- Main Library
- Digital Fabrication Lab
- Data Visualization Lab
- Business Learning Center
- Klai Juba Wald Architectural Studies Library
- NDSU Nursing at Sanford Health Library
- Research Assistance
- Special Collections
- Digital Collections
- Collection Development Policy
- Course Reserves
- Request Library Instruction
- Main Library Services
- Alumni & Community
- Academic Support Services in the Library
- Libraries Resources for Employees
- Book Equipment or Study Rooms
- Librarians by Academic Subject
- Germans from Russia Heritage Collection
- NDSU Archives
- Mission, Vision, and Strategic Plan 2022-2024
- Staff Directory
- Floor Plans
- The Libraries Magazine
- Accommodations for People with Disabilities
- Annual Report
- Donate to the Libraries
- Equity, Diversity and Inclusion
- Faculty Senate Library Committee
- Undergraduate Research Award

What is an original research article?
An original research article is a report of research activity that is written by the researchers who conducted the research or experiment. Original research articles may also be referred to as: “primary research articles” or “primary scientific literature.” In science courses, instructors may also refer to these as “peer-reviewed articles” or “refereed articles.”
Original research articles in the sciences have a specific purpose, follow a scientific article format, are peer reviewed, and published in academic journals.
Identifying Original Research: What to Look For
An "original research article" is an article that is reporting original research about new data or theories that have not been previously published. That might be the results of new experiments, or newly derived models or simulations. The article will include a detailed description of the methods used to produce them, so that other researchers can verify them. This description is often found in a section called "methods" or "materials and methods" or similar. Similarly, the results will generally be described in great detail, often in a section called "results."
Since the original research article is reporting the results of new research, the authors should be the scientists who conducted that research. They will have expertise in the field, and will usually be employed by a university or research lab.
In comparison, a newspaper or magazine article (such as in The New York Times or National Geographic ) will usually be written by a journalist reporting on the actions of someone else.
An original research article will be written by and for scientists who study related topics. As such, the article should use precise, technical language to ensure that other researchers have an exact understanding of what was done, how to do it, and why it matters. There will be plentiful citations to previous work, helping place the research article in a broader context. The article will be published in an academic journal, follow a scientific format, and undergo peer-review.
Original research articles in the sciences follow the scientific format. ( This tutorial from North Carolina State University illustrates some of the key features of this format.)
Look for signs of this format in the subject headings or subsections of the article. You should see the following:
Scientific research that is published in academic journals undergoes a process called "peer review."
The peer review process goes like this:
- A researcher writes a paper and sends it in to an academic journal, where it is read by an editor
- The editor then sends the article to other scientists who study similar topics, who can best evaluate the article
- The scientists/reviewers examine the article's research methodology, reasoning, originality, and sginificance
- The scientists/reviewers then make suggestions and comments to impove the paper
- The original author is then given these suggestions and comments, and makes changes as needed
- This process repeats until everyone is satisfied and the article can be published within the academic journal
For more details about this process see the Peer Reviewed Publications guide.
This journal article is an example. It was published in the journal Royal Society Open Science in 2015. Clicking on the button that says "Review History" will show the comments by the editors, reviewers and the author as it went through the peer review process. The "About Us" menu provides details about this journal; "About the journal" under that tab includes the statement that the journal is peer reviewed.
Review articles
There are a variety of article types published in academic, peer-reviewed journals, but the two most common are original research articles and review articles . They can look very similar, but have different purposes and structures.
Like original research articles, review articles are aimed at scientists and undergo peer-review. Review articles often even have “abstract,” “introduction,” and “reference” sections. However, they will not (generally) have a “methods” or “results” section because they are not reporting new data or theories. Instead, they review the current state of knowledge on a topic.
Press releases, newspaper or magazine articles
These won't be in a formal scientific format or be peer reviewed. The author will usually be a journalist, and the audience will be the general public. Since most readers are not interested in the precise details of the research, the language will usually be nontechnical and broad. Citations will be rare or nonexistent.
Tips for Finding Original research Articles
Search for articles in one of the library databases recommend for your subject area . If you are using Google, try searching in Google Scholar instead and you will get results that are more likely to be original research articles than what will come up in a regular Google search!
For tips on using library databases to find articles, see our Library DIY guides .
Tips for Finding the Source of a News Report about Science
If you've seen or heard a report about a new scientific finding or claim, these tips can help you find the original source:
- Often, the report will mention where the original research was published; look for sentences like "In an article published yesterday in the journal Nature ..." You can use this to find the issue of the journal where the research was published, and look at the table of contents to find the original article.
- The report will often name the researchers involved. You can search relevant databases for their name and the topic of the report to find the original research that way.
- Sometimes you may have to go through multiple articles to find the original source. For example, a video or blog post may be based on a newspaper article, which in turn is reporting on a scientific discovery published in another journal; be sure to find the original research article.
- Don't be afraid to ask a librarian for help!
Search The Site
Find Your Librarian
Phone: Circulation: (701) 231-8888 Reference: (701) 231-8888 Administration: (701) 231-8753
Email: Administration InterLibrary Loan (ILL)
- Online Services
- Phone/Email Directory
- Registration And Records
- Government Information
- Library DIY
- Subject and Course Guides
- Special Topics
- Collection Highlights
- Digital Horizons
- NDSU Repository (IR)
- Libraries Hours
- News & Events
- Privacy Policy

Home » Original Research – Definition, Examples, Guide
Original Research – Definition, Examples, Guide
Table of Contents

Original Research
Definition:
Original research refers to a type of research that involves the collection and analysis of new and original data to answer a specific research question or to test a hypothesis. This type of research is conducted by researchers who aim to generate new knowledge or add to the existing body of knowledge in a particular field or discipline.
Types of Original Research
There are several types of original research that researchers can conduct depending on their research question and the nature of the data they are collecting. Some of the most common types of original research include:
Basic Research
This type of research is conducted to expand scientific knowledge and to create new theories, models, or frameworks. Basic research often involves testing hypotheses and conducting experiments or observational studies.
Applied Research
This type of research is conducted to solve practical problems or to develop new products or technologies. Applied research often involves the application of basic research findings to real-world problems.
Exploratory Research
This type of research is conducted to gather preliminary data or to identify research questions that need further investigation. Exploratory research often involves collecting qualitative data through interviews, focus groups, or observations.
Descriptive Research
This type of research is conducted to describe the characteristics or behaviors of a population or a phenomenon. Descriptive research often involves collecting quantitative data through surveys, questionnaires, or other standardized instruments.
Correlational Research
This type of research is conducted to determine the relationship between two or more variables. Correlational research often involves collecting quantitative data and using statistical analyses to identify correlations between variables.
Experimental Research
This type of research is conducted to test cause-and-effect relationships between variables. Experimental research often involves manipulating one or more variables and observing the effect on an outcome variable.
Longitudinal Research
This type of research is conducted over an extended period of time to study changes in behavior or outcomes over time. Longitudinal research often involves collecting data at multiple time points.
Original Research Methods
Original research can involve various methods depending on the research question, the nature of the data, and the discipline or field of study. However, some common methods used in original research include:
This involves the manipulation of one or more variables to test a hypothesis. Experimental research is commonly used in the natural sciences, such as physics, chemistry, and biology, but can also be used in social sciences, such as psychology.
Observational Research
This involves the collection of data by observing and recording behaviors or events without manipulation. Observational research can be conducted in the natural setting of the behavior or in a laboratory setting.
Survey Research
This involves the collection of data from a sample of participants using questionnaires or interviews. Survey research is commonly used in social sciences, such as sociology, political science, and economics.
Case Study Research
This involves the in-depth analysis of a single case, such as an individual, organization, or event. Case study research is commonly used in social sciences and business studies.
Qualitative research
This involves the collection and analysis of non-numerical data, such as interviews, focus groups, and observation notes. Qualitative research is commonly used in social sciences, such as anthropology, sociology, and psychology.
Quantitative research
This involves the collection and analysis of numerical data using statistical methods. Quantitative research is commonly used in natural sciences, such as physics, chemistry, and biology, as well as in social sciences, such as psychology and economics.
Researchers may also use a combination of these methods in their original research depending on their research question and the nature of their data.
Data Collection Methods
There are several data collection methods that researchers can use in original research, depending on the nature of the research question and the type of data that needs to be collected. Some of the most common data collection methods include:
- Surveys : Surveys involve asking participants to respond to a series of questions about their attitudes, behaviors, beliefs, or experiences. Surveys can be conducted in person, over the phone, through email, or online.
- Interviews : Interviews involve asking participants open-ended questions about their experiences, beliefs, or behaviors. Interviews can be conducted in person, over the phone, or through video conferencing.
- Observations : Observations involve observing and recording participants’ behaviors or interactions in a natural or laboratory setting. Observations can be conducted using structured or unstructured methods.
- Experiments : Experiments involve manipulating one or more variables and observing the effect on an outcome variable. Experiments can be conducted in a laboratory or in the natural environment.
- Case studies: Case studies involve conducting an in-depth analysis of a single case, such as an individual, organization, or event. Case studies can involve the collection of qualitative or quantitative data.
- Focus groups: Focus groups involve bringing together a small group of participants to discuss a specific topic or issue. Focus groups can be conducted in person or online.
- Document analysis: Document analysis involves collecting and analyzing written or visual materials, such as reports, memos, or videos, to answer research questions.
Data Analysis Methods
Once data has been collected in original research, it needs to be analyzed to answer research questions and draw conclusions. There are various data analysis methods that researchers can use, depending on the type of data collected and the research question. Some common data analysis methods used in original research include:
- Descriptive statistics: This involves using statistical measures such as mean, median, mode, and standard deviation to describe the characteristics of the data.
- Inferential statistics: This involves using statistical methods to infer conclusions about a population based on a sample of data.
- Regression analysis: This involves examining the relationship between two or more variables by using statistical models that predict the value of one variable based on the value of one or more other variables.
- Content analysis: This involves analyzing written or visual materials, such as documents, videos, or social media posts, to identify patterns, themes, or trends.
- Qualitative analysis: This involves analyzing non-numerical data, such as interview transcripts or observation notes, to identify themes, patterns, or categories.
- Grounded theory: This involves developing a theory or model based on the data collected in the study.
- Mixed methods analysis: This involves combining quantitative and qualitative data analysis methods to provide a more comprehensive understanding of the research question.
How to Conduct Original Research
Conducting original research involves several steps that researchers need to follow to ensure that their research is valid, reliable, and produces meaningful results. Here are some general steps that researchers can follow to conduct original research:
- Identify the research question: The first step in conducting original research is to identify a research question that is relevant, significant, and feasible. The research question should be specific and focused to guide the research process.
- Conduct a literature review: Once the research question is identified, researchers should conduct a thorough literature review to identify existing research on the topic. This will help them identify gaps in the existing knowledge and develop a research plan that builds on previous research.
- Develop a research plan: Researchers should develop a research plan that outlines the methods they will use to collect and analyze data. The research plan should be detailed and include information on the population and sample, data collection methods, data analysis methods, and ethical considerations.
- Collect data: Once the research plan is developed, researchers can begin collecting data using the methods identified in the plan. It is important to ensure that the data collection process is consistent and accurate to ensure the validity and reliability of the data.
- Analyze data: Once the data is collected, researchers should analyze it using appropriate data analysis methods. This will help them answer the research question and draw conclusions from the data.
- Interpret results: After analyzing the data, researchers should interpret the results and draw conclusions based on the findings. This will help them answer the research question and make recommendations for future research or practical applications.
- Communicate findings: Finally, researchers should communicate their findings to the appropriate audience using a format that is appropriate for the research question and audience. This may include writing a research paper, presenting at a conference, or creating a report for a client or stakeholder.
Purpose of Original Research
The purpose of original research is to generate new knowledge and understanding in a particular field of study. Original research is conducted to address a research question, hypothesis, or problem and to produce empirical evidence that can be used to inform theory, policy, and practice. By conducting original research, researchers can:
- Expand the existing knowledge base: Original research helps to expand the existing knowledge base by providing new information and insights into a particular phenomenon. This information can be used to develop new theories, models, or frameworks that explain the phenomenon in greater depth.
- Test existing theories and hypotheses: Original research can be used to test existing theories and hypotheses by collecting empirical evidence and analyzing the data. This can help to refine or modify existing theories, or to develop new ones that better explain the phenomenon.
- Identify gaps in the existing knowledge: Original research can help to identify gaps in the existing knowledge base by highlighting areas where further research is needed. This can help to guide future research and identify new research questions that need to be addressed.
- Inform policy and practice: Original research can be used to inform policy and practice by providing empirical evidence that can be used to make decisions and develop interventions. This can help to improve the quality of life for individuals and communities, and to address social, economic, and environmental challenges.
How to publish Original Research
Publishing original research involves several steps that researchers need to follow to ensure that their research is accepted and published in reputable academic journals. Here are some general steps that researchers can follow to publish their original research:
- Select a suitable journal: Researchers should identify a suitable academic journal that publishes research in their field of study. The journal should have a good reputation and a high impact factor, and should be a good fit for the research topic and methods used.
- Review the submission guidelines: Once a suitable journal is identified, researchers should review the submission guidelines to ensure that their manuscript meets the journal’s requirements. The guidelines may include requirements for formatting, length, and content.
- Write the manuscript : Researchers should write the manuscript in accordance with the submission guidelines and academic standards. The manuscript should include a clear research question or hypothesis, a description of the research methods used, an analysis of the data collected, and a discussion of the results and their implications.
- Submit the manuscript: Once the manuscript is written, researchers should submit it to the selected journal. The submission process may require the submission of a cover letter, abstract, and other supporting documents.
- Respond to reviewer feedback: After the manuscript is submitted, it will be reviewed by experts in the field who will provide feedback on the quality and suitability of the research. Researchers should carefully review the feedback and revise the manuscript accordingly.
- Respond to editorial feedback: Once the manuscript is revised, it will be reviewed by the journal’s editorial team who will provide feedback on the formatting, style, and content of the manuscript. Researchers should respond to this feedback and make any necessary revisions.
- Acceptance and publication: If the manuscript is accepted, the journal will inform the researchers and the manuscript will be published in the journal. If the manuscript is not accepted, researchers can submit it to another journal or revise it further based on the feedback received.
How to Identify Original Research
To identify original research, there are several factors to consider:
- The research question: Original research typically starts with a novel research question or hypothesis that has not been previously explored or answered in the existing literature.
- The research design: Original research should have a clear and well-designed research methodology that follows appropriate scientific standards. The methodology should be described in detail in the research article.
- The data: Original research should include new data that has not been previously published or analyzed. The data should be collected using appropriate research methods and analyzed using valid statistical methods.
- The results: Original research should present new findings or insights that have not been previously reported in the existing literature. The results should be presented clearly and objectively, and should be supported by the data collected.
- The discussion and conclusions: Original research should provide a clear and objective interpretation of the results, and should discuss the implications of the research findings. The discussion and conclusions should be based on the data collected and the research question or hypothesis.
- The references: Original research should be supported by references to existing literature, which should be cited appropriately in the research article.
Advantages of Original Research
Original research has several advantages, including:
- Generates new knowledge: Original research is conducted to answer novel research questions or hypotheses, which can generate new knowledge and insights into various fields of study.
- Supports evidence-based decision making: Original research provides empirical evidence that can inform decision-making in various fields, such as medicine, public policy, and business.
- Enhances academic and professional reputation: Conducting original research and publishing in reputable academic journals can enhance a researcher’s academic and professional reputation.
- Provides opportunities for collaboration: Original research can provide opportunities for collaboration between researchers, institutions, and organizations, which can lead to new partnerships and research projects.
- Advances scientific and technological progress: Original research can contribute to scientific and technological progress by providing new knowledge and insights into various fields of study, which can inform further research and development.
- Can lead to practical applications: Original research can have practical applications in various fields, such as medicine, engineering, and social sciences, which can lead to new products, services, and policies that benefit society.
Limitations of Original Research
Original research also has some limitations, which include:
- Time and resource constraints: Original research can be time-consuming and expensive, requiring significant resources to design, execute, and analyze the research data.
- Ethical considerations: Conducting original research may raise ethical considerations, such as ensuring the privacy and confidentiality of research participants, obtaining informed consent, and avoiding conflicts of interest.
- Risk of bias: Original research may be subject to biases, such as selection bias, measurement bias, and publication bias, which can affect the validity and reliability of the research findings.
- Generalizability: Original research findings may not be generalizable to larger populations or different contexts, which can limit the applicability of the research findings.
- Replicability: Original research may be difficult to replicate, which can limit the ability of other researchers to verify the research findings.
- Limited scope: Original research may have a limited scope, focusing on a specific research question or hypothesis, which can limit the breadth of the research findings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Documentary Research – Types, Methods and...

Scientific Research – Types, Purpose and Guide

Humanities Research – Types, Methods and Examples

Historical Research – Types, Methods and Examples

Artistic Research – Methods, Types and Examples

- Become Involved |
- Give to the Library |
- Staff Directory |
- UNF Library
- Thomas G. Carpenter Library
Original Research
How can i tell if the article is original research.
- Glossary of Terms
- What are Peer Reviewed/Refereed Articles?
Chat With Us Text Us (904) 507-4122 Email Us Schedule a Research Consultation
Visit us on social media!
What is Original Research?
Original research is considered a primary source.
An article is considered original research if...
- it is the report of a study written by the researchers who actually did the study.
- the researchers describe their hypothesis or research question and the purpose of the study.
- the researchers detail their research methods.
- the results of the research are reported.
- the researchers interpret their results and discuss possible implications.
There is no one way to easily tell if an article is a research article like there is for peer-reviewed articles in the Ulrich's database. The only way to be sure is to read the article to verify that it is written by the researchers and that they have explained all of their findings, in addition to listing their methodologies, results, and any conclusions based on the evidence collected.
All that being said, there are a few key indicators that will help you to quickly decide whether or not your article is based on original research.
- Literature Review or Background
- Conclusions
- Read through the abstract (summary) before you attempt to find the full-text PDF. The abstract of the article usually contains those subdivision headings where each of the key sections are summarized individually.
- Use the checkbox with CINAHL's advanced search to only see articles that have been tagged as research articles.
- Next: Glossary of Terms >>
- Last Updated: Feb 7, 2022 11:44 AM
- URL: https://libguides.unf.edu/originalresearch
Research articles
Reverse total shoulder replacement versus anatomical total shoulder replacement for osteoarthritis, effect of combination treatment with glp-1 receptor agonists and sglt-2 inhibitors on incidence of cardiovascular and serious renal events, prenatal opioid exposure and risk of neuropsychiatric disorders in children, temporal trends in lifetime risks of atrial fibrillation and its complications, antipsychotic use in people with dementia, predicting the risks of kidney failure and death in adults with moderate to severe chronic kidney disease, impact of large scale, multicomponent intervention to reduce proton pump inhibitor overuse, esketamine after childbirth for mothers with prenatal depression, glucagon-like peptide 1 receptor agonist use and risk of thyroid cancer, use of progestogens and the risk of intracranial meningioma, delirium and incident dementia in hospital patients, derivation and external validation of a simple risk score for predicting severe acute kidney injury after intravenous cisplatin, quality and safety of artificial intelligence generated health information, large language models and the generation of health disinformation, 25 year trends in cancer incidence and mortality among adults in the uk, cervical pessary versus vaginal progesterone in women with a singleton pregnancy, comparison of prior authorization across insurers, diagnostic accuracy of magnetically guided capsule endoscopy with a detachable string for detecting oesophagogastric varices in adults with cirrhosis, ultra-processed food exposure and adverse health outcomes, added benefit and revenues of oncology drugs approved by the ema, exposure to air pollution and hospital admission for cardiovascular diseases, short term exposure to low level ambient fine particulate matter and natural cause, cardiovascular, and respiratory morbidity, optimal timing of influenza vaccination in young children, effect of exercise for depression, association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes, duration of cpr and outcomes for adults with in-hospital cardiac arrest, clinical effectiveness of an online physical and mental health rehabilitation programme for post-covid-19 condition, atypia detected during breast screening and subsequent development of cancer, publishers’ and journals’ instructions to authors on use of generative ai in academic and scientific publishing, effectiveness of glp-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes, neurological development in children born moderately or late preterm, invasive breast cancer and breast cancer death after non-screen detected ductal carcinoma in situ, all cause and cause specific mortality in obsessive-compulsive disorder, acute rehabilitation following traumatic anterior shoulder dislocation, perinatal depression and risk of mortality, undisclosed financial conflicts of interest in dsm-5-tr, effect of risk mitigation guidance opioid and stimulant dispensations on mortality and acute care visits, update to living systematic review on sars-cov-2 positivity in offspring and timing of mother-to-child transmission, perinatal depression and its health impact, christmas 2023: common healthcare related instruments subjected to magnetic attraction study, using autoregressive integrated moving average models for time series analysis of observational data, demand for morning after pill following new year holiday, christmas 2023: christmas recipes from the great british bake off, effect of a doctor working during the festive period on population health: experiment using doctor who episodes, christmas 2023: analysis of barbie medical and science career dolls, christmas 2023: effect of chair placement on physicians’ behavior and patients’ satisfaction, management of chronic pain secondary to temporomandibular disorders, christmas 2023: projecting complete redaction of clinical trial protocols, christmas 2023: a drug target for erectile dysfunction to help improve fertility, sexual activity, and wellbeing, christmas 2023: efficacy of cola ingestion for oesophageal food bolus impaction, conservative management versus laparoscopic cholecystectomy in adults with gallstone disease, social media use and health risk behaviours in young people, untreated cervical intraepithelial neoplasia grade 2 and cervical cancer, air pollution deaths attributable to fossil fuels, implementation of a high sensitivity cardiac troponin i assay and risk of myocardial infarction or death at five years, covid-19 vaccine effectiveness against post-covid-19 condition, association between patient-surgeon gender concordance and mortality after surgery, intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention, treatment of lower urinary tract symptoms in men in primary care using a conservative intervention, autism intervention meta-analysis of early childhood studies, effectiveness of the live zoster vaccine during the 10 years following vaccination, effects of a multimodal intervention in primary care to reduce second line antibiotic prescriptions for urinary tract infections in women, pyrotinib versus placebo in combination with trastuzumab and docetaxel in patients with her2 positive metastatic breast cancer, association of dcis size and margin status with risk of developing breast cancer post-treatment, racial differences in low value care among older patients in the us, pharmaceutical industry payments and delivery of low value cancer drugs, rosuvastatin versus atorvastatin in adults with coronary artery disease, clinical effectiveness of septoplasty versus medical management for nasal airways obstruction, ultrasound guided lavage with corticosteroid injection versus sham lavage with and without corticosteroid injection for calcific tendinopathy of shoulder, early versus delayed antihypertensive treatment in patients with acute ischaemic stroke, mortality risks associated with floods in 761 communities worldwide, interactive effects of ambient fine particulate matter and ozone on daily mortality in 372 cities, association between changes in carbohydrate intake and long term weight changes, future-case control crossover analysis for adjusting bias in case crossover studies, association between recently raised anticholinergic burden and risk of acute cardiovascular events, suboptimal gestational weight gain and neonatal outcomes in low and middle income countries: individual participant data meta-analysis, efficacy and safety of an inactivated virus-particle vaccine for sars-cov-2, effect of invitation letter in language of origin on screening attendance: randomised controlled trial in breastscreen norway, visits by nurse practitioners and physician assistants in the usa, non-erosive gastro-oesophageal reflux disease and oesophageal adenocarcinoma, venous thromboembolism with use of hormonal contraception and nsaids, food additive emulsifiers and risk of cardiovascular disease, balancing risks and benefits of cannabis use, promoting activity, independence, and stability in early dementia and mild cognitive impairment, effect of home cook interventions for salt reduction in china, cancer mortality after low dose exposure to ionising radiation, effect of a smartphone intervention among university students with unhealthy alcohol use, long term risk of death and readmission after hospital admission with covid-19 among older adults, mortality rates among patients successfully treated for hepatitis c, association between antenatal corticosteroids and risk of serious infection in children, the proportions of term or late preterm births after exposure to early antenatal corticosteroids, and outcomes, safety of ba.4-5 or ba.1 bivalent mrna booster vaccines, comparative effectiveness of booster vaccines among adults aged ≥50 years, third dose vaccine schedules against severe covid-19 during omicron predominance in nordic countries, private equity ownership and impacts on health outcomes, costs, and quality, healthcare disruption due to covid-19 and avoidable hospital admission, educational inequalities in mortality and their mediators among generations across four decades, prevalence and predictors of data and code sharing in the medical and health sciences, medicare eligibility and in-hospital treatment patterns and health outcomes for patients with trauma, follow us on, content links.
- Collections
- Health in South Asia
- Women’s, children’s & adolescents’ health
- News and views
- BMJ Opinion
- Rapid responses
- Editorial staff
- BMJ in the USA
- BMJ in South Asia
- Submit your paper
- BMA members
- Subscribers
- Advertisers and sponsors
Explore BMJ
- Our company
- BMJ Careers
- BMJ Learning
- BMJ Masterclasses
- BMJ Journals
- BMJ Student
- Academic edition of The BMJ
- BMJ Best Practice
- The BMJ Awards
- Email alerts
- Activate subscription
Information
- SpringerLink shop
Types of journal articles
It is helpful to familiarise yourself with the different types of articles published by journals. Although it may appear there are a large number of types of articles published due to the wide variety of names they are published under, most articles published are one of the following types; Original Research, Review Articles, Short reports or Letters, Case Studies, Methodologies.
Original Research:
This is the most common type of journal manuscript used to publish full reports of data from research. It may be called an Original Article, Research Article, Research, or just Article, depending on the journal. The Original Research format is suitable for many different fields and different types of studies. It includes full Introduction, Methods, Results, and Discussion sections.
Short reports or Letters:
These papers communicate brief reports of data from original research that editors believe will be interesting to many researchers, and that will likely stimulate further research in the field. As they are relatively short the format is useful for scientists with results that are time sensitive (for example, those in highly competitive or quickly-changing disciplines). This format often has strict length limits, so some experimental details may not be published until the authors write a full Original Research manuscript. These papers are also sometimes called Brief communications .
Review Articles:
Review Articles provide a comprehensive summary of research on a certain topic, and a perspective on the state of the field and where it is heading. They are often written by leaders in a particular discipline after invitation from the editors of a journal. Reviews are often widely read (for example, by researchers looking for a full introduction to a field) and highly cited. Reviews commonly cite approximately 100 primary research articles.
TIP: If you would like to write a Review but have not been invited by a journal, be sure to check the journal website as some journals to not consider unsolicited Reviews. If the website does not mention whether Reviews are commissioned it is wise to send a pre-submission enquiry letter to the journal editor to propose your Review manuscript before you spend time writing it.
Case Studies:
These articles report specific instances of interesting phenomena. A goal of Case Studies is to make other researchers aware of the possibility that a specific phenomenon might occur. This type of study is often used in medicine to report the occurrence of previously unknown or emerging pathologies.
Methodologies or Methods
These articles present a new experimental method, test or procedure. The method described may either be completely new, or may offer a better version of an existing method. The article should describe a demonstrable advance on what is currently available.
Back │ Next
- Enroll & Pay
- Prospective Students
- Current Students
- Degree Programs
Original Research
An original research paper should present a unique argument of your own. In other words, the claim of the paper should be debatable and should be your (the researcher’s) own original idea. Typically an original research paper builds on the existing research on a topic, addresses a specific question, presents the findings according to a standard structure (described below), and suggests questions for further research and investigation. Though writers in any discipline may conduct original research, scientists and social scientists in particular are interested in controlled investigation and inquiry. Their research often consists of direct and indirect observation in the laboratory or in the field. Many scientists write papers to investigate a hypothesis (a statement to be tested).
Although the precise order of research elements may vary somewhat according to the specific task, most include the following elements:
- Table of contents
- List of illustrations
- Body of the report
- References cited
Check your assignment for guidance on which formatting style is required. The Complete Discipline Listing Guide (Purdue OWL) provides information on the most common style guide for each discipline, but be sure to check with your instructor.
The title of your work is important. It draws the reader to your text. A common practice for titles is to use a two-phrase title where the first phrase is a broad reference to the topic to catch the reader’s attention. This phrase is followed by a more direct and specific explanation of your project. For example:
“Lions, Tigers, and Bears, Oh My!: The Effects of Large Predators on Livestock Yields.”
The first phrase draws the reader in – it is creative and interesting. The second part of the title tells the reader the specific focus of the research.
In addition, data base retrieval systems often work with keywords extracted from the title or from a list the author supplies. When possible, incorporate them into the title. Select these words with consideration of how prospective readers might attempt to access your document. For more information on creating keywords, refer to this Springer research publication guide.
See the KU Writing Center Writing Guide on Abstracts for detailed information about creating an abstract.
Table of Contents
The table of contents provides the reader with the outline and location of specific aspects of your document. Listings in the table of contents typically match the headings in the paper. Normally, authors number any pages before the table of contents as well as the lists of illustrations/tables/figures using lower-case roman numerals. As such, the table of contents will use lower-case roman numbers to identify the elements of the paper prior to the body of the report, appendix, and reference page. Additionally, because authors will normally use Arabic numerals (e.g., 1, 2, 3) to number the pages of the body of the research paper (starting with the introduction), the table of contents will use Arabic numerals to identify the main sections of the body of the paper (the introduction, literature review, methods, results, discussion, conclusion, references, and appendices).
Here is an example of a table of contents:
ABSTRACT..................................................iii
TABLE OF CONTENTS...............................iv
LIST OF ILLUSTRATIONS...........................v
LIST OF TABLES.........................................vii
INTRODUCTION..........................................1
LITERATURE REVIEW.................................6
METHODS....................................................9
RESULTS....................................................10
DISCUSSION..............................................16
CONCLUSION............................................18
REFERENCES............................................20
APPENDIX................................................. 23
More information on creating a table of contents can be found in the Table of Contents Guide (SHSU) from the Newton Gresham Library at Sam Houston State University.
List of Illustrations
Authors typically include a list of the illustrations in the paper with longer documents. List the number (e.g., Illustration 4), title, and page number of each illustration under headings such as "List of Illustrations" or "List of Tables.”
Body of the Report
The tone of a report based on original research will be objective and formal, and the writing should be concise and direct. The structure will likely consist of these standard sections: introduction, methods, results, discussion, and conclusion . Typically, authors identify these sections with headings and may use subheadings to identify specific themes within these sections (such as themes within the literature under the literature review section).
Introduction
Given what the field says about this topic, here is my contribution to this line of inquiry.
The introduction often consists of the rational for the project. What is the phenomenon or event that inspired you to write about this topic? What is the relevance of the topic and why is it important to study it now? Your introduction should also give some general background on the topic – but this should not be a literature review. This is the place to give your readers and necessary background information on the history, current circumstances, or other qualities of your topic generally. In other words, what information will a layperson need to know in order to get a decent understanding of the purpose and results of your paper? Finally, offer a “road map” to your reader where you explain the general order of the remainder of your paper. In the road map, do not just list the sections of the paper that will follow. You should refer to the main points of each section, including the main arguments in the literature review, a few details about your methods, several main points from your results/analysis, the most important takeaways from your discussion section, and the most significant conclusion or topic for further research.
Literature Review
This is what other researchers have published about this topic.
In the literature review, you will define and clarify the state of the topic by citing key literature that has laid the groundwork for this investigation. This review of the literature will identify relations, contradictions, gaps, and inconsistencies between previous investigations and this one, and suggest the next step in the investigation chain, which will be your hypothesis. You should write the literature review in the present tense because it is ongoing information.
Methods (Procedures)
This is how I collected and analyzed the information.
This section recounts the procedures of the study. You will write this in past tense because you have already completed the study. It must include what is necessary to replicate and validate the hypothesis. What details must the reader know in order to replicate this study? What were your purposes in this study? The challenge in this section is to understand the possible readers well enough to include what is necessary without going into detail on “common-knowledge” procedures. Be sure that you are specific enough about your research procedure that someone in your field could easily replicate your study. Finally, make sure not to report any findings in this section.
This is what I found out from my research.
This section reports the findings from your research. Because this section is about research that is completed, you should write it primarily in the past tense . The form and level of detail of the results depends on the hypothesis and goals of this report, and the needs of your audience. Authors of research papers often use visuals in the results section, but the visuals should enhance, rather than serve as a substitute, for the narrative of your results. Develop a narrative based on the thesis of the paper and the themes in your results and use visuals to communicate key findings that address your hypothesis or help to answer your research question. Include any unusual findings that will clarify the data. It is a good idea to use subheadings to group the results section into themes to help the reader understand the main points or findings of the research.
This is what the findings mean in this situation and in terms of the literature more broadly.
This section is your opportunity to explain the importance and implications of your research. What is the significance of this research in terms of the hypothesis? In terms of other studies? What are possible implications for any academic theories you utilized in the study? Are there any policy implications or suggestions that result from the study? Incorporate key studies introduced in the review of literature into your discussion along with your own data from the results section. The discussion section should put your research in conversation with previous research – now you are showing directly how your data complements or contradicts other researchers’ data and what the wider implications of your findings are for academia and society in general. What questions for future research do these findings suggest? Because it is ongoing information, you should write the discussion in the present tense . Sometimes the results and discussion are combined; if so, be certain to give fair weight to both.
These are the key findings gained from this research.
Summarize the key findings of your research effort in this brief final section. This section should not introduce new information. You can also address any limitations from your research design and suggest further areas of research or possible projects you would complete with a new and improved research design.
References/Works Cited
See KU Writing Center writing guides to learn more about different citation styles like APA, MLA, and Chicago. Make an appointment at the KU Writing Center for more help. Be sure to format the paper and references based on the citation style that your professor requires or based on the requirements of the academic journal or conference where you hope to submit the paper.
The appendix includes attachments that are pertinent to the main document but are too detailed to be included in the main text. These materials should be titled and labeled (for example Appendix A: Questionnaire). You should refer to the appendix in the text with in-text references so the reader understands additional useful information is available elsewhere in the document. Examples of documents to include in the appendix include regression tables, tables of text analysis data, and interview questions.
Updated June 2022

- University Libraries
- Research Guides
- Subject Guides
Biology 303L: Ecology and Evolution
- About Original Research
- Scientific Research Process
- Articles to Practice Identifying
- Reading Original Research Articles
- Citation-Based Searching
- Related Guides
- Review Tutorials
- Helpful Web Resources
Original Research Articles
Definition : An original research article communicates the research question, methods, results, and conclusions of a research study or experiment conducted by the author(s). These articles present original research data or findings generated through the course of the authors' study and an analysis of that data or information.
Published in Journals : Origingal research articles are published in scientific journals, also called scholarly or academic journals. These can be published in print and/or online. Journals are serial publications, meaning they publish volumes and issues on a schedule continually over time, similar to a magazine but for a scholarly audience. You can access journals through many of the library's databases. A list of recommended databases to use to search for original articles on biology subjects can be found through this link , accessible from the database "subject" dropdown on the library homepage.
Peer Reviewed : Prior to being published, original research articles undergo a process called peer review in an effort to ensure that published articles are based on sound research that adheres to established standards in the discipline. This means that after an article is first submitted to a journal, it is reviewed by other scientists who are experts in the article's subject area. These individuals review the article and provide unbiased feedback about the soundness of the background information, research methods, analysis, conclusions, logic, and reasoning of any conclusions; the author needs to incorporate and/or respond to recommended edits before an article will be published. Though it isn't perfect, peer review is the best quality control mechanism that scholars currently have in place to validate the quality of published research.
Peer reviewed articles will often be published with "Received", "Accepted", and "Published" dates, which indicates the timeline of the peer review process.
Structure : Traditionally, an original research article follows a standardized structure known by the acronym IMRD, which stands for Introduction, Methods, Results, & Discussion. Further information about the IMRD structure is available on the Reading Original Research Articles tab of this guide.
Other types of journal articles
Review Articles (usually peer reviewed) : Summarize and synthesize the current published literature on a certain topic. They do not involve original experiments or report new findings. The scope of a review article may be broad or narrow, depending on the publication record. Original research articles do incorporate literature review components, but a review article covers only review content.
Non Peer Reviewed Articles in Journals : Many journals publish the types of articles where peer review is not required. These differ by publication but may include research notes (brief reports of new research findings); responses to other articles; letters, commentaries, or opinion pieces; book reviews; and news. These articles are often more concise and will typically have a shorter reference list or no reference list at all. Many journals will indicate what genre these articles fall into on the article itself by using a label.
Why is Published Original Research Important?
Current information : Typical publication turnaround varies, but can be as quick as ~3 months.
Replicable : The studies published in original research articles contain enough methodological detail to be replicated so research can be verified (though this is a topic of recent debate ).
Contains Raw Data : The raw original research data, along with information about experimental conditions, allows for reuse of results for your own research or analysis.
Shows Logic : Using the provided data and methods, you can evaluate the logic of the authors' conclusions.
- << Previous: Scientific Research Process
- Next: Articles to Practice Identifying >>
- Last Updated: Sep 6, 2022 4:02 PM
- URL: https://libguides.unm.edu/biology303

Finding original (or "scientific") research articles: Where do I find these articles?
- Definition and description
- Where do I find these articles?
- How do I understand them?
- What's the point?
Quick answer...
Library research databases!
In databases, you can narrow down your options to research articles (also sometimes listed as "scholarly" and/or "peer reviewed" in databases) -- or choose a database that ONLY includes research articles. In the box below are a few good starting options, but you can also see all of the TCC library research databases via the link below.
- Complete List of TCC Library Research Databases Click here to view all of the research databases available at your TCC Library
Popular research databases
- EBSCOhost databases
- ScienceDirect
The Academic Search Complete, CINAHL, and PsycARTICLES databases (all published by EbscoHost) include many original research articles. (The direct links to these databases are at the bottom of this box.)
Search tips:
Type your topic into the first one or two search boxes and then use another box to type: "methods OR results OR study" as shown here in the second box. Use the drop down menu to choose the ABSTRACT search field for all boxes. Click search.
Limit to academic journals:
Limit the results list by checking the "Academic Journals" limiter on the left side of the results page
You will still need to examine the individual articles looking for the characteristics listed under the "Definition and description" tab on this guide to be sure of finding the right kind of article.
Explore these databases:

The ProQuest databases also include scientific research articles. There is a larger number and mix of article types in ProQuest, so you may need to look through a larger number of articles to find what you need.
Click on ADVANCED SEARCH, then enter your search terms in one or two search boxes and results OR methods in the second or third box. As in EbscoHost, change the search field to "Abstract".
Limit your results to scholarly journals:
Quite a bit further down the screen, limit the search as shown below:
As you look through your results, you will need to examine the characteristics of the individual articles to make certain they are what you need.
Explore the ProQuest database:

ScienceDirect is a database that only includes scholarly, scientific articles!
Advanced Search tips:
If you go to the "Advanced Search" option, you can see there is an option below the search boxes to narrow down to "research articles"

Explore ScienceDirect:

- << Previous: Definition and description
- Next: How do I understand them? >>
- Last Updated: Mar 21, 2024 10:25 AM
- URL: https://tacomacc.libguides.com/originalresearcharticles

Tacoma Community College Library - Building 7, 6501 South 19th Street, Tacoma, WA 98466 - P. 253.566.5087

Visit us on Instagram!
Scientific Manuscript Writing: Original Research, Case Reports, Review Articles
- First Online: 02 March 2024
Cite this chapter

- Kimberly M. Rathbun 5
12 Accesses
Manuscripts are used to communicate the findings of your work with other researchers. Writing your first manuscript can be a challenge. Journals provide guidelines to authors which should be followed closely. The three major types of articles (original research, case reports, and review articles) all generally follow the IMRAD format with slight variations in content. With planning and thought, manuscript writing does not have to be a daunting task.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Suggested Readings
Alsaywid BS, Abdulhaq NM. Guideline on writing a case report. Urol Ann. 2019;11(2):126–31.
Article PubMed PubMed Central Google Scholar
Cohen H. How to write a patient case report. Am J Health Syst Pharm. 2006;63(19):1888–92.
Article PubMed Google Scholar
Cooper ID. How to write an original research paper (and get it published). J Med Lib Assoc. 2015;103:67–8.
Article Google Scholar
Gemayel R. How to write a scientific paper. FEBS J. 2016;283(21):3882–5.
Article CAS PubMed Google Scholar
Gülpınar Ö, Güçlü AG. How to write a review article? Turk J Urol. 2013;39(Suppl 1):44–8.
PubMed PubMed Central Google Scholar
Huth EJ. Structured abstracts for papers reporting clinical trials. Ann Intern Med. 1987;106:626–7.
International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. http://www.ICMJE.org . Accessed 23 Aug 2022.
Liumbruno GM, Velati C, Pasqualetti P, Franchini M. How to write a scientific manuscript for publication. Blood Transfus. 2013;11:217–26.
McCarthy LH, Reilly KE. How to write a case report. Fam Med. 2000;32(3):190–5.
CAS PubMed Google Scholar
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
The Biosemantics Group. Journal/author name estimator. https://jane.biosemantics.org/ . Accessed 24 Aug 2022.
Weinstein R. How to write a manuscript for peer review. J Clin Apher. 2020;35(4):358–66.
Download references
Author information
Authors and affiliations.
Department of Emergency Medicine, AU/UGA Medical Partnership, Athens, GA, USA
Kimberly M. Rathbun
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kimberly M. Rathbun .
Editor information
Editors and affiliations.
Emergency Medicine, Penn State Milton S. Hershey Medical Center, Hershey, PA, USA
Robert P. Olympia
Elizabeth Barrall Werley
Jeffrey S. Lubin
MD Anderson Cancer Center at Cooper, Cooper Medical School of Rowan University, Camden, NJ, USA
Kahyun Yoon-Flannery
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Rathbun, K.M. (2023). Scientific Manuscript Writing: Original Research, Case Reports, Review Articles. In: Olympia, R.P., Werley, E.B., Lubin, J.S., Yoon-Flannery, K. (eds) An Emergency Physician’s Path. Springer, Cham. https://doi.org/10.1007/978-3-031-47873-4_80
Download citation
DOI : https://doi.org/10.1007/978-3-031-47873-4_80
Published : 02 March 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-47872-7
Online ISBN : 978-3-031-47873-4
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Sign up for alerts
- Perspective
- Published: 05 February 2007
Original research in pathology: judgment, or evidence-based medicine?
- James M Crawford 1
Laboratory Investigation volume 87 , pages 104–114 ( 2007 ) Cite this article
6931 Accesses
30 Citations
5 Altmetric
Metrics details
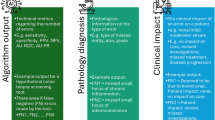
Pathology is both a medical specialty and an investigative scientific discipline, concerned with understanding the essential nature of human disease. Ultimately, pathology is accountable as well, as measured by the accuracy of our diagnoses and the resultant patient care outcomes. As such, we must consider the evidence base underlying our practices. Within the realm of Laboratory Medicine, extensive attention has been given to testing accuracy and precision. Critical examination of the evidence base supporting the clinical use of specific laboratory tests or technologies is a separate endeavor, to which specific attention must be given. In the case of anatomic pathology and more specifically surgical pathology, the expertise required to render a diagnosis is derived foremost from experience, both personal and literature-based. In the first instance, knowledge of the linkage between one's own diagnoses and individual patient outcomes is required, to validate the role of one's own interpretations in the clinical course of patients. Experience comes from seeing this linkage first hand, from which hopefully comes wisdom and, ultimately, good clinical judgment. In the second instance, reading the literature and learning from experts is required. Only a minority of the relevant literature is published in pathology journals to which one may subscribe. A substantial portion of major papers relevant to the practice of anatomic pathology are published in collateral clinical specialty journals devoted to specific disease areas or organs. Active effort is therefore required to seek out the literature beyond the domain of pathology journals. In examining the published literature, the essential question then becomes: Does the practice of anatomic pathology fulfill the tenets of ‘evidence-based medicine’ (EBM)? If the pinnacle of EBM is ‘systematic review of randomized clinical trials, with or without meta-analysis’, then anatomic pathology falls far short. Our published literature is largely observational in nature, with reports of case series (with or without statistical analysis) constituting the majority of our ‘evidence base’. Moreover, anatomic pathology is subject to ‘interobserver variation’, and potentially to ‘error’. Taken further, individual interpretation of tissue samples is not an objective endeavor, and it is not easy to fulfill the role of a ‘gold standard’. Both for rendering of an overall interpretation, and for providing the semi-quantitative and quantitative numerical ‘scores’ which support evidence-based clinical treatment algorithms, the Pathologist has to exercise a high level of interpretive judgment. Nevertheless, the contribution of anatomic pathology to ‘EBM’ is remarkably strong. To the extent that our judgmental interpretations become data, our tissue interpretations become the arbiters of patient care management decisions. In a more global sense, we support highly successful cancer screening programs, and play critical roles in the multidisciplinary management of complex patients. The true error is for the clinical practitioners of ‘EBM’ to forget the contribution to the supporting evidence base of the physicians that are Anatomic Pathologists. Finally, the academic productivity of pathology faculty who operate in the clinical realm must be considered. A survey of six North American academic pathology departments reveals that 26% of all papers published in 2005 came from ‘unfunded’ clinical faculty. While it is likely that their academic productivity is lower than that of ‘funded’ research faculty, the contribution of clinical faculty to the knowledge base for the practice of modern medicine, and to the academic reputation of the department, must not be overlooked. The ability of clinical faculty in academic departments of pathology to pursue original scholarship must be supported if our specialty is to retain its preeminence as an investigative scientific discipline in the age of EBM.

Similar content being viewed by others

An analysis of research biopsy core variability from over 5000 prospectively collected core samples
Understanding the errors made by artificial intelligence algorithms in histopathology in terms of patient impact
An end-to-end workflow for nondestructive 3D pathology
‘Pathology’ is the medical specialty concerned with the essential nature of disease, practiced by trained physicians for the benefit of patient care. ‘Pathology’ also is a scientific discipline, since the very process of understanding human disease is a scientific endeavor. However, defining the ‘discipline’ of pathology is problematic, since investigation of human disease spans all the scientific disciplines of biomedical research. Hence, the identity of ‘pathology’ is evanescent. On the one hand, medical students considering pathology for post-graduate training are given little opportunity to appreciate what Pathologists actually do—it is only the fortunate few who can appreciate the extraordinarily fulfilling nature of a potential career in the specialty of pathology. On the other hand, academic departments of pathology have to be all things to all people, owing both to the need to provide outstanding diagnostic expertise for all of the practicing clinical specialties and subspecialties, and to the need to have robust research programming in some or many realms of disease pathobiology. Further, one can wonder how ‘specialty’ and ‘discipline’ knit together into the concept of pathology as a ‘profession’. A profession is an occupation that requires extensive training, the study and mastery of specialized knowledge, and usually has an ethical code and process of certification or licensing. Corollaries are that one has to swear an oath to uphold the ethics of the profession, and ‘profess’ to a higher standard of accountability. To the extent that accountability ultimately is measured on the basis of patient outcomes, the ‘profession’ of pathology is subject to consideration of the evidence base underlying our practices.
From a historical perspective, what we might call ‘pathology’ emerged early in the practice of medicine. The technology of ancient times was straightforward, as exemplified by Hippocrates: the taking of a medical history and a bedside examination. A significant innovation in that era was examination of the urine. The dawn of rigorous medical science began with systematic dissection of the human body, beginning with Galen (129–200 AD). After a prolonged hiatus through medieval times, the Italian anatomists of the 15th century laid the groundwork for the publication in 1543 by Vesalius of the first books of morbid anatomy, De humani corporis fabrica libri septem (‘The Seven Books on the Structure of the Human Body’). Robert Hooke was the first to use the word ‘cell’ to name the small cavities in the honeycomb, in his 1665 book Micrographia (‘Small Drawings’). This work was soon followed by Malpighi's De viscerum structura exercitatio (1666), which essentially founded the fields of histology and microscopic anatomy, and Van Leeuwenhoek's remarkable advances in construction of microscopes, enabling his extensive studies of ‘animalcules’ (single-celled organisms) by 1675. Giovanni Morgagni of Padua published in 1761 the great work De sedibus et causis morborum (‘On the sites and Causes of Diseases’), followed ultimately by Rudolf Virchow's landmark publication in 1858 of Die Cellular-pathologie , which conceived of the cell as the center of all pathological changes. In the decades that followed, especially in Europe, the role of the Anatomic Pathologist as the final arbiter in human disease became well established.
The dawn of the 20th century saw an increasing role for the clinical laboratory in patient care world-wide, over-and-above the utilization of the microscope. By the early 1920s, physicians practicing in the clinical laboratories of the day recognized the need for elevation of this activity to full professional status, and in 1926 the American College of Surgeons revised its minimum standards for hospitals to require that ‘clinical laboratories be under the direction of MD physicians with special training in clinical pathology, with all tissue removed at operations to be examined in the laboratory and reports rendered thereon.’ The American Board of Pathology was instituted in 1936. Finally, in 1943 ‘pathology’ was recognized as the ‘practice of medicine’ by the House of Delegates of the American Medical Association.
Yet even the great Virchow was subject to error (whether it was sampling or interpretive error is a matter of speculation). In one of the most vituperative medical quarrels between opposing treating physicians in history, 1 the crown prince Frederick (1831–1888), son of William I, Emperor of Germany, fell ill with laryngeal hoarseness in January 1887. Although a laryngeal cancer was suspected from the outset, initial biopsies sent to Virchow for interpretation were either insufficient for diagnosis or interpreted as pachydermia verracosa (throat wart). Subsequent biopsies were necrotic or too purulent for diagnosis, despite overwhelming clinical evidence that the laryngeal process was malignant. Failure to obtain an anatomic diagnosis of malignancy, per Virchow , immobilized the clinical treatment team. Only when Frederick was in extremis (February 1888) did laryngeal biopsy finally reveal the ‘little bodies that brought it all about’ (to quote his son, the future Kaiser Wilhelm II).
Although Virchow's reputation appears to have been unharmed, this episode was illustrative of the potentially shaky status that pathology holds as the diagnostic bedrock for patient care. In this Editorial Perspective, consideration will be given to a number of pertinent issues, focusing primarily on the role of anatomic pathology. First, on what basis are diagnostic assessments made in anatomic pathology? Second, does this diagnostic exercise fulfill the requirements for EBM? Third, how well does anatomic pathology serve as a ‘gold standard’ for research being conducted on human tissues? Lastly, what is the academic productivity of pathology scholars who, operating in the clinical realm, are given opportunity to publish their original findings?
The basis of diagnosis in anatomic pathology
My first encounter with the diagnostic process in surgical pathology was on my second day of residency (1982), when my confident diagnosis of ‘axillary lymph nodes positive for metastatic breast carcinoma’ was countermanded by the attending pathologist: I was simply observing ‘sinus histiocytosis’ in the lymph nodes. My silent indignant reaction was, ‘On what basis do you know that you are right, and I am wrong?’ Put differently, how can it be that morphologic examination of devitalized human tissue that has been fixed in formalin, dehydrated and permeated with paraffin, sliced thinly and stained with biblical era colorizing agents (hematoxylin & eosin), covered in glue and sandwiched between glass, has any bearing on clinical management decisions made for the living patient? Potential answers include, ‘Because I am the authority here’, or ‘My experience’, or ‘This is what I was taught’, or ‘This is what the recent literature indicates’, or even ‘This is what is required for synoptic reporting.’ Then and now (24 years later), I am forced to conclude that, ultimately, it is the experience base of over a century-and-a-half of cellular Pathologists that enables us to have credibility in dictating the fortunes (and misfortunes) of living patients.
The question then immediately arises: how is this experience-base established, and what credibility does it have? In a 2006 Lab Invest editorial, 2 I asked this specific question for human liver biopsy interpretation. The methodology was to examine the top 150 most-cited publications in which human liver pathology was included, either as the primary focus of the publication or as contributing data. The dates of publication for these papers were from 1948 to 2003, with a heavy representation of published papers by the most honored leaders of our subspecialty. The results were remarkable: essentially half (48%) of the ‘classics’ in liver pathology were Case Series, which is the reporting of an assembled experience. Another 10% were case series that included additional research in the basic laboratory, so as to obtain insights into the molecular or structural basis of the disease. An additional 16% were ‘Position Papers’, either declarative by authorities in the field, reports by consensus panels, or reviews. So ¾ of the most cited papers in the liver pathology field were published predominantly on the basis of ‘experience’ or ‘opinion’. Only 26% of these 150 most-cited papers would qualify for ‘Evidence-Based Medicine (EBM)’ (to be discussed). These were exclusively within the realm of viral hepatitis, in which clinical outcomes clearly established that liver biopsy was a useful diagnostic investigation in the management of patients with Hepatitis B or C viral infection.
These findings in liver pathology are echoed in a 2005 article by Foucar and Wick 3 (actually published in February 2006). These authors performed a pilot observational analysis of a representative sample of the current pertinent literature on diagnostic tissue pathology. They show that most of such publications employ ‘observational’ research designs, most commonly ‘cross-sectional comparison’. Slightly more than 50% of the anatomic pathology observational studies employed statistical evaluations to support their final conclusions. Unfortunately, such research designs are not admired by advocates of EBM, since they are a distant second choice to ‘experimental’ clinical studies. Foucar and Wick advocate that the latter posture is unacceptable to pathologists, whose research advances are currently completely dependent upon well-conducted observational research. Rather, the challenge to anatomic pathology is to develop and adhere to standards for observational research—including the classification of error in anatomic pathology 4 —so as to realize the full potential of this time-tested approach for advancing clinical knowledge. 3
So how does one actually become a good diagnostic Surgical Pathologist? At the risk of being pedantic, I consider that, first and foremost, knowledge of the linkage between the anatomic pathology data and the patient is required: knowing the relationship between the disease process and clinical outcomes in the living patient. To this is added experience : seeing this linkage first hand. Wisdom hopefully follows: learning from experience (the cynic would observe that one is usually learning from bad experiences, with which I disagree). This requires communication , most especially validating the role of one's own histological interpretations in the clinical course of the patients one has influenced. Hopefully the final product is good clinical judgment , upon which weighs heavily the taking of responsibility for your decision-making. To be a great surgical pathologist, more extensive knowledge of the clinical presentations, course, and management of patients is required, along with authority . The first attribute (clinical knowledge) is constantly strengthened by obtaining the clinical information at the time of tissue evaluation: clinical presentation, clinical findings (including imaging characteristics), pertinent past and family history; and by constantly pursuing information about subsequent clinical course. Participation in multidisciplinary patient care conferences is a valuable way for pathologists to get feedback about clinical and therapeutic outcomes which were triggered by the histopathological interpretation of tissues. The latter attribute (authority) is gained especially by contributing new knowledge to the field of surgical pathology on the basis of one's own published scholarship.
In assembling knowledge , there is a challenge in finding the published literature—case series or otherwise. In the liver pathology field, at least, 2 only 16% of the 150 most-cited papers were published in ‘pathology’ journals; 84% were published in ‘Clinical’ journals (eg, Gastroenterology, Hepatology, J Hepatology ). If this is any reflection of surgical pathology in general, in order to find the most-cited published papers in surgical pathology, one has to put especial effort into reading the clinical journals of each related clinical specialty/subspecialty. One could also read textbooks, especially on a per case basis, relying upon the experience and authority of the writer. Very importantly, one can learn from local colleagues, so as to draw upon the collective expertise and experience of the local practice group. Fourth, it is worth taking opportunity to learn from ‘experts’, as at national meetings and at special courses. ‘Reading the literature’ is a given, but this is usually for the limited portfolio of journals to which one subscribes. I also recommend actively seeking out the published ‘evidence base’ (such as it may be) for the major disease categories of one's practice on a regular, pro-active basis; ‘Journal Club’ is one such impetus for so doing. One can seek out informative websites, such as ( www.uscap.org ). Lastly, we can now subscribe to ‘push’ electronic services, which post to our computer desktop listings of newly published papers which should be of interest to us. It does take reading them.
EBM in anatomic pathology
So we come to the essential question: is an Anatomic Pathologist rendering an ‘expert’ interpretation, or an ‘evidence-based’ interpretation. This begs the question, what is ‘EBM’? A brief history is appropriate. 5 In the first half of the 20th century, a fundamental premise of modern medicine was that ‘Each physician will think the right thoughts and do the right thing’. To quote Eddy, ‘The idea was that when a physician is faced with a patient, by some fundamentally human process called the ‘art of medicine’ or ‘clinical judgment’, the physician would synthesize all of the important information about the patient, relevant research, and experiences with previous patients to determine the best course of action’. 5 In the published literature and in medical textbooks, medical knowledge becomes a compendium of ‘if….then’ statements. As further detailed by Eddy, in 1973, Wennberg and Gittelson 6 documented that there were wide variations in clinical practice patterns, in the absence of apparent justification for such variations. In the 1980s, a group of physicians at the RAND Corporation changed the frame of reference entirely, by reporting that inappropriate medical procedures were being done by apparently well-informed physicians, such as the use of coronary angiography. 7 This was paralleled in 1985 by a highly influential Institute of Medicine report quoting a congressional committee statement that ‘only 15% of medical practices are based on clinical trial evidence’. 8 By the late 1980s, an increasing number of randomized clinical trials were documenting that certain current clinical practices were ineffective.
Such discussions made clear the need for more rigorous study of medical decision making, and the general concepts underlying EBM were evolving through the 1970s and 1980s. 9 David Eddy in 1990 10 discussed the different methods for designing clinical practice guidelines: preference-based; ‘global subjective judgment’; consensus; outcomes based, and evidence based —the first published use of this term. As summarized by Akobeng in 2005, 11 the ‘evidence base’ for the practice of clinical medicine follows a hierarchy ( Figure 1 ), in which ‘opinion’, ‘case reports’, and ‘case series’ are at the bottom. Unfortunately, this is precisely where anatomical pathology seems to live. At the peak of the pyramid are the randomized clinical trails, which are assembled into ‘systematic reviews of randomized clinical trials, with or without meta-analysis’. Indeed, it is the latter that find their ways into the lay press, when systematic reviews find that ‘fish oil may not reduce cancer risk’ or ‘soy has little effect on cholesterol’. 12 Such lay publicity arises chiefly because the medical literature has a propensity to publish positive results only, and not publish negative studies which refute exciting new clinical findings. It takes ‘systematic reviews’ not only to authenticate a growing body of positive clinical evidence, but also to refute nagging reports which won’t go away. The Wall Street Journal noted that ‘the scientific literature is biased towards positive results’, and ‘there is need for recognizing results which run counter to positive results’. 13 Two journals gaining visibility in that regard are the Journal of Spurious Results and the Journal of Negative Results in Biomedicine .
Hierarchy of evidence for questions about the effectiveness of an intervention or treatment. From Akobeng. 11 Adapted and reproduced with permission from the BMJ publishing group. The designation of ‘Anatomical Pathology’ is added. RCT, randomized clinical trial.
The founding goal of EBM was to enhance the teaching of medical residents, 5 especially as articulated in 1992 by the EBM Working Groups. 14 , 15 Although it was David Eddy who introduced the term, its use was irrevocably altered by a 1996 editorial by David Sackett (Associate Editor of the British Medical Journal ), who stated that EBM is ‘the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.’ 16 The practice of EBM means ‘integrating individual clinical expertise with the best available external clinical evidence’. The goal was to improve the quality of patient care through the identification and promotion of practices that work, and the elimination of ineffective or harmful ones. This requires clinicians to be open-minded and to try new methods that are scientifically proven to be effective, and to discard old methods that are not. The presumed benefits of EBM are: to help clinicians deal with ‘information overload’; to reduce inequalities in the delivery of healthcare (and distribute healthcare resources more equitably); to help reduce healthcare costs; and to justify treatment choices to the public. Vociferous criticisms are that EBM removes the ‘art’ from the practice of medicine. The rejoinder (articulated by Sackett himself in numerous follow-up letters) is that external ‘evidence’ is always to be tempered with internal experience. Eddy also observes that evidence in support of the practice of EBM still needs to be developed. 15
The practice of EBM would seem to presume that the practice of medicine is, in fact, a science. Arguably, ‘science’ is the formulation and attempted falsification of hypotheses using rigorous and reproducible methods. Applied to Medicine, ‘diagnostic inquiry’ is equated with ‘scientific inquiry’, with the premise that clinical observation is objective—including the diagnostic exercise that is anatomical pathology.
But clinical observation is not objective. As articulated by Trisha Greenhalgh, medicine is an ‘art’ because the ‘truths’ obtained from patient populations (ie, randomized controlled trials) cannot be reconciled with individual patients. 17 An ‘evidence base’ cannot substitute for clinical judgment, since the practicing physician has to interpret the patient's ‘narrative’ (their clinical course) before it can be placed in the context of the published literature. She states that the search for objective truths in a patient is a flight from interpretation, and is doomed to fail. Put differently, suspension of clinical judgment is a very bad idea. Genuine evidence-based practice presupposes an interpretive paradigm in which the patient experiences illness in a unique and contextual way. It is therefore the responsibility of the treating physician to reconcile individual patient ‘narratives’ with the ‘truths’ derived from population-based evidence. This can only be achieved through the use of clinical judgment.
We now return to anatomical pathology. Although our practices may be filled with routine diagnostic events, it is almost guaranteed that, with regularity, we encounter extraordinarily unusual cases. To deal with such a case, one must: become completely familiar with the clinical narrative; review the world's literature; consult with colleagues (beginning with local colleagues, then proceeding to national authorities and even international, as needed); and then render one's interpretation, complete with prognostic implications. Remarkably, one may then have to educate the clinical treatment teams on what you have actually identified, beginning with the disease process and moving on to the implications of the pathology interpretation. This may be termed ‘gaining a lifetime of experience with one case.’ It is hard to call this the ‘science’ of medicine. Rather, the pathologist is bringing all available information to bear on this one case, including most importantly experience. Ironically, this is much like David Eddy's description of the practice of medicine in the pre-‘evidence based’ era. 5
Specific interest in the role of EBM in pathology has only recently awoken. While Laboratory Medicine has long paid attention to testing accuracy and precision, 18 , 19 , 20 , 21 , 22 only recently has there been critical examination of the implicit assumption that an evidence-based culture underpins the use of laboratory medicine. The results are of concern, since the evidence base supporting use of a specific laboratory test procedure or technology in specific clinical situations may be quite limited or flawed. 23 , 24 Indeed, the failure to demonstrate the effective utilization of laboratory tests generated either in the clinical laboratory or in anatomic pathology puts the profession of pathology at risk of being deemed outside ‘best practices’, and hence ineligible for reimbursement. 25 It is hoped that there will be continued effort to advance ‘evidence-based laboratory medicine’. 26
In the case of anatomic pathology, in 1996 Baker et al 27 analyzed anatomic pathology databases to assess autopsy performance. The 2001 ‘consensus guidelines for the management of women with cervical cytological abnormalities’ 28 constitutes a tour-de-force analysis of the evidence base in support of cervical cytology examination and validated clinical outcomes. Nevertheless, in 2000 Sirota made clear that the 1999 Institute of Medicine report on ‘medical error’ should be applied to the practice of pathology. 29 Marchevsky and Wick 30 are the first to examine the broader role of EBM and ‘medical decision analysis’ (whereby mathematical tools are used to ‘reason with uncertainty’) in the practice of pathology. They noted that pathologists will be well served by becoming more familiar with the basic concepts of EBM and how pathology data can be better integrated into formal medical decision analysis.
The message evidently has been heard. Beginning with Zarbo and coworkers, 31 , 32 , 33 , 34 the role of error in the practice of anatomic pathology on patient management and clinical outcomes is now being rigorously examined. The entire May 2005 issue of Seminars in Diagnostic Pathology was devoted to the role of EBM in pathology. 3 , 4 , 25 , 35 , 36 , 37 , 38 , 39 A key point of these articles is that the practice guidelines of surgical pathologists, including our most revered classification schemes, are generally based on ‘expert opinion’, representing the weakest form of evidence. 39
Anatomic pathology as a ‘gold standard’
At what point does a Pathologist's ‘interpretation’ become a datum, to be used as a ‘gold standard’ in the practice of medicine? At the very least, the difficulties in reproducibility of histological and cytological diagnoses due to ‘interobserver variation’ challenge the concept of anatomic pathology as a gold standard. 40 , 41 , 42 , 43 In September 2004, I examined the PubMed citation index for the term ‘interobserver variation’. Subcategories were ‘…AND pathology’, ‘AND cancer’, and ‘AND pathology AND cancer’. In October 2006, I repeated this exercise ( Figure 2 ). In each search, the number of citations increased by 33 to 37%. In other words, the indexed world's biomedical literature in ‘interobserver variation’ increased by 1/3 between late 2004 and late 2006! Clearly, we have a problem. Among observers of the practice of pathology, we are viewed not as a ‘gold standard’ but rather as a ‘brass standard’, or a ‘tin standard’.
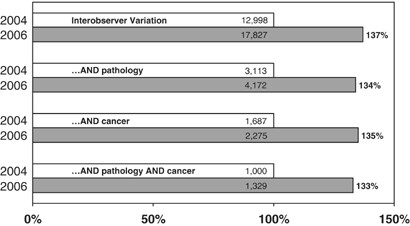
PubMed citations in four topic categories, as of September 18, 2004 (white bars) and October 29, 2006 (gray bars). PubMed ( www.pubmed.gov ) is a service of the National Library of Medicine and the National Institutes of Health.
A second issue arises from the practice of algorithmic EBM, in which semiquantitative or quantitative scores from anatomic pathology diagnoses are used to determine treatment interventions—pharmaceutical or surgical. In my experience (there we go), if the Treating Physician is making clinical management decisions based on a numerical ‘score’ from a morphological interpretation of biopsy tissue or an excised specimen, then the Pathologist is making the clinical treatment decision. This is based on the premise that the ‘score’ determines the treatment algorithm. The onus is very much on the Pathologist to know the treatment implications of their numerical scoring. Interestingly, it is precisely this arena where liver biopsy interpretation has been validated as an exercise in EBM. Peter Scheuer published in 1991 a critical opinion, stating that rigorous scoring of liver biopsies in viral hepatitis had to be changed, and providing a proposed scoring system for so doing. 44 A rash of echoing papers followed (reviewed in 2). Within five years, Poynard concluded on the basis of metanalysis of randomized clinical trials (the peak of the ‘EBM’ pyramid) that rigorous semi-quantitative scoring systems for liver biopsies in viral hepatitis are indeed valid for clinical management decisions. 45
But: a liver biopsy is not uniform. Interpretive judgment has to be used to ‘score’ the biopsy. Judgment can only be exercised on the basis of experience. So we are back to ‘experience’ underlying the ‘evidence base’ in anatomic pathology. Put differently, the Pathologist has remarkable power in an EBM setting, owing to the lower reproducibility of morphological evidence (as opposed to quantitative ‘scientific’ observation). Owing to the need for rigor and experience in the issuance of diagnostic reports, there is very definitely a role for ‘eminence’ in our field. Hence, no matter how ‘evidence based’ a clinical:pathologic paradigm for treatment, the Surgical Pathologist still has to interpret the histopathology. Judgment and experience remain regardless of how the objective criteria are to be used. Whether these issues apply across other anatomic pathology specialties (including cytopathology) will be left for others to examine.
The vagaries of obtaining rigorous pathology morphological interpretation are well documented elsewhere (as discussed in Marchevsky, 25 Raab 39 ). Recent examples abound, 34 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 regardless of whether the operative terms are ‘interobserver variation’ or outright ‘error’. 57 , 58 , 59 We thus encounter a fundamental challenge in the use of morphological data in EBM. The evidence base in the literature must be integrated with personal experience and judgment in order to render a successful morphological interpretation of human tissue. 25 Pathologists may have great difficulty in agreeing upon such interpretations and may be subject to ‘error’, particularly in those areas where rigor is needed most, such as the diagnosis of dysplasia. Nevertheless, such interpretations are inserted into algorithmic ‘evidence based’ paradigms for the treatment of patients. A most remarkable event then occurs: this process actually works! In the many subspecialty areas of anatomic pathology the rigorous efforts of Pathologists do indeed promote favorable outcomes in patient care. Beginning with cervical cytopathology, 28 screening programs for the discovery of cancer at its earliest stages—with the interpretive expertise of anatomic pathologists prominently featured—constitute one of the best examples of the practice of evidence-based medicine over the past several decades. 36 Ultimately, it is the integration of the pathologist's interpretation into the multidisciplinary management of the patient which promotes optimal patient care. 60 , 67 I should also note that inclusion of the pathologist as contributing author on such publications is imperative.
Nevertheless, there are numerous examples in which practices in anatomic pathology are inadequately supported by evidence, 35 and the rapidly increasing demand for molecular testing of tissue specimens can constitute a disconcerting departure from EBM if potential uses have not been adequately validated. 37 , 61 It is essential that both anatomic pathology and Laboratory Medicine continue to strive for both the highest standards for practice, and for the rigorous data sets that justify (or refute) our practices. 33 , 62 , 63
The academic productivity of pathology scholars
Returning to the concept that pathology research is all things to all people, one could argue that ‘pathology research’ has no specific identity. The ‘investigative pathologist’ is hard to define, although it is reasonable to state that essentially all of the vocabulary and much of the intellectual foundation of modern medicine is derived from research by ‘investigative pathologists’ in the 19th and 20th centuries. 64 In the first half of the 20th century, pathology played an extraordinary role in the advances of public health, with success in dealing with infectious diseases being the salient example. In the second half of the 20th century, ‘investigative pathologists’ drew ever increasingly upon the basic science laboratory to gain insights into human disease, with molecular and genetic medicine figuring ever more prominently. As a result, with the dawn of the 21st century, the research portfolio of academic pathology is encyclopedic. Each academic pathology department in turn must establish their optimal research portfolio and product, and define what their contribution to the expanse of medical knowledge should be.
To obtain insight into this issue, a sample of North American academic pathology departments were examined for their output of medical journal publications. Specifically, the six participating speakers at the summer 2006 meeting of the Association of Pathology Chairs (see Acknowledgement) gave permission for a 100% audit of their department's published papers for the calendar year 2005. Five of these departments were in the United States, one in Canada. The Tomson Science Citation Index ® was searched for publications from these six departments for calendar year 2005. Published papers were classified according to whether they were supported by extramural funding (based on the Acknowledgement) or were ‘unfunded’ and clinical in nature. For the ‘funded’ papers, the department and institutional affiliation of the corresponding author (based on the Title Page) also were identified. Lastly, the journal of publication was classified as a ‘pathology’ journal, a ‘top general’ journal (specifically, J Am Med Assoc , J Clin Invest , J Exp Med , Nature , New Eng J Med , Proc Natl Acad Sci USA , Science ), a ‘basic science’ journal (eg, J Biol Chem , J Immunol , J Virol ), or a ‘Disease/Organ’ journal (eg, Gastroenterology , Circulation , Nephron , Brain , Diabetes ).
There were a total of 922 papers published in calendar year 2005 from these six departments; of which 702 (74%) were supported by extramural funds, and 220 (26%) were ‘unfunded’ and clinical in nature ( Figure 3 ). For the published funded research, 323 papers (35% of the 922 total papers) had a member of the host Department of Pathology as corresponding author. On 184 papers (20% of total), a ‘pathology’ faculty member was contributing author for a paper submitted from the same institution, and for 175 papers (19% of total) the ‘pathology’ faculty member was a collaborator with a different institution. Two conclusions are drawn. First, funded faculty in these six departments of pathology make a major contribution to the academic productivity of each department, with collaborative work exceeding corresponding authorship. Collaborations are evenly split between the home institution and an outside institution. ‘Funded’ papers originating from the host departments are still impressive in number, constituting 1/3 of the academic productivity of these departments. Based on 2004 data from the Association of American Medical Colleges (AAMC), 45% of academic Pathology faculty in the US are classified as ‘clinical’, and 55% as ‘basic’. One might extrapolate that 55% of the faculty (‘basic’) produce 74% of the published papers, while 45% of the faculty (‘clinical’) produce 26% of the published papers.
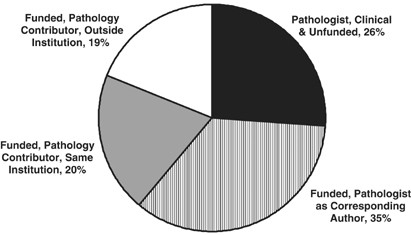
Classification of 2005 publications from six North American academic Departments of Pathology (five from the United States, one from Canada), according to funding status (per paper acknowledgement) and institutional affiliation of corresponding author. Data represent 992 publications.
This leads then to the second conclusion: clinical ‘unfunded’ faculty constitute ¼ of the academic productivity of these departments. This latter fact is neglected with risk, since the ability of unfunded clinical faculty to pursue scholarship may have a major impact on the overall productivity—and reputation—of a department. 68
The question then arises of where these papers are published. Figure 4 shows the distribution of published papers in ‘pathology’ journals (12%), ‘top general’ journals (4%), ‘basic science’ journals (37%), and ‘disease/organ’ journals (47%). The favorite journals outside the ‘pathology’ field were J Immunol (53 papers; 6% of total); J Biol Chem (26 papers; 3% of total); and J Virol (16 papers; 2% of total)—all classified as ‘basic science’ journals. The ‘disease/organ’ journal distribution was very broad, with only Infection and Immunity (10 papers; 1% of total) and Diabetes (9 papers, 1% of total) gaining a plurality. In keeping with a 2005 Lab Invest editorial, 65 it is clear that ‘pathology’ faculty seek a reading public largely outside the pathology literature. This supports the premise that defining the current identity of investigative pathology is somewhat difficult.
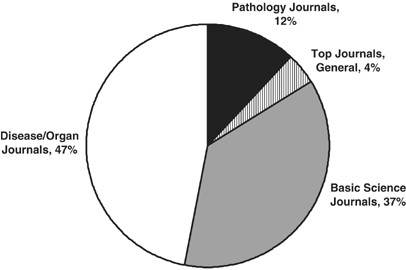
Classification of 2005 publications from six North American academic Departments of Pathology (five from the United States, one from Canada), according to journal. ‘Pathology Journals’ represent 28 pathology journals (see Table 1 ). ‘Top Journals’ refer to: J Am Med Assoc , J Clin Invest , J Exp Med , Nature , New Eng J Med , Proc Natl Acad Sci USA , and Science. ‘Basic Science’ journals are classified on the basis of scientific discipline; ‘Disease/Organ’ journals are classified on the basis of clinical specialty or disease interest. Data represent 992 publications.
Among the 28 major ‘pathology’ journals, Table 1 lists the journal of publication for the 111 papers published therein. More detailed data for 20 journals are presented, with Am J Clin Pathol, Am J Pathol , Am J Surg Pathol , Arch Pathol Lab Med, Mod Pathol , and Lab Invest having eight or more published papers from the 922 total. Inspection of these 111 papers revealed that 12 of the 12 papers published in Am J Pathol were extramurally funded research, as were seven of the eight papers published in Lab Invest (the only ‘unfunded’ paper was the aforementioned Lab Invest editorial; 2). For the remaining 18 journals in Table 1 , only 12 papers were extramurally funded, and 74 were ‘unfunded’ clinical scholarship. In this case, the 74 ‘unfunded’ clinical papers in ‘pathology’ journals represent 30% of the 240 papers published by ‘unfunded’ clinical faculty. Besides this evidence that clinical ‘pathology’ faculty largely publish outside the ‘pathology’ literature, it is also true that the preponderance of ‘pathology’ journals cater to ‘unfunded’ clinical pathology faculty.
This exercise has not attempted to determine the impact (scientific or otherwise) of the published papers. However, Table 2 continues the exercise of Crawford and Tykocinski in 2005, 65 examining the distribution of ‘pathology’ papers among the eight top Impact Factor journals, the eight top ‘disease/organ’ journals (identified on the basis of the top journal for each clinical specialty), and the eight top pathology journals, with updated data for the first months of 2006. Table 3 places these three journal groups in perspective to all journals in the Thomson Science Citation Index ® portfolio. For the past 3 years, over 95% of some 6000 journals have impact factors between 0.000 and 4.999. Only two of the 28 ‘pathology’ journals ( Am J Pathol , J Pathol ) fall into the top 5% of journals, with 2005 Impact Factors of 5.796 and 6.213, respectively. In aggregate, the Impact Factor of the eight top ‘pathology’ journals has not changed substantively since 1995, hovering around 3.6 (data not shown).
This exercise highlights the peculiar place of clinical scholarship in the practice of pathology. For the most part, original research in anatomic pathology is ‘observational’. Nevertheless, such observations constitute bedrock data for the practice of EBM. This exercise also brings up the issue of the visibility of the clinical ‘specialty’ of pathology. Pathology is a small specialty relative to the major clinical specialties. It is therefore unlikely that there will be global visibility for our publications in ‘pathology’ journals, since they are directed at the specific practice of our own profession by said Pathologists. To the extent that our clinical scholarship is directed towards ‘disease/organ’ journals, our clinical colleagues will be able to appreciate the impact of our work on their practice of medicine. The fundamental challenge remains nevertheless: to publish the highest quality original scholarship, both to enhance our ability to provide outstanding patient care and, ultimately, to better understand human disease.
This perspective has attempted to demonstrate that anatomic pathology has a unique place in the practice of EBM. On the one hand, the knowledge base in this specialty is predominantly observational. Moreover, at this point in time, a high level of experience and judgment is required on the part of the contributing pathologists in order to generate rigorous data from which publications emanate. On the other hand, there is growing evidence that the data provided by practitioners of anatomic pathology is highly relevant and valuable to the practice of EBM in the clinical arena. Although there is substantial concern about the vagaries of ‘error’ and ‘interobserver variation’ in anatomic pathology, pathologists exhibit a commendable ability to practice their observational art with rigor, so as to effect optimal patient outcomes locally on a case-by-case basis, and to contribute valuable data to the broader context of EBM trials. It remains to be seen whether more quantitative technologies for anatomic pathology will improve upon our time-honored practices. It also remains to be seen whether evidence bases can be obtained for the ever increasing introduction of molecular technologies into the interpretation of tissue samples.
The additional message of this perspective is that the scholarship emanating from clinical faculty in academic Departments of Pathology is crucial to the advancement of EBM. Although the productivity of such faculty may be less than colleagues with protected ‘research’ time, it is precisely their scholarship which helps advance clinical science and, ultimately, the validation (or not) of therapeutic interventions for human disease. If Impact Factor is any reflection of readership, the publication of clinical scholarship in the ‘pathology’ journals may lead to lower visibility of such scholarship than might be obtained from publication in higher impact organ- or disease-based journals. A dilemma therefore arises, in regards to the visibility of our specialty and discipline in the broader context of medicine. Whether the ‘pathology’ journals in specific, and our specialty in general, can maintain scientific preeminence remains an important challenge. The ultimate goal remains: to understand human disease for the benefit of mankind.
MacDonogh G . The Last Kaiser: The Life of Wilhelm II . St. Martin's Griffin: New York, NY, 2000, pp 192–213.
Google Scholar
Crawford JM . Evidence-based interpretation of liver biopsies. Lab Invest 2006; 86 :326–334.
Article Google Scholar
Foucar E, Wick MR . An observational examination of the literature in diagnostic anatomic pathology. Semin Diagn Pathol 2005; 22 :126–138.
Foucar E . Classification of error in anatomic pathology: a proposal for an evidence-based standard. Semin Diagn Pathol 2005; 22 :139–146.
Eddy DM . Evidence-based medicine: a unified approach. Health Affairs 2005; 24 :9–17.
Wennberg JE, Gittelsohn A . Small area variations in health care delivery. Science 1973; 82 :1102–1108.
Chassin MR, Kosekoff J, Solomon DH, et al . How coronary angiography is used: clinical determinants of appropriateness. J Am Med Assoc 1987; 258 :2543–2547.
Article CAS Google Scholar
Committee for Evaluating Medical Technologies in Clinical Use. The 15 per cent figure comes from an Institute of Medicine study, which cites the Congressional Office of Technology Assessment. Assessing Medical Technologies . National Academics Press: Washington, 1985, p 5.
Eddy DM . Variations in physician practice: the role of uncertainty. Health Affairs 1984; 3 :74–89.
Eddy DM . Practice policies: where do they come from? J Am Med Assoc 1990; 263 :1265, 1269, 1272, 1275.
Akobeng AK . Understanding randomised controlled trials. Arch Dis Child 2005; 90 :840–844.
Stengle J . Review casts doubt on soy's benefits. The Seattle Times 23 January 2006; Tanner L. Fish oil doesn't cut cancer risk, review finds. Seatlle Post-Intelligencer 25 January 2006.
Begley S . New journals bet negative results save time, money. Wall Street Journal 15 September 2006.
Mendelson D, Carino TV . Evidence-based medicine in the United States—de rigueur or dream deferred? Health Affairs 2005; 24 :133–136.
Pacy J . Evidence-based medicine and the search for a science of clinical care. Social Health Illness 2006; 28 :122–123.
Sackett DL, Rosenberg WMC, Gray JAM, et al . Evidence based medicine: what it is and what it isn't. Br Med J 1996; 312 :71–72.
Greenhalgh T . Narrative based medicine in an evidence-based world. Br Med J 1999; 318 :323–325.
Valenstein PN, Raab SS, Walsh MK . Identification errors involving clinical laboratories. A College of American Pathologists Q-Probes study of patient and specimen identification errors at 120 institutions. Arch Pathol Lab Med 2006; 130 :1106–1113.
PubMed Google Scholar
Zarbo RJ, Jones BA, Friedberg RC, et al . Q-Tracks. A College of American Pathologists program of continuous laboratory monitoring and longitudinal performance tracking. Arch Pathol Lab Med 2002; 126 :1036–1044.
Hollensead SC, Lockwood WB, Elin RJ . Errors in pathology and laboratory medicine: consequences and prevention. J Surg Oncol 2004; 88 :161–181.
Kalra J . Medical errors: an introduction to concepts. Clin Biochem 2004; 37 :1043–1051.
Kalra J . Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem 2004; 37 :1052–1062.
Price C . Evidence-based laboratory medicine: supporting decision-making. Clin Chem 2000; 46 :1041–1050.
CAS PubMed Google Scholar
McQueen MJ . Overview of evidence-based medicine: challenges for evidence-based laboratory medicine. Clin Chem 2001; 47 :1536–1546.
Marchevsky AM . Evidence-based medicine in pathology: an introduction. Semin Diagn Pathol 2005; 22 :105–115.
Trenti T . Evidence-based laboratory medicine as a tool for continuous professional improvement. Clin Chem Acta 2003; 333 :155–167.
Baker PB, Zarbo RJ, Howanitz PH . Quality assurance of autopsy face sheet reporting, final autopsy report turnaround time, and autopsy rates: a College of American Pathologists Q-Probes study of 10,003 autopsies from 418 institutions. Arch Pathol Lab Med 1996; 120 :1003–1008.
Wright Jr TC, Cox JT, Massad LS, et al . 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. J Am Med Assoc 2002; 287 :2120–2129.
Sirota RL . The Institute of Medicine's report on medical error: implications for pathology. Arch Pathol Lab Med 2000; 124 :1674–1678.
Marchevsky AM, Wick MR . Evidence-based medicine, medical decision analysis, and pathology. Hum Pathol 2004; 35 :1179–1188.
Zarbo RJ . Monitoring anatomic pathology practice through quality measures. Clin Lab Med 1999; 19 :713–742.
Zarbo RJ, Meier FA, Raab SS . Error detection in anatomic pathology. Arch Pathol Lab Med 2005; 129 :1237–1245.
Raab SS, Grzybicki DM, Zarbo RJ, et al . Anatomic pathology databases and patient safety. Arch Pathol Lab Med 2005; 129 :1246–1251.
Raab SS, Grzybicki DM, Janosky JE, et al . Clinical impact and frequency of anatomic pathology errors in cancer diagnoses. Cancer 2005; 104 :2205–2213.
Wick MR, Bourne D, Patterson JW, et al . Evidence-based principleas and practices in pathology: selected problem areas. Semin Diagn Pathol 2005; 22 :116–125.
Foucar E . Diagnostic precision and accuracy in interpretation of specimens from cancer screening programs. Semin Diagn Pathol 2005; 22 :147–155.
Marchevsky AM . The application of special technologies in diagnostic anatomic pathology: is it consistent with the principles of evidence-based medicine? Semin Diagn Pathol 2005; 22 :156–166.
Wick MR, Foucar E . Evidence-based medicine and tort law. Semin Diagn Pathol 2005; 22 :167–176.
Raab SS . Variability of practice in anatomic pathology and its effect on patient outcomes. Semin Diagn Pathol 2005; 22 :177–185.
Corley DE, Kirtland SH, Winterbauer RH, et al . Reproducibility of the histologic diagnosis of pneumonia among a panel of four pathologists: analysis of a gold standard. Chest 1997; 112 :458–465.
Rorke LB . Pathologic diagnosis as the gold standard. Cancer 1997; 79 :665–667.
Rushing L, Joste N . The surgical pathology report: Standardizing the ‘Gold Standard’. J Surg Oncol 1997; 65 :1–2.
Wells AU . Histopathologic diagnosis in diffuse lung disease: an ailing gold standard. Am J Resp Crit Care Med 2004; 170 :828–829.
Scheuer PJ . Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991; 13 :372–374.
Poynard T, Leroy V, Cohard M, et al . Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 1996; 24 :778–789.
Apple SK . Variability in gross and microscopic pathology reporting in excisional biopsies of breast cancer tissue. Breast J 2006; 12 :145–149.
Coffin CM, Spilker K, Zhou H, et al . Frozen section diagnosis in pediatric surgical pathology: a decade's experience in a children's hospital. Arch Pathol Lab Med 2005; 129 :1619–1625.
Coffin CM . Pediatric surgical pathology: pitfalls and strategies for error prevention. Arch Pathol Lab Med 2006; 130 :610–612.
Cooper K . Errors and error rates in surgical pathology: an Association of Directors of Anatomic and Surgical Pathology survey. Arch Pathol Lab Med 2006; 130 :607–609.
Grzybicki DM, Turcsanyi B, Becich MJ, et al . Database construction for improving patient safety by examining pathology errors. Am J Clin Pathol 2005; 124 :500–509.
Renshaw AA . Measuring and reporting errors in surgical pathology. Am J Clin Pathol 2001; 115 :338–341.
Renshaw AA, Pinnar NE, Jiroutek MR, et al . Blinded review as a method for quality improvement in surgical pathology. Arch Pathol Lab Med 2002; 126 :961–963.
Renshaw AA, Cartagena N, Granter SR, et al . Agreement and error rates using blinded review to evaluate surgical pathology of biopsy material. Am J Clin Pathol 2003; 119 :797–800.
Renshaw AA, Young ML, Jiroutek MR . How many cases need to be reviewed to compare performance in surgical pathology? Am J Clin Pathol 2003; 119 :388–391.
Renshaw AA, Gould EW . Measuring the value of review of pathology material by a second pathologist. Am J Clin Pathol 2006; 125 :737–739.
Renshaw AA . Comparing methods to measure error in gynecologic cytology and surgical pathology. Arch Pathol Lab Med 2006; 130 :626–629.
Sirota RL . Error and error reduction in pathology. Arch Pathol Lab Med 2005; 129 :1228–1233.
Sirota RL . Defining error in anatomic pathology. Arch Pathol Lab Med 2006; 130 :604–606.
Nakhleh RE . Error reduction in surgical pathology. Arch Pathol Lab Med 2006; 130 :630–632.
Flaherty KR, King TE, Raghu G, et al . Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Resp Crit Care Med 2004; 170 :904–910.
Altman DG, Riley RD . Primer: an evidence-based approach to prognostic markers. Nat Clin Pract Oncol 2005; 2 :466–472.
Raab SS . Improving patient safety through quality assurance. Arch Pathol Lab Med 2006; 130 :633–637.
Association of Directors of Anatomic and Surgical Pathology. Recommendations for quality assurance and improvement in surgical and autopsy pathology. Human Pathol 2006; 37 :985–988.
Korn D . Contribution of the Human Tissue Archive to the advancement of medical knowledge and the public health. A report to the National Bioethics Advisory Committee. US Government Printing Office: Bethesda, MD, January 1, 1998.
Crawford JM, Tykocinski M . Pathology as the enabler of human research. Lab Invest 2005; 85 :1058–1064.
Wagner RL (ed). In Pursuit of Excellence: The College of American pathologists, 1946–1996 . CAP Press: Northfield, IL, 1997.
Walker PD, Cavello T, Bonsib SM . Practice guidelines for the renal biopsy. Mod Pathol 2004; 17 :1555–1563.
Borkowski A, Berman JJ, Moore GW . Research by pathologists not funded by external grant agencies: a success story. Mod Pathol 1992; 5 :577–579.
Download references
Acknowledgements
Thanks are given to Catherine M Ketcham, PhD, Managing Editor for Laboratory Investigation , for her data and figure generation in support of this Editorial Perspective. Richard J Hausner, MD, provided valuable historical information on the 20th century foundations of the specialty of Pathology, drawing upon sources in Wagner 66 and the web pages of the American Society for Clinical Pathology ( www.ascp.org ) and the College of American Pathologists ( www.cap.org ). Concepts presented in the first and second sections of this perspective were presented in part at the Florida Society of Pathologists 2006 annual meeting, in Orlando, FL on January 28, 2006, and at the United States and Canadian Academy of Pathologists 2006 annual meeting, in Atlanta, GA on February 12, 2006. Concepts presented in the third section of this perspective were presented in part at an Optical Imaging Workshop hosted by the National Cancer Institute, on September 25, 2006 in Bethesda, MD. Concepts presented in the fourth section of this manuscript (‘academic productivity’) were presented in part at the summer 2006 annual meeting of the Association of Pathology Chairs, in Colorado Springs, CO on July 14, 2006. Participating speakers at the last meeting who gave permission for a 100% audit of 2005 published productivity of their departments were: James M Crawford, MD, PhD, Department of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL; Richard G Hegele, MD, PhD, Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada; Jay L Hess, MD, PhD, Department of Pathology, University of Michigan, Ann Arbor, MI; John B Lowe, MD, Department of Pathology, Case Western Reserve University School of Medicine, Cleveland U54 CA105480, OH; Tristram G Parslow, MD, PhD, Department of Pathology and Laboratory Medicine, Emory University Hospital, Atlanta, GA; and Kenneth L Rock, MD, Department of Pathology, University of Massachusetts School of Medicine, Worcester, MA. This work was supported in part by a grant from The National Cancer Institute, U54 CA105480, in support of the ‘Network for Translational Research in Optical Imaging’ (NTROI).
Author information
Authors and affiliations.
Department of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL, USA
James M Crawford
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to James M Crawford .
Additional information
NOTE ADDED IN PROOF
A recent publication documents that half of all renal practice evidence published between 1961–2005 also was published in non-renal journals (Barg AX, Iansavichius AV, Kastner M, et al. Kidney Int 2006;70:1995–2005). The issue of journal-of-publication is not limited to pathology.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Crawford, J. Original research in pathology: judgment, or evidence-based medicine?. Lab Invest 87 , 104–114 (2007). https://doi.org/10.1038/labinvest.3700511
Download citation
Accepted : 16 November 2006
Published : 05 February 2007
Issue Date : 01 February 2007
DOI : https://doi.org/10.1038/labinvest.3700511
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- surgical pathology
- clinical scholarship
- gold standard
This article is cited by
False-positive pathology: improving reproducibility with the next generation of pathologists.
- Benjamin L. Mazer
- Robert J. Homer
- David L. Rimm
Laboratory Investigation (2019)
Rethinking the doctor–patient relationship: toward a hermeneutically-informed epistemology of medical practice
Medicine, Health Care and Philosophy (2019)
Histology of adipose tissue inflammation in Dercum's disease, obesity and normal weight controls: a case control study
- Emma Hansson
- Henry Svensson
- Håkan Brorson
Journal of Inflammation (2011)
Diagnostische Studien
- Rosemarie Felder-Puig
- Philipp Mad
- Gerald Gartlehner
Wiener Medizinische Wochenschrift (2009)
Why microscopy will remain a cornerstone of surgical pathology
Laboratory Investigation (2007)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Healey Library
- Research Guides
Scholarly Articles and Journals
- Original Research
- Popular vs. Scholarly
Original Research Articles
Other types of articles.
- Reading Scholarly Articles
- Find Scholarly Articles
Research articles, empirical, research primary research, are based on original research. If you need to limit your sources to research articles, you must be able to tell the difference. Most research articles will contain the following:
A summary of the article. (Note: Abstracts appear in reviews or secondary articles as well.)
Sometimes called "methodology" or "materials and methods," this section describes the author's research methods and tools: experiment, survey, data sources, etc.
Results
Also called "findings," this is the section of the article in which raw data are presented.
Discussion
Sometimes called "analysis," this is the section in which the author analyzes the data.
The author's conclusions based on the analysis.
List of references to works cited in the article.
These standard parts of a research article may not always be labeled, and sometimes they are combined (for example, "Data and Methods"). Still, every research article indicates what methods and tools were used to conduct the research, what the results were, and how the author interprets those results.
Not every article in a scholarly journal contains research or analysis. Scholarly journals may also include:
- Literature reviews - often reviews original research
- Book reviews
- Meta-Analysis or systematic reviews - analysis of original research
- Editorials or commentaries
- Speeches and interviews
- Conference reports
These are not original or primary research articles.
- << Previous: Popular vs. Scholarly
- Next: Reading Scholarly Articles >>
- Last Updated: Apr 12, 2024 9:39 AM
- URL: https://umb.libguides.com/scholarlyjournals

A young researcher's guide to writing an original research article
Planning to Write
Types of articles: A guide for young researchers
Today, young researchers wish to start publishing articles early on in their career. However, they are often unsure of what type of article they wish to write and how to approach this task. This series provides detailed guidance to young researchers about different article types that journals publish and the standard requirements, procedures, and approach to each type.

Kakoli Majumder
February 20, 2015
826.9k views
6 Article types that journals publish: A guide…

March 1, 2015
98.4k views
A young researcher's guide to writing an original…

April 2, 2015
A young researcher's guide to writing a…

April 29, 2015
543.8k views
A young researcher's guide to a systematic review

May 11, 2015
14.1k views
Quiz: How much do you know about academic…

June 8, 2015
245.1k views
A young researcher's guide to writing a clinical…

July 24, 2015
18.9k views
A young researcher's guide to a clinical trial

September 2, 2015
230.4k views
A young researcher's guide to perspective, …

Journals publish different types of articles ; however, perhaps the most valued publications are original research articles. Original research articles are primary sources of scientific literature and present an original study. Authors have to conduct research on a particular topic through experiments, surveys, observation, etc. and report the findings of their study through original research articles. This post will help you understand what an original research article is and how to approach it.
What does an original research article mean?
For a manuscript to be considered an original research article, the following conditions need to be met :
- It should be written by the researchers who actually conducted the study.
- It should include the hypothesis or research question, the purpose of the study, and the details of the research methods.
- The research findings should be reported.
- These findings should be interpreted and possible implications discussed.
Note that even if a study does not produce positive results, it is regarded as original research and can be published. A study is said to have negative results when findings prove that the hypothesis was wrong. However, this is also an important learning that other researchers will benefit from. Hence negative results should also be published. Unfortunately, many authors and journal editors have a publication bias and do not prefer studies with negative findings . However, the scientific community has started realizing that not publishing negative results can slow down the progress of science. Hence, certain journals such as Journal of Negative Results in Biomedicine , PLOS ONE , and The All Results Journals are proactively countering publication biases by encouraging researchers to publish negative results.

How to approach an original research article?
Conducting original research and writing an article on it can indeed be a daunting task. In the beginning, most researchers feel lost and are unsure and confused about what they want to research on and how to go about it. However, if you proceed step by step, you will be able to form a clear idea of how to conduct your research and write the article. Here are a few important steps in the research writing process:
1. Choosing a research question
In order to begin your research, you first need to choose a research question . However, you cannot do this unless you have read a substantial amount of published literature in the field of your research. Develop a habit of regularly reading scientific literature . This will give you an idea of some of the existing problems in your field of interest. Think about these problems and discuss your ideas with your advisor before you decide which problem you would like to address. You need to consider both your interest and the feasibility of the idea before finalizing your research question.

2. Doing a literature search
Once you have finalized your research question, you need to do an extensive literature search . Plan your literature search well. You can choose a systematic approach by trying to search for all relevant material on the topic. You can also adopt a retrospective approach by finding the most recent material and working your way backward. You might also need to follow important citations that you come across. You will need to use books, journal articles, and other related sources of information such as government reports, online databases, etc. Keep a written record of your searches as this will be very helpful when writing the references and citations in your manuscript.
3. Structuring a research article
An original research article usually follows a specific structure . The most commonly used structure of a research paper includes the following sections: Introduction, Methods, Results, and Discussion. This is called the IMRaD structure .
- Introduction: The introduction provides background information and explains what your study is about and the purpose behind it.
- Methods: The methods section gives a detailed explanation of how you conducted your research and the materials you used. This is done so that other researchers can replicate your research and reproduce the findings.
- Results: This section presents your research findings in detail along with all the data .
- Discussion: This section interprets the findings and discusses the impact that your research may have on the field of study.
Make sure you cite all the references you have used in your paper. In the end, provide a detailed reference list of all the sources you have used.
4. Formatting the paper
Once you are done with writing the paper, it is time to format it. Generally, each journal has a house style, and you have to format your paper according to the style of the journal you are submitting to. This can be a tedious process, particularly if your paper is rejected and you have to re-format it when submitting it to another journal. One way to make this process less tedious is to follow a standard format based on a widely used style guide in your field. Format your paper using some general guidelines presented in that style guide. This will make it easier for you as you will only have to make a few modifications at the time of submission to suit the journal style.
If you have any doubts or questions, you can post them in the comments section below. Alternatively, you can also post a question on our Q&A forum , if you are facing a problem and need expert publication advice.

for this article
Published on: Mar 01, 2015
- Journal Article Types
- Original Article
You're looking to give wings to your academic career and publication journey. We like that!
Why don't we give you complete access! Create a free account and get unlimited access to all resources & a vibrant researcher community.
One click sign-in with your social accounts

Sign up via email
1536 visitors saw this today and 1210 signed up.
Subscribe to Conducting Research
Confirm that you would also like to sign up for free personalized email coaching for this stage.
Related Reading

The basics of converting your PhD thesis into journal articles

A young researcher's guide to writing a clinical case report
A young researcher's guide to writing an original research article 5 min read
How to write an effective title and abstract and choose appropriate keywords 11 min read
Our secret recipe (with 5 key ingredients) for a winning manuscript 9 min read
Guidelines for young researchers on tackling common problems in scientific publishing 15 min read
A young researcher's guide to a systematic review 6 min read
Trending Searches
- Statement of the problem
- Background of study
- Scope of the study
- Types of qualitative research
- Rationale of the study
- Concept paper
- Literature review
- Introduction in research
- Under "Editor Evaluation"
- Ethics in research
Recent Searches
- Review paper
- Responding to reviewer comments
- Predatory publishers
- Scope and delimitations
- Open access
- Plagiarism in research
- Journal selection tips
- Editor assigned
- Types of articles
- "Reject and Resubmit" status
- Decision in process
- Conflict of interest
- Chamberlain University
- Ask Us Page and Public FAQs
Frequently Asked Questions: FAQ Entry
- 1 Alumni Resources
- 3 APA Citation
- 1 APA Manual
- 1 Book Store
- 1 Center for Academic Success
- 12 Database
- 2 Download article
- 1 Drug Guides
- 1 Drug Information Portal
- 1 DynamedPlus
- 1 Full Text
- 2 How To Login
- 2 Library Home Page login
- 1 Micromedex
- 1 Nursing Theory
- 1 Paper Review
- 1 Pathophysiology
- 2 Peer Review
- 1 Permalinks
- 4 Search Everything
- 4 Search Strategy
- 1 Textbooks
Answered By: Last Updated: Feb 17, 2024 Views: 1546
What is an original research or single study article, and how do i find it in the library databases.
Original research, also known as a single study, primary study or empirical study, is one that reports results of a scientific study rather than summarizing other articles. Basically, original research is where the researchers do the study and report their findings.
Find an Original Research Article in the Library
- Start your search from the Search Everything box on the library homepage.
- Enter your keywords, and select Search .
- Under the Filter Results section on the left-hand side of the search results, select Peer-Reviewed Journals and Articles , and change the Publication Date range to reflect your assignment requirements.
- Read the abstract of the article(s) to determine if it is original research. The abstract of the article usually contains subdivision headings where each of the key sections are summarized individually such as Literature Review or Background, Methods, Results, Conclusions and Discussion. The methodology section will tell you what they are doing in the study.
Note! There is no way to limit the results to only show original research articles in this search system. If you are having trouble identifying the type of study, please contact your professor.
For more information on types of research, see our Finding Types of Research guide .
Watch the Tutorial Video
Select the image below to watch a video on how to determine if an article is original research.

- Share on Facebook
Was this helpful? Yes 2 No 0
- Search Website
- Library Tech Support
Chamberlain College of Nursing is owned and operated by Chamberlain University LLC. In certain states, Chamberlain operates as Chamberlain College of Nursing pending state authorization for Chamberlain University.

- Psychology Journals and research
- Original, Theoretical or Meta-analysis
Psychology Journals and research: Original, Theoretical or Meta-analysis
What is original research.
Original research is considered a primary source.
An article is considered original research if...
- it is the report of a study written by the researchers who actually did the study.
- the researchers describe their hypothesis or research question and the purpose of the study.
- the researchers detail their research methods.
- the results of the research are reported.
- the researchers interpret their results and discuss possible implications.
What is Theoretical Research?
Theoretical research is explanatory, and leads to the advancement of “knowledge for knowledge’s sake. This type of research attempts to gather knowledge about a phenomenon or idea whose conclusions may not have any immediate real-world application. It is sometimes referred to as "basic research."
How can I tell if the Article is Original Research?
There is no one way to easily tell if an article is a research article like there is for peer-reviewed articles in the Ulrich's database. The only way to be sure is to read the article to verify that it is written by the researchers and that they have explained all of their findings, in addition to listing their methodologies, results, and any conclusions based on the evidence collected.
All that being said, there are a few key indicators that will help you to quickly decide whether or not your article is based on original research.
- Literature Review or Background
- Conclusions
- Read through the abstract (summary) before you attempt to find the full-text PDF. The abstract of the article usually contains those subdivision headings where each of the key sections are summarized individually.
- Use the checkbox with CINAHL's advanced search to only see articles that have been tagged as research articles.
Examples of Theoretical Research
Examples of Theoretical research in psychology might include:
- An investigation looking at whether stress levels influence how often students engage in academic cheating
- A study looking at how caffeine consumption impacts the brain
- A study assessing whether men or women are more likely to be diagnosed with depression
- A study looking at how attachment styles among children of divorced parents compare to those raised by married parents
Notice in all of these examples, the goal of the research is merely to increase the amount of knowledge on a topic, not to come up with a practical solution to a problem.
What is Meta-Analysis?
Meta Analysis refers to a research strategy where instead of conducting new research with participants, the researchers examine the results of several previous studies. This is done with the purpose of gaining greater confidence in the results because of the larger pool of participants, as long as steps are taken to avoid errors that may have existed in the original studies.
Why study the studies?
Opposing theories and disparate findings populate the field of psychology; scientists must interpret the results of any single study in the context of its limitations. A researcher might study the studies by assimilating data across studies identified through a literature review. Meta-analysis has many strengths. First, meta-analysis provides an organized approach for handling a large number of studies. Second, the process is systematic and documented in great detail, which allows readers to evaluate the researchers’ decisions and conclusions. Third, meta-analysis allows researchers to examine an effect within a collection of studies in a more sophisticated manner than a qualitative summary.
- << Previous: Home
- Last Updated: Feb 5, 2021 2:36 PM
- URL: https://libraryguides.uwsp.edu/Neumann2
What does originality in research mean? A student's perspective
Affiliation.
- 1 University of South Wales Cardiff, UK.
- PMID: 25059081
- DOI: 10.7748/nr.21.6.8.e1254
Aim: To provide a student's perspective of what it means to be original when undertaking a PhD.
Background: A review of the literature related to the concept of originality in doctoral research highlights the subjective nature of the concept in academia. Although there is much literature that explores the issues concerning examiners' views of originality, there is little on students' perspectives.
Review methods: A snowballing technique was used, where a recent article was read, and the references cited were then explored. Given the time constraints, the author recognises that the literature review was not as extensive as a systematic literature review.
Discussion: It is important for students to be clear about what is required to achieve a PhD. However, the vagaries associated with the formal assessment of the doctoral thesis and subsequent performance at viva can cause considerable uncertainty and anxiety for students.
Conclusion: Originality in the PhD is a subjective concept and is not the only consideration for examiners. Of comparable importance is the assessment of the student's ability to demonstrate independence of thought and increasing maturity so they can become independent researchers.
Implications for research/practice: This article expresses a different perspective on what is meant when undertaking a PhD in terms of originality in the doctoral thesis. It is intended to help guide and reassure current and potential PhD students.
Keywords: PhD; Student perspectives; doctoral research; originality.
- Education, Nursing, Graduate
- Nursing Research*
- Students, Nursing / psychology*
- United Kingdom
- Open access
- Published: 18 April 2024
Research ethics and artificial intelligence for global health: perspectives from the global forum on bioethics in research
- James Shaw 1 , 13 ,
- Joseph Ali 2 , 3 ,
- Caesar A. Atuire 4 , 5 ,
- Phaik Yeong Cheah 6 ,
- Armando Guio Español 7 ,
- Judy Wawira Gichoya 8 ,
- Adrienne Hunt 9 ,
- Daudi Jjingo 10 ,
- Katherine Littler 9 ,
- Daniela Paolotti 11 &
- Effy Vayena 12
BMC Medical Ethics volume 25 , Article number: 46 ( 2024 ) Cite this article
1065 Accesses
6 Altmetric
Metrics details
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice. In this paper we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town, South Africa in November 2022.
The GFBR is an annual meeting organized by the World Health Organization and supported by the Wellcome Trust, the US National Institutes of Health, the UK Medical Research Council (MRC) and the South African MRC. The forum aims to bring together ethicists, researchers, policymakers, research ethics committee members and other actors to engage with challenges and opportunities specifically related to research ethics. In 2022 the focus of the GFBR was “Ethics of AI in Global Health Research”. The forum consisted of 6 case study presentations, 16 governance presentations, and a series of small group and large group discussions. A total of 87 participants attended the forum from 31 countries around the world, representing disciplines of bioethics, AI, health policy, health professional practice, research funding, and bioinformatics. In this paper, we highlight central insights arising from GFBR 2022.
We describe the significance of four thematic insights arising from the forum: (1) Appropriateness of building AI, (2) Transferability of AI systems, (3) Accountability for AI decision-making and outcomes, and (4) Individual consent. We then describe eight recommendations for governance leaders to enhance the ethical governance of AI in global health research, addressing issues such as AI impact assessments, environmental values, and fair partnerships.
Conclusions
The 2022 Global Forum on Bioethics in Research illustrated several innovations in ethical governance of AI for global health research, as well as several areas in need of urgent attention internationally. This summary is intended to inform international and domestic efforts to strengthen research ethics and support the evolution of governance leadership to meet the demands of AI in global health research.
Peer Review reports
Introduction
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice [ 1 , 2 , 3 ]. Beyond the growing number of AI applications being implemented in health care, capabilities of AI models such as Large Language Models (LLMs) expand the potential reach and significance of AI technologies across health-related fields [ 4 , 5 ]. Discussion about effective, ethical governance of AI technologies has spanned a range of governance approaches, including government regulation, organizational decision-making, professional self-regulation, and research ethics review [ 6 , 7 , 8 ]. In this paper, we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health research, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town, South Africa in November 2022. Although applications of AI for research, health care, and public health are diverse and advancing rapidly, the insights generated at the forum remain highly relevant from a global health perspective. After summarizing important context for work in this domain, we highlight categories of ethical issues emphasized at the forum for attention from a research ethics perspective internationally. We then outline strategies proposed for research, innovation, and governance to support more ethical AI for global health.
In this paper, we adopt the definition of AI systems provided by the Organization for Economic Cooperation and Development (OECD) as our starting point. Their definition states that an AI system is “a machine-based system that can, for a given set of human-defined objectives, make predictions, recommendations, or decisions influencing real or virtual environments. AI systems are designed to operate with varying levels of autonomy” [ 9 ]. The conceptualization of an algorithm as helping to constitute an AI system, along with hardware, other elements of software, and a particular context of use, illustrates the wide variety of ways in which AI can be applied. We have found it useful to differentiate applications of AI in research as those classified as “AI systems for discovery” and “AI systems for intervention”. An AI system for discovery is one that is intended to generate new knowledge, for example in drug discovery or public health research in which researchers are seeking potential targets for intervention, innovation, or further research. An AI system for intervention is one that directly contributes to enacting an intervention in a particular context, for example informing decision-making at the point of care or assisting with accuracy in a surgical procedure.
The mandate of the GFBR is to take a broad view of what constitutes research and its regulation in global health, with special attention to bioethics in Low- and Middle- Income Countries. AI as a group of technologies demands such a broad view. AI development for health occurs in a variety of environments, including universities and academic health sciences centers where research ethics review remains an important element of the governance of science and innovation internationally [ 10 , 11 ]. In these settings, research ethics committees (RECs; also known by different names such as Institutional Review Boards or IRBs) make decisions about the ethical appropriateness of projects proposed by researchers and other institutional members, ultimately determining whether a given project is allowed to proceed on ethical grounds [ 12 ].
However, research involving AI for health also takes place in large corporations and smaller scale start-ups, which in some jurisdictions fall outside the scope of research ethics regulation. In the domain of AI, the question of what constitutes research also becomes blurred. For example, is the development of an algorithm itself considered a part of the research process? Or only when that algorithm is tested under the formal constraints of a systematic research methodology? In this paper we take an inclusive view, in which AI development is included in the definition of research activity and within scope for our inquiry, regardless of the setting in which it takes place. This broad perspective characterizes the approach to “research ethics” we take in this paper, extending beyond the work of RECs to include the ethical analysis of the wide range of activities that constitute research as the generation of new knowledge and intervention in the world.
Ethical governance of AI in global health
The ethical governance of AI for global health has been widely discussed in recent years. The World Health Organization (WHO) released its guidelines on ethics and governance of AI for health in 2021, endorsing a set of six ethical principles and exploring the relevance of those principles through a variety of use cases. The WHO guidelines also provided an overview of AI governance, defining governance as covering “a range of steering and rule-making functions of governments and other decision-makers, including international health agencies, for the achievement of national health policy objectives conducive to universal health coverage.” (p. 81) The report usefully provided a series of recommendations related to governance of seven domains pertaining to AI for health: data, benefit sharing, the private sector, the public sector, regulation, policy observatories/model legislation, and global governance. The report acknowledges that much work is yet to be done to advance international cooperation on AI governance, especially related to prioritizing voices from Low- and Middle-Income Countries (LMICs) in global dialogue.
One important point emphasized in the WHO report that reinforces the broader literature on global governance of AI is the distribution of responsibility across a wide range of actors in the AI ecosystem. This is especially important to highlight when focused on research for global health, which is specifically about work that transcends national borders. Alami et al. (2020) discussed the unique risks raised by AI research in global health, ranging from the unavailability of data in many LMICs required to train locally relevant AI models to the capacity of health systems to absorb new AI technologies that demand the use of resources from elsewhere in the system. These observations illustrate the need to identify the unique issues posed by AI research for global health specifically, and the strategies that can be employed by all those implicated in AI governance to promote ethically responsible use of AI in global health research.
RECs and the regulation of research involving AI
RECs represent an important element of the governance of AI for global health research, and thus warrant further commentary as background to our paper. Despite the importance of RECs, foundational questions have been raised about their capabilities to accurately understand and address ethical issues raised by studies involving AI. Rahimzadeh et al. (2023) outlined how RECs in the United States are under-prepared to align with recent federal policy requiring that RECs review data sharing and management plans with attention to the unique ethical issues raised in AI research for health [ 13 ]. Similar research in South Africa identified variability in understanding of existing regulations and ethical issues associated with health-related big data sharing and management among research ethics committee members [ 14 , 15 ]. The effort to address harms accruing to groups or communities as opposed to individuals whose data are included in AI research has also been identified as a unique challenge for RECs [ 16 , 17 ]. Doerr and Meeder (2022) suggested that current regulatory frameworks for research ethics might actually prevent RECs from adequately addressing such issues, as they are deemed out of scope of REC review [ 16 ]. Furthermore, research in the United Kingdom and Canada has suggested that researchers using AI methods for health tend to distinguish between ethical issues and social impact of their research, adopting an overly narrow view of what constitutes ethical issues in their work [ 18 ].
The challenges for RECs in adequately addressing ethical issues in AI research for health care and public health exceed a straightforward survey of ethical considerations. As Ferretti et al. (2021) contend, some capabilities of RECs adequately cover certain issues in AI-based health research, such as the common occurrence of conflicts of interest where researchers who accept funds from commercial technology providers are implicitly incentivized to produce results that align with commercial interests [ 12 ]. However, some features of REC review require reform to adequately meet ethical needs. Ferretti et al. outlined weaknesses of RECs that are longstanding and those that are novel to AI-related projects, proposing a series of directions for development that are regulatory, procedural, and complementary to REC functionality. The work required on a global scale to update the REC function in response to the demands of research involving AI is substantial.
These issues take greater urgency in the context of global health [ 19 ]. Teixeira da Silva (2022) described the global practice of “ethics dumping”, where researchers from high income countries bring ethically contentious practices to RECs in low-income countries as a strategy to gain approval and move projects forward [ 20 ]. Although not yet systematically documented in AI research for health, risk of ethics dumping in AI research is high. Evidence is already emerging of practices of “health data colonialism”, in which AI researchers and developers from large organizations in high-income countries acquire data to build algorithms in LMICs to avoid stricter regulations [ 21 ]. This specific practice is part of a larger collection of practices that characterize health data colonialism, involving the broader exploitation of data and the populations they represent primarily for commercial gain [ 21 , 22 ]. As an additional complication, AI algorithms trained on data from high-income contexts are unlikely to apply in straightforward ways to LMIC settings [ 21 , 23 ]. In the context of global health, there is widespread acknowledgement about the need to not only enhance the knowledge base of REC members about AI-based methods internationally, but to acknowledge the broader shifts required to encourage their capabilities to more fully address these and other ethical issues associated with AI research for health [ 8 ].
Although RECs are an important part of the story of the ethical governance of AI for global health research, they are not the only part. The responsibilities of supra-national entities such as the World Health Organization, national governments, organizational leaders, commercial AI technology providers, health care professionals, and other groups continue to be worked out internationally. In this context of ongoing work, examining issues that demand attention and strategies to address them remains an urgent and valuable task.
The GFBR is an annual meeting organized by the World Health Organization and supported by the Wellcome Trust, the US National Institutes of Health, the UK Medical Research Council (MRC) and the South African MRC. The forum aims to bring together ethicists, researchers, policymakers, REC members and other actors to engage with challenges and opportunities specifically related to research ethics. Each year the GFBR meeting includes a series of case studies and keynotes presented in plenary format to an audience of approximately 100 people who have applied and been competitively selected to attend, along with small-group breakout discussions to advance thinking on related issues. The specific topic of the forum changes each year, with past topics including ethical issues in research with people living with mental health conditions (2021), genome editing (2019), and biobanking/data sharing (2018). The forum is intended to remain grounded in the practical challenges of engaging in research ethics, with special interest in low resource settings from a global health perspective. A post-meeting fellowship scheme is open to all LMIC participants, providing a unique opportunity to apply for funding to further explore and address the ethical challenges that are identified during the meeting.
In 2022, the focus of the GFBR was “Ethics of AI in Global Health Research”. The forum consisted of 6 case study presentations (both short and long form) reporting on specific initiatives related to research ethics and AI for health, and 16 governance presentations (both short and long form) reporting on actual approaches to governing AI in different country settings. A keynote presentation from Professor Effy Vayena addressed the topic of the broader context for AI ethics in a rapidly evolving field. A total of 87 participants attended the forum from 31 countries around the world, representing disciplines of bioethics, AI, health policy, health professional practice, research funding, and bioinformatics. The 2-day forum addressed a wide range of themes. The conference report provides a detailed overview of each of the specific topics addressed while a policy paper outlines the cross-cutting themes (both documents are available at the GFBR website: https://www.gfbr.global/past-meetings/16th-forum-cape-town-south-africa-29-30-november-2022/ ). As opposed to providing a detailed summary in this paper, we aim to briefly highlight central issues raised, solutions proposed, and the challenges facing the research ethics community in the years to come.
In this way, our primary aim in this paper is to present a synthesis of the challenges and opportunities raised at the GFBR meeting and in the planning process, followed by our reflections as a group of authors on their significance for governance leaders in the coming years. We acknowledge that the views represented at the meeting and in our results are a partial representation of the universe of views on this topic; however, the GFBR leadership invested a great deal of resources in convening a deeply diverse and thoughtful group of researchers and practitioners working on themes of bioethics related to AI for global health including those based in LMICs. We contend that it remains rare to convene such a strong group for an extended time and believe that many of the challenges and opportunities raised demand attention for more ethical futures of AI for health. Nonetheless, our results are primarily descriptive and are thus not explicitly grounded in a normative argument. We make effort in the Discussion section to contextualize our results by describing their significance and connecting them to broader efforts to reform global health research and practice.
Uniquely important ethical issues for AI in global health research
Presentations and group dialogue over the course of the forum raised several issues for consideration, and here we describe four overarching themes for the ethical governance of AI in global health research. Brief descriptions of each issue can be found in Table 1 . Reports referred to throughout the paper are available at the GFBR website provided above.
The first overarching thematic issue relates to the appropriateness of building AI technologies in response to health-related challenges in the first place. Case study presentations referred to initiatives where AI technologies were highly appropriate, such as in ear shape biometric identification to more accurately link electronic health care records to individual patients in Zambia (Alinani Simukanga). Although important ethical issues were raised with respect to privacy, trust, and community engagement in this initiative, the AI-based solution was appropriately matched to the challenge of accurately linking electronic records to specific patient identities. In contrast, forum participants raised questions about the appropriateness of an initiative using AI to improve the quality of handwashing practices in an acute care hospital in India (Niyoshi Shah), which led to gaming the algorithm. Overall, participants acknowledged the dangers of techno-solutionism, in which AI researchers and developers treat AI technologies as the most obvious solutions to problems that in actuality demand much more complex strategies to address [ 24 ]. However, forum participants agreed that RECs in different contexts have differing degrees of power to raise issues of the appropriateness of an AI-based intervention.
The second overarching thematic issue related to whether and how AI-based systems transfer from one national health context to another. One central issue raised by a number of case study presentations related to the challenges of validating an algorithm with data collected in a local environment. For example, one case study presentation described a project that would involve the collection of personally identifiable data for sensitive group identities, such as tribe, clan, or religion, in the jurisdictions involved (South Africa, Nigeria, Tanzania, Uganda and the US; Gakii Masunga). Doing so would enable the team to ensure that those groups were adequately represented in the dataset to ensure the resulting algorithm was not biased against specific community groups when deployed in that context. However, some members of these communities might desire to be represented in the dataset, whereas others might not, illustrating the need to balance autonomy and inclusivity. It was also widely recognized that collecting these data is an immense challenge, particularly when historically oppressive practices have led to a low-trust environment for international organizations and the technologies they produce. It is important to note that in some countries such as South Africa and Rwanda, it is illegal to collect information such as race and tribal identities, re-emphasizing the importance for cultural awareness and avoiding “one size fits all” solutions.
The third overarching thematic issue is related to understanding accountabilities for both the impacts of AI technologies and governance decision-making regarding their use. Where global health research involving AI leads to longer-term harms that might fall outside the usual scope of issues considered by a REC, who is to be held accountable, and how? This question was raised as one that requires much further attention, with law being mixed internationally regarding the mechanisms available to hold researchers, innovators, and their institutions accountable over the longer term. However, it was recognized in breakout group discussion that many jurisdictions are developing strong data protection regimes related specifically to international collaboration for research involving health data. For example, Kenya’s Data Protection Act requires that any internationally funded projects have a local principal investigator who will hold accountability for how data are shared and used [ 25 ]. The issue of research partnerships with commercial entities was raised by many participants in the context of accountability, pointing toward the urgent need for clear principles related to strategies for engagement with commercial technology companies in global health research.
The fourth and final overarching thematic issue raised here is that of consent. The issue of consent was framed by the widely shared recognition that models of individual, explicit consent might not produce a supportive environment for AI innovation that relies on the secondary uses of health-related datasets to build AI algorithms. Given this recognition, approaches such as community oversight of health data uses were suggested as a potential solution. However, the details of implementing such community oversight mechanisms require much further attention, particularly given the unique perspectives on health data in different country settings in global health research. Furthermore, some uses of health data do continue to require consent. One case study of South Africa, Nigeria, Kenya, Ethiopia and Uganda suggested that when health data are shared across borders, individual consent remains necessary when data is transferred from certain countries (Nezerith Cengiz). Broader clarity is necessary to support the ethical governance of health data uses for AI in global health research.
Recommendations for ethical governance of AI in global health research
Dialogue at the forum led to a range of suggestions for promoting ethical conduct of AI research for global health, related to the various roles of actors involved in the governance of AI research broadly defined. The strategies are written for actors we refer to as “governance leaders”, those people distributed throughout the AI for global health research ecosystem who are responsible for ensuring the ethical and socially responsible conduct of global health research involving AI (including researchers themselves). These include RECs, government regulators, health care leaders, health professionals, corporate social accountability officers, and others. Enacting these strategies would bolster the ethical governance of AI for global health more generally, enabling multiple actors to fulfill their roles related to governing research and development activities carried out across multiple organizations, including universities, academic health sciences centers, start-ups, and technology corporations. Specific suggestions are summarized in Table 2 .
First, forum participants suggested that governance leaders including RECs, should remain up to date on recent advances in the regulation of AI for health. Regulation of AI for health advances rapidly and takes on different forms in jurisdictions around the world. RECs play an important role in governance, but only a partial role; it was deemed important for RECs to acknowledge how they fit within a broader governance ecosystem in order to more effectively address the issues within their scope. Not only RECs but organizational leaders responsible for procurement, researchers, and commercial actors should all commit to efforts to remain up to date about the relevant approaches to regulating AI for health care and public health in jurisdictions internationally. In this way, governance can more adequately remain up to date with advances in regulation.
Second, forum participants suggested that governance leaders should focus on ethical governance of health data as a basis for ethical global health AI research. Health data are considered the foundation of AI development, being used to train AI algorithms for various uses [ 26 ]. By focusing on ethical governance of health data generation, sharing, and use, multiple actors will help to build an ethical foundation for AI development among global health researchers.
Third, forum participants believed that governance processes should incorporate AI impact assessments where appropriate. An AI impact assessment is the process of evaluating the potential effects, both positive and negative, of implementing an AI algorithm on individuals, society, and various stakeholders, generally over time frames specified in advance of implementation [ 27 ]. Although not all types of AI research in global health would warrant an AI impact assessment, this is especially relevant for those studies aiming to implement an AI system for intervention into health care or public health. Organizations such as RECs can use AI impact assessments to boost understanding of potential harms at the outset of a research project, encouraging researchers to more deeply consider potential harms in the development of their study.
Fourth, forum participants suggested that governance decisions should incorporate the use of environmental impact assessments, or at least the incorporation of environment values when assessing the potential impact of an AI system. An environmental impact assessment involves evaluating and anticipating the potential environmental effects of a proposed project to inform ethical decision-making that supports sustainability [ 28 ]. Although a relatively new consideration in research ethics conversations [ 29 ], the environmental impact of building technologies is a crucial consideration for the public health commitment to environmental sustainability. Governance leaders can use environmental impact assessments to boost understanding of potential environmental harms linked to AI research projects in global health over both the shorter and longer terms.
Fifth, forum participants suggested that governance leaders should require stronger transparency in the development of AI algorithms in global health research. Transparency was considered essential in the design and development of AI algorithms for global health to ensure ethical and accountable decision-making throughout the process. Furthermore, whether and how researchers have considered the unique contexts into which such algorithms may be deployed can be surfaced through stronger transparency, for example in describing what primary considerations were made at the outset of the project and which stakeholders were consulted along the way. Sharing information about data provenance and methods used in AI development will also enhance the trustworthiness of the AI-based research process.
Sixth, forum participants suggested that governance leaders can encourage or require community engagement at various points throughout an AI project. It was considered that engaging patients and communities is crucial in AI algorithm development to ensure that the technology aligns with community needs and values. However, participants acknowledged that this is not a straightforward process. Effective community engagement requires lengthy commitments to meeting with and hearing from diverse communities in a given setting, and demands a particular set of skills in communication and dialogue that are not possessed by all researchers. Encouraging AI researchers to begin this process early and build long-term partnerships with community members is a promising strategy to deepen community engagement in AI research for global health. One notable recommendation was that research funders have an opportunity to incentivize and enable community engagement with funds dedicated to these activities in AI research in global health.
Seventh, forum participants suggested that governance leaders can encourage researchers to build strong, fair partnerships between institutions and individuals across country settings. In a context of longstanding imbalances in geopolitical and economic power, fair partnerships in global health demand a priori commitments to share benefits related to advances in medical technologies, knowledge, and financial gains. Although enforcement of this point might be beyond the remit of RECs, commentary will encourage researchers to consider stronger, fairer partnerships in global health in the longer term.
Eighth, it became evident that it is necessary to explore new forms of regulatory experimentation given the complexity of regulating a technology of this nature. In addition, the health sector has a series of particularities that make it especially complicated to generate rules that have not been previously tested. Several participants highlighted the desire to promote spaces for experimentation such as regulatory sandboxes or innovation hubs in health. These spaces can have several benefits for addressing issues surrounding the regulation of AI in the health sector, such as: (i) increasing the capacities and knowledge of health authorities about this technology; (ii) identifying the major problems surrounding AI regulation in the health sector; (iii) establishing possibilities for exchange and learning with other authorities; (iv) promoting innovation and entrepreneurship in AI in health; and (vi) identifying the need to regulate AI in this sector and update other existing regulations.
Ninth and finally, forum participants believed that the capabilities of governance leaders need to evolve to better incorporate expertise related to AI in ways that make sense within a given jurisdiction. With respect to RECs, for example, it might not make sense for every REC to recruit a member with expertise in AI methods. Rather, it will make more sense in some jurisdictions to consult with members of the scientific community with expertise in AI when research protocols are submitted that demand such expertise. Furthermore, RECs and other approaches to research governance in jurisdictions around the world will need to evolve in order to adopt the suggestions outlined above, developing processes that apply specifically to the ethical governance of research using AI methods in global health.
Research involving the development and implementation of AI technologies continues to grow in global health, posing important challenges for ethical governance of AI in global health research around the world. In this paper we have summarized insights from the 2022 GFBR, focused specifically on issues in research ethics related to AI for global health research. We summarized four thematic challenges for governance related to AI in global health research and nine suggestions arising from presentations and dialogue at the forum. In this brief discussion section, we present an overarching observation about power imbalances that frames efforts to evolve the role of governance in global health research, and then outline two important opportunity areas as the field develops to meet the challenges of AI in global health research.
Dialogue about power is not unfamiliar in global health, especially given recent contributions exploring what it would mean to de-colonize global health research, funding, and practice [ 30 , 31 ]. Discussions of research ethics applied to AI research in global health contexts are deeply infused with power imbalances. The existing context of global health is one in which high-income countries primarily located in the “Global North” charitably invest in projects taking place primarily in the “Global South” while recouping knowledge, financial, and reputational benefits [ 32 ]. With respect to AI development in particular, recent examples of digital colonialism frame dialogue about global partnerships, raising attention to the role of large commercial entities and global financial capitalism in global health research [ 21 , 22 ]. Furthermore, the power of governance organizations such as RECs to intervene in the process of AI research in global health varies widely around the world, depending on the authorities assigned to them by domestic research governance policies. These observations frame the challenges outlined in our paper, highlighting the difficulties associated with making meaningful change in this field.
Despite these overarching challenges of the global health research context, there are clear strategies for progress in this domain. Firstly, AI innovation is rapidly evolving, which means approaches to the governance of AI for health are rapidly evolving too. Such rapid evolution presents an important opportunity for governance leaders to clarify their vision and influence over AI innovation in global health research, boosting the expertise, structure, and functionality required to meet the demands of research involving AI. Secondly, the research ethics community has strong international ties, linked to a global scholarly community that is committed to sharing insights and best practices around the world. This global community can be leveraged to coordinate efforts to produce advances in the capabilities and authorities of governance leaders to meaningfully govern AI research for global health given the challenges summarized in our paper.
Limitations
Our paper includes two specific limitations that we address explicitly here. First, it is still early in the lifetime of the development of applications of AI for use in global health, and as such, the global community has had limited opportunity to learn from experience. For example, there were many fewer case studies, which detail experiences with the actual implementation of an AI technology, submitted to GFBR 2022 for consideration than was expected. In contrast, there were many more governance reports submitted, which detail the processes and outputs of governance processes that anticipate the development and dissemination of AI technologies. This observation represents both a success and a challenge. It is a success that so many groups are engaging in anticipatory governance of AI technologies, exploring evidence of their likely impacts and governing technologies in novel and well-designed ways. It is a challenge that there is little experience to build upon of the successful implementation of AI technologies in ways that have limited harms while promoting innovation. Further experience with AI technologies in global health will contribute to revising and enhancing the challenges and recommendations we have outlined in our paper.
Second, global trends in the politics and economics of AI technologies are evolving rapidly. Although some nations are advancing detailed policy approaches to regulating AI more generally, including for uses in health care and public health, the impacts of corporate investments in AI and political responses related to governance remain to be seen. The excitement around large language models (LLMs) and large multimodal models (LMMs) has drawn deeper attention to the challenges of regulating AI in any general sense, opening dialogue about health sector-specific regulations. The direction of this global dialogue, strongly linked to high-profile corporate actors and multi-national governance institutions, will strongly influence the development of boundaries around what is possible for the ethical governance of AI for global health. We have written this paper at a point when these developments are proceeding rapidly, and as such, we acknowledge that our recommendations will need updating as the broader field evolves.
Ultimately, coordination and collaboration between many stakeholders in the research ethics ecosystem will be necessary to strengthen the ethical governance of AI in global health research. The 2022 GFBR illustrated several innovations in ethical governance of AI for global health research, as well as several areas in need of urgent attention internationally. This summary is intended to inform international and domestic efforts to strengthen research ethics and support the evolution of governance leadership to meet the demands of AI in global health research.
Data availability
All data and materials analyzed to produce this paper are available on the GFBR website: https://www.gfbr.global/past-meetings/16th-forum-cape-town-south-africa-29-30-november-2022/ .
Clark P, Kim J, Aphinyanaphongs Y, Marketing, Food US. Drug Administration Clearance of Artificial Intelligence and Machine Learning Enabled Software in and as Medical devices: a systematic review. JAMA Netw Open. 2023;6(7):e2321792–2321792.
Article Google Scholar
Potnis KC, Ross JS, Aneja S, Gross CP, Richman IB. Artificial intelligence in breast cancer screening: evaluation of FDA device regulation and future recommendations. JAMA Intern Med. 2022;182(12):1306–12.
Siala H, Wang Y. SHIFTing artificial intelligence to be responsible in healthcare: a systematic review. Soc Sci Med. 2022;296:114782.
Yang X, Chen A, PourNejatian N, Shin HC, Smith KE, Parisien C, et al. A large language model for electronic health records. NPJ Digit Med. 2022;5(1):194.
Meskó B, Topol EJ. The imperative for regulatory oversight of large language models (or generative AI) in healthcare. NPJ Digit Med. 2023;6(1):120.
Jobin A, Ienca M, Vayena E. The global landscape of AI ethics guidelines. Nat Mach Intell. 2019;1(9):389–99.
Minssen T, Vayena E, Cohen IG. The challenges for Regulating Medical Use of ChatGPT and other large Language models. JAMA. 2023.
Ho CWL, Malpani R. Scaling up the research ethics framework for healthcare machine learning as global health ethics and governance. Am J Bioeth. 2022;22(5):36–8.
Yeung K. Recommendation of the council on artificial intelligence (OECD). Int Leg Mater. 2020;59(1):27–34.
Maddox TM, Rumsfeld JS, Payne PR. Questions for artificial intelligence in health care. JAMA. 2019;321(1):31–2.
Dzau VJ, Balatbat CA, Ellaissi WF. Revisiting academic health sciences systems a decade later: discovery to health to population to society. Lancet. 2021;398(10318):2300–4.
Ferretti A, Ienca M, Sheehan M, Blasimme A, Dove ES, Farsides B, et al. Ethics review of big data research: what should stay and what should be reformed? BMC Med Ethics. 2021;22(1):1–13.
Rahimzadeh V, Serpico K, Gelinas L. Institutional review boards need new skills to review data sharing and management plans. Nat Med. 2023;1–3.
Kling S, Singh S, Burgess TL, Nair G. The role of an ethics advisory committee in data science research in sub-saharan Africa. South Afr J Sci. 2023;119(5–6):1–3.
Google Scholar
Cengiz N, Kabanda SM, Esterhuizen TM, Moodley K. Exploring perspectives of research ethics committee members on the governance of big data in sub-saharan Africa. South Afr J Sci. 2023;119(5–6):1–9.
Doerr M, Meeder S. Big health data research and group harm: the scope of IRB review. Ethics Hum Res. 2022;44(4):34–8.
Ballantyne A, Stewart C. Big data and public-private partnerships in healthcare and research: the application of an ethics framework for big data in health and research. Asian Bioeth Rev. 2019;11(3):315–26.
Samuel G, Chubb J, Derrick G. Boundaries between research ethics and ethical research use in artificial intelligence health research. J Empir Res Hum Res Ethics. 2021;16(3):325–37.
Murphy K, Di Ruggiero E, Upshur R, Willison DJ, Malhotra N, Cai JC, et al. Artificial intelligence for good health: a scoping review of the ethics literature. BMC Med Ethics. 2021;22(1):1–17.
Teixeira da Silva JA. Handling ethics dumping and neo-colonial research: from the laboratory to the academic literature. J Bioethical Inq. 2022;19(3):433–43.
Ferryman K. The dangers of data colonialism in precision public health. Glob Policy. 2021;12:90–2.
Couldry N, Mejias UA. Data colonialism: rethinking big data’s relation to the contemporary subject. Telev New Media. 2019;20(4):336–49.
Organization WH. Ethics and governance of artificial intelligence for health: WHO guidance. 2021.
Metcalf J, Moss E. Owning ethics: corporate logics, silicon valley, and the institutionalization of ethics. Soc Res Int Q. 2019;86(2):449–76.
Data Protection Act - OFFICE OF THE DATA PROTECTION COMMISSIONER KENYA [Internet]. 2021 [cited 2023 Sep 30]. https://www.odpc.go.ke/dpa-act/ .
Sharon T, Lucivero F. Introduction to the special theme: the expansion of the health data ecosystem–rethinking data ethics and governance. Big Data & Society. Volume 6. London, England: SAGE Publications Sage UK; 2019. p. 2053951719852969.
Reisman D, Schultz J, Crawford K, Whittaker M. Algorithmic impact assessments: a practical Framework for Public Agency. AI Now. 2018.
Morgan RK. Environmental impact assessment: the state of the art. Impact Assess Proj Apprais. 2012;30(1):5–14.
Samuel G, Richie C. Reimagining research ethics to include environmental sustainability: a principled approach, including a case study of data-driven health research. J Med Ethics. 2023;49(6):428–33.
Kwete X, Tang K, Chen L, Ren R, Chen Q, Wu Z, et al. Decolonizing global health: what should be the target of this movement and where does it lead us? Glob Health Res Policy. 2022;7(1):3.
Abimbola S, Asthana S, Montenegro C, Guinto RR, Jumbam DT, Louskieter L, et al. Addressing power asymmetries in global health: imperatives in the wake of the COVID-19 pandemic. PLoS Med. 2021;18(4):e1003604.
Benatar S. Politics, power, poverty and global health: systems and frames. Int J Health Policy Manag. 2016;5(10):599.
Download references
Acknowledgements
We would like to acknowledge the outstanding contributions of the attendees of GFBR 2022 in Cape Town, South Africa. This paper is authored by members of the GFBR 2022 Planning Committee. We would like to acknowledge additional members Tamra Lysaght, National University of Singapore, and Niresh Bhagwandin, South African Medical Research Council, for their input during the planning stages and as reviewers of the applications to attend the Forum.
This work was supported by Wellcome [222525/Z/21/Z], the US National Institutes of Health, the UK Medical Research Council (part of UK Research and Innovation), and the South African Medical Research Council through funding to the Global Forum on Bioethics in Research.
Author information
Authors and affiliations.
Department of Physical Therapy, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada
Berman Institute of Bioethics, Johns Hopkins University, Baltimore, MD, USA
Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Department of Philosophy and Classics, University of Ghana, Legon-Accra, Ghana
Caesar A. Atuire
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Phaik Yeong Cheah
Berkman Klein Center, Harvard University, Bogotá, Colombia
Armando Guio Español
Department of Radiology and Informatics, Emory University School of Medicine, Atlanta, GA, USA
Judy Wawira Gichoya
Health Ethics & Governance Unit, Research for Health Department, Science Division, World Health Organization, Geneva, Switzerland
Adrienne Hunt & Katherine Littler
African Center of Excellence in Bioinformatics and Data Intensive Science, Infectious Diseases Institute, Makerere University, Kampala, Uganda
Daudi Jjingo
ISI Foundation, Turin, Italy
Daniela Paolotti
Department of Health Sciences and Technology, ETH Zurich, Zürich, Switzerland
Effy Vayena
Joint Centre for Bioethics, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada
You can also search for this author in PubMed Google Scholar
Contributions
JS led the writing, contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. JA contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. CA contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. PYC contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. AE contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. JWG contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. AH contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. DJ contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. KL contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. DP contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. EV contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper.
Corresponding author
Correspondence to James Shaw .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shaw, J., Ali, J., Atuire, C.A. et al. Research ethics and artificial intelligence for global health: perspectives from the global forum on bioethics in research. BMC Med Ethics 25 , 46 (2024). https://doi.org/10.1186/s12910-024-01044-w
Download citation
Received : 31 October 2023
Accepted : 01 April 2024
Published : 18 April 2024
DOI : https://doi.org/10.1186/s12910-024-01044-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Artificial intelligence
- Machine learning
- Research ethics
- Global health
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
- Share full article
Advertisement
Supported by
Physical Fitness Linked to Better Mental Health in Young People
A new study bolsters existing research suggesting that exercise can protect against anxiety, depression and attention challenges.

By Matt Richtel
Physical fitness among children and adolescents may protect against developing depressive symptoms, anxiety and attention deficit hyperactivity disorder, according to a study published on Monday in JAMA Pediatrics.
The study also found that better performance in cardiovascular activities, strength and muscular endurance were each associated with greater protection against such mental health conditions. The researchers deemed this linkage “dose-dependent,” suggesting that a child or adolescent who is more fit may be accordingly less likely to experience the onset of a mental health disorder.
These findings come amid a surge of mental health diagnoses among children and adolescents, in the United States and abroad, that have prompted efforts to understand and curb the problem.

The new study, conducted by researchers in Taiwan, compared data from two large data sets: the Taiwan National Student Fitness Tests, which measures student fitness performance in schools, and the National Insurance Research Databases, which records medical claims, diagnoses prescriptions and other medical information. The researchers did not have access to the students’ names but were able to use the anonymized data to compare the students’ physical fitness and mental health results.
The risk of mental health disorder was weighted against three metrics for physical fitness: cardio fitness, as measured by a student’s time in an 800-meter run; muscle endurance, indicated by the number of situps performed; and muscle power, measured by the standing broad jump.
Improved performance in each activity was linked with a lower risk of mental health disorder. For instance, a 30-second decrease in 800-meter time was associated, in girls, with a lower risk of anxiety, depression and A.D.H.D. In boys, it was associated with lower anxiety and risk of the disorder.
An increase of five situps per minute was associated with lower anxiety and risk of the disorder in boys, and with decreased risk of depression and anxiety in girls.
“These findings suggest the potential of cardiorespiratory and muscular fitness as protective factors in mitigating the onset of mental health disorders among children and adolescents,” the researchers wrote in the journal article.
Physical and mental health were already assumed to be linked , they added, but previous research had relied largely on questionnaires and self-reports, whereas the new study drew from independent assessments and objective standards.
The Big Picture
The surgeon general, Dr. Vivek H. Murthy, has called mental health “the defining public health crisis of our time,” and he has made adolescent mental health central to his mission. In 2021 he issued a rare public advisory on the topic. Statistics at the time revealed alarming trends: From 2001 to 2019, the suicide rate for Americans ages 10 to 19 rose 40 percent, and emergency visits related to self-harm rose 88 percent.
Some policymakers and researchers have blamed the sharp increase on the heavy use of social media, but research has been limited and the findings sometimes contradictory. Other experts theorize that heavy screen use has affected adolescent mental health by displacing sleep, exercise and in-person activity, all of which are considered vital to healthy development. The new study appeared to support the link between physical fitness and mental health.
“The finding underscores the need for further research into targeted physical fitness programs,” its authors concluded. Such programs, they added, “hold significant potential as primary preventative interventions against mental disorders in children and adolescents.”
Matt Richtel is a health and science reporter for The Times, based in Boulder, Colo. More about Matt Richtel
Understanding A.D.H.D.
The challenges faced by those with attention deficit hyperactivity disorder can be daunting. but people who are diagnosed with it can still thrive..
Millions of children in the United States have received a diagnosis of A.D.H.D . Here is how their families can support them .
The condition is also being recognized more in adults . These are some of the behaviors that might be associated with adult A.D.H.D.
Since a nationwide Adderall shortage started, some people with A.D.H.D. have said their medication no longer helps with their symptoms. But there could be other factors at play .
Everyone has bouts of distraction and forgetfulness. Here is when psychiatrists diagnose it as something clinical .
The disorder can put a strain on relationships. But there are ways to cope .
Though meditation can be beneficial to those with A.D.H.D., sitting still and focusing on breathing can be hard for them. These tips can help .
- Australia edition
- International edition
- Europe edition

Healthy lifestyle may offset genetics by 60% and add five years to life, study says
Genetics alone can mean a 21% greater risk of early death, research finds, but people can improve their chances
A healthy lifestyle may offset the impact of genetics by more than 60% and add another five years to your life, according to the first study of its kind.
It is well established that some people are genetically predisposed to a shorter lifespan. It is also well known that lifestyle factors, specifically smoking, alcohol consumption, diet and physical activity, can have an impact on longevity.
However, until now there has been no investigation to understand the extent to which a healthy lifestyle may counterbalance genetics.
Findings from several long-term studies suggest a healthy lifestyle could offset effects of life-shortening genes by 62% and add as much as five years to your life. The results were published in the journal BMJ Evidence-Based Medicine .
“This study elucidates the pivotal role of a healthy lifestyle in mitigating the impact of genetic factors on lifespan reduction,” the researchers concluded. “Public health policies for improving healthy lifestyles would serve as potent complements to conventional healthcare and mitigate the influence of genetic factors on human lifespan.”
The study involved 353,742 people from the UK Biobank and showed that those with a high genetic risk of a shorter life have a 21% increased risk of early death compared with those with a low genetic risk, regardless of their lifestyle.
Meanwhile, people with unhealthy lifestyles have a 78% increased chance of early death, regardless of their genetic risk, researchers from Zhejiang University School of Medicine in China and the University of Edinburgh found.
The study added that having an unhealthy lifestyle and shorter lifespan genes more than doubled the risk of early death compared with people with luckier genes and healthy lifestyles.
However, researchers found that people did appear to have a degree of control over what happened. The genetic risk of a shorter lifespan or premature death may be offset by a favourable lifestyle by about 62%, they found.
They wrote: “Participants with high genetic risk could prolong approximately 5.22 years of life expectancy at age 40 with a favourable lifestyle.”
The “optimal lifestyle combination” for a longer life was found to be “never smoking, regular physical activity, adequate sleep duration and healthy diet”.
The study followed people for 13 years on average, during which time 24,239 deaths occurred. People were grouped into three genetically determined lifespan categories including long (20.1%), intermediate (60.1%) and short (19.8%), and three lifestyle score categories including favourable (23.1%), intermediate (55.6%) and unfavourable (21.3%).
Researchers used polygenic risk scores to look at multiple genetic variants to arrive at a person’s overall genetic predisposition to a longer or shorter life. Other scores looked at whether people smoked, drank alcohol, took exercise, their body shape, healthy diet and sleep.
Matt Lambert, the health information and promotion manager at the World Cancer Research Fund, said: “This new research shows that, despite genetic factors, living a healthy lifestyle, including eating a balanced nutritious diet and keeping active, can help us live longer.”
- Medical research
- Health & wellbeing
Most viewed
Can nasal Neosporin fight COVID? Surprising new research suggests it works
A potential treatment for covid-19 may have been hiding in our medicine cabinets, a new study in pnas has found, by nicole karlis.
Four years ago, when COVID-19 first began to spread globally, it didn't just damage our physical health, but also the health of our information ecosystem. Ever since, the internet has been rife with health misinformation on ways to treat or protect oneself against the coronavirus. First, internet healers falsely suggested that gargling salt water and vinegar could prevent a coronavirus infection. Then, despite multiple studies debunking the effectiveness of ivermectin , an anti-parasitic drug used in horses (and less commonly in humans), Joe Rogan fans continued to cling onto it as a potential treatment .
Health misinformation is a symptom of a lack of certainty. When there is no guaranteed preventative measure or treatment, people are bound to find solutions on their own. Thanks to cognitive biases like confirmation bias , they might even appear to work. But what if a way to reduce exposure to COVID-19, and treat it, was hiding in our medicine cabinets all along — and it wasn’t pseudoscience?
A new study published in the journal Proceedings of the National Academy of Sciences suggests that neomycin, an ingredient in the first aid ointment Neosporin , may prevent or treat a range of respiratory viral infections such as COVID-19 and influenza when applied to the nose.
In the study, researchers found that mice who had neomycin in their nostrils exhibited strong antiviral activity against both SARS-CoV- 2 and a highly virulent strain of influenza A virus. It also mitigated contact transmission of SARS-CoV- 2 between hamsters.
"When we compared the gene expression in the nose, Neosporin stimulated genes whereas those people who had Vaseline did not."
“We decided to see if neomycin applied into the nose can protect animals from infection with COVID as well as the flu,” Dr. Akiko Iwasaki , the lead author of the study and a professor of immunobiology at the Yale University School of Medicine, told Salon in a phone interview. “And what we found is that treatment with neomycin significantly prevented infection and also reduced disease burden in animals.”
Iwasaki described the work as “encouraging” because it shows that neomycin can trigger an antiviral response in animals by creating a localized immune response. “That’s resulting in this protection that we see,” Iwasaki said.
Want more health and science stories in your inbox? Subscribe to Salon's weekly newsletter Lab Notes .
The results are encouraging for mice and hamsters. But what about humans? The researchers proceeded to recruit healthy volunteers and asked them to apply Neosporin with a cotton swab to their nose, twice a day. The placebo for some was vaseline. The researchers measured their antiviral response and found similar results.
“When we compared the gene expression in the nose, Neosporin stimulated genes whereas those people who had Vaseline did not,” Iwasaki said. “So this suggests that we might be able to use Neosporin or neomycin in humans to induce this antiviral state that we also saw in animals.”
Does that mean we should all be applying Neosporin to our noses in high-risk situations? Not exactly, but it probably wouldn’t hurt either — as long as someone isn’t allergic to the cream, which is a combination of the antibiotics bacitracin, neomycin and polymyxin B. Notably, details around the dosage remain unclear.
“We know from the dose response that we did in animals that we probably need to give humans more Neosporin, or neomycin,” she said. “Because Neosporin has very little neomycin compared to what we were able to achieve in the animal model.”
"This could be a potential broad spectrum antiviral treatment and prophylaxis."
Iwasaki added they know that Neosporin can produce a similar effect in humans as it did in animals, but whether or not it can reduce transmission has yet to be determined.
“For that, we need different kinds of study and a much larger study to determine that,” she said.
Amesh Adalja, a senior scholar at the Johns Hopkins Center and infectious disease doctor who wasn’t involved in the study, told Salon via email that the research could have broader implications that extend beyond COVID-19.
“This could be a potential broad spectrum antiviral treatment and prophylaxis,”Adalja said. “The molecules in the topical antibiotic cream induce certain antiviral compounds to be made by cells where the ointment has been applied; these antiviral compounds produce non-specific immunity that impacts various viruses.”
Iwasaki cautioned against the idea that people swabbing their noses with Neosporin will be a cure-all in the future. Instead, she said she sees this as another possible layer of protection .
We need your help to stay independent
“We know how important it is to layer protection against infections,” Iwasaki said. “Vaccines and masks and other measures are very important, but this type of strategy where we can trigger the host to produce antiviral factors may be another layer that we can add on to the existing ones.”
The more layers a person has, Iwasaki said, the less likely a person is to get infected.
“And that's really important for preventing diseases like long COVID,” Iwasaki said, referring to a condition in which COVID symptoms last for months or even years . “So I think it's definitely worth kind of moving forward with an approach like this.”
An approach that was right under our noses all this time.
about COVID
- Do COVID-19 vaccines really have worse side effects than other vaccines? Here's what experts say
- Infectious desire: How the pandemic is still negatively impacting our sex lives
- Does your immune system need a workout? The bad science behind "immunity debt," explained
Nicole Karlis is a senior writer at Salon, specializing in health and science. Tweet her @nicolekarlis .
Related Topics ------------------------------------------
Related articles.
Time zones and tiredness strongly influence NBA results, study of 25,000 matches shows
The body clock has a significant impact on the performance of NBA players, according to study published in the peer-reviewed journal Chronobiology International .
The authors say their findings, from more than 25,000 matches, show elite basketball coaches and teams should consider the physical and mental effects of time zone travel when planning games and preparing for games.
A first of its kind, the research is based on the achievements at home and away of NBA (National Basketball Association) league players across 21 consecutive seasons. Considered the most competitive in the world, NBA athletes frequently travel to matches across the five US time zones used by NBA teams.
The findings show that there is a near 10% better win ratio difference for home teams from the western time zone area (PDT) when playing against a team from the eastern EDT time zone, compared to when an EDT team hosts a PDT team.
- When PDT teams play at home against EDT teams the winning percentage is 63.5%.
- When EDT teams host a PDT team, the winning percentage drops to 55.0%.
In addition, the findings also show that teams win more home games when players' sleep-wake cycles -- linked to their circadian rhythm (CR) -- are 'ahead' of the local time. This is after they have returned west from competing in a city further east where the local time is earlier.
For example, if the LA Lakers play an away match at Miami (EDT) and then return to Los Angeles (PDT) to play a home game without much CR adaptation time (CR is ahead of the local time), the Lakers play the next home game with a CR advantage against whomever their opponents are.
Teams do not have the same success when players' internal body clocks are either behind or synchronized with the local time where their home arena or stadium is based, according to the results.
Experts from Dokuz Eylül University and Yildiz Technical University, in Turkey, led the study. Dr Firat Özdalyan, a Sport Physiology expert from Dokuz Eylül, explains that they found NBA teams need to become used to the local time when they play away games to perform well.
"One of the most important results of this research for the home games of the NBA teams is that while traveling to the west increases the performance, traveling to the east decreases the performance," he states.
"Another notable finding is that the success of NBA teams increases when they are fully adapted to the local time for away games.
"Home teams who will be exposed to such a CR phase shift (traveling from west to east) should be mindful of these potential performance detriments when constructing game plans.
"It can be suggested that coaches (of away teams) should bear this (the low shooting success) in mind during the game preparation period."
A circadian rhythm (CR) is the body's sleep-wake pattern over a 24-hour day. A CR phase shift means bedtime and wake-up times move earlier or later in the day.
This means the body clock gets out of sync with the environment which can lead to sleeplessness, daytime tiredness and other issues. The body clock needs 24 hours to adapt for every one-hour time zone change.
The study investigated the effect of a CR shift on the performance of professional NBA athletes.
Data was analyzed from 25,016 regular games across 21 consecutive seasons between 2000 to 2021. Information included the date, location, game result and home or away team. Time zones of the cities where all games were played were identified to calculate the CR phase shifts of the teams.
The expert team say teams in the Pacific time zone may have an advantage in regular season home games such as the Los Angeles Lakers, Portland Trail Blazers, and Seattle Supersonics.
Anaerobic performance could explain why home teams who travel from east to west do better, say the authors. This type of activity which is crucial for scoring, defending and other feats peaks later in the day.
The authors add that the body clock adapts more easily to a long rather than a short day. The day becomes longer traveling east to west and a natural circadian rhythm is slightly longer than 24 hours. So this means basketball players are traveling in the direction their bodies want to go.
As for away teams, the authors say that travel fatigue is more likely to blame for poor performance than phase shifts in CR.
Players who have rest time between games or have not traveled across time zones for an away match are more able to synchronize their body with the local time. As such, they are not as tired and play better.
A limitation of this research is that the traveling schedules of the teams are not known. Since this information was not available, it was not possible to determine how long the teams stayed in which city/time zone; how much they adapted to the local UTC; and what extent they were exposed to a CR phase shift with real data. Therefore, the team used a predictive model for the traveling plans and CR adaptations of the teams by following the rules determined by previous research.
Another limitation is that the games were not separated according to teams' ability differences.
- Sleep Disorder Research
- Insomnia Research
- K-12 Education
- Child Development
- Sports Science
- Quantum Physics
- Travel and Recreation
- Circadian rhythm sleep disorder
- Runner's knee
- Chernobyl disaster
- Quantum entanglement
- Special relativity
Story Source:
Materials provided by Taylor & Francis Group . Note: Content may be edited for style and length.
Journal Reference :
- Fırat Özdalyan, Erhan Çene, Hikmet Gümüş, Osman Açıkgöz. Investigation of the effect of circadian rhythm on the performances of NBA teams . Chronobiology International , 2024; 1 DOI: 10.1080/07420528.2024.2325641
Cite This Page :
Explore More
- Far-Reaching Effects of Exercise
- Hidden Connections Between Brain and Body
- Novel Genetic Plant Regeneration Approach
- Early Human Occupation of China
- Journey of Inhaled Plastic Particle Pollution
- Earth-Like Environment On Ancient Mars
- A 'Cosmic Glitch' in Gravity
- Time Zones Strongly Influence NBA Results
- Climate Change and Mercury Through the Eons
- Iconic Horsehead Nebula
Trending Topics
Strange & offbeat.
- Main Library
- Reference Desk
- Digital Fabrication Lab
- Data Visualization Lab
- Business Learning Center
- Klai Juba Wald Architectural Studies Library
- NDSU Nursing at Sanford Health Library
- Research Assistance
- Special Collections
- Digital Collections
- Collection Development Policy
- Course Reserves
- Request Library Instruction
- Main Library Services
- Alumni & Community
- Academic Support Services in the Library
- Libraries Resources for Employees
- Book Equipment or Study Rooms
- Librarians by Academic Subject
- Germans from Russia Heritage Collection
- NDSU Archives
- Mission, Vision, and Strategic Plan 2022-2024
- Staff Directory
- Floor Plans
- The Libraries Magazine
- Accommodations for People with Disabilities
- Annual Report
- Donate to the Libraries
- Equity, Diversity and Inclusion
- Faculty Senate Library Committee
- Undergraduate Research Award
Finding and Identifying Original Research Articles in the Sciences: Home
What is an original research article.
An original research article is a report of research activity that is written by the researchers who conducted the research or experiment. Original research articles may also be referred to as: “primary research articles” or “primary scientific literature.” In science courses, instructors may also refer to these as “peer-reviewed articles” or “refereed articles.”
Original research articles in the sciences have a specific purpose, follow a scientific article format, are peer reviewed, and published in academic journals.
Identifying Original Research: What to Look For
Purpose, author, and audience.
An "original research article" is an article that is reporting original research about new data or theories that have not been previously published. That might be the results of new experiments, or newly derived models or simulations. The article will include a detailed description of the methods used to produce them, so that other researchers can verify them. This description is often found in a section called "methods" or "materials and methods" or similar. Similarly, the results will generally be described in great detail, often in a section called "results."
Since the original research article is reporting the results of new research, the authors should be the scientists who conducted that research. They will have expertise in the field, and will usually be employed by a university or research lab.
In comparison, a newspaper or magazine article (such as in The New York Times or National Geographic ) will usually be written by a journalist reporting on the actions of someone else.
An original research article will be written by and for scientists who study related topics. As such, the article should use precise, technical language to ensure that other researchers have an exact understanding of what was done, how to do it, and why it matters. There will be plentiful citations to previous work, helping place the research article in a broader context. The article will be published in an academic journal, follow a scientific format, and undergo peer-review.
Structure of an Original Research Article
Original research articles in the sciences follow the scientific format. ( This tutorial from North Carolina State University illustrates some of the key features of this format.)
Look for signs of this format in the subject headings or subsections of the article. You should see the following:
Peer Review
Scientific research that is published in academic journals undergoes a process called "peer review."
The peer review process goes like this:
- A researcher writes a paper and sends it in to an academic journal, where it is read by an editor
- The editor then sends the article to other scientists who study similar topics, who can best evaluate the article
- The scientists/reviewers examine the article's research methodology, reasoning, originality, and sginificance
- The scientists/reviewers then make suggestions and comments to impove the paper
- The original author is then given these suggestions and comments, and makes changes as needed
- This process repeats until everyone is satisfied and the article can be published within the academic journal
For more details about this process see the Peer Reviewed Publications guide.
This journal article is an example. It was published in the journal Royal Society Open Science in 2015. Clicking on the button that says "Review History" will show the comments by the editors, reviewers and the author as it went through the peer review process. The "About Us" menu provides details about this journal; "About the journal" under that tab includes the statement that the journal is peer reviewed.
Articles that are NOT Original Research Articles (But might look like one!)
Review articles.
There are a variety of article types published in academic, peer-reviewed journals, but the two most common are original research articles and review articles . They can look very similar, but have different purposes and structures.
Like original research articles, review articles are aimed at scientists and undergo peer-review. Review articles often even have “abstract,” “introduction,” and “reference” sections. However, they will not (generally) have a “methods” or “results” section because they are not reporting new data or theories. Instead, they review the current state of knowledge on a topic.
Search for articles in one of the library databases recommend for your subject area . If you are using Google, try searching in Google Scholar instead and you will get results that are more likely to be original research articles than what will come up in a regular Google search!
For tips on using library databases to find articles, see our Library DIY guides .
Tips for Finding the Source of a News Report about Science
If you've seen or heard a report about a new scientific finding or claim, these tips can help you find the original source:
- Often, the report will mention where the original research was published; look for sentences like "In an article published yesterday in the journal Nature ..." You can use this to find the issue of the journal where the research was published, and look at the table of contents to find the original article.
- The report will often name the researchers involved. You can search relevant databases for their name and the topic of the report to find the original research that way.
- Sometimes you may have to go through multiple articles to find the original source. For example, a video or blog post may be based on a newspaper article, which in turn is reporting on a scientific discovery published in another journal; be sure to find the original research article.
- Don't be afraid to ask a librarian for help!
Library Reference Staff

- Last Updated: Sep 18, 2023 10:57 AM
- URL: https://ndsu.libguides.com/findingoriginalresearch
- Online Services
- Phone/Email Directory
- Registration And Records
- Kreyòl Ayisyen

CFPB Finds 15 Million Americans Have Medical Bills on Their Credit Reports
People living in the South continue to be most likely to have medical bills on their reports
WASHINGTON, D.C. – The Consumer Financial Protection Bureau (CFPB) today released research showing that 15 million Americans still have medical bills on their credit reports despite changes by Equifax, Experian, and TransUnion. The 15 million Americans disproportionately live in the South and low-income communities. Collectively, they have more than $49 billion in outstanding medical bills in collections. This is the CFPB’s second analysis of the changes made by the three national credit reporting companies to reduce the number of medical bills on credit reports. Today’s report follows the start of a CFPB rulemaking that will consider options to restrict the reporting of allegedly unpaid medical bills on credit reports.
“Experian, Equifax, and TransUnion took steps to remove many medical bills in part because of the recognition that they hold little predictive value,” said CFPB Director Rohit Chopra. “Findings from our latest research reveal the impact of these changes and the need for further reforms.”
In early March 2022, a CFPB study found an estimated $88 billion in medical bills on Americans’ credit reports. Following that study, the three nationwide credit reporting companies – Equifax, Experian, and TransUnion – announced they would no longer report certain medical bills in collections. The companies announced they would increase the time before medical bills in collections can appear on credit reports – from 180 days to one year. Second, the companies would stop reporting medical bills that had been in collections but were resolved. Third, the companies would remove medical bills below $500 from credit reports.
Today’s research found the number of people with medical bills in collections on their credit reports has declined. As of June 2023, about 5% of Americans had unpaid medical bills on their credit reports – down from 14% in March 2022. Older Americans saw the largest improvement – 8.4% of older Americans had medical bills on their credit reports in March 2022 compared to below 3% in June 2023.
For the 15 million Americans with medical bills on their credit reports, today’s research finds:
- Many live in low-income communities and the southern United States: The credit reporting changes were slightly less likely to help Americans in lower-income communities compared to those in higher-income communities. The changes did not do as much for individuals residing in the South. On average, people living in the South continue to have the most medical bills in collections and for the largest dollar amounts.
- The average medical balance on credit reports increased from $2,000 to over $3,100: The credit reporting changes primarily removed smaller balances. As a result, the average balance of the remaining reported medical bills increased.
- Most medical collections balances stayed on credit reports: The three national credit reporting companies removed many bills, and many people now have no remaining medical bills on their credit reports. However, a majority of medical collections balances remain on credit reports.
The CFPB will continue to prioritize fixing the credit reporting market, including issues that involve the reporting of medical bills. In addition to the September 2023 announced rulemaking to address medical bills on credit reports, the CFPB launched an inquiry into costly credit cards and loans that are pushed onto patients to pay for health care costs. The CFPB also took action against illegal nursing home debt collection practices, as well as against medical debt collection and credit reporting practices that violate the No Surprises Act.
The CFPB has taken actions against entities engaged in illegal medical debt collection practices. The CFPB shut down Commonwealth Financial Systems for illegal medical debt collection practices. The CFPB also ordered Phoenix Financial Services to pay millions in redress and penalties for attempting to collect disputed medical debts through unlawful collection letters and misrepresentations.
Read the report, Recent Changes in Medical Collections on Consumer Credit Records .
Read more about the CFPB’s work on medical debt.
Read consumer complaints about medical bills and medical collections.
Consumers can submit complaints about medical billing and collections issues, as well as about other financial products and services, by visiting the CFPB’s website or by calling (855) 411-CFPB (2372) .
The Consumer Financial Protection Bureau is a 21st century agency that implements and enforces Federal consumer financial law and ensures that markets for consumer financial products are fair, transparent, and competitive. For more information, visit www.consumerfinance.gov .
- Arms and military expenditure
- Dual–use and arms trade control
- Emerging military and security technologies
- EU Non-Proliferation and Disarmament Consortium
- Weapons of mass destruction
- Middle East and North Africa
- Peace operations and conflict management
- Climate change and risk
- Environment of Peace
- Food, peace and security
- Governance and society
- Peacebuilding and resilience
- Publications
- Yearbook 2023
- Yearbook 2022
- Yearbook archive
- Yearbook summaries
- Yearbook translations
- Past News and Events
- Upcoming News and Events
- SIPRI Lecture
- Stockholm Forum on Peace and Development
- Stockholm Security Conference
- Press Releases
- SIPRI Experts
- SIPRI Films
- WritePeace Blog
- Expert Comments
- Backgrounders
- Governing Board
- Staff directory
- Support SIPRI
Trends in World Military Expenditure, 2023
https://doi.org/10.55163/BQGA2180
World military expenditure increased for the ninth consecutive year in 2023, reaching a total of $2443 billion. The 6.8 per cent increase in 2023 was the steepest year-on-year rise since 2009 and pushed global spending to the highest level SIPRI has ever recorded. The world military burden—defined as military spending as a percentage of global gross domestic product (GDP)—increased to 2.3 per cent in 2023. Average military expenditure as a share of government expenditure rose by 0.4 percentage points to 6.9 per cent in 2023 and world military spending per person was the highest since 1990, at $306.
The rise in global military spending in 2023 can be attributed primarily to the ongoing war in Ukraine and escalating geopolitical tensions in Asia and Oceania and the Middle East. Military expenditure went up in all five geographical regions, with major spending increases recorded in Europe, Asia and Oceania and the Middle East.
This SIPRI Fact Sheet highlights trends in military expenditure for 2023 and over the decade 2014–23. The data, which replaces all military spending data previously published by SIPRI, comes from the updated SIPRI Military Expenditure Database .
ABOUT THE AUTHOR(S)/EDITORS


IMAGES
VIDEO
COMMENTS
An original research article is a report of research activity that is written by the researchers who conducted the research or experiment. Original research articles may also be referred to as: "primary research articles" or "primary scientific literature." ... Should state the most important outcome of the study and to what extent the ...
The purpose of original research is to generate new knowledge and understanding in a particular field of study. Original research is conducted to address a research question, hypothesis, or problem and to produce empirical evidence that can be used to inform theory, policy, and practice. By conducting original research, researchers can:
it is the report of a study written by the researchers who actually did the study. the researchers describe their hypothesis or research question and the purpose of the study. the researchers detail their research methods. the results of the research are reported. the researchers interpret their results and discuss possible implications.
Therapeutic value of first versus supplemental indications of drugs in US and Europe. July 5, 2023. Can't find what you're looking for? Continue to all research articles. Original research studies that can improve decision making in clinical medicine, public health, health care policy, medical education, or biomedical research.
This is the most common type of journal manuscript used to publish full reports of data from research. It may be called an Original Article, Research Article, Research, or just Article, depending on the journal. The Original Research format is suitable for many different fields and different types of studies.
Original Research. An original research paper should present a unique argument of your own. In other words, the claim of the paper should be debatable and should be your (the researcher's) own original idea. Typically an original research paper builds on the existing research on a topic, addresses a specific question, presents the findings ...
Other tips to help you with the Results section: . If you need to cite the number in the text (not just in the table), and the total in the group is less than 50, do not include percentage. Write "7 of 34," not "7 (21%).". . Do not forget, if you have multiple comparisons, you probably need adjustment.
From these 163 publications, we identified 5 systematic reviews, 66 narrative reviews, 8 original research articles, 26 methodological studies, 4 study protocols, 37 conference abstracts, 4 commentaries, 2 discussion papers, 3 reports, 1 book chapter, 1 editorial, 1 guidance document and 5 links about trial registration (eg, ClinicalTrials.gov).
An "original" research article is a detailed account of research activity written by the scientists who did the research--not by someone else who is reporting on the research; it is a primary resource. Some instructors may refer to these as "scientific research" articles or as "empirical" research. ... Methods, Study, Results; Randomized ...
Definition: An original research article communicates the research question, methods, results, and conclusions of a research study or experiment conducted by the author(s).These articles present original research data or findings generated through the course of the authors' study and an analysis of that data or information.
The Academic Search Complete, CINAHL, and PsycARTICLES databases (all published by EbscoHost) include many original research articles. (The direct links to these databases are at the bottom of this box.) Search tips: Type your topic into the first one or two search boxes and then use another box to type: "methods OR results OR study" as shown ...
Journals provide guidelines to authors which should be followed closely. The three major types of articles (original research, case reports, and review articles) all generally follow the IMRAD format with slight variations in content. With planning and thought, manuscript writing does not have to be a daunting task. Download chapter PDF.
Laboratory Investigation - Original research in pathology: judgment, or evidence-based medicine? ... Such discussions made clear the need for more rigorous study of medical decision making, ...
Research articles, empirical, research primary research, are based on original research. If you need to limit your sources to research articles, you must be able to tell the difference. Most research articles will contain the following: Abstract. A summary of the article. (Note: Abstracts appear in reviews or secondary articles as well.) Methods
For a manuscript to be considered an original research article, the following conditions need to be met: It should be written by the researchers who actually conducted the study. It should include the hypothesis or research question, the purpose of the study, and the details of the research methods. The research findings should be reported.
In the second search box: research or study . Tips to identify empirical research. Original research studies are also known as primary or empirical studies. These studies report on research done by the author(s) of the study. They will include how the study was done, what was discovered, and what conclusions were drawn.
Original research, also known as a single study, primary study or empirical study, is one that reports results of a scientific study rather than summarizing other articles. Basically, original research is where the researchers do the study and report their findings. Find an Original Research Article in the Library
Original research is considered a primary source. An article is considered original research if... it is the report of a study written by the researchers who actually did the study. the researchers describe their hypothesis or research question and the purpose of the study. the researchers detail their research methods.
Aim: To provide a student's perspective of what it means to be original when undertaking a PhD. Background: A review of the literature related to the concept of originality in doctoral research highlights the subjective nature of the concept in academia. Although there is much literature that explores the issues concerning examiners' views of originality, there is little on students' perspectives.
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about: Your overall research objectives and approach. Whether you'll rely on primary research or secondary research. Your sampling methods or criteria for selecting subjects. Your data collection methods.
An original research paper is the one based on original research that produces new knowledge instead of summarizing what is already known in a new form.
Research that observes or applies an experiment to a group of people who have a shared characteristic. A cohort study is a type of longitudinal study that collects results over a period of time that may extend for months, years or decades. For example, a cohort study based on 5,000 babies all born this year in the same country that collects ...
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice [1,2,3].Beyond the growing number of AI applications being implemented in health care, capabilities of AI models such as Large Language Models (LLMs) expand the potential reach and significance of AI technologies across health ...
A new study bolsters existing research suggesting that exercise can protect against anxiety, depression and attention challenges. By Matt Richtel. April 29, 2024. Share full article. 30.
The study involved 353,742 people from the UK Biobank and showed that those with a high genetic risk of a shorter life have a 21% increased risk of early death compared with those with a low ...
A new study published in the journal Proceedings of the National Academy of Sciences suggests that neomycin, an ingredient in the first aid ointment Neosporin, may prevent or treat a range of ...
Time zones and tiredness strongly influence NBA results, study of 25,000 matches shows. ScienceDaily . Retrieved May 1, 2024 from www.sciencedaily.com / releases / 2024 / 05 / 240501091642.htm
An original research article is a report of research activity that is written by the researchers who conducted the research or experiment. Original research articles may also be referred to as: "primary research articles" or "primary scientific literature." ... Should state the most important outcome of the study and to what extent the ...
"Findings from our latest research reveal the impact of these changes and the need for further reforms." In early March 2022, a CFPB study found an estimated $88 billion in medical bills on Americans' credit reports. Following that study, the three nationwide credit reporting companies - Equifax, Experian, and TransUnion - announced ...
World military expenditure increased for the ninth consecutive year in 2023, reaching a total of $2443 billion. The 6.8 per cent increase in 2023 was the steepest year-on-year rise since 2009 and pushed global spending to the highest level SIPRI has ever recorded. The world military burden—defined as military spending as a percentage of global gross domestic product (GDP)—increased to 2.3 ...