- Search Menu
- Advance articles
- Editor's Choice
- Special Issues and Sections
- Methods Articles
- Trending Articles
- Cover Archive
- Author Guidelines
Submission Site
- Open Access Policy
- Self-Archiving Policy
- Reasons to Submit
- About Journal of Evolutionary Biology
- About the European Society for Evolutionary Biology
- Editorial Board
- Abstracting and Indexing
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic


Editor-in-Chief
Max Reuter, University College London, UK
Editorial Board
Managing Editor
Nicola Cook, University of St Andrews, UK
Editorial Office
About the journal
JEB is an international, peer-reviewed journal that covers diverse research areas in evolutionary biology. The journal prioritizes publishing significant advances from a broad taxonomic perspective.
Latest articles

Editorial team
Meet the editorial board for the Journal of Evolutionary Biology .
Learn more

Submit your work
Submit your manuscript for consideration to be included in a 2024 issue of the Journal of Evolutionary Biology .
Review Author Guidelines

Email alerts
Register to receive table of contents email alerts as soon as new issues of Journal of Evolutionary Biology are published online.

Keep up to date with JEB
Keep up with latest from the Journal of Evolutionary Biology and the wider evolutionary biology community.
Follow @jevbio.bsky.social on Bluesky
Follow @JEvBio on X
Read the JEB Blog
More from ESEB

Join us from 17-22 August 2025, in Barcelona, Spain, for the 2025 Congress of the European Society of Evolutionary Biology.
Visit the conference website

John Maynard Smith Prize
Every year the European Society for Evolutionary Biology distinguishes an outstanding young evolutionary biologist with the John Maynard Smith (JMS) Prize. In 2023, the winner is Dr Paul Jay, postdoctoral fellow at Copenhagen and Berkeley. Paul will give the 2023 JMS prize lecture at the ESEB Congress in 2025.
Visit the JMS Prize website

ESEB Social media
Never miss a call, deadline or news announcement from the Society!
Bluesky: @eseb.bsky.social
X: @eseb_org

Join our society!
ESEB brings together thousands of evolutionary biologists worldwide via our initiatives and international congresses.
Being an ESEB member qualifies you for benefits such as:
Reduced registration fee at ESEB congresses
30% discount on the Article Publication Charge for J ournal of Evolutionary Biology
ESEB travel awards, mobility grants, and prizes
Join now!

Author resources
Learn about how to submit your article, our publishing process, and tips on how to promote your article.
Find out more

Read and Publish deals
Authors interested in publishing in Journal of Evolutionary Biology may be able to publish their paper Open Access using funds available through their institution’s agreement with OUP.
Find out if your institution is participating

Open Access options for authors
The Journal of Evolutionary Biology welcomes submissions from authors wishing to submit open access papers.
Related Titles

- Advertising & Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1420-9101
- Print ISSN 1010-061X
- Copyright © 2024 European Society for Evolutionary Biology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Evolutionary Biology
- Committed to the view that evolutionary theory is a unifying framework for the biosciences.
- Offers a platform for broad syntheses, in-depth treatment and divergent views.
- Adapts the acclaimed format of the book serial to today's publication environment.
- Continues the vision and intent of the original series published in 1967.
- 100% of authors who answered a survey reported they would likely publish in the journal again.
This is a transformative journal , you may have access to funding.
- Kevin J Parsons

Latest issue
Volume 51, Issue 1
Latest articles
A graph-based mathematical model for more efficient dimensionality reduction of landmark data in geometric morphometrics.
- Lloyd A. Courtenay
- Julia Aramendi
- Diego González-Aguilera
The Evolution of Body Size in Terrestrial Tetrapods
- Fernanda S. Caron
- Marcio R. Pie

Urban Life Affects Differentiation and Phenotypic Variation but not Asymmetry in a Fully Terrestrial Salamander
- Lucía Alarcón-Ríos
- Antigoni Kaliontzopoulou
- Guillermo Velo-Antón

Analysis of Morphological Change during a Co-invading Assemblage of Lizards in the Hawaiian Islands
- John G. Phillips
- Travis J. Hagey
- Eben Gering
Colonization of a Novel Host Plant Reduces Phenotypic Variation
- Kalle J. Nilsson
- Masahito Tsuboi
- Anna Runemark

Journal information
- Astrophysics Data System (ADS)
- Google Scholar
- Japanese Science and Technology Agency (JST)
- Norwegian Register for Scientific Journals and Series
- OCLC WorldCat Discovery Service
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
- Zoological Record
Rights and permissions
Springer policies
© Springer Science+Business Media, LLC, part of Springer Nature
- Find a journal
- Publish with us
- Track your research
Evolutionary Biology

Evolutionary Biology seeks to understand how the astonishing diversity of life on our planet arose by integrating insights from genomes, developmental biology, natural population studies, and experimental analyses.
- Publications
We are a product of Evolution. Just like the plants that provide us with food and materials, the microorganisms that keep us healthy or make us ill, and the forests and plankton that produce our oxygen. By understanding evolution, we can begin to understand our place in the world, and address major challenges such as:
- Tackling the spread of infectious diseases
- Feeding the growing planet
- Predicting organism responses to environmental change
- Protecting natural resources
But evolution is still a subject where much remains unknown, where puzzles and problems are many, and where new approaches and new technologies are needed to unlock hidden mysteries. Oxford researchers are pushing back the boundaries of knowledge in many ways to uncover these mysteries.
Evolution requires inherited variation, so a key area of Oxford research is into studying the molecule of inheritance - DNA - to find all the genetic differences between and within species. These differences in DNA sequence cause changes in how cells and individuals behave, or how embryos develop. Oxford researchers are studying these links between ‘genotype’ and ‘phenotype’ across the Kingdoms, in organisms such as birds and bacteria, moths and mammals, plants and protists, tardigrades and tunicates, fish, flies and flatworms.
Organisms do not live in isolation. Oxford researchers are also studying the evolutionary consequences of interactions between and within species, both in the wild and using experimental evolution in the laboratory. Find out more about the work happening in this Section through our publications and recent news.
Professor Aziz Aboobaker | Section Head

"Evolution is the unifying principle of biology but there is still much we do not understand. New technologies are helping us to unlock the secrets of evolution, and put Oxford at the forefront of Evolutionary Biology research” Aziz Aboobaker
Section Administrator: Jess Bassett
Development, Genomes, & Evolution
Aziz Aboobaker, Peter Holland, Sebastian Shimeld, & Berta Verd

Ecological & Evolutionary Dynamics
Tim Coulson
Evolution & Biodiversity
Tim Barraclough

Evolutionary Ecology & Biogeography
Sonya Clegg
Evolutionary Genetics & Genomics
Dmitry Filatov

Macroecology, Population Ecology & Evolution
Rob Salguero-Gómez

Male Reproductive Ageing

Social Evolution
Ashleigh Griffin & Stuart West et al.
Recent section news
Articles on Evolutionary biology
Displaying 1 - 20 of 154 articles.

City mouse or country mouse? I collect mice from Philly homes to study how they got so good at urban living
Megan Phifer-Rixey , Drexel University
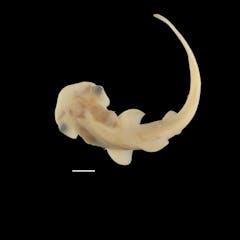
Rare access to hammerhead shark embryos reveals secrets of its unique head development
Gareth J. Fraser , University of Florida

Climate change is already forcing lizards, insects and other species to evolve – and most can’t keep up
Michael P. Moore , University of Colorado Denver and James T. Stroud , Georgia Institute of Technology

Two questions, hundreds of scientists, no easy answers: how small differences in data analysis make huge differences in results
Hannah Fraser , The University of Melbourne ; Elliot Gould , The University of Melbourne , and Timothy H. Parker , Whitman College

Curious Kids: what came first, the chicken or the egg?
Ellen K. Mather , Flinders University

How does fever help fight infections? There’s more to it than even some scientists realize
Edmund K. LeGrand , University of Tennessee and Joe Alcock , University of New Mexico

4 reasons not teaching evolution in schools is immoral
Peter Ellerton , The University of Queensland

Macaque monkeys shrink their social networks as they age – research suggests evolutionary roots of a pattern seen in elderly people, too
Erin Siracusa , University of Exeter and Noah Snyder-Mackler , Arizona State University

Racist and sexist depictions of human evolution still permeate science, education and popular culture today
Rui Diogo , Howard University

Power struggles in nature can be more subtle, nuanced and strategic than just dog-eat -dog
Lee Alan Dugatkin , University of Louisville

Human and Neanderthal brains have a surprising ‘youthful’ quality in common, new research finds
Stephen Wroe , University of New England ; Gabriele Sansalone , Institute of Marine Sciences , and Pasquale Raia , University of Naples Federico II

The study of evolution is fracturing – and that may be a good thing
Erik Svensson , Lund University

Celibacy: family history of Tibetan monks reveals evolutionary advantages in monasticism – podcast
Gemma Ware , The Conversation

‘Impressive rafting skills’: the 8-million -year old origin story of how rodents colonised Australia
Emily Roycroft , Australian National University

Axolotls can regenerate their brains – these adorable salamanders are helping unlock the mysteries of brain evolution and regeneration
Ashley Maynard , Swiss Federal Institute of Technology Zurich
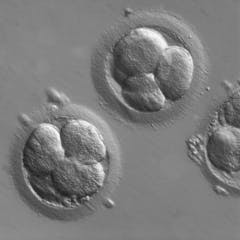
Most human embryos naturally die after conception – restrictive abortion laws fail to take this embryo loss into account
Kathryn Kavanagh , UMass Dartmouth

Neanderthals died out 40,000 years ago, but there has never been more of their DNA on Earth
Peter C. Kjærgaard , University of Copenhagen ; Mark Maslin , UCL , and Trine Kellberg Nielsen , Aarhus University

Slime is all around and inside you – new research on its origins offers insight into genetic evolution
Omer Gokcumen , University at Buffalo

Why are bigger animals more energy-efficient ? A new answer to a centuries-old biological puzzle
Craig White , Monash University and Dustin Marshall , Monash University

Why do animals have tails?
Michael A. Little , Binghamton University, State University of New York
Related Topics
- Charles Darwin
- Evolutionary genetics
- Human evolution
- Human evolutionary biology
- Natural selection
Top contributors
Honorary Associate Professor, UNSW Sydney
Strategic Professor in Palaeontology, Flinders University
Professor of Evolutionary Biology, The University of Melbourne
Postdoctoral Researcher , The University of Melbourne
PhD Researcher, Nature and Function of Human Nonverbal Vocalisations, University of Sussex
Adjunct Research Associate, Monash University
Associate Professor, The University of Queensland
Distinguished Professor of Genetics and Vice Chancellor's Fellow, La Trobe University
Senior Lecturer, Centre for Behaviour and Evolution, Newcastle University
Lecturer, Macquarie University
Sexual health researcher, The University of Melbourne
Research fellow, University of Sydney
Postdoctoral Researcher of Evolutionary Biology, University of Pennsylvania
Senior lecturer, School of Biological Sciences, University of Bristol, University of Bristol
Research Associate Professor in Evolutionary Biology, The University of Western Australia
- X (Twitter)
- Unfollow topic Follow topic
Volume 5 Supplement 2
Evolutionary Developmental Biology
- Open access
- Published: 08 June 2012
Evolutionary Developmental Biology (Evo-Devo): Past, Present, and Future
- Brian K. Hall 1
Evolution: Education and Outreach volume 5 , pages 184–193 ( 2012 ) Cite this article
40k Accesses
35 Citations
15 Altmetric
Metrics details
Evolutionary developmental biology (evo–devo) is that part of biology concerned with how changes in embryonic development during single generations relate to the evolutionary changes that occur between generations. Charles Darwin argued for the importance of development (embryology) in understanding evolution. After the discovery in 1900 of Mendel’s research on genetics, however, any relationship between development and evolution was either regarded as unimportant for understanding the process(es) of evolution or as a black box into which it was hard to see. Research over the past two decades has opened that black box, revealing how studies in evo–devo highlight the mechanisms that link genes (the genotype) with structures (the phenotype). This is vitally important because genes do not make structures. Developmental processes make structures using road maps provided by genes, but using many other signals as well—physical forces such as mechanical stimulation, temperature of the environment, and interaction with chemical products produced by other species—often species in entirely different kingdoms as in interactions between bacteria and squid or between leaves and larvae (Greene Science 243:643–666, 1989 ). Not only do genes not make structures (the phenotype), but new properties and mechanisms emerge during embryonic development: genes are regulated differentially in different cells and places; aggregations of similar cells provide the cellular resources (modules) from which tissues and organs arise; modules and populations of differently differentiated cells interact to set development along particular tracks; and organisms interact with their environment and create their niche in that environment. Such interactions are often termed “epigenetic,” meaning that they direct gene activity using mechanisms that are not encoded in the DNA of the genes. This paper reviews the origins of evo–devo, how the field has changed over the past 30 years, evaluates the recognition of the importance for development and evolution of mechanisms that are not encoded in DNA, and evaluates what the future might bring for evo–devo. Although impossible to know, history tells us that we might expect more of the same; expansion of evo–devo into other areas of biology (ecology, physiology, behavior); absorption of evo–devo by evolution or a unification of biology in which evo–devo plays a major role.
Introduction
Evolutionary developmental biology (evo–devo) is the name for that part of biology involved in understanding how alterations in the mechanisms of embryonic development influence or direct evolutionary changes in any and all stages of the life cycle. The field has become enormous in recent years; McCain ( 2009 ) analyzed thousands of papers published between 1975 and 2004 in her study of the impact of one early practitioner of evo–devo, Conrad Hal Waddington. Consequently, what follows is my perspective on the past, present, and future of evo–devo. I apologize in advance to those researchers whose contributions are underrepresented.
Although not usually regarded as a “founding father” of the field, the term “evolutionary developmental biology” appears first to have been used in print in 1983 by the zoologist and later environmentalist, Peter Calow, of the University of Sheffield in England. In an earlier book, Calow ( 1978 ) had provided an influential treatment of life cycles from developmental, evolutionary, and physiological points of view. Calow included a chapter on evolution and development (in itself unusual at the time Footnote 1 ) in his 1983 book Evolutionary Principles , published by Blackie in their Tertiary Level Biology Series. Calow described the field of evolution and development as “evolutionary developmental biology,” noting that “The area is a relatively new and complex one so the reader should not expect to find fully comprehensive treatments in the literature” (p. 80).
In his book of only 100 pages and 105 references and contrary to much prior opinion, Calow emphasized that natural selection could and did act at any stage in the life cycle of multicellular organisms, a conclusion reached by one of the founders of evolutionary embryology 109 years earlier. In his first paper on shark development, Francis Balfour argued for the role of selection in embryonic development as perhaps even more relevant for evolution than selection on the adult:
I see no reason for doubting that the embryo in the earliest periods of development is as subject to the laws of natural selection as is the animal at any other period. Indeed, there appear to me grounds for the thinking that it is more so. (Balfour 1874 , p. 343)
Although the roots of evo–devo are deep (Gould 1977 ; Bonner 1982 ; Arthur 1988 ; Hall and Olson 2003 ; Laubichler and Maienschein 2007 ; Olsson et al. 2009 ), evo–devo is just coming into its own. In the introduction to the publication of the Kowalevsky Medal winner symposium held in January 2003 (published in 2004) to recognize the research of the first recipients of the Kowalevsky medal, Laubichler and Wagner concluded that “By all accounts ‘evo–devo’ has arrived. It is now solidly entrenched in the conceptual framework of modern biology and has all the markings of a new discipline, such as representation in professional societies, scientific journals devoted to the field, academic programs and job searches, panels at funding agencies, textbooks, etc.” ( 2004 , p. 1) Eight years later, classes, courses, workshops, postdoctoral fellowships, faculty positions, university chairs, and research grant selection panels in evo–devo are widespread.
I took “evolutionary developmental biology” as the title for my 1992 book (Hall 1992 ), which set out to summarize what was then known about the origins of the field, its history, and its role in contributing to the evolutionary process. I will do a little of the same in the paper. Why? Because we can only appreciate the future prospects of evo–devo in the context of its past. What questions were being asked of embryos and of development? To which organisms were these questions addressed? Were we seeking a broad overarching theory of evo–devo that would apply to all animal life or differences that characterized grades of biological organization—kingdoms, phyla, classes, even individual species. What was missing from prevalent approaches to evolution that necessitated this new developmental approach?
Origins of Evo-Devo
I go back to the late nineteenth century when we find the origins of evo–devo in the research of individuals in England (largely Trinity College, Cambridge) and in Continental Europe. These evolutionary morphologists/evolutionary embryologists were attracted to this research following the publications of The Origin of Species by Charles Darwin (Darwin 1859 ) and Ernst Haeckel’s theory that ontogeny recapitulates phylogeny (Haeckel 1866 ). Paradoxically, the first published study testing Darwin’s theory using embryos and larvae—Fritz Müller’s study of crustacean life histories (Müller 1864 )—showed that ontogeny could be used to understand patterns of evolutionary history (phylogeny) and that mechanisms could be sought in ontogeny. So varied were crustacean life history strategies found to be that Müller found he could use the details and varieties of life history stages to construct a phylogeny of crustacean relationships. Haeckel took exactly the opposite position. Haeckel theorized that phylogeny explains ontogeny and erected his Biogenetic Law on this basis.
Embryos provided the way to study evolution. The fossil record was incomplete. Embryos, on the other hand, recorded in their development the history of their ancestors. This history had to be read with great care; there were gaps in the record, and secondary specializations such as the placenta could confuse the unwary (Bowler 1996 ; Hall 1999 ). Nevertheless, from the late 1860s or early 1870s until the mid-1880s, evolutionary embryology was the field that attracted the brightest and best zoologists. It attracted those who wanted to study embryos in the laboratory or field station and those who wanted to seek embryos of such ‘missing links’ as the platypus (thought to link reptiles and mammals), lungfish (thought to link fish and tetrapods), and the velvet worm Peripatus (thought to link insects and arthropods) in such exotic places as Australia, South America and Africa (Hall 1999 , 2001 ; MacLeod 1994 ; Bowler 1996 ; Laubichler and Maienschein 2007 ). William Bateson, the English zoologist who coined the name “genetics,” began his career as an evolutionary embryologist. Reminiscing on his career, Bateson commented that
Morphology was studied because it was the material believed to be the most favorable for the elucidation of the problems of evolution, and we all thought that in embryology the quintessence of morphological truth was most palpably presented. Therefore every aspiring zoologist was an embryologist, and the one topic of professional conversation was evolution. (Bateson 1922 , p. 56)
Frustration with reconstructing evolutionary trees from embryonic sequences, the rise of experimental and physiological approaches to embryonic development in the 1880s, and the rediscovery of Mendelian genetics in 1900 all cast evolutionary embryology into a backwater from which it would take a century to resurface. Mendel’s principles of segregation and assortment coupled with studies on the fruit fly Drosophila provided a powerful foundation upon which the new science of genetics was built. Publication of Dobzhansky’s ( 1937 ) influential book, “ Genetics and the Origin of Species ,” provided a basis for understanding evolution through population genetics, the mathematical models for which have been developed in the 1920.
By the middle of the twentieth century, maintenance of the features of organisms, variation in those features, and the origin of new features all seemed explicable by a fusion of Mendelian and population genetics. Paradoxically, the use of Drosophila as the model organism for genetics eliminated the roles of embryonic development and of the environment from evolutionary discussion and theory; inbred laboratory organisms display none of the variation and adaptability seen in nature.
The Twentieth Century
Despite the valiant attempts of a handful of individual researchers through the first six decades of the twentieth century, linking embryos and evolution did not make a comeback until Stephen J. Gould’s book Ontogeny and Phylogeny was published in 1977. Weighing in at 501 pages, this was no easy read, especially as the first half was a detailed history of the way in which development and evolution (ontogeny and phylogeny—the terms proposed by Haeckel) had been related in the past and when the past for Gould began at 450 BC. This historical tour de force was important; Gould reminded us, for indeed we had forgotten that we had been seeking relationships between development and evolution for millennia.
The second half of Gould’s book was equally important because it revisited a concept conceived by Haeckel in the 1860s, elaborated by Gavin de Beer in the 1930s (de Beer 1930 ), but then all but abandoned. Haeckel and de Beer’s concept is summed up in the word “heterochrony”—changes in the timing of developmental processes between a descendant and its ancestors (see Zelditch 2001 ; Willmore 2010 ). De Beer’s insight was to take studies showing that genes affected the rates of physiological processes in insects (Goldschmidt 1918 ) and to see that the rates of development must change during evolution. Here was a way in which comparative embryology could be investigated in an evolutionary (phylogenetic) framework). Heterochrony as a developmental mechanism operating during individual development could be selected for and so was important in evolutionary change; heterochrony was an “evolutionary developmental mechanism” (Hall and Olson 2003 ).
Julian Huxley, one of the founders of the modern synthesis of evolution, appreciated the importance for evolution of the study of genes in development—“a study of genes during development is as essential for an understanding of evolution as are the study of mutation and selection” (Huxley 1942 , p. 8)—but even Huxley neglected to incorporate development into evolutionary theory. Other founders of the modern synthesis such as Ernst Mayr spoke of an internal biology (to which development belonged) and an external biology (evolution, ecological interactions) as if the two were separate and non-overlapping/interacting one to the other (Mayr 1982 , 1997 ).
Because it was difficult to compare ancestors and their descendants, living organisms were compared with one another, initially across broad divisions such as phyla and classes—homology of the tissue interactions that initiate the development of Meckel’s cartilage in the lower jaw of amphibians, birds, and mammals (Goodwin et al. 1983 ), for example—but increasingly across smaller evolutionary gaps, to the point that pairs of species in the same genus (congeneric species) could be compared
—loss of the larval stage from one of a pair of congeneric sea urchins (Raff 1992 ), for example—
changes in the developmental processes in inbred strains of a single species of laboratory animal could be compared as experiments in evo–devo.
—changes in the timing (heterochrony) of the tissue interactions responsible for the induction of the lower jaw in three inbred strains of mice (C57BL, C3H/He, CBA/J) by MacDonald and Hall ( 2001 ), for example—
to the present day when variation in individuals of a single species are revealing the mechanisms underlying the maintenance of natural variation in developmental processes upon which natural selection can act
—as in the correlation of genetic distance between individuals in relation to variation in the timing of developmental events in the pond snail, Radix balthica by Tills et al. ( 2011 ).
Evo–devo exploded as heterochrony was found everywhere. Along the way, heterochrony became such a pervasive term that it lost some of its explanatory power; any change in timing became heterochrony, whether evolutionarily relevant or not (Zelditch 2001 , especially pp. vii–ix).
The next major impetus to evo–devo was not the resurrection of a previously known evolutionary developmental mechanisms but the discovery that all animals (subsequently shown for all plants and fungi too) share genes that contain a 180-bp sequence known as the homeobox and that these genes, known as homeobox, homeotic, or Hox genes, are responsible for determining that animals have an anterior and a posterior, a dorsal and a ventral side, and specific regions (often as repeated segments) along the body axis—head at one end tail at the other, thorax in front of abdomen, wings on a specific pair of segments, and so forth (Lewis 1978 ; Gehring 1985 , 1998 ; Averof 1997 ; Grenier et al. 1997 ; Carroll 2008 ).
“Master” genes, also known as developmental or regulatory genes, were discovered. One of the best understood of such genes is the paired-box protein gene known as Pax-6 in vertebrates and as eyeless ( ey ) in Drosophila . As determined from its DNA sequence, orthologues of Pax-6 are present throughout the Animal Kingdom. As determined from functional studies, the role of Pax-6 in anteriorizing the embryo and in the formation of anterior sensory structures also is conserved across the Animal Kingdom. Although best known as the major gene controlling eye development, Pax-6 functions in organisms that lack eyes, reflecting its ancient developmental role. Indeed, ey from fruit flies can initiate eyes in frogs and Pax-6 from frogs can initiate eyes in Drosophila (Dahl et al. 1997 ; Suga et al. 2010 ). Here is an astonishing and previously unthought-of genetic conservation across animals whose morphology varied enormously.
Heterochrony, homeotic genes, increasingly resolved relationships between organisms (phylogenetic trees), and an appreciation during the 1980s and 1990s of the importance of ecological and species interactions led evo–devo to the position where the aims of evo–devo could be stated as understanding:
The origination and evolution of embryonic development;
The role of modifications of developmental processes in the production of novel features;
How the adaptive plasticity of development facilitates the origin and maintenance of complex life cycles with embryos, larvae, and adults; and
How developing organisms interact with their ecological environment to facilitate evolutionary change (Hall 2000 ; West-Eberhard 2003 ).
Others had similar lists. Müller ( 2007a , b ) and Collins et al. ( 2007 ) listed seven approaches and aims of evo–devo:
The origin of developmental systems;
The evolution of developmental systems;
Modifications of timing and context of developmental processes;
Environment–development interactions;
Maintenance of phenotypic variation;
Origin of phenotypic novelty; and
Integration of genetics and epigenetic mechanisms.
Research programs in evo–devo are comparative and experimental (Hall 1999 ; Raff and Love 2004 ). Increasingly, they involve what Laubichler ( 2007 ) calls “evolutionary developmental genetics.” In pursuit of their analysis of the integration of development, evolution, and ecology, Collins et al. ( 2007 ) analyzed three model systems—breeding of the domestic dog Canis familiaris , research on a sea anemone Nematostella vectensis , and research into horn development/evolution in dung beetles in the genus Onthophagus —and the development/evolution of vertebrate limbs and mammalian teeth. In a very different approach, McCain ( 2010 ) identified trends in evo–devo between 1996 and 2008 by analyzing linkages between the literature in what she identified as three core journals of evo–devo [Evolution & Development; Development, Genes & Evolution; and the Journal of Experimental Zoology (Part B)]. Her analysis is worth a close examination, as is an earlier study in which the research of Conrad Waddington was used to trace the rise of evo–devo (McCain 2009 ).
Critical to our ability to answer these questions and evaluate these model systems are a sound basis to ensure that we are comparing the same organisms/processes/genes—the central biological concept of homology (Hall 1994 , 2012 )—and our ability to determine the direction of evolutionary change because of the development of robust phylogenetic trees of relationships (Valentine 2004 ; Erwin et al. 2011 ). We have the model systems and we have the methodology but still lack the required theory. In their analysis of the status of modeling in evo–devo, Collins et al. ( 2007 ) concluded that “these models are mostly diagrammatic and functional; very few analytical and predictive models exist within EvoDevo” (p. 373). In part, they see this deficiency residing in evo–devo’s reliance on developmental genetics rather than evolutionary theory, a position that is slowly changing.
The Present
Molecular genetics has revolutionized evo–devo over the past two decades. Integrating our expanding molecular understanding with mechanisms operating at the cell or other levels (tissues, organs, whole organism, organism–environment interactions; Box 1) has been and remains a major goal and a challenge for evo–devo. Essentially, it involves opening the black box between genotype and phenotype, taking out what is found in the box and how it fits together and then determining how to put the contents back in the box (Hall 1999 , 2003a , b ; West-Eberhard 2003 ; Carroll et al. 2005 ). One of the items found in the black box is known as epigenetics.
Box 1. A sample of evolutionary developmental mechanisms operating at various levels
Epigenetics
Many of the controls on gene regulation and function are subsumed under the term “epigenetics,” a term coined by the British geneticist and embryologist Conrad Waddington for the causal factors that control gene action during development (Waddington 1940 ; and see the papers in Hall and Laubichler 2009 ). Hall ( 1992 ) defined epigenetics as “the sum of the genetic and non-genetic factors acting upon cells to control selectively the gene expression that produces increasing phenotypic complexity during development” ( 1992 , p. 89). To this, I would only add “and evolution” at the end.
Epigenetics is still used with this original meaning but has increasingly come to be applied at the molecular level for heritable changes to the DNA other than changes to the nucleotide bases. Such changes include methylation, imprinting, and regulation of chromatin—which have been known for some time (Biémont 2010 ; Hallgrímsson and Hall 2011 ; Moazed 2011 ; Molaro et al. 2011 )—and regulation of genes by small RNA molecules, especially miRNAs (Kosik 2009 ; Hallgrímsson and Hall 2011 ). This now very well-characterized second inheritance system was appreciated in its infancy by John Maynard Smith, a leading twentieth century evolutionary theorist: “There is a second inheritance system—an epigenetic inheritance system—in addition to the system based on DNA sequence that links sexual generations (Maynard Smith 1989 , p. 11).
The heritable aspect of epigenetics has shown us that organisms do not start their lives as naked nuclear DNA. They possess DNA in their mitochondria, epigenetic “marks” in their nuclear DNA, and they inherit mRNA and proteins that were produced under the control of their mother’s DNA and deposited into the egg cytoplasm. Epigenetics provides another, but not the only other, means by which heritable information operates in organismal development. The ability to learn behaviors, interact with environments, and construct niches (Laland et al. 2008 ; Gissis and Jablonka 2011 ) are three further mechanisms introduced below.
Integrated Mechanisms
Integrated studies using molecular biology, molecular genetics, developmental biology, phylogenetics, paleontology, and molecular paleobiology are revealing previously unimagined information on how features change during evolution (Erwin and Wing 2000 ; Hall 2002 ; Wilkins 2002 , 2007 ; Carroll et al. 2005 ; Peterson et al. 2007 ; Raff 2007 ; Erwin et al. 2011 ).
One instance is exemplified by the rise of paleobiology as a discipline that brought evolutionary theory back into paleontology and incorporated developmental, phylogenetic, and environmental approaches into a biological perspective of fossils. The journal Paleobiology celebrated its 25th anniversary in 2000 with a special issue, Deep Time: Paleobiology’s Perspective , published as a supplement to volume 26. An edited volume of 26 essays by leading paleontologists and published in 2009 demonstrates the breadth and depth of insight achieved by paleobiology as “The Paleobiological Revolution” (Sepkoski and Ruse 2009 ).
We have long appreciated that development is responsible for introducing variation at the level of the individual (Darwin 1859 ; Thomson 1988 ; Hallgrímsson and Hall 2005 ; Salazar-Ciudad 2006 ; Fusco and Minelli 2010 ). Much of the research in evo–devo, however, has been conducted on features of organisms that characterize particular groups and whose evolutionary origin was a major departure (transition is the term often used for the change, novelty, or innovation for the character) from prior characters (Brylski and Hall 1988a , b ; Hall and Kerney 2012 ). Important examples include feathers and the origin of birds/flight (Prum and Brush 2002 ; Xu et al. 2011 ), flowers and the origin of land plants (Niklas 1997 ; Leliaert et al. 2011 ), wings and the origin of insects (Carroll et al. 1995 ; Abouheif and Wray 2002 ), and shells and the origin of turtles (Gilbert et al. 2001 ; Rieppel 2001 ; Willmore 2010 ). Indeed, some researchers see the major contribution of evo–devo (and its major contribution to expanding the modern synthesis of evolution) as being to provide a theoretical basis from developmental biology for the origin of novelties (Müller and Wagner 1991 ; Müller 2007a , b Pigliucci and Müller 2010 ).
New groups of organisms, as represented by new classes or orders, can arise slowly through the gradual accumulation of new characters or can arise more rapidly through key innovations (the origination of wings or lungs, for example) or through coordinated changes in different characters as seen in the origin of lungs, middle ear ossicles, and the transformation of fins to limbs at the origin of the tetrapods (Thomson 1988 ; Shubin et al. 1997 ). This is how microevolutionary changes within species are linked to macroevolutionary changes, as reflected in levels of classification: “the careful analyses of the differences in pathways between organisms of known phylogenetic relationship” (Thomson 1988 , p. 138).
The list of mechanisms summarized in Box 1 implies that evolutionary developmental mechanisms are not all found in the genes, although all have a genetic basis. This is because new mechanisms emerge as development proceeds. Evolutionary developmental mechanisms may be genetic, cellular, developmental, physiological, hormonal, or any combination of these. Embryonic development is hierarchical, with new properties and mechanisms emerging as development unfolds, each dependent on the stage/processes preceding them. The single cell that is the fertilized egg cannot show any of the cell-to-cell interactions that characterize the multicellular embryo, some of which come about because cells take up new positions in the embryo through active migration. The recent elucidation of gene networks is providing the regulatory link between the genotype and cellular modules (Davidson 2006 ; Davidson and Erwin 2006 ; Wagner et al. 2007 ; Wilkins 2007 ). Embryonic inductions, tissue, organ, and functional interactions link cellular activity to the phenotype. The onset of embryonic movement ushers in a new type of process, which is interactions between developing tissues and organs such as bones and muscles (Box 1; and see Hall 1999 , 2003a , b ; Wilkins 2002 ; Hall and Olson 2003 ).
Some of the most striking information has come from the discovery that different morphological types of some single species (often referred to as morphs) arise following interactions with individuals of other species. These range from species that live in societies with different life history forms [chemical signaling between individuals determines the balance of workers and soldiers in ant colonies (Nijhout 1999 ); chemicals produced by predatory tadpole shrimp induce the formation of a protective helmet and enlargement of the head in the water shrimp Daphnia (Petrusek et al. 2009 )]; the density of tadpoles and/or the amount of food promoting the development of large cannibalistic tadpoles in such species as the New Mexico spadefoot toad Scaphiopus multiplicatus (Pfennig 1992 ; Ledón-Rettig and Pfenning 2011 ); moth larvae-mimicking leaves of catkins in response to seasonal levels of tannin produced by oak trees when either catkins or leaves are on the trees (Greene 1989 , 1999 ); and interactions of bacterial viral and eukaryotic species in the human gut with one another and the role of this microbiota in maintaining the health of their human hosts (Clemente et al. 2012 ).
Of course, it is impossible to tell what the future of evo–devo will be or what evo–devo will bring to the future. Nevertheless, given the past dramatic history of links between development and evolution, it is interesting to speculate on the future.
It could be business as usual, with evo–devo continuing to inform us of how changes in development relate to changes in evolution. Or evo–devo may change.
Evo–devo Plus Endless Prefixes/Suffixes
The past decade has seen the addition of modifiers to evo–devo to reflect its embracing of other fields. Thus:
Eco-evo–devo brings ecology into evo–devo (Hall 2003a ; Gilbert and Apel 2008 ).
Evo–devo–niche construction links development to the evolutionary role of organisms constructing essential elements of their niche such as nests or tunnels (Laland et al. 2008 ).
Behav–evo–devo is the use of evolutionary developmental mechanisms to explain the origins of behaviors, learning, and language (Lickliter 2007 ; Bertossa 2011 ; Hoang et al. 2011 ; Hall 2012 ).
Evo–devo–medicine is the application of evo–devo to medical practice (Gluckman et al. 2009 ).
The future of evo–devo may be a continuation of this trend: evo–devo–physiology; evo–devo–life history evolution.
Or it could be that evo–devo will be replaced by what has been called developmental evolution or devel-evol (Hall 2000 ; Wagner 2000 ).
What is the difference between evo–devo and devo–evo? Evo–devo seeks to situate development within the study of evolution (Carroll 2008 ). Devo–evo seeks to generate a new theory of evolution based in development. Gunter Wagner and his colleagues (Wagner 2000 ; Wagner et al. 2000 ; Wagner and Larsson 2003 ), for example, maintain that understanding (1) the origin of innovations (novelties), (2) why development is constrained along particular paths, and (3) how new properties emerge in evolution requires an approach that is fundamentally evolutionary (devo–evo) rather than developmental (evo–devo).
Devo–evo would provide a new theory of the origin of novel structures and behaviors with evolution based on population genetics continuing to explain variation in existing phenotypes and different theories to explain the evolution of the new and the maintenance of the old (Hallgrímsson and Hall 2005 , 2011 ). Ken Weiss emphasized this in a discussion of the importance of niche construction and behavior-driven evolution when he concluded that “local divergence can be achieved by behavioral sorting by which organisms find or modify local niches according to their abilities, but this need not involve fitness differences nor even be based on genetic inheritance” (Weiss 2004 , p. 203).
Evo–devo = Evo
Or, it could be, as Scott Gilbert mused, “that sooner or later, the term ‘evo–devo’ will be abandoned, because at that time it will have become synonymous with ‘evolutionary biology” (Gilbert 2009 , p. 332). The Modern Synthesis forged in the 1930s and 1940s will become the “Expanded Modern Synthesis” or the “Extended Synthesis” of the twenty-first century. Although there were calls in the past for the replacement of the modern synthesis, the expectation now is an expanded synthesis that incorporates development (Müller 2007a , b ; Pigliucci and Müller 2010 ). Sommer ( 2009 ), who evaluated the various likely fates of evo–devo, demonstrated that “a synthesis of evo–devo with population genetics and evolutionary ecology is needed to meet future challenges” (p. 416), those challenges being to understand phenotypic change and novelty (his call for researchers to choose a limited number of model organisms goes against a major trend in evo–devo, which is to expand the numbers of organisms under investigation).
Unification
An expanded synthesis will be more than the modern synthesis plus evo–devo.
Laubichler ( 2010 ) argues that “the revolutionary nature of evo–devo lies precisely in its return to a more inclusive conception of phenotypic evolution, one that more closely resembles the conceptual framework of Darwin and the first few generations of evolutionists than the more narrowly focused interpretation of the Modern Synthesis” (p. 199). Laubichler is referring to the separation of the study of heredity, development, and evolution early in the twentieth century (Allen 1975 ; Laubichler and Maienschein 2007 ; Deichman 2011 ) and the need to reunify these three before a full account of development, evolution, or evo–devo can be given. Biology was integrated in the late nineteenth century. Evo–devo will be at center stage when we forge the integrated biology of the twenty-first century.
Conceptual unification will require much more than the addition of more and more suffixes and prefixes until all of biology is ensnared by evo–devo. As Laubichler ( 2007 ) points out, “any future synthesis of evo–devo will be conceptual rather than simply data driven…[and allow] the integration of developmental mechanisms into evolutionary explanations at a higher level of resolution than the current ideas about regulatory evolution and the evolution of the genetic toolkit suggest” (pp. 343, 359). At the very least, unification will involve molecular genetics, development, paleontology, systematics, the nature of heredity, and the role of ecology and the environment.
Experience from the past teaches us that unification could arise from the development of new techniques and methods, as occurred with the ability to identify biological species, decipher the genetic code in DNA, sequence genomes, and create phylogenetic trees. Experience also teaches us that unification may (is more likely to?) require unthought-of and perhaps revolutionary concepts and/or new ways to integrate signaling mechanisms operating at different levels (genes, cells, organs, organisms–environment). The gene regulatory networks discussed above provide such a conceptual and integrative advance at the level of gene action, but so far, they are not sufficient to link the activity of genes to the activities of cells. The phenotypic plasticity that links environment to phenotypic response is integrative at the level of environment–organism, but not at the level of genes–cells. We have traveled an enormously long way, but have an even longer uncharted road ahead.
As an exception, one of the acknowledged founders of evo-devo, N.J. Berrill (1903–1996), included a chapter on evolution and development in an undergraduate biology textbook (Berrill 1966 ) in which he saw the importance of development for understanding phylogenetic relationships, not because of recapitulation (see Origins of Evo-Devo) but because of developmental mechanisms such as neoteny.
Abouheif E, Wray G. Evolution of the gene network underlying wing polyphenism in ants. Science. 2002;297:249–52.
CAS PubMed Google Scholar
Allen G. Life science in the twentieth century. New York: Wiley; 1975.
Google Scholar
Arthur W. A theory of the evolution of development. Chichester: Wiley; 1988.
Averof M. Arthropod evolution: same Hox genes, different body plans. Curr Biol. 1997;7:R634–6.
Balfour FM. A preliminary account of the development of the elasmobranch fishes. Q J Microsc Sci. 1874;14:323–64.
Bateson W. Evolutionary faith and modern doubts. Science. 1922;55:53–61.
Berrill NJ. Biology in action: a beginning college textbook. New York: Dodd, Mead & Co.; 1966.
Bertossa RC. Morphology and behaviour: functional links in development and evolution. Philos Trans R Soc B. 2011;366:2056–68.
Biémont C. From genotype to phenotype. What do epigenetics and epigenomics tell us? Heredity. 2010;105:1–3.
PubMed Google Scholar
Bonner JT. Evolution and development. Report of the Dahlem Workshop on Evolution and Development, Berlin 1981, May 10–15. Life Sciences Research Report 22. Berlin: Springer; 1982.
Bowler PJ. Life's splendid drama. Evolutionary biology and the reconstruction of life's ancestry 1860–1940. Chicago: The University of Chicago Press; 1996.
Brylski P, Hall BK. Ontogeny of a macroevolutionary phenotype: the external cheek pouches of geomyoid rodents. Evolution. 1988a;42:391–5.
Brylski P, Hall BK. Epithelial behaviour and threshold effects in the development of external and internal cheek pouches in rodents. Zool Syst Evolutionsforsch. 1988b;26:144–54.
Calow P. Life cycles: an evolutionary approach to the physiology of reproduction, development and ageing. London: Wiley; 1978.
Calow P. Evolutionary principles. Blackie: Glasgow and London; 1983.
Carroll SB. Evo–devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36.
Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61.
Carroll SB, Grenier JK, Weatherbee SD. From DNA to diversity. Molecular genetics and the evolution of animal design. 2nd ed. Malden: Blackwell Science; 2005.
Clemente JC, Ursell LK, Palfrey LW, Knight B. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–70.
Collins JP, Gilbert S, Laubichler MD, Müller GB. Modeling in EvoDevo: how to integrate development, evolution, and ecology. In: Laubichler MD, editor. Roots of theoretical biology: the Prater Vivarium centenary. Cambridge: MIT Press; 2007. p. 355–78.
Dahl E, Koseki H, Balling R. Pax genes and organogenesis. BioEssays. 1997;19:755–65.
Darwin CR. The origin of species by means of natural selection. London: John Murray; 1859.
Davidson EH. The regulatory genome: gene regulatory networks in development and evolution. San Diego: Academic; 2006.
Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311:796–800.
de Beer GR. Embryos and evolution. Oxford: Clarendon; 1930.
Deichman U. Early 20th-century research at the interface of genetics, development, and evolution: reflections on progress and dead ends. Dev Biol. 2011;347:3–12.
Dobzhansky Th. Genetics and the origin of species. New York: Columbia University Press; 1937.
Erwin DH, Wing SL. Deep time: paleobiology’s perspective. Paleobiology (Suppl). 2000;26(4):1–371.
Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science. 2011;334:1091–7.
Fusco G, Minelli A. Phenotypic plasticity in development and evolution: facts and concepts. Philos Trans R Soc B. 2010;365:547–56.
Gehring WJ. The homeobox: a key to the understanding of development? Cell. 1985;40:3–5.
Gehring WJ. Master control genes in development and evolution: the homeobox story. New Haven: Yale University Press; 1998.
Gilbert SF. 2009 BIO. Evol Dev. 2009;11:331–2.
Gilbert SF, Apel D. Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sunderland: Sinauer Associates; 2008.
Gilbert SF, Loredo GA, Brukman A, Burke AC. Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev. 2001;3:47–58.
Gissis SB, Jablonka E. Transformations of Lamarckism: from subtle fluids to molecular biology. Cambridge: MIT Press; 2011.
Gluckman P, Beedle A, Hanson M. Principles of evolutionary medicine. Oxford: Oxford University Press; 2009.
Goldschmidt R. A preliminary report on some genetic experiments concerning evolution. Am Nat. 1918;52:28–50.
Goodwin BC, Holder NJ, Wylie CC, editors. Development and evolution. Cambridge: Cambridge University Press; 1983.
Gould SJ. Ontogeny and phylogeny. Cambridge: The Belknap Press of Harvard University Press; 1977.
Greene E. A diet-induced developmental polymorphism in a caterpillar. Science. 1989;243:643–66.
Greene E. Phenotypic variation in larval development and evolution: polymorphism, polyphenism, and developmental reaction norms. In: Hall BK, Wake MH, editors. The origin and evolution of larval form. San Diego: Academic; 1999. p. 379–410.
Grenier JK, Garber TL, Warren R, Whitington PM, Carroll SB. Evolution of the entire arthropod Hox gene set predated the origin and radiation of the onychophoran/arthropod clade. Curr Biol. 1997;7:547–53.
Haeckel E. Generelle morphologie der organismen: Allgemeine grundzüge der organischen formen-wissenschaft, mechanisch begründet durch die von Charles Darwin reformite descendenz-theorie (2 volumes). Berlin: Georg Reimer; 1866.
Hall BK. Evolutionary developmental biology. London: Chapman and Hall; 1992.
Hall BK. Homology: the hierarchical basis of comparative biology. San Diego: Academic; 1994.
Hall BK. Evolutionary developmental biology. 2nd ed. Dordrecht: Kluwer Academic; 1999.
Hall BK. Evo–devo or devo–evo—does it matter? Evol Dev. 2000;2:177–88.
Hall BK. John Samuel Budgett (1872–1904): in pursuit of Polypterus . BioScience. 2001;51:399–407.
Hall BK. Palaeontology and evolutionary developmental biology: a science of the 19th and 21st centuries. Palaeontology. 2002;45:647–69.
Hall BK. Evolution as the control of development by ecology. In: Hall BK, Pearson R, Müller GB, editors. Environment, evolution and development: towards a synthesis. Cambridge: MIT Press; 2003a. p. ix–xxiii.
Hall BK. Unlocking the black box between genotype and phenotype: cells and cell condensations as morphogenetic (modular) units. Biol Philos. 2003b;18:219–27.
Hall BK. Homology, homoplasy, novelty and behavior. Dev Psychobiol. 2012 (in press).
Hall BK, Kerney R. Levels of biological organization and the origin of novelty. J Exp Zool (Mol Dev Evol). 2012;314B. doi: 10.1002/jez.b.21425 .
Hall K, Olson WM (eds). Keywords and concepts in evolutionary developmental biology . Cambridge, MA: Harvard University Press; 2003.
Hall BK, Laubichler MD, (eds). Conrad Hal Waddington, theoretical biology, and evo–devo. Biol. Theory. 2009;3(3):185–289.
Hallgrîmsson B, Hall BK, editors. Epigenetics: linking genotype and phenotype in development and evolution. Berkeley: University of California Press; 2011.
Hallgrímsson B, Hall BK (eds). Variation: a central concept in biology . New York: Elsevier/Academic Press; 2005
Hoang T-H, McKay RI, Essam D, Hoai NX. On synergistic interactions between evolution, development and layered learning. IEEE Trans Evol Comput. 2011;15:287–311.
Huxley JS. Evolution: the modern synthesis. London: Allen and Unwin; 1942.
Kosik KS. MicroRNAs tell an evo–devo story. Nat Rev Neurosci. 2009;10:754–9.
Laland KN, Odling-Smee J, Gilbert SF. EvoDevo and niche construction: building bridges. J Exp Zool (Mol Dev Evol). 2008;310B:549–66.
Laubichler MD. Evolutionary developmental biology. In: Hull DL, Ruse M, editors. The Cambridge companion to the philosophy of biology. Cambridge: Cambridge University Press; 2007. p. 342–60.
Laubichler MD. Evolutionary developmental biology offers a significant challenge to the neo-Darwinian paradigm. In: Ayala FJ, Arp R, editors. Contemporary debates in philosophy of biology. Malden: Wiley-Blackwell; 2010. p. 199–212.
Laubichler MD, Maienschein J, editors. From Embryology to evo–devo: a history of developmental evolution. Cambridge: MIT Press; 2007.
Laubichler MD, Wagner GP. Introduction to the papers of the 2001 Kowalevsky Medal winner symposium. J Exp Zool (Mol Dev Evol). 2004;302B:1–4.
Ledón-Rettig CC, Pfenning DW. Emerging model systems in eco–evo–devo: the environmentally responsive spadefoot toad. Evol Dev. 2011;13:391–400.
Leliaert F, Verbruggen H, Zechman FW. Into the deep: new discoveries at the base of the green plant phylogeny. BioEssays. 2011;33:683–92.
Lewis EB. A gene complex controlling segmentation in Drosophila . Nature. 1978;276:565–70.
Lickliter R. The dynamics of development and evolution: insights from behavioral embryology. Dev Psychobiol. 2007;49:749–57.
MacDonald ME, Hall BK. Altered timing of the extracellular-matrix-mediated epithelial–mesenchymal interaction that initiates mandibular skeletogenesis in three inbred strains of mice: development, heterochrony, and evolutionary change in morphology. J Exp Zool. 2001;291:258–73.
Macleod R. Embryology and empire. In: Macleod R, Rehbock PF, editors. Darwin's laboratory. Evolutionary theory and natural history in the Pacific. Honolulu: University of Hawaii Press; 1994. p. 140–65.
Maynard Smith J. Weismann and modern biology. In: Harvey PH, Partridge L, editors. Oxford surveys in evolutionary biology, vol. 6. Oxford: Oxford University Press; 1989. p. 1–12.
Mayr E. The growth of biological thought. Diversity, evolution, and inheritance. Cambridge: The Belknap Press of Harvard University Press; 1982.
Mayr E. The establishment of evolutionary biology as a discrete biological discipline. BioEssays. 1997;19:263–6.
McCain KW. Using tricitation to dissect the citation image: Conrad Hal Waddington and the rise of evolutionary developmental biology. J Am Soc Inf Sci Technol. 2009;60:1301–19.
McCain KW. Core journal literatures and persistent research themes in an emerging interdisciplinary field: exploring the literature of evolutionary development biology. J Informet. 2010;4:157–65.
Moazed D. Mechanisms for the inheritance of chromatin states. Cell. 2011;146:510–7.
Molaro A, Hodges E, Fang F, Song Q, McCombie WR, Hannon GJ. Sperm methylation profiles reveal features of epigenetic inheritance and evolution in primates. Cell. 2011;146:1029–41.
Müller F. Für Darwin. Leipzig: Engelman; 1864 (Translated by W. S. Dallas as Facts and Arguments for Darwin , London: John Murray, 1869).
Müller GB. Six memos for EvoDevo. In: Laubichler MD, Maienschein J, editors. From Embryology to evo–devo: a history of developmental evolution. Cambridge: MIT Press; 2007a. p. 499–524.
Müller GB. Evo–devo” extending the evolutionary synthesis. Nat Rev Genet. 2007b;8:943–9.
Müller GB, Wagner GP. Novelty in evolution: restructuring the concept. Annu Rev Ecol Syst. 1991;22:229–56.
Nijhout HF. Hormonal control in larval development and evolution—insects. In: Hall BK, Wake MH, editors. The origin and evolution of larval form. San Diego: Academic; 1999. p. 217–54.
Niklas KJ. The evolutionary biology of plants. Chicago: The University of Chicago Press; 1997.
Olsson L, Hoßfeld U, Breidbach O. Preface. Between Ernst Haeckel and the homeobox: the role of developmental biology in evolution. Theory Biosci. 2009;128:1–5.
Peterson KJ, Summons RE, Donoghue PCJ. Molecular paleobiology. Palaeontology. 2007;50:775–809.
Petrusek A, Tollrian R, Schwenk K, Haas A, Laforsch C. A “crown of thorns” is an inducible defense that protects Daphnia against an ancient predator. Proc Natl Acad Sci USA. 2009;106:2248–52.
Pfennig DW. Polyphenism in spadefoot toad tadpoles as a locally adjusted evolutionarily stable strategy. Evolution. 1992;46:1408–20.
Pigliucci M, Müller GB, editors. Evolution—the extended synthesis. Cambridge: The MIT Press; 2010.
Prum RO, Brush AH. The evolutionary origin of diversification of feathers. Q Rev Biol. 2002;77:261–95.
Raff RA. Direct-developing sea urchins and the evolutionary reorganization of early development. BioEssays. 1992;14:211–8.
Raff RA. Written in stone: fossils, genes and evo–devo. Nat Rev Genet. 2007;8:911–20.
Raff RA, Love AC. Kowalevsky, comparative evolutionary embryology, and the intellectual lineage of evo–devo. J Exp Zool (Mol Dev Evol). 2004;302B:19–34.
Rieppel O. Turtles as hopeful monsters. BioEssays. 2001;23:987–91.
Salazar-Ciudad I. Developmental constraints vs. variational properties: how pattern formation can help to understand evolution and development. J Exp Zool (Mol Dev Evol). 2006;306B:107–25.
Sepkoski D, Ruse M. (eds). The paleobiological revolution: essays on the growth of modern paleontology. Chicago: The University of Chicago Press; 2009.
Shubin N, Tabin C, Carroll S. Fossils, genes, and the evolution of animal limbs. Nature. 1997;388:639–48.
Sommer RJ. The future of evo–devo: model systems and evolutionary theory. Nat Rev Genet. 2009;10:416–22.
Suga H, Tschopp P, Graziussi DF, Stierwald M, Schmid V, Gehring WJ. Flexibly deployed Pax genes in eye development at the early evolution of animals demonstrated by studies on a hydrozoan jellyfish. Proc Natl Acad Sci USA. 2010;107:14263–8.
Thomson KS. Morphogenesis and evolution. Oxford: Oxford University Press; 1988.
Tills O, Rundle SD, Salinger M, Haun T, Pfenninger M, Spicer JI. A genetic basis for intraspecific differences in developmental timing? Evol Dev. 2011;13:542–8.
Valentine JW. On the origin of phyla. Chicago: The university of Chicago Press; 2004.
Waddington CH. Organizers and genes. Cambridge: Cambridge University Press; 1940.
Wagner GP. What is the promise of developmental evolution? Part I: Why is developmental biology necessary to explain evolutionary innovations? J Exp Zool (Mol Dev Evol). 2000;288:95–8.
CAS Google Scholar
Wagner GP, Larsson HCE. What is the promise of developmental evolution? III. The crucible of developmental evolution. J Exp Zool (Mol Dev Evol). 2003;300B:1–4.
Wagner GP, Chiu C-H, Laubichler M. Developmental evolution as a mechanistic science: the inference from developmental mechanisms to evolutionary processes. Am Zool. 2000;40:819–31.
Wagner GP, Pavlicev M, Cheverud JM. The road to modularity. Nat Rev Genet. 2007;8:921–31.
Weiss KM. Thomas Henry Huxley (1825–1895) puts us in our place. J Exp Zool (Mol Dev Evol). 2004;302B:196–206.
West-Eberhard MJ. Developmental plasticity and evolution. Oxford: Oxford University Press; 2003.
Wilkins AS. The evolution of developmental pathways. Sunderland: Sinauer Associates; 2002.
Wilkins AS. Between “design” and “bricolage”: genetic networks, levels of selection, and adaptive evolution. Proc Natl Acad Sci USA. 2007;104:8590–6.
Willmore KE. Development influences evolution. Am Sci. 2010;98:220–7.
Xu X, You H, Du K, Han F. An Archaeopteryx -like theropod from China and the origin of Avialae. Nature. 2011;475:465–70.
Zelditch M. Beyond heterochrony: the evolution of development. New York: Wiley; 2001.
Download references
Acknowledgments
Research in my laboratory is supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada (grant A5056). I thank all past laboratory colleagues—students, postdoctoral fellows, sabbaticants, and collaborators—for discussions from which I have benefited enormously over the past 44 years. Jane Maienschein and an anonymous referee provided important comments on the manuscript.
Author information
Authors and affiliations.
Department of Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2
Brian K. Hall
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Brian K. Hall .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Hall, B.K. Evolutionary Developmental Biology (Evo-Devo): Past, Present, and Future. Evo Edu Outreach 5 , 184–193 (2012). https://doi.org/10.1007/s12052-012-0418-x
Download citation
Published : 08 June 2012
Issue Date : June 2012
DOI : https://doi.org/10.1007/s12052-012-0418-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Evolutionary developmental biology
- Gene regulation
- Evolutionary developmental mechanisms
- Modern synthesis
- Expanded modern synthesis
Evolution: Education and Outreach
ISSN: 1936-6434
- Submission enquiries: [email protected]
Loading metrics
Open Access
Essays articulate a specific perspective on a topic of broad interest to scientists.
See all article types »
Evolutionary Biology for the 21st Century
* E-mail: [email protected]
Affiliation Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts, United States of America
Affiliation Department of Zoology, Oregon State University, Corvallis, Oregon, United States of America
Affiliation Departments of Developmental Biology and Computer Science, Stanford University, Stanford, California, United States of America
Affiliation Department of Biology, University of Virginia, Charlottesville, Virginia, United States of America
Affiliation Department of Biology, Clark University, Worcester, Massachusetts, United States of America
Affiliations Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts, United States of America, Department of Molecular and Cellular Biology, Harvard University, Cambridge, Massachusetts, United States of America
Affiliation Department of Biochemistry and Biophysics, University of California, San Francisco, California, United States of America
Affiliation Department of Ecology and Evolutionary Biology, Yale University, New Haven, Connecticut, United States of America
Affiliations Museum of Vertebrate Zoology, University of California, Berkeley, California, United States of America, The Australian National University, Canberra, Australia
Affiliation Department of Biology, University of Rochester, Rochester, New York, United States of America
Affiliation Department of Biology, Stanford University, Stanford, California, United States of America
Affiliation Department of Biology, University of Munich, Munich, Germany
Affiliation Department of Biology, University of Missouri, St. Louis, Missouri, United States of America
Affiliation Florida Museum of Natural History, University of Florida, Gainesville, Florida, United States of America
Affiliation Department of Ecology, Evolution and Marine Biology, University of California, Santa Barbara, California, United States of America
- Jonathan B. Losos,
- Stevan J. Arnold,
- Gill Bejerano,
- E. D. Brodie III,
- David Hibbett,
- Hopi E. Hoekstra,
- David P. Mindell,
- Antónia Monteiro,
- Craig Moritz,

Published: January 8, 2013
- https://doi.org/10.1371/journal.pbio.1001466
- Reader Comments
Citation: Losos JB, Arnold SJ, Bejerano G, Brodie ED III, Hibbett D, Hoekstra HE, et al. (2013) Evolutionary Biology for the 21st Century. PLoS Biol 11(1): e1001466. https://doi.org/10.1371/journal.pbio.1001466
Copyright: © 2013 Losos et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The workshop that led to this report was funded by the National Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
We live in an exciting time for biology. Technological advances have made data collection easier and cheaper than we could ever have imagined just 10 years ago. We can now synthesize and analyze large data sets containing genomes, transcriptomes, proteomes, and multivariate phenotypes. At the same time, society's need for the results of biological research has never been greater. Solutions to many of the world's most pressing problems—feeding a global population, coping with climate change, preserving ecosystems and biodiversity, curing and preventing genetically based diseases—will rely heavily on biologists, collaborating across disciplines.
Theodosius Dobzhansky famously proclaimed that “nothing makes sense in biology except in the light of evolution." Though Dobzhansky's statement is sometimes dismissed by biologists in other fields as self-promotion, recent advances in many areas of biology have shown it to be prophetic. For example, genomics, which emerged mostly from molecular biology, is now steeped in evolutionary biology. Evolutionary theory helps to explain our origins, our history, and how we function as organisms and interact with other life forms, all of which are crucial to understanding our future (e.g., [1] – [5] ). Evolutionary approaches have helped reconstruct the history of human culture, including, for example, the history of human populations and languages [6] – [11] . And the impact of evolutionary biology is extending further and further into biomedical research and nonbiological fields such as engineering, computer sciences, and even the criminal justice system.
The pervasive relevance of evolution can be seen in the 2009 report commissioned by the National Research Council of the National Academies, A New Biology for the 21 st Century [12] , which identified four broad challenges for biology: develop better crops to feed the world, understand and sustain ecosystem function and biodiversity in a changing world, expand sustainable alternative energy sources, and understand individual health. In each of these areas, the report noted, evolutionary concepts and analyses have played—and will continue to play—an integral role.
It's hard to overstate evolutionary biology's power to explain the living world and our place in it. Many applications of evolutionary theory and methods—from animal and plant breeding to vaccine development to management of biological reserves and endangered species—affect society and promote human well-being [13] , [14] . Much human activity, however, is changing Earth's climate and habitats, with uncertain but potentially severe environmental stresses on many other species [15] – [18] , and the solutions to the many resulting problems may well require understanding evolutionary interactions among species and their mutual dependencies.
Our ability to apply evolutionary concepts to a wide range of problems has never been greater. Changes in the availability of data and an emerging scientific culture that embraces rapid, open access to many kinds of data (genomic, phenotypic, and environmental), along with a computational infrastructure that can connect these rich sources of data ( [19] , Figure 1 ), will transform the nature and scale of problems that can be addressed by evolutionary biology.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
All this can be connected to the Tree of Life (phylogeny), from populations to entire clades, and is enabled by new protocols and networks in biodiversity informatics.
https://doi.org/10.1371/journal.pbio.1001466.g001
Periodically, groups of scientists meet to identify new opportunities in evolutionary biology and associated disciplines (e.g., [12] , [20] – [23] ). Rather than set a specific research agenda for the future—clearly the charge of individual investigators—the aim has been to identify new themes and research directions that are already emerging in the field and to focus on the intersection of fundamental problems with new technologies and methods. In the following sections, we briefly highlight some key applications of evolutionary biology, provide examples of emerging research areas, and identify infrastructure and training needs.
Evolutionary Applications
Evolutionary medicine.
The new field of “evolutionary medicine" [24] – [26] posits that understanding our evolutionary past can inform us of the causes of perplexing common diseases. For instance, diabetes and autoimmune diseases such as asthma may represent mismatches between evolutionary adaptation to the environments in which humans evolved and current conditions. In addition, some age-related conditions, such as cancer, can be understood as the outcome of selection for early reproduction, when humans faced dying of disease or predation at an early age. This long-term selection on the cellular machinery to optimize growth and survival through early reproduction may now explain the prevalence of cancer late in life, a modern malaise that emerges because of the recent, rapid extension of postreproductive lifespan [27] . Aside from providing explanations for the occurrence of diseases, the field of evolutionary medicine is also concerned with suggesting strategies for slowing the evolution of resistance in pathogen populations [28] – [30] ; strategies to improve public health and reduce the incidence of common diseases [31] , [32] ; prediction of diseases that may emerge from recent host-shifts to humans [33] ; discovery, design, and enhancement of drugs and vaccines (e.g., [34] ); and understanding the role of the microbiome in human health [35] .
Feeding the Human Population
Feeding the rapidly growing human population, especially with increasing stress on agricultural systems from climate change, continues to be a major challenge. The green revolution, from the 1950s onwards, rested on selective plant breeding for larger yields and was underpinned by evolutionary theory [36] . Currently, the trend is to rely on biotechnology to introduce either herbicide resistance genes or herbivore-directed toxins, such as Bt, to combat crop competitors and herbivores, respectively, and thus promote increasing yields [37] . Unfortunately, genetically modified crops are genetically uniform and so do not represent a long-term solution against the evolution of either herbicide or Bt resistance. In addition, these herbicide resistance or toxin genes can be transferred to other nontarget species through pollen-mediated hybridization, rendering them resistant or toxic as well [38] . The agriculture of the future must incorporate evolutionary thinking to reduce the evolution of resistance and the risk of pathogen outbreaks. Maintaining genetic diversity in crop and animal production systems considerably reduces these risks [38] .
Sustaining Biological Diversity
Evolutionary approaches have often been applied to the conservation of species and ecosystems [13] , [39] – [42] . Linking spatial data on phenotypes, genomes and environments in a phylogenetic context allows us to identify and name Earth's diverse life forms. This linkage, in turn, helps to provide the basic units needed to quantify taxonomic diversity and to pursue its conservation. Determining phylogenetic relationships among species allows us to identify their unique adaptations and provides the historical context to understand how they arose [43] – [45] . Evolutionary approaches also can be used to determine the origins of invasive species [46] – [48] and to help design effective remediation [49] , [50] . Collectively, understanding the distribution of current biodiversity and its evolutionary response to past environmental change is fundamental to mitigating effects of ongoing habitat loss and climate change [51] . Given the rate of anthropogenic climate change, evolutionary theory and experiments can help predict vulnerability (i.e., inability to adapt) of species and thus improve conservation strategies [52] .
Computation and Design
Models of mutation, inheritance, and selection have inspired the development of computational evolutionary algorithms that are used to solve complex problems in many fields [53] , [54] . In particular, engineering and design processes have incorporated evolutionary computation, leading to improvements in design of cars, bridges, traffic systems robots, and wind turbine energy, among other applications [55] – [59] .
Evolution and Justice
Genealogical relationships bear on many court cases. Is the defendant really the parent of the plaintiff? Does the evidence (e.g., blood, semen, or skin cells) at the crime scene tend to exonerate or implicate a suspect? Evolutionary methods, particularly population genetics, are now used frequently in forensics and court cases to test the link of crime scene evidence to individuals [60] , and phylogenetic analyses have been vetted and accepted as valid and appropriate methods for determining facts of history in the United States criminal court system [61] .
Emerging Research and Future Challenges in Evolutionary Biology
Divining the direction of future scientific research is always fraught with difficulty. Nonetheless, we feel that it is possible to identify some general themes that will be important in coming years. We also present some examples of classic research problems that remain unsolved and that might well be the focus of future work, as well as new and important questions for which evolutionary approaches may be key.
The flood of data in all areas of evolutionary biology poses important theoretical challenges: new kinds of theory are sometimes required to make sense of new kinds of data. We can already point to certain broad areas of evolutionary biology that will likely demand sustained theoretical work. These include the elaboration of more formal theories for evolutionary developmental biology (e.g., analysis of gene network evolution and modification); the more complete incorporation of the roles of epigenetics, behavior, and plasticity in models of trait evolution; analysis of units of selection; and attempts to construct a quantitative and predictive theory that describes the genetic basis of adaptation. In other areas, problems will likely be more statistical than theoretical. Indeed, the enormous quantity of genome data poses serious statistical challenges even for fields that already possess strong theoretical foundations, such as evolutionary genetics.
The Explosion and Diversity of Data
DNA sequencing can now generate whole-genome data not only for single representatives of a few species but for multiple individuals from multiple conspecific populations and even from entire communities. Such multilevel data are giving rise to whole new fields of study (e.g., population genomics and metagenomics) as well as to new theoretical, computational, and data management challenges.
One particularly exciting avenue of research afforded by new genomic technology is the possibility of directly observing the dynamics of evolution. In the last few years, genomic analyses of experimental evolution have yielded new understanding of how RNA molecules, viruses, and bacteria evolve (bacteria: [62] , [63] ; virus: [64] ; RNA molecules: [65] ). This approach is now being applied to eukaryotic model systems such as C. elegans and yeast [66] – [68] . These efforts will continue to expand and will surely involve natural systems in field settings. Past evolution, for example, can be inferred from samples derived from ancient specimens, archived material in museum collections, lake sediments, and glacier cores. Contemporary evolution can be inferred from genomic sampling across seasons and years and can be detected in response to climatic disturbances such as El Niño events and to manmade environmental changes such as oil spills. In parallel with long-term ecological data (e.g., species abundance and distributions through time), we can now track genomic variation through ecological and evolutionary time. This capability, together with the realization that evolutionary change can occur on ecological timescales [69] , provides an important new window on real-time evolution. Evolution on contemporary time scales is likely to be especially important in the context of evolving pathogens, pest resistance, and the response of organisms to rapid environmental change.
While the explosion of data on genome sequences has received the most attention, supplementing these data with information on the natural history of individuals, species, and their environments will be important. Core information from field-collected specimens always includes species identity and place and time of collection, but increasingly, this information is being enriched with links to field notes and phenotypic (e.g., images), behavioral (e.g., sounds), and genomic data in a variety of databases (e.g., Morphbank— http://www.morphbank.net/ , Barcode of Life— http://www.barcodeoflife.org/ , Macaulay Library— http://macaulaylibrary.org/ ). Precise information on place, time, and reproductive stage can be integrated with data on local environmental conditions, often obtained from remote sensing [70] . The key is to connect information across repositories, such as natural history museums and genomic databases ( Figure 2 ). Such efforts will also include observational data provided by the broader public [71] .
Photo by Jeremiah Trimble, Department of Ornithology, Museum of Comparative Zoology, Harvard University.
https://doi.org/10.1371/journal.pbio.1001466.g002
Evolutionary Processes That Shape Genomic and Phenotypic Variation
The availability of genomic data from a remarkable range of species has allowed the alignment and comparison of whole genomes. These comparative approaches have been used to characterize the relative importance of fundamental evolutionary processes that cause genomic evolution and to identify particular regions of the genome that have experienced recent positive selection, recurrent adaptive evolution, or extreme sequence conservation [72] – [75] . Yet more recently, resequencing of additional individuals or populations is also allowing genome-wide population genetic analyses within species [76] – [82] . Such population-level comparisons will allow even more powerful study of the relative importance of particular evolutionary processes in molecular evolution as well as the identification of candidate genomic regions that are responsible for key evolutionary changes (e.g., sticklebacks [83] , butterflies [84] , Arabidopsis [85] ). These data, combined with theoretical advances, should provide insight into long-standing questions such as the prevalence of balancing selection, the relative frequency of strong versus weak directional selection, the role of hybridization, and the importance of genetic drift. A key challenge will be to move beyond documenting the action of natural selection on the genome to understanding the importance of particular selective agents. For example, what proportion of selection on genomes results from adaptation to the abiotic environment, coevolution of species, sexual selection, or genetic conflict? Finally, as sequencing costs continue to drop and analytical tools improve, these same approaches may be applied to organisms that present intriguing evolutionary questions but were not tractable methodologically just a few years ago. The nonmodel systems of today may well become the model systems of tomorrow [86] .
Earth–Biosphere Interactions Over Vast Stretches of Time and Space
We are in the midst of a massive perturbation of natural communities as species respond to human-driven changes in climate and land cover. Beyond the challenge of understanding the capacity of species to respond (e.g., [51] , [87] ) and the potential for dramatic state-shifts in the biosphere [17] lies the daunting problem of understanding the many interactions between community-scale ecological dynamics and evolution of traits within populations.
We now can also ask if evolution matters for ecosystem functioning. To date, most ecosystem studies have assumed that all individuals that compose a population within a community are equivalent ecologically. But individuals within a population are variable, and this variation may lead to ecological interactions that are in a continual state of evolutionary flux as ecologically driven evolutionary change feedbacks to alter the ongoing ecological interactions [88] – [90] . This evolutionary perspective on communities is an emerging area that will require the acquisition and analysis of large, temporal samples of genomic and phenotypic data, as well as the direct measurement of fitness. Samples that include paleo/historical DNA as well as contemporary DNA might be especially valuable by providing a temporal view on such questions.
Understanding Biological Diversification
A major and urgent challenge is to improve knowledge of the identity and distribution of species globally. While we need to retain the traditional focus on phenotypes, powerful new capabilities to obtain and interpret both genomic and spatial data can and should revolutionize the science of biodiversity. Building on momentum from single-locus “barcoding" efforts, new genome-level data can build bridges from population biology to systematics [91] . By establishing a comprehensive and robust “Tree of Life," we will improve understanding of both the distribution of diversity and the nature and timing of the evolutionary processes that have shaped it.
Studies of the biodiversity of Bacteria and Archaea are complicated by the widespread occurrence of lateral gene transfer. However, the phylogeny of these organisms and their genes remains critical to understanding their scope, origins, distributions, and change over time [92] . The advent of metagenomic sequencing of environmental microbial communities has revealed greater diversity and flux of genotypes than ever imagined, defying conventional species concepts and presenting a major challenge to applying traditional evolutionary and ecological theory to understanding microbial diversity [93] , [94] . Addressing this challenge will be necessary to advance microbial ecology beyond the descriptive stage. Moreover, it is only with such understanding that a natural history of microbes can be developed, leading to more meaningful exploration of genomic structure and function, the origin of novel genes, and increased knowledge of microbial influences at both the global and individual (microbiome) levels.
In addition to documenting biodiversity, more research is needed on the processes that produce this diversity. While research on speciation has seen a resurgence over the last two decades [95] – [97] , new tools—including genomic data—can support new approaches for a number of important questions, including discovering genomic signatures underlying the evolution of prezygotic reproductive isolation, and describing how hybridization, contact between incipient species, genome reorganization, and genome duplication, affect speciation.
Understanding the diversification of species and the origin of adaptations poses a number of challenges for evolutionary biologists, including integration of the fossil record with diversification inferred from the relationships among contemporary species; determining the relationship between lineage and phenotypic diversification; understanding the factors that lead to the replacement of clades over time; understanding the occupancy of ecological niche space through evolutionary diversification, adaptive radiation, and extinction; and assessing the role that evolving species interactions play in diversification.
All evolution has an ecological context that is essential to the interpretation of diversification. Consequently, we need to incorporate analyses of the environmental context of evolution, particularly species interactions that are likely to both set limits to diversification and promote evolutionary novelty. For all these reasons, further integration of paleontology with other fields of evolutionary biology, as well as development of genetic-evolutionary research programs on clades with excellent fossil records (e.g., foraminifera, diatoms, mollusks; Figure 3 ), will be important. More generally, uniting understanding of evolutionary pattern and process will require reductionist studies on evolutionary mechanisms of species formation and phenotypic change, as well as broadly historical studies that incorporate phylogenetic, paleontological, and geological data.
Genomic sequence data for stickleback fish is now providing insight into evolutionary patterns, such as the reduction in the pelvic skeleton, manifest both in the fossil record and in extant populations [83] . Photograph courtesy Peter J. Park.
https://doi.org/10.1371/journal.pbio.1001466.g003
As we address these challenges, the importance of natural history data cannot be overemphasized. Observations on the natural history of organisms, the basic building blocks of more detailed studies of ecology and evolution, are critical if we are to preserve and understand biological diversity [98] . Though few would argue against this point in principle, natural history research is rarely encouraged or supported. Making the acquisition of natural history data an integral part of hypothesis-driven science is now more important than ever.
Logistical Issues and Opportunities
To take full advantage of technological advances, especially the availability of new data types and databases, we must confront several challenges that involve community resources and how we use them. Some challenges concern infrastructure, while others involve aspects of scientific culture. Still others involve how we train the next generation of evolutionary biologists, who will need a better grasp of diverse disciplines—from natural history to developmental biology—as well as bioinformatics skills to handle immense datasets across multiple fields (see Text S1 and also Figure S2 ).
The infrastructure challenges center on creation of new kinds of databases—for instance, ones that focus on (continuous) phenotypic and not merely (discrete) DNA sequence data—as well as on integration across databases to allow synthesis of very different kinds of data (see Text S2 ). The cultural challenges center on the need for supporting a climate of scientific openness. Maintaining openness will require evolutionary biologists to make the results of their research available rapidly and in a form that is most useful to their colleagues. The scientific community has already made great strides in this direction (for instance by requiring deposition of data as a condition for publication and by founding open access journals), but additional steps are necessary. We strongly support the movement toward open access for the scientific literature to accelerate research and allow more investigators to participate. We also encourage provision of open software, data and databases, as well as their computational reuse and distillation, as outlined by Lathrop et al. (2011) [99] . These individual and community efforts will be increasingly necessary for development of new research programs and insights.
As noted at the outset, we live in an exciting time for evolutionary biology. The field has embraced the “omic" revolution, and answers to many classic questions, which have motivated research for a century, are now within reach. The study of evolution, which in the past was often equated with changes in gene frequencies in populations, has become more holistic and integrative. Researchers are increasingly interested in exploring how interactions among genes, individuals, and environments have shaped the evolutionary process, both at micro- and macrolevels. At the same time, large challenges such as global warming, novel infectious diseases, and threats to biodiversity are increasing, and the opportunity for evolutionary biologists to contribute to their resolution has never been greater.
Realizing the full potential inherent in evolutionary biology is, however, far from assured. The task of integrating evolutionary knowledge within and across scales of biological organization, as discussed above, requires development of many comparative databases and analytical tools. We would do well to collaborate broadly, cultivating new expertise, and to watch out for the unexpected, as analyses of new kinds of data can reveal that preconceptions are unfounded.
Because most of our science is supported by limited public funds, evolutionary biologists and ecologists should support and participate in efforts to help the public understand the issues and the value of scientific understanding. Science in general and evolutionary science in particular are often politicized, exactly because of their fundamental importance to human society. The next 20 years hold the promise of a golden age for evolutionary biology. Whether we realize that promise depends in part on how effectively we communicate that message.
Cyberinfrastructure —The research environments that support advanced data acquisition, data storage, data management, data integration, data mining, data visualization, and other computing and information processing services distributed over the Internet beyond the scope of a single institution. In scientific usage, cyberinfrastructure is a technological solution to the problem of efficiently connecting laboratories, data, computers, and people.
Evolutionary developmental biology —The study of the evolution of development, often by the comparative study of gene expression patterns through the course of development in different species.
Evolutionary genetics —Population and quantitative genetics.
Gene network —A flow diagram describing the interactions among genes during development that affect a particular phenotype or set of phenotypes.
Genomics —The study of the entire complement of DNA in organisms (Genome), including is sequence and organization.
GMO —Genetically modified organisms in which the genome has been deliberately changed; transgenic organisms resulting from DNA manipulations.
Lateral (horizontal) gene transfer —Genetic transfer between species, as opposed to vertical gene transmission from parents to offspring in a lineage.
Metadata —Data associated with individual DNA sequences or organismal specimens (e.g., the date and locality where the sample originated, its ecological context, etc.).
Model organism —Organisms whose genome has been sequenced and for which sophisticated tools for genetic manipulation are available.
Natural history —The entire description of an organism, including its phenotype, genome, and ecological context (i.e., abiotic niche as well as its biotic interactions with other species).
Nonmodel organism —Organisms whose genome has not been sequenced and/or for which sophisticated tools for genetic manipulation are not available.
Ontology —The naming of categories, especially of the functions of genes.
Population genetics —The study of the evolutionary forces that change the genetic composition of a population; the discipline is often concerned with evolution at one or a few genetic loci.
Quantitative genetics —The study of the inheritance and evolution of traits that are typically affected by many genetic loci.
Transgenic tools —Tools that enable the deliberate transfer of DNA sequences from one organism to another or the deletion or modification of DNA sequences, in every cell, in one organism.
Supporting Information
An example of the enormous phylogenetic trees that soon will represent the norm in phylogenetic analyses. This is the consensus tree of the maximum likelihood phylogenies for 55,473 species of seed plants with the location of significant shifts in species diversification rates marked in red across the tree. Adapted from [4] .
https://doi.org/10.1371/journal.pbio.1001466.s001
The Phenomobile, a remote sensing field buggy, and the Blimp, for remotely imaging an entire field. The Phenomobile integrates a variety of remote sensing technologies for measuring phenotypic variables on many plants simultaneously. The buggy straddles a plot and collects measurements of plant temperature, stress, chemistry, color, size and shape, as well as measures of senescence. The Blimp is designed to image all the plants in an entire field from a height of 30–80 m using both infrared and digital color cameras. These technologies were developed by David Deery of the High Resolution Plant Phenomics Centre at the Commonwealth Scientific and Industrial Research Organisation in Australia. Photo credit: Carl Davies, CSIRO Plant Industry.
https://doi.org/10.1371/journal.pbio.1001466.s002
Training to sustain evolutionary biology.
https://doi.org/10.1371/journal.pbio.1001466.s003
Infrastructure needs and opportunities in evolutionary biology.
https://doi.org/10.1371/journal.pbio.1001466.s004
Acknowledgments
The workshop that led to this report was funded by the National Science Foundation. We thank the American Society of Naturalists, the Society for the Study of Evolution, and the Society of Systematic Biologists for organizational and planning assistance. Many thanks to Melissa Woolley for invaluable assistance with logistics and manuscript preparation and to M. Bell, J. Borewitz, D. Jablonski, P. Parks, K. Roy, S. Smith, J. Trimble, and A. Weirman for help procuring images.
- 1. Wilson EO (2002) The future of life. New York: Alfred A. Knopf.
- 2. Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: synthesis. Washington, DC: Island Press.
- 3. Mindell DP (2006) The evolving world: evolution in everyday life. Cambridge, MA: Harvard University Press.
- 4. Chivian E, Bernstein A (2008) Sustaining life: how human health depends on biodiversity. Oxford; New York: Oxford University Press.
- 5. Held LI Jr (2009) Quirks of human anatomy: an evo-devo look at the human body. Cambridge: Cambridge University Press.
- View Article
- Google Scholar
- 11. Pagel M (2012) Wired for culture: origins of the human social mind. New York: W.W. Norton & Company.
- 12. National Research Council (US) Committee on a New Biology for the 21st Century: Ensuring the United States Leads the Coming Biology Revolution (2009) A new biology for the 21st century: ensuring the United States leads the coming biology revolution. Washington, DC: National Academies Press. Available: https://download.nap.edu/catalog.php?record_id=12764 . Accessed May 25, 2012.
- 20. National Science Foundation (1998) Frontiers in population biology: report of a population biology task force. Arlington, VA: National Science Foundation.
- 21. National Science Foundation (2005) Frontiers in evolutionary biology. Arlington, VA: National Science Foundation.
- 23. National Research Council. 2010. Research at the intersection of the physical and life sciences. Washington, DC: National Academies Press.
- 25. Gluckman P, Beedle A, Hanson M (2009) Principles of evolutionary medicine. Oxford; New York: Oxford University Press.
- 36. Kingsbury N (2009) Hybrid: the history and science of plant breeding. Chicago: University of Chicago Press. 512 p.
- 53. Poli R, Langdon WB, McPhee NF (2008) A field guide to genetic programming. Available: http://dces.essex.ac.uk/staff/rpoli/gp-field-guide/A_Field_Guide_to_Genetic_Programming.pdf . Accessed May 22, 2012.
- 54. Chiong R, Weise T, Michalewicz Z (2011) Variants of evolutionary algorithms for real-world applications. New York: Springer-Verlag.
- 58. Byrne J, Fenton M, Hemberg E, McDermott J, O'Neill M, et al.. (2011) Combining structural analysis and multi-objective criteria for evolutionary architectural design. In: Di Chio C, et al.. editors. Applications of evolutionary computation: EvoApplications 2011: EvoCOMNET, EvoFIN, EvoHOT, EvoMUSART, EvoSTIM, and EvoTRANSLOG, Torino, Italy, April 27–29, 2011, Proceedings, Part II.
- 95. Howard DJ, Berlocher SH, eds (1998) Endless forms: species and speciation. New York; Oxford: Oxford University Press.
- 96. Coyne JA, Orr HA (2004) Speciation. Sunderland: Sinauer Press.
- 97. Dieckmann U, Doebeli M, Metz JAJ, Tautz D, editors (2004) Adaptive speciation. Cambridge studies in adaptive dynamics. Cambridge, UK: Cambridge University Press.

Department of Ecology and Evolutionary Biology
In our department we value science and education grounded in the natural history of organisms, and strive to understand the patterns and processes that structure communities and ecosystems, and drive evolutionary change over all geographical and time scales. As new methods provide insight into ecological and evolutionary mechanism and function, we seek to refine fundamental concepts, integrate findings into novel theory, and address environmental challenges. As a department we are committed to diversity, equity, inclusion, justice and belonging - values that underlie all we do.
Explore and Support our Department

Research Spotlight
Cornell’s Experimental Ponds Facility is a research and teaching resource operated by our department. For over 50 years, a broad range of field and experimental projects have utilized the Ponds facility. Past and ongoing studies provide valuable insights and solutions into a variety of topics including: conservation of migratory birds; and a broadened understanding of nutrient and chemical pathways in aquatic environments. Research teams from EEB's Holgerson and Vitousek Labs are currently using the Ponds facility for their research programs.
Our Department in the News
Vertebrate 3D scan project opens collections to all
EEB's research and teaching resource, The Cornell Museum of Vertebrates (CUMV), one of 18 institutions taking part in the oVert (openVertebrate) Thematic Collection Network project: a venture to digitize vertebrate collections in museums and make them freely available online for anyone to access

Nabokov celebrated for crossing arts/science boundaries
An avid lepidopterist since childhood, Nabokov was known to spend most of his free time on campus in the Cornell University Insect Collection.

Grad student grants support sustainability, biodiversity
Thirty-one graduate students across three colleges, including A&S, have been awarded research grants from the Cornell Atkinson Center for Sustainability.

Support Arts & Sciences on Giving Day March 14
Your gift allows the College to fulfill our mission — to prepare our students to do the greatest good in the world.


Seminar explores Nabokov as writer and ‘butterfly man’
EEB's Anurag Agrawal, the James A. Perkins Professor of Environmental Studies helps uncover the ways in which human affairs and natural environments are inevitably and inextricably entangled in Nabokov’s imagination. An avid butterfly collector, Nabokov developed theories, recently proven accurate, ...
Ponds release more greenhouse gas than they store
Global climate models and predictions rely on accurate accounting of greenhouse gas emissions and carbon storage, reports EEB assistant prof Meredith Holgerson. Holgerson and her research team are beginning to quantify the significant effects that both human-made and natural ponds have on the global...

Adding crushed rock to farmland pulls carbon out of the air
CALS Dean and EEB prof Ben Houlton part of UC Davis/Cornell research team: Adding crushed volcanic rock to cropland could play a key role in removing carbon from the air. The process, called rock weathering, can take millions of years — too slow to offset global warming. But by crushing the rock int...

December A&S graduates share stories of growth
The College hosted a new pre-graduation reception in the Groos Family Atrium of Klarman Hall for December graduates and their families.
Integrative Biology
Evolutionary biology research.

How did the incredible diversity of living organisms on Earth arise and what principles have shaped this history? How do organisms adapt to specific environmental conditions or fluctuations in those conditions? It's these types of questions, and many others, that are being asked by evolutionary biologists in our department. Whether empirically or theoretically based, integrating from the microscopic sub-cellular levels of genes and genomes to those of entire populations and/or species is a hallmark of our department's approach to evolutionary research.
Our evolutionary biology faculty

Felipe S. Barreto

My research examines how marine species adapt and diversify across environmental gradients. We quantify how evolutionary forces of natural selection, genetic drift, and migration shape the genomes of populations in the process of adaptation.
View directory profile

Michael S. Blouin

We study host-parasite evolution and the genetics of susceptibility to parasites. We also study the causes and consequences of salmon adaptation to hatcheries.

Molly K. Burke

My group is generally interested in fundamental questions in genetics and evolution, which we test using controlled laboratory experiments. How do populations adapt to long-term environmental stresses? How does evolution shape complex traits, such as aging and fertility? Can we predict the course of future evolution? To address these and other questions, we primarily use the model organism Saccharomyces cerevisiae (yeast). Current projects in the lab integrate approaches in molecular biology, microbiology, and genomics. Please visit my lab's website for details.

Kathryn M. Everson

Members of our lab work on a variety of natural systems to understand how life on Earth evolves. Our research often addresses how species are related to one another (phylogenetics), how their evolutionary history has been shaped by landscape features (phylogeography) or ecology (molecular ecology), and how they are impacted by inter-species hybridization.

Evan Forsythe

The Forsythe Lab uses molecular evolution as a tool to understand the genetic interactions that underlie cellular function. This work includes developing new bioinformatic tools capable of predicting genome-wide interaction networks (i.e. the protein-protein interactome) from publicly available genome sequences. Funded research in the lab is largely focused on plant genome evolution, but our research bridges taxa and we have active projects probing molecular evolution in plants, animals, and bacteria.

David Kikuchi

His research explores how animals make sense of vast quantities of information and use it to make adaptive decisions, including learning and innate behaviors. He also studies how an animal's decision-making impacts the evolution of other species, as well as how adaptation and behavior create feedbacks with ecology. He focuses on predator-prey systems, communication, and social learning.

David A. Lytle

Our lab uses evolutionary ecology to understand how organisms and communities are shaped by disturbances such as floods, droughts, and dams. Much of our focus is on aquatic insects, but lab members also work on projects involving fish, frogs, and riparian plants. Current projects examine the evolution of species in regulated rivers, population dynamics and biodiversity estimation in desert streams, management of dammed rivers with prescribed flow regimes, and theory of interaction-neutral community models.

Christopher J. Marshall

My research area (Taxonomy and Systematics) is a cornerstone of evolutionary biology. Through comparative methods, I infer phylogenetic relationships among taxa that serves to inform both their taxonomic classifications and our understanding of evolutionary and biogeographic histories.

Mark A. Phillips

My work uses model systems to study microevolutionary processes at a fundamental level. In particular, I am interested in the underlying genetics of adaptation and the factors that determine whether or not populations will persist in the face of changing environmental conditions.

Rebecca C. Terry

Research in the Terry Lab explores macroevolutionary themes through the analysis of the fossil record. Specific areas of interest include morphological change over time and links between specialization, species duration, and extinction.
Sulochana K. Wasala
Latest Stories
Across the department, explore recent stories.

More progress needed on ocean protection, College of Science researchers tell global conference
Undergrad explores the avian world in paid summer research adventure

Connect with schools and programs specializing in health care at the Health Professions Fair!

Best marine biology program in Oregon links students to career networks
Unlocking the genetic mysteries behind plant adaptation: New insights into the evolution of a water-saving trait in the pineapple family (bromeliaceae)
Adaptation of the photosynthetic mechanism in air plants (tillandsia) occurs through gene duplication.
Researchers at the University of Vienna, along with collaborators from France, Germany, Switzerland and the USA, have achieved a major breakthrough in understanding how genetic drivers influence the evolution of a specific photosynthesis mechanism in Tillandsia (air plants). This sheds light on the complex actions that cause plant adaptation and ecological diversity. The results of their study are now published in Plant Cell.
Some plant species have evolved a water-saving trait called Crassulacean Acid Metabolism (CAM). CAM plants like most Tillandsia species -- the most species-rich genus in the pineapple family (Bromeliaceae) -- optimise their water use efficiency: While other plants normally open their stomata (tiny pores in their leaves) during the day to absorb carbon dioxide for photosynthesis, CAM plants do this at night and stash CO 2 away for later use, helping them survive with less water. This trait evolved independently several times across the plant kingdom. However, the evolution of the complex genetic basis of CAM has remained elusive, making it a focus of research in evolutionary biology.
Gene Regulation is Key
In this study, the research team focused on a Tillandsia species pair exhibiting divergent forms of photosynthetis- CAM vs. C3 -- meaning that the C3-species lacks the specialized adaptation to arid conditions. By using advanced techniques to study the plants' genetics and biochemistry -- e.g. analyses of gene arrangements, molecular and gene family evolution, temporal differential gene expression and metabolites -- they discovered that changes in gene regulation are mainly responsible for genomic mechanisms driving CAM evolution in Tillandsia.
Clara Groot Crego, Department of Botany and Biodiversity Research at University of Vienna and lead author of the study, explains: "Our findings reveal that while large-scale changes have influenced Tillandsia's genomes like other plants, the adjustment of how photosynthesis works mainly happens through how genes are regulated -- not by changing the sequences that code for proteins." Key insights from the study, funded by the Austrian Science Fund (FWF) and the University of Vienna, include the identification of CAM-related gene families undergoing accelerated expansion in CAM species. This highlights the critical role of gene family evolution in generating novel variation that drives CAM evolution.
Into New Niches by Repeated Evolution
"CAM repeatedly evolved in different Tillandsia species and has accelerated their ability to colonize new ecological niches, serving as a key driver of the rampant speciation observed within this group," says Ovidiu Paun, Department of Botany and Biodiversity Research at University of Vienna and principal investigator of the study. "Our research highlights the potential importance of genetic innovation, beyond mere base pair changes, in driving ecological diversification," Paun adds.
Thibault Leroy, principal investigator from INRAE Toulouse, France, emphasizes that this study has implications beyond basic science. "Understanding how CAM evolved can help develop strategies to make crops more resilient to water shortages and cope with climate change."
The research will be extended across more species of this and other plant groups in the framework of a newly collaborative project jointly funded by the Austrian Science Fund (FWF) and the French National Agency for Research (ANR).
- Evolutionary Biology
- Endangered Plants
- Exotic Species
- Environmental Issues
- Drought Research
- Biodiversity
- Timeline of evolution
- Photosynthesis
- Chlorophyll
- Chloroplast
- Hydroponics
- Plant sexuality
Story Source:
Materials provided by University of Vienna . Note: Content may be edited for style and length.
Journal Reference :
- Clara Groot Crego, Jaqueline Hess, Gil Yardeni, Marylaure de La Harpe, Clara Priemer, Francesca Beclin, Sarah Saadain, Luiz A Cauz-Santos, Eva M Temsch, Hanna Weiss-Schneeweiss, Michael H J Barfuss, Walter Till, Wolfram Weckwerth, Karolina Heyduk, Christian Lexer, Ovidiu Paun, Thibault Leroy. CAM evolution is associated with gene family expansion in an explosive bromeliad radiation . The Plant Cell , 2024; DOI: 10.1093/plcell/koae130
Cite This Page :
Explore More
- 'Doubling' in Origin of Cancer Cells
- New Catalyst for Using Captured Carbon
- Random Robots Are More Reliable
- Significant Discovery in Teleportation Research
- Orangutan Treats Wound With Pain-Relieving Plant
- 75,000-Year-Old Neanderthal from Burial Cave
- Anticoagulant With an On-Off Switch
- Sleep Resets Brain Connections -- At First
- Far-Reaching Effects of Exercise
- Hidden Connections Between Brain and Body
Trending Topics
Strange & offbeat.
- Utility Menu
oebfancylogo2_0.jpeg
- Contact OEB
Four broad research areas capture the core expertise of the members of OEB. Groundbreaking research in the life sciences is now more interdisciplinary than ever and many OEB faculty work in more than one of these areas. OEB also maintains expertise in a number of sub-fields of biology, including evolutionary developmental biology , neuroethology & behavioral ecology , paleobiology , population & evolutionary genetics , physiology , biodiversity & systematics , biology of marine systems , and mathematical & computational biology . Please visit individual faculty-group web sites for more specific information. Faculty are listed under the four broad research areas below according to their primary and, for some, secondary research interests. Please click on the images below to link to faculty in the field.

- Affiliated Institutions
- Grants & Funding
- Postdoctoral Fellows
- OEB Fieldwork and Travel Map
H3 Font Wt.

Vanessa Barone

Devaki Bhaya

Jonas Cremer

Barnabas Daru

Moises Exposito-Alonso

Marcus Feldman

Hunter Fraser

Tadashi Fukami

Benjamin Good

Elizabeth Hadly

Richard Klein

Andrew Leslie

Christopher Lowe

Lauren O'Connell

Stephen Palumbi

Jonathan Payne

Dmitri Petrov

Jonathan Pritchard

Noah Rosenberg

Molly Schumer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Evolutionary biology articles within Nature Reviews Genetics
Journal Club | 15 April 2024
Understanding human uniqueness in the pre-genomic era
In this Journal Club article, Jenny Tung reflects on a 1975 paper from King and Wilson that emphasized the importance of gene regulatory changes in human evolution.
Journal Club | 19 March 2024
The evolution of modifier genes
In this Journal Club, Yoav Ram recalls how he reconciled results from his own research with the reduction principle through the help of a paper published in PNAS by Altenberg et al.
Perspective | 31 January 2024
Selection on synonymous sites: the unwanted transcript hypothesis
Multiple mechanisms have evolved to prevent or trap deleterious unwanted transcripts. The unwanted transcript hypothesis proposes that selection at synonymous sites favours mutations that prevent the generation of unwanted transcripts or that make native transcripts appear ‘wanted’ by being GC-rich.
- Sofia Radrizzani
- , Grzegorz Kudla
- & Laurence D. Hurst
Research Highlight | 26 January 2024
Top predator decline has evolutionary as well as ecological effects
Beer et al. use multiple complementary approaches to show that declining densities of the Tasmanian devil have had evolutionary effects on gene flow and selection in the subordinate predator, the spotted-tail quoll.
- Kirsty Minton
Review Article | 23 January 2024
Horizontal gene transfer in eukaryotes: aligning theory with data
In this Review, Patrick Keeling proposes that the eukaryotic-specific processes of phagocytosis and endosymbiosis are unlikely to increase the frequency of horizontal gene transfers, because most of the transferred genes will be non-essential and will thus not be selected for the long term.
- Patrick J. Keeling
Journal Club | 20 December 2023
Swapping genes within and beyond our bodies
Mashaal Sohail reflects on a 2011 Nature study by Smillie et al., which analysed human microbiome data to show that microbial ecology, rather than phylogeny or geography, is a key driver of horizontal gene transfer.
- Mashaal Sohail
Journal Club | 25 September 2023
The evolutionary tale of lactase persistence in humans
Luis Barreiro highlights a 2007 paper by Tishkoff et al. that identified genetic variants associated with lactose persistence in East African populations, representing one of the first examples of convergent evolution in humans.
- Luis B. Barreiro
Review Article | 18 September 2023
More than a decade of genetic research on the Denisovans
Ancient DNA studies over the past decade have yielded a plethora of insights into the Denisovan archaic hominin group. The authors review our understanding of Denisovan population history and their interactions with other human groups, insights from studies of Denisovan ancestry in modern humans, what we know about the Denisovan phenotype and their impact on our own evolutionary history.
- Stéphane Peyrégne
- , Viviane Slon
- & Janet Kelso
Review Article | 03 July 2023
Steering and controlling evolution — from bioengineering to fighting pathogens
Control can be applied to alter the ecological or evolutionary trajectory of a target system towards a predefined objective. In this Review, the authors discuss the aims, applications, mechanisms and dynamics of eco-evolutionary control across different biological systems.
- Michael Lässig
- , Ville Mustonen
- & Armita Nourmohammad
Journal Club | 31 May 2023
Cell by cell: population genetics in the wild
Tanja Woyke highlights a 2014 study by Kashtan et al., who applied single-cell genomics to populations of the marine cyanobacterium Prochlorococcus , revealing hundreds of subpopulations with distinct genomic backbones of this wild uncultured microorganism.
- Tanja Woyke
Review Article | 04 January 2023
Current advances in primate genomics: novel approaches for understanding evolution and disease
In this Review, the authors describe how advances in comparative primate genomics — complemented by multi-layered omic resources and primate cell systems — are providing insights into the evolution of primates and the genetic underpinnings of key traits of developmental and biomedical importance.
- , Gabriel Santpere
- & Tomas Marques-Bonet
Research Highlight | 13 December 2022
Social shifts in spiders
A comparative genomics study published in Nature Communications provides new insight into the genomic changes underlying the convergent evolution of sociality in spiders.
- Dorothy Clyde
Journal Club | 05 December 2022
The Neanderthal inside us
Luis Saraiva recalls a 1997 paper by Krings et al., which reports the sequencing of mitochondrial Neanderthal DNA extracted from a 40,000-year-old bone, enabling the direct study of the relationship between ancient and modern humans.
- Luis R. Saraiva
Journal Club | 02 December 2022
Live long & prosper: evidence of evolutionary forces on lifespan
In this Journal Club, Morgan Levine discusses a publication by Rose and Charlesworth that provided direct evidence of the impact of natural selection on differential ageing rates.
- Morgan Levine
Research Highlight | 27 September 2022
Mapping vertebrate brain evolution
Four papers in Science use single-cell, single-nucleus and spatial transcriptomic profiling of reptilian and amphibian brain tissue to provide insights into the evolution of vertebrate forebrains.
Review Article | 15 August 2022
Sex-specific morphs: the genetics and evolution of intra-sexual variation
Sex-specific morphs exhibit phenotypes that differ between the sexes and typically include many different traits. Here, the author reviews recent genomic and transcriptomic studies that are yielding new insights into the evolutionary origin and development of sex-specific morphs in a wide range of animal species.
- Judith E. Mank
Perspective | 12 July 2022
Alternative splicing as a source of phenotypic diversity
In this Perspective, the authors discuss how regulated alternative splicing can generate phenotypic diversity and outline emerging evidence that alternative splicing contributes to adaptation and species divergence.
- Charlotte J. Wright
- , Christopher W. J. Smith
- & Chris D. Jiggins
Journal Club | 17 March 2022
The genome that fuelled a Mexican scientific revolution
Carla Daniela Robles-Espinoza celebrates a paper that inspired a new generation of Mexican scientists.
- Carla Daniela Robles-Espinoza
Journal Club | 10 March 2022
Genome-wide insights into human population structure
Irene Gallego Romero recalls a landmark publication by Rosenberg et al., which reported on the fine-scale structure within and between human populations.
- Irene Gallego Romero
Review Article | 08 February 2022
Genetic load: genomic estimates and applications in non-model animals
The reduction in individual and mean population fitness induced by novel deleterious genetic variation is known as the genetic load. Bertorelle et al. review the definition of the genetic load and its components as well as the impact of whole-genome sequencing on the theoretical and applied study of the genetic load.
- Giorgio Bertorelle
- , Francesca Raffini
- & Cock van Oosterhout
Expert Recommendation | 26 January 2022
Rethinking nomenclature for interspecies cell fusions
Cell fusion models containing genomes from different cell types or species in a single nucleus offer unique research benefits. Here, Pavlovic et al. advocate against describing such models as hybrids and propose a new nomenclature for interspecies cell fusions that lacks reproductive connotations.
- Bryan J. Pavlovic
- & Alex A. Pollen
Review Article | 21 October 2021
Disentangling host–microbiota complexity through hologenomics
Hologenomic studies aim to further our understanding of host–microbiota interactions through the integrated analysis of host genomes and microbiota metagenomes. Here, Alberdi and colleagues discuss key considerations for designing optimal hologenomic studies and outline important biological questions that these studies can address.
- Antton Alberdi
- , Sandra B. Andersen
- & M. Thomas P. Gilbert
Review Article | 18 August 2021
Opportunities and challenges of macrogenetic studies
Leigh and colleagues describe the potential of the emerging field of macrogenetics to improve conservation and biodiversity management. Challenges preventing the field from reaching its full promise are highlighted and possible solutions and a framework for future macrogenetic studies are proposed.
- Deborah M. Leigh
- , Charles B. van Rees
- & Ivan Paz-Vinas
Review Article | 13 August 2021
Genetic innovations in animal–microbe symbioses
The evolutionary persistence of animal symbioses depends on both host and symbiont innovations. Perreau and Moran review how genome sequencing and related experiments have clarified how these innovations arise under different symbiont population structures, categorized here as open, closed and mixed.
- Julie Perreau
- & Nancy A. Moran
Research Highlight | 28 April 2021
Bone-free ancient DNA
A new study in Science reports the extraction and analysis of ancient hominid nuclear DNA from Paleolithic sediments. This advance paves the way to a fuller picture of human evolution by bypassing the dependency on rare skeletal remains.
Review Article | 29 May 2020
From molecules to populations: appreciating and estimating recombination rate variation
Genetic recombination is a fundamental biological process generating genetic variation by shuffling combinations of alleles. In this Review, Peñalba and Wolf focus on how sequencing-based approaches are providing diverse insights into recombination rate variation across levels of biological organization and timescales, from individual gametes of single individuals to populations through evolutionary history.
- Joshua V. Peñalba
- & Jochen B. W. Wolf
Review Article | 16 April 2020
Harnessing genomics to fast-track genetic improvement in aquaculture
Genetic improvement of production traits in aquaculture has great potential to help meet the rising seafood demands driven by human population growth. The authors review how genomics is being applied to aquaculture species at all stages of the domestication process to optimize selective breeding.
- Ross D. Houston
- , Tim P. Bean
- & Diego Robledo
Research Highlight | 22 October 2019
Single-cell genomics illuminates human forebrain development
In a new study published in Nature , Kanton et al. shed light on the unique genetic features of human forebrain development and how they diverged from those in great apes.
- Conor A. Bradley
Research Highlight | 06 August 2019
Decoding dragon DNA
A study in Nature Ecology and Evolution reports the genome sequence of the Komodo dragon and describes genomic features that may underlie its distinct physiology.
Research Highlight | 10 May 2019
Ancient genomes shed light on dark horses
A study of ancient horse genomes, described in Cell , reveals the existence of two now-extinct horse lineages and shows that modern breeding practices reduced genetic diversity in horses.
- Katharine H. Wrighton
Research Highlight | 24 April 2019
Bug battles end in compromise
An experimental evolution study published in Science demonstrates that non-additive interactions between pollinators (bumblebees) and herbivores (caterpillars) drive rapid evolution in plants.
Perspective | 18 March 2019
The genetics of convergent evolution: insights from plant photosynthesis
Using the example of carbon concentrating mechanisms in plants, the authors of this Perspective provide evidence that broad comparative genomic analyses likely overestimate the genetic complexity underlying convergent evolution of complex traits.
- Karolina Heyduk
- , Jose J. Moreno-Villena
- & Erika J. Edwards
Review Article | 01 November 2018
The causes of evolvability and their evolution
In this article, Payne and Wagner discuss how recent experimental studies are complementing theoretical work to enhance our understanding of the evolvability of diverse biological systems. They highlight phenotypic heterogeneity, robustness and adaptive landscape topography as causes of evolvability, and they additionally discuss evidence for whether evolvability itself can evolve.
- Joshua L. Payne
- & Andreas Wagner
Review Article | 24 October 2017
Population genetics of sexual conflict in the genomic era
Sexual conflict is thought to increase population genetic diversity though balancing selection, which has important evolutionary implications. This Review discusses how population genomic approaches are contributing to a deeper understanding of sexual conflict and how it is resolved.
Review Article | 25 September 2017
Demographic history, selection and functional diversity of the canine genome
Despite being a single species, dogs represent nearly 400 breeds with substantial genetic, morphological and behavioural diversity. In this Review, Ostrander et al . discuss how genomics studies of dogs have enhanced our understanding of dog and human population history, the desired and unintended consequences of trait-based selective breeding, and potentially human-applicable insights into cancer, ageing, behaviour and neurological diseases.
- Elaine A. Ostrander
- , Robert K. Wayne
- & Brian W. Davis
Review Article | 15 May 2017
The evolutionary significance of polyploidy
Polyploidy occurs frequently but is usually detrimental to survival; thus, few polyploids survive in the long term. Here, evidence linking the short-term evolutionary success of polyploids to environmental upheaval is reviewed and possible longer-term evolutionary benefits of polyploidy are discussed.
- Yves Van de Peer
- , Eshchar Mizrachi
- & Kathleen Marchal
Review Article | 14 November 2016
Making sense of genomic islands of differentiation in light of speciation
To characterize the genetic underpinnings of speciation, genome scans can identify genomic regions that differ between divergent populations of wild organisms. In this Review, Wolf and Ellegren describe the methodological details of these approaches and how genomic islands of differentiation should be interpreted cautiously in the search for 'speciation genes'. They also discuss methodological best practice that takes into consideration genomic differentiation occurring through speciation-independent evolutionary processes.
- Jochen B. W. Wolf
- & Hans Ellegren
Review Article | 03 October 2016
Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications
Studies have demonstrated that paternal traits acquired in response to environmental conditions can be inherited by the offspring, sometimes persisting for multiple generations. In this Review, the authors discuss the accumulating evidence of a major role for sperm RNAs and RNA modifications in the inheritance of acquired traits and the mechanisms that may underlie this.
- & Enkui Duan
Review Article | 06 June 2016
Determinants of genetic diversity
The degree of genetic diversity differs greatly among species and across genomic loci within genomes. The wide ranges in genetic diversity have important implications, including for evolution, conservation and management of wild and domesticated species. In this Review, the authors discuss how genome-scale sequencing strategies are providing insight into the varied determinants of genetic variation both among species and across genomic regions.
- Hans Ellegren
- & Nicolas Galtier
Review Article | 30 November 2015
Evolution of vertebrate sex chromosomes and dosage compensation
The differentiation of sex chromosomes in vertebrates created a need for mechanisms that compensate for differences in dosage of gene expression between the sexes. The author reviews the diversity of these mechanisms, their effects on gene expression, and their origin and evolution across the major vertebrate groups.
- Jennifer A. Marshall Graves
Review Article | 10 November 2015
Methods and models for unravelling human evolutionary history
The rapid accumulation and increasing quality of human DNA sequence-variation data brought about by advances in genome-scale sequencing present opportunities to investigate human evolution. The authors discuss the statistical methods and models that can be used to gain insight into the evolution of human populations from analyses of large-scale genomic data sets, as well as the challenges associated with these approaches.
- Joshua G. Schraiber
- & Joshua M. Akey
Review Article | 09 June 2015
Reconstructing ancient genomes and epigenomes
Sequencing genomes of ancient specimens, including human ancestors, can provide rich insights into evolutionary histories. However, ancient DNA samples are frequently degraded, damaged and contaminated with ancient and modern DNA from various sources. This Review describes the methodological and bioinformatic advances that allow these challenges to be overcome in order to process and sequence ancient samples for genome reconstruction, as well as recent progress in characterizing ancient epigenomes.
- Ludovic Orlando
- , M. Thomas P. Gilbert
- & Eske Willerslev
Review Article | 11 November 2014
The RNA World: molecular cooperation at the origins of life
The RNA World concept is the idea that billions of years ago — before current life based on DNA, RNA and proteins — the primary living substance was RNA or something chemically similar. This Review highlights the challenges and solutions of this point of view, particularly for the synthesis and replication of RNA, and how various types of molecular cooperation probably had important roles.
- Paul G. Higgs
- & Niles Lehman
Review Article | 09 October 2014
Evolutionary dynamics of coding and non-coding transcriptomes
This Review provides insights obtained from comparative transcriptomic studies of mammalian species. The dynamics of gene expression evolution in coding and non-coding genes, as well as the regulatory basis of transcriptome evolution and future research avenues, are discussed.
- Anamaria Necsulea
- & Henrik Kaessmann
In Brief | 12 August 2014
Homing in on anthropoid evolution
- Bryony Jones
Sexual conflict in nematodes
- Isabel Lokody
Review Article | 11 March 2014
Cryptic genetic variation: evolution's hidden substrate
This Review discusses cryptic genetic variation and focuses particularly on empirical support for widespread cryptic genetic variation in natural populations, its potential role in human diseases and its contribution to evolution.
- Annalise B. Paaby
- & Matthew V. Rockman
Review Article | 18 February 2014
Genomics and the origin of species
Genomic approaches are an increasingly important aspect of speciation research. The authors review recent insights from speciation genomics and propose a roadmap for this field, which is aimed at addressing both long-standing and emerging questions about speciation.
- Ole Seehausen
- , Roger K. Butlin
- & Alex Widmer
Review Article | 09 October 2013
The genetic causes of convergent evolution
This Review distinguishes between three distinct routes by which similar genetic changes contribute to convergent evolution and discusses examples from diverse taxa. Convergent genetic evolution might result from the fact that some mutations both minimize pleiotropic effects and maximize adaptation.
- David L. Stern
Research Highlight | 11 September 2013
A complex case of resource management
- Louisa Flintoft
Browse broader subjects
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
- Departments and Units
- Majors and Minors
- LSA Course Guide
- LSA Gateway
Search: {{$root.lsaSearchQuery.q}}, Page {{$root.page}}
- News and Events
- Edwin S. George Reserve
- Museum of Zoology
- Undergraduates
- Alumni and Friends

- Scholarships
- EEB Major & Minor
- Biological Station
- Program in Biology
- Current Students
- Prospective Students
- Contact the Graduate Program
- Giving Opportunities
- Newsletters
- Graduate Alumni Directory
- Alumni News
- Search News
The Record: Bold Challenges awards $700K for eight research teams
- Grad Student News
- Outreach News
- Science Fun Facts
- Social Media
- Archived News

Bold Challenges, based in the Office of the Vice President for Research, launched its Accelerate Program last year to support the preliminary work needed to prepare for submitting proposals that have the potential to establish future initiatives and centers at U-M focused on the global challenges facing societies today.
Researchers are using the funding to cover costs associated with preliminary data collection, technical writers, workshops and support salaries. In addition to financial support, participants receive in-kind support, including team facilitation, project management, graphic design support, and proposal development and review.
The teams consist of researchers from nine U-M schools and colleges, as well as partners from peer institutions nationwide, including Boston University, Howard University and Cornell University.
Accelerate teams target grant values at least 25 times the funding they receive. The current cohort is writing proposals for grants ranging from $650,000 to $20 million from a broad spectrum of organizations, including the National Science Foundation, National Institutes of Health, U.S. Environmental Protection Agency, Belmont Forum and the Wellcome Trust.
“The current cohort of Accelerate researchers are advancing research related to some of the most difficult challenges affecting communities across the globe,” said Arthur Lupia, interim vice president for research and innovation. “Their work, and the support provided by Bold Challenges, exemplifies the ambitious, interdisciplinary projects the initiative aims to bolster.”
Accelerate applications are accepted on a rolling basis from teams that will soon be or are currently seeking large-scale funding opportunities. Applications for the 2024 cycle of Boost, the Bold Challenges’ program designed to connect and support researchers in the preliminary stages of large-scale projects, are due May 13.
The research projects and principal investigators for the teams that received the latest funding are:
International Network of Pathogen Prevention Biorepositories — Kelly Speer, assistant professor of ecology and evolutionary biology, LSA.

- Information For
- Faculty and Staff
- More about LSA
- How Do I Apply?
- LSA Magazine
- Student Resources
- Academic Advising
- Global Studies
- LSA Opportunity Hub
- Update Contact Info
- Privacy Statement
- Report Feedback

COMMENTS
Evolutionary biology is a subdiscipline of the biological sciences concerned with the origin of life and the diversification and adaptation of life forms over time. Latest Research and Reviews
About the journal. JEB is an international, peer-reviewed journal that covers diverse research areas in evolutionary biology. The journal prioritizes publishing significant advances from a broad taxonomic perspective. Find out more.
Evolutionary biology is the subfield of biology that studies the evolutionary processes (natural selection, common descent, ... Current research in evolutionary biology covers diverse topics and incorporates ideas from diverse areas, such as molecular genetics and computer science.
Evolutionary biology is the study of this process, which can occur through mechanisms including natural selection, sexual selection and genetic drift. ... Research Open Access 30 Apr 2024 Nature ...
Overview. Evolutionary Biology is a comprehensive forum dedicated to publishing critical reviews, original research, and controversial ideas in the field of evolutionary biology. Committed to the view that evolutionary theory is a unifying framework for the biosciences. Offers a platform for broad syntheses, in-depth treatment and divergent views.
Exciting times for evolutionary biology. Nature Ecology & Evolution 8 , 593-594 ( 2024) Cite this article. Evolutionary biologists should be proud of recent progress in their broad field. We ...
Periodically, groups of scientists meet to identify new opportunities in evolutionary biology and associated disciplines (e.g., , -).Rather than set a specific research agenda for the future—clearly the charge of individual investigators—the aim has been to identify new themes and research directions that are already emerging in the field and to focus on the intersection of fundamental ...
Evolutionary Biology. Evolutionary Biology seeks to understand how the astonishing diversity of life on our planet arose by integrating insights from genomes, developmental biology, natural population studies, and experimental analyses. We are a product of Evolution. Just like the plants that provide us with food and materials, the ...
A new answer to a centuries-old biological puzzle. Craig White, Monash University and Dustin Marshall, Monash University. A new theory linking metabolism and size shows how evolution, not physics ...
Apr. 30, 2024 — Researchers have achieved a breakthrough in understanding how genetic drivers influence the evolution of a specific photosynthesis mechanism in Tillandsia (air plants). This ...
Evolution & Genomics. Research in evolution and genomics addresses the formation and maintenance of biological diversity. We study the evolutionary process at multiple levels - from the molecular and cellular mechanisms that underlie changes in specific traits to the forces that shape patterns of variation across genomes, populations and species.
Section Information. The theory of evolution holds a pivotal position in biology, pervading and helping to explain a vast number of features, mechanisms, and processes studied by the different disciplines comprising the vast area of biological research. However, the theory itself can be subjected to reconsideration in the light of new advances ...
Click on the title to browse this journal
As such, evolutionary biology as a framing approach and methodological toolkit is a needed component of integrated disease control and prevention ... The role of evolutionary biology in research and control of liver flukes in Southeast Asia. Infection, Genetics and Evolution, 43, 381-397.
The OEB Department denounces all forms of racism, harassment and discrimination. Further, we recognize the painful legacy of discrimination in which scientists of color working in all aspects of our field have been traumatized, discouraged, and made to feel unwelcome. The OEB Diversity, Inclusion and Belonging (DIB) Committee is currently ...
A festschrift in honor of Daniel I. Rubenstein's 43 years of service in the department of Ecology and Evolutionary Biology was held on October 2-3, 2023 at Princeton University. EEB's chair Jonathan Levine introduced the event, highlighting Dan's career at Princeton and the Mpala Research Centre in Kenya, his scholarly work on decision…
Evolutionary developmental biology (evo-devo) is that part of biology concerned with how changes in embryonic development during single generations relate to the evolutionary changes that occur between generations. Charles Darwin argued for the importance of development (embryology) in understanding evolution. After the discovery in 1900 of Mendel's research on genetics, however, any ...
Periodically, groups of scientists meet to identify new opportunities in evolutionary biology and associated disciplines (e.g., , -).Rather than set a specific research agenda for the future—clearly the charge of individual investigators—the aim has been to identify new themes and research directions that are already emerging in the field and to focus on the intersection of fundamental ...
Evolutionary ecology articles from across Nature Portfolio. Evolutionary ecology is a field within both ecology and evolution that examines how interactions between and within species evolve. It ...
Department of Ecology and Evolutionary Biology. ... Research Spotlight. Cornell's Experimental Ponds Facility is a research and teaching resource operated by our department. For over 50 years, a broad range of field and experimental projects have utilized the Ponds facility. Past and ongoing studies provide valuable insights and solutions ...
It's these types of questions, and many others, that are being asked by evolutionary biologists in our department. Whether empirically or theoretically based, integrating from the microscopic sub-cellular levels of genes and genomes to those of entire populations and/or species is a hallmark of our department's approach to evolutionary research.
Evolutionary genetics is the study of how genetic variation leads to evolutionary change. It includes topics such as the evolution of genome structure, the genetic basis of speciation and ...
However, the evolution of the complex genetic basis of CAM has remained elusive, making it a focus of research in evolutionary biology. Gene Regulation is Key. In this study, ...
Research. Four broad research areas capture the core expertise of the members of OEB. Groundbreaking research in the life sciences is now more interdisciplinary than ever and many OEB faculty work in more than one of these areas. OEB also maintains expertise in a number of sub-fields of biology, including evolutionary developmental biology ...
Therefore, Bland Sutton and Krogh can be regarded as founders of model organism concepts in biology and biomedical research. More recently, studying animals with adaptive phenotypes, which may be considered devastating and morbid in humans, has become known as "evolutionary mutant models of human disease" (Albertson et al., 2009 ; Beck et ...
Gilbert Building 371 Jane Stanford Way Stanford, CA 94305 Phone: 650-723-2413 biologyinfo [at] stanford.edu (biologyinfo[at]stanford[dot]edu) Campus Map
The Fraser River once supported massive salmon returns, but now years with half of the recorded historical maximum are considered good. There is substantial interest from surrounding communities, governments, and other groups to increase salmon returns for both human use and for functional ecosystems. To help generate resources for this endeavour, we resequenced hundreds of genomes at moderate ...
This Review provides insights obtained from comparative transcriptomic studies of mammalian species. The dynamics of gene expression evolution in coding and non-coding genes, as well as the ...
In a paper published recently in ACS Synthetic Biology, researchers outline the potential opportunities that synthetic cell development could unlock and what challenges lie ahead in this groundbreaking research. They also present a roadmap to inspire and guide innovation in this intriguing field. "The potential for this field is incredible ...
The research projects and principal investigators for the teams that received the latest funding are: International Network of Pathogen Prevention Biorepositories — Kelly Speer, assistant professor of ecology and evolutionary biology, LSA. READ MORE. Tweet Email. Release Date: 05/01/2024 :