- SpringerLink shop

Types of journal articles
It is helpful to familiarise yourself with the different types of articles published by journals. Although it may appear there are a large number of types of articles published due to the wide variety of names they are published under, most articles published are one of the following types; Original Research, Review Articles, Short reports or Letters, Case Studies, Methodologies.
Original Research:
This is the most common type of journal manuscript used to publish full reports of data from research. It may be called an Original Article, Research Article, Research, or just Article, depending on the journal. The Original Research format is suitable for many different fields and different types of studies. It includes full Introduction, Methods, Results, and Discussion sections.
Short reports or Letters:
These papers communicate brief reports of data from original research that editors believe will be interesting to many researchers, and that will likely stimulate further research in the field. As they are relatively short the format is useful for scientists with results that are time sensitive (for example, those in highly competitive or quickly-changing disciplines). This format often has strict length limits, so some experimental details may not be published until the authors write a full Original Research manuscript. These papers are also sometimes called Brief communications .
Review Articles:
Review Articles provide a comprehensive summary of research on a certain topic, and a perspective on the state of the field and where it is heading. They are often written by leaders in a particular discipline after invitation from the editors of a journal. Reviews are often widely read (for example, by researchers looking for a full introduction to a field) and highly cited. Reviews commonly cite approximately 100 primary research articles.
TIP: If you would like to write a Review but have not been invited by a journal, be sure to check the journal website as some journals to not consider unsolicited Reviews. If the website does not mention whether Reviews are commissioned it is wise to send a pre-submission enquiry letter to the journal editor to propose your Review manuscript before you spend time writing it.
Case Studies:
These articles report specific instances of interesting phenomena. A goal of Case Studies is to make other researchers aware of the possibility that a specific phenomenon might occur. This type of study is often used in medicine to report the occurrence of previously unknown or emerging pathologies.
Methodologies or Methods
These articles present a new experimental method, test or procedure. The method described may either be completely new, or may offer a better version of an existing method. The article should describe a demonstrable advance on what is currently available.
Back │ Next

An Emergency Physician’s Path pp 557–563 Cite as
Scientific Manuscript Writing: Original Research, Case Reports, Review Articles
- Kimberly M. Rathbun 5
- First Online: 02 March 2024
Manuscripts are used to communicate the findings of your work with other researchers. Writing your first manuscript can be a challenge. Journals provide guidelines to authors which should be followed closely. The three major types of articles (original research, case reports, and review articles) all generally follow the IMRAD format with slight variations in content. With planning and thought, manuscript writing does not have to be a daunting task.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Suggested Readings
Alsaywid BS, Abdulhaq NM. Guideline on writing a case report. Urol Ann. 2019;11(2):126–31.
Article PubMed PubMed Central Google Scholar
Cohen H. How to write a patient case report. Am J Health Syst Pharm. 2006;63(19):1888–92.
Article PubMed Google Scholar
Cooper ID. How to write an original research paper (and get it published). J Med Lib Assoc. 2015;103:67–8.
Article Google Scholar
Gemayel R. How to write a scientific paper. FEBS J. 2016;283(21):3882–5.
Article CAS PubMed Google Scholar
Gülpınar Ö, Güçlü AG. How to write a review article? Turk J Urol. 2013;39(Suppl 1):44–8.
PubMed PubMed Central Google Scholar
Huth EJ. Structured abstracts for papers reporting clinical trials. Ann Intern Med. 1987;106:626–7.
International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. http://www.ICMJE.org . Accessed 23 Aug 2022.
Liumbruno GM, Velati C, Pasqualetti P, Franchini M. How to write a scientific manuscript for publication. Blood Transfus. 2013;11:217–26.
McCarthy LH, Reilly KE. How to write a case report. Fam Med. 2000;32(3):190–5.
CAS PubMed Google Scholar
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
The Biosemantics Group. Journal/author name estimator. https://jane.biosemantics.org/ . Accessed 24 Aug 2022.
Weinstein R. How to write a manuscript for peer review. J Clin Apher. 2020;35(4):358–66.
Download references
Author information
Authors and affiliations.
Department of Emergency Medicine, AU/UGA Medical Partnership, Athens, GA, USA
Kimberly M. Rathbun
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kimberly M. Rathbun .
Editor information
Editors and affiliations.
Emergency Medicine, Penn State Milton S. Hershey Medical Center, Hershey, PA, USA
Robert P. Olympia
Elizabeth Barrall Werley
Jeffrey S. Lubin
MD Anderson Cancer Center at Cooper, Cooper Medical School of Rowan University, Camden, NJ, USA
Kahyun Yoon-Flannery
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter.
Rathbun, K.M. (2023). Scientific Manuscript Writing: Original Research, Case Reports, Review Articles. In: Olympia, R.P., Werley, E.B., Lubin, J.S., Yoon-Flannery, K. (eds) An Emergency Physician’s Path. Springer, Cham. https://doi.org/10.1007/978-3-031-47873-4_80
Download citation
DOI : https://doi.org/10.1007/978-3-031-47873-4_80
Published : 02 March 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-47872-7
Online ISBN : 978-3-031-47873-4
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
How to write a good scientific review article
Affiliation.
- 1 The FEBS Journal Editorial Office, Cambridge, UK.
- PMID: 35792782
- DOI: 10.1111/febs.16565
Literature reviews are valuable resources for the scientific community. With research accelerating at an unprecedented speed in recent years and more and more original papers being published, review articles have become increasingly important as a means to keep up to date with developments in a particular area of research. A good review article provides readers with an in-depth understanding of a field and highlights key gaps and challenges to address with future research. Writing a review article also helps to expand the writer's knowledge of their specialist area and to develop their analytical and communication skills, amongst other benefits. Thus, the importance of building review-writing into a scientific career cannot be overstated. In this instalment of The FEBS Journal's Words of Advice series, I provide detailed guidance on planning and writing an informative and engaging literature review.
© 2022 Federation of European Biochemical Societies.
Publication types
- Review Literature as Topic*
- Enroll & Pay
- Prospective Students
- Current Students
- Degree Programs
Original Research
An original research paper should present a unique argument of your own. In other words, the claim of the paper should be debatable and should be your (the researcher’s) own original idea. Typically an original research paper builds on the existing research on a topic, addresses a specific question, presents the findings according to a standard structure (described below), and suggests questions for further research and investigation. Though writers in any discipline may conduct original research, scientists and social scientists in particular are interested in controlled investigation and inquiry. Their research often consists of direct and indirect observation in the laboratory or in the field. Many scientists write papers to investigate a hypothesis (a statement to be tested).
Although the precise order of research elements may vary somewhat according to the specific task, most include the following elements:
- Table of contents
- List of illustrations
- Body of the report
- References cited
Check your assignment for guidance on which formatting style is required. The Complete Discipline Listing Guide (Purdue OWL) provides information on the most common style guide for each discipline, but be sure to check with your instructor.
The title of your work is important. It draws the reader to your text. A common practice for titles is to use a two-phrase title where the first phrase is a broad reference to the topic to catch the reader’s attention. This phrase is followed by a more direct and specific explanation of your project. For example:
“Lions, Tigers, and Bears, Oh My!: The Effects of Large Predators on Livestock Yields.”
The first phrase draws the reader in – it is creative and interesting. The second part of the title tells the reader the specific focus of the research.
In addition, data base retrieval systems often work with keywords extracted from the title or from a list the author supplies. When possible, incorporate them into the title. Select these words with consideration of how prospective readers might attempt to access your document. For more information on creating keywords, refer to this Springer research publication guide.
See the KU Writing Center Writing Guide on Abstracts for detailed information about creating an abstract.
Table of Contents
The table of contents provides the reader with the outline and location of specific aspects of your document. Listings in the table of contents typically match the headings in the paper. Normally, authors number any pages before the table of contents as well as the lists of illustrations/tables/figures using lower-case roman numerals. As such, the table of contents will use lower-case roman numbers to identify the elements of the paper prior to the body of the report, appendix, and reference page. Additionally, because authors will normally use Arabic numerals (e.g., 1, 2, 3) to number the pages of the body of the research paper (starting with the introduction), the table of contents will use Arabic numerals to identify the main sections of the body of the paper (the introduction, literature review, methods, results, discussion, conclusion, references, and appendices).
Here is an example of a table of contents:
ABSTRACT..................................................iii
TABLE OF CONTENTS...............................iv
LIST OF ILLUSTRATIONS...........................v
LIST OF TABLES.........................................vii
INTRODUCTION..........................................1
LITERATURE REVIEW.................................6
METHODS....................................................9
RESULTS....................................................10
DISCUSSION..............................................16
CONCLUSION............................................18
REFERENCES............................................20
APPENDIX................................................. 23
More information on creating a table of contents can be found in the Table of Contents Guide (SHSU) from the Newton Gresham Library at Sam Houston State University.
List of Illustrations
Authors typically include a list of the illustrations in the paper with longer documents. List the number (e.g., Illustration 4), title, and page number of each illustration under headings such as "List of Illustrations" or "List of Tables.”
Body of the Report
The tone of a report based on original research will be objective and formal, and the writing should be concise and direct. The structure will likely consist of these standard sections: introduction, methods, results, discussion, and conclusion . Typically, authors identify these sections with headings and may use subheadings to identify specific themes within these sections (such as themes within the literature under the literature review section).
Introduction
Given what the field says about this topic, here is my contribution to this line of inquiry.
The introduction often consists of the rational for the project. What is the phenomenon or event that inspired you to write about this topic? What is the relevance of the topic and why is it important to study it now? Your introduction should also give some general background on the topic – but this should not be a literature review. This is the place to give your readers and necessary background information on the history, current circumstances, or other qualities of your topic generally. In other words, what information will a layperson need to know in order to get a decent understanding of the purpose and results of your paper? Finally, offer a “road map” to your reader where you explain the general order of the remainder of your paper. In the road map, do not just list the sections of the paper that will follow. You should refer to the main points of each section, including the main arguments in the literature review, a few details about your methods, several main points from your results/analysis, the most important takeaways from your discussion section, and the most significant conclusion or topic for further research.
Literature Review
This is what other researchers have published about this topic.
In the literature review, you will define and clarify the state of the topic by citing key literature that has laid the groundwork for this investigation. This review of the literature will identify relations, contradictions, gaps, and inconsistencies between previous investigations and this one, and suggest the next step in the investigation chain, which will be your hypothesis. You should write the literature review in the present tense because it is ongoing information.
Methods (Procedures)
This is how I collected and analyzed the information.
This section recounts the procedures of the study. You will write this in past tense because you have already completed the study. It must include what is necessary to replicate and validate the hypothesis. What details must the reader know in order to replicate this study? What were your purposes in this study? The challenge in this section is to understand the possible readers well enough to include what is necessary without going into detail on “common-knowledge” procedures. Be sure that you are specific enough about your research procedure that someone in your field could easily replicate your study. Finally, make sure not to report any findings in this section.
This is what I found out from my research.
This section reports the findings from your research. Because this section is about research that is completed, you should write it primarily in the past tense . The form and level of detail of the results depends on the hypothesis and goals of this report, and the needs of your audience. Authors of research papers often use visuals in the results section, but the visuals should enhance, rather than serve as a substitute, for the narrative of your results. Develop a narrative based on the thesis of the paper and the themes in your results and use visuals to communicate key findings that address your hypothesis or help to answer your research question. Include any unusual findings that will clarify the data. It is a good idea to use subheadings to group the results section into themes to help the reader understand the main points or findings of the research.
This is what the findings mean in this situation and in terms of the literature more broadly.
This section is your opportunity to explain the importance and implications of your research. What is the significance of this research in terms of the hypothesis? In terms of other studies? What are possible implications for any academic theories you utilized in the study? Are there any policy implications or suggestions that result from the study? Incorporate key studies introduced in the review of literature into your discussion along with your own data from the results section. The discussion section should put your research in conversation with previous research – now you are showing directly how your data complements or contradicts other researchers’ data and what the wider implications of your findings are for academia and society in general. What questions for future research do these findings suggest? Because it is ongoing information, you should write the discussion in the present tense . Sometimes the results and discussion are combined; if so, be certain to give fair weight to both.
These are the key findings gained from this research.
Summarize the key findings of your research effort in this brief final section. This section should not introduce new information. You can also address any limitations from your research design and suggest further areas of research or possible projects you would complete with a new and improved research design.
References/Works Cited
See KU Writing Center writing guides to learn more about different citation styles like APA, MLA, and Chicago. Make an appointment at the KU Writing Center for more help. Be sure to format the paper and references based on the citation style that your professor requires or based on the requirements of the academic journal or conference where you hope to submit the paper.
The appendix includes attachments that are pertinent to the main document but are too detailed to be included in the main text. These materials should be titled and labeled (for example Appendix A: Questionnaire). You should refer to the appendix in the text with in-text references so the reader understands additional useful information is available elsewhere in the document. Examples of documents to include in the appendix include regression tables, tables of text analysis data, and interview questions.
Updated June 2022
- Main Library
- Digital Fabrication Lab
- Data Visualization Lab
- Business Learning Center
- Klai Juba Wald Architectural Studies Library
- NDSU Nursing at Sanford Health Library
- Research Assistance
- Special Collections
- Digital Collections
- Collection Development Policy
- Course Reserves
- Request Library Instruction
- Main Library Services
- Alumni & Community
- Academic Support Services in the Library
- Libraries Resources for Employees
- Book Equipment or Study Rooms
- Librarians by Academic Subject
- Germans from Russia Heritage Collection
- NDSU Archives
- Mission, Vision, and Strategic Plan 2022-2024
- Staff Directory
- Floor Plans
- The Libraries Magazine
- Accommodations for People with Disabilities
- Annual Report
- Donate to the Libraries
- Equity, Diversity and Inclusion
- Faculty Senate Library Committee
- Undergraduate Research Award
What is an original research article?
An original research article is a report of research activity that is written by the researchers who conducted the research or experiment. Original research articles may also be referred to as: “primary research articles” or “primary scientific literature.” In science courses, instructors may also refer to these as “peer-reviewed articles” or “refereed articles.”
Original research articles in the sciences have a specific purpose, follow a scientific article format, are peer reviewed, and published in academic journals.
Identifying Original Research: What to Look For
An "original research article" is an article that is reporting original research about new data or theories that have not been previously published. That might be the results of new experiments, or newly derived models or simulations. The article will include a detailed description of the methods used to produce them, so that other researchers can verify them. This description is often found in a section called "methods" or "materials and methods" or similar. Similarly, the results will generally be described in great detail, often in a section called "results."
Since the original research article is reporting the results of new research, the authors should be the scientists who conducted that research. They will have expertise in the field, and will usually be employed by a university or research lab.
In comparison, a newspaper or magazine article (such as in The New York Times or National Geographic ) will usually be written by a journalist reporting on the actions of someone else.
An original research article will be written by and for scientists who study related topics. As such, the article should use precise, technical language to ensure that other researchers have an exact understanding of what was done, how to do it, and why it matters. There will be plentiful citations to previous work, helping place the research article in a broader context. The article will be published in an academic journal, follow a scientific format, and undergo peer-review.
Original research articles in the sciences follow the scientific format. ( This tutorial from North Carolina State University illustrates some of the key features of this format.)
Look for signs of this format in the subject headings or subsections of the article. You should see the following:
Scientific research that is published in academic journals undergoes a process called "peer review."
The peer review process goes like this:
- A researcher writes a paper and sends it in to an academic journal, where it is read by an editor
- The editor then sends the article to other scientists who study similar topics, who can best evaluate the article
- The scientists/reviewers examine the article's research methodology, reasoning, originality, and sginificance
- The scientists/reviewers then make suggestions and comments to impove the paper
- The original author is then given these suggestions and comments, and makes changes as needed
- This process repeats until everyone is satisfied and the article can be published within the academic journal
For more details about this process see the Peer Reviewed Publications guide.
This journal article is an example. It was published in the journal Royal Society Open Science in 2015. Clicking on the button that says "Review History" will show the comments by the editors, reviewers and the author as it went through the peer review process. The "About Us" menu provides details about this journal; "About the journal" under that tab includes the statement that the journal is peer reviewed.
Review articles
There are a variety of article types published in academic, peer-reviewed journals, but the two most common are original research articles and review articles . They can look very similar, but have different purposes and structures.
Like original research articles, review articles are aimed at scientists and undergo peer-review. Review articles often even have “abstract,” “introduction,” and “reference” sections. However, they will not (generally) have a “methods” or “results” section because they are not reporting new data or theories. Instead, they review the current state of knowledge on a topic.
Press releases, newspaper or magazine articles
These won't be in a formal scientific format or be peer reviewed. The author will usually be a journalist, and the audience will be the general public. Since most readers are not interested in the precise details of the research, the language will usually be nontechnical and broad. Citations will be rare or nonexistent.
Tips for Finding Original research Articles
Search for articles in one of the library databases recommend for your subject area . If you are using Google, try searching in Google Scholar instead and you will get results that are more likely to be original research articles than what will come up in a regular Google search!
For tips on using library databases to find articles, see our Library DIY guides .
Tips for Finding the Source of a News Report about Science
If you've seen or heard a report about a new scientific finding or claim, these tips can help you find the original source:
- Often, the report will mention where the original research was published; look for sentences like "In an article published yesterday in the journal Nature ..." You can use this to find the issue of the journal where the research was published, and look at the table of contents to find the original article.
- The report will often name the researchers involved. You can search relevant databases for their name and the topic of the report to find the original research that way.
- Sometimes you may have to go through multiple articles to find the original source. For example, a video or blog post may be based on a newspaper article, which in turn is reporting on a scientific discovery published in another journal; be sure to find the original research article.
- Don't be afraid to ask a librarian for help!
Search The Site
Find Your Librarian
Phone: Circulation: (701) 231-8888 Reference: (701) 231-8888 Administration: (701) 231-8753
Email: Administration InterLibrary Loan (ILL)
- Online Services
- Phone/Email Directory
- Registration And Records
- Government Information
- Library DIY
- Subject and Course Guides
- Special Topics
- Collection Highlights
- Digital Horizons
- NDSU Repository (IR)
- Libraries Hours
- News & Events

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
7 Writing a Literature Review
Hundreds of original investigation research articles on health science topics are published each year. It is becoming harder and harder to keep on top of all new findings in a topic area and – more importantly – to work out how they all fit together to determine our current understanding of a topic. This is where literature reviews come in.
In this chapter, we explain what a literature review is and outline the stages involved in writing one. We also provide practical tips on how to communicate the results of a review of current literature on a topic in the format of a literature review.
7.1 What is a literature review?

Literature reviews provide a synthesis and evaluation of the existing literature on a particular topic with the aim of gaining a new, deeper understanding of the topic.
Published literature reviews are typically written by scientists who are experts in that particular area of science. Usually, they will be widely published as authors of their own original work, making them highly qualified to author a literature review.
However, literature reviews are still subject to peer review before being published. Literature reviews provide an important bridge between the expert scientific community and many other communities, such as science journalists, teachers, and medical and allied health professionals. When the most up-to-date knowledge reaches such audiences, it is more likely that this information will find its way to the general public. When this happens, – the ultimate good of science can be realised.
A literature review is structured differently from an original research article. It is developed based on themes, rather than stages of the scientific method.
In the article Ten simple rules for writing a literature review , Marco Pautasso explains the importance of literature reviews:
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications. For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively. Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests. Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read. For such summaries to be useful, however, they need to be compiled in a professional way (Pautasso, 2013, para. 1).
An example of a literature review is shown in Figure 7.1.
Video 7.1: What is a literature review? [2 mins, 11 secs]
Watch this video created by Steely Library at Northern Kentucky Library called ‘ What is a literature review? Note: Closed captions are available by clicking on the CC button below.
Examples of published literature reviews
- Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review
- Traveler’s diarrhea: a clinical review
- Cultural concepts of distress and psychiatric disorders: literature review and research recommendations for global mental health epidemiology
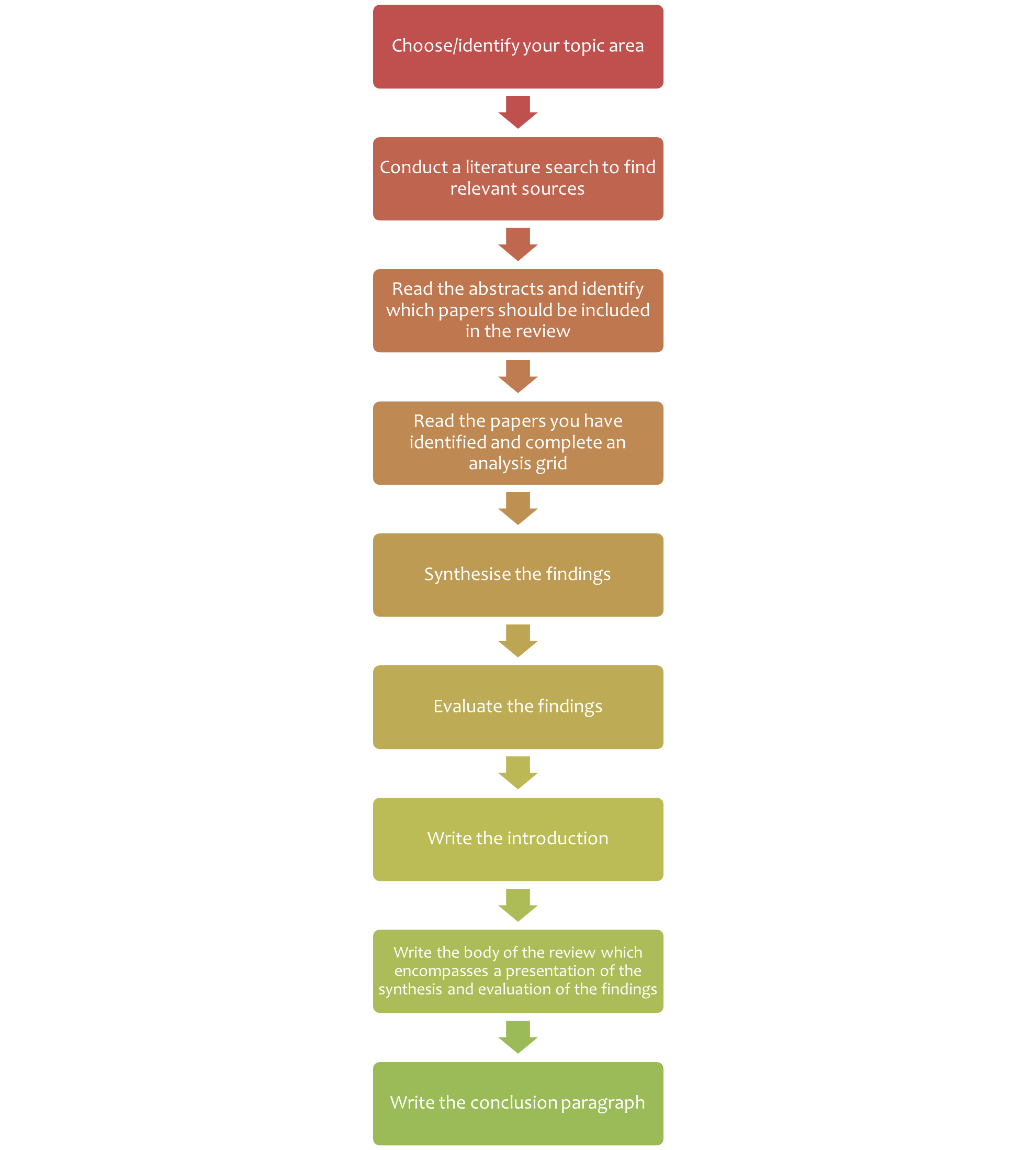
7.2 Steps of writing a literature review
Writing a literature review is a very challenging task. Figure 7.2 summarises the steps of writing a literature review. Depending on why you are writing your literature review, you may be given a topic area, or may choose a topic that particularly interests you or is related to a research project that you wish to undertake.
Chapter 6 provides instructions on finding scientific literature that would form the basis for your literature review.
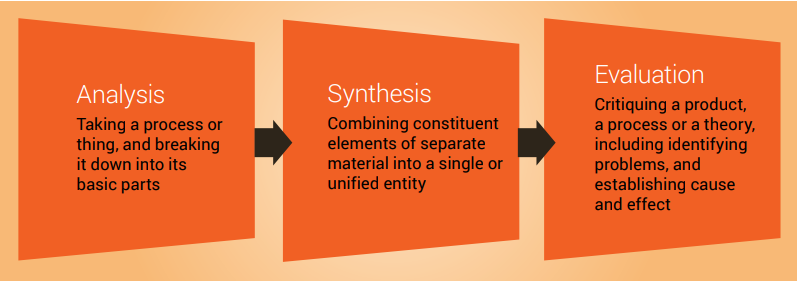
Once you have your topic and have accessed the literature, the next stages (analysis, synthesis and evaluation) are challenging. Next, we look at these important cognitive skills student scientists will need to develop and employ to successfully write a literature review, and provide some guidance for navigating these stages.
Analysis, synthesis and evaluation
Analysis, synthesis and evaluation are three essential skills required by scientists and you will need to develop these skills if you are to write a good literature review ( Figure 7.3 ). These important cognitive skills are discussed in more detail in Chapter 9.
The first step in writing a literature review is to analyse the original investigation research papers that you have gathered related to your topic.
Analysis requires examining the papers methodically and in detail, so you can understand and interpret aspects of the study described in each research article.
An analysis grid is a simple tool you can use to help with the careful examination and breakdown of each paper. This tool will allow you to create a concise summary of each research paper; see Table 7.1 for an example of an analysis grid. When filling in the grid, the aim is to draw out key aspects of each research paper. Use a different row for each paper, and a different column for each aspect of the paper ( Tables 7.2 and 7.3 show how completed analysis grid may look).
Before completing your own grid, look at these examples and note the types of information that have been included, as well as the level of detail. Completing an analysis grid with a sufficient level of detail will help you to complete the synthesis and evaluation stages effectively. This grid will allow you to more easily observe similarities and differences across the findings of the research papers and to identify possible explanations (e.g., differences in methodologies employed) for observed differences between the findings of different research papers.
Table 7.1: Example of an analysis grid
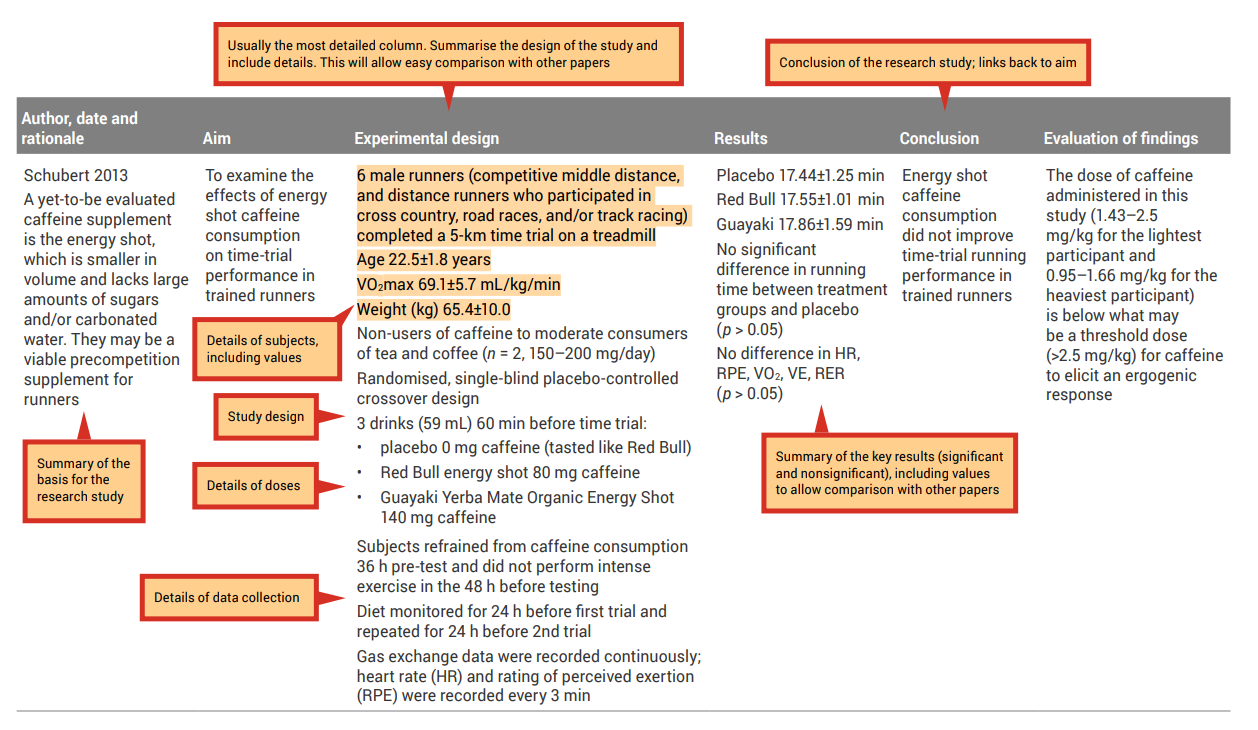
Table 7.3: Sample filled-in analysis grid for research article by Ping and colleagues
Source: Ping, WC, Keong, CC & Bandyopadhyay, A 2010, ‘Effects of acute supplementation of caffeine on cardiorespiratory responses during endurance running in a hot and humid climate’, Indian Journal of Medical Research, vol. 132, pp. 36–41. Used under a CC-BY-NC-SA licence.
Step two of writing a literature review is synthesis.
Synthesis describes combining separate components or elements to form a connected whole.
You will use the results of your analysis to find themes to build your literature review around. Each of the themes identified will become a subheading within the body of your literature review.
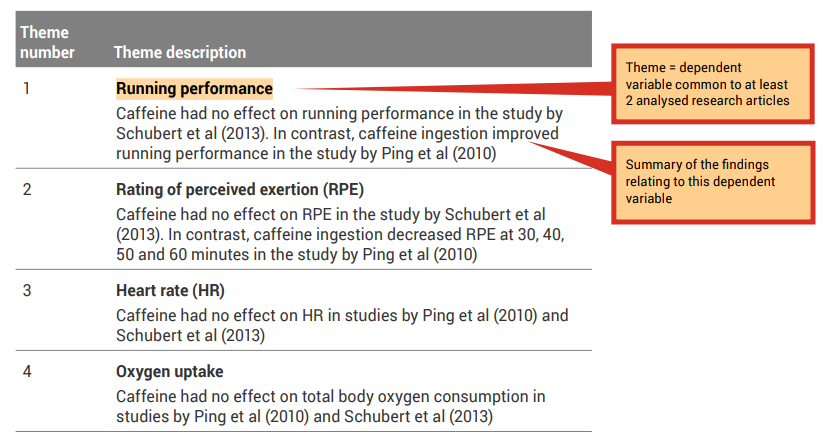
A good place to start when identifying themes is with the dependent variables (results/findings) that were investigated in the research studies.
Because all of the research articles you are incorporating into your literature review are related to your topic, it is likely that they have similar study designs and have measured similar dependent variables. Review the ‘Results’ column of your analysis grid. You may like to collate the common themes in a synthesis grid (see, for example Table 7.4 ).
Step three of writing a literature review is evaluation, which can only be done after carefully analysing your research papers and synthesising the common themes (findings).
During the evaluation stage, you are making judgements on the themes presented in the research articles that you have read. This includes providing physiological explanations for the findings. It may be useful to refer to the discussion section of published original investigation research papers, or another literature review, where the authors may mention tested or hypothetical physiological mechanisms that may explain their findings.
When the findings of the investigations related to a particular theme are inconsistent (e.g., one study shows that caffeine effects performance and another study shows that caffeine had no effect on performance) you should attempt to provide explanations of why the results differ, including physiological explanations. A good place to start is by comparing the methodologies to determine if there are any differences that may explain the differences in the findings (see the ‘Experimental design’ column of your analysis grid). An example of evaluation is shown in the examples that follow in this section, under ‘Running performance’ and ‘RPE ratings’.
When the findings of the papers related to a particular theme are consistent (e.g., caffeine had no effect on oxygen uptake in both studies) an evaluation should include an explanation of why the results are similar. Once again, include physiological explanations. It is still a good idea to compare methodologies as a background to the evaluation. An example of evaluation is shown in the following under ‘Oxygen consumption’.
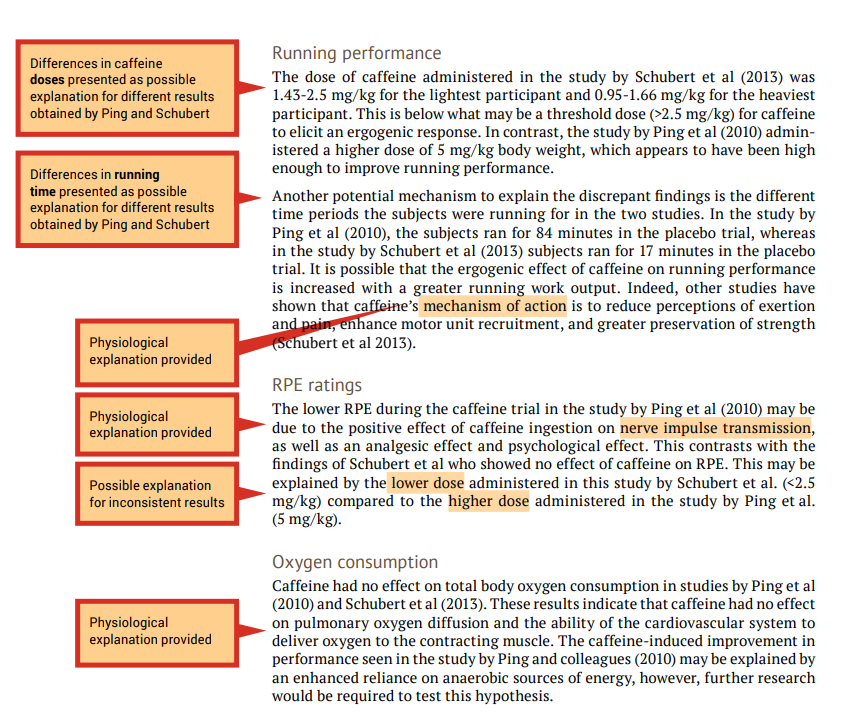
7.3 Writing your literature review
Once you have completed the analysis, and synthesis grids and written your evaluation of the research papers , you can combine synthesis and evaluation information to create a paragraph for a literature review ( Figure 7.4 ).
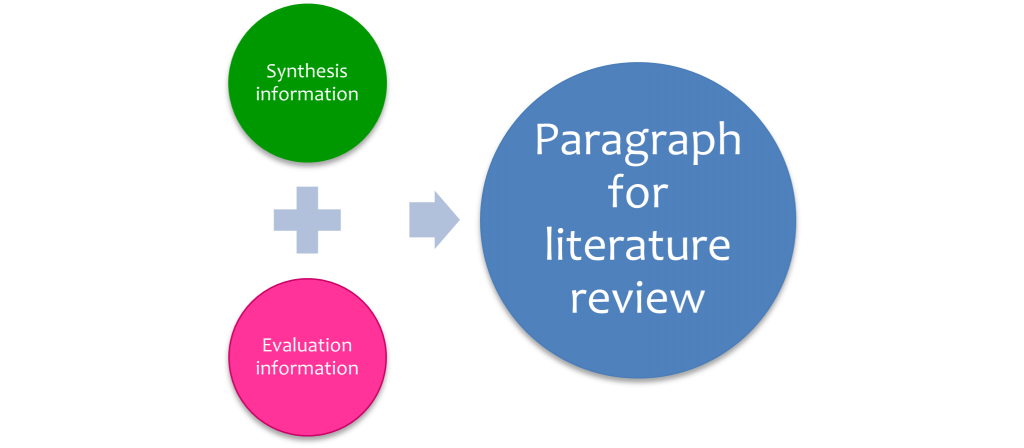
The following paragraphs are an example of combining the outcome of the synthesis and evaluation stages to produce a paragraph for a literature review.
Note that this is an example using only two papers – most literature reviews would be presenting information on many more papers than this ( (e.g., 106 papers in the review article by Bain and colleagues discussed later in this chapter). However, the same principle applies regardless of the number of papers reviewed.

The next part of this chapter looks at the each section of a literature review and explains how to write them by referring to a review article that was published in Frontiers in Physiology and shown in Figure 7.1. Each section from the published article is annotated to highlight important features of the format of the review article, and identifies the synthesis and evaluation information.
In the examination of each review article section we will point out examples of how the authors have presented certain information and where they display application of important cognitive processes; we will use the colour code shown below:

This should be one paragraph that accurately reflects the contents of the review article.
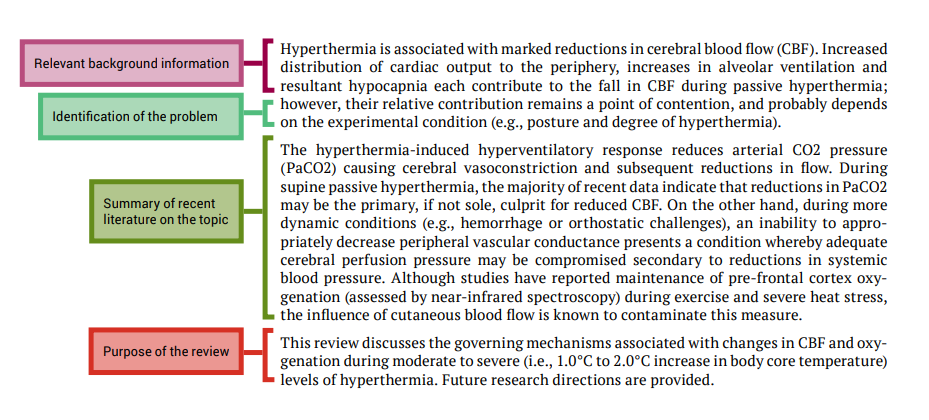
Introduction
The introduction should establish the context and importance of the review
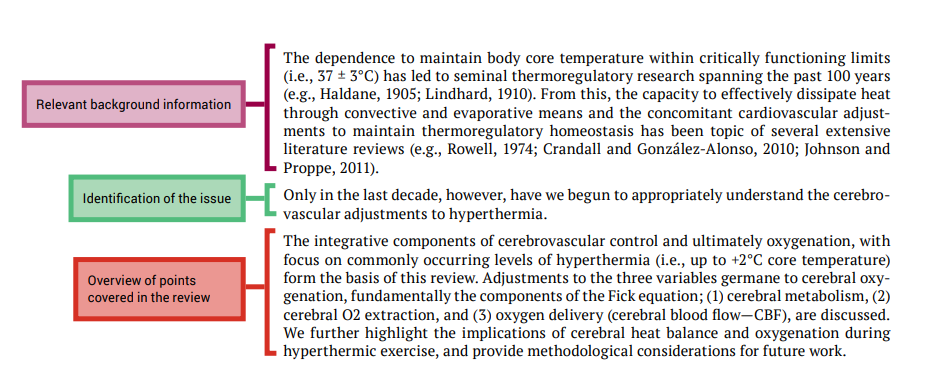
Body of literature review
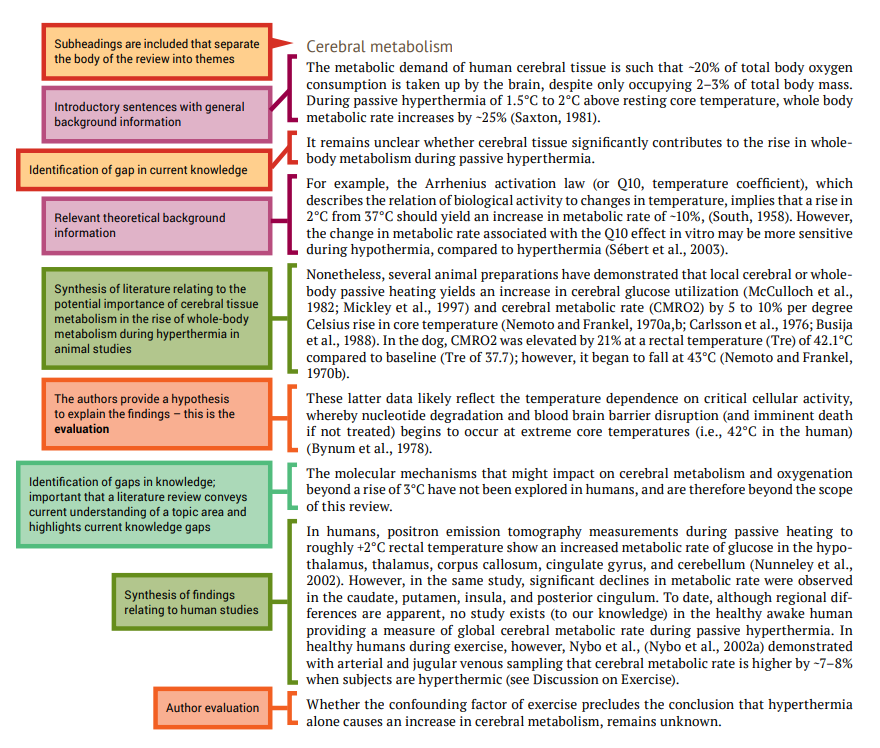
The reference section provides a list of the references that you cited in the body of your review article. The format will depend on the journal of publication as each journal has their own specific referencing format.
It is important to accurately cite references in research papers to acknowledge your sources and ensure credit is appropriately given to authors of work you have referred to. An accurate and comprehensive reference list also shows your readers that you are well-read in your topic area and are aware of the key papers that provide the context to your research.
It is important to keep track of your resources and to reference them consistently in the format required by the publication in which your work will appear. Most scientists will use reference management software to store details of all of the journal articles (and other sources) they use while writing their review article. This software also automates the process of adding in-text references and creating a reference list. In the review article by Bain et al. (2014) used as an example in this chapter, the reference list contains 106 items, so you can imagine how much help referencing software would be. Chapter 5 shows you how to use EndNote, one example of reference management software.
Click the drop down below to review the terms learned from this chapter.
Copyright note:
- The quotation from Pautasso, M 2013, ‘Ten simple rules for writing a literature review’, PLoS Computational Biology is use under a CC-BY licence.
- Content from the annotated article and tables are based on Schubert, MM, Astorino, TA & Azevedo, JJL 2013, ‘The effects of caffeinated ‘energy shots’ on time trial performance’, Nutrients, vol. 5, no. 6, pp. 2062–2075 (used under a CC-BY 3.0 licence ) and P ing, WC, Keong , CC & Bandyopadhyay, A 2010, ‘Effects of acute supplementation of caffeine on cardiorespiratory responses during endurance running in a hot and humid climate’, Indian Journal of Medical Research, vol. 132, pp. 36–41 (used under a CC-BY-NC-SA 4.0 licence ).
Bain, A.R., Morrison, S.A., & Ainslie, P.N. (2014). Cerebral oxygenation and hyperthermia. Frontiers in Physiology, 5 , 92.
Pautasso, M. (2013). Ten simple rules for writing a literature review. PLoS Computational Biology, 9 (7), e1003149.
How To Do Science Copyright © 2022 by University of Southern Queensland is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
- Privacy Policy
Buy Me a Coffee

Home » Original Research – Definition, Examples, Guide
Original Research – Definition, Examples, Guide
Table of Contents

Original Research
Definition:
Original research refers to a type of research that involves the collection and analysis of new and original data to answer a specific research question or to test a hypothesis. This type of research is conducted by researchers who aim to generate new knowledge or add to the existing body of knowledge in a particular field or discipline.
Types of Original Research
There are several types of original research that researchers can conduct depending on their research question and the nature of the data they are collecting. Some of the most common types of original research include:
Basic Research
This type of research is conducted to expand scientific knowledge and to create new theories, models, or frameworks. Basic research often involves testing hypotheses and conducting experiments or observational studies.
Applied Research
This type of research is conducted to solve practical problems or to develop new products or technologies. Applied research often involves the application of basic research findings to real-world problems.
Exploratory Research
This type of research is conducted to gather preliminary data or to identify research questions that need further investigation. Exploratory research often involves collecting qualitative data through interviews, focus groups, or observations.
Descriptive Research
This type of research is conducted to describe the characteristics or behaviors of a population or a phenomenon. Descriptive research often involves collecting quantitative data through surveys, questionnaires, or other standardized instruments.
Correlational Research
This type of research is conducted to determine the relationship between two or more variables. Correlational research often involves collecting quantitative data and using statistical analyses to identify correlations between variables.
Experimental Research
This type of research is conducted to test cause-and-effect relationships between variables. Experimental research often involves manipulating one or more variables and observing the effect on an outcome variable.
Longitudinal Research
This type of research is conducted over an extended period of time to study changes in behavior or outcomes over time. Longitudinal research often involves collecting data at multiple time points.
Original Research Methods
Original research can involve various methods depending on the research question, the nature of the data, and the discipline or field of study. However, some common methods used in original research include:
This involves the manipulation of one or more variables to test a hypothesis. Experimental research is commonly used in the natural sciences, such as physics, chemistry, and biology, but can also be used in social sciences, such as psychology.
Observational Research
This involves the collection of data by observing and recording behaviors or events without manipulation. Observational research can be conducted in the natural setting of the behavior or in a laboratory setting.
Survey Research
This involves the collection of data from a sample of participants using questionnaires or interviews. Survey research is commonly used in social sciences, such as sociology, political science, and economics.
Case Study Research
This involves the in-depth analysis of a single case, such as an individual, organization, or event. Case study research is commonly used in social sciences and business studies.
Qualitative research
This involves the collection and analysis of non-numerical data, such as interviews, focus groups, and observation notes. Qualitative research is commonly used in social sciences, such as anthropology, sociology, and psychology.
Quantitative research
This involves the collection and analysis of numerical data using statistical methods. Quantitative research is commonly used in natural sciences, such as physics, chemistry, and biology, as well as in social sciences, such as psychology and economics.
Researchers may also use a combination of these methods in their original research depending on their research question and the nature of their data.
Data Collection Methods
There are several data collection methods that researchers can use in original research, depending on the nature of the research question and the type of data that needs to be collected. Some of the most common data collection methods include:
- Surveys : Surveys involve asking participants to respond to a series of questions about their attitudes, behaviors, beliefs, or experiences. Surveys can be conducted in person, over the phone, through email, or online.
- Interviews : Interviews involve asking participants open-ended questions about their experiences, beliefs, or behaviors. Interviews can be conducted in person, over the phone, or through video conferencing.
- Observations : Observations involve observing and recording participants’ behaviors or interactions in a natural or laboratory setting. Observations can be conducted using structured or unstructured methods.
- Experiments : Experiments involve manipulating one or more variables and observing the effect on an outcome variable. Experiments can be conducted in a laboratory or in the natural environment.
- Case studies: Case studies involve conducting an in-depth analysis of a single case, such as an individual, organization, or event. Case studies can involve the collection of qualitative or quantitative data.
- Focus groups: Focus groups involve bringing together a small group of participants to discuss a specific topic or issue. Focus groups can be conducted in person or online.
- Document analysis: Document analysis involves collecting and analyzing written or visual materials, such as reports, memos, or videos, to answer research questions.
Data Analysis Methods
Once data has been collected in original research, it needs to be analyzed to answer research questions and draw conclusions. There are various data analysis methods that researchers can use, depending on the type of data collected and the research question. Some common data analysis methods used in original research include:
- Descriptive statistics: This involves using statistical measures such as mean, median, mode, and standard deviation to describe the characteristics of the data.
- Inferential statistics: This involves using statistical methods to infer conclusions about a population based on a sample of data.
- Regression analysis: This involves examining the relationship between two or more variables by using statistical models that predict the value of one variable based on the value of one or more other variables.
- Content analysis: This involves analyzing written or visual materials, such as documents, videos, or social media posts, to identify patterns, themes, or trends.
- Qualitative analysis: This involves analyzing non-numerical data, such as interview transcripts or observation notes, to identify themes, patterns, or categories.
- Grounded theory: This involves developing a theory or model based on the data collected in the study.
- Mixed methods analysis: This involves combining quantitative and qualitative data analysis methods to provide a more comprehensive understanding of the research question.
How to Conduct Original Research
Conducting original research involves several steps that researchers need to follow to ensure that their research is valid, reliable, and produces meaningful results. Here are some general steps that researchers can follow to conduct original research:
- Identify the research question: The first step in conducting original research is to identify a research question that is relevant, significant, and feasible. The research question should be specific and focused to guide the research process.
- Conduct a literature review: Once the research question is identified, researchers should conduct a thorough literature review to identify existing research on the topic. This will help them identify gaps in the existing knowledge and develop a research plan that builds on previous research.
- Develop a research plan: Researchers should develop a research plan that outlines the methods they will use to collect and analyze data. The research plan should be detailed and include information on the population and sample, data collection methods, data analysis methods, and ethical considerations.
- Collect data: Once the research plan is developed, researchers can begin collecting data using the methods identified in the plan. It is important to ensure that the data collection process is consistent and accurate to ensure the validity and reliability of the data.
- Analyze data: Once the data is collected, researchers should analyze it using appropriate data analysis methods. This will help them answer the research question and draw conclusions from the data.
- Interpret results: After analyzing the data, researchers should interpret the results and draw conclusions based on the findings. This will help them answer the research question and make recommendations for future research or practical applications.
- Communicate findings: Finally, researchers should communicate their findings to the appropriate audience using a format that is appropriate for the research question and audience. This may include writing a research paper, presenting at a conference, or creating a report for a client or stakeholder.
Purpose of Original Research
The purpose of original research is to generate new knowledge and understanding in a particular field of study. Original research is conducted to address a research question, hypothesis, or problem and to produce empirical evidence that can be used to inform theory, policy, and practice. By conducting original research, researchers can:
- Expand the existing knowledge base: Original research helps to expand the existing knowledge base by providing new information and insights into a particular phenomenon. This information can be used to develop new theories, models, or frameworks that explain the phenomenon in greater depth.
- Test existing theories and hypotheses: Original research can be used to test existing theories and hypotheses by collecting empirical evidence and analyzing the data. This can help to refine or modify existing theories, or to develop new ones that better explain the phenomenon.
- Identify gaps in the existing knowledge: Original research can help to identify gaps in the existing knowledge base by highlighting areas where further research is needed. This can help to guide future research and identify new research questions that need to be addressed.
- Inform policy and practice: Original research can be used to inform policy and practice by providing empirical evidence that can be used to make decisions and develop interventions. This can help to improve the quality of life for individuals and communities, and to address social, economic, and environmental challenges.
How to publish Original Research
Publishing original research involves several steps that researchers need to follow to ensure that their research is accepted and published in reputable academic journals. Here are some general steps that researchers can follow to publish their original research:
- Select a suitable journal: Researchers should identify a suitable academic journal that publishes research in their field of study. The journal should have a good reputation and a high impact factor, and should be a good fit for the research topic and methods used.
- Review the submission guidelines: Once a suitable journal is identified, researchers should review the submission guidelines to ensure that their manuscript meets the journal’s requirements. The guidelines may include requirements for formatting, length, and content.
- Write the manuscript : Researchers should write the manuscript in accordance with the submission guidelines and academic standards. The manuscript should include a clear research question or hypothesis, a description of the research methods used, an analysis of the data collected, and a discussion of the results and their implications.
- Submit the manuscript: Once the manuscript is written, researchers should submit it to the selected journal. The submission process may require the submission of a cover letter, abstract, and other supporting documents.
- Respond to reviewer feedback: After the manuscript is submitted, it will be reviewed by experts in the field who will provide feedback on the quality and suitability of the research. Researchers should carefully review the feedback and revise the manuscript accordingly.
- Respond to editorial feedback: Once the manuscript is revised, it will be reviewed by the journal’s editorial team who will provide feedback on the formatting, style, and content of the manuscript. Researchers should respond to this feedback and make any necessary revisions.
- Acceptance and publication: If the manuscript is accepted, the journal will inform the researchers and the manuscript will be published in the journal. If the manuscript is not accepted, researchers can submit it to another journal or revise it further based on the feedback received.
How to Identify Original Research
To identify original research, there are several factors to consider:
- The research question: Original research typically starts with a novel research question or hypothesis that has not been previously explored or answered in the existing literature.
- The research design: Original research should have a clear and well-designed research methodology that follows appropriate scientific standards. The methodology should be described in detail in the research article.
- The data: Original research should include new data that has not been previously published or analyzed. The data should be collected using appropriate research methods and analyzed using valid statistical methods.
- The results: Original research should present new findings or insights that have not been previously reported in the existing literature. The results should be presented clearly and objectively, and should be supported by the data collected.
- The discussion and conclusions: Original research should provide a clear and objective interpretation of the results, and should discuss the implications of the research findings. The discussion and conclusions should be based on the data collected and the research question or hypothesis.
- The references: Original research should be supported by references to existing literature, which should be cited appropriately in the research article.
Advantages of Original Research
Original research has several advantages, including:
- Generates new knowledge: Original research is conducted to answer novel research questions or hypotheses, which can generate new knowledge and insights into various fields of study.
- Supports evidence-based decision making: Original research provides empirical evidence that can inform decision-making in various fields, such as medicine, public policy, and business.
- Enhances academic and professional reputation: Conducting original research and publishing in reputable academic journals can enhance a researcher’s academic and professional reputation.
- Provides opportunities for collaboration: Original research can provide opportunities for collaboration between researchers, institutions, and organizations, which can lead to new partnerships and research projects.
- Advances scientific and technological progress: Original research can contribute to scientific and technological progress by providing new knowledge and insights into various fields of study, which can inform further research and development.
- Can lead to practical applications: Original research can have practical applications in various fields, such as medicine, engineering, and social sciences, which can lead to new products, services, and policies that benefit society.
Limitations of Original Research
Original research also has some limitations, which include:
- Time and resource constraints: Original research can be time-consuming and expensive, requiring significant resources to design, execute, and analyze the research data.
- Ethical considerations: Conducting original research may raise ethical considerations, such as ensuring the privacy and confidentiality of research participants, obtaining informed consent, and avoiding conflicts of interest.
- Risk of bias: Original research may be subject to biases, such as selection bias, measurement bias, and publication bias, which can affect the validity and reliability of the research findings.
- Generalizability: Original research findings may not be generalizable to larger populations or different contexts, which can limit the applicability of the research findings.
- Replicability: Original research may be difficult to replicate, which can limit the ability of other researchers to verify the research findings.
- Limited scope: Original research may have a limited scope, focusing on a specific research question or hypothesis, which can limit the breadth of the research findings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Documentary Research – Types, Methods and...

Scientific Research – Types, Purpose and Guide

Humanities Research – Types, Methods and Examples

Historical Research – Types, Methods and Examples

Artistic Research – Methods, Types and Examples

- Master Your Homework
- Do My Homework
Research and Review Papers: The Key Differences
Research and review papers are two of the most common types of scholarly works that contribute to a field’s progress. Despite their similarities, these two forms of writing have distinct differences in structure, content, purpose, and tone. This article will explore the distinctions between research papers and reviews by exploring each type separately before comparing them in terms of academic rigor, length, methodology used for data gathering or analysis (if applicable), formal language use in referencing sources as well as citing references throughout the paper itself. Finally we will discuss ways students can effectively differentiate between research papers versus review documents when engaging with either form while completing assignments or producing new work themselves.
1. Introduction: Investigating the Difference Between Research and Review Papers
2. types of scholarly papers commonly used in academia, 3. characteristics that distinguish a research paper from a review paper, 4. the role of original research within each type of paper, 5. structural elements unique to each kind of academic writing, 6. tips for differentiating between the two types when analyzing content, 7. conclusion: examining how understanding the differences can help students write better quality papers.
Exploring the Differences between Research and Review Papers The academic world is often abuzz with discourse on research and review papers. These two types of writing are distinct in their approach, structure, content and purpose. To begin, a research paper represents an original piece of work that involves comprehensive study into existing knowledge. It requires students to use scientific methodology to investigate topics by collecting data through experimentation or observation; it should then be analyzed thoroughly before any conclusions can be drawn. On the other hand, a review paper is more focused on existing literature: synthesizing different authors’ views together while pointing out various similarities/differences among them. The main goal is usually critiquing previous theories from an objective point-of-view rather than offering new ones for consideration.
In terms of composition style and formatting preferences – both types require rigorous proofreading but there are some nuances that distinguish one from another as well: research papers will typically feature introduction + body (with sections such as background info., materials & methods etc.)+ conclusion; reviews may also have this organization yet rely heavily on summarization techniques due to its nature of being secondary material based only off previously published works by others in the field at hand! Furthermore — references cited within research papers need to meet certain criteria whereas those included within review pieces can vary greatly depending upon particular topic under examination – so always remember these subtle distinctions when considering which type you’re trying write next time around!
When pursuing an academic career, it is important to be familiar with the different types of scholarly papers used in academia. Different areas of research often require different paper styles depending on the specifics and focus of the project. Here are two types that tend to be widely accepted.
Research papers and review papers are two distinct types of scholarly works, each with its own purpose. The primary difference between the two is that research papers aim to provide new insight into a given topic whereas review papers build upon existing knowledge by summarizing what has already been published on the subject.
- Purpose : Research paper typically focus on identifying or verifying an answer to a specific question or hypothesis through rigorous investigation of evidence from primary sources (e.g., empirical studies). Review articles, however, tend to evaluate and synthesize pre-existing information for readers’ benefit.
- Scope : While research articles often examine only one aspect of their chosen topic in great detail, reviews take a wider angle by combining multiple studies together under one umbrella theme. Reviews also incorporate other forms of data such as interviews, surveys and anecdotal accounts to offer more comprehensive insights.
Original research papers and review papers are two distinct types of academic writing, with each having its own set of specific requirements. Original research is the backbone behind any great paper as it allows for new facts to be discovered or revealed while providing an opportunity for interpretation and analysis. On the other hand, a review paper synthesizes previous work from multiple sources into one cohesive argument that can help provide greater insight into an issue.
- It provides a starting point by establishing evidence-based information on which to build further knowledge.
- It offers readers the chance to view your original insights in light of existing theories and literature.
- It establishes credibility when peers recognize contributions you have made based on careful investigation
Reviews allow researchers to combine ideas from several different studies into one more comprehensive overview . They enable readers—such as those studying at higher levels who lack time —to quickly familiarize themselves with current findings without needing additional primary data collection or synthesis .
Essay Writing
The hallmark of essay writing is the way it structures ideas into a coherent argument. Essays usually consist of an introduction, several body paragraphs and a conclusion. Each section can take up multiple pages as students may need to elaborate on key points or evidence from research studies to back up their point of view. It is essential that each paragraph logically transitions in order for readers to make sense out of them when they are read together.
Research Paper Writing A research paper requires much more extensive analysis than an essay does. In addition to discussing related literature, these papers often involve collecting data through surveys or experiments and providing detailed descriptions about study results and conclusions reached based on this information. Research papers may include charts, tables and diagrams in addition to written text which help illustrate any scientific processes or observations found during the course of investigations conducted by the student author.
- Compare & Contrast : A review paper will cover different aspects but unlike a research paper it won’t include gathering new data.
Distinguishing Content Analysis Types:
Analyzing content can be a daunting task without an understanding of the two types: research papers and review papers. Research papers are typically written to explain new results, while review papers offer insights on existing literature. Although both types focus on evidence-based arguments in their analysis, it is possible to differentiate between them through several tips.
Firstly, research paper should have a hypothesis that was tested with data or experiments. It is likely this work will include quantitative methods such as statistics or qualitative techniques like interviews; these elements make it easier to identify a paper’s type as one of empirical research rather than just opinion pieces. In contrast, review articles summarize what has already been published and assess current trends within the field instead of examining hypotheses.
- When researching scientific topics especially check if figures are included.
In general, primary sources from other researchers are necessary for creating original claims whereas reviews usually cite many secondary sources because they do not involve original findings.
- Consider which facts appear most frequently when analyzing whether something is a primary source.
Comprehending the Distinctions
Aspiring academic writers should be aware that research papers and review papers are fundamentally different. While each requires in-depth analysis, synthesis of existing literature, careful referencing and citation of sources, a research paper is intended to present original findings while a review paper provides an overview and synthesis of relevant sources. Therefore, an understanding of these distinctions can help students write better quality papers.
When writing either type of paper it’s important for students to understand their objectives. A successful research paper will require more time spent conducting independent investigation or experimentation than would a review paper which typically involves summarizing the conclusions drawn by other scholars on the topic being studied. Furthermore when formulating arguments in either type student must take into consideration how best to use evidence from reliable scholarly sources as support for their views.
- Research Papers: Involves gathering new data through primary investigation or experiments.
- Review Paper: Involves synthesising existing literature on particular subject.
In conclusion knowledge about both types can assist student authors craft higher caliber assignments as well as give them confidence that they have chosen appropriate approaches in tackling difficult topics within academia .
As a final thought, it is important to understand the differences between research and review papers in order to make sure that any work produced reflects an appropriate level of academic rigour. Research papers are typically more complex than reviews because they require original findings through empirical or theoretical study, whereas reviews rely on synthesizing existing literature from multiple sources. Knowing the key distinctions between these two types of writing can help ensure accuracy, clarity and ultimately success for both students and professionals alike when producing written works for submission or publication.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 15 April 2024
Revealed: the ten research papers that policy documents cite most
- Dalmeet Singh Chawla 0
Dalmeet Singh Chawla is a freelance science journalist based in London.
You can also search for this author in PubMed Google Scholar

Policymakers often work behind closed doors — but the documents they produce offer clues about the research that influences them. Credit: Stefan Rousseau/Getty
When David Autor co-wrote a paper on how computerization affects job skill demands more than 20 years ago, a journal took 18 months to consider it — only to reject it after review. He went on to submit it to The Quarterly Journal of Economics , which eventually published the work 1 in November 2003.
Autor’s paper is now the third most cited in policy documents worldwide, according to an analysis of data provided exclusively to Nature . It has accumulated around 1,100 citations in policy documents, show figures from the London-based firm Overton (see ‘The most-cited papers in policy’), which maintains a database of more than 12 million policy documents, think-tank papers, white papers and guidelines.
“I thought it was destined to be quite an obscure paper,” recalls Autor, a public-policy scholar and economist at the Massachusetts Institute of Technology in Cambridge. “I’m excited that a lot of people are citing it.”
The most-cited papers in policy
Economics papers dominate the top ten papers that policy documents reference most.
Data from Sage Policy Profiles as of 15 April 2024
The top ten most cited papers in policy documents are dominated by economics research; the number one most referenced study has around 1,300 citations. When economics studies are excluded, a 1997 Nature paper 2 about Earth’s ecosystem services and natural capital is second on the list, with more than 900 policy citations. The paper has also garnered more than 32,000 references from other studies, according to Google Scholar. Other highly cited non-economics studies include works on planetary boundaries, sustainable foods and the future of employment (see ‘Most-cited papers — excluding economics research’).
These lists provide insight into the types of research that politicians pay attention to, but policy citations don’t necessarily imply impact or influence, and Overton’s database has a bias towards documents published in English.
Interdisciplinary impact
Overton usually charges a licence fee to access its citation data. But last year, the firm worked with the London-based publisher Sage to release a free web-based tool that allows any researcher to find out how many times policy documents have cited their papers or mention their names. Overton and Sage said they created the tool, called Sage Policy Profiles, to help researchers to demonstrate the impact or influence their work might be having on policy. This can be useful for researchers during promotion or tenure interviews and in grant applications.
Autor thinks his study stands out because his paper was different from what other economists were writing at the time. It suggested that ‘middle-skill’ work, typically done in offices or factories by people who haven’t attended university, was going to be largely automated, leaving workers with either highly skilled jobs or manual work. “It has stood the test of time,” he says, “and it got people to focus on what I think is the right problem.” That topic is just as relevant today, Autor says, especially with the rise of artificial intelligence.
Most-cited papers — excluding economics research
When economics studies are excluded, the research papers that policy documents most commonly reference cover topics including climate change and nutrition.
Walter Willett, an epidemiologist and food scientist at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, thinks that interdisciplinary teams are most likely to gain a lot of policy citations. He co-authored a paper on the list of most cited non-economics studies: a 2019 work 3 that was part of a Lancet commission to investigate how to feed the global population a healthy and environmentally sustainable diet by 2050 and has accumulated more than 600 policy citations.
“I think it had an impact because it was clearly a multidisciplinary effort,” says Willett. The work was co-authored by 37 scientists from 17 countries. The team included researchers from disciplines including food science, health metrics, climate change, ecology and evolution and bioethics. “None of us could have done this on our own. It really did require working with people outside our fields.”
Sverker Sörlin, an environmental historian at the KTH Royal Institute of Technology in Stockholm, agrees that papers with a diverse set of authors often attract more policy citations. “It’s the combined effect that is often the key to getting more influence,” he says.

Has your research influenced policy? Use this free tool to check
Sörlin co-authored two papers in the list of top ten non-economics papers. One of those is a 2015 Science paper 4 on planetary boundaries — a concept defining the environmental limits in which humanity can develop and thrive — which has attracted more than 750 policy citations. Sörlin thinks one reason it has been popular is that it’s a sequel to a 2009 Nature paper 5 he co-authored on the same topic, which has been cited by policy documents 575 times.
Although policy citations don’t necessarily imply influence, Willett has seen evidence that his paper is prompting changes in policy. He points to Denmark as an example, noting that the nation is reformatting its dietary guidelines in line with the study’s recommendations. “I certainly can’t say that this document is the only thing that’s changing their guidelines,” he says. But “this gave it the support and credibility that allowed them to go forward”.

Broad brush
Peter Gluckman, who was the chief science adviser to the prime minister of New Zealand between 2009 and 2018, is not surprised by the lists. He expects policymakers to refer to broad-brush papers rather than those reporting on incremental advances in a field.
Gluckman, a paediatrician and biomedical scientist at the University of Auckland in New Zealand, notes that it’s important to consider the context in which papers are being cited, because studies reporting controversial findings sometimes attract many citations. He also warns that the list is probably not comprehensive: many policy papers are not easily accessible to tools such as Overton, which uses text mining to compile data, and so will not be included in the database.

The top 100 papers
“The thing that worries me most is the age of the papers that are involved,” Gluckman says. “Does that tell us something about just the way the analysis is done or that relatively few papers get heavily used in policymaking?”
Gluckman says it’s strange that some recent work on climate change, food security, social cohesion and similar areas hasn’t made it to the non-economics list. “Maybe it’s just because they’re not being referred to,” he says, or perhaps that work is cited, in turn, in the broad-scope papers that are most heavily referenced in policy documents.
As for Sage Policy Profiles, Gluckman says it’s always useful to get an idea of which studies are attracting attention from policymakers, but he notes that studies often take years to influence policy. “Yet the average academic is trying to make a claim here and now that their current work is having an impact,” he adds. “So there’s a disconnect there.”
Willett thinks policy citations are probably more important than scholarly citations in other papers. “In the end, we don’t want this to just sit on an academic shelf.”
doi: https://doi.org/10.1038/d41586-024-00660-1
Autor, D. H., Levy, F. & Murnane, R. J. Q. J. Econ. 118 , 1279–1333 (2003).
Article Google Scholar
Costanza, R. et al. Nature 387 , 253–260 (1997).
Willett, W. et al. Lancet 393 , 447–492 (2019).
Article PubMed Google Scholar
Steffen, W. et al. Science 347 , 1259855 (2015).
Rockström, J. et al. Nature 461 , 472–475 (2009).
Download references
Reprints and permissions
Related Articles

We must protect the global plastics treaty from corporate interference
World View 17 APR 24

UN plastics treaty: don’t let lobbyists drown out researchers
Editorial 17 APR 24

Smoking bans are coming: what does the evidence say?
News 17 APR 24

CERN’s impact goes way beyond tiny particles
Spotlight 17 APR 24

The economic commitment of climate change
Article 17 APR 24

Last-mile delivery increases vaccine uptake in Sierra Leone
Article 13 MAR 24
Postdoctoral Position
We are seeking highly motivated and skilled candidates for postdoctoral fellow positions
Boston, Massachusetts (US)
Boston Children's Hospital (BCH)
Qiushi Chair Professor
Distinguished scholars with notable achievements and extensive international influence.
Hangzhou, Zhejiang, China
Zhejiang University
ZJU 100 Young Professor
Promising young scholars who can independently establish and develop a research direction.
Head of the Thrust of Robotics and Autonomous Systems
Reporting to the Dean of Systems Hub, the Head of ROAS is an executive assuming overall responsibility for the academic, student, human resources...
Guangzhou, Guangdong, China
The Hong Kong University of Science and Technology (Guangzhou)
Head of Biology, Bio-island
Head of Biology to lead the discovery biology group.
BeiGene Ltd.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
EDITORIAL article
This article is part of the research topic.
Molecular Links between Mitochondrial Damage and Human Neurodegenerative Disorders - Volume II
Editorial: Molecular Links between Mitochondrial Damage and Human Neurodegenerative Disorders -Volume II Provisionally Accepted

- 1 Department of Research for Parkinson’s Disease, Juntendo University Graduate School of Medicine, Japan
The final, formatted version of the article will be published soon.
Mitochondria have various functions in cells, including energy production, iron and lipid metabolism, regulation of Ca2+ dynamics, and cell death and inflammatory signaling. Dysregulation and degeneration of mitochondria have been shown to cause a variety of neurodegenerative diseases. In 2021, we launched a research topic entitled 'Molecular Links between Mitochondrial Damage and Human Neurodegenerative Disorders', to which many excellent papers were contributed. This topic consists of two original articles and two review articles that were submitted after 2022 on the same research topic. These manuscripts cover mitochondrial metabolism, mitochondrial DNA, membrane potential, and mitochondrial membrane structure. Fragile X syndrome, one of the triplet repeat diseases, is a disease characterized by developmental delay/intellectual disability beginning in childhood due to mutations in the Fragile X messenger ribonucleoprotein 1 (FMR1) gene. The triplet repeat sequence (CGG) of the FMR1 gene on the long arm of the X chromosome is extended with each generation. When the number of CGG repeats exceeds 200, Fragile X syndrome develops. Reduced expression of the FMR1 gene product Fragile X Messenger Ribonucleoprotein (FMRP) is believed to be the core pathophysiology of fragile X syndrome. FMRP, an RNA-binding protein, binds to mRNA and negatively regulates local translation of mRNA in neurons. Using primary cortical neuronal cultures from FMR1 knockout mice, Bülow et al. report that neuronal activity-dependent changes in mitochondrial morphology and membrane potential differ in dendrites and axon initial segment. The mechanism by which FMRP regulates mitochondria has yet to be determined. FMR1 knockout mice exhibit elevated protein synthesis (Bhattacharya et al., 2012). Metformin inhibits protein translation by blocking mammalian/mechanistic target of rapamycin complex 1 (mTORC1) and suppresses an autism spectrum disorder-like phenotype in FMR1 knockout mice (Gantois et al., 2017). Metformin also inhibits mitochondrial respiratory chain complex I and activates AMP-activated protein kinase signaling (Foretz et al., 2014). This mechanism may provide clues for the elucidation of mitochondrial regulation by FMRP. Mitochondrial DNA deletion syndrome (MDS) is a group of diseases characterized by defects in mitochondrial DNA (mtDNA) maintenance (Basel, 2020). The mitochondrial genome encodes 13 proteins, all of which are important catalytic subunits of the electron transfer chain. Thus, depletion of mitochondrial DNA results in impaired oxidative phosphorylation. Dombi et al. determine the most effective nucleoside supplementation method (optimal concentration and nucleoside combination) based on improving mtDNA content, cell viability, and mitochondrial functions using MDS fibroblasts harboring mutations in POLG, DGUOK, and TWNK (genes encoding alpha subunits of mtDNA γ polymerase, deoxyguanosine kinase, and Twinkle mtDNA helicase, respectively) (Bulst et al., 2009). The findings of this study will be useful for future studies of nucleoside supplementation methods in MDS animal models. Mitophagy, autophagy of mitochondria, is a physiological phenomenon that is highly conserved from yeast to humans. Mitophagy is observed in metabolic changes during development and differentiation and in the quality control of defective mitochondria. The relationship between neurodegeneration and mitophagy was first discovered in a series of studies that showed that PRKN and PINK1, the genes responsible for juvenile Parkinson's disease (PD), are involved in mitophagy (Imai, 2020). Since then, many molecules associated with mitophagy have been identified and PINK1-Parkin (the gene product of PRKN)-independent mitophagy pathways have also been reported. Furthermore, mitophagy research continues to expand with reports of its involvement in neurodegenerative diseases such as Alzheimer's disease and amyotrophic lateral sclerosis (ALS), as well as Parkinson's disease. Jetto et al. provide an overview of mitophagy signaling in neurodegenerative diseases, including some unresolved issues. CHCHD2 and CHCHD10 have been identified as causative genes for PD and ALS/ frontotemporal lobe dementia (FTD), respectively (Bannwarth et al., 2014; Funayama et al., 2015). These two proteins are paralogous to each other, forming a complex in the mitochondrial intermembrane space. CHCHD2 and CHCHD10 have been implicated in maintaining mitochondrial respiratory chain complex function and matrix structure, as well as in the regulation of stress response and cell death. Despite this twin-like relationship, missense mutations in each gene cause different diseases. Ikeda et al. outlined the CHCHD2/CHCHD10 studies reported to date and aimed to provide clues to unravel this mystery. The multifaceted roles of mitochondria and the involvement of mitochondria in various diseases remain a fascinating research topic. Why do specific genes in ubiquitously distributed mitochondria cause specific diseases? To address this question, many researchers are expected to continue work in this field.
Keywords: Mitochondria, mtDNA, mitophagy, CHCHD2, CHCHD10, Fragile X Syndrome, Parkin (PARK2), FMR1
Received: 15 Apr 2024; Accepted: 19 Apr 2024.
Copyright: © 2024 Imai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Mx. Yuzuru Imai, Department of Research for Parkinson’s Disease, Juntendo University Graduate School of Medicine, Tokyo, Japan
People also looked at
AI Index Report
Welcome to the seventh edition of the AI Index report. The 2024 Index is our most comprehensive to date and arrives at an important moment when AI’s influence on society has never been more pronounced. This year, we have broadened our scope to more extensively cover essential trends such as technical advancements in AI, public perceptions of the technology, and the geopolitical dynamics surrounding its development. Featuring more original data than ever before, this edition introduces new estimates on AI training costs, detailed analyses of the responsible AI landscape, and an entirely new chapter dedicated to AI’s impact on science and medicine.
Read the 2024 AI Index Report
The AI Index report tracks, collates, distills, and visualizes data related to artificial intelligence (AI). Our mission is to provide unbiased, rigorously vetted, broadly sourced data in order for policymakers, researchers, executives, journalists, and the general public to develop a more thorough and nuanced understanding of the complex field of AI.
The AI Index is recognized globally as one of the most credible and authoritative sources for data and insights on artificial intelligence. Previous editions have been cited in major newspapers, including the The New York Times, Bloomberg, and The Guardian, have amassed hundreds of academic citations, and been referenced by high-level policymakers in the United States, the United Kingdom, and the European Union, among other places. This year’s edition surpasses all previous ones in size, scale, and scope, reflecting the growing significance that AI is coming to hold in all of our lives.
Steering Committee Co-Directors

Ray Perrault
Steering committee members.

Erik Brynjolfsson

John Etchemendy

Katrina Ligett

Terah Lyons

James Manyika

Juan Carlos Niebles

Vanessa Parli

Yoav Shoham

Russell Wald
Staff members.

Loredana Fattorini

Nestor Maslej
Letter from the co-directors.
A decade ago, the best AI systems in the world were unable to classify objects in images at a human level. AI struggled with language comprehension and could not solve math problems. Today, AI systems routinely exceed human performance on standard benchmarks.
Progress accelerated in 2023. New state-of-the-art systems like GPT-4, Gemini, and Claude 3 are impressively multimodal: They can generate fluent text in dozens of languages, process audio, and even explain memes. As AI has improved, it has increasingly forced its way into our lives. Companies are racing to build AI-based products, and AI is increasingly being used by the general public. But current AI technology still has significant problems. It cannot reliably deal with facts, perform complex reasoning, or explain its conclusions.
AI faces two interrelated futures. First, technology continues to improve and is increasingly used, having major consequences for productivity and employment. It can be put to both good and bad uses. In the second future, the adoption of AI is constrained by the limitations of the technology. Regardless of which future unfolds, governments are increasingly concerned. They are stepping in to encourage the upside, such as funding university R&D and incentivizing private investment. Governments are also aiming to manage the potential downsides, such as impacts on employment, privacy concerns, misinformation, and intellectual property rights.
As AI rapidly evolves, the AI Index aims to help the AI community, policymakers, business leaders, journalists, and the general public navigate this complex landscape. It provides ongoing, objective snapshots tracking several key areas: technical progress in AI capabilities, the community and investments driving AI development and deployment, public opinion on current and potential future impacts, and policy measures taken to stimulate AI innovation while managing its risks and challenges. By comprehensively monitoring the AI ecosystem, the Index serves as an important resource for understanding this transformative technological force.
On the technical front, this year’s AI Index reports that the number of new large language models released worldwide in 2023 doubled over the previous year. Two-thirds were open-source, but the highest-performing models came from industry players with closed systems. Gemini Ultra became the first LLM to reach human-level performance on the Massive Multitask Language Understanding (MMLU) benchmark; performance on the benchmark has improved by 15 percentage points since last year. Additionally, GPT-4 achieved an impressive 0.97 mean win rate score on the comprehensive Holistic Evaluation of Language Models (HELM) benchmark, which includes MMLU among other evaluations.
Although global private investment in AI decreased for the second consecutive year, investment in generative AI skyrocketed. More Fortune 500 earnings calls mentioned AI than ever before, and new studies show that AI tangibly boosts worker productivity. On the policymaking front, global mentions of AI in legislative proceedings have never been higher. U.S. regulators passed more AI-related regulations in 2023 than ever before. Still, many expressed concerns about AI’s ability to generate deepfakes and impact elections. The public became more aware of AI, and studies suggest that they responded with nervousness.
Ray Perrault Co-director, AI Index
Our Supporting Partners

Analytics & Research Partners

Stay up to date on the AI Index by subscribing to the Stanford HAI newsletter.
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Amanda Hoover
Students Are Likely Writing Millions of Papers With AI
Students have submitted more than 22 million papers that may have used generative AI in the past year, new data released by plagiarism detection company Turnitin shows.
A year ago, Turnitin rolled out an AI writing detection tool that was trained on its trove of papers written by students as well as other AI-generated texts. Since then, more than 200 million papers have been reviewed by the detector, predominantly written by high school and college students. Turnitin found that 11 percent may contain AI-written language in 20 percent of its content, with 3 percent of the total papers reviewed getting flagged for having 80 percent or more AI writing. (Turnitin is owned by Advance, which also owns Condé Nast, publisher of WIRED.) Turnitin says its detector has a false positive rate of less than 1 percent when analyzing full documents.
ChatGPT’s launch was met with knee-jerk fears that the English class essay would die . The chatbot can synthesize information and distill it near-instantly—but that doesn’t mean it always gets it right. Generative AI has been known to hallucinate , creating its own facts and citing academic references that don’t actually exist. Generative AI chatbots have also been caught spitting out biased text on gender and race . Despite those flaws, students have used chatbots for research, organizing ideas, and as a ghostwriter . Traces of chatbots have even been found in peer-reviewed, published academic writing .
Teachers understandably want to hold students accountable for using generative AI without permission or disclosure. But that requires a reliable way to prove AI was used in a given assignment. Instructors have tried at times to find their own solutions to detecting AI in writing, using messy, untested methods to enforce rules , and distressing students. Further complicating the issue, some teachers are even using generative AI in their grading processes.
Detecting the use of gen AI is tricky. It’s not as easy as flagging plagiarism, because generated text is still original text. Plus, there’s nuance to how students use gen AI; some may ask chatbots to write their papers for them in large chunks or in full, while others may use the tools as an aid or a brainstorm partner.
Students also aren't tempted by only ChatGPT and similar large language models. So-called word spinners are another type of AI software that rewrites text, and may make it less obvious to a teacher that work was plagiarized or generated by AI. Turnitin’s AI detector has also been updated to detect word spinners, says Annie Chechitelli, the company’s chief product officer. It can also flag work that was rewritten by services like spell checker Grammarly, which now has its own generative AI tool . As familiar software increasingly adds generative AI components, what students can and can’t use becomes more muddled.
Detection tools themselves have a risk of bias. English language learners may be more likely to set them off; a 2023 study found a 61.3 percent false positive rate when evaluating Test of English as a Foreign Language (TOEFL) exams with seven different AI detectors. The study did not examine Turnitin’s version. The company says it has trained its detector on writing from English language learners as well as native English speakers. A study published in October found that Turnitin was among the most accurate of 16 AI language detectors in a test that had the tool examine undergraduate papers and AI-generated papers.

Matt Burgess

Andrew Couts

Emily Mullin

C. Brandon Ogbunu
Schools that use Turnitin had access to the AI detection software for a free pilot period, which ended at the start of this year. Chechitelli says a majority of the service’s clients have opted to purchase the AI detection. But the risks of false positives and bias against English learners have led some universities to ditch the tools for now. Montclair State University in New Jersey announced in November that it would pause use of Turnitin’s AI detector. Vanderbilt University and Northwestern University did the same last summer.
“This is hard. I understand why people want a tool,” says Emily Isaacs, executive director of the Office of Faculty Excellence at Montclair State. But Isaacs says the university is concerned about potentially biased results from AI detectors, as well as the fact that the tools can’t provide confirmation the way they can with plagiarism. Plus, Montclair State doesn’t want to put a blanket ban on AI, which will have some place in academia. With time and more trust in the tools, the policies could change. “It’s not a forever decision, it’s a now decision,” Isaacs says.
Chechitelli says the Turnitin tool shouldn’t be the only consideration in passing or failing a student. Instead, it’s a chance for teachers to start conversations with students that touch on all of the nuance in using generative AI. “People don’t really know where that line should be,” she says.
You Might Also Like …
In your inbox: The best and weirdest stories from WIRED’s archive
Jeffrey Epstein’s island visitors exposed by data broker
8 Google employees invented modern AI. Here’s the inside story
The crypto fraud kingpin who almost got away
Listen up! These are the best podcasts , no matter what you’re into

Will Knight

Lauren Goode
Reece Rogers

Morgan Meaker

Tom Simonite
Joel Khalili

Benj Edwards, Ars Technica
This paper is in the following e-collection/theme issue:
Published on 19.4.2024 in Vol 26 (2024)
Investigating the Cost-Effectiveness of Telemonitoring Patients With Cardiac Implantable Electronic Devices: Systematic Review
Authors of this article:

- Sarah Raes 1 , MSc ;
- Andrea Prezzi 1 , MSc ;
- Rik Willems 2 , PhD ;
- Hein Heidbuchel 3 , PhD ;
- Lieven Annemans 1 , PhD
1 Department of Public Health and Primary Care, Ghent University, Gent, Belgium
2 Department of Cardiovascular Sciences, Universiteit Leuven, Leuven, Belgium
3 Department of Genetics, Pharmacology and Physiopathology of Heart, Blood Vessels and Skeleton (GENCOR), Antwerp University, Antwerp, Belgium
Corresponding Author:
Sarah Raes, MSc
Department of Public Health and Primary Care
Ghent University
Corneel Heymanslaan 10
Phone: 32 9 332 83 59
Email: [email protected]
Background: Telemonitoring patients with cardiac implantable electronic devices (CIEDs) can improve their care management. However, the results of cost-effectiveness studies are heterogeneous. Therefore, it is still a matter of debate whether telemonitoring is worth the investment.
Objective: This systematic review aims to investigate the cost-effectiveness of telemonitoring patients with CIEDs, focusing on its key drivers, and the impact of the varying perspectives.
Methods: A systematic review was performed in PubMed, Web of Science, Embase, and EconLit. The search was completed on July 7, 2022. Studies were included if they fulfilled the following criteria: patients had a CIED, comparison with standard care, and inclusion of health economic evaluations (eg, cost-effectiveness analyses and cost-utility analyses). Only complete and peer-reviewed studies were included, and no year limits were applied. The exclusion criteria included studies with partial economic evaluations, systematic reviews or reports, and studies without standard care as a control group. Besides general study characteristics, the following outcome measures were extracted: impact on total cost or income, cost or income drivers, cost or income drivers per patient, cost or income drivers as a percentage of the total cost impact, incremental cost-effectiveness ratios, or cost-utility ratios. Quality was assessed using the Consensus Health Economic Criteria checklist.
Results: Overall, 15 cost-effectiveness analyses were included. All studies were performed in Western countries, mainly Europe, and had primarily a male participant population. Of the 15 studies, 3 (20%) calculated the incremental cost-effectiveness ratio, 1 (7%) the cost-utility ratio, and 11 (73%) the health and cost impact of telemonitoring. In total, 73% (11/15) of the studies indicated that telemonitoring of patients with implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy ICDs was cost-effective and cost-saving, both from a health care and patient perspective. Cost-effectiveness results for telemonitoring of patients with pacemakers were inconclusive. The key drivers for cost reduction from a health care perspective were hospitalizations and scheduled in-office visits. Hospitalization costs were reduced by up to US $912 per patient per year. Scheduled in-office visits included up to 61% of the total cost reduction. Key drivers for cost reduction from a patient perspective were loss of income, cost for scheduled in-office visits and transport. Finally, of the 15 studies, 8 (52%) reported improved quality of life, with statistically significance in only 1 (13%) study ( P =.03).
Conclusions: From a health care and patient perspective, telemonitoring of patients with an ICD or a cardiac resynchronization therapy ICD is a cost-effective and cost-saving alternative to standard care. Inconclusive results were found for patients with pacemakers. However, telemonitoring can lead to a decrease in providers’ income, mainly due to a lack of reimbursement. Introducing appropriate reimbursement could make telemonitoring sustainable for providers while still being cost-effective from a health care payer perspective.
Trial Registration: PROSPERO CRD42022322334; https://tinyurl.com/puunapdr
Introduction
The implantation rates of cardiac implantable electronic devices (CIEDs), including pacemakers and implantable cardioverter-defibrillators (ICDs), have increased over the last decades due to expanded indications and a progressively aging population [ 1 ]. To evaluate the clinical status of the patient and device functioning, current guidelines recommend that older patients with pacemakers should be evaluated every 3 to 12 months and patients with ICDs should be evaluated every 3 to 6 months [ 2 ]. This regimen imposes a considerable burden on patients and physicians if the patient is required to be seen in person.
Telemonitoring, referring to the process of using telecommunication and information technology to monitor the health status of a patient and device function from a distance, can reduce this burden by replacing some in-office visits with transmissions from the patients’ home [ 3 ]. Existing research indicated that telemonitoring is safe (eg, experiencing equal major adverse events to standard care) [ 4 , 5 ]. The advantages of telemonitoring include fewer inappropriate shocks for patients with ICDs [ 4 , 6 ] and fewer hospitalizations for patients with atrial arrhythmias and strokes [ 4 , 6 , 7 ]. Moreover, there is a rapid detection of cardiovascular events and device malfunction [ 5 , 7 ], leading to a time reduction between clinical decision and intervention [ 8 ].
Besides the effectiveness of telemonitoring, patient experience is essential in high-quality health care services. Overall, patients with pacemakers on telemonitoring reported positive experiences comparable to the experience of patients with in-hospital monitoring [ 9 ]. Telemonitored patients with pacemakers tended to receive less information about their diagnosis but no significant differences were found in other items, such as confidence in clinicians, treatment decision involvement, treatment satisfaction, and waiting time before admission [ 9 ]. Another study indicated that telemonitoring of patients with a cardiac resynchronization therapy defibrillator (CRT-D) was time-saving for both patients and physicians [ 10 ].
Cost-effectiveness analyses are important to quantify the value of new interventions, informing both medical decision-making and public policy [ 11 ]. However, cost-effectiveness analyses depend on the perspective considered. The different perspectives are the health care payer perspective (eg, Medicare or Medicaid and British National Health Service), the patient perspective, the provider perspective (eg, physician), and the society perspective. The health care payer and societal perspectives differ from each other as the societal perspective includes indirect nonmedical costs (eg, transport) [ 12 ].
As cost-effectiveness analyses have shown heterogeneous results, it is still debatable whether telemonitoring is worth the investment relative to standard care. However, data on cost-effectiveness are important for health care payers to make decisions on the reimbursement of telemonitoring. Lack of reimbursement can be an important adoption barrier for new technology [ 13 , 14 ]. For these 2 reasons, this paper reviews the cost-effectiveness of telemonitoring, reviews how the results differ from different perspectives, and describes the key drivers of the cost-effectiveness of telemonitoring.
The review protocol was published by PROSPERO (International Prospective Register of Systematic Reviews; CRD42022322334). This systematic review was carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guideline of 2020 [ 15 ], and the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) [ 16 ], which can be found in the Multimedia Appendix 1 . Guidelines for preparing a systematic review of health economic evaluations were followed [ 17 ].
Literature Search
For this review, PubMed, Embase, EconLit, and Web of Science Core Collection were systematically searched. The last search was performed on July 7, 2022. No filters (eg, publication date or type of study) were applied. Search strategies for all electronic databases can be found in Multimedia Appendix 2 .
Search strings were developed based on explorations of databases and previous reviews. The following key concepts were translated into strings: (1) CIEDs, (2) telemonitoring, and (3) economic evaluations (eg, cost-effectiveness analyses and cost-utility analyses). The latter was based on a validated search filter, designed to identify economic evaluations, and was broadened for this study to maximize sensitivity [ 18 ]. The search terms for CIEDs and telemonitoring were based on existing reviews [ 19 - 21 ].
Study Selection
Studies were included if their primary focus was on the cost-effectiveness of telemonitoring patients with a CIED. The eligibility criteria were defined a priori for study selection ( Textbox 1 ). The population, intervention, comparator, and outcome strategy was applied to describe the criteria. Only complete and peer-reviewed studies were included. Specific exclusion criteria included partial economic evaluations, systematic reviews or reports, and studies without standard care as a control group. Only studies published in English, Dutch, French, or German were eligible for inclusion. The reference lists of the included studies were searched manually to identify relevant studies. Two reviewers (SR and AP) independently screened the titles and abstracts of all records using Rayyan (Rayyan Systems Inc) [ 22 ]. After the initial screening, full texts were retrieved and screened for a second time. The second screening round was independently performed by 2 reviewers (SR and AP). Reasons for exclusion were documented ( Figure 1 ). For both screening rounds, reviewers were blinded from each other’s decision, and disagreements were resolved through discussion.
Inclusion criteria
- Cardiac implantable electronic devices: pacemaker, implantable cardioverter-defibrillator, cardiac resynchronization therapy defibrillator, cardiac resynchronization therapy pacemaker, and loop recorder
- Standard care
- Complete health economic evaluations (within-trial and model-based)
- All settings
- English, French, German, or Dutch
Exclusion criteria
- Implantable pulmonary artery pressure monitor
- Partial health economic evaluations (outcomes related to costs or effectiveness only)
- Systematic reviews, reports, commentaries, congress abstracts, protocols, and animal studies

Quality Assessment
Two researchers (SR and AP) independently evaluated the original papers using the Consensus Health Economic Criteria (CHEC) checklist to assess the risk of bias [ 23 ]. The CHEC checklist included 19 items. Any disagreement was resolved by discussion and consensus. Interpretation of the CHEC list can be found in Multimedia Appendix 3 . The included studies were classified into 4 quality categories: excellent (score of 100%), good quality (score between 75% and 100%), moderate quality (score between 50% and 75%), and low quality (score <50%) [ 24 ].
Synthesis of Results
The study characteristics and main outcomes of the original papers are presented in the Results section. SR extracted all data. A data extraction sheet was developed using an existing template [ 17 ]. The following information was extracted from the included studies: study identification, general study characteristics, results, and authors’ conclusion. The principal outcome measures were health outcomes, cost or income outcomes (eg, the impact on total cost or income, cost or income drivers, cost or income drivers per patient, and cost or income drivers as a percentage of the total cost impact), and incremental cost-effectiveness ratios (ICERs) or cost-utility ratios.
To facilitate comparison across studies, the following adjustments and interpretations were made. First, the cost or income outcomes were presented per patient per year, and different currencies were converted to US Dollar (reference year: 2019 and reference country: United States) [ 25 ]. Second, perspectives were categorized into the health care payer perspective, patient perspective, provider perspective, and societal perspective. For the purpose of our study, the provider includes physicians who are directly involved in the care of patients with CIED.
The selection process is shown in Figure 1 . From a total of 3305 publications, 15 (0.45%) unique publications were reviewed. Studies were excluded because one of the following reasons: (1) intervention: the paper did not describe telemonitoring patients with a CIED; (2) outcome: the paper contained only a cost analysis and not a cost-effectiveness analysis; and (3) study design or publication: the paper was a partial health economic evaluation, congress abstract, protocol, systematic review, animal study, or with no peer review.
Characteristics of the included studies can be found in Table 1 . All 15 (100%) studies had a primarily male population, except for the Nordland study, which had an almost equal sex distribution ( Table 1 ) [ 26 ]. The mean age of the population with pacemakers was between 75 (SD 24.64) and 81 (SD 6.47) years. The mean age of patients with an ICD or CRT-D was between 61 (SD 12.6) and 69 (SD not calculated) years, except for the PREDICT RM study, where >50% of the population was aged >75 years [ 27 ]. Furthermore, of the 15 studies, 1 (7%) included only older patients (with a mean age of 81 years) with pacemakers [ 28 ], and 2 (13%) ICD or CRT-D studies only included patients with heart failure [ 11 , 29 ].
a CIED: cardiac implantable electronic device.
b N/A: not applicable.
c Age was a discrete variable in this study (higher of lower than 75 years old).
d ICD: implantable cardioverter-defibrillator.
e TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
f CRT-D: cardiac resynchronization therapy defibrillator.
g EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
h MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
i CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
j ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
k EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
l VVI-ICD: single-chamber ICD.
m DDD-ICD: dual-chamber ICD.
n SAVE-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
Study Designs
Tables 2 and 3 show the summary table of results. Of 15 studies, 11 (73%) were conducted in Europe [ 11 , 26 , 28 - 32 , 34 , 35 , 37 ], 3 (20%) in the United States [ 27 , 33 , 38 ], and 1 (7%) in Canada [ 36 ]. Of the 15 studies, 3 (20%) calculated the ICER [ 26 - 28 ], 1 (7%) calculated the cost-utility ratio [ 11 ], and 11 (73%) calculated the cost impact of telemonitoring. All studies analyzed the health care payer perspective, with 33% (5/15) analyzing the patient perspective [ 11 , 28 , 30 , 32 , 34 ], 13% (2/15) analyzing the societal perspective [ 33 , 35 ], and 13% (2/15) analyzing the provider perspective [ 13 , 30 ].
b RCT: randomized controlled trial.
c QALY: quality-adjusted life year.
d ICER: incremental cost-effectiveness ratio.
e SAVE-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
f N/A: not applicable.
g ICD: implantable cardioverter-defibrillator.
h CRT-D: cardiac resynchronization therapy defibrillator.
i TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
j EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
k The values are statistically significant.
l MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
m QOL: quality of life.
n CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
o ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
p SF-36: The 36-Item Short Form Survey.
q EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
a If the perspective is health care system or patient, then cost and if the perspective is provider, then income .
b pp: per patient (in health care and patient perspectives) or per physician (in provider perspective).
c The values are statistically significant.
d SAVE-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
e ED: emergency department.
f ICD: implantable cardioverter-defibrillator.
g CRT-D: cardiac resynchronization therapy defibrillator.
h TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
i EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
j MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
k Costs were recalculated per patient.
l CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
m ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
n EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
Intervention and Comparator
Telemonitoring entailed data transmission and data review. Table 4 shows the frequencies of data transmission, review, and in-office visits of the included studies. In 47% (7/15) of the studies, data were transmitted continuously or daily [ 26 , 28 , 30 , 31 , 34 , 35 ]; in 20% (3/15) studies, data were transmitted after a device alert [ 8 , 11 , 29 ]; and in 13% (2/15) studies, data were transmitted every 3 months [ 32 , 33 ]. In 20% (3/15) of the studies, data review was performed daily [ 28 , 34 , 35 ]; however, in 40% (6/15) of the studies, it was performed after a device alert was received [ 8 , 11 , 26 , 29 - 31 ]. Besides data transmission and review, telemonitoring included scheduled in-office visits. In 33% (5/15) of the studies, all scheduled in-office visits were based on the protocol [ 11 , 13 , 29 , 30 , 35 ]. In 7% (1/15) of the studies, at least 1 scheduled in-office visit was protocol based [ 37 ]. In 3 (20%) of the 15 studies, only 1 scheduled in-office visit was protocol based [ 32 - 34 ]. Protocol-based in-office visits are described in Table 4 .
b pp: per patient.
c TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
d EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
e MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
g ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
h EuroEco: European Health Economic Trial on Home Monitoring in ICD Patients.
i Save-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
j ICD: implantable-cardioverter defibrillator.
k N/A: not applicable.
Effectiveness
Effectiveness results of telemonitoring can be found in Table 2 . Of the 15 studies, 9 (60%) investigated a quality-adjusted life year (QALY) or quality of life (QOL) difference [ 11 , 26 - 30 , 33 , 34 ]. A total of 53% (8/15) of studies reported an increase in QALY or QOL [ 11 , 26 - 28 , 30 , 33 , 34 , 39 ], but the QALY or QOL increase was only statistically significant in 1 (13%; P =.03) of the 8 studies [ 26 - 28 , 30 , 33 , 34 ]. In contrast, only 1 (11%) of the 9 studies investigating QOL or QALY reported a significant decrease in QOL [ 29 ]. Comparing all studies, QALY differences ranged from 0.03 to 0.27 in patients with pacemakers and ranged from −1 to 0.64 in patients with ICD or CRT-D.
Besides QALY or QOL, several studies reported other health outcomes. Chew et al [ 36 ] indicated that the risk of death was lower with telemonitoring. Al-Khatib et al [ 33 ] reported that mortality and general patient satisfaction with telemonitoring were equal to those of standard care. Crossley et al [ 8 ] reported that the time between the clinical event and the clinical decision was 17.4 days shorter in patients with an ICD or CRT-D on telemonitoring than in those on standard care ( P <.001). Burri et al [ 31 ] indicated that telemonitoring patients with ICD or CRT-D led to fewer inappropriate shocks (−51%) and a reduction in battery exhaustion (−7%). Raatikainen et al [ 32 ] indicated that telemonitoring patients with an ICD reduced the average total time spent on device follow-up, with 17 minutes per patient per follow-up for physicians and 175 minutes per patient per follow-up for patients. Similarly, Dario et al [ 37 ] indicated that the time spent by physicians to treat the patient reduced by an average of 4.1 minutes per follow-up in patients with pacemakers and an average of 13.7 minutes per follow-up in patients with an ICD (SD was not reported).
Economic Impact
The results of the economic impact of telemonitoring are presented in Table 2 . Of the 15 studies, 4 (27%) investigated the cost impact of telemonitoring in patients with pacemakers [ 26 , 28 , 35 , 37 ]. From a health care payer perspective, 1 (25%) of the 4 pacemaker studies indicated that telemonitoring increased costs with US $2183 per patient per year (not statistically significant) mainly because of increased hospitalization costs [ 26 ]. A total of 2 (50%) of the 4 pacemaker studies indicated that telemonitoring reduced costs by US $8.9 and US $1054 per patient per year mainly because of a reduction in hospitalization and staff costs, respectively [ 28 , 37 ]. Therefore, hospitalizations reduced costs in the study by Dario et al [ 37 ] but increased costs in the study by Lopez-Villegas et al [ 26 ]. From a patient and societal perspective, the results indicated that telemonitoring reduced costs by US $11 and US $1113 per patient per year, respectively, mainly because of lower transport costs [ 28 , 35 ].
Of the 15 studies, 13 (87%) investigated the cost or income impact of telemonitoring in patients with an ICD or CRT-D [ 8 , 11 , 13 , 27 , 29 - 37 ]. A total of 11 (85%) of the 13 ICD or CRT-D studies investigated the cost impact of telemonitoring from a health care payer perspective, all indicating that telemonitoring reduced costs for patients with an ICD or CRT-D [ 8 , 11 , 13 , 27 , 29 - 32 , 34 , 36 , 37 ]. A total of 9 (82%) of the 11 health care payer perspective studies indicated that hospitalization was the largest driver for cost reduction for patients with an ICD or CRT-D [ 8 , 11 , 13 , 27 , 29 , 30 , 34 , 36 , 37 ]. The hospitalization cost reduced by up to US $912.3 per patient per year [ 34 ]. In addition, scheduled in-office visits were reported as a driver for cost reduction in 5 (45%) of the 11 health care payer perspective studies, as up to 61% of the total cost reduction was due to a decrease in the number of scheduled in-office visits [ 11 , 29 , 30 , 32 , 34 ]. Besides cost drivers that reduced costs, there were also drivers that increased costs. In 3 (27%) of the 11 health care payer perspective studies, unscheduled visits increased the total cost impact of telemonitoring [ 11 , 13 , 29 , 30 , 33 ]. A total of 3 (20%) of the 15 studies indicated that the cost reduction for scheduled in-office visits outweighed the cost increase for unscheduled in-office visits (−US $81.4 vs US $15.6, −US $45.4 vs US $7.8, and −US $44.1 vs US $14/patient/year) [ 11 , 29 , 30 ].
The results of 4 (31%) of the 13 ICD or CRT-D studies that investigated the cost impact of telemonitoring from the patients’ perspective [ 11 , 30 , 32 , 34 ] indicated that patient and caregiver loss of work or activity [ 30 ], scheduled in-office visits [ 11 ], and transport [ 34 ] were the largest drivers for cost reduction. The results of 2 (15%) of the 13 ICD or CRT-D studies that investigated the income impact of telemonitoring from a provider perspective indicated that the loss of reimbursed (scheduled) in-office visits was the most important factor for income loss due to telemonitoring [ 13 , 30 ], reducing income by up to €72.7 (US $77.21) per patient per year [ 30 ].
ICER and Cost-Utility Ratio
Results on ICER and the cost-utility ratio are presented in Table 2 . Of the 15 studies, 3 (20%) calculated the ICER from a health care payer perspective [ 26 - 28 ] and 1 (7%) calculated the cost-utility ratio from a health care payer perspective [ 11 ]. Of the 15 studies, 2 (13%) calculating ICER were conducted with patients with pacemakers [ 26 , 28 ]. Notably, of the 2 studies, 1 (50%) indicated that telemonitoring was cost-effective (ICER: US $270.09/QALY) [ 28 ], and 1 (50%) indicated that telemonitoring was not cost-effective (ICER: US $64,410/QALY) [ 26 ]. For patients with an ICD or CRT-D, of the 2 studies, 1 (50%) indicated that telemonitoring was cost-effective (ICER: US $12,069/QALY) [ 27 ] and 1 (50%) indicated that telemonitoring was dominant [ 11 ].
Critical Appraisal
The critical appraisal of the individual studies is provided in Tables 5 and 6 . Of the 15 studies, 1 (7%) was classified as excellent (score of 100%) [ 13 ], 8 (53%) had a good quality score (100%<score>75%) [ 26 , 28 , 30 , 31 , 33 , 34 , 36 , 37 ], and 6 (40%) had a moderate quality score (75%<score>50%) [ 8 , 11 , 27 , 29 , 32 , 35 ]. A total of 3 (20%) of the 15 studies scored the lowest, with 59% each [ 8 , 29 , 32 ]. More than 50% (>8/15) of the studies scored low for the items cost valuation (item 9) [ 11 , 27 - 32 , 34 , 35 , 37 ], discounting (item 14) [ 8 , 11 , 29 , 30 , 32 , 34 , 35 , 37 ], and no conflict of interest (item 18) [ 8 , 11 , 27 , 29 , 30 , 32 , 34 , 35 ]. All studies scored high on the items study population (item 1), study design (item 4), time horizon (item 10), outcome identification (item 11), outcome measurement (item 12), and ethics (item 19).
a EuroEco: European Health Economic Trial on Home Monitoring in ICD patients.
b TARIFF: Health Economics Evaluation Registry for Remote Follow-Up.
c ECOST: Effectiveness and Cost of ICD Follow-Up Schedule With Telecardiology.
d Sufficient attention was given to this aspect.
e Insufficient attention was given to this aspect.
a Save-HM: Socio-Economic Effects and Cost Saving Potential of Remote Patient Monitoring.
b EVOLVO: Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators.
c MORE-CARE: Monitoring Resynchronization Devices and Cardiac Patients.
d CONNECT: Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision.
e Sufficient attention is given to this aspect.
f Insufficient attention is given to this aspect.
g N/A: not applicable.
Principal Findings and Comparison With Prior Work
The primary aim of this study was to investigate the cost-effectiveness of telemonitoring patients with an ICD or CRT-D and a pacemaker from different perspectives.
From a health care payer perspective, most studies indicated that telemonitoring was a cost-saving and effective alternative to standard care. The most important driver for cost reduction was hospitalizations, both in patients with a pacemaker and those with an ICD or CRT-D. The cost of hospitalizations was reduced by up to US $912.3 per patient per year [ 34 ]. Moreover, the reduction of scheduled in-office visits was the second most important cost-saving factor in most ICD or CRT-D studies, with up to 61% of the total cost reduction. Previous research indicated that up to 55% of the device follow-ups were routine checks with no actionable events or device programming [ 35 , 40 , 41 ]. Several researchers pointed out that most scheduled in-office visits could be replaced by telemonitoring without affecting the quality of care [ 7 , 34 ] and with potentially diagnosing >99.5% of arrhythmia and device problems [ 41 ]. Although scheduled in-office visits decreased, our results show that unscheduled in-office visits increased because of telemonitoring patients with an ICD or CRT-D, probably because of the possible faster detection of arrhythmia and device malfunction by telemonitoring [ 8 ]. However, in all studies analyzing both scheduled and unscheduled in-office visits, the cost reduction for scheduled in-office visits outweighed the cost increase for unscheduled in-office visits [ 11 , 29 , 30 ].
From a patient perspective, our results indicated that the reduction of professional activity, transport time, and costs due to scheduled in-office visits are the most important factors for cost reduction.
The provider perspective was investigated less frequently in the included studies, although it is very relevant. Owing to the reduction of scheduled in-office visits, providers will lose income with telemonitoring if no reimbursement exists for telemonitoring but only for in-office visits. As a result, providers will be stimulated to maintain the classic follow-up instead of telemonitoring. Of the 15 studies, 1 (7%) observed that the total cost for insurance payers does not increase in countries where telemonitoring is reimbursed [ 13 ]. As telemonitoring decreases the overall costs from a health care payer perspective, there is room for proper compensation for providers to transition from in-office care to remote care. Hence, correct compensation (which is possible while still saving on the overall health care cost) will stimulate providers to switch to telemonitoring as the desired care path for patients with a CIED.
All studies reported the effectiveness of telemonitoring. Of the 15 studies, 9 (60%) indicated a QALY or QOL difference. Furthermore, 89% (8/9) of these studies indicated an increase in QALYs or QOL for telemonitoring patients with pacemakers or ICD or CRT-D, ranging from −1 to 0.64. Some studies (3/9, 33%) indicated this QALY or QOL increase was the result of the reduced routine in-office visits [ 7 , 34 ]. However, the QALY or QOL increase was only statistically significant (and positive) in 1 (11%) of the 9 studies [ 11 ]. Nevertheless, patient questionnaires have demonstrated a high acceptance of telemonitoring among patients with pacemakers and those with ICDs [ 39 ]. Moreover, telemonitoring is reported to lead to an increased sense of security [ 39 ]. Furthermore, the results indicated that telemonitoring leads to fewer inappropriate shocks, an important determinant of QALY, in patients with an ICD or CRT-D [ 31 ].
The cost-effectiveness analyses may be sensitive to the heterogeneity among the organization of telemonitoring in different hospitals. This may include different devices, the number of transmissions, the configuration of alerts, and hospital visit scheduling [ 26 ]. It seems reasonable to expect that the efficiency of telemonitoring not only depends on the technology but also on the organization of the service. If hospitals see telemonitoring as an additional service, on top of standard care, less cost-savings may be seen than if hospitals see telemonitoring as a substitute for standard care. A radical organizational change could lead to larger cost-savings, as suggested by an observational study by Facchin et al [ 42 ]. Moreover, such radical change may include a strategy involving other physicians, such as general practitioners, and referring cardiologists, that is, an integrated health care delivery [ 37 ].
Furthermore, the comparison between studies is challenged by differences in study design. The Poniente study by Bautista-Mesa et al [ 28 ] followed up patients with pacemakers for 12 months and indicated a QALY increase of 0.09 for telemonitoring. However, after 5 years of follow-up, the results indicated a QALY decrease of 0.20 for telemonitoring. Bautista-Mesa et al [ 28 ] indicated that some of the telemonitoring benefits (eg, reduction of in-office visits) may not be appreciated in the long term. Therefore, the evolution of utilities may be different depending on the follow-up time. In addition, the results indicated that hospitalizations reduced costs in the study by Dario et al [ 37 ] but increased costs in the study by Lopez-Villegas et al [ 26 ]. This discrepancy might be explained because significantly fewer patients were included in the study by Lopez-Villegas et al (50 vs 2101 patients). None of the 25 patients in the conventional follow-up group were hospitalized, whereas 12% (3/25) of the patients were hospitalized in the remotely monitored group (all for pacemaker problems) [ 26 ]. Furthermore, the included studies relied disproportionally on male participants, except for the Nordland study [ 26 ]. This may be explained by the significant sex disparity in ICD implantation rates, pointed out by Ingelaere et al [ 43 ]. Ingelaere et al [ 43 ] could not completely explain these differences by prevalence differences of cardiomyopathies and imply a possible undertreatment of women. Another study [ 44 ] observed an undertreatment of women with coronary heart disease, as they are less likely to undergo coronary angiography. Therefore, men may undergo more expensive treatments than women. This can explain why the included cost-effectiveness studies may present an overly positive result. In addition, time differences may impact the quality and cost-effectiveness of telemonitoring, as telemonitoring may evolve over time. However, our results did not provide meaningful insights in this respect.
The cost-effectiveness analyses may be sensitive to the heterogeneity among health care systems. From a provider perspective, our results indicated that telemonitoring generates lesser profit than standard care in the absence of reimbursement. Therefore, the lack of reimbursement is generally perceived as a major implementation barrier to telemonitoring, affecting 80% of the centers [ 45 ]. Consequently, providers tend to continue with standard care instead of telemonitoring. However, from a health care payer perspective, our results indicated that telemonitoring was still cost-saving even with reimbursement [ 13 , 34 ]. To stimulate providers to use telemonitoring, provider compensation should be provided based on overall health care cost-savings, making telemonitoring possible if it is preferred as the way to deliver CIED follow-up care.
Limitations
Because of the large discrepancies between health care systems’ organization, costs, access, delivery, quality, and reimbursement of cardiac care, any generalization may be perceived as inaccurate [ 37 , 46 ]. For instance, the included studies were mainly performed in Western countries. The results may not be generalizable to non-Western countries. Therefore, the cost-effectiveness results are contingent on the context in which they were analyzed [ 46 ]. Another limitation of this research is that 40% (6/15) of the included studies are not randomized controlled trials. These studies may have unobserved confounding factors that cannot be controlled for. Finally, cost analyses were excluded in this study because of our research objective. However, future cost analyses could draw a lot of information from analyzing these excluded studies.
Conclusions
Telemonitoring patients with CIED may be a cost-effective alternative to standard follow-up. Moreover, telemonitoring may lead to a cost reduction from a health care and patient perspective, mainly by the reduction of hospitalizations and scheduled in-office visits. Owing to the reduction in scheduled in-office visits, providers’ income tends to decrease when implementing telemonitoring without proper reimbursement. Introducing appropriate reimbursement could make telemonitoring sustainable for providers, while still being cost-effective from a health care payer perspective.
Acknowledgments
The authors would like to thank Dr Ingrid Kremer of Maastricht University for her help in the manuscript review. This work was supported by the Fund for Scientific Research Flanders (Fonds Wetenschappelijk Onderzoek Vlaanderen, grant 1SC9322N, 2021). RW is supported as a postdoctoral clinical researcher by the Fund for Scientific Research Flanders (Fonds Wetenschappelijk Onderzoek Vlaanderen).
Data Availability
All data generated or analyzed during this study are included in this published article and its multimedia appendices.
Authors' Contributions
SR and LA were responsible for the conceptualization of the manuscript. SR, LA, RW, and HH acquired the financial support necessary for this paper and developed the methodology. SR analyzed and investigated the data. AP, LA, RW, and HH validated the results. SR was responsible for the first and final drafts. LA, RW, and HH were involved in editing the drafts. All authors approved the final manuscript.
Conflicts of Interest
RW reports research funding from Abbott, Biotronik, Boston Scientific, and Medtronic and speakers and consultancy fees from Medtronic, Boston Scientific, Biotronik, and Abbott. None of these payments were personal; all were handled through the University of Leuven. HH received personal lecture and consultancy fees from Abbott, Biotronik, Daiichi-Sankyo, Pfizer-BMS, Medscape, and Springer Healthcare Limited. He received unconditional research grants through the University of Antwerp and the University of Hasselt from Abbott, Bayer, Biotronik, Biosense Webster, Boston Scientific, Boehringer Ingelheim, Daicchi-Sankyo, Fibricheck or Qompium, Medtronic, and Pfizer-BMS, all outside the scope of this work. All other authors declare no other conflicts of interest.
PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist.
Search strategy.
Interpretation Consensus Health Economic Criteria list.
- Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace. Apr 01, 2020;22(4):515-549. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. Jun 2008;5(6):907-925. [ CrossRef ] [ Medline ]
- de la Torre Díez I, Garcia-Zapirain B, Méndez-Zorrilla A, López-Coronado M. Monitoring and follow-up of chronic heart failure: a literature review of eHealth applications and systems. J Med Syst. Jul 2016;40(7):179. [ CrossRef ] [ Medline ]
- Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida J, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. Feb 2013;34(8):605-614. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace. Jan 2009;11(1):54-61. [ FREE Full text ] [ CrossRef ] [ Medline ]
- García-Fernández FJ, Osca Asensi J, Romero R, Fernández Lozano I, Larrazabal JM, Martínez Ferrer J, et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE). Eur Heart J. Jun 14, 2019;40(23):1837-1846. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. Jul 27, 2010;122(4):325-332. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. Mar 8, 2011;57(10):1181-1189. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Catalan-Matamoros D, Lopez-Villegas A, Tore-Lappegard K, Lopez-Liria R. Patients' experiences of remote communication after pacemaker implant: The NORDLAND study. PLoS One. 2019;14(6):e0218521. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Masella C, Zanaboni P, Di Stasi F, Gilardi S, Ponzi P, Valsecchi S. Assessment of a remote monitoring system for implantable cardioverter defibrillators. J Telemed Telecare. 2008;14(6):290-294. [ CrossRef ] [ Medline ]
- Zanaboni P, Landolina M, Marzegalli M, Lunati M, Perego GB, Guenzati G, et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res. 2013;15(5):e106. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Annemans L. Health Economics for Non-economists: An Introduction to the Concepts, Methods and Pitfalls of Health Economic Evaluations. Kalmthout, Belgium. Pelckmans Pro; 2018.
- Heidbuchel H, Hindricks G, Broadhurst P, Van Erven L, Fernandez-Lozano I, Rivero-Ayerza M, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. Jan 14, 2015;36(3):158-169. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Klersy C, Boriani G, De Silvestri A, Mairesse GH, Braunschweig F, Scotti V, et al. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail. Feb 2016;18(2):195-204. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Mar 29, 2021;372:n71. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. Oct 02, 2018;169(7):467-473. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. Dec 2016;16(6):723-732. [ CrossRef ] [ Medline ]
- Search strategies: the centre for reviews and dissemination. University of York. 2018. URL: http://www.crd.york.ac.uk/crdweb/searchstrategies.asp [accessed 2024-07-01]
- Auener SL, Remers TE, van Dulmen SA, Westert GP, Kool RB, Jeurissen PP. The effect of noninvasive telemonitoring for chronic heart failure on health care utilization: systematic review. J Med Internet Res. Sep 29, 2021;23(9):e26744. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lopez-Villegas A, Leal-Costa C, Perez-Heredia M, Villegas-Tripiana I, Catalán-Matamoros D. Knowledge update on the economic evaluation of pacemaker telemonitoring systems. Int J Environ Res Public Health. Nov 18, 2021;18(22):12120. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vehmeijer JT, Brouwer TF, Limpens J, Knops RE, Bouma BJ, Mulder BJ, et al. Implantable cardioverter-defibrillators in adults with congenital heart disease: a systematic review and meta-analysis. Eur Heart J. May 07, 2016;37(18):1439-1448. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Scott AM, Forbes C, Clark J, Carter M, Glasziou P, Munn Z. Systematic review automation tools improve efficiency but lack of knowledge impedes their adoption: a survey. J Clin Epidemiol. Oct 2021;138:80-94. [ CrossRef ] [ Medline ]
- Evers S, Goossens M, de VH, van TM, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240-245. [ Medline ]
- Jiang X, Ming W, You JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res. Jun 17, 2019;21(6):e13166. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Consumptieprijsindex. Statbel. URL: https://statbel.fgov.be/nl/themas/consumptieprijsindex/consumptieprijsindex#figures [accessed 2022-07-08]
- Lopez-Villegas A, Catalan-Matamoros D, Peiro S, Lappegard KT, Lopez-Liria R. Cost-utility analysis of telemonitoring versus conventional hospital-based follow-up of patients with pacemakers. The NORDLAND randomized clinical trial. PLoS One. 2020;15(1):e0226188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hummel JP, Leipold RJ, Amorosi SL, Bao H, Deger KA, Jones PW, et al. Outcomes and costs of remote patient monitoring among patients with implanted cardiac defibrillators: an economic model based on the PREDICT RM database. J Cardiovasc Electrophysiol. Jul 2019;30(7):1066-1077. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bautista-Mesa RJ, Lopez-Villegas A, Peiro S, Catalan-Matamoros D, Robles-Musso E, Lopez-Liria R, et al. Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: the PONIENTE study. BMC Geriatr. Nov 16, 2020;20(1):474. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. Mar 2017;19(3):416-425. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ricci RP, Vicentini A, D'Onofrio A, Sagone A, Rovaris G, Padeletti L, et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: results of the health economics evaluation registry for remote follow-up (TARIFF) study. Heart Rhythm. Jan 2017;14(1):50-57. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Burri H, Sticherling C, Wright D, Makino K, Smala A, Tilden D. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace. Nov 2013;15(11):1601-1608. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace. Oct 2008;10(10):1145-1151. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. May 2010;21(5):545-550. [ CrossRef ] [ Medline ]
- Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida J, et al. Costs of remote monitoring vs. ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace. Aug 2014;16(8):1181-1188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Perl S, Stiegler P, Rotman B, Prenner G, Lercher P, Anelli-Monti M, et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int J Cardiol. Nov 30, 2013;169(6):402-407. [ CrossRef ] [ Medline ]
- Chew DS, Zarrabi M, You I, Morton J, Low A, Reyes L, et al. Clinical and economic outcomes associated with remote monitoring for cardiac implantable electronic devices: a population-based analysis. Can J Cardiol. Jun 2022;38(6):736-744. [ CrossRef ] [ Medline ]
- Dario C, Delise P, Gubian L, Saccavini C, Brandolino G, Mancin S. Large controlled observational study on remote monitoring of pacemakers and implantable cardiac defibrillators: a clinical, economic, and organizational evaluation. Interact J Med Res. Jan 13, 2016;5(1):e4. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Crossley G, Boyle A, Vitense H, Sherfesee L, Mead RH. Trial design of the clinical evaluation of remote notification to reduce time to clinical decision: the clinical evaluation Of remote NotificatioN to rEduCe Time to clinical decision (CONNECT) study. Am Heart J. Nov 2008;156(5):840-846. [ CrossRef ] [ Medline ]
- Ricci RP, Morichelli L, Quarta L, Sassi A, Porfili A, Laudadio MT, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace. May 2010;12(5):674-679. [ CrossRef ] [ Medline ]
- Boriani G, Auricchio A, Klersy C, Kirchhof P, Brugada J, Morgan J, et al. Healthcare personnel resource burden related to in-clinic follow-up of cardiovascular implantable electronic devices: a European Heart Rhythm Association and Eucomed joint survey. Europace. Aug 2011;13(8):1166-1173. [ CrossRef ] [ Medline ]
- Heidbüchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, et al. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. Mar 2008;10(3):351-357. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Facchin D, Baccillieri MS, Gasparini G, Zoppo F, Allocca G, Brieda M, et al. Findings of an observational investigation of pure remote follow-up of pacemaker patients: is the in-clinic device check still needed? Int J Cardiol. Oct 01, 2016;220:781-786. [ CrossRef ] [ Medline ]
- Ingelaere S, Hoffmann R, Guler I, Vijgen J, Mairesse GH, Blankoff I, et al. Inequality between women and men in ICD implantation. Int J Cardiol Heart Vasc. Aug 2022;41:101075. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, et al. Sex differences in the management of coronary artery disease. Survival and Ventricular Enlargement Investigators. N Engl J Med. Jul 25, 1991;325(4):226-230. [ CrossRef ] [ Medline ]
- Mairesse GH, Braunschweig F, Klersy K, Cowie MR, Leyva F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: a survey from the health economics committee of the European Heart Rhythm Association. Europace. May 2015;17(5):814-818. [ CrossRef ] [ Medline ]
- Lucà F, Cipolletta L, Di Fusco SA, Iorio A, Pozzi A, Rao CM, et al. Remote monitoring: doomed to let down or an attractive promise? IJC Heart Vasculature. Sep 2019;24:100380. [ CrossRef ]
Abbreviations
Edited by T Leung; submitted 28.03.23; peer-reviewed by P Jeurissen, B Dechert; comments to author 07.09.23; revised version received 13.09.23; accepted 13.02.24; published 19.04.24.
©Sarah Raes, Andrea Prezzi, Rik Willems, Hein Heidbuchel, Lieven Annemans. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 19.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
Help | Advanced Search
Computer Science > Computation and Language
Title: leave no context behind: efficient infinite context transformers with infini-attention.
Abstract: This work introduces an efficient method to scale Transformer-based Large Language Models (LLMs) to infinitely long inputs with bounded memory and computation. A key component in our proposed approach is a new attention technique dubbed Infini-attention. The Infini-attention incorporates a compressive memory into the vanilla attention mechanism and builds in both masked local attention and long-term linear attention mechanisms in a single Transformer block. We demonstrate the effectiveness of our approach on long-context language modeling benchmarks, 1M sequence length passkey context block retrieval and 500K length book summarization tasks with 1B and 8B LLMs. Our approach introduces minimal bounded memory parameters and enables fast streaming inference for LLMs.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breathe (Sheff)
- v.18(4); 2022 Dec

How to peer review: practical advice for early career researchers
Alexander g. mathioudakis.
1 Division of Infection, Immunity and Respiratory Medicine, School of Biological Sciences, The University of Manchester, Manchester, UK
2 North West Lung Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK
Darcy Wagner
3 Lung Bioengineering and Regeneration, Department of Experimental Medical Sciences, Faculty of Medicine, Lund University, Lund, Sweden
4 Lund Stem Cell Center, Faculty of Medicine, Lund University, Lund, Sweden
5 Wallenberg Center for Molecular Medicine, Faculty of Medicine, Lund University, Lund, Sweden
Orianne Dumas
6 Université Paris-Saclay, UVSQ, Univ. Paris-Sud, Inserm, Équipe d'Épidémiologie respiratoire intégrative, CESP, Villejuif, France
Short abstract
Practical tips and guidance on peer review are provided by three scientists in the respiratory field, to help early career researchers who may be invited to review papers for respiratory journals https://bit.ly/3EuWpoH
Introduction
Peer review is an important part of scientists’ activities and the major system for evaluation of scientific reports [ 1 , 2 ]. Besides making an important contribution to science in terms of ensuring high standards in research reporting, conducting peer review has many values at an individual level: it can be seen as a way to keep updated with recent literature, including new findings and novel approaches; it is also an opportunity to read manuscripts in a particularly structured way and reflect on the hypotheses as well as how the data were obtained and how this might impact the conclusions of the paper; and it may even contribute to generating novel questions and ideas in your own research. Finally, evaluating papers is one of the best ways to improve your own paper writing.
There is no single way to conduct peer review, and the best practices for peer review are a topic of active research [ 1 ]. Guides to peer review, as well as editorials summarising thoughts on the peer review process, have been published in various biomedical fields, including in the respiratory field [ 2 – 4 ]. General views on peer review have been previously published in Breathe , in 2014, from both the editor's and reviewer's perspective [ 5 ]. However, peer review has changed rapidly over the past few years due to several factors: an increasing number of interdisciplinary papers covering a wider range of disparate scientific fields within one paper, the emergence of preprint repositories, and more rapid review processes which emerged during the coronavirus disease 2019 (COVID-19) pandemic in an effort to disseminate research findings to the broader scientific community with greater speed [ 6 ]. The current article includes practical tips and guidance from three scientists in the respiratory field, with different backgrounds (clinical research, epidemiology, and basic/translational research). It is particularly intended to help early career researchers who may be invited to review papers for European Respiratory Society (ERS) journals ( European Respiratory Journal ( ERJ ), European Respiratory Review ( ERR ), ERJ Open Research and Breathe ), and other respiratory journals. Although we focus on the review of original research papers, the advice given here is applicable to different contexts ( e.g. review articles and abstracts).
To write this article, we first established a list of questions that early career researchers may have when invited to review a manuscript, especially if it is their first time reviewing an article. Each of the co-authors provided his/her own answers to these questions. Because there was a large overlap in the responses, we combined and summarised them, but kept specific tips that may apply to different fields where applicable.
I'm invited to review a manuscript: should I accept?
If you are invited to review a manuscript, it is probably because you have recently published papers in a closely related field, and your records indicate that you have a certain expertise. Early career researchers might be invited to review a manuscript even if they only have a few publications as a first author. From the editors’ point of view, the best reviewers may be those who recently conducted a very similar study on a similar research question, and are aware of any potential limitations, but can also value novel/additional studies in the field.
However, such reviewers are not always available, and editors may extend their search to authors of closely related publications, even though they do not address the exact same research question. In addition, the editors may not know your exact competencies and field of expertise. For these reasons, it is important that you carefully read the abstract, which is generally attached to the “invitation to review” email, to check that you are confident enough in your capability to assess the value ( e.g. novelty, interest of the research question, added value to the field) and evaluate the adequacy of the methods and the potential limitations. The journal editors will expect that you are familiar with the relevant literature and research methods. If you only feel partially confident, you can consult the editor about the expertise that she/he is looking for, and clarify yours. This is particularly important in interdisciplinary studies or studies which use diverse methodological and analytical approaches. The editor may be looking for a reviewer with particular expertise concerning some aspect of the study and not necessarily a reviewer who can cover all areas of the paper.
Before accepting to peer review a paper, you should also ensure that you will have the time to complete it on time [ 7 ]. Delays are unpleasant for the editors and the authors, who want their work published. For the same reason, if you choose not to accept a peer review assignment, you should still respond to the journal as soon as possible, to avoid any delays.
Finally, you should evaluate your potential conflicts of interest in evaluating the manuscript. For example, you should refuse to review a manuscript if you have personal relationships or close collaborations with some of the authors (even though you have not yet been co-authors on published papers) or with the research group; if you receive fees or shares from the pharmaceutical industry with relevant vested interests; or if you are working on a competing study or group. For various reasons, you may also feel that you may not be able to provide an objective assessment of the research work (both positive and negative bias may exist). Therefore, to maintain scientific integrity in the peer review process, you should decline the invite.
Questions to consider when invited to peer review are summarised in figure 1 .

Questions to consider when invited to peer review.
What is the aim of the peer review process?
The simplest answer to this question is that peer review aims to ensure that only papers of a high methodological rigour, which support the conclusions stated by the authors, are accepted for publication in a peer-reviewed journal. Peer review also helps the authors to improve these manuscripts and better address their research questions. You should remember that the peer review process is a crucial component of the scientific discussion, where colleagues are invited to offer their expertise and their honest views. It can help authors to reflect on how strong their conclusions are given the data presented in their manuscript, as well as point out alternative conclusions which could be drawn from their data. As a result, peer review comments should not aim to diminish a manuscript (or its authors) but to improve it. A good peer review report could also serve as great educational material and a source of inspiration for future/follow-up studies. Finally, under no circumstances should the peer review be condemning or rough.
It's the first time I've been asked to peer review: where do I start?
An overview of the main steps of the peer review workflow is presented in figure 2 . Once you have accepted the invitation to review, you will receive a link to access all submitted files, and a deadline to send your review. First, you should plan the review work in your calendar. It will take several hours at a minimum, even if you have core expertise in the area of the paper, and it is best to avoid fragmented work for this task. Ask yourself when you will be able to devote significant time to your review with minimal interruptions. When starting the review, make sure to download all the files, not limited to the main manuscript, but also the figures and tables (sometimes provided separately) and if applicable, the supplementary material. It is always a good practice to read the journal's guidelines to authors and/or reviewers before reading the manuscript to have a general idea about the aims and scope of the journal as well as specific formatting requirements.

Main steps of the peer review workflow.
Then, although each reviewer may have their own way to proceed, most experienced reviewers advise reading the full manuscript a first time to understand the content and make a first general impression. Some reviewers initially skim through the paper to look for any major flaw that may lead to a quick rejection ( e.g. inappropriate methodology or significant, unaddressed bias). If you identify such a flaw, you can describe it in your comments and reject the manuscript without reading in more detail. A “major flaw” depends on the state of knowledge around a specific topic area as well as practical limitations when it comes to available methods to address basic research questions. For example, the first studies assessing risk factors for adverse COVID-19 outcomes were not adjusted for potential confounders and at the time this was acceptable, provided that it was highlighted as a limitation in the discussion [ 6 ]. However, a few months later it became very clear that factors like age, gender or specific comorbidities are strongly correlated with the adverse outcomes of COVID-19 and lack of adjustments in newer prognostic studies would be considered a major flaw leading to a quick rejection.
The first read through the manuscript can already be an active reading, highlighting some parts which seem important ( i.e. which will later help in evaluating the strengths and limitations of the manuscript), and taking quick notes. Although it remains a first impression, this first reading is already a time to ask yourself the following questions:
- Is it novel?
- Is the research question important and well introduced?
- Are the main findings clearly described?
- Do the methods seem adequate?
- Do you agree with the interpretation of the results?
On many occasions, the reviewer knows at the end of this first read through what his/her recommendation to the editors will probably be (acceptance, major/minor revision or rejection).
After this first overall impression of the manuscript, you should go back to each section for a very careful reading, taking detailed notes referring to specific sentences or paragraphs. This more thorough reading will be the basis for writing your reviewer report.
What should I check in each section of the manuscript?
We list below the main points of attention that are common to most research manuscripts. Research reporting guidelines, such as the CONSORT guidelines [ 8 ] for randomised controlled trials or the STROBE guidelines [ 9 ] for observational studies, provide useful checklists of items that need to be addressed in research papers. A large group of editors of respiratory journals have also provided specific guidance regarding the reporting of observational causal inference studies [ 10 ]. In basic and translational science, other guidelines, such as ARRIVE 2.0 [ 11 ] for animal work or FAIR Data Principles to allow for reuse of scholarly data [ 12 ], have become standard for most journals. Such material can be useful to reviewers who may refer to this as a list of good practices in their field. However, it is important to note that no exhaustive review checklist can be provided. The reviewer, as an expert in his/her field is expected to be able to evaluate the research beyond pre-established checklists and considering the various specificities of the research question.
The title should reflect the content of the article and its main findings. Generally, it should be short but informative. Some journals have specific requirements for the title in terms of length and style ( e.g. an “informative” rather than a “descriptive” title). It is important to read the title carefully before, but also after, reading the full manuscript, to check the consistency between what is stated, in terms of content and conclusion, and what was actually done and well supported by the findings of the paper. In particular, you should check that the title does not over-emphasise selected findings ( e.g. only positive fndings) or use overly strong statements as compared with findings reported in the abstract/manuscript.
The abstract should contain brief information about the rationale or hypothesis of the study, the main research methods used to address this, and the major findings. Ensure the key components of the methods and results are presented. For example, in epidemiological research, the abstract should include the main information about the study design and methods ( e.g. cross-sectional versus longitudinal study, number of subjects included in the analyses), and provide the main results with important estimates ( e.g. relative risks and confidence intervals rather than only statements such as “was significantly associated with”). Importantly, the abstract should reflect the main findings and conclusion from the manuscript. Often authors cannot summarise all their results in the abstract due to the word limit; however, it is important to ensure that the summary is balanced and does not only include the positive findings, but also pertinent negative findings. Similarly, you should carefully check that findings are not overinterpreted in the abstract compared with the manuscript, as the short format sometimes pushes authors to over-emphasise some findings. A recent systematic review found that the abstracts of systematic reviews are often inconsistent with their actual findings [ 13 ]. This suggests poor writing as well as a poor peer review process.
The introduction should include a balanced account of the available data/evidence around the authors’ research question. While the introduction should not be too long (an exhaustive description of all related data is not required), it should include all pertinent data and previous major research findings that will allow the reader to understand the current state of the research field, the remaining knowledge gaps, and the rationale of the research question. Moreover, the research question should be very clearly described, usually in the last paragraph of the introduction. You should also pay particular attention to the references provided in the introduction. Are they up to date? Do they appropriately reflect the field? Any omissions should be highlighted.
The methods may be the most scrutinised section in peer review. A first crucial question is whether the described methods can adequately address the question posed. It is also important to check that the methods are described with enough clarity. One way to do this is to ask yourself “If I had to replicate this research/experiment, do I have all the information I need to do so?”. Another good question is “If I wanted to address this research question with the same means ( e.g. the same data/study population/experimental material), how would I proceed?”. This generally implies a series of methodological choices at different steps of the study. Some of the authors’ choices may differ from yours. Do you find justification for their choices? How did they address uncertainties ( e.g. sensitivity analyses)? Are there any pertinent experiments or analyses missing?
What needs to be checked specifically differs in each research field. In epidemiological and clinical research studies, ensure adequate description is provided for the sample size selection, study population (including the main inclusion and exclusion criteria), as well as the outcomes of interest. Moreover, studies evaluating treatments should describe the interventions, comparators and co-interventions; prognostic studies should list the prognostic factors; and diagnostic studies the diagnostic tests and gold standards they are compared to. Especially, but not only, observational studies need to describe how they accounted for potential confounding factors. In epidemiology, the study design, exposure assessment and statistical methods should be additional points of attention. Any potential source of bias should be considered and appropriately addressed and/or discussed. Clinical trials and systematic reviews should be based on a prospectively registered protocol. Nowadays, high-quality observational studies may also be registered prospectively. This should be described in the methods. Also, any differences from the prospectively registered protocol should be described and justified. It is advisable to have a look at the study protocol while peer reviewing.
For basic and translational studies, it is crucial that the methods are described such that the research can be replicated. Therefore, information such as the source of all chemicals and biological reagents should be listed, including lot numbers or batch numbers when appropriate. If tools have been designed in the laboratory or given as a gift by another laboratory, such as antibodies, plasmids or primer sequences, then details or references to original papers describing these reagents should be listed. With the rapidly increasing use of bioinformatic analyses in biomedical research, papers which analyse or re-analyse large datasets should provide information regarding access to the raw data, all the software used, databases (including version numbers if applicable), as well as access to all the code used to analyse data or generate plots. External resources such as deposition of data in data repositories, such as the European life-sciences Infrastructure for biological Information (ELIXIR), as well as custom code should be provided during peer review and/or posted on resources such as GitHub.
Results (including figures/tables)
In the results section, the authors should describe in a balanced fashion all their results (positive and negative). Importantly, the presented results should correspond to the paper's main objectives, and to what is announced in the methods. While parsimony may be welcome ( e.g. authors should avoid presenting “side” results not related to their research question), any selective presentation or emphasis of the most significant results should be pointed out. For example, it is the responsibility of the peer reviewer to ensure that all outcome measures that are described in the study protocol and methods, or that are required to address the research question, are included in the results.
In terms of content and style, it is important to check that the authors report the absolute or relative effect estimates, along with their confidence intervals, and not only the p-values, which are less informative. The results should be described in an objective manner, without trying to predispose the reader ( e.g. “treatment X decreased the frequency of exacerbations by 2%” and not “treatment X decreased the frequency of exacerbations by ONLY 2%”). As a reviewer, you may also comment on the choice of tables and figures: are they clear and adequate? Are the figure legends and all labelling on the figures adequate for interpreting the findings presented? For basic and translational science: could the data be presented in a more transparent way to see the effects of batches or patient/animal/primary cell culture differences? Feel free to suggest alternative ways to present graphs or figures which will help to display the data more transparently or in a way that makes it easier for the reader to understand the main conclusions which can be drawn.
In all research papers, the discussion should contain the following components: a short description of the main study findings and their interpretation; a comparison of the study findings with other relevant studies, along with a potential explanation of the important similarities and differences; a description of the main strengths and limitations of the study; clinical and research implications of the study findings; followed by a conclusion. Besides checking that all these components are present, the main questions you should ask yourself to evaluate the paper at this stage are: “Is the conclusion supported by the findings?”, “Is the interpretation of the results balanced?”, and “Are the study limitations and alternative potential interpretations clearly stated?”. Comments on specific points in the discussion, e.g. if you disagree or do not fully agree with some statements made by the authors, can also be noted in your review.
All statements that the authors make should be justified either by their findings or by appropriate references. The references should include some of the most relevant, high-quality studies in the field. Generally, the most recent literature reviews or meta-analyses on a given topic, and papers published afterwards, should be cited. If you are aware of potential controversies in a field, you should check that papers reflecting different points of views are cited. You may also look at authors’ self-citations, which should appear justified and not be excessive. Moreover, you should not ask the authors to add references to your studies, unless they are directly linked to their work. It is not appropriate, and it does not look nice to the editor, or the authors (who may be able to identify you or your supervisor if you inappropriately ask them to cite your articles).
Funding/conflicts of interest
This section should not be neglected during peer review. If there are potential conflicts of interest, the authors should describe how these were managed during the study process. For example, if a study was funded by the pharmaceutical industry, the authors should explain what the role of the funder in the design, conduct and reporting of the study has been and how the impartiality of the study was maintained. If you think that that authors may have competing interests (explicitly declared or not), you should inform the editors.
Miscellaneous sections, e.g. “key points”
Several journals request additional summary sections ( e.g. key points, graphical abstracts or a “research in context” summary). They should also be reviewed carefully, as this may be the only part that some readers will read. The peer reviewer should ensure these are a balanced representation of the study and its findings. As in the title and abstract, authors may tend to present only the positive findings, or describe conclusions that do not directly result from their study.
How should I organise my report?
Once you are done with reading and taking notes on different parts of the manuscript, you should organise and format your report. The report is intended first for the editors to make a decision on the manuscript, and then for the authors either to appropriately revise their manuscript or if rejected, to understand the reasons for this decision and how the manuscript can be improved if submitted elsewhere. Keep in mind that the editor's final decision may be different from your recommendation, as she/he generally takes into account comments from several reviewers. Therefore, it is preferable to provide rather detailed comments regardless of the recommendation. Depending on the journal, some journals will have a section for comments to the editor and comments to the authors. You may provide more direct feedback about your thoughts to the editor in the “comments to the editor” box (see later). Comments to the authors should be written constructively, even if there are aspects which you perceived to be major flaws in the original submission.
A good report starts with a general assessment of the quality and interest of the manuscript, with 2–3 sentences describing the research question and main findings, focusing on the main strengths and limitations, for the benefit of the editor (see the example in Box 1 ). Comments on novelty and interest of the study can also be included.
BOX 1. Example of 2–3 sentences summary in the reviewer report
“This observational study explored predictors of the future risk of COPD exacerbations in two population cohorts totalling over 1 million eligible participants. All analyses were adjusted for multiple demographic parameters, important comorbidities and respiratory treatments. However, exacerbations were defined based on the prescription of systemic corticosteroids only. The investigators could not differentiate the use of steroids for an acute exacerbation, for another disease, or as a rescue pack that was perhaps never used. Moreover, hospitalisation data were lacking and it is not clear whether severe exacerbations were captured in this study.”
Then, most reports continue with a list of “major comments” and then “minor comments”. The former refers to major issues in the paper that require additional work to be resolved ( e.g. additional experiments, or additional dimensions to be described). The latter refers to small changes to improve the flow of the manuscript or add clarifications. The ordering of the comments depends on the reviewers and manuscripts. Reviewers may start with general points which apply to the manuscript as a whole and reflect general concerns that need to be addressed, and then provide detailed comments referring to specific parts of the manuscript. A common approach is to group the comments depending on the section of the manuscript they refer to ( e.g. abstract, introduction, methods). When making very specific comments ( e.g. when referring to a paragraph, sentence or word), it is useful to provide page and line numbering and to try to be as explicit as possible. Further, it may be helpful or appropriate to provide citations in your report if you would like to refute or question statements made by the authors in their original submission.
How long should my report be?
There is no requirement regarding the length of the reviewer report. Sometimes, you only need a few lines to state why you believe the manuscript has critical flaws that should lead to a rejection. Longer reports are usually required for papers that have some value, but could be improved significantly upon major revision.
In such cases, you should take the space that you need to make all your comments and to explain them clearly. This will help the authors to address them, and shorten the review process by avoiding going back and forth between the authors and reviewers ( i.e. having a second and sometimes third round of review). However, excessively long reports may also be counterproductive. The reviewer's role is not to redo the study or rewrite the manuscript. If very extensive changes are needed to make the manuscript suitable for publication, you should inform the editor of this assessment and summarise the major concerns in the comments to the authors.
What if I don't feel skilled to evaluate specific parts of the manuscript?
First, you should only accept to peer review manuscripts that are relevant to your expertise. Therefore, you should feel confident to evaluate most parts of the manuscripts that you peer review. If after accepting to review the manuscript, you realise that you are not skilled to have an expert opinion on some parts ( e.g. a specific statistical method), you can still write a report on the parts of the manuscript you are comfortable with, but you should inform the editor about the parts that you did not comment on because of lack of expertise ( e.g. in the “comments to the editor” section). However, depending on the journal and type of paper, your input as a “non-expert” can also be relevant. Highly interdisciplinary work will be read by diverse audiences and, therefore, it can be helpful to point out field-specific terminology to authors in the minor comments or some suggestions of areas to improve. It is important that you do not list these as major comments but specify that these may help non-specialists understand the paper, the methods used, as well as the conclusions which can be drawn.
How will the editor make his/her decision? What should I recommend?
Editors usually assess the comments of at least two, and sometimes more, peer reviewers. The full reports from the reviewers, and not only the actual recommendations ( e.g. “revision” or “rejection”) are important in the decision process. Editors usually review the manuscript as well, in order to make an informed decision. In cases with contradictory comments, they may invite additional peer reviewers.
Editors will make a decision considering: 1) the methodological rigour and all strengths and limitations of the manuscript, as described previously, 2) the pertinence and novelty of the findings, and 3) the relevance of the study to the scope and readership of the journal. If major issues are raised by the reviewer, the editor will also evaluate the chances that the authors can adequately revise the manuscript ( e.g. based on the material/data that appear available and the feasibility of the requested revisions).
Your recommendation should be supported by your comments. After carefully appraising a manuscript and considering the strengths and limitations in the methods, content and message, you should consider whether it is appropriate for the journal. Keep in mind that negative results may also be pertinent (but ideally a study needs to have the power to demonstrate lack of effect). Examples of types of studies which are generally considered suitable for different kinds of journals are presented in Box 2 .
BOX 2. Which kind of study would fit in which journal? Some examples
- If a study has methodological errors that significantly limit the confidence of the findings and cannot be addressed, then it should be rejected by any journal.
- A very preliminary report of pilot data around an innovative idea, a study confirming well-known and validated evidence, or an innovative study that is based on a shaky methodology or limited power are unlikely to fit the scope of a high-impact specialty journal, such as the European Respiratory Journal .
- Findings that are only relevant for researchers with a very narrow expertise are more likely to be published in more specialised or more inclusive journals ( e.g. ERJ Open Research ).
- Only studies of the highest rigour that are likely to imminently lead to changes in clinical practice would be accepted for publication at the New England Journal of Medicine or the Lancet .
What are the “confidential comments to the editor” for?
You can use the “confidential comments to the editor” to express any sensitive concern that you, or perhaps the editor, may not want to convey to the authors. Sometimes, it is also a space to justify your recommendation, or to moderate it. Keep in mind that you should not share your recommendation with the authors. You can also use this space to describe any relevant conflicts of interest that you may have or any concerns regarding ethical aspects of the study and manuscript, including data fabrication or plagiarism.
After revision, I'm not happy with the authors’ responses to my comments: what should I do?
Disagreements between the authors and peer reviewers are not unusual. First, you should ensure that you have reviewed the authors responses carefully and impartially. If you think that the methodology/results or any part of the manuscript is still significantly flawed and might be misinterpreted by the reader, then it may be appropriate to explain that to the authors and editor through an additional round of peer review. For this, you will need to write a new report, stating point by point the remaining issues and, if applicable, inadequate responses. Sometimes, the authors may not follow the advice of the reviewer but provide adequate justifications for this. In this case, it might be more appropriate to accept their responses.
I'm willing to peer review: how do I attract relevant peer review invitations?
To facilitate editors’ work in identifying suitable reviewers, and to avoid inadequate solicitations, it is recommended to have a personal or institutional webpage with a short description of your field of expertise, your current work, a list of publications and contact details. If you would like to maximise the likelihood that you will receive papers that fall within your expertise, you could register a profile at a few journals relevant to your field and add specific keywords ( e.g. “COPD”, or “COPD exacerbations”, rather than “Respiratory medicine”). Also, make sure to add keywords reflecting both your disease expertise and methods expertise ( e.g. clinical trials, epidemiology, or mouse models). There are also early career reviewer programmes at several of the respiratory journals, including the ERS and the American Thoracic Society family of journals. You can also typically attend sessions at all of the major respiratory meetings with editors where you can find out more details about which programmes they have for early career reviewers.
How do I get recognition for my work as a reviewer?
After submitting a peer review report, journals usually send you a confirmation email that you could include in your CV. There are online services, such as Web of Science Reviewer Recognition Service ( http://webofscience.help.clarivate.com/en-us/Content/wos-researcher-profile.html ), that can collect and validate all your peer review records. At any time, you can download a summary of your peer review activity for your CV. Some journals are directly linked with the service and your peer review records are updated by the journals. In other cases, you only have to forward the confirmation email that you receive from the journal. Additionally, some journals have an option for your name to be published with the final published article. This is a personal choice and optional in most cases. There are obviously pros and cons that go with having your name disclosed as a reviewer and so this should be carefully considered.
We strongly recommend all scientists, including early career researchers, dedicate time to peer review activities, which are valuable both at the community level and at an individual level. When engaging in peer review work, keep in mind what is valued in this process from the perspective of an author: receiving clear, fair and scientifically relevant reviews, and when limitations are pointed out, comments that help progress the work. When the peer review work is well conducted, editors often see respectful and positive exchanges between the authors and reviewers, in which authors honestly and warmly thank the anonymous reviewers who helped them improve their paper, and reviewers thank and congratulate the authors for their work. In general, peer review should aim to improve the overall quality of the paper for the team of authors and for the field. This speaks about how as a peer reviewer you can also make great contribution to science.
Conflict of interest: None declared.

IMAGES
VIDEO
COMMENTS
In our earlier editorials on research and medical writing, we have discussed the importance of research; we have given a roadmap for drafting an original research article and have also provided suggestions on how to publish research papers successfully.[1,2,3] In this editorial, we discuss the process of peer review and method for writing good reviews for original research papers.
Original Research: This is the most common type of journal manuscript used to publish full reports of data from research. It may be called an Original Article, Research Article, Research, or just Article, depending on the journal. The Original Research format is suitable for many different fields and different types of studies.
Literature reviews are valuable resources for the scientific community. With research accelerating at an unprecedented speed in recent years and more and more original papers being published, review articles have become increasingly important as a means to keep up-to-date with developments in a particular area of research.
Journals provide guidelines to authors which should be followed closely. The three major types of articles (original research, case reports, and review articles) all generally follow the IMRAD format with slight variations in content. With planning and thought, manuscript writing does not have to be a daunting task. Download chapter PDF.
With research accelerating at an unprecedented speed in recent years and more and more original papers being published, review articles have become increasingly important as a means to keep up to date with developments in a particular area of research. A good review article provides readers with an in-depth understanding of a field and ...
Generate a reading list by searching online citation databases such as PubMed ®, 3 which incorporates MEDLINE ®, 4 or Europe PubMed Central (PMC). 5 The text-mining capabilities of these sites allow you to identify peer-reviewed original research articles, review papers, book chapters and, in the case of Europe PMC, patents and NHS guidelines ...
Original Research. An original research paper should present a unique argument of your own. In other words, the claim of the paper should be debatable and should be your (the researcher's) own original idea. Typically an original research paper builds on the existing research on a topic, addresses a specific question, presents the findings ...
A researcher writes a paper and sends it in to an academic journal, where it is read by an editor; The editor then sends the article to other scientists who study similar topics, who can best evaluate the article ... Like original research articles, review articles are aimed at scientists and undergo peer-review. Review articles often even have ...
Analysis. The first step in writing a literature review is to analyse the original investigation research papers that you have gathered related to your topic. Analysis requires examining the papers methodically and in detail, so you can understand and interpret aspects of the study described in each research article.
"Original paper" is any research paper not falling into below categories. "Review paper" is that reporting a critical overview of recent articles in the field, can be very long, say, 30-40 journal pages. "Letter" is a short research paper, ca. 4 journal pages.
By the late 1960s, the review articles published in authoritative specialized journals had become the main locus for assessing the value and position of original research papers 4. Paradoxically ...
Scholarly literature can be of different types; some of which require that researchers conduct an original study, whereas others can be based on existing research. One of the most popular Q&As led us to conclude that of all the types of scholarly literature, researchers are most confused by the differences between a research paper and a review paper. This infographic explains the five main ...
Original research is the process of conducting research without relying on the work of others. This may include conducting experiments,survey ... significant, and feasible. The research question should be specific and focused to guide the research process. Conduct a literature review: ... This may include writing a research paper, presenting at ...
Original research papers and review papers are two distinct types of academic writing, with each having its own set of specific requirements. Original research is the backbone behind any great paper as it allows for new facts to be discovered or revealed while providing an opportunity for interpretation and analysis.
The research paper will be based on the analysis and interpretation of this data. A review article or review paper is based on other published articles. It does not report original research. Review articles generally summarize the existing literature on a topic in an attempt to explain the current state of understanding on the topic.
Formal Systematic reviews and meta-analyses should be formatted and submitted as original research papers. Review articles should not exceed 5000 words and 100 references. Additional data (eg Tables) can be provided as a Supplementary Appendix. The handling Editor may relocate tables or figures into a supplementary Appendix if the manuscript ...
JNIRS — Journal of Near Infrared Spectroscopy is a peer reviewed journal, publishing original research papers, technical notes, review articles and letters concerned with near infrared spectroscopy and technology, its application, new … | View full journal description. This journal is a member of the Committee on Publication Ethics (COPE).
Economics papers dominate the top ten papers that policy documents reference most. Title. Journal. Year. The impact of trade on intra-industry reallocations and aggregate industry productivity ...
In 2021, we launched a research topic entitled 'Molecular Links between Mitochondrial Damage and Human Neurodegenerative Disorders', to which many excellent papers were contributed. This topic consists of two original articles and two review articles that were submitted after 2022 on the same research topic.
An artificial systems, computational experiments and parallel execution-based surface electromyogram-driven anti-disturbance zeroing neurodynamic strategy for upper limb human-robot interaction control. Yongbai Liu, Keping Liu, Gang Wang, Jiliang Zhang, Yao Chou, Zhongbo Sun. , Pages: 511-525. First Published: 28 April 2023.
The AI Index report tracks, collates, distills, and visualizes data related to artificial intelligence (AI). Our mission is to provide unbiased, rigorously vetted, broadly sourced data in order for policymakers, researchers, executives, journalists, and the general public to develop a more thorough and nuanced understanding of the complex field ...
B ACKGROUND. The publication of original research in a peer-reviewed and indexed journal is the ultimate and most important step toward the recognition of any scientific work.However, the process starts long before the write-up of a manuscript. The journal in which the author wishes to publish his/her work should be chosen at the time of conceptualization of the scientific work based on the ...
Since then, more than 200 million papers have been reviewed by the detector, predominantly written by high school and college students. Turnitin found that 11 percent may contain AI-written ...
Background: Telemonitoring patients with cardiac implantable electronic devices (CIEDs) can improve their care management. However, the results of cost-effectiveness studies are heterogeneous. Therefore, it is still a matter of debate whether telemonitoring is worth the investment. Objective: This systematic review aims to investigate the cost-effectiveness of telemonitoring patients with ...
Leave No Context Behind: Efficient Infinite Context Transformers with Infini-attention. This work introduces an efficient method to scale Transformer-based Large Language Models (LLMs) to infinitely long inputs with bounded memory and computation. A key component in our proposed approach is a new attention technique dubbed Infini-attention.
Introduction. Peer review is an important part of scientists' activities and the major system for evaluation of scientific reports [1, 2].Besides making an important contribution to science in terms of ensuring high standards in research reporting, conducting peer review has many values at an individual level: it can be seen as a way to keep updated with recent literature, including new ...
Call for Papers Recent Advances on Modelling and Observations in Space and Earth Sciences. Submission deadline: Saturday, 31 August 2024. With the support of the larger international community, African Geophysical Society (AGS) successfully held its 6 th Annual International Conference on 2-4 October 2023 in Lusaka, Zambia. The AGS is an interdisciplinary organization established to enhance ...