Advertisement

- Search the Collections or Website

- Search the Collections or Website -->
- Healthy Living

Kidney Stones Over the Years: A Survivor Shares His Story
Posted on: 30 Jul 2021

Kim, a 75 year-old retired university professor, has lived with kidney stones for over 25 years.
In 1989, Kim had his first kidney stone surgery, shock wave lithotripsy (SWL). This was an old way to treat stones. It involved shock waves fired at his stones while he sat in a large tub of water. He says today’s SWL treatment is easier and more effective.
Many years later, in 2007, Kim was diagnosed with another kidney stone. This one was removed with ureteroscopy surgery (URS). In 2013, his stones returned. This time he needed a percutaneous nephrolithotomy (PCNL) surgery to treat a very large stone. It was almost the size of a baseball!
When Kim first heard about the surgery, he questioned how it would go. It involved making small cuts in his back, and inserting scopes into the center of his kidneys. Later, he said he was amazed at how smoothly the stones were removed.
Unfortunately, small pieces of stones still remain in Kim’s left kidney. Kim is now very careful about what he eats and drinks. He wishes he had known all along about how much your diet and fluids affect the way stones form. “I am much better educated today about how to prevent kidney stones,” says Kim. “I drink a lot of fluids and eat less salt and foods that form my type of stones. If I had some general education about stones and prevention 25 years ago, I would not have needed the care that I’ve had.”
Kim hopes his story will help the more than 1 million people diagnosed with kidney stones each year.
For more information on Kidney Stones, click here.
Check out more survivor stories like Kim's on our Survivor Story home page.
Explore Further

Share Your Story

Make a Differnece
This website uses cookies. We use cookies to enable you to more easily use our website, to monitor and analyze the use of our site to help improve our website and services, and to assist us with advertising reporting functions. By checking the “I Agree” box, you consent to our use of cookies as described in our Privacy Policy. I Agree You can learn more about our Cookie Policy here
Your Browser is Not Supported
This web site has been optimized for user experience and security, therefore Internet Explorer(IE) is not a recommended browser.Please use the latest version of Microsoft Edge, Chrome, Firefox or Safari(MacOS). Thank you.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
Contributor Disclosures
Please read the Disclaimer at the end of this page.
INTRODUCTION — Kidney stone disease (nephrolithiasis) is a common problem in primary care practice. Patients may present with the classic symptoms of renal colic and hematuria. Some patients may be asymptomatic or have atypical symptoms such as vague abdominal pain, while others will have more typical symptoms, such as acute abdominal or flank pain, nausea, urinary urgency or frequency, difficulty urinating, penile pain, or testicular pain.
This topic will review the evaluation of the patient with established symptomatic stone disease (either newly diagnosed or recurrent stones) or asymptomatic stones. Other aspects of kidney stones in adults are discussed separately:
● (See "Kidney stones in adults: Epidemiology and risk factors" .)
● (See "Kidney stones in adults: Diagnosis and acute management of suspected nephrolithiasis" .)
● (See "Kidney stones in adults: Prevention of recurrent kidney stones" .)
● (See "Kidney stones in adults: Surgical management of kidney and ureteral stones" .)
GOAL OF EVALUATION — In patients with established kidney stone disease, the goal of a diagnostic evaluation is to identify, as efficiently and economically as possible, the particular behavioral and physiologic differences present in a given patient so that effective therapy to prevent recurrent stones can be established and the prognosis can be better defined. Thus, the type and extent of evaluation depend in part upon the following:
● The severity and type of stone disease
● Whether it is a first or a recurrent stone
● Presence or absence of systemic disease and/or risk factors for recurrent stone formation
● Family history of nephrolithiasis
● The patient's interest in stone prevention
APPROACH TO EVALUATION
Patients with established stone disease — All patients presenting with established kidney stone disease (either newly diagnosed or recurrent stones) should undergo a focused history, radiologic imaging, stone analysis (if available), and at least a limited laboratory evaluation. The approach for patients with asymptomatic stones is discussed elsewhere in this topic. (See 'Patients with asymptomatic stones' below.)
Focused history for stone risk factors — The purpose of the focused history is to identify stone risk factors, such as a family history of stone disease and certain dietary habits ( table 1 ). These factors are described in detail elsewhere. (See "Kidney stones in adults: Epidemiology and risk factors" .)
Summarized briefly, adverse dietary habits include:
● Low fluid intake or a high fluid loss (eg, from sweating or gastrointestinal losses), which leads to a lower urine volume and, therefore, a higher concentration of lithogenic factors.
● A very high animal protein diet, which can lead to higher excretion of calcium and uric acid and lower excretion of urine citrate ( figure 1 ).
● Higher sodium diet, which increases urinary calcium excretion [ 1 ].
● Increased intake of higher oxalate-containing foods, particularly spinach. The magnitude of the contribution of dietary oxalate to urinary oxalate is controversial and likely varies considerably from person to person due to differences in gastrointestinal absorption.
● Lower calcium intake, which acts by increasing the absorption of dietary oxalate and subsequent higher excretion of oxalate in the urine due to decreased calcium oxalate complex formation within the intestinal lumen [ 2,3 ]. The effect of lower calcium intake on raising urine oxalate more than counterbalances the decrease in calcium absorption and excretion.
● Excessive vitamin C and D supplementation, which may increase urinary oxalate or calcium, respectively.
● Excessive sugar (sucrose and fructose) intake, which may increase calcium and/or oxalate excretion.
In addition, certain medications can occasionally crystallize in the urine and lead to stone formation. Examples include atazanavir , sulfadiazine , and triamterene . (See "Crystal-induced acute kidney injury" and "Triamterene nephrotoxicity" .)
Radiologic testing — If not yet performed during the initial evaluation, radiographic examination, preferably with noncontrast, low-dose computed tomography (CT), should be obtained to search for residual stones within the urinary tract. Diagnostic tests for the detection of nephrolithiasis are discussed in detail elsewhere. (See "Kidney stones in adults: Diagnosis and acute management of suspected nephrolithiasis", section on 'Diagnostic imaging' .)
Stone analysis — Analysis of the stone is an essential part of the evaluation [ 4-6 ]. Patients should be encouraged to retrieve stones they pass spontaneously for analysis, although novel CT imaging techniques may permit noninvasive discrimination among the main subtypes of urinary calculi. Similarly, stones that are surgically removed should also be submitted for analysis. (See "Kidney stones in adults: Diagnosis and acute management of suspected nephrolithiasis", section on 'Determination of stone composition' .)
Number of stones to analyze — At least one stone should be analyzed in every patient. Given sampling issues, analyzing two stones provides useful information about other possible and/or relevant components. If the two stone composition reports are sufficiently similar, then future stones do not need to be analyzed unless there has been a clinically relevant change. However, some patients produce more than one stone type. In a study of patients with bilateral stones, for example, the major stone component was discordant between the two kidneys in 25 percent of individuals [ 7 ]. In this setting, it would be reasonable to analyze more than one stone that is passed from each side. Also, if a new stone forms after many years, it should be sent for analysis if passed or surgically removed.
Occasionally, treatment to prevent one stone type may inadvertently lead to the formation of a different stone type (though this is uncommon). As an example, over-alkalinization of the urine of a patient with uric acid stones could increase the risk of calcium phosphate crystal formation. Thus, analysis of a newly formed stone after the initiation of treatment is indicated.
Individual crystalline components — The most common crystalline materials found in kidney stones are calcium oxalate, calcium phosphate, uric acid, and struvite (magnesium ammonium phosphate). It is not uncommon for a stone to contain more than one crystalline component.
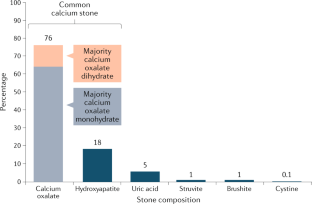
● Calcium oxalate – Calcium oxalate is the most common component found in kidney stones (approximately 70 to 80 percent). Calcium oxalate can be found in monohydrate and dihydrate forms ( picture 1A ). Calcium oxalate can also be present in combination with uric acid or calcium phosphate. Because calcium oxalate stones typically grow on a Randall's plaque (composed of calcium phosphate) on the papillary tip [ 8,9 ], a laboratory that examines the composition of the nidus may report a stone with an eccentric calcium phosphate nidus (usually 5 percent) and a calcium oxalate body (95 percent).
● Calcium phosphate – Calcium phosphate is found in approximately 15 percent of kidney stones and can be present in combination with calcium oxalate or struvite. Because of differences in solubility due to urine pH, calcium phosphate is not found mixed with uric acid. The two forms of calcium phosphate include apatite (sometimes reported as carbonate apatite), which is the crystal type found in bone, or calcium hydrogen phosphate (brushite); the frequency of apatite is much greater than brushite. Calcium phosphate crystals in the urine sediment are typically dark and amorphous.
● Uric acid – Uric acid is the most common crystal form that contains urate ( picture 2A ). Uric acid is present in approximately 8 percent of analyzed stones, sometimes in combination with calcium oxalate. Rare crystals that contain urate include sodium urate (which is present in the joint fluid of patients with gouty arthritis) and ammonium urate. (See "Kidney stones in adults: Uric acid nephrolithiasis" .)
● Struvite – Struvite is the crystal name for stones that form only in the presence of urease-producing bacteria (eg, Proteus mirabilis , Klebsiella pneumoniae , Corynebacterium species, Ureaplasma urealyticum ) in the upper urinary tract ( picture 3 ). Other names for this crystal type include "triple phosphate" (because the phosphate is in the triple-negative form) and magnesium ammonium phosphate carbonate apatite. Struvite is found in approximately 1 percent of analyzed stones and is much more common in females than in males (due to the higher risk of urinary tract infections in females). If a preexisting calcium-containing kidney stone is subsequently infected with a urease-producing bacterium, the stone analysis may report that the composition of the stone includes calcium oxalate or calcium phosphate in addition to struvite. (See "Kidney stones in adults: Struvite (infection) stones" .)
● Other crystal types – Rare crystal types include [ 10 ]:
• Cystine ( picture 4 ). (See "Cystinuria and cystine stones" .)
• 2,8-dihydroxyadenine (DHA) – DHA crystals form as a result of adenine phosphoribosyltransferase deficiency, a rare autosomal recessive disorder; DHA crystals may be incorrectly reported as uric acid by some laboratories [ 11 ].
• Triamterene . (See "Triamterene nephrotoxicity", section on 'Triamterene stones' .)
• Acyclovir . (See "Crystal-induced acute kidney injury", section on 'Acyclovir' .)
Clinical relevance — Knowing the composition of the stone assists with clinical decision-making for the treatment of existing stones and prevention of new stone formation. Examples of how this knowledge can impact clinical decision making include:
● Calcium oxalate monohydrate and brushite are hard stones and may not be fragmented as easily with shock wave lithotripsy.
● Uric acid stones form in acid urine, and alkalinization of the urine can both dissolve existing uric acid stones and prevent new stones from forming. (See "Kidney stones in adults: Uric acid nephrolithiasis", section on 'Urinary alkalinization' .)
● Calcium phosphate stones form in alkaline urine, and therefore, increasing the urine pH may increase the likelihood of calcium phosphate precipitation. While reducing the urine pH would be helpful, this is often not clinically possible for most patients who form calcium phosphate stones (for unclear reasons).
● The presence of certain stone types may indicate the existence of an underlying predisposing condition. As examples, calcium phosphate stones are more frequent in individuals with primary hyperparathyroidism and distal renal tubular acidosis, and struvite stones form only in the presence of an upper urinary tract infection. Uric acid stones may be more common in individuals with diabetes mellitus (due to impaired ammoniagenesis), metabolic syndrome [ 12 ], or gout. (See "Kidney stones in adults: Epidemiology and risk factors", section on 'Medical conditions' .)
When designing a preventive regimen for a patient, the individual components of the stone need to be considered:
● For pure stones, the focus is on modifying the urine composition to prevent the precipitation of that specific crystal type, even if the 24-hour urine composition suggests a high risk for precipitation of another crystal type. (See '24-hour urine collections' below.)
● For mixed stones, the treatment recommendations depend upon the specific components and the relative amounts present. As an example, for a stone that is reported to contain 95 percent calcium oxalate and 5 percent calcium phosphate, the focus should be on reducing the supersaturation of calcium oxalate. However, these two crystal types share risk factors (lower urine volume, higher urine calcium, and lower urine citrate), so modifying these components should reduce the risk of both crystal types. (See "Kidney stones in adults: Prevention of recurrent kidney stones" .)
Given the variability in reporting of mixed stones by commercial laboratories, it is important to keep in mind the clinical setting and other available information and to question the reliability of the stone composition report if it seems inconsistent with the patient's history. A report indicating the presence of struvite, for example, is probably inaccurate in a patient with no documented infection with a urease-producing bacterium. It is also important to examine the urine sediment for crystals as this might help identify the crystal type. (See 'Urinalysis' below.)
Laboratory testing
Approaches to laboratory testing — Three options have been proposed for laboratory evaluation after a first stone: a limited evaluation, a complete metabolic evaluation, or a targeted approach. Although there is disagreement whether a complete metabolic evaluation should be performed after the first kidney stone, there is general agreement that a complete metabolic evaluation is indicated in all patients with multiple stones at first presentation (including those who have passed a single stone but have other asymptomatic stones found in the kidney by imaging), patients with a strong family history of stones, individuals with active stone disease (defined as recurrent stone formation, enlargement of existing stones, or the recurrent passage of gravel), and patients with reduced bone mineral density. The decision about which option to pursue should be shared by the clinician and patient.
● Limited evaluation – A limited laboratory evaluation includes a urinalysis and routine blood chemistries. Some clinicians prefer this approach after a first stone because of the variable rate of stone recurrence and data suggesting that a comprehensive medical evaluation is not cost-effective for patients who have only formed one stone [ 13,14 ]. This approach is based upon the availability of nonoperative therapy for most symptomatic stones and avoids unnecessary therapy in those who would not have a recurrence. (See 'Urinalysis' below and 'Blood tests' below.)
● Complete metabolic evaluation – A complete metabolic evaluation consists of a urinalysis, routine blood chemistries, and at least two 24-hour urine collections for analysis of urine composition. Some clinicians recommend this approach after the first stone because of the potentially high rate of recurrence and potential morbidity from recurrent stones. Limited data suggest that single-stone formers have similar metabolic abnormalities as patients with recurrent nephrolithiasis [ 15,16 ]. In addition, there are several studies suggesting that the likelihood of stone formation can be predicted reasonably well from the 24-hour urine values [ 17-19 ]. This approach should be followed only in individuals willing to make changes to their diet or fluid intake or to take medical therapy if warranted by the work-up. (See 'Complete metabolic evaluation' below.)
● Targeted approach – A third approach is to base the extent of the laboratory evaluation upon an estimation of the risk for new stone formation [ 18 ]. A complete metabolic evaluation would be performed in patients at moderate to high risk for recurrent disease. Patients at high risk for recurrent disease include:
• Patients who have formed more than one kidney stone
• Patients with a family history of stones
• Patients with chronic diarrheal states and/or malabsorption, pathologic skeletal fractures, osteoporosis, urinary tract infection, diabetes, and/or gout
• Patients taking medication that may put them at higher risk (eg, topiramate , acetazolamide )
• Patients with stones composed of cystine, uric acid, or calcium phosphate
• Patients with dietary habits associated with higher risk of stone formation
Complete metabolic evaluation — The complete metabolic evaluation for nephrolithiasis consists of both blood and urine testing, including at least two 24-hour urine collections at baseline.
Urinalysis — A urinalysis should be performed on a voided urinary specimen. The urinalysis should include pH determination since a pH greater than 7.5 raises the possibility of a stone due to urease-producing bacteria, whereas a pH less than 5.5 favors uric acid lithiasis. (See "Urinalysis in the diagnosis of kidney disease" .)
The urine sediment should also be examined for crystalluria since particular crystal types may provide a clue as to the composition of stones (see "Urinalysis in the diagnosis of kidney disease" ):
● Uric acid crystals – Uric acid crystals are observed in acid urine (usually pH <5.5), a milieu that favors the conversion of the relatively soluble urate salt into the insoluble uric acid ( picture 2A-B ). (See "Uric acid kidney diseases" .)
● Calcium phosphate or calcium oxalate crystals – The formation of calcium oxalate crystals is not dependent upon the urine pH, while calcium phosphate crystals only form in a relatively alkaline urine (usually pH ≥6.5) ( picture 1A-B ). (See "Kidney stones in adults: Epidemiology and risk factors" .)
● Cystine crystals – Cystine crystals, with their characteristic hexagonal shape, are diagnostic of cystinuria ( picture 5 ). (See "Cystinuria and cystine stones" .)
● Magnesium ammonium phosphate crystals – Magnesium ammonium phosphate (struvite) and calcium carbonate apatite are the constituents of struvite stones ( picture 3 ) (see "Kidney stones in adults: Struvite (infection) stones" ). Normal urine is undersaturated with magnesium ammonium phosphate, and struvite stone formation occurs only when ammonia production is increased and the urine pH is elevated. Both of these requirements are only met when an upper urinary tract infection occurs with a urease-producing bacterium, such as Proteus or Klebsiella .
Blood tests — A routine chemistry profile should be obtained, including measurement of serum electrolytes, serum creatinine, and serum calcium. The results may help identify certain disorders, such as primary hyperparathyroidism, hyperuricemia, and distal renal tubular acidosis, that are associated with nephrolithiasis. (See "Primary hyperparathyroidism: Diagnosis, differential diagnosis, and evaluation" and "Kidney stones in adults: Uric acid nephrolithiasis" and "Nephrolithiasis in renal tubular acidosis" .)
● The serum calcium concentration should be measured looking for hypercalcemia; if high-normal (which we define as above the midpoint of the normal range), the serum calcium should be repeated. A measurement of intact parathyroid hormone is warranted in patients with serum calcium values in the high-normal range or if the urine calcium is high since primary hyperparathyroidism is often associated with only intermittent and mild elevations in the serum calcium concentration [ 20-22 ]. In one series of 48 patients with nephrolithiasis and primary hyperparathyroidism, 30 (63 percent) had serum calcium concentrations between 10.2 and 11 mg/dL (2.55 and 2.75 mmol/L) [ 20 ]. (See "Primary hyperparathyroidism: Diagnosis, differential diagnosis, and evaluation" .)
● Although usually in the middle of the reference range, the presence of a lower serum bicarbonate concentration raises the possibility of distal renal tubular acidosis or chronic diarrhea.
24-hour urine collections — An important component of the evaluation of patients at moderate to high risk for recurrent stone disease is the assessment of urine composition:
● Number of collections – At least two 24-hour urine collections should be obtained in the outpatient setting, with the patient following their usual diet, fluid intake, and physical activity. The differences among the collections are often substantial, so an important contributor may be missed in many patients if only one sample is collected ( figure 2 ) [ 23,24 ]. The validity of this approach was illustrated in the following studies:
• A study of 75 recurrent idiopathic calcium kidney stone formers examined the relative diagnostic utility of one, two, and three urine collections [ 23 ]. When compared with one or any combination of two urine collections, three urine collections were significantly associated with the highest yield of identifying a urinary abnormality ( figure 2 ).
• Similar findings were noted in another report of over 1000 stone formers in whom three 24-hour urine collections were obtained [ 24 ]. Differences in urinary biochemical risk factors among the three collections were substantial enough that an important metabolic abnormality would have been missed in many patients if only one sample had been collected.
Thus, we recommend that a minimum of two collections be performed as part of the initial evaluation.
● Timing of collections – The urine collections should be obtained while the patient is on their usual diet. Values should not be measured immediately after the acute stone episode; it is common practice to wait at least one to two months after a stone event to obtain the collections [ 23 ]. One should also wait at least one to two months after the patient has completely recovered from any interventions, such as shock wave lithotripsy, ureteroscopy, or percutaneous stone removal. Ideally, the patient should be free of pain, infection, and obstruction and following their "usual routine" when performing their urine collections.
● Tests to include – The urine volume and excretion of calcium, uric acid, citrate, oxalate, creatinine (to assess the completeness of the collection), pH, sodium, potassium, and magnesium should all be measured. Ideally, the supersaturation of lithogenic substances should be calculated. The results of the urine collections and stone analysis (if available) dictate subsequent evaluation and management. (See "Kidney stones in adults: Prevention of recurrent kidney stones" and "Kidney stones in adults: Struvite (infection) stones" and "Kidney stones in adults: Uric acid nephrolithiasis" and "Cystinuria and cystine stones" .)
A variety of definitions for "normal" are used by different laboratories for each of the urinary parameters. Below are some more common definitions:
• Calcium − Less than 200 mg (5.0 mmol) per day in females or less than 250 mg (6.25 mmol) per day in males
• Uric acid − Less than 750 mg (4.5 mmol) per day in females or less than 800 mg (4.8 mmol) per day in males
• Oxalate − Less than 40 mg (0.44 mmol) per day in both females and males
• Citrate − Greater than or equal to 450 mg per day in both females and males
However, the values for these definitions are arbitrary and additional data suggest a more linear relation for each of these factors. As an example, the risk continues to decrease as urinary calcium falls below 250 mg/day (6.25 mmol/day) in both males and females [ 25 ]. In addition, the concentration of lithogenic factors and the urinary supersaturation, as calculated in an experienced laboratory, are more important than the absolute amounts with respect to stone formation [ 25 ]. The definition of "normal" for calculated supersaturation is also arbitrary; the risk continues to decline even when the supersaturation is lower than the threshold [ 26 ].
Measurement of sodium excretion is also important. Higher sodium intake can contribute to increased calcium excretion and will affect the response to a thiazide diuretic when prescribed to reduce urine calcium. (See "Kidney stones in adults: Epidemiology and risk factors" .)
In many laboratories, two or three separate collections are required to obtain all of this information: uric acid is measured in a plain or alkaline solution, calcium and oxalate are measured in hydrochloric or nitric acid, and citrate is measured in an acidified solution. However, some specialized laboratories provide a kit that permits all of the above values to be measured and urinary supersaturation to be calculated from a single urine collection, thereby making it easier for the patient and more likely that the required information will be obtained [ 27-29 ].
Monitoring for new stones — Radiologic monitoring, usually with ultrasonography, abdominal radiography, low-dose, noncontrast CT, or digital tomosynthesis, is warranted for the detection of new stones. Monitoring should be performed initially at one year and, if negative, every two to four years thereafter depending upon the severity of the stone disease and the 24-hour urine values. (See "Kidney stones in adults: Diagnosis and acute management of suspected nephrolithiasis" .)
Several factors should be considered when choosing which modality to use for radiologic monitoring:
● If stones were previously visible by ultrasonography, then using this modality will minimize cumulative radiation exposure; this is particularly important in patients of childbearing age.
● To allow comparisons of stone burden over time, a modality should be selected that successfully detected the number and size of previous stones. As an example, ultrasound should not be used for radiologic monitoring if prior stones were only quantifiable with noncontrast CT, abdominal radiographs, or digital tomograms.
● Noncontrast CT remains the most sensitive test for detecting stones, particularly small stones, but is an expensive option for routine monitoring of stone burden [ 30 ]. Digital tomosynthesis, a high-resolution radiograph-based imaging technique that is used routinely for breast cancer screening, may be an effective and lower cost alternative to noncontrast CT. One retrospective study comparing digital tomosynthesis with noncontrast CT for the follow-up of nephrolithiasis found similar stone detection rates between the two imaging modalities [ 31 ]. Digital tomosynthesis has also been shown to have lower radiation exposure compared with noncontrast CT [ 32,33 ]. (See "Kidney stones in adults: Diagnosis and acute management of suspected nephrolithiasis", section on 'Digital tomosynthesis' .)
Patients with asymptomatic stones — Some patients are found to have an asymptomatic kidney stone or stones by imaging performed for a different indication [ 34 ]. Approximately one-third of such patients will develop symptoms related to their kidney stones within three years, and as many as one-half of these symptomatic patients may require surgical treatment for their stones [ 35,36 ]. Thus, asymptomatic patients may benefit from a metabolic evaluation and appropriate medical therapy to prevent growth of any existing stones and to prevent new stone formation. We perform a complete metabolic evaluation in all asymptomatic patients who have multiple stones since we consider such patients to be recurrent stone formers. In addition, we perform a complete metabolic evaluation in select asymptomatic patients with a single stone, based upon their occupation (airline pilots, frequent business travelers), complexity (neurologic disease, anatomic abnormalities of the urinary tract, such as urinary diversion or solitary kidney), or the need for surgical stone removal. (See 'Complete metabolic evaluation' above.)
In asymptomatic patients with a single stone who do not warrant a complete metabolic evaluation, active surveillance with repeat imaging within one to two years (see 'Monitoring for new stones' above) is a reasonable approach. If repeat imaging shows no evidence of stone growth, imaging can be repeated every two years; if there is no stone growth after four to five years, active surveillance may be discontinued. If repeat imaging shows an increase in stone size or new stones, we perform a complete metabolic evaluation.
Several studies have examined the natural history of asymptomatic kidney stones. As examples:
● A cohort of 110 patients with 160 asymptomatic kidney stones was followed with active surveillance (using kidney ultrasound performed every 6 to 12 months) [ 35 ]. During a mean follow-up of 3.4 years, 28 percent of stones produced symptoms and 17 percent required surgery for these symptoms; an additional 3 percent caused silent obstruction that required intervention. Lower pole stones were less likely to cause symptoms or pass spontaneously.
● Another study monitored 107 such patients for a mean of 32 months [ 36 ]. The likelihood of developing symptoms was approximately 32 percent at 2.5 years and 49 percent at 5 years; the risk was lowest in patients who had no history of previous stones. Roughly one-half of symptomatic patients required a procedure (such as shock wave lithotripsy) for removal of the stone, while the remaining symptomatic patients passed the stone spontaneously.
In addition to these findings, a number of studies have determined that patients with residual stones following shock wave lithotripsy or percutaneous stone removal are at increased risk for symptomatic stone episodes. However, these investigations also suggest that appropriate medical stone management can significantly reduce recurrent stone formation or growth of existing stones [ 37-39 ].
SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Kidney stones" .)
SUMMARY AND RECOMMENDATIONS
● Goal of evaluation – In patients with established kidney stone disease, the goal of a diagnostic evaluation is to identify, as efficiently and economically as possible, the particular behavioral and physiologic differences present in a given patient so that effective therapy to prevent recurrent stones can be established and the prognosis can be better defined. (See 'Goal of evaluation' above.)
● Approach to evaluation – All patients presenting with established kidney stone disease should undergo a focused history, radiologic imaging, stone analysis (if available), and at least a limited laboratory evaluation:
• Focused history – The purpose of the focused history is to identify stone risk factors, such as a family history of stone disease and certain dietary habits ( table 1 ). (See 'Focused history for stone risk factors' above.)
• Radiologic testing – If not yet performed during the initial evaluation, radiographic examination, preferably with noncontrast, low-dose computed tomography (CT), should be obtained to search for residual stones within the urinary tract. Diagnostic tests for the detection of nephrolithiasis are discussed in detail elsewhere. (See "Kidney stones in adults: Diagnosis and acute management of suspected nephrolithiasis", section on 'Diagnostic imaging' .)
• Stone analysis – Analysis of the stone is an essential part of the evaluation. Patients should be encouraged to retrieve stones they pass spontaneously for analysis, although novel CT imaging techniques may permit noninvasive discrimination among the main subtypes of urinary calculi. Similarly, stones that are surgically removed should also be submitted for analysis. The most common crystalline materials found in kidney stones are calcium oxalate, calcium phosphate, uric acid, and struvite (magnesium ammonium phosphate). It is not uncommon for a stone to contain more than one crystalline component. (See 'Stone analysis' above.)
• Laboratory testing – Three options have been proposed for laboratory evaluation after a first stone: a limited evaluation, a complete metabolic evaluation, or a targeted approach. Although there is disagreement whether a complete metabolic evaluation should be performed after the first kidney stone, a complete metabolic evaluation is indicated in all patients with multiple stones at first presentation, patients with a strong family history of stones, patients with low bone mineral density, and individuals with active stone disease (defined as recurrent stone formation, enlargement of existing stones, or the recurrent passage of gravel). (See 'Approaches to laboratory testing' above.)
• Complete metabolic evaluation – The complete metabolic evaluation for nephrolithiasis consists of both blood and urine testing, including at least two 24-hour urine collections. In each 24-hour urine collection, the urine volume, pH, and excretion of calcium, uric acid, citrate, oxalate, sodium, potassium, magnesium, and creatinine (to assess the completeness of the collection) should be measured. Also, urinary supersaturation should be calculated. Urine collections should not be performed if there is evidence of kidney/ureteral obstruction from existing stones or urinary tract infection. (See 'Complete metabolic evaluation' above.)
● Monitoring for new stones – Radiologic monitoring, usually with ultrasonography; abdominal radiography; noncontrast, low-dose CT; or digital tomosynthesis, is warranted for the detection of new stones. Monitoring should be performed initially at one year and, if negative, every two to four years thereafter depending upon the severity of the stone disease and the 24-hour urine values. (See 'Monitoring for new stones' above.)
● Asymptomatic stones – Some patients are found to have an asymptomatic kidney stone or stones by imaging performed for a different indication. Such patients may also benefit from a metabolic evaluation and appropriate medical therapy to prevent growth of any existing stones and to prevent new stone formation. (See 'Patients with asymptomatic stones' above.)
- Bayomy O, Zaheer S, Williams JS, et al. Disentangling the Relationships Between the Renin-Angiotensin-Aldosterone System, Calcium Physiology, and Risk for Kidney Stones. J Clin Endocrinol Metab 2020; 105.
- Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126:497.
- von Unruh GE, Voss S, Sauerbruch T, Hesse A. Dependence of oxalate absorption on the daily calcium intake. J Am Soc Nephrol 2004; 15:1567.
- Kourambas J, Aslan P, Teh CL, et al. Role of stone analysis in metabolic evaluation and medical treatment of nephrolithiasis. J Endourol 2001; 15:181.
- Pak CY, Poindexter JR, Adams-Huet B, Pearle MS. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med 2003; 115:26.
- Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med 2004; 350:684.
- Kadlec AO, Fridirici ZC, Acosta-Miranda AM, et al. Bilateral urinary calculi with discordant stone composition. World J Urol 2014; 32:281.
- Miller NL, Williams JC Jr, Evan AP, et al. In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randall's plaque. BJU Int 2010; 105:242.
- Miller NL, Gillen DL, Williams JC Jr, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall's plaque. BJU Int 2009; 103:966.
- Daudon M, Frochot V, Bazin D, Jungers P. Drug-Induced Kidney Stones and Crystalline Nephropathy: Pathophysiology, Prevention and Treatment. Drugs 2018; 78:163.
- Bollée G, Dollinger C, Boutaud L, et al. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol 2010; 21:679.
- Wong Y, Cook P, Roderick P, Somani BK. Metabolic Syndrome and Kidney Stone Disease: A Systematic Review of Literature. J Endourol 2016; 30:246.
- Chandhoke PS. When is medical prophylaxis cost-effective for recurrent calcium stones? J Urol 2002; 168:937.
- Parmar MS. Kidney stones. BMJ 2004; 328:1420.
- Strauss AL, Coe FL, Parks JH. Formation of a single calcium stone of renal origin. Clinical and laboratory characteristics of patients. Arch Intern Med 1982; 142:504.
- Pak CY. Should patients with single renal stone occurrence undergo diagnostic evaluation? J Urol 1982; 127:855.
- Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med 1995; 98:50.
- Preminger GM. The metabolic evaluation of patients with recurrent nephrolithiasis: a review of comprehensive and simplified approaches. J Urol 1989; 141:760.
- Mardis HK, Parks JH, Muller G, et al. Outcome of metabolic evaluation and medical treatment for calcium nephrolithiasis in a private urological practice. J Urol 2004; 171:85.
- Parks J, Coe F, Favus M. Hyperparathyroidism in nephrolithiasis. Arch Intern Med 1980; 140:1479.
- Yendt ER, Gagne RJ. Detection of primary hyperparathyroidism, with special reference to its occurrence in hypercalciuric females with "normal" or borderline serum calcium. Can Med Assoc J 1968; 98:331.
- Pierreux J, Bravenboer B. Normocalcemic Primary Hyperparathyroidism: A Comparison with the Hypercalcemic Form in a Tertiary Referral Population. Horm Metab Res 2018; 50:797.
- Hess B, Hasler-Strub U, Ackermann D, Jaeger P. Metabolic evaluation of patients with recurrent idiopathic calcium nephrolithiasis. Nephrol Dial Transplant 1997; 12:1362.
- Parks JH, Goldfisher E, Asplin JR, Coe FL. A single 24-hour urine collection is inadequate for the medical evaluation of nephrolithiasis. J Urol 2002; 167:1607.
- Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 2008; 73:489.
- Prochaska M, Taylor E, Ferraro PM, Curhan G. Relative Supersaturation of 24-Hour Urine and Likelihood of Kidney Stones. J Urol 2018; 199:1262.
- https://www.litholink.com/at-home-kit-registration (Accessed on April 26, 2019).
- https://www.labcorp.com/resource/urine-specimens (Accessed on April 26, 2019).
- https://www.questdiagnostics.com/home/physicians/testing-services/specialists/hospitals-lab-staff/specimen-handling/urine.html (Accessed on April 26, 2019).
- Zilberman DE, Tsivian M, Lipkin ME, et al. Low dose computerized tomography for detection of urolithiasis--its effectiveness in the setting of the urology clinic. J Urol 2011; 185:910.
- Cabrera FJ, Kaplan AG, Youssef RF, et al. Digital Tomosynthesis: A Viable Alternative to Noncontrast Computed Tomography for the Follow-Up of Nephrolithiasis? J Endourol 2016; 30:366.
- Liu S, Wang H, Feng W, et al. The value of X-ray digital tomosynthesis in the diagnosis of urinary calculi. Exp Ther Med 2018; 15:1749.
- Liu S, Nie P, Wang H, et al. Application of Digital Tomosynthesis in the Diagnosis of Urolithiasis: Comparison with MDCT. J Endourol 2020; 34:145.
- Bansal AD, Hui J, Goldfarb DS. Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol 2009; 4:680.
- Dropkin BM, Moses RA, Sharma D, Pais VM Jr. The natural history of nonobstructing asymptomatic renal stones managed with active surveillance. J Urol 2015; 193:1265.
- Glowacki LS, Beecroft ML, Cook RJ, et al. The natural history of asymptomatic urolithiasis. J Urol 1992; 147:319.
- Fine JK, Pak CY, Preminger GM. Effect of medical management and residual fragments on recurrent stone formation following shock wave lithotripsy. J Urol 1995; 153:27.
- Springhart WP, Maloney ME, Marguet CG, et al. Appropriate medical treatment after percutaneous nephrolithotomy can control active stone disease in the presence of residual calculi. J Urol 2004; 171:302.
- Osman MM, Alfano Y, Kamp S, et al. 5-year-follow-up of patients with clinically insignificant residual fragments after extracorporeal shockwave lithotripsy. Eur Urol 2005; 47:860.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 04 August 2020
Determining the true burden of kidney stone disease
- Charat Thongprayoon ORCID: orcid.org/0000-0002-8313-3604 1 ,
- Amy E. Krambeck ORCID: orcid.org/0000-0001-8255-598X 2 &
- Andrew D. Rule 1 , 3
Nature Reviews Nephrology volume 16 , pages 736–746 ( 2020 ) Cite this article
4135 Accesses
123 Citations
112 Altmetric
Metrics details
- Renal calculi
- Risk factors
The incidence and prevalence of kidney stones have increased over the past four decades. However, the diagnosis of ‘kidney stone’ can range from an incidental asymptomatic finding of limited clinical significance to multiple painful episodes of ureteral obstruction with eventual kidney failure. Some general strategies may be useful to prevent the recurrence of kidney stones. In particular, greater attention to kidney stone classification, approaches to assessing the risk of recurrence and individualized prevention strategies may improve the clinical care of stone formers. Although there have been some advances in approaches to predicting the recurrence of kidney stones, notable challenges remain. Studies of kidney stone prevalence, incidence and recurrence have reported inconsistent findings, in part because of the lack of a standardized stone classification system. A kidney stone classification system based on practical and clinically useful measures of stone disease may help to improve both the study and clinical care of stone formers. Any future kidney stone classification system should be aimed at distinguishing asymptomatic from symptomatic stones, clinically diagnosed symptomatic stone episodes from self-reported symptomatic stone episodes, symptomatic stone episodes that are confirmed from those that are suspected, symptomatic recurrence from radiographic recurrence (that is, with radiographic evidence of a new stone, stone growth or stone disappearance from presumed passage) and determine stone composition based on mutually exclusive categories.
Kidney stones can range from an asymptomatic incidental finding with limited clinical significance to a painful recurrent disorder with substantial morbidity.
The prevalence and incidence of kidney stones has increased worldwide, but some of this increase is due to improvements in medical imaging with increased utilization of CT.
Classifying stone formers according to their clinical presentation and stone composition can help to predict the risk of future symptomatic stone episodes and aid personalization of stone prevention strategies.
The wide range of recurrence rates reported between different studies might largely be due to the use of different definitions that include various degrees of symptomatic evidence of recurrence and/or radiographic manifestations of recurrence.
Risk factors for symptomatic kidney stone recurrence include younger age, male gender, family history of stones, obesity, pregnancy, rarer stone compositions, higher radiographic kidney stone burden, number of past symptomatic kidney stone episodes and fewer years since last kidney stone episode.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Development and validation of a new algorithm for improved cardiovascular risk prediction

Acute kidney injury

Chronic kidney disease and the global public health agenda: an international consensus
Scales, C. D. Jr., Smith, A. C., Hanley, J. M. & Saigal, C. S. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur. Urol. 62 , 160–165 (2012).
Article PubMed PubMed Central Google Scholar
Stamatelou, K. K., Francis, M. E., Jones, C. A., Nyberg, L. M. & Curhan, G. C. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 63 , 1817–1823 (2003).
Article PubMed Google Scholar
Trinchieri, A. et al. Increase in the prevalence of symptomatic upper urinary tract stones during the last ten years. Eur. Urol. 37 , 23–25 (2000).
Article CAS PubMed Google Scholar
Hesse, A., Brandle, E., Wilbert, D., Kohrmann, K. U. & Alken, P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur. Urol. 44 , 709–713 (2003).
Penniston, K. L., McLaren, I. D., Greenlee, R. T. & Nakada, S. Y. Urolithiasis in a rural Wisconsin population from 1992 to 2008: narrowing of the male-to-female ratio. J. Urol. 185 , 1731–1736 (2011).
Liu, Y. et al. Epidemiology of urolithiasis in Asia. Asian J. Urol. 5 , 205–214 (2018).
Sorokin, I. et al. Epidemiology of stone disease across the world. World J. Urol. 35 , 1301–1320 (2017).
Pearle, M. S., Calhoun, E. A. & Curhan, G. C. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J. Urol. 173 , 848–857 (2005).
Kittanamongkolchai, W. et al. The changing incidence and presentation of urinary stones over 3 decades. Mayo Clin. Proc. 93 , 291–299 (2018).
Dwyer, M. E. et al. Temporal trends in incidence of kidney stones among children: a 25-year population based study. J. Urol. 188 , 247–252 (2012).
Tasian, G. E. et al. Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. Clin. J. Am. Soc. Nephrol. 11 , 488–496 (2016).
Article CAS PubMed PubMed Central Google Scholar
Edvardsson, V. O., Indridason, O. S., Haraldsson, G., Kjartansson, O. & Palsson, R. Temporal trends in the incidence of kidney stone disease. Kidney Int. 83 , 146–152 (2013).
Edvardsson, V. O., Ingvarsdottir, S. E., Palsson, R. & Indridason, O. S. Incidence of kidney stone disease in Icelandic children and adolescents from 1985 to 2013: results of a nationwide study. Pediatr. Nephrol. 33 , 1375–1384 (2018).
Yasui, T., Iguchi, M., Suzuki, S. & Kohri, K. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology 71 , 209–213 (2008).
Krambeck, A. E. et al. Effect of age on the clinical presentation of incident symptomatic urolithiasis in the general population. J. Urol. 189 , 158–164 (2013).
Brisbane, W., Bailey, M. R. & Sorensen, M. D. An overview of kidney stone imaging techniques. Nat. Rev. Urol. 13 , 654–662 (2016).
Semins, M. J. & Matlaga, B. R. Kidney stones during pregnancy. Nat. Rev. Urol. 11 , 163–168 (2014).
Smith-Bindman, R. et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N. Engl. J. Med. 371 , 1100–1110 (2014).
Sternberg, K. M. et al. Is hydronephrosis on ultrasound predictive of ureterolithiasis in patients with renal colic? J. Urol. 196 , 1149–1152 (2016).
Chi, T., Miller, J. & Stoller, M. L. Randall plaque versus renal stone? Transl. Androl. Urol. 1 , 66–70 (2012).
PubMed PubMed Central Google Scholar
Dhondup, T. et al. Risk of ESRD and mortality in kidney and bladder stone formers. Am. J. Kidney Dis. 72 , 790–797 (2018).
Emamian, S. A., Nielsen, M. B., Pedersen, J. F. & Ytte, L. Sonographic evaluation of renal appearance in 665 adult volunteers. Correlation age obesity. Acta Radiol. 34 , 482–485 (1993).
Oshibuchi, M., Nishi, F., Sato, M., Ohtake, H. & Okuda, K. Frequency of abnormalities detected by abdominal ultrasound among Japanese adults. J. Gastroenterol. Hepatol. 6 , 165–168 (1991).
Buchholz, N. P. et al. The prevalence of silent kidney stones — an ultrasonographic screening study. J. Pak. Med. Assoc. 53 , 24–25 (2003).
CAS PubMed Google Scholar
Passerotti, C. et al. Ultrasound versus computerized tomography for evaluating urolithiasis. J. Urol. 182 , 1829–1834 (2009).
Brenner, D. J. & Hall, E. J. Computed tomography — an increasing source of radiation exposure. N. Engl. J. Med. 357 , 2277–2284 (2007).
Lorenz, E. C. et al. Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol. Dial. Transpl. 26 , 2695–2700 (2011).
Article Google Scholar
Durbin, J. M. et al. Genitourinary abnormalities in an asymptomatic screening population: findings on virtual colonoscopy. Clin. Nephrol. 77 , 204–210 (2012).
D’Costa, M. R. et al. Symptomatic and radiographic manifestations of kidney stone recurrence and their prediction by risk factors: a prospective cohort study. J. Am. Soc. Nephrol. 30 , 1251–1260 (2019).
Li, X. et al. Outcomes of long-term follow-up of asymptomatic renal stones and prediction of stone-related events. BJU Int. 123 , 485–492 (2019).
Goldsmith, Z. G. & Lipkin, M. E. When (and how) to surgically treat asymptomatic renal stones. Nat. Rev. Urol. 9 , 315–320 (2012).
Selby, M. G. et al. Quantification of asymptomatic kidney stone burden by computed tomography for predicting future symptomatic stone events. Urology 85 , 45–50 (2015).
Dropkin, B. M., Moses, R. A., Sharma, D. & Pais, V. M. Jr. The natural history of nonobstructing asymptomatic renal stones managed with active surveillance. J. Urol. 193 , 1265–1269 (2015).
Kang, H. W. et al. Natural history of asymptomatic renal stones and prediction of stone related events. J. Urol. 189 , 1740–1746 (2013).
Burgher, A., Beman, M., Holtzman, J. L. & Monga, M. Progression of nephrolithiasis: long-term outcomes with observation of asymptomatic calculi. J. Endourol. 18 , 534–539 (2004).
Assimos, D. et al. Surgical management of stones: American Urological Association/Endourological Society guideline, part I. J. Urol. 196 , 1153–1160 (2016).
Assimos, D. et al. Surgical management of stones: American Urological Association/Endourological Society guideline, part II. J. Urol. 196 , 1161–1169 (2016).
Curhan, G. C., Willett, W. C., Speizer, F. E. & Stampfer, M. J. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 59 , 2290–2298 (2001).
Chooi, Y. C., Ding, C. & Magkos, F. The epidemiology of obesity. Metabolism 92 , 6–10 (2019).
Taylor, E. N., Stampfer, M. J. & Curhan, G. C. Obesity, weight gain, and the risk of kidney stones. JAMA 293 , 455–462 (2005).
Geiss, L. S. et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 312 , 1218–1226 (2014).
Taylor, E. N., Stampfer, M. J. & Curhan, G. C. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 68 , 1230–1235 (2005).
Meyer, K. A. et al. Twenty-two-year population trends in sodium and potassium consumption: the Minnesota Heart Survey. J. Am. Heart Assoc. 2 , e000478 (2013).
Article PubMed PubMed Central CAS Google Scholar
Sorensen, M. D. et al. Impact of nutritional factors on incident kidney stone formation: a report from the WHI OS. J. Urol. 187 , 1645–1649 (2012).
Muldowney, F. P., Freaney, R. & Moloney, M. F. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 22 , 292–296 (1982).
Curhan, G. C., Willett, W. C., Speizer, F. E., Spiegelman, D. & Stampfer, M. J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann. Intern. Med. 126 , 497–504 (1997).
Daniel, C. R., Cross, A. J., Koebnick, C. & Sinha, R. Trends in meat consumption in the USA. Public. Health Nutr. 14 , 575–583 (2011).
Taylor, E. N., Stampfer, M. J. & Curhan, G. C. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J. Am. Soc. Nephrol. 15 , 3225–3232 (2004).
Breslau, N. A., Brinkley, L., Hill, K. D. & Pak, C. Y. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J. Clin. Endocrinol. Metab. 66 , 140–146 (1988).
Gross, L. S., Li, L., Ford, E. S. & Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am. J. Clin. Nutr. 79 , 774–779 (2004).
Curhan, G. C., Willett, W. C., Knight, E. L. & Stampfer, M. J. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 164 , 885–891 (2004).
Lemann, J. Jr., Piering, W. F. & Lennon, E. J. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N. Engl. J. Med. 280 , 232–237 (1969).
Bleich, S. N., Wang, Y. C., Wang, Y. & Gortmaker, S. L. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am. J. Clin. Nutr. 89 , 372–381 (2009).
Ferraro, P. M., Taylor, E. N., Gambaro, G. & Curhan, G. C. Soda and other beverages and the risk of kidney stones. Clin. J. Am. Soc. Nephrol. 8 , 1389–1395 (2013).
Fakheri, R. J. & Goldfarb, D. S. Ambient temperature as a contributor to kidney stone formation: implications of global warming. Kidney Int. 79 , 1178–1185 (2011).
Soucie, J. M., Coates, R. J., McClellan, W., Austin, H. & Thun, M. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am. J. Epidemiol. 143 , 487–495 (1996).
Soucie, J. M., Thun, M. J., Coates, R. J., McClellan, W. & Austin, H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 46 , 893–899 (1994).
Boscolo-Berto, R. et al. Do weather conditions influence the onset of renal colic? A novel approach to analysis. Urol. Int. 80 , 19–25 (2008).
Chen, Y. K., Lin, H. C., Chen, C. S. & Yeh, S. D. Seasonal variations in urinary calculi attacks and their association with climate: a population based study. J. Urol. 179 , 564–569 (2008).
Chauhan, V., Eskin, B., Allegra, J. R. & Cochrane, D. G. Effect of season, age, and gender on renal colic incidence. Am. J. Emerg. Med. 22 , 560–563 (2004).
Brikowski, T. H., Lotan, Y. & Pearle, M. S. Climate-related increase in the prevalence of urolithiasis in the United States. Proc. Natl Acad. Sci. USA 105 , 9841–9846 (2008).
Rule, A. D. et al. The ROKS nomogram for predicting a second symptomatic stone episode. J. Am. Soc. Nephrol. 25 , 2878–2886 (2014).
Vaughan, L. E. et al. Predictors of symptomatic kidney stone recurrence after the first and subsequent episodes. Mayo Clin. Proc. 94 , 202–210 (2019).
Singh, P. et al. Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clin. Proc. 90 , 1356–1365 (2015).
Lieske, J. C. et al. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. 9 , 2141–2146 (2014).
Knoll, T. et al. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J. Urol. 185 , 1304–1311 (2011).
Daudon, M. et al. Sex- and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urol. Res. 23 , 319–326 (1995).
Ye, Z. et al. The status and characteristics of urinary stone composition in China. BJU Int. 125 , 801–809 (2019).
Article CAS Google Scholar
Worcester, E. M., Bergsland, K. J., Gillen, D. L. & Coe, F. L. Mechanism for higher urine pH in normal women compared with men. Am. J. Physiol. Renal Physiol 314 , F623–F629 (2018).
Kohri, K. et al. Relationship between metabolic acidosis and calcium phosphate urinary stone formation in women. Int. Urol. Nephrol. 23 , 307–316 (1991).
Maalouf, N. M. Metabolic syndrome and the genesis of uric acid stones. J. Ren. Nutr. 21 , 128–131 (2011).
Sakhaee, K. & Maalouf, N. M. Metabolic syndrome and uric acid nephrolithiasis. Semin. Nephrol. 28 , 174–180 (2008).
Morgan, M. S. & Pearle, M. S. Medical management of renal stones. BMJ 352 , i52 (2016).
Pearle, M. S. et al. Medical management of kidney stones: AUA guideline. J. Urol. 192 , 316–324 (2014).
Qaseem, A. et al. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 161 , 659–667 (2014).
Skolarikos, A. et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur. Urol. 67 , 750–763 (2015).
D’Costa, M. R., Pais, V. M. & Rule, A. D. Leave no stone unturned: defining recurrence in kidney stone formers. Curr. Opin. Nephrol. Hypertens. 28 , 148–153 (2019).
Sternberg, K. M. et al. Ultrasonography significantly overestimates stone size when compared to low-dose, noncontrast computed tomography. Urology 95 , 67–71 (2016).
Williams, R. E. Long-term survey of 538 patients with upper urinary tract stone. Br. J. Urol. 35 , 416–437 (1963).
Marshall, V., White, R. H., De Saintonge, M. C., Tresidder, G. C. & Blandy, J. P. The natural history of renal and ureteric calculi. Br. J. Urol. 47 , 117–124 (1975).
Sutherland, J. W., Parks, J. H. & Coe, F. L. Recurrence after a single renal stone in a community practice. Min. Electrolyte Metab. 11 , 267–269 (1985).
CAS Google Scholar
Steyerberg, E. W. et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21 , 128–138 (2010).
Iremashvili, V. et al. External validation of the recurrence of kidney stone nomogram in a surgical cohort. J. Endourol. 33 , 475–479 (2019).
Emmott, A. S., Brotherhood, H. L., Paterson, R. F., Lange, D. & Chew, B. H. Complications, re-intervention rates, and natural history of residual stone fragments after percutaneous nephrolithotomy. J. Endourol. 32 , 28–32 (2018).
Chew, B. H. et al. Natural history, complications and re-intervention rates of asymptomatic residual stone fragments after ureteroscopy: a report from the EDGE research consortium. J. Urol. 195 , 982–986 (2016).
Alexander, C. E., Gowland, S., Cadwallader, J., Reynard, J. M. & Turney, B. W. Shock wave lithotripsy (SWL): outcomes from a national SWL database in New Zealand. BJU Int. 117 (Suppl. 4), 76–81 (2016).
Javanmard, B., Kashi, A. H., Mazloomfard, M. M., Ansari Jafari, A. & Arefanian, S. Retrograde intrarenal surgery versus shock wave lithotripsy for renal stones smaller than 2 cm: a randomized clinical trial. Urol. J. 13 , 2823–2828 (2016).
PubMed Google Scholar
Albala, D. M. et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J. Urol. 166 , 2072–2080 (2001).
Bozzini, G. et al. A prospective randomized comparison among SWL, PCNL and RIRS for lower calyceal stones less than 2 cm: a multicenter experience: a better understanding on the treatment options for lower pole stones. World J. Urol. 35 , 1967–1975 (2017).
Ferraro, P. M., Curhan, G. C., D’Addessi, A. & Gambaro, G. Risk of recurrence of idiopathic calcium kidney stones: analysis of data from the literature. J. Nephrol. 30 , 227–233 (2017).
Fink, H. A. et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians clinical guideline. Ann. Intern. Med. 158 , 535–543 (2013).
Download references
Author information
Authors and affiliations.
Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA
Charat Thongprayoon & Andrew D. Rule
Department of Urology, Indiana University, Indianapolis, IN, USA
Amy E. Krambeck
Division of Epidemiology, Mayo Clinic, Rochester, MN, USA
Andrew D. Rule
You can also search for this author in PubMed Google Scholar
Contributions
C.T. and A.D.R. researched data for the article. All authors contributed to discussion of the article’s content, writing and review/editing of the manuscript before submission.
Corresponding author
Correspondence to Andrew D. Rule .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Nephrology thanks D. Goldfarb and R. Pálsson for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Recurrence of Kidney Stone (ROKS) tool: https://qxmd.com/calculate/calculator_438/roks-recurrence-of-kidney-stone-2018
A procedure for treating stones in the kidney or ureter using a high-energy shock wave from outside the body to break stones into fragments that are small enough to spontaneously pass in urine.
A procedure in which a small scope is inserted into the ureter via the urethra and bladder to diagnose and treat a variety of problems in the urinary tract. In the case of urinary stones, it allows the urologist to actually look into the ureter or kidney, find the stone and remove or fragment the stone.
A procedure used to remove kidney stones from the body when they cannot pass spontaneously.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Thongprayoon, C., Krambeck, A.E. & Rule, A.D. Determining the true burden of kidney stone disease. Nat Rev Nephrol 16 , 736–746 (2020). https://doi.org/10.1038/s41581-020-0320-7
Download citation
Accepted : 23 June 2020
Published : 04 August 2020
Issue Date : December 2020
DOI : https://doi.org/10.1038/s41581-020-0320-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The sox4/ezh2/slc7a11 signaling axis mediates ferroptosis in calcium oxalate crystal deposition-induced kidney injury.
- Xinzhou Yan
Journal of Translational Medicine (2024)
Cymbopogon proximus and Petroselinum crispum seed ethanolic extract/Gum Arabic nanogel emulsion: Preventing ethylene glycol and ammonium chloride-induced urolithiasis in rats
- Hend A. Essa
- Alaa M. Ali
- Mona A. Saied
Urolithiasis (2024)
Surgical procedure and recurrence of upper urinary tract stone: a national-wide study based on hospitalized patients
- Ming-Hui Zhao
World Journal of Urology (2024)
Gaps in kidney stone disease management: From clinical theory to patient reality
- Agnieszka Pozdzik
- Viridiana Grillo
- Khashayar Sakhaee
N-acetylcysteine regulates oxalate induced injury of renal tubular epithelial cells through CDKN2B/TGF-β/SMAD axis
- Jingbo Zhang
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

VICTORIA J. SHARP, MD, DANIEL K. LEE, MD, AND ERIC J. ASKELAND, MD
A more recent article on office-based urinalysis is available.
Am Fam Physician. 2014;90(8):542-547
Author disclosure: No relevant financial affiliations.
Urinalysis is useful in diagnosing systemic and genitourinary conditions. In patients with suspected microscopic hematuria, urine dipstick testing may suggest the presence of blood, but results should be confirmed with a microscopic examination. In the absence of obvious causes, the evaluation of microscopic hematuria should include renal function testing, urinary tract imaging, and cystoscopy. In a patient with a ureteral stent, urinalysis alone cannot establish the diagnosis of urinary tract infection. Plain radiography of the kidneys, ureters, and bladder can identify a stent and is preferred over computed tomography. Asymptomatic bacteriuria is the isolation of bacteria in an appropriately collected urine specimen obtained from a person without symptoms of a urinary tract infection. Treatment of asymptomatic bacteriuria is not recommended in nonpregnant adults, including those with prolonged urinary catheter use.
Urinalysis with microscopy has proven to be an invaluable tool for the clinician. Urine dipstick testing and microscopy are useful for the diagnosis of several genitourinary and systemic conditions. 1 , 2 In 2005, a comprehensive review of urinalysis was published in this journal. 3 This article presents a series of case scenarios that illustrate how primary care physicians can utilize the urinalysis in common clinical situations.
Microscopic Hematuria: Case 1
Microscopic hematuria is common and has a broad differential diagnosis, ranging from completely benign causes to potentially invasive malignancy. Causes of hematuria can be classified as glomerular, renal, or urologic 3 – 5 ( Table 1 6 ) . The prevalence of asymptomatic microscopic hematuria varies among populations from 0.18% to 16.1%. 4 The American Urological Association (AUA) defines asymptomatic microscopic hematuria as three or more red blood cells per high-power field in a properly collected specimen in the absence of obvious causes such as infection, menstruation, vigorous exercise, medical renal disease, viral illness, trauma, or a recent urologic procedure. 5 Microscopic confirmation of a positive dipstick test for microscopic hematuria is required. 5 , 7
DIAGNOSTIC APPROACH
Case 1: microscopic hematuria.
A 58-year-old truck driver with a 30-year history of smoking one pack of cigarettes per day presents for a physical examination. He reports increased frequency of urination and nocturia, but does not have gross hematuria. Physical examination reveals an enlarged prostate. Results of his urinalysis with microscopy are shown in Table 2 .
Based on this patient's history, symptoms, and urinalysis findings, which one of the following is the most appropriate next step?
A. Repeat urinalysis in six months.
B. Obtain blood urea nitrogen and creatinine levels, perform computed tomographic urography, and refer for cystoscopy.
C. Treat with an antibiotic and repeat the urinalysis with microscopy.
D. Inform him that his enlarged prostate is causing microscopic hematuria, and that he can follow up as needed.
E. Perform urine cytology to evaluate for bladder cancer.
The correct answer is B .
For the patient in case 1 , because of his age, clinical history, and lack of other clear causes, the most appropriate course of action is to obtain blood urea nitrogen and creatinine levels, perform computed tomographic urography, and refer the patient for cystoscopy. 5 An algorithm for diagnosis, evaluation, and follow-up of patients with asymptomatic microscopic hematuria is presented in Figure 1 . 5 The AUA does not recommend repeating urinalysis with microscopy before the workup, especially in patients who smoke, because tobacco use is a risk factor for urothelial cancer ( Table 3 ) . 5
A previous article in American Family Physician reviewed the American College of Radiology's Appropriateness Criteria for radiologic evaluation of microscopic hematuria. 8 Computed tomographic urography is the preferred imaging modality for the evaluation of patients with asymptomatic microscopic hematuria. 5 , 8 It has three phases that can detect various causes of hematuria. The non–contrast-enhanced phase is optimal for detecting stones in the urinary tract; the nephrographic phase is useful for detecting renal masses, such as renal cell carcinoma; and the delayed phase outlines the collecting system of the urinary tract and can help detect urothelial malignancies of the upper urinary tract. 9 Although the delayed phase can detect some bladder masses, it should not replace cystoscopy in the evaluation for bladder malignancy. 9 After a negative microscopic hematuria workup, the patient should continue to be followed with yearly urinalysis until at least two consecutive normal results are obtained. 5
In patients with microscopic hematuria, repeating urinalysis in six months or treating empirically with antibiotics could delay treatment of potentially curable diseases. It is unwise to assume that benign prostatic hyperplasia is the explanation for hematuria, particularly because patients with this condition typically have risk factors for malignancy. Although urine cytology is typically part of the urologic workup, it should be performed at the time of cystoscopy; the AUA does not recommend urine cytology as the initial test. 5
Dysuria and Flank Pain After Lithotripsy: Case 2
After ureteroscopy with lithotripsy, a ureteral stent is often placed to maintain adequate urinary drainage. 10 The stent has one coil that lies in the bladder and another that lies in the renal pelvis. Patients with ureteral stents may experience urinary frequency, urgency, dysuria, flank pain, and hematuria. 10 They may have dull flank pain that becomes sharp with voiding. This phenomenon occurs because the ureteral stent bypasses the normal nonrefluxing uretero-vesical junction, resulting in transmission of pressure to the renal pelvis with voiding. Approximately 80% of patients with a ureteral stent experience stent-related pain that affects their daily activities. 11
POTENTIALLY MISLEADING URINALYSIS
Case 2: dysuria and flank pain after lithotripsy.
A 33-year-old woman with a history of nephrolithiasis presents with a four-week history of urinary frequency, urgency, urge incontinence, and dysuria. She recently had ureteroscopy with lithotripsy of a 9-mm obstructing left ureteral stone; she does not know if a ureteral stent was placed. She has constant dull left flank pain that becomes sharp with voiding. Results of her urinalysis with microscopy are shown in Table 4 .
A. Treat with three days of ciprofloxacin (Cipro), and tailor further antibiotic therapy according to culture results.
B. Treat with 14 days of ciprofloxacin, and tailor further antibiotic therapy according to culture results.
C. Obtain a urine culture and perform plain radiography of the kidneys, ureters, and bladder.
D. Perform a 24-hour urine collection for a metabolic stone workup.
E. Perform computed tomography.
The correct answer is C .
The presence of a ureteral stent causes mucosal irritation and inflammation; thus, findings of leukocyte esterase with white and red blood cells are not diagnostic for urinary tract infection, and a urine culture is required. In this setting, plain radiography of the kidneys, ureters, and bladder would be useful to determine the presence of a stent. If a primary care physician identifies a neglected ureteral stent, prompt urologic referral is indicated for removal. Retained ureteral stents may become encrusted, and resultant stone formation may lead to obstruction. 10
Flank discomfort and recent history of urinary tract manipulation suggest that this is not an uncomplicated urinary tract infection; therefore, a three-day course of antibiotics is inadequate. Although flank pain and urinalysis suggest possible pyelonephritis, this patient should not be treated for simple pyelonephritis in the absence of radiography to identify a stent. A metabolic stone workup may be useful for prevention of future kidney stones, but it is not indicated in the acute setting. Finally, although computed tomography would detect a ureteral stent, it is not preferred over radiography because it exposes the patient to unnecessary radiation. Typically, microscopic hematuria requires follow-up to ensure that there is not an underlying treatable etiology. In this case , the patient's recent ureteroscopy with lithotripsy is likely the etiology.
Urinalysis in a Patient Performing Clean Intermittent Catheterization: Case 3
Case 3: urinalysis in a patient performing clean intermittent catheterization.
A 49-year-old man who has a history of neurogenic bladder due to a spinal cord injury and who performs clean intermittent catheterization visits your clinic for evaluation. He reports that he often has strong-smelling urine, but has no dysuria, urge incontinence, fever, or suprapubic pain. Results of his urinalysis with microscopy are shown in Table 5 .
A. Inform the patient that he has a urinary tract infection, obtain a urine culture, and treat with antibiotics.
B. Refer him to a urologist for evaluation of a complicated urinary tract infection.
C. Perform computed tomography of the abdomen and pelvis to evaluate for kidney or bladder stones.
D. Inform him that no treatment is needed.
E. Obtain a serum creatinine level to evaluate for chronic kidney disease.
The correct answer is D .
Although the urinalysis results are consistent with a urinary tract infection, the clinical history suggests asymptomatic bacteriuria. Asymptomatic bacteriuria is the isolation of bacteria in an appropriately collected urine specimen obtained from a person without symptoms of a urinary tract infection. 12 The presence of bacteria in the urine after prolonged catheterization has been well described; one study of 605 consecutive weekly urine specimens from 20 chronically catheterized patients found that 98% contained high concentrations of bacteria, and 77% were polymicrobial. 13
Similar results have been reported in patients who perform clean intermittent catheterization; another study of 1,413 urine cultures obtained from 407 patients undergoing clean intermittent catheterization found that 50.6% contained bacteria. 14 Guidelines from the Infectious Diseases Society of America recommend against treatment of asymptomatic bacteriuria in nonpregnant patients with spinal cord injury who are undergoing clean intermittent catheterization or in those using a chronic indwelling catheter. 12
In the absence of symptoms of a urinary tract infection or nephrolithiasis, there is no need to culture the urine, treat with antibiotics, refer to a urologist, or perform imaging of the abdomen and pelvis. There is no reason to suspect acute kidney injury in this setting; thus, measurement of the serum creatinine level is also unnecessary.
Data Sources : Literature searches were performed in PubMed using the terms urinalysis review, urinalysis interpretation, microscopic hematuria, CT urogram, urinary crystals, indwelling ureteral stent, asymptomatic bacteriuria, and bacteriuria with catheterization. Guidelines from the American Urological Association were also reviewed. Search dates: October 2012 and June 2013.
Wu X. Urinalysis: a review of methods and procedures. Crit Care Nurs Clin North Am. 2010;22(1):121-128.
Hardy PE. Urinalysis interpretation. Neonatal Netw. 2010;29(1):45-49.
Simerville JA, Maxted WC, Pahira JJ. Urinalysis: a comprehensive review [published correction appears in Am Fam Physician . 2006;74(7):1096]. Am Fam Physician. 2005;71(6):1153-1162.
Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348(23):2330-2338.
American Urological Association. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults. http://www.auanet.org/education/asymptomatic-microhematuria.cfm . Accessed June 6, 2014.
Ahmed Z, Lee J. Asymptomatic urinary abnormalities. Hematuria and proteinuria. Med Clin North Am. 1997;81(3):641-652.
Rao PK, Jones JS. How to evaluate ‘dipstick hematuria’: what to do before you refer. Cleve Clin J Med. 2008;75(3):227-233.
Choyke PL. Radiologic evaluation of hematuria: guidelines from the American College of Radiology's Appropriateness Criteria. Am Fam Physician. 2008;78(3):347-352.
Sadow CA, Wheeler SC, Kim J, Ohno-Machado L, Silverman SG. Positive predictive value of CT urography in the evaluation of upper tract urothelial cancer. AJR Am J Roentgenol. 2010;195(5):W337-W343.
Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179(2):424-430.
Joshi HB, Stainthorpe A, MacDonagh RP, Keeley FX, Timoney AG, Barry MJ. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169(3):1065-1069.
Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults [published correction appears in Clin Infect Dis . 2005;40(10):1556]. Clin Infect Dis. 2005;40(5):643-654.
Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723.
Bakke A, Digranes A. Bacteriuria in patients treated with clean intermittent catheterization. Scand J Infect Dis. 1991;23(5):577-582.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2014 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

Like much of medicine, kidney stone disease is organized around a set of phenotypes, idiopathic calcium oxalate stone formers for example. A useful if medieval Platonic idealization, when looked at closely these phenotypes invariably disintegrate into what one might call medical atomism – the tyranny of the individual case.
When asked for an example of an idiopathic calcium oxalate stone former I never can find exactly the ‘perfect’ case but rather someone who fits well enough but with – how shall I put this? – rough edges, stray facts that do not fit the mold.
What this really means is obvious. Every physician practices ‘personalized medicine’ and, like most medieval intellectual conceptions, phenotypes have value more as metaphor than as a guide to the day’s work.
What it also means in that every patient is a node in the matrix of pathophysiology, clinical manifestations, and treatment complexities that make up this field, being a place in which all together give rise to the one single manifestation – crystal formation – which defines our work.
So I did not bring these cases, suitably disguised, from my precious museum but simply from recent outpatient sessions, as I find in almost every patient more or less a unique set of oddments that serve very well as special instances of general formulations – the reason cases are so important – concerning clinical evaluation, the technical business of pathophysiology, and the elaborate compromises of therapeutics. Every case is an ‘everyman’, and ‘everywoman’. You just have to look close and each one is a universe.
Ages are deliberately vague, dates for labs are presented from 0, the first one, upward in days or weeks or more. Occupations are similar but not the same as the real one – because people deserve privacy. Lab data, stone composition, rates of formation, treatments, and outcomes are rigorously exact.
Case 1: A Stone Former
As you will see, this is a person with considerable numbers of stone attacks who has certainly produced large stones in the past, but he posed major problems in deciding if stones were active and is therefore a perfect place to start. His many laboratory abnormalities are just wonderful for thinking about stone pathophysiology.
Case 3: A Success Story
This is a first for the site, and perhaps it should have been a feature long ago. After all the generalizations and reviews there is something wonderful about a single instance that contains all the elements of a topic in the kind of instructive detail we can get only in life itself. Pat – who has permitted me to use his name and data – forms calcium stones and has idiopathic hypercalciuria and a job that makes hydration a problem.
Case 5: Severe Hyperoxaluria
Severe hyperoxaluria – always worrisome, never something to dismiss or even wait a long time thinking about. The Vegetable Seller’ by Flemish painter Joachim Beuckelaer (c.1534-1574) seems a perfect image for this exercise in vegetable excess. He was never very famous but influential concerning food and kitchen scenes. Jill Harris (pictured right) co-authored this article with me. Kidney Stone History This 47 year old woman had her first manifest stone 12 years before I first met her.
Case 2: A Calcium Oxalate Stone Former
CLINICAL FINDINGS: A man in his fifties formed his first stone in the early 2000’s and his last 6 months ago. There was a single passage event a year or two after the first stone at which time he was given hydrochlorothiazide 25 mg daily.
Case 4: Medullary Sponge Kidney
Medullary sponge kidney (MSK) is more spoken about than witnessed, and more witnessed than accurately diagnosed. This patient adds to the 12 we have described in our publication, and adds also in having a very long and evolving history with one of us (FLC). We write for a general audience yet hope to include a level of detail that satisfies physicians and scientists. Here, we may fail of clarity to the one audience or of a sufficiency to the other because the disease is complex.
Case 6: Bariatric Surgery and Kidney Injury
Bariatric surgeries can injure kidneys by raising urine oxalate excretion. This latter causes kidney stones, and raises risk of acute and chronic oxalate nephropathy. Overall, their benefits far outweigh these risks, especially when patients and physicians take proper precautions.But risk lurks as if in shadows, and waits on accident. The patient here inadvertently raised her risk of injury. Like all instances this one is just that: Opportunity to inspect the details of an undesired outcome so as to reduce the chance it will happen to others.
- WEATHER ALERT High Surf Advisory Full Story
- Circle of Health

More kids are getting kidney stones, research shows. Here's why and how to fight them

LOS ANGELES (KABC) -- Most people think kidney stones are something that happens mainly in adults. But that's changed dramatically over the last 20 years.
They may be less common in children, but that's changing. And for kids, it can be a lifelong battle.
Four-year-old Alex Zellers may be a little guy, but he's been dealing with a very big problem.
Alex's parents say their son has had kidney stones, which have been very painful.
One stone in his kidney was the size of a golf ball. The other, in his bladder, was the size of a lacrosse ball.
"It's just like a giant dense egg. It's just a big mass," described Kate, Alex's mother.
Alex was born with a genetic disease called cystinuria.
"Your body doesn't absorb certain amino acids and that cystine accumulates and crystallizes in the urine forming stones early in life," said Dr. Greg Tasian, a pediatric urologist with Children's Hospital of Philadelphia.
Stones can cause higher risk of high blood pressure and heart attacks, as well as higher risk of fractures and loss of kidney function.
Alex's symptoms included recurring urinary tract infections and blood in his urine. There is no cure.
"You develop stones very early in life and that continues through the lifespan," said Dr. Tasian.
The stones Alex had were so large, they had to be surgically removed.
And although Alex's condition is rare, Dr. Tasian says he is seeing more and more kids with kidney stones.
The cause? A combination of factors, including kids eating more ultra processed foods, overuse of antibiotics and hotter temperatures causing dehydration.
"As the world becomes warmer through climate change, that is expected to increase the number of stones," Dr. Tasian said.
Common symptoms of kidney stones include abdominal, flank, or groin pain, blood in the urine, frequent urination, nausea and vomiting.
But experts point out that kidney stones can impact different children in different ways, making diagnosis challenging.
The three most important things you can do are: drink plenty of water, drink less sugary drinks, and decrease your salt intake.
As for Alex, while he'll always be at risk for developing stones, with careful watching and medication, they should be able to control them.
Studies show that both boys and girls are at risk of kidney stones, but they tend to happen more in teen girls.
Related Topics
- HEALTH & FITNESS
- CHILDREN'S HEALTH
- CIRCLE OF HEALTH
- MEDICAL RESEARCH
Circle Of Health

ADHD patients still face drug shortages due to increased demand

Black Maternal Health Week spotlights challenges faced amid pregnancy

What causes scary dreams and how can you prevent them?

SoCal families make medical history with organ donation swap
Top stories.

UCLA protesters violently clash before law enforcement intervenes
- 25 minutes ago

Santa Ana family mourns victims of alleged serial killer in Mexico

Fire damages fire station in Huntington Park

Tesla cuts entire Supercharger team in latest round of layoffs
- 2 hours ago

Man convicted in cartel-related murders that left 3 shot, burned in OC
- 13 minutes ago
Americans older than 60 lost $3.4 billion to scams in 2023: FBI
Police release new surveillance video in Long Beach killing of teen
Record number of sea lions swarm CA pier, highest in 15 years
- Patient Care & Health Information
- Diseases & Conditions
- Kidney stones
Female urinary system
Your urinary system includes the kidneys, ureters, bladder and urethra. The urinary system removes waste from the body through urine. The kidneys are located toward the back of the upper abdomen. They filter waste and fluid from the blood and produce urine. Urine moves from the kidneys through narrow tubes to the bladder. These tubes are called the ureters. The bladder stores urine until it's time to urinate. Urine leaves the body through another small tube called the urethra.
Male urinary system
Kidney stones (also called renal calculi, nephrolithiasis or urolithiasis) are hard deposits made of minerals and salts that form inside your kidneys.
Diet, excess body weight, some medical conditions, and certain supplements and medications are among the many causes of kidney stones. Kidney stones can affect any part of your urinary tract — from your kidneys to your bladder. Often, stones form when the urine becomes concentrated, allowing minerals to crystallize and stick together.
Passing kidney stones can be quite painful, but the stones usually cause no permanent damage if they're recognized in a timely fashion. Depending on your situation, you may need nothing more than to take pain medication and drink lots of water to pass a kidney stone. In other instances — for example, if stones become lodged in the urinary tract, are associated with a urinary infection or cause complications — surgery may be needed.
Your doctor may recommend preventive treatment to reduce your risk of recurrent kidney stones if you're at increased risk of developing them again.
Products & Services
- A Book: Mayo Clinic Book of Home Remedies
Kidney stones form in your kidneys. As stones move into your ureters — the thin tubes that allow urine to pass from your kidneys to your bladder — signs and symptoms can result. Signs and symptoms of kidney stones can include severe pain, nausea, vomiting, fever, chills and blood in your urine.
A kidney stone usually will not cause symptoms until it moves around within the kidney or passes into one of the ureters. The ureters are the tubes that connect the kidneys and bladder.
If a kidney stone becomes lodged in the ureters, it may block the flow of urine and cause the kidney to swell and the ureter to spasm, which can be very painful. At that point, you may experience these symptoms:
- Severe, sharp pain in the side and back, below the ribs
- Pain that radiates to the lower abdomen and groin
- Pain that comes in waves and fluctuates in intensity
- Pain or burning sensation while urinating
Other signs and symptoms may include:
- Pink, red or brown urine
- Cloudy or foul-smelling urine
- A persistent need to urinate, urinating more often than usual or urinating in small amounts
- Nausea and vomiting
- Fever and chills if an infection is present
Pain caused by a kidney stone may change — for instance, shifting to a different location or increasing in intensity — as the stone moves through your urinary tract.
When to see a doctor
Make an appointment with your doctor if you have any signs and symptoms that worry you.
Seek immediate medical attention if you experience:
- Pain so severe that you can't sit still or find a comfortable position
- Pain accompanied by nausea and vomiting
- Pain accompanied by fever and chills
- Blood in your urine
- Difficulty passing urine
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest health information from Mayo Clinic delivered to your inbox.
Subscribe for free and receive your in-depth guide to digestive health, plus the latest on health innovations and news. You can unsubscribe at any time. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth digestive health guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest health news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Kidney stones often have no definite, single cause, although several factors may increase your risk.
Kidney stones form when your urine contains more crystal-forming substances — such as calcium, oxalate and uric acid — than the fluid in your urine can dilute. At the same time, your urine may lack substances that prevent crystals from sticking together, creating an ideal environment for kidney stones to form.
Types of kidney stones
Knowing the type of kidney stone you have helps determine its cause, and may give clues on how to reduce your risk of getting more kidney stones. If possible, try to save your kidney stone if you pass one so that you can bring it to your doctor for analysis.
Types of kidney stones include:
Calcium stones. Most kidney stones are calcium stones, usually in the form of calcium oxalate. Oxalate is a substance made daily by your liver or absorbed from your diet. Certain fruits and vegetables, as well as nuts and chocolate, have high oxalate content.
Dietary factors, high doses of vitamin D, intestinal bypass surgery and several metabolic disorders can increase the concentration of calcium or oxalate in urine.
Calcium stones may also occur in the form of calcium phosphate. This type of stone is more common in metabolic conditions, such as renal tubular acidosis. It may also be associated with certain medications used to treat migraines or seizures, such as topiramate (Topamax, Trokendi XR, Qudexy XR).
- Struvite stones. Struvite stones form in response to a urinary tract infection. These stones can grow quickly and become quite large, sometimes with few symptoms or little warning.
- Uric acid stones. Uric acid stones can form in people who lose too much fluid because of chronic diarrhea or malabsorption, those who eat a high-protein diet, and those with diabetes or metabolic syndrome. Certain genetic factors also may increase your risk of uric acid stones.
- Cystine stones. These stones form in people with a hereditary disorder called cystinuria that causes the kidneys to excrete too much of a specific amino acid.
Risk factors
Factors that increase your risk of developing kidney stones include:
- Family or personal history. If someone in your family has had kidney stones, you're more likely to develop stones, too. If you've already had one or more kidney stones, you're at increased risk of developing another.
- Dehydration. Not drinking enough water each day can increase your risk of kidney stones. People who live in warm, dry climates and those who sweat a lot may be at higher risk than others.
- Certain diets. Eating a diet that's high in protein, sodium (salt) and sugar may increase your risk of some types of kidney stones. This is especially true with a high-sodium diet. Too much salt in your diet increases the amount of calcium your kidneys must filter and significantly increases your risk of kidney stones.
- Obesity. High body mass index (BMI), large waist size and weight gain have been linked to an increased risk of kidney stones.
- Digestive diseases and surgery. Gastric bypass surgery, inflammatory bowel disease or chronic diarrhea can cause changes in the digestive process that affect your absorption of calcium and water, increasing the amounts of stone-forming substances in your urine.
- Other medical conditions such as renal tubular acidosis, cystinuria, hyperparathyroidism and repeated urinary tract infections also can increase your risk of kidney stones.
- Certain supplements and medications, such as vitamin C, dietary supplements, laxatives (when used excessively), calcium-based antacids, and certain medications used to treat migraines or depression, can increase your risk of kidney stones.
Kidney stones care at Mayo Clinic
- Goldman L, et al., eds. Nephrolithiasis. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 20, 2020.
- Kidney stones. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/urologic-diseases/kidney-stones. Accessed Jan. 20, 2020.
- McKean SC, et al., eds. Kidney stones. In: Principles and Practice of Hospital Medicine. 2nd ed. McGraw-Hill Education; 2017. https://accessmedicine.mhmedical.com/. Accessed Jan. 20, 2020.
- What are kidney stones? American Urological Association. https://www.urologyhealth.org/urologic-conditions/kidney-stones. Accessed Jan. 20, 2020.
- Kellerman RD, et al. Nephrolithiasis. In: Conn's Current Therapy 2020. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 20, 2020.
- Braswell-Pickering EA. Allscripts EPSi. Mayo Clinic. Nov. 3, 2021.
- Curhan GC, et al. Diagnosis and acute management of suspected nephrolithiasis in adults. https://www.uptodate.com/search/contents. Accessed Jan. 20, 2020.
- Yu ASL, et al., eds. Diagnostic kidney imaging. In: Brenner & Rector's The Kidney. 11th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 20, 2020.
- Fontenelle LF, et al. Kidney stones: Treatment and prevention. American Family Physician. 2019. https://www.aafp.org/afp/2019/0415/p490.html. Accessed Jan. 20, 2020.
- Preminger GM. Options in the management of renal and ureteral stones in adults. https://www.uptodate.com/search/contents. Accessed Jan. 20, 2020.
- Preventing Kidney Stones
Associated Procedures
- Computerized tomography (CT) urogram
- Intravenous pyelogram
- Percutaneous nephrolithotomy
News from Mayo Clinic
- Preventing kidney stones before they form Oct. 11, 2023, 01:59 p.m. CDT
- Mayo Clinic Minute: Where is the kidney stone belt? July 03, 2023, 02:00 p.m. CDT
- Mayo Clinic Minute: What can you eat to avoid kidney stones? March 30, 2023, 03:30 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
The inexplicable rise of kidney disease in Sri Lanka’s farming communities
Residents of several ‘hotspots’ have been afflicted by kidney failure in the past few decades – and no one knows why.

Polonnaruwa, Sri Lanka – In the sleepy, verdant village of Ambagaswewa, in the Polonnaruwa district of Sri Lanka’s North Central province, 63-year-old TMH Gamini Sunil Thennakoon’s life is peaceful for the most part. On the brink of retirement, he still spends most days out working his rice paddies but is also content spending his days playing with his grandchildren and chatting with his wife and two daughters. Since boyhood, Thennakoon has farmed rice here across 2 hectares (20,000sqm). A majority-farming nation, agriculture plays a central role in Sri Lanka’s economy and constitutes 21.7 percent of total exports.
But for more than seven years, Thennakoon has been coping with unexplained kidney problems. The symptoms of his condition – abdominal and back pain – are not bad enough to require dialysis yet, but he does take tablets to keep the pain under control.
Keep reading
Deaf syrian boy hears for first time after life-changing operation, aslan, a little syrian boy’s journey to hear again, europe endured record number of ‘extreme heat stress’ days in 2023, deadly sahel heatwave caused by ‘human-induced’ climate change: study.
“I’m not sure what caused the issue, because the rest of my family seems fine,” he says calmly, his granddaughter straddling his lap. She reaches over to swipe at one of the puppies roaming the front porch of their home, where we’re sitting. Ambagaswewa, proliferated by rice paddies, is otherwise a jungle – birdsong twangs through the already humid morning air, luscious vines and creepers on the verge of overtaking farmers’ homes. It’s a peaceful place.
Every month, Thennakoon makes a round trip of more than 30km to a local government hospital for a check-up; during these trips, he has to hire labourers to work in the rice paddies and cover his absence.

Thennakoon is not the only one who has been affected in this way, here.
U Subasinha, a 60-year-old former rice farmer, is one of his neighbours. He has had a particularly hard life. One of his three children has been disabled since birth and, now aged 23, cannot walk. Seventeen years ago, Subasinha’s wife, Kamalavathi, now 54, started experiencing pain and was eventually diagnosed with chronic kidney disease.
Subasinha himself has suffered from acute kidney failure for the past eight years.
He is so frail that he can barely leave his cramped, hot bedroom most days, let alone work. But for the past seven years, he’s been going for dialysis four times a week at a government hospital, more than 25km away.
He has to find the money for the medicine he needs (16,000 rupees or $54) a month for himself and Kamalavathi), and for the hefty transportation costs – upwards of $16 for the round trip of a bumpy, 45-minute tuk-tuk ride each way to the hospital in Polonnaruwa.
None of this is covered by any sort of government-provided healthcare. It’s a huge sum for a household without an income.
The couple says they have no idea what made them sick and they seem surprised at the question. “No one has ever come to ask us this before,” says Kamalavathi.

The rise of kidney disease ‘hotspots’
According to statistics from the National Kidney Foundation in the United States, 10 percent of the world’s population is affected by chronic kidney disease and it is the 12th most common cause of death. Millions die annually due to a lack of access to affordable treatment.
Furthermore, according to an analysis by the Global Burden of Disease Study in 2019, chronic kidney disease (CKD) has increased by 40 percent over the past 30 years and is one of the fastest-rising major causes of death. Common precursors to CKD include diabetes and hypertension – diseases increasingly endemic to urbanising populations.
But across rural Sri Lanka, there’s a relatively new phenomenon; “chronic kidney disease of unknown aetiology (cause)” (CKDu). A flurry of scientific research studies has provided no concrete reason as to why as many as 22.9 percent of residents in several “hotspot” areas in the north-central districts of Polonnaruwa and Anuradhapura, plus some neighbouring districts, are suffering from acute kidney damage or failure.
On a national level, 10 to 15 percent of Sri Lankans are impacted by kidney diseases, according to Nishad Jayasundara, who is a researcher of global environmental health at Duke University in Durham, North Carolina, US. He grew up surrounded by rice fields in an area of Sri Lanka that was rapidly urbanising throughout his childhood and now researches the causes of CKDu.
“[The disease] disproportionately impacts farming communities,” he tells Al Jazeera. “The current estimates indicate that more than 20,000 people [in Sri Lanka] are at end-stage kidney failure, with no alternatives left, while 6 to 10 percent of the population in impacted communities are diagnosed with CDKu.”
Indeed, research published by the US government’s National Library of Medicine in 2016 states: “Geographical mapping indicates a relationship between CKDu and agricultural irrigation water sources [in Sri Lanka].”

A lack of early symptoms
While CKD has identifiable symptoms, such as weight loss and poor appetite, swollen ankles or hands, shortness of breath and itchy skin, early on, CKDu is asymptomatic until the latter stages of the disease, so early detection is nearly impossible, say doctors. By the time a patient receives a diagnosis, the disease is usually untreatable.
Even when symptoms do appear, they usually include back pain, swelling in the arms and legs and “body aches”, not uncommon for farmers and fishermen used to hard manual labour.
Dr S B A M Mujahith is a nephrologist – a doctor who specialises in treating kidney diseases – at Batticaloa Teaching Hospital on Sri Lanka’s eastern coast. He grew up just 50km down the coast from Batticaloa in the town of Nintavur and this played an important role in his career choice: “It was a community investment,” he tells Al Jazeera.
CKDu was first identified as an issue in Sri Lanka in the 1990s. There’s a geographical link, says Mujahith – some parts of the eastern and north-central provinces seemed especially hard hit. Many, like himself, wanted to investigate further and identify the causes.
A World Health Organisation (WHO) team even came to investigate the causes of CKDu in the 2010s, but ultimately the study was inconclusive.

Mujahith likes to use the term “chronic interstitial nephritis in agricultural communities” (CINAC) since the disease is rather specific to the nation’s agricultural workers. It affects mainly men – most patients live and work in poor agricultural communities and may be exposed to toxic agrochemicals through work, inhalation, and ingesting contaminated water and food, explains Mujahith.
Sri Lanka, a small tropical nation with a population of about 22 million people, is undergoing the fifth year of the worst economic crisis in its history. The result has been limited access to medicine and food which hinders treatment and management of the disease, particularly in remote and under-served places such as Ambagaswewa.
‘Education is key’
Jayasundara, who grew up in a farming village in southern Sri Lanka, is currently working to isolate the factors of CKDu in his research, which examines phenomena such as how agrochemical concentration increases during drought (due to evaporation), or how the economic decline has affected the rest of the country.
Chronic disease in one specific organ of the body – in this case, the kidneys – can be a telltale sign of environmental harm, he says. “Sri Lanka serves as a clear example of how environmental change leads to so many downstream effects that affect people’s lives.”

The confounding cause of CKDu means it’s difficult to prescribe solutions for villagers, although those with the means are switching from drinking groundwater to filtered water.
Filtered water is not an option for many, however.
“If you’re choosing between food and sending your kids to school, you’re not going to be spending money on filtered drinking water,” says Sumuthuni Sivanandarajah, a marine biologist working at Blue Resources Trust, a marine research and consultancy organisation based in Sri Lanka.
Her work focuses on the self-employed fishing communities along the coasts of Sri Lanka, among whom kidney disease is also on the rise.
Sameera Gunasekara is a research scientist at Theme Institute in Sri Lanka exploring how climate change and diverse environmental exposures affect public health – specifically kidney diseases.
He agrees that the economic crisis has made it harder for people in remote farming and fishing communities to buy water filters. “People know, are conscious that clean water helps,” he explains. “But there’s some misunderstanding. [People] think that chlorinated water, or boiling, will help. That does with bacteria, but not the removal of hazardous materials.” The need for more education in these underserved regions is key, says Gunasekara.

Across the afflicted north-central farming provinces, Gunasekara is working to help educate the local population on reducing agrochemical usage, not staying in the sun for a long time, and preventing dehydration.
“Farming and fishing people have a stereotype, they are hard groups to convince,” the researcher continues. To begin with, biomarkers for the initial stages of the disease – back pain and leg swelling – are very subtle; not everyone experiences them. But even those who do experience them may not pay them heed.
“They just take a painkiller and get back to the field – they tend to suffer for a long time without doing proper [kidney] screening.” For many of these households, says Gunasekara, since the father is the only person earning money, the whole family collapses when he falls ill.
An economic crisis and chronic dehydration
Batticaloa on Sri Lanka’s east coast, known for both its aquaculture and agricultural activities, in the form of shrimp farms and rice and fish processing facilities, was the site of a brutal massacre during the nation’s relatively recent, longrunning civil war between the Sinhalese and Tamils. It is also one of the hotspots identified for the prevalence of CKDu, he says.
The civil war was an ethnic conflict that lasted for 26 years, ending in 2009 after killing more than 100,000 civilians and 50,000 soldiers from both the Tamil and Sinhalese sides.
Christy PL Navil, 58, has been working as a fisherman here for 12 years – before that, he worked as a helper on the boats. Along Pasikuda beach near Batticaloa, a landing site where 106 fishermen work each day, Navil fishes for calamari from 5am, not returning until the afternoon.
“Sometimes it’s many fish, sometimes it’s no fish,” he says. On the boat, they bring very little water considering the conditions – just 5 litres for two people to last for more than nine hours in the tropical heat. “The sun is hot, but we are just used to it. Sometimes fishing is busy, we aren’t drinking water or eating,” the fisherman admits. “We want to catch the fish.”
With the economic crisis, many fishermen also have to cut back on food, only taking one meal a day.

The resulting chronic dehydration is a major problem, says Sivanandarajah. She points to a combination of hereditary issues, water sources and pollution, toxins in agrochemicals, anthropogenic factors (for example improper pesticide container disposal), and lifestyle issues as possible CKDu causes.
Some fishermen are accustomed to drinking local “arrack” – a form of liquor – to help manage seasickness, she adds. “This is wearing on the body, the kidneys. And with the rising temperatures, it may not be a root cause, but it’s definitely a stressor.”
The lack of formal fishing collectives or societies, the marine researcher continues, means that little is known about the impact of ocean resource depletion on these self-employed communities – or the subsequent health ramifications.
“Government officials lack the knowledge on how to communicate [with fishermen,] they don’t like being out in the field,” says Sivanandarajah. “Sri Lanka’s fisheries sector depends on politics, what the admin implements. No one knows about the fishermen’s income or situation on the ground. It’s very top down, and no one is actually doing anything with the data.”
Food scarcity is a major issue – particularly during the off-season and especially with the ongoing economic crisis, Sivanandarajah says.

There is also the high use of tube wells, inserted deep into the ground – deeper than wells – which extract very hard water as they break past phosphorus barriers in the earth which would normally act as a water softener, making the water easier on the human kidneys. “These became popular during the tsunami and monsoon seasons since ground wells are destroyed and contaminated by seawater,” Sivanandarajah explains.
Geological shifts linked to climate change can also increase the likelihood of earthquakes and volcanic eruptions, which in turn heighten the risk of tsunamis, say scientists. It is estimated that by the end of the 21st century, the global mean sea level will rise by at least 0.3 metres given current greenhouse gas emission rates, which would further inundate coastal communities with brackish water.
Crippling debt
Nadaraja Pereatambi, 62, has been working as a fisherman from Pasikuda beach since his youth. Two years ago, he was suffering from unexpected, acute kidney pain, culminating in an emergency operation and a 50-day hospital stay.
The treatment was largely successful – Pereatambi is cautiously back at work on the fishing boats. However, he had little choice but to take a 2 lakh loan (200,000 rupees, nearly $675 – an unthinkable sum for someone who makes as little as $4 a day, depending on the catch) to pay off the hospital bill.
“Six other fishermen working on this beach also have issues with kidneys,” he says. “Most have no money for hospital, even when suffering from kidney stones.”
It could be a water problem, he surmises. In the Pasikuda area, he continues, it is common knowledge that the water quality is poor: there’s too much calcium and fluoride, among other minerals: “It’s all very hard.”

Outside the government-funded District General Hospital in Negombo along Sri Lanka’s western coast, a little north of the capital city of Colombo, 48-year-old W Sirani Silva is easing into a tuk-tuk that her husband will drive her home in.
Two years ago, she found out she had acute kidney damage – with less than 10 percent function remaining – after experiencing nauseating back and stomach pain.
Each week, Silva makes the 20km journey twice for dialysis sessions in hospital, and is on the waiting list for a transplant. She is far too sick to take care of the house or her three children but is grateful that they are healthy. Since the onset of her illness, the family has switched to drinking filtered water, but still uses well water for cooking and other household needs.
Since Silva is so weak, her husband, K Usdesangar, 51, accompanies her to every dialysis visit, which means he loses income from working as a tuk-tuk driver – he was previously a fisherman – on those days.
“We have no idea where this comes from,” he says, since Silva had an otherwise clean medical history and never suffered from hypertension or diabetes, the main precursors for most kidney disease patients. “Perhaps, it just comes with the family.”

Study progresses of NK cell therapy in mCRPC
All patients have been successfully dosed in the first cohort of patients, and the Safety Review Committee has granted approval for progression of the study to the second dose level of INKmune.
Dosing and patient enrollment continue to progress in the phase 1/2 CaRe PC trial (NCT06056791), evaluating the natural killer (NK) cell targeted therapy INKmune in patients with metastatic castration-resistant prostate cancer (mCRPC), according to a news release from INmune Bio, the developer of the therapy. 1
INmune also announced that they have planned the process for FDA Biologic License Application standard validation in 2025.
According to the company, all patients have been successfully dosed in the first cohort of patients, and the Safety Review Committee (SRC) has granted approval for progression of the study to the second dose level (cohort 2). The first patient in the second cohort has been identified and will undergo screening in preparation for treatment.
“We are pleased with the safety of INKmune in men with mCRPC and feedback from the SRC to proceed with cohort 2. Our initial focus is on assessing the safety of INKmune in this group of patients, and the fact that the drug can be safely administered on an outpatient basis is appealing to both patients and clinical teams,” said Mark Lowdell, PhD, CSO of INmune Bio and inventor of INKmune, in the news release. 1 “Safety is just one aspect of the therapeutic process. The main objective of INKmune therapy is to transform resting NK cells into memory-like NK cells capable of attacking the tumor. Given that prostate cancer has numerous resting NK cells in the tumor microenvironment that do not eliminate cancer, we believe that INKmune, by transforming the patient’s NK cells into cancer-killing cells, could potentially be an optimal therapy for prostate cancer.”
INKmune is an NK cell-targeting therapy that is given as an outpatient therapy via intravenous infusion without the need for pre-medication or cytokine support.
INmune announced the launch of the CaRe PC trial in January 2024 with the dosing of the first patient. 2 To date, 9 doses of INKmune have been administered in the study, with no significant adverse events noted across any patients.
Overall, the open-label CaRe PC trial plans to enroll up to 30 adult patients with mCRPC. Patients may receive up to 3 doses of INKmune (given on days 1, 8, and 15) at a dose level of either low (1 x 10 8 INKmune), medium (3 x 10 8 INKmune), or high (5 x 10 8 INKmune), with each dose level including up to 10 patients.
Those enrolled in the study will be followed for 6 months after treatment to assess their immunologic and anti-cancer responses to therapy. Immune responses will be measured by the number of tumor killing memory-like NK cells in the patient’s blood and how long those specialized NK cells remain in circulation. The investigators will measure anti-tumor responses by monitoring the level of prostatic specific antigen in the blood, using artificial intelligence to quantify the number and size of metastatic lesions on PSMA imaging with piflufolastat F 18 (Pylarify), and by measuring circulating tumor DNA in the blood.
The primary goals of the study are to assess the safety of INKmune in patients with mCRPC and to determine a recommended dose level.
Following the phase 1 dose escalation portion of the trial, the phase 2 portion will be initiated with 12 patients, who will be enrolled in up to 2 candidate optimal dose levels for final dose determination. 3 According to INmune, the dose level determined to be the best following both phases of the study will be used in a blinded, randomized registration trial of the therapy.
1. INmune Bio Inc. completes first cohort and initiates second cohort of phase 1/2 study of INKmune natural killer cell therapy in patients with metastatic castration-resistant prostate cancer. News release. April 29, 2024. Accessed April 30, 2024. https://www.globenewswire.com/news-release/2024/04/29/2871192/0/en/INmune-Bio-Inc-Completes-First-Cohort-and-Initiates-Second-Cohort-of-Phase-1-2-Study-of-INKmune-Natural-Killer-Cell-Therapy-in-Patients-with-Metastatic-Castration-Resistant-Prostat.html
2. INmune Bio announces first patient dosed in a phase 1/2 study of INKmune in patients with metastatic castration-resistant prostate cancer. News release. INmune Bio Inc. January 2, 2024. Accessed January 3, 2024. https://www.biospace.com/article/releases/inmune-bio-announces-first-patient-dosed-in-a-phase-1-2-study-of-inkmune-in-patients-with-metastatic-castration-resistant-prostate-cancer-/
3. Study of INKmune in patients with mCRPC (CaRe Prostate) (CaRe). ClinicalTrials.gov. Last updated September 29, 2023. Accessed January 3, 2024. https://clinicaltrials.gov/study/NCT06056791
Dr. Tosoian on screening approaches and tools for prostate cancer
"I think in terms of the 2 large tool sets we have, biomarker testing through urine or blood tests and imaging. I think both are only going to get better," says Jeffrey Tosoian, MD, MPH.

Dr. Murphy on increasing diversity in cancer clinical trials
In this podcast episode, Adam B. Murphy, MD, MBA, MSCI, discusses the need to increase diversity in clinical trials, specifically focusing on studies in prostate cancer.
Trial of ONCT-534 in mCRPC progresses to higher dose level
"We look forward to announcing initial efficacy and safety data from the study, which we expect will be at the end of this quarter," says Salim Yazji, MD.
Dr. Schwen on focal therapies for prostate cancer
Zeyad Schwen, MD, a urologic oncologist at Cleveland Clinic, discusses how patient factors and cancer characteristics are key to deciding between focal therapies and whole gland treatment for prostate cancer.

Study finds disparities in management of mCSPC
“These findings suggest that guideline recommended treatment intensification remains low for patients with mCSPC in both Medicare and the Veterans Health Administration, but especially for Black patients,” says Daniel J. George, MD.
Addition of chemotherapy to ADT improves bPFS in prostate cancer
"Our clinical trial is the first to show a longer time to biochemical recurrence with chemotherapy plus standard hormone therapy for patients with locally advanced, high-risk prostate cancer," says Jiahua Pan, MD.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 7—July 2024
Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024
Suggested citation for this article
We report highly pathogenic avian influenza A(H5N1) virus in dairy cattle and cats in Kansas and Texas, United States, which reflects the continued spread of clade 2.3.4.4b viruses that entered the country in late 2021. Infected cattle experienced nonspecific illness, reduced feed intake and rumination, and an abrupt drop in milk production, but fatal systemic influenza infection developed in domestic cats fed raw (unpasteurized) colostrum and milk from affected cows. Cow-to-cow transmission appears to have occurred because infections were observed in cattle on Michigan, Idaho, and Ohio farms where avian influenza virus–infected cows were transported. Although the US Food and Drug Administration has indicated the commercial milk supply remains safe, the detection of influenza virus in unpasteurized bovine milk is a concern because of potential cross-species transmission. Continued surveillance of highly pathogenic avian influenza viruses in domestic production animals is needed to prevent cross-species and mammal-to-mammal transmission.
Highly pathogenic avian influenza (HPAI) viruses pose a threat to wild birds and poultry globally, and HPAI H5N1 viruses are of even greater concern because of their frequent spillover into mammals. In late 2021, the Eurasian strain of H5N1 (clade 2.3.4.4b) was detected in North America ( 1 , 2 ) and initiated an outbreak that continued into 2024. Spillover detections and deaths from this clade have been reported in both terrestrial and marine mammals in the United States ( 3 , 4 ). The detection of HPAI H5N1 clade 2.3.4.4b virus in severe cases of human disease in Ecuador ( 5 ) and Chile ( 6 ) raises further concerns regarding the pandemic potential of specific HPAI viruses.
In February 2024, veterinarians were alerted to a syndrome occurring in lactating dairy cattle in the panhandle region of northern Texas. Nonspecific illness accompanied by reduced feed intake and rumination and an abrupt drop in milk production developed in affected animals. The milk from most affected cows had a thickened, creamy yellow appearance similar to colostrum. On affected farms, incidence appeared to peak 4–6 days after the first animals were affected and then tapered off within 10–14 days; afterward, most animals were slowly returned to regular milking. Clinical signs were commonly reported in multiparous cows during middle to late lactation; ≈10%–15% illness and minimal death of cattle were observed on affected farms. Initial submissions of blood, urine, feces, milk, and nasal swab samples and postmortem tissues to regional diagnostic laboratories did not reveal a consistent, specific cause for reduced milk production. Milk cultures were often negative, and serum chemistry testing showed mildly increased aspartate aminotransferase, gamma-glutamyl transferase, creatinine kinase, and bilirubin values, whereas complete blood counts showed variable anemia and leukocytopenia.
In early March 2024, similar clinical cases were reported in dairy cattle in southwestern Kansas and northeastern New Mexico; deaths of wild birds and domestic cats were also observed within affected sites in the Texas panhandle. In > 1 dairy farms in Texas, deaths occurred in domestic cats fed raw colostrum and milk from sick cows that were in the hospital parlor. Antemortem clinical signs in affected cats were depressed mental state, stiff body movements, ataxia, blindness, circling, and copious oculonasal discharge. Neurologic exams of affected cats revealed the absence of menace reflexes and pupillary light responses with a weak blink response.
On March 21, 2024, milk, serum, and fresh and fixed tissue samples from cattle located in affected dairies in Texas and 2 deceased cats from an affected Texas dairy farm were received at the Iowa State University Veterinary Diagnostic Laboratory (ISUVDL; Ames, IA, USA). The next day, similar sets of samples were received from cattle located in affected dairies in Kansas. Milk and tissue samples from cattle and tissue samples from the cats tested positive for influenza A virus (IAV) by screening PCR, which was confirmed and characterized as HPAI H5N1 virus by the US Department of Agriculture National Veterinary Services Laboratory. Detection led to an initial press release by the US Department of Agriculture Animal and Plant Health Inspection Service on March 25, 2024, confirming HPAI virus in dairy cattle ( 7 ). We report the characterizations performed at the ISUVDL for HPAI H5N1 viruses infecting cattle and cats in Kansas and Texas.
Materials and Methods
Milk samples (cases 2–5) and fresh and formalin-fixed tissues (cases 1, 3–5) from dairy cattle were received at the ISUVDL from Texas on March 21 and from Kansas on March 22, 2024. The cattle exhibited nonspecific illness and reduced lactation, as described previously. The tissue samples for diagnostic testing came from 3 cows that were euthanized and 3 that died naturally; all postmortem examinations were performed on the premises of affected farms.
The bodies of 2 adult domestic shorthaired cats from a north Texas dairy farm were received at the ISUVDL for a complete postmortem examination on March 21, 2024. The cats were found dead with no apparent signs of injury and were from a resident population of ≈24 domestic cats that had been fed milk from sick cows. Clinical disease in cows on that farm was first noted on March 16; the cats became sick on March 17, and several cats died in a cluster during March 19–20. In total, >50% of the cats at that dairy became ill and died. We collected cerebrum, cerebellum, eye, lung, heart, spleen, liver, lymph node, and kidney tissue samples from the cats and placed them in 10% neutral-buffered formalin for histopathology.
At ISUVDL, we trimmed, embedded in paraffin, and processed formalin-fixed tissues from affected cattle and cats for hematoxylin/eosin staining and histologic evaluation. For immunohistochemistry (IHC), we prepared 4-µm–thick sections from paraffin-embedded tissues, placed them on Superfrost Plus slides (VWR, https://www.vwr.com ), and dried them for 20 minutes at 60°C. We used a Ventana Discovery Ultra IHC/ISH research platform (Roche, https://www.roche.com ) for deparaffinization until and including counterstaining. We obtained all products except the primary antibody from Roche. Automated deparaffination was followed by enzymatic digestion with protease 1 for 8 minutes at 37°C and endogenous peroxidase blocking. We obtained the primary influenza A virus antibody from the hybridoma cell line H16-L10–4R5 (ATCC, https://www.atcc.org ) and diluted at 1:100 in Discovery PSS diluent; we incubated sections with antibody for 32 minutes at room temperature. Next, we incubated the sections with a hapten-labeled conjugate, Discovery anti-mouse HQ, for 16 minutes at 37°C followed by a 16-minute incubation with the horse radish peroxidase conjugate, Discovery anti-HQ HRP. We used a ChromoMap DAB kit for antigen visualization, followed by counterstaining with hematoxylin and then bluing. Positive controls were sections of IAV-positive swine lung. Negative controls were sections of brain, lung, and eyes from cats not infected with IAV.
We diluted milk samples 1:3 vol/vol in phosphate buffered saline, pH 7.4 (Gibco/Thermo Fisher Scientific, https://www.thermofisher.com ) by mixing 1 unit volume of milk and 3 unit volumes of phosphate buffered saline. We prepared 10% homogenates of mammary glands, brains, lungs, spleens, and lymph nodes in Earle’s balanced salt solution (Sigma-Aldrich, https://www.sigmaaldrich.com ). Processing was not necessary for ocular fluid, rumen content, or serum samples. After processing, we extracted samples according to a National Animal Health Laboratory Network (NAHLN) protocol that had 2 NAHLN-approved deviations for ISUVDL consisting of the MagMax Viral RNA Isolation Kit for 100 µL sample volumes and a Kingfisher Flex instrument (both Thermo Fisher Scientific).
We performed real-time reverse transcription PCR (rRT-PCR) by using an NAHLN-approved assay with 1 deviation, which was the VetMAX-Gold SIV Detection kit (Thermo Fisher Scientific), to screen for the presence of IAV RNA. We tested samples along with the VetMAX XENO Internal Positive Control to monitor the possible presence of PCR inhibitors. Each rRT-PCR 96-well plate had 2 positive amplification controls, 2 negative amplification controls, 1 positive extraction control, and 1 negative extraction control. We ran the rRT-PCR on an ABI 7500 Fast thermocycler and analyzed data with Design and Analysis Software 2.7.0 (both Thermo Fisher Scientific). We considered samples with cycle threshold (Ct) values <40.0 to be positive for virus.
After the screening rRT-PCR, we analyzed IAV RNA–positive samples for the H5 subtype and H5 clade 2.3.4.4b by using the same RNA extraction and NAHLN-approved rRT-PCR protocols as described previously, according to standard operating procedures. We performed PCR on the ABI 7500 Fast thermocycler by using appropriate controls to detect H5-specific IAV. We considered samples with Ct values <40.0 to be positive for the IAV H5 subtype.
We conducted genomic sequencing of 2 milk samples from infected dairy cattle from Texas and 2 tissue samples (lung and brain) from cats that died at a different Texas dairy. We subjected the whole-genome sequencing data to bioinformatics analysis to assemble the 8 different IAV segment sequences according to previously described methods ( 8 ). We used the hemagglutinin (HA) and neuraminidase (NA) sequences for phylogenetic analysis. We obtained reference sequences for the HA and NA segments of IAV H5 clade 2.3.4.4 from publicly available databases, including GISAID ( https://www.gisaid.org ) and GenBank. We aligned the sequences by using MAFFT version 7.520 software ( https://mafft.cbrc.jp/alignment/server/index.html ) to create multiple sequence alignments for subsequent phylogenetic analysis. We used IQTree2 ( https://github.com/iqtree/iqtree2 ) to construct the phylogenetic tree from the aligned sequences. The software was configured to automatically identify the optimal substitution model by using the ModelFinder Plus option, ensuring the selection of the most suitable model for the dataset and, thereby, improving the accuracy of the reconstructed tree. We visualized the resulting phylogenetic tree by using iTOL ( https://itol.embl.de ), a web-based platform for interactive tree exploration and annotation.
Gross Lesions in Cows and Cats
All cows were in good body condition with adequate rumen fill and no external indications of disease. Postmortem examinations of the affected dairy cows revealed firm mammary glands typical of mastitis; however, mammary gland lesions were not consistent. Two cows that were acutely ill before postmortem examination had grossly normal milk and no abnormal mammary gland lesions. The gastrointestinal tract of some cows had small abomasal ulcers and shallow linear erosions of the intestines, but those observations were also not consistent in all animals. The colon contents were brown and sticky, suggesting moderate dehydration. The feces contained feed particles that appeared to have undergone minimal ruminal fermentation. The rumen contents had normal color and appearance but appeared to have undergone minimal fermentation.
The 2 adult cats (1 intact male, 1 intact female) received at the ISUVDL were in adequate body and postmortem condition. External examination was unremarkable. Mild hemorrhages were observed in the subcutaneous tissues over the dorsal skull, and multifocal meningeal hemorrhages were observed in the cerebrums of both cats. The gastrointestinal tracts were empty, and no other gross lesions were observed.
Microscopic Lesions in Cows and Cats
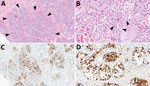
Figure 1 . Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary gland...
The chief microscopic lesion observed in affected cows was moderate acute multifocal neutrophilic mastitis ( Figure 1 ); however, mammary glands were not received from every cow. Three cows had mild neutrophilic or lymphocytic hepatitis. Because they were adult cattle, other observed microscopic lesions (e.g., mild lymphoplasmacytic interstitial nephritis and mild to moderate lymphocytic abomasitis) were presumed to be nonspecific, age-related changes. We did not observe major lesions in the other evaluated tissues. We performed IHC for IAV antigen on all evaluated tissues; the only tissues with positive immunoreactivity were mastitic mammary glands from 2 cows that showed nuclear and cytoplasmic labeling of alveolar epithelial cells and cells within lumina ( Figure 1 ) and multifocal germinal centers within a lymph node from 1 cow ( Table 1 ).
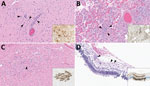
Figure 2 . Lesions in cat tissues in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Tissue sections were stained with...
Both cats had microscopic lesions consistent with severe systemic virus infection, including severe subacute multifocal necrotizing and lymphocytic meningoencephalitis with vasculitis and neuronal necrosis, moderate subacute multifocal necrotizing and lymphocytic interstitial pneumonia, moderate to severe subacute multifocal necrotizing and lymphohistiocytic myocarditis, and moderate subacute multifocal lymphoplasmacytic chorioretinitis with ganglion cell necrosis and attenuation of the internal plexiform and nuclear layers ( Table 2 ; Figure 2 ). We performed IHC for IAV antigen on multiple tissues (brain, eye, lung, heart, spleen, liver, and kidney). We detected positive IAV immunoreactivity in brain (intracytoplasmic, intranuclear, and axonal immunolabeling of neurons), lung, and heart, and multifocal and segmental immunoreactivity within all layers of the retina ( Figure 2 ).
PCR Data from Cows and Cats
We tested various samples from 8 clinically affected mature dairy cows by IAV screening and H5 subtype-specific PCR ( Table 3 ). Milk and mammary gland homogenates consistently showed low Ct values: 12.3–16.9 by IAV screening PCR, 17.6–23.1 by H5 subtype PCR, and 14.7–20.0 by H5 2.3.4.4 clade PCR (case 1, cow 1; case 2, cows 1 and 2; case 3, cow 1; and case 4, cow 1). We forwarded the samples to the National Veterinary Services Laboratory, which confirmed the virus was an HPAI H5N1 virus strain.
When available, we also tested tissue homogenates (e.g., lung, spleen, and lymph nodes), ocular fluid, and rumen contents from 6 cows by IAV and H5 subtype-specific PCR ( Table 3 ). However, the PCR findings were not consistent. For example, the tissue homogenates and ocular fluid tested positive in some but not all cows. In case 5, cow 1, the milk sample tested negative by IAV screening PCR, but the spleen homogenate tested positive by IAV screening, H5 subtype, and H5 2.3.4.4 PCR. For 2 cows (case 3, cow 1; and case 4, cow 1) that had both milk and rumen contents available, both samples tested positive for IAV. Nevertheless, all IAV-positive nonmammary gland tissue homogenates, ocular fluid, and rumen contents had markedly elevated Ct values in contrast to the low Ct values for milk and mammary gland homogenate samples.
We tested brain and lung samples from the 2 cats (case 6, cats 1 and 2) by IAV screening and H5 subtype-specific PCR ( Table 3 ). Both sample types were positive by IAV screening PCR; Ct values were 9.9–13.5 for brain and 17.4–24.4 for lung samples, indicating high amounts of virus nucleic acid in those samples. The H5 subtype and H5 2.3.4.4 PCR results were also positive for the brain and lung samples; Ct values were consistent with the IAV screening PCR ( Table 3 ).
Phylogenetic Analyses
We assembled the sequences of all 8 segments of the HPAI viruses from both cow milk and cat tissue samples. We used the hemagglutinin (HA) and neuraminidase (NA) sequences specifically for phylogenetic analysis to delineate the clade of the HA gene and subtype of the NA gene.

Figure 3 . Phylogenetic analysis of hemagglutinin gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate different...
For HA gene analysis, both HA sequences derived from cow milk samples exhibited a high degree of similarity, sharing 99.88% nucleotide identity, whereas the 2 HA sequences from cat tissue samples showed complete identity at 100%. The HA sequences from the milk samples had 99.94% nucleotide identities with HA sequences from the cat tissues, resulting in a distinct subcluster comprising all 4 HA sequences, which clustered together with other H5N1 viruses belonging to clade 2.3.4.4b ( Figure 3 ). The HA sequences were deposited in GenBank (accession nos. PP599465 [case 2, cow 1], PP599473 [case 2, cow 2], PP692142 [case 6, cat 1], and PP692195 [case 6, cat 2]).

Figure 4 . Phylogenetic analysis of neuraminidase gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate different...
For NA gene analysis, the 2 NA sequences obtained from cow milk samples showed 99.93% nucleotide identity. Moreover, the NA sequences derived from the milk samples exhibited complete nucleotide identities (100%) with those from the cat tissues. The 4 NA sequences were grouped within the N1 subtype of HPAI viruses ( Figure 4 ). The NA sequences were deposited in GenBank (accession nos. PP599467 [case 2, cow 1], PP599475 [case 2, cow 2], PP692144 [case 6, cat 1], and PP692197 [case 6, cat 2]).
This case series differs from most previous reports of IAV infection in bovids, which indicated cattle were inapparently infected or resistant to infection ( 9 ). We describe an H5N1 strain of IAV in dairy cattle that resulted in apparent systemic illness, reduced milk production, and abundant virus shedding in milk. The magnitude of this finding is further emphasized by the high death rate (≈50%) of cats on farm premises that were fed raw colostrum and milk from affected cows; clinical disease and lesions developed that were consistent with previous reports of H5N1 infection in cats presumably derived from consuming infected wild birds ( 10 – 12 ). Although exposure to and consumption of dead wild birds cannot be completely ruled out for the cats described in this report, the known consumption of unpasteurized milk and colostrum from infected cows and the high amount of virus nucleic acid within the milk make milk and colostrum consumption a likely route of exposure. Therefore, our findings suggest cross-species mammal-to-mammal transmission of HPAI H5N1 virus and raise new concerns regarding the potential for virus spread within mammal populations. Horizontal transmission of HPAI H5N1 virus has been previously demonstrated in experimentally infected cats ( 13 ) and ferrets ( 14 ) and is suspected to account for large dieoffs observed during natural outbreaks in mink ( 15 ) and sea lions ( 16 ). Future experimental studies of HPAI H5N1 virus in dairy cattle should seek to confirm cross-species transmission to cats and potentially other mammals.
Clinical IAV infection in cattle has been infrequently reported in the published literature. The first report occurred in Japan in 1949, where a short course of disease with pyrexia, anorexia, nasal discharge, pneumonia, and decreased lactation developed in cattle ( 17 ). In 1997, a similar condition occurred in dairy cows in southwest England leading to a sporadic drop in milk production ( 18 ), and IAV seroconversion was later associated with reduced milk yield and respiratory disease ( 19 – 21 ). Rising antibody titers against human-origin influenza A viruses (H1N1 and H3N2) were later again reported in dairy cattle in England, which led to an acute fall in milk production during October 2005–March 2006 ( 22 ). Limited reports of IAV isolation from cattle exist; most reports occurred during the 1960s and 1970s in Hungary and in the former Soviet Union, where H3N2 was recovered from cattle experiencing respiratory disease ( 9 , 23 ). Direct detection of IAV in milk and the potential transmission from cattle to cats through feeding of unpasteurized milk has not been previously reported.
An IAV-associated drop in milk production in dairy cattle appears to have occurred during > 4 distinct periods and within 3 widely separated geographic areas: 1949 in Japan ( 17 ), 1997–1998 and 2005–2006 in Europe ( 19 , 21 ), and 2024 in the United States (this report). The sporadic occurrence of clinical disease in dairy cattle worldwide might be the result of changes in subclinical infection rates and the presence or absence of sufficient baseline IAV antibodies in cattle to prevent infection. Milk IgG, lactoferrin, and conglutinin have also been suggested as host factors that might reduce susceptibility of bovids to IAV infection ( 9 ). Contemporary estimates of the seroprevalence of IAV antibodies in US cattle are not well described in the published literature. One retrospective serologic survey in the United States in the late 1990s showed 27% of serum samples had positive antibody titers and 31% had low-positive titers for IAV H1 subtype-specific antigen in cattle with no evidence of clinical infections ( 24 ). Antibody titers for H5 subtype-specific antigen have not been reported in US cattle.
The susceptibility of domestic cats to HPAI H5N1 is well-documented globally ( 10 – 12 , 25 – 28 ), and infection often results in neurologic signs in affected felids and other terrestrial mammals ( 4 ). Most cases in cats result from consuming infected wild birds or contaminated poultry products ( 12 , 27 ). The incubation period in cats is short; clinical disease is often observed 2–3 days after infection ( 28 ). Brain tissue has been suggested as the best diagnostic sample to confirm HPAI virus infection in cats ( 10 ), and our results support that finding. One unique finding in the cats from this report is the presence of blindness and microscopic lesions of chorioretinitis. Those results suggest that further investigation into potential ocular manifestations of HPAI H5N1 virus infection in cats might be warranted.
The genomic sequencing and subsequent analysis of clinical samples from both bovine and feline sources provided considerable insights. The HA and NA sequences derived from both bovine milk and cat tissue samples from different Texas farms had a notable degree of similarity. Those findings strongly suggest a shared origin for the viruses detected in the dairy cattle and cat tissues. Further research, case series investigations, and surveillance data are needed to better understand and inform measures to curtail the clinical effects, shedding, and spread of HPAI viruses among mammals. Although pasteurization of commercial milk mitigates risks for transmission to humans, a 2019 US consumer study showed that 4.4% of adults consumed raw milk > 1 time during the previous year ( 29 ), indicating a need for public awareness of the potential presence of HPAI H5N1 viruses in raw milk.
Ingestion of feed contaminated with feces from wild birds infected with HPAI virus is presumed to be the most likely initial source of infection in the dairy farms. Although the exact source of the virus is unknown, migratory birds (Anseriformes and Charadriiformes) are likely sources because the Texas panhandle region lies in the Central Flyway, and those birds are the main natural reservoir for avian influenza viruses ( 30 ). HPAI H5N1 viruses are well adapted to domestic ducks and geese, and ducks appear to be a major reservoir ( 31 ); however, terns have also emerged as an important source of virus spread ( 32 ). The mode of transmission among infected cattle is also unknown; however, horizontal transmission has been suggested because disease developed in resident cattle herds in Michigan, Idaho, and Ohio farms that received infected cattle from the affected regions, and those cattle tested positive for HPAI H5N1 ( 33 ). Experimental studies are needed to decipher the transmission routes and pathogenesis (e.g., replication sites and movement) of the virus within infected cattle.
In conclusion, we showed that dairy cattle are susceptible to infection with HPAI H5N1 virus and can shed virus in milk and, therefore, might potentially transmit infection to other mammals via unpasteurized milk. A reduction in milk production and vague systemic illness were the most commonly reported clinical signs in affected cows, but neurologic signs and death rapidly developed in affected domestic cats. HPAI virus infection should be considered in dairy cattle when an unexpected and unexplained abrupt drop in feed intake and milk production occurs and for cats when rapid onset of neurologic signs and blindness develop. The recurring nature of global HPAI H5N1 virus outbreaks and detection of spillover events in a broad host range is concerning and suggests increasing virus adaptation in mammals. Surveillance of HPAI viruses in domestic production animals, including cattle, is needed to elucidate influenza virus evolution and ecology and prevent cross-species transmission.
Dr. Burrough is a professor and diagnostic pathologist at the Iowa State University College of Veterinary Medicine and Veterinary Diagnostic Laboratory. His research focuses on infectious diseases of livestock with an emphasis on swine.
Acknowledgment
We thank the faculty and staff at the ISUVDL who contributed to the processing and analysis of clinical samples in this investigation, the veterinarians involved with clinical assessments at affected dairies and various conference calls in the days before diagnostic submissions that ultimately led to the detection of HPAI virus in the cattle, and the US Department of Agriculture National Veterinary Services Laboratory and NAHLN for their roles and assistance in providing their expertise, confirmatory diagnostic support, and communications surrounding the HPAI virus cases impacting lactating dairy cattle.
- Caliendo V , Lewis NS , Pohlmann A , Baillie SR , Banyard AC , Beer M , et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci Rep . 2022 ; 12 : 11729 . DOI PubMed Google Scholar
- Bevins SN , Shriner SA , Cumbee JC Jr , Dilione KE , Douglass KE , Ellis JW , et al. Intercontinental movement of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4 virus to the United States, 2021. Emerg Infect Dis . 2022 ; 28 : 1006 – 11 . DOI PubMed Google Scholar
- Puryear W , Sawatzki K , Hill N , Foss A , Stone JJ , Doughty L , et al. Highly pathogenic avian influenza A(H5N1) virus outbreak in New England seals, United States. Emerg Infect Dis . 2023 ; 29 : 786 – 91 . DOI PubMed Google Scholar
- Elsmo EJ , Wünschmann A , Beckmen KB , Broughton-Neiswanger LE , Buckles EL , Ellis J , et al. Highly pathogenic avian influenza A(H5N1) virus clade 2.3.4.4b infections in wild terrestrial mammals, United States, 2022. Emerg Infect Dis . 2023 ; 29 : 2451 – 60 . DOI PubMed Google Scholar
- Bruno A , Alfaro-Núñez A , de Mora D , Armas R , Olmedo M , Garcés J , et al. First case of human infection with highly pathogenic H5 avian influenza A virus in South America: a new zoonotic pandemic threat for 2023? J Travel Med. 2023 ;30:taad032.
- Pulit-Penaloza JA , Brock N , Belser JA , Sun X , Pappas C , Kieran TJ , et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg Microbes Infect . 2024 ; 2332667 . DOI PubMed Google Scholar
- United States Department of Agriculture Animal and Plant Health Inspection Service . Federal and state veterinary, public health agencies share update on HPAI detection in Kansas, Texas dairy herds. 2024 [ cited 2024 Mar 29 ]. https://www.aphis.usda.gov/news/agency-announcements/federal-state-veterinary-public-health-agencies-share-update-hpai
- Sharma A , Zeller MA , Souza CK , Anderson TK , Vincent AL , Harmon K , et al. Characterization of a 2016–2017 human seasonal H3 influenza A virus spillover now endemic to U.S. swine. MSphere . 2022 ; 7 : e0080921 . DOI PubMed Google Scholar
- Sreenivasan CC , Thomas M , Kaushik RS , Wang D , Li F . Influenza A in bovine species: a narrative literature review. Viruses . 2019 ; 11 : 561 . DOI PubMed Google Scholar
- Sillman SJ , Drozd M , Loy D , Harris SP . Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J Comp Pathol . 2023 ; 205 : 17 – 23 . DOI PubMed Google Scholar
- Klopfleisch R , Wolf PU , Uhl W , Gerst S , Harder T , Starick E , et al. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet Pathol . 2007 ; 44 : 261 – 8 . DOI PubMed Google Scholar
- Keawcharoen J , Oraveerakul K , Kuiken T , Fouchier RAM , Amonsin A , Payungporn S , et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis . 2004 ; 10 : 2189 – 91 . DOI PubMed Google Scholar
- Kuiken T , Rimmelzwaan G , van Riel D , van Amerongen G , Baars M , Fouchier R , et al. Avian H5N1 influenza in cats. Science . 2004 ; 306 : 241 . DOI PubMed Google Scholar
- Herfst S , Schrauwen EJA , Linster M , Chutinimitkul S , de Wit E , Munster VJ , et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science . 2012 ; 336 : 1534 – 41 . DOI PubMed Google Scholar
- Agüero M , Monne I , Sánchez A , Zecchin B , Fusaro A , Ruano MJ , et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill . 2023 ; 28 : 2300001 . DOI PubMed Google Scholar
- Leguia M , Garcia-Glaessner A , Muñoz-Saavedra B , Juarez D , Barrera P , Calvo-Mac C , et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun . 2023 ; 14 : 5489 . DOI PubMed Google Scholar
- Saito K . An outbreak of cattle influenza in Japan in the fall of 1949. J Am Vet Med Assoc . 1951 ; 118 : 316 – 9 . PubMed Google Scholar
- Gunning RF , Pritchard GC . Unexplained sporadic milk drop in dairy cows. Vet Rec . 1997 ; 140 : 488 . PubMed Google Scholar
- Brown IH , Crawshaw TR , Harris PA , Alexander DJ . Detection of antibodies to influenza A virus in cattle in association with respiratory disease and reduced milk yield. Vet Rec . 1998 ; 143 : 637 – 8 . PubMed Google Scholar
- Crawshaw TR , Brown I . Bovine influenza. Vet Rec . 1998 ; 143 : 372 . PubMed Google Scholar
- Gunning RF , Brown IH , Crawshaw TR . Evidence of influenza A virus infection in dairy cows with sporadic milk drop syndrome. Vet Rec . 1999 ; 145 : 556 – 7 . DOI PubMed Google Scholar
- Crawshaw TR , Brown IH , Essen SC , Young SCL . Significant rising antibody titres to influenza A are associated with an acute reduction in milk yield in cattle. Vet J . 2008 ; 178 : 98 – 102 . DOI PubMed Google Scholar
- Lopez JW , Woods GT . Influenza virus in ruminants: a review. Res Commun Chem Pathol Pharmacol . 1984 ; 45 : 445 – 62 . PubMed Google Scholar
- Jones-Lang K , Ernst-Larson M , Lee B , Goyal SM , Bey R . Prevalence of influenza A virus (H1N1) antibodies in bovine sera. New Microbiol . 1998 ; 21 : 153 – 60 . PubMed Google Scholar
- Briand FX , Souchaud F , Pierre I , Beven V , Hirchaud E , Hérault F , et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in domestic cat, France, 2022. Emerg Infect Dis . 2023 ; 29 : 1696 – 8 . DOI PubMed Google Scholar
- Frymus T , Belák S , Egberink H , Hofmann-Lehmann R , Marsilio F , Addie DD , et al. Influenza virus infections in cats. Viruses . 2021 ; 13 : 1435 . DOI PubMed Google Scholar
- Songserm T , Amonsin A , Jam-on R , Sae-Heng N , Meemak N , Pariyothorn N , et al. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis . 2006 ; 12 : 681 – 3 . DOI PubMed Google Scholar
- Thiry E , Zicola A , Addie D , Egberink H , Hartmann K , Lutz H , et al. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol . 2007 ; 122 : 25 – 31 . DOI PubMed Google Scholar
- Lando AM , Bazaco MC , Parker CC , Ferguson M . Characteristics of U.S. consumers reporting past year intake of raw (unpasteurized) milk: results from the 2016 Food Safety Survey and 2019 Food Safety and Nutrition Survey. J Food Prot . 2022 ; 85 : 1036 – 43 . DOI PubMed Google Scholar
- Fourment M , Darling AE , Holmes EC . The impact of migratory flyways on the spread of avian influenza virus in North America. BMC Evol Biol . 2017 ; 17 : 118 . DOI PubMed Google Scholar
- Guan Y , Smith GJD . The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res . 2013 ; 178 : 35 – 43 . DOI PubMed Google Scholar
- de Araújo AC , Silva LMN , Cho AY , Repenning M , Amgarten D , de Moraes AP , et al. Incursion of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus, Brazil, 2023. Emerg Infect Dis . 2024 ; 30 : 619 – 21 . DOI PubMed Google Scholar
- American Veterinary Medical Association . States with HPAI-infected dairy cows grows to six. USDA provides guidance for veterinarians, producers on protecting cattle from the virus. 2024 [ cited 2024 Apr 10 ]. https://www.avma.org/news/states-hpai-infected-dairy-cows-grows-six
- Figure 1 . Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary...
- Figure 2 . Lesions in cat tissues in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Tissue sections were stained...
- Figure 3 . Phylogenetic analysis of hemagglutinin gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate...
- Figure 4 . Phylogenetic analysis of neuraminidase gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate...
- Table 1 . Microscopic lesions observed in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
- Table 2 . Microscopic lesions observed in cats in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
- Table 3 . PCR results from various specimens in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
Suggested citation for this article : Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis. 2024 Jul [ date cited ]. https://doi.org/10.3201/eid3007.240508
DOI: 10.3201/eid3007.240508
Original Publication Date: April 29, 2024
Table of Contents – Volume 30, Number 7—July 2024
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eric R. Burrough, Iowa State University Veterinary Diagnostic Laboratory, 1937 Christensen Dr, Ames, IA 50011, USA
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Metric Details
What is the altmetric attention score.
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.

IMAGES
VIDEO
COMMENTS
Case study. History. ... The majority of kidney stones contain calcium (approximately 90% in men and 70% in women) while the remainder consist of cystine (<1%), pure uric acid (10-15%) and struvite (10-15%). 7 Calcium based stones are most commonly composed of calcium oxalate, calcium phosphate or both. Several factors can affect stone ...
Case. A 36-year-old woman with a 2-week history of left flank pain presented to the ED via emergency medical services. The patient, who had a history of nephrolithiasis, assumed her pain was due to another kidney stone. She stated that while waiting for the presumed stone to pass, the pain in her left flank worsened and she felt lightheaded and ...
Although a 24-hour urine study provides valuable information regarding the contents of a patient's urine and their metabolic status, it does not substitute for stone analysis. Per the most recent AUA guideline for the medical management of kidney stones, when a stone is available, it should be sent for analysis. 16
Urinary tract stones are a general term for stone disease in various parts of the urinary system and are common diseases of the urinary system. According to the location of the stone, it is divided into kidney stones, ureteral stones, bladder stones, urethra stones. Total urinary calculi occur less frequently, usually accompanied by severe ...
Kidney stone disease, also known as nephrolithiasis or urolithiasis, is a disorder in which urinary solutes precipitate to form aggregates of crystalline material in the urinary space. The incidence of nephrolithiasis has been increasing, and the demographics have been evolving. Once viewed as a limited disease with intermittent exacerbations that are simply managed by urologists ...
Kim, a 75 year-old retired university professor, has lived with kidney stones for over 25 years. In 1989, Kim had his first kidney stone surgery, shock wave lithotripsy (SWL). This was an old way to treat stones. It involved shock waves fired at his stones while he sat in a large tub of water.
Several studies have examined the natural history of asymptomatic kidney stones. As examples: A cohort of 110 patients with 160 asymptomatic kidney stones was followed with active surveillance (using kidney ultrasound performed every 6 to 12 months) . During a mean follow-up of 3.4 years, 28 percent of stones produced symptoms and 17 percent ...
1. Introduction. Kidney stone disease, also known as nephrolithiasis or urolithiasis, is one of the oldest diseases known to medicine. It is estimated that 1-15% individuals suffer from kidney stone formation at some point during their lifetime, and the prevalence and incidence of kidney stone is reported to be increasing worldwide (1,2).A recent study concluded that the prevalence of kidney ...
sound or abdominal (kidney-ureter-bladder, KUB) radiograph may be used. However, operator experience may affect ultrasound results; and some stones are radiolucent on a KUB radiograph. Additional studies such as serum uric acid, para-thyroid hormone, and 25-hydroxy vitamin D may be needed based on the results of these initial studies.
Case 5 • 73 year old male • 1st stone at age 24 • Prior R open pyelotomy, L PCNL, SWL x 12 • Previous stone CaOx, now CaP • Told previous needed to increase fluid hydration • Labs: Creatinine 1.09, calcium 8.2 • CT imaging: L hydronephrosis with 10, 17, 7 mm renal and 6mm ureteral stone, R 6, 9, 1 mm
A kidney stone classification system based on practical and clinically useful measures of stone disease may help to improve both the study and clinical care of stone formers. Any future kidney ...
Epidemiology of Cystinuria. Cystine stones represent 1-2% of total kidney stones. In children, up to 5% of total kidney stones. Cystinuria: Autosomal recessive Due to an inherited impairment of renal cystine transport Males more severely affected than females.
The primary components of the majority of kidney stones are calcium salts, uric acid, cystine, and struvite. 2 The incidence of urolithiasis is a significant financial burden on the healthcare ...
This case then highlights an important point of practice. It is futile to look for 'normal ranges' for supersaturations, as normal and stone forming patients have overlapping values - or can. Unfortunately i n his brilliant studies of urine stone risk, Curhan did not publish supersaturations, so we cannot link them stone stone risk in ...
For the patient in case 1 ... A metabolic stone workup may be useful for prevention of future kidney stones, but it is not indicated in the acute setting. ... one study of 605 consecutive weekly ...
Cases. Like much of medicine, kidney stone disease is organized around a set of phenotypes, idiopathic calcium oxalate stone formers for example. A useful if medieval Platonic idealization, when looked at closely these phenotypes invariably disintegrate into what one might call medical atomism - the tyranny of the individual case.
Kidney stone disease is a worldwide prevalent disease, and, due to various factors, especially diet- and climate-related, the prevalence across all ages, races, and sexes is showing an upward trend. At the same time, endourology is undergoing constant evolution, which in turn will alter the future management of urolithiasis.
X-ray examination on April 4, 1924, showed the condition seen in Figure 1—a much enlarged right kidney almost completely filled with overlapping masses of stones, arranged in two groups, that in the upper pole being somewhat smaller. No stones were seen in the ureters or the left kidney. Operation was performed one year after the initial X ...
Evidence based information: A case study Introduction Kidney stone disease also known as nephrolithiasis is a disease where there are hard deposits made of salt and minerals that form inside the kidney (Tang & Lieske, 2015). Some of the causes of kidney stones include certain medications, supplements, excess body weight, diet, and some medical ...
Common symptoms of kidney stones include abdominal, flank, or groin pain, blood in the urine, frequent urination, nausea and vomiting. ... Studies show that both boys and girls are at risk of ...
Javid M, Bhandari M, Parameshwari P, et al. Evaluation of ChatGPT for patient counseling in kidney stone clinic: A prospective study. J Endourol 2024; 38(4):377-383.
Kidney stone disease is a crystal concretion formed usually within the kidneys. It is an increasing urological disorder of human health, affecting about 12% of the world population. It has been associated with an increased risk of end-stage renal failure. The etiology of kidney stone is multifactorial. The most common type of kidney stone is ...
The mean age of patients at the start of the studies ranged from 39 to 68 years. The mean follow-up duration across studies ranged from 1.2 to 9.2 years.
Kidney stones (also called renal calculi, nephrolithiasis or urolithiasis) are hard deposits made of minerals and salts that form inside your kidneys. Diet, excess body weight, some medical conditions, and certain supplements and medications are among the many causes of kidney stones. Kidney stones can affect any part of your urinary tract ...
Furthermore, according to an analysis by the Global Burden of Disease Study in 2019, chronic kidney disease (CKD) has increased by 40 percent over the past 30 years and is one of the fastest ...
Introduction. The use of urine analysis as a guide to the diagnosis and treatment of kidney stones is recommended for at least some stone formers in all of the published international guidelines [1-4] (see Supplemental Table 1), but data suggest it is not generally utilized as widely as has been recommended.For example, a recent study of a large cohort within the United States (US) Veterans ...
Study progresses of NK cell therapy in mCRPC. May 1, 2024. Hannah Clarke. News. Article. All patients have been successfully dosed in the first cohort of patients, and the Safety Review Committee has granted approval for progression of the study to the second dose level of INKmune. Dosing and patient enrollment continue to progress in the phase ...
Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. ... lung, heart, spleen, liver, and kidney). We detected positive IAV immunoreactivity in brain (intracytoplasmic, intranuclear ... and 14.7-20.0 by H5 2.3.4.4 clade PCR ...
Two case studies in this article describe the diagnosis and management of cystinuria, the most common rare kidney stone disorder. Keywords: Genetic kidney disease, tiopronin, disorders of amino acids, kidney stones, nephrolithiasis, urolithiasis. Kidney stones, particularly those that present in childhood, may be due to rare inherited metabolic ...