Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 24 March 2021

A 20-year retrospective review of global aquaculture
- Rosamond L. Naylor ORCID: orcid.org/0000-0002-1260-3322 1 , 2 ,
- Ronald W. Hardy 3 ,
- Alejandro H. Buschmann ORCID: orcid.org/0000-0003-3246-681X 4 ,
- Simon R. Bush 5 ,
- Ling Cao 6 ,
- Dane H. Klinger ORCID: orcid.org/0000-0003-4178-8167 7 , 8 ,
- David C. Little 9 ,
- Jane Lubchenco ORCID: orcid.org/0000-0003-3540-5879 10 ,
- Sandra E. Shumway 11 &
- Max Troell ORCID: orcid.org/0000-0002-7509-8140 12 , 13
Nature volume 591 , pages 551–563 ( 2021 ) Cite this article
142k Accesses
859 Citations
626 Altmetric
Metrics details
- Environmental impact
- Environmental sciences
A Publisher Correction to this article was published on 06 July 2021
An Author Correction to this article was published on 26 April 2021
This article has been updated
The sustainability of aquaculture has been debated intensely since 2000, when a review on the net contribution of aquaculture to world fish supplies was published in Nature . This paper reviews the developments in global aquaculture from 1997 to 2017, incorporating all industry sub-sectors and highlighting the integration of aquaculture in the global food system. Inland aquaculture—especially in Asia—has contributed the most to global production volumes and food security. Major gains have also occurred in aquaculture feed efficiency and fish nutrition, lowering the fish-in–fish-out ratio for all fed species, although the dependence on marine ingredients persists and reliance on terrestrial ingredients has increased. The culture of both molluscs and seaweed is increasingly recognized for its ecosystem services; however, the quantification, valuation, and market development of these services remain rare. The potential for molluscs and seaweed to support global nutritional security is underexploited. Management of pathogens, parasites, and pests remains a sustainability challenge industry-wide, and the effects of climate change on aquaculture remain uncertain and difficult to validate. Pressure on the aquaculture industry to embrace comprehensive sustainability measures during this 20-year period have improved the governance, technology, siting, and management in many cases.
Similar content being viewed by others
Sustainable aquaculture through the One Health lens
Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030
A seaweed aquaculture imperative to meet global sustainability targets
Twenty years ago, Nature published a review characterizing aquaculture as a possible solution, and a contributing factor, to the decline in fisheries stocks worldwide 1 . At the time, the commercial aquaculture sector was flourishing, whereas the production of capture fisheries remained stagnant. The farmed (live-weight) production of fish and shellfish had almost tripled from 10 million tonnes (Mt) in 1987 to 29 Mt in 1997, and roughly 300 species of animals, plants, and algae were being cultivated worldwide 2 . The paper placed greater emphasis on fed marine species than on freshwater and molluscan species and cautioned that the net positive contribution of aquaculture to world fish supplies could not be sustained unless the sector reduced its use of wild fish in feed as well as its environmental impacts.
This Review covers global trends in aquaculture over the past 20 years, citing a selection of the most relevant papers (additional reviewed articles are listed in the Supplementary Information). In 2017, aquaculture supplied more than 80 Mt of fish and shellfish and 32 Mt of seaweeds, encompassing around 425 farmed species 2 . Three main patterns of aquaculture development have characterized the sector as it matured: continued growth in the volume and value chains of freshwater aquaculture; advances in fish nutrition, genetics, and alternative types of feed that reduce the use of wild fish in aquafeed formulations; and expanded culture of extractive bivalves and seaweeds with the potential to provide a wide range of food, industrial, and ecosystem services.
These trends reveal increasingly tight connections between land and sea. Continuing a long history of inland production, the share of freshwater fish raised on compound feed, which is made largely from terrestrial and some marine ingredients, has increased over the past two decades 3 . Meanwhile, the inclusion of plant-based ingredients in aquafeed has increased, and the production of extractive species (molluscs and seaweed) that filter nutrients from terrestrial and marine food systems has grown. Aquaculture has thus become more integrated into the global food system, with rapid growth in production and major transformations in feed ingredients, production technologies, farm management, and value chains. Through aquaculture growth, consumers from low- to high-income nations have benefited from year-round availability and access to aquatic foods, which are rich in protein and micronutrients 4 , 5 , 6 , 7 . The sector produces far more than fish, shellfish, and algae for direct human consumption. It also generates products used in food processing, feed, fuels, cosmetics, nutraceuticals, pharmaceuticals, and a variety of other industrial products, and it contributes to a range of ecosystem services 8 .
Despite impressive gains, the aquaculture sector still faces serious challenges that, in some cases, undermine its ability to achieve sustainable outcomes. The sector has generally embraced a business and societal expectation of environmentally and socially sound practices. Globally traded finfish and crustacean systems are progressively improving their environmental performances, either independently or in response to government regulation, private and public sector standards, and market incentives. Many aquaculture systems, however, still lack the motivation to meet sustainability criteria because their targeted markets do not reward producers through improved prices or access. At the same time, molluscs, filter-feeding finfish, and seaweeds have sustainable characteristics, particularly because they do not rely on aquafeed, but instead remove nutrients from the water column. In summary, as the global industry continues to expand, its contribution to economic social and environmental performance varies across a wide diversity of aquaculture systems.
Global expansion
Global aquaculture production more than tripled in live-weight volume from 34 Mt in 1997 to 112 Mt in 2017 (Fig. 1 ). The main species groups that contributed to the top 75% of aquaculture production in 2017 included seaweeds, carps, bivalves, tilapia, and catfish. Although the production of marine and diadromous fish species and crustaceans has also grown rapidly during this period, it has been dwarfed by the live-weight volume of marine bivalves and seaweeds, and by the production of freshwater aquaculture. Freshwater fish account for 75% of global edible aquaculture volume, reflecting their favourable conversion from live to edible weight in comparison to molluscs and crustaceans, which have high shell weights 9 . Because the previous review focused on marine-sourced feed in the production of high trophic marine and diadromous species, the dominant role of freshwater systems was only lightly covered 1 . The role of freshwater systems has gained attention in part because advances in feed technology and breeding, particularly for salmon and shrimp, are addressing earlier concerns regarding the effects of aquaculture on wild-capture fisheries.
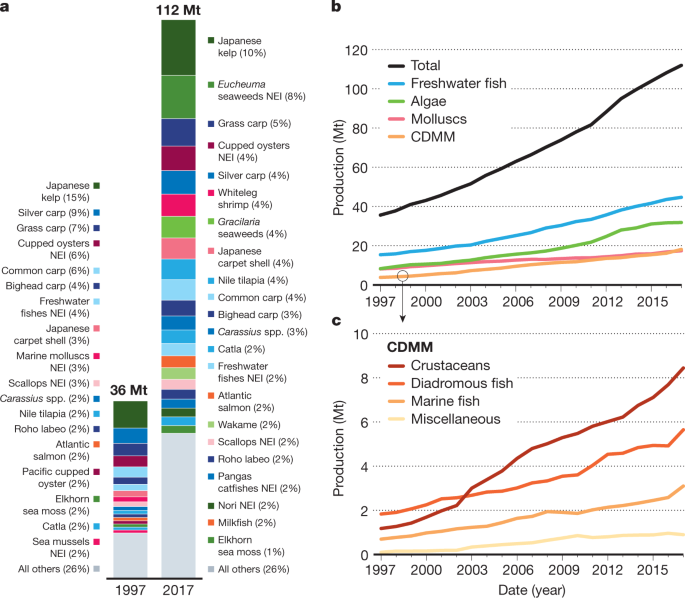
a , The species composition is shown for 1997 and 2017. Green, plants and algae; blue, freshwater fish; pink, shellfish; orange, diadromous fish. b , c , Growth is shown from 1997 to 2017 for the following production categories ( b ): total, freshwater fish, algae, molluscs and CDMM, which comprises crustaceans, diadromous fish, marine fish, and miscellaneous species and is expanded in c . Algae comprised more than 99% of the production weight of ‘algae and aquatic plants’ production in 2017. Data were obtained from the FAO 2 . National data are reported on the basis of the ASFIS List of Species ( http://www.fao.org/fishery/collection/asfis/en ). NEI, not elsewhere included for species identification in question.
Source data
Aquaculture is more diverse today, with 40% more fish, shellfish, aquatic plant, and algal species cultivated in a wide variety of marine, brackish, and freshwater systems globally 10 . Global production remains concentrated, however, with only 22 of all 425 species groups farmed in 2017 (5%) accounting for over 75% of global live-weight production 2 (Extended Data Fig. 1 ). A small fraction of the ‘aquatic plant and algae’ category (~32 Mt) consisted of aquatic plants (1,639 tonnes) in 2017 2 . Aquatic plants are listed by the Food and Agriculture Organization (FAO) under ‘aquatic plants NEI’ and are underreported given the informal nature of the harvests for household and local consumption.
Asia remains the largest aquaculture producer, accounting for 92% of the live-weight volume of animals and seaweeds in 2017 2 . Aquaculture in Asia is also more diverse than other regions in terms of production systems and cultivated species 11 . Nine of the top-ten ranked countries for aquaculture species diversity are in Asia, with China leading by a wide margin. As an example, China cultivated 86 different species of aquatic organisms in a variety of production systems in 2017, whereas Norway cultivated 13 different species, mainly in marine cage systems 10 .
China has an oversized role in nearly all areas of aquaculture production. Since 2000, the country has maintained its role as the largest global producer, processor, and trader of fish, crustaceans, and molluscs, and has emerged as a leading consumer owing to the rapid growth in income and domestic seafood demand 12 , 13 , 14 . China alone supplied 58% and 59% of the global aquaculture volume and value, respectively, for all categories combined in 2017 (Extended Data Table 1 ).
The role of China notwithstanding, the aquaculture sector has become increasingly global, with growth rates in South America and Africa exceeding Asia during the past two decades (albeit from a much smaller production base), and with relatively rapid expansion in South and Southeast Asia compared to East Asia 3 , 15 , 16 . The largest aquaculture producers outside Asia—each accounting for 1–2% of the global production—include Norway and Chile, which mainly produce Atlantic salmon ( Salmo salar ), and Egypt, which produces Nile tilapia ( Oreochromis niloticus ) 17 . Aquaculture in the Western Hemisphere has largely developed around single- or dual-species and single-production systems (for example, Atlantic salmon in cages, Nile tilapia and channel catfish ( Ictalurus punctatus ) in ponds). These systems and species have benefitted from targeted genetic and nutritional advances, but remain vulnerable to shocks related to market volatility, extreme climate events, and pandemics such as COVID-19 10 , 17 , 18 .
The growth of aquaculture has been fuelled by the expansion in global trade, declines in the availability of wild fish, competitive product pricing, rising incomes, and urbanization—all of which contribute to rising per capita consumption of seafood worldwide 11 , 19 . Global fish trade remains limited, however, to a relatively small number of species and countries: salmon, shrimp, catfish, and tilapia collectively represent approximately one-third of internationally traded seafood by value, but only 8% of global seafood production 17 . The process of globalization itself has been dynamic, with incomes and markets in the global South expanding more rapidly than the global North in recent decades 20 . The growing importance of domestic markets, particularly in Asia, means that over 89% of aquaculture output does not enter into international markets 21 .
Freshwater aquaculture
Freshwater aquaculture has been underrepresented in the proliferating literature on global environment and food system interactions since 2000 despite its dominant contribution to aquatic food supplies and nutrition security 21 , 22 . Of the 11,625 articles published in English between 2000 and 2020 with marine or freshwater aquaculture (or farming) in their titles (indexed in Web of Knowledge ( https://apps.webofknowledge.com/ )), three-quarters focused on mariculture and 68% on high-valued mariculture. These metrics do not include the vast literature published in Asia, particularly in China, where freshwater aquaculture has a long and vibrant tradition 23 .
Freshwater aquaculture consists of a wide diversity of systems across physical and economic scales, infrastructure configurations, species, ownership, and value chains. It consists predominantly of household-managed ponds and small- to medium-scale commercial enterprises that produce a variety of carps and other fish in polyculture systems for local and regional consumption 24 . Freshwater aquaculture is widely recognized for the production of tilapia and striped catfish ( Pangasianodon hypophthalmus ) that are produced mainly in earthen ponds for export and national consumption. It also includes the cultivation of freshwater and brackish-water crustaceans, produced intensively in monoculture (for example, whiteleg shrimp ( Litopenaeus vannamei )) or in polyculture systems (for example, black tiger shrimp ( Penaeus monodon )) with a wide variety of other fish, molluscs, and aquatic plants. Urbanization has increasingly shifted the demand from subsistence to marketed fish 25 .
A key characteristic of freshwater aquaculture growth during the past 20 years has been the proliferation of value chains in and across countries located in South and Southeast Asia, for example, in Andra Pradesh, India 26 , Bangladesh 24 , Myanmar 27 , Thailand, 28 and Vietnam 29 . China remains the single largest producer of freshwater fish—for export and domestic consumption—accounting for 56% of the global output in 2017 (Extended Data Table 1 ). The expansion of freshwater aquaculture in Asia (93% of global production) has been driven mainly by urban demand and the decline in wild inland fisheries that previously supported rural livelihoods and food security 30 .
Diverse value chains underpinning freshwater aquaculture in Asia have emerged with limited governmental support, spurred by economic development, rural transformation, and urbanization. These processes have boosted purchasing power and fuelled the demand for freshwater fish, paving the way for the expansion of private sector investment 27 , 31 . The development of aquaculture in small- to medium-scale commercial enterprises in South and Southeast Asia has helped to alleviate rural poverty, through direct benefits to consumers and other value chain participants 21 , 32 and broader ‘spillover’ benefits to labour and livelihoods in adjacent industries 33 . A similar process of the development of freshwater aquaculture is now occurring in parts of sub-Saharan Africa 15 , albeit shaped by different social and economic constraints to production, structures of the value chains, and consumer demand 16 , 34 , 35 .
Given the heterogeneity of freshwater aquaculture systems, much of the recent literature focuses on system diversity, nutrition security, and value chains, particularly within the Asian context. Generalizations regarding freshwater production practices, resource depletion, and environmental constraints are limited, but three lessons emerge.
First, over-intensification, particularly in cage aquaculture, has created problems of nutrient pollution and pathogen-related production declines in areas with unconstrained growth, such as Lake Taal, The Philippines 36 . Cage culture in deep lakes and reservoirs can be subject to turnover and related mortality due to sudden anoxic conditions 37 . In regions in which freshwater resource depletion, nutrient pollution, disease problems, and other constraints on the use of public waters have emerged, industry consolidation has often followed, forcing poor producers out of the sector 29 , 38 , 39 . In China, aquaculture pollution accounts for more than 20% of the total input of nutrient into freshwater environments in some provinces 40 , leading to prohibition in many public water bodies that are essential for drinking water and other important ecosystem services 41 . In other regions, in-pond raceway systems have been promoted to enhance feed-use efficiency and solid-waste removal (for example, channel catfish, carps and tilapia), but widespread adoption has been constrained by high capital costs 42 .
Second, and related to production intensification, compound feed use in freshwater systems has steadily increased, driven by local and international companies and certification initiatives operating across a range of production systems and countries 3 , 43 . An estimated 92% of tilapia, 81% of catfish and 57% of Chinese carps rely on some combination of commercially formulated pelleted feed and feed types made at the farm to supplement the naturally occurring nutrients produced in the culture systems 3 . Fertilization, combined with supplementary feeds, remains a key approach to producing low-cost tilapia, catfish, and carp in semi-intensive systems, and has underpinned the growth of commercial production in Asia.
Third, the steady emergence and proliferation of relatively low input–output culture-based fisheries through different forms of collective management has permitted access to, and control of, aquatic commons (for example, floodplains, reservoirs, and seasonal water bodies) 44 . Field studies show that productivity gains from non-fed, often exotic carp have generally been achieved in low-input systems while maintaining or enhancing nutrient balances and the biodiversity of indigenous species 45 .
These three trends result in a sector tightly integrated into terrestrial food systems via feed, nutrient cycling, and value chains. Scientific knowledge surrounding freshwater aquaculture and local resource use is extensive, especially in an Asian context. In comparison to ocean-based production, however, the global environmental impacts of freshwater aquaculture remain understudied. Specifically, the trend to intensify freshwater systems is increasingly linked to globally sourced feed ingredients that represent a critical area of the overall environmental impact of the aquaculture sector 46 .
Fish feed and wild fisheries
A major focus of the previous aquaculture review 1 was the increasing proportion of annual fishmeal and fish oil production for aquaculture feed, and the consequent potential future impacts on wild forage fish landings and stocks as well as marine ecosystems. In aggregate, global landings of forage fish have trended downward (Extended Data Fig. 2 ), reflecting full to overexploitation, and harvest restrictions (for example, in Peru) to prevent fishing above maximum sustainable yield levels.
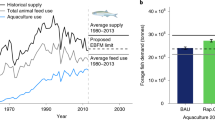
The aquaculture sector has made considerable progress in enhancing the efficiency of use of marine resources over the past 20 years. The global production of fed fish tripled between 2000 and 2017 3 while the annual catch of forage fish used to make fishmeal and fish oil decreased from 23 Mt to 16 Mt (refs. 47 , 48 ) (Extended Data Fig. 3 ). Global production of fishmeal from capture fisheries and trimmings decreased over the same period from 6.6 to 4.8 Mt (ref. 17 ). The production of fish oil declined from around 1.5 to 1.0 Mt and has been stable around 1.0 Mt during the past decade 49 , 50 , 51 .
Prices for fishmeal and fish oil have more than doubled during the 2000s and have remained consistently higher than plant-based alternatives since 2012 (Extended Data Fig. 4 ). Aquaculture producers have responded by reducing the use of fishmeal and fish oil in feed formulations, and these efforts have been reinforced by sustainability goals throughout the supply chain. Fishmeal and fish oil remain important ingredients of fish feed, suppling essential nutrients to support larval and fry performance and survival, but are now used at lower percentages in grow-out, broodstock, and finishing feeds. Nonetheless, the share of global fishmeal used by the aquaculture sector (versus livestock and non-food uses) increased from 33% in 2000 to 69% in 2016, while the share of global fish oil used by aquaculture rose from 55% to 75% (refs. 50 , 52 ). A continuation of this trend could push fishmeal and fish oil prices higher, creating further incentives for innovations in aquaculture feed.
Four major developments along the aquaculture supply chain have helped to reduce the dependence on wild fish resources since 2000: rapid growth in omnivorous species production; improved feed conversion ratios (FCRs) for all fed species; higher use of alternative protein and oil ingredients in feed; and increased production and use of fishmeal and fish oil from fish-processing wastes and bycatch. In addition, improvements in processing technologies have increased fishmeal recovery from anchovies and other pelagic species from 22.5% to 24% over the past few decades 53 . Fish oil recovery remains around 5% for anchovies and about 10% for fatty fish such as herring, capelin, and sand eel, which are used widely in the production of fish oil in Europe.
Between 1997 and 2017, the volume and share of freshwater fish produced with compound feeds, such as fed carps, tilapia, and catfish, increased substantially, but FCR also improved (Extended Data Table 2 ). Meanwhile, fishmeal inclusion rates dropped for these species to 1–2%, and there is almost no fish oil used in most types of freshwater aquafeed. Compound feed types for marine and brackish water finfish and crustaceans remain higher in fishmeal and fish oil, but their fishmeal and fish oil inclusion rates decreased by one-half to two-thirds over the period. For shrimp, there has been a major global shift in production away from black tiger shrimp to the more omnivorous whiteleg shrimp. Breeding strategies for salmon and trout and improvements in feed ingredient quality and formulations have permitted much higher inclusion of plant protein concentrates in feed 54 .
The increasing use of trimmings in fishmeal production, particularly for lower-valued freshwater species, has also had a critical role in lowering the use of wild fish in feed since 2000 (Table 1 ). The estimated use of trimmings is three times the use of wild fish in fishmeal for tilapia and catfish. Even high-valued marine and brackish species, such as salmon and shrimp, use equal ratios of fishmeal from trimmings and wild fish in their feed. Trimmings from both wild fisheries (for example, tuna in Thailand) and aquaculture (for example, salmon in Norway, pangasius in Vietnam) now comprise roughly one-third of global fishmeal production and one-half of fishmeal production in Europe 3 , 8 , 47 . Greater use of trimmings in fishmeal has been documented, in particular, in feed formulations for salmon production in Norway 55 and for shrimp and catfish production in Thailand 44 .
The combination of improved FCR, reduced fishmeal and fish oil inclusion ratios, and increased use of fishmeal from trimmings have lowered the ratio of wild fish inputs to farmed fish output (fish-in:fish-out ratio (FIFO)) (Extended Data Tables 2 , 3 ). On a global basis, FIFO was 0.28 in 2017 for the main aquaculture species groups that are dependent on feed (Table 1 ). FIFO exceeded 1.0 for shrimp, salmon, trout, and eels, but was still far below the FIFO calculated for these species 20 years ago. The previously published review of aquaculture and world fish supplies 1 calculated a global FIFO for fed aquaculture species of 1.9 using 1997 data (FIFO by species group was 2.81 for shrimp, 5.16 for marine fish, 3.16 for salmon, 2.46 for trout, 4.69 for eels, and below 1.0 for all freshwater fish). Calculations in Table 1 include the residual availability of fishmeal and fish oil from feed across different species groups, which can be used for global aquaculture feed production—thus addressing a point of contention related to earlier FIFO calculations 8 , 47 , 56 .
Despite the positive contribution of trimmings to global fishmeal production, aquaculture production in Asia—notably China, Thailand, and Vietnam—still relies on low-value feed-grade fish from non-targeted fisheries (including quasi-targeted bycatch) as an input for feeds 57 . In 2017, Asian aquaculture systems consumed more than 6.6 Mt of low-valued fish as direct or indirect feed inputs 17 . Roughly one-third of the Chinese domestic fish catch comprises low-valued fish (89% juveniles) that are used mainly in aquaculture feeds 57 . Such feed-grade focused fisheries can affect wild fish populations and marine ecosystems considerably through the capture of juvenile fish and loss of biodiversity 12 , 57 .
Feed from land and sea
Although marine resources continue to have an important role in aquafeed, the use of plant-based ingredients has been increasing steadily, creating tighter connections between land and sea. The aquafeed industry has become increasingly dependent on conventional animal feed ingredients from terrestrial systems that are widely traded in international markets (Fig. 2 ).
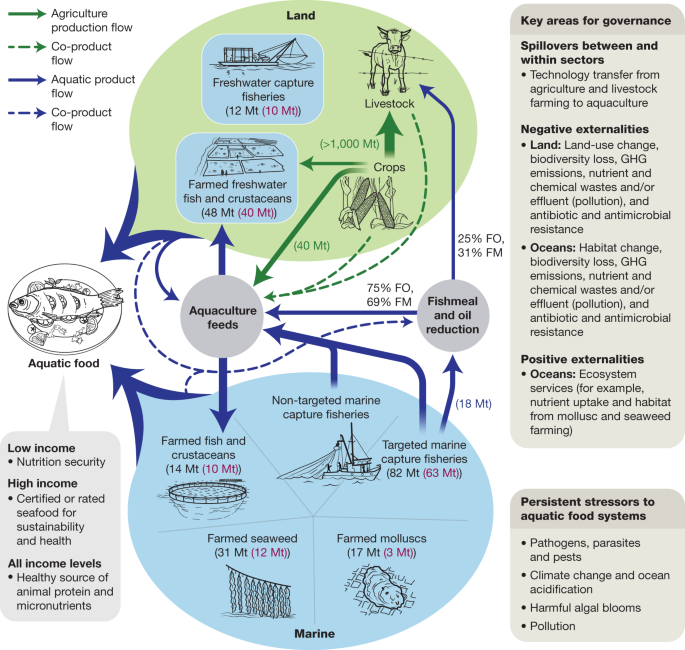
Blue arrows show the flows of aquatic products from freshwater and marine systems, including wild fish used as fishmeal and fish oil in animal feeds. Green arrows show the flows of terrestrial feed products. Co-products from terrestrial and aquatic processing used in feeds are indicated by dotted arrows. Live-weight and edible production from aquaculture sources are indicated in black and red numbers, respectively. Fishmeal (FM) and fish oil (FO) shares used in aquaculture and livestock production are also shown. Aquafeeds include a majority of ingredients from agriculture, and co-products from processing of terrestrial and aquatic foods are used as ingredients for fish feeds. Data sources: edible conversions 9 and production data 2 , 49 , 50 , 51 , 204 , 205 . GHG, greenhouse gas.
Three factors have contributed to the expanding role of terrestrial food systems in global aquaculture: feed ingredients tailored to fish; feed formulations based on accurate nutritional requirements; and breeding to enhance fish growth, feed efficiency, and animal health. Feed ingredients from grains and oilseeds are the basis of livestock feeding, but carnivorous fish have difficulty digesting starch, non-soluble carbohydrates, or fibre in these ingredients. They are also more sensitive than livestock to antinutrients and toxins in plant protein ingredients 58 . Additional processing steps have been introduced to increase the nutritional value of plant and land animal protein concentrates for fish 59 , 60 , 61 . Alternative oil sources—including rapeseed (canola) oil, palm oil, and poultry fat—are now commonly used substitutes for a portion of fish oil 62 . Although farmed salmon remain a good source of omega-3 fatty acids, replacing fish oil with terrestrial oils lowers the omega-3 content in fillets 63 . The use of high omega-3 oils from algae or genetically modified oilseeds can reduce fish oil use in salmon feed while maintaining the health benefits to consumers, but this remains economically inefficient and, in some markets, the latter is constrained by weak consumer acceptance 59 , 64 .
Replacing fishmeal and fish oil in feed with plant-sourced products affects the health of piscivorous aquaculture species through alterations of the microbiome, changes in gut morphology, modification of immune function, and interference with normal function of the endocrine system and maturation 65 , 66 . Moving towards full plant-based diets for these species thus increases disease risks. New tools, including high-throughput technologies (metabolomics and proteomics), RNA sequencing, polymerase chain reaction (PCR) and whole-genome sequencing, have been used since 2000 to detect and mitigate these problems 67 . Conventional breeding and marker-assisted selection have also been used to improve fish growth and health, and lessons from terrestrial animal breeding, especially poultry, have been used to advance breeding strategies for fish 68 , 69 . For example, genetically selected trout, which show improved weight gain of 10–15% per generation on fully plant-protein feeds 70 , are able to digest amino acids from plant proteins in a similar temporal pattern as fishmeal and do not develop distal enteritis in the intestine when fed high-soy diets 71 .These tools have thus far been applied to only a few high-valued aquaculture species.
The increasing share of plant-based ingredients in mariculture feed types, coupled with the steady growth in feed use in freshwater aquaculture, has led to a new set of controversies surrounding resource use and the environmental effects of terrestrial crop production for aquafeed. Life cycle analyses indicate that feed accounts for more than 90% of the environmental impact from fed aquaculture production 72 , 73 . Studies modelling fishmeal replacement with plant-based proteins (for example, soy protein concentrate) in shrimp 74 and salmon 75 show potential increases in ecotoxicity from fertilizer and pesticide use, rising pressure on freshwater and land resources, and heightened carbon emissions and biodiversity loss from forest clearing—particularly in Brazil.
Aquaculture producers seeking to market sustainable products are therefore faced with the unintended environmental and social consequences of their feeding practices. For example, between 2000 and 2016, the Norwegian salmon aquaculture industry cut its shares of marine protein in feed from 33.5% to 14.5% and marine oils from 31.1% to 10.4%, and increased the shares of plant proteins from 22.2% to 40.3% and terrestrial oils from 0 to 20.2% 76 . Despite its success in substituting fishmeal and fish oil with plant-based alternatives, including non-genetically engineered soy, the industry has been under pressure to identify new feed sources to eliminate the environmental damages associated with forest conversion to crop production in Brazil 77 , and parts of the industry have already banned the use of Brazilian soy in aquafeed.
Although certain segments of the aquaculture industry, such as salmon, face sustainability challenges with terrestrial feed sourcing, the share of global animal feed used as aquafeed is small—estimated at 4% (compared with roughly 40% for poultry, 30% for swine, and 25% for ruminants) 43 . Many terrestrial feed ingredients for aquaculture are by-products, such as oilseed protein concentrates extracted from the processing of food products, or protein meals and oils recovered from the processing of livestock and seafood (including aquaculture) 43 , 59 . Recycling processed by-products and food wastes into high protein feed ingredients contributes to the sustainable production of food globally, but life-cycle analysis is needed to measure the net environmental impact.
Nonetheless, terrestrial crop demand for aquafeed is expected to rise in the future as the production of finfishes and crustaceans expands in freshwater and marine systems 43 , 74 . Rising demand will probably place pressure on natural resources and feed prices. Research on new feed ingredients has proliferated recently 59 , 74 , 78 , 79 , 80 , 81 and will continue to expand. Single-cell proteins, insect meal, and microalgae represent early stage technologies with potential for replacing fishmeal and fish oil in aquaculture feed 81 .
Extractive species
Extractive species—molluscs and algae—have doubled in volume since 2000 (Fig. 1b ) and represent the third area of aquaculture development. Extractive filter-feeding bivalves and algae accounted for 43% of total (live-weight) aquacultural output in 2017 2 . On an edible-weight basis, however, molluscs and algae comprised only 6% and 7.6%, respectively, of total aquaculture output 9 . These groups also provide a wide range of ecosystem services and non-food products 8 , 82 , 83 , 84 , 85 .
Molluscan aquaculture includes approximately 65 reported species, mainly bivalves (clams, oysters, scallops, and mussels) 3 . Clams, for example, Japanese littleneck (carpet shell, Venerupis philippinarum ), and Pacific cupped oysters ( Crassostrea gigas ), account for two-thirds of the total. Bivalves do not require feed inputs, making them attractive candidates for the expansion of sustainable seafood—a point that was made in the previous review 1 and has been argued for more than 30 years 82 , 84 , 86 , 87 , 88 . Some high-value farmed molluscs, such as abalone and conchs, are herbivorous and reliant on feed, but they account for only 2.4% of cultivated molluscan output 3 .
The global production of farmed molluscs grew at an annual rate of 3.5% between 2000 and 2017, which is lower than that of farmed fish (5.7%) and crustaceans (9.9%) 3 . In China, however, bivalve culture expanded considerably in response to consumer demand. Between 2005 and 2014, the volume of scallops increased by 80.4%, clams by 40.8%, oysters by 30%, and mussels by 19% 84 . China is the largest consumer and producer of molluscs, accounting for 84% of global cultivated volume in 2017.
In addition to seafood, outputs from molluscan aquaculture are used in a variety of industrial products, such as fertilizers, construction materials, poultry grit, pharmaceuticals, and nutraceuticals 82 , 84 . Bivalves also provide important benthic and coastal ecosystem functions. By filtering phytoplankton and accumulating nitrogen and phosphorous, they remove nutrients from the ambient environment when harvested. In addition, molluscan aquaculture can provide habitat structure, shoreline stabilization, and local incomes for waterfront communities 82 , 84 , 87 , 89 . The role of bivalves as a carbon sink or source remains unclear, however, and research aimed at measuring carbon sequestration and system performance from these systems is ongoing 84 , 90 , 91 .
The most widely recognized ecosystem service of molluscan aquaculture is the assimilation of excess nutrients from human activities, for example, agriculture, aquaculture, and sewage discharge. Bivalves filter large volumes of water daily, and their abilities and impacts are species- and area-specific 82 , 84 , 92 . Nutrient extraction has two modes: harvest and removal of the bivalves, and increased denitrification near dense populations of wild or farmed bivalves. The ability of bivalves to mitigate coastal eutrophication fully requires large-scale production and a considerable reduction in nutrients at the source is also needed in most cases 93 . Efforts have been made to introduce new markets for bivalves that generate offset credits for non-point source pollution, but these markets have yet to develop at scale 84 , 94 , 95
Although bivalves can enhance water purification and water clarity, they also absorb viruses, bacteria, toxic algae, and polluted organic particles from the ambient environment. Food safety risks are therefore high for molluscs cultivated in polluted environments. Moreover, the introduction of large densities of filter-feeding bivalves to a habitat, whether in suspended or bottom culture, has the potential to impart negative changes in the water quality and benthic ecosystems (for example, depletion of phytoplankton and seston, and localized increases in sedimentation rates through bio-deposition) and can present serious disease risks 96 , 97 . Most negative impacts of bivalve production are site- and species-specific, and uncommon 98 . Negative environmental impacts may ensue if aquaculture systems are overstocked, inappropriately sited, or unsustainably managed, as indicated in certain cases in China 99 , 100 . Assessment of the influence of bivalve farming on the surrounding environment can be a complex process. As in many aquaculture systems, however, the application of carrying capacity models 101 , 102 , 103 , 104 and routinely modified best management practices 105 have continuously improved the sustainability of molluscan culture.
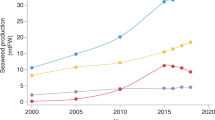
Since 2000, there has been a growing appreciation for algae (dominated by macroalgae or seaweed) for improved nutrition, industrial use, and ecosystem services, even in regions outside China, Japan, Korea, and parts of South America, where seaweeds have been consumed as food for centuries 83 , 106 , 107 . The global production of aquatic plants and algae has tripled from 10 Mt of wet biomass in 2000 to more than 32 Mt in 2017, with aquaculture contributing more than 97% of the current volume 17 , 106 . Of the 32 Mt of cultured algae—99% of which is produced in Asia—between 31% and 38% is consumed directly as food (Extended Data Table 4 ). The majority is used by the food industry sector as polysaccharide additives and functional food ingredients, and by the non-food sector as hydrocolloid products in nutraceuticals, pharmaceuticals and cosmetics, and to a lesser extent as fertilizers, feed ingredients, biofuels, bioplastics, and other industrial outputs 106 , 108 , 109 .
Research in recent decades has explored the potential for seaweeds to substitute for terrestrial crop and animal production in protein, fat (omega 3) and energy intake—alleviating pressure on freshwater and land resources and biodiversity—but there is little evidence to date that seaweeds can contribute substantially to human macronutrient intake 110 . Numerous studies have highlighted the micronutritional and sensory attributes of seaweeds for direct human consumption 111 or as functional foods 112 , but benefits are difficult to quantify because of variation across species, seasons, and coastal environments, and a lack of clear scientific evidence regarding nutritional bioavailability and metabolic processes associated with algal consumption 110 . Research has examined the use of microalgal biomass in aquaculture feed as a cost-competitive replacement for fishmeal and the use of macroalgae in dairy and cattle feed to reduce methane emissions 113 , but these types of feed have yet to develop commercially at scale.
Like molluscan aquaculture, seaweed culture is widely recognized for its ecosystem service values beyond the provision of food and feed, yet producers have not been able to capture this value in financial returns 114 . Bioremediation is the main ecological service reviewed in the literature. Some seaweed systems receive additional fertilizers, for example, in low-nutrient coastal zones, although fertilization is regulated in Japan and South Korea 115 . Ongoing research is also investigating the role of seaweed culture in mitigating ocean acidification, sequestering carbon, and enhancing biodiversity 116 , 117 , 118 . In China, studies suggest that large-scale seaweed aquaculture is effective in reducing nitrogen levels, controlling phytoplankton blooms, and limiting the frequency of toxic algal blooms 119 , 120 . Considerable variability exists, however, in the potential provision of seaweed ecosystem services across cultured systems, seasons, and scales.
Seaweed aquaculture lags behind other food sectors in breeding, pathogen management, and optimization of production systems for nutrient, light and temperature conditions 83 . Bacterial and viral outbreaks are especially high in intensively farmed seaweed systems, where disease management can account for up to 50% of farm-variable costs 106 , 121 . New seaweed cultivars with higher yield potential, disease resistance, nutritional qualities, and consumer attributes are needed to ensure production growth and increased value for the industry 108 , 122 .
Overall, progress in research and development for the seaweed industry has not met expectations in recent decades 108 . A few major exceptions include China’s success in cultivating alginate-bearing seaweeds ( Saccharina japonica , also known as Laminaria japonica ) and the expansion of agar-bearing seaweed aquaculture ( Gracilaria ) at scale. The industry remains fragmented outside Asia (mainly China and Indonesia), and competitive pricing constrains net revenues and incentives for innovation 108 . Value in the seaweed industry could be enhanced through the adoption of a ‘biorefinery’ approach to processing, in which the most valuable products from the algal biomass are extracted sequentially, leaving the remaining material for commodity uses and minimizing waste, energy inputs and environmental harm 123 . This approach has been successful in various segments of terrestrial agriculture. New global initiatives to promote seaweed production and use 124 will need to tackle critical social, economic, and regulatory constraints, including unethical supply chain activities 125 , food safety considerations, and limited consumer demand 83 , 106 , 126 .
Persistent challenges
Over the past 20 years, trends in the production and environmental performance of aquaculture have been positive. Destructive habitat conversion, particularly by shrimp farming in mangrove ecosystems raised in the previous review 1 , has declined markedly since 2000 127 , 128 . Challenges to the industry persist, however, including the effects of pathogens, parasites, and pests (PPP), pollution, harmful algal blooms, and climate change. The aquaculture industry has become increasingly vulnerable to these stressors given its rapid expansion, its reliance on the ambient environment, and the changing world in which all food systems operate 43 , 129 .
Pathogens, parasites and pests
Pathogens, parasites, and pests (PPP) are a chronic risk for the aquaculture sector, and the intensification of production and increased trade and supply chain integration since 2000 have amplified these risks 130 . Aquaculture species differ in their defences, and although invertebrates lack the adaptive immunity of finfish, their innate immune system—which is certainly not simple or homogenous—is not fully understood 131 , 132 , 133 . The gut is an important component of the immune system for finfish, which allows diet and alterations in the microbiome to influence the susceptibility and potential resistance of finfish to disease, whereas the external microbial communities are vitally important for the health status of invertebrates 134 . For most high-value and widely traded species, there have been substantial advances in PPP identification, diagnosis, and treatment over the past 20 years, derived in part from innovations in agriculture and human medicine 131 , 132 , 134 , 135 . Such science-led disease management options remain largely unavailable for many low-value aquaculture species and low-income regions owing to a lack of product development and prohibitive costs. Global networks, such as the World Organization for Animal Health, have emerged to facilitate the transfer of scientific knowledge.
The aquaculture industry has responded to PPP pressures in recent decades using a variety of approaches. Adoption of best management practices (for example, for site and system selection, stocking densities, species rotations, broodstock, and feed quality, filtration, pond, and cage cleanliness, parasite monitoring and removal, culling, zoning, and surveillance) has been the most important means of minimizing PPP risks across all types of production systems 25 , 134 . Once a pathogen, parasite, or pest is widely recognized in a given system, avoidance through biosecurity is the primary management action available to most aquaculture producers 136 . In some systems in which epizootics have caused boom-and-bust cycles, resistant species have been introduced, provided that viable markets exist 137 . For example, the aquaculture industry in Thailand transitioned from black tiger shrimp to whiteleg shrimp, largely because of problems with infectious diseases, specifically white spot disease and monodon slow growth syndrome 138 , 139 .
The use of therapeutants—chemical substances used to prevent and treat pathogens—including antimicrobials, has become a common practice in many aquaculture systems 140 . There are no comprehensive data on the nature and extent of therapeutic use in most aquaculture sectors, and both good and bad practices are found worldwide 141 , 142 , 143 , 144 . Although improper therapeutant use can pose risks to the health of consumers, workers, cultured organisms, and surrounding ecosystems (particularly in open production systems) 96 , 142 , the misuse of antimicrobials in aquaculture is especially problematic as it can lead to the emergence and transfer of antimicrobial-resistant genes and bacteria 140 .
As an alternative, large investments have been made in selective breeding for disease resistance in certain aquaculture species, but this avenue is costly and cannot easily be replicated across species 145 . Effective multivalent vaccines have also been introduced for some high-value species such as salmon and trout 146 , and show promise for replication in marine species aquaculture if efficient and cost-effective delivery systems (for example, oral or immersion) can be developed 147 . Vaccines developed for farmed salmon have led to reductions in antibiotic use of up to 95% in Norway, the UK, Ireland and Canada, but antibiotic use remains high in Chile 143 . Advanced water management through recirculating aquaculture systems, as discussed in the following section, represents another important, but relatively costly, technology for controlling PPP 148 . In addition, supplementation of feed with nutraceuticals, plant extracts, prebiotics, and probiotics is used to boost fish growth and immunity and serves as a promising alternative to antibiotics—mainly in high-value production systems, but also increasingly in lower-value freshwater systems in Southeast Asia 142 .
Even in sectors in which major investments and progress have been made in the detection, avoidance, and treatment of PPP, new threats frequently emerge. For example, the salmon aquaculture industry has successfully controlled some diseases, such as infectious pancreatic necrosis virus and infectious salmon anaemia, but other diseases and parasites (for example, salmon rickettsial syndrome and sea lice) remain costly for many producers and damaging to wild salmon as treatment options are either unavailable or the target organism has become resistant to treatment 131 , 143 , 149 , 150 . Similarly, despite the shift from black tiger shrimp to whiteleg shrimp, emerging diseases such as white spot disease, acute hepatopancreatic necrosis disease, shrimp hemocyte iridescent virus, and the microsporidian parasite ( Enterocytozoon hepatopenaei ) have resulted in substantial production losses and sustained economic costs to the shrimp industry 136 , 151 , 152 , 153 .
As aquaculture production expands into new geographies, PPP outbreaks and the risks to human health from therapeutic management approaches will probably increase, particularly in low-income regions. Studies also project increased risks of aquaculture disease incidence and antimicrobial resistance associated with disease management owing to global warming 154 , 155 , 160 . The quantification of trends in PPP is, however, complicated by variation between national and international disease monitoring and treatment regulations and by a lack data for most aquaculture species and production regions 157 . In the absence of reliable data, the incidence and management of PPP throughout the global aquaculture industry is and will remain highly unpredictable.
Harmful algal blooms and climate change
Harmful algal blooms are increasing globally with respect to frequency, magnitude, duration, geographical ranges, and species composition, and are driven largely by anthropogenic processes 98 . They occur in aquaculture areas worldwide, and their influences on production vary widely depending on species-specific effects 98 , 158 . Intensive and poorly managed finfish and crustacean systems can contribute to the emergence of harmful algal blooms, and shellfish, sea urchins, and sea cucumbers are common vectors for toxic microalgae 98 . Toxic blooms represent a large economic cost to parts of the industry for which monitoring and management are ineffective. Large blooms of Pseudochattonella and Karenia in southern Chile in 2016 caused salmon mortalities of 40,000 tonnes and required several salmon, mussel, and abalone operations to close for 2 years because of food safety risks, generating economic losses of around US$ 800 million 98 , 159 .
Climate-driven losses to aquaculture productivity and livelihoods stem mainly from suboptimal growing temperatures, sea-level rise (saltwater intrusion), infrastructure damage, droughts and freshwater shortages, and rising feed costs associated with lower crop yields and forage fish landings 156 , 160 . Risks to aquaculture infrastructure often drive investments to more protected geographies and systems. In addition, ocean acidification affects shellfish production, mainly at the larval life stage, and is managed through adjustments in pH within the hatchery 161 . The literature does not support generalizations of the damages of ocean acidification to shellfish aquaculture given the species-specific responses documented, sparse data, uneven and questionable experimentation, and the complexity of pathways through which species are affected 162 . Climate change also amplifies the uncertainties surrounding PPP and harmful algal blooms in aquaculture 159 , 160 , 163 and predictions remain uncertain 98 , 164 . In general, scientific studies on climate–aquaculture interactions are based on laboratory-based tolerance data and modelled, but not validated, for commercial aquaculture and thus remain speculative 165 , 166 , 167 , 168 . There are no comprehensive data on climate-driven production and economic losses in aquaculture at regional or global scales, and outcomes are contingent on adaptation responses 129 .
Responding to the challenges
Increased attention has been directed to ecosystem-based management, system design, and new forms of private and public sector governance to manage biological and climate risks, and encourage sustainable aquaculture production 86 , 169 , 170 . Integrated multi-trophic aquaculture has shown high bioremediation capacity in China 120 , 171 , but has demonstrated limited commercial success globally despite considerable research interest 172 , 173 . Recirculating aquaculture systems and offshore aquaculture have promising growth potential.
Recirculating aquaculture systems
Recirculating aquaculture systems are designed to control all environmental facets of production by continually filtering, treating, and reusing water, and thereby increasing operational efficiency and reducing risks from PPP and climate change. Recirculating aquaculture systems have lower direct land and water requirements than conventional aquaculture and enable higher stocking densities 174 but are constrained by large energy requirements, high production costs, waste disposal challenges, and risk of catastrophic disease failures 78 , 175 , 176 .
Recirculating aquaculture system technologies are typically used when advantages in fish performance outweigh the increased costs—for example, for broodstock and vulnerable early life stages 175 , 177 and recently for full-life cycle production of salmon. Applications of recirculating aquaculture systems within raceways and channelled pond systems for shrimp aquaculture are also cost-effective in many farming areas given high disease and water-quality risks 148 . Grow-out operations using recirculating aquaculture system technology are progressively focused on species with high market value, established production protocols, and production models that are large enough to realize the efficiency benefits of scale 177 , 178 . The competitiveness of recirculating aquaculture systems for full grow-out relative to other production systems remains uncertain, however, and there have been several failures in North America and Europe and few large-scale, commercial successes over multiple years 179 .
Offshore aquaculture
Offshore aquaculture in deep and open ocean waters is designed to produce large volumes of fish while minimizing land and freshwater constraints and coastal environmental impacts, such as nutrient pollution and sea lice infestations 78 , 180 . Prudent siting is required, however, to avoid conflicts with other marine uses and to ensure the effective dilution of wastes, particularly for large-scale systems 181 . Norway and China lead in offshore fish aquaculture with the introduction of massive submersible cages 182 , 183 , 184 . Given large capital costs and high risk-to-return ratios, offshore aquaculture in other countries has been confined mainly to small-scale pilot operations cultivating high-valued, carnivorous species. Offshore environments present a range of operational challenges (for example, water depth, strong currents and waves, and storms), which have induced several new design approaches 180 . Government regulations have constrained commercial development of offshore aquaculture, particularly in the USA and European Union, because of public controversy regarding its interactions with the marine environment, potential ecological damage, and competing uses of ocean and natural resources 185 , 186 .
Aspirations to improve the environmental and social performance of aquaculture practices and technologies have led to the emergence of new combinations of public and private regulation, codes and standards 187 ; however, the application of these governance instruments has struggled to match the expanded geographies, volumes, and diversity of aquaculture systems 188 . The uneven implementation of government regulation has led to regional disparities in production, growth and system design. Governments have facilitated aquaculture expansion in many Asian countries, Norway, and Chile, whereas in other regions—including the European Union and USA—governments have constrained growth 15 . In very few countries, such as Norway, has strict environmental regulation allowed the sector to expand by coordinating governing institutions to support planned aquaculture growth 15 . Uneven regulation has led to disparities in investment and trade, with only a few export nations selling into major net seafood importing markets such as the USA and European Union.
In response to public over- and under-regulation, several types of private governance arrangements have emerged with the intention of shaping demand for sustainable, ‘fair’, and organic aquaculture production. For example, 30–50 voluntary labelling, certification and rating schemes have been introduced by non-government organizations and private companies 189 , 190 .
Farm-level certification is setting new norms for sustainable aquaculture globally 191 , yet the role of certification remains limited by low (yet growing) levels of producer compliance. The two largest certification groups—the Aquaculture Stewardship Council (ASC) and the Global Aquaculture Alliance Best Aquaculture Practice (GAA-BAP) standards—account for 3% of global aquaculture production (Extended Data Fig. 5 ). Low levels of compliance have been attributed to insufficient finances, low demand for certified products, poor literacy levels, and inadequate administrative skills required for monitoring and reporting 192 , 193 , and environmental production risks beyond the control of the producer 194 . Consumer guides such as the US Seafood Watch have rated a further 53% of global production (Extended Data Fig. 5 ). These ratings are involuntary and based on broad-scale assessments at the sector or regional level.
Certified and rated production is skewed to major export species. Overall, 57% of salmon and trout, 17% of shrimp and prawns, 17% of pangasius and 11% of tilapia are certified (Extended Data Fig. 6 ), with higher levels of compliance observed in countries with a greater proportion of vertically integrated supply chains 38 , 195 , 196 . Domestic demand for sustainable products in Asian seafood markets appears to be increasing, driven by food safety concerns 197 , but considerable growth in domestic demand for sustainable seafood is needed to make aquaculture certification and rating systems effective globally 187 .
States can enhance the success of private governance arrangements by providing capabilities, resources, and minimum regulation to support improvements in farm practices. Both certification and consumer guides have now started shifting to ‘hybrid’ forms of governance 190 , which integrate private assessment tools into spatial management units that are managed in collaboration with buyers and states 198 . These ‘beyond farm’ forms of management aim to foster greater inclusion of large and small-holder producers in a given jurisdiction to minimize PPP, climate, and other ecological risks 169 . They are also increasingly aimed at avoiding spatial conflicts, promoting the trade in bio-derivatives, and creating new ecosystem and climate services markets 199 , 200 , 201 , 202 . They may also enable greater transparency and trust of aquaculture products exported from developing countries and create inclusive improvement pathways for the 90% of aquaculture output that is not directed towards export markets.
Over the past 20 years the aquaculture sector has evolved from having a relatively minor role to playing a mainstream part in the global food system. The aquaculture literature reflects the increased attention to food system outcomes, with consumers, value chains, and sustainability criteria progressively shaping the direction of the industry. Continued growth in the sector has important implications for achieving the United Nations Sustainable Development Goals.
Three key patterns emerge in this Review. First, freshwater fish have a central role in the global production, contributing more than any other aquaculture sub-sector to the total (live and edible) volume, rural livelihoods, and food security during the past two decades. Because most farmed freshwater fish do not enter the global market, however, there is currently little impetus for producers to engage in sustainable practices with recognized ratings or certification. Second, marked improvements have been made in the efficiency of marine resource use across all fed species and in the field of fish nutrition. Further gains in these areas may be more difficult and costly to achieve for carnivorous species, but the increasing costs of fishmeal and fish oil that are associated with marine resource limitation will provide continued incentives for innovation. Third, careful siting of aquaculture systems underpins the commercial and environmental success of the industry. Almost all freshwater and marine aquaculture systems interact with the ambient aquatic environment and both benefit from and provide environmental services to the ambient environment as a result. Prudent siting and scaling are essential for maximizing the ecosystem services provided by farmed extractive species and for mitigating critical challenges to the industry associated with PPP, coastal pollution, and climate change.
The wide diversity of aquaculture systems across species, geographies, producers, and consumers prevents the development of a single strategy to achieve sustainable and healthy products. Governance systems need to be designed with clearly articulated, science-informed goals, but without overly proscriptive standards and regulations for realizing those goals. Such flexibility is needed to support the abilities of industries, governments, and non-government organizations to innovate while still providing clear end points and requirements for monitoring, reporting, transparency, and accountability. The aquaculture sector will continue to face large uncertainties in the future, including climate change, evolving PPP pressures, pandemics, and market disruptions and changes in food systems more broadly.
Looking ahead, the effective spatial planning and regulation of aquaculture sites will be paramount for achieving positive environmental outcomes, especially as aquaculture systems increase in scale and production intensifies. The industry is investigating recirculating and offshore technologies to reduce its exposure to and impact on aquatic environments; however, these systems will require innovative financial and environmental management to have any chance of widespread success. In addition, investments are needed in an array of PPP prevention strategies across different aquaculture sub-sectors, recognizing that treatments after PPP problems emerge are largely futile. Finally, future policies and programmes to promote aquaculture will require a food systems approach that examines nutrition, equity, justice, and environmental outcomes and trade-offs across land and sea. Tools such as life cycle analysis will need to be refined and deployed to ensure comparability between terrestrial livestock and aquaculture production on the basis of nutritional value and global environmental outcomes. Research along these lines, as advanced through new studies including the ongoing Blue Food Assessment 203 , will undoubtedly be documented in the next 20-year retrospective review. Aquaculture systems can be designed and implemented to be highly sustainable. The human dimension presents both the opportunity and the challenge.
Change history
06 july 2021.
A Correction to this paper has been published: https://doi.org/10.1038/s41586-021-03736-4
26 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41586-021-03508-0
Naylor, R. L. et al. Effect of aquaculture on world fish supplies. Nature 405 , 1017–1024 (2000). This paper, the original study that motivated this 20-year retrospective Review, provides an analysis of the use of wild fish in aquafeeds and the contribution of fed aquaculture to the net balance of seafood supplies .
Article ADS CAS PubMed Google Scholar
FAO. Fisheries and Aquaculture Software. FishStatJ: Software for Fishery and Aquaculture Statistical Time Series http://www.fao.org/fishery/statistics/software/fishstatj/en (FAO Fisheries Division, 2019).
Tacon, A. G. J. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquacult . 28 , 43–56 (2020).
Article Google Scholar
Belton, B. & Thilsted, S. H. Fisheries in transition: food and nutrition security implications for the Global South. Glob. Food Secur . 3 , 59–66 (2014).
Béné, C. et al. Contribution of fisheries and aquaculture to food security and poverty reduction: assessing the current evidence. World Dev . 79 , 177–196 (2016).
Thilsted, S. H. et al. Sustaining healthy diets: The role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy 61 , 126–131 (2016).
Belton, B. et al. Farming fish in the sea will not nourish the world. Nat. Commun . 11 , 5804 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Stevens, J. R., Newton, R. W., Tlusty, M. & Little, D. C. The rise of aquaculture by-products: increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 90 , 115–124 (2018).
Edwards, P., Zhang, W., Belton, B. & Little, D. C. Misunderstandings, myths and mantras in aquaculture: its contribution to world food supplies has been systematically over reported. Mar. Policy 106 , 103547 (2019). This study provides a critical assessment of how aquaculture and fisheries compare to terrestrial livestock in terms of edible and live-weight production and growth in recent decades .
Metian, M., Troell, M., Christensen, V., Steenbeek, J. & Pouil, S. Mapping diversity of species in global aquaculture. Rev. Aquacult . 12 , 1090–1100 (2020).
Bush, S. R., Belton, B., Little, D. C. & Islam, M. S. Emerging trends in aquaculture value chain research. Aquaculture 498 , 428–434 (2019).
Cao, L. et al. China’s aquaculture and the world’s wild fisheries. Science 347 , 133–135 (2015).
Fabinyi, M. & Liu, N. The social context of the Chinese food system: an ethnographic study of the Beijing seafood market. Sustainability 8 , 244 (2016).
Crona, B. et al. China at a crossroads: an analysis of China’s changing seafood production and consumption. One Earth 3 , 32–44 (2020).
Article ADS Google Scholar
Garlock, T. et al. A global blue revolution: aquaculture growth across regions, species, and countries. Rev. Fish. Sci. Aquacult . 28 , 107–116 (2020).
Adeleke, B., Robertson-Andersson, D., Moodley, G. & Taylor, S. Aquaculture in Africa: a comparative review of Egypt, Nigeria and Uganda vis-à-vis South Africa. Rev. Fish. Sci. Aquacult . https://doi.org/10.1080/23308249.2020.1795615 (2020).
FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action http://www.fao.org/documents/card/en/c/ca9229en (FAO, 2020).
WorldFish. Addressing COVID-19 Impacts on Fish and Aquatic Food Systems https://mailchi.mp/worldfishcenter/covid-response (WorldFish, 2020).
Little, D. C., Newton, R. W. & Beveridge, M. C. M. Aquaculture: a rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc . 75 , 274–286 (2016).
Article CAS PubMed Google Scholar
Pieterse, J. N. Multipolar Globalization: Emerging Economies and Development (Routledge, 2017).
Belton, B., Bush, S. R. & Little, D. C. Not just for the wealthy: rethinking farmed fish consumption in the Global South. Glob. Food Secur . 16 , 85–92 (2018). This paper challenges the emerging view that aquaculture primarily benefits wealthy populations and shows that aquaculture improves food security for top producing low- and middle-income countries .
Belton, B. & Bush, S. R. Beyond net deficits: new priorities for an aquacultural geography. Geogr. J . 180 , 3–14 (2014).
Wang, Q. et al. Paradigm changes in freshwater aquaculture practices in China: moving towards achieving environmental integrity and sustainability. Ambio 47 , 410–426 (2018).
PubMed Google Scholar
Hernandez, R. et al. The “quiet revolution” in the aquaculture value chain in Bangladesh. Aquaculture 493 , 456–468 (2018). This study describes the extent and importance of freshwater aquaculture in stimulating societal benefits through employment generated by values chains in Bangladesh .
Little, D. C. & Bunting, S. W. in Emerging Technologies for Promoting Food Security: Overcoming the World Food Crisis (ed. Madramootoo, C.) 93–113 (Elsevier, 2016).
Belton, B., Padiyar, A., Ravibabu, G. & Gopal Rao, K. Boom and bust in Andhra Pradesh: development and transformation in India’s domestic aquaculture value chain. Aquaculture 470 , 196–206 (2017).
Belton, B. & Filipski, M. Rural transformation in central Myanmar: by how much, and for whom? J. Rural Stud . 67 , 166–176 (2019).
Belton, B. & Little, D. The development of aquaculture in central Thailand: domestic demand versus export-led production. J. Agrar. Change 8 , 123–143 (2008).
Loc, V. T. T., Bush, S. R., Sinh, L. X. & Khiem, N. T. High and low value fish chains in the Mekong Delta: challenges for livelihoods and governance. Environ. Dev. Sustain . 12 , 889–908 (2010).
Fluet-Chouinard, E., Funge-Smith, S. & McIntyre, P. B. Global hidden harvest of freshwater fish revealed by household surveys. Proc. Natl Acad. Sci. USA 115 , 7623–7628 (2018).
Article CAS PubMed PubMed Central Google Scholar
Belton, B. & Little, D. C. in World Small-Scale Fisheries: Contemporary Visions (ed. Chuenpagdee, R.) 151–170 (Eburon, 2011).
Toufique, K. A. & Belton, B. Is aquaculture pro-poor? Empirical evidence of impacts on fish consumption in Bangladesh. World Dev . 64 , 609–620 (2014).
Filipski, M. & Belton, B. Give a man a fishpond: modeling the impacts of aquaculture in the rural economy. World Dev . 110 , 205–223 (2018).
Beveridge, M. C. M. et al. Meeting the food and nutrition needs of the poor: the role of fish and the opportunities and challenges emerging from the rise of aquaculture. J. Fish Biol . 83 , 1067–1084 (2013).
Kaminski, A. M. et al. A review of inclusive business models and their application in aquaculture development. Rev. Aquacult . 12 , 1881–1902 (2020).
Google Scholar
Bestari, N., Edwards, P., Katon, B., Morales, A. & Pullin, R. An Evaluation of Small Scale Freshwater Rural Aquaculture Development for Poverty Reduction. Case Study 6: Tilapia Cage Farming in Lake Taal, Batangas, Philippines Report No. 091704, 110–127 https://www.adb.org/publications/evaluation-small-scale-freshwater-rural-aquaculture-development-poverty-reduction (Asian Development Bank, 2005).
Fakhrudin, M., Subehi, L., Jasalesmana, T. & Dianto, A. Dissolved oxygen and temperature stratification analysis for early warning system development in preventing mass mortality of fish in lake Maninjau, West Sumatera - Indonesia. IOP Conf. Ser. Earth Environ. Sci . 380 , 012002 (2019).
Ponte, S., Kelling, I., Jespersen, K. S. & Kruijssen, F. The blue revolution in Asia: upgrading and governance in aquaculture value chains. World Dev . 64 , 52–64 (2014).
Lebel, L., Lebel, P. & Chuah, C. J. Water use by inland aquaculture in Thailand: stakeholder perceptions, scientific evidence, and public policy. Environ. Manage . 63 , 554–563 (2019).
Article ADS PubMed Google Scholar
Wang, J., Beusen, A. H. W., Liu, X. & Bouwman, A. F. Aquaculture production is a large, spatially concentrated source of nutrients in Chinese freshwater and coastal seas. Environ. Sci. Technol . 54 , 1464–1474 (2020). This paper provides the first model-based estimate of the scale of total nutrient release from aquaculture to the freshwater and marine environment in China .
Article ADS PubMed CAS Google Scholar
Wu, Y., Shan, L., Guo, Z. & Peng, Y. Cultivated land protection policies in China facing 2030: dynamic balance system versus basic farmland zoning. Habitat Int . 69 , 126–138 (2017).
Brown, T. W., Chappell, J. A. & Boyd, C. E. A commercial-scale, in-pond raceway system for Ictalurid catfish production. Aquacult. Eng . 44 , 72–79 (2011).
Troell, M. et al. Does aquaculture add resilience to the global food system? Proc. Natl Acad. Sci. USA 111 , 13257–13263 (2014). This study assesses the resilience of the aquaculture sector using a portfolio approach that focuses on production and feed links between terrestrial and marine systems .
Leadbitter, D. Driving Change in South East Asian Trawl Fisheries, Fishmeal Supply and Aquafeed https://www.iffo.com/system/files/downloads/Full%20Report%20on%20South%20East%20Asia.pdf (IFFO, 2019).
Arthur, R. I. et al. Assessing impacts of introduced aquaculture species on native fish communities: Nile tilapia and major carps in SE Asian freshwaters. Aquaculture 299 , 81–88 (2010).
Henriksson, P. J. G., Belton, B., Jahan, K. M.-E. & Rico, A. Measuring the potential for sustainable intensification of aquaculture in Bangladesh using life cycle assessment. Proc. Natl Acad. Sci. USA 115 , 2958–2963 (2018).
Green, K. Fishmeal and Fish Oil Facts and Figures. March 2018 https://www.seafish.org/document/?id=1b08b6d5-75d9-4179-9094-840195ceee4b (SeaFish, 2018).
Pauly, D., Zeller, D. & Palomares, M. L. D. Sea Around Us Concepts, Design and Data http://www.seaaroundus.org/ (2020).
Davis, D. A. Feed and Feeding Practices in Aquaculture (Woodhead, 2015).
Bachis, E. Fishmeal and fish oil: a summary of global trends. 57th IFFO Annual Conference https://www.iffo.com/blog/day-2-summary-57th-iffo-annual-conference (2017).
Auchterlonie, N. A. The continuing importance of fishmeal and fish oil in aquafeeds. https://www.iffo.com/system/files/downloads/AquaFarm%20Feb18%20NA.pdf (2018).
Shepherd, J. Responsible marine ingredients for agriculture. https://www.iffo.com/system/files/downloads/JS%20IFFO%20presentation%20for%20GOAL.pdf (2011).
Péron, G., François Mittaine, J. & Le Gallic, B. Where do fishmeal and fish oil products come from? An analysis of the conversion ratios in the global fishmeal industry. Mar. Policy 34 , 815–820 (2010).
National Research Council. Nutrient Requirements of Fish and Shrimp (The National Academies Press, 2011).
Ytrestøyl, T., Aas, T. S. & Åsgård, T. Utilisation of feed resources in production of Atlantic salmon ( Salmo salar ) in Norway. Aquaculture 448 , 365–374 (2015).
Kok, B. et al. Fish as feed: using economic allocation to quantify the fish in: fish out ratio of major fed aquaculture species. Fish Fish . 528 , 735474 (2020).
CAS Google Scholar
Zhang, W. et al. Fishing for feed in China: facts, impacts and implications. Fish Fish . 21 , 47–62 (2020). This study provides field-based evidence on the extent of feed-grade, non-targeted fish catch in China for aquaculture feeds and its implications for marine food webs .
Krogdahl, Å., Penn, M., Thorsen, J., Refstie, S. & Bakke, A. M. Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquacult. Res . 41 , 333–344 (2010).
Article CAS Google Scholar
Naylor, R. L. et al. Feeding aquaculture in an era of finite resources. Proc. Natl Acad. Sci. USA 106 , 15103–15110 (2009). This perspective describes advances in fish nutrition with an emphasis on alternative protein sources to replace fishmeal and strategies to reduce fish oil levels in aquafeed .
Hua, K. et al. The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 1 , 316–329 (2019).
Drew, M. D., Borgeson, T. L. & Thiessen, D. L. A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim. Feed Sci. Technol . 138 , 118–136 (2007). This paper reviews the technologies used to improve the nutritional quality of plant protein concentrates and other alternative feed ingredients to support efficient fish growth when included in fish feeds .
Betancor, M. B. et al. A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish. Sci. Rep . 5 , 8104 (2015).
Sprague, M., Dick, J. R. & Tocher, D. R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep . 6 , 21892 (2016).
Turchini, G. M., Wing-Keong, N. & Tocher, D. R. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds (CRC, 2010). A thorough review of fish oil replacement in fish feeds .
Martin, S. A. M. & Król, E. Nutrigenomics and immune function in fish: new insights from omics technologies. Dev. Comp. Immunol . 75 , 86–98 (2017).
Simó-Mirabet, P. et al. Impact of low fish meal and fish oil diets on the performance, sex steroid profile and male–female sex reversal of gilthead sea bream ( Sparus aurata ) over a three-year production cycle. Aquaculture 490 , 64–74 (2018).
Caballero-Solares, A. et al. Changes in the liver transcriptome of farmed Atlantic salmon ( Salmo salar ) fed experimental diets based on terrestrial alternatives to fish meal and fish oil. BMC Genomics 19 , 796 (2018).
Gjedrem, T. & Rye, M. Selection response in fish and shellfish: a review. Rev. Aquacult . 10 , 168–179 (2018).
de Verdal, H. et al. Improving feed efficiency in fish using selective breeding: a review. Rev. Aquacult . 10 , 833–851 (2018).
Overturf, K., Barrows, F. T. & Hardy, R. W. Effect and interaction of rainbow trout strain ( Oncorhynchus mykiss ) and diet type on growth and nutrient retention. Aquacult. Res . 44 , 604–611 (2013).
Brezas, A. & Hardy, R. W. Improved performance of a rainbow trout selected strain is associated with protein digestion rates and synchronization of amino acid absorption. Sci. Rep . 10 , 4678 (2020).
Little, D. C. et al. Sustainable intensification of aquaculture value chains between Asia and Europe: a framework for understanding impacts and challenges. Aquaculture 493 , 338–354 (2018).
Newton, R. W. & Little, D. C. Mapping the impacts of farmed Scottish salmon from a life cycle perspective. Int. J. Life Cycle Assess . 23 , 1018–1029 (2018).
Malcorps, W. et al. The sustainability conundrum of fishmeal substitution by plant ingredients in shrimp feeds. Sustainability 11 , 1212 (2019).
Pelletier, N., Klinger, D. H., Sims, N. A., Yoshioka, J. R. & Kittinger, J. N. Nutritional attributes, substitutability, scalability, and environmental intensity of an illustrative subset of current and future protein sources for aquaculture feeds: joint consideration of potential synergies and trade-offs. Environ. Sci. Technol . 52 , 5532–5544 (2018). This paper provides a perspective on the shift from wild fish to terrestrial crop-based ingredients in aquafeeds .
Aas, T. S., Ytrestøyl, T. & Åsgård, T. Utilization of feed resources in the production of Atlantic salmon ( Salmo salar ) in Norway: An update for 2016. Aquacult. Rep . 15 , 100216 (2019).
Hansen, L. The weak sustainability of the salmon feed transition in Norway – a bioeconomic case study. Front. Mar. Sci . 6 , 764 (2019).
Klinger, D. & Naylor, R. Searching for solutions in aquaculture: charting a sustainable course. Annu. Rev. Environ. Resour . 37 , 247–276 (2012).
Wan, A. H. L., Davies, S. J., Soler-Vila, A., Fitzgerald, R. & Johnson, M. P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquacult . 11 , 458–492 (2019).
El Abbadi, S. H. & Criddle, C. S. Engineering the dark food chain. Environ. Sci. Technol . 53 , 2273–2287 (2019).
Cottrell, R. S., Blanchard, J. L., Halpern, B. S., Metian, M. & Froehlich, H. E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 1 , 301–308 (2020).
Shumway, S. E. Shellfish Aquaculture and the Environment (Wiley-Blackwell, 2011). This book presents a comprehensive review of shellfish aquaculture–environment interactions .
Buschmann, A. H. et al. Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol . 52 , 391–406 (2017). This paper describes the status and uses of capture and culture for seaweed production in the last decade, highlighting emerging trends and future avenues of research such as new pharmaceutical uses and carbon sequestration .
Smaal, A. C., Ferreira, J. G., Grant, J., Petersen, J. K. & Strand, Ø. Goods and Services of Marine Bivalves (Springer, 2019). This volume presents a comprehensive review of ecosystem services provided by marine bivalve molluscs .
Weitzman, J. Applying the ecosystem services concept to aquaculture: a review of approaches, definitions, and uses. Ecosyst. Serv . 35 , 194–206 (2019).
Costa-Pierce, B. A. Ecological Aquaculture: The Evolution of the Blue Revolution (Wiley-Blackwell, 2002).
Gentry, R. R. et al. Exploring the potential for marine aquaculture to contribute to ecosystem services. Rev. Aquacult . 12 , 499–512 (2020).
Costello, C. et al. The future of food from the sea. Nature 588 , 95–100 (2020).
van der Schatte Olivier, A. et al. A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquacult . 12 , 3–25 (2020).
Aubin, J., Fontaine, C., Callier, M. & Roque d’orbcastel, E. Blue mussel ( Mytilus edulis ) bouchot culture in Mont-St Michel Bay: potential mitigation effects on climate change and eutrophication. Int. J. Life Cycle Assess . 23 , 1030–1041 (2018).
Filgueira, R. et al. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar. Ecol. Prog. Ser . 518 , 281–287 (2015).
Article ADS CAS Google Scholar
Rosa, M., Ward, J. E. & Shumway, S. E. Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. J. Shellfish Res . 37 , 727–746 (2018).
Wilberg, M. J., Livings, M. E., Barkman, J. S., Morris, B. T. & Robinson, J. M. Overfishing, disease, habitat loss, and potential extirpation of oysters in upper Chesapeake Bay. Mar. Ecol. Prog. Ser . 436 , 131–144 (2011).
Lindahl, O. et al. Improving marine water quality by mussel farming: a profitable solution for Swedish society. Ambio 34 , 131–138 (2005).
Article PubMed Google Scholar
Parker, M. & Bricker, S. Sustainable oyster aquaculture, water quality improvement and ecosystem service value potential in Maryland, Chesapeake Bay. J. Shellfish Res . 39 , 269–281 (2020).
Lafferty, K. D. et al. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci . 7 , 471–496 (2015).
Fox, M. et al. Preventing and mitigating farmed bivalve disease: a Northern Ireland case study. Aquacult. Int . 28 , 2397–2417 (2020).
Shumway, S. E., Burkholder, J. M. & Morton, S. L. (eds) Harmful Algal Blooms: A Compendium Desk Reference (John Wiley & Sons, 2018). A comprehensive review of the causes, consequences, and dynamics of harmful algal blooms .
Liu, H. & Su, J. Vulnerability of China’s nearshore ecosystems under intensive mariculture development. Environ. Sci. Pollut. Res. Int . 24 , 8957–8966 (2017).
Wartenberg, R. et al. The impacts of suspended mariculture on coastal zones in China and the scope for integrated multi-trophic aquaculture. Ecosyst. Health Sustain . 3 , 1340268 (2017).
Ferreira, J. G., Hawkins, A. J. S. & Bricker, S. B. Management of productivity, environmental effects and profitability of shellfish aquaculture — the Farm Aquaculture Resource Management (FARM) model. Aquaculture 264 , 160–174 (2007).
Ferreira, J. G. et al. Integrated assessment of ecosystem-scale carrying capacity in shellfish growing areas. Aquaculture 275 , 138–151 (2008).
Ferreira, J. G. et al. Ecological carrying capacity for shellfish aquaculture—sustainability of naturally occurring filter-feeders and cultivated bivalves. J. Shellfish Res . 37 , 709–726 (2018).
Lavaud, R., Guyondet, T., Filgueira, R., Tremblay, R. & Comeau, L. A. Modelling bivalve culture - eutrophication interactions in shallow coastal ecosystems. Mar. Pollut. Bull . 157 , 111282 (2020).
Tucker, C. & Hargraeves, J. A. Environmental Best Management Practices for Aquaculture (Wiley-Blackwell, 2008).
Barbier, M. et al. PEGASUS - Phycomorph European Guidelines for a Sustainable Aquaculture of Seaweeds . COST Action FA1406 (eds Barbier, M. & Charrier, B.) https://doi.org/10.21411/2c3w-yc73 (COST, 2019).
Dillehay, T. D. et al. Monte Verde: seaweed, food, medicine, and the peopling of South America. Science 320 , 784–786 (2008).
Porse, H. & Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phycol . 29 , 2187–2200 (2017).
Shannon, E. & Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 58 , 563–577 (2019).
Wells, M. L. et al. Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol . 29 , 949–982 (2017). A review and critical analysis of the actual and purported benefits of seaweed for human nutrition .
Mouritsen, O. G., Rhatigan, P. & Pérez-Lloréns, J. L. The rise of seaweed gastronomy: phycogastronomy. Bot. Mar . 62 , 195–209 (2019).
Holdt, S. L. & Kraan, S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol . 23 , 543–597 (2011).
Li, X. et al. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci . 58 , 681–688 (2016).
Chopin, T. & Tacon, A. G. J. Importance of seaweeds and extractive species in global aquaculture production. Rev. Fish. Sci. Aquacult . https://doi.org/10.1080/23308249.2020.1810626 (2020). This paper provides a clear and comprehensive assessment of global seaweed aquaculture and shows the relevance of integrated multi-trophic aquaculture and other applications .
Hurd, C. L., Harrison, P. J., Bischof, K. & Lobban, C. S. Seaweed Ecology and Physiology 2nd edn (Cambridge Univ. Press, 2014).
Duarte, C. M., Wu, J., Xiao, X., Bruhn, A. & Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci . 4 , 100 (2017).
Krause-Jensen, D. et al. Sequestration of macroalgal carbon: the elephant in the blue carbon room. Biol. Lett . 14 , 20180236 (2018).
Article PubMed PubMed Central Google Scholar
Alleway, H. K. et al. The ecosystem services of marine aquaculture: valuing benefits to people and nature. Bioscience 69 , 59–68 (2019).
Yang, Y. et al. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res . 9 , 236–244 (2015).
Xiao, X. et al. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep . 7 , 46613 (2017).
Article ADS PubMed PubMed Central Google Scholar
Kim, G. H., Moon, K.-H., Kim, J.-Y., Shim, J. & Klochkova, T. A. A revaluation of algal diseases in Korean Pyropia (Porphyra) sea farms and their economic impact. Algae 29 , 249–265 (2014).
Hurtado, A. Q., Neish, I. C. & Critchley, A. T. Phyconomy: the extensive cultivation of seaweeds, their sustainability and economic value, with particular reference to important lessons to be learned and transferred from the practice of eucheumatoid farming. Phycologia 58 , 472–483 (2019).
Zollmann, M. et al. Green technology in green macroalgal biorefineries. Phycologia 58 , 516–534 (2019).
Doumeizel, V. et al. Seaweed Revolution: A Manifesto for a Sustainable Future . https://ungc-communications-assets.s3.amazonaws.com/docs/publications/The-Seaweed-Manifesto.pdf (UN Global Compact and Lloyd’s Register Foundation, 2020).
Fröcklin, S., de la Torre-Castro, M., Lindström, L., Jiddawi, N. S. & Msuya, F. E. Seaweed mariculture as a development project in Zanzibar, East Africa: a price too high to pay? Aquaculture 356–357 , 30–39 (2012).
van den Burg, S. W. K., Dagevos, H. & Helmes, R. J. K. Towards sustainable European seaweed value chains: a triple P perspective. ICES J. Mar. Sci. fsz183 (2019).
Herbeck, L. S., Krumme, U., Andersen, T. J. & Jennejahn, T. C. Decadal trends in mangrove and pond aquaculture cover on Hainan (China) since 1966: mangrove loss, fragmentation and associated biogeochemical changes. Estuar. Coast. Shelf Sci . 233 , 106531 (2020).
Nguyen, H. Q. et al. Socio-ecological resilience of mangrove-shrimp models under various threats exacerbated from salinity intrusion in coastal area of the Vietnamese Mekong Delta. Int. J. Sustain. Dev. World Ecol . 27 , 638–651 (2020).
Reid, G. K. et al. Climate change and aquaculture: considering adaptation potential. Aquacult. Environ. Interact . 11 , 603-624 (2019). This paper reviews potential adaptation strategies for reducing climate-induced impacts on the aquaculture sector.
Stentiford, G. D. et al. Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J. Invertebr. Pathol . 110 , 141–157 (2012).
Stentiford, G. D. et al. New paradigms to help solve the global aquaculture disease crisis. PLoS Pathog . 13 , e1006160 (2017).
Article PubMed PubMed Central CAS Google Scholar
Elaswad, A. & Dunham, R. Disease reduction in aquaculture with genetic and genomic technology: current and future approaches. Rev. Aquacult . 10 , 876–898 (2018).
Pernet, F., Lupo, C., Bacher, C. & Whittington, R. J. Infectious diseases in oyster aquaculture require a new integrated approach. Phil. Trans. R. Soc. Lond. B 371 , 20150213 (2016).
Austin, B. & Newaj-Fyzul, A. (eds) Diagnosis and Control of Diseases of Fish and Shellfish (John Wiley & Sons, 2017).
Luis, A. I. S., Campos, E. V. R., de Oliveira, J. L. & Fraceto, L. F. Trends in aquaculture sciences: from now to use of nanotechnology for disease control. Rev. Aquacult . 11 , 119–132 (2019).
Flegel, T. W. A future vision for disease control in shrimp aquaculture. J. World Aquacult. Soc . 50 , 249–266 (2019).
Leung, P., Lee, C. S. & O’Bryen, P. J. Species and System Selection for Sustainable Aquaculture (John Wiley & Sons, 2008). This paper presents a comprehensive review of the factors that affect species and system utilization in global aquaculture .
Shinn, A. P. et al. Asian shrimp production and the economic costs of disease. Asian Fish. Sci . 31S , 29–58 (2018).
You, W. & Hedgecock, D. Boom-and-bust production cycles in animal seafood aquaculture. Rev. Aquacult . 11 , 1045–1060 (2019).
Cabello, F. C. et al. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol . 15 , 1917–1942 (2013).
Cabello, F. C. & Godfrey, H. P. Salmon aquaculture, Piscirickettsia salmonis virulence, and One Health: dealing with harmful synergies between heavy antimicrobial use and piscine and human health. Aquaculture 507 , 451–456 (2019).
Rico, A. et al. Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev. Aquacult . 4 , 75–93 (2012).
Henriksson, P. J. G. et al. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustain. Sci . 13 , 1105–1120 (2018).
Lulijwa, R., Rupia, E. J. & Alfaro, A. C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev. Aquacult . 12 , 640–663 (2020).
Kumar, G. & Engle, C. R. Technological advances that led to growth of shrimp, salmon, and tilapia farming. Rev. Fish. Sci. Aquacult . 24 , 136–152 (2016).
Brudeseth, B. E. et al. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol . 35 , 1759–1768 (2013).
Plant, K. P. & Lapatra, S. E. Advances in fish vaccine delivery. Dev. Comp. Immunol . 35 , 1256–1262 (2011).
Boopathy, R. in Sustainable Aquaculture (eds Hai, F. I et al.) 301–322 (Springer, 2018).
Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol . 90 , 210–214 (2019).
Abolofia, J., Asche, F. & Wilen, J. E. The cost of lice: quantifying the impacts of parasitic sea lice on farmed salmon. Mar. Resour. Econ . 32 , 329–349 (2017).
Tangprasittipap, A. et al. The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus ( Litopenaeus ) vannamei . BMC Vet. Res . 9 , 139 (2013).
Kibenge, F. S. B. Emerging viruses in aquaculture. Curr. Opin. Virol . 34 , 97–103 (2019).
Santos, H. M. et al. Diagnosis and potential treatments for acute hepatopancreatic necrosis disease (AHPND): a review. Aquacult. Int . 28 , 169–185 (2020).
MacFadden, D. R., McGough, S. F., Fisman, D., Santillana, M. & Brownstein, J. S. Antibiotic resistance increases with local temperature. Nat. Clim. Change 8 , 510–514 (2018).
Reverter, M. et al. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun . 11 , 1870 (2020).
Reid, G. K. et al. Climate change and aquaculture: considering biological response and resources. Aquacult. Environ. Interact . 11 , 569–602 (2019). This paper reviews the science on climate impacts on the aquaculture sector.
Subasinghe, R. P., Delamare-Deboutteville, J., Mohan, C. V. & Phillips, M. J. Vulnerabilities in aquatic animal production. Rev. Sci. Tech . 38 , 423–436 (2019).
Matsuyama, Y. & Shumway, S. in New Technologies in Aquaculture: Improving Production Efficiency, Quality and Environmental Management (eds Burnell, G. & Allan, G.) 580–609 (Elsevier, 2009).
Díaz, P. A. et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol . 6 , 39–50 (2019).
Barange, M. et al. Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options . FAO Fisheries and Aquaculture Technical Paper 627 http://www.fao.org/3/i9705en/i9705en.pdf (FAO, 2018).
Barton, A. et al. Impacts of coastal acidification on the Pacific Northwest shellfish industry and adaptation strategies implemented in response. Oceanography 28 , 146–159 (2015).
Dupont, S., Dorey, N. & Thorndyke, M. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar. Coast. Shelf Sci . 89 , 182–185 (2010).
Burge, C. A. et al. Climate change influences on marine infectious diseases: implications for management and society. Annu. Rev. Mar. Sci . 6 , 249–277 (2014).
Wells, M. L. et al. Harmful algal blooms and climate change: learning from the past and present to forecast the future. Harmful Algae 49 , 68–93 (2015).
Handisyde, N., Telfer, T. C. & Ross, L. G. Vulnerability of aquaculture-related livelihoods to changing climate at the global scale. Fish Fish . 18 , 466–488 (2017).
Klinger, D. H., Levin, S. A. & Watson, J. R. The growth of finfish in global open-ocean aquaculture under climate change. Proc. R. Soc. B 284 , 20170834 (2017).
Froehlich, H. E., Gentry, R. R. & Halpern, B. S. Global change in marine aquaculture production potential under climate change. Nat. Ecol. Evol . 2 , 1745–1750 (2018).
Ellis, R. P., Urbina, M. A. & Wilson, R. W. Lessons from two high CO 2 worlds – future oceans and intensive aquaculture. Glob. Change Biol . 23 , 2141–2148 (2017).
Brugère, C., Aguilar-Manjarrez, J., Beveridge, M. C. M. & Soto, D. The ecosystem approach to aquaculture 10 years on – a critical review and consideration of its future role in blue growth. Rev. Aquacult . 11 , 493–514 (2019). This paper presents a critical overview of the advances and challenges of implementing the ecosystem approach to aquaculture .
Edwards, P. Aquaculture environment interactions: past, present and likely future trends. Aquaculture 447 , 2–14 (2015).
Fang, J., Zhang, J., Xiao, T., Huang, D. & Liu, S. Integrated multi-trophic aquaculture (IMTA) in Sanggou Bay, China. Aquacult. Environ. Interact . 8 , 201–205 (2016).
Hughes, A. D. & Black, K. D. Going beyond the search for solutions: understanding trade-offs in European integrated multi-trophic aquaculture development. Aquacult. Environ. Interact . 8 , 191–199 (2016).
Neori, A. et al. Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231 , 361–391 (2004).
Ebeling, J. M. & Timmons, M. B. in Aquaculture Production Systems (ed. Tidwell, J.) 245–277 (Wiley-Blackwell, 2012).
Badiola, M., Mendiola, D. & Bostock, J. Recirculating aquaculture systems (RAS) analysis: main issues on management and future challenges. Aquacult. Eng . 51 , 26–35 (2012).
Badiola, M., Basurko, O. C., Piedrahita, R., Hundley, P. & Mendiola, D. Energy use in recirculating aquaculture systems (RAS): a review. Aquacult. Eng . 81 , 57–70 (2018).
de Jong, B. Aquaculture 2.0: RAS Is Driving Change far.rabobank.com (2019).
Dalsgaard, J. et al. Farming different species in RAS in Nordic countries: current status and future perspectives. Aquacult. Eng . 53 , 2–13 (2013).
Cherry, D. & Mutter, R. Analysis: here’s a list of high-profile land-based aquaculture failures. IntraFish (27 November 2019).
Chu, Y. I., Wang, C. M., Park, J. C. & Lader, P. F. Review of cage and containment tank designs for offshore fish farming. Aquaculture 519 , 734928 (2020).
Dong, S. The development of aquaculture in the new era from a multi-dimensional perspective. Shuichan Xuebao 43 , 105–115 (2019).
Thomas, L. R., Clavelle, T., Klinger, D. H. & Lester, S. E. The ecological and economic potential for offshore mariculture in the Caribbean. Nat. Sustain . 2 , 62–70 (2019).
Gui, J. F., Tang, Q., Li, Z., Liu, J. & De Silva, S. Aquaculture in China: Success Stories and Modern Trends (John Wiley & Sons, 2018).
Harkell, L. Chinese firm to build second offshore salmon pen in 2019. Undercurrent News (18 February 2019).
Gentry, R. R. et al. Offshore aquaculture: spatial planning principles for sustainable development. Ecol. Evol . 7 , 733–743 (2017).
Ramos, J., Caetano, M., Himes-Cornell, A. & dos Santos, M. N. Stakeholders’ conceptualization of offshore aquaculture and small-scale fisheries interactions using a Bayesian approach. Ocean Coast. Manage . 138 , 70–82 (2017).
Bush, S. R. & Oosterveer, P. Governing Sustainable Seafood (Routledge, 2019). This paper provides a comprehensive overview of public and private governance initiatives for aquaculture within the global sustainable seafood movement .
Jonell, M., Tlusty, M., Troell, M. & Rönnbäck, P. Sustainability Certification Schemes in the Agricultural and Natural Resource Sectors (ed. Vogt, M.) 157–178 (Taylor & Francis, 2019).
Roheim, C. A., Bush, S. R., Asche, F., Sanchirico, J. N. & Uchida, H. Evolution and future of the sustainable seafood market. Nat. Sustain . 1 , 392–398 (2018).
Vince, J. & Haward, M. Hybrid governance of aquaculture: opportunities and challenges. J. Environ. Manage . 201 , 138–144 (2017).
Tlusty, M. F. Environmental improvement of seafood through certification and ecolabelling: theory and analysis. Fish Fish . 13 , 1–13 (2012).
Bush, S. R. et al. Certify sustainable aquaculture? Science 341 , 1067–1068 (2013).
Jonell, M., Phillips, M., Rönnbäck, P. & Troell, M. Eco-certification of farmed seafood: will it make a difference? Ambio 42 , 659–674 (2013).
Tlusty, M. F. & Tausig, H. Reviewing GAA-BAP shrimp farm data to determine whether certification lessens environmental impacts. Rev. Aquacult . 7 , 107–116 (2015).
Trifković, N. Certified standards and vertical coordination in aquaculture: the case of pangasius from Vietnam. Aquaculture 433 , 235–246 (2014).
Bush, S. R. Understanding the potential of eco-certification in salmon and shrimp aquaculture value chains. Aquaculture 493 , 376–383 (2018).
Swartz, W., Schiller, L., Sumaila, U. R. & Ota, Y. Searching for market-based sustainability pathways: challenges and opportunities for seafood certification programs in Japan. Mar. Policy 76 , 185–191 (2017).
Bottema, M. J. M. Institutionalizing area-level risk management: limitations faced by the private sector in aquaculture improvement projects. Aquaculture 512 , 734310 (2019).
Ferreira, J. G. & Bricker, S. in Goods and Services of Marine Bivalves (eds Smaal, A. C. et al.) 551–584 (Springer, 2019).
Stuiver, M. et al. The governance of multi-use platforms at sea for energy production and aquaculture: challenges for policy makers in European Seas. Sustainability 8 , 333 (2016).
Klinger, D. H., Eikeset, A. M., Davíðsdóttir, B., Winter, A.-M. & Watson, J. R. The mechanics of blue growth: management of oceanic natural resource use with multiple, interacting sectors. Mar. Policy 87 , 356–362 (2018).
Krause, G. & Stead, S. M. in Aquaculture Perspective of Multi-Use Sites in the Open Ocean (eds Buck, B. H. & Langan, R.) 149–162 (Springer, 2017).
BFA. The Blue Food Assessment . https://www.bluefood.earth (2020).
FAO. The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Goals http://www.fao.org/3/I9540EN/i9540en.pdf (FAO, 2018).
Froehlich, H. E., Runge, C. A., Gentry, R. R., Gaines, S. D. & Halpern, B. S. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natl Acad. Sci. USA 115 , 5295–5300 (2018).
Download references
Acknowledgements
We thank W. Falcon, A. Albalat, D. Battisti, G. Stentiford, P. Edwards, A. Hughes, F. Pernet, E. Heupel, D. Francks and J. Kaull for comments and assistance, and all authors from the 2000 review in Nature 1 for generating a constructive scientific discourse. Funding was provided through the Center on Food Security and the Environment, Stanford University. M.T. acknowledges Formas project SEAWIN (2016-00227).
Author information
Authors and affiliations.
Department of Earth System Science, Stanford University, Stanford, CA, USA
Rosamond L. Naylor
Center on Food Security and the Environment, Stanford University, Stanford, CA, USA
Aquaculture Research Institute, University of Idaho, Moscow, ID, USA
Ronald W. Hardy
Centro i-mar & CeBiB, Universidad de Los Lagos, Puerto Montt, Chile
Alejandro H. Buschmann
Environmental Policy Group, Wageningen University, Wageningen, The Netherlands
Simon R. Bush
School of Oceanography, Shanghai Jiao Tong University, Shanghai, China
Center for Oceans, Conservation International, Arlington, VA, USA
Dane H. Klinger
Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA
Institute of Aquaculture, University of Stirling, Stirling, UK
David C. Little
Department of Integrative Biology, Oregon State University, Corvallis, OR, USA
Jane Lubchenco
Department of Marine Sciences, University of Connecticut, Groton, CT, USA
Sandra E. Shumway
Beijer Institute, Royal Swedish Academy of Sciences, Stockholm, Sweden
Stockholm Resilience Centre, Stockholm University, Stockholm, Sweden
You can also search for this author in PubMed Google Scholar
Contributions
R.L.N. led, and R.W.H., A.H.B., S.R.B., L.C., D.H.K., D.C.L., J.L., S.E.S. and M.T. contributed to the conceptualization, analysis, literature review, writing, and responses to reviewer comments for this manuscript.
Corresponding author
Correspondence to Rosamond L. Naylor .
Ethics declarations
Competing interests.
R.L.N. is a member of the Forest Protection Advisory Panel at Cargill, and the Center on Food Security and the Environment (FSE) has received funding from the Cargill Foundation for visiting scholars and staff support, but not for research activities. She is also on the Scientific Advisory Board for Oceana and is the President of the Board of Directors for the Aspen Global Change Institute. She participates on the editorial board of Aquaculture Environment Interactions . D.H.K. is a member of the Technical Advisory Group for the Aquaculture Stewardship Council and a member of the Aquaculture Technical Advisory Committee of Monterey Bay Aquarium’s Seafood Watch Program. S.E.S. serves on the Advisory Committee on Aquaculture Science for DFO Canada ( http://www.dfo-mpo.gc.ca/aquaculture/advisory-comm-consultatif-eng.html ). She is currently working on two white papers for the United Nations Food and Agricultural Organization, and has previously chaired the Aquaculture Stewardship Council’s Technical Advisory Committee and the Monterey Bay Seafood Watch Advisory Committee. She also serves as Editor-in-Chief for the Journal of Shellfish Research , and Editor-in-Chief for Reviews in Fisheries Science & Aquaculture . A.H.B. is on the Standards Oversight Committee of the Global Aquaculture Alliance. He has no affiliation with any for-profit company; all of his research is supported by the Chilean National Science Agency (ANID) and therefore has no conflict of interest with any aquaculture activity. S.R.B. is a member of the Standards Oversight Committee of the Global Aquaculture Alliance, the Multi-Stakeholder Group of Monterey Bay Aquarium’s Seafood Watch programme, the Technical Advisory Committee of the Good Fish Foundation in the Netherlands, and the Technical Advisory Committee of the Aquaculture Program of the Sustainable Trade Initiative (IDH). He has received funding from the Monterey Bay Aquarium’s Seafood Watch programme for the development of Aquaculture Governance Indicators. R.W.H. is Editor-in-Chief of Aquaculture Research . In the past five years, he served as Chair of a Global Aquaculture Alliance committee that revised and updated best practices standards for fish feeds, a project that was completed in 2019, prior to his participation on this Review. In the past, also prior to this Review, he has been a principal investigator for grants and contracts awarded to the University of Idaho and received grants and contracts from industry or industry groups including the United Soybean Board, Enz-A-Bac, Midwest Ag Enterprises, Ajinomoto NA and Knipbio to assess feed ingredients for sustainable aquaculture. L.C. is a judge of the global F3 (fish-free feed) challenge. She was on the Scientific Advisory Board for the Aquaculture Stewardship Council between 2017 and 2019. She has no affiliations with for-profit companies. D.C.L. has received in-kind and financial support from a wide range of commercial and non-commercial entities, serves as a committee member for standards organizations and is a director of a commercial tilapia hatchery in Thailand. J.L. until recently served on the boards of The David and Lucile Packard Foundation, Oceano Azul Foundation, Prince Albert II of Monaco Foundation, the National Geographic Society, and Seafood Businesses for Ocean Stewardship (SeaBOS). She also co-chaired the Expert Group for the High Level Panel for a Sustainable Ocean Economy. She resigned from all of these roles in February 2021 when she took up her new position in the White House. M.T. is a member of the Program committee for The Marine and Coastal Science for Management (WIOMSA/MASMA), member of Action Areas and Solution Clusters Working Groups – Blue foods, United Nations Forum on Sustainability Standards (UNFSS), scientific lead for SeaBOS, and a Review Editor for Aquaculture Environment Interactions .
Additional information
Peer review information Nature thanks Peter Edwards, Adam Hughes, Fabrice Pernet, Grant Stentiford and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 number of species farmed for each production group (1950–2017)..
a – g , The numbers of species farmed for all aquaculture ( a ), freshwater fish ( b ), algae and aquatic plants ( c ), molluscs ( d ), crustaceans ( e ), diadromous fish ( f ) and marine fish ( g ) are shown. Solid lines indicate the total number of species farmed. Dashed lines show the number of species that comprise up to 75% of the total production in each group, by tonnage. Production in each group is dominated by a small number of species but each group also contains high diversity. Production according to ASFIS identification. Source: FAO 2 .
Extended Data Fig. 2 Global forage fish landings (1950–2017) for 315 species.
Global forage fish landings are sensitive to interannual climate variation associated with El Niño Southern Oscillation events. Orange line represents the trend in presence of interannual variation. Data source: FAO 2 .
Extended Data Fig. 3 Global landings of forage fish used for fishmeal and fish oil production.
Orange line represents the trend in presence of interannual variation. Data source: Sea Around Us 48 .
Extended Data Fig. 4 Nominal and real prices of fishmeal and fish oil versus plant-based meals and oils.
Prices deflated by the implicit GDP deflator. Data sources: FAO International Commodity Price Database (2020), http://www.fao.org/giews/food-prices ; Index mundi (2020), www.indexmundi.com ; National Sunflower Association (2020), www.sunflowernsa.com ; US Bureau of Economic Analysis (2020), https://www.bea.gov/ .
Extended Data Fig. 5 Proportion of global aquaculture production that is certified or rated.
Data from the Seafood Watch Sustainability of Global Seafood Data portal collating volumes certified from the Aquaculture Stewardship Council (ASC) (2020) and Global Aquaculture Alliance (GAA) Best Aquaculture Management (2020) and rated volumes from Seafood Watch (SFW) (2020). The ratings data represent the volume rates minus volumes certified based on internal assessments by SFW. The certification estimates may be overestimated as it was not possible to distinguish overlap between GAA- and ASC-certified volumes. A number of assumptions were made in these calculations as SFW does not recognize a number of species certified by ASC and GAA. These species include salmon, catfish, oysters, scallops, sturgeon, crawfish, and sea cucumber. In some cases, a surplus volume was created by adding GAA, ASC and SFW. This surplus volume was included in the ‘avoid’ category of SFW, under the assumption that cross-over between ratings and certification is more likely than certified and unrated production.
Extended Data Fig. 6 Proportion of aquaculture that is certified and rated by commodity group.
Data from the Seafood Watch Sustainability of Global Seafood Data portal collating volumes certified from the Aquaculture Stewardship Council (ASC) (2020) and Global Aquaculture Alliance (GAA) (2020) and rated volumes from Seafood Watch (SFW) (2020). The ratings data represent the volume rates minus volumes certified based on internal assessments by SFW. The certification estimates may be overestimated as it was not possible to distinguish overlap between GAA- and ASC-certified volumes. A number of assumptions were made in these calculations as SFW does not recognize a number of species certified by ASC and GAA. These species include salmon, catfish, oysters, scallops, sturgeon, crawfish and sea cucumber. In some cases, a surplus volume was created by adding GAA, ASC and SFW. Surplus volumes were added to certification and subtracted from ratings for the different regions. This calculation was assumes that a certified product is more likely to be rated than not.
Supplementary information
Supplemental information.
This file contains a list of references, including peer-reviewed articles, reports, and weblinks, that were used in the Review but not cited due to space constraints.
Source Data Fig. 1
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Naylor, R.L., Hardy, R.W., Buschmann, A.H. et al. A 20-year retrospective review of global aquaculture. Nature 591 , 551–563 (2021). https://doi.org/10.1038/s41586-021-03308-6
Download citation
Received : 21 August 2020
Accepted : 29 January 2021
Published : 24 March 2021
Issue Date : 25 March 2021
DOI : https://doi.org/10.1038/s41586-021-03308-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Dietary antarctic krill improves antioxidant capacity, immunity and reduces lipid accumulation, insights from physiological and transcriptomic analysis of plectropomus leopardus.
- Mengya Wang
- Shaoxuan Wu
BMC Genomics (2024)
Novel insights into the rhizosphere and seawater microbiome of Zostera marina in diverse mariculture zones
- Tianyu Wang
- Yanying Zhang
Microbiome (2024)
Deciphering the gut microbiome of grass carp through multi-omics approach
- Zhigang Zhou
Effect of trade on global aquatic food consumption patterns
- Kangshun Zhao
- Steven D. Gaines
Nature Communications (2024)
Wild fish consumption can balance nutrient retention in farmed fish
- David F. Willer
- Richard Newton
- James P. W. Robinson
Nature Food (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Microbiol
Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives
Seyed hossein hoseinifar.
1 Department of Fisheries, Faculty of Fisheries and Environmental Sciences, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran
Yun-Zhang Sun
2 Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture, Fisheries College, Jimei University, Xiamen, China
3 Key Laboratory for Feed Biotechnology of the Ministry of Agriculture, Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China
Zhigang Zhou
Along with the intensification of culture systems to meet the increasing global demands, there was an elevated risk for diseases outbreak and substantial loss for farmers. In view of several drawbacks caused by prophylactic administration of antibiotics, strict regulations have been established to ban or minimize their application in aquaculture. As an alternative to antibiotics, dietary administration of feed additives has received increasing attention during the past three decades. Probiotics, prebiotics, synbiotics and medicinal plants were among the most promising feed supplements for control or treatments of bacterial, viral and parasitic diseases of fish and shellfish. The present review summarizes and discusses the topic of potential application of probiotics as a means of disease control with comprehensive look at the available literature. The possible mode of action of probiotics (Strengthening immune response, competition for binding sites, production of antibacterial substances, and competition for nutrients) in providing protection against diseases is described. Besides, we have classified different pathogens and separately described the effects of probiotics as protective strategy. Furthermore, we have addressed the gaps of existing knowledge as well as the topics that merit further investigations. Overall, the present review paper revealed potential of different probiont to be used as protective agent against various pathogens.
The interactions between probiotics and diseases of fish and shellfish
Probiotics: definition and history.
Nowadays, several types of beneficial feed additive such as probiotics, prebiotics, and synbiotics are being used in aquaculture to improve growth performance, immune responses and disease resistance as well as an alternative to antibiotics (Irianto and Austin, 2002 ; Hoseinifar et al., 2016 , 2017b ; Sayes et al., 2018 ). The term “probiotics” arose from the Greek words “pro” and “bios” meaning “for life”; generally referred to microbial feed additives which confer host organism through modulation of intestinal microbiota. Parker ( 1974 ) was the first who defined probiotics as organisms and substances that affect microbial in intestine. According to the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), probiotics are live microorganisms which are used orally having some tangible health benefits to the host (Hotel and Córdoba, 2001 ). Considering the difference between environment in aquatic ecosystem and those terrestrial animals, a modified definition proposed for probiotics in aquaculture by Merrifield et al. ( 2010b ) as, “a probiotic organism can be regarded as a live, dead or component of a microbial cell, which is administered via the feed or to the rearing water, benefiting the host by improving disease resistance, health status, growth performance, feed utilization, stress response or general vigor, which is achieved at least in part via improving the hosts microbial balance or the microbial balance of the ambient environment.” The probiotics include different kinds of bacteria, bacteriophages, microalgae and yeast which have been widely used in aquaculture via water routine or feed supplement (Llewellyn et al., 2014 ) Currently, there are lots of commercially available probiotics in for of mono or multi-strains (Van Doan et al., 2017 ).
Mode of actions on disease resistance
The extensive literature on probiotics revealed beneficial effects on host's gut defenses which has vital importance in diseases prevention as well digestive tract inflammation treatment (Azimirad et al., 2016 ; Modanloo et al., 2017 ). Apart from immunomodulation, probioticmicroorganisms, such as lactic acid bacteria, Brevibacillus brevis, Vagococcus fluvialis , and Vibrio harveyi (Lazado et al., 2011 ; Sugimura et al., 2011 ; Korkea-aho et al., 2012 ; Mahdhi et al., 2012 ; Sorroza et al., 2012 ), stick with the mucosal epithelium of gastrointestinal tract and help to resist pathogens (Luis-Villaseñor et al., 2011 ). In another way, probiotics increase feed digestibility through elevation of different digestive enzymes such as alginate lyases, amylases, and proteases (Zokaeifar et al., 2012 ). They also produce organic acids, fatty acids, biotin and vitamin B12, hydrogen peroxide, antibiotics, bacteriocins, siderophores, lysozyme (Sugita et al., 1991 , 1992 ; Yan et al., 2002 ; Vine et al., 2006 ), which have positive effects on host health. Numerous studies have demonstrated that probiotics caused health benefits in aquatic organisms, such as Japanese flounder (Heo et al., 2013 ), black tiger prawns, Penaeus monodon (Rengpipat et al., 1998 ), whiteleg prawns (Chiu et al., 2007 ), and western king prawns (Hai et al., 2010 ).
Modulation of immune parameters
The first defense line against infections is innate immune responses (or non-specific immune responses) which include different cells and mechanisms that protect host organism from infectious diseases. It has been reported that probiotics can affect the elements of non-specific immune system such as mono-nuclear phagocytes (monocytes, macrophages) and polymorphonuclear leukocytes (neutrophils), natural killer (NK) cells etc. Previous studies revealed increment of leucocytes (Korkea-aho et al., 2012 ), monocytes (Aly et al., 2008b ), erythrocytes, granulocytes, macrophage, and lymphocytes in various fishes following treatment with probiotics (Kim and Austin, 2006a , b ; Nayak et al., 2007 ; Kumar et al., 2008 ). For instance, rainbow trout fed Clostridium butyricum showed increased resistance against vibriosis through affecting phagocytic activity of leukocytes (Sakai et al., 1995 ). Furthermore, dietary Bacillus sp. S11 positively affected cellular and humoral immunity in tiger shrimp ( Penaeus monodon ) which resulted in protection against disease (Rengpipat et al., 2000 ). Also, combined administration of Bacillus and Vibrio sp. in young white shrimp showed beneficial effects on growth performance, survival as well as resistance against V. harveyi and white spot syndrome virus (Antony et al., 2011 ). The authors attributed the protection to elevation of phagocytosis and antibacterial activity; indeed immunomodulation. Beside these results on shrimps, dietary Lactobacillus rhamnosus (ATCC 53103) (10 5 CFU g −1 ) increased the respiratory burst in rainbow trout ( Oncorhynchus mykiss )(Nikoskelainen et al., 2003 ). Therefore, probiotics are beneficial bacteria which not only capable of inhibiting pathogens, but also regulating the host immune system.Probiotics possess conserved microbe-associated molecular patterns (MAMPs), including peptidoglycan (PGN), lipoteichoic acids (LTA), S-layer protein A (SlpA), exopolysaccharides (EPS), flagellin and microbial nucleic acids which can be recognized by certain pattern recognition receptors (PRRs), and induces a signaling cascade that can result in the production of cytokines, chemokines, and other effector molecules thus activating the immune response in the host (Bron et al., 2012 ; Remus et al., 2012 ). During past years, there was increasing interests toward determination of mode of action of probiotics on intestinal immune system. In this regard, the researchers evaluated the possible relationship between TLR signaling-mediated recognition of probiotics and activation of the intestinal immunity. For example, it has been reported that TLR2 signaling pathway was involved in recognition of probiotic Psychrobacter sp. SE6 and inducing subsequently immune responses in grouper Epinephelus coioides (Sun et al., 2014 ).
Competition for binding sites
Competitive exclusion has been suggested as a mode of action of probiotic in prevention of pathogens (Mahdhi et al., 2012 ; Sorroza et al., 2012 ); achieved by colonization of probiotics in GI mucosal epithelium (Macey and Coyne, 2006 ; Merrifield et al., 2010a ; Lazado et al., 2011 ; Korkea-aho et al., 2012 ). Different types of surface determinants suggested to be involved in probiotis interaction with intestinal epithelial cells and mucus which per se prevents pathogens colonization (so called competitive exclusion). The primary reason for this could be competitions for adhesion receptors (Montes and Pugh, 1993 ) which can antagonize pathogens (Luis-Villaseñor et al., 2011 ) and reduce their colonization (Chabrillón et al., 2005 ). This clearly shows the potential of probiotics administration as a substitute for antibiotics and other chemicals (Cheng et al., 2014 ). It has been reported that passive forces, electrostatic interactions, hydrophobic, steric forces, lipoteichoic acids were among the factors which affect adhesion of probiotics to attachment sites (Wilson et al., 2011 ). Westerdahl et al. ( 1991 ) stated that competition for attachment sites and nutrients following occupying mucosal surfaces could be possible mode of action for protective effects of probiotic against pathogens.
Production of antibacterial substances
In aquaculture, probiotics are used as an alternative to antibiotics and chemicals (Decamp et al., 2008 ; Van Hai et al., 2009 ; Heo et al., 2013 ). Though the mode of action through which probiotics exert antibacterial effects remained to be determined, many studies indicated that probiotics produced antibiotic compounds (Moriarty, 1998 ). Besides, reduce in pH following production of organic acids can inhibits growth of pathogenic bacteria (Ma et al., 2009 ). For example, Ramesh et al. ( 2015 ) reported antibacterial activity of Bacillus licheniformis and B. pumilus ; which resist low pH and high bile concentrations. Another study with Bacillus licheniformis CPQB, revealed inhibition of Vibrio alginolyticus in whiteleg prawns (Ferreira et al., 2015 ). It has been demonstrated that Lactobacillus spp. (common probiotics) produce short chain fatty acids, diacetyl, hydro peroxide, and bactericidal proteins (Rengpipat et al., 1998 ; Verschuere et al., 2000 ; Faramarzi et al., 2011 ), which pre se improve immune responses as well as disease resistance (Raa, 1996 ; Gram et al., 1999 ). Consequently, probiotics can protect aquatic animals from challenge with pathogens by producing antibiotic compounds.
Competition for nutrients
The competition of nutrients has been considered among the mechanisms through which probiotics inhibit pathogens (Ringø et al., 2016 ). Previous study has reported that competition for iron is an essential element in marine bacteria (Verschuere et al., 2000 ). The majority of bacteria need Iron for their growth. However, there is limited available of iron in the tissues and body fluids of animals (Verschuere et al., 2000 ). The siderophores which are iron-binding agents, help bacteria to obtain the necessary amount of Iron for their growth. There is direct relation between production of siderophore and virulence of some pathogens (Gram et al., 1999 ).
The beneficial effects of Gram-positive genus Bacillus on water quality in culture environment has been reported in previous studies (Rafiee and Saad, 2005 ; El-Haroun et al., 2006 ; Hai, 2015 ; Dawood and Koshio, 2016 ). It seems that genus Bacillus is more effectual for converting organic matter to CO 2 as well as balancing phytoplankton production (Balcázar et al., 2006 ). It has been reported that supplemented F. vannamei feed with Bacillus sp., Saccharomyces cerevisiae, Nitrosomonas sp., and Nitrobacter sp. (a commercial product) could decrease the concent rations of inorganic nitrogen and phosphate from 3.74 to 1.79 mg/L and 0.1105 to 0.0364 mg/L, respectively (Li et al., 2006 ).
In addition, probiotics also enhanced growth performance and feed utilization in aquatic animals through increasing digestive enzymes activity (Yu et al., 2009 ; Zokaeifar et al., 2012 ; Hoseinifar et al., 2017a ). For example, Van Hai et al. reported that dietary probiotics ( Pseudomonas aeruginosa and Ps. Synxantha ) enhanced western king prawn growth performance (Van Hai et al., 2009 ; Hai et al., 2010 ). Recently research by Faturrahman et al. also revealed dietary probiotic ( Vibrio Alg3.1Rf R -Abn1.2Rf R -enriched protein) improved growth rate of Haliotis asinine (Rohyati, 2015 ). The incease of digestive enzyme activity and improvement of the digestive process following treatment with probiotic has been attributed to production of extracellular enzymes such as proteases, carbohydrolases and lipases (Arellano-Carbajal and Olmos-Soto, 2002 ; Leonel Ochoa-Solano and Olmos-Soto, 2006 ; Soleimani et al., 2012 ; Eshaghzadeh et al., 2015 ; Hoseinifar et al., 2015a , b ). Furthermore, considering provision of vital nutrients like fatty acids, biotin and vitamins, probiotics might be a complementary food source (Verschuere et al., 2000 ).
Probiotics and bacterial diseases in fish (Table (Table1 1 )
Overview of the effects of probiotics against pathogenic bacteria in fish.
Gram-positive bacteria
Lactic acid bacteria.
Lactic acid bacteria (LAB) Gram positive, usually non-motile and non-sporing bacteria which mainly produce lactic acid during fermentation (Stanier et al., 1975 ). They were among the mostly studied probiotics (Merrifield et al., 2014 ). The extensive available literature revealed beneficial effects of LABs as probiotic on growth performance, immune responses and disease resistance shellfish (Ringø et al., 2010 ; Merrifield et al., 2014 ). Another important feature of these probiont strains is disease protection which has been reviewed in this section.
Carnobacteria
Carnobacteria have been frequently isolated from fish intestine (Merrifield et al., 2014 ). It has shown antagonistic activity against different kinds of fish pathogens (Ringø et al., 2010 ). The C. inhibens K1 isolated from Atlantic salmon ( Salmo salar L.) digestive tract inhibited fish pathogens under in vitro condition (Jöborn et al., 1997 ), and subsequently study showed that dietary administration of 5 × 10 7 cells g −1 C. inhibens K1 for 14 days reduced mortalities caused by A. salmonicida, Vibrio ordalii and Yersinia ruckeri in Atlantic salmon and rainbow trout (Robertson et al., 2000 ). The C. divergens strain 6251, isolated from Artic charr ( Salvelinus alpinus L.) foregut, showed growth-inhibitory effects against both Aeromonas salmonicida and Vibrio anguillarum in vitro (Ringø et al., 2002 ; Ringø, 2008 ). Also, dietary administration of C. divergens for 3 weeks reduced vibriosis caused by V. anguillarum in Atlantic cod ( G. morhua) fry (Gildberg et al., 1997 ). Kim and Austin ( 2006a ) characterized two Carnobacteria isolates obtained from rainbow trout intestine ( C. maltaromaticum B26 and C. divergens B33). Both strains stimulated non-specific immunity and demonstrated effectiveness against A. salmonicida and Y. ruckeri in vitro . Løvmo Martinsen et al. ( 2011 ) reported that C. maltaromaticum which was previously isolated from Atlantic cod hindgut chamber could, to a certain extent, outcompete V. anguillarum in an unidentified mechanism.
Lactobacillus
The application of probiotic Lactobacillus spp. in fish aquaculture has been extensively studied (Merrifield et al., 2010a ; Merrifield and Carnevali, 2014 ). Lactobacillus ( Lb .) acidophilus improved immune responses and resistance against Pseudomonas fluorescens and Streptococcus iniae in Nile tilapia (Aly et al., 2008a , b ). Similarly, African catfish ( Clarias gariepinus ) juveniles were fed Lb. acidophilus (3 × 10 7 CFU g −1 ) for 12 weeks (Al-Dohail et al., 2011 ) and resistance against Staphylococcus xylosus, Aeromonas hydrophila gr2 and Streptococcus agalactiae (2 × 10 6 CFU ml −1 intraperitoneal injection) were tested which revealed elevated resistance. Likewise, feeding rainbow trout with Lb. rhamnosus ATCC 53101 (10 9 cells g −1 ) for 51 days resulting in a reduction of mortalities by A. salmonicida from ~ 53% to ~ 19% (Nikoskelainen et al., 2001 ). Furthermore, dietary supplemented with 10 8 CFU g −1 and 10 10 CFU g −1 Lb. rhamnosus for 14 days protected tilapia ( Oreochromis niloticus ) from acute septicemic death by experimental Edwardsiella tarda infection (Pirarat et al., 2011 ). Rainbow trout fed Lb. plantarum CLFP 238 at 10 7 CFU g −1 of feed for 30 days showed a dramatic reduction in mortalities when challenged with pathogenic Lactococcus ( Lc .) garvieae (Vendrell et al., 2008 ). Salinas et al. ( 2008 ) reported that Lactobacillus delbrueckii sp. lactis (CECT 287, Valencia, Spain) prevented A. salmonicida damaging effects in the foregut of Atlantic salmon. Likewise, a study on Gilthead seabream revealed Lb. rhamnosus and Bifidobacterium lactis notably reduced colonization of the pathogenic bacteria ( V. anguillarum, Photobacterium damselae ssp. piscicida, V. alginolyticus , and Vibrio harveyi ) (Chabrillon et al., 2006 ). It was also observed that L. plantarum , isolated from rainbow trout intestinal mucosa, could upregulate immune related genes expression and increase resistance against Lc. garvieae (Pérez-Sánchez et al., 2011 ). Feeding rock bream ( Oplegnathus fasciatus ) with Lb. sakei BK19 (2.2 × 10 7 CFU g −1 ) resulted in non-significant decrease of mortality after challenge with Edwardsiella tarda (Harikrishnan et al., 2011 ). Also, Lb. pentosus PL11 improved immune responses as well as resistance of Japanese eel ( Anguilla japonica) against Edwardsiella tarda (Lee et al., 2013 ). To test the protective effects of dietary supplementation of highly adhesive Lactobacillus brevis JCM 1170 (HALB) and less-adhesive Lb. acidophilus JCM 1132 (LALB) against the tilapia pathogen, A. hydrophila NJ-1, fish were immersed in strain NJ-1 for 14 days without supplemented feed. The results showed that diet containing 10 9 cells g −1 of strain HALB/g feed (B3) showed significantly lower mortality (Liu et al., 2013 ). Recently, Beck et al. ( 2015 ) evaluated the mixture or single application of two host associated probiotics include Lc. lactis BFE920 isolated from bean sprout and Lb. plantarum FGL0001 isolated from olive flounder ( Paralichthys olivaceus ) hindgut, in olive flounder. After challenge with Streptococcus iniae (log 10 6.0 CFU/fish), the survival rate in the groups fed mixed probiotics and Lb. plantarum FGL0001, and the control were 55, 45, 35, and 20%, respectively. In a comparative view, it seems that Lb. plantarum was the most efficient Lactobacillus species in terms of disease bio-control. As the -aforementioned studies revealed, this species substantially improved resistance against various pathogenic bacteria. Besides disease protection, the species showed beneficial effects on growth performance and immune parameters (Van Doan et al., 2017 ). Hence, Lb. plantarum can be suggested as a promising tool for disease control in aquaculture.
Lactococcus
Balcázar et al. ( 2007 ) isolated Lc. lactis subsp. lactis (CLFP 100) and Lc. lactis subsp. cremoris (CLFP 102) from rainbow trout intestine. Subsequently, in a separate study, they administered Lc. Lactis in rainbow trout diet and observed increased immune parameters as well as protection against furunculosis (Balcázar et al., 2007 ). The same results observed with brown trout ( Salmo trutta ) challenged with Aeromonas salmonicida (Balcázar et al., 2009 ). Kim et al. ( 2013 ) reported that Lc. lactis BFE920 inhibits the growth of different pathogens including Streptococcus iniae, S. parauberis, Enterococcus viikkiensis as well as Lactococcus garviae under in vitro condition. The same authors supplemented olive flounder ( Paralichthys olivaceus ) diet with Lc. lactis BFE920 and after 2 weeks feeding observed activated the innate immune system which resulted in protection against S. iniae infection in both in experimental condition and large scale field condition. In accordance, Heo et al. ( 2013 ) reported that dietary Lc. lactis (10 8 CFU g −1 ) elevated serum immune parameters (e.g., lysozyme, antiprotease, serum peroxidase, and blood respiratory burst activities) as well as resistance against S. iniae in olive flounder. Recently, Beck et al. ( 2015 ), in an study with olive flounder, observed that dietary administration of mixed probiotic Lb. plantarum FGL0001 and Lc. lactis BFE920, or single Lc. lactis BFE920 for 30 days could improve the survival rates after challenged with S. iniae . An overview of different Lactococcus spp. revealed that the main focus was on Lc. Lactis and this species was capable of protecting different fish species against bacterial pathogens.
Leuconostoc
Balcázar et al. ( 2007 ) reported that Lc. mesenteroides isolated from rainbow trout intestine inhibited the growth of various pathogens. The same research group supplemented rainbow trout and brown trout diets with Lc. mesenteroides (10 6 CFU g −1 ) and observed immunomodulation and increased resistance against furunculosis (Balcázar et al., 2007 ) and A. salmonicida infection (Balcázar et al., 2009 ). Dietary application of Lc. mesenteroides CLFP 196 to rainbow trout at 10 7 CFU g −1 of feed for 30 days dramatically reduced the mortalities following challenge with L. garvieae (Vendrell et al., 2008 ). However, Lc. mesenteroides subsp. Mesenteroides , obtained from rainbow trout intestine, failed to improve rainbow trout disease resistance to lactococcosis (Pérez-Sánchez et al., 2011 ). Although, there are limiting studies over Leuconostoc spp. potential to protect fish against diseases, but available results revealed beneficial effects of Luc. mesenteroides .
Pediococcus
Huang et al. ( 2014 ) isolated P. pentosaceus strain 4012 from cobia intestine and observed antagonistic effects on V. anguillarum under in vitro condition. Subsequently, dietary administration of P. pentosaceus 4012 significantly decreased the cumulative mortality of groupers after V. anguillarum infection (Huang et al., 2014 ). Dietary supplement with probiotic P. acidilactici increased resistance of rainbow trout fry against vertebral column compression syndrome (VCCS) (Aubin et al., 2005 ). Also, combined administration of galactooligosaccharides and P. acidilactici for 8 weeks improved the immune parameters and resistance against S. iniae in rainbow trout fingerlings. An overview of literature revealed increasing attentions toward administration of P. acidilactici as probiotic in aquaculture, recently. It seems that this species is capable to be considered as disease protection agent in aquaculture.
Enterococcus
Chang and Liu ( 2002 ) administered a commercial product containing E. faecium SF 68 in European eel, Anguilla anguilla diet and observed lower edwardsiellosis in fish exposed to Edwardsiella tarda . E. gallinarum showed a strong inhibitory effect against V. anguillarum in vitro , and under in vivo condition protected sea bass against V. anguillarum infection (Sorroza et al., 2013 ). Recently, Safari et al. ( 2016 ) evaluated the benefits of dietary administration of host-derived candidate probiotics E. casseliflavus in juvenile rainbow trout, and results showed that E. casseliflavus could improve growth performance and enhance disease resistance when challenged with S. iniae .
Sorroza et al. ( 2012 ) supplemented sea bass diet with Vagococcus fluvialis (10 9 cfu g −1 ) for 20 days and observed that probiotic fed fish had higher relative percent of survival (42.3%) than control group following challenge with V. anguillarum . This study showed the potential of Vagococcus spp. and highlighted the needs to additional research in future.
Bacillus sp.
Bacillus sp. as feed additives improves growth performance and immune response and disease resistance in fish has been extensively reviewed (Mingmongkolchai and Panbangred, 2018 ). Dietary administration of B. subtilis and B. licheniformis (BioPlus2B) improved trout resistance to infection with Y. ruckeri (Raida et al., 2003 ). Also, feeding Indian major carp, Labeo rohita with B. subtilis at 1.5 × 10 7 CFU g −1 increased resistance against A. hydrophila infection (Kumar et al., 2006 ). Newaj-Fyzul et al. ( 2007 ) administered different forms (viable, formalized or sonicated cells or cell-free supernatant) of B. subtilis AB1 in rainbow trout diet and observed higher resistance against Aeromonas (Newaj-Fyzul et al., 2007 ). Furthermore, B. subtilis (8 × 10 7 CFU g −1 ) reduced mortalities Ictalurus punctatus and striped catfish, Pangasianodon hypophthalmus following challenge with Edwardsiella ictaluri (Ran et al., 2012 ). Liu et al. ( 2012 ) proved that dietary B. subtilis (10 4 , 10 6 , and 10 8 CFU g −1 ) for 14 and 28 days was able to enhance the relative survival percentages of grouper, Epinephelus coioides challenged with Streptococcus sp. A diet supplemented with 0.1 or 0.3% B. subtilis enhanced prophylactic property of red hybrid tilapia, Oreochromis sp. against pathogenic Streptococcus agalactiae (Ng et al., 2014 ).
Aly et al. ( 2008b ) reported that feeding tilapia with 10 6 and 10 12 cells g −1 B. pumilus enhanced immune and health status and improve resistance against A. hydrophila . B. pumilus has also reported to dramatically improved survival of “Loco” Concholepas concholepas larvae (Leyton et al., 2012 ). Similarly, Sun et al. ( 2009 ) reported that B. pumilus SE5 and B. clausii DE5 obtained from orange-spotted grouper Epinephelus coioides , inhibited growth of pathogenic Staphylococcus aureus, V. harveyi and V. parahaemolyticus under in vitro condition. Also, feeding grouper E. coioides larvae with copepod ( P. annandalei ) enriched B. clausii DE5 and B. pumilus noticably larval survival (Sun et al., 2013 ).
Bandyopadhyay and Das Mohapatra ( 2009 ) isolated Bacillus circulans PB7 from Catla catla intestine, and subsequently added to Catla catla fingerlings diet at rate of 2 × 10 4 , 2 × 10 5 , or 2 × 10 6 cells per 100 g. After 60 days feeding elevated immune parameters as well as resistance against A. hydrophila infection. Likewise, feeding Olive flounder (Paralichthys olivaceus) with ( B. subtilis, B. pumilus , and B. licheniformis ) at rate of 10 10 CFU g −1 elevated resistance against S. iniae (Cha et al., 2013 ). Han et al. ( 2015 ) stated that feeding with commercial B. licheniformis improved the disease resistance against Streptococcus iniae infection in tilapia. Similarly, Nile tilapia fed with 1 × 10 6 and 1 × 10 4 CFU g −1 of B. amyloliquefaciens for 30 days showed higher resistance against pathogenic Yersinia ruckeri or Clostridium perfringens type D (Selim and Reda, 2015 ). Interestingly, intraperitoneally administration of cellular components (cell wall proteins and whole cell proteins) of Bacillus licheniformis and B. pumilus have been reported to improve immune parameters which per se protected rohu Labeo rohita (Hamilton) against A. hydrophila infection (Ramesh et al., 2015 ). The overview of literature regarding Bacillus spp. as probiotic aimed at elevation of disease resistance revealed more information on B. subtilis . The extensive research on this species revealed high potential for immunomodulation and disease protection. Indeed, B. subtilis can be considered as beneficial agent for disease bio-control.

Other gram-positive bacteria
Clostridium butyricum.
Sakai et al. ( 1995 ) demonstrated that dietary C. butyricum increased rainbow trout protection vibriosis. Pan et al. ( 2008a ) stated that C. butyricum CB2 showed strong antagonistic activity to pathogenic A. hydrophila and V. anguillarum . Subsequently, oral administration of live or dead C. butyricum CB2 at dose of 10 8 CFU g −1 feed enhanced the phagocytic activity of leucocytes and resistance to vibriosis in Chinese drum, Miichthys miiuy (Basilewsky) (Pan et al., 2008b ).
Micrococcus
Dietary application of probiotic Micrococcus luteus increased rainbow trout survival after A. salmonicida challenge (Irianto and Austin, 2002 ). Abd El-Rhman et al. ( 2009 ) reported that Nile tilapia fed M. luteus containing diets for 6-days per week for 90 days showed decreased mortality following A. hydrophila challenged.
Rhodococcus
It has reported that the cellular components (cell wall proteins and whole cell proteins) of Rhodococcus SM2 increased rainbow trout protection against V. anguillarum (Sharifuzzaman et al., 2011 ).
Brochothrix
Dietary administration of Brochothrix thermosphacta BA211 (10 10 cells g −1 ) protected rainbow trout against skin infections challenged with A. bestiarum , i.e., mortalities reduced from 88 to 22%, however, the probiotic had no effect against ichthyophthiriasis (caused by the parasite Ichthyophthirius multifiliis ) (Pieters et al., 2008 ).
Sharifuzzaman and Austin ( 2010 ) isolated Kocuria SM1 rainbow trout digestive tract and subsequently added to rainbow trout diet at rate of 10 8 cells g −1 . They observed higher protection against challenge with V. anguillarum and V. ordalii .
Gram-negative bacteria
Pseudomonas.
Rainbow trout exposed to P. fluorescens AH2 at rate of 10 5 CFU/ml for 5 days showed lower mortality after V. anguillarum challenge (Gram et al., 1999 ), while the probiotic did not confer protection of salmon against furunculosis (Gram et al., 2001 ). P. chlororaphis strain JF3835, obtained from perch ( Perca fluviatilis L.) intestine, has ability to control Aeromonas sobria infection in perch (Gobeli et al., 2009 ). Pseudomonas M162 showed in vitro inhibition to Flavobacterium psychrophilum , and dietary application of M162 increased rainbow trout resistance against F. psychrophilum infection (Korkea-aho et al., 2012 ). The same research group evaluated protection caused by various strains of Pseudomonas M174 in rainbow trout and observed highest protection against F. psychrophilum caused by M174 strain (Korkea-aho et al., 2011 ). Giri et al. ( 2012 ) fed Labeo rohita with 10 7 and 10 9 CFU g −1 P. aeruginosa VSG-2 and evaluated fish resistance against A. hydrophila. The results revealed that probiotic fed fish had significantly higher resistance against A. hydrophila infection (Giri et al., 2012 ). Similarly, oral administration of P. aeruginosa PsDAHP1 inhibited biofilm formation and increased defense mechanisms which per se elevated zebrafish protection from V. parahaemolyticus DAHV2 infection (Vinoj et al., 2015 ).
Dietary administration of A. hydrophila has been reported to reduce mortality caused by A. salmonicida in rainbow trout (Irianto and Austin, 2002 ). Similarly, Irianto et al. ( 2003 ) showed that feeding with formalin-inactivated A. hydrophila A3-51 increased goldfish (Carassius auratus) resistance against A. salmonicida. Likewise, rainbow trout fed A. sobria GC2 at rate of 5 × 10 7 cells g −1 showed improved resistance to L. garvieae and S. iniae (Brunt and Austin, 2005 ). Also, Pieters et al. ( 2008 ) demonstrated that feeding with 10 8 cells g −1 A . sobria GC2 and 10 10 cells g −1 Brochothrix thermosphacta BA211 improved rainbow trout resistance against causal agent of fin rot (i.e., A. bestiarum ). Recently, Chi et al. ( 2014 ) isolated A. veronii BA-1 from common carp (Cyprinus carpio) intestine. Dietary administration of isolated strain (1 × 10 8 cell g −1 ) beneficially affected immune parameters as well as resistance against A. hydrophila in carp.
Chabrillón et al. ( 2006 ) fed gilthead seabream (Sparus aurata) with S. putrefaciens (Pdp11) at rate of 10 8 CFU g −1 and then challenged with V. anguillarum DC11R2a. The results revealed significantly lower mortality in probiotic fed fish (10%) compared to control group (56%). In a study with Senegalese sole ( Solea senegalensis ), Diaz-Rosales et al. ( 2009 ) evaluated probiotic potential of S. putrefaciens Pdp11 and S. baltica Pdp13. They observed elevated immune responses as well as resistance against Photobacterium damselae sub sp. Piscicida. Also, De la Banda et al. ( 2012 ) evaluated effectiveness of different forms (fresh and lyophilized cells) of S. putrefaciens Pdp11, in juvenile Senegalese sole diet and observed improved growth and protection against P. damselae subsp. piscicida . Recently, the same group reported that dietary S. putrefaciens Pdp11 modulated immune related genes expression, intestinal microbiota as well as intestinal conditions which per se improved stress tolerance caused by crowding condition. (Tapia-Paniagua et al., 2014 ). Dietary application of 1 × 10 8 cell g −1 S. xiamenensis A-1 and S. xiamenensis A-2, which isolated from grass carp ( Ctenopharyngodon idellus ) intestine, for 28 days decrease the survival of grass carp after experimentally challenged with A. hydrophila (Wu et al., 2015 ).
Enterobacter
Capkin and Altinok ( 2006 ) isolated E. cloacae from rainbow digestive tract and supplemented trout diet with isolated strain at rate of 10 8 CFU g −1 . Interestingly, following challenge with Yersinia ruckeri, probiotic fed fish showed significantly higher survival (99.2%) compared those fed control diet (35%). Probiotic strains C6-6 and C6-8 which supposed to be E. amnigenus and Enterobacter sp., inhibited F. psychrophilum (Burbank et al., 2011 ). Moreover, supplementation of rainbow trout with 10 6 to 10 8 cells g −1 of those probiotic resulted in higher resistance to Flavobacterium psychrophilum infection (Burbank et al., 2011 ). Also, inclusion of inactivated E. faecalis in rainbow trout diet at rate of 5 g kg −1 decreased mortality caused by experimental A. salmonicida infection (Rodríguez-Estrada et al., 2013 ).
Roseobacter
It has been reported that rotifers enriched with Roseobacter 27-4 increased turbot, Scophthalmus maximus L., larvae protection against V. anguillarum infection (Planas et al., 2006 ). An isolate from seawater in scallop ( Pecten maximus ) identified as Phaeobacter ( Roseobacter ) gallaeciensis BS107 (DSM17395), which antagonized fish pathogenic bacteria in vitro and reduced the mortality by approximately 10% in cod larvae upon challenge with V. anguillarum (D'Alvise et al., 2012 ). Treatment of Turbot ( Scophthalmus maximus ) larvae with 10 7 CFU mL −1 Roseobacter sp. strain 27-4, decreased cumulative mortality following challenge with V. anguillarum (Hjelm et al., 2004 ). Likewise, feeding turbot larvae with Roseobacter sp. strain 27-4 enriched rotifers improved protection against V. anguillarum, (Planas et al., 2006 ).
Vibrio alginolyticus showed in vitro inhibition to V. ordalii, V. anguillarum, A. salmonicida and Y. ruckeri , and in vivo protection to Atlantic salmon challenged with A. salmonicida (Austin et al., 1995 ). Dietary administration of V. fluvialis resulted in higher survival in rainbow trout challenged with A. salmonicida (Irianto and Austin, 2002 ).
Zooshikella
Kim et al. ( 2010 ) showed that different levels (3.4 × 10 4 , 3.5 × 10 6 and 3.4 × 10 8 CFU g −1 ) of dietary Zooshikella sp. JE-34 helps to improve the innate immune response and control streptococcus inane infections in olive flounder (Paralichthys olivaceus).
Flavobacterium
Chi et al. ( 2014 ) supplemented carp diet with Flavobacterium sasangense BA-3 (1 × 10 8 cell g −1 ) isolated from the common carp intestine for 28 days. They observed enhanced immune parameters as well as resistance against A. hydrophila infection.
The potential of yeast as probiotic to improve disease resistance has been demonstrated in several studies. Abdel-Tawwab et al. ( 2008 ) reported that diets supplemented with baker's yeast S. cerevisiae at dose of 0.25, 0.50, 1.0, 2.0, or 5.0 g yeast/kg reduced mortality in tilapia after intraperitoneal injection pathogenic A. hydrophila . Subsequently, the same group observed that Baker's yeast improves the resistance against the water-borne Cu toxicity in Galilee tilapia Sarotherodon galilaeus (L.) (Abdel-Tawwab et al., 2010 ). Quentel et al. ( 2005 ) reported that singular or combined administration of P. acidilactici and S. cerevisiae var. boulardii improved rainbow trout resistance against Y. ruckeri . Reyes-Becerril et al. ( 2011 ) supplemented Leopard grouper ( Mycteroperca rosacea ) diet with Debaryomyces hansenii CBS 8339 (10 6 CFU g −1 ) for 5 weeks. At the end of feeding trial, probiotic fed fish ad noticeably higher immunoglobulin M (IgM) level, catalase (CAT) and superoxide dismutase (SOD) activities following A. hydrophila AH-315 challenge. Generally, the majority of studies performed on yeasts revealed beneficial effects on immune system (Hai, 2015 ). Hence, it seems that they can be considered as beneficial means of disease control and control.
Probiotics and bacterial diseases in shellfish (Table (Table2 2 )
Overview of the effects of probiotics against pathogenic bacteria in shellfish.
Ajitha et al. ( 2004 ) supplemented Indian white shrimp ( Penaeus indicus ) diet with as single dose (5 × 10 6 CFU g −1 ) of different probiotics including Lb. acidophilus, S. cremoris, Lb. bulgaricus 56 or L. bulgaricus 57 at doses of for 4 weeks and at the end of feeding trial shrimp exposed to experimental Vibrio alginolyticus infection. The results revealed substantially higher resistance (56–72%) compared control group (20%) (Ajitha et al., 2004 ). Also, dietary supplemented with 10 10 CFU kg −1 of Lb. plantarum upregulated proPO and PE genes, enhanced PO and SOD activities as well as resistance against V. alginolyticus in white shrimp (Chiu et al., 2007 ). Similarly, Vieira et al. ( 2010 ) reported that diet supplemented with probiotic Lb. plantarum modulated intestinal microbiota as well as resistance against V. harveyi . In addition, a Lactobacillus sp. has been reported to improve survival by 72% and performance of pearl oyster, P. mazatlanica (Aguilar-Macias et al., 2010 ). Furthermore, in a study with juvenile tiger shrimp ( Penaeus monodon ) Lb. acidophilus 04 (10 5 CFU g −1 ) was administered for 1 month and increased resistance (80% survival) was observed following exposure with pathogenic V. alginolyticus (Sivakumar et al., 2012 ). Interestingly, Dash et al. ( 2015 ) administered heat-killed form of Lb. plantarum at rate of 10 8 CFU g −1 in M. rosenbergii diet for 90 days. While no significant effects were observed on growth performance, feeding on probiotic supplemented diet noticeably enhanced immune responses and disease resistance.
Swain et al. ( 2009 ) reported that feeding with E. faecium MC13 and Lactococcus garvieae B49 protected post larval shrimp, P. monodon , against challenge with V. harveyi and V. parahaemolyticus . Similarly, feeding blue shrimp ( Litopenaeus stylirostris ) with probiotic P. acidilactici enhanced protection against V. nigripulchritudo SFn1; the mortality in probiotic and control group were 25 and 41.7%, respectively (Castex et al., 2010 ). Dietary administration of Lb. pentosus HC-2 and E. faecium NRW-2 noticeably enhanced resistance against pathogenic V. parahaemolyticus ATCC 17802 in L. vannamei (Sha et al., 2016 ).
To study protective effects of Bacillus subtilis BT23, Vaseeharan and Ramasamy ( 2003 ) treated black tiger shrimp with 10 6 -10 8 CFU ml −1 probiotic for 6 days and then challenged with V. harveyi . The results revealed significantly lower mortality in treated groups (Vaseeharan and Ramasamy, 2003 ). Similarly, Balcázar et al. ( 2007 ) fed L. vannamei juvenile with B. subtilis for 28 days and then exposed to pathogenic V. harveyi for 24 h. The results revealed substantially lower mortality in treated group (18.25%) compared to those in control (51.75%) in the control group (Balcázar et al., 2007 ). Also, Zokaeifar et al. ( 2012 ) tested combined administration of two probiotic strains ( B. subtilis L10 and G1) in juvenile white shrimp. Shrimps were fed with two levels of 10 5 and 10 8 CFU g −1 of selected probiotics for 8 weeks. At the end of feeding trial elevated growth performance, digestive enzyme activity, upregulated immune related genes as well as resistance against V. harveyi were observed (Zokaeifar et al., 2012 ). Liu et al. ( 2014 ) reported that dietary administration of B. subtilis strain S12 (isolated from L. vannamei digestive tract), beside in vitro antagonistic activity against aquatic animal pathogens, improved resistance against V. harveyi infection (Liu et al., 2014 ).
Rengpipat et al. ( 1998 ) reported that supplementation of black tiger shrimp with different forms (i.e., of fresh cells, fresh cells in normal saline solution and a lyophilized form) of Bacillus S11 for 100 days resulted in significantly higher growth performance and survival. Also, the authorsperformed experimental challenge V. harveyi at the end of feeding trial and surprisingly observed no mortality in probiotic fed shrimps, while survival rate was just 26% in control group (Rengpipat et al., 1998 ). Subsequently, the same research group studied possible effects of Bacillu s S11 and concluded limited improvement in resistance against V. harveyi (Rengpipat et al., 2003 ). In another routes of administration, Luis-Villaseñor et al. ( 2011 ) isolated four Bacillus strains from white shrimp digestive tract and added to white shrimp culture water at rate of 1 × 10 5 CFU mL −1 daily. Thereafter, the authors observed elevated overall survival of L. vannamei larvae (Luis-Villaseñor et al., 2011 ). In another study with post larvae, Ravi et al. ( 2007 ) claimed elevated resistance against V. harveyi following treatment of post larvae with Paenibacillus sp. {"type":"entrez-nucleotide","attrs":{"text":"EF012164","term_id":"116490062","term_text":"EF012164"}} EF012164 and Bacillus cereus {"type":"entrez-nucleotide","attrs":{"text":"DQ915582","term_id":"114154749","term_text":"DQ915582"}} DQ915582 (Ravi et al., 2007 ). The same results were also reported in case of Bacillus sp. P11 which resulted in substantially higher survival in comparison with control group (0%) following experimental challenge with V. harveyi (Utiswannakul et al., 2011 ). The literature review denote that, perhaps, the most studied and effective probiont in shrimp culture is B. subtilis . This species showed positive effects on shrimp resistance to various pathogens. Hence, can be considered as a means of disease control and control in shrimp aquaculture.
Swain et al. ( 2009 ) demonstrated that feeding P. monodon post larvae with Streptococcus phocae P180 significantly improved growth performance as well as protection against V. harveyi . However, the probiotic failed to protect the animals against V. parahaemolyticus (Swain et al., 2009 ). The probiotic Arthrobacter XE-7 was administered orally at four different doses of 0, 10 6 , 10 8 , and 10 10 CFU g −1 feed for 63 days in Pacific white shrimp, L. vannamei . Li et al. ( 2008 ) supplemented shrimp diet with Arthrobacter XE-7 and observed beneficial effects on intestinal microbiota, immune response as well as resistance against V. parahaemolyticus (Li et al., 2008 ).
Thompson et al. ( 2010 ) demonstrate in vitro growth inhibition of shrimp pathogens by probiotic V. gazogenes NCIMB 2250. Also, the same author revealed that feeding white shrimp with dietary V. gazogenes NCIMB 2250 elevated performance and health status as well as decreased of Vibrio sp. count in intestinal microbiota (Thompson et al., 2010 ). Likewise, Vibrio NE17 isolated from the egg samples improved performance as well as immune parameters of freshwater prawn, Macrobrachium rosenbergii (Mujeeb Rahiman et al., 2010 ). Also, a abalone, H. rufescens revealed that combined administration of three probiotics ( Vib rio sp. C21-UMA, Agarivorans albus F1-UMA and Vibrio sp. F15-UMA) using macroalgae M. integrifolia as vector increased significantly the survival of, in a period of 210 days (Silva-Aciares et al., 2011 ).
Streptomyces
In 2016, Tan et al. ( 2016 ) have reviewed the use of the genus Streptomyces bacteria as a probiotic in controlling diseases and improving the health and quality of aquaculture production. Das et al. ( 2010 ) used Marine Strepto myces strains (CLS-28, CLS-39) in Artemia culture and concluded that this probiotic significantly increased resistance of Artemia nauplii and adult against V. harveyi and V. proteolyticus (Das et al., 2010 ). Thereafter, they supplemented black tiger shrimp post larvae diet with 1% Strepto myces for 15 days. The results revealed improved resistance against V. harveyi and growth performance in probiotic fed shrimps (Das et al., 2010 ).
Van Hai et al. ( 2009 ) supplemented western king prawns (Penaeus latisulcatus) diet with a single dose (20 × 10 5 CFU kg −1 ) of P. aeruginosa and P. synxantha for 84 days and reported higher survival rate in P.aeruginosa fed group. Also, combined administration of those probiotic was more effective than singular. Pai et al. ( 2010 ) reported in vitro inhibition of V. harveyi MCCB 111 growth by Pseudomonas MCCB 102 and MCCB 103. Also, in vivo study revealed noticeable increase of tiger shrimp larvae survival against V. harveyi MCCB 111.
Alteromonas
Alteromonas macleodii 0444 has been reported to control of Vibrio splendidus infection in Greenshell mussel, Perna canaliculus , which per se caused increase in survival rate and natural Vibrios in the culture environment (Kesarcodi-Watson et al., 2010 ). Also, the same research group showed that A. macleodii 0444 protected scallop ( Pecten maximus ) and flat oyster ( Ostrea edulis ) larvae against V. coralliilyticus and V. splendidus, V. pectenicida infections (Kesarcodi-Watson et al., 2012 ).
Neptunomonas
Kesarcodi-Watson et al. ( 2010 ) demonstrated that Neptunomonas 0536 was capable of controlling infection caused by V. splendidus in Greenshell mussel ( P. canaliculus ). Also, the same research group highlighted the potential of this probiotic to protect scallop and flat oyster from larvae against V. coralliilyticus, V. splendidus and V. pectenicida (Kesarcodi-Watson et al., 2010 , 2012 ).
Phaeobacter
Phaeobacter gallaeciensis protected scallop larvae against V. coralliilyticus and V. splendidus . Also, the same probiotic strain protected flat oyster larvae against V. coralliilyticus and V. pectenicida , and Pacific oyster larvae against V. coralliilyticus but not V. pectenicida (Kesarcodi-Watson et al., 2012 ).
Pseudoalteromonas
Kesarcodi-Watson et al. ( 2012 ) reported that Pseudoalteromonas D41 as probiotic increased resistance of scallop larvae and Pacific oysters against V. splendidus and V. coralliilyticus , respectively.
To the best of our knowledge there is limited information regarding application of yeasts as probiotic in shellfish aquaculture. In an early study Scholz et al. ( 1999 ) supplemented white shrimp with 1% Phaffia rhodozyma and S. cerevisiae and reported elevation of protection against vibriosis. Furthermore, feeding pearl oyster, P. mazatlanica with marine yeast ( Yarrowia lipolytica ) enriched microalgae resulted in enhanced growth and survival (Aguilar-Macias et al., 2010 ).
Probiotics and viral diseases in fish
The occurrence of viral diseases causes mass mortality in aquaculture practice and considering still there is limited effective vaccine this could a bottleneck for aquaculture industry which resulted in substantial economic loss. In this regard, the potential of probiotics to be used as a means of controlling viral disease has been shown in few studies. For instance, Balcázar et al. ( 2007 ) in an in vitro study demonstrated antiviral activity of probiotic strains (including Vibrios spp., Pseudomonas spp., Aeromonas spp.) against infectious hematopoietic necrosis virus ( IHNV ). Likewise, Maeda et al. ( 1997 ) reported that Pseudoalteromonas undina , VKM-124 improved larval survival by giving the larvae a protection against Sima-aji Neuro Necrosis Virus ( SJNNV ) when added to Yellow Jack ( Carangoides bartholomaei ) larval tanks. Harikrishnan et al. ( 2010 ) studied antiviral activity of dietary two commercial probiotics (Lactobacil and/or Sporolac) in Olive flounder. The results revealed that both probiotics increased fish resistance against lymphocystis disease virus (LCDV) infection (Harikrishnan et al., 2010 ). The possible control of iridovirus in grouper ( Epinephelus coioides ) through dietary administration of probiotics ( Lb. plantarum ) was studied by Son et al. ( 2009 ). The results revealed higher survival in probiotic fed fish compared control group. In another study Liu et al. ( 2012 ) tested possible protection of grouper against iridovirus using dietary B. subtilis E20 and observed 50% higher survival than those in non-probiotic group. Likewise, dietary S. cerevisiae at rate of 5.3 × 10 7 CFU kg −1 protected grouper against iridovirus (GIV) infection (Chiu et al., 2010 ). Indeed, while fish fed control diet had 16.7% survival, probiotic fed fish survival was 43.3%. Although there are extensive literature regarding immunomodulatory effects of probiotics, they are not enough to speculate potential anti-viral effects of probiotics. Therefore, more studies should be conducted to illustrate the effect of probiotics on the viral diseases of fish and possible mechanisms.
Probiotics and viral diseases in shellfish
Unlike fish, shrimp aquaculture suffers from substantial economical loses due to occurrence and spread of different viral diseases like white spot syndrome virus (WSSV), lymphocystis disease virus (LCDV), infectious hypodermal and hematopoietic necrosis virus (IHHNV) etc. Treatment of shrimp culture environment or feed with probiotics has been suggested as efficient means of prevention and controlling viral diseases (Lakshmi et al., 2013 ). For instance, Vibrio spp. obtained from tiger shrimp hatchery showed strong antagonistic activity against IHNV and Oncorhynchus masou virus (OMV) (Direkbusarakom et al., 1998 ). The majority of studies practiced dietary administration of probiotics and tested anti-viral effect in different shrimp species. Rodríguez et al. ( 2007 ) stated that treatment of L. vannamei with 10 5 CFU mL −1 probiotic V. alginolyticus significantly increased resistance against WSSV compared to non-treated shrimps. Moreover, dietary administration of 10 10 CFU g −1 B. megaterium has resulted in higher survival and increased protection against WSSV (Li et al., 2009 ). Also, Leyva-Madrigal et al. ( 2011 ) reported that feeding white shrimp with either P. pentosaceus or Staphylococcus hemolyticus decrease WSSV infection. On the contrary, dietary supplemented with 10 5 CFU g −1 of a mixture lactic acid bacteria (BAL3, BAL7, BC1, and CIB1) had no significant effects on L. vannamei resistance against WSSV infections (Partida-Arangure et al., 2013 ). Recently, Chai et al. ( 2016 ) isolated Bacillus PC465 from Fenneropenaeus chinensis gut and evaluated its anti-viral effects via dietary administration. The results showed the application of Bacillus PC465 enhances the gut microbial structures, promotes the immune status of shrimp which per se protected against WSSV. Despite the needs for additional research to explain mechanisms, some researchers proposed immunomodulatory nature of probiotics as an important factor in observed protection against WSSV (Merrifield et al., 2010b ).
Probiotics and parasitic diseases in fish and shellfish
In general, available information about the probiotic control parasite diseases in fish and shellfish was limited. Dietary administration of Aeromonas sobria GC2 BA211 for 14 days at rate of 10 8 and 10 10 cells g −1 , respectively, protected rainbow trout against Ichthyophthirius multifiliis parasite and reduced the mortalities from 98 to 0%. On the other hand, Brochothrix thermosphacta at dose of 10 10 cells g −1 of feed failed to protect rainbow trout against the skin parasite (Pieters et al., 2008 ). Atira et al. ( 2012 ) assessed the inhibition of the growth of the parasitic Saprolegnia parasitica A3 on catfish ( Pangasius hypophthalamus ) using Lactobacillus plantarum FNCC 226 under in vivo and in vitro conditions. They concluded the potential of L. plantarum for inhibiting S. parasitica and therefore suggested as an environment-friendly means of parasite control in catfish aquaculture.
Concluding remarks and further perspectives
The review of available literature revealed the promising effects of probiotics on disease resistance of fish and shellfish. Therefore, it can be speculated that this environment friendly dietary supplement will receive increasing attention as an alternative for antibiotic in aquaculture. However, this fact should be kept mind that the results of previous researches revealed that the effects of probiotics are species specific. Therefore, optimum probiont, administration dose and dulactobacilli were among the most studied probiotics in shrimps. The studies reviewed here revealed the potential of lactobacilli to help in resolving the issue of diseases in shrimp culture. Given the primary nature of shrimp immune system as well as sensitivity to disease outbreak, development of such effective, environment-friendly means of disease bio-control is of high importance. The results of the mentioned above studies encourage further studies regarding bio-control of parasite in aquaculture using probiotics. However, the exact mode of actions remained to be clarified. Furthermore, despite promising effects obtained regarding probiotics as bio-control against viral and parasitic disease in aquatic animals, there is very limited research available compared with other immunostimulants. Consequently, extensive research should be performed regarding determination of antiviral nature of known probiotics. The last but not the least, present understanding on modes of action of probiotics effects on immune system is very limited and merit further research, especially the molecular mechanisms of the interactions between the probiotic and host.
Author contributions
SH and ZZ drafted the manuscript. Y-ZS performed the literature collection. AW participated in this review. All authors performed the critical revision of the article and approved the final version for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Abd El-Rhman A. M., Khattab Y. A. E., Shalaby A. M. E. (2009). Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immun. 27 , 175–180. 10.1016/j.fsi.2009.03.020 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Abdel-Tawwab M., Abdel-Rahman A. M., Ismael N. E. M. (2008). Evaluation of commercial live bakers' yeast, Saccharomyces cerevisiae as a growth and immunity promoter for fry nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila . Aquaculture 280 , 185–189. 10.1016/j.aquaculture.2008.03.055 [ CrossRef ] [ Google Scholar ]
- Abdel-Tawwab M., Mousa M. A. A., Mohammed M. A. (2010). Use of live baker's yeast, Saccharomyces cerevisiae , in practical diet to enhance the growth performance of Galilee tilapia, Sarotherodon galilaeus (L.), and its resistance to environmental copper toxicity . J. World Aquacult. Soc. 41 , 214–223. 10.1111/j.1749-7345.2010.00361.x [ CrossRef ] [ Google Scholar ]
- Aguilar-Macias O. L., Ojeda-Ramirez J. J., Campa-Cordova A. I., Saucedo P. E. (2010). Evaluation of natural and commercial probiotics for improving growth and survival of the pearl oyster, Pinctada mazatlanica , during late hatchery and early field culturing . J. World Aquacult. Soc. 41 , 447–454. 10.1111/j.1749-7345.2010.00386.x [ CrossRef ] [ Google Scholar ]
- Ajitha S., Sridhar M., Sridhar N., Singh I. S. B., Varghese V. (2004). Probiotic effects of lactic acid bacteria against Vibrio alginolyticus in Penaeus ( Fenneropenaeus indicus ) . Asian Fish. Sci. 17 , 71–80. [ Google Scholar ]
- Al-Dohail M. A., Hashim R., Aliyu-Paiko M. (2011). Evaluating the use of Lactobacillus acidophilus as a biocontrol agent against common pathogenic bacteria and the effects on the haematology parameters and histopathology in African catfish Clarias gariepinus juveniles . Aquac. Res. 42 , 196–209. 10.1111/j.1365-2109.2010.02606.x [ CrossRef ] [ Google Scholar ]
- Aly S. M., Ahmed Y. A. G., Ghareeb A. A. -A., Mohamed M. F. (2008a). Studies on Bacillus subtilis and Lactobacillus acidophilus , as potential probiotics, on the immune response and resistance of Tilapia nilotica ( Oreochromis niloticus ) to challenge infections . Fish Shellfish Immun. 25 , 128–136. 10.1016/j.fsi.2008.03.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aly S. M., Mohamed M. F., John G. (2008b). Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica ( Oreochromis niloticus ) . Aquac. Res. 39 , 647–656. 10.1111/j.1365-2109.2008.01932.x [ CrossRef ] [ Google Scholar ]
- Antony S. P., Singh I. B., Jose R. M., Kumar P. A., Philip R. (2011). Antimicrobial peptide gene expression in tiger shrimp, Penaeus monodon in response to gram-positive bacterial probionts and white spot virus challenge . Aquaculture 316 , 6–12. 10.1016/j.aquaculture.2011.03.025 [ CrossRef ] [ Google Scholar ]
- Arellano-Carbajal F., Olmos-Soto J. (2002). Thermostable α-1, 4-and α-1, 6-glucosidase enzymes from Bacillus sp. isolated from a marine environment . World. J. Microb. Biot. 18 , 791–795. 10.1023/A:1020433210432 [ CrossRef ] [ Google Scholar ]
- Atira N. J., Aryantha I. N. P., Kadek I. D. G. (2012). The curative action of Lactobacillus plantarum FNCC 226 to Saprolegnia parasitica A3 on catfish ( Pangasius hypophthalamus, Sauvage ) . Int. Food Res. J. 19 , 1723–1727. [ Google Scholar ]
- Aubin J., Gatesoupe F. J., Labbé L., Lebrun L. (2005). Trial of probiotics to prevent the vertebral column compression syndrome in rainbow trout (Oncorhynchus mykiss Walbaum) . Aquac. Res. 36 , 758–767. 10.1111/j.1365-2109.2005.01280.x [ CrossRef ] [ Google Scholar ]
- Austin B., Stuckey L. F., Robertson P. A. W., Effendi I., Griffith D. R. W. (1995). A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonas salmonicida, Vibrio anguillarum and Vibrio ordalii . J. Fish. Dis. 18 , 93–96. 10.1111/j.1365-2761.1995.tb01271.x [ CrossRef ] [ Google Scholar ]
- Azimirad M., Meshkini S., Ahmadifard N., Hoseinifar S. H. (2016). The effects of feeding with synbiotic ( Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish ( Pterophyllum scalare ) . Fish Shellfish Immun . 54 , 516–522. 10.1016/j.fsi.2016.05.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Balcázar J. L., De Blas I., Ruiz-Zarzuela I., Cunningham D., Vendrell D., Múzquiz J. L. (2006). The role of probiotics in aquaculture . Vet. Microbiol. 114 , 173–186. 10.1016/j.vetmic.2006.01.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Balcázar J. L., De Blas I., Ruiz-Zarzuela I., Vendrell D., Gironés O., Muzquiz J. L. (2007). Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout ( Oncorhynchus mykiss ) . FEMS Immunol. Med. Mic. 51 , 185–193. 10.1111/j.1574-695X.2007.00294.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Balcázar J. L., Vendrell D., De Blas I., Ruiz-Zarzuela I., Múzquiz J. L. (2009). Effect of Lactococcus lactis CLFP 100 and Leuconostoc mesenteroides CLFP 196 on Aeromonas salmonicida infection in brown trout ( Salmo trutta ) . J. Mol. Microb. Biotech. 17 , 153–157. 10.1159/000226588 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bandyopadhyay P., Das Mohapatra P. K. (2009). Effect of a probiotic bacterium Bacillus circulans PB7 in the formulated diets: on growth, nutritional quality and immunity of Catla catla (Ham.) . Fish Physiol. Biochem. 35 , 467–478. 10.1007/s10695-008-9272-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beck B. R., Kim D., Jeon J., Lee S. M., Kim H. K., Kim O. J., et al.. (2015). The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder ( Paralichthys olivaceus ) . Fish Shellfish Immun. 42 , 177–183. 10.1016/j.fsi.2014.10.035 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bron P. A., Tomita S., van Swam I. I., Remus D. M., Meijerink M., Wels M., et al.. (2012). Lactobacillus plantarum possesses the capability for wall teichoic acid backbone alditol switching . Microb. Cell. Fact. 11 :123. 10.1186/1475-2859-11-123 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brunt J., Austin B. (2005). Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum) . J. Fish. Dis. 28 , 693–701. 10.1111/j.1365-2761.2005.00672.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burbank D. R., Shah D. H., LaPatra S. E., Fornshell G., Cain K. D. (2011). Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout ( Oncorhynchus mykiss ) . Aquaculture 321 , 185–190. 10.1016/j.aquaculture.2011.09.004 [ CrossRef ] [ Google Scholar ]
- Capkin E., Altinok I. (2006). Effects of dietary probiotic suplementations on prevention/treatment of Yersiniosis disease . J. Appl. Microbiol. 106 , 1147–1153. 10.1111/j.1365-2672.2008.04080.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Castex M., Lemaire P., Wabete N., Chim L. (2010). Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative strees of Litopenaeus stylostris under Vibrio nigripulchritudo challenge . Fish Shellfish Immun. 28 , 622–631. 10.1016/j.fsi.2009.12.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cha J. H., Rahimnejad S., Yang S. Y., Kim K. W., Lee K. J. (2013). Evalutations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder ( Paralichthys olivaceus ) against Streptococcus iniae and as water additives . Aquaculture 402 , 50–57. 10.1016/j.aquaculture.2013.03.030 [ CrossRef ] [ Google Scholar ]
- Chabrillón M., Arijo S., Díaz-Rosales P., Balebona M. C., Moriñigo M. A. (2006). Interference of Listonella anguillarum with potential probiotic microorganisms isolated from farmed gilthead seabream ( Sparus aurata L.) . Aquac. Res. 37 , 78–86. 10.1111/j.1365-2109.2005.01400.x [ CrossRef ] [ Google Scholar ]
- Chabrillon M., Ouwehand A. C., Diaz-Rosales P., Arijo S., Martinez-Manzanares E., Balebona M. C., et al. (2006). Adhesion of lactic acid bacteria to mucus of farmed gilthead seabream, and interactions with fish pathogenic microorganisms . B. Eur Assoc. Fish. Pat. 26 , 202–210. [ Google Scholar ]
- Chabrillón M., Rico R., Arijo S., Díaz-Rosales P., Balebona M., Moriñigo M. (2005). Interactions of microorganisms isolated from gilthead sea bream, Sparus aurata L., on Vibrio harveyi , a pathogen of farmed Senegalese sole, Solea senegalensis (Kaup) . J. Fish. Dis. 28 , 531–537. 10.1111/j.1365-2761.2005.00657.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chai P. C., Song X. L., Chen G. F., Xu H., Huang J. (2016). Dietary supplementation of probiotic Bacillus PC465 isolated from the gut of Fenneropenaeus chinensis improves the health status and resistance of Litopenaeus vannamei against white spot syndrome virus . Fish Shellfish Immun. 54 , 602–611. 10.1016/j.fsi.2016.05.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chang C. I., Liu W. Y. (2002). An evaluation of two probiotic bacterial strains, Enterococcus faecium SF68 and Bacillus toyoi , for reducing edwardsiellosis in cultured European eel ( Anguilla anguilla ) . J. Fish. Dis. 25 , 311–315. 10.1046/j.1365-2761.2002.00365.x [ CrossRef ] [ Google Scholar ]
- Cheng G., Hao H., Xie S., Wang X., Dai M., Huang L., et al.. (2014). Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol . 5 :217. 10.3389/fmicb.2014.00217 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chi C., Jiang B., Yu X. B., Liu T. Q., Xia L., Wang G. X. (2014). Effects of three strains of intestinal autochthonous bacteria and their extracellular products on the immune response and disease resistance of common carp, Cyprinus carpio . Fish Shellfish Immun. 36 , 9–18. 10.1016/j.fsi.2013.10.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chiu C. H., Cheng C. H., Gua W. R., Guu Y. K., Cheng W. (2010). Dietary administration of the probiotic Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and diseases resistance of the grouper ( Epinephelus coioides ) . Fish Shellfish Immun. 29 , 1053–1059. 10.1016/j.fsi.2010.08.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chiu C. H., Guu Y. K., Liu C. H., Pan T. M., Cheng W. (2007). Immune responses and gene expression in white shrimp, Litopenaeus vannamei , induced by Lactobacillus plantarum . Fish Shellfish Immun. 23 , 364–377. 10.1016/j.fsi.2006.11.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- D'Alvise D. W., Lillebø S., Prol-Garcia M. J., Wergeland H. I., Nielsen K. F., Bergh Ø., et al.. (2012). Phaeobacter gallaeciensis reduces Vibrio anguillarum in cultures of microalgae and rotifers, and prevents vibriosis in cod larvae . PLoS ONE 7 :e43996. 10.1371/journal.pone.0043996 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Das S., Ward L. R., Burke C. (2010). Screening of marine Streptomyces spp. for potential use as probiotics in aquaculture . Aquaculture 305 , 32–41. 10.1016/j.aquaculture.2010.04.001 [ CrossRef ] [ Google Scholar ]
- Dash G., Raman R. P., Prasad K. P., Makesh M., Pradeep M. A., Sen S. (2015). Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879) . Fish Shellfish Immun. 43 , 167–174. 10.1016/j.fsi.2014.12.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dawood M. A. O., Koshio S. (2016). Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review . Aquaculture 454 , 243–251. 10.1016/j.aquaculture.2015.12.033 [ CrossRef ] [ Google Scholar ]
- De la Banda I. G., Lobo C., Chabrillon M., Leon-Rubio J. M., Arijo S., Pazos G., et al. (2012). Influence of dietary administration of a probiotic strain Shewanella putrefaciens on Senegalese sole ( Solea senegalensis , Kaup 1858) growth, body composition and resistance to Photobacterium damselae subsp piscicida . Aquac. Res. 43 , 662–669. 10.1111/j.1365-2109.2011.02871.x [ CrossRef ] [ Google Scholar ]
- Decamp O., Moriarty D. J., Lavens P. (2008). Probiotics for shrimp larviculture: review of field data from Asia and Latin America . Aquac. Res. 39 , 334–338. 10.1111/j.1365-2109.2007.01664.x [ CrossRef ] [ Google Scholar ]
- Diaz-Rosales P., Arijo S., Chabrillon M., Alarcón F. J., Tapia-Paniagua S. T., Martínez-Manzanares E., et al. (2009). Effects of two closely related probiotics on respiratory burst activity of Senegalese sole ( Solea senegalensis , Kaup) phagocytes, and protection against Photobacterium damselae subsp. piscicida . Aquaculture 293 , 16–21. 10.1016/j.aquaculture.2009.03.050 [ CrossRef ] [ Google Scholar ]
- Direkbusarakom S., Yoshimizu M., Ezura Y., Ruangpan L., Danayadol Y. (1998). Vibrio spp. the dominant flora in shrimp hatchery against some fish pathogenic viruses . J. Mar. Biotechnol. 6 , 266–267. [ PubMed ] [ Google Scholar ]
- El-Haroun E., Goda A. S., Chowdhury K. (2006). Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.) . Aquac. Res. 37 , 1473–1480. 10.1111/j.1365-2109.2006.01584.x [ CrossRef ] [ Google Scholar ]
- Eshaghzadeh H., Hoseinifar S. H., Vahabzadeh H., Ringø E. (2015). The effects ofdietary inulin on growth performances, survival and digestive enzyme activities of common carp ( Cyprinus carpio ) fry . Aquacult Nutr. 21 , 242–247. 10.1111/anu.12155 [ CrossRef ] [ Google Scholar ]
- Faramarzi M., Kiaalvandi S., Iranshahi F. (2011). The effect of probiotics on growth performance and body composition of common carp ( Cyprinus carpio ) . J. Anim. Vet. Adv. 10 , 2408–2413. 10.3923/javaa.2011.2408.2413 [ CrossRef ] [ Google Scholar ]
- Ferreira G. S., Bolívar N. C., Pereira S. A., Guertler C., do Nascimento Vieira F., Mouriño J. L. P., et al. (2015). Microbial biofloc as source of probiotic bacteria for the culture of Litopenaeus vannamei . Aquaculture 448 , 273–279. 10.1016/j.aquaculture.2015.06.006 [ CrossRef ] [ Google Scholar ]
- Gildberg A., Mikkelsen H., Sandaker E., Ringø E. (1997). Probiotic effect of lactic acid bacteria in the feed on growth and survival of fry of Atlantic cod ( Gadus morhua ) . Hydrobiologia 352 , 279–285. 10.1023/A:1003052111938 [ CrossRef ] [ Google Scholar ]
- Giri S. S., Sen S. S., Sukumaran V. (2012). Effects of dietary supplementation of potential probiotic Pseudomonas aeruginosa VSG-2 on the innate immunity and disease resistance of tropical freshwater fish, Labeo rohita . Fish Shellfish Immun. 32 , 1135–1140. 10.1016/j.fsi.2012.03.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gobeli S., Goldschmidt-Clermont E., Frey J., Burr S. E. (2009). Pseudomonas chlororaphis strain JF3835 reduces mortality of juvenile perch, Perca fluviatilis L., caused by Aeromonas sobria . J. Fish. Dis. 32 , 597–602. 10.1111/j.1365-2761.2009.01021.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gram L., Lovold T., Nielsen J., Melchiorsen J., Spanggaard B. (2001). In vitro antagonism of the probiont Pseudomonas fluorescens strain AH2 against Aeromonas salmonicida does not confer protection of salmon against furunculosis . Aquaculture 199 , 1–11. 10.1016/S0044-8486(01)00565-8 [ CrossRef ] [ Google Scholar ]
- Gram L., Melchiorsen J., Spanggaard B., Huber I., Nielsen T. F. (1999). Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish . Appl. Environ. Microb. 65 , 969–973. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hai N. V. (2015). Research findings from the use of probiotics in tilapia aquaculture: a review . Fish Shellfish Immun. 45 , 592–597. 10.1016/j.fsi.2015.05.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hai N. V., Buller N., Fotedar R. (2010). Effect of customized probiotics on the physiological and immunological responses of juvenile western king prawns ( Penaeus latisulcatus Kishinouye, 1896) challenged with Vibrio harveyi . J. Appl. Aquac. 22 , 321–336. 10.1080/10454438.2010.527580 [ CrossRef ] [ Google Scholar ]
- Han B., Long W. Q., He J. Y., Liu Y. J., Si Y. Q., Tian L. X. (2015). Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia ( Oreochromis niloticus ) to challenge infections . Fish Shellfish Immun. 46 , 225–231. 10.1016/j.fsi.2015.06.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harikrishnan R., Balasundaram C., Heo M. S. (2010). Effect of probiotics enriched diet on paralichthys olivaceus infected with lymphocystis disease virus (LCDV) . Fish Shellfish Immun. 29 , 868–874. 10.1016/j.fsi.2010.07.031 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harikrishnan R., Kim M. C., Kim J. S., Balasundaran C., Heo M. S. (2011). Protective effect of herbal and probiotics enriched diet on haematological and immunity status of Oplegnathus fasciatus (Temminck and Schlegel) against Edwarsiella tarda . Fish. Shellfish Immun. 30 , 886–893. 10.1016/j.fsi.2011.01.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heo W. S., Kim Y. R., Kim E. Y., Bai S. C., Kong I. S. (2013). Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder ( Paralichthys olivaceus ) . Aquaculture 376 , 20–24. 10.1016/j.aquaculture.2012.11.009 [ CrossRef ] [ Google Scholar ]
- Hjelm M., Bergh O., Riaza A., Nielsen J., Melchiorsen J., Jensen S., et al.. (2004). Selection and identification of autochthonous potential probiotic bacteria from turbot larvae ( Scophthalmus maximus ) rearing units . Syst. Appl. Microbiol. 27 , 360–371. 10.1078/0723-2020-00256 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoseinifar S. H., Dadar M., Ringø E. (2017a). Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: the functional feed additives scenario . Aquac. Res. 48 , 3987–4000. 10.1111/are.13368 [ CrossRef ] [ Google Scholar ]
- Hoseinifar S. H., Eshaghzadeh H., Vahabzadeh H., Peykaran Mana N. (2015a). Modulation of growth performances, survival, digestive enzyme activities and intestinal microbiota in common carp ( Cyprinus carpio ) larvae using short chain fructooligosaccharide . Aquac. Res. 47 , 3246–3253. 10.1111/are.12777 [ CrossRef ] [ Google Scholar ]
- Hoseinifar S. H., Mirvaghefi A., Amoozegar M. A., Sharifian M., Esteban M. Á. (2015b). Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout ( Oncorhynchus mykiss ) upon synbiotic feeding . Fish Shellfish Immun. 45 , 27–32. 10.1016/j.fsi.2015.03.029 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoseinifar S. H., Ringø E., Shenavar Masouleh A., Esteban M. Á. (2016). Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: a review . Rev. Aquacult. 8 , 89–102. 10.1111/raq.12082 [ CrossRef ] [ Google Scholar ]
- Hoseinifar S. H., Sun Y., Caipang C. M. (2017b). Short chain fatty acids as feed supplements for sustainable aquaculture: an updated view . Aquac. Res. 48 , 1380–1391. 10.1111/are.13239 [ CrossRef ] [ Google Scholar ]
- Hotel A., Córdoba A. (2001). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria . Prevention 5 , 1–10. [ Google Scholar ]
- Huang J., Wu Y., Chi S. (2014). Dietary supplementation of Pediococcus pentosaceus enhances innate immunity, physiological health and resistance to Vibrio anguillarum in orange-spotted grouper ( Epinephelus coioides) . Fish Shellfish Immun. 39 , 196–205. 10.1016/j.fsi.2014.05.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Irianto A., Austin B. (2002). Probiotics in aquaculture . Fish Shellfish Immun. 25 , 633–642. [ Google Scholar ]
- Irianto A., Robertson P. A. W., Austin B. (2003). Oral administration of formalin-inactivated cells of Aeromonas hydrophila A3-51 controls infection by a typical A. salmonicida in goldfish, ( Carassius auratus ) . Fish Shellfish Immun. 26 , 117–120. 10.1046/j.1365-2761.2003.00439.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jöborn A., Olsson J. C., Westerdahl A., Conway P. L., Kjelleberg S. (1997). Colonization in the fish intestinal tract and production of inhibitory substances in intestinal mucus and faecal extracts by Carnobacterium sp. strain K1 . Fish Shellfish Immun. 20 , 383–392. [ Google Scholar ]
- Kesarcodi-Watson A., Kaspar H., Lategan M. J., Gibson L. (2010). Alteromonas macleodii 0444 and Neptunomonas sp. 0536, two novel probiotics for hatchery-reared Greenshell (TM) mussel larvae, Perna canaliculus . Aquaculture 309 , 49–55. 10.1016/j.aquaculture.2010.09.019 [ CrossRef ] [ Google Scholar ]
- Kesarcodi-Watson A., Miner P., Nicolas J. L., Robert R. (2012). Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: pacific oyster ( Crassostrea gigas ), flat oyster ( Ostrea edulis ) and scallop ( Pecten maximus ) . Aquaculture 344 , 29–34. 10.1016/j.aquaculture.2012.02.029 [ CrossRef ] [ Google Scholar ]
- Kim D., Beck B. R., Heo S. -B., Kim J., Kim H. B., Lee S. -M., et al.. (2013). Lactococcus lactis BFE920 activates the innate immune system of olive flounder (Paralichthys olivaceus) , resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large scale field-studies . Fish Shellfish Immun. 35 , 1585–1590. 10.1016/j.fsi.2013.09.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim D. -H., Austin B. (2006a). Innate immune responses in rainbow trout ( Oncorhynchus mykiss , Walbaum) induced by probiotics . Fish Shellfish Immun. 21 , 513–524. 10.1016/j.fsi.2006.02.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim D. -H., Austin B. (2006b). Cytokine expression in leucocytes and gut cells of rainbow trout, Oncorhynchus mykiss Walbaum, induced by probiotics . Vet. Immunol. Immun. 114 , 297–304. 10.1016/j.vetimm.2006.08.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim J. S., Harikrishnan R., Kim M. C., Balasundaram C., Heo M. S. (2010). Dietary administration of Zooshikella sp. to enhance the innate immune response and disease resistance of Paralichthys olivaceus against Streptococcus iniae . Fish Shellfish Immun. 29 , 104–110. 10.1016/j.fsi.2010.02.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Korkea-aho T. L., Heikkinen J., Thompson K. D., von Wright A., Austin B. (2011). Pseudomonas sp. M174 inhibits the fish pathogen Flavobacterium psychrophilum . J. Appl. Microbiol. 111 , 266–277. 10.1111/j.1365-2672.2011.05044.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Korkea-aho T. L., Papadopoulou A., Heikkinen J., von Wright A., Adams A., Austin B., et al.. (2012). Pseudomonas M162 confers protection against rainbow trout fry syndrome by stimulating immunity . J. Appl. Microbiol. 113 , 24–35. 10.1111/j.1365-2672.2012.05325.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar R., Mukherjee S., Ranjan R., Nayak S. (2008). Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis . Fish Shellfish Immun. 24 , 168–172. 10.1016/j.fsi.2007.10.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar R., Mukherjee S. C., Pani Prasad K., Pal A. K. (2006) Evaluation of Bacillus subtilis as a probiotic to Indian major carp, Labeo rohita (Ham) . Aquac. Res. 37 , 1215–1221. 10.1111/j.1365-2109.2006.01551.x [ CrossRef ] [ Google Scholar ]
- Lakshmi B., Viswanath B., SaiGopal D. V. R. (2013). Probiotics as antiviral agents in shrimp aquaculture . J. Pathogens 2013 :424123. 10.1155/2013/424123 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lazado C. C., Caipang C. M. A., Brinchmann M. F., Kiron V. (2011). In vitro adherence of two candidate probiotics from Atlantic cod and their interference with the adhesion of two pathogenic bacteria . Vet. Microbiol. 148 , 252–259. 10.1016/j.vetmic.2010.08.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee J. S., Cheng H., Damte D., Lee S. J., Kim J. C., Rhee M. H., et al.. (2013). Effects of dietary supplementation of Lactobacillus pentosus PL11 on the growth performance, immune and antioxidant systems of Japanese eel Anguilla japonica challenged with Edwardsiella tarda . Fish Shellfish Immun. 34 , 756–761. 10.1016/j.fsi.2012.11.028 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leonel Ochoa-Solano J., Olmos-Soto J. (2006). The functional property of Bacillus for shrimp feeds . Food Microbiol. 23 , 519–525. 10.1016/j.fm.2005.10.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leyton Y. E., Varas Psijas R. A., Riquelme C. E. (2012). Probiotic activity of bacteria associated with egg capsules of Concholepas concholepas (common name ‘Loco') . Aquacult. Res. 43 , 1089–1095. 10.1111/j.1365-2109.2011.02912.x [ CrossRef ] [ Google Scholar ]
- Leyva-Madrigal K. Y., Luna-González A., Escobedo-Bonilla C. M., Fierro-Coronado J. A., Maldonado-Mendoza I. E. (2011). Screening for potential probiotic bacteria to reduce prevalence of WSSV and IHHNV in whiteleg shrimp ( Litopenaeus vannamei ) under experimental conditions . Aquaculture 322 , 16–22. 10.1016/j.aquaculture.2011.09.033 [ CrossRef ] [ Google Scholar ]
- Li J., Tan B., Mai K. (2009). Dietary probiotic Bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp ( Litopenaeus vannamei ) . Aquaculture 291 , 35–40. 10.1016/j.aquaculture.2009.03.005 [ CrossRef ] [ Google Scholar ]
- Li J., Tan B., Mai K., Ai Q., Zhang W., Liufu Z., et al. (2006). Comparative study between probiotic bacterium Arthrobacter XE-7 and chloramphenicol on protection of Penaeus chinensis post-larvae from pathogenic vibrios . Aquaculture 253 , 140–147. 10.1016/j.aquaculture.2005.07.040 [ CrossRef ] [ Google Scholar ]
- Li J., Tan B., Mai K., Ai Q., Zhang W., Liufu Z., et al. (2008). Immune responses and resistance against Vibrio parahaemolyticus induced by probiotic bacterium Arthrobacter XE-7 in Pacific White Shrimp, Litopenaeus vannamei . J. World. Aquacult. Soc. 39 , 477–489. 10.1111/j.1749-7345.2008.00188.x [ CrossRef ] [ Google Scholar ]
- Liu C. H., Chiu C. H., Wang S. W., Cheng W. (2012). Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides . Fish Shellfish Immun. 33 , 699–706. 10.1016/j.fsi.2012.06.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu H., Li Z., Tan B., Lao Y., Duan Z., Sun W., et al.. (2014). Isolation of a putative probiotic strain S12 and its effect on growth performance, non-specific immunity and disease-resistance of white shrimp, Litopenaeus vannamei . Fish Shellfish Immun. 41 , 300–307. 10.1016/j.fsi.2014.08.028 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu W., Ren P., He S., Xu L., Yang Y., Gu Z., et al.. (2013). Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains . Fish Shellfish Immun. 35 , 54–62. 10.1016/j.fsi.2013.04.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Llewellyn M. S., Boutin S., Hoseinifar S. H., Derome N. (2014). Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries . Front. Microbiol. 5 :207. 10.3389/fmicb.2014.00207 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Løvmo Martinsen L., Salma W., Myklebust R., Mayhew T. M., Ringø E. (2011). Carnobacterium maltaromaticum vs . Vibrio (Listonella) anguillarum in the midgut of Atlantic cod (Gadus morhua L.): an ex vivo study. Aquac. Res . 42 , 1830–1839. 10.1111/j.1365-2109.2010.02784.x [ CrossRef ] [ Google Scholar ]
- Luis-Villaseñor I. E., Macías-Rodríguez M. E., Gómez-Gil B., Ascencio-Valle F., Campa-Córdova Á. I. (2011). Beneficial effects of four Bacillus strains on the larval cultivation of Litopenaeus vannamei . Aquaculture 321 , 136–144. 10.1016/j.aquaculture.2011.08.036 [ CrossRef ] [ Google Scholar ]
- Ma C. -W., Cho Y. -S., Oh K. -H. (2009). Removal of pathogenic bacteria and nitrogens by Lactobacillus spp. JK-8 and JK-11 . Aquaculture 287 , 266–270. 10.1016/j.aquaculture.2008.10.061 [ CrossRef ] [ Google Scholar ]
- Macey B. M., Coyne V. E. (2006). Colonization of the gastrointestinal tract of the farmed South African abalone Haliotis midae by the probionts Vibrio midae SY9, Cryptococcus sp. SS1, and Debaryomyces hansenii AY1 . Mar. Biotechnol. 8 , 246–259. 10.1007/s10126-005-0113-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maeda M., Nogami K., Kanematsu M., Hirayama K. (1997). The concept of biological control methods in aquaculture . Hydrobiologia 358 , 285–290. [ Google Scholar ]
- Mahdhi A., Kamoun F., Messina C., Santulli A., Bakhrouf A. (2012). Probiotic properties of Brevibacillus brevis and its influence on sea bass ( Dicentrarchus labrax ) larval rearing . Afr. J. Microbiol. Res. 6 , 6487–6495. 10.5897/AJMR12.1201 [ CrossRef ] [ Google Scholar ]
- Merrifield D. L., Balcazar J. L., Daniels C. L., Zhou Z., Carnevali O., Sun Y. Z., et al. (2014). Indigenous lactic acid bacteria in fish and crustaceans , in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics [M] , eds Merrifield D. L., Ringø E. (West Sussex: Wiley-Blackwell; ), 128–168. [ Google Scholar ]
- Merrifield D. L., Bradley G., Baker R., Davies S. (2010a). Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment . Aquacult. Nutr . 16 , 496–503. 10.1111/j.1365-2095.2009.00688.x [ CrossRef ] [ Google Scholar ]
- Merrifield D. L., Carnevali O. (2014). Probiotic Modulation of the Gut Microbiota of Fish. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics . Hoboken, NJ: John Wiley and Sons, Ltd. [ Google Scholar ]
- Merrifield D. L., Dimitroglou A., Foey A., Davies S. J., Baker R. T., Bøgwald J., et al. (2010b). The current status and future focus of probiotic and prebiotic applications for salmonids . Aquaculture 302 , 1–18. 10.1016/j.aquaculture.2010.02.007 [ CrossRef ] [ Google Scholar ]
- Mingmongkolchai S., Panbangred W. (2018). Bacillus probiotics: an alternative to antibiotics for livestock production . J. Appl. Microbiol. 124 , 1334−1346. 10.1111/jam.13690 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Modanloo M., Soltanian S., Akhlaghi M., Hoseinifar S. H. (2017). The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp ( Cyprinus carpio ) fingerlings . Fish Shellfish Immun. 70 , 391–397. 10.1016/j.fsi.2017.09.032 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Montes A., Pugh D. (1993). The use of probiotics in food-animal practice . Vet. Med . 88 , 282–288. [ Google Scholar ]
- Moriarty D. (1998). Control of luminous Vibrio species in penaeid aquaculture ponds . Aquaculture 164 , 351–358. 10.1016/S0044-8486(98)00199-9 [ CrossRef ] [ Google Scholar ]
- Mujeeb Rahiman K. M., Jesmi Y., Thomas A. P., Mohamed Hatha A. A. (2010). Probiotic effect of Bacillus NL110 and Vibrio NE17 on the survival, growth performance and immune response of Macrobrachium rosenbergii (de Man) . Aquac. Res. 41 , e120–e134. 10.1111/j.1365-2109.2009.02473.x [ CrossRef ] [ Google Scholar ]
- Nayak S., Swain P., Mukherjee S. (2007). Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.) . Fish Shellfish Immun. 23 , 892–896. 10.1016/j.fsi.2007.02.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Newaj-Fyzul A., Adesiyun A. A., Mutani A., Ramsubhag A., Brunt J., Austin B. (2007). Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout ( Oncorhynchus mykiss , Walbaum) . J. Appl. Microb. 103 , 1699–1706. 10.1111/j.1365-2672.2007.03402.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ng W. K., Kim Y. C., Romano N., Koh C. B., Yang S. Y. (2014). Effects of dietary probiotics on the growth and feeding efficiency of red hybrid Tilapia, Oreochromis sp., and subsequent resistance to Streptococcus agalactiae . J. Appl. Aquac. 26 , 22–31. 10.1080/10454438.2013.874961 [ CrossRef ] [ Google Scholar ]
- Nikoskelainen S., Ouwehand A. C., Bylund G., Salminen S., Lilius E. -M. (2003) Immune enhancement in rainbow trout ( Oncorhynchus mykiss ) by potential probiotic bacteria ( Lactobacillus rhamnosus ) . Fish Shellfish Immun . 15 , 443–452. 10.1016/S1050-4648(03)00023-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nikoskelainen S., Salminen S., Bylund G., Ouwehand A. C. (2001). Characterization of the properties of human and dairy derived probiotics for prevention of infectious diseases in fish . Appl. Environ. Micro. 67 , 2430–2435. 10.1128/AEM.67.6.2430-2435.2001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pai S. S., Anas A., Jayaprakash N. S., Priyaja P., Sreelakshmi B., Preetha R., et al.. (2010). Penaeus monodon larvae can be protected from Vibrio harveyi infection by pre-emptive treatment of a rearing system with antagonistic or non-antagonistic bacterial probiotics . Aquac. Res. 41 , 847–860. 10.1111/j.1365-2109.2009.02362.x [ CrossRef ] [ Google Scholar ]
- Pan X., Wu T., Song Z., Tang H., Zhao Z. (2008b). Immune responses and enhanced disease resistance in Chinese drum, Miichthys miiuy (Basilewsky), after oral administration of live or dead cells of Clostridium butyricum CB2 . J. Fish. Dis. 31 , 679–686. 10.1111/j.1365-2761.2008.00955.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pan X., Wu T., Zhang L., Song Z., Tang H., Zhao Z. (2008a). In vitro evaluation on adherence and antimicrobial properties of a candidate probiotic Clostridium butyricum CB2 for farmed fish . J. Appl. Microbiol. 105 , 1623–1629. 10.1111/j.1365-2672.2008.03885.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Parker R. (1974). Probiotics, the other half of the antibiotic story . Animal Nutr. Health 29 , 4–8. [ Google Scholar ]
- Partida-Arangure B. O., Luna-González A., Fierro-Coronado J. A., Flores-Miranda C., González-Ocampo H. A. (2013). Effect of inulin and probiotic bacteria on growth, survival, immune response, and prevalence of white spot syndrome virus (WSSV) in Litopenaeus vannamei cultured under laboratory conditions . Afr. J. Bio. 12 , 3366–3375. 10.5897/AJB12.1569 [ CrossRef ] [ Google Scholar ]
- Pérez-Sánchez T., Balcázar J. L., Merrifield D. L., Carnevali O., Gioacchini G., de Blas I., et al.. (2011). Expression of immune-related genes in rainbow trout ( Oncorhynchus mykiss ) induced by probiotic bacteria during Lactococcus garvieae infection . Fish Shellfish Immun. 31 , 196–201. 10.1016/j.fsi.2011.05.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pieters N., Brunt J., Austin B., Lyndon A. R. (2008). Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliisskin infections in rainbow trout ( Oncorhynchus mykiss , Walbaum) . J. Appl. Microb. 105 , 723–732. 10.1111/j.1365-2672.2008.03817.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pirarat N., Kobayashi T., Katagiri T., Maita M., Endo M. (2006). Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia ( Oreochromis niloticus ) . Vet. Immunol. Immunop. 113 , 339–347. 10.1016/j.vetimm.2006.06.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pirarat N., Pinpimai K., Endo M., Katagiri T., Ponpornpisit A., Chansue N., et al.. (2011). Modulation of intestinal morphology and immunity in nile tilapia ( Oreochromis niloticus ) by Lactobacillus rhamnosus GG . Res. Vet. Sci. 91 , e92–e97. 10.1016/j.rvsc.2011.02.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Planas M., Pérez-Lorenzo M., Hjelm M., Gram L., Fiksdal I. U., Bergh Ø., et al. (2006). Probiotic effect in vivo of Roseobacter strain 27-4 against Vibrio (Listonella) anguillarum infections in turbot ( Scophthalmus maximus L.) larvae . Aquaculture 255 , 323–333. 10.1016/j.aquaculture.2005.11.039 [ CrossRef ] [ Google Scholar ]
- Quentel C., Gatesoupe F. J., Aubin J., Lamour F., Abiven A., Baud M., et al. (2005). Ofimer probiotic study on rainbow trout. I. Resistance against Yersinia ruckeri and humoral immune response of rainbow trout (Oncorhynchus mykiss) submitted to probiotic treatment with Saccharomyces cerevisiae var. boulardii , in Lessons from the Past to Optimise the Future. Aquaculture Europe 2005, Trondheim, EAS Special Publication , Vol 35 eds Howell B., Flos R. (Oostende: European Aquaculture Society; ), 380–381. [ Google Scholar ]
- Raa J. (1996). The use of immunostimulatory substances in fish and shellfish farming . Rev. Fish. Sci. 4 , 229–288. 10.1080/10641269609388587 [ CrossRef ] [ Google Scholar ]
- Rafiee G., Saad C. R. (2005). Nutrient cycle and sludge production during different stages of red tilapia ( Oreochromis sp.) growth in a recirculating aquaculture system . Aquaculture 244 , 109–118. 10.1016/j.aquaculture.2004.10.029 [ CrossRef ] [ Google Scholar ]
- Raida M. K., Larsen J. L., Nielsen M. E., Buchmann K. (2003). Enhanced resistance of rainbow trout ( Oncorhynchus myskiss ) against Yersinia ruckeri challenge following oral administration of Bacillus subtilis and B. licheniformis (Bio plus 2B) . J. Fish. Dis. 26 , 495–498. 10.1046/j.1365-2761.2003.00480.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramesh D., Vinothkanna A., Rai A. K., Vignesh V. S. (2015). Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila . Fish Shellfish Immun . 45 , 268–276. 10.1016/j.fsi.2015.04.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ran C., Carrias A., Williams M. A., Capps N., Dan B. C. T. (2012). Identification of Bacillus strains for biological control of catfish pathogens . PLoS ONE 7 :e45793. 10.1371/journal.pone.0045793 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ravi A., Musthafa K., Jegathammbal G., Kathiresan K., Pandian S. (2007). Screening and evaluation of probiotics as a biocontrol agent against pathogenic Vibrios in marine aquaculture . Lett. Appl. Microbiol. 45 , 219–223. 10.1111/j.1472-765X.2007.02180.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Remus D. M., van Kranenburg R., van Swam I. I., Taverne N., Bongers R. S., Wels M., et al.. (2012). Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling . Microb. Cell. Fact. 11 :149. 10.1186/1475-2859-11-149 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rengpipat S., Phianphak W., Piyatiratitivorakul S., Menasveta P. (1998). Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth . Aquaculture 167 , 301–313. 10.1016/S0044-8486(98)00305-6 [ CrossRef ] [ Google Scholar ]
- Rengpipat S., Rukpratanporn S., Piyatiratitivorakul S., Menasaveta P. (2000). Immunity enhancement in black tiger shrimp ( Penaeus monodon ) by a probiont bacterium ( Bacillus S11) . Aquaculture 191 , 271–288. 10.1016/S0044-8486(00)00440-3 [ CrossRef ] [ Google Scholar ]
- Rengpipat S., Tunyanun A., Fast A. W., Piyatiratitivorakul S., Menasveta P. (2003). Enhanced growth and resistance to Vibrio challenge in pond-reared black tiger shrimp Penaeus monodon fed a Bacillus probiotic . Dis. Aquat. Organ. 55 , 169–173. 10.3354/dao055169 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reyes-Becerril M., Tovar-Ramírez D., Ascensio-Valle F., Civera-Cerecedo R., Gracia-López V., Barbosa-Solomieu V., et al. (2011). Effects of dietary supplementation with probiotic live yeast Debaryomyces hansenii on the immune and antioxidant systems of leopard grouper Mycteroperca rosacea infected with Aeromonas hydrophil a . Aquac. Res. 42 , 1676–1686. 10.1111/j.1365-2109.2010.02762.x [ CrossRef ] [ Google Scholar ]
- Ringø E. (2008). The ability of carnobacteria isolated from fish intestine to inhibit growth of fish pathogenic bacteria: a screening study . Aquac. Res. 39 , 171–180. 10.1111/j.1365-2109.2007.01876.x [ CrossRef ] [ Google Scholar ]
- Ringø E., Løvmo L., Kristiansen M., Bakken Y., Salinas I., Myklebust R., et al. (2010). Lactic acid bacteria vs . pathogens in the gastrointestinal tract of fish: a review . Aquac. Res. 41 , 451–467. 10.1111/j.1365-2109.2009.02339.x [ CrossRef ] [ Google Scholar ]
- Ringø E., Sepploa M., Berg A., Olsen R. E., Schillinger U., Holzapfel W. (2002). Characterization of Carnobacterium divergens strain 6251 isolated from intestine of Arctic charr ( Salvelinus alpinus L.) . Syst. Appl. Microbiol. 25 , 120–129. 10.1078/0723-2020-00080 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ringø E., Zhou Z., Vecino J. L. G., Wadsworth S., Romero J., Krogdahl Å., et al. (2016). Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquacult. Nutr. 22 , 219–282. 10.1111/anu.12346 [ CrossRef ] [ Google Scholar ]
- Robertson P. A. W., O'Dowd C., Burrells C., Williams P., Austin B. (2000). Use of Carnobacterium sp. as a probiotic for Atlantic salmon ( Salmo salar L.) and rainbow trout ( Oncorhynchus mykiss , Walbaum) . Aquaculture 185 , 235–243. 10.1016/S0044-8486(99)00349-X [ CrossRef ] [ Google Scholar ]
- Rodríguez J., Espinosa Y., Echeverria F., Cárdenas G., Román R., Stern S. (2007). Exposure to probiotics and β-1,3/1,6-glucans in larviculture modifies the immune response of Penaeus vannamei juveniles and both the survival to White Spot Syndrome Virus challenge and pond culture . Aquaculture 273 , 405–415. 10.1016/j.aquaculture.2007.10.042 [ CrossRef ] [ Google Scholar ]
- Rodríguez-Estrada U., Satoh S., Haga Y., Fushimi H., Sweetman J. (2013). Effects of inactivated Enterococcus faecalis and mannan oligosaccharide and their combination on growth, immunity, and disease protection in rainbow trout . North Am. J. Aquacult. 75 , 416–428. 10.1080/15222055.2013.799620 [ CrossRef ] [ Google Scholar ]
- Rohyati I. S. (2015). Improved of growth rate of abalone haliotis asinine fed pudding probiotic-enriched protein . Procedia Environ. Sci. 23 , 315–322. 10.1016/j.proenv.2015.01.046 [ CrossRef ] [ Google Scholar ]
- Safari R., Adel M., Lazado C. C., Caipang C. M., Dadar M. (2016). Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout ( Oncorhynchus mykiss ) via immunomodulation . Fish Shellfish Immun. 52 , 198–205. 10.1016/j.fsi.2016.03.020 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sakai M., Yoshida T., Atsuta S., Kobayashi M. (1995). Enhancement of resistance to vibriosis in rainbow trout, Oncorhynchus mykiss (Walbaum), by oral administration of Clostridium butyricum bacterin . J. Fish. Dis. 18 , 187–190. 10.1111/j.1365-2761.1995.tb00276.x [ CrossRef ] [ Google Scholar ]
- Salinas I., Myklebust R., Esteban M. A., Olsen R. E., Meseguer J., Ringø E. (2008). In vitro studies of Lactobacillus delbrueckii subsp. lactis in Atlantic salmon ( Salmo salar L.) foregut: tissue responses and evidence of protection against Aeromonas salmonicida subsp. salmonicida epithelial damage . Vet. Microbiol . 128 , 167–177. 10.1016/j.vetmic.2007.10.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sayes C., Leyton Y., Riquelme C. (2018). Probiotic bacteria as an healthy alternative for fish aquaculture , in Antibiotic Use in Animals , ed Savic S. (London: Intech Open; ) 115–132. [ Google Scholar ]
- Scholz U., Garcia Diaz G., Ricque D., Cruz Suarez L. E., Vargas Albores F., Latchford J. (1999). Enhancement of vibriosis resistance in juvenile Penaeus vannamei by supplementation of diets with different yeast products . Aquaculture 176 , 271–283. [ Google Scholar ]
- Selim K. M., Reda R. M. (2015). Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus , by dietary supplementation with Bacillus amyloliquefaciens . Fish Shellfish Immun. 44 , 496–503. 10.1016/j.fsi.2015.03.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sha Y., Wang L., Liu M., Jiang K., Xin F., Wang B. (2016). Effects of lactic acid bacteria and the corresponding supernatant on the survival, growth performance, immune response and disease resistance of Litopenaeus vannamei . Aquaculture 452 , 28–36. 10.1016/j.aquaculture.2015.10.014 [ CrossRef ] [ Google Scholar ]
- Sharifuzzaman S. M., Abbass A., Tinsley J. W., Austin B. (2011). Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout ( Oncorhynchus mykiss , Walbaum) against Vibrio anguillarum . Fish Shellfish Immun. 30 , 347–353. 10.1016/j.fsi.2010.11.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharifuzzaman S. M., Austin B. (2010). Development of protection in rainbow trout ( Oncorhynchus mykiss , Walbaum) to Vibrio anguillarum following use of the probiotic Kocuria SM1 . Fish Shellfish Immun. 29 , 212–216. 10.1016/j.fsi.2010.03.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Silva-Aciares F. R., Carvajal P. O., Mejias C. A., Riquelme C. E. (2011). Use of macroalgae supplemented with probiotics in the Haliotis rufescens (Swainson, 1822) culture in Northern Chile . Aquac. Res. 42 , 953–961. 10.1111/j.1365-2109.2010.02678.x [ CrossRef ] [ Google Scholar ]
- Sivakumar N., Sundararaman M., Selvakumar G. (2012). Probiotic effect of Lactobacillus acidophilus against Vibriosis in juvenile shrimp ( Penaeus monodon ) . Afr. J. Biotechnol. 11 , 15811–15818. 10.5897/AJB12.1328 [ CrossRef ] [ Google Scholar ]
- Soleimani N., Hoseinifar S. H., Merrifield D. L., Barati M., Abadi Z. H. (2012). Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach ( Rutilus rutilus ) fry . Fish Shellfish Immun. 32 , 316–321. 10.1016/j.fsi.2011.11.023 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Son V. M., Chang C. C., Wu M. C., Guu Y. K., Chiu C. H., Cheng W. (2009). Dietary administration of the probiotic, Lactobacillusplantarum , enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides . Fish Shellfish Immun . 26 , 691–698. 10.1016/j.fsi.2009.02.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sorroza L., Padilla D., Acosta F., Román L., Grasso V., Vega J., et al.. (2012). Characterization of the probiotic strain Vagococcus fluvialis in the protection of European sea bass ( Dicentrarchus labrax ) against vibriosis by Vibrio anguillarum . Vet. Microbiol. 155 , 369–373. 10.1016/j.vetmic.2011.09.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sorroza L., Real F., Acosta F., Acosta B., Deniz S, Roman L., et al. (2013). A probiotic potential of Enterococcus gallinarum against Vibrio anguillarum infection . Fish Pathol. 48 , 9–12. 10.3147/jsfp.48.9 [ CrossRef ] [ Google Scholar ]
- Stanier R. Y., Doudoroff M., Adelberg E. A. (1975). General Microbiology , 3rd Edn New York, NY: The MacMillan Press. [ Google Scholar ]
- Sugimura Y., Hagi T., Hoshino T. (2011). Correlation between in vitro mucus adhesion and the in vivo colonization ability of lactic acid bacteria: screening of new candidate carp probiotics . Biosci. Biotech. Bioch. 75 , 511–515. 10.1271/bbb.100732 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sugita H., Miyajima C., Deguchi Y. (1991) The vitamin B 12-producing ability of the intestinal microflora of freshwater fish . Aquaculture 92 , 267–276. 10.1016/0044-8486(91)90028-6 [ CrossRef ] [ Google Scholar ]
- Sugita H., Takahashi J., Deguchi Y. (1992). Production and consumption of biotin by the intestinal microflora of cultured freshwater fishes . Biosci. Biotechnol. Biochem. 56 , 1678–1679. 10.1271/bbb.56.1678 [ CrossRef ] [ Google Scholar ]
- Sun Y. Z., Xia H. Q., Yang H. L., Wang Y. L., Zou W. C. (2014). TLR2 signaling may play a key role in the probiotic modulation of intestinal microbiota in grouper Epinephelus coioides . Aquaculture 430 , 50–56. 10.1016/j.aquaculture.2014.03.042 [ CrossRef ] [ Google Scholar ]
- Sun Y. Z., Yang H. L., Huang K. P., Ye J. D., Zhang C. X. (2013). Application of autochthonous Bacillus bioencapsulated in copepod to grouper Epinephelus coioides larvae . Aquaculture 392– 395 , 44–50. 10.1016/j.aquaculture.2013.01.037 [ CrossRef ] [ Google Scholar ]
- Sun Y. Z., Yang H. L., Ling Z. C., Chang J. B., Ye J. D. (2009). Gut microbiota of fast and slow growing grouper Epinephelus coioides . Afr. J. Microbiol. Res. 3 , 713–720. [ Google Scholar ]
- Swain S. M., Singh C., Arul V. (2009). Inhibitory activity of probiotics Streptococcus phocae PI80 and Enterococcus faecium MC13 against vibriosis in shrimp Penaeus monodon . World J. Microbiol. Biotechnol. 25 , 697–703. 10.1007/s11274-008-9939-4 [ CrossRef ] [ Google Scholar ]
- Tan L. T., Chan K. -G., Lee L. -H., Goh B. -H. (2016). Streptomyces bacteria as potential probiotics in aquaculture . Front. Microbiol . 7 :79. 10.3389/fmicb.2016.00079 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tapia-Paniagua S. T., Vidal S., Lobo C., Prieto-Álamo M. J., Jurado J., Cordero H., et al.. (2014). The treatment with the probiotic Shewanella putrefaciens Pdp11 of specimens of Solea senegalensis exposed to high stocking densities to enhance their resistance to disease . Fish Shellfish Immun. 41 , 209–221. 10.1016/j.fsi.2014.08.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thompson J., Gregory S., Plummer S., Shields R. J., Rowley A. F. (2010). An in vitro and in vivo assessment of the potential of Vibrio spp. as probiotics for the Pacific white shrimp, Litopenaeus vannamei . J. Appl. Microbiol. 109 , 1177–1187. 10.1111/j.1365-2672.2010.04743.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Utiswannakul P., Sangchai S., Rengpipat S. (2011). Enhanced growth of black tiger shrimp Penaeus monodon by dietary supplementation with Bacillus (BP11) as a probiotic . J. Aquac. Res. Dev. 2 :006 10.4172/2155-9546.S1-006 [ CrossRef ] [ Google Scholar ]
- Van Doan H., Hoseinifar S. H., Dawood M. A. O., Chitmanat C., Tayyamath K. (2017). Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia ( Oreochromis niloticus ) . Fish Shellfish Immun . 70 ( Supplement C ):87–94. 10.1016/j.fsi.2017.09.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Hai N., Buller N., Fotedar R. (2009). The use of customised probiotics in the cultivation of western king prawns ( Penaeus latisulcatus Kishinouye, 1896) . Fish Shellfish Immun. 27 , 100–104. 10.1016/j.fsi.2009.05.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vaseeharan B., Ramasamy P. (2003). Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon . Lett. Appl. Microbiol. 36 , 83–87. 10.1046/j.1472-765X.2003.01255.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vendrell D., Balcazar J. L., Blas I. D., Ruiz-Zzarzuela I., Girones O., Muzquiz J. L. (2008). Protection of Rainbow trout ( Oncorhynchus mykiss ) from lactococcosis by probiotic bacteria . Comp. Immunol. Microb. 31 , 337–345. 10.1016/j.cimid.2007.04.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Verschuere L., Rombaut G., Sorgeloos P., Verstraete W. (2000). Probiotic bacteria as biological control agents in aquaculture . Microbiol. Mol. Biol. R. 64 , 655–671. 10.1128/MMBR.64.4.655-671.2000 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vieira F. N., Buglione C. C., Mourino J. P. L., Jatoba A., Martins M. L., Schleder D. D., et al. (2010). Effect of probiotic supplemented diet on marine shrimp survival after challenge with Vibrio harveyi . Br. J. Veterinary Res. Animal Sci. 62 , 631–638. 10.1590/S0102-09352010000300019 [ CrossRef ] [ Google Scholar ]
- Vine N. G., Leukes W. D., Kaiser H. (2006). Probiotics in marine larviculture . FEMS Microbiol. Rev. 30 , 404–427. 10.1111/j.1574-6976.2006.00017.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vinoj G., Jayakumar R., Chen J. C., Withyachumnarnkul B., Shanthi S., Vaseeharan B. (2015). N-hexanoyl-L-homoserine lactone-degrading Pseudomonas aeruginosa PsDAHP1 protects zebrafish against Vibrio parahaemolyticus infection . Fish Shellfish Immun. 42 , 204–212. 10.1016/j.fsi.2014.10.033 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Westerdahl A., Olsson J. C., Kjelleberg S., Conway P. L. (1991). Isolation and characterization of turbot ( Scophtalmus maximus )-associated bacteria with inhibitory effects against Vibrio anguillarum . Appl. Environ. Microb. 57 , 2223–2228. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilson B. A., Salyers A. A., Whitt D. D., Winkler M. E. (eds.). (2011). Bacterial Pathogenesis: A Molecular Approach . Washington: American Society for Microbiology(ASM). [ Google Scholar ]
- Wu Z., Jiang C., Ling F., Wang G. X. (2015). Effects of dietary supplementation of intestinal autochthonous bacteria on the innate immunity and disease resistance of grass carp ( Ctenopharyngodon idellus ) . Aquaculture 438 , 105–114. 10.1016/j.aquaculture.2014.12.041 [ CrossRef ] [ Google Scholar ]
- Yan L., Boyd K. G., Burgess J. G. (2002). Surface attachment induced production of antimicrobial compounds by marine epiphytic bacteria using modified roller bottle cultivation . Mar. Biotechnol. 4 , 356–366. 10.1007/s10126-002-0041-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yu M., Li Z., Lin H., Wen G., Ma S. (2009). Effects of dietary medicinal herbs and Bacillus on survival, growth, body composition, and digestive enzyme activity of the white shrimp Litopenaeus vannamei . Aquacult. Int. 17 , 377–384. 10.1007/s10499-008-9209-3 [ CrossRef ] [ Google Scholar ]
- Zokaeifar H., Balcázar J. L., Saad C. R., Kamarudin M. S., Sijam K., Arshad A., et al.. (2012). Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei . Fish Shellfish Immun. 33 , 683–689. 10.1016/j.fsi.2012.05.027 [ PubMed ] [ CrossRef ] [ Google Scholar ]
Learn more about how the Cal Poly Humboldt Library can help support your research and learning needs.
Stay updated at Campus Ready .

- Cal Poly Humboldt Library
- Research Guides
Fisheries Biology Research Guide
- Literature of Fisheries
- Finding Books
- Finding Articles
- Internet Resources
- Reference Resources
- AFS Publications Style Guide
- Canadian Journal of Fisheries and Aquatic Sciences: Style Guide
- Zotero This link opens in a new window
- Introduction
Fisheries literature is part of the larger scientific literature and is derived from basic research in related disciplines and applied research in fisheries. Scientific literature is the principal medium for communicating the results of scientific research and represents a permanent record of the collective achievements of the scientific community. It is composed of the individual "end products" of scientific research and continues to expand as new research builds on earlier research.
Scientific literature is divided into two basic categories - "primary" and "secondary". Publications that report the results of original scientific research constitute the "primary" literature and include journal papers, conference papers, monographic series, technical reports, theses, and dissertations. The "primary" literature is eventually compacted into "secondary" sources which synthesize and condense what is known on specific topics. These include reviews, monographs, textbooks, treatises, handbooks, and manuals.
Availability of scientific literature varies depending upon its publication format. Some formats are widely available, e.g., journal papers, while others have limited distribution and are difficult to identify and acquire. This "gray literature" commonly includes technical reports, theses, and dissertations.
Back to the Top
- Scientific Research/Publication Cycle
The following chart illustrates common steps involved in the scientific research process (inner circle), the dissemination of research results through the primary and secondary literature (outer circle), and the personal assimilation of this information resulting in new ideas and research (inner circle):
Fisheries Serials: Journals, Magazines, Monograhic Series
Fisheries serials can be grouped into the following three categories:
- Journals - regularly issued publications that contain papers reporting the results of scholarly research in the discipline
- Magazines & Newsletters - contain popular reports on developments in the discipline
- Monographic Series - irregularly issued publications that in most cases contain the results of scholarly research
The research paper published in a scientific journal represents the most important "primary" source of information for the fisheries scientist and manager. Papers published in journals generally go through a "peer review" process before acceptance and publication. Seventy-five percent of the fisheries research literature is published in this format.To find fisheries journals ranked by impact see SCImago Journal & Country Rank or Eigenfactor.org - Ranking and Mapping Scientific Knowledge .
Another thing to note is that databases and cited literature typically abbreviate journal titles. Databases can be used to find individual research papers by author, subject, taxonomic category, habitat, time period, chemical compound, or geographic area. In addition many journal publisher websites now maintain a searchable database of articles that have been published in their journals.
The following list contains many of the print and online journals available which publish research of interest to fisheries scientists and managers.
Magazines and Newsletters
Articles appearing in these publications tend to be popular in format and scope. They may contain news and perspectives of professional societies and environmental organizations, report on research published in scholarly journals, report on environmental problems and new political initiatives, or contain articles aimed at the layperson.
Monographic Series
While most fisheries research is published in journals, perhaps 10% is published as individual issues of monographic series. Longer contributions resulting from scientific research are often published in this format. Monographic series typically have the following characteristics:
- They are published by government agencies, major universities or professional organizations.
- Individual issues are collectively published in a continuing series which has a distinctive name. Typical names include Bulletin, Special Report, Special Paper, Technical Report, and Technical Paper.
- Individual issues in the series are consecutively numbered, e.g. Technical Paper No. 36.
- Each issue has a distinctive author and title.
- There is no regular publication schedule in contrast to a journal.
- Individual issues contain the completed results of a single research project.
- Individual issues range from several pages to several hundred pages.
A typical example is:
Serns, S.L.(a) 1984. An 8-Inch Length Limit on Smallmouth Bass: Effects on the Sport Fisheries and Populations of Smallmouth Bass and Yellow Perch in Nebish Lake, Wisconsin(b). Wisconsin Department of Natural Resources(c) Technical Bulletin(d) No. 148(e). where a=individual author; b=individual title; c=series author; d=series title; e=series number
To locate monographic series in the Library you need to consult the following two sources:
- For federal and California State agency series use the catalogs and indexes listed in Natural Resources Agency Government Documents and Reports .
- For all other monographic series use the Library's catalog, OneSearch. The key is to look for the series of which an individual issue is a part. You must look under either the series title (Technical Bulletin in the above example) or the sponsoring organization (Wisconsin Department of Natural Resources in the above example). In the above example there is no listing under the author "Serns" or the title "An 8-Inch..." since these are the author and title of the individual issue.
As with individual journal papers the Finding Aticles tab in the Fisheries Guide can be used to identify research published in this format.
The following monographic series of interest to fisheries are found in the regular bookstacks of the Library. Again, those published by federal and California state agencies are listed in Natural Resources Agency Government Documents and Reports and are physically located in the separate Documents Collection.
- Alaska Department of Fish & Game
- Special Publication Series
- Canadian Bulletin of Fisheries & Aquatic Sciences
- Canadian Manuscript Report of Fisheries & Aquatic Sciences
- Canadian Technical Report of Fisheries & Aquatic Sciences
- Division Report
- Outdoor Facts: Fishery Information Leaflet
- Special Report
- Technical Publication
- EIFAC Technical Paper
- Special Scientific Report
- Folia Limnologica Scandinavica
- FAO Fisheries Circular
- FAO Fisheries Reports
- FAO Fisheries Technical Paper
- FAO Fisheries Synopsis
- Scotland Fisheries Research Report
- Fishery Investigations
- Biological Notes
- Bulletin Fishery Status Report
- Stock Assessment Report
- Research & Development Series
- CM Documents (papers presented at ICES Annual Science Conferences)
- Cooperative Research Reports
- Scientific Report
- Technical Report
- Progress Report
- Technical Bulletin
- Fisheries Research Reports
- Fisheries Technical Reports
- Fisheries Research Bulletin
- Fisheries Technical Report
- Nordic Journal of Freshwater Research (formerly Swedish Board of Fisheries (Drottningholm). Institute of Freshwater Research. Report)
- Information Reports
- Publication
- Theses and Dissertations
The outcome of graduate study conducted at universities is commonly a master's thesis or doctoral dissertation. In addition to the formal thesis or dissertation, research results are often communicated in other "primary" literature formats, such as the journal paper.
See Theses and Dissertations for how to find and acquire 1) HSU masters theses; and 2) theses and dissertations produced at other universities that are available in other libraries and on the Internet. In addition the following are specialized directories and databases to theses and dissertations in fisheries:
- Bibliography of Theses on Fishery Biology and its Supplement (QL 615 S66) Lists approximately 3,000 fisheries theses and dissertations completed through 1971.
- California Cooperative Fish & Wildlife Research Unit Masters of Science Theses Lists current and completed masters projects with links to the fulltext of completed master's theses.
- Fish, Fisheries and Aquatic Biodiversity Worldwide (CPH users only) Includes dissertations and theses in fisheries.
- Conferences Papers
Papers presented at national and international conferences, symposia, and workshops are another source of "primary" scientific information in fisheries. For many of these meetings the presented papers are eventually published in a "proceedings" or "transactions" volume. Those available in the Library are listed in the OneSearch catalog under author (generally the name of the conference, individual editor or sponsoring organization) and title.
Subject, taxonomic, geographic, and author access to individual conference papers also is provided by databases listed in the Finding Articles tab of the Fisheries Guide.
Following are some of the regularly recurring fisheries conferences received by the Library. In addition there are many other one-time speciality conferences listed in the catalog.
- American Fisheries Society, Bonneville Chapter. Proceedings
- California Salmon and Steelhead Restoration Conference. Reports
- Canada Department of Fisheries & Oceans. Annual Aquatic Toxicology Workshop Proceedings (appear in issues of Canadian Technical Report of Fisheries and Aquatic Sciences)
- Desert Fishes Council. Proceedings
- Federal-Provincial Wildlife Conference. Transactions
- Indo-Pacific Fisheries Council. Proceedings
- International Association of Fish & Wildlife Agencies. Proceedings
- International Association of Theoretical & Applied Limnology (Internationale Vereiniguing Fur Theoretische und Augewandte Limnologie). Proceedings (Verhandlungen)
- International Council for the Exploration of the Sea (Conseil International Pour L'Exploration de la Mer).ICES Marine Science Symposia (formerly Rapports et Proces-Verbaux des Reunion)
- International Commission for the Conservation of Atlantic Tunas. Collection Volume of Scientific Papers
- International Symposium on Regulated Rivers
- Marine Recreational Fisheries Symposium. Marine Recreational Fisheries
- National Shellfisheries Association. Proceedings (now Journal of Shellfish Research)
- North American Wildlife & Natural Resources Conference. Transactions
- Northwest Fish Culture Conference. Proceedings
- Southeastern Association of Fish & Wildlife Agencies. Proceedings
- Symposium on Aquatic Toxicology. Aquatic Toxicology
- Western Association of Fish & Wildlife Agencies. Proceedings
- Wildlife Society. Western Division. Transactions (formerly Cal-Neva Wildlife)
- World Mariculture Society. Proceedings (now Journal of the World Aquaculture Society)
- Monographs (Books)
Monographs generally are not part of the "primary" literature of science, but rather are "secondary" sources of information. They may be either scholarly contributions or popularizations on specific topics. Through scholarly monographs the "primary" literature on specific topics is condensed, summarized or reviewed. Most include references back to the "primary" literature. They may take the format of textbooks, treatises, taxonomic works, or a multitude of reference works, such as encyclopedias or handbooks. Monographs are listed in the Library catalog, OneSearch. For guidance in use of the HSU Library Catalog and other library catalogs see Finding Books on Fish and Fisheries .
- << Previous: Reference Resources
- Next: AFS Publications Style Guide >>
Contents of this Page
- Fisheries Serials: Journals, Magazines and Newsletters, Monographic Series
Related Guides
Natural Resource Agency Government Documents and Reports
Content Attribution
Some of the content of this page was created in another format by Robert Sathrum, HSU Librarian, retired 06/2013.
Rice field ecology and fish culture — an overview
- Published: May 1993
- Volume 259 , pages 91–113, ( 1993 )
Cite this article

- C. H. Fernando 1
932 Accesses
98 Citations
3 Altmetric
Explore all metrics
Rice fields are an integral part of the landscape throughout most of the tropics. Rice is also grown widely in higher latitudes. Most rice cultivation is done in flooded fields where a temporary aquatic fauna is generated. Rice cultivation has sustained some of the oldest civilizations but the use of the aquatic phase for raising a crop of fish has not been practiced widely although fragmentary records indicate that rice and fish have been cultivated concurrently but rarely over 2 or 3 millennia. We have more reliable records of rice and fish culture in rice fields during the past 150 years.
Rice cultivation is now very highly mechanized and uses high fertilizer and pesticide inputs and extensive irrigation facilities have been constructed to increase the area of rice cultivation and enhance yields. Rice cultivation also provides a suitable habitat for the breeding of mosquitoes, some of which are vectors for diseases. It appears that in regions outside the tropics aquatic pests of rice are also encountered. In the tropics indigenous fishes and other organisms including copepods act as biological control agents for mosquitoes and aquatic rice pests.
The rice field is usually a successor of shallow marshes or a lowland area which can be supplied with adequate water. In addition deep water rice is grown in permanent marshes and rice is also grown in terraced hillsides, not to mention relatively dry localities where dry-land rice is cultivated. The marsh habitat is usually rich in plant and animal species. Some of these survive in rice fields. The water supplied to rice fields come via irrigation systems which bring a complement of plants, animals, and other organisms seasonally to colonize the rice field. The rice field is thus a new habitat, like a reservoir, with some similarities to a marsh but manipulated for cultivation of rice. This creates a unique, temporary and rapidly changing habitat which is often very productive and can be used to raise fish on an artisanal or intensive scale.
Fish culture in rice fields has had a checkered history during the past 150 years when records are available. Its earlier history is obscure. Long-term records of fish culture activities are not available from any part of the world although apparently thriving enterprises seem to have existed in Japan, Italy, USSR and China. Attempts to culture fish in rice fields have been made on all continents except Australasia and Antarctica of course. At the present time the focus of rice-cultivation seems to have shifted to China, Indonesia, and Thailand. Whether this enterprise will endure even in these countries cannot be predicted with any degree of certainty.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Column: Rice-Fish Culture: The Contemporary Significance of a Traditional Practice
Does intensification of the rice cultivation cycle influence anuran diversity in rice fields.
Rodent damage to rice crops is not affected by the water-saving technique, alternate wetting and drying
Abul-Nasr, S., A. I. Isa & A. M. El Tantaway, 1970. Control measures of the blood-worms, Chironomus sp., in rice nurseries (Diptera). Bull. ent. Soc. Egypt Econ. Ser. 4: 127–133.
Google Scholar
Aleshin, E. P., A. P. Smetanin & N. N. Elagin, 1972. Progressive ways of rice cultivation. Kolos, Moscow, 152 pp. (Russian).
Andryushin, M. A., 1977. Rice irrigation. Kolos, Moscow, 128 pp. (Russian).
Angladette, A., 1966. Le Riz. Collections techniques agricoles in production tropicale. Edit. Maisonneuve et Larose, Paris, 930 pp.
Anon., 1957. Fish culture in rice fields — a preliminary review. Proc. Indo-Pacif. Fish. Counc. 7: 193–206.
Anon., 1975. Arroz. Instituto Cubano del Libro, La Habana, 502 pp.
Anon., 1977. Literature review of insect pests and diseases of rice in Bangladesh. Bangladesh Rice Research Institute, Dacea, 131 pp.
Anon., 1983. Weed control in rice. Publ. IRRI, Los Baños, Philippines, 421 pp.
Anon., 1987. Fish culture in rice fields in Northern Thailand. Publ. Northeast Fishery Project, Thailand — Canada, 20 pp. (Thai).
Anon., 1990. IRRI Rice facts. Manila (pamphlet).
Anon., (undated). Rice fields are our life (Thai), Bangkok.
Ardiwinata, R. O., 1957. Fish culture in paddy fields in Indonesia. Proc. Indo-Pacif. Fish. Counc. 7: 119–154.
Baharin, B. K., K. G.Ang & C. E.Tan, 1979. A review of the status of research and development activities in rice-cumfish culture in Asia. Publ. Univ. Pertanian, Malaysia, 49 pp.
Berczik, A., 1970. Schädigung eines Reisfeldes durch Chironomiden und seine ökologischen Umstände. Opusc. Zool. (Budapest) 10: 221–230.
Berka, R., 1992. (pers. comm.).
Berti, A. L. & M. Montesinos, 1946. Cultivo de arroz en relación conel problema de malaria de Venezuela. Third Conf. Agric. Interamericana, (abstract).
Bezerra e Silva, J. W., 1988. Outros sistemas de cultivo em piscicultura. In Manual sobre manejo de reservatorios para a produção de peixes. FAO-DNOCS Publ. Brasilia GCP/ RLA/075/ITA 9: 131–158.
Botnariuc, N. & P. Albu, 1966. Cricotopus silvertris Fabr. Chironomide nuisible au riz. Gewässer und Abwässer 41/42: 64–69.
Bowling, C. C., 1961. Chemical control of rice water weevil. J. econ. Ent. 54: 710–712.
Ca, R. K., 1983. Rice field fish culture. Agricultural Publisher, Peking, 70 pp.
Cavassa, F., 1914. Ricerche intorno alle specie dannose alla coltivazione del riso e specialmente intorno al Chironomus cavassai . Bol. Lab. Zool. Portici 8: 228–239.
Chang, T. T., 1976a. The origin, evolution, cultivation, dissemination and diversification of Asian and African rices. Euphytica 25: 425–441.
Chang, T. T., 1976b. The rice culture in the early history of agriculture. Phil. Trans. r. Soc., Lond.: 143–157.
Chapman, G. C., 1991. The diet and feeding habits of Oreochromis niloticus (Linnaeus) and Cyprinus carpio (Linnaeus) in lowland rice fields in Northern Thailand. M.Sc. Thesis, University of Waterloo, 131 pp.
Čižov, N. I. & A. I. Anošin, 1969. Fish culture in rice fields under fallow. Trudy Vsesoyuznogo nauchno issledovatelskogo instituta rybnogo khozyaistva 16: 187–193 (Russian).
Clement, S. L., A. A. Grigarick & M. D. Way, 1977. Conditions associated with rice plant injury by chironomid midges in California. Envir. Ent. 6: 91–96.
Coche, A. G., 1967. Fish culture in rice fields: A world-wide synthesis: Hydrobiologia 30: 1–44.
Coosemans, M. H., 1985. Comparaison de l'endémie malarienne dans une zone de riziculture et dans une zone de culture de coton dans la plaine de la Ruzizi, Burundi. Ann. Soc. Belge Méd. trop. 65: 187–200.
Crossland, N. O., 1965. The pest status and control of the tadpole shrimp, Triops granarius and the snail Lanistes ovum in Swaziland rice fields. J. appl. Ecol. 2: 115–120.
Darby, R. E., 1962. Midges associated with California rice fields with special reference to their ecology (Diptera, Chironomidae). Hilgardia 32: 1–206.
deBont, A. G., 1956. Lutte contre les mollusques dans les eaux africaines. Bull. Agric. Congo Belge 47: 377–380.
DeDutta, S. K., 1987. Principles and practices of rice production. Krieger, Malabar, Florida, 618 pp.
deMora, F., 1923. Un destructor de plantales de arroz. Bol. Soc. Esp. Hist. Nat. Madrid 23: 313–314.
delGuercio, 1913. Le tipule e i tafani nocivi nelle risaie di Molinella (Bologna). Redia 9: 11.
Denishenko, M. (ed.), 1954. Rice cultivation, Bratislava, 173 pp. (Slovak).
Fagi, A. M., S. Suriapermana & I. Syamsiah, 1989. Rice field farming systems in lowland areas. The West Java Case. Pap. Second Asian Reg. Workshop, Rice-Fish Farming Res. Develop., Neuva Ecija, Philippines, 36 pp. (mimeo).
Fedoruk, A. & W. Leelapatra, 1985. Rice field fisheries in Thailand. Fisheries Dept. Publ., Bangkok, 64 pp. (mimeo).
Fernando, C. H., 1956a. The fish fauna of paddy fields and small irrigation ditches in the Western lowlands of Ceylon and a bibliography to references to fish in paddy fields. Ceylon J. Sci. 7: 223–227.
Fernando, C. H., 1956b. The food of four common freshwater fishes of Ceylon. Ceylon J. Sci. 7: 201–217.
Fernando, C. H., 1958. Some aspects of the evolution of animal life histories. In Symp. Darwin-Wallace Centenary. Proc. Ceylon Assn. Adv. Sci.: 225—228.
Fernando, C. H., 1960. Colonization of freshwater habitats with special reference to aquatic insects. In R. D. Purchon (ed.), Proc. Cent. Bicent. Congr. Biol. Singapore: 182–186.
Fernando, C. H., 1962. Freshwater crabs — useful scavengers and food for wild animals, but some damage paddy bunds. Loris 9: 189–191.
Fernando, C. H., 1977. Investigations on the aquatic fauna of tropical rice fields with special reference to South East Asia. GEO-ECO-TROP 3: 169–188.
Fernando, C. H., 1990. Freshwater fauna and fisheries of Sri Lanka. NARESA, Colombo, 444 pp.
Fernando, C. H., J. I. Furtado & R. P. Lim, 1979. The aquatic fauna of the world's rice fields. Wallaceana Suppl. (Kuala Lumpur) 2: 1–105.
Fernando, C. H. & J. Holĉik, 1982. The nature of fish communities: A factor influencing the fishery potential and yields of tropical lakes and reservoirs. Hydrobiologia 97: 127–140.
Fernando, C. H. & J. Holcîk, 1991. Fish in reservoirs. Int. Revue ges. Hydrobiol. 76: 149–167.
Fernando, C. H., C. Tudorancea & Seyoum Mengestou, 1990. Invertebrate zooplankton predator composition and diversity in tropical lentic waters. In H. J. Dumont, J. G. Tundisi & K. Roche (eds), Intrazooplankton Predation. Developments in Hydrobiology 60. Kluwer Academic Publishers, Dordrecht: 13–31. Reprinted from Hydrobiologia 198.
Gosh, C. C., 1923. Crabs damaging paddy in Burma. Proc. Ent. Mtgs. Pusa 5: 141–146.
Grigarick, A. A., W. H. Lange, & D. C. Finfrock, 1961. Control of tadpole shrimp, Triops longicaudatus in California rice fields. J. econ. Ent. 54: 30–40.
Grist, D. H., 1975. Rice. Longman, London, 601 pp.
Grist, D. H. & R. J. A. W. Lever, 1969. Pests of rice. Longmans, Green, London, 520 pp.
Haroon, A. K. Y, S. Dewan & S. M. R. Karin, 1989. Advances in rice-fish production in irrigated rice fields in Bangladesh. Pap. Second Asian Regional Workshop, Rice-Fish Farming Res. Develop., Neuva Ecija, Philippines 11 pp. (mimeo).
Heckman, C. W., 1974. The seasonal succession of species in a rice paddy in Vientianne, Laos. Int. Revue ges. Hydrobiol. 59: 489–507.
Heckman, C. W., 1979. Rice field ecology in Northeastern Thailand. Monographiae Biologicae 34: 1–228, Dr W. Junk Publishers, The Hague.
Hickling, C. F., 1961. Fish culture. Faber and Faber, London, 295 pp.
Hicks, C. S., 1976. Man and natural resources. Croom Helm, London, 122 pp.
Hora, S. L., 1929. Mud fishing in lower Bengal. J. Asiat. Soc. Beng. 30: 197.
Huner, J. V. & H. K. Dupree, 1984. Multiple use of land and water. In H. K. Dupree & J. V. Huner (ed.), The Status of warm water fish farming and progress in fish farming research. U.S. Fish and Wildlife Service Publ.: 220–223.
IRRI, 1988. Vector borne disease control in humans through rice agroecosystem management. Proc. Workshop on research training needs of integrated vector borne disease control in riceland agroecosystems of developing countries. WHO/FAO/UNEP Panel of experts on environmental management for vector control. Manila, 237 pp.
Jangua, N. A., 1957. Insect pests of paddy in Pakistan. Agric. Pakistan 8: 5–21.
Jhingran, V. G., 1982. Fish and fisheries in India. Hindustan Press, Delhi, 666 pp.
Johnson, D. S., 1967. Distributional patterns of Malaysian freshwater fish. Ecology 48: 722–730.
Jones, E. L., 1968. Bloodworms, pests of rice. Agric. Gaz. 79: 477–478.
Jones, E. L., 1975. Aquatic snails attack rice. Farm. Newsl. (Australia) 95: 20–21.
Jordan, H. D., 1957. Crabs as pests of rice in tidal swamps. Emp. J. Exptl. Agric. (Oxford) 25: 197–206.
Kafuku, T., 1989. Paddy-cum-rice culture in Japan, 10 pp. (mimeo).
Kerfoot, W. C. & M. Lynch, 1987. Branchiopod communities: Associations with planktivorous fish in space and time. In W. C. Kerfoot & A. Sih (ed.), Predation, direct and indirect impacts on aquatic communities. University Press, New England, Hanover, 367–378.
Kurasawa, H., 1956. The weekly succession in the standing crop of plankton and zoobenthos in a paddy field. Part 1. Misc. Rep. Inst. Nat. Res. Tokyo 41: 86–98 (Japanese).
Kurasawa, H., 1957. The weekly succession in the standing crop of plankton and zoobenthos in a paddy field. Part 2. Misc. Rep. Inst. Nat. Res. Tokyo 45: 74–89.
Kurihara, Y., 1989. Ecology of some rice fields in Japan as exemplified by some benthic fauna, with notes on management. Int. Revue ges. Hydrobiol. 74: 507–548.
Kuronuma, K., 1954. Carp culture in rice fields as a side work of Japanese farmers. Publ. Freshw. Lab. Min. Agric. Forestry, Government of Japan, 27 pp.
Li, K., 1988a. Rice-fish farming systems, past, present and future. 21 pp. (mimeo).
Li, K., 1988b. Rice-fish culture in China: A review. Aquaculture 71: 173–186.
Lord, L., 1927. Land crabs in paddy fields. Trop. Agric. 69: 141–146.
Lowe-McConnell, R. H., 1975. Fish communities in tropical freshwaters. Longman, London, 337 pp.
Margraf, J. & M. Voggesberger, 1986. Traditionelle Agrarökosysteme der Ifugaos (Provinz, Ifugao, Phillipinen) aus biologischer Sicht. In H. Vogtmann, E. Boechncke & I. Fricke (ed.), Öko-Landbau eine weltweite Notwendigkeit, Verlag C. F. Müller, Karlsruhe: 306–319.
Marten, G. G., 1989. A survey of cyclopoid copepods for control of Aedes albopictus larvae. Bull. Soc. Vector Ecol. 14:232–236.
Masaki, J., 1959. Studies on the crane fly ( Tipula aino Alexander) with special reference to its ecology and control. J. Kanto-Tosan Agric. Exptl. Sta. 13: 1–202 (Japanese).
Matsuo, T., 1955. Rice cultivation in Japan. Yokénda Publ. Japan, 128 pp. (Japanese).
Megyeri, J., 1956. The common horseshoe crab Triops cancriformis , a micro rice pest. Szed. Pedag. Fiosk. Eukon. 7: 33–44 (Hungarian).
Meijen, V. A., 1940. Fish culture in rice fields. Food Industry Publishers. Moscow, 96 pp. (Russian).
Moormann, F. R. & N. Van Breemen, 1978. Rice, soil, water, land. Publ. IRRI, Los Baños, Philippines, 185 pp.
Moretti, G. P., 1955. Biological control exercised by carp on insects in paddy fields. FAO Cons. Gen. de pêches pour la Méditerranée, Rome. Proc. Tech. Pap. 3: 179–185.
Moretti, G. P. & A. Mauri, 1946. Esperimenti di lotta contro l'idrocampa delle risaie. Boll. Zool. Agric. Bach Univ. Milano 13: 1–36.
Moroni, A., 1961. L'ecosistema di risaia (Monografia). Ann. Fac. Agr. dell'U.C.S.C. 31: 8–53.
Mukhemediev, A. M., 1960. Contributions to the hydrobiology of rice fields of the Fergana Valley. Uchenye zapisky uzbekskogo gosudarstvennogo pedagogitcheskogo instituta 6: 1–82. (Russian).
Munro, A. D., 1990. Tropical freshwater fishes. In A. D. Munro, A. P. Scott & T. J. Cam (ed.), Reproductive seasonality in teleosts: Environmental influences. CRC Press, Florida: 145–239.
Muzarov, A. M., M. A. Kucharova & E. K. Mikhailova, 1980. Prospects for the use of bluegreen algae in rice cultivation. Vsb. Kul'tivirovanie i primenenie mikrovodoroslei v narodnom khozyaistve, Tashkent: 8–20 (Russian).
Nalinda, M. A. K., 1988. Checklist of fishes (Pisces) of the Bellanwila-Attidiya marshes. Young Zoologists Assn. Sri Lanka Occ. Pap. 3: 1–4.
Nishida, T. & T. Torii, 1970. A handbook of field methods for research on rice stem borers and their natural enemies. IBP Handbook, 14: 1–132.
Novelli, N., 1926. Rizicultura e malaria. Riz et Rizic. 1: 409–414.
Onderiková, V., 1955. Contributions to the hydrobiology of rice fields. Sbornik Polnohos. Vied.: 32–55 (Slovak).
Onderiková, V., 1957. The food of common carp in rice fields. Biologia, Bratislava, 12: 693–698 (Slovak).
Paddock, J., N. Paddock & C. Bly, 1986. Soil and survival. Sierra Club, San Francisco, 217 pp.
Pantastico, J. B. & Z. A. Suayan, 1973. Algal succession in the rice fields of college and Bay Laguna. Philipp. Agric. J. 57: 313–326.
Pao-Ling, L., 1984. The wet irrigation method of mosquito control in rice fields. An experience of intermittent irrigation in China. In T. H. Mather (ed.), Environmental management for vector control in rice fields. FAO Irrigation and Drainage Paper 41: 133–143.
Patil, S. S., 1958. Crab control in paddy fields. The Farmer 9: 19–22.
Pazhitnova, Z. A., 1929. Materialien zur Erforschung der Mikrofauna der Reisfelder und die Biologie der Anopheles — Larvae auf den Reisfeldern. Trudy sredneaziatskogo Universiteta (Zool.), 10: 1–40 (Russian, German summary).
Pazhitnova, Z. A., 1935. Microfauna of a typical rice field in Samarkand Oasis. Trudy sredneaziatskogo Universiteta (Zool.), 18: 1–64. (Russian, German summary).
Pont, D., 1977. Structure et évolution saisonnière de populations de copépodes, cladocères et ostracodes des rizières de Camargue. Ann. Limnol., 13: 15–28.
Ramakrishna Ayyar, T. V., 1935. A fish pest of paddy-fields along the Coromandel coast ( Ophichthys boro ). J. Bombay Nat. Hist. Soc. 36: 276–278.
Risbec, J., 1952. Les insectes nuisibles au riz dans le midi de la France. Les Riziculteurs de France. Bull. Etud. Rech. Tech., 18: 14–19.
Risbec, J. & H. Mallamaire, 1949. Les animaux prédateurs et les insectes parasites du riz cultivé en Afrique occidentale. Agron. trop. (Paris) 4: 70–76.
Rivière, F., B. H. Kay, J. M. Klein & Y. Sechan, 1987. Mesocyclops aspericornis (Copepoda) and Bacillus thuringiensis var. israelensis for the biological control of Aedes and Culex vectors (Diptera: Culicidae) breeding in crab holes, and artificial containers. J. Med. Ent. 24: 425–430.
Roger, P. A. & S. A. Kulasooriya, 1980. Blue green algae and rice. Publ. IRRI, Los Baños, Philippines, 112 pp.
Roger, P. A. & Y. Kurihara, 1988. Floodwater biology of tropical wetland rice fields. Pap. Int. Symp. Paddy Soil and Fertility, Chiangmai, Thailand, 1988, 28 pp. (mimeo).
Rosenberg, L. E., 1947. Apus as a pest in California rice fields. Calif. Agric. Dept. Bull. 36: 42–48.
Ruddle, K., 1982. Traditional integrated farming systems and rural development. The example of rice field fisheries in Southeast Asia. Agric. Admin., 10: 1–11.
Sachlan, M. & Zahir, 1942. Pemriksaan fauna dan flora di Sawah (quoted in Ardiwinata, 1957) (Indonesia Bahasa).
Schachter, D. & M. Conat, 1951. Note préliminairè sur la faune de rizières. Bull. Soc. Zool. Fr. 74: 365–371.
Service, M. W., 1989a. Rice, a challenge to health. Parasitology today. 5: 162–165.
Service, M. W., 1989b. Irrigation: Boon or bane? In M. W. Service (ed.), Demography and vector borne disease. Boca Raton, Florida. CRC Press. 237–254.
Service, M. W., 1991. Agricultural development and Arthropod-borne disease: A review. Rev. Saúde Publ. S. Paulo. 25:
Sevilleja, R. C., 1988. Rice-fish farming development in the Philippines, past, present and future. ICLARM Workshop Rice-Fish Farming Research Development, Ubon, Thailand, 40 pp. (mimeo).
Šilenko, J. V., 1981. Culture of fish in rice irrigation systems under conditions of modern technology of rice culture. Proc. State Symp. Complex Fishery Management, Krasnodar: 50–52 (Russian).
Sivickis, P. B., 1929. Modes of distribution of the mud fishes of the Philippines. Nature 123: 493.
Soong, M. K., 1949. Fishes of the Malayan padi-fields 1. Sepat Siam (gourami). Malay. Nat. J. 2: 1–3.
Soong, M. K., 1949. Fishes of the Malayan padi-fields 2. Aruan (snakeheads). Malay. Nat. J. 4: 29–31.
Soong, M. K., 1950. Fishes of the Malayan padi-fields 3. Keli (catfishes). Malay. Nat. J. 5: 88–91.
Stanley, N. F. & M. P. Alpers (ed.), 1975. Man-made lakes and human health. Academic Press, New York, 495 pp.
Suchoverchov, F. M., 1946. Fisheries in rice fields. Moscow, 116 pp. (Russian).
Surtees, G., 1975., Mosquitoes, arboviruses and vertebrates. In N. F. Stanley & M. P. Alpers (ed.), Man-made lakes and human health. Academic Press, New York: 21–34.
Swaminathan, M. S., 1984. Rice. Scientific American250: 81–93.
Szabó, G. M., 1949. Qualitative und quantitative Verteilung der Planktonorganismen auf Reisfeldern. Revue Suisse Hydrobiol.: 618–622.
Szilvássy, L., 1959. New observations on the damage due to the summer horse-shoe crab ( Triops cancriformis ). Növénytermelés 8: 361–363 (Hungarian).
Szilvássy, L., 1964. Die Arthropodenschädlinge der ungarischen Reisfelder und Massnahmen zu ihrer Bekämpfung. Beitr. trop. und subtrop. Landwirschaft und Tropenveterinärmed., 1: 29–44.
Szilvássy, C. & A. Szító, 1960. Der Schaden von Hydrellia griseola Fall. an Reis in Ungarn im Jahre 1959. Növenytermelés 9: 171–173 (Hungarian).
Szító, A., 1972. Insect pests of rice and new methods of protection against them. Önt. Gazd., 9: 107–116 (Hungarian).
Szító, A., 1976. On the overwintering niches of the rice fly Hydrellia griseola Fall. Folia Ent. Hung. (NS), 19: 103–106. (Hungarian).
Tamura, T., 1961. Carp cultivation in Japan. In G. Borgstrom (ed.), Fish as food. Academic Press, New York: 103–120.
Tan, C. E., B. J. Chong, H. K. Sier & T. Moulton, 1973. A report on paddy-field fish production in Krian, Perak. Min. Agric. Fish. Malaysia Bull. No. 128: 57 pp.
Temprosa, R. M. & Z. H. Shehadeh, 1980. Preliminary bibliography of rice-fish culture. ICLARM (Manila) 20 pp.
Timon-David, V., 1949. Une menace pour la riziculture en Camargue. La mouche du riz. C.R. Acad. Agric. Fr. 35: 382.
Ting, Y., 1961. Chinese culture of lowland rice. Agricultural Publishing Society, Peking (Chinese).
Tonolli, V., 1955. Carp culture in rice fields. Proc. Tech. Pap. No. 3. Gen. Fish. Counc. Medit. FAO, Rome: 151–159.
Vranovský, M., 1955. Zooplankton of two rice fields at Okoch in Zitný Ostrov. Diploma Thesis, Comenius University, Bratislava, 77 pp. (Slovak).
Washino, P. R., K. G. Whitesell, E. J. Sherman & R. J. McKenna, 1972. Rice field mosquito control studies with low volume Dursban sprays in Colusa county, California. 3. Effects on target organisms. Mosquito News. 32: 357–382.
Watanabe, I. & C. Furusaka, 1980. Microbial ecology of flooded rice fields. Adv. Micro. Ecol. 4: 125–168.
Weeraratna, S. & C. H. Fernando, 1984. Some aspects of the ecology of rice fields. In C. H. Fernando (ed.), Ecology and Biogeography of Sri Lanka. Monographiae Biologicae 57: 133–144, Dr W. Junk Publishers, The Hague.
Weerekoon, A. C. J., 1957. Some animals of the paddy field. Loris 7: 1–8.
Weerekoon, A. C. J. & E. L. Samarasinghe, 1958. Mesofauna of the soil of a paddy field in Ceylon. Preliminary survey. Ceylon J. Sci. (Bio. Sci.) 1: 155–170.
Welcomme, R. L., 1979. Fisheries ecology of floodplain rivers. Longman, London, 317 pp.
Welcomme, R. L., 1985. River Fisheries. FAO Fish. Tech. Pap. 262: 330 pp.
Williams, W. D., 1988. Limnological imbalances: An antipodean viewpoint. Freshwater Biol. 20: 407–420.
Yunus, A. & G. S. Lim, 1971. A problem in the use of insecticides in paddy fields in Western Malaysia: A case study. Malay. Agric. J. 48: 168–178.
Zazou, M. H., A. K. M. el-Nahial & M. A. Bishara, 1971. Studies on the control of the rice field fly, Ephydra macellaria Egger (Ephydridae) and the blood worm Chironomus sp. (Chironomidae) (Diptera). Bull. ent. soc. Egypt Econ. Ser. 5: 13–17.
Zozaya, C., 1943. Paludismo y arrozales. Rev. Fac. Med. 11: 448–476.
Download references
Author information
Authors and affiliations.
Department of Biology, University of Waterloo, N2L 3G1, Waterloo, Ontario, Canada
C. H. Fernando
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Fernando, C.H. Rice field ecology and fish culture — an overview. Hydrobiologia 259 , 91–113 (1993). https://doi.org/10.1007/BF00008375
Download citation
Received : 05 March 1992
Revised : 13 October 1992
Accepted : 29 October 1992
Issue Date : May 1993
DOI : https://doi.org/10.1007/BF00008375
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Rice fields
- fish culture
- Find a journal
- Publish with us
- Track your research
- Skip to main content
- Keyboard shortcuts for audio player
- Latest Show
- Terry Gross
- Tonya Mosley
- Contact Fresh Air
- Subscribe to Breaking News Alerts

Author Interviews
Barbara walters forged a path for women in journalism, but not without paying a price.
Walters was the first woman to co-anchor a national news show on prime time television. "The path she cut is one that many of us have followed," says biographer Susan Page, author of The Rulebreaker.

by Tonya Mosley
Music Reviews
Taylor swift's confessional 'tortured poets' would have benefitted from an edit.
by Ken Tucker
- See Fresh Air sponsors and promo codes
- Open access
- Published: 23 April 2024
Designing feedback processes in the workplace-based learning of undergraduate health professions education: a scoping review
- Javiera Fuentes-Cimma 1 , 2 ,
- Dominique Sluijsmans 3 ,
- Arnoldo Riquelme 4 ,
- Ignacio Villagran ORCID: orcid.org/0000-0003-3130-8326 1 ,
- Lorena Isbej ORCID: orcid.org/0000-0002-4272-8484 2 , 5 ,
- María Teresa Olivares-Labbe 6 &
- Sylvia Heeneman 7
BMC Medical Education volume 24 , Article number: 440 ( 2024 ) Cite this article
217 Accesses
Metrics details
Feedback processes are crucial for learning, guiding improvement, and enhancing performance. In workplace-based learning settings, diverse teaching and assessment activities are advocated to be designed and implemented, generating feedback that students use, with proper guidance, to close the gap between current and desired performance levels. Since productive feedback processes rely on observed information regarding a student's performance, it is imperative to establish structured feedback activities within undergraduate workplace-based learning settings. However, these settings are characterized by their unpredictable nature, which can either promote learning or present challenges in offering structured learning opportunities for students. This scoping review maps literature on how feedback processes are organised in undergraduate clinical workplace-based learning settings, providing insight into the design and use of feedback.
A scoping review was conducted. Studies were identified from seven databases and ten relevant journals in medical education. The screening process was performed independently in duplicate with the support of the StArt program. Data were organized in a data chart and analyzed using thematic analysis. The feedback loop with a sociocultural perspective was used as a theoretical framework.
The search yielded 4,877 papers, and 61 were included in the review. Two themes were identified in the qualitative analysis: (1) The organization of the feedback processes in workplace-based learning settings, and (2) Sociocultural factors influencing the organization of feedback processes. The literature describes multiple teaching and assessment activities that generate feedback information. Most papers described experiences and perceptions of diverse teaching and assessment feedback activities. Few studies described how feedback processes improve performance. Sociocultural factors such as establishing a feedback culture, enabling stable and trustworthy relationships, and enhancing student feedback agency are crucial for productive feedback processes.
Conclusions
This review identified concrete ideas regarding how feedback could be organized within the clinical workplace to promote feedback processes. The feedback encounter should be organized to allow follow-up of the feedback, i.e., working on required learning and performance goals at the next occasion. The educational programs should design feedback processes by appropriately planning subsequent tasks and activities. More insight is needed in designing a full-loop feedback process, in which specific attention is needed in effective feedforward practices.
Peer Review reports
The design of effective feedback processes in higher education has been important for educators and researchers and has prompted numerous publications discussing potential mechanisms, theoretical frameworks, and best practice examples over the past few decades. Initially, research on feedback primarily focused more on teachers and feedback delivery, and students were depicted as passive feedback recipients [ 1 , 2 , 3 ]. The feedback conversation has recently evolved to a more dynamic emphasis on interaction, sense-making, outcomes in actions, and engagement with learners [ 2 ]. This shift aligns with utilizing the feedback process as a form of social interaction or dialogue to enhance performance [ 4 ]. Henderson et al. (2019) defined feedback processes as "where the learner makes sense of performance-relevant information to promote their learning." (p. 17). When a student grasps the information concerning their performance in connection to the desired learning outcome and subsequently takes suitable action, a feedback loop is closed so the process can be regarded as successful [ 5 , 6 ].
Hattie and Timperley (2007) proposed a comprehensive perspective on feedback, the so-called feedback loop, to answer three key questions: “Where am I going? “How am I going?” and “Where to next?” [ 7 ]. Each question represents a key dimension of the feedback loop. The first is the feed-up, which consists of setting learning goals and sharing clear objectives of learners' performance expectations. While the concept of the feed-up might not be consistently included in the literature, it is considered to be related to principles of effective feedback and goal setting within educational contexts [ 7 , 8 ]. Goal setting allows students to focus on tasks and learning, and teachers to have clear intended learning outcomes to enable the design of aligned activities and tasks in which feedback processes can be embedded [ 9 ]. Teachers can improve the feed-up dimension by proposing clear, challenging, but achievable goals [ 7 ]. The second dimension of the feedback loop focuses on feedback and aims to answer the second question by obtaining information about students' current performance. Different teaching and assessment activities can be used to obtain feedback information, and it can be provided by a teacher or tutor, a peer, oneself, a patient, or another coworker. The last dimension of the feedback loop is the feedforward, which is specifically associated with using feedback to improve performance or change behaviors [ 10 ]. Feedforward is crucial in closing the loop because it refers to those specific actions students must take to reduce the gap between current and desired performance [ 7 ].
From a sociocultural perspective, feedback processes involve a social practice consisting of intricate relationships within a learning context [ 11 ]. The main feature of this approach is that students learn from feedback only when the feedback encounter includes generating, making sense of, and acting upon the information given [ 11 ]. In the context of workplace-based learning (WBL), actionable feedback plays a crucial role in enabling learners to leverage specific feedback to enhance their performance, skills, and conceptual understandings. The WBL environment provides students with a valuable opportunity to gain hands-on experience in authentic clinical settings, in which students work more independently on real-world tasks, allowing them to develop and exhibit their competencies [ 3 ]. However, WBL settings are characterized by their unpredictable nature, which can either promote self-directed learning or present challenges in offering structured learning opportunities for students [ 12 ]. Consequently, designing purposive feedback opportunities within WBL settings is a significant challenge for clinical teachers and faculty.
In undergraduate clinical education, feedback opportunities are often constrained due to the emphasis on clinical work and the absence of dedicated time for teaching [ 13 ]. Students are expected to perform autonomously under supervision, ideally achieved by giving them space to practice progressively and providing continuous instances of constructive feedback [ 14 ]. However, the hierarchy often present in clinical settings places undergraduate students in a dependent position, below residents and specialists [ 15 ]. Undergraduate or junior students may have different approaches to receiving and using feedback. If their priority is meeting the minimum standards given pass-fail consequences and acting merely as feedback recipients, other incentives may be needed to engage with the feedback processes because they will need more learning support [ 16 , 17 ]. Adequate supervision and feedback have been recognized as vital educational support in encouraging students to adopt a constructive learning approach [ 18 ]. Given that productive feedback processes rely on observed information regarding a student's performance, it is imperative to establish structured teaching and learning feedback activities within undergraduate WBL settings.
Despite the extensive research on feedback, a significant proportion of published studies involve residents or postgraduate students [ 19 , 20 ]. Recent reviews focusing on feedback interventions within medical education have clearly distinguished between undergraduate medical students and residents or fellows [ 21 ]. To gain a comprehensive understanding of initiatives related to actionable feedback in the WBL environment for undergraduate health professions, a scoping review of the existing literature could provide insight into how feedback processes are designed in that context. Accordingly, the present scoping review aims to answer the following research question: How are the feedback processes designed in the undergraduate health professions' workplace-based learning environments?
A scoping review was conducted using the five-step methodological framework proposed by Arksey and O'Malley (2005) [ 22 ], intertwined with the PRISMA checklist extension for scoping reviews to provide reporting guidance for this specific type of knowledge synthesis [ 23 ]. Scoping reviews allow us to study the literature without restricting the methodological quality of the studies found, systematically and comprehensively map the literature, and identify gaps [ 24 ]. Furthermore, a scoping review was used because this topic is not suitable for a systematic review due to the varied approaches described and the large difference in the methodologies used [ 21 ].
Search strategy
With the collaboration of a medical librarian, the authors used the research question to guide the search strategy. An initial meeting was held to define keywords and search resources. The proposed search strategy was reviewed by the research team, and then the study selection was conducted in two steps:
An online database search included Medline/PubMed, Web of Science, CINAHL, Cochrane Library, Embase, ERIC, and PsycINFO.
A directed search of ten relevant journals in the health sciences education field (Academic Medicine, Medical Education, Advances in Health Sciences Education, Medical Teacher, Teaching and Learning in Medicine, Journal of Surgical Education, BMC Medical Education, Medical Education Online, Perspectives on Medical Education and The Clinical Teacher) was performed.
The research team conducted a pilot or initial search before the full search to identify if the topic was susceptible to a scoping review. The full search was conducted in November 2022. One team member (MO) identified the papers in the databases. JF searched in the selected journals. Authors included studies written in English due to feasibility issues, with no time span limitation. After eliminating duplicates, two research team members (JF and IV) independently reviewed all the titles and abstracts using the exclusion and inclusion criteria described in Table 2 and with the support of the screening application StArT [ 25 ]. A third team member (AR) reviewed the titles and abstracts when the first two disagreed. The reviewer team met again at a midpoint and final stage to discuss the challenges related to study selection. Articles included for full-text review were exported to Mendeley. JF independently screened all full-text papers, and AR verified 10% for inclusion. The authors did not analyze study quality or risk of bias during study selection, which is consistent with conducting a scoping review.
The analysis of the results incorporated a descriptive summary and a thematic analysis, which was carried out to clarify and give consistency to the results' reporting [ 22 , 24 , 26 ]. Quantitative data were analyzed to report the characteristics of the studies, populations, settings, methods, and outcomes. Qualitative data were labeled, coded, and categorized into themes by three team members (JF, SH, and DS). The feedback loop framework with a sociocultural perspective was used as the theoretical framework to analyze the results.
The keywords used for the search strategies were as follows:
Clinical clerkship; feedback; formative feedback; health professions; undergraduate medical education; workplace.
Definitions of the keywords used for the present review are available in Appendix 1 .
As an example, we included the search strategy that we used in the Medline/PubMed database when conducting the full search:
("Formative Feedback"[Mesh] OR feedback) AND ("Workplace"[Mesh] OR workplace OR "Clinical Clerkship"[Mesh] OR clerkship) AND (("Education, Medical, Undergraduate"[Mesh] OR undergraduate health profession*) OR (learner* medical education)).
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were used (Table 1 ):
Data extraction
The research group developed a data-charting form to organize the information obtained from the studies. The process was iterative, as the data chart was continuously reviewed and improved as necessary. In addition, following Levac et al.'s recommendation (2010), the three members involved in the charting process (JF, LI, and IV) independently reviewed the first five selected studies to determine whether the data extraction was consistent with the objectives of this scoping review and to ensure consistency. Then, the team met using web-conferencing software (Zoom; CA, USA) to review the results and adjust any details in the chart. The same three members extracted data independently from all the selected studies, considering two members reviewing each paper [ 26 ]. A third team member was consulted if any conflict occurred when extracting data. The data chart identified demographic patterns and facilitated the data synthesis. To organize data, we used a shared Excel spreadsheet, considering the following headings: title, author(s), year of publication, journal/source, country/origin, aim of the study, research question (if any), population/sample size, participants, discipline, setting, methodology, study design, data collection, data analysis, intervention, outcomes, outcomes measure, key findings, and relation of findings to research question.
Additionally, all the included papers were uploaded to AtlasTi v19 to facilitate the qualitative analysis. Three team members (JF, SH, and DS) independently coded the first six papers to create a list of codes to ensure consistency and rigor. The group met several times to discuss and refine the list of codes. Then, one member of the team (JF) used the code list to code all the rest of the papers. Once all papers were coded, the team organized codes into descriptive themes aligned with the research question.
Preliminary results were shared with a number of stakeholders (six clinical teachers, ten students, six medical educators) to elicit their opinions as an opportunity to build on the evidence and offer a greater level of meaning, content expertise, and perspective to the preliminary findings [ 26 ]. No quality appraisal of the studies is considered for this scoping review, which aligns with the frameworks for guiding scoping reviews [ 27 ].
The datasets analyzed during the current study are available from the corresponding author upon request.
A database search resulted in 3,597 papers, and the directed search of the most relevant journals in the health sciences education field yielded 2,096 titles. An example of the results of one database is available in Appendix 2 . Of the titles obtained, 816 duplicates were eliminated, and the team reviewed the titles and abstracts of 4,877 papers. Of these, 120 were selected for full-text review. Finally, 61 papers were included in this scoping review (Fig. 1 ), as listed in Table 2 .
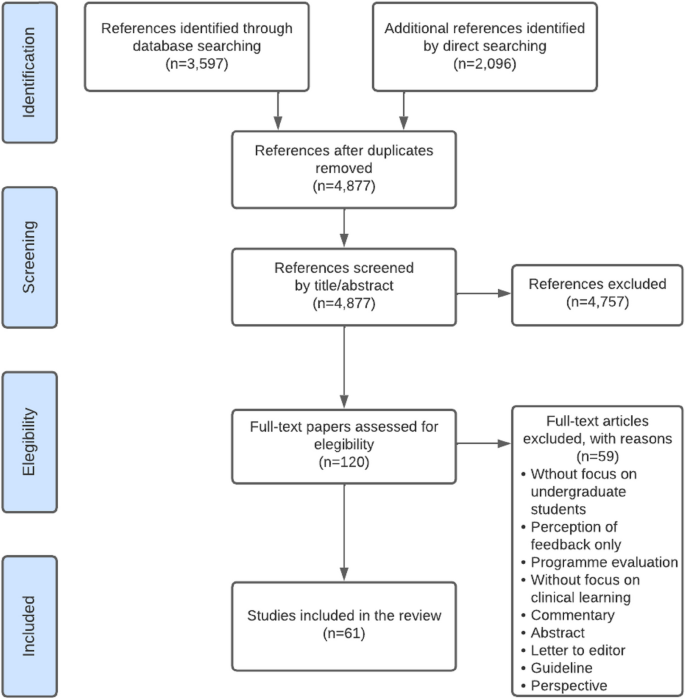
PRISMA flow diagram for included studies, incorporating records identified through the database and direct searching
The selected studies were published between 1986 and 2022, and seventy-five percent (46) were published during the last decade. Of all the articles included in this review, 13% (8) were literature reviews: one integrative review [ 28 ] and four scoping reviews [ 29 , 30 , 31 , 32 ]. Finally, fifty-three (87%) original or empirical papers were included (i.e., studies that answered a research question or achieved a research purpose through qualitative or quantitative methodologies) [ 15 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 ].
Table 2 summarizes the papers included in the present scoping review, and Table 3 describes the characteristics of the included studies.
The thematic analysis resulted in two themes: (1) the organization of feedback processes in WBL settings, and (2) sociocultural factors influencing the organization of feedback processes. Table 4 gives a summary of the themes and subthemes.
Organization of feedback processes in WBL settings.
Setting learning goals (i.e., feed-up dimension).
Feedback that focuses on students' learning needs and is based on known performance standards enhances student response and setting learning goals [ 30 ]. Discussing goals and agreements before starting clinical practice enhances students' feedback-seeking behavior [ 39 ] and responsiveness to feedback [ 83 ]. Farrell et al. (2017) found that teacher-learner co-constructed learning goals enhance feedback interactions and help establish educational alliances, improving the learning experience [ 50 ]. However, Kiger (2020) found that sharing individualized learning plans with teachers aligned feedback with learning goals but did not improve students' perceived use of feedback [ 64 ]
Two papers of this set pointed out the importance of goal-oriented feedback, a dynamic process that depends on discussion of goal setting between teachers and students [ 50 ] and influences how individuals experience, approach, and respond to upcoming learning activities [ 34 ]. Goal-oriented feedback should be embedded in the learning experience of the clinical workplace, as it can enhance students' engagement in safe feedback dialogues [ 50 ]. Ideally, each feedback encounter in the WBL context should conclude, in addition to setting a plan of action to achieve the desired goal, with a reflection on the next goal [ 50 ].
Feedback strategies within the WBL environment. (i.e., feedback dimension)
In undergraduate WBL environments, there are several tasks and feedback opportunities organized in the undergraduate clinical workplace that can enable feedback processes:
Questions from clinical teachers to students are a feedback strategy [ 74 ]. There are different types of questions that the teacher can use, either to clarify concepts, to reach the correct answer, or to facilitate self-correction [ 74 ]. Usually, questions can be used in conjunction with other communication strategies, such as pauses, which enable self-correction by the student [ 74 ]. Students can also ask questions to obtain feedback on their performance [ 54 ]. However, question-and-answer as a feedback strategy usually provides information on either correct or incorrect answers and fewer suggestions for improvement, rendering it less constructive as a feedback strategy [ 82 ].
Direct observation of performance by default is needed to be able to provide information to be used as input in the feedback process [ 33 , 46 , 49 , 86 ]. In the process of observation, teachers can include clarification of objectives (i.e., feed-up dimension) and suggestions for an action plan (i.e., feedforward) [ 50 ]. Accordingly, Schopper et al. (2016) showed that students valued being observed while interviewing patients, as they received feedback that helped them become more efficient and effective as interviewers and communicators [ 33 ]. Moreover, it is widely described that direct observation improves feedback credibility [ 33 , 40 , 84 ]. Ideally, observation should be deliberate [ 33 , 83 ], informal or spontaneous [ 33 ], conducted by a (clinical) expert [ 46 , 86 ], provided immediately after the observation, and clinical teacher if possible, should schedule or be alert on follow-up observations to promote closing the gap between current and desired performance [ 46 ].
Workplace-based assessments (WBAs), by definition, entail direct observation of performance during authentic task demonstration [ 39 , 46 , 56 , 87 ]. WBAs can significantly impact behavioral change in medical students [ 55 ]. Organizing and designing formative WBAs and embedding these in a feedback dialogue is essential for effective learning [ 31 ].
Summative organization of WBAs is a well described barrier for feedback uptake in the clinical workplace [ 35 , 46 ]. If feedback is perceived as summative, or organized as a pass-fail decision, students may be less inclined to use the feedback for future learning [ 52 ]. According to Schopper et al. (2016), using a scale within a WBA makes students shift their focus during the clinical interaction and see it as an assessment with consequences [ 33 ]. Harrison et al. (2016) pointed out that an environment that only contains assessments with a summative purpose will not lead to a culture of learning and improving performance [ 56 ]. The recommendation is to separate the formative and summative WBAs, as feedback in summative instances is often not recognized as a learning opportunity or an instance to seek feedback [ 54 ]. In terms of the design, an organizational format is needed to clarify to students how formative assessments can promote learning from feedback [ 56 ]. Harrison et al. (2016) identified that enabling students to have more control over their assessments, designing authentic assessments, and facilitating long-term mentoring could improve receptivity to formative assessment feedback [ 56 ].
Multiple WBA instruments and systems are reported in the literature. Sox et al. (2014) used a detailed evaluation form to help students improve their clinical case presentation skills. They found that feedback on oral presentations provided by supervisors using a detailed evaluation form improved clerkship students’ oral presentation skills [ 78 ]. Daelmans et al. (2006) suggested that a formal in-training assessment programme composed by 19 assessments that provided structured feedback, could promote observation and verbal feedback opportunities through frequent assessments [ 43 ]. However, in this setting, limited student-staff interactions still hindered feedback follow-up [ 43 ]. Designing frequent WBA improves feedback credibility [ 28 ]. Long et al. (2021) emphasized that students' responsiveness to assessment feedback hinges on its perceived credibility, underlining the importance of credibility for students to effectively engage and improve their performance [ 31 ].
The mini-CEX is one of the most widely described WBA instruments in the literature. Students perceive that the mini-CEX allows them to be observed and encourages the development of interviewing skills [ 33 ]. The mini-CEX can provide feedback that improves students' clinical skills [ 58 , 60 ], as it incorporates a structure for discussing the student's strengths and weaknesses and the design of a written action plan [ 39 , 80 ]. When mini-CEXs are incorporated as part of a system of WBA, such as programmatic assessment, students feel confident in seeking feedback after observation, and being systematic allows for follow-up [ 39 ]. Students suggested separating grading from observation and using the mini-CEX in more informal situations [ 33 ].
Clinical encounter cards allow students to receive weekly feedback and make them request more feedback as the clerkship progresses [ 65 ]. Moreover, encounter cards stimulate that feedback is given by supervisors, and students are more satisfied with the feedback process [ 72 ]. With encounter card feedback, students are responsible for asking a supervisor for feedback before a clinical encounter, and supervisors give students written and verbal comments about their performance after the encounter [ 42 , 72 ]. Encounter cards enhance the use of feedback and add approximately one minute to the length of the clinical encounter, so they are well accepted by students and supervisors [ 72 ]. Bennett (2006) identified that Instant Feedback Cards (IFC) facilitated mid-rotation feedback [ 38 ]. Feedback encounter card comments must be discussed between students and supervisors; otherwise, students may perceive it as impersonal, static, formulaic, and incomplete [ 59 ].
Self-assessments can change students' feedback orientation, transforming them into coproducers of learning [ 68 ]. Self-assessments promote the feedback process [ 68 ]. Some articles emphasize the importance of organizing self-assessments before receiving feedback from supervisors, for example, discussing their appraisal with the supervisor [ 46 , 52 ]. In designing a feedback encounter, starting with a self-assessment as feed-up, discussing with the supervisor, and identifying areas for improvement is recommended, as part of the feedback dialogue [ 68 ].
Peer feedback as an organized activity allows students to develop strategies to observe and give feedback to other peers [ 61 ]. Students can act as the feedback provider or receiver, fostering understanding of critical comments and promoting evaluative judgment for their clinical practice [ 61 ]. Within clerkships, enabling the sharing of feedback information among peers allows for a better understanding and acceptance of feedback [ 52 ]. However, students can find it challenging to take on the peer assessor/feedback provider role, as they prefer to avoid social conflicts [ 28 , 61 ]. Moreover, it has been described that they do not trust the judgment of their peers because they are not experts, although they know the procedures, tasks, and steps well and empathize with their peer status in the learning process [ 61 ].
Bedside-teaching encounters (BTEs) provide timely feedback and are an opportunity for verbal feedback during performance [ 74 ]. Rizan et al. (2014) explored timely feedback delivered within BTEs and determined that it promotes interaction that constructively enhances learner development through various corrective strategies (e.g., question and answers, pauses, etc.). However, if the feedback given during the BTEs was general, unspecific, or open-ended, it could go unnoticed [ 74 ]. Torre et al. (2005) investigated which integrated feedback activities and clinical tasks occurred on clerkship rotations and assessed students' perceived quality in each teaching encounter [ 81 ]. The feedback activities reported were feedback on written clinical history, physical examination, differential diagnosis, oral case presentation, a daily progress note, and bedside feedback. Students considered all these feedback activities high-quality learning opportunities, but they were more likely to receive feedback when teaching was at the bedside than at other teaching locations [ 81 ].
Case presentations are an opportunity for feedback within WBL contexts [ 67 , 73 ]. However, both students and supervisors struggled to identify them as feedback moments, and they often dismissed questions and clarifications around case presentations as feedback [ 73 ]. Joshi (2017) identified case presentations as a way for students to ask for informal or spontaneous supervisor feedback [ 63 ].
Organization of follow-up feedback and action plans (i.e., feedforward dimension).
Feedback that generates use and response from students is characterized by two-way communication and embedded in a dialogue [ 30 ]. Feedback must be future-focused [ 29 ], and a feedback encounter should be followed by planning the next observation [ 46 , 87 ]. Follow-up feedback could be organized as a future self-assessment, reflective practice by the student, and/or a discussion with the supervisor or coach [ 68 ]. The literature describes that a lack of student interaction with teachers makes follow-up difficult [ 43 ]. According to Haffling et al. (2011), follow-up feedback sessions improve students' satisfaction with feedback compared to students who do not have follow-up sessions. In addition, these same authors reported that a second follow-up session allows verification of improved performances or confirmation that the skill was acquired [ 55 ].
Although feedback encounter forms are a recognized way of obtaining information about performance (i.e., feedback dimension), the literature does not provide many clear examples of how they may impact the feedforward phase. For example, Joshi et al. (2016) consider a feedback form with four fields (i.e., what did you do well, advise the student on what could be done to improve performance, indicate the level of proficiency, and personal details of the tutor). In this case, the supervisor highlighted what the student could improve but not how, which is the missing phase of the co-constructed action plan [ 63 ]. Whichever WBA instrument is used in clerkships to provide feedback, it should include a "next steps" box [ 44 ], and it is recommended to organize a long-term use of the WBA instrument so that those involved get used to it and improve interaction and feedback uptake [ 55 ]. RIME-based feedback (Reporting, Interpreting, Managing, Educating) is considered an interesting example, as it is perceived as helpful to students in knowing what they need to improve in their performance [ 44 ]. Hochberg (2017) implemented formative mid-clerkship assessments to enhance face-to-face feedback conversations and co-create an improvement plan [ 59 ]. Apps for structuring and storing feedback improve the amount of verbal and written feedback. In the study of Joshi et al. (2016), a reasonable proportion of students (64%) perceived that these app tools help them improve their performance during rotations [ 63 ].
Several studies indicate that an action plan as part of the follow-up feedback is essential for performance improvement and learning [ 46 , 55 , 60 ]. An action plan corresponds to an agreed-upon strategy for improving, confirming, or correcting performance. Bing-You et al. (2017) determined that only 12% of the articles included in their scoping review incorporated an action plan for learners [ 32 ]. Holmboe et al. (2004) reported that only 11% of the feedback sessions following a mini-CEX included an action plan [ 60 ]. Suhoyo et al. (2017) also reported that only 55% of mini-CEX encounters contained an action plan [ 80 ]. Other authors reported that action plans are not commonly offered during feedback encounters [ 77 ]. Sokol-Hessner et al. (2010) implemented feedback card comments with a space to provide written feedback and a specific action plan. In their results, 96% contained positive comments, and only 5% contained constructive comments [ 77 ]. In summary, although the recommendation is to include a “next step” box in the feedback instruments, evidence shows these items are not often used for constructive comments or action plans.
Sociocultural factors influencing the organization of feedback processes.
Multiple sociocultural factors influence interaction in feedback encounters, promoting or hampering the productivity of the feedback processes.
Clinical learning culture
Context impacts feedback processes [ 30 , 82 ], and there are barriers to incorporating actionable feedback in the clinical learning context. The clinical learning culture is partly determined by the clinical context, which can be unpredictable [ 29 , 46 , 68 ], as the available patients determine learning opportunities. Supervisors are occupied by a high workload, which results in limited time or priority for teaching [ 35 , 46 , 48 , 55 , 68 , 83 ], hindering students’ feedback-seeking behavior [ 54 ], and creating a challenge for the balance between patient care and student mentoring [ 35 ].
Clinical workplace culture does not always purposefully prioritize instances for feedback processes [ 83 , 84 ]. This often leads to limited direct observation [ 55 , 68 ] and the provision of poorly informed feedback. It is also evident that this affects trust between clinical teachers and students [ 52 ]. Supervisors consider feedback a low priority in clinical contexts [ 35 ] due to low compensation and lack of protected time [ 83 ]. In particular, lack of time appears to be the most significant and well-known barrier to frequent observation and workplace feedback [ 35 , 43 , 48 , 62 , 67 , 83 ].
The clinical environment is hierarchical [ 68 , 80 ] and can make students not consider themselves part of the team and feel like a burden to their supervisor [ 68 ]. This hierarchical learning environment can lead to unidirectional feedback, limit dialogue during feedback processes, and hinder the seeking, uptake, and use of feedback [ 67 , 68 ]. In a learning culture where feedback is not supported, learners are less likely to want to seek it and feel motivated and engaged in their learning [ 83 ]. Furthermore, it has been identified that clinical supervisors lack the motivation to teach [ 48 ] and the intention to observe or reobserve performance [ 86 ].
In summary, the clinical context and WBL culture do not fully use the potential of a feedback process aimed at closing learning gaps. However, concrete actions shown in the literature can be taken to improve the effectiveness of feedback by organizing the learning context. For example, McGinness et al. (2022) identified that students felt more receptive to feedback when working in a safe, nonjudgmental environment [ 67 ]. Moreover, supervisors and trainees identified the learning culture as key to establishing an open feedback dialogue [ 73 ]. Students who perceive culture as supportive and formative can feel more comfortable performing tasks and more willing to receive feedback [ 73 ].
Relationships
There is a consensus in the literature that trusting and long-term relationships improve the chances of actionable feedback. However, relationships between supervisors and students in the clinical workplace are often brief and not organized as more longitudinally [ 68 , 83 ], leaving little time to establish a trustful relationship [ 68 ]. Supervisors change continuously, resulting in short interactions that limit the creation of lasting relationships over time [ 50 , 68 , 83 ]. In some contexts, it is common for a student to have several supervisors who have their own standards in the observation of performance [ 46 , 56 , 68 , 83 ]. A lack of stable relationships results in students having little engagement in feedback [ 68 ]. Furthermore, in case of summative assessment programmes, the dual role of supervisors (i.e., assessing and giving feedback) makes feedback interactions perceived as summative and can complicate the relationship [ 83 ].
Repeatedly, the articles considered in this review describe that long-term and stable relationships enable the development of trust and respect [ 35 , 62 ] and foster feedback-seeking behavior [ 35 , 67 ] and feedback-giver behavior [ 39 ]. Moreover, constructive and positive relationships enhance students´ use of and response to feedback [ 30 ]. For example, Longitudinal Integrated Clerkships (LICs) promote stable relationships, thus enhancing the impact of feedback [ 83 ]. In a long-term trusting relationship, feedback can be straightforward and credible [ 87 ], there are more opportunities for student observation, and the likelihood of follow-up and actionable feedback improves [ 83 ]. Johnson et al. (2020) pointed out that within a clinical teacher-student relationship, the focus must be on establishing psychological safety; thus, the feedback conversations might be transformed [ 62 ].
Stable relationships enhance feedback dialogues, which offer an opportunity to co-construct learning and propose and negotiate aspects of the design of learning strategies [ 62 ].
Students as active agents in the feedback processes
The feedback response learners generate depends on the type of feedback information they receive, how credible the source of feedback information is, the relationship between the receiver and the giver, and the relevance of the information delivered [ 49 ]. Garino (2020) noted that students who are most successful in using feedback are those who do not take criticism personally, who understand what they need to improve and know they can do so, who value and feel meaning in criticism, are not surprised to receive it, and who are motivated to seek new feedback and use effective learning strategies [ 52 ]. Successful users of feedback ask others for help, are intentional about their learning, know what resources to use and when to use them, listen to and understand a message, value advice, and use effective learning strategies. They regulate their emotions, find meaning in the message, and are willing to change [ 52 ].
Student self-efficacy influences the understanding and use of feedback in the clinical workplace. McGinness et al. (2022) described various positive examples of self-efficacy regarding feedback processes: planning feedback meetings with teachers, fostering good relationships with the clinical team, demonstrating interest in assigned tasks, persisting in seeking feedback despite the patient workload, and taking advantage of opportunities for feedback, e.g., case presentations [ 67 ].
When students are encouraged to seek feedback aligned with their own learning objectives, they promote feedback information specific to what they want to learn and improve and enhance the use of feedback [ 53 ]. McGinness et al. (2022) identified that the perceived relevance of feedback information influenced the use of feedback because students were more likely to ask for feedback if they perceived that the information was useful to them. For example, if students feel part of the clinical team and participate in patient care, they are more likely to seek feedback [ 17 ].
Learning-oriented students aim to seek feedback to achieve clinical competence at the expected level [ 75 ]; they focus on improving their knowledge and skills and on professional development [ 17 ]. Performance-oriented students aim not to fail and to avoid negative feedback [ 17 , 75 ].
For effective feedback processes, including feed-up, feedback, and feedforward, the student must be feedback-oriented, i.e., active, seeking, listening to, interpreting, and acting on feedback [ 68 ]. The literature shows that feedback-oriented students are coproducers of learning [ 68 ] and are more involved in the feedback process [ 51 ]. Additionally, students who are metacognitively aware of their learning process are more likely to use feedback to reduce gaps in learning and performance [ 52 ]. For this, students must recognize feedback when it occurs and understand it when they receive it. Thus, it is important to organize training and promote feedback literacy so that students understand what feedback is, act on it, and improve the quality of feedback and their learning plans [ 68 ].
Table 5 summarizes those feedback tasks, activities, and key features of organizational aspects that enable each phase of the feedback loop based on the literature review.
The present scoping review identified 61 papers that mapped the literature on feedback processes in the WBL environments of undergraduate health professions. This review explored how feedback processes are organized in these learning contexts using the feedback loop framework. Given the specific characteristics of feedback processes in undergraduate clinical learning, three main findings were identified on how feedback processes are being conducted in the clinical environment and how these processes could be organized to support feedback processes.
First, the literature lacks a balance between the three dimensions of the feedback loop. In this regard, most of the articles in this review focused on reporting experiences or strategies for delivering feedback information (i.e., feedback dimension). Credible and objective feedback information is based on direct observation [ 46 ] and occurs within an interaction or a dialogue [ 62 , 88 ]. However, only having credible and objective information does not ensure that it will be considered, understood, used, and put into practice by the student [ 89 ].
Feedback-supporting actions aligned with goals and priorities facilitate effective feedback processes [ 89 ] because goal-oriented feedback focuses on students' learning needs [ 7 ]. In contrast, this review showed that only a minority of the studies highlighted the importance of aligning learning objectives and feedback (i.e., the feed-up dimension). To overcome this, supervisors and students must establish goals and agreements before starting clinical practice, as it allows students to measure themselves on a defined basis [ 90 , 91 ] and enhances students' feedback-seeking behavior [ 39 , 92 ] and responsiveness to feedback [ 83 ]. In addition, learning goals should be shared, and co-constructed, through a dialogue [ 50 , 88 , 90 , 92 ]. In fact, relationship-based feedback models emphasize setting shared goals and plans as part of the feedback process [ 68 ].
Many of the studies acknowledge the importance of establishing an action plan and promoting the use of feedback (i.e., feedforward). However, there is yet limited insight on how to best implement strategies that support the use of action plans, improve performance and close learning gaps. In this regard, it is described that delivering feedback without perceiving changes, results in no effect or impact on learning [ 88 ]. To determine if a feedback loop is closed, observing a change in the student's response is necessary. In other words, feedback does not work without repeating the same task [ 68 ], so teachers need to observe subsequent tasks to notice changes [ 88 ]. While feedforward is fundamental to long-term performance, it is shown that more research is needed to determine effective actions to be implemented in the WBL environment to close feedback loops.
Second, there is a need for more knowledge about designing feedback activities in the WBL environment that will generate constructive feedback for learning. WBA is the most frequently reported feedback activity in clinical workplace contexts [ 39 , 46 , 56 , 87 ]. Despite the efforts of some authors to use WBAs as a formative assessment and feedback opportunity, in several studies, a summative component of the WBA was presented as a barrier to actionable feedback [ 33 , 56 ]. Students suggest separating grading from observation and using, for example, the mini-CEX in informal situations [ 33 ]. Several authors also recommend disconnecting the summative components of WBAs to avoid generating emotions that can limit the uptake and use of feedback [ 28 , 93 ]. Other literature recommends purposefully designing a system of assessment using low-stakes data points for feedback and learning. Accordingly, programmatic assessment is a framework that combines both the learning and the decision-making function of assessment [ 94 , 95 ]. Programmatic assessment is a practical approach for implementing low-stakes as a continuum, giving opportunities to close the gap between current and desired performance and having the student as an active agent [ 96 ]. This approach enables the incorporation of low-stakes data points that target student learning [ 93 ] and provide performance-relevant information (i.e., meaningful feedback) based on direct observations during authentic professional activities [ 46 ]. Using low-stakes data points, learners make sense of information about their performance and use it to enhance the quality of their work or performance [ 96 , 97 , 98 ]. Implementing multiple instances of feedback is more effective than providing it once because it promotes closing feedback loops by giving the student opportunities to understand the feedback, make changes, and see if those changes were effective [ 89 ].
Third, the support provided by the teacher is fundamental and should be built into a reliable and long-term relationship, where the teacher must take the role of coach rather than assessor, and students should develop feedback agency and be active in seeking and using feedback to improve performance. Although it is recognized that institutional efforts over the past decades have focused on training teachers to deliver feedback, clinical supervisors' lack of teaching skills is still identified as a barrier to workplace feedback [ 99 ]. In particular, research indicates that clinical teachers lack the skills to transform the information obtained from an observation into constructive feedback [ 100 ]. Students are more likely to use feedback if they consider it credible and constructive [ 93 ] and based on stable relationships [ 93 , 99 , 101 ]. In trusting relationships, feedback can be straightforward and credible, and the likelihood of follow-up and actionable feedback improves [ 83 , 88 ]. Coaching strategies can be enhanced by teachers building an educational alliance that allows for trustworthy relationships or having supervisors with an exclusive coaching role [ 14 , 93 , 102 ].
Last, from a sociocultural perspective, individuals are the main actors in the learning process. Therefore, feedback impacts learning only if students engage and interact with it [ 11 ]. Thus, feedback design and student agency appear to be the main features of effective feedback processes. Accordingly, the present review identified that feedback design is a key feature for effective learning in complex environments such as WBL. Feedback in the workplace must ideally be organized and implemented to align learning outcomes, learning activities, and assessments, allowing learners to learn, practice, and close feedback loops [ 88 ]. To guide students toward performances that reflect long-term learning, an intensive formative learning phase is needed, in which multiple feedback processes are included that shape students´ further learning [ 103 ]. This design would promote student uptake of feedback for subsequent performance [ 1 ].
Strengths and limitations
The strengths of this study are (1) the use of an established framework, the Arksey and O'Malley's framework [ 22 ]. We included the step of socializing the results with stakeholders, which allowed the team to better understand the results from another perspective and offer a realistic look. (2) Using the feedback loop as a theoretical framework strengthened the results and gave a more thorough explanation of the literature regarding feedback processes in the WBL context. (3) our team was diverse and included researchers from different disciplines as well as a librarian.
The present scoping review has several limitations. Although we adhered to the recommended protocols and methodologies, some relevant papers may have been omitted. The research team decided to select original studies and reviews of the literature for the present scoping review. This caused some articles, such as guidelines, perspectives, and narrative papers, to be excluded from the current study.
One of the inclusion criteria was a focus on undergraduate students. However, some papers that incorporated undergraduate and postgraduate participants were included, as these supported the results of this review. Most articles involved medical students. Although the authors did not limit the search to medicine, maybe some articles involving students from other health disciplines needed to be included, considering the search in other databases or journals.
The results give insight in how feedback could be organized within the clinical workplace to promote feedback processes. On a small scale, i.e., in the feedback encounter between a supervisor and a learner, feedback should be organized to allow for follow-up feedback, thus working on required learning and performance goals. On a larger level, i.e., in the clerkship programme or a placement rotation, feedback should be organized through appropriate planning of subsequent tasks and activities.
More insight is needed in designing a closed loop feedback process, in which specific attention is needed in effective feedforward practices. The feedback that stimulates further action and learning requires a safe and trustful work and learning environment. Understanding the relationship between an individual and his or her environment is a challenge for determining the impact of feedback and must be further investigated within clinical WBL environments. Aligning the dimensions of feed-up, feedback and feedforward includes careful attention to teachers’ and students’ feedback literacy to assure that students can act on feedback in a constructive way. In this line, how to develop students' feedback agency within these learning environments needs further research.
Boud D, Molloy E. Rethinking models of feedback for learning: The challenge of design. Assess Eval High Educ. 2013;38:698–712.
Article Google Scholar
Henderson M, Ajjawi R, Boud D, Molloy E. Identifying feedback that has impact. In: The Impact of Feedback in Higher Education. Springer International Publishing: Cham; 2019. p. 15–34.
Chapter Google Scholar
Winstone N, Carless D. Designing effective feedback processes in higher education: A learning-focused approach. 1st ed. New York: Routledge; 2020.
Google Scholar
Ajjawi R, Boud D. Assessment & Evaluation in Higher Education Researching feedback dialogue: an interactional analysis approach. 2015. https://doi.org/10.1080/02602938.2015.1102863 .
Carless D. Feedback loops and the longer-term: towards feedback spirals. Assess Eval High Educ. 2019;44:705–14.
Sadler DR. Formative assessment and the design of instructional systems. Instr Sci. 1989;18:119–44.
Hattie J, Timperley H. The Power of Feedback The Meaning of Feedback. Rev Educ Res. 2007;77:81–112.
Zarrinabadi N, Rezazadeh M. Why only feedback? Including feed up and feed forward improves nonlinguistic aspects of L2 writing. Language Teaching Research. 2023;27(3):575–92.
Fisher D, Frey N. Feed up, back, forward. Educ Leadersh. 2009;67:20–5.
Reimann A, Sadler I, Sambell K. What’s in a word? Practices associated with ‘feedforward’ in higher education. Assessment evaluation in higher education. 2019;44:1279–90.
Esterhazy R. Re-conceptualizing Feedback Through a Sociocultural Lens. In: Henderson M, Ajjawi R, Boud D, Molloy E, editors. The Impact of Feedback in Higher Education. Cham: Palgrave Macmillan; 2019. https://doi.org/10.1007/978-3-030-25112-3_5 .
Bransen D, Govaerts MJB, Sluijsmans DMA, Driessen EW. Beyond the self: The role of co-regulation in medical students’ self-regulated learning. Med Educ. 2020;54:234–41.
Ramani S, Könings KD, Ginsburg S, Van Der Vleuten CP. Feedback Redefined: Principles and Practice. J Gen Intern Med. 2019;34:744–53.
Atkinson A, Watling CJ, Brand PL. Feedback and coaching. Eur J Pediatr. 2022;181(2):441–6.
Suhoyo Y, Schonrock-Adema J, Emilia O, Kuks JBM, Cohen-Schotanus JA. Clinical workplace learning: perceived learning value of individual and group feedback in a collectivistic culture. BMC Med Educ. 2018;18:79.
Bowen L, Marshall M, Murdoch-Eaton D. Medical Student Perceptions of Feedback and Feedback Behaviors Within the Context of the “Educational Alliance.” Acad Med. 2017;92:1303–12.
Bok HGJ, Teunissen PW, Spruijt A, Fokkema JPI, van Beukelen P, Jaarsma DADC, et al. Clarifying students’ feedback-seeking behaviour in clinical clerkships. Med Educ. 2013;47:282–91.
Al-Kadri HM, Al-Kadi MT, Van Der Vleuten CPM. Workplace-based assessment and students’ approaches to learning: A qualitative inquiry. Med Teach. 2013;35(SUPPL):1.
Dennis AA, Foy MJ, Monrouxe LV, Rees CE. Exploring trainer and trainee emotional talk in narratives about workplace-based feedback processes. Adv Health Sci Educ. 2018;23:75–93.
Watling C, LaDonna KA, Lingard L, Voyer S, Hatala R. ‘Sometimes the work just needs to be done’: socio-cultural influences on direct observation in medical training. Med Educ. 2016;50:1054–64.
Bing-You R, Hayes V, Varaklis K, Trowbridge R, Kemp H, McKelvy D. Feedback for Learners in Medical Education: What is Known? A Scoping Review Academic Medicine. 2017;92:1346–54.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–73.
Colquhoun HL, Levac D, O’brien KK, Straus S, Tricco AC, Perrier L, et al. Scoping reviews: time for clarity in definition methods and reporting. J Clin Epidemiol. 2014;67:1291–4.
StArt - State of Art through Systematic Review. 2013.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: Advancing the methodology. Implementation Science. 2010;5:1–9.
Peters MDJ, BPharm CMGHK, Parker PMD, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141–6.
Bing-You R, Varaklis K, Hayes V, Trowbridge R, Kemp H, McKelvy D, et al. The Feedback Tango: An Integrative Review and Analysis of the Content of the Teacher-Learner Feedback Exchange. Acad Med. 2018;93:657–63.
Ossenberg C, Henderson A, Mitchell M. What attributes guide best practice for effective feedback? A scoping review. Adv Health Sci Educ. 2019;24:383–401.
Spooner M, Duane C, Uygur J, Smyth E, Marron B, Murphy PJ, et al. Self -regulatory learning theory as a lens on how undergraduate and postgraduate learners respond to feedback: A BEME scoping review: BEME Guide No. 66. Med Teach. 2022;44:3–18.
Long S, Rodriguez C, St-Onge C, Tellier PP, Torabi N, Young M. Factors affecting perceived credibility of assessment in medical education: A scoping review. Adv Health Sci Educ. 2022;27:229–62.
Bing-You R, Hayes V, Varaklis K, Trowbridge R, Kemp H, McKelvy D. Feedback for Learners in Medical Education: What is Known? A Scoping Review: Lippincott Williams and Wilkins; 2017.
Schopper H, Rosenbaum M, Axelson R. “I wish someone watched me interview:” medical student insight into observation and feedback as a method for teaching communication skills during the clinical years. BMC Med Educ. 2016;16:286.
Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behaviour: a literature review. Med Educ. 2013;47:232–41.
Adamson E, Kinga L, Foy L, McLeodb M, Traynor J, Watson W, et al. Feedback in clinical practice: Enhancing the students’ experience through action research. Nurse Educ Pract. 2018;31:48–53.
Al-Mously N, Nabil NM, Al-Babtain SA, et al. Undergraduate medical students’ perceptions on the quality of feedback received during clinical rotations. Med Teach. 2014;36(Supplement 1):S17-23.
Bates J, Konkin J, Suddards C, Dobson S, Pratt D. Student perceptions of assessment and feedback in longitudinal integrated clerkships. Med Educ. 2013;47:362–74.
Bennett AJ, Goldenhar LM, Stanford K. Utilization of a Formative Evaluation Card in a Psychiatry Clerkship. Acad Psychiatry. 2006;30:319–24.
Bok HG, Jaarsma DA, Spruijt A, Van Beukelen P, Van Der Vleuten CP, Teunissen PW, et al. Feedback-giving behaviour in performance evaluations during clinical clerkships. Med Teach. 2016;38:88–95.
Bok HG, Teunissen PW, Spruijt A, Fokkema JP, van Beukelen P, Jaarsma DA, et al. Clarifying students’ feedback-seeking behaviour in clinical clerkships. Med Educ. 2013;47:282–91.
Calleja P, Harvey T, Fox A, Carmichael M, et al. Feedback and clinical practice improvement: A tool to assist workplace supervisors and students. Nurse Educ Pract. 2016;17:167–73.
Carey EG, Wu C, Hur ES, Hasday SJ, Rosculet NP, Kemp MT, et al. Evaluation of Feedback Systems for the Third-Year Surgical Clerkship. J Surg Educ. 2017;74:787–93.
Daelmans HE, Overmeer RM, Van der Hem-Stokroos HH, Scherpbier AJ, Stehouwer CD, van der Vleuten CP. In-training assessment: qualitative study of effects on supervision and feedback in an undergraduate clinical rotation. Medical education. 2006;40(1):51–8.
DeWitt D, Carline J, Paauw D, Pangaro L. Pilot study of a ’RIME’-based tool for giving feedback in a multi-specialty longitudinal clerkship. Med Educ. 2008;42:1205–9.
Dolan BM, O’Brien CL, Green MM. Including Entrustment Language in an Assessment Form May Improve Constructive Feedback for Student Clinical Skills. Med Sci Educ. 2017;27:461–4.
Duijn CC, Welink LS, Mandoki M, Ten Cate OT, Kremer WD, Bok HG. Am I ready for it? Students’ perceptions of meaningful feedback on entrustable professional activities. Perspectives on medical education. 2017;6:256–64.
Elnicki DM, Zalenski D. Integrating medical students’ goals, self-assessment and preceptor feedback in an ambulatory clerkship. Teach Learn Med. 2013;25:285–91.
Embo MP, Driessen EW, Valcke M, Van der Vleuten CP. Assessment and feedback to facilitate self-directed learning in clinical practice of Midwifery students. Medical teacher. 2010;32(7):e263-9.
Eva KW, Armson H, Holmboe E, Lockyer J, Loney E, Mann K, et al. Factors influencing responsiveness to feedback: On the interplay between fear, confidence, and reasoning processes. Adv Health Sci Educ. 2012;17:15–26.
Farrell L, Bourgeois-Law G, Ajjawi R, Regehr G. An autoethnographic exploration of the use of goal oriented feedback to enhance brief clinical teaching encounters. Adv Health Sci Educ. 2017;22:91–104.
Fernando N, Cleland J, McKenzie H, Cassar K. Identifying the factors that determine feedback given to undergraduate medical students following formative mini-CEX assessments. Med Educ. 2008;42:89–95.
Garino A. Ready, willing and able: a model to explain successful use of feedback. Adv Health Sci Educ. 2020;25:337–61.
Garner MS, Gusberg RJ, Kim AW. The positive effect of immediate feedback on medical student education during the surgical clerkship. J Surg Educ. 2014;71:391–7.
Bing-You R, Hayes V, Palka T, Ford M, Trowbridge R. The Art (and Artifice) of Seeking Feedback: Clerkship Students’ Approaches to Asking for Feedback. Acad Med. 2018;93:1218–26.
Haffling AC, Beckman A, Edgren G. Structured feedback to undergraduate medical students: 3 years’ experience of an assessment tool. Medical teacher. 2011;33(7):e349-57.
Harrison CJ, Könings KD, Dannefer EF, Schuwirth LWTT, Wass V, van der Vleuten CPMM. Factors influencing students’ receptivity to formative feedback emerging from different assessment cultures. Perspect Med Educ. 2016;5:276–84.
Harrison CJ, Könings KD, Schuwirth LW, Wass V, Van der Vleuten CP, Konings KD, et al. Changing the culture of assessment: the dominance of the summative assessment paradigm. BMC medical education. 2017;17:1–4.
Harvey P, Radomski N, O’Connor D. Written feedback and continuity of learning in a geographically distributed medical education program. Medical teacher. 2013;35(12):1009–13.
Hochberg M, Berman R, Ogilvie J, Yingling S, Lee S, Pusic M, et al. Midclerkship feedback in the surgical clerkship: the “Professionalism, Reporting, Interpreting, Managing, Educating, and Procedural Skills” application utilizing learner self-assessment. Am J Surg. 2017;213:212–6.
Holmboe ES, Yepes M, Williams F, Huot SJ. Feedback and the mini clinical evaluation exercise. Journal of general internal medicine. 2004;19(5):558–61.
Tai JHM, Canny BJ, Haines TP, Molloy EK. The role of peer-assisted learning in building evaluative judgement: opportunities in clinical medical education. Adv Health Sci Educ. 2016;21:659–76.
Johnson CE, Keating JL, Molloy EK. Psychological safety in feedback: What does it look like and how can educators work with learners to foster it? Med Educ. 2020;54:559–70.
Joshi A, Generalla J, Thompson B, Haidet P. Facilitating the Feedback Process on a Clinical Clerkship Using a Smartphone Application. Acad Psychiatry. 2017;41:651–5.
Kiger ME, Riley C, Stolfi A, Morrison S, Burke A, Lockspeiser T. Use of Individualized Learning Plans to Facilitate Feedback Among Medical Students. Teach Learn Med. 2020;32:399–409.
Kogan J, Shea J. Implementing feedback cards in core clerkships. Med Educ. 2008;42:1071–9.
Lefroy J, Walters B, Molyneux A, Smithson S. Can learning from workplace feedback be enhanced by reflective writing? A realist evaluation in UK undergraduate medical education. Educ Prim Care. 2021;32:326–35.
McGinness HT, Caldwell PHY, Gunasekera H, Scott KM. ‘Every Human Interaction Requires a Bit of Give and Take’: Medical Students’ Approaches to Pursuing Feedback in the Clinical Setting. Teach Learn Med. 2022. https://doi.org/10.1080/10401334.2022.2084401 .
Noble C, Billett S, Armit L, Collier L, Hilder J, Sly C, et al. ``It’s yours to take{’’}: generating learner feedback literacy in the workplace. Adv Health Sci Educ. 2020;25:55–74.
Ogburn T, Espey E. The R-I-M-E method for evaluation of medical students on an obstetrics and gynecology clerkship. Am J Obstet Gynecol. 2003;189:666–9.
Po O, Reznik M, Greenberg L. Improving a medical student feedback with a clinical encounter card. Ambul Pediatr. 2007;7:449–52.
Parkes J, Abercrombie S, McCarty T, Parkes J, Abercrombie S, McCarty T. Feedback sandwiches affect perceptions but not performance. Adv Health Sci Educ. 2013;18:397–407.
Paukert JL, Richards ML, Olney C. An encounter card system for increasing feedback to students. Am J Surg. 2002;183:300–4.
Rassos J, Melvin LJ, Panisko D, Kulasegaram K, Kuper A. Unearthing Faculty and Trainee Perspectives of Feedback in Internal Medicine: the Oral Case Presentation as a Model. J Gen Intern Med. 2019;34:2107–13.
Rizan C, Elsey C, Lemon T, Grant A, Monrouxe L. Feedback in action within bedside teaching encounters: a video ethnographic study. Med Educ. 2014;48:902–20.
Robertson AC, Fowler LC. Medical student perceptions of learner-initiated feedback using a mobile web application. Journal of medical education and curricular development. 2017;4:2382120517746384.
Scheidt PC, Lazoritz S, Ebbeling WL, Figelman AR, Moessner HF, Singer JE. Evaluation of system providing feedback to students on videotaped patient encounters. J Med Educ. 1986;61(7):585–90.
Sokol-Hessner L, Shea J, Kogan J. The open-ended comment space for action plans on core clerkship students’ encounter cards: what gets written? Acad Med. 2010;85:S110–4.
Sox CM, Dell M, Phillipi CA, Cabral HJ, Vargas G, Lewin LO. Feedback on oral presentations during pediatric clerkships: a randomized controlled trial. Pediatrics. 2014;134:965–71.
Spickard A, Gigante J, Stein G, Denny JC. Automatic capture of student notes to augment mentor feedback and student performance on patient write-ups. J Gen Intern Med. 2008;23:979–84.
Suhoyo Y, Van Hell EA, Kerdijk W, Emilia O, Schönrock-Adema J, Kuks JB, et al. nfluence of feedback characteristics on perceived learning value of feedback in clerkships: does culture matter? BMC medical education. 2017;17:1–7.
Torre DM, Simpson D, Sebastian JL, Elnicki DM. Learning/feedback activities and high-quality teaching: perceptions of third-year medical students during an inpatient rotation. Acad Med. 2005;80:950–4.
Urquhart LM, Ker JS, Rees CE. Exploring the influence of context on feedback at medical school: a video-ethnography study. Adv Health Sci Educ. 2018;23:159–86.
Watling C, Driessen E, van der Vleuten C, Lingard L. Learning culture and feedback: an international study of medical athletes and musicians. Med Educ. 2014;48:713–23.
Watling C, Driessen E, van der Vleuten C, Vanstone M, Lingard L. Beyond individualism: Professional culture and its influence on feedback. Med Educ. 2013;47:585–94.
Soemantri D, Dodds A, Mccoll G. Examining the nature of feedback within the Mini Clinical Evaluation Exercise (Mini-CEX): an analysis of 1427 Mini-CEX assessment forms. GMS J Med Educ. 2018;35:Doc47.
Van De Ridder JMMM, Stokking KM, McGaghie WC, ten Cate OTJ, van der Ridder JM, Stokking KM, et al. What is feedback in clinical education? Med Educ. 2008;42:189–97.
van de Ridder JMMM, McGaghie WC, Stokking KM, ten Cate OTJJ. Variables that affect the process and outcome of feedback, relevant for medical training: a meta-review. Med Educ. 2015;49:658–73.
Boud D. Feedback: ensuring that it leads to enhanced learning. Clin Teach. 2015. https://doi.org/10.1111/tct.12345 .
Brehaut J, Colquhoun H, Eva K, Carrol K, Sales A, Michie S, et al. Practice feedback interventions: 15 suggestions for optimizing effectiveness. Ann Intern Med. 2016;164:435–41.
Ende J. Feedback in clinical medical education. J Am Med Assoc. 1983;250:777–81.
Cantillon P, Sargeant J. Giving feedback in clinical settings. Br Med J. 2008;337(7681):1292–4.
Norcini J, Burch V. Workplace-based assessment as an educational tool: AMEE Guide No. 31. Med Teach. 2007;29:855–71.
Watling CJ, Ginsburg S. Assessment, feedback and the alchemy of learning. Med Educ. 2019;53:76–85.
van der Vleuten CPM, Schuwirth LWT, Driessen EW, Dijkstra J, Tigelaar D, Baartman LKJ, et al. A model for programmatic assessment fit for purpose. Med Teach. 2012;34:205–14.
Schuwirth LWT, der Vleuten CPM. Programmatic assessment: from assessment of learning to assessment for learning. Med Teach. 2011;33:478–85.
Schut S, Driessen E, van Tartwijk J, van der Vleuten C, Heeneman S. Stakes in the eye of the beholder: an international study of learners’ perceptions within programmatic assessment. Med Educ. 2018;52:654–63.
Henderson M, Boud D, Molloy E, Dawson P, Phillips M, Ryan T, Mahoney MP. Feedback for learning. Closing the assessment loop. Framework for effective learning. Canberra, Australia: Australian Government, Department for Education and Training; 2018.
Heeneman S, Pool AO, Schuwirth LWT, van der Vleuten CPM, Driessen EW, Oudkerk A, et al. The impact of programmatic assessment on student learning: theory versus practice. Med Educ. 2015;49:487–98.
Lefroy J, Watling C, Teunissen P, Brand P, Watling C. Guidelines: the do’s, don’ts and don’t knows of feedback for clinical education. Perspect Med Educ. 2015;4:284–99.
Ramani S, Krackov SK. Twelve tips for giving feedback effectively in the clinical environment. Med Teach. 2012;34:787–91.
Telio S, Ajjawi R, Regehr G. The, “Educational Alliance” as a Framework for Reconceptualizing Feedback in Medical Education. Acad Med. 2015;90:609–14.
Lockyer J, Armson H, Könings KD, Lee-Krueger RC, des Ordons AR, Ramani S, et al. In-the-Moment Feedback and Coaching: Improving R2C2 for a New Context. J Grad Med Educ. 2020;12:27–35.
Black P, Wiliam D. Developing the theory of formative assessment. Educ Assess Eval Account. 2009;21:5–31.
Download references
Author information
Authors and affiliations.
Department of Health Sciences, Faculty of Medicine, Pontificia Universidad Católica de Chile, Avenida Vicuña Mackenna 4860, Macul, Santiago, Chile
Javiera Fuentes-Cimma & Ignacio Villagran
School of Health Professions Education, Maastricht University, Maastricht, Netherlands
Javiera Fuentes-Cimma & Lorena Isbej
Rotterdam University of Applied Sciences, Rotterdam, Netherlands
Dominique Sluijsmans
Centre for Medical and Health Profession Education, Department of Gastroenterology, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
Arnoldo Riquelme
School of Dentistry, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
Lorena Isbej
Sistema de Bibliotecas UC (SIBUC), Pontificia Universidad Católica de Chile, Santiago, Chile
María Teresa Olivares-Labbe
Department of Pathology, Faculty of Health, Medicine and Health Sciences, Maastricht University, Maastricht, Netherlands
Sylvia Heeneman
You can also search for this author in PubMed Google Scholar
Contributions
J.F-C, D.S, and S.H. made substantial contributions to the conception and design of the work. M.O-L contributed to the identification of studies. J.F-C, I.V, A.R, and L.I. made substantial contributions to the screening, reliability, and data analysis. J.F-C. wrote th e main manuscript text. All authors reviewed the manuscript.
Corresponding author
Correspondence to Javiera Fuentes-Cimma .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1, supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fuentes-Cimma, J., Sluijsmans, D., Riquelme, A. et al. Designing feedback processes in the workplace-based learning of undergraduate health professions education: a scoping review. BMC Med Educ 24 , 440 (2024). https://doi.org/10.1186/s12909-024-05439-6
Download citation
Received : 25 September 2023
Accepted : 17 April 2024
Published : 23 April 2024
DOI : https://doi.org/10.1186/s12909-024-05439-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical clerkship
- Feedback processes
- Feedforward
- Formative feedback
- Health professions
- Undergraduate medical education
- Undergraduate healthcare education
- Workplace learning
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on FISH CULTURE. Find methods information, sources, references or conduct a literature review on FISH ...
The previously published review of aquaculture and world fish supplies 1 calculated a global FIFO for fed aquaculture species of 1.9 using 1997 data (FIFO by species group was 2.81 for shrimp, 5. ...
The effect of feeding rate on FCR and TGC is well described in the literature (e.g. review by de Verdal et al. 2018). As in terrestrial animals, protein plays a vital role in fish. It constitutes about 65-75% of fish body weight on dry matter basis (Halver & Hardy 2002). Fish require protein for growth, development and reproduction.
Tilapia continued its rapid increase in global production. Recent production figures reported by. various sources, our global producti on estimate for 2015 is 5,576,800 m t. Tilapias are now one ...
The systematic literature review further revealed multiple knowledge gaps in our understanding of the habitat role of bivalve and seaweed aquaculture for fish and mobile invertebrates that should be addressed through future research. We provide an overview of knowledge gaps, future research needs and recommendations within Table 2.
1. Introduction. Aquaculture has become the most rapidly growing agricultural production system in the world over the last 40 years (FAO, 2012).Production of both fish and crustaceans has boomed, with an annual growth rate of 7.8% worldwide between 1990 and 2010 (Troell et al., 2014).This growth was enabled by the expansion of the area dedicated to aquaculture production and the ...
We undertook a systematic review (Arksey and Malley 2005; Levac et al. 2010) to assess the extent to which the development policy literature on freshwater fisheries and aquaculture in South and Southeast Asia reflects a shift to food systems thinking.We acknowledge that this literature does not provide a complete picture of how fish has been taken up in food systems thinking.
Tilapia is the common name of several cichlid species, which are farmed worldwide, with a global annual production that has been constantly growing, from 379,169 t in 1990 to 6,100,719 t in 2020 1. Amongst the different farmed tilapia species, Nile tilapia ( Oreochromis niloticus ), which is native of northern Africa, is the most popular ...
1. Introduction. Due to the rapid growth in the world economy and population, global fish demand shows an increment annually. Based on the Food and Agriculture Organization of the United Nations, the global fish production in the year 2018 had reached 178.5 million tonnes (including inland and marine fish) with total capture of 82.1 million tonnes and 96.4 million tonnes from aquaculture.
A literature review Nozomi Kawarazuka . This publication should be cited as: Kawarazuka N. (2010). The contribution of fish intake, aquaculture, and small-scale fisheries to improving ... rice-fish, cage culture, netting, fingerling trade, fish trade and marketing, conducted full-time, part-time and with seasonal participation. The resources
Along with the intensification of culture systems to meet the increasing global demands, there was an elevated risk for diseases outbreak and substantial loss for farmers. ... The literature review denote that, perhaps, ... Lactic acid bacteria vs. pathogens in the gastrointestinal tract of fish: a review. Aquac. Res. 41, 451-467. 10.1111/j ...
The reviewed 104 articles investigated MP ingestion by fish in 44 countries and regions, with most of the research papers distributed in five countries, i.e., China (23%), Iran (8%), Thailand (5%), Turkey (4%), Indonesia (4%), while half of them pertained to 39 other countries and regions (Fig. 3 A).Similarly, Sequeira et al. (2020) indicated that their selected works (from March 2019 to March ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on FISH CULTURE. Find methods information, sources, references or conduct a literature review on FISH ...
Scientific literature is the principal medium for communicating the results of scientific research and represents a permanent record of the collective achievements of the scientific community. ... Papers published in journals generally go through a "peer review" process before acceptance and publication. ... Northwest Fish Culture Conference ...
A. The state of World and EU Aquaculture. World food fish aquaculture production expanded at an. average annual rate of 6.2% in the period 2000-2012, slower than in the pe riods 1980-1990 (10. ...
ations and the fish used are domesticated and semi-domesticated species. However, fish ponds are designed and used only for fish culture while rice fields are for growing rice and fish culture is a secondary enterprise. This makes it necessary to balance the priorities for rice and fish cultiva- tion in the same habitat. Literature review
Where fish culture is concerned, any characteristic of water that affects the survival, reproduction, growth, production, or man-agement of fish in any way is a water quality variable. Obviously, there are many water quality variables in pond fish culture. Fortunately, only a few of these normally play an
The identification of isolates combined with culture-independent methods showed that the fermentative process of fish-based products was ... The present literature review could serve as comprehensive database for the scientific community, and as a reference for the food industry in order to formulate tailored starter or adjunctive cultures for ...
1994). In the integrated fish farming system, wastes, leftovers and by-product from other farm activities are used to raise fish and so produce highly valued fish protein. Other wastes such as pond silt are used as fertilizer for plant crops (Wang, 1994). In India, fish culture has undergone gradual change during the past few
affecting the fish culture. With RAS, 30-50 times more fish . ... To answer this question, first, a systematic literature review on RAS in sub-Saharan Africa is conducted. Second, the RAS ...
2.1. Definition of organizational culture. OC is a set of norms, values, beliefs, and attitudes that guide the actions of all organization members and have a significant impact on employee behavior (Schein, Citation 1992).Supporting Schein's definition, Denison et al. (Citation 2012) define OC as the underlying values, protocols, beliefs, and assumptions that organizational members hold, and ...
Archival and literature review, followed by site observation, showed that there are still several fishing communities and towns in Brunswick County, among which four have been selected here for closer analysis: ... Fishing material culture, including fish houses, boats, docks, etc., are significant for fishermen and their communities in sense ...
Author Interviews Barbara Walters forged a path for women in journalism, but not without paying a price. Walters was the first woman to co-anchor a national news show on prime time television.
The cage culture of fish utilizes existing water resources but encloses the fish in a cage or basket, which allows water to pass freely between the fish and the pond, permitting water exchange and ...
A scoping review was conducted using the five-step methodological framework proposed by Arksey and O'Malley (2005) [], intertwined with the PRISMA checklist extension for scoping reviews to provide reporting guidance for this specific type of knowledge synthesis [].Scoping reviews allow us to study the literature without restricting the methodological quality of the studies found ...
The aim of this review is to assess the current state of knowledge regarding two areas of key. importance in fish welfare: physiological stress a nd pain perception, with particular reference to ...