An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmacological Reviews


New Aspects of Diabetes Research and Therapeutic Development
Both type 1 and type 2 diabetes mellitus are advancing at exponential rates, placing significant burdens on health care networks worldwide. Although traditional pharmacologic therapies such as insulin and oral antidiabetic stalwarts like metformin and the sulfonylureas continue to be used, newer drugs are now on the market targeting novel blood glucose–lowering pathways. Furthermore, exciting new developments in the understanding of beta cell and islet biology are driving the potential for treatments targeting incretin action, islet transplantation with new methods for immunologic protection, and the generation of functional beta cells from stem cells. Here we discuss the mechanistic details underlying past, present, and future diabetes therapies and evaluate their potential to treat and possibly reverse type 1 and 2 diabetes in humans.
Significance Statement
Diabetes mellitus has reached epidemic proportions in the developed and developing world alike. As the last several years have seen many new developments in the field, a new and up to date review of these advances and their careful evaluation will help both clinical and research diabetologists to better understand where the field is currently heading.
I. Introduction
Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020 ). Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing pancreatic beta cells. The much more prevalent T2D arises in conjunction with peripheral tissue insulin resistance and beta cell failure and is estimated to increase to 21%–33% of the US population by the year 2050 (Boyle et al., 2010 ). To combat this growing health threat and its cardiac, renal, and neurologic comorbidities, new and more effective diabetes drugs and treatments are essential. As the last several years have seen many new developments in the field of diabetes pharmacology and therapy, we determined that a new and up to date review of these advances was in order. Our aim is to provide a careful evaluation of both old and new therapies ( Fig. 1 ) in a manner that we hope will be of interest to both clinical and bench diabetologists. Instead of the usual encyclopedic approach to this topic, we provide here a targeted and selective consideration of the underlying issues, promising new treatments, and a re-examination of more traditional approaches. Thus, we do not discuss less frequently used diabetes agents, such as alpha-glucosidase inhibitors; these were discussed in other recent reviews (Hedrington and Davis, 2019 ; Lebovitz, 2019 ).

Pharmacologic targeting of numerous organ systems for the treatment of diabetes. Treatment of diabetes involves targeting of various organ systems, including the kidney by SGLT2 inhibitors; the liver, gut, and adipose tissue by metformin; and direct actions upon the pancreatic beta cell. Beta cell compounds aim to increase secretion or mass and/or to protect from autoimmunity destruction. Ultimately, insulin therapy remains the final line of diabetes treatment with new technologies under development to more tightly regulate blood glucose levels similar to healthy beta cells. hESC, human embryonic stem cell.
II. Diabetes Therapies
A. metformin.
Metformin is a biguanide originally based on the natural product galegine, which was extracted from the French lilac (Bailey, 1992 ; Rojas and Gomes, 2013 ; Witters, 2001 ). A closely related biguanide, phenformin, was also used initially for its hypoglycemic actions. Based on its successful track record as a safe, effective, and inexpensive oral medication, metformin has become the most widely prescribed oral agent in the world in treating T2D (Rojas and Gomes, 2013 ; He and Wondisford, 2015 ; Witters, 2001 ), whereas phenformin has been largely bypassed due to its unacceptably high association with lactic acidosis (Misbin, 2004 ). Unlike sulfonylureas, metformin lowers blood glucose without provoking hypoglycemia and improves insulin sensitivity (Bailey, 1992 ). Despite these well known beneficial metabolic actions, metformin’s mechanism of action and even its main target organ remain controversial. In fact, metformin has multiple mechanisms of action at the organ as well as the cellular level, which has hindered our understanding of its most important molecular effects on glucose metabolism (Witters, 2001 ). Adding to this, a specific receptor for metformin has never been identified. Metformin has actions on several tissues, although the primary foci of most studies have been the liver, skeletal muscle, and the intestine (Foretz et al., 2014 ; Rena et al., 2017 ). Metformin and phenformin clearly suppress hepatic glucose production and gluconeogenesis, and they improve insulin sensitivity in the liver and elsewhere (Bailey, 1992 ). The hepatic actions of metformin have been the most exhaustively studied to date, and there is little doubt that these actions are of some importance. However, several of the studies remain highly controversial, and there are still open questions.
One of the first reported specific molecular targets of metformin was mitochondrial complex I of the electron transport chain. Inhibition of this complex results in reduced oxidative phosphorylation and consequently decreased hepatic ATP production (El-Mir et al., 2008 ; Evans et al., 2005 ; Owen et al., 2000 ). As is the case in many other studies of metformin, however, high concentrations of the drug were found to be necessary to depress metabolism at this site (El-Mir et al., 2000 ; He and Wondisford, 2015 ; Owen et al., 2000 ). Also controversial is whether metformin works by activating 5′ AMP-activated protein kinase (AMPK), a molecular energy sensor that is known to be a major metabolic sensor in cells, or if not AMPK directly, then one of its upstream regulators such as liver kinase B2 (Zhou et al., 2001 ). Although metformin was shown to activate AMPK in several excellent studies, other studies directly contradicted the AMPK hypothesis. Most dramatic were studies showing that metformin’s actions to suppress hepatic gluconeogenesis persisted despite genetic deletion of the AMPK’s catalytic domain (Foretz et al., 2010 ). More recent studies identified additional or alternative targets, such as cAMP signaling in the liver (Miller et al., 2013 ) or glycogen synthase kinase-3 (Link, 2003 ). Other work showed that the phosphorylation of acetyl-CoA carboxylase and acetyl-CoA carboxylase 2 are involved in regulating lipid homeostasis and improving insulin sensitivity after exposure to metformin (Fullerton et al., 2013 ).
Although there are strong data to support each of these pathways, it is not entirely clear which signaling pathway(s) is most essential to the actions of metformin in hepatocytes. Metformin clearly inhibits complex I and concomitantly decreases ATP and increases AMP. The latter results in AMPK activation, reduced fatty acid synthesis, and improved insulin receptor activation, and increased AMP has been shown to inhibit adenylate cyclase to reduce cAMP and thus protein kinase A activation. Downstream, this reduces the expression of phosphoenolpyruvate carboxykinase and glucose 6-phosphatase via decreased cAMP response element-binding protein, the cAMP-sensitive transcription factor. Decreased PKA also promotes ATP-dependent 6-phosphofructokinase, liver type activity via fructose 2,6-bisphosphate and reduces gluconeogenesis, as fructose-bisphosphatase 1 is inhibited by fructose 2,6-bisphosphate, along with other mechanisms (Rena et al., 2017 ; Pernicova and Korbonits, 2014 ).
More recent work has shown that metformin at pharmacological rather than suprapharmacological doses increases mitochondrial respiration and complex 1 activity and also increases mitochondrial fission, now thought to be critical for maintaining proper mitochondrial density in hepatocytes and other cells. This improvement in respiratory activity occurs via AMPK activation (Wang et al., 2019 ).
Although the liver has historically been the major suspected site of metformin action, recent studies have suggested that the gut instead of the liver is a major target, a concept supported by the increased efficacy of extended-release formulations of metformin that reside for a longer duration in the gut after their administration (Buse et al., 2016 ). An older, but in our view an important observation, is that the intravenous administration of metformin has little or no effect on blood glucose, whereas, in contrast, orally administered metformin is much more effective (Bonora et al., 1984 ). Recent imaging studies using labeled glucose have shown directly that metformin stimulates glucose uptake by the gut in patients with T2D to reduce plasma glucose concentrations (Koffert et al., 2017 ; Massollo et al., 2013 ). Additionally, it is possible that metformin may exert its effect in the gut by inducing intestinal glucagon-like peptide-1 (GLP-1) release (Mulherin et al., 2011 ; Preiss et al., 2017) to potentiate beta cell insulin secretion and by stimulating the central nervous system (CNS) to exert control over both blood glucose and liver function. Indeed, CNS effects produced by metformin have been proposed to occur via the local release of GLP-1 to activate intestinal nerve endings of ascending nerve pathways that are involved in CNS glucose regulation (Duca et al., 2015 ). Lastly, several papers have now implicated that metformin may act by altering the gut microbiome, suggesting that changes in gut flora may be critical for metformin’s actions (McCreight et al., 2016 ; Wu et al., 2017 ; Devaraj et al., 2016 ). A new study proposed that activation of the intestinal farnesoid X receptor may be the means by which microbiota alter hyperglycemia (Sun et al., 2018 ). However, these studies will require more mechanistic detail and confirmation before they can be fully accepted by the field. In addition to the action of metformin on gut flora, the production of imidazole propionate by gut microbes in turn has been shown to interfere with metformin action through a p38-dependent mechanism and AMPK inhibition. Levels of imidazole propionate are especially higher in patients with T2D who are treated with metformin (Koh et al., 2020 ).
In summary, the combined contribution of these various effects of metformin on multiple cellular targets residing in many tissues may be key to the benefits of metformin treatment on lowering blood glucose in patients with type 2 diabetes (Foretz et al., 2019 ). In contrast, exciting new work showing metformin leads to weight loss by increasing circulating levels of the peptide hormone growth differentiation factor 15 and activation of brainstem glial cell-derived neurotropic factor family receptor alpha like receptors to reduce food intake and energy expenditure works independently of metformin’s glucose-lowering effect (Coll et al., 2020 ).
B. Sulfonylureas and Beta Cell Burnout
The class of compounds known as sulfonylureas includes one of the oldest oral antidiabetic drugs in the pharmacopoeia: tolbutamide. Tolbutamide is a “first generation” oral sulfonylurea secretagogue whose clinical usefulness is due to its prompt stimulation of insulin release from pancreatic beta cells. “Second generation” sulfonylureas include drugs such as glyburide, gliclazide, and glipizide. Sulfonylureas act by binding to a high affinity sulfonylurea binding site, the sulfonylurea receptor 1 subunit of the K(ATP) channel, which closes the channel. These drugs mimic the physiologic effects of glucose, which closes the K(ATP) channel by raising cytosolic ATP/ADP. This in turn provokes beta cell depolarization, resulting in increased Ca 2+ influx into the beta cell (Ozanne et al., 1995 ; Ashcroft and Rorsman, 1989 ; Nichols, 2006 ). Importantly, sulfonylureas, and all drugs that directly increase insulin secretion, are associated with hypoglycemia, which can be severe, and which limits their widespread use in the clinic (Yu et al., 2018 ). Meglitinides are another class of oral insulin secretagogues that, like the sulfonylureas, bind to sulfonylurea receptor 1 and inhibit K(ATP) channel activity (although at a different site of action). The rapid kinetics of the meglitinides enable them to effectively blunt the postprandial glycemic excursions that are a hallmark (along with elevated fasting glucose) of T2D (Rosenstock et al., 2004). However, the need for their frequent dosing (e.g., administration before each meal) has limited their appeal to patients.
The efficacy of sulfonylureas is known to decrease over time, leading to failure of the class for effective long-term treatment of T2D (Harrower, 1991 ). More broadly, it is now widely accepted that the number of functional beta cells in humans declines during the progression of T2D. Thus, one would expect that due to this decline, all manner of oral agents intended to target the beta cell and increase its cell function (and especially insulin secretion) will fail over time (RISE Consortium, 2019 ), a process referred to as “beta cell failure” (Prentki and Nolan, 2006 ). Currently, treatments that can expand beta cell mass or improve beta cell function or survival over time are not yet available for use in the clinic. As a result, treatments that may be able to help patients cope with beta cell burnout such as islet cell transplantation, insulin pumps, or stem cell therapy are alternatives that will be discussed below.
C. Ca 2+ Channel Blockers and Type 1 Diabetes
Strategies to treat and prevent T1D have historically focused on ameliorating the toxic consequences of immune dysregulation resulting in autoimmune destruction of pancreatic beta cells. More recently, a concerted focus on alleviating the intrinsic beta cell defects (Sims et al., 2020 ; Soleimanpour and Stoffers, 2013 ) that also contribute to T1D pathogenesis have been gaining traction at both the bench and the bedside. Several recent preclinical studies suggest that Ca 2+ -induced metabolic overload induces beta cell failure (Osipovich et al., 2020 ; Stancill et al., 2017 ; Xu et al., 2012 ), with the potential that excitotoxicity contributes to beta cell demise in both T1D and T2D, similar to the well known connection between excitotoxicity and, concomitantly, increased Ca 2+ loading of the cells and neuronal dysfunction. Indeed, the use of the phenylalkylamine Ca 2+ channel blocker verapamil has been successful in ameliorating beta cell dysfunction in preclinical models of both T1D and T2D (Stancill et al., 2017 ; Xu et al., 2012 ). Verapamil is a well known blocker of L-type Ca 2+ channels, and, in normally activated beta cells, it limits Ca 2+ entry into the beta cell (Ohnishi and Endo, 1981 ; Vasseur et al., 1987 ). This would be expected to, in turn, alter the expression of many Ca 2+ influx–dependent beta cell genes (Stancill et al., 2017 ), and the evidence to date suggests it is likely that verapamil preserves beta cell function in diabetes models by repressing thioredoxin-interacting protein (TXNIP) expression and thus protecting the beta cell. This is somewhat surprising given the physiologic role of Ca 2+ is to acutely trigger insulin secretion; this process would be expected to be inhibited by L-type Ca 2+ channel blockers (Ashcroft and Rorsman, 1989 ; Satin et al., 1995 ).
Hyperglycemia is a well known inducer of TXNIP expression, and a lack of TXNIP has been shown to protect against beta cell apoptosis after inflammatory stress (Chen et al., 2008a ; Shalev et al., 2002 ; Chen et al., 2008b ). Excitingly, the use of verapamil in patients with recent-onset T1D improved beta cell function and improved glycemic control for up to 12 months after the initiation of therapy, suggesting there is indeed promise for targeting calcium and TXNIP activation in T1D. Use of verapamil for a repurposed indication in the preservation of beta cell function in T1D is attractive due its well known safety profile as well as its cardiac benefits (Chen et al., 2009 ). Although the long-term efficacy of verapamil to maintain beta cell function in vivo is unclear, a recently described TXNIP inhibitor may also show promise in suppressing the hyperglucagonemia that also contributes to glucose intolerance in T2D (Thielen et al., 2020 ). As there is a clear need for increased Ca 2+ influx into the beta cell to trigger and maintain glucose-dependent insulin secretion (Ashcroft and Rorsman, 1990 ; Satin et al., 1995 ), it remains to be seen how well regulated insulin secretion is preserved in the presence of L-type Ca 2+ channel blockers like verapamil in the system. One might speculate that reducing but not fully eliminating beta cell Ca 2+ influx might reduce TXNIP levels while preserving enough influx to maintain glucose-stimulated insulin release. Alternatively, these two phenomena may operate on entirely different time scales. At present, these issues clearly will require further investigation.
D. GLP-1 and the Incretins
Studies dating back to the 1960s revealed that administering glucose in equal amounts via the peripheral circulation versus the gastrointestinal tract led to dramatically different amounts of glucose-induced insulin secretion (Elrick et al., 1964 ; McIntyre et al., 1964 ; Perley and Kipnis, 1967 ). Gastrointestinal glucose administration greatly increased insulin secretion versus intravenous glucose, and this came to be known as the “incretin effect” (Nauck et al., 1986a ; Nauck et al., 1986b ). Subsequent work showed that release of the gut hormone GLP-1 mediated this effect such that food ingestion induced intestinal cell hormone secretion. GLP-1 so released would then circulate to the pancreas via the blood to prime beta cells to secrete more insulin when glucose became elevated because these hormones stimulated beta cell cAMP formation (Drucker et al., 1987 ). The discovery that a natural peptide corresponding to GLP-1 could be found in the saliva of the Gila monster, a desert lizard, hastened progress in the field, and ample in vitro studies subsequently confirmed that GLP-1 potentiated insulin secretion in a glucose-dependent manner. GLP-1 has little or no significant action on insulin secretion in the absence of elevated glucose (such as might typically correspond to the postprandial case or during fasting), thus minimizing the likelihood of hypoglycemia provoked by GLP-1 in treated patients (Kreymann et al., 1987 ). Although not completely understood, the glucose dependence of GLP-1 likely reflects the requirement for adenine nucleotides to close glucose-inhibited K(ATP) channels and thus subsequently activate Ca 2+ influx–dependent insulin exocytosis. Besides potentiating GSIS at the level of the beta cell, glucagon-like peptide-1 receptor (GLP-1R) agonists also decrease glucagon secretion from pancreatic islet alpha cells, reduce gastric emptying, and may also increase beta cell proliferation, among other cellular actions (reviewed in Drucker, 2018 ; Muller et al., 2019).
Intense interest in the incretins by basic scientists, clinicians, and the pharma community led to the rapid development of new drugs for treating primarily T2D. These drugs include a range of GLP-1R agonists and inhibitors of the incretin hormone degrading enzyme dipeptidyl peptidase 4 (DPP4), whose targeting increases the half-lives of GLP-1 and gastric inhibitory polypeptide (GIP) and thereby increases protein hormone levels in plasma. GLP-1R agonists have been associated with not only a lowering of plasma glucose but also weight loss, decreased appetite, reduced risk of cardiovascular events, and other favorable outcomes (Gerstein et al., 2019; Hernandez et al., 2018; Husain et al., 2019; Marso et al., 2016a; Marso et al., 2016b ; Buse et al., 2004). Regarding their untoward actions, although hypoglycemia is not a major concern, there have been reports of pancreatitis and pancreatic cancer from use of GLP-1R agonists. However, a recent meta-analysis covering four large-scale clinical trials and over 33,000 participants noted no significantly increased risk for pancreatitis/pancreatic cancer in patients using GLP-1R agonists (Bethel et al., 2018).
Ongoing and future developments in the use of proglucagon-derived peptides such as GLP-1 and glucagon include the use of combined GLP-1/GIP, glucagon/GLP-1, and agents targeting all three peptides in combination (reviewed in Alexiadou and Tan, 2020 ). Although short-term infusions of GLP-1 with GIP failed to yield metabolic benefits beyond those seen with GLP-1 alone (Bergmann et al., 2019 ), several GLP-1/GIP dual agonists are currently in development and have shown promising metabolic results in clinical trials (Frias et al., 2017 ; Frias et al., 2020 ; Frias et al., 2018 ). At the level of the pancreatic islet, beneficial effects of dual GLP-1/GIP agonists may be related to imbalanced and biased preferences of these agonists for the gastric inhibitory polypeptide receptor over the GLP-1R (Willard et al., 2020 ) and possibly were not simply to dual hormone agonism in parallel. Dual glucagon/GLP-1 agonist therapy has also been shown to have promising metabolic effects in humans (Ambery et al., 2018 ; Tillner et al., 2019 ). Oxyntomodulin is a natural dual glucagon/GLP-1 receptor agonist and proglucagon cleavage product that is also secreted from intestinal enteroendocrine cells, which has beneficial effects on insulin secretion, appetite regulation, and body weight in both humans and rodents (Cohen et al., 2003 ; Dakin et al., 2001 ; Dakin et al., 2002 ; Shankar et al., 2018 ; Wynne et al., 2005 ). Interestingly, alpha cell crosstalk to beta cells through the combined effects of glucagon and GLP-1 is necessary to obtain optimal glycemic control, suggesting a potential pathway for therapeutic dual glucagon/GLP-1 agonism within the islets of patients with T2D (Capozzi et al., 2019a ; Capozzi et al., 2019b ). Although the early results appear promising, more studies will be necessary to better understand the mechanistic and clinical impacts of these multiagonist agents.
E. DPP4 Inhibitors
Inhibition of DPP4, the incretin hormone degrading enzyme, is one of the most common T2D treatments to increase GLP-1 and GIP plasma hormone levels. These DPP4 inhibitors or “gliptins” are generally used in conjunction with other T2D drugs such as metformin or sulfonylureas to obtain the positive benefits discussed above (Lambeir et al., 2008 ). DPP4 is a primarily membrane-bound peptidase belonging to the serine peptidase/prolyl oligopeptidase gene family, which cleaves a large number of substrates in addition to the incretin hormones (Makrilakis, 2019 ). DPP4 inhibitors provide glucose-lowering benefits while being generally well tolerated, and the variety of available drugs (including sitagliptin, saxagliptin, vildagliptin, alogliptin, and linagliptin) with slightly different dosing frequency, half-life, and mode of excretion/metabolism allows for use in multiple patient populations (Makrilakis, 2019 ). This includes the elderly and individuals with renal or hepatic insufficiency (Makrilakis, 2019 ).
Although hypoglycemia is not a concern for DPP4 inhibitor use, other considerations should be made. DPP4 inhibitors tend to be more expensive than metformin or other second-line oral drugs in addition to having more modest glycemic effects than GLP-1R agonists (Munir and Lamos, 2017 ). Finally, meta-analysis of randomized and observational studies concluded that heart failure in patients with T2D was not associated with use of DPP4 inhibitors; however, this study was limited by the short follow-up and lack of high-quality data (Li et al., 2016 ). Thus, the US Food and Drug Administration (FDA) did recommend assessing risk of heart failure hospitalization in patients with pre-existing cardiovascular disease, prior heart failure, and chronic kidney disease when using saxagliptin and alogliptin (Munir and Lamos, 2017 ).
F. Sodium Glucose Cotransporter 2 Inhibitors
A recent development in the field of T2D drugs are sodium glucose cotransporter 2 (SGLT2) inhibitors, which have an interesting and very different mechanism of action. Within the proximal tubule of the nephron, SGLT2 transports ingested glucose into the lumen of the proximal tubule between the epithelial layers, thereby reclaiming glucose by this reabsorption process (reviewed in Vallon, 2015 ). SGLT2 inhibitors target this transporter and increase glucose in the tubular fluid and ultimately increase it in the urine. In patients with diabetes, SGLT2 inhibition results in a lowering of plasma glucose with urine glucose content rising substantially (Adachi et al., 2000 ; Vallon, 2015 ). These drugs, although they are relatively new, have become an area of great interest for not only patients with T2D (Grempler et al., 2012 ; Imamura et al., 2012 ; Meng et al., 2008 ; Nomura et al., 2010 ) but also for patients with T1D (Luippold et al., 2012 ; Mudaliar et al., 2012 ). Part of their appeal also rests on reports that their use can lead to a statistically significant decline in cardiac events that are known to occur secondarily to diabetes, possibly independently of plasma glucose regulation (reviewed in Kurosaki and Ogasawara, 2013 ). Although the long-term consequences of their clinical use cannot yet be determined, raising the glucose content of the urogenital tract leads to an increased risk of urinary tract infections and other related infections in some patients (Kurosaki and Ogasawara, 2013 ).
Another recent concern about the use of SGLT2 inhibitors has been the development of normoglycemic diabetic ketoacidosis (DKA). Despite the efficacy of SGLT2 inhibitors, observations of hyperglucagonemia in patients with euglycemic DKA has led to a number of recent studies focused on SGLT2 actions on pancreatic islets. Initial studies of isolated human islets treated with small interfering RNA directed against SGLT2 and/or SGLT2 inhibitors demonstrated increased glucagon release. These studies were complemented by the finding of elevations in glucagon release in mice that were administered SGLT2 inhibitors in vivo (Bonner et al., 2015 ). Insights into the possible mechanistic links between SGLT2 inhibition, DKA frequency, and glucagon secretion in humans may relate to the observation of heterogeneity in SGLT2 expression, as SGLT2 expression appears to have a high frequency of interdonor and intradonor variability (Saponaro et al., 2020 ). More recently, both insulin and GLP-1 have been demonstrated to modulate SGLT2-dependent glucagon release through effects on somatostatin release from delta cells (Vergari et al., 2019 ; Saponaro et al., 2019 ), suggesting potentially complex paracrine effects that may affect the efficacy of these compounds.
On the other hand, several recent studies question that the development of euglycemic DKA after SGLT2 inhibitor therapy may be through alpha cell–dependent mechanisms. Three recent studies found no effect of SGLT2 inhibitors to promote glucagon secretion in mouse and/or rat models and could not detect SGLT2 expression in human alpha cells (Chae et al., 2020 ; Kuhre et al., 2019 ; Suga et al., 2019 ). A fourth study demonstrated only a brief transient effect of SGLT2 inhibition to raise circulating glucagon concentrations in immunodeficient mice transplanted with human islets, which returned to baseline levels after longer exposures to SGLT2 inhibitors (Dai et al., 2020 ). Furthermore, SGLT2 protein levels were again undetectable in human islets (Dai et al., 2020 ). These results could suggest alternative islet-independent mechanisms by which patients develop DKA, including alterations in ketone generation and/or clearance, which underscore the additional need for further studies both in molecular models and at the bedside. Nevertheless, SGLT2 inhibitors continue to hold promise as a valuable therapy for T2D, especially in the large segment of patients who also have superimposed cardiovascular risk (McMurray et al., 2019; Wiviott et al., 2019; Zinman et al., 2015).
G. Thiazolidinediones
Once among the most commonly used oral agents in the armamentarium to treat T2D, thiazolidinediones (TZDs) were clinically popular in their utilization to act specifically as insulin sensitizers. TZDs improve peripheral insulin sensitivity through their action as peroxisome proliferator-activated receptor (PPAR) γ agonists, but their clinical use fell sharply after studies suggested a connection between cardiovascular toxicity with rosiglitazone and bladder cancer risk with pioglitazone (Lebovitz, 2019 ). Importantly, an FDA panel eventually removed restrictions related to cardiovascular risk with rosiglitazone in 2013 (Hiatt et al., 2013 ). Similarly, concerns regarding use of bladder cancer risk with pioglitazone were later abated after a series of large clinical studies found that pioglitazone did not increase bladder cancer (Lewis et al., 2015 ; Schwartz et al., 2015 ). However, usage of TZDs had already substantially decreased and has not since recovered.
Although concerns regarding edema, congestive heart failure, and fractures persist with TZD use, there have been several studies suggesting that TZDs protect beta cell function. In the ADOPT study, use of rosiglitazone monotherapy in patients newly diagnosed with T2D led to improved glycemic control compared with metformin or sulfonylureas (Kahn et al., 2006). Later analyses revealed that TZD-treated subjects had a slower deterioration of beta cell function than metformin- or sulfonylurea-treated subjects (Kahn et al., 2011). Furthermore, pioglitazone use improved beta cell function in the prevention of T2D in the ACT NOW study (Defronzo et al., 2013; Kahn et al., 2011). Mechanistically, it is unclear if TZDs lead to beneficial beta cell function through direct effects or through indirect effects of reduced beta cell demand due to enhanced peripheral insulin sensitivity. Indeed, a beta cell–specific knockout of PPAR γ did not impair glucose homeostasis, nor did it impair the antidiabetic effects of TZD use in mice (Rosen et al., 2003 ). However, other reports demonstrated PPAR-responsive elements within the promoters of both glucose transporter 2 and glucokinase that enhance beta cell glucose sensing and function, which could explain beta cell–specific benefits for TZDs (Kim et al., 2002 ; Kim et al., 2000 ). Furthermore, TZDs have been shown to improve beta cell function by upregulating cholesterol transport (Brunham et al., 2007 ; Sturek et al., 2010 ). Additionally, use of TZDs in the nonobese diabetic (NOD) mouse model of T1D augmented the beta cell unfolded protein response and prevented beta cell death, suggesting potential benefits for TZDs in both T1D and T2D (Evans-Molina et al., 2009 ; Maganti et al., 2016 ). With a now refined knowledge of demographics in which to avoid TZD treatment due to adverse effects, together with genetic approaches to identify candidates more likely to respond effectively to TZD therapy (Hu et al., 2019 ; Soccio et al., 2015 ), it remains to be seen if TZD therapy will return to more prominent use in the treatment of diabetes.
H. Insulin and Beyond: The Use of “Smart” Insulin and Closed Loop Systems in Diabetes Treatment
Due to recombinant DNA technology, numerous insulin analogs are now available in various forms ranging from fast acting crystalline insulin to insulin glargine; all of these analogs exhibit equally effective insulin receptor binding. Most are generated by altering amino acids in the B26–B30 region of the molecule (Kurtzhals et al., 2000 ). The American Diabetes Association delineates these insulins by their 1) onset or time before insulin reaches the blood stream, 2) peak time or duration of maximum blood glucose–lowering efficacy, and 3) the duration of blood glucose–lowering time. Insulin administration is independent of the residuum of surviving and/or functioning beta cells in the patient and remains the principal pharmacological treatment of both T1D and T2D. The availability of multiple types of delivery methods, i.e., insulin pens, syringes, pumps, and inhalants, provides clinicians with a solid and varied tool kit with which to treat diabetes. The downsides, however, are that 1) hypoglycemia is a constant threat, 2) proper insulin doses are not trivial to calculate, 3) compliance can vary especially in children and young adults, and 4) there can be side effects of a variety of types. Nonetheless, insulin therapy remains a mainstay treatment of diabetes.
To eliminate the downsides of insulin therapy, research in the past several decades has worked toward generating glucose-sensitive or “smart” insulin molecules. These molecules change insulin bioavailability and become active only upon high blood glucose using glucose-binding proteins such as concanavalin A, glucose oxidase to alter pH sensitivity, and phenylboronic acid (PBA), which forms reversible ester linkages with diol-containing molecules including glucose itself (reviewed in Rege et al., 2017 ). Indeed, promising recent studies included various PBA moieties covalently bonded to an acylated insulin analog (insulin detemir, which contains myristic acid coupled to Lys B29 ). The detemir allows for binding to serum albumin to prolong insulin’s half-life in the circulation, and PBA provided reversible glucose binding (Chou et al., 2015 ). The most promising of the PBA-modified conjugates showed higher potency and responsiveness in lowering blood glucose levels compared with native insulin in diabetic mouse models and decreased hypoglycemia in healthy mice, although the molecular mechanisms have not yet been determined (Chou et al., 2015 ).
An additional active area of research includes structurally defining the interaction between insulin and the insulin receptor ectodomain. Importantly, a major conformational change was discovered that may be exploited to impair insulin receptor binding under hypoglycemic conditions (Menting et al., 2013 ; Rege et al., 2017 ). Challenges in the design, testing, and execution of glucose-responsive insulins may be overcome by the adaptation of novel modeling approaches (Yang et al., 2020 ), which may allow for more rapid screening of candidate compounds.
Technologies have also progressed in the field of artificial pancreas design and development. Currently two “closed loop” systems are now available: Minimed 670G from Medtronic and Control-IQ from Tandem Diabetes Care. Both systems use a continuous glucose monitor, insulin pump, and computer algorithm to predict correct insulin doses and administer them in real time. Such algorithm systems also take into account insulin potency, the rate of blood glucose increase, and the patient’s heart rate and temperature to adjust insulin delivery levels during exercise and after a meal. In addition, so-called “artificial pancreas” systems have also been clinically tested, which use both insulin and glucagon and as such result in fewer reports of hypoglycemic episodes (El-Khatib et al., 2017 ). These types of systems will continue to become more popular as the development of room temperature–stable glucagon analogs continue, such as GVOKE by Xeris Pharmaceuticals (currently available in an injectable syringe) and Baqsimi, a nasally administered glucagon from Eli Lilly.
I. Present and Future Therapies: Beta Cell Transplantation, Replication, and Immune Protection
1. islet transplantation.
The idea to use pancreatic allo/xenografts to treat diabetes remarkably dates back to the late 1800s (Minkowski, 1892 ; Pybus, 1924 ; Williams, 1894 ). Before proceeding to the discovery of insulin (together with Best, MacLeod, and Collip), Frederick Banting also postulated the potential for transplantation of pancreatic tissue emulsions to treat diabetes in dog models in a notebook entry in 1921 (Bliss, 1982 ). Decades later, Paul Lacy, David Scharp, and colleagues successfully isolated intact functional pancreatic islets and transplanted them into rodent models (Kemp et al., 1973 ). These studies led to the initial proof of concept studies for humans, with the first successful islet transplant in a patient with T1D occurring in 1977 (Sutherland et al., 1978 ). A rapid expansion of islet transplantation, inspired by these original studies led to key observations of successfully prolonged islet engraftment by the “Edmonton protocol” whereby corticosteroid-sparing immunosuppression was applied, and islets from at least two allogeneic donors were used to achieve insulin independence (Shapiro et al., 2000 ). More recent work has focused on improving upon the efficiency and long-term engraftment of allogeneic transplants leading to more prolonged graft function (to the 5-year mark) and successful transplantation from a single islet donor (Hering et al., 2016; Hering et al., 2005 ; Rickels et al., 2013 ). Critical to these efforts to improve the success rate was the recognition that the earlier generation of immunosuppressive agents to counter tissue rejection was toxic to islets (Delaunay et al., 1997 ; Paty et al., 2002 ; Soleimanpour et al., 2010 ) and that more appropriate and less toxic agents were needed (Hirshberg et al., 2003 ; Soleimanpour et al., 2012 ).
Certainly, islet transplantation as a therapeutic approach for patients with T1D has been scrutinized due to several challenges, including (but not limited to) the lack of available donor supply to contend with demand, limited long-term functional efficacy of islet allografts, the potential for re-emergence of autoimmune islet destruction and/or metabolic overload-induced islet failure, and significant adverse effects of prolonged immunosuppression (Harlan, 2016 ). Furthermore, although islet transplantation is not currently available for individuals with T2D, simultaneous pancreas-kidney transplantation in T2D had similar favorable outcomes to simultaneous pancreas-kidney transplantation in T1D; therefore, islet-kidney transplantation may eventually be a feasible option to treat T2D, as patients will already be on immunosuppressors (Sampaio et al., 2011 ; Westerman et al., 1983 ). An additional significant obstacle is the tremendous expense associated with islet transplantation therapy. Indeed, the maintenance, operation, and utilization of an FDA-approved and Good Manufacturing Practice–compliant islet laboratory can lead to operating costs at nearly $150,000 per islet transplant, which is not cost effective for the vast majority of patients with T1D (Naftanel and Harlan, 2004 ; Wallner et al., 2016 ). At present, the focus has been to obtain FDA approval for islet allo-transplantation as a therapy for T1D to allow for insurance compensation (Hering et al., 2016; Rickels and Robertson, 2019 ). In the interim, the islet biology, stem cell, immunology, and bioengineering communities have continued the development of cell-based therapies for T1D by other approaches to overcome the challenges identified during the islet transplantation boom of the 1990s and 2000s.
2. Pharmacologic Induction of Beta Cell Replication
Besides transplantation, progress in islet cell biology and especially in developmental biology of beta cells over several decades raised the additional possibility that beta cell mass reduction in diabetes might be countered by increasing beta cell number through mitogenic means. A key method to expand pancreatic beta cell mass is through the enhancement of beta cell replication. Although the study of pancreatic beta cell replication has been an area of intense focus in the beta cell biology field for several decades, only recently has this seemed truly feasible. Seminal studies identified that human beta cells are essentially postmitotic, with a rapid phase of growth occurring in the prenatal period that dramatically tapers off shortly thereafter (Gregg et al., 2012 ; Meier et al., 2008 ). The plasticity of rodent beta cells is considerably higher than that of human beta cells (Dai et al., 2016 ), which has led to a renewed focus on validation of pharmacologic agents to enhance rodent beta cell replication using isolated and/or engrafted human islets (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). Indeed, a large percentage of agents that were successful when applied to rodent systems were largely unsuccessful at inducing replication in human beta cells (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). However, several recent studies have begun to make significant progress on successfully pushing human beta cells to replicate.
Several groups have reported successful human beta cell proliferation, both in vitro and in vivo, in response to inhibitors of the dual specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). These inhibitors include harmine, INDY, GNF4877, 5-iodotubericidin, leucettine-42, TG003, AZ191, CC-401, and more specific, recently developed DYRK1A inhibitors (Ackeifi et al., 2020 ). Although DYRK1A is conclusively established as the important mediator of human beta cell proliferation, comprehensively determining other cellular targets and if additional gene inhibition amplifies the proliferative response is still in process. New evidence from Wang and Stewart shows dual specificity tyrosine phosphorylation-regulated kinase 1B to be an additional mitogenic target and also describes variability in the range of activated kinases within cells and/or levels of inhibition for the many DYRK1A inhibitors listed above (Ackeifi et al., 2020 ). Interestingly, opposite to these human studies, earlier mouse studies from the Scharfmann group demonstrated that Dyrk1a haploinsufficiency leads to decreased proliferation and loss of beta cell mass (Rachdi et al., 2014b ). In addition, overexpression of Dyrk1a in mice led to beta cell mass expansion with increased glucose tolerance (Rachdi et al., 2014a ).
Although important differences in beta cell proliferative capacity have been shown between human and rodent species, there are also significant differences in the mitogenic capacity of beta cells from juvenile, adult, and pregnant individuals. This demonstrates that proliferative stimuli appear to act within the complex islet, pancreas, and whole-body environments unique to each time point. For example, the administration of the hormones platelet-derived growth factor alpha or GLP-1 result in enhanced proliferation in juvenile human beta cells yet are ineffective in adult human beta cells (Chen et al., 2011 ; Dai et al., 2017 ). This has been shown to be due to a loss of platelet-derived growth factor alpha receptor expression as beta cells age but appears to be unrelated to GLP-1 receptor expression levels (Chen et al., 2011 ). Indeed, the GLP-1 receptor is highly expressed in adult beta cells, and GLP-1 secretion increases insulin secretion, as detailed previously; however, the induction of proliferative factors such as nuclear factor of activated T cells, cytoplasmic 1; forkhead box protein 1; and cyclin A1 is only seen in juvenile islets (Dai et al., 2017 ). Human studies using cadaveric pancreata from pregnant donors also showed increased beta cell mass, yet lactogenic hormones from the pituitary or placenta (prolactin, placental lactogen, or growth hormone) are unable to stimulate proliferation in human beta cells despite their ability to produce robust proliferation in mouse beta cells (reviewed in Baeyens et al., 2016 ). Experiments overexpressing mouse versus human signal transducer and activator of transcription 5, the final signaling factor inducing beta cell adaptation, in human beta cells allows for prolactin-mediated proliferation revealing fundamental differences in prolactin pathway competency in human (Chen et al., 2015 ). Overcoming the barrier of recapitulating human pregnancy’s effect on beta cells through isolating placental cells or blood serum during pregnancy may result in the discovery of a factor(s) that facilitates the increase in beta cell mass observed during human pregnancy.
Mechanisms that stimulate beta cell proliferation have also been discovered from studying genetic mutations that result in insulinomas, spontaneous insulin-producing beta cell adenomas. The most common hereditary mutation occurs in the multiple endocrine neoplasia type 1 (MEN1) gene. Indeed, administration of a MEN1 inhibitor in addition to a GLP-1 agonist (which cannot induce proliferation alone) is able to increase beta cell proliferation in isolated human islets through synergistic activation of KRAS proto-oncogene, GTPase downstream signals (Chamberlain et al., 2014 ). Interestingly, MEN1 mutations are uncommon in sporadic insulinomas, yet assaying genomic and epigenetic changes in a large cohort of non-MEN1 insulinomas found alterations in trithorax and polycomb chromatin modifying genes that were functionally related to MEN1 (Wang et al., 2017 ). Stewart and colleagues hypothesized that changes in histone 3 lysine 27 and histone 3 lysine 4 methylation status led to increased enhancer of zeste homolog 2 and lysine demethylase 6A, decreased cyclin-dependent kinase inhibitor 1C, and thereby increased beta cell proliferation, among other phenotypes. They also proposed that these findings help to explain why increased proliferation always occurs despite broad heterogeneity of mutations found between individual insulinomas (Wang et al., 2017 ).
Although factors that induce proliferation are continuing to be discovered, there are drawbacks that still limit their clinical application. Harmine and other DYRK1A inhibitors are not beta cell specific, nor have all their cellular targets been determined (Ackeifi et al., 2020 ). Targeting other pathways to induce human beta cell proliferation such as modulation of prostaglandin E2 receptors (i.e., inhibition of prostaglandin E receptor 3 alone or in combination with prostaglandin E receptor 4 activation) showed promising increases in proliferative rate yet suffers from the same lack of specificity (Carboneau et al., 2017 ). Induction of proliferation may also come at the expense of glucose sensing as in insulinomas, which have an increased expression of “disallowed genes” and alterations in glucose transporter and hexokinase expression (Wang et al., 2017 ). A further untoward consequence that must be avoided is the production of cancerous cells through unchecked proliferation. Finally, increasing beta cell mass through low rates of proliferation may increase the pool of functional insulin-secreting cells in T2D, but without additional measures, these beta cells will still ultimately be targeted for immune cell destruction in T1D.
3. Beta Cell Stress Relieving Therapies
Metabolic, inflammatory, and endoplasmic reticulum (ER) stress contribute to beta cell dysfunction and failure in both T1D and T2D. Although reduction of metabolic overload of beta cells by early exogenous insulin therapy or insulin sensitizers can temporarily reduce loss of beta cell mass/function early in diabetes, a focus on relieving ER and inflammatory stress is also of interest to preserve beta cell health.
ER stress is a well known contributor to beta cell demise both in T1D and T2D (Laybutt et al., 2007 ; Marchetti et al., 2007 ; Marhfour et al., 2012 ; Tersey et al., 2012 ) and a target of interest in the prevention of beta cell loss in both diseases. Preclinical studies suggest that the use of chemical chaperones, including 4-phenylbutyric acid and tauroursodeoxycholic acid (TUDCA), to alleviate ER stress improves beta cell function and insulin sensitivity in mouse models of T2D (Cnop et al., 2017 ; Ozcan et al., 2006 ). Furthermore, TUDCA has been shown to preserve beta cell mass and reduce ER stress in mouse models of T1D (Engin et al., 2013 ). Interestingly, TUDCA has shown promise at improving insulin action in obese nondiabetic human subjects, yet beta cell function and insulin secretion were not assessed (Kars et al., 2010 ). A clinical trial regarding the use of TUDCA for humans with new-onset T1D is also ongoing ( {"type":"clinical-trial","attrs":{"text":"NCT02218619","term_id":"NCT02218619"}} NCT02218619 ). However, a note of caution regarding use of ER chaperones is that they may prevent low level ER stress necessary to potentiate beta cell replication during states of increased insulin demand (Sharma et al., 2015 ), suggesting that the broad use of ER chaperone therapies should be carefully considered.
The blockade of inflammatory stress has long been an area of interest for treatments of both T1D and T2D (Donath et al., 2019 ; Eguchi and Nagai, 2017 ). Indeed, use of nonsteroidal anti-inflammatory drugs (NSAIDs), which block cyclooxygenase, have been observed to improve metabolic control in patients with diabetes since the turn of the 20th century (Williamson, 1901 ). Salicylates have been shown to improve insulin secretion and beta cell function in both obese human subjects and those with T2D (Fernandez-Real et al., 2008; Giugliano et al., 1985 ). However, another NSAID, salsalate, has not been shown to improve beta cell function while improving other metabolic outcomes (Kim et al., 2014 ; Penesova et al., 2015 ), possibly suggesting distinct mechanisms of action for anti-inflammatory compounds. The regular use of NSAIDs to enhance metabolic outcomes is also often limited to the tolerability of long-term use of these agents due to adverse effects. Recently, golilumab, a monoclonal antibody against the proinflammatory cytokine tumor necrosis factor alpha, was demonstrated to improve beta cell function in new-onset T1D, suggesting that targeting the underlying inflammatory milieu may have benefits to preserve beta cell mass and function in T1D (Quattrin et al., 2020). Taken together, both new and old approaches to target beta cell stressors still remain of long-term interest to improve beta cell viability and function in both T1D and T2D.
3. New Players to Induce Islet Immune Protection
Countless researchers have expended intense industry to determine T1D disease etiology and treatments focused on immunotherapy and tolerogenic methods. Multiple, highly comprehensive reviews are available describing these efforts (Goudy and Tisch, 2005 ; Rewers and Gottlieb, 2009 ; Stojanovic et al., 2017 ). Here we will focus on the protection of beta cells through programmed cell death protein-1 ligand (PD-L1) overexpression, major histocompatibility complex class I, A, B, C (HLA-A,B,C) mutated human embryonic stem cell–derived beta cells, and islet encapsulation methods.
Cancer immunotherapies that block immune checkpoints are beneficial for treating advanced stage cancers, yet induction of autoimmune diseases, including T1D, remains a potential side effect (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). A subset of these drugs target either the programmed cell death-1 protein on the surface of activated T lymphocytes or its receptor PD-L1 (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). PD-L1 expression was found in insulin-positive beta cells from T1D but not insulin-negative islets or nondiabetic islets, leading to the hypothesis that PD-L1 is upregulated in an attempt to drive immune cell attenuation (Osum et al., 2018 ; Colli et al., 2018 ). Adenoviral overexpression of PD-L1 specifically in beta cells rescued hyperglycemia in the NOD mouse model of T1D, but these animals eventually succumbed to diabetes by the study’s termination (El Khatib et al., 2015 ). A more promising report from Ben Nasr et al. ( 2017 ) demonstrated that pharmacologically or genetically induced overexpression of PD-L1 in hematopoietic stem and progenitor cells inhibited beta cell autoimmunity in the NOD mouse as well as in vitro using human hematopoietic stem and progenitor cells from patients with T1D.
As mentioned above, islet transplantation to treat T1D is limited by islet availability, cost, and the requirement for continuous immunosuppression. Islet cells generated by differentiating embryonic or induced pluripotent stem (iPS) cells could circumvent these limitations. Ideally, iPS-derived beta cells could be manipulated to eliminate the expression of polymorphic HLA-A,B,C molecules, which were found to be upregulated in T1D beta cells (Bottazzo et al., 1985 ; Richardson et al., 2016 ). These molecules allow peptide presentation to CD8+ T cells or cytotoxic T lymphocytes and may lead to beta cell removal. Interestingly, remaining insulin-positive cells in T1D donor pancreas are not HLA-A,B,C positive (Nejentsev et al., 2007; Rodriguez-Calvo et al., 2015 ). However, current differentiation protocols are still limited in their ability to produce fully glucose-responsive beta cells without transplantation into animal models to induce mature characteristics. Additionally, use of iPS-derived beta cells will still lead to concerns regarding DNA mutagenesis resulting from the methods used to obtain pluripotency or teratoma formation from cells that have escaped differentiation.
Encapsulation devices would protect islets or stem cells from immune cell infiltration while allowing for the proper exchange of nutrients and hormones. Macroencapsulation uses removable devices that would help assuage fears surrounding mutation or tumor formation; indeed, the first human trial using encapsulated hESC-derived beta cells will be completed in January 2021 ( {"type":"clinical-trial","attrs":{"text":"NCT02239354","term_id":"NCT02239354"}} NCT02239354 ). Macroencapsulation of islets prior to transplantation using various alginate-based hydrogels has historically been impeded by a strong in vivo foreign body immune response (Desai and Shea, 2017 ; Doloff et al., 2017 ; Pueyo et al., 1993 ). More recently, chemically modified forms of alginate that avoid macrophage recognition and fibrous deposition have been successfully used in rodents and for up to 6 months in nonhuman primates (Vegas et al., 2016 ). Indeed, Bochenek et al. ( 2018 ) successfully transplanted alginate protected islets for 4 months without immunosuppression in the bursa omentalis of nonhuman primates demonstrating the feasibility for this approach to be extended to humans. It remains to be seen if these devices will be successful for long-term use, perhaps decades, in patients with diabetes.
III. Summary
Although existing drug therapies using classic oral antidiabetic drugs like sulfonylureas and metformin or injected insulin remain mainstays of diabetes treatment, newer drugs based on incretin hormone actions or SGLT2 inhibitors have increased the pharmacological armamentarium available to diabetologists ( Fig. 1 ). However, the explosion of progress in beta cell biology has identified potential avenues that can increase beta cell mass in sophisticated ways by employing stem cell differentiation or enhancement of beta cell proliferation. Taken together, there should be optimism that the increased incidence of both T1D and T2D is being matched by the creativity and hard work of the diabetes research community.
Abbreviations
Authorship contributions.
Wrote and contributed to the writing of the manuscript: Satin, Soleimanpour, Walker
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [Grant R01-DK46409] (to L.S.S.), [Grant R01-DK108921] (to S.A.S.), and [Grant P30-DK020572 pilot and feasibility grant] (to S.A.S.), the Juvenile Diabetes Research Foundation (JDRF) [Grant CDA-2016-189] (to L.S.S. and S.A.S.), [Grant SRA-2018-539] (to S.A.S.), and [Grant COE-2019-861] (to S.A.S.), and the US Department of Veterans Affairs [Grant I01 BX004444] (to S.A.S.). The JDRF Career Development Award to S.A.S. is partly supported by the Danish Diabetes Academy and the Novo Nordisk Foundation.
https://doi.org/10.1124/pharmrev.120.000160
- Ackeifi C, Swartz E, Kumar K, Liu H, Chalada S, Karakose E, Scott DK, Garcia-Ocaña A, Sanchez R, DeVita RJ, et al. (2020) Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors . JCI Insight 5 :e132594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Adachi T, Yasuda K, Okamoto Y, Shihara N, Oku A, Ueta K, Kitamura K, Saito A, Iwakura I, Yamada Y, et al. (2000) T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats . Metabolism 49 :990–995. [ PubMed ] [ Google Scholar ]
- Alexiadou K, Tan TM (2020) Gastrointestinal peptides as therapeutic targets to mitigate obesity and metabolic syndrome . Curr Diab Rep 20 :26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai LF, Robertson D, Jain M, Petrone M, et al. (2018) MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study . Lancet 391 :2607–2618. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell . Prog Biophys Mol Biol 54 :87–143. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1990) ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion . Biochem Soc Trans 18 :109–111. [ PubMed ] [ Google Scholar ]
- Baeyens L, Hindi S, Sorenson RL, German MS (2016) β-Cell adaptation in pregnancy . Diabetes Obes Metab 18 ( Suppl 1 ):63–70. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bailey CJ (1992) Biguanides and NIDDM . Diabetes Care 15 :755–772. [ PubMed ] [ Google Scholar ]
- Ben Nasr M, Tezza S, D’Addio F, Mameli C, Usuelli V, Maestroni A, Corradi D, Belletti S, Albarello L, Becchi G, et al. (2017) PD-L1 genetic overexpression or pharmacological restoration in hematopoietic stem and progenitor cells reverses autoimmune diabetes . Sci Transl Med 9 :eaam7543. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bergmann NC, Lund A, Gasbjerg LS, Meessen ECE, Andersen MM, Bergmann S, Hartmann B, Holst JJ, Jessen L, Christensen MB, et al. (2019) Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study . Diabetologia 62 :665–675. [ PubMed ] [ Google Scholar ]
- Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A (2014) Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map . Diabetes 63 :819–831. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, et al.; EXSCEL Study Group (2018) Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis . Lancet Diabetes Endocrinol 6 :105–113. [ PubMed ] [ Google Scholar ]
- Bliss M (1982) Banting’s, Best’s, and Collip’s accounts of the discovery of insulin . Bull Hist Med 56 :554–568. [ PubMed ] [ Google Scholar ]
- Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, et al. (2018) Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques . Nat Biomed Eng 2 :810–821. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion . Nat Med 21 :512–517. [ PubMed ] [ Google Scholar ]
- Bonora E, Cigolini M, Bosello O, Zancanaro C, Capretti L, Zavaroni I, Coscelli C, Butturini U (1984) Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects . Curr Med Res Opin 9 :47–51. [ PubMed ] [ Google Scholar ]
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR (1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis . N Engl J Med 313 :353–360. [ PubMed ] [ Google Scholar ]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence . Popul Health Metr 8 :29. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, et al. (2007) Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment . Nat Med 13 :340–347. [ PubMed ] [ Google Scholar ]
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M (2016) The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies . Diabetes Care 39 :198–205. [ PubMed ] [ Google Scholar ]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes . Diabetes Care 27 :2628–2635. [ PubMed ] [ Google Scholar ]
- Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, Jaffe JL, Coch RW, Haldeman JM, MacDonald PE, et al. (2019a) β Cell tone is defined by proglucagon peptides through cAMP signaling . JCI Insight 4 :e126742. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Capozzi ME, Wait JB, Koech J, Gordon AN, Coch RW, Svendsen B, Finan B, D’Alessio DA, Campbell JE (2019b) Glucagon lowers glycemia when β-cells are active . JCI Insight 5 :e129954. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M (2017) Opposing effects of prostaglandin E 2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation . Mol Metab 6 :548–559. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chae H, Augustin R, Gatineau E, Mayoux E, Bensellam M, Antoine N, Khattab F, Lai BK, Brusa D, Stierstorfer B, et al. (2020) SGLT2 is not expressed in pancreatic α- and β-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans . Mol Metab 42 :101071. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chamberlain CE, Scheel DW, McGlynn K, Kim H, Miyatsuka T, Wang J, Nguyen V, Zhao S, Mavropoulos A, Abraham AG, et al. (2014) Menin determines K-RAS proliferative outputs in endocrine cells . J Clin Invest 124 :4093–4101. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK (2011) PDGF signalling controls age-dependent proliferation in pancreatic β-cells . Nature 478 :349–355. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, et al. (2015) Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells . Diabetes 64 :3784–3797. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Cha-Molstad H, Szabo A, Shalev A (2009) Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein . Am J Physiol Endocrinol Metab 296 :E1133–E1139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, Lusis AJ, Davis RA, Shalev A (2008a) Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes . FASEB J 22 :3581–3594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A (2008b) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis . Diabetes 57 :938–944. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG (2015) Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates . Proc Natl Acad Sci USA 112 :2401–2406. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells . Mol Metab 6 :1024–1039. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR (2003) Oxyntomodulin suppresses appetite and reduces food intake in humans . J Clin Endocrinol Metab 88 :4696–4701. [ PubMed ] [ Google Scholar ]
- Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, et al. (2020) GDF15 mediates the effects of metformin on body weight and energy balance . Nature 578 :444–448. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Colli ML, Hill JLE, Marroquí L, Chaffey J, Dos Santos RS, Leete P, Coomans de Brachène A, Paula FMM, Op de Beeck A, Castela A, et al. (2018) PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction . EBioMedicine 36 :367–375. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- RISE Consortium (2019) Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes . Diabetes Care 42 :1742–1751. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, Phillips N, Levy SE, Greiner DL, Shultz LD, et al. (2017) Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling . J Clin Invest 127 :3835–3844. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Kayton NS, Shostak A, Poffenberger G, Cyphert HA, Aramandla R, Thompson C, Papagiannis IG, Emfinger C, Shiota M, et al. (2016) Stress-impaired transcription factor expression and insulin secretion in transplanted human islets . J Clin Invest 126 :1857–1870. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Walker JT, Shostak A, Bouchi Y, Poffenberger G, Hart NJ, Jacobson DA, Calcutt MW, Bottino R, Greiner DL, et al. (2020) Dapagliflozin does not directly affect human α or β cells . Endocrinology 161 :bqaa080. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR (2001) Oxyntomodulin inhibits food intake in the rat . Endocrinology 142 :4244–4250. [ PubMed ] [ Google Scholar ]
- Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR (2002) Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats . Am J Physiol Endocrinol Metab 283 :E1173–E1177. [ PubMed ] [ Google Scholar ]
- Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Gastaldelli A, Henry RR, Kitabchi AE, et al.; ACT NOW Study (2013) Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates . Diabetes 62 :3920–3926. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S (1997) Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids . J Clin Invest 100 :2094–2098. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Desai T, Shea LD (2017) Advances in islet encapsulation technologies . Nat Rev Drug Discov 16 :338–350. [ PubMed ] [ Google Scholar ]
- Devaraj S, Venkatachalam A, Chen X (2016) Metformin and the gut microbiome in diabetes . Clin Chem 62 :1554–1555. [ PubMed ] [ Google Scholar ]
- Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, et al. (2017) Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates . Nat Mater 16 :671–680. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Donath MY, Dinarello CA, Mandrup-Poulsen T (2019) Targeting innate immune mediators in type 1 and type 2 diabetes . Nat Rev Immunol 19 :734–746. [ PubMed ] [ Google Scholar ]
- Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1 . Cell Metab 27 :740–756. [ PubMed ] [ Google Scholar ]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF (1987) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line . Proc Natl Acad Sci USA 84 :3434–3438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats . Nat Med 21 :506–511. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eguchi K, Nagai R (2017) Islet inflammation in type 2 diabetes and physiology . J Clin Invest 127 :14–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El Khatib MM, Sakuma T, Tonne JM, Mohamed MS, Holditch SJ, Lu B, Kudva YC, Ikeda Y (2015) β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection . Gene Ther 22 :430–438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, et al. (2017) Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial . Lancet 389 :369–380. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X (2008) Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons . J Mol Neurosci 34 :77–87. [ PubMed ] [ Google Scholar ]
- El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I . J Biol Chem 275 :223–228. [ PubMed ] [ Google Scholar ]
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y (1964) Plasma Insulin Response to Oral and Intravenous Glucose Administration . J Clin Endocrinol Metab 24 :1076–1082. [ PubMed ] [ Google Scholar ]
- Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, Hotamisligil GS (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes [published correction appears in Sci Transl Med (2013) 5 :214er11] . Sci Transl Med 5 :211ra156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients . BMJ 330 :1304–1305. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, et al. (2009) Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure . Mol Cell Biol 29 :2053–2067. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fernández-Real JM, López-Bermejo A, Ropero AB, Piquer S, Nadal A, Bassols J, Casamitjana R, Gomis R, Arnaiz E, Pérez I, et al. (2008) Salicylates increase insulin secretion in healthy obese subjects . J Clin Endocrinol Metab 93 :2523–2530. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies . Cell Metab 20 :953–966. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus . Nat Rev Endocrinol 15 :569–589. [ PubMed ] [ Google Scholar ]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state . J Clin Invest 120 :2355–2369. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JPBastyr EJ 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RHDiMarchi RD (2017) The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes . Cell Metab 26 :343–352.e2. [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, Urva S, Haupt A, Robins DA (2020) Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens . Diabetes Obes Metab 22 :938–946. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, et al. (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial . Lancet 392 :2180–2193. [ PubMed ] [ Google Scholar ]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, et al. (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin . Nat Med 19 :1649–1654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al.; REWIND Investigators (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial . Lancet 394 :121–130. [ PubMed ] [ Google Scholar ]
- Giugliano D, Ceriello A, Saccomanno F, Quatraro A, Paolisso G, D’Onofrio F (1985) Effects of salicylate, tolbutamide, and prostaglandin E2 on insulin responses to glucose in noninsulin-dependent diabetes mellitus . J Clin Endocrinol Metab 61 :160–166. [ PubMed ] [ Google Scholar ]
- Goudy KS, Tisch R (2005) Immunotherapy for the prevention and treatment of type 1 diabetes . Int Rev Immunol 24 :307–326. [ PubMed ] [ Google Scholar ]
- Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ (2012) Formation of a human β-cell population within pancreatic islets is set early in life . J Clin Endocrinol Metab 97 :3197–3206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P (2012) Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors . Diabetes Obes Metab 14 :83–90. [ PubMed ] [ Google Scholar ]
- Harlan DM (2016) Islet transplantation for hypoglycemia unawareness/severe hypoglycemia: caveat emptor . Diabetes Care 39 :1072–1074. [ PubMed ] [ Google Scholar ]
- Harrower AD (1991) Efficacy of gliclazide in comparison with other sulphonylureas in the treatment of NIDDM . Diabetes Res Clin Pract 14 ( Suppl 2 ):S65–S67. [ PubMed ] [ Google Scholar ]
- He L, Wondisford FE (2015) Metformin action: concentrations matter . Cell Metab 21 :159–162. [ PubMed ] [ Google Scholar ]
- Hedrington MS, Davis SN (2019) Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes . Expert Opin Pharmacother 20 :2229–2235. [ PubMed ] [ Google Scholar ]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al.; Clinical Islet Transplantation Consortium (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia . Diabetes Care 39 :1230–1240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, et al. (2005) Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes . JAMA 293 :830–835. [ PubMed ] [ Google Scholar ]
- Hernandez AFGreen JBJanmohamed SD’Agostino RB Sr , Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MCet al.; Harmony Outcomes committees and investigators (2018) Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial . Lancet 392 :1519–1529. [ PubMed ] [ Google Scholar ]
- Hiatt WR, Kaul S, Smith RJ (2013) The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience . N Engl J Med 369 :1285–1287. [ PubMed ] [ Google Scholar ]
- Hirshberg B, Preston EH, Xu H, Tal MG, Neeman Z, Bunnell D, Soleimanpour S, Hale DA, Kirk AD, Harlan DM (2003) Rabbit antithymocyte globulin induction and sirolimus monotherapy supports prolonged islet allograft function in a nonhuman primate islet transplantation model . Transplantation 76 :55–60. [ PubMed ] [ Google Scholar ]
- Hu W, Jiang C, Guan D, Dierickx P, Zhang R, Moscati A, Nadkarni GN, Steger DJ, Loos RJF, Hu C, et al. (2019) Patient adipose stem cell-derived adipocytes reveal genetic variation that predicts antidiabetic drug response . Cell Stem Cell 24 :299–308.e6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al.; PIONEER 6 Investigators (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 381 :841–851. [ PubMed ] [ Google Scholar ]
- Imamura M, Nakanishi K, Suzuki T, Ikegai K, Shiraki R, Ogiyama T, Murakami T, Kurosaki E, Noda A, Kobayashi Y, et al. (2012) Discovery of Ipragliflozin (ASP1941): a novel C-glucoside with benzothiophene structure as a potent and selective sodium glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes mellitus . Bioorg Med Chem 20 :3263–3279. [ PubMed ] [ Google Scholar ]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, et al.; ADOPT Study Group (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy . N Engl J Med 355 :2427–2443. [ PubMed ] [ Google Scholar ]
- Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Viberti G, Holman RR; ADOPT Study Group (2011) Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT . Diabetes 60 :1552–1560. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, et al. (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women . Diabetes 59 :1899–1905. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF (1973) Transplantation of isolated pancreatic islets into the portal vein of diabetic rats . Nature 244 :447. [ PubMed ] [ Google Scholar ]
- Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH (2002) Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells . Diabetes 51 :676–685. [ PubMed ] [ Google Scholar ]
- Kim HI, Kim JW, Kim SH, Cha JY, Kim KS, Ahn YH (2000) Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter . Diabetes 49 :1517–1524. [ PubMed ] [ Google Scholar ]
- Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, Tomasso V, Ochoa H, Reaven G (2014) Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study . Diabetes Care 37 :1944–1950. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koffert JP, Mikkola K, Virtanen KA, Andersson AD, Faxius L, Hällsten K, Heglind M, Guiducci L, Pham T, Silvola JMU, et al. (2017) Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial . Diabetes Res Clin Pract 131 :208–216. [ PubMed ] [ Google Scholar ]
- Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, Perkins R, Smith JG, Bäckhed F (2020) Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation . Cell Metab 32 :643–653.e4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7-36: a physiological incretin in man . Lancet 2 :1300–1304. [ PubMed ] [ Google Scholar ]
- Kuhre RE, Ghiasi SM, Adriaenssens AE, Wewer Albrechtsen NJ, Andersen DB, Aivazidis A, Chen L, Mandrup-Poulsen T, Ørskov C, Gribble FM, et al. (2019) No direct effect of SGLT2 activity on glucagon secretion . Diabetologia 62 :1011–1023. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF (2012) Human β-cell proliferation and intracellular signaling: driving in the dark without a road map . Diabetes 61 :2205–2213. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kurosaki E, Ogasawara H (2013) Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data . Pharmacol Ther 139 :51–59. [ PubMed ] [ Google Scholar ]
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, Trüb T (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use . Diabetes 49 :999–1005. [ PubMed ] [ Google Scholar ]
- Lambeir AM, Scharpé S, De Meester I (2008) DPP4 inhibitors for diabetes--what next? Biochem Pharmacol 76 :1637–1643. [ PubMed ] [ Google Scholar ]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes . Diabetologia 50 :752–763. [ PubMed ] [ Google Scholar ]
- Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications . Curr Diab Rep 19 :151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ, et al. (2015) Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes . JAMA 314 :265–277. [ PubMed ] [ Google Scholar ]
- Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, et al. (2016) Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies . BMJ 352 :i610. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Link JT (2003) Pharmacological regulation of hepatic glucose production . Curr Opin Investig Drugs 4 :421–429. [ PubMed ] [ Google Scholar ]
- Luippold G, Klein T, Mark M, Grempler R (2012) Empagliflozin, a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus . Diabetes Obes Metab 14 :601–607. [ PubMed ] [ Google Scholar ]
- Maganti AV, Tersey SA, Syed F, Nelson JB, Colvin SC, Maier B, Mirmira RG (2016) Peroxisome proliferator-activated receptor-γ activation augments the β-cell unfolded protein response and rescues early glycemic deterioration and β cell death in non-obese diabetic mice . J Biol Chem 291 :22524–22533. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Makrilakis K (2019) The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect . Int J Environ Res Public Health 16 :2720. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M (2007) The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients . Diabetologia 50 :2486–2494. [ PubMed ] [ Google Scholar ]
- Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, Morgan NG, Eizirik DL (2012) Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes . Diabetologia 55 :2417–2420. [ PubMed ] [ Google Scholar ]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al.; SUSTAIN-6 Investigators (2016a) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 375 :1834–1844. [ PubMed ] [ Google Scholar ]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al.; LEADER Steering Committee; LEADER Trial Investigators (2016b) Liraglutide and cardiovascular outcomes in type 2 diabetes . N Engl J Med 375 :311–322. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Massollo M, Marini C, Brignone M, Emionite L, Salani B, Riondato M, Capitanio S, Fiz F, Democrito A, Amaro A, et al. (2013) Metformin temporal and localized effects on gut glucose metabolism assessed using 18F-FDG PET in mice . J Nucl Med 54 :259–266. [ PubMed ] [ Google Scholar ]
- McCreight LJ, Bailey CJ, Pearson ER (2016) Metformin and the gastrointestinal tract . Diabetologia 59 :426–435. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McIntyre N, Holdsworth CD, Turner DS (1964) New interpretation of oral glucose tolerance . Lancet 2 :20–21. [ PubMed ] [ Google Scholar ]
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al.; DAPA-HF Trial Committees and Investigators (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction . N Engl J Med 381 :1995–2008. [ PubMed ] [ Google Scholar ]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans . Diabetes 57 :1584–1594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, et al. (2008) Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes . J Med Chem 51 :1145–1149. [ PubMed ] [ Google Scholar ]
- Menting JG, Whittaker J, Margetts MB, Whittaker LJ, Kong GK, Smith BJ, Watson CJ, Záková L, Kletvíková E, Jiráček J, et al. (2013) How insulin engages its primary binding site on the insulin receptor . Nature 493 :241–245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP . Nature 494 :256–260. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Minkowski O (1892) Weitere Mitteilungen über den Diabetes mellitus nach Extirpation des Pankreas . Berliner Klinische Wochenschrift 29 :90–93. [ Google Scholar ]
- Misbin RI (2004) The phantom of lactic acidosis due to metformin in patients with diabetes . Diabetes Care 27 :1791–1793. [ PubMed ] [ Google Scholar ]
- Mudaliar S, Armstrong DA, Mavian AA, O’Connor-Semmes R, Mydlow PK, Ye J, Hussey EK, Nunez DJ, Henry RR, Dobbins RL (2012) Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes . Diabetes Care 35 :2198–2200. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL (2011) Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell . Endocrinology 152 :4610–4619. [ PubMed ] [ Google Scholar ]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. (2019) Glucagon-like peptide 1 (GLP-1) . Mol Metab 30 :72–130. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Munir KM, Lamos EM (2017) Diabetes type 2 management: what are the differences between DPP-4 inhibitors and how do you choose? Expert Opin Pharmacother 18 :839–841. [ PubMed ] [ Google Scholar ]
- Naftanel MA, Harlan DM (2004) Pancreatic islet transplantation . PLoS Med 1 :e58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W (1986a) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes . Diabetologia 29 :46–52. [ PubMed ] [ Google Scholar ]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W (1986b) Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses . J Clin Endocrinol Metab 63 :492–498. [ PubMed ] [ Google Scholar ]
- Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, et al.; Wellcome Trust Case Control Consortium (2007) Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A . Nature 450 :887–892. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nichols CG (2006) KATP channels as molecular sensors of cellular metabolism . Nature 440 :470–476. [ PubMed ] [ Google Scholar ]
- Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, et al. (2010) Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus . J Med Chem 53 :6355–6360. [ PubMed ] [ Google Scholar ]
- Ohnishi ST, Endo M, editors. (1981) The Mechanism of Gated Calcium Transport Across Biological Membranes , Academic Press, New York. [ Google Scholar ]
- Osipovich AB, Stancill JS, Cartailler JP, Dudek KD, Magnuson MA (2020) Excitotoxicity and overnutrition additively impair metabolic function and identity of pancreatic β-cells . Diabetes 69 :1476–1491. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, Pauken KE, Papas K, Appakalai B, Spanier JA, et al. (2018) Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes . Sci Rep 8 :8295. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain . Biochem J 348 :607–614. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ozanne SE, Guest PC, Hutton JC, Hales CN (1995) Intracellular localization and molecular heterogeneity of the sulphonylurea receptor in insulin-secreting cells . Diabetologia 38 :277–282. [ PubMed ] [ Google Scholar ]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes . Science 313 :1137–1140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paty BW, Harmon JS, Marsh CL, Robertson RP (2002) Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT-T15 cells and Wistar rat islets . Transplantation 73 :353–357. [ PubMed ] [ Google Scholar ]
- Penesova A, Koska J, Ortega E, Bunt JC, Bogardus C, de Courten B (2015) Salsalate has no effect on insulin secretion but decreases insulin clearance: a randomized, placebo-controlled trial in subjects without diabetes . Diabetes Obes Metab 17 :608–612. [ PubMed ] [ Google Scholar ]
- Perdigoto AL, Quandt Z, Anderson M, Herold KC (2019) Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome . Lancet Diabetes Endocrinol 7 :421–423. [ PubMed ] [ Google Scholar ]
- Perley MJ, Kipnis DM (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects . J Clin Invest 46 :1954–1962. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pernicova I, Korbonits M (2014) Metformin--mode of action and clinical implications for diabetes and cancer . Nat Rev Endocrinol 10 :143–156. [ PubMed ] [ Google Scholar ]
- Preiss D, Dawed A, Welsh P, Heggie A, Jones AG, Dekker J, Koivula R, Hansen TH, Stewart C, Holman RR, et al.; DIRECT consortium group (2017) Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes . Diabetes Obes Metab 19 :356–363. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes . J Clin Invest 116 :1802–1812. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pueyo ME, Darquy S, Capron F, Reach G (1993) In vitro activation of human macrophages by alginate-polylysine microcapsules . J Biomater Sci Polym Ed 5 :197–203. [ PubMed ] [ Google Scholar ]
- Pybus F (1924) Notes on suprarenal and pancreatic grafting . Lancet 204 :550–551. [ Google Scholar ]
- Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, et al.; T1GER Study Investigators (2020) Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes . N Engl J Med 383 :2007–2017. [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Aïello V, Herault Y, Janel N, Delabar JM, Polak M, Scharfmann R (2014a) Dyrk1A induces pancreatic β cell mass expansion and improves glucose tolerance . Cell Cycle 13 :2221–2229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Guez F, Aïello V, Arbonés ML, Janel N, Delabar JM, Polak M, Scharfmann R (2014b) Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass . Diabetologia 57 :960–969. [ PubMed ] [ Google Scholar ]
- Rege NK, Phillips NFB, Weiss MA (2017) Development of glucose-responsive ‘smart’ insulin systems . Curr Opin Endocrinol Diabetes Obes 24 :267–278. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin . Diabetologia 60 :1577–1585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rewers M, Gottlieb P (2009) Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future . Diabetes Care 32 :1769–1782. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jørgensen K, et al. (2016) Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes . Diabetologia 59 :2448–2458. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, et al. (2013) Improvement in β-cell secretory capacity after human islet transplantation according to the c7 protocol . Diabetes 62 :2890–2897. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Robertson RP (2019) Pancreatic islet transplantation in humans: recent progress and future directions . Endocr Rev 40 :631–668. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rodriguez-Calvo T, Suwandi JS, Amirian N, Zapardiel-Gonzalo J, Anquetil F, Sabouri S, von Herrath MG (2015) Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase . J Histochem Cytochem 63 :626–636. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rojas LB, Gomes MB (2013) Metformin: an old but still the best treatment for type 2 diabetes . Diabetol Metab Syndr 5 :6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, et al. (2003) Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis . Mol Cell Biol 23 :7222–7229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM; Repaglinide Versus Nateglinide Comparison Study Group (2004) Repaglinide versus nateglinide monotherapy: a randomized, multicenter study . Diabetes Care 27 :1265–1270. [ PubMed ] [ Google Scholar ]
- Sampaio MS, Kuo HT, Bunnapradist S (2011) Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients . Clin J Am Soc Nephrol 6 :1198–1206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, Coddeville A, Quenon A, Daoudi M, Hubert T, et al. (2019) The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin via somatostatin release . Cell Rep 28 :1447–1454.e4. [ PubMed ] [ Google Scholar ]
- Saponaro C, Mühlemann M, Acosta-Montalvo A, Piron A, Gmyr V, Delalleau N, Moerman E, Thévenet J, Pasquetti G, Coddeville A, et al. (2020) Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets . Diabetes 69 :902–914. [ PubMed ] [ Google Scholar ]
- Satin LS, Tavalin SJ, Kinard TA, Teague J (1995) Contribution of L- and non-L-type calcium channels to voltage-gated calcium current and glucose-dependent insulin secretion in HIT-T15 cells . Endocrinology 136 :4589–4601. [ PubMed ] [ Google Scholar ]
- Schwartz AV, Chen H, Ambrosius WT, Sood A, Josse RG, Bonds DE, Schnall AM, Vittinghoff E, Bauer DC, Banerji MA, et al. (2015) Effects of TZD use and discontinuation on fracture rates in ACCORD Bone Study . J Clin Endocrinol Metab 100 :4059–4066. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM (2002) Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway . Endocrinology 143 :3695–3698. [ PubMed ] [ Google Scholar ]
- Shankar SS, Shankar RR, Mixson LA, Miller DL, Pramanik B, O’Dowd AK, Williams DM, Frederick CB, Beals CR, Stoch SA, et al. (2018) Native oxyntomodulin has significant glucoregulatory effects independent of weight loss in obese humans with and without type 2 diabetes . Diabetes 67 :1105–1112. [ PubMed ] [ Google Scholar ]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen . N Engl J Med 343 :230–238. [ PubMed ] [ Google Scholar ]
- Sharma RB, O’Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Arvan P, Alonso LC (2015) Insulin demand regulates β cell number via the unfolded protein response . J Clin Invest 125 :3831–3846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sims EK, Mirmira RG, Evans-Molina C (2020) The role of beta-cell dysfunction in early type 1 diabetes . Curr Opin Endocrinol Diabetes Obes 27 :215–224. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soccio RE, Chen ER, Rajapurkar SR, Safabakhsh P, Marinis JM, Dispirito JR, Emmett MJ, Briggs ER, Fang B, Everett LJ, et al. (2015) Genetic variation determines PPARγ function and anti-diabetic drug response in vivo . Cell 162 :33–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, et al. (2010) Calcineurin signaling regulates human islet beta-cell survival . J Biol Chem 285 :40050–40059. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Hirshberg B, Bunnell DJ, Sumner AE, Ader M, Remaley AT, Rother KI, Rickels MR, Harlan DM (2012) Metabolic function of a suboptimal transplanted islet mass in nonhuman primates on rapamycin monotherapy . Cell Transplant 21 :1297–1304. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Stoffers DA (2013) The pancreatic β cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab 24 :324–331. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, et al. (2018) Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors . Diabetes 67 :1471–1480. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stancill JS, Cartailler JP, Clayton HW, O’Connor JT, Dickerson MT, Dadi PK, Osipovich AB, Jacobson DA, Magnuson MA (2017) Chronic β-cell depolarization impairs β-cell identity by disrupting a network of Ca 2+ -regulated genes . Diabetes 66 :2175–2187. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stewart AF, Hussain MA, García-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, Kulkarni RN (2015) Human β-cell proliferation and intracellular signaling: part 3 . Diabetes 64 :1872–1885. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stojanovic I, Dimitrijevic M, Vives-Pi M, Mansilla MJ, Pujol-Autonell I, Rodríguez-Fernandez S, Palova-Jelínkova L, Funda DP, Gruden-Movsesijan A, Sofronic-Milosavljevic L, et al. (2017) Cell-based tolerogenic therapy, experience from animal models of multiple sclerosis, type 1 diabetes and rheumatoid arthritis . Curr Pharm Des 23 :2623–2643. [ PubMed ] [ Google Scholar ]
- Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC (2010) An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells . J Clin Invest 120 :2575–2589. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, et al. (2019) SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels . Mol Metab 19 :1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin . Nat Med 24 :1919–1929. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sutherland DE, Matas AJ, Najarian JS (1978) Pancreatic islet cell transplantation . Surg Clin North Am 58 :365–382. [ PubMed ] [ Google Scholar ]
- Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model . Diabetes 61 :818–827. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thielen LA, Chen J, Jing G, Moukha-Chafiq O, Xu G, Jo S, Grayson TB, Lu B, Li P, Augelli-Szafran CE, et al. (2020) Identification of an anti-diabetic, orally available small molecule that regulates TXNIP expression and glucagon action . Cell Metab 32 :353–365.e8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, Keil S, Haack T, Wagner M, Bossart M, et al. (2019) A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials . Diabetes Obes Metab 21 :120–128. [ PubMed ] [ Google Scholar ]
- Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus . Annu Rev Med 66 :255–270. [ PubMed ] [ Google Scholar ]
- Vasseur M, Debuyser A, Joffre M (1987) Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine . Fundam Clin Pharmacol 1 :95–113. [ PubMed ] [ Google Scholar ]
- Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, et al. (2016) Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates . Nat Biotechnol 34 :345–352. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, Asterholm IW, Benrick A, Briant LJB, Chibalina MV, et al. (2019) Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion . Nat Commun 10 :139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wallner K, Shapiro AM, Senior PA, McCabe C (2016) Cost effectiveness and value of information analyses of islet cell transplantation in the management of ‘unstable’ type 1 diabetes mellitus . BMC Endocr Disord 16 :17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang H, Bender A, Wang P, Karakose E, Inabnet WB, Libutti SK, Arnold A, Lambertini L, Stang M, Chen H, et al. (2017) Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas . Nat Commun 8 :767. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O’Rourke B, He L (2019) Metformin improves mitochondrial respiratory activity through activation of AMPK . Cell Rep 29 :1511–1523.e5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Westerman J, Wirtz KW, Berkhout T, van Deenen LL, Radhakrishnan R, Khorana HG (1983) Identification of the lipid-binding site of phosphatidylcholine-transfer protein with phosphatidylcholine analogs containing photoactivable carbene precursors . Eur J Biochem 132 :441–449. [ PubMed ] [ Google Scholar ]
- World Health Organization (2020) World Health Organization Diabetes Fact Sheet . [ Google Scholar ]
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, et al. (2020) Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist . JCI Insight 5 :e140532. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Williams P (1894) Notes on diabetes treated with extract and by grafts of sheep’s pancreas . BMJ 2 :1303–1304. [ Google Scholar ]
- Williamson RT (1901) On the treatment of glycosuria and diabetes mellitus with sodium salicylate . BMJ 1 :760–762. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Witters LA (2001) The blooming of the French lilac . J Clin Invest 108 :1105–1107. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al.; DECLARE–TIMI 58 Investigators (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes . N Engl J Med 380 :347–357. [ PubMed ] [ Google Scholar ]
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug . Nat Med 23 :850–858. [ PubMed ] [ Google Scholar ]
- Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, et al. (2005) Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial . Diabetes 54 :2390–2395. [ PubMed ] [ Google Scholar ]
- Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers . Diabetes 61 :848–856. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yang JF, Gong X, Bakh NA, Carr K, Phillips NFB, Ismail-Beigi F, Weiss MA, Strano MS (2020) Connecting rodent and human pharmacokinetic models for the design and translation of glucose-responsive insulin . Diabetes 69 :1815–1826. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu O, Azoulay L, Yin H, Filion KB, Suissa S (2018) Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglycemia . Am J Med 131 :317.e11–317.e22. [ PubMed ] [ Google Scholar ]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action . J Clin Invest 108 :1167–1174. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al.; EMPA-REG OUTCOME Investigators (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes . N Engl J Med 373 :2117–2128. [ PubMed ] [ Google Scholar ]
- Reference Manager
- Simple TEXT file
People also looked at
Hypothesis and theory article, type 2 diabetes mellitus: a pathophysiologic perspective.

- Department of Medicine, Duke University, Durham, NC, United States
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5–10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years, and their effectiveness may simply be due to lowering the dietary contribution to glucose and insulin levels, which then leads to improvements in hyperglycemia and hyperinsulinemia. Treatments for T2DM that lead to improvements in glycemic control and reductions in blood insulin levels are sensible based on this pathophysiologic perspective. In this article, a pathophysiological argument for using carbohydrate restriction to treat T2DM will be made.
Introduction
Type 2 Diabetes Mellitus (T2DM) is characterized by a persistently elevated blood glucose, or an elevation of blood glucose after a meal containing carbohydrate ( 1 ) ( Table 1 ). Unlike Type 1 Diabetes which is characterized by a deficiency of insulin, most individuals affected by T2DM have elevated insulin levels (fasting and/or post glucose ingestion), unless there has been beta cell failure ( 2 , 3 ). The term “insulin resistance” (IR) has been used to explain why the glucose levels remain elevated even though there is no deficiency of insulin ( 3 , 4 ). Attempts to determine the etiology of IR have involved detailed examinations of molecular and intracellular pathways, with attribution of cause to fatty acid flux, but the root cause has been elusive to experts ( 5 – 7 ).
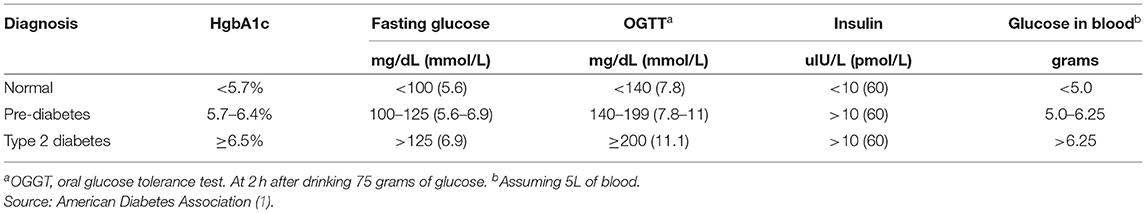
Table 1 . Definition of type 2 diabetes mellitus.
How Much Glucose Is in the Blood?
Keeping in mind that T2DM involves an elevation of blood glucose, it is important to understand how much glucose is in the blood stream to begin with, and then the factors that influence the blood glucose—both exogenous and endogenous factors. The amount of glucose in the bloodstream is carefully controlled—approximately 5–10 grams in the bloodstream at any given moment, depending upon the size of the person. To calculate this, multiply 100 milligrams/deciliter × 1 gram/1,000 milligrams × 10 deciliters/1 liter × 5 liters of blood. The “zeros cancel” and you are left with 5 grams of glucose if the individual has 5 liters of blood. Since red blood cells represent about 40% of the blood volume, and the glucose is in equilibrium, there may be an extra 40% glucose because of the red blood cell reserve ( 8 ). Adding the glucose from the serum and red blood cells totals about 5–10 grams of glucose in the entire bloodstream.
Major Exogenous Factors That Raise the Blood Glucose
Dietary carbohydrate is the major exogenous factor that raises the blood glucose. When one considers that it is common for an American in 2021 to consume 200–300 grams of carbohydrate daily, and most of this carbohydrate is digested and absorbed as glucose, the body absorbs and delivers this glucose via the bloodstream to the cells while attempting to maintain a normal blood glucose level. Thinking of it in this way, if 200–300 grams of carbohydrates is consumed in a day, the bloodstream that holds 5–10 grams of glucose and has a concentration of 100 milligrams/deciliter, is the conduit through which 200,000–300,000 milligrams (200 grams = 200,000 milligrams) passes over the course of a day.
Major Endogenous Factors That Raise the Blood Glucose
There are many endogenous contributors that raise the blood glucose. There are at least 3 different hormones that increase glucose levels: glucagon, epinephrine, and cortisol. These hormones increase glucose levels by increasing glycogenolysis and gluconeogenesis ( 9 ). Without any dietary carbohydrate, the normal human body can generate sufficient glucose though the mechanism of glucagon secretion, gluconeogenesis, glycogen storage and glycogenolysis ( 10 ).
Major Exogenous Factors That Lower the Blood Glucose
A reduction in dietary carbohydrate intake can lower the blood glucose. An increase in activity or exercise usually lowers the blood glucose ( 11 ). There are many different medications, employing many mechanisms to lower the blood glucose. Medications can delay sucrose and starch absorption (alpha-glucosidase inhibitors), slow gastric emptying (GLP-1 agonists, DPP-4 inhibitors) enhance insulin secretion (sulfonylureas, meglitinides, GLP-1 agonists, DPP-4 inhibitors), reduce gluconeogenesis (biguanides), reduce insulin resistance (biguanides, thiazolidinediones), and increase urinary glucose excretion (SGLT-2 inhibitors). The use of medications will also have possible side effects.
Major Endogenous Factors That Lower the Blood Glucose
The major endogenous mechanism to lower the blood glucose is to deliver glucose into the cells (all cells can use glucose). If the blood glucose exceeds about 180 milligrams/deciliter, then loss of glucose into the urine can occur. The blood glucose is reduced by cellular uptake using glut transporters ( 12 ). Some cells have transporters that are responsive to the presence of insulin to activate (glut4), others have transporters that do not require insulin for activation. Insulin-responsive glucose transporters in muscle cells and adipose cells lead to a reduction in glucose levels—especially after carbohydrate-containing meals ( 13 ). Exercise can increase the glucose utilization in muscle, which then increases glucose cellular uptake and reduce the blood glucose levels. During exercise, when the metabolic demands of skeletal muscle can increase more than 100-fold, and during the absorptive period (after a meal), the insulin-responsive glut4 transporters facilitate the rapid entry of glucose into muscle and adipose tissue, thereby preventing large fluctuations in blood glucose levels ( 13 ).
Which Cells Use Glucose?
Glucose can used by all cells. A limited number of cells can only use glucose, and are “glucose-dependent.” It is generally accepted that the glucose-dependent cells include red blood cells, white blood cells, and cells of the renal papilla. Red blood cells have no mitochondria for beta-oxidation, so they are dependent upon glucose and glycolysis. White blood cells require glucose for the respiratory burst when fighting infections. The cells of the inner renal medulla (papilla) are under very low oxygen tension, so therefore must predominantly use glucose and glycolysis. The low oxygen tension is a result of the countercurrent mechanism of urinary concentration ( 14 ). These glucose-dependent cells have glut transporters that do not require insulin for activation—i.e., they do not need insulin to get glucose into the cells. Some cells can use glucose and ketones, but not fatty acids. The central nervous system is believed to be able to use glucose and ketones for fuel ( 15 ). Other cells can use glucose, ketones, and fatty acids for fuel. Muscle, even cardiac muscle, functions well on fatty acids and ketones ( 16 ). Muscle cells have both non-insulin-responsive and insulin-responsive (glut4) transporters ( 12 ).
Possible Dual Role of an Insulin-Dependent Glucose-Transporter (glut4)
A common metaphor is to think of the insulin/glut transporter system as a key/lock mechanism. Common wisdom states that the purpose of insulin-responsive glut4 transporters is to facilitate glucose uptake when blood insulin levels are elevated. But, a lock serves two purposes: to let someone in and/or to keep someone out . So, one of the consequences of the insulin-responsive glut4 transporter is to keep glucose out of the muscle and adipose cells, too, when insulin levels are low. The cells that require glucose (“glucose-dependent”) do not need insulin to facilitate glucose entry into the cell (non-insulin-responsive transporters). In a teleological way, it would “make no sense” for cells that require glucose to have insulin-responsive glut4 transporters. Cells that require glucose have glut1, glut2, glut3, glut5 transporters—none of which are insulin-responsive (Back to the key/lock metaphor, it makes no sense to have a lock on a door that you want people to go through). At basal (low insulin) conditions, most glucose is used by the brain and transported by non-insulin-responsive glut1 and glut3. So, perhaps one of the functions of the insulin-responsive glucose uptake in muscle and adipose to keep glucose OUT of the these cells at basal (low insulin) conditions, so that the glucose supply can be reserved for the tissue that is glucose-dependent (blood cells, renal medulla).
What Causes IR and T2DM?
The current commonly espoused view is that “Type 2 diabetes develops when beta-cells fail to secrete sufficient insulin to keep up with demand, usually in the context of increased insulin resistance.” ( 17 ). Somehow, the beta cells have failed in the face of insulin resistance. But what causes insulin resistance? When including the possibility that the environment may be part of the problem, is it possible that IR is an adaptive (protective) response to excess glucose availability? From the perspective that carbohydrate is not an essential nutrient and the change in foods in recent years has increased the consumption of refined sugar and flour, maybe hyperinsulinemia is the cause of IR and T2DM, as cells protect themselves from excessive glucose and insulin levels.
Insulin Is Already Elevated in IR and T2DM
Clinical experience of most physicians using insulin to treat T2DM over time informs us that an escalation of insulin dose is commonly needed to achieve glycemic control (when carbohydrate is consumed). When more insulin is given to someone with IR, the IR seems to get worse and higher levels of insulin are needed. I have the clinical experience of treating many individuals affected by T2DM and de-prescribing insulin as it is no longer needed after consuming a diet without carbohydrate ( 18 ).
Diets Without Carbohydrate Reverse IR and T2DM
When dietary manipulation was the only therapy for T2DM, before medications were available, a carbohydrate-restricted diet was used to treat T2DM ( 19 – 21 ). Clinical experience of obesity medicine physicians and a growing number of recent studies have demonstrated that carbohydrate-restricted diets reverse IR and T2DM ( 18 , 22 , 23 ). Other methods to achieve caloric restriction also have these effects, like calorie-restricted diets and bariatric surgery ( 24 , 25 ). There may be many mechanisms by which these approaches may work: a reduction in glucose, a reduction in insulin, nutritional ketosis, a reduction in metabolic syndrome, or a reduction in inflammation ( 26 ). Though there may be many possible mechanisms, let's focus on an obvious one: a reduction in blood glucose. Let's assume for a moment that the excessive glucose and insulin leads to hyperinsulinemia and this is the cause of IR. On a carbohydrate-restricted diet, the reduction in blood glucose leads to a reduction in insulin. The reduction in insulin leads to a reduction in insulin resistance. The reduction in insulin leads to lipolysis. The resulting lowering of blood glucose, insulin and body weight reverses IR, T2DM, AND obesity. These clinical observations strongly suggest that hyperinsulinemia is a cause of IR and T2DM—not the other way around.
What Causes Atherosclerosis?
For many years, the metabolic syndrome has been described as a possible cause of atherosclerosis, but there are no RCTs directly targeting metabolic syndrome, and the current drug treatment focuses on LDL reduction, so its importance remains controversial. A recent paper compared the relative importance of many risk factors in the prediction of the first cardiac event in women, and the most powerful predictors were diabetes, metabolic syndrome, smoking, hypertension and BMI ( 27 ). The connection between dietary carbohydrate and fatty liver is well-described ( 28 ). The connection between fatty liver and atherosclerosis is well-described ( 29 ). It is very possible that the transport of excess glucose to the adipose tissue via lipoproteins creates the particles that cause the atherosclerotic damage (small LDL) ( Figure 1 ) ( 30 – 32 ). This entire process of dietary carbohydrate leading to fatty liver, leading to small LDL, is reversed by a diet without carbohydrate ( 26 , 33 , 34 ).
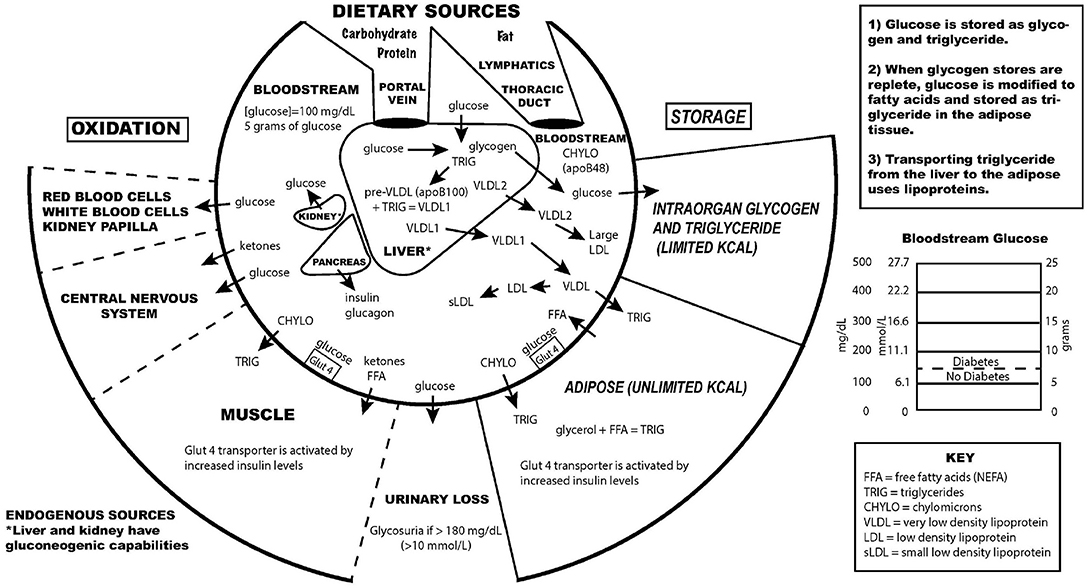
Figure 1 . Key aspects of the interconnection between glucose and lipoprotein metabolism.
Reducing dietary carbohydrate in the context of a low carbohydrate, ketogenic diet reduces hyperglycemia and hyperinsulinemia, IR and T2DM. In the evaluation of an individual for a glucose abnormality, measure the blood glucose and insulin levels. If the insulin level (fasting or after a glucose-containing meal) is high, do not give MORE insulin—instead, use an intervention to lower the insulin levels. Effective ways to reduce insulin resistance include lifestyle, medication, and surgical therapies ( 23 , 35 ).
The search for a single cause of a complex problem is fraught with difficulty and controversy. I am not hypothesizing that excessive dietary carbohydrate is the only cause of IR and T2DM, but that it is a cause, and quite possibly the major cause. How did such a simple explanation get overlooked? I believe it is very possible that the reductionistic search for intracellular molecular mechanisms of IR and T2DM, the emphasis on finding pharmaceutical (rather than lifestyle) treatments, the emphasis on the treatment of high total and LDL cholesterol, and the fear of eating saturated fat may have misguided a generation of researchers and clinicians from the simple answer that dietary carbohydrate, when consumed chronically in amounts that exceeds an individual's ability to metabolize them, is the most common cause of IR, T2DM and perhaps even atherosclerosis.
While there has historically been a concern about the role of saturated fat in the diet as a cause of heart disease, most nutritional experts now cite the lack of evidence implicating dietary saturated fat as the reason for lack of concern of it in the diet ( 36 ).
The concept of comparing medications that treat IR by insulin-sensitizers or by providing insulin itself was tested in the Bari-2D study ( 37 ). Presumably in the context of consuming a standard American diet, this study found no significant difference in death rates or major cardiovascular events between strategies of insulin sensitization or insulin provision.
While lifestyle modification may be ideal to prevent or cure IR and T2DM, for many people these changes are difficult to learn and/or maintain. Future research should be directed toward improving adherence to all effective lifestyle or medication treatments. Future research is also needed to assess the effect of carbohydrate restriction on primary or secondary prevention of outcomes of cardiovascular disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
EW receives royalties from popular diet books and is founder of a company based on low-carbohydrate diet principles (Adapt Your Life, Inc.).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care . (2016) 39 (Suppl. 1):S13–22. doi: 10.2337/dc16-S005
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. (1984) 74:1238–46. doi: 10.1172/JCI111533
3. Reaven GM. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. (2005) 34:49–62. doi: 10.1016/j.ecl.2004.12.001
4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. (1991) 14:173–94. doi: 10.2337/diacare.14.3.173
5. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365:1415–28. doi: 10.1016/S0140-6736(05)66378-7
6. Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
7. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. (2000) 106:171–6. doi: 10.1172/JCI10583
8. Guizouarn H, Allegrini B. Erythroid glucose transport in health and disease. Pflugers Arch. (2020) 472:1371–83. doi: 10.1007/s00424-020-02406-0
9. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. (2017) 13:572–87. doi: 10.1038/nrendo.2017.80
10. Tondt J, Yancy WS, Westman EC. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr Res Rev. (2020) 33:260–70. doi: 10.1017/S0954422420000050
11. Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. (2013) 36:e177. doi: 10.2337/dc13-0965
12. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. (2013) 34:121–38. doi: 10.1016/j.mam.2012.07.001
13. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. (2002) 3:267–77. doi: 10.1038/nrm782
14. Epstein FH. Oxygen and renal metabolism. Kidney Int. (1997) 51:381–5. doi: 10.1038/ki.1997.50
15. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. (2006) 26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258
16. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. (2020) 370:364–8. doi: 10.1126/science.abc8861
17. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. (2017) 66:241–55. doi: 10.2337/db16-0806
18. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:36. doi: 10.1186/1743-7075-5-36
CrossRef Full Text | Google Scholar
19. Allen F. The treatment of diabetes. Boston Med Surg J. (1915) 172:241–7. doi: 10.1056/NEJM191502181720702
20. Osler W, McCrae T. The Principles and Practice of Medicine . 9th ed. New York and London: Appleton & Company (1923).
21. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. (2021) 131:e142246. doi: 10.1172/JCI142246
22. Steelman GM, Westman EC. Obesity: Evaluation and Treatment Essentials . 2nd ed. Boca Raton: CRC Press, Taylor & Francis Group (2016). 340 p.
23. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
24. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. (2011) 54:2506–14. doi: 10.1007/s00125-011-2204-7
25. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. (2010) 33:1438–42. doi: 10.2337/dc09-2107
26. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol. (2018) 17:56. doi: 10.1186/s12933-018-0698-8
27. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. (2021) 6:437–47. doi: 10.1001/jamacardio.2020.7073
28. Duwaerts CC, Maher JJ. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. (2019) 7:749–61. doi: 10.1016/j.jcmgh.2019.02.001
29. Zhang L, She Z-G, Li H, Zhang X-J. Non-alcoholic fatty liver disease: a metabolic burden promoting atherosclerosis. Clin Sci Lond Engl. (1979) 134:1775–99. doi: 10.1042/CS20200446
30. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. (1995) 62:19–29. doi: 10.1093/ajcn/62.1.19
31. Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol. (2000) 74 (Suppl. 1):S17–22. doi: 10.1016/S0167-5273(99)00107-2
32. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
33. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769. doi: 10.7326/0003-4819-140-10-200405180-00006
34. Tendler D, Lin S, Yancy WS, Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. (2007) 52:589–93. doi: 10.1007/s10620-006-9433-5
35. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. (1995) 222:339–50. doi: 10.1097/00000658-199509000-00011
36. Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:844–57. doi: 10.1016/j.jacc.2020.05.077
37. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med . (2009) 360:2503–15. doi: 10.1056/NEJMoa0805796
Keywords: type 2 diabetes, insulin resistance, pre-diabetes, carbohydrate-restricted diets, hyperinsulinemia, hyperglycemia
Citation: Westman EC (2021) Type 2 Diabetes Mellitus: A Pathophysiologic Perspective. Front. Nutr. 8:707371. doi: 10.3389/fnut.2021.707371
Received: 09 May 2021; Accepted: 20 July 2021; Published: 10 August 2021.
Reviewed by:
Copyright © 2021 Westman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric C. Westman, ewestman@duke.edu
This article is part of the Research Topic
Carbohydrate-restricted Nutrition and Diabetes Mellitus
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 February 2021
To tackle diabetes, science and health systems must take into account social context
- Jacqueline A. Seiglie ORCID: orcid.org/0000-0001-9278-4516 1 , 2 ,
- Devaki Nambiar ORCID: orcid.org/0000-0001-5682-6109 3 , 4 , 5 , 6 ,
- David Beran 7 , 8 &
- J. Jaime Miranda ORCID: orcid.org/0000-0002-4738-5468 6 , 9 , 10 , 11 , 12
Nature Medicine volume 27 , pages 193–195 ( 2021 ) Cite this article
4694 Accesses
10 Citations
31 Altmetric
Metrics details
- Developing world
- Endocrinology
An increasing amount of publications are recognizing that a person’s risk of diabetes and diabetes outcomes are influenced largely by social determinants of health. This renewed understanding of disease should influence health provision and diabetes research, but will it?
In a year marked by the centenary of the discovery of insulin 1 and outstanding scientific progress in the therapeutic management of diabetes mellitus 2 , 3 , these advances have been contrasted by the sobering disparities that, although present before the COVID-19 pandemic, have been unmasked by this crisis for people living with diabetes. While diabetes is now well recognized as an important biological risk factor for poor COVID-19 outcomes, the disproportionate impact of this pandemic on socially vulnerable people with diabetes has laid bare the profound importance of the social determinants of health in the tackling of health inequalities 4 , 5 . These disparities are particularly alarming when one considers that among the nearly half-billion people with diabetes globally, three out of every four people with diabetes live in low- and middle-income countries (LMICs) 6 . The renewed attention on the social determinants of health in relation to diabetes in the wake of the COVID-19 pandemic has revealed the sheer complexity of tackling health inequalities and the inadequacy of existing metrics, frameworks and approaches 7 . The question is: amid the astounding scientific progress accomplished in the management of diabetes, what does a social-determinants framework add to the field, particularly in LMICs?
It has long been recognized that health is strongly influenced by social determinants—the conditions in which people are born, grow, live, work, and age, including the health system 8 . In fact, the concept of the social determinants of health was woven into the very foundations of modern public health, which at the turn of the 19th century inspired pioneers such as Rudolf Virchow to posit that social medicine was inextricably intertwined with the politics of social justice 9 , 10 . Further evidence on the strong linkage between the social determinants and their impact on disease emerged throughout the 20th century 11 and, in 2008, culminated in the report of the World Health Organization’s Commission on the Social Determinants of Health 8 , which called for a global movement to recognize their importance in the tackling of health inequalities, with the goal of closing the health gap within a generation. Yet more than a decade since that call for action was put forth, the COVID-19 crisis has abruptly highlighted the lack of progress achieved in tackling inequalities, making visible the linkages and the gross weaknesses of living conditions, health and well-being, and the impact of this on multiple facets of long-term chronic conditions 12 , 13 .
In their timely article ‘Social determinants of health and diabetes: a scientific review’, published in Diabetes Care 4 , the authors provide an overview of key definitions and social-determinant frameworks of diabetes and outline detailed recommendations for their implementation into community sectors, diabetes research and clinical practice in the USA. The review builds on pragmatic scientific and consensus statements published before the COVID-19 pandemic 14 , 15 and categorizes social determinants of diabetes into the following five domains: socioeconomic status; neighborhood and physical environment; food environment; healthcare; and social context 4 . Each of these domains encompass aspects of life circumstances that influence how a person’s risk of diabetes and diabetes outcomes are shaped—a phenomenon that occurs largely outside of the health system 16 . While there is robust evidence to support this framework, much of this evidence has been generated in high-income contexts. Less is known about the social determinants of diabetes in LMICs, which are highly heterogeneous and may be subject to additional societal pressures, such as socio-political and protracted conflicts 17 , environmental pollutants 18 , and corporate and commercial power 19 , 20 , as well as constrained health systems with limited capacity to manage chronic conditions that rely on continuity of care 21 .
Whereas much of the scientific progress in diabetes has gravitated toward the domain of pharmacological interventions, diabetes ‘exists’ within a much broader context, with factors that influence its development throughout the life course. For instance, an adverse intrauterine and postnatal environment may influence the development of insulin resistance and the onset of metabolic disease later in life 22 . When these early life disadvantages are coupled with social and commercial determinants that catalyze the development of overweight and obesity 19 , the cumulative burden of these life-course circumstances, which disproportionately affect vulnerable segments of the population, can be difficult to overcome even within equitable health systems. Notably, solutions for mitigating the risk of diabetes may very well lie outside the sphere of health systems, as demonstrated by a housing experiment showing that the opportunity to move to a neighborhood with a lower poverty rate was associated with a lower prevalence of severe obesity and poorly controlled diabetes 16 . As contended by others, “a diagnosis is rarely a solution to problems caused by poverty and inequality” 23 . Furthermore, there is a strong socioeconomic patterning of diabetes and other non-communicable diseases at the population level, whose rise in LMICs is occurring in the context of a complex interplay of epidemiological, demographic and nutritional transitions 24 , 25 . Within this framework, diabetes and other non-communicable diseases are posited to rise initially among affluent groups before shifting to groups of low socioeconomic status 26 , 27 . It is critical to recognize this socioeconomic patterning of diabetes, ideally before the burden of diabetes shifts among populations facing socioeconomic and other vulnerabilities, which requires that additional obstacles, manifold in quantity and complexity, be overcome.
While the social-determinants framework is fundamental for understanding elements largely outside of the health system that contribute to the risk of diabetes and its outcomes, insightful medical anthropologists have long proposed the broader framework of ‘syndemic’ (‘synergistic epidemic’) theory 28 , 29 . A syndemic refers to the clustering of two or more diseases within a population that contributes to, and results from, persistent social and economic inequalities 28 , 30 —a concept that is particularly relevant in the context of the intersection between COVID-19 and diabetes and that highlights social determinants as core components of the effort to eradicate disparities 30 . Indeed, it has been proposed that in tackling COVID-19 and its interaction with non-communicable diseases, “the syndemic nature of the threat we face means that a more nuanced approach is needed if we are to protect the health of our communities” 30 . A ‘syndemic lens’ can both lead to a deeper understanding of the reasons for the clustering of diabetes in certain populations and inform targeted policies to address broader structural and political forces that impact both the development of diabetes and diabetes-related outcomes 31 . This framework requires a nuanced understanding of context-dependent factors that influence disease development and progression, as well as investment in research that can tackle the biosocial complexities of diabetes 28 , 29 , 32 .
The strengthening of health systems for the provision of diabetes care in LMICs is critical. However, the development of health systems that solely prioritize short-term care delivery, relying on patient–provider interaction or health-system inputs, is likely to fall short in tackling both the rising burden of diabetes and its associated disparities. This core challenge, of recognizing the limitations of the current models of care, is poignantly captured by this reflection on patients’ real-world experience of living with type 2 diabetes in Peru: “a patient’s agency to manage their [type 2 diabetes] is affected by a multiplicity of factors acting together: the burden of treatment, the chronicity of poverty, the immediate social context, the deficiencies in the health system, as well as the financial burden of dietary change and medication adherence…the interaction among all these factors in an individual’s vulnerability to ill-health have been recognized by critical medical anthropologists who developed the syndemics framework to acknowledge the role of broader political, economic, and social structures in the individual lives of people, their health, and responses to health” 33 .
How can the COVID-19 crisis be leveraged to revisit the approach to diabetes care in LMICs through a syndemic framework? Diabetes has been long considered a suitable ‘tracer’ condition of international benchmarking of health systems, since it is well defined, fairly easy to diagnose and common 34 . Given the enormous and rapidly growing prevalence of diabetes in LMICs, as well as its social patterning, we argue that diabetes is also a suitable tracer of health disparities 35 . Through its recognition as such, the approach to understanding the growing burden of diabetes in LMICs can be broadened beyond its biology and within its tightly linked social, economic and structural context 29 , 32 .
The publication of scientific statements on the importance of social-determinant frameworks is a great step forward 4 , 14 , 15 . However, will this translate into research efforts and changes in this arena beyond the exploration of disparities in high-income contexts? The incorporation of a social-determinants framework will require both a conceptual shift in the collective approach to the prevention and management of diabetes, beyond pathophysiology and pharmacotherapy, and concomitant investment in upstream factors as an integral aspect of the diabetes field. Funders, governments, the private sector and researchers should consider the critical importance of the social determinants of diabetes as the world moves toward the ‘new normal’ after COVID-19, to ensure a more equitable society in which health is not vitiated by underlying social and economic factors. The centenary of the discovery of insulin is a reminder not only of the impact science and medicine can have on health but also that if governments and civil society, including academia, do not play a role in addressing disparities, advances will be sequestered among the privileged.
And we return to our question: what does a social-determinants ‘lens’ add to the field, particularly in the LMIC context? The multi-faceted challenges imposed by COVID-19 and the neglected rising burden of diabetes in LMICs present a key opportunity for remembering the caution and the bold call to action put forward by the World Health Organization’s Commission on the Social Determinants of Health in 2008: social injustice is killing people on a grand scale, and the health gap must be closed within a generation. It follows that ‘building back better and fairer’ 36 will require rather more than advances in pharmacotherapy and technologies. Life course, the whole of society and intergenerational approaches ought to guide responses, attempting to tackle multiple drivers at once. Indeed, contending with the global, national and local legacies of power (asymmetries) lies at the heart of addressing diabetes—and the ailing health systems and societies its burden reflects—in the coming decades.
Hegele, R. A. & Maltman, G. M. Lancet Diabetes Endocrinol. 8 , 971–977 (2020).
Article CAS PubMed Google Scholar
Sheahan, K. H., Wahlberg, E. A. & Gilbert, M. P. Postgrad. Med. J. 96 , 156–161 (2020).
Article PubMed Google Scholar
Zannad, F. et al. Lancet 396 , 819–829 (2020).
Hill-Briggs, F. et al. Diabetes Care https://doi.org/10.2337/dci20-0053 (2021).
Sosa-Rubí, S.G. et al. Diabetes Care https://doi.org/10.2337/dc20-2192 (2020).
International Diabetes Federation. https://www.diabetesatlas.org/en/resources/ (accessed 18 December 2019).
Gianella, C., Gideon, J. & Romero, M. J. Can. J. Dev. Stud . https://doi.org/10.1080/02255189.2020.1843009 (2020).
World Health Organization. https://www.who.int/publications-detail-redirect/WHO-IER-CSDH-08.1 (2008).
Nambiar, D. & Muralidharan, A. (eds.) The Social Determinants of Health in India: Concepts, Processes, and Indicators https://doi.org/10.1007/978-981-10-5999-5 (Springer Singapore, 2017).
Reese, D. M. West. J. Med. 169 , 105–108 (1998).
CAS PubMed PubMed Central Google Scholar
Marmot, M. G., Rose, G., Shipley, M. & Hamilton, P. J. J. Epidemiol. Community Health 32 , 244–249 (1978).
Article CAS PubMed PubMed Central Google Scholar
Nambiar, D., Bhaumik, S., Pal, A. & Ved, R. BMC Health Serv. Res. 20 , 1077 (2020).
Article PubMed PubMed Central Google Scholar
Pesantes, M. A. et al. Rev. Peru. Med. Exp. Salud Publica 37 , 541–546 (2020).
Hill, J. O. et al. Diabetes Care 36 , 2430–2439 (2013).
Thornton, P. L. et al. Ann. NY Acad. Sci. 1461 , 5–24 (2020).
Ludwig, J. et al. N. Engl. J. Med. 365 , 1509–1519 (2011).
Boulle, P., Kehlenbrink, S., Smith, J., Beran, D. & Jobanputra, K. Lancet Diabetes Endocrinol. 7 , 648–656 (2019).
Liu, F. et al. Environ. Pollut. 252 , 1235–1245 (2019).
McKee, M. & Stuckler, D. Am. J. Public Health 108 , 1167–1170 (2018).
de Lacy-Vawdon, C. & Livingstone, C. BMC Public Health 20 , 1022 (2020).
Beran, D. Curr. Diab. Rep. 15 , 20 (2015).
Lawlor, D. A., Davey Smith, G. & Ebrahim, S. Diabetes Care 26 , 97–103 (2003).
Burgess, R. Nature https://doi.org/10.1038/d41586-020-01313-9 (2020).
Popkin, B. M. Public Health Nutr. 1 , 5–21 (1998).
Omran, A. R. Milbank Mem. Fund Q. 49 , 509–538 (1971).
Jiwani, S. S. et al. Lancet Glob. Health 7 , e1644–e1654 (2019).
Jaacks, L. M. et al. Lancet Diabetes Endocrinol. 7 , 231–240 (2019).
Singer, M., Bulled, N., Ostrach, B. & Mendenhall, E. Lancet 389 , 941–950 (2017).
Mendenhall, E., Kohrt, B. A., Norris, S. A., Ndetei, D. & Prabhakaran, D. Lancet 389 , 951–963 (2017).
Horton, R. Lancet 396 , 874 (2020).
Mendenhall, E. Lancet 396 , 1731 (2020).
Saxena, A. & Mendenhall, E. Soc. Sci. Med . https://doi.org/10.1016/j.socscimed.2020.113503 (2020).
Pesantes, M. A., Tetens, A., Valle, A. D. & Miranda, J. J. Hum. Organ. 78 , 85–96 (2019).
Article Google Scholar
Nolte, E., Bain, C. & McKee, M. Diabetes Care 29 , 1007–1011 (2006).
Taype-Rondan, A., Lazo-Porras, M., Moscoso-Porras, M., Moreano-Sáenz, M. & Miranda, J. J. Br. J. Gen. Pract. 66 , 197–197 (2016).
Marmot, M. The BMJ Opinion https://blogs.bmj.com/bmj/2020/12/15/michael-marmot-post-covid-19-we-must-build-back-fairer/ (15 December 2020).
Download references
Author information
Authors and affiliations.
Diabetes Unit, Massachusetts General Hospital, Boston, MA, USA
Jacqueline A. Seiglie
Department of Medicine, Harvard Medical School, Boston, MA, USA
The George Institute for Global Health, New Delhi, Delhi, India
Devaki Nambiar
Faculty of Medicine, University of New South Wales, Sydney, Australia
Prasanna School of Public Health, Manipal Academy of Higher Education, Karnataka, India
The Bernard Lown Scholars in Cardiovascular Health Program, Harvard TH Chan School of Public Health, Boston, MA, USA
Devaki Nambiar & J. Jaime Miranda
Division of Tropical and Humanitarian Medicine, Hôpitaux Universitaires de Genève, Geneva, Switzerland
David Beran
Faculty of Medicine, University of Geneva, Geneva, Switzerland
Department of Medicine, School of Medicine, Universidad Peruana Cayetano Heredia, Lima, Peru
J. Jaime Miranda
CRONICAS Center of Excellence in Chronic Diseases, Universidad Peruana Cayetano Heredia, Lima, Peru
The George Institute for Global Health, Sydney, Australia
Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, London, UK
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Jacqueline A. Seiglie or J. Jaime Miranda .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Seiglie, J.A., Nambiar, D., Beran, D. et al. To tackle diabetes, science and health systems must take into account social context. Nat Med 27 , 193–195 (2021). https://doi.org/10.1038/s41591-021-01231-x
Download citation
Published : 01 February 2021
Issue Date : February 2021
DOI : https://doi.org/10.1038/s41591-021-01231-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
How could taxes on sugary drinks and foods help reduce the burden of type 2 diabetes.
- Alan Reyes-García
- Isabel Junquera-Badilla
- Ana Basto-Abreu
Current Diabetes Reports (2023)
Multimorbidity
- Søren T. Skou
- Frances S. Mair
- Susan M. Smith
Nature Reviews Disease Primers (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
271 Diabetes Essay Topics, Research Questions, & Presentation Titles
If you’re looking for diabetes-related research topics, you’re at the right place! StudyCorgi has prepared a list of interesting diabetes thesis topics, presentation titles, and essay ideas to write about. Read on to discover the most engaging diabetes project titles and research questions!
👨⚕️ TOP 7 Diabetes Thesis Topics
🏆 best essay topics on diabetes, ❓ diabetes research questions, 👍 good diabetes research topics & essay examples, 🔎 current research questions about diabetes, 🌶️ hot diabetes topics for presentations, 📝 catchy titles for diabetes research paper, 🎓 most interesting diabetes project ideas, 💡 simple diabetes research paper topics, ✍️ diabetes essay topics for college.
- Pathophysiology of Type 2 Diabetes
- The Ageing and Diabetes Care
- Reflection on Diabetes Program
- Diabetes Mellitus Type 1 Discussion
- Diabetes Management in Primary Care
- Clinical Narrative: Conversation With a 30-Years-Old Woman With Diabetes
- Diabetes: Types, Causes, Symptoms and Cures
- Caring for Patients With Diabetes This paper contains recommendations on caring for patients with diabetes mellitus, mainly providing a warm welcome to the patient in the clinic.
- Diabetes: Causes and Effects of Disease Diabetes is a common disease that is found in all parts of the world. Its defining feature is the accumulation of excessive sugar {glucose} in the bloodstream.
- Diabetes Type 2 Treatment and Health Promotion The purpose of this paper is to develop evidence-based management and a plan for a patient with diabetes type 2 and describe health promotion and possible follow-up.
- Diabetes and Diabetic Foot Diabetes mellitus is a disease that occurs when the body fails to regulate the level of glucose in the blood. There are two types of diabetes mellitus, type I, and II.
- Diabetes Mellitus and Its Pathophysiology Diabetes mellitus is a disease that results in the increase of sugar level in the blood due to the inability of the body to produce sufficient insulin.
- Metformin for Type 2 Diabetes Patients Metformin is not metabolized by the organism: research in patients shows that the drug is excreted unaltered in the urine with not metabolites identified.
- Nutrition Impact in Developing Type II Diabetes Mellitus Type II diabetes is a chronic metabolic disease that affects the production and use of insulin in the body facilitating the uptake of glucose (blood sugar).
- Diabetes Type 2 Self-Management Education The concept of diabetes self-management comprises several activities aimed to alleviate disease symptoms: medication intake, physical exercise, and diet.
- Type 1 Diabetes in Children Type 1 diabetes is a major problem among young members of the population because they become infringed from their earliest years.
- Type 2 Diabetes Patients Care Plan The current paper dwells on the elaboration of a care plan for type 2 diabetes patients. The mortality rates connected to type 2 diabetes grow bigger with every other year.
- Gestational Diabetes Mellitus in Nursing Practice Gestational diabetes mellitus is widely debated as one of the controversial and less-researched medical conditions. Nurses play an important role in the treatment of GDM.
- Improving Diabetes Lifestyle Diabetes is one of the health conditions affecting many people in different corners of the world. Individuals and family members affected by the condition should lead healthy lives.
- Type 2 Diabetes in a 50-Year-Old Male This paper contains a description and analysis of vulnerability and an appropriate holistic care plan for a 50-year-old male with type 2 diabetes.
- Diabetes Type 1 and Type 2 and Its Causes This paper analyzes the causes of diabetes. They vary depending on genetic makeup, family history, ethnicity, health, and environmental factors.
- Comparison of Type 1 and 2 Diabetes The primary difference between different types of diabetes is that type one diabetes is more serious, as people continuously rely on particular medications to continue living.
- Prevention of Type II Diabetes This article is devoted to the prevention of type 2 diabetes: the factors that can trigger the disease, as well as the categories of people who are at risk, are considered.
- Diabetes in Children: The Prevalence and Prevention The Canadian Pediatric Association has laid down several recommendations in order to prevent the spread of the disease: encouraging physical activities, and a healthy diet, etc.
- Role of Physical Activity in the Management of Type 2 Diabetes The paper analyzes facts on the interlinkages between metabolic outcomes, physical activity levels in patients with type 2 diabetes, and strategies.
- Diabetes: Anatomy and Physiology This paper analyses how diabetes affects the various body parts and the treatment and prevention methods. It is a condition that causes difficulties.
- Awareness on Diabetes Causes and Treatment The need to increase awareness of diabetes causes and treatment is the reason why precisely this disease is chosen for this study.
- Type 1 Diabetes Mellitus Treatment and Management Type 1 diabetes mellitus is associated with different complications. The disease can occur naturally and make it impossible for many patients to lead quality lives.
- Diabetes Mellitus Type II: Diagnosis and Treatment Type II diabetes is caused by a combination of amplified tissue struggle to insulin, scarce insulin emission, or the surplus secretion of glucagon.
- Annotated Bibliography to Health Literacy, Self-Care and Patients With Diabetes This annotated bibliography covers topics related to health literacy, self-care, and glycemic control among others in patients with diabetes.
- Reduction of Kidney Failure Due to Diabetes This proposal aims to outline the project’s matter for future advanced practice because of the prevalence of chronic kidney disease and its vast economic and health consequences.
- A Peer Group Support in Intervention for Adolescent With Type One Diabetes Adolescents with diabetes usually experience difficulties in their physical, emotional, and social stress emerging from the complex medication regimen they have to comply with.
- Prevalence of Diabetes Mellitus in Low-Income Communities: An Ethical Aspect Poor quality of life leads to widespread diabetes mellitus, especially among low-income communities. It creates an ethical dilemma that requires the attention of the authorities.
- Impacts of Nutrition on the Development of Type 2 Diabetes Mellitus The purpose of this article is to highlight the contribution of nutrition to Type 2 diabetes mellitus – the most prevalent type of diabetes amid adults.
- Diabetes Mellitus II: Screening and Statistics Diabetes is a health problem that bothers many people around the whole world despite their race, gender, and age.
- Foot Ulcers Management in Diabetes Patients The guideline “Assessment and management of foot ulcers for people with diabetes” comprises various recommendations for advanced practice nurses.
- Diabetes in Evidence-Based Nursing Practice The paper analyzes “Diabetic Foot Ulcer: An Evidence-Based Treatment Update” and “Assessment of Diabetes-Related Knowledge among Nursing Staff in a Hospital Setting”.
- Which Type of Diabetes Begins in Childhood or Adolescence?
- Can Coffee Reduce the Risk of Diabetes?
- How Does a Child Get Childhood Diabetes?
- What Factor Is Most Predictive of Successful Compliance With Diabetes Treatment?
- Can Exercising and Dieting Prevent People From Type 2 Diabetes?
- What Is a Health Promotion Strategy for Diabetes?
- How Can We Prevent Diabetes in Children?
- What Are the Risk Factors and Complications of Diabetes?
- Can Food Stamps Help to Reduce Medicare Spending on Diabetes?
- How Is Childhood Diabetes Effectively Managed?
- What Affects the Quality of Life for People With Type 1 Diabetes?
- Does Diabetes Affect Cardiovascular Health?
- Can a Child Get Diabetes From Eating Too Much Sugar?
- What Are the 7 Steps to Control Diabetes?
- How Can Diabetes Management Be Improved?
- What Are the Components of a Successful Diabetes Care Team?
- Can Diabetes Go Away if You Lose Weight?
- When Does Type 1 Diabetes Need Insulin?
- What Is the Average Lifespan of a Person With Type 2 Diabetes?
- Can Type 2 Diabetes Be Controlled Without Medication?
- What Are Some of the Latest Advances in the Treatment of Diabetes?
- Does Stress Cause Gestational Diabetes?
- How Has Treatment for Diabetes Changed Over the Years?
- Can a Child Live a Normal Life With Diabetes?
- What Is the Best Way to Manage Diabetes?
- Diabetes and Its Economic Cost in the United States Governments in developing nations should lobby for resources from developed economies to deliver inexpensively and quality care to diabetes patients.
- A Diabetes-Related PICOT (Research) Question The study of the PICOT question involves the search and systematization of sources to find the most relevant evidence.
- Standards of Medical Care in Diabetes A nurse should not recommend medical treatment for excess weight to a patient who has not tried traditional methods of treatment.
- The Role Exercise Plays in Diabetes Prevention The paper states that exercise has a critical role in preventing diabetes. It helps control cholesterol, weight, blood glucose, and blood pressure.
- Evidence-Based Practice in Diabetes Nursing Care While all nurses should be familiar with the importance of evidence-based practice in enhancing patient outcomes, few have received formal education on how to implement it.
- Ketones Diet and Insulin in Type 2 Diabetes Mellitus The paper synthesizes evidence-based practices in nursing that can equip care providers with the necessary knowledge to educate diabetic patients.
- Healthy Lifestyle Program Impact on Type 2 Diabetes Patients The purpose of the paper is comparing the clinical results of exercise program implementation and conventional therapy in terms of type 2 diabetes interventions.
- Homeostatic Imbalance and Diabetes Symptoms The choice of treatment methods for diabetes mellitus depends on the type of disease, but any form of the disease requires a compulsory diet, exercise, and physical activity.
- Aspects of the Epidemiology of Diabetes The paper discusses the epidemiology of diabetes. It provides information about diabetes mellitus, explains the types of it, and shows how it varies.
- Diabetes Mellitus Self-Management The paper indicates a rising trend in diabetes mellitus diagnosis. Individuals who were diagnosed with type 2 diabetes need information on self-management.
- Significance of the Diabetes Issue The paper states that diabetes is a severe health issue characterized by a high spread level and a range of symptoms that require constant monitoring.
- Diabetes Disease, Its Prevention and Treatment This paper states that the critical element of achieving success in the precluding of diabetes and its complications appears to be the prevention of diabetes.
- The Diabetes Epidemic in the United States Diabetes is one of the most common autoimmune diseases in the United States. This is a pressing issue for the nation, especially for nurses and doctors.
- Diabetes: Types, Causes, and Complications Diabetes is a serious and dangerous disease that, if untreated, can cause severe health problems or lead to death. There are several types of diabetes.
- Diabetes: Causes, Treatment, and Magnitude The importance of such problem as diabetes is obvious. This disease is the fastest growing in the world at the moment, taking a significant burden on healthcare professionals.
- Hypertension and Type 2 Diabetes The development of hypertension and diabetes stems from the use of Glucocorticoid medications. Glucocorticoids increases blood glucose production in the liver.
- Social Epidemiology of Type 2 Diabetes: Ecosocial Perspective The diaTribe Foundation aims to address the urgent issue of increased diabetes prevalence among racial minorities, who have poor awareness of diabetes.
- Habits to Prevent Epidemiology of Type 2 Diabetes The paper aims to raise awareness among various racial groups in Las Vegas about good preventive habits that prevent the development of type 2 diabetes.
- Type 2 Diabetes Prevention in Racial Minorities: Lifestyle Changes To help diabetic patients control weight loss, well-trained nurses need to promote education on healthy diets and physical activities.
- Incretin Mimetic Drugs for Type 2 Diabetes In patients with type 2 diabetes, there is a significant decrease in the incretin effect and a decrease in insulin secretion in response to an oral load.
- Discussion: Diabetes in the United States Diabetes diagnoses are more common in individuals who have completed high school or earned a GED, or have some college education than in those who hold a bachelor’s degree or higher.
- Care for Diabetes African-American Patients in Nevada There is an urgent need to promote the professional role of nurses for diabetes African-American patients in Nevada.
- Diabetes Patient Case Study: Endocrinology Mr. X’s situation can be analyzed through the lens of social determinants. The first determinant is health care access and quality.
- The Current Trends of Patients With Diabetes The study aims to observe the current trends of patients with diabetes aged sixty-five or older within the selected healthcare setting.
- Interventions Preventing Diabetes Development The patient was diagnosed with prediabetes three months ago. Possible interventions to prevent the development of diabetes type two were unable to succeed.
- Diabetes Health Care Information Collection This work aims at proposing a method of collecting information associated with diabetes, such as demographics, medications used, and other data.
- Reaching Optimal Health With Type 2 Diabetes To combat the symptoms of this disease and its consequences, it is necessary to adjust nutrition, which will normalize the level of insulin in the human body.
- Effects of Diabetes on Quality of Care in Massachusetts The patient involved was a male relative who is diabetic and receives treatment at the Massachusetts General Hospital.
- Epidemic of Type 2 Diabetes Among Hispanic Males Diabetes is a disease with a very high prevalence of 19% among Hispanic males. It is more common among Hispanics than all other races.
- Role of Genes in Diabetes Development Diabetes is a global pandemic whose effects cause immeasurable burden to the globe. About ten percent of the world’s population suffers from diabetes currently.
- Type 2 Diabetes: Diabetes in Canada Type 2 diabetes is a disease that presents a great danger to the life of a human being, and there is currently no cure for it.
- Type 1 Diabetes in Children: Genetic and Environmental Factors The prevalence rate of type 1 diabetes in children raises the question of the role of genetic and environmental factors in the increasing cases of this illness.
- Symptoms of Type I Diabetes The paper discusses the possible symptoms inherent to diabetes. They are unmotivated weak, have drowsiness, persistent thirst, and have dry mouths.
- Diabetes Mellitus: Information Collection This work aims at reviewing legislative considerations, collecting information and its life cycle, which are associated with diabetes.
- Type 1 Diabetes and Appropriate Therapeutic Diet The food intake and knowledge needed can be related to the education and subsequent application of the therapeutic diet.
- Type 2 Diabetes: Study Purpose, Design and Results Study results showed the developed glucose control and prompted variations in the muscle that are associated with improvement in metabolic wellbeing in type two diabetes patients.
- Evidence-Based Practices to Reduce the Risks of Diabetes A person’s lifestyle can directly affect their health in various ways. An unhealthy lifestyle can lead to a diverse range of diseases later in life.
- What is the role of gut microbiota in the development of insulin resistance?
- How to improve the accuracy of continuous glucose monitoring systems?
- What are the long-term effects of bariatric surgery for diabetes management?
- How does sleep quality influence glycemic control?
- How can telemedicine enhance diabetes care?
- What is the impact of diabetes on cognitive function and brain health?
- What are the best practices for diabetes prevention in children?
- What are the barriers to older adults’ diabetes self-management?
- How does gestational diabetes affect maternal and fetal health?
- How does continuous glucose monitoring impact patients’ quality of life?
- Supporting Patients With Diabetes in U.S. The central problem that this essay raises describes the characteristics of the U.S. health care system in supporting patients with diabetes.
- Type 2 Diabetes Mellitus and Its Etiology Decreased insulin sensitivity in the muscle, tissue, and liver leads to increased insulin production by beta cells of the pancreas.
- Diabetes: Community Teaching The community setting is the Adult Day Care Center, yet this teaching plan also applies to hospitals and clinics with a high influx of diabetes patients.
- Diabetes: Etiology and Expected Treatment Options This paper is a diabetes case study of a patient who has type I diabetes and has not been managing her blood sugars since she’s been ill and unable to keep any food down.
- Type 2 Diabetes Management in Primary School Children The care plan for children with type 2 diabetes implies meeting specific objectives for managing the condition of this population group in the context of educational facilities.
- Diabetes Mellitus: Cost-Effective Solution for India At present, a large number of people are experiencing health complications due to a sedentary lifestyle, lack of physical activities, poor nutritional habits, and SUD.
- Type 2 Diabetes Mellitus Among Children and Adolescents The increase of T2DM among children and adolescents in the last five years has surfaced in parallel with a surprising rise in the number of young people who are obese.
- Diabetes: Overview of the Problem and Treatment The percentage of people who have diabetes has increased lately due to the sedentary lifestyle that many individuals select.
- The Importance of Diabetes Prevention Education Diabetes has become a significant threat to society that’s why the annotated bibliography was selected to ensure that readers can acquire information regarding the disease.
- Scientific Method: The Risk of Contracting Diabetes The paper discusses that drinking coffee may reduce the risk of contracting diabetes. The control group produced a significant increase in blood sugar levels.
- Problem of Diabetes in the Elderly Despite the efforts made by health care organizations around the world, the number of people with diabetes is expected to grow.
- Digital Health Interventions for Adults With Type 2 Diabetes “Digital health interventions for adults with type 2 diabetes” is aimed to determine the patients’ perceptions about the diabetes self-management education (DSME) limitation.
- Educating the Client on Diabetes Medications In order to ascertain the reasons for polypharmacy, an interview was conducted with a client who takes several medications at once.
- Diabetes in African Americans and Effectiveness of Educational Sessions According to the Diabetes Research Institute Foundation, over a tenth of the population has diabetes and related conditions, and the number of new cases continues to rise rapidly.
- Diabetes Mellitus of Type I vs. Type II Unhealthy eating habits, obesity, and an inactive lifestyle are the most common associations with diabetes. These factors are among the main reasons for developing Type 2 diabetes.
- Management of Type 2 Diabetes Metformin is an antihyperglycemic drug prescribed to patients with type 2 diabetes to increase their glucose tolerance.
- Australian Government Policy Response to Diabetes Mellitus Type II It is highly necessary to inform the health officer trainees about the main constraints and challenges that should be considered to handle the problem of diabetes pandemic.
- Diabetes Community Health Programs in Florida The discussion examines how the quality of life in Florida correlates with diabetes and proposed a powerful program for dealing with diabetes in children.
- Obesity, Diabetes and Self-Care The paper discusses being overweight or obese is a high-risk factor for diabetes mellitus and self-care among middle-aged diabetics is a function of education and income.
- Diabetes Prevention Lessons in the Community This paper discusses the problem of diabetes prevention in the community, elaborates the teaching plans to help all stakeholders affected by the diabetes problem.
- Understanding Biostatiscal Principles with Diabetes This paper is meant to review the effectiveness of Biostatistics applied by the information/news medium in communicating diabetes mellitus type 1 and 2 related information.
- Patient Engagement in Type 2 Diabetes The presented research is not exactly in line with the existing literature since it does not demonstrate a statistically significant effect of the selected method by Smith et al.
- Community Obesity and Diabetes: Mississippi Focus Study The paper provides a detailed discussion of the correct method to be used in the state of Mississippi to control and avoid obesity and diabetes issues.
- Pathophysiology of Nephrogenic Diabetes Insipidus Diabetes insipidus is a type of diabetes that is characterized by a reduced production of the ADH (antidiuretic hormone) also known as vasopressin in the body.
- Childhood Diabetes in Saudi Arabia Saudi Arabia has one of the highest diabetes prevalence rates in the world. Five-year research determines that Saudi Arabia has an adult diabetes prevalence rate of 23.7%.
- Endocrine Disorders: Diabetes Mellitus This artticle describes Diabetes Mellitus, its etiology, pathophysiology, signs and symptoms, diagnosis, treatment and nursing considerations.
- Weight Training and Risk of Type 2 Diabetes in Men Should research in media if the claim that little exercise is adequate to minimize the risk of type two diabetes.
- Evidence-Based Practice Project on Diabetes A fundamental component of early Type 2 diabetes mellitus (T2DM) treatment is patient education, which in turn sets the foundation for effective treatment.
- Childhood Diabetes in Saudi Arabia: The Prevalence of Type 1 Diabetes Among Children Diabetes is one of the major chronic ailments facing children in Saudi Arabia. This trend has been observed in recent years.
- Community Prevention: Type 2-Diabetes in California Community-based programs on prevention and control of type 2-diabetes will incorporate approximately four intensive and core sessions that will be offered every year.
- Diabetes Education Skills for Low Grade Literacy Patients This article is a guide for nurses to help them explain diabetes to patients with low medical literacy in simple terms.
- Reducing the Incidence of Diabetes Mellitus and Diabetic Foot in the Veteran Population The research proposes to use a comprehensive education program to reduce the incidence of diabetes mellitus and diabetic foot in the Veteran population.
- Type 1 Diabetes: Characteristics, Epidemiology Type 1 diabetes exhibits different characteristics depending on the person suffering from the disease, place, and time.
- Smartphone Role in Type 2 Diabetes Self-Management The current research paper endeavors to explore mat-analysis studies and past research studies on the role played by smartphones in type 2 diabetes self-management.
- Smartphone Application and Diabetes Reminder Management The proposed intervention implies the implementation of smartphone applications aimed at managing diabetes, the intervention has a lot of advantages.
- Diabetes Insipidus: Causes, Treatment, Pathophysiology The lack of sufficient antidiuretic hormone in the body results in diabetes insipidus. Diabetes insipidus can be managed by taking high amounts of fluids to keep the body hydrated.
- Type II Diabetes: Pathophysiology, Initial Signs, Symptoms, This paper discusses pathophysiology associated with type 2 diabetes, initial signs, symptoms, and type of vascular changes that occur early in type II diabetes.
- Microbiome matters: the link between gut microbiota and diabetes.
- Diabetes and the aging brain: cognitive impacts on older adults.
- Addressing cultural disparities in diabetes care.
- The complex relationship between diabetes and heart health.
- The influence of stress on diabetes development.
- Beyond blood sugar: the multi-organ effects of diabetes.
- The benefits of a plant-based diet for diabetes.
- Can type 2 diabetes be reversed?
- Unique challenges of adults with latent autoimmune diabetes.
- Diabetes and reproductive health: the impact on fertility.
- Social Epidemiology: Diabetes Mellitus in Australian Indigenous People People are advised to engage in physical activity, take a balanced diet, avoid stress, and reduce food and drinks with high levels of sugar.
- Reducing Diabetic Foot Incidence and Its Related Complications Complications arising from the diabetic foot are caused by deep infections and gangrene, which increase the risk of the amputation of the lower limb.
- Type II Diabetes: Disease Analysis Diabetes is one of the diseases that can cause several complications on patients. Evidence has revealed that diabetic complication range from stroke, heart disease or death.
- Standards of Medical Care in Diabetes Guidelines The research paper recommends that ADA and other health bodies should customize DSME so that it can suit the needs of diverse patients in different unique communities.
- Type 1 and 2 Diabetes Mellitus: The Inhaled Insulin Therapy The paper will focus on glycaemic control for patients with diabetes mellitus and will attempt to identify whether the use of inhaled insulin is beneficial for these patients.
- The Diabetes Study of Northern California The population-based study shows that Latinos in the United States are disproportionately affected by diabetes type-2 and have poor glycemic control.
- Lived Experience of Diabetes Among Older, Rural People The implied research question is, “what are the most significant issues associated with the self-management of diabetes among the elderly?”
- Diabetes Diagnosis: The Use of Magnetic Nanoparticles This paper will discuss the use of magnetic nanoparticles in the diagnosis of diabetes, its application in the children population, and its relevance to the nursing profession.
- “Bariatric Surgery v. Conventional Medical Therapy for Type 2 Diabetes” by Mingrone This paper critiqued a study “Bariatric surgery versus conventional medical therapy for type 2 diabetes” that aimed to compare traditional medical therapy and bariatric surgery.
- Depression Intervention Among Diabetes Patients The research examines the communication patterns used by depression care specialist nurses when communicating with patients suffering from diabetes.
- The New Jersey Diabetes Prevention and Control Program The aim of the New Jersey Diabetes Prevention and Control Program is to mitigate the high level of type II diabetes in the target population, through education on lifestyle.
- Type 2 Diabetes Patients and Self-Administer Insulin The importance of patient education to facilitate primary health care skills and knowledge in vulnerable populations has been broadly addressed in scholarly literature.
- Type 1 Diabetes Mellitus Pathophysiology This paper explains Type 1 diabetes. The causes, symptoms, therapeutic procedures, and management procedures of the disease are also explained.
- Diabetes and Tuberculosis: Review of Articles in Nursing This paper discusses articles in nursing about different issues related to diabetes, trends in prevalence and control, and also about tuberculosis treatment.
- Metabolic Syndrome and Type 2 Diabetes Mellitus This article discusses in detail how type 2 diabetes develops over time in patients with metabolic syndrome, focusing on the pathophysiological changes that occur.
- Diabetes Type 2 and Related Lifestyle Challenges Diabetes is continuously becoming a big challenge. There is more than one type of diabetes: type 2 seems to be the most challenging one.
- The Type II Diabetes in Obese Children Approximately 10% of school-going children aged between 5 years and 17 years can be described to be obese; a quarter of them are at a heightened risk of developing type 2 diabetes.
- Diabetes Mellitus and Diabetic Foot Evaluation The research proposes to use a comprehensive education program to reduce incidences of diabetes mellitus and diabetic foot in the population.
- Screening and Management of Diabetes: Standards of Medical Care in Diabetes Guidelines Health care systems across the world are employing diverse screening strategies and criteria in the diagnosis of diabetes mellitus among the population.
- Prevalence of Diabetes Mellitus in Low-Income Communities The present paper offers a review of literature on the major reasons for diabetes prevalence in low-income communities.
- Diabetes: Danger Factors Analysis Diabetes is one of the most common diseases that older people are most affected by it. Danger factors include many points.
- Nursing Diabetes and Obesity Patients Nursing diabetes and obese patients are regarded as one of the most serious problems of contemporary nursing practices.
- Increasing Diabetes Infections Among the Hispanic Populations The article’s objective is centered around establishing whether chronic stress makes US Hispanics more susceptible to diabetes.
- Study of the Diabetes Mellitus Type 1 Diabetes mellitus is a disease that affects many systems in the body; it has both anatomical and biochemical consequences, which are manifested in various ways.
- Type II Diabetes: Treatments Metformin is the most common drug recommended for treating type II diabetes. This drug lowers blood glucose level by reducing the production of insulin.
- Diabetes in American Society To get prepared for diabetes, it is important to learn diabetes triggers, causes, complications, and other characteristics.
- Diabetes Mellitus Overview and Analysis Diabetes which is medically referred to as diabetes mellitus, is a metabolic disorder that occurs due to the lack of production or action of insulin in the body.
- Chronic Bronchitis, Heart Failure, Hypertension, and Diabetes Mellitus This paper discusses the symptoms and causes of such diseases as chronic bronchitis, heart failure, hypertension, and diabetes mellitus.
- Diabetes Type 1 and 2 Preventive Measures Diabetes is a common disease that can lead to adverse consequences for humans’ overall health if not treated properly.
- Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus Blood pressure in diabetic patients remains controversial. The uncertainty surrounding its control complicates patients’ care as the risk of future cardiac events grows.
- The Prevalence of Diabetes in the Elderly From 2000 to 2009 The endeavor of this review is to determine the occurrence of diabetes in the American populace for the period spanning 2000 to 2009.
- The Connection Between Apoptosis and Diabetes The purpose of this study is to investigate the existing body of information regarding apoptosis processes and their connection with diabetes mellitus.
- Mobile Apps for Diabetes Mellitus Patients Research To address people with diabetes mellitus issues, researchers advocate that mobile health services might help them manage their life with the disease better.
- Diabetes: Preventive Measures and Diagnostics To prevent the risk of developing diabetes, the diet of the patient should include healthy fats, fruits and vegetables, high-fiber bread and cereals, and seafood.
- Diabetes Care Team Best Practices Successful diabetes care requires the systematic collaboration of professionals from different branches of medicine.
- Type II Diabetes Mellitus Overview The prevalence of type II diabetes mellitus is anticipated to rise gradually with aging and decreased life expectancy.
- The Role of Significant Others in Adolescent Diabetes, A Qualitative Study by Carroll and Marrero The work “The Role of Significant Others in Adolescent Diabetes” by Carroll and Marrero, from a scholarly approach, demonstrates that the two researchers achieved their aim.
- Care Plan For the Patient With the Type 2 Diabetes
- Incidence of Diabetes in the United States
- How Diabetes Works: Medical Analysis
- Mexican American Children and Type 2 Diabetes
- Diabetes Chronic Condition Management
- Mindful Eating Intervention and Diabetes Self-Management Intervention
- Patient Education Technology: MySugr Diabetes Logbook
- Diabetes Mellitus and Self-Care Education
- Insulin Pump Therapy in Children with Diabetes
- Evaluation of the Clinical Outcomes of Telehealth for Managing Diabetes
- Impact of Establishing a Communication Network of Family Physicians on Level of Hba1c and FBS in Patients With Diabetes
- Integrative Review on Adherence in Haitians With Diabetes
- Preventing Diabetes and Heart Failure Hospitalizations
- Diabetes Issues in the United States and Florida
- Diabetes Control and Education: Four-Week Project
- Cardiovascular Autonomic Neuropathy in Diabetes Patients
- Diabetes Conference as a Scholarly Activity
- Bariatric Surgery in Type 2 Diabetes Management
- The Use of Diabetes Self-Management Apps by African-American Women
- Older Rural People with Diabetes: Life Expectancy
- EHR Database Management: Diabetes Prevention
- Diabetes in African American Patients
- Diabetes Management for Older Adults in Long-Term Care
- Type 2 Diabetes: Possible Interventions and Prevention
- Type 2 Diabetes, Risk Factors, Medical Intervention
- Developmental Care for Type 1 Diabetes
- Evidence-Based Practice: Diabetes Prevalence
- Cardiovascular Autonomic Neuropathy and Diabetes
- Educational Programs for Hispanic Patients with Diabetes
- Diabetes Negligence in the Pediatric Population
- Diabetes in Children: Symptoms and Diagnostics
- Diabetes Self-Management Education in Elderly
- Type-2 Diabetes: Condition and Resources Analysis
- Insulin Pump Therapy in Diabetes
- Transition’s Impact for Patients With Diabetes
- Diabetes in Adolescents, Social and Medical Issues
- Insulin Pharmacological Effects in Diabetes Management
- Education Strategies for Elderly Patients with Diabetes
- Diabetes Interventions for Aging African Americans
- Chronic Fatigue in Diabetes
- Diabetes Diagnosis and Classification
- Type II Diabetes Treatment
- Type II Diabetes in Evidence-Based Pharmacology
- Diabetes and High Blood Pressure Patient Teaching
- Diabetes 2 Complications: Neuropathy and Retinopathy
- Weight Gain, Atherosclerosis, Diabetes Relationship
- Prevention or Delay of Type 2 Diabetes
- Risk Assessment Models for Diabetes Complications
- Diabetes Among Hispanics in Miami: Risk Factors
- Patients with Type 2 Diabetes Mellitus in China
- Diabetes and Status among Immigrants in California
- Diabetes Patient and Holistic Nursing Intervention
- Chronic Disease: Diabetes Mellitus
- Type 2 Diabetes Mellitus in Adults
- Diabetes Genetic Risks in Diagnostics
- Patients With Diabetes and Concomitant Diseases’ Risk
- Prevention and Management of Type 2 Diabetes
- Using Dulaglutide in the Treatment of Patients with Diabetes
- Diabetic Nutritional Plan For a 15-Year-Old Type 1 Diabetes Mellitus Patient
- Diabetes Treatment: Computer-Based Intervention
- Diabetes: Country Walk Community’s Health Problem
- Wound Care Tests in Diabetes
- Treatment and Advances in Diabetes
- Type II Diabetes: Patient Case Study
- Types of Diabetes Mellitus: Role of Insulin
- Diabetes Mellitus Patients and Supporting Resources
- Food Diversion as a Type-2 Diabetes Treatment
- The Most Acute Problems With Patients With Diabetes
- Diabetes in American Adolescents and Its Effects
- Vitamin D Deficiency and Diabetes Mellitus Type 2
- Diabetes and Possible Interventions
- Diabetes and Dementia Relationships and Nursing
- Type 1 Diabetes Mellitus in Adolescents
- Type 2 Diabetes: Disease Process and Screening
- Overweight Diabetes Patients With Cardiovascular Risk
- Type 2 Diabetes Mellitus Integrated Management
- Diabetes Mellitus Type 2
- “Prandial Inhaled Insulin Plus Basal Insulin Glargine Versus Twice Daily Biaspart Insulin for Type 2 Diabetes: A Multicentre Randomised Trial”: Article Review
Cite this post
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2021, September 9). 271 Diabetes Essay Topics, Research Questions, & Presentation Titles. https://studycorgi.com/ideas/diabetes-essay-topics/
"271 Diabetes Essay Topics, Research Questions, & Presentation Titles." StudyCorgi , 9 Sept. 2021, studycorgi.com/ideas/diabetes-essay-topics/.
StudyCorgi . (2021) '271 Diabetes Essay Topics, Research Questions, & Presentation Titles'. 9 September.
1. StudyCorgi . "271 Diabetes Essay Topics, Research Questions, & Presentation Titles." September 9, 2021. https://studycorgi.com/ideas/diabetes-essay-topics/.
Bibliography
StudyCorgi . "271 Diabetes Essay Topics, Research Questions, & Presentation Titles." September 9, 2021. https://studycorgi.com/ideas/diabetes-essay-topics/.
StudyCorgi . 2021. "271 Diabetes Essay Topics, Research Questions, & Presentation Titles." September 9, 2021. https://studycorgi.com/ideas/diabetes-essay-topics/.
These essay examples and topics on Diabetes were carefully selected by the StudyCorgi editorial team. They meet our highest standards in terms of grammar, punctuation, style, and fact accuracy. Please ensure you properly reference the materials if you’re using them to write your assignment.
This essay topic collection was updated on January 22, 2024 .
- How it works
Useful Links
How much will your dissertation cost?
Have an expert academic write your dissertation paper!
Dissertation Services

Get unlimited topic ideas and a dissertation plan for just £45.00
Order topics and plan
Get 1 free topic in your area of study with aim and justification
Yes I want the free topic

45 of the Best Diabetes Dissertation Topics
Published by Owen Ingram at January 2nd, 2023 , Revised On August 16, 2023
The prevalence of diabetes among the world’s population has been increasing steadily over the last few decades, thanks to the growing consumption of fast food and an increasingly comfortable lifestyle. With the field of diabetes evolving rapidly, it is essential to base your dissertation on a trending diabetes dissertation topic that fills a gap in research.
Finding a perfect research topic is one of the most challenging aspects of dissertation writing in any discipline . Several resources are available to students on the internet to help them conduct research and brainstorm to develop their topic selection, but this can take a significant amount of time. So, we decided to provide a list of well-researched, unique and intriguing diabetes research topics and ideas to help you get started.
Other Subject Links:
- Evidence-based Practice Nursing Dissertation Topics
- Child Health Nursing Dissertation Topics
- Adult Nursing Dissertation Topics
- Critical Care Nursing Dissertation Topics
- Palliative Care Nursing Dissertation Topics
- Mental Health Nursing Dissertation Topics
- Nursing Dissertation Topics
- Coronavirus (COVID-19) Nursing Dissertation Topics
List of Diabetes Dissertation Topics
- Why do people recently diagnosed with diabetes have such difficulty accepting reality and controlling their health?
- What are the reactions of children who have recently been diagnosed with diabetes? What can be done to improve their grasp of how to treat the disease?
- In long-term research, people getting intensive therapy for the condition had a worse quality of life. What role should health professionals have in mitigating this effect?
- Why do so many individuals experience severe depression the months after their diagnosis despite displaying no other signs of deteriorating health?
- Discuss some of the advantages of a low-carbohydrate, high-fat diet for people with diabetes
- Discuss the notion of diabetes in paediatrics and why it is necessary to do this research regularly.
- Explain the current threat and difficulty of childhood obesity and diabetes, stressing some areas where parents are failing in their position as guardians to avoid the situation
- Explain some of the difficulties that persons with diabetes have, particularly when obtaining the necessary information and medical treatment
- Explain some of the most frequent problems that people with diabetes face, as well as how they affect the prevalence of the disease. Put out steps that can be implemented to help the problem.
- Discuss the diabetes problem among Asian American teens
- Even though it is a worldwide disease, particular ethnic groups are more likely to be diagnosed as a function of nutrition and culture. What can be done to improve their health literacy?
- Explain how self-management may be beneficial in coping with diabetes, particularly for people unable to get prompt treatment for their illness
- Discuss the possibility of better management for those with diabetes who are hospitalized
- What current therapies have had the most influence on reducing the number of short-term problems in patients’ bodies?
- How have various types of steroids altered the way the body responds in people with hypoglycemia more frequently than usual?
- What effects do type 1, and type 2 diabetes have on the kidneys? How do the most widely used monitoring approaches influence this?
- Is it true that people from specific ethnic groups are more likely to acquire heart disease or eye illness due to their diabetes diagnosis?
- How has the new a1c test helped to reduce the detrimental consequences of diabetes on the body by detecting the condition early?
- Explain the difficulty of uncontrolled diabetes and how it can eventually harm the kidneys and the heart
- Discuss how the diabetic genetic strain may be handed down from generation to generation
- What difficulties do diabetic people have while attempting to check their glucose levels and keep a balanced food plan?
- How have some individuals with type 1 or type 2 diabetes managed to live better lives than others with the disease?
- Is it true that eating too much sugar causes diabetes, cavities, acne, hyperactivity, and weight gain?
- What effect does insulin treatment have on type 2 diabetes?
- How does diabetes contribute to depression?
- What impact does snap participation have on diabetes rates?
- Why has the number of persons who perform blood glucose self-tests decreased? Could other variables, such as social or environmental, have contributed to this decrease?
- Why do patients in the United States struggle to obtain the treatment they require to monitor and maintain appropriate glucose levels? Is this due to increased healthcare costs?
- Nutrition is critical to a healthy lifestyle, yet many diabetic patients are unaware of what they should consume. Discuss
- Why have injuries and diabetes been designated as national health priorities?
- What factors contribute to the growing prevalence of type ii diabetes in adolescents?
- Does socioeconomic status influence the prevalence of diabetes?
- Alzheimer’s disease and type 2 diabetes: a critical assessment of the shared pathological traits
- What are the effects and consequences of diabetes on peripheral blood vessels?
- What is the link between genetic predisposition, obesity, and type 2 diabetes development?
- Diabetes modifies the activation and repression of pro- and anti-inflammatory signalling pathways in the vascular system.
- Understanding autoimmune diabetes through the tri-molecular complex prism
- Does economic status influence the regional variation of diabetes caused by malnutrition?
- What evidence is there for using traditional Chinese medicine and natural products to treat depression in people who also have diabetes?
- Why was the qualitative method used to evaluate diabetes programs?
- Investigate the most common symptoms of undiagnosed diabetes
- How can artificial intelligence help diabetes patients?
- What effect does the palaeolithic diet have on type 2 diabetes?
- What are the most common diabetes causes and treatments?
- What causes diabetes mellitus, and how does it affect the United Kingdom?
Hire an Expert Writer
Orders completed by our expert writers are
- Formally drafted in an academic style
- Free Amendments and 100% Plagiarism Free – or your money back!
- 100% Confidential and Timely Delivery!
- Free anti-plagiarism report
- Appreciated by thousands of clients. Check client reviews
You can contact our 24/7 customer service for a bespoke list of customized diabetes dissertation topics , proposals, or essays written by our experienced writers . Each of our professionals is accredited and well-trained to provide excellent content on a wide range of topics. Getting a good grade on your dissertation course is our priority, and we make sure that happens. Find out more here .
Free Dissertation Topic
Phone Number
Academic Level Select Academic Level Undergraduate Graduate PHD
Academic Subject
Area of Research
Frequently Asked Questions
How to find diabetes dissertation topics.
To find diabetes dissertation topics:
- Study recent research in diabetes.
- Focus on emerging trends.
- Explore prevention, treatment, tech, etc.
- Consider cultural or demographic aspects.
- Consult experts or professors.
- Select a niche that resonates with you.

You May Also Like
In this article, we suggest some topics regarding USA’s Withdrawal From Afghanistan so you can kick start your dissertation without any delay.
Urban geography is a growing field of study that provides learners with a comprehensive understanding of how cities, towns and other human settlements develop and change over time.
Here is a list of Geography Dissertation Topics to help you choose the one studies anyone as per your requirements.
USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

- How It Works

- Previous Article
- Next Article
Acknowledgments
Connected content.
In a special series of the ADA Journals' podcast Diabetes Core Update , host Dr. Neil Skolnik interviews special guests and authors of this clinical compendium issue. Listen now at Special Podcast Series: Focus on Diabetes or view the interviews on YouTube at A Practice Guide to Diabetes-Related Eye Care .
Summary and Conclusion
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Thomas W. Gardner; Summary and Conclusion. ADA Clinical Compendia 1 July 2022; 2022 (3): 20. https://doi.org/10.2337/db20223-20
Download citation file:
- Ris (Zotero)
- Reference Manager
Diabetes is a multifactorial disease process, and its long-term management requires the active involvement of people with diabetes and their families, as well as a large multidisciplinary care team to ensure optimal health, quality of life, and productivity. Keeping up with new medications, emerging technology, and evolving treatment recommendations can be challenging, and the language and care processes commonly used by practitioners in one discipline may be less familiar to other diabetes care professionals.
In the realm of diabetes-related eye care, our ability to prevent the progression of diabetes-related retinal disease and thereby preserve vision has never been greater. However, far too many people with diabetes still are not receiving appropriate screening to identify eye disease early and ensure its timely treatment.
It is our hope that this compendium has provided information and guidance to improve communication and encourage collaboration between eye care professionals and other diabetes health care professionals and allow them to more effectively cooperate to reduce barriers to care and improve both the ocular and systemic health of their shared patients.
Editorial and project management services were provided by Debbie Kendall of Kendall Editorial in Richmond, VA.
Dualities of Interest
B.A.C. is a consultant for Genentech and Regeneron. S.A.R. is a speaker for Allergan, Inc., and VSP Vision Care. No other potential conflicts of interest relevant to this compendium were reported.
Author Contributions
All authors researched and wrote their respective sections. Lead author T.W.G. reviewed all content and is the guarantor of this work.
The opinions expressed are those of the authors and do not necessarily reflect those of VSP Vision Care, Regeneron, or the American Diabetes Association. The content was developed by the authors and does not represent the policy or position of the American Diabetes Association, any of its boards or committees, or any of its journals or their editors or editorial boards.
Email alerts
- Online ISSN 2771-6880
- Print ISSN 2771-6872
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Essay on Diabetes
Introduction
Diabetes is a healthcare condition that has continued to affect so many people, both young and old. Understanding more about Diabetes will help people live a healthy lifestyle by avoiding all the possible things that might cause it. In this assignment, I will assess why Diabetes is a significant health issue to individuals and the world. I will discuss the background of Diabetes, its definitions, and the types of Diabetes. Besides, I will discuss what is needed to promote individual and group health for people who have Diabetes. By the end of the assignment, one will have better knowledge about Diabetes since I will also discuss the causes and preventive measures that can be undertaken to prevent the disease. Towards the end of the assignment, I will describe three achievable health promotion goals, hence helping fight against Diabetes. I will also describe some of the interventions and roles that different people, groups, and organizations play to reduce the high cases of Diabetes in the world.
During the medieval ages, being diagnosed with Diabetes was like a death sentence. The pioneers of diabetes treatment were Thomas Willis, Sushruta, and Arataeus (Mandal, 2021). The three were Greek physicians who encouraged people to exercise on horsebacks to prevent excess urination. They also described other therapies like overfeeding and taking wine to reduce starvation and excessive loss of fluids (Mandal, 2021). On the other hand, the ancient Indians would test for Diabetes by taking ants near a person’s urine. If the human urine attracted the ants, then the person would be diagnosed with urine (Mandal, 2021). Diabetes is a disease that is the leading cause of high blood sugar levels. People who have Diabetes have bodies that cannot make enough insulin, or their bodies cannot use the insulin they have effectively (Healthline, 2021). Insulin is the hormone that moves sugars from the blood to the body cells. There are several types of Diabetes, including type 1 diabetes, type 2 diabetes, gestational diabetes, prediabetes, and Diabetes insipidus (Healthine, 2021). All these types affect our bodies differently, and they all have different effects, hence different coping strategies.
The rationale for Choosing Diabetes
Diabetes is among the most severe health issues in the world. This is the reason why I chose to discuss it to create awareness about it. The bad thing with Diabetes is that one can get it and not know that they have it. By the time they realize that they have Diabetes, the condition is worse, and the person is highly affected. According to Genesis Medical Associates (2015), one out of three adults have higher blood sugar levels; a condition referred to as prediabetes. If the persons do not change their lifestyles, the sugar levels increase, leading to other types of Diabetes (Genesis Medical Associates, 2015). Learning about Diabetes will allow people to support each other in the fight against Diabetes. This includes eating healthy meals and maintaining a healthy lifestyle through exercising (Dowshen, 2021). Another reason why I chose to discuss Diabetes is to learn more about the causes and how to manage the disease. Since most people do not know about the condition, it is crucial to educate them so that in case they feel any symptoms, and they can get the treatment as early as possible (Dowshen, 2021). It is easy to deal with Diabetes as long as the signs are detected early enough and the patient follows the given guidelines on healthy living.
Epidemiology
Diabetes is a significant health concern since it affects so many people in the world. Diabetes can affect any person. However, some ethnic groups are affected more than others. The Alaska Natives and the American Indians are more affected by Diabetes as compared to all other ethnic groups. In terms of age, more than sixty-five years are more prone to getting diabetes than young people. According to Shaikh (2021), % of the people who are more than 65 years have diabetes. However, the young people are also affected but at a meager percentage compared to the older people.
The risk factors for Type 1 diabetes are hereditary, hence easily transferred from parents to children. Type 1 diabetes primarily affects young children and teenagers. Also, white Americans are at a higher risk of getting the disease than African Americans and Latino Americans (Shaikh, 2021). Type 2 diabetes affects middle and old age persons. Also, other risk factors for type 2 diabetes include genes, being overweight, a history of gestational pregnancy, and giving birth to a baby that is more than 9lbs (Shaikh, 2021).
It is important to note that diabetes is more prone in rural areas where people do not have access to health services and education. In the United Kingdom, 28% of the people with diabetes have issues obtaining medication due to a lack of health services and knowledge on how to go about diabetes treatment (Whicher et al., 2019 p.243). Besides, most of the people who are in the rural do not go for annual health checkups; hence their conditions get worse daily.
Assessment and assessment tools for Diabetes
Different tools are used during the assessment of diabetes. Assessing diabetes is very important as it helps differentiate between different types of diabetes and the extent of the condition. The Diabetes Prevention Screening Tool helps identify the persons at risk of getting diabetes (Diabetes Education Services, 2021). Such people are encouraged to join the CDC prevention program. There is also the Risk Test for Pre Diabetes patients to understand the risks they face as pre-diabetics (Diabetes Education Services, 2021).
The Diabetes Risk calculator is a tool that is used to detect undiagnosed diabetes and prediabetes. The social Support Assessment Tool helps diabetic patients to have a support system (Diabetes Initiative, 2020). Patients who have Diabetes need a lot of support from family and friends. The support shown will help them adhere to the doctor’s instructions, hence improving the chances of being better. Another assessment is the Mental Health Progress Report. The report is filled up during the patient’s follow-up visits. The assessment involves questions determining if the patient is affected by the condition mentally (Diabetes Initiative, 2020). It helps the doctors to guide the patient on how they can cope mentally with Diabetes.
Health Promotion Goals that you will like to Achieve
One of the goals that I would like to achieve is to reduce the high number of people diagnosed with Diabetes. I will encourage people to ensure they exercise at least thirty minutes a day to become physically fit. To make this goal achievable, I will create small groups that will act as support systems. This will help push people towards healthy living, preventing them from being diagnosed with the condition (Cecelia Health, 2021). My goal is realistic since it is easy to adopt a good eating habit and exercise at least thirty minutes daily. Still, it becomes easier when these activities are done in groups so that members feel motivated. To ensure that the goal is achieved, I will set a time frame of three months. Each member must have dropped at least 10 pounds within three months and managed to exercise at least 30 minutes daily, consistently.
The second goal is to enhance a better diabetes management program. Most people who have diabetes do not know what they should avoid, while others ignore the advice given to them by the doctors. In this case, I will form a group of people of different ages who are diabetic. The group formed will be a support system that will help each other cope with Diabetes. I will encourage the group members to remain healthy by eating the right food and exercising daily (McDermott, 2020). For those that are older, they can do simple exercises like jogging and walking a few kilometers daily. After five months, I will assess each patient’s changes in sugar levels and the general healthcare status (McDermott, 2020). I expect the sugar levels to be expected or close to normal for most patients within this period. Besides, the patients will have adapted to the new lifestyle since they got used to it.
Interventions for your health promotion goals
As indicated above, the first goal is to reduce the high numbers of people diagnosed with diabetes. The first health intervention is by ensuring that people are engaging in vigorous activities and exercises. Before one retires to bed, they must ensure that they have done a bit of practice to increase the metabolic activities of their bodies (Harvard T.H CHAN, 2021). Exercising helps maintain a moderate weight; hence, the high obesity and overweight people will reduce significantly. Besides, exercise helps increase insulin sensitivity in the body. As a result, the body cells can consume the sugars that are in the bloodstream.
For this intervention to work, both individuals and groups work together. A person must know that they have a personal responsibility to ensure that they maintain healthy body weight. Besides, organizations can play a significant role by ensuring that they create team-building activities (Harvard T.H CHAN, 2021). Organizations can set a day or two per month whereby all the employees and employers are involved in various team-building activities. This will help to ensure that at least all members keep fit, even if some of the members might not be keeping fit at a personal level. Since young people are also at a very high risk of getting diabetes, schools should develop a schedule to see all the students engage in exercise activities (John Muir Health, 2021). For example, the school can decide to have a physical exercise lesson after every two days.
Another intervention that will see few people being diagnosed with diabetes is maintaining a healthy eating lifestyle. Most people, especially teenagers, eat food that is full of calories. First, one should ensure they increase the fiber intake (Science Daily, 2018). Fiber is essential as it helps to slow down the digestion of carbs and sugars. Foods that contain more fibers include legumes, vegetables, and whole grains. Too many carbs place a person at a very high risk of getting diabetes. Another healthy eating habit is taking plenty of water to stay hydrated at all times (John Muir Health, 2021). When one takes a lot of water, it also helps the kidney eliminate excess sugars through the urine (Science Daily, 2018). A well-hydrated person is at a lower risk of getting diabetes. However, one should avoid sugar-sweetened drinks as they raise the level of glucose in the blood.
Both individuals and organizations have a role to play when it comes to maintaining a healthy eating lifestyle. Families should ensure that they prepare meals that are balanced diet. As an individual, one has a choice to eat whatever they want. Following this, one should avoid taking foods with high carb content instead of increasing the intake of high fiber meals. Organizations should also participate in this intervention by preparing healthy meals for their employees (Science Daily, 2018). Communities should be encouraged to grow more fibers and take the origin foods rather than rely on ready-made foods with high calories. Also, schools can be involved by ensuring that they have a reasonable timetable for all the meals, and the fiber intake for each student should be higher than the carb intake.
The second goal is enhancing better management for people who are living with diabetes. Individuals have a tremendous responsibility to ensure that they follow the given guidelines to stabilize sugar levels efficiently. As a diabetic patient, one should know the type of diabetes they are suffering from and the measures they are supposed to take to become better (NIH, 2021). The first step that a diabetic person should take is to ensure that they are not stressed. Stress triggers sugar levels, hence raising them. To reduce stress triggers, one can listen to their favorite music, take a walk, breathing in and out, or doing their favorite activities (Diabetes UK, 2021). Also, a person needs to have a support system to reach out in case they feel stressed.
The second step that one can take to deal with diabetes is ensuring that they eat well. After being assessed by the doctor, a health care team should help the sick person come up with a meal plan (Diabetes UK, 2021). The meal plan should contain fewer calories, fewer sugars and salt, and high saturated fats. Also, a diabetic person should eat foods that have high fiber, like rice and bread. Instead of drinking sweetened juices, a diabetic person should ensure that they take plenty of clean drinking water. This helps to keep the body hydrated at all times.
Both individuals and groups have a significant role in ensuring that diabetic persons are taken care of. They have the necessary things needed for them to reduce sugar levels. Health facilities should make sure that they do follow-ups so that if a patient has forgotten to go for checkups, they can go upon being reminded. Besides, other organizations like NGOs should develop fiber for needy people who might not afford such things.
Evaluation of your Health Promotion Care
Maintaining a healthy lifestyle through exercise is not hard to achieve as long as the people involved know the benefits of exercising. Exercising is an effective strategy that will help prevent diabetes and prevent other diseases like heart attack and stroke (Diabetes UK, 2021). However, people should be allowed to choose the kind of exercise that they want to do. Instead of going for a run, one can engage in other activities like playing football, netball, or swimming (Harvard T.H CHAN, 2021). Since people are not the same, one should not be forced to go for a morning jog, yet they like swimming. If this is done, the exercises will be more effective since people will be doing them willingly. I would recommend that the government makes it paramount for organizations to have different days from engaging in other activities like swimming, running, jogging, etc. Also, schools should ensure that there are various exercises for all the students to have one or two activities that they can engage in easily.
The second promotion of care was encouraging people to eat healthy meals. From the above discussion, it is evident that people need to engage in healthy lifestyles. Whether a person has diabetes or not, engaging in a healthy lifestyle is very important (Science Daily, 2018. Following this, one should ensure that they avoid high calories and have high fibers. This healthcare plan can be effective only if the government and other non-governmental organizations are willing to provide the proper meals for the people in need. Some diabetic people do not have access to medical care; hence they cannot do follow-ups about their conditions. As a result, the health care plan will become hard to achieve if the doctors and health care workers do not follow up on their patients to ensure they have taken the right medicines and that the sugar levels are not increasing (John Muir Health, 2021. For this, I would recommend that treatment of diabetes becomes free of charge in all public healthcare institutions. This will make it easy for the poor diabetic people to go for checkups since they know they will not be asked for any money to get the services they need. During the Diabetes Awareness week in the country, the government led by the health care sector should ensure that people are educated about diabetes. This will help people learn more about it and engage in activities that will help reduce diseases.
Tannahill Health Promotion Model
The Tannahill Health Promotion Model helps in the prevention of diabetes and protection of people who have diabetes. As discussed above, diabetes can be prevented through eating the right foods and ensuring that one is physically fit. The Tannahill Health promotion strategy also suggests a good communication flow between the patient and the health care providers (Queens University Belfast, 2021). In this case, the healthcare providers should do the follow up’s for their patients. The third aspect of the Tannahill Health promotion program is that the citizens should be given health protection through the legislature, social measures, and financial measures (Queens University Belfast, 2021). This includes helping needy people eat healthy meals and ensuring that organizations and companies give their employees the proper meals. Besides, Companies, organizations, and schools should set aside specific days where each person is engaged in other activities like swimming, ring, and playing their favorite games.
Diabetes is indeed one of the most severe diseases in the world. Diabetes affects both the young and the old and people of all ages. Although people at the age of 65 and older are more prone to being diagnosed with diabetes, other factors also determine if a person is prone to getting diabetes (Healthline, 2021). For example, a child can get diabetes from their parents; hence they get hereditary diabetes. Women who have experienced gestational diabetes are also at a very high risk of contracting the disease again (Shaikh, 2021). People who are not physically fit are also prone to getting diabetes. Following this, it is evident that although some people are more prone to getting diabetes, several other factors play a significant role.
Although diabetes is a severe condition worldwide, it can be controlled and the high rates reduced. This can be achieved through two maintaining it; exercising and eating suitable meals. Since some people cannot afford the healthy diet recommended for diabetic people, the government and other non-governmental organizations can provide such meals to the people (Whicher et al., 2019 p.243. Also, ensuring that the medication services are accessible at the public hospitals will encourage most people to go for follow-ups. Exercising is easy since there are so many activities that help burn calories (Shaikh, 2021). That is why it is essential to let the person choose activities they are good at and concentrate on them. Generally, although diabetes is a serious condition, it is easy to prevent and manage it if all resources are available.
Cecelia Health, 2021. How to Set and Achieve SMART Goals — in Life and Diabetes – Cecelia Health . [online] Cecelia Health. Available at: <https://www.ceceliahealth.com/how-to-set-and-achieve-smart-goals-in-life-and-diabetes/> [Accessed 1 June 2021].
Diabetes Education Services, 2021. Screening Tools for Diabetes – Diabetes Education Services . [online] Diabetes Education Services. Available at: <https://diabetesed.net/screening-tools-for-diabetes/> [Accessed 1 June 2021].
Diabetes Initiative, 2020. Tools: Assessment Instruments . [online] Diabetesinitiative.org. Available at: <http://www.diabetesinitiative.org/resources/type/assessmentInstruments.html> [Accessed 1 June 2021].
Diabetes UK, 2021. 10 Tips for Healthy Eating with Diabetes . [online] Diabetes UK. Available at: <https://www.diabetes.org.uk/guide-to-diabetes/enjoy-food/eating-with-diabetes/10-ways-to-eat-well-with-diabetes> [Accessed 1 June 2021].
Dowshen, S., 2021. Diabetes Control: Why It’s Important (for Teens) – Nemours KidsHealth . [online] Kidshealth.org. Available at: <https://kidshealth.org/en/teens/diabetes-control.html> [Accessed 1 June 2021].
Genesis Medical Associates, 2015. The Importance Of Understanding And Preventing Diabetes – Genesis Medical Associates, Inc . [online] Genesismedical.org. Available at: <https://www.genesismedical.org/blog/the-importance-of-understanding-and-preventing-diabetes> [Accessed 1 June 2021].
Harvard T.H CHAN, 2021. Simple Steps to Preventing Diabetes . [online] The Nutrition Source. Available at: <https://www.hsph.harvard.edu/nutritionsource/disease-prevention/diabetes-prevention/preventing-diabetes-full-story/> [Accessed 1 June 2021].
Healthline, 2021. Everything You Need to Know About Diabetes . [online] Healthline. Available at: <https://www.healthline.com/health/diabetes#:~:text=Diabetes%20mellitus%2C%20commonly% 20known%20as,the%20insulin%20it%20does%20make.> [Accessed 1 June 2021].
John Muir Health, 2021. Preventing Diabetes . [online] Johnmuirhealth.com. Available at: <https://www.johnmuirhealth.com/health-education/conditions-treatments/diabetes-articles/preventing-diabetes.html> [Accessed 1 June 2021].
Mandal, A., 2021. History of Diabetes . [online] News Medical. Available at: <https://www.news-medical.net/health/History-of-Diabetes.aspx#:~:text=The%20term%20diabetes%20was%20probably,sweet%20taste%20of%20the%20urine.> [Accessed 1 June 2021].
McDermott, A., 2020. 7 Long-Term Goals for Better Diabetes Management . [online] Healthline. Available at: <https://www.healthline.com/health/type-2-diabetes/living-better-with-type-2-diabetes/long-term-goals-everyone-with-type-2-diabetes-should-make> [Accessed 1 June 2021].
NIH, 2021. 4 Steps to Manage Your Diabetes for Life | NIDDK . [online] National Institute of Diabetes and Digestive and Kidney Diseases. Available at: <https://www.niddk.nih.gov/health-information/diabetes/overview/managing-diabetes/4-steps> [Accessed 1 June 2021].
Queens University Belfast, 2021. Health Promotion. [online] Queens University Belfast. Available at https://www.qub.ac.uk/elearning/public/HealthyEating/HealthPromotion/ [Accessed 1 June 2021]
Science Daily, 2018. Physical exercise reduces the risk of developing diabetes, study shows . [online] ScienceDaily. Available at: <https://www.sciencedaily.com/releases/2018/02/180220102420.htm> [Accessed 1 June 2021].
Shaikh, J., 2021. What Population Is Most Affected by Diabetes? . [online] MedicineNet. Available at: <https://www.medicinenet.com/what_population_is_most_affected_by_diabetes/article.htm> [Accessed 1 June 2021].
Whicher, C., O’Neill, S., and Holt, R., 2019. Diabetes in the UK: 2019. Diabetic Medicine , [online] 37(2), pp.242-247. Available at: <https://onlinelibrary.wiley.com/doi/epdf/10.1111/dme.14225> [Accessed 1 June 2021].
Cite this page
Similar essay samples.
- Journal article.
- The Doctor Movie Review
- Formula One constructor attributes – a case study
- Focus on the Learner – English as a Foreign Language: Student Case S...
- Essay on Literature Review on Marketing Strategy and Firm Performance
- Handling and Managing Commercial Projects and Contracts in the Second ...
Advertisement
Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes
- Open access
- Published: 23 August 2023
- Volume 12 , pages 679–694, ( 2023 )
Cite this article
You have full access to this open access article
- Janet Antwi ORCID: orcid.org/0000-0002-8270-7717 1
2944 Accesses
2 Citations
2 Altmetric
Explore all metrics
Purpose of Review
Existing dietary and lifestyle interventions and recommendations, to improve the risk factors of obesity and type 2 diabetes with the target to mitigate this double global epidemic, have produced inconsistent results due to interpersonal variabilities in response to these conventional approaches, and inaccuracies in dietary assessment methods. Precision nutrition, an emerging strategy, tailors an individual’s key characteristics such as diet, phenotype, genotype, metabolic biomarkers, and gut microbiome for personalized dietary recommendations to optimize dietary response and health. Precision nutrition is suggested to be an alternative and potentially more effective strategy to improve dietary intake and prevention of obesity and chronic diseases. The purpose of this narrative review is to synthesize the current research and examine the state of the science regarding the effect of precision nutrition in improving the risk factors of obesity and type 2 diabetes.
Recent Findings
The results of the research review indicate to a large extent significant evidence supporting the effectiveness of precision nutrition in improving the risk factors of obesity and type 2 diabetes. Deeper insights and further rigorous research into the diet-phenotype-genotype and interactions of other components of precision nutrition may enable this innovative approach to be adapted in health care and public health to the special needs of individuals.
Precision nutrition provides the strategy to make individualized dietary recommendations by integrating genetic, phenotypic, nutritional, lifestyle, medical, social, and other pertinent characteristics about individuals, as a means to address the challenges of generalized dietary recommendations. The evidence presented in this review shows that precision nutrition markedly improves risk factors of obesity and type 2 diabetes, particularly behavior change.
Similar content being viewed by others
Precision nutrition in diabetes: when population-based dietary advice gets personal
Jordi Merino

Nutrition in Lifestyle Medicine: Overview

Metabotyping for Precision Nutrition and Weight Management: Hype or Hope?
Kristina Pigsborg & Faidon Magkos
Avoid common mistakes on your manuscript.
Introduction
Obesity and diabetes have emerged as enormous public health problems not only in the USA but also globally. Diabetes is a significant global challenge to the health and well-being of individuals and societies [ 1 ]. With a continued global increase in diabetes, the current prevalence of 537 million adults living with diabetes is projected to rise to 643 million by 2030 [ 1 ]. In the USA, an estimated 37.3 million people have diabetes, of which 90–95% of cases, including children, adolescents, and young adults are attributed to type 2 diabetes [ 2 , 3 , 4 ]. Diabetes data and trends for 2019 available at the Centers for Disease Control and Prevention indicated that diabetes is the sixth leading cause of death, and number one cause of kidney failure and lower limb amputation [ 3 , 4 ]. Obesity is the strongest risk factor for the development of type 2 diabetes [ 5 , 6 , 7 ]. Thus, the burden of type 2 diabetes is increasing in parallel to increasing cases of obesity [ 8 ]. Clinical data show that of the people diagnosed with type 2 diabetes, about 80–90% are highly likely to be diagnosed as obese [ 9 , 10 , 11 , 12 ]. The associated medical expenses of obesity and type 2 diabetes are steep. Obesity costs the US health care system nearly $173 billion a year [ 13 , 14 ], while the total estimated economic burden of type 2 diabetes was $327 billion in medical costs and lost productivity [ 15 ].
Both obesity and type 2 diabetes have related multifactorial etiology, making them highly complex diseases and investment in their effective prevention and management has become necessary to tackle this global epidemic. While obesity and type 2 diabetes have traditionally been studied to be diseases of energy imbalance, other risk factors such as high body weight and fat, dyslipidemia, high blood glucose, and insulin resistance are also involved in the etiology [ 16 , 17 , 18 ]. Unhealthy diet characterized by foods high in fat, sugars, and calories, but low in plant-based sources, and lack of physical activity are now considered top risk factors for the development and progression of obesity and type 2 diabetes [ 19 ]. Thus, improving dietary intake and physical activity is a global priority [ 20 ].
Dietary recommendations and public health campaigns for tackling risk factors of obesity and type 2 diabetes have focused on using population averages, have been based on generalized advice, or have been poorly adhered to [ 21 , 22 , 23 , 24 ]. Moreover, there have been great challenges with the validity, consistency, and reproducibility of dietary assessments [ 25 ]. Because obesity and type 2 diabetes are heterogeneous diseases from the pathophysiological, genetic, and clinical perspectives, and there is dramatic inter-individual variability in response to any therapeutic diet or physical activity regime, there is a need to shift to or complement the population perspective with patient-centric interventions [ 26 , 27 , 28 ]. These variabilities are attributed to differences in genetics, biomarkers of metabolic pathways, gut microbiome, environmental, physiological, behavioral, social, and economic factors. Given the substantial burden of obesity and its related comorbidities, research and practice efforts should adopt a holistic approach for sustainable solutions in preventing and treating the obesity and type 2 diabetes epidemic [ 9 ].
Precision nutrition (or personalized nutrition) has emerged as a new area of lifestyle intervention that allows dietary recommendations to be tailored at the individual level through integration of demographic information, lifestyle-based information (e.g., dietary intake, and physical activity), phenotype-based information (e.g., anthropometrics, and standard clinical biomarkers of disease risk), and gene- and omics-based information (e.g., genetic testing of single nucleotide polymorphisms, and gut microbiome) (Fig. 1 ) [ 29 , 30 ]. The current use of nutrigenetics, metabolomics, and metagenomics in precision nutrition enables the holistic interrogation of dietary and lifestyle factors to objectively assess risk factors of obesity and type 2 diabetes. The identification of various genes and polymorphisms has been determined as the basis for the interpersonal variability in metabolic response to specific diets [ 31 , 32 , 33 ]. Metabolomics investigates, among other things, the effect of food-derived biomarkers metabotypes variation among individuals in metabolizing the same diets in health and disease states for customized dietary interventions through metabolic patterns [ 34 ]. The identification of metabolites of food intake to serve as target of nutrition intervention makes metabolomics have potential to improve the accuracy of dietary assessment [ 35 ]. Metagenomics is vital in precision nutrition because it can be used to comprehensively analyze the diet-microbiome interaction to identify various metabotypes that characterize metabolic risk and tailor dietary intervention approaches for improved health [ 36 ].
Components of the precision nutrition approach. The individual characteristics of demographic, phenotype, lifestyle, genetic, and omics information are incorporated into the precision nutrition intervention to address the interpersonal variabilities in response to general nutrition intervention and recommendations to improve the risk factors of obesity and type 2 diabetes
It is suggested that precision nutrition interventions could result in greater weight loss and blood glucose control than non-personalized strategies [ 37 , 38 ]. In personalizing nutritional advice, there is evidence that people are more motivated to make appropriate behavioral changes [ 39 , 40 ]. The interest in precision nutrition has not only significantly increased in the scientific community [ 41 ], but is already becoming more accessible to consumers, largely through self-administered test-kits coupled with diet plans and subscription programs [ 41 , 42 , 43 ]. Thus, precision nutrition has been identified as the individualized solution to prevent and manage obesity and type 2 diabetes in lieu of the population-based dietary interventions, whose effectiveness in reducing the risks of these conditions using the “one-way diet” approach for all individuals is questionable [ 44 ].
The purpose of this review is to examine the current state of the science regarding precision nutrition in improving the risk factors of obesity and type 2 diabetes with emphasis on studies that included more than one component of precision nutrition and not only genetic testing to provide individualized/personalized dietary advice. While progress has been made on the quantity of research focused on precision nutrition, reviews discussing particularly behavior change and changes in nutrient/diet quality and physical activity as part of a comprehensive analysis of the utility of precision nutrition intervention and its outcomes are lacking.
- Nutrigenetics
Nutrigenetics is considered the foundation of precision nutrition (Table 1 ) [ 45 , 46 ]. Genetic variation in the form of single nucleotide polymorphisms (SNPs) is considered to account for the heterogeneity in individual dietary response and risk for obesity and type 2 diabetes [ 47 , 48 ]. Nutrigenetic research has investigated the interactions between SNPs influencing body composition, insulin signaling, and dietary factors in relation to adiposity and glucose homeostasis in obesity and type 2 diabetes. In an observational study, a genetic risk score-diet interaction used to provide precision nutrition based on 16 SNPs related to obesity or lipid metabolism demonstrated its value in obesity prediction. Specifically, in individuals carrying > 7 risk alleles, there was higher body mass index (BMI), body fat mass, waist circumference, and waist-to-hip ratio more than the individuals with ≤ 7 risk alleles [ 49 ]. Additionally, there was a significant interaction between genetic risk score and the macronutrient intake used in personalized intervention. Similarly, a systematic review and meta-analyses and two observational studies reported genetic interactions with specific macronutrients, that is, carbohydrate [ 50 ], fat [ 51 ], and protein intakes, respectively [ 52 ]. SNPs in the apolipoprotein A1 and C3 ( APOA1 and APOC3 ) genes and cluster of differentiation 36 ( CD36 ) gene led to increased risk of metabolic syndrome in subjects with Western dietary pattern and dyslipidemia in individuals who consumed high amounts of fat, respectively. Two randomized controlled trials (RCT) showed that personalized prescription of energy-restricted diets (low-fat and moderately high-protein) based on 95 different genetic variants related to energy homeostasis, phenotypic, and environmental factors was associated with differential adiposity outcomes, with waist circumference and total body fat loss particularly among obese subjects who carried the Peroxisome Proliferator Activator Receptor Gamma Coactivator 1 ( PPARGC1A Gly482Gly) genotype [ 53 ••, 54 ]. In an observational prospective cohort design from the RCT, Prevención con Dieta Mediterránea (PREDIMED), the investigators concluded that genetic predisposition to type 2 diabetes associated with the Transcription Factor 7-Like 2 Gene [ TCF7L2 gene (rs790314 TT)] homozygosity could be counteracted through precision nutrition interventions with the Mediterranean diet [ 55 ]. While precision nutrition effectively addresses the genetic variability in nutrient metabolism, and other physiological processes among individuals, it was found in a parallel-group, pragmatic, RCT that providing nutrigenetic information and advice for management could help reduce body fat percentage up to 6 months, and reductions in body fat were similar to the standard weight loss intervention after 12 months. The clinical implications of this study are that the genetic-based precision nutrition approach should be considered for use for clinical cases which require short- to long-term body fat loss, particularly for individuals needing that to undergo surgery or transplant [ 56 ]. The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) RCT was conducted to determine the impact of precision nutrition on fasting glucose, fasting insulin, hemoglobin A1C (HbA1C), insulin resistance, and β cell function. The precision nutrition diet varied in macronutrient composition and was investigated with type 2 diabetes genetic risk scores on these parameters of glucose metabolism. At 2 years of intervention, low-protein diet responses significantly interacted with lower genetic risk score and greater decreases in fasting insulin, HbA1C, insulin resistance, and a lesser increase in β cell function, compared to those with a higher genetic risk score [ 57 ]. A post hoc analysis of the POUNDS LOST RCT showed that in response to high-fat diets, participants with the highest genetic risk score showed increased fasting glucose, insulin resistance, and decreased insulin sensitivity at 6-month follow-up than those with low-fat diets [ 58 ]. The influence of genetic factors and nutrient-gene interactions in precision nutrition applications has been indicated by twin studies. In the Personalized Responses to Dietary Composition Trial (PREDICT) RCT [ 59 ••], a large inter-individual variability in postprandial blood glucose and insulin responses was observed following the same meals among 1002 twins and unrelated healthy adults in the UK. Genetic variants had modest impact on predictions of glucose, triglycerides, and C-peptide. These results were independently validated among 100 US adults. In addition, a machine learning algorithm predicted these variabilities to precision nutrition. An observational retrospective pre/post comparison of digital twin-enabled precision nutrition therapy was used to examine diabetes reversal [ 60 ••]. The authors reported diabetes reversal (that is, achieving HbA1C < 6.5% at least 3 months after stopping antidiabetic medications) during 90 days of precision nutrition therapy at varying rates of subgroups of obese and non-obese type 2 diabetes patients. Baseline data showed that only 9.5% of patients were in reversal stage 4 or better; however, over the first 90 days, 82.1% achieved advanced stages of reversal with improved clinical outcomes and fewer pharmacotherapy. Furthermore, a retrospective study reported that there was a decrease in HbA1C, body weight, fasting blood glucose, and insulin resistance at 90-day follow-up assessment [ 61 ]. In contrast, a prospective RCT [ 62 ] that randomized overweight or obese individuals to receive a nutrigenetic-based precision nutrition diet or standard balanced diet reported no difference in weight loss between the two groups. However, the results highlight the need for larger macronutrient differences between groups and adherence to the recommended intervention diet plan. Further research should be conducted to provide new data and make the use of genetic-based precision nutrition management in the clinical setting more effective [ 62 ]. Studies on diet-gene interactions among non-Caucasians are limited. In a prospective cohort study of Hispanics of Caribbean origin who were genotyped for the Perilipin SNP [ PLIN 11482G > A (rs894160)] to determine whether dietary macronutrients modulated the associations of the SNP with obesity (measured as BMI, waist and hip circumference), the investigators found that the minor allele was protective against obesity for subjects who consumed higher complex carbohydrate, whereas among those with lower complex carbohydrate intake, the minor allele was linked with increased risk of obesity [ 63 ].
- Metabolomics
Metabolomics, an emerging technology which encompasses comprehensive analysis of metabolites, holds promise to inform precision nutrition recommendations (Table 1 ) [ 64 ]. The various metabolites produced from metabolism of dietary factors have been used to characterize metabolic phenotypes or biomarkers that can be used for individual stratification. This metabolic specificity enables precision nutrition to resolve metabolic derangements that underlie obesity and type 2 diabetes [ 34 ]. Additionally, metabotyping which stratifies individuals with metabolic similarity into metabotype subgroups using their metabolic and phenotype patterns could be used for population stratification to customize dietary interventions [ 65 ]. Earlier studies that paved the way for the use of metabolomics in precision nutrition showed that dietary intake patterns were revealed in metabolomic profiles [ 66 ], and were associated with biomarkers such as high levels of lipid metabolites, amino acids, and ferritin that mediated red meat consumption and risk of type 2 diabetes [ 67 ]. Recently, a study analyzed blood metabolites using metabolomics among normoglycemic healthy adults to predict the risk of developing type 2 diabetes. A web-based platform interventional study was used to deliver precision nutrition intervention based on the blood metabolites health risk score to lower the blood metabolites to normal levels for 40 participants. A follow-up assessment of the blood metabolites showed significant reductions in the health risks associated with the development of type 2 diabetes, insulin resistance, and related comorbidities [ 68 •]. A replication of the study through observational longitudinal analysis in a larger cohort of 1000 US adults demonstrated similar positive results with the precision nutrition intervention given based on biomarkers measured through metabolomics [ 69 ]. Bouwman et al. [ 70 ] in a double-blind placebo-controlled cross-over design used a health space model to visualize the effect of personalized nutrition intervention on metabolic stress profile including inflammatory and oxidative processes associated with obesity and type 2 diabetes. After following the recommendations for 5 weeks, the 145 metabolites and 79 proteins measured prior and before treatment were able to distinguish modulation of metabolic stress and specific oxidative and inflammatory response to treatment. Fiamoncini et al. [ 71 ] in an experimental design identified 2 metabotype clusters and tested their responses to a personalized nutrition intervention over a 12-week weight loss program. The researchers reported that only the study participants with higher disease-linked metabotype demonstrated improvements in glucose and insulin levels when fed a low caloric diet. They concluded that through the application of metabolomics in precision nutrition advice, a responsive and non-responsive metabotype was revealed. In the DIRECT (Dietary Intervention Randomized Controlled Trial) trial, personalized weight-loss diets decreased circulating amino acid metabolites that were associated with risk of type 2 diabetes, and improved insulin resistance. In addition, the reduction in the level of circulating amino acid metabolites which is indicative of an increase in insulin sensitivity was independent of weight loss [ 72 ]. Walford and colleagues performed plasma metabolite profiling to elucidate new pathways of type 2 diabetes incidence and the role of personalized nutrition interventions in a nested case–control design [ 73 ]. Dietary and lifestyle modifications based on the metabolites effectively raised betaine concentration from baseline to 2-year follow-up, which predicted lower risk of type 2 diabetes. Interestingly, a 10-week RCT that allocated 100 overweight and obese adults to a personalized diet and control diet based on their metabolomic and genetic information did not show significant difference between groups in fat mass; however, the individual diets produced significant improvements in insulin resistance and lipid profile, which was not significantly different between groups. The soundness of various precision nutrition approaches is required to translate such findings into clinical relevance [ 74 •].
- Metagenomics
Metagenomics is the comprehensive study of host microbial and their genetic material (Table 1 ) [ 75 ]. The role of the gut microbiota in obesity and type 2 diabetes has been underscored, and this has been an area of immense research [ 76 ]. It is believed that the metabolism of dietary compounds into other metabolites by the gut microbiota, which is associated with disease risk, mediates the impact of the gut microbiota on human health [ 77 , 78 , 79 ]. For example, the metabolism of dietary fibers and resistant starches into bacterial metabolites of short-chain fatty acids such as acetate, propionate, and butyrate presents a mechanism that modulates the pathways involved in obesity, insulin resistance, and type 2 diabetes [ 80 ]. Studies show that the diet-gut microbiota interactions vary in composition and functionality among individuals [ 81 ], and this appears to be a determinant to integrate metagenomics into precision nutrition [ 36 ]. Pioneering work by Zeevi et al. [ 82 ] in an observational study and blinded randomized controlled dietary intervention showed that postprandial glucose responses have high interpersonal variability even when individuals consumed identical standardized diets. The authors further used a machine learning algorithm that integrated dietary habits, blood parameters, anthropometrics, physical activity, and gut microbiota features for precision nutrition recommendations in the 800 person cohort. The precision nutrition recommendations accurately predicted personalized postprandial glucose response to the recommendations and resulted in significantly lower glucose levels and consistent alterations in gut microbiome. In modifying and extending the model created by Zeevi and colleagues, two cohort studies that evaluated the utility of such precision nutrition approaches to predict postprandial glucose responses found that across the cohort of non-diabetic adults that were examined, a personalized model was more predictive than current models of carbohydrate content [ 83 , 84 ]. Similarly, Kovatcheva-Datchary et al. [ 85 ] in a cross-over study demonstrated that among 39 healthy Swedes, improved postprandial glucose metabolism was in those with statistically significant higher ratio of Prevotella/Bacteroides spp., following an intervention of 3-day consumption of barley kernel bread diet. Another RCT demonstrated through metagenomic analysis and a dietary weight loss intervention that compared to individuals with a low bacterial ratio, subjects with a high Prevotella/Bacteroides genera ratio lost more weight and body fat in response to high-fiber diets [ 86 ]. In a sub-study of a larger RCT, researchers examined whether the baseline composition and diversity of gut microbiota was associated with weight loss in a sample of 49 participants. Findings from the study showed that baseline gut microbiota composition was not associated with weight loss; however, there were substantial changes in gut microbiota in response to each diet, 3 months after initiating the intervention. The changes were attributed specifically to the healthy low-carbohydrate diet used in the intervention, although the changes were attenuated after 12 months [ 87 ]. Another important step in the use of metagenomics in precision nutrition was the work conducted by Vangay et al. [ 88 ] in an observational study that provided valuable insight into differences in population groups that requires racial considerations and sociocultural influences when employing precision nutrition approaches. In this study, Karen and Hmong natives residing in Thailand and the USA as well as European Americans born in the USA were assessed for the impact of migration to the USA on the gut microbiota in development of metabolic diseases such as obesity. After metagenomic DNA sequencing, the investigators found that US immigration rapidly depleted gut microbiota diversity and function and was replaced by US-associated strains and functions, and was exacerbated by obesity. These results were confirmed in a prospective cohort study that used similar metagenomic approaches of 16S and deep shotgun DNA sequencing among 144 Chinese individuals in Shanghai. A long-term healthy diet intervention was associated with greater diversity of Tenericutes , Firmicutes , and Actinobacteria , with or without adjustment for BMI [ 89 ]. Data from an RCT of an integrative model using gut microbiota and genetic information to personalize weight loss prescription among 190 Spanish overweight and obese participants suggested that the mixed models’ microbiota scores facilitated the selection of the optimal diet in 84% of men and 72% of women for weight loss [ 90 ••].
Behavioral (Dietary Patterns, and Physical Activity) Aspects of Precision Nutrition
Healthy behaviors (e.g., consuming a healthy diet and engaging in regular physical activity) are associated with the incidence of morbidity and mortality of chronic diseases including obesity and type 2 diabetes [ 91 ]. Behavior change components that may be beneficial to improve adoption of healthier options are goal setting, social interactions, and customized messages [ 92 , 93 ]. Diet and physical activity behaviors are the strongest risk factors for obesity and type 2 diabetes prevention and outcomes [ 94 ]. Given this crucial role of behavior in preventing and treating chronic diseases, it is important to assess behavior change in dietary patterns and physical activity for improvement. The 2019 global burden of disease study reported that among the 3 largest increases in risk exposure for disability-adjusted life years (DALYs) lost across the world, 2 were high BMI and high fasting plasma glucose, and 6 of the top 10 causes of DALYs are due to poor health behaviors, including unhealthy dietary patterns and low physical activity levels [ 95 ]. Diet quality which represents the nutritional adequacy of a diet with varied nutrient composition, measured by how closely dietary patterns are within core nutrient-dense food groups, is a higher priority than the quantity of dietary intake [ 96 , 97 , 98 , 99 ]. In a systematic review of prospective cohort studies, a strong association was found between poor diet quality and greater weight gain, irrespective of gender [ 100 ]. In addition, higher diet quality is demonstrated in several studies to be associated with chronic disease risk, cause-specific mortality, and all-cause mortality [ 101 , 102 , 103 ]. Diet quality in the USA remains far from optimal and for all Americans, the average diet quality measured by the Healthy Eating Index (HEI) score is 58, which is far from the maximum of 100 points [ 104 ]. The top dietary risk factors in the USA are diets low in fruits, vegetables, whole grains, nuts, and legumes, and high in refined grains, red or processed meats, sodium, saturated and trans fats, and sugar-sweetened beverages [ 21 , 105 , 106 , 107 ]. The transition from heavy labor to sedentary livelihoods, increased screen time, decrease in school physical education, and improved transportation has been implicated in the decline in physical activity levels [ 18 , 107 ]. Studies show that moderate to vigorous-intensity physical activity such as walking or running is necessary for optimal health. A systematic review and meta-analysis of prospective cohort studies [ 108 ] reported that individuals who engaged in the minimum recommended amount of physical activity had potentially significant benefits to reduce the risk for type 2 diabetes by 26%, compared with inactive individuals. Thus, improvement in diet and physical activity signifies a huge potential for obesity and type 2 diabetes reduction either directly or indirectly through improvements in weight gain and blood glucose levels. It has been suggested that conventional dietary advice does not have as big of an impact on improving dietary health as expected [ 109 , 110 ].
Precision nutrition interventions have demonstrated encouraging changes in dietary behaviors (Table 1 ). Precision nutrition studies that reported on behavior changes observed as healthy dietary patterns found that optimizing dietary patterns through individualized care improves management of obesity and type 2 diabetes [ 111 , 112 , 113 ]. For example, a randomized controlled trial that provided personalized nutrition advice using individualized information on diet and lifestyle, phenotype and/or genotype, produced larger, more appropriate, and sustained changes in dietary behavior to healthier diet as food groups compared to a conventional approach. Study participants in the precision nutrition group consumed less red meat, salt, and saturated fat, increased folate intake, and had higher HEI scores [ 114 ]. In line with these results, another RCT [ 115 ] that considered application of a dietary pattern technique instead of individual food items in isolation has reported that the use of precision nutrition enhanced dietary behavior changes associated with higher Mediterranean-style diet scores. The Mediterranean diet, characterized by high intakes of fruit and vegetables and low intakes of sugar-sweetened beverages and snacks, has been consistently linked with a beneficial effect on health, including obesity and type 2 diabetes [ 116 , 117 , 118 ]. Thus, it is strongly suggested that changing dietary intakes so as to align more appropriately with the Mediterranean diet would yield extensive public health benefit [ 119 ]. Through post hoc analyses, findings of the study further supported the importance of personalized nutritional advice which, when done with increased frequency, promoted sustained changes in dietary behavior and larger improvements in overall diet quality [ 120 ]. The changes in behavior of dietary patterns through the implementation of precision nutrition recommendations have also been associated with reduced intake of calories, carbohydrates, sugar, total fat, and saturated fat which correlated with significant weight loss, reduced waist circumference, and increased high density lipoprotein (HDL), decreased total cholesterol and low density lipoprotein (LDL) with improved glucose levels through observational studies, single-arm, multi-phase, open-label exploratory trial, and retrospective analysis of an RCT [ 121 , 122 ••, 123 , 124 ]. A pretest–posttest pilot study that organized a personalized dietary advice in a real-life setting found that dietary quality measured by the Dutch Healthy Diet Index was significantly improved compared with baseline. In addition, this research revealed that personalized dietary advice resulted in positive effects in self-perceived health in motivated pre-metabolic syndrome adults. Because the study was performed in the real-life setting (do-it-yourself), it highlighted the potential of at-home health behavior improvement through dietary changes [ 125 ]. The EatWellUK is another RCT that attests to the advancement of precision nutrition research beyond the USA. The authors of this research reported that an automated precision nutrition advice via a mobile web app was effective to elicit beneficial dietary change, improve diet quality, and increase engagement in healthy dietary behaviors in UK adults, relative to general population-based dietary guidelines [ 126 ••]. Similarly, other precision nutrition interventions found behavior change in dietary intake which favored healthier choices and increase in diet quality irrespective of the setting and/or platform used for delivery of the intervention, as well as measure used to assess diet quality score [ 127 , 128 ••]. Short-term dietary behavior changes are usually very short lived, thus long-term compliance to dietary behavior change should not be compromised because it is crucial in maintaining body weight and blood glucose levels [ 129 ]. Generally, long-term dietary changes are difficult when it comes to consistency; however with the application of precision nutrition, there is a potential to optimize dietary behavior change by motivating greater adherence and change in dietary intake for the long-term for improved weight and glucose management [ 130 , 131 , 132 ]. The nutrigenomics overweight/obesity and weight management (NOW) trial was an RCT that shed more light on long-term dietary behavior change and adherence. More specifically, the investigators described that the use of precision nutrition increased motivation to long-term reduction in total fat intake, and long-term adherence to total fat and saturated fat advice [ 133 ].
Evidence shows that fixed step goals that are not personalized can discourage individuals, leading to unchanged behavior or even reduced physical activity levels [ 134 , 135 , 136 ]. There are findings, however, that show that the effect of precision nutrition to promote behavior change in physical inactivity and improve physical activity levels is not as consistent as observed for behavior changes in dietary patterns and diet quality. The findings of an RCT that included 1279 participants in 7 European countries to determine the effects of personalized advice on physical activity showed that while self-report-based physical activity levels increased to a greater extent with more personalized nutrition advice, there was no difference between the effect of personalized advice to promote changes in physical activity levels and conventional guidelines when physical activity was objectively measured. The authors concluded that it is vital to measure physical activity objectively in any physical activity intervention study [ 137 ]. Studies that analyzed objective measurement of physical activity levels in personalized advice support this theory as they found association between personalized and adaptive goal-setting intervention and steady daily steps, but not with constant steps in the control group, thus promoting behavior change in physical activity [ 138 ]. These data are in contrast with the results of an RCT that reported no changes in physical activity behavior after a precision nutrition intervention using objectively measured physical activity [ 139 ]. Nevertheless, an observational study found that precision nutrition significantly increased strength exercise frequency which was attributed to direct motivation of their personal genetic testing results to make behavior changes [ 140 ]. However, genetic results were not consistently associated with physical activity changes. Together these studies provide important insights into the precision nutrition effects on physical activity behavior changes, which highlights the need for further research.
The current review provides evidence that although the application of precision nutrition is emerging, it is to a large extent associated with obesity and type 2 diabetes and may be effective approach in improving the risks factors including dietary patterns, physical activity, body weight and fat, blood lipids, blood glucose, and insulin resistance. This advancement has been enabled through the use of cutting-edge omics technologies which provide genetic, biomarkers, and microbiome insights into variabilities in individual metabolic pathways in response to dietary intakes that may impact health. It is worth noting as presented in this review that the evidence for precision nutrition is stronger for behavior change than for actual hard endpoints but maintaining the behavior changes in the long term is important for the hard endpoints to change, and this is challenging. The choosing of genetic and phenotypic parameters as a rational basis for individual-level, precision nutrition advice is a key factor that motivates people to make appropriate behavioral changes. However, individual health aspirations, food preferences, and barriers/facilitators to behavior change need to be considered and integrated more using a biopsychosocial model in developing precision nutrition approaches to maintain long-term behavior change and promote sustainability for better health outcomes [ 141 ]. In addition, there are still methodological challenges in the design and application of precision nutrition in clinical settings and scale up to the population level in addressing obesity and type 2 diabetes. While sensitivity and specificity issues of the omics technologies exist, some studies do not incorporate all the sources of individual variability in their assessment, and others do not have relevant behavior change techniques, are of short duration in their intervention, low diet quality, and of small sample sizes to observe an effect. More rigorous and well-executed RCTs are required to reinforce the evidence base for precision nutrition to be widely and effectively used in clinical setting and the public health domain. Moreover, increasing the reliability and reducing the cost of cutting-edge omics technologies and new frontiers in machine learning will undoubtedly pave the way for comprehensive and integrated framework of big data to combine multi-omics approaches with lifestyle and behavioral, phenotype, sociocultural, and demographic factors. This will help apprise the optimal design of precision nutrition interventions in clinical settings, and improve population diets at scale in improving the risk factors of obesity and type 2 diabetes. The vast majority of present knowledge and research on precision nutrition has been derived from developed countries [ 142 ]. It is crucial to conduct original research in other populations with different dietary habits, disease susceptibility, genetic makeup, socioeconomic characteristics, and health-related lifestyles. Extending precision nutrition research and application by examining and understanding a wider array of multi-race population health, technological and digital landscape, and political will are needed to ensure that there is equity prior to implementation of such approaches.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
International Diabetes Federation. Diabetes around the world in 2021. 2021. https://diabetesatlas.org/ . Accessed 5 Apr 2022.
Centers for Disease Control and Prevention. National diabetes statistics report. 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html . Accessed 9 Jul 2022.
Centers for Disease Control and Prevention. Type 2 diabetes. 2021. https://www.cdc.gov/diabetes/basics/type2.html . Accessed 5 Apr 2022.
American Diabetes Association. Statistics about diabetes. 2022. https://www.diabetes.org/about-us/statistics/about-diabetes . Accessed 9 Jul 2022.
Centers for Disease Control and Prevention. Prevalence of overweight and obesity among adults with diagnosed diabetes–United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep. 2004;19:1066–8.
Google Scholar
American Diabetes Association. Extra Weight, Extra Risk. 2022. https://diabetes.org/healthy-living/weight-loss/extra-weight-extra-risk . Accessed 5 Aug 2022.
Koren D, Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metab. 2018;84:67–75.
Article CAS Google Scholar
Cameron NA, Petito LC, McCabe M, Allen NB, O'Brien MJ, Carnethon MR, N. et al. Quantifying the sex-race/ethnicity-specific burden of obesity on incident diabetes mellitus in the United States, 2001 to 2016: MESA and NHANES. J Am Heart Assoc. 2021;10:e018799–e018810.
Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118:1723–35.
Article CAS PubMed PubMed Central Google Scholar
American Society for Metabolic and Bariatric Surgery. Type 2 diabetes and metabolic surgery. 2018. https://asmbs.org/resources/type-2-diabetes-and-metabolic-surgery-fact-sheet . Accessed 5 Apr 2022.
Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44–58.
Article PubMed Google Scholar
Ganz ML, Wintfeld N, Li Q, Alas V, Langer J, Hammer M. The association of body mass index with the risk of type 2 diabetes: a case–control study nested in an electronic health records system in the United States. Diabetol Metab Syndr. 2014;6:50–8.
Article PubMed PubMed Central Google Scholar
Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS ONE. 2021;16:e0247307–11.
Cawley J, Biener A, Meyerhoefer C, Ding Y, Zvenyach T, Smolarz BG, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27:354–66.
PubMed Google Scholar
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–28.
Yoo S. Dynamic energy balance and obesity prevention. J Obes Metab Syndr. 2018;27:203–12.
Gassasse Z, Smith D, Finer S, Gallo V. Association between urbanisation and type 2 diabetes: an ecological study. BMJ Glob Health. 2017;2:e000473–7.
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;1:3–21.
Article Google Scholar
Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;10:954–64.
Interagency Committee on Human Nutrition Research. National Nutrition Research Roadmap 2016‒2021: advancing nutrition research to improve and sustain health. 2016. Interagency Committee on Human Nutrition Research, Washington (DC). 2019. https://www.nal.usda.gov/sites/default/files/page-files/2016-03-30-%20ICHNR%20NNRR.pdf . Accessed 7 Apr 2022.
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. 2020. https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf . Accessed 20 Apr 2022.
Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–42.
Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–8.
Banfield EC, Liu Y, Davis JS, Chang S, Frazier-Wood AC. Poor adherence to US dietary guidelines for children and adolescents in the national health and nutrition examination survey population. J Acad Nutr Diet. 2016;116(1):21–7.
Trepanowski JF, Ioannidis JPA. Perspective: limiting dependence on nonrandomized studies and improving randomized trials in human nutrition research: why and how. Adv Nutr. 2018;9:367–77.
Florez JC. Precision medicine in diabetes: is it time? Diabetes Care. 2016;39:1085–8.
Article CAS PubMed Google Scholar
Scheen AJ. Precision medicine: the future in diabetes care? Diabetes Res Clin Pract. 2016;117:12–21.
Reddy SS. Evolving to personalized medicine for type 2 diabetes. Endocrinol Metab Clin North Am. 2016;45:1011–20.
Bush CL, Blumberg JB, El-Sohemy A, Minich DM, Ordovás JM, Reed DG, et al. Toward the definition of personalized nutrition: a proposal by the American Nutrition Association. J Am Coll Nutr. 2020;39:5–15.
Ordovas JM, Ferguson LR, Tai ES, Mathers JC. Personalised nutrition and health. BMJ. 2018;361:2173–83.
Rudkowska I, Pérusse L, Bellis C, Blangero J, Després J-P, Bouchard C, et al. Interaction between common genetic variants and total fat intake on low-density lipoprotein peak particle diameter: a genome-wide association study. Lifestyle Genomics. 2015;8:44–53.
Tremblay BL, Cormier H, Rudkowska I, Lemieux S, Couture P, Vohl M-C. Association between polymorphisms in phospholipase A 2 genes and the plasma triglyceride response to an n-3 PUFA supplementation: a clinical trial. Lipids Health Dis. 2015;14:12.
Ferguson LR. Genome-wide association studies and diet. World Rev Nutr Diet. 2010;101:8–14.
Tebani A, Bekri S. Paving the way to precision nutrition through metabolomics. Front Nutr. 2019;6:41–7.
Brouwer-Brolsma EM, Brennan L, Drevon CA, van Kranen H, Manach C, Dragsted LO, et al. Combining traditional dietary assessment methods with novel metabolomics techniques: present efforts by the Food Biomarker Alliance. Proc Nutr Soc. 2017;76(4):619–27.
European Joint Programming Initiative, “A Healthy Diet for a Healthy Life.” JPI-HDHL INTIMIC. 2020. https://www.healthydietforhealthylife.eu/index.php/ec-partnerships/hdhl-intimic . Accessed 5 Aug 2022.
Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69: 110549.
Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser M, Rigdon J, Loannidis JPA, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–9.
Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O'Donovan CB, et al. Food4Me Study. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46(2):578–8.
de Roos B. Personalised nutrition: ready for practice? Proc Nutr Soc. 2013;72:48–52.
Garwood G. U.S.-backed study draws renewed focus on precision nutrition. The Food Institute. 2022. https://foodinstitute.com/focus/u-s-backed-study-draws-renewed-focus-on-precisionnutrition/ . Accessed 5 Aug 2022.
Poínhos R, Oliveira BMPM, van der Lans IA, Fischer ARH, Berezowska A, Rankin A, et al. Providing personalised nutrition: consumers’ trust and preferences regarding sources of information, service providers and regulators, and communication channels. Public Health Genom. 2017;20:218–28.
Stewart-Knox BJ, Poínhos R, Fischer ARH, Chaudhrey M, Rankin A, Davison J, et al. Sex and age differences in attitudes and intention to adopt personalised nutrition in a UK sample. Z Gesundh Wiss. 2021;14:1–7.
Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;11:1–10.
Livingstone KM, Brayner B, Celis-Morales C, Ward J, Mathers JC, Bowe SJ. Dietary patterns, genetic risk, and incidence of obesity: application of reduced rank regression in 11,735 adults from the UK Biobank study. Prev Med. 2022;158: 107035.
Marcum JA. Nutrigenetics/nutrigenomics, personalized nutrition, and precision healthcare. Curr Nutr Rep. 2020;9:338–45.
Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, Dam RMD, et al. Variant of transcription factor #7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55(9):2645–48.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Goni L, Cuervo M, Milagro FI, Martínez JA. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr. 2015;10:445–51.
Martinez JA, Navas-Carretero S, Saris WH, Astrup A. Personalized weight loss strategies — the role of macronutrient distribution. Nat Rev Endocrinol. 2014;10:749–60.
Celis-Morales CA, Lyall DM, Gray SR, Steell L, Anderson J, Iliodromiti S, et al. Dietary fat and total energy intake modifies the association of genetic profile risk score on obesity: evidence from 48,170 UK Biobank participants. Int J Obes. 2017;41:1761–8.
Merritt DC, Jamnik J, El-Sohemy A. FTO genotype, dietary protein intake, and body weight in a multiethnic population of young adults: a cross-sectional study. Genes Nutr. 2018;13:4–11.
•• Ramos-Lopez O, Cuervo M, Goni L, Milagro FI, Riezu-Boj JI, Martinez JA. Modeling of an integrative prototype based on genetic, phenotypic, and environmental information for personalized prescription of energy-restricted diets in overweight/obese subjects. Am J Clin Nutr. 2020;1:459–70. This article examined an integrative genetic, phenotype, and environmental information for personalized prescription of energy-restricted diets and the effect on BMI in overweight/obese subjects. The use of the model revealed how personalized dietary advice based on genetic, phenotypic, and environmental profile had impact on management of excessive body weight.
Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Cuervo M, Goni L, Martinez JA. Models integrating genetic and lifestyle interactions on two adiposity phenotypes for personalized prescription of energy-restricted diets with different macronutrient distribution. Front Genet. 2019;30:686–93.
Corella D, Coltell O, Sorlí JV, Estruch R, Quiles L, Martínez-González MÁ, et al. Polymorphism of the transcription factor 7-like 2 gene (TCF7L2) interacts with obesity on type-2 diabetes in the PREDIMED study emphasizing the heterogeneity of genetic variants in type-2 diabetes risk prediction: time for obesity-specific genetic risk scores. Nutrients. 2016;8(12):793–8.
Horne JR, Gilliland JA, O’Connor CP, Seabrook JA, Madill J. Change in weight, BMI, and body composition in a population-based intervention versus genetic-based intervention: the NOW trial. Obesity. 2020;28:1419–27.
Huang T, Ley SH, Zheng Y, Wang T, Bray GA, Sacks FM, et al. Genetic susceptibility to diabetes and long-term improvement of insulin resistance and β cell function during weight loss: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Am J Clin Nutr. 2016;104:198–204.
Wang T, Huang T, Zheng Y, Rood J, Bray GA, Sacks FM, et al. Genetic variation of fasting glucose and changes in glycemia in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Int J Obes (Lond). 2016;40:1164–9.
• Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:964–73. This large-scale high-resolution study investigated postprandial metabolic responses in identical meal intake using person-specific factors such as gut microbiome and genetics, and machine learning model. Gut microbiome had greater influence for postprandial lipemia, and the model predicted both triglyceride and glycemic response.
•• Shamanna P, Joshi S, Shah L, Dharmalingam M, Saboo B, Mohammed J, et al. Type 2 diabetes reversal with digital twin technology-enabled precision nutrition and staging of reversal: a retrospective cohort study. Clin Diabetes Endocrinol. 2021;7:21–27. This retrospective cohort study assessed type 2 diabetes reversal and measure changes in reversal stages before and after 90 days of digital twin-enabled precision nutrition therapy. It revealed how precision nutrition had effect in reversing high levels of HbA1C, weight, BMI without diabetes medication.
Shamanna P, Saboo B, Damodharan S, Mohammed J, Mohamed M, Poon T, et al. Reducing HbA1c in type 2 diabetes using digital twin technology-enabled precision nutrition: a retrospective analysis. Diabetes Ther. 2020;11:2703–14.
Frankwich KA, Egnatios J, Kenyon ML, Rutledge TR, Liao PS, Gupta S, et al. Differences in weight loss between persons on standard balanced vs nutrigenetic diets in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1625–32.
Smith CE, Tucker KL, Yiannakouris N, Garcia-Bailo B, Mattei J, Lai CQ, et al. Perilipin polymorphism interacts with dietary carbohydrates to modulate anthropometric traits in Hispanics of Caribbean origin. J Nutr. 2008;138(10):1852–8.
O’Donovan CB, Walsh MC, Gibney MJ, Gibney ER, Brennan L. Can metabotyping help deliver the promise of personalised nutrition? Proc Nutr Soc. 2015;75:106–14.
Allam-Ndoul B, Guénard F, Garneau V, Cormier H, Barbier O, Pérusse L, et al. Association between metabolite profiles, metabolic syndrome and obesity status. Nutrients. 2016;8:324–8.
O’Sullivan A, Gibney MJ, Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr. 2010;93:314–21.
Wittenbecher C, Mühlenbruch K, Kröger J, Jacobs S, Kuxhaus O, Floegel A, et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am J Clin Nutr. 2015;101:1241–50.
• Anwar MA, Barrera-Machuca AA, Calderon N, Wang W, Tausan D, Vayali T, et al. Value-based healthcare delivery through metabolomics-based personalized health platform. Healthc Manage Forum. 2020;33(3):126–34. This article discusses the use of a metabolomics-based personalized lifestyle recommendations in type 2 diabetes, insulin resistance, and associated comorbidities. Results indicated reductions in health risks associated with type 2 diabetes and associated diseases.
Westerman K, Reaver A, Roy C, Ploch M, Sharoni E, Nogal B, et al. Longitudinal analysis of biomarker data from a personalized nutrition platform in healthy subjects. Sci Rep. 2018;8:14685–710.
Bouwman J, Vogels JT, Wopereis S, Rubingh CM, Bijlsma S, Ommen B. Visualization and identification of health space, based on personalized molecular phenotype and treatment response to relevant underlying biological processes. BMC Med Genomics. 2012;5:1–6.
Fiamoncini J, Rundle M, Gibbons H, Thomas EL, Geillinger-Kästle K, Bunzel D, et al. Plasma metabolome analysis identifies distinct human metabotypes in the postprandial state with different susceptibility to weight loss-mediated metabolic improvements. FASEB J. 2018;32(10):5447–58.
Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, et. al.: Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr. 2016;103:505–11.
Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE; Diabetes Prevention Program Research Group. Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes. 2016;65(5):1424–33.
• Aldubayan MA, Pigsborg K, Gormsen SMO, Serra F, Palou M, Galmés S, et al. A double-blinded, randomized, parallel intervention to evaluate biomarker-based nutrition plans for weight loss: the PREVENTOMICS study. Clin Nutr. 2022;41(8):1834–44. This double-blinded randomized intervention examined biomarker-based personalized diet and control group diet on fat mass. There was no change in fat mass, and while the individual diets produced significant improvements in insulin resistance and lipid profile, there was no significant differences between groups. This article is an example of studies that did not show an effect of precision nutrition approach as compared to general healthy diet on weight loss, and warrants further research on the topic.
Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341–55.
Harsch IA, Konturek PC. The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: new insights into “old” diseases. Med Sci (Basel). 2018.17;6(2):32.
Wilson K, Situ C. Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J Clin Nutr Metab. 2017;1(2).
Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019. 22;11(12):2862.
Dahl WJ, Mendoza DR, Lambert JM. Diet, nutrients and the microbiome. Prog Mol Biol Transl Sci. 2020;171:237–63.
Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23(3):1105.
Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64.
Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–94.
Mendes-Soares H, Raveh-Sadka T, Azulay S, Edens K, Ben-Shlomo Y, Cohen Y, et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw Open. 2019;2: e188102.
Mendes-Soares H, Raveh-Sadka T, Azulay S, Ben-Shlomo Y, Cohen Y, Ofek T, S, et al. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am J Clin Nutr. 2019;1:63–75.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22(6):971–82.
Hjorth MF, Roager HM, Larsen TM, Poulsen SK, Licht TR, Bahl MI, et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes (Lond). 2018;42(3):580–3.
Fragiadakis GK, Wastyk HC, Robinson JL, Sonnenburg ED, Sonnenburg JL, Gardner CD. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am J Clin Nutr. 2020;111(6):1127–36.
Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, et al. US Immigration Westernizes the Human Gut Microbiome. Cell. 2018;175(4):962–72.
Yu D, Nguyen SM, Yang Y, Xu W, Cai H, Wu J, et al. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am J Clin Nutr. 2021;113(3):684–94.
•• Cuevas-Sierra A, Milagro FI, Guruceaga E, Cuervo M, Goni L, García-Granero M, et al. A weight-loss model based on baseline microbiota and genetic scores for selection of dietary treatments in overweight and obese population. Clin Nutr. 2022;41(8):1712–1723. This article determined the use of an integrative model using gut microbiota and genetic information to personalize weight loss prescription. The use of the model facilitated the selection of diet in 84% of men and 72% of women for weight loss.
Bacon SL, Lavoie K, Ninot G, Czajkowski SM, Freedland KE, Michie S, et al. An international perspective on improving the quality and potential of behavioral clinical trials. Curr Cardiovasc Risk Rep. 2013;9:1–6.
Shilts M, Horowitz M, Townsend M. Goal setting as a strategy for dietary and physical activity behavior change: a review of the literature. Am J Health Promot. 2004;19:81–93.
Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215.
Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, et al. Trends in dietary quality among adults in the united states, 1999 through 2010. JAMA Intern Med. 2014;174:1587–95.
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;17:1223–49.
Collins CE, Morgan PJ, Jones P, Fletcher K, Martin J, Aguiar EJ, et al. A 12-week commercial web-based weight-loss program for overweight and obese adults: randomized controlled trial comparing basic versus enhanced features. J Med Internet Res. 2012;14:e57–62.
Hutchesson MJ, Collins CE, Morgan PJ, Watson JF, Guest M, Callister R. Changes to dietary intake during a 12-week commercial web-based weight loss program: a randomized controlled trial. Eur J Clin Nutr. 2013;68:64–70.
Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care. 2016;39:833–46.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Collins CE, Young AF, Hodge A. Diet quality is associated with higher nutrient intake and self-rated health in mid-aged women. J Am Coll Nutr. 2008;27:146–57.
Collins CE, Morgan PJ, Jones P, Fletcher K, Martin J, Aguiar EJ, et al. Evaluation of a commercial web-based weight loss and weight loss maintenance program in overweight and obese adults: a randomized controlled trial. BMC Public Health. 2010;10:669–76.
O’Brien KM, Hutchesson MJ, Jensen M, Morgan P, Callister R, Collins CE. Participants in an online weight loss program can improve diet quality during weight loss: a randomized controlled trial. Nutr J. 2014;9:82–6.
Collins CE, Boggess MM, Watson JF, Guest M, Duncanson K, Pezdirc K, et al. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin Nutr. 2014;33:906–14.
United States Department of Agriculture. Food and Nutrition Service. Healthy Eating Index. 2022. https://www.fns.usda.gov/hei-scores-americans . Accessed 6 Aug 2022.
The US, Burden of Disease Collaborators. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–72.
Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007.
World Health Organization. Physical Activity. 2020. https://www.who.int/news-room/fact-sheets/detail/physical-activity#:~:text=Levels%20of%20physical%20activity%20globally&text=Globally%2C%2028%25%20of%20adults%20aged,intensity%20physical%20activity%20per%20week . Accessed 5 Apr 2022.
Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527–45.
Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev. 2010;6:CD007275-CD007280.
Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285:1172–7.
Celis-Morales C, Lara J, Mathers JC. Personalising nutritional guidance for more effective behaviour change. Proc Nutr Soc. 2015;74:130–218.
Hietaranta-Luoma HL, Tahvonen R, Iso-Touru T, Puolijoki H, Hopia A. An intervention study of individual, apoE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2014;7:161–74.
CAS PubMed Google Scholar
Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. PLoS ONE. 2014;9:e112665–21126.
Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O’Donovan CB, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578–88.
Livingstone KM, Celis-Morales C, Navas-Carretero S, San-Cristobal R, Macready AL, Fallaize R, et al. Effect of an Internet-based, personalized nutrition randomized trial on dietary changes associated with the Mediterranean diet: the Food4Me Study. Am J Clin Nutr. 2016;104:288–97.
Rodríguez-Rejón AI, Castro-Quezada I, Ruano-Rodríguez C, Ruiz-López MD, Sánchez-Villegas A, Toledo E, et al. Effect of a Mediterranean diet intervention on dietary glycemic load and dietary glycemic index: the PREDIMED study. J Nutr Metab. 2014;2014:985373–83.
Pérez-Martínez P, García-Ríos A, Delgado-Lista J, Pérez-Jiménez F, López-Miranda J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr Pharm Des. 2011;17:769–77.
Beunza JJ, Toledo E, Hu FB, Bes-Rastrollo M, Serrano-Martínez M, Sánchez-Villegas A, et al. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2010;92:1484–93.
Hu EA, Toledo E, Diez-Espino J, Estruch R, Corella D, Salas-Salvado J, et al. Lifestyles and risk factors associated with adherence to the Mediterranean diet: a baseline assessment of the PREDIMED trial. PLoS ONE. 2013;8: e60166.
Celis-Morales C, Livingstone KM, Petermann-Rocha F, Navas-Carretero S, San-Cristobal R, O’Donovan CB, et al. Frequent nutritional feedback, personalized advice, and behavioral changes: findings from the European Food4Me Internet-based RCT. Am J Prev Med. 2019;57:209–19.
Héroux M, Watt M, McGuire KA, Berardi JM. A personalized, multi-platform nutrition, exercise, and lifestyle coaching program: a pilot in women. Internet Interv. 2016;28:16–22.
•• de Hoogh IM, Winters BL, Nieman KM, Bijlsma S, Krone T, van den Broek TJ, et al. A novel personalized systems nutrition program improves dietary patterns, lifestyle behaviors and health-related outcomes: results from the habit study. Nutrients. 2021;22:1763–70. This single-arm exploratory study evaluated the impact of personalized systems nutrition program on lifestyle behavior and health outcomes. Results showed that the personalized nutrition recommendation improves lifestyle habits and reduces body weight, BMI and other health-related outcomes.
Ritz C, Astrup A, Larsen TM, Hjorth MF. Weight loss at your fingertips: personalized nutrition with fasting glucose and insulin using a novel statistical approach. Eur J Clin Nutr. 2019;73:1529–35.
Hjorth MF, Astrup A, Zohar Y, Urban LE, Sayer RD, Patterson BW, et al. Personalized nutrition: pretreatment glucose metabolism determines individual long-term weight loss responsiveness in individuals with obesity on low-carbohydrate versus low-fat diet. Int J Obes (Lond). 2019;43:2037–44.
van der Haar S, Hoevenaars FPM, van den Brink WJ, van den Broek T, Timmer M, Boorsma A, et al. Exploring the potential of personalized dietary advice for health improvement in motivated individuals with premetabolic syndrome: pretest-posttest study. JMIR Form Res. 2021;24: e25043.
•• Zenun Franco R, Fallaize R, Weech M, Hwang F, Lovegrove JA. Effectiveness of web-based personalized nutrition advice for adults using the eNutri web app: evidence from the EatWellUK randomized controlled trial. J Med Internet Res. 2022;25:e29088. This randomized controlled trial investigated the effectiveness of an automated precision nutrition advice for improving diet quality compared to general dietary guidelines. The precision nutrition recommendation was found to improve short-term diet quality and increased healthy eating behaviors.
Marmash D, Ha K, Sakaki JR, Hair R, Morales E, Duffy VB, et al. A feasibility and pilot study of a personalized nutrition intervention in mobile food pantry users in Northeastern Connecticut. Nutrients. 2021;25:2939–45.
•• Rollo ME, Haslam RL, Collins CE. Impact on dietary intake of two levels of technology-assisted personalized nutrition: a randomized trial. nutrients. 2020;29: 3334–3339. This study of a randomized controlled trial compared the impact of low personalized nutrition feedback and high personalized nutrition feedback on dietary intake. The results allowed for differences between the two groups to emerge.
Horne J, Madill J, O’Connor C, Shelley J, Gilliland J. A systematic review of genetic testing and lifestyle behaviour change: are we using high-quality genetic interventions and considering behaviour change theory? Lifestyle Genom. 2018;11:49–63.
Nielsen DE, Shih S, El-Sohemy A. Perceptions of genetic testing for personalized nutrition: a randomized trial of DNA-based dietary advice. J Nutrigenet Nutrigenomics. 2014;7:94–104.
Horne J, Madill J, Gilliland J. Incorporating the ‘Theory of Planned Behavior’ into personalized healthcare behavior change research: a call to action. Per Med. 2017;14:521–9.
Drabsch T, Holzapfel C. A scientific perspective of personalised gene-based dietary recommendations for weight management. Nutrients. 2019;11:617–24.
Horne J, Gilliland J, O’Connor C, Seabrook J, Madill J. Enhanced long-term dietary change and adherence in a nutrigenomics-guided lifestyle intervention compared to a population-based (GLB/DPP) lifestyle intervention for weight management: results from the NOW randomised controlled trial. BMJ Nutr Prev Health. 2020;21:49–59.
Adams MA, Hurley JC, Todd M, Bhuiyan N, Jarrett CL, Tucker WJ, et al. Adaptive goal setting and financial incentives: a 2 × 2 factorial randomized controlled trial to increase adults’ physical activity. BMC Public Health. 2017;29:286–92.
Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA. 2016;20:1161–71.
Joseph R, Keller C, Adams M, Ainsworth B. Print versus a culturally-relevant Facebook and text message delivered intervention to promote physical activity in African American women: a randomized pilot trial. BMC Womens Health. 2015;27:30–6.
Marsaux CF, Celis-Morales C, Fallaize R, Macready AL, Kolossa S, Woolhead C, et al. Effects of a web-based personalized intervention on physical activity in European adults: a randomized controlled trial. J Med Internet Res. 2015;14:e231–7.
Zhou M, Fukuoka Y, Mintz Y, Goldberg K, Kaminsky P, Flowers E, et al. Evaluating machine learning-based automated personalized daily step goals delivered through a mobile phone app: randomized controlled trial. JMIR Mhealth Uhealth. 2018;25:e28–36.
Godino JG, van Sluijs EM, Marteau TM, Sutton S, Sharp SJ, Griffin SJ. Lifestyle advice combined with personalized estimates of genetic or phenotypic risk of type 2 diabetes, and objectively measured physical activity: a randomized controlled trial. PLoS Med. 2016;29:e1002185–91.
Nielsen DE, Carere DA, Wang C, Roberts JS, Green RC, PGen Study Group. Diet and exercise changes following direct-to-consumer personal genomic testing. BMC Med Genomics. 2017;2:24–30.
National Academies of Sciences, Engineering, and Medicine. Challenges and Opportunities for Precision and Personalized Nutrition: Proceedings of a Workshop—in Brief. 1st ed. Washington, DC: The National Academies Press. 2021.
Sight and Life. Precision nutrition for low- and middle-income countries: hype or hope? Special Report. 2022. https://sightandlife.org/10.52439/PRCJ5543.pdf . Accessed 27 Feb 2023.
Download references
Acknowledgements
The author would like to thank the Cooperative Agriculture Research Center of the College of Agriculture and Human Sciences at Prairie View A&M University for supporting Open Access publication of this work.
Open Access publication support was received from the Cooperative Agriculture Research Center of the College of Agriculture and Human Sciences at Prairie View A&M University.
Author information
Authors and affiliations.
Department of Agriculture, Nutrition and Human Ecology, Prairie View A&M University, Prairie View, USA
Janet Antwi
You can also search for this author in PubMed Google Scholar
Contributions
JA conceptualized the review, conducted literature searches, and wrote the paper. The sole author had responsibility for all parts of the manuscript.
Corresponding author
Correspondence to Janet Antwi .
Ethics declarations
Conflict of interest.
The author declares no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Antwi, J. Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes. Curr Nutr Rep 12 , 679–694 (2023). https://doi.org/10.1007/s13668-023-00491-y
Download citation
Accepted : 07 August 2023
Published : 23 August 2023
Issue Date : December 2023
DOI : https://doi.org/10.1007/s13668-023-00491-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Precision nutrition
- Personalized nutrition
- Risk factors
- Behavior change
- Dietary patterns
- Type 2 diabetes
- Find a journal
- Publish with us
- Track your research
Type 2 Diabetes Essay
Introduction.
Diabetes is a health condition that is developed when sugar level in the blood increases above normal levels. The two major types of diabetes are type 1 diabetes and type 2 diabetes. Type 2 diabetes is more prevalent than type 1 diabetes. This essay discusses some of the most frequently asked questions about type 2 diabetes through a sample dialogue between a patient and a doctor.
Patient: What is type 2 Diabetes and how is it developed?
Doctor: Type 2 diabetes can be described as a complication in the metabolic processes characterized by a relative shortage of insulin and high levels of glucose in the blood (Barnett, 2011). It differs from type 1 diabetes where there is a complete deficiency of insulin caused by destruction of pancreatic islet cells.
In addition, type 2 diabetes is more common in adults unlike type 1 diabetes which is prevalent amongst young people. The typical symptoms of type 2 diabetes include: recurrent urination, excessive thirst, and persistent hunger (Wilson &Mehra, 1997).
Type 2 diabetes is caused by a mixture of lifestyle and hereditary factors. Even though some factors, like nutrition and obesity, are under individual control, others like femininity, old age, and genetics are not. Sedentary lifestyle, poor nutrition and stress are the major causes of Type 2 diabetes.
Particularly, excessive consumption of sugar and fats increases the risk of infection. Genetic factors have been linked to this condition. For instance, research indicates that if one identical twin is infected, there is a 90% probability of the other twin getting infected. Nutritional condition of a mother for the period of fetal growth can as well lead to this condition. Inadequate sleep is associated with Type 2 diabetes since it affects the process of metabolism (Hawley & Zierath, 2008).
Patient: How is type 2 Diabetes transmitted?
Doctor: Type 2 diabetes cannot be transmitted from one individual to another, since it is not caused by micro-organisms that can be spread. Instead, it is a health condition where the body is unable to create sufficient insulin to maintain the blood sugar level.
Nevertheless, a child from diabetic parents is likely to develop the complication due to genetic inheritance. According to Hanas & Fox (2007), there are some genes that may result in diabetes. As in 2011, research showed that there are more than thirty-six genes that increase the risk of type 2 diabetes infection.
These genes represent 10 per cent of the entire hereditary component of the complication. For instance, a gene referred to as TCF7L2 allele, increases the probability of diabetes occurrence by 1.5 times. It is the greatest threat amongst the genetic invariants. Children from diabetic parents are, therefore, likely to get infected since genes are transferrable from parents to the offspring.
Patient: How is type 2 Diabetes treated?
Doctor: The first step in the treatment of type 2 diabetes is consumption of healthy diet. This involves avoiding excessive consumption of foods that contain sugar and fats as they are likely to increase the levels of sugar in the blood. In addition, getting involved in physical activity and losing excessive weight are also important.
These management practices are recommended because they lower insulin resistance and improve the body cells’ response to insulin. Eating healthy food and physical activity also lower the level of sugar in the blood. There are also pills and other medications that can be injected when these lifestyle changes do not regulate the blood sugar (Roper, 2006).
Type2 diabetes pills function in different ways. Some pills work by lowering insulin resistance while some raise the level of insulin in the blood or decrease the rate of food digestion. Even though the non-insulin injected medicines for this condition work in complex ways, essentially, they lower the levels of blood glucose after injection.
Insulin injection treatment basically raises the insulin level in the blood. Another treatment for type 2 diabetes is weight loss surgery that is recommended for obese people. This treatment has been proved effective since most of the patients can maintain regular levels of sugar in their blood after surgery (Codario, 2011).
Multiple prescriptions can be applied in controlling the levels of blood sugar. Actually, combination treatment is a popular remedy for Type 2 diabetes. If a single therapy is not sufficient, a health care provider may prescribe two or more different kinds of pills.
For instance, individuals with type 2 diabetes have high fat levels in the blood and high blood pressure. Therefore, doctors can prescribe medicines for treatment of these conditions at the same time. The kind of medication prescribed depends on the health condition of the patient (Ganz, 2005).
Patient: What are the chances of survival?
Doctor: Diabetes is one of the major causes of deaths in the United States each year. Statistics indicates that it contributes to approximately 100,000 deaths every year. In the United States, there are over 20 million reported cases of diabetes, the majority being Type 2 diabetes. Proper remedy including change of lifestyle and medications is known to improve the health condition of a patient. If properly used together, lifestyle changes and medication can increase the chances of survival of a patient by up to 85 per cent (Rosenthal, 2009).
Barnett, H. (2011). Type 2 diabetes. Oxford: Oxford University Press.
Codario, A. (2011). Type 2 diabetes, pre-diabetes, and the metabolic syndrome. Totowa, N.J: Humana Press.
Ganz, M. (2005). Prevention of Type 2 Diabetes . Chichester: John Wiley & Sons.
Hanas, R., & Fox, C. (2007). Type 2 diabetes in adults of all ages. London: Class Health.
Hawley, A., & Zierath, R. (2008). Physical activity and type 2 diabetes: Therapeutic effects and mechanisms of action. Champaign, IL: Human Kinetics.
Roper, R. (2006). Type 2 diabetes: The adrenal gland disease : the cause of type 2 diabetes and a nutrition program that takes control! . Bloomington, IN: AuthorHouse.
Rosenthal, S. (2009). The Canadian type 2 diabetes sourcebook. Mississauga, Ont: J. Wiley & Sons Canada.
Wilson, L., & Mehra, V. (1997). Managing the patient with type II diabetes . Gaithersburg, Md: Aspen Publishers.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2019, February 4). Type 2 Diabetes. https://ivypanda.com/essays/type-2-diabetes-2/
"Type 2 Diabetes." IvyPanda , 4 Feb. 2019, ivypanda.com/essays/type-2-diabetes-2/.
IvyPanda . (2019) 'Type 2 Diabetes'. 4 February.
IvyPanda . 2019. "Type 2 Diabetes." February 4, 2019. https://ivypanda.com/essays/type-2-diabetes-2/.
1. IvyPanda . "Type 2 Diabetes." February 4, 2019. https://ivypanda.com/essays/type-2-diabetes-2/.
Bibliography
IvyPanda . "Type 2 Diabetes." February 4, 2019. https://ivypanda.com/essays/type-2-diabetes-2/.
- Insulin Injection: Drug Properties and Injection Process
- Doctors' Reluctance to Prescribe Birth Control Pills to Early Adolescents
- Endocrine Disorders: The Diabetic
- The Diabetic Plate and Healthy Nutrition
- How to Self-Administer Insulin Injection
- The History of the Pill and Feminism
- Management of Patients With Diabetic Ketoacidosis
- Stontioum-90 in Plasma
- Diabetic Diet and Food Restrictions
- R-insulin: Article Critique
- Type I Diabetes: Pathogenesis and Treatment
- Human Body Organ Systems Disorders: Diabetes
- Informative Speech on Hyperthyroidism
- Are there Occasions in the Delivery of Health Care when Deception is Warranted?
- Xtra Mile: Roles of Medical Directors
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
Healthy Living with Diabetes
- Español
On this page:
How can I plan what to eat or drink when I have diabetes?
How can physical activity help manage my diabetes, what can i do to reach or maintain a healthy weight, should i quit smoking, how can i take care of my mental health, clinical trials for healthy living with diabetes.
Healthy living is a way to manage diabetes . To have a healthy lifestyle, take steps now to plan healthy meals and snacks, do physical activities, get enough sleep, and quit smoking or using tobacco products.
Healthy living may help keep your body’s blood pressure , cholesterol , and blood glucose level, also called blood sugar level, in the range your primary health care professional recommends. Your primary health care professional may be a doctor, a physician assistant, or a nurse practitioner. Healthy living may also help prevent or delay health problems from diabetes that can affect your heart, kidneys, eyes, brain, and other parts of your body.
Making lifestyle changes can be hard, but starting with small changes and building from there may benefit your health. You may want to get help from family, loved ones, friends, and other trusted people in your community. You can also get information from your health care professionals.
What you choose to eat, how much you eat, and when you eat are parts of a meal plan. Having healthy foods and drinks can help keep your blood glucose, blood pressure, and cholesterol levels in the ranges your health care professional recommends. If you have overweight or obesity, a healthy meal plan—along with regular physical activity, getting enough sleep, and other healthy behaviors—may help you reach and maintain a healthy weight. In some cases, health care professionals may also recommend diabetes medicines that may help you lose weight, or weight-loss surgery, also called metabolic and bariatric surgery.
Choose healthy foods and drinks
There is no right or wrong way to choose healthy foods and drinks that may help manage your diabetes. Healthy meal plans for people who have diabetes may include
- dairy or plant-based dairy products
- nonstarchy vegetables
- protein foods
- whole grains
Try to choose foods that include nutrients such as vitamins, calcium , fiber , and healthy fats . Also try to choose drinks with little or no added sugar , such as tap or bottled water, low-fat or non-fat milk, and unsweetened tea, coffee, or sparkling water.
Try to plan meals and snacks that have fewer
- foods high in saturated fat
- foods high in sodium, a mineral found in salt
- sugary foods , such as cookies and cakes, and sweet drinks, such as soda, juice, flavored coffee, and sports drinks
Your body turns carbohydrates , or carbs, from food into glucose, which can raise your blood glucose level. Some fruits, beans, and starchy vegetables—such as potatoes and corn—have more carbs than other foods. Keep carbs in mind when planning your meals.
You should also limit how much alcohol you drink. If you take insulin or certain diabetes medicines , drinking alcohol can make your blood glucose level drop too low, which is called hypoglycemia . If you do drink alcohol, be sure to eat food when you drink and remember to check your blood glucose level after drinking. Talk with your health care team about your alcohol-drinking habits.

Find the best times to eat or drink
Talk with your health care professional or health care team about when you should eat or drink. The best time to have meals and snacks may depend on
- what medicines you take for diabetes
- what your level of physical activity or your work schedule is
- whether you have other health conditions or diseases
Ask your health care team if you should eat before, during, or after physical activity. Some diabetes medicines, such as sulfonylureas or insulin, may make your blood glucose level drop too low during exercise or if you skip or delay a meal.
Plan how much to eat or drink
You may worry that having diabetes means giving up foods and drinks you enjoy. The good news is you can still have your favorite foods and drinks, but you might need to have them in smaller portions or enjoy them less often.
For people who have diabetes, carb counting and the plate method are two common ways to plan how much to eat or drink. Talk with your health care professional or health care team to find a method that works for you.
Carb counting
Carbohydrate counting , or carb counting, means planning and keeping track of the amount of carbs you eat and drink in each meal or snack. Not all people with diabetes need to count carbs. However, if you take insulin, counting carbs can help you know how much insulin to take.
Plate method
The plate method helps you control portion sizes without counting and measuring. This method divides a 9-inch plate into the following three sections to help you choose the types and amounts of foods to eat for each meal.
- Nonstarchy vegetables—such as leafy greens, peppers, carrots, or green beans—should make up half of your plate.
- Carb foods that are high in fiber—such as brown rice, whole grains, beans, or fruits—should make up one-quarter of your plate.
- Protein foods—such as lean meats, fish, dairy, or tofu or other soy products—should make up one quarter of your plate.
If you are not taking insulin, you may not need to count carbs when using the plate method.

Work with your health care team to create a meal plan that works for you. You may want to have a diabetes educator or a registered dietitian on your team. A registered dietitian can provide medical nutrition therapy , which includes counseling to help you create and follow a meal plan. Your health care team may be able to recommend other resources, such as a healthy lifestyle coach, to help you with making changes. Ask your health care team or your insurance company if your benefits include medical nutrition therapy or other diabetes care resources.
Talk with your health care professional before taking dietary supplements
There is no clear proof that specific foods, herbs, spices, or dietary supplements —such as vitamins or minerals—can help manage diabetes. Your health care professional may ask you to take vitamins or minerals if you can’t get enough from foods. Talk with your health care professional before you take any supplements, because some may cause side effects or affect how well your diabetes medicines work.
Research shows that regular physical activity helps people manage their diabetes and stay healthy. Benefits of physical activity may include
- lower blood glucose, blood pressure, and cholesterol levels
- better heart health
- healthier weight
- better mood and sleep
- better balance and memory
Talk with your health care professional before starting a new physical activity or changing how much physical activity you do. They may suggest types of activities based on your ability, schedule, meal plan, interests, and diabetes medicines. Your health care professional may also tell you the best times of day to be active or what to do if your blood glucose level goes out of the range recommended for you.

Do different types of physical activity
People with diabetes can be active, even if they take insulin or use technology such as insulin pumps .
Try to do different kinds of activities . While being more active may have more health benefits, any physical activity is better than none. Start slowly with activities you enjoy. You may be able to change your level of effort and try other activities over time. Having a friend or family member join you may help you stick to your routine.
The physical activities you do may need to be different if you are age 65 or older , are pregnant , or have a disability or health condition . Physical activities may also need to be different for children and teens . Ask your health care professional or health care team about activities that are safe for you.
Aerobic activities
Aerobic activities make you breathe harder and make your heart beat faster. You can try walking, dancing, wheelchair rolling, or swimming. Most adults should try to get at least 150 minutes of moderate-intensity physical activity each week. Aim to do 30 minutes a day on most days of the week. You don’t have to do all 30 minutes at one time. You can break up physical activity into small amounts during your day and still get the benefit. 1
Strength training or resistance training
Strength training or resistance training may make your muscles and bones stronger. You can try lifting weights or doing other exercises such as wall pushups or arm raises. Try to do this kind of training two times a week. 1
Balance and stretching activities
Balance and stretching activities may help you move better and have stronger muscles and bones. You may want to try standing on one leg or stretching your legs when sitting on the floor. Try to do these kinds of activities two or three times a week. 1
Some activities that need balance may be unsafe for people with nerve damage or vision problems caused by diabetes. Ask your health care professional or health care team about activities that are safe for you.

Stay safe during physical activity
Staying safe during physical activity is important. Here are some tips to keep in mind.
Drink liquids
Drinking liquids helps prevent dehydration , or the loss of too much water in your body. Drinking water is a way to stay hydrated. Sports drinks often have a lot of sugar and calories , and you don’t need them for most moderate physical activities.
Avoid low blood glucose
Check your blood glucose level before, during, and right after physical activity. Physical activity often lowers the level of glucose in your blood. Low blood glucose levels may last for hours or days after physical activity. You are most likely to have low blood glucose if you take insulin or some other diabetes medicines, such as sulfonylureas.
Ask your health care professional if you should take less insulin or eat carbs before, during, or after physical activity. Low blood glucose can be a serious medical emergency that must be treated right away. Take steps to protect yourself. You can learn how to treat low blood glucose , let other people know what to do if you need help, and use a medical alert bracelet.
Avoid high blood glucose and ketoacidosis
Taking less insulin before physical activity may help prevent low blood glucose, but it may also make you more likely to have high blood glucose. If your body does not have enough insulin, it can’t use glucose as a source of energy and will use fat instead. When your body uses fat for energy, your body makes chemicals called ketones .
High levels of ketones in your blood can lead to a condition called diabetic ketoacidosis (DKA) . DKA is a medical emergency that should be treated right away. DKA is most common in people with type 1 diabetes . Occasionally, DKA may affect people with type 2 diabetes who have lost their ability to produce insulin. Ask your health care professional how much insulin you should take before physical activity, whether you need to test your urine for ketones, and what level of ketones is dangerous for you.
Take care of your feet
People with diabetes may have problems with their feet because high blood glucose levels can damage blood vessels and nerves. To help prevent foot problems, wear comfortable and supportive shoes and take care of your feet before, during, and after physical activity.

If you have diabetes, managing your weight may bring you several health benefits. Ask your health care professional or health care team if you are at a healthy weight or if you should try to lose weight.
If you are an adult with overweight or obesity, work with your health care team to create a weight-loss plan. Losing 5% to 7% of your current weight may help you prevent or improve some health problems and manage your blood glucose, cholesterol, and blood pressure levels. 2 If you are worried about your child’s weight and they have diabetes, talk with their health care professional before your child starts a new weight-loss plan.
You may be able to reach and maintain a healthy weight by
- following a healthy meal plan
- consuming fewer calories
- being physically active
- getting 7 to 8 hours of sleep each night 3
If you have type 2 diabetes, your health care professional may recommend diabetes medicines that may help you lose weight.
Online tools such as the Body Weight Planner may help you create eating and physical activity plans. You may want to talk with your health care professional about other options for managing your weight, including joining a weight-loss program that can provide helpful information, support, and behavioral or lifestyle counseling. These options may have a cost, so make sure to check the details of the programs.
Your health care professional may recommend weight-loss surgery if you aren’t able to reach a healthy weight with meal planning, physical activity, and taking diabetes medicines that help with weight loss.
If you are pregnant , trying to lose weight may not be healthy. However, you should ask your health care professional whether it makes sense to monitor or limit your weight gain during pregnancy.
Both diabetes and smoking —including using tobacco products and e-cigarettes—cause your blood vessels to narrow. Both diabetes and smoking increase your risk of having a heart attack or stroke , nerve damage , kidney disease , eye disease , or amputation . Secondhand smoke can also affect the health of your family or others who live with you.
If you smoke or use other tobacco products, stop. Ask for help . You don’t have to do it alone.
Feeling stressed, sad, or angry can be common for people with diabetes. Managing diabetes or learning to cope with new information about your health can be hard. People with chronic illnesses such as diabetes may develop anxiety or other mental health conditions .
Learn healthy ways to lower your stress , and ask for help from your health care team or a mental health professional. While it may be uncomfortable to talk about your feelings, finding a health care professional whom you trust and want to talk with may help you
- lower your feelings of stress, depression, or anxiety
- manage problems sleeping or remembering things
- see how diabetes affects your family, school, work, or financial situation
Ask your health care team for mental health resources for people with diabetes.
Sleeping too much or too little may raise your blood glucose levels. Your sleep habits may also affect your mental health and vice versa. People with diabetes and overweight or obesity can also have other health conditions that affect sleep, such as sleep apnea , which can raise your blood pressure and risk of heart disease.

NIDDK conducts and supports clinical trials in many diseases and conditions, including diabetes. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for healthy living with diabetes?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care professionals and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of healthy living for people with diabetes, such as
- how changing when you eat may affect body weight and metabolism
- how less access to healthy foods may affect diabetes management, other health problems, and risk of dying
- whether low-carbohydrate meal plans can help lower blood glucose levels
- which diabetes medicines are more likely to help people lose weight
Find out if clinical trials are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical trials for healthy living with diabetes are looking for participants?
You can view a filtered list of clinical studies on healthy living with diabetes that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe for you. Always talk with your primary health care professional before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
NIDDK would like to thank: Elizabeth M. Venditti, Ph.D., University of Pittsburgh School of Medicine.

IMAGES
COMMENTS
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
357 Diabetes Essay Topics & Examples. Updated: Feb 25th, 2024. 26 min. When you write about the science behind nutrition, heart diseases, and alternative medicine, checking titles for diabetes research papers can be quite beneficial. Below, our experts have gathered original ideas and examples for the task. We will write.
The research could lead to a better availability of beta cells for future research purposes. Type 1 and type 2 diabetes results when beta cells in the pancreas fail to produce enough insulin, the ...
Diabetes drug slows development of Parkinson's disease. The drug, which is in the same family as blockbuster weight-loss drugs such as Wegovy, slowed development of symptoms by a small but ...
The systematic review widely focused on diabetes and the developments in nutrition and diets required to prevent or control all types of diabetes. Diabetes is characterized by high blood sugar ...
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5-10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years ...
An increasing amount of publications are recognizing that a person's risk of diabetes and diabetes outcomes are influenced largely by social determinants of health. This renewed understanding of ...
New estimates published this week in The Lancet indicate that more than 1·31 billion people could be living with diabetes by 2050 worldwide. That's 1·31 billion people living with a disease that causes life-altering morbidity, high rates of mortality, and interacts with and exacerbates many other diseases. The increase in prevalence (up from 529 million in 2021) is expected to be driven by ...
According to Ekoé, Rewers, Williams and Zimmet (2008), the major effects of diabetes include long term damage, dysfunction, and failure of various body organs. Other chronic diseases include cancer, arthritis, asthma, and obesity. In a definition by the World Health Organization, diabetes is a state of chronic hyperglycemia resulting from many ...
C. Manrique-Acevedo, I.B. Hirsch, and R.H. EckelN Engl J Med 2024;390:1207-1217. More than half of newly diagnosed cases of type 1 diabetes occur in adulthood. This review focuses on the ...
Conclusion. The DCCT, the DPP, and the Look AHEAD are three interventions, which have proved that nutrition and physical activity are central in the treatment and management of diabetes among the population. The DCCT intervention aims at aiding a diabetic patient to understand how to manage body weight and blood glucose levels.
The central problem that this essay raises describes the characteristics of the U.S. health care system in supporting patients with diabetes. Type 2 Diabetes Mellitus and Its Etiology. Decreased insulin sensitivity in the muscle, tissue, and liver leads to increased insulin production by beta cells of the pancreas.
28 Mar 2024. 20 Mar 2024. 18 Mar 2024. 18 Mar 2024. 18 Mar 2024. 16 Mar 2024. Journal of Diabetes Research publishes articles related to type 1 and type 2 diabetes. Topics include etiology, pathogenesis, management, and prevention of diabetes, as well as associated complications such as nephropathy.
45 of the Best Diabetes Dissertation Topics. Published by Owen Ingram at January 2nd, 2023 , Revised On August 16, 2023. The prevalence of diabetes among the world's population has been increasing steadily over the last few decades, thanks to the growing consumption of fast food and an increasingly comfortable lifestyle.
setting and goal achievement in patients with type 2 diabetes. Nature of the research project This dissertation research is a theory based cross-sectional study using a patient self-administered questionnaire. The exploration of the relationships between support group participation and goal behavior is guided by Social identity theory.
Summary and Conclusion. Diabetes is a multifactorial disease process, and its long-term management requires the active involvement of people with diabetes and their families, as well as a large multidisciplinary care team to ensure optimal health, quality of life, and productivity. Keeping up with new medications, emerging technology, and ...
According to Shaikh (2021), % of the people who are more than 65 years have diabetes. However, the young people are also affected but at a meager percentage compared to the older people. The risk factors for Type 1 diabetes are hereditary, hence easily transferred from parents to children.
Nutrigenetics is considered the foundation of precision nutrition (Table 1) [45, 46].Genetic variation in the form of single nucleotide polymorphisms (SNPs) is considered to account for the heterogeneity in individual dietary response and risk for obesity and type 2 diabetes [47, 48].Nutrigenetic research has investigated the interactions between SNPs influencing body composition, insulin ...
Type 2 diabetes is more prevalent than type 1 diabetes. This essay discusses some of the most frequently asked questions about type 2 diabetes through a sample dialogue between a patient and a doctor. ... For instance, research indicates that if one identical twin is infected, there is a 90% probability of the other twin getting infected ...
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public.
A total of 3260 patients were assigned to receive empagliflozin and 3262 to receive placebo. During a median follow-up of 17.9 months, a first hospitalization for heart failure or death from any ...