Recent advances in lung cancer research: unravelling the future of treatment
- Review Article
- Published: 06 April 2024

Cite this article
- Luca Bertolaccini ORCID: orcid.org/0000-0002-1153-3334 1 ,
- Monica Casiraghi 1 , 2 ,
- Clarissa Uslenghi 1 ,
- Sebastiano Maiorca 1 &
- Lorenzo Spaggiari 1 , 2
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine, enabling targeted therapies that minimise harm to healthy tissues while effectively combating cancer cells. The relationship between pulmonary tuberculosis and lung cancer is examined, shedding light on potential mechanisms linking these two conditions. Early detection methods, notably low-dose computed tomography scans, have significantly improved patient outcomes, emphasising the importance of timely interventions. There has been a growing interest in segmentectomy as a surgical intervention for early-stage lung cancer in recent years. Immunotherapy has emerged as a transformative approach, harnessing the body's immune system to recognise and eliminate cancer cells. Combining immunotherapy with traditional treatments, such as chemotherapy and targeted therapies, has shown enhanced efficacy, addressing the disease's heterogeneity and overcoming drug resistance. Precision medicine, guided by genomic profiling, has enabled the development of targeted therapies like tyrosine kinase inhibitors, offering personalised treatments tailored to individual patients. Challenges such as drug resistance and limited accessibility to advanced therapies persist, emphasising the need for collaborative efforts and innovative technologies like artificial intelligence. Despite challenges, ongoing interdisciplinary collaborations and technological advancements offer hope for a future where lung cancer is treatable and preventable, reducing the burden on patients and healthcare systems worldwide.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
Not applicable.
Cheng B, Xiong S, Li C, Liang H, Zhao Y, Li J et al (2020) An annual review of the remarkable advances in lung cancer clinical research in 2019. J Thorac Dis 12(3):1056–1069
Article PubMed PubMed Central Google Scholar
Ibodeng GO, Uche IN, Mokua R, Galo M, Odigwe B, Galeas JN, Dasgupta S (2023) A snapshot of lung cancer: where are we now?-a narrative review. Ann Transl Med 11(6):261
Article CAS PubMed PubMed Central Google Scholar
Bertolaccini L, Casiraghi M, Petrella F, Rampinelli C, Tessitore A, Spaggiari L (2022) A methodological quality evaluation of the published guidelines and recommendations about the lung cancer screening. Eur J Cancer Prev 31(1):19–25
Article PubMed Google Scholar
Duma N, Santana-Davila R, Molina JR (2019) Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 94(8):1623–1640
Article CAS PubMed Google Scholar
Hwang SY, Kim JY, Lee HS, Lee S, Kim D, Kim S et al (2022) Pulmonary tuberculosis and risk of lung cancer: a systematic review and meta-analysis. J Clin Med 11(3):765
Yaegashi LB, Baldavira CM, Prieto TG, Machado-Rugolo J, Velosa APP, da Silveira LKR et al (2021) In situ overexpression of matricellular mechanical proteins demands functional immune signature and mitigates non-small cell lung cancer progression. Front Immunol 12:714230
Bourgot I, Primac I, Louis T, Noel A, Maquoi E (2020) Reciprocal interplay between fibrillar collagens and collagen-binding integrins: implications in cancer progression and metastasis. Front Oncol 10:1488
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA et al (2011) Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 171(1):1–5
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S et al (2017) Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376(22):2109–2121
Oliver AL (2022) Lung cancer: epidemiology and screening. Surg Clin North Am 102(3):335–344
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547(7662):222–226
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Franzi S, Mattioni G, Rijavec E, Croci GA, Tosi D (2022) Neoadjuvant chemo-immunotherapy for locally advanced non-small-cell lung cancer: a review of the literature. J Clin Med 11(9):2629
Szeto CH, Shalata W, Yakobson A, Agbarya A (2021) Neoadjuvant and adjuvant immunotherapy in early-stage non-small-cell lung cancer, past, present, and future. J Clin Med 10(23):5614
Chai Y, Wu X, Bai H, Duan J (2022) Combined immunotherapy with chemotherapy versus bevacizumab with chemotherapy in first-line treatment of driver-gene-negative non-squamous non-small cell lung cancer: an updated systematic review and network meta-analysis. J Clin Med 11(6):1655
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohe C et al (2023) Overall Survival with Osimertinib in Resected EGFR-mutated NSCLC. N Engl J Med 389(2):137–147
Dohopolski M, Iyengar P (2021) Oligometastatic non-small cell lung cancer: a narrative review of stereotactic ablative radiotherapy. Ann Palliat Med 10(5):5944–5953
Yuan Z, Wang Y, Zhang J, Zheng J, Li W (2019) A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol 16(3):302–314
Chen Y, Luo H, Liu R, Tan M, Wang Q, Wu X et al (2023) Efficacy and safety of particle therapy for inoperable stage II–III non-small cell lung cancer: a systematic review and meta-analysis. Radiat Oncol 18(1):86
Harada H, Suefuji H, Mori K, Ishikawa H, Nakamura M, Tokumaru S et al (2023) Proton and carbon ion radiotherapy for operable early-stage lung cancer: 3-year results of a prospective nationwide registry. Int J Radiation Oncol Biol Phys 117(2):23
Article Google Scholar
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA et al (2020) reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382(6):503–513
Huo B, Manos D, Xu Z, Matheson K, Chun S, Fris J et al (2023) Screening criteria evaluation for expansion in pulmonary neoplasias (SCREEN). Semin Thorac Cardiovasc Surg 35(4):769–780
Passiglia F, Cinquini M, Bertolaccini L, Del Re M, Facchinetti F, Ferrara R et al (2021) Benefits and harms of lung cancer screening by chest computed tomography: a systematic review and meta-analysis. J Clin Oncol 39(23):2574–2585
Qi SA, Wu Q, Chen Z, Zhang W, Zhou Y, Mao K et al (2021) High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci Rep 11(1):11805
Madama D, Martins R, Pires AS, Botelho MF, Alves MG, Abrantes AM, Cordeiro CR (2021) Metabolomic profiling in lung cancer: a systematic review. Metabolites 11(9):630
Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F et al (2016) Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 17(5):642–650
Araujo DC, Veloso AA, Borges KBG, Carvalho MDG (2022) Prognosing the risk of COVID-19 death through a machine learning-based routine blood panel: a retrospective study in Brazil. Int J Med Inform 165:104835
Chiu HY, Chao HS, Chen YM (2022) Application of artificial intelligence in lung cancer. Cancers (Basel) 14(6):1370
Christie JR, Lang P, Zelko LM, Palma DA, Abdelrazek M, Mattonen SA (2021) Artificial intelligence in lung cancer: bridging the gap between computational power and clinical decision-making. Can Assoc Radiol J 72(1):86–97
Goncalves S, Fong PC, Blokhina M (2022) Artificial intelligence for early diagnosis of lung cancer through incidental nodule detection in low- and middle-income countries-acceleration during the COVID-19 pandemic but here to stay. Am J Cancer Res 12(1):1–16
CAS PubMed PubMed Central Google Scholar
Goldsmith I, Chesterfield-Thomas G, Toghill H (2021) Pre-treatment optimization with pulmonary rehabilitation in lung cancer: making the inoperable patients operable. EClinicalMedicine 31:100663
Shields MD, Chen K, Dutcher G, Patel I, Pellini B (2022) Making the rounds: exploring the role of circulating tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci 23(16):9006
Abbosh C, Frankell AM, Harrison T, Kisistok J, Garnett A, Johnson L et al (2023) Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616(7957):553–562
Zaman FY, Subramaniam A, Afroz A, Samoon Z, Gough D, Arulananda S, Alamgeer M (2023) Circulating tumour DNA (ctDNA) as a predictor of clinical outcome in non-small cell lung cancer undergoing targeted therapies: a systematic review and meta-analysis. Cancers (Basel) 15(9):2425
Jaffee EM, Dang CV, Agus DB, Alexander BM, Anderson KC, Ashworth A et al (2017) Future cancer research priorities in the USA: a lancet oncology commission. Lancet Oncol 18(11):e653–e706
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617
Nakada T, Noda Y, Kato D, Shibasaki T, Mori S, Asano H et al (2019) Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 10(10):1945–1952
Bedetti B, Bertolaccini L, Rocco R, Schmidt J, Solli P, Scarci M (2017) Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 9(6):1615–1623
Bertolaccini L, Prisciandaro E, Bardoni C, Cara A, Diotti C, Girelli L, Spaggiari L (2022) Minimally invasive anatomical segmentectomy versus lobectomy in stage IA non-small cell lung cancer: a systematic review and meta-analysis. Cancers (Basel) 14(24):6157
Wang P, Fu YH, Qi HF, He P, Wang HF, Li C, Liu XC (2023) Evaluation of the efficacy and safety of robot-assisted and video assisted thoracic surgery for early non-small cell lung cancer: a meta-analysis. Technol Health Care 32(2):511–523
Casiraghi M, Galetta D, Borri A, Tessitore A, Romano R, Diotti C et al (2019) Ten years’ experience in robotic-assisted thoracic surgery for early stage lung cancer. Thorac Cardiovasc Surg 67(7):564–572
Wang P, Wang S, Liu Z, Sui X, Wang X, Li X et al (2022) Segmentectomy and wedge resection for elderly patients with stage I non-small cell lung cancer: a systematic review and meta-analysis. J Clin Med 11(2):294
Bertolaccini L, Cara A, Chiari M, Diotti C, Glick N, Mohamed S et al (2023) Real-world survival outcomes of wedge resection versus lobectomy for cT1a/b cN0 cM0 non-small cell lung cancer: a single center retrospective analysis. Front Oncol 13:1226429
Bertolaccini L, Spaggiari L (2023) Is it time to cross the pillars of evidence in favor of segmentectomies in early-stage non-small cell lung cancer? Cancers (Basel) 15(7):1993
Zaraca F, Kirschbaum A, Pipitone MD, Bertolaccini L, Group PS (2023) Prospective randomized study on the efficacy of three-dimensional reconstructions of bronchovascular structures on preoperative chest CT scan in patients who are candidates for pulmonary segmentectomy surgery: the patches (prospective randomized study efficacy of three-dimensional reconstructions segmentecomy) study protocol. Trials 24(1):594
Komarnicki P, Musialkiewicz J, Stanska A, Maciejewski A, Gut P, Mastorakos G, Ruchala M (2022) Circulating neuroendocrine tumor biomarkers: past, present and future. J Clin Med 11(19):5542
Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyo D et al (2018) Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 24(10):1559–1567
Biesinger M, Eicken N, Varga A, Weber M, Brndiar M, Erd G et al (2022) Lymph but not blood vessel invasion is independent prognostic in lung cancer patients treated by VATS-lobectomy and might represent a future upstaging factor for early stages. Cancers 14(8):1893
Asamura H, Nishimura KK, Giroux DJ, Chansky K, Hoering A, Rusch V, et al (2023) IASLC Lung Cancer Staging Project The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 18(5): 564–575
Hardenberg MC, Patel B, Matthews C, Califano R, Garcia Campelo R, Grohe C et al (2022) The value of disease-free survival (DFS) and osimertinib in adjuvant non-small-cell lung cancer (NSCLC): an international Delphi consensus report. ESMO Open 7(5):100572
Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M et al (2020) Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383(18):1711–1723
Xu H, Baidoo AAH, Su S, Ye J, Chen C, Xie Y et al (2019) A comparison of EGFR mutation status in tissue and plasma cell-free DNA detected by ADx-ARMS in advanced lung adenocarcinoma patients. Transl Lung Cancer Res 8(2):135–143
Zou PC, Wang L, Liu B, Zhang HZ, Liu HC (2011) EGFR-targeted therapies combined with chemotherapy for treating advanced non-small-cell lung cancer: a meta-analysis. Diagnostics 9:38
Google Scholar
Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F et al (2023) Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 11(4):354–366
Hotta K, Hida T, Nokihara H, Morise M, Kim YH, Azuma K et al (2022) Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naive Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 7(4):100527
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G et al (2020) First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383(21):2018–2029
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Isaacs J, Stinchcombe TE (2022) Neoadjuvant and adjuvant systemic therapy for early-stage non-small-cell lung cancer. Drugs 82(8):855–863
John AO, Ramnath N (2023) Neoadjuvant versus adjuvant systemic therapy for early-stage non-small cell lung cancer: the changing landscape due to immunotherapy. Oncologist 28(9):752–764
Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S et al (2023) Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 389(6):491–503
Kogure Y, Hashimoto H, Oki M (2021) A randomized phase iii study of pembrolizumab versus pembrolizumab-carboplatin-pemetrexed for locally advanced or metastatic nonsquamous non-small-cell lung cancer with PD-L1 50% or more (LAPLACE-50): study protocol. Clin Lung Cancer 11:921–924
Download references
Acknowledgements
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Ministero della Salute, 5 × 1000, Ricerca Corrente.
Author information
Authors and affiliations.
Department of Thoracic Surgery, IEO, European Institute of Oncology IRCCS, Via Ripamonti 435, 20141, Milan, Italy
Luca Bertolaccini, Monica Casiraghi, Clarissa Uslenghi, Sebastiano Maiorca & Lorenzo Spaggiari
Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
Monica Casiraghi & Lorenzo Spaggiari
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Luca Bertolaccini .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Bertolaccini, L., Casiraghi, M., Uslenghi, C. et al. Recent advances in lung cancer research: unravelling the future of treatment. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01841-3
Download citation
Received : 06 March 2024
Accepted : 24 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s13304-024-01841-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
- Comprehensive review
- Find a journal
- Publish with us
- Track your research
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
- Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
- Commenting Guidelines
- Guest Posting Policies
Cancer.Net Podcasts
What to know about peer-reviewed cancer information and its role in cancer research.

Christopher Flowers, MD, MS, FASCO, is Chair of the Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center and was appointed Division Head ad interim of Cancer Medicine in August 2020. He is the 2022 Cancer.Net Associate Editor for Lymphoma . Vicki Keedy, MD, is an Associate Professor of Medicine in the Division of Hematology/Oncology at the Vanderbilt University Medical Center. She is the 2022 Cancer.Net Associate Editor for Sarcoma . Daniel Mulrooney, MD, MS, is an Associate Member in the Division of Cancer Survivorship at St. Jude Children’s Research Hospital. He is the 2022 Cancer.Net Associate Editor for Pediatric Cancers . Sumanta (Monty) Pal, MD, FASCO, is the co-director of City of Hope's Kidney Cancer Program and is the head of the kidney and bladder cancer disease team at the institution. He is the 2022 Cancer.Net Associate Editor for Genitourinary Cancers . Disclosure information for the authors can be found in their individual biographies linked to above.
Doctors and scientists conduct research studies to find better ways to prevent, diagnose, and treat cancer. When searching for cancer information online, you may come across articles about research studies. However, it can sometimes be difficult to tell whether articles you find online are reliable or potential misinformation . One important way to tell if an article you find online is dependable is if it is “peer reviewed.” Here, we break down what to know about peer-reviewed information and the types of peer-reviewed cancer research you may find when searching online.
What are peer-reviewed articles?
For articles published in scientific journals, “peer reviewed" means that the article has undergone a process in which qualified experts have reviewed it, provided feedback, and the authors have responded to that feedback to improve the scientific quality and integrity of the article.
Articles that have not been peer reviewed have not been reviewed by experts in the field for scientific validity or quality. For example, research studies that are published in non-peer-reviewed journals are not heavily scrutinized and have not been reviewed for the quality of the methods performed in the study or for the integrity of the data reported from the study.
One example of a non-peer-reviewed article is called a “pre-print.” Pre-prints are rapid reports of study results that are often found in the online version of a journal that is published before peer review is performed. Pre-prints allow for rapid distribution of important information. However, the final article for the study may change significantly after it goes through peer review. Other non-peer-reviewed sources can include book chapters, blogs, or medical articles in a magazine.
If you come across an article online that you’re not sure has been peer reviewed, bring it up with your health care team. They can point you toward sources of peer-reviewed research and other cancer information that is helpful and dependable. Learn more about evaluating cancer information online.

“‘Peer reviewed’ means that the article has undergone a process in which qualified experts have reviewed it, provided feedback, and the authors have responded to that feedback to improve the scientific quality and integrity of the article.” – Sumanta (Monty) Pal, MD, FASCO, co-director of City of Hope's Kidney Cancer Program, head of the kidney and bladder cancer disease team at the institution, and the 2022 Cancer.Net Associate Editor for Genitourinary Cancers
What is peer-reviewed cancer research, and why is it important?
Peer-reviewed cancer research studies are evaluated and critiqued by an independent group of scientific experts in the same field before that research is performed. The purpose of this review is to ensure the scientific merit of the study by asking if the research question being studied is important and based on sound science. Peer review also ensures that the methods used to answer the research question are safe and appropriate. Often, research proposals must go through a rigorous peer review before they receive funding from research organizations.
However, not all research studies go through a peer review prior to the research being performed, particularly if it is not required for the funding of the project. These often are smaller pilot projects or projects that are privately funded. There is a risk that non-peer-reviewed studies may not use reliable research techniques and may not hold as much scientific value, because they do not undergo a formal critique. It is important when evaluating studies to identify whether they are peer reviewed, as peer review is crucial to ensuring scientific integrity. If you are unsure whether a study you have found is peer reviewed, your health care team can help you answer this question.

“Peer review also ensures that the methods used to answer the research question are safe and appropriate. Often, research proposals must go through a rigorous peer review before they receive funding from research organizations.” – Vicki Keedy, MD, Associate Professor of Medicine in the Division of Hematology/Oncology at the Vanderbilt University Medical Center and the 2022 Cancer.Net Associate Editor for Sarcoma
What does "levels of evidence" mean in cancer research?
Cancer research studies are assigned a level of evidence based on how strong the evidence is in a particular study. It is simplest to think about levels of evidence in cancer research like a school report card, with “A” being the highest level of evidence. When the level of evidence is “A,” that means we have the best information to provide clarity about what the right way is to diagnose or treat a given type of cancer.
The other important feature to understand when considering levels of evidence is the experimental design of the study. The best type of experimental design for testing whether one diagnostic or treatment option is better than another is a called a randomized controlled trial. In a randomized controlled trial, participants in the trial are randomly assigned to receive a particular intervention, such as Treatment 1 versus Treatment 2, based on the belief that we do not know which treatment is more likely to benefit patients in general. High-quality randomized controlled trials provide an A-grade level of evidence.
When multiple randomized controlled trials have been done to answer the same research question, then researchers may perform something called a “systematic review” of these trials to combine and provide a summary of all the evidence from the multiple trials. The summary from a systematic review highlights the best available research on a specific topic and can be a way to improve grade A evidence.

“It is simplest to think about levels of evidence in cancer research like a school report card, with “A” being the highest level of evidence. When the level of evidence is “A,” that means we have the best information to provide clarity about what the right way is to diagnose or treat a given type of cancer.” – Christopher Flowers, MD, MS, FASCO, Chair of the Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center and the 2022 Cancer.Net Associate Editor for Lymphoma
Other forms of studies have lower levels of evidence, including cohort studies, which are when researchers study the event as it occurs, and case-control studies, which is when we know what outcome has occurred for 2 different groups of people and compare the results. Both types of studies would be considered different forms of “grade B” evidence. When we do not have data to provide information on a particular research question, we rely on expert opinion, which would also be a lower level of evidence.
What are the differences between research studies in cell lines, animals, and humans?
Cancer research can be performed on several different sources, including cell lines, animals, or humans. The choice often depends on the research question being asked and available resources.
In cell line studies, the cells can be isolated from animals or humans and preserved or grown in culture dishes. These cells may come from different tissue, such as the bone, brain, or muscle of animals or humans. Laboratories can change these cells to grow indefinitely, which are called “immortalized cell lines.” Immortalized cells provide a long-lasting resource for future studies. Cells can also be changed into induced pluripotent stem cells (iPSCs), which are important to research because under the correct conditions, they can grow into nearly any cell type.
Animals can also be used as models for studying human disease because there are many biologic and genetic similarities between animals and humans. Mice and rats are the most common animals used in research. However, depending on the disease being studied, different animals may be used, ranging from zebrafish to larger animals. Many animals have a shorter lifespan than humans, which allows diseases like cancer to be studied over an entire lifecycle. Animals are used to test how safe and effective new drugs and medical treatments are, and they can also help identify potential side effects. In fact, new drugs must first be tested in animals before human studies can begin.
While research into new medical treatments often begins in cell lines and animals, studying humans is important to truly understand the effects on the various organ systems in the human body. There is no substitute for the complexity of human behavior and the interactions new treatments can have within our bodies. Studies conducted in humans are watched very closely for safety and may identify new risks and benefits that have not yet been shown in other studies.
How are research studies in humans conducted?
Medical treatments studied in humans, including those for cancer, are called “clinical trials” and are conducted in 4 phases . A phase I clinical trial is designed to test the safety of a new drug or procedure and identify potential side effects. A phase II clinical trial is focused on determining if the drug or procedure is effective. Phase III clinical trials are conducted in large populations and test the treatment compared to other treatments or the standard of care, which is the best treatment currently known. Phase III clinical trials are required for a drug or procedure to be approved by the U.S. Food and Drug Administration (FDA) for regular use. Finally, phase IV clinical trials are conducted after the FDA has approved a new treatment and are used to monitor that treatment over a longer period of time and in diverse populations. Learn more about patient safety in clinical trials.

“While research into new medical treatments often begins in cell lines and animals, studying humans is important to truly understand the effects on the various organ systems in the human body. There is no substitute for the complexity of human behavior and the interactions new treatments can have within our bodies.” – Daniel Mulrooney, MD, MS, Associate Member in the Division of Cancer Survivorship at St. Jude Children’s Research Hospital and the 2022 Cancer.Net Associate Editor for Pediatric Cancers
Research studies are an important way for doctors to identify new ways to care for and treat people with cancer, and peer review is an important tool for ensuring that research is safe and reliable. Talk with your doctor if you have any questions about finding trustworthy cancer research studies or other cancer information online.
Category:
- Cancer Research
Tags:
- expert information
- clinical trials
Related Resources:
- Understanding Cancer Research Study Design and How to Evaluate Results
- Understanding the Publication and Format of Cancer Research Studies
- What to Know When Searching for Cancer Information Online: An Expert Perspective
- About Cancer Clinical Trials
Share your thoughts on this blog post on Cancer.Net's Facebook and Twitter .
Archives by Month:
- communication
- decision making
- health care team
- patient perspective
- survivorship
- View all episodes
- Listen on Apple Podcasts
- Listen on Spotify
Policies and Guidelines:
- Cancer.Net Terms and Conditions
Contact Us:
Email: [email protected]
ASCO's toll-free patient information line: 571-483-1780 or 888-651-3038
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
- Search by keyword
- Search by citation
Page 1 of 75
HSPA4 upregulation induces immune evasion via ALKBH5/CD58 axis in gastric cancer
Gastric cancer (GC) is one of the leading causes of cancer-related death worldwide. Recently, targeted therapies including PD1 (programmed cell death 1) antibodies have been used in advanced GC patients. Howev...
- View Full Text
GPR65 sensing tumor-derived lactate induces HMGB1 release from TAM via the cAMP/PKA/CREB pathway to promote glioma progression
Lactate has emerged as a critical regulator within the tumor microenvironment, including glioma. However, the precise mechanisms underlying how lactate influences the communication between tumor cells and tumo...
RBM10 C761Y mutation induced oncogenic ASPM isoforms and regulated β-catenin signaling in cholangiocarcinoma
Cholangiocarcinoma (CCA) comprises a heterogeneous group of biliary tract cancer. Our previous CCA mutation pattern study focused on genes in the post-transcription modification process, among which the altern...
Mesothelin promotes brain metastasis of non-small cell lung cancer by activating MET
Brain metastasis (BM) is common among cases of advanced non-small cell lung cancer (NSCLC) and is the leading cause of death for these patients. Mesothelin (MSLN), a tumor-associated antigen expressed in many ...
Oncolytic adenovirus encoding apolipoprotein A1 suppresses metastasis of triple-negative breast cancer in mice
Dysregulation of cholesterol metabolism is associated with the metastasis of triple-negative breast cancer (TNBC). Apolipoprotein A1 (ApoA1) is widely recognized for its pivotal role in regulating cholesterol ...
Investigating the impact of regulatory B cells and regulatory B cell-related genes on bladder cancer progression and immunotherapeutic sensitivity
Regulatory B cells (Bregs), a specialized subset of B cells that modulate immune responses and maintain immune tolerance in malignant tumors, have not been extensively investigated in the context of bladder ca...
A phase I open-label, dose-escalation study of NUC-3373, a targeted thymidylate synthase inhibitor, in patients with advanced cancer (NuTide:301)
5-fluorouracil (5-FU) is inefficiently converted to the active anti-cancer metabolite, fluorodeoxyuridine-monophosphate (FUDR-MP), is associated with dose-limiting toxicities and challenging administration sch...
Transcriptional regulation of cancer stem cell: regulatory factors elucidation and cancer treatment strategies
Cancer stem cells (CSCs) were first discovered in the 1990s, revealing the mysteries of cancer origin, migration, recurrence and drug-resistance from a new perspective. The expression of pluripotent genes and ...
Tumor suppressor role of the complement inhibitor CSMD1 and its role in TNF-induced neuroinflammation in gliomas
The complement inhibitor CSMD1 acts as a tumor suppressor in various types of solid cancers. Despite its high level of expression in the brain, its function in gliomas, malignant brain tumors originating from ...
A high-content screen of FDA approved drugs to enhance CAR T cell function: ingenol-3-angelate improves B7-H3-CAR T cell activity by upregulating B7-H3 on the target cell surface via PKCα activation
CAR T cell therapy is a promising approach to improve outcomes and decrease toxicities for patients with cancer. While extraordinary success has been achieved using CAR T cells to treat patients with CD19-posi...
Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation
Lung cancer stands as the most prevalent form of cancer globally, posing a significant threat to human well-being. Due to the lack of effective and accurate early diagnostic methods, many patients are diagnose...
Identification of genetic modifiers enhancing B7-H3-targeting CAR T cell therapy against glioblastoma through large-scale CRISPRi screening
Glioblastoma multiforme (GBM) is a highly aggressive brain tumor with a poor prognosis. Current treatment options are limited and often ineffective. CAR T cell therapy has shown success in treating hematologic...
Analysis of the effect of CCR7 on the microenvironment of mouse oral squamous cell carcinoma by single-cell RNA sequencing technology
Studies have shown that CCR7, an important inflammatory factor, can promote the proliferation and metastasis of oral squamous cell carcinoma (OSCC), but its role in the tumor microenvironment (TME) remains unc...
Correction: A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle
The original article was published in Journal of Experimental & Clinical Cancer Research 2024 43 :74
Bispecific aptamer-decorated and light-triggered nanoparticles targeting tumor and stromal cells in breast cancer derived organoids: implications for precision phototherapies
Based on the established role of cancer-stroma cross-talk in tumor growth, progression and chemoresistance, targeting interactions between tumor cells and their stroma provides new therapeutic approaches. Dual...
PELI1: key players in the oncogenic characteristics of pancreatic Cancer
Pancreatic cancer (PC) is a highly malignant gastrointestinal tumor, which is characterized by difficulties in early diagnosis, early metastasis, limited therapeutic response and a grim prognosis. Therefore, i...
Ropivacaine as a novel AKT1 specific inhibitor regulates the stemness of breast cancer
Ropivacaine, a local anesthetic, exhibits anti-tumor effects in various cancer types. However, its specific functions and the molecular mechanisms involved in breast cancer cell stemness remain elusive.
CDKL1 potentiates the antitumor efficacy of radioimmunotherapy by binding to transcription factor YBX1 and blocking PD-L1 expression in lung cancer
The evasion of the immune response by tumor cells through programmed death-ligand 1 (PD-L1) has been identified as a factor contributing to resistance to radioimmunotherapy in lung cancer patients. However, th...
Auranofin repurposing for lung and pancreatic cancer: low CA12 expression as a marker of sensitivity in patient-derived organoids, with potentiated efficacy by AKT inhibition
This study explores the repurposing of Auranofin (AF), an anti-rheumatic drug, for treating non-small cell lung cancer (NSCLC) adenocarcinoma and pancreatic ductal adenocarcinoma (PDAC). Drug repurposing in on...
Cross-reactive CD8 + T cell responses to tumor-associated antigens (TAAs) and homologous microbiota-derived antigens (MoAs)
We have recently shown extensive sequence and conformational homology between tumor-associated antigens (TAAs) and antigens derived from microorganisms (MoAs). The present study aimed to assess the breadth of ...
Unveiling CXCR2 as a promising therapeutic target in renal cell carcinoma: exploring the immunotherapeutic paradigm shift through its inhibition by RCT001
In clear cell renal cell carcinoma (ccRCC), first-line treatment combines nivolumab (anti-PD-1) and ipilimumab (anti-CTLA4), yielding long-term remissions but with only a 40% success rate. Our study explored t...
Anaplastic thyroid cancer spheroids as preclinical models to test therapeutics
Anaplastic thyroid cancer (ATC) is the most aggressive thyroid cancer. Despite advances in tissue culture techniques, a robust model for ATC spheroid culture is yet to be developed. In this study, we created a...
The localization, origin, and impact of platelets in the tumor microenvironment are tumor type-dependent
How platelets interact with and influence the tumor microenvironment (TME) remains poorly characterized.
Gli1-mediated tumor cell-derived bFGF promotes tumor angiogenesis and pericyte coverage in non-small cell lung cancer
Tumor angiogenesis inhibitors have been applied for non-small cell lung cancer (NSCLC) therapy. However, the drug resistance hinders their further development. Intercellular crosstalk between lung cancer cells...
Blood-based biomarkers in patients with non-small cell lung cancer treated with immune checkpoint blockade
The paradigm of non-small cell lung cancer (NSCLC) treatment has been profoundly influenced by the development of immune checkpoint inhibitors (ICI), but the range of clinical responses observed among patients...
Validation of a multiomic model of plasma extracellular vesicle PD-L1 and radiomics for prediction of response to immunotherapy in NSCLC
Immune-checkpoint inhibitors (ICIs) have showed unprecedent efficacy in the treatment of patients with advanced non-small cell lung cancer (NSCLC). However, not all patients manifest clinical benefit due to th...
Correction: RNA-binding protein RPS7 promotes hepatocellular carcinoma progression via LOXL2-dependent activation of ITGB1/FAK/SRC signaling
The original article was published in Journal of Experimental & Clinical Cancer Research 2024 43 :45
C/EBPα-p30 confers AML cell susceptibility to the terminal unfolded protein response and resistance to Venetoclax by activating DDIT3 transcription
Acute myeloid leukemia (AML) with biallelic ( CEBPA bi ) as well as single mutations located in the bZIP region is associated with a favorable prognosis, but the underlying mechanisms are still unclear. Here, we pro...
Correction: The epigenetic downregulation of LncGHRLOS mediated by RNA m6A methylase ZCCHC4 promotes colorectal cancer tumorigenesis
The original article was published in Journal of Experimental & Clinical Cancer Research 2024 43 :44
Functionally-instructed modifiers of response to ATR inhibition in experimental glioma
The DNA damage response (DDR) is a physiological network preventing malignant transformation, e.g. by halting cell cycle progression upon DNA damage detection and promoting DNA repair. Glioblastoma are incurab...
Combined IL6 and CCR2 blockade potentiates antitumor activity of NK cells in HPV-negative head and neck cancer
While T cell-activating immunotherapies against recurrent head and neck squamous cell carcinoma (HNSCC) have shown impressive results in clinical trials, they are often ineffective in the majority of patients....
TRF2 as novel marker of tumor response to taxane-based therapy: from mechanistic insight to clinical implication
Breast Cancer (BC) can be classified, due to its heterogeneity, into multiple subtypes that differ for prognosis and clinical management. Notably, triple negative breast cancer (TNBC) – the most aggressive BC ...
A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle
Glutamine metabolism plays a pivotal role in cancer progression, immune cell function, and the modulation of the tumor microenvironment. Dysregulated glutamine metabolism has been implicated in cancer developm...
The Correction to this article has been published in Journal of Experimental & Clinical Cancer Research 2024 43 :93
TGF-β-activated circRYK drives glioblastoma progression by increasing VLDLR mRNA expression and stability in a ceRNA- and RBP-dependent manner
The TGF-β signalling pathway is intricately associated with the progression of glioblastoma (GBM). The objective of this study was to examine the role of circRNAs in the TGF-β signalling pathway.
S100A9 + CD14 + monocytes contribute to anti-PD-1 immunotherapy resistance in advanced hepatocellular carcinoma by attenuating T cell-mediated antitumor function
The paucity of reliable biomarkers for predicting immunotherapy efficacy in patients with advanced hepatocellular carcinoma (HCC) has emerged as a burgeoning concern with the expanding use of immunotherapy. Th...
Biological mechanisms and clinical significance of endoplasmic reticulum oxidoreductase 1 alpha (ERO1α) in human cancer
A firm link between endoplasmic reticulum (ER) stress and tumors has been wildly reported. Endoplasmic reticulum oxidoreductase 1 alpha (ERO1α), an ER-resident thiol oxidoreductase, is confirmed to be highly u...
Inhibition of MER proto-oncogene tyrosine kinase by an antisense oligonucleotide enhances treatment efficacy of immunoradiotherapy
The combination of radiotherapy and immunotherapy (immunoradiotherapy) has been increasingly used for treating a wide range of cancers. However, some tumors are resistant to immunoradiotherapy. We have previou...

KLF7 regulates super-enhancer-driven IGF2BP2 overexpression to promote the progression of head and neck squamous cell carcinoma
Head and neck squamous carcinoma (HNSCC) is known for its high aggressiveness and susceptibility to cervical lymph node metastasis, which greatly contributes to its poor prognosis. During tumorigenesis, many t...
MUC20 regulated by extrachromosomal circular DNA attenuates proteasome inhibitor resistance of multiple myeloma by modulating cuproptosis
Proteasome inhibitors (PIs) are one of the most important classes of drugs for the treatment of multiple myeloma (MM). However, almost all patients with MM develop PI resistance, resulting in therapeutic failu...
Single-cell deconvolution algorithms analysis unveils autocrine IL11-mediated resistance to docetaxel in prostate cancer via activation of the JAK1/STAT4 pathway
Docetaxel resistance represents a significant obstacle in the treatment of prostate cancer. The intricate interplay between cytokine signalling pathways and transcriptional control mechanisms in cancer cells c...


GMP-manufactured CRISPR/Cas9 technology as an advantageous tool to support cancer immunotherapy
CRISPR/Cas9 system to treat human-related diseases has achieved significant results and, even if its potential application in cancer research is improving, the application of this approach in clinical practice...
Tight junction protein cingulin variant is associated with cancer susceptibility by overexpressed IQGAP1 and Rac1-dependent epithelial-mesenchymal transition
Cingulin (CGN) is a pivotal cytoskeletal adaptor protein located at tight junctions. This study investigates the link between CGN mutation and increased cancer susceptibility through genetic and mechanistic analy...
JAK/STAT3 represents a therapeutic target for colorectal cancer patients with stromal-rich tumors
Colorectal cancer (CRC) is a heterogenous malignancy underpinned by dysregulation of cellular signaling pathways. Previous literature has implicated aberrant JAK/STAT3 signal transduction in the development an...
IL6-STAT3-C/EBPβ-IL6 positive feedback loop in tumor-associated macrophages promotes the EMT and metastasis of lung adenocarcinoma
Lung cancer is one of the most common tumors in the world, and metastasis is one of the major causes of tumor-related death in lung cancer patients. Tumor-associated macrophages (TAMs) are a major component of...

ThermomiR-377-3p-induced suppression of Cirbp expression is required for effective elimination of cancer cells and cancer stem-like cells by hyperthermia
In recent years, the development of adjunctive therapeutic hyperthermia for cancer therapy has received considerable attention. However, the mechanisms underlying hyperthermia resistance are still poorly under...
Organoids as a biomarker for personalized treatment in metastatic colorectal cancer: drug screen optimization and correlation with patient response
The inability to predict treatment response of colorectal cancer patients results in unnecessary toxicity, decreased efficacy and survival. Response testing on patient-derived organoids (PDOs) is a promising b...
Targeting HDAC6 improves anti-CD47 immunotherapy
Cancer cells can overexpress CD47, an innate immune checkpoint that prevents phagocytosis upon interaction with signal regulatory protein alpha (SIRPα) expressed in macrophages and other myeloid cells. Several...
Tumor-derived exosomal ADAM17 promotes pre-metastatic niche formation by enhancing vascular permeability in colorectal cancer
Hematological metastasis has been recognized as a crucial factor contributing to the high rates of metastasis and mortality observed in colorectal cancer (CRC). Notably, exosomes derived from cancer cells part...
Splicing targeting drugs highlight intron retention as an actionable vulnerability in advanced prostate cancer
Advanced prostate cancer (PC) is characterized by insensitivity to androgen deprivation therapy and chemotherapy, resulting in poor outcome for most patients. Thus, advanced PC urgently needs novel therapeutic...
Essential role of PLD2 in hypoxia-induced stemness and therapy resistance in ovarian tumors
Hypoxia in solid tumors is an important source of chemoresistance that can determine poor patient prognosis. Such chemoresistance relies on the presence of cancer stem cells (CSCs), and hypoxia promotes their ...

Owned by the Association for International Promotion & Study in Tumors (APSIT)

Official journal of the Regina Elena National Cancer Institute , Scientific Director Gennaro Ciliberto, Rome, Italy
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Submit manuscript
Annual Journal Metrics
2022 Citation Impact 11.3 - 2-year Impact Factor 11.5 - 5-year Impact Factor 1.870 - SNIP (Source Normalized Impact per Paper) 2.413 - SJR (SCImago Journal Rank)
2023 Speed 4 days submission to first editorial decision for all manuscripts (Median) 100 days submission to accept (Median)
2023 Usage 3,003,080 downloads 3,022 Altmetric mentions
- More about our metrics
Journal of Experimental & Clinical Cancer Research
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Advertisement
Categories of Articles
« Return to General AACR Journals' Instructions for Authors
Cancer Research welcomes submission of the following article types to be considered for publication in the journal, subject to peer review as noted below for each type. Please see About Cancer Research for information about the journal, and Instructions for Authors for important policies and instructions relevant to all authors submitting to an AACR journal. Where applicable, the journal’s editorial team reserves the right to edit the title, abstract, and any article summary statement to improve the article's clarity, brevity, and quality prior to publication.
Please note: The article size recommendations below, and most formatting requirements for final publication, are not enforced for initial submissions and do not impact the manuscript’s assessment for potential publication. Word counts are exclusive of cover page, abstract, methods section, tables, and references.
Research Articles
Original studies offering broad impact across the fields of basic, preclinical, clinical, prevention, and epidemiologic cancer research. The reports should be well-documented, novel, and significant to the field as a whole.
- 250-word abstract
- 32-word statement of significance
- 5,000 words of text
- 8 tables and/or figures (Please keep the number of panels per figure to an absolute minimum)
- 50 references
Priority Reports
Short, definitive reports of findings of high impact, significance, and timeliness. The format for Priority Reports is the same as for Research Articles with the exception that the Results and Discussion may be combined.
- 2,500 words of text
- 4 tables and/or figures (Please keep the number of panels per figure to an absolute minimum)
- 20 references
Articles that review a timely subject important to cancer researchers. Reviews must be written as concisely as possible. All Reviews, whether invited or not, will be subject to peer review. Non-invited Reviews are subject to publication fees. Reviews not within the length restrictions below will be considered only at the discretion of the Deputy Editor for Reviews.
- Maximum of 200 words abstract
- 5,000-6,000 words of text
- Maximum of 150 references
- Limit of a total of two display items—whether figures or tables—with a 3-5 sentence legend. While figures with multiple panels are possible, please avoid overly complex or overly detailed submissions.
Letters to the Editor
In the spirit of open scientific dialogue, the Editors invite the submission of correspondence that presents considered opinions in response to articles published in the journal. Letters to the Editor will be peer reviewed and, if found to meet the requisite publication criteria (scholarly commentary on a subject of importance and interest to the broad readership), the Letter may be sent to the author(s) of the originally published article and possibly to other interested parties for a response to be published in the same issue of the journal as the Letter. Please note that the journal will not consider Letters to the Editor regarding Cancer Research that were published more than 3 months prior. Correspondence concerning articles that have not been published in Cancer Research will not be considered.
- 400 words of text
- 5 references, the first of which must be the citation for the original article under discussion
- Letters may contain figures or tables only if they show data that refute the conclusions of the originating article. Figures or tables showing unpublished data in support of the conclusions of the originating article will not be considered
- Include a title: "Running title of the original article — Letter"
Resource Reports
Concise descriptions of a new technology, research database, software tool, or internet-based service that represents a useful resource for cancer researchers. A practical application of the resource must be clearly described.
For manuscripts describing a software tool or internet-based service, a strong emphasis is placed on the usability of the resource, and reviewers will be asked to assess this through hands-on testing, including any required installation procedure. The resource must be freely available for non-commercial use, and at least a minimum level of support provided for no less than two years.
- Online First
- Collections
- Online ISSN 1538-7445
- Print ISSN 0008-5472
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
REVIEW article
This article is part of the research topic.
Tumor Cell Mechanosensitivity: Molecular Basis
The Hidden Messengers: Cancer Associated Fibroblasts -Derived Exosomal miRNAs as Key Regulators of Cancer Malignancy Provisionally Accepted

- 1 Department of Bethune First Clinical School of Medicine, First Affiliated Hospital of Jilin University, China
- 2 Department of General Surgery, Second Affiliated Hospital of Jilin University, China
- 3 Department of Otolaryngology Head and Neck Surgery, China-Japan Union Hospital, Jilin University, China
- 4 Department of Clinical Pharmacy, First Affiliated Hospital of Jilin University, China
The final, formatted version of the article will be published soon.
Cancer-associated fibroblasts (CAFs), a class of stromal cells in the tumor microenvironment (TME), play a key role in controlling cancer cell invasion and metastasis, immune evasion, angiogenesis, and resistance to chemotherapy. CAFs mediate their activities by secreting soluble chemicals, releasing exosomes, and altering the extracellular matrix (ECM). Exosomes contain various biomolecules, such as nucleic acids, lipids, and proteins. microRNA (miRNA), a 22-26 nucleotide non-coding RNA, can regulate the cellular transcription processes. Studies have shown that miRNA-loaded exosomes secreted by CAFs engage in various regulatory communication networks with other TME constituents. This study focused on the roles of CAF-derived exosomal miRNAs in generating cancer malignant characteristics, including immune modulation, tumor growth, migration and invasion, epithelial-mesenchymal transition (EMT), and treatment resistance. This study thoroughly examines miRNA's dual regulatory roles in promoting and suppressing cancer. Thus, changes in the CAF-derived exosomal miRNAs can be used as biomarkers for the diagnosis and prognosis of patients, and their specificity can be used to develop newer therapies. This review also discusses the pressing problems that require immediate attention, aiming to inspire researchers to explore more novel avenues in this field.
Keywords: Cancer-associated fibroblasts-derived exosomal miRNA, Cancer malignant characteristics, Dual regulatory functions, diagnosis, prognosis, therapy
Received: 29 Jan 2024; Accepted: 08 Apr 2024.
Copyright: © 2024 Gou, Li, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: MD. Na Yang, First Affiliated Hospital of Jilin University, Department of Clinical Pharmacy, Changchun, China
People also looked at
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Res Policy Syst

Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment
Catherine r. hanna.
1 CRUK Clinical Trials Unit, Institute of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom
Kathleen A. Boyd
2 Health Economics and Health Technology Assessment, Institute of Health and Wellbeing, University of Glasgow, Glasgow, United Kingdom
Robert J. Jones
Associated data.
Additional files included. No primary research data analysed.
Performing cancer research relies on substantial financial investment, and contributions in time and effort from patients. It is therefore important that this research has real life impacts which are properly evaluated. The optimal approach to cancer research impact evaluation is not clear. The aim of this study was to undertake a systematic review of review articles that describe approaches to impact assessment, and to identify examples of cancer research impact evaluation within these reviews.
In total, 11 publication databases and the grey literature were searched to identify review articles addressing the topic of approaches to research impact assessment. Information was extracted on methods for data collection and analysis, impact categories and frameworks used for the purposes of evaluation. Empirical examples of impact assessments of cancer research were identified from these literature reviews. Approaches used in these examples were appraised, with a reflection on which methods would be suited to cancer research impact evaluation going forward.
In total, 40 literature reviews were identified. Important methods to collect and analyse data for impact assessments were surveys, interviews and documentary analysis. Key categories of impact spanning the reviews were summarised, and a list of frameworks commonly used for impact assessment was generated. The Payback Framework was most often described. Fourteen examples of impact evaluation for cancer research were identified. They ranged from those assessing the impact of a national, charity-funded portfolio of cancer research to the clinical practice impact of a single trial. A set of recommendations for approaching cancer research impact assessment was generated.
Conclusions
Impact evaluation can demonstrate if and why conducting cancer research is worthwhile. Using a mixed methods, multi-category assessment organised within a framework, will provide a robust evaluation, but the ability to perform this type of assessment may be constrained by time and resources. Whichever approach is used, easily measured, but inappropriate metrics should be avoided. Going forward, dissemination of the results of cancer research impact assessments will allow the cancer research community to learn how to conduct these evaluations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12961-020-00658-x.
Cancer research attracts substantial public funding globally. For example, the National Cancer Institute (NCI) in the United States of America (USA) had a 2020 budget of over $6 billion United States (US) dollars. In addition to public funds, there is also huge monetary investment from private pharmaceutical companies, as well as altruistic investment of time and effort to participate in cancer research from patients and their families. In the United Kingdom (UK), over 25,000 patients were recruited to cancer trials funded by one charity (Cancer Research UK (CRUK)) alone in 2018 [ 1 ]. The need to conduct research within the field of oncology is an ongoing priority because cancer is highly prevalent, with up to one in two people now having a diagnosis of cancer in their lifetime [ 2 , 3 ], and despite current treatments, mortality and morbidity from cancer are still high [ 2 ].
In the current era of increasing austerity, there is a desire to ensure that the money and effort to conduct any type of research delivers tangible downstream benefits for society with minimal waste [ 4 – 6 ]. These wider, real-life benefits from research are often referred to as research impact. Given the significant resources required to conduct cancer research in particular, it is reasonable to question if this investment is leading to the longer-term benefits expected, and to query the opportunity cost of not spending the same money directly within other public sectors such as health and social care, the environment or education.
The interest in evaluating research impact has been rising, partly driven by the actions of national bodies and governments. For example, in 2014, the UK government allocated its £2 billion annual research funding to higher education institutions, in part based on an assessment of the impact of research performed by each institution in an assessment exercise known as the Research Excellence Framework (REF). The proportion of funding dependent on impact assessment will increase from 20% in 2014, to 25% in 2021[ 7 ].
Despite the clear rationale and contemporary interest in research impact evaluation, assessing the impact of research comes with challenges. First, there is no single definition of what research impact encompasses, with potential differences in the evaluation approach depending on the definition. Second, despite the recent surge of interest, knowledge of how best to perform assessments and the infrastructure for, and experience in doing so, are lagging [ 6 , 8 , 9 ]. For the purposes of this review, the definition of research impact given by the UK Research Councils is used (see Additional file 1 for full definition). This definition was chosen because it takes a broad perspective, which includes academic, economic and societal views of research impact [ 10 ].
There is a lack of clarity on how to perform research impact evaluation, and this extends to cancer research. Although there is substantial interest from cancer funders and researchers [ 11 ], this interest is not accompanied by instruction or reflection on which approaches would be suited to assessing the impact of cancer research specifically. In a survey of Australian cancer researchers, respondents indicated that they felt a responsibility to deliver impactful research, but that evaluating and communicating this impact to stakeholders was difficult. Respondents also suggested that the types of impact expected from research, and the approaches used, should be discipline specific [ 12 ]. Being cognisant of the discipline specific nature of impact assessment, and understanding the uniqueness of cancer research in approaching such evaluations, underpins the rationale for this study.
The aim of this study was to explore approaches to research impact assessment, identify those approaches that have been used previously for cancer research, and to use this information to make recommendations for future evaluations. For the purposes of this study, cancer research included both basic science and applied research, research into any malignant disease, concerning paediatric or adult cancer, and studies spanning nursing, medical, public health elements of cancer research.
The study objectives were to:
- i. Identify existing literature reviews that report approaches to research impact assessment and summarise these approaches.
- ii. Use these literature reviews to identify examples of cancer research impact evaluations, describe the approaches to evaluation used within these studies, and compare them to those described in the broader literature.
This approach was taken because of the anticipated challenge of conducting a primary review of empirical examples of cancer research impact evaluation, and to allow a critique of empirical studies in the context of lessons learnt from the wider literature. A primary review would have been difficult because examples of cancer research impact evaluation, for example, the assessment of research impact on clinical guidelines [ 13 ], or clinical practice [ 14 – 16 ], are often not categorised in publication databases under the umbrella term of research impact. Reasons for this are the lack of medical subject heading (MeSH) search term relating to research impact assessment and the differing definitions for research impact. In addition, many authors do not recognise their evaluations as sitting within the discipline of research impact assessment, which is a novel and emerging field of study.
General approach
A systematic search of the literature was performed to identify existing reviews of approaches to assess the impact of research. No restrictions were placed on the discipline, field, or scope (national/global) of research for this part of the study. In the second part of this study, the reference lists of the literature reviews identified were searched to find empirical examples of the evaluation of the impact of cancer research specifically.
Data sources and searches
For the first part of the study, 11 publication databases and the grey literature from January 1998 to May 2019 were searched. The electronic databases were Medline, Embase, Health Management and Policy Database, Education Resources Information Centre, Cochrane, Cumulative Index of Nursing and Allied Health Literature, Applied Social Sciences Index and Abstract, Social Services Abstracts, Sociological Abstracts, Health Business Elite and Emerald. The search strategy specified that article titles must contain the word “impact”, as well as a second term indicating that the article described the evaluation of impact, such as “model” or “measurement” or “method”. Additional file 1 provides a full list of search terms. The grey literature was searched using a proforma. Keywords were inserted into the search function of websites listed on the proforma and the first 50 results were screened. Title searches were performed by either a specialist librarian or the primary researcher (Dr. C Hanna). All further screening of records was performed by the primary researcher.
Following an initial title screen, 800 abstracts were reviewed and 140 selected for full review. Articles were kept for final inclusion in the study by assessing each article against specific inclusion criteria (Additional file 1 ). There was no assessment of the quality of the included reviews other than to describe the search strategy used. If two articles drew primarily on the same review but contributed a different critique of the literature or methods to evaluate impact, both were kept. If a review article was part of a grey literature report, for example a thesis, but was also later published in a journal, the journal article only was kept. Out of 140 articles read in full, 27 met the inclusion criteria and a further 13 relevant articles were found through reference list searching from the included reviews [ 17 ].
For the second part of the study, the reference lists from the literature reviews were manually screened [ 17 ] ( n = 4479 titles) by the primary researcher to identify empirical examples of assessment of the impact of cancer research. Summary tables and diagrams from the reviews were also searched using the words “cancer” and “oncology” to identify relevant articles that may have been missed by reference list searching. After removal of duplicates, 57 full articles were read and assessed against inclusion criteria (Additional file 1 ). Figure 1 shows the search strategy for both parts of the study according to the guidelines for preferred reporting items for systematic reviews and meta-analysis (PRISMA) [ 18 ].

Search strategies for this study
Data extraction and analysis
A data extraction form produced in Microsoft ® Word 2016 was used to collect details for each literature review. This included year of publication, location of primary author, research discipline, aims of the review as described by the authors and the search strategy (if any) used. Information on approaches to impact assessment was extracted under three specific themes which had been identified from a prior scoping review as important factors when planning and conducting research impact evaluation. These themes were: (i) categorisation of impact into different types depending on who or what is affected by the research (the individuals, institutions, or parts of society, the environment), and how they are affected (for example health, monetary gain, sustainability) (ii) methods of data collection and analysis for the purposes of evaluation, and (iii) frameworks to organise and communicate research impact. There was space to document any other key findings the researcher deemed important. After data extraction, lists of commonly described categories, methods of data collection and analysis, and frameworks were compiled. These lists were tabulated or presented graphically and narrative analysis was used to describe and discuss the approaches listed.
For the second part of the study, a separate data extraction form produced in Microsoft ® Excel 2016 was used. Basic information on each study was collected, such as year of publication, location of primary authors, research discipline, aims of evaluation as described by the authors and research type under assessment. Data was also extracted from these empirical examples using the same three themes as outlined above, and the approaches used in these studies were compared to those identified from the literature reviews. Finally, a set of recommendations for future evaluations of cancer research impact were developed by identifying the strengths of the empirical examples and using the lists generated from the first part of the study to identify improvements that could be made.
Part one: Identification and analysis of literature reviews describing approaches to research impact assessment
Characteristics of included literature reviews.
Forty literature reviews met the pre-specified inclusion criteria and the characteristics of each review are outlined in Table Table1. 1 . A large proportion (20/40; 50%) were written by primary authors based in the UK, followed by the USA (5/40; 13%) and Australia (5/40; 13%), with the remainder from Germany (3/40; 8%), Italy (3/40; 8%), the Netherlands (1/40; 3%), Canada (1/40; 3%), France (1/40; 3%) and Iran (1/40; 3%). All reviews were published since 2003, despite the search strategy dating from 1998. Raftery et al. 2016 [ 19 ] was an update to Hanney et al. 2007 [ 20 ] and both were reviews of studies assessing research impact relevant to a programme of health technology assessment research. The narrative review article by Greenhalgh et al. [ 21 ] was based on the same search strategy used by Raftery et al. [ 19 ].
Summary of literature reviews
Approximately half of the reviews (19/40; 48%) described approaches to evaluate research impact without focusing on a specific discipline and nearly the same amount (16/40; 40%) focused on evaluating the impact of health or biomedical research. Two reviews looked at approaches to impact evaluation for environmental research and one focused on social sciences and humanities research. Finally, two reviews provided a critique of impact evaluation methods used by different countries at a national level [ 22 , 23 ]. None of these reviews focused specifically on cancer research.
Twenty-five reviews (25/40; 63%) specified search criteria and 11 of these included a PRISMA diagram. The articles that did not outline a search strategy were often expert reviews of the approaches to impact assessment methods and the authors stated they had chosen the articles included based on their prior knowledge of the topic. Most reviews were found by searching traditional publication databases, however seven (7/40; 18%) were found from the grey literature. These included four reports written by an independent, not-for-profit research institution (Research and Development (RAND) Europe) [ 23 – 26 ], one literature review which was part of a Doctor of Philosophy (Ph.D) thesis [ 27 ], a literature review informing a quantitative study [ 28 ] and a review that provided background information for a report to the UK government on the best use of impact metrics [ 29 ].
Key findings from the reviews: approaches to research impact evaluation
Nine reviews attempted to categorise the type of research impact being assessed according to who or what is affected by research, and how they are affected. In Fig. 2 , colour coding was used to identify overlap between impact types identified in these reviews to produce a summary list of seven main impact categories.

Categories of impact identified in the included literature reviews
The first two categories of impact refer to the immediate knowledge produced from research and the contribution research makes to driving innovation and building capacity for future activities within research institutions. The former is often referred to as the academic impact of research. The academic impact of cancer research may include the knowledge gained from conducting experiments or performing clinical trials that is subsequently disseminated via journal publications. The latter may refer to securing future funding for cancer research, providing knowledge that allows development of later phase clinical trials or training cancer researchers of the future.
The third category identified was the impact of research on policy. Three of the review articles included in this overview specifically focused policy impact evaluation [ 30 – 32 ]. In their review, Hanney et al. [ 30 ] suggested that policy impact (of health research) falls into one of three sub-categories: impact on national health policies from the government, impact on clinical guidelines from professional bodies, and impact on local health service policies. Cruz Rivera and colleagues [ 33 ] specifically distinguished impact on policy making from impact on clinical guidelines, which they described under health impact. This shows that the lines between categories will often blur.
Impact on health was the next category identified and several of the reviews differentiated health sector impact from impact on health gains. For cancer research, both types of health impact will be important given that it is a health condition which is a major burden for healthcare systems and the patients they treat. Economic impact of research was the fifth category. For cancer research, there is likely to be close overlap between healthcare system and economic impacts because of the high cost of cancer care for healthcare services globally.
In their 2004 article, Buxton et al. [ 34 ] searched the literature for examples of the evaluation of economic return on investment in health research and found four main approaches, which were referenced in several later reviews [ 19 , 25 , 35 , 36 ]. These were (i) measuring direct cost savings to the health-care system, (ii) estimating benefits to the economy from a healthy workforce, (iii) evaluating benefits to the economy from commercial development and, (iv) measuring the intrinsic value to society of the health gain from research. In a later review [ 25 ], they added an additional approach of estimating the spill over contribution of research to the Gross Domestic Product (GDP) of a nation.
The final category was social impact. This term was commonly used in a specific sense to refer to research improving human rights, well-being, employment, education and social inclusion [ 33 , 37 ]. Two of the reviews which included this category focused on the impact of non-health related research (social sciences and agriculture), indicating that this type of impact may be less relevant or less obvious for health related disciplines such as oncology. Social impact is distinct from the term societal impact, which was used in a wider sense to describe impact that is external to traditional academic benefits [ 38 , 39 ]. Other categories of impact identified that did not show significant overlap between the reviews included cultural and technological impact. In two of the literature reviews [ 33 , 40 ], the authors provided a list of indicators of impact within each of their categories. In the review by Thonon et al. [ 40 ], only one (1%) of these indicators was specific to evaluating the impact of cancer research.
In total, 36 (90%) reviews discussed methods to collect or analyse the data required to conduct an impact evaluation. The common methods described, and the strengths and weaknesses of each approach, are shown in Additional file 2 : Table S1. Many authors advocated using a mixture of methods and in particular, the triangulation of surveys, interviews (of researchers or research users), and documentary analysis [ 20 , 30 – 32 ]. A large number of reviews cautioned against the use of quantitative metrics, such as bibliometrics, alone [ 29 , 30 , 41 – 48 ]. Concerns included that these metrics were often not designed to be comparable between research programmes [ 49 ], their use may incentivise researchers to focus on quantity rather than quality [ 42 ], and these metrics could be gamed and used in the wrong context to make decisions about researcher funding, employment and promotion [ 41 , 43 , 45 ].
Several reviews explained that the methods for data collection and analysis chosen for impact evaluation depended on both the unit of research under analysis and the rationale for the impact analysis [ 23 , 24 , 26 , 31 , 36 , 50 , 51 ]. Specific to cancer research, the unit of analysis may be a single clinical trial or a programme of trials, research performed at a cancer centre or research funded by a specific institution or charity. The rationale for research impact assessment was categorised in multiple reviews under four headings (“the 4 As”): advocacy, accountability, analysis and allocation [ 19 , 20 , 23 , 24 , 30 – 33 , 36 , 46 , 52 , 53 ]. Finally, Boaz and colleagues found that there was a lack of information on the cost-effectiveness of research impact evaluation methods but suggested that pragmatic, but often cheaper approaches to evaluation, such as surveys, were least likely to give in depth insights into the processes through which research impact occurred [ 31 ].
Applied to research impact evaluation, a framework provides a way of organising collected data, which encourages a more objective and structured evaluation than would be possible with an ad hoc analysis. In total, 27 (68%) reviews discussed the use of a framework in this context. Additional file 2 : Table S2 lists the frameworks mentioned in three or more of the included reviews. The most frequently described framework was the Payback Framework, developed by Buxton and Hanney in 1996 [ 54 ], and many of the other frameworks identified reported that they were developed by adapting key elements of the Payback framework. None of the frameworks identified were specifically developed to assess the impact of cancer research, however several were specific to health research. The unit of cancer research being evaluated will dictate the most suitable framework to use in any evaluation. The unit of research most suited to each framework is outlined in Additional file 2 : Table S2.
Additional findings from the included reviews
The challenges of research impact evaluation were commonly discussed in these reviews. Several mentioned that the time lag [ 24 , 25 , 33 , 35 , 38 , 46 , 50 , 53 , 55 ] between research completion and impact occurring should influence when an impact evaluation is carried out: too early and impact will not have occurred, too late and it is difficult to link impact to the research in question. This overlapped with the challenge of attributing impact to a particular piece of research [ 24 , 26 , 33 – 35 , 37 – 39 , 46 , 50 , 56 ]. Many authors argued that the ability to show attribution was inversely related to the time since the research was carried out [ 24 , 25 , 31 , 46 , 53 ].
Part II: Empirical examples of cancer research impact evaluation
Study characteristics.
In total, 14 empirical impact evaluations relevant to cancer research were identified from the references lists of the literature reviews included in the first part of this study. These empirical studies were published between 1994–2015 by primary authors located in the UK (7/14; 50%), USA (2/14; 14%), Italy (2/14; 14%), Canada (2/14; 14%) and Brazil (1/14; 14%). Table Table2 2 lists these studies with the rationale for each assessment (defined using the “4As”), the unit of analysis of cancer research evaluated and the main findings from each evaluation. The categories of impact evaluated, methods of data collection and analysis, and impact frameworks utilised are also summarised in Table Table2 2 and discussed in more detail below.
Examples of primary studies assessing the impact of cancer research
Approaches to cancer research impact evaluation used in empirical studies
Several of the empirical studies focused on academic impact. For example, Ugolini and colleagues evaluated scholarly outputs from one cancer research centre in Italy [ 57 ] and in a second study looked at the academic impact of cancer research from European countries [ 58 ]. Saed et al. [ 59 ] used submissions to an international cancer conference (American Society of Clinical Oncology (ASCO)) to evaluate the dissemination of cancer research to the academic community, and Lewison and colleagues [ 60 – 63 ] assessed academic, as well as policy impact and dissemination of cancer research findings to the lay media.
The category of the health impact was also commonly evaluated, with particular focus on the assessment of survival gains. Life years gained or deaths averted [ 64 ], life expectancy gains [ 65 ] and years of extra survival [ 66 ] were all used as indicators of the health impact attributable to cancer research. Glover and colleagues [ 67 ] used a measure of health utility, the quality adjusted life year (QALY), which combines both survival and quality of life assessments. Lakdawalla and colleagues [ 66 ] considered the impact of both research on cancer screening and treatments, and concluded that survival gains were 80% attributable to treatment improvement. In contrast, Glover and colleagues [ 67 ] acknowledged the importance of improved cancer therapies due to research but also highlight the major impacts from research around smoking cessation, as well as cervical and bowel cancer screening. Several of these studies that assessed health impact, also used the information on health gains to assess the economic impact of the same research [ 64 – 67 ].
Finally, two studies [ 68 , 69 ] performed multi-dimensional research impact assessments, which incorporated nearly all of the seven categories of impact identified from the previous literature (Fig. 2 ). In their assessment of the impact of research funded by one breast cancer charity in Australia, Donovan and colleagues [ 69 ] evaluated academic, capacity building, policy, health, and wider economic impacts. Montague and Valentim [ 68 ] assessed the impact of one randomised clinical trial (MA17) which investigated the use of a hormonal medication as an adjuvant treatment for patients with breast cancer. In their study, they assessed the dissemination of research findings, academic impact, capacity building for future trials and international collaborations, policy citation, and the health impact of decreased breast cancer recurrence attributable to the clinical trial.
Methods for data collection and analysis used in these studies aligned with the categories of impact assessed. For example, studies assessing academic impact used traditional bibliometric searching of publication databases and associated metrics. Ugolini et al. [ 57 ] applied a normalised journal impact factor to publications from a cancer research centre as an indicator of the research quality and productivity from that centre. This analysis was adjusted for the number of employees within each department and the scores were used to apportion 20% of future research funding. The same bibliometric method of analysis was used in a second study by the same authors to compare and contrast national level, cancer research efforts across Europe [ 58 ]. They assessed the quantity and the mean impact factor of the journals for publications from each country and compared this to the location-specific population and GDP. A similar approach was used for the manual assessment of 10% of cancer research abstracts submitted to an international conference (ASCO) between 2001–2003 and 2006–2008 [ 59 ]. These authors examined if the location of authors affected the likelihood of the abstract being presented orally, as a face-to-face poster or online only.
Lewison and colleagues, who performed four of the studies identified [ 60 – 63 ], used a different bibliometric method of publication citation count to analyse the dissemination, academic, and policy impact of cancer research. The authors also assigned a research level to publications to differentiate if the research was a basic science or clinical cancer study by coding the words in the title of each article or the journal in which the paper was published. The cancer research types assessed by these authors included cancer research at a national level for two different countries (UK and Russia) and research performed by cancer centres in the UK.
To assess policy impact these authors extracted journal publications from cancer clinical guidelines and for media impact they looked at publications cited in articles stored within an online repository from a well-known UK media organisation (British Broadcasting Co-operation). Interestingly, most of the cancer research publications contained in guidelines and cited in the UK media were clinical studies whereas a much higher proportion published by UK cancer centres were basic science studies. These authors also identified that funders of cancer research played an critical role as commentators to explain the importance of the research in the lay media. The top ten most frequent commentators (commenting on > 19 media articles (out of 725) were all representatives from the UK charity CRUK.
A combination of clinical trial findings and documentary analysis of large data repositories were used to estimate health system or health impact. In their study, Montague and Valentim [ 68 ] cited the effect size for a decrease in cancer recurrence from a clinical trial and implied the same health gains would be expected in real life for patients with breast cancer living in Canada. In their study of the impact of charitable and publicly funded cancer research in the UK, Glover et al. [ 67 ] used CRUK and Office for National Statistics (ONS) cancer incidence data, as well as national hospital databases listing episodes of radiotherapy delivered, number of cancer surgeries performed and systemic anti-cancer treatments prescribed, to evaluate changes in practice attributable to cancer research. In their USA perspective study, Lakdawalla et al. [ 66 ] used the population-based Surveillance, Epidemiology and End Results Program (SEER) database to evaluate the number of patients likely to be affected by the implementation of cancer research findings [ 66 ]. Survival calculations from clinical trials were also applied to population incidence estimates to predict the scale of survival gain attributable to cancer research [ 64 , 66 ].
The methods of data collection and analysis used for economic evaluations aligned with the categories of assessment identified by Buxton in their 2004 literature review [ 34 ]. For example, three studies [ 65 – 67 ] estimated direct healthcare cost savings from implementation of cancer research. This was particularly relevant in one ex-ante assessment of the potential impact of a clinical trial testing the equivalence of using less intensive follow up for patients following cancer surgery [ 65 ]. These authors assessed the number of years it would take (“years to payback”) of implementing the hypothetical clinical trial findings to outweigh the money spent developing and running the trial. The return on investment calculation was performed by estimating direct cost savings to the healthcare system by using less intensive follow up without any detriment to survival.
The second of Buxton’s categories was an estimation of productivity loss using the human capital approach. In this method, the economic value of survival gains from cancer research are calculated by measuring the monetary contribution from patients surviving longer who are of working age. This approach was used in two studies [ 64 , 66 ] and in both, estimates of average income (USA) were utilised. Buxton’s fourth category, an estimation of an individual’s willingness to pay for a statistical life, was used in two assessments [ 65 , 66 ], and Glover and colleagues [ 67 ] adapted this method, placing a monetary value on the opportunity cost of QALYs forgone in the UK health service within a fixed budget [ 70 ]. One of the studies that used this method identified that there may be differences in how patients diagnosed with distinct cancer types value the impact of research on cancer specific survival [ 66 ]. In particular, individuals with pancreatic cancer seemed to be willing to spend up to 80% of their annual income for the extra survival attributable to implementation of cancer research findings, whereas this fell to below 50% for breast and colorectal cancer. Only one of the studies considered Buxton’s third category of benefits to the economy from commercial development [ 66 ]. These authors calculated the gain to commercial companies from sales of on-patent pharmaceuticals and concluded that economic gains to commercial producers were small relative to gains from research experienced by cancer patients.
The cost estimates used in these impact evaluations came from documentary analysis, clinical trial publications, real-life data repositories, surveys, and population average income estimates. For example, in one study, cost information from NCI trials was supplemented by using a telephone phone survey to pharmacies, historical Medicare documents and estimates of the average income from the 1986 US Bureau of the Census Consumer Income [ 64 ]. In their study, Coyle et al. [ 65 ] costed annual follow up and treatment for cancer recurrence based on the Ontario Health Insurance plan, a cost model relevant to an Ottawa hospital and cost estimates from Statistics Canada [ 71 ]. The data used to calculate the cost of performing cancer research was usually from funding bodies and research institutions. For example, charity reports and Canadian research institution documents were used to estimate that it costs the National Cancer Institute in Canada $1500 per patient accrued to a clinical trial [ 65 ]. Government research investment outgoings were used to calculate that $300 billion was spent on cancer research in the USA from 1971 to 2000, 25% of which was contributed by the NCI [ 66 ] and that the NCI spent over $10 million USD in the 1980s to generate the knowledge that adjuvant chemotherapy was beneficial to colorectal cancer patients [ 64 ]. Charity and research institution spending reports, along with an estimation of the proportion of funds spent specifically on cancer research, were used to demonstrate £15 billion of UK charity and public money was spent on cancer research between 1970 and 2009 [ 67 ].
Lastly, the two studies [ 68 , 69 ] which adopted a multi-category approach to impact assessment used the highest number and broadest range of methods identified from the previous literature (Additional file 2 : Table S1). The methods utilised included surveys and semi-structured telephone interviews with clinicians, documentary analysis of funding and project reports, case studies, content analysis of media release, peer review, bibiliometrics, budget analysis, large data repository review, and observations of meetings.
Only two of the empirical studies identified used an impact framework. Unsurprisingly, these were also the studies that performed a multi-category assessment and used the broadest range of methods within their analyses. Donovan et al. [ 69 ] used the Payback framework (Additional file 2 : Table S2) to guide the categories of impact assessed and the questions in their researcher surveys and interviews. They also reported the results of their evaluation using the same categories: from knowledge production, through capacity building to health and wider economic impacts. Montague and Valentim [ 68 ] used the Canadian Academy Health Services (CAHS) Framework (Additional file 2 : Table S2). Rather than using the framework in it is original form, they arranged impact indicators from the CAHS framework within a hierarchy to illustrate impacts occurring over time. The authors distinguished short term, intermediate and longer-term changes resulting from one clinical cancer trial, aligning with the concept of categorising impacts based on when they occur, which was described in one of the literature reviews identified in the first part of this study [ 33 ].
Lastly, the challenges of time lags and attribution of impact were identified and addressed by several of these empirical studies. Lewison and colleagues tracked the citation of over 3000 cancer publications in UK cancer clinical guidelines over time [ 61 ], and in their analysis Donovan et al. [ 69 ] explicitly acknowledged that the short time frame between their analysis and funding of the research projects under evaluations was likely to under-estimate the impact achieved. Glover et al. [ 67 ] used bibliometric analysis of citations in clinical cancer guidelines to estimate the average time from publication to clinical practice change (8 years). They added 7 years to account for the time between funding allocation and publication of research results giving an overall time lag from funding cancer research to impact of 15 years. The challenge of attribution was addressed in one study by using a time-line to describe impacts occurring at different time-points but linking back to the original research in question [ 68 ]. The difficultly of estimating time lags and attributing impact to cancer research were both specifically addressed in a companion study [ 72 ] to the one conducted by Glover and colleagues. In this study, instead of quantifying the return on cancer research investment, qualitative methods of assessment were used. This approach identified factors that enhanced and accelerated the process of impact occurring and helped to provide a narrative to link impacts to research.
This study has identified several examples of the evaluation of the impact of cancer research. These evaluations were performed over three decades, and mostly assessed research performed in high-income countries. Justification for the approach to searching the literature used in this study is given by looking at the titles of the articles identified. In only 14% (2/14) was the word “impact” included, suggesting that performing a search for empirical examples of cancer research impact evaluation using traditional publication databases would have been challenging. Furthermore, all the studies identified were included within reviews of approaches to research impact evaluation, which negated the subjective decision of whether the studies complied with a particular definition of research impact.
Characteristics of research that were specifically relevant to cancer studies can be identified from these impact assessments. Firstly, many of these evaluations acknowledged the contribution of both basic and applied studies to the body of cancer research, and several studies categorised research publications based on this distinction. Second, the strong focus on health impact and the expectation that cancer research will improve health was not surprising. The focus on survival in particular, especially in economic studies looking at the value of health gains, reflects the high mortality of cancer as a disease entity. This contrasts with similar evaluations of musculoskeletal or mental health research, which have focused on improvements in morbidity [ 73 , 74 ]. Third, several studies highlighted the distinction between research looking at different aspects of the cancer care continuum; from screening, prevention and diagnosis, to treatment and end of life care. The division of cancer as a disease entity by the site of disease was also recognised. Studies that analysed the number of patients diagnosed with cancer, or population-level survival gains, often used site-specific cancer incidence and other studies evaluated research relating to only one type of cancer [ 64 , 65 , 68 , 69 ]. Lastly, the empirical examples of cancer research impact identified in this study confirm the huge investment into cancer research that exists, and the desire by many research organisations and funders to quantify a rate of return on that investment. Most of these studies concluded that cancer research investment far exceeded expectations of the return on investment. Even using the simple measure of future research grants attracted by researchers funded by one cancer charity, the monetary value of these grants outweighed the initial investment [ 69 ].
There were limitations in the approaches to impact evaluation used in these studies which were recognised by reflecting on the findings from the broader literature. Several studies assessed academic impact in isolation, and studies using the journal impact factor or the location of authors on publications were limited in the information they provided. In particular, using the journal impact factor (JIF) to allocate funding research which was used in one study, is now outdated and controversial. The policy impact of cancer research was commonly evaluated by using clinical practice guidelines, but other policy types that could be used in impact assessment [ 30 ], such as national government reports or local guidelines, were rarely used. In addition, using cancer guidelines as a surrogate for clinical practice change and health service impact could have drawbacks. For example, guidelines can often be outdated, irrelevant or simply not used by cancer clinicians and in addition, local hospitals often have their own local clinical guidelines, which may take precedent over national documents. Furthermore, the other aspects of policy impact described in the broader literature [ 30 ], such as impact on policy agenda setting and implementation, were rarely assessed. There were also no specific examples of social, environmental or cultural impacts and very few of the studies mentioned wider economic benefits from cancer research, such as spin out companies and patents. It may be that these types of impact were less relevant to cancer research being assessed, however unexpected impacts may have be identified if they were considered at the time of impact evaluation.
Reflecting on how the methods of data collection and analysis used in these studies aligned with those listed in Additional file 2 : Table S1 bibliometrics, alternative metrics (media citation), documentary analysis, surveys and economic approaches were often used. Methods less commonly adopted were interviews, using a scale and focus groups. This may have been due to the time and resource implications of using qualitative techniques and more in depth analysis, or a lack of awareness by authors regarding the types of scales that could be used. An example of a scale that could be used to assess the impact of research on policy is provided in one of the literature reviews identified [ 30 ]. The method of collecting expert testimony from researchers was utilised in the studies identified, but there were no obvious examples of testimony about the impact of cancer research from stakeholders such as cancer patients or their families.
Lastly, despite the large number of examples identified from the previous literature, a minority of the empirical assessments used an impact framework. The Payback Framework, and an iteration of the CAHS Framework were used with success and these studies are excellent examples of how frameworks can be used for cancer research impact evaluation in future. Other frameworks identified from the literature (Additional file 2 : Table S2) that may be appropriate for the assessment of cancer research impact in future include Anthony Weiss’s logic model [ 75 ], the research impact framework [ 76 ] and the research utilisation ladder [ 77 ]. Weiss’s model is specific to medical research and encourages evaluation of how clinical trial publication results are implemented in practice and lead to health gain. He describes an efficacy-efficiency gap [ 75 ] between clinical decision makers becoming aware of research findings, changing their practice and this having impact on health. The Research Impact Framework, developed by the Department of Public Health and Policy at the UK London School of Hygiene and Tropical Medicine [ 76 ], is an aid for researchers to self-evaluate their research impact, and offers an extensive list of categories and indicators of research which could be applied to evaluating the impact of cancer research. Finally, Landry’s Research Utilisation Ladder [ 77 ] has similarities to the hierarchy used in the empirical study by Montegue and Valentim [ 68 ], and focuses on the role of the individual researcher in determining how research is utilised and its subsequent impact.
Reflecting on the strengths and limitations of the empirical approaches to cancer research impact identified in this study, Fig. 3 outlines recommendations for the future. One of these recommendations refers to improving the use of real-life data to assess the actual impact of research on incidence, treatment, and outcomes, rather than predicting these impacts by using clinical trial results. Databases for cancer incidence, such as SEER (USA) and the Office of National Statistics (UK), are relatively well established. However, those that collect data on treatments delivered and patient outcomes are less so, and when they do exist, they have been difficult to establish and maintain and often have large quantities of missing data [ 78 , 79 ]. In their study, Glover et al. [ 67 ] specifically identified the lack of good quality data documenting radiotherapy use in the UK in 2012.

Suggestions for approaching cancer research impact evaluation.
1 Thonon F, Boulkedid R, Teixeira M, Gottot S, Saghatchian M, Alberti C. Identifying potential indicators to measure the outcome of translational cancer research: a mixed methods approach. Health Res Policy Syst. 2015;13:72
The recommendations also suggest that impact assessment for cancer and other health research could be made more robust by giving researchers access to cost data linked to administrative datasets. This type of data was used in empirical impact assessments performed in the USA [ 64 , 66 ] because the existing Medicare and Medicaid health service infrastructure collects and provides access to this data. In the UK, hospital cost data is collected for accounting purposes but this could be unlocked as a resource for research impact assessments going forward. A good example of where attempts are being made to link resource use to cost data for cancer care in the UK is through the UK Colorectal Cancer Intelligence Hub [ 80 ].
Lastly, several empirical examples highlighted that impact from cancer research can be increased when researchers or research organisations advocate, publicise and help to interpret research findings for a wider audience [ 60 , 72 ]. In addition, it is clear from these studies that organisations that want to evaluate the impact of their cancer research must also appreciate that research impact evaluation is a multi-disciplinary effort, requiring the skills and input from individuals with different skill sets, such as basic scientists, clinicians, social scientists, health economists, statisticians, and information technology analysts. Furthermore, the users and benefactors from cancer research, such as patients and their families, should not be forgotten, and asking them which impacts from cancer research are important will help direct and improve future evaluations.
The strengths of this study are the broad, yet systematic approach used to identify existing reviews within the research impact literature. This allowed a more informed assessment of cancer research evaluations than would have been possible if a primary review of these empirical examples had been undertaken. Limitations of the study include the fact that the review protocol was not registered in advance and that one researcher screened the full articles for review. The later was partly mitigated by using pre-defined inclusion criteria.
Impact assessment is a way of communicating to funders and patients the merits of undertaking cancer research and learning from previous research to develop better studies that will have positive impacts on society in the future. To the best of our knowledge, this is the first review to consider how to approach evaluation of the impact of cancer research. At the policy level, a lesson learned from this study for institutions, governments, and funders of cancer research, is that an exact prescription for how to conduct cancer research impact evaluation cannot be provided, but a multi-disciplinary approach and sufficient resources are required if a meaningful assessment can be achieved. The approach to impact evaluation used to assess cancer research will depend on the type of research being assessed, the unit of analysis, rationale for the assessment and the resources available. This study has added to an important dialogue for cancer researchers, funders and patients about how cancer research can be evaluated and ultimately how future cancer research impact can be improved.
Acknowledgements
We would like to acknowledge the help of Ms Lorraine MacLeod, specialist librarian from the Beatson West of Scotland Cancer Network in NHS Greater Glasgow and Clyde for her assistance in formulating the search strategy. We would like to acknowledge that Professor Stephen Hanney provided feedback on an earlier version of this review.
Abbreviations
Authors’ contributions.
All authors contributed to the concept and design of the study. CH was responsible for the main data analysis and writing of the manuscript. KAB and RJJ responsible for writing, editing and final approval of the manuscript. All authors read and approved the final manuscript.
Dr. Catherine Hanna has a CRUK and University of Glasgow grant. Grant ID: {"type":"entrez-nucleotide","attrs":{"text":"C61974","term_id":"2420679","term_text":"C61974"}} C61974 /A2429.
Availability of data and materials
Ethics approval and consent to participate.
No ethical approval required.
Consent for publication
No patient level or third party copyright material used.
Competing interests
Nil declared.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catherine R. Hanna, Email: [email protected] .
Kathleen A. Boyd, Email: [email protected] .
Robert J. Jones, Email: [email protected] .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Review Articles
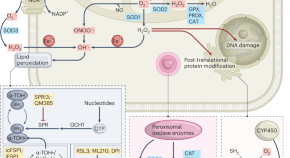
The pleiotropic functions of reactive oxygen species in cancer
Papagiannakopoulos and colleagues discuss the roles of reactive oxygen species in cancer and the ways in which redox mechanisms may be exploited for cancer therapy.
- Katherine Wu
- Ahmed Ezat El Zowalaty
- Thales Papagiannakopoulos
Clinical and translational attributes of immune-related adverse events
Suijkerbuijk et al. summarize the clinical manifestation and classification of immune-related adverse events, discuss the immunopathogenesis of immune-related adverse events and provide key insights into their management and future therapeutic directions.
- Karijn P. M. Suijkerbuijk
- Mick J. M. van Eijs
- Alexander M. M. Eggermont
Aneuploidy and complex genomic rearrangements in cancer evolution
Van Loo and colleagues review the mechanistic underpinnings of large genomic alterations and their roles in tumor development and evolution.
- Toby M. Baker
- Peter Van Loo
Lipids as mediators of cancer progression and metastasis
Schulze and colleagues discuss the latest advances in understanding the role of lipids in cancer progression and metastasis and reflect on opportunities to target lipid metabolism in tumors.
- Felix C. E. Vogel
- Adriano B. Chaves-Filho
- Almut Schulze

Harnessing progress in radiotherapy for global cancer control
Jaffray and co-authors discuss progress in radiotherapy that has been driven by technological advances and ways to enable broad access to innovative radiation-based treatment modalities by aligning individualized therapy with global access needs.
- David A. Jaffray
- Felicia Knaul
- Mary Gospodarowicz
Clinical and translational advances in ovarian cancer therapy
In this Review, Konstantinopoulos and Matulonis discuss recent clinical advances in the treatment of ovarian cancer and reflect on persisting challenges.
- Panagiotis A. Konstantinopoulos
- Ursula A. Matulonis
Cancer cell plasticity during tumor progression, metastasis and response to therapy
Blanpain and colleagues review the current knowledge on cancer cell plasticity and its impact on tumor initiation and progression, the metastatic cascade and resistance to therapy.
- Andrea Pérez-González
- Kevin Bévant
- Cédric Blanpain
Understanding the mechanisms and translational implications of the microbiome for cancer therapy innovation
Sears and colleagues discuss the latest advances in the understanding of host and microbiota mechanisms involved in the relationship between the microbiome and cancer and emerging avenues for applying these insights to cancer therapy.
- Jessica Queen
- Fyza Shaikh
- Cynthia L. Sears
Leveraging translational insights toward precision medicine approaches for brain metastases
Brastianos and colleagues discuss the molecular and microenvironmental features of brain metastases, as well as promising avenues in translational research to overcome challenges for effective treatments.
- Albert E. Kim
- Edwin Nieblas-Bedolla
- Priscilla K. Brastianos
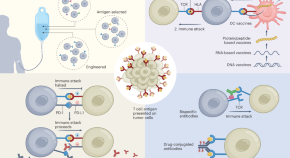
The landscape of T cell antigens for cancer immunotherapy
Samuels and colleagues review the landscape of cancer peptide antigens, focusing on emerging concepts and the latest translational and clinical advances.
- Aviyah Peri
- Nadja Salomon
- Yardena Samuels
Impact of context-dependent autophagy states on tumor progression
Kimmelman and colleagues discuss the role of autophagy in tumor cells and of cell-nonautonomous autophagy in the microenvironment and host cells in supporting tumor growth and reflect on open questions in the field.
- Mohamad Assi
- Alec C. Kimmelman
Single-cell profiling to explore pancreatic cancer heterogeneity, plasticity and response to therapy
Saur and colleagues provide an overview of single-cell analyses in pancreatic cancer and discuss their implications for our understanding of heterogeneity and plasticity in the tumor and its microenvironment.
- Stefanie Bärthel
- Chiara Falcomatà
- Dieter Saur
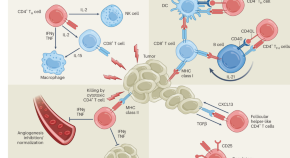
CD4 + T cells in cancer
Speiser and colleagues review the latest advances in understanding of the biological roles of CD4 + T cells in cancer immunology and applications for immunotherapy.
- Daniel E. Speiser
- Obinna Chijioke
- Christian Münz
Advances in antibody-based therapy in oncology
In this review article, Christ and colleagues discuss the recent developments in antibody-based cancer therapies.
- Rodrigo Vazquez-Lombardi
- Daniel Christ
ALK-positive lung cancer: a moving target
Shaw and colleagues discuss the oncogenic roles of ALK in lung cancer, targeting approaches and the mechanisms underlying acquired resistance to ALK-directed therapy.
- Jaime L. Schneider
- Jessica J. Lin
- Alice T. Shaw
YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches
Piccolo and colleagues discuss the current knowledge on YAP/TAZ biology in cancer, highlighting recent progress in the field and discussing open questions, as well as potential clinical implications.
- Stefano Piccolo
- Tito Panciera
- Michelangelo Cordenonsi
The cGAS–STING pathway and cancer
Samson and Ablasser review the roles of the cGAS–STING pathway in cancer and efforts to target this signaling axis therapeutically.
- Natasha Samson
- Andrea Ablasser
Artificial intelligence in histopathology: enhancing cancer research and clinical oncology
Schmatko et al. review the application of artificial intelligence to digitized histopathology for cancer diagnosis, prognosis and classification and discuss its potential utility in the clinic and broader implications for cancer research and care.
- Artem Shmatko
- Narmin Ghaffari Laleh
- Jakob Nikolas Kather
Cancer vaccines: the next immunotherapy frontier
Brody and colleagues discuss the current status and potential of cancer vaccines, highlighting challenges and opportunities to advance promising candidates to the clinic.
- Matthew J. Lin
- Judit Svensson-Arvelund
- Joshua D. Brody
Cancer-associated fibroblasts in the single-cell era
Lavie et al. review the recent advances in the field of cancer-associated fibroblasts, including their tissue-specific complexity and overall plasticity, as revealed by single-cell technologies.
- Aviad Ben-Shmuel
- Ruth Scherz-Shouval
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Extrachromosomal DNA (ecDNA) is now accepted as a major contributor to cancer pathogenesis. In this Review, Yan, Mischel and Chang highlight the recent advancements in ecDNA research, providing ...
Cancer therapies have evolved considerably in recent decades, substantially improving the quality of life and survival of patients with cancer. In this issue, we launch our Series on Cancer ...
Extrachromosomal DNA (ecDNA) is now accepted as a major contributor to cancer pathogenesis. In this Review, Yan, Mischel and Chang highlight the recent advancements in ecDNA research, providing ...
Cancer Research publishes impactful original studies, reviews, and opinion pieces of high significance to the broad cancer research community. Cancer Research seeks manuscripts that offer conceptual or technological advances leading to basic and translational insights into cancer biology. Read More About the Journal.
In this systematic review and meta-analysis, we show that advanced cancer patients who have exhausted standard of care treatments and participate in a randomized clinical trial with a control arm without any anticancer drug overall benefited from the experimental treatment when randomized into the experimental arms with a 42% decrease in the risk of disease progression and a 18% decrease in ...
In this review, we will provide an in-depth analysis of the most innovative advances in basic and applied cancer research. Keywords: cancer, ... In recent years, research into cancer medicine has taken remarkable steps towards more effective, precise and less invasive cancer treatments (Figure 1). While nanomedicine, combined with targeted ...
Explore Cancer articles from The New England Journal of Medicine ... Research 166; Commentary 155; Review 48; Other 47; ... Review Article; VOL. 389 NO. 14; Oct 05, 2023 ...
In this narrative review, a general overview of the most advanced and novel cancer therapies was provided. In addition, also new strategies currently under investigation at the research stage that should overwhelm the drawbacks of standard therapies; different strategies to cancer diagnosis and therapy; and their current status in the clinical ...
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed ...
In this study, we reviewed cancer from the perspective of the molecular level, in order to get a closer look at this disease. 2. Review method. Initially, we searched research papers using keywords such as cancer and molecular process, cancer and treatment and molecular aspects.
AIMS AND SCOPE OF JOURNAL: The Annual Review of Cancer Biology reviews a range of subjects in cancer research that represent important and emerging areas in the field. With recent advances in our understanding of the basic mechanisms of cancer development and the translation of an increasing number of these findings into the clinic in the form of targeted treatments for the disease, the Annual ...
Black voices in cancer research and oncology. ... In their Review article, Fuchs and colleagues discuss how a single or a few mutations in adult cells can lead to invasive cancers without a high ...
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine ...
About 10 million men are presently living with a diagnosis of prostate cancer, and approximately 700 000 of these are living with metastatic disease. 1. , 2. Metastatic prostate cancer accounts for more than 400 000 deaths annually, and this mortality is expected to more than double by 2040.
Organoid biology will further develop with a goal of translating the research into personalized therapy. These research areas may result in the creation of new cancer treatments in the future. Keywords: exosomes, immunotherapy, microbiome, organoid. Cancer research has made remarkable progress and new discoveries are beginning to be made.
Peer-reviewed cancer research studies are evaluated and critiqued by an independent group of scientific experts in the same field before that research is performed. The purpose of this review is to ensure the scientific merit of the study by asking if the research question being studied is important and based on sound science.
Cancer, an international interdisciplinary journal of the American Cancer Society, publishes high-impact, peer-reviewed original articles and solicited content on the latest clinical research findings.Spanning the breadth of oncology disciplines, Cancer delivers something for everyone involved in cancer research, risk reduction, treatment, and patient care.
Ke Chen, Jingcheng Zhang, Lei Meng, Lingshang Kong, Ming Lu, Zhengguang Wang and Wenbin Wang. Journal of Experimental & Clinical Cancer Research 2024 43 :78. Correction Published on: 12 March 2024. The original article was published in Journal of Experimental & Clinical Cancer Research 2024 43 :44. Full Text.
Overview of Checkpoint Inhibitors. Cancer immuno-editing is the process by which various immune system components protect the host against primary tumour development or enhance tumour escape, or both, either by sculpting tumour immunogenicity or attenuating antitumour immune responses 7.The process is tightly regulated by immune checkpoints, which are immune-cell surface receptors controlling ...
This Review discusses the metabolic alterations and vulnerabilities across multiple types of cancer, and describes how these could potentially be targeted using diet in conjunction with ...
Categories of Articles. Cancer Research welcomes submission of the following article types to be considered for publication in the journal, subject to peer review as noted below for each type. Please see About Cancer Research for information about the journal, and Instructions for Authors for important policies and instructions relevant to all ...
Tel: +91-7834871362, +91-7055064227. 1. Cancer: An Overview. Garima Mathur, Sumitra Nain and Pramod Kumar Sha rma. 12 1. School of Medical and Allied Scien ces, Galgotia university, Greater Noida ...
In this review article, we try to shed a light on various cancer-causing factors, type of cancer, how the cancer starts, sign or symptom of cancer, diagnosing tests, the treatments of cancer and ...
Cancer-associated fibroblasts (CAFs), a class of stromal cells in the tumor microenvironment (TME), play a key role in controlling cancer cell invasion and metastasis, immune evasion, angiogenesis, and resistance to chemotherapy. CAFs mediate their activities by secreting soluble chemicals, releasing exosomes, and altering the extracellular matrix (ECM). Exosomes contain various biomolecules ...
The optimal approach to cancer research impact evaluation is not clear. The aim of this study was to undertake a systematic review of review articles that describe approaches to impact assessment, and to identify examples of cancer research impact evaluation within these reviews.
Related article As colorectal cancer rises among younger adults, some seek colonoscopies earlier "We all know cancer is an aging disease. However, it is really coming to a younger population.
Speiser and colleagues review the latest advances in understanding of the biological roles of CD4 + T cells in cancer immunology and applications for immunotherapy. Daniel E. Speiser. Obinna ...
Cancer is becoming increasingly prevalent among younger people. New evidence presented at AACR suggests why that may be the case: On a cellular level, younger generations seem to be aging faster ...