- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case NPS+ Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Surveys Academic Research

Research Summary: What is it & how to write one

The Research Summary is used to report facts about a study clearly. You will almost certainly be required to prepare a research summary during your academic research or while on a research project for your organization.
If it is the first time you have to write one, the writing requirements may confuse you. The instructors generally assign someone to write a summary of the research work. Research summaries require the writer to have a thorough understanding of the issue.
This article will discuss the definition of a research summary and how to write one.
What is a research summary?
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article’s structure in which it is written.
You must know the goal of your analysis before you launch a project. A research overview summarizes the detailed response and highlights particular issues raised in it. Writing it might be somewhat troublesome. To write a good overview, you want to start with a structure in mind. Read on for our guide.
Why is an analysis recap so important?
Your summary or analysis is going to tell readers everything about your research project. This is the critical piece that your stakeholders will read to identify your findings and valuable insights. Having a good and concise research summary that presents facts and comes with no research biases is the critical deliverable of any research project.
We’ve put together a cheat sheet to help you write a good research summary below.
Research Summary Guide
- Why was this research done? – You want to give a clear description of why this research study was done. What hypothesis was being tested?
- Who was surveyed? – The what and why or your research decides who you’re going to interview/survey. Your research summary has a detailed note on who participated in the study and why they were selected.
- What was the methodology? – Talk about the methodology. Did you do face-to-face interviews? Was it a short or long survey or a focus group setting? Your research methodology is key to the results you’re going to get.
- What were the key findings? – This can be the most critical part of the process. What did we find out after testing the hypothesis? This section, like all others, should be just facts, facts facts. You’re not sharing how you feel about the findings. Keep it bias-free.
- Conclusion – What are the conclusions that were drawn from the findings. A good example of a conclusion. Surprisingly, most people interviewed did not watch the lunar eclipse in 2022, which is unexpected given that 100% of those interviewed knew about it before it happened.
- Takeaways and action points – This is where you bring in your suggestion. Given the data you now have from the research, what are the takeaways and action points? If you’re a researcher running this research project for your company, you’ll use this part to shed light on your recommended action plans for the business.
LEARN ABOUT: Action Research
If you’re doing any research, you will write a summary, which will be the most viewed and more important part of the project. So keep a guideline in mind before you start. Focus on the content first and then worry about the length. Use the cheat sheet/checklist in this article to organize your summary, and that’s all you need to write a great research summary!
But once your summary is ready, where is it stored? Most teams have multiple documents in their google drives, and it’s a nightmare to find projects that were done in the past. Your research data should be democratized and easy to use.
We at QuestionPro launched a research repository for research teams, and our clients love it. All your data is in one place, and everything is searchable, including your research summaries!
Authors: Prachi, Anas
MORE LIKE THIS
Data Information vs Insight: Essential differences
May 14, 2024

Pricing Analytics Software: Optimize Your Pricing Strategy
May 13, 2024

Relationship Marketing: What It Is, Examples & Top 7 Benefits
May 8, 2024

The Best Email Survey Tool to Boost Your Feedback Game
May 7, 2024
Other categories
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Uncategorized
- Video Learning Series
- What’s Coming Up
- Workforce Intelligence
- Research Summary: What Is It & How To Write One

Introduction
A research summary is a requirement during academic research and sometimes you might need to prepare a research summary during a research project for an organization.
Most people find a research summary a daunting task as you are required to condense complex research material into an informative, easy-to-understand article most times with a minimum of 300-500 words.
In this post, we will guide you through all the steps required to make writing your research summary an easier task.
What is a Research Summary?
A research summary is a piece of writing that summarizes the research of a specific topic into bite-size easy-to-read and comprehend articles. The primary goal is to give the reader a detailed outline of the key findings of a research.
It is an unavoidable requirement in colleges and universities. To write a good research summary, you must understand the goal of your research, as this would help make the process easier.
A research summary preserves the structure and sections of the article it is derived from.
Research Summary or Abstract: What’s The Difference?
The Research Summary and Abstract are similar, especially as they are both brief, straight to the point, and provide an overview of the entire research paper. However, there are very clear differences.
To begin with, a Research summary is written at the end of a research activity, while the Abstract is written at the beginning of a research paper.
A Research Summary captures the main points of a study, with an emphasis on the topic, method , and discoveries, an Abstract is a description of what your research paper would talk about and the reason for your research or the hypothesis you are trying to validate.
Let us take a deeper look at the difference between both terms.
What is an Abstract?
An abstract is a short version of a research paper. It is written to convey the findings of the research to the reader. It provides the reader with information that would help them understand the research, by giving them a clear idea about the subject matter of a research paper. It is usually submitted before the presentation of a research paper.
What is a Summary?
A summary is a short form of an essay, a research paper, or a chapter in a book. A research summary is a narration of a research study, condensing the focal points of research to a shorter form, usually aligned with the same structure of the research study, from which the summary is derived.
What Is The Difference Between an Abstract and a Summary?
An abstract communicates the main points of a research paper, it includes the questions, major findings, the importance of the findings, etc.
An abstract reflects the perceptions of the author about a topic, while a research summary reflects the ideology of the research study that is being summarized.
Getting Started with a Research Summary
Before commencing a research summary, there is a need to understand the style and organization of the content you plan to summarize. There are three fundamental areas of the research that should be the focal point:
- When deciding on the content include a section that speaks to the importance of the research, and the techniques and tools used to arrive at your conclusion.
- Keep the summary well organized, and use paragraphs to discuss the various sections of the research.
- Restrict your research to 300-400 words which is the standard practice for research summaries globally. However, if the research paper you want to summarize is a lengthy one, do not exceed 10% of the entire research material.
Once you have satisfied the requirements of the fundamentals for starting your research summary, you can now begin to write using the following format:
- Why was this research done? – A clear description of the reason the research was embarked on and the hypothesis being tested.
- Who was surveyed? – Your research study should have details of the source of your information. If it was via a survey, you should document who the participants of the survey were and the reason that they were selected.
- What was the methodology? – Discuss the methodology, in terms of what kind of survey method did you adopt. Was it a face-to-face interview, a phone interview, or a focus group setting?
- What were the key findings? – This is perhaps the most vital part of the process. What discoveries did you make after the testing? This part should be based on raw facts free from any personal bias.
- Conclusion – What conclusions did you draw from the findings?
- Takeaways and action points – This is where your views and perception can be reflected. Here, you can now share your recommendations or action points.
- Identify the focal point of the article – In other to get a grasp of the content covered in the research paper, you can skim the article first, in a bid to understand the most essential part of the research paper.
- Analyze and understand the topic and article – Writing a summary of a research paper involves being familiar with the topic – the current state of knowledge, key definitions, concepts, and models. This is often gleaned while reading the literature review. Please note that only a deep understanding ensures efficient and accurate summarization of the content.
- Make notes as you read – Highlight and summarize each paragraph as you read. Your notes are what you would further condense to create a draft that would form your research summary.
How to Structure Your Research Summary
- Title – This highlights the area of analysis, and can be formulated to briefly highlight key findings.
- Abstract – this is a very brief and comprehensive description of the study, required in every academic article, with a length of 100-500 words at most.
- Introduction – this is a vital part of any research summary, it provides the context and the literature review that gently introduces readers to the subject matter. The introduction usually covers definitions, questions, and hypotheses of the research study.
- Methodology –This section emphasizes the process and or data analysis methods used, in terms of experiments, surveys, sampling, or statistical analysis.
- Results section – this section lists in detail the results derived from the research with evidence obtained from all the experiments conducted.
- Discussion – these parts discuss the results within the context of current knowledge among subject matter experts. Interpretation of results and theoretical models explaining the observed results, the strengths of the study, and the limitations experienced are going to be a part of the discussion.
- Conclusion – In a conclusion, hypotheses are discussed and revalidated or denied, based on how convincing the evidence is.
- References – this section is for giving credit to those who work you studied to create your summary. You do this by providing appropriate citations as you write.
Research Summary Example 1
Below are some defining elements of a sample research summary.
Title – “The probability of an unexpected volcanic eruption in Greenwich”
Introduction – this section would list the catastrophic consequences that occurred in the country and the importance of analyzing this event.
Hypothesis – An eruption of the Greenwich supervolcano would be preceded by intense preliminary activity manifesting in advance, before the eruption.
Results – these could contain a report of statistical data from various volcanic eruptions happening globally while looking critically at the activity that occurred before these events.
Discussion and conclusion – Given that Greenwich is now consistently monitored by scientists and that signs of an eruption are usually detected before the volcanic eruption, this confirms the hypothesis. Hence creating an emergency plan outlining other intervention measures and ultimately evacuation is essential.
Research Summary Example 2
Below is another sample sketch.
Title – “The frequency of extreme weather events in the UK in 2000-2008 as compared to the ‘60s”
Introduction – Weather events bring intense material damage and cause pain to the victims affected.
Hypothesis – Extreme weather events are more frequent in recent times compared to the ‘50s
Results – The frequency of several categories of extreme events now and then are listed here, such as droughts, fires, massive rainfall/snowfalls, floods, hurricanes, tornadoes, etc.
Discussion and conclusion – Several types of extreme events have become more commonplace in recent times, confirming the hypothesis. This rise in extreme weather events can be traced to rising CO2 levels and increasing temperatures and global warming explain the rising frequency of these disasters. Addressing the rising CO2 levels and paying attention to climate change is the only to combat this phenomenon.
A research summary is the short form of a research paper, analyzing the important aspect of the study. Everyone who reads a research summary has a full grasp of the main idea being discussed in the original research paper. Conducting any research means you will write a summary, which is an important part of your project and would be the most read part of your project.
Having a guideline before you start helps, this would form your checklist which would guide your actions as you write your research summary. It is important to note that a Research Summary is different from an Abstract paper written at the beginning of a research paper, describing the idea behind a research paper.

Connect to Formplus, Get Started Now - It's Free!
- abstract in research papers
- abstract writing
- action research
- research summary
- research summary vs abstract
- research surveys
- Angela Kayode-Sanni

You may also like:
The McNamara Fallacy: How Researchers Can Detect and to Avoid it.
Introduction The McNamara Fallacy is a common problem in research. It happens when researchers take a single piece of data as evidence...

Action Research: What it is, Stages & Examples
Introduction Action research is an evidence-based approach that has been used for years in the field of education and social sciences....
How to Write An Abstract For Research Papers: Tips & Examples
In this article, we will share some tips for writing an effective abstract, plus samples you can learn from.
Research Questions: Definitions, Types + [Examples]
A comprehensive guide on the definition of research questions, types, importance, good and bad research question examples
Formplus - For Seamless Data Collection
Collect data the right way with a versatile data collection tool. try formplus and transform your work productivity today..
Please enable JavaScript in your browser to enjoy a better experience.
A Complete Guide to Writing a Research Summary
A summary is a key part of any research. So, how should you go about writing one?
You will find many guides on the Internet about writing research. But, any article seldom covers the prospect of writing a research summary. While many things are shortened versions of the original article, there’s much more to research summaries.
From descriptive statistics to writing scientific research, a summary plays a vital role in describing the key ideas within. So, it begs a few questions, such as:
- What exactly is a research summary?
- How do you write one?
- What are some of the tips for writing a good research summary ?
In this guide, we’ll answer all of these questions and explore a few essential factors about research writing. So, let’s jump right into it.
What is a Research Summary?
A research summary is a short, concise summary of an academic research paper. It is often used to summarize the results of an experiment, summarize the major findings and conclusions, and provide a brief overview of the methods and procedures used in the study.
The purpose of a research summary is to provide readers with enough information about an article to decide whether they want to read it in its entirety. It should be no more than two paragraphs long and should include:
- A brief introduction summarizing why the article was written
- The main idea of the article
- The major findings and conclusions
- An overview of how the study was conducted
In order to write effective research summaries, it is important that you can capture the essential points of the research and provide a concise overview. The key step in writing a good summary is to read through the article and make notes of the key points.
This can be done by underlining or highlighting key phrases in the article. One essential thing is to organize these points into an outline format, which includes an introduction and conclusion paragraph.
Another best and quick way to generate a precise summary of your research paper is to take assistance from the online text summarizer, like Summarizer.org .
The online summarizing tool gets the research paper and creates a precise summary of it by taking the important points.
Finally, you must edit your work for grammar and spelling errors before submitting it for grading.
The purpose of the research summary is to provide a comprehensive sum of everything that’s in the research. This includes a summarization of scientific/literal research, as well as of the writer’s aim and personal thoughts.
As for the summary length, it shouldn’t be more than 10% of the entire content. So, if your research is around 1000-words or so, then your summary should be 100-words. But, considering how most research papers are around 3000-4000 words, it should be 300-400 words.
Key pillars of a Research Summary
The summary of any research doesn’t just include the summarized text of the entire research paper. It includes a few other key things, which we’ll explore later on in this article. But, the purpose of a summary is to give proper insights to the reader, such as:
- The writer’s intention
- sources and bases of research
- the purpose & result.
That’s why it’s important to understand that the summary should tell your reader all these elements. So, the fundamentals of any summary include:
- Write a section and state the importance of the research paper from your perspective. In this section, you will have to describe the techniques, tools, and sources you employed to get the conclusion.
- Besides that, it’s also meant to provide a brief and descriptive explanation of the actionable aspect of your research. In other words, how it can be implemented in real life.
- Treat your research summary like a smaller article or blog. So, each important section of your research should be written within a subheading. However, this is highly optional to keep things organized.
- As mentioned before, the research summary shouldn’t exceed 300-400 words. But, some research summaries are known to surpass 10000-words. So, try to employ the 10% formula and write one-tenth of the entire length of your research paper.
These four main points allow you to understand how a research summary is different from the research itself. So, it’s like a documentary where research and other key factors are left to the science (research paper), while the narration explains the key points (research summary)
How do you write a Research Summary?
Writing a research summary is a straightforward affair. Yet, it requires some understanding, as it’s not a lengthy process but rather a tricky and technical one. In a research summary, a few boxes must be checked. To help you do just that, here are 6 things you should tend to separately:
A summary’s title can be the same as the title of your primary research. However, putting separate titles in both has a few benefits. Such as:
- A separate title shifts attention towards the conclusion.
- A different title can focus on the main point of your research.
- Using two different titles can provide a better abstract.
Speaking of an abstract, a summary is the abstract of your research. Therefore, a title representing that very thought is going to do a lot of good too. That’s why it’s better if the title of your summary differs from the title of your research paper.
2. Abstract
The abstract is the summarization of scientific or research methods used in your primary paper. This allows the reader to understand the pillars of the study conducted. For instance, there has been an array of astrological research since James Webb Space Telescope started sending images and data.
So, many research papers explain this Telescope’s technological evolution in their abstracts. This allows the reader to differentiate from the astrological research made by previous space crafts, such as Hubble or Chandra .
The point of providing this abstract is to ensure that the reader grasps the standards or boundaries within which the research was held.
3. Introduction
This is the part where you introduce your topic. In your main research, you’d dive right into the technicalities in this part. However, you’ll try to keep things mild in a research summary. Simply because it needs to summarize the key points in your main introduction.
So, a lot of introductions you’ll find as an example will be extensive in length. But, a research summary needs to be as concise as possible. Usually, in this part, a writer includes the basics and standards of investigation.
For instance, if your research is about James Webb’s latest findings , then you’ll identify how the studies conducted by this Telescope’s infrared and other technology made this study possible. That’s when your introduction will hook the reader into the main premise of your research.
4. Methodology / Study
This section needs to describe the methodology used by you in your research. Or the methodology you relied on when conducting this particular research or study. This allows the reader to grasp the fundamentals of your research, and it’s extremely important.
Because if the reader doesn’t understand your methods, then they will have no response to your studies. How should you tend to this? Include things such as:
- The surveys or reviews you used;
- include the samplings and experiment types you researched;
- provide a brief statistical analysis;
- give a primary reason to pick these particular methods.
Once again, leave the scientific intricacies for your primary research. But, describe the key methods that you employed. So, when the reader is perusing your final research, they’ll have your methods and study techniques in mind.
5. Results / Discussion
This section of your research needs to describe the results that you’ve achieved. Granted, some researchers will rely on results achieved by others. So, this part needs to explain how that happened – but not in detail.
The other section in this part will be a discussion. This is your interpretation of the results you’ve found. Thus, in the context of the results’ application, this section needs to dive into the theoretical understanding of your research. What will this section entail exactly? Here’s what:
- Things that you covered, including results;
- inferences you provided, given the context of your research;
- the theory archetype that you’ve tried to explain in the light of the methodology you employed;
- essential points or any limitations of the research.
These factors will help the reader grasp the final idea of your research. But, it’s not full circle yet, as the pulp will still be left for the actual research.
6. Conclusion
The final section of your summary is the conclusion. The key thing about the conclusion in your research summary, compared to your actual research, is that they could be different. For instance, the actual conclusion in your research should bring around the study.
However, the research in this summary should bring your own ideas and affirmations to full circle. Thus, this conclusion could and should be different from the ending of your research.
5 Tips for writing a Research Summary
Writing a research summary is easy once you tend to the technicalities. But, there are some tips and tricks that could make it easier. Remember, a research summary is the sum of your entire research. So, it doesn’t need to be as technical or in-depth as your primary work.
Thus, to make it easier for you, here are four tips you can follow:
1. Read & read again
Reading your own work repeatedly has many benefits. First, it’ll help you understand any mistakes or problems your research might have. After that, you’ll find a few key points that stand out from the others – that’s what you need to use in your summary.
So, the best advice anyone can give you is to read your research again and again. This will etch the idea in your mind and allow you to summarize it better.
2. Focus on key essentials in each section
As we discussed earlier, each section of your research has a key part. To write a thoroughly encapsulating summary, you need to focus on and find each such element in your research.
Doing so will give you enough leverage to write a summary that thoroughly condenses your research idea and gives you enough to write a summary out of it.
3. Write the research using a summarizing tool
The best advice you can get is to write a summary using a tool. Condensing each section might be a troublesome experience for some – as it can be time-consuming.
To avoid all that, you can simply take help from an online summarizer. It gets the lengthy content and creates a precise summary of it by using advanced AI technology.
As you can see, the tool condenses this particular section perfectly while the details are light.
Bringing that down to 10% or 20% will help you write each section accordingly. Thus, saving precious time and effort.
4. Word count limit
As mentioned earlier, word count is something you need to follow thoroughly. So, if your section is around 200-word, then read it again. And describe it to yourself in 20-words or so. Doing this to every section will help you write exactly a 10% summary of your research.
5. Get a second opinion
If you’re unsure about quality or quantity, get a second opinion. At times, ideas are in our minds, but we cannot find words to explain them. In research or any sort of creative process, getting a second opinion can save a lot of trouble.
There’s your guide to writing a research summary, folks. While it’s not different from condensing the entire premise of your research, writing it in simpler words will do wonders. So, try to follow the tips, tools, and ideas provided in this article, and write outstanding summaries for your research.
- Privacy Policy

Home » Research Findings – Types Examples and Writing Guide
Research Findings – Types Examples and Writing Guide
Table of Contents

Research Findings
Definition:
Research findings refer to the results obtained from a study or investigation conducted through a systematic and scientific approach. These findings are the outcomes of the data analysis, interpretation, and evaluation carried out during the research process.
Types of Research Findings
There are two main types of research findings:
Qualitative Findings
Qualitative research is an exploratory research method used to understand the complexities of human behavior and experiences. Qualitative findings are non-numerical and descriptive data that describe the meaning and interpretation of the data collected. Examples of qualitative findings include quotes from participants, themes that emerge from the data, and descriptions of experiences and phenomena.
Quantitative Findings
Quantitative research is a research method that uses numerical data and statistical analysis to measure and quantify a phenomenon or behavior. Quantitative findings include numerical data such as mean, median, and mode, as well as statistical analyses such as t-tests, ANOVA, and regression analysis. These findings are often presented in tables, graphs, or charts.
Both qualitative and quantitative findings are important in research and can provide different insights into a research question or problem. Combining both types of findings can provide a more comprehensive understanding of a phenomenon and improve the validity and reliability of research results.
Parts of Research Findings
Research findings typically consist of several parts, including:
- Introduction: This section provides an overview of the research topic and the purpose of the study.
- Literature Review: This section summarizes previous research studies and findings that are relevant to the current study.
- Methodology : This section describes the research design, methods, and procedures used in the study, including details on the sample, data collection, and data analysis.
- Results : This section presents the findings of the study, including statistical analyses and data visualizations.
- Discussion : This section interprets the results and explains what they mean in relation to the research question(s) and hypotheses. It may also compare and contrast the current findings with previous research studies and explore any implications or limitations of the study.
- Conclusion : This section provides a summary of the key findings and the main conclusions of the study.
- Recommendations: This section suggests areas for further research and potential applications or implications of the study’s findings.
How to Write Research Findings
Writing research findings requires careful planning and attention to detail. Here are some general steps to follow when writing research findings:
- Organize your findings: Before you begin writing, it’s essential to organize your findings logically. Consider creating an outline or a flowchart that outlines the main points you want to make and how they relate to one another.
- Use clear and concise language : When presenting your findings, be sure to use clear and concise language that is easy to understand. Avoid using jargon or technical terms unless they are necessary to convey your meaning.
- Use visual aids : Visual aids such as tables, charts, and graphs can be helpful in presenting your findings. Be sure to label and title your visual aids clearly, and make sure they are easy to read.
- Use headings and subheadings: Using headings and subheadings can help organize your findings and make them easier to read. Make sure your headings and subheadings are clear and descriptive.
- Interpret your findings : When presenting your findings, it’s important to provide some interpretation of what the results mean. This can include discussing how your findings relate to the existing literature, identifying any limitations of your study, and suggesting areas for future research.
- Be precise and accurate : When presenting your findings, be sure to use precise and accurate language. Avoid making generalizations or overstatements and be careful not to misrepresent your data.
- Edit and revise: Once you have written your research findings, be sure to edit and revise them carefully. Check for grammar and spelling errors, make sure your formatting is consistent, and ensure that your writing is clear and concise.
Research Findings Example
Following is a Research Findings Example sample for students:
Title: The Effects of Exercise on Mental Health
Sample : 500 participants, both men and women, between the ages of 18-45.
Methodology : Participants were divided into two groups. The first group engaged in 30 minutes of moderate intensity exercise five times a week for eight weeks. The second group did not exercise during the study period. Participants in both groups completed a questionnaire that assessed their mental health before and after the study period.
Findings : The group that engaged in regular exercise reported a significant improvement in mental health compared to the control group. Specifically, they reported lower levels of anxiety and depression, improved mood, and increased self-esteem.
Conclusion : Regular exercise can have a positive impact on mental health and may be an effective intervention for individuals experiencing symptoms of anxiety or depression.
Applications of Research Findings
Research findings can be applied in various fields to improve processes, products, services, and outcomes. Here are some examples:
- Healthcare : Research findings in medicine and healthcare can be applied to improve patient outcomes, reduce morbidity and mortality rates, and develop new treatments for various diseases.
- Education : Research findings in education can be used to develop effective teaching methods, improve learning outcomes, and design new educational programs.
- Technology : Research findings in technology can be applied to develop new products, improve existing products, and enhance user experiences.
- Business : Research findings in business can be applied to develop new strategies, improve operations, and increase profitability.
- Public Policy: Research findings can be used to inform public policy decisions on issues such as environmental protection, social welfare, and economic development.
- Social Sciences: Research findings in social sciences can be used to improve understanding of human behavior and social phenomena, inform public policy decisions, and develop interventions to address social issues.
- Agriculture: Research findings in agriculture can be applied to improve crop yields, develop new farming techniques, and enhance food security.
- Sports : Research findings in sports can be applied to improve athlete performance, reduce injuries, and develop new training programs.
When to use Research Findings
Research findings can be used in a variety of situations, depending on the context and the purpose. Here are some examples of when research findings may be useful:
- Decision-making : Research findings can be used to inform decisions in various fields, such as business, education, healthcare, and public policy. For example, a business may use market research findings to make decisions about new product development or marketing strategies.
- Problem-solving : Research findings can be used to solve problems or challenges in various fields, such as healthcare, engineering, and social sciences. For example, medical researchers may use findings from clinical trials to develop new treatments for diseases.
- Policy development : Research findings can be used to inform the development of policies in various fields, such as environmental protection, social welfare, and economic development. For example, policymakers may use research findings to develop policies aimed at reducing greenhouse gas emissions.
- Program evaluation: Research findings can be used to evaluate the effectiveness of programs or interventions in various fields, such as education, healthcare, and social services. For example, educational researchers may use findings from evaluations of educational programs to improve teaching and learning outcomes.
- Innovation: Research findings can be used to inspire or guide innovation in various fields, such as technology and engineering. For example, engineers may use research findings on materials science to develop new and innovative products.
Purpose of Research Findings
The purpose of research findings is to contribute to the knowledge and understanding of a particular topic or issue. Research findings are the result of a systematic and rigorous investigation of a research question or hypothesis, using appropriate research methods and techniques.
The main purposes of research findings are:
- To generate new knowledge : Research findings contribute to the body of knowledge on a particular topic, by adding new information, insights, and understanding to the existing knowledge base.
- To test hypotheses or theories : Research findings can be used to test hypotheses or theories that have been proposed in a particular field or discipline. This helps to determine the validity and reliability of the hypotheses or theories, and to refine or develop new ones.
- To inform practice: Research findings can be used to inform practice in various fields, such as healthcare, education, and business. By identifying best practices and evidence-based interventions, research findings can help practitioners to make informed decisions and improve outcomes.
- To identify gaps in knowledge: Research findings can help to identify gaps in knowledge and understanding of a particular topic, which can then be addressed by further research.
- To contribute to policy development: Research findings can be used to inform policy development in various fields, such as environmental protection, social welfare, and economic development. By providing evidence-based recommendations, research findings can help policymakers to develop effective policies that address societal challenges.
Characteristics of Research Findings
Research findings have several key characteristics that distinguish them from other types of information or knowledge. Here are some of the main characteristics of research findings:
- Objective : Research findings are based on a systematic and rigorous investigation of a research question or hypothesis, using appropriate research methods and techniques. As such, they are generally considered to be more objective and reliable than other types of information.
- Empirical : Research findings are based on empirical evidence, which means that they are derived from observations or measurements of the real world. This gives them a high degree of credibility and validity.
- Generalizable : Research findings are often intended to be generalizable to a larger population or context beyond the specific study. This means that the findings can be applied to other situations or populations with similar characteristics.
- Transparent : Research findings are typically reported in a transparent manner, with a clear description of the research methods and data analysis techniques used. This allows others to assess the credibility and reliability of the findings.
- Peer-reviewed: Research findings are often subject to a rigorous peer-review process, in which experts in the field review the research methods, data analysis, and conclusions of the study. This helps to ensure the validity and reliability of the findings.
- Reproducible : Research findings are often designed to be reproducible, meaning that other researchers can replicate the study using the same methods and obtain similar results. This helps to ensure the validity and reliability of the findings.
Advantages of Research Findings
Research findings have many advantages, which make them valuable sources of knowledge and information. Here are some of the main advantages of research findings:
- Evidence-based: Research findings are based on empirical evidence, which means that they are grounded in data and observations from the real world. This makes them a reliable and credible source of information.
- Inform decision-making: Research findings can be used to inform decision-making in various fields, such as healthcare, education, and business. By identifying best practices and evidence-based interventions, research findings can help practitioners and policymakers to make informed decisions and improve outcomes.
- Identify gaps in knowledge: Research findings can help to identify gaps in knowledge and understanding of a particular topic, which can then be addressed by further research. This contributes to the ongoing development of knowledge in various fields.
- Improve outcomes : Research findings can be used to develop and implement evidence-based practices and interventions, which have been shown to improve outcomes in various fields, such as healthcare, education, and social services.
- Foster innovation: Research findings can inspire or guide innovation in various fields, such as technology and engineering. By providing new information and understanding of a particular topic, research findings can stimulate new ideas and approaches to problem-solving.
- Enhance credibility: Research findings are generally considered to be more credible and reliable than other types of information, as they are based on rigorous research methods and are subject to peer-review processes.
Limitations of Research Findings
While research findings have many advantages, they also have some limitations. Here are some of the main limitations of research findings:
- Limited scope: Research findings are typically based on a particular study or set of studies, which may have a limited scope or focus. This means that they may not be applicable to other contexts or populations.
- Potential for bias : Research findings can be influenced by various sources of bias, such as researcher bias, selection bias, or measurement bias. This can affect the validity and reliability of the findings.
- Ethical considerations: Research findings can raise ethical considerations, particularly in studies involving human subjects. Researchers must ensure that their studies are conducted in an ethical and responsible manner, with appropriate measures to protect the welfare and privacy of participants.
- Time and resource constraints : Research studies can be time-consuming and require significant resources, which can limit the number and scope of studies that are conducted. This can lead to gaps in knowledge or a lack of research on certain topics.
- Complexity: Some research findings can be complex and difficult to interpret, particularly in fields such as science or medicine. This can make it challenging for practitioners and policymakers to apply the findings to their work.
- Lack of generalizability : While research findings are intended to be generalizable to larger populations or contexts, there may be factors that limit their generalizability. For example, cultural or environmental factors may influence how a particular intervention or treatment works in different populations or contexts.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
How To Write The Results/Findings Chapter
For qualitative studies (dissertations & theses).
By: Jenna Crossley (PhD). Expert Reviewed By: Dr. Eunice Rautenbach | August 2021
So, you’ve collected and analysed your qualitative data, and it’s time to write up your results chapter. But where do you start? In this post, we’ll guide you through the qualitative results chapter (also called the findings chapter), step by step.
Overview: Qualitative Results Chapter
- What (exactly) the qualitative results chapter is
- What to include in your results chapter
- How to write up your results chapter
- A few tips and tricks to help you along the way
- Free results chapter template
What exactly is the results chapter?
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and discuss its meaning), depending on your university’s preference. We’ll treat the two chapters as separate, as that’s the most common approach.
In contrast to a quantitative results chapter that presents numbers and statistics, a qualitative results chapter presents data primarily in the form of words . But this doesn’t mean that a qualitative study can’t have quantitative elements – you could, for example, present the number of times a theme or topic pops up in your data, depending on the analysis method(s) you adopt.
Adding a quantitative element to your study can add some rigour, which strengthens your results by providing more evidence for your claims. This is particularly common when using qualitative content analysis. Keep in mind though that qualitative research aims to achieve depth, richness and identify nuances , so don’t get tunnel vision by focusing on the numbers. They’re just cream on top in a qualitative analysis.
So, to recap, the results chapter is where you objectively present the findings of your analysis, without interpreting them (you’ll save that for the discussion chapter). With that out the way, let’s take a look at what you should include in your results chapter.

What should you include in the results chapter?
As we’ve mentioned, your qualitative results chapter should purely present and describe your results , not interpret them in relation to the existing literature or your research questions . Any speculations or discussion about the implications of your findings should be reserved for your discussion chapter.
In your results chapter, you’ll want to talk about your analysis findings and whether or not they support your hypotheses (if you have any). Naturally, the exact contents of your results chapter will depend on which qualitative analysis method (or methods) you use. For example, if you were to use thematic analysis, you’d detail the themes identified in your analysis, using extracts from the transcripts or text to support your claims.
While you do need to present your analysis findings in some detail, you should avoid dumping large amounts of raw data in this chapter. Instead, focus on presenting the key findings and using a handful of select quotes or text extracts to support each finding . The reams of data and analysis can be relegated to your appendices.
While it’s tempting to include every last detail you found in your qualitative analysis, it is important to make sure that you report only that which is relevant to your research aims, objectives and research questions . Always keep these three components, as well as your hypotheses (if you have any) front of mind when writing the chapter and use them as a filter to decide what’s relevant and what’s not.
Need a helping hand?
How do I write the results chapter?
Now that we’ve covered the basics, it’s time to look at how to structure your chapter. Broadly speaking, the results chapter needs to contain three core components – the introduction, the body and the concluding summary. Let’s take a look at each of these.
Section 1: Introduction
The first step is to craft a brief introduction to the chapter. This intro is vital as it provides some context for your findings. In your introduction, you should begin by reiterating your problem statement and research questions and highlight the purpose of your research . Make sure that you spell this out for the reader so that the rest of your chapter is well contextualised.
The next step is to briefly outline the structure of your results chapter. In other words, explain what’s included in the chapter and what the reader can expect. In the results chapter, you want to tell a story that is coherent, flows logically, and is easy to follow , so make sure that you plan your structure out well and convey that structure (at a high level), so that your reader is well oriented.
The introduction section shouldn’t be lengthy. Two or three short paragraphs should be more than adequate. It is merely an introduction and overview, not a summary of the chapter.
Pro Tip – To help you structure your chapter, it can be useful to set up an initial draft with (sub)section headings so that you’re able to easily (re)arrange parts of your chapter. This will also help your reader to follow your results and give your chapter some coherence. Be sure to use level-based heading styles (e.g. Heading 1, 2, 3 styles) to help the reader differentiate between levels visually. You can find these options in Word (example below).
Section 2: Body
Before we get started on what to include in the body of your chapter, it’s vital to remember that a results section should be completely objective and descriptive, not interpretive . So, be careful not to use words such as, “suggests” or “implies”, as these usually accompany some form of interpretation – that’s reserved for your discussion chapter.
The structure of your body section is very important , so make sure that you plan it out well. When planning out your qualitative results chapter, create sections and subsections so that you can maintain the flow of the story you’re trying to tell. Be sure to systematically and consistently describe each portion of results. Try to adopt a standardised structure for each portion so that you achieve a high level of consistency throughout the chapter.
For qualitative studies, results chapters tend to be structured according to themes , which makes it easier for readers to follow. However, keep in mind that not all results chapters have to be structured in this manner. For example, if you’re conducting a longitudinal study, you may want to structure your chapter chronologically. Similarly, you might structure this chapter based on your theoretical framework . The exact structure of your chapter will depend on the nature of your study , especially your research questions.
As you work through the body of your chapter, make sure that you use quotes to substantiate every one of your claims . You can present these quotes in italics to differentiate them from your own words. A general rule of thumb is to use at least two pieces of evidence per claim, and these should be linked directly to your data. Also, remember that you need to include all relevant results , not just the ones that support your assumptions or initial leanings.
In addition to including quotes, you can also link your claims to the data by using appendices , which you should reference throughout your text. When you reference, make sure that you include both the name/number of the appendix , as well as the line(s) from which you drew your data.
As referencing styles can vary greatly, be sure to look up the appendix referencing conventions of your university’s prescribed style (e.g. APA , Harvard, etc) and keep this consistent throughout your chapter.
Section 3: Concluding summary
The concluding summary is very important because it summarises your key findings and lays the foundation for the discussion chapter . Keep in mind that some readers may skip directly to this section (from the introduction section), so make sure that it can be read and understood well in isolation.
In this section, you need to remind the reader of the key findings. That is, the results that directly relate to your research questions and that you will build upon in your discussion chapter. Remember, your reader has digested a lot of information in this chapter, so you need to use this section to remind them of the most important takeaways.
Importantly, the concluding summary should not present any new information and should only describe what you’ve already presented in your chapter. Keep it concise – you’re not summarising the whole chapter, just the essentials.
Tips for writing an A-grade results chapter
Now that you’ve got a clear picture of what the qualitative results chapter is all about, here are some quick tips and reminders to help you craft a high-quality chapter:
- Your results chapter should be written in the past tense . You’ve done the work already, so you want to tell the reader what you found , not what you are currently finding .
- Make sure that you review your work multiple times and check that every claim is adequately backed up by evidence . Aim for at least two examples per claim, and make use of an appendix to reference these.
- When writing up your results, make sure that you stick to only what is relevant . Don’t waste time on data that are not relevant to your research objectives and research questions.
- Use headings and subheadings to create an intuitive, easy to follow piece of writing. Make use of Microsoft Word’s “heading styles” and be sure to use them consistently.
- When referring to numerical data, tables and figures can provide a useful visual aid. When using these, make sure that they can be read and understood independent of your body text (i.e. that they can stand-alone). To this end, use clear, concise labels for each of your tables or figures and make use of colours to code indicate differences or hierarchy.
- Similarly, when you’re writing up your chapter, it can be useful to highlight topics and themes in different colours . This can help you to differentiate between your data if you get a bit overwhelmed and will also help you to ensure that your results flow logically and coherently.
If you have any questions, leave a comment below and we’ll do our best to help. If you’d like 1-on-1 help with your results chapter (or any chapter of your dissertation or thesis), check out our private dissertation coaching service here or book a free initial consultation to discuss how we can help you.

Psst... there’s more!
This post was based on one of our popular Research Bootcamps . If you're working on a research project, you'll definitely want to check this out ...
You Might Also Like:

20 Comments
This was extremely helpful. Thanks a lot guys
Hi, thanks for the great research support platform created by the gradcoach team!
I wanted to ask- While “suggests” or “implies” are interpretive terms, what terms could we use for the results chapter? Could you share some examples of descriptive terms?
I think that instead of saying, ‘The data suggested, or The data implied,’ you can say, ‘The Data showed or revealed, or illustrated or outlined’…If interview data, you may say Jane Doe illuminated or elaborated, or Jane Doe described… or Jane Doe expressed or stated.
I found this article very useful. Thank you very much for the outstanding work you are doing.
What if i have 3 different interviewees answering the same interview questions? Should i then present the results in form of the table with the division on the 3 perspectives or rather give a results in form of the text and highlight who said what?
I think this tabular representation of results is a great idea. I am doing it too along with the text. Thanks
That was helpful was struggling to separate the discussion from the findings
this was very useful, Thank you.
Very helpful, I am confident to write my results chapter now.
It is so helpful! It is a good job. Thank you very much!
Very useful, well explained. Many thanks.
Hello, I appreciate the way you provided a supportive comments about qualitative results presenting tips
I loved this! It explains everything needed, and it has helped me better organize my thoughts. What words should I not use while writing my results section, other than subjective ones.
Thanks a lot, it is really helpful
Thank you so much dear, i really appropriate your nice explanations about this.
Thank you so much for this! I was wondering if anyone could help with how to prproperly integrate quotations (Excerpts) from interviews in the finding chapter in a qualitative research. Please GradCoach, address this issue and provide examples.
what if I’m not doing any interviews myself and all the information is coming from case studies that have already done the research.
Very helpful thank you.
This was very helpful as I was wondering how to structure this part of my dissertation, to include the quotes… Thanks for this explanation
This is very helpful, thanks! I am required to write up my results chapters with the discussion in each of them – any tips and tricks for this strategy?
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Jump to navigation

Cochrane Training
Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.
Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Key Points:
- A ‘Summary of findings’ table for a given comparison of interventions provides key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.
- ‘Summary of findings’ tables include a row for each important outcome (up to a maximum of seven). Accepted formats of ‘Summary of findings’ tables and interactive ‘Summary of findings’ tables can be produced using GRADE’s software GRADEpro GDT.
- Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of a body of evidence.
- The GRADE approach specifies four levels of the certainty for a body of evidence for a given outcome: high, moderate, low and very low.
- GRADE assessments of certainty are determined through consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. For evidence from non-randomized studies and rarely randomized studies, assessments can then be upgraded through consideration of three further domains.
Cite this chapter as: Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
14.1 ‘Summary of findings’ tables
14.1.1 introduction to ‘summary of findings’ tables.
‘Summary of findings’ tables present the main findings of a review in a transparent, structured and simple tabular format. In particular, they provide key information concerning the certainty or quality of evidence (i.e. the confidence or certainty in the range of an effect estimate or an association), the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Cochrane Reviews should incorporate ‘Summary of findings’ tables during planning and publication, and should have at least one key ‘Summary of findings’ table representing the most important comparisons. Some reviews may include more than one ‘Summary of findings’ table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). In the Cochrane Database of Systematic Reviews (CDSR), all ‘Summary of findings’ tables for a review appear at the beginning, before the Background section.
14.1.2 Selecting outcomes for ‘Summary of findings’ tables
Planning for the ‘Summary of findings’ table starts early in the systematic review, with the selection of the outcomes to be included in: (i) the review; and (ii) the ‘Summary of findings’ table. This is a crucial step, and one that review authors need to address carefully.
To ensure production of optimally useful information, Cochrane Reviews begin by developing a review question and by listing all main outcomes that are important to patients and other decision makers (see Chapter 2 and Chapter 3 ). The GRADE approach to assessing the certainty of the evidence (see Section 14.2 ) defines and operationalizes a rating process that helps separate outcomes into those that are critical, important or not important for decision making. Consultation and feedback on the review protocol, including from consumers and other decision makers, can enhance this process.
Critical outcomes are likely to include clearly important endpoints; typical examples include mortality and major morbidity (such as strokes and myocardial infarction). However, they may also represent frequent minor and rare major side effects, symptoms, quality of life, burdens associated with treatment, and resource issues (costs). Burdens represent the impact of healthcare workload on patient function and well-being, and include the demands of adhering to an intervention that patients or caregivers (e.g. family) may dislike, such as having to undergo more frequent tests, or the restrictions on lifestyle that certain interventions require (Spencer-Bonilla et al 2017).
Frequently, when formulating questions that include all patient-important outcomes for decision making, review authors will confront reports of studies that have not included all these outcomes. This is particularly true for adverse outcomes. For instance, randomized trials might contribute evidence on intended effects, and on frequent, relatively minor side effects, but not report on rare adverse outcomes such as suicide attempts. Chapter 19 discusses strategies for addressing adverse effects. To obtain data for all important outcomes it may be necessary to examine the results of non-randomized studies (see Chapter 24 ). Cochrane, in collaboration with others, has developed guidance for review authors to support their decision about when to look for and include non-randomized studies (Schünemann et al 2013).
If a review includes only randomized trials, these trials may not address all important outcomes and it may therefore not be possible to address these outcomes within the constraints of the review. Review authors should acknowledge these limitations and make them transparent to readers. Review authors are encouraged to include non-randomized studies to examine rare or long-term adverse effects that may not adequately be studied in randomized trials. This raises the possibility that harm outcomes may come from studies in which participants differ from those in studies used in the analysis of benefit. Review authors will then need to consider how much such differences are likely to impact on the findings, and this will influence the certainty of evidence because of concerns about indirectness related to the population (see Section 14.2.2 ).
Non-randomized studies can provide important information not only when randomized trials do not report on an outcome or randomized trials suffer from indirectness, but also when the evidence from randomized trials is rated as very low and non-randomized studies provide evidence of higher certainty. Further discussion of these issues appears also in Chapter 24 .
14.1.3 General template for ‘Summary of findings’ tables
Several alternative standard versions of ‘Summary of findings’ tables have been developed to ensure consistency and ease of use across reviews, inclusion of the most important information needed by decision makers, and optimal presentation (see examples at Figures 14.1.a and 14.1.b ). These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). They are available through GRADE’s official software package developed to support the GRADE approach: GRADEpro GDT (www.gradepro.org).
Standard Cochrane ‘Summary of findings’ tables include the following elements using one of the accepted formats. Further guidance on each of these is provided in Section 14.1.6 .
- A brief description of the population and setting addressed by the available evidence (which may be slightly different to or narrower than those defined by the review question).
- A brief description of the comparison addressed in the ‘Summary of findings’ table, including both the experimental and comparison interventions.
- A list of the most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes.
- A measure of the typical burden of each outcomes (e.g. illustrative risk, or illustrative mean, on comparator intervention).
- The absolute and relative magnitude of effect measured for each (if both are appropriate).
- The numbers of participants and studies contributing to the analysis of each outcomes.
- A GRADE assessment of the overall certainty of the body of evidence for each outcome (which may vary by outcome).
- Space for comments.
- Explanations (formerly known as footnotes).
Ideally, ‘Summary of findings’ tables are supported by more detailed tables (known as ‘evidence profiles’) to which the review may be linked, which provide more detailed explanations. Evidence profiles include the same important health outcomes, and provide greater detail than ‘Summary of findings’ tables of both of the individual considerations feeding into the grading of certainty and of the results of the studies (Guyatt et al 2011a). They ensure that a structured approach is used to rating the certainty of evidence. Although they are rarely published in Cochrane Reviews, evidence profiles are often used, for example, by guideline developers in considering the certainty of the evidence to support guideline recommendations. Review authors will find it easier to develop the ‘Summary of findings’ table by completing the rating of the certainty of evidence in the evidence profile first in GRADEpro GDT. They can then automatically convert this to one of the ‘Summary of findings’ formats in GRADEpro GDT, including an interactive ‘Summary of findings’ for publication.
As a measure of the magnitude of effect for dichotomous outcomes, the ‘Summary of findings’ table should provide a relative measure of effect (e.g. risk ratio, odds ratio, hazard) and measures of absolute risk. For other types of data, an absolute measure alone (such as a difference in means for continuous data) might be sufficient. It is important that the magnitude of effect is presented in a meaningful way, which may require some transformation of the result of a meta-analysis (see also Chapter 15, Section 15.4 and Section 15.5 ). Reviews with more than one main comparison should include a separate ‘Summary of findings’ table for each comparison.
Figure 14.1.a provides an example of a ‘Summary of findings’ table. Figure 15.1.b provides an alternative format that may further facilitate users’ understanding and interpretation of the review’s findings. Evidence evaluating different formats suggests that the ‘Summary of findings’ table should include a risk difference as a measure of the absolute effect and authors should preferably use a format that includes a risk difference .
A detailed description of the contents of a ‘Summary of findings’ table appears in Section 14.1.6 .
Figure 14.1.a Example of a ‘Summary of findings’ table
Summary of findings (for interactive version click here )
a All the stockings in the nine studies included in this review were below-knee compression stockings. In four studies the compression strength was 20 mmHg to 30 mmHg at the ankle. It was 10 mmHg to 20 mmHg in the other four studies. Stockings come in different sizes. If a stocking is too tight around the knee it can prevent essential venous return causing the blood to pool around the knee. Compression stockings should be fitted properly. A stocking that is too tight could cut into the skin on a long flight and potentially cause ulceration and increased risk of DVT. Some stockings can be slightly thicker than normal leg covering and can be potentially restrictive with tight foot wear. It is a good idea to wear stockings around the house prior to travel to ensure a good, comfortable fit. Participants put their stockings on two to three hours before the flight in most of the studies. The availability and cost of stockings can vary.
b Two studies recruited high risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for the seven studies that excluded high risk participants was 1.45% and the incidence for the two studies that recruited high-risk participants (with at least one risk factor) was 2.43%. We have used 10 and 30 per 1000 to express different risk strata, respectively.
c The confidence interval crosses no difference and does not rule out a small increase.
d The measurement of oedema was not validated (indirectness of the outcome) or blinded to the intervention (risk of bias).
e If there are very few or no events and the number of participants is large, judgement about the certainty of evidence (particularly judgements about imprecision) may be based on the absolute effect. Here the certainty rating may be considered ‘high’ if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
f None of the other studies reported adverse effects, apart from four cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in one study.
Figure 14.1.b Example of alternative ‘Summary of findings’ table
14.1.4 Producing ‘Summary of findings’ tables
The GRADE Working Group’s software, GRADEpro GDT ( www.gradepro.org ), including GRADE’s interactive handbook, is available to assist review authors in the preparation of ‘Summary of findings’ tables. GRADEpro can use data on the comparator group risk and the effect estimate (entered by the review authors or imported from files generated in RevMan) to produce the relative effects and absolute risks associated with experimental interventions. In addition, it leads the user through the process of a GRADE assessment, and produces a table that can be used as a standalone table in a review (including by direct import into software such as RevMan or integration with RevMan Web), or an interactive ‘Summary of findings’ table (see help resources in GRADEpro).
14.1.5 Statistical considerations in ‘Summary of findings’ tables
14.1.5.1 dichotomous outcomes.
‘Summary of findings’ tables should include both absolute and relative measures of effect for dichotomous outcomes. Risk ratios, odds ratios and risk differences are different ways of comparing two groups with dichotomous outcome data (see Chapter 6, Section 6.4.1 ). Furthermore, there are two distinct risk ratios, depending on which event (e.g. ‘yes’ or ‘no’) is the focus of the analysis (see Chapter 6, Section 6.4.1.5 ). In the presence of a non-zero intervention effect, any variation across studies in the comparator group risks (i.e. variation in the risk of the event occurring without the intervention of interest, for example in different populations) makes it impossible for more than one of these measures to be truly the same in every study.
It has long been assumed in epidemiology that relative measures of effect are more consistent than absolute measures of effect from one scenario to another. There is empirical evidence to support this assumption (Engels et al 2000, Deeks and Altman 2001, Furukawa et al 2002). For this reason, meta-analyses should generally use either a risk ratio or an odds ratio as a measure of effect (see Chapter 10, Section 10.4.3 ). Correspondingly, a single estimate of relative effect is likely to be a more appropriate summary than a single estimate of absolute effect. If a relative effect is indeed consistent across studies, then different comparator group risks will have different implications for absolute benefit. For instance, if the risk ratio is consistently 0.75, then the experimental intervention would reduce a comparator group risk of 80% to 60% in the intervention group (an absolute risk reduction of 20 percentage points), but would also reduce a comparator group risk of 20% to 15% in the intervention group (an absolute risk reduction of 5 percentage points).
‘Summary of findings’ tables are built around the assumption of a consistent relative effect. It is therefore important to consider the implications of this effect for different comparator group risks (these can be derived or estimated from a number of sources, see Section 14.1.6.3 ), which may require an assessment of the certainty of evidence for prognostic evidence (Spencer et al 2012, Iorio et al 2015). For any comparator group risk, it is possible to estimate a corresponding intervention group risk (i.e. the absolute risk with the intervention) from the meta-analytic risk ratio or odds ratio. Note that the numbers provided in the ‘Corresponding risk’ column are specific to the ‘risks’ in the adjacent column.
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding intervention risk is obtained as:
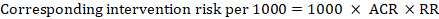
As an example, in Figure 14.1.a , the meta-analytic risk ratio for symptomless deep vein thrombosis (DVT) is RR = 0.10 (95% CI 0.04 to 0.26). Assuming a comparator risk of ACR = 10 per 1000 = 0.01, we obtain:
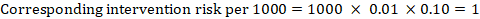
For the meta-analytic odds ratio (OR) and assumed comparator risk, ACR, the corresponding intervention risk is obtained as:
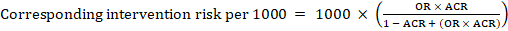
Upper and lower confidence limits for the corresponding intervention risk are obtained by replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.10 with 0.04, then with 0.26, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
When dealing with risk ratios, it is critical that the same definition of ‘event’ is used as was used for the meta-analysis. For example, if the meta-analysis focused on ‘death’ (as opposed to survival) as the event, then corresponding risks in the ‘Summary of findings’ table must also refer to ‘death’.
In (rare) circumstances in which there is clear rationale to assume a consistent risk difference in the meta-analysis, in principle it is possible to present this for relevant ‘assumed risks’ and their corresponding risks, and to present the corresponding (different) relative effects for each assumed risk.
The risk difference expresses the difference between the ACR and the corresponding intervention risk (or the difference between the experimental and the comparator intervention).
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding risk difference is obtained as (note that risks can also be expressed using percentage or percentage points):
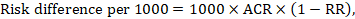
As an example, in Figure 14.1.b the meta-analytic risk ratio is 0.41 (95% CI 0.29 to 0.55) for diarrhoea in children less than 5 years of age. Assuming a comparator group risk of 22.3% we obtain:
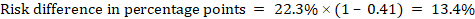
For the meta-analytic odds ratio (OR) and assumed comparator risk (ACR) the absolute risk difference is obtained as (percentage points):
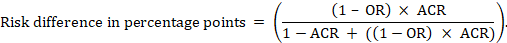
Upper and lower confidence limits for the absolute risk difference are obtained by re-running the calculation above while replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.41 with 0.28, then with 0.55, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
14.1.5.2 Time-to-event outcomes
Time-to-event outcomes measure whether and when a particular event (e.g. death) occurs (van Dalen et al 2007). The impact of the experimental intervention relative to the comparison group on time-to-event outcomes is usually measured using a hazard ratio (HR) (see Chapter 6, Section 6.8.1 ).
A hazard ratio expresses a relative effect estimate. It may be used in various ways to obtain absolute risks and other interpretable quantities for a specific population. Here we describe how to re-express hazard ratios in terms of: (i) absolute risk of event-free survival within a particular period of time; (ii) absolute risk of an event within a particular period of time; and (iii) median time to the event. All methods are built on an assumption of consistent relative effects (i.e. that the hazard ratio does not vary over time).
(i) Absolute risk of event-free survival within a particular period of time Event-free survival (e.g. overall survival) is commonly reported by individual studies. To obtain absolute effects for time-to-event outcomes measured as event-free survival, the summary HR can be used in conjunction with an assumed proportion of patients who are event-free in the comparator group (Tierney et al 2007). This proportion of patients will be specific to a period of time of observation. However, it is not strictly necessary to specify this period of time. For instance, a proportion of 50% of event-free patients might apply to patients with a high event rate observed over 1 year, or to patients with a low event rate observed over 2 years.
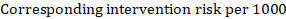
As an example, suppose the meta-analytic hazard ratio is 0.42 (95% CI 0.25 to 0.72). Assuming a comparator group risk of event-free survival (e.g. for overall survival people being alive) at 2 years of ACR = 900 per 1000 = 0.9 we obtain:
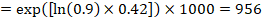
so that that 956 per 1000 people will be alive with the experimental intervention at 2 years. The derivation of the risk should be explained in a comment or footnote.
(ii) Absolute risk of an event within a particular period of time To obtain this absolute effect, again the summary HR can be used (Tierney et al 2007):
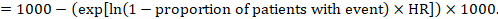
In the example, suppose we assume a comparator group risk of events (e.g. for mortality, people being dead) at 2 years of ACR = 100 per 1000 = 0.1. We obtain:
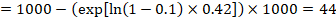
so that that 44 per 1000 people will be dead with the experimental intervention at 2 years.
(iii) Median time to the event Instead of absolute numbers, the time to the event in the intervention and comparison groups can be expressed as median survival time in months or years. To obtain median survival time the pooled HR can be applied to an assumed median survival time in the comparator group (Tierney et al 2007):
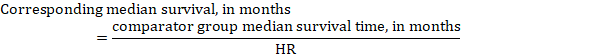
In the example, assuming a comparator group median survival time of 80 months, we obtain:
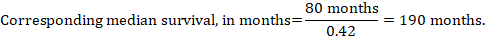
For all three of these options for re-expressing results of time-to-event analyses, upper and lower confidence limits for the corresponding intervention risk are obtained by replacing HR by its upper and lower confidence limits, respectively (e.g. replacing 0.42 with 0.25, then with 0.72, in the example). Again, as for dichotomous outcomes, such confidence intervals do not incorporate uncertainty in the assumed comparator group risks. This is of special concern for long-term survival with a low or moderate mortality rate and a corresponding high number of censored patients (i.e. a low number of patients under risk and a high censoring rate).
14.1.6 Detailed contents of a ‘Summary of findings’ table
14.1.6.1 table title and header.
The title of each ‘Summary of findings’ table should specify the healthcare question, framed in terms of the population and making it clear exactly what comparison of interventions are made. In Figure 14.1.a , the population is people taking long aeroplane flights, the intervention is compression stockings, and the control is no compression stockings.
The first rows of each ‘Summary of findings’ table should provide the following ‘header’ information:
Patients or population This further clarifies the population (and possibly the subpopulations) of interest and ideally the magnitude of risk of the most crucial adverse outcome at which an intervention is directed. For instance, people on a long-haul flight may be at different risks for DVT; those using selective serotonin reuptake inhibitors (SSRIs) might be at different risk for side effects; while those with atrial fibrillation may be at low (< 1%), moderate (1% to 4%) or high (> 4%) yearly risk of stroke.
Setting This should state any specific characteristics of the settings of the healthcare question that might limit the applicability of the summary of findings to other settings (e.g. primary care in Europe and North America).
Intervention The experimental intervention.
Comparison The comparator intervention (including no specific intervention).
14.1.6.2 Outcomes
The rows of a ‘Summary of findings’ table should include all desirable and undesirable health outcomes (listed in order of importance) that are essential for decision making, up to a maximum of seven outcomes. If there are more outcomes in the review, review authors will need to omit the less important outcomes from the table, and the decision selecting which outcomes are critical or important to the review should be made during protocol development (see Chapter 3 ). Review authors should provide time frames for the measurement of the outcomes (e.g. 90 days or 12 months) and the type of instrument scores (e.g. ranging from 0 to 100).
Note that review authors should include the pre-specified critical and important outcomes in the table whether data are available or not. However, they should be alert to the possibility that the importance of an outcome (e.g. a serious adverse effect) may only become known after the protocol was written or the analysis was carried out, and should take appropriate actions to include these in the ‘Summary of findings’ table.
The ‘Summary of findings’ table can include effects in subgroups of the population for different comparator risks and effect sizes separately. For instance, in Figure 14.1.b effects are presented for children younger and older than 5 years separately. Review authors may also opt to produce separate ‘Summary of findings’ tables for different populations.
Review authors should include serious adverse events, but it might be possible to combine minor adverse events as a single outcome, and describe this in an explanatory footnote (note that it is not appropriate to add events together unless they are independent, that is, a participant who has experienced one adverse event has an unaffected chance of experiencing the other adverse event).
Outcomes measured at multiple time points represent a particular problem. In general, to keep the table simple, review authors should present multiple time points only for outcomes critical to decision making, where either the result or the decision made are likely to vary over time. The remainder should be presented at a common time point where possible.
Review authors can present continuous outcome measures in the ‘Summary of findings’ table and should endeavour to make these interpretable to the target audience. This requires that the units are clear and readily interpretable, for example, days of pain, or frequency of headache, and the name and scale of any measurement tools used should be stated (e.g. a Visual Analogue Scale, ranging from 0 to 100). However, many measurement instruments are not readily interpretable by non-specialist clinicians or patients, for example, points on a Beck Depression Inventory or quality of life score. For these, a more interpretable presentation might involve converting a continuous to a dichotomous outcome, such as >50% improvement (see Chapter 15, Section 15.5 ).
14.1.6.3 Best estimate of risk with comparator intervention
Review authors should provide up to three typical risks for participants receiving the comparator intervention. For dichotomous outcomes, we recommend that these be presented in the form of the number of people experiencing the event per 100 or 1000 people (natural frequency) depending on the frequency of the outcome. For continuous outcomes, this would be stated as a mean or median value of the outcome measured.
Estimated or assumed comparator intervention risks could be based on assessments of typical risks in different patient groups derived from the review itself, individual representative studies in the review, or risks derived from a systematic review of prognosis studies or other sources of evidence which may in turn require an assessment of the certainty for the prognostic evidence (Spencer et al 2012, Iorio et al 2015). Ideally, risks would reflect groups that clinicians can easily identify on the basis of their presenting features.
An explanatory footnote should specify the source or rationale for each comparator group risk, including the time period to which it corresponds where appropriate. In Figure 14.1.a , clinicians can easily differentiate individuals with risk factors for deep venous thrombosis from those without. If there is known to be little variation in baseline risk then review authors may use the median comparator group risk across studies. If typical risks are not known, an option is to choose the risk from the included studies, providing the second highest for a high and the second lowest for a low risk population.
14.1.6.4 Risk with intervention
For dichotomous outcomes, review authors should provide a corresponding absolute risk for each comparator group risk, along with a confidence interval. This absolute risk with the (experimental) intervention will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the absolute effect in the same format as the risks with comparator intervention (see Section 14.1.6.3 ), for example as the number of people experiencing the event per 1000 people.
For continuous outcomes, a difference in means or standardized difference in means should be presented with its confidence interval. These will typically be obtained directly from a meta-analysis. Explanatory text should be used to clarify the meaning, as in Figures 14.1.a and 14.1.b .
14.1.6.5 Risk difference
For dichotomous outcomes, the risk difference can be provided using one of the ‘Summary of findings’ table formats as an additional option (see Figure 14.1.b ). This risk difference expresses the difference between the experimental and comparator intervention and will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the risk difference in the same format as assumed and corresponding risks with comparator intervention (see Section 14.1.6.3 ); for example, as the number of people experiencing the event per 1000 people or as percentage points if the assumed and corresponding risks are expressed in percentage.
For continuous outcomes, if the ‘Summary of findings’ table includes this option, the mean difference can be presented here and the ‘corresponding risk’ column left blank (see Figure 14.1.b ).
14.1.6.6 Relative effect (95% CI)
The relative effect will typically be a risk ratio or odds ratio (or occasionally a hazard ratio) with its accompanying 95% confidence interval, obtained from a meta-analysis performed on the basis of the same effect measure. Risk ratios and odds ratios are similar when the comparator intervention risks are low and effects are small, but may differ considerably when comparator group risks increase. The meta-analysis may involve an assumption of either fixed or random effects, depending on what the review authors consider appropriate, and implying that the relative effect is either an estimate of the effect of the intervention, or an estimate of the average effect of the intervention across studies, respectively.
14.1.6.7 Number of participants (studies)
This column should include the number of participants assessed in the included studies for each outcome and the corresponding number of studies that contributed these participants.
14.1.6.8 Certainty of the evidence (GRADE)
Review authors should comment on the certainty of the evidence (also known as quality of the body of evidence or confidence in the effect estimates). Review authors should use the specific evidence grading system developed by the GRADE Working Group (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011a), which is described in detail in Section 14.2 . The GRADE approach categorizes the certainty in a body of evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ by outcome. This is a result of judgement, but the judgement process operates within a transparent structure. As an example, the certainty would be ‘high’ if the summary were of several randomized trials with low risk of bias, but the rating of certainty becomes lower if there are concerns about risk of bias, inconsistency, indirectness, imprecision or publication bias. Judgements other than of ‘high’ certainty should be made transparent using explanatory footnotes or the ‘Comments’ column in the ‘Summary of findings’ table (see Section 14.1.6.10 ).
14.1.6.9 Comments
The aim of the ‘Comments’ field is to help interpret the information or data identified in the row. For example, this may be on the validity of the outcome measure or the presence of variables that are associated with the magnitude of effect. Important caveats about the results should be flagged here. Not all rows will need comments, and it is best to leave a blank if there is nothing warranting a comment.
14.1.6.10 Explanations
Detailed explanations should be included as footnotes to support the judgements in the ‘Summary of findings’ table, such as the overall GRADE assessment. The explanations should describe the rationale for important aspects of the content. Table 14.1.a lists guidance for useful explanations. Explanations should be concise, informative, relevant, easy to understand and accurate. If explanations cannot be sufficiently described in footnotes, review authors should provide further details of the issues in the Results and Discussion sections of the review.
Table 14.1.a Guidance for providing useful explanations in ‘Summary of findings’ (SoF) tables. Adapted from Santesso et al (2016)
14.2 Assessing the certainty or quality of a body of evidence
14.2.1 the grade approach.
The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) has developed a system for grading the certainty of evidence (Schünemann et al 2003, Atkins et al 2004, Schünemann et al 2006, Guyatt et al 2008, Guyatt et al 2011a). Over 100 organizations including the World Health Organization (WHO), the American College of Physicians, the American Society of Hematology (ASH), the Canadian Agency for Drugs and Technology in Health (CADTH) and the National Institutes of Health and Clinical Excellence (NICE) in the UK have adopted the GRADE system ( www.gradeworkinggroup.org ).
Cochrane has also formally adopted this approach, and all Cochrane Reviews should use GRADE to evaluate the certainty of evidence for important outcomes (see MECIR Box 14.2.a ).
MECIR Box 14.2.a Relevant expectations for conduct of intervention reviews
For systematic reviews, the GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessing the certainty of a body of evidence involves consideration of within- and across-study risk of bias (limitations in study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, imprecision of the effect estimates and risk of publication bias (see Section 14.2.2 ), as well as domains that may increase our confidence in the effect estimate (as described in Section 14.2.3 ). The GRADE system entails an assessment of the certainty of a body of evidence for each individual outcome. Judgements about the domains that determine the certainty of evidence should be described in the results or discussion section and as part of the ‘Summary of findings’ table.
The GRADE approach specifies four levels of certainty ( Figure 14.2.a ). For interventions, including diagnostic and other tests that are evaluated as interventions (Schünemann et al 2008b, Schünemann et al 2008a, Balshem et al 2011, Schünemann et al 2012), the starting point for rating the certainty of evidence is categorized into two types:
- randomized trials; and
- non-randomized studies of interventions (NRSI), including observational studies (including but not limited to cohort studies, and case-control studies, cross-sectional studies, case series and case reports, although not all of these designs are usually included in Cochrane Reviews).
There are many instances in which review authors rely on information from NRSI, in particular to evaluate potential harms (see Chapter 24 ). In addition, review authors can obtain relevant data from both randomized trials and NRSI, with each type of evidence complementing the other (Schünemann et al 2013).
In GRADE, a body of evidence from randomized trials begins with a high-certainty rating while a body of evidence from NRSI begins with a low-certainty rating. The lower rating with NRSI is the result of the potential bias induced by the lack of randomization (i.e. confounding and selection bias).
However, when using the new Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al 2016), an assessment tool that covers the risk of bias due to lack of randomization, all studies may start as high certainty of the evidence (Schünemann et al 2018). The approach of starting all study designs (including NRSI) as high certainty does not conflict with the initial GRADE approach of starting the rating of NRSI as low certainty evidence. This is because a body of evidence from NRSI should generally be downgraded by two levels due to the inherent risk of bias associated with the lack of randomization, namely confounding and selection bias. Not downgrading NRSI from high to low certainty needs transparent and detailed justification for what mitigates concerns about confounding and selection bias (Schünemann et al 2018). Very few examples of where not rating down by two levels is appropriate currently exist.
The highest certainty rating is a body of evidence when there are no concerns in any of the GRADE factors listed in Figure 14.2.a . Review authors often downgrade evidence to moderate, low or even very low certainty evidence, depending on the presence of the five factors in Figure 14.2.a . Usually, certainty rating will fall by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one domain (e.g. when assessing risk of bias, all studies were unconcealed, unblinded and lost over 50% of their patients to follow-up), evidence may fall by two levels due to that factor alone. It is not possible to rate lower than ‘very low certainty’ evidence.
Review authors will generally grade evidence from sound non-randomized studies as low certainty, even if ROBINS-I is used. If, however, such studies yield large effects and there is no obvious bias explaining those effects, review authors may rate the evidence as moderate or – if the effect is large enough – even as high certainty ( Figure 14.2.a ). The very low certainty level is appropriate for, but is not limited to, studies with critical problems and unsystematic clinical observations (e.g. case series or case reports).
Figure 14.2.a Levels of the certainty of a body of evidence in the GRADE approach. *Upgrading criteria are usually applicable to non-randomized studies only (but exceptions exist).

14.2.2 Domains that can lead to decreasing the certainty level of a body of evidence
We now describe in more detail the five reasons (or domains) for downgrading the certainty of a body of evidence for a specific outcome. In each case, if no reason is found for downgrading the evidence, it should be classified as 'no limitation or not serious' (not important enough to warrant downgrading). If a reason is found for downgrading the evidence, it should be classified as 'serious' (downgrading the certainty rating by one level) or 'very serious' (downgrading the certainty grade by two levels). For non-randomized studies assessed with ROBINS-I, rating down by three levels should be classified as 'extremely' serious.
(1) Risk of bias or limitations in the detailed design and implementation
Our confidence in an estimate of effect decreases if studies suffer from major limitations that are likely to result in a biased assessment of the intervention effect. For randomized trials, these methodological limitations include failure to generate a random sequence, lack of allocation sequence concealment, lack of blinding (particularly with subjective outcomes that are highly susceptible to biased assessment), a large loss to follow-up or selective reporting of outcomes. Chapter 8 provides a discussion of study-level assessments of risk of bias in the context of a Cochrane Review, and proposes an approach to assessing the risk of bias for an outcome across studies as ‘Low’ risk of bias, ‘Some concerns’ and ‘High’ risk of bias for randomized trials. Levels of ‘Low’. ‘Moderate’, ‘Serious’ and ‘Critical’ risk of bias arise for non-randomized studies assessed with ROBINS-I ( Chapter 25 ). These assessments should feed directly into this GRADE domain. In particular, ‘Low’ risk of bias would indicate ‘no limitation’; ‘Some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘High’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. ‘Critical’ risk of bias on ROBINS-I would indicate extremely serious limitations in GRADE. Review authors should use their judgement to decide between alternative categories, depending on the likely magnitude of the potential biases.
Every study addressing a particular outcome will differ, to some degree, in the risk of bias. Review authors should make an overall judgement on whether the certainty of evidence for an outcome warrants downgrading on the basis of study limitations. The assessment of study limitations should apply to the studies contributing to the results in the ‘Summary of findings’ table, rather than to all studies that could potentially be included in the analysis. We have argued in Chapter 7, Section 7.6.2 , that the primary analysis should be restricted to studies at low (or low and unclear) risk of bias where possible.
Table 14.2.a presents the judgements that must be made in going from assessments of the risk of bias to judgements about study limitations for each outcome included in a ‘Summary of findings’ table. A rating of high certainty evidence can be achieved only when most evidence comes from studies that met the criteria for low risk of bias. For example, of the 22 studies addressing the impact of beta-blockers on mortality in patients with heart failure, most probably or certainly used concealed allocation of the sequence, all blinded at least some key groups and follow-up of randomized patients was almost complete (Brophy et al 2001). The certainty of evidence might be downgraded by one level when most of the evidence comes from individual studies either with a crucial limitation for one item, or with some limitations for multiple items. An example of very serious limitations, warranting downgrading by two levels, is provided by evidence on surgery versus conservative treatment in the management of patients with lumbar disc prolapse (Gibson and Waddell 2007). We are uncertain of the benefit of surgery in reducing symptoms after one year or longer, because the one study included in the analysis had inadequate concealment of the allocation sequence and the outcome was assessed using a crude rating by the surgeon without blinding.
(2) Unexplained heterogeneity or inconsistency of results
When studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. For instance, drugs may have larger relative effects in sicker populations or when given in larger doses. A detailed discussion of heterogeneity and its investigation is provided in Chapter 10, Section 10.10 and Section 10.11 . If an important modifier exists, with good evidence that important outcomes are different in different subgroups (which would ideally be pre-specified), then a separate ‘Summary of findings’ table may be considered for a separate population. For instance, a separate ‘Summary of findings’ table would be used for carotid endarterectomy in symptomatic patients with high grade stenosis (70% to 99%) in which the intervention is, in the hands of the right surgeons, beneficial, and another (if review authors considered it relevant) for asymptomatic patients with low grade stenosis (less than 30%) in which surgery appears harmful (Orrapin and Rerkasem 2017). When heterogeneity exists and affects the interpretation of results, but review authors are unable to identify a plausible explanation with the data available, the certainty of the evidence decreases.
(3) Indirectness of evidence
Two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomized trials are available, but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to indirect comparisons between A and B. Where indirect comparisons are undertaken within a network meta-analysis context, GRADE for network meta-analysis should be used (see Chapter 11, Section 11.5 ).
Second, a review may find randomized trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. For example, suppose that in a review addressing an intervention for secondary prevention of coronary heart disease, most identified studies happened to be in people who also had diabetes. Then the evidence may be regarded as indirect in relation to the broader question of interest because the population is primarily related to people with diabetes. The opposite scenario can equally apply: a review addressing the effect of a preventive strategy for coronary heart disease in people with diabetes may consider studies in people without diabetes to provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had conducted few if any randomized trials in the target population (e.g. people with diabetes). Other sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical intervention was implemented by expert, highly trained specialists in specialist centres, then evidence on the effects of the intervention outside these centres may be indirect), comparators used (e.g. if the comparator groups received an intervention that is less effective than standard treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes when data on patient-important outcomes are not available, or when investigators seek data on quality of life but only symptoms are reported). Review authors should make judgements transparent when they believe downgrading is justified, based on differences in anticipated effects in the group of primary interest. Review authors may be aided and increase transparency of their judgements about indirectness if they use Table 14.2.b available in the GRADEpro GDT software (Schünemann et al 2013).
(4) Imprecision of results
When studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. The confidence intervals included in the ‘Summary of findings’ table will provide readers with information that allows them to make, to some extent, their own rating of precision. Review authors can use a calculation of the optimal information size (OIS) or review information size (RIS), similar to sample size calculations, to make judgements about imprecision (Guyatt et al 2011b, Schünemann 2016). The OIS or RIS is calculated on the basis of the number of participants required for an adequately powered individual study. If the 95% confidence interval excludes a risk ratio (RR) of 1.0, and the total number of events or patients exceeds the OIS criterion, precision is adequate. If the 95% CI includes appreciable benefit or harm (an RR of under 0.75 or over 1.25 is often suggested as a very rough guide) downgrading for imprecision may be appropriate even if OIS criteria are met (Guyatt et al 2011b, Schünemann 2016).
(5) High probability of publication bias
The certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non-reporting bias). Selective reporting of outcomes from among multiple outcomes measured is assessed at the study level as part of the assessment of risk of bias (see Chapter 8, Section 8.7 ), so for the studies contributing to the outcome in the ‘Summary of findings’ table this is addressed by domain 1 above (limitations in the design and implementation). If a large number of studies included in the review do not contribute to an outcome, or if there is evidence of publication bias, the certainty of the evidence may be downgraded. Chapter 13 provides a detailed discussion of reporting biases, including publication bias, and how it may be tackled in a Cochrane Review. A prototypical situation that may elicit suspicion of publication bias is when published evidence includes a number of small studies, all of which are industry-funded (Bhandari et al 2004). For example, 14 studies of flavanoids in patients with haemorrhoids have shown apparent large benefits, but enrolled a total of only 1432 patients (i.e. each study enrolled relatively few patients) (Alonso-Coello et al 2006). The heavy involvement of sponsors in most of these studies raises questions of whether unpublished studies that suggest no benefit exist (publication bias).
A particular body of evidence can suffer from problems associated with more than one of the five factors listed here, and the greater the problems, the lower the certainty of evidence rating that should result. One could imagine a situation in which randomized trials were available, but all or virtually all of these limitations would be present, and in serious form. A very low certainty of evidence rating would result.
Table 14.2.a Further guidelines for domain 1 (of 5) in a GRADE assessment: going from assessments of risk of bias in studies to judgements about study limitations for main outcomes across studies
Table 14.2.b Judgements about indirectness by outcome (available in GRADEpro GDT)
Intervention:
Comparator:
Direct comparison:
Final judgement about indirectness across domains:
14.2.3 Domains that may lead to increasing the certainty level of a body of evidence
Although NRSI and downgraded randomized trials will generally yield a low rating for certainty of evidence, there will be unusual circumstances in which review authors could ‘upgrade’ such evidence to moderate or even high certainty ( Table 14.3.a ).
- Large effects On rare occasions when methodologically well-done observational studies yield large, consistent and precise estimates of the magnitude of an intervention effect, one may be particularly confident in the results. A large estimated effect (e.g. RR >2 or RR <0.5) in the absence of plausible confounders, or a very large effect (e.g. RR >5 or RR <0.2) in studies with no major threats to validity, might qualify for this. In these situations, while the NRSI may possibly have provided an over-estimate of the true effect, the weak study design may not explain all of the apparent observed benefit. Thus, despite reservations based on the observational study design, review authors are confident that the effect exists. The magnitude of the effect in these studies may move the assigned certainty of evidence from low to moderate (if the effect is large in the absence of other methodological limitations). For example, a meta-analysis of observational studies showed that bicycle helmets reduce the risk of head injuries in cyclists by a large margin (odds ratio (OR) 0.31, 95% CI 0.26 to 0.37) (Thompson et al 2000). This large effect, in the absence of obvious bias that could create the association, suggests a rating of moderate-certainty evidence. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. However, if the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0, then some hesitation would be appropriate in the decision to rate up for a large effect. Another situation allows inference of a strong association without a formal comparative study. Consider the question of the impact of routine colonoscopy versus no screening for colon cancer on the rate of perforation associated with colonoscopy. Here, a large series of representative patients undergoing colonoscopy may provide high certainty evidence about the risk of perforation associated with colonoscopy. When the risk of the event among patients receiving the relevant comparator is known to be near 0 (i.e. we are certain that the incidence of spontaneous colon perforation in patients not undergoing colonoscopy is extremely low), case series or cohort studies of representative patients can provide high certainty evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events.
- Dose-response The presence of a dose-response gradient may increase our confidence in the findings of observational studies and thereby enhance the assigned certainty of evidence. For example, our confidence in the result of observational studies that show an increased risk of bleeding in patients who have supratherapeutic anticoagulation levels is increased by the observation that there is a dose-response gradient between the length of time needed for blood to clot (as measured by the international normalized ratio (INR)) and an increased risk of bleeding (Levine et al 2004). A systematic review of NRSI investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate (RR) with rofecoxib was 1.33 (95% CI 1.00 to 1.79) with doses less than 25mg/d, and 2.19 (95% CI 1.64 to 2.91) with doses more than 25mg/d. Although residual confounding is likely to exist in the NRSI that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect
- Plausible confounding On occasion, all plausible biases from randomized or non-randomized studies may be working to under-estimate an apparent intervention effect. For example, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is larger than the data suggest. For instance, a rigorous systematic review of observational studies including a total of 38 million patients demonstrated higher death rates in private for-profit versus private not-for-profit hospitals (Devereaux et al 2002). One possible bias relates to different disease severity in patients in the two hospital types. It is likely, however, that patients in the not-for-profit hospitals were sicker than those in the for-profit hospitals. Thus, to the extent that residual confounding existed, it would bias results against the not-for-profit hospitals. The second likely bias was the possibility that higher numbers of patients with excellent private insurance coverage could lead to a hospital having more resources and a spill-over effect that would benefit those without such coverage. Since for-profit hospitals are likely to admit a larger proportion of such well-insured patients than not-for-profit hospitals, the bias is once again against the not-for-profit hospitals. Since the plausible biases would all diminish the demonstrated intervention effect, one might consider the evidence from these observational studies as moderate rather than low certainty. A parallel situation exists when observational studies have failed to demonstrate an association, but all plausible biases would have increased an intervention effect. This situation will usually arise in the exploration of apparent harmful effects. For example, because the hypoglycaemic drug phenformin causes lactic acidosis, the related agent metformin was under suspicion for the same toxicity. Nevertheless, very large observational studies have failed to demonstrate an association (Salpeter et al 2007). Given the likelihood that clinicians would be more alert to lactic acidosis in the presence of the agent and over-report its occurrence, one might consider this moderate, or even high certainty, evidence refuting a causal relationship between typical therapeutic doses of metformin and lactic acidosis.
14.3 Describing the assessment of the certainty of a body of evidence using the GRADE framework
Review authors should report the grading of the certainty of evidence in the Results section for each outcome for which this has been performed, providing the rationale for downgrading or upgrading the evidence, and referring to the ‘Summary of findings’ table where applicable.
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the ‘Summary of Findings’ table (see Section 14.1.6.10 ).
Chapter 15, Section 15.6 , describes in more detail how the overall GRADE assessment across all domains can be used to draw conclusions about the effects of the intervention, as well as providing implications for future research.
Table 14.3.a Framework for describing the certainty of evidence and justifying downgrading or upgrading
14.4 Chapter information
Authors: Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Acknowledgements: Andrew D Oxman contributed to earlier versions. Professor Penny Hawe contributed to the text on adverse effects in earlier versions. Jon Deeks provided helpful contributions on an earlier version of this chapter. For details of previous authors and editors of the Handbook , please refer to the Preface.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health.
14.5 References
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF, Guyatt G. Meta-analysis of flavonoids for the treatment of haemorrhoids. British Journal of Surgery 2006; 93 : 909-920.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011; 64 : 401-406.
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004; 170 : 477-480.
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Annals of Internal Medicine 2001; 134 : 550-560.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P, Meerpohl JJ, Vandvik PO, Brozek JL, Akl EA, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Garner P, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. Journal of Clinical Epidemiology 2016; 74 : 7-18.
Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context . 2nd ed. London (UK): BMJ Publication Group; 2001. p. 313-335.
Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schünemann HJ, Haines T, Lavis JN, Grant BJ, Haslam DR, Bhandari M, Sullivan T, Cook DJ, Walter SD, Meade M, Khan H, Bhatnagar N, Guyatt GH. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. Canadian Medical Association Journal 2002; 166 : 1399-1406.
Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Statistics in Medicine 2000; 19 : 1707-1728.
Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002; 31 : 72-76.
Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine 2007; 32 : 1735-1747.
Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann H. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 3.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011b; 64 : 1283-1293.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schünemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350 : h870.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, Heus P, Lasserson T, Moustgaard R, Brozek J, Schünemann HJ. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. Journal of Clinical Epidemiology 2016; 74 : 19-27.
Levine MN, Raskob G, Landefeld S, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 : 287S-310S.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017; 6 : CD001081.
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007; 4 : CD002967.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Best D, Vist G, Oxman AD, Group GW. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Canadian Medical Association Journal 2003; 169 : 677-680.
Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, Manthous CA, Maurer JR, McNicholas WT, Oxman AD, Rubenfeld G, Turino GM, Guyatt G. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006; 174 : 605-614.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr., Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008a; 336 : 1106-1110.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP Journal Club 2008b; 149 : 2.
Schünemann HJ, Mustafa R, Brozek J. [Diagnostic accuracy and linked evidence--testing the chain]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2012; 106 : 153-160.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2018.
Spencer-Bonilla G, Quinones AR, Montori VM, International Minimally Disruptive Medicine W. Assessing the Burden of Treatment. Journal of General Internal Medicine 2017; 32 : 1141-1145.
Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012; 345 : e7401.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355 : i4919.
Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database of Systematic Reviews 2000; 2 : CD001855.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8 .
van Dalen EC, Tierney JF, Kremer LCM. Tips and tricks for understanding and using SR results. No. 7: time‐to‐event data. Evidence-Based Child Health 2007; 2 : 1089-1090.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Write the Results/Findings Section in Research
What is the research paper Results section and what does it do?
The Results section of a scientific research paper represents the core findings of a study derived from the methods applied to gather and analyze information. It presents these findings in a logical sequence without bias or interpretation from the author, setting up the reader for later interpretation and evaluation in the Discussion section. A major purpose of the Results section is to break down the data into sentences that show its significance to the research question(s).
The Results section appears third in the section sequence in most scientific papers. It follows the presentation of the Methods and Materials and is presented before the Discussion section —although the Results and Discussion are presented together in many journals. This section answers the basic question “What did you find in your research?”
What is included in the Results section?
The Results section should include the findings of your study and ONLY the findings of your study. The findings include:
- Data presented in tables, charts, graphs, and other figures (may be placed into the text or on separate pages at the end of the manuscript)
- A contextual analysis of this data explaining its meaning in sentence form
- All data that corresponds to the central research question(s)
- All secondary findings (secondary outcomes, subgroup analyses, etc.)
If the scope of the study is broad, or if you studied a variety of variables, or if the methodology used yields a wide range of different results, the author should present only those results that are most relevant to the research question stated in the Introduction section .
As a general rule, any information that does not present the direct findings or outcome of the study should be left out of this section. Unless the journal requests that authors combine the Results and Discussion sections, explanations and interpretations should be omitted from the Results.
How are the results organized?
The best way to organize your Results section is “logically.” One logical and clear method of organizing research results is to provide them alongside the research questions—within each research question, present the type of data that addresses that research question.
Let’s look at an example. Your research question is based on a survey among patients who were treated at a hospital and received postoperative care. Let’s say your first research question is:
“What do hospital patients over age 55 think about postoperative care?”
This can actually be represented as a heading within your Results section, though it might be presented as a statement rather than a question:
Attitudes towards postoperative care in patients over the age of 55
Now present the results that address this specific research question first. In this case, perhaps a table illustrating data from a survey. Likert items can be included in this example. Tables can also present standard deviations, probabilities, correlation matrices, etc.
Following this, present a content analysis, in words, of one end of the spectrum of the survey or data table. In our example case, start with the POSITIVE survey responses regarding postoperative care, using descriptive phrases. For example:
“Sixty-five percent of patients over 55 responded positively to the question “ Are you satisfied with your hospital’s postoperative care ?” (Fig. 2)
Include other results such as subcategory analyses. The amount of textual description used will depend on how much interpretation of tables and figures is necessary and how many examples the reader needs in order to understand the significance of your research findings.
Next, present a content analysis of another part of the spectrum of the same research question, perhaps the NEGATIVE or NEUTRAL responses to the survey. For instance:
“As Figure 1 shows, 15 out of 60 patients in Group A responded negatively to Question 2.”
After you have assessed the data in one figure and explained it sufficiently, move on to your next research question. For example:
“How does patient satisfaction correspond to in-hospital improvements made to postoperative care?”
This kind of data may be presented through a figure or set of figures (for instance, a paired T-test table).
Explain the data you present, here in a table, with a concise content analysis:
“The p-value for the comparison between the before and after groups of patients was .03% (Fig. 2), indicating that the greater the dissatisfaction among patients, the more frequent the improvements that were made to postoperative care.”
Let’s examine another example of a Results section from a study on plant tolerance to heavy metal stress . In the Introduction section, the aims of the study are presented as “determining the physiological and morphological responses of Allium cepa L. towards increased cadmium toxicity” and “evaluating its potential to accumulate the metal and its associated environmental consequences.” The Results section presents data showing how these aims are achieved in tables alongside a content analysis, beginning with an overview of the findings:
“Cadmium caused inhibition of root and leave elongation, with increasing effects at higher exposure doses (Fig. 1a-c).”
The figure containing this data is cited in parentheses. Note that this author has combined three graphs into one single figure. Separating the data into separate graphs focusing on specific aspects makes it easier for the reader to assess the findings, and consolidating this information into one figure saves space and makes it easy to locate the most relevant results.
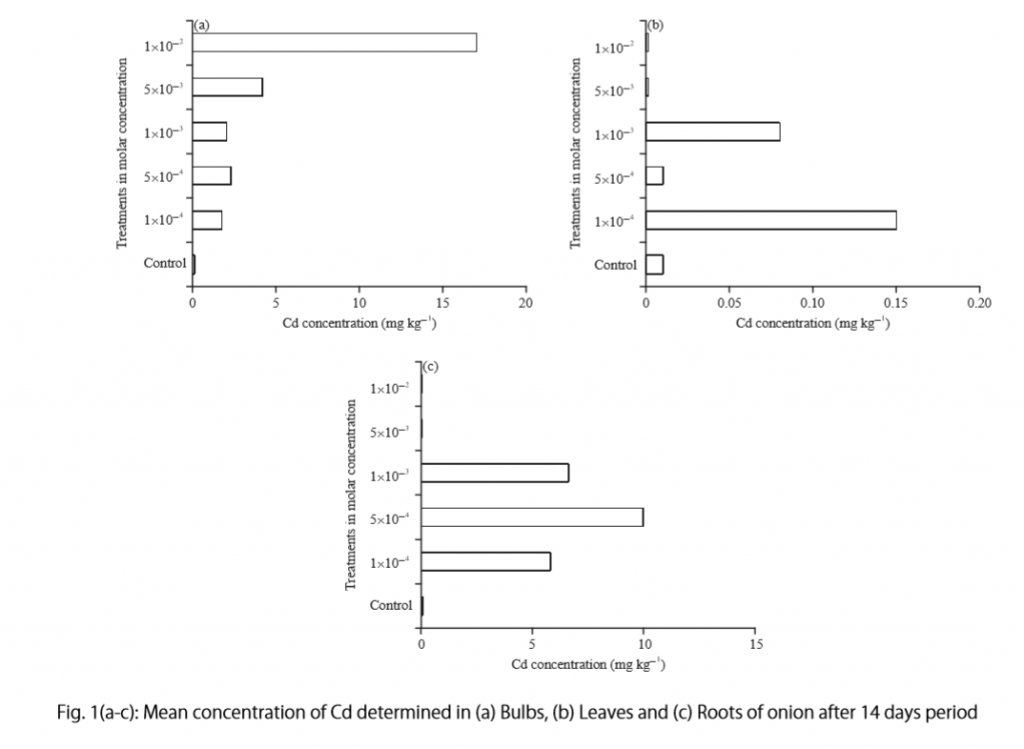
Following this overall summary, the relevant data in the tables is broken down into greater detail in text form in the Results section.
- “Results on the bio-accumulation of cadmium were found to be the highest (17.5 mg kgG1) in the bulb, when the concentration of cadmium in the solution was 1×10G2 M and lowest (0.11 mg kgG1) in the leaves when the concentration was 1×10G3 M.”
Captioning and Referencing Tables and Figures
Tables and figures are central components of your Results section and you need to carefully think about the most effective way to use graphs and tables to present your findings . Therefore, it is crucial to know how to write strong figure captions and to refer to them within the text of the Results section.
The most important advice one can give here as well as throughout the paper is to check the requirements and standards of the journal to which you are submitting your work. Every journal has its own design and layout standards, which you can find in the author instructions on the target journal’s website. Perusing a journal’s published articles will also give you an idea of the proper number, size, and complexity of your figures.
Regardless of which format you use, the figures should be placed in the order they are referenced in the Results section and be as clear and easy to understand as possible. If there are multiple variables being considered (within one or more research questions), it can be a good idea to split these up into separate figures. Subsequently, these can be referenced and analyzed under separate headings and paragraphs in the text.
To create a caption, consider the research question being asked and change it into a phrase. For instance, if one question is “Which color did participants choose?”, the caption might be “Color choice by participant group.” Or in our last research paper example, where the question was “What is the concentration of cadmium in different parts of the onion after 14 days?” the caption reads:
“Fig. 1(a-c): Mean concentration of Cd determined in (a) bulbs, (b) leaves, and (c) roots of onions after a 14-day period.”
Steps for Composing the Results Section
Because each study is unique, there is no one-size-fits-all approach when it comes to designing a strategy for structuring and writing the section of a research paper where findings are presented. The content and layout of this section will be determined by the specific area of research, the design of the study and its particular methodologies, and the guidelines of the target journal and its editors. However, the following steps can be used to compose the results of most scientific research studies and are essential for researchers who are new to preparing a manuscript for publication or who need a reminder of how to construct the Results section.
Step 1 : Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study.
- The guidelines will generally outline specific requirements for the results or findings section, and the published articles will provide sound examples of successful approaches.
- Note length limitations on restrictions on content. For instance, while many journals require the Results and Discussion sections to be separate, others do not—qualitative research papers often include results and interpretations in the same section (“Results and Discussion”).
- Reading the aims and scope in the journal’s “ guide for authors ” section and understanding the interests of its readers will be invaluable in preparing to write the Results section.
Step 2 : Consider your research results in relation to the journal’s requirements and catalogue your results.
- Focus on experimental results and other findings that are especially relevant to your research questions and objectives and include them even if they are unexpected or do not support your ideas and hypotheses.
- Catalogue your findings—use subheadings to streamline and clarify your report. This will help you avoid excessive and peripheral details as you write and also help your reader understand and remember your findings. Create appendices that might interest specialists but prove too long or distracting for other readers.
- Decide how you will structure of your results. You might match the order of the research questions and hypotheses to your results, or you could arrange them according to the order presented in the Methods section. A chronological order or even a hierarchy of importance or meaningful grouping of main themes or categories might prove effective. Consider your audience, evidence, and most importantly, the objectives of your research when choosing a structure for presenting your findings.
Step 3 : Design figures and tables to present and illustrate your data.
- Tables and figures should be numbered according to the order in which they are mentioned in the main text of the paper.
- Information in figures should be relatively self-explanatory (with the aid of captions), and their design should include all definitions and other information necessary for readers to understand the findings without reading all of the text.
- Use tables and figures as a focal point to tell a clear and informative story about your research and avoid repeating information. But remember that while figures clarify and enhance the text, they cannot replace it.
Step 4 : Draft your Results section using the findings and figures you have organized.
- The goal is to communicate this complex information as clearly and precisely as possible; precise and compact phrases and sentences are most effective.
- In the opening paragraph of this section, restate your research questions or aims to focus the reader’s attention to what the results are trying to show. It is also a good idea to summarize key findings at the end of this section to create a logical transition to the interpretation and discussion that follows.
- Try to write in the past tense and the active voice to relay the findings since the research has already been done and the agent is usually clear. This will ensure that your explanations are also clear and logical.
- Make sure that any specialized terminology or abbreviation you have used here has been defined and clarified in the Introduction section .
Step 5 : Review your draft; edit and revise until it reports results exactly as you would like to have them reported to your readers.
- Double-check the accuracy and consistency of all the data, as well as all of the visual elements included.
- Read your draft aloud to catch language errors (grammar, spelling, and mechanics), awkward phrases, and missing transitions.
- Ensure that your results are presented in the best order to focus on objectives and prepare readers for interpretations, valuations, and recommendations in the Discussion section . Look back over the paper’s Introduction and background while anticipating the Discussion and Conclusion sections to ensure that the presentation of your results is consistent and effective.
- Consider seeking additional guidance on your paper. Find additional readers to look over your Results section and see if it can be improved in any way. Peers, professors, or qualified experts can provide valuable insights.
One excellent option is to use a professional English proofreading and editing service such as Wordvice, including our paper editing service . With hundreds of qualified editors from dozens of scientific fields, Wordvice has helped thousands of authors revise their manuscripts and get accepted into their target journals. Read more about the proofreading and editing process before proceeding with getting academic editing services and manuscript editing services for your manuscript.
As the representation of your study’s data output, the Results section presents the core information in your research paper. By writing with clarity and conciseness and by highlighting and explaining the crucial findings of their study, authors increase the impact and effectiveness of their research manuscripts.
For more articles and videos on writing your research manuscript, visit Wordvice’s Resources page.
Wordvice Resources
- How to Write a Research Paper Introduction
- Which Verb Tenses to Use in a Research Paper
- How to Write an Abstract for a Research Paper
- How to Write a Research Paper Title
- Useful Phrases for Academic Writing
- Common Transition Terms in Academic Papers
- Active and Passive Voice in Research Papers
- 100+ Verbs That Will Make Your Research Writing Amazing
- Tips for Paraphrasing in Research Papers
Bulk Content Generator
Brand Voice
AI Text Editor
Research Summary Generator
Craft a detailed research synopsis utilizing the given data for an all-encompassing understanding.
Try Research Summary for free →
Research Summary
Learn how to provide the key points, main findings, and any other relevant information for the research you want to summarize
1 variation
What is a Research Summary?
Have you ever found yourself drowning in a sea of research articles, struggling to make sense of it all? Well, fear not! A research summary is here to save the day. But what exactly is a research summary, and how can it help you navigate the vast ocean of information?
A research summary is a concise and informative overview of a research article, report, or thesis. It aims to provide the reader with a clear understanding of the study's purpose, methods, results, and conclusions without having to read the entire document. Think of it as a mini-version of the original work that highlights its most important aspects.
Now that we know what a research summary is let's dive into why they're so beneficial.
The Benefits of Research Summaries
Research summaries offer several advantages for both readers and writers:
Time-saving : Reading a well-written research summary can save you hours of sifting through dense academic papers. It allows you to quickly grasp the key points and decide if you want to explore the full document further.
Improved comprehension : By breaking down complex ideas into digestible chunks, research summaries make it easier for readers to understand the material. This is particularly helpful for those who are new to a topic or have limited knowledge in the field.
Enhanced communication : Research summaries enable researchers to share their findings with a wider audience, including non-experts and industry professionals. This can lead to increased collaboration and knowledge exchange across disciplines.
Better decision-making : For professionals who rely on evidence-based practices, research summaries provide an accessible way to stay informed about the latest developments in their field. This enables them to make better decisions based on up-to-date information.
With these benefits in mind, let's explore some tips for writing an effective research summary.
Tips for Writing a Great Research Summary
Creating an engaging and informative research summary doesn't have to be a daunting task. Here are some tips to help you craft the perfect summary:
Know your audience : Consider who will be reading your summary and tailor your language and content accordingly. If you're writing for a general audience, avoid jargon and technical terms. If your readers are experts in the field, focus on the most relevant and novel aspects of the research.
Be concise : A research summary should be brief yet informative. Aim to capture the essence of the study without getting bogged down in unnecessary details.
Use clear language : Write in simple, straightforward sentences that are easy to understand. Avoid flowery language or complex sentence structures that may confuse readers.
Highlight key points : Focus on the main elements of the study, such as its purpose, methods, results, and conclusions. Make sure these points stand out by using headings, bullet points, or bold text.
Stay objective : Present the information in a neutral tone and avoid expressing personal opinions or biases. Stick to the facts and let your readers draw their own conclusions.
Proofread : Before submitting your research summary, take the time to proofread it carefully for grammar, spelling, and punctuation errors. A polished summary will make a better impression on your readers.
Generate the Perfect Research Summary with Our Research Summary Generator
Now that we've covered what a research summary is, its benefits, and tips for writing one – wouldn't it be great if there was a tool that could generate a perfect research summary every single time? Well, guess what? There is!
With our Research Summary Generator, you can create an engaging and informative summary in just a few clicks. Say goodbye to hours spent poring over dense academic papers and hello to quick, easy-to-understand summaries tailored to your needs.
Give it a try today and see how our Research Summary Generator can revolutionize your research process!
Example outputs
This Research Summary Generator saves you time and effort by summarizing your research findings in a clear and concise manner, allowing you to easily communicate your results to others.
The Effects of Exercise on Mental Health
A study was conducted to investigate the effects of exercise on mental health. Participants were randomly assigned to either an exercise group or a control group. The exercise group engaged in moderate-intensity aerobic exercise for 30 minutes, three times per week for eight weeks. The results showed that the exercise group had significantly lower levels of depression and anxiety compared to the control group.
Keywords: exercise, mental health, depression, anxiety
The impact of social media on body image.
This study aimed to examine the impact of social media on body image. A sample of young adults completed surveys assessing their use of social media and their perceptions of their own body image. Results indicated that individuals who spent more time on social media reported greater dissatisfaction with their bodies. Additionally, exposure to images of thin and fit individuals on social media was associated with increased body dissatisfaction.
Keywords: social media, body image, young adults, body dissatisfaction
The benefits of meditation for stress reduction.
This meta-analysis aimed to evaluate the effectiveness of meditation for stress reduction. A total of 18 randomized controlled trials were included in the analysis. Results showed that meditation was effective in reducing perceived stress, with larger effect sizes observed for mindfulness-based interventions. Furthermore, the benefits of meditation appeared to be maintained over time.
Keywords: meditation, stress reduction, mindfulness, meta-analysis
What other amazing things can this template help you create.
✔ Meta Title
✔ Meta Description
✔ Extract keywords
✔ Feature Image
✔ Soon Internal Linking
Who needs Research Summary Generator?
Researchers
Graduate students
Business professionals
Frequently asked questions
- How does the Research Summary Generator work? Simply input your research findings into the generator, and it will automatically summarize them in a clear and concise manner.
- Can I customize the generated summary? Yes, you can edit the summary as needed to ensure it accurately reflects your research findings.
- Is the Research Summary Generator free to use? Yes, the generator is completely free to use with no limitations.
- +254728776317
- info@masomomsingi.com
Masomo Msingi Publishers Mobile App

MASOMO MSINGI PUBLISHERS
KASNEB|KNEC|KISM
SUMMARY OF FINDINGS, CONCLUSIONS AND RECOMMENDATIONS
This chapter summarizes the whole research process. It first provides a brief summary of the whole study with particular reference to the research problem, research methodology, results, the main contributions of the research and recommendations for future work. It provides a summary of the main findings of the study, conclusions and recommendations. This chapter should be reasonably short.
The readers would want to know whether the objectives of the study were achieved, and whether the work has contributed to knowledge. Therefore, when compiling this chapter, a researcher should focus on answering these questions.
Any conclusions drawn should be those resulting from the study. A researcher should make relevant references to chapters that support the listed findings and may also refer to the work of others for comparison. However, one should not discuss the stu1y’s results here.
Summary of the Main Findings
In summarizing, a researcher should identify the findings of the study and discuss them briefly. In addition, the methodological problems encountered should be outlined so that future/other researchers may take the relevant precautions. The researcher should clearly pinpoint if the study objectives were achieved or not. An effective summary has the following qualities:
- It bases on results from the study.
- It is brief, all statements are concise, and pinpoint to the contributions that the researcher has made.
Recommendations
- All statements are factual.
One way to present the summary is to use one paragraph for each idea. Alternatively, the researcher can use a point-by-point format.
The Conclusion section should be very brief, about half a page. It should indicate what the study results reaffirm. It should also briefly discuss some of the strategies highlighted by the respondents. In this section, the researcher should clearly state how the study has contributed to knowledge.
The recommendations section is important in research. This section often exposes further problems and introduces more questions. As a researcher, there is a time limit to the research project, so it is unlikely that the study would have solved all the problems associated with the area of study. The researcher is therefore expected to make suggestions about how his/her work can be improved, and also based on the study findings, point out whether there are areas that deserve further investigation. This section will indicate whether a researcher has a firm appreciation of his/her work, and whether he/ she has given sufficient thought to its implications, not only within the narrow confines of the research topic but to related fields. This section reflects the researcher’s foresightedness and creativity.
Written by MJ
How to Summarize Research Papers and articles using AI?
Shaurya Bedi
Research papers can be difficult due to their inherent complexity and technical nature. The volume of technical terms and jargon may be a barrier, making it more difficult for readers to understand the content.
Sometimes, you must feel overwhelmed, not knowing everything!

Furthermore, research papers usually delve into intricate theories, models, and statistical analyses, necessitating a solid foundational knowledge of the field to guarantee appropriate comprehension. What complicates the problem further is the size of the research papers and the need to assess the supplied data critically.
Understanding the insights from a research paper doesn't have to feel like deciphering a cryptic code.
Thanks to the strides in artificial intelligence, distilling the essence of a research article has become remarkably straightforward.
Whether you're a student crafting your paper, an individual eager to unravel the depths of a lengthy article, or someone intrigued by a complex subject, using AI study assistants to summarize article is a good idea and offers a simplified solution to grasp the core ideas without going through intricate scientific jargon.
Let's dive in to see how to summarize research article.

Overview of AI for research papers and articles
AI tools are like competent helpers for researchers. They're trained to understand language and do things like humans. AI makes things easier in literature reviews by processing information, finding important studies, and making quick summaries.
It's a significant change, helping researchers read, analyze, and compare articles effortlessly. Using language skills, AI simultaneously spots vital ideas and findings from many articles, saving researchers time and effort. It's like a super helper for understanding lots of information in literature.

In simple terms, old summarizing methods can make mistakes, like getting things wrong or leaving out important stuff. But with the AI research paper summarizer, using super-intelligent algorithms, it can find and pull out the main points from papers without making mistakes.
This makes sure the AI summarizer research paper is right on the money, making it an excellent tool for getting research paper summaries spot-on.
Benefits of Summarizing Research Papers and Articles Using AI
Using AI to summarize research papers and articles is a game-changer. It saves time, makes things easier to understand, and helps students and professionals quickly get the important stuff. Now, let's see how.

Time saved in information processing
Reading tons of articles the old-fashioned way takes forever! Researchers spend weeks or months sorting through articles, reading them, and picking out the important stuff. It's a big job, especially with lots of studies.
But guess what? AI makes it way faster and easier; with excellent tools and intelligent algorithms, AI can zip through many research articles and give short summaries. That means less time slogging through details and more time for researchers to dig into meaningful sections.
Better understanding and retention
Keeping up with tons of new research is challenging. There's so much out there; staying on top of it all is hard.
But guess what? AI is here to help! With smart tools, it can check out new sections for you. That way, researchers can always be in the loop with the latest and greatest in their field. Thanks to article summarizer AI, keeping your brains filled with the newest information is more accessible.
Simplified information retrieval
AI gets the tricks in understanding language and learning from machines. It snags info from many places, organizes it, and finds the perfect answers for you, even if your search could be clearer.
Personalized and precise summaries
Incorporating AI tools into literature reviews decreases the likelihood of human errors in traditional methods or manual summarization. By doing so, these AI tools enhance the efficiency and accuracy of literature reviews, enabling researchers to access pertinent information while minimizing errors swiftly.
Comprehensive content insights
AI takes the heavy lifting out of content analysis. Instead of spending hours dissecting articles and papers, researchers can rely on AI to swiftly analyze and provide comprehensive insights. It digs into the details, identifies key themes, and helps researchers grasp the complete picture without exhaustive manual effort.
Streamlined decision-making
For research, quick decision-making is crucial. AI-powered summarization accelerates this process by distilling complex information into concise summaries. Researchers can make informed decisions faster, whether it's about the relevance of a paper, the direction of their own research, or the incorporation of new findings into their work.
Language and context adaptability
Different fields have their own jargon and nuances, and navigating and understanding diverse research papers is challenging. AI comes to the rescue with its ability to adapt to various languages and contexts. It breaks down barriers by providing summaries catering to the researcher's specific language and context, ensuring seamless information integration across disciplines.
Steps to Summarize Research Papers and Articles Using AI
Here are some steps you need to know if you are wondering how to summarize a research article using AI:

Selecting the Right AI-Powered Tool or Software
Choosing the right AI tool is the first step to efficient summarization. Researchers should explore and select a tool that aligns with their specific needs and the nature of their research. For example, tools like SummarizeBot or IBM Watson can be tailored to different domains, ensuring a customized and effective summarization process.
Inputting and processing text or documents
Once you select the tool, the next step involves feeding it with the relevant text or documents. Researchers can upload the research papers or articles they want to summarize.
This input is then processed through the AI algorithms, which work their magic to extract critical information and generate concise summaries. It's a straightforward process that significantly reduces the manual effort required for traditional document analysis.
Adjusting settings and preferences
AI to summarize article often comes with customizable settings to tailor the summarization output. Researchers can adjust preferences based on factors such as the desired summary length or the level of detail required.
For instance, settings can be adjusted to generate shorter summaries if a quick overview is needed. This flexibility allows researchers to fine-tune the summarization process according to their preferences, enhancing the tool's adaptability to individual needs.
Generating and reviewing summaries
The final step involves generating and reviewing the AI-generated summaries. Researchers receive concise overviews of the key points from the inputted documents. It's a time-saving approach that lets them quickly grasp the core ideas without delving into the entire content.
After receiving the summaries, researchers can review and ensure the accuracy and relevance of the information. This step ensures that the AI-generated summaries align with their research goals and objectives.
Why Outsourcing AI-Driven Summarization to a Virtual Assistant is Beneficial
Virtual assistants possess expertise in utilizing various AI tools, ensuring optimal selection and configuration for effective summarization.

Let's see why it is beneficial:
Expertise in AI tools and applications
Virtual assistants are well-versed in utilizing a variety of AI tools and applications. Their expertise ensures optimal selection and configuration of tools, aligning with the specific requirements of the research.
For example, a virtual assistant proficient in tools like OpenAI's GPT-3 can deliver advanced summarization capabilities, enhancing the overall quality of the process.
Quality control and accuracy
Virtual assistants bring a human touch to AI-driven summarization by overseeing quality control and accuracy. They can review and refine the AI-generated summaries, ensuring they meet the desired standards. This extra layer of human scrutiny enhances the reliability of the summarized content, addressing any potential inaccuracies that may arise during the automated process.
Handling large volumes of information
Dealing with a large volume of research papers can overwhelm individual researchers. Virtual assistants excel in managing such volumes efficiently. Their ability to process information at scale ensures that researchers can obtain summaries for numerous documents without compromising accuracy or speed.
Customization and personalization
Virtual assistants offer a personalized touch to the AI to summarize article process. They can adapt to researchers' preferences, adjusting settings and tailoring summaries to meet specific criteria.
This customization ensures that the summarization output aligns with the unique needs and goals of the researcher, providing a tailored and efficient solution.
Focus on core research goals
Outsourcing summarization allows researchers to concentrate on their core research goals and activities, enhancing overall productivity and output.
Other AI-Related Tasks a Virtual Assistant Can Help You With
Virtual assistants are versatile and can assist with various AI-related tasks, including the following:
Data Analysis and Interpretation
Virtual assistants adept in data analytics can assist researchers in interpreting complex datasets. They streamline the analysis process, extracting meaningful insights and trends.
For example, a virtual assistant skilled in tools like Tableau or Python can facilitate data-driven decision-making, enhancing the researcher's understanding of their findings.
AI Model Training and Optimization
Proficient in AI model training, virtual assistants contribute to refining and optimizing models for specific research needs. They can work with frameworks like TensorFlow or PyTorch, ensuring that the AI models align with the desired outcomes. This collaborative effort enhances the accuracy and performance of AI applications within the research domain.
Travel Planning
Beyond technical tasks, virtual assistants excel in practical aspects like travel planning. When you use AI algorithms, they can identify optimal travel routes, accommodations, and schedules. This capability is particularly beneficial for researchers attending conferences or fieldwork, ensuring seamless and efficient travel logistics.
Proofreading
Ensuring the quality of written content is crucial in research. Virtual assistants equipped with language processing capabilities can aid in proofreading, identifying grammatical errors, and enhancing the clarity of research papers. This attention to detail contributes to the overall professionalism and impact of the research output.
Content and Marketing Strategy
Virtual assistants with knowledge of AI in content creation and marketing can assist in developing strategies. They can analyze trends, identify target audiences, and recommend content optimization techniques.
For example, a virtual assistant familiar with SEO principles can enhance the visibility of research outputs, contributing to a broader impact.
Why is Wishup the best place to hire a Virtual Assistant?
Choosing the right place for hiring a virtual assistant is crucial for optimal support.

Wishup stands out as the premier choice for several compelling reasons.
We hire only the top 0.1% of applicants
Wishup's stringent selection process ensures that only the top 1% of applicants are hired as virtual assistants. This commitment to excellence guarantees that clients receive assistance from highly skilled and competent professionals.
Onboarding in 24 hours
Time is of the essence, and Wishup recognizes this. With a swift onboarding process, clients can have a virtual assistant ready to support them within just 24 hours of initiating the hiring process.
Choose from US-based or Indian VAs
Wishup offers the flexibility to choose virtual assistants based on preferences, whether clients prefer US-based or Indian professionals. This versatility caters to diverse needs and ensures a seamless collaboration experience.
Pre-trained and upskilled professionals
Wishup's virtual assistants come pre-trained and continually upskilled to stay abreast of the latest trends and technologies. This dedication to ongoing professional development ensures that clients benefit from the most proficient and updated virtual assistant support.
Dedicated Account Manager at your service
Clients enjoy personalized assistance with a dedicated Account Manager from Wishup. This point of contact ensures effective communication, understanding of client needs, and seamless coordination for a more tailored and responsive virtual assistant experience.
Instant replacement policy
Wishup prioritizes client satisfaction with its instant replacement policy. In case of any concerns or preferences for change, clients can swiftly access a replacement, ensuring uninterrupted support and a hassle-free engagement.

Conclusion
In conclusion, AI summarization tools revolutionize academic research by simplifying the literature review. Researchers benefit from staying current with field advancements and making well-informed decisions.
Delegating the task to a Virtual Assistant enhances efficiency and precision, primarily through platforms like Wishup. The advantages are clear: time-saving, better understanding, and personalized summaries.
Ready to experience these benefits? Take the next step by scheduling a free consultation or emailing [email protected] . Elevate your research game with AI and virtual assistance – your academic journey just got much smoother!
AI to summarize article: Frequently Asked Questions
Can i use ai to summarize articles.
Yes, AI excels at summarizing articles, offering a quick and efficient way to distill key information from lengthy content.
Can ChatGPT summarize articles?
Yes, ChatGPT, powered by OpenAI, is adept at summarizing articles. Its language understanding capabilities make it a valuable tool for concise content extraction.
Can OpenAI summarize an article?
Yes, indeed. OpenAI provides powerful models like GPT-3, which can effectively summarize articles, offering a comprehensive overview of the content.
Which AI is good for summarizing?
Several AI models, including GPT-3, BERT, and SummarizeBot, excel in summarization. The choice relies on your specific requirements, such as language nuances, length preferences, and application context.
Request consultation has been made.
Virtual Assistants | Software Testers | Bookkeepers
* Valid for new signups only
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Broad Public Support for Legal Abortion Persists 2 Years After Dobbs
By more than 2 to 1, americans say medication abortion should be legal, table of contents.
- Other abortion attitudes
- Overall attitudes about abortion
- Americans’ views on medication abortion in their states
- How statements about abortion resonate with Americans
- Acknowledgments
- The American Trends Panel survey methodology
Pew Research Center conducted this study to understand Americans’ views on the legality of abortion, as well as their perceptions of abortion access. For this analysis, we surveyed 8,709 adults from April 8 to 14, 2024. Everyone who took part in this survey is a member of the Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology .
Here are the questions used for the report and its methodology .
Nearly two years after the Supreme Court overturned the 1973 Roe v. Wade decision guaranteeing a national right to abortion, a majority of Americans continue to express support for abortion access.
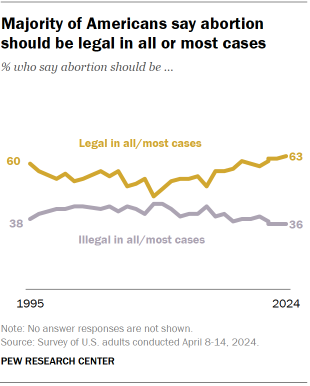
About six-in-ten (63%) say abortion should be legal in all or most cases. This share has grown 4 percentage points since 2021 – the year prior to the 2022 decision in Dobbs v. Jackson Women’s Health Organization that overturned Roe.
The new Pew Research Center survey, conducted April 8-14, 2024, among 8,709 adults, surfaces ongoing – and often partisan – divides over abortion attitudes:
- Democrats and Democratic-leaning independents (85%) overwhelmingly say abortion should be legal in all or most cases, with near unanimous support among liberal Democrats.
- By comparison, Republicans and Republican leaners (41%) are far less likely to say abortion should be legal in all or most cases. However, two-thirds of moderate and liberal Republicans still say it should be.
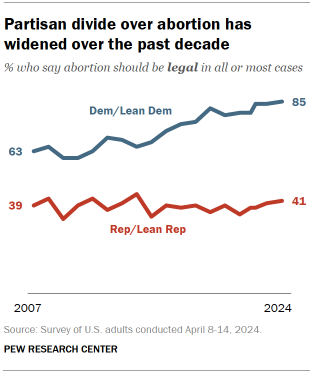
Since before Roe was overturned, both parties have seen a modest uptick in the share who say abortion should be legal.
As in the past, relatively few Americans (25%) say abortion should be legal in all cases, while even fewer (8%) say it should be illegal in all cases. About two-thirds of Americans do not take an absolutist view: 38% say it should be legal in most cases, and 28% say it should be illegal in most cases.
Related: Americans overwhelmingly say access to IVF is a good thing
Women’s abortion decisions
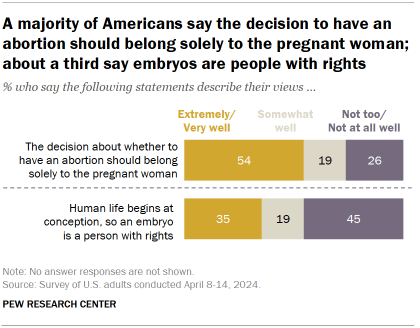
A narrow majority of Americans (54%) say the statement “the decision about whether to have an abortion should belong solely to the pregnant woman” describes their views extremely or very well. Another 19% say it describes their views somewhat well, and 26% say it does not describe their views well.
Views on an embryo’s rights
About a third of Americans (35%) say the statement “human life begins at conception, so an embryo is a person with rights” describes their views extremely or very well, while 45% say it does not describe their views well.
But many Americans are cross-pressured in their views: 32% of Americans say both statements about women’s decisions and embryos’ rights describe their views at least somewhat well.
Abortion access
About six-in-ten Americans in both parties say getting an abortion in the area where they live would be at least somewhat easy, compared with four-in-ten or fewer who say it would be difficult.
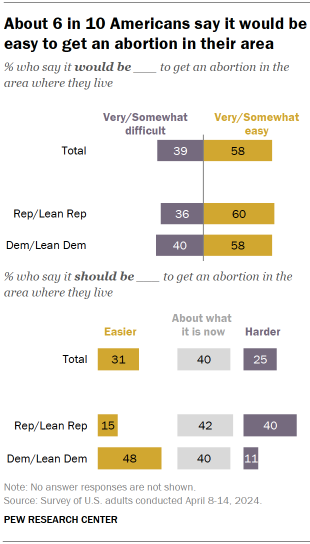
However, U.S. adults are divided over whether getting an abortion should be easier or harder:
- 31% say it should be easier for someone to get an abortion in their area, while 25% say it should be harder. Four-in-ten say the ease of access should be about what it is now.
- 48% of Democrats say that obtaining an abortion should be easier than it is now, while just 15% of Republicans say this. Instead, 40% of Republicans say it should be harder (just 11% of Democrats say this).
As was the case last year, views about abortion access vary widely between those who live in states where abortion is legal and those who live in states where it is not allowed.
For instance, 20% of adults in states where abortion is legal say it would be difficult to get an abortion where they live, but this share rises to 71% among adults in states where abortion is prohibited.
Medication abortion
Americans say medication abortion should be legal rather than illegal by a margin of more than two-to-one (54% vs. 20%). A quarter say they are not sure.
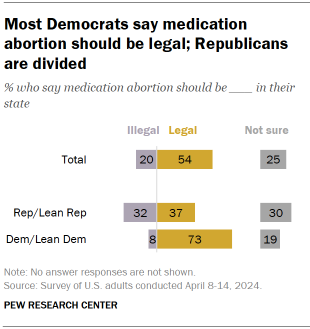
Like opinions on the legality of abortion overall, partisans differ greatly in their views of medication abortion:
- Republicans are closely split but are slightly more likely to say it should be legal (37%) than illegal (32%). Another 30% aren’t sure.
- Democrats (73%) overwhelmingly say medication abortion should be legal. Just 8% say it should be illegal, while 19% are not sure.
Across most other demographic groups, Americans are generally more supportive than not of medication abortion.
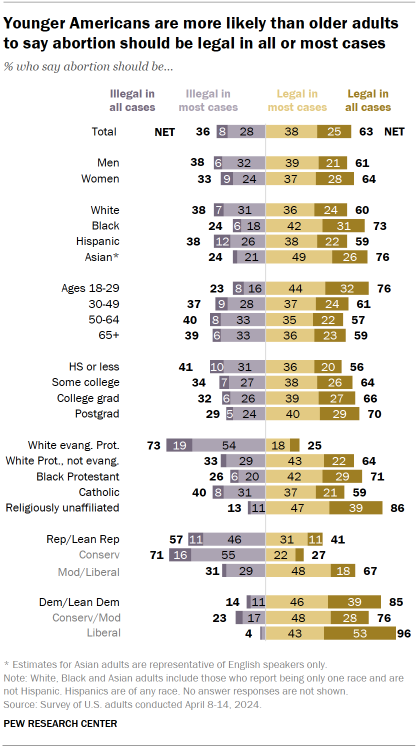
Across demographic groups, support for abortion access has changed little since this time last year.
Today, roughly six-in-ten (63%) say abortion should be legal in all (25%) or most (38%) cases. And 36% say it should be illegal in all (8%) or most (28%) cases.
While differences are only modest by gender, other groups vary more widely in their views.
Race and ethnicity
Support for legal abortion is higher among Black (73%) and Asian (76%) adults compared with White (60%) and Hispanic (59%) adults.
Compared with older Americans, adults under 30 are particularly likely to say abortion should be legal: 76% say this, versus about six-in-ten among other age groups.
Those with higher levels of formal education express greater support for legal abortion than those with lower levels of educational attainment.
About two-thirds of Americans with a bachelor’s degree or more education (68%) say abortion should be legal in all or most cases, compared with six-in-ten among those without a degree.
White evangelical Protestants are about three times as likely to say abortion should be illegal (73%) as they are to say it should be legal (25%).
By contrast, majorities of White nonevangelical Protestants (64%), Black Protestants (71%) and Catholics (59%) say abortion should be legal. And religiously unaffiliated Americans are especially likely to say abortion should be legal (86% say this).
Partisanship and ideology
Democrats (85%) are about twice as likely as Republicans (41%) to say abortion should be legal in all or most cases.
But while more conservative Republicans say abortion should be illegal (76%) than legal (27%), the reverse is true for moderate and liberal Republicans (67% say legal, 31% say illegal).
By comparison, a clear majority of conservative and moderate Democrats (76%) say abortion should be legal, with liberal Democrats (96%) overwhelmingly saying this.
Views of abortion access by state
About six-in-ten Americans (58%) say it would be easy for someone to get an abortion in the area where they live, while 39% say it would be difficult.
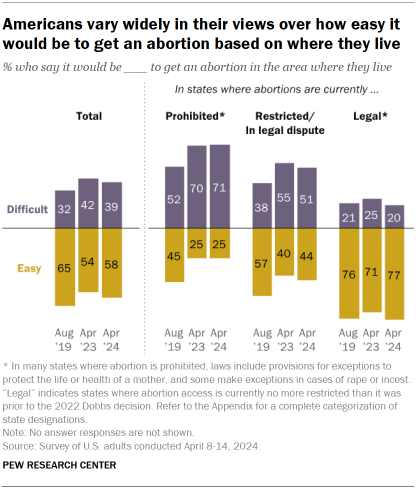
This marks a slight shift since last year, when 54% said obtaining an abortion would be easy. But Americans are still less likely than before the Dobbs decision to say obtaining an abortion would be easy.
Still, Americans’ views vary widely depending on whether they live in a state that has banned or restricted abortion.
In states that prohibit abortion, Americans are about three times as likely to say it would be difficult to obtain an abortion where they live as they are to say it would be easy (71% vs. 25%). The share saying it would be difficult has risen 19 points since 2019.
In states where abortion is restricted or subject to legal challenges, 51% say it would be difficult to get an abortion where they live. This is similar to the share who said so last year (55%), but higher than the share who said this before the Dobbs decision (38%).
By comparison, just 20% of adults in states where abortion is legal say it would be difficult to get one. This is little changed over the past five years.
Americans’ attitudes about whether it should be easier or harder to get an abortion in the area where they live also varies by geography.
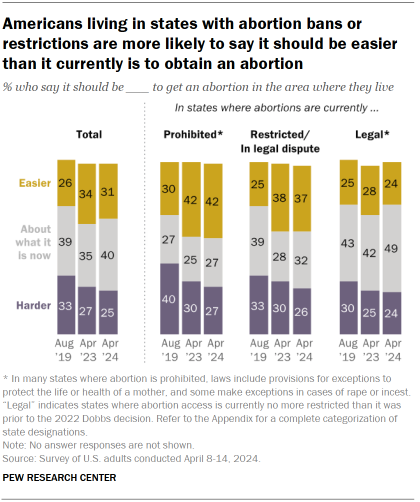
Overall, a decreasing share of Americans say it should be harder to obtain an abortion: 33% said this in 2019, compared with 25% today.
This is particularly true of those in states where abortion is now prohibited or restricted.
In both types of states, the shares of Americans saying it should be easier to obtain an abortion have risen 12 points since before Roe was overturned, as the shares saying it should be harder have gradually declined.
By comparison, changes in views among those living in states where abortion is legal have been more modest.
While Americans overall are more supportive than not of medication abortion (54% say it should be legal, 20% say illegal), there are modest differences in support across groups:
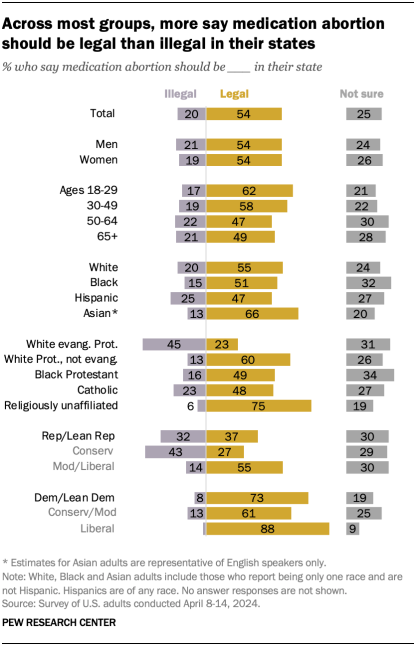
- Younger Americans are somewhat more likely to say medication abortion should be legal than older Americans. While 59% of adults ages 18 to 49 say it should be legal, 48% of those 50 and older say the same.
- Asian adults (66%) are particularly likely to say medication abortion should be legal compared with White (55%), Black (51%) and Hispanic (47%) adults.
- White evangelical Protestants oppose medication abortion by about two-to-one (45% vs. 23%), with White nonevangelicals, Black Protestants, Catholics and religiously unaffiliated adults all being more likely than not to say medication abortion should be legal.
- Republicans are closely divided over medication abortion: 37% say it should be legal while 32% say it should be illegal. But similar to views on abortion access overall, conservative Republicans are more opposed (43% illegal, 27% legal), while moderate and liberals are more supportive (55% legal, 14% illegal).
Just over half of Americans (54%) say “the decision about whether to have an abortion should belong solely to the pregnant woman” describes their views extremely or very well, compared with 19% who say somewhat well and 26% who say not too or not at all well.
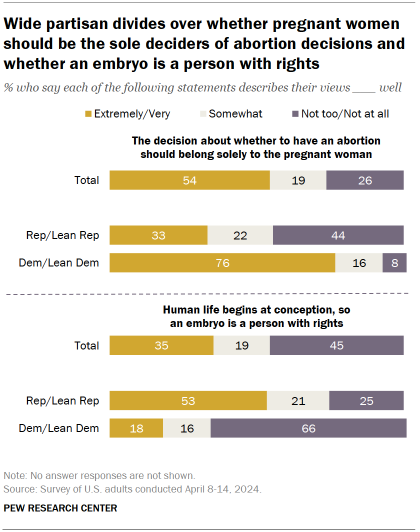
Democrats (76%) overwhelmingly say this statement describes their views extremely or very well, with just 8% saying it does not describe their views well.
Republicans are more divided: 44% say it does not describe their views well while 33% say it describes them extremely or very well. Another 22% say it describes them somewhat well.
Fewer Americans (35%) say the statement “human life begins at conception, so an embryo is a person with rights” describes their views extremely or very well. Another 19% say it describes their views somewhat well while 45% say it describes them not too or not at all well.
(The survey asks separately whether “a fetus is a person with rights.” The results are roughly similar: 37% say that statement describes their views extremely or very well.)
Republicans are about three times as likely as Democrats to say “an embryo is a person with rights” describes their views extremely or very well (53% vs. 18%). In turn, Democrats (66%) are far more likely than Republicans (25%) to say it describes their views not too or not at all well.
Some Americans are cross-pressured about abortion
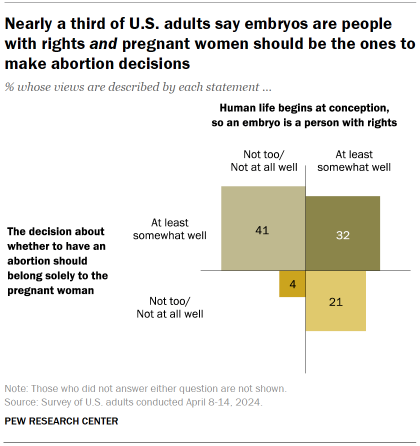
When results on the two statements are combined, 41% of Americans say the statement about a pregnant woman’s right to choose describes their views at least somewhat well , but not the statement about an embryo being a person with rights. About two-in-ten (21%) say the reverse.
But for nearly a third of U.S. adults (32%), both statements describe their views at least somewhat well.
Just 4% of Americans say neither statement describes their views well.
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
- Partisanship & Issues
Support for legal abortion is widespread in many places, especially in Europe
Public opinion on abortion, americans overwhelmingly say access to ivf is a good thing, what the data says about abortion in the u.s., nearly a year after roe’s demise, americans’ views of abortion access increasingly vary by where they live, most popular, report materials.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
- Frontiers in Plant Science
- Functional Plant Ecology
- Research Topics
Ecophysiological Traits-Based Community Assembly and Maintenance of Ecosystem Functioning in Tropical Rainforests
Total Downloads
Total Views and Downloads
About this Research Topic
Human activities, such as agriculture and mining, have caused extensive deforestation and degradation of tropical rainforests, reducing them to less than 50% of their pre-industrial land cover. With their restoration emerging as a global challenge, gaining a comprehensive understanding of community assembly and maintenance of ecosystem functioning across various types of (primary, secondary, and degraded) tropical rainforests is critical for generating a roadmap on how to successfully restore degraded tropical rainforests. Functional traits have been widely proven effective for uncovering community assembly and ecosystem function maintenance in tropical rainforests. However, most studies gravitate toward easily measurable morphological traits, such as specific leaf area and leaf dry matter content. Such traits are not always a proxy for ecophysiological traits, which can directly determine community assembly and ecosystem function maintenance of a tropical rainforest across diverse environments and ecosystems. Therefore, it is urgent to use ecophysiological traits to better understand community assembly and maintenance of ecosystems functioning across varied tropical rainforests. This research topic aims to provide a platform for communication and integration of findings from different fields (i.e., community ecology, restoration ecology, functional ecology, and conservation biology), deepen and expand the understanding of community assembly and ecosystem functioning in tropical rainforests, and unravel the significant roles that ecophysiological traits play in directing community assembly and maintaining ecosystem functions across different tropical rainforests. We encourage the submission of theoretical and experimental studies on different tropical rainforest ecosystems, such as tropical lowland rainforests, tropical cloud forests, tropical island forests, and so on. Submissions are encouraged on, but not limited to, the following topics: • Ecophysiological trait-based community assembly and maintenance of ecosystem functioning across primary and secondary tropical rainforests. • Ecophysiological traits-based community assembly and maintenance of ecosystem functioning in degraded and restored areas of tropical rainforests. • Identification of ecophysiological traits that can effectively disentangle community assembly and ecosystem function maintenance in different types of tropical rainforests. • Trait-based or phylogeny-based conservation strategy for highly endangered keystone species in tropical rainforests.
Keywords : Community assembly, functional trait, ecophysiological traits, ecosystem functioning maintenance, restoration, tropical rainforest.
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, submission deadlines, participating journals.
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
Browse through the Interactive 2024 Report of the UN Sustainable Development Group
UN in Action
- Global Level
- Regional Level
- Countries and Territories
Regional Teams
Rcp: africa, rcp: arab states, rcp: asia and the pacific, rcp: europe and central asia, rcp: latin america and the caribbean, featured content.
- Resources Library
- Executive Board Session Documents
- UNCT Key Documents Library
- Annual UNCT Results Reports
- UNSDG Chair Report on DCO
- System-Wide Contribution to the SDGs
- UNSDG Data Portal
- How We Work (Our Common Premises)
- Resident Coordinator Statistics
2030 Agenda
- 2030 Agenda and the SDGs
- RCs and the Decade of Action
- About the Goals
- SDG Action Campaign
- Joint SDG Fund
Quick links
Read more about the UNSDG members
How We Work
Read more about how the UNSDG works
- The UN Reform
- UN Development Coordination Office
Informal Summary of Findings and Recommendations: 2024 Report of the Chair of the UN Sustainable Development Group

Read through an informal summary of the key findings and recommendations from the 2024 Report of the Chair of the UN Sustainable Development Group.
Five years into the UN reforms and the implementation of the reinvigorated resident coordinator system, the Report provides details of results achieved in 2023 to foster a more impactful, efficient, effective and accountable UN development system that responds to country needs.
Browse through the interactive version of the 2024 Report here .

An official website of the United States government
Here’s how you know
The .gov means it’s official.
Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure.
The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Investigative Summary: Findings of Misconduct by a then Federal Bureau of Investigation Senior Official for Numerous Comments to a Subordinate in Violation of the Department’s Zero Tolerance Policy on Harassment and FBI Policies
Posted Date May 15, 2024 Report Number 24-066 Component Federal Bureau of Investigation Report Type Investigation

IMAGES
VIDEO
COMMENTS
A research summary is a brief yet concise version of the research paper for a targeted audience. Read more to find out about structure of a research summary, tips to write a good research summary, and common mistakes to write a research summary. ... Additionally, there needs to be a short but thorough explanation of how the findings of the ...
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
A summary must be coherent and cogent and should make sense as a stand-alone piece of writing. It is typically 5% to 10% of the length of the original paper; however, the length depends on the length and complexity of the article and the purpose of the summary. Accordingly, a summary can be several paragraphs or pages, a single paragraph, or ...
Checklist: Research results 0 / 7. I have completed my data collection and analyzed the results. I have included all results that are relevant to my research questions. I have concisely and objectively reported each result, including relevant descriptive statistics and inferential statistics. I have stated whether each hypothesis was supported ...
Having summed up your key arguments or findings, the conclusion ends by considering the broader implications of your research. This means expressing the key takeaways, practical or theoretical, from your paper—often in the form of a call for action or suggestions for future research. Argumentative paper: Strong closing statement
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article's structure in which it is written. You must know the goal of your analysis before you launch a project.
A research summary is a piece of writing that summarizes the research of a specific topic into bite-size easy-to-read and comprehend articles. The primary goal is to give the reader a detailed outline of the key findings of a research. It is an unavoidable requirement in colleges and universities. To write a good research summary, you must ...
A lay summary that describes and communicates your research findings in a clear, simple way leaves little room for misrepresentation. Improve engagement: A succinct lay summary makes research findings easier to understand and highlights its significance. This means that audiences can engage more actively with your work, leading to an increase ...
A research summary is a short, concise summary of an academic research paper. It is often used to summarize the results of an experiment, summarize the major findings and conclusions, and provide a brief overview of the methods and procedures used in the study.
Creating an effective research paper summary requires finesse, precision, and the art of distilling complex information into bite-sized pieces of knowledge. Here's an infographic explaining the 3 key things you must keep in mind as you write a research paper summary. Paperpal is an AI academic writing assistant that can help researchers ...
Qualitative Findings. Qualitative research is an exploratory research method used to understand the complexities of human behavior and experiences. Qualitative findings are non-numerical and descriptive data that describe the meaning and interpretation of the data collected. Examples of qualitative findings include quotes from participants ...
Research Summary Example 2. Below is another sample sketch, also from an imaginary article. Title - "The frequency of extreme weather events in US in 2000-2008 as compared to the '50s". Introduction - Weather events bring immense material damage and cause human victims.
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and ...
These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). ... Summary of findings (for interactive version click here) Compression stockings compared with no compression stockings for people taking long flights.
Draft Introduction for Summary of Findings: In the introduction for the Summary of Findings, assert that you have answered your research questions. At a minimum you would tell the reader how many findings emerged and describe them in a sentence each. Most important is the findings you present in chapter 5 reflect and match what is significant ...
Step 1: Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study. The guidelines will generally outline specific requirements for the results or findings section, and the published articles will ...
A research summary is a concise and informative overview of a research article, report, or thesis. It aims to provide the reader with a clear understanding of the study's purpose, methods, results, and conclusions without having to read the entire document. Think of it as a mini-version of the original work that highlights its most important ...
5.3 Summary of Findings The findings showed a distinct tendency by the five participants to use similar words and phrases in their communication both through online written forums and face- to -
This chapter summarizes the whole research process. It first provides a brief summary of the whole study with particular reference to the research problem, research methodology, results, the main contributions of the research and recommendations for future work. It provides a summary of the main findings of the study, conclusions and ...
The result is an instrument with a degree of feasibility, validity and reliability suitable for comparative research as was found in a Spanish macro-study involving 6398 secondary school students. Preliminary results for the Spanish context are offered.
Our findings indicate that tonic alertness continues to develop from ages 6 to 13 in both deaf and typical hearing children (Dye and Hauser, 2014; Dye and Terhune-Cotter, 2023). This aligns with previous research on typical hearing children using the same task, which showed a specific development ceiling at 10 years old (Betts et al., 2006).
Like an abstract in a published research article, the purpose of an article summary is to give the reader a brief, structured overview of the study. To write a good summary, identify what information is important and condense that information for your reader. The better you understand a subject, the easier it is to explain it thoroughly and ...
AI to summarize article often comes with customizable settings to tailor the summarization output. Researchers can adjust preferences based on factors such as the desired summary length or the level of detail required. For instance, settings can be adjusted to generate shorter summaries if a quick overview is needed.
Nearly two years after the Supreme Court overturned the 1973 Roe v. Wade decision guaranteeing a national right to abortion, a majority of Americans continue to express support for abortion access. About six-in-ten (63%) say abortion should be legal in all or most cases. This share has grown 4 percentage points since 2021 - the year prior to ...
This research topic aims to provide a platform for communication and integration of findings from different fields (i.e., community ecology, restoration ecology, functional ecology, and conservation biology), deepen and expand the understanding of community assembly and ecosystem functioning in tropical rainforests, and unravel the significant ...
Read through an informal summary of the key findings and recommendations from the 2024 Report of the Chair of the UN Sustainable Development Group. Five years into the UN reforms and the implementation of the reinvigorated resident coordinator system, the Report provides details of results achieved in 2023 to foster a more impactful, efficient ...
Investigative Summary: Findings of Misconduct by a then Federal Bureau of Investigation Senior Official for Numerous Comments to a Subordinate in Violation of the Department's Zero Tolerance Policy on Harassment and FBI Policies. Posted Date. May 15, 2024. Report Number. 24-066. Component. Federal Bureau of Investigation.