Advertisement

Trends in insomnia research for the next decade: a narrative review
- Review Article
- Published: 06 April 2020
- Volume 18 , pages 199–207, ( 2020 )
Cite this article

- Daniel Ruivo Marques 1 , 2 ,
- Ana Allen Gomes 2 , 3 ,
- Vanda Clemente 2 , 4 ,
- José Moutinho dos Santos 4 ,
- Joana Serra 4 &
- Maria Helena Pinto de Azevedo 5
641 Accesses
10 Citations
Explore all metrics
Insomnia disorder has known striking developments over the last few years. Partly due to advances in neuroimaging techniques and brain sciences, our understanding of insomnia disorder has become more fine-tuned. Besides, developments within psychological and psychiatric fields have contributed to improve conceptualization, assessment, and treatment of insomnia. In this paper, we present a list of promising 10 key “hot-topics” that we think in the next 10 years will continue to stimulate researchers in insomnia’s domain: increasing of systematic reviews and meta-analyses; improvement of existing self-report measures; increasing of genetic and epigenetic investigation; research on new pharmacological agents; advances in neuroimaging studies and methods; new psychological clinical approaches; effectiveness studies of e-treatments and greater dissemination of evidence-based therapies for insomnia; call for integrative models; network approach using in insomnia; and assessment of insomnia phenotypes. The breadth of all these topics demands the collaboration of researchers from different scientific fields within sleep medicine. In summarizing, in the next decade, it is predictable that insomnia’s research still benefit from different scientific disciplines.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways
Insomnia and the Orexinergic Pathway in the Link with Psychopathology: Effects of DORAs in Insomnia Comorbid with Mental Disturbances
American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 3rd ed. Westchester: American Academy of Sleep Medicine; 2014.
Google Scholar
American Psychiatric Association. Diagnostic and statistical manual of mental disorders-5. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Book Google Scholar
Baglioni C, Regen W, Teghen A, Spiegelhalder K, Feige B, Nissen C, Riemann D. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18(3):195–21313. https://doi.org/10.1016/j.smrv.2013.04.001 .
Article PubMed Google Scholar
Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. https://doi.org/10.1016/S1389-9457(00)00065-4 .
Berman M, Jonides J, Nee D. Studying mind and brain with fMRI. Soc Cogn Affect Neurosci. 2006;1(2):158–61. https://doi.org/10.1093/scan/nsl019 .
Article PubMed PubMed Central Google Scholar
Bheemsain T, Kar S. An overview of insomnia management. Delphi Psychiatry J. 2012;15(2):294–301.
Blanken T, Benjamins J, Borsboom D, Vermunt J, Paquola C, Ramautar J, Van Someren E. Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry. 2019;6(2):151–63. https://doi.org/10.1016/S2215-0366(18)30464-4 .
Borsboom D, Cramer A. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9(1):91–121. https://doi.org/10.1146/annurev-clinpsy-050212-185608 .
Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. https://doi.org/10.1002/wps.20375 .
Bragantini D, Sivertsen B, Gehrman P, Lydersen S, Güzey IC. Genetic polymorphisms associated with sleep-related phenotypes; relationships with individual nocturnal symptoms of insomnia in the HUNT study. BMC Med Genet. 2019;20(1):179. https://doi.org/10.1186/s12881-019-0916 .
Broomfield N, Espie C. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14(4):401–7. https://doi.org/10.1111/j.1365-2869.2005.00481.x .
Busto U, Sykora K, Sellers E. A clinical scale to assess benzodiazepine withdrawal. J Clin Psychopharmacol. 1989;9(6):412–6. https://doi.org/10.1097/00004714-198912000-00005 .
Article CAS PubMed Google Scholar
Buysse D, Ancoli-Israel S, Edinger J, Lichstein K, Morin C. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–73. https://doi.org/10.1093/sleep/29.9.1155 .
Buysse D, Germain A, Hall M, Monk T, Nofzinger E. A neurobiological model of insomnia. Drug Discov Today Dis Models. 2011;8(4):129–37. https://doi.org/10.1016/j.ddmod.2011.07.002 .
Buysse D, Harvey A. Insomnia: recent developments and future directions. In: Kryger M, Roth T, Dement W, editors. Principles and practices of sleep medicine. 6th ed. Philadelphia: Elsevier; 2017. p. 757–760.
Chapter Google Scholar
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–21313. https://doi.org/10.1016/0165-1781(89)90047-4 .
Cassano GB, Petracca A, Cesana BM. A new scale for the evaluation of benzodiazepine withdrawal symptoms: sessb. Curr Therapeutic Res. 1994;55(3):275–89. https://doi.org/10.1016/s0011-393x(05)80171-7 .
Article Google Scholar
Dekker K, Blanken T, Van Someren E. Insomnia and personality: a network approach. Brain Sci. 2017;7(3):28. https://doi.org/10.3390/brainsci7030028 .
Article PubMed Central Google Scholar
Edinger J, Leggett M, Carney C, Manber R. Psychological and behavioral treatments for insomnia II: implementation and specific populations. In: Kryger M, Roth T, Dement W, editors. Principles and practices of sleep medicine. 6th ed. Philadelphia: Elsevier; 2017. p. 814–831.
Eisai Global. U.S. FDA approves Eisai’s Dayvigo TM (Lemborexant) for treatment of insomnia in adult patients. 2019. https://www.eisai.com/news/2019/news201993.html . Accessed 31 Jan 2020.
Espie C, Kyle S, Williams C, Ong J, Douglas N, Hames P, Brown J. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–81. https://doi.org/10.5665/sleep.1872 .
Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Curr Opin Psychiatry. 2017;30(1):56–63. https://doi.org/10.1097/YCO.0000000000000292 .
Field A, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63:665–94. https://doi.org/10.1348/000711010X502733 .
Gehrman P, Pfeiffenberger C, Byrne E. The role of genes in the insomnia phenotype. Sleep Med Clin. 2013;8(3):323–31. https://doi.org/10.1016/j.jsmc.2013.04.005 .
Gilbert P. The origins and nature of compassion focused therapy. Br J Clin Psychol. 2014;53(1):6–41. https://doi.org/10.1111/bjc.12043 .
Gregory AM, Rijsdijk FV, Eley TC, Buysse DJ, Schneider MN, Parsons M, Barclay NL. A longitudinal twin and sibling study of associations between insomnia and depression symptoms in young adults. Sleep. 2016;39(11):1985–92. https://doi.org/10.5665/sleep.6228 .
Hadian S, Jabalameli S. The effectiveness of compassion-focused therapy (CFT) on rumination in students with sleep disorders: a quasi-experimental research, before and after. J Urmia Univ Med Sci. 2019;30(2):86–96.
Hein M, Lanquart J-P, Loas G, Hubain P, Linkowski P. Similar polysomnographic pattern in primary insomnia and major depression with objective insomnia: a sign of common pathophysiology?. BMC Psychiatry. 2017. https://doi.org/10.1186/s12888-017-1438-4
Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Baglioni C. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. https://doi.org/10.1016/j.smrv.2018.10.006 .
Jansen P, Watanabe K, Stringer S, Skene N, Bryois J, Posthuma D. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51:394–403. https://doi.org/10.1038/s41588-018-0333-3 .
Jones S, van Hees V, Mazzotti D, Marques-Vidal P, Sabia S, van der Spek A, Wood A. Genetic studies of accelerometer-based sleep measures in 85,670 individuals yield new insights into human sleep behaviour. Nat Commun. 2018;10(1):1585. https://doi.org/10.1038/s41467-019-09576-1 .
Article CAS Google Scholar
Kobayashi M, Okajima I, Narisawa H, Kikuchi T, Matsui K, Inada K, Inoue Y. Development of a new benzodiazepine hypnotics withdrawal symptom scale. Sleep Biol Rhythm. 2018;16(3):263–71. https://doi.org/10.1007/s41105-018-0151-0 .
Lane J, Jones S, Dashti H, Wood A, Aragam K, Saxena R. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51:387–93. https://doi.org/10.1038/s41588-019-0361-7 .
Article CAS PubMed PubMed Central Google Scholar
Lilenfeld S. What is “evidence” in psychotherapies? World Psychiatry. 2019;18(3):245–6. https://doi.org/10.1002/wps.20654 .
Lind MJ, Gehrman PR. Genetic pathways to insomnia. Brain Sci. 2016;6(4):64. https://doi.org/10.3390/brainsci6040064 .
Article CAS PubMed Central Google Scholar
Ma Z-R, Shi L-J, Deng M-H. Efficacy of cognitive behavioral therapy in children and adolescents with insomnia: a systematic review and meta-analysis. Braz J Med Biol Res. 2018;51(6):e7070. https://doi.org/10.1590/1414-431X20187070 .
Marques D. Do we need neuroimaging to treat insomnia effectively? Sleep Med. 2019;53:205. https://doi.org/10.1016/j.sleep.2017.08.005 .
Marques D. “Time to relax”: considerations on relaxation training for insomnia disorder. Sleep Biol Rhythm. 2019;17(2):263–4. https://doi.org/10.1007/s41105-018-00203-y .
Marques D. Self-report measures as complementary exams in the diagnosis of insomnia. Revista Portuguesa de Investigação Comportamental e Social. 2020. [Accepted for publication] .
Marques D, Azevedo MH. Potentialities of network analysis for sleep medicine. J Psychosom Res. 2018;111:89–90. https://doi.org/10.1016/j.jpsychores.2018.05.019 .
Marques D, Clemente V, Gomes A, Azevedo MH. Profiling insomnia using subjective measures: where are we and where are we going. Sleep Med. 2018;43:103–4. https://doi.org/10.1016/j.sleep.2017.12.006 .
Marques D, Gomes A, Azevedo MH. Utility of studies in community-based populations. 2019. [Manuscript submitted for publication] .
Marques D, Gomes A, Caetano G, Castelo-Branco M. Insomnia disorder and brain’s default-mode network. Curr Neurol Neurosci Rep. 2018;18(8):45. https://doi.org/10.1007/s11910-018-0861-3 .
Marques D, Gomes A, Clemente V, Santos J, Castelo-Branco M. Default-mode network activity and its role in comprehension and management of psychophysiological insomnia: a new perspective. New Ideas Psychol. 2015;36:30–7. https://doi.org/10.1016/j.newideapsych.2014.08.001 .
Marques D, Gomes A, Clemente V, Santos J, Duarte I, Caetano G, Castelo-Branco M. Unbalanced resting-state networks activity in psychophysiological insomnia. Sleep Biol Rhythm. 2017;15(2):167–77. https://doi.org/10.1007/s41105-017-0096-8 .
Marques D, Gomes AA, Clemente V, Moutinho J, Caetano G, Castelo-Branco M. An overview regarding insomnia disorder: conceptualization, assessment and treatment. In: Columbus AM, editor. Advances in psychology research. New York: Nova Science Publishers Inc; 2016. p. 81–116.
Marques D, Gomes A, Clemente V, Santos J, Caetano G, Castelo-Branco M. Neurobiological correlates of psychological treatments for insomnia: a review. Eur Psychol. 2016;21(3):195–205. https://doi.org/10.1027/1016-9040/a000264 .
McNally R. Can network analysis transform psychopathology? Behav Res Ther. 2016;86:95–104. https://doi.org/10.1016/j.brat.2016.06.006 .
Morales-Lara D, De-la-Peña C, Murillo-Rodríguez E. Dad’s snoring may have left molecular scars in your DNA: the emerging role of epigenetics in sleep disorders. Mol Neurobiol. 2018;55(4):2713–24. https://doi.org/10.1007/s12035-017-0409-6 .
Morin C. Contributions of cognitive-behavioral approaches to the clinical management of insomnia. Prim Care Companion J Clin Psychiatry. 2002;4(1):21–6.
Morin C, Vallières A, Ivers H. Dysfunctional Beliefs and Attitudes about Sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–54. https://doi.org/10.1093/sleep/30.11.1547 .
Ong J, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt J. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–63. https://doi.org/10.5665/sleep.4010 .
Owen M, Cardno A, O’Donovan M. Psychiatric genetics: back to the future. Mol Psychiatry. 2000;5(1):22–31. https://doi.org/10.1038/sj.mp.4000702 .
Päivi L, Sitwat L, Harri O-K, Joona M, Raimo L. ACT for sleep—internet-delivered self-help ACT for sub-clinical and clinical insomnia: a randomized controlled trial. J Context Behav Sci. 2019;12:119–27. https://doi.org/10.1016/j.jcbs.2019.04.001 .
Palagini L, Biber K, Riemann D. The genetics of insomnia–evidence for epigenetic mechanisms? Sleep Med Rev. 2014;18(3):225–35. https://doi.org/10.1016/j.smrv.2013.05.002 .
Perlis M, Ellis J, Kloss J, Riemann D. Etiology and pathophysiology of insomnia. In: Kryger M, Roth T, Dement W, editors. Principles and practices of sleep medicine. 6th ed. Philadelphia: Elsevier; 2017. p. 769–784.
Perlis M, Shaw P, Cano G, Espie C. Models of insomnia. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 5th ed. Missouri: Elsevier Saunders; 2011. p. 850–865.
Qureshi I, Mehler M. Epigenetics of sleep and chronobiology. Curr Neurol Neurosci Rep. 2014;14(3):432. https://doi.org/10.1007/s11910-013-0432-6 .
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis J, Spiegelhalder K. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. https://doi.org/10.1111/jsr.12594 .
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–311. https://doi.org/10.1016/j.smrv.2009.04.002 .
Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. REM sleep instability: a new pathway for insomnia? Pharmacopsychiatry. 2012;45(5):167–76. https://doi.org/10.1055/s-0031-1299721 .
Rodriguez M, Maeda Y. Meta-analysis of coefficient alpha. Psychol Methods. 2006;11(3):306–22. https://doi.org/10.1037/1082-989X.11.3.306 .
Schulz H, Salzarulo P. The development of sleep medicine: a historical sketch. J Clin Sleep Med. 2016;12(7):1041–52. https://doi.org/10.5664/jcsm.5946 .
Siddaway A, Wood A, Hedges L. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019;70:747–70. https://doi.org/10.1146/annurev-psych-010418-102803 .
Sirois F, Nauts S, Molnar D. Self-compassion and bedtime procrastination: an emotion regulation perspective. Mindfulness. 2019;10(3):434–45. https://doi.org/10.1007/s12671-018-0983-3 .
Spiegelhalder K, Regen W, Baglioni C, Nissen C, Riemann D, Kyle S. Neuroimaging insights into insomnia. Curr Neurol Neurosci Rep. 2015;15(3):9. https://doi.org/10.1007/s11910-015-0527-3 .
Stein M, McCarthy M, Chen C, Jain S, Gelernter J, He F, Ursano R. Genome-wide analysis of insomnia disorder. Mol Psychiatry. 2018;23(11):2238–50. https://doi.org/10.1038/s41380-018-0033-5 .
Stepanski E. Behavioral sleep medicine: a historical perspective. Behav Sleep Med. 2003;1(1):4–21. https://doi.org/10.1207/S15402010BSM0101_3 .
Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–84. https://doi.org/10.1038/s41576-019-0127 .
Tolin D, McKay D, Forman E, Klonsky E, Thombs B. Empirically supported treatment: recommendations for a new model. Clin Psychol Sci Pract. 2015;22(4):317–38. https://doi.org/10.1111/cpsp.12122 .
Trauer J, Qian M, Doyle J, Rajaratnam S, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. https://doi.org/10.7326/M14-2841 .
van Dalfsen J, Markus C. The involvement of sleep in the relationship between the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and depression: a systematic review. J Affect Disord. 2019;256:205–12. https://doi.org/10.1016/j.jad.2019.05.047 .
van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin C, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3–16. https://doi.org/10.1016/j.smrv.2017.02.001 .
Walsh J, Roth T. Pharmacologic treatment of insomnia: benzodiazepine receptor agonists. In: Kryger M, Roth T, Dement W, editors. Principles and practices of sleep medicine. 6th ed. Philadelphia: Elsevier; 2017. p. 832–841.
Wang Y, Wang F, Zheng W, Zhang L, Ng C, Unqvari G, Xiang Y. Mindfulness-based interventions for insomnia: A meta-analysis of randomized controlled trials. Behav Sleep Med. 2018. https://doi.org/10.1080/15402002.2018.1518228 [Epub ahead of print] .
Whitfield-Gabrieli S, Ford J. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. https://doi.org/10.1146/annurev-clinpsy-032511-143049 .
Wilson B. Cutting edge developments in neuropsychological rehabilitation and possible future directions. Brain Impair. 2011;12(1):33–42. https://doi.org/10.1375/brim.12.1.33 .
Wilson B. Neuropsychological rehabilitation: state of the science. S Afr J Psychol. 2013;43(3):267–77. https://doi.org/10.1177/0081246313494156 .
Winkler A, Rief W. Effect of placebo conditions on polysomnographic parameters in primary insomnia: a meta-analysis. Sleep. 2015;38(6):925–31. https://doi.org/10.5665/sleep.4742 .
Wittchen H, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Steinhausen H. The size and burden of mental disorders and other disorders of the brain in Europe. Eur Neuropsychopharmacol. 2011;21(9):655–79. https://doi.org/10.1016/j.euroneuro.2011.07.018 .
Download references
Acknowledgements
The authors would like to express their gratitude to the reviewers for their important comments and suggestions.
Author information
Authors and affiliations.
Department of Education and Psychology, University of Aveiro, Campus Universitário de Santiago, 3810-193, Aveiro, Portugal
Daniel Ruivo Marques
Faculty of Psychology and Educational Sciences, CINEICC-Center for Research in Neuropsychology and Cognitive Behavioral Intervention, University of Coimbra, Coimbra, Portugal
Daniel Ruivo Marques, Ana Allen Gomes & Vanda Clemente
Faculty of Psychology and Educational Sciences, University of Coimbra, Rua Do Colégio Novo, 3000-115, Coimbra, Portugal
Ana Allen Gomes
Sleep Medicine Centre, Coimbra University Hospital Centre (CHUC), Coimbra, Portugal
Vanda Clemente, José Moutinho dos Santos & Joana Serra
Faculty of Medicine, University of Coimbra, Rua Larga, 3004-504, Coimbra, Portugal
Maria Helena Pinto de Azevedo
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Daniel Ruivo Marques .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Marques, D.R., Gomes, A.A., Clemente, V. et al. Trends in insomnia research for the next decade: a narrative review. Sleep Biol. Rhythms 18 , 199–207 (2020). https://doi.org/10.1007/s41105-020-00269-7
Download citation
Received : 20 August 2019
Accepted : 28 March 2020
Published : 06 April 2020
Issue Date : July 2020
DOI : https://doi.org/10.1007/s41105-020-00269-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sleep disorder
- Find a journal
- Publish with us
- Track your research
HYPOTHESIS AND THEORY article
Conceptual framework for insomnia: a cognitive model in practice.

- 1 Neurocognitive Engineering Laboratory (NEL), Institute of Mathematics and Computer Science, University of São Paulo, São Carlos, Brazil
- 2 Department of Neuroscience and Behavioural Sciences, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil
- 3 Neuroscience Center, Instituto de Investigaciones Científicas Servicios de Alta Tecnología (INDICASAT AIP), Panama City, Panama
- 4 Department of Neuroscience, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
- 5 Dana Brain Health Institute, Iranian Neuroscience Society-Fars Chapter, Shiraz, Iran
- 6 Academy of Health, Senses Cultural Foundation, Sacramento, CA, United States
- 7 Department of Cognitive Neuroscience, Institute for Cognitive Science Studies (ICSS), Pardis, Iran
- 8 Reconfigurable Computing Laboratory, Institute of Mathematics and Computer Science, University of São Paulo, São Carlos, Brazil
- 9 Department of Ophthalmology, Otorhinolaryngology, Head and Neck Surgery, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil
Insomnia is a widespread neuropsychological sleep-related disorder known to result in various predicaments including cognitive impairments, emotional distress, negative thoughts, and perceived sleep insufficiency besides affecting the incidence and aggravation of other medical disorders. Despite the available insomnia-related theoretical cognitive models, clinical studies, and related guidelines, an evidence-based conceptual framework for a personalized approach to insomnia seems to be lacking. This study proposes a conceptual cognitive framework (CCF) providing insight into cognitive mechanisms involved in the predisposition, precipitation, and perpetuation of insomnia and consequent cognitive deficits. The current CCF for insomnia relies on evaluative conditional learning and appraisal which generates negative valence (emotional value) and arousal (cognitive value). Even with the limitations of this study, the suggested methodology is well-defined, reproducible, and accessible can help foster future high-quality clinical databases. During clinical insomnia but not the neutral one, negative mood (trait-anxiety) causes cognitive impairments only if mediating with a distorted perception of insomnia ( Ind-1 = 0.161, 95% CI 0.040–0.311). Further real-life testing of the CCF is intended to formulate a meticulous, decision-supporting platform for clinical interventions. Furthermore, the suggested methodology is expected to offer a reliable platform for CCF-development in other cognitive impairments and support the causal clinical data models. It may also improve our knowledge of psychological disturbances and complex comorbidities to help design rehabilitation interventions and comprehensive frameworks in line with the “preventive medicine” policies.
Introduction
Behavioral sleep disturbances are classified into various types of insomnia, excessive daytime somnolence (EDS), sleep phase disorders, and parasomnias. These are potentially rooted in psychophysiological, cognitive, emotional, and behavioral abnormalities resulting in impaired sleep efficacy, disintegrated sleep cycles, and/or arousal instability ( Cormier, 1990 ; Sateia, 2014 ). Insomnias are characterized by poor subjective sleep quality, difficulty in falling asleep and maintaining sleep at bed-time, wakes after sleep onset (WASO), or unprompted early morning awakening. The consequent diurnal symptoms may then present as inadequate cognitive functions, declined cognitive aptitude, fatigue, hampered productivity, depression or irritability, impaired decision-making, low motivation, and mood dysregulation ( Mai and Buysse, 2008 ; Nami, 2014 ).
Recently, cognitive-vulnerability models, which theoretically justify the interrelation between sleeplessness and mood dysregulation or cognitive insufficiencies, have drawn the attention of the research community. When insomnia becomes a chief complaint, the vicious cycle of insomnia-anxiety-insomnia starts to emerge. Undeniably, affective dysregulation, impulsivity, restlessness, EDS, disrupted vigilance, and cognitive decline are some consequences of long-term sleep insufficiency in many instances ( Nami, 2014 ).
Among the theoretical and cognitive-computational models related to insomnia, the cognitive vulnerability model for insomnia induced mood disturbances (CVMIMD), the sleep-specific cognitive vulnerability (SSCV), the behaviorally induced insufficient sleep syndrome with restricted and extended sleep opportunity (BIISS-RESO), and the global cognitive vulnerability to insomnia (GCVI) ( Bei et al., 2015 ) are the main highlights. These models are addressed subsequently.
Cognitive Vulnerability Model for Insomnia Induced Mood Disturbances
From the neurocognitive standpoint, the prefrontal cortex (PFC), which plays a pivotal role in affect-regulation and cognitive-control, develops intensely throughout the neurodevelopment phase and adolescence owing to neuroplasticity. When the hypnic tone is decreased either due to poor sleep hygiene or socio-behavioral and psychophysiological stressors, a proposed explanation is the activation of PFC’s maladaptive processes as a potential neurocognitive mechanism underlying the affective consequences of insomnia and inefficient sleep, in general ( Freeman et al., 2005 ).
The body of psycho-behavioral and neurocognitive empirical evidence describing the precise mechanisms that underlie the link between insomnia and negative mood is thin. However, subjective sleep insufficiencies and dysregulated mood observations exhibited more robust relationship as compared to objective findings from polysomnography or even full-setup sleep electroencephalography data. This points to the fact that psychological factors that hinder sleep efficiency might play significant roles in justifying the sleep-mood crosstalk. Yet some of these insomnia-related cognitive vulnerability factors are now acknowledged as erroneous beliefs, cognitive biases, and thought patterns that increase the likelihood of the predisposed individuals toward psychopathology ( Freeman et al., 2005 ).
Sleep Specific Cognitive Vulnerability
In some instances, the erroneous beliefs and attitudes represent exclusive sleep-related problems in which case, the distressing worries related to insomnia-continuation are usually evaluated using the dysfunctional beliefs and attitudes about sleep (DBAS) Scale. Harvey’s cognitive model ( Harvey, 2002 ) described the impact of the DBAS-related cognitive vulnerability on insomnia complaints. According to this model, insomniacs are generally worried about poor sleep and its daytime consequences, and such strong, negatively toned thoughts trigger selective attentional-emotional bias, wherein individuals over-monitor their sleep-related threat cues. Previous investigations proposed a strong connection between DBAS and poor sleepers, which happens to play a key role in DBAS-driven disturbances in sleep perception and sleep safety behaviors such as napping ( Harvey, 2002 ).
Behaviorally Induced Insufficient Sleep Syndrome With Restricted and Extended Sleep Opportunity
This condition refers to a typical complaint reported by the patients as “ at nights I cannot sleep, in the morning, I cannot wake up .” Habitual sleep episodes are usually shorter (confirmed by history, sleep log, or actigraphy) for patients experiencing initial or maintenance insomnia compared to the normative values from age-adjusted groups. Such patients also report sleep-inertia in the morning and complain about EDS for a minimum of 3 months before the interview. However, they tend to sleep considerably longer on weekends or during vacation. In general, the reported objective sleep efficiency as detected by polysomnography is below 80%, besides the mean initial nocturnal sleep latency which takes a longer time, more than 45 min. Also, these patients report repeated WASOs ( Bastien et al., 2008 ).
Global Cognitive Vulnerability to Insomnia
Cognitive vulnerability is defined as global when the dysfunctional beliefs and attitudes are general and not necessarily focused on a distinct behavioral or experiential area. According to Beck’s cognitive model, psycho-traumas in the early years of life combined with a complicated past can foster negative attitudes and biases concerning both self, world and the future. Such beliefs yield maladaptive schemas that may trigger cognitive vulnerabilities and negative tendencies based on depression later in life ( Beck, 2008 ). A few studies on GCVI suggest strong links between sleep predicaments (mainly insomnias) and negatively toned cognitive constructs. For instance, complaints of chronic insomnia in young adults were found to be associated with anxiety and depression-related cognitive factors ( Alfano et al., 2009 ). In the same vein, Sadler et al. (2013) claimed hopelessness, a global cognitive-vulnerability factor in older adults, can amplify the effects of insomnia on depressive symptoms ( Sadler et al., 2013 ).
The various types of insomnia (more than 10) require personalized treatment approaches. Some of the broadly described types include adjustment insomnia, drug or substance-induced insomnia, comorbid insomnia, onset insomnia, middle insomnia, late insomnia, conditioned, or psychophysiological insomnia, behavioral insomnia of childhood, idiopathic insomnia, paradoxical insomnia, and sleep hygiene insomnia ( Dzierzewski et al., 2018 ). Based on the severity of sleep insufficiency, we have categorized insomnia patients into Neutral (with a mild to moderate perception of sleep difficulties) and Clinical (with a severe perception of sleep difficulty) types.
Gross (1998) designed the modal model of emotion as a conceptual framework to illustrate how emotions can be generated and evolve over time. The emotion-generation process begins with internal or external goal-relevant situations that draw attention to specific features of the situation, appraisals emerge to make meaning of the situation resulting in multi-faceted emotional responses and feedback to modulate the current situation perpetually. Collectively, the modal model reflects the dynamic nature of the emotions and suggests possible emotion-regulation strategies comprising of situation selection, situation modification, attention deployment, cognitive change, and response modulation ( Gross, 2013 ).
To the best of our knowledge, the aforementioned studies have little mention of causality (mediation) relationships, which can easily mislead interpretations of the findings. Thus, necessitating the design of an approach to conceptualize these theories and hypotheses. A novel insomnia theoretical-conceptual framework would enable the drawing of data models for testing mediational relationships between independent variables and outcomes within retrospective studies. Besides, it may also help suggest research strategies and predictions designing prospective studies on insomnia. The present study aims to fill this void in the literature by proposing and validating a novel conceptual cognitive framework (CCF) for insomnia in light of the above-mentioned models. The CCF illustrates how cognitive processes and their interactions can generate annoyance-distress reactions, which in turn, lead to the development or maintenance of insomnia. The insomnia numerical model is also demonstrated through multi-mediatory (causality) modeling approaches.
Proposed Conceptual Cognitive Framework
Fundamental ideas and postulations of the conceptual cognitive framework.
• Conceptual cognitive framework aims at illustrating the interaction between cognitive processes that cause annoyance-distress reactions in insomnia.
• Conceptual cognitive framework rests mainly on evaluative conditioning, assuming a conscious attended awareness perception (CAAP) to both unconditioned stimulus (US) and conditioned stimulus (CS), and their contingencies essential for attitude formation.
• Either or both, cognitive-value and emotional-value, can cause annoyance; however, they can also affect each other merely through annoyance. Furthermore, annoyance distorts the corresponding perception of sleep quality by affecting cognitive and emotional values.
• Lower levels of cognitive-emotional values such as those encountered in the Neutral stage might generate annoyance, yet not sufficient enough to trigger distress reactions. Consequently, annoyance and distress are considered two different concepts in the current framework.
• Cognitive processes for sleep-initiation and sleep-maintenance difficulties are presumed to occur analogously.
Hypothetically, CCF compartments include situation, attention bias, cognitive value (arousal), emotional value (valence), annoyance-distress reaction, and distorted perception. The proposed CCF aims at illustrating how the interaction among cognitive processes contributes to distress reactions.
This paper focuses on insomnia experienced before sleep, and the associated “situation” is restricted to the night-time silence period. According to the CCF, when insomnia-related stimuli capture attentional resources, either directly or through corresponding cognitive and emotional values, distress is triggered resulting in a distorted perception. Distress, in turn, feeds back and influences the situation. Similarly, distress reaction fuels back corresponding cognitive and emotional values. The proposed CCF is illustrated in Figure 1 .
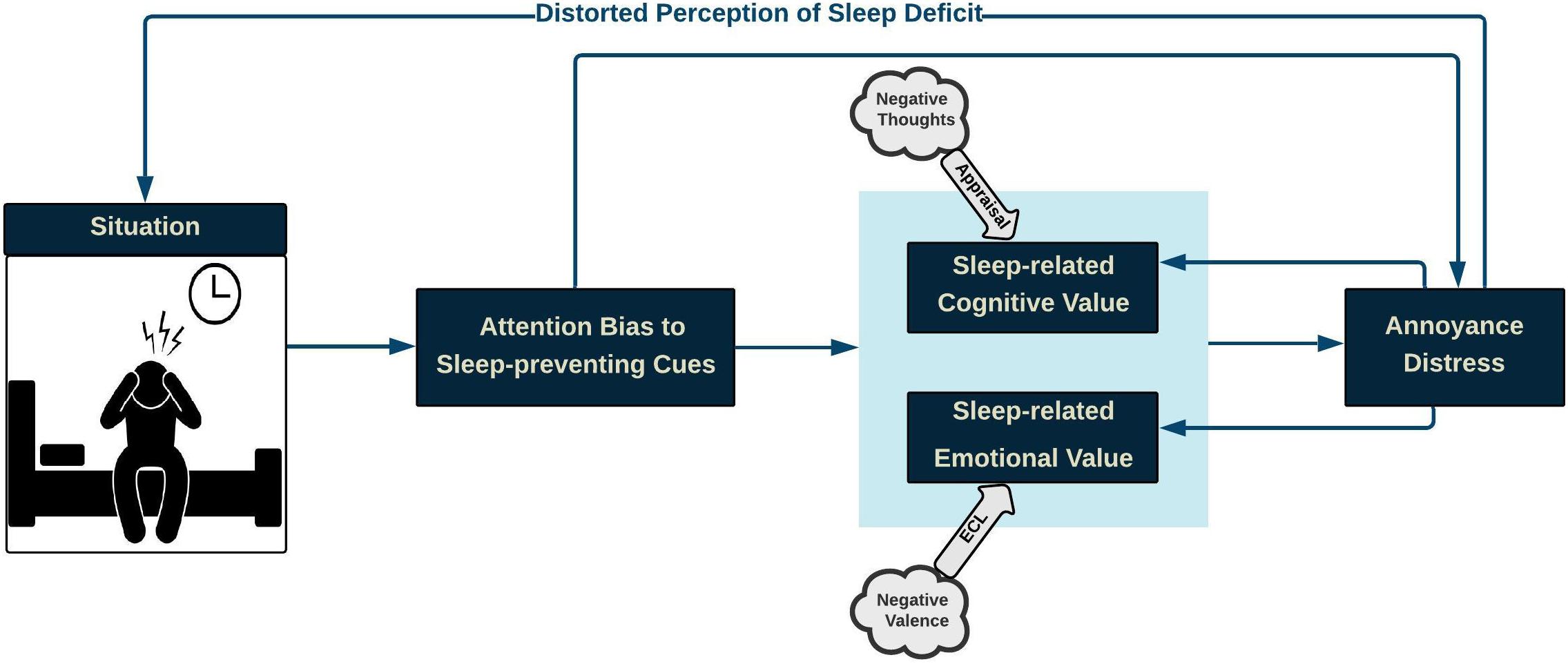
Figure 1. Conceptual Cognitive Framework of Insomnia: During the pre-sleep situation, when attentional resources are captured by insomnia-related stimuli, either directly or through insomnia-related cognitive and emotional values, distress is triggered, thus resulting in a distorted perception of sleep quality, which, in turn, worsens the sleep-initiation process. Likewise, insomnia distress strengthens the negative cognitive-emotional value of difficulty in sleep.
Toward providing a proof of concept for the proposed CCF, we primarily present some supporting evidence from the Insomnia literature.
Compartments and Cognitive Processes
Nighttime silence in the pre-sleep period can facilitate CAAP of internal (body sensation or thoughts) and external (environmental sounds, light, and heat) stimuli. Bootzin and Rider (1997) noted that “bedtime may often be the first quiet time during the day available to think about the day’s events and to worry and plan for the next day.” Therefore, bed and bedtime tend to be cues for arousal rather than for sleep.
Attention Bias
Consciously attended internal and external stimuli develop an individual’s predictions and expectations from the pre-sleep situation. Therefore, an attention bias takes place if any novelty or change occurs in the features of such stimuli ( Horstmann and Herwig, 2016 ). Similarly, Roberts et al. (2013) have supported the notion that “discrepancy between an expectation and upcoming stimuli can bias attention” ( Horstmann and Herwig, 2016 ). Additionally, emotion-laden or threat-related stimuli would be prioritized over other stimuli leading to an attentional bias. The same rationale applies to cognitive theories of anxiety disorders ( Beck and Clark, 1997 ), according to which prioritized attention-allocation to threat cues would trigger the development and maintenance of anxiety ( Dalgleish and Watts, 1990 ). Threat cues for patients with insomnia might be related to sleep quality (arising secondarily to bodily sensations such as palpitation, muscle tension, or attention bias toward noises outside and inside the house), which impairs the process of falling asleep.
One of the commonly used paradigms for experimental assessment of attentional bias is the Dot-Probe task. In this, a pair of stimuli (e.g., words or pictures) are presented simultaneously at different locations (up/down or top/bottom) on the screen. The stimuli pair disappear after a fixed time window and a probe appears in the location of emotional (congruent presentations) or neutral (incongruent presentations) stimuli. Subjects are asked to detect and respond to the location of the probe as fast as possible, and the attentional bias is measured through their reaction time in responding to the probe location. A faster probe detection for congruent trials is believed to indicate vigilance, and a slower probe detection for the incongruent trials is suggestive of difficulty in disengaging attention from emotional stimuli ( Koster et al., 2004 ).
Several studies have investigated the impact of the emotional-attentional bias on sleep-related threatening cues through different attentional paradigms, including Dot-probe ( MacMahon et al., 2006 ; Jansson-Fröjmark et al., 2012 ), flicker ( Jones et al., 2005 ), Posner ( Woods et al., 2009 ), emotional Stroop ( Barclay and Ellis, 2013 ), and eye-tracking ( Woods et al., 2013 ). Most of these studies have endorsed the notion that poor sleepers display attentional bias to sleep-related cues compared with controls. Jansson-Fröjmark et al. (2012) used a dot-probe task to demonstrate that individuals with primary insomnia had a considerably prolonged reaction-time when shifting attention away from insomnia-associated pictures paired with neutral pictures, in comparison to neutral-neutral paired picture presentations as control. Their findings suggest that insomniacs have more difficulty in shifting attention away from insomnia-related stimuli, but are not more vigilant to those stimuli than normal sleepers ( Jansson-Fröjmark et al., 2012 ). However, results reported by Spiegelhalder et al. (2010) yielded no statistically significant preferential attentional-allocation to sleep-related stimuli. Inconsistent results from studies on insomnia may have emerged due to confounding factors and possible bias, impeding their methodologies and study design.
Emotional Value
The emotional value gets shaped through the evaluative conditional learning (ECL) mechanism which plays a crucial role in liking and disliking stimuli ( Ghodratitoostani et al., 2016a , b ). Based on ECL, neutral stimuli (CS) can obtain either positive or negative valence after being repeatedly paired with emotion-laden stimuli (US) ( De Houwer et al., 2001 ). Valence represents emotional states varying along a spectrum, ranging from positive to negative feelings with a neutral center-point ( Bradley and Lang, 1994 ). Based on the CCF, CAAP of both CS and US, and their contingencies are required at the time of EC-learning formation. Additionally, evaluative conditioning is an accumulative procedure through which different valenced USs can add to CS valence after being repeatedly paired ( Stahl and Unkelbach, 2009 ). Therefore, EC-learning is resistant to extinction so that neither individual CS/US presence alone, nor pairing CS with different USs would cause the extinction of previously shaped evaluative conditioning ( De Houwer et al., 2001 ). Applying the CCF, the ECL mechanism suggests that the negative valence of other USs fuels a negative sleep-related emotional-value leading to annoyance or distress reaction. Different negative USs can also frequently get paired with internal (bodily sensations) and external (environmental sounds, light, or heat) sleep-preventing stimuli. Thereafter, attending to sleep-preventing cues alone might trigger distress reactions due to the learned USs’ valence.
Cognitive Value
The cognitive value related to internal and external stimuli is built through an appraisal process. This process initiates when the meaning of an object or event is evaluated in a particular situation according to pre-existing beliefs, desires, and intentions ( Scherer et al., 2001 ). However, not all information but that relevant to individuals’ concerns ( Frijda, 1987 ), can trigger a cognitively aroused state followed by the appraisal. Accordingly, attention bias to sleep-preventing cues (as concern-relevant stimuli) can trigger a cognitively aroused state with subsequent appraisals about insomnia, “ I am never going to get to sleep ,” “ I am not coping with the amount of sleep I get ,” and “ I am going to lose my job ” ( Harvey, 2002 ). Negative thoughts through this appraisal mechanism further fuel the negative sleep-related cognitive value, leading to annoyance or distress reaction.
Self-reported questionnaires are widely used for collecting patients’ thoughts and beliefs about events, situations, or objects that require conscious appraisals of conditions, and their corresponding consequences. Pre-Sleep Arousal Scale ( Nicassio et al., 1985 ), Sleep Disturbance Questionnaire ( Espie et al., 1989 ), and DBAS Scale ( Morin, 1993 ) are commonly applied for assessing thoughts and beliefs related to insomnia. The latter is greatly helpful in clinical practice since it distinguishes salient irrational, and often emotionally loaded thoughts that disturb sleep onset. Nicassio et al. (1985) and Lichstein and Rosenthal (1980) evaluated the intensity of cognitive and somatic arousal at bedtime through the Pre-Sleep Arousal Scale and reported cognitive arousal was more strongly associated with sleeping difficulty. Similarly, Espie et al. (1989) used the Sleep Disturbance Questionnaire and observed “ My mind keeps turning things over ” and “ I am unable to empty my mind ” were the most often endorsed statements among insomniacs ( Espie et al., 1989 ).
Several authors have assessed characteristics of pre-sleep thoughts in terms of content ( Harvey, 2000 ; Wicklow and Espie, 2000 ), frequency ( Barclay and Gregory, 2010 ), and valence ( Kuisk et al., 1989 ). For instance, Wicklow and Espie (2000) conducted an experimental study on people with clinically significant sleep difficulties using audiotape to record their pre-sleep thoughts and wrist-actigraphy to obtain sleep patterns. The authors indicated that the more frequent thoughts were related to “rehearsing, planning and problem-solving” and “sleep and its consequences,” which strongly correlated with unpleasant emotions and could predict objective sleep latency. Contrarily, Barclay and Gregory (2010) observed that the orientation of catastrophic thoughts in poor sleepers may not be necessarily sleep-specific, instead it was linked to a general tendency to be in an iterative manner regardless of the content or emotional valence. Sleepers were asked to catastrophize their thoughts into three topics namely sleep quality, current personal worries, and hypothetical positive topics. Poor sleepers exhibited greater catastrophic thoughts on every single topic in comparison with good ones, however, no difference was observed in occurrence of catastrophic worry about each topic among poor sleepers. Davey and Levy (1998) suggested that the tendency for repetitive thinking in insomniacs is similar to that of worriers who hold dysfunctional beliefs about the benefits of worrying. In other words, insomniacs believe the ongoing worry helps them find solutions and prevent adverse outcomes. Using DBAS, Morin et al. (1993) reported that not only excessive cognitive activity, but the valence of thoughts also plays a crucial role in provoking emotional reactions to sleep impairment.
Annoyance-Distress Reaction
Consistent with many cognitive-behavioral studies, the CCF suggests that negative appraisals of insomnia trigger the annoyance-distress reactions. According to the cognitive model of insomnia, excessively negative thinking in the pre-sleep time provokes autonomic arousal, and emotional distress ( Harvey, 2002 ). Tang and Harvey (2004a) have reported that the manipulation of psychological and physiological arousal produces adverse effects on the perception of sleep quality. For illustrative purposes, Baglioni et al. (2010) presented five blocks showing neutral, negative, positive, sleep-related negative and sleep-related positive pictures to evaluate the psychophysiological reactivity to emotional stimuli, both related and unrelated to sleep, in people with primary insomnia and normal sleepers. facial electromyography, heart rate, and cardiac vagal tone were recorded during the picture presentation. The insomnia group indicated an enhanced physiological “craving” response for positive sleep stimuli (e.g., picture of a person asleep in bed), prolonged physiological arousal in response to all stimuli, and increased subjective arousal for negative sleep stimuli (e.g., picture of a person lying awake in bed) when compared to normal sleepers ( Baglioni et al., 2010 ).
Distorted Perception
According to the CCF, valence and cognitive-arousal as two components of emotion can affect patients’ judgment about sleep quality perception. The following findings lend support to this proposal.
Yoo and Lee (2015) explored the effect of modulating arousal and valence on time-perception in subjects with social anxiety, comparing the time duration of the presented stimuli with the standard duration in training sessions. The perceived duration of negative-stimuli against positive-stimuli was longer with high arousal, but shorter with low arousal levels, suggesting that modifications in the type and magnitude of both valence and arousal modulate time-perception ( Yoo and Lee, 2015 ). This may also be analogous to the distortion in sleep quality-perception in insomniacs.
Using self-reported subjective sleep quality, Tang and Harvey (2004a) observed that experiencing anxious cognitive and physiological arousal in the pre-sleep period resulted in the perception of a longer sleep-onset latency and shorter total sleep time. Moreover, actigraphy results showed contradictions to the reported subjective sleep quality, thus corroborating distorted perception ( Tang and Harvey, 2004a ).
On the contrary, Herbert et al. (2017) inspected the psychophysiological predictors of subjective/objective sleep discrepancy in Total Sleep Time ( Manconi et al., 2010 ) and Sleep Onset Latency ( Herbert et al., 2017 ) indices among poor sleepers. They reported that excessive pre-sleep cognitive activity and lower mood at the awakening time of the following day are predictors of distortion in time estimation.
Hypotheses of Conceptual Cognitive Framework
The primary speculation was that the CAAP of internal and external sleep-preventing stimuli captures attentional resources preferentially and triggers the appraisal process, ending with annoyance and distress in the pre-sleep situation. A secondary hypothesis was that intermittent distress experienced in the Clinical stage leads to a misperception about sleep quality.
We applied the multi-mediation insomnia model based on clinical data toward putting the CCF into practice and provide supporting evidence for the proposed causality relationship between the cognitive processes in different stages of insomnia.
For the CCF assessment, data were collected from the participants of (1) a randomized crossover three-session double-blind study and (2) an observational prospective cohort study. Both studies were approved by the Ethics Committee for Analysis of Research Projects, Specialized Center of Otorhinolaryngology and Speech Therapy, Hospital das Clínicas de Ribeirão Preto, University of São Paulo, Brazil (HCRP no 55716616.1.1001.5440, and HCRP no 09813519.1.0000.5440; internationally registered with U1111-1236-5441). All participants gave written informed consent.
Two-hundred fifty-three participants (123 female, 130 male) aged 27–72 years (54.43 ± 10.31 years) were recruited for this study. Before the sessions in both studies, participants filled up a Portuguese version of a battery of questionnaires that included (a) a six-item state-trait anxiety inventory (STAI) ( Gorenstein and Andrade, 1996 ) for measuring the presence and severity of anxiety symptoms in the current moment (State anxiety) and a generalized predisposition to be anxious (Trait anxiety), and (b) a mini-sleep questionnaire (MSQ) ( Falavigna et al., 2011 ), i.e., a short screening for sleep disturbances in clinical populations for the assessment of insomnia and sleep difficulties ( Table 1 ). Table 1 shows the items selected from each questionnaire for the development of the insomnia Mediator-Causality model.
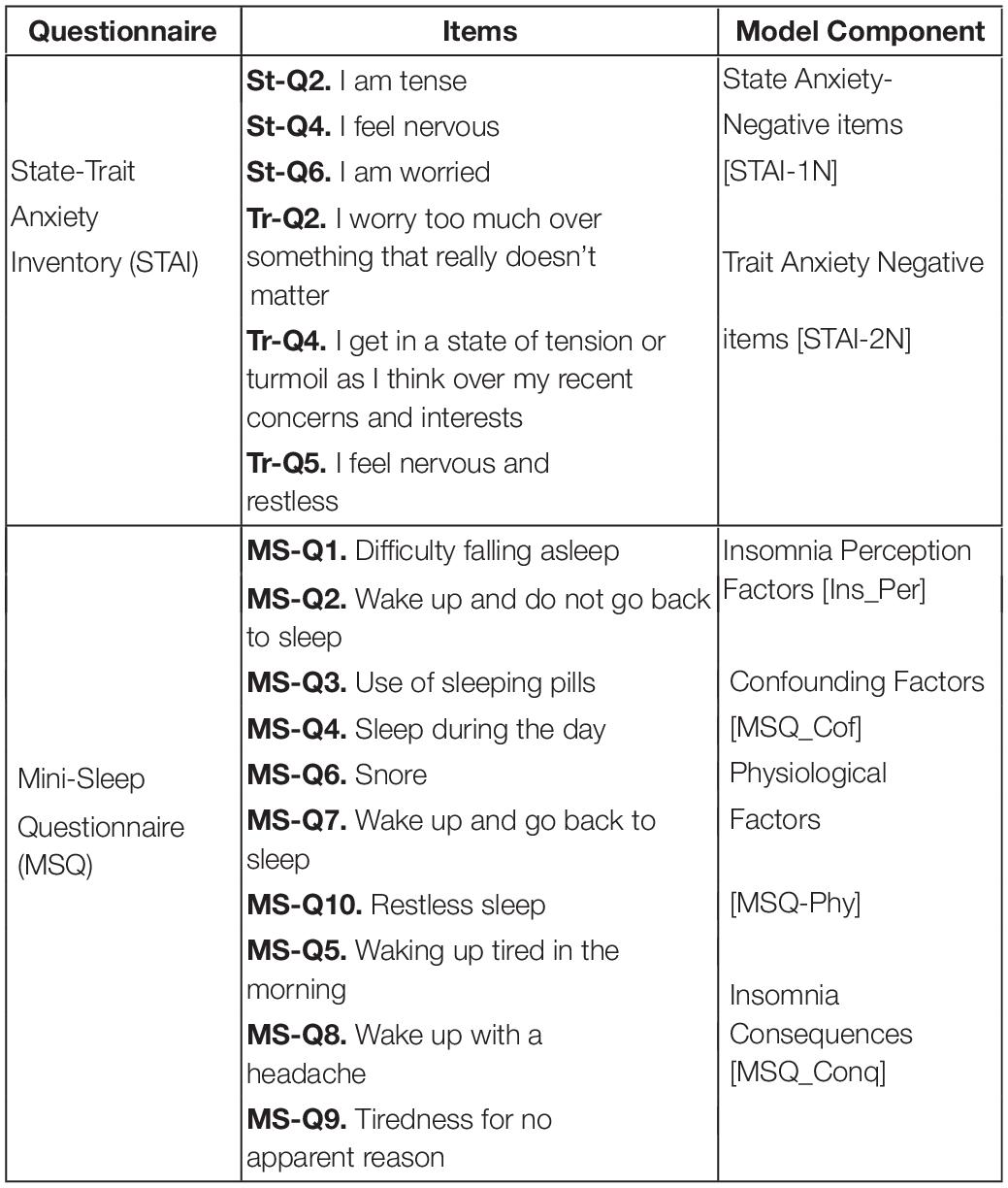
Table 1. List of selected questions from state-trait anxiety inventory ( Gorenstein and Andrade, 1996 ) and mini-sleep questionnaire ( Falavigna et al., 2011 ) for each model’s component.
Pre-processing of the Data
The data were anonymized to ensure blinding. Initially, those with missing values were omitted, which resulted in 112 and 134 session-wised questionnaires from the first and second studies, respectively. The datasets were then aggregated and segmented based on the insomnia severity stage. For insomnia, scores of the MSQ-questionnaire lower than 30 (MSQ-R < 30; mild to moderate sleep difficulties) were labeled as Neutral insomnia, and MSQ-R ≥ 30 (severe sleep difficulty) were denoted Clinical insomnia ( Natale et al., 2014 ). Such segmentations provided two sub-datasets (Neutral Insomnia, and Clinical Insomnia) for the statistical analysis. Figure 2 illustrates the correlation matrix of the variables in the mediator model.
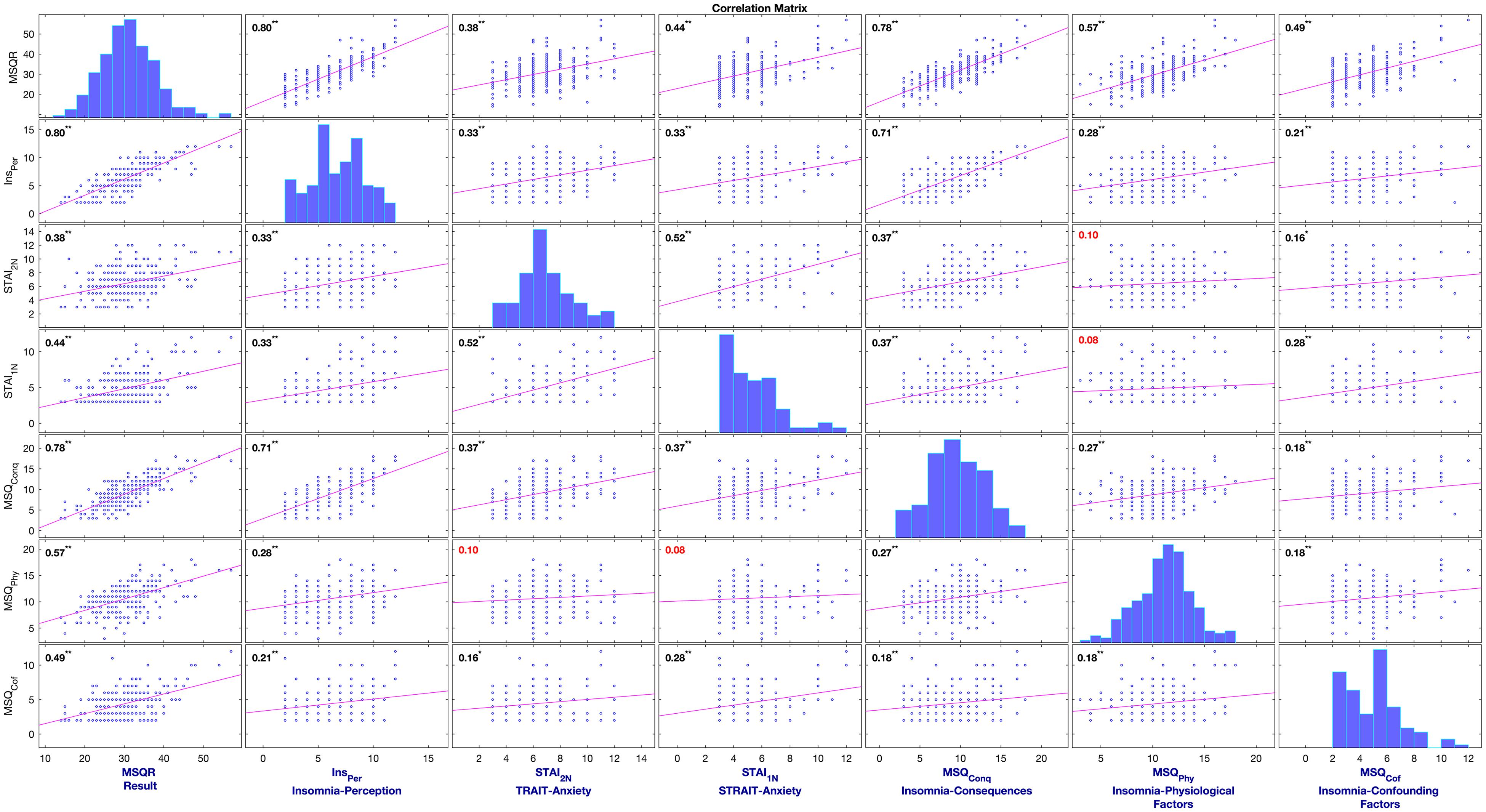
Figure 2. Correlation Matrix of variables used in the multi-mediation model of insomnia to support CCF of insomnia.
Statistical Analysis
Every segment of the dataset was tested for multicollinearity/autocorrelation by the Durbin-Watson test and showed independence in residuals in general. SPSS v.26 and PROCESS macro ( Hayes, 2017 ) were used for the data analysis. Within the macro, customized models and 5.000 bias-corrected bootstrap samples were set for all tests with the fixed random-seed (“ 12020 ”). A 95% confidence level was chosen, with significance at for P < 0.05 was set. A hierarchical regression analysis investigated the evidence for insomnia CCF within the data-segments, and multiple mediation models were constructed for determining the mediating effects of insomnia-related cognitive items and emotional factors for insomnia. PROCESS macro generated standard errors, P -values, and confidence intervals for direct effects, as well as bootstrap confidence intervals for conditional indirect effects.
Datasets and analyzed details are available on “Zenodo” repository with the doi: http://doi.org/10.5281/zenodo.4145224 .
Fundamental Ideas and Postulations for Mediator Models
• Insomnia-perception-factors (Ins_Per) variable contains difficulty in sleep initiation and maintenance.
• The employed dataset was unable to test hypotheses related to the distorted perception of sleep quality.
Proposed Mediator Model
The insomnia mediator model aims to illustrate that negative trait-anxiety can affect the perception of deficits in sleep quality. Concurrently, Insomnia-perception-factors can directly or through state-anxiety affect insomnia consequences . The insomnia model is depicted in Figure 3 .
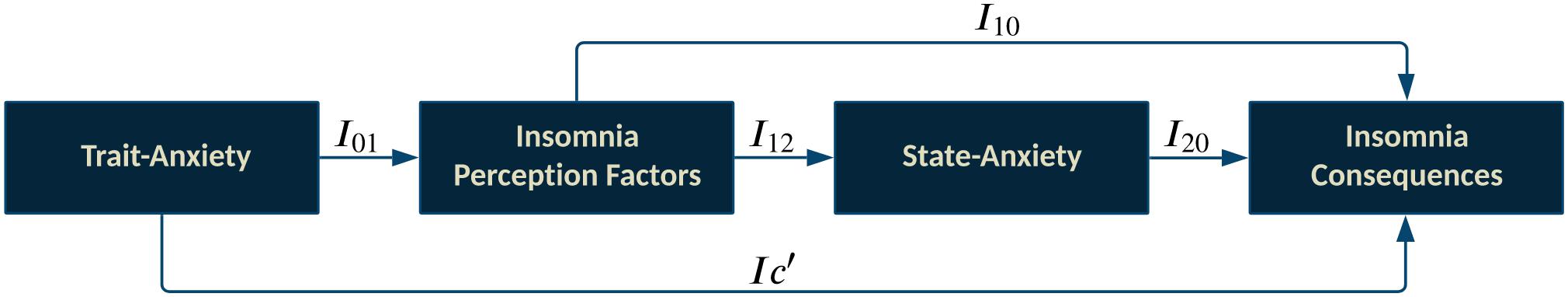
Figure 3. Insomnia mediator model includes the direct effect of trait-anxiety on Insomnia consequences (I c’ ); Ind-1 [ I 01 → I 10 ]: Trait-anxiety → Insomnia perception factors → Insomnia consequences; Ind-2 [ I 01 → I 12 → I 20 ]: Trait-anxiety → Insomnia perception factors→ State-anxiety→ Insomnia consequences; Covariates: Confounding factors and Physiological problems.
Several studies introduced trait-anxiety as an important predisposing factor for both the development and maintenance of insomnia ( Sadigh et al., 2014 ; Bavafa et al., 2018 ; Lauriola et al., 2019 ). Harvey (2002) argued that anxious individuals tend to interpret ambiguous situations in a threat-related fashion which, in turn, promotes over-thinking about sleep-related threat cues. This process maintains individuals in a cognitively aroused state which is in contradiction to the relaxed state needed for getting to sleep ( Harvey, 2002 ; Lancee et al., 2017a ).
Multi-mediation regression analysis with the conventional least-squares method revealed that trait-anxiety can only indirectly influence the insomnia consequences. As shown in Figure 3 and Table 2 , in the full-dataset, trait-anxiety can lead to insomnia consequences through either insomnia perception, or cascade mediators from insomnia perception to state-anxiety. The 95% confidence interval of bootstrap results revealed “ Ind-1 ” [ I 01 × I 10 = 0.303] and “ Ind-2 ” [ I 01 × I 12 × I 20 = 0.025] were significantly different from zero (0.183–0.432) and (0.006–0.057), respectively, but there was not enough evidence for trait-anxiety ( I c ′ = 0.088, P = 0.32) that might directly lead to insomnia consequences.
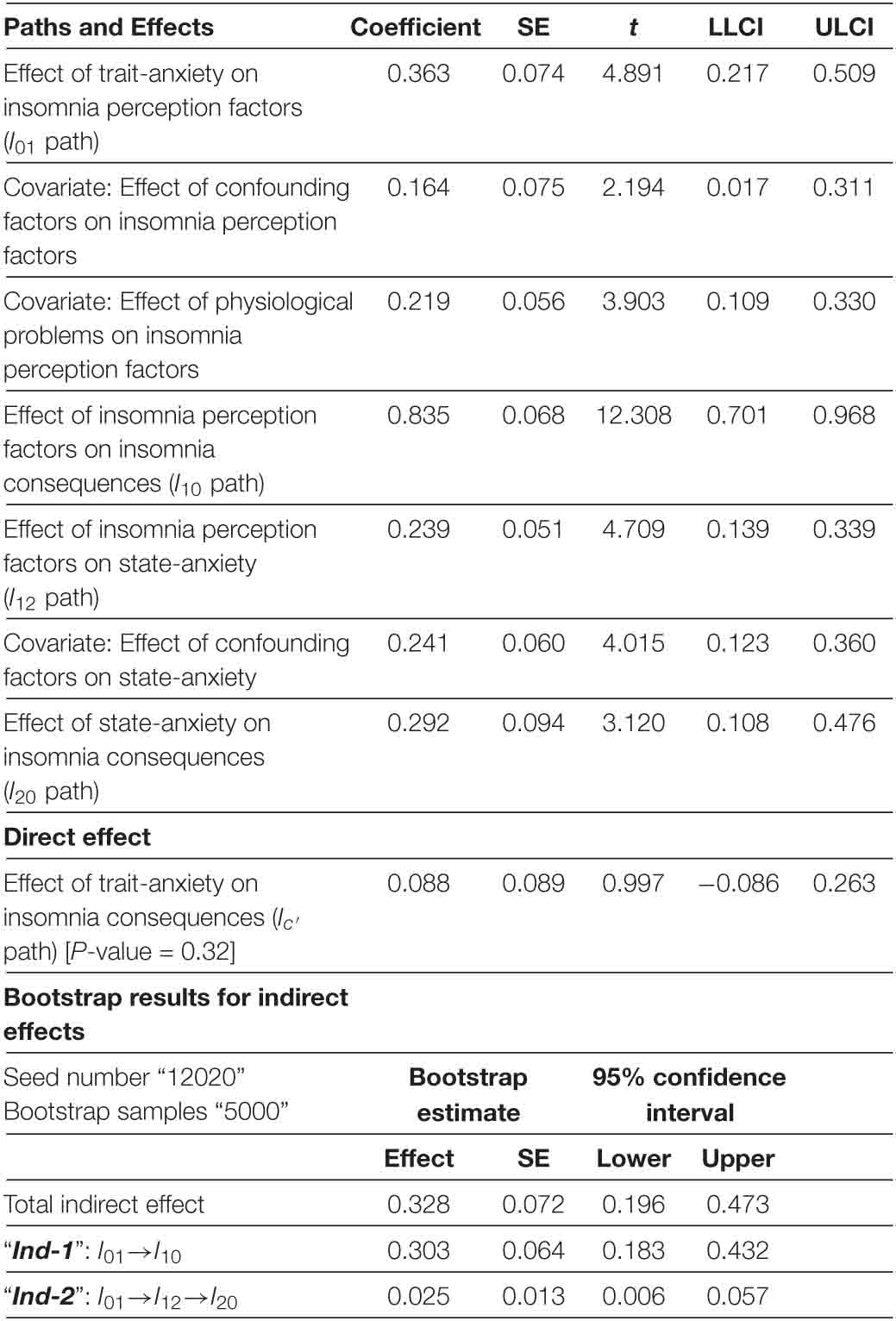
Table 2. Mediator model of insomnia in full-dataset.
According to Table 3 , trait-anxiety in the Clinical insomnia segment leads to insomnia consequences only through insomnia perception. The 95% confidence interval of bootstrap results revealed a significant difference in “ Ind-1 ” [ I 01 × I 10 = 0.161] different from zero (0.040–0.311), but not in “ Ind-2 .” Moreover, there was not enough evidence of trait-anxiety ( I c ′ = 0.2, P -value = 0.104) might directly lead to insomnia consequences.
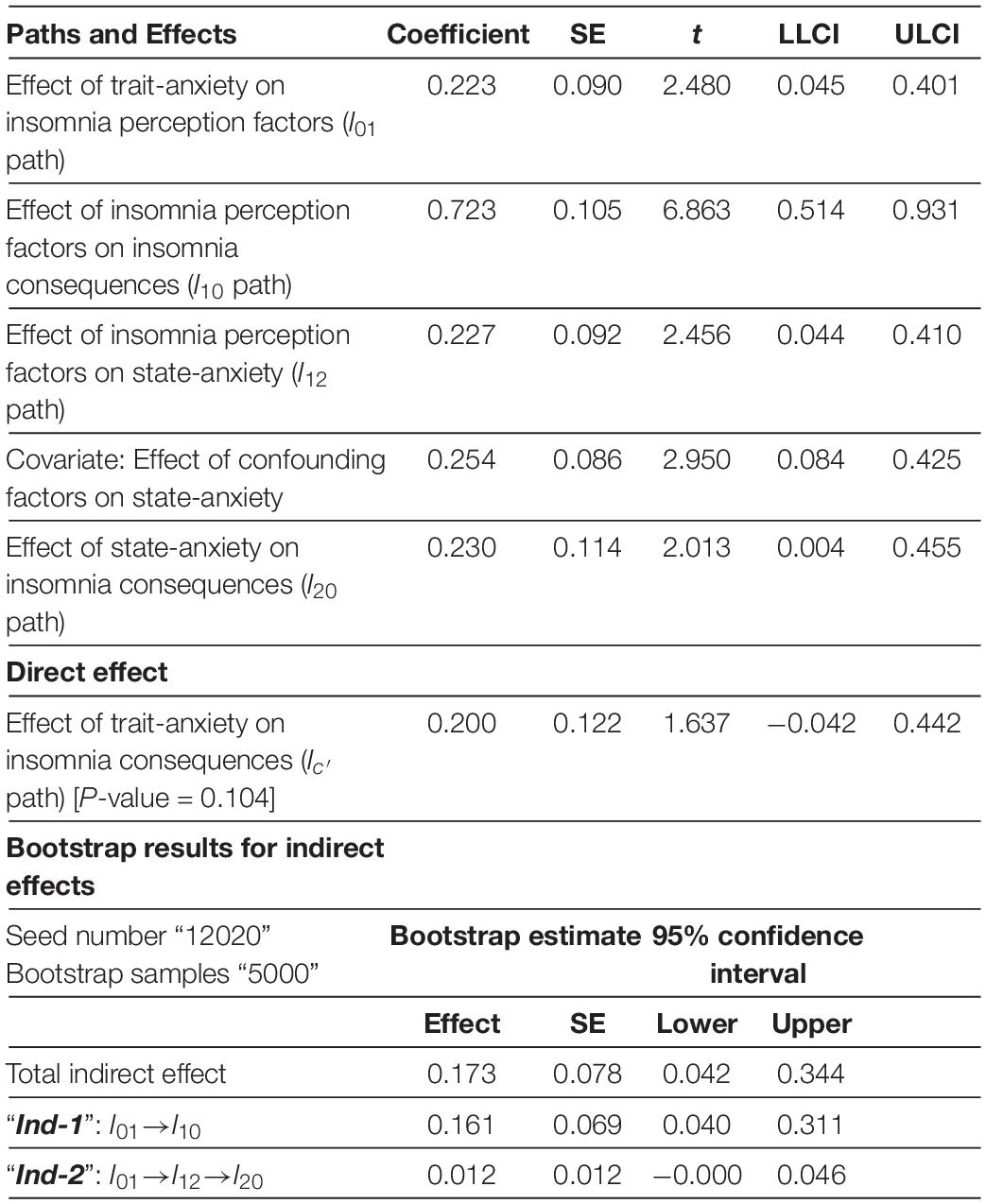
Table 3. Mediator model of insomnia in clinical insomnia segment.
Table 4 shows trait-anxiety neither directly, nor indirectly can lead to insomnia consequences within the Neutral insomnia segment.
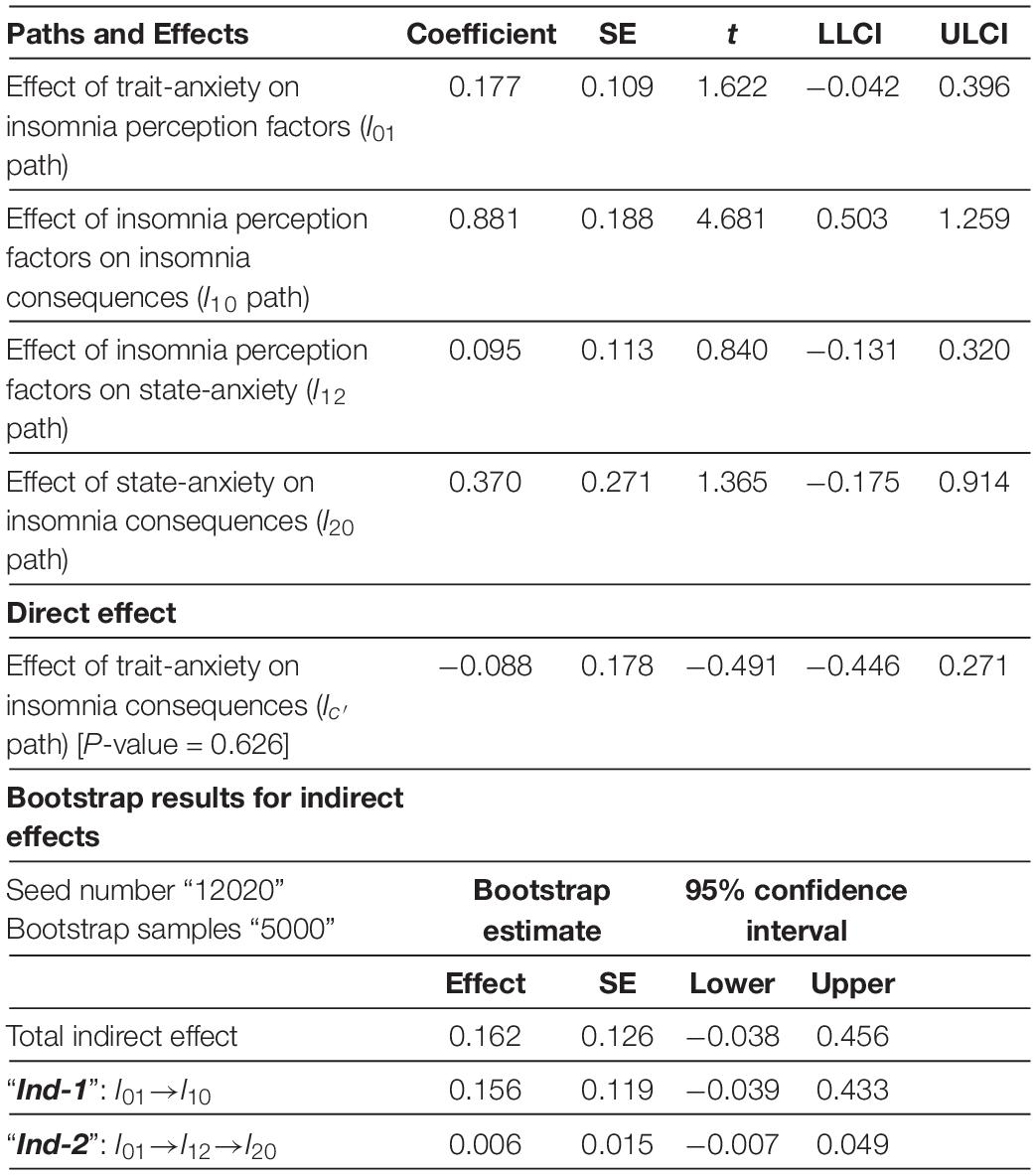
Table 4. Mediator model of insomnia in neutral insomnia segment.
Clinical Implications
Insomnia per se is a clinical issue with short-term and long-term consequences affecting both physiological and psychological systems. It has been associated (at least partly in terms of duration and severity) with increasing the incidence and worsening the state of many pre-existing clinical conditions ( Schutte-Rodin et al., 2008 ). Hence, the need for formulating a conceptual and concurrently pragmatic framework toward an individualized approach for people suffering from insomnia.
The proposed CCF, together with insomnia mediator models, explained the contribution of cognitive processes to the development and maintenance of clinical insomnia. The CCF proposes the following predictions and target-oriented clinical implications:
Decreasing Attentional Bias
Conceptual cognitive framework predicts that attentional bias modification (ABM) training can decrease the attentional bias to insomnia. In practice, the ABM treatment encourages the insomniacs to shift attention away from the negative sleep-related words toward a neutral one, thus reducing attention bias toward sleep-related threatening cue. Such a simple task enables patients to consciously and repeatedly select unbiased information over negative information, thereby progressively help to develop a tendency to not focus on negative information related to insomnia in their daily life ( Clarke et al., 2016 ).
Milkins et al. (2016) conducted a crossover study in which 18 insomniacs alternatively fulfilled an ABM task and a non-ABM control task before sleep across six successive nights. At nights on which the subjects performed the ABM task, they reported shorter sleep-onset latencies and lower pre-sleep worry, than the nights on which they performed the control task. Likewise, in a parallel design ( Clarke et al., 2016 ), 36 students with sleep problems underwent ABM or control training sessions across five nights. Compared with the control condition, subjects who underwent ABM training reported less pre-sleep arousal, fell asleep faster, woke less often during the night, reported better overall sleep quality, and had significant reductions in sleep-related anxiety ( Clarke et al., 2016 ). These findings support the above-mentioned proposition.
In contrast, the results of Lancee et al. (2017b) showed no added benefit of the ABM training over the placebo training on sleep-related indices and outcome measures. The authors believe it was probably due to the absence of attentional bias at baseline and hence no change could be deduced after training ( Lancee et al., 2017b ).
Employing Attention-Distraction Techniques Can Help Deviate Attention From Concerns-Relevant Topics to Neutral Ones
Addressing the issue of attentional bias toward relevant topics, Haynes et al. (1981) observed that engagement with a challenging mental arithmetic problem reduced subjective sleep latency among insomniacs ( Haynes et al., 1981 ). Similarly, practicing crossword puzzles, reading, and listening to audiobooks could provide sufficient distraction so that the patient would no longer attend to or think about their inability to sleep. Troxel et al. (2012) recommended patients should keep doing those activities until they feel sleepy enough to return to bed. And, if they cannot fall asleep after returning to bed, the process should be repeated ( Troxel et al., 2012 ).
Preventing Annoyance and Distress-Reaction
Conceptual cognitive framework draws attention to the crucial role of appraisal and ECL mechanisms in reducing negative cognitive and emotional value.
Cognitive-behavioral therapy (CBT) to reduce the negative cognitive-value related to insomnia
Sleep difficulties are commonly accompanied by dysfunctional beliefs, unrealistic expectations, and worries, which contribute to distress and maladaptive sleep habits producing an anxious state opposite to the relaxation required for sleeping. Therefore, patients’ beliefs regarding sleep and insomnia must be explored and attempts be made to change them eventually. Cognitive therapy aims at the identification of dysfunctional beliefs and attitudes related to sleep and their replacement with more adaptive substitutes. Cognitive therapies also address catastrophizing the consequences of poor sleep to help patients reconceptualize the realities of their beliefs, thereby reducing the upcoming distress and arousal that impedes sleeping ( Perlis et al., 2006 ). Through cognitive-behavioral therapy (CBT) a combination of cognitive reconstruction and behavioral techniques are delivered to encourage patients to develop more adaptive coping skills and stop self-criticizing ( Perlis et al., 2006 ). The European guideline for diagnosis and treatment of insomnia ( Riemann et al., 2017 ) recommends CBT for insomnia (CBT-I) as the first-line of treatment for chronic insomnia.
Furthermore, several systematic reviews and meta-analyses ( Taylor and Pruiksma, 2014 ; Mitchell et al., 2019 ) have reported strong empirical support for CBT-I on different subjective and objective sleep parameters. CBT-I’s common approaches for non-comorbid insomnia were cognitive therapy, stimulus control, sleep restriction, sleep hygiene, and relaxation. The results indicated that CBT-I improved sleep onset latency, wake after sleep onset, total sleep time, and sleep efficiency. The changes persisted over time alleviating the symptoms ( Wang et al., 2005 ; Trauer et al., 2015 ).
Mindfulness-based cognitive therapy (MBCT) for reducing the negative cognitive and emotional value related to insomnia
Mindfulness-based cognitive therapy (MBCT), as an emotion-regulation based psychotherapy, is a purposeful and unbiased form of therapy directing attention to the present moment as a way of self-regulation that promotes mind-body relaxation ( Ludwig and Kabat-Zinn, 2008 ). The approach educates people toward changing their relationship with their thoughts and negative emotions. Patients must be aware of their thoughts and are inspired to take a non-judgmental perspective on them rather than a negative, self-referential assessment that intensifies both negative thoughts and emotions ( Ludwig and Kabat-Zinn, 2008 ). In concordance with suggestions put forth by the CCF, Shallcross and Visvanathan (2016) have explained that experiential awareness, attentional control, and acceptance techniques used in MBCT interventions improve rumination, arousal, selective attention, and the distorted perception involved in the development and maintenance of insomnia ( Shallcross and Visvanathan, 2016 ).
The MBCT protocol tailored for insomniacs showed significant pre–post improvements in self-reported total sleep time and various thought-control domains, along with reductions in sleep-related monitoring and worry ( Heidenreich et al., 2006 ). MBCT was also effective for individuals with a history of depression or anxiety accompanied by sleep difficulty or insomnia ( Ree and Craigie, 2007 ; Yook et al., 2008 ; Britton et al., 2010 ). Ree and Craigie (2007) reported decreased scores of insomnia severity symptoms lasting for about 3 months with the MBCT. Similarly, MBCT protocol in older adults showed a 14.5% improvement in self-reported sleep problems ( Foulk et al., 2014 ). Self-regulation of attention and orientation to experience to achieve better sleep are the proposed mechanisms of actions for MBCT ( Larouche et al., 2014 ). A recent comprehensive meta-analysis reported significantly improved insomnia symptoms as measured by the Pittsburgh Sleep Quality Index ( Wang et al., 2020 ).
ECL mechanism for modifications in negative emotional-value related to insomnia
Positive emotion-induction techniques can reduce the negative valence of insomnia when paired with positively valenced and high arousal pictures, films ( Uhrig et al., 2016 ), audio ( Bergman et al., 2016 ), music, and video clips ( Lazar and Pearlman-Avnion, 2014 ; Siedlecka and Denson, 2019 ). Game-like design, app-based format, goggles of virtual reality, or a screen are different ways to present stimuli to provide cost-effective home-based individualized treatments.
Rectifying the Distorted Perception of the Quality of Sleep Deficit
Digital-technology approaches are believed to provide an online measurement of sleep duration and correct the distorted perception of sleep deficit. Since we have established that negative emotions might influence the perception of insomniacs about their sleep deficit, interventions aiming at emotion-regulation or modifications of dysfunctional beliefs may help prevent the formation of the distorted perception. Furthermore, Holter monitoring of rest/activity cycle of sleep, smartphone gadgets ( Izmailova et al., 2018 ), actigraphy, and sleep diary ( Tang and Harvey, 2004b ) might help insomniacs correct misperception. In contrast, parts of the literature studying the time-perception concept ( Thomas and Cantor, 1975 , 1976 ) have revealed that when more information is processed, time is perceived as longer. A high level of cognitive arousal and repetitive thought patterns distorts time perception for insomniacs, leading to an overestimation of sleep onset latency ( Tang and Harvey, 2004a ). Another implication is that during sleep onset, cognitive arousal maintains an enhanced sensory and memory processing level obscuring the distinction between sleep and wakefulness ( Perlis et al., 1997 ).
Future Trends
The clinical recommendations provided in this paper can be applied separately or in combination, to plan treatment for individuals with insomnia. The CCF builds upon a general assumption that patients should be consciously and actively involved in the rehabilitation process. Subsequently, new treatments can be developed aimed at encouraging patients to be consciously aware of their negative thoughts related to sleep-difficulty and contingencies for intervention. Moreover, the inclusion of surrogate measurements is recommended for guaranteeing the patient’s conscious attended-awareness. Collectively, the CCF can provide a decision-support platform for clinicians to deliver more targeted interventions, and eventually, the methodologies suggested can provide a reliable platform to build a CCF for other cognitive disorders and support the causal clinical data models. This novel approach can improve our knowledge of psychological disturbances and complex comorbidities toward the design of rehabilitation interventions and suggestions in line with the “preventive medicine” policies.
The CCF of insomnia, its predictions, and the corresponding suggested interventions do not include patients with organic sleep disorders, general cognitive distortion, and psychotic problems. MSQ was obtained from patients with complaints of tinnitus at the clinic during the day, and not before sleep. Despite their importance, daytime cognitive processes were not taken into account in the presented framework. Lastly, to achieve clinical endpoints, repeated measures and longitudinal studies are required to improve predictabilities.
Data Availability Statement
The datasets used and analyzed during this study are available from the corresponding author on reasonable request and filling out NEL-Consent redirecting to “Zenodo” with the doi: http://doi.org/10.5281/zenodo.4145224 .
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee for Analysis of Research Projects, Specialized Center of Otorhinolaryngology and Speech Therapy, Hospital das Clínicas de Ribeirão Preto, University of São Paulo, Brazil (HCRP no. 55716616.1.1001.5440, and HCRP no. 09813519.1.0000.5440; internationally registered with U1111-1236-5441). All subjects gave written informed consent prior to participation in the study.
Author Contributions
ZV: leading author responsible for manuscript development, concept and study design, conceptual modeling participation, and data acquisition in the clinic. MN: collaborating in manuscript development and concept. JL: supervising clinical data acquisition and conceptual development, and collaborating in manuscript development. AD: collaborating in manuscript development and supervising data mining and modeling. MH: monitoring clinical data acquisition, and collaborating in manuscript development. IG: manuscript development, concept and study design, analogy, numerical methodology design and implementation, and data acquisition, and data pre-processing. All authors read and approved the final manuscript.
This research is part of a Multidisciplinary Cognitive Rehabilitation (MCR) Platform and was supported (Grant number: 2013/07375-0) by Innovation and Diffusion of Mathematical Sciences Center Applied to Industry (CEPID-CeMEAI) of São Paulo Research Foundation (FAPESP). It also supported with Centro de Engenharia Aplicada a Saúde (CEAS) of the University of São Paulo, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for student’s scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to appreciate all efforts of employees and clinicians in CEOF who supported the current study voluntarily.
Abbreviations
ABM, attentional bias modification; BIISS-RESO, behaviorally induced insufficient sleep syndrome with restricted and extended sleep opportunity; CAAP, conscious attended awareness perception; CBT, cognitive-behavioral therapy; CBT-I, cognitive-behavioral therapy for insomnia; CCF, conceptual cognitive framework; CEOF, Centro Especializado de Otorrinolaringologia e Fonoaudiologia; CS, conditioned stimulus; CVMIMD, cognitive vulnerability model for insomnia induced mood disturbances; DBAS, dysfunctional beliefs and attitudes about sleep; ECL, evaluative conditional learning; EDS, excessive daytime somnolence; GCVI, global cognitive vulnerability to insomnia; Ind, Indirect; MBCT, mindfulness-based cognitive therapy; MSQ, mini-sleep questionnaire; MSQ-R, mini-sleep questionnaire result; PFC, prefrontal cortex; SSCV, sleep-specific cognitive vulnerability; STAI, state-trait anxiety inventory; US, unconditioned Stimulus; WASO, wakes after sleep onset.
Alfano, C. A., Zakem, A. H., Costa, N. M., Taylor, L. K., and Weems, C. F. (2009). Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depression Anxiety 26, 503–512. doi: 10.1002/da.20443
PubMed Abstract | CrossRef Full Text | Google Scholar
Baglioni, C., Lombardo, C., Bux, E., Hansen, S., Salveta, C., Biello, S., et al. (2010). Psychophysiological reactivity to sleep-related emotional stimuli in primary insomnia. Behav. Res. Ther. 48, 467–475. doi: 10.1016/j.brat.2010.01.008
Barclay, N. L., and Ellis, J. G. (2013). Sleep-related attentional bias in poor versus good sleepers is independent of affective valence. J. Sleep Res. 22, 414–421. doi: 10.1111/jsr.12035
Barclay, N. L., and Gregory, A. M. (2010). The presence of a perseverative iterative style in poor vs. good sleepers. J. Behav. Ther. Exp. Psychiatry 41, 18–23. doi: 10.1016/j.jbtep.2009.08.003
Bastien, C. H., St-Jean, G., Morin, C. M., Turcotte, I., and Carrier, J. (2008). Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep 31, 887–898.
Google Scholar
Bavafa, A., Akbar Foroughi, A., Khaledi-Paveh, B., Abbas Taheri, A., Fehrest, F., and Amiri, S. (2018). The comparison of effects of state and trait anxiety on the components of sleep quality. J. Sleep Sci. 3, 95–101.
Beck, A. T. (2008). The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 165, 969–977. doi: 10.1176/appi.ajp.2008.08050721
Beck, A. T., and Clark, D. A. (1997). An information processing model of anxiety: automatic and strategic processes. Behav. Res. Ther. 35, 49–58. doi: 10.1016/s0005-7967(96)00069-1
CrossRef Full Text | Google Scholar
Bei, B., Wiley, J. F., Allen, N. B., and Trinder, J. A. (2015). Cognitive vulnerability model of sleep and mood in adolescents under naturalistically restricted and extended sleep opportunities. Sleep 38, 453–461. doi: 10.5665/sleep.4508
Bergman, P., Vastfjall, D., Tajadura-Jimenez, A., and Asutay, E. (2016). Auditory-induced emotion mediates perceptual categorization of everyday sounds. Front. Psychol. 7:1565. doi: 10.3389/fpsyg.2016.01565
Bootzin, R. R., and Rider, S. P. (1997). “Behavioral techniques and biofeedback for insomnia,” in Understanding Sleep: The Evaluation and Treatment of Sleep Disorders , eds M. R. Pressman and C. William (Washington, DC: American Psychological Association), 315–338.
Bradley, M. M., and Lang, P. J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 25, 49–59. doi: 10.1016/0005-7916(94)90063-9
Britton, W. B., Haynes, P. L., Fridel, K. W., and Bootzin, R. R. (2010). Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psych. Med. 72, 539–548. doi: 10.1097/PSY.0b013e3181dc1bad
Clarke, P. J., Bedford, K., Notebaert, L., Bucks, R. S., Rudaizky, D., Milkins, B. C., et al. (2016). Assessing the therapeutic potential of targeted attentional bias modification for insomnia using smartphone delivery. Psychother. Psych. 85, 187–189. doi: 10.1159/000442025
Cormier, R. E. (1990). “Sleep disturbances,” in Clinical Methods: The History, Physical, and Laboratory Examinations , eds H. K. Walker, W. D. Hall, and J. W. Hurst (Boston: Butterworths).
Dalgleish, T., and Watts, F. N. (1990). Biases of attention and memory in disorders of anxiety and depression. Clin. Psychol. Rev. 10, 589–604. doi: 10.1016/0272-7358(90)90098-U
Davey, G. C. L., and Levy, S. (1998). Catastrophic worrying: personal inadequacy and a perseverative iterative style as features of the catastrophizing process. J. Abnormal Psychol. 107, 576–586. doi: 10.1037/0021-843x.107.4.576
De Houwer, J., Thomas, S., and Baeyens, F. (2001). Associative learning of likes and dislikes: a review of 25 years of research on human evaluative conditioning. Psychol. Bull. 127, 853–869. doi: 10.1037/0033-2909.127.6.853
Dzierzewski, J. M., Dautovich, N., and Ravyts, S. (2018). Sleep and COGNITION IN OLDER ADULTS. Sleep Med. Clin. 13, 93–106. doi: 10.1016/j.jsmc.2017.09.009
Espie, C. A., Brooks, D. N., and Lindsay, W. R. (1989). An evaluation of tailored psychological treatment of insomnia. J. Behav. Ther. Exp. Psychiatry 20, 143–153. doi: 10.1016/0005-7916(89)90047-5
Falavigna, A., de Souza Bezerra, M. L., Teles, A. R., Kleber, F. D., Velho, M. C., da Silva, R. C., et al. (2011). Consistency and reliability of the Brazilian Portuguese version of the Mini-Sleep Questionnaire in undergraduate students. Sleep Breath 15, 351–355. doi: 10.1007/s11325-010-0392-x
Foulk, M. A., Ingersoll-Dayton, B., Kavanagh, J., Robinson, E., and Kales, H. C. (2014). Mindfulness-based cognitive therapy with older adults: an exploratory study. J. Gerontol. Soc. Work. 57, 498–520. doi: 10.1080/01634372.2013.869787
Freeman, A., Felgoise, S., Nezu, A., Nezu, C., and Reinecke, M. (2005). Encyclopedia of Cognitive Behavior Therapy. New York, NY: Springer Science+ Business Media.
Frijda, N. H. (1987). Comment on Oatley and Johnson-Laird’s “Towards a cognitive theory of emotions”. Cogn. Emotion 1, 51–58.
Ghodratitoostani, I., Delbem, A. C. B., Torabi-Nami, M., Makkiabadi, B., Jalilvand, H., and Sanchez, T. G. (2016a). Theoretical tinnitus multimodality framework: a neurofunctional model. J. Adv. Med. Sci. Appl. Technol. 2, 181–189.
Ghodratitoostani, I., Zana, Y., Delbem, A. C., Sani, S. S., Ekhtiari, H., and Sanchez, T. G. (2016b). Theoretical tinnitus framework: a neurofunctional model. Front. Neurosci. 10:370. doi: 10.3389/fnins.2016.00370
Gorenstein, C., and Andrade, L. (1996). Validation of a portuguese version of the beck depression inventory and the state-trait anxiety inventory in Brazilian subjects. Braz. J. Med. Biol. 29, 453–457.
Gross, J. J. (1998). The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 2:271.
Gross, J. J. (2013). Handbook of Emotion Regulation , Second Edn. New York, NY: Guilford Publications.
Harvey, A. G. (2000). Pre-sleep cognitive activity: a comparison of sleep-onset insomniacs and good sleepers. Br. J. Clin. Psychol. Br. Psychol. Soc. 39, 275–286. doi: 10.1348/014466500163284
Harvey, A. G. (2002). A cognitive model of insomnia. Behav. Res. Ther. 40, 869–893. doi: 10.1016/s0005-7967(01)00061-4
Hayes, A. F. (2017). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford publications.
Haynes, S. N., Adams, A., and Franzen, M. (1981). The effects of presleep stress on sleep-onset insomnia. J. Abnormal Psychol. 90, 601–606. doi: 10.1037//0021-843x.90.6.601
Heidenreich, T., Tuin, I., Pflug, B., Michal, M., and Michalak, J. (2006). Mindfulness-based cognitive therapy for persistent insomnia: a pilot study. Psychother. Psych. 75, 188–189. doi: 10.1159/000091778
Herbert, V., Pratt, D., Emsley, R., and Kyle, S. D. (2017). Predictors of nightly subjective-objective sleep discrepancy in poor sleepers over a seven-day period. Brain Sci. 7:29. doi: 10.3390/brainsci7030029
Horstmann, G., and Herwig, A. (2016). Novelty biases attention and gaze in a surprise trial. Atten. Percep. Psychophys. 78, 69–77. doi: 10.3758/s13414-015-0995-1
Izmailova, E. S., Wagner, J. A., and Perakslis, E. D. (2018). Wearable devices in clinical trials: hype and hypothesis. Clin. Pharmacol. Ther. 104, 42–52. doi: 10.1002/cpt.966
Jansson-Fröjmark, M., Bermås, M., and Kjellén, A. (2012). Attentional bias in insomnia: the dot-probe task with pictorial stimuli depicting daytime fatigue/malaise. Cogn. Ther. Res. 37, 534–546. doi: 10.1007/s10608-012-9486-z
Jones, B. T., Macphee, L. M., Broomfield, N. M., Jones, B. C., and Espie, C. A. (2005). Sleep-related attentional bias in good, moderate, and poor (primary insomnia) sleepers. J. Abnormal Psychol. 114, 249–258. doi: 10.1037/0021-843X.114.2.249
Koster, E. H., Crombez, G., Verschuere, B., and De Houwer, J. (2004). Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav. Res. Ther. 42, 1183–1192. doi: 10.1016/j.brat.2003.08.001
Kuisk, L. A., Bertelson, A. D., and Walsh, J. K. (1989). Presleep cognitive hyperarousal and affect as factors in objective and subjective insomnia. Perceptual Motor Skills 69, 1219–1225. doi: 10.2466/pms.1989.69.3f.1219
Lancee, J., Eisma, M. C., van Zanten, K. B., and Topper, M. (2017a). When thinking impairs sleep: trait, daytime and nighttime repetitive thinking in insomnia. Behav. Sleep Med. 15, 53–69. doi: 10.1080/15402002.2015.1083022
Lancee, J., Yasiney, S. L., Brendel, R. S., Boffo, M., Clarke, P. J. F., and Salemink, E. (2017b). Attentional bias modification training for insomnia: a double-blind placebo controlled randomized trial. PLoS One 12:e0174531. doi: 10.1371/journal.pone.0174531
Larouche, M., Cote, G., Belisle, D., and Lorrain, D. (2014). Kind attention and non-judgment in mindfulness-based cognitive therapy applied to the treatment of insomnia: state of knowledge. Pathol. Biol. (Paris) 62, 284–291. doi: 10.1016/j.patbio.2014.07.002
Lauriola, M., Carleton, R. N., Tempesta, D., Calanna, P., Socci, V., Mosca, O., et al. (2019). A correlational analysis of the relationships among intolerance of uncertainty, anxiety sensitivity, subjective sleep quality, and insomnia symptoms. Int. J. Environ. Res. Public Health 16:3253. doi: 10.3390/ijerph16183253
Lazar, J. N., and Pearlman-Avnion, S. (2014). Effect of affect induction method on emotional valence and arousal. Psychology 5:595.
Lichstein, K. L., and Rosenthal, T. L. (1980). Insomniacs’ perceptions of cognitive versus somatic determinants of sleep disturbance. J. Abnormal Psychol. 89, 105–107. doi: 10.1037//0021-843x.89.1.105
Ludwig, D. S., and Kabat-Zinn, J. (2008). Mindfulness in medicine. Jama J. Am. Med. Assoc. 300, 1350–1352. doi: 10.1001/jama.300.11.1350
MacMahon, K. M., Broomfield, N. M., and Espie, C. A. (2006). Attention bias for sleep-related stimuli in primary insomnia and delayed sleep phase syndrome using the dot-probe task. Sleep 29, 1420–1427.
Mai, E., and Buysse, D. J. (2008). Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med. Clin. 3, 167–174. doi: 10.1016/j.jsmc.2008.02.001
Manconi, M., Ferri, R., Sagrada, C., Punjabi, N. M., Tettamanzi, E., Zucconi, M., et al. (2010). Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J. Sleep Res. 19, 478–486. doi: 10.1111/j.1365-2869.2009.00801.x
Milkins, B., Notebaert, L., MacLeod, C., and Clarke, P. J. F. (2016). The potential benefits of targeted attentional bias modification on cognitive arousal and sleep quality in worry-related sleep disturbance. Clin. Psychol. Sci. 4, 1015–1027. doi: 10.1177/2167702615626898
Mitchell, L. J., Bisdounis, L., Ballesio, A., Omlin, X., and Kyle, S. D. (2019). The impact of cognitive behavioural therapy for insomnia on objective sleep parameters: a meta-analysis and systematic review. Sleep Med. Rev. 47, 90–102. doi: 10.1016/j.smrv.2019.06.002
Morin, C. M. (1993). Insomnia: Psychological Assessment and Management. New York, NY: Guilford press.
Morin, C. M., Stone, J., Trinkle, D., Mercer, J., and Remsberg, S. (1993). Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol. Aging 8, 463–467. doi: 10.1037/0882-7974.8.3.463
Nami, M. (2014). Chronic insomnia, pharmacotherapy and the cognitive behavioural approaches. J. Sleep Disord. Ther. 3:1000151.
Natale, V., Fabbri, M., Tonetti, L., and Martoni, M. (2014). Psychometric goodness of the Mini Sleep Questionnaire. Psychiatry Clin. Neurosci. 68, 568–573. doi: 10.1111/pcn.12161
Nicassio, P. M., Mendlowitz, D. R., Fussell, J. J., and Petras, L. (1985). The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav. Res. Ther. 23, 263–271. doi: 10.1016/0005-7967(85)90004-x
Perlis, M. L., Giles, D. E., Mendelson, W. B., Bootzin, R. R., and Wyatt, J. K. (1997). Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J. Sleep Res. 6, 179–188. doi: 10.1046/j.1365-2869.1997.00045.x
Perlis, M. L., Jungquist, C., Smith, M. T., and Posner, D. (2006). Cognitive Behavioral Treatment of Insomnia: A Session-By-Session Guide. Berlin: Springer Science & Business Media.
Ree, M. J., and Craigie, M. A. (2007). Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav. Change 24, 70–86. doi: 10.1375/bech.24.2.70
Riemann, D., Baglioni, C., Bassetti, C., Bjorvatn, B., Dolenc Groselj, L., Ellis, J. G., et al. (2017). European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 26, 675–700. doi: 10.1111/jsr.12594
Roberts, L. E., Husain, F. T., and Eggermont, J. J. (2013). Role of attention in the generation and modulation of tinnitus. Neurosci. Biobehav. Rev. 37, 1754–1773. doi: 10.1016/j.neubiorev.2013.07.007
Sadigh, M. R., Himmanen, S. A., and Scepansky, J. A. (2014). An investigation of the prevalence of insomnia in college students and its relationship to trait anxiety. College Student J. 48, 397–406.
Sadler, P., McLaren, S., and Jenkins, M. (2013). A psychological pathway from insomnia to depression among older adults. Int. Psychogeriatr. 25, 1375–1383. doi: 10.1017/S1041610213000616
Sateia, M. J. (2014). International classification of sleep disorders-third edition: highlights and modifications. Chest 146, 1387–1394. doi: 10.1378/chest.14-0970
Scherer, K. R., Schorr, A., and Johnstone, T. (2001). Appraisal Processes in Emotion: Theory, Methods, Research. Oxford: Oxford University Press.
Schutte-Rodin, S., Broch, L., Buysse, D., Dorsey, C., and Sateia, M. (2008). Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 4, 487–504.
Shallcross, A. J., and Visvanathan, P. D. (2016). “Mindfulness-based cognitive therapy for insomnia,” in Mindfulness-Based Cognitive Therapy , ed. S. J. Eisendrath (Berlin: Springer), 19–29.
Siedlecka, E., and Denson, T. F. (2019). Experimental methods for inducing basic emotions: a qualitative review. Emotion Rev. 11, 87–97. doi: 10.1177/1754073917749016
Spiegelhalder, K., Kyle, S. D., Feige, B., Prem, M., Nissen, C., Espie, C. A., et al. (2010). The impact of sleep-related attentional bias on polysomnographically measured sleep in primary insomnia. Sleep 33, 107–112. doi: 10.1093/sleep/33.1.107
Stahl, C., and Unkelbach, C. (2009). Evaluative learning with single versus multiple unconditioned stimuli: the role of contingency awareness. J. Exp. Psychol. Animal Behav. Proc. 35, 286–291. doi: 10.1037/a0013255
Tang, N. K., and Harvey, A. G. (2004b). Correcting distorted perception of sleep in insomnia: a novel behavioural experiment? Behav. Res. Ther. 42, 27–39. doi: 10.1016/s0005-7967(03)00068-8
Tang, N. K., and Harvey, A. G. (2004a). Effects of cognitive arousal and physiological arousal on sleep perception. Sleep 27, 69–78. doi: 10.1093/sleep/27.1.69
Taylor, D. J., and Pruiksma, K. E. (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int. Rev. Psychiatry 26, 205–213. doi: 10.3109/09540261.2014.902808
Thomas, E. A., and Cantor, N. E. (1975). On the duality of simultaneous time and size perception. Percept. Psychophys. 18, 44–48.
Thomas, E. A. C., and Cantor, N. E. (1976). Simultaneous time and size perception. Percept. Psychophys. 19, 353–360. doi: 10.3758/Bf03204243
Trauer, J. M., Qian, M. Y., Doyle, J. S., Rajaratnam, S. M., and Cunnington, D. (2015). Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann. Intern. Med. 163, 191–204. doi: 10.7326/M14-2841
Troxel, W. M., Germain, A., and Buysse, D. J. (2012). Clinical management of insomnia with brief behavioral treatment (BBTI). Behav. Sleep Med. 10, 266–279. doi: 10.1080/15402002.2011.607200
Uhrig, M. K., Trautmann, N., Baumgartner, U., Treede, R. D., Henrich, F., Hiller, W., et al. (2016). Emotion elicitation: a comparison of pictures and films. Front. Psychol. 7:180. doi: 10.3389/fpsyg.2016.00180
Wang, M. Y., Wang, S. Y., and Tsai, P. S. (2005). Cognitive behavioural therapy for primary insomnia: a systematic review. J. Adv. Nursing 50, 553–564. doi: 10.1111/j.1365-2648.2005.03433.x
Wang, Y. Y., Wang, F., Zheng, W., Zhang, L., Ng, C. H., Ungvari, G. S., et al. (2020). Mindfulness-based interventions for insomnia: a meta-analysis of randomized controlled trials. Behav. Sleep Med. 18, 1–9. doi: 10.1080/15402002.2018.1518228
Wicklow, A., and Espie, C. A. (2000). Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav. Res. Ther. 38, 679–693. doi: 10.1016/S0005-7967(99)00136-9
Woods, H., Marchetti, L. M., Biello, S. M., and Espie, C. A. (2009). The clock as a focus of selective attention in those with primary insomnia: an experimental study using a modified Posner paradigm. Behav. Res. Ther. 47, 231–236. doi: 10.1016/j.brat.2008.12.009
Woods, H. C., Scheepers, C., Ross, K. A., Espie, C. A., and Biello, S. M. (2013). What are you looking at? moving toward an attentional timeline in insomnia: a novel semantic eye tracking study. Sleep 36, 1491–1499. doi: 10.5665/sleep.3042
Yoo, J. Y., and Lee, J. H. (2015). The effects of valence and arousal on time perception in individuals with social anxiety. Front. Psychol. 6:1208. doi: 10.3389/fpsyg.2015.01208
Yook, K., Lee, S. H., Ryu, M., Kim, K. H., Choi, T. K., Suh, S. Y., et al. (2008). Usefulness of mindfulness-based cognitive therapy for treating insomnia in patients with anxiety disorders: a pilot study. J. Nervous Mental Dis. 196, 501–503. doi: 10.1097/NMD.0b013e31817762ac
Keywords : cognitive model, insomnia, evaluative conditional learning, mediator model, distorted perception, appraisal, valence, conceptual cognitive framework
Citation: Vaziri Z, Nami M, Leite JP, Delbem ACB, Hyppolito MA and Ghodratitoostani I (2021) Conceptual Framework for Insomnia: A Cognitive Model in Practice. Front. Neurosci. 15:628836. doi: 10.3389/fnins.2021.628836
Received: 13 November 2020; Accepted: 03 June 2021; Published: 22 July 2021.
Reviewed by:
Copyright © 2021 Vaziri, Nami, Leite, Delbem, Hyppolito and Ghodratitoostani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iman Ghodratitoostani, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Neuropsychopharmacology Reviews
- Published: 23 August 2019
Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms
- Anne Richards 1 , 2 na1 ,
- Jennifer C. Kanady 1 , 2 na1 &
- Thomas C. Neylan 1 , 2
Neuropsychopharmacology volume 45 , pages 55–73 ( 2020 ) Cite this article
12k Accesses
95 Citations
33 Altmetric
Metrics details
- Neuroscience
A Correction to this article was published on 07 October 2019
This article has been updated
The current report provides an updated review of sleep disturbance in posttraumatic stress disorder and anxiety-related disorders. First, this review provides a summary description of the unique and overlapping clinical characteristics and physiological features of sleep disturbance in specific DSM anxiety-related disorders. Second, this review presents evidence of a bidirectional relationship between sleep disturbance and anxiety-related disorders, and provides a model to explain this relationship by integrating research on psychological and neurocognitive processes with a current understanding of neurobiological pathways. A heuristic neurobiological framework for understanding the bidirectional relationship between abnormalities in sleep and anxiety-related brain pathways is presented. Directions for future research are suggested.
You have full access to this article via your institution.
Similar content being viewed by others
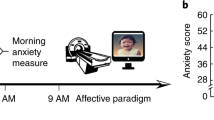
Overanxious and underslept
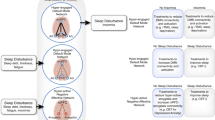
Integrating sleep, neuroimaging, and computational approaches for precision psychiatry
Investigating the effect of a nap following experimental trauma on analogue PTSD symptoms
Introduction.
Sleep is a vital physiological process, disruptions of which affect performance in multiple domains of functioning [ 1 ], including but not restricted to cognitive [ 2 , 3 ], emotional [ 4 ], metabolic [ 5 ], and immunologic [ 6 ]. Focusing on posttraumatic stress disorder (PTSD) and other anxiety-related disorders specifically, published research points to a bidirectional relationship between disturbances in sleep and anxiety-related disorders. Neurobiological research ranging from animal research to human neuroimaging and polysomnography-based research, alongside clinical treatment research, provide insights about this relationship, while also highlighting questions that remain to be answered. A heuristic neurobiological framework for this relationship is proposed to contribute to ongoing research in this area.
The study of sleep–wake regulation and the neurobiology of sleep disturbance in humans: an introductory overview
Both human and basic animal research have driven a surge in our understanding of sleep neurobiology in recent decades. In the 1950’s and 1960’s, researchers coined the terms REM and NREM sleep (REMS, NREMS) after observing that humans cycled through periods of sleep with unique electroencephalography (EEG) signatures combined with distinct eye movement patterns and muscle activity levels [ 7 , 8 , 9 ]. Since then, the field of sleep research has started to unravel the significance of sleep and its different visually scored stages (NREM N1, N2, N3, and REMS) for various essential functions, including but not limited to synaptic homeostasis [ 10 , 11 ], cognitive function [ 2 , 12 ], emotion regulation [ 4 ], memory processing and consolidation [ 10 , 13 , 14 , 15 ], glucose metabolism [ 5 ], immunity [ 6 ], and more [ 1 ]. Since the mid-20th century as well, numerous studies have also established that circadian and homeostatic processes regulate sleep in humans [ 16 , 17 ]. Although understanding the functional neuroanatomy of sleep regulation in humans is still impeded by the challenges of imaging the human brain during sleep, animal research has greatly advanced our grasp of sleep/wake neurocircuitry in mammals, and in combination with pharmacological, EEG, and imaging studies in humans, provides clues regarding the relevance of different brain nuclei and circuits in human sleep.
The existing research points to distinct nuclei and brain regions with prominent and preferential roles in promoting NREMS (which includes slow-wave sleep (SWS/N3 sleep), REMS, and the wake state. Although the most-recent evidence challenges the relative simplicity of sleep/wake neurocircuitry models developed in recent decades (e.g., see [ 18 ]), the extant research strongly indicates that specific brain regions and cell types are preferentially active in NREMS, REMS, and/or the wake state, and that these nuclei and cell types influence each other via positive and negative feedback mechanisms in the service of regulating sleep and wake. Figure 1 depicts a current understanding of important brain nuclei involved in NREMS, REMS, and wake regulation. Mutually inhibitory network interactions between NREMS promoting neurons in the ventrolateral preoptic area (VLPO in rodent model; intermediate nucleus (IN) in humans) and wake-promoting neurons in the brainstem and hypothalamus regulate sleep–wake rhythms in a fashion analogous to the engineering concept of a flip–flop switch [ 19 , 20 , 21 , 22 , 23 ]. This model was recently updated to emphasize the importance of fast neurotransmitters, glutamate and GABA [ 24 ]. In addition to the VLPO, GABAergic neurons in the medullary parafacial zone (PZ) are also critical for slow-wave sleep (SWS) homeostasis, shown by anatomic, electrophysiologic, chemogenetic and optogenetic studies in mice [ 25 , 26 ]. For example, lesions in GABAergic neurons in PZ increased wakefulness by 50% and severely disrupted sleep, including a marked reduction of SWS [ 25 ]. Brain regions specifically or preferentially active during REMS have also been identified (Fig. 1 ). Of critical importance in the context of a discussion of anxiety disorders is that arousal/wake centers also broadly innervate the cerebral cortex, and the cerebral cortex reciprocally innervates arousal centers [ 24 , 27 ]. This means that in the context of salient information to the cortex, for example from the amygdala, arousal may be elicited despite competing sleep-promoting signals from sleep-specific centers of the brain.
Important wake, NREM sleep, and REM sleep-regulating brain structures and/or nuclei, and associated neuromodulatory milieus. Wake-promoting regions, in green 1 a – i ; NREMS (including SWS) promoting regions in blue 2a – b ; REM sleep-regulating regions in orange ( 3a –d). Wake (green): 1 a : lateraldorsal tegmentum (LDT; Ach); 1b : pedunculopontine tegmentum (PPT; Ach); 1c : locus coeruleus (LC; NE); 1d : dorsal raphe nucleus (DRN; 5HT); 1e : tuberomammillary nucleus (TMN; Hist); 1f : hypocretin neurons of Lateral Hypothalamus (LH; Hct); 1g : cholinergic neurons of basal forebrain (BF; Ach); 1h : ventrolateral periaqueductal gray (vlPAG; DA); 1i : ventral tegmental area (VTA, DA). NREMS (blue): 2a : ventrolateral preoptic nucleus (VLPO; GABA, galanin; human homolog: intermediate nucleus (IN)); 2b : parafacial zone (PZ; GABA). REMS (orange): 3a : sublaterodorsal tegmental nucleus (SDT; Glu); 3b : ventral gigantocellular reticular nuclei (mediate REM-related muscle atonia; GABA/glycine); 3c : MCH neurons of lateral hypothalamus (LH; MCH/GABA); 3d : ventrolateral periaqueductal gray (vlPAG; GABA), *which is REM-suppressing. **LDT and PPT Ach neurons are also active during and may promote REMS, but may not play a central regulatory role
With respect to the study of sleep in psychiatric disorders, scalp-EEG-based measurement of cortical physiology remains the most-commonly utilized tool for studying the neurophysiology of sleep disturbance. Some researchers have also successfully probed the deeper functional neuroanatomy of human sleep disturbance in anxiety-related disorders utilizing neuroimaging techniques [ 28 , 29 , 30 ], but such studies are still relatively few. Figure 2 depicts the polysomnographic characteristics associated with different stages of sleep, along with a list of their associated clinical features and proposed or evidence-based functions, selected for their pertinence to anxiety disorders. Anxiety-disorder-related findings that will be discussed in greater detail in the following sections include reductions in sleep duration and sleep continuity, reductions in SWS, increases in lighter stages of sleep (NREM N1 and/or N2), and a variety of abnormalities in REMS, including disrupted REMS in anxiety subjects relative to controls. Based on the proposed functions of these various stages of sleep, one can infer numerous neurocognitive and psychological consequences relevant to anxiety, including disturbances in cognitive control, emotion regulation, memory consolidation, and emotional memory processing.
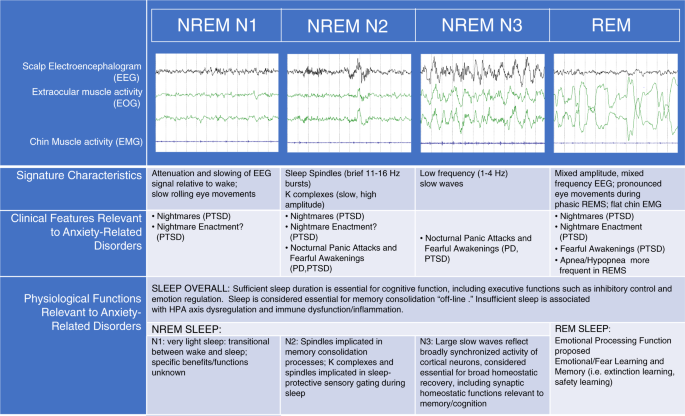
Polysomnographic characteristics of different sleep stages in humans, along with a list of their associated clinical features and proposed or evidence-based functions selected for their pertinence to anxiety disorders. Some studies have studied the effects of sleep overall (especially sleep duration) on performance in various domains, such as executive cognitive function and emotion regulation, whereas other studies have examined the role of specific sleep stages on performance of various functions more specifically
Neurobiology of anxiety-related disorders: a brief overview of neurocircuitry
An overview of the neurobiology of anxiety-related disorders must begin with a definition of these disorders. Given the ever-changing landscape of DSM-defined anxiety disorders, and given that all mental disorders may contain or produce a pronounced anxiety component (take paranoid schizophrenia, for example, in which patients may experience core psychotic symptoms resulting in profound and debilitating anxiety), we refer here to anxiety-related disorders as disorders in which fear and/or anxiety are considered primary and core features of the disorder. As such, we limit our detailed review of sleep disturbance to PTSD, Generalized Anxiety Disorder (GAD), Panic Disorder (PD), Social Phobia (SP), and Specific Phobias (SP). We also include obsessive–compulsive disorder (OCD) owing to its recent (DSM-IV) categorization as an anxiety disorder, because there are significant anxiety components to this disorder, and because we believe this will be of interest to readers.
With these definitions in mind, neuroimaging research in humans has provided relatively greater insight (relative to sleep disturbance in humans) into the functional neuroanatomy and neurocircuitry of anxiety-related disorders [ 31 , 32 , 33 , 34 , 35 ]. Figure 3 depicts the main brain regions broadly implicated in anxiety-related disorders. The amygdala and insula, key structures involved in the processing of aversive stimuli and in the expression of fear and anxiety, most consistently display hyperactivation in (aversive) task-based neuroimaging studies of anxiety-related disorders (see Duval et al., [ 34 ] for a review including disorders of interest minus OCD). In contrast, the medial prefrontal cortex (mPFC) tends to display hypoactivation in anxiety-related disorders, especially PTSD and GAD, and treatment of anxiety-related disorders has been associated with increases in mPFC activation that relate to symptom improvement [ 34 ]. In addition, the dorsal anterior cingulate cortex (dACC), with some exceptions, shows increased activation in anxiety-related disorders, which is thought to reflect the role of dACC in processing and expression of fear and anxiety. In contrast, the rostral ACC (rACC) is proposed to have a fear-modulating role based on neuroimaging findings. Functional connectivity studies in anxiety-related disorders generally indicate decreased connectivity between regions that modulate negative emotions (mPFC/rACC) and emotion-processing regions (amygdala, insula).
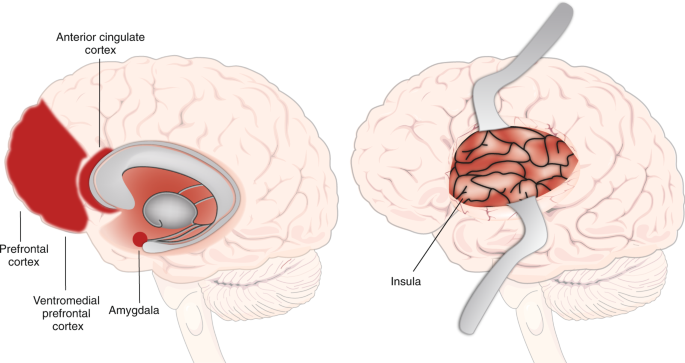
Important brain regions involved in fear, threat, and anxiety expression and modulation. Neuroimaging research indicates that the amygdala and insula are involved in the expression of fear, threat and anxiety. The dorsal anterior cingulate cortex (dACC) is more involved in the processing and expression of anxiety and fear, whereas the rostral ACC (rACC) is more involved in their modulation. The medial prefrontal cortex (mPFC) is involved in their modulation, especially the ventromedial PFC (vmPFC)
Other regions are considered to be relevant to anxiety-related disorders, some of which are less well understood or are associated with less consistent or broadly generalizable findings [ 34 ]. For example, the hippocampus is considered to play an emotion-modulating role in fear and anxiety, and often shows hyperactivation in imaging studies of anxiety-related disorders. The dorsolateral prefrontal cortex (dlPFC), implicated in emotion modulation and attention control, has shown patterns of hyper- and hypoactivation in anxiety-related disorders. Finally, the thalamus has been shown to display excess activation in task-based neuroimaging studies in PTSD, Social Anxiety Disorder (SAD), and SD relative to controls. The thalamus is also a key structure involved in the generation of SWS as well as the downregulation of sensory processing during sleep.
Anxiety and sleep circuitry: partially overlapping, intercommunicating neurocircuits
Although we describe two distinct sets of brain regions implicated in sleep/wake regulation and anxiety states, there is clear evidence of interaction between them, the extent and details of which are only beginning to be uncovered. There are obvious ways in which these communicate, including the aforementioned tight connection between the arousal centers and the cerebral cortex. This shared broad connectivity with the cerebral cortex provides a concrete neurobiological pathway for interconnectivity, but also provides an anatomical context for the cognitive hyperarousal that may link anxiety to sleep disturbance. In addition, the locus coeruleus, the main generator of norepinephrine in the central nervous system, plays both an important role in sleep/wake regulation as well as in anxiety-related processes through its inputs to and from the amygdala as well as the cerebral cortex. We will return to these neurobiological pathways later. A closer look at the clinical and sleep-physiological findings pertaining to the disorders of interest will create a phenomenological background for a subsequent discussion of these neurobiological pathways.
Sleep disturbance in anxiety-related disorders: a review of clinical features
The term “sleep disturbance” in published research on sleep and anxiety-related disorders refers to a range of subjective complaints, sleep disorder diagnoses, and alterations in subjective and objective measures of sleep when clinical samples are compared with controls. Several types of clinical sleep disturbance stand out in this literature; including DSM insomnia disorder diagnoses or symptoms per se, more broadly defined disturbances in subjective sleep quality as measured using various self-report surveys, nightmares, fearful awakenings, nocturnal panic attacks, obstructive sleep apnea (OSA), and abnormal movements during sleep ranging from periodic limb movements to dream enactment behaviors. Certain types of sleep disturbance are more common across anxiety-related disorders (e.g., insomnia and/or broadly defined subjective sleep quality disturbances [ 36 , 37 , 38 , 39 , 40 ] than others (e.g., nightmares) and individuals with anxiety-related disorders often experience more than one type of sleep disturbance. A recent comprehensive publication by Cox and Olatunji provides a highly informative review of sleep disturbance in anxiety-related disorders and the clinical features are given relatively brief review here, where we focus on elements that are less emphasized in that review [ 41 ].

Clinical features of sleep disturbance: PTSD
Subjective sleep disturbance is highly comorbid with PTSD and is often considered a “hallmark” feature of the disorder [ 42 , 43 ]. Subjective sleep disturbance and recurrent nightmares are listed as diagnostic criteria with prevalence rates as high as 90% [ 36 , 44 , 45 , 46 ]. It is therefore rare to encounter a patient with clinically significant non-sleep symptoms of PTSD who does not also report non-restorative and/or disrupted sleep, or distressing dreams. Sleep disturbance in PTSD may also be the most well-studied as compared with sleep disturbance in other anxiety-related disorders, and therefore may provide the best clues for understanding the sleep–anxiety relationship [ 41 ].
Although comorbid insomnia symptoms and poor subjective sleep quality are pervasive features of PTSD, nightmares stand out as a particularly unique feature of this disorder. Prevalence rates for nightmares vary widely, ranging from 19 to 96% [ 39 , 47 , 48 ], however ample research indicates that the prevalence of nightmares is significantly higher in individuals with PTSD than the general population [ 45 , 49 ] and other psychiatric conditions [ 47 , 48 , 50 , 51 ]. The wide range is likely attributable to differences in data collection methods, differences in study sample characteristics, and differences in trauma nightmare definitions. For example, most research uses retrospective measures, which are likely to underestimate nightmare frequency compared with prospective log assessments [ 52 ]. In addition, the definition of trauma nightmares has changed with various iterations of the DSM and varies widely across studies. Research has demonstrated higher rates of trauma nightmares in females compared with males [ 53 , 54 ] and nightmares may be more frequent in the context of recent trauma compared with chronic PTSD [ 55 ].
In addition to the highly prevalent disturbances of insomnia and nightmares, it is now well-established that other sleep disturbances also occur following trauma and/or in PTSD [ 56 , 57 , 58 , 59 , 60 ]. For example, several studies have indicated that panicked awakenings from sleep with poor or no recall of dream content are common in PTSD [ 59 ]. Several studies have also reported increases in physical movement during sleep when compared with controls, including periodic leg movements [ 59 ] (although see Woodward et al. [ 61 ] reporting decreased movement in PTSD sleep). Nightmare enactment is also commonly reported in clinical practice, although prevalence rates are poorly established. Nightmare enactment has typically been observed in younger individuals and is more common immediately following trauma. However, most studies have been conducted in combat veterans, for whom age and time since trauma are difficult to disentangle (e.g., military personnel are exposed at a young age), and thus warrants study in other populations.
Based on the unique combination of sleep symptoms following trauma, Mysliwiec et al. [ 57 , 62 ] have proposed a new disorder, “trauma-associated sleep disorder”. The proposed disorder encompasses disruptive nocturnal behaviors such as thrashing movements panic-like, or startled awakenings, and dream enactment that are proposed to occur out of both REM and NREM sleep. Further research is necessary to determine whether these sleep symptoms represent an early and severe form of sleep response to trauma that later evolves into the more typically described sleep presentation (e.g., insomnia and nightmares), or whether this trajectory is distinct, and possibly even related to neurodegenerative risk. For example, dream enactment is well-described in REM Behavior Disorder (RBD) and is considered a prodromal marker of Parkinson’s Disease. It remains to be seen whether dream enactment following trauma is unique or is related to the dream enactment characteristic of RBD.
Clinical features of sleep disturbance: GAD
GAD is the other anxiety-related disorder whose DSM diagnostic criteria specifically include sleep disturbance [ 46 ]. Despite the inclusion of sleep disturbance in the nosological definition, empirical investigations of sleep disturbance in GAD are relatively limited. Nonetheless, existing published research fairly consistently indicates that subjects with GAD report worse subjective sleep than healthy controls [ 41 ]. Sleep disturbance has been reported in up to 75% of individuals with GAD [ 63 , 64 ]. Adults with GAD report poorer subjective sleep quality [ 65 , 66 , 67 ] and have been found to be more likely to have a sleep disorder (vaguely defined, as per chart review) when compared with control groups [ 68 ].
Clinical features of sleep disturbance: PD
The extant research has demonstrated that subjective sleep complaints are common in PD, with controlled studies indicating greater difficulties with sleep initiation, sleep maintenance, early morning awakenings and more general sleep quality disturbances in PD subjects than healthy controls [ 41 , 69 , 70 , 71 , 72 , 73 ].
A PD-specific sleep disturbance that may overlap with panicked awakenings seen in PTSD are nocturnal panic attacks. Nocturnal panic attacks are characterized by abrupt awakenings from sleep with panic attack symptoms [ 74 ]. These can be distinguished from sleep terrors, which are a parasomnia involving severe autonomic arousal and fearful behavior during NREM sleep, and which are generally not recalled subsequently [ 75 ]. Although epidemiological studies have not been conducted, survey data indicate that lifetime prevalence rates for at least one nocturnal panic attack in individuals with PD are as high as 71% [ 74 , 76 , 77 , 78 , 79 ]. Based on one report with a small sample, it appears that nocturnal panic attacks in PD emerge during the transition from lighter to deeper NREM sleep, either in visually scored N2 preceded by EEG slowing (indicating a transition to deeper sleep) or in early N3 sleep [ 80 ]. There is some evidence to suggest that individuals with PD and nocturnal panic suffer from higher rates of depression [ 80 ] and suicidality [ 81 ] compared with individuals with PD without nocturnal panic. Individuals with nocturnal panic may also suffer from more-frequent daytime panic attacks and greater somatic symptoms during daytime attacks [ 76 ]. These findings have led researchers to postulate that nocturnal panic attacks may represent a more severe variant of PD.
Clinical features of sleep disturbance: specific phobia and SAD
Limited research exists examining sleep disturbance in phobias and SAD [ 41 ]. To summarize, two studies using community samples found that the presence of phobias and SAD was associated with increased likelihood of having sleep disturbances, as measured by the PSQI [ 40 ] and WHO Composite International Diagnostic Interview (WHO CIDI) [ 82 ].
Clinical features of sleep disturbance: OCD
Several studies have examined subjective sleep disturbance in adults with OCD. One study found no differences in sleep parameters when comparing an OCD and controls [ 83 ]. The second study found greater subjective reports of sleep disorders and poorer subjective sleep quality in individuals with OCD compared with healthy controls [ 84 ]. Similar to other anxiety-related disorders, greater OCD symptom severity may be associated with greater sleep disturbance severity; a cross-sectional study demonstrated that short sleep duration predicted OCD in a Korean community sample (Park et al. [ 85 ]) and another cross-sectional study found that greater OCD symptom severity was associated with shorter sleep duration and decreased sleep efficiency in 13 adults with an OCD diagnosis [ 86 ]. Conversely, Marcks et al. [ 87 ] failed to find an association between OCD diagnosis and sleep disturbance in a sample of primary care patients. In a nationally representative sample, Cox and Olatunji [ 41 ] found that individuals reporting insomnia symptoms reported increased OCD symptoms relative to those who did not.
Obstructive sleep apnea
Recently, there has been a growing interest in the relationship between OSA and anxiety-related disorders, particularly PTSD. OSA is a sleep disorder characterized by repeated upper airway obstruction during sleep, leading to breathing interruptions and sleep fragmentation [ 88 ]. The underlying pathophysiology of OSA is multifactorial and complex and may vary considerably across individuals. Important components of OSA pathophysiology include upper airway anatomy, the ability of upper airway dilator muscles to respond to respiratory challenges during sleep, arousal thresholds during sleep, the stability of the respiratory control system, and state-related changes in lung volume [ 89 , 90 ]. Major risk factors for OSA include obesity, older age, male sex, smoking, and use of central nervous system depressants (e.g., alcohol). The apnea hypopnea index (AHI) is the number of apneas or hypopneas recorded per hour of sleep. The AHI is often used as a diagnostic tool and is a marker of OSA severity.
OSA prevalence rates appear to be higher in PTSD samples compared with the general population [ 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 ]. Greater PTSD symptom severity increases the risk of screening positive for OSA [ 91 ]. On the other hand, including both veteran and non-veteran studies, prevalence rates of OSA in PTSD vary considerably [ 92 , 100 , 101 , 102 , 103 , 104 , 105 ]. Differences may result from differences in diagnostic criteria and sample characteristics such as sample size, sex, age, ethnicity, and health status compositions. It is also important to note that some studies generated prevalence estimates from samples specifically recruited for OSA-high-risk features (e.g., sleep disturbance), which is likely to artificially inflate prevalence estimates.
There is insufficient evidence to conclude that OSA rates in non-PTSD anxiety disorders differ relative to controls. One cross-sectional study using data from outpatient records of the Veteran Health Administration found that an OSA diagnosis increased the odds of having an anxiety disorder diagnosis in veterans [ 106 ]. With respect to PD, the majority of work elucidating associations between PD and OSA has been limited to case studies [ 107 , 108 , 109 ], studies with small sample sizes [ 110 , 111 ], and studies without an appropriate control group [ 111 ]. To our knowledge, only one study has examined OSA prevalence rates in PD and GAD; this study was conducted in a community sample of OSA-high-risk participants and reported an OSA prevalence rate of 58.8% in PD and 57.1% in GAD [ 112 ]. Another study demonstrated that individuals with PD demonstrated significantly greater rates of microapneas during sleep than controls [ 110 ].
In summary, although high rates of OSA are reported in PTSD, these are not convincingly demonstrated in other anxiety-related disorders, and the mechanism explaining the relationship between OSA and PTSD remains to be clarified [ 98 ]. The increasingly high rates of obesity in veterans [ 113 ] and people with PTSD [ 114 ] may be a major factor contributing to this surge in OSA prevalence.
Physiological and functional neuroanatomical features of sleep disturbance in anxiety-related disorders
A number of polysomnography (PSG) studies along with a few neuroimaging studies have begun to identify physiological and functional neuroanatomical features that may underly the subjective complaints of sleep disturbance in anxiety-related disorders. We focus here on polysomnography-based studies, and also include a brief presentation of neuroimaging and neuroendocrine research. Of note, we do not discuss actigraphy-based findings in detail here, and instead focus on direct measures of physiology during sleep in anxiety disorders. Overall, actigraphy-based findings demonstrate objective sleep abnormalities in anxiety-related disorders, although there are inconsistencies in results within and across disorders [ 41 , 84 ].
Sleep physiological findings: PTSD
Several studies, including two meta-analyses, now provide convincing evidence of PSG-measured sleep abnormalities in PTSD [ 41 , 115 , 116 ]. In a meta-analysis incorporating 20 studies, Kobayashi and colleagues found that PTSD was associated with more stage N1 percent, and lower SWS percent as compared with a mix of controls, including healthy subjects, trauma-exposed, or non-exposed [ 116 ]. Authors of a more-recent meta-analysis, which only incorporated 13 studies owing to more-restrictive inclusion criteria, and which incorporated new studies not reviewed by Kobayashi et al., also reported less SWS duration in PTSD as compared with a mix of controls [ 115 ]. The latter study found no differences in N1 or N2 sleep duration. On the other hand, they report that PTSD was associated with a reduction in PSG sleep continuity as compared with controls, as defined by a composite measure including sleep latency, sleep efficiency, and/or number of awakenings [ 115 ].
Needless to say, the above meta-analyses were performed in the context of inconsistencies in the literature. Some clinical studies observed no differences in PSG-based sleep measures in PTSD vs. control groups (all trauma exposed) [ 99 , 117 ], whereas other studies have found reduced SWS, increased stage 1 sleep, reduced sleep efficiency, reduced sleep duration, and/or greater number of awakenings in PTSD relative to trauma-exposed [ 118 ] or healthy and depressed controls [ 119 ]. Inconsistencies have been attributed to moderating third variables such as age, sex [ 116 , 120 ], comorbid diagnoses, and substance use disorders [ 116 ]. For example, the Kobayashi et al. meta-analysis also found an effect of PTSD on total sleep time (shorter in PTSD) in studies with male samples only. Finally, laboratory-based studies of PTSD subjects suggest that PTSD subjects may in fact sleep better in the supervised environment of the sleep laboratory. This may obfuscate differences in objective sleep quality in PTSD relative to controls.
In terms of REM sleep, both of the above-cited meta-analyses reported that PTSD was associated with greater REM density (REMD), an index of eye movements during REM sleep, than controls [ 115 , 116 ]. The implications of REMD are not entirely clear, although some research indicates that REMD is an indicator of phasic hyperarousal during sleep. Interruptions or fragmentation of REMS have also been reported in PTSD and/or trauma survivors relative to controls [ 56 , 121 , 122 ], including PTSD-positive vs. PTSD-negative veterans [ 56 ], and PTSD-positive vs. normal healthy civilians [ 121 ]. In a subsample of a representative community-based sample Breslau et al. reported that more frequent arousals from REMS was associated with a history of lifetime PTSD [ 122 ]. Mellman and colleagues [ 123 ] have also found that REMS fragmentation in the early aftermath of trauma predicted subsequent development of PTSD symptoms. Complicating the REM/PTSD story, Mellman et al. [ 124 ] also reported that duration of PTSD was associated with higher REM percent, longer REM duration, and shorter REM latency. Mellman et al. [ 124 ] suggested that the linear association between REM parameters and the course of PTSD may reflect an adaptive process in chronic patients, as REM sleep is implicated in emotional processing. On the other hand, Ross suggests that this “reconstituted” REM sleep in chronic PTSD is actually pathological [ 125 ]. Richards et al. found longer duration of REMS in PTSD relative to exposed controls in women only [ 120 ]. Studies in animal models have fairly consistently demonstrated alterations in REMS in rodent models of acute and chronic stress, which may be relevant across anxiety-related disorders, but these have included both disruptions and prolongations of REMS. Altogether, these findings provoke ongoing interest in REMS but limited definitive conclusions about patterns of disturbance [ 126 , 127 , 128 , 129 , 130 , 131 ].
Although few neuroimaging studies have been performed to understand sleep disturbance in anxiety-related disorders, a number of studies have been conducted in PTSD. For example, Germain and colleagues [ 132 ] performed FDG PET imaging in military veterans with and without PTSD and found hypermetabolism in both wake and REM sleep in PTSD-positive, relative to PTSD-negative subjects, in regions involved in emotion expression and emotion regulation. These included the mPFC, the (right) amygdala, and hippocampus as well as brainstem regions overlapping with the raphe nuclei and (right) locus coeruleus. They also saw elevated activity in the thalamus in PTSD relative to controls. In another study, Germain and colleagues reported hyperactivity in NREMS in PTSD relative to control subjects, as well as increased elevation in NREMS relative to wake, in PTSD more than controls [ 29 ]. Although these reports indicate hyperarousal of both cortical and subcortical brain regions during sleep, other studies contribute to a more complex story with less straightforward interpretation [ 30 , 133 , 134 ].
Other approaches have been utilized to examine the evidence for physiological hyperarousal during sleep in PTSD by measuring peripheral indicators of neuroendocrine activity. Indices of hyperarousal such as nighttime excretion of norepinephrine metabolites and hypothalamic-pituitary-adrenal (HPA) axis activity have indeed been associated sleep disturbance in PTSD [ 135 , 136 , 137 , 138 ].
Sleep physiological findings: GAD
Studies examining physiological sleep characteristics in GAD are limited and therefore it is difficult to draw conclusions [ 41 ]. Nonetheless, PSG-based differences in GAD vs. controls are reported. compared with control groups, individuals with GAD exhibit decreased sleep duration [ 139 , 140 ], increased sleep onset latency [ 139 ], increased wake after sleep onset and reduced sleep efficiency [ 140 ], reduced NREM N2 sleep and reduced SWS [ 140 ]. Two studies found no differences in objective sleep efficiency between GAD and controls [ 139 , 141 ]. A separate study found that individuals with GAD exhibit increased REM latency compared with individuals without GAD [ 141 ].
Sleep physiological findings: PD
Objective reports of PSG sleep abnormalities in PD are less clear. Some studies have found that individuals with PD exhibit decreased sleep efficiency [ 80 , 142 , 143 , 144 ], increased onset latency [ 80 , 142 , 143 ], and reduced sleep duration [ 78 , 80 ] compared with controls. Another study found no differences in PSG measures of sleep disturbance, despite subjective differences in sleep quality across a PD and control group [ 73 ]. A recent meta-analysis based on four studies concluded that PD is associated with poorer sleep efficiency and marginally significant longer sleep onset latency when compared with individuals without PD [ 115 ].
An older and small body of research exists examining sleep physiological characteristics in PD [ 41 ]. The existing literature has demonstrated decreased REM latency [ 142 , 145 ], decreased number of REM periods [ 80 ], decreased REM density [ 145 ], increased stage 1% [ 146 ], decreased stage 2 duration [ 143 ], and deceased SWS % [ 78 , 144 ]. Contrarily, Dube et al. [ 147 ] found no differences in sleep physiology when comparing PD to healthy controls.
Sleep physiological findings in OCD
A small number of studies have found differences in NREM sleep parameters in individuals with OCD compared with healthy controls [ 41 ]. More specifically, studies have reported decreased sleep duration [ 148 , 149 ], reduced sleep efficiency [ 149 , 150 ], and greater number of nocturnal and early morning awakenings [ 148 , 149 , 150 ] in individuals with OCD compared with healthy controls. Studies also indicate increased N1 sleep [ 148 ], decreased N2 sleep [ 148 ], and decreased SWS [ 148 , 151 ] in individuals with OCD. There is also evidence of altered REM parameters in OCD, though results are mixed. Some studies have demonstrated reduced REM latency [ 148 ], higher REM density in the first REM period [ 149 ], and a trend towards reduced REM efficiency ( p = 0.06; [ 148 ]) in individuals with OCD. In contrast, other studies have found no differences in REM parameters between OCD and control groups [ 86 , 150 ]. A recent meta-analysis that combined data from PSG, self-report and observer reports found that sleep duration was also reduced in OCD relative to healthy controls [ 152 ].
Summary of Sleep Physiological Findings
In summary, published sleep-physiological studies overall indicate hyperarousal during sleep in anxiety disorders, with effects on sleep architecture and continuity that may impact some important functions of sleep. It is possible that publication bias has reduced the number of negative published results, favoring publications detecting differences between anxiety disorders and controls. On the flip-side, the generalized but subtle pattern of disruption may indicate that measuring hyperarousal during sleep is more complex than current methods are able to discern, and more sophisticated methods of measurement and analysis such as quantitative EEG analysis [ 120 , 153 , 154 , 155 ] and brain imaging during sleep [ 156 ] are indicated.
Evidence for a bidirectional relationship between sleep disturbance and anxiety-related disorders
Most studies demonstrating high levels of sleep disturbance in anxiety-related disorders are cross-sectional, therefore the causal relationship between sleep disturbance and anxiety-related disorders cannot be discerned. However, a number of studies strongly indicate that sleep disturbance enhances risk for future anxiety-related disorders, especially PTSD, and there is also empirical evidence from human studies demonstrating that that anxiety-related disturbances can precede and increase risk for sleep disturbances. We briefly review studies indicating this bidirectional relationship here.
Prospective studies have convincingly demonstrated that sleep disturbance both prior to and following trauma is an important predictor of subsequent PTSD development [ 117 , 157 , 158 ]. Van Liempt and colleagues [ 159 ] found that nightmares, although not insomnia symptoms, prior to military deployment predicted PTSD subsequent to deployment. Furthermore, treatment research indicates that treatment of insomnia using standard cognitive-behavioral therapy for insomnia (CBT-I) results in an improvement in daytime PTSD symptoms [ 160 , 161 ], indicating that insomnia may be a driver of PTSD symptoms, which, when treated, results in a diminution of PTSD symptoms. A recent meta-analysis by Hertenstein et al. [ 162 ] also demonstrated that insomnia symptoms prospectively predicted anxiety symptoms in the short-term (12–24 months) and long-term (>24 months). In the other direction, Wright et al., cited above, found that PTSD symptoms 4 months after trauma did not predict insomnia symptoms at 12 months. In contrast, an interesting recent study used ecological momentary assessments of PTSD and insomnia symptoms and demonstrated that daytime anxiety predicted (temporally preceded) poorer sleep quality and efficiency, even when controlling for baseline PTSD symptoms [ 163 ]. This study also found that daytime PTSD symptoms and fear of sleep predicted subsequent nightmares. Findings from a large community-based sample of adolescents also showed that having any anxiety disorder was prospectively associated with an increased risk of insomnia [ 164 ]. In that study, insomnia did not predict the future development of anxiety disorder. Although our review is mostly focused on sleep and anxiety in adults, research in children and adolescents, when these disorders first present, may be particularly informative with respect to the chicken-and-egg question.
To our knowledge, studies that prospectively examine the relationship between sleep and non-PTSD anxiety disorders is limited. As described above, in a study in adolescents aged 13–17, anxiety disorder predicted future insomnia, whereas insomnia did not predict future anxiety [ 164 ]. One prospective study utilizing self-report measures found that sleep disturbance was associated with anxiety symptoms (though not GAD specifically) or a diagnosis of PD 4 years later [ 165 ]. CBT for GAD reduces sleep disturbance symptoms, but one study demonstrated that 33% of individuals had an insomnia disorder diagnosis and 63% had clinically significant sleep disturbance following treatment, indicating that insomnia is not a result of GAD alone, and/or that insomnia that may have resulted from GAD has taken on a life of its own, and is therefore unresponsive to GAD treatment. Several laboratory studies using total sleep deprivation paradigms have demonstrated that sleep deprivation increases the vulnerability to subsequent daytime panic attacks [ 166 , 167 ]. It is not clear how these experimental results generalize to sleep disruption in the real world. Additional work has demonstrated that sleep disturbance symptoms persist following successful PD treatment [ 168 , 169 ], which suggests that sleep disturbance is not a secondary symptom of PD or that sleep disturbance has become independent of original PD precipitants.
Explaining the relationship between sleep disturbance and anxiety-related disorders: psychological and psychophysiological models
Although the extant research indicates that sleep disturbances and anxiety-related processes mutually influence each other, the mechanisms through which this occurs is not yet clear. The dominant psychological and psychophysiological models of insomnia disorder certainly provide clues about these mechanisms.
Insomnia disorder is a sleep disorder characterized by subjective difficulties with sleep onset and/or sleep maintenance, despite adequate opportunity to sleep, with associated daytime impairment or distress [ 46 ]. A wealth of research has advanced our understanding of insomnia as a condition resulting from and reinforced by maladaptive, sleep-focused cognitions and maladaptive behaviors and coping strategies. Genetic and biological/physiological traits may predispose individuals to the development of insomnia, and physiological disturbances also result from maladaptive cognitions and behaviors. The interaction of cognitive, behavioral, and physiological factors all contribute to a vicious cycle of sleep disturbance and associated distress. The profound value of these models and testimony to their validity is that the CBT-I that is based on these models is the first-line therapy for insomnia disorder, with proven efficacy in insomnia alone and comorbid with a multitude of psychiatric and medical conditions [ 170 , 171 ]. CBT-I has also demonstrated benefits for anxiety symptoms in several studies [ 161 , 172 ].
The diathesis-stress model states that there are three interrelated and sequential factors involved in the pathogenesis of insomnia: predisposing, precipitating, and perpetuating factors [ 173 ]. Predisposing factors are individual genetic, physiological, or psychological traits that produce differing levels of susceptibility for developing insomnia (e.g., sex, chronotype, genetic polymorphisms affecting arousal regulation or cognition). Precipitating factors are physiological, environmental, or psychological stressors that trigger the onset of disrupted sleep patterns and include factors ranging from physical injury, work-related stress, or a traumatic stressor. They may or may not be psychological in nature. Perpetuating factors are maladaptive strategies for coping with disrupted sleep that contribute to insomnia symptoms and result in chronic insomnia if sustained. They generally reflect efforts to compensate for insufficient nighttime sleep and are comprised of behaviors such as sleeping in on weekends, taking daytime naps, and increasing time in bed in an effort to obtain the desired amount of sleep. The perpetuating influence of the latter behaviors is explained by the stimulus-control model proposed by Bootzin [ 174 ], and is anchored in classical conditioning theory. Increased time in bed in the absence of actual sleep eventually results in increased frustration in bed (i.e., cognitive and physiological arousal), which effectively reduces the likelihood of sleep and results in a further perpetuation of wakefulness rather than sleep. Their perpetuating effect is enhanced by the fact that the compensatory mechanisms result in efforts to sleep at physiologically suboptimal times (i.e., in the absence of homeostatic and circadian pressures to sleep).
Cognitive models of insomnia focus on thoughts, beliefs, and/or feelings that may interfere with sleep and lead to maladaptive coping behaviors [ 175 ]. According to cognitive models of insomnia, excessive worry and rumination lead to arousal and distress, all of which precipitate and perpetuate insomnia symptoms [ 175 ]. Harvey hypothesized that insomnia is driven by inappropriate worry about sleep and sleep-related consequences. This worry subsequently leads to physiological arousal, selective attention to sleep-related threats (e.g., ambient noise), and the adoption of maladaptive safety behaviors. Another cognitive model proposed by Espie proposes that psychological and/or physiological stress leads to selective attention towards stressors, which inhibits the natural “de-arousal” that is necessary to initiate sleep [ 176 ].
Riemann and colleagues have advanced a hyperarousal model of insomnia that incorporates the elements of the above models while also emphasizing a fundamental contribution of genetic and physiological vulnerability to sleep/wake regulatory problems and to hyperarousal at a physiological as well as cognitive level. In their words, they conceptualize insomnia as “a final common pathway resulting from the interplay between a genetic vulnerability for an imbalance between arousing and sleep-inducing brain activity, psychosocial/medical stressors and perpetuating mechanisms including dysfunctional sleep-related behavior, learned sleep preventing associations, and other cognitive factors like tendency to worry/ruminate” [ 177 ]. They cite evidence indicating that hyperarousal processes from the molecular to higher system levels play a role in the pathophysiology of insomnia [ 177 ]. Several other researcher groups have demonstrated that hyperarousal in insomnia exists in cognitive (e.g., sleep-related worries [ 178 , 179 ], somatic/physiologic (e.g., increased heart rate; [ 178 , 179 ]), and cortical (e.g., high frequency EEG) [ 179 ] domains. Riemann et al. [ 177 ] have pointed out that while standard visual scoring of EEG has often failed to show differences between insomnia and controls, more sophisticated approaches to EEG analysis are pointing to subtle and dynamic features that distinguish good from poor sleepers. These methods are now often used in insomnia disorder research but have only been used in a small number of studies of sleep in anxiety-related disorders [ 120 , 153 , 154 ].
Altogether, research on insomnia provides strong evidence for an interplay between biological predispositions, sleep-disrupting life events, and maladaptive coping behaviors that result in cognitive and physiological hyperarousal, that then perpetuate the cycle of sleep disturbance. The model described by Riemann et al. proposes both cognitive-behavioral and neurobiological pathways through which biological and cognitive risk factors may lead to insomnia as well as anxiety disorders, that then positively reinforce sleep-disturbance in a vicious-cyclic pattern.
Applicability of the dominant insomnia models to PTSD
Consistent with the above models, a traumatic event amounts to a classic and severe insomnia “precipitating factor” that may result in disrupted sleep owing to a variety of factors [ 180 ]. From a psychological perspective, nighttime arousal that occurs immediately following trauma may be adaptive in settings of increased predatory threat (e.g., combat), or owing to one’s increased vulnerability when asleep [ 181 , 182 ]. Unfortunately, whether initially adaptive or not, compensatory behaviors (e.g., naps) to compensate for nighttime sleep deficits will result in mistimed sleep, and therefore wakefulness in bed even in the absence of psychological distress, and thereby contribute to conditioned arousal. On top of this, nighttime trauma-related psychological distress, such as distressing trauma memories and ruminations, and frightful awakenings occurring either spontaneously or from nightmares, are likely to contribute to conditioned arousal in the sleep environment. Although the insomnia models emphasize sleep-related cognitions as a precipitant of hyperarousal, the essential ingredient is that worry in the bedroom, at night, and in the sleep environment precipitates hyperarousal. In this context, the application of insomnia models to PTSD is therefore straightforward, and the success of standard CBT-I for treating sleep disturbance in PTSD provides strong support for its relevance to PTSD [ 160 ].
Fear of sleep is common in PTSD and distinguishes sleep-related cognitions in PTSD from insomnia (in which individuals desire rather than fear sleep). Evidence suggests that fear of sleep is driven by both fear of having a nightmare and fear of the vulnerability that occurs during sleep [ 163 , 183 , 184 , 185 ]. Fear of sleep may result in individuals avoiding sleep and/or in difficulties with sleep initiation. Some studies have elucidated an association between fear of sleep and insomnia symptoms [ 163 , 185 , 186 ], whereas other studies have found no such association [ 183 ]. One highly credible explanation for the latter findings is that over time, insomnia is no longer driven by fear of sleep and is instead maintained by a pattern of learned maladaptive coping behaviors that are no longer dependent on the initial precipitating causes [ 183 ].
Applicability of the dominant insomnia models to GAD
Cognitive and hyperarousal models of insomnia may be particularly applicable for insomnia pathogenesis in GAD. A cardinal feature of GAD is excessive cognitive activity, specifically worry and rumination. Although not performed specifically in GAD subject, one study demonstrated that sleep-related rumination was associated with both trait and state arousal in insomnia disorder [ 187 ]. Several studies have also demonstrated associations between worry, rumination, and sleep disturbance, though most of these studies are limited to undergraduate samples and results may not be generalizable [ 188 , 189 , 190 , 191 , 192 ]. On the other hand, some studies examining trait worry and rumination failed to find significant associations with sleep disturbance [ 193 ]. O’Kearney et al. [ 194 ] found that worry was associated with insomnia symptoms as measured by the ISI, but not specific sleep continuity parameters.
Applicability of Dominant Insomnia Models to PD
Within the insomnia model framework, nocturnal panic attacks can be considered a precipitating factor for insomnia, disrupting sleep, and contributing to sleep–environment arousal and fear of having another panic attack [ 79 ]. Repeated nocturnal panic attacks produce conditioned fear (e.g., hyperarousal) and avoidance of sleep [ 79 ], which results in the adoption of maladaptive sleep-related behaviors (e.g., sleeping in brightly lit and very loud environments to obtain rest but avoid deep sleep). Conditioned fear, avoidance of sleep, and maladaptive safety behaviors are perpetuating factors and maintain insomnia symptoms in the absence of nocturnal panic attacks.
Two studies have demonstrated the presence of insomnia symptoms in the absence of nocturnal panic attacks, suggesting that other factors may be involved in insomnia pathogenesis in PD [ 72 , 195 , 196 ]. Anxiety sensitivity—a tendency to experience excessive fear of anxiety-related sensations based on the fear that these sensations are harmful [ 197 ] has been associated with sleep onset difficulties in PD [ 198 ]. Anxiety sensitivity may amplify the conditioned hyperarousal characteristic of insomnia by leading to a hyper-focus on physiological symptoms. Related to this, two studies manipulating pre-sleep appraisal of sleep physiology support this theory by demonstrating that individuals with PD and nocturnal panic report more distress when they believe that physiological signals are abnormal [ 199 , 200 ].
Contrasting with this idea of anxiety sensitivity, Craske and Tsao [ 74 ] also propose that nocturnal panic in PD is driven in part by fear of loss of vigilance. Evidence cited to support this model included studies demonstrating higher physiological and self-reported emotional reactivity to relaxation and imagery exercises, greater self-reports of panic attacks following relaxation and fatigue, and greater self-reported discomfort during relaxation exercises in individuals with PD and nocturnal panic compared with individuals with PD without nocturnal panic [ 80 , 201 , 202 ].
Applicability of dominant insomnia models to OCD
A recent study exploring associations between insomnia symptoms and OCD symptoms in an undergraduate sample found that insomnia symptoms were associated with obsessions, but not compulsions [ 203 ]. This finding suggests that similar to other anxiety-related disorders, hyperarousal and cognitive activity may be driving insomnia in individuals with OCD as obsessions are characterized by heightened arousal and compulsions serve to alleviate anxiety.
In summary, the above-described findings implicate fear, threat, and anxiety-related cognitions occurring at night or in the sleep environment, or in a trait-like fashion and therefore likely to occur at night, in sleep disturbance associated with anxiety-related disorders. Whether they are focused on anxiety about missed sleep (insomnia) or any other range of concerns may be less important than the fact that they reflect overall cognitive hyperarousal in the sleep environment and as such result in physiological arousal as well as all the downstream compensatory and conditioning phenomena typical of insomnia disorder. Overall, this reflects a transdiagnostic process, consistent with a transdiagnostic model of insomnia relevant across psychiatric disorders.
Affective and cognitive neuroscience of sleep inform the understanding of sleep disturbance in anxiety-related disorders: emotion regulation and emotional memory
Insomnia models satisfactorily explain many of the subjective sleep complaints and insomnia manifestations per se in anxiety-related disorders, but they do not fully or explicitly incorporate the effects of objective disruptions of sleep integrity on two aspects of brain functioning that may be elemental to the disorders discussed here: emotion regulation and emotional memory.
Sleep disturbance and emotion regulation
Emotion regulation generally refers to the ability to modulate emotional experiences in a way that is responsive to the context of a situation as well as an organism’s long-term objectives [ 187 ]. In anxiety disorders, emotion-regulatory problems manifest as the maladaptive expression of fearful and anxious emotions and emotional responses. Emotion regulation may be considered an aspect of cognitive executive function [ 4 ]. The prefrontal cortex (PFC) has been demonstrated to play an important role in emotion regulation [ 204 , 205 ], and the frontal lobes may be particularly vulnerable to sleep loss. Gruber and Cassoff [ 4 ] recently published an informative review of empirical findings and a conceptual framework for understanding the importance of sleep for executive functioning and emotion regulation more specifically.
Related to this, it has been reported that deficits in cognitive inhibition (e.g.,“tuning out” irrelevant stimuli) [ 206 ] and emotion regulation [ 187 ] were associated with sleep-related rumination in individuals with insomnia disorder. Particular to GAD, one study found that difficulties with emotion regulation mediated the association between GAD diagnosis and insomnia symptoms [ 207 ]. A recent study in PD found that poorer performance on a cognitive inhibition task was associated with longer sleep onset latency and reduced sleep quality in individuals with PD [ 208 ].
A recent imaging study provides neurobiological evidence for the role of emotion regulation in the anxiety–sleep relationship. Pace-Schott et al. found that compared with GAD and insomnia disorder groups, a good sleeper group exhibited greater resting-state functional connectivity between the left amygdala and a bilateral region of the rACC. As described earlier, the rACC is part of a prefrontal network believed to exert top-down control over amygdala activity and may constitute an emotion-regulatory circuit. These findings support the idea that deficits in emotion regulation may play a role in both anxiety and insomnia pathogenesis [ 209 ].
These studies do not explain causal relationships, and do not necessarily distinguish between objective sleep disruption and insomnia symptoms/disorder per se, yet they provide evidence that deficits in cognitive functions, which may result from sleep disturbance or be a manifestation of an anxiety disorder, are mechanistically involved in the sleep-disturbance-anxiety disorder cycle.
Sleep and memory: relevance to anxiety-related disorders
A sizable body of research now provides strong evidence that sleep has an important role in memory consolidation [ 10 , 11 , 210 , 211 , 212 , 213 ]. The “off-line” state of sleep provides a unique opportunity to consolidate information considered relevant to the organism through the reinforcement of key synaptic connections in the relative absence of environmental inputs and high energy-expenditure needs [ 10 ]. Although the most-compelling evidence currently points to NREMS as the major driver of sleep-dependent memory consolidation, both NREMS and REMS have been shown to be relevant for consolidation of memory of various sorts [ 11 , 210 , 214 ]. Research on sleep in anxiety-related disorders, and PTSD in particular, has honed in on REMS as an important player in the processing of fearful, threatening, or distressing experiences in a way that results in selective and adaptive consolidation of emotional memories [ 215 , 216 ].
Fear conditioning and related protocols in both animal models and human subjects constitute the dominant approach for studying the role of sleep and REMS in emotional learning relevant to anxiety-related disorders [ 13 , 217 , 218 ]. Fear conditioning involves the transformation of a neutral stimulus, such as a neutral visual image or context, into a frightening stimulus through its repetitive pairing with an aversive experience, such as an electric shock. Via classical conditioning, the neutral stimulus becomes a conditioned stimulus eliciting a fearful emotional response, usually measured using electrophysiology techniques such as skin conductance response or electromyography responses in humans. Once established, conditioned fear can be extinguished through subsequent repetitive presentation of the conditioned stimulus in the absence of the aversive stimulus. Modifications of the simplest fear-conditioning protocols exist, including addition of “safety” stimuli that are never paired with an aversive stimulus, the responses to which can be compared with conditioned fear responses to assess the subject’s ability to discriminate between fear and safety cues. Neuroimaging research indicates that fear extinction is mediated in large part by the mPFC, in particular the ventromedial PFC and rACC, which are structures implicated in anxiety-related disorders [ 13 , 219 ].
Although findings are not entirely consistent across studies, which utilize different measures of REMS consolidation and/or different outcome measures, recent findings in humans indicate that intact REMS is important for adaptive extinction of conditioned fear, including retention of extinction over time, and the ability to distinguish between safety and fear signals [ 218 , 220 ]. This has important implications for psychotherapies since extinction and safety learning are psychological models underlying psychotherapy for anxiety. Although not focused on REMS, there is indeed some evidence indicating that sleep may enhance the efficacy of phobia and SAD treatment [ 221 ]. Exposure therapy followed by a period of sleep is associated with decreases in heart rate [ 222 ], skin conductance [ 222 ], and self-reported fear [ 223 ] when confronted with the feared stimulus compared with sessions followed by wake. Poorer sleep at baseline was associated with poorer outcome following CBT treatment for SAD and more restful sleep following sessions was associated with a greater reduction in SAD symptoms [ 224 ].
Although fear conditioning is a form of implicit emotional learning, some researchers have also found REMS associations with declarative forms of emotional memory [ 14 , 225 , 226 ]. These studies may be most pertinent to PTSD, the symptoms of which revolve around a traumatic emotional memory and include persistent, intrusive emotional memories [ 227 ]. For example, Walker et al. found that higher REMS duration correlated with enhanced emotional, vs. neutral, recall in a nap paradigm involving recall for emotionally distressing vs. neutral images [ 225 ]. Walker and others have proposed an emotional processing function to REMS, wherein REMS enhances the declarative recall of emotional memories while diffusing the negative emotional valence [ 216 ]. On the other hand, several studies reported findings indicating that REMS in healthy subjected either heightened, or did not affect, the emotional tone associated with memories [ 15 , 226 , 228 , 229 ]. Furthermore, a REM-deprivation study found that selective REMS deprivation did not impact emotional memory. Another study found that while sleep after learning did enhance emotional memory recall relative to a non-sleep group, selective REM deprivation had no impact on negative vs. neutral recall [ 230 ]. These varied findings leave researchers with many unanswered puzzles, and suggest that REM/NREM distinctions may be inadequate for pinpointing the complexities of sleep effects on emotional memory.
Sleep spindles deserve brief mention because sleep spindles reflect memory consolidation processes involving the coordinated oscillations of the thalamo-cortical circuitry, the hippocampus and the cerebral cortex that together result in the transfer of information from temporary storage in the hippocampus to more durable cortical storage [ 211 , 231 ]. In a recent laboratory study in which subjects viewed a trauma film prior to either overnight sleep, a day of wake, or sleep deprivation, Kleim and colleagues [ 232 ] found that greater N2 sleep and more parietal spindles (13–15 Hz) in the sleep group were associated with fewer intrusive memories in the week following the laboratory exposure. They also found that increased N1, wake-after-sleep-onset, and REMD were associated with higher intrusions, and that sleep was protective relative to both the other groups. Although few studies have examined spindles in emotional memory, these findings may be relevant in the context of anxiety-related disorders, especially PTSD.
What about nightmares?
Nightmares continue to be an elusive and intriguing phenomenon, which have generated a host of theories regarding etiology and possible function. A long history of dream theory based primarily in psychoanalytic work, but also in peer-reviewed dream research, has espoused a psychological, sleep-protective, and/or emotional processing function for dreams [ 57 , 233 ]. These theories are generally anchored in the notion that emotional dreams are REMS phenomena [ 234 ], that REMS is important for emotional processing (Walker, [ 212 ]; Walker & van der Helm, [ 216 ]), and that trauma nightmares result from a disruption in the normal emotional processing and consolidation functions of REMS [ 235 , 236 , 237 ]. These conceptualizations are grounded in compelling reasoning, but require bolstering by empirical evidence. Further, early research increasingly indicates that trauma nightmares are not an exclusively REMS phenomenon; nightmare awakenings occur out of both REM and NREM sleep [ 59 , 238 , 239 ].
In the context of REM sleep models, it has also been proposed that abnormally elevated adrenergic tone during REMS contributes to the distress of REMS dreams and/or awakenings from REMS [ 240 ]. Our current understanding of central nervous system pathways involved in sleep and arousal are generally consistent with this hypothesis: norepinephrine-generating locus coeruleus activity is low during normal sleep, and essentially absent during normal REMS. Excess activation of the locus coeruleus could perturb REMS. The hypothesis that excessive adrenergic tone during sleep contributes to nightmares seems reasonable and could apply regardless of sleep stage context. We are not aware of studies that have examined this hypothesis. Results from one recent study demonstrated that reduced respiratory sinus arrhythmia during sleep, a measure of parasympathetic tone, was a predictor of nightmare endorsement the following morning [ 241 ]. This may indicate that a disruption in the normal balance of parasympathetic vs. sympathetic tone contributes to nightmares, but is not direct evidence of enhanced sympathetic activity.
Despite our limited understanding of the neurobiology underlying the generation of nightmares, nightmares likely contribute to the maintenance of PTSD and sleep disturbance in a manner consistent with the insomnia processes discussed previously. Nightmares are likely to contribute to fear of sleep and sleep avoidance, factors that contribute to altered sleep-related behaviors and insomnia. Short et al. also demonstrated that daytime PTSD symptoms and fear of sleep were prospectively related to nightmares [ 163 ]. The effects of repeated activation of trauma-related thoughts during sleep are unknown, but one might speculate that they contribute to reinforcement of memory-containing neural networks (the PTSD “fear network”), thereby consolidating those memories in association with distressing emotions.
Abnormal movements during sleep: from muscle twitches to nightmare enactment
Abnormal movements during sleep in anxiety-related disorders are also poorly understood phenomena, and are primarily reported in PSTD, as opposed to other anxiety-related disorders. They are grouped together here because they involve abnormal motor behavior during sleep, rather than due to a clearly understood neurobiological overlap. Muscle twitching in the context of REMS atonia is normal phenomena, and there is even evidence that it plays a role in sensorimotor development [ 214 ]. However, indications that muscle movements are increased in PTSD relative to normal are consistent with the overall concept of hyperarousal during sleep. Carwile and colleagues proposed that RBD-like phenomena in PTSD (nightmare enactment) may be due to abnormalities in LC NE firing. However, in contrast with the idea that NE activity is elevated in REMS, they propose that it is a high “turnover” and resulting depletion of NE in the LC of PTSD patients that disrupts REM atonia, just like degeneration of LC neurons occurs in neurogenerative diseases [ 242 ]. Further research is clearly indicated to better understand the neurobiology of these phenomena and their implications for outcomes in anxiety-related disorders. Regardless of etiology, like other sleep-disrupting events described in this report, they are likely to contribute to the many downstream effects of objective sleep disturbance and the cycle of behavioral, cognitive, and physiological problems that characterize the sleep–anxiety relationship.
Sleep disturbance and anxiety-related disorders: underlying neuromodulatory context
Figure 4 provides a schematic diagram modeling the psychological and neurocognitive factors that link sleep disturbance and anxiety-related disorders in a bidirectional fashion. This relationship occurs in the setting of heightened activity in known neuromodulatory pathways. Animal and pharmacology studies point to important neuromodulators, such as norepinephrine and hypocretin, that play an important role in this dynamic.
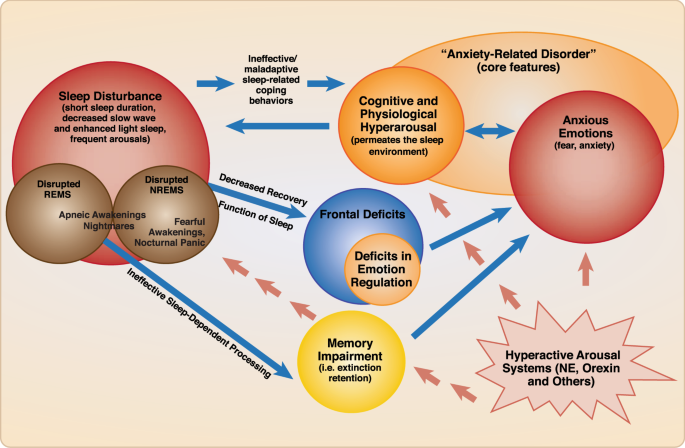
Schematic diagram including the psychological and neurocognitive factors that link sleep disturbance and anxiety-related disorders in a bidirectional fashion. Sleep disturbance, regardless of cause, may result in maladaptive sleep-related compensatory behaviors, resulting in sleep timing that is out-of-synchrony with sleep drive, and therefore wake time in bed. This results in hyperarousal (i.e., cognitive and emotional) in the sleep environment, promoting a vicious cycle of sleep disturbance, compensatory behaviors, hyperarousal cognitions and anxious emotions. Sleep disturbance also has deleterious effects on cognitive functions, due to insufficient sleep-dependent recovery of neural substrates that carry out said functions during wake; this results in widespread deficits including deficits in emotion regulation (i.e., reduced ability to modulate anxious emotions). Sleep disturbance also disrupts processes thought to be carried out during sleep , such as adaptive memory functions (e.g., fear extinction retention; safety learning); this also promotes or maintains anxiety/fear emotions. Anxious emotions and cognitive hyperarousal are mutually reinforcing, and feed back into the sleep disturbance cycle. The specific role of REMS and NREMS in recovery and sleep-dependent processes is a topic of investigation. The nature of mechanistic connections between anxiety-related events such as nightmares and panicked awakenings, and NREM or REM sleep-dependent processes, remains to be demonstrated with empirical evidence. At the very least, their promotion of cognitive hyperarousal and anxious emotions can be inferred from their contribution to sleep disruption and the fearful emotions they generate. A neuromodulatory milieu involving heightened arousal signals, e.g., norepinephrine, and/or hypocretin, drives and underlies this relationship
Major neuromodulators in sleep–wake regulation and anxiety-related disorders
Norepinephrine.
Norepinephrine is released both peripherally (adrenal medulla) and in the central nervous system as a neurotransmitter and plays a central role in both sleep regulation and anxiety (See Fig. 1 ). Although its nearly exclusive CNS source, the locus coeruleus, seems to be too small to be captured by classic neuroimaging techniques (although see PET studies above and fMRI findings by Naegeli et al. [ 243 ] in PTSD), it is clearly central to anxiety- and fear-related processes in the CNS through its direct innervation of the amygdala and its inhibitory inputs to the VLPO, among a multitude of other projections (e.g., cerebral cortex). Normally, NE activity is high during wake, low during NREMS, and nearly completely quiescent during REMS [ 244 ]. Human studies have provided some evidence of elevated sympathetic tone and/or LC activity in anxiety-disordered sleep [ 29 , 136 , 245 ].
Pharmacological research has studied various noradrenergic blockers for treatment of anxiety-related disorders. For example, the selective alpha-1-adrenergic blocker prazosin has demonstrated benefits in multiple small to medium-sized RCTs for PTSD symptoms and trauma nightmares [ 246 , 247 , 248 ]. Disappointingly, a recent large RCT of prazosin for PTSD in veterans demonstrated no benefit relative to placebo and no benefit overall for either PTSD symptoms overall or for nightmares [ 249 , 250 ]. One recent study indicates that higher baseline systolic blood pressure predicts greater therapeutic response to prazosin for nightmares and sleep disturbance [ 251 ]. Doxazosin, a medication with the same alpha-1 blocking effects of prazosin, but with a longer half-life, has shown some benefit in preliminary studies and is currently being studied as an alternative to prazosin [ 252 ]. Interestingly, it is thought that doxazosin is less able to cross the blood–brain barrier than prazosin. This raises questions about whether PTSD is associated with greater blood–brain barrier permeability, thus allowing entry of less lipophilic agents, or whether some of the CNS benefits are mediated via peripheral adrenergic nervous system responses. Finally, the alpha-2-agonist clonidine has shown some benefit in PTSD nightmares, however it has not been extensively studied [ 253 ].
The neurotransmitter serotonin (5-hydroxytryptophan, 5HT) is a neurotransmitter that has an important role in arousal (see Fig. 1 ) and which also plays an important role in the regulation of anxiety and mood states. It is produced in the dorsal raphe nucleus, which has broad axonal projections including projections to the VLPO (inhibitory) and the cerebral cortex. The arousal effect of 5HT is mediated in part by 5HT-2A receptor activity, based on animal studies as well as clinical research demonstrating that agents with high 5HT-2A blocking effects are sedating (i.e., trazodone, quetiapine, amitriptyline). Agents that selectively inhibit the reuptake of serotonin into neurons (selective serotonin reuptake inhibitors, (SSRI)) and thereby increase 5HT in the synapse, are first-line agents for anxiety and mood-related disorders, including all of the disorders described in this review. Serotonin levels are high during wake, low during NREMS and at their lowest during REMS [ 244 ]. One can therefore see how agents that enhance 5HT across 24 hours (SSRI’s and SNRI’s, which also block norepinephrine reuptake) might have untoward effects on sleep even if beneficial for anxiety. On the other hand, downregulation of 5HT-2A receptors with prolonged SSRI administration may be associated with resolution of initial activating effects of these drugs.
The role of histamine in sleep/wake circuitry is demonstrated in basic research and is supported by the broad use, though limited controlled research, of various drugs with anti-histaminergic properties (e.g., trazodone, quetiapine, amitriptyline, doxepin, diphenhydramine, and cetirizine). It has been proposed that histamine release is under circadian control, and is released earlier than other neurotransmitters. Although circadian disruptions are a major topic that is hardly covered in this review, the possibility the role of circadian timing of histamine release may be relevant to early morning awakening in anxiety-related disorders. Anti-histamines are commonly used off-label for the treatment of anxiety, although evidence for specificity as an anxiolytic, as opposed to a sedating agent, is weak.
Acetylcholine
Acetylcholine is a neurotransmitter synthesized in several brain regions/nuclei, including the pedunculopontine tegmentum (pons), the lateraldorsal tegmentum (pons) and the basal forebrain and is also an important arousal neurotransmitter. In contrast to the other major neuromodulators described above, CNS cholinergic activity is normally high in both wake and REMS, although decreased in NREMS [ 244 ]. Walker and colleagues [ 212 , 216 ] have postulated that elevated cholinergic activity in REMS, combined with maximal suppression of noradrenergic activity during this stage, contributes to the emotion and emotional memory processing function of REMS. Although this remains highly theoretical, Walker and colleagues propose that the memory-promoting function of acetylcholine in REMS acts to consolidate the declarative memories (an adaptive process) in the absence of the NE-promoted emotional “tag”.
Several other medications with effects on these neurotransmitters systems have been used to treat sleep disturbance in anxiety-related disorders. Trazodone, for example, is used widely in public health settings [ 254 ] despite limited controlled data on efficacy and is known to block receptors for histamine, serotonin (5HT-2A/2C), and alpha-adrenergic receptors, [ 255 ]. A recent study of cyclobenzaprine, a tricyclic molecule structurally similar to amitriptyline, which like trazodone blocks histamine, alpha-adrenergic, and serotonergic 5-HT2A receptors, was halted during a Phase III development trial because of inadequate separation from placebo for the primary sleep endpoint in military-related PTSD (Clinicaltrials.gov: NCT03062540).
Dopamine, produced in the ventral tegmental area and the ventrolateral periaqueductal gray, is also an important arousal-promoting neurotransmitter. While mostly known for its central involvement in reward neurocircuitry and disorders of addiction, a recent study of quetiapine for PTSD lends some support for dopamine-receptor blockade in the treatment of anxiety-related disorders, and quetiapine is often used off-label for the treatment of insomnia [ 256 , 257 ]. Quetiapine’s broad inhibition of multiple arousal-promoting neurotransmitters, including serotonin (5HT-2A), histamine, and NE (alpha-1) do raise questions about dopamine-specific mechanisms.
Hypocretin/orexin
The hypocretins, also known as orexins, including hypocretin-1 and 2, are neuropeptides with a well-established role in promoting and stabilizing arousal [ 258 , 259 , 260 ]. Recent publications present the growing evidence implicating the hypocretins in anxiety-related disorders, and the pathways through which they act [ 261 , 262 ]. Hypocretin is produced in the hypothalamus, and hypocretin-producing neurons have widespread excitatory projections, including to the main wake-promoting centers (see Fig. 1a ), the cerebral cortex, and the amygdala. Overall, the research indicates that hyperactivity of the orexin system contributes to anxiety-related behavior. While most research has been performed in animal models, one recent study demonstrated elevated CSF orexin in patients with PD. An interesting recent study found that hypocretin was related to panicked awakenings in a rodent model. These findings may be highly relevant to understanding nocturnal panic attacks and frightful awakenings in the context of PTSD and PD and deserve further study.
The dual-orexin antagonist suvorexant is FDA-approved for the treatment of insomnia. At present there are no published trials of hypocretin/orexin antagonists in anxiety-related disorders, though a few studies are underway. Theoretically, hypocretin/orexin antagonists would target more-discrete pathways than commonly used GABAergic hypnotics, and ideally would be suitable for individuals who demonstrate abnormally increased hypocretin activity [ 263 ]. There is preclinical evidence that orexin antagonism can block stress-induced sleep disturbance but have no effect on baseline autonomic and autonomic activity [ 264 ]. The implication is that it is possible to develop compounds that only affect stress-related over-activation of arousal pathways without interfering with normal activity in healthy individuals.
GABA receptors are ubiquitous in the CNS and GABA-mediated CNS inhibition has a huge role in sleep promotion through specific pathways. On the other hand, endogenous GABA-mediated promotion of sleep involves a targeted suppression of arousal-promoting nuclei, rather than a more global inhibition of the cerebral cortex (See Fig. 1 ). Despite some theoretical value of targeting GABAergic function in anxiety-related disorders, the potential for adverse effects (tolerance, dependence, cognitive side-effects, ataxia, respiratory depression) are substantial given widespread distribution of GABAergic inhibitory neurons in the brainstem, cerebellum, and cortex [ 265 ]. At least in PTSD, there is also limited evidence of diminished GABA brain activity [ 266 ]. Furthermore, GABA also plays a role in arousal through inhibition of inhibitory interneurons in the cerebral cortex. This may contribute to paradoxical disinhibition in response to GABAergic drugs in some individuals. Despite concerns, benzodiazepines have demonstrated benefits for anxiety symptoms, and their use continues to be widespread [ 267 ]. There are only a few published controlled studies focused on GABAergic hypnotics in PTSD [ 268 , 269 ], and the extant evidence is insufficient for recommending a GABAergic approach.
The so-called non-benzodiazepine receptor agonists, or “z-drugs,” were developed to specifically target subtypes of the GABA-A receptor, with the aim of reducing adverse effects. One study of eszopiclone, for example, which targets the alpha-3 subunit specifically, provides preliminary evidence of benefit for both sleep disturbance and PTSD [ 269 ]. The advantages of z-drugs over the benzodiazepines in sleep disturbance in anxiety-related disorders has not been well-established.
The HPA axis and CRF
Research in humans and rodent models indicate that stress-induced alterations in corticotropin-releasing factor (CRF) and the HPA axis may result in objective sleep quality disturbances, including decreased delta sleep in human subjects, a quantitative measure of slow-wave activity [ 137 , 153 ] and REMS disturbances in rodents [ 127 ]. CRF functions as a neurotransmitter in the amygdala, locus coeruleus, and bed nucleus of the stria terminalis, and has an arousing effect on the cortex. Theoretically, CRF antagonism would be a good target for treating anxiety-related sleep disturbance, however trials of such agents in psychiatric disorders, including PTSD, have been disappointing [ 270 ]. Nonetheless, these studies represent an attempt to specifically engage in a target pathway implicated in fear and anxiety states. Future trials with agents that are more specifically targeted to abnormal pathways in PTSD and other anxiety disorders await much-needed advances for understanding the underpinning neurobiology [ 256 ].
Other diverse and emerging players: cytokines, adenosine, cannabinoids
Research is beginning to cover the role of multiple other players in sleep-regulatory processes that may be relevant to the relationship reviewed here. Adenosine is a major player in the homeostatic sleep drive (the drive to sleep that increases as a function of time awake and/or physiologic recovery need), and cytokines IL-1 and TNF influence adenosine-mediated effects [ 271 ]. Likewise, overnight cytokine activity has been demonstrated to be altered in anxiety, including PTSD [ 272 ], indicating that abnormalities in these inflammatory factors may contribute to sleep disturbances in anxiety.
One example of an approach that targets a signaling pathway outside the intrinsic sleep or wake-promoting networks is a small trial of nabilone, a synthetic cannabinoid with agonist properties for the CB1 receptor. Cannabis, which contains hundreds of cannabinoids, is widely used as self-medication for anxiety and sleep problems, but the existing evidence of long-term therapeutic benefit is scarce and overall unfavorable, at least for PTSD [ 273 , 274 ]. On the other hand, cannabidiol, as opposed to tetrahydrocannabinol, shows some promise in preliminary research for sleep disturbance [ 273 ]. A trial of nabilone was predicated on evidence from PET imaging for elevated brain cannabinoid CB1 receptor availability in the amygdala of PTSD, which was associated with attentional bias to threat [ 275 ]. This small trial, which showed some efficacy for improving sleep in military-related PTSD represents a proof of concept trial of a sleep-related therapeutic targeting a pathway putatively known to be abnormal in the specific clinical population. Another potential target in the endocannabinoid system is fatty acid amide hydrolase, which metabolizes endogenous cannabinoids [ 276 ].
Summary of Important Neuromodulators in Sleep and Anxiety
The above-described CNS neuromodulators have well-described roles in sleep regulation and/or anxiety, and may be targets for pharmacotherapy of sleep disturbance in anxiety-related disorders. Norepinephrine and hypocretin stand out as obvious targets that are currently under study, but novel research is pointing to a host of other neuromodulators worthy of study as potential drug targets.
A heuristic neurobiological framework for understanding sleep disturbance in anxiety disorders
Given what is known about the brain pathways (see introduction) and neuromodulators regulating sleep and anxiety, a logical assumption is that sleep disturbance and anxiety-related disorders can emanate from distinct innate or acquired neurobiological abnormalities which can then impact each other in mutually reinforcing ways [ 196 ]. Figure 5 presents a heuristic model for considering the neurobiological dynamics underlying the sleep–anxiety relationship modeled in Fig. 4 . For example, the emergence of anxiety-driven sleep disturbance (i.e., anxiety first) results from the input of anxiety circuitry on sleep–wake regulatory neurocircuitry. These anxiety-related arousal pathways are likely to emanate from brain regions involved in fear and/or threat responses. The end result is disruption of NREM-generating circuitry, especially nodes most important for the generation of deep, restorative (i.e., N3/slow-wave) sleep, or the over-riding of sleep-promoting signals [ 277 ]. The effects of anxiety circuitry inputs on REMS circuitry remain to be clarified, as research in humans and animals indicates disruptions as well as increases in REMS in response to stress or in PTSD subjects [ 120 , 125 , 127 , 128 , 278 , 279 ].
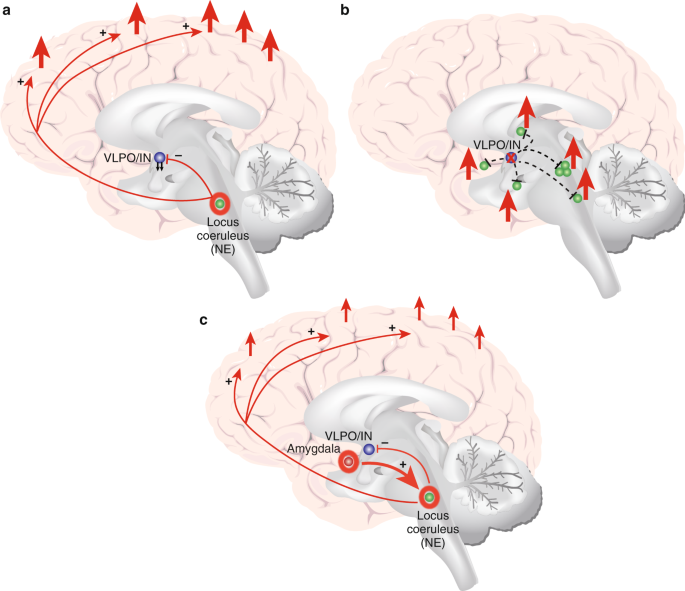
Heuristic neurobiological framework for the sleep disturbance and anxiety disorder relationship. Abnormal activation in wake, NREMS, and/or anxiety regions may generate sleep disturbance in anxiety-related disorders and promote a bidirectional sleep-disturbance-anxiety relationship. a Sleep disturbance emerges from hyperactivation in intrinsic wake circuitry. For example, here, excess arousal in NE-generating LC (green with orange halo) results in inhibition of VLPO/Intermediate Nucleus (IN) (blue) and hyperactivation of cerebral cortex. b Sleep disturbance emerges from dysfuncton in central (NREM) sleep-promoting region. For example, here failure of VLPO GABA signaling (blue, with orange “X”) disinhibits arousal centers (green). c Sleep disturbance emerges from hyperactivation of fear/threat/anxiety regions. For example, here amygdala hyperactivation (red, with orange halo) sends excitatory inputs to LC, which then inhibits VLPO-mediated sleep promotion and sends excitatory inputs to cerebral cortex, resulting in cortical hyperarousal, as in a . A common node in these examples is LC and NE signaling, but aberrant activity in other wake, NREMS, REMS, and anxiety regions may produce analogous effects
In the other direction, abnormalities in sleep–wake regulatory circuitry in the form of heightened arousal signals or deficits in NREM sleep circuitry may impact anxiety-related neurocircuitry directly. They will also act indirectly via the cerebral cortex to generate cortical hyperarousal during wake and sleep, while at the same time impeding sleep-recovery processes that reinforce frontal-lobe-mediated functions such as emotion regulation and adaptive emotional memory processes
Finally, disruptions in neurocircuitry and modulators with fundamental roles in both sleep regulation and anxiety-related circuitry, such as NE, CRF, and hypocretin, can affect both types of disturbance. Regardless of the source cause, pathological processes emanating from either source influence each other and can become mutually reinforcing.
Summary, important undiscussed topics, future directions
Summarizing the above findings, the clinical and epidemiological research indicates that anxiety disorders and subjective/clinical sleep disturbance are highly co-occurring. The objective physiological data provide a mixed picture, but overall suggest cortical hyperarousal during sleep in anxiety-related disorders, characterized by interrupted or shortened sleep, increased lighter stages of sleep (NREM N1 and N2), reductions in SWS (N3), and higher REM density (in PTSD). More-sophisticated analysis of existing data that move beyond simple sleep-stage analysis are likely necessary to better understand hyperarousal in anxiety-related sleep disturbance. The dominant psychological models of insomnia, which underscore the mutually reinforcing effects of fear and anxiety-based cognitive hyperarousal in the sleep environment and maladaptive behavioral coping, go a long way toward explaining bidirectional mechanisms driving the sleep-disturbance anxiety–disorder relationship. Insomnia models emphasizing multi-domain hyperarousal as a risk factor and endpoint in insomnia are consistent with various sleep neurobiological indicators of arousal in insomnia and are consistent with findings in anxiety-disordered sleep. Beyond insomnia models, abnormalities in cognitive inhibition, emotion regulation, and memory consolidation result from sleep disturbance and are features of anxiety disorders, and therefore have implications for bidirectional mechanisms in the anxiety–disorder–sleep–disturbance relationship. Finally, animal and human translational research as well as pharmacological treatment research provide clues to important neuromodulatory pathways that may be relevant to this relationship. A heuristic neurobiological framework indicates how sleep/wake regulatory centers may be affected during sleep in anxiety-related disorders, and suggests direct and indirect pathways through which sleep and anxiety neurobiology may affect each other.
In this review, we broadly defined anxiety-related disorders as DSM disorders in which fear and/or anxiety are considered to play a core and primary role. This reflects an attempt to maintain a faithful connection to the clinical phenomena and the human research, which has been conducted, until recently, using case/controls approaches, while also recognizing that an approach guided by current knowledge of neurocircuitry (i.e., mediating arousal, fear and anxiety processes) in psychopathology has many advantages. The Research Domain Criteria (RDoC) encourages researchers to focus on transdiagnostic mental processes that correspond better with known neurocircuitry than do DSM disorders because they are more likely to advance our understanding of psychopathology. Therefore, rather than recruiting uniquely for a specific anxiety-related disorder (e.g., PTSD) or sleep disorder (e.g., insomnia), investigators should consider recruiting for a specific domain or symptom presentation (e.g., individuals who report high-anxiety sensitivity, individuals with poor sleep continuity). The RDoC also emphasizes the importance of integrating many levels of information (e.g., genetics, neurobiological data from human and animal subjects, subjective-report data). In line with this model, future studies will benefit from multimodal assessments in order to better understand the intricacies of relevant mechanisms.
Although reviewing this vast topic, it is important to acknowledge items not covered in this review that are important when considering sleep and anxiety-related disorders. First, the effects of circadian rhythm and circadian disruption on sleep and anxiety-related disorders are outside of the scope of this review, but are fundamental contributors to the disturbances in question. For example, there is evidence that circadian factors impact anxiety symptom expression, extinction memory, and exposure therapy efficacy, although discrimination between circadian and homeostatic contributors is not clear in most studies [ 280 , 281 ]. We refer readers to existing studies which address this topic [ 83 , 152 , 282 , 283 , 284 , 285 , 286 , 287 ] and encourage the consideration of circadian factors in sleep research, and anxiety research, in general. In addition, we have focused on anxiety disorders, but the comorbidity and overlap with mood disorders is considerable [ 288 , 289 ]. The effects reviewed in this report often, but not always, accounted for the effects of mood disorders such as major depressive disorder. Finally, the genetics underlying the above-described relationships are outside of the scope of the current review but genetics research is essential for understanding shared and overlapping etiologies.
The future of therapeutic progress in improving outcomes for individuals with sleep disturbance in anxiety-related disorders will involve expanding upon existing excellent cognitive-behavioral treatments and integrating these with tools developed based on a deeper grasp of neurobiology. Dias and colleagues [ 290 ] predict that advanced technologies that target specific disturbed pathways in the human body, in the way that optogenetic manipulations already affect anxiety in animal models, are on the horizon. Combining the least invasive biological methods with the many advantages of psychological treatments should be the objective of medical science in sleep and anxiety-related disorders into the future.
Funding and Disclosure
Dr. Neylan is a consultant for Jazz Pharmaceuticals. Dr. Kanady and Dr. Richards declare nopotential conflicts of interest.
Change history
07 october 2019.
The HTML and PDF versions of this Article have been updated to replace Figure 3 and Figure 5 and amend the Figure 3 legend.
A Correction to this paper has been published: https://doi.org/10.1038/s41386-019-0529-y
Van Someren, EJW et al. Disrupted sleep: from molecules to cognition. J Neurosci. 2015;35:13889–95.
Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339.
Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-29.
Gruber R, Cassoff J. The interplay between sleep and emotion regulation: conceptual framework empirical evidence and future directions. Curr Psychiatry Rep. 2014;16:500.
Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66.
Ibarra-Coronado EG, et al. The Bidirectional Relationship between Sleep and Immunity against Infections. J Immunol Res. 2015;2015:678164.
Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–74
Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–90.
Jouvet M. The States of Sleep. Sci Am. 1967;216:62–8.
Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34.
Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62.
Richards A, et al. Sleep and cognitive performance from teens to old age: more is not better. Sleep. 2017;40: https://doi.org/10.1093/sleep/zsw029 .
Pace-Schott EF, Germain A, Milad MR. Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull. 2015; https://doi.org/10.1037/bul0000014 .
Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013; https://doi.org/10.1016/j.nlm.2012.10.006 .
Groch S, Zinke K, Wilhelm I, Born J. Dissociating the contributions of slow-wave sleep and rapid eye movement sleep to emotional item and source memory. Neurobiol Learn Mem. 2015; https://doi.org/10.1016/j.nlm.2014.08.013 .
Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81.
CAS PubMed Google Scholar
Borbely AA. A two process model of sleep regulation. Hum Neurobiol 1982;1:195–204.
Oh J. et al. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2018; https://doi.org/10.1038/s41380-018-0291-2 .
Schwartz MD, Kilduff TS. The neurobiology of sleep and wakefulness. Psychiatr Clin North Am. 2015; https://doi.org/10.1016/j.psc.2015.07.002 .
Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63.
Behn CGD, Brown EN, Scammell TE, Kopell NJ. Mathematical model of network dynamics governing mouse sleep–wake behavior. J Neurophysiol 2007;97:3828–40.
PubMed Google Scholar
Phillips AJK, Robinson PA. A quantitative model of sleep-wake dynamics based on the physiology of the brainstem ascending arousal system. J Biol Rhythms. 2007;22:167–79.
Rempe MJ, Best J, Terman D. A mathematical model of the sleep/wake cycle. J Math Biol 2010;60:615–44.
Saper CB, Fuller PM. wake-sleep circuitry: an overview. Curr Opin Neurobiol 2017;44:186–92.
CAS PubMed PubMed Central Google Scholar
Anaclet C, et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci 2012;32:17970–6.
Anaclet C, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci 2014;17:1217–24.
Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron. 2010; https://doi.org/10.1016/j.neuron.2010.11.032 .
Nofzinger EA, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry. 2004; https://doi.org/10.1176/appi.ajp.161.11.2126 .
Germain A, Stocker R, Ebdlahad S, Mammen O. Functional neuroimaging of NREM sleep in combat veterans with and without posttraumatic stress disorder (PTSD). Sleep. 2013;211:176–79.
Ebdlahad S, Stocker R, Mammen O, Nofzinger E, Germain A. Neural correlates of insomnia comorbid with PTSD during wakefulness and nrem sleep. Sleep. 2013;36:A303.
Etkin A, Gyurak A, O’Hara, R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin. Neurosci. 2013;15:419–429.
Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007; https://doi.org/10.1176/appi.ajp.2007.07030504 .
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010; https://doi.org/10.1038/npp.2009.83 .
Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 2015; https://doi.org/10.2147/TCRM.S48528 .
Norton PJ, Paulus DJ. Transdiagnostic models of anxiety disorder: theoretical and empirical underpinnings. Clin Psychol Rev 2017;56:122–37.
Neylan TC, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam Veterans. Am J Psychiatry. 1998;155:929–33.
Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med Rev 2000;4:263–76.
Stewart R, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7.
Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res 2002;36:449–52.
Ramsawh HJ, Stein MB, Belik SL, Jacobi F, Sareen J. Relationship of anxiety disorders, sleep quality, and functional impairment in a community sample. J Psychiatr Res 2009;43:926–33.
Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord 2016;37:104–29.
Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–82.
PubMed PubMed Central Google Scholar
Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the Hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707.
Roszell DK, McFall ME, Malas KL. Frequency of symptoms and concurrent psychiatric disorder in Vietnam veterans with chronic PTSD. Hosp Community Psychiatry. 1991;42:293–6.
Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41:469–78.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc. 2013; https://doi.org/10.1176/appi.books.9780890425596.744053
Creamer JL, Brock MS, Mysliwiec V. Nightmares in United States military personnel are multifactorial and require further astudy. J Clin Sleep Med 2018;14:1275–6.
Sandman N, et al. Nightmares: prevalence among the Finnish general adult population and war veterans during 1972-2007. Sleep. 2013;36:1041–50.
Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–62.
Bjorvatn B, Grønli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med 2010;11:1031–4.
Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. 2010;33:774–80.
Robert G, Zadra A. Measuring nightmare and bad dream frequency: Impact of retrospective and prospective instruments. J Sleep Res 2008;17:132–9.
Kobayashi I, Delahanty DL. Gender differences in subjective sleep after trauma and the development of posttraumatic stress disorder symptoms: sa pilot study. J Trauma Stress. 2013;26:467–74.
Milanak ME, et al. Traumatic event exposure, posttraumatic stress disorder, and sleep disturbances in a national sample of U.S. adults. J Trauma Stress. 2019;32:14–22.
Woodward SH, Arsenault NJ, Murray C, Bliwise DL. Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol Psychiatry. 2000;48:1081–7.
Insana SP, Hall M, Buysse DJ, Germain A. Validation of the pittsburgh sleep quality index addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J Trauma Stress. 2013;26:192–200.
Mysliwiec V, et al. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev 2018;37:94–104.
Brown TM, Boudewyns PA. Periodic limb movements of sleep in combat veterans with posttraumatic stress disorder. J Trauma Stress. 1996;9:129–36.
Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:110–5.
Ross RJ, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17:723–32.
Woodward SH, Michell G, Santerre C. The psychophysiology of PTSD nightmares. in Sleep and Combat-Related Post Traumatic Stress Disorder (2017). https://doi.org/10.1007/978-1-4939-7148-0_20 .
Mysliwiec V, et al. Trauma associated sleep disorder: sa proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med 2014;10:1143–8.
Bélanger L, Morin CM, Langlois F, Ladouceur R. Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for gad on insomnia symptoms. J Anxiety Disord 2004;18:561–71.
Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–36.
Brenes GA, et al. Insomnia in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2009;17:465–72.
Tempesta D, et al. Neuropsychological functioning in young subjects with generalized anxiety disorder with and without pharmacotherapy. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;45:236–41.
CAS Google Scholar
Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18:622–7.
Berger A, et al. Patterns of healthcare utilization in patients with generalized anxiety disorder in general practice in Germany. Eur J Psychiat. 2009; 23:90–100.
Mellman TA, Uhde TW. Patients with frequent sleep panic: clinical findings and response to medication treatment. J Clin Psychiatry. 1990;51:513–26.
Park HJ, et al. Association between sleep disorder and panic disorder in South Korea: nationwide nested case-control study of data from 2004 to 2013. Psychiatry Res 2018;260:286–91.
Sheehan DV, Ballenger J, Jacobsen G. Treatment of endogenous anxiety with phobic, hysterical, and hypochondriacal symptoms. Arch Gen Psychiatry. 1980;37:51–59.
Overbeek T, Van Diest R, Schruers K, Kruizinga F, Griez E. Sleep complaints in panic disorder patients. J Nerv Ment Dis 2005;193:488–93.
Todder D, Baune BT. Quality of sleep in escitalopram-treated female patients with panic disorder. Hum Psychopharmacol. 2010;25:167–73.
Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Med Rev 2005;9:173–84.
Castelnovo A, Lopez R, Proserpio P, Nobili L, Dauvilliers Y. NREM sleep parasomnias as disorders of sleep-state dissociation. Nat Rev Neurol. 2018; https://doi.org/10.1038/s41582-018-0030-y .
Craske MG, Barlow DH. Nocturnal panic. J Nerv Ment Dis 1989;173:160–7.
Google Scholar
Krystal JH, Woods SW, Hill CL, Charney DS. Characteristics of panic attack subtypes: sassessment of spontaneous panic, situational panic, sleep panic, and limited symptom attacks. Compr Psychiatry. 1991;32:474–80.
Stein MB, Enns MW, Kryger MH. Sleep in nondepressed patients with panic disorder: II. Polysomnographic assessment of sleep architecture and sleep continuity. J Affect Disord 1993;28:1–6.
Uhde TW, Anxiety Disorders. in Principles and Practice of Sleep Medicine,. Kryger Meir H; Roth, TDWC (ed.) 871–98 (Saunders Co., 1994).
Mellman TA, Uhde TW. Electroencephalographic sleep in panic disorder: a focus on sleep-related panic attacks. Arch Gen Psychiatry. 1989;46:178–84.
Aǧargün MY, Kara H. Recurrent sleep panic, insomnia, and suicidal behavior in patients with panic disorder. Compr Psychiatry. 1998;39:149–51.
Roth T, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60:1364–71.
Bobdey M, Fineberg N, Gale TM, Patel A, Davies HA. Reported sleep patterns in obsessive compulsive disorder (OCD). Int J Psychiatry Clin Pract 2002;6:15–21.
Donse L, Sack AT, Fitzgerald PB, Arns M. Sleep disturbances in obsessive-compulsive disorder: association with non-response to repetitive transcranial magnetic stimulation (rTMS). J Anxiety Disord. 2017; https://doi.org/10.1016/j.janxdis.2017.03.006 .
Park S, et al. Relationships of sleep duration with sociodemographic and health-related factors, psychiatric disorders and sleep disturbances in a community sample of Korean adults. J Sleep Res 2010;19:567–77.
Robinson D, Walsleben J, Pollack S, Lerner G. Nocturnal polysomnography in obsessive-compulsive disorder. Psychiatry Res 1998;80:257–63.
Marcks BA, Weisberg RB, Edelen MO, Keller MB. The relationship between sleep disturbance and the course of anxiety disorders in primary care patients. Psychiatry Res 2010;178:487–92.
Daroff RB. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. Neurology. 2012; https://doi.org/10.1212/wnl.41.1.160 .
Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; https://doi.org/10.1513/pats.200707-114mg .
Younes M. Pathogenesis of obstructive sleep Apnea. Clin Chest Med. 2019; https://doi.org/10.1016/j.ccm.2019.02.008 .
Colvonen PJ, et al. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med 2015;11:513–8.
Yesavage JA, et al. Sleep-disordered breathing in vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry. 2012;20:199–204.
Krakow B, et al. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis 2002;190:442–52.
Krakow B, et al. Complex insomnia: Insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49:948–53.
Ocasio-Tascón ME, Alicea-Colón E, Torres-Palacios A, Rodríguez-Cintrón W. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 2006;10:70–75.
Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: saecondary symptom or core feature? Sleep Med Rev 2008;12:169–84.
Youakim JM, Doghramji K, Schutte SL. Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics. 1998;39:168–71.
Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. 2015; https://doi.org/10.1016/j.smrv.2014.11.001 .
Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biol Psychiatry. 2000;47:520–5.
Kinoshita LM, et al. Modeling the effects of obstructive sleep apnea and hypertension in Vietnam veterans with PTSD. Sleep Breath 2012;16:1201–9.
Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med 2015;11:165–75.
Mysliwiec V, et al. Sleep disorders and sassociated medical comorbidities in sactive duty military personnel. Sleep. 2013;36:167–74.
Mysliwiec V, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144:549–57.
van Liempt, S, Westenberg, HG, Arends, J, Vermetten, E. Obstructive sleep apnea in combat-related posttraumatic stress disorder: a controlled polysomnography study. Eur J Psychotraumatol 2011;2:10.3402/ejpt.v2i0.8451.
Zhang Y, Weed JG, Ren R, Tang X, Zhang W. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: a meta-analysis. Sleep Med 2017;36:125–32.
Babson KA, Del Re AC, Bonn-Miller MO, Woodward SH. The comorbidity of sleep apnea and mood, anxiety, and substance use disorders among obese military veterans within the veterans health administration. J Clin Sleep Med 2013;9:1253–8.
Pevernagie D, et al. Behavioural hyperventilation as a novel clinical condition associated with central sleep apnoea: a report of three cases. Sleep Med 2012;13:1317–20.
Trajanovic NN, Rasool MS, Voloh I, Shapiro CM. Sleep-disordered breathing, cardiac arrhythmia, and panic disorder. J Clin Psychol Med Settings. 2005;1:288–9.
Enns MW, Stein M, Kryger M. Successful treatment of comorbid panic disorder and sleep apnea with continuous positive airway pressure. Psychosomatics. 1995;36:585–6.
Stein MB, Millar TW, Larsen DK, Kryger MH. Irregular breathing during sleep in patients with panic disorder. Am J Psychiatry. 1995;152:1168–73.
Edlund MJ, McNamara ME, Millman RP. Sleep apnea and panic attacks. Compr Psychiatry. 1991;32:130–2.
Hrubos-Strøm H, et al. Sleep apnoea, anxiety, depression and somatoform pain: sa community-based high-risk sample. Eur Respir J 2012;40:400–7.
Breland JY, et al. The obesity epidemic in the veterans health administration: prevalence samong key populations of women and men veterans. J Gen Intern Med. 2017; https://doi.org/10.1007/s11606-016-3962-1 .
Rosenbaum S, et al. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism 2015;64:926–33.
Baglioni C, et al. Sleep and mental disorders: sa meta-analysis of polysomnographic research. Psychol Bull 2016;142:969–90.
Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9.
Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7.
Fuller KH, Waters WF, Scott O. An investigation of slow-wave sleep processes in chronic PTSD patients. J Anxiety Disord 1994;8:227–36.
Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20:46–51.
Richards A, et al. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J Sleep Res 2013;22:679–87.
Habukawa M, Uchimura N, Maeda M, Kotorii N, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol Psychiatry. 2007;62:1179–82.
Breslau N, et al. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61:508–16.
Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002; https://doi.org/10.1176/appi.ajp.159.10.1696 .
Mellman TA, Kobayashi I, Lavela J, Wilson B, Hall Brown TS. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. 2014;1:1321–6.
Ross RJ. The changing REM sleep signature of posttraumatic satress disorder. Sleep. 2014;37:1281–2.
Sanford LD, Suchecki D, Meerlo P. Stress, arousal, and sleep. Curr Top Behav Neurosci. 2015; https://doi.org/10.1007/7854_2014_314 .
Wellman LL, Yang L, Ambrozewicz MA, Machida M, Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. Sleep. 2013; https://doi.org/10.5665/sleep.2526 .
Vanderheyden WM, et al. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp Brain Res 2015;233:2335–46.
Vanderheyden W, Urpa L, Poe G. Increase in rem sleep following trauma exposure. Sleep Med. 2014; https://doi.org/10.1016/j.sleep.2013.11.718 .
Suchecki D, Tiba PA, Machado RB. REM sleep rebound as an adaptive response to stressful situations. Front Neurol. 2012; https://doi.org/10.3389/fneur.2012.00041 .
Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008; https://doi.org/10.1016/j.neubiorev.2007.06.001 .
Germain A, James JA, Mammen O, Price J, Nofzinger E. Functional neuroimaging of rem sleep in returning veterans with ptsd: san [18F]-FDG PET study. Sleep. 2011;A242:34.
Suter D, Mammen O, Insana S, Nofzinger E, Germain A. Neurobiological effects of prazosin on NREM sleep in Veterans with PTSD. in SLEEP - Abstract Supplement, Volume 37 (2014).
Germain A, et al. Prazosin increases brain glucose metabolism in regions involved in fear extinction learning and memory during REM sleep in combat exposed Veterans with PTSD. in SLEEP - Abstract Supplement, Volume 37 (2014).
Van Wyk M, Thomas KGF, Solms M, Lipinska G. Prominence of hyperarousal symptoms explains variability of sleep disruption in posttraumatic stress disorder. Psychol Trauma. 2016;8:688–96.
Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–9.
Inslicht SS, et al. Sleep and hypothalamic pituitary adrenal axis responses to metyrapone in posttraumatic stress disorder. Psychoneuroendocrinology. 2018;88:136–43.
Van Liempt S, et al. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38:155–65.
Papadimitriou GN, Kerkhofs M, Kempenaers C, Mendlewicz J. EEG sleep studies in patients with generalized anxiety disorder. Psychiatry Res 1988;26:183–90.
Arriaga F, Paiva T. Clinical and EEG sleep changes in primary dysthymia and generalized anxiety: a comparison with normal controls. Neuropsychobiology. 1990;24:109–14.
Lund HG, Bech P, Eplov L, Jennum P, Wildschiødtz G. An epidemiological study of REM latency and psychiatric disorders. J Affect Disord 1991;23:107–12.
Lauer CJ, Krieg JC, Garcia-Borreguero D, Özdaglar A, Holsboer F. Panic disorder and major depression: a comparative electroencephalographic sleep study. Psychiatry Res 1992;44:41–54.
Lydiard RB, et al. Electroencephalography during sleep of patients with panic disorder. J Neuropsychiatry Clin Neurosci 1989;1:372–6.
Sloan EP, et al. Nocturnal and daytime panic attacks comparison of sleep architecture, heart rate variability, and response to sodium lactate challenge. Biol Psychiatry. 1999;45:1313–20.
Uhde TW, et al. The sleep of patients with panic disorder: sa preliminary report. Psychiatry Res 1984;12:251–9.
Ferini-Strambi L, et al. Cyclic alternating pattern of sleep electroencephalogram in patients with panic disorder. Biol Psychiatry. 1996;40:225–6.
Dubé S, et al. Interface of panic and depression: clinical and sleep EEG correlates. Psychiatry Res 1986;19:119–33.
Insel TR, et al. The sleep of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1982;39:1372–7.
Voderholzer U, et al. Sleep in obsessive compulsive disorder: Polysomnographic studies under baseline conditions and after experimentally induced serotonin deficiency. Eur Arch Psychiatry Clin Neurosci 2007;257:173–82.
Hohagen F, et al. Sleep EEG of patients with obsessive-compulsive disorder. Eur Arch Psychiatry Clin Neurosci 1994;243:273–8.
Kluge M, et al. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J Psychiatr Res 2007;41:928–33.
Nota JA, Sharkey KM, Coles ME. Sleep, arousal, and circadian rhythms in adults with obsessive-compulsive disorder: sa meta-analysis. Neurosci Biobehav Rev.2015; https://doi.org/10.1016/j.neubiorev.2015.01.002 .
Otte C, et al. Effects of Metyrapone on hypothalamic-pituitary-adrenal axis and sleep in women with post-traumatic stress disorder. Biol Psychiatry. 2007, https://doi.org/10.1016/j.biopsych.2006.08.018 .
Woodward SH, Murburg MM, Bliwise DL. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000, https://doi.org/10.1016/S0031-9384(00)00271-7 .
Wei Y, et al. Sleep stage transition dynamics reveal specific stage 2 vulnerability in insomnia. Sleep 2017; https://doi.org/10.1093/sleep/zsx117 .
Nofzinger EA, et al. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Res Protoc. 1998; https://doi.org/10.1016/S1385-299X(97)00042-1 .
Gehrman P, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36:1009–18.
Wright KM, et al. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol 2011;67:1240–58.
Van Liempt S, Van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: sa prospective longitudinal cohort study. Depress Anxiety. 2013; https://doi.org/10.1002/da.22054 .
Talbot LS, et al. Cognitive behavioral therapy for insomnia in posttraumatic satress disorder: a randomized controlled trial. Sleep. 2014;37:327–41.
Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016; https://doi.org/10.1016/j.cpr.2015.09.005 .
Hertenstein E, et al. Insomnia as a predictor of mental disorders: sa systematic review and meta-analysis. Sleep Med Rev. 2019;. https://doi.org/10.1016/j.smrv.2018.10.006 .
Short NA, Allan NP, Stentz L, Portero AK, Schmidt NB. Predictors of insomnia symptoms and nightmares among individuals with post-traumatic stress disorder: san ecological momentary assessment study. J Sleep Res 2017;27:64–72.
Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006; https://doi.org/10.1016/j.jpsychires.2006.07.008 .
Batterham PJ, Glozier N, Christensen H. Sleep disturbance, personality and the onset of depression and anxiety: prospective cohort study. Aust N Z J Psychiatry. 2012;46:1089–98.
Roy Byrne PP, Uhde TW, Post RM. Effects of one night’s sleep deprivation on mood and behavior in panic disorder: patients with panic disorder compared with depressed patients and normal controls. Arch Gen Psychiatry. 1986;43:895–9.
Babson KA, Feldner MT, Trainor CD, Smith RC. An experimental investigation of the effects of acute sleep deprivation on panic-relevant biological challenge responding. Behav Ther 2009;40:239–50.
Cousineau H, et al. Insomnia symptoms following treatment for comorbid panic disorder with agoraphobia and generalized anxiety disorder. J Nerv Ment Dis 2016;204:267–73.
Cervena K, Matousek M, Prasko J, Brunovsky M, Paskova B. Sleep disturbances in patients treated for panic disorder. Sleep Med 2005;6:149–53.
Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R. National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans. Int J Geriatr Psychiatry. 2015; https://doi.org/10.1002/gps.4143 .
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med 2016;165:125–33.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme M-E. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev 2011; https://doi.org/10.1016/j.cpr.2011.02.004 .
Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541–53.
Bootzin R. Stimulus control treatment for insomnia. Proc Am Psychol Assoc 1972;7:395–6.
Harvey AG. A cognitive model of insomnia. Behav Res Ther 2002;40:869–93.
Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol 2002;53:215–43.
Riemann D et al. The hyperarousal model of insomnia: sa review of the concept and its evidence. Sleep Med Rev. 2010; https://doi.org/10.1016/j.smrv.2009.04.002 .
Perlis ML, Smith MT, Pigeon WR. Etiology and Pathophysiology of Insomnia. in Principles and Practice of Sleep Medicine, 714–25 (Elsevier Inc., 2005). https://doi.org/10.1016/B0-72-160797-7/50067-7 .
Pigeon WR. Diagnosis, prevalence, pathways, consequences, treatment of insomnia. Indian J Med Res 2010;131:321–32.
Sinha SS. Trauma-induced insomnia: sa novel model for trauma and sleep research. Sleep Med Rev 2016;25:74–83.
Lima SL, Rattenborg NC, Lesku JA, Amlaner CJ. Sleeping under the risk of predation. Anim Behav 2005;70:723–36.
Eban-Rothschild A, Giardino WJ, de Lecea L. To sleep or not to sleep: neuronal and ecological insights. Curr Opin Neurobiol 2017;44:132–8.
Kanady JC, et al. Cognitive behavioral therapy for insomnia reduces fear of sleep in individuals with posttraumatic stress disorder. J Clin Sleep Med 2018;14:1193–203.
Krakow B, et al. An open-label trial of evidence-based cognitive behavior therapy for nightmares and insomnia in crime victims with PTSD. Am J Psychiatry. 2001;158:2043–7.
Pruiksma KE, et al. A psychometric study of the Fear of Sleep Inventory-Short Form (FoSI-SF). J Clin Sleep Med 2014;10:551–8.
Hall Brown T, Mellman TA. The influence of PTSD, sleep fears, and neighborhood stress on insomnia and short sleep duration in urban, young adult, african americans. Behav Sleep Med 2014;12:198–206.
Palagini L, Moretto U, Dell’Osso L, Carney C. Sleep-related cognitive processes, arousal, and emotion dysregulation in insomnia disorder: the role of insomnia-specific rumination. Sleep Med 2017;30:97–104.
Palagini L, et al. Daytime rumination as a feature of insomnia disorder: sleep related cognition is not merely a problem of the night. Arch Ital Biol 2015;153:239–47.
Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Heal. 2010;46:124–32.
Takano K, Iijima Y, Tanno Y. Repetitive thought and self-reported sleep disturbance. Behav Ther 2012;43:779–89.
Thomsen DK, Mehlsen MY, Christensen S, Zachariae R. Rumination - relationship with negative mood and sleep quality. Pers Individ Dif. 2003;34:1293–301.
Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med 2009;71:771–5.
Lancee J, Eisma MC, van Zanten KB, Topper M. When thinking impairs sleep: trait, daytime and nighttime repetitive thinking in insomnia. Behav Sleep Med 2017;15:53–69.
O’Kearney R, Pech M. General and sleep-specific worry in insomnia. Sleep Biol Rhythms. 2014;12:212–5.
Singareddy R, Uhde TW. Nocturnal sleep panic and depression: relationship to subjective sleep in panic disorder. J Affect Disord 2009;112:262–6.
Uhde TW, Cortese BM, Vedeniapin A. Anxiety and sleep problems: emerging concepts and theoretical treatment implications. Curr Psychiatry Rep 2009;11:269–76.
Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther 1986;24:1–8.
Hoge EA, et al. The role of anxiety sensitivity in sleep disturbance in panic disorder. J Anxiety Disord 2011;25:536–8.
Craske MG, et al. Presleep attributions about arousal during sleep: nocturnal panic. J Abnorm Psychol 2002;111:53–62.
Craske MG, Freed S. Expectations about sarousal and nocturnal panic. J Abnorm Psychol 1995;104:567–75.
Craske MG, Lang AJ, Tsao JC, Mystkowski JL, Rowe MK. Reactivity to interoceptive cues in nocturnal panic. J Behav Ther Exp Psychiatry. 2001;32:173–90.
Tsao JCI, Craske MG. Reactivity to imagery and nocturnal panic attacks. Depress Anxiety. 2003;18:205–13.
Timpano KR, Carbonella JY, Bernert RA, Schmidt NB. Obsessive compulsive symptoms and sleep difficulties: exploring the unique relationship between insomnia and obsessions. J Psychiatr Res 2014;57:101–7.
Del Río-Casanova L, González A, Páramo M, Van Dijke A, Brenlla J. Emotion regulation strategies in trauma-related disorders: Pathways linking neurobiology and clinical manifestations. Rev Neurosci. 2016; https://doi.org/10.1515/revneuro-2015-0045 .
Cohen Kadosh K, et al. Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. Neuroimage. 2016; https://doi.org/10.1016/j.neuroimage.2015.09.070 .
Ballesio A, Ottaviani C, Lombardo C. Poor cognitive inhibition predicts rumination about insomnia in a clinical sample. Behav Sleep Med 2018;20:1–10.
Tsypes A, Aldao A, Mennin DS. Emotion dysregulation and sleep difficulties in generalized anxiety disorder. J Anxiety Disord 2013;27:197–203.
Hovland A, et al. Subjective sleep quality in relation to inhibition and heart rate variability in patients with panic disorder. J Affect Disord 2013;150:152–5.
Pace-Schott EF, et al. Resting state functional connectivity in primary insomnia, generalized anxiety disorder and controls. Psychiatry Res - Neuroimaging. 2017;265:26–34.
Diekelmann S, Born J. Slow-wave sleep takes the leading role in memory reorganization. Nat. Rev. Neurosci. 2010; https://doi.org/10.1038/nrn2762-c2 .
McDevitt EA, Krishnan GP, Bazhenov M, Mednick SC. Cognitive Neuroscience of Memory Consolidation, Studies in Neuroscience, Psychology and Behavioral Economics. 2017; https://doi.org/10.1007/978-3-319-45066-7_13 .
Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci 2009;1156:168–97.
Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; https://doi.org/10.1152/physrev.00032.2012 .
Peever J, Fuller PM. The biology of REM sleep. Curr Biol. 2017; https://doi.org/10.1016/j.cub.2017.10.026 .
Colvonen PJ, Straus LD, Acheson D, Gehrman P. A Review of the relationship between emotional learning and memory, sleep, and PTSD. Curr Psychiatry Rep. 2019; https://doi.org/10.1007/s11920-019-0987-2 .
Walker MP, van der Helm E. Overnight Therapy? The Role of Sleep in Emotional Brain Processing. Psychol Bull 2009;135:731–48.
Pace-Schott EF, Germain A, Milad MR. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord. 2015; https://doi.org/10.1186/s13587-015-0018-9 .
Straus LD, Norman SB, Risbrough VB, Acheson DT, Drummond SPA. REM sleep and safety signal learning in posttraumatic stress disorder: a preliminary study in military veterans. Neurobiol Stres. 2018; https://doi.org/10.1016/j.ynstr.2018.07.001 .
Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci. 2011; https://doi.org/10.3389/fnbeh.2011.00044 .
Marshall AJ, Acheson DT, Risbrough VB, Straus LD, Drummond, SPA. Fear conditioning, safety learning, and sleep in humans. J Neurosci. 2014; https://doi.org/10.1523/jneurosci.0478-14.2014 .
Pace-Schott EF et al. Effects of post-exposure naps on exposure therapy for social anxiety. Psychiatry Res. 2018; https://doi.org/10.1016/j.psychres.2018.10.015 .
Pace-Schott EF, Verga PW, Bennett TS, Spencer RMC. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res 2012;46:1036–44.
Kleim B, et al. Sleep enhances exposure therapy. Psychol Med 2014;44:1511–9.
Zalta AK, et al. Sleep quality predicts treatment outcome in CBT for social anxiety disorder. Depress Anxiety. 2013;30:1114–20.
Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009; https://doi.org/10.1093/cercor/bhn155 .
Wiesner CD, et al. The effect of selective REM-sleep deprivation on the consolidation and affective evaluation of emotional memories. Neurobiol Learn Mem. 2015; https://doi.org/10.1016/j.nlm.2015.02.008 .
van Marle H. PTSD as a memory disorder. Eur J Psychotraumatol. 2015; https://doi.org/10.3402/ejpt.v6.27633 .
Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002; https://doi.org/10.1097/01.PSY.0000021940.35402.51 .
Baran B, Pace-Schott EF, Ericson C, Spencer RMC, Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012; https://doi.org/10.1523/JNEUROSCI.2532-11.2012 .
Morgenthaler J, et al. Selective REM-sleep deprivation does not diminish emotional memory consolidation in young healthy subjects. PLoS ONE. 2014;9:e89849.
Latchoumane CFV, Ngo HVV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95:424–35.e6.
Kleim, B, Wysokowsky, J, Schmid, N, Seifritz, E, Rasch, B. Effects of sleep after experimental trauma on intrusive emotional memories. Sleep. 2016;39:2125–32.
Fisher C, Byrne J, Edwards A, Kahn E. A psychophysiological study of nightmares. J Am Psychoanal Assoc 1970;18:747–82.
Ross RJ, et al. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202.
Kobayashi I, Mellman TA, Altaee D, Howell MK, Lavela J. Sleep and processing of trauma memories. J Trauma Stress. 2016;29:568–71.
Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull 2007;133:482–528.
Rothbaum BO, Mellman TA. Dreams and exposure therapy in PTSD. J Trauma Stress. 2001;14:481–90.
Phelps AJ, et al. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep. 2018;41: https://doi.org/10.1093/sleep/zsx188 .
Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology, psychophysiology and treatment. Psychother Psychosom 2007;76:25–39.
Spoormaker VI. Sleep and combat-related post traumatic stress disorder (Springer, 2018).
Miller KE, Jamison AL, Gala S, Woodward SH. Two independent predictors of nightmares in posttraumatic stress disorder. J Clin Sleep Med 2018;14:1921–7.
Husain AM, Miller PP, Carwile ST. REM sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol 2001;18:148–57.
Naegeli C, et al. Locus coeruleus activity mediates hyperresponsiveness in posttraumatic satress disorder. Biol Psychiatry 2018;83:254–62.
Nir Y, Tononi G. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn Sci. 2010;14:88–100.
Germain A, et al. A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Res. 2013;211:176–9.
Germain A, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res 2012;72:89–96.
Raskind MA, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–3.
Taylor FB, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–32.
Raskind MA, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med 2018;378:507–17.
Morgenthaler T, et al. Position paper for the treatment of nightmare disorder in adults: an american academy of sleep medicine position paper. J Clin Sleep Med 2018;14:1041–55.
Raskind MA, et al. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016;80:736–42.
Richards A, et al. No access an open-label study of doxazosin extended-release for PTSD: findings and recommendations for future research on doxazosin. J lifelong Learn psychiatry. 2018;16:67–73.
Aurora RN, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6:389–401.
Krystal JH, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82:e51–e59.
Hertzberg MA, Feldman ME, Beckham JC, Davidson JRT. Trial of trazodone for posttraumatic stress disorder using a multiple baseline group design. J Clin Psychopharmacol. 1996;16:294–8.
Villarreal G, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173:1205–12.
Kozaric-Kovacic D, Pivac N. Quetiapine treatment in an open trial in combat-related post-traumatic stress disorder with psychotic features. Int J Neuropsychopharmacol. 2007;10:253–61.
De Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res. 2012;198:15–24.
Tao R, et al. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101-5.
Bonnavion P, De Lecea L. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 2010;10:174–9.
Grafe LA, Bhatnagar S. Orexins and stress. Front Neuroendocrinol. 2018;51:132–45.
Carter M, De Lecea L. Hyperarousal and post-traumatic stress disorder: a role for the hypocretin system. In: LeDoux J., Keane T., Shiromani P. (eds) Post-Traumatic Stress Disorder (Humana Press, 2009). https://doi.org/10.1007/978-1-60327-329-9_9 .
Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekkar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–61.
Bonaventure P, et al. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther 2015;352:590–601.
Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther 2002;300:2–8.
Quadrelli S, Mountford C, Ramadan S. Systematic review of in-vivo neuro magnetic resonance spectroscopy for the assessment of posttraumatic stress disorder. Psychiatry Res. 2018;282:110–25.
Starcevic V. The reappraisal of benzodiazepines in the treatment of anxiety and related disorders. Expert Rev Neurother. 2014;14:1275–86.
Guina J, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract 2015;21:281–303.
Pollack MH, et al. Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72:892–7.
Dunlop BW, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82:866–74.
Davis CJ, Krueger JM. Sleep and cytokines. Sleep Medicine Clinics. 2012;7:517–527.
Küffer A, et al. Altered overnight levels of pro-inflammatory cytokines in men and women with posttraumatic stress disorder. Psychoneuroendocrinology. 2019;102:114–20.
Babson KA, Sottile J, Morabito D. Cannabis, Cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:23.
Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn-Miller MO. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict. 2013;22:277–84.
Pietrzak RH, et al. Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology. 2014;39:2519–28.
Ney LJ, Matthews A, Bruno R, Felmingham KL. Cannabinoid interventions for PTSD: where to next? Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;93:124–40.
Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J. Neurosci. 2008;28:10167–84.
Mellman TA, David D, Kulick-Bell R, Hebding J, Nolan B. Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. Am J Psychiatry. 1995;152:1659–63.
Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901.
Zuj DV, et al. Impaired fear extinction associated with ptsd increases with hours-since-waking. Depress Anxiety (2016). https://doi.org/10.1002/da.22463 .
Pace-Schott EF, et al. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J Psychiatr Res. 2013;47:1776–84.
Schuch JB, Genro JP, Bastos CR, Ghisleni G, Tovo-Rodrigues L. The role of CLOCK gene in psychiatric disorders: evidence from human and animal research. Am J Med Genet Part B Neuropsychiatr Genet. 2018;177:181–98.
Liu C, Chung M. Genetics and epigenetics of circadian rhythms and their potential roles in neuropsychiatric disorders. Neurosci Bull. 2015;31:141–59.
Mukhopadhyay S, et al. Delayed sleep phase in severe obsessive-compulsive disorder: a systematic case-report survey. CNS Spectr 2008;13:406–13.
Turner J, et al. A prospective study of delayed sleep phase syndrome in patients with severe resistant obsessive-compulsive disorder. World Psychiatry. 2007;6:108–11.
Coles ME, Schubert JR, Sharkey KM. Delayed bedtimes and obsessive-compulsive symptoms. Behav Sleep Med 2012;10:258–65.
Cox RC, Tuck B, Olatunji BO. The role of eveningness in obsessive-compulsive symptoms: cross-sectional and prospective approaches. J Affect Disord. 2018;235:448–55.
Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000. https://doi.org/10.1002/1520-6394 (2000)12:1+<69::AID-DA9>3.0.CO;2-K.
Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61:22–32.
Dias BG, Banerjee SB, Goodman JV, Ressler KJ. Towards new approaches to disorders of fear and anxiety. Curr Opin Neurobiol. 2013;23:346-52.
Download references
Author information
These authors contributed equally: Dr. Richards, Dr. Kanady
Authors and Affiliations
The San Francisco VA Health Care System, San Francisco, CA, USA
Anne Richards, Jennifer C. Kanady & Thomas C. Neylan
The University of California, San Francisco, San Francisco, CA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anne Richards .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Richards, A., Kanady, J.C. & Neylan, T.C. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacol. 45 , 55–73 (2020). https://doi.org/10.1038/s41386-019-0486-5
Download citation
Received : 17 March 2019
Revised : 09 August 2019
Accepted : 12 August 2019
Published : 23 August 2019
Issue Date : 01 January 2020
DOI : https://doi.org/10.1038/s41386-019-0486-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Associations between 24-h movement behaviors and indicators of mental health and well-being across the lifespan: a systematic review.
- Claire I. Groves
- Christopher Huong
- Denver M. Y. Brown
Journal of Activity, Sedentary and Sleep Behaviors (2024)
Investigating the Leisure Patterns and the Well-being of Healthcare Workers in High-Risk Environments: Insights from a Pandemic Landscape
- Hsiao-Hsien Lin
- Yi-Han Tseng
- Chih-Hsiang Hung
Journal of the Knowledge Economy (2024)
The relationship between living alone or not and depressive symptoms in older adults: a parallel mediation effect of sleep quality and anxiety
- Zhanpeng Guo
BMC Geriatrics (2023)
From childhood adversity to latent stress vulnerability in adulthood: the mediating roles of sleep disturbances and HPA axis dysfunction
Neuropsychopharmacology (2023)
Rate and correlates of post-traumatic stress disorder (PTSD) following the Beirut blast and the economic crisis among Lebanese University students: a cross-sectional study
- Christian-Joseph El Zouki
- Abdallah Chahine
- Souheil Hallit
BMC Psychiatry (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders
Affiliations.
- 1 Sleep Disorders and Research Center, Henry Ford Hospital, Detroit, Michigan.
- 2 Department of Psychological Sciences, Kent State University, Kent, Ohio.
- PMID: 29797753
- PMCID: PMC7045300
- DOI: 10.1111/jsr.12710
Sleep reactivity is the trait-like degree to which stress exposure disrupts sleep, resulting in difficulty falling and staying asleep. Individuals with highly reactive sleep systems experience drastic deterioration of sleep when stressed, whereas those with low sleep reactivity proceed largely unperturbed during stress. Research shows that genetics, familial history of insomnia, female gender and environmental stress influence how the sleep system responds to stress. Further work has identified neurobiological underpinnings for sleep reactivity involving disrupted cortical networks and dysregulation in the autonomic nervous system and hypothalamic-pituitary-adrenal axis. Sleep reactivity is most pathologically and clinically pertinent when in excess, such that high sleep reactivity predicts risk for future insomnia disorder, with early evidence suggesting high sleep reactivity corresponds to severe insomnia phenotypes (sleep onset insomnia and short sleep insomnia). High sleep reactivity is also linked to risk of shift-work disorder, depression and anxiety. Importantly, stress-related worry and rumination may exploit sensitive sleep systems, thereby augmenting the pathogenicity of sleep reactivity. With the development of cost-effective assessment of sleep reactivity, we can now identify individuals at risk of future insomnia, shift-work disorder and mental illness, thus identifying a target population for preventive intervention. Given that insomniacs with high sleep reactivity tend to present with severe insomnia phenotypes, patient sleep reactivity may inform triaging to different levels of treatment. Future research on sleep reactivity is needed to clarify its neurobiology, characterize its long-term prospective associations with insomnia and shift-work disorder phenotypes, and establish its prognostic value for mental illness and other non-sleep disorders.
Keywords: Ford insomnia response to stress test; mental health; preventive treatment.
© 2018 European Sleep Research Society.
Publication types
- Research Support, N.I.H., Extramural
- Anxiety / epidemiology
- Anxiety / metabolism
- Anxiety / psychology
- Depression / epidemiology
- Depression / metabolism
- Depression / psychology
- Hypothalamo-Hypophyseal System / metabolism
- Pituitary-Adrenal System / metabolism
- Prospective Studies
- Sleep / physiology
- Sleep Disorders, Circadian Rhythm / epidemiology*
- Sleep Disorders, Circadian Rhythm / metabolism
- Sleep Disorders, Circadian Rhythm / psychology*
- Sleep Initiation and Maintenance Disorders / epidemiology*
- Sleep Initiation and Maintenance Disorders / metabolism
- Sleep Initiation and Maintenance Disorders / psychology*
- Stress, Psychological / epidemiology*
- Stress, Psychological / metabolism
- Stress, Psychological / psychology*
Grants and funding
- R01 MH082785/MH/NIMH NIH HHS/United States
- R01 NR013959/NR/NINR NIH HHS/United States
- R56 MH115150/MH/NIMH NIH HHS/United States
- T32 HL110952/HL/NHLBI NIH HHS/United States

10 Tips for Couples Navigating Menopause
New research highlights how to maintain good mental health through menopause..
Posted April 29, 2024 | Reviewed by Devon Frye
- New research challenges the notion that the time surrounding menopause is synonymous with poor mental health.
- Still, there are factors that make women vulnerable to clinical depression during menopause transition.
- Left unchecked, stress and poor social support can contribute to mental health problems.
- It's vital that women access the support they need to manage their stress.
A recent review paper published in the British journal The Lancet pooled data from 12 studies investigating the relationship between clinical depression and the menopause transition—the time starting with the onset of menstrual changes and ending with the final menstrual period.
The researchers analyzed data from 600 women globally and “found no compelling evidence for a universal or uniform increased risk of depressive symptoms over the menopause transition.” Indeed, their analysis went further and studied other mental health conditions such as anxiety , bipolar , and psychosis and found no evidence that menopause universally elevates the risk of those mental health conditions either.
This study challenges the widely held notion that the time surrounding menopause is synonymous with poorer mental health.

A Vulnerable Subgroup at Risk for Depression
While these findings are encouraging, the study did identify some of the factors that did make women vulnerable to clinical depression during the menopause transition. There was a higher risk of depression in those who experienced:
- A longer menopause transition. (This period can last anywhere from 4-10 years)
- Sleep disruption e.g., frequent hot flashes at night that contribute to chronic insomnia .
- A prior history of clinical depression, which made them more vulnerable to experiencing a recurrence during the menopausal transition.
- Stressful life events and low social support
Stress and Social Support: Modifiable Risk Factors
I’d like to home in on these last factors that put menopausal women at risk for poorer mental health. When it comes to psychological stress, women, more than men, consistently report higher levels with each passing year. As a women’s health psychiatrist, I’ve witnessed how stress is ubiquitous and frequent in the daily lives of women and how this situation is further complicated by lives that have quickened and intensified in the digital era.
What’s more, the average age of menopause transition is 47—a time of life that is often associated with stressful events. For example, it’s not uncommon for women of this age to be dealing with other health conditions (e.g., heart disease or cancer) or the loss of loved ones such as parents, siblings, or close friends. They may be going through many transitions, such as facing an empty nest or changes in the workplace. Often, they are spending many hours in unpaid labor caring for elderly parents and dependent children.
To add insult to injury, we live in a culture that does not value women past a certain age, who may not meet a standard of youthful beauty and are no longer capable of reproduction. And in my experience, it is hit or miss if women get the support they need when dealing with stressful situations. This is unfortunate when considering the downstream consequences for their mental health.
The bad news, then, is that stressful life events appear to be an integral part of the stage of life that also coincides with the menopausal transition. Left unchecked, this can contribute to mental health problems.
The good news is that stress is a modifiable risk factor—meaning it can be managed—as is increasing your social support network. If women can get the support they need to manage their stress, its harmful impacts on their physical and mental health could be mitigated.

What Women Going Through Menopause Can Do
1. Be proactive about managing stress. Establish and practice a daily self-care routine. At a minimum, this should include exercise, eating healthfully, and allowing sufficient time for sleep. Now is also the time to think about cutting out unhealthy ways of coping with stress, such as excessive alcohol consumption or nicotine use.
2. Be proactive about building a social support network. Look at the people who make up your social support network. Who is there for you when you need them? Who do you feel you can trust? Who do you feel is invested in you and your well-being?
Now is a good time to re-evaluate the time and energy you give to one-sided or unhealthy relationships. If you’re someone who finds it hard to ask for help or verbalize when you’re overwhelmed, now is also a good time to start working on developing that skill.
3. Don’t assume that depression or poor mental health is part and parcel of menopause. In fact, if you’ve never had clinical depression before, then you’re unlikely to have it during menopause.
4. Seek professional evaluation if necessary. If you notice significant signs and symptoms of depression, it’s important that you don’t chalk it up to menopause and seek professional attention . Find a therapist near you in the Psychology Today Therapy Directory.

What Partners Can Do
People whose partners are going through menopause can be a key source of support. It’s important to:
5. Get educated. There are many good and reliable resources available on the internet from which to get educated about menopause. It is helpful to have knowledge about what menopause is, what common signs and symptoms are, and how they may change over the different stages of menopause.
6. Act rather than speak. If you know your spouse is overloaded or taking on a disproportionate burden of certain duties—including childcare, caregiving , and household tasks—say directly, “Tell me what I can do to help,” and then be ready to follow through.
7. Prioritize kindness, compassion, and outward displays of affection or affirmation. Anything that fosters optimism , a healthy self-image , and perceived control has been found to be protective of mental health across the menopause transition.
8. Get proactive about self-care. Mid-life is a time when consistent self-care is nonnegotiable for anyone. If you are not doing so already, now is the time to make basic self-care a priority; that includes exercise, healthy eating, and high-quality sleep. If you know your spouse is struggling in these areas, create a buddy system: Sync your calendars so you can exercise together, divvy up tasks (i.e., shopping, preparation, and clean up) associated with healthy home cooking, and set up a similar time for going to bed and waking up so you can keep each other accountable when it comes to sleep wellness.
9. Schedule regular communication or check-ins: The menopause transition is an ever-evolving situation that may take place over many years. Make sure you make time to stay connected on this issue. Keep this time free of distractions or interruptions.
10. Cultivate a “We’re in this together” philosophy : Viewing this as a “you problem” is inherently unhelpful and insensitive and can leave your spouse feeling isolated and lonely . Be a partner on this journey. Advocate for your spouse if they need help navigating or accessing the healthcare system. If you feel they are delaying getting help and putting the needs of others before their own, nudge them to prioritize their own health and well-being.

Shaili Jain, M.D., is an Adjunct Clinical Professor of Psychiatry and Behavioral Sciences at the Stanford University School of Medicine.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience

IMAGES
VIDEO
COMMENTS
Introduction. The prevalence of insomnia in the general population ranges between 8-40%, depending on the definition used. While 20-30% of the general population has poor sleep (i.e., insomnia symptoms of difficulty initiating or maintaining sleep, early morning awakening, or non-restorative sleep at any given time), another 8-10% of the population suffers from chronic insomnia. 1,2 Also ...
This paper reviews the state of the art for optimally diagnosing and treating insomnia based on the available research evidence. DIAGNOSTIC CRITERIA FOR INSOMNIA The clinical diagnosis of insomnia is based on the complaint of trouble falling asleep, trouble staying asleep, or early morning awakening, and resultant daytime dysfunction 1 , 2 .
1.1. The Definition of Insomnia. This review addresses insomnia disorder (ID), by far the most common sleep disorder, as well as the second most common neuropsychiatric disorder, only outnumbered by the Diagnostic and Statistical Manual of Mental Disorders comprehensive category of all anxiety disorders (1, 2).ID is defined by symptoms that we may all have experienced: difficulties initiating ...
Health Psychology: Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age: 322: ... In conclusion, to our knowledge, this is the first bibliometric study to identify the 100 most cited papers in insomnia research. Our results suggest that the outbreak of ...
This article is part of the Research Topic Insomnia: ... Faculty of Psychology, University of Bergen, Bergen, Norway; 5 Department of Psychology, University of California, ... (2009). A comparison of web-based and paper-based survey methods: testing assumptions of survey mode and response cost. Eval. Rev. 33, 464-480. doi: 10.1177 ...
Insomnia. Dear readers of the Journal of Sleep Research, This virtual issue of JSR brings together ten articles published in 2019 and 2020 dealing with different aspects of insomnia. Insomnia, especially in its chronic form, is a frequent sleep disorder, probably the most frequent sleep disorder of all, afflicting approximately 10% of the adult ...
Insomnia disorder has known striking developments over the last few years. Partly due to advances in neuroimaging techniques and brain sciences, our understanding of insomnia disorder has become more fine-tuned. Besides, developments within psychological and psychiatric fields have contributed to improve conceptualization, assessment, and treatment of insomnia. In this paper, we present a list ...
Insomnia is defined as sleep continuity disturbance. associated with daytime complaints related to sleepiness, fatigue, somatic symptoms (eg, head or body aches), mood disturbance, compromised cognitive or occupational function, concerns about sleep, or dissatisfaction with sleep.
insomnia results from insuf ficient overnight adaptation to emotional distress (sect. 11) and concludes with sugges-tions for research (sect. 12). Importantly, the model can be evaluated not only in humans, but also by using the amaz-ing manipulation and assessment tools that have become available in animal research. It is hoped that the testable
To our knowledge, this is the largest randomised controlled trial of a psychological intervention for a mental health problem. It provides strong evidence that insomnia is a causal factor in the occurrence of psychotic experiences and other mental health problems. Whether the results generalise beyond a student population requires testing. The treatment of disrupted sleep might require a ...
A novel insomnia theoretical-conceptual framework would enable the drawing of data models for testing mediational relationships between independent variables and outcomes within retrospective studies. Besides, it may also help suggest research strategies and predictions designing prospective studies on insomnia.
Altogether, research on insomnia provides strong evidence for an interplay between biological predispositions, sleep-disrupting life events, and maladaptive coping behaviors that result in ...
The use of qualitative approaches (e.g., ethnography, phenomenology, grounded theory) may help gain a better understanding of this sleep disorder. The present paper summarizes the evidence derived from insomnia studies using a qualitative research methodology (e.g., focus group, semi-structured interviews).
As a universal, evolutionarily conserved phenomenon, sleep serves many roles, with an integral role in memory. This interplay has been examined in a variety of research. The purpose of this article will be to review the literature of sleep, aging, cognition, and the impact of two common clinical conditions (obstructive sleep apnea and insomnia ...
Charles M. Morin, School of Psychology and CERVO/BRAIN Research Center, Université Laval, Québec City, QC, Canada. Email: [email protected] Contribution: Conceptualization, Writing - review & editing, Methodology, Writing - original draft. Search for more papers by this author
Research shows that genetics, familial history of insomnia, female gender and environmental stress influence how the sleep system responds to stress. Further work has identified neurobiological underpinnings for sleep reactivity involving disrupted cortical networks and dysregulation in the autonomic nervous system and hypothalamic-pituitary ...
Insomnia is highly prevalent in clinical practice, occurring in up to 50% of primary care patients. Insomnia can present independently or alongside other medical conditions or mental health disorders and is a risk factor for the development and exacerbation of these other disorders if not treated. In 2016, the American College of Physicians recommended that insomnia be specifically targeted ...
This again recognizes insomnia disorder as a possible transdiagnostic process in psychopathology, with consequent important clinical implications. Nevertheless, more longitudinal studies are needed evaluating the link between insomnia disorder and mental disorders.
When defined as a sleep disorder, insomnia is characterise d by a. difficulty in falling asleep or remaining asleep, which may represe nt problems with sleep. mainte nance or early morning ...
Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262. Link Google Scholar; 53. Meltzer LJ, Phillips C, Mindell JA. Clinical psychology training in sleep and sleep disorders. J Clin Psychol. 2009;65(3):305-318.
Insomnia is thought to be a disorder of hyperarousal experienced throughout the entire day. This hyperarousal may exhibit itself as a state of hypervigilance during the day and difficulty initiating and maintaining sleep at night. This arousal is currently explained by both cognitive and physiological models of insomnia.
Introduction. As one of the most common sleep disorders, insomnia has become a significant public health problem. While the prevalence of insomnia varies considerably across countries, its global prevalence of insomnia as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria is estimated to be ~6-10% (1-3).
Key points. New research challenges the notion that the time surrounding menopause is synonymous with poor mental health. Still, there are factors that make women vulnerable to clinical depression ...
Abstract. Since ancient times it is known that melancholia and sleep disturbances co-occur. The introduction of polysomnography into psychiatric research confirmed a disturbance of sleep continuity in patients with depression, revealing not only a decrease in Slow Wave Sleep, but also a disinhibition of REM (rapid eye movement) sleep ...