- Business Plan for Investors
- Bank/SBA Business Plan
- Operational/Strategic Planning Services
- L1 Visa Business Plan
- E1 Treaty Trader Visa Business Plan
- E2 Treaty Investor Visa Business Plan
- EB-1 Business Plan
- EB-2 NIW Business Plan
- EB-5 Business Plan
- Innovator Founder Visa Business Plan
- Start-Up Visa Business Plan
- Expansion Worker Visa Business Plan
- Manitoba MPNP Visa Business Plan
- Nova Scotia NSNP Visa Business Plan
- British Columbia BC PNP Visa Business Plan
- Self-Employed Visa Business Plan
- OINP Entrepreneur Stream Business Plan
- LMIA Owner Operator Business Plan
- ICT Work Permit Business Plan
- LMIA Mobility Program – C11 Entrepreneur Business Plan
- USMCA (ex-NAFTA) Business Plan
- Franchise Business Plan
- Landlord business plan
- Nonprofit Start-Up Business Plan
- USDA Business Plan
- Cannabis business plan
- Ecommerce business plan
- Online boutique business plan
- Mobile application business plan
- Daycare business plan
- Restaurant business plan
- Food delivery business plan
- Real estate business plan
- Business Continuity Plan
- Pitch Deck Consulting Services
- Financial Due Diligence Services
- ICO whitepaper
- ICO consulting services
- Confidential Information Memorandum
- Private Placement Memorandum
- Feasibility study
- Fractional CFO
- How it works
- Business Plan Examples

Medical Device Business Plan
NOV.06, 2023

Medical Device Business Plan Sample
A medical device business plan is a document that outlines how to start and run a successful company that produces and sells products that diagnose, treat, or prevent diseases or injuries. Navigating the vast and expanding medical device sector presents thrilling opportunities alongside complex hurdles. A well-crafted business plan illuminates the route to success. Articulate your vision, milestones, tactics, and budgetary forecasts.
A business plan should also demonstrate how you will stand out from the crowd, satisfy users, adhere to regulations, and uphold ethical standards. A medical billing business plan is a specific type of medical device business plan that focuses on how to provide billing and coding services for healthcare providers.
In this article, we will provide you with a medical device business plan sample that you can use as a template or a reference for your business plan. We will cover the following sections:
- Executive Summary
- Company Overview
- Industry Analysis
- Customer Analysis
- Competitive Analysis
- Marketing Plan
- Operations Plan
Management Team
- Financial Plan
Executive Summary Section of Our Medical Device Business Plan
Business overview.
Medix is a medical device company that develops and sells innovative and affordable devices for diabetes management. We aim to enhance the well-being and health results of those managing diabetes. We aim to offer user-friendly and dependable products that assist in tracking and regulating blood sugar levels.
Products and Services
Medix offers two main products:
- Medix Glucometer – A smart glucose meter that connects to a mobile app via Bluetooth and provides accurate and instant readings of blood glucose levels.
- Medix Patch – A wearable patch that continuously measures blood glucose levels through the skin without needing finger pricks or test strips.
Customer Focus
Medix focuses on serving people with diabetes, seeking convenient and affordable solutions to manage their condition. According to the IDF Diabetes Atlas 10th edition report , 537 million adults (20-79 years) live with diabetes – 1 in 10. Experts predict that this number will rise to 643 million by 2030 and 783 million by 2045. Therefore, there is a huge demand for effective and accessible diabetes care products.
Leo Clark and Aria Bennett, two experienced entrepreneurs with biomedical engineering and business administration backgrounds, founded Medix. Leo is the CEO and head of product development, while Aria is the COO and head of marketing and sales. A team of qualified engineers, designers, developers, marketers, salespeople, and advisors supports them.
Success Factors
Medix has several competitive advantages that will enable it to succeed in the medical device industry:
- Innovation with cutting-edge technology to create novel devices
- High standards of quality and safety in every aspect of devices
- Customer satisfaction by providing user-friendly devices
- Social impact by addressing a major health problem globally
Financial Highlights
Medix seeks $5 million in seed funding to launch its products and scale its operations. The company projects to generate $1.2 million in revenue in the first year, $3.6 million in the second year, and $10.8 million in the third year, with a gross margin of 60% and a net profit margin of 20%. The company expects to break even in the second year and reach a valuation of $50 million by the end of the third year.
Company Overview Section of Our Medical Device Sales Business Plan

Who is Medix Medical Supply?
Medix dedicates itself to developing and selling innovative, affordable, and reliable devices for diabetes management. Our products help people with diabetes to monitor and control their blood glucose levels with ease and effectiveness, leading to better health outcomes and an improved quality of life.
Medix Medical Supply History
Medix is a company that provides innovative solutions for diabetes care. It was founded by Leo Clark and Aria Bennett in 2023, who both personally experienced the challenges and frustrations of living with diabetes. These challenges included frequent finger pricks, expensive test strips, inaccurate readings, and complicated insulin injections.
They started Medix with their personal funds and an incubator grant to address these issues. Medix developed two products – the Medix Glucometer and the Medix Patch – to make diabetes monitoring and treatment easier, more accurate, and more affordable.
The Medix products have received regulatory approvals from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). They are now ready for launch in the US and European markets. For more information, please refer to our dentistry business plan .
Legal Structure
Medix, an LLC registered in Delaware, USA, has obtained ownership by Leo Clark (60%) and Aria Bennett (40%). Additionally, the company has applied for a patent for its products in the US Patent and Trademark Office (USPTO).
Industry Analysis Section of Our Medical Device Business Plan
The medical device industry is one of the world’s most innovative and dynamic sectors. Fortune Business Insights reported that the global medical device market was valued at $512.29 billion in 2022 and can grow from $536.12 billion in 2023 to $799.67 billion by 2030, at a CAGR of 5.9%.
The medical device industry is driven by several factors, such as:
- The increasing prevalence of diseases and the aging population
- The rising demand for minimally invasive and personalized treatments
- The advancement of technology and digitalization
- The emergence of new markets and segments
Customer Analysis Section of Our Medical Supply Business Plan
Demographic profile of target market.
Medix’s target market is the US market, which ranks third for the highest number of people with diabetes. We target diabetic people looking for convenient, affordable solutions to manage their condition.
According to the National Diabetes Statistics Report by CDC, here are some interesting stats about why the US market is best for Medix:
- 37.3 million people have diabetes (11.3% of the US population)
- 28.7 million people are diagnosed, including 28.5 million adults
- 8.5 million people are undiagnosed (23.0% of adults)
- 96 million people aged 18 years or older have prediabetes (38.0% of the adult US population)
- 26.4 million people aged 65 years or older (48.8%) have prediabetes
The demographic profile of our target market is as follows:
- Age – We target all ages, mainly the young and middle-aged, who are tech-savvy and have more money to spend. A CDC report says 34.1 million adults aged 18 years or older—or 13.0% of all US adults—have diabetes.
- Gender – We target both males and females, as diabetes does not discriminate by gender. A NIDDK (NIH) report says a higher percentage of men (41%) than women (32%) have prediabetes.
- Income – We target all income levels, mainly the low and middle-income who need better healthcare solutions. An NCBI (NIH) report says 80% of the adults worldwide with diabetes live in low- and middle-income countries (LMICs).
Customer Segmentation
Based on our market research and customer feedback, we have identified four main customer segments for our products:
- Segment A – Tech-savvy innovators who value quality, performance, and convenience. They share their views online.
- Segment B – Cost-conscious buyers who seek affordable and effective products. They trust their peers’ recommendations.
- Segment C – Health-conscious improvers who want products that motivate and support them. They join online health communities.
- Segment D – Compliance-driven users need products that ensure safety, security, and simplicity. They depend on their health providers and caregivers.
The table below summarizes our findings:
Based on the table, we have decided to target segments A and B as our primary segments, and segments C and D as our secondary segments.
Competitive Analysis Section of Our Medical Equipment Producer Business Plan
Direct and indirect competitors.
Our direct competitors are other medical device companies that offer similar or substitute surgical medical equipment for diabetes management. Some of the major players in this category are:
1. Abbott – A global healthcare company that offers a range of products for diabetes care with mobile apps for real-time data and insights.
- Strong brand recognition
- Global presence
- Innovation capabilities
- Customer loyalty
Weaknesses:
- Limited availability
- Technical issues
2. Dexcom – A medical device company specializing in CGMs for diabetes management. These devices use sensors to record and transmit data to a receiver or a smartphone.
- High accuracy
- Reliability
- Convenience
- Customer satisfaction
- Short sensor lifespan
- Skin irritation
3. Medtronic – A medical technology company that offers a range of durable medical equipment for diabetes care, such as insulin pumps, CGMs, and APSs. The system connects to a mobile app to monitor and control settings.
- Leadership position
- Advanced technology
- Clinical evidence
- Customer support
- Safety concerns
- Regulatory hurdles
- Competition
Our indirect competitors are other healthcare providers or solutions that offer alternative or complementary ways to manage diabetes, such as medications, diet plans, exercise programs, coaching services, etc. Refer to our hospital business plan to learn more.
Competitive Advantage
Medix’s unique value proposition and competitive advantage over its competitors are:
- Medix is more innovative
- Medix is more convenient
- Medix is more versatile
- Medix is more affordable
- Medix is more user-friendly
Marketing Plan Section of Our Medical Device Business Plan
Promotions strategy.
We will promote our products using online and offline channels to attract and retain customers. Our promotional mix consists of:
- Advertising – Online platforms (e.g., Google Ads, Facebook Ads) and offline media (e.g., newspapers, billboards) to deliver relevant and engaging messages.
- Public Relations – Press releases, media interviews, podcasts, webinars, etc., to generate positive publicity and exposure. Social media platforms (e.g., Facebook, Twitter) to interact and communicate with customers and stakeholders.
- Sales Promotion – Discounts, coupons, free samples, free trials, referrals, loyalty programs, etc., to stimulate sales and repeat purchases. Contests, sweepstakes, giveaways, etc., to create excitement and buzz.
- Personal Selling – Direct sales, telemarketing, email marketing, etc., to contact and persuade customers to buy our products. Online platforms (e.g., Amazon, eBay, Shopify) to sell our products directly.
We will use a value-based pricing strategy that reflects the value and benefits of our products and our competitive advantage. We will also offer competitive pricing that matches or undercuts our competitors’ prices.
We will charge $100 for each Medix Glucometer and $50 for each Medix Patch. We will also generate recurring revenue from the sales of test strips ($0.5 each) and insulin cartridges ($10 each). We estimate that each customer will use an average of 100 test strips and 12 insulin cartridges per year.
Operations Plan Section of Our Medical Device Business Plan
Operation functions.
We do these core activities to offer our products and services to our customers:
- Product Development – We research, design, test, and improve our products using agile methods, customer feedback, market trends, and tools like GitHub, Jira, Figma, etc.
- Manufacturing – We produce our products on a large scale and high quality by outsourcing to a reliable contract manufacturer.
- Distribution – We deliver our products to our customers quickly and cheaply using direct and indirect channels in different regions or countries.
- Customer Service – We support and assist our customers before, during, and after their purchase using various channels and methods.
Milestones and Timeline
We have these specific goals and objectives to track our progress and success in our operation functions:
- June 2024: Complete R&D, testing, prototyping of products
- September 2024: Obtain regulatory approvals and certifications
- December 2024: Launch marketing campaign and product launch in the US
- March 2025: Market research for Europe entry
- December 2025: Launch Europe marketing, market entry
- March 2026: Invest in production capacity
- June 2026: Expand manufacturing workforce
- December 2026: Evaluate production, increase to 100k units/month
Management Team Section of Our Medical Device Business Plan
Founders and co-founders.
Leo Clark, a biomedical engineer with type 1 diabetes, and Aria Bennett, the daughter of a type 2 diabetic and a business administrator, founded Medix. Leo is responsible for the product development function, while Aria leads the marketing and sales function. Both have several years of experience working in their respective fields and personal and professional experience with diabetes.
Other Key Team Members
- Alice Lee – Our chief engineer
- Bob Chen – Our chief developer
- Carol Wang – Our chief designer
- Dave Jones – Our chief marketer
- Emma Smith – Our chief salesperson
Financial Plan Section of Our Medical Device Business Plan
Key revenue and costs.
Medix’s main sources of revenue, along with pricing, are:
- Medix Glucometer – $100 for each Glucometer
- Medix Patch – $50 for each Patch
- Test Strips – $0.5 for each test strip
- Insulin Cartridge – $10 for each cartridge
We estimate that each customer will use an average of 100 test strips and 12 insulin cartridges per year.
Medix’s main categories of expenses are:
- Cost of Goods Sold (COGS) – Our main cost of goods sold is the cost of materials, components, parts, and additional supplies. We estimate that the COGS per unit is $40 for the Medix Glucometer, $20 for the Medix Patch, $0.1 for the test strip, and $2 for the insulin cartridge.
- Operating Expenses (OPEX) – Our main operating expenses are the costs we incur for running and operating our business, such as salaries, rent, utilities, marketing, advertising, R&D, etc. Our OPEX will be 40% of our revenue in the first year, 35% in the second year, and 30% in the third year.
Funding Requirements and Use of Funds
Funding Requirements – We seek $5 million in seed funding to launch our products and scale our operations. We have already raised $500,000 from our savings and a small grant from a local incubator. We need an additional $4.5 million to cover our expenses for the next 18 months until we reach the break-even point.
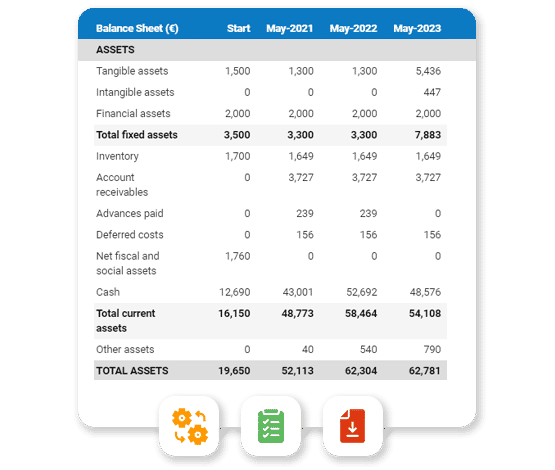
Use of Funds – We will use the funds for the following purposes as highlighted in the below chart:
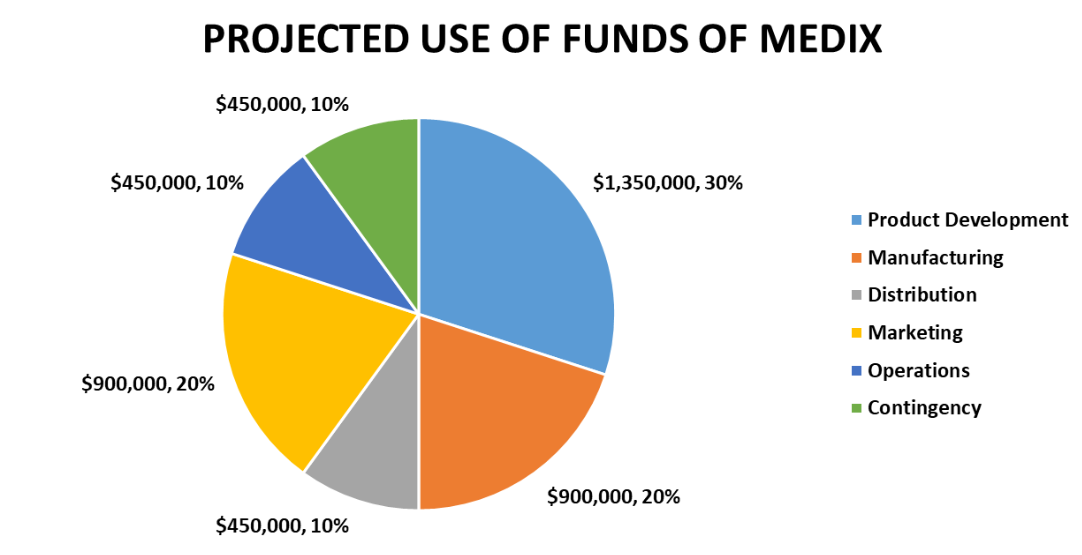
Key Assumptions
- Market size for our products is 10% of the total number of people with diabetes in the US and Europe
- Market share is projected to grow from 107,000 customers in 2024 to 444,000 customers in 2026
- Sales volume is projected to grow from 321,000 units in 2024 to 1.33 million units in 2026
- Gross margin is projected to be 60% in all three years
- Net margin is projected to grow from 20% in 2024 to 30% in 2026
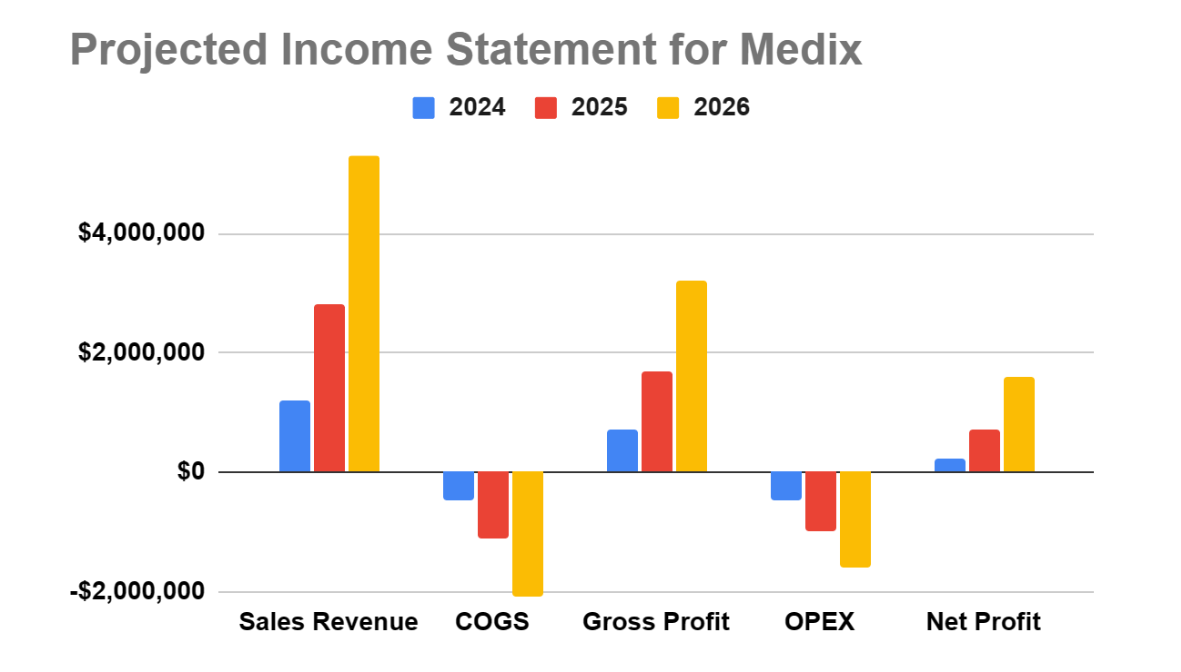
Financial Projections
Based on the above assumptions, we have prepared the following financial projections for the next three years:
Income Statement
OGSCapital – Your Partner for Medical Device Startup Success
With over a decade of experience, at OGSCapital, we have helped various entrepreneurs craft winning business plans. Our consultants provide end-to-end support – from market research and competitor analysis to realistic profitability forecasts. We understand the medical device industry inside-out, including regulations, manufacturing, and distribution.
Whether you need help with your hospital feasibility study , medical equipment manufacturing business plan, or medical supply store business plan, we tailor our approach to your specific product and goals. Partner with us to launch your startup on the path to profitability and rapid growth.
Frequently Asked Questions
How to start a medical device business.
A strategic business plan is a key ingredient in a startup medical device company. But that alone won’t cut it – the company also requires a talented group of professionals, structured product development procedures, a plan for meeting regulatory guidelines, and effective marketing tactics. A distributor or a medical equipment supplier can help distribute the devices.
How profitable are medical devices?
The medical equipment industry is booming with high growth potential. The average operating margin for medical equipment and supplies companies averages 2.87%. The medical device market will grow at a CAGR of 5.5% to 5.9% from 2022 to 2030.
How do I market my medical device?
As highlighted in our Medical Clinic Business Plan , some popular marketing channels to market a medical device include online platforms, social media, trade shows, conferences, webinars, publications, referrals, and testimonials. A medical equipment rental company can also help market the device.
OGSCapital’s team has assisted thousands of entrepreneurs with top-rate business plan development, consultancy and analysis. They’ve helped thousands of SME owners secure more than $1.5 billion in funding, and they can do the same for you.

Add comment
E-mail is already registered on the site. Please use the Login form or enter another .
You entered an incorrect username or password
Comments (0)
mentioned in the press:
Search the site:
OGScapital website is not supported for your current browser. Please use:

Medical Device Business Plan Template
Written by Dave Lavinsky
Medical Device Business Plan
You’ve come to the right place to create your Medical Device business plan.
We have helped over 1,000 entrepreneurs and business owners create business plans and many have used them to start or grow their Medical Device businesses.
Below is a template to help you create each section of your Medical Device business plan.
Executive Summary
Business overview.
MediTech LLC is a medical device company that sells Class I medical devices to hospitals, clinics, and other establishments in the medical industry. We manufacture a long list of devices including surgical instruments, syringes, and bandages. We know that patients can’t receive quality care if medical professionals don’t have good tools. Therefore, our mission is to provide the best medical devices in the industry so that all hospitals and clinics can provide the best care possible.
MediTech LLC is founded by Sarah Nelson. Sarah has considerable experience as a surgeon and used hundreds of medical devices throughout her career. She knows exactly what it takes to make high quality medical products and has made it her mission to create the best medical devices in the industry. Her expertise and knowledge of the industry will give us a considerable advantage over the competition.
Product Offering
MediTech LLC sells a long list of Class I medical devices. Class I medical devices are low risk devices and are unlikely to cause any harm to users. These include bandages, surgical tools, bedpans, gloves, and surgical masks. Our product list will grow and change depending on which devices are in high demand.
Customer Focus
MediTech LLC will primarily serve hospitals, clinics, and other medical organizations. Some products will be sold in stores to the public, including bandages, gloves, and face masks.
Management Team
MediTech LLC was founded by Sarah Nelson, a licensed and experienced surgeon. While working in the medical industry, she was frustrated by the quality of the medical devices she used. Her hospital routinely purchases low quality devices to save costs and this would affect the quality of her care. She researched what it would take to make higher quality versions of these products and decided to start a company that provides better quality devices for an affordable cost.
Success Factors
MediTech LLC will be able to achieve success by offering the following competitive advantages:
- We will provide the best quality medical devices in the industry. Our devices will help improve the quality of care that our clients give their patients.
- MediTech will price all of its products moderately so all of our clients and customers can afford them.
- Our founder has years of experience as a surgeon in the medical industry, bringing a vast amount of medical knowledge to the table. This will help us create perfect medical devices and products that all medical professionals will be eager to use.
Financial Highlights
MediTech LLC is currently seeking $1,400,000 to launch. The funding will be dedicated to the facility build out, purchase of initial equipment, working capital, marketing costs, and startup overhead expenses. The breakout of the funding is below:
- Facility design/build: $500,000
- Equipment: $200,000
- Six months of overhead expenses (payroll, rent, utilities): $400,00
- Initial supplies and inventory: $100,000
- Marketing and advertising: $100,000
- Working capital: $100,000
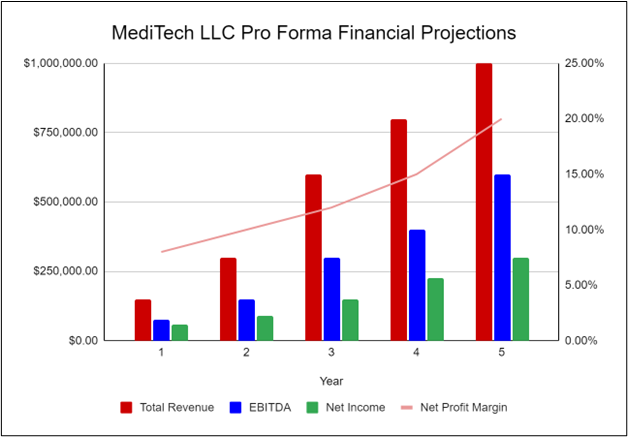
The following graph below outlines the pro forma financial projections for MediTech LLC.
Company Overview
Who is meditech llc.
MediTech LLC sells high-quality Class I medical devices to the medical industry. Our management team knows from experience that patients can’t receive the best care possible if physicians aren’t using the best tools. However, many medical organizations order lower quality devices in order to save on costs. At MediTech LLC, we are committed to making the best medical devices in the industry that are more affordable than the competition.
MediTech LLC produces a long list of medical devices for the medical industry. These include bandages, surgical masks, gloves, surgical instruments, and bedpans. All of our products are Class I devices, meaning they present a low risk to the user.
MediTech LLC is founded by Sarah Nelson. Sarah has considerable experience as a surgeon and used hundreds of medical devices throughout her career. She knows exactly what it takes to make high quality medical products and has made it her mission to create the best medical devices in the industry. Her expertise and connections in the industry will ensure that MediTech LLC achieves its mission.
MediTech LLC History
Sarah Nelson founded and incorporated MediTech LLC as an LLC in June 2023. Though the company is currently running out of a small rented office, it will move to a large warehouse once the lease is finalized.
Since incorporation, MediTech LLC has achieved the following milestones:
- Developed the company’s name, logo, and website
- Determined equipment and fixture requirements
- Identified and established relationships with potential clients and suppliers
- Begun recruiting key employees
MediTech LLC Services
MediTech LLC manufactures and sells Class I medical devices. These include (but are not limited to) the following:
- Surgical instruments
- Non-electric wheelchairs
- Stethoscopes
- Surgical masks
Industry Analysis
The medical industry is dependent on the access to high-quality medical devices and products. From gloves and masks to EKG machines, every device used in the care of patients needs to be high quality and always in working order. Devices that are poor quality or don’t work properly can cause significant problems when being used to care for patients.
Medical devices are categorized into three classes. Class I devices are devices that pose very little risk to the user. These items include bandages, surgical instruments, and gloves. Class II devices are intermediate risk devices. These include intravenous pumps and CT machines. Class III devices are high risk and require a great amount of regulation. These devices are also critical to sustaining life. These include pacemakers and brain stimulators.
According to Fortune Business Insights, the medical device industry is valued at $539 billion and is expected to grow at a CAGR of 5.9%. Medical devices are constantly in high demand and are essential for the success of the medical industry. Therefore, now is a great time to start a new medical device company.
Customer Analysis
Demographic profile of target market, customer segmentation.
The company will primarily target the following customer segments:
- Medical clinics
Competitive Analysis
Direct and indirect competitors.
MediTech LLC will face competition from other companies with similar business profiles. A description of each competitor company is below.
Smith & Smith
Smith & Smith is a large corporation that sells thousands of products, including cosmetics, hygiene products, and certain medical devices. The medical devices they primarily produce include bandages, ointments, and low risk surgical and physician instruments. They sell many of their products to the general public (such as simple wound care devices) but also sell some devices to the medical industry. They will be a major competitor since they sell primarily Class I devices. However, they currently do not produce as many medical devices as MediTech LLC plans to produce, which gives us an advantage in the market.
MedMonitor is a medical device company that manufactures Class III medical devices. Some of their products include breast implants, pacemakers, implanted prosthetics, and defibrillators. They do sell some Class I and Class II products, such as gloves, wound care items, and surgical masks, but they are not a major manufacturer of these products. As such, we expect that MedMonitor will only be a minor competitor in the market.
MedSource is the source for most of the medical industry’s Class II medical devices. They produce a long list of devices including syringes, testing kits, contact lenses, and blood pressure cuffs. They do produce some products that can be categorized as Class I devices, but their product list does not overlap too much with ours. As such, we expect that MedSource will only be a minor competitor.
Competitive Advantage
MediTech LLC enjoys several advantages over its competitors. These advantages include the following:
- Management : Sarah Nelson has been extremely successful working in the medical industry and will be able to use her previous experience to design and manufacture the best medical devices in the industry.
- Relationships : Sarah knows many of the local leaders, business managers, and other influencers in the medical industry. These relationships will help her have access to quality materials and create an initial clientbase.
- Affordability : Thanks to Sarah’s connections within the industry, we are able to access high-quality materials for our products for an affordable cost. As a result, we can price all our products more moderately than the competition.
Marketing Plan
Brand & value proposition.
The MediTech LLC brand will focus on the company’s unique value proposition:
- High quality medical devices
- Affordable pricing
- Client-focused service
Promotions Strategy
The promotions strategy for MediTech LLC is as follows:
Social Media Marketing
Social media is one of the most cost-effective and practical marketing methods for improving brand visibility. MediTech LLC will use social media to develop engaging content in terms of the company’s product offerings. Engaging with prospective consumers and businesses on social media platforms like Facebook, Instagram, Twitter, and LinkedIn will also help understand changing customer needs.
Website/SEO
MediTech LLC will invest in developing a professional website that displays all of the products offered by the company. It will also invest in SEO so that the company’s website will appear at the top of search engine results.
Direct Mail
MediTech LLC will blanket businesses with direct mail pieces. These pieces will provide general information on MediTech LLC, offer discounts, and/or provide other incentives for companies to buy our products.
Advertisement
Advertisements in print publications like newspapers, magazines, etc., are an excellent way for businesses to connect with their audience. MediTech LLC will advertise its products in popular magazines and news dailies. Obtaining relevant placements in industry magazines and journals will also help in increasing brand visibility.
MediTech LLC’s pricing will be moderate, so clients feel they receive great value when purchasing our products.
Operations Plan
The following will be the operations plan for MediTech LLC. Operation Functions:
- Sarah Nelson will be the CEO of MediTech LLC. She will oversee the general operations and executive aspects of the business.
- Sarah is joined by Rebecca Smith who will act as the warehouse manager. She will train and manage the staff as well as oversee general production of our products.
- Sarah will hire an Administrative Assistant, Marketing Manager, and Accountant, to handle the administrative, marketing, and bookkeeping functions of the company.
- Sarah will also hire several employees to manufacture our products and maintain the equipment and machinery.
Milestones:
MediTech LLC will have the following milestones completed in the next six months.
- 02/202X Finalize lease agreement
- 03/202X Design and build out MediTech LLC
- 04/202X Hire and train initial staff
- 05/202X Kickoff of promotional campaign
- 06/202X Launch MediTech LLC
- 07/202X Reach break-even
Sarah Nelson is a former surgeon who is familiar with the most popular medical devices in the industry. She knows better than anyone that low quality products means low quality care for patients. As a surgeon, she was often disappointed with the quality of the medical devices she used. Her hospital would routinely choose the cheapest options to save costs. This resulted in more problems and low quality care being delivered to her patients. She is now passionate about starting her own company that provides high quality medical devices for an affordable cost.
Though Sarah has never run a business of her own, she has worked in the medical industry long enough to gain an in-depth knowledge of the operations (e.g., running day-to-day operations) and the business (e.g., staffing, marketing, etc.) sides of the industry. She will also hire several professionals to help her run other aspects of the business she is unfamiliar with.
Financial Plan
Key revenue & costs.
The key revenues for MediTech LLC will come from the sale of our medical devices and products.
The major cost drivers for the company will include manufacturing costs, overhead expenses, labor expenses, and marketing costs.
Funding Requirements and Use of Funds
- Six months of overhead expenses (payroll, rent, utilities): $400,000
Key Assumptions
The following outlines the key assumptions required in order to achieve the revenue and cost numbers in the financials and pay off the startup business loan.
- Number of wholesale contracts:
- Year 5: 100
- Average order value: $5,000
Financial Projections
Income statement, balance sheet, cash flow statement, medical device business plan faqs, what is a medical device business plan.
A medical device business plan is a plan to start and/or grow your medical device business. Among other things, it outlines your business concept, identifies your target customers, presents your marketing plan and details your financial projections.
You can easily complete your Medical Device business plan using our Medical Device Business Plan Template here .
What are the Main Types of Medical Device Businesses?
There are a number of different kinds of medical device businesses , some examples include: Class 1 medical device, Class 2 medical device, and Class 3 medical device.
How Do You Get Funding for Your Medical Device Business Plan?
Medical Device businesses are often funded through small business loans. Personal savings, credit card financing and angel investors are also popular forms of funding.
What are the Steps To Start a Medical Device Business?
Starting a medical device business can be an exciting endeavor. Having a clear roadmap of the steps to start a business will help you stay focused on your goals and get started faster.
1. Develop A Medical Device Business Plan - The first step in starting a business is to create a detailed medical device business plan that outlines all aspects of the venture. This should include potential market size and target customers, the services or products you will offer, pricing strategies and a detailed financial forecast.
2. Choose Your Legal Structure - It's important to select an appropriate legal entity for your medical device business. This could be a limited liability company (LLC), corporation, partnership, or sole proprietorship. Each type has its own benefits and drawbacks so it’s important to do research and choose wisely so that your medical device business is in compliance with local laws.
3. Register Your Medical Device Business - Once you have chosen a legal structure, the next step is to register your medical device business with the government or state where you’re operating from. This includes obtaining licenses and permits as required by federal, state, and local laws.
4. Identify Financing Options - It’s likely that you’ll need some capital to start your medical device business, so take some time to identify what financing options are available such as bank loans, investor funding, grants, or crowdfunding platforms.
5. Choose a Location - Whether you plan on operating out of a physical location or not, you should always have an idea of where you’ll be based should it become necessary in the future as well as what kind of space would be suitable for your operations.
6. Hire Employees - There are several ways to find qualified employees including job boards like LinkedIn or Indeed as well as hiring agencies if needed – depending on what type of employees you need it might also be more effective to reach out directly through networking events.
7. Acquire Necessary Medical Device Equipment & Supplies - In order to start your medical device business, you'll need to purchase all of the necessary equipment and supplies to run a successful operation.
8. Market & Promote Your Business - Once you have all the necessary pieces in place, it’s time to start promoting and marketing your medical device business. This includes creating a website, utilizing social media platforms like Facebook or Twitter, and having an effective Search Engine Optimization (SEO) strategy. You should also consider traditional marketing techniques such as radio or print advertising.
Learn more about how to start a successful medical device business:
- How to Start a Medical Device Company
How to write a business plan for a medical device manufacturer?

Writing a business plan for a medical device manufacturer can be an intimidating task, especially for those just starting.
This in-depth guide is designed to help entrepreneurs like you understand how to create a comprehensive business plan so that you can approach the exercise with method and confidence.
We'll cover: why writing a medical device manufacturer business plan is so important - both when starting up, and when running and growing the business - what information you need to include in your plan, how it should be structured, and what tools you can use to get the job done efficiently.
Let's get started!
In this guide:
Why write a business plan for a medical device manufacturer?
- What information is needed to create a business plan for a medical device manufacturer?
- What goes in the financial forecast for a medical device manufacturer?
- What goes in the written part of a medical device manufacturer business plan?
- What tool can I use to write my medical device manufacturer business plan?
Being clear on the scope and goals of the document will make it easier to understand its structure and content. So before diving into the actual content of the plan, let's have a quick look at the main reasons why you would want to write a medical device manufacturer business plan in the first place.
To have a clear roadmap to grow the business
It's rarely business as usual for small businesses. The economy follows cycles where years of growth are followed by recessions, and the business environment is always changing with new technologies, new regulations, new competitors, and new consumer behaviours appearing all the time...
In this context, running a business without a clear roadmap is like driving blindfolded: it's dangerous at best. That's why writing a business plan for a medical device manufacturer is essential to create successful and sustainable businesses.
To write an effective business plan, you will need to take stock of where you are (if you are already in business) and where you want the business to go in the next three to five years.
Once you know where you want your medical device manufacturer to be, you'll have to identify:
- what resources (human, equipment, and capital) are needed to get there,
- at what pace the business needs to progress to get there in time,
- and what risks you'll face along the way.
Going through this process regularly is beneficial, both for startups and existing companies, as it helps make informed decisions about how best to allocate resources to ensure the long-term success of the business.
To anticipate future cash flows
Regularly comparing your actual financial performance to the projections in the financial forecast of your medical device manufacturer's business plan gives you the ability to monitor your business's financial health and make necessary adjustments as needed.
This practice allows you to detect potential financial issues, such as unexpected cash shortfalls before they escalate into major problems. Giving you time to find additional financing or put in place corrective measures.
Additionally, it helps you identify growth opportunities, like excess cash flow that could be allocated to launch new products and services or expand into new markets.
Staying on track with these regular comparisons enables you to make well-informed decisions about the amount of financing your business might require, or the excess cash flow you can expect to generate from your main business activities.
To secure financing
Whether you are a startup or an existing business, writing a detailed medical device manufacturer business plan is essential when seeking financing from banks or investors.
This makes sense given what we've just seen: financiers want to ensure you have a clear roadmap and visibility on your future cash flows.
Banks will use the information included in the plan to assess your borrowing capacity (how much debt your business can support) and your ability to repay the loan before deciding whether they will extend credit to your business and on what terms.
Similarly, investors will review your plan carefully to assess if their investment can generate an attractive return on investment.
To do so, they will be looking for evidence that your medical device manufacturer has the potential for healthy growth, profitability, and cash flow generation over time.
Now that you understand why it is important to create a business plan for a medical device manufacturer, let's take a look at what information is needed to create one.
Need a convincing business plan?
The Business Plan Shop makes it easy to create a financial forecast to assess the potential profitability of your projects, and write a business plan that’ll wow investors.
Information needed to create a business plan for a medical device manufacturer
Drafting a medical device manufacturer business plan requires research so that you can project sales, investments and cost accurately in your financial forecast, and convince the reader that there is a viable commercial opportunity to be seized.
Below, we'll focus on three critical pieces of information you should gather before starting to write your plan.
Carrying out market research for a medical device manufacturer
Carrying out market research before writing a business plan for a medical device manufacturer is essential to ensure that the financial projections are accurate and realistic.
Market research helps you gain insight into your target customer base, competitors, pricing strategies and other key factors which can have an impact on the commercial success of your business.
In particular, it is useful in forecasting revenue as it provides valuable data regarding potential customers’ spending habits and preferences.
1. Your medical device manufacturer may discover a trend in consumer preferences for more convenient or user-friendly products. 2. Market research might reveal a trend in demand for products that offer additional features or higher performance than those currently on the market.
This information can then be used to create more accurate financial projections which will help investors make informed decisions about investing in your medical device manufacturer.
Developing the sales and marketing plan for a medical device manufacturer
Budgeting sales and marketing expenses is essential before creating a medical device manufacturer business plan.
A comprehensive sales and marketing plan should provide an accurate projection of what actions need to be implemented to acquire and retain customers, how many people are needed to carry out these initiatives, and how much needs to be spent on promotions, advertising, and other aspects.
This helps ensure that the right amount of resources is allocated to these activities in order to hit the sales and growth objectives forecasted in your business plan.
The staffing and equipment needs of a medical device manufacturer
As you embark on starting or expanding your medical device manufacturer, having a clear plan for recruitment and capital expenditures (investment in equipment and real estate) is essential for ensuring your business's success.
Both the recruitment and investment plans must align with the timing and level of growth projected in your forecast, and they require appropriate funding.
Staffing costs may include salaries for engineers, technicians, and other personnel who are responsible for designing, producing, and testing the medical device. Equipment costs may include the purchase of materials and supplies such as raw materials, components, and machinery needed to produce the medical device. Other costs may include the cost of renting space for production, purchasing additional machinery, and the cost of maintenance and repairs.
To create a realistic financial forecast, you also need to consider other operating expenses associated with the day-to-day running of your business, such as insurance and bookkeeping.
With all the necessary information at hand, you are ready to begin crafting your business plan and developing your financial forecast.
What goes into your medical device manufacturer's financial forecast?
The financial forecast of your medical device manufacturer will enable you to assess the profitability potential of your business in the coming years and how much capital is required to fund the actions planned in the business plan.
The four key outputs of a financial forecast for a medical device manufacturer are:
- The profit and loss (P&L) statement ,
- The projected balance sheet ,
- The cash flow forecast ,
- And the sources and uses table .
Let's take a closer look at each of these.
The projected P&L statement
The projected P&L statement for a medical device manufacturer shows how much revenue and profits your business is expected to generate in the future.
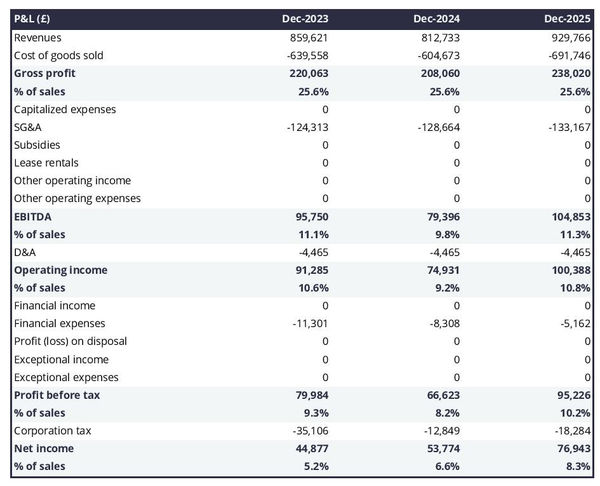
Ideally, your medical device manufacturer's P&L statement should show:
- Healthy growth - above inflation level
- Improving or stable profit margins
- Positive net profit
Expectations will vary based on the stage of your business. A startup will be expected to grow faster than an established medical device manufacturer. And similarly, an established company should showcase a higher level of profitability than a new venture.
The forecasted balance sheet of your medical device manufacturer
The projected balance sheet of your medical device manufacturer will enable the reader of your business plan to assess the overall financial health of your business.
It shows three elements: assets, liabilities and equity:
- Assets: are productive resources owned by the business, such as equipment, cash, and accounts receivable (money owed by clients).
- Liabilities: are debts owed to creditors, lenders, and other entities, such as accounts payable (money owed to suppliers).
- Equity: includes the sums invested by the shareholders or business owners and the profits and losses accumulated by the business to date (which are called retained earnings). It is a proxy for the value of the owner's stake in the business.
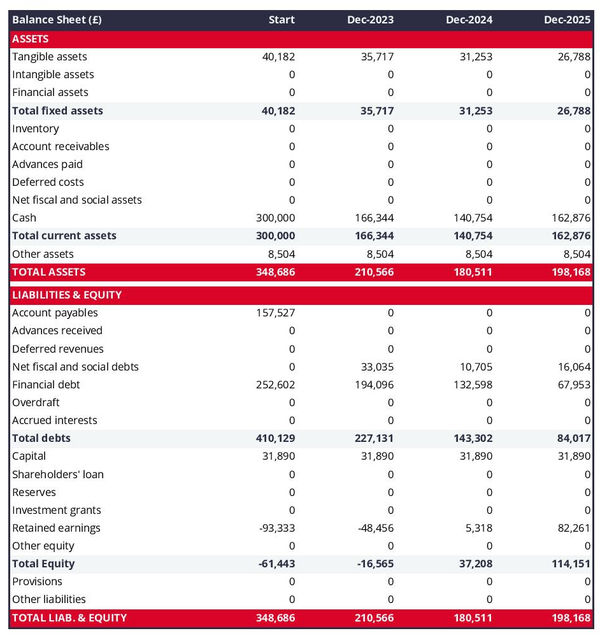
Analysing your medical device manufacturer projected balance sheet provides an understanding of your medical device manufacturer's working capital structure, investment and financing policies.
In particular, the readers of your plan can compare the level of financial debt on the balance sheet to the equity value to measure the level of financial risk (equity doesn't need to be reimbursed, while financial debt must be repaid, making it riskier).
They can also use your balance sheet to assess your medical device manufacturer's liquidity and solvency:
- A liquidity analysis: focuses on whether or not your business has sufficient cash and short-term assets to cover its liabilities due in the next 12 months.
- A solvency analysis: takes and longer view to assess whether or not your business has the capacity to repay its debts over the medium-term.
The cash flow forecast
A projected cash flow statement for a medical device manufacturer is used to show how much cash the business is generating or consuming.
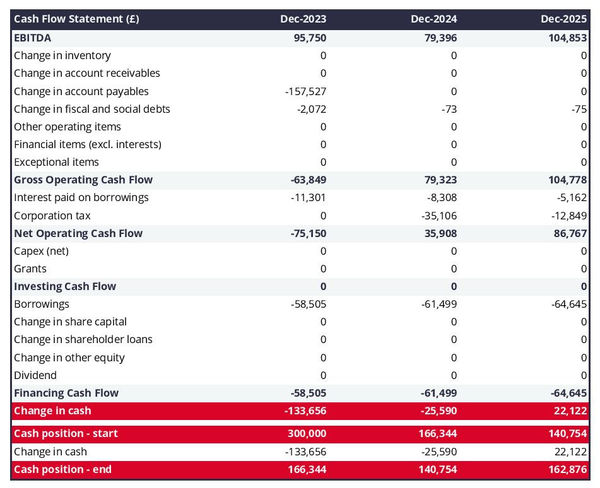
The cash flow forecast is usually organized by nature to show three key metrics:
- The operating cash flow: do the core business activities generate or consume cash?
- The investing cash flow: how much is the business investing in long-term assets (this is usually compared to the level of fixed assets on the balance sheet to assess whether the business is regularly maintaining and renewing its equipment)?
- The financing cash flow: is the business raising new financing or repaying financiers (debt repayment, dividends)?
As we discussed earlier, cash is king and keeping an eye on future cash flows an imperative for running a successful business. Therefore, you can expect the reader of your medical device manufacturer business plan to pay close attention to your cash flow forecast.
Also, note that it is customary to provide both yearly and monthly cash flow forecasts in a business plan - so that the reader can analyze seasonal variation and ensure the medical device manufacturer is appropriately funded.
The initial financing plan
The initial financing plan - also called a sources and uses table - is an important tool when starting a medical device manufacturer.
It shows where the money needed to set up the business will come from (sources) and how it will be allocated (uses).
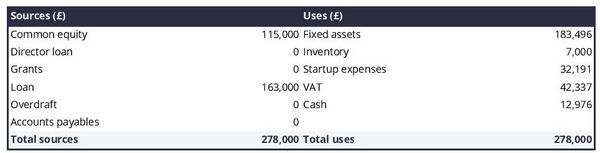
Having this table helps understand what costs are involved in setting up the medical device manufacturer, how the risks are distributed between the shareholders and the lenders, and what will be the starting cash position (which needs to be sufficient to sustain operations until the business breaks even).
Now that the financial forecast of a medical device manufacturer business plan is understood, let's focus on what goes into the written part of the plan.
Need inspiration for your business plan?
The Business Plan Shop has dozens of business plan templates that you can use to get a clear idea of what a complete business plan looks like.

The written part of a medical device manufacturer business plan
The written part of a medical device manufacturer business plan is composed of 7 main sections:
- The executive summary
- The presentation of the company
- The products and services
- The market analysis
- The strategy
- The operations
- The financial plan
Throughout these sections, you will seek to provide the reader with the details and context needed for them to form a view on whether or not your business plan is achievable and your forecast a realistic possibility.
Let's go through the content of each section in more detail!
1. The executive summary
In your medical device manufacturer's business plan, the first section is the executive summary — a captivating overview of your plan that aims to pique the reader's interest and leave them eager to learn more about your business.
When crafting the executive summary, start with an introduction to your business, including its name, concept, location, how long it has been running, and what sets it apart. Briefly mention the products and services you plan to offer and your target customer profile.
Following that, provide an overview of the addressable market for your medical device manufacturer, current trends, and potential growth opportunities.
Next, include a summary of key financial figures like projected revenues, profits, and cash flows.
Finally, in the "ask" section, detail any funding requirements you may have.
2. The presentation of the company
The second section in your medical device manufacturer's business plan should focus on the structure and ownership, location, and management team of the company.
The structure and ownership part provides an overview of the legal structure of the business, who the owners are and how much each has invested and owns. If you are seeking financing it is important that the reader gets a clear picture of which legal entity is receiving the funds, and who controls the business.
The location part should give an overview of the premises from which the company is operating, and why that location is of particular interest (catchment area, accessibility, amenities nearby, etc.).
When describing the location of your medical device manufacturer, you may want to emphasize its access to a highly-educated workforce, competitive costs, and strong transportation networks. You could point out its proximity to a major metropolitan area, which could provide access to potential customers and other resources. Additionally, you might want to highlight that the region has a long history of successful manufacturing operations and is home to a wide range of industries. All of these factors could make it an attractive option for a third party financier.
Finally, you should introduce the management team. Explain each member's role, background, and experience.
It is also important to emphasize any past successes that the members of the management team have achieved, and how long they've been working together, as this will help potential lenders or investors understand why they should trust in their leadership.
3. The products and services section
The products and services section of your business plan should include a detailed description of what your company offers, who are the target customers, and what distribution channels are part of your go-to-market.
For example, your medical device manufacturer might offer customers a variety of medical devices such as hearing aids, catheters, and wheelchairs. They might also provide customers with repair services and product maintenance in order to ensure the product is functioning properly and safely. Additionally, they might offer educational resources about their products to teach customers how to safely and properly use the device. These services and products help customers stay healthy and safe while using medical devices.
4. The market analysis
When you present your market analysis in your medical device manufacturer business plan, it's crucial to include detailed information about customers' demographics and segmentation, target market, competition, barriers to entry, and any relevant regulations.
The main objective of this section is to help the reader understand the size and attractiveness of the market while demonstrating your solid understanding of the industry.
Begin with the demographics and segmentation subsection, providing an overview of the addressable market for your medical device manufacturer, the key trends in the marketplace, and introducing different customer segments along with their preferences in terms of purchasing habits and budgets.
Next, focus on your target market, zooming in on the specific customer segments your medical device manufacturer aims to serve and explaining how your products and services fulfil their distinct needs.
For example, your target market might include elderly individuals who need medical devices to help them maintain their independence. This segment includes people who need wheelchairs, walkers, hearing aids, and other medical devices. Additionally, they often have insurance plans that cover these medical devices, making them a viable target market for a medical device manufacturer.
Then proceed to the competition subsection, where you introduce your main competitors and highlight what sets you apart from them.
Finally, conclude your market analysis with an overview of the key regulations applicable to your medical device manufacturer.
5. The strategy section
When you write the strategy section of your medical device manufacturer business plan, remember to cover key elements such as your competitive edge, pricing strategy, sales & marketing plan, milestones, and risks and mitigants.
In the competitive edge subsection, elaborate on what makes your company stand out from competitors. This becomes especially important if you're a startup, aiming to carve a place for yourself amidst established players in the marketplace.
The pricing strategy subsection should demonstrate how you plan to maintain profitability while offering competitive prices to attract customers.
Outline your sales & marketing plan, detailing how you'll reach out to new customers and retain existing ones through loyalty programs or special offers.
For the milestones subsection, outline your company's achievements to date and your main objectives for the future, complete with specific dates to set clear expectations for progress.
Lastly, the risks and mitigants subsection should address the main risks that could affect your plan's execution. Explain the measures you've put in place to minimize these risks, assuring potential investors or lenders.
Your medical device manufacturer could face the risk of product failure or recalls. This could be due to a defect in the device itself, or it could be caused by user error or misuse. In either case, this could result in large financial losses, as well as serious damage to the manufacturer's reputation and brand. Another risk your medical device manufacturer may face is increased competition from other manufacturers. This could result in lower profits as well as a decrease in market share for the product. It could also lead to price wars and other competitive tactics, which may be costly and time consuming.
6. The operations section
In your business plan, it's also essential to provide a detailed overview of the operations of your medical device manufacturer.
Start by covering your team, highlighting key roles and your recruitment plan to support the expected growth. Outline the qualifications and experience required for each role and your intended recruitment methods, whether through job boards, referrals, or headhunters.
Next, clearly state your medical device manufacturer's operating hours, allowing the reader to assess staffing levels adequately. Additionally, mention any plans for varying opening times during peak seasons and how you'll handle customer queries outside normal operating hours.
Then, shift your focus to the key assets and intellectual property (IP) necessary for your business. If you rely on licenses, trademarks, physical structures like equipment or property, or lease agreements, make sure to include them in this section.
You might have key assets such as proprietary technology and brand recognition. For example, the manufacturer could have patented technology that is used in the medical devices they produce, or they could have a well-known brand name that is associated with quality and trust. Additionally, they could have valuable intellectual property such as designs, trademarks, and trade secrets that they could use to protect their products from being copied or counterfeited.
Lastly, include a list of suppliers you plan to work with, detailing their services and main commercial terms, such as price, payment terms, and contract duration. Investors are interested in understanding why you've chosen specific suppliers, which may be due to higher-quality products or established relationships from previous ventures.
7. The presentation of the financial plan
The financial plan section is where we will present the financial forecast we talked about earlier in this guide.
Now that you have a clear idea of what goes in your medical device manufacturer business plan, let's look at the solutions you can use to draft yours.
What tool should I use to write my medical device manufacturer's business plan?
In this section, we will be reviewing the two main solutions for creating a medical device manufacturer business plan:
- Using specialized online business plan software,
- Outsourcing the plan to the business plan writer.
Using an online business plan software for your medical device manufacturer's business plan
The modern and most efficient way to write a medical device manufacturer business plan is to use business plan software .
There are several advantages to using specialized software:
- You can easily create your financial forecast by letting the software take care of the financial calculations for you without errors
- You are guided through the writing process by detailed instructions and examples for each part of the plan
- You can access a library of dozens of complete business plan samples and templates for inspiration
- You get a professional business plan, formatted and ready to be sent to your bank or investors
- You can easily track your actual financial performance against your financial forecast
- You can create scenarios to stress test your forecast's main assumptions
- You can easily update your forecast as time goes by to maintain visibility on future cash flows
- You have a friendly support team on standby to assist you when you are stuck
If you're interested in using this type of solution, you can try The Business Plan Shop for free by signing up here .
Need a solid financial forecast?
The Business Plan Shop does the maths for you. Simply enter your revenues, costs and investments. Click save and our online tool builds a three-way forecast for you instantly.
Hiring a business plan writer to write your medical device manufacturer's business plan
Outsourcing your medical device manufacturer business plan to a business plan writer can also be a viable option.
These writers possess valuable experience in crafting business plans and creating accurate financial forecasts. Additionally, enlisting their services can save you precious time, enabling you to concentrate on the day-to-day operations of your business.
It's important to be mindful, though, that hiring business plan writers comes with a cost. You'll be paying not just for their time but also for the software they use, and their profit margin.
Based on experience, a complete business plan usually requires a budget of at least £1.5k ($2.0k) excluding tax, and more if revisions are needed after initial meetings with lenders or investors - changes often arise following these discussions.
When seeking investment, be cautious about spending too much on consulting fees. Investors prefer their funds to contribute directly to business growth. Thus, the amount you spend on business plan writing services and other consulting services should be negligible compared to the amount you raise.
Another aspect to consider is that while you'll receive the output of the business plan, you usually won't own the actual document. It will be saved in the consultant's business plan software, which will make updating the plan challenging without retaining the consultant on a retainer.
Given these factors, it's essential to carefully weigh the pros and cons of outsourcing your medical device manufacturer business plan to a business plan writer and decide what best suits your business's unique needs.
Why not create your medical device manufacturer's business plan using Word or Excel?
Using Microsoft Excel and Word (or their Google, Apple, or open-source equivalents) to write a medical device manufacturer business plan is a terrible idea.
For starters, creating an accurate and error-free financial forecast on Excel (or any spreadsheet) is very technical and requires both a strong grasp of accounting principles and solid skills in financial modelling.
As a result, it is unlikely anyone will trust your numbers unless - like us at The Business Plan Shop - you hold a degree in finance and accounting and have significant financial modelling experience in your past.
The second reason is that it is inefficient. Building forecasts on spreadsheets was the only option in the 1990s and early 2000s, nowadays technology has advanced and software can do it much faster and much more accurately.
And with the rise of AI, software is also becoming smarter at helping us detect mistakes in our forecasts and helping us analyse the numbers to make better decisions.
Also, using software makes it easy to compare actuals vs. forecasts and maintain our forecasts up to date to maintain visibility on future cash flows - as we discussed earlier in this guide - whereas this is a pain to do with a spreadsheet.
That's for the forecast, but what about the written part of my medical device manufacturer business plan?
This part is less error-prone, but here also software brings tremendous gains in productivity:
- Word processors don't include instructions and examples for each part of your business plan
- Word processors don't update your numbers automatically when they change in your forecast
- Word processors don't handle the formatting for you
Overall, while Word or Excel may be viable options for creating a medical device manufacturer business plan for some entrepreneurs, it is by far not the best or most efficient solution.
- Using business plan software is a modern and cost-effective way of writing and maintaining business plans.
- A business plan is not a one-shot exercise as maintaining it current is the only way to keep visibility on your future cash flows.
- A business plan has 2 main parts: a financial forecast outlining the funding requirements of your medical device manufacturer and the expected growth, profits and cash flows for the next 3 to 5 years; and a written part which gives the reader the information needed to decide if they believe the forecast is achievable.
We hope that this in-depth guide met your expectations and that you now have a clear understanding of how to write your medical device manufacturer business plan. Do not hesitate to contact our friendly team if you have questions additional questions we haven't addressed here.
Also on The Business Plan Shop
- How to write a business plan to secure a bank loan?
- Key steps to write a business plan?
- Top mistakes to avoid in your business plan
Do you know entrepreneurs interested in starting or growing a medical device manufacturer? Share this article with them!

Founder & CEO at The Business Plan Shop Ltd
Guillaume Le Brouster is a seasoned entrepreneur and financier.
Guillaume has been an entrepreneur for more than a decade and has first-hand experience of starting, running, and growing a successful business.
Prior to being a business owner, Guillaume worked in investment banking and private equity, where he spent most of his time creating complex financial forecasts, writing business plans, and analysing financial statements to make financing and investment decisions.
Guillaume holds a Master's Degree in Finance from ESCP Business School and a Bachelor of Science in Business & Management from Paris Dauphine University.
Create a convincing business plan
Assess the profitability of your business idea and create a persuasive business plan to pitch to investors
500,000+ entrepreneurs have already tried our solution - why not join them?
Not ready to try our on-line tool ? Learn more about our solution here
Need some inspiration for your business plan?
Subscribe to The Business Plan Shop and gain access to our business plan template library.

Need a professional business plan? Discover our solution
Write your business plan with ease!

It's easy to create a professional business plan with The Business Plan Shop
Want to find out more before you try? Learn more about our solution here

How to Start a Medical Device Company

Starting a medical device business can be very profitable. With proper planning, execution and hard work, you can enjoy great success. Below you will learn the keys to launching a successful medical device company.
Importantly, a critical step in starting a medical device company is to complete your business plan. To help you out, you should download Growthink’s Ultimate Business Plan Template here .
Download our Ultimate Business Plan Template here
15 Steps To Start a Medical Device Company:
- Choose the Name for Your Medical Device Company
- Determine the Type of Medical Device Company You Will Launch
- Develop Your Medical Device Company Plan
- Choose the Legal Structure for Your Medical Device Company
- Secure Startup Funding for Your Medical Device Company (If Needed)
- Secure a Location for Your Company
- Register Your Medical Device Company with the IRS
- Open a Company Bank Account
- Get a Company Credit Card
- Get the Required Company Licenses and Permits
- Get Company Insurance for Your Medical Device Company
- Buy or Lease the Right Medical Device Company Equipment
- Develop Your Medical Device Company Marketing Materials
- Purchase and Setup the Software Needed to Run Your Medical Device Company
- Open for Company
1. Choose the Name for Your Medical Device Company
The first step to starting a medical device business is to choose your business’ name.
This is a very important choice since your company name is your brand and will last for the lifetime of your business. Ideally you choose a name that is meaningful and memorable. Here are some tips for choosing a name for your medical device business:
- Make sure the name is available . Check your desired name against trademark databases and your state’s list of registered business names to see if it’s available. Also check to see if a suitable domain name is available.
- Keep it simple . The best names are usually ones that are easy to remember, pronounce and spell.
- Think about marketing . Come up with a name that reflects the desired brand and/or focus of your medical device business.
2. Determine the Type of Medical Device Company You Will Launch
The next step is to determine the type of medical device business you will launch. The four main types of medical device businesses are:
- Manufacturers – These companies create medical devices from scratch, using materials and designs they have developed.
- Distributors – Distributors purchase products from manufacturers and resell them to buyers such as hospitals, doctors’ offices, surgical centers, etc.
- Retailers – Retailers sell medical devices directly to consumers, such as over-the-counter products like thermometers and blood pressure monitors.
- Service Providers – These businesses provide installation, repair and maintenance services for medical devices.
3. Develop Your Medical Device Company Plan
One of the most important steps in starting a medical device business is to develop your medical device company business plan . The process of creating your plan ensures that you fully understand your market and your business strategy. The plan also provides you with a roadmap to follow and if needed, to present to funding sources to raise capital for your business.
Your business plan should include the following sections:
- Executive Summary – this section should summarize your entire business plan so readers can quickly understand the key details of your medical device business.
- Company Overview – this section tells the reader about the history of your medical device business and what type of medical device business you operate. For example, are you a manufacturer, distributor, retailer or service provider?
- Industry Analysis – here you will document key information about the medical device industry. Conduct market research and document how big the industry is and what trends are affecting it.
- Customer Analysis – in this section, you will document who your ideal or target customers are and their demographics. For example, how old are they? Where do they live? What do they find important when purchasing products like the ones you will offer?
- Competitive Analysis – here you will document the key direct and indirect competitors you will face and how you will build competitive advantage.
- Marketing Plan – your marketing plan should address the 4Ps: Product, Price, Promotions and Place.
- Product : Determine and document what products you will offer
- Prices : Document the prices of your products/services
- Place : Where will your business be located and how will that location help you increase sales?
- Promotions : What promotional methods will you use to attract customers to your medical device business? For example, you might decide to use pay-per-click advertising, public relations, search engine optimization and/or social media marketing.
- Operations Plan – here you will determine the key processes you will need to run your day-to-day operations. You will also determine your staffing needs. Finally, in this section of your plan, you will create a projected growth timeline showing the milestones you hope to achieve in the coming years.
- Management Team – this section details the background of your company’s management team.
- Financial Plan – finally, the financial plan answers questions including the following:
- What startup costs will you incur?
- How will your medical device business make money?
- What are your projected sales and expenses for the next five years?
- Do you need to raise funding to launch your business?
Finish Your Business Plan Today!
4. choose the legal structure for your medical device company.
Next you need to choose a legal structure for your medical device business and register it and your business name with the Secretary of State in each state where you operate your business.
Below are the five most common legal structures:
1) Sole proprietorship
A sole proprietorship is a business entity in which the owner of the medical device business and the business are the same legal person. The owner of a sole proprietorship is responsible for all debts and obligations of the business. There are no formalities required to establish a sole proprietorship, and it is easy to set up and operate. The main advantage of a sole proprietorship is that it is simple and inexpensive to establish. The main disadvantage is that the owner is liable for all debts and obligations of the business.
2) Partnerships
A partnership is a legal structure that is popular among small businesses. It is an agreement between two or more people who want to start a medical device business together. The partners share in the profits and losses of the business.
The advantages of a partnership are that it is easy to set up, and the partners share in the profits and losses of the business. The disadvantages of a partnership are that the partners are jointly liable for the debts of the business, and disagreements between partners can be difficult to resolve.
3) Limited Liability Company (LLC)
A limited liability company, or LLC, is a type of business entity that provides limited liability to its owners. This means that the owners of an LLC are not personally responsible for the debts and liabilities of the business. The advantages of an LLC for a medical device business include flexibility in management, pass-through taxation (avoids double taxation as explained below), and limited personal liability. The disadvantages of an LLC include lack of availability in some states and self-employment taxes.
4) C Corporation
A C Corporation is a business entity that is separate from its owners. It has its own tax ID and can have shareholders. The main advantage of a C Corporation for a medical device business is that it offers limited liability to its owners. This means that the owners are not personally responsible for the debts and liabilities of the business. The disadvantage is that C Corporations are subject to double taxation. This means that the corporation pays taxes on its profits, and the shareholders also pay taxes on their dividends.
5) S Corporation
An S Corporation is a type of corporation that provides its owners with limited liability protection and allows them to pass their business income through to their personal income tax returns, thus avoiding double taxation. There are several limitations on S Corporations including the number of shareholders they can have among others.
Once you register your medical device business, your state will send you your official “Articles of Incorporation.” You will need this among other documentation when establishing your banking account (see below). We recommend that you consult an attorney in determining which legal structure is best suited for your company.
Incorporate Your Business at the Guaranteed Lowest Price
We are proud to have partnered with Business Rocket to help you incorporate your business at the lowest price, guaranteed.
Not only does BusinessRocket have a 4.9 out of 5 rating on TrustPilot (with over 1,000 reviews) because of their amazing quality…but they also guarantee the most affordable incorporation packages and the fastest processing time in the industry.
5. Secure Startup Funding for Your Medical Device Company (If Needed)
In developing your medical device business plan , you might have determined that you need to raise funding to launch your business.
If so, the main sources of funding for a medical device business to consider are personal savings, family and friends, credit card financing, bank loans, crowdfunding and angel investors. Angel investors are individuals who provide capital to early-stage businesses. Angel investors typically will invest in a medical device business that they believe has high potential for growth.
6. Secure a Location for Your Company
Having the right space can be important for your medical device business, particularly if you’d like to meet clients there. You may need a place to store and assemble products, or you might need to rent office space. Additionally, if your product requires the use of large equipment, such as 3D printers or milling machines, it will be important for you to find a space large enough to accommodate these needs.
To find the right space, consider:
- Driving around to find the right areas while looking for “for lease” signs
- Contacting a commercial real estate agent
- Doing commercial real estate searches online
- Telling others about your needs and seeing if someone in your network has a connection that can help you find the right space
7. Register Your Medical Device Company with the IRS
Next, you need to register your business with the Internal Revenue Service (IRS) which will result in the IRS issuing you an Employer Identification Number (EIN).
Most banks will require you to have an EIN in order to open up an account. In addition, in order to hire employees, you will need an EIN since that is how the IRS tracks your payroll tax payments.
Note that if you are a sole proprietor without employees, you generally do not need to get an EIN. Rather, you would use your social security number (instead of your EIN) as your taxpayer identification number.
8. Open a Company Bank Account
It is important to establish a bank account in your medical device business’s name. This process is fairly simple and involves the following steps:
- Identify and contact the bank you want to use
- Gather and present the required documents (generally include your company’s Articles of Incorporation, driver’s license or passport, and proof of address)
- Complete the bank’s application form and provide all relevant information
- Meet with a banker to discuss your business needs and establish a relationship with them
9. Get a Company Credit Card
You should get a business credit card for your medical device business to help you separate personal and business expenses.
You can either apply for a business credit card through your bank or apply for one through a credit card company.
When you’re applying for a business credit card, you’ll need to provide some information about your business. This includes the name of your business, the address of your business, and the type of business you’re running. You’ll also need to provide some information about yourself, including your name, Social Security number, and date of birth.
Once you’ve been approved for a business credit card, you’ll be able to use it to make purchases for your business. You can also use it to build your credit history which could be very important in securing loans and getting credit lines for your business in the future.
10. Get the Required Company Licenses and Permits
The types of licenses and permits you need for your medical device business will vary depending on the state or jurisdiction you are doing business in. However, some of the most common licenses and permits you may need include:
Company License – A business license is generally required to operate a business in a particular jurisdiction. This license allows you to operate your business under a certain name and conduct certain activities.
Tax Identification Number – You will need to register with the IRS and get an Employer Identification Number (EIN). This number is used to track your payroll tax payments.
Professional License – If you are providing professional services as part of your medical device business, you may need to obtain a professional license. For example, if you are a doctor providing medical consultation services, you may need to obtain a medical license.
Health Department Permit – If your medical device business involves selling or distributing products that come into contact with human skin, you will likely need to obtain a health department permit from the relevant authority.
Nearly all states, counties and/or cities have license requirements including:
Zoning Approval : typically at the city or county level, this provides authorization for construction or use of a building or land for a particular purpose
Fire Department Approval : a process by which the local fire department reviews and approves the installation of a fire alarm system.
Depending on the type of medical device business you launch, you will have to obtain the necessary state, county and/or city licenses.
11. Get Company Insurance for Your Medical Device Company
Medical device businesses face a variety of risks that can potentially lead to costly losses. This is why it’s important for medical device businesses to have business insurance.
There are a variety of business insurance policies that medical device businesses can purchase, including:
Product Liability Insurance – This insurance policy provides coverage for damages that may be incurred as a result of a defective product.
General Liability Insurance – This insurance policy provides coverage for damages that may be incurred as a result of accidents or injuries that occur in your business.
Professional Liability Insurance – This insurance policy provides coverage for damages that may be incurred as a result of malpractice or negligence by your employees.
Find an insurance agent, tell them about your business and its needs, and they will recommend policies that fit those needs.
12. Buy or Lease the Right Medical Device Company Equipment
Medical device businesses need a variety of equipment in order to operate, including:
- Medical devices
- Test equipment
- Lab equipment
- Packaging and shipping supplies
- Office supplies
- Marketing materials
When purchasing or leasing medical device business equipment, it’s important to consider the following factors:
- What type of medical device business you have
- The size of your business
- Your budget
- The amount of space you have available for equipment
- The type of equipment you need
When purchasing medical device business equipment, it’s important to shop around and compare prices. You may also be able to get discounts by buying equipment in bulk or through a supplier. Leasing medical device business equipment can be a more affordable option than purchasing equipment outright, but it’s important to make sure you understand the terms of the lease agreement.
Some common pieces of medical device business equipment include:
Lab Equipment: This includes centrifuges, incubators, ovens and autoclaves.
Test Equipment : This includes pH meters, microscopes and spectrophotometer.
Packaging and Shipping Supplies : This includes boxes, packing material, labels and tape.
Office Supplies : This includes computers, printers, fax machines and office furniture.
Marketing Materials : This includes brochures, website design and trade show displays.
13. Develop Your Medical Device Company Marketing Materials
Marketing materials will be required to attract and retain customers to your medical device business.
The key marketing materials you will need are as follows:
- Logo : Spend some time developing a good logo for your medical device business. Your logo will be printed on company stationery, business cards, marketing materials and so forth. The right logo can increase customer trust and awareness of your brand.
- Website : Likewise, a professional medical device business website provides potential customers with information about the products you offer, your company’s history, and contact information. Importantly, remember that the look and feel of your website will affect how customers perceive you.
- Social Media Accounts : establish social media accounts in your company’s name. Accounts on Facebook, Twitter, LinkedIn and/or other social media networks will help customers and others find and interact with your medical device business.
14. Purchase and Setup the Software Needed to Run Your Medical Device Company
To run a medical device business, you will need accounting software to manage your finances, a CRM system to keep track of customer data, and a manufacturing or inventory management system to track your product line.
There are a variety of software programs available for each of these tasks, so it’s important to research the options and find the system that best suits your needs. Make sure to ask your software provider about any special requirements the software may have in order to run on a Mac or PC.
Research the software that best suits your needs, purchase it, and set it up.
15. Open for Company
You are now ready to open your medical device business. If you followed the steps above, you should be in a great position to build a successful business. Below are answers to frequently asked questions that might further help you.

How to Finish Your Medical Device Company Business Plan in 1 Day!
Don’t you wish there was a faster, easier way to finish your Medical Device Company business plan?
With Growthink’s Ultimate Business Plan Template you can finish your plan in just 8 hours or less!
How to Start a Medical Device Company FAQs
Where can i download a medical device business plan pdf.
You can download our medical device business plan PDF here. This is a business plan template you can use in PDF format.
How much does it cost to start a medical device business?
The cost of starting a medical device business can vary significantly, depending on the type of business, the products offered, and the location. The average startup costs for a medical device company vary. These costs include the cost of licensing and permits, research and development expenses, equipment costs, marketing costs, website design and hosting fees, personnel costs, and more.
In addition to these startup costs, you should also factor in ongoing operational expenses such as employee salaries and benefits; rent or lease payments.
How can I start a medical device business with no experience?
If you are starting a medical device business with no experience, it is important to do your research and learn as much as you can about the industry. There are a variety of resources available, including books, articles, and online courses.
Another important thing to keep in mind is that you don't need to know everything about the medical device industry to start a business. You can always partner with other companies or individuals who have more experience in this field.
Finally, make sure to contact your local Small Company Association (SBA) office for help and advice on starting a medical device business.
What type of medical device business is most profitable?
Some of the most profitable medical device businesses are those that offer innovative and cutting-edge products. These businesses often have higher research and development costs, but can also reap greater rewards if their products are successful.
Another factor that can affect the profitability of a medical device business is the size of the market it serves. Companies that serve a large market have the potential to be more profitable than those that serve a smaller market.
Is it hard to start a medical device business?
There are many resources available to help you get started.
However, if you follow the steps above, you should be able to start your medical device business without too much difficulty.
What are the ongoing expenses for a medical device business?
The ongoing expenses for a medical device business can vary significantly, depending on the type of business, the products offered, and the location. The average ongoing expenses for a medical device company range. This includes the cost of licensing and permits, research and development expenses, equipment costs, marketing costs, website design and hosting fees, personnel costs, and more.
In addition to these ongoing expenses, you should also factor in regular updates and upgrades to your products, as well as the cost of maintaining your inventory. You may also want to consider purchasing advertising or marketing materials, or investing in training or certification courses.
How does a medical device business make money?
There are a few different ways that medical device businesses can make money. One way is to sell the products that they develop to hospitals or other healthcare providers. Another way is to provide contract manufacturing services to other medical device companies. Medical device businesses can also make money by providing consulting services or licensing their patents and intellectual property.
Medical device businesses often have high research and development costs, which can be a major expense for the company. However, these businesses also have the potential to reap greater rewards if their products are successful. By selling their products to healthcare providers or providing contract manufacturing services, medical device businesses can generate steady income and profit over time.
A third way for medical device businesses to make money is by providing consulting services or licensing their patents and intellectual property.
Is owning a medical device business profitable?
Yes, owning a medical device business can be very profitable.
A medical device business owner can earn between $50,000 and $250,000 a year in profit. However, this depends on the size of the business and the type of products or services offered. Additionally, some medical device businesses may require significant upfront investments in equipment and personnel before they become profitable. The potential for success is also greatly affected by factors such as existing competition and market demand.
Some of the key things you can do to make your medical device business more profitable include:
- Invest in marketing and advertising to attract clients
- Offer products that are in high demand
- Provide quality products and services that meet or exceed your clients' expectations
- Keep your operating costs as low as possible
- Make sure you are registered with the FDA and other relevant agencies
- Maintain a good reputation in the industry
- Optimizing your website for SEO to increase online visibility
- Creating a unique selling proposition
- Building references from clients who are willing to recommend you to their friends
Why do medical device businesses fail?
There are a number of reasons why medical device businesses can fail, such as:
- Lack of capital – It can be expensive to start and run a medical device business, so it's important to have enough financial resources in place to sustain you during the early stages.
- Lack of experience – If you don't have the necessary experience or knowledge in the medical device industry, it will be difficult to succeed.
- Poor marketing – If you don't invest in marketing and advertising, you'll likely find it difficult to attract new clients.
One of the main reasons that medical device businesses fail is a lack of planning. This can include not having a detailed business plan, not doing research on the industry, and not targeting the right customers.
Another reason is a lack of marketing and sales skills. This can include not creating a sales process and not have a clear and strong value proposition.
The last main reason is a lack of financial management skills. This can include not having a realistic budget, not tracking expenses, and not investing in the business.
Who are key players in the medical device market?
The medical device market is made up of a variety of different players, including small businesses, large enterprises, and even individuals.
Some of the key players in the market include:
- GE Healthcare
- Stryker Corporation
- Philips Health
However, there are many other players in your specific target market, and it is important to research the market to identify the key players that may have the most direct influence on the success of your business.
How much should I charge for my medical device products?
Medical device fees can vary depending on the type of medical device products being offered.
However, some common medical device fees include:
- Heart Monitors - $300-$2,000
- CT Scanners - $20,000-$150,000
- Ultrasound Machines - $10,000-$50,000
- MRI Machines - $200,000+
The best way to determine the right fee for your medical device products is to research the rates of similar businesses in your industry, and to also consider the value that you will be providing to the client.
Other Helpful Business Plan Articles & Templates


More than a Quality Management System: Tools for the entire MedTech Lifecycle.
Featured Capabilities:
Experience the #1 QMS software for medical device companies first-hand. Click through an interactive demo.
Accelerate development with integrated design control and risk software.
Schedule a custom demo of Greenlight Guru Product now.
Data collection and management designed for MedTech clinical trials.
Get a personalized demo of Greenlight Guru Clinical today.
- By Initiative
- Migrating From Paper
- Managing and Assessing Risk
- Preparing for Regulatory Submissions
- Becoming Audit Ready
- Managing Postmarket Quality
- Small Business
- By Function
- Product / R&D
- ROI Calculator
- Customer Success
- Case Studies
- Checklists & Templates
- eBooks & Guides
- Content Hub
- Live & Virtual Events
- True Quality Roadshow
- Quality Pricing
- Clinical Pricing

How to Build a Medical Device Business Case in 5 Easy Steps

In business, so much of success depends on timing. A product launches too early and is misunderstood by the market; a product launches too late and becomes another face in the crowd.
The margins for error are already thin, and in the heavily competitive and regulated medical device industry they become almost nonexistent. So making the right decision isn’t something to take lightly.
That’s why the most agile companies use a medical device business case to make and present their decisions to company stakeholders, such as product teams, C-suite executives, or investors.
While there is much to consider when developing medical device business cases, thankfully it doesn’t have to be a shot in the dark. Let’s take a look at some foundational info about medical device business cases, and five easy steps to develop your own.
FREE DOWNLOAD: Click here to download your free PDF copy of our checklist for what medical device investors want to see.
What is a Medical Device Business Case?
When medtech organizations spot a potential market opportunity, they’ll often use a carefully constructed business case as a decision-making tool. When carefully constructed, the business case provides a framework to explore the pros, cons, and potential value for a proposed solution to an ongoing or forthcoming opportunity.
It should be noted that a business case is not the same as a business plan. A business plan is used to outline decisions at the organizational level, such as how and why a business should exist. Business cases, on the other hand, support product level decisions, such as how and why a new line of products should be developed as a solution to a specific market opportunity.
Business cases are a highly effective and efficient way to communicate a decision to stakeholders. This is largely thanks to its format, in which each step is built atop insights gleaned from the one preceding it, providing the audience with the necessary context as to why the decision should be made.
However, this iterative process places much more emphasis on the research—faulty data means inaccurate insights and conclusions. So, when working through these five steps for building a medical device business case, be sure to dedicate significant time and attention to each step.
You’ll also want to consider the following medical device business case best practices:
Rely heavily on input from subject matter experts (SMEs) and end users
Keep risk and regulations top of mind
Insights should always be grounded in data
Stay realistic about your team’s capabilities and time
Customize your presentation to your audience
Step 1: Define the Current Situation or Opportunity
Key Questions to Answer:
Who is the customer? Who is our customer’s customer?
What is our problem or opportunity?
How do I best characterize the current situation?
What is the current cost of the situation?
In this first step, you’ll need to clearly articulate the situation, whether it’s a problem to be solved or an opportunity to be leveraged. Try to keep your focus centered on the impact and needs of the customer — and that means all customers, external and internal.
Keep in mind that all companies are unique, with their own culture, mission, and values. A solution that fits with one company may be a nonstarter with another. Be sure you’re validating your definition of the opportunity or problem with management.
In order to keep track of so many internal and external pain points, speak with as many people as possible — this means talking to different departments and subject matter experts like doctors and surgeons, and device users, if possible.
Step 2: Develop Success Criteria
What is the value proposition?
What are the benefits and timing of value creation?
What will it look like if we implement the plan?
Another critical definition in a business case is how you’ll determine whether the solution is successful. To do so, you’ll need to find ways to quantify the value to the business once the opportunity is realized or the problem is solved.
Organizing your thoughts around this issue can be tough, especially when they feel fairly nebulous. A good strategy to ground your thinking is to set parameters like timeframe, profit/loss amounts, or budget restrictions. You may find that a situation is more or less dire than you anticipated, and therefore requires a different approach altogether.
You’ll be looking at various KPIs that impact various departments and areas of business. When doing so, don’t forget to prioritize the areas that matter most. As with the previous step, validate the criteria with all department heads, especially those in finance and marketing.
Step 3: Research Alternatives
What alternatives do we have?
How do we deliver the benefits to our customer
What is the cost of doing nothing?
What relationships do I need to consider?
Great solutions don’t happen without great alternatives. Why? Because great decision making depends on you to be unbiased and properly informed. When we enter the process with a predetermined solution in mind, it’s easy to slip the blinders on and ignore other, more potentially valuable alternatives.
Of course, just as every business is unique, so too are the scenarios and protected impacts of alternative solutions. You’ll also need to refer back to the success criteria outlined in the previous step. These will help you prioritize the KPIs that matter most to your business.
For instance, an acquisition option of a rival technology may have a shorter timeframe but it also has high upfront costs. Developing a “me-too” product may take longer, but has potentially lower costs in the long run. There may even be hybrid options which combine multiple alternatives. The point here is to ensure you fully explore the spectrum of choices for your business case.
Step 4: Analyze and Select a Path Forward
What key resources are needed to implement the plan?
What activities are most important and what is the timing?
How do we quantify the financial impact to the company?
Now comes the hardest part of decision-making: deciding. No one wants to end up making the wrong choice, especially when it could result in lower quality medical devices , increased risk for your patients, or the loss of jobs for your internal department. Even for the most confident individuals, that’s a heavy load to bear.
Selecting a path forward should focus on the following areas:
Financial impact
Personnel impact
Resources Needed
External relationships required
Any changes to key activities of the business
For medical device industry veterans, it’s a good chance to adopt a risk-based approach . When considering potential impacts, be as specific as possible — the extent of these impacts may not be apparent on a surface level.
You may even tap your QA/RA department for assistance with the risk management approaches laid out in ISO 14971 , or using SWOT techniques to identify strengths, weaknesses, opportunities, and threats.
Step 5: Present the Plan
What is most important to convey?
What is best left for the appendix?
Who is my audience and what is the most effective way to present my case?
All the work you’ve put in has led to this moment: a carefully constructed argument that’s communicated clearly. But even with all the analysis, conversations, and ruminating, a poorly prepared presentation could cause the stakeholders to lose faith in your plan..
Your primary focus should be on the why of the business case, and that means demonstrating value to the business. Don’t get muddled down in the weeds of methodology, or in-depth statistical analysis; save extensive data for an appendix provided to attendees. Instead, highlight the basics, and bridge the gap from the current situation to the vision that was cast.
Finally, consider your audience. What are they like? How do they communicate? How many people are involved in approval? How familiar are they with the current scenario? All of these factors should help you in creating a presentation that actually sells them on the idea without feeling like you’re selling anything at all.
Understand the impact of every decision with Greenlight Guru
It’s clear that making decisions isn’t something that’s done in a single moment. These are choices that will have a major impact on all areas of your business and the quality of life for all users. So, while you’re considering your next move, here’s another question: Can you leverage your eQMS platform to ensure you’re making an informed decision?
Greenlight Guru helps your entire team achieve closed-loop traceability by allowing you to efficiently navigate through your quality system, making connections and understanding the relationships within your QMS and how they affect your business.
Contact us today for your free, personalized demo of Greenlight Guru!
Looking for an all-in-one QMS solution to advance the success of your in-market devices and integrates your quality processes with product development efforts? Click here to take a quick tour of Greenlight Guru's Medical Device QMS software →
Etienne Nichols
Etienne Nichols is a Medical Device Guru and Mechanical Engineer who loves learning and teaching how systems work together. He has both manufacturing and product development experience, even aiding in the development of combination drug-delivery devices, from startup to Fortune 500 companies and holds a Project...
Related Posts
Paper systems are the riskiest way to manage medical device projects, making the business case for an eqms solution for your company, 7 tips to attract investors and raise funds for your medical device, subscribe to our blog.
Join 200,000+ other medical device professionals outperforming their peers.

Get your free resource
What medical device investors want to see.
- Checklists/Templates
- Request a Demo
- Content Title Description
Business Plan for Medical Innovation
A robust business plan provides a clear roadmap to achieve commercial success and clearly articulates a lifescience technology’s value proposition and projected adoption trajectory.
A compelling business plan is the foundation to achieve durable differentiation by outlining for investors and the company alike the pathway for an innovation to realize forecasts, capture market share and maximize ROI. Companies with a carefully constructed business plan are more likely to achieve their targets within the projected budget as the steps are clearly outlined. Clear market analysis with the anticipated target market ensures that the innovation under development will meet or even anticipate market needs. Confirmation of market needs is an iterative process, as market trends evolve with competitive product offerings and technology advancements.
Early stage angel, venture capital and corporate investors alike pursue medical technology innovators with a clear, thoroughly developed strategy that fully considers market potential, the competitive opportunity, and prepares for anticipated potential hurdles to adoption. Furthermore, a company’s business plan should be regularly updated to reflect changing markets, product lines and company objectives over time.
Essential to guiding a successful company and product launch, a well-defined Business Plan includes market segmentation, competitive positioning and development of a robust medical device business model with assumption-driven financial projections. Realistic capital needs are identified per outlined development requirements. Metrics provided by an outline of a sustainable growth strategy with well-defined milestones can further help insure follow-on funding for the medical device startup.
The Strategy Inc. Business Plan is essential for:
- Medtech developers with a novel technology seeking funding
- Lifescience companies preparing for exit
- Entities attempting to prioritize opportunities, technologies or target clinical applications and determine those with the highest potential ROI
- Companies seeking a commercialization road map
- Startups and medical device innovators building their go-to-market strategy
- OUS entities developing a strategy for US market entry
- Established companies seeking to redefine company goals, targets or strategy to achieve
A Strategy Inc. Business Plan is a comprehensive assessment that incorporates a broad scope of global business analyses:
- The Market Assessment defines the potential for commercial success within each of the identified target markets
- The Competitive Analysis facilitates the identification and assessment of a potential market space, product positioning and feature set identification for a selected product
- The Voice of the Customer determines projected clinical adoption based on one-on-one interviews with global opinion leaders and in-the-trenches physicians
- The Risk Analysis outlines the most direct path to successful commercialization while mitigating the known and potential risks of product development
- The Financial Valuation includes a ProForma with five to ten-year revenue projections based on a risk adjusted bottoms-up sales analysis which includes a defensible projected product adoption, a pricing matrix and projected cost of goods forecasts
Strategy Inc. offers a range of financial services to facilitate fund raising activities at the highest valuation, identify acquisition targets and outline exit strategy.
Medical Design and Outsourcing
Starting a medical device company: everything you need to know
June 27, 2016 By Rogene Evans

Once you’ve got some scratch, you’ll need to think about outsourcing product design and development and prototyping services.
All set? Now you need to think about getting your device on the market. Once you’ve gotten that figured, you should figure out how to keep your FDA or CE Mark approval through your quality systems.
And you still need to get paid, right? You need to build a reimbursement strategy into your plan.
Finally, to protect the intellectual property underlying all of this effort, you need to understand U.S. patent law.
Raising capital in a VC-light environment
Venture capital hasn’t completely deserted the medical device sector, but most analysts agree that VCs are simply not as interested in medtech as they are in other sectors like biotech. Medtech attracted less than $5 billion in 2014, only 5.9% of all U.S. venture dollars that year, according to the Ernst & Young Pulse of the Industry report.
And it’s startups that are feeling the pressure most deeply. VC backing of earlier-stage medtechs now makes up a smaller share of a smaller pie “due to the retreat of several stalwart medtech-focused VCs at a time when corporate venture investors have yet to fill the gap,” according to the report. Seed, Series A and Series B rounds dropped 8% in 2014-2015 from fiscal 2013-2014 and made up only 29% of medtech venture investments in 2014-15.
Think small
Although VC has diminished, other funding sources are out there – the biggest change is that they’re just much smaller:
• Angel investors – Often individuals with deep pockets and an interest in a specific disease or condition. Get busy networking with as many high-net-worth people as you can. • Private placement – Use a private placement – the sale of securities to a relatively small number of select investors – to fund your vision by raising money from individual donors. • Incubators – Public, private or academic incubators offer subsidized business accommodation, academic support and business mentoring, and connections with resources for prototyping, testing and clinical trials. • Entrepreneurial competitions – Universities or large companies, hoping to attract technology partners, often offer technology contests with sometimes significant payouts in cash and in-kind services. • Crowdfunding – Most effective after the early development phase, when the funds are needed for validation or prototyping. The same high risk and high capital needs medical technology that made VCs chary of medical technology make the crowd a less optimal source of cash. • City, state, & county resources – Local governments often use tax breaks and other incentives to draw entrepreneurs to development zones. And your area likely has technical associations formed to boost the industry, some with their own tech incubators. Trade associations offer access to universities or hospitals for clinical testing and connections to local angel investors and competitions. They can also provide opportunities for networking and mentorship.
As Aum Cardiovascular CEO Marie Johnson puts it, “They don’t give you money because they’re in love with the story.” (Johnson raised $5 million for Aum via private placement.) The best advice for any medical device startup is that today, no medtech investment pitch, no matter the source receiving it, succeeds without compelling clinical date, a clear unmet and demonstrable cost savings over current treatments.
Are you ready for an outsourcer? Here’s how to find out.
Outsourcing your product development doesn’t have to mean losing control of your technology. Although it’s one of the biggest decisions a startup will make, working with a contract supplier can deliver unexpected benefits.
Gary Boseck, VP of technical operations at Vention Medical, says there are ways for startups to prepare for the challenges that come with working with an outsourcing partner.
And partnership, he says, is really the key word. “CMOs want their clients to succeed. They have a vested interest in helping develop a technology that has potential in the market and their expertise can contribute to the likelihood of success.”
Getting a trusted CMO involved early in the process could even have some unexpected benefits. Boseck says some CMOs will provide funding for their most promising startup clients. Others hold contests to attract the best startup technologies and assist in developing those platforms for the market.
Readiness is all
It’s not an easy road from ideation to production, Boseck notes. “As the saying goes, ‘If it were easy, someone would have already done it.’” That’s precisely the point: Contract manufacturers have done it, and they’re willing and able to help startups do it as well.
There are a few questions medtech startups should ask themselves when considering whether to look outside the company for product development:
• Can you provide well-defined and stable product requirements? Although adjustments are expected and often necessary, keep in mind that mission creep can kill deadlines, Boseck notes. • Do you have clear priorities? Whatever the challenges, your end goals should be very well defined. • Can you provide timely feedback to the team? Manufacturing can’t take place in a vacuum. Startups, which often have limited staff, should commit to having a dedicated liaison with their CMO partner. • Will you actively engage with the CMO development team? This might be as easy as getting a team member on site frequently. • Do you understand the development process? If not, ask more questions. • What are the terms? Make sure IP and ownership of the work product is well defined.
Another important aspect of engaging with a CMO is the selection process, often a rigorous and challenging one, Boseck says. Some CMOs actively try to entice startups, but just because they claim to be experts “they may not meet your specific requirements,” he cautions. Boseck advises budding medtech entrepreneurs to evaluate CMOs based on the following criteria:
• Their expertise matches the project need. This should encompass design expertise, clinical familiarity, and component and assembly experience. • The CMO offers the full spectrum of needed services. These should include concept ideation and prototyping, clinical production and scalable commercial production. • The CMO is accessible and responsible. This is the due diligence portion of the analysis. You’ll need to talk to a variety of clients, and conduct some online research, just to start. A good CMO has a reputation for building good working relationships, with an emphasis on trust and transparency.
Quality and compliance for startups: What you can ignore and what you can’t
Quality systems might be the last thing on your startup’s mind, as other activities take precedence. But it’s not a good idea to overlook the development of your quality system at the beginning.
“I think it’s a good idea to put process and product planning in place right from the start, simply because once things get going, they get going fast,” notes Timothy Lozier, director of marketing for ETQ. “You might be too worried about R&D, product and supply chain, and then market approval, to think about compliance. But it will come to you, and you don’t want to get caught off-guard.”
“Establish a quality management system that suits the company size and expected growth,” adds Christine Santagate, client solutions advisor with Regulatory Quality & Solutions. “Make sure that it’s something that the current staff can manage and maintain. When a company institutes a [quality management system] that’s too large, they set themselves up for failure to comply with their own system.”
Implementing and maintaining a QMS is a crucial part of regulatory compliance. The medical device quality system is primarily concerned with production and post-production. FDA 21 CFR Part 820 defines the quality regulations for the U.S. market. Otherwise, ISO 13485 can be used to build a quality system for global markets.
Although it’s time-consuming and expensive, establishing a total quality management system need not be as challenging as it sounds. The key is to build the system as you develop, focusing on the relevant aspects of quality and ignoring the others until they’re needed.
“You have to find something you can scale with,” says Lozier. “There are systems out there that let you start off small-scale and simple (yet effective), but as you grow you can grow the solution with your business.”
Startups should begin building their quality and compliance programs during the development phase, he advises, by focusing on design controls, risk management, document control and record management, and supplier management.
Design controls, essential for a QMS, can also help with the design process by capturing key aspects of development to prove your product meets user needs and is safe and effective. Likewise, risk management works with design controls to create documents and records throughout product development, to demonstrate that you’ve considered the risks and are doing something about them. Document management is the process that helps those pieces of the puzzle stay together.
“The concept of planning and gaining control over the process is that as you grow, you’ll always have them to lean on,” Lozier explains. “People can follow a process, but if it’s not documented, then you run into issues.”
Lozier emphasizes centralizing documentation, process and product planning.
“Too often, companies take that first step towards documenting their processes, but it resides in spreadsheets, file systems, and other, more decentralized, often manual methods. Having a system that’s able to manage and track these things, as well as serve as a central resource, is important to ensuring consistency in the operation. One source of the truth outweighs the risks associated with errant copies floating around the operation.”
Lozier cites tools that offer free or scalable methods to handle compliance events, issue actions, and launch corrective & preventive actions (CAPAs). ETQ, for example, offers traqpath, a free download, and VERSE, a cloud-based quality management system that brings in document control/training and CAPA. In addition, both tools have a supplier component to them, which can help startups send actions and CAPAs to suppliers through secure external assignments.
Many vendors have programs that are specifically designed to help companies scale compliance.
“These aren’t expensive tools, but are built with startups in mind,” says Lozier. “The benefit is that if you’re a startup and you’re looking to just put the pieces in place, you can do that for a low cost to your business, or even for free. That way, you’re not ignoring it, or factoring in major investment on something you may not need now.”
Committing assets to compliance is a “future-proofing” investment, he says. “You’re going to have to meet the compliance standards and regulations as you grow, and, just like documenting your processes and controlling and tracking your quality and compliance operations, you don’t want to be caught in growth mode and playing catch-up.”
How to plan your regulatory strategy
The regulatory burden for startup medical device businesses might be the most rigorous of any industry, and with good reason – making products for implantation or use in the human body requires hurdling a pretty high safety and efficacy bar.
So building a regulatory strategy from the very beginning is crucial, and there some key considerations every medtech startup should keep in mind, according to Christine Santagate, client solutions advisor at Regulatory Quality & Solutions.
First off, Santagate advises, startups should remember to budget for the testing and related costs associated with regulatory processes.
“The regulatory strategy is really an extension of the overall business plan and the costs associated should roll up into the overall budget. Unfortunately, testing and registration costs are sometimes overlooked when creating a business plan,” she explains. “The testing times and costs of sterilization validation, environmental testing, aging, biocompatibility and possibly clinical trials are sometimes not fully understood, as the initial focus is usually on technology and not on the entire life cycle.”
Another early consideration is where to pursue approval first. The relative ease of obtaining a CE Mark in Europe compared with the FDA’s more stringent requirements has been an attraction in the past, but Santagate says that’s changing.
“It used to be easier to gain approval in Europe, but things are changing quickly. With the updated ISO 13485 and pending IVDR updates, notified bodies [in Europe] are going to have their hands full,” she says. “Small startups should focus on gaining approval in the country that allows the most opportunity and is manageable and sustainable for their small staff.”
If the U.S. is the first target for commercialization, planning a detailed approach ahead of time helps determine which pathway to pursue, depending on the type of device being developed. It can help to find a trusted partner with the expertise in this area, to develop a regulatory strategy up front and identify potential paths to market – and its associated risks.
“This strategy will assess applicable FDA regulations, device classification options, potential predicate device and product claims, indications and contra-indication options, and potential regulatory risks based on the company’s marketing claims, product requirements, risk analysis, etc.,” Santagate notes. “The advantage in working with a consulting group is that they stay up to date on all regulation changes. A group like R&Q has 80+ full time consultants with experience spanning nearly all FDA device classes and they can provide an experienced, independent review.”
If your device is Class III, requiring the FDA’s most-stringent pre-market approval path, establishing a relationship with the agency and keeping the lines open should be your primary considerations.
“Make sure that indications are clear and supported and that any study design is robust and appropriate data points are collected to support the submission,” Santagate advises. “It’s all in the preparation – this is a long process and up-front focus and attention to detail will be well worth the effort.”
Santagate says a sound regulatory strategy can be an unexpected benefit for startups on the funding trail.
“Potential investors are able to see that the company has a planned path forward, based on risk. It provides an additional layer of preparedness and overall understanding of the full funding requirements – and how those funds will be used,” she says.
Reimbursement tops the ‘must-do’ list
Five years ago, if you asked a medical device executive about their top worry, regulatory – specifically, the cost and uncertainty around winning favor with the FDA – nearly always came first.
Today, those same executives are more likely to cite reimbursement as their top issue. Driven by the declining number of physicians owing their own practices – 70% in 2002, compared with about 25% in 2011, according to the U.S. Medical Group Management Assn. – and the resulting shift to group purchasing organizations, reimbursement has vaulted to the top of the list of must-do items for new medical device concerns.
“Reimbursement comes up in just about every discussion I’ve ever had with investors,” says David Rosa, the former president & CEO of Sunshine Heart in Eden Prairie, Minn. “Today, they’re all looking to de-risk their investment, and they all want to know, up front, the likelihood of reimbursement.”
“The hardest questions you’re going to get are on the reimbursement side,” adds Preceptis Medical president & CEO Steve Anderson. “It goes to the heart of everything we’ve been doing. Five years ago, we would’ve said, ‘Oh, reimbursement, I don’t need to worry about that.’ But this is the big issue today. It’s a big issue, and a big opportunity.
“It really is all about the money. The first real money we spent was on an outside analysis on reimbursement strategy. So it was fundamental to everything we’ve done,” Anderson says.
In fact, Minneapolis-based Preceptis chose to develop its therapy to help children with hearing problems in part because of its relatively simple reimbursement path, he adds.
Bob Thompson, president of Gahanna, Ohio-based Comprehensive Reimbursement Solutions, says the key is targeting a truly unmet need.
“There’s a lot of opportunity out there,” Thompson says. “If you direct your products toward unmet medical needs, you’ll see the benefits – particularly if you deal with the quality and cost-control issues that hospitals and payers are feeling.”
The challenges vary by country, so it’s important to become familiar with the ins and outs of local reimbursement policies in your target markets before you start spending on product development. One challenge common to most markets is the gap between reimbursement rates and the actual value of the technology. In many countries, rates are set using cost-based formulae by device or procedure type.
Because winning reimbursement hinges on clinical data, startups should plot their reimbursement strategy in parallel with their clinical and regulatory plans. That’s because collecting Class I data from randomly controlled trials and post-approval Class II data is key to a favorable review from a payer. Startups should also have a strategy for disseminating that data to peer-reviewed publications and at medical conferences once it’s collected.
In the U.S., an increasing number of payers are exploring value-based reimbursement models, not least the Centers for Medicare & Medicaid Services. Last July Medicare announced a program to bundle reimbursement payments for hip and knee replacement procedures, saying it wants to “hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries.”
Startups that can demonstrate that their devices both improve outcomes and can be reimbursed based on that improvement stand a better chance of winning a favorable decision from payers.
February 1, 2018 at 9:00 am
Nice write up.
My question is, whether does a medical start-up needs to be ISO13485 or just their contract supplier needs to be?
If the company is already ISO9001 certified but intends to diverse into a medical device design company (but gets contract manufacturers to manufacture and control the quality), is the ISO9001 good enough? Pls advise. Thanks.
February 21, 2018 at 5:07 pm
Good question, Raymond. I don’t have the answer. Any readers able to help Raymond on this?
Related Articles Read More >

E Tech Group secures new investment from Graham Partners

Wytech buys Silvertip & Associates to launch Minneapolis grinding division

eXeX uses Apple Vision Pro to manage reverse total shoulder arthroplasty

4 megatrends driving the future of medtech
Search medical design & outsourcing.
- Mergers & Acquisitions
- Cardiovascular
- Implantables
- Medical Equipment
- Supplies and Components Index
- Contract Manufacturing
- Electronics
- Motion Control
- Prototyping
- Medtech Events in 2024
- Subscribe to Print Magazine
- DeviceTalks Tuesdays
- Digital Editions
- Manufacturer Search
- Medical Device Handbook
- MedTech 100 Index
- Print Subscription
- The 2023 Big 100
- Webinars / Digital Events
- Whitepapers
- 2023 Leadership
- 2022 Leaders
- 2021 Leaders
- Women in Medtech

- Medical Device business plan
The medical device field has several unique aspects that are important to address when writing a business plan; for example, clinical & regulatory paths, (including clinical trials, FDA & CE approval) and medical insurance refunds.
Below is a table of contents, for example:
Executive Summary
1. Overview of the business concept
2. Brief description about the industry / market
3. Brief description about the products, strategies, & competition
4. A summary of what is needed to do in order to reach the target (e.g regulatory approvals)
5. Business Model: How are you going to make money?
6. Financial plan – How much capital has been drafted and how much capital is required in the current round?
7. Management’s CV
* This should not be more than three or four pages. You may wish to send the executive summary before sending the full business plan to generate more interest.
Condition Insights
1. Background on the problem/ illness ; What are the current possibilities for diagnostics/ therapy; characterization of the target audience
Market size and opportunity
1. Describe the market
2. Market size in numbers & geographic description (US / world)
3. Competitors, their market share (if applicable), strengths & weaknesses , and prices
4. Distribution channels & the sale process
5. Current reimbursement for expenses
Product and Production Processes of the Company
1. A detailed description of the product and a brief description on future development
2. How “far along” product development is and does the company have proof of the feasibility
4. A clear description of why the product is special, which problem it solves, who will buy it & why, and what prevents others from developing the same product
5. If possible, present a specific economic report that shows value (e.g customer manufacturing efficiency)
6. What changes will the customer/distributor/health provider have to make in order to adopt the product, and how are you going to ease this behavioral transition
Clinical and Regulatory
1. Which regulatory approvals are needed (FDA, CE, PMPA, etc…)
2. Timetable
3. The nature of the clinical trial, an estimated number of participants, the time window followed by the experimenters, and the necessary end points in order to get a regulatory approval
Intellectual Property
1. Patents filed & their status, etc.
2. Licensing agreements & royalties, etc.
3. Is there a similar patent? If so, how are we different?
Key Personnel
1. The management team & their CVs: relevant past experience, and previous successes (include names of companies)
2. Which key positions are still unmanned and are there any possible candidates?
3. Scientific Advisory Committee, Board of Directors, and other key partners (e.g lawyers, accountants, etc.)
Current Status of the Project and Investment needs
1. The company’s achievements to date (including product development & trials, etc.)
2. The amount of investment required and use of funds
3. The financial history and current investors (capitalization table showing how much each investor holds %, including options for employees)
4. Key milestones that will be achieved until the next fund raising round
5. How much money will be required in order to reach a stage where the company is self-sustaining?
Financial projections
1. Projected revenues as a % of the market
2. Projected income and expenses of the Company with the investment required
3. The expected pricing of products
Footnotes and references
1. Relevant clinical articles
2. Renderings or photos of the product
3. References to relevant information used for various forecast
for startup valuation

Do you have a brand on Amazon? You can make an exit!
Background: Ecommerce has had a staggering rise since internet broke into our lives. Many people switched to online shopping and companies such as Amazon, eBay,

Revealing the 3 top myths on successful startups
Why success is independent of your business idea, product quality, or your capital? Every year, 350 million new startups are established. 90% of these startups

What is unit economics and why it’s important?
In the early phases of a startup, decision making guided by data is the key to securing the necessary information in order to understand the

The 5 Phases of your business life cycle, and in which step are you at?
Once the decision of building a business is set, you enter the business life cycle. This cycle will bring you from the idea stage of
Tel Aviv Menachem Begin road 154 Floor: 3 Zipcode: 2065246
Phone : +972-1-700-707-404 Email: [email protected]
- Investors Zone
- Business Plan
- A business plan for performance improvement
- Business plan for a Blockchain product
- Business plan for a LegalTech product
- Business Plan for a Social Platform
- Business Plan for a Travel Tech Product
- Business Plan for an Information Security Cyber Product
- Business Plan for online platforms
- Business Plan for the Fintech Product
- Consumer Product Business Plan
- Mobile App Business Plan
- Software product business plan (SaaS, Freeware, shareware)
- Business Plan for a Cannabis Product
Business Services
- Building a Team
- MVP Definition
- Preparation for a meeting with an investor
- Feasibility test
- Business Consulting
- Business Model
- Go-to-Market
- Investor’s Presentation
- One Pager for Investor
- From Idea to Startup
- Start-up Valuation
Financing Solutions
- Bank Financing
- Crowd-Funding
- Grants For Technological Projects – R&D in Early Stages
- Grants For Technological Projects – Tnufa
- Private Investors – Angels
- Technological Incubators
- Venture Capital Funds
- Fund Raising
Targo Consulting © all rights reserved

Medical Device Development Business Plan [Sample Template]
By: Author Tony Martins Ajaero
Home » Business Plans » Medical and Healthcare
Are you about starting a medical device development company? If YES, here is a complete sample medical device development business plan template & feasibility report you can use for FREE .
Okay, so we have considered all the requirements for starting a medical device development company. We also took it further by analyzing and drafting a sample medical device development marketing plan template backed up by actionable guerrilla marketing ideas for medical device development companies. So let’s proceed to the business planning section .
In order to become a business player in the healthcare industry, you don’t necessarily need to become a medical doctor, a pharmacist or a nurse. If you are interested in the healthcare industry, one of the many businesses that you can successfully start is a medical device development business ; it is a business that is promising and profitable in the united states of America.
The Medical device development business basically develops and manufactures various devices that are used in the healthcare industry. Just like most businesses, the medical device development business is open to as many people that are interested in the industry as long as they have the required experience and qualifications.
If you are sure this type of business is what you truly want to do after you must have conducted your feasibility studies and market research, then the next step to follow is to write a good business plan.
The truth is that it is one thing to have a fantastic idea cum business plan, and another thing for the business plan to translate to money (profits), and that is why it is important to assemble a team of dedicated staff to work with you if you want to be successful in your business.
Below is a sample medical device development business plan template that will help you successfully write yours with little effort.
A Sample Medical Device Development Business Plan Template
1. industry overview.
Medical device development business is under the Medical Device manufacturing industry and players in this industry include manufacturers of electromedical and electrotherapeutic apparatuses such as magnetic resonance imaging equipment, medical ultrasound equipment, pacemakers, hearing aids, electrocardiographs and electromedical endoscopic equipment.
The industry also engages in the manufacturing irradiation apparatus and tubes for applications such as medical diagnostic, medical therapeutic, industrial, research, and scientific evaluation among others. The Medical Device manufacturing industry revenue has increased steadily due to demand for effective and efficient devices. Rising product prices have also supported industry growth.
Revenue is projected to rise over the five years to 2022 as healthcare providers continue to invest in new equipment to keep up with growing demand for healthcare services. New product development by medical device manufacturers will also contribute to industry growth by offering new solutions to health issues.
The Medical Device Manufacturing industry is indeed a thriving line of business in most countries of the world. In the United States of America, the industry generates over $43 billion annually from more than 993 medical device development and manufacturing companies scattered all around the United States of America.
The industry is responsible for the employment of over 94,106 people. Experts project that the industry will grow at a 1.0 percent annual rate between 2012 and 2017. The establishments in this industry that has dominant market shares in the United States of America are Abbott Laboratories, General Electric Company, Johnson & Johnson and Medtronic.
A recent report published by IBISWORLD shows that the Medical Device Manufacturing industry has a moderately low level of capital intensity; for every $1.00 an average industry company spends on labor, they invest $0.06 into capital improvements.
Although the cost of assets can be high for industry operators, not all products can be manufactured on an automated production line.
The report further stated that medical device manufacturers produce a range of products for highly specialized applications, and the production of many of these devices requires hands-on work, which keeps industry labor costs high.
Skilled specialists are needed to research and develop medical devices, and these employees command salaries well above the manufacturing sector’s average wage.
Over and above, medical device development and manufacturing business is a profitable business and it is open for any aspiring entrepreneur to come in and establish his or her business; you can choose to start on a small scale manufacturing limited devices or you can choose to start on a large scale manufacturing various medical devices not just for the US market, but for international market.
2. Executive Summary
Eden® Medical Device Development Company, Inc. is a registered medical device development and manufacturing business that will be located in one of the industrial estates in Dover – Delaware, United States of America. We have been able to lease a facility that is big enough to fit into the design of the kind of standard medical device Development Company that we intend launching.
Eden® Medical Device Development Company, Inc. will design, develop and manufacture medical devices; we will engage in fabricating medical diagnostic and therapeutic apparatus, marketing and distribution. We are set to sell our products to a wide range of clientele not just in the United States of America, but also all across the world.
We are aware that there are several large and small medical device development companies, which is why we spent time and resources to conduct a thorough feasibility studies and market survey so as to be well positioned to favorably compete with all our competitors.
We will ensure that we engage in continuous improvement of our products so as to suite the ever – changing trends in the healthcare industry .
Eden® Medical Device Development Company, Inc. will ensure that all our customers are given first class treatment whenever they purchase any of our products. We have a CRM software that will enable us manage a one on one relationship with our customers no matter how large the number of our customer base may grow to.
We will ensure that we get our customers involved in the design and manufacturing of medical devices and also when making some business decisions.
Eden® Medical Device Development Company, Inc. will at all times demonstrate her commitment to sustainability, both individually and as a firm, by actively participating in our communities and integrating sustainable business practices wherever possible.
We will ensure that we hold ourselves accountable to the highest standards by meeting our customers’ needs precisely and completely whenever they patronize our products.
Eden® Medical Device Development Company, Inc. is a family business that is owned by Engr. David Eden and his immediate family members. Engr. David Eden has a B.Engr. in Electronic Electrical Engineering, with well over 5 years of experience in the medical devices development industry, working for some of the leading brands in the United States.
Although the business is launching out with just one outlet in Dover – Delaware, but we will ensure that we market and distribute our products all across the United States of America and other countries of the world.
3. Our Products and Services
Eden® Medical Device Development Company, Inc. is in the medical devices development industry to sell our products to a wide range of clients and of course to make profits, which is why we will ensure we go all the way to design, develop and manufacture various medical equipment.
We will ensure that we do all that is permitted by the law of the United States to achieve our aim and ambition of starting the business. Our product offerings are listed below;
- Neuromodulation and spinal devices
- Cardiovascular devices
- Diabetes devices
- Irradiation devices
- Patient recovery and noninvasive devices
- Surgical instruments
- Dental instruments
- Hospital beds and other specialized hospital furniture
- Personal safety equipment
4. Our Mission and Vision Statement
- Our vision is to become one of the leading brands in the medical device development industry in the United States of America and Canada.
- Our mission is to establish a world – class medical device development company that will manufacture a wide range of medical devices and build a brand that will become a household name not just in the United States of America, but also other parts of the world.
Our Business Structure
Eden® Medical Device Development Company, Inc. knows the standards expected from companies that are into the manufacturing of medical devices hence the need for us to conform to industrial best practices. We will ensure that we hire people that are qualified, honest, customer centric and are ready to work to help us build a prosperous business that will benefit all our stake holders.
As a matter of fact, profit-sharing arrangement will be made available to all our senior management staff and it will be based on their performance for a period of ten years or more. In view of that, we have decided to hire qualified and competent hands to occupy the following positions;
- Chief Executive Officer (Owner)
- Production Manager
- Human Resources and Amin Manager
- Engineers (Production Engineers)
Sales and Marketing Manager
- Accountants/Cashiers
- Customer Services Executive
5. Job Roles and Responsibilities
Chief Executive Officer – CEO:
- Increases management’s effectiveness by recruiting, selecting, orienting, training, coaching, counseling, and disciplining managers; communicating values, strategies, and objectives; assigning accountabilities; planning, monitoring, and appraising job results; developing incentives; developing a climate for offering information and opinions; providing educational opportunities.
- Responsible for fixing prices and signing business deals
- Responsible for signing checks and documents on behalf of the company
- Evaluates the success of the organization
- Responsible for providing direction for the business
- Reports to the board
Admin and HR Manager
- Responsible for overseeing the smooth running of HR and administrative tasks for the organization
- Sustains office supplies by checking stocks; placing and expediting orders; evaluating new products
- Ensures operation of equipment by completing preventive maintenance requirements; calling for repairs
- Updates job knowledge by participating in educational opportunities; reading professional publications; maintaining personal networks; participating in professional organizations.
- Improves department and organization reputation by accepting ownership for accomplishing new and different requests; exploring opportunities to add value to job accomplishments.
- Outlines job positions for recruitment and managing interviewing process
- Carries out staff induction for new team members
- Responsible for training, evaluation and assessment of employees
- Responsible for arranging travel, meetings and appointments
- Oversee the smooth running of the daily office activities
Production Manager:
- In charge of approving designs for the organization
- Responsible for managing the daily activities in the production facility
- Ensure that the production facility is in top shape and goods are properly arranged and easy to locate
- Interfaces with third-party suppliers (vendors)
- Controls medical equipment and instrument manufacturing, distribution and supply chain inventory
- Supervises the workforce in the production facility
- Manages external research and coordinate all the internal sources of information to retain the organizations’ best customers and attract new ones
- Models demographic information and analyze the volumes of transactional data generated by customer purchases
- Identifies, prioritizes, and reaches out to new partners, and business opportunities et al
- Identifies development opportunities; follows up on development leads and contacts; participates in the structuring and financing of projects; assures the completion of development projects
- Responsible for supervising implementation, advocate for the customer’s needs, and communicate with clients
- Develops, executes and evaluates new plans for expanding increase sales
- Documents all customer contact and information
- Represent the company in strategic meetings
- Aids the increase sales and growth for the company
Accountant/Cashier:
- Responsible for preparing financial reports, budgets, and financial statements for the organization
- Provides managements with financial analyses, development budgets, and accounting reports; analyzes financial feasibility for the most complex proposed projects; conducts market research to forecast trends and business conditions
- Responsible for financial forecasting and risks analysis
- Performs cash management, general ledger accounting, and financial reporting
- Responsible for developing and managing financial systems and policies
- Responsible for administering payrolls
- Ensuring compliance with taxation legislation
- Handles all financial transactions for the organization
- Serves as internal auditor for the organization
Medical Devices Production Engineers
- Responsible for designing, fabricating, developing and manufacturing medical devices such as Neuromodulation and spinal devices, Cardiovascular devices, Diabetes devices, Irradiation devices, noninvasive devices, Surgical appliances, Surgical instruments, Dental instruments, Hospital beds and other specialized hospital furniture, and Personal safety equipment
- Responsible for the manufacturing electromedical and electrotherapeutic apparatuses such as magnetic resonance imaging equipment, medical ultrasound equipment, pacemakers, hearing aids, electrocardiographs and electromedical endoscopic equipment
Client Service Executive
- Ensures that all contacts with clients (e-mail, walk-In center, SMS or phone) provides the client with a personalized customer service experience of the highest level
- Through interaction with customers on the phone, uses every opportunity to build client’s interest in the company’s products and services
- Manages administrative duties assigned by the human resources and admin manager in an effective and timely manner
- Consistently stays abreast of any new information on the organizations’ products, promotional campaigns etc. to ensure accurate and helpful information is supplied to customers when they make enquiries
- Enquires about customers’ needs, recommend, select and help locate the right merchandise, describe a product’s features and benefits.
- make suggestions and encourage purchase of products
- Provides information about warranties, manufacturing specifications, care and maintenance of merchandise and delivery options
6. SWOT Analysis
Eden® Medical Device Development Company, Inc. is in business to become one of the leading medical devices development companies in the United States of America and we are fully aware that it will take the right business concept, management and organization structure to achieve our goal.
We are quite aware that there are several medical device development companies all over the United States of America and even in the same location where we intend locating ours, which is why we are following the due process of establishing a business. We know that if a proper SWOT analysis is conducted for our business, we will be able to position our business to maximize our strength, leverage on the opportunities that will be available to us, mitigate our risks and be welled equipped to confront our threats.
Eden® Medical Device Development Company, Inc. employed the services of an expert HR and Business Analyst with bias in manufacturing to help us conduct a thorough SWOT analysis and to help us create a Business model that will help us achieve our business goals and objectives.
This is the summary of the SWOT analysis that was conducted for Eden® Medical Device Development Company, Inc.
Our strength lies in the quality of our finished medical devices, the power of our team and the state of the art medical device production plant that we own. We have a team of highly trained and experienced engineers and support staff that can go all the way to design and produce top notch medical devices. We are well positioned in the heart of Dover – Delaware and we know we will attract loads of clients from the first day we open our medical device development company for business.
Our major weakness is that we are a new medical devices development and manufacturing company and we don’t have the financial capacity to compete with multi – billion dollars medical device development companies such as Abbott Laboratories, General Electric Company, Johnson & Johnson and Medtronic et al when it comes to manufacturing and selling medical devices at a rock bottom prices. So also, we may not have enough cash reserve to promote our brand the way we would want to do.
- Opportunities:
Our well-equipped medical device development company provides us with unlimited opportunities to sell our products to a large number of hospitals and health facilities. We have been able to conduct thorough feasibility studies and market survey and we know what our potential clients will be looking for when they visit our show room and production plant; we are well positioned to take on the opportunities that will come our way.
Just like any other business, one of the major threats that we are likely going to face is economic downturn. It is a fact that economic downturn affects purchasing/spending power. Another threat that may likely confront us is the arrival of a new medical device development company in same location where ours is located. So also, unfavorable government policies may also pose a threat for businesses such as ours.
7. MARKET ANALYSIS
- Market Trends
If you keep tabs with happening in the medical devices manufacturing industry, you will agree that technological advances, the legislative expansion of healthcare access and the improving economy have stimulated demand for medical devices over the last five years, and the aging US population has further contributed to revenue growth due to the high incidence of health issues requiring medical devices among the elderly.
A report published by IBISWorld shows that industry revenue will grow going forward. Over the five years to 2022, this growth is expected to continue, with revenue increasing at a slightly faster rate. The aging baby-boomer population and technological developments will continue to bolster industry growth, but the changing regulatory environment will likely hamper profitability.
So also, technological advances, expansion of healthcare and the improving economy have stimulated demand. Industry operators often have the weak hand in price negotiations and recent healthcare legislation has created a degree of uncertainty for medical device companies.
Lastly, as part of marketing strategies, medical device development companies now ensure that they have showrooms at different locations where they display their products. As a matter of fact, it is even cheaper to purchase directly from these showrooms established by medical devices development companies as against purchasing from medical device retail stores. It is a strategy that helps them increase sales and income for their business.
8. Our Target Market
We have positioned our medical device development and manufacturing company to service businesses in the healthcare industry in and around Dover – Delaware and every other location where our show rooms will be located all over key cities in the United States of America and other countries of the world.
We have conducted our market research and feasibility studies and we have ideas of what our target market would be expecting from us. We are in business to design, develop and manufacture a wide range of medical equipment and instruments for the following customers;
- Medical laboratories
- Medical colleges
- Dental clinics
- Optical centers
Our competitive advantage
Eden® Medical Device Development Company, Inc. is launching a standard medical device development and manufacturing company that will become the preferred choice for hospitals and other healthcare facilities in the United States of America and other countries of the world. Our production plant is located in very good locations.
One thing is certain; we will ensure that we design, develop and manufacture a wide range of medical equipment and instruments that are in high demand in the healthcare industry. We will also go all the way to ensure that we work with some of our high – profile clients in designing, developing and manufacturing of customized medical devices that suits their business.
Lastly, our employees will be well taken care of, and their welfare package will be among the best within our category in the industry meaning that they will be more than willing to build the business with us and help deliver our set goals and achieve all our aims and objectives. We will also give good working conditions and commissions to freelance sales agents that we will recruit from time to time.
9. SALES AND MARKETING STRATEGY
- Sources of Income
Eden® Medical Device Development Company, Inc. is in business to design, develop and manufacture a wide range of medical equipment and instruments. We are in the medical device development industry to maximize profits and we are going to go all the way out to ensure that we achieve our business goals and objectives.
In essence, our source of income will be the supplying and selling of a wide range of medical equipment and instruments at affordable prices . Eden® Medical Device Development Company, Inc. will generate income by selling the following products;
10. Sales Forecast
We are set to go into the designing, developing and manufacturing of various types of medical equipment and instruments and we have put plans in in place that will help us always attract customers cum sales and that will sure translate to increase in revenue generation for the business.
We are well positioned to take on the available market in the United States of America and we are quite optimistic that we will meet our set target of generating enough income/profits from the first six months of operation and grow the business and our clientele base.
We have been able to critically examine the medical device development industry and we have analyzed our chances in the industry and we have been able to come up with the following sales forecast. The sales projection is based on information gathered on the field and some assumptions that are peculiar to startups in Dover – Delaware.
- First Fiscal Year: $750,000
- Second Fiscal Year: $1.2 million
- Third Fiscal Year: $3 Million
N.B : This projection is done based on what is obtainable in the industry and with the assumption that there won’t be any major economic meltdown and there won’t be any major competitor manufacturing same medical equipment and instruments as we do within the same location. Please note that the above projection might be lower and at the same time it might be higher.
- Marketing Strategy and Sales Strategy
Before choosing a location for Eden® Medical Device Development Company, Inc. we conducted a thorough market survey and feasibility studies in order for us to be able to penetrate the available market and become the preferred choice for hospitals and healthcare facilities in and around Dover – Delaware. We have detailed information and data that we were able to utilize to structure our business to attract the number of customer we want to attract per time and the number of products to be sold monthly.
We hired experts who have good understanding of the medical device development and manufacturing industry to help us develop marketing strategies that will help us achieve our business goal of winning a larger percentage of the available market in the United States of America.
In other to continue to be in business and grow, we must continue to sell the medical equipment and instruments that we developed and manufacture which is why we will go all out to empower or sales and marketing team to deliver.
In summary, Eden® Medical Device Development Company, Inc. will adopt the following sales and marketing approach to win customers over;
- Open our medical device development plant in a grand style with a party
- Introduce our business by sending introductory letters alongside our brochure to hospitals, dental clinics, optical centers , medical laboratories and key stake holders in the United States of America
- Ensure that we design, develop and manufacture a wide range of medical equipment and instruments needed in the healthcare sector
- Make use of attractive hand bills to create awareness and also to give direction to our show rooms
- Position our signage/flexi banners at strategic places around Dover – Delaware
- Position our greeters to welcome and direct potential customers
- Create a loyalty plan that will enable us reward our regular customers
- List our business and products on yellow pages ad (local directories)
- Leverage on the internet to promote our business
- Engage in direct marketing and sales
- Encourage the use of Word of mouth marketing (referrals)
- Join local chambers of commerce and industries to network and market our products
11. Publicity and Advertising Strategy
Despite the fact that our medical device development plant is well located, we will still go ahead to intensify publicity for the business. We are going to explore all available means to promote our medical devices development company.
Eden® Medical Device Development Company, Inc. has a long-term plan of exporting our medical devices to other countries of the world and to become a household name in the healthcare industry which is why we will deliberately build our brand to be well accepted in Dover – Delaware before venturing out. As a matter of fact, our publicity and advertising strategy is not solely for winning customers over but to effectively communicate our brand.
Here are the platforms we intend leveraging on to promote and advertise Eden® Medical Device Development Company, Inc.;
- Place adverts on community based newspapers, radio stations and TV stations
- Encourage the use of word of mouth publicity from our loyal customers
- Leverage on the internet and social media platforms like YouTube, Instagram, Facebook, Twitter, LinkedIn, Snapchat, Google+ and other platforms to promote our business.
- Ensure that our we position our banners and billboards in strategic positions all around Dover – Delaware
- Distribute our fliers and handbills in target areas in and around our neighborhood
- Contact hospitals, dental clinics, optical center, medical laboratories and other health facilities by calling them up and informing them of Eden® Medical Device Development Company, Inc. and the products we sell
- Advertise our medical equipment and instruments in our official website and employ strategies that will help us pull traffic to the site
- Brand all our official cars and vans and ensure that all our staff members and management staff wear our branded shirt or cap at regular intervals
12. Our Pricing Strategy
Aside from quality, pricing is one of the key factors that gives leverage to players in the medical device development and manufacturing industry, it is normal for customers to go to places where they can get quality medical equipment and instruments at cheaper price which is why big players in the medical device development and manufacturing industry like Abbott Laboratories, General Electric Company, Johnson & Johnson and Medtronic et al will attract loads of corporate and individual clients.
We know we don’t have the capacity to compete with the market leaders in the industry, but we will ensure that the prices and quality of all the medical equipment and instruments that we develop are competitive with what is obtainable amongst medical device development companies within our level.
- Payment Options
The payment policy adopted by Eden® Medical Device Development Company, Inc. is all inclusive because we are aware that different customers prefer different payment options as it suits them but at the same time, we will ensure that we abide by the financial rules and regulation of the United States of America.
Here are the payment options that Eden® Medical Device Development Company, Inc. will make available to her clients;
- Payment via bank transfer
- Payment with cash
- Payment via credit cards/Point of Sale Machines (POS Machines)
- Payment via online bank transfer
- Payment via check
- Payment via mobile money transfer
- Payment via bank draft
In view of the above, we have chosen banking platforms that will enable our client make payment for all purchases without any stress on their part. Our bank account numbers will be made available on our website and promotional materials.
13. Startup Expenditure (Budget)
In setting up any business, the amount or cost will depend on the approach and scale you want to undertake. If you intend to go big by renting/leasing a big facility, then you would need a good amount of capital as you would need to ensure that your employees are well taken care of, and that your facility is conducive enough for workers to be creative and productive.
The tools and equipment that will be used are nearly the same cost everywhere, and any difference in prices would be minimal and can be overlooked. As for the detailed cost analysis for starting a medical device development and manufacturing business; it might differ in other countries due to the value of their money.
These are the key areas where we will spend our startup capital on;
- The total fee for registering the business in the United States of America – $750
- Legal expenses for obtaining licenses and permits as well as the accounting services (software, P.O.S machines and other software) – $3,300
- Marketing promotion expenses for the grand opening of Eden® Medical Device Development Company, Inc. in the amount of $3,500 and as well as flyer printing (2,000 flyers at $0.04 per copy) for the total amount of $3,580
- The total cost for hiring Business Consultant – $2,500
- The total cost for payment of insurance policy covers (general liability, workers’ compensation and property casualty) coverage at a total premium – $9,400
- The total cost for long – term leasing of a standard warehouse and showroom – $250,000
- The total cost for remodeling the production facility and showroom – $20,000
- Other start-up expenses including stationery ( $500 ) and phone and utility deposits – ( $2,500 )
- Operational cost for the first 3 months (salaries of employees, payments of bills et al) – $60,000
- The total cost for Start-up inventory (purchase of medical device making tools and equipment and the purchase of raw materials inclusive) – $250,000
- The total cost for store equipment (cash register, security, ventilation, signage) – $13,750
- The total cost for the purchase and installation of CCTVs – $10,000
- The cost for the purchase of office furniture and gadgets (Computers, Printers, Telephone, TVs, Sound System, tables and chairs et al) – $4,000
- The total cost of launching a Website – $600
- The total cost for our opening party – $7,000
- Miscellaneous – $10,000
We would need an estimate of $950,000 to successfully set up our medical devices development plant in Dover – Delaware.
Generating Startup Capital for Eden® Medical Device Development Company, Inc.
Eden® Medical Device Development Company, Inc. is a private registered business that is solely owned and financed by Engr. David Eden and his immediate family members. They do not intend to welcome any external business partner which is why he has decided to restrict the sourcing of the startup capital to 3 major sources.
- Generate part of the startup capital from personal savings
- Source for soft loans from family members and friends
- Apply for loan from my Bank
N.B: We have been able to generate about $350,000 ( Personal savings $200,000 and soft loan from family members $150,000 ) and we are at the final stages of obtaining a loan facility of $600,000 from our bank. All the papers and documents have been signed and submitted, the loan has been approved and any moment from now our account will be credited with the amount.
14. Sustainability and Expansion Strategy
The future of a business lies in the number of loyal customers that they have, the capacity and competence of the employees, their investment strategy and the business structure. If all of these factors are missing from a business, then it won’t be too long before the business close shop.
One of our major goals of starting Eden® Medical Device Development Company, Inc. is to build a business that will survive off its own cash flow without the need for injecting finance from external sources once the business is officially running.
We know that one of the ways of gaining approval and winning customers over is to retail our wide range of quality medical equipment and instrument a little bit cheaper than what is obtainable in the market and we are well prepared to survive on lower profit margin for a while.
Eden® Medical Device Development Company, Inc. will make sure that the right foundation, structures and processes are put in place to ensure that our staff welfare are well taken of. Our company’s corporate culture is designed to drive our business to greater heights and training and re – training of our workforce is at the top burner.
We know that if that is put in place, we will be able to successfully hire and retain the best hands we can get in the industry; they will be more committed to help us build the business of our dreams.
Check List/Milestone
- Business Name Availability Check: Completed
- Business Registration: Completed
- Opening of Corporate Bank Accounts: Completed
- Securing Point of Sales (POS) Machines: Completed
- Opening Mobile Money Accounts: Completed
- Opening Online Payment Platforms: Completed
- Application and Obtaining Tax Payer’s ID: In Progress
- Application for business license and permit: Completed
- Purchase of Insurance for the Business: Completed
- Leasing of facility and remodeling the factory: In Progress
- Conducting Feasibility Studies: Completed
- Generating capital from family members: Completed
- Applications for Loan from the bank: In Progress
- Writing of Business Plan: Completed
- Drafting of Employee’s Handbook: Completed
- Drafting of Contract Documents and other relevant Legal Documents: In Progress
- Design of The Company’s Logo: Completed
- Graphic Designs and Printing of Promotional Materials: In Progress
- Recruitment of employees: In Progress
- Purchase of the needed machines, tools, furniture, racks, shelves, computers, electronic appliances, office appliances and CCTV: In progress
- Creating Official Website for the Company: In Progress
- Creating Awareness for the business both online and around the community: In Progress
- Health and Safety and Fire Safety Arrangement (License): Secured
- Opening party/launching party planning: In Progress
- Compilation of our list of products that will be available in our store: Completed
- Establishing business relationship with vendors: In Progress
Related Posts:
- Concierge Medicine Business Plan [Sample Template]
- Private Ambulance Service Business Plan [Sample Template]
- Dental Handpiece Repair Business Plan [Sample Template]
- Maple Syrup Production Business Plan [Sample Template]
- DNA Testing Business Plan [Sample Template]

Medical Device Business Plan Template [Updated 2024]
Medical Device Business Plan Template
If you want to start a medical device business or expand your current medical device business, you need a business plan.
The following Medical Device business plan template gives you the key elements to include in a winning Medical Device business plan.
You can download our Business Plan Template (including a full, customizable financial model) to your computer here.
Below are links to each of the key sections of your Medical Device business plan: I. Executive Summary II. Company Overview III. Industry Analysis IV. Customer Analysis V. Competitive Analysis VI. Marketing Plan VII. Operations Plan VIII. Management Team IX. Financial Plan
Comments are closed.
Medical Device Business Plan Home I. Executive Summary II. Company Overview III. Industry Analysis IV. Customer Analysis V. Competitive Analysis VI. Marketing Plan VII. Operations Plan VIII. Management Team IX. Financial Plan

Don't bother with copy and paste.
Get this complete sample business plan as a free text document.
Medical Software Business Plan
Start your own medical software business plan
AgaMatrix, Inc.
Executive summary executive summary is a brief introduction to your business plan. it describes your business, the problem that it solves, your target market, and financial highlights.">.
Overview AgaMatrix is a development stage venture based in Boston offering proprietary Digital Signal Processing (DSP) technology that dramatically improves the functionality and performance of biosensor devices. AgaMatrix’s core DSP algorithms solve a number of immediate problems in the medical devices market by significantly boosting the performance of biosensors without costly specialized hardware and additional chemicals. Initially, AgaMatrix will sell to medical device makers, specifically, home blood glucose monitors and hospital point-of-care blood analyzers. AgaMatrix anticipates achieving positive cash flow by year three with future target healthcare segments to include the large immunoassay and implantable biosensor sectors; as well as other vertical industries that heavily rely on biosensors, such as the military chemical agent detection, environmental air/water quality monitoring, and industrial processing sectors.
Problem – Glucose Monitors Are Burdensome, Painful To Use Many diabetic patients fail to use home blood glucose devices as prescribed because the regimen is too burdensome or too physically painful. Four to seven times a day, a patient must puncture his or her finger to draw blood onto a test strip for insertion into the glucose biosensor. The average compliance rate for testing is less than 1.5 times a day, resulting in the acceleration of complications caused by diabetes, such as blindness, stroke, and heart and kidney failure. In fact, diabetes is the leading cause of blindness in individuals aged 20-74 and better glucose monitoring compliance is the single biggest key to prevention. Device makers have identified the physical pain of using existing devices as the root cause of non-compliance, and they are seeking ways to reduce the sample size required by their devices. AgaMatrix technology will enable less invasive drawing mechanisms to meet the overwhelming demand for less painful alternatives.
Problems in the hospital blood analyzer market are more related to the lack of the comprehensiveness and accuracy of the devices, which results in reduced adoption levels. AgaMatrix’s value proposition to this market is very clear: devices that are more accurate and sensitive will stand a higher chance of being more readily adopted.
A Software Solution for a Hardware Problem Historically, the biosensor device industry has attempted to overcome problems related to accuracy, sensitivity, and robustness by enhancing the chemical (hardware) aspects of the devices, such as the biological and chemical design of their sensors. By contrast, AgaMatrix is pioneering a software approach based on digital signal processing (DSP) algorithms that has a number of distinct practical advantages, including lower cost, easier/faster upgrade capability, and complementarity with respect to a wide variety of chemistry/hardware-based biosensor technologies.
AgaMatrix’s solution, consisting of a suite of software modules, enables new functionality and dramatically improves the performance of biosensor devices. Performance improvements include the ability to leverage increases in signal-to-noise ratio to reduce blood sample requirements. For the professional healthcare market, AgaMatrix offers the ability to improve the overall accuracy and sensitivity of hospital point-of-care analyzers. Boosting accuracy removes a major roadblock hindering widespread adoption of portable blood analyzers in place of conventional laboratory equipment.
Software DSP solutions have been vital to the success of many other industries where physical limitations would have impaired their growth. For example, in the 1980s, makers of CD players relied on oversampling and error-correction algorithms to compensate for low quality hardware filters and to overcome disk-skipping problems. AgaMatrix’s algorithms provide analogous solutions in the biosensor space.
Business Model – Software Licensing and Royalty Fees from Device Makers Initially, AgaMatrix will operate as a technology licensing company, deriving royalty revenue streams based on device makers’ consumables sales (i.e., disposable test strips and cartridges used in the devices). Revenues will be acquired from the sale of the technology to home blood glucose device makers, hospital point-of-care blood analyzer makers, and minimally invasive and implantable blood glucose biosensor developers.
Therasense – an Illustration of How Disruptable the Glucose Market Is Just a few years ago, the blood glucose market was dominated by four major players (numbers represent annual revenues from test strips): Roche ($1.27B), J & J ($1.09B), Bayer ($650M), and Abbot ($450M). These companies have been around since the 1980’s. Therasense (THER) was founded in 1996, rolled out their first product in June 2000, and leveraged their key differentiator (very similar to what AgaMatrix is offering): the ability to reduce blood sample volume to make glucose testing less painful. In the span of less than two years since their product roll-out, they have achieved $200 million in annual revenues, gone public, and now have a market capitalization of over $800 million. Bottom line: this is a market that is very open to new technological entrants, especially when they are able to reduce pain for the user.
Competitive Advantages There are no direct competitors pursuing our highly unique and proprietary approach, developed over the past seven years by our world-class scientific team. AgaMatrix technology will be complementary to potential indirect competition from the in-house laboratories of major medical device makers. The sustainable competitive advantages that AgaMatrix commands include:
- Superior software paradigm, complementary to chemical (hardware) advances in biosensors.
- Expertise developed over the course of seven years of biosensor research.
- Monopolization of the scientific team responsible for the original paradigm innovation.
- Development lead time of at least two years over potential competition.
- Intellectual property strategy involving two core utility patents (filed) and three defensive utility patents.
Customer Traction We have approached two blood glucose monitor makers and one hospital point-of-care device manufacturer as potential customer targets. There are over 20 other major potential target companies we have not yet approached. The following is a summary of the current status of the companies we have reached:
- Strong interest to partner from two blood glucose monitor companies (discussions with Presidents); details are confidential at this point, but we believe we will be able to close a deal by June 2002.
- Strong interest from a leading blood glucose monitor maker (J & J – discussions with Director-level staff) and the leading hospital point-of-care device maker (i-STAT – discussions with Vice President and Director-level staff).
The Team A current team composed of:
- The three leading scientists pioneering the use of digital signal processing to improve biosensor technology, with an aggregate of over 40 years of direct DSP/biosensor research experience.
- Entrepreneurs who have founded, built and run an enterprise software company.
- An expanding board of veteran advisors made up of medical doctors who have healthcare business experience.
- An additional technical team of three committed to joining the company post-seed financing, composed of engineers from MIT and Tufts, with technical management experience and an aggregate of over 25 years of commercial engineering experience.
Financing AgaMatrix has been self-funded by the principals of the company since its founding. The company recently closed a seed round of $500K from a number of healthcare angel investors and IncTANK, an early stage venture capital fund. A Series A round is expected in four to five months of approximately $1 million.
Brought to you by
Create a professional business plan
Using ai and step-by-step instructions.
Secure funding
Validate ideas
Build a strategy
- A world-class scientific team consisting of Dr. Sridhar Iyengar (CTO), Dr. Justin Gooding, and Dr. Ian Harding, the engineering team, and an aggressive business team with start-up and management experience.
- Technology applicable to a number of other vertical markets and protected by a rigorous IP strategy.
- External validation from existing relationships with potential customers and advancement to final rounds in a number of national business plan competitions.
1.1 Mission
AgaMatrix develops solutions to power next-generation biological and chemical sensor systems. The value that AgaMatrix delivers to this market is the ability to dramatically improve the accuracy, sensitivity, and robustness of a range of different sensors for the purpose of making medical diagnostic devices more effective. AgaMatrix’s technology enables the development of devices that will be essentially painless to patients and that will meet the demand for better accuracy in medical diagnostics. It is committed to providing software solutions for a critical hardware problem that affects millions of diabetic patients and hospital patients worldwide.
1.2 Objectives
- Develop technology solutions that will increase the adoption and compliance rates of diagnostic medical devices by improving the functionality and performance of biosensors, specifically for home blood glucose monitors and hospital point-of-care blood analyzers.
- Achieve positive cash flow by year three.
- Reach $50 million in annual revenues by year four.
- Expand into other industries that heavily rely on biosensors, including industrial processing, environmental monitoring, and military sectors.
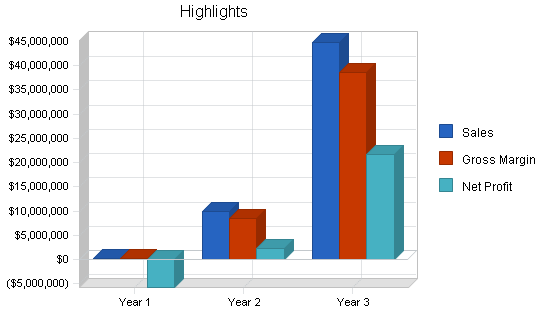
Company Summary company overview ) is an overview of the most important points about your company—your history, management team, location, mission statement and legal structure.">
AgaMatrix, Inc., which was incorporated in Delaware in 2001, is an early-stage venture offering proprietary Digital Signal Processing (DSP) technology that dramatically improves the functionality and performance of biosensor devices. AgaMatrix’s core DSP algorithms solve a number of immediate problems in the medical devices market by significantly boosting the performance of biosensors without costly specialized hardware and additional chemicals. Initially, the company will sell technology solutions to diagnostic medical device makers, specifically, manufacturers of home blood glucose monitors and hospital point-of-care blood analyzers. Future target healthcare segments include the immunoassay and implantable biosensor sectors. Its headquarters are located in Cambridge, Massachusetts.
2.1 Start-up Summary
AgaMatrix’s start-up costs amount to $4,900, which covers the initial expenses for opening the first office. These costs include incorporation of the company, design of the company logo and website, purchase of office and IT equipment, and other miscellaneous expenses. The start-up costs are financed by direct owner investment. The assumptions are detailed in the following table and illustration.

2.2 Company Ownership
AgaMatrix is a privately-held Delaware corporation, subchapter C. It was created in 2001. Sonny Vu and Sridhar Iyengar, the company’s founders, own the majority of equity. Members of the board of directors and advisors also hold minority stock positions. All employees of the company are rewarded with stock compensation packages.
AgaMatrix will develop a set of software products that provides critically needed diagnostic functionality to current and next-generation medical biosensors. Optimized for computational efficiency, they are designed to be easily incorporated into a number of leading biosensor platforms. All products that we develop will be powered by our core DSP algorithms with certain features configured and optimized for the relevant applications. Our algorithms will be delivered in a format that is convenient and useful to our customers; as such, each AgaMatrix Product Suite will consist of a core DSP engine supplemented with integration tools, application-specific expansion modules, and professional services. Since our initial product focus is OEM technology, we will work closely with our customers and partners in the development and deployment of our products. The core DSP algorithms will be encoded as a platform technology in modular components that can be rapidly configured as needed for various customers’ applications.
Initially, we are marketing one product for the glucose biosensor market and one product for the hospital POC market:
Suite Name: AccuMatrix
- Target Customer: Smaller Home Glucose Biosensor Manufacturers
- Why Needed: These customers need a method of suppressing interference from other known chemicals in the blood (vitamin C, Tylenol, and uric acid) that can react at the biosensor and give erroneous readings. They need to be able to suppress these interferences without the use of chemical mediators or expensive membranes.
Suite Name: PosiMatrix
- Target Customer: Hospital Point-of-Care Manufacturers
- Why Needed: These customers need a method of alerting the user when unknown chemicals in the blood interfere with the biosensor and give false positives. E.g., the FDA regularly approves new anesthetic gases that are used in surgery; however, the POC makers cannot keep up with these new “unknown” chemicals that may be present in the patient’s blood and can interfere with the device. For one POC maker, it is “medically imperative” for them to avoid reporting false readings; thus, they need a method of identifying when the reading is corrupted by unknown chemical interference so that they may indicate to the user that the reading is not valid.
The core DSP algorithm engine contains all the needed functionality. Using a Configuration Tool, we can rapidly integrate the appropriate Data Modules that are appropriate for the target customer. These Modules contain a library of information that is needed to configure and optimize the core algorithms for the chemicals that are relevant to the customer’s device. Once configured, the algorithms will be delivered in the appropriate software or ASIC-design version for the target device and can be deployed with our Integration Tools by our Professional Services Deployment Team or by the customer’s engineering team. The basic components of each AgaMatrix Suite will include the following:
Core Engine Core DSP technology software and firmware code base will consist of a major portion of the algorithms that AgaMatrix develops. These algorithms will be activated as needed for each customer’s requirements by the Configuration Tools.
Data Modules These are libraries that contain empirical data needed to optimize the core DSP engine for the detection of different chemicals in various operating environments. The Configuration Tools will in part use the data from these Data Modules to customize the software for customers’ various products. We will initially offer the following 2 modules:
- Blood Glucose Data Module: Library of empirical information of the standard chemicals that are commonly encountered in blood glucose measurements. This data is used to optimize the core algorithms for detection of glucose and suppression of common interfering chemicals.
- Point-of-Care Data Module(s): Library of empirical information of the standard chemicals that are commonly encountered in various blood chemistry measurements routinely performed by point-of-care devices. This data is used to optimize the core algorithms for detection of each of the relevant chemicals and suppression of common interfering chemicals.
- Implantables Data Module: Library of empirical information on quantities that are relevant to implantable glucose sensors. This data is used to optimize the core algorithms to correct for sensor deterioration (fouling) effects and suppress signals from interfering chemicals while boosting the signal from glucose.
- Immunoassay Data Module(s): Library of empirical information of the standard chemicals that are commonly encountered in relevant immunoassays. Immunoassay Data Modules will be developed for each immunoassay that is of interest to the customer. This data is used to optimize the core algorithms for detection of the relevant chemicals for the given immunoassay.
Configuration Tools Configuration tools are the front-end interface of the software. This set of tools will allow for the rapid optimization and configuration of the core algorithms for various functionalities and chemicals. These tools are used to select which Data Modules and algorithms are needed for the customer’s application and generate the end product, which can be delivered either as software/firmware for the target device or be delivered in the form of an ASIC design.
Code Integration Tools This set of tools facilitates the integration and customization of software and firmware code base into customers’ products. These tools may be used by our Professional Services Deployment Team when integrating the product into the customers’ end-device, or they may be used by the customer’s in-house engineering teams themselves. Initially, we will include:
- Software Integration Tools: Tools that facilitate the integration of our technology onto microprocessor-based devices.
- Firmware Integration Tools: Tools that facilitate the integration of our technology onto firmware-based devices.
Technology and Development Tutorials These are in-depth, easy-to-use online tutorials consisting of scientific and engineering guides to help quickly bring a development and integration team up to speed on AgaMatrix’s DSP technology. The tutorials will consist of code examples, customization, and integration tutorials.
Professional Support Services Package A set of professional services, including software/firmware development and QA/QC testing, designed to assist in supporting the use and maintenance of the AgaMatrix Product Suites for customers and partners.
Based on initial discussions with potential customers, we believe that we can deliver our product in a format that will be readily usable by their development and integration teams. We will use established processes analogous to those used in the deployment of enterprise software solutions where a Deployment Team will assist the customer in the integration of our product into their devices, as indicated by the professional services component of our product offering.
Market Analysis Summary how to do a market analysis for your business plan.">
Medical diagnostics has the greatest existing opportunity from an industry size perspective as well as the degree of match between existing needs and AgaMatrix’s technology capability. The sub-segments in this market that the AgaMatrix product line is addressing in the short term (within the next two to three years) are the large, high-margin consumer blood glucose monitor market and the now quickly growing hospital point-of-care device market, i.e., customers are makers of these devices. Even by a conservative estimate, the value proposed by AgaMatrix to the glucose market alone would be enough to sustain a viable standalone venture. However, given the minimal incremental effort that would be needed, we will deliver the product to both sub-markets for the benefit of augmenting and diversifying our revenue streams.
The market that we are concerned with consists of advancing medical devices and technologies that allow healthcare professionals and home users to acquire medical diagnostic data such as blood glucose levels (e.g., for diabetics) and various other blood chemistry data (e.g., for emergency care situations) instantly, easily, cheaply, and accurately without having to send blood samples to centralized lab facilities which have longer turnaround times and are more costly. The conclusion that this market should be the company’s initial focus is substantiated by the fact that it has all the relevant characteristics of a market we found to be desirable. These characteristics are discussed below:
- Large existing, robust, high-growth market.
- Converging market forces sustaining industry growth.
- Clear, immediate need for benefits provided by technology.
- Technology delivered would be strongly positioned to participate in emerging trends.
In the medium and long term (three to four years from now), AgaMatrix aims to address needs in the emerging electrochemical immunoassay and implantable biosensor markets.
The Home Blood Glucose Monitoring is the largest, immediately addressable market, over $4.1 billion in size today and growing 13% CAGR. Based on preliminary discussions with several potential customers, AgaMatrix believes that a significant portion of this market can eventually be captured. We expect device makers to pass on the modest costs of AgaMatrix’s technology through the high margins currently enjoyed by the consumable reagents (test strips) they sell. While AgaMatrix technology does not directly improve the test strips themselves, potential customers will incorporate the cost of such technology as part of the total solution cost; development costs of their test devices are already paid for in this way. Diabetics generally are not price sensitive to test strips since insurance usually covers the costs of the strips.
4.1 Market Segmentation
Home Blood Glucose Monitors AgaMatrix will initially target home blood glucose monitoring device makers. One primary dimension along which these device makers compete is the reduction of pain and discomfort from testing that involves pricking fingers to extract blood. By improving device sensitivity, AgaMatrix allows blood glucose device OEMs to reduce the required blood sample size enabling the use of less painful blood extraction mechanisms, a major competitive advantage for such devices according to customer surveys. In one foreseeable application, diabetics would be able to painlessly extract a small amount of blood using automated AgaMatrix-enabled microneedles to test blood glucose levels.
Hospital Point-of-Care Blood Analyzers AgaMatrix will also initially target the hospital blood analyzer market ($300 million in 2001, 25% CAGR) by providing increased accuracy and increased types of tests for these devices. Based on a bottom-up analysis of end user (physicians) and device maker surveys, we believe market penetration for these players has been hindered by the relatively low accuracy (when compared to tests done by centralized labs) and by the limited number of available tests. Physicians are thus forced to wait several hours for results from blood sent to centralized labs, and only use portable blood analyzers in acute emergency situations.
AgaMatrix solves the problem of low accuracy for portable hospital blood analyzers, allowing physicians to use portable analyzers in more situations, thus increasing quality of care, increasing patient turnover, and reducing hospital costs. Our technology could also help boost the yield on current cartridge products, eliminate future cartridge production steps, and provide a broader menu of tests on portable devices, delivering a suite of offerings comparable to traditional large and expensive lab equipment analyzers.
With almost all of the major players trying to develop an “artificial pancreas,” commercializing implantable glucose biosensors that can regulate an implanted insulin pump has been the Holy Grail for the industry. The artificial pancreas allows diabetics to lead a near normal lifestyle without the constant pain and inconvenience of finger pricking and insulin injections. One of the key challenges in the development of implantable sensors is eliminating the use of toxic chemicals currently needed to correct for cross-sensitivity effects that reduce the accuracy of the sensor. AgaMatrix’s technology minimizes these effects without having to use toxic chemicals, thus eliminating a key barrier to the development of complete implantable glucose monitoring and insulin pump systems. Such breakthroughs could lead to adoption of implantable devices on the order of today’s cardiac pacemaker. These large players have expressed initial interest in using AgaMatrix’s technology in these next-generation implantable devices.
Electrochemical Immunoassays Immunoassays are tests that measure biological and chemical species associated with the body’s immune system. Currently, the majority of immunoassays are performed via color-changing tests strips (for simple non-critical applications like home pregnancy tests), or via time-consuming laboratory procedures for more critical tests (like cardiac markers). In hospitals and clinical labs alone, millions of these immunoassays are performed daily. These laboratory assays are based on complicated optical and radioactive detection instrumentation. Leaders in the industry are developing electrochemical immunoassays because electrochemical technologies are generally recognized to be more cost effective, robust, and possibly faster than optical methods given the fact that no complicated sample pre-treatment processes are needed. One of the main challenges to commercializing this new technology is achieving the low detection levels needed for such measurements. AgaMatrix’s technology can be eventually embedded in these devices to overcome the sensitivity issues that currently hinder their commercialization.
4.2 Industry Analysis
The medical diagnostics industry is prone to disruptions because of technological innovations. We have found the following current industry needs are immediately addressable by AgaMatrix’s technology based on a survey of potential customers in the blood glucose and Hospital point-of-care (POC) market:
Near future market needs prompted by emerging trends
We have also identified a number of needs for which AgaMatrix is aligned to be a major technology provider. For the sake of conservatism and maintaining a focused company positioning in the healthcare arena, we are not pursuing available applications in environmental monitoring, industrial processing, and military biological/chemical warfare agent detection. Instead, we consider our primary expansion markets to be other segments in the healthcare market including the immunoassay and implantable/minimally invasive biosensor markets, with some initial penetration into the latter in Y2 and Y3.
4.2.1 Competition and Buying Patterns
Based on a prior art search and our cross-disciplinary technology expertise, we believe we are the only solution provider of our kind to medical device manufacturers. However, AgaMatrix indirectly competes against other biosensor-enhancing technologies. Rival technologies include advances in physical designs such as improvements in chemical reagents used in these devices and the integration of permselective membranes that are intended to materially filter out interfering chemicals from contacting the sensor. We have identified the research efforts of the following companies as potential competition due to their efforts to solve the same problems, albeit through very different approaches.
Strategy and Implementation Summary
AgaMatrix’s strategy will be built upon sustainable advantages from superior software technology, in-house expertise, monopolization of a scientific team, and development lead time over competitors. In addition, the company will deploy a strong intellectual property strategy of defensive and offensive patents to create an IP minefield to make litigation for competitors as costly as possible. Coupled with an aggressive marketing and sales strategy, AgaMatrix is positioned to be the leading provider of technology that enables biosensor devices used in medical and other life science applications.
5.1 Competitive Edge
Advantage #1: Superior and Complementary Software Paradigm Potential indirect competition could lie within R&D departments of medical device OEMs who are striving to create both incrementally higher-performing biosensors for existing products. The R&D teams are also striving to make revolutionary advances which enable implantable biosensors such as blood glucose monitors. However, based on secondary market research and on first-hand conversations with potential customers/partners, the observed historical trend in this industry has been to approach chemical problems with chemical experts. We believe that our multi-disciplinary software approach fills a missing piece in the development of these devices.
From a technological standpoint, our software-based solutions achieve the same goals of interference suppression as rival chemical solutions; however, because we obviate the need for these chemicals, most of which are toxic, products deployed with AgaMatrix technology will be suited for in vivo applications, such as some minimally invasive and implantable glucose monitors. Furthermore, our technology can simultaneously monitor multiple chemicals, both the target analyte and any interfering chemicals, engendering low-cost multi-analyte sensors which are not readily viable with current chemical-based sensor enhancements.
From a marketing standpoint, our products have the advantage of being software-based, engendering many of the potential benefits that other software-based products traditionally enjoy. One of the principle advantages that end users would have is the ability to upgrade the software as new, better algorithms are developed, a benefit that cannot be as easily realized with other physical and chemical technological advances. From a cost-saving standpoint, many of the permselective membranes that are currently designed to be used in biosensor devices are too expensive to be used in all applications. As such, our software solutions would provide a cost benefit advantage to our customers.
Regardless of other traditional technology advances in sensor design, our DSP technologies will ultimately prove to be complementary. Our noise-filtering algorithms will increase the signal-to-noise ratio enabling greater sensitivity and lowering detection limits. In many applications, membrane filters are not fully effective; as such, our interference suppression algorithms can compensate for the limitations of such membranes. Additionally, rival empirical improvements cannot address other limitations of these devices, such as sensor deterioration, where our technology may be applied to auto-correct for such sources of error.
Advantage #2: In-House Expertise As is the case with chemical and life science research, one of the most resource-intensive aspects of the development time lies in optimizing empirical protocols and avoiding unforeseen pitfalls; most of the knowledge comes from “hands-on” experience, not only theoretical background. Furthermore, expertise in multi-disciplinary areas as ours requires specialized knowledge. AgaMatrix’s scientific team has been involved in biosensor research for an aggregate of over 40 years. The foundational research for our current technology was initiated seven years ago, and our scientists have developed and optimized many of the techniques that are vital to the continuing development and validation of AgaMatrix’s products. To date, we have developed the groundbreaking technology approach, the experimental protocols, the validation mechanisms, and the core algorithms. Our extensive in-house expertise in working on bridging biosensor systems and DSP technologies represents a significant barrier to any potential competitor.
Advantage #3: Monopolization of Scientific Team The technology that AgaMatrix is built upon has been inspired by research performed throughout the past decade at the University of Cambridge. The original scientific team that achieved these breakthroughs boasts inimitable credentials and has remained intact to form the current AgaMatrix R&D team. In the ensuing years, AgaMatrix has developed new technologies and is moving towards its commercialization. We believe that our virtual monopoly on the intellectual resources that have been responsible for the technological advances that AgaMatrix owns represents a significant competitive advantage over potential competitors. As is the case with any empirical endeavor, much of the in-house expertise comes in the form of a close working knowledge of the practical aspects of technology development. With the current R&D team already experienced in the relevant technologies, and having worked together in the past, much of this knowledge has already been acquired.
Advantage #4: Development Lead Time Over Potential Competition In sharp contrast to the typical chemistry-based approach, AgaMatrix’s technology is based upon a multi-disciplinary core competency. Our competitive capabilities are derived from a unique confluence of electrical engineering and life science disciplines, a roadblock for potential competitors entrenched in traditional “wet chemistry” research paradigms.
5.2 Marketing Strategy
Presenting compelling value through superior technology Because of AgaMatrix’s revolutionary, proprietary technology, we are positioned to be the market leader in biosensor enhancing solutions. AgaMatrix offers a novel approach that clearly provides significant value to our customer and ultimately the end user. The AgaMatrix solution delivers value in two ways: by improving the performance of their product against competing products and by increasing customers’ market share and revenue. Communicating this value to the device manufacturers, as well as branding our technology to defend market share, is the fundamental philosophy behind our marketing strategy.
Becoming a competitive standard The core AgaMatrix technology is a unique approach to improving biosensors systems in a way that substantially increases performance and adds value to the end user. In a competitive marketplace, we will present our technology to medical device makers as an industry standard that they must adopt to be able to compete. Examples of this kind of standard-setting technology include the adoption of Windows platforms on PCs and auto-focus and red-eye reduction capabilities on cameras. Specific selling points include elimination of cross-interference, improvement in accuracy, improvement in signal to noise ratio, improvement in device robustness, reduction in sample requirements, and increase in market acceptance of product. The value proposition will differ depending on the needs of each customer.
Marketing to end users by marketing the end product AgaMatrix will market to end users through partnership with the device makers; to add value, our technology must increase their bottom line profit. AgaMatrix can do this if end users appreciate the advantages AgaMatrix enhancements bring and if they require our products for a healthcare “standard of care.” The desire for end users, such as doctors and patients, to use the best and most effective technology for diagnosis and treatment of health problems will drive demand for AgaMatrix-enabled devices. Therefore, AgaMatrix will develop a marketing plan with our partners to increase the awareness of the clinical advantages of our devices. The AgaMatrix-enabled label will become a moral imperative to clinicians in the same way that advanced digital imaging technology is used by radiologists and cardiovascular specialists.
In summary, AgaMatrix will market both to the device makers we sell to and end customers, who will drive demand. Because device makers are concerned about increasing their bottom line through value-add to their products as well as through production cost reductions, AgaMatrix will sell to them on the basis of value rather than on any other consumer-based premise. End users, such as healthcare professionals and customers, demand standard of care. Therefore, AgaMatrix will co-market its brand as a necessary technology for healthcare diagnostics.
5.3 Sales Strategy
Phase 1: Sales to medical device manufacturer partners In the first stage of bringing AgaMatrix technology to market, the company will approach and partner with medical device manufacturers. Such partnerships have the added advantages of product development that is supported by the partner’s engineering, finance, marketing, and management.
Phase 2: Becoming the “competitive standard” With a base of customers who can vouch for the product value, AgaMatrix aims to become the competitive standard that all players must adopt. Specific marketing tactics in this stage include: increasing market awareness through trade shows (e.g., Medical Device Expo, SensorExpo) and technical conferences, advertising in trade journals and publications (e.g., Sensor Magazine, Medical Device and Diagnostics Magazine), and retaining “Thought Leaders” from industry and academia who will corroborate our claims.
Phase 3: Branding for mind share and market domination AgaMatrix will brand its proprietary DSP technology to associate enhanced solutions with our identity. A consistent, strong, and clearly defined brand will add yet another barrier to entry and market penetration. Increased awareness of the advantages we deliver will give rise to increased demand for the end product.
5.3.1 Sales Forecast
The sales forecast is based on a royalties pricing model. However, a revenue model based on licensing fees is also described and provided for comparison purposes, below.
Charging on a royalty-based per-use fee, AgaMatrix will initially sell OEM technology solutions to manufacturers of biosensor-based medical devices that will enhance their products’ performance. Because AgaMatrix technology is software-based and is optimized for minimal hardware requirements, it can be easily integrated into existing sensor devices, boosting functionality on a cost-effective basis. By embedding our technology within their devices, OEMs will realize substantial gains (20% to over 100%) in performance dimensions such as accuracy, sensitivity, and robustness. Technology OEM royalty-based business models are not new in this business. Our ability to quickly provide performance upgrades in the form of easy-to-integrate software/firmware updates provides a number of technical and sales advantages over the existing development paradigm, which relies on “wet” chemistry approaches.
5.3.2 Pricing Model and Revenues
The primary value proposition that AgaMatrix presents to medical device manufacturers is increased revenues through increased market share from product advantages over other competing devices and from premium pricing for increased functionality and performance of their products. Because the new product offering from the manufacturer contains “best of” technology and is in the healthcare space, they can charge a premium for their product, which will translate into revenues to AgaMatrix. Another value proposition which a potential customer (i-STAT) actually brought to our attention is that our technology could very likely reduce production costs for them by allowing them to eliminate the need to use costly membranes in their products.
The pricing for our product can be either “value-added” pricing on the price of the medical device or based on device usage, depending on the revenue model used by our customers. In the case of the blood glucose market, revenues are driven not by the device, but rather by recurring revenues from consumable test strips. For example, the test strips that LifeScan sells retail for approximately $0.70 each. These test strips are supposed to be used three to four times a day, although the pain associated with testing has reduced compliance to about 1.5 tests per day per patient. AgaMatrix will share in the revenues this model generates. For example, every time a test strip is analyzed by the device that LifeScan sells, AgaMatrix technology will be utilized to provide a more accurate reading. Therefore, AgaMatrix will enter into a royalty-based fee agreement with device manufacturers, such as those in glucose monitoring, where consumables generate revenue. Preliminary conversations with Hypoguard indicate a general willingness to this type of pricing model.
Another example of how our royalty will work could be through the partnership with Company X. Company X manufactures and sells a point-of-care device for approximately $5,000. Test cartridges are priced at around $3.40 each and can perform 5-6 different tests once. For Company X, our product would solve an existing problem with the performance and reliability of their cartridges. Cartridges would be priced approximately $4.00 – $5.00. Company X manufactures these cartridges for $0.12-$0.16 each and should be amenable to sharing the increased margins. For cases in which consumables are not used, premium pricing of about +20% will be used depending on the added value that can be delivered to the end user. The following table summarizes a conservative revenue forecast based on royalties.
An alternative pricing model would be to charge an annual licensing fee for each device enabling AgaMatrix technology. The value proposition to customers is the same: devices enabled with AgaMatrix technology will be more accurate and therefore require smaller blood samples and result in less pain, which will increase device and test strip sales. Pricing structures and terms of the company’s software modules and services will ultimately be determined by negotiations with customers. The most likely scenario will be a hybrid pricing model of flat licensing fees on devices and royalties on test strip sales. The following table summarizes a conservative revenue forecast based on a licensing structure.
5.4 Intellectual Property Strategy
With a fully developed IP strategy consisting of core utility patents (currently filed as provisional applications) and defensive utility patents to be filed imminently, and based on our technological leadership, we believe that it would be far more beneficial for potential customers to purchase our technology than to develop it in-house.
5.5 Milestones
The following tables summarize the company’s developmental goals (month-to-month for Y1). Product Milestones are listed separately, below.
5.5.1 Year 2 and 3 Milestones
- Business Milestones
- Secure two major paying POC customers
- Secure another small paying glucose customer
- Secure one major glucose beta customer
- Operational Milestones
- Move to larger office
- Hire 6 scientists, 8 engineers
- Hire product management and operations staff
Year 3 Milestones
- Secure one major paying glucose customer
- Secure one major implantable beta
- Expand to other POC players
5.5.2 Product Milestones
The following table summarizes the product development vision. Future products will all contain updated core DSP algorithm software, associated tools, and documentation of performance results, ensuring that we maximally leverage our existing technology base as productization evolves.
5.5.3 Summary of Current Accounts
Web plan summary.
AgaMatrix’s website will be a dynamic marketing tool for the company that serves the needs of business development, sales, and recruiting. The company site will provide information about AgaMatrix’s products and services for target customers and potential business partners, such as marketing collateral, technical white papers, and new product updates. As the company grows, its recruiting needs can be addressed by posting career opportunities and FAQs about the company. AgaMatrix.com will also communicate company news to create and maintain positive public relations with the community and investors. The goal will be to implement a functional and professionally designed website that can be adapted to meet the company’s growing needs.
6.1 Development Requirements
Creation of future versions of the AgaMatrix website will continue to be outsourced to Nathan Bailey, a professional graphics designer with over 15 years of experience. The contractor will work with the marketing department to conceptualize the company’s logo and overall design. It will be maintained in-house and major site redesigning will be made through a contractor.
Management Summary management summary will include information about who's on your team and why they're the right people for the job, as well as your future hiring plans.">
The following are the current members of the AgaMatrix Team. Once a permanent CEO is on board, Sonny will transfer to a Director of Product Management role.
Sonny Vu, Chief Executive Officer and Founder Sonny brings management and entrepreneurial experience from having worked in several of Microsoft’s product groups and having launched and built FireSpout, an enterprise software company. At Microsoft, he worked in a number of product development groups, including the natural language group responsible for shipping linguistic technologies to over 16 applications in 22 languages. While at FireSpout, Sonny created the original technology vision, recruited the technical teams, developed and managed the technology development and various operational processes, and developed the intellectual property strategy. Originally a mathematician by training, Sonny was a Ph.D. candidate at MIT prior to working in the software industry.
Dr. Sridhar Iyengar, Chief Technology Officer and Founder With 10 years of research and engineering experience in DSP and mathematical modeling of chemical systems, Sridhar drives and directs the implementation of AgaMatrix’s technology vision. He is the leading expert in the core DSP/electrochemistry interdisciplinary approach used by AgaMatrix. Combining his background in electrical engineering and biological sciences, Sridhar conceived and pioneered the concept of using a DSP approach to enhance biosensor performance. His work in the years following his breakthrough Ph.D. research is the cornerstone for AgaMatrix’s intellectual property with two key patents filed under his name and another three defensive patents to be filed during the summer of 2002. Sridhar obtained his Ph.D. from the University of Cambridge as a Marshall Scholar.
Craig Bolon, Vice President of Engineering With more than 35 years of management and technical experience in software and hardware engineering, Craig is responsible for executing AgaMatrix’s product development initiatives. He brings his leadership experiences from being a hands-on development engineer, team leader, general manager, engineering director, and entrepreneur. Craig has a proven track record of delivering on-time, on-budget projects while working on commercial product development in software and instrumentation for organizations such as Schlumberger, Polaroid, Betagen, Exxon, and MIT. His commercial product development work has spanned the fields of molecular biology, chemical analysis, electronic imaging, speech recognition, and mechanical design software. Craig has invented key technologies and holds a number of software and hardware patents. He holds a degree in particle physics from MIT.
Dr. Paul J. Kelly, Advisor Paul is the founder and former CEO of Gemini Genomics plc, until its merger in 2001. A physician who specialized in endocrinology, he has more than 25 years of experience in medicine, and research in clinical and commercial settings. He has published extensively in over 90 publications, has an issued patent, and has held faculty appointments at the University of New South Wales and St. Vincent’s Hospital in Sydney, Australia. After launching Gemini Genomics in Cambridge, England, Paul went on to list the company on NASDAQ, in the most successful IPO of 2000 in the UK. He has served on national governmental advisory bodies, as well as on the boards of public and private companies, and non-profit institutions. Paul graduated in Medicine from the University of New South Wales, Sydney, and received his Doctor of Medicine degree for his thesis in the genetics of osteoporosis also from the University of New South Wales. He is a Fellow of the Australasian College of Physicians.
7.1 Personnel Plan
The personnel table assumes steady growth in employees over the next year. We expect head count to reach 14 employees by end of year one. We are in the process of implementing a strong benefits policy (with fully-paid medical, dental, and life insurance, plus a profit sharing and 401K plan). Employees generally earn competitive salaries and receive generous equity packages.
Financial Plan investor-ready personnel plan .">
The following subtopics highlight the financial plan for AgaMatrix.
8.1 Break-even Analysis
The break-even analysis demonstrates that AgaMatrix will have a sales level running comfortably above break-even starting in year two. Depending on which pricing model is used – either royalties, licensing, or both – average revenue could vary significantly, but the table shows a fair estimate given our revenue projections.
The business will have very few fixed costs – most laboratory equipment can be leased, as will the real estate for our offices. All costs are expected to be variable for modeling purposes, giving the company flexibility to adapt as needs and environmental conditions may change. Because AgaMatrix technology is software-based and is optimized for minimal hardware requirements, it can be easily distributed and integrated into biosensor devices with advantages of economies of scale. As volume increases, average variable costs will significantly decrease.

8.2 Important Assumptions
The financial plan depends on important assumptions, most of which are shown in the following table. The key underlying assumptions are:
- We assume a slow-growth economy, without major recession.
- We assume of course that there are no unforeseen changes in technology to make our products immediately obsolete.
- We assume access to equity capital and financing sufficient to maintain our financial plan as shown in the tables.
Financial projections are predicated upon targeting the life sciences vertical exclusively. Within the life sciences market, blood glucose will drive the majority of revenue. However, the point-of-care testing market will contribute modest revenue in the near term, accompanied by a substantial contribution from the implantable market in the medium and long term.
8.3 Projected Profit and Loss
Gross and operating margins
Gross margins will be approximately 85% on the core product offering, which will be delivered in the form of software. Such margins are typical in the software industry; we have not modeled in support revenue streams for our products, assuming this will be handled entirely by our OEM customers. In year one, we expect a loss, as we grow the business from a small base by conserving cash. Beginning in year two (post-institutional funding), as we ramp up the business more aggressively, operating expenses as a percent of revenue will fall as we hire a critical mass of personnel for marketing, sales, and research and development. By the end of the forecast horizon, operating margins will once again exceed 30%.
Profit potential and durability
AgaMatrix is expected to be net income positive beginning in its second full year of operations. Profitability is expected to grow rapidly following year two, once the business is able to leverage the investment from the year two ramp-up. AgaMatrix has the potential to be an enduring standalone business, supported by a diversified revenue stream within the life sciences vertical (blood glucose, point-of-care testing and minimally invasive/implantable devices), with the opportunity to expand into other sub-segments in the healthcare sector and new verticals for long-term growth.

8.4 Projected Cash Flow
The financial outlook is positive as the company rolls out and meets its milestones. After financing, cash flow will be negative for year one. By year two, AgaMatrix expects to be cash flow positive.
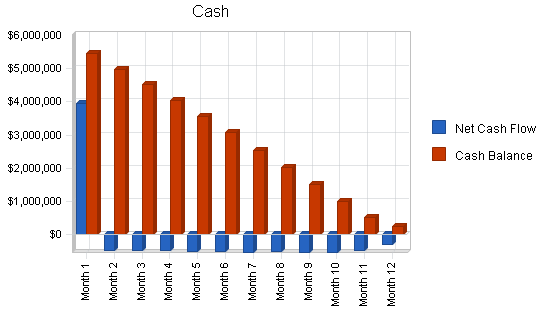
8.5 Projected Balance Sheet
Our projected balance sheet shows an increase in net worth. The monthly projections for the first year are in the appendix. Net worth is negative initially because the company does not expect to secure its first paying customer until end of year one.
8.6 Financial Risks and Contingencies
We have identified several critical risks and assumptions that must be addressed to ensure AgaMatrix’s success.
Market Risks
Risk #1: Corporate R&D labs of our customers/partners may develop competing DSP-based technologies to enhance their own electrochemical sensors based products.
See section 4 for a detailed discussion of competition and AgaMatrix’s sustainable competitive advantages.
Risk #2: Other technologies may be developed to improve sensor performance.
Other technology solutions designed to improve sensor performance have been generally hardware-based introducing additional costs and at times toxic chemicals. For example, MEMS-based infrared sensors, being developed as an alternative to electrochemical sensors, are expected to be much more costly despite increased performance. Similarly, mediators such as ferrocene are used to deliver accurate readings, but are toxic and less effective than AgaMatrix’s solution. AgaMatrix software-based solution improves performance while being cost effective and safe.
Risk #3: As a pioneer in electrochemical applications for DSP algorithms, AgaMatrix may not be able to convince customers to adopt such a revolutionary solution.
Developers of blood glucose monitors and portable blood analyzers have never considered using a software-based approach to solving their accuracy and cross-interference problems. There is thus a psychological barrier that we believe can be overcome through a simple, concrete demonstration of low-cost performance gains which we can provide.
Risk #4: There may not be enough computing power and memory on blood glucose monitoring devices and portable blood analyzers to support AgaMatrix’s software.
The algorithms have been optimized for computational speed and are designed for use on devices with very little CPU resources. Initial customer feed back shows that AgaMatrix’s algorithms can be incorporated in next-generation ASICS designs for blood glucose monitoring devices, as well as into current microprocessor-powered portable blood analyzers.
Risk #5: Implantable blood glucose sensors may be prolonged from the marketplace indefinitely.
Although most blood glucose monitoring device companies are trying to develop implantable sensors, other technical and marketing issues may prevent the eventual adoption of the artificial pancreas. AgaMatrix’s technology will accelerate the development of the artificial pancreas by not requiring toxic mediators. However, AgaMatrix cannot solely depend on this market’s development, and has thus chosen to focus on existing markets to drive short to medium term revenue.
Risk #6: AgaMatrix must prove out the technology on blood samples.
Despite a high confidence in the technology, we must still create experimental data sets created from tests using actual blood samples. These data sets will be shown to customers as proof of the technology’s effectiveness. AgaMatrix is confident that after initial funding, lab space and equipment can be quickly secured to produce these data sets.
Risk #7: AgaMatrix may face regulatory delays from FDA approval.
We will work with our customers to ensure that the technologies that are deployed into their devices will incur minimal regulatory risks thereby complying with the FDA’s less onerous regulations for a “derivative device” (compared to the approval process for a completely new device).
Risk #8: AgaMatrix needs to determine customer willingness to pay and secure concrete deals with customers.
Several conversations with potential customers have already reached the level of discussing potential pricing structures so we believe there is some genuine interest.
Risk #9: Each OEM customer will require a custom-built version of the AgaMatrix software.
The software suite will be designed to be a modular and scalable platform technology. We will construct a set of configuration and integration tools designed to translate our core technology into suitable deployment formats.
Risk #10: University of Cambridge may have claims to AgaMatrix’s technologies.
The technology is based on 3rd generation algorithms that AgaMatrix alone has been developing for two years. 1st and 2nd generation technologies were developed at the University of Cambridge and validated the proof of concept of using a DSP approach to solving many of the outstanding problems in biosensors. Our 3rd generation technology is fundamentally different from the earlier technologies and has overcome a number of critical limitations, on both the theoretical and empirical sides, that prevent commercialization. AgaMatrix owns all rights to these 3rd generation technologies. The 1st and 2nd generation technologies, while illustrative of the concept, do not pose any commercial threat due to fundamental technological limitations
Financial Risks
Risk #1: Working Capital Management – We expect to be running a significant working capital deficit because of the time it will take to establish payment schedules (e.g. quarterly royalties from partners) and receive payments from large OEM vendors while, as an early-stage company, we will simultaneously have to make payments on our supplies on a short-term basis. Managing the cash conversion cycle will be critical to ensuring liquidity and solvency.
Risk #2: Seasonal, Cyclical, or Highly Volatile Cash Flows – at this time, we expect there to be volatility in our cash flows based primarily on the new product introduction cycles of major medical devices manufacturers. Therefore our revenue and cash flow streams will not be smooth throughout the year, but will be stronger during times of new product introduction. By targeting three different market segments early on (blood glucose, point-of-care, and implantable devices) we aim to mitigate this risk.
Risk #3: Concentration of Customers – The blood glucose market and portable blood analyzer markets are dominated by an oligopoly of a handful of companies. It may be difficult to diversify our customer base sufficiently to prevent large swings in our revenue and cash flow based upon the actions of a small number of customers. To diminish this risk, we will initially target smaller players who will move more quickly and provide us with greater leverage when we go to negotiate with larger customers.
8.7 Business Ratios
Business ratios for the years of this plan are shown below. Industry profile ratios based on the Standard Industrial Classification (SIC) code 7373 or NAICS code 541512, Computer Systems Design Services, are shown for comparison.
The quickest way to turn a business idea into a business plan
Fill-in-the-blanks and automatic financials make it easy.
No thanks, I prefer writing 40-page documents.
Discover the world’s #1 plan building software

IMAGES
VIDEO
COMMENTS
The medical device industry is one of the world's most innovative and dynamic sectors. Fortune Business Insights reported that the global medical device market was valued at $512.29 billion in 2022 and can grow from $536.12 billion in 2023 to $799.67 billion by 2030, at a CAGR of 5.9%. The medical device industry is driven by several factors ...
Your medical device business plan is a living document that should be updated annually as your company grows and changes. Sources of Funding for Medical Device Businesses. With regards to funding, the main sources of funding for a medical device business are personal savings, credit cards, bank loans, and angel investors. ...
Develop A Medical Device Business Plan - The first step in starting a business is to create a detailed medical device business plan that outlines all aspects of the venture. This should include potential market size and target customers, the services or products you will offer, pricing strategies and a detailed financial forecast.
MedNexis, Inc. is a start-up medical device company that has designed and patented devices to aid in atrophy treatment/prevention. Medical Equipment Developer Business Plan Medquip, Inc. is a start-up business that will develop and market endoscopic medical devices through multiple distribution channels, both foreign and domestic.
MedNexis, Inc. (the company) is a medical device development company that has designed and patented medical devices which it plans to produce and market. A magnetic muscle stimulator/field generator has been designed with the participation of leading medical personnel and biomedical engineers. One patent is initially incorporated.
Executive Summary. Medquip, Inc. is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche markets. Three devices have already been designed with the participation of leading physicians and surgeons in gastroenterology. Seven patents are initially incorporated.
A business plan has 2 main parts: a financial forecast outlining the funding requirements of your medical device manufacturer and the expected growth, profits and cash flows for the next 3 to 5 years; and a written part which gives the reader the information needed to decide if they believe the forecast is achievable.
Learn how to start a medical equipment manufacturing business with a solid business plan sample that covers all important sections and details. Download a free template and use Upmetrics business plan software to write your own plan in no time.
Learn the keys to launching a successful medical device business, from choosing the name and type of company to developing your plan and choosing the legal structure. Download Growthink's Ultimate Business Plan Template to complete your plan in hours.
A Medical Device Business Plan is a strategic document that outlines the goals, objectives, and operational details of a venture involved in designing, developing, manufacturing, and distributing ...
You'll also want to consider the following medical device business case best practices: Rely heavily on input from subject matter experts (SMEs) and end users. Keep risk and regulations top of mind. Insights should always be grounded in data. Stay realistic about your team's capabilities and time.
A Strategy Inc. Business Plan is a comprehensive assessment that incorporates a broad scope of global business analyses: Strategy Inc. offers a range of financial services to facilitate fund raising activities at the highest valuation, identify acquisition targets and outline exit strategy. A robust Business Plan provides a clear roadmap to ...
The medical device quality system is primarily concerned with production and post-production. FDA 21 CFR Part 820 defines the quality regulations for the U.S. market. Otherwise, ISO 13485 can be used to build a quality system for global markets. Although it's time-consuming and expensive, establishing a total quality management system need ...
Lanzor Medical's Business. We design, develop, and are selling unique solutions for the $4.2 billion global market for catheter-based equipment to diagnose and treat cardiovascular disease. Our clinically proven and FDA and EU approved SmartFlow® products represent the next generation in interventional cardiovascular.
Surgical Medical Equipment Business Plan. Bioring SA, is a manufacturer of heart valve surgical replacement parts. Medical equipment manufacturing is a competitive marketplace, but that doesn't mean there isn't an opportunity to start a business. If you start small, find a niche category, or identify a weakness in current manufacturers, you ...
Cayenne Consulting offers business plan preparation services for medical device, biotech, and healthcare companies. See how they prepared a pitch deck and business plan for Florence Medical, a company that developed interventional applications of computational fluid dynamics.
Medical Device business plan. The medical device field has several unique aspects that are important to address when writing a business plan; for example, clinical & regulatory paths, (including clinical trials, FDA & CE approval) and medical insurance refunds. Below is a table of contents, for example:
Marketing promotion expenses for the grand opening of Eden® Medical Device Development Company, Inc. in the amount of $3,500 and as well as flyer printing (2,000 flyers at $0.04 per copy) for the total amount of $3,580. The total cost for hiring Business Consultant - $2,500.
This business plan has been developed to present our company to prospective supplier partners, employers, and investors. Zenergy Medical Industries is a start-up company focused initially on distribution of leading brands of therapeutic systems for use by residents of Homecare and Assisted Living facilities at risk of complications from X disease.
Medical Device Business Plan Template. If you want to start a medical device business or expand your current medical device business, you need a business plan. The following Medical Device business plan template gives you the key elements to include in a winning Medical Device business plan.
Healthcare Business Plan Consulting. Our healthcare business plan consultants have hands-on experience founding, funding, and scaling ventures. We're much more than an ordinary business plan writing service. Think of us as your co-founder for the duration of our project. Are you looking for a skilled healthcare, biotech or medical device ...
Explore a real-world medical software business plan example and download a free template with this information to start writing your own business plan. ... Phase 1: Sales to medical device manufacturer partners In the first stage of bringing AgaMatrix technology to market, the company will approach and partner with medical device manufacturers ...