- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
What Are the Different Types of Clinical Research?
Different types of clinical research are used depending on what the researchers are studying. Below are descriptions of some different kinds of clinical research.
Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
Genetic studies aim to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies seek to identify the patterns, causes, and control of disorders in groups of people.
An important note: some clinical research is “outpatient,” meaning that participants do not stay overnight at the hospital. Some is “inpatient,” meaning that participants will need to stay for at least one night in the hospital or research center. Be sure to ask the researchers what their study requires.
Phases of clinical trials: when clinical research is used to evaluate medications and devices Clinical trials are a kind of clinical research designed to evaluate and test new interventions such as psychotherapy or medications. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose and help scientists answer different questions.
Phase I trials Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
Phase II trials The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III trials The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
Phase IV trials Post-marketing studies, which are conducted after a treatment is approved for use by the FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
Examples of other kinds of clinical research Many people believe that all clinical research involves testing of new medications or devices. This is not true, however. Some studies do not involve testing medications and a person’s regular medications may not need to be changed. Healthy volunteers are also needed so that researchers can compare their results to results of people with the illness being studied. Some examples of other kinds of research include the following:
A long-term study that involves psychological tests or brain scans
A genetic study that involves blood tests but no changes in medication
A study of family history that involves talking to family members to learn about people’s medical needs and history.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, finding a clinical trial, around the nation and worldwide.

NIH conducts clinical research trials for many diseases and conditions, including cancer , Alzheimer’s disease , allergy and infectious diseases , and neurological disorders . To search for other diseases and conditions, you can visit ClinicalTrials.gov.
ClinicalTrials.gov [ How to Use Search ] This is a searchable registry and results database of federally and privately supported clinical trials conducted in the United States and around the world. ClinicalTrials.gov gives you information about a trial's purpose, who may participate, locations, and phone numbers for more details. This information should be used in conjunction with advice from health care professionals.
Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read the disclaimer on ClinicalTrials.gov for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits.
At the NIH Clinical Center in Bethesda, Maryland

Search NIH Clinical Research Studies The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as studies for healthy volunteers. Visitors can search by diagnosis, sign, symptom or other key words.
Join a National Registry of Research Volunteers

ResearchMatch This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the future.
This page last reviewed on November 6, 2018
Connect with Us
- More Social Media from NIH

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Clinical Research Study Investigator’s Toolbox

Supporting Clinical Research
The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies.
Study Startup
- NIA Guidance on Clinical Trials
Forms and Templates
- Glossary of Terms
Data Safety and Monitoring
As depicted in the NIA Guidance on Clinical Trials , NIA is responsible for overseeing the data and safety monitoring of the clinical research it supports. Data and safety monitoring of a clinical trial is commensurate with the risks posed to the study participants and with the size and complexity of the study.
Applicants requesting support for any intervention study must complete "PHS Human Subjects and Clinical Trials Information" form of the SF424 (R&R), describe a data and safety monitoring plan (DSMP), which discusses the need for an independent data and safety monitoring body or justifies why such a body is not needed to monitor the study and proposes an alternative safety monitoring mechanism. For example, for a single-site, low risk study, the PI may propose a local safety monitor, while a multi-site, higher risk study might propose a Data and Safety Monitoring Board (DSMB).
- For behavioral and social clinical trials, consider using the adapted DSMP Template (MS Word, 62K) .
- Guideline for Budgeting for Data and Safety Monitoring Activities (MS Word, 25K) aids investigators in budgeting for an independent DSMB or a Safety Officer when preparing the budget section of a grant application.
Data Sharing
The National Institutes of Health (NIH) advocates making available to the public the results and accomplishments of the activities that it funds. NIH assures that research resources developed with public funds become readily available to the broader research community in a timely manner for further research, development, application, and secondary data analysis. The expectation is that this will lead to products and knowledge of benefit to public health. To ensure that future research can build on previous efforts and discoveries, the National Institutes of Health (NIH) has developed a data sharing policy effective October 1, 2003, for applicants seeking NIH funding of $500,000 or more in direct costs in any one year. The policy expects final research data, especially unique data, from NIH-supported research efforts be made available to the investigators. The NIH policy on data sharing applies to:
- Basic research, clinical studies, surveys, and other types of research supported by the NIH.
- Human subjects and laboratory research.
- Data not produced with NIH funding but used in an NIH-supported activity in some instances.
Investigators are expected to include in their grant application a brief description of how final research data will be shared, or explain why data-sharing is not possible (for example: human subject protection concerns). Please see NIH’s Example Plan (MS Word, 55K) for a template you may modify to fit the data you plan to share.
Initial Proposal Concept Form (MS Word, 39K) - This form should be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology (DGCG) for a clinical trial or trials that exceed $2 million in direct costs in any year of funding. DGCG Clinical Trials Advisory Panel, a task force of the National Advisory Council on Aging (NACA), will evaluate the concept proposals in October – November of each Fiscal Year and will provide its recommendations to DGCG, NACA, and to the NIA Director on initiatives for large clinical trials.
Back to top
The clinical protocol is a document that describes how a clinical study will be conducted by detailing the objective(s), design, methodology, statistical considerations and organization of a clinical study, and describes methods used to ensure the safety of the study participants and integrity of the data collected.
Protocol (MS Word, 93K) - The Clinical Intervention Study Protocol Template outlines a clinical study protocol and provides guidance on important content to include in each section. The template can be downloaded as an MS Word file for adaptation by the study investigator.
Manual of Procedures
A Manual of Procedures (MOP) is a handbook that details a study’s conduct and operations as well as facilitates consistency in protocol implementation and data collection across study participants and sites. It operationalizes the study protocol and describes each step of the study and how it is to be executed. A copy of the MOP should be provided to each member of the Study Team. Ideally, the MOP would contain an adequate amount of detail that any individual(s) at any site(s) could run the study consistently with only the information contained in the MOP and its appendices.
The NIA recognizes the importance of a MOP and has developed documents to assist principal investigators in writing their study MOP. Investigators with a multi-site study are required to submit a MOP, while single-site study investigators are strongly encouraged to review the MOP and determine which sections are necessary in order to ensure the study procedures are performed as intended. The Guidelines below provide details on each section of the MOP, while the MOP Outlines are an overview listing the sections that are most relevant in those types of studies.
- Manual of Procedures (MOP) Outline – Multi-Site (MS Word, 30K)
- Manual of Procedures (MOP) Guidelines – Multi-Site (MS Word, 2.9M)
- Manual of Procedures (MOP) Outline – Single-Site (MS Word, 27K)
- Manual of Procedures (MOP) Guidelines - Single-Site (MS Word, 170K)
The following documents can also be found within the MOP template:
- Schedule of Events presents the activities that take place at each contact with the participant.
- Protocol Deviation Log provides participant-specific documentation of missed visits and other actions that deviate from the protocol.
Informed Consent
The consent process provides individuals with sufficient information for making informed decisions about participation in a clinical research study. The following documents are provided as a tool to assist NIA investigators for developing a comprehensive informed consent:
- Informed Consent Template (MS Word, 63K) provides a general outline of a study specific informed consent form (ICF). It is critical that investigators consult with their local IRB for any institution-specific templates and/or requirements regarding the format and content of the consent form.
- Informed Consent Checklist (MS Word, 54K) presents required and additional elements of the consent forms as set forth in Code of Federal Regulations.
- Informed Consent Version Tracker (MS Excel, 20K) provides a template with two examples of tools that sites may use to track informed consent versions; this helps minimize the use of expired versions and the occurrence of consent deviations.
- Informed Consent for Secondary Research with Data and Specimens (PDF, 736K) , from NIH's Office of Science Policy, provides points to consider and sample language for informed consent documents for research studies that plan to store and share data and/or biospecimens for future use.
Data Safety and Monitoring Boards
The Data and Safety Monitoring Board (DSMB) is an independent group of experts that advises the NIA Director and the study investigators. The members of the DSMB serve in an individual capacity and provide their expertise and recommendations. The need for DSMB oversight is based on assessment of the study’s overall risk. Investigators may propose a DSMB in their grant application, or NIA may require that a DSMB be established following consideration of review panel’s comments, NIA’s National Advisory Council on Aging (NACA) advice, and/or input from NIA staff.
- Sample Data and Safety Monitoring Board Charter (MS Word, 25.8K) The DSMB Charter describes the responsibilities of the DSMB to ensure ongoing, independent study review and assure the study is conducted according to the highest scientific and ethical standards.
- DSMB Conflict of Interest and Confidentiality Statement (MS Word, 22K) and DSMB Conflict of Interest and Confidentiality Statement (PDF, 130K) - All members of the DSMB are required to be independent of the studies being reviewed and need to certify this by signing a DSMB Conflict of Interest and Confidentiality statement.
- DSMB Report - Single Site Open (MS Word, 323K)
- DSMB Report - Single Site Closed (MS Word, 342K)
- DSMB Report - Multi Site Open (MS Word, 449K)
- DSMB Report - Multi Site Closed (MS Word, 348K)
Additional Startup Tools
- Recruitment and Retention Tips (MS Word, 33K) describe approaches to recruitment and retention of older individuals from diverse ethnic and racial groups in clinical research studies.
- Data Management Tips (MS Word, 30K) help to ensure adequate data management processes and procedures in a clinical study. Investigators are encouraged to use Data Management Tips to describe how data will be handled in the study.
- Best Practices for Data Coordinating Centers – This Compendium, developed by the National Heart Lung and Blood Institute (NHLBI) provides helpful tips for clinical researchers and other stakeholders for developing large, multisite clinical trial programs.
Investigators must include in their application proposed adverse event (AE) and serious adverse event (SAE) definitions and discuss their monitoring and reporting. All clinical trials of drugs and biological products conducted under an Investigational New Drug Application (IND) must use definitions of adverse events and adverse reactions and follow the reporting requirements established by 21 Code of Federal Regulations (CFR) Part 312.32. Trials of medical devices conducted under an Investigational Device Exemption (IDE) must use the definitions and reporting requirements established by 21 CFR 812. All other interventional studies must propose their definitions of adverse events and their reporting procedures. See the NIA Guidance on Clinical Trials for additional information .
- Adverse Event Form ( MS Word , 38K or screen-readable PDF , 69K) provides a template for a study form for collecting information about adverse events that is reviewed by safety monitoring bodies.
- Serious Adverse Event Form ( MS Word , 31K or screen-readable PDF , 769K) provides a template for a study form for collecting information about serious adverse events. The form includes major components of the Food and Drug Administration (FDA) Form 3500.
- AE/SAE Process Flow ( PDF , 119K) illustrates how adverse events and serious adverse events are handled within a study.
The NIA Safety Training Course (available below), an online training venue, provides an overview of human subject safety surveillance and reporting requirements in clinical research studies. The intent of the course is to help clinical study investigators and staff understand and implement NIA and regulatory requirements for safe, high quality clinical research. The topics covered include Good Clinical Practice (GCP), Human Subject Protections, Adverse Events and Unanticipated Problems, Safety Monitoring and Reporting Requirements, Safety Monitoring and Oversight: Data and Safety Monitoring Boards (DSMBs) and Safety Officers, Regulatory Requirements and Responsibilities of Principal Investigators, and Data and Safety Monitoring Plans. The course requires about 40 minutes to complete.
Administrative Forms
Screening Log ( MS Excel, 47K ) Provides documentation of all individuals who were evaluated for participation in a research study. The log typically contains a unique identification number for each person screened along with individuals’ date of birth, gender, race and ethnicity, screening date, and eligibility status.
Site Signature Log - Delegation of Authority Log ( MS Excel, 47K or screen-readable PDF, 294K ) A record of all study personnel and their specific responsibilities, signatures, and dates of involvement during the conduct of a clinical research study.
Note to File Template (MS Word, 20K) - Used by clinical site staff to document protocol deviations or other discrepancies identified during the conduct of the clinical research study and plans for resolution/prevention.
Sample Visit Flow and Schedule (MS Word, 25K) – The visit schedule tracks an individual participant’s progress through the study and helps to ensure that visits take place during the protocol-specified timeframe. The visit flow provides an overview of the activities that take place at each study visit, and may be customized for each study site.
Study Drug/Investigational Product Tracker (MS Excel, 12K) - Used to track study drug/investigational product disposition and accountability by the clinical research site. For multi-site studies under an investigational new drug (IND) application, this tracker could be used by coordinating centers to track the overall distribution of investigational product.
Study Drug/Investigational Product Compliance Log (MS Word, 30K) - Used to track study drug/investigational product disposition and accountability for each individual participant. This form may be used to track protocol adherence via amount dispensed and returned and is designed to be used in conjunction with the Study Drug/Investigational Product Tracker. May also be used to track study drug/investigational return or destruction.
Study-wide Forms
Adverse Events Form ( MS Word, 38K or screen-readable PDF, 68K )
Prior and Concomitant Medications ( MS Word, 34K or screen-readable PDF, 58K )
Protocol Deviations Form ( MS Word, 46K or screen-readable PDF, 80K )
Serious Adverse Events Form ( MS Word, 31K or screen-readable PDF, 769K )
Study Disposition Form ( MS Word, 32K or screen-readable PDF, 56K )
Baseline Visit Forms
Visit Checklist ( MS Word, 34K or screen-readable PDF, 53K )
Eligibility Form ( MS Word, 29K or screen-readable PDF, 184K )
Demographics Form ( MS Word, 32K or screen-readable PDF, 661K )
Medical History Form ( MS Word, 50K or screen-readable PDF, 87K )
Medical History Conventional ( MS Word, 54K or screen-readable PDF,184 K )
Vital Signs Form ( MS Word, 33K or screen-readable PDF, 101K )
Physical Exam Form ( MS Word, 73K or screen-readable PDF, 193K )
Randomization and Enrollment Form ( MS Word, 32K or screen-readable PDF, 806K )
Sign up for NIA training and career development updates
nia.nih.gov
An official website of the National Institutes of Health
Clinical Trials
Displaying 40 studies
To evaluate the efficacy and safety of Botulinum Toxin Type A versus placebo (normal saline) as headache prophylaxis in adolescents (children 12 to 17) with chronic migraine.
The purpose of the study is to determine whether monthly subcutaneous administration of LBR-101 is safe and provides migraine prevention in patients with chronic migraine.
Hemiplegic migraine is a rare subtype of migraine with aura, with symptoms affecting vision, speech and language, hearing, and sensory and motor disturbances. Muscle weakness and hemiplegia are defining characteristics of hemiplegic migraines. Compared to patients with typical migraine with aura, patients with hemiplegic migraine tend to have more prolonged aura symptoms. In some cases, reversible unilateral weakness may last days to weeks. The primary objective of this project is to evaluate the response of hemiplegic migraine patients evaluated at Mayo Clinic to onabotulinumtoxinA treatments.
There are two commonly used treatment strategies for treating patients who have chronic migraine with medication overuse. This study will compare the outcomes amongst patients randomized to one of the two treatment strategies.
The primary objective of this study is to evaluate the safety and efficacy of occipital nerve stimulation (ONS) using the Boston Scientific Corporation (BSC) Precision™ System in the management of intractable chronic migraine, when used in conjunction with anti-migraine medications.
Migraine is the third most prevalent disease in the world. Preventive treatment is indicated in about 40% of individuals with episodic migraine. Although 4 treatments are approved by the US Food and Drug Administration for prevention of episodic migraine, none were designed to prevent migraine, efficacy is modest, and all have significant adverse-event profiles. As a result, less than 1/3 of migraine sufferers with who are candidates for prevention receive drug treatment and of those who are treated, more than 85% have discontinued the preventive drug within one year. Migraine pain is associated with the activation and sensitization of specific ...
The purpose of this study is to determine whether monthly subcutaneous administration of LBR-101 is safe and provides migraine prevention in subjects with high frequency episodic migraine.
The purpose of this study is to investigate the accuracy of predicting migraine attacks in individual patients using a mobile phone app and a Fitbit to collect daily headache diary data, exposure/trigger data and physiologic data. It is believed the data will predict the occurrence of migraine attacks with high accuracy via the mobile phone app.
The purpose of the study is to investigate if the use of gammaCore Sapphire™ device reduces the number of migraines preventatively.
The purpose of this study is to find sex-based differences in migraine-related functional connectivity. To achieve this, men with migraine will be compared to men healthy controls and women with migraine will be compared to women healthy controls.
Men and women with migraine will then be compared, using region-pairs that differed in the single-sex comparisons. Identification of migraine-related, sex-specific, functional imaging aberrations will provide insights into sex-specific migraine pathophysiology and will inform future imaging studies
The purpose of this study is to predict migraine headache onset from changes in speech in people who have a history of migraines.
The purpose of this study is to create individualized prediction models utilizing sensor data and cognitive function which will accurately identify the premonitory phase of the migraine attack and predict an oncoming migraine headache.
This study will seek to determine which measures differ between migraine attack phases (e.g., interictal vs. premonitory), develop an initial migraine prediction algorithm, and to provide insights into how long prior to headache onset premonitory changes begin. It will also look for migraine phenotypes associated with changes in sensor-measured data during the preictal vs. ictal or interictal periods.
The purpose of this study is to look at the natural history of VM and learn more about common symptoms. Investigators also want to learn the effects, both positive and negative, of the commonly used migraine drug, rizatriptan, when it is used for spells of dizziness in people with VM.
Patients may be eligible to participate if:
- Patients are between the ages of 18 & 65
- Patients have a history of vestibular migraine
- Patients are able to maintain a vestibular symptom diary
The study includes 3 visits with compensation. All participants must ...
The purpose of this study is to assess the safety, tolerability, and optimal dose of the COOLSTAT Transnasal Thermal Regulating Device for acute treatment of migraine. The hypothesis is that evaporative cooling induced by the CoolStat using only ambient, dry air will reduce the pain and other symptoms of migraine headaches during an acute migraine episode.
The purpose of this study is to evaluate the long-term safety of Fremanezumab in all patients with migraine through evaluation of incidence of all adverse events.
The purpose of this study is to identify changes in brain function and structure that correlate with response to erenumab, and to develop models using imaging data to predict which patients will respond to erenumab.
This study will evaluate the efficacy, safety, and tolerability of 2 doses of ubrogepant (25 and 50 mg) compared to placebo for the acute treatment of a single migraine attack.
The purpose of this study is to assess ALD403 in the prevention of migraine headache in chronic migraineurs.
This research study is being done to help determine if King-Devick test (KDT) and Visual Contrast Sensitivity (VCS) can detect migraine-induced cerebral function changes.
Changes in vision are commonly reported by patients during migraine attacks. Even in patients that do not report changes in vision, we have noted objective changes as demonstrated by changes in the King-Devick Test (KDT), a quantitative measure of rapid eye movement; which was 6.5 seconds slower (SD 7.6 seconds, p
Chronic dizziness and recurrent vertigo are frequent complaints in primary and specialty medical care settings. Two common causes of these symptoms are vestibular migraine (VM) and chronic subjective dizziness (CSD), which may be seen in up to 25% of patients examined in tertiary neurotology centers. However, VM and CSD are relatively new diagnoses that have not yet been validated. Furthermore, recent research found that they co-exist 30% of the time with overlap in several features. From a clinical standpoint, this makes it difficult to diagnose and treat them well. From a research standpoint, it confounds subject selection for mechanistic investigations.
The purpose of this study is to investigate the use of erenumab 140mg IV in patients with status migrainosus. The specific aims of the study are to evaluate early endpoints of response including pain freedom at 2 hours, absence of the most bothersome symptom (MBS) at 2 hours, change in pain severity at 2 hours, and sustained pain freedom at 24 hours, and to evaluate late endpoints of response including change in migraine days at 4 weeks compared to baseline report, change in headache days at 4 weeks compared to baseline report, and change in acute medication use.
The purpose of this study is to accurately track biomarkers of headache coupled with patient reporting of severity with minimal effort by the patient using a custom phone app integrating patient and devices. Also, to accurately forecast periods of increased headache likelihood to allow patients earlier and more effective treatments.
The study sponsor has developed a non-invasive treatment for reducing migraine pain. The Theranova Migraine Treatment System uses electrical stimulation applied to the skin.
The purpose of this study is to use structural and functional neuroimaging to compare what regional brain changes are similar or distinctly different between migraines and TAC ( trigeminal autonomic cephalagias).
This study will evaluate the long-term safety and tolerability of intermittent treatment with ubrogepant for the acute treatment of migraine over 1 year.
This study will assess the efficacy of a neuromodulation device for the treatment of episodic migraine headache.
The purpose of this study is to test the accuracy of a previously developed algorithm for predicting patient outcomes to migraine preventive treatments. Also, to optimize the performance of a previously developed algorithm for predicting patient outcomes to migraine preventive treatments.
The purpose of this study is to prospectively determine what method of assessment predicts headache directionality using three standardized methods: 1) pictorial representation, 2) standardized patient survey and 3) standardized headache neurology investigator assessment.
The purpose of this study is to develop an MRI based model to accurately identify persistent posttraumatic headache related to mild traumatic brain injury, as different from chronic migraine.
The purpose of this study is to study the length of the QTc interval in patients receiving the standard of care dose of intravenous droperidol for headache or migraine, nausea, pain, and indications other than agitation over 30 minutes.
The purpose of this study is to identify whether there is a difference in prolactin levels between patients with chronic migraine and healthy controls. This may help to explain the sensitivity to pain those patients with chronic migraine experience and may also help to establish a target for new treatments of chronic migraine
The purpose of this study is to assess the efficacy and safety of ALD403 in the prevention of migraine headache in chronic migraineurs.
The primary purpose of this study is determine the frequency of positive visual symptoms including visual snow in pediatric patients seen for general pediatric care as well as for neurologic care.
The secondary purpose is to determine if positive visual symptoms are more common in pediatric patients who also have a self-reported history of headache or migraine.
The purpose of this study is to compare eye glass lenses that have been treated with tints, to lenses that have been treated with thin-films to reduce light sensitivity and headache in patients who have chronic migraine. It is unknown if there is an advantage to either spectacle lens treatment. Both treatments could be a novel, non-invasive addition in the treatment of chronic migraine.
The purpose of this research is to better understand brain white matter hyperintensities (WMH) in women with migraine. We will accomplish this by comparing brain imaging and clinical assessments of women with migraine, small vessel ischemic (SVI) disease, and multiple sclerosis (MS).
The purpose of this study is to evaluate Patent Foramen Ovale (PFO) closure for migraine headache relief using the GORE® CARDIOFORM Septal Occluder after a trial of oral anticoagulation therapy vs. placebo for a 6 week period.
The purpose of this research study is to test two medicines for migraine prevention in children and adolescents. The investigators want to see if amitriptyline and/or topiramate are better than placebo (sugar pill) in reducing headache frequency in children and adolescents ages 8 to 17 with migraines. At this time, there are no FDA approved medicines approved in the US for the prevention treatment of migraine headaches in children and adolescents.
The purpose of this study is to enroll in a registry patients who have headache disorders, collecting detailed headache characteristics, biospecimens, and brain imaging data, to allow for more research into accurate diagnosis, prognosis, and treatments.
Headache is the most commonly reported symptom after trauma to the head. Although posttraumatic headache (PTH) shares some clinical characteristics with primary headache disorders, such as migraine, there are many unique features including head trauma and the exacerbation of headache with physical and cognitive stressors. The purpose of this study is to determine if subjects who have recently sustained a mild TBI and have PTH will have lower pain thresholds (i.e. hyperalgesia) and greater allodynia compared to healthy controls. The study will also determine if exposure to a bright light stressor will exacerbate hyperalgesia in subjects with mild TBI and PTH ...
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

Advanced Search
See studies by topic, see studies on map, how to search, how to use search results, how to find results of studies, how to read a study record.

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
Find Studies
The Find Studies section of this site describes the options for finding studies on ClinicalTrials.gov, how to use those options to find the studies you are looking for, and how to read study records.
Options for Finding Studies
Conduct a focused search of studies by matching one or more words entered in specific fields. See also How to Use Advanced Search .
Find studies in categories such as condition, drug intervention, sponsor, or location. See also How to Find Studies by Topic or on a Map .
Find studies by clicking on a country or region on a map. See also How to Find Studies by Topic or on a Map .
How to Search and Use the Results
Learn how to use the site's search features to find studies:
- How to Use Search
- How to Use Advanced Search
- How to Modify a Search
- How to Find Studies by Topic or on a Map
Learn about the different features of the search results list, including how to customize your display.
Learn how to find studies that have been updated with study results, including studies with results that have been published in medical journals.
Learn about the information available in a study record and the different ways to view a record.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.11(2); 2019 Feb

Planning and Conducting Clinical Research: The Whole Process
Boon-how chew.
1 Family Medicine, Universiti Putra Malaysia, Serdang, MYS
The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice changes and improvements. The research idea is further informed through a systematic literature review, clarified into a conceptual framework, and defined into an answerable research question. Engagement with clinical experts, experienced researchers, relevant stakeholders of the research topic, and even patients can enhance the research question’s relevance, feasibility, and efficiency. Clinical research can be completed in two major steps: study designing and study reporting. Three study designs should be planned in sequence and iterated until properly refined: theoretical design, data collection design, and statistical analysis design. The design of data collection could be further categorized into three facets: experimental or non-experimental, sampling or census, and time features of the variables to be studied. The ultimate aims of research reporting are to present findings succinctly and timely. Concise, explicit, and complete reporting are the guiding principles in clinical studies reporting.
Introduction and background
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices. There are many ways a clinical research's findings can become invalid or less impactful including ignorance of previous similar studies, a paucity of similar studies, poor study design and implementation, low test agent efficacy, no predetermined statistical analysis, insufficient reporting, bias, and conflicts of interest [ 1 - 4 ]. Scientific, ethical, and moral decadence among researchers can be due to incognizant criteria in academic promotion and remuneration and too many forced studies by amateurs and students for the sake of research without adequate training or guidance [ 2 , 5 - 6 ]. This article will review the proper methods to conduct medical research from the planning stage to submission for publication (Table (Table1 1 ).
a Feasibility and efficiency are considered during the refinement of the research question and adhered to during data collection.
Epidemiologic studies in clinical and medical fields focus on the effect of a determinant on an outcome [ 7 ]. Measurement errors that happen systematically give rise to biases leading to invalid study results, whereas random measurement errors will cause imprecise reporting of effects. Precision can usually be increased with an increased sample size provided biases are avoided or trivialized. Otherwise, the increased precision will aggravate the biases. Because epidemiologic, clinical research focuses on measurement, measurement errors are addressed throughout the research process. Obtaining the most accurate estimate of a treatment effect constitutes the whole business of epidemiologic research in clinical practice. This is greatly facilitated by clinical expertise and current scientific knowledge of the research topic. Current scientific knowledge is acquired through literature reviews or in collaboration with an expert clinician. Collaboration and consultation with an expert clinician should also include input from the target population to confirm the relevance of the research question. The novelty of a research topic is less important than the clinical applicability of the topic. Researchers need to acquire appropriate writing and reporting skills from the beginning of their careers, and these skills should improve with persistent use and regular reviewing of published journal articles. A published clinical research study stands on solid scientific ground to inform clinical practice given the article has passed through proper peer-reviews, revision, and content improvement.
Systematic literature reviews
Systematic literature reviews of published papers will inform authors of the existing clinical evidence on a research topic. This is an important step to reduce wasted efforts and evaluate the planned study [ 8 ]. Conducting a systematic literature review is a well-known important step before embarking on a new study [ 9 ]. A rigorously performed and cautiously interpreted systematic review that includes in-process trials can inform researchers of several factors [ 10 ]. Reviewing the literature will inform the choice of recruitment methods, outcome measures, questionnaires, intervention details, and statistical strategies – useful information to increase the study’s relevance, value, and power. A good review of previous studies will also provide evidence of the effects of an intervention that may or may not be worthwhile; this would suggest either no further studies are warranted or that further study of the intervention is needed. A review can also inform whether a larger and better study is preferable to an additional small study. Reviews of previously published work may yield few studies or low-quality evidence from small or poorly designed studies on certain intervention or observation; this may encourage or discourage further research or prompt consideration of a first clinical trial.
Conceptual framework
The result of a literature review should include identifying a working conceptual framework to clarify the nature of the research problem, questions, and designs, and even guide the latter discussion of the findings and development of possible solutions. Conceptual frameworks represent ways of thinking about a problem or how complex things work the way they do [ 11 ]. Different frameworks will emphasize different variables and outcomes, and their inter-relatedness. Each framework highlights or emphasizes different aspects of a problem or research question. Often, any single conceptual framework presents only a partial view of reality [ 11 ]. Furthermore, each framework magnifies certain elements of the problem. Therefore, a thorough literature search is warranted for authors to avoid repeating the same research endeavors or mistakes. It may also help them find relevant conceptual frameworks including those that are outside one’s specialty or system.
Conceptual frameworks can come from theories with well-organized principles and propositions that have been confirmed by observations or experiments. Conceptual frameworks can also come from models derived from theories, observations or sets of concepts or even evidence-based best practices derived from past studies [ 11 ].
Researchers convey their assumptions of the associations of the variables explicitly in the conceptual framework to connect the research to the literature. After selecting a single conceptual framework or a combination of a few frameworks, a clinical study can be completed in two fundamental steps: study design and study report. Three study designs should be planned in sequence and iterated until satisfaction: the theoretical design, data collection design, and statistical analysis design [ 7 ].
Study designs
Theoretical Design
Theoretical design is the next important step in the research process after a literature review and conceptual framework identification. While the theoretical design is a crucial step in research planning, it is often dealt with lightly because of the more alluring second step (data collection design). In the theoretical design phase, a research question is designed to address a clinical problem, which involves an informed understanding based on the literature review and effective collaboration with the right experts and clinicians. A well-developed research question will have an initial hypothesis of the possible relationship between the explanatory variable/exposure and the outcome. This will inform the nature of the study design, be it qualitative or quantitative, primary or secondary, and non-causal or causal (Figure (Figure1 1 ).

A study is qualitative if the research question aims to explore, understand, describe, discover or generate reasons underlying certain phenomena. Qualitative studies usually focus on a process to determine how and why things happen [ 12 ]. Quantitative studies use deductive reasoning, and numerical statistical quantification of the association between groups on data often gathered during experiments [ 13 ]. A primary clinical study is an original study gathering a new set of patient-level data. Secondary research draws on the existing available data and pooling them into a larger database to generate a wider perspective or a more powerful conclusion. Non-causal or descriptive research aims to identify the determinants or associated factors for the outcome or health condition, without regard for causal relationships. Causal research is an exploration of the determinants of an outcome while mitigating confounding variables. Table Table2 2 shows examples of non-causal (e.g., diagnostic and prognostic) and causal (e.g., intervention and etiologic) clinical studies. Concordance between the research question, its aim, and the choice of theoretical design will provide a strong foundation and the right direction for the research process and path.
A problem in clinical epidemiology is phrased in a mathematical relationship below, where the outcome is a function of the determinant (D) conditional on the extraneous determinants (ED) or more commonly known as the confounding factors [ 7 ]:
For non-causal research, Outcome = f (D1, D2…Dn) For causal research, Outcome = f (D | ED)
A fine research question is composed of at least three components: 1) an outcome or a health condition, 2) determinant/s or associated factors to the outcome, and 3) the domain. The outcome and the determinants have to be clearly conceptualized and operationalized as measurable variables (Table (Table3; 3 ; PICOT [ 14 ] and FINER [ 15 ]). The study domain is the theoretical source population from which the study population will be sampled, similar to the wording on a drug package insert that reads, “use this medication (study results) in people with this disease” [ 7 ].
The interpretation of study results as they apply to wider populations is known as generalization, and generalization can either be statistical or made using scientific inferences [ 16 ]. Generalization supported by statistical inferences is seen in studies on disease prevalence where the sample population is representative of the source population. By contrast, generalizations made using scientific inferences are not bound by the representativeness of the sample in the study; rather, the generalization should be plausible from the underlying scientific mechanisms as long as the study design is valid and nonbiased. Scientific inferences and generalizations are usually the aims of causal studies.
Confounding: Confounding is a situation where true effects are obscured or confused [ 7 , 16 ]. Confounding variables or confounders affect the validity of a study’s outcomes and should be prevented or mitigated in the planning stages and further managed in the analytical stages. Confounders are also known as extraneous determinants in epidemiology due to their inherent and simultaneous relationships to both the determinant and outcome (Figure (Figure2), 2 ), which are usually one-determinant-to-one outcome in causal clinical studies. The known confounders are also called observed confounders. These can be minimized using randomization, restriction, or a matching strategy. Residual confounding has occurred in a causal relationship when identified confounders were not measured accurately. Unobserved confounding occurs when the confounding effect is present as a variable or factor not observed or yet defined and, thus, not measured in the study. Age and gender are almost universal confounders followed by ethnicity and socio-economic status.

Confounders have three main characteristics. They are a potential risk factor for the disease, associated with the determinant of interest, and should not be an intermediate variable between the determinant and the outcome or a precursor to the determinant. For example, a sedentary lifestyle is a cause for acute coronary syndrome (ACS), and smoking could be a confounder but not cardiorespiratory unfitness (which is an intermediate factor between a sedentary lifestyle and ACS). For patients with ACS, not having a pair of sports shoes is not a confounder – it is a correlate for the sedentary lifestyle. Similarly, depression would be a precursor, not a confounder.
Sample size consideration: Sample size calculation provides the required number of participants to be recruited in a new study to detect true differences in the target population if they exist. Sample size calculation is based on three facets: an estimated difference in group sizes, the probability of α (Type I) and β (Type II) errors chosen based on the nature of the treatment or intervention, and the estimated variability (interval data) or proportion of the outcome (nominal data) [ 17 - 18 ]. The clinically important effect sizes are determined based on expert consensus or patients’ perception of benefit. Value and economic consideration have increasingly been included in sample size estimations. Sample size and the degree to which the sample represents the target population affect the accuracy and generalization of a study’s reported effects.
Pilot study: Pilot studies assess the feasibility of the proposed research procedures on small sample size. Pilot studies test the efficiency of participant recruitment with minimal practice or service interruptions. Pilot studies should not be conducted to obtain a projected effect size for a larger study population because, in a typical pilot study, the sample size is small, leading to a large standard error of that effect size. This leads to bias when projected for a large population. In the case of underestimation, this could lead to inappropriately terminating the full-scale study. As the small pilot study is equally prone to bias of overestimation of the effect size, this would lead to an underpowered study and a failed full-scale study [ 19 ].
The Design of Data Collection
The “perfect” study design in the theoretical phase now faces the practical and realistic challenges of feasibility. This is the step where different methods for data collection are considered, with one selected as the most appropriate based on the theoretical design along with feasibility and efficiency. The goal of this stage is to achieve the highest possible validity with the lowest risk of biases given available resources and existing constraints.
In causal research, data on the outcome and determinants are collected with utmost accuracy via a strict protocol to maximize validity and precision. The validity of an instrument is defined as the degree of fidelity of the instrument, measuring what it is intended to measure, that is, the results of the measurement correlate with the true state of an occurrence. Another widely used word for validity is accuracy. Internal validity refers to the degree of accuracy of a study’s results to its own study sample. Internal validity is influenced by the study designs, whereas the external validity refers to the applicability of a study’s result in other populations. External validity is also known as generalizability and expresses the validity of assuming the similarity and comparability between the study population and the other populations. Reliability of an instrument denotes the extent of agreeableness of the results of repeated measurements of an occurrence by that instrument at a different time, by different investigators or in a different setting. Other terms that are used for reliability include reproducibility and precision. Preventing confounders by identifying and including them in data collection will allow statistical adjustment in the later analyses. In descriptive research, outcomes must be confirmed with a referent standard, and the determinants should be as valid as those found in real clinical practice.
Common designs for data collection include cross-sectional, case-control, cohort, and randomized controlled trials (RCTs). Many other modern epidemiology study designs are based on these classical study designs such as nested case-control, case-crossover, case-control without control, and stepwise wedge clustered RCTs. A cross-sectional study is typically a snapshot of the study population, and an RCT is almost always a prospective study. Case-control and cohort studies can be retrospective or prospective in data collection. The nested case-control design differs from the traditional case-control design in that it is “nested” in a well-defined cohort from which information on the cohorts can be obtained. This design also satisfies the assumption that cases and controls represent random samples of the same study base. Table Table4 4 provides examples of these data collection designs.
Additional aspects in data collection: No single design of data collection for any research question as stated in the theoretical design will be perfect in actual conduct. This is because of myriad issues facing the investigators such as the dynamic clinical practices, constraints of time and budget, the urgency for an answer to the research question, and the ethical integrity of the proposed experiment. Therefore, feasibility and efficiency without sacrificing validity and precision are important considerations in data collection design. Therefore, data collection design requires additional consideration in the following three aspects: experimental/non-experimental, sampling, and timing [ 7 ]:
Experimental or non-experimental: Non-experimental research (i.e., “observational”), in contrast to experimental, involves data collection of the study participants in their natural or real-world environments. Non-experimental researches are usually the diagnostic and prognostic studies with cross-sectional in data collection. The pinnacle of non-experimental research is the comparative effectiveness study, which is grouped with other non-experimental study designs such as cross-sectional, case-control, and cohort studies [ 20 ]. It is also known as the benchmarking-controlled trials because of the element of peer comparison (using comparable groups) in interpreting the outcome effects [ 20 ]. Experimental study designs are characterized by an intervention on a selected group of the study population in a controlled environment, and often in the presence of a similar group of the study population to act as a comparison group who receive no intervention (i.e., the control group). Thus, the widely known RCT is classified as an experimental design in data collection. An experimental study design without randomization is referred to as a quasi-experimental study. Experimental studies try to determine the efficacy of a new intervention on a specified population. Table Table5 5 presents the advantages and disadvantages of experimental and non-experimental studies [ 21 ].
a May be an issue in cross-sectional studies that require a long recall to the past such as dietary patterns, antenatal events, and life experiences during childhood.
Once an intervention yields a proven effect in an experimental study, non-experimental and quasi-experimental studies can be used to determine the intervention’s effect in a wider population and within real-world settings and clinical practices. Pragmatic or comparative effectiveness are the usual designs used for data collection in these situations [ 22 ].
Sampling/census: Census is a data collection on the whole source population (i.e., the study population is the source population). This is possible when the defined population is restricted to a given geographical area. A cohort study uses the census method in data collection. An ecologic study is a cohort study that collects summary measures of the study population instead of individual patient data. However, many studies sample from the source population and infer the results of the study to the source population for feasibility and efficiency because adequate sampling provides similar results to the census of the whole population. Important aspects of sampling in research planning are sample size and representation of the population. Sample size calculation accounts for the number of participants needed to be in the study to discover the actual association between the determinant and outcome. Sample size calculation relies on the primary objective or outcome of interest and is informed by the estimated possible differences or effect size from previous similar studies. Therefore, the sample size is a scientific estimation for the design of the planned study.
A sampling of participants or cases in a study can represent the study population and the larger population of patients in that disease space, but only in prevalence, diagnostic, and prognostic studies. Etiologic and interventional studies do not share this same level of representation. A cross-sectional study design is common for determining disease prevalence in the population. Cross-sectional studies can also determine the referent ranges of variables in the population and measure change over time (e.g., repeated cross-sectional studies). Besides being cost- and time-efficient, cross-sectional studies have no loss to follow-up; recall bias; learning effect on the participant; or variability over time in equipment, measurement, and technician. A cross-sectional design for an etiologic study is possible when the determinants do not change with time (e.g., gender, ethnicity, genetic traits, and blood groups).
In etiologic research, comparability between the exposed and the non-exposed groups is more important than sample representation. Comparability between these two groups will provide an accurate estimate of the effect of the exposure (risk factor) on the outcome (disease) and enable valid inference of the causal relation to the domain (the theoretical population). In a case-control study, a sampling of the control group should be taken from the same study population (study base), have similar profiles to the cases (matching) but do not have the outcome seen in the cases. Matching important factors minimizes the confounding of the factors and increases statistical efficiency by ensuring similar numbers of cases and controls in confounders’ strata [ 23 - 24 ]. Nonetheless, perfect matching is neither necessary nor achievable in a case-control study because a partial match could achieve most of the benefits of the perfect match regarding a more precise estimate of odds ratio than statistical control of confounding in unmatched designs [ 25 - 26 ]. Moreover, perfect or full matching can lead to an underestimation of the point estimates [ 27 - 28 ].
Time feature: The timing of data collection for the determinant and outcome characterizes the types of studies. A cross-sectional study has the axis of time zero (T = 0) for both the determinant and the outcome, which separates it from all other types of research that have time for the outcome T > 0. Retrospective or prospective studies refer to the direction of data collection. In retrospective studies, information on the determinant and outcome have been collected or recorded before. In prospective studies, this information will be collected in the future. These terms should not be used to describe the relationship between the determinant and the outcome in etiologic studies. Time of exposure to the determinant, the time of induction, and the time at risk for the outcome are important aspects to understand. Time at risk is the period of time exposed to the determinant risk factors. Time of induction is the time from the sufficient exposure to the risk or causal factors to the occurrence of a disease. The latent period is when the occurrence of a disease without manifestation of the disease such as in “silence” diseases for example cancers, hypertension and type 2 diabetes mellitus which is detected from screening practices. Figure Figure3 3 illustrates the time features of a variable. Variable timing is important for accurate data capture.

The Design of Statistical Analysis
Statistical analysis of epidemiologic data provides the estimate of effects after correcting for biases (e.g., confounding factors) measures the variability in the data from random errors or chance [ 7 , 16 , 29 ]. An effect estimate gives the size of an association between the studied variables or the level of effectiveness of an intervention. This quantitative result allows for comparison and assessment of the usefulness and significance of the association or the intervention between studies. This significance must be interpreted with a statistical model and an appropriate study design. Random errors could arise in the study resulting from unexplained personal choices by the participants. Random error is, therefore, when values or units of measurement between variables change in non-concerted or non-directional manner. Conversely, when these values or units of measurement between variables change in a concerted or directional manner, we note a significant relationship as shown by statistical significance.
Variability: Researchers almost always collect the needed data through a sampling of subjects/participants from a population instead of a census. The process of sampling or multiple sampling in different geographical regions or over different periods contributes to varied information due to the random inclusion of different participants and chance occurrence. This sampling variation becomes the focus of statistics when communicating the degree and intensity of variation in the sampled data and the level of inference in the population. Sampling variation can be influenced profoundly by the total number of participants and the width of differences of the measured variable (standard deviation). Hence, the characteristics of the participants, measurements and sample size are all important factors in planning a study.
Statistical strategy: Statistical strategy is usually determined based on the theoretical and data collection designs. Use of a prespecified statistical strategy (including the decision to dichotomize any continuous data at certain cut-points, sub-group analysis or sensitive analyses) is recommended in the study proposal (i.e., protocol) to prevent data dredging and data-driven reports that predispose to bias. The nature of the study hypothesis also dictates whether directional (one-tailed) or non-directional (two-tailed) significance tests are conducted. In most studies, two-sided tests are used except in specific instances when unidirectional hypotheses may be appropriate (e.g., in superiority or non-inferiority trials). While data exploration is discouraged, epidemiological research is, by nature of its objectives, statistical research. Hence, it is acceptable to report the presence of persistent associations between any variables with plausible underlying mechanisms during the exploration of the data. The statistical methods used to produce the results should be explicitly explained. Many different statistical tests are used to handle various kinds of data appropriately (e.g., interval vs discrete), and/or the various distribution of the data (e.g., normally distributed or skewed). For additional details on statistical explanations and underlying concepts of statistical tests, readers are recommended the references as cited in this sentence [ 30 - 31 ].
Steps in statistical analyses: Statistical analysis begins with checking for data entry errors. Duplicates are eliminated, and proper units should be confirmed. Extremely low, high or suspicious values are confirmed from the source data again. If this is not possible, this is better classified as a missing value. However, if the unverified suspicious data are not obviously wrong, they should be further examined as an outlier in the analysis. The data checking and cleaning enables the analyst to establish a connection with the raw data and to anticipate possible results from further analyses. This initial step involves descriptive statistics that analyze central tendency (i.e., mode, median, and mean) and dispersion (i.e., (minimum, maximum, range, quartiles, absolute deviation, variance, and standard deviation) of the data. Certain graphical plotting such as scatter plot, a box-whiskers plot, histogram or normal Q-Q plot are helpful at this stage to verify data normality in distribution. See Figure Figure4 4 for the statistical tests available for analyses of different types of data.

Once data characteristics are ascertained, further statistical tests are selected. The analytical strategy sometimes involves the transformation of the data distribution for the selected tests (e.g., log, natural log, exponential, quadratic) or for checking the robustness of the association between the determinants and their outcomes. This step is also referred to as inferential statistics whereby the results are about hypothesis testing and generalization to the wider population that the study’s sampled participants represent. The last statistical step is checking whether the statistical analyses fulfill the assumptions of that particular statistical test and model to avoid violation and misleading results. These assumptions include evaluating normality, variance homogeneity, and residuals included in the final statistical model. Other statistical values such as Akaike information criterion, variance inflation factor/tolerance, and R2 are also considered when choosing the best-fitted models. Transforming raw data could be done, or a higher level of statistical analyses can be used (e.g., generalized linear models and mixed-effect modeling). Successful statistical analysis allows conclusions of the study to fit the data.
Bayesian and Frequentist statistical frameworks: Most of the current clinical research reporting is based on the frequentist approach and hypotheses testing p values and confidence intervals. The frequentist approach assumes the acquired data are random, attained by random sampling, through randomized experiments or influences, and with random errors. The distribution of the data (its point estimate and confident interval) infers a true parameter in the real population. The major conceptual difference between Bayesian statistics and frequentist statistics is that in Bayesian statistics, the parameter (i.e., the studied variable in the population) is random and the data acquired is real (true or fix). Therefore, the Bayesian approach provides a probability interval for the parameter. The studied parameter is random because it could vary and be affected by prior beliefs, experience or evidence of plausibility. In the Bayesian statistical approach, this prior belief or available knowledge is quantified into a probability distribution and incorporated into the acquired data to get the results (i.e., the posterior distribution). This uses mathematical theory of Bayes’ Theorem to “turn around” conditional probabilities.
The goal of research reporting is to present findings succinctly and timely via conference proceedings or journal publication. Concise and explicit language use, with all the necessary details to enable replication and judgment of the study applicability, are the guiding principles in clinical studies reporting.
Writing for Reporting
Medical writing is very much a technical chore that accommodates little artistic expression. Research reporting in medicine and health sciences emphasize clear and standardized reporting, eschewing adjectives and adverbs extensively used in popular literature. Regularly reviewing published journal articles can familiarize authors with proper reporting styles and help enhance writing skills. Authors should familiarize themselves with standard, concise, and appropriate rhetoric for the intended audience, which includes consideration for journal reviewers, editors, and referees. However, proper language can be somewhat subjective. While each publication may have varying requirements for submission, the technical requirements for formatting an article are usually available via author or submission guidelines provided by the target journal.
Research reports for publication often contain a title, abstract, introduction, methods, results, discussion, and conclusions section, and authors may want to write each section in sequence. However, best practices indicate the abstract and title should be written last. Authors may find that when writing one section of the report, ideas come to mind that pertains to other sections, so careful note taking is encouraged. One effective approach is to organize and write the result section first, followed by the discussion and conclusions sections. Once these are drafted, write the introduction, abstract, and the title of the report. Regardless of the sequence of writing, the author should begin with a clear and relevant research question to guide the statistical analyses, result interpretation, and discussion. The study findings can be a motivator to propel the author through the writing process, and the conclusions can help the author draft a focused introduction.
Writing for Publication
Specific recommendations on effective medical writing and table generation are available [ 32 ]. One such resource is Effective Medical Writing: The Write Way to Get Published, which is an updated collection of medical writing articles previously published in the Singapore Medical Journal [ 33 ]. The British Medical Journal’s Statistics Notes series also elucidates common and important statistical concepts and usages in clinical studies. Writing guides are also available from individual professional societies, journals, or publishers such as Chest (American College of Physicians) medical writing tips, PLoS Reporting guidelines collection, Springer’s Journal Author Academy, and SAGE’s Research methods [ 34 - 37 ]. Standardized research reporting guidelines often come in the form of checklists and flow diagrams. Table Table6 6 presents a list of reporting guidelines. A full compilation of these guidelines is available at the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network website [ 38 ] which aims to improve the reliability and value of medical literature by promoting transparent and accurate reporting of research studies. Publication of the trial protocol in a publicly available database is almost compulsory for publication of the full report in many potential journals.
Graphics and Tables
Graphics and tables should emphasize salient features of the underlying data and should coherently summarize large quantities of information. Although graphics provide a break from dense prose, authors must not forget that these illustrations should be scientifically informative, not decorative. The titles for graphics and tables should be clear, informative, provide the sample size, and use minimal font weight and formatting only to distinguish headings, data entry or to highlight certain results. Provide a consistent number of decimal points for the numerical results, and with no more than four for the P value. Most journals prefer cell-delineated tables created using the table function in word processing or spreadsheet programs. Some journals require specific table formatting such as the absence or presence of intermediate horizontal lines between cells.
Decisions of authorship are both sensitive and important and should be made at an early stage by the study’s stakeholders. Guidelines and journals’ instructions to authors abound with authorship qualifications. The guideline on authorship by the International Committee of Medical Journal Editors is widely known and provides a standard used by many medical and clinical journals [ 39 ]. Generally, authors are those who have made major contributions to the design, conduct, and analysis of the study, and who provided critical readings of the manuscript (if not involved directly in manuscript writing).
Picking a target journal for submission
Once a report has been written and revised, the authors should select a relevant target journal for submission. Authors should avoid predatory journals—publications that do not aim to advance science and disseminate quality research. These journals focus on commercial gain in medical and clinical publishing. Two good resources for authors during journal selection are Think-Check-Submit and the defunct Beall's List of Predatory Publishers and Journals (now archived and maintained by an anonymous third-party) [ 40 , 41 ]. Alternatively, reputable journal indexes such as Thomson Reuters Journal Citation Reports, SCOPUS, MedLine, PubMed, EMBASE, EBSCO Publishing's Electronic Databases are available areas to start the search for an appropriate target journal. Authors should review the journals’ names, aims/scope, and recently published articles to determine the kind of research each journal accepts for publication. Open-access journals almost always charge article publication fees, while subscription-based journals tend to publish without author fees and instead rely on subscription or access fees for the full text of published articles.
Conclusions
Conducting a valid clinical research requires consideration of theoretical study design, data collection design, and statistical analysis design. Proper study design implementation and quality control during data collection ensures high-quality data analysis and can mitigate bias and confounders during statistical analysis and data interpretation. Clear, effective study reporting facilitates dissemination, appreciation, and adoption, and allows the researchers to affect real-world change in clinical practices and care models. Neutral or absence of findings in a clinical study are as important as positive or negative findings. Valid studies, even when they report an absence of expected results, still inform scientific communities of the nature of a certain treatment or intervention, and this contributes to future research, systematic reviews, and meta-analyses. Reporting a study adequately and comprehensively is important for accuracy, transparency, and reproducibility of the scientific work as well as informing readers.
Acknowledgments
The author would like to thank Universiti Putra Malaysia and the Ministry of Higher Education, Malaysia for their support in sponsoring the Ph.D. study and living allowances for Boon-How Chew.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The materials presented in this paper is being organized by the author into a book.
Clinical research studies
For certain qualifying clinical research studies, Medicare Part A (Hospital Insurance) and/or Medicare Part B (Medical Insurance) cover some costs, like office visits and tests.
Your costs in Original Medicare
You may pay 20% of the Medicare-Approved Amount , depending on the treatment you get. The Part B deductible may apply.
Clinical research studies test different types of medical care, including new treatments, to find out how well they work and if they’re safe. For example, a clinical research study might test how well a new cancer drug works. Clinical research studies may involve diagnostic tests, surgical treatments, medicine, or new types of patient care.
Related resources
- National Institutes of Health's ClinicalTrials.gov
- National Cancer Institute's (NCI) Clinical Trials website

Is my test, item, or service covered?
- Open access
- Published: 18 March 2024
Utilization of EHRs for clinical trials: a systematic review
- Leila R. Kalankesh 1 , 3 &
- Elham Monaghesh 2 , 3
BMC Medical Research Methodology volume 24 , Article number: 70 ( 2024 ) Cite this article
249 Accesses
1 Altmetric
Metrics details
Background and objective
Clinical trials are of high importance for medical progress. This study conducted a systematic review to identify the applications of EHRs in supporting and enhancing clinical trials.
Materials and methods
A systematic search of PubMed was conducted on 12/3/2023 to identify relevant studies on the use of EHRs in clinical trials. Studies were included if they (1) were full-text journal articles, (2) were written in English, (3) examined applications of EHR data to support clinical trial processes (e.g. recruitment, screening, data collection). A standardized form was used by two reviewers to extract data on: study design, EHR-enabled process(es), related outcomes, and limitations.
Following full-text review, 19 studies met the predefined eligibility criteria and were included. Overall, included studies consistently demonstrated that EHR data integration improves clinical trial feasibility and efficiency in recruitment, screening, data collection, and trial design.
Conclusions
According to the results of the present study, the use of Electronic Health Records in conducting clinical trials is very helpful. Therefore, it is better for researchers to use EHR in their studies for easy access to more accurate and comprehensive data. EHRs collects all individual data, including demographic, clinical, diagnostic, and therapeutic data. Moreover, all data is available seamlessly in EHR. In future studies, it is better to consider the cost-effectiveness of using EHR in clinical trials.
Peer Review reports
Introduction
Clinical trials are of high importance for medical progress [ 1 ]. Well designed and well-executed clinical trial studies provide the foundational data for evidence-based medicine [ 2 ], which are the standard for evaluating the benefits and harms of medical interventions [ 3 ]. Numerous factors lead to the success of clinical trials, such as appropriate trial design(e.g. randomization, blinding, and controls), thorough training of research staff, and recruitment of an adequate sample size by identifying and enrolling qualified participants in a timely manner [ 4 , 5 ] and maintaining good participation through study completion [ 2 , 6 ].
Strategic selection of study sites with access to suitable patient populations can optimize recruitment. Moreover, developing practical yet scientifically sound protocols through careful planning and analysis helps ensure trials are completed in an accurate and cost-effective manner [ 2 ]. Traditionally, many trials have relied heavily on physician referrals to identify and attract potential participants [ 7 ]. While essential, sole dependence on this approach has limitations including referral bias and logistical challenges that could hamper recruitment. To strengthen the recruiting process, manually reviewing patient’s electronic records to identify and diagnose eligible candidates for clinical trials has become a standard practice [ 8 ]. However, this manual chart review method is often time-consuming and resource-intensive [ 9 ].
To modernize clinical recruitment and conduct, new tools have been developed that enable data-driven insights into patient populations within EHR systems [ 10 ]. In fact, to digitalize processes, the TransCelerate e-Resource initiative, launched in January 2016, aims to facilitate understanding the e-resource landscape and the optimal use of electronic data resources to improve clinical science and clinical trial implementation for stakeholders. The eSource initiative also aligns well with other TransCelerate initiatives designed to help modernize trial execution and ways to enroll patients in clinical trials [ 38 ].
EHR systems contain comprehensive demographic, medical and treatment history collected during routine care, which offer potential to efficiently pre-scan, identify and recruit appropriate patients for clinical trials [ 11 , 12 , 13 , 14 ]. Specifically, recruiting patients through the EHR allows pre-assessment of eligibility criteria, selection of targeted population, and automated outreach to participants [ 15 ]. EHRs also provide ongoing access to detailed patient data that may decrease redundant measurements and data collection during trials [ 12 ]. Overall, EHR-enabled recruitment and workflow processes have potential to make clinical trials more cost-effective and feasible [ 11 ]. This study conducted a systematic review to identify the applications of EHR in supporting and enhancing clinical trials.
Study design
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature search
A systematic search of PubMed was conducted on 12/3/2023 to identify relevant studies on the use of EHRs in clinical trials. The search included a combination of Medical Subject Headings (MeSH terms) and keywords related to electronic health records (EHR OR electronic medical record) AND clinical trials. The search was limited to title and abstract fields. No date or language limits were applied. The specific Boolean search syntax was:
("EHR"[Title/Abstract] OR "Electronic health record"[Title/Abstract] OR "Electronic health records"[Title/Abstract] OR "EMR"[Title/Abstract] OR "Electronic medical record"[Title/Abstract] OR "Electronic medical records"[Title/Abstract]) AND (clinical trial* [Title/Abstract]).
Reference lists of included studies were hand-searched to identify additional relevant articles. The search was performed without any time limit.
Eligibility criteria
Studies were included if they (1) were full-text journal articles, (2) were written in English, (3) examined applications of EHR data to support clinical trial processes (e.g. recruitment, screening, data collection). Reviews, letters, abstracts, editorials and other non-research studies were excluded.
Study selection and data extraction
Two researchers (EM and LRK) independently screened titles and abstracts of retrieved records to identify potentially eligible studies. After obtaining full texts of potential articles, the two investigators independently assessed eligibility based on predefined criteria. Disagreements were resolved through discussion and consensus. A form was used by two reviewers to extract data on: study design, EHR-enabled process(es), related outcomes, and limitations.
Evidence synthesis
A qualitative synthesis was conducted summarizing key outcomes and limitations of included studies grouped by the EHR-enabled process examined. The study authors met regularly to discuss consensus on findings.
The systematic literature search yielded 2161 records, out of which 312 were selected for full-text review after screening titles and abstracts. After conducting a thorough review of the full-texts and resolving disagreements regarding 2 articles, a total of 19 studies that met the predefined eligibility criteria were included in the final qualitative synthesis (Fig. 1 ).
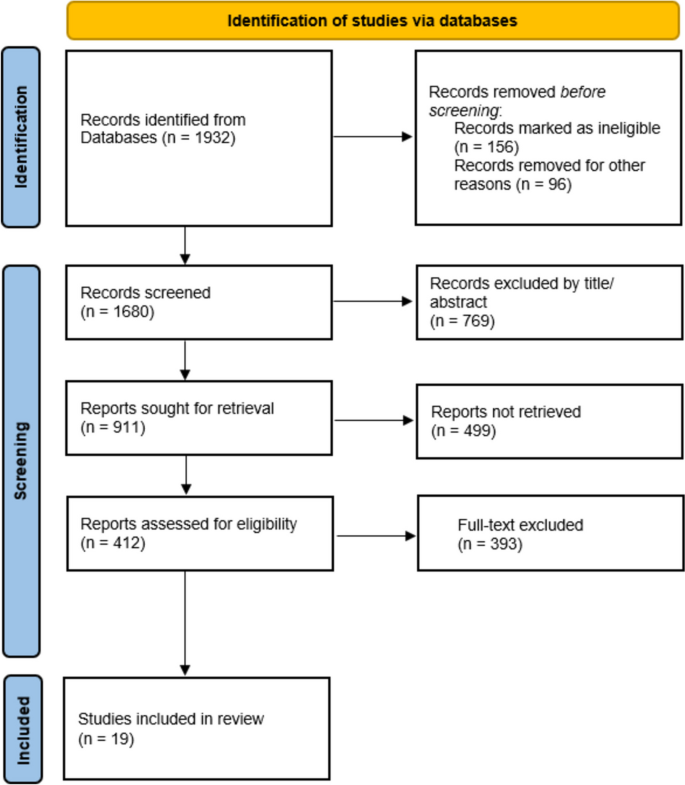
PRISMA flow diagram illustrating study selection for utilization of EHRs in Clinical Trials
Characteristics of included studies
The key characteristics of the 19 included studies are summarized in Table 1 . The studies were published in a variety of international journals, with the majority (14/19) from the United States. The remaining studies originated from China, Switzerland, Germany, Belgium, and Finland. The sample sizes ranged from 165 to 5,529,407 patients.
Clinical Trial Processes and Outcomes
Nineteen studies examined the impacts of EHR use on clinical trial processes and outcomes. Table 2 summarizes the key findings on EHR applications for recruitment, screening, data collection, and trial design. Overall, the included studies consistently demonstrated that utilization of EHR data improved clinical trial feasibility and efficiency in the following ways:
Recruitment: 19 studies evaluating EHR-enabled recruitment have reported increased enrollment efficiency compared to standard practices.
Screening: In 5 studies, EHR pre-screening excluded patients prior to full eligibility screening, reducing unnecessary procedures.
Data collection: In 3 studies using EHR data reduced data collection costs compared to standard methods.
Trial Design: In one study examining this application, EHR data informed optimization of eligibility criteria to improve statistical power for a COVID-19 trial.
Purposes of using EHR
The most frequent application of EHR data was to identify and recruit eligible participants into clinical trials. By containing diverse information on demographics, clinical history, diagnoses, and more, EHRs allowed pre-screening and outreach to potential candidates that met enrollment criteria. In several studies, EHR data was leveraged for secondary research purposes including data collection, data analysis and optimizing trial design [ 16 , 27 , 28 , 29 , 30 , 38 ]. Specifically, one study utilized EHR data from COVID-19 patients to inform eligibility criteria selection and improve statistical power for COVID-19 trials [ 23 ]. Overall, the primary use case was to enable secondary research applications of EHR data beyond routine clinical care to facilitate clinical trial processes. Key limitations of these applications included potential for selection bias, generalizability concerns in single health system populations, and heterogeneity in methods and endpoints assessed across studies. Further investigation using standardized methodology is needed to realize the full potential of EHR-enabled clinical research.
This systematic review aimed to identify applications and impacts of electronic health record (EHR) use in clinical trials. The included studies demonstrated EHR data has been leveraged to serve various key functions, including identifying eligible participants, facilitating recruitment, enabling data collection and analysis, and optimizing trial design.
In one study, EHR data was from 59639 patients who encountered health care system. The results showed that the EHR data could be used as a promising clinical tool to assist physicians in early identification of patients suitable for palliative care counseling [ 35 ]. Although this study used EHR for therapeutic purposes, it can be concluded that EHR data is very effective in identifying individuals with any target.
Another study found that primary care electronic health record data could be used effectively to identify patients who have been prescribed specific medications and patients who are potentially experiencing drug side effects [ 36 ]. In general, based on the results of this study, EHR can also be utilized in clinical trials for purposes other than patient care and in particular for the secondary use of this tool. In fact, according to the studies [ 20 , 28 , 29 , 30 , 31 , 32 ], the use of EHR serves various purposes in clinical trials, including identifying eligible participants, facilitating their recruitment and analyzing patient data to assess outcomes and measure the safety and efficacy of the intervention.
EHRs can be used as a database for the use of data needed in clinical trials. For example, a study in Brazil used EHR data to obtain benchmark for stroke patients [ 37 ].
According to the results of the present study, the use of EHR in conducting clinical trials is very helpful. Therefore, it is better for researchers to use EHR in their studies for easy access to more accurate and comprehensive data. EHRs collects all individual data, including demographic, clinical, diagnostic, and therapeutic data. So that all data is available seamlessly. Real-time access to patient data directly from EHRs could eliminate the need for manual data entry, minimizing errors and ensuring data integrity.
Moreover, EHRs enable the seamless integration of clinical trial data with other relevant health information, providing a more comprehensive picture of patient health and facilitating the evaluation of long term outcomes. In future studies, it is better to consider the cost-effectiveness of using EHR in clinical trials. Because due to the increasing use and effectiveness of using EHR in clinical trials, its cost-effectiveness should also be determined. Also, conducting such research would be useful for the wider scientific community. Also, in future studies, many metrics can be investigated and reported to reflect the effectiveness of EHR for patient registration. Also, some statistics can be shown to illustrate this.
One of the limitations of the present study was the lack of access to some databases due to sanctions. Another limitation is the lack of a similar study that comprehensively examines the role and effectiveness of EHR in clinical trials. There are also a small number of studies that have examined the effectiveness, how the EHR is used, and its uses in clinical trials.
Another limitation is related to the comparison of the studies included in this study, considering that the EHR system used in different countries, even in each country, is very different in many aspects, including the type of system used, the culture of each country, the level of EHR implementation, technical infrastructure, etc. Therefore, the comparison between systems was one of the limitations of this study.
According to the results of the present study, it can be concluded that EHR in clinical trials is used for various purposes. While promising, several limitations should be considered when interpreting the evidence. Many EHRs may rely on single health system populations, limiting generalizability of findings. Heterogeneity in methods and endpoints used to evaluate the same EHR processes is another issue to be considered. Additional limitations included potential for selection and referral bias. More research is needed to develop standardized methodology and reporting for EHR-enabled clinical trials. Future directions of the research should be to optimize EHRs for supporting clinical trials. This may be realized through enhanced interoperability and data sharing between EHR systems to facilitate multi-site and diverse patient populations trials and expand access to diverse patient populations beyond single health systems. Standardization of data formats, development of shared platforms, and policies enabling access are needed. Integration of clinical trial-specific modules into EHRs is required to simplify participant screening, recruitment, enrollment, and data collection. This could include dashboards, automated alerts, and documentation templates. Advanced analytics and machine learning applied to EHR data can also be a part of agenda for future research. Stronger privacy protections and cybersecurity measures should be in place to securely operationalize EHR data for research while maintaining patient confidentiality.
There is also gap in cost-effectiveness studies to quantify financial benefits and guide investments in EHR-enabled research infrastructure.
Availability of data and materials
Not applicable.
Kohl CD, Garde S, Knaup P. Facilitating secondary use of medical data by using openEHR archetypes. Stud Health Technol Inform. 2010;160(Pt 2):1117–21.
PubMed Google Scholar
Laaksonen N, Varjonen J-M, Blomster M, Palomäki A, Vasankari T, Airaksinen J, et al. Assessing an electronic health record research platform for identification of clinical trial participants. Contemp Clin Trials Commun. 2021;21:100692.
Article PubMed Google Scholar
Bothwell L, Greene J, Podolsky S, Jones D. Assessing the gold standard-lessons from the history of RCTs. N Engl J Med. 2016;374(22):2175–81.
Article PubMed CAS Google Scholar
Foster JM, Sawyer SM, Smith L, Reddel HK, Usherwood T. Barriers and facilitators to patient recruitment to a cluster randomized controlled trial in primary care: lessons for future trials. BMC Med Res Methodol. 2015;15(1):1–9.
Article Google Scholar
Farrell B, Kenyon S, Shakur H. Managing clinical trials. Trials. 2010;11(1):1–6.
Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthcare Policy. 2011;4:47.
Mapstone J, Elbourne DD, Roberts IG. Strategies to improve recruitment to research studies. Cochrane Database of Systematic Reviews. 2002(3).
Vickers AJ. How to improve accrual to clinical trials of symptom control 2: design issues. J Soc Integr Oncol. 2007;5(2):61.
Article PubMed PubMed Central Google Scholar
Doods J, Bache R, McGilchrist M, Daniel C, Dugas M, Fritz F. Piloting the EHR4CR feasibility platform across Europe. Methods Inf Med. 2014;53(04):264–8.
Kellar E, Bornstein SM, Caban A, Célingant C, Crouthamel M, Johnson C, et al. Optimizing the use of electronic data sources in clinical trials: the landscape, part 1. Ther Innov Reg Sci. 2016;50(6):682–96.
Mc Cord KA, Ewald H, Ladanie A, Briel M, Speich B, Bucher HC, et al. Current use and costs of electronic health records for clinical trial research: a descriptive study. CMAJ Open. 2019;7(1):E23.
Zuidgeest MG, Goetz I, Groenwold RH, Irving E, van Thiel GJ, Grobbee DE, et al. Series: pragmatic trials and real world evidence: paper 1 introduction. J Clin Epidemiol. 2017;88:7–13.
Mc Cord KA, Salman RAS, Treweek S, Gardner H, Strech D, Whiteley W, et al. Routinely collected data for randomized trials: promises, barriers, and implications. Trials. 2018;19(1):1–9.
Beaver JA, Howie LJ, Pelosof L, Kim T, Liu J, Goldberg KB, et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–56.
Li G, Sajobi TT, Menon BK, Korngut L, Lowerison M, James M, et al. Registry-based randomized controlled trials-what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16–24.
Ateya MB, Delaney BC, Speedie SM. The value of structured data elements from electronic health records for identifying subjects for primary care clinical trials. BMC Med Inform Decis Mak. 2016;16:1.
Beresniak A, Schmidt A, Proeve J, Bolanos E, Patel N, Ammour N, et al. Cost-benefit assessment of using electronic health records data for clinical research versus current practices: Contribution of the Electronic Health Records for Clinical Research (EHR4CR) European Project. Contemp Clin Trials. 2016;46:85–91.
Bruland P, McGilchrist M, Zapletal E, Acosta D, Proeve J, Askin S, et al. Common data elements for secondary use of electronic health record data for clinical trial execution and serious adverse event reporting. BMC Med Res Methodol. 2016;16(1):159.
Carrion J. Improving the patient-clinician interface of clinical trials through health informatics technologies. J Med Syst. 2018;42(7):120.
De Moor G, Sundgren M, Kalra D, Schmidt A, Dugas M, Claerhout B, et al. Using electronic health records for clinical research: the case of the EHR4CR project. J Biomed Inform. 2015;53:162–73.
Embi PJ, Jain A, Clark J, Bizjack S, Hornung R, Harris CM. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165(19):2272–7.
Ernecoff NC, Wessell KL, Gabriel S, Carey TS, Hanson LC. A novel screening method to identify late-stage dementia patients for palliative care research and practice. J Pain Symptom Manage. 2018;55(4):1152–8.e1.
Kim JH, Ta CN, Liu C, Sung C, Butler AM, Stewart LA, et al. Towards clinical data-driven eligibility criteria optimization for interventional COVID-19 clinical trials. J American Med Inform Assoc. 2021;28(1):14–22.
Kirshner J, Cohn K, Dunder S, Donahue K, Richey M, Larson P, et al. Automated electronic health record-based tool for identification of patients with metastatic disease to facilitate clinical trial patient ascertainment. JCO Clin Cancer Inform. 2021;5:719–27.
Laaksonen N, Varjonen JM, Blomster M, Palomäki A, Vasankari T, Airaksinen J, et al. Assessing an electronic health record research platform for identification of clinical trial participants. Contemp Clin Trials Commun. 2021;21:100692.
Li M, Cai H, Nan S, Li J, Lu X, Duan H. A Patient-screening tool for clinical research based on electronic health records using openEHR: development study. JMIR Med Inform. 2021;9(10):e33192.
Meystre SM, Heider PM, Kim Y, Aruch DB, Britten CD. Automatic trial eligibility surveillance based on unstructured clinical data. Int J Med Informatics. 2019;129:13–9.
Miotto R, Weng C. Case-based reasoning using electronic health records efficiently identifies eligible patients for clinical trials. J American Med Inform Assoc. 2015;22(e1):e141–50.
Nelson SJ, Drury B, Hood D, Harper J, Bernard T, Weng C, et al. EHR-based cohort assessment for multicenter RCTs: a fast and flexible model for identifying potential study sites. Journal of the American Medical Informatics Association : JAMIA. 2021.
Ni Y, Bermudez M, Kennebeck S, Liddy-Hicks S, Dexheimer J. A real-time automated patient screening system for clinical trials eligibility in an emergency department: design and evaluation. JMIR Med Inform. 2019;7(3):e14185.
O’Brien EC, Raman SR, Ellis A, Hammill BG, Berdan LG, Rorick T, et al. The use of electronic health records for recruitment in clinical trials: a mixed methods analysis of the harmony outcomes electronic health record ancillary study. Trials. 2021;22(1):465.
Rogers JR, Liu C, Hripcsak G, Cheung YK, Weng C. Comparison of clinical characteristics between clinical trial participants and nonparticipants using electronic health record data. JAMA Netw Open. 2021;4(4):e214732.
Sun Y, Butler A, Diallo I, Kim JH, Ta C, Rogers JR, et al. A framework for systematic assessment of clinical trial population representativeness using electronic health records data. Appl Clin Inform. 2021;12(4):816–25.
Zimmerman LP, Goel S, Sathar S, Gladfelter CE, Onate A, Kane LL, et al. A novel patient recruitment strategy: patient selection directly from the community through linkage to clinical data. Appl Clin Inform. 2018;9(1):114–21.
Guo A, Foraker R, White P, Chivers C, Courtright K, Moore N. Using electronic health records and claims data to identify high-risk patients likely to benefit from palliative care. American J Managed Care. 2021;27(1):e7–15.
Cole AM, Stephens KA, West I, Keppel GA, Thummel K, Baldwin L-M. Use of electronic health record data from diverse primary care practices to identify and characterize patients’ prescribed common medications. Health Informatics J. 2020;26(1):172–80.
Valêncio RFZ, Souza JTd, Winckler FC, Modolo GP, Ferreira NC, Bazan SGZ, et al. Semi-automated data collection from electronic health records in a stroke unit in Brazil. Arquivos de Neuro-Psiquiatria. 2021.
US Food and Drug Administration. Guidance for industry: electronic source data in clinical investigations. Silver Spring MD. 2013;16:15.
Google Scholar
Download references
Acknowledgements
The research protocol was approved & Supported by Student Research Committee, Tabriz University of Medical Sciences (grant number: IR.TBZMED.VCR.REC.1401.152).
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and affiliations.
Tabriz Health Services Management Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Leila R. Kalankesh
Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
Elham Monaghesh
Department of Health Information Technology, School of Management and Medical Informatics, Tabriz University of Medical Sciences, Tabriz, Iran
Leila R. Kalankesh & Elham Monaghesh
You can also search for this author in PubMed Google Scholar
Contributions
E.M. Writing the main manuscript text, Data curation, prepared figures, writing – review & editing. L.K. Validation, Investigation, Conceptualization, Methodology, Supervision, All authors reviewed the manuscript.
Corresponding author
Correspondence to Elham Monaghesh .
Ethics declarations
Ethics approval and consent to participate.
The study is approved by ethical committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1401.152).
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kalankesh, L.R., Monaghesh, E. Utilization of EHRs for clinical trials: a systematic review. BMC Med Res Methodol 24 , 70 (2024). https://doi.org/10.1186/s12874-024-02177-7
Download citation
Received : 03 September 2023
Accepted : 08 February 2024
Published : 18 March 2024
DOI : https://doi.org/10.1186/s12874-024-02177-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Electronic Health Record
- Clinical trials
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
In two early trials, blood cancer treatment appears promising for deadly brain tumor
Two early trials published Wednesday showed promise in treating one of the deadliest types of cancer, glioblastoma .
The aggressive brain cancer, which took the lives of John McCain and Beau Biden , is only diagnosed at stage 4, and the five-year survival rate is around 10% .
The disease has no cure and, according to Dr. Michael Vogelbaum, chief of neurosurgery and program leader of neuro-oncology at Moffitt Cancer Center in Tampa, Florida, there have been no new drug approvals in the past two decades that have extended the lives of patients with glioblastoma.
The two clinical trials published Wednesday were extremely small, conducted on just nine patients in total, and much more research is needed, with larger trials, to determine how effective the therapy might be in the long run.
“All of these results are preliminary but encouraging,” said Vogelbaum, who wasn’t involved with either trial.
In the two unrelated trials, a novel take on an existing treatment for blood cancer was shown to be safe and it shrank tumors –– at least temporarily.
Both studies looked at the effects of a personalized immunotherapy called chimeric antigen receptor T-cell therapy — CAR-T therapy for short — in patients whose glioblastoma had returned after their initial treatment.
CAR-T therapy involves harvesting a person’s own immune cells and modifying them in a lab to seek out specific tumor proteins. The cells are then reintroduced into the body where they replicate, creating a surge of cancer-fighting immune cells.
The treatment is highly effective for certain blood cancers , but scientists are still studying whether modified versions of CAR-T therapy can be used for solid tumors like glioblastoma. These tumors, which account for the majority of cancers, present challenges that blood cancers do not.
Many blood cancers are homogeneous, meaning their cells are uniform. This gives CAR-T therapy a clear target to latch onto and attack. But solid tumors tend to have a variety of different cell types that can differ within individual tumors. This is particularly true for glioblastoma, which contains a large number of abnormal-looking cells.
“We had previous experience using a regular CAR in brain tumors but it wasn’t enough,” said Dr. Marcela Maus, director of the Cellular Immunotherapy Program at the Massachusetts General Cancer Center in Boston. Maus led one of the new studies, the results of which were published in The New England Journal of Medicine .
The original studies testing CAR-T therapy for glioblastoma only had one target, which is how the therapy has worked in blood cancers.
The cells targeted a protein with a specific mutation, but Maus said that not everyone with glioblastoma had the mutation. What’s more, even in patients who had the mutation, not every one of their tumor cells necessarily had it. “Even if we got the right cells, we didn’t get all of them because other tumors had other targets,” she said.
Expanding an existing therapy
Both phase 1 clinical trials used CAR-T cells that were programmed to attack two targets instead of one, with the hope that multiple targets would better equip the cells to destroy solid tumors.
“It gives you more shots on goal, at targeting the protein, because these are not completely overlapping targets on any given tumor,” said Dr. Vincent Lam, an assistant professor of oncology at the Johns Hopkins Cancer Center, who specializes in immunotherapies and wasn’t involved with either trial.
In Maus’ clinical trial, which included three patients, T-cells were engineered to seek out and attack a protein called EGRF that’s often found in abundance in glioblastoma tumors but is not present in healthy brain tissue. The second target was a variant of EGRF that’s also commonly found in the tumors.
When used to treat blood cancer, CAR-T cells are transferred back into the body intravenously. Maus’ team chose a more targeted approach for their experimental therapy: injecting the cells directly into the cerebrospinal fluid that surrounds the brain and spinal cord.
This prompted more of the cancer-fighting cells to stick around the site of the brain tumors and, the researchers hypothesized, would reduce the amount of the immunotherapy elsewhere in the body.
Confining the therapy to the brain was important: While the target, EGFR, is not found in healthy brain tissue, it is found in healthy cells elsewhere in the body. If the CAR-T cells went beyond the brain, they could potentially attack these cells.
To further prevent the CAR-T cells from escaping, the researchers bulked them up by binding them to an antibody, which made it more difficult for the cells to cross the blood-brain barrier and enter the bloodstream.
All three patients — two who were in their 70s and one in her late 50s — responded quickly to the treatment. Brain scans showed their tumors shrunk significantly within a day of receiving the therapy. In the 57-year-old woman, an MRI taken five days after her infusion of the modified cells showed her tumor was nearly gone.
The results, however, were temporary.
“We’re still in the early phases of the study. Two patients had their disease recur in the first six months and we want to aim for something better,” Maus said.
While some CAR-T cells did pass beyond the brain, they didn’t do so in large enough numbers to cause damage, the trial found.
Striking a balance
The other trial , published in Nature Medicine, included six patients with recurrent glioblastoma. Their CAR-T cells also sought out EGFR, but used another protein, called IL13Rα2, which is found in 75% of glioblastoma tumors, as their second target.
All six patients underwent radiation to shrink their tumors before they started the immunotherapy, and each was given a single injection of the cells.
The team also delivered the immunotherapy locally, injecting it directly into the cerebrospinal fluid. All of the patients saw a reduction in their tumor size within the first two days of treatment, and they also experienced a significant spike in active CAR-T cells in their spinal fluid for several weeks after injection, meaning the cells were successfully dividing as well as concentrating in the area surrounding tumors.
“That was striking to us. We didn’t expect that kind of expansion, proliferation and maintenance in the spinal fluid,” said Dr. Donald O’Rourke, director of the Glioblastoma Translational Center of Excellence at the Abramson Cancer Center at Penn Medicine in Philadelphia, who co-led the trial.
O’Rourke and his team also used two different doses to get closer to an understanding of what the ideal number of CAR-T cells is for an infusion.
The ideal number would provide the most potent therapeutic effect without causing side effects so severe they negate the cancer-killing benefits, but striking a balance is tricky.
CAR-T therapy is different from a regular drug; it’s considered a “living drug” because the modified cells keep dividing once they’re in the body, meaning the amount in the initial infusion isn’t the final amount a patient will have, O’Rourke said.
All immunotherapies come with risk of neurological side effects, including confusion, language difficulties and sleepiness. The trial found that these side effects came on more quickly when CAR-T cells were injected into the cerebrospinal fluid, but that starting with a lower dose may be able to remedy this. The first three patients were given a lower dose of CAR-T. While they did experience signs of neurotoxicity, it was milder than the neurotoxicity in the three who received the higher dose.
Participants in both trials did experience at least some side effects of CAR-T therapy, which included fever and vomiting as well as neurological effects such as aphasia .
The two trials come just a week after the results of another CAR-T therapy clinical trial for glioblastoma were published in Nature Medicine. That trial used a single target, IL-13Rα2 — also used in the Penn Medicine trial — in 65 patients and also determined that CAR-T therapy is safe and could be an effective treatment for glioblastoma.
Lam, of Johns Hopkins, said that CAR-T has shown promise in glioblastoma in studies over the past five years. The biggest takeaway the two newest trials bring to the table, he said, is that CAR-T therapy appears to be able to safely target two proteins commonly found in glioblastoma tumors.
The question now is whether the results are durable. The results of the early trials don’t necessarily mean the therapy will work long term.
Both teams plan to continue with their ongoing phase 1 trials and modify their approach to home in on the best combination of treatments for glioblastoma.
Maus said she believes CAR-T therapy may be more effective if a tumor is first weakened by radiation and chemotherapy. Other researchers are also exploring cancer-fighting vaccines , which may be able to be used in tandem with CAR-T therapy.
“We’re learning from each other. I think that’s really a tremendous model, and what we are all seeing is that this sort of therapy has legs for brain tumor patients,” Maus said. “It may not be the final version yet, but we’re onto something.”
Kaitlin Sullivan is a contributor for NBCNews.com who has worked with NBC News Investigations. She reports on health, science and the environment and is a graduate of the Craig Newmark Graduate School of Journalism at City University of New York.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Estimated Lifetime Gained With Cancer Screening Tests : A Meta-Analysis of Randomized Clinical Trials
- 1 Clinical Effectiveness Research Group, Institute of Health and Society, University of Oslo, Department for Transplantation Medicine, Oslo University Hospital, Oslo, Norway
- 2 Department of Gastroenterology, Hepatology and Clinical Oncology, Centre of Postgraduate Medical Education, Warsaw, Poland
- 3 Department of Cancer Prevention and Oncological Gastroenterology, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 4 Medical Library, University of Oslo and Oslo University Hospital, Oslo, Norway
- 5 Digestive Disease Center, Showa University Northern Yokohama Hospital, Yokohama, Japan
- 6 Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts
- 7 Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- Comment & Response Lifetime Gained With Cancer Screening—Reply Michael Bretthauer, MD, PhD; Michal F. Kaminski, MD, PhD; Mette Kalager, MD, PhD JAMA Internal Medicine
- Comment & Response Lifetime Gained With Cancer Screening Hermann Brenner, MD, MPH; Michael Hoffmeister, PhD JAMA Internal Medicine
- Comment & Response Lifetime Gained With Cancer Screening Ernst J. Kuipers, MD, PhD; Manon C. W. Spaander, MD, PhD JAMA Internal Medicine
- Comment & Response Lifetime Gained With Cancer Screening Jennifer A. Watt, MD, PhD; Areti-Angeliki Veroniki, PhD; Sharon E. Straus, MD, MSc JAMA Internal Medicine
Question Cancer screening tests are promoted to save lives, but how much is life extended due to commonly used cancer screening tests?
Findings In this systematic review and meta-analysis of 18 long-term randomized clinical trials involving 2.1 million individuals, colorectal cancer screening with sigmoidoscopy prolonged lifetime by 110 days, while fecal testing and mammography screening did not prolong life. An extension of 37 days was noted for prostate cancer screening with prostate-specific antigen testing and 107 days with lung cancer screening using computed tomography, but estimates are uncertain.
Meaning The findings of this meta-analysis suggest that colorectal cancer screening with sigmoidoscopy may extend life by approximately 3 months; lifetime gain for other screening tests appears to be unlikely or uncertain.
Importance Cancer screening tests are promoted to save life by increasing longevity, but it is unknown whether people will live longer with commonly used cancer screening tests.
Objective To estimate lifetime gained with cancer screening.
Data Sources A systematic review and meta-analysis was conducted of randomized clinical trials with more than 9 years of follow-up reporting all-cause mortality and estimated lifetime gained for 6 commonly used cancer screening tests, comparing screening with no screening. The analysis included the general population. MEDLINE and the Cochrane library databases were searched, and the last search was performed October 12, 2022.
Study Selection Mammography screening for breast cancer; colonoscopy, sigmoidoscopy, or fecal occult blood testing (FOBT) for colorectal cancer; computed tomography screening for lung cancer in smokers and former smokers; or prostate-specific antigen testing for prostate cancer.
Data Extraction and Synthesis Searches and selection criteria followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline. Data were independently extracted by a single observer, and pooled analysis of clinical trials was used for analyses.
Main Outcomes and Measures Life-years gained by screening was calculated as the difference in observed lifetime in the screening vs the no screening groups and computed absolute lifetime gained in days with 95% CIs for each screening test from meta-analyses or single randomized clinical trials.
Results In total, 2 111 958 individuals enrolled in randomized clinical trials comparing screening with no screening using 6 different tests were eligible. Median follow-up was 10 years for computed tomography, prostate-specific antigen testing, and colonoscopy; 13 years for mammography; and 15 years for sigmoidoscopy and FOBT. The only screening test with a significant lifetime gain was sigmoidoscopy (110 days; 95% CI, 0-274 days). There was no significant difference following mammography (0 days: 95% CI, −190 to 237 days), prostate cancer screening (37 days; 95% CI, −37 to 73 days), colonoscopy (37 days; 95% CI, −146 to 146 days), FOBT screening every year or every other year (0 days; 95% CI, −70.7 to 70.7 days), and lung cancer screening (107 days; 95% CI, −286 days to 430 days).
Conclusions and Relevance The findings of this meta-analysis suggest that current evidence does not substantiate the claim that common cancer screening tests save lives by extending lifetime, except possibly for colorectal cancer screening with sigmoidoscopy.
Read More About
Bretthauer M , Wieszczy P , Løberg M, et al. Estimated Lifetime Gained With Cancer Screening Tests : A Meta-Analysis of Randomized Clinical Trials . JAMA Intern Med. 2023;183(11):1196–1203. doi:10.1001/jamainternmed.2023.3798
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Advertisement

Abstract 1013: Strengths, weaknesses, opportunities, and threats of conducting clinical trials in Nigeria: The IRONMAN study
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record March 22 2024
Opeyemi Oreoluwa Bolajoko , Parisa Fathi , Oluwaseyi Toye , Dottington D. Fulwood , Ademola Popoola , Chidiebere Ogo , Hassan Dogo , Omolara Fatiregun , Anthonia Sowumi , Paul Jibrin , Mutiu A. Jimoh , Faruk Mohammed , Folakemi Odedina , Prostate Cancer Transatlantic Consortium; Abstract 1013: Strengths, weaknesses, opportunities, and threats of conducting clinical trials in Nigeria: The IRONMAN study. Cancer Res 15 March 2024; 84 (6_Supplement): 1013. https://doi.org/10.1158/1538-7445.AM2024-1013
Download citation file:
- Ris (Zotero)
- Reference Manager
Background: The need to increase understanding of prostate cancer (CaP) is ever increasing. Unfortunately, there is limited understanding of how CaP affects men of African ancestry (MAA) despite significant disparities in CaP prevention, diagnosis, and treatment due to genetic and sociobehavioral factors. Conducting CaP clinical trials (CTs) in Africa presents multiple opportunities to improve upon collective knowledge of CaP in MAA experiences, expand patient access to CTs, and provide opportunities for African scientists and urologists to contribute to research on the global stage. The International Registry of Men with Advanced Prostate Cancer (IRONMAN) seeks to reduce CaP disparities by sponsoring sites throughout Africa. Four Prostate Cancer Transatlantic Consortium (CaPTC) institutions in Nigeria currently participate in IRONMAN: University of Ilorin Teaching Hospital, Federal Medical Center, University of Maiduguri, and Lagos State University Teaching Hospital. Because CTs of this scale are infrequently conducted in Africa, there are special considerations when conducting CTs in low-middle-income countries (LMICs) like Nigeria.
Methods: Hourlong interviews dedicated to site staff were conducted via Zoom in Spring 2023 (four total). Interviews utilized a Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis approach. Each interview was recorded, transcribed, and validated by study staff for accuracy. Themes from each were inductively coded and then collated across all four sites.
Results: The urologists, oncologists, phlebotomists, pathologists, nurses, research coordinators that make up the study teams identified the holistics of conducting the IRONMAN study at their sites. Strengths included a large patient population to recruit from and appropriate resources to conduct the study (ex., lab space, clinical knowledge, staffing levels). Weaknesses included the social determinants of health that negatively impact patient participation, limited biorepository space to store samples, and the need for patient-physician trust. The greatest opportunity presented was Nigerian institutions joining more CTs in the future, particularly multi-site global trials. Described threats included staff turnover, national stability, and increased economic disparities.
Discussion: While increasing the overall number of CTs in Africa is a noble cause, sponsors and institutions should note the unique circumstances in implementing CTs in Africa when designing CTs and recruiting sites. Listening to the experiences of study teams is pivotal in ensuring CTs are Africa-minded - they will be complimentary to established research infrastructure, appropriately funded, and culturally responsive all in the name of equitable research. By doing so, ethical compliance and quality data collection will ensue and reduce disparities at the micro, mezzo, and macro levels.
Citation Format: Opeyemi Oreoluwa Bolajoko, Parisa Fathi, Oluwaseyi Toye, Dottington D. Fulwood, Ademola Popoola, Chidiebere Ogo, Hassan Dogo, Omolara Fatiregun, Anthonia Sowumi, Paul Jibrin, Mutiu A. Jimoh, Faruk Mohammed, Folakemi Odedina, Prostate Cancer Transatlantic Consortium. Strengths, weaknesses, opportunities, and threats of conducting clinical trials in Nigeria: The IRONMAN study [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2024; Part 1 (Regular Abstracts); 2024 Apr 5-10; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2024;84(6_Suppl):Abstract nr 1013.
Citing articles via
Email alerts.
- Online First
- Collections
- Online ISSN 1538-7445
- Print ISSN 0008-5472
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account

IMAGES
VIDEO
COMMENTS
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe.
Clinical trials are conducted according to a plan, called a protocol, which describes: what the researchers hope to learn from the study. Volunteers who participate in the study must agree to the ...
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study: New drugs or new combinations of drugs; New ways of doing surgery; New medical devices; New ways to use existing treatments; New ways to change behaviors to ...
A clinical study is conducted according to a research plan known as the protocol. The protocol is designed to answer specific research questions and safeguard the health of participants. It contains the following information: The reason for conducting the study. Who may participate in the study (the eligibility criteria)
What is Clinical Research? From a US national research authority. Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances. This page last reviewed on September 29, 2016. Clinical research occurs in many formats and can involve anyone.
The term "clinical research" refers to the entire process of studying and writing about a drug, a medical device or a form of treatment, which includes conducting interventional studies ( clinical trials) or observational studies on human participants. [1] [3] Clinical research can cover any medical method or product from its inception in the ...
Below are descriptions of some different kinds of clinical research. Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to ...
The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as ...
Explore 487,073 research studies in all 50 states and in 223 countries. See listed clinical studies related to the coronavirus disease (COVID-19) ClinicalTrials.gov is a resource provided by the U.S. National Library of Medicine. IMPORTANT: Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Supporting Clinical Research The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies.
ClinicalTrials.gov is a place to learn about clinical studies from around the world. The U.S. government does not review or approve the safety and science of all studies listed on this website. Read our full disclaimer for details. Focus Your Search (all filters optional) Condition/disease. Other terms.
The Biology of Aging and Age-Related Diseases Lab studies molecular and cellular mechanisms behind dementia and other aging disorders. Areas of Research. Discover research activities, initiatives and projects. ... Clinical Trials Clinical trials testing the effects and safety of new drugs, devices and therapies. Find active clinical trials ...
Efficacy, Safety, and Tolerability of Oral Ubrogepant in the Acute Treatment of Migraine Scottsdale/Phoenix, AZ. This study will evaluate the efficacy, safety, and tolerability of 2 doses of ubrogepant (25 and 50 mg) compared to placebo for the acute treatment of a single migraine attack. A Multicenter Assessment of ALD403 in Chronic Migraine ...
How to Find Studies by Topic or on a Map; How to Use Search Results. Learn about the different features of the search results list, including how to customize your display. How to Find Results of Studies. Learn how to find studies that have been updated with study results, including studies with results that have been published in medical journals.
Abstract. The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice ...
What it is. Clinical research studies test different types of medical care, including new treatments, to find out how well they work and if they're safe. For example, a clinical research study might test how well a new cancer drug works. Clinical research studies may involve diagnostic tests, surgical treatments, medicine, or new types of ...
Background and objective Clinical trials are of high importance for medical progress. This study conducted a systematic review to identify the applications of EHRs in supporting and enhancing clinical trials. Materials and methods A systematic search of PubMed was conducted on 12/3/2023 to identify relevant studies on the use of EHRs in clinical trials. Studies were included if they (1) were ...
When test performance was evaluated on the basis of clinical stage, sensitivity for clinical stage I colorectal cancer was 55% (95% CI, 35 to 73) (12 of 22 cancers) and sensitivity for clinical ...
The two trials come just a week after the results of another CAR-T therapy clinical trial for glioblastoma were published in Nature Medicine. That trial used a single target, IL-13Rα2 — also ...
One dose of LSD in a clinical trial significantly improved anxiety and lasted for 12 weeks, convincing the FDA to give the drug a breakthrough therapy designation.
Importance Cancer screening tests are promoted to save life by increasing longevity, but it is unknown whether people will live longer with commonly used cancer screening tests.. Objective To estimate lifetime gained with cancer screening.. Data Sources A systematic review and meta-analysis was conducted of randomized clinical trials with more than 9 years of follow-up reporting all-cause ...
AIMS AND SCOPE OF JOURNAL: The Annual Review of Clinical Psychology provides comprehensive reviews of significant developments in the field of clinical psychology and psychiatry. The journal covers research, theory, and the application of psychological principles to address recognized disorders, including schizophrenia, mood, anxiety, childhood, substance use, cognitive, and personality disorders.
Abstract. Background: The need to increase understanding of prostate cancer (CaP) is ever increasing. Unfortunately, there is limited understanding of how CaP affects men of African ancestry (MAA) despite significant disparities in CaP prevention, diagnosis, and treatment due to genetic and sociobehavioral factors. Conducting CaP clinical trials (CTs) in Africa presents multiple opportunities ...