Fuel Cell: Working Principle, Characteristics, Systems, Advantages and Disadvantages
The article provides an overview of fuel cells, describing their basic working principles, historical development, characteristics, and applications. It touches on topics such as oxidation-reduction reactions, fuel cell systems, hydrogen production, and the role of nanotechnology in enhancing fuel cell efficiency. Additionally, the advantages and disadvantages of fuel cells are discussed, emphasizing their reliability, clean operation, and challenges related to hydrogen storage and costly catalysts.
Fuel cells combine a fuel (usual hydrogen in some form) with an oxidizing agent (usually oxygen). In the hydrogen fuel cell, hydrogen and oxygen react to form water as a by-product.
Electrical current is produced when electrons are freed during the process, which is clean, quiet, and more efficient than burning fuels.
A fuel cell is a device that converts electrochemical energy into DC, much like a battery.
One difference is that a battery stores its chemicals inside; a fuel cell has a constant flow of fuel into the system from an outside source.

Fuel Cell History
Early major users of fuel cells were NASA and the military because of their very specialized requirements and because the high cost of manufacture was not the main issue.
Fuel cells were first used by NASA in 1962 in the Gemini space program, a manned space program.
Fuel cells replaced battery power as a power source on the shorter flights of the Mercury space program, which preceded Gemini.
Improved alkaline fuel cells were used for the longer flights to the moon on the Apollo missions, and later on the space shuttle.
NASA went on to fund 200 research contracts for fuel cell technology. Today, renewable energy systems are able to take advantage of this research.
Fuel Cell Working Principle
This section covers the operating mechanism of fuel cells, providing insights into their fundamental processes and functionality.
Today fuel cells are used to produce electrical power for newer spacecraft; remote undersea stations; and mobile vehicles such as automobiles, trucks, buses, forklifts, and tractors.
Some larger fuel cells provide power to buildings or small cities as stationary electrical plants. These units are highly reliable and can bring power closer to the end user, and thus save on distribution costs.
Oxidation-Reduction Reactions
Like all physical systems, chemical reactions tend to go from a higher energy state to a lower state (energy decreases).
Hydrogen gas, when in the presence of oxygen, can react explosively, producing light and heat after the addition of a small amount of activation energy (heat from a flame). The reaction of burning hydrogen is
2 H 2 (g) +O 2 (g) ↔2 H 2 O (l) +Energy
This chemical equation shows that two molecules of hydrogen can react with one molecule of oxygen to produce two molecules of water plus a net change in energy (called free energy).
The process involves breaking the chemical bonds in the gases (H 2 and O 2 ), which absorb energy. New bonds are formed in the water molecule, which releases energy, and the system becomes stable at a lower energy.
The free energy of the system has decreased and has appeared as heat and light from the reaction. Not all of the energy in the reaction is available for work—some go into an increase in entropy, a quantity that measures the randomness of disorder in a system. This energy is not available for work, so there is an automatic limit on the efficiency of a fuel cell.
Sir William Grove, a British scientist who is often referred to as the father of the fuel cell, performed research in 1839 on reversing the electrolysis of water.
Groves found that it was possible to free electrons in the process, and the free electrons could power a small electrical load.
In 1889, Charles Langer and Ludwig Mond completed further research in Britain and coined the term fuel cell.
In 1932, Francis Bacon, at Cambridge University in England, developed what was perhaps the first successful fuel cell device, with a hydrogen-oxygen cell using alkaline electrolytes and nickel electrodes.
The chemical reaction for the combustion of hydrogen is called an oxidation reaction (in this case, combining hydrogen with oxygen).
The process involves transferring electrons from hydrogen to oxygen; hydrogen loses electrons and oxygen gains electrons.
Oxidation is the process in which one reactant loses one or more electrons, and reduction is the process in which a reactant gains one or more electrons.
The two processes always occur together, so the type of reaction is referred to as a redox (from reduction-oxidation) reaction.
The reaction described for oxidation of hydrogen is one of the most common reactions used for fuel cells.
In a fuel cell, the free energy is turned into electricity and heat. The reactions vary from one cell to another, but all fuel cells use a redox reaction in which electrons are separated from a fuel (oxidation), travel in an external circuit, and combine (reduction) with oxygen to complete the process.
Like fuel cells , batteries also use redox reactions in which electrons travel through the external circuit to complete the reaction. The main difference is that, in a fuel cell, a constant supply of fuel is supplied, whereas, in a battery, the reactants are stored within the battery.
You may have noticed that the arrow in the equation for the oxidation of hydrogen is double-headed, implying that the reaction is reversible .
To separate the resulting water molecules back into hydrogen gas and oxygen gas, energy must be added.
Electrolysis is the breaking down of a substance that contains ions by applying an electric current between two electrodes and separating the substance into its components.
The electrolysis of liquid water breaks it down into hydrogen and oxygen gases, which is one way to obtain pure hydrogen fuel for use in a fuel cell.
Although hydrogen is the most common element in the universe, it is not available in its elemental form on earth and must be generated from some other source. For this reason, it is not considered to be a primary source.
It is important to understand that the energy to produce hydrogen is never fully recovered.
If an electric power plant that burns fossil fuel is used to create the hydrogen used in a fuel cell, the net effect is more steps in the process, and each step loses a little of the available energy.
Fuel Cells Characteristics
This section discusses the key characteristics of fuel cells, exploring their fundamental attributes and functionalities.
Figure 1 shows a simplified diagram of a typical fuel cell, which has three basic elements :
(1) An anode that separates the hydrogen fuel into positive ions and electrons;
(2) A cathode that forms oxygen ions from oxygen molecules and electrons and combines negative oxygen ions and the positive hydrogen ions to form water; and
3) An electrolyte, which is a conductive substance that passes hydrogen ions from the anode to the cathode.
The type of material for the electrolyte differs in various types of fuel cells, but it must meet certain requirements, such as mechanical strength and resistance to impurities, as well as being a good conductor.
Within the cell, hydrogen ions migrate across a barrier and combine at the cathode to complete the reaction.
The anode and cathode provide the electrical connection to the external electrical circuit. The electrolyte material is sandwiched between the anode and cathode.
Together, the components in a fuel cell, which includes the anode and cathode electrodes, the electrolyte, and the catalyst, form an assembly called the membrane electrode assembly, where the chemical reactions occur.
The anode is made of porous material such as carbon and is coated by platinum. It must be capable of allowing hydrogen ions to pass while discriminating against electrons and hydrogen molecules.
The catalyst in the cathode breaks apart oxygen molecules, forming two oxygen ions.
The cathode is made of porous material that is coated by a material such as platinum or nickel and allows the hydrogen ions to combine with the oxygen ions to form water molecules (H 2 O), which are eliminated from the cell as a by-product.
Both the anode and cathode are constructed with a catalyst. This means that both the anode and cathode must be able to facilitate the reactions within the fuel cell; ideally, they should have high electrical conductivity as well.
The best materials for the electrodes depend on the type of fuel cell.

Figure 1 Simplified Diagram of a Typical Fuel Cell
Water is removed from the fuel cell at the bottom right side in Figure 1.
Some fuel cells also create extreme heat during the process, so the heat can be used to heat buildings or to create steam, which can be used to power a steam turbine to make electrical power.
By controlling the means by which such a reaction occurs and directing the reaction through a heat exchanger, it is possible to harvest the heat energy.
Some fuel cells must operate on pure hydrogen and pure oxygen, while others have been designed to operate on hydrocarbon fuels such as methane, butane, natural gas, coal, or gasoline. These fuel cells use a reformer, which separates the hydrogen from the hydrocarbon fuel.
Hydrocarbons are especially useful for fuel cells in vehicles because transporting pure hydrogen is expensive and dangerous.
Fuel Cell Systems
The fuel cell itself is but one part of the overall fuel cell system. Fuel cell systems are used for applications such as stationary power units and for transportation, that is, electric vehicles.
A fuel cell system has three basic parts: the fuel cell stack ; the fuel processing unit ; and a heat recovery system that processes the excess heat that is a by-product of the fuel cell operation.
Systems that have AC output for the grid have a standard electrical inverter to change the DC output to AC. Some systems also have a water containment subsystem.
Fuel Cell Stack
The fuel cell stack consists of a group of fuel cells that are connected and bound together to provide increased electrical power.
The output voltage of a single fuel cell is very small (about 0.7 V), so fuel cells are connected (stacked) in series to increase the voltage and in parallel to increase the amount of current they can provide.
The stack is typically rated by the total wattage it can provide. The exact number of cells and how they are connected depends on the current, voltage, and power requirement of the load.
The system includes an inverter if the load requires AC. Figure 2 shows a 5-kW fuel cell stack and a physically smaller 25 W fuel cell stack.

Figure 2 Five kW Fuel Cell Stack with a Smaller 25 W Three-Cell Stack Diagram
Fuel Processing Unit
The fuel processing unit is the portion of a fuel cell system that converts the input fuel into a form usable by the fuel cell.
If hydrogen is fed to the system, a processor may not be required, or it may be needed only to filter impurities out of the hydrogen gas.
In solar energy systems , the system may be used to prepare pure hydrogen and oxygen from water; in which case, the fuel processor is not needed.
Larger fuel cell systems frequently use methane or another hydrocarbon to produce electrical power, so the fuel processor is required to extract the hydrogen from the hydrocarbon.
Heat Recovery System
Fuel cells typically operate at high temperatures (up to 600° C to 700° C), so they produce heat as a by-product.
The heat recovery system collects excess heat for another use, which increases the overall energy efficiency of the fuel cell system.
The excess heat can be used for hot water and/or steam generation. Steam can be used to drive a turbine and generator to produce electricity.
Hot water can be used to provide heat for buildings or power an absorption air-conditioning system.
Fuel Cell Example
How many 25-watt fuel cell stacks are needed to produce 5 kW?
The total number of 25-watt fuel cell stacks needed to produce 5 kW: 5 kWy0.025 kW = 200 fuel cell stacks.
Regenerative Fuel Cells
Regenerative fuel cells produce electricity from hydrogen and oxygen, and they generate heat and water as by-products, just like other fuel cells.
The regenerative fuel cell systems also receive extra hydrogen and oxygen from water that is processed from electrical power produced by a solar panel or wind turbine.
Electricity from a solar cell or wind generator is used to recycle water from the fuel cell into hydrogen and oxygen through electrolysis. This process is unique because all the energy is produced through renewable energy systems.
It has been used by NASA on space flights that get electrical power from solar panels as well as fuel cells. In this case, the hydrogen and oxygen can be stored for use in the fuel cell when solar energy is not available.
Obtaining Hydrogen
Hydrogen is the basic fuel for most fuel cells. As mentioned previously, hydrogen is not available naturally and must be produced from another source, so it is not considered to be a primary source.
Electrolysis of water has been mentioned as a useful method for obtaining hydrogen from any source of electricity, including renewable sources.
Electrolysis can produce very pure hydrogen, but it is simply the reverse fuel cell reaction, so it requires a source of electric power and always requires more energy to produce the fuel that can be obtained from the fuel cell.
If the source of electricity for producing the fuel is a renewable source, such as solar or wind , the hydrogen may serve as an energy storage mechanism, available whenever needed and without depleting nonrenewable sources.
Another method used to produce hydrogen is a reaction called steam reformation in which methane (CH 4 ) and steam are passed over a catalyst to produce carbon monoxide (CO) and hydrogen (H 2 ).
The thermal or catalytic process of breaking down a large molecule into smaller ones is called reforming (such as what happens when gasoline is separated from oil).
The carbon monoxide can be burned to create additional hydrogen and carbon dioxide. The chemical reactions involved in steam reformation are:
CH 4 (g)+H 2 O(g)→CO(g)+3H 2 (g)CO(g)+H 2 O(g)→CO 2 (g)+H 2 (g)
A problem with steam reformation is that the resulting hydrogen is not as pure as with electrolysis, so is not suitable for all types of fuel cells.
Also, using a fossil fuel such as methanol, natural gas, propane, gasoline, coal gas, or even coal powder to obtain hydrogen is not carbon-neutral except in the case of biofuels, so this may not be the most desirable way to obtain hydrogen.
No matter how it is obtained, the hydrogen normally is stored as a cryogenic liquid. Hydrogen liquifies, boils, and condenses at −252.5° C (−422° F) at atmospheric pressure.
If hydrogen is stored under pressure, its temperature can be higher before it changes from a liquid to a vapor.
Generally, hydrogen is transported in vehicles as a liquid at cryogenic temperatures, which is one of the problems associated with its use. Converting gaseous hydrogen to a liquid requires a significant amount of energy.
Hydrogen is highly flammable and an accident involving the exposure of liquid hydrogen to the environment means immediate evaporation into a gaseous state, which can be explosive. Thus, stringent safety measures need to be in place for transporting liquid hydrogen and detecting any leaks.
Hydrogen gas must be under very high pressure, but gaseous transport by pipeline offers advantages over other forms of moving it.
No matter how it is stored, hydrogen can enter the microstructure of many metals, so special tanks and pipelines need to be constructed.
Newer hydrogen storage tanks are reinforced with composite carbon fibers that allow hydrogen to be stored at high pressure. To reduce costs, most hydrogen is prepared close to the place where it will be used.
Hydrogen Production Using Electricity from Wind or Solar
Hydrogen for fuel cells can be produced easily by using electricity to separate hydrogen from water or other materials. The biggest drawback to this process is that, in many cases, the electricity used for this process was produced by burning hydrocarbon fuels such as coal or natural gas.
It is possible to use electricity produced by wind turbines or solar panels to make hydrogen. This process is considered a renewable form of energy because the electrical power to isolate the hydrogen comes from a renewable energy system, and it may help by providing a way to store the energy from solar or wind for use as needed.
Carbon Nanotube as a Catalyst in Fuel Cells
Nanotechnology is being used in several ways with fuel cells. One way is to reduce the amount of expensive platinum that is used as the catalyst in certain types of fuel cells.
Nanotechnology allows platinum nanoparticles to be produced. The nanoparticles are used to provide a thinner coating on the anode, which reduces the amount of platinum in the fuel cell and reduces the cost of production.
Nanotechnology is also used to create lighter, more efficient fuel cell membranes. Researchers at the University of Dayton in Ohio found that an array of carbon nanotubes could be used as a catalyst in some fuel cells.
This new material makes the fuel cell more durable, and the material can be made less expensive than more traditional materials.
Advantages and Disadvantages of Fuel Cells
Fuel cells are very reliable. Their advantages for producing electricity, particularly in remote locations, include no moving parts, quiet operation, and heat as a by-product. They also produce clean water as a by-product.
A disadvantage of some fuel cells is that they use expensive platinum catalysts. Platinum is expensive because it is in high demand for many other applications.
Fuel cells that use hydrogen gas as a fuel also have an issue with storing the hydrogen gas safely. As we noted, hydrogen gas must be stored under high pressure.
Fuel Cell Review Questions
- List the three basic parts of a fuel cell. Explain what each part does.
- What is the difference between oxidation and reduction?
- Explain how a fuel cell produces an electrical current.
- Explain what a reformer does.
- What are the benefits of carbon nanotubes for certain fuel cells?
- The basic parts of a fuel cell are the anode, the cathode, and the electrolyte. The anode separates hydrogen into ions and electrons, the cathode forms oxygen ions that combine with hydrogen ions to form water, and the electrode is a conductor that passes hydrogen ions to the cathode.
- Oxidation is a loss of electrons; reduction is a gain in electrons.
- A fuel cell, like a battery, completes an oxidation-reduction reaction, which is a transfer of electrons between reactants. The electrons are transferred in the external circuit, forming a current.
- A reformer converts a hydrocarbon fuel to hydrogen fuel.
- Carbon nanotubes can reduce the amount of platinum required for the fuel cell and can serve as a catalyst in some fuel cells.
Browse Course Material
Course info.
- Prof. Martin Bazant
Departments
- Chemical Engineering
As Taught In
- Electronic Materials
- Analytical Chemistry
- Physical Chemistry
Learning Resource Types
Electrochemical energy systems, lecture notes.
Topics covered in lectures in 2014 are listed below. In some cases, links are given to new lecture notes by student scribes. All scribed lecture notes are used with the permission of the anonymous student author. The recommended reading refers to the lectures notes and exam solutions from previous years or to the books listed below. Lecture notes from previous years are also found in the study materials section.
[Newman] = Newman, John, and Karen E. Thomas-Alyea. Electrochemical Systems . 3rd ed. Wiley-Interscience, 2004. ISBN: 9780471477563. [Preview with Google Books ]
[Bard] = Bard, Allen J., and Larry R. Faulkner. Electrochemical Methods: Fundamentals and Applications . 2nd ed. Wiley, 2000. ISBN: 9780471043720.
[O’ Hayre] = O’ Hayre, Ryan, Suk-Won Cha, et al. Fuel Cell Fundamentals . 2nd ed. Wiley, 2009. ISBN: 9780470258439.
[Huggins] = Huggins, Robert A. Advanced Batteries: Materials Science Aspects . Springer, 2008. ISBN: 9780387764238. [Preview with Google Books ]

You are leaving MIT OpenCourseWare
- Chemical Engineering
- Fuel Cell Technology (Web)
- Co-ordinated by : IIT Delhi
- Available from : 2012-11-26
- Why we need fuel cell?
- Overview - History;Principle of fuel cell technology
- Basic electrochemistry for all the fuel cell
- Gibbs free energy; reversible and irreversible losses; Fuel cell efficiency
- Nernst equation; Effect of temperature, pressure, concentration on Nernst potential
- Concept of electrochemical potential
- Activation polarization
- Concentration polarization
- Ohmic polarization
- Modelling of fuel cell: current-voltage predictions
- Electrolytes
- Current collector/ bipolar plate
- Why characterization needed? Possible ways of characterization
- In-situ characterization especially I-V characteristics and electrochemical impedance spectroscopy; Cyclic voltammetry; Current interruption technique
- Ex-situ characterization especially electrolyte and bipolar plate
- SOFC - High Temperature Fuel Cell
- Comparison of low and high temperature fuel cells
- Hydrogen production
- Hydrogen Storage
- Balance of Plant and Power electronics and system integration
- Web Content
- CODA Coriolis
- Mass Flow Meters
- Mass Flow Controllers
- Liquid Flow Meters
- Liquid Flow Controllers
- Accessories
- Pressure Transducers
- Pressure Controllers for Flowing Processes
- Pressure Controllers for Closed Volumes
- Technical Documentation
- Part Number Decoder
- Certifications
- Software and Drivers
- Choosing an Instrument
- Tutorials and FAQs
- Case Studies and Apps
- Aerospace and Defense
- Bioreactors and Fermenters
- Calibration and Metrology
- Environmental and Air Monitoring
- Food and Beverage
- Gas Chromatography
- Glass and Optical Fiber
- Leak Testing
- Research Laboratories
- Thin Film Deposition
- What’s New?
- Careers at Alicat
- Request Service
Introduction to hydrogen fuel cells
Hydrogen fuel cell example
Hydrogen fuel cells are a clean, emissions-free alternative to traditional combustion processes, and use hydrogen to generate energy via electrochemical reactions. These cells are made even more environmentally friendly when the hydrogen is produced via sustainable means.
There are many variations in fuel cell technology, but they share similar features. All fuel cells house an anode and cathode on either side of an electrolyte. In a proton-conducting fuel cell, hydrogen is fed to the anode and a catalyst is used to generate positively charged ions that flow through the electrolyte to the cathode. This induces a current, thereby generating electricity. Meanwhile at the cathode, air is fed into the system and combines with a catalyst, hydrogen ions, and electrons to produce heat and water as byproducts.
Types of fuel cells
The fundamental principle of the fuel cell was first proposed by Sir William Grove in 1838, and many deviations of the fuel cell have developed since then. Here we highlight some of the most prominent fuel cells and their respective applications.
Alkaline fuel cells (AFCs)

Apollo mission lunar module
Alkaline fuel cells are the oldest commercialized fuel cell, first invented by Francis Bacon in 1932. AFCs contain porous electrodes saturated with alkaline solution that are separated by a hydroxide electrolyte. Since they are highly sensitive to CO 2 , their reactions use pure oxygen or highly purified air. These are famous for their use by NASA in the 1960s to generate electricity and water for the crew of the Apollo and Space Shuttle programs. Because they are so well developed, alkaline fuel cells are currently the cheapest variant to manufacture.
Solid oxide fuel cells (SOFCs)
Solid oxide fuel cells contain a solid ceramic electrolyte layer that is heated to very high temperatures (1000°C, 1800°F) and conducts oxygen ions. Their primary use is in stationary power generation systems for small residential and business applications. While SOFCs are compatible with many fuel types and offer long-term stability, the high operating temperature creates challenges in terms of material compatibility and lengthy start-up times.
Proton exchange membrane fuel cells (PEMFCs)

PEM fuel cell stack testing
Proton exchange membrane fuel cells use a special electrolyte membrane that conducts protons between the anode and cathode. PEMFCs have higher power density, lower weight, and smaller volume than other fuel cells, making them ideal for vehicle and transport applications in addition to stationary applications. These are a fairly new development, and research efforts are focused on increasing viability by decreasing cost and increasing efficiency.
Molten carbonate fuel cells (MCFCs)
Molten carbonate fuel cells operate at high-temperatures like SOFCs, although they use a molten carbonate salt mixture electrolyte. MCFCs are being developed for electrical utility, military, and industrial applications.
Phosphoric acid fuel cells (PAFCs)
Phosphoric acid fuel cells have utilized phosphoric acid as the electrolyte since their introduction in 1961. These cells are primarily used in stationary power generation applications, particularly those within an output power range of 100-400 kW. An advantage is their ability to handle high levels of impurities compared to PEMFCs.
More on fuel cell electric vehicles
Fuel cell electric vehicles (FCEVs) are a major area of developmental focus, discussed frequently in popular media. These vehicles operate on fuel cell generated energy rather than standard combustion engine technology. FCEVs like the Toyota Mirai have competitive driving ranges compared to electric vehicles, and are very quick to refill. However, some challenges must be overcome before FCEVs are able to scale effectively – high price tags and insufficient hydrogen infrastructure.
Fun Fact! October 8th is National Hydrogen and Fuel Cell Day in the United States. The date was chosen based on the atomic weight of hydrogen: 1.008 amu.
- 13 tools to use when planning for pharma scale-up Battery vs hydrogen fuel cell vehicles
Privacy Overview
- IAS Preparation
- Topic of the Day
Fuel Cell - UPSC Science & Technology Notes
A fuel cell is a device that produces electric energy, through a chemical reaction. Science & Technology is one of the subjects included in the civil services examination. Many questions circling this subject have been asked both in the UPSC Prelims and the Mains exams.
Fuel Cell is an important chemistry concept in Science & Technology, which is a part of the General Studies Paper-3 in the UPSC Syllabus . In this article, one can learn about the types and working of fuel cells. We have also mentioned topics that are related to Fuel Cells for the UPSC Exam .
What is a Fuel cell?
A fuel cell is a device that produces electric energy, through a chemical reaction.
- Fuel cells use a positively charged ion (Hydrogen) and an oxidizing agent (oxygen).
- There are many types of fuel cells, but they all consist of a cathode, an anode, and an electrolyte that allows positively charged (hydrogen) ions to move between the two sides of the fuel cell.
- They differ from batteries as they (fuel cells) require a continuous supply of fuel.
- Both batteries and fuel cells produce direct current (D.C).
Working of a Fuel Cell
The working of this fuel cell involved the passing of hydrogen and oxygen into a concentrated solution of sodium hydroxide via carbon electrodes. The cell reaction can be written as follows:
Cathode Reaction: O 2 + 2H 2 O + 4e – → 4OH –
Anode Reaction: 2H 2 + 4OH – → 4H 2 O + 4e –
Net Cell Reaction: 2H 2 + O 2 → 2H 2 O
However, the reaction rate of this electrochemical reaction is quite low. This issue is overcome with the help of a catalyst such as platinum or palladium. To increase the effective surface area, the catalyst is finely divided before being incorporated into the electrodes.
A block diagram of this fuel cell is provided below.
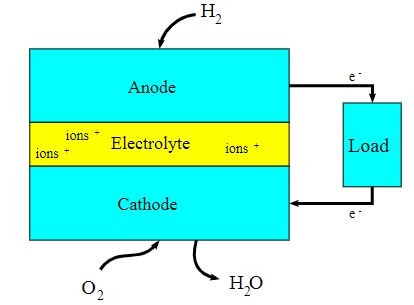
- The first fuel cell was used by NASA in its satellites and space capsules.
- Fuel cells can be used for power backup in commercial and residential buildings.
- Fuel cells can be arranged in stacks like series and parallel connections depending on the requirement of higher voltage (series) and current (parallel).
National Electric Mobility Mission Plan 2020
The National Electric Mobility Mission Plan (NEMMP) 2020 was launched by the Government of India in 2013 to achieve national fuel security by promoting electric and hybrid vehicles. The target is to achieve sales of 6 – 7 million in the hybrid and electric vehicles sector from 2020.
The government will provide fiscal and monetary incentives for this industry. The expectation is that crude oil worth Rs.62000 crore will be saved due to this.
FAME and National Electric Mobility Mission Plan
Under the NEMMP, the government has launched the Faster Adoption and Manufacturing of (Hybrid &) Electric Vehicles, FAME India Scheme.
- This scheme had an initial outlay of Rs. 75 crore.
- This scheme is expected to provide a major thrust towards the early adoption of electric and hybrid technologies.
Read more about the National Electric Mobility Mission Plan
Fuel Cells UPSC:- Download PDF Here
FAQ about Fuel Cell
What are the advantages of fuel cells, what are fuel cells used for.
For more articles and UPSC preparation material follow the links given in the table below:
For information on various Government Exams visit the given link.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
IAS 2024 - Your dream can come true!
Download the ultimate guide to upsc cse preparation.
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- Crown Signia
- Grand Highlander
- Land Cruiser
- Concept Vehicles
- Historic Vehicles
- 2025 Toyota 4Runner
- CALTY 50th Anniversary
- 2024 Toyota Land Cruiser
- 2024 Toyota Tacoma
- New Product Showcase
- Los Angeles
- Global Shows
- What’s New for 2025
- What’s New for 2024
- What’s New for 2023
- What’s New for 2022
- Images & Videos
- Latest News
- Sales & Financial
- Voluntary Recalls
- Takata Info
- Environmental
- Community Engagement
- Diversity & Inclusion
- Finance, Insurance & Banking
- Advanced Technology
- Research & Development
- Safety Technology
- Motorsports
- Company History
- Executive Bios
Media Contacts
- Takata Recall
Home > Innovation > Mobility


Toyota Establishes Hydrogen Headquarters to Accelerate Advancement of Fuel Cell Technology
- H2HQ teams to lead NA region’s efforts to research, develop, commercialize and sell fuel cell and hydrogen-related products
- Renovated facility provides a refreshed office workspace, with plans to add features in the future, including a flexible microgrid, customer education center and more
GARDENA, Calif. (May 1, 2024) – Reaffirming its commitment to support fuel cell and additional hydrogen-related products and technology toward a hydrogen economy, Toyota Motor North America (TMNA) today announced that it is renaming the TMNA R&D California office as its new North American Hydrogen Headquarters (H2HQ). The office workspace at the new H2HQ was recently redesigned for its teams working from research and development to commercialization planning and sales of hydrogen-related products and technologies. There are plans to add key features to the H2HQ campus in the future such as a flexible microgrid, sustainable customer education center and more.
“Toyota has developed hydrogen fuel cell electric solutions for more than three decades, and we will continue to advance this scalable, zero-emission technology as part of our electrified portfolio,” said Ted Ogawa, President and CEO, Toyota Motor North America. “Renaming this facility as North American Hydrogen Headquarters represents our leadership in fuel cell development creating real-world products to help reduce carbon emissions.”
Last year, Toyota Motor Corporation reorganized its hydrogen business in Japan to create what it calls “Hydrogen Factory” with the idea to bring all hydrogen-related work under one location and accelerate customer-oriented product development and production in fuel cell or hydrogen-related products. Then, Toyota Motor Europe announced its own “Hydrogen Factory” with the aim to further grow Toyota’s hydrogen business and stimulate wider roll-out of hydrogen ecosystems and infrastructure across Europe.
H2HQ will drive North American-led hydrogen initiatives and support the localization of global hydrogen-related technologies and products that include light-duty fuel cell applications, heavy-duty fuel cell opportunities, stationary fuel cell power generation, port vehicle applications and more. The facility already provides impressive research and development assets, including Toyota’s largest dynamometer (1.2 MW), a scalable test bench for stationary applications, and a hydrogen fueling station capable of providing fuel for both light- and heavy-duty vehicles. Moreover, as part of its plans to remain and grow fuel cell leadership, NA H2HQ will be home to several new projects in the coming years.
“I’m very pleased that Toyota is building on its longstanding commitment to California by locating its North American hydrogen headquarters here in the Golden State,” said California State Senator, District 29, Josh Newman. “The work done there, along with green hydrogen initiatives throughout the state, is propelling California toward a dynamic, clean-energy economy which will also reduce carbon emissions and foster environmental stewardship while extending California’s leadership in this important space.”
Construction has begun on a flexible microgrid that features energy sources available today, including a 230-kW solar photovoltaic system, a 1-MW stationary proton exchange membrane (PEM) fuel cell generator, 325-kW solid oxide fuel cell (SOFC), and an onsite 500-kWh battery energy storage system. The microgrid is designed to support the campus’ energy needs, allowing it the ability to operate off-grid. The system is expected to be fully operational by 2026.
“California has ambitious goals to achieve clean air, carbon neutrality and a vibrant economy. Toyota’s investment to expand their research and development of hydrogen fuel cell technology in our state is an example of the innovation that will accelerate the development and deployment of zero-emissions transportation options, particularly as we decarbonize the goods movement sector,” said CARB Chair Liane Randolph.
In the future, Toyota’s plans for the new North American Hydrogen HQ will include a sustainable education center, available for tours by reservation. The center will be a place for people to learn more about Toyota’s vision of sustainability and the role that hydrogen will play.
30+ Years of Development From creating one of the world’s first mass market passenger fuel cell electric vehicles in the Mirai , to applying and scaling the technology now to other applications that can benefit from zero-emissions, including heavy-duty transport , power generation systems, and others, Toyota’s research and development with hydrogen fuel cell technology spans more than 30 years.
For much of that time, the Gardena office supported or initiated a wide range of fuel cell electric projects. To share some recent examples, the Fuel Cell Development (FCD) team was instrumental in supporting the development of Toyota’s light-duty Mirai, launched back in 2015, and the team collaborated with industry partners to help support infrastructure growth through the state of California.
In 2017, to address decarbonization efforts at local ports, Toyota’s FCD team helped prove the scalability of fuel cell technology after it acquired a Class 8 truck and fitted it with a fuel cell electric powertrain consisting of two Mirai fuel-cell stacks. This effort then led to a collaboration with PACCAR’s Kenworth brand to build 10 proof-of-concept trucks, trucks used to support the “Shore to Store” ZANZEFF project that proved the viability of hydrogen-powered fuel cells as a zero-emission powertrain in heavy-duty applications. PACCAR and Toyota later agreed in 2023 to pursue commercialization of the project, with Toyota supplying the fuel cell powertrain kits from its Kentucky plant as a Tier 1 supplier.
Most recently, Toyota has demonstrated a non-automotive opportunity for hydrogen-powered fuel cell technology in stationary power generation. Toyota and TRD partnered to build a stationary unit to provide electricity at events where it was not readily available, launching the first public activation at an LPGA Tour stop where the unit supported the power needs of the event stage and sound system. The solution provided clean, quiet power that was proven to capably replace traditional diesel generators. Last year, Toyota built a 1MW fuel cell electric generator for the National Renewable Energy Laboratory in Colorado to support microgrid testing at the facility. And finally, earlier this year, Toyota collaborated with Kohler on a prototype stationary generator to provide backup emergency power for Klickitat Valley Health hospital in Goldendale, Washington.
Related Media
Related images.
About Toyota Toyota (NYSE:TM) has been a part of the cultural fabric in North America for more than 65 years, and is committed to advancing sustainable, next-generation mobility through our Toyota and Lexus brands, plus our more than 1,800 dealerships.
Toyota directly employs more than 63,000 people in North America who have contributed to the design, engineering, and assembly of nearly 47 million cars and trucks at our 13 manufacturing plants. By 2025, Toyota’s 14th plant in North Carolina will begin to manufacture automotive batteries for electrified vehicles. With more electrified vehicles on the road than any other automaker, Toyota currently offers 27 electrified options.
For more information about Toyota, visit www.ToyotaNewsroom.com .
Joshua Burns [email protected]
- + Select All
- - DeSelect All

H2HQ_New_5-1-24
Related stories.

Four Reasons Why You Should Subscribe to Newsroom Connection

Cirba Solutions Helps Toyota Expand Battery Recycling Network to Nationwide Program

FuelCell Energy and Toyota Announce Completion of World’s First “Tri-gen” Production System
Email sign up.
Enter your email address below to sign up for email alerts.
* Indicates Required
Thank you for subscribing. Please check your email to validate your sign up.
You are already subscribed, your mailing lists have been updated.
Copyright Notice
All materials on this site are for editorial use only. The use of these materials for advertising, marketing or any other commercial purpose is prohibited. They may be cropped but not otherwise modified. To download these materials, you must agree to abide by these terms.
Energy & Environmental Science
Utilizing three-terminal, interdigitated back contact si solar cells as a platform to study the durability of photoelectrodes for solar fuel production †.

* Corresponding authors
a Materials, Chemical, and Computational Science Center (MCCS), National Renewable Energy Laboratory, Golden, Colorado 80401, USA E-mail: [email protected] , [email protected]
b Colorado School of Mines, Advanced Energy Systems Graduate Program, Golden, CO, USA
c International Solar Energy Research Center (ISC) Konstanz, Rudolf–Diesel–Straße 15, Konstanz, Germany
Unassisted photoelectrochemical (PEC) reactions, such as H 2 generation and CO 2 reduction, are limited by the durability of the immersed photoelectrode. Small band gap semiconductors, like Si, are efficient at utilizing a large portion of the solar spectrum but are not stable in aqueous environments without protection. While great strides have been made to improve stability under constant illumination, dark stability remains relatively unexamined and presents great challenges for durable PEC systems. Cathodic protection is an established electrochemical method for preventing metal electrode degradation in harsh conditions. Similar protection strategies cannot be applied to traditional two-terminal (2T) semiconductor photoelectrodes because of their inability to pass reverse bias current in the dark. New, three-terminal (3T) photovoltaic (PV) architectures introduce additional degrees of freedom in traditional 2T PEC operations by adding an extra electrical contact for an alternative low resistance path to protect the photoelectrode and drive electrochemical reactions, even in the dark. Here, we investigate bare 3T Si PV devices operating as photocathodes in aqueous methyl viologen electrolyte. The 3T architecture provides additional capabilities to PEC systems such as cathodic protection, the ability to drive reactions with or without illumination, and in situ switching between different operational modes. We show that 3T-based Si photocathodes maintain PEC activity after several hours of light/dark cycling. This work helps advance PEC use in real-world conditions where variable illumination must be considered.
Supplementary files
- Supplementary information PDF (1636K)
Article information
Download Citation
Permissions.
Utilizing three-terminal, interdigitated back contact Si solar cells as a platform to study the durability of photoelectrodes for solar fuel production
D. K. Collins, Z. G. Schichtl, N. T. Nesbitt, A. L. Greenaway, V. D. Mihailetchi, D. Tune and E. L. Warren, Energy Environ. Sci. , 2024, Advance Article , DOI: 10.1039/D4EE00349G
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence . You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication , please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
- NURBURGRING WEBCAMS
- WORK FOR US

Hyundai Chasing Pikes Peak EV Glory With Four Ioniq 5 Ns

First Look: New Toyota 4Runner Trailhunter Is A Turn-Key Overlanding Machine

2025 Toyota 4Runner Is The Tacoma Of SUVs And It’s Coming For The Bronco
Editor's picks.

Dodge Hornet Outsells Alfa Tonale 10:1, Stellantis Sees 10% Drop In Q1 Sales
Hyundai Expands Hydrogen Fuel Cell Truck Fleet Across California
The Hyundai XCIENT is operating in eight countries, including Australia, Saudi Arabia, and the U.S.
by Brad Anderson
- There are already 30 XCIENT Fuel Cell trucks being used in Oakland.
- The Class 8 hydrogen fuel cell trucks will be refueled at a FirstElement Fuel station.
- Hyundai believes the program can save over 24,000 metric tons of carbon emissions.
Hyundai has brought zero-emission freight transportation to San Francisco and California’s Central Valley with the rollout of its NorCAL Zero Project featuring 30 Class 8 XCIENT Fuel Cell trucks.
This project is the largest single commercial deployment of Class 8 hydrogen fuel cell trucks in the United States. Each of the 30 XCIENT Fuel Cell trucks has a 6×4 drive axle configuration and will be refueled at a new hydrogen refueling station recently opened by FirstElement Fuel. This station can fuel up to 200 heavy-duty trucks per day.
Read: Hyundai Brings XCIENT Hydrogen Truck To The U.S.
The project was launched at a special event at the Oakland refueling station and attended by local political representatives, the Port of Oakland, the City of Oakland, and the companies involved. Hyundai says the program can reduce carbon emissions by over 24,000 metric tons compared to diesel-powered trucks.
“The NorCAL ZERO Project in Oakland marks a significant step forward in realizing Hyundai’s vision for a global hydrogen society,” executive vice president and head of Global Commercial Vehicle & Hydrogen Business at Hyundai Motor Company, Ken Ramirez said. “The project demonstrates how the transport energy transition is achievable today and will serve as one of the building blocks for Hyundai’s port decarbonization initiatives worldwide.”

These are not the first XCIENT Fuel Cell trucks to hit U.S. roads. Last year, the carmaker supplied 30 examples to the freight transport business of Glovis America, G.E.T Freight Corp, and these vehicles have been hauling containers from the Port of Oakland and transportation vehicles from the Port of Richmond.
Hyundai believes hydrogen can serve as a clean energy solution for commercial vehicles across the country. The XCIENT Fuel Cell is the first mass-produced, heavy-duty truck powered solely by hydrogen and has also been deployed in Switzerland, Germany, New Zealand, Australia, Korea, Israel , and Saudi Arabia.


Logistics: Hyundai delivers fuel cell trucks to NorCAL ZERO Project
Hyundai delivered 30 of its fuel cell trucks to Northern California. The 30 Hyundai XCIENT Class 8 hydrogen fuel cell electric trucks will hit the road as part of the NorCAL ZERO Project to push for emission-free freight transportation.

- Electric trucks
- NorCAL Zero
Glovis America will operate the trucks around the Port of Oakland. FirstElement Fuel set up a hydrogen filling station in Oakland, where the trucks will be refuelled. The station can support up to 200 H2 trucks per day by design. The fuel comes from project partner Air Liquide.
The project is also known as “Zero-Emission Regional Truck Operations with Fuel Cell Electric Trucks.” It is led by the Center for Transportation and the Environment (CTE) and kicked off in August 2021. It is funded with $29 million by the California Air Resources Board (CARB) and The California Energy Commission (CEC) to purchase the above-mentioned hydrogen-powered trucks, infrastructure and maintenance facilities.
But back to the trucks: Hyundai launched the Xcient fuel cell in the US in Mai 2023 . Even before that, specifically in July 2021 , the manufacturer secured the order for the fuel cell trucks for the NorCAL ZERO project. The Class 8 vehicle has two 90 kW hydrogen fuel cell systems (total 180 kW power) and a 350 kW electric motor. According to Hyundai, the 6×4 tractor has a range of more than 450 miles (724 km) when fully loaded.
“The NorCAL ZERO Project in Oakland marks a significant step forward in realizing Hyundai’s vision for a global hydrogen society,” said Ken Ramirez, Executive Vice President and Head of Global Commercial Vehicle & Hydrogen Business at Hyundai Motor Company. “The project demonstrates how the transport energy transition is achievable today and will serve as one of the building blocks for Hyundai’s port decarbonization initiatives worldwide.”
“The deployment of these trucks in Northern California provides a groundbreaking opportunity to demonstrate the exceptional performance of fuel cell electric trucks for an industry that has traditionally relied on conventional diesel and CNG-powered vehicles,” said CTE Executive Director Dan Raudebaugh. “We look forward to being a part of a new era for trucking across the U.S. – one marked by zero-emission vehicles that won’t force fleets to compromise on performance.”
hyundai.com , cte.tv (NorCAL ZERO Project)
- Port of Oakland
- North America
- First Element Fuel
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
I agree with the Privacy policy

Gillig partners with BAE Systems and Ballard on H2 bus model

Joby Aviation completes pre-production flight tests

Stellantis significantly expands FCEV production

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.5: Batteries and Fuel Cells
- Last updated
- Save as PDF
- Page ID 210170

Learning Objectives
- Classify batteries as primary or secondary
- List some of the characteristics and limitations of batteries
- Provide a general description of a fuel cell
A battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used as a battery. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Real batteries strike a balance between ideal characteristics and practical limitations. For example, the mass of a car battery is about 18 kg or about 1% of the mass of an average car or light-duty truck. This type of battery would supply nearly unlimited energy if used in a smartphone, but would be rejected for this application because of its mass. Thus, no single battery is “best” and batteries are selected for a particular application, keeping things like the mass of the battery, its cost, reliability, and current capacity in mind. There are two basic types of batteries: primary and secondary. A few batteries of each type are described next.
Visit this site to learn more about batteries.
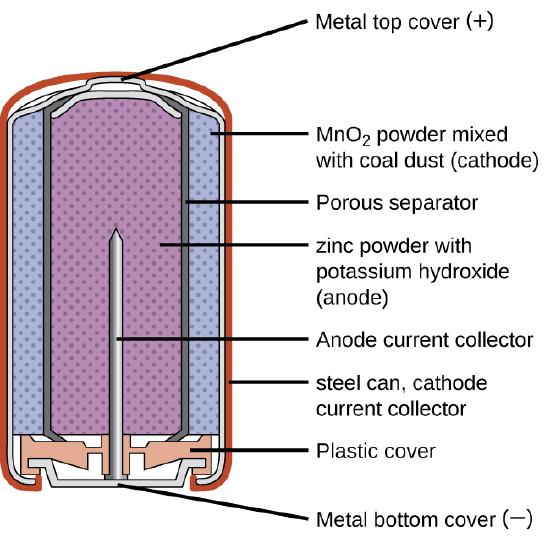
Primary Batteries
Primary batteries are single-use batteries because they cannot be recharged. A common primary battery is the dry cell (Figure \(\PageIndex{1}\)). The dry cell is a zinc-carbon battery. The zinc can serves as both a container and the negative electrode. The positive electrode is a rod made of carbon that is surrounded by a paste of manganese(IV) oxide, zinc chloride, ammonium chloride, carbon powder, and a small amount of water. The reaction at the anode can be represented as the ordinary oxidation of zinc:
\[\ce{Zn}(s)⟶\ce{Zn^2+}(aq)+\ce{2e-} \hspace{20px} E^\circ_{\ce{Zn^2+/Zn}}=\mathrm{−0.7618\: V} \nonumber \]
The reaction at the cathode is more complicated, in part because more than one reaction occurs. The series of reactions that occurs at the cathode is approximately
\[\ce{2MnO2}(s)+\ce{2NH4Cl}(aq)+\ce{2e-}⟶\ce{Mn2O3}(s)+\ce{2NH3}(aq)+\ce{H2O}(l)+\ce{2Cl-} \nonumber \]
The overall reaction for the zinc–carbon battery can be represented as
\[\ce{2MnO2}(s) + \ce{2NH4Cl}(aq) + \ce{Zn}(s) ⟶ \ce{Zn^2+}(aq) + \ce{Mn2O3}(s) + \ce{2NH3}(aq) + \ce{H2O}(l) + \ce{2Cl-} \nonumber \]
with an overall cell potential which is initially about 1.5 V, but decreases as the battery is used. It is important to remember that the voltage delivered by a battery is the same regardless of the size of a battery. For this reason, D, C, A, AA , and AAA batteries all have the same voltage rating. However, larger batteries can deliver more moles of electrons. As the zinc container oxidizes, its contents eventually leak out, so this type of battery should not be left in any electrical device for extended periods.

Alkaline batteries (Figure \(\PageIndex{2}\)) were developed in the 1950s partly to address some of the performance issues with zinc–carbon dry cells. They are manufactured to be exact replacements for zinc-carbon dry cells. As their name suggests, these types of batteries use alkaline electrolytes, often potassium hydroxide. The reactions are
\[\begin{align*} &\textrm{anode: }\ce{Zn}(s)+\ce{2OH-}(aq)⟶\ce{ZnO}(s)+\ce{H2O}(l)+\ce{2e-} \hspace{40px} E^\circ_\ce{anode}=\mathrm{−1.28\: V}\\ &\underline{\textrm{cathode: }\ce{2MnO2}(s)+\ce{H2O}(l)+\ce{2e-}⟶\ce{Mn2O3}(s)+\ce{2OH-}(aq) \hspace{40px} E^\circ_\ce{cathode}=\mathrm{+0.15\: V}}\\ &\textrm{overall: }\ce{Zn}(s)+\ce{2MnO2}(s)⟶\ce{ZnO}(s)+\ce{Mn2O3}(s) \hspace{40px} E^\circ_\ce{cell}=\mathrm{+1.43\: V} \end{align*} \nonumber \]
An alkaline battery can deliver about three to five times the energy of a zinc-carbon dry cell of similar size. Alkaline batteries are prone to leaking potassium hydroxide, so these should also be removed from devices for long-term storage. While some alkaline batteries are rechargeable, most are not. Attempts to recharge an alkaline battery that is not rechargeable often leads to rupture of the battery and leakage of the potassium hydroxide electrolyte.
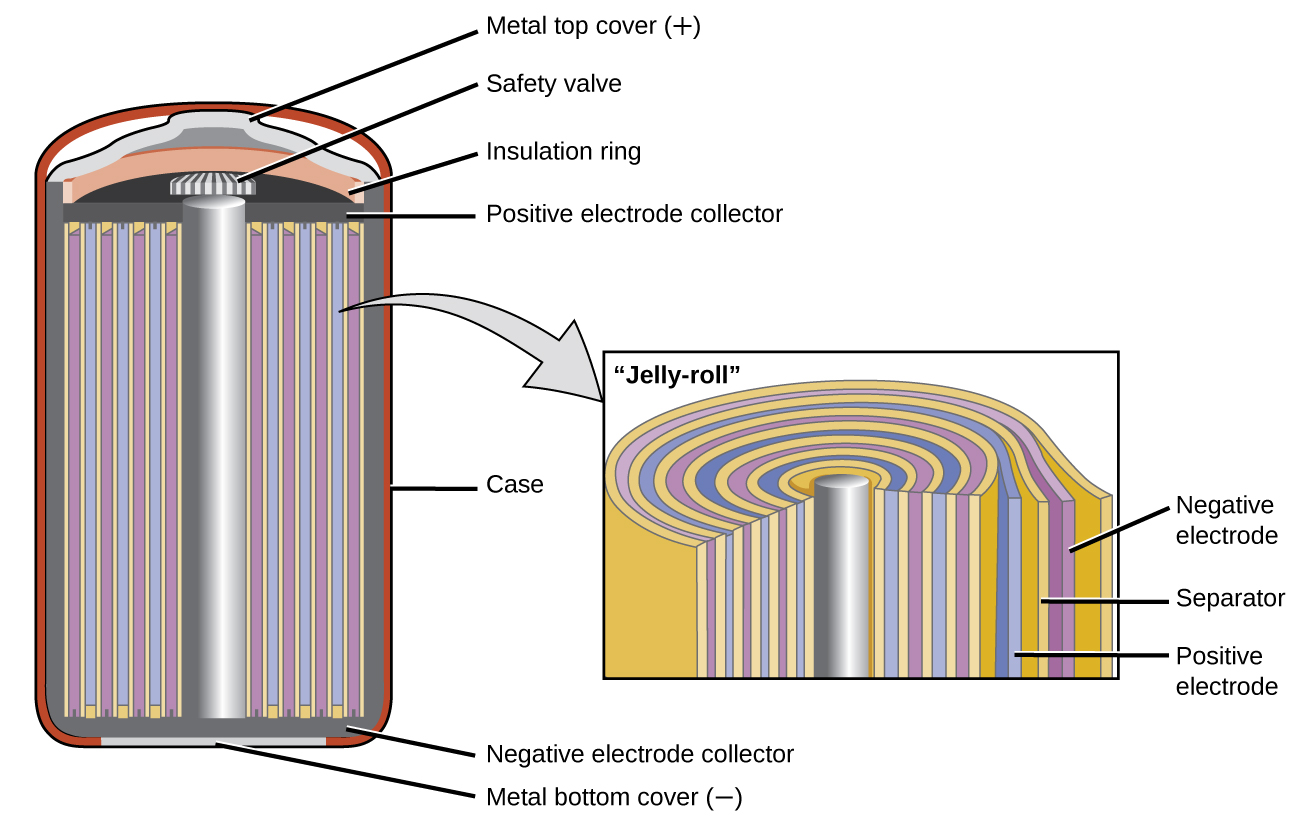
Secondary Batteries
Secondary batteries are rechargeable. These are the types of batteries found in devices such as smartphones, electronic tablets, and automobiles.
Nickel-cadmium, or NiCd, batteries (Figure \(\PageIndex{3}\)) consist of a nickel-plated cathode, cadmium-plated anode, and a potassium hydroxide electrode. The positive and negative plates, which are prevented from shorting by the separator, are rolled together and put into the case. This is a “jelly-roll” design and allows the NiCd cell to deliver much more current than a similar-sized alkaline battery. The reactions are
\[\begin{align*} &\textrm{anode: }\ce{Cd}(s)+\ce{2OH-}(aq)⟶\ce{Cd(OH)2}(s)+\ce{2e-}\\ &\underline{\textrm{cathode: }\ce{NiO2}(s)+\ce{2H2O}(l)+\ce{2e-}⟶\ce{Ni(OH)2}(s)+\ce{2OH-}(aq)}\\ &\textrm{overall: }\ce{Cd}(s)+\ce{NiO2}(s)+\ce{2H2O}(l)⟶\ce{Cd(OH)2}(s)+\ce{Ni(OH)2}(s) \end{align*} \nonumber \]
The voltage is about 1.2 V to 1.25 V as the battery discharges. When properly treated, a NiCd battery can be recharged about 1000 times. Cadmium is a toxic heavy metal so NiCd batteries should never be opened or put into the regular trash.
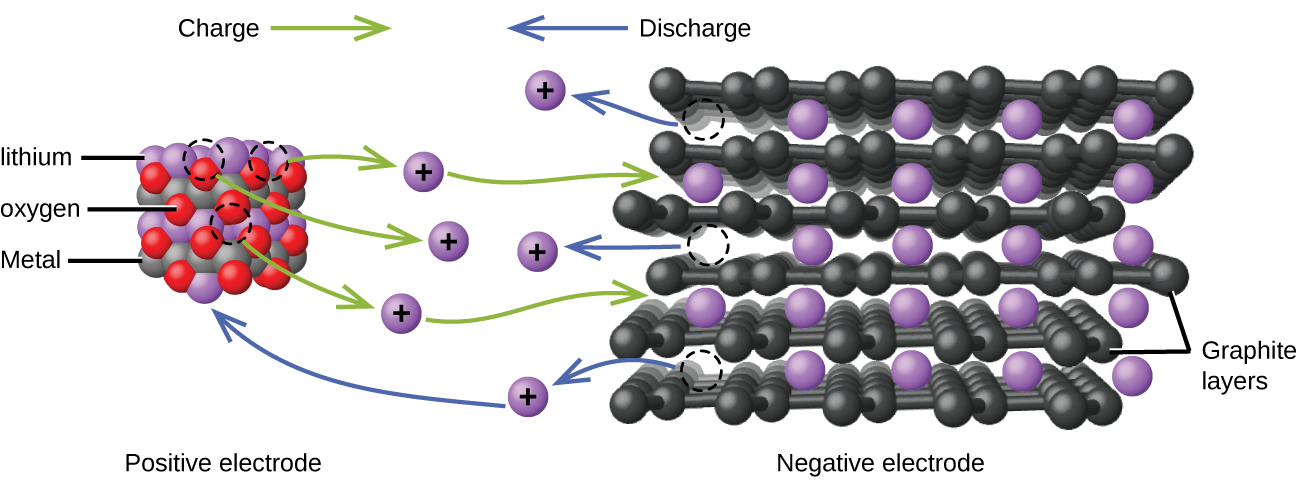
Lithium ion batteries (Figure \(\PageIndex{4}\)) are among the most popular rechargeable batteries and are used in many portable electronic devices. The reactions are
\[\begin{align*} &\textrm{anode: }\ce{LiCoO2}⇌\ce{Li}_{1-x}\ce{CoO2}+x\ce{Li+}+x\ce{e-}\\ &\textrm{cathode: }x\ce{Li+}+x\ce{e-}+x\ce{C6}⇌x\ce{LiC6}\\ &\overline{\textrm{overall: }\ce{LiCoO2}+x\ce{C6}⇌\ce{Li}_{1-x}\ce{CoO2}+x\ce{LiC6}} \end{align*} \nonumber \]
With the coefficients representing moles, x is no more than about 0.5 moles. The battery voltage is about 3.7 V. Lithium batteries are popular because they can provide a large amount current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored.
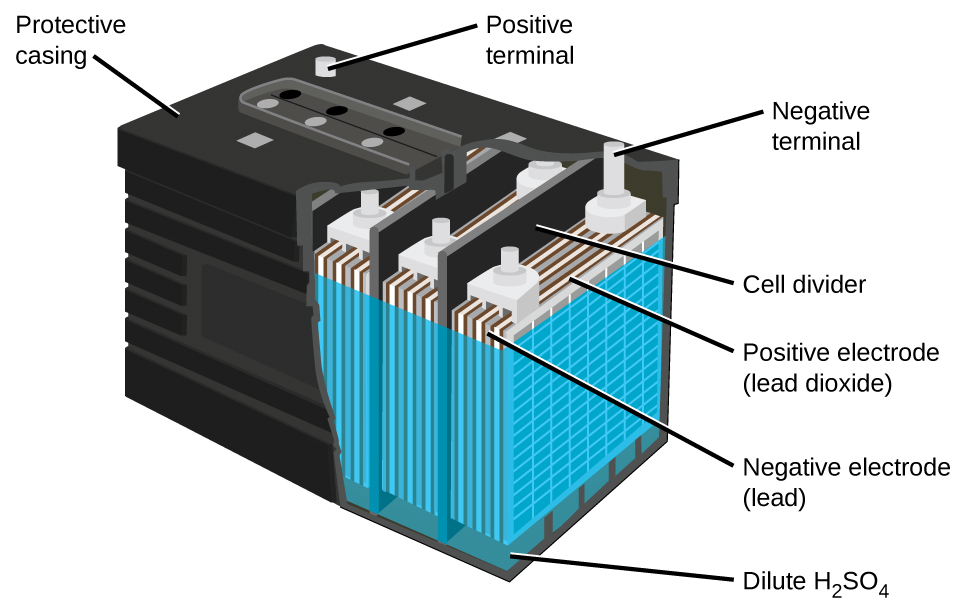
The lead acid battery (Figure \(\PageIndex{5}\)) is the type of secondary battery used in your automobile. It is inexpensive and capable of producing the high current required by automobile starter motors. The reactions for a lead acid battery are
\[\begin{align*} &\textrm{anode: }\ce{Pb}(s)+\ce{HSO4-}(aq)⟶\ce{PbSO4}(s)+\ce{H+}(aq)+\ce{2e-}\\ &\underline{\textrm{cathode: } \ce{PbO2}(s)+\ce{HSO4-}(aq)+\ce{3H+}(aq)+\ce{2e-}⟶\ce{PbSO4}(s)+\ce{2H2O}(l)}\\ &\textrm{overall: }\ce{Pb}(s)+\ce{PbO2}(s)+\ce{2H2SO4}(aq)⟶\ce{2PbSO4}(s)+\ce{2H2O}(l) \end{align*} \nonumber \]
Each cell produces 2 V, so six cells are connected in series to produce a 12-V car battery. Lead acid batteries are heavy and contain a caustic liquid electrolyte, but are often still the battery of choice because of their high current density. Since these batteries contain a significant amount of lead, they must always be disposed of properly.
A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to batteries but require a continuous source of fuel, often hydrogen. They will continue to produce electricity as long as fuel is available. Hydrogen fuel cells have been used to supply power for satellites, space capsules, automobiles, boats, and submarines (Figure \(\PageIndex{6}\)).
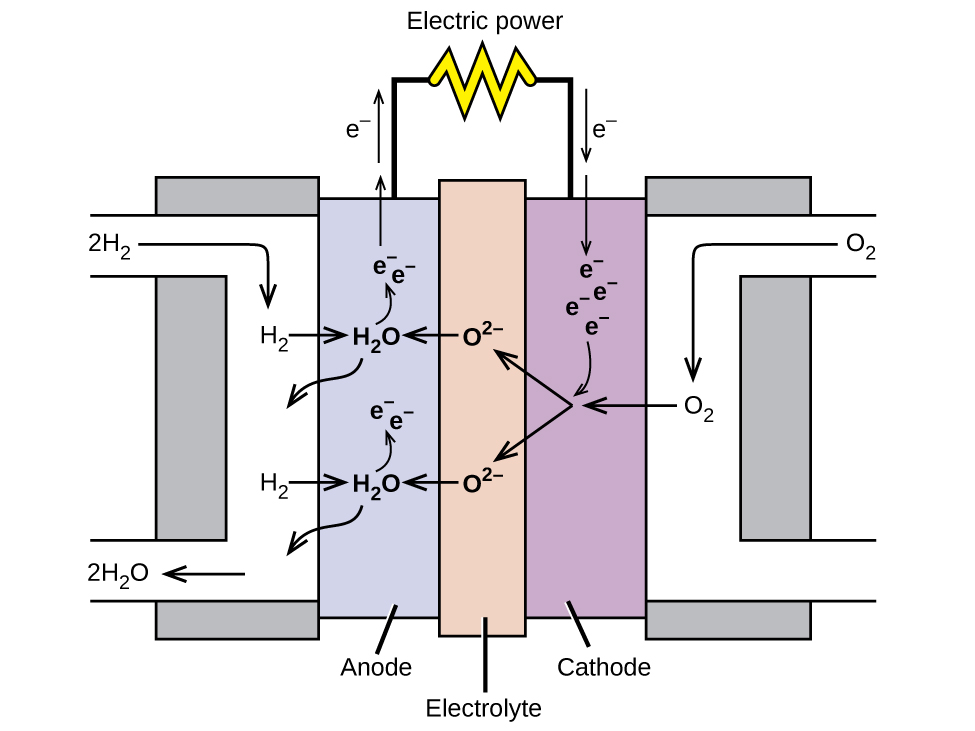
In a hydrogen fuel cell, the reactions are
\[\begin{align*} &\textrm{anode: }\ce{2H2 + 2O^2- ⟶ 2H2O + 4e-}\\ &\underline{\textrm{cathode: }\ce{O2 + 4e- ⟶ 2O^2-}\hspace{55px}}\\ &\textrm{overall: }\ce{2H2 + O2 ⟶ 2H2O} \end{align*} \nonumber \]
The voltage is about 0.9 V. The efficiency of fuel cells is typically about 40% to 60%, which is higher than the typical internal combustion engine (25% to 35%) and, in the case of the hydrogen fuel cell, produces only water as exhaust. Currently, fuel cells are rather expensive and contain features that cause them to fail after a relatively short time.
Batteries are galvanic cells, or a series of cells, that produce an electric current. When cells are combined into batteries, the potential of the battery is an integer multiple of the potential of a single cell. There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery. Examples of secondary batteries include nickel-cadmium (NiCd), lead acid, and lithium ion batteries. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. The hydrogen fuel cell uses hydrogen and oxygen from the air to produce water, and is generally more efficient than internal combustion engines.

Apr 25, 2024
Products & Solutions / Press Release
Panasonic Launches 10 kW Pure Hydrogen Fuel Cell Generator in Europe, Australia, and China
- Multiple connections allow a higher output, while the output adjustment function enables flexible power generation plans -
- Panasonic GREEN IMPACT
- Hydrogen Energy Solutions
- Europe and CIS
- Asia and Oceania
- Business Solution
Osaka, Japan - Panasonic Corporation (hereinafter referred to as Panasonic) today announced that its Electric Works Company will launch a new pure hydrogen fuel cell generator that generates power through a chemical reaction between high-purity hydrogen and oxygen in the air in Oct. 2024 in Europe *1 , Australia, and China.
In October 2021, Panasonic launched a 5 kW type pure hydrogen fuel cell generator PH1 (single-phase three-wire type). The company will now launch a new product PH3 (DC type) for overseas markets to fully scale up its hydrogen business outside Japan.
Since the PH3 to be launched is a cogeneration system, it can output a maximum of 10 kW of DC power, and generate approximately 8.2 kW of heat, which can be used to heat water to approximately 60ºC. The product is designed to output DC power in order to support power voltage in a wide variety of countries and areas. In addition to a higher output by connecting multiple units (up to 250 modules), the power output can be adjusted *2 , allowing for the formulation of flexible power generation plans. Furthermore, maintenance access points are located in the front, and the product is designed to withstand cold temperatures, as well as high altitudes, enabling it to be proposed to customers with various installation environments. In addition, the product achieves an electrical efficiency of 57% (DC, LHV), heat recovery efficiency of 47% (LHV), and a total efficiency of 104% *3 , contributing to reduced running costs. The overhaul stop period is designed to be 15 years to ensure a long operating life *4 , which can reduce life cycle costs.
Panasonic, based on knowledge obtained through H2 KIBOU FIELD *5,6 , the demonstration facility for RE100 solutions in Japan, plans to carry out similar demonstrations in its own factories in Wales, as well as in Germany.
Panasonic will offer a new option, full-scale use of hydrogen, in order to expand the introduction of renewable energy based on the Panasonic GREEN IMPACT formulated by the Group, contributing to the realization of a decarbonized society.
*1: The planned target markets are Germany, the UK, Belgium, Switzerland, France, Austria, Netherlands, Spain, and Italy.
*2: Power output can be set in 1 kW increments between 4 kW and 10 kW per module.
*3: The new product can recover condensation latent heat. Since its heat recovery rate includes condensation latent heat, its total efficiency is over 100%.
*4: The operating life is depended on customer’s power generation plan. (Whichever comes first energization time 15 years, cumulative hours of power generation 100,000 hours and cumulative start & stop cycle 4,500 times)
*5: The H2 KIBOU and H2 KIBOU logos have been registered as trademarks of Panasonic Corporation.
*6: In April 2022, Panasonic began operations at its RE100 solution demonstration facility in Japan, which generates 100% of the power used by its fuel cell factory from renewable resources using a self-sustaining power system that combines generation from both pure hydrogen fuel cell generators and photovoltaic generators. As a result, high environmental performance (a power purchase reduction rate of 98%) was achieved. Although hydrogen from renewable energy is not used during demonstration, Panasonic aims for the achievement of RE100 by using hydrogen derived from renewable energy in the future.
<Main Features>
1. cogeneration system with a maximum 10 kw of dc power output, 2. multiple 10 kw modules can be connected. furthermore, the output adjustment function *2 enables more flexible power generation plans., 3. highly-flexible installation facilitates proposals to a wide range of customers, 4. high efficiency and long-designed life cycle reduce total costs.
A pure hydrogen fuel cell generator PH3 can generate a maximum of 10 kW of DC power, as well as approximately 8.2 kW of heat through a chemical reaction between high-purity hydrogen and oxygen in the air. By connecting a hot water storage to the product, heat generated from the fuel cell can be converted into hot water (approx. 60ºC) for use in a factory or building.
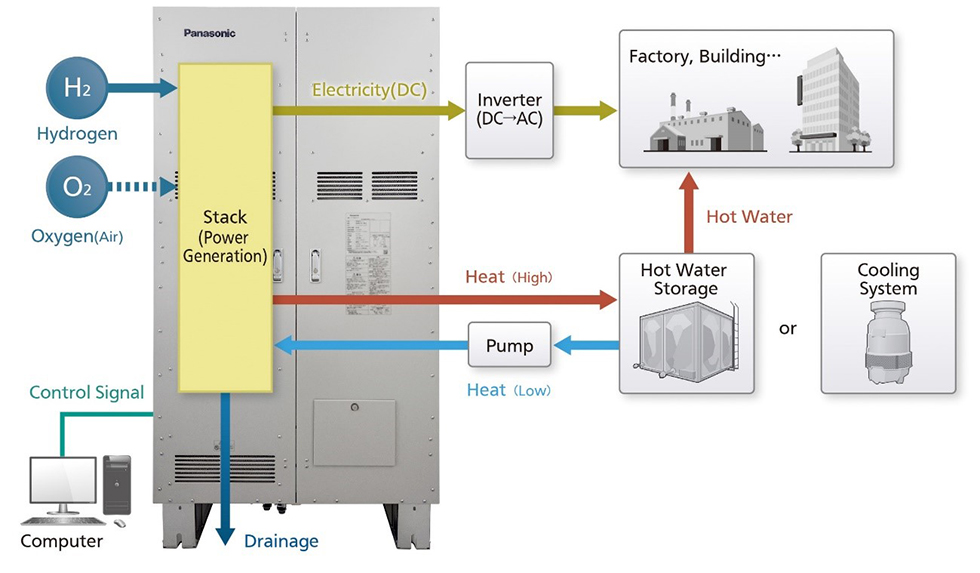
Power generation mechanism
By using the dedicated PH3 app, the power output can be adjusted in 1 kW increments between 4 kW and 10 kW per module. Since up to 250 pure hydrogen fuel cell generators can be connected, flexible power generation plans can be formulated for factories and buildings where various types of demand fluctuations are expected.
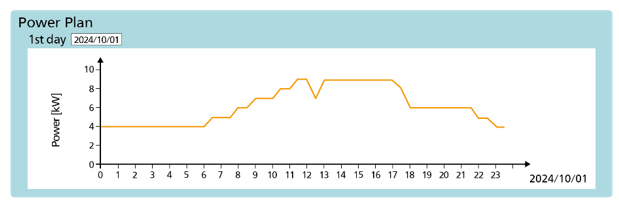
Screen for setting a power generation plan (1 module)
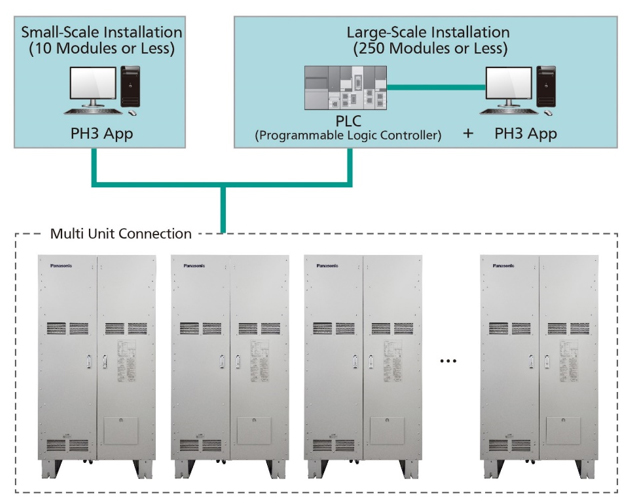
Connection image
The PH3 can be installed even in small spaces, since the maintenance access points are located in the front *7 . It can also be provided to a wide range of customers as it is resistant to cold (lower limit: -15ºC), as well as high altitude (1,000 m).
*7: Since installation regulations differ depending on the country, please confirm them before installation.
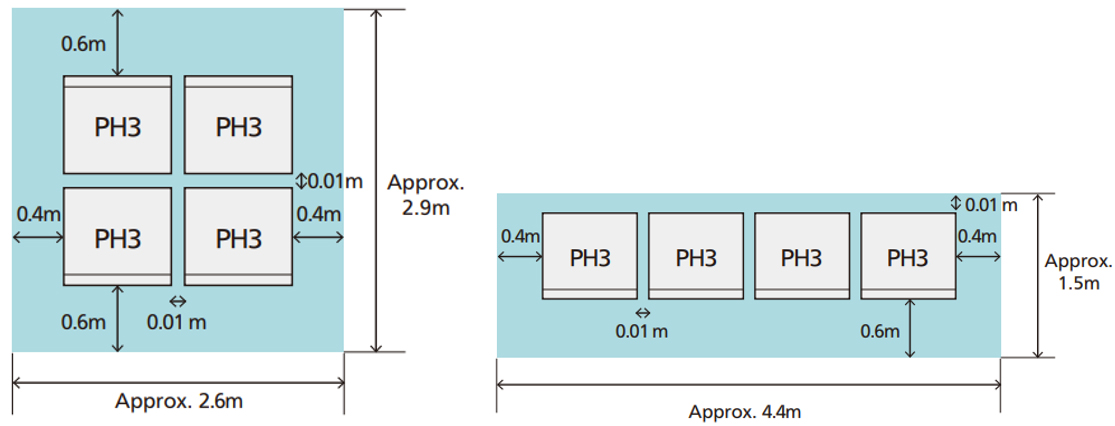
Installation examples for 40 kW power output (In case of without a design panel)
High efficiency rates such as an electrical efficiency of 57% (DC, LHV), heat recovery efficiency of 47% (LHV), and total efficiency of 104% have been achieved. High efficiency leads to reduced hydrogen usage, contributing to reduced running costs. The overhaul stop period is designed to be 15 years to ensure a long operating life *4 , which can reduce life cycle costs.
Specifications
*8: The inverter is required conforming to the application note.
*9: The computer used to install the app must be provided by the customer. The computer and fuel cell unit must be connected via LAN.
*10: The computer and PLC used to install the app must be provided by the customer. The computer and PLC, as well as the PLC and fuel cell unit, must be connected via LAN (hub relay).
*11 : On the side of the front face of the main unit, 400 mm space is required to open the door.
*12 : In case of attaching a design panel, 70 mm space is required for left and right sides.
Contact: [email protected]
The content in this website is accurate at the time of publication but may be subject to change without notice. Please note therefore that these documents may not always contain the most up-to-date information. Please note that German, French and Chinese versions are machine translations, so the quality and accuracy may vary.
- Business Solutions
Downloads (Images)

Sustainability

Featured news

Apr 09, 2024
- Products & Solutions
- Press Release
- Media and Entertainment

Apr 08, 2024
- North America
- Operating company

Mar 26, 2024
- China and Northeast Asia

Mar 14, 2024
- Air Conditioning

IMAGES
VIDEO
COMMENTS
Study with Quizlet and memorize flashcards containing terms like Construct an argument for or against the immediate introduction of fuel-cell cars. Include likely points of objection from those with opposing views (such as environmental and economic impacts) as well as counterarguments to those objections., If a stack of 40 solid oxide fuel cells in series generates 1,000 W of power, and each ...
Figure 17.12.1 17.12. 1: A hydrogen-oxygen fuel cell. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is silver. The electrolyte is usually a warm solution of potassium hydroxide, and the two electrode reactions can be written as.
A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates hydrogen atoms into protons and electrons, which take ...
The Polymer Electrolyte Membrane (PEM) Fuel Cell. These cells are also known as proton exchange membrane fuel cells (or PEMFCs). The temperature range that these cells operate in is between 50 o C to 100 o C. The electrolyte used in PEMFCs is a polymer which has the ability to conduct protons. A typical PEM fuel cell consists of bipolar plates ...
A fuel cell (actually a group of cells) has essentially the same kinds of components as a battery. As in the latter, each cell of a fuel cell system has a matching pair of electrodes. These are the anode, which supplies electrons, and the cathode, which absorbs electrons.Both electrodes must be immersed in and separated by an electrolyte, which may be a liquid or a solid but which must in ...
Figure 17.5.1: The diagram shows a cross section of a flashlight battery, a zinc-carbon dry cell. A diagram of a cross section of a dry cell battery is shown. The overall shape of the cell is cylindrical. The lateral surface of the cylinder, indicated as a thin red line, is labeled "zinc can (electrode).".
Direct Methanol Fuel Cells (DMFC): This fuel cell is similar to the Proton Exchange Membrane Fuel Cell; however, instead of using gaseous hydrogen as the fuel, liquid methanol is used. Solid Oxide Fuel Cells (SOFC): SOFC's can operate from ~ 550 °C to 1000 °C. SOFC's are able to do so because they use a solid ceramic electrolyte between their ...
A 150 year-old technology, hydrogen fuels cells are attractive alternatives for a number of reasons. One reason is efficiency. An internal combustion engine is limited by Carnot Efficiency, while a fuel cell converts chemical energy directly to electrical energy. Hydrogen fuel cells have the added benefit of producing nothing but water.
Attach a plugged piece of tubing on to the top pin of the hydrogen side of the fuel cell. Hydrate the electrolyte material in the fuel cell. Take the small syringe and suck water into it by placing the tip of the syringe into the distilled water and pulling the plunger. Suck a few milliliters (mL) into the syringe.
Fuel cell systems are used for applications such as stationary power units and for transportation, that is, electric vehicles. A fuel cell system has three basic parts: the fuel cell stack; the fuel processing unit; and a heat recovery system that processes the excess heat that is a by-product of the fuel cell operation.
A fuel cell course has been developed for junior/senior mechanical engineering students. The focus of the course is on systems level modeling of the fuel cell stack and the balance of plant. Lectures, assignments, and labs are geared toward introducing students to fuel cells and developing the basics of thermodynamics, electrochemistry, and ...
10.626 Lecture Notes, Fuel cells and lead-acid batteries Download File DOWNLOAD. Course Info Instructor Prof. Martin Bazant; Departments Chemical Engineering ... assignment Problem Sets. grading Exams with Solutions. notes Lecture Notes. co_present Instructor Insights. Download Course.
2.60 S2020 Lecture 9: Fuel Cells at Finite Current Download File DOWNLOAD. Course Info Instructor Prof. Ahmed F. Ghoniem; Departments Mechanical Engineering ... assignment_turned_in Problem Sets with Solutions. notes Lecture Notes. group_work Projects with Examples. Download Course.
The tools in this lesson plan will enable students to: • learn about the basics of electrochemistry, oxidation states, redox reactions, half-cells, and cell potentials. • describe electrolysis, electrolytic cells, and their applications. • explain what fuel cells are, describe their types, and how they work.
Fuel Cells and Batteries 2011 Lecture 9: Fuel Cells and Lead-Acid Batteries (PDF) [O'Hayre] Chapter 2. 11 Pourbaix Diagram (PDF) 2011 Lecture 9: Fuel Cells and Lead-Acid Batteries (PDF) Prentice, Geoffrey A. Chapter 3 in Electrochemical Engineering Principles. Prentice Hall, 1990. ISBN: 9780132490382. 12 Metal Acid Batteries, Lemon Battery ...
A query submitted for the term 'microbial fuel cells' on the Web of Science™ platform of Clarivate Analytics (Fig. (Fig.3) 3) showed a gradual increase in the number of research articles on MFCs that were published in the years 2004-2020 in scientific, peer-reviewed journals.It must be noted that this figure serves to only emphasize the growth trend and that the output of a similar ...
This Fuel cell uses hydrogen and oxygen to produce potable water, heat and electricity. This cell is also known as Bacon Fuel cell. Q3. Name the different types of Fuel cells. Answer: There are 6 types of Fuel cells namely: Proton exchange membrane fuel cell (PEMFC) Phosphoric acid fuel cell (PAFC) Solid acid fuel cell.
Thermodynamics of the fuel cell: 225: Irreversible losses in fuel cell: FAQ of Module3: Irreversible losses in fuel cell: 161: Components of fuel cell: FAQ of Module4: Components of fuel cell: 84: Fuel cell characterization: FAQ of Module5: Fuel cell characterization: 109: High temperature fuel cell: FAQ of Module6: High temperature fuel cell: 85
These types of fuel cells generally produce voltages of approximately 1.2 V. Compared to an internal combustion engine, the energy efficiency of a fuel cell using the same redox reaction is typically more than double (~20%-25% for an engine versus ~50%-75% for a fuel cell). Hydrogen fuel cells are commonly used on extended space missions ...
Alkaline fuel cells (AFCs) Alkaline fuel cells are the oldest commercialized fuel cell, first invented by Francis Bacon in 1932. AFCs contain porous electrodes saturated with alkaline solution that are separated by a hydroxide electrolyte. Since they are highly sensitive to CO 2, their reactions use pure oxygen or highly purified air.
A fuel cell is a device that produces electric energy, through a chemical reaction. Fuel cells use a positively charged ion (Hydrogen) and an oxidizing agent (oxygen). There are many types of fuel cells, but they all consist of a cathode, an anode, and an electrolyte that allows positively charged (hydrogen) ions to move between the two sides ...
Hydrogen and Fuel Cell Technologies Office; Draft Responses to Frequently Asked Questions and Common Concerns About Clean Hydrogen; A wide range of stakeholders, including environmental justice groups, have raised important questions about hydrogen's opportunities and limitations as a decarbonization solution, along with questions and concerns ...
GARDENA, Calif. (May 1, 2024) - Reaffirming its commitment to support fuel cell and additional hydrogen-related products and technology toward a hydrogen economy, Toyota Motor North America (TMNA) today announced that it is renaming the TMNA R&D California office as its new North American Hydrogen Headquarters (H2HQ). The office workspace at the new H2HQ was recently redesigned for its teams ...
Solid oxide cells (SOCs) have recently gained attention as an efficient energy conversion system for a smart-grid that can resolve the intermittency of renewable energy. In particular, a symmetric configuration of the electrode has been developed to minimize fabrication cost and relieve compatibility issues. In thi
Aside from the single hydrogen fuel cell Tundra it is testing in Japan, Toyota also showcased an FCV version of the Hilux pickup created by its UK arm (pictured). It features the Mirai's 182 ...
Unassisted photoelectrochemical (PEC) reactions, such as H2 generation and CO2 reduction, are limited by the durability of the immersed photoelectrode. Small band gap semiconductors, like Si, are efficient at utilizing a large portion of the solar spectrum but are not stable in aqueous environments without protecti
The Class 8 hydrogen fuel cell trucks will be refueled at a FirstElement Fuel station. Hyundai believes the program can save over 24,000 metric tons of carbon emissions. Hyundai has brought zero ...
Even before that, specifically in July 2021, the manufacturer secured the order for the fuel cell trucks for the NorCAL ZERO project. The Class 8 vehicle has two 90 kW hydrogen fuel cell systems (total 180 kW power) and a 350 kW electric motor. According to Hyundai, the 6×4 tractor has a range of more than 450 miles (724 km) when fully loaded.
Figure 5.5.6: In this hydrogen fuel-cell schematic, oxygen from the air reacts with hydrogen, producing water and electricity. In a hydrogen fuel cell, the reactions are. anode: 2H 2 + 2O2 − 2H 2O + 4e − cathode: O 2 + 4e − 2O2 − _ overall: 2H 2 + O 2 2H 2O. The voltage is about 0.9 V.
Osaka, Japan - Panasonic Corporation (hereinafter referred to as Panasonic) today announced that its Electric Works Company will launch a new pure hydrogen fuel cell generator that generates power through a chemical reaction between high-purity hydrogen and oxygen in the air in Oct. 2024 in Europe *1, Australia, and China.. In October 2021, Panasonic launched a 5 kW type pure hydrogen fuel ...