
- Jun 23, 2023

Understanding Your Pathology Report: A Comprehensive Step-By-Step Guide

If you’ve had surgery or a biopsy, the biological sample from your procedure will be sent to a pathologist working in a laboratory. The pathologist will study your sample and create a pathology report providing important information about what was found in your sample. Your pathology report will be reviewed carefully by your oncology team, and you should discuss it with your doctors to ensure you understand your specific situation.
Before you receive or review your pathology report, however, you may be looking for more information about what exactly this report is and what you can expect it to contain. You may feel some anxiety waiting for your pathology report, and deciphering the medical jargon and dense information can be daunting. Continue reading to understand the process and your pathology report in a simple, step-by-step format.
What Is a Pathology Report?
A pathology report is a document that contains the findings of a pathologist who has examined a patient’s biological samples under a microscope. The samples are usually obtained through biopsy, surgery, or a medical examination and can include findings from body tissues, fluids, or cells.
Your pathology report provides valuable insight into the nature of your disease. It includes detailed information about the type, grade, and extent of your cancer, as well as the margins of the removed tissue. Pathologists play a critical role in diagnosing diseases like cancer, and their reports guide physicians in determining the appropriate course of treatment.
Why Understanding Your Pathology Report Matters
Understanding your pathology report empowers you to participate actively in your healthcare decisions with your oncology team. It helps you grasp the severity and nature of your condition and set expectations for your treatment. For more insights on why understanding your pathology report is crucial, refer to resources provided by The American Cancer Society.
Understanding the Structure of Your Pathology Report
A pathology report usually contains the following sections:
Patient Information: Your name, patient ID, date of birth, and the name of the physician who requested the test.
Specimen Information: The type and location of the sample, how and when pathology received it, and who provided it. It may include information about the margins — the edges of tissue that was removed during the biopsy or surgery. Since the goal of surgery is to remove all of the cancerous tissue, it’s vital to ensure that the margins are clear or negative (meaning that no cancer cells are detected on the edges). If any lymph nodes were removed during the procedure, the pathology report will also state whether cancer cells were found in them.
Gross Description: What the pathologist observed with the naked eye, including the color, size, and weight of the sample.
Microscopic Description: A description of what the pathologist observed under the microscope, including cell structures and abnormalities.
Diagnosis: The pathologist’s interpretation of the findings and the final diagnosis.
Reading Your Pathology Report
Now that you know what a pathology report includes, here’s a guide to understanding your report:
Verify Patient Information: Ensure that the patient details are correct. Mistakes, although rare, can happen.
Understand the Specimen: Knowing what type of specimen was taken and where it was taken from can give context to the report.
Gross Description: While this section can be technical, look for descriptors of size, shape and color, which can give you an idea of the sample’s normality.
Microscopic Description: This section might be complex, containing detailed observations of cellular structures. Look for terms like benign, malignant, normal, abnormal, etc. to get a sense of what was found.
Interpret the Diagnosis: Again, the final diagnosis as stated by the pathologist might be technical and challenging to understand. You can use reliable medical dictionaries like MedlinePlus to understand them.
Remember, it’s essential to discuss these findings with your healthcare provider to fully understand your condition and to determine the next steps in your care.
Count On Us for Information, Resources, and Support
Understanding your pathology report is a critical step in managing your health. The knowledge contained in your report can help you better understand your diagnosis. This will empower you to navigate and make informed decisions about your treatment. Consult with your healthcare provider for clarifications and concerns. Please contact us if you have questions about speaking with your doctor regarding your pathology report!
Whether you’re newly diagnosed with breast cancer , are navigating survivorship, or are the loved one of someone experiencing breast cancer, you can count on SurvivingBreastCancer.org to keep you informed. We provide educational information to help you better understand symptoms , testing, treatment options , surgery, etc., and podcasts that feature professionals, advocates, and caregivers that share valuable information.
Your donations enable SurvivingBreastCancer.org to offer resources and support every day, every month, and every year.
SBC is here for you!
Learn more:
What is Breast Cancer?
Breast Cancer Symptoms
Newly Diagnosed. Now What?
Note: This article is designed to provide general information and not meant to replace professional medical advice. Always discuss your pathology reports with your healthcare provider.
SurvivingBreastCancer.org Resources & Support:
Weekly Meetup s
Free Events
Recent Posts
The Waiting Room
The Last Thing I Told My Mom Was a Lie (Part 2)
This article makes so much sense to me. Every word is so true. As challenging as it may be... knowing ones specifics helps one to make informed decisions thru out their BC journey.
So glad to hear that you enjoyed the article, Gloria! Thanks for reading 😀
Meditation Mondays:
Chakra Chanting with Gloria
Mondays at 10:00 a.m. ET
Thursday Night Thrivers:
All Stages Support Group
Thursdays at 7:00 p.m. ET
Metastatic Breast Cancer Support Group
First and third Thursdays
of the month at 7:00 p.m. ET
Inflammatory Breast Cancer Support Group
Second Thursday
of the month at 7:00 p.m. ET
Tuesday Night Thrivers
Después de un Diagnóstico:
Grupo de Apoyo en Español
2do y Cuarto Martes de cada mes
7:00 p.m. ET
Encourage and Empower
For Newly Diagnosed
September 10, 11:00 a.m. ET
Breast Cancer Book Club
The first Sunday of the month
Restorative Yoga:
The secret Garden
April 22, 6:00 p.m. ET
April 23, 11:30 a.m. ET
Art Therapy
May 6, 6:00 p.m. ET
Forest Bathing
May 7, 6:00 p.m. ET
Reflect & Recharge
Expressive Writing
May 13, 6:00 p.m. ET
Yoga Fitness with Chair Assist
May 14, 11:30 a.m. ET
Yoga Stretching for DIEP flap
May 14, 6:00 p.m. ET
Más eventos en español
Upcoming Events

Events and Webinars
Surviving Breast Cancer provides breast cancer support, events, and webinars at no cost to you! Whether you are looking to gain more knowledge on a particular topic or meet up with other breast cancer survivors, we have something for everyone.
Thursday Night Thrivers MeetUp
Our standing appointment on Thursdays is for all stages. We also host specific breakout groups once a month for specific stages and subtypes such as Metastatic breast cancer, and Inflammatory Breast Cancer, etc.
The Book Club meets the first Sunday of every month at 11 am ET. You are welcome to join each month or pick and choose your month based on your availability and the book we are reading.
Healing Through The Arts
Through art, writing, and other creative modalities, we hold the power to manage our stress, make sense of our now, and relax into moments of stillness.
Movement Mondays
Free, monthly, online classes in restorative yoga, yoga for breast cancer, and Zumba.
Program in Spanish
Después de un Diagnóstico

Toxicologic Pathology for Non-Pathologists pp 45–77 Cite as
The Pathology Report, Peer Review, and Pathology Working Group
- Ted A. Birkebak 4 &
- Peter C. Mann 5
- First Online: 31 October 2019
895 Accesses
The pathology report is the mechanism by which the important results of the pathology assessment are communicated to the interested parties, which range from study directors, sponsors, regulators, and investors. In this chapter, we review the structure of a pathology report and what should be expected to be in each section. The sections of a pathology report generally include a summary, description of methods, and results. The results section consists of a discussion on organ weights, macroscopic findings, and microscopic findings and should identify test article-related findings and address their significance. There is a review of terminology that is often used in pathology reports and which can be confusing to a non-pathologist. Interpretation of adversity is also discussed. The chapter expands on pathology reporting by discussing quality assessment of the pathology report through peer review and pathology working groups. The types of peer reviews and the methodology for performing them are presented as is a discussion on conducting a pathology working group.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
With a few exceptions, a complete review is an examination of all protocol-listed tissue samples collected at necropsy. A few samples on the protocol list, such as nasal cavity, spinal cord, or bone marrow smears, may be collected for conditional review and are not always part of a complete review. Complete reviews are typically conducted on all (main study and recovery) high-dose and control group animals in rodent studies and on all animals in all groups in nonrodent studies. Recommendations on the tissue sampling and examination and references to relevant regulatory guidance are presented in the following best practice article: Bregman, C. L., Adler, R. R., Morton, D. G., Regan, K. S., & Yano, B. L. (2003). Recommended Tissue List for Histopathologic Examination in Repeat-Dose Toxicity and Carcinogenicity Studies: A Proposal of the Society of Toxicologic Pathology (STP). Toxicologic Pathology , 31 (2): 252–253.
Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol 32:448–466
Article Google Scholar
Dorato MA, Engelhardt JA (2005) The no-observed-adverse-effect level in drug safety evaluations: use, issues, and definition(s). Regul Toxicol Pharmacol 42:265–274
Article CAS Google Scholar
Environmental Protection Agency (1994) Pesticide registration (PR) notice94–5: requests for re-considerations of carcinogenicity peer review decisions based on changes in pathology diagnoses. http://www.epa.gov/PR_Notices/pr94-5.html . Last Accessed 2 Oct 2011
European Medicines Agency Committee for Proprietary Medicinal Products (2002) Note for guidance on carcino- genic potential. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/
Haley PJ (2017) The lymphoid system: a review of species differences. Toxicol Pathol 30:111–123
Keller DA, Juberg DR, Catlin N, Farland WH, Hess FG, Wolf DC, Doerrer NG (2012) Identification and characterization of adverse effects in 21st century toxicology. Toxicol Sci 126:291–297
Kerlin R, Bolon B, Burkhardt J, Francke S, Greaves P, Meador V, Popp J (2016) Scientific and Regulatory Policy Committee: recommended (“Best”) practices for determining, communicating, and using adverse effect data from nonclinical studies. Toxicol Pathol 44:147–162
Lewis RW, Billington R, Debryune E, Debryune Gamer A, Lang B, Carpanini F (2002) Recognition of adverse and nonadverse effects in toxicity studies. Toxicol Pathol 30:66–74
Mann PC, Vahle J, Charlotte M, Keenan JF, Baker AE, Bradley DG, Goodman TH, Herbert R, Kaufmann W, Kellner R, Nolte T, SusanneRittinghausen TT (2012) International harmonization of toxicologic pathology nomenclature: an overview and review of basic principles. Toxicol Pathol 40(4):7S–13S
Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K (2007) Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol 35:742–750
Morton D, Sellers RS, Barale-Thomas E, Bolon B, George C, Hardisty JF, Irizarry A, McKay JS, Odin M, Teranishi M (2010) Recommendations for pathology peer review. Toxicol Pathol 38:1118–1127
Palazzi X, Burkhardt J, Caplain H, Dellarco V, Fant P, Foster J, Francke S, Germann P, Groeters S, Harada T, Harleman J, Inui K, Kaufmann W, Lenz B, Nagai H, Pohlmeyer-Esch G, Schulte A, Skydsgaard M, Tomlinson L, Wood CAND, Yoshida M (2016) Characterizing “Adversity” of pathology findings in nonclinical toxicity studies: results from the 4th ESTP international expert workshop. Toxicol Pathol 44:810–824
Vishwanathan CT (2005) FDA perspectives on current issues in GLP. Presentation at Society for Quality Assurance Regulatory Forum, Baltimore, MD
Google Scholar
Download references
Author information
Authors and affiliations.
Experimental Pathology Laboratories, Inc., Redwood City, CA, USA
Ted A. Birkebak
Experimental Pathology Laboratories, Inc., Seattle, WA, USA
Peter C. Mann
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ted A. Birkebak .
Editor information
Editors and affiliations.
North Carolina Laboratory, Experimental Pathology Laboratories, Inc., Durham, NC, USA
Thomas J. Steinbach
Charles River Laboratories, Inc., Mattawan, MI, USA
Daniel J. Patrick
MEC Regulatory and Toxicology Consulting, LLC, Moorpark, CA, USA
Mary Ellen Cosenza
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter.
Birkebak, T.A., Mann, P.C. (2019). The Pathology Report, Peer Review, and Pathology Working Group. In: Steinbach, T., Patrick, D., Cosenza, M. (eds) Toxicologic Pathology for Non-Pathologists. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9777-0_2
Download citation
DOI : https://doi.org/10.1007/978-1-4939-9777-0_2
Published : 31 October 2019
Publisher Name : Humana, New York, NY
Print ISBN : 978-1-4939-9776-3
Online ISBN : 978-1-4939-9777-0
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- Previous Article
- Next Article
FIRST-PAGE REPORT HEADER
Subsequent-page header and footer, diagnosis field, gross/macroscopic description, intraoperative consultation, microscopic description, comments/notes, addendum reports, amended reports, other data elements, conclusions, acknowledgments, reporting guidelines for clinical laboratory reports in surgical pathology.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Guest Access
- Get Permissions
- Cite Icon Cite
- Search Site
Jeffrey D. Goldsmith , Gene P. Siegal , Saul Suster , Thomas M. Wheeler , Richard W. Brown; Reporting Guidelines for Clinical Laboratory Reports in Surgical Pathology. Arch Pathol Lab Med 1 October 2008; 132 (10): 1608–1616. doi: https://doi.org/10.5858/2008-132-1608-RGFCLR
Download citation file:
- Ris (Zotero)
- Reference Manager
Context. —The surgical pathology report (SPR) is an essential part of patient care because it documents the pathologic findings in tissues removed from patients for diagnostic or therapeutic reasons. Despite the importance of the SPR, exhaustive guidelines outlining the various elements of the SPR have not, to our knowledge, been published.
Objectives. —To outline recommendations delineating the required, preferred, and optional elements that should be included in the SPR. These guidelines, if implemented, will bring uniformity to the reporting of surgical pathology specimens.
Data Sources. —The Surgical Pathology Resource Committee of the College of American Pathologists compiled and prioritized the elements that have been included in various institutional SPRs. Additional data sources include the College of American Pathologists Laboratory Accreditation Program checklists and the recommendations of the Association of Directors of Anatomic and Surgical Pathology. Each element was assigned a priority of required, preferred , or optional. These priorities were discussed and consensus was reached. This report does not address issues of formatting or style substantively.
Conclusions. —These recommendations afford a framework for the creation of an SPR containing all of the components that are required or optimal for patient care.
The surgical pathology report (SPR) is the final written product of the surgical pathology laboratory, and it contains critical information that drives patient care, especially in the oncologic setting. A variety of individuals, including physicians, nurses, statisticians, epidemiologists, support personnel, and patients, use the information contained in the SPR. Because of the variety of people who access the SPR and the importance of the information contained therein, the report must contain a minimal amount of standard content and must be presented in such a way as to efficiently and accurately convey its information.
The College of American Pathologists (CAP) has developed guidelines for the content of SPRs. Similar guidelines were developed in the past by the Association of Directors of Anatomic and Surgical Pathology (see http://www.adasp.org/papers/position/Standardization.htm , last accessed March 14, 2008); additionally, various organizations have called for report standardization. 1 In this article, we have compiled an exhaustive list of elements to be included in the SPR. Some of these recommendations are contained in the CAP Laboratory Accreditation Program checklists (see http://www.cap.org , last accessed March 14, 2008). This article is meant to convey guiding principles regarding the elements that should be contained in the SPR. Additionally, this article serves to rank these elements by degree of importance.
A summary of the body of the recommendations is contained in Tables 1 through 10 . These tables are organized by the various sections of a typical SPR. It is not the intent of this article to dictate the format of the report, which might include properties such as font, type size, and position of the various elements on the typed page, nor is it our intent to dictate that the various elements be located in the particular sections delineated in the tables. However, it is clear that a well-designed SPR more effectively conveys critical information to the reader. 2 The location of the constituents within the SPR is ultimately at the discretion of its creators. The exception to this is text color, which should always be black because many methods of duplication (eg, copy machines, facsimile) do not reproduce nonblack colors well. It is our intent, however, to provide a standardized list of the content elements to be included in the various sections of the SPR so that all readers, be they certified coders, billing clerks, or paramedical or medical personnel, can be assured that all required information will be present in the SPR, regardless of the laboratory of origin.
First-Page Report Header
The SPR is being generated with ever-increasing frequency in electronic format. These guidelines should be applied to the SPR, regardless of the mode in which it is published; however, certain elements, such as the subsequent-page header or footer ( Table 2 ), might be eliminated if SPRs are generated in a purely electronic format. In the tables of this manuscript, some elements are not preceded by a symbol. These are deemed required elements that must be included in all reports. Other elements are considered preferred , as designated by the * symbol. The preferred entries are considered important to convey to the reader and should be included in some fashion, unless their inclusion is precluded by hospital policy or some other extenuating circumstance. A few of the elements are considered optional and are designated by a dagger (†).
Subsequent-Page Header and/or Footer§
The content that follows includes a detailed description of the SPR elements and the reasoning behind our decisions regarding the necessity of the various constituents.
The header on the first page of the SPR ( Table 1 ) contains critical information, including information about the laboratory, the patient, and the type of report. As such, a delimiter, such as a box or line, below the header is often used to separate its contents from the remainder of the report. Within this header, one group of elements is the detailed laboratory identifying information that must, minimally, include the laboratory's name, address, and main phone number. If the laboratory has multiple phone numbers, which might include customer service, billing, or reporting offices, these may be included as well. These identifying elements must be present on every report for easy recognition of the responsible laboratory by the reader. Additionally, the laboratory's or hospital's identifying logo or trademark, if available, should appear on every report. We recognize that some laboratory information systems may not support the inclusion of such graphics on reports; however, we feel that this is an element that provides easy identification of the origin of the SPR, and thus, we have categorized this element as preferred. Additional optional elements that provide contact information or data regarding the laboratory's credentials include facsimile phone numbers, uniform resource locator (URL) for electronic access via the World Wide Web, and Clinical Laboratory Improvement Amendments of 1988 (CLIA) number. As a preferred element, the name of the laboratory's medical director should also appear in the portion of the header that contains the laboratory's contact information. We consider this an important component of the SPR because it provides the reader with supervisory contact information, should the pathologist-of-record be unavailable, or if a discussion with the laboratory's leadership is required.
If a secondary laboratory is involved in the routine processing of tissue or ancillary testing, such as immunohistochemistry or histochemical staining, and no separate report is issued by that laboratory, that reference laboratory's contact information, including address and phone number(s), should also appear in the report. The inclusion of this information is essential for quality assurance because it may provide the only documentation of the site at which those technical services were performed. The information regarding the laboratory that provides those services need not be included in the first-page report header, but it must be included somewhere within the report.
The patient's identifying information is, without doubt, one of the most crucial aspects of the report. As such, this information should be displayed prominently within the header. The patient's name is often formatted as last name, first name, middle initial. However, other formatting conventions are acceptable as hospital or laboratory policy dictates. Additionally, it is common in Hispanic cultures for patients to use both the paternal and maternal last names. When that is the case, the paternal and maternal last names should be joined by a hyphen so the paternal surname is not confused with the middle name (eg, Jose Luis Martinez-Rendon). The inclusion of the patient's full middle name is regarded as an optional element because it may help resolve some identity issues. Additional patient identifying information must include the patient's medical record number (if applicable) assigned by the institution and date of birth (see GEN.40750 in the CAP Laboratory General Checklist, available at http://www.cap.org ). Some institutions incorporate a billing or fiscal number in addition to the medical record number to track individual physician visits. If such a tracking system is in place, that number may also be included. Many institutions also use other patient identifiers, such as age and sex. Both age and sex are considered preferred elements because they give pathologists additional, readily accessible information that often has a clinical bearing on the case. Additionally, conspicuous reporting of age and sex may occasionally serve to highlight and resolve patient identity discrepancies. The name of the submitting physician must be included in the report; the submitting physician's office phone number, however, is a preferred element. Inclusion of the phone number is preferable because it gives readers direct, efficient access to the submitting clinician's contact information; however, some laboratories may elect not to include it because of clinician preference. Similarly, the submitting physician's pager information, URL, and facsimile number may be included; these data are considered less important than the clinician's direct office phone number. In addition to the submitting clinician, other clinicians who are to receive additional copies of the report must be included. This gives readers quick access to other physicians who are directly involved in the patient's care.
Clearly, the surgical pathology accession number is one of the most critical elements contained in the report because it serves as a unique identifier for the case. As such, the accession number should be prominently placed on the first-page header of the report. Additionally it must be placed on the header or footer of subsequent pages (see below).
The various elements that constitute the history portion of the report include the patient's clinical history provided by the clinician, preoperative diagnosis, postoperative diagnosis, and clinical laboratory data. Of these components, all but the relevant clinical laboratory data must be included in the report because they provide documentation of the information that is conveyed to the laboratory by our clinical colleagues and give the clinicians important feedback that the time they took to write a clinical history on the requisition slip was noted by the pathologist. These clinical data are often critical in the proper interpretation of the case. Relevant clinical laboratory data are often included in the SPR from particular anatomic sites, such as liver, renal, and bone marrow biopsies; inclusion of these data adds a more comprehensive view of the patient's underlying pathologic condition and obviates the need to look in multiple places for sometimes disparate laboratory and anatomic pathology results. In such cases, only the laboratory data relevant to the pathologic specimen should be included. Many modern laboratory information systems contain functionality that automatically imports particular laboratory results into reports to decrease the possibility of transcription errors.
The date of specimen collection and the date the specimen was received in the laboratory are critical data in the tracking of specimens and the creation of turnaround time reports; these data are also included in information that is sent to insurance companies for billing. As such, these elements are required. The time of specimen collection and the time that the specimen arrived in the laboratory are categorized as preferred elements. Tracking these times is important for accurate construction of turnaround time reports and may help resolve issues of specimen tracking in medical centers where ancillary personnel transport specimens to the surgical pathology laboratory. Additionally, inclusion of these times on the report more accurately communicates issues of turnaround time and chain of custody to the reader. Lastly, these data are becoming more critical as time of fixation and similar measures are needed to maximize standardization for biomarker interpretation. Prefixation ischemic time and fixation time are regarded as essential data elements by the CAP; however, they are categorized as preferred with the knowledge that these data may not be attainable in all circumstances and that some laboratories prefer to record these data internally rather than in the body of the report. Optionally, the site of specimen collection may be included as well and serves to inform the reader which department within the institution collected the specimen. This information might assist in specimen resolution or identity discrepancies.
The title of the report is an important component because it gives the reader a conceptual framework from which to interpret the report. Most laboratories simply use the title “Surgical Pathology Report.” However, some laboratory information systems allow various types of reports to create different formats that cater to various clinician subsets, for example, “Dermatopathology Report” or “Hematopathology Report.” The report type should be preceded or followed by the report status indicator. Report status indicators might include “Final Report” and “Addendum Report.” These designators inform the reader about the report's contents; for example, “Addendum Report” notifies the reader that the report's contents are intended to convey additional information that expounds on the original final diagnosis (see below for further details).
Both the page number and the total number of pages must be displayed on every page of the report, but they do not need to appear in the header on the first page (see below). The time and date that the original report was printed is a required element. This information gives the reader a time point from which further copies of the report can be referenced and, thus, can be instrumental in accounting for changes in the report's content over time, especially for addendums or amended reports. The actual time that a particular hard copy of the report is printed (“Report print date and time” in Table 1 ) is considered a preferred element; its presence on the report provides a comparison to the original report date and time and may, in certain circumstances, provide data to account for changes in the report's content.
Certain critical identifying elements must appear in the header or footer ( Table 2 ) of each page of the SPR after the first page. These elements include the patient's name, preferably in the same format as in the first-page header (see above), medical record number, and surgical pathology accession number. These components serve to inform readers of the patient's identifying information on each page and obviate potential identification issues should a multipage report be separated into individual pages. The report page number and total number of pages must appear so that the reader can account for every page in the report and can be informed of the total number of pages. The presence of the report print date and time could be useful to include because they tell the reader the age of the hard copy. If the print date is quite removed from the current day, it might alert the reader that a more current version of the report may exist.
In general, the diagnosis field ( Table 3 ) contains all the information that pertains to the pathologic diagnosis. Not surprisingly, this is perhaps the most highly examined portion of the report, and as such, it is often positioned directly after the header on the first page of the report.
Diagnosis Field
The SPR, by definition, gives readers diagnoses on all specimens that are delivered to the pathology department from one operation or patient visit to a single clinician on a particular day. The diagnoses for each specimen should be separated by a specimen designator, which may be arabic numerals, roman numerals, alphabetic characters, or should be clearly linked back to a summary table, such as the block summary or the list of submitted blocks in the gross description. Following this specimen designator, the specimen title appears, which includes the body site, body subsite, and surgical procedure and is typically formatted as “Body site, subsite, surgical procedure,” for example, Right breast, upper inner quadrant, excisional biopsy for calcifications.
If a portion of the specimen title is quoted directly from the surgical pathology requisition, it may be contained in quotation marks. The use of this format standardizes the specimen-labeling syntax and allows readers of SPRs to expect the same format from institution to institution. We understand that the inclusion of the surgical procedure portion of the specimen title might not be desirable because, in some institutions, the exact surgical procedure might not be known and may lead to discrepancies in the medical record should the procedure be assumed by the pathologist of record. Because of this possibility, we have classified the surgical procedure as a preferred element. The pathologic diagnosis is self-explanatory; however, there must be a diagnosis for each specimen submitted, and it should be placed in the report in such a way as to facilitate its easy identification by readers.
The use of cancer-reporting checklists has received much attention of late. These checklists have been created by several national and international associations and serve to standardize the reporting of key pathologic findings in definitive cancer resections. These checklists also ensure inclusion of all relevant pathologic parameters. Therefore, all information from the relevant cancer-reporting checklist should be included in appropriate cases. Laboratories may use any nationally or internationally approved reporting scheme, which might include those developed by the CAP ( http://www.cap.org , last accessed March 14, 2008), the Association of Directors of Anatomic and Surgical Pathology ( http://www.adasp.org/Checklists/checklists.htm , last accessed March 14, 2008), or the International Federation of Gynecology and Obstetrics ( http://www.figo.org/docs/staging_booklet.pdf , last accessed January 4, 2008) for gynecologic malignancies. It should be noted, however, that the use of the CAP templates is mandated in the standards for accreditation of tumor registries. Alternatively, institutions may elect to create one or more documents that fulfill their own particular clinical or individual needs. These homemade checklists should, at minimum, contain the mandatory elements present in nationally or internationally accepted checklists. Although a synoptic report format is not required, provided that all the checklist elements are included in the report, use of synoptic reports is strongly preferred because they have been shown to increase both the completeness of the reporting and the comprehension by the reader. 3 If reported synoptically, the checklist may appear in another field of the SPR; however, it should be referenced in the diagnostic fields (eg, “see tumor summary”).
Some laboratories use pictorial diagrams to visually identify the biopsy site, especially for the prostate or the gastrointestinal tract. These diagrams are purely optional and might appear within the diagnostic section or elsewhere in the report.
Most surgical pathology laboratories accession cases that have been previously interpreted by other laboratories, when second opinions are requested by pathologists or clinicians from other institutions. Additionally, when patients are referred from other institutions, their relevant pathology is often reexamined by the pathology department at the receiving institution. In these cases, the date of original specimen procurement, outside accession number, block/slide designations, and the name and location of the outside laboratory should be contained within the diagnostic field, for example, Left thigh, core biopsy (Beth Israel Deaconess Medical Center Department of Pathology, Boston, MA; S07-XXXXX, slide A; 1/1/06). This information must be included in every consultation report to ensure accurate documentation of the material examined. However, some laboratories might elect to include these data in the gross description field (see below) and, therefore, inclusion of these elements in the diagnosis field is regarded as preferred.
If applicable, the transcriptionist's name and initials are optional. The presence of the transcriptionist's initials on the report can aid in the resolution of typographical or transcription errors and would help in quality assurance issues surrounding report accuracy. However, this element is not mandatory because many laboratory information systems automatically store this information within its databases so that it can be accessed easily without requiring these initials on the report.
The gross/macroscopic description section ( Table 4 ) contains the written description of all tissue or removed foreign materials received by the surgical pathology laboratory; it also includes vital documentation of the specimen's handling within the laboratory and the tissue's disposition.
Gross/Macroscopic Description
The specimen title or designation is typically reproduced verbatim from the information contained on the surgical pathology requisition form. A list of these specimen designations following the specimen number might be organized in a tabular format that precedes or follows the gross description. Alternatively, it might be incorporated into the gross description itself.
The body of the macroscopic description is most commonly written in a prose style with each specimen described separately and separated into paragraphs. When series of similarly sized biopsies are described, most commonly in the case of prostate or gastrointestinal biopsies, some laboratories elect to condense the gross description of all specimens into one paragraph that aggregates the specimen measurements into one measurement (eg, “the specimens measure from 0.1 cm to 0.3 cm in greatest dimension”). Although this approach does simplify the gross description of specimens, we feel that the creation of separate gross descriptions for each specimen, with distinct sets of specimen dimensions, is preferable because this information can more accurately be used to resolve block or slide labeling discrepancies.
The body of the gross description begins with the specimen number, which is immediately followed by the designation written on the specimen label. As noted above, the specimen label should be reproduced verbatim from the label written on the specimen container. When there is no site designated on the specimen container or when there is a discrepancy between the sites designated on the specimen container and on the requisition, that information should be clearly stated in the gross description. Most often, the specimen label is followed by the state of the specimen upon receipt in the laboratory, which might include, for example, fresh or fixed in formalin.
The macroscopic description follows the specimen number, specimen label, and specimen state. The description should be a concise, yet substantive, description of the type of specimen, salient macroscopic findings, processing information, and the disposition of the tissue. A comprehensive description of the appropriate treatment and description of gross specimens is beyond the scope of this article, and many texts have been published on the subject. 4 , 5 However, all gross descriptions must contain the size and weight of the specimens, as appropriate. A description is required for the specimen's orientation, or lack thereof, and the presence of clinically important surgical margins. A detailed description of the of specimen's inking in relation to the specimen's orientation is a required element.
Other optional elements that might be included within this portion of the report include specimen diagrams. These graphics can be quite helpful in orientation of complex resection specimens. The inclusion of these diagrams clarifies the origin of microscopic sections taken from the specimen. However, the integration of specimen diagrams or photographs is difficult for some laboratory information systems, and thus, these materials may be kept in the paper record. If gross photographs were taken, a statement to that effect should appear in the report because it informs the reader about the existence of gross photographs; the same holds true for specimen radiographs. The name or initials of the grossing personnel must appear as a separate element in the report because it informs the reader of the person(s) who was responsible for grossing the case and is important for quality assurance and tracking of the specimen's chain of custody. As an optional element, the transcriptionist's name or initials may appear in the report. Again, the inclusion provides ready access to this information for all readers of the report. However, with most modern-day laboratory information systems, this information is easily accessed by laboratory staff; in which case, the transcriptionist's identifying information does not need to be present on the report.
One of the key portions of the gross description is the method by which the specimen was processed. Mandatory elements include the type of fixative or preservative in which the tissue was submitted and the presence or absence of decalcification. Inclusion of the type of fixative or preservative is critical in informing the reader about the state of the tissue and the existence of tissue fixed by other means. This information is particularly important as molecular testing becomes more commonplace because that testing often requires tissue be treated differently from routine formalin fixation. The presence or absence of decalcification treatment has direct billing implications because charging for decalcification ( Current Procedural Terminology code 88311) requires the process be documented in the SPR. Although decalcification performed must be documented in the report, the type of decalcification and the estimated time for decalcification are optional elements. The various types of commercially available decalcification solutions are known to variably affect the antigenicity of proteins in the tissue, which may significantly affect the results of immunohistochemistry. As such, the inclusion of the type of decalcification solution might inform the reader as to the state of the decalcified tissue. Similarly, the time the tissue was exposed to preservative or fixative can be an optional element in the report. Again, the time the tissue is exposed to fixative affects the results of the assays performed on that tissue. It is known that excessive or inadequate exposure to formalin fixation alters the reactivity of the tissue to certain antigens, 6 , 7 and thus, the documentation of fixation time will likely increase in importance as initiatives that call for the standardization of tissue-based assays increase. 8
Another fundamental portion of the gross description section is the documentation of the tissue's disposition. Critical elements of this portion include a key to the paraffin blocks submitted; a tabular format is preferred because it is easier to read. However, many laboratories integrate the block key into the gross description. Further, a statement in the gross description or specimen key must include whether the tissue was submitted in its entirety or if it was sampled in a representative fashion. If additional blocks are submitted after the case has been signed out, they must be added to the report, typically, as an addendum report (see below); if the diagnosis is modified as a result of the findings in these additional blocks, that must be changed as well, usually in the format of an amended report (see below). Also, if tissue is procured for additional studies within the laboratory, such as flow cytometric, cytogenetic, or molecular testing, that must be mentioned within the gross description.
Increasingly, tissue is sent to ancillary laboratories for additional testing, stored for possible future research purposes, or sent to biorepositories. If any of these activities are performed, they must be documented in the report. In the case of additional testing done at an outside laboratory, the name of the outside laboratory must be included. Optionally, the preservation method for this retained material or tissue sent to outside laboratories might be included.
The format of the gross description is modified when slides from outside surgical pathology laboratories are examined. In this situation, required elements include the name and full address of the submitting laboratory (if available), the original date of service, and the outside accession number(s). The nature of the material received must be explicitly documented in this type of report, which includes the quantity of slides received, slide labels, type of stain present on the slides (eg, hematoxylin-eosin, trichrome stain), and paraffin block designators (if applicable). In the case of immunohistochemically stained slides, the stain name and antibody clone, if available, should also be included in the gross description. A sentence documenting that the patient's name and slide labeling information matches the accompanying original SPR is a preferred element. Addition of this statement explicitly confirms to the reader that this information was checked and verified by the accessioning personnel. Additionally, portions of the original gross description might be included in cases that require that information for full pathologic interpretation. For example, the tumor size should be included in cases of gastrointestinal stromal tumors because tumor size is a major determinant of prognosis. Although that information should be added to the gross description, it may appear in another area of the report, as necessary. Lastly, the disposition of the consult slides or blocks after the consult is an optional element (eg, “all slides and blocks were returned to the submitting institution”); that information provides explicit documentation of the handling of consult materials after the consultation is complete.
The intraoperative consultation (IOC; Table 5 ) is defined as a service performed on tissue resected in an operative setting that results in immediate information that affects the procedure as it is being performed. The elements that pertain to the IOC can be placed into a separate section of the report or, more commonly, can be integrated into the gross description. As described above in the diagnosis section, the IOC diagnosis should be preceded by the specimen designator, body site, and the surgical procedure. However, in the IOC section, the possibility of typographical errors or discrepancies in the specimen designator might result in unnecessary confusion when compared with the final diagnosis; as a result, some laboratories elect to exclude specimen designators from the IOC section, and they are, therefore, regarded as preferred elements. Possible intraoperative procedures include frozen section, gross examination, intraoperative cytology (eg, fine-needle aspirations, smear preparations), and dissection with the intent of procurement of tissue for ancillary procedures, including flow cytometry, cytogenetics, molecular studies, and electron microscopy. The exact procedure performed must be included in the report for both documentation and billing purposes. The IOC diagnosis is self-explanatory; however, at least one diagnosis must appear for each specimen examined in the intraoperative setting. Documentation about the pathologist(s) who rendered the IOC diagnosis must also appear. The time, date, and name of the physician or staff member who received the diagnosis are optional elements. The inclusion of this information in the report ensures its formal documentation in the patient's medical record.
Intraoperative Consultation (IOC)
Classically, the microscopic description section ( Table 6 ) describes the salient histopathologic findings of the case. However, in the modern era of high-volume surgical pathology laboratories, the microscopic description is becoming less common. Because this portion of the report does little to assist readers in the interpretation of the clinical impact of the diagnoses, the section is optional. However, many laboratories use this section to record the results of histochemical and immunohistochemical stains. If such results are entered here, the documentation of appropriate positive and negative control staining, as well as all pertinent information regarding the stain method or antibody clone used, should be included; if reference ranges are applicable, they might also be documented. The results of each special stain must be included in the report, although they need not be present in the microscopic description section. As mandated by the CAP Laboratory Accreditation Program, Anatomic Pathology Checklist, ANP.12425, a disclaimer regarding the use of class I analyte-specific reagents, if applicable, must be stated in reports that use these reagents. This disclaimer may appear in the microscopic description section or elsewhere in the report.
Microscopic Description (Optional)
The comments/notes field ( Table 7 ) is optional and typically includes supplementary information that documents or expounds on additional information that is directly relevant to the pathologic diagnoses rendered in the diagnosis section. These notes are usually added when subtleties of differential diagnosis or when correlation with the clinical, laboratory, or radiologic data is required. Further, this area might be used to document specimen discrepancies or to provide an explanation of disagreements in the interpretation of frozen section diagnoses.
Comments/Notes (Optional)
If previous pathology was reviewed in the course of interpretation of the case, the fact that this material was reviewed must be documented in the report (see also CAP Laboratory Accreditation Program, Anatomic Pathology Checklist, Item ANP.10050). When clinicians are contacted regarding the pathologic findings or to clarify aspects of the case, these conversations must be documented in the report. Potential scenarios where clinicians might be contacted regarding pathologic diagnoses include an unexpected finding of malignancy, the lack of chorionic villi in a missed abortion sample, or the unexpected finding of infectious organisms. In addition to the name of the clinician or medical staff, the time and date of the conversation might be included. Lastly, references to the relevant medical literature may be included. Many laboratories include this informational material in the case of rare or diagnostically difficult cases. The comment field may also contain recommendations for further treatment or biopsy from the pathologist.
An addendum report ( Table 8 ) is a type of ancillary report that contains additional information, typically the results of ancillary diagnostic studies completed after the original SPR has been released; addendum reports, by definition, only add information to a report that has been previously finalized. If the intent of this ancillary report is to change a previously rendered diagnosis or to change other content, then the report should be titled “Amended Report” (see below). For example, an addendum report might be issued to report the results of adjunct studies (eg, flow cytometry, electron microscopy, histochemistry, immunochemistry, immunofluorescence, molecular/genetic studies, cytogenetics, or microbiologic cultures of tissue), microscopic findings in additional or decalcified tissue sections, results of deeper sections if they do not change the diagnosis, the findings on slides from reprocessed or re-embedded blocks, and the results of extradepartmental consultations. If any of the ancillary studies were performed at an outside laboratory, the name and location of this laboratory must be included.
Addendum Reports
The name of the pathologist who signed out the addendum report, the original pathologist of record, and the date/time of addendum sign-out must also be included. However, the original sign-out pathologist need not be reproduced in the addendum if the original, final SPR is included when the addendum is printed or displayed electronically. If the results contained in the addendum were discussed directly with a physician or other medical staff, the name of the physician must, and the date and time of this conversation should, be included in the report for the purposes of documentation. Additionally, the name of the transcriptionist, if applicable, is optional.
Like addenda, amended reports ( Table 9 ) are added after the completion of the final report. However, amended reports are created to correct errors or discrepancies in the original final report. Typical reasons to create an amended report include correction of typographical errors, modification of the final diagnosis, or documentation of the resolution of a specimen-labeling discrepancy. In the case of typographical errors, an amended report must be issued only if that error could potentially lead to a misinterpretation of the diagnosis. When amended reports are created, the amended portion of the report must also include a reproduction of the original, unamended entry, or a reference to the incorrect information that was originally reported. It is generally desirable to provide an explanation as to why the amendment was created. The remaining elements listed in Table 9 are identical to those in the addendum section ( Table 8 ); see above for additional explanation.
Amended Reports
These other data elements ( Table 10 ) do not have an intuitive location within the report; however, that does not discount their importance. The critical elements in this table include the name of the sign-out pathologist(s) with the sign-out date and time. Although these data must appear somewhere in the report, the location of these entries varies. Some laboratories place these data into the header on the first page or immediately following the diagnosis, whereas others place the sign-out pathologist at the very end of the report. Most laboratories use electronic signature technology and, if applicable, the phrase “Electronically Signed by” must also be included. The name of the diagnosing pathologist should be preceded or followed by “Diagnosing Pathologist,” “Sign-out Pathologist” or similar wording. The pathologist's full name and credentials (eg, MD; MD, PhD; or DO) should be included. Some institutions may choose to add the name or initials of pathologists who are involved in specific portions of the pathologic examination. For example, if one pathologist performed the macroscopic examination and another examined the microscopic slides, the name or initials of the grossing pathologist might optionally be included in the report. The date of sign-out is a required element and is typically located adjacent to the sign-out pathologist's name. The time of sign-out is optional and might be included as data to facilitate quality assurance documentation, such as turnaround time reports. Many laboratories also include a statement attesting that the pathologist of record personally reviewed the pathologic material referenced in the report; this statement is an optional element because it only emphasizes the implicit responsibility of the sign-out pathologist. In laboratories that support pathology-training programs, the name or initials of the responsible trainee should be present. The inclusion of these data assist clinical staff in contacting the resident or fellow for additional information, if this is in agreement with hospital or departmental policy. Information pertaining to the patient's previous pathology testing may be included as an optional element. The inclusion of these data on the report gives readers quick access to information on the patient's previous pathology results. Additionally, the amount of information included in this element varies. Many laboratories simply list previous accession numbers, whereas others include the specimen designators as well.
Other Data Elements
Representative reproductions of photomicrographs or other images are included in the reports by some surgical pathology laboratories; however, their inclusion is optional. Additionally, some laboratories include educational or prognostic information, in certain instances. For example, some laboratories include the Partin tables, which are prognostic nomograms for prostatic adenocarcinoma, whereas others add diagrams and biopsy-site labels for both prostate biopsies and luminal biopsies of the gastrointestinal tract. Although the inclusion of this material might add some ancillary information to the interpretation of the report, it is considered strictly optional. Lastly, some laboratories include billing and diagnostic coding information in the report to more readily track that data. Possible codes that might be added include the International Statistical Classification of Diseases and Related Health Problems ( ICD-9 ), Current Procedural Terminology ( CPT ), and the Systematized Nomenclature of Medicine (SNOMED); again the inclusion of this information is optional.
The SPR is a critical document, used by many parties in medical care; it has the potential to guide diagnosis, treatment, and prognosis in a variety of disease processes. As such, this report must be clearly constructed and must include a variety of elements in pursuit of this goal. In this article, the Surgical Pathology Committee of the CAP puts forth its recommendations regarding the required, preferred, and optional elements that should be included in SPRs. Adoption of these guidelines will go far in standardizing these reports, which should minimize errors, and thus, improve patient care.
To provide feedback, please forward your comments to [email protected] or [email protected] . These and updated versions of these guidelines will be posted on the CAP Web site at http://www.cap.org .
These guidelines were developed by the College of American Pathologists' Surgical Pathology Resource Committee. We would like to thank Paul Valenstein, MD, for his thoughtful review of the manuscript. Additionally, we thank Douglas Murphy and Patricia Vasalos, for their able support of this effort, and Kumarasen Cooper, MBChB, for his insightful comments.
Additional members of the Surgical Pathology Committee include Mary Beth Beasley, MD; Hagen Blaszyk, MD; Daniel John Carter, MD; Liang Cheng, MD; Arthur R. Cohen, MD; Michael T. Deavers, MD; Megan K. Dishop, MD; Andrew L. Folpe, MD; Sanjay Kakar, MD; Robert H. Knapp, MD; Dylan V. Miller, MD; Talat M. Nazir, MBBS; Marisa R. Nucci, MD; Vijaya B Reddy, MD; Mary S. Richardson, MD DDS; and Marie E. Robert, MD.
The authors have no relevant financial interest in the products or companies described in this article.
Author notes
Reprints: Jeffrey D. Goldsmith, MD, Pathology and Laboratory Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215 ( [email protected] )
Recipient(s) will receive an email with a link to 'Reporting Guidelines for Clinical Laboratory Reports in Surgical Pathology' and will not need an account to access the content.
Subject: Reporting Guidelines for Clinical Laboratory Reports in Surgical Pathology
(Optional message may have a maximum of 1000 characters.)
Citing articles via
Get email alerts.
- eISSN 1543-2165
- ISSN 0003-9985
- Privacy Policy
- Get Adobe Acrobat Reader
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Overview on ordering and evaluation of laboratory tests.
Zoia E. Khattak ; Husam El Sharu ; Beenish S. Bhutta .
Affiliations
Last Update: August 17, 2023 .
- Continuing Education Activity
The laboratory medicine service in a hospital is concerned with relevant investigations of patient’s presenting complaints and sometimes with detection and potential prevention of disease. It involves a systematic approach of clinical advice or request for a particular investigation, analysis of the collected specimen, interpretation of results, and appropriate reporting in a timely fashion. This activity reviews the ordering and evaluation of laboratory tests and highlights the interprofessional team's role in evaluating and treating patients with the help of laboratory tests.
- Describe the typical presentation of a patient with anemia.
- Outline the most definitive test for diabetes mellitus.
- Identify the patient history associated with diabetes mellitus.
- Outline the classic presentation of a patient with anemia.
- Introduction
Laboratory medicine service in a hospital is concerned with relevant investigations of patient’s presenting complaints and sometimes with detection and potential prevention of disease. It involves a systematic approach of clinical advice or request for a particular investigation, analysis of the collected specimen, interpretation of results, and appropriate reporting in a timely fashion. These functions are carried out in a specified and designated area inside the hospital premises called the pathology laboratory, supervised by a physician who specializes in pathology. A pathologist is responsible for issuing a report and providing guidance to physician colleagues on the best utilization of laboratory services. The size and facilities of a laboratory depend upon the population of the community it serves, bed strength of the hospital, and diversity of cases seen in the hospital. [1] [2]
- Etiology and Epidemiology
Laboratory investigations play a pivotal role in diagnosing and management of diseases. The number of tests ordered daily rises with the number and complexity of diagnoses at discharge. Patients with a single diagnosis were found to undergo 7.5 tests per day. A study observed that the daily average for tests in the internal medicine department was 9.5. Similar results were found by another study performed at an American hospital stating an average of 9.67 tests per inpatient day. The average for CBC (complete blood count), an important initial test frequently ordered, was 1.07. The most frequently ordered analytes were serum electrolytes levels, particularly sodium. [3] [4]
- Specimen Requirements and Procedure
Blood Specimen
After identifying the patient, a phlebotomist takes a blood sample in a syringe from an appropriate vein (preferably antecubital). Blood is collected in properly labeled containers. Serological tests are performed on a clotted blood specimen. It should be protected from extreme temperatures during transport and refrigerated at -20 degrees celsius or lower and sent frozen to the laboratory. A serum for serology, on the other hand, is kept at 2 to 8 degrees celsius.
For blood cultures, contact the laboratory staff for appropriate media, as it depends upon the type of pathogens. Change the syringe needle before injecting blood into the culture bottle. The rubber bung of the culture bottle needs to be cleaned with iodine solution before and after injecting blood into it. The quantity of blood injected is 10% of the liquid medium volume (for 30 mL medium, 3 mL of blood is required). If possible, specimens for culture are obtained before giving antibiotics. Specimens are placed in sterile containers, and sufficient quantities are obtained to allow complete examination. The site of material depends upon the nature of illness and the site where the likelihood of isolation of organism is high. Proper labeling of samples with the patient’s name, age, type of test, date, and site of the collection is an important prerequisite. The specimens for viral cultures are transported in a frozen state at -70 degrees celsius before culture initiation.
Throat and Nasal Swab
These swabs are taken under direct and good visibility and lighting. The preferred sites are areas of exudation, inflammatory membranes, or tonsillar crypts. For nasal specimens, make the patient sit up, bend head slightly backward facing the light source, and then insert and repeatedly rotate the swab into one of the nasal cavities. Spread the isolate on 2 or 3 evenly spread smears. Air-dry the slides and send them to the laboratory for examination.
Sputum Specimen
For certain diseases like mycobacterium tuberculosis ( M. tuberculosis ), a series of 3 early morning specimens, 8-10 mL in quantity, are collected and stored in a refrigerator. In uncooperative patients, M. tuberculosis can also be recovered from gastric contents by stomach aspiration.
Fecal Specimen
Feces are passed directly into a clean container with a tight cover. They are then transferred to another container. The sample should contain pus, mucus, blood, or any formed elements passed along with stool and include the representative of the first, last, and middle of the stool. Approximately 1 ml (10%) specimen is added to 10 ml alkaline peptone water in diarrhea with cholera suspicion. In viral cases, 1 ml feces are mixed with 9 ml sterile buffered saline and sedimented/centrifuged for half an hour. The supernatant is frozen and stored below – 40 degrees centigrade until further processed.
Physical examination of feces comments on color, odor, consistency, parasites, and pH. Microscopically, we can see food residues, muscle fibers, fats, fatty acid crystals, starch particles, cellulose residues, cells (RBCs, leukocytes, and epithelial), crystals (triple phosphate, calcium oxalate, cholesterol), ova and cysts of parasites, motile amoeba (containing ingested RBC, pseudopodia), mucus, and foreign bodies (hair, wool, etc.).
Urine Specimen
Proper instructions are given to the patients who collect the specimen themselves in a sterile container. An uncontaminated mid-stream urine sample is the best for reporting purposes. Physical examination involves volume, odor, color, appearance, pH, and specific gravity estimation. Chemical examination of urine comments on quantities of normal or abnormal elements such as proteins, glucose, reducing sugars, bile pigments (bilirubin), bile salts, blood, nitrites, ketone bodies, urobilinogen, and Bence Jones proteins, etc. Microscopic examination is an important urinalysis component and can detect ova, parasites, red blood cells, leucocytes, casts, epithelial cells, and crystals. Many other cells can be seen with light-microscopy, like malignant cells, bacteria, yeast cells, trichomonas, filaria, spermatozoa, mucous threads, fat, and other artifacts. Automated equipment like Clinitek 100 and Urilux are used for routine urine examination. They are convenient, accurate, eliminates dependency on visual interpretation, allow compute readings, and decrease the need for re-testing.
- Diagnostic Tests
There are hundreds of laboratory tests ordered by physicians that help diagnose, follow-up, and prognosis of various diseases. Herein, we will focus on the most commonly reported groups of tests.
Complete Blood Count (CBC)
Common terms in CBC and their normal values are as follows
Red Blood Cell (RBC) Count: The number of RBCs per volume of blood (normal value: 4.2 to 6.9 x 109/mm3
Hemoglobin (Hb): Amount of oxygen-carrying capacity of blood (normal value: 130 to 180 g/L in males, 120 to 160 g/L in females)
Hematocrit (Hct): Percentage of whole blood occupied by packed RBCs (normal value: 45% to 62% in males, 37% to 48% in females)
Mean Corpuscular Volume (MCV): The measure of RBC size (normal value: 80 to 100 micromillimeter)
Mean Corpuscular Hemoglobin (MCH): Amount of oxygen-carrying hemoglobin inside RBCs (normal value: 27 to 32 pg/cell)
Mean Corpuscular Hemoglobin Concentration (MCHC): Average concentration of Hb inside RBCs (normal value: 32% to 36%)
White Blood Cell (WBC) Count: The number of WBCs per volume of blood (normal value: 4.3 to 11 x 109/mm3)
WBC differential:
- Neutrophils (normal value: 1.8 to 7.8 x 103/mm^3)
- Lymphocytes (normal value: 0.7 to 4.5 x 103/mm^3)
- Monocytes (normal value: 0.1 to 1.0 x 103/mm^3)
- Eosinophils (normal value: 0 to 0.4 x103/mm^3)
- Basophils (normal value: 0 to 0.2 x 103/mm^3)
Platelet Count: The number of platelets per volume of blood (normal value: 150 to 400 x 109/mm^3)
Reticulocytes: The number of immature RBCs in circulating blood (normal value: 1% of total RBC count)
Peripheral Smear
Peripheral smear gives information regarding abnormality in size, shape, color, counts, and composition (inclusions) of cells compared to normal.
Coagulation Profile
Commonly used tests for hemostasis are as follows.
Platelet Count: it gives an account of the number of platelets in the volume of blood (normal value: 150 to 400 x109/L). Low platelet count occurs in thrombocytopenia. It could be primary (isolated thrombocytopenia) without any other underlying cause, or it could be secondary with associated conditions such as HIV, HCV, SLE, CLL. Certain drugs cause drug-induced thrombocytopenia, e.g., aspirin, ethanol, and NSAIDs.
Prothrombin Time (PT): It is also reported along with laboratory control (normal range: 11 to 24s). It measures the extrinsic pathway (factor VII) and common pathway. It is prolonged in vitamin K deficiency, vitamin K antagonist therapy (warfarin), and factor VII deficiency.
International Normalized Ratio (INR): Normal range is 0.9 to 1.2. It is used to monitor warfarin therapy and for the assessment of hepatic function.
Activated Partial Thromboplastin Time (aPTT): It is reported against a normal control (normal range: 22 to 35s). It measures the activity of the common pathway and intrinsic pathway (factors VIII, IX, XI, XII). It is also used to monitor heparin therapy. It is prolonged in hemophilia A (Factor VIII deficiency) and B (Factor IX deficiency).
Other Tests
- Specific factor assays (Factor VIII)
- Lupus anticoagulant
- Tests for thrombophilias (activated protein C resistance)
- Von Willebrand tests (vWF antigen, Ristocetin cofactor activity, factor VIII)
Diagnostic Tests in Diabetes Mellitus
- Fasting Blood Glucose (FBG): normal range is <126 mg/dL
- Random Blood Glucose (RBG): normal range is <200 mg/dL
- Glycosylated Hemoglobin (HbA1c): normal value is <6.5 %
- 2 hour 75 gm Oral Glucose Tolerance Test (OGTT): normal value is < 200 mg/dL
Diagnostic Tests in Hepatobiliary Disease
The most commonly ordered tests to evaluate liver function and detect hepatocytes injury are as follows:
- PT/INR: normal range 0.9 to 1.2
- Serum Albumin: normal range 3.5 to 5.0 g/dL
- Serum Bilirubin (total): normal range 0.1 to 1.0 mg/dL
- Serum Bilirubin (direct): normal range 0 to 0.3 mg/dL
- Alanine aminotransferase (ALT): 8 to 20 U/L
- Aspartate aminotransferase (AST): 8 to 20 U/L
- Alkaline phosphatase (ALP): 35 to 100 U/L
- Gamma-glutamyl transferase (GGT): 0 to 30 U/L
- Testing Procedures
Microbiological work requires culture media and containers free of all contaminating microorganisms. There are two methods used to kill microorganisms in a sample.
Sterilization
Sterilization is defined as removing all organisms, such as viruses, bacteria, bacterial spores, fungi, and fungal spores, be it pathogenic or non-pathogenic in nature. It is used for culture media, suspended fluids, chemical reagents, containers, and other laboratory equipment.
Methods of Sterilisation
The methods used to achieve an absolute germ-free state are heat (dry heat and moist heat), ionizing radiation (beta electrons and gamma photons irradiation), filtration, and chemical disinfection (gases: ethylene oxide and liquids: glutaraldehydes).
Articles in an autoclave are exposed to moist heat (steam) at temperatures higher than 100 degrees centigrade (mostly 121 degrees centigrade) and pressure greater than the atmospheric pressure. This causes denaturation of protein, destroying the bacterial endospores as well as vegetative cells. Autoclaving is an important method to sterilize most bacteriological culture media, linen, and surgical instruments. The efficiency of an autoclave is checked with chemical and biological controls. The chemical controls include Browne's tube and Bowie Dick tape. The spores of bacillus stereothermophillus are employed as biological control and tested for viability after autoclaving.
Filtration is another method of sterilization and is used for attaining bacteria-free solutions and fluids. Filtration is used for materials that are heat-sensitive such as antisera and toxins. There are two types of filtration methods.
- Surface filtration
- Depth filtration
In surface filtration, a medium with pores (filter papers, membranes, sieves) is used, and liquids are run through it. Particles that are bigger than the pores' size are retained on the surface of the medium, and the remaining filtrate is collected at the bottom.
Depth filtration follows a similar mechanism as surface filtration, but the particles are retained on the surface and the body of the filter. The fibers network is arranged in such a manner that they retain bi particles. Examples include earthenware (Berkfield and Chamberland), asbestos (Seitz), sintered glass, and cellulose membrane.
Disinfection
Disinfection implies killing most vegetative forms of bacteria, viruses, fungi, and parasites but does not completely eradicate spores and non-vegetative forms. They kill by destroying proteins, lipids, or nucleic acids in the cell itself or its cell membrane. They are particularly used to disinfect surfaces that contact body fluids, tissues, pathological tissue, or samples and microbiological cultures. They are mainly classified into antiseptics and disinfectants.
Antiseptics are diluted forms of disinfectants and are non-toxic for living tissue. Examples include spirit, povidone, alcohol, etc.
Disinfectants are concentrated forms of chemicals and are corrosive to living tissues. They disinfect non-living objects. Examples include phenolic compounds (phenol, Lysol), halogen compounds (chlorine), metallic salts (mercuric chloride, silver nitrate 1%), formaldehyde, volatile solvents (ethyl alcohol, acetone), gaseous disinfectants (gentian violet, potassium permanganate), soaps and detergents.
It is a procedure used to identify the quantity of acid or base in a solution with an indicator's help. It helps find an estimate of acids and bases in body fluids such as gastric juice HCl.
Electrophoresis
It is based on the mechanism of the mobility of ions in an electric field. Positively charged ions attract o negative electrode (cathode), and negatively charged ions migrate towards a positive electrode (anode). It is used for the identification and quantification of abnormal patterns of proteins in disease processes. It delineates normal and abnormal protein bands, normal and abnormal hemoglobin, quantification of lipoproteins and isoenzymes.
Chromatography
This is a technique that involves separating pure substances from mixtures. It is based on the fact that different substances follow moving solvents at different speeds. There are two main types.
- Column chromatography
- Layer chromatography
- Thin-layer chromatography
- Liquid chromatography
- High-performance liquid chromatography
Some of the chromatography applications include identifying, quantifying, and analyzing drugs, drug metabolites, toxic materials, hemoglobin variants, amino acids and carbohydrates, hormones, etc., in a sample.
Molecular Techniques
Molecular biology involves amplifying small genetic material (DNA/RNA) and its detection using electrophoresis, ELISA (enzyme-linked immunosorbent assay), and chemiluminescence. Examples of amplification techniques are as under:
- Polymerase chain reaction (PCR)
- Ligase chain reaction (LCR)
- Nucleic acid-based amplification (NASBA)
- Strand displacement assays (SDA)
PCR has useful applications in cases of infectious diseases (tuberculosis, hepatitis B and C, EBV, HIV), genetic disorders (thalassemia, inborn errors of metabolism, insulin gene mutations, cystic fibrosis, muscle dystrophies), antenatal diagnosis, oncogenes, medicolegal science, and tissue typing.
- Interfering Factors
There are preset protocols on how to approach, order, and evaluate laboratory tests. Clinical reasoning is necessary before ordering particular laboratory tests. The following queries should be answered before ordering laboratory tests:
- Is the test logically consistent with the diagnostic hypothesis in question?
- Would any change in the clinical condition of the patient justify the test or its repetition?
- Can the test outcome change treatment decisions?
- How safe is the test?
- Is there any harmful consequence if the test is not ordered? [3]
- Results, Reporting, and Critical Findings
Approach to Interpretation of CBC
To understand complete blood count, it is important to understand the process of hemopoiesis. Hemopoiesis involves forming mature blood cells from immature cells and consists of a series of distinctively recognizable cells. The production of RBCs, WBCs, platelets, and lymphocytes are named erythropoiesis, granulopoiesis, thrombopoiesis, and lymphopoiesis, respectively. There is a balance between the production and destruction of cells, and each cell has a normal range of count in normal human blood.
If all cell lines are decreased, it is called pancytopenia. Pancytopenia occurs in viral infections, malignancies, bone marrow disorders, autoimmune diseases, a side effect of drugs, and after chemotherapy or radiotherapy.
If RBCs and platelets are decreased, the cause is MAHA/TPA
If a single line is affected, then the approach is different, e.g., low RBCs is called anemia, and a specific approach is followed. Similarly, raised WBCs and/or the presence of different immature cells of white blood cell lineage in the blood are referred to as leukemia.
A decrease in hemoglobin level or total circulating red cell mass for a specified age and sex of a person is called anemia. Clinical features include fatigue, weakness, shortness of breath, palpitations, dizziness, headache, and decreased exercise tolerance.
Diagnostic tests for anemias:
The following initial investigations are performed if anemia is suspected:
- CBC with differential
- Retic count
- Peripheral blood smear
Morphologically, anemias are classified into three major types
- Microcytic hypochromic anemia: RBCs are smaller and in size and contain less hemoglobin than normal cells. Examples: Fe deficiency anemia, thalassemia, sideroblastic anemia, and anemia of chronic disease.
- Macrocytic anemia: RBCs are larger than normal, and the amount of hemoglobin may be normal or below normal. Examples: B12 deficiency anemia, folate deficiency anemia, drugs, alcoholism, liver disease, and hypothyroidism.
- Normocytic normochromic anemia: RBCs are normal in size, although the hemoglobin concentration in blood is reduced. Examples: acute blood loss, leukemias, bone marrow infiltration, chronic renal failure, and chronic infections.
Leukemia is defined as the uncontrolled proliferation, abnormal maturation, and accumulation of various intermediate cells. These are divided into acute and chronic leukemia based on disease and the maturation of malignant cells in blood and bone marrow.
Acute Myeloid Leukemia (AML)
It is a rapidly progressive hematological malignancy characterized by the failure of myeloid cells to differentiate beyond the blast stage. The classic finding is circulating blast with Auer rods (azurophilic granules) on peripheral blood film.
Chronic Myeloid Leukemia (CML)
It is an increased proliferation of the granulocytic cell line without loss of their differentiation ability. There is a leukoerythroblastic picture on peripheral blood film with a predominance of immature red cells and granulocytes. Diagnostic test for CML is the presence of bcr-abl fusion gene in more than 90% of patients.
Acute Lymphoblastic Leukemia (ALL)
In ALL, the early lymphoid precursor cells proliferate and replace normal progenitor cells in the bone marrow. Leukemic lymphoblasts lack specific morphological features; therefore, diagnosis requires immunophenotyping. Cytogenetics shows Philadelphia chromosome (Ph) in approximately 25% of adult cases.
Chronic Lymphocytic Leukemia (CLL)
It is a clonal malignancy of mature B-cells. CBC detects population of B lymphocytes >5 x 10/L. On the peripheral blood picture, there are small mature lymphocytes and numerous smudge cells.
Approach to Disorders of Hemostasis
In a patient suspected of coagulation disorder, the investigations should be pre-planned. History and physical examination findings are significant and help decide whether the patient has a vascular defect, platelet defect, or a defect in one of the coagulation factors. It also gives clues of the hereditary or acquired nature of the disorder and narrows down the tests performed. The preliminary tests ordered include platelet count, PT, and aPTT. Further course of action is decided based on the results of these tests.
Approach to Suspected Diabetes Mellitus
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia with disturbances in carbohydrate, protein, and fat metabolism resulting from defects in insulin secretion, its action, or both. Typical DM symptoms are polyuria, polydipsia, weight loss, polyphagia, blurred vision, and increased susceptibility to infections. There are two major types of DM.
- Type 1 diabetes mellitus (immune-mediated, idiopathic)
- Type 2 diabetes mellitus
Any one of the following is diagnostic
- FBG ≥ 126 gm/dL OR
- 2 hr OGTT ≥ 200 mg/dL OR
- RBG ≥ 200mg/dL along with classic symptoms of DM OR
- HbA1c ≥ 6.5 %
Impaired fasting glucose:
Patients who have FBG between 109 to 126 mg/dL are labeled to have impaired fasting glucose and are then subjected to 2 hr 75 gm OGTT to confirm DM.
Approach to Liver Function Tests
An increase in PT/INR, serum albumin, serum direct bilirubin, and disproportionately high ALT or AST implies hepatocellular dysfunction. ALT is more specific to the liver; AST comes from multiple sources (especially muscles). AST>ALT is suggestive of alcoholic liver disease.
Serum transaminases >1000 signify direct hepatocyte injury, and the differentials include viral hepatitis, drugs/toxins, autoimmune hepatitis, and hepatic ischemia.
When ALP is elevated alone, rule out the bone disease by fractionating ALP. A disproportionately high ALP and GGT are evident of cholestatic liver diseases (e.g., cholelithiasis, cholangiocarcinoma, sclerosing cholangitis).
- Clinical Significance
Laboratory tests are beneficial for patients if an illness is detected, and the monitoring is timely and might have negative effects if the patient complaint is not diagnosed and monitored. Some unnecessary consequences include but are not limited to unwanted phlebotomies leading to phlebitis, pain, journeys, delay in important tests, and improper use of patient and technician time.
A campaign named “Choosing Wisely” by the American Board of Internal Medicine in North America advocates for choosing clinical value tests and encourages patients and healthcare workers to mobilize laboratory resources wisely. Tests that are ordered sometimes have major side effects: they are responsible for a <5% increase in healthcare costs but affect 70% of major decisions regarding admission, treatment, and discharge plan. A study defined by clinical guideline’s recommendations demonstrated that repeat testing is unnecessary in about 25 to 30% of cases. Among medical specialties, family practitioners represent the largest group of doctors ordering laboratory tests, followed by the internal medicine department. [5] [6] [7] [1] [2]
- Quality Control and Lab Safety
There are two types of quality control methods
Internal Quality Control
It ensures the accuracy and precision of all procedures and equipment by daily checking by the laboratory staff. The following tools are utilized.
Levy-Jennings Chart (LJ Chart)
Levy-Jennings chart estimates accuracy and precision. Serial daily measurements of controls are used to calculate the mean values of standard deviation (SD). The values are then plotted on a graph paper representing mean +-1SD, +-2SD, and +-3SD in horizontal lines. Both normal and abnormal values of controls are plotted on the LJ chart and are monitored daily using Westguard rules.
Five Cycle Quality Control Charts
This chart facilitated the recording of daily values by running controls 5 times repeatedly, giving a wide range of values. Horizontal lines are drawn at mean and +-2SD from standard reference. Control is randomly introduced in daily runs, and the mean is calculated at the end of the day. The results are then plotted on the graph. If the numbers keep increasing or decreasing on one side of the mean value, it is denoted as the upward or downward trend.
Moving Two Standard Deviation Charts
These charts comprise four columns. The left-most column is for recording daily changes from mean assigned to a control. The mean value in the control run during a particular day is calculated, and the difference from the assigned mean is deduced. The values are entered in the assigned slots for respective days. At the end of the week, the values are added and divided by two, which gives 2SD reading. The weekly 2SD values should not differ remarkably from the assigned 2SD value of the control.
Youden Plots
These are specially designed to compare results on two controls. One set is plotted on the x-axis and another set on the y-axis. Zero lines are drawn joining crossing points of axes and +-2SD values. It is inferred that 95% of all results fall in 2SD squares on sides of zero lines.
Results on Patient’s Sample
Specific patient’s sample from the previous day is selected and run to check between run precisions. Mean is calculated from patient results within a defined range and should be relatively constant. The values could be plotted in a graph format. Laboratory errors and non-analytical sampling, and transportation errors affect this method.
Cumulative Sum (CUSUM) Charts
It is applied to daily means or control values. The values are graphically plotted such that the SD value of a given day is added or subtracted from previous day results to obtain a CUSUM chart rather than plotting mean values daily. The outcome should be a straight line of cusum values if accuracy remains unchanged.
2. External Quality Control
This ensures quality control by merging the performance of different laboratories. Samples are distributed to participating laboratories, and results are analyzed critically and statistically. These techniques also allow comparison of various methodologies used by different laboratories and infer recommendations for standard methods.
Laboratory Safety Practices
- Proper handwashing before and after work
- Use personal protective equipment, e.g., gloves, mask, goggles, gowns, etc., while handling reagents and testing materials.
- Do not eat, drink, or smoke on laboratory premises
- Never pipette with mouth
- The work area should be free of sharps, glassware, and chemicals
- Read manufacturer instructions before storage of all laboratory equipment and chemicals
- Properly label all containers in bold letters and always read labels before using any chemicals
- Put warning signs at hazardous places
- Learn proper skills before using unfamiliar equipment and unknown techniques
- Use caution during transferring and mixing chemicals.
- Enhancing Healthcare Team Outcomes
It is the responsibility of basic medical science teachers to teach future clinicians how to approach patients, exercise careful clinical thinking, and apply general principles before requesting laboratory tests. This process begins in medical school by teaching the benefits of high-value, cost-effective laboratory testing. Janssens et al. reviewed various studies and found options to halt the unreasonable demand for laboratory tests. Education and awareness play a vital role, particularly in a teaching university hospital with residents under training. However, it has been found to have limited success due to these institutions' short-term educational opportunities.
Strict protocols need to be followed in the light of clinical reasoning, and proper rationale should be supplied for ordering multiple laboratory tests. The size of the population used to be the predictor of overall laboratory test demand previously. However, an increasing number of primary care physicians might reduce this demand due to fewer visits of patients to emergency departments and urgent care centers. This discourages the practice of defensive medicine and also decreases hospital admissions, surgical operations, and emergency department visits. [8] [9] [10] [7]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Zoia Khattak declares no relevant financial relationships with ineligible companies.
Disclosure: Husam El Sharu declares no relevant financial relationships with ineligible companies.
Disclosure: Beenish Bhutta declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Khattak ZE, El Sharu H, Bhutta BS. Overview on Ordering and Evaluation of Laboratory Tests. [Updated 2023 Aug 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- [Consultation on laboratory information--from the perspectives of clinical pathologists and medical technologists]. [Rinsho Byori. 1998] [Consultation on laboratory information--from the perspectives of clinical pathologists and medical technologists]. Shimetani N. Rinsho Byori. 1998 Oct; 46(10):987-93.
- [A role of clinical pathologists in Laboratory Information (Consulting) Center]. [Rinsho Byori. 1999] [A role of clinical pathologists in Laboratory Information (Consulting) Center]. Shimetani N, Ohtani H. Rinsho Byori. 1999 Oct; 47(10):926-32.
- Critical Care Network in the State of Qatar. [Qatar Med J. 2019] Critical Care Network in the State of Qatar. Hijjeh M, Al Shaikh L, Alinier G, Selwood D, Malmstrom F, Hassan IF. Qatar Med J. 2019; 2019(2):2. Epub 2019 Nov 7.
- Review Evidence Brief: Effectiveness of Intensive Primary Care Programs [ 2013] Review Evidence Brief: Effectiveness of Intensive Primary Care Programs Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. 2013 Feb
- Review Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses [ 2014] Review Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses McCleery E, Christensen V, Peterson K, Humphrey L, Helfand M. 2014 Sep
Recent Activity
- Overview on Ordering and Evaluation of Laboratory Tests - StatPearls Overview on Ordering and Evaluation of Laboratory Tests - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Call timings: 10:30AM - 5:30PM, Mon.-Fri.
Call timings 10:30AM - 5:30PM, Mon.-Fri.
WhatsApp +91-9318313723
Call us on +91-9318313723
Free business site for labs is coming soon. Apply now for early access
Pathology Report Format
A standard pathology report consists of basic patient details, TAT information, registration number, referring doctor name and degree, signature of lab in-charge, signature of pathologist in-charge along with the test results and page number information.
All the reports are printed in the standardized template with all the information mentioned before except for the test results. Presentation of the test results vary depending upon the nature of test being conducted.
It is the latest trend to provide QR coded lab reports which helps in verifying the authenticity of the report. Thus increasing the confidence of the patient and the doctor in diagnostic and helps in performing the treatment efficiently and accurately.
Package Report Formats -->
What are packages?
Packages are set of individual tests and panels.
Panel Report Formats -->
What are panels?
Panels are set of individual tests.
Haematology Test Report Formats -->
Biochemistry test report formats -->, clinical pathology test report formats -->, cytology test report formats -->, endocrinology test report formats -->, histopathology test report formats -->, microbiology test report formats -->, serology and immunology test report formats -->, miscellaneous test report formats -->.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Interpretation of Pathology Report (ppt)

Related Papers
IP Archives of Cytology and Histopathology Research
PREMA LATHA Y
Laboratory Medicine
Michael Becich
Andrea Vingiani
An accurate pathological assessment of core biopsies or resection specimens provides important information on the major features of breast cancer, such as tumor type, size, biological characteristics, lymph node status, stage, and extent of residual disease in case of neoadjuvant chemotherapy, and is crucial for ensuring an appropriate patient management. In the era of molecular medicine and tailored therapies, the pathologic assessment of primary tumor still represents an essential guide for oncologists and surgeons to inform the choice of the best treatment options available for individual patients. Therefore, the management of patients with breast cancer detected through imaging or symptomatic presentation depends heavily on the quality of the pathology service.
Jon Plumley
Cancer Imaging
Rodney Hicks
Quality of Life Research
Purva Abhyankar
Caro Sluijter
Abstract Pathology reporting is evolving from a traditional narrative report to a more structured synoptic report. Narrative reporting can cause misinterpretation due to lack of information and structure. In this systematic review, we evaluate the impact of synoptic reporting on completeness of pathology reports and quality of pathology evaluation for solid tumours. Pubmed, Embase and Cochrane databases were systematically searched to identify studies describing the effect of synoptic reporting implementation on completeness of reporting and quality of pathology evaluation of solid malignant tumours. Thirty-three studies met the inclusion criteria. All studies, except one, reported an increased overall completeness of pathology reports after introduction of synoptic reporting (SR). Most frequently studied cancers were breast (n = 9) and colorectal cancer (n = 16). For breast cancer, narrative reports adequately described ‘tumour type’ and ‘nodal status’. Synoptic reporting resulted in improved description of ‘resection margins’, ‘DCIS size’, ‘location’ and ‘presence of calcifications’. For colorectal cancer, narrative reports adequately reported ‘tumour type’, ‘invasion depth’, ‘lymph node counts’ and ‘nodal status’. Synoptic reporting resulted in increased reporting of ‘circumferential margin’, ‘resection margin’, ‘perineural invasion’ and ‘lymphovascular invasion’. In addition, increased numbers of reported lymph nodes were found in synoptic reports. Narrative reports of other cancer types described the traditional parameters adequately, whereas for ‘resection margins’ and '(lympho)vascular/perineural invasion’, implementation of synoptic reporting was necessary. Synoptic reporting results in improved reporting of clinical relevant data. Demonstration of clinical impact of this improved method of pathology reporting is required for successful introduction and implementation in daily pathology practice.
Archives of pathology & laboratory medicine
Carolyn Compton
World Journal of Nuclear Medicine
Ethel Belho
RELATED PAPERS
Mountain Research and Development
Ben Campbell
Ewa Gregoraszczuk
Vie et Milieu
Sharon Smulders
Dorota Gołek-Sepetliewa
Cadernos EBAPE.BR
Carla Winter Afonso
Jurnal Ilmiah Kesehatan
Amran Anwar
General Topology and its Applications
Paul Bankston
Psychiatry Journal
yonas tesfaye
Transportation Letters
Tomás Ruiz Sánchez
Jadwiga Laska
Jurnal_Kebidanan
Elise Putri
Contemporary Theatre Review
Ryuta Suzuki
Lecture Notes in Computer Science
Maryam Arab
IOP Conference Series: Materials Science and Engineering
Evgenii Strizhakov
Annals of Otology, Rhinology & Laryngology
Andrew Thamboo
Review of Scientific Instruments
Burak Yedierler
KnE Social Sciences
safitri hariani
Entomologia Experimentalis et Applicata
richard shelby
Said Dermime
Clinical vision sciences
Arnold J Wilkins
Resistenze Quotidiane
Jason W. Moore
Monteagudo: revista de literatura española, hispanoamericana y teoría de la literatura
Francisco Florit Durán
Stephen Hammill
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Pathology Lab Report Format - Lab SAAS
India's First AI based Smart Report Providing Lab Software Trusted By 20,000+ Doctors
Drlogy Pathology Software is a Complete Pathology Lab & Diagnostic Management system, with an integrated information system design to manage the Medical Imaging Centers, Medical Laboratories & Pathology Centers.
Our cloud-based pathology lab management software with modern AI technologies and innovation helps even the busiest pathology labs run more efficiently by automating & digitizing every task to grow more.

Pathology Lab Report
- A standard pathology report typically includes basic patient details such as name, identification number, and relevant medical history, as well as information on the type of specimen, the source of the specimen, and any relevant details about the collection and handling of the specimen.
- The report also includes the name and degree of the referring doctor and the signature of the lab in charge and the pathologist in charge.
- It also includes the TAT (Turn Around Time) information and registration number.
- The test results are presented in a standardized template, with the layout and format depending on the nature of the test being conducted.
- It is common to see results presented in tables, graphs, or images, depending on the type of test.
- It is the latest trend to provide QR-coded lab reports, which helps in verifying the authenticity of the report and increasing the confidence of the patient and the doctor in the diagnostic process.
- This can help to ensure that the treatment is performed efficiently and accurately.
Lab Software Handle report format for all Departments
Qr coded lab reports.

Phlebo Collection & Sharing Management


Pathology Lab Report Format
A pathology lab report typically includes the following sections:
1. Patient Information
- This section includes the patient's name, identification number, age, sex, and relevant medical history.
2. Specimen Information
- This section includes the type of specimen, the source of the specimen, and any relevant details about the collection and handling of the specimen.
3. Clinical Information
- This section includes information provided by the ordering physician, such as the reason for the test and any relevant clinical findings.
4. Laboratory Results
- This section includes the results of the laboratory tests performed on the specimen, including any relevant measurements or observations.
5. Interpretation
- This section includes the pathologist's interpretation of the laboratory results and their relationship to the patient's clinical condition.
6. Conclusion
- This section includes a summary of the main findings and any recommendations for further testing or treatment.
7. Signature
- This section includes the signature of the pathologist who reviewed and interpreted the results.
Fully Customization in Lab Report
Fully Report Customization at your end

Pathology Lab Report Example
Here is an example of a pathology lab report

Pathology Lab Report Types
There are several different types of pathology reports, depending on the type of specimen and the tests being performed. Some scientific pathology report common types include:

Panel Report Formats
What are panels.
The panel typically refers to a group of laboratory tests that are ordered and conducted together to gather comprehensive information about a particular medical condition or to assess multiple aspects of a patient's health.
For example, a lipid panel is a group of tests that measure different types of cholesterol and triglycerides in the blood, providing insight into a person's cardiovascular health. Similarly, a metabolic panel includes tests to evaluate kidney function, electrolyte balance, and blood sugar levels.
Here is an example of a Panel Report Formats.

Hematology Test Report Formats
What is hematology test report.
A hematology test report is a document generated by a laboratory as a result of various tests performed on a blood sample. These tests are collectively known as hematology tests and focus on the analysis of blood components, including red blood cells, white blood cells, and platelets, as well as other parameters like hemoglobin levels, hematocrit, and more.
Here is an example of a Hematology Test Report Formats.
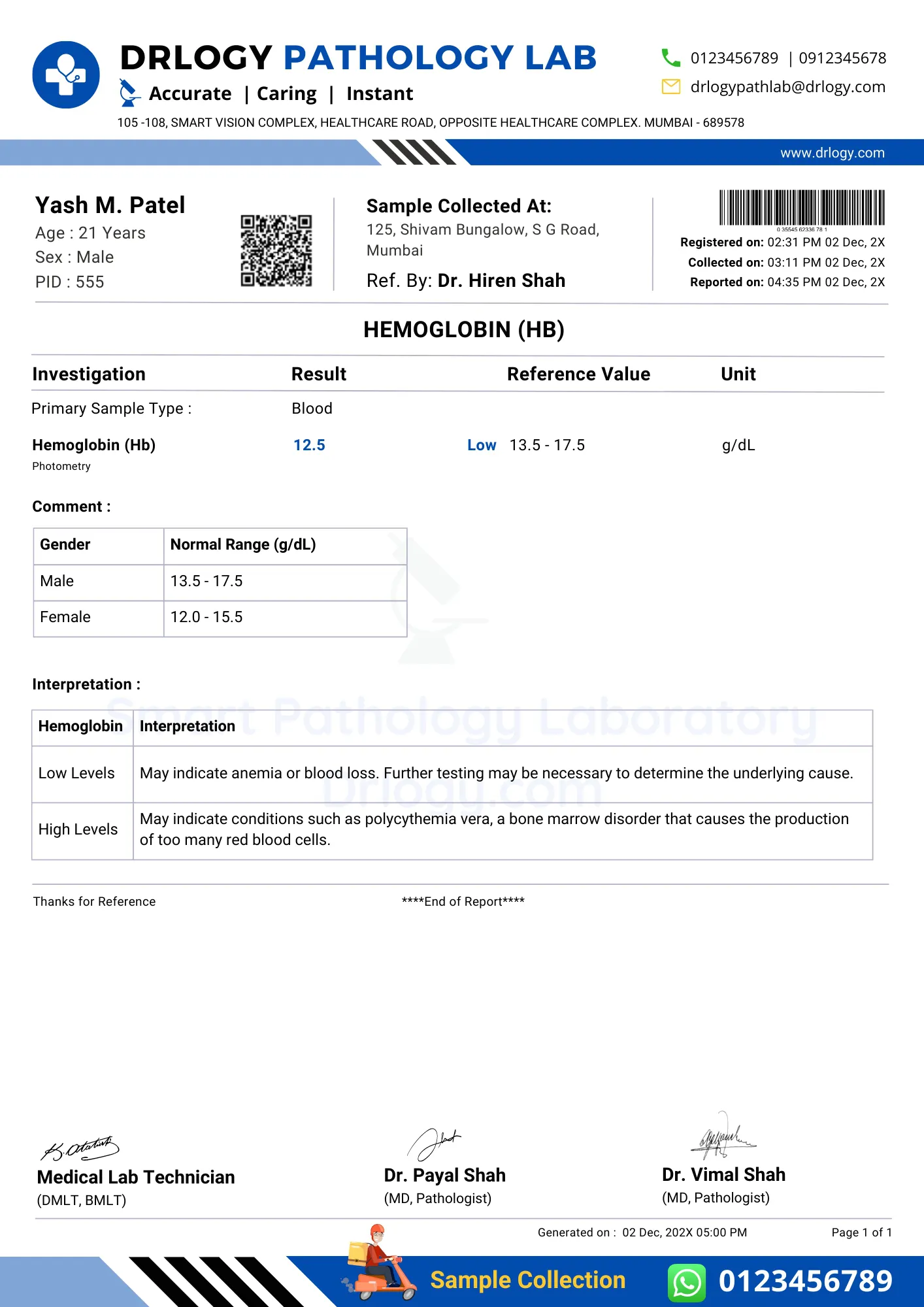
Biochemistry Test Report Formats
What is biochemistry test report.
A biochemistry test report, also known as a clinical chemistry report, is a document generated by a medical laboratory after conducting a battery of tests to analyze various chemical components present in a person's blood, urine, or other body fluids.
Here is an example of a Biochemistry Test Report Formats.

Clinical Pathology Test Report Formats
What is clinical pathology test.
A Clinical Pathology Test Report is a document generated by a clinical pathology laboratory that provides the results of various laboratory tests conducted on a patient's samples, such as blood, urine, or other bodily fluids.
Here is an example of a Clinical Pathology Test Report Formats.

Histopathology Test Report Formats
What is histopathology test report.
A histopathology test report is a document generated by a pathology laboratory that provides detailed information about the microscopic examination of tissue samples removed from a patient's body.
Here is an example of a Histopathology Test Report Formats.

Cytopathology Test Report Formats
What is cytopathology test report.
Cytopathology is a branch of pathology that deals with the study of cells, particularly those that are shed or extracted from various parts of the body. A cytopathology test report provides information about the microscopic examination of cells obtained from body fluids, lesions, or tissues.
Here is an example of a Cytopathology Test Report Formats.

Microbiology Test Report Formats
What is microbiology test report.
Microbiology tests are laboratory procedures performed to study microorganisms such as bacteria, viruses, fungi, and parasites. These tests are conducted to identify, characterize, and understand the properties of microorganisms and their interactions with human health and the environment.
Here is an example of a Microbiology Test Report Formats.

Serology and Immunology Test Report Formats
What is serology and immunology test report.
A serology and immunology test report is a document that provides the results of laboratory tests related to the study of antibodies and immune responses in the body.
Here is an example of a Serology and Immunology Test Report Formats.

Endocrinology Test Report Formats
What is endocrinology test report.
An endocrinology test report is a document that presents the results of various medical tests related to the endocrine system.
Here is an example of a Endocrinology Test Report Formats.

Imaging & Radiology Report Formats
What is imaging & radiology report.
An Imaging & Radiology report is a document generated by a radiologist or medical imaging specialist after analyzing the results of various diagnostic imaging tests. These reports provide detailed information about the findings from imaging procedures such as X-rays, CT scans, MRIs, ultrasounds, and more.

Pathology Lab Report Format in Word
- Instead of using Pathology Lab Report Format in Word, You should use the online pathology lab report format.
- The report would be written in paragraphs and may include headings, tables, and images to present the information in a clear and easy-to-understand format.
Here is an example of how such information may be presented in a word format:
Patient Information:
- Name: Yash Patel
- Date of Birth: 01/01/1976
- Medical Record Number: 123456
- Specimen Information:
- Type of Specimen: Skin biopsy
- Date and time of collection: 01/01/2021 12:00 PM
- Test Requested: Biopsy of suspicious skin lesion
- Results: Microscopic examination revealed a proliferation of atypical cells consistent with malignant melanoma. Immunohistochemical stains were positive for S-100 and Melan-A.
- Interpretation: The findings are consistent with a diagnosis of malignant melanoma, a potentially deadly form of skin cancer. The patient should be referred to a dermatologist or a surgical oncologist for further management.
Pathology Lab Report Format in Excel
A pathology report in excel format would typically include the following information organized in columns and rows
Here is an example of how such information may be organized in excel:
It's worth noting that the example above is a simple representation of how the data could be organized, and the actual format of a pathology report in excel may vary depending on the lab, the specific test, and the data being collected.
But Instead of Pathology Lab Report Format in Excel & Word or pathology lab report pdf, you should use the Online pathology lab report format. Here are some benefits of that.
Pathology Lab Report Benefits
There are several benefits to having online pathology reports, including
- Convenience : Online pathology reports can be accessed from anywhere and at any time, making it easier for healthcare professionals and patients to access and review test results.
- Faster turnaround time : Online pathology reports can be generated and shared more quickly than traditional paper reports, which can lead to faster diagnosis and treatment.
- Improved communication : Online pathology reports can be shared easily among healthcare professionals, allowing for better coordination of care.
- Reduced errors : Online pathology reports can be designed to include built-in quality control checks, which can help reduce the risk of errors.
- Reduced costs : Electronic pathology reports can be less expensive to produce and distribute than paper reports, which can lead to cost savings for healthcare organizations.
- Better data management : Online pathology reports can be stored and organized electronically, which makes it easier to track and analyze patient data over time.
- Easy to share with patients : patients can access their own reports, which can lead to better patient engagement and education about their health.
- Improved data security : online pathology reports can be more secure than paper reports, as they can be password-protected and stored on secure servers.
Overall, the use of online pathology reports can improve the efficiency, accuracy, and communication of pathology results and enhance the quality of patient care.
Pathology Software Lab Report FAQ
What is a lab report in the pathology lab.
- A lab report in a pathology laboratory is a document that describes the results of tests and examinations performed on samples of tissue, blood, or other bodily fluids.
- The report typically includes information about the patient, such as their demographic information and relevant medical history, as well as the results of the tests or examinations that were performed.
- This information can include the results of microscopy, histology, and various molecular assays.
- The report should also include a summary of the findings, a diagnosis if one is made, and any relevant clinical information or recommendations.
- The purpose of a lab report in a pathology lab is to provide accurate and detailed information about the patient's condition to the physician or other healthcare provider, who will use this information to make a diagnosis and plan the appropriate treatment.
What are the 7 sections of a lab report?
A typical lab report includes the following sections:
Please note that, depending on the experiment, the professor may require additional or fewer sections, and may also change the names of the sections.
What is the most important part of a lab report?
- The most important part of a lab report is the discussion or interpretation section, where the results of the experiment are interpreted, analyzed, and explained in relation to the research question or hypothesis.
- This is where the results are explained in detail and their significance is discussed.
- The discussion should clearly link the results to the hypothesis being tested, should explain the patterns and trends in the data, and should provide an explanation of any unexpected results.
- In addition, the discussion should also be the place where the researcher highlights the limitations and any sources of error in the experiment.
- It is also the place where the researcher can make recommendations for future research.
- It is also important that all sections of the lab report are well-written, clear, and concise.
- The introduction should provide enough background and context, the methods should be clearly described and the results should be accurately presented and clearly discussed.
- The conclusion should summarize the main findings and link them back to the research question or hypothesis.
Overall, the use of QR codes in lab software can help increase efficiency, improve patient care, and reduce errors in laboratory environments by providing quick and secure access to information.
What is a pathology report?
- A pathology report is a document that describes the findings of a pathological examination of a tissue sample.
- It is usually prepared by a pathologist, a medical doctor who specializes in diagnosing diseases by examining tissue samples under a microscope.
- The report typically includes information about the type of tissue sampled, any abnormal cells or structures present, and a diagnosis or a list of possible diagnoses.
- Pathology reports are important for guiding treatment decisions and monitoring the progression of a disease.
What is on a pathology report?
A pathology report typically includes several key pieces of information, including
It is important to note that the content of the pathology report may vary depending on the type of tissue sample and the reason for the examination. The report is usually signed by the pathologist who performed the examination.
Why Drlogy Pathology Lab Management Software?
Speed Up Your Growth
Good Return on Investment
Surplus Profit Achieve
Speed up your Operations
Reduce Daily Expenses
Productivity
Staff Work Efficiency
Why Drlogy Lab SAAS?

Fully Secure
Easy To Use

Start in Second

Universal App

Regular Updates

24*7 Support
Best pathology lab software ideal features, lab report format, smart reports, smart billing, radiology reports, qr code report, phlebo management, reference doctor portal, patient portal, request free pathology lab software demo.

Drlogy Healthcare Software - SAAS

Drlogy Hospital Management Software
Powerful Hospital Management system includes OPD, IPD, ICU, OT, Labs, Pharmacy, Store, Inventory, MRD, Billing, Patient Portal, Online Appointments, Telehealth & other 30 Modules to manage small to large scale Hospitals.

Drlogy Pathology Lab Software
Drlogy Pathology Software is a Complete Pathology Lab & Diagnostic Management system, with AI-based integrated information system design to manage the Medical Imaging Centers, Medical Laboratories & Pathology Centers.

Drlogy Clinic Management Software
Drlogy Clinic SaaS helps to speed up & digitize every process of medical practice including the history, e-prescriptions, investigation, procedures, billing, etc. without compromising the accuracy & improve the patient experience.

Drlogy Dental Software
Dental Dental Software (Dental SaaS) is an advanced AI-based powerful affordable Dental Clinic Software that helps Dentist to improve the efficiency of a clinic with good patient satisfaction.

Drlogy Ophthalmology Software
Drlogy Ophthalmology EHR Software specifically designed for Ophthalmology Practices, advanced AI-based solutions help to early detection of eyes medical conditions & enhance the patient experience.
- Pathology Lab Software >
Drlogy Healthcare Software
- Hospital Management Software
- Clinic Software
- Ophthalmology Software
- Dental Software
- Pathology Lab Software
- Pharmacy Software
- Agency Software
Copyright © 2024 Drlogy Technologies Pvt. Ltd. All rights reserved.
Get A Free Software Demo !

IMAGES
VIDEO
COMMENTS
PATHOLOGY LABORATORY GOOD PRACTICES MAAL GIDELIES 8 The pathology laboratory should have records of all procedures performed, in addition to all stages of laboratory tests, from collection and reception of specimens to delivery of reports and results. These records must be an integral part of the quality manual and/or SOPs, validated by the
To ensure that the report is about you and your specimen, each pathology report contains your patient identi˜ers—speci˜c information that relates directly to you and includes your name, birth date, and hospital or medical record number. In addition, your pathologist's name and signature and the laboratory's name
pathology report that are not explained in this booklet, don't be afraid to ask your doctor what they mean. three lab reports from one surgery. Together, the lab reports make up your pathology report. Try to keep all your reports in one place, so that when you go for your treatment evaluations, the doctors will have all the information they ...
Pathology Report Patient Companion Guide Pathology Reports can be overwhelming. They contain scientific terms that are unfamiliar and might be a bit scary. This companion Patient Guide to Pathology Reports is designed to help patients understand what the various tests mean and how they help guide treatment planning. Together with other ...
In 2007 an estimated 27 million surgical pathology reports and 55 million cytology reports were issued in the United States.1 The anatomic pathology market in the United States has been increasing at an annual rate of 8.7%,2 and in 2007 approximately $6.7 billion will be spent to reimburse US providers for the professional and technical services required to produce these 82 million reports.1 ...
1. Clear and Concise Introduction. Provide background information on the patient and their medical history. Describe the purpose of the lab test. Highlight any relevant symptoms or clinical findings. State the hypothesis or expected outcomes. Establish the context for the report. 2. Proper Sample Collection and Handling.
Understanding your pathology report is a critical step in managing your health. The knowledge contained in your report can help you better understand your diagnosis. This will empower you to navigate and make informed decisions about your treatment. Consult with your healthcare provider for clarifications and concerns.
Pathologic staging. An important piece of information in the pathology report is the overall stage of the cancer. Staging is a method for determining how much cancer there is in the body and where it is located. It indicates the extent or severity of can-cer based on an analysis of the original or primary tumor.
The chapter expands on pathology reporting by discussing quality assessment of the pathology report through peer review and pathology working groups. The types of peer reviews and the methodology for performing them are presented as is a discussion on conducting a pathology working group. Download chapter PDF.
Abstract. Context.—The surgical pathology report (SPR) is an essential part of patient care because it documents the pathologic findings in tissues removed from patients for diagnostic or therapeutic reasons. Despite the importance of the SPR, exhaustive guidelines outlining the various elements of the SPR have not, to our knowledge, been published.Objectives.—To outline recommendations ...
Laboratory medicine service in a hospital is concerned with relevant investigations of patient's presenting complaints and sometimes with detection and potential prevention of disease. It involves a systematic approach of clinical advice or request for a particular investigation, analysis of the collected specimen, interpretation of results, and appropriate reporting in a timely fashion ...
1. You can always add more tests in Labsmart Software with the help of Add Test feature. View report formats for all kinds of pathology lab tests and panels like LFT, KFT, CBC etc. Also report formats are included for all departments like Haematology, Biochemistry Serology and Immunology, Clinical pathology, Microbiology, Endocrinology ...
Introduction. Your lab report introduction should set the scene for your experiment. One way to write your introduction is with a funnel (an inverted triangle) structure: Start with the broad, general research topic. Narrow your topic down your specific study focus. End with a clear research question.
A central function of the chemical pathology or clinical chemistry laboratory is to provide biochemical informa-tion for the management of patients. Such information will be of value only if it is accurate and relevant, and if its signifi cance is appreciated by the clinician so that it can be used appropriately to guide clinical decision-making.
Introduction. This Guide is designed to be used in preparing laboratory reports for all general science and engineering courses at IIT. It describes the structure of a good laboratory report, outlines the different sections of the report, and explains the need for each of them. It also introduces some standard conventions and rules for writing ...
Specimens must be placed in appropriate fixative as specified in collection/handling and submission procedure. Volume of fixative to tissue ratio must be included in the collection/handling and submission procedures. i.e. 10% neutral buffered formalin volume should be 15-20 times the volume of the specimen.
What is pathology report? A pathology report is a document that contains the diagnosis determined by examining cells and tissues under a microscope. Created by a pathologist; who is a doctor who does this examination and writes the pathology report. (According to National Cancer Institute.) 1) Definition of pathology report.
Pathology Lab Report Format. A pathology lab report typically includes the following sections: 1. Patient Information. This section includes the patient's name, identification number, age, sex, and relevant medical history. 2. Specimen Information. This section includes the type of specimen, the source of the specimen, and any relevant details ...
trips or on your own. Homework is due 1 week after the disease symptoms observation lab. Each student must bring a bound notebook to the lab to record observations. Learning goals assessment: Measures of assessment include: • Lab notebook reports and answering pathogen group questions on exams (learning goal 1)
1. Name the two main types of cells present in nervous tissue. Describe their functions. 2. Create an illustration of a neuron from the images in Chapter 5. Label the cell body, axon, dendrites, and nucleus.
an anatomic pathology laboratory section or department. Do NOT use this Checklist if the laboratory does NOT perform any on-site preparation or examination of anatomic pathology specimens, but refers all submitted material to an outside laboratory, or if the laboratory's involvement in anatomic pathology is limited to filing of reports and/or ...
*Assignments Dpharma part 2 according to Pharmacy council of India*The students shall be asked to submit written assignments on the following topics (One ass...
BIOCHEMISTRY & CLINICAL PATHOLOGY ASSIGNMENT Name of the College: Name of the Student: Academic Year of the Student: Name of the Subject: ... The students shall be asked to submit written assignments on Various Pathology Lab Reports (One assignment per student per sessional period. i.e., a minimum of THREE assignments per student) Page No