Gastroesophageal Reflux Disease: A Review
Affiliations.
- 1 Upper Gastrointestinal Surgery, Department of Molecular Medicine and Surgery, Karolinska Institutet, and Karolinska University Hospital, Stockholm, Sweden.
- 2 Centre for Clinical Research Sormland, Uppsala University, Eskilstuna, Sweden.
- 3 Department of Surgery and Cancer, Imperial College London, London, United Kingdom.
- 4 School of Cancer and Pharmaceutical Sciences, King's College London, London, United Kingdom.
- PMID: 33351048
- DOI: 10.1001/jama.2020.21360
Importance: Gastroesophageal reflux disease (GERD) is defined by recurrent and troublesome heartburn and regurgitation or GERD-specific complications and affects approximately 20% of the adult population in high-income countries.
Observations: GERD can influence patients' health-related quality of life and is associated with an increased risk of esophagitis, esophageal strictures, Barrett esophagus, and esophageal adenocarcinoma. Obesity, tobacco smoking, and genetic predisposition increase the risk of developing GERD. Typical GERD symptoms are often sufficient to determine the diagnosis, but less common symptoms and signs, such as dysphagia and chronic cough, may occur. Patients with typical GERD symptoms can be medicated empirically with a proton pump inhibitor (PPI). Among patients who do not respond to such treatment or if the diagnosis is unclear, endoscopy, esophageal manometry, and esophageal pH monitoring are recommended. Patients with GERD symptoms combined with warning symptoms of malignancy (eg, dysphagia, weight loss, bleeding) and those with other main risk factors for esophageal adenocarcinoma, such as older age, male sex, and obesity, should undergo endoscopy. Lifestyle changes, medication, and surgery are the main treatment options for GERD. Weight loss and smoking cessation are often useful. Medication with a PPI is the most common treatment, and after initial full-dose therapy, which usually is omeprazole 20 mg once daily, the aim is to use the lowest effective dose. Observational studies have suggested several adverse effects after long-term PPI, but these findings need to be confirmed before influencing clinical decision making. Surgery with laparoscopic fundoplication is an invasive treatment alternative in select patients after thorough and objective assessments, particularly if they are young and healthy. Endoscopic and less invasive surgical techniques are emerging, which may reduce the use of long-term PPI and fundoplication, but the long-term safety and efficacy remain to be scientifically established.
Conclusions and relevance: The clinical management of GERD influences the lives of many individuals and is responsible for substantial consumption of health care and societal resources. Treatments include lifestyle modification, PPI medication, and laparoscopic fundoplication. New endoscopic and less invasive surgical procedures are evolving. PPI use remains the dominant treatment, but long-term therapy requires follow-up and reevaluation for potential adverse effects.

Publication types
- Research Support, Non-U.S. Gov't
- Adenocarcinoma / etiology
- Barrett Esophagus / etiology
- Diagnosis, Differential
- Esophageal Neoplasms / etiology
- Fundoplication*
- Gastroesophageal Reflux / complications
- Gastroesophageal Reflux / diagnosis*
- Gastroesophageal Reflux / therapy*
- Omeprazole / administration & dosage
- Proton Pump Inhibitors / administration & dosage
- Proton Pump Inhibitors / adverse effects
- Proton Pump Inhibitors / therapeutic use*
- Risk Reduction Behavior
- Proton Pump Inhibitors
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Gastro-oesophageal reflux disease articles from across Nature Portfolio
Gastro-oesophageal reflux disease is a condition in which contents of the stomach (gastric acid) flow back into the oesophagus, causing injury to the oesophagus and problematic symptoms such as heartburn or regurgitation. The condition usually arises as a result of dysfunction of the lower oesophageal sphincter.
Latest Research and Reviews
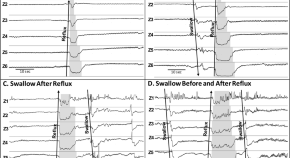
Characteristics of esophageal refluxate and symptoms in infants compared between pre-treatment and on treatment with proton pump inhibitors
- Zakia Sultana
- Vedat O. Yildiz
- Sudarshan R. Jadcherla
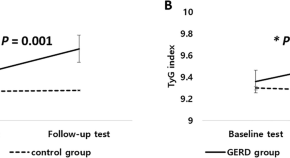
Triglyceride-glucose index is associated with gastroesophageal reflux disease and erosive reflux disease: a health checkup cohort study
- Young Min Kim
- Hyojin Park
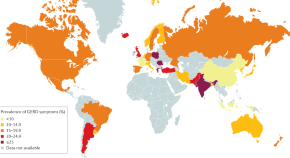
Gastro-oesophageal reflux disease
Gastro-oesophageal reflux disease is common in adults and children. This Primer covers current knowledge of the different aspects of the disease in these populations, including its epidemiology, pathophysiology, diagnosis, treatment and prognosis as well as the quality of life of patients.
- Ronnie Fass
- Guy E. Boeckxstaens
- Michael F. Vaezi
Use of a non-invasive accelerometric method for diagnosing gastroesophageal reflux in premature infants
- Ira H. Gewolb
- Frank L. Vice
GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression
Genetic factors contribute to peptic ulcer disease (PUD). Here, the authors perform a genome-wide association analysis on PUD in the UK Biobank, highlighting shared architecture with other gastrointestinal disorders and possible causal links with depression.
- Graham K. Murray
- Naomi R. Wray
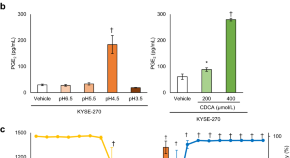
Weak acids induce PGE 2 production in human oesophageal cells: novel mechanisms underlying GERD symptoms
- Daichi Sadatomi
- Naoki Fujitsuka
News and Comment
Transpyloric feeding in severe bpd: a call for prospective trials.
- Christopher D. Baker
- Deborah R. Liptzin
- Laurie C. Eldredge
No excess cancer risk for non-erosive disease
- Caroline Barranco
Increasing global burden of gastro-oesophageal reflux disease
- Iain Dickson
A new candidate for the progenitor cell of Barrett metaplasia
At the squamocolumnar junction of mice and humans,a new study has identified a unique population of transitional basal cells that express molecular markers of both oesophageal stratified squamous epithelium and gastrointestinal columnar epithelium. These transitional basal cells are an attractive candidate for the cell of origin for Barrett oesophagus.
- Rhonda F. Souza
- Stuart J. Spechler
Symptom perception in patients with NERD: do nerves matter?
Various mechanisms eliciting symptoms in GERD and its most common phenotypic presentation, nonerosive reflux disease (NERD), have been suspected and investigated. One study now suggests that superficial nerves in the oesophageal epithelium might have a key role in the pathogenesis of NERD and could represent a potential target for topical therapies.
- Edoardo Savarino
- Marco della Coletta
Novel oesophageal cancer risk loci identified
- Peter Sidaway
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
The latest advances from Vanderbilt University Medical Center
GERD Drug Offers New Approach to Relieve Chronic Symptoms

An investigational drug that binds to bile in the stomach may provide relief for patients with difficult-to-treat cases of gastroesophageal reflux (GERD). The drug acts differently from protein-pump inhibitors (PPIs), which are the mainstay of GERD therapy today. PPIs inhibit the harmful effects of digestive acids, but do not inhibit the effects of bile.
The promising findings emerged in a study in Gastroenterology led by Michael F. Vaezi, M.D. , director of the Center for Swallowing and Esophageal Disorders at Vanderbilt University Medical Center. Vaezi’s earlier research has shown that the more severe the case of GERD, the more likely it is that bile reflux is a major contributor . “PPIs are really good at suppressing acid reflux but don’t do much to address bile reflux. This drug offers us a novel way of treating patients with refractory GERD, not just to keep going with more and more acid suppression but to intervene through bile suppression,” Vaezi said.
“This drug offers us a novel way of treating patients with refractory GERD, not just to keep going with more and more acid suppression but to intervene through bile suppression.”
An Alternative Treatment Approach
PPIs, most of which are FDA-approved for once-a-day dosing , work by impeding stomach enzymes with major roles in acid secretion. However, approximately 30 percent of GERD patients remain symptomatic despite the use of once-a-day PPI therapy. Vaezi says this often prompts clinicians to try off-label twice-daily dosing of the PPI or adding another sort of reflux medicine, such as an H 2 receptor antagonist.
“These add-on agents are only targeting acid suppression, and not addressing the problem with bile reflux,” Vaezi said.
Vaezi’s new study evaluated the safety and efficacy of three different doses of a medication called IW-3718 as an adjunct therapy to PPIs in patients who either failed to respond to PPI treatment or had only a partial response. Vanderbilt was one of 62 U.S. sites that gathered data on a total of 280 patients with refractory GERD between March 2016 and April 2017.
The drug is a special, extended-release formulation taken as a tablet. “It sits in the stomach and slowly releases a bile sequestrant, thus making the bile much less available to reflux into the esophagus,” Vaezi explained.

Promising Results
Patients were randomized to receive placebo or IW-3718 twice daily (500 mg, 1000 mg, or 1500 mg). All patients continued to take their once-daily PPI along with the study-provided medication (or placebo) for eight weeks. The primary endpoint was the percent change in a weekly heartburn severity score, with a secondary endpoint being the percent change in the patient’s weekly regurgitation score. The researchers observed a dose-response trend across treatment groups. Weekly heartburn severity scores were reduced by 58 percent in the 1500 mg treatment group. Patients in the two groups taking lower doses of the IW-3718 experienced intermediate heartburn improvements (55 percent in the 1000 mg group and 49 percent change in the 500 mg group), as compared to 46 percent in the placebo group.
Among patients who experienced regurgitation at baseline, forty-six percent of patients taking 1500 mg of the investigational drug responded with improvements, while 34 percent of the patients on the placebo improved with regard to regurgitation frequency.
“Our results are positive. They confirmed our hypothesis, that this novel way of treating patients with refractory GERD through bile sequestration would make them feel better.”
“Our results are positive. They confirmed our hypothesis, that this novel way of treating patients with refractory GERD through bile sequestration would make them feel better,” Vaezi said.
Chronic GERD: Widespread and Dangerous
With no drug-related serious adverse events in the new study, IW-3718 appears promising to treat a major patient population. About 20 percent of U.S. adults report having GERD-related symptoms weekly and many report having them daily. Symptoms include burning sensations or pain in the chest, problems swallowing, and the regurgitation of food or sour liquid.
Chronic GERD can contribute to problems that include chronic cough, laryngitis, and esophageal inflammation and ulcers. Chronic GERD is also a precursor to Barrett’s esophagus , a condition that raises the risk of esophageal cancer. “With treatments that may allow us to intervene sooner and more effectively to reduce the severity of reflux, we can reduce the likelihood of that progression,” Vaezi said.

Michael Vaezi, M.D.
Refer a patient.
To refer a patient to Vanderbilt University Medical Center, call (615) 343-4444 . Visit here for more information.
Innovation in Your Inbox
Get the latest stories about research and clinical innovation from Vanderbilt University Medical Center.
A new podcast on the ideas and innovations transforming the future of care from Vanderbilt Health.
About the Expert
Related articles.
6 Degrees to a Better Dual-Organ Transplant
Robotic System Aims to Democratize Colonoscopy

Innovation in Colonoscopy: Creating a Robotic Solution
New Developments in the Diagnosis and Management of Gastroesophageal Reflux
- Esophagus (P Iyer, Section Editor)
- Published: 19 February 2020
- Volume 18 , pages 69–81, ( 2020 )
Cite this article

- Yan Jiang MD 1 &
- John O. Clarke MD 1 , 2
676 Accesses
Explore all metrics
Purpose of review
To examine recent key developments in the pathophysiology, diagnosis, and treatment of gastroesophageal reflux disease (GERD).
Recent findings
Newer research has suggested cytokine-mediated inflammation may play a role in the physiology of GERD, implying that the underlying mechanism may not be entirely related to chemical damage due to acid. Aided by novel technologies, diagnostic testing is also moving toward elucidating individual mechanisms and better defining specific GERD phenotypes with the goal of providing directed therapy. This is especially important in current times given the increase in coverage of adverse events reportedly linked to long-term proton pump inhibitor use.
As patients are looking for potential alternatives, we highlight the key recent updates in pathophysiology and understanding of GERD and current medical and endoscopic/surgical options and explore the exciting treatments in the pipeline.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Gastroesophageal reflux disease: diagnosis and patient selection.

Gastroesophageal Reflux Disease (GERD) Overview and Introduction
Gastro-oesophageal reflux disease
References and recommended reading.
El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–80. https://doi.org/10.1136/gutjnl-2012-304,269 .
Article PubMed Google Scholar
Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–28; quiz 29. https://doi.org/10.1038/ajg.2012.444 .
Vela MF. Diagnostic work-up of GERD. Gastrointestinal endoscopy clinics of North America. 2014;24(4):655–66. https://doi.org/10.1016/j.giec.2014.07.002 .
Article Google Scholar
Schumock GT, Li EC, Suda KJ, Wiest MD, Stubbings J, Matusiak LM, et al. National trends in prescription drug expenditures and projections for 2016. Am J Health-Syst Pharm: AJHP: official journal of the American Society of Health-System Pharmacists. 2016;73(14):1058–75. https://doi.org/10.2146/ajhp160205 .
Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35–48. https://doi.org/10.1053/j.gastro.2017.04.047 A review of studies examing PPI related adverse effects that concludes there is inadequate evidence to establish causal relationships of many reported effects. Recommends continued use in those with proven indication for PPI but discourages dose escalation and chronic therapy for those unresponsive to initial empiric trial.
Article PubMed CAS Google Scholar
Dunbar KB, Agoston AT, Odze RD, Huo X, Pham TH, Cipher DJ, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. Jama. 2016;315(19):2104–12. https://doi.org/10.1001/jama.2016.5657 A study examining esophageal biopsies of those with reflux esophagitis which showed an increase in T lymphocytes, providing evidence to suggest that cytokine mediated inflammation plays a role in mucosal injury.
Article PubMed PubMed Central CAS Google Scholar
Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137(5):1776–84. https://doi.org/10.1053/j.gastro.2009.07.055 .
Huo X, Agoston AT, Dunbar KB, Cipher DJ, Zhang X, Yu C, et al. Hypoxia-inducible factor-2alpha plays a role in mediating oesophagitis in GORD. Gut. 2017;66(9):1542–54. https://doi.org/10.1136/gutjnl-2016-312,595 .
Woodland P, Shen Ooi JL, Grassi F, Nikaki K, Lee C, Evans JA, et al. Superficial esophageal mucosal afferent nerves may contribute to reflux hypersensitivity in nonerosive reflux disease. Gastroenterology. 2017;153(5):1230–9. https://doi.org/10.1053/j.gastro.2017.07.017 .
Corning B, Copland AP, Frye JW. The esophageal microbiome in health and disease. Curr Gastroenterol Rep. 2018;20(8):39. https://doi.org/10.1007/s11894-018-0642-9 .
Okereke I, Hamilton C, Wenholz A, Jala V, Giang T, Reynolds S, et al. Associations of the microbiome and esophageal disease. J Thorac Disease. 2019;11(Suppl 12):S1588–s93. https://doi.org/10.21037/jtd.2019.05.82 .
Hirano I, Pandolfino JE, Boeckxstaens GE. Functional lumen imaging probe for the management of esophageal disorders: expert review from the clinical practice updates committee of the AGA Institute. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association. 2017;15(3):325–34. https://doi.org/10.1016/j.cgh.2016.10.022 .
Scarpulla G, Camilleri S, Galante P, Manganaro M, Fox M. The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol. 2007;102(12):2642–7. https://doi.org/10.1111/j.1572-0241.2007.01461.x .
Barrett C, Choksi Y, Vaezi MF. Mucosal impedance: a new approach to diagnosing gastroesophageal reflux disease and eosinophilic esophagitis. Curr Gastroenterol Rep. 2018;20(7):33. https://doi.org/10.1007/s11894-018-0639-4 .
Bakalar N Heartburn drugs can lead to fatal heart or kidney disease. New York Times. 2019 .
Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53(7):1024–31. https://doi.org/10.1136/gut.2003.033290 .
Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout A, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. 2018;67(7):1351–62. https://doi.org/10.1136/gutjnl-2017-314,722 Consensus statement defining specific GERD populations. Highlights conclusive endoscopic criteria for GERD, pH testing indications on/off PPI, abnormal pH testing parameters and current role of high resolution manometry.
Article PubMed PubMed Central Google Scholar
Chae S, Richter JE. Wireless 24, 48, and 96 hour or impedance or oropharyngeal prolonged pH monitoring: which test, when, and why for GERD. Curr Gastroenterol Rep. 2018;20(11):52. https://doi.org/10.1007/s11894-018-0659-0 .
Hirano I, Zhang Q, Pandolfino JE, Kahrilas PJ. Four-day Bravo pH capsule monitoring with and without proton pump inhibitor therapy. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association. 2005;3(11):1083–8.
Garrean CP, Zhang Q, Gonsalves N, Hirano I. Acid reflux detection and symptom-reflux association using 4-day wireless pH recording combining 48-h periods off and on PPI therapy. Am J Gastroenterol. 2008;103(7):1631–7. https://doi.org/10.1111/j.1572-0241.2008.01829.x .
Patel RCS, Jacobs J, Kumar A, Richter J. 96-h esophageal pH monitoring: the tiebreaker for abnormal DeMeester score and symptom index. Gastroenterology. 2018;154(6):S-487-7.
Google Scholar
Patel DA, Vaezi MF. Utility of esophageal mucosal impedance as a diagnostic test for esophageal disease. Curr Opin Gastroenterol. 2017;33(4):277–84. https://doi.org/10.1097/mog.0000000000000367 .
Frazzoni M, de Bortoli N, Frazzoni L, Tolone S, Furnari M, Martinucci I et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil: the official journal of the European Gastrointestinal Motility Society. 2017;29(3). doi: https://doi.org/10.1111/nmo.12947 .
Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44(8):890–8. https://doi.org/10.1111/apt.13777 .
Saritas Yuksel E, Higginbotham T, Slaughter JC, Mabary J, Kavitt RT, Garrett CG, et al. Use of direct, endoscopic-guided measurements of mucosal impedance in diagnosis of gastroesophageal reflux disease. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association. 2012;10(10):1110–6. https://doi.org/10.1016/j.cgh.2012.05.018 .
Ates F, Yuksel ES, Higginbotham T, Slaughter JC, Mabary J, Kavitt RT, et al. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology. 2015;148(2):334–43. https://doi.org/10.1053/j.gastro.2014.10.010 .
Patel DA, Higginbotham T, Slaughter JC, Aslam M, Yuksel E, Katzka D, et al. Development and validation of a mucosal impedance contour analysis system to distinguish esophageal disorders. Gastroenterology. 2019;156(6):1617–26.e1. https://doi.org/10.1053/j.gastro.2019.01.253 .
Tucker E, Sweis R, Anggiansah A, Wong T, Telakis E, Knowles K, et al. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil: the official journal of the European Gastrointestinal Motility Society. 2013;25(11):904–10. https://doi.org/10.1111/nmo.12218 .
Article CAS Google Scholar
Rinsma NF, Smeets FG, Bruls DW, Kessing BF, Bouvy ND, Masclee AA, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc. 2014;28(3):941–9. https://doi.org/10.1007/s00464-013-3250-7 .
Ilczyszyn A, Botha AJ. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Diseases Esophagus: official journal of the International Society for Diseases of the Esophagus. 2014;27(7):637–44. https://doi.org/10.1111/dote.12130 .
Oshima T, Miwa H. Potent potassium-competitive acid blockers: a neweEra for the treatment of acid-related diseases. J Neurogastroenterol Motil. 2018;24(3):334–44. https://doi.org/10.5056/jnm18029 .
Ashida K, Sakurai Y, Hori T, Kudou K, Nishimura A, Hiramatsu N, et al. Randomized clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43(2):240–51. https://doi.org/10.1111/apt.13461 .
Di Stefano M, Papathanasopoulos A, Blondeau K, Vos R, Boecxstaens V, Farre R, et al. Effect of buspirone, a 5-HT1A receptor agonist, on esophageal motility in healthy volunteers. Diseases Esophagus: official journal of the International Society for Diseases of the Esophagus. 2012;25(5):470–6. https://doi.org/10.1111/j.1442-2050.2011.01275.x .
Karamanolis GP, Panopoulos S, Denaxas K, Karlaftis A, Zorbala A, Kamberoglou D, et al. The 5-HT1A receptor agonist buspirone improves esophageal motor function and symptoms in systemic sclerosis: a 4-week, open-label trial. Arthritis Res Ther. 2016;18:195. https://doi.org/10.1186/s13075-016-1094-y .
Aggarwal N, Thota PN, Lopez R, Gabbard S. A randomized double-blind placebo-controlled crossover-style trial of buspirone in functional dysphagia and ineffective esophageal motility. Neurogastroenterol Motil: the official journal of the European Gastrointestinal Motility Society. 2018;30(2). doi: https://doi.org/10.1111/nmo.13213 .
Thomas E, Wade A, Crawford G, Jenner B, Levinson N, Wilkinson J. Randomized clinical trial: relief of upper gastrointestinal symptoms by an acid pocket-targeting alginate-antacid (Gaviscon Double Action) - a double-blind, placebo-controlled, pilot study in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2014;39(6):595–602. https://doi.org/10.1111/apt.12640 .
Wilkinson JWA, Thomas SJ, Jenner B, Hodgkinson V, Coyle C. Randomized clinical trial: a double-blind, placebo-controlled study to assess the clinical efficacy and safety of alginate-antacid (Gaviscon Double Action) chewable tablets in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2019;31(1):86–93.
Clarke JO, Fernandez-Becker NQ, Regalia KA, Triadafilopoulos G. Baclofen and gastroesophageal reflux disease: seeing the forest through the trees. Clin Transl Gastroenterol. 2018;9(3):137. https://doi.org/10.1038/s41424-018-0010-y .
Lei WY, Hung JS, Liu TT, Yi CH, Chen CL. Influence of GABA-B agonist baclofen on capsaicin-induced excitation of secondary peristalsis in humans. Clin Transl Gastroenterol. 2017;8(10):e120. https://doi.org/10.1038/ctg.2017.46 .
Vaezi MF, Fass R, Vakil N, Mittleman R, Hall M, Reasner DS, et al. 875 - Iw-3718, a novel gastric-retentive bile acid sequestrant, improved heartburn and regurgitation symptoms in patients with persistent gerd despite PPI treatment: a double-blind, placebo-controlled study. Gastroenterology. 2018;154(6):S-174. https://doi.org/10.1016/S0016-5085(18)30995-8 .
Lipka S, Kumar A, Richter JE. No evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association. 2015;13(6):1058–67.e1. https://doi.org/10.1016/j.cgh.2014.10.013 .
Fass R, Cahn F, Scotti DJ, Gregory DA. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc. 2017;31(12):4865–82. https://doi.org/10.1007/s00464-017-5431-2 .
Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, De Vault KR, et al. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association. 2016;14(5):671–7. https://doi.org/10.1016/j.cgh.2015.05.028 Five year follow up of an initial multicenter trial of patients receiving MSA which showed a significant decrease in GERD-HRQL scores, moderate to severe regurgitation symptoms and PPI use (100% of patients initially to 15.3% of patients at follow up).
Skubleny D, Switzer NJ, Dang J, Gill RS, Shi X, de Gara C, et al. LINX((R)) magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc. 2017;31(8):3078–84. https://doi.org/10.1007/s00464-016-5370-3 .
Tatum JM, Lipham JC. Extraluminal approaches to gastroesophageal reflux disease. Thorac Surg Clin. 2018;28(4):521–6. https://doi.org/10.1016/j.thorsurg.2018.07.003 .
Hunter JG, Kahrilas PJ, Bell RC, Wilson EB, Trad KS, Dolan JP, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. 2015;148(2):324–33.e5. https://doi.org/10.1053/j.gastro.2014.10.009 .
Testoni PA, Testoni S, Mazzoleni G, Vailati C, Passaretti S. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc. 2015;29(9):2770–80. https://doi.org/10.1007/s00464-014-4008-6 .
Trad KS, Barnes WE, Prevou ER, Simoni G, Steffen JA, Shughoury AB, et al. The TEMPO Trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov. 2018;25(2):149–57. https://doi.org/10.1177/1553350618755214 .
Satodate H, Inoue H, Yoshida T, Usui S, Iwashita M, Fukami N, et al. Circumferential EMR of carcinoma arising in Barrett’s esophagus: case report. Gastrointest Endosc. 2003;58(2):288–92. https://doi.org/10.1067/mge.2003.361 .
Inoue H, Ito H, Ikeda H, Sato C, Sato H, Phalanusitthepha C, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol. 2014;27(4):346–51.
PubMed PubMed Central Google Scholar
Inoue H, Sumi K, Tatsuta T, Ikebuchi Y, Tuason J. 998 clinical results of antireflux mucosectomy (ARMS) for refractory Gerd. Gastrointest Endosc. 2017;85(5):AB120. https://doi.org/10.1016/j.gie.2017.03.196 Research describing experience and results of a novel technique currently used in Japan for refractory GERD. This 67 patient cohort study, expanding on an initial pilot study, shows significant decrease in reflux symptoms, endoscopic flap valve grade, percent time clearance pH, and PPI use 1 year post procedure .
Kappelle WF, Bredenoord AJ, Conchillo JM, Ruurda JP, Bouvy ND, van Berge Henegouwen MI, et al. Electrical stimulation therapy of the lower oesophageal sphincter for refractory gastro-oesophageal reflux disease - interim results of an international multicentre trial. Aliment Pharmacol Ther. 2015;42(5):614–25. https://doi.org/10.1111/apt.13306 .
Download references
Author information
Authors and affiliations.
Division of Gastroenterology and Hepatology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, 94305, USA
Yan Jiang MD & John O. Clarke MD
Redwood City, USA
John O. Clarke MD
You can also search for this author in PubMed Google Scholar
Ethics declarations
Conflict of interest.
JC is a consultant for Sanofi, Medtronic, and Isothrive; YJ has no conflicts to report.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Esophagus
Rights and permissions
Reprints and permissions
About this article
Jiang, Y., Clarke, J.O. New Developments in the Diagnosis and Management of Gastroesophageal Reflux. Curr Treat Options Gastro 18 , 69–81 (2020). https://doi.org/10.1007/s11938-020-00275-1
Download citation
Published : 19 February 2020
Issue Date : March 2020
DOI : https://doi.org/10.1007/s11938-020-00275-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gastroesophageal reflux disease
- Functional lumen imaging probe (FLIP)
Advertisement
- Find a journal
- Publish with us
- Track your research
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, trends in gastroesophageal reflux disease research: a bibliometric and visualized study.

- 1 Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2 Department of Gastroenterology, Xiyuan Hospital, China Academy of Traditional Chinese Medical Sciences, Beijing, China
- 3 Traditional Chinese Medicine Research Institute of Spleen and Stomach Diseases, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 4 National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Gastroesophageal reflux disease (GERD), a disorder resulting from the retrograde flow of gastric contents into the esophagus, affects an estimated 10–30% of the Western population, which is characterized by multifactorial pathogenesis. Over the past few decades, there have been many aspects of uncertainty regarding GERD leading to an ongoing interest in the field as reflected by a large number of publications, whose heterogeneity and variable quality may present a challenge for researchers to measure their scientific impact, identify scientific collaborations, and to grasp actively researched themes in the GERD field. Accordingly, we aim to evaluate the knowledge structure, evolution of research themes, and emerging topics of GERD research between 2012 and 2022 with the help of bibliometric approaches.
Methods: The literature focusing on GERD from 2012 to 2022 was retrieved from the Science Citation Index Expanded of the Web of Science Core Collection. The overall publication performance, the most prolific countries or regions, authors, journals and resources-, knowledge- and intellectual-networking, as well as the co-citation analysis of references and keywords, were analyzed through Microsoft Office Excel 2019, CiteSpace, and VOSviewer.
Results: A total of 8,964 publications were included in the study. The USA published the most articles (3,204, 35.74%). Mayo Clin ranked first in the number of articles published (201, 2.24%). EDOARDO SAVARINO was the most productive author (86, 0.96%). The most productive journal in this field was SURGICAL ENDOSCOPY AND OTHER INTERVENTIONAL TECHNIQUES (304, 3.39%). AMERICAN JOURNAL OF GASTROENTEROLOGY had the most co-citations (4,953, 3.30%). Keywords with the ongoing strong citation bursts were transoral incision less fundoplication, eosinophilic esophagitis, baseline impedance, and functional heartburn.
Conclusion: For the first time, we obtained deep insights into GERD research through bibliometric analysis. Findings in this study will be helpful for scholars seeking to understand essential information in this field and identify research frontiers.
Introduction
Gastroesophageal reflux disease (GERD) is a chronic, progressive, and relapsing condition, affecting up to 33% of the population worldwide ( 1 , 2 ). It is estimated that up to 40% of the US population report GERD symptoms monthly and 20% weekly ( 3 ). GERD can be divided classically into non-erosive esophageal reflux disease (NERD) and erosive esophagitis (EE) ( 3 ). GERD occurs due to abnormal reflux of gastric contents into the esophagus, provoking symptoms or complications ( 4 ). Manifestations of GERD include esophageal syndrome consisting of heartburn, regurgitation, and reflux chest pain syndrome, or non-cardiac chest pain, and potentially, extra-esophageal syndrome ( 5 ). Esophageal exposure to gastric acid can be severe enough to cause ulcer or peptic stricture, Barrett’s esophagus (BE), and even esophageal adenocarcinoma (EAC) ( 6 , 7 ). The management of GERD is complex as the clinical presentation is highly heterogeneous, and the pathophysiology involves an interplay of chemical, mechanical, psychologic, and neurologic mechanisms ( 8 ).
Although proton pump inhibitors (PPIs) are considered the mainstay of therapy for GERD and its complications, up to 40% of patients with NERD remain symptomatic while on standard therapy, and approximately 10–15% of patients with EE fail to achieve complete healing after 8 weeks of treatment ( 9 – 11 ).
In addition, GERD brings a heavy financial burden, negatively affects the quality of life, and reduces work productivity, especially in patients with severe and frequent symptoms ( 12 – 14 ). In the US alone, the financial burden is $9 to $10 billion per year in direct costs, mainly related to the use of PPIs as the first-line medication ( 2 ). GERD is also associated with 8,863,568 physician visits, 65,634 hospitalizations, and an estimated $12.3 billion in upper endoscopies in a year ( 15 ).
Despite decades of scientific work, there remain many unanswered aspects related to GERD, contributing to considerable attention from scholars. Hence, the disorder has been a subject of continued research interest, with a large body of literature published per year concerning epidemiology, etiology, pathophysiology, and therapeutics.
However, a mass of literature also represents great challenges for a researcher to identify the quality of the scientific publications, measure their scientific impact, or evaluate the research activity and scientific importance of countries, institutions, and scholars as well as gauge their scientific collaborations.
In this context, bibliometric approaches and visualization techniques are used to assess and visualize the qualitative, quantitative, and chronological aspects related to distinct areas of research ( 16 ).
The goal of the present study is to display the current status, knowledge components, research trends, and emerging topics of GERD research during the last decade by using knowledge maps. This article provides an overview of the studies and the scholarly contributions involved in this field and identifies the new developments.
Materials and methods
Source of the data and search strategy.
Data were obtained from the Science Citation Index Expanded of the Web of Science Core Collection (WOSCC) of Clarivate Analytics on a single day, February 20, 2022. The full search strategy has been presented in Supplementary material . Articles or reviews which were published in English from 2012 through 2022 were included. The bibliographic records of the retrieved publications, including titles, abstracts, authors, affiliations, keywords, source, publication year, and cited references were downloaded in plain text and imported into CiteSpace and VOSviewer for analysis.
Data analysis
CiteSpace ( 17 ), a freely available software developed by Professor Chaomei Chen from Drexel University in the USA, enjoys great popularity for visualizing the knowledge structure, distribution, and evolution of a given field. VOSviewer ( 18 ), another bibliometric software developed by Professor Nees Jan van Eck and Ludo Waltman from Leiden University in the Netherlands, has text mining capabilities to extract key elements from a large pool of scientific publications for constructing and visualizing co-authorship, co-citation, and co-occurrence network. Detailed definitions of each indicator, calculated in this study using CiteSpace and VOSviewer software are available elsewhere ( 19 – 23 ).
In the present study, CiteSpace was to (1) visualize collaborations among countries, institutions, and authors using knowledge maps; (2) perform a co-citation analysis of references; (3) generate the keyword co-occurrence cluster map; (4) depict the timeline view of co-occurring keywords; and (5) determine references and keywords with strong citation bursts. VOSviewer was applied to construct a keyword co-occurrence network. Microsoft Excel was used to demonstrate the temporal trends of publications.
Publication output
The output of research related to GERD per year from 2012 through 2021 reached a stable range, resulting in a total of 8,964 documents, including 7,550 (84.23%) articles and 1,414 (15.77%) reviews.
The number of literature annually since 2012 is shown in Figure 1 . The overall trend of publications roughly fell into two stages. In the first phase from 2012 to 2019, the annual number of articles ranging from 820 to 921, resulting in a total of 6,844 documents. In the second stage between 2019 and 2021, the annual publication output was in a period of rapid growth, with a total of 2,081 publications.
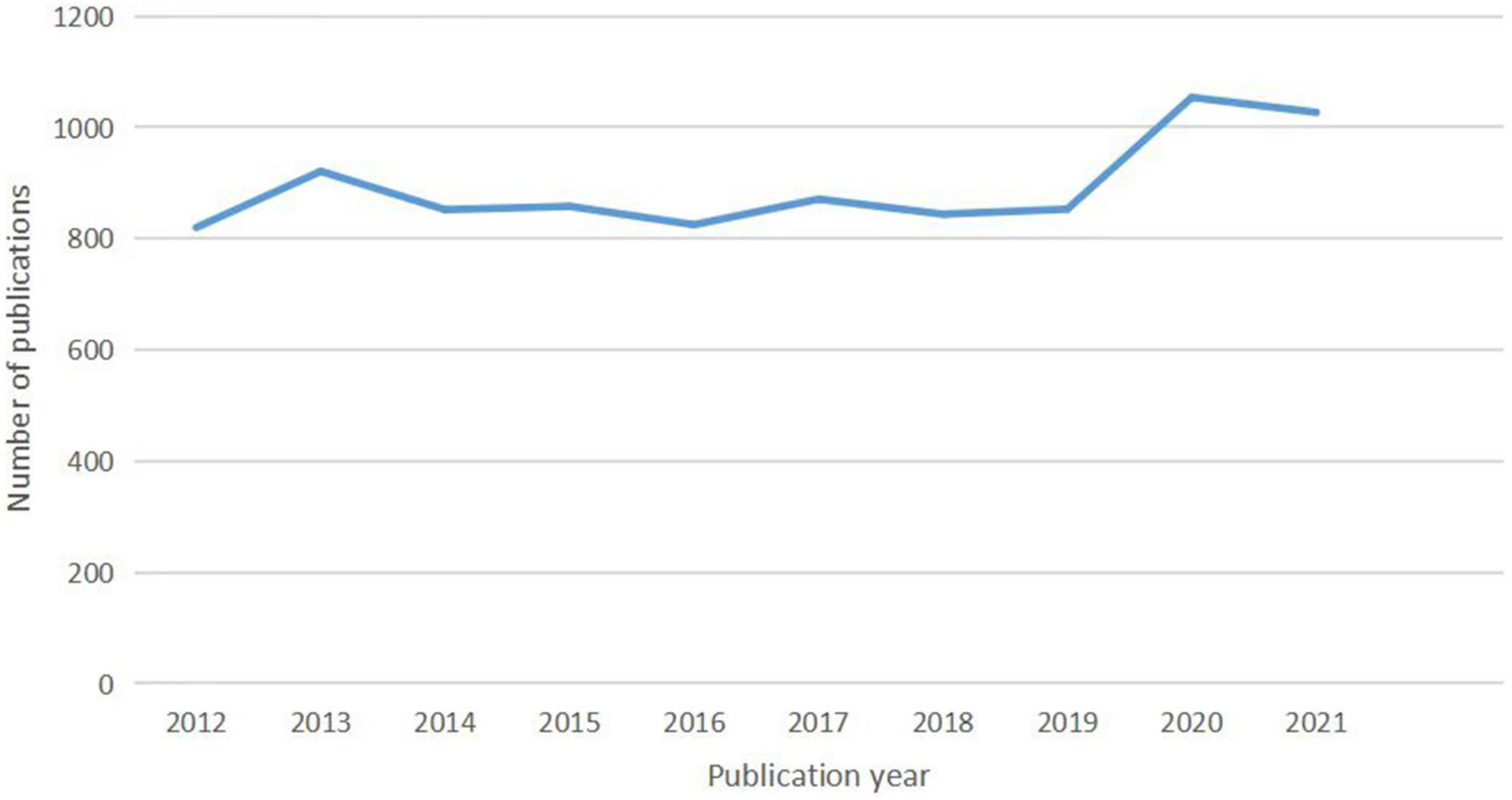
Figure 1. The number of articles published annually in GERD research.
Countries or regions and institutions analysis
GERD-related documents were published by 529 institutions from 138 countries or regions. The top 10 countries or regions and institutions are shown in Table 1 according to the publication number and the betweenness centrality. The USA was the leading producer of publications with 3,204 (35.74%) documents. Concerning productive institutions, Mayo Clin was the leader in publishing documents at 201 (2.24%).
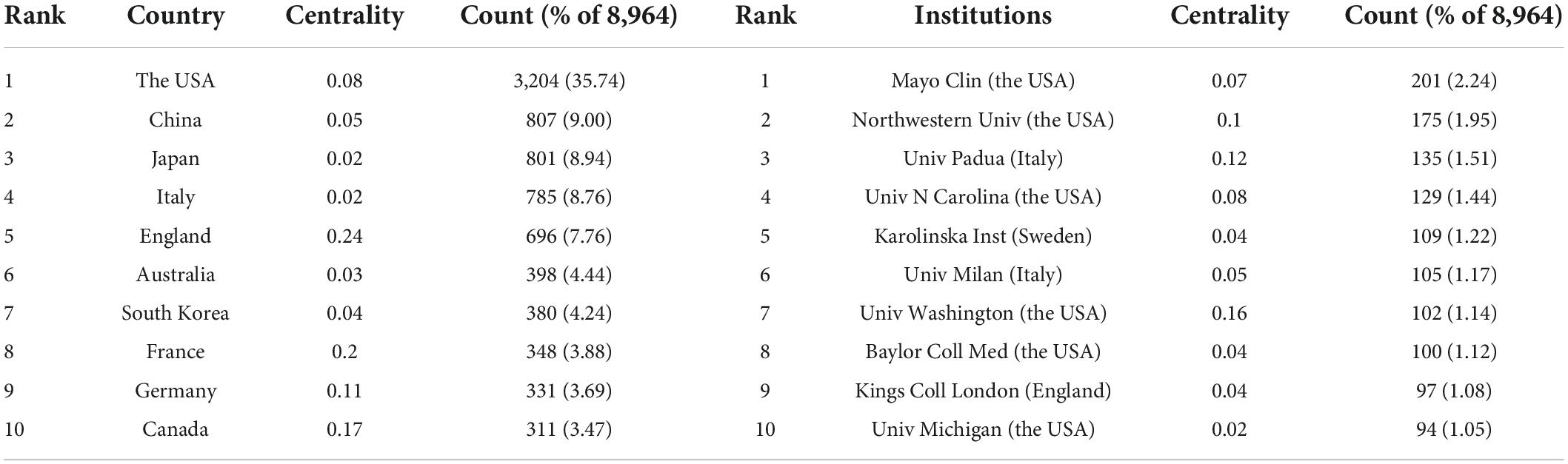
Table 1. The top 10 countries or regions and institutions involved in GERD research.
In general, an article with authors from more than one country or institution denotes possible scientific partnerships ( 24 ). The visualization map of cooperation among countries or regions is presented in Figure 2 . England, France, Canada, Germany, and Sweden, which were highlighted with purple rims had extensive ties and played a vital role in each of their partnerships.
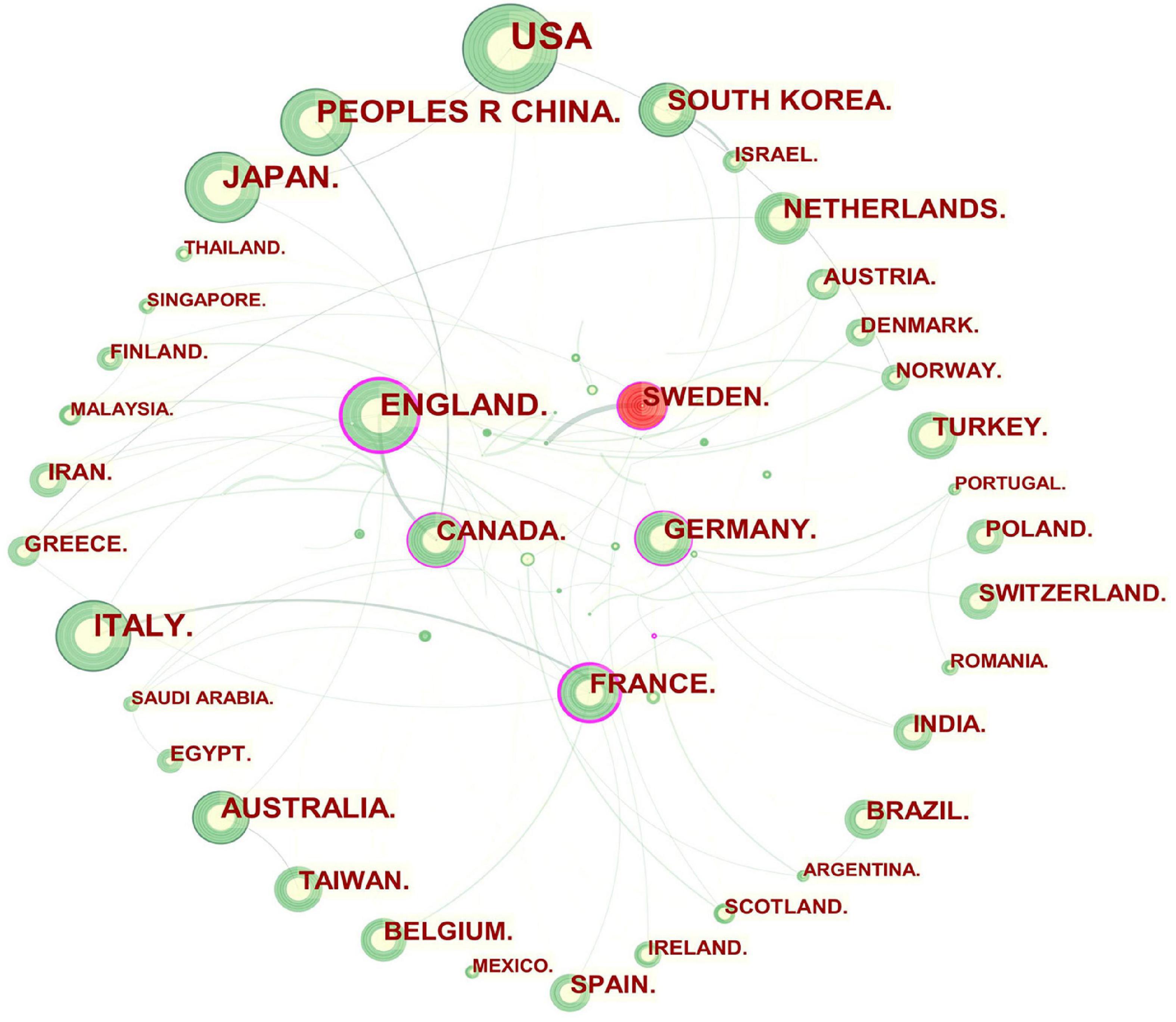
Figure 2. Network of countries and regions engaged in GERD research. In the network map, a node represents a country or region. The larger the area of the node is, the larger the number of publications. The thicker the curved line connecting nodes indicates the frequency with which they co-occur, as they indicate collaborative relationships. An isolated node without any connection is devoid of all collaboration. A node with a high betweenness centrality links two or more large groups of nodes. A node with a high betweenness centrality score exerts a strong influence on the network. A purple trim indicates a high degree of betweenness centrality. Red tree rings indicate bursts of citation. The greater the thickness of the red tree rings, the greater the bursts for the corresponding node.
The collaboration network of institutions is shown in Figure 3 . The landmark nodes included Univ Padua and Univ Washington. They might be seen as the linking bridge between North American and European groups of collaborators, respectively.
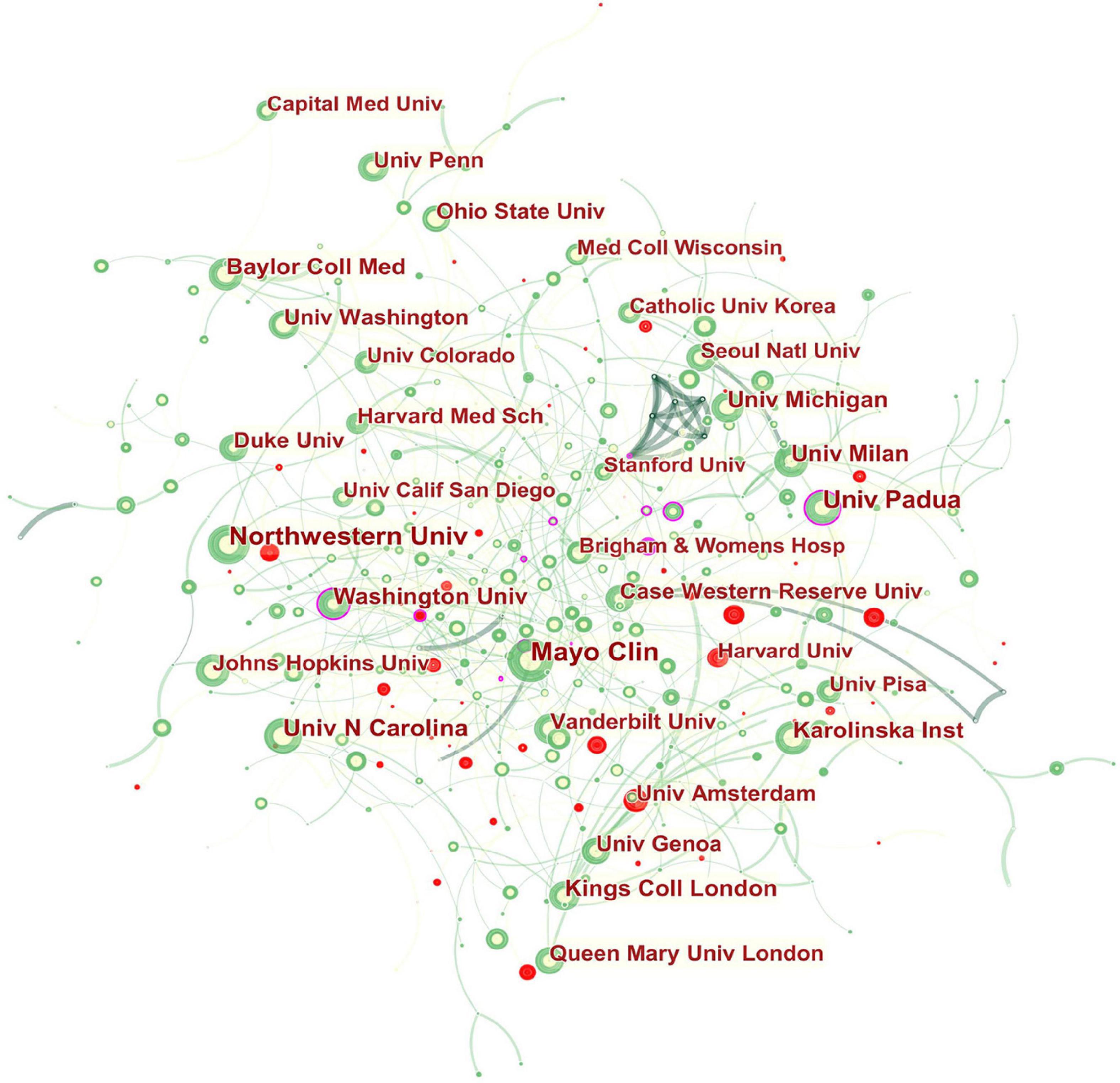
Figure 3. Network of institutions engaged in GERD research. In the network map, a node represents an institution. The volume of each node (institution) corresponds to the number of publications, and connecting lines between nodes indicate bidirectional relationships between the institutions; the thickness of the line indicates the strength of the bidirectional collaborative relationships. Isolated institutions lack any collaboration. A node with a high betweenness centrality links two or more large groups of nodes. A node with a high betweenness centrality score exerts a strong influence on the network. A purple trim indicates a high betweenness centrality. Red tree rings indicate bursts of citation. The greater the thickness of the red tree rings, the greater the bursts for the corresponding node.
A total of 452 authors were included in the GERD studies. The top 10 active researchers are presented in Table 2 . Among them, researchers from institutions in the USA authored most of the GERD literature. EDOARDO SAVARINO occupied the first position with 86 (0.96%) documents.
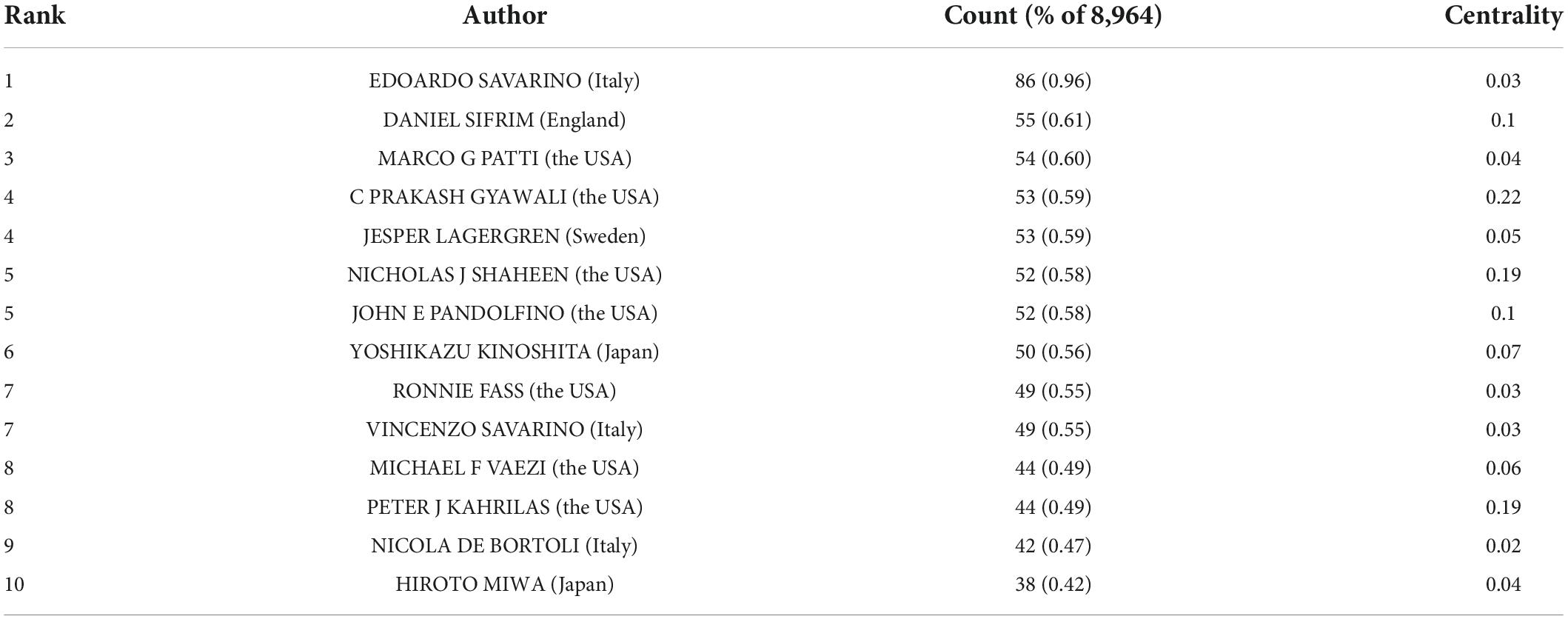
Table 2. The top 10 authors in GERD research.
According to Figure 4 , the networks of partnerships are centered on DANIEL SIFRIM, C PRAKASH GYAWALI, NICHOLAS J SHAHEEN, JOHN E PANDOLFINO, PETER J KAHRILAS, MOTOYASU KUSANO, JOHN C LIPHAM, DAVID A KATZKA, THOMAS L VAUGHAN, DOUGLAS A CORLEY, and FRANK ZERBIB, suggesting they are most likely to initiate collaborative relationships and provide support for funding and resources in their respective communities.
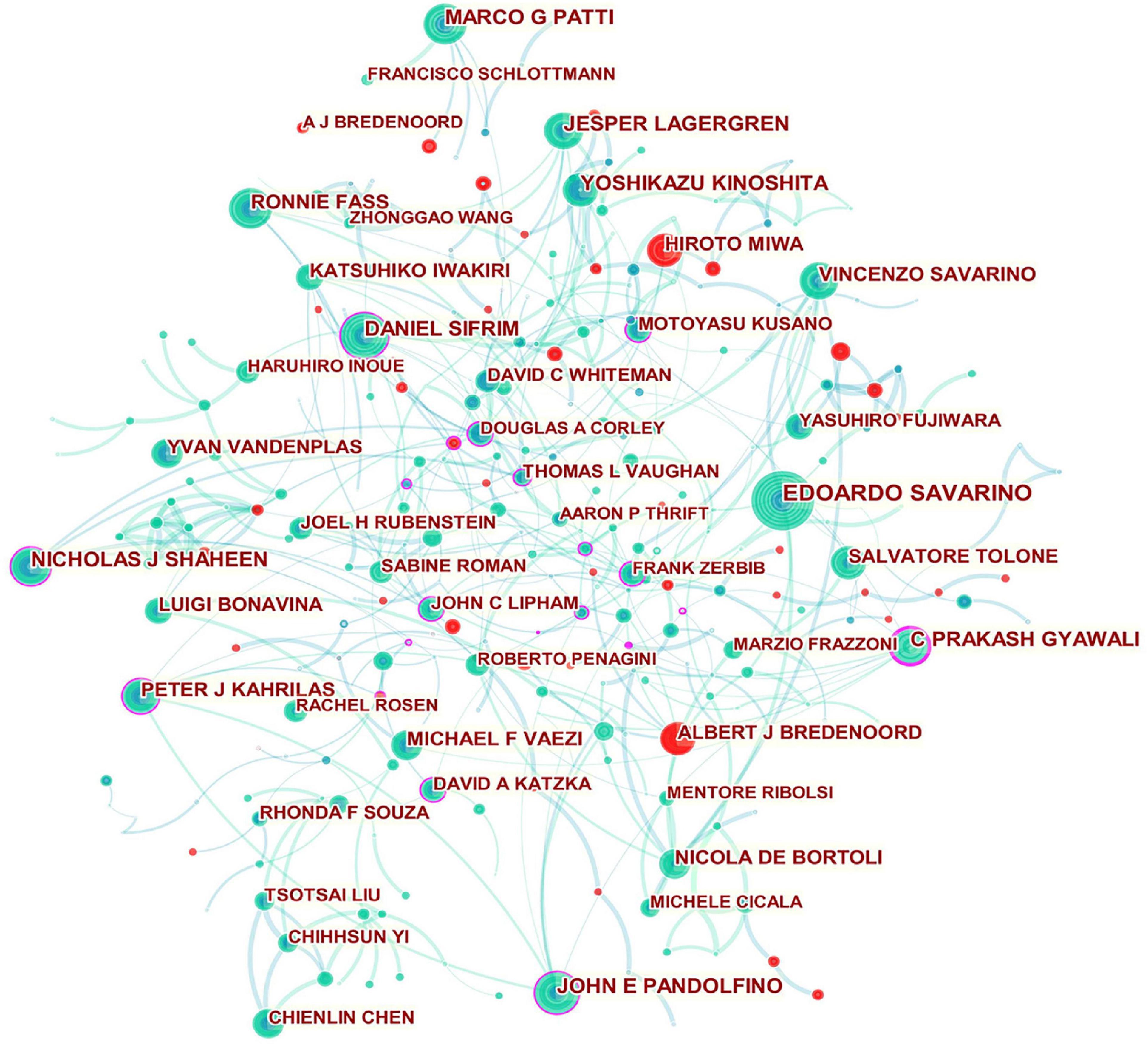
Figure 4. Network of authors in GERD research. In the network map, a node represents an author. The volume of each node (author) corresponds to the number of publications, and connecting lines between nodes indicate bidirectional relationships between the authors; the thickness of the line indicates the strength of the bidirectional collaborative relationships. Isolated authors lack any collaboration. A node with a high betweenness centrality links two or more large groups of nodes. A node with a high betweenness centrality score exerts a strong influence on the network. A purple trim indicates a high betweenness centrality. Red tree rings indicate bursts of citation. The greater the thickness of the red tree rings, the greater the bursts for the corresponding node.
Journals and co-cited academic journals
There were 1,524 academic journals publishing articles related to GERD research. The top 10 journal outlets with high publication output are shown in Supplementary Table 1 . Of these, SURGICAL ENDOSCOPY AND OTHER INTERVENTIONAL TECHNIQUES had the highest output (304, 3.39%).
In 1973, co-citation in the scientific literature was proposed by Marshakova I and Small H, which refers to the relationship between two documents that appear simultaneously in the bibliography of a third citing document ( 25 , 26 ). Journal co-citation refers to the frequency with which any documents of two journals are cited together by the documents of other journals ( 27 ). A total of 1,244 co-cited scholarly journals published articles on GERD research. As shown in Supplementary Table 1 , AMERICAN JOURNAL OF GASTROENTEROLOGY had the most co-citations (4,953, 3.30%).

Co-cited references and references with citation bursts
Supplementary Table 2 presents the top 10 co-cited articles. These documents which were viewed as the knowledge base of GERD research deal primarily with the epidemiology of GERD, guidelines and consensus on the diagnosis and treatment of GERD, classification of esophageal motility diseases, and differential diagnoses for GERD [e.g., the functional esophageal disorders and eosinophilic esophagitis (EoE)].
As shown in Supplementary Table 3 , the top co-cited references with the highest betweenness centrality were published from 2014 to 2020. They are considered to be key components in the intellectual base.
Burst detection is applied to detect publications or keywords that had a surge of their occurrences or citations, thus allowing for identifying subjects that have received intensive attention during a specific period ( 28 ). A citation burst has two attributes: strength and duration ( 29 ). As shown in Supplementary Figure 1 , 25 references with the strongest citation bursts reflected research topics that received much attention over time. The document entitled “ Modern diagnosis of GERD: the Lyon Consensus ” written by Gyawali et al. ( 30 ) has received the strongest burst. Eleven articles ( 2 , 30 – 39 ) still hold busts and their topics are considered research fronts in the field of GERD.
Keywords analysis
Keyword co-occurrence.
Essentially, a keyword describes the subject of a particular document succinctly and accurately and is used for indexing and cataloging purposes ( 40 ). Another prevalent way to track research topics in bibliometrics is keyword co-occurrence analysis ( 41 ). Keyword co-occurrence is based on the relevance of keywords which is determined by the number of documents in which they occur together. That is, when two keywords that reflect the research theme of an article appear in the same document, it is considered that there exist co-occurring relationships between the two terms. The higher the number of co-occurrences of two terms, the closer their relationship is.
A map of keyword co-occurrence is generated based on the frequency of occurrence of paired keywords. The visualization map of keyword co-occurrence by VOSviewer is shown in Figure 5 . Each bubble represents a keyword. Bubble size indicates the frequency of occurrence of a keyword in publications, while the color of the bubble specifies the cluster to which it belongs. There were six clusters of keywords. Each cluster represents a distinct area of GERD research.
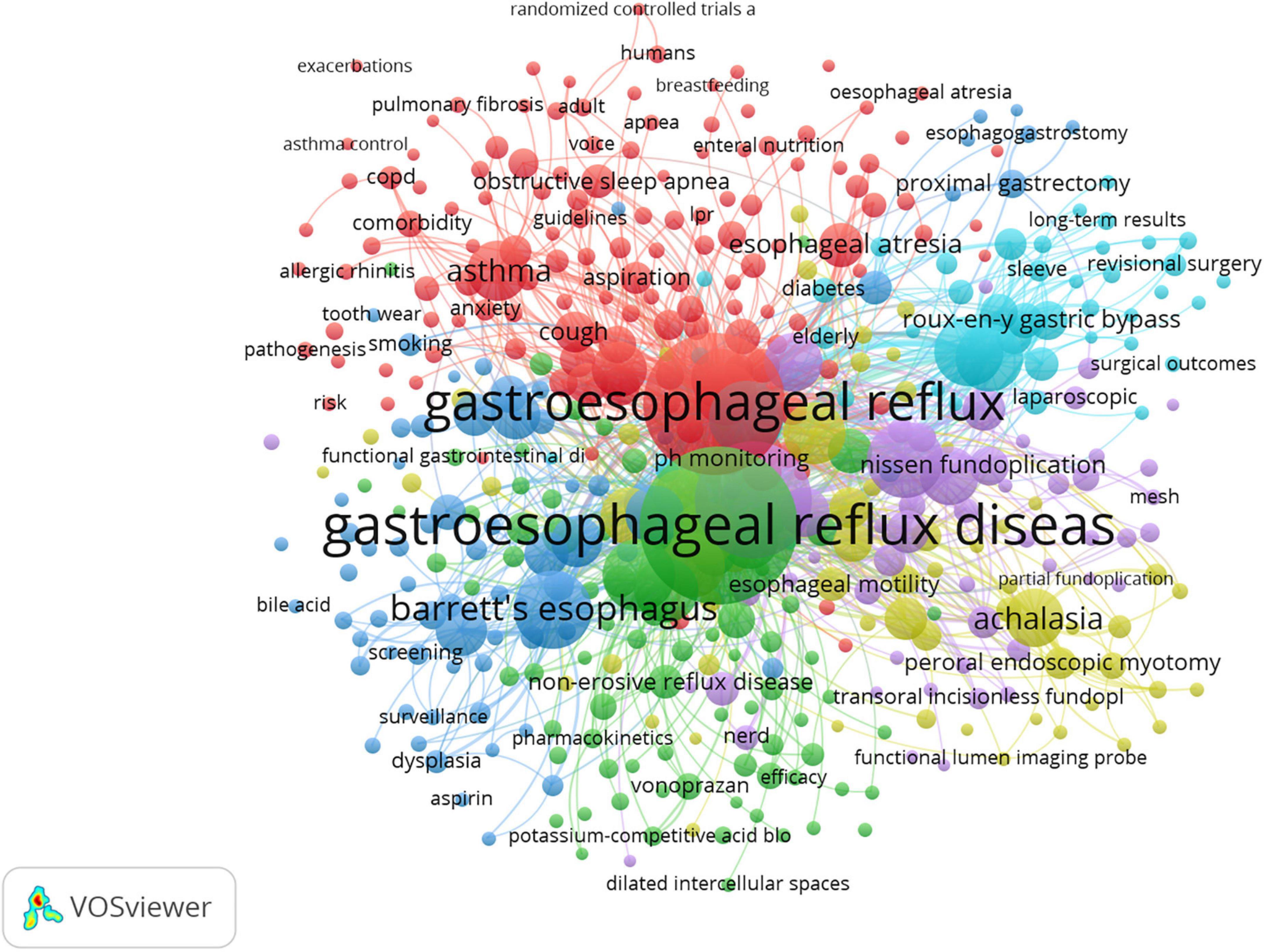
Figure 5. Map of keyword clustering with a minimum of 10 occurrences in GERD research. Minimum number of occurrence of a keyword = 10, minimum links strength = 10. There are 6 clusters of keywords.
Cluster 1 (Yellow): The cluster demonstrated conditions mimicking GERD, particularly in regard to achalasia. Achalasia is considered the most studied and best-described primary motility disorder of the esophagus, characterized by abnormal relaxation of the lower esophageal sphincter (LES) and the absence of peristalsis. Swallowing rather than peristalsis causes simultaneous pressure waves in the esophagus (pressurization) that result in esophageal emptying in patients with this disorder, though the emptying is incomplete in these patients. In cases of advanced achalasia (types 1 and 2), the myenteric ganglia are fully replaced by fibrous tissue. Type 3 (spastic) achalasia is characterized by ganglia surrounded by chronic inflammatory cells, T (primarily), and B lymphocytes.
Patients with achalasia commonly complain of heartburn which may be mistaken for GERD ( 42 ). The cause of heartburn in achalasia is not fully understood. Perhaps the sensation occurs due to abnormal esophageal motor activity, or perhaps acid refluxes into the esophagus when the LES finally relaxes. It has been observed that patients with achalasia exhibit abnormal esophageal acid exposure, although it is still unclear whether the acid is hydrochloric acid refluxed from the stomach or lactic acid produced by carbohydrate fermentation in the esophagus after retention ( 43 ). Achalasia patients may experience heartburn resistant to PPI treatment, a condition which has occasionally led to Nissen fundoplications being performed to treat heartburn misattributed to GERD. In this context, Nissen fundoplication can result in debilitating dysphagia ( 44 ).
It is estimated that achalasia spectrum disorders are identified in 1–2.5% of patients who undergo esophageal manometry prior to anti-reflux surgery ( 45 ), and this test should be considered if esophageal symptoms fail to improve after acid suppressive therapy. To prevent this potentially catastrophic mistake, a preoperative esophageal manometry is recommended for patients considering fundoplication as a treatment for GERD ( 46 ).
Cluster 2 (Purple): This cluster is centered on anti-reflux surgery. The first fundoplication for EE was performed by Dr. Rudolf Nissen in 1955, which was then published in 1956 ( 47 , 48 ). Following an initial period of success and widespread adoption, this revolutionary procedure has begun to experience significant declines owing primarily to high complications ( 48 , 49 ). With the introduction of PPIs in the 1980s, patients who did not receive adequate symptomatic relief or had advanced esophagitis despite optimal medical treatment were only considered for traditional open surgery ( 49 , 50 ). In 1991, Dallemagne et al. ( 50 ) reported their first experience with a laparoscopic Nissen fundoplication (LNF) and noted symptomatic improvement and no mortality from the procedure. The laparoscopic anti-reflux surgery (LARS), which includes LNF and partial wraps, has resulted in significantly less morbidity and mortality and a shorter recovery period than the open approach ( 51 ). It has since become the preferred procedure among patients with complications resulting from GERD. Additionally, this may be a more cost-effective procedure for those in the younger age group.
Despite the initial report by Dallemagne et al. ( 50 ) indicating no mortality, it is notable that there are serious adverse events, which may include mortality. In the LOTUS trial, which is a randomized, open, parallel-group trial conducted in Europe, it was noted that, at 5 years, although heartburn and regurgitation were better controlled in the LARS group, a higher prevalence of dysphagia, bloating and flatulence were observed in the LARS group than those in the esomeprazole group ( 52 ). In addition, there was a 3% in-hospital mortality rate and a 26.8% rate of serious adverse events in the LARS group ( 52 ). Consequently, laparoscopic surgery is underutilized due to its perceived side effects, generally indicated for patients with long-standing severe diseases and large hiatal hernias. In light of such experience and results, further investigation into procedural strategies that minimize post-operative side effects while maximizing LARS therapeutic benefits continues.
The results of a landmark prospective study by Spechler SJ et al. ( 53 ) demonstrated the benefit of surgical treatment in patients with reflux hypersensitivity (RH). LNF or medical therapy was administered to patients with refractory heartburn who had RH or pathological acid reflux on esophageal function testing. Reflux surgery proved superior to medical therapy; 71% of patients with RH had success, compared to 62% of patients with abnormal acid reflux ( 53 ). This study demonstrates that RH considered a functional esophageal disorder until recently, responds to fundoplication. Yet one-third of the patients in the surgical arm did not respond, and serious risks of fundoplication need to be carefully evaluated against the benefits for each patient.
Cluster 3 (Light blue): With its emphasis on laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB), this cluster has primarily focused on bariatric surgery. Despite the fact that weight loss after bariatric surgery improves GERD symptoms, the degree of symptom relief varies according to the type of procedure. There was evidence to suggest that patients undergoing LRYGB, adjustable gastric band, and LSG all showed significant relief in GERD symptom scores after 6 months of follow-up ( 54 ). A maximum GERD score improvement occurred in patients with LRYGB, followed by an adjustable gastric band and LSG ( 54 ). Prachand et al. ( 55 ) reported that GERD resolution was still higher after RYGB (76.9%) compared to biliopancreatic diversion and duodenal switch (48.6%) in the super-obese patients, even though the latter procedure offered better weight loss, better control of diabetes mellitus, hypertension, and hyperlipidemia.
The Swiss Multicenter Bypass or Sleeve Study (SM-BOSS) reported that GERD remission was greater after RYGB, at 60.4% than LSG, at 25% after 5 years ( 34 ). Further, GERD symptoms worsened in 31.8% of patients with LSG compared to 6.3% with RYGB ( 34 ). De novo GERD developed in 31.6% of LSG patients after 5 years, versus 10.7% of patients with RYGB ( 34 ). The results indicated that GERD symptoms were more prevalent after LSG compared to RYGB.
Because of the LSG’s robust weight loss effect and simple surgical procedure, it has grown increasingly popular among the bariatric community ( 56 ). However, the development of de novo GERD following LSG has recently gained attention, which is estimated to occur in 73% of patients ( 57 , 58 ). Compared to other bariatric procedures like LRYGB, LSG has been estimated to increase the risk of developing de novo GERD by five times ( 59 ). An increased risk of GERD can also result in adverse consequences, such as BE and perhaps even adenocarcinoma ( 60 , 61 ). Some mechanisms are proposed to account for de novo GERD development, such as altered anatomy of the gastroesophageal junction, disruption of the sling fibers, altered LES function, narrowing of the stomach, dilation of the fundus, increased intragastric pressure, and the concomitant presence of a hiatal hernia ( 62 – 65 ).
Cluster 4 (Dark blue): This cluster mainly pertains to BE. In response to gastroesophageal reflux (GER), BE develops from an acquired metaplastic epithelial change in the esophagus. Based on a systematic review and meta-analysis of 19 studies involving 43,017 subjects, GER symptoms are significantly associated with a higher risk of endoscopically suspected and histologically confirmed BE ( 66 ). This risk increases further when patients exhibit weekly symptoms ( 66 ).
In fact, up to half of the patients with BE do not complain of reflux symptoms, although the magnitude of the risk for BE and EAC has been reported to be higher in patients with prolonged symptoms of GER and a younger age of reflux onset ( 67 , 68 ). Additionally, symptom relief is a poor indicator of acid control, with pH normalization seen in 85% of patients on PPIs ( 67 ). There has been evidence that acid reflux can cause inflammation in the distal esophagus via activation of cyclooxygenase-2 (COX-2), c-myc, and mitogen-activated protein kinase ( 69 , 70 ). Ex vivo studies have demonstrated the over-expression of COX-2 in Barrett’s epithelium and in the EAC ( 69 , 70 ).
In addition, recent research has shown indirect effects of acid on the epithelium of the esophagus. Some pro-inflammatory mediators have been implicated in animal and human models, such as interleukin (IL)-8, platelet-activating factor, and interferon-γ ( 71 – 73 ). In response to the release of these mediators, immune cells are recruited to the esophageal mucosa, leading to a cascade of inflammatory pathways that produce reactive oxygen species (ROS) and further cell injury ( 71 – 73 ). Research has demonstrated that acidic media induces pro-proliferative and anti-apoptotic effects in esophageal cells ( 74 ).
It has been proposed that bile reflux or duodenogastric reflux also contributes to BE with bile acids in the refluxed material of patients with BE. Bile acids’ effects on the mucosa of the esophagus have been linked to both cytotoxic mechanisms and the activation of proto-oncogene and c-myc, which contribute to inflammation-related carcinogenesis ( 75 ). Bile acids, as detergent molecules, have the potential to solubilize cell membranes, but their ability to penetrate cell membranes depends on being neutralized and therefore lipophilic. When exposed to acidic pH, bile acids become non-ionized, enter cells, and cause mucosal injury and inflammation ( 67 , 76 ). The conjugation of bile acid with gastric acid increases the viability of acid hydrolase, destroys lysosomal membranes, and leads to reverse hydrogen ion diffusion ( 77 ). Additionally, previous studies have suggested that acid and duodenogastric reflux are synergistic, with the latter contributing to mucosal damage rather than reflux symptoms ( 77 ).
Bile acids may trigger the release of inflammatory mediators, which may contribute to the development of BE, as well as carcinogenesis in patients with BE. This may involve an increase in levels of IL-6, IL-8, COX-2, and tumor necrosis factor-α, along with the recruitment of inflammatory cells ( 78 ). The increased pro-inflammatory cytokines and cells were not observed in an acid-only cohort, indicating that bile acids directly contribute to esophageal damage ( 78 ). Moreover, there is evidence that bile acids, when present in an acidic environment, may induce the release of ROS that can, in turn, cause oxidative stress, which leads to DNA damage, and increases the risk of cellular metaplasia ( 79 , 80 ).
The esophagus is bathed in up to 3 mg/ml pepsin (up to 73 times per day) during chronic GERD that persists for years. A study by Samuels et al. ( 81 ) established the expression of pepsinogen mRNA in BE mucosa; acute non-acid pepsin, on the other hand, was able to induce pro-inflammatory mechanisms and cell migration in esophageal cells in vitro . Furthermore, mitochondrial cristae degeneration and mitochondrial dysfunction are also associated with non-acid pepsin-mediated injuries and may be caused by the suppression of B cell lymphoma-2 family proteins involved in the maintenance of mitochondrial structure and homeostasis ( 82 – 85 ). Mitochondrial dysfunction is characterized by ROS causing mitochondrial DNA damage, a common event during chronic inflammation, and is associated with the metaplastic and preneoplastic responses including BE ( 86 , 87 ). As reported in a study by Samuels et al. ( 88 ), chronic (2–4 weeks) exposure to pepsin induced the production of IL-8, a neutrophil chemoattractant and a trigger for cellular proliferation and angiogenesis and, therefore, facilitates inflammation-mediated tumor initiation. In patients with GERD, IL-8 levels are elevated and are highest in patients with BE and EAC; following anti-reflux surgery, IL-8 levels are reduced in patients with BE ( 89 ). Chronic pepsin treatment also results in a shift from the normal esophageal cytokeratin profile of KRT10 high and KRT8 low to the BE-type cytokeratin profile of KRT10 low and KRT8 high ( 88 , 90 ).
Pepsin is erosive to esophageal tissue when it is present in acid reflux. When weak and non-acidic reflux occurs, as seen in patients taking PPIs, the enzyme activity of pepsin is temporarily inhibited, allowing interaction with an unidentified receptor, endocytosis, and retention in acidic intracellular vesicles where its activity can be restored ( 82 , 83 ). Mechanistically, in BE, there are diverse cell types including the oxyntocardiac mucosa, which contains parietal cells that secrete gastric acid, and chief cells that secrete digestive enzymes ( 91 ). Oxyntocardiac mucosa is a precursor to intestinal metaplasia and EAC ( 92 ) and often occurs in the context of EAC ( 93 ). Further, in GERD-associated metaplasia and dysplasia, pepsinogen and proton pump mRNA were exclusively expressed; no expression was observed in the non-cancerous esophagus and squamous cell carcinomas are not associated with GERD ( 88 ). In this manner, there is a hypothesis that the refluxed or locally produced acid may facilitate the conversion of locally synthesized pepsinogen to active pepsin and reactivate intracellularly deposited refluxed pepsin, which might account for the association of GERD with metaplastic changes and neoplastic lesions ( 88 ).
Cluster 5 (Red cluster): With “asthma,” “cough,” and “obstructive sleep apnea-hypopnea syndrome,” GERD-induced respiratory disease is the focus of this cluster. Taking asthma as an example, its frequent coexistence with GERD raises the possibility that these diseases may have shared mechanisms.
The prevalence of asthma among GERD populations has been compared in many studies, with results showing a modest or no correlation ( 3 , 94 , 95 ). As reported in a systematic review involving 28 studies, the average prevalence of asthma among patients with GERD was 4.6% (compared to 3.9% among controls), with an overall ratio of 2.3 [95% confidence interval (CI): 1.8–2.8] ( 96 ). Even if the evidence indicates an increased prevalence of asthma among patients with GERD, most studies were cross-sectional or case-control studies; therefore, the causal direction of the association could not be determined ( 96 ).
Studies have also examined the relationship between GERD and asthma severity and exacerbation; GERD is clearly associated with asthma exacerbations. For example, a significant correlation has been reported between the presence of reflux disease and an increase in exacerbations [odds ratio (OR) = 4.9; 95% CI: 1.4–17.8] and hospitalizations in asthmatic patients ( 97 , 98 ). Further evidence for this association was presented in a recent meta-analysis involving 32 studies of 1,612,361 patients of all ages ( 99 ). The study found that GERD was associated with asthma exacerbations (OR = 1.27; 95% CI: 1.18–1.35) and exacerbations requiring oral corticosteroid therapy (OR = 1.24; 95% CI: 1.09–1.41) ( 99 ).
There are two main mechanisms involved in GERD associated with asthma severity and exacerbations, which include (1) aspiration of gastric contents directly affecting pulmonary parenchyma and aspiration of contents up to the pharynx causing the symptoms by stimulating irritant receptors ( 100 , 101 ) and (2) gastric contents reflux into the lower esophagus, which may cause symptoms by increasing bronchial reactivity, or by causing bronchoconstriction as a result of the vagal nerve stimulation ( 102 , 103 ). Furthermore, asthmatics often experience hyperinflation, and with hyperinflation, there is an increase in intrathoracic pressure as a result of an increased lung capacity, which may induce descent of the diaphragm in this setting, increasing the gradient between the abdomen and the chest, leading to herniation of the LES into the chest and thus affecting LES tone ( 104 ). Bronchodilators used for asthma can also relax the LES, causing gastric contents to reflux. Therefore, asthma and GERD seem to be closely related physiologically, as asthma may cause GER and GERD may aggravate asthma symptoms, although the causal relationships are not well understood and demonstrated.
Cluster 6 (Green cluster): T his cluster focused on potassium-competitive acid blockers (P-CAB) in GERD, particularly in relation to NERD, the most common type.
Currently, P-CABs are primarily approved in Asia, and they were thought to be an attractive option for patients with NERD due to their quick onset of action on gastric acid secretion. Vonoprazan (TAK-438) is the most extensively studied of this new class of acid suppressants, which has been available to the Japanese market since 2015 ( 105 ). According to the first randomized, double-blind, placebo-controlled, multicenter study conducted in patients with NERD with no mucosal changes (Grade N) or minimal mucosal changes, such as mucosal redness, or turbidity (Grade M) and recurrent acid reflux symptoms, the number of heartburn-free days with vonoprazan (10 or 20 mg daily) was not superior to placebo, even though it was found that both doses of vonoprazan significantly decreased the mean severity of heartburn ( 106 ). As the results of the first trial were close to showing a significant difference, another phase III trial using vonoprazan 10 mg in NERD patients was conducted ( 107 ). The proportion of days without heartburn was not significantly different between the vonoprazan and placebo groups among patients with NERD in the full analysis ( 107 ). In the per-protocol-set analysis, however, the complete heartburn resolution in the fourth week of treatment was significantly higher in the vonoprazan group compared with the placebo group ( p = 0.0023) ( 107 ). Therefore, it remains surprising that vonoprazan failed to achieve superiority in the primary efficacy outcome compared with placebo and therefore failed to receive approval for the NERD indication.
These low response rates suggest that other factors are responsible for symptom generation in this group of patients. The problem has been noted because NERD patients have been indicated as having inferior treatment outcomes with PPIs compared with EE patients ( 108 ). The partial response seen with vonoprazan monotherapy can be attributed to weakly acidic reflux or functional heartburn (FH), according to Kawami N et al. ( 109 ) and Abe et al. ( 110 ).
In spite of these results, the drug appears to be effective in the treatment of PPI-resistant NERD. A small retrospective study found that 69.2% of PPI-resistant GERD patients reported an improvement in self-reported GER symptoms and quality of life measured by GERD-Q score following their medication from a PPI to vonoprazan ( 111 ). Similarly, vonoprazan was reported to be effective in relieving the GER symptoms in patients with PPI-resistant NERD by Shinozaki et al. ( 112 ). Accordingly, these studies do not eliminate the possibility that P-CAB might be useful in NERD; however, larger randomized controlled trials are required to confirm these findings.
Tegoprazan (CJ-12420) is a benzimidazole derivative, approved in 2018 for treating gastric ulcers and GERD in South Korea. In South Korea, tegoprazan has been approved as the first P-CAB for NERD. According to the study by Kim et al. ( 113 ), which assessed complete relief of heartburn and regurgitation in patients with NERD following 4-week tegoprazan therapy; 42.5, 48.5, and 24.2% of patients had completely resolved major symptoms receiving tegoprazan 50 mg, tegoprazan 100 mg, and placebo, respectively. Both tegoprazan groups experienced higher rates of complete heartburn resolution and heartburn-free days than the placebo group ( p < 0.05 for all) ( 113 ). The authors concluded that tegoprazan relieved NERD symptoms in an effective and sustained manner, thus providing a viable therapeutic option for the treatment of NERD ( 113 ). In this study, patients with NERD were defined as those who frequently experienced heartburn and regurgitation and had a normal endoscopy results. However, the clinical relevance of this definition is still open to question today given its heterogeneity. Despite statistically significant improvement over placebo, there appears to be no clinically significant advantage over PPIs ( 114 , 115 ), although no PPIs were compared with this drug. Therefore, unless negative endoscopy with positive 24-h-pH testing was used to define confirmed NERD, it remained unclear how much better tegoprazan and other PCABs may be at reducing GER symptoms. Furthermore, large-scale, prospective, randomized controlled studies using PPI as a comparator may further inform research on symptomatic control in patients with NERD.
Figure 6 shows the timeline view of keyword co-occurrence, from which the evolution of research topics can be examined with time.
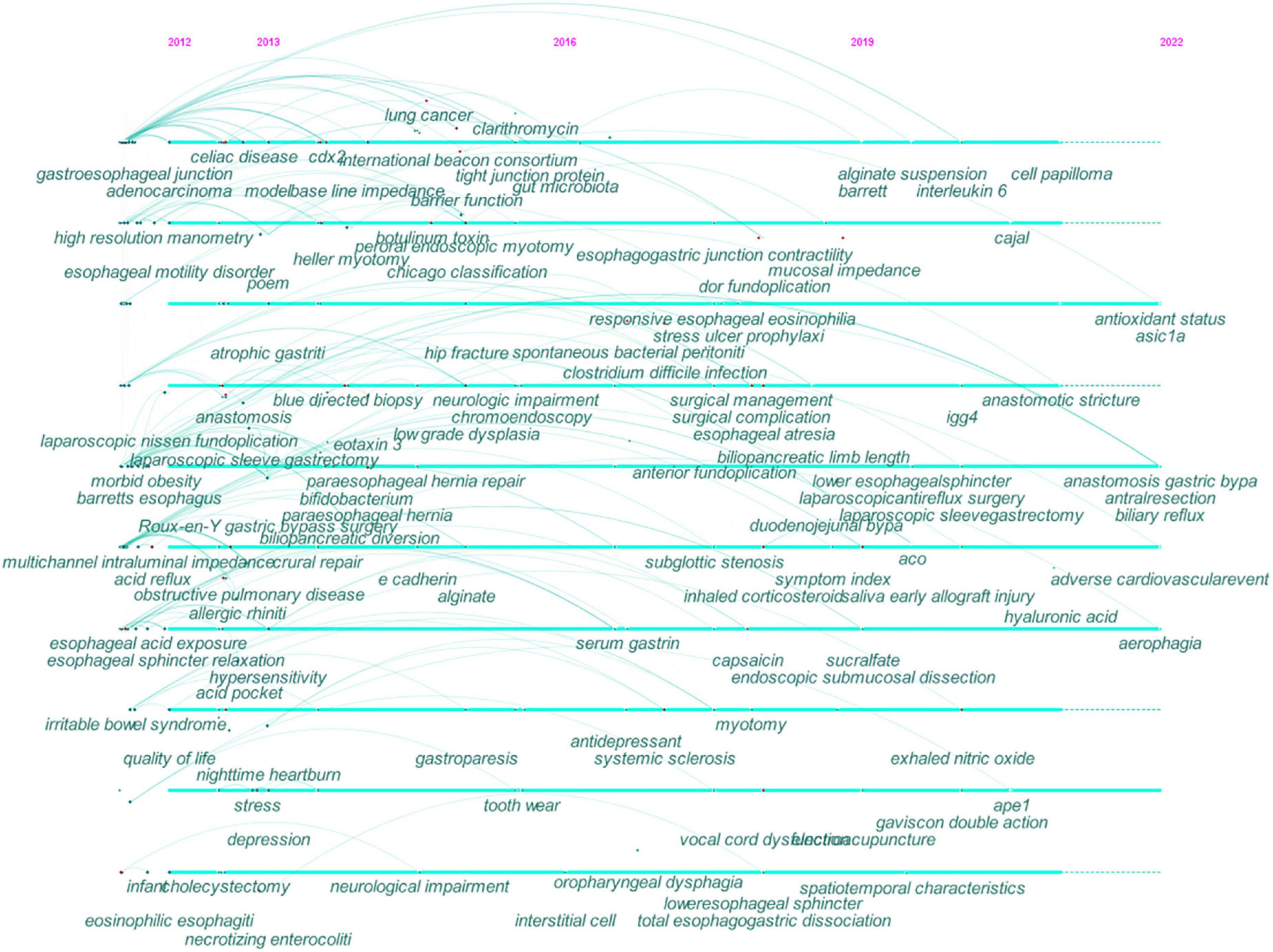
Figure 6. Timeline view of co-occurring keywords map in GERD research. The year placed at the top of the view corresponds to the earliest year when each keyword appeared. Each node represents a keyword. The links represent the co-occurrence of keywords and the colors represent the average year of publication for each node. Each cross corresponds to the bursts of keyword co-occurrence.
During the early years from 2012 to 2016, GERD research began to focus on (1) EoE, BE, EAC, and esophageal motility disorders; (2) morbid obesity, stress, and depression; (3) nighttime heartburn; (4) gastroparesis, celiac disease, and irritable bowel syndrome; (5) chronic obstructive pulmonary disease and allergic rhinitis, (6) high-resolution manometry, multichannel intraluminal impedance-pH monitoring, and baseline impedance; (7) botulinum toxin and Bifidobacterium ; (8) alginate; (9) RYGB and LSG; (10) Heller myotomy, per-oral endoscopic myotomy, LNF, and para-esophageal hiatal hernia repair; (11) esophageal sphincter relaxation, esophageal hypersensitivity, and acid pocket; (12) caudal-type homeobox transcription factor 2, eotaxin-3, and E-cadherin.
From 2016 to 2019, research in this field focused on (1) hip fracture, Clostridium difficile infection, and spontaneous bacterial peritonitis; (2) systemic sclerosis; (3) subglottic stenosis; (4) antidepressant; (5) Toupet fundoplication; (6) capsaicin; (7) interstitial cell.
Recent research trends from 2019 to 2022 included (1) IgG4-related disease, aerophagia, and early allograft injury; (2) the symptom index and exhaled nitric oxide; (3) biliary reflux; (4) adverse cardiovascular event; (5) Gaviscon double action; (6) hyaluronic acid; (7) antioxidant status; (8) acid-sensing ion channel 1a; (9) apyrimidinic endonuclease I and IL-6;
Burstness of keywords
The strength and duration of the 25 keywords with the strongest citation bursts are shown in Figure 7 . The most intense one is “transoral incisionless fundoplication (TIF)” (30.8), followed by “EOE” (14.76), “baseline impedance” (12.71), and “functional heartburn” (11.22). In addition, these keywords had ongoing bursts, representing the research frontiers in this field.
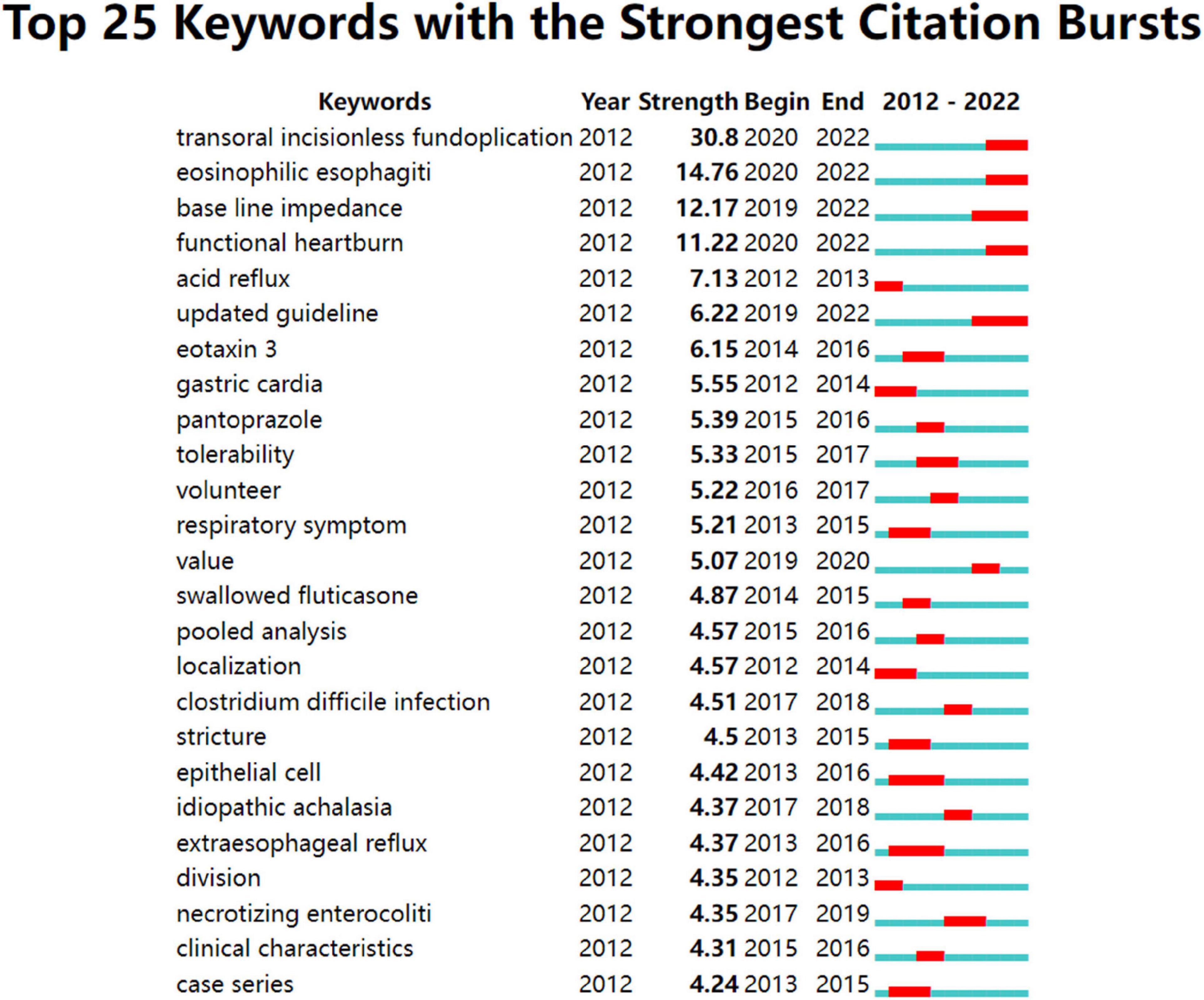
Figure 7. Top 25 keywords with strong citation bursts in GERD research. A blue bar represents the period from 2012 to 2022, whereas red line segments represent the time slices during which keyword bursts occur, i.e., rapid increases in citation counts.
General information
As shown in Table 1 and Figure 2 , Europe (Italy, England, France, and Germany), Asia (China, Japan, and South Korea), North America (the USA and Canada), and Oceania (Australia) were active in GERD research. The betweenness centrality of a node refers to the ability to connect to other parts of the network. A source with a high betweenness centrality is more likely to broker information flows, proving its diversity in collaboration and revolutionary potential ( 116 ). It is demonstrated that collaboration exerts a positive effect on research quality, with an increase in the number of coauthors correlating with citation impact, especially when international collaboration is involved ( 117 , 118 ). Thus, England, France, Canada, Germany, and Sweden were pivotal in bridging global cooperation and influence in the GERD field.
As shown in Table 1 and Figure 3 , high-yield institutions are concentrated in North America and Europe. Overall, the USA-based institutions had limited collaborative relationships globally. For example, the most prolific institution, Mayo Clinic, actively collaborated with Mahidol Univ, Northwestern Univ, Univ Newcastle, and AstraZeneca, indicating a lack of intra-continental cooperation. Northwestern Univ which ranked second in scientific output, frequently cooperated with Univ Calif San Diego, Texas A&M Univ, Mayo Clinic, Univ Colorado, and Lyon I Univ, whose collaboration, therefore, tended to be an intra-country phenomenon.
As shown in Table 2 and Figure 4 , scholars from the USA and Italy stood out as the leading force. However, Asian researchers were scarce in the top ranking, especially Chinese and Korean ones, which was similar to the landscape of top institutions without any Asian institutions included. It is possible that GERD research in Asia was hampered by inadequate resource-, intellectual- and knowledge-sharing. Thus, Asian regions such as Japan, China, and South Korea are encouraged to pursue international scientific cooperation while increasing research productivity, which is closely linked to improved research quality and enhanced research capacity.
In addition, GERD research showed an overall trend of specialization, with most productive researchers interested in esophageal gastroenterology and upper gastrointestinal surgery. This was also reflected in the author’s collaboration network. For example, C PRAKASH GYAWALI, who had the highest centrality indicator, possessed strong research collaboration and held significant scientific impact in this domain, whose co-authorship community consisted of researchers with expertise in esophageal motility, esophageal pathology, and esophageal surgery, including FRANK ZERBIB (France), RONNIE FASS (the USA), SABINE ROMAN (France), BENJAMIN D ROGERS (the USA), CHRISTINA BROCK (Denmark), JOHN E PANDOLFINO (the USA), AMIT PATEL (the USA), and MICHAEL F VAEZI (the USA).
Based on Supplementary Table 1 , studies on GERD have primarily been published in journals dealing with gastrointestinal motility, esophageal surgery, gastrointestinal endoscopy, pediatric gastroenterology, and metabolic and bariatric surgery, which is indicative of GERD being multifactorial and affecting almost all age groups.
The number of co-citations was generally applied to appraise the academic performance of a researched subject and its impact on the scientific community ( 119 ). Journals with high co-citations are referred to as mainstream journals in this field. There were primarily high co-citations found in journals with high IF and journals located in Q1, indicating that GERD research published in top-tier journals has consistently drawn attention.
Furthermore, there is a concurrence of SURGICAL ENDOSCOPY AND OTHER INTERVENTIONAL TECHNIQUES , DISEASES OF THE ESOPHAGUS , WORLD JOURNAL OF GASTROENTEROLOGY , and DIGESTIVE DISEASES AND SCIENCES in the top productive journals and highly co-cited ones, implying they were deemed core journals in the GERD field.
Knowledge base
In addition to the conceptual structure using the keyword co-occurrences to identify clusters of research themes that extensive research has been dedicated to shown in Figure 5 , we took a closer look at the top co-cited references ( 36 , 60 , 120 – 124 ) with the highest betweenness centrality listed in Supplementary Table 3 to shed light on the key components of the intellectual base of GERD research. The topics of these references were closely related to cluster 3 (bariatric surgery) and cluster 4 (BE) displayed in Figure 5 . In addition, potential risks associated with PPI use in the long term are also of concern. These references with their key findings are summarized in Supplementary Table 4 to provide an overview.
Research frontiers
Figure 7 shows keywords with continuing strong citation bursts that denoted research frontiers within this field. The emerging topics mostly focus on endoscopic treatments, such as TIF, as well as the differential diagnosis of GERD including EOE and FH, utilizing emerging modalities such as mucosal impedance.
Endoscopic treatments
Even though many patients with GERD experience relief following dietary and medical management alone; however, a substantial percentage of these patients remain resistant to medical management or reject long-term pharmacotherapy, citing concerns regarding possible side effects. In terms of surgical treatment for GERD, the Nissen fundoplication is currently the gold standard and the mainstay of surgical management for GERD; however, the invasive nature of the procedure makes it less appealing to many patients, particularly those with less severe symptoms.
With regard to endoscopic treatments for GERD, they offer a true minimally invasive option with less pain and shorter hospital stays as well as the ability to alleviate post-operative dysphagia and the inability to vomit or belch. These mainly include radiofrequency therapy, TIF, and endoscopic suturing. As identified in our analysis, TIF has generated great interest for research; we came closer to this emerging approach.
There have been multiple non-comparative studies as well as randomized controlled trials that compare TIF with PPI controls ( 124 – 128 ). Based on a meta-analysis of 963 patients across 18 studies, the relative risk of response to TIF therapy compared with PPIs or sham treatment was 2.44 (95% CI: 1.25–4.79; p = 0.0009) ( 129 ). Even though the total number of reflux events decreased after TIF compared with either the PPIs or the sham group, the AET and the number of acid refluxes did not decrease significantly ( 129 ). The long-term follow-up further revealed that PPIs usage increased with time after the procedure, although at a lower dose ( 129 ).
Another meta-analysis was conducted using data from randomized studies evaluating TIF compared to sham or PPI therapy ( 130 ). This study aimed to evaluate the efficacy and long-term outcomes of TIF therapy in patients with refractory GERD who were on optimized PPI therapy ( 130 ). It was found that the TIF subjects at 3 years showed significantly improved esophageal pH, a decrease in PPI utilization, and improved quality of life ( 130 ). Recent studies have also demonstrated favorable long-term outcomes at 5 years, and preliminary results at 10 years ( 131 – 133 ).
With TIF, patients with GERD who have a hiatal hernia under 2 cm and a Hill grade of less than 3 can be safely and effectively treated. The American College of Gastroenterology’s 2021 Clinical Guideline for the Diagnosis and Management of GERD also recommends using TIF in patients with troublesome symptoms who refuse surgery or have a mild form of GERD, including those with hiatal hernias of less than 2 cm and esophagitis with a LA grade of A or B ( 134 ). Interestingly, TIF is being performed in combination with laparoscopic hiatal hernia repair to increase accessibility to TIF for patients with GERD and hiatal hernias larger than 2 cm ( 135 ).
Esophageal electrical impedance technologies
Despite the absence of macroscopic signs of injury, mucosal impedance reflects the degree of permeability of the mucosa and is correlated with its integrity, with low values indicative of an alteration in the intercellular spaces and tight junctions found in GERD ( 136 – 140 ).
There are several methods available for measuring esophageal mucosal impedance, including mean nocturnal baseline impedance (MNBI), high-resolution impedance manometry (HRIM)-derived measurements, and mucosal impedance probes (single-channel or balloon).
The MNBI may serve as a surrogate marker of pathological reflux, whereas the AET is subject to significant day-to-day variability ( 30 ). MNBI has been extensively studied in the field of reflux disease for its role in diagnosis and phenotyping.
The established cut-off value for MNBI was 2,292 Ω, and it has been shown to potentially distinguish GERD patients from those with FH ( 138 , 141 ). The MNBI levels are proportionally higher, moving from EE to NERD to RH, whereas the values are normal in FH and healthy controls ( 138 , 141 ). Further, MNBI results have been linked to medical and surgical outcomes. A low MNBI is shown to be an independent predictor of response to anti-reflux therapy even in patients with borderline AET (4-6%) ( 142 , 143 ). The MNBI value improves with the healing of EE, making it an interesting adjunctive measure of acid control ( 144 , 145 ). The MNBI values were also found to distinguish PPI-responsive from PPI-refractory patients with heartburn and normal conventional impedance-pH variables, with a normalization of the MNBI values following anti-reflux surgery ( 139 , 146 ). However, Ribolsi et al. ( 147 ) failed to identify the difference in MNBI between PPI responders and non-responders in NERD patients and they concluded baseline impedance didn’t predict PPI response and has no association with reflux perception. To date, the role of MNBI in NERD patients who are PPI-refractory has not been thoroughly examined.
However, despite its increasing popularity as an assessment of mucosal integrity, MNBI requires overnight catheter placement and manual software manipulation. To simplify impedance recording further, it has been suggested that baseline impedance be measured during HRIM. Ravi et al. ( 148 ) compared baseline impedance obtained from HRIM with MNBI and AET among 29 GERD patients and 26 controls. Baseline impedance measurements were made during the 15 s landmark phase of HRIM ( 148 ). There was a good correlation between baseline impedance via HRIM and MNBI ( r = 0.59, p < 0.01) and the low baseline impedance via HRIM had high diagnostic accuracy for GERD ( 148 ). The authors concluded that the HRIM baseline impedance provided the potential for accurate, cost-effective, and less-invasive diagnostics for GERD ( 148 ). Although HREMI has limited evidence for this purpose, available data regarding HRIM-derived impedance seem promising ( 148 , 149 ).
In a pioneering effort, Vaezi MF and colleagues have developed a mucosal impedance catheter to ensure the impedance sensors onto the esophageal mucosa through the working channel of the gastroscope. An initial study was carried out using a single-channel mucosal impedance catheter that was inserted through the accessory channel of an endoscope to measure the direct mucosal impedance of 19 patients with EE, 23 with NERD, and 27 controls ( 150 ). GERD patients had a significantly lower distal esophageal impedance than non-GERD patients ( 150 ). The axis of the distal-proximal esophagus showed a significant and graded increase in mucosal impedance in GERD patients ( 150 ). Further, in 61 patients with EE, 81 patients with NERD, 18 patients with achalasia, 15 patients with EoE, and 93 controls, Ates et al. ( 151 ) evaluated the utility of mucosal impedance utilizing a single-channel probe through the endoscope. A significant decrease in mucosal impedance values was observed in patients with GERD and EoE when compared with non-GERD or achalasia patients ( 151 ). EoE also had a distinct mucosal impedance pattern compared with GERD, with low values observed along the length of the esophagus at 2, 5, and 10 cm above the squamocolumnar junction, rather than a clear distal to proximal mucosal impedance gradient ( 151 ).
Although the single-channel mucosal impedance catheter introduced in these studies as a proof of concept was attractive due to its simplicity, some were concerned that a point impedance measurement could result in significant inter-provider variability due to intraluminal air or liquid, catheter movement, and insufficient mucosal contact. Consequently, a novel balloon mucosal impedance catheter system composed of 10 cm axial columns of impedance sensors mounted on a balloon that is inflated to enable a 360-degree measurement of mucosal impedance across the tubular esophagus was developed in order to eliminate these concerns. Mucosal impedance patterns acquired by the balloon-mounted mucosal impedance catheter system have been proven safe and reliable for differentiating patients of GERD, EoE, and non-GERD ( 152 ). Despite the need for more data, the initial results from the mucosal impedance balloon catheter are promising; furthermore, its ease of data acquisition, simplicity of interpretation, and rapidity of the test make it appealing in comparison to MNBI interpretation which entails cumbersome ambulatory catheter monitoring and manual analysis of tracings ( 153 ).
During upper endoscopy, biopsies may be obtained to rule out structural abnormalities that may cause heartburn, such as EoE. However, the distribution of eosinophilia in EoE is segmental or patchy, requiring multiple biopsies for detection, and even aggressive biopsies may only cover a small section of the entire esophagus. Histologically, in addition to eosinophil infiltrates, one of the key histologic characteristics of EoE involves the appearance of dilated intercellular spaces (DIS) between epithelial cells, thus leading to esophageal epithelial permeability ( 154 ). Accordingly, mucosal impedance is a valuable method for measuring the conductivity of the esophageal epithelium and can reliably separate EoE from GERD and other non-GERD conditions.
Katzka et al. ( 155 ) evaluated 10 active and 10 inactive EoE patients and compared mucosal impedance measurements with biopsy results in a study using the single-channel mucosal impedance probe. An inverse correlation was reported between mucosal impedance measurements and eosinophil/high-power field and spongiosis on esophageal biopsies in EoE ( 155 ). Patients with active disease had a lower mucosal impedance, and a cut-off value of 2,300 Ω identified patients with active EoE with 90% sensitivity and 91% specificity, and high-grade DIS with 89% sensitivity and 82% specificity ( 155 ). Furthermore, this measurement was validated in the pediatric population, showing that patients with active EoE have lower mucosal impedance than those with inactive EoE, NERD, or controls ( 156 ). Another study by Alexander et al. ( 157 ) compared mucosal impedance using a balloon catheter and eosinophil counts in endoscopic biopsies in 10 controls, 18 patients with active EoE, and 5 patients with inactive EoE. In analyzing individual impedance measurements (18 per patient), it was found that control patients had normal mucosal impedance values 95.6% of the time as compared to 29.2% in EoE patients, and no EoE patient had uniformly normal mucosal impedance ( 157 ). However, a poor correlation was reported between peak esophageal eosinophil counts, EoE activity, and mucosal impedance ( 157 ).
Overall, mucosal impedance, measured with a variety of techniques, either ambulatory setting or single-channel and balloon-based, appears promising and may be able to differentiate GERD from EoE as well as track the histologic progression of EoE ( 151 , 155 – 159 ). Currently, however, data regarding mucosal impedance measurements in EoE are limited. It appears that mucosal impedance, peak eosinophil counts, and EoE disease activity in individual patients do not correlate well, according to Alexander et al. ( 157 ); even with a balloon-probe mucosal impedance catheter system, mucosal impedance measurements do not assess the whole esophagus, which may not accurately evaluate the patchy epithelial change associated with EoE. Therefore, it is still necessary to obtain biopsies for EoE assessment and mucosal impedance cannot currently replace biopsy acquisition for assessing EoE disease activity ( 153 ).
PPI-resistant heartburn can be caused by functional heartburn, and approximately 50% of patients with refractory heartburn are attributed to functional heartburn ( 160 , 161 ). Furthermore, about half of patients with NERD who fail to improve after treatment with P-CABs, in fact, have functional heartburn ( 109 , 162 ). In a recent survey, FH was cited by healthcare providers as the most common reason for incomplete response to PPI therapy despite following dosing suggestions ( 163 ). It is, therefore, necessary to distinguish GERD, in particular NERD, from FH, as FH is not reflux-related and PPIs lack therapeutic value, except in cases of proven GERD that overlap with functional heartburn ( 164 ).
Savarino et al. ( 165 ) found that the prevalence of DIS was quite low among patients with FH which was comparable to healthy controls when compared with patients with NERD. Also, in the study by Vela et al. ( 166 ), the mean intracellular space diameter in patients with GERD was significantly greater than in those with FH and controls, and there was no evidence of esophageal epithelial dilatation in those with FH or controls.
Accordingly, DIS is the most common histopathological feature in the basal epithelial layer of the esophageal mucosa in all patients with GERD, and its presence is considered the most important difference between patients with NERD and those with FH. Since the underlying premise of mucosal impedance is that it is a marker of mucosal integrity, these results suggest that this tool will likely distinguish patients with FH from those with GERD and EoE.
However, likely, a mucosal impedance measurement will not be able to distinguish patients with FH and RH from controls, since recent studies reported that mucosal impedance was lower among patients with GERD, but was similar between those with FH and RH ( 167 ). In other words, the presence of normal mucosal impedance in patients with heartburn is suggestive of a functional disorder rather than GERD, which would call for sensory modulation for treatment. Although it has previously been shown that lower baseline impedance was found in RH versus FH, it appears that this is inconsistent with the idea that low baseline impedance levels of the esophagus may be associated with high reflux burdens or increased esophageal acid exposure ( 124 ). Therefore, further studies are needed.
In the present study, we conducted a bibliometric analysis of 8,964 publications on GERD research retrieved from the WOSCC of Clarivate Analytics from 2012 to 2022. As a result of the analysis, it was found that current studies in this field focused on the differential diagnosis for GERD, including esophageal motility disorders and functional esophageal disorders, anti-reflux surgery, the reflux-inflammation-Barrett’s cascade, treatment options for GERD in obese patients, and P-CAB, most of which were clinically relevant, and the basic research was insufficient.
Basic research on GERD appears to be lagging, which may be explained by the high focus placed on the role of acid in its pathogenesis for years. Following the degradation of glycoproteins, H + penetrates the esophageal epithelial layer along the intercellular spaces ( 168 ). In the presence of DIS, H + can enter the submucosa and interact with sensory afferents, thus leading to symptoms associated with reflux ( 169 ). However, an alternative mechanism was proposed by Souza et al. ( 170 ), who suggested that bile salts enhanced the stabilization of hypoxia-inducible factor-1a, increasing T lymphocyte-derived chemokines that facilitate the development of esophagitis. This highlights that T lymphocytes mediate the primary mode of injury ( 171 ). The GERD pathogenesis, therefore, can be quite complex; it should be viewed as a disorder that goes far beyond acid reflux. Furthermore, the paradox of patients with BE exhibiting few symptoms compared with FH patients having esophageal pain sensitivity indicates the complexity of heartburn generation mechanisms. Research remains to be conducted on the relationship between acid or non-acid reflux and sensory afferents.
From the clinical perspective, as identified in our analysis, there is an increasing trend toward endoscopic- and surgical specialization in GERD research. However, multiple studies that compared GERD patients to non-GERD patients have suggested that GERD and psychological disorders may be interlinked ( 172 ). Further, the number of patients with GERD and overlapping functional disorders is increasing, and their clinical and psychological characteristics are comparable to those of conventional functional disorders ( 173 ). It is, therefore, necessary for GERD research to involve a collaborative research network consisting of esophagologist, endoscopists, surgeons, endocrinologists, pathologists, and psychiatrists.
Moreover, as mucosal impedance is an emerging technology that has entered the arsenal of the esophageal provider, it has raised awareness that mucosal integrity impairment is present in 68.2–83% of patients with NERD, and 48–100% of those with EE ( 174 ). Thus, even though acid has been investigated for many years as a fundamental factor in the pathogenesis of GERD, the availability of other treatments, including hyaluronic acid plus chondroitin sulfate ( 175 ), alginates ( 176 ) and Rikkushito ( 177 ), that are able to strengthen esophageal defenses, has sparked new research in this relevant field and offers opportunities for future research.
Data availability statement
The original contributions presented in this study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding author.
Author contributions
XT, FW, and BZ led the team and were responsible for all aspects of the project. TZ, WT, XY, XW, YW, and JL substantially contributed to the methods, data acquisition, results, and interpretation. WT, YW, and XY participated in designing and writing the manuscript. TZ, BZ, WT, FW, and XW revised this manuscript critically for important intellectual content. XT gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Key R&D Program in the thirteenth Five-year Plan (No. 2019YFC1709600) from the Chinese Ministry of Science and Technology, and National Natural Science Foundation of China (No. 81804089), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202010), and Special Fund for Basic Scientific Research Business of Central Public Welfare Scientific Research Institute (No. ZZ13-YQ-003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.994534/full#supplementary-material
1. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. (2014) 63:871–80. doi: 10.1136/gutjnl-2012-304269
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology. (2018) 154:302–18. doi: 10.1053/j.gastro.2017.07.049
3. Locke GR III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ III. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. (1997) 112:1448–56. doi: 10.1016/s0016-5085(97)70025-8
CrossRef Full Text | Google Scholar
4. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group. The montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. (2006) 101:1900–20; quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x
5. Maneerattanaporn M, Pittayanon R, Patcharatrakul T, Bunchorntavakul C, Sirinthornpanya S, Pitisuttithum P, et al. Thailand guideline 2020 for medical management of gastroesophageal reflux disease. J Gastroenterol Hepatol . (2022) 37:632–643. doi: 10.1111/jgh.15758
6. Ronkainen J, Talley NJ, Storskrubb T, Johansson SE, Lind T, Vieth M, et al. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community-based endoscopic follow-up study. Am J Gastroenterol. (2011) 106:1946–52. doi: 10.1038/ajg.2011.326
7. Hunt R, Armstrong D, Katelaris P, Afihene M, Bane A, Bhatia S, et al. World gastroenterology organisation global guidelines: GERD global perspective on gastroesophageal reflux disease. J Clin Gastroenterol. (2017) 51:467–78. doi: 10.1097/MCG.0000000000000854
8. Vaezi MF, Pandolfino JE, Vela MF, Shaheen NJ. White paper AGA: optimal strategies to define and diagnose gastroesophageal reflux disease. Clin Gastroenterol Hepatol. (2017) 15:1162–72. doi: 10.1016/j.cgh.2017.03.021
9. Horn J. The proton-pump inhibitors: similarities and differences. Clin Ther. (2000) 22:266–80; discussion 265. doi: 10.1016/S0149-2918(00)80032-6
10. Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. (1997) 112:1798–810. doi: 10.1053/gast.1997.v112.pm9178669
11. Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease–where next? Aliment Pharmacol Ther. (2005) 22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x
12. Hongo M, Kinoshita Y, Miwa H, Ashida K. The demographic characteristics and health-related quality of life in a large cohort of reflux esophagitis patients in Japan with reference to the effect of lansoprazole: the REQUEST study. J Gastroenterol. (2008) 43:920–7. doi: 10.1007/s00535-008-2257-7
13. Toghanian S, Wahlqvist P, Johnson DA, Bolge SC, Liljas B. The burden of disrupting gastro-oesophageal reflux disease: a database study in US and European cohorts. Clin Drug Investig. (2010) 30:167–78. doi: 10.2165/11531670-000000000-00000
14. Bruley des Varannes S, Löfman HG, Karlsson M, Wahlqvist P, Ruth M, Furstnau ML, et al. Cost and burden of gastroesophageal reflux disease among patients with persistent symptoms despite proton pump inhibitor therapy: an observational study in France. BMC Gastroenterol. (2013) 13:39. doi: 10.1186/1471-230X-13-39
15. Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. (2012) 143:1179–87.e3. doi: 10.1053/j.gastro.2012.08.002
16. PenDlebury DA. Whitepaper Using Bibliometrics: A Guide to Evaluating Research Performance with Citation Data. Toronto, ON: Thomson Reuters (2008).
Google Scholar
17. Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 3:359–77. doi: 10.1002/asi.20317
18. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
19. Chen C, Chen Y, Hou J, Liang Y. CiteSpace I : detecting and visualizing emerging trends and transient patterns in scientific literature. J Chin Soc Sci Tech Inform. (2009) 28:401–21. doi: 10.3772/j.issn.1000-0135.2009.03.012
20. Freeman LC. A set of measures of centrality based on betweenness. Sociometry. (1977) 40:35–41.
21. Okhovati M, Arshadi H. COVID-19 research progress: bibliometrics and visualization analysis. Med J Islam Repub Iran. (2021) 35:20. doi: 10.47176/mjiri.35.20
22. Zhang T, Zhang B, Tian W, Ma X, Wang F, Wang P, et al. A bibliometric analysis of atrophic gastritis from 2011 to 2021. Front Med. (2022) 9:843395.
23. Zhang T, Ma X, Tian W, Zhang J, Wei Y, Zhang B, et al. Global research trends in irritable bowel syndrome: a bibliometric and visualized study. Front Med. (2022) 9:922063. doi: 10.3389/fmed.2022.922063
24. Katz JS, Martin BR. What is research collaboration? Res Policy. (1997) 26:1–18. doi: 10.1016/S0048-7333(96)00917-1
25. Marshakova I. System of document connections based on references. Sci Tech Inf Ser VINITI. (1973) 2:3–8.
26. Small H. Co-citation in the scientific literature: a new measure of the relationship between two documents. J Am Soc Inf Sci. (1973) 24:265–9.
27. Tsay M-Y, Xu H, Wu C-W. Journal co-citation analysis of semiconductor literature. Scientometrics. (2003) 57:7–25.
28. Dennis S, Yunyue Z. Elastic Burst Detection. New York, NY: Springer (2004).
29. Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. (2012) 12:593–608. doi: 10.1517/14712598.2012.674507
30. Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. (2018) 67:1351–62. doi: 10.1136/gutjnl-2017-314722
31. Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. (2018) 66:516–54. doi: 10.1097/MPG.0000000000001889
32. Roman S, Gyawali CP, Savarino E, Yadlapati R, Zerbib F, Wu J, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. (2017) 29:1–15. doi: 10.1111/nmo.13067
33. Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. (2018) 67:430–40. doi: 10.1136/gutjnl-2016-313589
34. Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. (2018) 319:255–65. doi: 10.1001/jama.2017.20897
35. Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. (2018) 154:267–76. doi: 10.1053/j.gastro.2017.07.045
36. Genco A, Soricelli E, Casella G, Maselli R, Castagneto-Gissey L, Di Lorenzo N, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. (2017) 13:568–74. doi: 10.1016/j.soard.2016.11.029
37. Sebastianelli L, Benois M, Vanbiervliet G, Bailly L, Robert M, Turrin N, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of barrett’s esophagus: results of a multicenter study. Obes Surg. (2019) 29:1462–9. doi: 10.1007/s11695-019-03704-y
38. Felsenreich DM, Kefurt R, Schermann M, Beckerhinn P, Kristo I, Krebs M, et al. Reflux, sleeve dilation, and Barrett’s esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. (2017) 27:3092–101. doi: 10.1007/s11695-017-2748-9
39. Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. (2016) 51:751–67. doi: 10.1007/s00535-016-1227-8
40. Dotsika F, Watkins A. Identifying potentially disruptive trends by means of keyword network analysis. Technol Forecast Soc Change. (2017) 119:114–27. doi: 10.1016/j.techfore.2017.03.020
41. Kim MC, Chen C. A scientometric review of emerging trends and new developments in recommendation systems. Scientometrics. (2015) 104:239–63. doi: 10.1007/s11192-015-1595-5
42. Spechler SJ, Souza RF, Rosenberg SJ, Ruben RA, Goyal RK. Heartburn in patients with achalasia. Gut. (1995) 37:305–8. doi: 10.1136/gut.37.3.305
43. Smart HL, Foster PN, Evans DF, Slevin B, Atkinson M. Twenty four hour oesophageal acidity in achalasia before and after pneumatic dilatation. Gut. (1987) 28:883–7. doi: 10.1136/gut.28.7.883
44. Kessing BF, Bredenoord AJ, Smout AJ. Erroneous diagnosis of gastroesophageal reflux disease in achalasia. Clin Gastroenterol Hepatol. (2011) 9:1020–4. doi: 10.1016/j.cgh.2011.04.022
45. Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. (2011) 25:2943–9. doi: 10.1007/s00464-011-1646-9
46. Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. (2007) 293:G878–85. doi: 10.1152/ajpgi.00252.2007
47. Schein M, Schein H, Wise L. Rudolf Nissen: the man behind the fundoplication. Surgery. (1999) 125:347–53.
PubMed Abstract | Google Scholar
48. Nissen R. Eine einfache operation zur beeinflussung der refluxoesophagitis [A simple operation for control of reflux esophagitis]. Schweiz Med Wochenschr. (1956) 86(Suppl. 20):590–2.
49. Bammer T, Hinder RA, Klaus A, Klingler PJ. Five- to eight-year outcome of the first laparoscopic Nissen fundoplications. J Gastrointest Surg. (2001) 5:42–8. doi: 10.1016/s1091-255x(01)80012-3
50. Dallemagne B, Weerts JM, Jehaes C, Markiewicz S, Lombard R. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. (1991) 1:138–43.
51. Lin DC, Chun CL, Triadafilopoulos G. Evaluation and management of patients with symptoms after anti-reflux surgery. Dis Esophagus. (2015) 28:1–10. doi: 10.1111/dote.12103
52. Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. (2011) 305:1969–77. doi: 10.1001/jama.2011.626
53. Spechler SJ, Hunter JG, Jones KM, Lee R, Smith BR, Mashimo H, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. (2019) 381:1513–23. doi: 10.1056/NEJMoa1811424
54. Pallati PK, Shaligram A, Shostrom VK, Oleynikov D, McBride CL, Goede MR. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: review of the bariatric outcomes longitudinal database. Surg Obes Relat Dis. (2014) 10:502–7. doi: 10.1016/j.soard.2013.07.018
55. Prachand VN, Ward M, Alverdy JC. Duodenal switch provides superior resolution of metabolic comorbidities independent of weight loss in the super-obese (BMI > or = 50 kg/m2) compared with gastric bypass. J Gastrointest Surg. (2010) 14:211–20. doi: 10.1007/s11605-009-1101-6
56. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. (2015) 21:10348–57. doi: 10.3748/wjg.v21.i36.10348
57. Tutuian R. Effects of bariatric surgery on gastroesophageal reflux. Curr Opin Gastroenterol. (2014) 30:434–8. doi: 10.1097/MOG.0000000000000083
58. Borbély Y, Schaffner E, Zimmermann L, Huguenin M, Plitzko G, Nett P, et al. De novo gastroesophageal reflux disease after sleeve gastrectomy: role of preoperative silent reflux. Surg Endosc. (2019) 33:789–93. doi: 10.1007/s00464-018-6344-4
59. Gu L, Chen B, Du N, Fu R, Huang X, Mao F, et al. Relationship between bariatric surgery and gastroesophageal reflux disease: a systematic review and meta-analysis. Obes Surg. (2019) 29:4105–13. doi: 10.1007/s11695-019-04218-3
60. Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: a systematic review and meta-analysis. Ann Surg. (2020) 271:257–65. doi: 10.1097/SLA.0000000000003275
61. Wright FG, Duro A, Medici JR, Lenzi S, Beskow AF, Cavadas D. Esophageal adenocarcinoma five years after laparoscopic sleeve gastrectomy. A case report. Int J Surg Case Rep. (2017) 32:47–50. doi: 10.1016/j.ijscr.2017.01.054
62. Mion F, Tolone S, Garros A, Savarino E, Pelascini E, Robert M, et al. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. (2016) 26:2449–56. doi: 10.1007/s11695-016-2127-y
63. Braghetto I, Lanzarini E, Korn O, Valladares H, Molina JC, Henriquez A. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg. (2010) 20:357–62. doi: 10.1007/s11695-009-0040-3
64. Hutopila I, Copaescu C. Hiatal hernia is more frequent than expected in bariatric patients. intraoperative findings during laparoscopic sleeve gastrectomy. Chirurgia. (2019) 114:779–89. doi: 10.21614/chirurgia.114.6.779
65. Keidar A, Appelbaum L, Schweiger C, Elazary R, Baltasar A. Dilated upper sleeve can be associated with severe postoperative gastroesophageal dysmotility and reflux. Obes Surg. (2010) 20:140–7. doi: 10.1007/s11695-009-0032-3
66. Eusebi LH, Telese A, Cirota GG, Haidry R, Zagari RM, Bazzoli F, et al. Effect of gastro-esophageal reflux symptoms on the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2022) 37:1507–16. doi: 10.1111/jgh.15902
67. Jankowski JA, Anderson M. Review article: management of oesophageal adenocarcinoma – control of acid, bile and inflammation in intervention strategies for Barrett’s oesophagus. Aliment Pharmacol Ther. (2004) 20(Suppl. 5):71–80; discussion 95–6. doi: 10.1111/j.1365-2036.2004.02143.x
68. Green JA, Amaro R, Barkin JS. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. Dig Dis Sci. (2000) 45:2367–8. doi: 10.1023/a:1005638924929
69. Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. (2000) 60:5767–72.
70. Souza RF, Shewmake K, Pearson S, Sarosi GA Jr, Feagins LA, Ramirez RD, et al. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett’s adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. (2004) 287:G743–8. doi: 10.1152/ajpgi.00144.2004
71. Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. (2002) 50:451–9. doi: 10.1136/gut.50.4.451
72. Ma J, Altomare A, de la Monte S, Tong M, Rieder F, Fiocchi C, et al. HCl-induced inflammatory mediators in esophageal mucosa increase migration and production of H2O2 by peripheral blood leukocytes. Am J Physiol Gastrointest Liver Physiol. (2010) 299:G791–8. doi: 10.1152/ajpgi.00160.2010
73. Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, et al. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. (2003) 98:551–6. doi: 10.1111/j.1572-0241.2003.07303.x
74. Leedham S, Jankowski J. The evidence base of proton pump inhibitor chemopreventative agents in Barrett’s esophagus–the good, the bad, and the flawed! Am J Gastroenterol. (2007) 102:21–3. doi: 10.1111/j.1572-0241.2006.01033.x
75. Raj A, Jankowski J. Acid suppression and chemoprevention in Barrett’s oesophagus. Dig Dis. (2004) 22:171–80. doi: 10.1159/000080316
76. Batzri S, Harmon JW, Schweitzer EJ, Toles R. Bile acid accumulation in gastric mucosal cells. Proc Soc Exp Biol Med. (1991) 197:393–9. doi: 10.3181/00379727-197-43272
77. Yang N, Xu J, Wang X, Chen N, Su L, Liu Y. The spatial landscape of the bacterial community and bile acids in the digestive tract of patients with bile reflux. Front Microbiol. (2022) 13:835310. doi: 10.3389/fmicb.2022.835310
78. Sun D, Wang X, Gai Z, Song X, Jia X, Tian H. Bile acids but not acidic acids induce Barrett’s esophagus. Int J Clin Exp Pathol. (2015) 8:1384–92.
79. McQuaid KR, Laine L, Fennerty MB, Souza R, Spechler SJ. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment Pharmacol Ther. (2011) 34:146–65. doi: 10.1111/j.1365-2036.2011.04709.x
80. Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. (2007) 56:763–71. doi: 10.1136/gut.2006.103697
81. Samuels T, Hoekzema C, Gould J, Goldblatt M, Frelich M, Bosler M, et al. Local synthesis of pepsin in Barrett’s esophagus and the role of pepsin in esophageal adenocarcinoma. Ann Otol Rhinol Laryngol. (2015) 124:893–902. doi: 10.1177/0003489415590657
82. Johnston N, Wells CW, Blumin JH, Toohill RJ, Merati AL. Receptor-mediated uptake of pepsin by laryngeal epithelial cells. Ann Otol Rhinol Laryngol. (2007) 116:934–8. doi: 10.1177/000348940711601211
83. Johnston N, Wells CW, Samuels TL, Blumin JH. Rationale for targeting pepsin in the treatment of reflux disease. Ann Otol Rhinol Laryngol. (2010) 119:547–58. doi: 10.1177/000348941011900808
84. Southwood JE, Hoekzema CR, Samuels TL, Wells C, Poetker DM, Johnston N, et al. The impact of pepsin on human nasal epithelial cells in vitro: a potential mechanism for extraesophageal reflux induced chronic rhinosinusitis. Ann Otol Rhinol Laryngol. (2015) 124:957–64. doi: 10.1177/0003489415593556
85. Johnston N, Yan JC, Hoekzema CR, Samuels TL, Stoner GD, Blumin JH, et al. Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells. Laryngoscope. (2012) 122:1317–25. doi: 10.1002/lary.23307
86. O’Farrell NJ, Feighery R, Picardo SL, Lynam-Lennon N, Biniecka M, McGarrigle SA, et al. Changes in mitochondrial stability during the progression of the Barrett’s esophagus disease sequence. BMC Cancer. (2016) 16:497. doi: 10.1186/s12885-016-2544-2
87. Tan BH, Skipworth RJ, Stephens NA, Wheelhouse NM, Gilmour H, de Beaux AC, et al. Frequency of the mitochondrial DNA 4977bp deletion in oesophageal mucosa during the progression of Barrett’s oesophagus. Eur J Cancer. (2009) 45:736–40. doi: 10.1016/j.ejca.2009.01.013
88. Samuels TL, Altman KW, Gould JC, Kindel T, Bosler M, MacKinnon A, et al. Esophageal pepsin and proton pump synthesis in Barrett’s esophagus and esophageal adenocarcinoma. Laryngoscope. (2019) 129:2687–95. doi: 10.1002/lary.28051
89. Oh DS, DeMeester SR, Vallbohmer D, Mori R, Kuramochi H, Hagen JA, et al. Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett’s esophagus with antireflux surgery. Arch Surg. (2007) 142:554–9; discussion 559–60. doi: 10.1001/archsurg.142.6.554
90. van Baal JW, Bozikas A, Pronk R, Ten Kate FJ, Milano F, Rygiel AM, et al. Cytokeratin and CDX-2 expression in Barrett’s esophagus. Scand J Gastroenterol. (2008) 43:132–40. doi: 10.1080/00365520701676575
91. Biswas S, Quante M, Leedham S, Jansen M. The metaplastic mosaic of Barrett’s oesophagus. Virchows Arch. (2018) 472:43–54. doi: 10.1007/s00428-018-2317-1
92. Chandrasoma P, Wijetunge S, Demeester SR, Hagen J, Demeester TR. The histologic squamo-oxyntic gap: an accurate and reproducible diagnostic marker of gastroesophageal reflux disease. Am J Surg Pathol. (2010) 34:1574–81. doi: 10.1097/PAS.0b013e3181f06990
93. Takubo K, Aida J, Naomoto Y, Sawabe M, Arai T, Shiraishi H, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol. (2009) 40:65–74. doi: 10.1016/j.humpath.2008.06.008
94. Ruigómez A, Rodríguez LA, Wallander MA, Johansson S, Thomas M, Price D. Gastroesophageal reflux disease and asthma: a longitudinal study in UK general practice. Chest. (2005) 128:85–93. doi: 10.1378/chest.128.1.85
95. Jaspersen D, Kulig M, Labenz J, Leodolter A, Lind T, Meyer-Sabellek W, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD study. Aliment Pharmacol Ther. 2003. 17(12):1515-20. Erratum Aliment Pharmacol Ther. (2003) 18:355. doi: 10.1046/j.1365-2036.2003.01606.x</arttitle<
96. Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. (2007) 56:1654–64. doi: 10.1136/gut.2007.122465
97. ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. (2005) 26:812–8. doi: 10.1183/09031936.05.00037905
98. Diette GB, Krishnan JA, Dominici F, Haponik E, Skinner EA, Steinwachs D, et al. Asthma in older patients: factors associated with hospitalization. Arch Intern Med. (2002) 162:1123–32. doi: 10.1001/archinte.162.10.1123
99. Mallah N, Turner JM, González-Barcala FJ, Takkouche B. Gastroesophageal reflux disease and asthma exacerbation: a systematic review and meta-analysis. Pediatr Allergy Immunol. (2022) 33:e13655. doi: 10.1111/pai.13655
100. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. (2011) 39:818–26. doi: 10.1097/CCM.0b013e31820a856b
101. Tuchman DN, Boyle JT, Pack AI, Scwartz J, Kokonos M, Spitzer AR, et al. Comparison of airway responses following tracheal or esophageal acidification in the cat. Gastroenterology. (1984) 87:872–81.
102. Wright RA, Miller SA, Corsello BF. Acid-induced esophagobronchial-cardiac reflexes in humans. Gastroenterology. (1990) 99:71–3. doi: 10.1016/0016-5085(90)91231-t
103. Wu DN, Tanifuji Y, Kobayashi H, Yamauchi K, Kato C, Suzuki K, et al. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest. (2000) 118:1553–6. doi: 10.1378/chest.118.6.1553
104. Parsons JP, Mastronarde JG. Gastroesophageal reflux disease and asthma. Curr Opin Pulm Med. (2010) 16:60–3. doi: 10.1097/MCP.0b013e328332ca2f
105. Garnock-Jones KP. Vonoprazan: first global approval. Drugs. (2015) 75:439–43. doi: 10.1007/s40265-015-0368-z
106. Kinoshita Y, Sakurai Y, Shiino M, Kudou K, Nishimura A, Miyagi T, et al. Evaluation of the efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a phase III, randomized, double-blind, placebo-controlled, multicenter study. Curr Ther Res Clin Exp. (2016) 81–2:1–7. doi: 10.1016/j.curtheres.2016.12.001
107. Kinoshita Y, Sakurai Y, Takabayashi N, Kudou K, Araki T, Miyagi T, et al. Efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a randomized, placebo-controlled, phase 3 study. Clin Transl Gastroenterol. (2019) 10:e00101. doi: 10.14309/ctg.0000000000000101
108. Chey WD. Endoscopy-negative reflux disease: concepts and clinical practice. Am J Med. (2004) 117(Suppl. 5A):36S–43S. doi: 10.1016/j.amjmed.2004.07.016
109. Kawami N, Hoshino S, Hoshikawa Y, Takenouchi N, Umezawa M, Hanada Y, et al. Pathogenesis of potassium-competitive acid blocker-resistant non-erosive reflux disease. Digestion. (2018) 98:194–200. doi: 10.1159/000488530
110. Abe Y, Koike T, Saito M, Okata T, Nakagawa K, Hatta W, et al. The ameliorating effect of switching to vonoprazan: a novel potassium-competitive acid blocker in patients with proton pump inhibitor refractory non-erosive reflux disease. Digestion. (2021) 102:480–8. doi: 10.1159/000506152
111. Niikura R, Yamada A, Hirata Y, Hayakawa Y, Takahashi A, Shinozaki T, et al. Efficacy of vonoprazan for gastroesophageal reflux symptoms in patients with proton pump inhibitor-resistant non-erosive reflux disease. Intern Med. (2018) 57:2443–50. doi: 10.2169/internalmedicine.0492-17
112. Shinozaki S, Osawa H, Hayashi Y, Sakamoto H, Kobayashi Y, Lefor AK, et al. Vonoprazan 10 mg daily is effective for the treatment of patients with proton pump inhibitor-resistant gastroesophageal reflux disease. Biomed Rep. (2017) 7:231–5. doi: 10.3892/br.2017.947
113. Kim SH, Cho KB, Chun HJ, Lee SW, Kwon JG, Lee DH, et al. Randomised clinical trial: comparison of tegoprazan and placebo in non-erosive reflux disease. Aliment Pharmacol Ther. (2021) 54:402–11. doi: 10.1111/apt.16477
114. Katz PO, Castell DO, Levine D. Esomeprazole resolves chronic heartburn in patients without erosive oesophagitis. Aliment Pharmacol Ther. (2003) 18:875–82. doi: 10.1046/j.1365-2036.2003.01771.x
115. Katz P. Editorial: non-erosive reflux disease is often not GERD-time to change the definition or abandon the term? Aliment Pharmacol Ther. (2021) 54:493–4. doi: 10.1111/apt.16480
116. Yan E, Ding Y. Applying centrality measures to impact analysis: a coauthorship network analysis. J Am Soc Inform Sci Technol. (2009) 60:2107–18. doi: 10.3389/fdata.2019.00039
117. Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. (2007) 316:1036–9. doi: 10.1126/science.1136099
118. Adams J, Potter R, Szomszor M. Global Research Report: Multi-Authorship and Research Analytics. London: Institute for Scientific Information, Clarivate (2019).
119. Moed HF. New developments in the use of citation analysis in research evaluation. Arch Immunol Ther Exp. (2009) 57:13–8. doi: 10.1007/s00005-009-0001-5
120. Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. (2014) 63:1229–37. doi: 10.1136/gutjnl-2013-305997
121. Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori : a population-based study. Gut. (2018) 67:28–35. doi: 10.1136/gutjnl-2017-314605
122. Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. (2015) 42:649–63. doi: 10.1111/apt.13324
123. Csendes A, Orellana O, Martínez G, Burgos AM, Figueroa M, Lanzarini E. Clinical, endoscopic, and histologic findings at the distal esophagus and stomach before and late (10.5 years) after laparoscopic sleeve gastrectomy: results of a prospective study with 93% follow-up. Obes Surg. (2019) 29:3809–17. doi: 10.1007/s11695-019-04054-5
124. Martinucci I, de Bortoli N, Savarino E, Piaggi P, Bellini M, Antonelli A, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. (2014) 26:546–55. doi: 10.1111/nmo.12299
125. Håkansson B, Montgomery M, Cadiere GB, Rajan A, Bruley des Varannes S, Lerhun M, et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther. (2015) 42:1261–70. doi: 10.1111/apt.13427
126. Trad KS, Barnes WE, Simoni G, Shughoury AB, Mavrelis PG, Raza M, et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO randomized clinical trial. Surg Innov. (2015) 22:26–40. doi: 10.1177/1553350614526788
127. Hunter JG, Kahrilas PJ, Bell RC, Wilson EB, Trad KS, Dolan JP, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. (2015) 148:324–33.e5. doi: 10.1053/j.gastro.2014.10.009
128. Barnes WE, Hoddinott KM, Mundy S, Williams M. Transoral incisionless fundoplication offers high patient satisfaction and relief of therapy-resistant typical and atypical symptoms of GERD in community practice. Surg Innov. (2011) 18:119–29. doi: 10.1177/1553350610392067
129. Wilson EB, Barnes WE, Mavrelis PG, Carter BJ, Bell RC, Sewell RW, et al. The effects of transoral incisionless fundoplication on chronic GERD patients: 12-month prospective multicenter experience. Surg Laparosc Endosc Percutan Tech. (2014) 24:36–46. doi: 10.1097/SLE.0b013e3182a2b05c
130. Huang X, Chen S, Zhao H, Zeng X, Lian J, Tseng Y, et al. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: a systematic review with meta-analysis. Surg Endosc. (2017) 31:1032–44. doi: 10.1007/s00464-016-5111-7
131. Gerson L, Stouch B, Lobonţiu A. Transoral incisionless fundoplication (TIF 2.0): a meta-analysis of three randomized, controlled clinical trials. Chirurgia. (2018) 113:173–84. doi: 10.21614/chirurgia.113.2.173
132. Stefanidis G, Viazis N, Kotsikoros N, Tsoukalas N, Lala E, Theocharis L, et al. Long-term benefit of transoral incisionless fundoplication using the esophyx device for the management of gastroesophageal reflux disease responsive to medical therapy. Dis Esophagus. (2017) 30:1–8. doi: 10.1111/dote.12525
133. Trad KS, Barnes WE, Prevou ER, Simoni G, Steffen JA, Shughoury AB, et al. The TEMPO trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov. (2018) 25:149–57. doi: 10.1177/1553350618755214
134. Richter JE, Kumar A, Lipka S, Miladinovic B, Velanovich V. Efficacy of laparoscopic nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology. (2018) 154:1298–308.e7. doi: 10.1053/j.gastro.2017.12.021
135. Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. (2022) 117:27–56. doi: 10.14309/ajg.0000000000001538
136. Ramai D, Shapiro A, Barakat M, Facciorusso A, Dull A, Chandan S, et al. Adverse events associated with transoral incisionless fundoplication (TIF) for chronic gastroesophageal reflux disease: a MAUDE database analysis. Surg Endosc. (2022) 36:4956–9. doi: 10.1007/s00464-021-08851-x
137. Farré R, Blondeau K, Clement D, Vicario M, Cardozo L, Vieth M, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. (2011) 60:885–92. doi: 10.1136/gut.2010.233049
138. Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJ, Loots CM, Smout AJ. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. (2011) 106:2093–7. doi: 10.1038/ajg.2011.276
139. de Bortoli N, Martinucci I, Savarino E, Tutuian R, Frazzoni M, Piaggi P, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. (2015) 13:1082–8.e1. doi: 10.1016/j.cgh.2014.11.035
140. Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. (2015) 13:1075–81. doi: 10.1016/j.cgh.2014.11.033
141. Frazzoni M, Savarino E, de Bortoli N, Martinucci I, Furnari M, Frazzoni L, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. (2016) 14:40–6. doi: 10.1016/j.cgh.2015.06.026
142. Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. (2016) 44:890–8. doi: 10.1111/apt.13777
143. Rengarajan A, Savarino E, Della Coletta M, Ghisa M, Patel A, Gyawali CP. Mean nocturnal baseline impedance correlates with symptom outcome when acid exposure time is inconclusive on esophageal reflux monitoring. Clin Gastroenterol Hepatol. (2020) 18:589–95. doi: 10.1016/j.cgh.2019.05.044
144. van Rhijn BD, Weijenborg PW, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. (2014) 12:1815–23.e2. doi: 10.1016/j.cgh.2014.02.037
145. Frazzoni M, Penagini R, Frazzoni L, de Bortoli N, Mauro A, Tolone S, et al. Role of reflux in the pathogenesis of eosinophilic esophagitis: comprehensive appraisal with off- and on PPI impedance-pH monitoring. Am J Gastroenterol. (2019) 114:1606–13. doi: 10.14309/ajg.0000000000000379
146. Frazzoni M, De Bortoli N, Frazzoni L, Tolone S, Furnari M, Martinucci I, et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil . (2017) 29:e12947.
147. Ribolsi M, Emerenziani S, Borrelli O, Balestrieri P, Addarii MC, Petitti T, et al. Impedance baseline and reflux perception in responder and non-responder non-erosive reflux disease patients. Scand J Gastroenterol. (2012) 47:1266–73. doi: 10.3109/00365521.2012.722674
148. Ravi K, Geno DM, Vela MF, Crowell MD, Katzka DA (2017). Baseline impedance measured during high-resolution esophageal impedance manometry reliably discriminates GERD patients. Neurogastroenterol Motil . 29:e12974.
149. Hung JS, Wong MW, Liu TT, Yi CH, Lei WY, Chen CL. Evaluation of baseline impedance during water-perfused high resolution impedance manometry in patients with symptomatic GERD. J Clin Gastroenterol. (2019) 53:350–4. doi: 10.1097/MCG.0000000000001147
150. Saritas Yuksel E, Higginbotham T, Slaughter JC, Mabary J, Kavitt RT, Garrett CG, et al. Use of direct, endoscopic-guided measurements of mucosal impedance in diagnosis of gastroesophageal reflux disease. Clin Gastroenterol Hepatol. (2012) 10:1110–6. doi: 10.1016/j.cgh.2012.05.018
151. Ates F, Yuksel ES, Higginbotham T, Slaughter JC, Mabary J, Kavitt RT, et al. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology. (2015) 148:334–43. doi: 10.1053/j.gastro.2014.10.010
152. Patel DA, Higginbotham T, Slaughter JC, Aslam M, Yuksel E, Katzka D, et al. Development and validation of a mucosal impedance contour analysis system to distinguish esophageal disorders. Gastroenterology. (2019) 156:1617–26.e1. doi: 10.1053/j.gastro.2019.01.253
153. Clarke JO, Ahuja NK, Chan WW, Gyawali CP, Horsley-Silva JL, Kamal AN, et al. Mucosal impedance for esophageal disease: evaluating the evidence. Ann N Y Acad Sci. (2020) 1481:247–57. doi: 10.1111/nyas.14414
154. Katzka DA, Tadi R, Smyrk TC, Katarya E, Sharma A, Geno DM, et al. Effects of topical steroids on tight junction proteins and spongiosis in esophageal epithelia of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. (2014) 12:1824–9.e1. doi: 10.1016/j.cgh.2014.02.039
155. Katzka DA, Ravi K, Geno DM, Smyrk TC, Iyer PG, Alexander JA, et al. Endoscopic mucosal impedance measurements correlate with eosinophilia and dilation of intercellular spaces in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. (2015) 13:1242–8.e1. doi: 10.1016/j.cgh.2014.12.032
156. Lowry MA, Vaezi MF, Correa H, Higginbotham T, Slaughter JC, Acra S. Mucosal impedance measurements differentiate pediatric patients with active versus inactive eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2018) 67:198–203. doi: 10.1097/MPG.0000000000001943
157. Alexander JA, Ravi K, Geno DM, Tholen CJ, Higginbotham TC, Wildhorn S, et al. Comparison of mucosal impedance measurements throughout the esophagus and mucosal eosinophil counts in endoscopic biopsy specimens in eosinophilic esophagitis. Gastrointest Endosc. (2019) 89:693–700.e1. doi: 10.1016/j.gie.2018.08.031
158. Eldredge JA, Omari TI, Moore DJ. Pediatric eosinophilic esophagitis is associated with low baseline impedance. J Pediatr Gastroenterol Nutr. (2022) 74:621–5. doi: 10.1097/MPG.0000000000003396
159. Choksi Y, Lal P, Slaughter JC, Sharda R, Parnell J, Higginbotham T, et al. Esophageal mucosal impedance patterns discriminate patients with eosinophilic esophagitis from patients with GERD. Clin Gastroenterol Hepatol. (2018) 16:664–71.e1. doi: 10.1016/j.cgh.2017.12.020
160. Roman S, Keefer L, Imam H, Korrapati P, Mogni B, Eident K, et al. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil. (2015) 27:1667–74. doi: 10.1111/nmo.12666
161. Kawami N, Takenouchi N, Umezawa M, Hoshino S, Hanada Y, Hoshikawa Y, et al. Pathogenesis of double-dose proton pump inhibitor-resistant non-erosive reflux disease, and mechanism of reflux symptoms and gastric acid secretion-suppressive effect in the presence or absence of Helicobacter pylori infection. Digestion. (2017) 95:140–5. doi: 10.1159/000455834
162. Hamada S, Ihara E, Ikeda H, Muta K, Ogino H, Chinen T, et al. Clinical characterization of vonoprazan-refractory gastroesophageal reflux disease. Digestion. (2021) 102:197–204. doi: 10.1159/000503340
163. Armstrong D, Hungin AP, Kahrilas PJ, Sifrim D, Sinclair P, Vaezi MF, et al. (2022). Knowledge gaps in the management of refractory reflux-like symptoms: Healthcare provider survey. Neurogastroenterol Motil . e14387. doi: 10.1111/nmo.14387
164. Ribolsi M, Cicala M, Zentilin P, Neri M, Mauro A, Efthymakis K, et al. Prevalence and clinical characteristics of refractoriness to optimal proton pump inhibitor therapy in non-erosive reflux disease. Aliment Pharmacol Ther. (2018) 48:1074–81. doi: 10.1111/apt.14986
165. Savarino E, Zentilin P, Mastracci L, Dulbecco P, Marabotto E, Gemignani L, et al. Microscopic esophagitis distinguishes patients with non-erosive reflux disease from those with functional heartburn. J Gastroenterol. (2013) 48:473–82. doi: 10.1007/s00535-012-0672-2
166. Vela MF, Craft BM, Sharma N, Freeman J, Hazen-Martin D. Refractory heartburn: comparison of intercellular space diameter in documented GERD vs. functional heartburn. Am J Gastroenterol. (2011) 106:844–50. doi: 10.1038/ajg.2010.476
167. Kim YG, Noh CK, Lee KJ. The usefulness of esophageal baseline impedance levels for the diagnosis of nonerosive reflux disease and the proper time for measurement in endoscopy-negative Korean patients with esophageal or supraesophageal symptoms. J Neurogastroenterol Motil. (2020) 26:463–70. doi: 10.5056/jnm20019
168. Woodland P, Sifrim D. Esophageal mucosal integrity in nonerosive reflux disease. J Clin Gastroenterol. (2014) 48:6–12. doi: 10.1097/MCG.0b013e318299f181
169. Tobey NA, Gambling TM, Vanegas XC, Carson JL, Orlando RC. Physicochemical basis for dilated intercellular spaces in non-erosive acid-damaged rabbit esophageal epithelium. Dis Esophagus. (2008) 21:757–64. doi: 10.1111/j.1442-2050.2008.00841.x
170. Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. (2009) 137:1776–84. doi: 10.1053/j.gastro.2009.07.055
171. Huo X, Agoston AT, Dunbar KB, Cipher DJ, Zhang X, Yu C, et al. Hypoxia-inducible factor-2α plays a role in mediating oesophagitis in GORD. Gut. (2017) 66:1542–54. doi: 10.1136/gutjnl-2016-312595
172. He M, Wang Q, Yao D, Li J, Bai G. Association between psychosocial disorders and gastroesophageal reflux disease: a systematic review and meta-analysis. J Neurogastroenterol Motil. (2022) 28:212–21. doi: 10.5056/jnm21044
173. Rengarajan A, Pomarat M, Zerbib F, Gyawali CP. Overlap of functional heartburn and reflux hypersensitivity with proven gastroesophageal reflux disease. Neurogastroenterol Motil. (2021) 33:e14056. doi: 10.1111/nmo.14056
174. van Malenstein H, Farré R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. (2008) 103:1021–8.
175. Tang M, Dettmar P, Batchelor H. Bioadhesive oesophageal bandages: protection against acid and pepsin injury. Int J Pharm. (2005) 292:169–77. doi: 10.1016/j.ijpharm.2004.11.039
176. Kwiatek MA, Roman S, Fareeduddin A, Pandolfino JE, Kahrilas PJ. An alginate-antacid formulation (Gaviscon double action liquid) can eliminate or displace the postprandial ‘acid pocket’ in symptomatic GERD patients. Aliment Pharmacol Ther. (2011) 34:59–66. doi: 10.1111/j.1365-2036.2011.04678.x
177. Miwa H, Koseki J, Oshima T, Kondo T, Tomita T, Watari J, et al. Rikkunshito, a traditional Japanese medicine, may relieve abdominal symptoms in rats with experimental esophagitis by improving the barrier function of epithelial cells in esophageal mucosa. J Gastroenterol. (2010) 45:478–87.
Keywords : gastroesophageal reflux disease, VOSviewer, CiteSpace, bibliometrics, trends
Citation: Zhang T, Zhang B, Tian W, Wei Y, Wang F, Yin X, Wei X, Liu J and Tang X (2022) Trends in gastroesophageal reflux disease research: A bibliometric and visualized study. Front. Med. 9:994534. doi: 10.3389/fmed.2022.994534
Received: 14 July 2022; Accepted: 06 September 2022; Published: 29 September 2022.
Reviewed by:
Copyright © 2022 Zhang, Zhang, Tian, Wei, Wang, Yin, Wei, Liu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Tang, [email protected]
† These authors have contributed equally to this work and share first authorship
This article is part of the Research Topic
Global Excellence in Gastroenterology: Europe
Clinical Trials
Gastroesophageal reflux disease (gerd).
Displaying 22 studies
The aim of this study is to provide pilot data on the possible gastrointestinal predictors of respiratory hyper-responsiveness and how these relate to the clinical sub-types of GERD and visceral acid hypersensitivity.
In patients with gastroesophageal reflux and/or laryngopharyngeal reflux with cricopharyngeal hypertrophy or Zenker's diverticulum, does cricopharyngeal myotomy increase the amount of laryngopharyngeal reflux?
The purpose of this study is to determine if an artificial intelligence (AI) program can efficiently determine whether patients have gastro-esophageal reflux disease (GERD) via voice recordings and AI training.
This registry is intended as an observational, post-marketing surveillance tool. The purpose of this registry is to collect baseline demographics and user experience of the commercially available MUSE (Medigus Ultrasonic Endostapler) system for the treatment of gastroesophageal reflux disease. The data collection aims to gather a minimum of 3 years follow-up on a large number of subjects during use of the system outside of a prescribed clinical study.
The purpose of this study is to evaluate and compare esophageal high resolution impedance manometry (HRIM) with direct mucosal impedance catheter diagnostic measurements in patients with gastroesophageal reflux disorder.
This study aims to understand why patients have predominantly upright gastroesophageal reflux disease by comparing such patients to healthy persons AND whether a behavioral intervention (diaphragmatic breathing) will impact this disease
The purpose of this study is to gather information and determine if one achalasia procedure at Mayo Clinic Rochester is superior to the other.
The purpose of this study is to identify a predictive model for worsening of preexisting gastroesophageal reflux disease (GERD) or development of de novo GERD after laparoscopic sleeve gastrectomy (LSG) by using EndoFLIP, upper endoscopy and esophageal manometry to assess patients preoperatively in a discovery cohort. This prospective predictive model will then identify sensitivity, specificity, positive and negative predictive values for prediction of worsening or de novo GERD.
This study will use a 22 gene pharmacogenomics panel on 30 children with persistent Gastroesophageal Reflux Disease (GERD) who have not responded to therapy.
The purpose of this study is to establish if abdominal breathing exercises can reduce symptoms associated with reflux.
The purpose of this study is to demonstrate the safety and effiectiveness of Aluvra for the treatment of GERD.
The purpose of this study is to evaluate the accuracy of balloon catheter based mucosal impedance for the diagnosis of GERD in patients who have undergone Peroral Endoscopic Myotomy (POEM).
This study is being done to determine if people with and without GERD or trouble swallowing have increased esophageal mucosa impedance (food getting into the esophageal tissue).
This is a mechanistic research study to evaluate the relationship between cough, reflux, and aspiration in patients with systemic sclerosis (scleroderma).
Is waist to hip ratio (WHR), waist circumference (WC), (as markers of visceral adiposity) associated with an increase in acidic and non acidic reflux as well as systemic inflammation involving esophageal mucosa, thereby increasing esophageal injury and predisposing to subsequent development of Barrett's esophagus (BE)?
The purpose of this study is to compare treatment effectiveness and safety between Transoral Incisionless Fundoplication (TIF) and Laparoscopic Nissen Fundoplication (LNF) in GERD patients with hiatal hernia undergoing hernia repair.
Prospective registry comparing outcomes after laparoscopic treatment of gastroesophageal reflux disease and hiatal hernia.
The purpose of this study is to learn more about the prevalence of gastroesophageal reflux among patients with esophagitis using long term proton-pump-inhibitor use.
The investigators would like to determine if there are patients with PPI responsive Eosinophilic Esophagitis Infiltration that have significant loss of esophageal distensibility suggestive of esophageal fibrosis typical of classic Eosniophilic Esophagitis.
If this group of patients exists, the investigators would like to determine if they have the typical endoscopic features of EoE rather than those of GERD.
This study will evaluate if the sponge capsule device can accurately detect the presence of Barrett's Esophagus and prevalent dysplasia/adenocarcinoma detection, in a screening population, with and without chronic gastroesophageal reflux disease.
The purpose of this study is to evaluate the safety and effectiveness of IW-3718 administered to patients with GERD who continue to have persistent symptoms, such as heartburn and regurgitation, while receiving once-daily (QD), standard-dose proton pump inhibitors (PPIs).
The purpose of this study is to collect data related to patients undergoing diagnostic evaluation and treatment of Gastroesophageal Reflux Disease (GERD) and its associated diseases in multiple academic and community settings.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Digestive Diseases
- Acid Reflux (GER & GERD) in Adults
- Clinical Trials
- Español
Clinical Trials for GER & GERD
The NIDDK conducts and supports clinical trials in many diseases and conditions, including digestive diseases. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for GER & GERD?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help doctors and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of gastroesophageal reflux disease (GERD), such as
- how diet affects symptoms
- new ways to diagnose the disease
- new treatments for GERD
- risk factors for GERD complications, such as Barrett’s esophagus and esophageal cancer
Find out if clinical studies are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical studies for GER & GERD are looking for participants?
You can view a filtered list of clinical studies on GERD that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the NIH does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
- Patient Care & Health Information
- Diseases & Conditions
- Gastroesophageal reflux disease (GERD)
How heartburn and GERD occur
Acid reflux occurs when the sphincter muscle at the lower end of your esophagus relaxes at the wrong time, allowing stomach acid to back up into your esophagus. This can cause heartburn and other signs and symptoms. Frequent or constant reflux can lead to GERD .
Gastroesophageal reflux disease (GERD) occurs when stomach acid repeatedly flows back into the tube connecting your mouth and stomach (esophagus). This backwash (acid reflux) can irritate the lining of your esophagus.
Many people experience acid reflux from time to time. However, when acid reflux happens repeatedly over time, it can cause GERD .
Most people are able to manage the discomfort of GERD with lifestyle changes and medications. And though it's uncommon, some may need surgery to ease symptoms.
Products & Services
- A Book: Mayo Clinic on Digestive Health
- A Book: Mayo Clinic on Healthy Aging
Common signs and symptoms of GERD include:
- A burning sensation in your chest (heartburn), usually after eating, which might be worse at night or while lying down
- Backwash (regurgitation) of food or sour liquid
- Upper abdominal or chest pain
- Trouble swallowing (dysphagia)
- Sensation of a lump in your throat
If you have nighttime acid reflux, you might also experience:
- An ongoing cough
- Inflammation of the vocal cords (laryngitis)
- New or worsening asthma
When to see a doctor
Seek immediate medical care if you have chest pain, especially if you also have shortness of breath, or jaw or arm pain. These may be signs and symptoms of a heart attack.
Make an appointment with your doctor if you:
- Experience severe or frequent GERD symptoms
- Take over-the-counter medications for heartburn more than twice a week
More Information
Gastroesophageal reflux disease (GERD) care at Mayo Clinic
- GERD: Can certain medications make it worse?
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest health information from Mayo Clinic delivered to your inbox.
Subscribe for free and receive your in-depth guide to digestive health, plus the latest on health innovations and news. You can unsubscribe at any time. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth digestive health guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest health news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
GERD is caused by frequent acid reflux or reflux of nonacidic content from the stomach.
When you swallow, a circular band of muscle around the bottom of your esophagus (lower esophageal sphincter) relaxes to allow food and liquid to flow into your stomach. Then the sphincter closes again.
If the sphincter does not relax as it should or it weakens, stomach acid can flow back into your esophagus. This constant backwash of acid irritates the lining of your esophagus, often causing it to become inflamed.
Risk factors
Hiatal hernia
A hiatal hernia occurs when the upper part of your stomach bulges through your diaphragm into your chest cavity.
Conditions that can increase your risk of GERD include:
- Bulging of the top of the stomach up above the diaphragm (hiatal hernia)
- Connective tissue disorders, such as scleroderma
- Delayed stomach emptying
Factors that can aggravate acid reflux include:
- Eating large meals or eating late at night
- Eating certain foods (triggers) such as fatty or fried foods
- Drinking certain beverages, such as alcohol or coffee
- Taking certain medications, such as aspirin
Complications
Over time, chronic inflammation in your esophagus can cause:
- Inflammation of the tissue in the esophagus (esophagitis). Stomach acid can break down tissue in the esophagus, causing inflammation, bleeding, and sometimes an open sore (ulcer). Esophagitis can cause pain and make swallowing difficult.
- Narrowing of the esophagus (esophageal stricture). Damage to the lower esophagus from stomach acid causes scar tissue to form. The scar tissue narrows the food pathway, leading to problems with swallowing.
- Precancerous changes to the esophagus (Barrett esophagus). Damage from acid can cause changes in the tissue lining the lower esophagus. These changes are associated with an increased risk of esophageal cancer.
- What causes laryngospasm?
- Maret-Ouda J, et al. Gastroesophageal reflux disease: A review. JAMA. 2020; doi:10.1001/jama.2020.21360.
- Katz PO, et al. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. American Journal of Gastroenterology. 2022; doi:10.14309/ajg.0000000000001538.
- Acid reflux (GER and GERD) in adults. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/digestive-diseases/acid-reflux-ger-gerd-adults. Accessed April 13, 2022.
- Kahrilas PJ. Clinical manifestations and diagnosis of gastroesophageal reflux in adults. https://www.uptodate.com/contents/search. Accessed April 13, 2022.
- AskMayoExpert. Gastroesophageal reflux disease (GERD) (adult). Mayo Clinic. 2021.
- Townsend CM Jr, et al. Gastroesophageal reflux disease and hiatal hernia. In: Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 21st ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed April 13, 2022.
- Feldman M, et al., eds. Gastroesophageal reflux disease. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed April 13, 2022.
- Kahrilas PJ. Medical management of gastroesophageal reflux in adults. https://www.uptodate.com/contents/search. Accessed April 13, 2022.
- Winter HS. Management of gastroesophageal reflux disease in children and adolescents. https://www.uptodate.com/contents/search. Accessed April 13, 2022.
- Ami TR. Allscripts EPSi. Mayo Clinic. April 7, 2022.
- Khanna S (expert opinion). Mayo Clinic. May 1, 2022.
- GERD surgery
- Heartburn medicines and B-12 deficiency
- Opera Star's Surgery
- Substitute for esophageal sphincter
Associated Procedures
- Needle biopsy
- Upper endoscopy
News from Mayo Clinic
- Mayo Clinic Minute: GERD is not 'just' heartburn Nov. 24, 2023, 04:00 p.m. CDT
- Mayo Clinic Healthcare expert explains why reflux disease isn't `just' heartburn Jan. 12, 2023, 04:28 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Share full article
Advertisement
Subscriber-only Newsletter
5 Gut Facts Experts Want You to Know
We asked gastroenterologists every mortifying question we could think of. Here’s straight talk on constipation, heartburn and gas.

By Jancee Dunn
Flatulence, constipation and diarrhea can be embarrassing. But you don’t need to be shy about discussing them with a gastroenterologist, a doctor who treats conditions of the digestive system.
“We’ve heard it all,” said Sophie Balzora, a gastroenterologist and clinical professor of medicine at NYU Langone Health. “And we want to normalize talking about it.”
So I took her advice and plunged in, asking her and other experts what they want you to know about your gut.
Acid reflux and heartburn are not the same thing.
People often use the terms “acid reflux” and “heartburn” interchangeably, but it’s important not to mix them up, said Christine Lee, a gastroenterologist at Cleveland Clinic.
Acid reflux, which affects nearly a third of U.S. adults weekly, is the backward flow of stomach acid into the esophagus. Sometimes it progresses to a more serious condition called gastroesophageal reflux disease, or GERD.
Heartburn, on the other hand, is a symptom, not a condition. A burning pain in the chest can be caused by acid reflux or GERD. It can also be a sign of other problems, including heart trouble or a peptic ulcer, so it’s worth visiting a doctor if you have persistent heartburn, Dr. Lee said.
And if you think you have GERD, check in with your doctor to help you get it under control, Dr. Lee said, not only because you can get some relief, but because it has been associated with esophageal cancer .
Taking antacids regularly can cause (or mask) problems.
Just because antacids are often sold next to gum and mints at the drugstore doesn’t mean that you should eat them like candy, Dr. Balzora said. If you’re taking any antacids daily, or even a few times a week, that’s a sign that something could be wrong, she added.
Overusing these medications, she said, can cause additional problems. Regularly downing calcium-based antacids, for instance, can increase your risk for kidney stones.
You don’t have to poop every day.
Some people worry about not having a daily bowel movement, but constipation is generally defined as having fewer than three bowel movements a week .
“There’s a wide range of what’s considered normal,” Dr. Balzora said. If you’re a “three-a-weeker,” as she puts it, and you’re not having pain or difficulty passing or any other symptoms, she said, “then that’s fine.”
What’s more noteworthy is a sudden change in habits. If “you used to be a one-a-dayer and now you’re a once-a-weeker, or vice versa,” Dr. Balzora said, “then that needs to be evaluated.”
Constipated? Check your medicine cabinet.
If you have constipation, your first strategy should be to make lifestyle changes such as adding more water and fiber to your diet and exercising a little more, said Xavier Llor, a gastroenterologist at Yale Medicine.
But many people overlook how often medications can be a culprit, Dr. Llor said. “You’ll hear an ad with potential side effects, and get bored and stop listening, but so many medications are constipating,” he said.
Prescription medications like antidepressants and blood pressure medication can cause constipation, according to the National Institute on Aging. So can over-the-counter drugs like pain killers, antihistamines and some antacids , as well as calcium and iron supplements.
If you are using a medication that stops you up, “ask your doctor if there’s a nonconstipating alternative,” Dr. Llor said.
Healthy habits may lower your risk of I.B.S.
Irritable bowel syndrome, a gastrointestinal condition that brings on frequent episodes of diarrhea, constipation or cramping, is one of the most commonly diagnosed digestive disorders .
There’s no cure , but there are habits that may lower your overall risk of developing it, Dr. Balzora said. A recent study that followed almost 65,000 people for over 12 years looked at five healthy behaviors: never smoking, regular exercise, moderate alcohol consumption, a healthy diet and at least seven hours of sleep. Those who did at least three of those things had a 42 percent lower risk of developing I.B.S.
If you think you have I.B.S., don’t self-diagnose, said Natasha Chhabra, a gastroenterologist at Gastroenterology Associates of New Jersey. A doctor will perform specific tests, she said, as well as screen for conditions, like celiac disease.
As I wound up my chat with Dr. Balzora, she stressed the importance of having open conversations around bowel habits, and signed off with a possibly liberating fact: The average healthy person passes gas 10 to 20 times a day.
“I’m on a crusade to normalize flatulence, too,” she said.
Protect yourself from melanoma, a dangerous type of skin cancer.
About 100,000 people are diagnosed with melanoma each year in the United States. Ted Alcorn explores simple ways to reduce your risk and to catch possible cases early, while they are most curable.
Read the article: How to Avoid One of the Deadliest Forms of Skin Cancer
To get the best care at the dentist, speak up.
A visit to the dentist can be confusing or intimidating. Knvul Sheikh reports on how to demystify what’s going on in your mouth, and figure out what treatments are necessary.
Read the article: How to Advocate for Yourself at the Dentist
The Week in Well
Here are some stories you don’t want to miss:
Holly Burns finds that a little bit of dirt is good for you .
Amanda Loudin explores how to turn a bike ride into a bike workout .
Christina Caron reports on the growing practice of prescribing social activities for mental health.
Why do we have tick medication for dogs but not for people? Dana Smith investigates .
Let’s keep the conversation going. Follow Well on Instagram , or write to us at [email protected] . And check out last week’s newsletter about decluttering projects you can do in half an hour .
Jancee Dunn , who writes the weekly Well newsletter for The Times, has covered health and science for more than 20 years. More about Jancee Dunn
Use of acid reflux drugs linked to higher risk of migraine
People who take acid-reducing drugs may have a higher risk of migraine and other severe headache than people who do not take these medications, according to a study published in the April 24, 2024, online issue of Neurology ® Clinical Practice , an official journal of the American Academy of Neurology. The acid-reducing drugs includeproton pump inhibitors such as omeprazole and esomeprazole, histamine H2-receptor antagonists, or H2 blockers, such as cimetidine and famotidine, and antacid supplements.
The study does not prove that acid-reducing drugs cause migraine; it only shows an association.
Acid reflux is when stomach acid flows into the esophagus, usually after a meal or when lying down. People with acid reflux may experience heartburn and ulcers. People with frequent acid reflux may develop gastroesophageal reflux disease, or GERD, which can lead to cancer of the esophagus.
"Given the wide usage of acid-reducing drugs and these potential implications with migraine, these results warrant further investigation," said study author Margaret Slavin, PhD, RDN, of the University of Maryland in College Park. "These drugs are often considered to be overprescribed, and new research has shown other risks tied to long-term use of proton pump inhibitors, such as an increased risk of dementia."
For the study, researchers looked at data on 11,818 people who provided information on use of acid-reducing drugs and whether they had migraine or severe headache in the past three months.
A total of 25% of participants taking proton pump inhibitors had migraine or severe headache, compared to 19% of those who were not taking the drugs. A total of 25% of those taking H2 blockers had severe headache, compared to 20% of those who were not taking those drugs. And 22% of those taking antacid supplements had severe headache, compared to 20% of those not taking antacids.
When researchers adjusted for other factors that could affect the risk of migraine, such as age, sex and use of caffeine and alcohol, they found that people taking proton pump inhibitors were 70% more likely to have migraine than people not taking proton pump inhibitors. Those taking H2 blockers were 40% more likely and those taking antacid supplements were 30% more likely.
"It's important to note that many people do need acid-reducing medications to manage acid reflux or other conditions, and people with migraine or severe headache who are taking these drugs or supplements should talk with their doctors about whether they should continue," Slavin said.
Slavin noted that the study looked only at prescription drugs. Some of the drugs became available for over-the-counter use at non-prescription strength during the study period, but use of these over-the-counter drugs was not included in this study.
Other studies have shown that people with gastrointestinal conditions may be more likely to have migraine, but Slavin said that relationship is not likely to fully explain the tie between acid-reducing drugs and migraine found in the study.
A limitation of the study is that a small number of people were taking the drugs, especially the H2 blockers.
- Headache Research
- Gastrointestinal Problems
- Disorders and Syndromes
- Anti-obesity drug
- Antiretroviral drug
- Antioxidant
- Cells of the stomach
- Antihistamine
Story Source:
Materials provided by American Academy of Neurology . Note: Content may be edited for style and length.
Journal Reference :
- Margaret Slavin, Cara L. Frankenfeld, Alexander B. Guirguis, Elizabeth K. Seng. Use of Acid-Suppression Therapy and Odds of Migraine and Severe Headache in the National Health and Nutrition Examination Survey . Neurology Clinical Practice , 2024; 14 (3) DOI: 10.1212/CPJ.0000000000200302
Cite This Page :
Explore More
- Sustainable Jet Fuel from Landfill Emissions
- Bacterial Spores in Bioplastic Make It 'Green'
- Genetic Signals Linked to Blood Pressure
- Double-Fangs of Adolescence Saber-Toothed Cats
- Microarray Patches for Vaccinating Children
- Virus to Save Billions of Gallons of Wastewater
- Weather Report On Planet 280 Light-Years Away
- Trotting Robots and Animal Gait Transitions
- Where Have All the Fireflies Gone?
- Cardio-Fitness Cuts Death and Disease by 20%
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
The phenotypic presentations of GERD include nonerosive reflux disease (in 60 to 70% of patients), erosive esophagitis (in 30%), and Barrett's esophagus (in 5 to 12%). 9 The focus of the present ...
Gastroesophageal reflux disease (GERD) is a chronic gastrointestinal disorder characterized by the regurgitation of gastric contents into the esophagus. It is one of the most commonly diagnosed digestive disorders in the US with a prevalence of 20%, resulting in a significant economic burden in direct and indirect costs and adversely affects the quality of life[1][2]. GERD is caused by ...
The pathogenesis of GERD involves impaired EGJ barrier, esophageal hypomotility, refluxate and acid burden, psychiatric comorbidity, and hypervigilance. Acid-inhibition therapy is the mainstay of medical treatment for GERD. Recent advances in diagnostic testing for GERD have shed light on tailored management.
Importance: Gastroesophageal reflux disease (GERD) is defined by recurrent and troublesome heartburn and regurgitation or GERD-specific complications and affects approximately 20% of the adult population in high-income countries. Observations: GERD can influence patients' health-related quality of life and is associated with an increased risk of esophagitis, esophageal strictures, Barrett ...
ing this time, scrutiny of proton pump inhibitors (PPIs) has increased considerably. Although PPIs remain the medical treatment of choice for GERD, multiple publications have raised questions about adverse events, raising doubts about the safety of long-term use and increasing concern about overprescribing of PPIs. New data regarding the potential for surgical and endoscopic interventions have ...
A systematic review demonstrated that the prevalence of GERD ranged from 18.1% to 27.8% in North America, 8.8% to 25.9% in Europe, 2.5% to 7.8% in East Asia, 8.7% to 33.1% in the Middle East, 11.6% in Australia, and 23.0% in South America. 3. The cardinal symptoms of GERD are heartburn and regurgitation. 4 However, GERD may present with a ...
The Lyon Consensus 2.0 updates oesophageal test parameters that either conclusively establish or rule out the presence of GERD (figure 3). The existing Lyon Consensus criteria have been updated based on new research, and parameters that have not functioned as diagnostic criteria have been retired (table 3).
Gastro-oesophageal reflux disease (GERD) is a common disorder in adults and children. The global prevalence of GERD is high and increasing. Non-erosive reflux disease is the most common phenotype ...
From the inside of the stomach, several H-fasteners are placed under endoscopic visualization to create an anti-reflux flap valve (center). The new anti-reflux barrier is typically about 3 centimeters in length, and at least 270 degrees in circumference, preserving the ability to belch and vomit, and minimizing dysphagia and gas-bloat (right).
Gastro-oesophageal reflux disease is a condition in which contents of the stomach (gastric acid) flow back into the oesophagus, causing injury to the oesophagus and problematic symptoms such as ...
GERD Drug Offers New Approach to Relieve Chronic Symptoms. March 25, 2020. Rather than doubling down on acid reflux, compound targets bile reflux. An investigational drug that binds to bile in the stomach may provide relief for patients with difficult-to-treat cases of gastroesophageal reflux (GERD). The drug acts differently from protein-pump ...
Purpose of review To examine recent key developments in the pathophysiology, diagnosis, and treatment of gastroesophageal reflux disease (GERD). Recent findings Newer research has suggested cytokine-mediated inflammation may play a role in the physiology of GERD, implying that the underlying mechanism may not be entirely related to chemical damage due to acid. Aided by novel technologies ...
April 25, 2024 - Treatments for acid reflux may increase the risk of experiencing migraines or severe headaches, a new study shows. Acid reflux affects as many as 1 in 3 adults on a weekly basis ...
Introduction. Evidence-based clinical practice guidelines for gastroesophageal reflux disease (GERD) 2015 (revised 2nd edition) were published in October 2015 [].New findings on the management of GERD were subsequently reported, and vonoprazan, a potassium-competitive acid blocker (P-CAB), became available in Japan in February 2015 for the first time as a treatment for reflux esophagitis (RE) [].
To confirm a diagnosis of GERD, or to check for complications, your doctor might recommend: Upper endoscopy. Your doctor inserts a thin, flexible tube equipped with a light and camera (endoscope) down your throat. The endoscope helps your provider see inside your esophagus and stomach. Test results may not show problems when reflux is present ...
Background: Gastroesophageal reflux disease (GERD), a disorder resulting from the retrograde flow of gastric contents into the esophagus, affects an estimated 10% to 30% of the Western population, which is characterized by multifactorial pathogenesis. ... , that are able to strengthen esophageal defenses, has sparked new research in this ...
This is a mechanistic research study to evaluate the relationship between cough, reflux, and aspiration in patients with systemic sclerosis (scleroderma). Anthropometry in Gastroesophageal Reflux Disease and Esophageal Injury Rochester, MN. Is waist to hip ratio (WHR), waist circumference (WC), (as markers of visceral adiposity) associated with ...
Research published in The New England Journal of Medicine suggests a new course of diagnosis and treatment for the estimated one in five adults with symptoms of gastroesophageal reflux disease, or GERD.. Heartburn is the most commonly reported symptom of the disease. However, the new research suggests that many patients who are unresponsive to treatment by proton pump inhibitors, a standard ...
Heartburn, GERD and Acid Reflux help. Read the latest medical research on acid reflux symptoms and the causes for heartburn as well as heartburn remedies and new treatment options.
The trials look to find new ways to prevent, detect, or treat disease and improve quality of life. What are clinical trials for GER & GERD? Clinical trials—and other types of clinical studies—are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help doctors and researchers learn ...
Gastroesophageal reflux disease (GERD) is a chronic, progressive, and relapsing condition, affecting up to 33% of the population worldwide ( 1, 2 ). It is estimated that up to 40% of the US population report GERD symptoms monthly and 20% weekly ( 3 ). GERD can be divided classically into non-erosive esophageal reflux disease (NERD) and erosive ...
Gastroesophageal reflux disease (GERD) occurs when stomach acid repeatedly flows back into the tube connecting your mouth and stomach (esophagus). This backwash (acid reflux) can irritate the lining of your esophagus. ... You will also receive emails from Mayo Clinic on the latest health news, research, and care. If you don't receive our ...
Acid reflux, which affects nearly a third of U.S. adults weekly, is the backward flow of stomach acid into the esophagus. Sometimes it progresses to a more serious condition called ...
People with frequent acid reflux may develop gastroesophageal reflux disease, or GERD, which can lead to cancer of the esophagus. ... and new research has shown other risks tied to long-term use ...
Abstract. Gastroesophageal reflux disease (GERD) is a chronic condition which has a high global prevalence. Dietary intake is considered to be a contributing factor for GERD. However, scientific evidence about the effect of diet on the risk of GERD is controversial. This systematic review was conducted to address this issue.
Introduction. Gastroesophageal reflux disease (GERD) is a very common digestive disorder worldwide with an estimated prevalence of 18.1-27.8% in North America. 1 Approximately half of all adults will report reflux symptoms at some time. 2 According to the Montreal definition, GERD is a condition of troublesome symptoms and complications that result from the reflux of stomach contents into ...