Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
- Developmental biology
- Blastocysts
- Blastomeres
- Embryo development
- Umbilical cord
- Get an email alert for Embryology
- Get the RSS feed for Embryology
Showing 1 - 13 of 3,573
View by: Cover Page List Articles
Sort by: Recent Popular

Determinants of stillbirth among women who delivered in hospitals of North Wollo Zone, Northeast Ethiopia: A case-control study
Atnaf Alem Abriham, Eyob Shitie, [ ... ], Asmamaw Demis Bizuneh
C-phycocyanin improves the developmental potential of cryopreserved human oocytes by minimizing ROS production and cell apoptosis
Lu Wang, Hao-Ran Liu, [ ... ], Cheng-Guang Liang
THC and sperm: Impact on fertilization capability, pre-implantation in vitro development and epigenetic modifications
Alexander G. Kuzma-Hunt, Reem Sabry, [ ... ], Laura A. Favetta
Immune dysfunction mediated by the competitive endogenous RNA network in fetal side placental tissue of polycystic ovary syndrome
Ningning Xie, Fangfang Wang, [ ... ], Fan Qu
Application of artificial neural networks to evaluate femur development in the human fetus
Anna Badura, Mariusz Baumgart, [ ... ], Adam Buciński
Biochemical profiling of the follicular environment to predict oocyte competence in cattle
Nayara Ribeiro Kussano, Mauricio Machaim Franco, Margot Alves Nunes Dode
Sexual dimorphic miRNA-mediated response of bovine elongated embryos to the maternal microenvironment
Dessie Salilew-Wondim, Michael Hoelker, [ ... ], Dawit Tesfaye
Ultrasonographic assessment of abnormal fetal growth related to uteroplacental-fetal biometrics and Doppler (U-AID) indices: Protocol for multicenter retrospective cohort study trial
Eun-Saem Choi, Hwasun Lee, [ ... ], Ki Hoon Ahn
Factors affecting the efficiency of equine embryo transfer (EET) in polo mares under subtropical conditions of Pakistan
Khalid Mahmood, Aijaz Ali Channa, Aamir Ghafoor, Amjad Riaz
Xenopus gastrula chordamesoderm">Cell contacts and pericellular matrix in the Xenopus gastrula chordamesoderm
Olivia Luu, Debanjan Barua, Rudolf Winklbauer
Metabolomics analysis of the yolk of Zhijin white goose during the embryogenesis based on LC-MS/MS
Zhonglong Zhao, Hong Yang, [ ... ], Yong Zhang
Non-invasive cumulus cell analysis can be applied for oocyte ranking and is useful for countries with legal restrictions on embryo generation or freezing
Tom Adriaenssens, Inge Van Vaerenbergh, [ ... ], Johan Smitz
C . elegans hermaphrodites">Sperm function is required for suppressing locomotor activity of C . elegans hermaphrodites
Satoshi Suo
Connect with Us
- PLOS ONE on Twitter
- PLOS on Facebook
New insight into formation of the human embryo
Pioneering research led by experts from the University of Exeter's Living Systems Institute has provided new insight into formation of the human embryo.
The team of researchers discovered an unique regenerative property of cells in the early human embryo.
The first tissue to form in the embryo of mammals is the trophectoderm, which goes on to connect with the uterus and make the placenta. Previous research in mice found that trophectoderm is only made once.
In the new study, however, the research team found that human early embryos are able to regenerate trophectoderm. They also showed that human embryonic stem cells grown in the laboratory can similarly continue to produce trophectoderm and placental cell types.
These findings show unexpected flexibility in human embryo development and may directly benefit assisted conception (IVF) treatments. In addition, being able to produce early human placental tissue opens a door to finding causes of infertility and miscarriage.
The study is published in the leading international peer-review journal Cell Stem Cell on Wednesday, April 7th 2021.
Dr Ge Guo, lead author of the study from the Living Systems Institute said: "We are very excited to discover that human embryonic stem cells can make every type of cell required to produce a new embryo."
Professor Austin Smith, Director of the Living Systems Institute and co-author of the study added, said: "Before Dr Guo showed me her results, I did not imagine this should be possible. Her discovery changes our understanding of how the human embryo is made and what we may be able do with human embryonic stem cells"
Human naïve epiblast cells possess unrestricted lineage potential is published in Cell Stem Cell . The research was funded by the Medical Research Council (MRC) .
- Pregnancy and Childbirth
- Human Biology
- Medical Topics
- Skin Cancer
- Pancreatic Cancer
- Introduction to genetics
- Amniotic sac
- Mammalian embryogenesis
- Double blind
- Umbilical cord
- Embryonic stem cell
- Positron emission tomography
Story Source:
Materials provided by University of Exeter . Note: Content may be edited for style and length.
Journal Reference :
- Ge Guo, Giuliano Giuseppe Stirparo, Stanley E. Strawbridge, Daniel Spindlow, Jian Yang, James Clarke, Anish Dattani, Ayaka Yanagida, Meng Amy Li, Sam Myers, Buse Nurten Özel, Jennifer Nichols, Austin Smith. Human naive epiblast cells possess unrestricted lineage potential . Cell Stem Cell , 2021; DOI: 10.1016/j.stem.2021.02.025
Cite This Page :
Explore More
- Quantum Effects in Electron Waves
- Star Trek's Holodeck Recreated Using ChatGPT
- Cloud Engineering to Mitigate Global Warming
- Detecting Delayed Concussion Recovery
- Genes for Strong Muscles: Healthy Long Life
- Brightest Gamma-Ray Burst
- Stellar Winds of Three Sun-Like Stars Detected
- Fences Causing Genetic Problems for Mammals
- Ozone Removes Mating Barriers Between Fly ...
- Parkinson's: New Theory On Origins and Spread
Trending Topics
Strange & offbeat.
The Future of IVF: The New Normal in Human Reproduction
- Infertility: Perspective, Opinions and Commentaries
- Open access
- Published: 03 January 2022
- Volume 29 , pages 849–856, ( 2022 )
Cite this article
You have full access to this open access article
- Vitaly A. Kushnir ORCID: orcid.org/0000-0002-0637-1166 1 ,
- Gary D. Smith 2 &
- Eli Y. Adashi 3
18k Accesses
24 Citations
78 Altmetric
10 Mentions
Explore all metrics
Increased demand for in vitro fertilization (IVF) due to socio-demographic trends, and supply facilitated by new technologies, converged to transform the way a substantial proportion of humans reproduce. The purpose of this article is to describe the societal and demographic trends driving increased worldwide demand for IVF, as well as to provide an overview of emerging technologies that promise to greatly expand IVF utilization and lower its cost.
Similar content being viewed by others

In Vitro Fertilization Research is Translational Research
Alan H. DeCherney & Rebecca L. Barnett
Revisiting selected ethical aspects of current clinical in vitro fertilization (IVF) practice
Anja von Schondorf-Gleicher, Lyka Mochizuki, … Norbert Gleicher

Assisted Reproductive Technology: Clinical Aspects
Avoid common mistakes on your manuscript.
Introduction
Since its clinical introduction in 1978, in vitro fertilization (IVF) has redefined the ability of the human species to procreate. Initially developed to aid the infertile couple, clinical indications for IVF have since rapidly expanded to include medical and genetic conditions, as well as fertility preservation. While IVF access and utilization vary widely globally, the practice now accounts for the conception of over 5% of all newborns in some European countries where IVF is more affordable and/or is covered by insurance [ 1 ]. The corresponding figure presently stands at 4.1% in Australia and New Zealand, 1.9% in the USA, and 1.7% in China and is rapidly rising in all regions of the world [ 2 , 3 ]. Infertility, which affects approximately 10% of couples, remains the main driver of IVF utilization. These simple statistics suggest that IVF utilization may significantly grow in the coming decades if barriers to its utilization are lowered; this is without even considering an increasing number of indications for IVF beyond infertility.
Changing demographics and societal norms are driving increased IVF utilization. Improved access of women to educational and career opportunities, as well as effective contraception has contributed to progressively delayed childbearing and overall lower fertility rates worldwide. In many countries and in virtually all US states, fertility rates are now substantially below population replacement levels of 2100 births per 1000 women. In a growing number of metropolitan areas as well as in entire highly developed countries, the average age at first birth now exceeds 30 years, that is, well beyond peak fertility which occurs in the mid 20s. Inadvertently, a growing proportion of women is delaying childbearing to a point where age-related fertility decline contributes to the prevalence of infertility and to increased demand for fertility treatments including IVF and oocyte cryopreservation. These trends will likely accelerate due to the socio-economic impact of the COVID-19 pandemic, which has forestalled new family formation. Indeed, preliminary data from Chinese cities indicate that birth rates declined between 9 and 32.6% in the second half of 2020 compared with 2019, reflecting effects of the COVID-19 lockdowns [ 4 ]. Declining fertility rates in China have prompted its government to reverse a decades old one-child policy in favor of a two-child policy in 2016, and to a three-child policy in 2021.
The utilization of IVF is closely linked to its affordability and accessibility [ 5 ]. Indeed, a growing number of countries and US states are adopting various policies intended to reverse declining fertility rates. These policies range from legally mandated insurance coverage for fertility treatments to subsidies intended to ease the burdens of child-rearing. The concept that fertility is a basic human right is just starting to gain traction and is sure to accelerate wider adoption of such policies [ 6 ]. Another recent development is the growing number of prominent corporations opting to fund fertility benefits as a part of their social mission and as a means of attracting and retaining employees. Combined, the various policies that promote improved insurance coverage are bound to lower the cost of IVF to patients and increase its utilization.
The distribution of established fertility clinics thus closely corresponds to affluent metropolitan areas with the lowest fertility rates and the most advanced maternal ages at birth. Conversely, less densely populated and less affluent areas are characterized by relatively poor IVF access. Moreover, racial and ethnic disparities in the utilization of IVF, largely due to socio-economic factors, are inversely correlated with fertility rates [ 7 ]. An additional driver of IVF utilization is the growing societal acceptance of non-traditional families including single and same-sex parents. Finally, third-party IVF that includes the use of donor oocytes, sperm, or embryo and gestational carrier is rapidly growing, now accounting for over 20% of all birth conceived through IVF in the USA [ 8 ].
The IVF process is complex and stressful, it consists of multiple steps which can take up to several months to complete. The main reasons patient prematurely drop-out of IVF prior to achieving a pregnancy are the financial, physical, and psychological burdens of the treatment regimen [ 9 ]. Here, we describe promising future approaches and technological innovations which might improve IVF accessibility while reducing its costs and burden of care.
Medical Advancements
Controlled ovarian hyperstimulation (COH) is performed to increase the number of oocytes available for IVF. COH involves multiple injections of gonadotropins and serial visits to the fertility clinic for the conduct of transvaginal ultrasound evaluations and the measurement of circulating hormone levels. It follows that COH is complex, time sensitive, and intensive. Various strategies intent on reducing the number of injections by utilizing long-acting gonadotropins or oral medications are already available and are gaining increased acceptance in the field for the treatment of select patient populations [ 10 , 11 ]. Similarly, an emerging strategy to measure salivary estradiol levels may help decrease the need for blood draws during COH [ 12 ]. Recent advancements in portable lower cost ultrasound devices may further simplify follicular and endometrial monitoring by way of convenient mobile facilities and potentially even self-operated endovaginal telemonitoring [ 13 ]. Combined, these approaches may greatly simplify COH by rendering it less invasive and by decreasing the time commitment required. Finally, interventions which may further decrease the treatment burden may include screening of patients for psychological issues as well as offering counseling and coping interventions such as e-therapy as an integral part of IVF [ 14 , 15 ].
Technological Advancements
Perhaps the most promising technological development that might democratize IVF access in the near-term is the automation and miniaturization of the IVF laboratory. Building, staffing, and manually operating an IVF laboratory account for much of the high cost, maldistribution in access, and variability of outcomes. The basic steps in the IVF laboratory include:
identification and separation of sperm and oocytes
fertilization
embryo culture
embryo selection for transfer
cryopreservation of surplus embryos and gametes
Great progress has already been made towards the automation of these individual steps by way of new technologies. Still, the IVF process in its entirety remains highly manual. The altogether novel IVF lab-on-a-chip concept has the potential to revolutionize IVF by enabling the automation of virtually all of the steps involved in a single system [ 16 , 17 , 18 ].
Microfluidics is defined as a multidisciplinary field of study and design whereby fluid behaviors are accurately controlled and manipulated with small scale geometric constraints that yields dominance of surface forces over volumetric counterparts. Past procedures in the IVF laboratory, though successful, apply a macroscale approaches to microscale cellular biological events [ 18 ]. Integration of microfluidics into the IVF laboratory may give rise to at least four foreseeable advantages: (1) precisely controlled fluidic gamete/embryo manipulations; (2) providing biomimetic environments for culture; (3) facilitating microscale genetic and molecular bioassays; and (4) enabling miniaturization and automation. The basic utility and advantages of individual microfluidic devices for gamete and preimplantation embryo isolation, manipulation, and assessment have been demonstrated [ 18 ]. Current efforts are focused on integrating extant individualized microfluidic procedural components into a future IVF lab-on-a-chip.
Microfluidic sperm-sorting devices [ 19 , 20 , 21 ] and automated sperm analyzers [ 22 ] are already being introduced into routine IVF practice. Indeed, microfluidics has been used for the isolation of sperm from semen and testicular biopsies [ 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. These novel sperm-isolating microfluidics devices provide for the collection of highly motile sperm populations replete with enriched normal morphology, and most importantly, reduced DNA fragmentation relative to conventional methods of sperm isolation [ 19 , 27 , 30 , 31 ].
Microfluidic in vitro insemination has been demonstrated [ 32 ], whereas conventional fertilization is suitable for the vast majority of IVF patients, microfluidic systems may further decrease the need for Intracytoplasmic Sperm Injection (ICSI). Such outcomes may even be possible in the setting of oligospermia, because even a low concentration of sperm may still be sufficient to achieve fertilization [ 32 ]. As ICSI has become a dominant method of insemination in human clinical IVF, the importance of precise microfluidic push/pull cumulus-oocyte-complex cumulus cell removal has been shown to yield good visualization of the oocyte cytoplasm/orientation [ 33 ]. The fertilization step by ICSI is perhaps the most technically difficult step to achieve on a commercial scale, but feasibility of one such system has been demonstrated [ 34 ]. Future automated ICSI will likely involve a combination of microfluidics, robotics, and refined optics [ 34 , 35 ].
Embryo culture has already been fully automated with use of time-lapse incubators which allow continuous monitoring of embryo development. Data generated from time-lapse incubators can be analyzed with machine learning to aid in the selection of embryos with the highest pregnancy potential [ 36 , 37 , 38 ]. Additional information about embryo viability may be gleaned from other omics technologies which can either sample the embryo directly or indirectly via its culture media. The technologies in question include genomic, transcriptomic, proteomic, and metabolomic analyses [ 39 ]. Although the use of preimplantation genetic testing (PGT) of trophectoderm cells of blastocyst stage embryos is quite common in clinical practice, the utility of such testing for the ascertainment of aneuploidy remains controversial on both biological and technical grounds [ 40 ]. Microfluidics technology has been successfully used to culture mammalian preimplantation embryos from the zygote to the blastocyst stage both individually and in groups [ 41 , 42 , 43 , 44 , 45 , 46 ]. These experiments have proven informative to overcoming the hurdles of microenvironment manipulations in microfluidic devices involving microchannels [ 42 ], microfunnels [ 45 ], microwells [ 44 ], and microdroplets [ 46 ] that can induce shear stresses and osmotic shifts that can be detrimental to embryo development [ 45 , 47 ]. The importance of individual embryo culture in microfluidic devices can be appreciated when one considers the desire to integrate real-time imaging and morphometrics [ 48 ], molecular [ 49 ], and/or metabolomic [ 50 , 51 ] bioassays, biomechanics [ 52 ], and non-invasive PGT of cell-free DNA in spent media [ 53 ]. Noninvasive PGT, which utilizes cell-free DNA released into the spent embryo culture media, is likely to become the first omics technology used clinically in conjunction with a microfluidic system [ 53 ].
Finally, cryopreservation of sperm, oocytes, and embryos has become the standard of care. Vitrification has become the dominant method for oocyte and embryo cryopreservation. While semi-automated/automated systems for oocyte/embryo vitrification have been reported and are now in early stages of clinical adoption [ 54 , 55 , 56 ], these devices do not necessarily use or require microfluidics. If one looks to the future of a microfluidic automated lab-on-a-chip, the question arises of whether or not microfluidics is useful and/or beneficial in the vitrification process? Microfluidics can be used to precisely control cryoprotectant exposures (gradual versus step-wise exposure) to oocytes/zygotes/embryos and thus reduce osmotic strain, reduce sub-lethal membrane damage, and improve subsequent development [ 57 , 58 , 59 , 60 ]. Future potential benefits of integrating microfluidics with vitrification and automation have been carefully enumerated in recent reviews [ 59 , 61 , 62 ]. Integrated microfluidics for vitrification with automation is promising. Such a system/device will reduce reagent consumption, decrease labor intensity, facilitate ease of use, offer medium to high throughput, and may foster point-of-care cryopreservation and/or promote in-office cryopreservation procedures that require less in the way of technical/personnel expertise and sophisticated laboratory/equipment needs.
Figure 1 illustrates the future IVF lab-on-a-chip concept, including all of the laboratory steps performed during IVF while integrating emerging non-invasive techniques of embryo assessment. Adoption of automated IVF systems offers multiple potential advantages: standardization of workflows, reduction in errors, reduction in cost, reduction in contamination, and the potential for incremental system improvement via machine learning. Additionally, miniaturization and automation of the IVF laboratory can greatly improve accessibility to IVF treatment for underserved communities, especially those who are economically disadvantaged and who reside in rural areas. Regulatory approval will doubtlessly be required if automated systems are to be adequately validated to produce clinical outcomes superior to those attained with the current manual process in the IVF laboratory. Furthermore, automation will likely significantly decrease the staffing requirements and alter the type of skills required to operate fertility centers. It is likely that the technical aspects of IVF will be gradually assumed by machines. This may well increase the emphasis placed on human interactions which supports the medical and psychological needs of patients during their fertility journey.
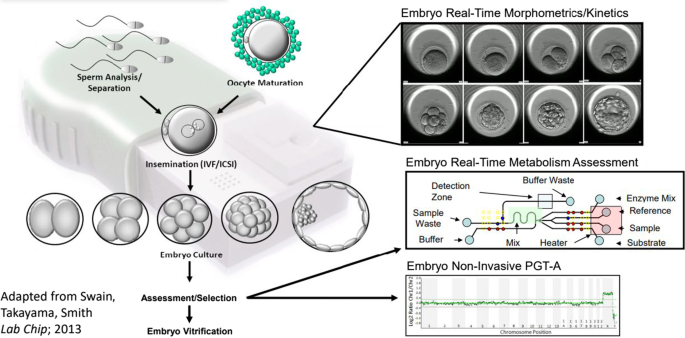
Future IVF lab-on-a-chip concept displaying the integration of all the steps performed during the IVF process and of emerging non-invasive techniques of embryo assessment
Scientific Advancements
Fertility preservation research has steadily increased our understanding of the mechanisms that govern folliculogenesis [ 63 ]. The development of in vitro culture systems for follicles provided insights into the relationship between oocytes and their surrounding somatic cells, as well as the requisite hormones and growth factors. Multi-step culture systems have advanced to a point where primordial follicles residing in ovarian cortical tissue can undergo activation, growth, and in vitro maturation (IVM) to yield metaphase II (MII) oocytes [ 64 ]. These advancements are expanding fertility preservation via ovarian tissue cryopreservation and subsequent chance at parenthood via IVF to pre-pubertal girls and young women at-risk to develop primary ovarian insufficiency (POI) due to gonadotoxic chemotherapy for cancer or due to other serious diseases. Intriguing extensions of this technology may enable the isolation of oocytes from patients who have already developed POI or have entered natural menopause so long as some dormant follicles remain within their ovarian cortex. Another avenue of research is to develop an artificial ovary as has been achieved in a murine model using 3D printed scaffolds for tissue engineering [ 65 , 66 ]. Microfluidic culture systems may also be utilized to support follicle development while mimicking the natural menstrual cycle [ 67 ].
In Vitro Gametogenesis (IVG)
Perhaps the most revolutionary concept in modern reproductive science is that of in vitro gametogenesis (IVG). IVG comprises various approaches, including organ culture systems, embryonic stem cells (ESC), induced pluripotent stem cells (iPSC), and spermatogonial stem cells (SSCs). Several of these approaches led to the creation of functional gametes in rodent models [ 68 ]. Japanese scientists, who have been at the forefront of IVG research, have recently succeeded in extending these techniques to the generation of human oogonia from iPSCs [ 69 ]. Yet, another approach to IVG involves reconstruction of functional oocytes by nuclear transfer of the first polar body genome from an MII oocyte into an enucleated donor MII cytoplasm [ 70 ]. This latter technique may well increase the number of oocytes available for the treatment of infertility of women with few or poor-quality autologous oocytes.
The existence of human oogonial stem cells (OSCs) capable of giving rise to new oocytes has been an area of some controversy for nearly a decade. Reports to the effect that cells isolated from human ovarian tissue using fluorescence-activated cell sorting and an antibody against the DDX4 protein constituted OSCs challenged the long-standing dogma that the ovarian reserve is finite [ 71 , 72 ]. Multiple follow up studies by several groups were unable to confirm the presence of OSCs in the human ovary. Recently, single-cell analysis of the human ovarian cortex failed to identify OSCs [ 73 ]. Instead, cells captured by the DDX4-directed antibody proved to be perivascular cellular elements [ 73 ].
SSCs constitute the progenitor cells in the process of spermatogenesis. As such, these cells are the focus of in vitro spermatogenesis (IVS) and in vivo restoration of male fertility. While IVS has been achieved in rodent models, it has proven far more difficult to realize in primate counterparts [ 74 ]. One recent approach to IVS involved the culture of SSCs with immortalized Sertoli cells. Meiosis and the production of spermatid-like cells followed, albeit in the face of improper activation of cognate meiotic checkpoints [ 75 ]. In yet another approach, sperm nuclear transfer allowed production of androgenetic haploid embryonic stem cells which were able to “fertilize” oocytes and support early embryonic development, diploid blastocysts, and ESC generation [ 76 ]. Once fully realized, IVS is destined to offer genetic parenthood via IVF to infertile men diagnosed with azoospermia and pre-pubertal boys undergoing gonadotoxic treatments.
Reproductive Genetics
The convergence of IVF with reproductive genetics has been at the forefront of the field for the past few decades. The development of next generation sequencing has expedited the adoption of PGT of embryos with an eye toward detecting the presence of chromosomal abnormalities. Moreover, increased use of carrier screening of infertile couples has increased the use of PGT for monogenic diseases. As cost of carrier screening decreases and the number of detected mutations expands, a substantial new population of patients identified as carriers may pursue IVF with PGT to build their families. Indeed, population genomic screening of young adults may offer significant healthcare savings through the prevention of rare disorders and cancers [ 77 ]. Future applications of PGT may expand to multifactorial diseases and whole-exome screening, though current attempts at introduction of embryo selection based on polygenic scores into clinical practice seem premature and fraught with ethical challenges [ 78 ]. Recent improvements in micromanipulation techniques and the development of CRISPR-Cas9 gene editing tools [ 79 ] raise the prospect of germline genome modification (GGM) for severe monogenic disorders. Indeed, GGM has already been achieved in human embryos [ 80 ]. Mitochondrial replacement therapy (MRT) for the prevention of heritable mitochondrial DNA diseases is even further developed than GGM, with clinical trials already underway in the UK [ 81 ].
The growing utilization of IVF will transform the way a substantial proportion of the human species procreates. It is likely that in the near future, as many as 10% of all children will be conceived through IVF in many parts of the world. Given the rapid scientific and technological evolution of IVG and of reproductive genetics, it is imperative that both the public and regulatory bodies be engaged in establishing a framework for the ethical evaluation of emerging technologies [ 82 , 83 , 84 ]. Such public engagement is critical. The absence of such may well result in reactionary bans against clinical research as has been the case for GGM and MRT in the USA [ 85 ]. Moreover, the introduction of innovative technologies into clinical practice must be rooted in science and supported by well-designed clinical trials [ 86 ]. Premature commercialization of costly and unproven “add-ons” to IVF has been an ongoing issue in the field, ranging from procedures to medicines to laboratory techniques [ 87 , 88 ]. Collectively, routine application and marketing of unproven IVF add-ons may erode the public trust in the reproductive medicine field. Thus, it is imperative for the field to prioritize requiring confirmation of safety and efficacy of technologies before allowing them to be offered routinely to IVF patients. Reproductive medicine, and especially IVF, is rapidly transforming human reproduction and is thus bound to remain of fundamental importance to both science and society.
Data availability
Not applicable.
Code Availability
Abbreviations.
Controlled ovarian hyperstimulation
Embryonic stem cells
Germline stem cells
Germline genome modification
Induced pluripotent stem cells
Intracytoplasmic sperm injection
- In vitro fertilization
- In vitro gametogenesis
In vitro maturation
In vitro spermatogenesis
Metaphase II
Mitochondrial replacement therapy
Oogonial stem cells
Preimplantation genetic testing
Primary ovarian insufficiency
Spermatogonial stem cells
European IVF-monitoring consortium (EIM)‡ for the European society of human reproduction and embryology (ESHRE), Wyns C, Bergh C, et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020(3):hoaa032. https://doi.org/10.1093/hropen/hoaa032
De Geyter C, Wyns C, Calhaz-Jorge C, et al. 20 years of the European IVF-monitoring consortium registry: what have we learned? A comparison with registries from two other regions. Hum Reprod. 2020;35(12):2832–49. https://doi.org/10.1093/humrep/deaa250 .
Article PubMed PubMed Central Google Scholar
Bai F, Wang DY, Fan YJ, et al. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum Reprod. 2020;35(2):446–52. https://doi.org/10.1093/humrep/dez245 .
Article CAS PubMed Google Scholar
Instead of a desperately needed baby boom, China gets a COVID-19 baby bust - SupChina. https://supchina.com/2021/02/09/instead-of-a-desperately-needed-baby-boom-china-gets-a-covid-19-baby-bust/ . Accessed Apr 2, 2021.
Chambers GM, Adamson GD, Eijkemans MJC. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–27. https://doi.org/10.1016/j.fertnstert.2013.06.017 .
Article PubMed Google Scholar
Kawwass JF, Penzias AS, Adashi EY. Fertility-a human right worthy of mandated insurance coverage: the evolution, limitations, and future of access to care. Fertil Steril. 2021;115(1):29–42. https://doi.org/10.1016/j.fertnstert.2020.09.155 .
Shapiro AJ, Darmon SK, Barad DH, Albertini DF, Gleicher N, Kushnir VA. Effect of race and ethnicity on utilization and outcomes of assisted reproductive technology in the USA. Reprod Biol Endocrinol. 2017;15(1):44. https://doi.org/10.1186/s12958-017-0262-5 .
Kushnir VA, Darmon SK, Shapiro AJ, Albertini DF, Barad DH, Gleicher N. Utilization of third-party in vitro fertilization in the United States. Am J Obstet Gynecol. 2017;216(3):266.e1-266.e10. https://doi.org/10.1016/j.ajog.2016.11.1022 .
Article Google Scholar
Verberg MFG, Eijkemans MJC, Heijnen EMEW, et al. Why do couples drop-out from IVF treatment? A prospective cohort study Hum Reprod. 2008;23(9):2050–5. https://doi.org/10.1093/humrep/den219 .
Pouwer AW, Farquhar C, Kremer JAM. Long-acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database Syst Rev. 2015;(7):CD009577. https://doi.org/10.1002/14651858.CD009577.pub3
Datta AK, Maheshwari A, Felix N, Campbell S, Nargund G. Mild versus conventional ovarian stimulation for IVF in poor responders: a systematic review and meta-analysis. Reprod Biomed Online. 2020;41(2):225–38. https://doi.org/10.1016/j.rbmo.2020.03.005 .
Sakkas D, Howles CM, Atkinson L, et al. A multi-centre international study of salivary hormone oestradiol and progesterone measurements in ART monitoring. Reprod Biomed Online. 2020. https://doi.org/10.1016/j.rbmo.2020.10.012 .
Gerris J, Delvigne A, Dhont N, et al. Self-operated endovaginal telemonitoring versus traditional monitoring of ovarian stimulation in assisted reproduction: an RCT. Hum Reprod. 2014;29(9):1941–8. https://doi.org/10.1093/humrep/deu168 .
Rich CW, Domar AD. Addressing the emotional barriers to access to reproductive care. Fertil Steril. 2016;105(5):1124–7. https://doi.org/10.1016/j.fertnstert.2016.02.017 .
van Dongen AJCM, Nelen WLDM, IntHout J, Kremer JAM, Verhaak CM. e-therapy to reduce emotional distress in women undergoing assisted reproductive technology (ART): a feasibility randomized controlled trial. Hum Reprod. 2016;31(5):1046–57. https://doi.org/10.1093/humrep/dew040 .
Weng L. IVF-on-a-chip: recent advances in microfluidics technology for in vitro fertilization. SLAS Technol. 2019;24(4):373–85. https://doi.org/10.1177/2472630319851765 .
Swain JE, Lai D, Takayama S, Smith GD. Thinking big by thinking small: application of microfluidic technology to improve ART. Lab Chip. 2013;13(7):1213–24. https://doi.org/10.1039/c3lc41290c .
Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod. 2017;23(4):257–68. https://doi.org/10.1093/molehr/gaw076 .
Article CAS PubMed PubMed Central Google Scholar
Quinn MM, Jalalian L, Ribeiro S, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33(8):1388–93. https://doi.org/10.1093/humrep/dey239 .
Parrella A, Keating D, Cheung S, et al. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J Assist Reprod Genet. 2019;36(10):2057–66. https://doi.org/10.1007/s10815-019-01543-5 .
Marzano G, Chiriacò MS, Primiceri E, et al. Sperm selection in assisted reproduction: a review of established methods and cutting-edge possibilities. Biotechnol Adv. 2020;40:107498. https://doi.org/10.1016/j.biotechadv.2019.107498 .
Lammers J, Chtourou S, Reignier A, Loubersac S, Barrière P, Fréour T. Comparison of two automated sperm analyzers using 2 different detection methods versus manual semen assessment. J Gynecol Obstet Hum Reprod. 2021;50(8):102084. https://doi.org/10.1016/j.jogoh.2021.102084 .
Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem. 2003;75(7):1671–5. https://doi.org/10.1021/ac020579e .
Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod Biomed Online. 2003;7(1):75–81. https://doi.org/10.1016/s1472-6483(10)61732-4 .
Nosrati R, Vollmer M, Eamer L, et al. Rapid selection of sperm with high DNA integrity. Lab Chip. 2014;14(6):1142–50. https://doi.org/10.1039/c3lc51254a .
Wu J-K, Chen P-C, Lin Y-N, Wang C-W, Pan L-C, Tseng F-G. High-throughput flowing upstream sperm sorting in a retarding flow field for human semen analysis. Analyst. 2017;142(6):938–44. https://doi.org/10.1039/c6an02420c .
Nagata MPB, Endo K, Ogata K, et al. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc Natl Acad Sci USA. 2018;115(14):E3087–96. https://doi.org/10.1073/pnas.1717974115 .
Mangum CL, Patel DP, Jafek AR, et al. Towards a better testicular sperm extraction: novel sperm sorting technologies for non-motile sperm extracted by microdissection TESE. Transl Androl Urol. 2020;9(Suppl 2):S206–14. https://doi.org/10.21037/tau.2019.08.36 .
Samuel R, Feng H, Jafek A, Despain D, Jenkins T, Gale B. Microfluidic-based sperm sorting & analysis for treatment of male infertility. Transl Androl Urol. 2018;7(Suppl 3):S336–47. https://doi.org/10.21037/tau.2018.05.08 .
Schulte RT, Chung YK, Ohl DA, Takayama S, Smith GD. Microfluidic sperm sorting device provides a novel method for selecting motile sperm with higher DNA integrity. Fertil Steril. 2007;88:S76. https://doi.org/10.1016/j.fertnstert.2007.07.254 .
Shirota K, Yotsumoto F, Itoh H, et al. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil Steril. 2016;105(2):315-21.e1. https://doi.org/10.1016/j.fertnstert.2015.10.023 .
Suh RS, Zhu X, Phadke N, Ohl DA, Takayama S, Smith GD. IVF within microfluidic channels requires lower total numbers and lower concentrations of sperm. Hum Reprod. 2006;21(2):477–83. https://doi.org/10.1093/humrep/dei323 .
Zeringue HC, Beebe DJ. Microfluidic removal of cumulus cells from Mammalian zygotes. Methods Mol Biol. 2004;254:365–74. https://doi.org/10.1385/1-59259-741-6:365 .
Lu Z, Zhang X, Leung C, Esfandiari N, Casper RF, Sun Y. Robotic ICSI (intracytoplasmic sperm injection). IEEE Trans Biomed Eng. 2011;58(7):2102–8. https://doi.org/10.1109/TBME.2011.2146781 .
Mor A, Zhang M, Esencan E, et al. A step towards the automation of intracytoplasmic sperm injection: real time confirmation of mouse and human oocyte penetration and viability by electrical resistance measurement. Fertil Steril. 2020;113(1):234–6. https://doi.org/10.1016/j.fertnstert.2019.09.023 .
Tran D, Cooke S, Illingworth PJ, Gardner DK. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum Reprod. 2019;34(6):1011–8. https://doi.org/10.1093/humrep/dez064 .
Fishel S, Campbell A, Montgomery S, et al. Time-lapse imaging algorithms rank human preimplantation embryos according to the probability of live birth. Reprod Biomed Online. 2018;37(3):304–13. https://doi.org/10.1016/j.rbmo.2018.05.016 .
Khosravi P, Kazemi E, Zhan Q, et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. npj Digital Med. 2019;2(1):21. https://doi.org/10.1038/s41746-019-0096-y
Horcajadas JA, Gosálvez J, eds. Reproductomics: the -omics revolution and its impact on human reproductive medicine.
Practice committees of the American society for reproductive medicine and the society for assisted reproductive technology. Electronic address: [email protected], practice committees of the American society for reproductive medicine and the society for assisted reproductive technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429–436. https://doi.org/10.1016/j.fertnstert.2018.01.002
Raty S, Walters EM, Davis J, et al. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip. 2004;4(3):186–90. https://doi.org/10.1039/b316437c .
Walters EM, Clark SG, Beebe DJ, Wheeler MB. Mammalian embryo culture in a microfluidic device. Methods Mol Biol. 2004;254:375–82. https://doi.org/10.1385/1-59259-741-6:375 .
Han C, Zhang Q, Ma R, et al. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip. 2010;10(21):2848–54. https://doi.org/10.1039/c005296e .
Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev. 2010;22(1):32–9. https://doi.org/10.1071/RD09219 .
Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod. 2010;25(3):613–22. https://doi.org/10.1093/humrep/dep449 .
Esteves TC, van Rossem F, Nordhoff V, Schlatt S, Boiani M, Le Gac S. A microfluidic system supports single mouse embryo culture leading to full-term development. RSC Adv. 2013;3(48):26451. https://doi.org/10.1039/c3ra44453h .
Article CAS Google Scholar
Heo YS, Cabrera LM, Song JW, et al. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal Chem. 2007;79(3):1126–34. https://doi.org/10.1021/ac061990v .
Gardner DK, Balaban B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and “OMICS”: is looking good still important? Mol Hum Reprod. 2016;22(10):704–18. https://doi.org/10.1093/molehr/gaw057 .
Rosàs-Canyelles E, Modzelewski AJ, Geldert A, He L, Herr AE. Assessing heterogeneity among single embryos and single blastomeres using open microfluidic design. Sci Adv. 2020;6(17):eaay1751. https://doi.org/10.1126/sciadv.aay1751 .
Urbanski JP, Johnson MT, Craig DD, Potter DL, Gardner DK, Thorsen T. Noninvasive metabolic profiling using microfluidics for analysis of single preimplantation embryos. Anal Chem. 2008;80(17):6500–7. https://doi.org/10.1021/ac8010473 .
Heo YS, Cabrera LM, Bormann CL, Smith GD, Takayama S. Real time culture and analysis of embryo metabolism using a microfluidic device with deformation based actuation. Lab Chip. 2012;12(12):2240–6. https://doi.org/10.1039/c2lc21050a .
Yanez LZ, Camarillo DB. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol Hum Reprod. 2017;23(4):235–47. https://doi.org/10.1093/molehr/gaw071 .
Huang L, Bogale B, Tang Y, Lu S, Xie XS, Racowsky C. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci USA. 2019;116(28):14105–12. https://doi.org/10.1073/pnas.1907472116 .
Roy TK, Brandi S, Tappe NM, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29(11):2431–8. https://doi.org/10.1093/humrep/deu214 .
Arav A, Natan Y, Kalo D, et al. A new, simple, automatic vitrification device: preliminary results with murine and bovine oocytes and embryos. J Assist Reprod Genet. 2018;35(7):1161–8. https://doi.org/10.1007/s10815-018-1210-9 .
Canto MD, Moutier C, Brambillasca F, et al. Automated vitrification for embryo cryopreservation: preliminary comparative results and first live birth in Europe. Fertil Steril. 2019;112(3):e116–7. https://doi.org/10.1016/j.fertnstert.2019.07.425 .
Heo YS, Lee H-J, Hassell BA, et al. Controlled loading of cryoprotectants (CPAs) to oocyte with linear and complex CPA profiles on a microfluidic platform. Lab Chip. 2011;11(20):3530–7. https://doi.org/10.1039/c1lc20377k .
Pyne DG, Liu J, Abdelgawad M, Sun Y. Digital microfluidic processing of mammalian embryos for vitrification. PLoS One. 2014;9(9):e108128. https://doi.org/10.1371/journal.pone.0108128 .
Lai D, Ding J, Smith GW, Smith GD, Takayama S. Slow and steady cell shrinkage reduces osmotic stress in bovine and murine oocyte and zygote vitrification. Hum Reprod. 2015;30(1):37–45. https://doi.org/10.1093/humrep/deu284 .
Guo Y, Yang Y, Yi X, Zhou X. Microfluidic method reduces osmotic stress injury to oocytes during cryoprotectant addition and removal processes in porcine oocytes. Cryobiology. 2019;90:63–70. https://doi.org/10.1016/j.cryobiol.2019.08.005 .
Zhao G, Fu J. Microfluidics for cryopreservation. Biotechnol Adv. 2017;35(2):323–36. https://doi.org/10.1016/j.biotechadv.2017.01.006 .
Smith GD, Takayama S. Cryopreservation and microfluidics: a focus on the oocyte. Reprod Fertil Dev. 2018;31(1):93–104. https://doi.org/10.1071/RD18326 .
Yang Q, Zhu L, Jin L. Human follicle in vitro culture including activation, growth, and maturation: a review of research progress. Front Endocrinol (Lausanne). 2020;11:548. https://doi.org/10.3389/fendo.2020.00548 .
McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24(3):135–42. https://doi.org/10.1093/molehr/gay002 .
Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. https://doi.org/10.1038/ncomms15261 .
Salama M, Woodruff TK. From bench to bedside: current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet Gynecol Scand. 2019;98(5):659–64. https://doi.org/10.1111/aogs.13552 .
Xiao S, Coppeta JR, Rogers HB, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. https://doi.org/10.1038/ncomms14584 .
Nagamatsu G, Hayashi K. Stem cells, in vitro gametogenesis and male fertility. Reproduction. 2017;154(6):F79–91. https://doi.org/10.1530/REP-17-0510 .
Yamashiro C, Sasaki K, Yabuta Y, et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science. 2018;362(6412):356–60. https://doi.org/10.1126/science.aat1674 .
Ma H, O’Neil RC, Marti Gutierrez N, et al. Functional human oocytes generated by transfer of polar body genomes. Cell Stem Cell. 2017;20(1):112–9. https://doi.org/10.1016/j.stem.2016.10.001 .
White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–21. https://doi.org/10.1038/nm.2669 .
Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8(5):966–88. https://doi.org/10.1038/nprot.2013.047 .
Wagner M, Yoshihara M, Douagi I, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. https://doi.org/10.1038/s41467-020-14936-3 .
Ibtisham F, Honaramooz A. Spermatogonial stem cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells. 2020;9(3). https://doi.org/10.3390/cells9030745
Lei Q, Lai X, Eliveld J, Chuva de Sousa Lopes SM, van Pelt AMM, Hamer G. In vitro meiosis of male germline stem cells. Stem Cell Rep. 2020;15(5):1140–1153. https://doi.org/10.1016/j.stemcr.2020.10.006
Zhang XM, Wu K, Zheng Y, et al. In vitro expansion of human sperm through nuclear transfer. Cell Res. 2020;30(4):356–9. https://doi.org/10.1038/s41422-019-0265-1 .
Zhang L, Bao Y, Riaz M, et al. Population genomic screening of all young adults in a health-care system: a cost-effectiveness analysis. Genet Med. 2019;21(9):1958–68. https://doi.org/10.1038/s41436-019-0457-6 .
Turley P, Meyer MN, Wang N, et al. Problems with using polygenic scores to select embryos. N Engl J Med. 2021;385(1):78–86. https://doi.org/10.1056/NEJMsr2105065 .
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. https://doi.org/10.1126/science.1225829 .
Ma H, Marti-Gutierrez N, Park S-W, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–9. https://doi.org/10.1038/nature23305 .
Kang E, Wu J, Gutierrez NM, et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540(7632):270–5. https://doi.org/10.1038/nature20592 .
Adashi EY, Cohen IG, Hanna JH, Surani AM, Hayashi K. Stem cell-derived human gametes: the public engagement imperative. Trends Mol Med. 2019;25(3):165–7. https://doi.org/10.1016/j.molmed.2019.01.005 .
Adashi E, Cohen IG. Heritable genome editing-edited eggs and sperm to the rescue? JAMA. 2019;322(18):1754–5. https://doi.org/10.1001/jama.2019.17538 .
Cohen IG, Daley GQ, Adashi EY. Disruptive reproductive technologies. Sci Transl Med. 2017;9(372). https://doi.org/10.1126/scitranslmed.aag2959
Cohen GI, Adashi EY. The FDA is prohibited from going germline. Science. August 2016.
Mastenbroek S, de Wert G, Adashi EY. The imperative of responsible innovation in reproductive medicine. N Engl J Med. 2021;385(22):2096–100. https://doi.org/10.1056/NEJMsb2101718 .
Harper J, Jackson E, Sermon K, et al. Adjuncts in the IVF laboratory: where is the evidence for “add-on” interventions? Hum Reprod. 2017;32(3):485–91. https://doi.org/10.1093/humrep/dex004 .
Special collection - in vitro fertilisation – effectiveness of add-ons | Cochrane Library. https://www.cochranelibrary.com/collections/doi/SC000046/full . Published July 15, 2021. Accessed Dec 6, 2021.
Download references
Author information
Authors and affiliations.
Department of Obstetrics and Gynecology, University of California Irvine, 333 City Blvd, W. Ste. 1400, Orange, CA, 92868, USA
Vitaly A. Kushnir
Departments of Molecular and Integrative Physiology, Obstetrics and Gynecology, Urology, University of Michigan, 4422 MS1 1301 E, Catherine St. Ann Arbor, MI, 48109, USA
Gary D. Smith
Medical Science, Medicine and Biological Sciences, Brown University, 222 Richmond Street, Providence, RI, 02903, USA
Eli Y. Adashi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Vitaly A. Kushnir .
Ethics declarations
Ethics approval, consent to participate, consent for publication.
The authors consent.
Conflict of Interest
Dr. Kushnir is an inventor on patents related to clinical use of recombinant anti-müllerian hormone and a consultant for medical insurance companies. Professor Smith is an inventor and jointly holds patents with the University of Michigan related to microfluidics and gamete isolation, in vitro fertilization, embryo culture and cryopreservation. Dr. Smith served as consultant for Overture Life.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Kushnir, V.A., Smith, G.D. & Adashi, E.Y. The Future of IVF: The New Normal in Human Reproduction. Reprod. Sci. 29 , 849–856 (2022). https://doi.org/10.1007/s43032-021-00829-3
Download citation
Received : 08 September 2021
Accepted : 09 December 2021
Published : 03 January 2022
Issue Date : March 2022
DOI : https://doi.org/10.1007/s43032-021-00829-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Microfluidics
- Reproductive genetics
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 20 November 2023
Changing the public perception of human embryology
- Nicolas C. Rivron ORCID: orcid.org/0000-0003-1590-5964 1 ,
- Alfonso Martinez-Arias ORCID: orcid.org/0000-0002-1781-564X 2 ,
- Karen Sermon 3 , 4 ,
- Christine Mummery ORCID: orcid.org/0000-0002-4549-6535 5 ,
- Hans R. Schöler 6 ,
- James Wells ORCID: orcid.org/0000-0002-1398-848X 7 , 8 ,
- Jenny Nichols 9 ,
- Anna-Katerina Hadjantonakis ORCID: orcid.org/0000-0002-7580-5124 10 ,
- Madeline A. Lancaster ORCID: orcid.org/0000-0003-2324-8853 11 ,
- Naomi Moris 12 ,
- Jianping Fu ORCID: orcid.org/0000-0001-9629-6739 13 , 14 , 15 ,
- Roger G. Sturmey 16 ,
- Kathy Niakan ORCID: orcid.org/0000-0003-1646-4734 17 , 18 , 19 , 20 , 21 ,
- Janet Rossant ORCID: orcid.org/0000-0002-3731-5466 22 &
- Kazuto Kato 23 , 24
Nature Cell Biology volume 25 , pages 1717–1719 ( 2023 ) Cite this article
2627 Accesses
42 Altmetric
Metrics details
- Embryonic stem cells
An Author Correction to this article was published on 27 November 2023
This article has been updated
Human embryology is flourishing thanks to an impetus provided by embryo models formed from stem cells. These scientific advances require meticulous experimental work and a refined ethical framework, but also sensible public communication. Securing public support is essential to achieve societal impact.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Change history
27 november 2023.
A Correction to this paper has been published: https://doi.org/10.1038/s41556-023-01319-1
Ball, P. Book. Unnatural: The Heretical Idea of Making People (Random House, 2011).
Braude, P. et al. BJOG 126 , 135–137 (2019).
Lovell-Badge, R. et al. Stem Cell Rep. 16 , 1398–1408 (2021).
Article Google Scholar
ISSCR. Guidelines for Stem Cell Research and Clinical Translation https://www.isscr.org/guidelines (accessed September 2023).
Zhao, C. et al. Genome Res. 32 , 1627–1641 (2022).
Article PubMed PubMed Central Google Scholar
Rivron, N. et al. Nature 564 , 183–185 (2018).
Article CAS PubMed Google Scholar
Hyun, I., Munsie, M., Pera, M. F., Rivron, N. C. & Rossant, J. Stem Cell Rep. 14 , 169–174 (2020).
Article CAS Google Scholar
Clark, A. T. et al. Stem Cell Rep. 16 , 1416–1424 (2021).
Rivron, N. C., Martinez Arias, A., Pera, M. F., Moris, N. & M’hamdi, H. I. Cell 186 , 3548–3557 (2023).
Foreman, A. L. et al. Curr. Opin. Genet. Dev. 82 , 102103 (2023).
Blasimme, A. & Sugarman, J. Cell Stem Cell 30 , 1008–1012 (2023).
Rossant, J. & Fu, J. Nature 622 , 454–456 (2023).
Agence de la Biomédicine. https://go.nature.com/3u9aghe (11 October 2023).
Landecker, H. L. & Clark, A. T. Cell Stem Cell 30 , 1290–1293 (2023).
ISSCR. The ISSCR Statement on New Research with Embryo Models https://go.nature.com/49vrHc4 (26 June 2023).
Download references
Author information
Authors and affiliations.
Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA), Vienna BioCenter (VBC), Vienna, Austria
Nicolas C. Rivron
Systems Bioengineering, MELIS, Universidad Pompeu Fabra and Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain
Alfonso Martinez-Arias
Research Group Reproduction and Genetics, Vrije Universiteit Brussel, Brussels, Belgium
Karen Sermon
European Society for Human Reproduction and Embryology (ESHRE), Strombeek-Bever, Belgium
Leiden University Medical Center, Leiden, the Netherlands
Christine Mummery
Max Planck Institute for Molecular Biomedicine, Münster, Germany
Hans R. Schöler
Center for Stem Cell and Organoid Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
James Wells
Division of Developmental Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
MRC Human Genetics Unit, Institute of Genetics and Cancer, The University of Edinburgh, Crewe Road, Edinburgh, UK
Jenny Nichols
Developmental Biology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Anna-Katerina Hadjantonakis
MRC Laboratory of Molecular Biology, Cambridge, UK
Madeline A. Lancaster
The Francis Crick Institute, London, UK
Naomi Moris
Department of Mechanical Engineering, University of Michigan, Ann Arbor, MI, USA
Jianping Fu
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA
Department of Cell & Developmental Biology, University of Michigan Medical School, Ann Arbor, MI, USA
Biomedical Institute for Multimorbidity, Hull York Medical School, University of Hull, Hull, UK
Roger G. Sturmey
Cambridge Reproduction, University of Cambridge, Cambridge, UK
Kathy Niakan
The Centre for Trophoblast Research, Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge, UK
Human Embryo and Stem Cell Laboratory, The Francis Crick Institute, London, UK
Wellcome Trust–Medical Research Council Stem Cell Institute, University of Cambridge, Jeffrey Cheah Biomedical Centre, Cambridge, UK
Epigenetics Programme, Babraham Institute, Cambridge, UK
The Hospital for Sick Children, Toronto, ON, Canada
Janet Rossant
Department of Biomedical Ethics and Public Policy, Graduate School of Medicine, Osaka University, Suita, Japan
Kazuto Kato
Ethics Committee, International Society for Stem Cell Research, Evanston, IL, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nicolas C. Rivron .
Ethics declarations
Competing interests.
N.C.R. is an inventor on the patents “Blastoid, cell line based artificial blastocyst” (EP2986711) and “Blastocyst-like cell aggregate and methods” (EP21151455.9), which are both licensed to dawn-bio, a company he co-founded. A.M.A. and N.M. are inventors on the patents “Polarised three-dimensional cellular aggregates” (PCT/GB2019/052668) and “Human polarised three-dimensional cellular” (PCT/GB2019/052670), maintained by Cambridge Enterprise.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rivron, N.C., Martinez-Arias, A., Sermon, K. et al. Changing the public perception of human embryology. Nat Cell Biol 25 , 1717–1719 (2023). https://doi.org/10.1038/s41556-023-01289-4
Download citation
Published : 20 November 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s41556-023-01289-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Help | Advanced Search
Computer Science > Computation and Language
Title: leave no context behind: efficient infinite context transformers with infini-attention.
Abstract: This work introduces an efficient method to scale Transformer-based Large Language Models (LLMs) to infinitely long inputs with bounded memory and computation. A key component in our proposed approach is a new attention technique dubbed Infini-attention. The Infini-attention incorporates a compressive memory into the vanilla attention mechanism and builds in both masked local attention and long-term linear attention mechanisms in a single Transformer block. We demonstrate the effectiveness of our approach on long-context language modeling benchmarks, 1M sequence length passkey context block retrieval and 500K length book summarization tasks with 1B and 8B LLMs. Our approach introduces minimal bounded memory parameters and enables fast streaming inference for LLMs.

Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
With inspiration from “Tetris,” MIT researchers develop a better radiation detector
Press contact :, media download.

*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."

Previous image Next image
The spread of radioactive isotopes from the Fukushima Daiichi Nuclear Power Plant in Japan in 2011 and the ongoing threat of a possible release of radiation from the Zaporizhzhia nuclear complex in the Ukrainian war zone have underscored the need for effective and reliable ways of detecting and monitoring radioactive isotopes. Less dramatically, everyday operations of nuclear reactors, mining and processing of uranium into fuel rods, and the disposal of spent nuclear fuel also require monitoring of radioisotope release.
Now, researchers at MIT and the Lawrence Berkeley National Laboratory (LBNL) have come up with a computational basis for designing very simple, streamlined versions of sensor setups that can pinpoint the direction of a distributed source of radiation. They also demonstrated that by moving that sensor around to get multiple readings, they can pinpoint the physical location of the source. The inspiration for their clever innovation came from a surprising source: the popular computer game “Tetris.”
The team’s findings, which could likely be generalized to detectors for other kinds of radiation, are described in a paper published in Nature Communications , by MIT professors Mingda Li, and Benoit Forget, senior research scientist Lin-Wen Hu, and principal research scientist Gordon Kohse; graduate students Ryotaro Okabe and Shangjie Xue; research scientist Jayson Vavrek SM ’16, PhD ’19 at LBNL; and a number of others at MIT and Lawrence Berkeley.
Radiation is usually detected using semiconductor materials, such as cadmium zinc telluride, that produce an electrical response when struck by high-energy radiation such as gamma rays. But because radiation penetrates so readily through matter, it’s difficult to determine the direction that signal came from with simple counting. Geiger counters, for example, simply provide a click sound when receiving radiation, without resolving the energy or type, so finding a source requires moving around to try to find the maximum sound, similarly to how handheld metal detectors work. The process requires the user to move closer to the source of radiation, which can add risk.
To provide directional information from a stationary device without getting too close, researchers use an array of detector grids along with another grid called a mask, which imprints a pattern on the array that differs depending on the direction of the source. An algorithm interprets the different timings and intensities of signals received by each separate detector or pixel. This often leads to a complex design of detectors.
Typical detector arrays for sensing the direction of radiation sources are large and expensive and include at least 100 pixels in a 10 by 10 array. However, the group found that using as few as four pixels arranged in the tetromino shapes of the figures in the “Tetris” game can come close to matching the accuracy of the large, expensive systems. The key is proper computerized reconstruction of the angles of arrival of the rays, based on the times each sensor detects the signal and the relative intensity each one detects, as reconstructed through an AI-guided study of simulated systems.
Of the different configurations of four pixels the researchers tried — square, or S-, J- or T-shaped — they found through repeated experiments that the most precise results were provided by the S-shaped array. This array gave directional readings that were accurate to within about 1 degree, but all three of the irregular shapes performed better than the square. This approach, Li says, “was literally inspired by ‘Tetris.’”
Key to making the system work is placing an insulating material such as a lead sheet between the pixels to increase the contrast between radiation readings coming into the detector from different directions. The lead between the pixels in these simplified arrays serves the same function as the more elaborate shadow masks used in the larger-array systems. Less symmetrical arrangements, the team found, provide more useful information from a small array, explains Okabe, who is the lead author of the work.
“The merit of using a small detector is in terms of engineering costs,” he says. Not only are the individual detector elements expensive, typically made of cadmium-zinc-telluride, or CZT, but all of the interconnections carrying information from those pixels also become much more complex. “The smaller and simpler the detector is, the better it is in terms of applications,” adds Li.
While there have been other versions of simplified arrays for radiation detection, many are only effective if the radiation is coming from a single localized source. They can be confused by multiple sources or those that are spread out in space, while the “Tetris”-based version can handle these situations well, adds Xue, co-lead author of the work.
In a single-blind field test at the Berkeley Lab with a real cesium radiation source, led by Vavrek, where the researchers at MIT did not know the ground-truth source location, a test device was performed with high accuracy in finding the direction and distance to the source.
“Radiation mapping is of utmost importance to the nuclear industry, as it can help rapidly locate sources of radiation and keep everyone safe,” says co-author Forget, an MIT professor of nuclear engineering and head of the Department of Nuclear Science and Engineering.
Vavrek, another co-lead-author, says that while in their study they focused on gamma-ray sources, he believes the computational tools they developed to extract directional information from the limited number of pixels are “much, much more general.” It isn’t restricted to certain wavelengths, it can also be used for neutrons, or even other forms of light, such as ultraviolet light. Using this machine learning-based algorithm and aerial radiation detection “will allow real-time monitoring and integrated emergency planning of radiological accidents,” adds Hu, a senior scientist at the MIT Nuclear Reactor Lab.
Nick Mann, a scientist with the Defense Systems branch at the Idaho National Laboratory, says, "This work is critical to the U.S. response community and the ever-increasing threat of a radiological incident or accident.”
Additional research team members include Ryan Pavlovsky, Victor Negut, Brian Quiter, and Joshua Cates at Lawrence Berkely National Laboratory, and Jiankai Yu, Tongtong Liu, Stephanie Jegelka at MIT. The work was supported by the U.S. Department of Energy.
Share this news article on:
Related links.
- Benoit Forget
- Quantum Measurement Group
- Department of Nuclear Science and Engineering
- Nuclear Reactor Laboratory
Related Topics
- Computer science and technology
- Electronics
- Nuclear science and engineering
- Nuclear Reactor Lab
- Department of Energy (DoE)
Related Articles

A faster experiment to find and study topological materials
Researchers harness 2D magnetic materials for energy-efficient computing

Physicists trap electrons in a 3D crystal for the first time
Making more magnetism possible with topology
Previous item Next item
More MIT News

From neurons to learning and memory
Read full story →

A biomedical engineer pivots from human movement to women’s health

MIT tops among single-campus universities in US patents granted

A new way to detect radiation involving cheap ceramics

A crossroads for computing at MIT

Growing our donated organ supply
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
About 1 in 4 u.s. teachers say their school went into a gun-related lockdown in the last school year.
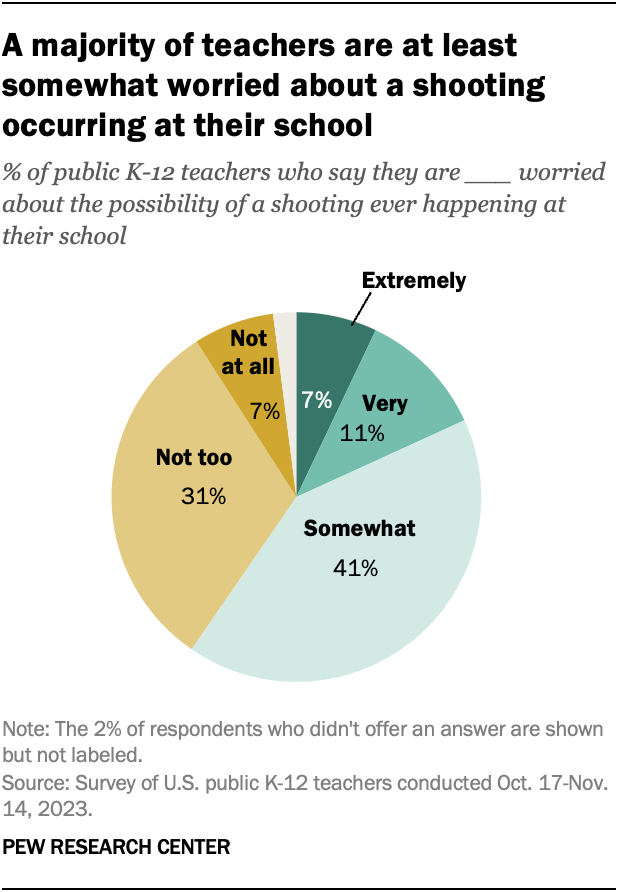
Twenty-five years after the mass shooting at Columbine High School in Colorado , a majority of public K-12 teachers (59%) say they are at least somewhat worried about the possibility of a shooting ever happening at their school. This includes 18% who say they’re extremely or very worried, according to a new Pew Research Center survey.
Pew Research Center conducted this analysis to better understand public K-12 teachers’ views on school shootings, how prepared they feel for a potential active shooter, and how they feel about policies that could help prevent future shootings.
To do this, we surveyed 2,531 U.S. public K-12 teachers from Oct. 17 to Nov. 14, 2023. The teachers are members of RAND’s American Teacher Panel, a nationally representative panel of public school K-12 teachers recruited through MDR Education. Survey data is weighted to state and national teacher characteristics to account for differences in sampling and response to ensure they are representative of the target population.
We also used data from our 2022 survey of U.S. parents. For that project, we surveyed 3,757 U.S. parents with at least one child younger than 18 from Sept. 20 to Oct. 2, 2022. Find more details about the survey of parents here .
Here are the questions used for this analysis , along with responses, and the survey methodology .
Another 31% of teachers say they are not too worried about a shooting occurring at their school. Only 7% of teachers say they are not at all worried.
This survey comes at a time when school shootings are at a record high (82 in 2023) and gun safety continues to be a topic in 2024 election campaigns .
Teachers’ experiences with lockdowns
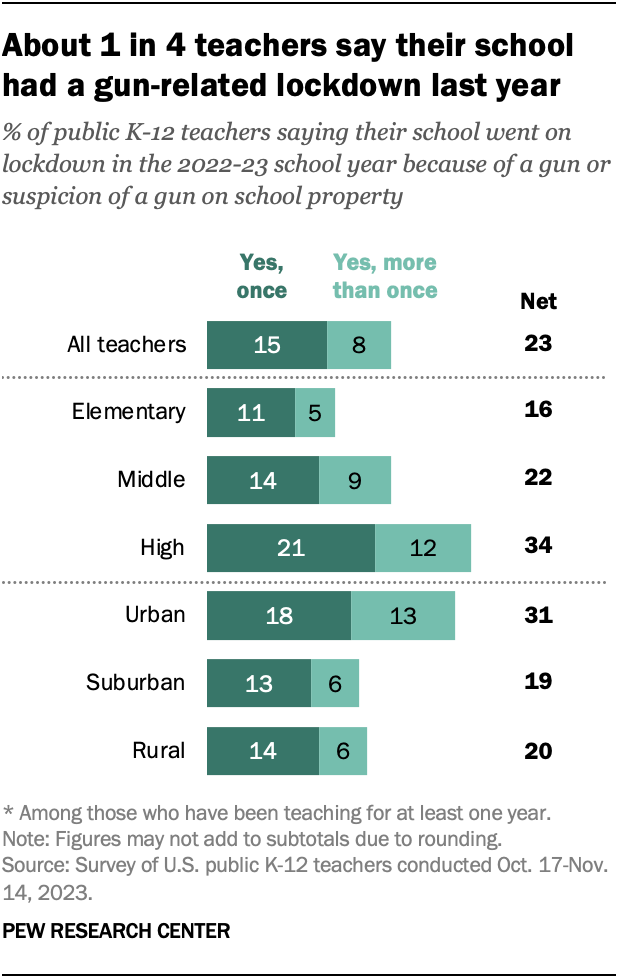
About a quarter of teachers (23%) say they experienced a lockdown in the 2022-23 school year because of a gun or suspicion of a gun at their school. Some 15% say this happened once during the year, and 8% say this happened more than once.
High school teachers are most likely to report experiencing these lockdowns: 34% say their school went on at least one gun-related lockdown in the last school year. This compares with 22% of middle school teachers and 16% of elementary school teachers.
Teachers in urban schools are also more likely to say that their school had a gun-related lockdown. About a third of these teachers (31%) say this, compared with 19% of teachers in suburban schools and 20% in rural schools.
Do teachers feel their school has prepared them for an active shooter?
About four-in-ten teachers (39%) say their school has done a fair or poor job providing them with the training and resources they need to deal with a potential active shooter.
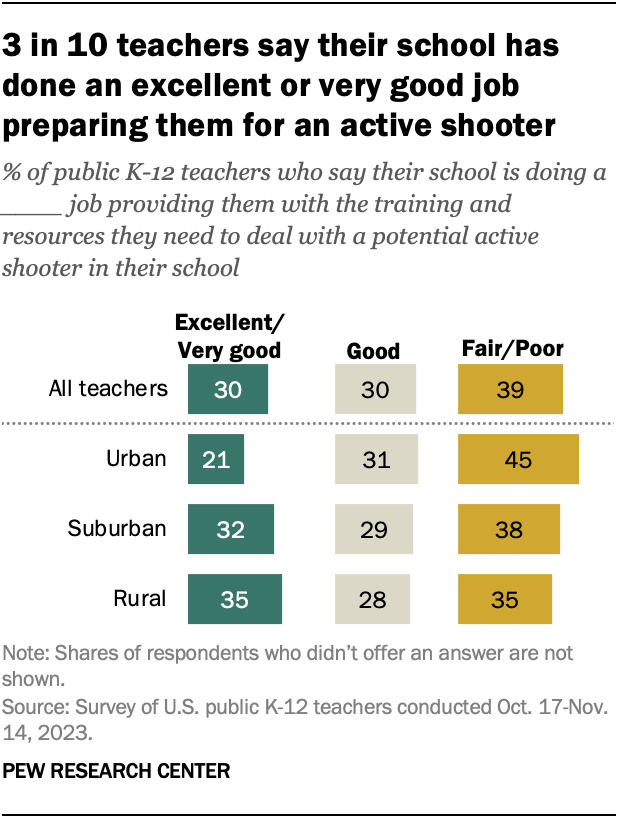
A smaller share (30%) give their school an excellent or very good rating, and another 30% say their school has done a good job preparing them.
Teachers in urban schools are the least likely to say their school has done an excellent or very good job preparing them for a potential active shooter. About one-in-five (21%) say this, compared with 32% of teachers in suburban schools and 35% in rural schools.
Teachers who have police officers or armed security stationed in their school are more likely than those who don’t to say their school has done an excellent or very good job preparing them for a potential active shooter (36% vs. 22%).
Overall, 56% of teachers say they have police officers or armed security stationed at their school. Majorities in rural schools (64%) and suburban schools (56%) say this, compared with 48% in urban schools.
Only 3% of teachers say teachers and administrators at their school are allowed to carry guns in school. This is slightly more common in school districts where a majority of voters cast ballots for Donald Trump in 2020 than in school districts where a majority of voters cast ballots for Joe Biden (5% vs. 1%).
What strategies do teachers think could help prevent school shootings?
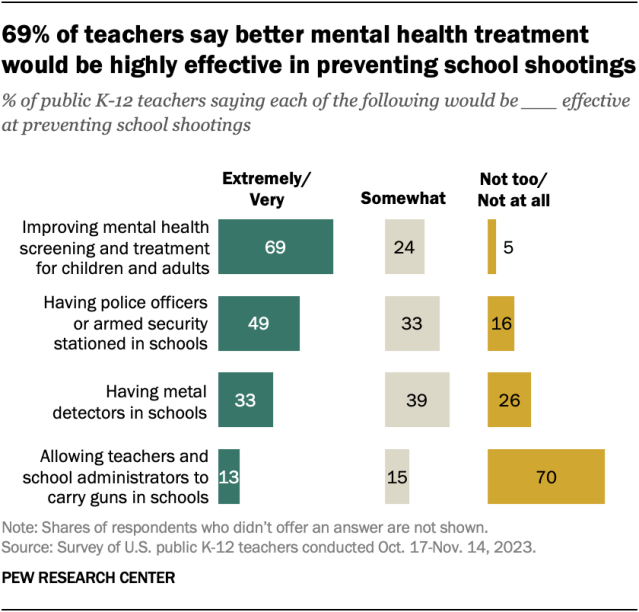
The survey also asked teachers how effective some measures would be at preventing school shootings.
Most teachers (69%) say improving mental health screening and treatment for children and adults would be extremely or very effective.
About half (49%) say having police officers or armed security in schools would be highly effective, while 33% say the same about metal detectors in schools.
Just 13% say allowing teachers and school administrators to carry guns in schools would be extremely or very effective at preventing school shootings. Seven-in-ten teachers say this would be not too or not at all effective.
How teachers’ views differ by party
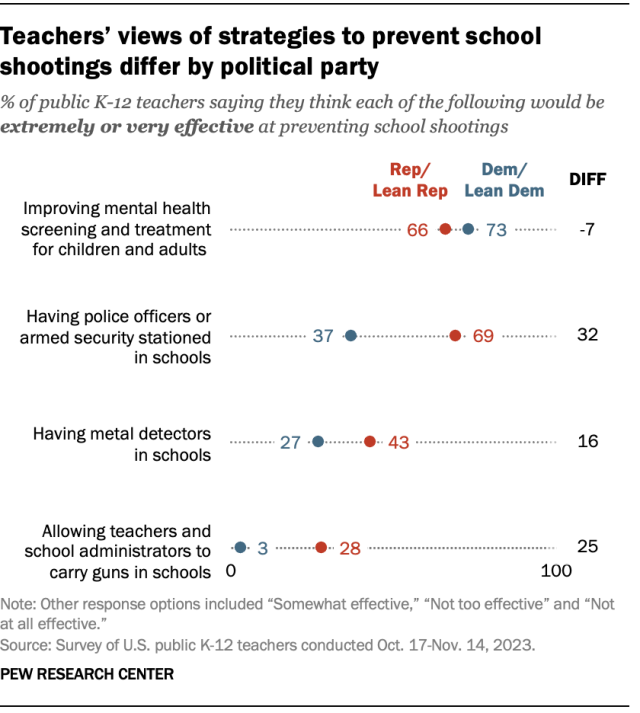
Republican and Republican-leaning teachers are more likely than Democratic and Democratic-leaning teachers to say each of the following would be highly effective:
- Having police officers or armed security in schools (69% vs. 37%)
- Having metal detectors in schools (43% vs. 27%)
- Allowing teachers and school administrators to carry guns in schools (28% vs. 3%)
And while majorities in both parties say improving mental health screening and treatment would be highly effective at preventing school shootings, Democratic teachers are more likely than Republican teachers to say this (73% vs. 66%).
Parents’ views on school shootings and prevention strategies
In fall 2022, we asked parents a similar set of questions about school shootings.
Roughly a third of parents with K-12 students (32%) said they were extremely or very worried about a shooting ever happening at their child’s school. An additional 37% said they were somewhat worried.
As is the case among teachers, improving mental health screening and treatment was the only strategy most parents (63%) said would be extremely or very effective at preventing school shootings. And allowing teachers and school administrators to carry guns in schools was seen as the least effective – in fact, half of parents said this would be not too or not at all effective. This question was asked of all parents with a child younger than 18, regardless of whether they have a child in K-12 schools.
Like teachers, parents’ views on strategies for preventing school shootings differed by party.
Note: Here are the questions used for this analysis , along with responses, and the survey methodology .

Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
‘Back to school’ means anytime from late July to after Labor Day, depending on where in the U.S. you live
Among many u.s. children, reading for fun has become less common, federal data shows, most european students learn english in school, for u.s. teens today, summer means more schooling and less leisure time than in the past, about one-in-six u.s. teachers work second jobs – and not just in the summer, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Stem Cell Res Ther
- PMC10441753

Ethical, legal, regulatory, and policy issues concerning embryoids: a systematic review of the literature
Ana s. iltis.
1 Center for Bioethics, Health and Society and Department of Philosophy, Wake Forest University, Winston-Salem, NC 27106 USA
Grace Koster
Emily reeves, kirstin r. w. matthews.
2 Baker Institute for Public Policy, Rice University, Houston, TX 77005 USA
Associated Data
Datasets generated and analyzed during the current study are available from the first author upon request.
Recent advances in methods to culture pluripotent stem cells to model human development have resulted in entities that increasingly have recapitulated advanced stages of early embryo development. These entities, referred to by numerous terms such as embryoids, are becoming more sophisticated and could resemble human embryos ever more closely as research progresses. This paper reports a systematic review of the ethical, legal, regulatory, and policy questions and concerns found in the literature concerning human embryoid research published from 2016 to 2022. We identified 56 papers that use 53 distinct names or terms to refer to embryoids and four broad categories of ethical, legal, regulatory, or policy considerations in the literature: research justifications/benefits, ethical significance or moral status, permissible use, and regulatory and oversight challenges. Analyzing the full range of issues is a critical step toward fostering more robust ethical, legal, and social implications research in this emerging area and toward developing appropriate oversight.
Methods to culture stem cells to model early human development have been reported since 2014 [ 1 , 2 ]. Recent advances have resulted in entities that model different stages of early embryo development—from the blastocyst stage at 5 days post-fertilization (dpf), to gastrulation at 17 dpf, to later stages of organogenesis [ 3 – 8 ]. These entities are referred to by numerous terms, including embryoids, synthetic embryos, gastruloids, and blastoids [ 9 ]. For ease, we will refer to them as embryoids, a general name for all types of cell models of early development.
As science and technology advance, researchers anticipate embryoids will become more sophisticated and resemble human embryos ever more closely [ 8 , 9 ]. In 2022, researchers were able to grow a mouse embryoid in culture from a cell line to a synthetic embryo that had the early formation of organs and limbs [ 10 ]. This new technology, which has only been used on mouse cells, raises concerns regarding how far scientists can and should grow human embryos and embryoids in culture [ 8 , 9 ].
Embryoid, embryo, and human–animal chimera research raise a number of sometimes-overlapping ethical, regulatory, and policy issues, though they merit separate attention. Numerous scholars have written about ethical questions or concerns associated with embryoid research or that might arise as the science of embryoids advances [ 11 , 12 ]. Others have highlighted policy or regulatory issues that such research prompts both regarding the status of embryoid research and the relationship between such research and human embryo research. Two recent systematic reviews examined ethical issues associated with organoids [ 11 , 12 ]. We know of no systematic review regarding ethical, legal, regulatory, and policy issues regarding embryoids. Analyzing the full range of issues identified in the literature pertaining to research on human embryoids (or any stem cell-based models of early human development, regardless of the names used to describe them) is an important step toward fostering more robust ethical, legal, and social implications research in this area and developing appropriate oversight.
This systematic review followed PRISMA reporting guidelines [ 13 , 14 ]. The protocol for this paper was published in Open Science Forum and registered on June 16, 2022. In consultation with reference librarians from the Z. Smith Reynolds Library at Wake Forest University, J. Denice Lewis and Kathy Shields, we designed a search strategy that included three databases: PubMed, Embase, and Web of Science. The search strategy for each database is in Appendix 1. No date limits were used. Language limits were applied to restrict publications to English. All searches were completed on January 10, 2022. Searches were imported into Rayyan for de-duplication and then imported to Zotero. Through consultation with authors and review of reference sections of included publications, additional possible publications were identified.
To be eligible, publications had to:
- Be discoverable using our search strategy or identified by an author;
- Be published in English;
- Be accessible via full text to us either online, through the Wake Forest University or Rice University Library, or through Interlibrary Loan; and
- Identify at least one ethical, regulatory, or policy question or issue related to research involving embryoids (or any term used to describe a stem cell-based model of human embryos).
Consistent with published recommendations for systematic reviews, we included abstracts and dissertations [ 15 , 16 ]. We also included commentaries, editorials, and other types of publications to maximize data collection.
Following PRISMA reporting guidelines, Fig. 1 presents the selection process [ 13 , 14 ]. Initial screening of titles and abstracts was done by one author (ASI) using Rayyan web-based systematic review software. If the abstract did not clearly indicate that an article should be excluded, it was tagged as a “Maybe” and advanced to the next round. In the second phase, three authors (ASI, GK, ER) screened 20 of the same publications to promote consistency and inter-rater reliability. Assessments were compared after screening 10 entries and differences discussed. The same process was repeated with 10 more entries. After that, two authors (either ASI and GK or ASI and ER) screened all remaining titles and abstracts. All articles were excluded based on the highest ranking exclusion criterion in our exclusion hierarchy (see Table Table1), 1 ), included, or tagged “Maybe” for full-text screening. We obtained full texts for all publications marked “Maybe” or “Include.” Full texts were stored and reviewed in Zotero. Two authors (either ASI and GK or ASI and ER) reviewed all full-text publications and either included or excluded each one. Where necessary, we consulted with other authors to make inclusion and exclusion decisions. At each stage, all differences were resolved through discussion and review of the material, allowing us to have 100% consensus. Two duplicates that had not been previously detected were found during the screening process and removed manually.

PRISMA flow diagram [ 92 ]
Exclusion hierarchy
The reference section of each included publication was reviewed for additional publications to assess. Authors were invited to recommend additional reports or publications for screening. These were screened by two authors (either ASI and GK or ASI and ER), and often in consultation with KRWM, they were marked for inclusion or exclusion.
Data extraction and synthesis
For each included publication, data were extracted independently by two authors (either ASI and GK or ASI and ER) and entered into a data extraction form created using Google Sheets. Data items extracted were:
- Complete citation
- Publication type
- Year published
- Terms used to refer to embryoids
- Background information
- Ethical issues identified
- Commentary on ethical issues
- Policy or Regulatory issues identified
- Commentary on policy or ethical issues
Three authors (ASI, GK, and ER) reviewed the extracted data to combine information, resulting in one comprehensive data sheet. The results were shared with all authors. Through qualitative content analysis, authors identified the themes and identified categories and sub-categories reported here [ 17 ].
The initial search yielded 6536 publications. These were imported into Rayyan for de-duplication. After removing 1794 duplicates, 4472 records were screened. After screening titles and abstracts, 4623 publications were excluded because they did not address human embryoids or synthetic embryos. These articles concerned animal models or addressed only “embryoid bodies,” “organoids,” or other models that were not models of human embryos. Full texts were retrieved for the remaining 119 publications. Two additional duplicates were found and removed manually, leaving 117 publications for full-text screening. These were added to Zotero, and full texts were obtained. Of those 117 publications, 83 were excluded because they were not about human models ( n = 2), they were not about embryoids ( n = 65) or they did not address any ethical, regulatory, oversight, or policy issues ( n = 16), and 34 were included. The references of the 34 included publications were reviewed for additional publications to screen, and all authors were invited to share information regarding other possible publications or reports to consider. An additional 22 publications were identified this way and screened. All 22 met our inclusion criteria. A total of 56 publications were included in the review. One of these was an erratum for another included publication [ 18 , 19 ]. Included publications are listed in Table Table2 2 .
Included publications
We identified 53 distinct names or terms used to refer to embryoids (see Table Table3). 3 ). There are three different types of terms used to identify embryoids previously described: general, time-based, and cell-based [ 9 ]. Some terms, such as embryoid or cell-based embryo model, describe the field as a whole. Other terms, such as blastoid or gastruloid, identify a subset of entities at a specific biological moment that they are recapitulating, the blastocyst or gastrulation, respectively. Still other terms identify the cell types used in the model, for example, ETX embryos which describe embryoids using embryonic, trophoblast, and extra-embryonic endoderm stem cells [ 20 ].
Names identified
Through an iterative inductive process, we identified four broad categories of ethical, legal, regulatory, or policy considerations found in the literature, each of which is discussed in more detail below: research justifications/potential benefits, ethical significance or moral status, permissible use, and regulatory and oversight challenges. As depicted in Fig. 2 , the majority of papers included discussions about oversight, policies and regulations (Policies, n = 45), and the ethical significance or moral status of embryoids (Status, n = 40). Fewer publications discussed potential benefits (Benefits, n = 28) and uses and applications of embryoid research (Uses, n = 25). One publication stated that embryoid research raises no ethical or regulatory considerations (None, n = 1).

Major themes identified
Justifications and potential benefits of embryoid research
Some authors noted that embryoid research requires justification and many indicated that potential benefits associated with the research could justify it ( n = 28) [ 9 , 21 – 47 ]. Over one-third of the publications ( n = 19) noted that embryoid research could avoid some of the ethical concerns or practical problems associated with human embryo research [ 9 , 21 – 37 , 47 ]. Several scholars also believed that embryoid research could offer an ethical alternative to animal research and reduce reliance on animals ( n = 8) [ 22 , 26 , 30 , 31 , 35 , 38 , 39 , 47 ]. A third potential benefit noted is that embryoid research could improve scientific knowledge in ways that advance human health ( n = 12) [ 9 , 21 , 22 , 24 , 26 , 30 , 36 , 40 – 42 , 45 , 46 ]. Specific examples of potential health benefits include knowledge that could improve understanding of early pregnancy loss, management of early pregnancy, and treating early developmental disorders [ 22 ]. Although embryoid research can raise concerns over the destruction of human embryos, it was suggested that when scientists use induced pluripotent stem cells (which are derived from adult cells) rather than embryonic stem cells, they can avoid these issues ( n = 3) [ 9 , 29 , 33 ]. Interestingly, the view that embryoid research requires justification based on potential benefits might suggest that such research raises ethical considerations. This contrasts with the view expressed in one publication that embryoid research raises no ethical considerations: “iBlastoids [embryo-like structures] do not pose any serious ethical concern for several reasons and would not need a robust ethical framework that thoughtfully foresees unintended and unanticipated consequences” [ 48 ]. The full list of potential benefits that could justify embryoid research are also summarized in Table Table4 4 .
Main ethical, legal, regulatory, and policy issues and themes identified in journal articles
Ethical significance or moral status of embryoids
Several authors mentioned issues related to assessing the ethical significance or moral status of embryoids (summarized in Table Table4). 4 ). At the most general level, some authors ( n = 5) noted that the possibility of creating synthetic human life raises concerns and suggested that embryoids are or could become sufficiently complex that they constitute synthetic human life [ 38 , 49 – 52 ]. Three publications noted the importance of determining the features of an entity that are morally relevant and how those features relate to an entity’s moral status [ 49 , 53 , 54 ]. A related claim was that there could be ethically significant differences among different types of embryoids based on their features ( n = 4) [ 9 , 47 , 49 , 55 ]. Many other publications addressed the issue of morally relevant features in more detail by addressing specific features. Some authors noted that the level of complexity of embryoids would affect the ethical issues such research raises ( n = 9) [ 41 , 49 , 51 , 54 , 56 – 60 ]. Specific morally relevant or potentially morally relevant features identified in the publications reviewed included the possibility of experiencing pain ( n = 7) [ 18 , 36 , 43 , 44 , 53 , 57 , 61 ], the possibility of sentience ( n = 5) [ 18 , 36 , 43 , 57 , 62 ], and human organismic potential ( n = 22) [ 9 , 18 , 21 , 23 , 26 , 35 – 37 , 41 , 49 , 50 , 52 – 54 , 59 – 66 ].
In addition, authors discussed the ethical significance or status of embryoids in relation to human embryos and clones. According to some authors, the ethical significance or status of embryoids could be assessed by first determining which features of embryos are morally relevant and then determining which of those features also appear in embryoids ( n = 9) [ 18 , 30 , 31 , 37 , 41 , 44 , 57 , 63 , 64 ]. Many publications addressed the relationship between ethical assessments of embryos and embryoids. Some suggested that different accounts of the moral significance of human embryos likely would result in different assessments of embryoids ( n = 9) [ 18 , 21 , 28 , 38 , 41 , 55 , 61 , 62 , 67 ]. Several publications raised the question of whether embryos and embryoids should be treated the same or differently ( n = 7) [ 9 , 37 , 41 , 55 , 57 , 60 , 61 ]. Two publications noted that embryoid research might lead to reconsideration of the question of what respect is owed to embryos [ 54 , 63 ]. Another issue that draws on the connection between embryo and embryoid research was the observation that embryoid research could raise the same concerns that human embryonic stem cell (hESC) research raises insofar as both involve embryo destruction ( n = 2) [ 28 , 68 ]. A final set of issues regarding the ethical significance of embryoids was the question of whether they are clones and, if so, what concerns that might raise ( n = 10) [ 21 , 39 , 45 , 48 , 52 , 53 , 61 , 62 , 65 , 69 ].
Permissible uses of embryoid research
A third category of ethical issues concerns permissible uses or applications of embryoid research (summarized in Table Table4). 4 ). While several publications noted the need to set some limits on the use of embryoids ( n = 3) [ 34 , 50 , 51 ], one publication questioned whether it would be possible to effectively draw lines limiting such research [ 49 ]. Three publications noted that while much attention is paid to the uncertain moral status of embryoids, resolving those questions will not necessarily resolve the question of whether embryoid research is permissible and, if so, which research is permissible ( n = 3) [ 41 , 55 , 62 ]. Other articles questioned whether the intentions of researchers, such as the absence of reproductive intentions, were relevant to assessing the permissibility of embryoid research ( n = 7) [ 21 , 35 , 37 , 41 , 48 , 57 , 61 ]. One publication raised concerns regarding the commercialization of human tissue [ 40 ]. Finally, some authors raised questions about banning the use embryoids for reproduction ( n = 10) [ 22 , 37 , 39 , 47 , 48 , 52 , 53 , 60 , 67 , 69 ] or to create chimeras ( n = 3) [ 18 , 52 , 53 ].
Regulatory, and policy considerations regarding embryoid research
A significant number of publications addressed issues related to regulations, oversight mechanisms, guidelines, or policies pertaining to embryoid research (Table (Table4). 4 ). These fell into three sub-categories. The first sub-category concerns the relationship between regulation or oversight of embryoid research and previously existing guidelines for human embryo and hESC research. A large group of publications acknowledge that existing human embryo research and cloning laws, policies, and regulations have unclear implications for embryoid research ( n = 23) [ 9 , 18 , 21 , 23 , 27 , 28 , 33 , 34 , 41 , 44 , 50 – 52 , 55 , 57 , 60 , 62 , 64 , 66 , 67 , 70 – 72 ]. Some stated that the regulations do not apply directly, creating a regulatory gap ( n = 3) [ 41 , 60 , 62 ]. Scholars suggest that the major reason for a regulatory gap is that embryoids do not follow canonical embryogenesis, making references to the 14-day rule or appearance of the primitive streak irrelevant ( n = 13) [ 9 , 18 , 27 , 31 , 34 , 37 , 47 , 53 , 60 , 66 , 67 , 69 , 70 ].
Others were concerned with how embryoid research impacts for regulations, guidelines, policies, or oversight practices of human embryo and hESC research. Several publications indicated that embryoid research motivates revisiting the 14-day rule ( n = 6) [ 18 , 41 , 49 , 54 , 62 , 64 ] or has implications for the 14-day rule ( n = 4) [ 18 , 27 , 41 , 54 ] that has governed human embryo research for more than 40 years. Two publications noted that future decisions regarding embryoid research oversight or regulation could have implications for other types of stem cell research [ 65 , 67 ]. Finally, several publications noted the many different definitions of embryos and fetuses used in existing policies, guidelines, laws, and regulations, and authors suggested that embryoid research points to the need to revisit those definitions ( n = 9) [ 41 , 45 , 46 , 55 , 57 , 60 , 62 , 64 , 67 ].
The second sub-category concerns development of new guidelines, policies, regulations, or oversight mechanisms for embryoid research. A group of publications raised questions regarding the need for separate ethical guidelines, oversight procedures, or regulatory framework for embryoid research ( n = 12) [ 18 , 21 , 26 – 29 , 34 , 44 , 47 , 52 , 55 , 67 ]. Insofar as separate ethical and regulatory frameworks are necessary, several publications addressed the overall goals and priorities that should inform them ( n = 9) [ 9 , 18 , 21 , 41 , 51 , 59 – 61 , 66 ]. Some noted that an ethical framework for embryoid research should first be developed and then appropriate regulations and oversight procedures should be based on that framework ( n = 6) [ 18 , 22 , 39 , 47 , 54 , 67 ]. One publication raised the question of whether and how judgments about the moral status of embryoids should shape a regulatory framework and of how policies, guidelines, and regulations should treat entities whose ontological status is unclear [ 53 ]. One raised the question of how differences among embryoid types should inform regulation [ 41 ]. Another questioned the appropriate role of the precautionary principle in shaping policy and practice [ 39 ]. Several possible overarching goals or concerns regarding the development of guidelines, policies, regulations, or oversight practices were noted. One concern was that implementing new policies or applying existing rules and regulations to embryoid research could undermine important research ( n = 3) [ 39 , 41 , 70 ]. Several publications noted the importance of maintaining public trust in science and scientific institutions ( n = 3) [ 43 , 52 , 55 ], or promoting transparency about science ( n = 4) [ 21 , 27 , 41 , 69 ].
The third sub-category concerns the scope of any new policies, guidelines, regulations, or oversight mechanisms that might developed. Various publications addressed questions of how they should be developed and who should be involved ( n = 9) [ 21 , 27 , 42 , 52 , 55 , 57 , 59 , 60 , 66 ]. In particular, one publication noted the importance of including different perspectives and securing international collaboration to avoid disrupting science [ 53 ]. Many publications indicated that public consultation, engagement, and deliberation were necessary for the process to be legitimate ( n = 11) [ 18 , 21 , 27 , 36 , 39 , 42 , 45 , 46 , 53 , 60 , 70 ].
There were also several publications that addressed the scope of new guidelines, policies, oversight mechanisms, or regulations. According to some authors, they should address donor rights and interests such as informed consent for gamete or embryo donors ( n = 4) [ 43 , 51 , 64 , 73 ]; privacy ( n = 3) [ 51 , 61 , 73 ]; and benefit sharing ( n = 1) [ 73 ]. Publications also noted that given the evolving nature of embryoid research, any regulations, guidelines, or policies would need to be sufficiently flexible to adapt to changes in science ( n = 8) [ 21 , 23 , 39 , 41 , 56 , 59 , 60 , 67 ].
Systematic review of ethical concerns, questions, reasons, and arguments regarding emerging technologies and practices often reveals additional questions for investigation and can help to advance ethical research, guideline development, and practice by providing an overview of the relevant issues [ 11 , 12 , 74 , 75 ]. Our findings reveal several areas for further assessment regarding embryoid research.
Names and definitions
There is no consensus regarding the term that should be used to identify these new entities [ 9 ]. Scientists have used both broad and specific names in their publications. Some use complicated jargon-laden names that refer to what these entities are scientifically and what they are derived from. For example, a 2019 paper created a system to make entities that “recapitulate developmental events reflecting epiblast and amniotic ectoderm development in the post-implantation human embryo” [ 6 ]. This approach raises at least two concerns. First, it likely makes it more challenging for non-experts to understand what was created, rendering science less rather than more transparent. Second, similar general names are used often for both two- and three-dimensional embryoids, which have significantly different potential to recapitulate an embryo faithfully and precisely.
Decisions about what to call embryoids are important for multiple reasons. First, names and descriptions can affect ethical perceptions of embryoids, a point that others have made regarding organoids [ 76 ]. For instance, the term “synthetic human embryo” could immediately generate concerns about destruction of these entities or the possibility of gestating these entities to live birth. In contrast, a term such as gastruloid is much less likely to generate those questions, particularly among the lay public that would not associate it with gastrulation or an early embryo. Second, names can have implications for funding, oversight, and ethical assessment. For instance, if they are referred to in terms of their stem cell origin, oversight of such research might be delegated to stem cell research oversight committees. Using the term synthetic human embryos might trigger review by a committee overseeing embryo research. Depending on how they are described and treated, embryoid research could face different funding or other restrictions. For instance, ISSCR categorizes embryoids into two types: “integrated” embryoids that include all cell or tissue types (and which are to receive full reviews) and “non-integrated” embryoids, which are missing extra-embryonic cells or tissues (which do not require full reviews and instead only needed to be reported to an oversight committee) [ 47 ]. Researchers can ensure that their work is viewed as non-integrated and receives reduced oversight by specifying the lack of a cell-type.
There is also no consistent definition of embryoids [ 9 ]. No criteria that an entity must meet to be considered an embryoid have been established. There are no shared mechanisms for differentiating between simpler models and more sophisticated models that have greater capacity to develop more fully. Other than the distinction between “integrated” and “non-integrated” embryoids that some scientists use, no additional work to understand what embryoids are has been conducted [ 9 , 47 ].
It may be helpful to think of questions regarding which entities should be classified as embryoids in terms of long-standing debates in the philosophy of science regarding essentialist, pluralist, and cluster concept approaches to classifying species. One possibility is that we must identify a list of necessary and sufficient conditions an entity must meet to be an embryoid. Attempting to articulate the essence of what is an embryoid could prove impossible, much as essentialist approaches to defining species have faced serious challenges and fallen out of favor [ 77 , 78 ]. Alternatively, there might be multiple different ways to think about when an entity is an embryoid. Although this still would require identifying the plurality of ways such entities might be classified, it would alleviate the need to identify a single set of criteria, which is what the many varieties of pluralist accounts of species definition offer biologists [ 77 – 79 ]. Finally, borrowing from Wittgenstein, “embryoid” might be a cluster concept [ 80 ]. The entities share a family resemblance because of various properties that they have without having to possess all of the properties to “count,” much as some philosophers of science have suggested is the case for the concept “species” [ 81 ]. Defining the “complicated network of similarities overlapping and criss-crossing” (Wittgenstein §66) that are pertinent to embryoids requires further analysis and re-assessment as research advances.
In establishing criteria for embryoids, important questions related to embryos likely will surface. These include questions about when an embryoid is sufficiently similar to an embryo to be treated as such and, in turn, how embryos ought to be treated, issues we address below.
Fundamental philosophical questions
Numerous metaphysical, epistemological, and ethical questions associated with human embryo research continue to be debated [ 27 ]. Embryoids raise many questions similar to those associated with embryo research as well as new ones. We expect disagreements and uncertainties comparable to the human embryo and hESC research debates to ensue and likely to be unsolved as embryoid research advances.
Our answers to these and other fundamental questions likely will lead to different judgments about what research may and may not be done using embryoids. If we were to assume that embryoids are or ought to be treated as if they were equivalent to human embryos, ongoing disagreements about the permissibility of embryo research would apply here as well, with some advocating for different limits or restrictions and others advocating to expand such research.
Public trust, engagement, transparency and research hype
Many publications mention the importance of trust, public or stakeholder engagement, and/or transparency. However, they rarely define engagement or transparency or indicate how it could be achieved. Often, they include little or no discussion of who counts as a stakeholder and how they understand “the public” with whom they recommend engagement. There is no clarity about the type and scope of engagement they recommend nor the purpose of engagement. In addition, there is little to no discussion on how such engagement should inform research and policy decisions. For instance, Lovell-Badge et al. describe new guidelines banning genetic editing of embryos and note that “[i]t will also require meaningful public engagement, political support, and proper oversight within the relevant jurisdiction” [ 69 ]. However, neither the paper nor the ISSCR guidelines it references offers any details regarding who this public engagement should include, what it should address, the approach, methods, or models for such engagement, or how it should be used. It is also unclear how researchers would respond if public engagement results in recommendations for limiting or even restricting research.
Identifying stakeholders is an important first step toward a more robust account of public engagement regarding embryoid research. Stakeholders might include scientists, public and private funders of research, donors, patients and their families, patient advocacy organizations, policy makers, and regulators. At a broader level, members of the public in general are stakeholders since a large portion of biomedical research, especially in the USA, is publicly funded [ 28 ]. As a result, many believe that this research should be accountable to the public and that researchers should justify the use of limited resources for their work [ 28 , 82 ]. For example, in the USA there is no stated priority list for research. However, the fact that the federal government invests significant resources into biomedical research, compared to other area of science, suggests that it is a major research priority. In contrast, human embryo research is not a public funding priority area in the USA and federal funding has been explicitly banned for several decades on all human embryo research [ 28 ]. The goals of public and stakeholder engagement often are not articulated, yet engagement goals should guide the methods and scope for such engagement [ 82 ].
Goals for stakeholder engagement regarding embryoid research remain unclear. ISSCR promotes public engagement for human embryo and embryoid research and has a Public Engagement Task Force [ 69 , 83 ]. While ISSCR had limited stakeholder engagement when developing their guidelines, others, such as the American College of Obstetric and Gynecology, removed their 14-day limit recommendation without public comment [ 84 ]. However, engagement implies two-way communication—all parties both express their views and listen to others. If public input was not an important part of establishing policies, guidelines, or recommendations, it seems that they may be using the term “engagement” to refer to what might best be described as “public outreach.” Outreach involves scientists informing the public about the new policies and research, not listening to and considering various views [ 82 ]. True public engagement could help uncover broader social concerns related to research and ways to address them. These discussions, in turn, could play a key role in guiding future research or fostering public acceptance of new research.
As the science of embryoid research advances and these entities become more sophisticated, new questions likely will emerge. Recent work using mouse cells to create embryoids that grew in culture to the point limbs and organs was developing, including a beating primitive heart structure [ 10 ]. This work could challenge claims that embryoids will not be able to further develop in culture [ 10 ].
Overall, the process of assessing embryoid research should be iterative and therefore public and stakeholder engagement should be iterative. Scientists should consider engaging the public before the work becomes discussed extensively in the public forum, including the lay press, to foster an informed narrative. Ongoing engagement is important, particularly in light of recent significant changes to human embryo research guidelines that were adopted without robust public engagement [ 27 , 57 ].
One possible explanation for the failure to engage the public is fear. Transparency, while often hailed as an important factor in building and maintaining public trust, could undermine scientists’ interest in garnering public support for embryoid research. Transparency about the significant uncertainty regarding potential future benefits of the research and whether it will yield knowledge that transforms and saves lives could dampen public support for the research. Perhaps to secure support for their work, scientists sometimes “overpromise” or hype as some scholars have suggested happened with hESC research [ 85 ]. Transparency, rather than inflated speculation, could weaken rather than strengthen public support. Similarly, transparency about the potential to develop sophisticated entities that could be models of later stage embryos as well as fetuses could interfere with public support or result in unwanted regulatory oversight.
Thus far, scientists have emphasized that embryoids do not have the potential to develop and model later stage human embryos and fetuses [ 47 ]. These remarks might be meant, in part, to assuage fears or concerns about this research that could undermine support for it. However, we do not know the true potential of many models until their limits are tested. Significant developments in the area of human embryo research as well as developments in mouse embryoid research suggest that these entities will continue to become more sophisticated [ 10 ]. There is no mechanism of accountability in place to avoid the creation of embryoids that mimic later developmental stages or with advanced neurological systems. ISSCR’s recent decision to remove the 14-day limit on human embryo and embryoid research in its guidelines results in having no developmental or time limit for such work. Given the recent incident developing humans with permanent germline edits prior to public or scientific acceptance of this work, guidelines without enforcement are likely to do little to limit researchers interested in expanding to new areas [ 86 ]. It is therefore plausible that scientists would see no reason to set a firm limit on the developmental stages or features of embryoids.
Despite numerous calls for public and stakeholder engagement and transparency regarding embryoid research in the publications we reviewed, very little substantive work has been done in this area.
Guidance, oversight, or regulation
Many ( n = 21, 37%) publications called for “something” to guide embryoid research. Some authors suggest that ethical guidance will suffice, while others indicate that oversight is or will become necessary as science advances and embryoids resemble embryos more closely. Although some scholars offered recommendations for how embryoid research should be conducted, the scope of any possible guidance remains largely undefined. Existing stem cell research guidelines either do not mention embryoids [ 87 ] or provide significant flexibility regarding recommended oversight [ 69 ]. One’s understanding of what embryoids are and the values and goals one prioritizes likely will shape the kind of guidance, oversight, or regulation one deems appropriate.
To determine whether and how embryoid research should be regulated or overseen and what such oversight or regulation should require, permit, or prohibit requires, clarity regarding the purpose of regulation and oversight as well as clear definition of what is and is not acceptable will be necessary. Oversight could aim at building and maintaining trust, avoiding wrongdoing, facilitating research, or avoiding liability and ensuring compliance with funders’ and publishers’ policies. Many unanswered questions remain about who should develop regulations or policies, what they should permit and prohibit, how they should be enforced, and who should oversee such research and how.
Understandings of what kinds of entities embryoids are and their moral significance could shape assessments of appropriate regulatory and oversight mechanisms. Someone who sees them as no different from any other collection of cells or biological tissue will answer these questions differently from someone who views sophisticated embryoids as human embryos. Among the latter, views about oversight and limits will turn on their understanding of permissible research on human embryos.
Lack of consensus
Research in this field has been rapidly expanding and the number of publications increased over time. Different areas of concerns were addressed, and recommendations were made by various authors on how to respond to concerns. We did not see any consensus-building efforts, nor did we see any consensus emerging within the field. Authors associated with the 2021 ISSCR guidelines promoted specific recommendations by ISSCR, including how to name and regulate embryoids [ 69 ]. However, several of these recommendations were challenged in subsequent literature, including what to call embryoids and how to define them [ 9 , 88 – 90 ]. For example, a 2023 ISSCR statement expressed concern over the use of terms that suggest that embryo models are embryos [ 91 ].This lack of consensus suggests that scientists working in this area themselves do not agree on standards or how to move forward in uncertain regulatory climates.
Limitations
There are several ways in which this systematic review did not capture every relevant publication on this topic. First, we searched only for publications in English, missing conversations on the subject, especially by Chinese researchers who are conducting embryo and embryoid research. Second, embryoid research is developing rapidly and this review reflects a snapshot of the literature up to the date on which we closed our search. Newer material that addresses more recent developments is not represented here. Finally, although the search was carefully designed by a multidisciplinary team that included research librarians, it is possible that we failed to locate some relevant publications. This is due in part to the wide range of terms that have been used to describe embryoids. Due to the nature of this review, we could not assess the quality or significance of the publications reviewed.
Much work remains to be done to address the ethical, legal, regulatory, and policy considerations relevant to embryoid research. As this review revealed, thus far discussion of the risks or potential harms associated with this research has been quite limited and very little attention has been given to this topic from various religious perspectives. As this work becomes more widely known, it will be important to engage people of various faiths to understand how various religious frameworks view embryoids and to expand our understanding of the full range of relevant considerations. At this time, a critical question is how to operate with respect to embryoid research in the face of uncertainty and ongoing scientific developments.
Acknowledgements
We would like to acknowledge and thank Denice Lewis and Kathy Shields, librarians in the Z. Smith Reynolds Library at Wake Forest University, for their assistance in designing the search strategy. We also acknowledge Sally Anglin for help with preparing figures.
Abbreviations
Appendix 1: search strategy.
(“Artificial embryo” OR embryoid OR “embryonic organoid” OR “SHEEF” OR “synthetic human entity with embryo-life features” OR “SHEEFs” OR “Synthetic embryo” OR “Blastoid” OR “Gastruloid” OR “PASE” OR “post-implantation amniotic sac embryoid” OR “Micropatterned hESC colony” OR “Micropatterned hESC colonies” OR “ETS Embryo” OR “ETX Embryo” OR “human cell-culture models of early development” OR “hCCMED”) AND (Ethics OR ethic OR ethical OR moral OR Policy OR policies OR Guideline OR Guidelines OR.
Recommendation OR Recommendations OR Law OR Legal OR Regulation OR Regulatory OR.
Oversight OR Governance).
Limits: English.
Web of Science
Author contributions.
ASI contributed to all aspects of data collection, screening, analysis, and writing. ASI, together with KRWM, wrote the first draft of the manuscript. GK contributed to all aspects of data collection, screening, analysis, and manuscript revision. ER contributed to all aspects of data collection, screening, analysis, and manuscript revision. KRWM supported the data screening process and participated fully in analysis and writing. She, together with ASI, wrote the first draft of the manuscript.
KRWM’s work on this manuscript was supported by the State of Qatar Endowment for International Stem Cell Policy.
Availability of data and materials
Declarations.
Not applicable.
The authors have no competing interests to declare.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Human embryology is flourishing thanks to an impetus provided by embryo models formed from stem cells. These scientific advances require meticulous experimental work and a refined ethical ...
The field of human embryology has seen major scientific breakthroughs in the past decade. In 2016, researchers were able to culture human embryos for up to 14 days for the first time, whereas previous techniques could reach only 7 days. And in recent months, scientists have shown that models mimicking human embryo development up to day 14 can be created from stem cells. Although the models are ...
The study of the development of an organism during the embryonic and fetal stages of life. | Explore the latest full-text research PDFs, articles, conference papers, preprints and more on EMBRYOLOGY.
Sexual dimorphic miRNA-mediated response of bovine elongated embryos to the maternal microenvironment. Dessie Salilew-Wondim, Michael Hoelker, [ ... ], Dawit Tesfaye. Ultrasonographic assessment of abnormal fetal growth related to uteroplacental-fetal biometrics and Doppler (U-AID) indices: Protocol for multicenter retrospective cohort...
The day 14 rule limits human embryo research to the first 14 days of development and, thus, to the period prior to the formation of the primitive streak (see Glossary, Box 1). Consequently, gastrulation cannot be studied in human embryos. ... In New Discoveries in Embryology (ed. Wu B.), pp. 97-124 IntechOpen. [Google Scholar]
The aim is to bring insight regarding the identity of clinical embryology research, introducing concerns when solely relying on the methodology of bibliometric analysis. ... (3 studies), the 80′s (2 studies) and the 70′s (1 study). Among the top-10, three papers served to introduce and establish the respective fields, two papers present ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on CLINICAL EMBRYOLOGY. Find methods information, sources, references or conduct a literature review on ...
Apr. 6, 2023 — Human embryo development and early organ formation remain largely unexplored due to ethical issues surrounding the use of embryos for research as well as limited availability of ...
Increased demand for in vitro fertilization (IVF) due to socio-demographic trends, and supply facilitated by new technologies, converged to transform the way a substantial proportion of humans reproduce. The purpose of this article is to describe the societal and demographic trends driving increased worldwide demand for IVF, as well as to provide an overview of emerging technologies that ...
Feature papers represent the most advanced research with significant potential for high impact in the field. ... contributing to this important area of study by submitting an article to this Special Issue on the state-of-the-art research on clinical embryology and reproductive medicine currently underway. ... The latest trend and the apparent ...
A new chapter in human embryo research High scientific and ethical standards have become even more important recently given forays into modelling the early stages of development with stem cells.
The United Kingdom includes the fourteen‐day limit in the Human Fertilisation and Embryology Act of 1990, which permits human embryo research after approval by a regulatory authority. Other countries (including Canada, South Korea, and Sweden) allow certain human embryo research but have enshrined the fourteen‐day limit into law. Still ...
A human embryo's legal definition and its entitlement to protection vary greatly worldwide. Recently, human pluripotent stem cells have been used to form in vitro models of early embryos that have challenged legal definitions and raised questions regarding their usage. In this light, we propose a refined legal definition of an embryo, suggest "tipping points" for when human embryo models ...
White Papers; More... MediKnowledge; ... Embryology News and Research RSS. ... Ph.D. of Harvard Medical School, about their latest research, revealing the surprising properties of pain.
A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the ...
unified text-to-text transformer.The Journal of Machine Learning Research, 21(1):5485-5551, 2020. Nir Ratner, Yoav Levine, Yonatan Belinkov, Ori Ram, Omri Abend, Ehud Karpas, Amnon Shashua, Kevin Leyton-Brown, and Yoav Shoham. Parallel context windows improve in-context learning of large language models. arXiv preprint arXiv:2212.10947, 2022.
This paper focuses on the development of embryo engineering technology. The second to fourth sections of this paper describe the work that scientists have done to date in terms of the three high-risk nodes mentioned above and explain how the application of new techniques can improve the efficiency of embryo engineering technology and the survival rate through these high-risk nodes.
Leave No Context Behind: Efficient Infinite Context Transformers with Infini-attention. This work introduces an efficient method to scale Transformer-based Large Language Models (LLMs) to infinitely long inputs with bounded memory and computation. A key component in our proposed approach is a new attention technique dubbed Infini-attention.
The MIT technique reported in 2022 worked with UV light. Would it work with other wavelengths of light, potentially opening up new applications? Yes, the team found. In the current paper they show that gamma rays also modify the grain boundaries resulting in a faster flow of ions that, in turn, can be easily detected.
Traditionally, prolonged medical therapy has been needed in most cases since a conservative approach to surgery has usually been taken, especially in young women. In 2022, new European Society of Human Reproduction and Embryology (ESHRE) guidelines were published that present different directions for diagnosis and treatment from the past.
The team's findings, which could likely be generalized to detectors for other kinds of radiation, are described in a paper published in Nature Communications, by MIT professors Mingda Li, and Benoit Forget, senior research scientist Lin-Wen Hu, and principal research scientist Gordon Kohse; graduate students Ryotaro Okabe and Shangjie Xue ...
Pew Research Center conducted this analysis to better understand public K-12 teachers' views on school shootings, how prepared they feel for a potential active shooter, and how they feel about policies that could help prevent future shootings. To do this, we surveyed 2,531 U.S. public K-12 teachers from Oct. 17 to Nov. 14, 2023.
Background. Methods to culture stem cells to model early human development have been reported since 2014 [1, 2].Recent advances have resulted in entities that model different stages of early embryo development—from the blastocyst stage at 5 days post-fertilization (dpf), to gastrulation at 17 dpf, to later stages of organogenesis [3-8].These entities are referred to by numerous terms ...