

The GlobalReady CRO
GlobalReady the full-service biotech Contract Research Organisation service for Australia and North America
Globally accepted data
Rapid start-up, extend budget by 43.5%, latest technology, meet our team at the next industry conference/event book a meeting, latest news videos, the contract research organization, how we work with medidata, how we work with oracle argus, recent features and awards, talk to our team contact us, established & proven over two decades, agile and solutions-focussed.
Avance Clinical is the largest full-service Australian CRO delivering quality clinical trials with globally accepted data in Australia and New Zealand for international biotechs.
Frost & Sullivan awards
Avance Clinical, a Frost & Sullivan Asia-Pacific CRO Best Practices Award recipient for the past two years, has been providing CRO services in the region for more than two decades.
The company’s clients are biotechs in their early phases of drug development that need fast, agile, and adaptive solution-oriented clinical research services.
Pre-clinical through to Phase 1 and 2
Avance Clinical offers pre-clinical services with their experienced ClinicReady team right through to Phases 1 and 2 leveraging significant Government incentive rebates of up to 43.5% and rapid start-up regulatory processes.
With experience across more than 105 indications, the CRO can deliver world-class results and high-quality internationally accepted data for FDA and EMA review.
Avance Clinical delivers customized solutions designed around specific client needs rather than a one size fits all approach.
As a company, Avance Clinical has focused on state-of-the-art technology and systems across all functional areas to provide clients with the most effective processes. Medidata, Oracle, and Medrio are just some of the technology partners.
Experienced Team
More than 300 in-house cro specialists.
Avance Clinical had its genesis in the University of South Australia in the early 1980s as the Center for Pharmaceutical Research, when a group of academic scientists began providing clinical research and bioanalytical services to the local pharmaceutical industry.
Since then, it has grown to become the leading mid-sized CRO servicing the international drug development industry.
Avance Clinical offers some of the most extensive in-house clinical services including:
- ClinicReady – pre-clinical services
- Clinical Monitoring Management
- Data Management
- eClinical Solutions
Safety and Pharmacovigilance
- Medical Writing
- Project Management
- Statistics and Pharmacokinetics
- Quality Assurance
Pre-clinical ClinicReady
The ClinicReady range of services constitutes a natural progression in Avance Clinical’s role as the premier provider of early drug development support to the biotechnology sector. The company recognizes the major challenges faced by small biotechnology companies, particularly start-up companies who seek to drive their novel therapeutics to demonstration of safety and preliminary clinical Proof of Concept.
The Avance Clinical biostatistics and pharmacokinetics experts provide specialist guidance for your clinical research trial design and analysis.
Avance Clinical’s data management team has gained a world-class reputation for accuracy, precision, and quality of their biostatistics and pharmacokinetic (PK) analysis.
Clinical trials safety monitoring and reporting is vital to ensure participant safety, data integrity, and regulatory compliance.
Regulatory guidelines around clinical trial safety monitoring and reporting demand strict safety management procedures, from a highly-skilled, and experienced safety team.
The Avance Clinical team works with eClinical leaders such as Medrio, Medidata, and Oracle to offer regulatory compliant and patient-centric tools for rapid start-up and continual data flow.
Data quality is at the heart of everything at Avance Clinical. Delivered by our highly experienced staff we support our clients with their clinical trial audit to achieve their quality objectives for their clinical research trials.
Avance Clinical has experienced clinical monitoring staff across all phases of clinical trials and can collaboratively support the Australian CRO arm of multinational trials.
Avance Clinical provides each clinical research trial with a dedicated Project Manager to ensure that milestones are reached, timelines are met and that our clients receive effective communication and ongoing status reports.
“ “It has been a great pleasure working with you on this project. Your flexibility, pragmatic approaches and focus on the work has been exceptional. I am looking forward to have the next compound entering the clinic with you.” Head of Drug Discovery, Pharmaxis Ltd
“ “Working with the Avance Team has helped us to develop and complete high quality and compliant clinical studies. I especially appreciate their penchant for communication, quality and pragmatism.” Sr. Vice President, Regulatory, Quality & Clinical Affairs, Atossa Genetics, Inc.
“ “Having conducted several Phase I studies in Australia it made sense to continue in the country with our Phase II program, particularly given the high-quality support we have received from our CRO.” Atossa Therapeutics Inc.
“ “Avance Clinical has proven themselves to be my “Champions”, anticipating problems and always acting in the interest of getting the trial done professionally, resourcefully, and in alignment with the goals of the Sponsor and well-being of the volunteers.” Chairman of the Board and Chief Medical Officer, BioFactura Inc
“ “Medrio has supported numerous study starts in Australia, over 150 with Avance Clinical alone, and we’re always impressed with the speed of their timelines.” CEO Mike Novotny (former), Medrio
“ “We reached out to Avance to refine and polish a substantive and comprehensive drug study clinical trial ethics application. The Avance Team were remarkably quick to respond to our inquiry. Avance provided us within a very short turn-around period a comprehensive appraisal of the documentation covering all elements including protocol, ethics, investigator brochure and patient information and consent. Avance were highly engaged with the research investigator team and suggested changes were clear and well rationalized. The services provided were cost-effective and well justified. I have no hesitation to recommend Avance as a quality CRO”. Curtin Health Innovation Research Institute
“This overall customer-first approach offers immense value to existing and new customers and solidifies the company’s position in the market. Avance Clinical remains a trusted partner, earning a reputation for delivering the overall best in the CRO industry. With its strong overall performance, Avance Clinical earns Frost & Sullivan’s 2022 Asia-Pacific Customer Value Leadership Award in the contract research organization industry.” Azza Fazar, Best Practices Research Analyst, Frost & Sullivan
Read the News Announcement
Latest news.
View our latest client and company
Avance Clinical at World Vaccine Congress to Share Latest Vaccine Clinical Trial News Including an HIV-1 Study
Avance clinical ceo talks with scrip intelligence about a new cro report and us biotech sector “green shoots” , avance clinical and ryght partner to bring novel genai technologies to clinical research networks, partner with the globalready contract research organisation.
- Avance Clinical at World Vaccine Congress to Share Latest Vaccine Clinical Trial News Including an HIV-1 Study April 3, 2024
- Avance Clinical CEO Talks with SCRIP Intelligence about a New CRO Report and US Biotech Sector “Green Shoots” March 4, 2024
- Avance Clinical and Ryght Partner to Bring Novel GenAI Technologies to Clinical Research Networks February 22, 2024
- Frost & Sullivan Analysis Reveals 65% of US Biotechs Struggle to Identify Suitable CRO Partner January 8, 2024
Australian HQ
213 Glynburn Road, Firle, South Australia, Australia 5070.
Phone: +61 8 8249 4788 US Toll Free: 1 (206) 686-4644 Media Inquiries: [email protected] Web: www.avancecro.com
Office Hours: Monday to Friday: 8.00 am to 5.30 pm
Avance Clinical is a full-service CRO that leverages the entire suite of eClinical technology, and an expert team of more than 300 CRO specialists, to deliver clinical drug development services for international biotechs.
The largest Australian CRO for international biotechs
Avance Clinical is the largest specialist Australian CRO delivering quality clinical trials in Australia and New Zealand for international biotechs for over two decades.
TERMS & CONDITIONS
© 2024 Avance Clinical. All Rights Reserved.
- About Avance Clinical
- Meet the Team
- The Australian Advantage
- Industry Awards
- Avance Clinical GlobalReady
- ClinicReady Latest Case Study
- QAReady – data quality is at the heart of everything
- Statistical and Pharmacokinetics Services
- Safety and Pharmacovigilance Services
- We are Hiring – Join the Team
Privacy Overview
Founded in 2010 and headquartered in Australia, Southern Star Research is a full-service clinical research organisation (CRO) helping sponsors to navigate the complexities of bringing new medical products to market. Since commencing operations in Sydney, we have grown to become an international team of specialists, managing studies across the globe.
With a focus on the biotechnology and medical device industries, we offer flexible and bespoke solutions that meet the unique needs of each project. While we have experience across all study phases, we are early phase specialists (Phase 1 and 2) with particular expertise in designing and managing clinical trials that ensure your asset is ready for larger Phase 3 and 4 studies, licensing, partnering and acquisition.
We believe a customer-first approach, transparent relationships and a proactive team of experts are the best path to achieving successful commercial outcomes for our clients.
Get in touch now to discuss how our agile and dedicated team can help you run a successful clinical trial.
Our Leadership team
Our partnerships.

Our mission is to help sponsors bring safe and effective medicines and products to market in an efficient and streamlined way. We believe in:
Agile, proactive and bespoke solutions that help you reach your commercial goals faster
The very best quality at a predictable price - no unexpected add-ons
Transparent, close relationships, with a focus on your unique needs
Simplifying the clinical trial process – we’ll help you navigate each stage with confidence
- Professionalism
- Honesty & integrity
- Excellence in customer service
- Focus on timelines and budget
- Agility & flexibility
- Dependability
- Spirit of altruism
- Commitment to exceeding expectations
Our work in the community

request a proposal
“What a stellar job you’re doing with the SAD study – so calm, confident and professional. In my 30+ years training and managing project managers you stand out by far as one of the best I have seen. I am proud to have you as part of our team.”
Phase 1 study, local Biotech company
“Thank you, your professional attitude and constant support are much appreciated.”
CEO, Australian Medical Device Company
“Their expertise is second to none, they are on the ground working with sites intimitely with close attention to detail to ensure that the job gets done. With Southern Star you dont feel like you’re dealing with big organisation – you get a face to face personal approach.”
Director of Regulatory Affairs
“SSR take time to find out how we work and are solutions focused suggesting things that might help our business. They invest in great outcomes and are willing to think about the long term relationship and how they might work better with their partners.”
Head of Trial Management & Monitoring
“Working with Southern Star Research gives us a level of security, you know your trial will be delivered as expected without surprises. Communication is easy and they are flexible, we talk through issues and adapt continuously to fine tune and tailor to what we need.”
Clinical Trials Program Manager
- Phone: +61 (0)2 9011 6266
- Connect with us
- Clinical Trials in Australia
- News & Resources
- Legal Notice
- Privacy Policy
Sign up to our newsletter
Keep up to date with the latest news, updates and promotions

Quick links
- Climate change
- COVID-19 research
- Staff profiles
We work with biomedical companies to deliver new medical treatments and technologies that benefit millions of people in Australia and overseas, helping them live longer, healthier and more productive lives.
Work with CSIRO's biomedical researchers
Our biologics capabilities.
CSIRO’s biologics facilities play a vital role in the biomedical research industry. Our esteemed scientists and world-class facilities provide companies and other research organisations the opportunity to produce biologics at the scale and quality required for pre-clinical and clinical applications.
Biologics capabilities
Our researchers and unique facilities play a vital role in the biomedical ecosystem. We partner with businesses and produce biologics at the scale and quality required for pre-clinical and clinical applications.
Manufacturing a COVID-19 vaccine
How do we produce a COVID-19 vaccine that is safe, effective and can be mass-produced? We take you into our labs to find out!
Our drug discovery capabilities
Our highly skilled biomedical researchers work with industry to develop, validate and optimise drug discovery. Read more about our capabilities in the infographic below.
Melbourne biotech start-up MecRx is working with CSIRO researcher Dr Rohan Volpe to fast track drug discovery.
Developing medicinal cannabis products
Our new licence to manufacture medicinal cannabis products will help drive the development of novel cannabis-based therapeutics in Australia.
CSIRO starts work on medicinal psychedelics
CSIRO, Australia’s national science agency, is aiming to develop new psychedelics to help people with a variety of mental health issues including depression, addiction, end of life anxiety and post-traumatic stress disorder.
This form of B3 can make blood cells more youthful
Our scientists helped find that blood cells can become “more youthful” through exposure to nicotinamide riboside – a form of vitamin B3.
Hitting a nerve: how antidepressants treat nerve pain
Researchers at Australia’s national science agency CSIRO, have shown for the first time how tricyclic antidepressants (TCAs) work against nerve pain, paving the way for further research and new therapies to treat the debilitating condition.
Biomedical Materials Translation Facility
CSIRO's Biomedical Materials Translational Facility (BMTF) helps Medtech companies turn new discoveries into market ready products. It has the capability and equipment needed to develop a biomedical product though prototyping, scale-up, pre-clinical testing and industry evaluation and offers access to ISO 9001, ISO 17025 and GLP.
Medtech to market
CSIRO's Biomedical Materials Translational Facility (BMTF) helps medtech companies turn new discoveries into market ready products.
Sidelining delays in diagnosing concussion
We're working on a game-changing new device that could assist first responders to diagnose traumatic brain injuries on the spot.
How we're using gold nano-particles to help treat age-related macular degeneration.
3D printed biomedical implants at Lab22
We innovate additive manufacturing (3D printing). Our research and development underpins a growing Australian metallic additive manufacturing industry.
How do you mend a broken heart
Our latest polymer, LifePolymer™, was recently transplanted into a man in the US in the form of a novel heart valve, to save his life. Talk about Australian technology making a difference to global heart health!
There are no news releases to show.
More biomedical research
Find out how we can help you and your business. Get in touch using the form below and our experts will get in contact soon!
CSIRO will handle your personal information in accordance with the Privacy Act 1988 (Cth) and our Privacy Policy .
Enter a valid email address, for example [email protected]
A Country value must be provided
First name must be filled in
Surname must be filled in
Please choose an option
Organisation must be filled in
Please provide a subject for the enquriy
We'll need to know what you want to contact us about so we can give you an answer
We have received your enquiry and will reply soon.
We're Sorry
The contact form is currently unavailable. Please try again later. If this problem persists, please call us with your enquiry on 1300 363 400 or +61 3 9545 2176. We are available from 9.00 am to 4.00 pm AEST Monday - Friday.
31 May 2022
Australia: A go-to destination for clinical trials
Australia’s world-leading clinical trials infrastructure, streamlined regulatory approval system, and grants and incentives attract both pharmaceutical giants and red-hot startups.
Chinese biotech giant BeiGene has enrolled thousands of patients in clinical trials in Australia since 2014. The company has over 2,000 patients at more than 300 research sites across Australia testing BeiGene’s products. It has 120 staff across the country and an in-house research team. In 2020, it invested more than A$35 million on clinical trials in Australia.[1]
US biotech startup Earli chose Australia to research and test its cancer detection technology at sites in Sydney, Melbourne and Adelaide. The company says the speed of Australia’s approvals process shaved at least six months off its development timelines.
‘We came to Australia because the local approval cycles are much shorter here,’ says Cyriac Roeding, Earli’s Chief Executive. ‘Approvals are happening at the local level, not at the federal government level. Australia is world leading in this regard.’[2]
A large and thriving clinical trials network
Australia has more than 50 clinical trial networks offering Phase I – IV clinical trials. Many clinical trial sites are located in biomedical precincts close to universities, research institutions and private industry. The sector boasts an 8,000-strong workforce with capabilities across all life sciences.
Australia offers clinical trials for several biologics sub-sectors, including gene therapies, cell therapies, antibody-based therapies, CAR-T therapies and RNA therapies. Clinical trials for several COVID-19 vaccine candidates, including for CSL and Novavax, were conducted in Australia.
Novavax engaged Australia’s Nucleus Network to conduct Phase I and II clinical trials for its COVID-19 vaccine candidate. Nucleus Network had previously worked with Novavax on Phase 1 clinical trials for an Ebola treatment. Nucleus Network recruited 200 participants for the Novavax trial over 2020.
‘The urgent global race to develop a vaccine against the COVID-19 pandemic drove our rapid identification and selection of an optimal, highly immunogenic vaccine candidate,’ says Stanley C. Erck, President and CEO of Novavax. ‘We are pleased that Nucleus, our long-time partner, was able to accommodate our accelerated timeline.’[3]
Strong, pro-innovation and globally recognised regulatory system
Australia is a highly competitive clinical trials destination, due to a streamlined approval process and a globally recognised regulatory system. Its excellent public and private health systems are open to trialling and adopting new technologies.
Australia’s large, multicultural population also ensures easy access to ethnically diverse patient cohort. Australians are highly engaged with healthcare, which facilitates recruitment. In 2019, 95,000 Australians took part in over 1,800 clinical trials.[4]
Australia is a particularly receptive market for the development of genomics and precision medicine. Three-quarters of Australians would be willing to use genetic testing to identify the most effective drug to treat their disease. Ninety-five per cent are willing for their results to be used to improve treatments for future patients.
Data from clinical trials conducted in Australia is accepted by key jurisdictions, including the US Food and Drug Administration (FDA) and European Medicines Evaluation Agency (EMEA). This ensures research does not lose momentum. Clinical trials undertaken in Australia do not require US FDA Investigational New Drug (IND) application approval.
‘On average in Australia, the first patient into a clinical trial [after approvals] is about three months, whereas in the US or the EU, it’s six to nine months — it’s efficient,’ says BeiGene’s commercial vice president and head of APAC, Adam Roach.[5]
The Sydney Children’s Hospital Network was the only Australian site selected to participate in the global SPR1NT trial. The trial investigated the use of Zolgensma®, a novel viral vector-based gene replacement therapy, to treat children diagnosed with Spinal Muscular Atrophy. Results from the Australian trials fed into the global study.
Australia’s legal system also provides robust protection for intellectual property, encourages innovation and safeguards investment. Australia’s IP regime consistently ranks in the top tier of international IP systems, and is aligned with international standards.
Thriving research and industry ecosystem
Australia’s large research and industry ecosystem offers opportunities to partner with Australian companies and institutions on clinical trials. Australia has over 1,200 pharmaceutical and medical technology companies, 55 medical research institutes and 40 universities focused on clinical research.
UK company Gyroscope Therapeutics is partnering with the Centre for Eye Research Australia (CERA) to deliver Australia’s first experimental gene therapy for dry aged-related macular degeneration. The Phase II trials are testing the safety and effectiveness of Gyroscope’s experimental gene therapy. CERA performed the first surgeries to deliver the therapy in 2021.
Singapore-based biopharmaceutical company Cerecin chose Australia to conduct Phase II clinical trials for an experimental migraine prevention drug. The study was conducted exclusively in Australia at 9 sites across the country. Cerecin worked with a specialist neurologist from the Department of Neuroscience at Monash University as lead investigator.
R&D grants and incentives
Australia offers grants and incentives that can help reduce clinical trial costs. The National Health Medical Research Council Fund is the largest single source of direct government funding for clinical trials in Australia. It provides around A$100 million in funding annually through a wide range of programs.
Australia’s grants and incentives for R&D activities include:
- The R&D Tax Incentive offers tax offsets for eligible R&D expenditure, reducing the cost to businesses of undertaking R&D activities
- The Patent Box is a A$206 million scheme to reduce taxes on income from Australian medical and biotech patents. It aims to encourage businesses to undertake their R&D in Australia and keep patents here
- The Medical Research Future Fund includes A$750 million over 10 years from 2022–23 for Clinical Trials Activity
- The mRNA Clinical Trials Enabling Infrastructure Grant Opportunity supports medical research and medical innovation projects that use emerging technologies, platforms, equipment and infrastructure to conduct clinical trials of mRNA-based vaccines and therapeutics
- The Biomedical Translation Fund is a A$500 million fund for promising biomedical discoveries sponsored by the government and matched by private sector investors.
[1] Sydney Morning Herald, Cancer-fighting firm BeiGene expands operations in Australia (smh.com.au) , 3 May 2022 [2] Sydney Morning Herald, Cancer detection: Australia’s ‘high quality’ clinical trials lures Silicon Valley cancer fighter (smh.com.au) , 5 April 2022 [3] Nucleus Network (May 2020), Novavax to commence COVID-19 vaccine trial with Nucleus Network , media release [4] MTPConnect (2021), Australia’s clinical trials sector. [5] Sydney Morning Herald, Cancer-fighting firm BeiGene expands operations in Australia (smh.com.au) , 3 May 2022
Invest in Australia
Global Australia offers tailored support for high-value foreign businesses to expand to Australia.
Visit Global Australia
Related analysis
Australia’s a$7 billion cyber security opportunity.
Australia is looking for new solutions to bolster its cyber security capability and fight cybercrimes.
Making modern medicines in Australia
Global pharmaceutical giants engage Australian contract development and manufacturing organisations to make life-saving therapies. Read about partnerships.
Australia’s multibillion-dollar 5G opportunities
Australia offers multibillion-dollar investment opportunities in 5G.
Footer content
Australian Government Department of Health and Aged Care
Medical Research Commercialisation initiative
The Medical Research Commercialisation initiative will provide $450 million over 10 years from 2022-23 to support innovative early stage health and medical research in Australia. It will help researchers transform ideas into life-saving medicines, devices and treatments to help future patients.
About the Medical Research Commercialisation initiative
The Medical Research Commercialisation initiative will provide $450 million over 10 years between 2022-23 and 2031-32.
The initiative will build on previous support for projects with commercial potential (particularly from small and medium-sized enterprises). It will focus on supporting research discoveries, including for novel or repurposed drugs, devices and digital health technologies, as they progress from proof-of-concept through to clinical implementation.
This initiative supports:
- BioMedTech Horizons , which funds innovative and collaborative health technologies. It drives discoveries that address key health challenges towards proof-of-concept and commercialisation, maximising entrepreneurship and idea potential ($45 million over four years from 2017-18; closed for new funding support)
- the Biomedical Translation Bridge , which funds and nurtures early stage health and medical research to reach proof-of-concept with the potential to attract further capital and support. Research will have secured at least matched funding from third-party sponsorship or co-investment ($22.3 million over four years from 2018-19; closed for new funding support)
- Early Stage Translation and Commercialisation Support, a $79 million activity providing funds to support early stage Australian medical research and medical innovation projects with commercial potential ($79 million over three years from 2020-21; $19.75 million per Stream)
- BioMedTech Incubator, a $50 million activity intended to improve the health and wellbeing of Australians by increasing the number of innovations, novel drugs, novel uses for existing drugs, innovative medical devices and/or digital health technologies that have progressed through the early stages of research and development to the point where they are ‘de-risked’ and attractive to private investment or commercialisation ($50 million over two years from 2022-23).
Why it is important
It is hard to turn a scientific discovery into a medical product that’s ready for clinical use. Taking a project from an idea to a final product is a long and expensive process.
This initiative is important because it develops Australia’s world-class research into real treatments, drugs and devices to benefit Australians.
The objective of this initiative is to support early stage health and medical research and innovation in Australia through to proof-of-concept and beyond, providing opportunities for commercialisation.
Meeting our objectives
We will monitor the initiative in accordance with the principles and approach detailed in the MRFF Monitoring, evaluation and learning strategy .
Who we work with
The Medical Research Future Fund (MRFF) funds the initiative.
Our Health and Medical Research Office oversees this and other MRFF initiatives.
MTPConnect delivers the BioMedTech Horizons and Biomedical Translation Bridge.
The Early Stage Translation and Commercialisation Support activity is delivered through four Streams by the Medical Research Commercialisation Fund, ANDHealth and MTPConnect.
Apply for funding
View the MRFF grants calendar to see which grants are open, when applications close and when we expect to award funding.
Register with GrantConnect to receive notifications about future funding opportunities under this initiative.
Monitoring, evaluation and learning
In 2020, we commissioned a review of the medical research commercialisation landscape in Australia, to map existing government initiatives and understand the sector’s views on:
- existing government funding support for the commercialisation of medical research and any perceived gaps
- the impact of MRFF initiatives on improving the commercialisation of research
- how institutions have positioned themselves to make the most of medical research and innovation commercial opportunities
- other barriers to research commercialisation.
Read the outcomes of the Medical Research Commercialisation Landscape report .
Learn more about MRFF monitoring, evaluation and learning .
Grants awarded
See a list of all MRFF grant recipients .
For more information contact us.

Medical Research Future Fund (MRFF) contact
MTPConnect contact
- Health data and medical research
- Medical Research Future Fund
Is there anything wrong with this page?
Help us improve health.gov.au
If you would like a response please use the enquiries form instead.

- Case Studies
- Get in touch

Our mission
Research Australia uses its unique convening power to position health and medical research as a significant driver of a healthy population and contributor to a healthy economy.

Engage Australians in a conversation about the health benefits and economic value of its investment in health and medical research

Connect researchers, funders and consumers to increase investment in health and medical research from all sources

Research Australia continues to influence government policies that support effective health and medical research
Research Australia Advocacy
Research Australia is in the unique position of being the voice of health and medical research participants rights across the health and medical research pipeline.It is because of this unique position we have broad insight into what patients and consumers, funders, researchers and commercial groups can contribute and require from it.
Health and medical research has made a significant contribution in the last century to improved health outcomes around the world, and it holds the promise for even greater advances in the future. Australia has world-leading expertise in health and medical research, and Australian governments, companies, and individuals make significant investments in health and medical research.

Research in perimenopause and menopause
Pre Budget Submission calls for greater investment in health and medical research and innovation
Research australia appoints two new board members university of wollongong vice-chancellor professor patricia m. davidson and csl’s dr michael wilson.

Research Australia Appoints New Chair of Board Martin Bowles AO PSM
Congratulations to the data innovation award winner: dr craig dalton.
The future of National Digital Research Infrastructure
Home » Medical Research Institutes
Medical Research Institutes
AAMRI’s 58 member organisations are internationally recognised leaders in health and medical research that work on a broad spectrum of human health issues. These include areas such as preventive health, chronic disease, mental health, immunology and Indigenous health. Their research ranges from fundamental biomedical discovery through to clinical research and the translation of research findings from bench to bedside. The majority of our members are independent Medical Research Institutes; that is they are mission-driven charities that operate independently from a university or hospital.

Australia’s Medical Research Institutes have a combined annual turnover of over $1 billion. As non-profit organisations, Medical Research Institutes receive a large proportion of their research funding through competitive grants for research projects—primarily through the Federal Government’s National Health and Medical Research Council (NHMRC). Other income for Medical Research Institutes is from a variety of sources including state and federal government funding for research support and infrastructure, competitive grants from foundations and trusts, commercial income, and philanthropic donations.
Australia’s Medical Research Institutes are well placed to achieve high level of research impact as their research is focused on improving health outcomes and are closely linked with clinical and health services. Primarily based on hospital campuses, Medical Research Institutes have a distinct and vital role in the health and medical research sector, providing a direct interface between laboratory-based research and clinical practice. Through affiliations with leading universities, medical research institutes also provide a cohesive training environment, attracting many of Australia’s best graduates and training the next generation of world-class researchers and scientists. For more information on what health and medical research is underway at AAMRI’s member institutes, refer to the filtering tool on our Members page .
Medical Research Institutes are all not-for-profit charities endorsed as Deductible Gift Recipients (DGRs). This allows individuals and organisations to provide donations to Medical Research Institutes to help them achieve their objectives to improve human health and lessen suffering across the globe.
AAMRI’s member organisations together employ 19,000 staff and higher degree students and range in size from 20 to more than 1,000 FTE.
View a list of our members , and search for members by research topic or state.
Information on becoming an AAMRI member organisation .
Stay Up To Date
Sign up to AAMRI e-news for regular updates

Research Organisations
Comprising of more than 13 major medical research institutes, eleven teaching hospitals and nine universities, Victoria is home to Australia’s largest bioscience research community.
A number of well-known national and international research organisations are based in Victoria.
Medical Research Organisations in Victoria
Australian Genome Research Facility – is an efficient state-of-the-art facility for the collection of molecular genetic information covering large-scale DNA sequencing, genotyping, microarraying, agricultural genomic services and other resources for the genetic and physical mapping of chromosomes, mutation detection and associated bioinformatic analysis.
Baker IDI Heart and Diabetes Institute – tackles the deadly trio of obesity, diabetes and cardiovascular disease through research, education and patient care.
Bio21 Institute for Molecular Science & Biotechnology – is a multidisciplinary research centre, specialising in medical, agricultural and environmental biotechnology.
Bionics Institute – is an independent, non-profit, medical research organisation.
Burnet Institute – The Burnet Institute is a leading Australian medical research and public health organisation focused on improving the health of disadvantaged and marginalised groups.
Centre for Eye Research Australia – The Centre brings together a body of dedicated medical researchers to improve the living conditions and lifestyles of thousands of people both in Australia and abroad who are now, or may in the future, be affected by vision impairment.
Epworth Research Institute – Epworth Research Institute (ERI) has built a reputation as a high quality medical research institute within the private teaching hospital environment. It fosters a vibrant research culture that is committed to excellence and values clinically relevant research that translates into better outcomes for patients here and around the world.
European Molecular Biology Laboratory – EMBL Australia was launched on 29th March 2010, making Australia the first Associate Member of EMBL. EMBL Australia provides Australian researchers access to EMBL through activities such as funded research positions, collaborative ventures and the formation of research institutes.
Institute for Breathing and Sleep – The Institute for Breathing and Sleep (IBAS) is an incorporated not-for-profit organisation designed to facilitate and coordinate research, education and public advocacy in respiratory and sleep health.
Institute for Safety, Compensation and Recovery Research – Established in April 2009, the Institute for Safety, Compensation and Recovery Research (ISCRR) is a joint initiative of WorkSafe Victoria, the Transport Accident Commission (TAC) and Monash University.
Ludwig Institute for Cancer Research – is a worldwide network of nine branches in seven countries dedicated to basic and clinical cancer research.
Mental Health Research Institute – mission is to further knowledge in mental health, behaviour and neuroscience.
Monash Institute of Medical Research – is committed to excellence in the performance of medical and biological research into the science of reproduction, development and growth.
Murdoch Childrens Research Institute (MCRI) – conducts life-saving medical research and community health research for babies, children and adolescents.
National Ageing Research Institute Inc. – strives to be the centre of excellence in medical research (biological, clinical and service delivery) into the causes and consequences of ageing and its social accompaniments.
National Health and Medical Research Council (NHMRC) – Supporting health and medical research.
National Trauma Research Institute – The NTRI aims to prevent or limit the disabling effects of traumatic injuries through research and education in trauma care. National Vision Research Institute – Founded in 1972, NVRI pursues research in vision and the disorders of vision.
O’Brien Institute of Microsurgery – The Institute has made striking advances in the delicate craft of replantation surgery and the transfer by microsurgical techniques of body parts and tissue to reconstruct people maimed by trauma, cancer, burns and congenital deformity.
Orygen Youth Health – Orygen Youth Health Research Centre (OYH-RC) is Australia’s largest youth mental health research centre. Our research aims to understand the biological, psychological and social factors that influence mental illnesses in order to find better ways to prevent and/or reduce the impact of mental disorders for young people.
Peter MacCallum Cancer Centre – a premier resource for cancer patients in the provision of integrated treatment, research and education.
Prince Henry’s Institute of Medical Research – has maintained a reputation for excellence in the field of endocrinology (the study of hormones).
St Vincent’s Institute of Medical Research – an enviable reputation within the global scientific community as one of the world’s premier medical research institutes, and is a shining example of Australian intellectual excellence and achievement.
Walter and Eliza Hall Institute of Medical Research – is one of Australia’s foremost medical research establishments, its mission being “mastery of disease through discovery”.
Teaching Hospitals in Victoria
The Alfred – Alfred Health is a leader in health care delivery, improvement, research and education. We strive to achieve the best possible health outcomes for our patients and our community by integrating clinical practice with research and education.
Austin Health – Austin Health is the major provider of tertiary health services, health professional education and research in the northeast of Melbourne. Austin Health is world-renowned for its research and specialist work in cancer, liver transplantation, spinal cord injuries, neurology, endocrinology, mental health and rehabilitation.
Barwon Health (Geelong Hospital) – The Geelong Hospital has a full suite of medical and surgical services, including cardiothoracic surgery and is one of the busiest hospitals in the state. It is a 406 bed general medical and surgical teaching hospital affiliated with The University of Melbourne and Deakin University with obstetric, paediatric and psychiatric beds.
Monash Medical Centre – Monash Medical Centre Clayton is a 640 bed teaching and research hospital of international standing providing a comprehensive range of specialist surgical, medical, allied health and mental health services to our community.
Peter MacCallum Cancer Centre – Peter MacCallum Cancer Centre is a world leader in cancer treatment, research and education. At Peter Mac we treat more cancer patients each year than any other hospital and our highly skilled medical, nursing and allied health team is backed by the largest cancer research group in Australia.
Royal Children’s Hospital – As the major paediatric hospital in Victoria, the Royal Children’s Hospital provides clinical, academic and advocacy services for children and young people throughout the state. It is internationally recognised as a leading centre for research and education. In its role as a leading paediatric teaching centre, the hospital has affiliations with the University of Melbourne and LaTrobe University and links with other universities in post graduate studies.
Royal Melbourne Hospital – The Royal Melbourne Hospital is one of Victoria’s leading public teaching hospitals, and operates across two campuses. The Royal Melbourne Hospital is a privileged member of Melbourne’s world-leading Parkville Precinct, and enjoys strong relationships with many of the city’s major universities and research institutes.
Royal Women’s Hospital – The Royal Women’s Hospital is Australia’s largest specialist obstetric, gynaecological and neonatal paediatric hospital. We have an outstanding international reputation for research advances and clinical developments.
Royal Victorian Eye and Ear – Proudly serving Victorians since 1863, the Royal Victorian Eye and Ear Hospital is a state-wide teaching, training and research health service. Specialising in eye, ear, nose and throat (ENT) medicine, it is Australia’s pre-eminent specialty eye and ear hospital.
St Vincent ’s Health – Education is one of the three core roles of St. Vincent’s, along with patient care and research. Most senior staff play an active role in teaching and training. A number of our senior staff hold professorial appointments with the University of Melbourne. While educating health professionals is a priority, St. Vincent’s supports staff at all levels to complete further study and training in their field of expertise.
Western Hospital – Western Health aims to provide work based education and training for Western Health staff and students which will support excellence in practice and career progression opportunity. Some of our training programs are also available as open programs for members of the health and local community.
Universities
Deakin University – By 2012, Deakin aims to have improved its research performance so that it is in the top third of the Australian higher education sector. The staff of Deakin University are responsible for its success. Their dedication to our students, to the research we conduct and to the communities we serve, will ensure the continued success of Deakin University.
Federation University Australia – Federation University Australia is an institution that combines teaching and research and especially one where teaching is informed by research. The university has moved, in a comparatively short period of time, to being research active. This is a credit to the University’s leaders, the staff and the students. The quest is now to increase the University’s comparative performance. The University continues to focus its research in those areas that both serve and benefit the region and the Institute for Regional and Rural Research and Innovation (IRRRI) leads that focus through its designated research centres.
La Trobe University – La Trobe’s research strategy encourages innovation, specialisation and collaboration. La Trobe is affiliated with the Biosciences Research Centre, the CRC for Plant Biosecurity, the CRC for Molecular Plant Breeding, the Bushfire Cooperative Research Centre, the Centre for Sustainable Regional Communities, as well as Departments/Programs including Botany and Zoology.
Monash University – Monash University is committed to finding solutions for 21st Century challenges such as climate change, water shortage, cancer, diabetes, obesity and those posed by terrorism. With campuses and partnerships in Australia and overseas, Monash pursues knowledge and solutions from a global perspective.
RMIT University – We focus on applied research that is delivered in partnership with leading organisations and individuals who are capable of using research outcomes to create products and services that are leading edge. The passion, energy and intellect of our researchers is used to make a difference to the economic, social, environmental and cultural well-being of the communities we operate within here in Australia and internationally through innovative research and research excellence.
Swinburne University of Technology – A commitment to high quality, high impact research is ingrained in Swinburne University. Swinburne has made remarkable progress in research and intends to continue to foster its various areas of research focus – on its way to becoming an internationally respected research-intensive University of Technology. Swinburne actively encourages and values its international and industry connections and recognises that it is this that enriches and enhances the research efforts and careers of our academic staff and students alike.
University of Melbourne – The University of Melbourne is Australia’s second largest research organisation after the CSIRO. The Melbourne Research Office and the School of Graduate Studies are the two major central academic support groups which facilitate the responsible conduct of research through educational, preventive, and service activities.
Victoria University – At Victoria University we provide quality research training, and undertake specialised and cross-disciplinary research that is recognised in Australia and overseas. Our staff and research students are part of an innovative research community made up of University institutes, research centres and faculty research units that make a substantial contribution in their field. We actively pursue opportunities to collaborate with other researchers and organisations to develop innovative technologies that can also be commercialised and transferred for use by business, industry and the broader community.
NHMRC Centres of Clinical Research Excellence (CCREs)
Diabetes CCRE – Based at the University of Melbourne, the Diabetes CCRE seeks to encourage and promote clinical research in diabetes and its complications, with the ultimate goal of improving the treatment and prevention of this disease.
CCRE for Translational Clinical Research in Major Eye Diseases – This CCRE will fund a world-leading, broad-based, clinical and translational research program in Melbourne and Sydney to tackle the four eye diseases that cause the majority of vision loss in Australia: age-related macular degeneration, diabetic retinopathy, cataract and glaucoma eye diseases. The CCRE will be headquartered at the Centre for Eye Research Australia in Victoria.
CCRE in Newborn Medicine – Based at the Murdoch Childrens Research Institute, the CCRE in Newborn Medicine will study adverse outcomes for the brains and lungs of newborn babies.
Biosciences Research Centre – AgriBio, the Centre for AgriBioscience.
The Victorian Government, through the Department of Primary Industries (DPI), and La Trobe University (La Trobe) are investing in a world-class centre for agricultural biosciences research and development through a AUD$288 million public-private partnership.
The building will be known as AgriBio, the Centre for AgriBioscience . AgriBio will focus on cutting-edge research to improve productivity, fight disease and reduce environmental impact, to protect Victoria’s $11.8 billion agricultural sector.
CRCs are an incorporated or unincorporated organisation, formed through collaborative partnerships between publicly funded researchers and end users. CRCs must comprise at least one Australian end-user (either from the private, public or community sector) and one Australian higher education institution (or research institute affiliated with a university).
Cooperative Research Centre
There are currently seven broad CRC sectors.
CRC for Biomarker Translation (CRC-BT) – The CRC-BT focuses on the development of diagnostic and therapeutic biomarkers for the treatment of cancer and autoimmune diseases. The CRC-BT is headquartered at La Trobe University, Bundoora, Victoria. CRC for Biomedical Imaging Development (CRC BID) – The primary aims of the CRC BID are to develop new and advanced imaging techniques and equipment for application in biomedicine; and to foster the growth of Australian expertise in biomedical imaging. The CRC BID is headquartered in Bundoora, Victoria.
Cancer Therapeutics CRC (CTx) – The focus of the CTx is working on the discovery and early development of small molecule drugs or enabling therapeutics for the treatment of cancer. Ctx is headquartered at the Walter and Eliza Hall Institute’s Biotechnology Centre in Bundoora, Victoria.
CRC for Mental Health – The CRC for Mental Health researches early detection and treatment of neurodegenerative diseases and psychoses. The CRC’s research includes areas such as Alzheimer’s and Parkinson’s Disease and psychoses such as schizophrenia and mood disorders.
CRC for Oral Health Science – The CRC for Oral Health Science brings together scientists, dentists, population health experts and manufacturers to find new and efficient ways of reducing the burden of oral disease in Australia. The CRC for Oral Health Science is headquartered at the Royal Dental Hospital, Carlton, Victoria.
CRC for Polymers – The research focus of the CRC for Polymers is on developing ‘functional’ (i.e. responsive to physical or biological stimuli) and higher value-added specialty polymers for emerging high growth opportunities. The Polymer CRC is headquartered in Notting Hill, Victoria. The HEARing CRC – The HEARing CRC brings together an internationally unique consortium dedicated to the common purpose of ‘creating sound value’ through research – to prevent and better remediate the lost productivity resulting from hearing loss in children and adults. The HEARing CRC is headquartered at the University of Melbourne, Parkville, Victoria.
News & opinion
Member Directory
BioMelbourne Network respectfully acknowledges the Traditional Custodians of the unceded lands and waters of Victoria, the Bunurong, Eastern Maar, Gunaikurnai, Wadawurrung and Wurundjeri Woi-wurrung peoples, on which BioMelbourne Network lives and works. We pay respect to their Elders past and present. We acknowledge the significant contribution that they and other Aboriginal and Torres Strait Islander people have made and continue to make to the research and knowledge systems that inform our community and our sector. We acknowledge that Aboriginal and Torres Strait Islander people continue to live in a spiritual and sacred relationship with this country and recognise our responsibility to continue to work towards reconciliation.

Copyright © BioMelbourne Network Network
- Privacy Policy
Website by Silver Lane Studio
Top 49 medical and healthcare startups in Australia

Contact an Austrade specialist as an investor as a buyer
Health and life sciences
State ecosystems
Australia’s fast-growing life sciences sector is one of the largest in the southern hemisphere. It’s worth more than $250 billion and home to more than 2,600 organisations. Our high-quality and innovative healthcare system is envied around the world. It’s underpinned by a world-class medical research sector, nurtured in our universities and hospitals, medical research institutes and life science companies.
Thinking about entering the Australian market?
- Investors typically establish a new company, register as a foreign company or acquire an existing company. Assess your options with our Investor Guide .
- Austrade is Australia’s national investment promotion agency. We attract and facilitate game-changing foreign direct investment into Australia. Our team of business and investment specialists can connect investors to early-stage opportunities in Australia and provide direct and tailored professional assistance. Find out how we can help .
Australia’s progressive environment fosters research translation, manufacturing and clinical adoption with ease. Businesses considering expanding to Australia will find:
- An established and early-adopting healthcare system
- A vibrant innovation ecosystem with world-class medical research expertise and infrastructure.
- Outstanding clinical trial capabilities, with streamlined and globally-recognised regulatory pathways to expedite studies.
- Significant government support, including an R&D tax offset of up to 43.5% and more than A$21.5 billion in support funds for life sciences.
- An export-driven economy with 18 free trade agreements
- Strong intellectual property protections
Advanced Therapeutics in Australia 2023
Australia has full bench to bedside capabilities. Find out why Australia is an outstanding location to research, develop and scale your life sciences innovations.
Source: AusBiotech Snapshot 2022
A culture of innovation
Australia has a strong culture of innovation and investment in medical research. It makes us a key development hub for cutting-edge medical procedures and life-saving medicines.
We consistently rank among Nature Index’s top 10 contributors to life sciences research globally – including ninth in 2022 .
World-leading medical research institutes are found across the country, including the Walter and Eliza Hall Institute in Melbourne, Queensland’s Translational Research Institute , the South Australia Health and Medical Research Institute , the Harry Perkins Institute of Medical Research in Perth and the Garvan Institute of Medical Research in Sydney.
Our world-class universities top the leaderboards in multiple health and medical research fields, such as oncology, neurology, regenerative medicine, medical devices, tropical diseases, diabetes, cardiovascular disease and diagnostics.
Collaborating with an Australian university provides access to some of the world's best minds. The Australian government is investing in university-industry partnerships through the A$2.2 billion University Research Commercialisation Package .
Top Australian innovations
With a history of Nobel Prize-winning research, Australia has made significant contributions to global medical science:
- GARDASIL® – the HPV vaccine that is significantly lowering the risk of HPV-related cancers for women and men around the world
- Electronic pacemaker
- Ultrasound for pregnancy
- Bionic ear – the cochlear implant is one of the earliest examples of improving wellbeing through digital technology
- Spray-on skin
- Prevention of spina bifida with folate
- Penthrox® inhaler (green whistle) – a pain relief inhaler used in trauma and emergency settings
- CPAP – a successful, non-invasive breathing support device – invented in 1981 and used by patients during the COVID-19 pandemic
- EVestG – a diagnostic tool that detects mental and neurological illnesses.
- Australia ranks 3rd among high-income countries for healthcare choice and fifth for quality (Source: 2022 FREOPP World Index of Healthcare Innovation )
- We’re 2nd among OECD countries for health (Source: OECD Better Life Index )
- Australia spends US$176 billion on health - the 6th highest health expenditure in the world. (Source: Fitch Solutions Group, Worldwide medical devices market factbook 2022 )
- Australia is a “go-to” clinical trial destination, with the world’s 3rd highest rate of industry-initiated early phase clinical trials (Source: ABPI 2022 )
- 11% of global cell and gene therapies clinical trials were conducted in Australia (Source: AusBiotech 2021 )
- More than four in five Australians support patients’ medical records being used for research ( Research Australia 2020 ). More than nine in ten would share their de-identified personal health information to advance medical research, support patient care, develop new diagnostics or treatments or track diseases ( Research Australia 2021 )
- Australia ranks 3rd for intellectual property rights on the International Property Rights Index 2022
Our vibrant industries
Aged care is a growing industry in Australia. The Australian population is ageing rapidly: by 2066, it’s estimated that 22% of the Australian population will be aged 65 years or over ( Australian Bureau of Statistics, 2018 ). The Australian Government is forecast to spend A$36 billion nationwide in 2023-24 .
The Australian biotech sector has grown by 40% in the last two years. It’s valued at almost A$250 billion market capitalisation (Source: Stockhead 2022). With the establishment of a new Moderna mRNA vaccine manufacturing facility in Victoria further expanding Australia's vaccine manufacturing capability, the sector is expected to generate over A$8 billion in gross value added and $12 billion in manufacturing exports. With a vibrant research ecosystem and established clinical trials sector, there is significant demand that are creating opportunities for new investment into Australia.
Clinical Trials
Australia’s clinical trial capabilities, skilled workforce and specialised infrastructure are second to none. Our globally recognised regulator, the Therapeutic Goods Administration (TGA), provides fast-track regulatory pathways. Quality data from Australian studies can be used to support international submissions, including to the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).
Digital Health
Australia is at the forefront of adopting innovative digital health technologies such as telehealth, electronic patient records and wearable devices. This is partly due to the development of connected national health infrastructure. It’s supported by multi-billion-dollar government investments and initiatives to establish data standards and drive interoperability.
Our health consumer ecosystems, market dynamics and regulatory frameworks are similar to the US and EU. It makes us an ideal location to pilot new digital health solutions before scaling to global markets.
The total market for medical devices in Australia is valued over US$4.9 billion (Source: Medical Technology Association of Australia 2021/22 ). Australia’s strengths include bionic devices; implantables; nanotechnology; wearable devices; digital smart devices; digital therapeutics and 3D-printed body parts. Australia provides a low-risk testbed to develop and test medtech products, with quality clinical trials data accepted in other major markets.
Grants, incentives and support
- the A$450 million , which supports innovative early-stage health and medical research in Australia.
- the A$47 million MRFF Targeted Translation Research Accelerator , which is improving the prevention and treatment of diabetes and cardiovascular disease.
- The Clinical Trials Activity initiative. This will provide $750 million over 10 years from 2022–23 to help Australian researchers and patients test new treatments through national and international clinical trials.
- A new A$50 million Australian Government-funded incubator program is due to launch in 2023, providing grants of up to $5 million for emerging biomedical and digital health companies. The new BioMedTech Incubator (BMTI) program will be jointly delivered by venture firm Brandon Capital’s BioCatalyst program and digital health accelerator ANDHealth.
- The National Reconstruction Fund (NRF) has A$1.5 billion earmarked for medical manufacturing through co-investment by the Australian Government via debt or equity support or guarantees.
- The Biomedical Translation Fund is A$501 million co-investment program helping translate biomedical discoveries into products and services.
- The Research and Development Tax Incentive makes Australia globally competitive for companies looking to innovate. Companies can access an R&D tax offset of up to 43.5% for eligible R&D expenditure greater than A$20,000.
- MTPConnect is an independent Australia Government-supported industry growth centre that for the medtech, biotech and pharmaceutical sectors.
- The National Health and Medical Research Council supports investigator-led research activities across all areas of health and medicine, providing grants in excess of A$850 million a year.
- The Australian Research Council funds fundamental and applied research across a broad range of health and medical science fields.
- AusBiotech has launched a directory of accelerators and incubators relevant to Australian life sciences companies in the early stages of their development. The majority of programmes listed provide funding.
- Australia boasts a number of health precincts that connect hospitals, universities, research institutes and industry partners support collaboration and knowledge transfer such as the QEII Medical Centre and Adelaide BioMed City .
Life science capability map
Explore Australia’s capabilities and innovation in the life sciences sector.
Australia’s national science agency, CSIRO, is one of the world's largest mission-driven multidisciplinary science and research organisations.
CSIRO consistently ranks in the top 1% of the world’s scientific institutions in 15 of 22 research fields (CSIRO Annual Report 2021-22 ).
Its expertise includes molecular diagnostic solutions and precision health and medicine research. Facilities include:
- National Vaccine and Therapeutics Lab
- Recombinant Protein Production and Purification Facility
- Australian Centre for Disease Preparedness
- Biomedical Materials Translational Facility
Success stories

SK bioscience partners with Vaxxas on needle-free typhoid vaccine
SK bioscience is partnering with Australian biotech company Vaxxas to develop a needle-free, second-generation typhoid vaccine. The partnership is supported by a US$3.67 million grant from global charitable foundation Wellcome.
Under the partnership agreement, SK bioscience will supply the antigen used by its typhoid conjugate vaccine, SKYTyphoid™. The vaccine was developed by SK bioscience and the International Vaccine Institute, with funding support from the Bill and Melinda Gates Foundation.
Vaxxas will reformulate the antigen so it can be “printed” onto its high-density microarray patch (HD-MAP) technology. The technology uses a patch with thousands of vaccine-coated micro-projections. The patch is applied to the skin for a few seconds to deliver the vaccine to the immune cells immediately below the skin’s surface.
The antigen will also be reformulated to be more stable at high temperatures than required for needle and syringe vaccination. The vaccines could then be potentially stored and distributed at room temperature, making them more accessible to lower- and middle-income countries with limited cold-chain distribution infrastructure.
Vaxxas will conduct preclinical studies on the reformulated vaccine. If successful, the studies will be followed by a Phase I human clinical trial. The project is expected to be completed within two years from initiation to reporting the data from the Phase I clinical trial.
Fighting influenza with the Doherty Institute
SK bioscience has also signed a research collaboration agreement with the Peter Doherty Institute for Infection and Immunity ( Doherty Institute ) in Melbourne, Australia.
The agreement will include:
testing anti-influenza compounds to identify new antivirals
capacity building in low- and middle-income countries of the region
developing a new influenza vaccine platform.
The Doherty Institute is one of seven WHO Collaborating Centres for Reference and Research Influenza (WHOCCRRI). The Doherty Institute has more than 700 staff who work on infection and immunity through a broad spectrum of activities. This includes discovery research; diagnosis, surveillance and investigation of infectious disease outbreaks; and the development of ways to prevent, treat and eliminate infectious diseases.
Australia’s healthcare system is underpinned by a world-class medical research sector, nurtured in our universities and hospitals, medical research institutes and life science companies. Find out more about Australia’s thriving health and life sciences sector.
How Austrade helped
Austrade will continue to provide SK bioscience with insights on the Australian life sciences market and introduce the company to collaborative research opportunities.
Austrade will support international delegates attending the AusBiotech 2023 conference (1–3 November 2023) in Brisbane. The Seoul Bio Hub will host the Korea-themed session on 2 November followed by the Korea night networking event.
Go further, faster with Austrade
Contact Austrade for more information about investment opportunities in Australia
Subscribe to the Investment Update newsletter to find out about new investment opportunities, insights and investor success stories across Australia.
Published: 25 September 2023

Spinogenix to conduct world-first clinical trial in Australia
US biotech company Spinogenix is conducting a world-first clinical trial in Australia. The company is investigating a therapy that could slow the progression of motor neurone disease.
Spinogenix is recruiting 112 participants for the trial, set to begin in 2024. It is seeking healthy volunteers and patients with Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s Disease.
Nucleus Network will conduct the trials at its facilities in Melbourne, Victoria. The company is one of Australia’s largest Phase 1 clinical trials organisations. It has conducted over 1,000 trials for global biotechnology and pharmaceutical companies.
‘We are extremely pleased to receive approval from [Australia’s] Human Research Ethics Committee to proceed with human clinical trials,’ says Spinogenix Founder and CEO Dr. Stella Sarraf. ‘These human clinical trials will help us determine safety and tolerability in healthy volunteers. They will also provide early signals of efficacy of our novel, first-in-class drug to help improve the lives of people with ALS.
A new therapy to treat ALS
Founded in 2016, Spinogenix develops transformative therapeutics for diseases involving synaptic loss and dysfunction.
Spinogenix’s SPG302 treatment candidate is designed to increase the number of synapses in nerve cells. Synapses are the key connections between neurons that allow people to think, plan, remember and control motor functions. These faculties are diminished in neurodegenerative diseases like ALS.
In tests on mice, SPG302 was found to slow the progression of symptoms. It was also found to prolong life span by restoring broken neuron connections in the brain.
The current therapies for ALS provide only a modest extension of life and are not well tolerated by all patients. ALS is almost invariably fatal within 3 to 5 years of diagnosis.
A top destination for clinical trials
Australia has more than 50 clinical trial networks offering Phase I – IV clinical trials. Many clinical trial sites are in biomedical precincts close to universities, research institutions and private industry. Australia offers clinical trials for several biologics sub-sectors, including gene therapies, cell therapies, antibody-based therapies, CAR-T therapies and RNA therapies.
Data from clinical trials conducted in Australia is accepted by the US Food and Drug Administration (FDA) and European Medicines Evaluation Agency (EMEA). Australia’s streamlined regulatory approval system reduces the time it takes start clinical trials.
Australia also offers grants and incentives that can help reduce clinical trial costs.
Find out more about Australia’s clinical trials capabilities .
How Austrade helped
Austrade provided Spinogenix with information about Australia’s clinical trials ecosystem and facilitated introductions to local partners. Additionally, Spinogenix participated in Austrade’s Clinical Trials Roadshow in October 2022, which was delivered in partnership with IQVIA, 360 Biolabs, Bentleys and Nucleus Network.
Go further, faster with Austrade
Contact Austrade for more information about investment opportunities in Australia
- Subscribe to the Investment Update newsletter to find out about new investment opportunities, insights and investor success stories across Australia.
Published: 15 September 2023

Myeloid and NSW Government partner on A$96m manufacturing facility dedicated to RNA immunotherapies
Myeloid Therapeutics is building a state-of-the-art RNA manufacturing and research facility in Australia. The US company is partnering with the New South Wales Government and Macquarie University on the project.
The NSW Government is investing A$96 million in the facility. It will be the only site in Australia and one of a handful in the world where a wide range of RNA therapeutics and potential delivery technologies will be independently produced.
The facility will accelerate the commercialisation of Myeloid’s RNA-based therapeutics and enable the company to secure its supply chain for clinical development. The investment and collaboration will also enhance the growth potential of other biotechnology companies and researchers in Australia.
Speeding the development and commercialisation of RNA therapeutics
Myeloid is a clinical stage mRNA-immunotherapy company. It integrates the fields of RNA, immunology and medicine to develop novel therapies for cancer and autoimmune diseases.
The state-of-the-art RNA manufacturing and research facility will be at the Macquarie University campus in the Connect Macquarie Park Innovation District . The facility will include laboratories and other support spaces to support the development of products evaluated in Australian clinical trials and across the globe.
The facility will leverage NSW’s world-class cell and gene therapy expertise. It will also leverage the NSW RNA Production & Research Network, the UNSW RNA Institute and Australia’s first Viral Vector Manufacturing Facility at the Westmead Health and Innovation District. Myeloid’s CEO and co-founder Dr. Daniel Getts is an Australian researcher.
‘By enabling control of our manufacturing processes, combined with our proven bioengineering capabilities and mRNA expertise, we have secured a unique, non-dilutive path to delivering our product portfolio to patients,’ he says.
‘We are proud to partner with NSW, who have demonstrated foresight and a willingness to innovate, by deploying capital for the facility and more broadly, for the health benefit of its citizens. We and NSW share the goal to bring innovative, cost-effective immunotherapies to patients who need them in a timely manner and on a consistent basis.’ Find out more about Australia’s thriving health and life sciences sector.
Austrade has supported Myeloid since May 2022. Austrade’s advice and introductions ensured Myeloid secured the best location and partners for its investment.
Austrade has:
introduced Myeloid to Australian state governments to discuss opportunities
invited Myeloid to various conferences and industry events where the company could meet Australian industry and build its network
facilitated meetings with potential partners including medical research institutes and pharmaceutical companies
arranged introductions to potential co-investors and financial sector leaders
advised on Australian Government funding including the Medical Research Future Fund and Cooperative Research Centre grants
invited Myeloid to join Austrade’s BIO23 delegation, where the company participated in panel discussions and attended Austrade’s investment roundtable.
Published: 3 September 2023

Singapore Success Story - Cerecin
Global pharmaceutical company Cerecin, which develops therapeutics for neurological disorders, is undertaking clinical trials and expanding its research capabilities in Melbourne, Australia. Charles Stacey, Cerecin’s President and CEO, explains why it has chosen Australia to expand its global footprint. Benefits include its diverse population mix, R&D incentives and human capital.
Contact an Austrade Investment Specialist in your region for more information about the pharmaceutical industry in Australia.
Published: 13 April 2023

Singapore Success Story - Homage
Singapore’s Homage is bringing its personalised aged care and support services platform to the Australian market. Homage co-founder and CEO Gillian Tee explains why Australia is a strategic location for growth.
Read more about Australia's growing aged care industry.
Contact an Austrade Investment Specialist in your region for more information about aged care in Australia.

Agility and innovation make Australia’s Linear a top clinical trials host
There was a time when Jayden Rogers had to explain to potential clients where Australia was and why they should conduct clinical trials here. Today, Australia is known as one of the best places in the world for clinical trials. This is in part due to the high-quality work undertaken by Rogers’ company, Linear Clinical Research.
Linear is an Australian not-for-profit organisation offering Phase I to Phase III clinical trials. To date, Linear has carried out over 420 studies in 20 therapeutic areas. It has worked with more than 300 sponsors from 18 countries. During the pandemic, the company saw record patient enrolments. It undertook over a dozen trials for COVID-19 therapeutics, two of which are now approved.
‘Australia has a well-deserved reputation as a world leader in clinical trials,’ says Rogers, Linear’s CEO. ‘We have a high-quality, advanced healthcare system, brilliant scientists and clinicians, and excellent R&D. Our streamlined regulatory approval process and generous research incentives also support international companies to conduct trials here.’
Tech innovation supports world-class trials
Founded in 2010, Linear has 2 state-of-the-art facilities in Western Australia. One of these is in the QEII Medical Centre, the largest medical precinct in the Southern Hemisphere.
Linear has a strong focus on Phase I healthy volunteer and Phase I cancer trials. The company has more than 100 early-phase cancer trials in its portfolio. It has one of the most active cancer trials team in the Asia-Pacific region.
Later in 2023, Linear will open a dedicated clinical trial private hospital for early-phase cancer trials. This will be the only one of its kind in Australia and it will triple Linear’s existing capacity.
Innovation has also played a part in Linear’s success. In 2017, the company was the first in Australia to install the eSource platform to capture data at the point of entry. It has also invested in telemetry and technology to monitor vital signs remotely. This investment in technology came to the fore during the pandemic.
‘Because we had electronic systems in place, we were able to switch to remote monitoring in just 7 days,’ says Rogers. ‘Our clinicians in Australia and clients in the US, Europe and China could view trial data in real time. It’s a collaborative way to run clinical trials.’
Clinical trials during COVID-19
Finding clinical trial participants during the pandemic was not an issue. In Western Australia, there is strong reach into many patient groups which are often consolidated into a small number of hospitals. This supports strong patient recruitment for clinical trials.
Linear also has over 40,000 healthy volunteer participants in its database. Australia’s multicultural population – 29% of citizens are born overseas – ensures a diverse patient cohort for clinical trials.
‘We delivered record enrolments for our cancer trials,’ says Rogers. ‘These patients would not have received treatment in other facilities. And we’re seeing amazing response rates. Up to 75% of patients observe some form of response with a small number experiencing complete responses (full remission) as a result of new therapeutics. It’s lifechanging.’
Linear also deployed its capabilities to support COVID-19 vaccine and therapeutic trials. The company worked with Chinese biotech Clover to undertake Phase I clinical trials for a vaccine candidate. It had the trial up and running in two weeks following ethics submission. The Phase I trial recruited 140 volunteers as a single site. This enabled a subsequent global Phase II/III trial that went on to demonstrate successful protection against COVID-19.
‘The Clover vaccine is now among the group of vaccines used in China,’ says Rogers. ‘It was thrilling to play a role in its development.’
Australia’s accelerated trial approval process
Linear undertook more than a dozen COVID-19 trials and prophylactic studies across a range of therapeutic modalities from 2020–2022. One of these was with Stanford University to trial an antibody to prevent COVID-19.
‘We worked around the clock with the Stanford researchers to help design the protocol,’ says Rogers. ‘Then we obtained ethics committee approval within 15 days, recruited patients and had the trial running within 20 days. It was the quickest clinical trial Linear has ever done. It is probably one of the quickest clinical trials in history, from the point of engagement to clinical execution and the delivery of data.’
Linear works with Bellberry, an organisation providing scientific and ethical review of human research projects. During the pandemic, Bellberry had an accelerated approval process for COVID-19 therapeutics and vaccines.
‘Australia’s pragmatic regulatory environment is one of our key strengths,’ says Rogers. ‘The Therapeutic Goods Administration delegates trial reviews to human-research ethics committees like Bellberry. This speeds approval times so clinical trials can be set up quickly but safely.’
Austrade paves entry into China
Almost all of Linear’s clients are international pharmaceutical or biotech firms. Most of them are from the US, followed by China and the EU. Rogers says Austrade was instrumental in helping Linear enter the Chinese market in 2017.
‘We’ve had tremendous support from Austrade over years,’ says Rogers. ‘The on-the-ground support in China was an important aspect of our growth in that market. Their staff went above and beyond. I would happily recommend Austrade to any business wanting to expand their overseas markets.’
Keeping innovation on the agenda
The past few years have seen major changes in therapeutics and approaches to clinical trials.
‘We’re seeing new molecules being tested, including bi-specific antibodies and drugs that target certain mutations based on genomics,’ say Rogers. ‘COVID-19 saw a shift to new treatments for infectious diseases and respiratory viruses. We’re seeing more targeted therapeutics, particularly mRNA therapeutics and cell therapies.
‘The world made a vaccine for COVID-19 in 12 months, thanks to research, industry and government working together,’ says Rogers. ‘At Linear, we want to keep pushing the innovation agenda. It’s the only way we can keep creating drugs that save lives.’
Contact an Austrade Investment Specialist in your region for more information about conducting clinical trials in Australia.
Published: 30 March 2023

- Promoted Listings
- Purchase Data
- Drug Pipelines
- Data Licensing
Australia - Biotech Companies


AAMRI showcases Australia’s Medical Research Discoveries
Showcasing breakthroughs from australian medical research institutes.

The Association of Australian Medical Research Institutes (AAMRI) has today launched a new website, Medical Research Discoveries, to bring together the best stories and discoveries from medical research institutes to one centralised place.
“Being part of AMMRI gives us all a collective voice to the Government” said Sandra Bellekom, CEO Ear Science Institute Australia. “As a group and through this website, it helps us communicate the value of medical research institutes to the general public. They can see and read about all the exceptional science being conducted right here in Australia” she said
“We designed this showcase to demonstrate to Australians what our sector does – and how much amazing work comes out of our institutes,” said Professor Kathryn North AC, AAMRI President .
“It makes me feel immense pride to see all the discoveries arising across Australia – a new affordable dialysis system that will help millions, a novel trial for diabetes, to the entire NatureBank in Queensland for biodiscovery on Australian flora and fauna,” said Professor North. “At our Institutes, we are talking about these things every day, and now we have the opportunity for people to get the full picture of what medical research institutes really do.”
When AAMRI decided to bring together new discoveries from across our medical research institutes, the response to the call was overwhelming. The website has started with 20 discoveries today, with another 50 going up over the next few weeks, building continuously.
The site also profiles all 58 Institutes contributing to the site and lists 25 research areas all linked back to each discovery and institute.
“When asked about medical discoveries from Australia, the cervical cancer vaccine and the cochlear ear often spring to mind. Amazing, life-changing science. With this showcase, we hope to expand the conversation, and lift the lid on medical research institutes so people can really see the impact of our research,” said Professor North.
https://www.medicalresearchdiscoveries.org.au/
About AAMRI AAMRI is the peak body representing medical research institutes across Australia. Our 58 member organisations are international leaders in health and medical research, addressing practically every aspect of human health and disease. Collectively, AAMRI’s members represent more than 19,000 staff and students.
Related Posts:

Largest Australian Companies in the Biotechnology industry by Market Cap
This is the list of the largest public listed companies in the Biotechnology industry from Australia by market capitalization with links to their reference stock.
Top 1-year algo backtest: +287.69%
$10,000 in March 2023 would now be $38,769 by following this algorithm daily at market close.
Boost your stocks returns with Disfold AI... Now!
Try Disfold AI for FREE

Top Biotechnology Companies from Australia as of Jan. 01, 2024
1. csl limited.
Company Profile: CSL Limited Market Cap (USD): $94.74 B Country: Australia Sector: Healthcare Industry: Biotechnology
2. Neuren Pharmaceuticals Limited
Company Profile: Neuren Pharmaceuticals Limited Market Cap (USD): $2.13 B Country: Australia Sector: Healthcare Industry: Biotechnology
3. Telix Pharmaceuticals Limited
Company Profile: Telix Pharmaceuticals Limited Market Cap (USD): $2.11 B Country: Australia Sector: Healthcare Industry: Biotechnology
4. PolyNovo Limited
Company Profile: PolyNovo Limited Market Cap (USD): $759.2 M Country: Australia Sector: Healthcare Industry: Biotechnology
5. Imugene Limited
Company Profile: Imugene Limited Market Cap (USD): $585.9 M Country: Australia Sector: Healthcare Industry: Biotechnology
6. Clinuvel Pharmaceuticals Limited
Company Profile: Clinuvel Pharmaceuticals Limited Market Cap (USD): $548.0 M Country: Australia Sector: Healthcare Industry: Biotechnology
7. Immutep Limited
Company Profile: Immutep Limited Market Cap (USD): $279.4 M Country: Australia Sector: Healthcare Industry: Biotechnology
8. Opthea Limited
Company Profile: Opthea Limited Market Cap (USD): $277.6 M Country: Australia Sector: Healthcare Industry: Biotechnology
9. PYC Therapeutics Limited
Company Profile: PYC Therapeutics Limited Market Cap (USD): $236.5 M Country: Australia Sector: Healthcare Industry: Biotechnology
10. Botanix Pharmaceuticals Limited
Company Profile: Botanix Pharmaceuticals Limited Market Cap (USD): $200.2 M Country: Australia Sector: Healthcare Industry: Biotechnology
11. Mesoblast Limited
Company Profile: Mesoblast Limited Market Cap (USD): $172.4 M Country: Australia Sector: Healthcare Industry: Biotechnology
12. Paradigm Biopharmaceuticals Limited
Company Profile: Paradigm Biopharmaceuticals Limited Market Cap (USD): $108.9 M Country: Australia Sector: Healthcare Industry: Biotechnology
13. Race Oncology Limited
Company Profile: Race Oncology Limited Market Cap (USD): $93.0 M Country: Australia Sector: Healthcare Industry: Biotechnology
14. Recce Pharmaceuticals Ltd
Company Profile: Recce Pharmaceuticals Ltd Market Cap (USD): $72.1 M Country: Australia Sector: Healthcare Industry: Biotechnology
15. Proteomics International Laboratories Limited
Company Profile: Proteomics International Laboratories Limited Market Cap (USD): $70.8 M Country: Australia Sector: Healthcare Industry: Biotechnology
16. Biotron Limited
Company Profile: Biotron Limited Market Cap (USD): $70.7 M Country: Australia Sector: Healthcare Industry: Biotechnology
17. Dimerix Limited
Company Profile: Dimerix Limited Market Cap (USD): $65.3 M Country: Australia Sector: Healthcare Industry: Biotechnology
18. Orthocell Limited
Company Profile: Orthocell Limited Market Cap (USD): $56.3 M Country: Australia Sector: Healthcare Industry: Biotechnology
19. Mawson Infrastructure Group, Inc.
Company Profile: Mawson Infrastructure Group, Inc. Market Cap (USD): $53.9 M Country: Australia Sector: Healthcare Industry: Biotechnology
20. AnteoTech Limited
Company Profile: AnteoTech Limited Market Cap (USD): $53.9 M Country: Australia Sector: Healthcare Industry: Biotechnology
21. Starpharma Holdings Limited
Company Profile: Starpharma Holdings Limited Market Cap (USD): $49.1 M Country: Australia Sector: Healthcare Industry: Biotechnology
22. Actinogen Medical Limited
Company Profile: Actinogen Medical Limited Market Cap (USD): $34.6 M Country: Australia Sector: Healthcare Industry: Biotechnology
23. Prescient Therapeutics Limited
Company Profile: Prescient Therapeutics Limited Market Cap (USD): $33.5 M Country: Australia Sector: Healthcare Industry: Biotechnology
24. PharmAust Limited
Company Profile: PharmAust Limited Market Cap (USD): $32.8 M Country: Australia Sector: Healthcare Industry: Biotechnology
25. Bionomics Limited
Company Profile: Bionomics Limited Market Cap (USD): $20.6 M (July 1, 2023) Country: Australia Sector: Healthcare Industry: Biotechnology
26. Kazia Therapeutics Limited
Company Profile: Kazia Therapeutics Limited Market Cap (USD): $19.1 M (July 1, 2023) Country: Australia Sector: Healthcare Industry: Biotechnology
27. Chimeric Therapeutics Limited
Company Profile: Chimeric Therapeutics Limited Market Cap (USD): $16.3 M Country: Australia Sector: Healthcare Industry: Biotechnology
28. Noxopharm Limited
Company Profile: Noxopharm Limited Market Cap (USD): $16.1 M Country: Australia Sector: Healthcare Industry: Biotechnology
29. Living Cell Technologies Limited
Company Profile: Living Cell Technologies Limited Market Cap (USD): $15.6 M Country: Australia Sector: Healthcare Industry: Biotechnology
30. Cynata Therapeutics Limited
Company Profile: Cynata Therapeutics Limited Market Cap (USD): $15.3 M Country: Australia Sector: Healthcare Industry: Biotechnology
Top 1-year algo backtest: +287.69% - Don't wait, try Disfold AI for FREE now!
The Disfold Newsletter
Join 3,000 subscribers who stay in touch with Disfold.
- attach_money Pricing
- lock_outline Login
- stars Try Disfold AI Free
- wb_incandescent Algorithms Backtests
- swap_vert Trading Signals
- visibility Watchlist
- Stock Indices
- dashboard World Dashboard
- format_list_numbered Top 1000 Companies
- public Countries
- class Sectors & Industries
- first_page Close Menu
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Operational excellence
- Delivery models
- Building patient insights into assets, profile and claims
- Portfolio Optimization
- Asset Valuation and Indication Prioritization
- Early Evidence Review
- Model-Based Drug Development
- Integrated Development Strategy and Planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Leveraging AI and digital in clinical development
- Biotech Clinical Trial Solutions
- Gain an advantage through FSP

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
- Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- Exploring ICH Q5A revision 2
- Potency assurance for CGT products: FDA's new draft guidance
- The FDA’s final guidance: CAR-T product development
Latest Report

New Medicines, Novel Insights: Achieving patient-guided drug development

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Management team
- Global reach
- Our DE&I strategy
- Compliance, Tax & Privacy
- Meet us at an event
- Jamie Macdonald
- Peyton Howell
- Michael Crowley
- Jonathan Shough
- Sheri McCoy
- Michael Bruun
- John Groetelaars
- Susan Salka
- Global Reach
- Our DE&I Strategy
- Our ESG Strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
- World Vaccine Congress
- Patients as Partners U.S.
- MAPS Americas
- ASCPT 2024 Annual Meeting
- Managing Complexity in Cell and Gene Trials: Establishing an RBQM Strategy that Drives Better Performance
- EDI in clinical research: The case for adaptive strategies at every step
- 17th ISCR ANNUAL CONFERENCE 2024
- IncluDE Site Solutions Summit
- AAN Annual Meeting
- ASGCT Annual Meeting
- Dermatology Drug Development Summit EU
- World Orphan Drug Congress US
- AACR Annual Meeting
- BIO International Convention
- DIA Global Annual Meeting
- International Society for Cell & Gene Therapy
- Final countdown: How to transition your trials under EU-CTR
- SCRS: Oncology Site Solutions Summit
- Accelerating Vaccine Development Through Operational Excellence
- Alzheimer’s Disease vs Multiple Sclerosis Drug Development: Similarities, Differences & New Perspectives
What can we help you find today?
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
Patient Story
- Cell & Gene Therapy
- Rare Diseases
- Inflammation & Immunology
- Neurosciences
One night, while watching TV with her husband, Robyn felt a lump in her neck.
She had large B-cell lymphoma — an aggressive cancer diagnosed in 150,000 patients each year globally.
As a doctor, she knew the risk. She got a CAT scan, looked at the images, and her world stopped.
Four years later the lymphoma returned. Robyn’s care team prescribed a further six cycles of a brutal chemotherapy, followed by an autologous stem cell transplant (ASCT) and external beam radiation. Robyn’s ASCT treatment was further complicated by septic shock requiring a stay in intensive care.
Robyn entered remission again, but it was short-lived, with the lymphoma returning nine months later. Treatment options for Robyn were now bleak and limited.
Robyn immediately sought treatment. With her husband by her side, she underwent many rounds of chemotherapy and went into remission.
Feeling defeated, she found a clinical trial for car t-cell therapy, a new treatment that reengineers white blood cells to target and eradicate cancer., she thought this was her last chance., a week after receiving treatment, her lymph nodes shrunk. within three months, there was no evidence of the disease., today, she’s cancer free — and sharing her experiences to help us better meet the needs of patients like her in our cell and gene therapy trials., lives can change when you design cell and gene therapy trials with speed and precision..
- Utilize the right experts, focused on the right indications
- Access even hard-to-find patient populations
- Pinpoint the perfect sites for your trial
- Satisfy global regulations, to get your treatment to market safely and quickly
What we do, we do
Our Experts
Our cell and gene therapy specialists collaborate to help get your innovative treatments to patients like Robyn faster.
MORE EXPERTS
Steve Winitsky
Vice President, Tech...
Stefan Zietze
Executive Director, ...
Hicham El Bariqi
Director, Cell & Gen...
Jingke Yang, M.D., Ph.D.
Global TA Section He...
Chris Learn, Ph.D., PMP

Vice President, Cell and Gene Therapy, Center of Excellence
With 20+ years of trial execution and team management experience, Chris leads development for our cell and gene therapeutic area. He reinforces our patient-first focus to help ensure your trials are designed to meet patient needs.
"Being able to capture and understand the patient’s perspective — not just as a patient, but as a person — and use their insight to guide my decisions is what it means to work With Heart™."
Our diverse experiences ensure you get the expertise you need, no matter the indication.
Our experience with CAGT clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Middle East & Africa
Access to global EMR data enables us to pinpoint the perfect sites for your trial.

Our cross-functional team overcomes any technical, logistical, and strategic challenges.
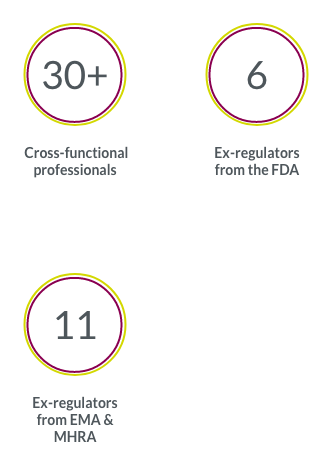
Our highly tailored recruitment and retention strategies drive access to even hard-to-find patient populations.
What can we do to help you change patient lives?
See CAGT capabilities Visit all therapeutic areas Explore CAGT careers
Want our latest insights for cell and gene therapy?
Ready to speak to someone on our team?
Are you a patient interested in a clinical trial?
We focus on patients, because they inspire us to deliver better trials, faster than ever . So we can make a difference for more patients like Robyn.
Who we are,, parexel is proudly among the world’s largest clinical research organizations.
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise , our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind , to make clinical research a care option for anyone, anywhere.
Australia Healthcare Research
- Veterinary Equipment
- Animal Drugs
- Animal Vaccines
- Purified Proteins
- Bioservices
- Biotechnology Research Equipment
- Protein Engineering
- Electro Therapy
- Nuclear Medicine
- Cardiovascular
- Dental Care
- Diabetes Care
- Ophthalmology
- Pulmonology
- Drug Filled Devices With Needle
- Clinical IT
- Healthcare Analytics
- Aesthetic Devices
- Clinical Diagnostic Instrument
- Immune System Diagnostics
- Infectious Disease Testing
- Handheld Monitoring Devices
- Single time Monitoring Devices
- Molecular Diagnostic Devices
- Medical Optical Imaging
- Nuclear Medicine Imaging
- Fluoroscopy
- Mammography
- Surgical Imaging
- Joint Reconstruction
- Drug Delivery Products
- Personal Protective Equipment
- Dental Medical Instruments
- Hospital and Clinical Use
- Ablation Devices
- General Surgical Devices
- Minimally Invasive Surgery Devices
- Bariatric Surgery Devices
- Surgical Access Devices
- Wound Care Devices
- Contraceptive Devices
- Anesthesia Equipment
- Insulin Pumps
- Hyperglycemics
- Behavioural Disorder Drugs
- Diabetes Care Drugs
- Oncology Drugs
- Developmental Anomalities
- External Condition Drugs
- Immune System Drugs
- Infectious Disease Drugs
- OB/GYN Drugs
Filter Reports
2316 Australia Healthcare Reports
Country Covered: Australia
Study Period: 2021 - 2029
Major Players: Abbott , Bio-Rad Laboratories, Inc, BD, Roche Diagnostics , bioMérieux SA
Study Period: 2019 - 2029
Major Players: Carestream Health Inc., Dentsply Sirona, Envista Holdings Corporation, GC Corporation, 3M Company

Major Players: Siemens AG, Koninklijke Philips NV, GE Healthcare, Carestream Health, Canon Australia Pty Ltd

Major Players: Boston Scientific Corporation, Medtronic PLC, Olympus Corporation, Stryker Corporation , Zimmer Biomet

Major Players: B. Braun Melsungen AG, Boston Scientific Corporation, Medtronic PLC, Johnson & Johnson, Conmed Corporation

Major Players: Natus Medical Incorporated, GE Healthcare, Koninklijke Philips NV, Getinge AB, Vyaire Medical

Major Players: Novartis AG, GlaxoSmithKline Plc, Becton, Dickinson and Company, Johnson and Johnson, Pfizer, Inc.

Major Players: Zoetis Inc, Vetoquinol SA, Elanco, Merck Co. Inc., Boehringer Ingelheim

Major Players: Advanced Sterilization Products Services Inc., 3M Healthcare, B. Braun Melsungen AG, Becton, Dickinson and Company, Cardinal Health Inc.

Major Players: Lumenis Inc., Cutera, Inc., Bausch Health Companies Inc. (Solta Medical, Inc.), Candela Corporation, Alma Lasers (Sisram Medical Ltd)

Major Players: Reckitt Benckiser, HERO, Bayer Healthcare, Lifestyles Healthcare, Merck & Co. Inc.

Major Players: Koninklijke Philips N.V, Mindray Medical International Co. Ltd., Nihon Kohden Corporation, Baxter International Inc., Siemens Healthcare GmbH

Study Period: 2018 - 2029
Major Players: Abbott Diabetes Care, Novo Nordisk A/S, Medtronic PLC, Dexcom Inc., Roche Diabetes Care

Major Players: Apollo Endosurgery Inc., Intuitive Surgical Inc., B. Braun SE, Medtronic, Johnson & Johnson

Major Players: Stryker Corporation, B. Braun Melsungen AG, Anatomics Pty Ltd, Nihon Kohden Corporation, Penumbra, Inc.

Major Players: Abbott Laboratories, Boston Scientific Corporation, Medtronic PLC, B. Braun SE, Cardinal Health Inc.

Major Players: Depuy Synthes Spine Inc. (Johnson & Johnson), Medtronic PLC, NuVasive Inc, Zimmer Holdings Inc, Orthofix Holdings Inc

Major Players: Abiomed Inc, LivaNova PLC, Bionic Vision Technologies, Ossur, Cochlear Ltd

Major Players: Stryker Corporation, FUJIFILM Holdings Corporation, Olympus Corporation, Medtronic Plc., Hoya Group (PENTAX Medical)

Major Players: Alcon Inc, Bausch Health Companies Inc, Johnson and Johnson, Carl Zeiss Meditec AG, Ziemer Ophthalmic Systems AG

Major Players: GSK PLC, GE Healthcare Inc., Drive Medical (DeVilbiss Healthcare LLC), Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Ltd

Major Players: 3M Health Care Ltd, Medtronic PLC, Medline Industries Inc., Smith & Nephew PLC, Johnson & Johnson

Major Players: Novo Nordisk, Dexcom, Abbott, Sanofi, Medtronic

Major Players: Abbvie Inc., Amgen Inc., AstraZeneca plc, Eli Lilly & Co., Pfizer Inc.

Major Players: Siemens AG, Planmed OY, Fujifilm Holdings Corporation, Hologic Inc., GE Healthcare

Major Players: GE Healthcare, Siemens Healthineers AG, Fujifilm Holdings Corporation, Koninklijke Philips NV, Mindray Medical International Limited

Major Players: Siemens AG, Canon Medical Systems, GE Healthcare, Fujifilm Holding Corporatio, Koninklijke Philips N.V.

Major Players: Eli Lilly, Boehringer Ingelheim, Astrazeneca, Sanofi, NovoNordisk

Major Players: GE Healthcare, Siemens Healthineers AG, Koninklijke Philips N.V., Canon Inc. (Canon Medical Systems Corporation), Bayer AG

Major Players: Carestream Health, GE Healthcare, Siemens Healthineers AG, Fujifilm Holdings Corporation , Koninklijke Philips NV

Please be sure to check your spam folder too.

Get a free sample of this report
Please enter your name
Business Email
Please enter a valid email
Please enter your phone number

Sorry! Payment Failed. Please check with your bank for further details.
Want to use this image? X
Please copy & paste this embed code onto your site:
Images must be attributed to Mordor Intelligence. Learn more
About The Embed Code X
Mordor Intelligence's images may only be used with attribution back to Mordor Intelligence. Using the Mordor Intelligence's embed code renders the image with an attribution line that satisfies this requirement.
In addition, by using the embed code, you reduce the load on your web server, because the image will be hosted on the same worldwide content delivery network Mordor Intelligence uses instead of your web server.
- Claim $100 of Free Data

Top 15 Clinical Research Companies: Leaders in Medical Innovation

In the healthcare industry, the number of contract research organizations in the US has reached 2,823 in 2023. This marks a subtle but significant increase of 0.9% compared to the previous year.
This increase signals a vital trend: the growing complexity of finding the best clinical research companies in a crowded field. These organizations aren’t just businesses; they’re important in advancing medicine and developing drugs and therapies.
With such an important task, choosing the right company becomes essential. In this guide, we’ve looked closely at many companies along with their strengths and weaknesses and made a list of the top clinical research organizations.
By the end, you’ll know which company is the best fit for your needs.
Table of Contents
Quick List of Top 15 Clinical Research Companies
Here is a quick overview of the best companies of clinical research:
- IQVIA: Best for data-driven insights and advanced analytics in healthcare research.
- ICON: Best for comprehensive clinical development services and therapeutic expertise.
- Parexel: Best for global biopharmaceutical services, emphasizing regulatory and clinical trial excellence.
- Syneos Health: Best for integrated biopharmaceutical solutions and clinical-commercial capabilities.
- PPD: Best for drug development services with innovative, technology-enhanced trial strategies.
- Labcorp: Best for comprehensive clinical testing and diagnostics services with global reach.
- Medpace: Best for expertise in clinical research and regulatory affairs for pharmaceutical companies.
- Charles River Laboratories: Best for preclinical research and development services, including animal testing and research models.
- PRA Health Sciences: Best for clinical trial expertise and integrated solutions for biopharmaceutical development.
- AdvanCell: Best for innovative cell and tissue-based research solutions for life science industries.
- Dynata: Best for data-driven insights and market research services for informed decision-making.
- Covance: Best for end-to-end drug development solutions, from preclinical to post-marketing.
- MedNet: Best for technology solutions and eClinical platforms for streamlined clinical trials.
- Fisher Clinical Services Inc: Best for global logistics and supply chain services for clinical trial materials.
- Worldwide Clinical Trials: Best for specialized CRO offering personalized clinical research solutions.
3 Best Clinical Research Organizations: Comparison Chart
Here’s a comparison table to highlight the key features and differences among the best companies of clinical research. This table aims to provide a quick overview of each company’s unique strengths and areas of expertise in the pharmaceutical and healthcare research sector.
3 Top Clinical Research Organization List For Advanced Medical Discoveries

Now, we’ll explore the top clinical research organizations (CROs) dedicated to advancing medical discoveries. Let’s jump into the details of these exceptional organizations.
IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.
Why is IQVIA among the best? Their strength lies in their vast database and advanced technology, which enable them to analyze complex healthcare data efficiently. This leads to a better understanding of diseases, more effective treatments, and faster drug development.
IQVIA’s work is essential because it speeds up the process of bringing new medicines to the market, ultimately benefiting patients worldwide. In short, IQVIA is a key catalyst in advancing global healthcare.

About IQVIA
- Founding Team: Dennis Gillings
- Founding Year: 1982
- Company Size: 86,000
Features of IQVIA
IQVIA, a prominent player in the life sciences sector, is dedicated to advancing healthcare through connected intelligence. Here are some key features of IQVIA in the world of clinical research:
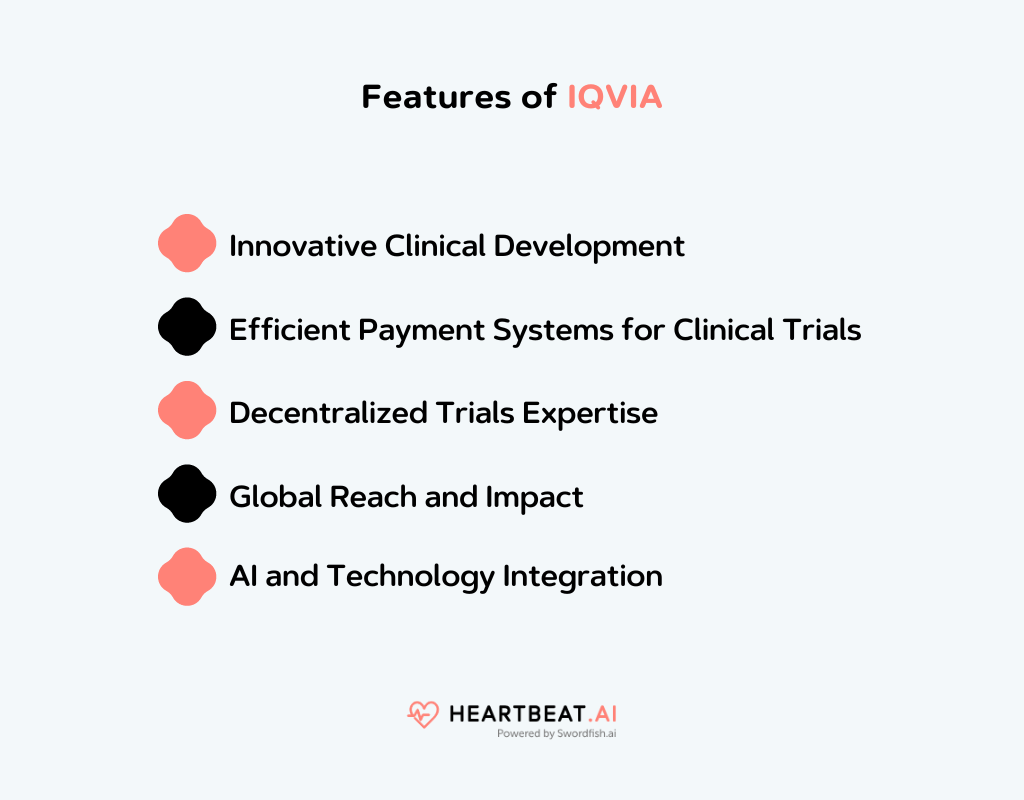
Innovative Clinical Development
IQVIA is reimagining clinical development by intelligently connecting data, technology, and analytics. This approach leads to faster decision-making and reduced risk, enabling the delivery of life-changing therapies more quickly.
Efficient Payment Systems for Clinical Trials
They have simplified the process of paying sites involved in clinical trials. IQVIA offers the capability to make payments within 30 days, even in challenging locations. This significantly reduces the administrative burden of managing clinical trial payments by up to 90%.
Decentralized Trials Expertise
The company has conducted over 500 studies in more than 75 countries, covering over 30 indications using decentralized trial methodologies. This demonstrates their capability in managing complex, multinational clinical trials.
Global Reach and Impact
With a presence in various regions, including Australia, New Zealand, the Middle East, and Africa, IQVIA’s global footprint allows it to drive healthcare innovations worldwide.
AI and Technology Integration
The company is at the forefront of integrating AI and other technologies in healthcare. Their Healthcare-grade AI promises precision, speed, scale, trust, and reliability, essential for advancing health and improving patient outcomes.
- Extensive, reliable healthcare data enhances market research quality.
- Utilizes AI and machine learning for advanced healthcare insights.
- Specialized focus yields a deep understanding of healthcare dynamics.
- Broad international presence enables diverse and large-scale studies.
- Offers advanced tools for insightful healthcare data analysis.
- Advanced tools can be challenging to use without training.
- Handling sensitive health data raises privacy and security issues.
Our Review of IQVIA
IQVIA, a prominent player in the healthcare and life sciences industry, presents a mixed bag of strengths and weaknesses. On the positive side, we appreciate IQVIA’s extensive expertise in data analytics and healthcare consulting.
Their comprehensive research and analysis have undoubtedly driven valuable insights and innovations in the sector. Moreover, their global presence allows for diverse perspectives and access to critical healthcare data.
However, we must also acknowledge some shortcomings. IQVIA’s services can be prohibitively expensive for smaller organizations, limiting accessibility. Additionally, the sheer volume of data can sometimes lead to information overload, making it challenging to extract actionable insights.
ICON is a prominent company in the field of clinical research, playing a significant role in advancing medical science. They specialize in designing and conducting clinical trials for new medicines and treatments.
The work of ICON is crucial because they help determine the safety and effectiveness of these potential medical breakthroughs. They are considered one of the best in clinical research due to their high standards of accuracy, reliability, and ethical practices.
ICON’s expertise ensures that the clinical trials they manage are conducted efficiently and effectively, leading to faster approval of new treatments. This directly impacts patient care, as it allows quicker access to new, potentially life-saving medicines.
In essence, ICON’s contribution is vital in driving forward medical innovations.
- Founding Team: John Climax and Ronan Lambe
- Founding Year: 1990
- Company Size: 41,160
Features of ICON
Here are some of the key features of ICON in clinical research:
Diverse Clinical and Scientific Operations
ICON offers a wide range of clinical and scientific operations services, ensuring comprehensive support for various aspects of clinical trials. This includes everything from study design to execution and data analysis.
Decentralized Clinical Trial Solutions
They provide end-to-end services, operational models, and technology to deliver customized solutions for decentralized clinical trials. This approach is increasingly important in today’s clinical research landscape, offering flexibility and efficiency.
Specialized Therapeutic Areas
ICON has expertise across multiple therapeutic areas including cardiovascular, central nervous system, endocrine & metabolic disorders, infectious diseases, internal medicine & immunology, oncology, and more. This broad expertise allows them to handle a wide range of clinical research projects.
Innovative Solutions for Biotech
ICON provides full-service outsourcing and flexible support customized to the specific needs of biotech companies. This includes due diligence and asset valuation, which are critical for biotech firms navigating the complex landscape of drug development.
Advanced Medical Imaging Solutions
Their expert medical imaging solutions support all stages of clinical research, improving decision-making, increasing efficiency, and reducing trial costs.
- Decades of expertise ensure high-quality clinical research.
- Offers wide-reaching capabilities for multi-regional clinical studies.
- Deep understanding of global regulations enhances compliance and efficiency.
- Invests in new technologies for more efficient trial processes.
- Broad range of specialties contributes to comprehensive service offerings.
- Managing multi-regional trials can lead to logistical challenges.
- Rapid growth may strain resources and affect service quality.
Our Review of ICON
When we researched ICON, we found both commendable aspects and areas for improvement. On the positive side, we appreciate their commitment to clinical research and their global presence, which allows for diverse study options. Their experienced team and advanced technology contribute to reliable data collection and analysis.
However, there are some drawbacks to consider. We have noticed occasional delays in project timelines, which can be frustrating. Additionally, the cost of their services tends to be on the higher side, making it a potential barrier for smaller research endeavors.
Parexel is a globally recognized company in clinical research, known for its important role in developing new medical treatments. They are one of the biggest clinical research organizations. Parexel conducts clinical trials, crucial steps in testing the safety and effectiveness of new drugs.
The work of Parexel is essential because it bridges the gap between medical research and the availability of new treatments to patients. One of the reasons they stand out as one of the best in this field is their rigorous approach to research.
Their commitment to quality and their global network also enables diverse and large-scale studies, setting them apart from others in the field. These strengths allow Parexel to deliver reliable and valuable data, accelerating the process of bringing new, effective medicines to the market.
Simply put, Parexel is a key player in transforming medical research into real-world health solutions.
About Parexel
- Founding Team: Josef von Rickenbach and Anne B. Sayigh
- Company Size: 18,900
Features of Parexel
Parexel, a global biopharmaceutical services organization, offers a range of features in clinical research. Here are some key aspects of their approach:
Patient-Centric Approach
Parexel emphasizes a patient-first strategy in their clinical trials. This approach results in deeper and more relevant insights for trial design and execution. This ensures that the trials are more aligned with patient needs and experiences.
Innovative Trial Designs
Parexel employs innovative trial designs to optimize trials for maximum impact. This includes advanced modeling and simulation to predict drug effects ahead of time, which can save time, money, and resources.
Regulatory Compliance and Market Access
Parexel designs studies and endpoints with market access in mind, ensuring that they satisfy global regulations. This approach helps in getting treatments to patients safely and quickly.
Patient Advocacy and Engagement
The company includes patient advocates in their council, using their experiences to improve trial designs. This inclusion demonstrates their commitment to understanding and incorporating patient perspectives in clinical research.
Focus on Speed and Precision
Parexel aims to design neuroscience trials with speed and precision, utilizing the right experts and specializations. This focus is crucial in delivering effective treatments on time.
- Provides advanced technology and analytics for efficient data management.
- Extensive network provides global insights with regional knowledge.
- Expertise in navigating complex regulatory environments worldwide.
- Broad experience across various therapeutic areas ensures versatile solutions.
- Focuses on patient engagement for more effective trial outcomes.
- Rapid expansion can lead to challenges in resource management.
- Concentration in specific areas could pose risks in market shifts.
Our Review of Parexel
Parexel is a notable player in the field of clinical research and pharmaceutical services. We’ve thoroughly analyzed their offerings and found both strengths and areas that need improvement.
On the positive side, Parexel excels in its commitment to innovation and technology. We appreciate their continuous efforts to simplify clinical trials and drug development processes, making them more efficient.
However, we also noticed some downsides. Communication with clients could be more transparent, with clearer updates on project progress. Additionally, there’s room for improvement in terms of ensuring consistency in service quality across different projects.
Other 12 Companies of Clinical Research

In the world of clinical research, beyond the well-known names, there are 12 other companies making significant contributions. Let’s explore their vital role in advancing healthcare.
1. Syneos Health
Syneos Health helps develop medicines by managing clinical trials for new drugs. They’re essential because they ensure medicines are safe and effective. Syneos Health stands out in clinical research for its comprehensive services and global reach, making drug development smoother and faster.
About Syneos Health
- Founding Team: Colin Shannon
- Founding Year: 1980
- Company Size: 28,000
PPD is a group that tests new drugs to see if they’re good and safe. This is crucial for getting new treatments to people. They stand out for their thorough research and global reach.
- Founding Team: Fred Eshelman
- Founding Year: 1985
- Company Size: 40,000+3
Labcorp does important tests and research for health. They’re needed because they help find out if new treatments are good. They’re among the best for their big labs and fast results.
About Labcorp
- Founding Team: Matthew Benger
- Founding Year: 1978
- Company Size: 75,5000
Medpace focuses on making sure new health treatments are safe. This is key for better medicine. They’re a top choice because of their focus on quality and detail in research.
About Medpace
- Founding Team: August Troendle
- Founding Year: 1992
- Company Size: 5,400
5. Charles River Laboratories
Charles River Laboratories tests drugs and does research to help pets and people stay healthy. They’re essential for safe, new treatments. Their expertise makes them a leader in the field.
About Charles River Laboratories
- Founding Team: Henry Foster
- Founding Year: 1947
- Company Size: 21,400
6. PRA Health Science
PRA Health Science works on finding out if new medicines are safe. This helps everyone get better treatments. They’re known for their excellent research and care in studies.
About PRA Health Science
- Founding Year: 1976
- Company Size: 17,000+
7. AdvanCell
AdvanCell specializes in new treatments, checking if they’re safe and working. Their work is vital for progress in medicine. They’re recognized for their innovation in research.
About AdvanCell
- Founding Team: Andrew Adamovich
Dynata gathers data for health studies. They’re needed for understanding what works in healthcare. They’re a top name for their accurate and wide-reaching data collection.
About Dynata
- Founding Team: Mike Petrullo
- Founding Year: 1940
- Company Size: 5000-10000
Covance helps with drug tests and research to fight diseases. Their role is key for new treatments. They’re celebrated for their comprehensive services and global impact.
About Covance
- Founding Team: Fred Cummings
- Founding Year: 1981
- Company Size: 50,000
MedNet provides software for managing clinical trials. This helps in making research easier and faster. They’re among the best for their tech solutions in research.
About MedNet
- Founding Team: John “Rob” Robertson
- Founding Year: 1996
- Company Size: 51-200
11. Fisher Clinical Services Inc.
Fisher Clinical Services Inc. manages the logistics of clinical trials, ensuring that treatments are tested efficiently. Their work is crucial for the progress of medicine, and they are renowned for their reliability and global network.
About Fisher Clinical Services Inc.
- Founding Team: John Pickering
- Founding Year: 1989
12. Worldwide Clinical Trials
Worldwide Clinical Trials conducts essential research to evaluate new medical treatments. Their work is critical for advancing healthcare. They are distinguished by their global expertise and commitment to innovation in clinical research.
About Worldwide Clinical Trials
- Founding Team: Neal Cutler
- Founding Year: 1986
- Company Size: 3,147
What To Consider When Choosing the Best Clinical Research Companies?
Choosing the right clinical research company (CRC) is crucial for the success of any clinical trial. Here’s a detailed guide on what to consider:
Expertise and Specialization
Always ensure the CRC has expertise in your specific therapeutic area. Companies with experience in similar drug trials or medical devices can better navigate the complexities of your project.
Regulatory Compliance
The CRC must adhere to regulatory guidelines like FDA (US) , EMA (Europe), and others. Check their track record in meeting these standards to avoid compliance issues.
Reputation and Track Record
You should research the company’s history. Look for testimonials, case studies, and reviews from past clients. A company with a strong reputation is likely to deliver quality results.
Project Management Capabilities
Effective project management is key. Assess their ability to manage timelines, budgets, and communication. A CRC that provides transparent, regular updates is preferable.
Patient Recruitment Strategies
Patient recruitment can be challenging. Evaluate their strategies for participant recruitment and retention. Consider their demographic reach and methods for ensuring a diverse participant pool.
Data Management and Analysis
The CRC should have strong systems for data collection, management, and analysis. Ask about their use of Electronic Data Capture (EDC) systems and how they handle data security and confidentiality.
Cost and Financial Terms
Get a clear understanding of the cost structure. Consider the value for money rather than just the lowest cost. Ensure there are no hidden fees and clarify what is included in the quoted price.
How Heartbeat AI Can Help You Get the Best List of Clinical Research Organizations?
Heartbeat AI, with its advanced features, can significantly assist in identifying the best list of clinical research organizations (CROs). Here’s how its various features contribute to this process:
Data Analysis and Processing
Heartbeat AI excels in analyzing vast amounts of data. When it comes to selecting CROs, it can process and analyze information from numerous sources, including past performance records, clinical trial reports, and regulatory compliance data. This thorough analysis helps in identifying CROs with a proven track record of success and reliability.
Machine Learning Algorithms
These algorithms enable Heartbeat AI to learn from historical data and improve its recommendations over time. By understanding trends and patterns in the successful execution of clinical trials, it can better predict which CROs are likely to meet your specific needs.
Predictive Analytics
Heartbeat AI uses predictive models to forecast future trends and outcomes based on historical data. This can be invaluable in predicting the success rate of CROs in upcoming projects, thus aiding in making more informed choices.
Customization and Personalization
The AI can be customized to your specific requirements. If you’re focusing on a specific therapeutic area or clinical trial phase, Heartbeat AI can prioritize specialized CROs in these fields.
Real-time Data Updates
The healthcare and pharmaceutical landscapes are constantly changing. Heartbeat AI’s ability to integrate and analyze real-time data ensures that the recommendations are based on the most current information available.
Integration with External Databases
Heartbeat AI can integrate with various external databases and platforms. This enables it to pull in comprehensive information about CROs from diverse sources, enhancing the accuracy of its recommendations.
Claim $500 of Free Data
Summing up, we’ve explored the best clinical research companies, diving into their features, strengths, weaknesses, and more. Clinical research is vital in healthcare; it’s key for advancing medical knowledge and developing new treatments.
With this guide, you’re equipped to find the right clinical research company that meets your specific needs. Whether it’s for innovative therapies, drug development, or medical advancements, choosing the right partner is crucial. This guide serves as a valuable resource to help you make an informed decision in the complex world of clinical research.
Frequently Asked Question
What services do companies of clinical research offer.
Clinical research organizations offer a wide range of services, including protocol development, patient recruitment, data collection and analysis, regulatory compliance, and more.
What is the role of a clinical research coordinator?
A clinical research coordinator is responsible for managing various aspects of a clinical trial, including patient recruitment, data collection, and ensuring compliance with protocols.
What is informed consent in clinical research?
Informed consent is the process by which participants in a clinical trial are fully informed about the study’s purpose, risks, and benefits. They voluntarily agree to participate based on this information.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Enter your account information
- Access 11m+ Healthcare providers
- 100% Risk Free Trial
- No credit card required
Enter your phone number

COMMENTS
iNGENū is the FDA-centric Australian CRO championing disruptive, innovative biotech firms globally. Our core mission is to create access to high quality clinical research globally, for early to mid-stage biotechs by removing financial and other unnec... 💻 Website ↗ 📞 +1300 633 226 View all details.
The company's clients are biotechs in their early phases of drug development that need fast, agile, and adaptive solution-oriented clinical research services. ... Avance Clinical had its genesis in the University of South Australia in the early 1980s as the Center for Pharmaceutical Research, when a group of academic scientists began ...
Register today! Established in 1993 CMAX is one of Australia's largest and most experienced clinical trial centres. Our expert team is committed to supporting innovative medical research in partnership with our national and international sponsors. Centrally positioned in Adelaide, CMAX is located adjacent to The Royal Adelaide Hospital in ...
Australia's Original Full Service CRO. Datapharm has earned an enviable reputation for detail and quality through involvement in hundreds of clinical trials in all phases (Phase I to IV) for clients ranging from large international pharmaceutical companies to local and international biotechnology companies, device manufacturers, producers of alternative therapies, private, hospital and ...
About us. Founded in 2010 and headquartered in Australia, Southern Star Research is a full-service clinical research organisation (CRO) helping sponsors to navigate the complexities of bringing new medical products to market. Since commencing operations in Sydney, we have grown to become an international team of specialists, managing studies ...
Mobius Medical is a top tier, full service clinical research partner offering a customized service with unparalleled responsiveness. Owner operated, Mobius has operations throughout the United States, Australia and New Zealand, and staff based globally. With a well-established, ISO:9001-certified Quality Management System, you can rely on our ...
CSIRO's Biomedical Materials Translational Facility (BMTF) helps Medtech companies turn new discoveries into market ready products. It has the capability and equipment needed to develop a biomedical product though prototyping, scale-up, pre-clinical testing and industry evaluation and offers access to ISO 9001, ISO 17025 and GLP.
The Patent Box is a A$206 million scheme to reduce taxes on income from Australian medical and biotech patents. It aims to encourage businesses to undertake their R&D in Australia and keep patents here. The Medical Research Future Fund includes A$750 million over 10 years from 2022-23 for Clinical Trials Activity.
The Biomedical Translation Fund invests in companies to help them turn medical research into products and services. Learn more about funding. Find information about health and medical research funding opportunities from GrantConnect, the NHMRC and the MRFF grant opportunities calendar. See more about Australia's health status and medical ...
The Medical Research Commercialisation initiative will provide $450 million over 10 years from 2022-23 to support innovative early stage health and medical research in Australia. It will help researchers transform ideas into life-saving medicines, devices and treatments to help future patients.
Australia has over 50 pharmaceutical companies, 400 biotechnology companies and 500 medical technology companies, where over 100 are listed on the Australian Stock Exchange (MTP Connect, 2016). Australia's Medical Technology and Pharmaceutical sector is the 8th most valuable exporter for the nation, worth $8.2 billion in 2019 (MTPConnect, 2020).
Molecule2Market is a team of expert clinical research professionals dedicated to advancing Australia's contribution to the research and development of innovative medical treatments. Our directors have accumulated over 50 years experience in the development of life-changing medical innovations. Our team of expert clinical trial consultants and ...
Health and medical research has made a significant contribution in the last century to improved health outcomes around the world, and it holds the promise for even greater advances in the future. Australia has world-leading expertise in health and medical research, and Australian governments, companies, and individuals make significant ...
Australia's Medical Research Institutes are well placed to achieve high level of research impact as their research is focused on improving health outcomes and are closely linked with clinical and health services. Primarily based on hospital campuses, Medical Research Institutes have a distinct and vital role in the health and medical research ...
Bionics Institute - is an independent, non-profit, medical research organisation. Burnet Institute - The Burnet Institute is a leading Australian medical research and public health organisation focused on improving the health of disadvantaged and marginalised groups. Centre for Eye Research Australia - The Centre brings together a body of ...
Upvio. Upvio is a revolutionary healthcare technology company that builds software designed to empower medical, health, and wellness professionals to tech-enable and adopt groundbreaking tech tools that redefine hybrid, virtual, and remote care. 46.
Grants, incentives and support. Australia's A$20 billion Medical Research Future Fund (MRFF) aims to transform health and medical research and innovation. It includes: the A$450 million , which supports innovative early-stage health and medical research in Australia. the A$47 million MRFF Targeted Translation Research Accelerator, which is ...
Australia - Biotech Companies. Order by: Company Name - Location. Australia - Cannon Hill: ... Medical Device Clinical Research: AtCor Medical : Australia - Barangaroo: Cardiovascular diagnostic devices: Crux Biolabs : Australia - Bayswater: Specialist Immunology Contract Research. PBMC processing, Flow Cytometry, ELIspot & Custom Immunoassays ...
AAMRI is the peak body representing medical research institutes across Australia. Our 58 member organisations are international leaders in health and medical research, addressing practically every aspect of human health and disease. Collectively, AAMRI's members represent more than 19,000 staff and students.
Top Biotechnology Companies from Australia as of Jan. 01, 2024. Rank Company Market Cap (USD) Country Sector Industry; 1: CSL Limited: $94.74 B Australia Healthcare: Biotechnology: 2: Neuren Pharmaceuticals Limited: $2.13 B ... Actinogen Medical Limited
Parexel is proudly among the world's largestclinical research organizations. A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical ...
Australia Healthcare Research. 2316 comprehensive market analysis studies and industry reports on the Healthcare sector, offering an industry overview with historical data since 2019 and forecasts up to 2029. This includes a detailed market research of 13449 research companies, enriched with industry statistics, industry insights, and a ...
1. IQVIA. IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.