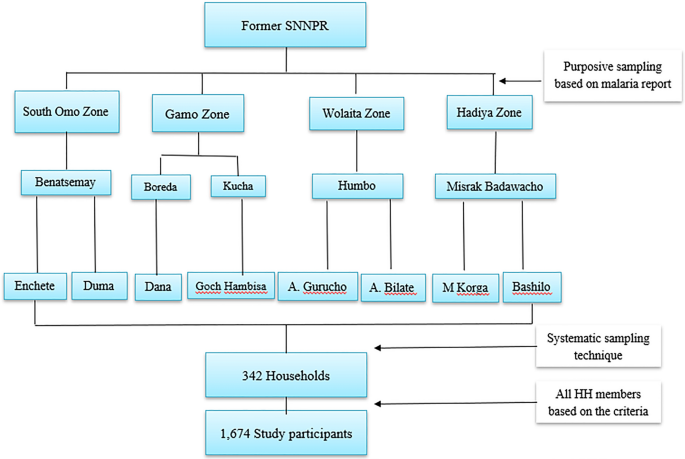
Sampling framework of the study sites and households
According to Ethiopian population and housing census of 2007, average family size for the region was 4.9 [ 18 ]; and hence the calculated household was 345 (Table 1 ). The total sample size (1674) was allocated to HHs proportionally to individual kebeles based on entire population of study sites as indicated in Table 1 . Systematic sampling was carried out using the lists of households in each kebele health post to select the households. The first household was selected randomly by lottery method and every k th household was included in the study. Where K is calculated by the formula of K = \(\frac{N}{n}, {\text{K}}=\frac{4729}{345}\) , Where, K = the gap between every household, N = total number of households in the study kebeles and n = sample size of households was calculated from individual sample size. Therefore, K = 13, thus every thirteenth household was included. Few houses were replaced by nearby houses when the selected household heads were absent or did not volunteer to participate in the study.
Sample collection and processing
Blood sample collection and processing.
Capillary blood sample was collected using sterile blood lancets from participants after obtaining written consent during house-to-house visits. Blood sample collection was done by senior medical laboratory technicians, following standard guidelines [ 19 ]. Thick and thin blood smears were prepared at field and dried by air. The air dried blood thin and thick smears were transported to nearby health centres’ laboratories using slide boxes. The smears were fixed using 99.8% methanol, dried, and stained with a 10% Giemsa solution for 10 min. Then, microscopy was employed by experienced laboratory technicians to detect and identify Plasmodium parasite species according to laboratory guidelines. Slides were declared negative for Plasmodium parasites after thorough examination of 100 fields and no Plasmodium parasite is detected by microscopy.
Sociodemographic data collection
Sociodemographic data were collected from 345 households based on structured questionnaire. The questioner prepared in local language was sought information on sociodemographic characteristics, and malaria prevention and control practices. After having the written consents, both individual and household-level factors associated with malaria transmission was obtained from the participant. During the time of sample collection, fever of study participants was checked and signs and symptoms of malaria such as headache, chills, sweating were asked. Fever of individuals was measured using thermometers (Hanimax) and auxiliary body temperature (> 37.5 ℃) were considered as febrile.
Data quality assurance
Data quality was maintained using various approaches. First, training was given for field assistants (data collectors) to have a common understanding to collect the appropriate demographic information. Second, blood sample collection and microscopy were done by senior laboratory technologists and discussion was held to apply standard operational diagnostic procedures during laboratory work. Each questioner and the collected sample were cross-checked for completeness, accuracy, and consistency by the group members and corrective measures taken. Moreover, all houses were coordinated using geographical position system and study individuals were coded during blood sample collection. All positive slides and 10% of negative slides were re-examined by another senior laboratory technologist blinded to previous slide results.

Study variables
The outcome variable for examination of blood films was Plasmodium infection status.
Independent variables included house structure (the roof material, floor material, presence of visible holes on wall), IRS spraying in the last 12 months, LLINs ownership (presence of bed nets, total number of nets, access to LLINs and use of mosquito nets), presence of mosquito breeding site. The variables like sex, age, and fever (auxiliary temperature) were considered as individual level for analysis of data.
Data analysis
Data was entered into Microsoft Excel spreadsheets and analysed using SPSS version 20.0. Descriptive statistics were used to determine the frequencies of variables. Bivariate logistic regression analysis was conducted to examine the association between Plasmodium infections with associated risk factors. Multivariate logistic regression analysis was conducted to test potential predicators’ variable that was the main risk factor for Plasmodium infection. The goodness of model fit was checked by Hosmer-Leme show-test and the logistic regression was fit for the test. Data normality was checked by non-parametric test of one-sample Kolmogorove-Smirnov test (1-sample K-S). Logistic regression statistical method of multivariate logistic regression was used with a 95% confidence interval and odds ratio was used to control confounders with the level of statistical significance was taken as P -value < 0.05 for analysis of independent and outcome variables. During binary logistic regression if the p ≤ 0.025 was considered as a candidate for multivariate logistic regression.
Sociodemographic characteristics
The sociodemographic characteristic of the study participants was summarized in Table 2 . From the total of 1674 participants, 748 (44.7%) were males and 926 (55.3%) were female. With regard to the age, 346 (20.7%), 531 (31.7%) and 797 (47.6%) were in the age groups < 5, 5–14 and > 14 age groups, respectively. Of the total, 1638 (97.8%) were non-febrile and the rest 36 (2.2%) were febrile cases.
Overall, and site-specific prevalence of malaria
The overall prevalence of malaria was 4.5% (76/1674) confirmed by microscopy (Table 3 ). The Plasmodium infection was more prevalent in Bashilo kebele 14.6% (33/226) followed by Mehal Korga 12.1% (26/214). Plasmodium infection was detected in seven study kebeles and no malaria cases were detected in Gocho Hambisa kebele .
Among the confirmed malaria cases, P. falciparum was dominant species accounting 65.8% (50/76), while P. vivax was 18.4% (14/76). Mixed infections with P. falciparum and P. vivax were accounted 15.8% (12/76). Higher prevalence of P. falciparum 10.18% (23/226) was observed in Bashilo kebele . Among study kebeles , Mehal Korga had the high prevalence of P. vivax 3.74% (8/214) (Table 3 ).
Sex and age-related prevalence of malaria
Of the study participants, 5.2% (39/748) males and 4% (37/926) females were found positive for Plasmodium parasite (Table 4 ). The prevalence of Plasmodium parasites among age groups were 7.8% (27/346) in under five children, 7.5% (40/531) in 5–14 years and 1.1 (9/797) in > 14 years. The greatest malaria prevalence was observed among under five children followed by school age groups.
Malaria-associated factors analysis
A total of eight independent variables were considered for bivariate logistic regression analysis of individuals and household associated risk factors for malaria parasite infections (Table 5 ). The variables associated with individual and household-level risk factors of malaria parasite infection was age, fever during survey time, LLINs utilization, IRS spray status, house structure (main roof material), main wall material, presence of visible hole on the wall, and living proximity to breeding sites. Among those variables, the age of individuals, fever, LLINs utilization and living proximity to the breeding site were a candidate for multivariate analysis.
In the multivariate logistic regression analysis, the predictors of Plasmodium infections after controlling confounders of the variables were the age of individuals (AOR = 0.14, 95% CI 0.02–0.82) and fever during survey time (AOR = 0.37, 95% CI0.19–0.72). Household-level predictor variables of Plasmodium infections were LLINs utilization (AOR = 0.37, 95% CI 0.19–0.72) and proximity mosquito breeding sites (AOR = 6.17, 95% CI 2.66–14.3) were a significant association with Plasmodium infection.
The individuals who’s aged < 5 was 86% more likely to have a malaria as compared with individuals whose age > 14 with the p-value = 0.029 (IC = 0.02-0.82). Individuals who do not have a fever during study time were 28.4 times more likely have Plasmodium parasite as compared to individuals with fever with the p-value = 0.001 (CI 11.4–70.06).
LLINs utilization was significantly associated with Plasmodium species. The individuals that have not to use LLINs during a sleeping time were 63% more likely have a chance to Plasmodium parasite infection as compared with their counterparts with the p-value = 0.003 (CI 0.19–0.72) (Table 5 ). Those individuals who live proximity to the breeding site were 6.17 times more likely have a chance to develop malaria as compared to individuals do not live around breeding site with the p-value = 0.001 (CI 2.66–14.3).
Malaria affects the lives of almost all people living in sub-Saharan African countries. In Ethiopia, malaria remains a major public health problem despite continuous control and preventive strategies in place. The overall prevalence of malaria in this study was 4.5% with varying prevalence in different study sites in South Ethiopia. Both P. falciparum and P. vivax has been identified with P. falciparum dominant species accounted for 65.8% (50/76). It was also observed that lower age group, non-febrile case, and individuals who live proximity to mosquito breeding site had higher Plasmodium infection.
The overall prevalence of malaria in this study (4.5%) was in line with reports from various parts of Ethiopia including 4.4% in Butajira, 6.1% in Benatsemay district (South Omo), 6.7% in Dembia districts, 6.8% in Sanja town, and 4% in Jimma zone [ 20 , 21 , 22 , 23 ]. This finding is higher than the prevalence reported in another study in Butajira and national malaria indicator survey 2015 result, with prevalence of 0.9% and 0.5%, respectively [ 24 , 25 ]. On the other hand, the present finding is much lower than the prevalence reported in Kisumu country in the Kenya with 28% [ 26 ], Armachiho districts, North West Ethiopia with 18.4% [ 27 ], and Dilla town and surrounding areas with 16.0% [ 17 ]. The difference in findings might be associated with sociodemographic, socioeconomic and environmental factors that could affect the epidemiology of malaria.
Prevalence of Plasmodium infection was relatively high in Bashilo (14.6%) and Mehal Korga kebeles (12.6%) as compared to Enchete, Duma, Dana, Gocho Hambisa, Abaya Gurucho and Abaya Bilate. The same holds true in other studies conducted in different parts of Ethiopia [ 20 , 28 , 29 ]. The heterogeneity of Plasmodium infection in the present study settings might be because of ecologic and environmental factors, host and vector characteristics, social, biological and socio demographic factors.
Plasmodium falciparum and P. vivax were identified as co-endemic species in study areas while P. falciparum was dominant species of parasite. The dominance of P. falciparum was consistent with the study conducted in Benatsemay districts in South Omo, Ethiopia [ 23 ]. In addition, the national community-based malaria indicator surveys conducted during peak malaria transmission season in the 2007 and 2011 reported the dominance of P. falciparum as 83% and 77%, respectively [ 30 , 31 ]. The dominance of P. falciparum species might be more widely distributed in many parts of Ethiopia. This might be associated to the capacity of P. falciparum parasite to develop resistance against anti-malarial drugs represents a central challenge in the global control and elimination of malaria [ 32 ]. In contrast to this finding, other studies conducted in different geographical settings in Ethiopia [ 28 , 29 ] monitoring changing of the epidemiology of malaria beyond Gark projects [ 33 ] and the facility-based cross-sectional study in Hadiya Zone [ 34 ] the P. vivax dominates over P. falciparum . One possible reason for predominance of P. vivax might be improper management of primaquine that lead to the relapse of hyponozoites.
Regarding the age groups, the likelihood of having higher malaria cases was found among under five children and school age children than other age groups. This finding was in line with malaria prevalence in Ethiopian on malaria indicator survey [ 25 ], in Arba Minch Zuria district [ 35 ] children this age groups are more vulnerable and had have Plasmodium parasite infections. The reason why high malaria cases in this age groups might be due to immunity status, more exposed to mosquito bites before bedding, and less awareness of self-care for utilization of malaria preventive measures.
Non-febrile Plasmodium infection was common in endemic areas. In malaria-endemic areas, people may develop partial immunity, allowing the non-febrile infection to occur. The odds of Plasmodium infection were higher in individuals that do not have fevers than those who have fever. The result consistent with the study conducted in Senegal that indicated P. falciparum was dominant species in asymptomatic cases [ 36 ]. In other way, in low transmission settings, asymptomatic cases are common and most of the asymptomatic infections are sub-microscopic [ 28 , 37 ]. Study showed that asymptomatic cases could serve as reservoirs of infections to the mosquito vectors [ 38 ]. Thus, they could serve as a major source of gametocytes and contributed to residual transmissions of malaria as asymptomatic carriers do not visit health facility for treatment. In many countries P. falciparum is asymptomatic or sub-clinical. In very low transmission settings, sub-microscopic carriers may contribute up to 50% of humans to mosquito transmission [ 39 ].
Appropriate use of the utilization of LLINs is one of the key interventions for the prevention of malaria [ 40 ]. In this study, ownership of LLINs was 76.9%. This finding was higher than the previous findings in Hadiya zones with LLINs ownership of 41.6% [ 34 ]. On the other hand, national malaria indicator survey conducted in 2011 and 2015 showed 55% and 64% of households have at least one LLINs of any type [ 25 , 30 ] and a community-based cohort study in South Central Ethiopia [ 41 ]. However, the accesses to LLINs were not significantly associated with Plasmodium infection in study sites.
The utilization of LLINs has an association with malaria cases among study participants. The current study showed that participants who use LLINs had lower malaria cases than those do not use. This findings is in line with the study conducted Dilla and surroundings areas, Dembia districts, and Hadiya zones where participants do not use bed nets were 0.2, 0.2 and 4.6 times more likely developed Plasmodium parasite infections, respectively [ 17 , 22 , 34 ]. The finding speculates the proper usage of LLINs protects from malaria through protecting mosquito bites depending on biting activity. It is noticeable that the proper utilization of LLINs will prevent mosquito that in turn prevent Plasmodium parasite infection. These findings might the implication of possession and efficacy of LLINs utilization in the community and less attention to frequent utilization in different local settings.
Another important factor that determines the odds of Plasmodium infection is living proximity to the breeding site. In this study, a participant who live proximity to mosquito breeding sites was at high risk of Plasmodium infections. The study participants those lives proximity to the stagnant water of mosquito the breeding sites 6.17 times more likely have a chance to develop Plasmodium infection as compared to individuals do not live around the breeding site. This finding in agreement with the study conducted in Dilla and surrounding areas and Dembia districts [ 17 , 22 ] by increasing the probability of having Plasmodium infection. This is because proximity mosquito breeding sites give more chances to exposure mosquito bites in the community.
This study has some limitations. One of the limitations of this study is the laboratory diagnosis which is limited to microscopy only, a low sensitive tool. The second limitation is seasonality of transmission was not determined. The community-based nature of the study can be viewed as one of the strengths of this study as it enables us to screen the non-febrile cases who could serve as potential reservoir of malaria parasite. High response rate of study participants can also be viewed as another strength of this study.
Malaria is still important public health problems, although the prevalence of disease was varying in the study sites . Lower age children, non-febrile cases and those who reside proximity to mosquito breeding sites were at higher risk of Plasmodium infection. Thus, malaria prevention and control strategies addressing communities at high risk of infection should be in place to reduce malaria associated morbidity and mortality in the study localities.
Availability of data and materials
The data supporting the conclusions conferred in this article is presented in the main paper.
WHO. World Malaria Report 2023. Geneva: World Health Organization; 2023.
Google Scholar
FDRoE. National Strategic Plan for Malaria Prevention, Control and Elimination in Ethiopia 2011–2015. Addis Ababa, Ethiopia. Federal Democratic Republic of Ethiopia Ministry of Health, 2010.
President’s Malaria Initiative Ethiopia. Malaria operational plan. 2019. https://reliefweb.int/sites/reliefweb.int/files/resources/fy-2019-ethiopia-malaria-operational-plan.pdf .
Carter Center. Annual malaria control program review enhancing impact through integrated strategies malaria programs Ethiopia and Nigeria. Atlanta, Georgia. 2012.
Yewhalaw D, Legesse W, Bortel WV, GebreSelassie S, Kloos H, Duchateau L, et al. Malaria and water resource development: the case of Gilgel-Gibehydroelectric dam in Ethiopia. Malar J. 2009;8:21.
Article PubMed PubMed Central Google Scholar
Ketema TG, Bacha K. Therapeutic efficacy of chloroquine for treatment of Plasmodium vivax malaria cases in Halaba district, South Ethiopia. Parasit Vectors. 2011;4:46.
WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
Fedral Minstry of Health (FMoH). National Malaria Elimination Strategic Plan: 2021–2025, Addis Ababa, Ethiopia. 2020.
Bugssa G, Tedla K. Feasibility of malaria elimination in Ethiopia. Ethiop J Health Sci. 2020;30:607–14.
PubMed PubMed Central Google Scholar
WHO. World Malaria Report 2018. Geneva: World Health Organization; 2018.
Gueye CS, Sanders KC, Galappaththy GN, Rundi C, Tobgay T, Sovannaroth S, et al. Active case detection for malaria elimination: a survey among Asia Pacific countries. Malar J. 2013;12:358.
Article Google Scholar
Cotter C, Sturrock HW, Hsiang MS, Llu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11.
Kokwaro G. Ongoing challenges in the management of malaria. Malar J. 2009;8:S2.
Eligo N, Wegayehu T, Pareyn M, Tamiru G, Lindtjørn B, Massebo F. Anopheles arabiensis continues to be the primary vector of Plasmodium falciparum after decades of malaria control in southwestern Ethiopia. Malar J. 2023;23:14.
Loha E, Lindtjørn B. Model variation in predicting incidence of Plasmodium falciparium malaria using 1998–2007 morbidity and metereological data from South Ethiopia. Malar J. 2010;9:166.
Fink AKJ. How to conduct surveys: a step by step guide. Thousand Oaks: Sage Publications; 1995.
Molla E, Ayele B. Prevalence of malaria and associated factors in Dilla Town and the surrounding rural areas, Gedeo Zone, Southwest Ethiopia. J Bacteriol Parasitol. 2015;6:5.
Central Statistical Agency (CSA). 2007 Population and housing census of Ethiopia. Addis Ababa, Ethiopia: 2007.
WHO. Basic malaria microscopy–Part I: Learner’s guide. 2nd ed. Geneva: World Health Organization; 2010.
Zemene E, Koepfli C, Tiruneh A, Yeshiwondim AK, Seyoum D, Lee MC, et al. Detection of foci of residual malaria transmission through reactive case detection in Ethiopia. Malar J. 2018;17:390.
Tesfaye S, Belyhun Y, Teklu T, Mengesha T, Petros B. Malaria prevalence pattern observed in the highland fringe of Butajira, Southwest Ethiopia: a longitudinal study from parasitological and entomological survey. Malar J. 2011;10:153.
Fekadu M, Yenit MK, Lakew AA. The prevalence of asymptomatic malaria parasitemia and associated factors among adults in Dembia district, northwest Ethiopia, 2017. Arch Public Health. 2018;76:74.
Debo GW, Kassa DH. Prevalence of malaria and associated factors in Benna Tsemay district of pastoralist community, Southwest Ethiopia. Travel Med Vacc. 2016;2:16.
Woyessa A, Deressa W, Ali A, Lindtjørn AB. Prevalence of malaria infection in Butajira area, south-central Ethiopia. Malar J. 2012;11:84.
Ethiopia National Malaria Indicator Survey (EMIS). Ethiopia National Malaria Indicator Survey 2015. Addis Ababa: Ethiopian Public Health Institute; 2016.
Jenkins R, Omollo R, Ongecha M, Sifuna P, Othieno C, Ongeri L, et al. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar J. 2015;14:26.
Aschale Y, Mengist A, Bitew A, Kassie B, Talie A. Prevalence of malaria and associated risk factors among asymptomatic migrant laborers in Armachiho District, Northwest Ethiopia. Res Rep Trop Med. 2018;9:95–101.
Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018;66:1883–91.
Article CAS PubMed Google Scholar
Haji Y, Fogarty AW, Deressa W. Prevalence and associated factors of malaria among febrile children in Ethiopia: a cross-sectional health facility-based study. Acta Trop. 2015;155:63–70.
Article PubMed Google Scholar
Ethiopia National Malaria Indicator Survey (EMIS). Ethiopia National Malaria Indicator Survey 2011: Technical Summary. Addis Ababa: The Ethiopian Health and Nutrition Research Institute & Partners; 2012.
Ethiopia National Malaria Indicator Survey (EMIS). Ethiopian National Malaria Indicator Survey (2007). Addis Ababa: Technical Summary Ethiopian; 2008.
Gil JP, Fançony C. Plasmodium falciparum multidrug resistance proteins (pfMRPs). Front Pharmacol. 2021;12: 759422.
Article CAS PubMed PubMed Central Google Scholar
Abeku TA, Helinski ME, Kirby JM, Kefyalew T, Awano T, Batisso E, et al. Monitoring changes in malaria epidemiology and effectiveness of interventions in Ethiopia and Uganda: beyond Garki Project baseline survey. Malar J. 2015;14:337.
Delil R, Dileba TK, Habtu YA, Gone T, Leta TJ. Magnitude of malaria and factors among febrile cases in low transmission areas of Hadiya Zone, Ethiopia: a facility based crosssectional study. PLoS ONE. 2016;5:11.
Abose T, Ye Y, Olana D, Alamirew D, Beyene Y, Regassa L, et al. Re-orientation and definition of the role of malaria vector control in Ethiopia: the epidemiology and control of malaria with special emphasis on the distribution, behaviour and susceptibility of insecticides of anopheline vectors and chloroquine resistance in Zwai, Central Ethiopia and other areas Geneva: World Health Organization. 1998
Niang M, Thiam LG, Sane R, Diagne N, Talla C, Doucoure S, et al. Substantial asymptomatic submicroscopic Plasmodium carriage during dry season in low transmission areas in Senegal: implications for malaria control and elimination. PLoS ONE. 2017;12: e0182189.
Federal Ministry of Health (FMOH). Annual Performance Report 2015 (HSDP IV) for EFY 2007 (2014/15) version 1. Addis abeba, Ethiopia. 2015.
Kiattibutr K, Roobsoong W, Sriwichai P, Saeseu T, Rachaphaew N, Suansomjit C, et al. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector. Anopheles dirus Int J Parasitol. 2017;47:163–70.
Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med. 2016;13: e1001942.
Fedal Ministry of Health (FMoH). National Malaria Guidelines. 3 rd edn. Accessed at www.medbox.org . Addis Abeba, Ethiopia. 2012.
Solomon T, Loha E, Deressa W, Gari T, Overgaard HJ, Lindtjørn B. Low use of long-lasting insecticidal nets for malaria prevention in south-central Ethiopia: a community-based cohort study. PLoS ONE. 2019;14: e0210578.
Download references
Acknowledgements
We would like to thank Arba Minch University for its financial support and study participants for taking parts in the study. We would also like to thank all the health centers and laboratory technicians of study sites for their cooperation during sample collection and processing. We are also grateful to South Omo, Gamo, Wolaita and Hadiya zonal and districts health departments and Kebele administrators for their technical support.
Financial support was obtained from Arba Minch University.
Author information
Authors and affiliations.
Malaria and Neglected Tropical Diseases, Gorche Health District, Hawassa, Sidama Regional State, Ethiopia
Girma Yutura
Department of Biology, College of Natural and Competitional Sciences, Arba Minch University, Arba Minch, Ethiopia
Fekadu Massebo, Nigatu Eligo & Teklu Wegayehu
Malaria and Neglected Tropical Diseases, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Abena Kochora
You can also search for this author in PubMed Google Scholar
Contributions
G.Y., F.M. and T.W. Conception and design of the study. G.Y. and N.E. Data accusation and management. G.Y., F.M. and T.W. Analyzed and interpreted data. G.Y., F.M., AK and T.W. Drafted the work and substantively revised it.
Corresponding author
Correspondence to Teklu Wegayehu .
Ethics declarations
Ethics approval and consent to participate.
The study was reviewed and approved by the Ethical Review Committee of Arba Minch University (Ref.No.CMHS/12033592/111). Prior to the study, permission letter was obtained from selected Zonal Health Departments. Written consent (assent for children) was obtained from head of the household before undertaking the data collection and official letter was sought from the respective district’s health office. For children and younger participants’ consents were obtained from their parents/guardians. The purpose of the study and procedure of blood sample collection were explained to the participants. The Study participants those positive for P. falciparum and P. vivax was treated free of charge at nearby health facilities.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yutura, G., Massebo, F., Eligo, N. et al. Prevalence of malaria and associated risk factors among household members in South Ethiopia: a multi-site cross-sectional study. Malar J 23 , 143 (2024). https://doi.org/10.1186/s12936-024-04965-4
Download citation
Received : 21 December 2023
Accepted : 25 April 2024
Published : 12 May 2024
DOI : https://doi.org/10.1186/s12936-024-04965-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Risk factors
- South Ethiopia
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Plasmodium falciparum malaria in pregnancy and fetal, newborn, and maternal outcomes among a cohort of pregnant women in coastal Kenya, 2006 - 2009
Add to collection, downloadable content.
- March 22, 2019
- Affiliation: Gillings School of Global Public Health, Department of Epidemiology
- Plasmodium falciparum malaria in pregnancy causes adverse pregnancy outcomes, most notably reduced birth weight and maternal anemia. Preventive treatment that is safe during pregnancy has been shown to effectively reduce rates of malaria in pregnancy, yet in malaria-endemic regions rates of adverse pregnancy outcomes remain high. We sought to explore the association of malaria in pregnancy and other risk factors with poor outcomes, among a cohort of pregnant women who received the recommended preventative treatment for malaria at antenatal care. The prevalence of malaria at the first antenatal care visit was 11%, and malaria infection was associated with lower measures of fetal growth, as measured by ultrasound. Among live, term births, the mean birth weight was not significantly different for malaria-positive vs. malaria-negative women. However, among women with under-nutrition, as measured by low body-mass-index, malaria exposure was associated with significantly decreased birth weight (mean difference -370 grams, 95% CI -728, -12 g). The rates of maternal anemia (hemoglobin <11.0 g/dL) and moderate/severe anemia (hemoglobin < 9.0 g/dL) at antenatal care were 70% and 27%, respectively. Moderate/severe maternal anemia at first antenatal care was associated with malaria as diagnosed by microscopy (aRR 2.06, 95% CI 1.24, 3.44) as was high-intensity hookworm infection in multivariate regression (aRR 2.37, 95% CI 1.44, 3.91). Our findings suggest the importance of good preventative treatment for malaria in pregnancy to minimize the impact of exposure to malaria on fetal and newborn growth. However, under-nutrition has an important role and research and programs to improve maternal nutritional health may be important to important to further improving birth outcomes in low-resource settings. Furthermore, given the high prevalence of anemia seen in our study, also associated with under-nutrition, as well as hookworm, and malaria, further research is needed to optimize interventions around pregnancy to improve maternal and newborn health in malaria-endemic regions.
- Epidemiology
- https://doi.org/10.17615/1jfw-vm95
- Dissertation
- In Copyright
- Meshnick, Steven R.
- Doctor of Philosophy
- University of North Carolina at Chapel Hill
This work has no parents.
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.

Malaria: Obstacles and Opportunities (1991)
Chapter: 1. conclusions and recommendations, conclusions and recommendations, defining the problem.
The outlook for malaria control is grim. The disease, caused by mosquito-borne parasites, is present in 102 countries and is responsible for over 100 million clinical cases and 1 to 2 million deaths each year. Over the past two decades, efforts to control malaria have met with less and less success. In many regions where malaria transmission had been almost eliminated, the disease has made a comeback, sometimes surpassing earlier recorded levels. The dream of completely eliminating malaria from many parts of the world, pursued with vigor during the 1950s and 1960s, has gradually faded. Few believe today that a global eradication of malaria will be possible in the foreseeable future.
Worldwide, the number of cases of malaria caused by Plasmodium falciparum , the most dangerous species of the parasite, is on the rise. Drug-resistant strains of P. falciparum are spreading rapidly, and there have been recent reports of drug resistance in people infected with P. vivax , a less virulent form of the parasite. Furthermore, mosquitoes are becoming increasingly resistant to insecticides, and in many cases, have adapted so as to avoid insecticide-treated surfaces altogether.
In large part because of the spread of drug and insecticide resistance, there are fewer tools available today to control malaria than there were 20 years ago. In many countries, the few remaining methods are often ap-
plied inappropriately. The situation in many African nations is particularly dismal, exacerbated by a crumbling health infrastructure that has made the implementation of any disease control program difficult.
Malaria cases among tourists, business travelers, military personnel, and migrant workers in malarious areas have been increasing steadily in the last several years, posing new concerns that the disease will be introduced to currently nonmalarious areas. Recent epidemics have claimed tens of thousands of lives in Africa, and there is an increasing realization that malaria is a major impediment to socioeconomic development in many countries. Unless practical, cost-effective strategies can be developed and successfully implemented, malaria will continue to exact a heavy toll on human life and health around the world.
Although often considered a single disease, malaria is more accurately viewed as many diseases, each shaped by subtle interactions of biologic, ecologic, social, and economic factors. The species of parasite, the behavior of the mosquito host, the individual's immune status, the climate, human activities, and access to health services all play important roles in determining the intensity of disease transmission, who will become infected, who will get sick, and who will die.
Gem miners along the Thailand-Cambodia border, American tourists on a wildlife safari in East Africa, villagers living on the central highlands in Madagascar, residents of San Diego County, California, a young pregnant woman in Malawi, Swiss citizens living near Geneva International Airport, children in Africa south of the Sahara, and a U.S. State Department secretary in Tanzania seem to have little in common, yet they are all at risk of contracting malaria. Because of the disease's variable presentations, each will be affected differently, as illustrated below.
For the hundreds of thousands of Thai seasonal agricultural workers who travel deep into the forest along the Thailand-Cambodia border to mine for gems, malaria is the cost of doing business. These young men are exposed to aggressive forest mosquitoes, and within two to three weeks after arriving, almost every miner will get malaria. Many gem miners seek medications to prevent and self-treat mild cases of the disease. But because malaria in this part of the world is resistant to most antimalarial drugs, the few effective drugs are reserved for the treatment of confirmed cases of malaria. To complicate matters, there are no health services in the forest to treat patients, and the health clinics in Thailand are overburdened by the high demand for treating those with severe malaria, most of whom are returning gem miners. A similar scenario involving over 400,000 people exists among gold miners in Rondonia, Brazil.
Each year, over seven million U.S. citizens visit parts of the world
where malaria is present. Many, at the recommendation of their travel agent or physician, take antimalarial medications as a preventive measure, but a significant number do not. Tourists and other travelers who have never been exposed to malaria, and therefore have never developed protective immunity, are at great risk for contracting severe disease. Ironically, it is not the infection itself that poses the biggest danger, but the chance that treatment will be delayed because of misdiagnosis upon the individual's return to the United States. Most U.S. doctors have never seen a patient with malaria, are often confused by the wide array of symptoms, and are largely unaware that malaria in a nonimmune person can be a medical emergency, sometimes rapidly fatal.
Prior to 1950, malaria was the major cause of death in the central highlands of the African island nation of Madagascar. In the late 1950s, an aggressive program of indoor insecticide spraying rid the area of malaria-carrying mosquitoes, and malaria virtually disappeared. By the 1970s, confident of a victory in the battle against malaria, Madagascar began to phase out its spraying program; in some areas spraying was halted altogether. In the early 1980s, the vector mosquitoes reinvaded the central highlands, and in 1986 a series of devastating epidemics began. The older members of the population had long since lost the partial immunity they once had, and the younger island residents had no immunity at all. During the worst of the epidemics, tens of thousands of people died in one three-month period. The tragedy of this story is that it could have been prevented. A cheap antimalarial drug, chloroquine, could have been a powerful weapon in Madagascar, where drug resistance was not a significant concern. Because of problems in international and domestic drug supply and delivery, however, many people did not receive treatment and many died. In the last 18 months, surveillance has improved, spraying against the mosquito has resumed, and more effective drug distribution networks have been established. Malaria-related mortality has declined sharply as a result.
Malaria, once endemic in the southern United States, occurs relatively infrequently. Indeed, there have been only 23 outbreaks of malaria since 1950, and the majority of these occurred in California. But for each of the past three years, the San Diego County Department of Health Services has had to conduct an epidemiologic investigation into local transmission of malaria. An outbreak in the late summer of 1988 involved 30 persons, the largest such outbreak in the United States since 1952. In the summer of 1989, three residents of San Diego County—a migrant worker and two permanent residents—were diagnosed with malaria; in 1990, a teenager living in a suburb of San Diego County fell ill with malaria. All of the cases were treated successfully, but these incidents raise questions about the possibility of new and larger outbreaks in the future. Malaria
transmission in San Diego County (and in much of California) is attributed to the presence of individuals from malaria-endemic regions who lack access to medical care, the poor shelter and sanitation facilities of migrant workers, and the ubiquitous presence of Anopheles mosquitoes in California.
A 24-year-old pregnant Yao woman from the Mangochi District in Malawi visited the village health clinic monthly to receive prenatal care. While waiting to be seen by the health provider, she and other women present listened to health education talks which were often about the dangers of malaria during pregnancy, and the need to install screens around the house to keep the mosquitoes away, to sleep under a bednet, and to take a chloroquine tablet once a week. Toward the end of her second trimester of pregnancy, the woman returned home from her prenatal visit with her eight tablets of chloroquine wrapped in a small packet of brown paper. She promptly gave the medicine to her husband to save for the next time he or one of their children fell ill. The next week she developed a very high malarial fever and went into labor prematurely. The six-month-old fetus was born dead.
Over a two-week period in the summer of 1989, five Swiss citizens living within a mile of Geneva International Airport presented at several hospitals with acute fever and chills. All had malaria. Four of the five had no history of travel to a malarious region; none had a history of intravenous drug use or blood transfusion. Apart from their symptoms, the only thing linking the five was their proximity to the airport. A subsequent epidemiologic investigation suggested that the malaria miniepidemic was caused by the bite of stowaway mosquitoes en route from a malaria-endemic country. The warm weather, lack of systematic spraying of aircraft, and the close proximity of residential areas to the airport facilitated the transmission of the disease.
Malaria is a part of everyday life in Africa south of the Sahara. Its impact on children is particularly severe. Mothers who bring unconscious children to the hospital often report that the children were playing that morning, convulsed suddenly, and have been unconscious ever since. These children are suffering from the most frequently fatal complication of the disease, cerebral malaria. Other children succumb more slowly to malaria, becoming progressively more anemic with each subsequent infection. By the time they reach the hospital, they are too weak to sit and are literally gasping for breath. Many children are brought to hospitals as a last resort, after treatment given for “fever” at the local health center has proved ineffective. Overall, children with malaria account for a third of all hospital admissions. A third of all children hospitalized for malaria die. In most parts of Africa, there are no effective or affordable options to prevent the
disease, so children are at high risk until they have been infected enough times to develop a partial immunity.
A 52-year-old American woman, the secretary to the U.S. ambassador in Tanzania, had been taking a weekly dose of chloroquine to prevent malaria since her arrival in the country the year before. She arrived at work one morning complaining of exhaustion, a throbbing headache, and fever. A blood sample was taken and microscopically examined for malaria parasites. She was found to be infected with P. falciparum , and was treated immediately with high doses of chloroquine. That night, she developed severe diarrhea, and by morning she was found to be disoriented and irrational. She was diagnosed as having cerebral malaria, and intravenous quinine treatment was started. Her condition gradually deteriorated—she became semicomatose and anemic, and approximately 20 percent of her red blood cells were found to be infected with malaria parasites. After continued treatment for several days, no parasites were detected in her blood. Despite receiving optimal care, other malaria-related complications developed and she died just nine days after the illness began. The cause of death: chloroquine-resistant P. falciparum .
These brief scenarios give a sense of the diverse ways that malaria can affect people. So fundamental is this diversity with respect to impact, manifestation, and epidemiology that malaria experts themselves are not unanimous on how best to approach the disease. Malariologists recognize that malaria is essentially a local phenomenon that varies greatly from region to region and even from village to village in the same district. Consequently, a single global technology for malaria control is of little use for specific conditions, yet the task of tailoring strategies to each situation is daunting. More important, many malarious countries do not have the resources, either human or financial, to carry out even the most meager efforts to control malaria.
These scenarios also illustrate the dual nature of malaria as it affects U.S. policy. In one sense, it is a foreign aid issue; a devastating disease is currently raging out of control in vast, heavily populated areas of the world. In another sense, malaria is of domestic public health concern. The decay of global malaria control and the invasion of the parasite into previously disease-free areas, coupled with the increasing frequency of visits to such areas by American citizens, intensify the dangers of malaria for the U.S. population. Tourists, business travelers, Peace Corps volunteers, State Department employees, and military personnel are increasingly at risk, and our ability to protect and cure them is in jeopardy. What is desperately needed is a better application of existing malaria control tools and new methods of containing the disease.
In most malarious regions of the world, there is inadequate access to malaria treatment. Appropriate health facilities may not exist; those that do exist may be inaccessible to affected populations, may not be supplied with effective drugs, or may be staffed inappropriately. In many countries, the expansion of primary health care services has not proceeded according to expectations, particularly in the poorest (and most malarious) nations of the tropical world.
In some countries, antimalarial interventions are applied in broad swaths, without regard to underlying differences in the epidemiology of the disease. In other countries, there are no organized interventions at all. The malaria problem in many regions is compounded by migration, civil unrest, poorly planned exploitation of natural resources, and their frequent correlate, poverty.
During the past 15 years, much research has focused on developing vaccines for malaria. Malaria vaccines are thought to be possible in part because people who are naturally exposed to the malaria parasite acquire a partial immunity to the disease over time. In addition, immunization of animals and humans by the bites of irradiated mosquitoes infected with the malaria parasite can protect against malaria infection. Much progress has been made, but current data suggest that effective vaccines are not likely to be available for some time.
Compounding the difficulty of developing more effective malaria prevention, treatment, and control strategies is a worldwide decline in the pool of scientists and health professionals capable of conducting field research and organizing and managing malaria control programs at the country level. With the change in approach from malaria eradication to malaria control, many malaria programs “lost face,” admitting failure and losing the priority interest of their respective ministries of health. As external funding agencies lost interest in programs, they reduced their technical and financial support. As a consequence, there were fewer training opportunities, decreased contacts with international experts, and diminished prospects for improving the situation. Today, many young scientists and public health specialists, in both the developed and developing countries, prefer to seek higher-profile activities with better defined opportunities for career advancement.
It is against this backdrop of a worsening worldwide malaria situation that the Institute of Medicine was asked to convene a multidisciplinary committee to assess the current status of malaria research and control and to make recommen-
dations to the U.S. government on promising and feasible strategies to address the problem. During the 18-month study, the committee reviewed the state of the science in the major areas of malariology, identified gaps in knowledge within each of the major disciplines, and developed recommendations for future action in malaria research and control.
Organization
Chapter 2 summarizes key aspects of the individual state-of-the-science chapters, and is intended to serve as a basic introduction to the medical and scientific aspects of malaria, including its clinical signs, diagnosis, treatment, and control. Chapter 3 provides a historical overview of malaria, from roughly 3000 B.C. to the present, with special emphasis on efforts in this century to eradicate and control the disease. The state-of-the-science reviews, which start in Chapter 4 , begin with a scenario titled “Where We Want To Be in the Year 2010.” Each scenario describes where the discipline would like to be in 20 years and how, given an ideal world, the discipline would have contributed to malaria control efforts. The middle section of each chapter contains a critical review of the current status of knowledge in the particular field. The final section lays out specific directions for future research based on a clear identification of the major gaps in scientific understanding for that discipline. The committee urges those agencies that fund malaria research to consult the end of each state-of-the-science chapter for suggestions on specific research opportunities in malaria.
Sponsorship
This study was sponsored by the U.S. Agency for International Development, the U.S. Army Medical Research and Development Command, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
CONCLUSIONS AND RECOMMENDATIONS
A major finding of the committee is the need to increase donor and public awareness of the growing risk presented by the resurgence of malaria. Overall, funding levels are not adequate to meet the problem. The committee believes that funding in the past focused too sharply on specific technologies and particular control strategies (e.g., indiscriminate use of insecticide spraying). Future support must be balanced among the needs outlined in this report. The issue for prioritization is not whether to select specific technologies or control strategies, but to raise the priority for solv-
ing the problem of malaria. This is best done by encouraging balanced research and control strategies and developing a mechanism for periodically adjusting support for promising approaches.
This report highlights those areas which the committee believes deserve the highest priority for research or which should be considered when U.S. support is provided to malaria control programs. These observations and suggestions for future action, presented below in four sections discussing policy, research, control, and training, represent the views of a multidisciplinary group of professionals from diverse backgrounds and with a variety of perspectives on the problem.
The U.S. government is the largest single source of funds for malaria research and control activities in the world. This investment is justified by the magnitude of the malaria problem, from both a foreign aid and a public health perspective. The increasing severity of the threat of malaria to residents of endemic regions, travelers, and military personnel, and our diminishing ability to counter it, should be addressed by a more comprehensive and better integrated approach to malaria research and control. However, overall U.S. support for malaria research and control has declined over the past five years. The committee believes that the amount of funding currently directed to malaria research and control activities is inadequate to address the problem.
Over the past 10 years, the majority of U.S. funds available for malaria research have been devoted to studies on immunity and vaccine development. Although the promise of vaccines remains to be realized, the committee believes that the potential benefits are enormous. At the same time, the relative paucity of funds available for research has prevented or slowed progress in other areas. Our incomplete knowledge about the basic biology of malaria parasites, how they interact with their mosquito and human hosts, and how human biology and behavior affect malaria transmission and control remains a serious impediment to the development and implementation of malaria control strategies. The committee believes that this situation must be addressed without reducing commitment to current research initiatives. The committee further believes that such research will pay long-term dividends in the better application of existing tools and the development of new drugs, vaccines, and methods for vector control.
The committee recommends that increased funds be made available so that U.S. research on malaria can be broadened according to the priorities addressed in this report, including laboratory and field research on the biology of malaria parasites, their mosquito vectors, and their interaction with humans.
The committee believes that the maximum return on investment of funds devoted to malaria research and control can be achieved only by rigorous review of project proposals. The committee further believes that the highest-quality review is essential to ensure that funding agencies spend their money wisely. The committee believes that all U.S.-supported malaria field activities, both research and control, should be of the highest scientific quality and relevance to the goals of malaria control.
The committee recommends decisions on funding of malaria research be based on scientific merit as determined by rigorous peer review, consistent with the guidelines of the National Institutes of Health or the United Nations Development Program/World Bank/ World Health Organization Special Programme for Research and Training in Tropical Diseases, and that all U.S.-supported malaria field projects be subject to similar rigorous review to ensure that projects are epidemiologically and scientifically sound.
Commitment and Sustainability
For malaria control, short-term interventions can be expected to produce only short-term results. The committee believes that short-term interventions are justified only for emergency situations. Longer-term interventions should be undertaken only when there is a national commitment to support sustained malaria surveillance and control.
The committee recommends that malaria control programs receive sustained international and local support, oriented toward the development of human resources, the improvement of management skills, the provision of supplies, and the integration of an operational research capability in support of an epidemiologically sound approach to malaria control.
Surveillance
During the major effort to eradicate malaria from many parts of the world that began in the late 1950s and ended in 1969, it was important to establish mechanisms to detect all malaria infections. As a result, systems were established in many countries to collect blood samples for later microscopic examination for the presence of parasites. Each year, the results from more than 140 million slides are reported to the World Health Organization, of which roughly 3 to 5 percent are positive for malaria. This approach seeks to answer the question posed 30 years ago: How many people are infected with the malaria parasite? It does not answer today's questions: Who is sick? Where? Why? The committee concludes that the mass collection of blood slides requires considerable resources, poses seri-
ous biosafety hazards, deflects attention from the treatment of ill individuals, and has little practical relevance for malaria control efforts today.
Instead of the mass collection of slides, the committee believes that the most effective surveillance networks are those that concurrently measure disease in human populations, antimalarial drug use, patterns of drug resistance, and the intensity of malaria transmission by vector populations. The committee believes that malaria surveillance practices have not received adequate recognition as an epidemiologic tool for designing, implementing, and evaluating malaria control programs.
The committee recommends that countries be given support to orient malaria surveillance away from the mass collection and screening of blood slides toward the collection and analysis of epidemiologically relevant information that can be used to monitor the current situation on an ongoing basis, to identify high-risk groups, and to detect potential epidemics early in their course.
Inter-Sectoral Cooperation
The committee believes that insufficient attention has been paid to the impact that activities in non-health-related sectors, such as construction, industry, irrigation, and agriculture, have on malaria transmission. Conversely, there are few assessments of the impact of malaria control projects on other public health initiatives, the environment, and the socioeconomic status of affected populations. Malaria transmission frequently occurs in areas where private and multinational businesses and corporations (e.g., hotel chains, mining operations, and industrial plants) have strong economic interests. Unfortunately and irresponsibly, some local and multinational businesses contribute few if any resources to malaria control in areas in which they operate.
The committee recommends greater cooperation and consultation between health and nonhealth sectors in the planning and implementation of major development projects and malaria activities. It also recommends that all proposed malaria control programs be analyzed for their potential impact on other public health programs, the environment, and social and economic welfare, and that local and multinational businesses be recruited by malaria control organizations to contribute substantially to local malaria control efforts.
New Tools for Malaria Control
The committee believes that, as a policy directive, it is important to support research activities to develop new tools for malaria control. The
greatest momentum for the development of new tools exists in vaccine and drug development, and the committee believes it essential that this momentum be maintained. The committee recognizes that commendable progress has been made in defining the characteristics of antigens and delivery systems needed for effective vaccines, but that the candidates so far tested fall short of the goal. Much has been learned which supports the hope that useful vaccines can be developed. To diminish activity in vaccine development at this stage would deal a severe blow to one of our best chances for a technological breakthrough in malaria control.
The committee recommends that vaccine development continue to be a priority of U.S.-funded malaria research.
Only a handful of drugs are available to prevent or treat malaria, and the spread of drug-resistant strains of the malaria parasite threatens to reduce further the limited pool of effective drugs. The committee recognizes that there is little economic incentive for U.S. pharmaceutical companies to undertake antimalarial drug discovery activities. The committee is concerned that U.S. government support of these activities, based almost entirely at the Walter Reed Army Institute of Research (WRAIR), has decreased and is threatened with further funding cuts. The committee concludes that the WRAIR program in antimalarial drug discovery, which is the largest and most successful in the world, is crucial to international efforts to develop new drugs for malaria. The benefits of this program in terms of worldwide prevention and treatment of malaria have been incalculable.
The committee strongly recommends that drug discovery and development activities at WRAIR receive increased and sustained support.
The next recommendation on policy directions reflects the committee 's concern about the lack of involvement in malaria research by the private sector. The committee believes that the production of candidate malaria vaccines and antimalarial drugs for clinical trials has been hampered by a lack of industry involvement. Greater cooperation and a clarification of the contractual relationships between the public and private sectors would greatly enhance the development of drugs and vaccines.
The committee recommends that mechanisms be established to promote the involvement of pharmaceutical and biotechnology firms in the development of malaria vaccines, antimalarial drugs, and new tools for vector control.
Coordination and Integration
The committee is concerned that there is inadequate joint planning and coordination among U.S.-based agencies that support malaria research and
control activities. Four government agencies and many nongovernmental organizations in the United States are actively involved in malaria-related activities. There are also numerous overseas organizations, governmental and nongovernmental, that actively support such activities worldwide.
The complexity and variability of malaria, the actual and potential scientific advances in several areas of malariology, and most important the worsening worldwide situation argue strongly for an ongoing mechanism to assess and influence current and future U.S. efforts in malaria research and control.
The committee strongly recommends the establishment of a national advisory body on malaria.
In addition to fulfilling a much needed coordinating function among U.S.-based agencies and between the U.S. and international efforts, the national advisory body could monitor the status of U.S. involvement in malaria research and control, assess the relevant application of knowledge, identify areas requiring further research, make recommendations to the major funding agencies, and provide a resource for legislators and others interested in scientific policy related to malaria. The national advisory body could convene specific task-oriented scientific working groups to review research and control activities and to make recommendations, when appropriate, for changes in priorities and new initiatives.
The committee believes that the national advisory body should be part of, and appointed by, a neutral and nationally respected scientific body and that it should actively encourage the participation of governmental and nongovernmental organizations, industry, and university scientists in advising on the direction of U.S. involvement in malaria research and control.
The increasing magnitude of the malaria problem during the past decade and the unpredictability of changes in human, parasite, and vector determinants of transmission and disease point strongly to the need for such a national advisory body, which can be responsive to rapidly changing problems, and advances in scientific research, relating to global efforts to control malaria.
Malaria Research Priorities
Malaria control is in crisis in many areas of the world. People are contracting and dying of severe malaria in unprecedented numbers. To address these problems, the committee strongly encourages a balanced research agenda. Two basic areas of research require high priority. Research that will lead to improved delivery of existing interventions for malaria, and the development of new tools for the control of malaria.
Research in Support of Available Control Measures
Risk Factors for Severe Malaria People who develop severe and complicated malaria lack adequate immunity, and many die from the disease. Groups at greatest risk include young children and pregnant women in malaria endemic regions; nonimmune migrants, laborers, and visitors to endemic regions; and residents of regions where malaria has been recently reintroduced. For reasons that are largely unknown, not all individuals within these groups appear to be at equal risk for severe disease. The committee believes that the determinants of severe disease, including risk factors associated with a population, the individual (biologic, immunologic, socioeconomic, and behavioral), the parasite, or exposure to mosquitoes, are likely to vary considerably in different areas.
The committee recommends that epidemiologic studies on the risk factors for severe and complicated malaria be supported.
Pathogenesis of Severe and Complicated Malaria Even with optimal care, 20 to 30 percent of children and adults with the most severe form of malaria—primarily cerebral malaria—die. The committee believes that a better understanding of the disease process will lead to improvements in preventing and treating severe forms of malaria. The committee further believes that determining the indications for treatment of severe malarial anemia is of special urgency given the risk of transmitting the AIDS virus through blood transfusions, the only currently available treatment for malarial anemia. Physicians need to know when it is appropriate to transfuse malaria patients.
The committee recommends greater support for research on the pathogenesis of severe and complicated malaria, on the mechanisms of malarial anemia, and on the development of specific criteria for blood transfusions in malaria.
Social Science Research The impact of drugs to control disease or programs to reduce human-mosquito contact is mediated by local practices and beliefs about malaria and its treatment. Most people in malaria-endemic countries seek initial treatment for malaria outside of the formal health sector. Programs that attempt to influence this behavior must understand that current practices satisfy, at some level, local concerns regarding such matters as access to and effectiveness of therapy, and cost. These concerns may lead to practices at odds with current medical practice. Further, many malaria control programs have not considered the social, cultural, and behavioral dimensions of malaria, thereby limiting the effectiveness of measures undertaken. The committee recognizes that control programs often fail to incorporate household or community concerns and resources
into program design. In most countries, little is known about how the demand for and utilization of health services is influenced by such things as user fees, location of health clinics, and the existence and quality of referral services. The committee concludes that modern social science techniques have not been effectively applied to the design, implementation, and evaluation of malaria control programs.
The committee recommends that research be conducted on local perceptions of malaria as an illness, health-seeking behaviors (including the demand for health care services), and behaviors that affect malaria transmission, and that the results of this research be included in community-based malaria control interventions that promote the involvement of communities and their organizations in control efforts.
Innovative Approaches to Malaria Control Malaria control programs will require new ideas and approaches, and new malaria control strategies need to be developed and tested. There is also a need for consistent support of innovative combinations of control technologies and for the transfer of new technologies from the laboratory to the clinic and field for expeditious evaluation. Successful technology transfer requires the exchange of scientific research, but more importantly, must be prefaced by an improved understanding of the optimal means to deliver the technology to the people in need (see Chapter 11 ).
The committee recommends that donor agencies provide support for research on new or improved control strategies and into how new tools and technologies can be better implemented and integrated into on-going control efforts.
Development of New Tools
Antimalarial Immunity and Vaccine Development Many people are able to mount an effective immune response that can significantly mitigate symptoms of malaria and prevent death. The committee believes that the development of effective malaria vaccines is feasible, and that the potential benefits of such vaccines are enormous. Several different types of malaria vaccines need to be developed: vaccines to prevent infection (of particular use for tourists and other nonimmune visitors to endemic countries), prevent the progression of infection to disease (for partially immune residents living in endemic areas and for nonimmune visitors), and interrupt transmission of parasites by vector populations (to reduce the risk of new infections in humans). The committee believes that each of these directions should be pursued.
The committee recommends sustained support for research to identify mechanisms and targets of protective immunity and to exploit the
use of novel scientific technologies to construct vaccines that induce immunity against all relevant stages of the parasite life cycle.
Drug Discovery and Development Few drugs are available to prevent or treat malaria, and the spread of drug-resistant strains of malaria parasites is steadily reducing the limited pool of effective chemotherapeutic agents. The committee believes that an inadequate understanding of parasite biochemistry and biology impedes the process of drug discovery and slows studies on the mechanisms of drug resistance.
The committee recommends increased emphasis on screening compounds to identify new classes of potential antimalarial drugs, identifying and characterizing vulnerable targets within the parasite, understanding the mechanisms of drug resistance, and identifying and developing agents that can restore the therapeutic efficacy of currently available drugs.
Vector Control Malaria is transmitted to humans by the bites of infective mosquitoes. The objective of vector control is to reduce the contact between humans and infected mosquitoes. The committee believes that developments are needed in the areas of personal protection, environmental management, pesticide use and application, and biologic control, as well as in the largely unexplored areas of immunologic and genetic approaches for decreasing parasite transmission by vectors.
The committee recommends increased support for research on vector control that focuses on the development and field testing of methods for interrupting parasite transmission by vectors.
Malaria Control
Malaria is a complex disease that, even under the most optimistic scenario, will continue to be a major health threat for decades. The extent to which malaria affects human health depends on a large number of epidemiologic and ecologic factors. Depending on the particular combination of these and other variables, malaria may have different effects on neighboring villages and people living in a single village. All malaria control programs need to be designed with a view toward effectiveness and sustainability, taking into account the local perceptions, the availability of human and financial resources, and the multiple needs of the communities at risk. If community support for health sector initiatives is to be guaranteed, the public needs to know much more about malaria, its risks for epidemics and severe disease, and difficulties in control.
Unfortunately, there is no “magic bullet” solution to the deteriorating worldwide malaria situation, and no single malaria control strategy will be applicable in all regions or epidemiologic situations. Given the limited available financial and human resources and a dwindling pool of effective
antimalarial tools, the committee suggests that donor agencies support four priority areas for malaria control in endemic countries.
The committee believes that the first and most basic priority in malaria control is to prevent infected individuals from becoming severely ill and dying. Reducing the incidence of severe morbidity and malaria-related mortality requires a two-pronged approach. First, diagnostic, treatment, and referral capabilities, including the provision of microscopes, training of technicians and other health providers, and drug supply, must be enhanced. Second, the committee believes that many malaria-related deaths could be averted if individuals and caretakers of young children knew when and how to seek appropriate treatment and if drug vendors, pharmacists, physicians, nurses, and other health care providers were provided with up-to-date and locally appropriate treatment and referral guidelines. The development and implementation of an efficient information system that provides rapid feedback to the originating clinic and area is key to monitoring the situation and preventing epidemics.
The committee believes that the second priority should be to promote personal protection measures (e.g., bednets, screens, and mosquito coils) to reduce or eliminate human-mosquito contact and thus to reduce the risk of infection for individuals living in endemic areas. At the present time, insecticide-treated bednets appear to be the most promising personal protection method.
In many environments, in addition to the treatment of individuals and use of personal protection measures, community-wide vector control is feasible. In such situations, the committee believes that the third priority should be low-cost vector control measures designed to reduce the prevalence of infective mosquitoes in the environment, thus reducing the transmission of malaria to populations. These measures include source reduction (e.g., draining or filling in small bodies of water where mosquito larvae develop) or the application of low-cost larval control measures. In certain environments, the use of insecticide-impregnated bednets by all or most members of a community may also reduce malaria transmission, but this approach to community-based malaria control remains experimental.
The committee believes that the fourth priority for malaria control should be higher cost vector control measures such as large-scale source reduction or widespread spraying of residual insecticides. In certain epidemiologic situations, the use of insecticides for adult mosquito control is appropriate and represents the method of choice for decreasing malaria transmission and preventing epidemics (see Chapter 7 and Chapter 10 ).
The committee recommends that support of malaria control programs include resources to improve local capacities to conduct prompt diagnosis, including both training and equipment, and to ensure the availability of antimalarial drugs.
The committee recommends that resources be allocated to develop and disseminate malaria treatment guidelines for physicians, drug vendors, pharmacists, village health workers, and other health care personnel in endemic and non-endemic countries. The guidelines should be based, where appropriate, on the results of local operational research and should include information on the management of severe and complicated disease. The guidelines should be consistent and compatible among international agencies involved in the control of malaria.
The committee recommends that support for malaria control initiatives include funds to develop and implement locally relevant communication programs that provide information about how to prevent and treat malaria appropriately (including when and how to seek treatment) and that foster a dialogue about prevention and control.
Organization of Malaria Control
One of the major criticisms of malaria control programs during the past 10 to 15 years has been that funds have been spent inappropriately without an integrated plan and without formal evaluation of the efficacy of control measures instituted. In many instances, this has led to diminished efforts to control malaria.
The committee strongly encourages renewed commitment by donor agencies to support national control programs in malaria-endemic countries.
The committee recommends that U.S. donor agencies develop, with the advice of the national advisory body, a core of expertise (either in-house or through an external advisory group) to plan assistance to malaria control activities in endemic countries.
The committee believes that the development, implementation, and evaluation of such programs must follow a rigorous set of guidelines. These guidelines should include the following steps:
Identification of the problem
Determine the extent and variety of malaria. The paradigm approach described in Chapter 10 should facilitate this step.
Analyze current efforts to solve malaria problems.
Identify and characterize available in-country resources and capabilities.
Development of a plan
Design and prioritize interventions based on the epidemiologic situation and the available resources.
Design a training program for decision makers, managers, and technical staff to support and sustain the interventions.
Define specific indicators of the success or failure of the interventions at specific time points.
Develop a specific plan for reporting on the outcomes of interventions.
Develop a process for adjusting the program in response to successes and/or failures of interventions.
Review of the comprehensive plan by a donor agency review board
Modification of the plan based on comments of the review board
Implementation of the program
Yearly report and analysis of outcome variables
To guide the implementation of the activities outlined above, the committee has provided specific advice on several components, including an approach to evaluating malaria problems and designing control strategies (the paradigm approach), program management, monitoring and evaluation, and operational research.
Paradigm Approach
Given the complex and variable nature of malaria, the committee believes that the epidemiologic paradigms (see Chapter 10 ), developed in conjunction with this study, may form the basis of a logical and reasoned approach for defining the malaria problems and improving the design and management of malaria control programs.
The committee recommends that the paradigm approach be field tested to determine its use in helping policymakers and malaria program managers design and implement epidemiologically appropriate and cost-effective control initiatives.
The committee recognizes that various factors, including the local ecology, the dynamics of mosquito transmission of malaria parasites, genetically determined resistance to malaria infection, and patterns of drug use, affect patterns of malaria endemicity in human populations and need to be considered when malaria control strategies are developed. In most endemic countries, efforts to understand malaria transmission through field studies of vector populations are either nonexistent or so limited in scope that they have minimal impact on subsequent malaria control efforts. The committee recognizes that current approaches to malaria control are clearly inadequate. The committee believes, however, that malaria control strategies are sometimes applied inappropriately, with little regard to the underlying differences in the epidemiology of the disease.
The committee recommends that support for malaria control programs include funds to permit a reassessment and optimization of antimalarial tools based on relevant analyses of local epidemiologic, parasitologic, entomologic, socioeconomic, and behavioral determinants of malaria and the costs of malaria control.
Poor management has contributed to the failure of many malaria control programs. Among the reasons are a chronic shortage of trained managers who can think innovatively about health care delivery and who can plan, implement, supervise, and evaluate malaria control programs. Lack of incentives, the absence of career advancement options, and designation of responsibility without authority often hinder the effectiveness of the small cadre of professional managers that does exist. The committee recognizes that management technology is a valuable resource that has yet to be effectively introduced into the planning, implementation, and evaluation of most malaria control programs.
The committee recommends that funding agencies utilize management experts to develop a comprehensive series of recommendations and guidelines as to how basic management skills and technology can be introduced into the planning, implementation, and evaluation of malaria control programs.
The committee recommends that U.S. funding of each malaria control program include support for a senior manager who has responsibility for planning and coordinating malaria control activities. Where such an individual does not exist, a priority of the control effort should be to identify and support a qualified candidate. The manager should be supported actively by a multidisciplinary core group with expertise in epidemiology, entomology, the social sciences, clinical medicine, environmental issues, and vector control operations.
Monitoring and Evaluation
Monitoring and evaluation are essential components of any control program. For malaria control, it is not acceptable to continue pursuing a specific control strategy without clear evidence that it is effective and reaching established objectives.
The committee recommends that support for malaria control programs include funds to evaluate the impact of control efforts on the magnitude of the problem and that each program be modified as necessary on the basis of periodic assessments of its costs and effectiveness.
Problem Solving (Operational Research) and Evaluation
At the outset of any malaria prevention or control initiative and during the course of implementation, gaps in knowledge will be identified and problems will arise. These matters should be addressed through clearly defined, short-term, focused studies. Perhaps the most difficult aspects of operational research are to identify the relevant problem, formulate the appropriate question, and design a study to answer that question.
The committee recommends that a problem-solving (operational research) component be built into all existing and future U.S.-funded malaria control initiatives and that support be given to enhance the capacity to perform such research. This effort will include consistent support in the design of focused projects that can provide applicable results, analysis of data, and dissemination of conclusions.
The committee concludes that there is a need for additional scientists actively involved in malaria-related research in the United States and abroad. To meet this need, both short- and long-term training at the doctoral and postdoctoral levels must be provided. This training will be of little value unless there is adequate long-term research funding to support the career development of professionals in the field of malaria.
The committee recommends support for research training in malaria.
Whereas the curricula for advanced degree training in basic science research and epidemiology are fairly well defined, two areas require attention, especially in the developing world: social sciences and health management and training.
The committee recommends that support be given for the development of advanced-degree curricula in the social sciences, and in health management and training, for use in universities in developing and developed countries.
The availability of well-trained managers, decision makers, and technical staff is critical to the implementation of any malaria prevention and control program. The development of such key personnel requires a long term combination of formal training, focused short courses, and a gradual progression of expertise.
The committee recommends support for training in management, epidemiology, entomology, social sciences, and vector control. Such training for malaria control may be accomplished through U.S.-funded grant programs for long-term cooperative relationships
between institutions in developed and developing countries; through the encouragement of both formal and informal linkages among malaria-endemic countries; through the use of existing training courses; and through the development of specific training courses.
The committee recommends further that malaria endemic countries be supported in the development of personnel programs that provide long-term career tracks for managers, decision makers, and technical staff, and that offer professional fulfillment, security, and competitive financial compensation.
Malaria is making a dramatic comeback in the world. The disease is the foremost health challenge in Africa south of the Sahara, and people traveling to malarious areas are at increased risk of malaria-related sickness and death.
This book examines the prospects for bringing malaria under control, with specific recommendations for U.S. policy, directions for research and program funding, and appropriate roles for federal and international agencies and the medical and public health communities.
The volume reports on the current status of malaria research, prevention, and control efforts worldwide. The authors present study results and commentary on the:
- Nature, clinical manifestations, diagnosis, and epidemiology of malaria.
- Biology of the malaria parasite and its vector.
- Prospects for developing malaria vaccines and improved treatments.
- Economic, social, and behavioral factors in malaria control.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.

- Report Highlights
- EU-Summaries

- About the publications
Malaria status & challenges of the epidemic
- Level 1: Summary
- Level 2: Details
- 1. Introduction

Malaria is one of the most common infectious diseases and a great public health problem worldwide, particularly in Africa and south Asia. About three billion people are at risk of infection in 109 countries. Each year, there are an estimated 250 million cases of malaria leading to approximately one million deaths, mostly in children under five years of age. The organism that causes the most dangerous form of malaria is a microscopic parasite called Plasmodium falciparum .
This parasite is transmitted by mosquito species belonging to the Anopheles genus and only by females of those species.
There is growing international agreement on how best to use prevention and treatment methods that are available. The most effective prevention measures include the use of mosquito bed nets treated with long-lasting insecticides – to avoid the mosquito bites and to kill the mosquitoes – and spraying the inside walls of houses with similar insecticides to kill malaria-carrying mosquitoes. The most effective treatment for malaria consists in using a combination of several anti-malarial drugs, one of which is a derivative of artemisinin . Preventive treatment of pregnant women with anti-malarial drugs can also reduce the harmful effects of malaria both on the mother and on the unborn child.
Several international organisations have set up ambitious objectives for large-scale malaria control. The target set by the Word Health Organization ( WHO ) in 2005 is to offer malaria prevention and treatment services by 2010 to at least 80% of the people who need them. By doing so, it aims to reduce at least by half the proportion of people who become ill or die from malaria by 2010 and at least by three quarters by 2015 compared to 2005.
It is vital to monitor malaria trends to see if malaria control campaigns are being effective, and to make improvements.
The WHO World Malaria Report 2008 estimates the number of malaria cases and deaths for the period 2001-2006 in affected countries and investigates whether or not WHO recommendations are being implemented. It evaluates progress made against the disease it also describes the sources of funding and reviews the impact of malaria control programmes. The aim of the report is to support the development of effective national malaria control programmes.
- 2. Which strategies were adopted to prevent and treat malaria?
- 3. How many people were affected by malaria in 2006?
- 4. What is being done to prevent and treat malaria?
- 5. How much funding is allocated to malaria control?
- 6. How effective is malaria control?
- 7. Can malaria be completely eradicated?
- Accidental poisoning
- Acrylamide in food
- Acupuncture
- Agriculture
- Aids Epidemic
- Air Pollution Europe
- Air quality in Europe
- Allergenic fragrances
- Aluminium exposure
- Animal testing
- Antibiotic resistance
- Antibiotics Research
- Antimicrobial resistance
- Aquatic environment
- Arctic Climate Change
- Artificial Light
- Artificial Light and Health
- Aspartame Reevaluation
- Aspirin & Cancer
- Benzodiazepines
- Biodiversity
- Biological Diversity
- Biosecurity
- Bisphenol A
- CO 2 Capture & Storage
- Cancer rates and mortality, types and causes
- Chemical Mixtures
- Children & Screens
- Chlorine Sodium Hypochlorite
- Chlorpyrifos pesticide
- Chronic Diseases on Labour Practices
- Circular Economy
- Climate Change
- Climate Change Mitigation
- Climate impact of shale gas
- Climate impacts adaptation
- Dental Amalgams
- Dental Fillings
- Desertification
- Diet & Nutrition
- Ecosystem Change
- Effects of cannabis
- Electromagnetic Fields
- Electronic Cigarettes
- Endocrine Disruptors
- Endocrine disrupting properties of pesticides
- Endocrine disruptors risks
- Energy Saving Lamps
- Energy Technologies
- Epidemic diseases
- Estrogen-progestogen cancer risk
- Europe Green Deal
- Evaluation of endocrine disruptors
- Exposure to chemical mixtures
- Fisheries and aquaculture
- Fluorinated gases
- Food & Agriculture
- Food Wastage
- Forests & Energy
- Forests & agriculture land use
- Fukushima Consequences
- Fukushima accident
- Genetically Modified Crops
- Geothermal Energy
- Global Biodiversity Outlook 4
- Global Public Health Threats
- Global Warming
- Gluten intolerance
- Glyphosate and cancer
- Hazardous chemicals
- Health Effects of Electromagnetic Fields
- Health Environment Management
- Illicit drugs in Europe
- Impacts of a 4°C global warming
- India Millennium Development Goals
- Indonesian forests
- Indoor Air Quality
- Land Degradation and Desertification
- Lyme Disease
- Marine Litter
- Marine litter
- Mercury from dental amalgam
- Mercury in CFL
- Metal-on-Metal hip implants
- Methylene glycol
- Mineral extraction risks
- Multiple vaccinations
- Nano-silica
- Nanomaterials
- Nanotechnologies
- Neonicotinoids
- Nitrogen Dioxide
- Non-human primates
- Organic Food
- Ozone layer depletion
- Parabens used in cosmetics
- Particulate Matter
- Perfluorooctanoic acid (PFOA)
- Personal Music Players & Hearing
- Pesticides occupational risks
- Pharmaceuticals environment
- Phosphate resources
- Phthalates Comparison
- Phthalates in school supplies
- Poly brominated flame retardant decaBDE
- Power lines
- Psychoactive Drugs
- Radiological nuclear emergency
- Respiratory Diseases
- Safety of Cosmetics
- Safety of sunscreens
- Sand Extraction
- Security Scanners
- Silver Nanoparticles
- Single-use plastics
- Soils degradation
- Solar Energy
- State of the European Environment
- Static Fields
- Substitution of harmful chemicals
- Sulfaxoflor Pesticide
- Sunbeds & UV radiation
- Sustainable oceans
- Synthetic Biology
- Thorium nuclear fuel
- Tidal Energy
- Titanium dioxide nanoparticles
- Tooth Whiteners
- Transgenic salmon
- Tuberculosis
- Wastewater management
- Water Disinfectants
- Water Resources
- Water Resources Assessments
- Water resources
- Wind Resources
- X-Ray Full-Body Scanners

Get involved!
This summary is free and ad-free, as is all of our content. You can help us remain free and independant as well as to develop new ways to communicate science by becoming a Patron!
- Terms & Conditions

Graduate College
Postdoc examines impact of fungal priority pathogen cryptococcus.
The World Health Organization lists Cryptococcus as a fungal priority pathogen to guide research, development, and public health action.

In extreme cases, Cryptococcus is breathed into the lungs before traveling to the spinal cord and brain, causing c ryptococcal meningitis -- an infection that is fatal if untreated.
Andrew Jezewski, a postdoctoral fellow in the Krysan Lab in the Stead Family Department of Pediatrics at the University of Iowa, studies how Cryptococcus grows in the human host. Specifically, Jezewski investigates how the fungus grows in the brain and how it tolerates carbon dioxide. Jezewski estimates that carbon dioxide levels are 100-fold higher in the human body than in the environment.
“Most strains of Cryptococcus don’t grow well in the human body,” Jezewski says. “If that strain gets in your lungs, chances are it won’t survive. It’s a stress on Cryptococcus to encounter substantial amounts of carbon dioxide. But a few strains can resist that stress from carbon dioxide. I am trying figure out how those strains can tolerate that carbon dioxide stress because those are the strains that cause severe disease.”
Cryptococcus isn’t passed from human to human and almost never causes disease in immunocompetent individuals. This alleviates concern when Jezewski and his advisor, Damian Krysan, a professor of pediatric-infectious diseases, work with Cryptococcus that has been isolated from an individual patient.
People with cryptococcal meningitis can experience seizures and may require cerebral spinal fluid drainage. They are treated intravenously with the antifungal medication amphotericin B . Jezewski says there around 200,000 cases of cryptococcal meningitis worldwide, with 150,000 resulting in death.
Since the World Health Organization lists cryptococcus as a fungal priority pathogen, resources are being increased in resource-impoverished areas, so amphotericin B can be more widely prescribed to patients. Researchers like Krysan and Jezewski are also working to identify novel therapeutic strategies.
Grant writing perfection
Jezewski received a rare perfect score on his National Institutes of Health K22 grant application, which will support his research as a faculty member.
“ I circulated that grant with a lot of people who had won K awards before and got helpful feedback,” Jezewski says. “I took all the feedback to heart to try and improve my grant. My grant received a perfect score in the first round, so I didn’t have to do a resubmission.”
Jezewski plans to take his K22 award, worth $250,000 over two years, with him to his faculty position.
“This shows, as proof of concept, I am competitive in writing grants. When I apply for positions, which are dependent on writing grants, I can at least show I have a record of that,” Jezewski says. “You can take that money to help get your research off the ground in combination with any start-up package you get. When you get offered a start-up package from an institution, (the employer) must show the NIH that the (new hire) is receiving the same level of support that they would give a faculty member who is not receiving a grant.”
Krysan believes that new faculty members doing research must learn to write and communicate their ideas effectively.
“Andrew has expansive curiosity and creativity. However, one must be able to convince others that what you are interested in and how you will study it are significant and feasible,” Krysan says. “He has written grant proposals at multiple times during his training, culminating with a perfect score on his NIH K22 application; the evolution of his writing and his ability to express his creativity and insights have been gratifying to watch and read.”
In the upcoming academic year, Jezewski will be joining the Department of Genetics and Biochemistry at Clemson University as an assistant professor affiliated with the nationally renowned Eukaryotic Pathogens Innovation Center (EPIC). His research will focus on Cryptococcus, but he also has prior experience working with other eukaryotic pathogens, including Plasmodium, the causative agent of malaria, and the brain parasite Toxoplasma.
“I am excited to bring my expertise to this new role and collaborate with a distinguished group of faculty," Jezewski says. "Together, we aim to advance our understanding of neglected and complex pathogens, ultimately working toward novel therapeutic strategies.”

IMAGES
VIDEO
COMMENTS
Abstract and Figures. SUPERVISOR BY: Dr. HAMZE ALI ABDILLAHI. .5 your occupations. 13 IS malaria a common disease in your community. 19 shows the 52% answer to yes ,33.8% n0 and 13.8%l don't know ...
ii Dissertation Abstract Background: Recently, malaria has become a major global health priority.As a result there has been renewed interest in malaria control, elimination, and eradication. Zambia is one of the Elimination 8 countries and one of the President's Malaria Initiative focus
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
First, I focus on the ecology that serves as a backdrop to transmission, and focus on the role agriculture may play. In doing so, I attempt to understand how agriculture affects both mosquito behavior, as well as malaria risk in under-5 children in the Democratic Republic of Congo (DRC), a country with one of the world's highest malaria burdens.
Mr AB Mapossa. PhD, Chemical Engineering. Thesis. Slow-release of mosquito repellents from microporous polyolefin strands. Prof WW Focke. Mr M Mpofu. PhD, Environmental Health. Thesis. Effectiveness of community larval source management (LSM) as an additional vector control intervention for malaria elimination.
ABSTRACT In this study, a novel deterministic mathematical model for the transmis- sion and control of malaria is formulated. The main innovation in the model is that, in addition to the natural death rate of the vector (mosquito) population, a proportion of the prevention effort also contributes to a reduction of the vector population. The ...
"Like chess, (malaria) is played with a few pieces, but is capable of an infinite variety of situations" (Hackett 1937).Four pieces comprise the malaria system: the vector, the parasite, the human, and the environment (Table 1).The triad human-vector-parasite exists within (and interacts with) the environment, resulting in a variety of unique local patterns of malaria transmission that ...
HEL-3950 Master's thesis in Public Health August 2016 Supervisor: Ranjan Parajuli, PhD . iii ... Malaria is Primarily a vector-borne disease caused by the protozoan Plasmodium. Protozoa Plasmodium is unicellular eukaryote parasites. The parasite is then transmitted to people through the bites of an infected Anopheles mosquitos, called ...
Malaria is unique among diseases because its roots lie so deep within human communities. The most dangerous vectors of malaria thrive mainly within the village environment. Logically, the adult vectors remain close to their noctur-nal source of human blood, and the developmental stages of these mosquitoes
The Relationship Between Malaria Status in Under-five Children and Some Household Demographic, Socioeconomic and Environmental Factors Associated with the Disease in Sierra ... Authors Statement Page . In presenting this thesis as a partial fulfillment of the requirements for an advanced degree
The outlook for malaria control is grim. The disease, caused by mosquito-borne parasites, is present in 102 countries and is responsible for over 100 million clinical cases and 1 to 2 million deaths each year. Over the past two decades, efforts to control malaria have met with less and less success. In many regions where malaria transmission had been almost eliminated, the disease has made a ...
Public health strategies for malaria in endemic countries aim to prevent transmission of the disease and control the vector. This historical analysis considers the strategies for vector control developed during the first four decades of the twentieth century. In 1925, policies and technological advances were debated internationally for the first time after the outbreak of malaria in Europe ...
Background Malawi is a malaria-endemic country and approximately 6 million cases are reported annually. Improving knowledge of malaria causes and symptoms, and the overall perception towards malaria and its preventive measures is vital for malaria control. The current study investigated the levels of knowledge of the causes, symptoms and prevention of malaria among Malawian women. Methods Data ...
Each year there are more than 200 million new cases of malaria, a preventable and treatable disease. According to the World Health Organization's (WHO) World malaria report 2019, there were no global gains in reducing new infections between 2014 and 2018, and nearly as many people died from malaria in 2018 as in the previous year. TDR's malaria research focuses on helping low- and middle ...
assessment of malaria prevention practices and associated factors among households in pigniwudo town, gambella region, south west ethiopia: community- ... based cross-sectional study, 2021 by: temesgen mulu (b.sc.) a research thesis to be submitted to jimma university, institute of health, faculty of public health, ... statement of problem ...
Gametocytes are the sexual stage of the Plasmodia life cycle which render malaria cases infectious to mosquitoes. The proportion of P. falciparum malaria cases with gametocytemia and the duration of gametocytemia are varied. Interventions for detecting and treating gametocytemia also differ from those used against asexual parasitemia.
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period ...
Malaria contributes indirectly to many additional deaths, mainly of young children, through synergy with other infections and illnesses. It is a major cause of anaemia in children and pregnant women and of low birth weight, premature births and infant mortality. In endemic African countries, 25% to 35% of all outpatient visits, 20% to 45% of ...
MALARIA RESEARCH. Contents [ hide] 1 Within Host Diversity of Malaria Infections. 2 Spatial Epidemiology of Malaria. 3 Diagnosis Resistant Malaria. 4 Malaria Drug Resistance. 4.1 Molecular Epidemiology of Drug Resistance. 4.2 Chloroquine Resistance in Plasmodium vivax. 4.3 Impacts of ACT Partner Drugs on Population Structure.
Background Despite continuous prevention and control strategies in place, malaria remains a major public health problem in sub-Saharan Africa including Ethiopia. Moreover, prevalence of malaria differs in different geographical settings and epidemiological data were inadequate to assure disease status in the study area. This study was aimed to determine the prevalence of malaria and associated ...
Among live, term births, the mean birth weight was not significantly different for malaria-positive vs. malaria-negative women. However, among women with under-nutrition, as measured by low body-mass-index, malaria exposure was associated with significantly decreased birth weight (mean difference -370 grams, 95% CI -728, -12 g).
The committee recommends decisions on funding of malaria research be based on scientific merit as determined by rigorous peer review, consistent with the guidelines of the National Institutes of Health or the United Nations Development Program/World Bank/ World Health Organization Special Programme for Research and Training in Tropical Diseases ...
An Essay on Malaria and Its Consequences - PMC. Journal List. Glasgow Med J. v.45 (2); 1896 Feb. PMC5950432. As a library, NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health.
1. Introduction. Malaria is one of the most common infectious diseases and a great public health problem worldwide, particularly in Africa and south Asia. About three billion people are at risk of infection in 109 countries. Each year, there are an estimated 250 million cases of malaria leading to approximately one million deaths, mostly in ...
World Health Organization. This position paper supersedes the 2022 WHO position paper on malaria vaccines.6 It includes the updated WHO recommendations on the use of the RTS,S/AS01 and R21/Matrix-M vaccines for the reduction of malaria morbidity and mortality in children living in endemic areas, prioritizing areas of moderate and high malaria ...
Written by. John Riehl. The World Health Organization lists Cryptococcus as a fungal priority pathogen to guide research, development, and public health action. In extreme cases, Cryptococcus is breathed into the lungs before traveling to the spinal cord and brain, causing c ryptococcal meningitis -- an infection that is fatal if untreated.