Internet Book of Critical Care (IBCC)
Online Medical Education on Emergency Department (ED) Critical Care, Trauma, and Resuscitation


Posterior Reversible Encephalopathy Syndrome (PRES)
June 24, 2022 by Josh Farkas
- Pathogenesis
- Clinical presentation
- PRES-RCVS (Reversible Cerebral Vasoconstriction Syndrome) overlap
- Lumbar puncture
- EEG & seizure semiology
- Differential diagnosis
- Questions & discussion
(back to contents)
- Brain injury is not always reversible.
- Involvement is not always localized to the posterior regions of the brain, nor to the white matter.
- PRES is a clinicoradiologic diagnosis that was not discovered until the widespread application of brain imaging modalities such as CT and MRI.
- PRES often occurs in the context of a hypertensive emergency, in which case PRES is equivalent to “hypertensive encephalopathy.”
- The exact incidence of PRES is unclear, but this is commonly encountered within the ICU.
three dimensions of pathogenesis
- Note: High-quality evidence on the pathogenesis of PRES is lacking. Consequently, the following is largely hypothetical in nature.
- Normally, the cerebral arterioles will vasoconstrict in the context of hypertension, thereby shielding the brain tissue from experiencing hypertension.
- At extremely high blood pressures, autoregulation may fail, causing the brain tissue to experience very high blood pressures. Hypertension may lead to fluid exudation and tissue edema.
- Both the absolute blood pressure and the rate of blood pressure rise are important. Patients with chronic hypertension can tolerate extremely high blood pressures without developing PRES. Alternatively, patients with baseline hypotension or highly labile blood pressures may be more likely to develop PRES.
- Posterior regions of the brain may be more susceptible to failed autoregulation, because the vasculature has less sympathetic innervation. ( 33630183 )
- Dysfunction of the vascular endothelium may promote exudation of fluid from the vasculature and tissue edema.
- Endothelial dysfunction may be especially relevant in the context of cytotoxic chemotherapies or preeclampsia.
- In occasional patients, dysfunctional attempts at autoregulation may result in reactive focal vasoconstriction. This may lead to focal hypoperfusion and infarction.
- When focal vasoconstriction occurs, this may represent a combined syndrome involving both PRES and reversible cerebral vasoconstriction syndrome (RCVS) – more on this below .
varying pathogenesis in different patients
- In some patients, the primary mechanism of PRES may be failure of autoregulation (#1 above). For example, this may be the case in patients with hypertensive emergency.
- In other patients, endothelial dysfunction alone could be the cause of PRES. This may explain how PRES can occur in patients who are not hypertensive.
hypertension is the most common contributing factor
- The key contributing factor is a rapid increase in blood pressure above the patient's baseline that overwhelms cerebral autoregulation.
- ⚠️ ~25% of patients lack any documented hypertension. Thus, a normal or low blood pressure does not exclude PRES. ( 35046115 )
- (1) Hypertensive emergency due to uncontrolled chronic hypertension. “Hypertensive encephalopathy” is one subgroup of PRES patients.
- (2) Preeclampsia/eclampsia . 98% of patients with eclampsia in one series had PRES on MRI scan. ( 33630183 ) Eclampsia thus represents a subgroup of patients with PRES.
- (3) Renal failure (e.g., glomerulonephritis). Renal failure may cause both hypertension and endothelial dysfunction.
- (4) Guillain-Barre syndrome with autonomic instability. 📖
- (5) Paroxysmal sympathetic hyperactivity (PSH). 📖
medications that cause hypertension and/or endothelial dysfunction
- Anthracyclines: adriamycin, daunorubicin.
- Antimetabolites : azathioprine, capecitabine, cytarabine, gemcitabine, nelarabine.
- Alkylating agent: ifosfamide, cyclophosphamide.
- Folate antagonists : 5-fluorouracil, methotrexate.
- Platinum analogues : cisplatin, carboplatin, oxaliplatin.
- Proteasome inhibitor: bortezomib.
- Taxenes (paclitaxel).
- Vinca alkaloids: vincristine, vinblastine, vinorelbine.
- Multidrug regimens for acute leukemia: L-asparaginase and intrathecal methotrexate.
- Intravenous immunoglobulin (IVIG).
- Calcineurin inhibitors ( tacrolimus , sirolimus, cyclosporine ).
- Mycophenolate mofetil.
- Rituximab .
- Steroids, especially high-dose.
- Checkpoint inhibitor ipilimumab and CAR T-cell therapy. ( 32487905 )
- Granulocyte colony stimulating factor (G-CSF).
- Interferon therapy. ( 35046115 )
- VEGF inhibitors (e.g., bevacizumab ).
- Tyrosine kinase inhibitors : sorafenib, sunitinib, erlotinib, vandetanib, pazopanib, lenvatinib.
- mTOR kinase inhibitor: temsirolimus.
- Sympathomimetics (therapeutic or illicit).
- Carbamazepine.
- LSD intoxication. ( 35046115 )
systemic inflammation & endothelial dysfunction
- Sepsis (septic patients with encephalopathy may have a prevalence of 9%). ( 35133605 )
- Autoimmune diseases , (e.g., lupus, scleroderma, Sjogren's disease, rheumatoid arthritis, ANCA vasculitides, neuromyelitis optica spectrum disorder). ( 35046115 )
- Thrombotic microangiopathies 📖 (e.g., hemolytic uremic syndrome, thrombotic thrombocytopenic purpura).
other risk factors
- Renal failure , both acute and chronic (promotes endothelial dysfunction & volume overload).
- Hypomagnesemia , hypercalcemia. ( 24476076 )
- Fluid overload .
- Sickle cell disease.
- Nephrotic syndrome. ( 35419136 )
Course: clinical deterioration is acute or subacute (may evolve over 1-2 days).
clinical features
- ⚠️ Absence of hypertension does not exclude PRES. ( 34618761 )
- Seizure (~70%) is very common (especially among ICU PRES cohorts). ( 35046115 ) More on seizure semiology below 📖 .
- Encephalopathy (~70%): Ranges from somnolence to coma.
- Usually described as constant, dull, diffuse, gradual onset, and difficult to treat.
- If a thunderclap headache occurs, this should suggest reversible cerebral vasoconstriction syndrome (RCVS) as either an alternative or superimposed diagnosis. 📖
- Auras, blurred vision, color vision abnormality, diplopia.
- Visual field deficits.
- Cortical blindness, visual hallucinations (Anton syndrome).
- Vary depending on the site of involvement.
- May include hemiparesis, aphasia, or ataxia. ( 33630183 )
basics of PRES-RCVS (Reversible Cerebral Vasoconstriction Syndrome) overlap
- PRES involves failure of autoregulation, with excess blood flow through the arterioles.
- RCVS involves excessive vasospasm, causing inadequate blood flow through the arterioles.
- (1) The most common form of PRES-RCVS overlap appears to occur in patients who initially present with PRES. Over time, cerebral vasculature responds to hypertension and endothelial damage with vasospasm , thereby causing RCVS to be superimposed on top of PRES . RCVS might complicate the majority of patients with PRES. ( 29274685 )
- (2) Less commonly, ~20% of RCVS may be complicated by the subsequent occurrence of PRES. ( 29274685 ) In these patients, the primary insult is RCVS – which leads to subsequent hypertension and sympathetic activation. This hypertension may overwhelm autoregulation in posterior areas of the brain which aren't experiencing vasoconstriction – leading to PRES. Essentially, the body is trying to overcome the cerebral vasoconstriction of RCVS, but this leads to an excessive blood pressure.
clinical features of PRES-RCVS overlap
- Papers on “RCVS” often note that some patients have cerebral edema.
- Papers on “PRES” often note that some patients have convexity subarachnoid hemorrhage.
- The table above shows more classic features of PRES and RCVS. Patients with substantial symptomatology and imaging features of both syndromes may have PRES-RCVS overlap.
treatment implications
- Patients with PRES-RCVS overlap may theoretically benefit from consideration of treating both disease processes.

the hallmark finding is vasogenic edema
- CT scan may be normal, but in more severe cases the edema will also be visible on CT scanning as hypodense areas within the white matter.
- Vasogenic should be seen in 100% of cases on MRI, as this is part of the definition of PRES.
- Contrast enhancement (~40%) may occur in a leptomeningeal pattern, a cortical pattern within regions of altered FLAIR signal, or a combination. ( 35046115 )
various patterns of edema distribution
- Neither the pattern nor severity of vasogenic edema are related to the severity of clinical symptoms. (Busl 2022)
- Parieto-occipital pattern (~50%) – Edema is predominantly along the MCA-PCA watershed, located within the parietal and occipital lobes. This edema usually spares the paramedian parts of the occipital lobe (which may help differentiate it from ischemic stroke)( figure a, below). Even when edema is present in unusual areas such as the brainstem, a parieto-occipital pattern is generally present as well.
- Superior frontal sulcus pattern (~25%) – Edema is predominantly along the ACA-MCA watershed, located in the depth of the superior frontal sulcus ( figure b, below).
- Holohemispheric watershed pattern (~25%) – Edema is located along anterior, posterior, medial, and lateral watershed zones ( figure c , below).
- Central pattern (~10%) – Edema is in the deep white matter, basal ganglia, thalami, brainstem, pons, and cerebellum ( figure d , below).

MRI findings of superimposed infarction
- PRES typically causes vasogenic edema, with a characteristic appearance on different MRI sequences as shown in the top row below.
- In ~20% of patients with PRES, small areas of brain tissue may become ischemic. This causes cytotoxic edema, which is marked on MRI by hyperintensity on DWI and hypointensity on ADC (the red circle in the figure below). ( 31582040 ) Areas of hypointensity on ADC have greater specificity for ischemia that will progress to tissue infarction. This is a poor prognostic sign. ( 33630183 )

intracranial hemorrhage
- Intracranial hemorrhage is found in ~10-25% of patients. ( 35046115 )
- Parenchymal hemorrhage may occur (either lobar hematoma or punctate microhemorrhages). GRE/SWI sequences may reveal microhemorrhages in up to 65% of patients. ( 35046115 )
- Convexity subarachnoid hemorrhage may occur. This may suggest the coexistence of RCVS.
CT angiography (CTA) or MR angiography (MRA)
- These may be performed if there is concern for superimposed RCVS (e.g., based on MRI findings suggesting large areas of infarction, convexity subarachnoid hemorrhage, or focal neurologic deficits).
- Lumbar puncture is generally not required for the diagnosis of PRES. However, this may be necessary in some scenarios to exclude alternative diagnoses.
- Protein elevation may correlate with edema, as a marker of blood-brain barrier dysfunction.
- Opening pressure may be elevated.
clinical findings
- Seizures occur in ~70% of patients (and perhaps higher rates among patients sick enough to be in the ICU).
- Complex partial seizure may be the presenting symptom of PRES. ( 33630183 )
- Status epilepticus occurs in ~10%. This may be the presenting symptom. ( 35046115 )
- ⚠️ There should be a very low threshold to obtain continuous EEG monitoring for any patients with PRES who have altered mental status (especially if mental status abnormality is fluctuating, or seems disproportionate to the MRI abnormalities).(More on the indications for EEG here: 📖 )
- Focal sharp-wave discharges.
- Lateralized Periodic Discharges (LPDs 📖 ), often with a posterior distribution.
- Bilateral Independent Posterior Discharges (BIPDs 📖 ).
- Diffuse theta slowing is the most common finding. (Busl 2022)
management – see below 📖
- No single diagnostic test proves PRES (although MRI may be strongly suggestive).
- The above figure shows how this diagnosis is often approached, using a combination of clinical features, supportive evidence, and exclusion of other possibilities (section below). ( 28190431 )
The differential diagnosis will vary depending on any specific patient's imaging and clinical findings. However, the following list may include some reasonable considerations: ( 35046115 ; 34618761 )
- Reversible cerebral vasoconstriction syndrome (RCVS) – PRES and RCVS may coexist, so this differentiation may be impossible. 📖
- Demyelination (e.g., acute disseminated encephalomyelitis) – gadolinium enhancement in a ring-configuration may favor demyelination; microhemorrhages don't occur with demyelination; CSF with oligoclonal bands may favor demyelination. ( 35046115 )
- Progressive multifocal leukoencephalopathy (PML) 📖 – may be favored by immunosuppression, lesion asymmetry.
- Autoimmune or paraneoplastic encephalitis – may be favored by CSF pleocytosis, detection of autoantibodies.
- Viral encephalitis .
- SMART syndrome 📖 – favored by unilateral involvement, prominent gyral enhancement, history of radiation exposure.
- Toxic leukoencephalopathy (e.g., heroin).
- Acute hepatic encephalopathy – may cause FLAIR hyperintensity with reduced diffusion in the thalami, posterior limb of the internal capsule, and periventricular white matter. ( 35046115 )
- Osmotic demyelination syndrome 📖 – may be suggested by epidemiological risk factors, changes in osmolarity, prominent hyperintensity on diffusion weighted imaging (DWI).
- Cerebral venous thrombosis 📖 – may be suggested by edema, epidemiological factors, abnormal vascular imaging.
Treatment obviously focuses on management of blood pressure and seizures. However, the full treatment package may include five items:
(#1/5) Bp control
- MAP should be lowered by ~20-25% within 1-2 hours. ( 35046115 )
- A MAP target of 105-125 mm is often reasonable, although this may need to be personalized. ( 35046115 )
- Intravenous agents are initially preferred: Nicardipine infusion 💉 or clevidipine infusion 💉 .
- Oral calcium channel blockers may subsequently be utilized: Isradipine or nifedipine ER 💉 .
- ⚠️ Nitroglycerine should be avoided, as this may aggravate PRES. ( 30531559 )
(#2/5) seizure management
- Consider EEG monitoring in patients with altered consciousness (discussed above: 📖 )
- For most patients with PRES, a general antiseizure medication may be utilized (e.g., levetiracetam).
- Antiseizure medication may be tapered off as patients improve, often within a 1-2 week timeframe. (Busl 2022) However, 1-4% of patients may develop epilepsy and require longer term antiseizure medication. ( 35046115 )
- In some cases, these patterns combined with clinical features might reflect an electroclinical diagnosis of nonconvulsive status epilepticus (NCSE), which would warrant therapy. 📖
(#3/5) medication review & treatment of underlying cause(s)
- 💡PRES may result from a combination of several synergistic causes.
- This predominantly involves a medication review, to ensure that the patient isn't on any medications which may be causing PRES (listed above: 📖 ).
(#4/5) treat hypomagnesemia if present
- (a) Hypomagnesemia may contribute to PRES. ( 28054130 )
- (b) Magnesium is the only medication supported by RCT-level data for patients with PRES (noting that eclampsia is a subset of PRES).
- (c) Many patients may have an overlap of both PRES and RCVS; these patients may benefit from magnesium for treatment of RCVS.
- It's unknown whether intravenous magnesium could benefit most patients with PRES, but aggressive management of any hypomagnesemia seems sensible. More on the management of hypomagnesemia here: 📖 .
(#5/5) treat volume overload if present
- Among patients with hypertension and volume overload, the most effective “antihypertensive” agent is often furosemide. Blood pressure can be extremely difficult to manage in the face of uncontrolled volume overload.
- PRES involves tissue edema formation in the brain. Theoretically, edema resolution might be hastened if volume overload is managed.
- More on the management of volume overload & diuresis here: 📖 .
malignant PRES
- Malignant PRES is defined based on the presence of coma, deterioration despite standard management for elevated intracranial pressure, and radiological evidence of edema. ( 35046115 ) This isn't the usual trajectory for PRES, so alternative diagnoses should be considered (e.g., cerebral venous thrombosis, acute disseminated encephalomyelitis). 📖
- Management of intracranial pressure elevation. 📖 Rarely, severe swelling in the posterior fossa may cause obstructive hydrocephalus requiring temporary placement of an external ventricular drain.
- Aggressive treatment of any underlying condition (e.g., steroid for patients with underlying autoimmune disease).
- As a general rule, patients with PRES can look awful initially (e.g., due to brainstem involvement), yet subsequently make excellent recoveries. Recovery can take several days, so patience is required. Unfortunately, PRES can occasionally cause irreversible brain injury.
- Secondary intracranial hemorrhage in addition to PRES.
- Restricted diffusion on MRI, suggestive of cerebral infarction.
- Extensive cerebral edema. ( 35046115 )
- Patients may have recurrent episodes of PRES, especially if they have a persistent risk factor (e.g., sickle cell anemia, renal failure, or hypertension). ( 35046115 )

Follow us on iTunes
To keep this page small and fast, questions & discussion about this post can be found on another page here .
- 20% of patients with PRES do not have hypertension – so don't assume that simply because a patient is normotensive that they do not have PRES.
- The management of PRES is more than simply reduction in blood pressure. For example, it is important to carefully consider why the patient has PRES and whether any contributory factors can be reversed.
Acknowledgement: Thanks to Dr. Richard Choi (@rkchoi) for thoughtful comments on this chapter.
Guide to emoji hyperlinks
- 24476076 Camara-Lemarroy CR, Gonzalez-Moreno EI, Ortiz-Corona Jde J, Yeverino-Castro SG, Sanchez-Cardenas M, Nuñez-Aguirre S, Villarreal-Alarcon MA, Galarza-Delgado DA. Posterior reversible encephalopathy syndrome due to malignant hypercalcemia: physiopathological considerations. J Clin Endocrinol Metab. 2014 Apr;99(4):1112-6. doi: 10.1210/jc.2013-3487 [ PubMed ]
- 28054130 Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017 Aug;264(8):1608-1616. doi: 10.1007/s00415-016-8377-8 [ PubMed ]
- 28190431 Toledano M, Fugate JE. Posterior reversible encephalopathy in the intensive care unit. Handb Clin Neurol. 2017;141:467-483. doi: 10.1016/B978-0-444-63599-0.00026-0 [ PubMed ]
- 29274685 Arrigan MT, Heran MKS, Shewchuk JR. Reversible cerebral vasoconstriction syndrome: an important and common cause of thunderclap and recurrent headaches. Clin Radiol. 2018 May;73(5):417-427. doi: 10.1016/j.crad.2017.11.017 [ PubMed ]
- 31582040 Levitt A, Zampolin R, Burns J, Bello JA, Slasky SE. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome: Distinct Clinical Entities with Overlapping Pathophysiology. Radiol Clin North Am. 2019 Nov;57(6):1133-1146. doi: 10.1016/j.rcl.2019.07.001 [ PubMed ]
- 32487905 Santomasso BD. Anticancer Drugs and the Nervous System. Continuum (Minneap Minn). 2020 Jun;26(3):732-764. doi: 10.1212/CON.0000000000000873 [ PubMed ]
- 32596758 Thakkar JP, Prabhu VC, Rouse S, Lukas RV. Acute Neurological Complications of Brain Tumors and Immune Therapies, a Guideline for the Neuro-hospitalist. Curr Neurol Neurosci Rep. 2020 Jun 29;20(8):32. doi: 10.1007/s11910-020-01056-0 [ PubMed ]
- 33273175 Lee EQ. Neurologic Complications in Patients With Cancer. Continuum (Minneap Minn). 2020 Dec;26(6):1629-1645. doi: 10.1212/CON.0000000000000937 [ PubMed ]
- 33630183 Gewirtz AN, Gao V, Parauda SC, Robbins MS. Posterior Reversible Encephalopathy Syndrome. Curr Pain Headache Rep. 2021 Feb 25;25(3):19. doi: 10.1007/s11916-020-00932-1 [ PubMed ]
- 34618761 Singhal AB. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome as Syndromes of Cerebrovascular Dysregulation. Continuum (Minneap Minn). 2021 Oct 1;27(5):1301-1320. doi: 10.1212/CON.0000000000001037 [ PubMed ]
- 35046115 Triplett JD, Kutlubaev MA, Kermode AG, Hardy T. Posterior reversible encephalopathy syndrome (PRES): diagnosis and management. Pract Neurol. 2022 Jun;22(3):183-189. doi: 10.1136/practneurol-2021-003194 [ PubMed ]
- Busl KM, Dangayach N, Maciel CB (2022) : Neurointensive Care: The essentials . Presentation at the American Academy of Neurology Conference, Seattle 2022.
- 35419136 Niznick N, Lun R, Lelli DA, Fantaneanu TA. Clinical Problem Solving: Decreased Level of Consciousness and Unexplained Hydrocephalus. Neurohospitalist. 2022 Apr;12(2):312-317. doi: 10.1177/19418744211056781 [ PubMed ]
The Internet Book of Critical Care is an online textbook written by Josh Farkas ( @PulmCrit ), an associate professor of Pulmonary and Critical Care Medicine at the University of Vermont.
We are the EMCrit Project , a team of independent medical bloggers and podcasters joined together by our common love of cutting-edge care, iconoclastic ramblings, and FOAM.
Resus Leadership Academy

Subscribe by Email
Insert/edit link.
Enter the destination URL
Or link to existing content

- Posterior reversible encephalopathy syndrome
- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Frank Gaillard had no recorded disclosures.
At the time the article was last revised Rohit Sharma had no financial relationships to ineligible companies to disclose.
- Reversible posterior leukoencephalopathy syndrome
- Reversible posterior cerebral edema syndrome
- Hyperperfusion encephalopathy
- Occipito-parietal encephalopathy
- Reversible leukoencephalopathy
- Reversible posterior cerebral oedema syndrome
- PRES with spinal cord involvement
- Posterior eversible encephalopathy syndrome with spinal cord involvement
- Central PRES
- Central posterior reversible encephalopathy syndrome
- Posterior reversible encephalopathy syndrome (PRES)
Posterior reversible encephalopathy syndrome (PRES) , also known as reversible posterior leukoencephalopathy syndrome (RPLS) , is a neurotoxic state that occurs secondary to the inability of the posterior circulation to autoregulate in response to acute changes in blood pressure . Hyperperfusion with resultant disruption of the blood-brain barrier results in vasogenic edema , usually without infarction, most commonly in the parieto-occipital regions.
On this page:
Terminology, clinical presentation, radiographic features, treatment and prognosis, history and etymology, differential diagnosis.
- Cases and figures
- Imaging differential diagnosis
The term posterior reversible encephalopathy syndrome may be a misnomer as the syndrome:
can involve or extend beyond the posterior cerebrum
can progress to develop permanent cerebral injury and residual neurological defects, although most cases involve a resolution of changes and a clinical recovery with the treatment of the precipitating cause
may not present with encephalopathy in all patients
It should not be confused with chronic hypertensive encephalopathy , also known as hypertensive microangiopathy, which results in microhemorrhages in the basal ganglia, pons, and cerebellum.
ADVERTISEMENT: Supporters see fewer/no ads
Common presenting clinical features include 16,19 :
encephalopathy ( acute confusion or altered mental state or decreased level of consciousness)
visual disturbance, including reversible cortical blindness 20
However, the presentation can be quite varied, and may include other neurological symptoms such as ataxia, focal neurological deficits, vertigo, or tinnitus 19 .
Although posterior reversible encephalopathy syndrome is most commonly thought of occurring as secondary to marked hypertension , this does not appear to be a necessary or sufficient explanation, given the very large and heterogeneous clinical scenarios that precipitate the development of posterior reversible encephalopathy syndrome and the fact that hypertension is not present or does not reach the upper limits of self-regulation (140-160 mmHg) in 25% of patients.
The underlying mechanisms involved are not well understood but is thought to culminate in altered integrity of the blood-brain barrier . Three main precipitant theories have been proposed, that are not mutually exclusive 19 :
high blood pressure (breakthrough theory) leads to loss of self-regulation, hyperperfusion with endothelial damage and vasogenic edema
vasospasm theory results in local ischemia and hypoperfusion
endothelial dysfunction secondary to circulating endogenous or exogenous toxins
severe hypertension
eclampsia / pre-eclampsia
acute glomerulonephritis
hemolytic-uremic syndrome (HUS)
thrombocytopenic thrombotic purpura (TTP)
systemic lupus erythematosus (SLE)
drug toxicity
cyclophosphamide 10
erythropoietin
cyclosporine
azathioprine
L-asparaginase
filgrastim 15
ustekinumab 17,18
bone marrow or stem cell transplantation
solid organ transplantation
hyperammonemia
sickle cell disease 11
ventriculoperitoneal shunt insertion/overshunting 12
alcohol hepatitis 22
Microscopic appearance
during the acute course of PRES: vasogenic edema, without inflammation, ischemia, or neuronal damage 3
during the late course of PRES: demyelination and myelin pallor along with evidence of ischemia, anoxic neuronal damage, laminar necrosis, or older hemorrhage in the white matter and cortex 3
Typical posterior reversible encephalopathy syndrome manifests as bilateral vasogenic edema within the occipital and parietal regions (70-90% of cases), perhaps relating to the posterior cerebral artery supply. Despite its name, however, posterior reversible encephalopathy syndrome can be found in a non-posterior distribution, mainly in watershed areas, including within the frontal, inferior temporal, cerebellar, and brainstem regions 2,19 . Both cortical and subcortical locations are affected.
Uncommon patterns of posterior reversible encephalopathy syndrome in <5% include:
purely unilateral
central ("central PRES"): brainstem or basal ganglia involvement without cortical or subcortical white matter involvement
spinal cord involvement ("PRES-SCI")
Ischemic stroke , intracerebral hemorrhage , and subarachnoid hemorrhage are associated with posterior reversible encephalopathy syndrome in ~11%, ~10% and 7% of cases respectively 23 . The presence of contrast enhancement, no matter the pattern or how avid, does not portend the clinical outcome.
The affected regions, as outlined above, are hypoattenuating.
Angiography (DSA)
There may be signs of vasospasm or arteritis 3 :
diffuse vasoconstriction
focal vasoconstriction
vasodilatation
string-of-beads appearance
Signal characteristics of affected areas usually reflect vasogenic edema, with some exceptions.
T1: hypointense in affected regions
T1 C+ (Gd): patchy variable enhancement can be seen in ~35% of patients, in either a leptomeningeal or cortical pattern
T2: hyperintense in affected regions
DWI: usually normal, sometimes hyperintense due to edema ( T2 shine-through ) or true restricted diffusion
ADC: usually increased signal due to increased diffusion, but restricted diffusion is present in a quarter of cases 5
GRE/SWI: may show hemorrhages (including microhemorrhages ) in 9-50% 5
MRA: may show patterns of vasculopathy with vessel irregularity consistent with focal vasoconstrictions/vasodilatation and diffuse vasoconstriction 3
MRV: tends to be normal 3
Management is supportive, with discontinuation of any offending medication, gradual lowering of blood pressure, and antiseizure medications if appropriate 20 .
Posterior reversible encephalopathy syndrome was described for the first time as a distinct entity in 1996 by an American neurologist Judy Hinchey et al. 13 . Although others had previously described similar reversible CT and MRI findings in hypertension back to the 1980s 14 .
General imaging differential considerations include:
inflammatory cerebral amyloid angiopathy
edema usually centered on microhemorrhages
progressive multifocal leukoencephalopathy (PML)
periventricular and subcortical involvement, sparing the cortex
little or no mass effect or enhancement
severe hypoglycemia
posterior circulation infarct
occipital and cerebellar involvement
acute infarct demonstrates restricted diffusion; PRES with vasogenic edema alone does not restrict
hypertensive brainstem encephalopathy
absence of parieto-occipital involvement
gliomatosis cerebri
more asymmetric
sagittal sinus thrombosis
hypoxic-ischemic encephalopathy
SMART syndrome
Quiz questions
- 1. Foocharoen C, Tiamkao S, Srinakarin J et-al. Reversible posterior leukoencephalopathy caused by azathioprine in systemic lupus erythematosus. J Med Assoc Thai. 2006;89 (7): 1029-32. Pubmed citation
- 2. Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28 (7): 1320-7. doi:10.3174/ajnr.A0549 - Pubmed citation
- 3. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29 (6): 1036-42. doi:10.3174/ajnr.A0928 - Pubmed citation
- 4. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29 (6): 1043-9. doi:10.3174/ajnr.A0929 - Pubmed citation
- 5. Bartynski WS, Tan HP, Boardman JF et-al. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol. 2008;29 (5): 924-30. doi:10.3174/ajnr.A0960 - Pubmed citation
- 6. Fugate JE, Claassen DO, Cloft HJ et-al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin. Proc. 2010;85 (5): 427-32. doi:10.4065/mcp.2009.0590 - Free text at pubmed - Pubmed citation
- 7. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. (2007) AJR. American journal of roentgenology. 189 (4): 904-12. doi:10.2214/AJR.07.2024 - Pubmed
- 8. McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. (2012) AJNR. American journal of neuroradiology. 33 (5): 896-903. doi:10.3174/ajnr.A2886 - Pubmed
- 9. Karia SJ, Rykken JB, McKinney ZJ, Zhang L, McKinney AM. Utility and Significance of Gadolinium-Based Contrast Enhancement in Posterior Reversible Encephalopathy Syndrome. (2016) AJNR. American journal of neuroradiology. 37 (3): 415-22. doi:10.3174/ajnr.A4563 - Pubmed
- 10. Jayaweera JL, Withana MR, Dalpatadu CK, Beligaswatta CD, Rajapakse T, Jayasinghe S, Chang T. Cyclophosphamide-induced posterior reversible encephalopathy syndrome (PRES): a case report. (2014) Journal of medical case reports. 8: 442. doi:10.1186/1752-1947-8-442 - Pubmed
- 11. Thust SC, Burke C, Siddiqui A. Neuroimaging findings in sickle cell disease. (2014) The British journal of radiology. 87 (1040): 20130699. doi:10.1259/bjr.20130699 - Pubmed
- 12. Merola J, Magdum S. An Unusual Complication following Ventriculoperitoneal Shunting. (2017) Journal of pediatric neurosciences. 12 (1): 61-63. doi:10.4103/1817-1745.205653 - Pubmed
- 13. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. (1996) The New England journal of medicine. 334 (8): 494-500. doi:10.1056/NEJM199602223340803 - Pubmed
- 14. Hauser RA, Lacey DM, Knight MR. Hypertensive encephalopathy. Magnetic resonance imaging demonstration of reversible cortical and white matter lesions. (1988) Archives of neurology. 45 (10): 1078-83. doi:10.1001/archneur.1988.00520340032007 - Pubmed
- 15. Stübgen J. Posterior Reversible Encephalopathy Syndrome (PRES) After Granulocyte-Colony Stimulating Factor (G-CSF) Therapy: A Report of 2 Cases. J Neurol Sci. 2012;321(1-2):35-8. doi:10.1016/j.jns.2012.07.028 - Pubmed
- 16. Sudulagunta SR, Sodalagunta MB, Kumbhat M, Settikere Nataraju A. Posterior reversible encephalopathy syndrome(PRES). (2017) Oxford medical case reports. 2017 (4): omx011. doi:10.1093/omcr/omx011 - Pubmed
- 17. Gratton D, Szapary P, Goyal K, Fakharzadeh S, éronique Germain V, Saltiel P. Reversible Posterior Leukoencephalopathy Syndrome in a Patient Treated With Ustekinumab: Case Report and Review of the Literature. Arch Dermatol. 2011 Oct 1;147(10):1197–202. Available at https://jamanetwork.com/journals/jamadermatology/fullarticle/1105163
- 18. Mishra A & Seril D. Posterior Reversible Encephalopathy Syndrome Following Ustekinumab Induction for Crohn's Disease. Case Rep Gastroenterol. 2018;12(2):521-7. doi:10.1159/000492462 - Pubmed
- 19. Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: A review with emphasis on neuroimaging characteristics. (2019) Journal of the neurological sciences. 404: 72-79. doi:10.1016/j.jns.2019.07.018 - Pubmed
- 20. Bandyopadhyay S, Mondal K, Das S et al. Reversible Cortical Blindness: Posterior Reversible Encephalopathy Syndrome. J Indian Med Assoc. 2010;108(11):778-80. - Pubmed
- 21. Anderson R, Patel V, Sheikh-Bahaei N et al. Posterior Reversible Encephalopathy Syndrome (PRES): Pathophysiology and Neuro-Imaging. Front Neurol. 2020;11. doi:10.3389/fneur.2020.00463
- 22. John E, Sedhom R, Dalal I, Sharma R. Posterior Reversible Encephalopathy Syndrome in Alcoholic Hepatitis: Hepatic Encephalopathy a Common Theme. World J Gastroenterol. 2017;23(2):373-6. doi:10.3748/wjg.v23.i2.373 - Pubmed
- 23. Kaufmann J, Buecke P, Meinel T et al. Frequency of Ischaemic Stroke and Intracranial Haemorrhage in Patients with Reversible Cerebral Vasoconstriction Syndrome (RCVS) and Posterior Reversible Encephalopathy Syndrome (PRES) – A Systematic Review. Euro J of Neurology. 2024;:e16246. doi:10.1111/ene.16246 - Pubmed
Incoming Links
- Perivascular spaces
- Cerebral hyperperfusion syndrome
- Cerebral cortical restricted diffusion
- Eosinophilic granulomatosis with polyangiitis (neurological manifestations)
- Uraemic encephalopathy
- Postpartum angiopathy
- Bilateral middle cerebellar peduncle lesions
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Anton-Babinski syndrome
- Vasogenic cerebral oedema
- Amyloid related imaging abnormalities (ARIA)
- Thrombotic thrombocytopenic purpura
- Cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome
- Reversible cerebral vasoconstriction syndrome
- Demyelination
- Gyral enhancement
- Lesions of the corpus callosum
- Levamisole-induced leukoencephalopathy
- Progressive multifocal leukoencephalopathy
- PRES with underlying thrombotic thrombocytopenic purpura
- Diabetic ketoacidosis-induced posterior reversible encephalopathy syndrome (PRES)
- PRES with cerebellar involvement and haemorrhage
- Posterior reversible encephalopathy syndrome with microhaemorrhage (importance of susceptibility-weighted imaging)
- Posterior reversible encephalopathy syndrome with subarachnoid haemorrhage
- Severe posterior reversible encephalopathy syndrome (PRES) complicated by cerebellar hemorrhage
- Question 2695
- Question 2591
Promoted articles (advertising)
By section:.
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Search Menu
- Advance Articles
- Editors Choice
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Author Resources
- Read & Publish
- Reasons to Publish With Us
- About Postgraduate Medical Journal
- About the Fellowship of Postgraduate Medicine
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction.
- < Previous
Posterior reversible encephalopathy syndrome (PRES): presentation, diagnosis and treatment
- Article contents
- Figures & tables
- Supplementary Data
Anant Parasher, Rajat Jhamb, Posterior reversible encephalopathy syndrome (PRES): presentation, diagnosis and treatment, Postgraduate Medical Journal , Volume 96, Issue 1140, October 2020, Pages 623–628, https://doi.org/10.1136/postgradmedj-2020-137706
- Permissions Icon Permissions
Posterior reversible encephalopathy syndrome (PRES) is a neurological disorder which is characterised by variable symptoms, which include visual disturbances, headache, vomiting, seizures and altered consciousness. The exact pathophysiology of PRES has not been completely explained, but hypertension and endothelial injury seem to be almost always present. Vasoconstriction resulting in vasogenic and cytotoxic edema is suspected to be responsible for the clinical symptoms as well as the neuro-radiological presentation. On imaging studies, Symmetrical white matter abnormalities suggestive of edema are seen in the computer tomography (CT) and magnetic resonance imaging (MRI) scans, commonly but not exclusively in the posterior parieto-occipital regions of the cerebral hemispheres. The management is chiefly concerned with stabilization of the patient, adequate and prompt control of blood pressure, prevention of seizures and timely caesarean section in obstetric cases with pre-eclampsia/eclampsia. In conclusion, persistently elevated blood pressures remain the chief culprit for the clinical symptoms as well as the neurological deficits. Early diagnosis by diffusion weighted MRI scans, and differentiation from other causes of altered sensorium i.e. seizures, meningitis and psychosis, is extremely important to initiate treatment and prevent further complications. Although most cases resolve successfully and carry a favorable prognosis, patients with inadequate therapeutic support or delay in treatment may not project a positive outcome.
Posterior reversible encephalopathy syndrome (PRES) is a neurological disorder which is characterised by variable symptoms, which include visual disturbances, headache, vomiting, seizures and altered consciousness. 1 Its association is seen with a number of conditions including hypertension, pre-eclampsia and eclampsia, renal failure, systemic lupus erythematosus (SLE) and the use of some immunosuppressive agents. 2 3 PRES was first described in 1996 by Hinchey et al and shortly after the description, two other case series were published. 2 4 This condition has been known by various names previously (reversible posterior leukoencephalopathy syndrome, reversible posterior cerebral oedema syndrome and reversible occipital parietal encephalopathy), but PRES is now the widely accepted term. 5 6 It is commonly, but not always associated with acute hypertension and is now increasingly being diagnosed, because of increased availability and improvement of brain imaging techniques. 7
PRES can be considered to be the basis of the neurological manifestations of preeclampsia/eclampsia. 8 Cases can present in very early pregnancy (before the 20th week of gestation), as well as rarely in the late stages of pregnancy with intrauterine death. 9 Severe pre-eclampsia (defined as arterial blood pressure >170/110 mm Hg) is common in most women, but rare cases of PRES in pregnant women with normal blood pressure and without pre-eclampsia have also been described. 10 The major clinical conditions associated with PRES are represented in box 1 .
Immunosuppressive/cytotoxic drugs.
Hypertensive encephalopathy.
Pre-eclampsia/eclampsia/HELLP syndrome.
Autoimmune disorders, for example, SLE.
Acute or chronic renal diseases.
High dose steroids.
Liver failure/transplantation.
Endocrine dysfunction.
Hypercalcemia/hyperparathyroidism.
Bone marrow transplant.
Massive blood transfusion.
Porphyrias.
PRES, posterior reversible encephalopathy syndrome; SLE, systemic lupus erythematosus; HELLP, Hemolysis, Elevated Liver Enzymes and Low Platelet count; HUS/TTP, Hemolytic Uraemic Syndrome/ Thrombotic Thrombocytopenic Purpura.
Pathophysiology
The exact pathophysiology of PRES has not been completely explained, but hypertension and endothelial injury seem to be almost always present. Vasoconstriction resulting in vasogenic and cytotoxic oedema is suspected to be responsible for the clinical symptoms and the neuroradiological presentation. 11 Barring cerebral ischaemia or haemorrhage which can result in permanent damage, PRES is usually reversible. 1 Hypertension is the most common precipitating factor, with endothelial dysfunction playing an important role. 12 The various mechanisms explaining the pathophysiology of PRES include (i) failure of cerebral autoregulation causing vasogenic oedema, (ii) cerebral vasoconstriction and (iii) disruption of the blood brain barrier due to endothelial disruption. 5 Among the various theories that have been proposed for PRES, failure of brain autoregulation causing vasogenic oedema is presently the most accepted one.
Once the cerebral autoregulation, which maintains a constant blood flow to the brain despite alterations in the systemic pressures gets disrupted, increased, perfusion pressure causes extravasation of fluid by overcoming the blood brain barrier. 13–15 This can be briefly explained as follows. Cerebral blood flow is usually regulated by dilatation and constriction of vessels to maintain adequate tissue perfusion 15 which also avoids excessive increase in the intracerebral pressure. Sustained mean arterial pressure more than 150–160 mm Hg results in the breakdown of autoregulation mechanisms leading to hyperperfusion and cerebral vessel damage, resulting in interstitial extravasation of proteins and fluid, causing vasogenic oedema. Above 200 mm Hg mean arterial pressure (MAP), the changes start to become irreversible. 15 Chronic hypertension and atherosclerosis, which usually accompany PRES, are known to reduce the effectiveness of autoregulation. 16
Although this theory explains why control of hypertension benefits these patients, it does not explain few things such as the occurrence of PRES in the absence of hypertension and the correlation of extent of the oedema and the severity of hypertension. Also, some positron-emission tomography based studies have actually demonstrated cerebral hypoperfusion instead of hyperperfusion. 7 14–17
Another theory has implicated a systemic inflammatory state causing endothelial dysfunction as the cause of PRES. 15 Systemic inflammatory process such as sepsis, eclampsia, transplantation and autoimmune disease are usually associated with PRES, which can lead to reversible focal and diffuse abnormalities seen on angiographic studies. Vasoconstriction that occurs during cerebral autoregulation has a propensity to worsen pre-existing inflammatory endothelial dysfunction. This leads to further hypoxia and subsequent vasogenic oedema. 15 Although this theory explains well the role of endothelial dysfunction due to inflammation, it still does not explain the occurrence of PRES in the absence of inflammation. 16 17
A simplified flowchart describing the pathogenesis of PRES has been shown in figure 1 .

The pathogenesis of PRES. 6 18 19 PRES, posterior reversible encephalopathy syndrome.
Breakdown of the blood brain barrier and endothelial dysfunction occurs in PRES with fluid and macromolecule extravasation into the interstitium. Increased concentrations of circulating cytokines (eg, tumour necrosis factor α, interleukin 1 and endothelin 1) activate endothelial cells and allow interaction and adhesion of circulating leucocytes( figure 2 ). The tight junctions are disrupted and vascular endothelial growth factor expression is increased, leading to increased vascular permeability and vasogenic oedema.

A representational diagram showing the pathophysiology of PRES. PRES, posterior reversible encephalopathy syndrome.
To complicate the matter further, not all the patients with PRES have hypertension, and cytotoxicity is thought to be the mechanism underlying cerebral oedema in these patients. The associated conditions include cytotoxic therapies (eg, ciclosporin, tacrolimus), infection/sepsis/shock, autoimmune disease and exposure to toxic agents. 6 18 19 The mechanism might be direct toxicity to vascular endothelium leading to capillary leakage and breakdown of the blood brain barrier, which triggers vasogenic oedema. 2 The damage may also be seen with non-toxic levels of these drugs.
Severe anaemia can be a predisposing factor for PRES due to the endothelial dysfunction caused by insufficient oxygen supply. This can further damage and disrupt the blood brain barrier. 20 Rapid blood transfusion in these patients may cause a rapid increase in total blood volume, with resultant cerebral blood flow overload. This acute cerebral hyperperfusion disrupts cerebral autoregulation and might result in the vasogenic oedema found in PRES. 5
Clinical presentation
The symptoms of PRES are variable, ranging from visual disturbances which may present as blurred vision, homonymous hemianopsia and cortical blindness, to altered consciousness presenting as mild confusion, agitation or coma. Other symptoms may include nausea, vomiting and seizures. Status epilepticus is common, which may be generalised. Non-convulsive status can be prolonged and last for days in PRES and should be carefully observed. Drug intoxication and psychosis should be ruled out in these cases, so that treatment can initiated as early as possible. 5
The most common symptoms seen in obstetric patients are seizures (45%), visual disturbances (34%), alteration of consciousness (19%) 1 and focal deficits (4%). 21 The degree of hypertension is not associated with the extent of cerebral lesions and oedema can also occur at lower levels of arterial blood pressure. This is chiefly due to ongoing endothelium damage, as indicated by the high lactic acid dehydrogenase (LDH) levels in laboratory tests. 22 23
Imaging studies
The most common location of the lesions in PRES is the parietal-occipital lobe or ‘posterior’ area of the brain. Lesions may also be observed in the anterior regions, basal ganglia, brainstem and the cerebellum. 1 24 25 The characteristic imaging patterns in PRES are represented in box 2 . 26 Symmetrical white matter abnormalities suggestive of oedema may be seen in the CT and MRI scans, but not exclusively in the posterior parieto-occipital regions of the cerebral hemispheres. 1 27 28
Holo-hemispheric watershed.
Superior frontal sulcus.
Dominant parietal/occipital.
Partial and/or asymmetric PRES.
PRES, posterior reversible encephalopathy syndrome.
Diffusion-weighted imaging is essential to distinguish between vasogenic and cytotoxic oedema. 1 29 Diffusion-weighted MRI is the modality of choice for confirming the diagnosis of PRES( figure 3 ) and to differentiate between reversible vasogenic and irreversible cytotoxic oedema, as compared with a CT scan, which can be normal in some cases of PRES. Radiologically detectable cerebral lesions may persist in some cases in spite of intensive monitoring and prompt aggressive therapy. 1

MRI with T2-flair-weighted images showing the typically hyperintense bilateral lesions indicating vasogenic oedema in the parieto-occipital regions as well as less common lesions in the frontal regions and brain stem (arrows). 27 28
The key thing to remember in the management of PRES is early diagnosis and initiation of therapy. Many patients may require intensive care unit (ICU) care for aggressive management of their symptoms such as seizures, encephalopathy and status epilepticus. 30 The important points of therapy include: 31
Prompt induction of labour in cases of pre-eclampsia/eclampsia and HELLP.
Immediate removal of the offending cytotoxic drugs/immunosuppressants.
Stabilisation of the patient with adequate hydration, along with correction of acidosis and electrolyte abnormalities, if any.
Gradual reduction of blood pressure in patients with hypertension to avoid sudden hypoperfusion of vital organs.
Prevention and management of seizures in pregnant women by magnesium sulfate. For seizures in non-pregnant patients presenting with PRES, first-line drugs used are diazepam, phenobarbital and fosphenytoin. Refractory cases can be started on propofol or midazolam.
Dialysis for patients presenting with renal failure.
Airway management and intubation in altered patients with a poor Glasgow Coma Score, as per the standard protocol.
PRES in non-obstetric cases
In cases of PRES caused by factors other than pre-eclampsia and eclampsia, the most effective therapy includes withdrawal of the offending agent, immediate control of blood pressure, anticonvulsive therapy and temporary renal replacement therapy (haemodialysis/peritoneal dialysis) if required. Aggressive treatment with corticosteroids and cyclophosphamide is effective in cases of SLE-related PRES. 5
PRES in pre-eclampsia/eclampsia
The majority of obstetric cases with pre-eclampsia and eclampsia are treated with a similar protocol. Initially, the mother needs to be stabilised by means of antihypertensive and antiepileptic drugs, especially labetalol, nifedipine and magnesium sulfate. 32 The underlying cause has to be removed without delay, and a caesarean section has to be performed to reduce feto-maternal stress. General anaesthesia is preferred if there are complications such as coagulopathy, seizures or thrombocytopenia. Neuroaxial anaesthesia should always be given for the majority of patients without any complications as due to the antihypertensive effect of sympathetic blockade, it is the least risky for the mother and fetus. Rapid reduction of blood pressure by more than 15%–25% should be avoided as it can worsen the cytotoxic oedema and compromise uteroplacental perfusion. 1 Magnesium sulfate can prevent convulsions and reduce cerebral oedema. 33 The use of thiopental, valproate or phenytoin has been reported only for status epilepticus in these patients. 34 Specific cerebral antioedema therapy with steroids or mannitol has not been found to be superior to magnesium sulfate in achieving neurological recovery. 35
A concise overview of the management of PRES has been described in figure 4 .

Management of PRES. PRES, posterior reversible encephalopathy syndrome.
Prognosis and outcomes
PRES usually has a favourable prognosis among pregnant women, with resolution being rapid and complete after adequate therapy. 36 Permanent damage can persist in a few cases (6%) and death due to haemorrhage has been described in a couple of patients. 37–39 ICU care is advisable for postcaesarean patients to allow monitoring and sufficient recovery. 1 Recurrence of PRES is not uncommon in patients presenting with repeated episodes/flares of hypertensive crisis, renal failure, autoimmune conditions and multiorgan failure. 31
Although prognosis is good for most patients, delayed diagnosis and treatment may lead to mortality or irreversible neurological deficits. Poor prognosis is associated with factors such as severe encephalopathy, chronic hypertension, neoplastic aetiology, delayed diagnosis of causative factor, multiple comorbidities, elevated C-reactive protein (CRP) and coagulopathy. 40 41 Involvement of the corpus callosum, extensive cerebral oedema or haemorrhage, restrictive diffusion and subarachnoid haemorrhage are the MRI features which predict a worse prognosis. 42–44
PRES has been increasingly recognised in recent years and has been the cause of recurrent physician consultations for obstetric pre-eclamptic and eclamptic cases. In majority of patients, persistently elevated blood pressures remain the chief culprit for the clinical symptoms as well as the neurological deficits. Early diagnosis by diffusion weighted MRI scans, and differentiation from other causes of altered sensorium, that is, seizures, meningitis and psychosis, is extremely important to initiate treatment and prevent further complications. Reduction of blood pressure and seizure control remain the mainstays of therapy after prompt stabilisation of the patient and removal of any known toxic insult. Although most cases resolve successfully and carry a favourable prognosis, patients with inadequate therapeutic support or delay in treatment may not project a positive outcome.
Posterior reversible encephalopathy syndrome is increasingly being recognised now due to better imaging techniques.
Pathophysiology not completely elucidated, but hypertension, vasoconstriction and endothelial dysfunction seen to be important inciting factors.
Management protocols need to be specific and well defined, especially in obstetric cases.
Can posterior reversible encephalopathy syndrome (PRES) be predicted from early signs and symptoms in high-risk cases?
What is the pathophysiology of PRES?
Do early intervention, treatment and intensive care unit care have any effect on the prognosis of patients with PRES?
Poma S, Delmonte MP, Gigliuto C et al . Management of posterior reversible syndrome in preeclamptic women. Case Rep Obstet Gynecol 2014;2014:928079. https://doi.org/10.1155/2014/928079 . (Ref. 1)
Sudulagunta SR, Sodalagunta MB, Kumbhat M, Nataraju AS. Posterior reversible encephalopathy syndrome (PRES). Oxf Med Case Rep 2017;2017(4):omx011. doi: 10.1093/omcr/omx011 . (Ref. 5)
Bartynski W. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic oedema. Am J Neuroradiol 2008;29:1043–9. (Ref. 16)
Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914–25. (Ref. 19)
Hinduja A, Habetz K, Raina SK, Fitzgerald RT. Predictors of intensive care unit utilisation in patients with posterior reversible encephalopathy syndrome. Acta Neurol Belg 2017;117:201–6. doi: 10.1007/s13760-016-0703-5. (Ref. 31)
Hinduja A. Posterior reversible encephalopathy syndrome: clinical features and outcome. Front Neurol 2020;11:71. doi: 10.3389/fneur.2020.00071. (Ref. 32)
AP did the research and final submission. RJ reviewed and edited the manuscript.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
None declared.
Not required.
Not commissioned; externally peer reviewed.
Poma S , Delmonte MP , Gigliuto C , et al. Management of posterior reversible syndrome in preeclamptic women . Case Rep Obstet Gynecol 2014 ; 2014 : 1 – 6 . doi:10.1155/2014/928079
Google Scholar
Hinchey J , Chaves C , Appignani B , et al. A reversible posterior leukoencephalopathy syndrome . N Engl J Med 1996 ; 334 : 494 – 500 . doi:10.1056/NEJM199602223340803
Onder AM , Lopez R , Teomete U , et al. Posterior reversible encephalopathy syndrome in the pediatric renal population . Pediatr Nephrol 2007 ; 22 : 1921 – 9 . doi:10.1007/s00467-007-0578-z
Schwartz RB , Jones KM , Kalina P , et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases . AJR Am J Roentgenol 1992 ; 159 : 379 – 83 . doi:10.2214/ajr.159.2.1632361
Sudulagunta SR , Sodalagunta MB , Kumbhat M , et al. Posterior reversible encephalopathy syndrome(PRES) . Oxf Med Case Reports 2017 ; 2017 :omx011. doi:10.1093/omcr/omx011
Bartynski WS . Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features . AJNR Am J Neuroradiol 2008 ; 29 : 1036 – 42 . doi:10.3174/ajnr.A0928
McKinney AM , Short J , Truwit CL , et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings . AJR Am J Roentgenol 2007 ; 189 : 904 – 12 . doi:10.2214/AJR.07.2024
Zeeman GG , Cunningham FG . Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia . Am J Obstet Gynecol 2014 ; 210 : 378 – 9 . doi:10.1016/j.ajog.2013.11.025
Pratap JN , Down JF . Posterior reversible encephalopathy syndrome: a report of a case with atypical features . Anaesthesia 2008 ; 63 : 1245 – 8 . doi:10.1111/j.1365-2044.2008.05587.x
Fujiwara Y , Higaki H , Yamada T , et al. Two cases of reversible posterior leukoencephalopathy syndrome, one with and the other without pre-eclampsia . J Obstet Gynaecol Res 2005 ; 31 : 520 – 6 . doi:10.1111/j.1447-0756.2005.00345.x
Staykov D , Schwab S . Posterior reversible encephalopathy syndrome . J Intensive Care Med 2012 ; 27 : 11 – 24 . doi:10.1177/0885066610393634
Ponciano A . e-Pearl of the week: posterior reversible encephalopathy syndrome (PRES , 2018 .
Google Preview
Mehta P , Rani GU . Posterior reversible encephalopathy syndrome in pregnancy: experience in 2 cases . Chennai, India : Sri Ramachandra University .
Roth C , Ferbert A . The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Pract Neurol 2011 ; 11 : 136 – 44 . doi:10.1136/practneurol-2011-000010
Bartynski WS . Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema . AJNR Am J Neuroradiol 2008 ; 29 : 1043 – 9 . doi:10.3174/ajnr.A0929
Fugate JE , Claassen DO , Cloft HJ , et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings . Mayo Clin Proc 2010 ; 85 : 427 – 32 . doi:10.4065/mcp.2009.0590
Hobson EV , Craven I , Blank SC . Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness . Perit Dial Int 2012 ; 32 : 590 – 4 . doi:10.3747/pdi.2012.00152
Fugate JE , Rabinstein AA . Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions . Lancet Neurol 2015 ; 14 : 914 – 25 . doi:10.1016/S1474-4422(15)00111-8
Legriel S , Pico F , Azoulay E . Understanding posterior reversible encephalopathy syndrome . Annu Update Intensive Care Emerg Med 2011 : 631 – 53 .
Wada K-ichiro , Kano M , Machida Y , et al. Posterior reversible encephalopathy syndrome induced after blood transfusion for severe anemia . CRCM 2013 ; 02 : 332 – 4 . doi:10.4236/crcm.2013.25089
Pizon AF , Wolfson AB . Postpartum focal neurologic deficits: posterior leukoencephalopathy syndrome . J Emerg Med 2005 ; 29 : 163 – 6 . doi:10.1016/j.jemermed.2005.02.006
Wagner SJ , Acquah LA , Lindell EP , et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control . Mayo Clin Proc 2011 ; 86 : 851 – 6 . doi:10.4065/mcp.2011.0090
Gao B , Liu F-li , Zhao B . Association of degree and type of edema in posterior reversible encephalopathy syndrome with serum lactate dehydrogenase level: initial experience . Eur J Radiol 2012 ; 81 : 2844 – 7 . doi:10.1016/j.ejrad.2011.12.010
Peng W-X , Nakaii M , Matsushima T , et al. Atypical case of reversible posterior leucoencephalopathy syndrome associated with puerperal HELLP syndrome . Arch Gynecol Obstet 2008 ; 278 : 269 – 71 . doi:10.1007/s00404-008-0578-7
Mueller-Mang C , Mang T , Pirker A , et al. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 2009 ; 51 : 373 – 83 . doi:10.1007/s00234-009-0504-0
Bartynski WS , Boardman JF . Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome . AJNR Am J Neuroradiol 2007 ; 28 : 1320 – 7 . doi:10.3174/ajnr.A0549
Posterior reversible encephalopathy syndrome postpartum - Scientific Figure on ResearchGate . Available: https://www.researchgate.net/figure/Magnetic-resonance-imaging-with-T2-flair-weighted-images-showing-the-typically_fig1_273390342 [Accessed 23 Jan 2020].
Nielsen LH , Grøn BS , Ovesen PG . Posterior reversible encephalopathy syndrome postpartum . Clin Case Rep 2015 ; 3 : 266 – 70 . doi:10.1002/ccr3.218
Doelken M , Lanz S , Rennert J , et al. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI . Diagn Interv Radiol 2007 ; 13 : 125 – 8 .
Hinduja A , Habetz K , Raina SK , et al. Predictors of intensive care unit utilization in patients with posterior reversible encephalopathy syndrome . Acta Neurol Belg 2017 ; 117 : 201 – 6 . doi:10.1007/s13760-016-0703-5
Hinduja A . Posterior reversible encephalopathy syndrome: clinical features and outcome . Front Neurol 2020 ; 11 :71. doi:10.3389/fneur.2020.00071
Dean C , Douglas J . Magnesium and the obstetric anaesthetist . Int J Obstet Anesth 2013 ; 22 : 52 – 63 . doi:10.1016/j.ijoa.2012.10.003
Schwartz RB , Feske SK , Polak JF , et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy . Radiology 2000 ; 217 : 371 – 6 . doi:10.1148/radiology.217.2.r00nv44371
Demirel I , Ozer AB , Bayar MK , et al. Anesthesia and intensive care management in a pregnant woman with PRES: a case report . Case Rep Anesthesiol 2012 ; 2012 : 1 – 5 . doi:10.1155/2012/745939
Demir BC , Ozerkan K , Ozbek SE , et al. Comparison of magnesium sulfate and mannitol in treatment of eclamptic women with posterior reversible encephalopathy syndrome . Arch Gynecol Obstet 2012 ; 286 : 287 – 93 . doi:10.1007/s00404-012-2268-8
Liman TG , Bohner G , Heuschmann PU , et al. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases . Eur J Neurol 2012 ; 19 : 935 – 43 . doi:10.1111/j.1468-1331.2011.03629.x
Striano P , Striano S , Tortora F , et al. Clinical spectrum and critical care management of posterior reversible encephalopathy syndrome (PRES) . Med Sci Monit 2005 ; 11 : 549 – 53 .
Servillo G , Striano P , Striano S , et al. Posterior reversible encephalopathy syndrome (PRES) in critically ill obstetric patients . Intensive Care Med 2003 ; 29 : 2323 – 6 . doi:10.1007/s00134-003-1901-1
Goetzl LM , Bulletin AP . ACOG practice Bulletin. clinical management guidelines for Obstetrician-Gynecologists number 36, July 2002. obstetric analgesia and anesthesia . Obstet Gynecol 2002 ; 100 : 177 – 91 . doi:10.1016/s0029-7844(02)02156-7
Alhilali LM , Reynolds AR , Fakhran S . A multi-disciplinary model of risk factors for fatal outcome in posterior reversible encephalopathy syndrome . J Neurol Sci 2014 ; 347 : 59 – 65 . doi:10.1016/j.jns.2014.09.019
Siebert E , Bohner G , Liebig T , et al. Factors associated with fatal outcome in posterior reversible encephalopathy syndrome: a retrospective analysis of the Berlin preS study . J Neurol 2017 ; 264 : 237 – 42 . doi:10.1007/s00415-016-8328-4
Karia SJ , Rykken JB , McKinney ZJ , et al. Utility and significance of gadolinium-based contrast enhancement in posterior reversible encephalopathy syndrome . AJNR Am J Neuroradiol 2016 ; 37 : 415 – 22 . doi:10.3174/ajnr.A4563
Chen Z , Zhang G , Lerner A , et al. Risk factors for poor outcome in posterior reversible encephalopathy syndrome: systematic review and meta-analysis . Quant Imaging Med Surg 2018 ; 8 : 421 – 32 . doi:10.21037/qims.2018.05.07
Schweitzer AD , Parikh NS , Askin G , et al. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome . Neuroradiology 2017 ; 59 : 379 – 86 . doi:10.1007/s00234-017-1815-1
Servillo G , Bifulco F , De Robertis E , et al. Posterior reversible encephalopathy syndrome in intensive care medicine . Intensive Care Med 2007 ; 33 : 230 – 6 . doi:10.1007/s00134-006-0459-0
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1469-0756
- Print ISSN 0032-5473
- Copyright © 2024 Fellowship of Postgraduate Medicine
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement
Posterior Reversible Encephalopathy Syndrome
- Secondary Headache (M Robbins, Section Editor)
- Published: 25 February 2021
- Volume 25 , article number 19 , ( 2021 )
Cite this article
- Alexandra N. Gewirtz ORCID: orcid.org/0000-0002-1807-9226 1 ,
- Virginia Gao 1 ,
- Sarah C. Parauda 1 &
- Matthew S. Robbins 1
12k Accesses
54 Citations
10 Altmetric
Explore all metrics
Purpose of Review
This review provides an updated discussion on the clinical presentation, diagnosis and radiographic features, mechanisms, associations and epidemiology, treatment, and prognosis of posterior reversible encephalopathy syndrome (PRES). Headache is common in PRES, though headache associated with PRES was not identified as a separate entity in the 2018 International Classification of Headache Disorders. Here, we review the relevant literature and suggest criteria for consideration of its inclusion.

Recent Findings
COVID-19 has been identified as a potential risk factor for PRES, with a prevalence of 1–4% in patients with SARS-CoV-2 infection undergoing neuroimaging, thus making a discussion of its identification and treatment particularly timely given the ongoing global pandemic at the time of this writing.
PRES is a neuro-clinical syndrome with specific imaging findings. The clinical manifestations of PRES include headache, seizures, encephalopathy, visual disturbances, and focal neurologic deficits. Associations with PRES include renal failure, preeclampsia and eclampsia, autoimmune conditions, and immunosuppression. PRES is theorized to be a syndrome of disordered autoregulation and endothelial dysfunction resulting in preferential hyperperfusion of the posterior circulation. Treatment typically focuses on treating the underlying cause and removal of the offending agents.
Similar content being viewed by others

Status epilepticus in the ICU
Andrea O. Rossetti, Jan Claassen & Nicolas Gaspard

Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients
Aaron M. Cook, G. Morgan Jones, … Lori Shutter

Guidelines for the Neurocritical Care Management of Aneurysmal Subarachnoid Hemorrhage
Miriam M. Treggiari, Alejandro A. Rabinstein, … Stavropoula Tjoumakaris
Avoid common mistakes on your manuscript.
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological entity that was first described in 1996 in a series of fifteen patients with acute neurological symptoms including headache, seizures, visual disturbances, and other focal neurological deficits [ 1 ]. Though it was initially described as a reversible posterior leukoencephalopathy syndrome, PRES has since been the name most commonly applied to this entity. Here, we discuss the clinical presentation, diagnosis and radiographic features, mechanisms, associations and epidemiology, treatments, and prognosis of PRES. In addition, we propose criteria for a PRES-associated headache syndrome for consideration by the International Classification of Headache Disorders [ 2 ].
Clinical Presentation
PRES is associated with a wide array of clinical presentations including headaches, focal neurological deficits, seizures, visual disturbances, and encephalopathy. The severity and acuity of clinical symptoms vary, although typically occur with rapid onset.
PRES-associated headache was first characterized by Hinchey et al. as sudden in onset, with or without associated neurologic deficits or seizures. It is typically described as constant and dull, at times intractable, and has been reported in 50% of patients [ 3 , 4 ]. A subset of patients, however, describe a “thunderclap headache” as a harbinger of associated reversible cerebral vasoconstriction syndrome (RCVS) [ 5 ]. Headache in conjunction with PRES-associated disease states has been reported. Postpartum headache has been described as a recurrent syndrome associated with PRES. [ 6 ] In one prospective study of eclampsia patients, the presence of headache predicted abnormal imaging, with 95.6% of those with headache having abnormal imaging as compared to 12.5% of those without headache [ 7 ]. The most common characterizations of these eclampsia-associated headaches were throbbing or pounding pain (53%) and pressure like (40%). Of note, abnormal imaging findings in this study were not limited to PRES, but 16 of the 40 patients in the study had FLAIR abnormalities, a hallmark feature of the syndrome. One study demonstrated 58% prevalence of headache in preeclampsia/eclampsia patients with PRES. [ 8 ] Headache attributed to arterial hypertension is yet another syndrome associated with PRES and presents as severe in onset in conjunction with notably high blood pressures [ 9 ]. Prior reports have described headache in patients with PRES receiving associated medications; however, these patients are usually hypertensive at time of diagnosis [ 10 , 11 ].
Seizures are common in PRES, seen in up to 81% of patients [ 4 ] and most often manifesting as generalized tonic-clonic episodes [ 12 ] with a propensity for recurrence. In a retrospective review of 49 patients with PRES, (17.6%) had recurrent generalized tonic-clonic seizures [ 13 ]. The semiology of PRES-associated seizures varies, however, and also includes convulsive status epilepticus (SE), complex partial seizures, and nonconvulsive SE. SE has been observed in up to 17% of patients with PRES in a larger case series [ 5 ]. It occasionally represents symptomatic onset of PRES, as seen in a series of 10 patients with the majority exhibiting focal-onset complex partial SE as the initial presenting complaint [ 14 ]. In another study of 11 pediatric patients with PRES undergoing stem cell transplantation, there was a high rate of SE with 12 episodes of SE in 10 patients, 8 cases of convulsive SE and 4 cases of nonconvulsive SE [ 15 ]. Seizures, when present, tend to occur early after disease onset in PRES. In a study of 38 patients with PRES and seizures, 100% had clinical seizures the first day after diagnosis, with no subsequent seizures [ 13 ]. Occipital lobe involvement has been identified as a significant predictor of the occurrence of seizures. On multivariate analysis in one study, occipital lobe involvement alone was significantly associated with the occurrence of PRES-related seizure development (OR: 9.63, p = 0.02) [ 16 ]. In one prospective study of 40 women with eclampsia, which is defined by the presence of seizures, of the 22 women with MR imaging of the brain, all had T2 abnormalities including 11 with high parietal and 8 with occipital pole abnormalities [ 7 ].
Encephalopathy
PRES can present with encephalopathy and, in one study, was the presenting complaint in 28% of patients [ 17 ]. Encephalopathy is present in most cases with a variable severity and can range from mild confusion to disordered consciousness [ 18 ••]. Practically, since seizures are so common in PRES, encephalopathy may also be associated with an ictal or postictal state.
Visual Disturbances
Visual disturbances are common in PRES and were seen in 39% of patients in one study [ 19 ]. The symptoms can present as cortical blindness, various types of visual field deficits, visual neglect, hallucinations, and blurred vision. Ocular examination in patients with PRES is often unrevealing, although may reveal papilledema on fundoscopic examination along with nonspecific hemorrhages and exudates [ 20 ]. In one study of patients with PRES, visual complaints included bilateral vision loss in the majority of patients (64%), diplopia in 27%, and unilateral vision loss, color desaturation, and pain with extraocular movements each individually found in 9% of patients. Of note, of those patients with ocular complaints, 100% had a history of hypertension [ 21 ]. Visual symptoms have been theorized to occur with higher frequency with certain associations of PRES. A retrospective review observed that visual disturbances such as cortical blindness, blurred vision, and hemianopia are more common in eclampsia-related PRES. [ 8 ]
Visual recovery appears to be favorable in PRES. In a review of PRES in systemic lupus erythematosus (SLE), visual impairment was found in 15 of 26 episodes, with 87% of patients completely recovering their vision [ 22 ]. In two case reports of PRES and associated visual loss, follow-up results of visual field examination and peripapillary retinal nerve fiber lining were normal [ 23 ].
Focal Neurologic Deficits
Focal neurologic deficits are varied and correlate with location of edema. In the literature, focal neurological symptoms have been reported in 10–15% of patients with PRES. [ 17 ] In one review of 71 patients with PRES, focal deficits were characterized as hemiparesis in 8.5%, followed by hemiplegia and speaking difficulty each in 4.2% of patients [ 18 ••].
Diagnosis and Radiographic Features
The diagnosis of PRES is typically made with magnetic resonance imaging (MRI) of the brain. Imaging characteristically shows focal regions of symmetric hyperintensities on T2-weighted studies most commonly in the parietal and occipital lobes, followed by the frontal lobes and the cerebellum. In a large cohort study by Bartynski et al., some degree of involvement of the parieto-occipital regions was seen in 98% of cases. In their review, imaging findings also included a holohemispheric watershed pattern with involvement of the frontal lobes in 22.8% of patients as well as a superior frontal sulcus watershed pattern in 27.2% of patients [ 24 ]. Apart from the aforementioned patterns, the less common but well-described additional regions of involvement may include the midbrain, pons, medulla, and basal ganglia. PRES has rarely been associated with spinal cord involvement [ 25 ]. PRES is also associated with hemorrhage, including small volume hemorrhage, subarachnoid hemorrhage, and hematomas, seen in 15.1% of cases in one study. Not surprisingly, there was a higher frequency of hemorrhage in those patients on therapeutic anticoagulation or undergoing bone marrow transplant [ 26 ]. Susceptibility weighted images (SWI) have also been utilized to assess for the presence of hemorrhage, seen in 64.5% of patients in another review [ 27 ]. Abnormal apparent diffusion coefficient is seen in approximately 20% of cases [ 28 ••] and has been associated with poor outcomes.
Vasculopathy is a common finding in patients with PRES. Angiography, if performed, can show evidence of constriction of the blood vessels, which suggests a possible overlap with reversible cerebral vasoconstriction syndrome (RCVS). Conversely, typical PRES imaging findings have been reported in 17–38% patients with RCVS [ 29 ].
In a series of 99 cases with PRES, 38% of patients had extensive vasogenic edema, 21% had brainstem edema, and 37% had evidence of intracranial hemorrhage—all classified as advanced radiologic PRES. Of the 94 cases with available MRIs, 16% had restricted diffusion on T2, also considered an advanced radiologic sign of PRES. Extensive vasogenic edema and the presence of hemorrhage were both associated with a low modified Rankin Scale (mRS) score at discharge ( p = 0.047 and 0.021, respectively). The presence of diffusion restriction also showed a trend towards association with poor mRS at discharge ( p = 0.074). Overall, the presence of advanced radiologic PRES was associated with both poor discharge disposition ( p = 0.021) and poor mRS at discharge ( p = 0.008) [ 30 ].
PRES is a disorder of dysregulated perfusion, leading to usually reversible vasogenic edema. There are several theories as to why the cerebrovasculature becomes dysregulated in PRES. There is no single mechanism that explains the development of PRES in all cases, and multiple nonexclusive mechanisms likely contribute.
In cases where hypertension is a key feature, hyperperfusion is thought to play a critical role. In response to fluctuations in systemic blood pressure, cerebrovascular autoregulation preserves cerebral blood flow, leading to vasodilation during systemic hypotension and vasoconstriction during systemic hypertension. Rapid development of hypertension can exceed the capacity of cerebral blood flow autoregulation leading to hyperperfusion. Consistent with the clinical and radiographic features of the disease, posterior brain regions are thought to be more vulnerable to hyperperfusion because there is less sympathetic innervation to the posterior circulation, potentially through reduced opposition to parasympathetic reflex vasodilation. Whether the systemic blood pressures seen in patients with PRES can truly overwhelm autoregulatory mechanisms has been questioned [ 18 ••]. Among patients who have PRES and hypertension, less than 50% have a mean arterial pressure (MAP) above the upper limit of cerebral blood flow autoregulation derived from physiological studies, MAP > 140–150 [ 31 ]. However, there may be individual and circumstantial variability. For example, arterial hypertension, acute fluctuations in blood pressure, and autonomic activity can all shift autoregulatory thresholds. Autoregulatory curves may also be shifted in pregnancy, making it a particularly vulnerable time for PRES despite only modest elevations in blood pressure.
On a mechanistic level, blood-brain barrier breakdown can result from hyperperfusion and increased cerebral perfusion pressure leading to extravasation of plasma and macromolecules into the interstitial space through tight junction proteins [ 32 ]. Other mechanisms also contribute to the loss of the integrity of the vascular endothelium, which can lead to vasoconstriction in and of itself. The release of vasoactive substances including nitric oxide, thromboxane A2, or endothelin-1 from the vascular endothelium contributes to cerebral autoregulation [ 33 ]. Thromboxane A2 and endothelin-1 can mediate cerebral vasospasm and lead to blood pressure elevations. It has further been proposed that hypertension may also result from hyperperfusion caused by endothelial dysfunction or another systemic process. However, as development of hypertension usually precedes development of symptoms, this does not provide a unifying explanation. Indeed, hyperperfusion due to hypertension cannot explain the development of PRES in the 15–20% of patients who have normotension or hypotension [ 32 ].
Endothelial damage is also implicated in PRES. Vascular integrity is normally preserved by inter-endothelial adhesion molecules, and circulating toxins can trigger vascular leakage and edema. In immune disorders and other systemic disorders, release of cytokines including tumor necrosis factor alpha (TNFα), interleukin-1 (IL1), and interferon gamma (IFNγ) activates the secretion of vasoactive factors from endothelial cells that increase vascular permeability leading to interstitial edema. The release of these cytokines can also influence downstream gene expression cascades. TNFα and IL1 induce the expression of adhesion molecules, including intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion protein 1 (VCAM-1), and E-selectin, that interact with circulating leukocytes and lead to endothelial activation [ 34 ]. Similarly, in infection, polymorphonuclear leukocytes are activated, marginate to the vessel wall, adhere to the vascular endothelium, and increase vascular permeability. TNFα also induces the expression of vascular endothelial growth factor (VEGF), which is thought to independently increase vascular permeability [ 33 ]. Elevated levels of VEGF have been implicated in a number of disease states associated with PRES, most notably SLE. Kuryliszyn-Moskal et al. showed that patients with SLE have significantly higher levels of VEGF than healthy controls ( p < 0.05) [ 35 ]. In addition, in patients with SLE and PRES, brain biopsies were performed that showed evidence of endothelial activation and subsequent VEGF expression, suggesting a possible relationship between PRES and VEGF levels [ 36 ]. Hypoxia has also been associated with the development of PRES. Release of endothelial factors in response to hypoxia promotes angiogenesis (including VEGF) and this breakdown of the blood-brain barrier has been postulated to contribute to vasogenic edema.
Taken together, and depending on the clinical situation, all these mechanisms may contribute to the development of PRES pathophysiology (Fig. 1 ).
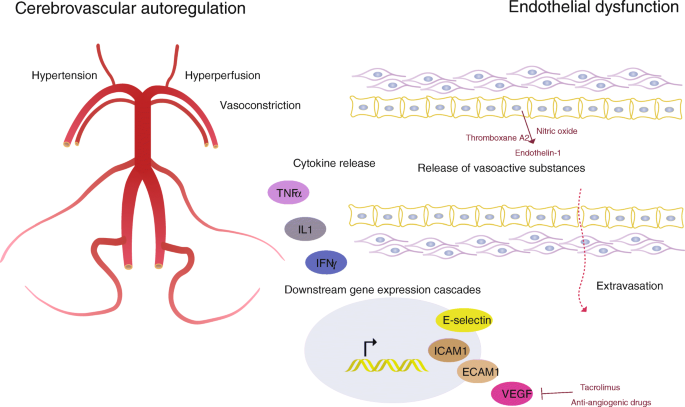
Mechanisms contributing to the development of PRES. Disordered cerebrovascular autoregulation in hypertension can lead to hyperperfusion. Blood-brain barrier breakdown results from increased cerebral perfusion pressure leading to extravasation of plasma and macromolecules into the interstitial space through tight junction proteins. Release of cytokines activates the secretion of vasoactive factors from endothelial cells that increase vascular permeability leading to interstitial edema. Release of these cytokines can also influence downstream gene expression cascades
Epidemiology and Associations
PRES is seen in all age groups with a range from 4 to 90 years [ 37 ] and a mean age of 45 [ 4 ]. In adults, there is a female predominance of cases even after exclusion of patients with eclampsia [ 17 ]. The prevalence in the pediatric population has not been well established, although a prior study of 2588 admissions to a pediatric critical care unit reported a rate of 0.4% [ 38 ]. Another study of 825 pediatric hospitalizations for PRES-associated conditions such as bone marrow transplant, hypertension, and connective tissue disorders found the rate of PRES to be 0.04% [ 39 ].
Renal Injury/Failure
Many studies suggest renal injury is the strongest predictor of the development of PRES—up to 55% of cases are associated with renal failure [ 17 , 18 ••, 40 ]. In a retrospective study of 63 SLE patients, renal failure was the only independent predictor of the development of PRES. [ 40 ] Similarly, a case series involving thrombotic thrombocytopenic purpura (TTP) and PRES revealed glomerular filtration rate to be the only laboratory value with a significant association with the presence of PRES on brain MRI [ 41 ]. The mechanisms tying kidney injury to PRES have yet to be fully elucidated but may involve disruption in the renin-angiotensin-aldosterone system (RAAS). Another theory suggests that upregulation and accumulation of vasopressin in kidney failure may play a role in arteriole vasoconstriction and failure of autoregulation in PRES. [ 42 ]
The combination of disordered cerebrovascular autoregulation, acute renal failure, acute hypertension, hypoxia, inflammation, and endothelial injury can characterize SARS-CoV-2 infection and its sequelae. Coincidence of PRES and COVID-19 disease has been reported, making recognition and diagnosis of PRES particularly timely. Along with the common use of immunomodulatory medications, these associated characteristics make this pandemic disease a perfect storm for the development of PRES. [ 43 , 44 ] A larger study of 278 patients with confirmed COVID-19 undergoing imaging demonstrated a 1.1% prevalence of PRES. MRI as an imaging modality had a higher yield in detecting cases, demonstrating a 3.9% prevalence of PRES. [ 45 ]
Preeclampsia/Eclampsia
The obstetric complications of preeclampsia and eclampsia are closely linked with PRES. In one study, 97.9% of patients with eclampsia had confirmed PRES on imaging [ 46 ], suggesting that the disease entities are essentially one and the same, with the eclampsia diagnosis existing long before imaging modalities were available. Widespread endothelial dysfunction in combination with abrupt elevations in blood pressure is thought to be the key features that drive PRES in preeclampsia and eclampsia [ 47 ]. In women with preeclampsia or eclampsia, those with PRES also have a higher prevalence of thrombocytopenia and proteinuria as compared to those without PRES. [ 48 ] However, PRES associated with these etiologies appears to have some key differences compared to PRES attributed to other etiologies. One study showed that patients with PRES associated with these conditions have a significantly higher prevalence of headaches (58% vs. 18%) and lower incidence of altered mental status (12.5% vs. 45%) as compared to patients with PRES associated with non-obstetric conditions [ 8 ]. Additionally, this study found that in this population, imaging showed less involvement of T2 hyperintensities in the thalamus, midbrain, and pons; lower incidence of hemorrhage and diffusion restriction; as well as less severe edema when compared to those patients with PRES associated with other etiologies. However, other studies have not found such differences in imaging findings [ 49 , 50 ].
Autoimmune Conditions and Hemoglobinopathies
Almost half of PRES patients have an associated autoimmune condition, including SLE, TTP, Crohn’s disease, and scleroderma, among others [ 18 ••]. However, it is difficult to determine if the root of the association is the autoimmune disease itself or rather the high incidence of renal injury as well as the immunosuppressive medications frequently used in these conditions. PRES has also been reported in a series of patients with neuromyelitis optica, and it has been postulated that disordered fluid shifts play a role in pathogenesis in these patients with abnormal function of aquaporin 4 [ 51 ]. Both solid organ and stem cell transplant patients have high incidence of PRES, with incidence in solid organ transplantation varying from 0.4 and 6% [ 52 ] and in hematopoietic stem cell transplantation between 1.1 and 22% [ 53 – 55 ]. Graft-versus-host disease associated with transplant also appears to increase risk for PRES. [ 55 ] PRES has been reported in individuals with sickle cell disease (SCD), typically in those with acute chest syndrome as well as post stem cell transplant patients on calcineurin inhibitors. In the pediatric population, PRES has been seen in children independently of acute chest syndrome. In a retrospective review of MRI findings in 80 children with SCD, 8 patients had radiologic and clinical characteristics consistent with PRES. [ 39 ] It is not clear whether PRES is independently associated with SCD or whether the underlying vasculopathy, hypertension, and endothelial damage contribute [ 56 ].
Immunosuppression and Other Associated Medications
Classically, the immunosuppressive medications highly associated with PRES are the calcineurin inhibitors tacrolimus and cyclosporine, though this may be via induction of hypertension and/or impaired autoregulation as blood levels do not seem to correlate with incidence [ 18 ••, 42 ]. Steroids have both been implicated in the improvement of PRES-associated vasogenic edema and in the development of PRES itself [ 57 ]. Tocilizumab has also been reported in association with PRES. [ 57 , 58 ] Hydroxychloroquine has also been implicated in PRES, though it is unclear if this is an independent risk factor or simply associated via its common use as a treatment for SLE [ 17 , 59 , 60 ]. Finally, several VEGF antagonists have been associated with PRES—bevacizumab, sunitinib, and sorafenib [ 18 ••, 61 ].
Treatment and Prognosis
There are no clinical trials to date regarding the treatment of PRES. Withdrawal or management of the offending trigger such as hypertension, impaired renal function, or immunosuppressive therapies appears to improve outcomes. Systemic blood pressure abnormalities, if untreated, can lead to development or exacerbation of cerebral edema. Correspondingly, aggressive blood pressure management in cases of hypertension-induced PRES has been shown to reduce associated morbidity [ 62 ]. In cases of pregnancy-associated PRES, management includes expeditious delivery of the infant. In pregnant women with PRES and preeclampsia, magnesium sulfate is indicated to prevent seizures [ 5 ].
In cases of calcineurin inhibitor-associated PRES, a common strategy is the replacement of the offending calcineurin inhibitor with other immunosuppressive agents (43.7% in one study). Other strategies include replacement with another calcineurin inhibitor (26.8%); replacement with sirolimus, everolimus, mycophenolate mofetil, or hydrocortisone (16.9%); or lowering the dose of the offending agent (22.5%). In only 9.9% of cases, discontinuation of the agent with no replacement was pursued [ 63 ]. Other studies suggest that continuation of the same calcineurin inhibitor or changing to alternative calcineurin inhibitors is well tolerated. In one study of patients with hematologic disorders, the majority of patients (77%) tolerated continuation or alternative calcineurin inhibitor therapy. An additional 11% tolerated conversion from a calcineurin inhibitor to sirolimus, with only 8% of patients experiencing recurrent PRES with continuation of calcineurin or sirolimus therapy [ 64 ].
Steroid therapy has not been shown to play a role in the routine treatment of PRES-associated vasogenic edema. In a study of 99 cases of PRES, corticosteroid therapy frequently preceded the onset of PRES and was not significantly associated with the extent of vasogenic edema, suggesting that it does not lessen edema and may in fact contribute to the development of PRES. [ 56 ]
Treatment of malignant complications of PRES is also paramount, such as IV anticonvulsants in status epilepticus and aggressive management of hemorrhagic PRES and increased intracranial pressure. Management of PRES often requires an ICU setting; in one study, 70% of patients with PRES required ICU care for PRES-associated complications including status epilepticus [ 65 ]. The optimal agent and length of treatment in PRES-associated seizures, however, remains controversial [ 66 ].
Prognosis is generally favorable and the majority of patients make a full recovery, though some diagnostic criteria require clinical and radiographic resolution [ 18 ••] which does not include the entire spectrum of the disease. When reversibility is not mandated, complete recovery is reported in 75–90% of cases, and most patients will recover within a week, though in some patients, recovery occurs over a longer interval and neurological sequelae have been reported in 10–20% of patients [ 9 ]. PRES can also be quite severe, and mortality has been reported in 3–6% of cases [ 67 ]. Etiology of severe neurologic injury and death include intracranial hemorrhage, edema in the posterior fossa leading to hydrocephalus or brainstem compression, or increased intracranial pressure. The underlying cause of PRES, time to treatment, and underlying imaging characteristics have all been shown to contribute to prognosis [ 4 , 68 ]. As previously mentioned, a retrospective review of 99 cases with PRES showed that the presence of advanced radiologic PRES was associated with both an unfavorable discharge disposition and poor mRS at discharge [ 30 ].
Proposed New Criteria for PRES-Associated Headache
While some cases of PRES are likely captured under ICHD-3 “10.3.3. Headache attributed to hypertensive encephalopathy,” hypertensive encephalopathy and PRES are not equivalent diagnoses. About 30% of PRES cases are associated with normal or only mildly elevated blood pressure values [ 69 , 70 ]. Many of these PRES cases end up being attributed to other known associations including immunosuppressive and cytotoxic medications, sepsis, autoimmune disorders, and renal failure [ 70 ]. Also, while the majority of patients with hypertensive encephalopathy will have altered mental status, this is not so with a significant portion of PRES cases [ 71 ]. In particular, PRES cases associated with preeclampsia and eclampsia have lower incidence of altered mental status and higher incidence of headache compared to PRES cases associated with other causes [ 8 ]. While some cases of PRES-associated headache may be captured under ICHD-3 10.3.4-Headache attributed to preeclampsia or eclampsia, these are again overwhelmingly entwined with hypertension [ 8 ]. As the ICHD-3 currently stands, there is still a significant diagnostic gap for headache that is associated with PRES but not associated with either hypertensive encephalopathy or preeclampsia/eclampsia.
Conclusions
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome with a complex clinical presentation. Early recognition of classic radiographic features is vital to prompt recognition and treatment, as the symptoms are relatively nonspecific and the differential diagnosis is broad.
Fifty percent of patients will present with nonspecific headache as previously discussed. To date, headache secondary to PRES is not listed as a distinct entity in the ICHD-3, though related conditions are including headache attributed to arterial hypertension and headache attributed to preeclampsia or eclampsia. We propose that PRES-associated headache should be its own unique diagnosis in the ICHD-3 under “6.7: Headache attributed to other acute intracranial arterial disorder” as seen in Table 1 .
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500.
Article CAS PubMed Google Scholar
Arnold M. Headache classification committee of the international headache society (ihs) the international classification of headache disorders. Cephalalgia. 2018;38(1):1–211.
Article Google Scholar
Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, et al. Posterior reversible encephalopathy syndrome—insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. 2015;14(9):830–6.
Legriel S, Schraub O, Azoulay E, Hantson P, Magalhaes E, Coquet I, et al. Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One. 2012;7(9):e44534.
Article CAS PubMed PubMed Central Google Scholar
Hinduja A. Posterior reversible encephalopathy syndrome: clinical features and outcome. Front Neurol. 2020;11:71.
Article PubMed PubMed Central Google Scholar
Effendi M, Rashidi A, Ahmad MZ, Yusoff HM, bin Amir Hamzah, A. Postpartum headache: an unexpected manifestation of posterior reversible encephalopathy syndrome. Eurasian Journal of Emergency Medicine. 2016;15(2):108–10.
Shah AK, Rajamani K, Whitty JE. Eclampsia: a neurological perspective. J Neurol Sci. 2008;271(1–2):158–67.
Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol. 2012;19(7):935–43.
Frick D, Huecker M, Shoff H. Posterior reversible encephalopathy syndrome presenting as stroke mimic. Clinical practice and cases in emergency medicine. 2017;1(3):171–4.
Hamid M, Ghani A, Micaily I, Sarwar U, Lashari B, Malik F. Posterior reversible encephalopathy syndrome (PRES) after bevacizumab therapy for metastatic colorectal cancer. Journal of community hospital internal medicine perspectives. 2018;8(3):130–3.
Macdonald DR. Posterior reversible encephalopathy syndrome: case report. Reactions. 2014;1528:162–22.
Google Scholar
Spencer D. PRES-ing for answers about long-term seizure risk in patients with posterior reversible encephalopathy syndrome: PRES-ing for answers about long-term seizure risk. Epilepsy currents. 2015;15(6):317–8.
Kastrup O, Gerwig M, Frings M, Diener HC. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012;259(7):1383–9.
Article PubMed Google Scholar
Kozak OS, Wijdicks EFM, Manno EM, Miley JT, Rabinstein AA. Status epilepticus as initial manifestation of posterior reversible encephalopathy syndrome. Neurology. 2007;69(9):894–7.
Cordelli DM, Masetti R, Bernardi B, Barcia G, Gentile V, Biagi C, et al. Status epilepticus as a main manifestation of posterior reversible encephalopathy syndrome after pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2012;58(5):785–90.
Hinduja A, Habetz K, Raina SK, Fitzgerald RT, Sahaya K. Predictors of seizures in patients with posterior reversible encephalopathy syndrome. Epilepsy Behav. 2016;61:97–101.
Fugate, J. E., Claassen, D. O., Cloft, H. J., Kallmes, D. F., Kozak, O. S., & Rabinstein, A. A. (2010, May). Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. In Mayo Clinic Proceedings (Vol. 85, no. 5, pp. 427-432). Elsevier.
•• Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. The Lancet Neurology . 2015; 14 (9):914–25 This study provides a thorough analysis of PRES and discusses the main radiologic patterns, current theories of pathophysiology, and clinical outcomes.
Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–10.
Chou MCY, Lee CY, Chao SC. Temporary visual loss due to posterior reversible encephalopathy syndrome in the case of an end-stage renal disease patient. Neuro-ophthalmology. 2018;42(1):35–9.
Lifson N, Pasquale A, Salloum G, Alpert S. Ophthalmic manifestations of posterior reversible encephalopathy syndrome. Neuro-Ophthalmology. 2019;43(3):180–4.
Lai CC, Chen WS, Chang YS, Wang SH, Huang CJ, Guo WY, et al. Clinical features and outcomes of posterior reversible encephalopathy syndrome in patients with systemic lupus erythematosus. Arthritis care & research. 2013;65(11):1766–74.
Baranowski D, Rejdak K, Kiszka A, Nowomiejska K, Rejdak R. Vision deterioration in posterior reversible encephalopathy syndrome (PRES). Ophthalmology Journal. 2017;2(2):61–7.
Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am J Neuroradiol. 2007;28(7):1320–7.
Saad AF, Chaudhari R, Wintermark M. Imaging of atypical and complicated posterior reversible encephalopathy syndrome. Front Neurol. 2019;10:964.
Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. Am J Neuroradiol. 2009;30(7):1371–9.
McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. Am J Neuroradiol. 2012;33(5):896–903.
•• Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: a review with emphasis on neuroimaging characteristics. Journal of the neurological sciences . 2019; 404 :72–9 This reference focuses on the imaging characteristics of PRES and discusses the various MRI patterns found in the disease.
Pilato F, Distefano M, Calandrelli R. Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome: clinical and radiological considerations. Front Neurol. 2020;11:34.
Schweitzer AD, Parikh NS, Askin G, Nemade A, Lyo J, Karimi S, et al. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology. 2017;59(4):379–86.
Li Y, Gor D, Walicki D, Jenny D, Jones D, Barbour P, et al. Spectrum and potential pathogenesis of reversible posterior leukoencephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21(8):873–82.
Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate JE. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21(4):254–8.
Creager, M., Loscalzo, J., & Beckman, J. A. (2012). Vascular medicine E-book: A companion to Braunwald's heart disease. Elsevier Health Sciences.
Pruitt JH, Copeland EM III, Moldawer LL. Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock. 1995;3(4):235–51.
Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S, Ciołkiewicz M. Vascular endothelial growth factor in systemic lupus erythematosus: relationship to disease activity, systemic organ manifestation, and nailfold capillaroscopic abnormalities. Arch Immunol Ther Exp. 2007;55(3):179–85.
Article CAS Google Scholar
Carvalho JF, Blank M, Shoenfeld Y. Vascular endothelial growth factor (VEGF) in autoimmune diseases. J Clin Immunol. 2007;27(3):246–56.
Chen TH. Childhood posterior reversible encephalopathy syndrome: Clinicoradiological characteristics, managements, and outcome. Front Pediatr. 2020;8.
Raj S, Overby P, Erdfarb A, Ushay HM. Posterior reversible encephalopathy syndrome: incidence and associated factors in a pediatric critical care population. Pediatr Neurol. 2013;49(5):335–9.
Khademian Z, Speller-Brown B, Nouraie SM, Minniti CP. Reversible posterior leuko-encephalopathy in children with sickle cell disease. Pediatr Blood Cancer. 2009;52(3):373–5.
Jung SM, Moon SJ, Kwok SK, Ju JH, Park KS, Park SH, et al. Posterior reversible encephalopathy syndrome in Korean patients with systemic lupus erythematosus: risk factors and clinical outcome. Lupus. 2013;22(9):885–91.
Burrus TM, Mandrekar J, Wijdicks EF, Rabinstein AA. Renal failure and posterior reversible encephalopathy syndrome in patients with thrombotic thrombocytopenic purpura. Arch Neurol. 2010;67(7):831–4.
Largeau B, Le Tilly O, Sautenet B, Gandonnière CS, Barin-Le Guellec C, Ehrmann S. Arginine vasopressin and posterior reversible encephalopathy syndrome pathophysiology: the missing link? Mol Neurobiol. 2019;56(10):6792–806.
Parauda SC, Gao V, Gewirtz AN, Parikh NS, Merkler AE, Lantos J, et al. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416:117019.
Anand P, Lau KV, Chung DY, Virmani D, Cervantes-Arslanian AM, Mian AZ, et al. Posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019: two cases and a review of the literature. J Stroke Cerebrovasc Dis. 2020;29(11):105212.
Lin E, Lantos JE, Strauss SB, Phillips CD, Campion TR, Navi BB, et al. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. Am J Neuroradiol. 2020;41:2001–8.
Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208(6):468–e1.
McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018;49(3):524–30.
Fisher N, Saraf S, Egbert N, Homel P, Stein EG, Minkoff H. Clinical correlates of posterior reversible encephalopathy syndrome in pregnancy. The Journal of Clinical Hypertension. 2016;18(6):522–7.
Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology. 2009;51(6):373–83.
Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: is there a difference between pregnant and non-pregnant patients? Eur Neurol. 2009;62(3):142–8.
Magaña SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72(8):712–7.
Staykov D, Schwab S. Posterior reversible encephalopathy syndrome. J Intensive Care Med. 2012;27(1):11–24.
Gaziev J, Marziali S, Paciaroni K, Isgrò A, Di Giuliano F, Rossi G, et al. Posterior reversible encephalopathy syndrome after hematopoietic cell transplantation in children with hemoglobinopathies. Biology of Blood and Marrow Transplantation. 2017;23(9):1531–40.
Hammerstrom AE, Howell J, Gulbis A, Rondon G, Champlin RE, Popat U. Tacrolimus-associated posterior reversible encephalopathy syndrome in hematopoietic allogeneic stem cell transplantation. Am J Hematol. 2013;88(4):301–5.
Chen Q, Zhao X, Fu HX, Chen YH, Zhang YY, Wang JZ, et al. Posterior reversible encephalopathy syndrome (PRES) after haploidentical haematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant. 2020:1–8.
Farooq S, Testai FD. Neurologic complications of sickle cell disease. Current Neurology and Neuroscience Reports. 2019;19(4):17.
Parikh NS, Schweitzer AD, Young RJ, Giambrone AE, Lyo J, Karimi S, et al. Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. J Neurol Sci. 2017;380:11–5.
Rosa Júnior M, Borges ÉI, Fonseca APA, Fiorot JL, Balarini L, Valim V. Posterior reversible encephalopathy syndrome during treatment with tocilizumab in juvenile idiopathic arthritis. Arq Neuropsiquiatr. 2018;76(10):720–1.
Leroux G, Sellam J, Costedoat-Chalumeau N, Le Thi Huong D, Combes A, Tieulié N, et al. Posterior reversible encephalopathy syndrome during systemic lupus erythematosus: four new cases and review of the literature. Lupus. 2008;17(2):139–47.
Gatla N, Annapureddy N, Sequeira W, Jolly M. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2013;19(6):334–40.
Tlemsani C, Mir O, Boudou-Rouquette P, Huillard O, Maley K, Ropert S, et al. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target Oncol. 2011;6(4):253–8.
Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, et al. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med. 2007;33(2):230–6.
Song T, Rao Z, Tan Q, Qiu Y, Liu J, Huang Z, et al. Calcineurin inhibitors associated posterior reversible encephalopathy syndrome in solid organ transplantation: report of 2 cases and literature review. Medicine. 2016;95(14):e3173.
Cerejo, M. C., Barajas, R. F., Cha, S., & Logan, A. C. (2014). Management strategies for posterior reversible encephalopathy syndrome (PRES) in patients receiving calcineurin-inhibitor or sirolimus therapy for hematologic disorders and allogeneic transplantation.
Hinduja A, Habetz K, Raina SK, Fitzgerald RT. Predictors of intensive care unit utilization in patients with posterior reversible encephalopathy syndrome. Acta Neurol Belg. 2017;117(1):201–6.
Skiba V, Etienne M, Miller JA. Development of chronic epilepsy after recurrent episodes of posterior reversible encephalopathy syndrome associated with periodic lateralized epileptiform discharges. Seizure. 2011;20(1):93–5.
Liman TG, Bohner G, Endres M, Siebert E. Discharge status and in-hospital mortality in posterior reversible encephalopathy syndrome. Acta Neurol Scand. 2014;130(1):34–9.
Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23:1038–48.
PubMed Google Scholar
Feske SK. Posterior reversible encephalopathy syndrome: a review. Semin Neurol. 2011;31(2):202–15.
Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017;264(8):1608–16.
Miller JB, Suchdev K, Jayaprakash N, Hrabec D, Sood A, Sharma S, et al. New developments in hypertensive encephalopathy. Curr Hypertens Rep. 2018;20(2):13.
Download references
Author information
Authors and affiliations.
Department of Neurology, New York-Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065, USA
Alexandra N. Gewirtz, Virginia Gao, Sarah C. Parauda & Matthew S. Robbins
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Alexandra N. Gewirtz .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Secondary Headache
Rights and permissions
Reprints and permissions
About this article
Gewirtz, A.N., Gao, V., Parauda, S.C. et al. Posterior Reversible Encephalopathy Syndrome. Curr Pain Headache Rep 25 , 19 (2021). https://doi.org/10.1007/s11916-020-00932-1
Download citation
Accepted : 29 December 2020
Published : 25 February 2021
DOI : https://doi.org/10.1007/s11916-020-00932-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Reversible posterior encephalopathy syndrome
- Immunosuppressive drugs
- Vasogenic edema
- Hypertensive encephalopathy
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Posterior reversible encephalopathy syndrome
Marlene fischer.
1 Department of Anesthesiology, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany
Erich Schmutzhard
2 Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
The posterior reversible encephalopathy syndrome (PRES) is a neurological disorder of (sub)acute onset characterized by varied neurological symptoms, which may include headache, impaired visual acuity or visual field deficits, disorders of consciousness, confusion, seizures, and focal neurological deficits. In a majority of patients the clinical presentation includes elevated arterial blood pressure up to hypertensive emergencies. Neuroimaging, in particular magnetic resonance imaging, frequently shows a distinctive parieto-occipital pattern with a symmetric distribution of changes reflecting vasogenic edema. PRES frequently develops in the context of cytotoxic medication, (pre)eclampsia, sepsis, renal disease or autoimmune disorders. The treatment is symptomatic and is determined by the underlying condition. The overall prognosis is favorable, since clinical symptoms as well as imaging lesions are reversible in most patients. However, neurological sequelae including long-term epilepsy may persist in individual cases.
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a neurological disorder characterized by a range of neurological signs and symptoms and distinctive neuroimaging findings reflecting vasogenic edema [ 1 ]. Both clinical and imaging characteristics are usually reversible [ 2 ]. On average, about 40% of all patients diagnosed with PRES require intensive care monitoring and treatment due to severe complications such as status epilepticus, cerebral ischemia, intracerebral hemorrhage or intracranial hypertension [ 3 ].
The syndrome was first described in 1996 by Hinchey and colleagues who reported on a series of 15 patients with neurological signs and symptoms including headache, seizures, visual disturbance and other focal neurological deficits [ 4 ]. Moreover, computed tomographic (CT) or magnetic resonance imaging (MRI) alterations suggestive of cerebral edema were observed predominantly in the posterior regions [ 4 ]. Since this first description of PRES numerous case reports and case series, as well as retrospective observational studies describing the syndrome have been published. Importantly, no randomized controlled studies have been performed, a fact that has to be taken into account when discussing epidemiological data, diagnostic criteria and treatment recommendations.
Epidemiological data in particular should be interpreted with caution, since the syndrome may still be significantly underdiagnosed as the condition can be hard to confirm. PRES has been reported in almost all age groups, from children to older adults, but most frequently in young- or middle-aged adults with a preponderance of female patients, which might be attributable to etiological aspects [ 5 , 6 ].
Etiology and pathophysiological considerations
There are two leading theories regarding the pathophysiology of PRES (Fig. 1 ) [ 7 ]. The first hypothesis proposes a rapid increase of arterial blood pressure up to a hypertensive crisis or emergency, which has been observed in a majority of patients at PRES onset [ 1 ]. According to this hypothesis, elevation of blood pressure levels above the upper autoregulatory limit leads to cerebral hyperperfusion, which may cause vascular leakage and vasogenic edema [ 8 ]. Increased cerebral perfusion pressure contributes to additional blood–brain barrier dysfunction causing extravasation of plasma and macromolecules through tight-junction proteins [ 7 ].

The two main hypotheses explaining the pathophysiology of posterior reversible encephalopathy and associated conditions
Cerebrovascular autoregulation is supposed to preserve a continuous cerebral blood flow independently of systemic blood pressure fluctuations [ 9 ]. This is ensured by vasodilation of the cerebral arteries during hypotensive episodes. In contrast, during periods of hypertension, this results in cerebral vasoconstriction. This adaptive mechanism is mainly regulated by pressure and carbon dioxide reactivity, as well as the release of vasoactive substances such as nitric oxide, thromboxane A 2 or endothelin-1 from the vascular endothelium [ 1 ].
In healthy individuals a continuous cerebral blood flow can be maintained between the lower and upper autoregulatory limits, usually a cerebral perfusion pressure between 50 and 150 mmHg [ 10 ]. Various conditions such as arterial hypertension, acute fluctuations of blood pressure or autonomic activity may induce changes of these autoregulatory thresholds. This may lead to increased vulnerability of the cerebral circulation and predispose to cerebral ischemia during periods of hypotension on the one hand, or cerebral hyperperfusion and vascular leakage on the other, when blood pressure rises above the upper autoregulatory limit [ 11 , 12 ]. The “hyperperfusion theory” is supported by observations of elevated or fluctuating blood pressure, or hypertensive episodes in a majority of patients with PRES at disease onset [ 3 ].
The posterior areas of the cerebral hemispheres seem to be particularly susceptible, which is supported by clinical as well as imaging findings. This might be caused by a reduced density of sympathetic innervation in the posterior, compared to the anterior, circulation, the latter being more densely innervated by the superior cervical ganglion [ 1 ]. This may prevent excessive vasodilation, which could reduce the risk of cerebral hyperperfusion in these areas compared to the posterior regions.
However, arguing against this hypothesis is that about 30% of patients with PRES show normal or only slightly elevated blood pressure values that do not necessarily exceed the normal upper autoregulatory limit, as would be expected in the context of cerebral hyperperfusion [ 13 ]. Thus, the theory of hypertensive episodes and cerebral hyperperfusion as the underlying pathological condition in PRES is still a matter of controversy.
The second theory regarding the cause of PRES is that the syndrome is triggered by endothelial dysfunction caused by circulating endogenous or exogenous toxins [ 7 ]. Arguing for this hypothesis, PRES is frequently observed in patients with (pre)eclampsia, sepsis or during treatment regimens with immunosuppressive agents or cytotoxic medication [ 14 – 16 ]. The common factor in these diverse conditions is the presence of endogenic (preeclampsia, sepsis) or exogenic (chemotherapy, immunosuppressive agents) toxins causing endothelial dysfunction [ 17 ]. One of the key features of the vascular endothelium is the preservation of vascular integrity by inter-endothelial adhesion molecules. Circulating toxins could trigger vascular leakage and edema formation, and additionally lead to endothelial activation resulting in the release of immunogenic and vasoactive substances [ 17 ]. Vasoconstrictive agents released by vascular endothelial cells are thought to mediate cerebral vasospasm, which is frequently observed in PRES patients [ 2 ]. In this “toxic” theory, blood pressure elevations occur as a consequence of primary endothelial dysfunction. A variation on the “toxic/immunogenic” theory is that the trigger is the excessive release of pro-inflammatory cytokines resulting in endothelial activation, release of vasoactive agents, increased vascular permeability and edema formation. This mechanism is regarded as the key feature causing PRES in patients with autoimmune disorders or sepsis [ 17 ].
Apart from arterial hypertension, a variety of conditions have been linked to the diagnosis of PRES. Etiologies may be manifold; however, a clear correlation between clinical signs and symptoms, lesion site or specific trigger factors has not been observed [ 2 , 5 ]. PRES has been frequently reported in patients receiving immunosuppressive medication after solid organ, bone marrow or stem cell transplantation [ 18 , 19 ]. The incidence of PRES after solid organ transplantation is reported to be between 0,4 and 6%, whereas up to 8% of patients after bone marrow transplantation may be affected [ 18 , 20 ].
Compared to solid organ transplantation immunosuppressive medication is usually administered at a higher dose with bone marrow or stem cell transplantation, possibly explaining the higher incidence of PRES after non-solid organ transplantation. However, it is unclear whether PRES is linked to the dose of causative agents. Plasma levels of immunosuppressive substances do not necessarily correlate with the severity of clinical signs or imaging findings [ 20 , 21 ]. Moreover, PRES has been observed up to several months after administration of cytotoxic agents [ 20 ]. Adding to this controversy, there are numerous reports of PRES in patients with plasma concentrations of immunosuppressants within the therapeutic range. Nevertheless, tapering off or reducing the dosage of causative agents usually leads to clinical improvement and/or a reduction in lesion size [ 20 ]. This observation supports a positive correlation between the dose of the offending agent and the neurological/radiological manifestations.
The exact mechanism of how specific substances may cause this form of encephalopathy is unknown. Numerous authors have reported calcineurin inhibitors to be linked with PRES development [ 22 – 24 ]. These substances are well-known for their neurotoxic properties, which have been attributed to the release of vasoconstrictive substances, aggravation of hypomagnesemia, and arterial hypertension [ 25 , 26 ]. In a retrospective study, Hammerstrom and colleagues observed an average increase of 35% in the mean arterial blood pressure under a Tacrolimus regimen [ 21 ]. Adding to the reported effects, polymorphisms in the multidrug resistance protein 1 gene may allow central nervous system dissemination of these substances [ 27 ]. Importantly, Tacrolimus but also antiangiogenic drugs such as Bevacizumab, Sunitinib or Sorafenib may mediate increased vascular permeability, thereby contributing to edema formation [ 1 ].
Autoimmune disorders have been frequently reported in the context of PRES. Fugate and colleagues report a history of autoimmune disease in 45% of patients in a retrospective study of 120 cases [ 5 ]. Several explanations have been provided for this linkage [ 5 , 17 ]. As is the case in post-transplant patients, immunosuppressive medication may play an important role. Additionally, (auto)immunologic reactions may trigger endothelial activation by excessive cytokine release followed by vascular leakage of proteins and fluid into the interstitial space.
Renal disease and preeclampsia have also been linked to PRES. Impaired renal function has been reported in 55% of all patients with PRES [ 1 ]. However, it is unclear whether accompanying arterial hypertension or renal dysfunction itself is the primary causal factor.
PRES occurs frequently in the setting of preeclampsia or eclampsia [ 28 ]. In a retrospective study, PRES was found in more than 90% of eclamptic and about 20% of preeclamptic patients with neurological symptoms [ 16 ]. Compared with pregnant women with eclampsia or preeclampsia without PRES, significant elevations of hematocrit, serum creatinine, aspartate transaminase, alanine transaminase and lactate dehydrogenase values were noted [ 16 ].
Clinical findings
PRES is characterized by a variety of neurological symptoms, usually going along with elevated arterial blood pressure. The onset may be acute or subacute, with symptoms developing within a few hours up to several days or even weeks [ 1 ].
Patients may present with signs of encephalopathy, including quantitative and qualitative disorders of consciousness such as cognitive deficits or stupor, somnolence or coma [ 2 ]. Epileptic seizures, focal as well as generalized, are very common, and have been observed in about two third of all patients [ 3 , 29 ]. In 3–13% of cases seizures may result in status epilepticus, which is one of the most severe and potentially life threatening complications of PRES [ 3 , 30 ].
In accordance with the frequent involvement of the occipital lobes, visual disturbances such as a deterioration of visual acuity, visual field deficits including hemianopia and cortical blindness or visual hallucinations can be observed in about two third of all PRES patients [ 4 ]. Less specific neurological symptoms include headache, nausea, vomiting and disorders of consciousness. Depending on the location of the lesions, focal neurological deficits have been reported in 5–15% [ 1 , 31 , 32 ]. Some case reports have described myelopathic symptoms in patients with spinal cord involvement [ 33 ]. An overview of the most common clinical findings is provided in Fig. 2 .

Incidence of neurological signs in patients with posterior reversible encephalopathy syndrome
Established diagnostic criteria have been lacking so far and clinical as well as imaging findings are often not specific (Table 1 ). Therefore, the diagnosis of PRES can often only be made after excluding important other diagnoses. The presence of neurological symptoms of acute onset, concurrent blood pressure fluctuations, vasogenic edema as the leading neuroimaging finding and a clinical context of associated comorbidities or trigger factors are suggestive of PRES. Fugate et al. suggested the following criteria for the diagnosis of PRES: neurological symptoms of acute onset, neuroimaging abnormalities of (focal) vasogenic edema and the reversibility of clinical and/or radiological findings (see Fig. 3 ) [ 5 ].
Table 1
Diagnostic findings in patients with posterior reversible encephalopathy syndrome
EEG electroencephalogram, CT computed tomography, MRI magnetic resonance imaging, FLAIR fluid-attenuated inversion recovery, DWI diffusion-weighted imaging, ADC apparent diffusion coefficient

Suggested criteria for the diagnosis of posterior reversible encephalopathy syndrome
Modified after Fugate et al. (2010) [ 5 ]
Neuroimaging, in particular MRI, is the most important diagnostic tool. Therefore, characteristic neuroimaging findings are discussed in more detail below. Electroencephalography (EEG) may be necessary for the detection of (non convulsive) epileptic seizures, status epilepticus and may also help in the evaluation of encephalopathy [ 29 ]. Lumbar puncture is of major importance to exclude encephalitis or leptomeningeal spread in patients with hemato-oncological disease. However, pathological alterations in cerebrospinal fluid (CSF) that are specific for PRES have not been observed. Elevated CSF levels of albumin and an elevated CSF/serum albumin quotient as a manifestation of blood–brain barrier disruption have been reported in a series of 87 patients, whereas pleocytosis was rare [ 34 ]. This is in line with a retrospective review of 73 patients with PRES undergoing lumbar puncture [ 35 ]. Mild albuminocytologic dissociation was found in all patients with median protein levels of 58 mg/dl [ 35 ].
Serum findings are usually not specific. Hypomagnesemia during the first 48 h after onset was reported in a cohort of patients with PRES of varying etiology [ 36 ]. Both Gao and Pirker et al. observed decreased serum albumin in up to 85% of patients with PRES of miscellaneous etiology [ 37 , 38 ].
CT scans usually show vasogenic edema with a bihemispheric distribution [ 2 ]. MRI is more sensitive displaying hyperintense lesions in T2-weighted or fluid-attenuated inversion recovery (FLAIR) sequences [ 2 ]. MRI lesions reflecting vasogenic edema frequently follow a parieto-occipital pattern [ 15 ]. Though usually bihemispheric, lesions may be distributed asymmetrically (Fig. 4 ). Due to the lower density of the white matter, subcortical areas are affected predominantly. However, cortical involvement has also been described [ 2 ]. While the parieto-occipital distribution occurs in about 70% of all patients, a frontal sulcus or watershed pattern is also frequently seen [ 39 ]. Lesions in other areas such as the cerebellum, brain stem, basal ganglia or the spinal cord are less common [ 32 ].

a – c Axial MR image (fluid-attenuated inversion recovery sequence) demonstrates extensive vasogenic edema in the occipital region bilaterally and right insular hemorrhage
Though rare, diffusion abnormalities can be found as small lesions surrounded by edematous zones. The presence of larger areas of restricted diffusion may be indicative of ischemic stroke. Increased apparent diffusion coefficient (ADC) values on diffusion-weighted imaging (DWI) are characteristic and reflect vasogenic edema [ 15 ]. ADC imaging can be of prognostic relevance: higher values have been associated with a reversibility of lesions [ 15 ]. By contrast, attenuated ADC values indicate cerebral ischemia and a poor neurological outcome [ 15 ]. Contrast enhanced lesions have been found in about 20% of all patients. However, there does not seem to be a clear link to clinical severity or functional outcome [ 40 ].
In a retrospective observational study, the presence of microbleeds in susceptibility weighted imaging has been reported as an initial presentation and on follow-up scans [ 41 ]. The authors found microhemorrhages in 65% of all cases [ 41 ]. As with contrast enhancement, there is no clear correlation with clinical symptoms. Therefore, the clinical relevance of microbleeds in PRES has yet to be determined.
Imaging studies on cerebral perfusion in PRES patients have reported conflicting results. Increased perfusion has been observed in edematous zones confirming the hypothesis of cerebral hyperperfusion as a result of a blood pressure that is above the upper limit of cerebral autoregulation [ 42 ]. In contrast, single photon emission computed tomography (SPECT) and MR perfusion demonstrate cerebral hypoperfusion in lesion zones in PRES of varying etiology [ 43 , 44 ].
Different perfusion patterns in PRES may be explained by the variety of etiological aspects, causing a diverse pathophysiological response. In line with the main hypotheses for PRES pathophysiology, conflicting perfusion patterns may be a result of primary hypertension and cerebral perfusion on the one hand or endothelial dysfunction, cerebral vasoconstriction and/or vasospasm followed by cerebral hypoperfusion on the other hand. Vasculopathy has been observed in MR or conventional angiography. Vasculopathic findings are usually reversible and include cerebral vasoconstriction, vasospasm (both diffuse or focal) and string-of-beads appearances which are usually located in the posterior circulation [ 2 ].
One of the most important differential diagnoses of PRES is reversible cerebral vasoconstriction syndrome (RCVS). Both conditions have similar clinical and angiographic findings. As with PRES, RCVS is frequently diagnosed postpartum or after administration of vasoactive substances and vasculopathic alterations may follow a similar distribution pattern [ 45 , 46 ]. Interestingly, PRES-like lesions have been observed in patients with RCVS, suggesting that both conditions may reflect different manifestations of the same pathology.
The treatment of PRES is symptomatic, since no specific therapeutic strategy is currently available. The management of the underlying disease or pathology leading to PRES development is of major importance.
The management of hypertensive episodes and maintenance of normal blood pressure is an essential component of PRES treatment [ 1 , 15 , 47 ]. However, there is no evidence, based on prospective controlled studies, that strict blood pressure control limits neurologic injury, or results in a regression of clinical or imaging findings. The choice of antihypertensive drugs per se is based on general recommendations for the management of hypertensive crisis or hypertensive emergency [ 48 , 49 ]. A reduction of blood pressure levels by 25% from baseline values is recommended. As with other conditions, blood pressure fluctuations should be avoided and the continuous administration of antihypertensive drugs under hemodynamic monitoring should be considered [ 50 ].
Anticonvulsive treatment is frequently required. There is no general recommendation for the use of specific drugs. Moreover, the optimal duration of antiepileptic drug treatment is unclear. Usually, anticonvulsive medication can be tapered off as soon as the patient is asymptomatic and the imaging lesions have fully reversed [ 15 , 29 ].
Whenever possible, the elimination of the triggering factor or management of the underlying pathology should be initiated early during the course of the disease [ 1 , 13 , 15 ]. In many cases of PRES, immunosuppressive or cytotoxic medication is identified as the substance responsible for the neurological manifestations. It is still a matter of controversy whether tapering off or immediate discontinuation of the triggering agent is required, or whether reducing the dosage with strict control of serum levels within the therapeutic range is sufficient. Adding to this issue, the most beneficial therapeutic regimen after the neurological symptoms have resolved is unknown. In a retrospective analysis, Hammerstrom et al. compared three interventions after Tacrolimus-induced PRES in pediatric patients following stem cell transplantation [ 21 ]. They either: (1) continued Tacrolimus in the same dosage as before the onset of PRES; (2) interrupted Tacrolimus administration for a mean of 12 days; or (3) suspended treatment with Tacrolimus but switched to a different immunosuppressant immediately. Interestingly, there was no difference in mortality between the three groups in this retrospective analysis. Singer et al. continued treatment with the agent in question in 7 out of 17 cancer patients and did not find recurring PRES [ 51 ]. In patients with autoimmune disease, further administration of immunosuppressive medication may require a different management than in patients after solid organ or stem cell transplantation. In a review of patients with PRES associated with systemic lupus erythematosus, active disease was found as the initiating trigger and intensification of immunosuppressive therapy was suggested to control neurological manifestations [ 52 ].
Due to the fact that magnesium levels are reduced in a high number of patients with PRES, coupled to its known prophylactic anticonvulsive and vasodilating effects, hypomagnesemia should be avoided and serum levels be maintained in the high normal range [ 15 , 36 ].
In case of cerebral vasospasm or cerebral vasoconstriction, treatment of the vasospasm (either systemically or, if required, through local intra-arterial administration of calcium antagonists) may be initiated at an early stage.
Prognosis and outcome
The prognosis of PRES is mainly determined by the underlying condition, since the neurological manifestations are reversible in the majority of patients. However, since PRES is often accompanied by severe complications, neurological sequelae may persist.
In a recent retrospective chart review, poor neurological outcome, as defined by a modified Rankin scale score between 2 and 6, was reported in 36% of all patients at hospital discharge [ 53 ]. The authors found that preexisting diabetes mellitus and corpus callosum involvement of the PRES-associated lesions were strong predictors of poor outcome. Singer and colleagues observed a complete resolution of neurological signs and symptoms in 84% of cancer patients with PRES [ 51 ]. In 81% of cases, neuroimaging findings were reversible on follow-up MRI or CT scans. Mortality rate in their cohort was reported to be 19%. However, no death was directly associated with PRES. In a review of 111 pediatric cases with hematological disease, 19 patients (17%) died of a PRES-associated mortality [ 54 ]. Neurological sequelae including epilepsy, motor deficits and mydriasis were observed in another 17 patients. This is in line with a retrospective study in 35 pediatric cases of PRES triggered by cancer treatment, which also reported a long-term requirement for anticonvulsive treatment due to persistent epilepsy in 19% of patients [ 55 ]. Persisting epilepsy with seizures occurring one year after PRES onset was reported in two patients out of a cohort of 75 [ 56 ]. Heo and colleagues reviewed 102 cases of PRES and found long-term epilepsy in four patients [ 57 ]. In contrast, Kastrup et al. described a cohort of 49 patients, 38 of them presenting with seizures during the acute phase [ 29 ]. At follow-up none of their patients suffered from persisting epilepsy.
So far factors such as serum markers, CSF or neuroimaging findings in PRES have not been identified as useful in the risk stratification of patients nor as a measure of prognostic relevance. However, in a recent study by Karia et al., MR imaging severity (as defined by McKinney et al. 2007) correlated with clinical outcomes in 135 patients [ 40 ].
Future directions
Although numerous case reports and observational studies have been published since the first description in 1996, many aspects of PRES, in particular on pathophysiology and treatment, remain unclear [ 4 ]. Findings on cerebral perfusion in PRES patients are conflicting, since hyperperfusion as well as decreased perfusion have been reported after PRES [ 42 – 44 ]. Future neuroimaging studies should focus on angiographic imaging and perfusion patterns to characterize cerebral hemodynamics during PRES that may vary depending on etiological aspects or disease progress. Further, non-invasive continuous monitoring of the cerebrovascular autoregulation may aid in the optimal hemodynamic management and the definition of individual blood pressure targets maintaining a constant cerebral blood flow within the limits of cerebral autoregulation [ 58 ].
Although there is consensus on the elimination of the etiological factor in PRES induced by cytotoxic medication, further management of immunosuppressants or chemotherapy remains a challenging issue that is usually decided on an individual basis. For clarification, future studies should address several questions: (1) Does the medication causing PRES symptoms have to be eliminated persistently? (2) If not, what is the optimal duration for treatment interruption? (3) Are patients at risk for recurring PRES? (4) Is there a linear correlation between clinical symptoms and substance dose?
In conclusion, PRES-associated clinical signs and symptoms and neuroimaging lesions are reversible in the majority of patients. The prognosis is mainly determined by the underlying pathology. However, neurological sequelae, in particular epilepsy, may persist in individual cases and may require long-term treatment. So far, specific prognostic factors have not been identified. The severity of MR imaging lesions including ADC values may be an important parameter determining long-term prognosis.
Compliance with ethical standards
Conflicts of interest.
The authors declare that they have no conflict of interest.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Winter 2024 | VOL. 36, NO. 1 CURRENT ISSUE pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Psychiatric Morbidity and Its Prognosis in Posterior Reversible Encephalopathy Syndrome
- Roxanne C. Keynejad , M.R.C.P. , M.R.C.Psych. , and
- Anthony S. David , M.D. , F.R.C.Psych .
Search for more papers by this author
Posterior reversible encephalopathy syndrome (PRES) is a clinically and radiologically diagnosed disorder distinguished by subcortical vasogenic cerebral edema. To date, its presentation has been described through summarized neurological categories, such as seizures, headaches, “confusion,” and “altered mental function.” This retrospective case series identified all cases of clinically confirmed, radiologically diagnosed PRES resulting in treatment in a large teaching hospital from 2010 to 2019. The authors conducted a search for the term “reversible encephalopathy” in the hospital clinical radiology information system, followed by an audit of scan reports and clinical records. The most common reasons for psychiatric referral were addictions, acute psychosis, depression, suicidality, and treatment refusal. Multidisciplinary staff should consider PRES as a rare, organic differential diagnosis for acute mental state changes. Physicians should be aware of elevated rates of post-PRES psychiatric symptoms and consider whether psychiatric consultation may enhance recovery.
Posterior reversible encephalopathy syndrome (PRES) is clinically and radiologically diagnosed and characterized by subcortical vasogenic cerebral edema, with characteristic neuroimaging appearances. Its commonest known precipitants are drug toxicity ( 1 ), hypertension, sepsis ( 2 ), preeclampsia, and autoimmune disease. In a review of the literature of the past 20 years, Gao et al. ( 3 ) highlighted unanswered questions about the underlying pathophysiology, diagnostic criteria, and prognosis of PRES. The importance of clinical suspicion has been emphasized ( 4 ); however, presenting symptom descriptions have been confined to summarized neurological categories, such as seizures, headaches, “confusion,” and “altered mental function” ( 5 – 7 ). Studies of the psychiatric sequelae of PRES and its mental health comorbidity are, to our knowledge, absent from the literature.
Following the description of new neuropsychiatric syndromes, such as anti- N -methyl- d -aspartate receptor encephalitis ( 8 ), interest in potentially reversible “organic” etiologies of psychiatric presentations has expanded markedly ( 9 , 10 ). Research on psychiatric outcomes of acute neurological disorders is well established, but recent work on encephalitides is noteworthy, reporting high rates of anger, anxiety, mood swings, and low mood ( 11 ). For example, a large population-based retrospective cohort study revealed elevated risk ratios for bipolar affective disorder (risk ratio=6.34) as well as for psychotic (risk ratio=3.48), depressive (risk ratio=1.88), and anxiety (risk ratio=1.46) disorders postencephalitis ( 12 ). Comorbid psychiatric disorders are associated with worse quality of life in a range of neurological disorders, including multiple sclerosis ( 13 ), epilepsy ( 14 ), and migraine ( 15 ).
Based on clinical experience, we hypothesized that a proportion of patients diagnosed with PRES would develop psychiatric symptoms during and after the acute illness. We aimed to identify all cases of PRES radiologically diagnosed in a large teaching hospital over a 10-year period to audit psychiatric precipitants, symptoms, and sequelae, alongside clinical and etiological correlates.
In a retrospective case series conducted in the liaison neuropsychiatry service of a large South London teaching hospital serving four boroughs, we identified patients with PRES presenting between April 2010 and April 2019. The study was authorized by the South London and Maudsley National Health Service Foundation Trust Clinical Audit and Effectiveness Team on June 16, 2016. We retrieved records from the hospital’s clinical radiology information system using the search term “reversible encephalopathy” to address disambiguation problems with “PRES.” For each patient for whom a scan report mentioned reversible encephalopathy, we reviewed clinical and radiological records to determine whether PRES was ultimately the confirmed diagnosis. We audited the clinical records of these patients using a piloted data collection form and entered pseudonymized data into a spreadsheet stored in password-protected hard drives.
Case Detection
Of an initial 122 results, 69 patients were excluded at initial screening (duplicates, N=18; no scan recorded, N=13; and PRES ruled out radiologically, N=38; Figure 1 ). On the basis of 53 clinical notes reviewed, we excluded seven cases in which the ultimate clinical or radiological impression was not PRES. Forty-seven case subjects were included in the final study sample, including one whose diagnosis was reported by the neurology team.
FIGURE 1. Flow diagram of study subjects with posterior reversible encephalopathy syndrome (PRES) a
a CRIS=clinical radiology information system.
Demographic Characteristics
Of the 47 included case subjects, 33 (70%) were female, with a median age of 39 years at presentation (range, 5–74 years).
Neuroimaging
Radiological reports revealed that abnormalities were bilateral in 44 (94%) case subjects; of these, 25 (57%) were asymmetrical. Gray matter involvement was mentioned in 23 (49%) reports, and abnormalities were more pronounced in posterior regions compared with anterior regions in 40 patients (85%). Involved brain regions were occipital (N=40; 85%), parietal (N=37; 79%), frontal (N=20; 43%), temporal (N=17; 36%), cerebellar (N=12; 26%), basal ganglia (N=7; 15%), and brainstem (N=4; 9%).
Presenting Symptoms
The most common presenting symptoms were seizures (N=38; 81%), reduced consciousness (N=29; 62%), headache (N=12; 26%), visual abnormalities (N=12; 26%), and nausea and vomiting (N=11; 23%). Focal neurological signs were documented for 10 (21%) patients, with confusion and agitation present in seven (15%) cases each.
Acute hypertension was identified in 34 (72%) patients. When blood pressure at the time of presenting symptoms was electronically documented, the mean systolic pressure was 185 mmHg (SD=24; range, 140–234 mmHg; N=22), and the mean diastolic pressure was 109 mmHg (SD=19; range, 71–140 mmHg; N=17). Possible drug toxicity was identified in 27 (57%) patients, including for tacrolimus (N=8; 30%), cyclosporine (N=6; 22%), and a range of other agents, which included cancer chemotherapeutic agents (N=4; 15%), anti-inflammatory drugs (N=4; 15%), and antibiotics (N=2; 7%). Infection or sepsis was present in 22 (47%) patients; of these cases, 10 (46%) involved chest sepsis, and each of three (14%) involved both HIV opportunistic infections and skin infections, while other cases involved bacterial infections, including clostridium difficile diarrhea (N=2).
Medical Comorbidities
The commonest medical comorbidities were hematological (N=14; 30%), including aplastic anemia (N=3), acute leukemias (N=3), myelodysplastic syndrome (N=2), and sickle cell anemia (N=2). The second most common comorbidities were autoimmune disorders (N=13; 28%), including systemic lupus erythematosus (N=5; 38%), sarcoidosis (N=2; 15%), and autoimmune hepatitis (N=2; 15%). Chronic liver disease affected 11 (23%) patients, while eight (17%) patients had chronic kidney disease, with seven (88%) requiring renal replacement therapy. Seven patients were diagnosed with malignancy (15%), and four (9%) had a history of seizures. Three PRES cases (6%) occurred during a pregnancy or postpartum period.
The most frequently prescribed treatments were antiepileptic medications (N=13; 27%), antihypertensive medications (N=13; 27%), or a combination of both (N=8; 17%). Five patients (11%) received treatment for potential CNS infection, and in 11% of these, no specific treatment for PRES was provided beyond management of comorbid disorders or withdrawal of the suspected toxic agent.
Seven patients (15%) died during the index hospital admission, one patient was discharged to palliative care, and five patients died after the index hospital admission, resulting in a mortality rate of 28% during the follow-up period. Age of death ranged from 5 to 74 years old, with a median age of 41 years.
Psychiatric Contact
Eighteen (38%) case subjects had some form of contact with local psychiatric services. This subgroup of patients had a higher median age (44 years old versus 39 years old), a higher proportion of females (83% versus 70%), and a higher mortality rate (33% versus 28%) compared with the total sample. Psychiatric services contact comprised pre-PRES consultation (N=7; 15% of all cases), liaison psychiatry referrals during the index admission (21%), and post-PRES consultation (21%). The median duration of psychiatric services consultation from PRES onset until hospital discharge was 677 days (range, 7–3,123 days).
Premorbid Conditions
Reasons for psychiatric treatment before PRES diagnosis were assessments of low mood and suicidality (29%), regular consultation from addictions services (29%), mental capacity assessments due to dialysis refusal (29%), and routine assessment pretransplant surgery (14%).
Psychiatric PRES Symptoms
Mental state abnormalities were documented in 12 PRES case records (26%). These abnormalities were speech disturbance (67%), confusion (58%), agitation (58%), hallucinations (33%), disinhibition (25%), low mood (25%), delusions (17%), bad or vivid dreams (17%), and religious preoccupation, self-harm, and anxiety (8%). Of the 10 patients who were referred to liaison psychiatry, only one was evaluated for symptoms directly related to PRES, and three were evaluated for symptoms identified once PRES had been treated. The remaining referrals were for medication overdoses that precipitated hospital admission (20%), cognitive or mental capacity assessment (20%), or substance-use nurse review (20%). The one patient who was referred to psychiatric services during PRES onset and treatment was evaluated for grandiose and persecutory delusions and auditory hallucinations. The three patients who were evaluated for post-PRES symptoms were referred for agitated depression and suicidality, low mood and vivid dreams, and advice regarding antipsychotic medication.
Psychiatric Symptoms Post-PRES Hospital Admission
Of the 10 patients who received psychiatric services post-PRES hospital admission, four were referred for low mood, along with acute delusions, suicidality, erratic behavior, or reduced oral intake. In addition, four patients were referred regarding their capacity to refuse treatment or self-discharge; of these, comorbid addictions were present in three. Two patients were referred for acute confusion, along with persecutory delusions or rapid cognitive decline.
In this study, we found that psychiatric symptoms were not reported in all cases of PRES, although confusion and agitation were common. However, psychiatric symptoms often occurred before, during, or following PRES onset, which is consistent with evidence of psychiatric comorbidities in neurological disorders, including epilepsy, migraine, stroke, and traumatic brain injury ( 16 ). The subgroup of patients who received psychiatric consultation was a median of 5 years older than the total sample, with a higher proportion of females and a higher mortality rate (33% versus 28%).
Physicians treating PRES and liaison psychiatrists should be alert to psychiatric comorbidities. In addition, multidisciplinary staff should consider PRES as a rare, organic differential diagnosis for acute mental state changes, especially when PRES coincides with neurological symptoms, hypertension, or medical comorbidities. Physicians treating PRES should assess for post-PRES psychiatric symptoms, to consider whether consultation-liaison psychiatry, specialist substance use, or community psychiatric follow-up may enhance recovery.
Given these rates of psychiatric comorbidity, it is striking that mental health comorbidity is near-absent from literature on PRES, which focuses on neuroimaging, neurological sequelae, treatment, and prognosis. This is surprising, given the association between neuropsychiatric symptoms and temporo-limbic network lesions, which are not uncommon in PRES. Despite the term reversible, residual infarcts and subsequent leukomalacia are recognized sequelae of PRES ( 17 ), supporting the likelihood of longer-term psychiatric symptoms in a proportion of patients, which is well recognized in acute neurological disorders such as encephalitis ( 11 , 12 ).
As expected, our study sample had high rates of underlying medical comorbidity. Worldwide, depression prevalence is higher among people with chronic conditions ( 18 ), including neurological ( 19 ) disorders. The most common reasons for psychiatric services referral in our sample were alcohol and intravenous drug addictions, acute psychosis, depression, suicidality, and refusal of medical treatment due to delirium or agitation. There were few or no referrals for severe anxiety, long-standing psychotic illnesses, bipolar illness, intellectual disability, and personality or eating disorders. Perhaps during critical illness treatment and follow-up, only markedly acute psychiatric symptoms were detected by physicians and referred for assessment. It is not possible to definitively quantify the extent to which some psychiatric morbidity predating PRES onset may have gone undetected. Such morbidity would be expected to be increased over the population baseline in the context of chronic medical illness. The United Kingdom National Health Service provides open access to family health practitioners, and therefore most moderate to severe mental disorders are recorded. Substance use disorders, however, may not always be identified, treated, and recorded by health care services.
To our knowledge, this is the first study to provide preliminary support for our clinically informed hypothesis of elevated psychiatric morbidity among individuals following PRES onset, which requires more widespread, prospective investigation. One strength of our study is its relatively large sample size, drawn from a broad, diverse, densely populated region of South London in a teaching hospital with expertise in a range of relevant medical disorders. One limitation is its pragmatic, retrospective audit design, dependent on routinely documented free-text clinical records. Future research and PRES case series should investigate psychiatric comorbidity and sequelae systematically, including the premorbid period, to elaborate on these findings. Perspectives of patients and their care givers on pre- and post-PRES mental states, as well as longitudinal follow-up of neuropsychiatric outcomes, would be particularly informative.
Dr. Keynejad has received a National Institute of Health Research (NIHR) Academic Clinical Fellowship, and her Ph.D. research is funded by King’s College London and a King’s IoPPN Clinician Investigator Scholarship. Dr. David is supported by the University College London Hospitals NIHR Biomedical Research Centre.
The authors report no financial relationships with commercial interests.
The authors thank Mr. Mark Allin for assistance in record searching and Dr. James Gratwicke for clinical discussion.The study funders played no role in the study design, data collection, data analysis, data interpretation, or writing of this article.
The views expressed in this article are those of the authors and not necessarily those of the National Health Service or the Department of Health and Social Care.
1 Hategan A, Bourgeois JA : Tacrolimus neurotoxicity and the role of the Renin-Angiotensin system . J Neuropsychiatry Clin Neurosci 2015 ; 27:e140–e141 Link , Google Scholar
2 Lamar CD, Hurley RA, Taber KH : Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome . J Neuropsychiatry Clin Neurosci 2011 ; 23:237–241 Link , Google Scholar
3 Gao B, Lyu C, Lerner A, et al. : Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry 2018 ; 89:14–20 Crossref , Medline , Google Scholar
4 Fugate JE, Rabinstein AA : Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions . Lancet Neurol 2015 ; 14:914–925 Crossref , Medline , Google Scholar
5 Legriel S, Pico F, Azoulay E : Understanding Posterior Reversible Encephalopathy Syndrome , in Annual Update in Intensive Care and Emergency Medicine . Edited by Vincent JL . Berlin, Springer, 2011 , pp 631–653 Google Scholar
6 Liman TG, Siebert E, Endres M : Posterior reversible encephalopathy syndrome . Curr Opin Neurol 2019 ; 32:25–35 Crossref , Medline , Google Scholar
7 Roth C, Ferbert A : Posterior reversible encephalopathy syndrome: long-term follow-up . J Neurol Neurosurg Psychiatry 2010 ; 81:773–777 Crossref , Medline , Google Scholar
8 Dalmau J, Gleichman AJ, Hughes EG, et al. : Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies . Lancet Neurol 2008 ; 7:1091–1098 Crossref , Medline , Google Scholar
9 Lennox BR, Tomei G, Vincent S-A, et al. : Study of immunotherapy in antibody positive psychosis: feasibility and acceptability (SINAPPS1) . J Neurol Neurosurg Psychiatry 2019 ; 90:365–367 Crossref , Medline , Google Scholar
10 Prüss H, Lennox BR : Emerging psychiatric syndromes associated with antivoltage-gated potassium channel complex antibodies . J Neurol Neurosurg Psychiatry 2016 ; 87:1242–1247 Crossref , Medline , Google Scholar
11 Dowell E, Easton A, Solomon T : Consequences of encephalitis . Malton, United Kingdom, Encephalitis Society, 2000 Google Scholar
12 Granerod J, Davies NWS, Ramanuj PP, et al. : Increased rates of sequelae post-encephalitis in individuals attending primary care practices in the United Kingdom: a population-based retrospective cohort study . J Neurol 2017 ; 264:407–415 Crossref , Medline , Google Scholar
13 Fruehwald S, Loeffler-Stastka H, Eher R, et al. : Depression and quality of life in multiple sclerosis . Acta Neurol Scand 2001 ; 104:257–261 Crossref , Medline , Google Scholar
14 Johnson EK, Jones JE, Seidenberg M, et al. : The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy . Epilepsia 2004 ; 45:544–550 Crossref , Medline , Google Scholar
15 Antonaci F, Nappi G, Galli F, et al. : Migraine and psychiatric comorbidity: a review of clinical findings . J Headache Pain 2011 ; 12:115–125 Crossref , Medline , Google Scholar
16 Kanner AM : Management of psychiatric and neurological comorbidities in epilepsy . Nat Rev Neurol 2016 ; 12:106–116 Crossref , Medline , Google Scholar
17 Lee VH, Wijdicks EFM, Manno EM, et al. : Clinical spectrum of reversible posterior leukoencephalopathy syndrome . Arch Neurol 2008 ; 65:205–210 Medline , Google Scholar
18 Moussavi S, Chatterji S, Verdes E, et al. : Depression, chronic diseases, and decrements in health: results from the World Health Surveys . Lancet 2007 ; 370:851–858 Crossref , Medline , Google Scholar
19 Hesdorffer DC : Comorbidity between neurological illness and psychiatric disorders . CNS Spectr 2016 ; 21:230–238 Crossref , Medline , Google Scholar
- Posterior Reversible Encephalopathy Syndrome Presenting as Delirium With Psychosis and Agitation in the Postpartum Period Cureus, Vol. 65
- Multiorgan dysfunction precipitated by disulfiram use and posterior reversible encephalopathy syndrome with atypical presentation: a case report 6 December 2022 | Journal of Substance Use, Vol. 29, No. 2

- Posterior Reversible Encephalopathy Syndrome (PRES)
- Hypertensive Encephalopathy
- Liaison Psychiatry
- Cerebral Disorders
- Consultation-Liaison Neuropsychiatry

IMAGES
VIDEO
COMMENTS
Posterior reversible encephalopathy syndrome (PRES) is an illness in which a person can present with acutely altered mentation, drowsiness or sometimes stupor, visual impairment (e.g., visual hallucinations, cortical blindness, hemianopia, quadrantanopia, and diplopia), seizures (focal or general tonic-clonic), and sudden or constant, non-localized headaches.[1] PRES can unfold acutely or ...
CONTENTS Basics Pathogenesis Causes Clinical presentation PRES-RCVS (Reversible Cerebral Vasoconstriction Syndrome) overlap Imaging Lumbar puncture EEG & seizure semiology Diagnosis Differential diagnosis Treatment Prognosis Podcast Questions & discussion Pitfalls PRES refers to reversible, vasogenic edema which occurs predominantly in the posterior brain. PRES is less commonly known as ...
Introduction. Posterior reversible encephalopathy syndrome (PRES) is a clinicoradiological diagnosis that is based on a combination of typical clinical features and risk factors, and supported by magnetic resonance (MR) brain scan findings. Neurological symptoms can be multiple or occur in isolation and may evolve over the course of the acute ...
40. Reversible cerebral vasoconstriction syndrome • Thunderclap headache • PRES quickly progresses over a few hours, complications may occur for several days with the RCVS • Imaging PRES- Bilateral parieto-occipital lesions on MRI, typical for PRES • Imaging RCVS- classic pattern of 'string of beads' on Angiography, at least two narrowings per artery on two different cerebral ...
Posterior reversible encephalopathy syndrome (PRES) 1,2 is an acute or subacute cerebral syndrome, the main manifestations of which are headache, encephalopathy, seizures, or visual disturbances ...
Posterior reversible encephalopathy syndrome (PRES), also known as reversible posterior leukoencephalopathy syndrome (RPLS), is a neurotoxic state that occurs secondary to the inability of the posterior circulation to autoregulate in response to acute changes in blood pressure . Hyperperfusion with resultant disruption of the blood-brain ...
Posterior. reversible encephalopathy syndrome (PRES)1,2 is an. acute or subacute cerebral syndrome, the main manifestations of which are headache, encephalopathy, seizures, or visual disturbances ...
Introduction. Posterior reversible encephalopathy syndrome (PRES) is a neurological disorder which is characterised by variable symptoms, which include visual disturbances, headache, vomiting, seizures and altered consciousness. 1 Its association is seen with a number of conditions including hypertension, pre-eclampsia and eclampsia, renal failure, systemic lupus erythematosus (SLE) and the ...
Initially recognized in association with eclampsia, cyclosporine after transplantation, and in the setting of severe hypertension, posterior reversible encephalopathy syndrome (PRES) has become synonymous with a unique pattern of brain vasogenic edema seen in the setting of neurotoxicity. 1-14 On CT or MR imaging studies, the edema is often widespread but predominates in the parietal and ...
Posterior reversible encephalopathy syndrome (PRES), also known as reversible posterior leukoencephalopathy syndrome (RPLS), is a rare condition in which parts of the brain are affected by swelling, usually as a result of an underlying cause.Someone with PRES may experience headaches, changes in vision, and seizures, with some developing other neurological symptoms such as confusion or ...
Although posterior reversible encephalopathy syndrome (PRES) has gained substantial recognition since its initial description by Hinchey et al ... Status epilepticus as a clinical presentation of PRES was not uncommon. Brain MRIs reviewed independently by 2 neuroradiologists revealed the parieto-occipital head region to be the region most ...
sical presentation is a combination of visual loss, headache, altered mental func-tion, seizures and nausea, but may include other focal deficits including weakness, sensory disturbance or speech disturbance. The syndrome of PRES has many under-lying causes and may result from medical treatments (eg, antineoplastic therapy) or
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome with a complex clinical presentation. Early recognition of classic radiographic features is vital to prompt recognition and treatment, as the symptoms are relatively nonspecific and the differential diagnosis is broad.
Jennifer Fugate and Alejandro Rabinstein2 summarised the main clinical symptoms of PRES in adults: encephalopathy (50-80%), seizures (60-75%), headache (50%), visual disturbances (33%), focal neurological defi cit (10-15%), and status epilepticus (5-15%). In the paediatric population, seizures are reported to be the most common ...
CLINICAL PRESENTATION AND TREATMENT. The diagnosis is often missed. The clinical presentation typically consists of headaches, visual disturbances, seizures, and altered mental status. 4 Features most commonly observed on CT or MRI are edema or swelling in the parieto-occipital white matter. On MRI, the syndrome usually manifests as a T2 hyperintensity with normal diffusion-weighted imaging.
Introduction. Posterior reversible encephalopathy syndrome (PRES) is a neurological disorder characterized by a range of neurological signs and symptoms and distinctive neuroimaging findings reflecting vasogenic edema [].Both clinical and imaging characteristics are usually reversible [].On average, about 40% of all patients diagnosed with PRES require intensive care monitoring and treatment ...
Posterior reversible encephalopathy syndrome (PRES) is a clinically and radiologically diagnosed disorder distinguished by subcortical vasogenic cerebral edema. To date, its presentation has been described through summarized neurological categories, such as seizures, headaches, "confusion," and "altered mental function." This retrospective case series identified all cases of clinically ...