Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.

What’s New in Breast Cancer
This section gives an overview of new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, and prevent, detect, diagnose and treat breast cancer. Advances in breast cancer care are evaluated through a rigorous process that includes clinical trials and regulatory approvals before being considered standards of care and included in breast cancer care guidelines. Komen’s research team monitors the rapidly evolving breast cancer landscape, and here we will highlight new breast cancer treatment breakthroughs, innovations in technology or key advances that may be added or are new to guidelines. We will share these research advancements to empower patients with knowledge to help them make informed decisions with their doctors.
Use these links to jump to the topics below.
- Emerging Areas in Metastatic Breast Cancer Treatment
- Clinical Trials
Treatments and Drugs
For patients, new treatments can mean more options and more hope. Researchers are working to develop new breast cancer treatment breakthroughs, such as more effective drugs that will specifically target breast cancer cells, minimize side effects and prevent breast cancer cells from coming back. While some treatments increase the effectiveness of existing drugs, others may offer new, innovative strategies for attacking tumor cells.
As of August 2023, the following new treatments and drugs are currently in clinical trials and have not yet received FDA approval:
- A new antibody-drug conjugate called datopotamab deruxtecan (Dato-DXd) is currently being evaluated in three Phase 3 clinical trials for advanced estrogen receptor-positive (ER+) [2] breast cancer, metastatic triple negative [ 3 ] breast cancer and early triple negative [ 4 ] breast cancer (TNBC). Dato-DXd specifically targets a protein called TROP2, a biomarker that can be used to target cancer cells instead of healthy cells. Another TROP2-targeting therapy called sacituzumab govitecan has already been approved for TNBC and estrogen-receptor-positive breast cancer. Dato-DXd uses a different chemotherapy drug and delivery system compared to sacituzumab govitecan.
- HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results for a Phase 2 clinical trial studying HER3-DXd, a new HER3-targeting antibody-drug conjugate for people with metastatic breast cancer . [ 1 ]. While the study found that 35% of patients responded positively to HER3-DXd, researchers will continue to evaluate which patients could benefit most from this drug through future Phase 3 clinical trials.
- CDK4/6 inhibitors are commonly used to treat estrogen receptor-positive breast cancer, but a new CDK4/6 inhibitor called trilaciclib is being tested to treat TNBC. Results from a Phase 2 clinical trial showed that trilaciclib improved outcomes for people with advanced TNBC, and the drug is currently being evaluated in the Phase 3 PRESERVE 2 clinical trial [ 5 ]. Researchers believe that unlike currently available CDK4/6 inhibitors, trilaciclib may improve response to immunotherapy and mitigate some of the side effects of chemotherapy . If this clinical trial is successful, this would be the first CDK4/6 inhibitor approved for people with TNBC.
New and improved technologies may be able to increase the speed and accuracy of detecting, diagnosing or monitoring breast cancer for progression and response to treatment.
- Doctors may use PET scans, or positron emission tomography, to scan for evidence that breast cancer has spread or metastasized. Once breast cancer has spread, the metastases may have evolved to a different type of breast cancer than the original tumor. These differences mean the metastases and the original tumor may not respond to the same treatments. A diagnostic imaging agent called Cerianna (fluoroestradiol F-18 or FES PET) allows doctors to use PET scans to learn if estrogen receptors are present in metastatic lesions. If a person has metastatic lesions that are estrogen receptor-positive, they may respond well to hormone therapy. This agent was recently incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [ 6 ] as an option for some people with metastatic or recurrent estrogen receptor-positive breast cancer to consider [ 7 ].
- Ovarian suppression increases the effectiveness of hormone therapy in some premenopausal women but comes with additional side effects that can affect quality of life. A study presented at the 2022 San Antonio Breast Cancer Symposium [ 8 ] suggests that the Breast Cancer Index , a tumor profiling test that looks at genes to predict how likely a cancer is to metastasize, may be able to identify premenopausal women that would benefit most from ovarian suppression. This test would give doctors a new tool to personalize treatment for premenopausal women with estrogen receptor-positive breast cancer. More data are needed to confirm these results.
- Doctors are getting closer to identifying which patients with early HER2-positive breast cancer can safely avoid chemotherapy by using the HER2DX genomic test. HER2DX is the first test specifically designed to identify HER2-positive patients at high and low risk for recurrence . For some people, being able to avoid chemotherapy without comprising long-term outcomes will lead to a better quality of life.

Research can take decades to reach the bedside, but what discoveries are just around the corner for patients? Susan G. Komen shares all of this and more through Breast Cancer Breakthroughs, a virtual education series focusing on the new science and technology advancements that are poised to make a difference for patients in the near future. Sign up for Breast Cancer Breakthroughs to never miss an episode.

Kimberly’s Story: Finding Joy in the Midst of a Metastatic Breast Cancer Diagnosis
After Kimberly Reinika’s mother passed away in 2019 from ovarian cancer, she worried that it would ultimately take her life, too. “That was the cancer I was checking for,” she said.
Approaches to Care
With knowledge gained from clinical trials, researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining certain drugs, finding which patients can skip certain elements of treatment or changing the order of their treatments to maximize effectiveness or minimize side effects.
- Patients with early estrogen receptor-positive breast cancer generally have a good prognosis, but some people have a higher risk of recurrence for as long as 20 years. Researchers are seeking new strategies to reduce this risk of recurrence. CDK4/6 inhibitors are used to treat advanced breast cancer, but the Phase 3 NATALEE clinical trial, presented at the 2023 American Society of Clinical Oncology Annual Meeting [ 9 ], found that using the CDK4/6 inhibitor ribociclib for two years in the adjuvant setting reduced the risk of recurrence for people with estrogen receptor-positive breast cancer.
- Inflammatory breast cancer is difficult to diagnose because its symptoms often mimic infections. Additionally, because some medical professionals don’t see it often, they may lack experience in recognizing and treating inflammatory breast cancer. In partnership with the Inflammatory Breast Cancer Research Foundation and the Milburn Foundation, Susan G. Komen launched a first-of-its kind diagnostic tool for inflammatory breast cancer. Through this scoring system, the tool considers the defining features of inflammatory breast cancer and provides data that can help providers accurately determine whether a person has inflammatory breast cancer. The goal of this tool is to increase the accuracy of diagnosing inflammatory breast cancer so that people will receive the appropriate care they need to treat this aggressive disease.
- Immunotherapy targets the immune system to help the body fight off tumors. Immunotherapy is currently only available for some patients with triple negative breast cancer, but researchers are aiming to bring this cutting edge therapy to more people. In a recent announcement [ 10 ], positive results were announced for a clinical trial that evaluated the immunotherapy drug pembrolizumab in patients with early estrogen receptor-positive breast cancer. Komen will be closely monitoring the results of this study at upcoming scientific conferences and hopes to see more promising data suggesting that a new treatment option may soon be available for patients with early estrogen receptor-positive breast cancer.
- Clinical trials are often designed using the maximum tolerated dose of a drug. However, many drugs may give the same effect with a smaller dose that results in fewer side effects for the patient. The X-7/7 clinical trial, which was presented at the 2023 ASCO Annual Meeting, tested the impact of a new treatment schedule for the chemotherapy drug capecitabine to treat metastatic breast cancer. Researchers found that people who took a higher dose of capecitabine over fewer days had fewer side effects and were able to remain on their treatment longer compared to the standard regimen. This new approach can improve the quality of life for those living with metastatic breast cancer without compromising the effectiveness of their treatments.
Komen will be closely monitoring the results of these studies and more at upcoming scientific conferences and hopes to see more promising data regarding new ways to prevent, detect, diagnose and treat breast cancer.

It Looks Promising: Uncovering New Possibilities in Breast Cancer Prevention
Is breast cancer prevention possible? Komen Scientific Advisory Board Member Dr. Kornelia Polyak is exploring a new strategy to identify and eliminate cell precursors from which tumors can grow.

Help discover cures to breast cancer, faster. New treatment breakthroughs for breast cancer come from researchers learning from people who have breast cancer, but our current data sources only represent a small portion of the breast cancer community. Help us discover the cures to breast cancer, faster, by joining ShareForCures.
What’s New in Breast Cancer References
- Hamilton, E. P., et al. (2023). “A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC).” Journal of Clinical Oncology 41(16_suppl): 1004-1004. https://meetings.asco.org/abstracts-presentations/219699
- https://classic.clinicaltrials.gov/ct2/show/NCT05104866
- https://clinicaltrials.gov/study/NCT05374512
- https://classic.clinicaltrials.gov/ct2/show/NCT05629585
- https://classic.clinicaltrials.gov/ct2/show/NCT04799249
- https://www.gehealthcare.com/about/newsroom/press-releases/ge-healthcare-announces-fes-pet-imaging-recommendation-in-nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines
- https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf (page 16)
- https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
- Stroyakovskiy, D., et al. (2023). “Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial.” Journal of Clinical Oncology 41(17_suppl): LBA500-LBA500.
- https://www.merck.com/news/merck-announces-phase-3-keynote-756-trial-met-primary-endpoint-of-pathological-complete-response-pcr-rate-in-patients-with-high-risk-early-stage-er-her2-breast-cancer/
TOOLS & RESOURCES
NEED HELP OR MORE INFORMATION?
1-877 GO KOMEN (1-877-465-6636)
Educational Resources
Komen Financial Assistance Program

Search form
- Find Stories
- For Journalists
Researchers uncover on/off switch for breast cancer metastasis
New research from Stanford and the Arc Institute could lead to a new and more effective immunotherapy and help clinicians better predict patient response to existing medicines.

Songnan Wang (left) and Lingyin Li (right) found that a protein called ENPP1 acts as an on/off switch for breast cancer metastases. High protein levels lead to a high chance of metastasis (as seen by cells growing in the dish on the left), while low levels lead to no metastasis (as seen by no cells growing in the dish on the right). (Image credit: Lingyin Li and Songnan Wang)
Despite their promise, immunotherapies fail to treat many cancers, including over 80% of some of the most advanced breast cancers. And many of those patients who do respond still experience metastases eventually. New research from Stanford University and the Arc Institute has revealed a better way to predict and improve patient responses.
A team led by Lingyin Li , associate professor of biochemistry at Stanford and Arc Core Investigator, found that a protein called ENPP1 acts as an on/off switch that controls breast cancer’s ability to both resist immunotherapy and metastasize. The study, published on Dec. 20 in the Proceedings of the National Academy of Sciences , showed that ENPP1 is produced by cancer cells and by healthy cells in and around the tumor, and that high patient ENPP1 levels are linked to immunotherapy resistance and subsequent metastases. The research could lead to new, more effective immunotherapies and help clinicians better predict patient response to existing medicines.
“Our study should offer hope for everyone,” said Li, who is also an institute scholar at Sarafan ChEM-H .
Thawing cold tumors
Immunotherapies, like pembrolizumab (Keytruda), work by blocking an immune-dampening interaction between a cancer cell and a T cell, a kind of immune cell. For this to be effective, though, T cells need to permeate the tumor. So-called “hot” tumors, like those in melanoma and a subset of lung cancer, are treatable through immunotherapies, but many others, like breast and pancreatic cancers, are “cold,” devoid of T cell infiltration.
In her quest to turn cold tumors hot, Li started with cGAMP, a molecule that cells produce when their DNA is damaged, which happens when a cell becomes cancerous. If left intact, cGAMP activates an immune response through what is known as the STING pathway, which can help make a tumor hot. Li previously discovered that cGAMP is exported outside the cells but often, before it can trigger a response, a protein called ENPP1 chews up these molecular “danger” signals. ENPP1, she proposed, helped keep cold tumors cold.
High levels of ENPP1 correlate with poor prognosis in many cancers, but the protein can perform many actions in the body, so Li set out to determine if its cGAMP-chewing ability is behind its clinical significance.
An on/off switch
Li began collaborating with two professors at the University of California, San Francisco: Hani Goodarzi, also an incoming Arc Institute Core Investigator, and Laura Van’t Veer, a clinician who leads the I-SPY 2 Trial, a groundbreaking breast cancer trial. ENPP1 levels naturally vary across individuals, so the team looked at data from patients in the I-SPY 2 Trial to see how responses to pembrolizumab varied with ENPP1 levels at the time of diagnosis.
The results were astounding. Patients with high ENPP1 levels had low response to pembrolizumab and high chance of metastases. Those with low ENPP1 levels had a high response to pembrolizumab and no metastases. ENPP1 predicted both response to immunotherapy and likelihood of relapse.
Two things were suddenly clear: that ENPP1 was critical in metastases, not just in primary tumors; and that they should be looking at ENPP1 in healthy cells, not only in cancer cells.
“Using the finest molecular scalpels developed in our lab, I was excited to dig deeper and figure out exactly how ENPP1 has such a dramatic influence on clinical outcomes,“ said Songnan Wang , an MD-PhD student in biochemistry, Arc researcher, and first author on the paper.
In a series of mouse studies, Wang proved that removing ENPP1 entirely or eliminating only its cGAMP-chewing ability in normal and cancer cells yielded exactly the same result: decreased tumor growth and decreased metastases. And the team proved that it resulted directly from suppressing the STING pathway. They found an on/off switch.
On top of the waterfall
Immune pathways are often described as “cascades” with a series of signals that trigger downstream actions that eventually lead to a response.
“For cancers to stop the immune system from detecting them, they need to build dams that block the signal from flowing,” said Li. “We have shown that ENPP1 acts like a big dam at the top of the waterfall.”
This means that clinicians can use ENPP1 levels to better determine appropriate treatment for breast cancer patients. It also means that drugs that destroy the ENPP1 dam could make existing therapies more effective – and several ENPP1 inhibitors are already in clinical development.
While this work focused on breast cancer, Li believes that ENPP1 plays a critical role in other kinds of “cold” tumors.
“I hope to inspire clinicians who treat cancers – including lung cancer, glioblastoma, and pancreatic cancer – to investigate ENPP1’s role in patient outcomes,” said Li.
Li is also a member of Stanford Bio-X and the Stanford Cancer Institute . Other Stanford co-authors include Alby Joseph and Valentino Sudyaryo (of Stanford and Arc); Volker Böhnert, Gemini Skariah, and Xuchao Lyu (of Stanford). Additional co-authors are from the University of California, San Francisco, and Arc.
This work was supported by the Arc Institute, the Stanford Chi-Li Pao Foundation Alpha Omega Alpha Student Research Fellowship, the Stanford Medical Scholars Research Program, a Stanford Graduate Fellowship, the Chemistry/Biology Interface (CBI) Predoctoral Training Program at Sarafan ChEM-H, the NSF Graduate Research Fellowship Program, an Era of Hope Scholar Award, an NIH New Innovator Award, a Pew-Steward Scholars for Cancer Research award, and the National Institutes of Health.
Lingyin Li and Volker Böhnert have filed two patents on ENPP1 inhibitors (PCT/US2020/015968 and PCT/US2018/050018) that are licensed to Angarus Therapeutics. Li is a co-founder of Angarus Therapeutics.
- International edition
- Australia edition
- Europe edition

Breast cancer drug cuts risk of most common form returning by 25%
Trial results presented at US oncology conference suggest ribociclib could be gamechanging and boost survival rate significantly
Thousands of women with the world’s most common form of breast cancer could benefit from a blockbuster drug that helps them live longer and cuts the risk of the disease returning by a quarter.
More than 2 million people globally are diagnosed each year with the disease, which is the world’s most prevalent cancer. Although treatments have improved in recent decades, many patients will later experience the cancer returning. If a recurrence does occur, it is often at a more advanced stage.
Now, “very promising” research presented at the American Society of Clinical Oncology (Asco) annual meeting, the world’s largest cancer conference, suggests a new targeted therapy drug, ribociclib, could be gamechanging. Trial results show it can boost survival and significantly slash the chances of cancer coming back.
Ribociclib has previously shown survival benefits in breast cancer patients whose disease has spread. But in the new study, researchers discovered it may also boost outcomes for patients with much earlier-stage disease, including those with cancer that has not yet spread to the lymph nodes.
The findings excited researchers and oncologists at Asco’s annual meeting in Chicago because the data suggests the drug, also known as Kisqali, could ward off the threat of cancer returning in a broad population, and change global practice.
Ribociclib is a targeted therapy called a small molecule inhibitor. It works by targeting proteins in breast cancer cells called CDK4 and CDK6, which modulate cell growth, including the growth of cancer cells.
The late-stage trial of the drug showed it cut the risk of recurrence by 25% when used with standard hormone therapy, rather than hormone therapy alone, after traditional treatments.
It has already been approved by regulators, including in the UK and US, to treat breast cancer that has spread to other body parts. But the earlier-stage setting, when tumours can still be surgically removed, is seen as a much bigger breakthrough due to the huge numbers of patients it could help.
Breast cancer patients are typically offered surgery and chemotherapy or radiation treatment before taking hormone blocking drugs to try to stop the disease recurring.
Adding ribociclib to hormone therapy showed a “significant improvement” in disease-free survival times for patients with hormone receptor-positive, HER2-negative early-stage breast cancer, the study found.
Hormone receptor-positive, HER2-negative breast cancer is the most common subtype of the disease, making up nearly 70% of all breast cancer cases in the US.
“Currently, approved targeted treatments can only be used in a small population of patients diagnosed with hormone receptor-positive, HER2-negative early breast cancer, leaving many without an effective treatment option for reducing risk of the cancer returning,” said lead author Dr Dennis Slamon, the director of clinical and translational research at the UCLA Jonsson Comprehensive Cancer Center in Los Angeles.
About one-third of those with stage two hormone receptor-positive, HER2-negative disease experience a recurrence after standard treatment and more than half of people with stage three disease will see their cancer return, said Slamon.
“Thus, there is a significant unmet need for both reducing the risk of recurrence and providing a tolerable treatment option that keeps patients cancer-free without disrupting their daily life.”
The Natalee study involved 5,101 patients who were given either ribociclib for three years alongside five years of hormonal therapy or the hormonal therapy alone.
after newsletter promotion
After three years, 90.4% of those taking ribociclib remained free of disease, compared with 87.1% in the hormonal therapy alone group. Ribociclib also showed more favourable outcomes in overall survival, recurrence-free survival, and distant disease-free survival, according to the researchers.
“While early, these results are very promising and suggest that there will be a role for adjuvant ribociclib for stage two and higher hormone receptor-positive, HER2-negative breast cancer,” said Dr Rita Nanda, an Asco expert in Chicago, who was not involved with the study.
Dr Kotryna Temcinaite, the head of research communications at Breast Cancer Now, hailed the results as positive news for patients. “Researchers found that when combined with hormone therapy, ribociclib significantly reduced the chances of the disease returning in women with oestrogen receptor-positive, HER2-negative primary breast cancer.
“We know many women and their loved ones worry about breast cancer returning after treatment so new treatments like ribociclib, which can reduce this risk, are incredibly welcome.
“This treatment must now be swiftly submitted for licensing, and assessed for use on the NHS , so this group of primary breast cancer patients have the chance to benefit from it as soon as possible.”
Dr Catherine Elliott, director of research and partnerships at Cancer Research UK, said: “While more research is required, the initial early results from the ongoing Natalee trial are promising.
“The combination of ribociclib and hormonal therapy could provide a new treatment option for people with this type of early-stage breast cancer, reducing the risk of the disease coming back and improving survival.”
- Cancer research
- Breast cancer
- Medical research
Medics design AI tool to predict side-effects in breast cancer patients

Labour MP Foy says she has breast cancer and urges others to get checked
Cost of breast cancer to UK economy could reach £3.6bn by 2034, study shows
Consumer genetic test results ‘causing unnecessary breast cancer alarm’
Most early-stage breast cancer patients will be long-term survivors – study

Should I worry about the cancer risk from hormonal contraceptives?
Advising older patients against breast cancer surgery is ‘age bias’, UK study finds
Drug may help more women survive hereditary breast cancer

NHS England rolls out five-minute treatment for some breast cancers
Most viewed.
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Mechanics of breast cancer metastasis discovered, offering target for treatment
The most lethal feature of any cancer is metastasis, the spread of cancer cells throughout the body. New research, led by Penn State, reveals for the first time the mechanics behind how breast cancer cells may invade healthy tissues. The U.S. National Science Foundation -supported discovery, showing that a motor protein called dynein powers the movement of cancer cells in soft tissue models, offers new clinical targets against metastasis and has the potential to fundamentally change how cancer is treated.
"This discovery marks a paradigm shift in many ways," said Erdem Tabdanov, a pharmacologist at Penn State and a lead co-corresponding author on the study, published in the journal Advanced Science . "Until now, dynein has never been caught in the business of providing the mechanical force for cancer cell motility, which is their ability to move themselves. Now we can see that if you target dynein, you could effectively stop motility of those cells and, therefore, stop metastatic dissemination."
The researchers used live microscopy to watch the migration of breast cancer cells in two different systems modeled after the human body. The first system, a 2D network of collagen fibers, revealed how cancer cells move through an extracellular matrix that surrounds tumors and showed that dynein was key.
The second system was a 3D model developed by a team led by Amir Sheikhi, a chemical and biomedical engineer at Penn State. This system was designed to mimic soft tissue using a network of microscopic hydrogel particles or microgels linked together in tumor-like shapes. Like in the 2D model, the researchers found in the 3D model that dynein was "indispensable" in the spread or metastasis of cancer cells.
"Using these three-dimensional models that partially mimic a tumor, we discovered that if we block the dynein, the cancer cells cannot effectively move and infiltrate solid tissues," Sheikhi said. "In both models, we found that dynein is extremely important for cell locomotion, which suggests a whole new method for cancer management. Instead of killing the cancer cells with radiation or chemotherapy, we are showing how to paralyze them.
"This is great news because you don't really have to kill the cells, which is a harsh approach that targets both cancerous and healthy cells. Instead, you just must stop the cancer cells from moving."
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- ADVERTISEMENT FEATURE Advertiser retains sole responsibility for the content of this article
New studies shed light on breast cancer in young women
Produced by
Breast cancer in young women is not a different disease than in older counterparts, but it does present unique challenges for patients and providers Credit: Roger Harris/SPL/Getty Images
In 2016, 21-year-old Jessica Florence found a lump in her right breast. The architectural student at Florida A&M University soon found out that she had stage 3 breast cancer. Despite treatment, her disease later turned into stage 4 metastatic breast cancer. By age 28, the cancer had spread to Florence’s brain and spine.
Breast cancer is a devastating disease at any point in a patient’s life, but for patients under 40, it can present unique challenges.
“It’s not that we think breast cancer is a completely different disease in younger women,” says Ann Partridge, professor of medicine at Harvard Medical School, but it tends to be more aggressive and frequently goes undiagnosed for longer periods. Current screening for breast cancer suggests that women get their first mammogram at age 40 or even later. “So, most young women are not being screened for breast cancer,” Partridge says.
Perhaps most troubling, cases of breast cancer in women under 40 are on the rise , for reasons that remain unclear .
Historically, researchers have directed comparatively little effort to understanding this segment of breast cancer cases, and key questions remain partially unanswered. The impact of pathogenic variants in breast cancer predisposition genes on prognosis and the efficacy of anticancer therapies requires more research. So do survivorship issues, including fertility preservation strategies and the safety of pregnancy after treatment completion.
A series of new studies examines breast cancer in young women, and the results provide much needed insights into the nature of the disease, prognosis and treatments, and the safety of pregnancy.
Looking for molecular differences
To better understand the age-related specificities of breast cancer in young women, scientists first need to characterize the molecular biology of this disease. Although some of the genetic differences between breast cancer in younger and older patients are known, many are not.
Some of the most studied pathogenic variants in breast cancer predisposition genes occur in the BRCA1 and BRCA2 genes. One study found that women with a BRCA1 or BRCA2 variant face breast-cancer risks of 72% and 69%, respectively, and Partridge says, “The prevalence of having a BRCA1 or BRCA2 mutation is more common in our youngest patients.”
Still, BRCA pathogenic variants only appear in 10% to 15% of younger women with breast cancer. Partridge wondered which others might play a role.
In January, Partridge, Nikhil Wagle, a medical oncologist also at Harvard, and a research team published a study in which they applied whole genome sequencing to tumors from 93 breast-cancer patients under the age of 35. They compared the genomes to those from breast-cancer patients over 45, recorded in The Cancer Genome Atlas.
The results showed that younger women with breast cancer more frequently had mutations in two genes: GATA3 and ARID1A . Both of these genes play a role in tumour suppression, and mutations in them could allow the initiation and progression of breast cancer.
In other, previous studies, mutations in GATA3 and ARID1A show an association with cancer. Other studies point to potential treatments. For GATA3 mutations, for example, one study showed that the small molecule, milademetan could be a beneficial adjuvant treatment for these patients. For breast cancer patients with mutations in ARID1A , immune checkpoint inhibitors bear further investigation.
Testing more patients
Despite the rise in breast cancer in young women in the western world, these cases represent only 5% of total breast cancer diagnoses. That can make it difficult for researchers to gather enough data to draw clear conclusions.
To expand the search, Matteo Lambertini, associate professor at the University of Genova, in Italy, and consultant in medical oncology at IRCCS Policlinico San Martino Hospital, and his colleagues worked with 30 healthcare centres around the world to study the results of breast cancer treatment in patients under the age of 40 with a BRCA pathogenic variant. The researchers also collected data on a patient’s hormone-receptor status, positive or negative, which indicates if their cancer can be stimulated by estrogen or progesterone.
Lambertini and his colleagues recently reported their findings from 1,236 female subjects in npj Breast Cancer . The results showed that a recurrence of breast or another form of cancer—usually ovarian—was more likely in patients with a BRCA1 pathogenic variant. Also, patients with hormone-receptor positive disease were more likely to experience a recurrence, and they did not have better prognosis, compared to those with hormone receptor-negative breast cancer.
Based on such findings, “We need to pay more attention to secondary tumours, particularly in the BRCA1 patient population,” Lambertini says. “We may need to counsel a BRCA1 patient more aggressively on the need for preventive measures including risk-reducing strategies.”
Focusing on fertility
While a breast cancer diagnosis will upend anyone’s life, the issues that accompany diagnoses in younger patients can be particularly daunting. “Fertility is particularly pertinent to our youngest patients with breast cancer,” Partridge says.
For those who planned to have children, learning that some oncologists advise against pregnancy after breast cancer treatment can be nearly unbearable.
Young women with a BRCA pathogenic variant may be less fertile.Lambertini and his colleagues also found that fewer oocytes are collected after ovarian stimulation than in a comparable population of breast cancer patients without BRCA pathogenic variants.
But a lot more research remains. Lambertini, Partridge, and a team of researchers recently studied more t han 1,250 young women with breast cancer and a germline BRCA pathogenic variant. They found that pregnancy had no impact on a woman’s likelihood of recurrence and no increased concern of birth defects.
As work continues, Partridge sees some cause for optimism both about the issue of fertility andsurvival. “We know that most young women who get breast cancer will survive,” she says. “We also know that women with a BRCA1 or BRCA2 mutation do just as well as women who don’t have that, as long as they get appropriate therapy.”
To learn more about how the Breast Cancer Research Foundation is supporting research into breast cancer in younger patients, visit www.bcrf.org
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Português Br
- Journalist Pass
New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Kelley Luckstein
Share this:

ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.
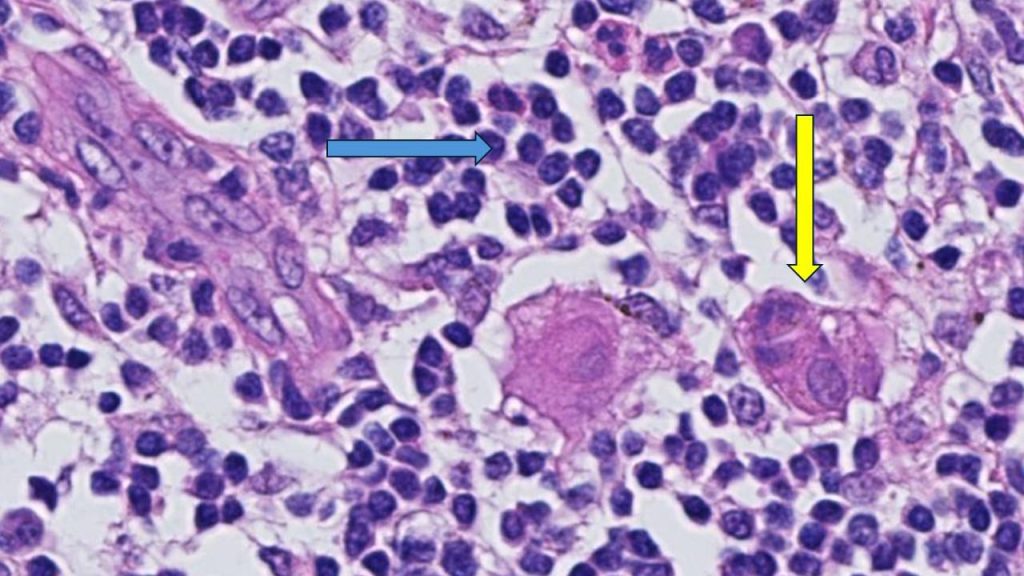
"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."
"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group, led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors taken into consideration to determine the course of chemotherapy treatment for each person are the tumor size and the presence of lymph node metastases. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
About Mayo Clinic Comprehensive Cancer Center Designated as a comprehensive cancer center by the National Cancer Institute , Mayo Clinic Comprehensive Cancer Center is defining new boundaries in possibility, focusing on patient-centered care, developing novel treatments, training future generations of cancer experts and bringing cancer research to communities. At Mayo Clinic Comprehensive Cancer Center, a culture of innovation and collaboration is driving research breakthroughs that are changing approaches to cancer prevention, screening and treatment, and improving the lives of cancer survivors.
About Mayo Clinic Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
About Gustave Roussy Ranked as the leading French and European Cancer Centre and fourth in the world, Gustave Roussy is a centre with comprehensive expertise and is devoted entirely to patients suffering with cancer. The Institute is a founding member of the Paris Saclay Cancer Cluster. It is a source of diagnostic and therapeutic advances. It caters for almost 50,000 patients per year and its approach is one that integrates research, patient care and teaching. It is specialized in the treatment of rare cancers and complex tumors and it treats all cancers in patients of any age. Its care is personalized and combines the most advanced medical methods with an appreciation of the patient’s human requirements. In addition to the quality of treatment offered, the physical, psychological and social aspects of the patient’s life are respected. 4,100 professionals work on its two campuses: Villejuif and Chevilly-Larue. Gustave Roussy brings together the skills, which are essential for the highest quality research in oncology: 40% of patients treated are included in clinical studies. For further information: www.gustaveroussy.fr/en , Twitter , Facebook , LinkedIn , Instagram
Media contact:
- Kelley Luckstein, Mayo Clinic Communications, [email protected]
- CAR-T cell therapy helps man continue community advocacy Protect those eyes on the sky
Related Articles
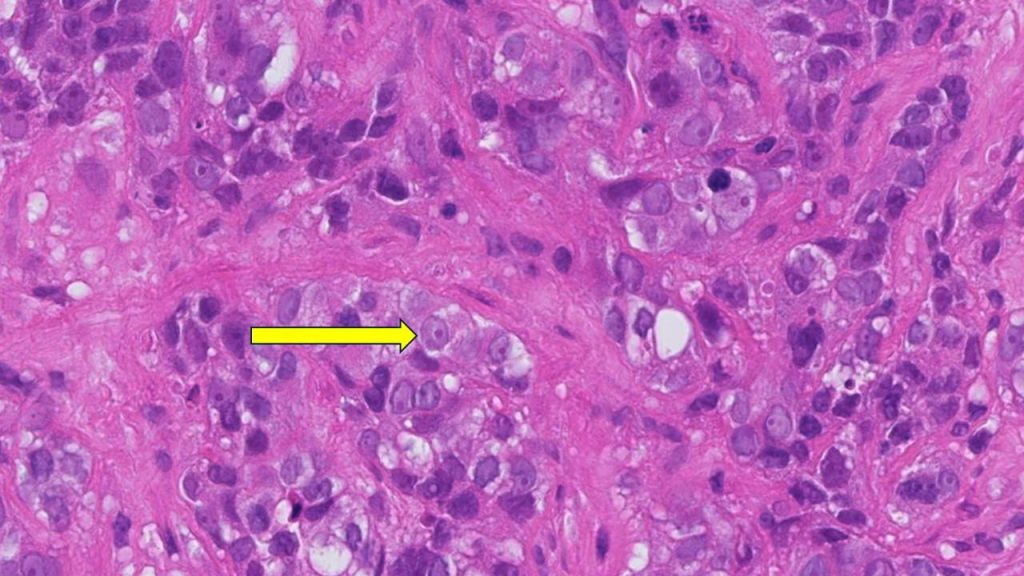
Clinical Trials
Breast cancer.
Displaying 356 studies
The purpose of this study is to show that improvements in the molecular breast imaging (MBI) technology will allow reduction of the administered dose of Tc-99m sestamibi while maintaining a sensitivity of 90% for tumor detection.
The purpose of this study is to determine whether treatment with alpelisib plus fulvestrant will lengthen progression-free survival compared to fulvestrant and a placebo in men and postmenopausal women who have hormone receptor positive (HR+), HER2-negative advanced breast cancer.
This study aims to compare an additional support program (text message reminders and/or telephone-based counseling) with usual care in making sure breast cancer patients take their endocrine therapy medication as prescribed (medication adherence).
This randomized phase II trial studies how well carboplatin and paclitaxel with or without atezolizumab before surgery works in treating patients with newly diagnosed, stage II-III triple negative breast cancer. Monoclonal antibodies, such as atezolizumab, may block tumor growth in different ways by targeting certain cells. Drugs used in chemotherapy, such as carboplatin and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving carboplatin and paclitaxel with or without atezolizumab before surgery may make the tumor smaller and reduce the ...
The purpose of this study is to identify subtype-specific signatures for breast cancer using genomic positioning of plasma DNA fragments, and to validate changes in ctDNA levels as a biomarker for treatment monitoring in patients with metastatic breast cancer.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate, goserelin acetate, leuprolide acetate, anastrozole, letrozole, or exemestane, may fight breast cancer by lowering the amount of estrogen the body makes. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet know whether hormone therapy is more effective when given with or without everolimus in treating breast cancer.
PURPOSE: This randomized phase III trial studies how well giving hormone therapy together with or without everolimus work in treating patients with breast cancer.
The purpose of this study is to further advance the ability to practice personalized medicine by learning which new drug agents are most effective with which types of breast cancer tumors and by learning more about which early indicators of response (tumor analysis prior to surgery via magnetic resonance imaging (MRI) images along with tissue and blood samples) are predictors of treatment success.
The purpose of this study is to to evaluate the integration of cancer genetic testing into a mammography practice aimed toward women at intermediate- to-high lifetime risk of breast cancer. If successful, this will provide an opportunity for cancer risk stratification and individualized screening.
The primary purpose of this study is to evaluate the incremental invasive cancer yield in patients with a negative mammogram who are intermediate or high-risk for breast cancer and get supplemental screening with Contrast-Enhanced Digital Mammography (CEDM).
RATIONALE: Estrogen can stimulate the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by reducing the production of estrogen.
PURPOSE: This randomized phase III trial is studying letrozole to see how well it works in treating women with breast cancer who have received tamoxifen for at least 5 years.
The goal of this clinical research study is to learn how often breast cancer recurs (returns after treatment) in the breast in patients who have been treated with chemotherapy and have had follow-up radiation therapy (but not surgery) and are in complete remission (no evidence of disease). This is an investigational study. Radiation therapy is delivered using FDA-approved and commercially available methods. The study doctor can explain how radiation therapy is designed to work. About 120 participants will be enrolled on this multicenter study. Up to 90 may take part at MD Anderson.
The purpose of this registry is to collect and maintain samples of breast tissue from women and men undergoing surgery for a breast related concern at Mayo Clinic Rochester, to create a biospecimen resource for the study of benign and cancerous breast conditions.
This randomized phase III trial studies how well aspirin works in preventing the cancer from coming back (recurrence) in patients with human epidermal growth factor receptor 2 (HER2) breast cancer after chemotherapy, surgery, and/or radiation therapy. Aspirin is a drug that reduces pain, fever, inflammation, and blood clotting. It is also being studied in cancer prevention. Giving aspirin may reduce the rate of cancer recurrence in patients with breast cancer.
The purpose of this study is to determine the recommended Phase 2 dose twice weekly of berzosertib administered concurrently with conventionally fractionated radiation therapy to the breast/chest wall and regional nodes.
This is a 3 arm Phase 3 study to evaluate the safety and efficacy of the addition of veliparib plus carboplatin versus the addition of carboplatin to standard neoadjuvant chemotherapy versus standard neoadjuvant chemotherapy in subjects with early stage TNBC.
The primary purpose of this study is to examine the effects of abemaciclib on the CD8/FOXP3 ratio in chemotherapy-resistant triple negative breast cancer (TNBC) patients following neoadjuvant chemotherapy.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug and giving them in different ways after surgery may kill more tumor cells. It is not yet known which chemotherapy regimen is more effective in treating older women with breast cancer. PURPOSE: This randomized phase III trial is studying different combination chemotherapy regimens to see how well they work in treating older women who have undergone surgery for breast cancer.
The purpose of this study is designed to evaluate the effectiveness and safety of AL101 monotherapy in subjects with Notch-activated recurrent or metastatic Triple Negative Breast Cancer (TNBC); Notch activation will be determined by a Next Generation Sequencing (NGS) test.
This study will evaluate the efficacy, safety and tolerability of trastuzumab deruxtecan compared with investigator's choice chemotherapy in human epidermal growth factor receptor (HER)2-low, hormone receptor (HR) positive breast cancer patients whose disease has progressed on endocrine therapy in the metastatic setting.
The purpose of this study is to evaluate an investigational drug as a possible treatment for breast cancer that is positive for the protein Human Epidermal Growth Factor Receptor 2, also known as HER2-positive breast cancer. The drug involved in this study is: -ado-trastuzumab emtansine (T-DM1)
RATIONALE: Drugs used in chemotherapy, such as doxorubicin, cyclophosphamide, and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Monoclonal antibodies, such as trastuzumab, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry tumor-killing substances to them. Lapatinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving combination chemotherapy together with trastuzumab and lapatinib after surgery may kill ...
This randomized phase III trial studies combination chemotherapy and trastuzumab to see how well they work compared to combination chemotherapy alone in treating women with breast cancer. Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Monoclonal antibodies such as trastuzumab can locate tumor cells and either kill them or deliver tumor-killing substances to them without harming normal cells. It is not yet known whether combination chemotherapy is more effective with or without trastuzumab in treating breast cancer.
This study is an open label, randomized, multicenter trial of MM-302 plus trastuzumab. The trial is designed to demonstrate whether MM-302 plus trastuzumab is more effective than the chemotherapy of physician's choice (CPC) plus trastuzumab in locally advanced/metastatic HER2-positive breast cancer patients. Patients may not have been previously treated with an anthracycline in any setting. Patients must have received prior treatment with trastuzumab in any setting, have either progressed or are intolerant to ado-trastuzumab emtansine in the metastatic or locally advanced setting, have either progressed or are intolerant to pertuzumab in the metastatic or locally advanced setting or had disease ...
The purpose of this study is to compare the safety and efficacy of nab-paclitaxel in combination with either gemcitabine or carboplatin to the combination of gemcitabine and carboplatin as first line treatment in female subjects with triple negative metastatic breast cancer (TNMBC) or metastatic triple negative breast cancer.
The purpose of this study is to compare the progression-free survival (PFS) between sacituzumab govitecan-hziy (SG) and pembrolizumab versus treatment of physician's choice (TPC) and pembrolizumab in participants with previously untreated, locally advanced inoperable or metastatic triple-negative breast cancer, whose tumors express programmed cell death ligand 1 (PD-L1).
The primary purpose of the Safety-Run-in Cohort 1 of this study is to evaluate the safety, tolerability, and recommended Phase 2 dose of magrolimab in combination with nab-paclitaxel or paclitaxel. In Phase 2 Cohort 1, the study will compare the efficacy of magrolimab in combination with nab-paclitaxel or paclitaxel versus nab-paclitaxel or paclitaxel alone as determined by progression-free survival (PFS) by investigator assessment
The primary purpose of the Safety-Run-in Cohort 2 of this study is to evaluate the safety, tolerability, and recommended Phase 2 dose of magrolimab in combination with sacituzumab govitecan. In Cohort 2 (Safety Run-in Cohort 2 and Phase 2 Cohort 2) the study will evaluate the efficacy of magrolimab in combination ...
This randomized phase II trial studies how well cisplatin works with or without veliparib in treating patients with triple-negative breast cancer and/or BRCA mutation-associated breast cancer that has come back or has or has not spread to the brain. Drugs used in chemotherapy, such as cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Veliparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known if cisplatin is more ...
The purpose of this trial is to determine how well estradiol works in treating patients with estrogen receptor beta (ER beta) positive, triple negative breast cancer that has spread to nearby tissue or lymph nodes (locally advanced) or other places in the body (metastatic). Hormone receptors like ER beta allow the body to respond appropriately to hormones. Triple negative means that the breast cancer does not express other hormone receptors called ER alpha, progesterone, and HER2. In some people with triple negative breast cancer, ER beta is overexpressed. Tumor cells that overexpress ER beta grow slower in the laboratory and ...
The purpose of this study is to determine the correlation between HER2 specific T-cell response in HER2-positive breast cancer patients with stage I-IV who receive anti-HER2 therapies, such as trastuzumab, pertuzumab, lapatinib, or neratinib and clinical responses.
This partially randomized phase Ib/II trial studies the side effects and best dose of taselisib when given together with enzalutamide and to see how well they work in treating patients with androgen receptor positive triple-negative breast cancer that has spread to other places in the body. Taselisib is a PI3K inhibitor. The PI3K pathway is involved is cancer growth. Androgen may cause the growth of tumor cells. Enzalutamide may stop the growth of tumor cells by blocking the androgen receptor from working. Giving taselisib with enzalutamide may be a better treatment for patients with breast cancer.
This randomized phase II trial studies how well olaparib and atezolizumab work either alone or in combination in treating patients with stage III-IV triple negative breast cancer. Olaparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as atezolizumab, may interfere with the ability of tumor cells to grow and spread. It is not known whether giving olaparib and atezolizumab either alone or in combination would work better in patients with triple negative breast cancer.
The purpose of this study is to evaluate if changes made to the design of the table used specifically for breast Positron Emission Mammography (PEM), will help improve the images of breast tissue close to the chest wall.
This study will examine the safety and effectiveness of pertuzumab combined with high-dose trastuzumab in adult patients who have HER2-positive breast cancer that has spread to the central nervous system and the brain following radiation therapy.
The purpose of this study is to evaluate T cell and antibody immunity in patients with HER2+ breast cancer who will receive trastuzumab with standard chemotherapy in order to address whether the immunity is associated with the patient’s response to treatment.
The purpose of this study is to evaluate a new treatment using a potent Poly polymerase (PARP) inhibitor known as Niraparib. Niraparib will be combined with trastuzumab, a HER2-targeted agent, to evaluate the safety and tolerability in patients with metastatic HER2 positive breast cancer. It is anticipated that the combination of drugs will improve survival and have few side effects.
The human epidermal growth factor receptor 2 (HER2) regulates cell growth and survival. Approximately 15-20% of all breast cancers are HER2-positive, which are an aggressive and fast-growing subtype of breast cancer.
The purpose of this study is to determine if tucatinib works better than placebo when given with other drugs to treat participants with HER2-positive breast cancer. A placebo is a pill that looks the same as tucatinib but has no medicine in it. This study will also test what side effects happen when participants take this combination of drugs. A side effect is anything a drug does to the body besides treating your disease. Participants will have cancer that has spread in the body near where it started (locally advanced) and cannot be removed (unresectable) or has spread through the body ...
The purpose of this study is to examine the effectiveness and safety of pembrolizumab as first line or above treatment in patients with metastatic triple-negative breast cancer.
The primary purposes of this study are to is to assess safety and tolerability of IV administration of TVH vaccine alone and in combination with HER2 antibodies in patients with advanced cancer.
The purpose of this study is to determine if the invasive disease-free survival (iDFS) with T-DM1 and tucatinib is superior to the iDFS in the control arm (T-DM1 + placebo) when administered to high risk patients with HER2-positive breast cancer and residual disease after neoadjuvant HER2-directed therapy.
The purpose of this study is to evaluate combining endocrine therapy with CDK4/6 inhibition along with trastuzumab in ER+/ human epidermal growth factor receptor 2 (HER2)+ early stage breast cancer in order to influence estrogen receptor (ER) signaling.
This randomized, double-blind, placebo-controlled, two-arm study will assess the safety and efficacy of pertuzumab in addition to chemotherapy plus Herceptin (t rastuzumab) as adjuvant therapy in patients with operable HER2-positive primary breast cancer. After surgery, patients will be randomized to receive either pertuzumab or placebo intravenously (iv) every 3 weeks for one year, in addition to 6-8 cycles of chemotherapy and 1 year of Herceptin (trastuzumab) iv every 3 wee ks. Anticipated time on study treatment is 52 weeks. This study will be carried out in collaboration with the Breast International Group (BIG).
This research is being done to determine if early changes on a type of imaging procedure called PET (Positron Emission Tomography) can predict which patients are most likely to respond to the combination of trastuzumab and pertuzumab when given prior to surgery.
The primary objective of this study is to compare progression-free survival (PFS) in patients with advanced HER2-positive breast cancer treated with T-DM1 and abemaciclib vs. T-DM1 monotherapy.
This phase II trial studies how well FASN inhibitor TVB-2640 and trastuzumab plus either paclitaxel or endocrine therapy with an aromatase inhibitor work in treating patients with HER2 positive breast cancer that has spread to other places in the body. FASN inhibitor TVB-2640 may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Drugs used in chemotherapy, such as paclitaxel and trastuzumab, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Endcocrine therapy helps reduce ...
The objective of this study is to provide preliminary data to support the development of selected technologies for the efficient and reliable analyses of cell free DNA (cfDNA) and circulating tumor cells (CTCs) in the setting of metastatic breast cancer.
This research study is a Phase II clinical trial. Phase II clinical trials test the effectiveness of an investigational drug to learn whether the drug works in treating a specific cancer. "Investigational" means that the drug is still being studied and that research doctors are trying to find out more about it-such as the safest dose to use, the side effects it may cause, and if the drug is effective for treating different types of cancer. It also means that the FDA has not approved this drug for use patients undergoing adjuvant treatment for HER2+ breast cancer. Trastuzumab emtansine (T-DM1) ...
The purpose of this study is to look at the safety and immune response to a vaccine used in patients previously treated for HER2 (human epidermal growth factor receptor 2) positive breast cancer.
The purpose of this study is to evaluate how well paclitaxel, trastuzumab, and pertuzumab with or without atezolizumab works in treating patients with breast cancer that has spread to other parts of the body. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Immunotherapy with trastuzumab, pertuzumab, and atezolizumab, may induce changes in body's immune system and may interfere with the ability of tumor cells to grow and spread. It is not yet known whether giving ...
The objective of this study is to assess efficacy and safety of radium-223 dichloride in subjects with human epidermal growth factor receptor 2 negative (HER2 negative) hormone receptor positive breast cancer with bone metastases treated with hormonal treatment background therapy.
The no show rate for mammography screening is high among Navajo women. One barrier to preventive screening is a lack of cancer literacy including low knowledge and cultural attitudes (e.g., fatalism) about screening. The investigators will examine the potential feasibility and acceptability of a cancer literacy intervention for families of Navajo women who have no showed for three consecutive times to mammography screening who have never or rarely been screened in the past.
The purpose of this study is to evaluate the safety and effectiveness of the study drug abemaciclib in participants with high risk, node positive, early stage, hormone receptor positive (HR+), human epidermal receptor 2 negative (HER2-), breast cancer.
The purpose of this study is to evaluate invasive disease-free survival (iDFS) of multi-epitope HER2 vaccine vs. placebo in combination with ado-trastuzumab emtansine (TTT-DM1) in patients with stage II-III HER2+ breast cancer with residual disease post-neoadjuvant chemotherapy, and to evaluate the safety of multi-epitope HER2 vaccine given concurrently with T-DM1 maintenance therapy.
The purpose of this study is to assess the effectiveness and safety of pembrolizumab (MK-3475) versus placebo in combination with neoadjuvant (pre-surgery) chemotherapy and adjuvant (post-surgery) endocrine therapy in the treatment of adults who have high-risk early-stage estrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER+/HER2-) breast cancer.
This trial studies how well the drug tucatinib works when given with trastuzumab deruxtecan. It will also look at what side effects happen when these drugs are given together. A side effect is anything a drug does besides treating cancer. Participants in this trial have HER2-positive (HER2+) breast cancer that has either spread to other parts of the body (metastatic) or cannot be removed completely with surgery (unresectable). All participants will get both tucatinib and trastuzumab deruxtecan.
This phase II trial studies how well trastuzumab emtansine works in treating older patients with human epidermal growth factor receptor 2 (HER2)-positive stage I-III breast cancer. HER2 is a protein found on the surface of cancer cells that helps them to grow and spread. Trastuzumab emtansine may kill cancer cells by binding to HER2-positive on the surface of the tumor cells and blocking their ability grow and spread.
This phase II trial studies how well rifaximin works for the treatment of gastrointestinal toxicities related to pertuzumab-based therapy in patients with stage I-III HER2 positive breast cancer. Rifaximin may reduce the incidence and severity of pertuzumab induced gastrointestinal toxicities without interrupting or delaying the chemotherapy schedule.
The purpose of this study is to identify a (Z)-endoxifen dose that achieves (Z)-endoxifen steady-state plasma concentrations (Css) between 500-1000 ng/mL. Dosing will begin with the (Z)-endoxifen 40 mg/day dose and may additionally explore either a lower (20 mg/day) or higher (80 mg/day) dose level based on (Z)-endoxifen Css as well as toxicity.
The purpose of this study is to collect user and subject feedback on the design, use and operation of Affirm® Contrast Biopsy.
Utilizing CellSearch® technology, the ability to both enumerate and reliably and reproducibly characterize circulating tumor cells (CTC) for tumor markers that predict endocrine sensitivity (estrogen receptor [ER] and Bcl-2) and resistance (HER2 and Ki67) has been demonstrated. An algorithm for a CTC-Endocrine Therapy Index (CTC-ETI) has been constructed that can be calculated for each patient using the CTC enumeration and marker results. The primary goal of this study is to determine a CTC-ETI in ER positive, HER2 negative metastatic breast cancer patients before the initiation of a new endocrine therapy for the identification of patients that will progress rapidly.
The purpose of this retrospective study is to evaluate the complication rate of prophylactic open NSM procedures through 42 days follow-up from retrospective chart review at the same investigators and institutions as those included under IDE Study protocol G190065/A001.
Olaparib treatment in patients with germline BRCA1/2 mutations and high risk HER2 negative primary breast cancer who have completed definitive local treatment and neoadjuvant or adjuvant chemotherapy
The purpose of this study is to study to collect tissue samples from patients with early stage hormone receptor-positive HER2-negative breast cancer.
The objective of this study is to determine if the diagnostic performance of a dedicated breast-specific positron emission mammography (PEM) system, is superior to that obtained with a conventional PET/CT scanner and capable of producing acceptable image quality at a low-radiation dose level.
The purpose of this research study is to better understand the reasons why or why not breast cancers are destroyed by standard chemotherapy. This information will be used to develop new and better cancer therapies.
This is an open label phase II study to determine the safety and efficacy of a novel 3 fraction daily dosing regimen for accelerated partial breast irradiation (APBI) for early invasive and noninvasive breast cancer. The three techniques utilized are recognized as standard options for the delivery of APBI, and there is no evidence that either technique is superior or inferior to any other. The APBI technique utilized will be at the physician's discretion and will be based on technical considerations, availability at the treating radiation facility, insurance coverage, as well as patient preference.
The purpose of this study is to compare three different combination chemotherapy regimens and evaluate how well they work in treating women who have undergone surgery for node-positive breast cancer. Drugs used in chemotherapy, such as docetaxel, doxorubicin, cyclophosphamide, paclitaxel, and gemcitabine work in different ways to stop tumor cells from dividing so they stop growing or die. Giving combination chemotherapy after surgery may kill any remaining tumor cells.
The purpose of this study to assess the effectiveness of proton vs. photon therapy in reducing major cardiovascular events (MCE), defined as atherosclerotic coronary heart disease or other heart disease death, myocardial infarction, coronary revascularization, or hospitalization for major cardiovascular event (heart failure, valvular disease, arrhythmia, or unstable angina or other major cardiovascular event) in patients with locally-advanced breast cancer.
The purpose of the study is to determine whether treatment with a PI3K inhibitor plus letrozole leads to an increase in pathologic clinical response and Objective Response Rate compared to treatment with placebo plus letrozole in patients with Breast cancer
The purpse of this study is to evaluate the safety and tolerability of the lasofoxifene and abemaciclib combination for the treatment of pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer who have disease progression on first and/or 2nd lines of hormonal treatment for metastatic disease and have an ESR1 mutation.
The purpose of this study is to collect (i) pre-treatment (at the time of breast cancer surgery) normal skin and (ii) on-treatment (around the third week of treatment with breast radiation) irradiated skin with clinical hallmarks of radiation dermatitis (erythema and/or dry desquamation, documented by a photograph and graded by clinical criteria).
The purpose of this study is to determine the usefulness and effectiveness of Muse, a brain sensing headband intervention, to affect quality of life, stress, fatigue and sleep in newly diagnosed breast cancer patients who are undergoing surgical treatment.
The primary objective of this study is to determine the correlation between the distribution of F-18 FES within ER+ breast tumors as seen on Positron Emission Mammography (PEM) images of the breast, and the distribution of cells stained ER+ within the tumor by immunohistochemistry (IHC) measurements at surgical pathology. The secondary aim is to determine if the correlation (or lack of) between F-18 FES uptake and F-18 FDG uptake as imaged by PEM, is an accurate representation of the heterogeneity of ER expression in the tumor.
The primary purposes of this trial are to determine the safety and tolerability of RBX7455 given for at least 2 weeks and not more than 4 weeks prior to surgery, and to evaluate intratumoral immune system resonse, including TILs, CD4, and CD8 T cells, in operable breast cancer patients.
This is a combination Phase I and Phase II study, with an aim to evaluate the combination of GSK525762 and fulvestrant in women with advanced or metastatic ER+ breast cancer, who have disease that has progressed after prior treatment with at least one line of endocrine therapy. The objectives of the study are to first identify, in open-label single-arm Phase I, a recommended Phase II dose of GSK525762 that may be combined safely with fulvestrant. Phase I will follow a modified toxicity probability interval (mTPI) design, and a sentinel group will be evaluated first for dose-limiting toxicity and further expanded ...
This is a phase 1b/2 study of the safety and efficacy of MLN0128 in combination with exemestane or fulvestrant therapy in women with estrogen receptor positive/human epidermal growth factor receptor 2 negative (ER+/HER2-) advanced or metastatic breast cancer that has progressed on treatment with everolimus in combination with exemestane or fulvestrant.
The treatment for postmenopausal women diagnosed with hormone receptor positive (HR+) metastatic breast cancer (mBr) includes endocrine therapy However, de novo or acquired resistance to endocrine therapy remains an important clinical problem. Many hormone receptor positive breast cancers demonstrate overexpression of cyclin D. Cyclin D interacts directly with cyclin-dependent kinases 4 and 6 (CDK4/6) in an active protein complex that promotes cell proliferation; and consequently, CDK4/6 represents a potential therapeutic target for HR+ breast cancers. LY2835219 represents a selective and potent small molecule inhibitor of CDK4/6. LY2835219 demonstrates suitable physical and pharmacokinetic (PK) properties, an acceptable toxicity profile in ...
RATIONALE: It is not yet know whether higher per daily radiation therapy is equally as effective as standard per daily radiation therapy in treating breast cancer.
PURPOSE: This randomized phase III trial studies how well an accelerated course of higher per daily radiation therapy with concomitant boost works compared to standard per daily radiation therapy with a sequential boost in treating patients with early-stage breast cancer that was removed by surgery.
In the light of the pandemic, institutions have had to take greater precautions and instigate procedures to aim to improve safety and reduce risk for patients undergoing surgery. One intiative was designed to implement a same day discharge for patients undergoing mastectomy with or without alloplastic reconstruction. This study aims to evaluate the outcomes and patient satisfaction with same day mastectomy with or without alloplastic reconstruction following COVID-19 and compare satisfaction and outcomes (e.g complications) with patients pre-COVID 19. This is part of a quality improvement project.
The purpose of this study is to see if having different kinds of bacteria genes in breast tissue may be connected to the risk of getting breast cancer.
The purpose of this study is to understand the causes of breast cancer, in particular the connection between genetic variations and breast density or how tissue is distributed on a mammogram.
The purpose of this study is to evaluate the efficacy of venetoclax in combination with fulvestrant compared with fulvestrant alone for HER2-negative breast cancer.
The study is intended to show superiority of AZD9833 in combination with CDK4/6 inhibitor (palbociclib, abemaciclib or ribociclib) versus aromatase inhibitors (anastrozole or letrozole) in combination with CDK4/6 inhibitor in patients with hormone receptor-positive (HR-positive), human epidermal growth factor receptor 2-negative (HER2-negative) metastatic breast cancer with detectable ESR1 mutation.
The investigators hypothesize that knowledge of the functional behavior of areas of mammographic density will enable more specific identification of dense tissue at-risk for breast cancer, ultimately providing predictive information on an individual's risk of developing breast cancer.
The purpose of this study is to assess whether elevated fasting plasma proneurotensin levels are common in patients genetically predisposed to breast cancer.
This pilot study involves very frequent monitoring of breast cancer patient blood levels of hs-cTnT Troponin and n-t-BNP (Brain Natriuretic Peptide) before and after initiation of chemotherapy with either adriamycin or trastuzumab in order to define the kinetics of both biomarkers during the first two cycles of chemotherapy. Cardiac troponins and BNP are frequently elevated after experimental chemotherapy in animal models. Their behavior in humans has been inconsistent, with occasional elevations seen, usually within 30 days of therapy. Assays for troponin with sensitivity into the pg/ml range have now been introduced. A majority of patients greater than age 50 have ...
The purpose of this trial is to assess the safety and effectiveness of the combination of dapagliflozin plus metformin extended release (XR) compared with metformin XR during treatment with alpelisib plus fulvestrant in participants with HR-positive, HER2-negative advanced breast cancer with a PIK3CA mutation following progression on or after endocrine-based therapy.
This study will use proteomic and genomic profiling to analyze tumor tissue to see if treatment selected by this analysis will benefit patients.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. It is not yet known which regimen of chemotherapy is more effective for breast cancer.
PURPOSE: Randomized phase III trial to compare the effectiveness of two different regimens of combination chemotherapy in treating women who have stage II or stage IIIA breast cancer that has spread to the lymph nodes.
RATIONALE: Testosterone may help relieve moderate or severe arthralgia associated with the use of aromatase inhibitors, such as anastrozole or letrozole.
PURPOSE: This randomized phase III trial studies testosterone to see how well it works compared to placebo in treating postmenopausal patients with arthralgia caused by anastrozole or letrozole.
Researchers at Mayo Clinic are developing a Biobank of adult stem cell-rich breast organoids, a new research resource to facilitate normal and cancer stem cell research. Subjects in the Biobank will provide samples of excess breast tissue, complete a health questionnaire, and allow access to medical records now and in the future. The Biobank serves as a library for researchers; instead of having to look for volunteers for each new project, researchers can use samples from the Biobank as well as share information already collected.
This randomized phase III trial studies standard or comprehensive radiation therapy in treating patients with early-stage breast cancer who have undergone surgery. Radiation therapy uses high-energy x rays to kill tumor cells. It is not yet known whether comprehensive radiation therapy is more effective than standard radiation therapy in treating patients with breast cancer.
The purpose of this study is to recruit 2000 incident cases of primary breast cancer in order to perform laboratory assays to measure frequencies of genetic polymorphisms for genes that encode enzymes involved in candidate gene pathways, including: estrogen and catecholestrogen formation, bioactivation and inactivation, cellular proliferation and apoptosis, nuclear factor kappa-beta; to compare genotype frequencies for polymorphisms of genes in breast cancer cases and controls, and to evaluate possible interactions among common polymorphisms in candidate genes.
This is an open-label, single-arm pilot study evaluating the antitumor activity and safety of niraparib as neoadjuvant therapy in patients with HER2 negative and BRCAmut localized breast cancer (primary tumor >1 cm).
The purpose of this study is to measure DEL-1 levels and develop and validate methods for measurement of the exosomal T-RNAs( miR 1274b and mirR 720) as well as four miRs ( miR 21, let-7a, miR 125b and miR 100) in pre and post tumor resection plasma samples from women with newly diagnosed localized breast cancer.
The purpose of this study is to compare the effects on low-risk breast cancer receiving usual care that includes regional radiation therapy, with receiving no regional radiation therapy. Researchers want to see if not giving this type of radiation treatment works as well at preventing breast cancer from coming back.
This randomized phase II trial studies how well tamoxifen citrate works compared with z-endoxifen hydrochloride in treating patients with breast cancer that has spread to nearby tissue or lymph nodes or other parts of the body and has estrogen receptors but not human epidermal growth factor receptor 2 (HER2) receptors on the surface of its cells. Estrogen can cause the growth of tumor cells. Hormone therapy using tamoxifen citrate or z-endoxifen hydrochloride may fight breast cancer by lowering the amount of estrogen the body makes. It is not yet known whether tamoxifen citrate or z-endoxifen hydrochloride is more effective in ...
The purpose of this study is to obtain additional high resolution images of your breast cancer using a Positron Emission Mammography (PEM) system. This system only allows us to image the breast, but provides higher quality and better resolution than those images we obtain with the PET/CT scanner.
The purpose of this study is to determine the feasibility and the effect of a wellness coaching intervention (WCI) on quality of life, weight, and healthy lifestyle in overweight breast cancer survivors.
The major purpose of this study is to evaluate a laboratory developed test that measures multiple breast cancer-specific biomarker proteins and multiple antibodies in your blood samples. The biomarker and antibody results along with your personal medical profile will be evaluated to determine your risk for the presence of a malignancy in the breast as compared to your breast evaluation assessment conducted by your physician.
This phase I trial studies the side effects and best dose of alisertib when given together with fulvestrant in treating patients with hormone positive breast cancer that has spread to other parts of the body or is locally advanced and cannot be removed by surgery. Alisertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Estrogen and progesterone are type of hormones made by the body and they can cause the growth of breast cancer cells. Hormone therapy using fulvestrant may fight breast cancer by lowering the amount of estrogen or progesterone ...
The purpose of this study is to evaluate a low-cost Contrast Enhanced Digital Mammogram (CEDM) protocol as a supplemental screening method to standard mammographic screening in women at intermediate lifetime-risk (and not undergoing annual MR surveillance) for breast cancer.
The purpose of this study is to evaluate the safety and effectiveness of the da Vinci Surgical Systems in Nipple Sparing Mastectomy procedures.
The aim of this study is to use the combine clinical risk assessment models that are already used in routine clinical practice with information derived from polygenic risk score (PRS) testing in women of racial minorities to see if this can improve adherence to recommended breast cancer screening and prevention strategies.
The purposes of this study are (i) to obtain and study biospecimens from patients with breast cancer that has either spread out of the breast or recurred after initial treatment(s), such as surgery, chemotherapy, and/or radiation, and (ii) to collect information about patients, treatments, and the behavior of the underlying cancer. Research involving biospecimens that are linked to related medical information is one way to learn more about diseases. In this case, we seek to understand the mechanism of tumor spread and determine why people respond differently to specific cancer treatments. In general terms, scientists will study the cells, DNA, ...
Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate may fight breast cancer by blocking the use of estrogen by the tumor cells
This phase IIb trial studies how well low-dose tamoxifen citrate works in reducing breast cancer risk in radiation-induced cancer survivors.
The purpose of this study is to determine whether a supervised exercise-training program, initiated prior to chemotherapy induction (pre-conditioning) and continued throughout chemotherapy treatment, can preserve short- and long-term cardiovascular performance, skeletal muscle function, cognitive ability and quality of life better than current standard or care recommendations for exercise during chemotherapy.
The objective of this study is to assess efficacy and safety of radium 223 dichloride in subjects with human epidermal growth factor receptor 2 (HER2) negative hormone receptor positive breast cancer with bone metastases treated with exemestane and everolimus.
This trial studies how well paclitaxel, trastuzumab, and pertuzumab work in eliminating further chemotherapy after surgery in patients with HER2-positive stage II-IIIa breast cancer who have no cancer remaining at surgery (either in the breast or underarm lymph nodes) after pre-operative chemotherapy and HER2-targeted therapy. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Trastuzumab and pertuzumab are both a form of "targeted therapy" because they work by attaching themselves to specific molecules (receptors) on ...
The purpose of this study is to investigate the mechanical properties of breast tissue on MRE (including stiffness, elasticity, viscosity, and volumetric stain) to find any correlation between these variables with breast density and background parenchymal enhancement, both of which are considered independent risk factors for breast carcinoma. We can also assess any suspicious lymph nodes noted on diagnostic MRI and correlate with stiffness values of the lymph nodes on MRI.
The purpose of this study is to assess the effectiveness of capivasertib + fulvestrant vs placebo + fulvestrant for the treatment of patients with locally advanced (inoperable) or metastatic HR+/HER2- breast cancer following recurrence or progression on or after Aromatase Inhibitor (AI) therapy.
The purpose of this study is to evaluate the addition of 2 years of palbociclib to standard adjuvant endocrine therapy for patients with HR+ / HER2- early breast cancer to determine whether the addition of palbociclib will improve outcomes over endocrine therapy alone.
To determine the maximally tolerated dose (MTD) of intratumoral administration of an Edmonston strain measeles virus genetically engineered to express NAP (MV-s-NAP) in patients with metastatic breast cancer; to determine the safety and toxicity of on-time and serial administration of MV-s-NAP in patients with metastic breast cancer.
This research study is being done to determine if a low vitamin D level and mammographic density may be risk factors for developing breast cancer.
The purpose of this study is to compare the detection sensitivity of positron emission mammography to contrast-enhanced breast MRI in women with a high suspicion of breast cancer.
The primary purpose of this study is to investigate the relationship between a technology-assisted diet and exercise program which is easily implemented in an outpatient setting and the levels of biomarkers that have been associated with breast cancer recurrence risk in overweight women with stage 0, I, or II breast cancer.
The purpose of this study is to determine the feasibility and perceived effectiveness of acupuncture for breast cancer-related symptoms.
This randomized phase III trial has several primary objectives. One primary objective is to compare the efficacy of 3 different endocrine therapies, the estrogen receptor down regulator fulvestrant and the aromatase inhibitor anastrozole, either alone or in combination, in reducing cancer growth before surgery (neoadjuvant) in postmenopausal women with clinical stage II-III estrogen receptor positive and HER2 negative breast cancer. Another primary objective is to evaluate whether patients who achieved a modified PEPI (Preoperative Endocrine Prognostic Index) score of 0, defined by tumor size
The purpose of this study is to evaluate digital tomosynthesis (3-D) mammography and digital mammography in screening patients for breast cancer. Screening for breast cancer with tomosynthesis mammography may be superior to digital mammography for breast cancer screening and may help reduce the need for additional imaging or treatment.
The purpose of this study is to explore the effectiveness of massage therapy combined with acupuncture in breast cancer patients recovering from autologous tissue reconstruction with the hope that the combination will augment the benefit obtained by massage therapy alone.
The optimal dose and fractionation regimen for whole breast irradiation, whole breast and regional nodal irradiation, and postmastectomy radiotherapy remains unknown. The goal of this phase II randomized controlled trial is to determine whether the hypofractionated proton regimens proposed are non-inferior compared with standard fractionated proton radiotherapy and therefore worthy of further investigation.
This research study is studying Ruxolitinib as possible treatment for Inflammatory Breast Cancer (IBC). The Following drugs will be use in combination with Ruxolinitinib. - Paclitaxel (also called Taxol) - Doxorubicin also called Adriamycin - Cyclophosphamide, also called Cytoxan
This phase II trial studies F-18 16 alpha-fluoroestradiol (FES) positron emission tomography (PET)/computed tomography (CT) in predicting response to endocrine therapy in patients with newly diagnosed breast cancer that has spread to other parts of the body. FES is a radioactive form of the hormone estrogen and may "light up" where cancer is in the body. Diagnostic procedures using FES, such as FES PET/CT, may help measure the FES and help doctors predict how well the cancer will respond to treatment.
The purpose of this research is to optimize and evaluate the efficacy of a hybrid imaging and quantitative viscoelasticity measurement tool for breast cancer detection and monitoring.
This randomized phase III trial studies axillary lymph node dissection to see how well it works compared to axillary radiation therapy in treating patients with node-positive breast cancer treated with neoadjuvant chemotherapy followed by surgery. Lymph node dissection may remove cancer cells that have spread to nearby lymph nodes in patients with breast cancer. Radiation therapy uses high-energy x-rays to kill tumor cells. This study will evaluate whether radiation therapy is as effective as lymph node dissection.
This phase II trial studies how well anastrozole and letrozole after surgery work in treating patients with stage I-III breast cancer. Drugs, such as anastrozole and letrozole may stop the growth of tumor cells by decreasing the amount of estrogen made by the body. Giving anastrozole and letrozole after surgery may prevent breast cancer from coming back (recurrence).
The purpose of this study is to continue with a long term follow-up of previously enrolled people treated with doxorubicin-cyclophosphamid followed by paclitaxel or docetaxel for axillary node-positive or high risk node-negative breast cancer.
The purpose of this study is to evaluate the effectiveess of a new 3D ultrasound imaging technology combining B-mode, microvessel imaging, shear wave elastography, and machine learning for breast lesion diagnosis.
The purpose of this study is:
- To assess whether a team based care model applied to distressed breast cancer patients will result in lower distress at 3, 6, 9 & 12 months compared to treatment as usual.
- To assess whether health promotion tools such as psychoeducation applied to non-distressed breast cancer patients will result in lower distress at 3, 6, 9 & 12 months compared to treatment as usual.
The purpose of this study is to evaluate how well multi-epitope folate receptor alpha peptide vaccine, sargramostim, and cyclophosphamide work in treating patients with triple negative breast cancer. Vaccines made from a person's white blood cells mixed with tumor proteins may help the body build an effective immune response to kill tumor cells. Drugs used in chemotherapy, such as cyclophosphamide, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving multi-epitope folate receptor alpha peptide vaccine, sargramostim, and cyclophosphamide may work better ...
The purpose of this study is to test if a culturally tailored virtual navigation program (Second-Life) meet the needs of Black women in learning more about breast health and care for cancer prevention, and mammography screening. We are asking for feedback on the Second-Life experience to determine if the information is easy to understand, relevant, and valuable to the community. The goal is to develop virtual community navigation that helps Black women make informed decisions about their breast healthcare since Black women have high mortality rates from breast cancer compared to other races or ethnicities.
The purpose of this study is to assess the effect of acculturation, socio-economic status (SES) and place of residence (urban vs. rural) on the level of participation in breast cancer screening programs and on the breast cancer knowledge and beliefs among Asian American women in Olmsted and surrounding counties.
This phase II trial studies how well alisertib with or without fulvestrant works in treating patients with endocrine-resistant breast cancer that has spread to other places in the body. Alisertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Hormone therapy using fulvestrant may fight breast cancer by blocking the use of estrogen by the tumor cells or reducing the amount of estrogen made by the body. Giving alisertib with or without fulvestrant may be better in treating patients with breast cancer.
This phase II study will test cancer to see if it has a HER2 mutation and, if so, see how HER2 mutated cancer responds to treatment with neratinib.
The purpose of this research study is to understand the views and experiences of Non-Hispanic Black women with a diagnosis or who support a family member with breast or ovarian cancer. We also want to know participant thoughts on genetic testing for cancer risk and research participation.
This is a qualitative interview study. Study participation involved talking on the phone or videoconference (e.g., Zoom) for about 1 - 1 1/2 hours with a researcher.
The goal of this project is to develop an AI system for early cancer detection on routine screening tomosynthesis mammograms. There will be significant impact on clinical practices if a mammography AI tool can be built. Such a tool will improve efficiency and decrease false positives and false negatives in practice.
This protocol captures the details for the reader study involving Aidoc, ScreenPoint, and iCAD. Mayo Clinic Breast Imaging Radiologists will evaluate the software tools that both ScreenPoint and iCAD have developed for assisting in reading tomosynthesis scans. Aidoc is facilitating this study by providing their user ...
The goal of this research is to identify risk profiles of women (with particular emphasis on Hispanic women) for breast cancer based on family history, breast density and other factors known to impact risk such as age, weight, age at menarche, age at birth of first child, etc.
The purpose of this study is to assess the antitumor activity of TAS-120 as monotherapy or in combination with Fulvestrant in the treatment of patients with metastatic breast cancer harboring fibroblast growth factor receptor (FGFR) amplifications.
The purpose of this study is to evaluate pre-operative therapy that is specifically targeted for breast cancer in individuals with BRCA mutations. The drugs are Niraparib (Zejula) and Dostarlimab.
The purpose of the study is to alleviate the occurrence of chemotherapy-induced nausea (CIN) and to improve chemotherapy treatment outcomes. Recent research has shown that changes in the functions performed by the gut microbiome can cause the occurrence of chemotherapy-induced symptoms that include chemotherapy-induced nausea.
The purpose of this study is to evaluate whether or not treatment with alternating 17B-estradiol / anti-estrogen therapies on a defined 8-week / 16-week schedule will more effectively prevent cancer growth than continuous treatment with either type of therapy in patients with metastatic anti-estrogen-resistant ER+ breast cancer.
The purpose of this study is to evaluate the change of vulvovaginal symptoms score from baseline to 6 months in premenopausal women with stage 0-III breast cancer treating with ovarian suppression in combination with aromatase inhibitors.
This study is being performed to better understand the mechanisms behind severe radiation toxicity of a patient with severe fibrosis after breast radiation.
The purpose of this trial is to determine the safety of 15 fraction vs 25 fraction pencil beam scanning proton radiotherapy after mastectomy in patients requiring regional nodal irradiation. Proton therapy is recognized as a standard option for the delivery of radiotherapy for breast cancer.
The investigators are conducting a longitudinal cohort study of young women with breast cancer. The investigators identify women age 40 and younger with newly-diagnosed breast cancer from academic and community healthcare institutions. After women consent to the study, they fill-out surveys and give blood samples, and the investigators collect tissue from their breast cancer tumor after it is removed. Women are surveyed every 6 months for the first 3 years after diagnosis, then yearly thereafter for an additional 7 years (for a total follow-up of at least 10 years following diagnosis). The study investigates short and long-term disease and treatment ...
A Phase II study to investigate the potential utility of PD 0332991 in the treatment of early stage ER+ Human epidermal growth factor receptor 2 (HER2)- breast cancer, to investigate whether the combination of PD 0332991 and anastrozole is able to: 1) improve the pathologic complete response rate when compared to the historical control of single agent aromatase inhibitors, 2) result in fewer patients with on therapy Ki67>10% compared to historical control.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by lowering the amount of estrogen the body makes. It is not yet known whether letrozole is more effective than a placebo in treating patients with hormone receptor-positive breast cancer.
PURPOSE: This randomized phase III trial is studying letrozole to see how well it works compared with a placebo in treating postmenopausal women who have received hormone therapy for hormone receptor-positive breast cancer.
This study is being done to determine the long-term effects of different kinds of breast surgeries on women’s health-related quality of life from the patient’s perspective. The information will also provide information to assist in improving the quality of care given to patients.
Collection of blood to track serial circulating tumor cells (CTCs) in subjects with metastatic breast cancer (MBC). Study will also collect data from investigators are Mayo Clinic and the Mayo Clinic Health Systems to determine effectiveness of the proposed process.
The purpose of this study is to culture human mammary cells to identify cellular characteristics associated with lobular involution status.
The purpose of this study is to determine if exercise improves cardiac remodeling as a way to mitigate the negative effects of chemotherapy and radiation therapy which lead to reduced exercise tolerance.
The purpose of this study is to measure the expression and frequency of the tumor tissue biomarkers (the genetics) of breast cancer, specifically the decreased presence and amount of a specific protein (Human epidermal growth factor receptor-2 [HER2]), how often genetic mutations occur, and why the cancer might or might not respond to monoclonal antibody therapy, such as trastuzumab emtansine (T-DM1) and/or pertuzumab.
RATIONALE: Drugs used in chemotherapy work in different ways to stop the growth of breast cancer cells, either by killing the cells or by stopping them from dividing. Giving the drugs in different combinations may kill more breast cancer cells. Giving combination chemotherapy after surgery may kill any tumor cells that remain after surgery.
PURPOSE: This randomized phase III trial is studying different combination chemotherapy regimens and their side effects and comparing how well they work in treating women with non-metastatic breast cancer.
Increased mammographic density is recognized as an important risk factor for developing breast cancer, however, the underlying mechanism explaining this relationship is unclear. The investigators hypothesize that Molecular Breast Imaging (MBI) can more accurately distinguish dense tissue on mammography which is at high risk from dense tissue at low risk by indicating cellular activity in dense tissue as radiotracer uptake (functional density) in the breast. In this pilot study, the investigators want to compare the histological characteristics of breast tissue in patients with who have similar density on mammography but different levels of functional density on MBI.
PURPOSE: This randomized phase III trial studies how well giving hormone therapy together with or without everolimus works in treating patients with breast cancer.
The purpose of this trial is to determine the patient's pathological response after hypofractionated radiotherapy to the whole breast based on a specimen after surgery. The analysis of tumor mutation may lead to a better understanding of the effect of radiotherapy in breast cancer.
The purpose of this study is to assess whether patients undergoing a breast MRI (magnetic resonance imaging) before breast surgery will have better results after the surgery. Breast tumors are routinely evaluated using mammograms and ultrasound before surgery. This study would like to find out if using MRI in addition to mammography before surgery improves the ability to evaluate tumors and decide what kind of surgery is best for the patient.
This randomized phase III trial studies how well hypofractionated radiation therapy works in preventing recurrence in patients with stage IIa-IIIa cancer who have undergone mastectomy. Hypofractionated radiation therapy delivers higher doses of radiation therapy over a shorter period of time and may kill more tumor cells that remain after surgery and have fewer side effects.
The purpose of this study is to evaluate the accuracy of diagnosis with contrast enhanced digital mammography when used in addition to standard mammography or ultrasound in patients with suspicious findings.
The purpose of this study is to determine the ability to capture PROs during patients’ radiation and during 3 months after.
Additionally, the study plans to conduct a retrospective chart review of prospectively accrued patients and ask subjects to complete a questionnaire.
The purpose of this study is to evaluate if Magseed is a viable alternative to radioactive seed as a localization method for biopsy proven metastatic breast carcinoma following neoadjuvant chemotherapy.
A pragmatic randomized clinical trial of patients with locally advanced breast cancer randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. Quality of life is the outcome measure for the estimated primary completion date of November, 2020.
The purpose of this study is to evaluate the use of molecular breast imaging as an accurate way to assess the response of breast cancer tumors to chemotherapy or hormone therapy that is newly supplemental to the standard treatment.
Efficacy and safety of treatment with alpelisib plus endocrine therapy in patients with HR+, HER2-negative aBC, with PIK3CA mutations, whose disease has progressed on or after CDK 4/6 treatment with an aromatase inhibitor (AI) or fulvestrant
The purpose of this study is to:
- To assess patient feelings and opinions on their variants
- To evaluate how the discovery of this variant has impacted relatives
- To evaluate how individuals would incorporate this knowledge into reproductive planning
- To create practice guidelines for handling this information in the future
The purpose of this study is to compare the effectiveness and safety of elacestrant to the standard of care (SoC) options of fulvestrant or an aromatase inhibitor (AI) in women and men with breast cancer whose disease has advanced on at least one endocrine therapy including a CDK4/6 inhibitor in combination with fulvestrant or an aromatase inhibitor (AI) .
The purpose of this study is to evaluate the impact of blood collection tube type and processing methods on ctDNA, evaluate the impact of long-term storage of plasma and extracted DNA, and evaluate ctDNA levels at baseline and during treatment for patients with Stage I-III breast cancer.
GRAIL is using high-intensity sequencing of circulating cell-free nucleic acids (cfNAs) to develop blood tests to detect cancer early. The purpose of this study is to train and validate an assay to detect invasive breast cancer in patients undergoing mammography.
There is evidence that mindfulness-based interventions (MBIs) such as meditation, mindfulness-based stress reduction (MBSR) and yoga might improve Quality of Life (QOL) and reduce stress in breast cancer survivors. These interventions are becoming increasingly popular in cancer survivors. However, little is known about the feasibility and effect of MBIs administered during the interval of time of chemotherapy, on QOL and stress. The investigators are planning a MBI intervention study developed specifically for breast cancer survivors receiving chemotherapy (usually 4-5 months) at the investigators institution, for at least 8 sessions combined with at least 8 weeks of home-practice, in 25 women ...
This study is being done to:
• Test an investigational stiffness measurement and imaging method on the lymph node found in the underarm area.
• Compare investigational imaging to sonography images.
• Compare investigational information to FDA approved US elasticity imaging conducted by SuperSonic Imaging (SSI) machine on the same lymph node in your underarm area.
• Compare to FDA approved ultrasound stiffness imaging system (GE Logiq E9)
• Compare to ultrasound images using Alpinion clinical ultrasound platform, FDA approved ECUBE 12and a non-FAD approved ECUBE 12R
The aim of this study is to examine alterations in the skin microbiome that occur during radiation therapy. The study design will examine changes secondary to ionizing radiation, and correlate these changes with the development and severity of radiation dermatitis. The goal is to improve understanding of the mechanism of radiation dermatitis.
The overall goal of this project is to study a new 3D ultrasound imaging technology for evaluation of axillary lymph nodes in patients with breast cancer.
RATIONALE: Breast-conserving surgery is a less invasive type of surgery for breast cancer and may have fewer side effects and improve recovery. Radiation therapy uses high-energy x rays to kill tumor cells. Giving radiation therapy after surgery may kill any tumor cells that remain after surgery.
PURPOSE: This phase II trial studies how well breast-conserving surgery and radiation therapy work in treating patients with multiple ipsilateral breast cancer
The purpose of this study is to analyze the impact of active participation of patients in producing tones in combination with breathing technique; i.e., TBT to reduce aromatase inhibitor induced musculoskeletal symptoms.
Tonation Breathing Techniques (TBT) is a set of diverse, mostly non-strenuous, specialized breathing techniques with the addition of Tonation; i.e., controlled exhalation through nostrils or lips while producing and sustaining a constant sound frequency as is comfortable to the participant as instructed in Musopathy sessions and/or videos.
This randomized phase II/III trial studies how well standard of care therapy with stereotactic radiosurgery and/or surgery works and compares it to standard of care therapy alone in treating patients with breast cancer that has spread to one or two locations in the body (limited metastatic) that are previously untreated. Standard of care therapy comprising chemotherapy, hormonal therapy, biological therapy, and others may help stop the spread of tumor cells. Radiation therapy and/or surgery is usually only given with standard of care therapy to relieve pain; however, in patients with limited metastatic breast cancer, stereotactic radiosurgery, also known as stereotactic ...
This project will investigate whether ctDNA analysis in newly diagnosed stage I, II, III breast cancer patients treated with neoadjuvant systemic therapy can predict pathological Complete Response (pCR).
The purpose of this study is to interview patients and providers at Phoenix Indian Medical Center and Mayo Clinic Arizona to identify perceptions, experiences, and perceived factors influencing referrals and enrollment on clinical trials in the Department of Radiation Oncology at Mayo Clinic Arizona.
The overall goal of this line of research is to enhance the hospitality, cultural responsiveness, and efficiency with which a leading cancer center can collaborate with a neighboring treatment hub for an important, underserved population within that cancer center’s catchment area.
American Indian and Alaska Native people experience higher rates of cancer ...
The purpose of this study is to extend the follow up on the BEAUTY study (MC1137) cohort and collect additional blood samples to evaluate for minimal residual disease and tissue at the time of any breast cancer recurrence.
The main purpose of this study is to learn more about the safety, side effects, and effectiveness of LOXO-783. LOXO-783 may be used to treat breast cancer and other solid tumors that have a change in a particular gene (known as the PIK3CA gene). Participation could last up to 36 months (3 years) and possibly longer if the disease does not get worse.
This phase II trial studies how well Akt inhibitor MK-2206 (MK-2206) and anastrozole with or without goserelin acetate works in treating patients with stage II-III breast cancer. MK-2206 may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Estrogen can cause the growth of breast cancer cells. Hormone therapy using anastrozole and goserelin acetate may fight breast cancer by blocking the use of estrogen by the tumor cells. Giving MK-2206, anastrozole, and goserelin acetate together may kill more cancer cells.
The purpose of this study is to assess genetic predictors of chemotherapy-related amenorrhea in breast cancer survivors of both European and non-European ancestry.
The primary aim of this study is to determine if the addition of an individual polygenic risk score (PRS), in addition to the standard National Cancer Institute's Breast Cancer Risk Assessment Tool (BCRAT) or Tyrer-Cuzick (IBIS) score, will aid women at risk of breast cancer in making a decision to take (or not take) medications to prevent breast cancer.
The purpose of this study is to collect blood and tissue samples for research of cancer.
Collection of tissue and blood from patients with residual disease after neoadjuvant systemic therapy for breast cancer. We hope to use these samples to find out why some patients still have cancer after they have completed neoadjuvant anticancer therapy.
The purpose of this study is to estimate the performance (sensitivity, specificity, accuracy) of dual-energy CT of the breast for detection of breast cancer in the ipsilateral and contralateral breast compared to standard multihance breast MR imaging. To determine the optimal workflow and images needed (multiplanar reconstructions, color maps, etc.) needed for routine interpretation of DE or quantitative CT imaging of contrast enhanced breast DECT.
This is a Phase 0 clinical trial to evaluate a twinkling biopsy marker (Patent Application Title: Non-Metallic Ultrasound-Detectable Markers Patent Application No.: 62/903,078, Application Type: Provisional) for ultrasound conspicuity in patients with breast cancer and locally advanced disease involving the axillary lymph nodes.
The purpose of this study is to utilize the systematic application of transcriptome-wide microarray to measure differential gene expression in banked breast implant associated-anaplastic large cell lymphoma (BIA-ALCL) tumor specimens and healthy control tissue.
The purpose of this study is to determine the overall prevalence and types of germline genetic mutations in a contemporary multi-institutional cohort of women diagnosed with a phyllodes (connective tissue) tumor of the breast, to compare the overall rate and types of germline genetic mutations observed in a multi-institutional cohort of women with phyllodes tumors to the average population and women with breast cancer, and to compare the rate and types of germline genetic mutations identified between each of the histologic subtypes (grade) of phyllodes tumors (benign, borderline, malignant).
The purposes of this study are to evaluate whether pre-NAC peripheral blood immune phenotypes (defined by mass cytometry) are associated with pathologic complete response (pCR) after neoadjuvant chemotherapy in patients with operable breast cancer, and to evaluate whether the baseline peripheral blood immune phenotype differs between patients with breast cancer and age-matched healthy controls.
Each year, the number of breast cancer survivors who choose post-mastectomy breast reconstruction keeps rising. Among women who elect to pursue breast reconstruction, approximately 75% will choose prosthetic breast reconstruction. Implant-based breast reconstruction is frequently achieved in two-stages. The first stage consists of the placement of a tissue expander after mastectomy. This is followed by a period of weekly tissue expansions that can last several months. In the second stage, the tissue expander is removed in a surgical procedure and replaced with a permanent breast implant. Tissue expansion is a well-established breast reconstruction technique characterized by high success rates and ...
This randomized phase III trial studies whether weight loss in overweight and obese women may prevent breast cancer from coming back (recurrence). Previous studies have found that women who are overweight or obese when their breast cancer is found (diagnosed) have a greater risk of their breast cancer recurring, as compared to women who were thinner when their cancer was diagnosed. This study aims to test whether overweight or obese women who take part in a weight loss program after being diagnosed with breast cancer have a lower rate of cancer recurrence as compared to women who do not take ...
This is a two arm Phase III trial in first and second-line HER2 negative patients with locally recurrent or metastatic breast cancer. The primary endpoint is overall survival (OS), and the objective is to test for the superiority of eribulin mesylate over standard weekly paclitaxel. Patients will be randomized between the experimental and control arm with equal allocation (1:1) within strata defined by prior adjuvant taxanes, hormone receptor status, and line of therapy. Subjects will continue protocol directed therapy until documentation of disease progression, development of unacceptable toxicity, or withdrawal of consent. Those who discontinue study treatment without radiological progression ...
The purpose of this study is to gather qualitative information about patient comfort during MBI examinations. The primary aim is to assess patient comfort during MBI, relative to comfort during a mammogram. We also wish to identify factors that contribute to discomfort and patients’ willingness to have MBI in the future.
The short-term goal of the proposed research is to develop a new method for viscoelasticity imaging of breast that can work with any type of wave, and not restricted to plane shear waves.
The long-term goal of this project is to develop an ultrasound-based breast imaging technique to improve the diagnostic specificity in breast cancer.
The purpose of this study is to determine whether contrast-enhanced ultrasound (CEUS) can be used in diagnostic evaluation of breast lesions that cannot be seen using contrast-enhanced MRI (CEMR) and contrast- enhanced dual energy mammography (CEDM). If so, patients can undergo US guided biopsy which is more comfortable for patients and more cost effective.
The purpose of this research is to evaluate the efficacy of a new breast tissue assessment tool that provides new diagnostic information about breast masses and potential for early classification of malignant masses.
• Test an investigational breast imaging system;
• Image the breast and see if we can differentiate lesions by using the investigational imaging system;
• Test an investigational breast stiffness measurement method
• Test and compare to an FDA approved ultrasound stiffness imaging system (GE LOGIQ E9 (LE9)
• Compare to ultrasound images using Alpinion ...
The purpose of this study is to test whether a short course of aspirin can change the markers of inflammation in patients who have a benign finding within five years of their last pregnancy, and possibly reduce their risk of future breast cancer.
A Phase I, Multicenter, Open-label, Dose-Escalation, Safety, Pharmacokinetic and Pharmacodynamic Study of Minnelide™Capsules given daily for 21 days followed by 7 days off schedule in patients with Advanced Solid Tumors
The purpose of this study is to evaluate whether engineering gut microbiome using probiotics will alter host immunological response to breast and lung cancers.
This study proposes to combine data from six large existing epidemiology studies to examine the association of MD and clinical risk factors with breast cancer subtypes.
This is an open-label, two-part, multiple study to evaluate the safety and tolerability of DS-8201a in patients with advanced solid malignant tumors.
The purpose of this study is to find out if ginseng decreases fatigue in people who were treated for cancer.
The purpose of this study is to gather measurements of mechanical properties of BI-RADS 4 and 5 lesions in order to test the effectiveness of MR elastography (MRE) in differentiating benign versus malignant disease. We will also test the mechanical properties of bilateral breast tissue on MRE to find any correlation with breast density on mammograms.
The purpose of this study is to help women in their late 30s and in their 40s, make decisions regarding breast cancer screening that align with each women’s values and preferences.
The purpose of this study is to to bring molecular risk prediction for breast cancer into the clinical arena through: the establishment of a large tissue repository from a retrospective cohort of women with benign breast disease with complete and long-term clinical follow-up to identify those who developed breast cancer (cases) and those who did not (controls); the application of potential biomarkers of risk to this archival tissue set; and, the discovery of new, potentially relevant biomarkers of risk in fresh and frozen specimens of benign breast disease.
The purpose of this research study is to understand the views and experiences of African American women with a diagnosis or who support a family member with breast or ovarian cancer. We also want to know participant thoughts on genetic testing for cancer risk.
This is a qualitative interview study. Study participation involves talking on the phone or videoconference (e.g., Zoom) for about 1 hour with a researcher.
The purpose of this study is to identify the recommended Phase 2 dose (RP2D) of LY3484356 administered as monotherapy and in combination with other anticancer therapies in patients with locally advanced or metastatic ER+ breast cancer or ER+ recurrent, persistent, or metastatic endometrial endometrioid cancer (EEC).
The purpose of this study is to offer pre-approval drug access of iniparib combined with gemcitabine and carboplatin, in order to provide potential clinical benefit to patients who have ER-, PR-, and HER2-negative metastatic breast cancer.
The purpose of this study is to evaluate whether breast conservation surgery and endocrine therapy results in a non-inferior rate of invasive or non-invasive ipsilateral breast tumor recurrence (IBTR) compared to breast conservation with breast radiation and endocrine therapy.
The primary objective of this study is to determine if exercise, fasting, or eating prior to the molecular breast imaging study will have an effect on the uptake of the tracer in the breast tissue.
This phase I/II trial studies the best dose of pembrolizumab and binimetinib and how well it works when giving together with pembrolizumab in treating patients with triple negative breast cancer that has spread to other parts of the body. Monoclonal antibodies, such as pembrolizumab, may block tumor growth in different ways by targeting certain cells. Binimetinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving pembrolizumab and binimetinib may work better in treating patients with triple negative breast cancer.
The purpose of this study is to evaluate the safety and effectiveness of combined treatment with niraparib and pembrolizumab (MK-3475) in patients who have triple-negative breast cancer that is advanced or has spread, or ovarian cancer that has returned after previous treatment.
The goal of the project is to develop and test a new 3D Doppler technology, with enhanced vessel sensitivity than conventional Doppler, to employ tumor vascularity as a differential biomarker for improved cancer diagnosis and reduced unnecessary biopsy on benign tumors
The purpose of this research study is to see if Atorvastatin(Lipitor) 40 mg by mouth daily decreases the chance of developing heart problems in women who are receiving anthracycline-based chemotherapy for breast cancer.
The purpose of this study is to explore, through surveys of providers and patients, the frequency and nature of shared decision making aspects in the cardiovascular care of cancer patients seen in the cardio-oncology clinic referred by an oncology provider.
This study will examine the safety and tolerability of SGN-LIV1A in patients with metastatic breast cancer. SGN-LIV1A will be given every 3 weeks alone or in combination with trastuzumab.
An Open-Label, First-in-Human Study of the Safety, Tolerability, and Pharmacokinetics (PK) of M6620 in Combination With Cytotoxic Chemotherapy in Subjects With Advanced Solid Tumors
This phase II trial is studying giving veliparib together with carboplatin to see how well they work compared to veliparib alone in treating patients with stage III or stage IV breast cancer. Veliparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Drugs used in chemotherapy, such as carboplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known whether veliparib is more effective with or without carboplatin in treating breast cancer.
The purpose of this study is to determine the safety and tolerability of OBT076, and to define the maximum tolerated dose (MTD) and/or the RP2D of OBT076.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug may kill more tumor cells. It is not yet known which combination chemotherapy regimen is more effective for breast cancer.
PURPOSE: Randomized phase III trial to compare the effectiveness of two combination chemotherapy regimens in treating women with breast cancer who have undergone surgery to remove the tumor.
The purpose of this study is to evaluate how well the combination of avelumab with liposomal doxorubicin with or without binimetinib, or the combination of avelumab with sacituzumab govitecan works in treating patients with triple negative breast cancer that is stage IV or is not able to be removed by surgery (unresectable) and has come back (recurrent).
The purpose of this research is to determine if previously adding a medication by the name of bevacizumab to the current standard chemotherapy of cancer-reducing medications, namely doxorubicin, cyclophosphamide and paclitaxel, reduces the risk of recurrence (called disease-free survival) compared to standard chemotherapy alone.
The purpose of this study is to learn about potential side effects facing people who are undergoing treatments for their cancer, specifically, hair loss. While this is not a well-documented side effect of hormone-blocking medications (such as tamoxifen, letrozole, anastrozole, or exemestane), we have preliminary evidence that it is a problem for some patients getting this treatment. This study will include some patients receiving the hormone therapy and some patients who are not, so we can better understand whether patients getting the hormonal therapy have more hair loss than patients who are not getting such.
The purpose of this study is to collect and correlate the data from various cardiovascular risk asessments to successfully measure the risk of heart toxicity in patients receiving chemotherapy for breast cancer.
This phase III clinical trial is studying how well giving tamoxifen citrate, anastrozole, letrozole, or exemestane with or without chemotherapy works in treating patients with invasive breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy, using tamoxifen citrate, may fight breast cancer by blocking the use of estrogen by the tumor cells. Aromatase inhibitors, such as anastrozole, letrozole, and exemestane, may fight breast cancer by lowering the amount of estrogen the body makes. Drugs used in chemotherapy work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping ...
The purpose of this study is to examine how well axillary reverse mapping works in preventing lymphedema in patients with breast cancer undergoing axillary lymph node dissection. Axillary reverse mapping may help to preserve the lymph node drainage system around the breast so as to prevent lymphedema after surgery.
The purpose of this study is to determine if Brain Natriuretic Peptide (NT-pro-BNP) values increase over time in breast cancer survivors and correlate with cardiac dysfunction. This study will define the average NT-pro-B-natriuretic peptide values in female breast cancer patients 1, 2, 3, 4, and 5 years out from anthracycline-based chemotherapy.
The best available evidence suggests that pregnancy after breast cancer does not increase a woman's risk of developing a recurrence from her breast cancer. In particular, the most recent data suggest that this is the case also in women with a hormone receptor-positive breast cancer. There is also no indication of increased risk for delivery complications or for the newborn. The aim of the study is to investigate if temporary interruption of endocrine therapy, with the goal to permit pregnancy, is associated with a higher risk of breast cancer recurrence.The study aims also to evaluate different specific indicators related to ...
This is an international, multi-center, open-label, randomized, Phase III study in patients with metastatic TNBC refractory or relapsing after at least 2 prior chemotherapies (including a taxane) for their metastatic disease. Patients meeting eligibility will be randomized 1:1 to receive either sacituzumab govitecan or treatment of physician choice (TPC), which needs to be selected prior to randomization from one of the 4 allowed regimens. Randomization will be stratified by number of prior chemotherapies for advanced disease (2-3 vs > 3) and geographical location (North America vs Europe). Patients will be treated until progression, unacceptable toxicity, study withdrawal, or death, whichever ...
Lymphaticovenous anstomosis is an effective surgery to treat lymphedema in the upper extremities secondary to cancer treatment. A crucial step is to identify patent lymphatic channels. Contrast enhanced ultrasound (CEUS) with intradermal injection of microbubbles is a promising method for lymphatic mapping in the upper extrmeities with lymphedema. The goals of the study are(1) to establish the preferred FDA approved microbubble agent (Lumason, Optison, Definity) for CEUS lymphatic mapping, (2) to identify lymphatic channels with CEUS and high-frequency ultrasound in patients receiving lymphaticovenous anastomosis surgery for upper extremity lymphedema, (3) and to validate the use of shear wave elastography for detecting improving in ...
The purpose of this study is to better understand the anatomy of the lymphatic structure and the molecular process that leads to the over production of lymph fluid. This proposal will begin intense lymphedema screening and identify baseline characteristics potentially predisposing someone to lymphedema, and identify molecular markers that might be altered to prevent lymphedema.
The purpose of this studyis to assess how well tamoxifen citrate works in patients with metastatic or recurrent breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate may fight cancer by blocking the use of estrogen by tumor cells.
The purpose of this study is to collect blood samples and fresh tissue from biopsies of metastatic lesions from Mayo Clinic patients with metastatic breast cancer. The biospecimens will be used for analysis of genetic alterations in germline and tumor DNA and for tracking of response to therapy using blood-based liquid biopsy approaches.
The purpose of this study is to examine the capability of contrast enhanced breast PCD-CT in staging breast cancer within the breasts and regional nodes of human subjects. Developing and using a PCD-CT imaging technique and postprocessing algorithms, dedicated for breast cancer detection.
The main purpose of this study is to evaluate how effective nonsteroidal aromatase inhibitors (NSAI) plus abemaciclib are in postmenopausal women with breast cancer.
The purpose of this study is to evaluate the effectiveness of abemaciclib plus trastuzumab with or without fulvestrant or chemotherapy in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 positive (HER2+) locally advanced or metastatic breast cancer after prior exposure to at least two HER2-directed therapies for advanced disease.
This phase I trial studies the side effects and the best dose of Z-endoxifen hydrochloride in treating patients with estrogen receptor-positive (ER+) breast cancer that has spread to other places in the body (metastatic) or has come back at or near the same place as the original tumor (locally recurrent). Estrogen can cause the growth of breast cancer cells. Hormone therapy using Z-endoxifen hydrochloride may fight breast cancer by blocking the use of estrogen by tumor cells.
This research trial studies genetic profiles in blood and tumor samples from patients with estrogen receptor positive and HER2 negative breast cancer that has spread to other places in the body who are receiving palbociclib and endocrine therapy. Examine the genetic changes associated with the cancer and comparing the genetic material from the cancer tissue with the genetic material found in the blood may help doctors to develop customized treatment for breast cancer.
The Primary Aim of this study is to quantify BDTC in breast cancer patients at different stages of cancer. As part of this aim we will establish the proliferation status of the tumor cells. We will in parallel examine CTC to determine the correlation between BDTC and CTC. The Second Aim is to determine role of tumor associated immune responses in maintaining tumor dormancy. Knowledge gained will provide the rationale for an in depth study of breast cancer tumor dormancy and immune response. Ultimately, the information gained will help us to design of immune intervention strategies that prevent cancer recurrence.
Objective: To determine the Overall Response Rate (ORR) to Imprime PGG + pembrolizumab in subjects with advanced melanoma or metastatic TNBC
Safety: To characterize the safety of Imprime PGG + pembrolizumab given in combination
Hypothesis: Restore (for melanoma) or enhance (for TNBC) sensitivity to checkpoint inhibitors (CPI) by appropriate and effective stimulation of the subject's innate and adaptive immune systems in those subjects who have failed 1st line therapy
The study will incorporate Simon's optimal 2-stage design with sample size fixed at 12 subjects each in Stage 1 for advanced melanoma and for Triple Negative Breast Cancer ...
The treating physician/investigator contacts Lilly when, based on their medical opinion, a patient meets the criteria for inclusion in the expanded access program.
The purpose of this study is to correlate the parathyroid hormone-related peptide (PTHrp) levels in the current and new assays in patients with known disease and calcium status.
The purpose of this study is to look at the effects cancer and melanoma have on the immune cells found in lymph nodes.
The purpose of this study is to evaluate different strategies of cardiovascular therapy with Carvedilol, aiming to reduce the incidence of left ventricular ejection fraction (LVEF) decline and heart failure (HF) in patients undergoing curative intent Trastuzumab for breast cancer.
A pragmatic randomized clinical trial of patients with locally advanced breast cancer randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. Quality of life is the outcome measure for the estimated primary completion date of November, 2020."
The purpose of this study is to assess the side effects and effectiveness of s-adenosyl-L-methionine for treating hot flashes in women who have a history of breast cancer or who do not wish to take estrogen due to a perceived increased risk of breast cancer.
The goal of this study is to perform a quantitative measure of lobular involution (qLI) from breast biopsy samples obtained at baseline (surgery) and 12 months or at time of study discontinuation. The study will determine breast density (MMG) and correlate with qLI. The study will also determine the influence of chemoprevention therapies such as tamoxifen and letrozole on qLI.
The purpose of this study is to assess the side effects and best dose of ruxolitinib phosphate when given together with pembrolizumab for treating patients with stage IV triple negative breast cancer that has spread to other places in the body. Monoclonal antibodies, such as pembrolizumab, may interfere with the ability of tumor cells to grow and spread. Ruxolitinib phosphate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving pembrolizumab and ruxolitinib phosphate together may work better in treating patients with stage IV triple negative breast cancer.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug may kill more tumor cells. Monoclonal antibodies such as trastuzumab can locate tumor cells and either kill them or deliver tumor-killing substances to them without harming normal cells.
PURPOSE: Phase II trial to study the effectiveness of combining paclitaxel, carboplatin, and trastuzumab in treating women who have metastatic breast cancer that overexpresses HER2.
This phase I trial studies the side effects and best dose of olaparib and onalespib when given together in treating patients with solid tumors that have spread to other places in the body or cannot be removed by surgery or ovarian, fallopian tube, primary peritoneal, or triple-negative breast cancer that has come back. Olaparib and onalespib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
The purpose of this research is to see how well fruquintinib works in combination with tislelizumab in participants with metastatic colorectal cancer (mCRC).
The purpose of part one of this study is to determine the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and safety profile of Q901 monotherapy when administered via intravenous (IV) infusion once-weekly (QW) for 4 weeks and once every 2 weeks (Q2W) thereafter. Also, to establish for future clinical development the recommended Phase 2 dose (RP2D) of Q901 monotherapy when administered via IV infusion QW for 4 weeks and Q2W thereafter.
The purpose of part two of this study is to evaluate safety and tolerability and evidence of anticancer activity of Q901 as monotherapy and in combination with pembrolizumab. In Part 2 Cohort 1, ...
The purpose of this study is to compare conventional assessment of systolic ventricular function on a 2D heart echo with an assessment of more immediate changes in heart mechanics using 2D and 3D measurements of deformation and strain in patients undergoing infusion of Trastuzumab for breast cancer.
To evaluate the safety and tolerability of AMG 650 in adult participants and to determine the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D).
The purpose of this study is to evaluate the safety and tolerability of LYL797, a ROR1-targeted CAR T-cell therapy, in patients with ROR1+ relapsed or refractory triple negative breast cancer (TNBC) or non-small cell lung cancer (NSCLC). The first part of the study will determine the safe dose for the next part of the study, and will enroll TNBC patients only. The second part of the study will test that dose in additional TNBC patients and NSCLC patients.
The overall goal is to investigate the value of ultrasound imaging of small vasculature as a new biomarker for cancer characterization and early treatment evaluation.
The purpose of this study is to evaluate SV-BR-1-GM in metastatic or locally recurrent breast cancer patients, in combination with the PD-1 inhibitor INCMGA00012 and the IDO inhibitor epacadostat. Patients who with advanced breast cancer who have failed prior therapies will be eligible to enroll in this study. The study will evaluate SV-BR-1-GM in combination with INCMGA00012 and epacadostat. Treatment cycles will be every 3 weeks with evaluations for tumor progression or response every 6-12 weeks.
The purpose of this study is to evaluate the safety and feasibility of the spinal array in treatment of patients with leptomeningeal metastases within the spine
The median survival of patients with LM with treatment is generally less than 5 months. There are four FDA-approved drugs for intra-CSF use in LM, but all have shown limited activity with no clear increase in survival outcome with treatment. Intra-CSF treatment is also invasive, involving either surgical placement of an intraventricular reservoir, or treatment (intrathecal) via repetitive lumbar punctures, and there is risk of adverse events including vomiting, headache, arachnoiditis and ...
The purpose of this study is to analyze the effect of sacituzumab govitecan in treating patients with HER2-negative breast cancer that has spread to the brain (brain metastases). Sacituzumab govitecan is a monoclonal antibody, called sacituzumab, linked to a chemotherapy drug, called govitecan. Sacituzumab is a form of targeted therapy because it attaches to specific molecules on the surface of cancer cells, known as Trop-2 receptors, and delivers govitecan to kill them. Giving sacituzumab govitecan may shrink the cancer in the brain and/or extend the time until the cancer gets worse.
The purpose of this study is to evaluate different strategies of cardiovascular therapy with carvedilol aiming to reduce the incidence of heart function declines and heart failure in at-risk breast cancer patients while on trastuzumab therapy.
The purpose of this study is to compare the effectiveness of two different combinations of chemotherapy in treating women who have stage II or stage IIIA breast cancer that has spread to the lymph nodes. Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. It is not yet known which regimen of chemotherapy is more effective for breast cancer.
The purpose of this study is to analyze the prevalence of mood disorders in newly-diagnosed breast cancer patients with use of specific questionnaires, aimed to diagnose clinically significant depression and anxiety, at a rural community hospital.
This study is being done to gather information. The study will provide important information related to the safety and the effect of the vaccine on a patient's immune system. What researchers learn from this study could possibly be used in the future to prevent or delay recurrence of breast or ovarian cancers.
The purpose of this study is to evaluate dose escalation and expansion of MT-5111 (a recombinant fusion protein) in subjects with HER2-positive solid tumors.
The purpose of this study is to assess the safety of PH FDC SC when administered at home by an HHNP. Patients will be assessed for safety by regular evaluation of AEs, vital signs, routine clinical laboratory tests (hematology, blood chemistry), LVEF assessments, and by physical examinations.
This study is a process evaluation of a digital storytelling (DST) intervention. Because the DST intervention has not yet been developed, it is appropriate to use qualitative methods to assess the process.
This study will recruit gifted storytellers for a digital storytelling intervention on breast, cervical, and colorectal cancer. A digital storytelling intervention will be developed to improve breast, cervical, and colorectal cancer screening rates among Hispanic, Spanish speaking individuals. Additionally, to conduct a qualitative assessment of the digital storytelling workshop.
The purpose of this study is to determine the maximum dose of LDE225 and BKM120 that can be safely given together to patients and/or the dose that will be used in future studies. This study will also learn more about how the combination of these two investigational drugs may work for patients with certain cancers (specifically metastatic breast cancer, advanced pancreatic adenocarcinoma, metastatic colorectal cancer and recurrent glioblastoma multiforme).
The purpose of this study is to estimate the circulating tumor DNA (ctDNA)detection rate and mutational load in breast cancer patients with indications for regional nodal irradiation.
The purpose of this study is to analyze breast tissue changes after a short course of Tamoxifen (Tam).
The purpose of this study is to to map out the extent of Skin Angiosarcoma disease using ultrasound. This will be compared to the MRI and or PET/CT and with clinical and photographic determination of disease extent confirmed with clinically requested punch biopsies. The patient will be scanned with a commercially avilable GE Logiq 10 machine and then with the 25-30 MHz linear microvessel transducer for microvessel imaging. These scans will be obtained pretreatment, after 2 cylces of neoadjuvant chemotherapy and after completion of neoadjuvant chemotherapy and radiation therapy prior to surgery.
This phase II trial studies how well biopsy of breast after chemotherapy works in predicting pathologic response in patients with stage II-IIIA breast cancer undergoing breast conserving surgery. Tumor tissue collected from biopsy before surgery may help to check if chemotherapy destroyed the breast cancer cells and may be compared to the tumor removed during surgery to check if they are the same.
The purpose of this research study is to determine how well neratinib works in treating breast cancer that has spread to the brain. Neratinib is a recently discovered oral drug that may stop breast cancer cells from growing abnormally by inhibiting (or blocking) members of a family of proteins that include Human Epidermal Growth Factor Receptor 2 (HER2).
In this research study, the investigators are looking to see how well neratinib works to decrease the size of or stabilize breast cancer that has spread to the brain. The investigators are also looking at how previous treatments have affected your ...
The purpose of this study is to compare the use of Bioimpedance Spectroscopy versus tape measurements for follow-up arm measurements after regional treatment for breast cancer. Catching the smallest increases in fluid buildup and intervening early may result in a decrease in the rate of progressions to chronic lymphedema.
The purpose of this study is to determine the safety, effcicacy and tolerability of H2NVAC in patients with HER2-expressing DCIS in order to prevent future invasive breast cancer among patients who are diagnosed with DCIS.
The purpose of this study is to assess the use of an audio recording containing positive suggestion as a means to provide needed psychological support to critically ill patients in a feasible and reliable manner.
We aim to determine if Molecular Breast Imaging (a new nuclear medicine technique developed at Mayo) can identify malignant breast lesions in women who have atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ.
The purpose of this study is to investigate the antitumor activity of navicixizumab monotherapy or in combination with paclitaxel or irinotecan in patients with advanced solid tumors including:
- Cohort A: Colorectal cancer (CRC);
- Cohort B: Gastric and gastroesophageal junction (GEJ) cancer;
- Cohort C: Triple-negative breast cancer (TNBC);
- Cohort D: Platinum-resistant/refractory epithelial ovarian, primary peritoneal, or fallopian tube cancer (ovarian cancer).
A Phase 1a/1b Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of PY314 as a Single Agent and In Combination with Pembrolizumab in Subjects with Advanced Solid Tumors
The purposes of this study are to to determine the physical, cognitive, emotional, and social health outcomes and trajectory of recovery in a population of children post-critical illness, to determine the baseline health, presenting problem, and PICU factors associated with impaired physical, cognitive, emotional, and social outcomes among PICU survivors, and to determine the emotional and social health outcomes in parents and siblings of PICU survivors. Our primary goal is to explicate the impact of pediatric critical illness over a two-year period of time to guide future intervention research to optimize child and family outcomes. Our overall goal is to improve ...
The purpose of this study is to evaluate the timing of AVB-620 administration relative to surgery on the fluorescence and accuracy of the AVB-620 imaging data to distinguish between malignant and nonmalignant tissues in women undergoing surgery with primary, nonrecurrent and nonmetastatic breast cancer.
This study looks at the risks and benefits of active surveillance (AS) compared to guideline concordant care (GCC) in the setting of a pragmatic prospective randomized trial for low risk DCIS. Our overarching hypothesis is that management of low-risk Ductal Carcinoma in Situ (DCIS) using an AS approach does not yield inferior cancer or quality of life outcomes compared to GCC.
This randomized phase IIB trial studies how well tamoxifen or afimoxifene works in treating patients with estrogen receptor positive breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate or afimoxifene may fight breast cancer by blocking the use of estrogen by the tumor cells.
In this research study, the investigators are testing a new type of breast camera, called Molecular Breast Imaging, to see if it can find tumors in the subject's breast.
The purpose of this study is to assess the safety of in vivo in host drug sensitivity testing in patients with breast cancer and patients with lymphoma (nodal, extranodal masses, or cutaneous lesions).
The purpose of this study is to determine the recommended Phase 2 dose (RP2D) of TBio-6517 when administered by direct injection into tumor(s) alone and when combined with pembrolizumab in patients with solid tumors (RIVAL-01).
Over a third of those who survive critical illness suffer from symptoms of anxiety, depression, or post-traumatic stress disorder (PTSD) after leaving the intensive care unit (ICU). This is twice as high as the rates of PTSD in combat veterans. The strongest risk factor is memories of frightening experiences and delusions (something that is very common during critical illness, when patients feel that something is real when it is actually not). Patients can hear speech even when sedated, yet there is no systematic communication with the critically ill while they receive life-saving medical treatments. We think that lack of real-time communication ...
The purpose of this study is to evaluate whether post-ICU assessment predicts PICS at 3 months and to validate a screening tool to identify patients at risk for PICS.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by blocking the use of estrogen by the tumor cells or by lowering the amount of estrogen the body makes.
PURPOSE: This phase II trial is studying how well letrozole works in treating women with ductal carcinoma in situ.
The purpose of this study is to develop a breast cancer survivor (BCS) multiscale omics database that includes a complete fecal metagenome (gut microbiome) and fecal metabolome characterization.
RATIONALE: Giving calcium together with magnesium may stop or delay the development of peripheral neuropathy in patients with cancer who are receiving treatment with ixabepilone. It is not yet known whether calcium and magnesium are more effective than a placebo in preventing peripheral neuropathy caused by ixabepilone.
PURPOSE: This randomized phase III trial is studying calcium given together with magnesium to see how well it works compared with a placebo in preventing peripheral neuropathy caused by ixabepilone in patients with breast cancer.
This is a Phase I study to understand the biodistribution of MM-398 and to determine the feasibility of using Ferumoxytol as a tumor imaging agent.
The purpose of this study is to evaluate the safety, tolerability, feasibility, and preliminary effectiveness of the administration of genetically-modified autologous T cells (CART-TnMUC1 cells) engineered to express a chimeric antigen receptor (CAR) capable of recognizing the tumor antigen, TnMUC1 and activating the T cell (CART- TnMUC1 cells).
The purpose of this trial is to evaluate the safety of GEN1046 in patients with malignant solid tumors.
This randomized phase II study aims to investigate whether the addition of bevacizumab to standard corticosteroid therapy results in greater improvement in symptoms and less treatment-induced symptoms compared with standard corticosteroid therapy for patients with symptomatic brain radionecrosis following radiosurgery. It is hypothesized that the addition of bevacizumab to standard care corticosteroids will reduce treatment-induced toxicities and improve neurologic impairments in patients with brain radionecrosis following radiosurgery for brain metastases.
The purpose of this study is to determine if counseling patients about low dose tamoxifen will influence the decision to take (or not take) preventive therapy among women at increased risk for breast cancer.
This is a Phase 1 multi-center study to evaluate the clinical safety and immune response of ID-LV305 when injected intradermally in patients with advanced cancer. ID-LV305 is a novel immunotherapy agent designed to target dendritic cells and stimulate the body's immune system to fight the spread and growth of cancer for patients whose tumors express the NY-ESO-1 protein. Patients with melanoma, sarcoma, ovarian cancer, or non-small cell lung cancer that express NY-ESO-1 may be considered for the trial. Selected sites will be evaluating ID-LV305 with pembrolizumab for patients with melanoma who have inadequately responded to anti-PD-1 therapy.
The purpose of this research study is to test whether metformin, a drug commonly used to treat diabetes, is able to get rid of atypia (early cell changes that are thought to be a marker of breast cancer risk) in women at increased risk for breast cancer. There will be testing for the presence of atypia in the breast after metformin is given to see if it can get rid of atypia. The study will compare the effects, good and/or bad, of metformin or placebo on atypia to find out which is better. Note: The standard drug used for the ...
The purpose of this study is to test the safety and side effects of a drug called SGN-B7H4V in participants with solid tumors. A side effect is anything a drug does to the body besides treating the disease. Participants will have cancer that has spread in the body near where it started (locally advanced) and cannot be removed (unresectable) or has spread through the body (metastatic). This study will have three parts. Parts A and B of the study will find out how much SGN-B7H4V should be given to participants. Part C will use the dose found in Parts A ...
This phase I trial studies the side effects and the best dose of stereotactic body radiation therapy in treating patients with breast cancer, non-small cell lung cancer, or prostate cancer that has spread to other parts of the body. Stereotactic body radiation therapy delivers fewer, tightly-focused, high doses of radiation therapy to all known sites of cancer in the body while minimizing radiation exposure of surrounding normal tissue.
The purpose of this trial is to compare the effectiveness of psychological support based on positive suggestions (PSBPS) vs. usual care on mental health morbidity and cognitive function in survivors of critical illness.
The purpose of this study is to determine the safety, tolerability, activity, and drug/body interactions of Oradoxel for the treatment of patients who have advanced malignancies.
The purpose of this study is to compare the effectiveness of fulvestrant to anastrozole or tamoxifen in treating invasive lobular breast cancer, by measuring the level of the biomarker Ki67 found in tumor tissue before and then after treatment.
The purpose of this study is to determine if the use of Gilenya® can reduce neuropathy caused by paclitaxel.
The primary purpose of this study is to obtain de-identified, clinically characterized, whole blood specimens to evaluate biomarkers associated with cancer for diagnostic assay development.
This randomized phase III trial studies how well oxybutynin chloride works in managing hot flashes in patients who are not candidates for, or not interested in hormone replacement therapy. Previous studies have shown that oxybutynin is effective in managing hot flashes, however doses used in prior studies have resulted in side effects. This trial is evaluating lower doses of oxybutynin with the goal of determining if they are efficacious with less side effects.
The primary objectives for this study are:
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 6 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 12 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 18 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 24 months of HLA-A LOH status
- Percentage of screened subjects experiencing loss ...
The ultimate goal of this biobank will be to provide the resource to initiate an exploration of human saliva as a potential liquid biopsy for cancer detection and surveillance.
THe purpose of this study is to examine the current and (potential) future therapeutic relevance of pharmacogenomics (PGx) testing for a cohort of cancer patients in order to improve quality of life (QOL) in patients receiving clinical care at Mayo Clinic.
The purpose for this study is to find out if MEDI5395 and durvalumab will work and be safe for the treatment of solid tumors.
This study will recruit breast cancer patients and survivors to take part in an ongoing study of the issues and concerns surrounding breast cancer survivorship. We will recruit both newly diagnosed patients as well as patients diagnosed within the past 5 years. Those who consent to the study will be asked to provide a series of questionnaires and blood samples over time. These data/samples will create a repository that will enable us to address many specific hypotheses both now and in the future. As part of the study DNA samples will be genotyped for common genetic variants ...
The purpose of this study is to demonstrate that rivaroxaban is superior to placebo for reducing the risk of lower extremity proximal deep vein thrombosis (DVT), asymptomatic lower extremity proximal DVT, symptomatic upper extremity DVT, symptomatic non-fatal pulmonary embolism (PE), incidental PE, and venous thromboembolism (VTE)-related death in ambulatory adult patients with various cancer types receiving systemic cancer therapy who are at high risk of developing a VTE.
Tinostamustine (EDO-S101) is a new chemical entity, an AK-DAC (a first-in-class alkylating deacetylase inhibiting molecule) that, in preclinical studies, has been shown to simultaneously improve access to the DNA strands within cancer cells, break them and block damage repair. This Phase 1/2 study will enroll patients with various advanced solid tumors.
The purpose of this multicenter prospective observational case-control study is to train and validate Adela’s cfMeDIP-seq based methylome profiling platform to detect and differentiate multiple cancer subtypes. In addition, this study includes longitudinal follow-up for a subset of participants to train and validate the methylome profiling platform to detect minimal residual disease and recurrence.
The purpose of this study is to assess the safety, tolerability and pharmacokinetics (PK) of CYT-0851 in patients with relapsed/refractory B-cell malignancies and advanced solid tumors and to identify a recommended Phase 2 dose for evaluation in these patients.
The goal of this study is to further evaluate the effect of magnesium on the symptoms of menopause, specifically vasomotor symptoms (VMS) in breast cancer patients and/or women at an elevated risk of breast cancer.
The purpose of this study is to collect clinical and biomarker data from patients receiving neurotoxic chemotherapy who are at risk for developing Chemotherapy-induced Peripheral Neuropathy (CIPN).
The purpose of this study is to collect blood and tissue samples from patients with and without cancer to evaluate laboratory tests for early cancer detection which may help researchers develop tests for the early detection of cancers.
The purpose of this study is to characterize TRPC6 risk variants for doxorubicin-related cardiotoxicity in prospectively collected samples from breast cancer patients.
Breast cancer patients are more than three times at risk for developing congestive heart failure (CHF), compared with patients who did not have cancer. The increased risk of HF is observed as early as one year from diagnosis of cancer and overall, 7% of patients develop CHF (median follow-up 8.5 years)
GRAIL is using deep sequencing of circulating cell-free nucleic acids (cfNAs) to develop assays to detect cancer early in blood. The purpose of this study is to collect biological samples from donors with a new diagnosis of cancer (blood and tumor tissue) and from donors who do not have a diagnosis of cancer (blood) in order to characterize the population heterogeneity in cancer and non-cancer subjects and to develop models for distinguishing cancer from non-cancer.
The goal of the study is to create a database of clinical information and a repository of biological specimens for genetic, molecular and microbiological research to better understand hereditary cancer and help develop new therapies and preventive strategies.
The purpose of this study is to develop a biorepository of blood samples from cancer patients participating in the Gemini (IRB 19-006717) protocol. These samples will be used for future biomarker discovery and other translational studies.
This is a Phase I, open label study to evaluate the safety, tolerability, and immunogenicity of INO-1400 alone or in combination with INO-9012, delivered by electroporation in subjects with high-risk solid tumor cancer with no evidence of disease after surgery and standard therapy. Subjects will be enrolled into one of six treatment arms. Subjects will be assessed according to standard of care. Restaging and imaging studies will be performed to assess disease relapse per NCCN guidelines. RECIST will be used to validate the findings in cases of relapse.
The purpose of this study is to sequence patient germline and tumor samples, and nominate top neoantigen candidates using an in-house developed bioinformatics pipeline, and to validate the neoantigen candidates by laboratory assays using patient peripheral blood immune cells or serum.
The purpose of this study is to provide a large database and platform for prospective sub-studies and eventually develop additional collaborations with a platform for clinical studies and trials following the initial pilot phase.
The purpose of this study is to evaluate the challenges, behavioral patterns, and preferences of minority patient participation in clinical trials. Also, to develop and validate a personalized clinical trial educational platform to boost participation among underserved cancer patients.
The purpose of this study is to evaluate the safety, tolerability, and preliminary anti-tumor activity of SAR444881 alone and in combination with pembrolizumab or with cetuximab. The study will enroll advanced cancer patients with unresectable or metastatic disease who are refractory to or are not candidates for standard approved therapy and will be comprised of two parts - an initial "3 + 3" dose escalation phase (Part 1) with Sub-Parts 1A (monotherapy SAR444881), 1B (SAR444881 in combination with pembrolizumab) and 1C (SAR444881 in combination with cetuximab) followed by a dose optimization/expansion phase (Part 2), including Sub-Part 2A (Dose Optimization) with Cohorts A1 (SAR444881 in ...
Falls are common and catastrophic in cancer patients. Cancer patients are vulnerable to falls due to muscle loss. In prescribing exercise in a data driven manner to cancer patients, our hypothesis is this "prescription" for exercise will eventually be demonstrated to reduce the occurrence of injurious falls.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Outcomes of sentinel node biopsy according to MRI response in an association with the subtypes in cN1–3 breast cancer after neoadjuvant systemic therapy, multicenter cohort study
Authors: Soong June Bae, Jung Whan Chun, Sae Byul Lee, Jai Min Ryu, Seok Jin Nam, Joon Jeong, Hyung Seok Park and Sung Gwe Ahn
Meeting Abstracts from the British Society of Breast Radiology annual scientific meeting 2023
Selective omission of sentinel lymph node biopsy in mastectomy for ductal carcinoma in situ: identifying eligible candidates.
Authors: Soong June Bae, Yoonwon Kook, Ji Soo Jang, Seung Ho Baek, Sohyun Moon, Jung Hyun Kim, Seung Eun Lee, Min Ji Kim, Sung Gwe Ahn and Joon Jeong
Metabolomics assisted by transcriptomics analysis to reveal metabolic characteristics and potential biomarkers associated with treatment response of neoadjuvant therapy with TCbHP regimen in HER2 + breast cancer
Authors: Ningning Zhang, Yuxin Huang, Guanwen Wang, Yimei Xiang, Zhouhong Jing, Junjie Zeng, Feng Yu, Xianjun Pan, Wenqi Zhou and Xiaohua Zeng
Chitin-mediated blockade of chitinase-like proteins reduces tumor immunosuppression, inhibits lymphatic metastasis and enhances anti-PD-1 efficacy in complementary TNBC models
Authors: Robbe Salembier, Caro De Haes, Julie Bellemans, Kristel Demeyere, Wim Van Den Broeck, Niek N. Sanders, Steven Van Laere, Traci R. Lyons, Evelyne Meyer and Jonas Steenbrugge
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .


Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .

A new paper in JNCI Cancer Spectrum finds that following a healthy diet lowers the risk of cardiovascular disease in breast cancer survivors.
Cardiovascular disease is the top non-breast cancer related cause of death in women with breast cancer. There are more than 3.8 million female breast cancer survivors in the United States. These women are at higher risk for cardiovascular disease than women who have not had breast cancer.
This is likely due to the cardiotoxic effects of breast cancer treatment, as well as common risk factors for both breast cancer and cardiovascular disease, such as aging, lack of exercise, and smoking. Dietary guidance for breast cancer survivors is limited and until recently has been based primarily on research related to cancer prevention.
Researchers used data from the Pathways Study, a prospective cohort study of women diagnosed with invasive breast cancer, to examine associations between diet quality and cardiovascular-related events. The analysis included 3,415 women diagnosed with invasive breast cancer at Kaiser Permanente Northern California between 2005 and 2013 and monitored through 2021.
To assess diet quality , researchers used a scoring system based on the Dietary Approaches to Stop Hypertension (DASH) diet which was developed in the 1990s to manage and treat hypertension. The diet emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy. It also limits sodium, red and processed meats, and sugar sweetened beverages.
The diet is similar to that recommended by the American Cancer Society, but also encourages consumption of low-fat dairy and nuts, and discourages sodium. The study evaluated heart health tied to these two diets as well as a plant-based diet , the 2020 Healthy Eating Index, and the alternate Mediterranean diet.
The researchers found that women whose diets were most similar to DASH at the time of their breast cancer diagnosis had a 47% lower risk of heart failure, a 23% lower risk of arrhythmia, a 23% lower risk of cardiac arrest, a 21% lower risk of valvular heart disease, and a 25% lower risk of venous thromboembolic disease than the women whose diets were least aligned with DASH.
In a closer examination, the researchers found that higher consumption of low-fat dairy reduced the risk for cardiovascular disease-related death, after adjusting for all other food groups. They also found that the relationship between DASH and cardiovascular disease appeared to be modified by the type of chemotherapy treatment a woman received.
For example, women whose treatment included an anthracycline and had diets closely aligned with the diet had a lower risk of cardiovascular disease than women least aligned with DASH, a relationship that was not apparent among women on other types of chemotherapy regimens.
"Our findings suggest that we need to begin talking to breast cancer survivors about the potential heart benefits of the DASH diet," said the paper's lead author, Isaac J. Ergas, Ph.D., a staff scientist at the Kaiser Permanente Division of Research. "We know that breast cancer survivors have an elevated risk for cardiovascular disease, and the diet might be able to help improve the overall health of this population."
The paper is titled " Diet quality and cardiovascular disease risk among breast cancer survivors in the Pathways Study ."
Explore further
Feedback to editors

AI speeds up drug design for Parkinson's by ten-fold
3 hours ago

Researchers find ethnic minorities are underrepresented in studies into multiple long-term health conditions
13 hours ago

Bacteria behind meningitis in babies explained
16 hours ago

Antibiotics reveal a new way to fight cancer

A urine-based test that detects tumor DNA fragments could offer early reliable screening for head and neck cancer
17 hours ago

Multidisciplinary research team creates computational models to predict heart valve leakage in children

Research uncovers new reasons to target neutrophils for tuberculosis therapy

New treatment method using plasma irradiation promotes faster bone healing
18 hours ago

Good blood pressure control could prevent fibroids
19 hours ago

Study suggests the brain's reward system works to make others happy, not just ourselves
Related stories.

Drinking alcohol not likely to increase risk of a breast cancer recurrence
Aug 9, 2023
Survivors of breast cancer face increased risk of heart disease
Jun 18, 2019

Assessing breast cancer risk
Mar 18, 2024

African American breast cancer survivor cardiovascular disease risk high but knowledge low
Mar 5, 2021

Can eating nuts have health-protective effects for breast cancer survivors?
Oct 20, 2021

AHA: breast cancer patients at increased risk for CV disease
Feb 1, 2018
Recommended for you

An effective drug delivery system for next-generation treatments to hitch a ride in cancer cells
20 hours ago

New insights could unlock immunotherapy for rare, deadly eye cancer
Apr 16, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Skip to main content
- Keyboard shortcuts for audio player
The many roadblocks that keep women from getting mammograms

Yuki Noguchi
CDC research finds that in addition to cost and access, other factors of daily life keep many women from getting screened for breast cancer. ( Story aired on All Things Considered on 4/9/24 .)
MICHEL MARTIN, HOST:
Mammograms are critical in detecting breast cancer early, but everyday life challenges can get in the way of people getting that screening. New research from the Centers for Disease Control and Prevention points to some of the challenges people face in getting screened more often. NPR's Yuki Noguchi has more.
YUKI NOGUCHI, BYLINE: Guidelines recommend women over 40 get mammograms every other year. The new CDC report shows just over three-quarters of women aged 50 to 74 get their breast cancer screening. But if you look at those who don't, often they lack the money for a copay or transportation, or no one's reminded them to.
Deb Houry is chief medical officer at the CDC. She says the data show how economic hardships and emotional challenges are common barriers that prevent people from getting their mammograms.
DEB HOURY: We really see a cumulative impact. So what we saw was if you had three or more of these health-related social needs, that was when you really saw a difference in who wasn't getting the screening mammogram.
NOGUCHI: That difference is pretty stark - 65% with three or more of these social needs, like food insecurity, were able to get screened. That's compared to 83% of those not facing such challenges. Houry says the analysis shows the importance of physicians understanding more about how their patients' lives are shaped by various kinds of struggles, like paying for food and utilities.
HOURY: You're going to choose food over paying for a mammogram.
NOGUCHI: She says the research also shows that emotional factors also play a role in who seeks preventative care.
HOURY: I think if you're not feeling connected to others, you may not be going to medical care on a regular basis.
NOGUCHI: Houry says all of this new data should inform patient outreach.
HOURY: Providers can refer patients who have low income and are uninsured to those programs and health departments, as well, for free mammograms.
NOGUCHI: She says, in many cases, there are state and philanthropic programs offering free transportation or free mammograms, including ones funded by the CDC.
Yuki Noguchi, NPR News.
Copyright © 2024 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
Trial Tests Abemaciclib As New Option for Early-Stage Breast Cancer
January 6, 2021 , by Carmen Phillips
Results from a clinical trial point to a possible new treatment choice for some women with the most common form of breast cancer.
UPDATE: On October 12, 2021, the Food and Drug Administration (FDA) approved abemaciclib (Verzenio) in combination with endocrine therapy (either an aromatase inhibitor or tamoxifen) following surgery to treat some people with HR-positive, HER2-negative breast cancer. Under the approval, patients’ cancers must have spread to nearby lymph nodes and exhibit certain traits that make the cancer more likely to come back, including high levels of a protein called Ki-67.
The approval was based on 3-year survival results from the monarchE trial (described below). Those results show more than 86% of people treated with abemaciclib and endocrine therapy had not experienced a recurrence after 3 years compared with 79% of those treated with endocrine therapy alone.
FDA also approved a companion diagnostic test called Ki-67 IHC MIB-1 pharmDx for this indication.
Updated results from a large study suggest that the drug abemaciclib (Verzenio) may be a new treatment option for people with the most common type of breast cancer.
Nearly 90% of people diagnosed with breast cancer have early-stage disease. And most of the time the cancer is classified as HR positive and HER2 negative. Although the available therapies for this type of breast cancer are very effective, a portion of patients are at particularly high risk of their cancer returning, or relapsing , in the years following treatment.
The new findings from the study—a clinical trial called monarchE—suggest that for people at high risk of relapse, adding abemaciclib to their treatment regimen may decrease the chances of their cancer coming back.
Participants in the trial who received abemaciclib for 2 years along with standard postsurgical, or adjuvant, hormone therapy were about 30% less likely to have their cancer come back in an invasive form than participants who received the standard adjuvant hormone therapy alone.
The study’s lead investigator, Priya Rastogi, M.D., of the University of Pittsburgh Department of Medicine, reported the findings on December 9 at the San Antonio Breast Cancer Symposium (SABCS).
Overall, the more than 5,600 participants in the trial had good survival outcomes regardless of whether they received abemaciclib. The vast majority experienced no return of their cancer during the short time they were followed. Participants treated with abemaciclib did have a higher risk of some side effects, including a very small number of women who experienced blood clots or inflammation in the lungs.
While oncologists who specialize in treating breast cancer largely agreed that the trial findings are promising, they also noted that it’s not yet clear if abemaciclib is ready for widespread use in patients with early-stage disease.
The early results from the monarchE trial “are clearly encouraging,” said Ruth O’Regan, M.D., of the University of Wisconsin Carbone Cancer Center, who was not involved in the study. “But longer follow-up [of study participants] is crucial,” Dr. O’Regan stressed during an SABCS session on the trial’s findings.
Larissa Korde, M.D., M.P.H., head of Breast Cancer and Melanoma Therapeutics in NCI’s Division of Cancer Treatment and Diagnosis , said that she believes abemaciclib could become an important treatment for some women with high-risk disease. But some significant unknowns remain, Dr. Korde added.
“One unanswered question is whether the treatment is actually preventing recurrences or just delaying them,” she said.
Testing CDK4/6 Inhibitors in Early-Stage Breast Cancer
Largely due to advances in treatment over the past several decades, the long-term prognosis for people diagnosed with early-stage, HR-positive, HER2-negative cancer is generally very good.
Standard treatment includes surgery and radiation, followed by adjuvant treatment with hormone-blocking drugs (also called endocrine therapy ) and, for many patients, chemotherapy. In some instances, patients will receive a short course of chemotherapy before surgery, also known as neoadjuvant therapy , to shrink the tumor and improve the chances that it can be completely removed.
The chances of the cancer returning under this general treatment approach are modest. But several factors are known to increase the risk, including whether the cancer has spread to several lymph nodes near the breast (in the armpit), having a large tumor in the breast, and having a higher “ grade ” cancer, meaning there are certain physical features of tumor tissue (under a microscope) that are indicative of more aggressive cancer.
Another factor that doctors have begun to use in this risk calculation is the percentage of tumor cells in a biopsy sample that express the Ki-67 protein , which is associated with cell division. However, this marker—called the Ki-67 index—is not routinely determined for all patients.
There is an “unmet need” for new treatment options for patients with high-risk disease, Dr. Rastogi said. And that’s where abemaciclib entered the picture.
Abemaciclib is one of three drugs approved by the Food and Drug Administration (FDA) that block the activity of two proteins on cancer cells, CDK4 and CDK6. The two others are palbociclib (Ibrance) and ribociclib (Kisqali) . All three drugs are approved to treat advanced or metastatic breast cancer, based on data from large clinical trials showing that they can substantially improve how long patients live without their cancer getting worse.
Based on their effectiveness in treating advanced breast cancer, several clinical trials were launched to see if CDK4/6 inhibitors might fill a treatment gap for people with high-risk early-stage breast cancer.
Positive Results, But Limited Follow-Up
To participate in the monarchE trial, participants had to have evidence of cancer in the lymph nodes nearest to their tumor, as well as at least one of several other disease characteristics linked to an increased risk of the cancer recurring. The trial was funded by Eli Lilly, which manufactures abemaciclib.
Participants were assigned at random to receive standard adjuvant hormone therapy alone or in combination with abemaciclib. Many trial participants also received some form of adjuvant chemotherapy. The median patient follow-up thus far in the trial is approximately 19 months, and only about a quarter of participants have completed 2 years of adjuvant therapy.
The findings reported at the San Antonio meeting included a 3-month update of a planned early (interim) analysis of the trial published in September .
Along with the nearly 30% reduction in the risk of developing invasive cancer in participants who received abemaciclib (called invasive disease-free survival), there was also a similar decrease in the risk of cancer returning as metastatic disease (called distant relapse-free survival).
The development of metastatic cancer is an important indicator for people with early-stage breast cancer, Dr. Korde said, because when it returns in another part of the body, such as the liver or lungs, the cancer is no longer considered to be curable.
Dr. Rastogi also presented results for the subgroup of patients with a high Ki-67 index score. (See Table)
† Only includes the 2,498 patients for which Ki-67 was assessed at a central laboratory.
Overall, side effects were more common in the abemaciclib group, leading more of these participants to skip doses of the drug or take lower doses. Nearly 17% of participants in the abemaciclib group stopped taking the drug because of side effects, compared with fewer than 1% of participants in the hormone therapy–only group.
Most of the participants who stopped taking abemaciclib did so within the first 5 months of starting it, Dr. Rastogi explained. And most of those who changed to a reduced dose or skipped doses of the drug have been able to continue taking it, she said.
Blood clots and lung inflammation, both of which can be fatal, occurred in fewer than 3% of people in the abemaciclib group, compared with fewer than 1% in the hormone therapy–only group.
Ready for Prime Time?
Abemaciclib is currently approved by FDA to treat advanced or metastatic breast cancer (alone and in combination with an aromatase inhibitor ). It’s not approved to treat early-stage breast cancer but, according to Eli Lilly, the company recently filed for that approval.
However, several breast cancer experts noted that it’s not yet clear how abemaciclib would be used to treat early-stage breast cancer.
In part, that’s because in two other clinical trials involving a similar group of patients, adding the CDK4/6 inhibitor palbociclib to adjuvant hormone therapy failed to improve invasive disease-free survival. Results from one of those trials, called Penelope-B, also were presented at SABCS .
Dr. O’Regan noted several key differences between Penelope-B and monarchE, including different treatment durations: 1 year of palbociclib versus 2 years of abemaciclib.
In addition, the Penelope-B trial results covered a median of 4 years of patient follow-up. However, Dr. O’Regan pointed out that, after 2 years of follow-up in the trial, the magnitude of the difference in invasive disease-free survival between the treatment groups was very similar to the difference reported in monarchE.
That raises the question of whether the improvement in invasive disease-free survival could disappear after the monarchE participants are followed for a longer time, said C. Kent Osborne, M.D., of the Dan L. Duncan Comprehensive Cancer Center at Baylor College of Medicine, during a press briefing.
Dr. Osborne agreed that longer follow-up of monarchE participants will be important, particularly since the cancer in people with early-stage disease can return 5 or 10 years after they’ve completed treatment.
In addition, he noted that CDK4/6 inhibitors are thought to work by stopping or slowing the growth of cancer cells, not by killing them. That’s important, he said, because when these drugs are used in people with advanced cancer, there have been some reports that “tumors seem to grow faster when you stop [treatment].”
If FDA’s approval of abemaciclib is expanded to include early-stage breast cancer, Dr. Korde said, oncologists will need to discuss the pros and cons of this treatment option with their patients.
“As with any therapy, I think the risks of the drugs need to be carefully weighed against the risk of recurrence,” she said. “One could argue that in a very high-risk population, the potential benefit outweighs the risk, but I think this decision needs to be made on an individual basis.”
Featured Posts
February 22, 2024, by Carmen Phillips
January 23, 2024, by Elia Ben-Ari
January 12, 2024, by Shana Spindler
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
Komen Tissue Bank co-founder: ‘We’re going to make a big difference’ in breast cancer research
Thanks to more than 5,000 women and men who donated breast tissue to the Kome n Tissue Bank at the Indiana University Melvin and Bren Simon Comprehensive Cancer Center , researchers are making progress to better understand and find a cure for breast cancer.

The tissue bank, the world’s only biorepository for normal breast tissue, started with an idea sparked by the annual Amelia Project conference in Indianapolis in 2004.
“One of the scientists got up and said, ‘I’m really close to getting the answer I need, but I need some normal breast tissue as the control.’ And the conference presenter looked at her and said, ‘Well you’re going to wait a long time for that because it doesn’t exist,’” said Dr. Anna Maria Storniolo, an oncologist at the IU Simon Comprehensive Cancer Center. “So I’m sitting there next to a dear friend and patient advocate, Connie Rufenbarger, and she would not let this go. She said, ‘What do you mean we don’t have normal breast tissue? All you have to do is ask women to have a breast biopsy.’”
The trick was finding out whether women would be willing to make such breast biopsy donations. Storniolo first polled people at one of her children’s soccer games to see if it was possible.
“One Saturday I’m out on the field and I just said, ‘I’m going to do this,’ and I walked around and asked about 10 to 15 women, that I made sure I did not know, if they would have a breast biopsy for breast cancer research,” Storniolo said. “Everybody but one said yes.”
Storniolo and Rufenbarger started with doing blood drives for research use, and after a few small breast tissue collection events, their idea for the Komen Tissue Bank officially became a reality in 2007. Seventeen years later, normal tissue samples from the bank are being used in 216 research projects around the world and have led to more than 90 published breast cancer research manuscripts.

“What we’ve learned thus far is that the normal breast tissue in women of African descent differs from the Northern European women, differs from Asian women, differs from Native American women,” Storniolo said.
“In several of those cases there are clear links to pathways that are accentuated in the normal breast and then changed in their cancer. That leads us to really emphasize the importance of the Komen Tissue Bank because we’re looking at normal breast tissue to find the clues and cues to what happens when it becomes abnormal and cancerous. You need to start with normal. You can’t know abnormal if you don’t know normal.”
In her 30 years of breast cancer work, Storniolo said, it has also become more common for young people to be diagnosed with breast cancer . The youngest patient she treated for cancer was 19 years old. More have been in their 20s, and an even greater number have been younger than 40.
“The devastating thing is it has the capability of completely altering their lives,.” Storniolo said. “In general, younger women tend to develop more aggressive breast cancers at more advanced stages. In addition, women with familial genetic abnormalities develop breast cancer at a younger age.
“As you can imagine, you’re 19; it interrupts your education, and it changes relationships. It’s hard, and it breaks your heart.”
That’s why donations to the Komen Tissue Bank are crucial . When volunteers come to donate, information from their individual medical history and from their healthy breast tissue inform the research and make treatments possible.
The donation process is simple. Women and men ages 18 and older can participate in the organization’s tissue collection events, at least one of which is held every year at the cancer center in downtown Indianapolis. Participants sign up for an appointment, and the process takes 60 to 90 minutes total.
Donors start by filling out paperwork, then small vials of blood are taken before they extract the tissue in an examination room. Storniolo was the first to donate breast tissue to the tissue bank and donated again a few years later.
“We make a little nick in your skin, but the area is numbed first so it won’t be felt. You are likely to bruise a bit, though,” Storniolo said.
The tissue bank will hold its next breast tissue donation event April 27 at the IU Simon Comprehensive Cancer Center in Indianapolis. Those interested in donating can make an appointment or email [email protected] for more information or to ask any questions.
“The Komen Tissue Bank allows women to be actively part of the solution,” Storniolo said. “These women understand that their tissue is going to make a difference and get us a cure faster. You don’t have to be a millionaire and give enough money to fund a building; you can give a little piece of yourself, which in many ways is much more precious.
“When a huge breakthrough is made in breast cancer and we actually crack the code, the women and men in Indiana are going to be able to say, ‘I helped do that.’ It’s been incredibly rewarding, and I think we’re going to make a huge difference.”

Elizabeth Cotter
Filed under:, more stories.

Cybersecurity and Global Policy Program personalizes support for students pursuing in-demand careers

Hamilton Lugar faculty share Lunar New Year traditions ahead of celebration
Social media.
- Facebook for IU
- Linkedin for IU
- Twitter for IU
- Instagram for IU
- Youtube for IU
Additional resources
Indiana university.
- About Email at IU
- People Directory
- Non-discrimination Notice
- Email Newsletters & Press Releases
- Announcements
- Suggest A Story
Texas A&M, University Of Colorado Research Collaboration Wins Federal Grant To Help Turn Off ‘Breast Cancer Switch’
Story by courtney price, vmbs communications.

The five-year study aims to identify more effective treatments.
Researchers at the Texas A&M School of Veterinary Medicine & Biomedical Sciences (VMBS) and the University of Colorado Cancer Center have received a $3.3 million grant from the National Institutes of Health to study how a pair of molecules that regulate certain types of metastatic breast cancer interact with a new drug therapy.
One molecule — semaphorin 7A, or SEMA7A — appears to grant drug therapy resistance to some breast cancers; the other — single-minded two, or SIM2 — helps determine whether SEMA7A is switched on or off.
Over the next five years, the researchers hope to combine knowledge of SEMA7A and SIM2 with a new drug to help women with metastatic cancer receive more effective treatment that will prolong their lives.
How Two Molecules Make The ‘Breast Cancer Switch’
“SEMA7A is a unique gene,” said Dr. Weston Porter , a professor in the VMBS’ Department of Veterinary Physiology & Pharmacology and co-primary investigator on the project. “My colleague at the University of Colorado and lead PI on the grant, Dr. Traci Lyons, discovered that it’s associated with increased metastasis — or cancer spread — especially in postpartum breast cancers, which do not respond to therapy.
“We found that invasive breast cancers are associated with a loss of SIM2, which leads to an increase in the expression of SEMA7A,” he said. “So we started asking whether we could use these molecules to identify patients with metastatic breast cancer and determine the best treatment regimen to use.
“We’ve found that certain cancer drug therapies are less effective when SIM2 is lost and SEMA7A is present. However, we have identified a new drug that we believe can be used instead, and so Dr. Lyons and I are working with Dr. Virginia Borges, a professor of medical oncology at Colorado, to look at patient samples and see how this new drug might be more effective,” he said.
Postpartum breast cancers like the ones affected by SEMA7A and SIM2 are often estrogen receptor positive (ER+) breast cancers, which represent about three-quarters of all breast cancer cases. The name means that the cancer cells have receptors — proteins on the cells — that can respond to estrogen hormones, which tell the cell to grow.
According to Lyons, there are targeted therapies for ER+ breast cancers, yet more than two-thirds of breast cancer deaths are attributed to metastatic ER+ disease, partly because of the therapy resistance promoted by SEMA7A.
“SEMA7A promotes pretty much every hallmark of cancer. It increases cell growth, cell migration, cell invasion, the ability to survive in harsh conditions — such as what a cancer cell will encounter while traveling from one site in the body to the other — and ultimately it promotes metastasis,” she said.
“When SEMA7A is on, it promotes all the things that are advantageous for the tumor and bad for the patient,” she said. “And SIM2s turns it off by preventing SEMA from being made in the first place. So, we’re looking at how we can use the switch to turn SEMA7A off.”
“Theoretically, if we can look at a patient’s samples and see their levels for both of these molecules, we would know what kind of treatment that they need much sooner, possibly giving them more time.” Dr. Weston Porter
Getting To The Heart Of The Problem
To find out how the two molecules react to the new drug and whether it will be helpful to breast cancer patients, the team will perform laboratory tests to uncover the mechanism behind SEMA7A and SIM2’s interactions in patient samples. Then, they can correlate their findings with data showing how each patient responded to therapy and what the outcome was.
“When someone has ER+ metastatic breast cancer, they are often given a drug that targets one of the mutated proteins caused by the cancer. However, we found that a significant number of women have cancer that isn’t affected by the primary drugs used for this purpose. We wanted to know why,” Porter said.
“What we found when we looked into the details, all the way to the protein level, was that there are actually different variants of this mutated protein, and there are other drugs out there that can target these other variants,” he explained. “The new drug that we’re testing is actually already approved by the FDA for treatment of lymphomas and leukemias, and it can be used for breast cancer treatment.”
The mutated protein targeted by the drug therapies is deeply connected to SEMA7A and SIM2.
“Theoretically, if we can look at a patient’s samples and see their levels for both of these molecules, we would know what kind of treatment that they need much sooner, possibly giving them more time,” Porter said.
For more information about the Texas A&M School of Veterinary Medicine & Biomedical Sciences, please visit our website at vetmed.tamu.edu or join us on Facebook , Instagram , and Twitter .
Contact Information: Jennifer Gauntt, Director of VMBS Communications, Texas A&M School of Veterinary Medicine & Biomedical Sciences, [email protected], 979-862-4216
Admissions & Academics
- DVM Professional Program
- Financial Aid & Scholarships
- Internships & Residencies
- Graduate Programs
Departments
- Large Animal Clinical Sciences (VLCS)
- Small Animal Clinical Sciences (VSCS)
- Veterinary Integrative Biosciences (VIBS)
- Veterinary Pathobiology (VTPB)
- Veterinary Physiology & Pharmacology (VTPP)
- Dean's Office
Global Scope
- Outreach & Service
- Global One Health
- International Programs
- Education Abroad
- VERO (VMBS + West Texas A&M Partnership)
- Veterinary Emergency Team (VET)
- Youth STEM Promotion (PEER)
- Contact Information
- Continuing Education (CE)
- Finance & Business Services
- Human Resources
- Marketing & Communications
- Technology Services (IT)
- UHS Counseling for Veterinary Students
Donors | Alumni | Clients
- Alumni Relations
- Stevenson Companion Animal Life-Care Center
- Veterinary Medical Teaching Hospital (VMTH)
- Employment/Recruitment Opportunities
- HireAggies (Texas A&M Career Center)
- Veterinary Job & Externship Fair
- Careers at the VMBS
- Code of Professional Conduct


Every year, BCC invites Canadians to participate in a purposeful walk, raising money for scientific advancements to end breast cancer. Many participants are survivors themselves, including Lauren Kehl of Toronto, who received a breast cancer diagnosis last year. At the age of 37, in October, a lump was discovered in Lauren’s left breast during an ultrasound, later diagnosed as stage two hormone-positive breast cancer (estrogen and progesterone positive). Under the care of Princess Margaret Cancer Centre, Lauren underwent a bilateral mastectomy in December, followed by radiation therapy. Throughout this challenging period, she has been fortunate to receive support from her family and friends, many of whom will be accompanying her on the upcoming walk.
“My best friend told me about Breast Cancer Canada's Walk-a-Thon to Mother's Day, raising funds for research. For me, it was the perfect event to turn a negative experience into a positive,” said Lauren Kehl. “I’ve witnessed firsthand the progress that has been made in treatment over the past 10 years and I want to do my part to further breast cancer research. It's my wish that anyone else who has to undergo breast cancer treatment in the future will receive the best care and most personalized treatment possible. Breast cancer isn't one diagnosis - there are over 50 different types. As a patient, I know we need more discoveries to continue the progress being made for the 1 in 8 women who will have breast cancer in their lifetime.”
Long-standing BCC partner and women’s fashion retailer, Cleo will return as the presenting sponsor. “This year marks over two decades of Cleo supporting us in their mission to fund patient-focused breast cancer research," Kimberly Carson continues. “We are honoured to have Cleo's ongoing and consistent partnership year after year."
Cleo, is offering individuals a $25 gift voucher for every $250 they raise, up to a maximum of $500 in vouchers per participant.
Canadians can also take part in BCC's spring fundraising by supporting their first National Silent Auction. From captivating artwork to coveted sports memorabilia and vacations, there's something for every taste and passion.
To register for the walk or participate in the online silent auction, please visit: mothersdaywalk.ca .
ABOUT BREAST CANCER CANADA Breast Cancer Canada is a national charity dedicated to saving lives through breast cancer research. With a focus on precision oncology (personalized care), it is the only national breast cancer organization in Canada that has a clear mandate to raise money for research. The organization receives no government funding, meaning all research is funded through the generosity of donors. For more information visit, breastcancerprogress.ca.
ABOUT CLEO Cleo is a Canadian national specialty brand featuring apparel – with a focus on workwear – for women in sizes XS-XXL and offers the largest Petite selection in Canada. Based out of Mississauga, Ontario, Cleo is part of a group of affiliate brands: Ricki’s, Bootlegger, Warehouse One and Suzanne's. Please visit Cleo.ca
Lindsay Silverberg Business Director, Talk Shop Media [email protected]

Researchers discover urine-based test to detect head and neck cancer
At-home test can detect tumor dna fragments in urine samples, providing a non-invasive alternative to traditional blood-based biomarker tests.
Researchers from the University of Michigan Health Rogel Cancer Center have created a urine-based test that detects pieces of DNA fragments released by head and neck tumors. The test could potentially facilitate early detection of this cancer type, which currently does not have a reliable screening method.
Human papillomavirus (HPV) is widely recognized for causing cervical cancer, but is increasingly found to cause cancers in the mouth, throat and other head and neck regions.
Early detection is critical because detecting a cancer at an earlier stage can lead to better outcomes for patients.
Using whole genome sequencing, the Rogel group showed that cell-free DNA fragments released by tumor cells, which are passed on from the bloodstream into urine through the kidneys, are predominantly ultra-short, with fewer than 50 base pairs. Given their small size, these fragments are likely to be missed using conventional urine or blood-based liquid biopsy tests in detecting circulating tumor DNA (ctDNA).
The research was led by Muneesh Tewari, M.D., Ph.D., professor of hematology and oncology, J. Chad Brenner, Ph.D., associate professor of otolaryngology-head and neck surgery, and Paul L. Swiecicki, M.D., associate medical director for the Oncology Clinical Trials Support Unit at Rogel. Initial results are published in JCI Insight .
"In this study we provide evidence to support the hypothesis that conventional assays do not detect ultrashort fragments found in urine, since they are designed to target longer DNA fragments. Our team used an unconventional approach to develop a urine test for HPV-positive head and neck cancer ctDNA detection," said study co-first author and research specialist Chandan Bhambhani, Ph.D.
Still in the discovery phase, this mail-in test has already been distributed for research purposes to patients within a hundred-plus miles from Ann Arbor, allowing scientists to gather significant data on the efficacy of the at-home kit. Participants collect a urine sample and have it shipped back to the U-M laboratory, where the testing can be done to detect the presence or absence of head and neck cancer.
"One of the most remarkable outcomes of this study is that the test that has been developed has detected cancer recurrences far earlier than would typically happen based on clinical imaging. As such, these promising results have given us the confidence to broaden the scope of the study, seeking to expanding distribution even further," said Brenner, co-senior author of the study.
While initial studies have focused on head and neck cancer, the paper also describes a new method that could be applied to expand the test to detect other cancers as well. For example, the authors show that the test can detect ctDNA in the urine of patients with breast cancer and acute myeloid leukemia. This suggests new opportunities to also study the application of urine-based testing for these additional cancers.
"Many people are not aware that urine carries information about many different cancer types, although it is made in the kidneys. Our findings about the difference in ctDNA fragment sizes and the test we developed for HPV-positive head and neck cancer detection provide crucial information on how urine-based diagnostic assays can be developed for different cancers," Bhambhani said. "Further, these types of tests are likely to have a much higher compliance in patients requiring follow-up testing post treatment, due to the convenience of self-collection of samples, when compared to blood-based assays."
Funding: NIH grants R33 CA229023, R21 CA225493; NIH/National Cancer Institute grants U01 CA183848, R01 CA184153, and P30CA046592; American Cancer Society RSG-18-062-01-TBG; American Cancer Society Mission Boost grant MBGI-22-056-01-MBG; and the A. Alfred Taubman Medical Research Institute.
Authors: Chandan Bhambhani, Qing Kang, Daniel H. Hovelson, Erin Sandford, Mary Olesnavich, Sarah M. Dermody, Jenny Wolfgang, Kirsten L. Tuck, Collin Brummel, Apurva D. Bhangale, Kuang He, Marc G. Gutierrez, Ryan H. Lindstrom, Chia-Jen Liu, Melissa Tuck, Malathi Kandarpa, Michelle Mierzwa, Keith Casper, Mark E. Prince, John C. Krauss, Moshe Talpaz, N. Lynn Henry, Maria D. Giraldez, Nithya Ramnath, Scott A. Tomlins, Paul L. Swiecicki, J. Chad Brenner, and Muneesh Tewari
- Brain Tumor
- Breast Cancer
- Colon Cancer
- K-12 Education
- Intelligence
- Alzheimer's
- Brain Injury
- Mammography
- Breast cancer
- Mirror test
- Cervical cancer
Story Source:
Materials provided by Michigan Medicine - University of Michigan . Original written by Anna Megdell. Note: Content may be edited for style and length.
Journal Reference :
- Chandan Bhambhani, Qing Kang, Daniel H. Hovelson, Erin Sandford, Mary Olesnavich, Sarah M. Dermody, Jenny Wolfgang, Kirsten L. Tuck, Collin Brummel, Apurva D. Bhangale, Kuang He, Marc G. Gutierrez, Ryan H. Lindstrom, Chia-Jen Liu, Melissa Tuck, Malathi Kandarpa, Michelle Mierzwa, Keith Casper, Mark E. Prince, John C. Krauss, Moshe Talpaz, N. Lynn Henry, Maria D. Giraldez, Nithya Ramnath, Scott A. Tomlins, Paul L. Swiecicki, J. Chad Brenner, Muneesh Tewari. ctDNA transiting into urine is ultrashort and facilitates noninvasive liquid biopsy of HPV+ oropharyngeal cancer . JCI Insight , 2024; 9 (6) DOI: 10.1172/jci.insight.177759
Cite This Page :
Explore More
- Plastic Pollution Kills Ocean Embryos
- Most Massive Stellar Black Hole in Our Galaxy
- Coffee's Prehistoric Origin and It's Future
- Can Animals Count? New Rat Study
- A Single Atom Layer of Gold: Goldene
- Fool's Gold May Contain Valuable Lithium
- Exercise Cuts Stress-Related Brain Activity
- Microplastics Go from Gut to Other Organs
- Epilepsy Drug May Prevent Brain Tumors
- Evolution's Recipe Book
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results ...
This page highlights what's new and recent research in the detection and treatment of breast cancer. New methods to detect breast cancer, such as 3-D mammography, are described. Treatments tailored to breast cancer subtypes are discussed, including targeted therapies.
A new way to boost breast cancer treatment. In early 2023, our researchers found a way to potentially improve treatment effectiveness, by targeting a different form of a common protein. Dr Ahmet Ucar and this team from the University of Manchester found that a protein called RAC1B can help the disease become resistant to treatment, spread and ...
Possible environmental causes of breast cancer have also received more attention in recent years. While much of the science on this topic is still in its earliest stages, this is an area of active research. Breast cancer prevention. Researchers are looking for ways to help reduce breast cancer risk, especially for women who are at high risk.
An experimental form of immunotherapy that uses an individual's own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute's (NCI) Center for Cancer Research, part of the National Institutes of Health.
Patients with new metastatic triple-negative breast cancer at initial diagnosis were also eligible for the trial. Patients who received taxane, gemcitabine, or platinum agents as neoadjuvant or ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
Address reprint requests to Dr. Tutt at the Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research, 237 Fulham Rd., London SW3 6JB, United Kingdom, or at [email protected].
The long-sought discovery of HER2 as an actionable and highly sensitive therapeutic target was a major breakthrough for the treatment of highly aggressive HER2-positive breast cancer, leading to ...
Sep. 10, 2019 — Researchers have discovered that a cell adhesion protein, E-cadherin, allows breast cancer cells to survive as they travel through the body and form new tumors, a process termed ...
December 20, 2023 Researchers uncover on/off switch for breast cancer metastasis. New research from Stanford and the Arc Institute could lead to a new and more effective immunotherapy and help ...
Ribociclib is a targeted therapy called a small molecule inhibitor. It works by targeting proteins in breast cancer cells called CDK4 and CDK6, which modulate cell growth, including the growth of ...
Small nucleolar RNAs (snoRNAs) play key roles in ribosome biosynthesis. However, the mechanism by which snoRNAs regulate cancer stemness remains to be fully elucidated. Wenrong Zhang, Xinyue Song, Zining Jin, Yiqi Zhang, Shan Li, Feng Jin and Ang Zheng. Breast Cancer Research 2024 26 :60.
Early detection of breast cancer: Study confirms the effectiveness of a new approach Further 'TOSYMA' analysis underpins advantages of DBT+SM use compared to the previous screening standard
New research, led by Penn State, reveals for the first time the mechanics behind how breast cancer cells may invade healthy tissues. The U.S. National Science Foundation -supported discovery, showing that a motor protein called dynein powers the movement of cancer cells in soft tissue models, offers new clinical targets against metastasis and ...
The world's leading breast cancer research meeting, SABCS 2023 drew approximately 10,000 attendees, with researchers sharing more than 1,700 new scientific abstracts and, hopefully, far fewer germs. Physician-scientists and others from Fred Hutchinson Cancer Center presented via podium and poster, with findings on everything from new drug ...
Breast cancer cases in patients under 40 are on the rise. New research sheds light on the comparatively rare cancer, for both oncologists and patients. In 2016, 21-year-old Jessica Florence found ...
ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
In that study, patients with high-risk, early-stage triple-negative breast cancer benefited from pembrolizumab given with chemotherapy before surgery, and then continued as a single agent as an additional, or adjuvant, treatment after surgery. "This is an exciting time" for research on triple-negative breast cancer, said Dr. Lee.
Researchers at Mayo Clinic are developing a Biobank of adult stem cell-rich breast organoids, a new research resource to facilitate normal and cancer stem cell research. Subjects in the Biobank will provide samples of excess breast tissue, complete a health questionnaire, and allow access to medical records now and in the future.
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic, biochemical, and cellular basis of breast cancer.
A large international group of researchers used a recently published gene aggregation method to uncover new genes implicated in the development of breast cancer. Two of the coauthors are with the Population Science team at the American Cancer Society (ACS): Lauren Teras, PhD, and Alpa Patel, PhD. They published their results in Genome Medicine.
In the European Organization for Research and Treatment of Cancer (EORTC) 10981-22023 Comparison of Complete Axillary Lymph Node Dissection with Axillary Radiation Therapy in Treating Women with ...
A new paper in JNCI Cancer Spectrum finds that following a healthy diet lowers the risk of cardiovascular disease in breast cancer survivors. Cardiovascular disease is the top non-breast cancer ...
CDC research finds that in addition to cost and access, other factors of daily life keep many women from getting screened for breast cancer. (Story aired on All Things Considered on 4/9/24.)
Updated results from a large study suggest that the drug abemaciclib (Verzenio) may be a new treatment option for people with the most common type of breast cancer. Nearly 90% of people diagnosed with breast cancer have early-stage disease. And most of the time the cancer is classified as HR positive and HER2 negative.
Seventeen years later, normal tissue samples from the bank are being used in 216 research projects around the world and have led to more than 90 published breast cancer research manuscripts. Connie Rufenbarger, left, and Dr. Anna Maria Storniolo at a Komen Tissue Bank donation event in 2010.
The five-year study aims to identify more effective treatments. Researchers at the Texas A&M School of Veterinary Medicine & Biomedical Sciences (VMBS) and the University of Colorado Cancer Center have received a $3.3 million grant from the National Institutes of Health to study how a pair of molecules that regulate certain types of metastatic breast cancer interact with a new drug therapy.
This is the exact research that Breast Cancer Canada is funding, and Canadians can be part of this progress by participating in the walk or auction for Mother's Day this year," said Kimberly ...
Funding: NIH grants R33 CA229023, R21 CA225493; NIH/National Cancer Institute grants U01 CA183848, R01 CA184153, and P30CA046592; American Cancer Society RSG-18-062-01-TBG; American Cancer Society ...