A Heat Transfer Textbook, 5th edition

Solutions manual
Solutions to more than 490 problems are on the following links.
Solutions for Chapter 1 (v1.01, 16 MB, February 2023)
Solutions for Chapter 2 (v1.0, 13 MB, August 2020)
Solutions for Chapter 3 (v1.0, 15 MB, August 2020)
Partial solutions for Chapters 4-11 (v1.05, 24 MB, 24 March 2023) Includes solutions for all problems in Chapters 4, 5, 6, 10 & 11
If additional solutions become available, they will be posted here.
Most of the handwritten solutions were prepared many years ago, and some use property data that don’t precisely match today’s Appendix A. In most instances, the differences are small.
Between them, the authors have spent more than 100 years using various textbook solutions manuals. In our experience, none are without errors. If you happen to find one in our solutions manual, do let us know.
Heat Transfer: Homework Solutions

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

15: Thermodynamics (Exercises)
- Last updated
- Save as PDF
- Page ID 4199
Conceptual Questions
15.1: the first law of thermodynamics.
1. Describe the photo of the tea kettle at the beginning of this section in terms of heat transfer, work done, and internal energy. How is heat being transferred? What is the work done and what is doing it? How does the kettle maintain its internal energy?
2. The first law of thermodynamics and the conservation of energy, as discussed in Conservation of Energy, are clearly related. How do they differ in the types of energy considered?
3. Heat transfer \(\displaystyle Q\) and work done \(\displaystyle W\) are always energy in transit, whereas internal energy \(\displaystyle U\) is energy stored in a system. Give an example of each type of energy, and state specifically how it is either in transit or resides in a system.
4. How do heat transfer and internal energy differ? In particular, which can be stored as such in a system and which cannot?
5. If you run down some stairs and stop, what happens to your kinetic energy and your initial gravitational potential energy?
6. Give an explanation of how food energy (calories) can be viewed as molecular potential energy (consistent with the atomic and molecular definition of internal energy).
7. Identify the type of energy transferred to your body in each of the following as either internal energy, heat transfer, or doing work:
(a) basking in sunlight;
(b) eating food;
(c) riding an elevator to a higher floor.
15.2: The First Law of Thermodynamics and Some Simple Processes
8. A great deal of effort, time, and money has been spent in the quest for the so-called perpetual-motion machine, which is defined as a hypothetical machine that operates or produces useful work indefinitely and/or a hypothetical machine that produces more work or energy than it consumes. Explain, in terms of heat engines and the first law of thermodynamics, why or why not such a machine is likely to be constructed.
9. One method of converting heat transfer into doing work is for heat transfer into a gas to take place, which expands, doing work on a piston, as shown in the figure below.
(a) Is the heat transfer converted directly to work in an isobaric process, or does it go through another form first? Explain your answer.
(b) What about in an isothermal process?
(c) What about in an adiabatic process (where heat transfer occurred prior to the adiabatic process)?
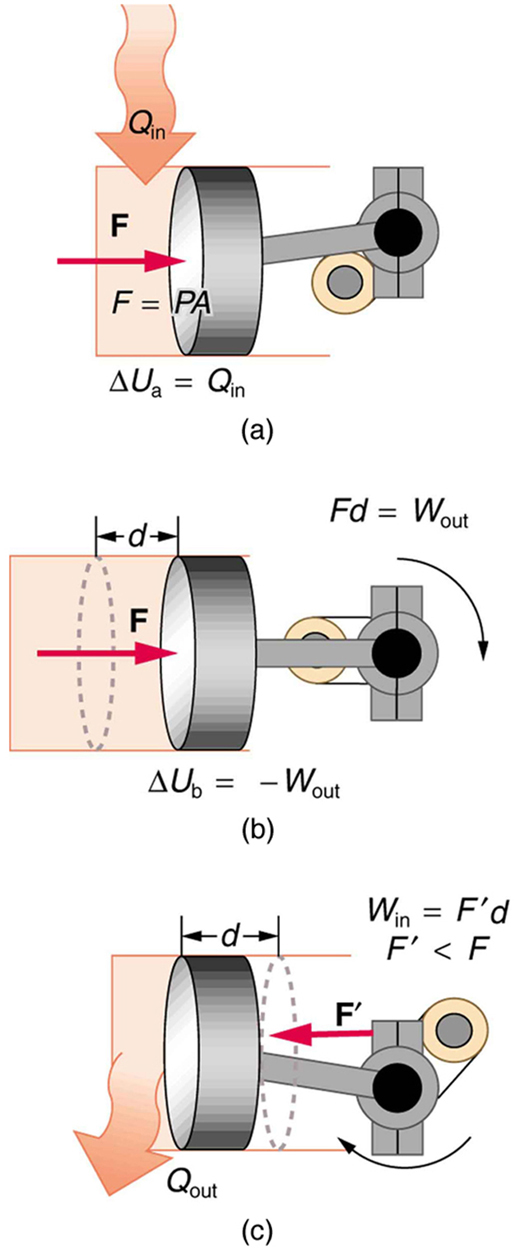
10. Would the previous question make any sense for an isochoric process? Explain your answer.
11. We ordinarily say that \(\displaystyle ΔU=0\) for an isothermal process. Does this assume no phase change takes place? Explain your answer.
12. The temperature of a rapidly expanding gas decreases. Explain why in terms of the first law of thermodynamics. (Hint: Consider whether the gas does work and whether heat transfer occurs rapidly into the gas through conduction.)
13. Which cyclical process represented by the two closed loops, ABCFA and ABDEA, on the \(\displaystyle PV\) diagram in the figure below produces the greatest net work? Is that process also the one with the smallest work input required to return it to point A? Explain your responses.
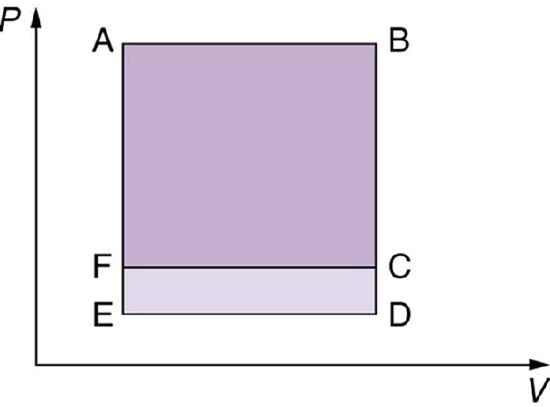
14. A real process may be nearly adiabatic if it occurs over a very short time. How does the short time span help the process to be adiabatic?
15. It is unlikely that a process can be isothermal unless it is a very slow process. Explain why. Is the same true for isobaric and isochoric processes? Explain your answer.
15.3 Introduction to the Second Law of Thermodynamics: Heat Engines and Their Efficiency
16. Imagine you are driving a car up Pike’s Peak in Colorado. To raise a car weighing 1000 kilograms a distance of 100 meters would require about a million joules. You could raise a car 12.5 kilometers with the energy in a gallon of gas. Driving up Pike's Peak (a mere 3000-meter climb) should consume a little less than a quart of gas. But other considerations have to be taken into account. Explain, in terms of efficiency, what factors may keep you from realizing your ideal energy use on this trip.
17. Is a temperature difference necessary to operate a heat engine? State why or why not.
18. Definitions of efficiency vary depending on how energy is being converted. Compare the definitions of efficiency for the human body and heat engines. How does the definition of efficiency in each relate to the type of energy being converted into doing work?
19. Why—other than the fact that the second law of thermodynamics says reversible engines are the most efficient—should heat engines employing reversible processes be more efficient than those employing irreversible processes? Consider that dissipative mechanisms are one cause of irreversibility.
15.4 Carnot’s Perfect Heat Engine: The Second Law of Thermodynamics Restated
20. Think about the drinking bird at the beginning of this section (Figure). Although the bird enjoys the theoretical maximum efficiency possible, if left to its own devices over time, the bird will cease “drinking.” What are some of the dissipative processes that might cause the bird’s motion to cease?
21. Can improved engineering and materials be employed in heat engines to reduce heat transfer into the environment? Can they eliminate heat transfer into the environment entirely?
22. Does the second law of thermodynamics alter the conservation of energy principle?
15.5: Applications of Thermodynamics: Heat Pumps and Refrigerators
23. Explain why heat pumps do not work as well in very cold climates as they do in milder ones. Is the same true of refrigerators?
24. In some Northern European nations, homes are being built without heating systems of any type. They are very well insulated and are kept warm by the body heat of the residents. However, when the residents are not at home, it is still warm in these houses. What is a possible explanation?
25. Why do refrigerators, air conditioners, and heat pumps operate most cost-effectively for cycles with a small difference between \(\displaystyle T_h\) and \(\displaystyle T_c\)? (Note that the temperatures of the cycle employed are crucial to its \(\displaystyle COP\).)
26. Grocery store managers contend that there is less total energy consumption in the summer if the store is kept at a low temperature. Make arguments to support or refute this claim, taking into account that there are numerous refrigerators and freezers in the store.
27. Can you cool a kitchen by leaving the refrigerator door open?
15.6: Entropy and the Second Law of Thermodynamics: Disorder and the Unavailability of Energy
28. A woman shuts her summer cottage up in September and returns in June. No one has entered the cottage in the meantime. Explain what she is likely to find, in terms of the second law of thermodynamics.
29. Consider a system with a certain energy content, from which we wish to extract as much work as possible. Should the system’s entropy be high or low? Is this orderly or disorderly? Structured or uniform? Explain briefly.
30. Does a gas become more orderly when it liquefies? Does its entropy change? If so, does the entropy increase or decrease? Explain your answer.
31. Explain how water’s entropy can decrease when it freezes without violating the second law of thermodynamics. Specifically, explain what happens to the entropy of its surroundings.
32. Is a uniform-temperature gas more or less orderly than one with several different temperatures? Which is more structured? In which can heat transfer result in work done without heat transfer from another system?
33. Give an example of a spontaneous process in which a system becomes less ordered and energy becomes less available to do work. What happens to the system’s entropy in this process?
34. What is the change in entropy in an adiabatic process? Does this imply that adiabatic processes are reversible? Can a process be precisely adiabatic for a macroscopic system?
35. Does the entropy of a star increase or decrease as it radiates? Does the entropy of the space into which it radiates (which has a temperature of about 3 K) increase or decrease? What does this do to the entropy of the universe?
36. Explain why a building made of bricks has smaller entropy than the same bricks in a disorganized pile. Do this by considering the number of ways that each could be formed (the number of microstates in each macrostate).
15.7: Statistical Interpretation of Entropy and the Second Law of Thermodynamics: The Underlying Explanation
37. Explain why a building made of bricks has smaller entropy than the same bricks in a disorganized pile. Do this by considering the number of ways that each could be formed (the number of microstates in each macrostate).

Problems & Exercises
38. What is the change in internal energy of a car if you put 12.0 gal of gasoline into its tank? The energy content of gasoline is \(\displaystyle 1.3×10^8J/gal\). All other factors, such as the car’s temperature, are constant.
Solution \(\displaystyle 1.6×10^9J\)
39. How much heat transfer occurs from a system, if its internal energy decreased by 150 J while it was doing 30.0 J of work?
40. A system does \(\displaystyle 1.80×10^8J\) of work while \(\displaystyle 7.50×10^8J\) of heat transfer occurs to the environment. What is the change in internal energy of the system assuming no other changes (such as in temperature or by the addition of fuel)?
Solution \(\displaystyle −9.30×10^8J\)
41. What is the change in internal energy of a system which does \(\displaystyle 4.50×10^5J\) of work while \(\displaystyle 3.00×10^6J\) of heat transfer occurs into the system, and \(\displaystyle 8.00×10^6J\) of heat transfer occurs to the environment?
42. Suppose a woman does 500 J of work and 9500 J of heat transfer occurs into the environment in the process.
(a) What is the decrease in her internal energy, assuming no change in temperature or consumption of food? (That is, there is no other energy transfer.)
(b) What is her efficiency?
Solution (a) \(\displaystyle −1.0×10^4J\), or \(\displaystyle −2.39 kcal\) (b) 5.00%
43. (a) How much food energy will a man metabolize in the process of doing 35.0 kJ of work with an efficiency of 5.00%?
(b) How much heat transfer occurs to the environment to keep his temperature constant? Explicitly show how you follow the steps in the Problem-Solving Strategy for thermodynamics found in Problem-Solving Strategies for Thermodynamics.
44. (a) What is the average metabolic rate in watts of a man who metabolizes 10,500 kJ of food energy in one day?
(b) What is the maximum amount of work in joules he can do without breaking down fat, assuming a maximum efficiency of 20.0%?
(c) Compare his work output with the daily output of a 187-W (0.250-horsepower) motor.
Solution (a) 122 W (b) \(\displaystyle 2.10×10^6J\) (c) Work done by the motor is \(\displaystyle 1.61×10^7\); thus the motor produces 7.67 times the work done by the man
45. (a) How long will the energy in a 1470-kJ (350-kcal) cup of yogurt last in a woman doing work at the rate of 150 W with an efficiency of 20.0% (such as in leisurely climbing stairs)?
(b) Does the time found in part (a) imply that it is easy to consume more food energy than you can reasonably expect to work off with exercise?
46. (a) A woman climbing the Washington Monument metabolizes \(\displaystyle 6.00×10^2kJ\) of food energy. If her efficiency is 18.0%, how much heat transfer occurs to the environment to keep her temperature constant?
(b) Discuss the amount of heat transfer found in (a). Is it consistent with the fact that you quickly warm up when exercising?
Solution (a) 492 kJ (b) This amount of heat is consistent with the fact that you warm quickly when exercising. Since the body is inefficient, the excess heat produced must be dissipated through sweating, breathing, etc.
47. A car tire contains \(\displaystyle 0.0380m^3\) of air at a pressure of \(\displaystyle 2.20×10^5N/m^2\) (about 32 psi). How much more internal energy does this gas have than the same volume has at zero gauge pressure (which is equivalent to normal atmospheric pressure)?
Solution \(\displaystyle 6.77×10^3J\)
48. A helium-filled toy balloon has a gauge pressure of 0.200 atm and a volume of 10.0 L. How much greater is the internal energy of the helium in the balloon than it would be at zero gauge pressure?
49. Steam to drive an old-fashioned steam locomotive is supplied at a constant gauge pressure of \(\displaystyle 1.75×10^6N/m^2\) (about 250 psi) to a piston with a 0.200-m radius.
(a) By calculating \(\displaystyle PΔV\), find the work done by the steam when the piston moves 0.800 m. Note that this is the net work output, since gauge pressure is used.
(b) Now find the amount of work by calculating the force exerted times the distance traveled. Is the answer the same as in part (a)?
Solution (a) \(\displaystyle W=PΔV=1.76×10^5J\) (b) \(\displaystyle W=Fd=1.76×10^5J\). Yes, the answer is the same.
50. A hand-driven tire pump has a piston with a 2.50-cm diameter and a maximum stroke of 30.0 cm. (a) How much work do you do in one stroke if the average gauge pressure is \(\displaystyle 2.40×10^5N/m^2\) (about 35 psi)?
(b) What average force do you exert on the piston, neglecting friction and gravitational force?
51. Calculate the net work output of a heat engine following path ABCDA in the figure below.
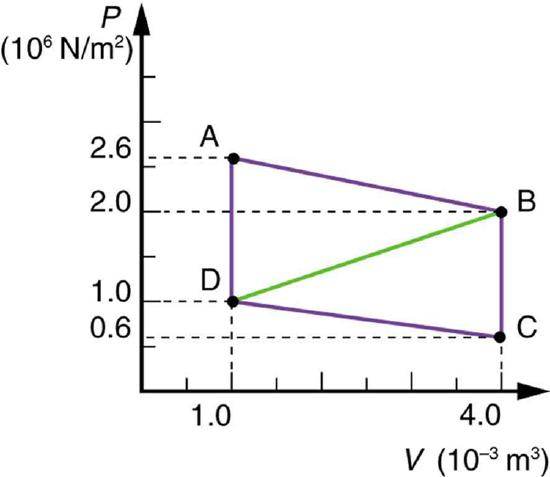
Solution \(\displaystyle W=4.5×10^3J\)
52. What is the net work output of a heat engine that follows path ABDA in the figure above, with a straight line from B to D? Why is the work output less than for path ABCDA? Explicitly show how you follow the steps in the Problem-Solving Strategies for Thermodynamics.
53. Unreasonable Results
What is wrong with the claim that a cyclical heat engine does 4.00 kJ of work on an input of 24.0 kJ of heat transfer while 16.0 kJ of heat transfers to the environment?
Solution \(\displaystyle W\) is not equal to the difference between the heat input and the heat output.
54. (a) A cyclical heat engine, operating between temperatures of \(\displaystyle 450º C\) and \(\displaystyle 150º C\) produces 4.00 MJ of work on a heat transfer of 5.00 MJ into the engine. How much heat transfer occurs to the environment?
(b) What is unreasonable about the engine?
(c) Which premise is unreasonable?
55. Construct Your Own Problem
Consider a car’s gasoline engine. Construct a problem in which you calculate the maximum efficiency this engine can have. Among the things to consider are the effective hot and cold reservoir temperatures. Compare your calculated efficiency with the actual efficiency of car engines.
56. Construct Your Own Problem
Consider a car trip into the mountains. Construct a problem in which you calculate the overall efficiency of the car for the trip as a ratio of kinetic and potential energy gained to fuel consumed. Compare this efficiency to the thermodynamic efficiency quoted for gasoline engines and discuss why the thermodynamic efficiency is so much greater. Among the factors to be considered are the gain in altitude and speed, the mass of the car, the distance traveled, and typical fuel economy.
57. A certain heat engine does 10.0 kJ of work and 8.50 kJ of heat transfer occurs to the environment in a cyclical process.
(a) What was the heat transfer into this engine?
(b) What was the engine’s efficiency?
Solution (a) \(\displaystyle 18.5kJ\) (b) \(\displaystyle 54.1%\)
58. With \(\displaystyle 2.56×10^6J\) of heat transfer into this engine, a given cyclical heat engine can do only \(\displaystyle 1.50×10^5J\) of work.
(a) What is the engine’s efficiency?
(b) How much heat transfer to the environment takes place?
59. (a) What is the work output of a cyclical heat engine having a 22.0% efficiency and \(\displaystyle 6.00×10^9J\) of heat transfer into the engine?
(b) How much heat transfer occurs to the environment?
Solution (a) \(\displaystyle 1.32 × 10^9J\) (b) \(\displaystyle 4.68 × 10^9J\)
60. (a) What is the efficiency of a cyclical heat engine in which 75.0 kJ of heat transfer occurs to the environment for every 95.0 kJ of heat transfer into the engine?
(b) How much work does it produce for 100 kJ of heat transfer into the engine?
61. The engine of a large ship does \(\displaystyle 2.00×10^8J\) of work with an efficiency of 5.00%.
(a) How much heat transfer occurs to the environment?
(b) How many barrels of fuel are consumed, if each barrel produces \(\displaystyle 6.00×10^9J\) of heat transfer when burned?
Solution (a) \(\displaystyle 3.80 × 10^9J\) (b) 0.667 barrels
62. (a) How much heat transfer occurs to the environment by an electrical power station that uses \(\displaystyle 1.25×10^{14}J\) of heat transfer into the engine with an efficiency of 42.0%?
(b) What is the ratio of heat transfer to the environment to work output?
(c) How much work is done?
63. Assume that the turbines at a coal-powered power plant were upgraded, resulting in an improvement in efficiency of 3.32%. Assume that prior to the upgrade the power station had an efficiency of 36% and that the heat transfer into the engine in one day is still the same at \(\displaystyle 2.50×10^{14}J\).
(a) How much more electrical energy is produced due to the upgrade?
(b) How much less heat transfer occurs to the environment due to the upgrade?
Solution (a) \(\displaystyle 8.30 × 10^{12}J\), which is 3.32% of \(\displaystyle 2.50 × 10^{14}J\). (b) \(\displaystyle –8.30 × 10^{12}J\), where the negative sign indicates a reduction in heat transfer to the environment.
64. This problem compares the energy output and heat transfer to the environment by two different types of nuclear power stations—one with the normal efficiency of 34.0%, and another with an improved efficiency of 40.0%. Suppose both have the same heat transfer into the engine in one day, \(\displaystyle 2.50×10^{14}J\).
(a) How much more electrical energy is produced by the more efficient power station?
(b) How much less heat transfer occurs to the environment by the more efficient power station? (One type of more efficient nuclear power station, the gas-cooled reactor, has not been reliable enough to be economically feasible in spite of its greater efficiency.)
65. A certain gasoline engine has an efficiency of 30.0%. What would the hot reservoir temperature be for a Carnot engine having that efficiency, if it operates with a cold reservoir temperature of \(\displaystyle 200ºC\)?
Solution \(\displaystyle 403ºC\)
66. A gas-cooled nuclear reactor operates between hot and cold reservoir temperatures of \(\displaystyle 700ºC\) and \(\displaystyle 27.0ºC\).
(a) What is the maximum efficiency of a heat engine operating between these temperatures?
(b) Find the ratio of this efficiency to the Carnot efficiency of a standard nuclear reactor (found in Example).
67. (a) What is the hot reservoir temperature of a Carnot engine that has an efficiency of 42.0% and a cold reservoir temperature of \(\displaystyle 27.0ºC\)?
(b) What must the hot reservoir temperature be for a real heat engine that achieves 0.700 of the maximum efficiency, but still has an efficiency of 42.0% (and a cold reservoir at \(\displaystyle 27.0ºC\)?
(c) Does your answer imply practical limits to the efficiency of car gasoline engines?
Solution (a) \(\displaystyle 244ºC\) (b) \(\displaystyle 477ºC\) (c) Yes, since automobiles engines cannot get too hot without overheating, their efficiency is limited.
68. Steam locomotives have an efficiency of 17.0% and operate with a hot steam temperature of \(\displaystyle 425ºC\).
(a) What would the cold reservoir temperature be if this were a Carnot engine?
(b) What would the maximum efficiency of this steam engine be if its cold reservoir temperature were \(\displaystyle 150ºC\)?
69. Practical steam engines utilize \(\displaystyle 450ºC\) steam, which is later exhausted at \(\displaystyle 270ºC\).
(a) What is the maximum efficiency that such a heat engine can have?
(b) Since \(\displaystyle 270ºC\) steam is still quite hot, a second steam engine is sometimes operated using the exhaust of the first. What is the maximum efficiency of the second engine if its exhaust has a temperature of \(\displaystyle 150ºC\)?
(c) What is the overall efficiency of the two engines?
(d) Show that this is the same efficiency as a single Carnot engine operating between \(\displaystyle 450ºC\) and \(\displaystyle 150ºC\). Explicitly show how you follow the steps in the Problem-Solving Strategies for Thermodynamics.
Solution (a) \(\displaystyle Eff_1=1−\frac{T_{c,1}}{T_{h,1}}=1−\frac{543K}{723K}=0.249\) or \(\displaystyle 24.9%\) (b) \(\displaystyle Eff_2=1−\frac{423K}{543K}=0.221\) or \(\displaystyle 22.1%\) (c) \(\displaystyle Eff_1=1−\frac{T_{c,1}}{T_{h,1}}⇒T_{c,1}=T_{h,1}(1,−,eff_1)\) similarly, \(\displaystyle T_{c,2}=T_{h,2}(1−Eff_2)\) using \(\displaystyle T_{h,2}=T_{c,1} \text{in above equation gives} T_{c,2}=T_{h,1}(1−Eff_1)(1−Eff_2)≡T_{h,1}(1−Eff_{overall})\) \(\displaystyle ∴(1−Eff_{overall})=(1−Eff_1)(1−Eff_2)\) \(\displaystyle Eff_{overall}=1−(1−0.249)(1−0.221)=41.5%\) (d) \(\displaystyle Eff_{overall}=1−\frac{423K}{723K}=0.415\) or \(\displaystyle 41.5%\)
70. A coal-fired electrical power station has an efficiency of 38%. The temperature of the steam leaving the boiler is \(\displaystyle 550ºC \). What percentage of the maximum efficiency does this station obtain? (Assume the temperature of the environment is \(\displaystyle 20ºC\).)
71. Would you be willing to financially back an inventor who is marketing a device that she claims has 25 kJ of heat transfer at 600 K, has heat transfer to the environment at 300 K, and does 12 kJ of work? Explain your answer.
Solution The heat transfer to the cold reservoir is \(\displaystyle Q_c=Q_h−W=25kJ−12kJ=13kJ\), so the efficiency is \(\displaystyle Eff=1−\frac{Q_c}{Q_h}=1−\frac{13kJ}{25kJ}=0.48\). The Carnot efficiency is \(\displaystyle Eff_C=1−\frac{T_c}{T_h}=1−\frac{300K}{600K}=0.50\). The actual efficiency is 96% of the Carnot efficiency, which is much higher than the best-ever achieved of about 70%, so her scheme is likely to be fraudulent.
72. Unreasonable Results
(a) Suppose you want to design a steam engine that has heat transfer to the environment at 270ºC and has a Carnot efficiency of 0.800. What temperature of hot steam must you use?
(b) What is unreasonable about the temperature?
73. Unreasonable Results
(a) Calculate the cold reservoir temperature of a steam engine that uses hot steam at \(\displaystyle 450ºC\) and has a Carnot efficiency of 0.700.
Solution (a) –56.3ºC (b) The temperature is too cold for the output of a steam engine (the local environment). It is below the freezing point of water. (c) The assumed efficiency is too high.
74. What is the coefficient of performance of an ideal heat pump that has heat transfer from a cold temperature of \(\displaystyle −25.0ºC\) to a hot temperature of \(\displaystyle 40.0ºC\)?
Solution 4.82
75. Suppose you have an ideal refrigerator that cools an environment at \(\displaystyle −20.0ºC\) and has heat transfer to another environment at \(\displaystyle 50.0ºC\). What is its coefficient of performance?
76. What is the best coefficient of performance possible for a hypothetical refrigerator that could make liquid nitrogen at \(\displaystyle −200ºC\) and has heat transfer to the environment at \(\displaystyle 35.0ºC\)?
Solution 0.311
77. In a very mild winter climate, a heat pump has heat transfer from an environment at \(\displaystyle 5.00ºC\) to one at \(\displaystyle 35.0ºC\). What is the best possible coefficient of performance for these temperatures? Explicitly show how you follow the steps in the Problem-Solving Strategies for Thermodynamics.
78. (a) What is the best coefficient of performance for a heat pump that has a hot reservoir temperature of \(\displaystyle 50.0ºC\) and a cold reservoir temperature of \(\displaystyle −20.0ºC\)?
(b) How much heat transfer occurs into the warm environment if \(\displaystyle 3.60×10^7J\) of work (10.0kW⋅h) is put into it?
(c) If the cost of this work input is \(\displaystyle 10.0 cents/kW⋅h\), how does its cost compare with the direct heat transfer achieved by burning natural gas at a cost of 85.0 cents per therm. (A therm is a common unit of energy for natural gas and equals \(\displaystyle 1.055×10^8J\).)
Solution (a) 4.61 (b) \(\displaystyle 1.66×10^8J\) or \(\displaystyle 3.97×10^4kcal\) (c) To transfer \(\displaystyle 1.66×10^8J\), heat pump costs $1.00, natural gas costs $1.34.
79. (a) What is the best coefficient of performance for a refrigerator that cools an environment at \(\displaystyle −30.0ºC\) and has heat transfer to another environment at \(\displaystyle 45.0ºC\)?
(b) How much work in joules must be done for a heat transfer of 4186 kJ from the cold environment?
(c) What is the cost of doing this if the work costs 10.0 cents per \(\displaystyle 3.60×10^6J\)?
(d) How many kJ of heat transfer occurs into the warm environment?
(e) Discuss what type of refrigerator might operate between these temperatures.
80. Suppose you want to operate an ideal refrigerator with a cold temperature of \(\displaystyle −10.0ºC\), and you would like it to have a coefficient of performance of 7.00. What is the hot reservoir temperature for such a refrigerator?
Solution 27.6ºC
81. An ideal heat pump is being considered for use in heating an environment with a temperature of \(\displaystyle 22.0ºC\). What is the cold reservoir temperature if the pump is to have a coefficient of performance of 12.0?
82. A 4-ton air conditioner removes \(\displaystyle 5.06×10^7J\) (48,000 British thermal units) from a cold environment in 1.00 h.
(a) What energy input in joules is necessary to do this if the air conditioner has an energy efficiency rating (\(\displaystyle EER\)) of 12.0?
(b) What is the cost of doing this if the work costs 10.0 cents per \(\displaystyle 3.60×10^6J \) (one kilowatt-hour)?
(c) Discuss whether this cost seems realistic. Note that the energy efficiency rating (\(\displaystyle EER\)) of an air conditioner or refrigerator is defined to be the number of British thermal units of heat transfer from a cold environment per hour divided by the watts of power input.
Solution (a) \(\displaystyle 1.44×10^7J\) (b) 40 cents (c) This cost seems quite realistic; it says that running an air conditioner all day would cost $9.59 (if it ran continuously).
83. Show that the coefficients of performance of refrigerators and heat pumps are related by \(\displaystyle COP_{ref}=COP_{hp}−1\).
Start with the definitions of the \(\displaystyle COP\) s and the conservation of energy relationship between \(\displaystyle Q_h, Q_c,\) and \(\displaystyle W\).
84. (a) On a winter day, a certain house loses \(\displaystyle 5.00×10^8J\) of heat to the outside (about 500,000 Btu). What is the total change in entropy due to this heat transfer alone, assuming an average indoor temperature of \(\displaystyle 21.0º C\) and an average outdoor temperature of \(\displaystyle 5.00º C\)?
(b) This large change in entropy implies a large amount of energy has become unavailable to do work. Where do we find more energy when such energy is lost to us?
Solution (a) \(\displaystyle 9.78×10^4J/K\) (b) In order to gain more energy, we must generate it from things within the house, like a heat pump, human bodies, and other appliances. As you know, we use a lot of energy to keep our houses warm in the winter because of the loss of heat to the outside.
85. On a hot summer day, \(\displaystyle 4.00×10^6J\) of heat transfer into a parked car takes place, increasing its temperature from \(\displaystyle 35.0º C\) to \(\displaystyle 45.0º C\). What is the increase in entropy of the car due to this heat transfer alone?
86. A hot rock ejected from a volcano’s lava fountain cools from \(\displaystyle 1100º C\) to \(\displaystyle 40.0º C\), and its entropy decreases by 950 J/K. How much heat transfer occurs from the rock?
Solution \(\displaystyle 8.01×10^5J\)
87. When \(\displaystyle 1.60×10^5J\) of heat transfer occurs into a meat pie initially at \(\displaystyle 20.0º C\), its entropy increases by 480 J/K. What is its final temperature?
88. The Sun radiates energy at the rate of \(\displaystyle 3.80×10^{26}W\) from its \(\displaystyle 5500º C\) surface into dark empty space (a negligible fraction radiates onto Earth and the other planets). The effective temperature of deep space is \(\displaystyle −270º C\).
(a) What is the increase in entropy in one day due to this heat transfer?
(b) How much work is made unavailable?
Solution (a) \(\displaystyle 1.04×10^{31}J/K\) (b) \(\displaystyle 3.28×10^{31}J\)
89. (a) In reaching equilibrium, how much heat transfer occurs from 1.00 kg of water at \(\displaystyle 40.0º C\) when it is placed in contact with 1.00 kg of \(\displaystyle 20.0º C\) water in reaching equilibrium?
(b) What is the change in entropy due to this heat transfer?
(c) How much work is made unavailable, taking the lowest temperature to be \(\displaystyle 20.0º C\)? Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy.
90. What is the decrease in entropy of 25.0 g of water that condenses on a bathroom mirror at a temperature of \(\displaystyle 35.0º C\), assuming no change in temperature and given the latent heat of vaporization to be 2450 kJ/kg?
Solution 199 J/K
91. Find the increase in entropy of 1.00 kg of liquid nitrogen that starts at its boiling temperature, boils, and warms to \(\displaystyle 20.0º C\) at constant pressure.
92. A large electrical power station generates 1000 MW of electricity with an efficiency of 35.0%.
(a) Calculate the heat transfer to the power station, \(\displaystyle Q_h\), in one day.
(b) How much heat transfer \(\displaystyle Q_c\) occurs to the environment in one day?
(c) If the heat transfer in the cooling towers is from \(\displaystyle 35.0º C\) water into the local air mass, which increases in temperature from \(\displaystyle 18.0º C\) to \(\displaystyle 20.0º C\), what is the total increase in entropy due to this heat transfer?
(d) How much energy becomes unavailable to do work because of this increase in entropy, assuming an \(\displaystyle 18.0º C\) lowest temperature? (Part of \(\displaystyle Q_c\) could be utilized to operate heat engines or for simply heating the surroundings, but it rarely is.)
Solution (a) \(\displaystyle 2.47×10^{14}J\) (b) \(\displaystyle 1.60×10^{14}J\) (c) \(\displaystyle 2.85×10^{10}J/K\) (d) \(\displaystyle 8.29×10^{12}J\)
93. (a) How much heat transfer occurs from 20.0 kg of \(\displaystyle 90.0º C\) water placed in contact with 20.0 kg of \(\displaystyle 10.0º C\) water, producing a final temperature of \(\displaystyle 50.0º C\)?
(b) How much work could a Carnot engine do with this heat transfer, assuming it operates between two reservoirs at constant temperatures of \(\displaystyle 90.0º C\) and \(\displaystyle 10.0º C\)?
(c) What increase in entropy is produced by mixing 20.0 kg of \(\displaystyle 90.0º C\) water with 20.0 kg of \(\displaystyle 10.0º C\) water?
(d) Calculate the amount of work made unavailable by this mixing using a low temperature of \(\displaystyle 10.0º C\), and compare it with the work done by the Carnot engine. Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy.
(e) Discuss how everyday processes make increasingly more energy unavailable to do work, as implied by this problem.
94. Using Table, verify the contention that if you toss 100 coins each second, you can expect to get 100 heads or 100 tails once in \(\displaystyle 2×10^{22}\) calculate the time to two-digit accuracy.
Solution It should happen twice in every \(\displaystyle 1.27×10^{30}s\) or once in every \(\displaystyle 6.35×10^{29}s\) \(\displaystyle (6.35×10^{29}s)(\frac{1 h}{3600 s})=(\frac{1 d}{24 h})(\frac{1 y}{365.25 d})=2.0×10^{22}y\)
95. What percent of the time will you get something in the range from 60 heads and 40 tails through 40 heads and 60 tails when tossing 100 coins? The total number of microstates in that range is \(\displaystyle 1.22×10^{30}\). (Consult Table.)
96. (a) If tossing 100 coins, how many ways (microstates) are there to get the three most likely macrostates of 49 heads and 51 tails, 50 heads and 50 tails, and 51 heads and 49 tails?
(b) What percent of the total possibilities is this? (Consult Table.)
Solution (a) \(\displaystyle 3.0×10^{29}\) (b) 24%
97. (a) What is the change in entropy if you start with 100 coins in the 45 heads and 55 tails macrostate, toss them, and get 51 heads and 49 tails?
(b) What if you get 75 heads and 25 tails?
(c) How much more likely is 51 heads and 49 tails than 75 heads and 25 tails?
(d) Does either outcome violate the second law of thermodynamics?
98. (a) What is the change in entropy if you start with 10 coins in the 5 heads and 5 tails macrostate, toss them, and get 2 heads and 8 tails?
(b) How much more likely is 5 heads and 5 tails than 2 heads and 8 tails? (Take the ratio of the number of microstates to find out.)
(c) If you were betting on 2 heads and 8 tails would you accept odds of 252 to 45? Explain why or why not.
Solution (a) \(\displaystyle −2.38×10^{–23}J/K\) (b) 5.6 times more likely (c) If you were betting on two heads and 8 tails, the odds of breaking even are 252 to 45, so on average you would break even. So, no, you wouldn’t bet on odds of 252 to 45.
99. (a) If you toss 10 coins, what percent of the time will you get the three most likely macrostates (6 heads and 4 tails, 5 heads and 5 tails, 4 heads and 6 tails)?
(b) You can realistically toss 10 coins and count the number of heads and tails about twice a minute. At that rate, how long will it take on average to get either 10 heads and 0 tails or 0 heads and 10 tails?
100. (a) Construct a table showing the macrostates and all of the individual microstates for tossing 6 coins. (Use Table as a guide.)
(b) How many macrostates are there?
(c) What is the total number of microstates?
(d) What percent chance is there of tossing 5 heads and 1 tail?
(e) How much more likely are you to toss 3 heads and 3 tails than 5 heads and 1 tail? (Take the ratio of the number of microstates to find out.)
Solution (b) 7 (c) 64 (d) 9.38% (e) 3.33 times more likely (20 to 6)
101. In an air conditioner, 12.65 MJ of heat transfer occurs from a cold environment in 1.00 h.
(a) What mass of ice melting would involve the same heat transfer?
(b) How many hours of operation would be equivalent to melting 900 kg of ice?
(c) If ice costs 20 cents per kg, do you think the air conditioner could be operated more cheaply than by simply using ice? Describe in detail how you evaluate the relative costs.
Contributors and Attributions
Paul Peter Urone (Professor Emeritus at California State University, Sacramento) and Roger Hinrichs (State University of New York, College at Oswego) with Contributing Authors: Kim Dirks (University of Auckland) and Manjula Sharma (University of Sydney). This work is licensed by OpenStax University Physics under a Creative Commons Attribution License (by 4.0) .

FREE K-12 standards-aligned STEM
curriculum for educators everywhere!
Find more at TeachEngineering.org .
- TeachEngineering
- Heat Transfer: From Hot to Not
Lesson Heat Transfer: From Hot to Not
Grade Level: 11 (10-12)
Time Required: 30 minutes
Lesson Dependency: None
Subject Areas: Chemistry, Physics
NGSS Performance Expectations:

- Print lesson and its associated curriculum
Activities Associated with this Lesson Units serve as guides to a particular content or subject area. Nested under units are lessons (in purple) and hands-on activities (in blue). Note that not all lessons and activities will exist under a unit, and instead may exist as "standalone" curriculum.
- Counting Calories
- Hot Potato, Cool Foil
TE Newsletter
Engineering connection, learning objectives, worksheets and attachments, more curriculum like this, pre-req knowledge, introduction/motivation, associated activities, lesson closure, vocabulary/definitions, user comments & tips.

Among many other things that we use every day, engineers design industrial plants and processes that make usable products from chemicals, such as food products, medicines, materials, and fuels. To safely and efficiently apply and control these processes, engineers must know how much heat will be generated in a given reaction. If too much heat is generated, proteins denature, products burn or decompose, or a reactor might explode. If too little heat is generated, the chemicals do not react, enough energy might not be created, and the wrong products are produced.
In addition to reaction temperatures, an engineer must also have an understanding of the specific heat capacity of various substances. Heat capacity refers to how much energy is required to change the temperature of a substance by one unit temperature. Engineers need to understand heat capacity for a variety of reasons, such as determining how hot metal parts in an engine will get or how much energy must be added to a chemical reactor to raise or lower the contents to the desired temperature.
After this lesson, students should be able to:
- Describe that specific heat capacity is the amount of energy an object can absorb before changing in temperature by one unit temperature.
- Explain how heat capacity, heat of reaction and heat transfer can be applied in engineering to understand and control chemical processes and physical systems.
- Identify exothermic reactions as heat generating, and endothermic reactions as heat consuming.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards. All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN) , a project of D2L (www.achievementstandards.org). In the ASN, standards are hierarchically structured: first by source; e.g. , by state; within source by type; e.g. , science or mathematics; within type by subtype, then by grade, etc .
Ngss: next generation science standards - science, common core state standards - math.
View aligned curriculum
Do you agree with this alignment? Thanks for your feedback!
International Technology and Engineering Educators Association - Technology
State standards, colorado - science.
Algebra: Students need to be aware of basic algebraic manipulation of equations and substitution techniques.
Chemistry: Students should be aware that chemicals interact in reactions that change the chemical and/or physical properties of a system.
Physical Science: Students should be familiar with the concept of energy, that it can be exchanged, and that it comes in different forms.
Some of the most interesting demonstrations in science and engineering involve energy and heat. Think of a balloon full of hydrogen being ignited with a match, a cold-weather hand warmer releasing warmth, or salt melting ice. Heat exchanges such as these are only a small sample of the broad applications in engineering and science of heat transfer. The explosive ignition of the fuel in a rocket that provides energy for lift-off, the tough material on the surface of the space shuttle, the lining inside of a high-temperature reactor, or even the chemical processes that go into making ice-cream are all drawing from our knowledge of heat transfer.
Engineers use the concept of heat of reaction to know how much energy we can expect from burning rocket fuel, or how much energy will be absorbed by reactions that make everything from plastic to cookies. Knowledge of heat capacity allows engineers to predict how much energy a material can hold before reaching a certain temperature, and then can design a product accordingly.
What is heat? It may seem hard to describe exactly what heat is. Heat could be described as hotness by some people, but that is already described by temperature. For scientists and engineers, heat is simply a term referring to thermal energy transferred between two bodies. Heat can also be energy released in a reaction. There are many applications for knowledge of heat and heat transfer. As we will see, engineers use knowledge of heat of reaction to predict how much energy will be produced in a chemical system, which is important for keeping the reactor safe and efficient. Beyond chemical reactions, heat is exchanged for physical reactions, too. Some examples include dissolving one chemical in another, or phase changes between solid, liquid and gas.
Heat of reaction is the amount of heat energy generated or absorbed for a given physical or chemical reaction. Reactions can either give off heat or they can absorb heat. When something gives off heat, it is called exothermic . Examples of exothermic reactions are easy to name. They include burning wood, lighting a hydrogen balloon, and ice freezing. On the other hand, endothermic reactions are reactions that absorb heat. Baking a cake, boiling water, and dissolving certain salts in water are examples of endothermic reactions. Heat of reaction is also often called the enthalpy of reaction . Heat/enthalpy of solution is another important concept. This is the same idea, but instead of chemical reactions, it refers to dissolving one chemical in another (as a simple definition). Other terms to keep in mind are heat of vaporization and heat of fusion . These describe the energy inputs and outputs in boiling and freezing.
Another important concept is heat capacity . A chemical engineer needs to know how much a given amount of energy will raise the temperature of the reaction components and the reactor itself. An aerospace engineer needs to understand the tolerances of a spaceship's building materials so that it can successfully survive the extreme temperatures of space. Even in cooking it is important to understand heat capacity in order to determine how long and how hot to cook a turkey, for instance. Heat capacity can be thought of as how much energy must be put into something before it will get one degree hotter. This is different for different substances. The heat capacity for different substances depends on complex atomic and molecular interactions, such as the way in which atoms are connected to one another, the atomic bond strength, and how quickly the atoms transfer energy among themselves. Refer to the associated activity Hot Potato, Cool Foil to have students investigate different material properties and the basic principles of heat transfer using calorimeters to determine the specific heat of several substances.
Finally, how heat transfers between two systems is an important part of engineering. The knowledge of how quickly and how much heat will be conducted is important for controlling reactions as well as keeping important materials and components in a device within operating conditions. For example, a chemical engineer might need to know how much heat will be transferred from the outside of a reactor to the surrounding air, or a computer engineer would require a certain type and size of cooling fins and fans to keep the processor from overheating.
Lesson Background and Concepts for Teachers
Heat of Reaction and More!
What causes an energy change in a reaction, dissolution or phase change? It has to do with the rearrangement of atoms and molecules. For a chemical reaction, energy is either absorbed or released to form chemical bonds between atoms. If the new bond arrangement is more stable than the original arrangement, then is less energetic than before and releases the extra energy it does not need. Thus, it is an exothermic reaction .
If the bond is less stable and requires more energy to exist, it absorbs energy from its surroundings until it has stabilized. This is an endothermic reaction . The same principle applies for dissolution. When you dissolve table salt NaCl in water, you break it into sodium and chloride ions surrounding molecules. Since this requires an input of energy, it is labeled endothermic. Some salts are exothermic and release energy in the same situation.
Finally, boiling water is an endothermic reaction because the water molecules need to absorb energy to break their inter-molecular interaction with each other and become a gas. That is why we boil water on a hot stove!
The amount of heat transferred in a given reaction can be predicted if we know certain things about what is going on. First, we have to identify if it is a chemical or a physical process. Is there a chemical change, such as burning wood to get ash and CO2? Or is it physical, such as melting ice to get water? After we have identified the type of reaction, we can look up the standard heat of reaction. Negative heats of reaction are exothermic, whereas endothermic reactions are positive. These standards are on a per mole basis. Therefore, the heat of reaction is the amount of heat produced for the number of moles of product in the base chemical equation.
Heat Transfer
Heat transfer between two systems is governed by a relatively simple equation that relates energy exchanged to the heat capacity and quantity of the substance. The equation is as follows: Q=mC P ∆T
In this equation, Q refers to the amount of energy transferred, m is the mass of the object in question, C P is its heat capacity, and ∆ T is the change in temperature of the substance between the starting temperature before heat is transferred and the temperature after heat transferred. Students can illustrate this idea with the Counting Calories associated activity by constructing constant pressure calorimeters to determine the heat of solution of potassium chloride in water.
We can set the heat that exits the system equal to the heat entering the surroundings, which can be the air surrounding an object, or something the object is touching. The idea of energy conservation is known as the first law of thermodynamics . Another way of saying this is that energy can neither be created nor destroyed, just changed into different forms. So if energy is leaving the system, it must be entering another system because it cannot disappear.
In the case of two substances/objects originally at different temperatures which are brought into contact (e.g., two fluids mixed together, hot ball bearings quenched in cool water), the energy equation can be used to set up a mathematical model to determine the energy change in one substance/object when the energy change in the other is known. For instance, if a hot cube of aluminum is submerged in cool water, the energy equation can be utilized to show that the energy lost be the aluminum is gained by the water.
Q lost by Al = Q gained by water
m Al C p,Al (T f,Al - T i,Al ) = m w C p,w (T f,w - T i,w )
If the aluminum cube is left in the water for a long enough period, the water and aluminum will reach the same temperature. Assuming that we know the masses, specific heats, and initial temperatures of the water and aluminum, the energy balance above can be rearranged to solve for the final temperature. This final temperature can then be used to solve for the energy change of the water and aluminum.
T f,Al = T f,w
T f = (m Al C p,Al T i,Al - m w C p,w T i,w )/(m Al C p,Al - m w C p,w )
- Counting Calories - Students discover the basics of heat transfer by constructing constant pressure calorimeters to determine the heat of solution of potassium chloride in water.
- Hot Potato, Cool Foil - Students explore material properties and the basic principles of heat transfer using calorimeters to determine the specific heat of several substances.
Heat transfer is an extremely important aspect of nearly all fields of engineering. Whether it be cooling fins on a computer component or the cooling system in a car's engine, engineers apply their knowledge of heat transfer in many situations. Heat capacity describes how much heat a substance can hold when increased by one degree of temperature. This is important in applications such as industrial-scale cooking, chemical reactors and material tolerances for machines, such as cars and spacecraft heat shielding. Heats of reaction, solution and phase changes describe the energy absorbed or released in the rearrangement of atoms and molecules in their interactions. Applications of this include everything from formulating powerful rocket fuels to creating an effective road de-icer. Finally, understanding that reactions such as burning rocket fuel are considered exothermic because they release energy, and baking a cake or boiling water are endothermic because they require energy input to be considered "complete."
endothermic : A process or reaction which absorbs energy.
enthalpy: The enthalpy change is the amount of heat released or absorbed when a chemical reaction occurs at constant pressure.
exothermic: A process or reaction which releases energy.
heat: Energy transferred between two systems as a result of a temperature difference.
heat capacity: The amount of energy transfer required to raise or lower a given amount of a substance by one unit temperature at a constant pressure
heat of reaction: The amount of energy released or absorbed for a given amount of reacting chemicals.
Pre-Lesson Assessment
Discussion: Gather and discuss student ideas.
- What is energy? (Answer: The capacity of a system for doing work; kinetic energy, potential energy, electrical energy, etc.)
- What is heat? (Answer: Energy transferred between two systems as a result of a temperature difference.)
- What is the difference between heat and energy? (Answer: Heat is a form of energy; it has the same units but is specifically the transferable energy across a temperature gradient.)
Post-Introduction Assessment
Everyday Examples: As a class, think of examples of heat transfer and heat of reaction and heat of solution in everyday life and list them on the board. (Answers may include: ovens, road salt for ice melt, furnaces, etc.)
Lesson Summary Assessment
Applying Concepts to Problem Solving: Have students work in small groups of 2-3 to complete the Heat Transfer Problem Sheet . Go through the solutions as a class, allowing groups to show their solutions on the board if desired. The problems ask students to use the concepts they learn in this lesson to develop computational models to calculate change in energy in the form of heat. Solutions are available for the teacher's use in the Heat Transfer Problem Sheet Answer Key .
Brainstorming: Put students in small groups to brainstorm ideas about how knowledge of heat of reaction and heat transfer might be useful. This should include applications for everyday life, industry, science/engineering and at least one other category (cooking, fixing cars, etc).
- Have students present their ideas to the class.
- Have students think of ways they would explain the causes behind heat of reaction, dissolution and phase changes to someone else. They should come up with good analogies, or even act out the roles of the atoms for these situations.

Learn the basics of the analysis of forces engineers perform at the truss joints to calculate the strength of a truss bridge known as the “method of joints.” Find the tensions and compressions to solve systems of linear equations where the size depends on the number of elements and nodes in the trus...

Blair, John, National Institute of Standards and Technology, NIST Tech Beat, January 23, 2008, accessed November 12, 2009. http://www.nist.gov/public_affairs/techbeat/tb2008_0123.htm.
U.S. Department of Energy, Energy Efficiency and Renewable Energy, Petroleum Refining Industry for the Future, 5/16/2007, accessed November 15, 2009. http://www1.eere.energy.gov/industry/petroleum_refining/profile.html
Contributors
Supporting program, acknowledgements.
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: May 14, 2020
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
A cooling system is being used in order to decrease heat gradually from a house at a rate of 900 kJ/min while drawing electric power at a rate of 75 kW. Determine the COP of this system and the rate of heat transfer to the outside weather.
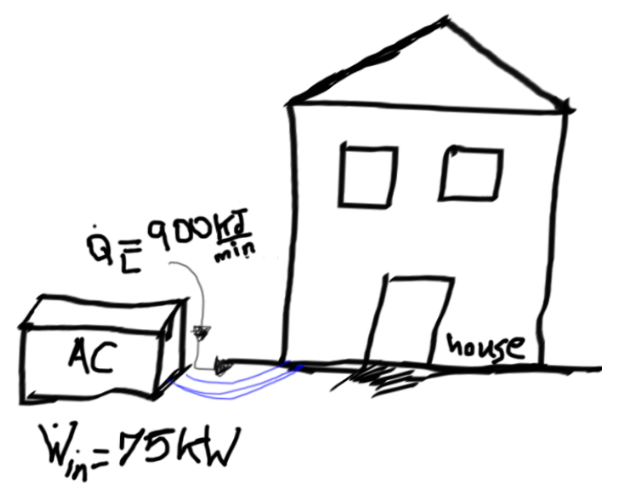
Determine the COP of this system and the rate of heat transfer for the outside weather.
The COP of this system
The rate of heat transfer for the outside weather.
Determine the entropy change of the working fluid, the entropy change of the source, and the total entropy change for the process.
Thermodynamics Copyright © by Diana Bairaktarova (Adapted from Engineering Thermodynamics - A Graphical Approach by Israel Urieli and Licensed CC BY NC-SA 3.0) is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

IMAGES
VIDEO
COMMENTS
Download site for A Heat Transfer Textbook. Solutions to more than 490 problems are on the following links. Solutions for Chapter 1 (v1.01, 16 MB, February 2023). Solutions for Chapter 2 (v1.0, 13 MB, August 2020). Solutions for Chapter 3 (v1.0, 15 MB, August 2020). Partial solutions for Chapters 4-11 (v1.05, 24 MB, 24 March 2023) Includes solutions for all problems in Chapters 4, 5, 6, 10 & 11
Step-by-step solution. Step 1 of 3. Calculate the area of insulation. Here, d is the side length of the sheet. Substitute 2 m for d. Step 2 of 3. (a) Calculate the heat flux through the insulated sheet. Here, is the temperature difference, is the length of the insulated sheet along the direction of heat flow, and k is the thermal conductivity.
Now, with expert-verified solutions from Heat Transfer 10th Edition, you'll learn how to solve your toughest homework problems. Our resource for Heat Transfer includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can ...
Solutions Manuals are available for thousands of the most popular college and high school textbooks in subjects such as Math, Science (Physics, Chemistry, Biology), Engineering (Mechanical, Electrical, Civil), Business and more. Understanding Fundamentals of Heat and Mass Transfer 8th Edition homework has never been easier than with Chegg Study.
In certain large geographic regions, the underlying rock is hot. Wells can be drilled and water circulated through the rock for heat transfer for the generation of electricity. (a) Calculate the heat transfer that can be extracted by cooling 1.00km3 1.00 k m 3 of granite by 100ºC 100 º C.
Heat Transfer: Homework Solutions. 1-015. 1-016. 1-017. 1-020. 1-021. 1-022. 1-027. 1-028. 1-029. 1-031. 1-054. 1-055. 1-056. 1-057. 1-059
Step-by-step solution. Step 1 of 5. Draw the schematic diagram and the thermal circuit of the heat transfer between the hot surface and the two insulation materials in series. Step 2 of 5. Assume that steady state exists, neglect effects at the corners and edges of the hot surface, and assume that the surface temperatures are uniform.
REASONING The heat Q conducted along the bar is given by the relation Q =. L (Equation 13.1). We can determine the temperature difference between the hot end of the bar and a point 0.15 m from that end by solving this equation for ∆T and noting that the heat conducted per second is Q/t and that L = 0.15 m.
Textbook solutions for Heat and Mass Transfer: Fundamentals and Applications… 5th Edition Yunus A. Cengel Dr. and others in this series. View step-by-step homework solutions for your homework. Ask our subject experts for help answering any of your homework questions!
ME 375 - Heat Transfer 4 19 Transient 1D Convection Figure 4-11 in Çengel, Heat and Mass Transfer All problems have similar chart solutions 20 Slab Center-line (x = 0) Temperature Chart Figure 4-15(a) in Çengel, Heat and Mass Transfer 21 Chart II • Can find T at any x/L from this chart once T at x = 0 is found from previous chart • See ...
Steam to drive an old-fashioned steam locomotive is supplied at a constant gauge pressure of 1.75 × 106N / m2 (about 250 psi) to a piston with a 0.200-m radius. (a) By calculating PΔV, find the work done by the steam when the piston moves 0.800 m. Note that this is the net work output, since gauge pressure is used.
Heat transfer homework solution problem known: dimensions, thermal conductivity and surface temperatures of concrete slab. efficiency of gas furnace and cost of Skip to document Ask an Expert
With Expert Solutions for thousands of practice problems, you can take the guesswork out of studying and move forward with confidence. Science. Engineering. Fundamentals of Heat and Mass Transfer. 8th Edition. ISBN: 9781118989173. Adrienne S Lavine, David P. Dewitt, Frank P. Incropera, Theodore L. Bergman. Textbook solutions.
Heat Transfer. Heat transfer between two systems is governed by a relatively simple equation that relates energy exchanged to the heat capacity and quantity of the substance. The equation is as follows: Q=mC P ∆T. In this equation, Q refers to the amount of energy transferred, m is the mass of the object in question, C P is its heat capacity ...
978-1-107-17953- — Introduction to Engineering Heat Transfer G. F. Nellis , S. A. Klein Frontmatter ... from the solution and exploration steps to encourage a deep and practical understanding. Numerous exercises are provided for homework and self-study and include standard hand calculations as well as more advanced
Here is the equation: Q = mcΔT. Q is thermal energy in joules (J) m is the mass of the object in grams (g) c is the caloric requirement for the phase of matter the object is in (J/g⋅°C) - for ...
A cooling system is being used in order to decrease heat gradually from a house at a rate of 900 kJ/min while drawing electric power at a rate of 75 kW. Determine the COP of this system and the rate of heat transfer to the outside weather. Given: Find: Determine the COP of this system and the rate of heat transfer for the outside weather. Solution:
Example 4: Heat flux in a cylindrical shell - Newton's law of cooling. Example 5: Heat conduction with generation. Example 6: Wall heating of laminar flow. Conclusion: When we can simplify geometry, assume steady state, assume symmetry, the solutions are easily obtained. 3 Faith A. Morrison, Michigan Tech U. State.
Heat And Mass Transfer | 5th Edition. ISBN-13: 9780073398181 ISBN: 0073398181 Authors: Yunus A. Cengel Rent | Buy. This is an alternate ISBN. View the primary ISBN for: Heat And Mass Transfer: Fundamentals And Applications 5th Edition Textbook Solutions.
Author. Massoud Kaviany, University of Michigan, Ann Arbor Massoud Kaviany is a Professor in the Department of Mechanical Engineering and in the Applied Physics Program at the University of Michigan, where he has been since 1986. His area of teaching and research is heat transfer physics, with a particular interest in porous media. His current projects include atomic structural metrics in high ...
Step-by-step solution. Step 1 of 3. Calculate the area of insulation. Here, d is the side length of the sheet. Substitute 2 m for d. Step 2 of 3. (a) Calculate the heat flux through the insulated sheet. Here, is the temperature difference, is the length of the insulated sheet along the direction of heat flow, and k is the thermal conductivity.
Solutions Manuals are available for thousands of the most popular college and high school textbooks in subjects such as Math, Science (Physics, Chemistry, Biology), Engineering (Mechanical, Electrical, Civil), Business and more. Understanding Principles of Heat Transfer homework has never been easier than with Chegg Study.
Step-by-step solution. Step 1 of 2. Write the Fourier's law of heat conduction equation. Here, is the rate of heat transfer, A is the cross-sectional area normal to the direction of heat flow, k is the thermal conductivity, is the temperature difference, and is the thickness of the material. The minus sign indicates, heat flow will be in the ...