Methodology
Methodology articles should present a new experimental or computational method, test or procedure. The method described may either be completely new, or may offer an improved version of an existing method. The article must describe an advance on what is currently available and the advantages and disadvantages of the method compared to other alternatives should be discussed. The method needs to have been tested, validated and ideally, but not necessarily, used in a way that proves its value.
Methodology articles do not include a step-by-step description of the method. If appropriate, authors are encouraged to submit the step-by-step protocol as a Protocol article or to reference a previously published step-by-step protocol in the Methods section. More details about the Protocol article type can be found here .
BMC Methods strongly supports open research, including transparency and openness in reporting. Further details of our Data availability policy can be found here .

Preparing your manuscript
The title page should:
- present a title that includes, if appropriate, the research design
- if a collaboration group should be listed as an author, please list the Group name as an author and include the names of the individual members of the Group in the “Acknowledgements” section in accordance with the instructions below
- Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript
- indicate the corresponding author
The Abstract should not exceed 350 words. Please minimise the use of abbreviations and do not cite references in the abstract. The abstract must include the following separate sections:
- Background: the context and purpose of the study
- Methods: a concise description of the methodology used including any statistical tests
- Results: the main findings
- Discussion: potential implications and limitations
Three to ten keywords representing the main content of the article.
Introduction
The Introduction section should explain the background to the study, its aims, a summary of the existing literature and why this study was necessary.
This section should include:
- the aim, design and setting of the study
- the characteristics of participants and any inclusion/exclusion criteria
- a detailed list of all biological materials, reagents, solutions (with recipes if appropriate), laboratory supplies and equipment required for the successful execution of a method, including manufacturer catalogue numbers, instructions on how to acquire/produce items that are not commercially available, and storage conditions/shelf-life for critical reagents
- a clear description of all processes and methodologies employed
- a clear description of any previously unreported software or custom code (provide version and release date). Provide references for items that are not commercially available
- the type of statistical analysis used, including a power calculation if appropriate
- studies involving human participants, data or tissue or animals must include statement on ethics approval and consent
If a step-by-step protocol for a method has already been published on protocols.io, a link to the protocol on protocols.io can be provided in the Methods section. The following format should be used: “The step-by-step protocol of the method described in this peer-reviewed article is published on protocols.io, Updated February 9 2021 https://dx.doi.org/10.17504/protocols.io.xxxxxxx.”
The results section should include validation data to demonstrate performance, reproducibility and applicability of the method. If appropriate, results of statistical analysis must be provided either in the text or as tables and figures.
This section should provide an explanation of the importance and relevance of the study to the field, discussing the advantages and disadvantages of the method compared to other alternatives, highlight the limitations of the study, report any practical or operational issues involved in performing the study and any issues not covered in other sections.
Conclusions
This should state clearly the main conclusions and provide an explanation of the importance and relevance of the study to the field.
List of abbreviations
If abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations should be provided.
Declarations
All manuscripts must contain the following sections under the heading 'Declarations':
Ethics approval and consent to participate
Consent for publication, availability of data and materials, competing interests, authors' contributions, acknowledgements.
- Authors' information (optional)
Please see below for details on the information to be included in these sections.
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Manuscripts reporting studies involving human participants, human data or human tissue must:
- include a statement on ethics approval and consent (even where the need for approval was waived)
- include the name of the ethics committee that approved the study and the committee’s reference number if appropriate
Studies involving animals must include a statement on ethics approval and for experimental studies involving client-owned animals, authors must also include a statement on informed consent from the client or owner.
See our editorial policies for more information.
If your manuscript does not report on or involve the use of any animal or human data or tissue, please state “Not applicable” in this section.
If your manuscript contains any individual person’s data in any form (including any individual details, images or videos), consent for publication must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent for publication.
You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication).
See our editorial policies for more information on consent for publication.
If your manuscript does not contain data from any individual person, please state “Not applicable” in this section.
All manuscripts must include an ‘Availability of data and materials’ statement. Data availability statements should include information on where data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study. By data we mean the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article. We recognise it is not always possible to share research data publicly, for instance when individual privacy could be compromised, and in such instances data availability should still be stated in the manuscript along with any conditions for access.
Authors are also encouraged to preserve search strings on searchRxiv https://searchrxiv.org/ , an archive to support researchers to report, store and share their searches consistently and to enable them to review and re-use existing searches. searchRxiv enables researchers to obtain a digital object identifier (DOI) for their search, allowing it to be cited.
Data availability statements can take one of the following forms (or a combination of more than one if required for multiple datasets):
- The datasets generated and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
- The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- All data generated or analysed during this study are included in this published article [and its supplementary information files].
- The datasets generated and/or analysed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
- The data that support the findings of this study are available from [third party name] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [third party name].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
More examples of template data availability statements, which include examples of openly available and restricted access datasets, are available here .
BioMed Central strongly encourages the citation of any publicly available data on which the conclusions of the paper rely in the manuscript. Data citations should include a persistent identifier (such as a DOI) and should ideally be included in the reference list. Citations of datasets, when they appear in the reference list, should include the minimum information recommended by DataCite and follow journal style. Dataset identifiers including DOIs should be expressed as full URLs. For example:
Hao Z, AghaKouchak A, Nakhjiri N, Farahmand A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare. 2014. http://dx.doi.org/10.6084/m9.figshare.853801
With the corresponding text in the Availability of data and materials statement:
The datasets generated during and/or analysed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]. [Reference number]
If you wish to co-submit a data note describing your data to be published in BMC Research Notes , you can do so by visiting our submission portal . Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support ( example ).
All financial and non-financial competing interests must be declared in this section.
See our editorial policies for a full explanation of competing interests. If you are unsure whether you or any of your co-authors have a competing interest please contact the editorial office.
Please use the authors initials to refer to each authors' competing interests in this section.
If you do not have any competing interests, please state "The authors declare that they have no competing interests" in this section.
All sources of funding for the research reported should be declared. If the funder has a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript, this should be declared.
The individual contributions of authors to the manuscript should be specified in this section. Guidance and criteria for authorship can be found in our editorial policies .
Please use initials to refer to each author's contribution in this section, for example: "FC analyzed and interpreted the patient data regarding the hematological disease and the transplant. RH performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript."
Please acknowledge anyone who contributed towards the article who does not meet the criteria for authorship including anyone who provided professional writing services or materials.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
See our editorial policies for a full explanation of acknowledgements and authorship criteria.
If you do not have anyone to acknowledge, please write "Not applicable" in this section.
Group authorship (for manuscripts involving a collaboration group): if you would like the names of the individual members of a collaboration Group to be searchable through their individual PubMed records, please ensure that the title of the collaboration Group is included on the title page and in the submission system and also include collaborating author names as the last paragraph of the “Acknowledgements” section. Please add authors in the format First Name, Middle initial(s) (optional), Last Name. You can add institution or country information for each author if you wish, but this should be consistent across all authors.
Please note that individual names may not be present in the PubMed record at the time a published article is initially included in PubMed as it takes PubMed additional time to code this information.
Authors' information
This section is optional.
You may choose to use this section to include any relevant information about the author(s) that may aid the reader's interpretation of the article, and understand the standpoint of the author(s). This may include details about the authors' qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Examples of the Vancouver reference style are shown below.
See our editorial policies for author guidance on good citation practice
Web links and URLs: All web links and URLs, including links to the authors' own websites, should be given a reference number and included in the reference list rather than within the text of the manuscript. They should be provided in full, including both the title of the site and the URL, as well as the date the site was accessed, in the following format: The Mouse Tumor Biology Database. http://tumor.informatics.jax.org/mtbwi/index.do . Accessed 20 May 2013. If an author or group of authors can clearly be associated with a web link, such as for weblogs, then they should be included in the reference.
Example reference style:
Article within a journal
Smith JJ. The world of science. Am J Sci. 1999;36:234-5.
Article within a journal (no page numbers)
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Medicine. 2013;11:63.
Article within a journal by DOI
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. Dig J Mol Med. 2000; doi:10.1007/s801090000086.
Article within a journal supplement
Frumin AM, Nussbaum J, Esposito M. Functional asplenia: demonstration of splenic activity by bone marrow scan. Blood 1979;59 Suppl 1:26-32.
Book chapter, or an article within a book
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. p. 251-306.
OnlineFirst chapter in a series (without a volume designation but with a DOI)
Saito Y, Hyuga H. Rate equation approaches to amplification of enantiomeric excess and chiral symmetry breaking. Top Curr Chem. 2007. doi:10.1007/128_2006_108.
Complete book, authored
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Online document
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Online database
Healthwise Knowledgebase. US Pharmacopeia, Rockville. 1998. http://www.healthwise.org. Accessed 21 Sept 1998.
Supplementary material/private homepage
Doe J. Title of supplementary material. 2000. http://www.privatehomepage.com. Accessed 22 Feb 2000.
University site
Doe, J: Title of preprint. http://www.uni-heidelberg.de/mydata.html (1999). Accessed 25 Dec 1999.
Doe, J: Trivial HTTP, RFC2169. ftp://ftp.isi.edu/in-notes/rfc2169.txt (1999). Accessed 12 Nov 1999.
Organization site
ISSN International Centre: The ISSN register. http://www.issn.org (2006). Accessed 20 Feb 2007.
Dataset with persistent identifier
Zheng L-Y, Guo X-S, He B, Sun L-J, Peng Y, Dong S-S, et al. Genome data from sweet and grain sorghum (Sorghum bicolor). GigaScience Database. 2011. http://dx.doi.org/10.5524/100012 .
Figures, tables and additional files
See General formatting guidelines for information on how to format figures, tables and additional files.
Submit manuscript
In partnership with:

BMC Methods works in partnership with protocols.io .
Discover more here .
Important information
For authors
For editorial board members
For reviewers
- Editorial Board
- Manuscript editing services
BMC Methods
ISSN: 3004-8729
- Submission enquiries: [email protected]
- General enquiries: [email protected]
BMC Medical Research Methodology

Subject Area and Category
- Epidemiology
- Health Informatics
BioMed Central Ltd.
Publication type
Information.
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2022. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Editorial Board Members
Our Editorial Board Members work closely with our in-house editors to ensure that all manuscripts are subject to the same editorial standards and journal policies. Editorial Board Members are active researchers recognized as experts in their field. Our Editorial Board Members handle manuscripts within their areas of expertise, overseeing all aspects of the peer review process from submission to acceptance.
Cardiovascular Medicine
Fares al-ahdab, md, msc.
orcid.org/0000-0001-5481-696X Research interests: Global burden of cardiovascular disease, machine learning, preventive cardiology, nuclear cardiology, systematic review, meta-analysis, echocardiography, cardiac computed tomography angiography

Shiu Lun Ryan Au Yeung, PhD
orcid.org/0000-0001-6136-1836 Research interests: Cardiovascular diseases, Type 2 diabetes, Mendelian randomization, alcohol, epidemiology

Anping Cai, MD, PhD
orcid.org/0000-0003-3953-5881 Research interests: Hypertension, heart failure, health disparities

Tao Chen, PhD

Chengming Fan, MD, PhD
orcid.org/0000-0003-0497-1798 Research interests: Clinic practice, cardiovascular surgery, treatment of ischemic heart disease with human induced pluripotent stem cells (hiPSC)-related strategy and with preclinical animal models

Min Gao, PhD

Frederick Ho, PhD

Costan Magnussen, PhD
orcid.org/0000-0002-6238-5730 Research interests: Atherosclerosis, obesity, lipids, blood pressure, chronic disease epidemiology, early-life determinants of cardiovascular and metabolic diseases, cardiometabolic conditions with paediatric origins
Dr. Costan Magnussen is a chronic disease epidemiologist and Associate Professor, specialising in the investigation of early-life determinants of cardiovascular and metabolic (cardiometabolic) diseases. Harnessing the power of long-term observational studies, randomised controlled trials, and biorepositories, Dr. Magnussen’s research has advanced our understanding of the development and progression of cardiometabolic conditions with paediatric origins. Currently serving as an Editorial Board Member at BMC Medicine and an Associate Editor at Annals of Medicine, Dr. Magnussen has also provided his expertise as a reviewer for over 50 journals. As a journal editor, he is committed to upholding research standards and cultivating an open and proactive environment for the exchange of ideas toward important discoveries. With a passion for nurturing the next generation of scientists, Dr. Magnussen is engaged with the professional development of early-career researchers, providing guidance and support to help them excel in their chosen fields. Webpage .
Giulio Francesco Romiti, MD
orcid.org/0000-0002-3788-8942 Research interests: Cardiovascular Diseases; Atrial Fibrillation; Epidemiology; Systematic Review

Gaetano Santulli, MD, PhD, FAHA
orcid.org/0000-0001-7231-375X Research interests: Coronary artery disease, hypertension, cardiometabolic, cardiac arrhythmia, biomarkers, cardiac remodelling, blood pressure

Alessandro Spirito, MD
orcid.org/0000-0002-7473-6577 Research interests: Atherosclerosis, percutaneous coronary intervention, coronary arterty bypass grafting, coronary artery disease, ischemic heart disease, antiplatelet therapy, oral anticoagulation

Xiaoqiang Tang, PhD

Guo-Wei Tu, MD
orcid.org/0000-0001-7861-7631 Research interests: ICU, Mechanical Ventilation, Respiratory Physiology, Critical Care Medicine, Intensive Care Medicine, Sepsis, Hemodynamics

Hideki Uosaki, MD, PhD
orcid.org/0000-0002-8964-8609 Research interests: iPS cell-derived cardiomyocytes, cardiomyopathy disease modeling, gene therapy, genome editing, transcriptome analysis, cardiomyocyte physiology, mitochondrial function
Hideki Uosaki, M.D., Ph.D. is an Associate Professor at Jichi Medical University. He joined the Center for Molecular Medicine at Jichi Medical University, after completing postdoctoral training in heart development and cardiology at Johns Hopkins University School of Medicine (2016). He earned his M.D. and Ph.D. from Hokkaido University (2004) and Kyoto University (2011), respectively. He is primarily focusing on deciphering the molecular mechanisms and finding treatment for cardiomyopathy. His research includes 1) iPS cell-based disease models, 2) animal models created with genome editing, 3) gene therapy (adeno-associated virus), and 4) transcriptome analysis. Webpage .
orcid.org/0000-0003-0491-5585 Research interests: Obesity, Hypertension, Cardiovascular disease, Lifestlyle, Tobacco, Children, Women

Junjie Xiao, PhD
orcid.org/0000-0002-9202-0003 Research interests: Cardiovascular diseases; Heart failure; Cardiac Hypertrophy; Myocardial infarction; Exercise training; Biomarker; Extracellular vesicles; Non-coding RNAs

Hongsong Zhang, PhD
orcid.org/0000-0003-4251-7609 Research interests: Atherosclerosis, vascular calcification, heart failure, endothelial function, coronary artery disease, vascular biology, cardio-metabolomics, CVD epigenetics

Jie Jane Zhao, PhD

Endocrinology and Metabolism
Ling-wei chen, phd.
orcid.org/0000-0002-2661-8752 Research interests: Nutritional epidemiology, diet, dietary pattern, chrono-nutrition, DOHaD

Marloes Dekker Nitert, PhD
orcid.org/0000-0002-1909-8920 Research interests: metabolic and cardiovascular complications of pregnancy and the role of the microbiome

Huaidong Du, PhD

Yuanqing Fu, PhD
orcid.org/0000-0002-3955-9376 Research interests: Precision nutrition, gut microbiome, nutritional epidemiology, public health nutrition

Raúl González-Domínguez, PhD
orcid.org/0000-0002-7640-8833 Research interests: Metabolomics, nutrition, gut microbiota, exposome, Alzheimer's disease, cognitive decline

Jiaqi Huang, PhD
orcid.org/0000-0002-7808-9477 Research interests: Precision nutrition, nutritional epidemiology, metabolomics, metabolic epidemiology, chronic diseases, biostatistics

Jingjing Jiao, PhD
orcid.org/0000-0003-4167-1567 Research interests: Nutrition and disease, precise nutrition, nutritional epidemiology, cardiovascular metabolic disease, type 2 diabetes, public health nutrition

Lee-Ling Lim, MBBS, PhD, FRCP
orcid.org/0000-0002-6214-6924 Research interests: Diabetes mellitus, cardiometabolic medicine, heart failure, diabetic kidney disease, integrated care, precision medicine, implementation science

Sartaj Ahmad Mir, PhD
orcid.org/0000-0001-6032-0936 Research interests: Lipido mics, metabolomics, metabolism, lipoproteins, fatty acids, maternal and child health, insulin resistance, diabetes

Noah Peeri, PhD
orcid.org/0000-0003-4278-650X Research interests: Epidemiology, health disparities, genetic epidemiology, nutritional epidemiology, cancer survivorship, cancer epidemiology

Catarina Schiborn, PhD
orcid.org/0000-0002-9556-4540 Research interests: Risk prediction, risk scores, type 2 diabetes, cardiovascular diseases, observational studies, population-based cohorts

Gabriela Telo, PhD
orcid.org/0000-0001-9093-383X Research interests: Evidence-Based Practice; Type 1 Diabetes; Type 2 Diabetes; Obesity; Adherence; Mental Health; Cognitive Bias; Multiprofessional Care

Thaddäus Tönnies, Dr. PH
orcid.org/0000-0003-1577-4212 Research interests: Diabetes Epidemiology, Epidemiologic Methods, Prevalence, Incidence, Years of Life Lost, Modelling, Projections

Hongjiang Wu, PhD
orcid.org/0000-0003-2193-1114 Research interests: Diabetes, Epidemiology, Noncommunicable disease, Socioeconomic status, Trend analysis

Ju-Sheng Zheng, PhD
orcid.org/0000-0001-6560-4890 Research interests: Nutrition epidemiology, type 2 diabetes, genetic epidemiology

Gastroenterology
Ina bergheim, phd.
orcid.org/0000-0002-3356-4115 Research interests: Liver, nutrition, alcohol, endotoxin, intestinal barrier, obesity, aging

Huarong Chen, PhD
orcid.org/0000-0003-2192-1864 Research interests: Gastrointestinal cancer, cancer immunology, epitranscriptomicsm colorectal cancer, RNA modification, tumor immune microenvironment, cancer biology, cancer biomarkers, cancer diagnostics, targeted therapy for cancer, molecular oncology, signal transduction

Arunkumar Krishnan, MD

Dabin Liu, PhD
orcid.org/0000-0002-3811-8317 Research interests: Non-alcoholic fatty liver disease, medicine, liver cancer, oncology, cholesterol metabolism, obesity, nutrition, disorders of adipocyte-liver interaction

Alexandre Nuzzo, MD, PhD
orcid.org/0000-0002-8952-7620 Research interests: Gastroenterology and hepatology, enteral and parenteral nutrition, short bowel syndrome, abdominal pain, mesenteric ischemia, IBD (crohn’s disease and ulcerative colitis). Intensive care medicine, GI emergencies.

Andrea Shin, MD
orcid.org/0000-0001-7284-3398 Research interests: disorders of gut-brain interaction, functional gastrointestinal disorders, irritable bowel syndrome, chronic constipation, dyssynergic defecation, gastroparesis, gastrointestinal motility disorders, functional diarrhea

Guoxiang Xie, MD
orcid.org/0000-0002-0951-4150 Research interests: Metabolomics, biomarker discovery, gut microbial-host co-metabolism, metabolic disease biomarkers, gastrointestinal cancer, gastrointestinal carcinogenesis, bile acid metabolism

Santosh Yadav, PhD
orcid.org/0000-0001-9728-4561 Research interests: Gut microbiome and dysbiosis, pancreatitis, IBD and colon cancer, barrier proteins, PTSD, oxidative stress and Inflammation, cardiovascular disease, diabetes cardiomyopathy, inflammatory and non-inflammatory cell death mechanisms

Xiaotao Zhang, PhD
orcid.org/0000-0002-3968-5030 Research interests: Microbiome, epidemiology, nutrition, cancer epidemiology, liver disease, gastrointestinal disease, cancer prevention, clinical trial

Geriatric Medicine
Prasad nishtala, phd.
orcid.org/0000-0002-4155-8540 Research interests: Pharamcoepidemiology, geriatrics, polypharmacy, deprescribing

Yao Yao, MD
orcid.org/0000-0002-6723-6152 Research interests: Healthy aging, fall prevention, compression of dependency, health management, cohort study

Global, Public and Environmental Health
Loai albarqouni, md, msc, phd.

Steven Bell, PhD

Jean Joel Bigna, MD
orcid.org/0000-0001-8018-6279 Research interests: Epidemiology, Systematic reviews, Meta-analysis, Clinical Trials, Metabolic syndrome, Diabetes, Hypertension, Cardiovascular risk factors, Dyslipidemias, Obesity, Pulmonary Hypertension, HIV, hepatitis, Tuberculosis, Public Health, Global Health

Emelda Okiro, PhD

Petteri Oura, MD, PhD

Carmen Piernas, PhD
orcid.org/0000-0002-7536-922X Research interests: nutritional epidemiology, dietary interventions, NCD prevention

Zhirong Yang, PhD

Zhenyu Zhang, PhD

Health Services Research
Mohamed estai, phd.
orcid.org/0000-0001-7109-0267 Research interests: Health services research, RCTs, systematic review, diagnostic accuracy, surveys, population health, e-health, epidemiology, digital health, qualitative research, disease screening, prevention, artificial intelligence, rural and remote health, user behaviour, dental public health, global health, social determinants, health promotion
I hold a PhD degree from the University of Western Australia (2018). I have over 10 years of experience in research, writing, and academic publishing. My research background lies in digital health and population health. I have been working with researchers from the USA, Singapore, Thailand and Saudi Arabia on different projects. I have a good track record in funding and publication. I secured over $500,000 in research funding over the past 4 years. Throughout my career, I have actively engaged with the scholarly community as both an author and an editor. With over 40 research papers published in reputable journals, I have gained invaluable firsthand knowledge of the publication process. Additionally, I have served as a reviewer for numerous journals, honing my attention to detail and comprehensive understanding of academic standards. My editorial contributions extend to prestigious journals such as BMC Public Health (Q2) and Scientific Reports (Q2), where I serve on the editorial board. Currently, I hold the position of Research Program Officer at Western Australia Country Health Services (WACHS) since November 2022. I also maintain the role of Honorary Research Fellow at the University of Western Australia since 2019. I am a member of the HREC at the North Metropolitan Health Services, Graylands Hospital. Webpage .
Yixue Shao, PhD
orcid.org/0000-0003-1410-5455 Research interests: Health economics and outcomes research, health policy, health services research, endocrinology and metabolism

Jason Wasiak, PhD
orcid.org/0000-0002-5194-1586 Research interests: clinical expertise (burns, plastics, intensive care); clinical epidemiology; evidence-based medicine; evidence synthesis; randomised and non-randomised studies; medical writing; retracted publications.

Infectious Diseases
Samuel akech, phd.
orcid.org/0000-0001-5126-1225 Research interests: malaria, sepsis, antimicrobial resistance, paediatrics, quality of care, dehydration, malnutrition

Andrei R. Akhmetzhanov, PhD
orcid.org/0000-0003-3269-7351 Research interests: modeling, infectious diseases, outbreaks, COVID-19, Bayesian, infection control, reproduction number, emerging infectious diseases, vaccines, public health, global health

Marion Brunck, PhD
orcid.org/0000-0001-8994-8276 Research interests: Neutrophils, granulocyte transfusions, breastmilk, flow cytometry

Godfrey Bwire, MBCHB, MPH, PhD
orcid.org/0000-0002-8376-2857 Research interests: Cholera, infectious diseases, public health, epidemiology

Emily Edwards, PhD
orcid.org/0000-0002-0240-4370 Research interests: Infectious diseases, primary immunodeficiency, immunology

Kwadwo Kusi, PhD
orcid.org/0000-0001-5483-9985 Research interests: Cellular and humoral immunology of parasite and viral infections; Vaccine candidate discovery, design and testing; Pathogen biology

Bartholomew Ondigo, PhD

Matthew Quaife, PhD
orcid.org/0000-0001-9291-1511 Research interests: Health economics, infectious disease modelling, TB, HIV, cost-effectiveness analyses, choice modelling

I am a health economist specialising in the prevention of infectious diseases, working at the interface of mathematical modelling and health economics. I enjoy the challenge of representing complex human behaviours in models and understanding how to encourage equitable and efficient health. My work mainly focuses on HIV and TB. Webpage .
Max Carlos Ramírez-Soto, BSc, MPH, FRSPH, PhD
orcid.org/0000-0003-0471-6746 Research interests: Sporotrichosis, subcutaneous mycoses, fungal infections, epidemiology, viral hepatitis (A, B, C, D and E), dengue, SARS-CoV-2

Shuo Su, PhD

Lin Wang, PhD
orcid.org/0000-0002-5371-2138 Research Interests: Infectious disease epidemiology, mathematical modelling, Bayesian statistics, machine learning, serology, immunology, arbovirus, dengue, influenza, SARS-CoV-2

Courtney Yuen, PhD
orcid.org/0000-0002-5219-2599 Research interests: Tuberculosis, TB/HIV, community-based interventions, patient-centered care, implementation science, mixed-methods research, global health

Medical Genomics
Sadhana gaddam, msc.

Chani Hodonsky, PhD
orcid.org/0000-0001-8566-5877 Research interests: Genomics, atherosclerosis, blood traits, epidemiology, ancestry, diversity, gene expression, TWAS, GWAS, somatic clonality

Liang-Dar Hwang, PhD
orcid.org/0000-0002-5535-2199 Research interests: Personalized nutrition, sensory nutrition, twin modelling, causal modelling, mendelian randomization, genetic epidemiology, statistical genetics, taste perception

Boyang Ji, PhD
orcid.org/0000-0002-7269-4342 Research interests: Systems biology; Genomic Medicine; Omics integration; Modeling; Microbiome; Biomarker

Dries Martens, PhD

Nagarajan Raju, PhD
orcid.org/0000-0003-3851-5520 Research interests: Bioinformatics and computational biology, anti-HIV-1/covid/viral antibody discovery, B-cell repertoire analysis

Chao Xu, PhD
orcid.org/0000-0002-3821-6187 Research interests: multi-omics; genetic epidemiology; bioinformatics; biostatistics

Jie Zheng, PhD
orcid.org/0000-0002-6623-6839 Research interests: Genetic epidemiology, bioinformatics, genetic tools and platform development, drug development research, multi-omics Mendelian randomization, phenome-wide proteome research

Medical Informatics and Mathematical Modelling
Nan liu, phd.
orcid.org/0000-0003-3610-4883 Research interests: AI, machine learning, data science, medical informatics, health services research, deep learning, heart rate variability, out-of-hospital cardiac arrest

Medical Statistics
Bhaskar thakur, phd.
orcid.org/0000-0001-6869-8968 Research interests: cardiovascular disease epidemiology, rehabilitation research, nutrition and liver disease epidemiology, cancer epidemiology, hypertension, diabetes, mental health disorder, maternal and child health

Guangyu Tong, PhD
orcid.org/0000-0002-7697-5029 Research interests: Trial design, causal inference, treatment heterogenity, meta-analysis, machine learning, developmental psychiatry, mental health, gun violent crime, life-course research

James Fotheringham, MBChB, PhD
orcid.org/0000-0002-8980-2223 Research interests: Health-related quality of life, Health Economics, Health Services Research, Causal Inference, haemodialysis, dialysis, kidney transplantation, chronic kidney disease, acute kidney injury, kidney

Sam Kant, MD
orcid.org/0000-0001-5863-3562 Research interests: Glomerulonephritis, Immunosuppression, Transplant, nephrology, kidney, anti–neutrophil cytoplasmic antibody–associated vasculitis Dr. Kant is a transplant and general nephrologist at the Johns Hopkins University School of Medicine. He has completed internal medicine residency and chief residency at University of Maryland, with subsequent general and transplant nephrology fellowship at Johns Hopkins. His research interests include immunosuppression, transplantation, immunology and glomerulonephritis. He is also chair of the American College of Physicians-Maryland Young Physicians Council. Webpage.
Ashish Verma, MBSS
orcid.org/0000-0001-9497-6327 Research interests: Chronic kidney disease, diabetic kidney disease, renal amyloidosi, acid base and electrolytes disorder, aldosterone

Pablo Andrade, PhD, MD
orcid.org/0000-0002-2596-7301 Research interests: Neuromodulation, deep brain stimulation, psychosurgery, spinal cord stimulation, functional neurosurgery, stereotactic neurosurgery, neuroprosthetics

Sherief Ghozy, MD
orcid.org/0000-0001-5629-3023 Research interests: stroke prevention and treatment, endovascular and interventional neurology, neuroimaging in stroke and neurovascular diseases, health services, clinical trial methodology, meta-analysis, medical education, e-learning, telemedicine, and digital health technologies

Panteleimon Giannakopoulos, MD
orcid.org/0000-0003-4624-7386 Research interests: Cognitive and Affective Neurosciences, Psychiatry, Neuropsychology, Psychotherapy

Lana Ruvolo Grasser, PhD
orcid.org/0000-0002-7168-5524 Research interests: PTSD, trauma, anxiety, development, children and adolescents, dance/movement therapy, art therapy, inflammation, psychophysiology

Wenting Guo, PhD
orcid.org/0000-0003-3585-7728 Research interests: neurodegenerative diseases (with special expertise in Amyotrophic lateral sclerosis, Frontotemporal Dementia and Charcot–Marie–Tooth disease), regenerative medicine, translational research

Niklas Joisten, PhD
orcid.org/0000-0002-9947-8746 Research interests: Exercise, tryptophan metabolism, kynurenine, inflammation, autoimmune diseases, rehabilitation, physical activity

Feng Sha, PhD

Shashank Shekhar, PhD
orcid.org/0000-0001-8720-6690 Research interests: Neuroscience, neuronal circuit-signaling regulating homeostatic responses, UPR, autophagy, stress reponses, neurodegeneration

Yuzhen Xu, MD, PhD
orcid.org/0000-0002-1289-3438 Research interests: Cognitive, dementia, stroke, biomarker, rehabilitation, diabetes, complementary and alternative medicine, Traditional Chinese Medicine, sports medicine

Obstetrics and Gynecology
Francesco dessole, md, phd.
orcid.org/0000-0003-3581-2503 Research interests: Obstetrics, urogynecology, urinary incontinence, sexual dysfunction, delivery and pelvic damage, bi-quadri radiofrequency treatment

Jon Huang, PhD, MPH
orcid.org/0000-0002-5901-8403 Research interests: Reproductive, perinatal, pediatric epidemiology, causal inference, molecular epidemiology, environmental health, life course, birth cohorts

Kimiyo Kikuchi, PhD
orcid.org/0000-0002-9397-4327 Research interests: global health, maternal and child health

Mengmeng Li, MD, MSPH
orcid.org/0000-0001-7518-1641 Research interests: Reproductive medicine, in-vitro fertilization, environmental exposure and child health, neurodevelopmental disorder, adolescent gender norm, adolescent mental health

Johannes Ott, PhD

Alex Ridout, MBBS, BA, MRCOG, MD
orcid.org/0000-0003-0988-7229 Research interests: Preterm birth, pre-eclampsia, global health, maternal mortality

Natalie Suff, PhD
orcid.org/0000-0002-0736-7046 Research interests: Pregnancy, preterm birth, immunology, reproductive health, animal models, infection

Shengzhi Sun, PhD
orcid.org/0000-0002-3708-1225 Research interests: Environmental epidemiology; climate change and health; air pollution and health; built environment and health; cohort study; time-series study

Kilian Vomstein, MD
orcid.org/0000-0003-2981-4211 Research interests: Infertility, recurrent pregnancy loss, embryo implantation, endometriosis, endometrium, reproductive immunology, early pregnancy

Jian Zhao, PhD
orcid.org/0000-0002-1306-1676 Research interests: Early life determinants of endocrinology & metabolic disorders, Mendelian randomization, integrative analysis of multi-omics data, aetiological epidemiology of reproductive and perinatal outcomes, prospective cohort studies

Yeyi Zhu, PhD
orcid.org/0000-0002-7296-738X Research interests: Pregnancy complications, gestational diabetes, gestational hypertension, preterm birth, nutrition, epidemiology, obesity, children

Kilan Ashad-Bishop, PhD
orcid.org/0000-0001-6846-3499 Research interests: cancer biology, cancer disparities, social determinants of health, structural determinants of health

Melvin L.K. Chua, MBBS, FRCR, PhD
orcid.org/0000-0002-1648-1473 Research interests: Nasopharyngeal carcinoma, head and neck cancer, prostate cancer, genomics, biomarkers, clinical trials

Alexis Combes, PhD
orcid.org/0000-0002-9110-6542 Research interests: Tumor immunology, cancer, system immunology, multi-dimensional single cell technologies

Narendranath Epperla, MD, MS

Kelly Hirko, PhD, MPH
orcid.org/0000-0002-0050-655X Research interests: cancer, epidemiology, obesity, diet, disparities, rural, implementation science

Jaidip Jagtap, PhD
orcid.org/0000-0003-1839-862X Research interests: biomedical engineering, lasers & optics, physics, and Radiology. Computer assisted quantification, Cancer, Translational medicine, Bioinformatics, Radiomics, Radiation oncology, Medical Imaging (MRI, CT, Ultrasound, Digital imaging), Nanoparticles

Jianguang Ji, PhD
orcid.org/0000-0003-0324-9496 Research interests: Cancer epidemiology, pharmacoepidemiology, colorectal cancer, stomach sancer, mendelian randomization

Christiana Kartsonaki, PhD, DPhil
orcid.org/0000-0002-3981-3418 Research interests: Cancer, statistics, epidemiology, COVID-19, case-cohort studies, fine-mapping, breast cancer, pancreatic cancer, proteomics

Victor Kok, MD, PhD

Can Küçük, PhD
orcid.org/0000-0001-5540-9012 Research interests: Lymphoma, mutiple myeloma, hematological malignancies, cancer biology, oncology, molecular biology and genetics, genomics, transcriptomics, epigenomics

Shuai Li, PhD
orcid.org/0000-0002-8696-8594 Research interests: Genetic epidemiology, epigenetic epidemiology, twin research, family research, cancer risk prediction, breast cancer

Suping Ling, PhD
orcid.org/0000-0002-7207-5656 Research interests: Diabetes, cancer, inequalities, electronic health records data

Huai Liu, MD
orcid.org/0000-0001-7854-8082 Research interests: Nasopharyngeal carcinoma, head and neck cancer, lung cancer, radiotherapy

Tanimola Martins, PhD

Yoshiaki Nagatani, PhD
orcid.org/0009-0000-6153-6456 Research interests: Chemotherapy, gastrointestinal cancer, oncology, immuno-oncology

Hirak Patra, PhD
orcid.org/0000-0002-6142-5489 Research interests: Regenerative medicines, drug delivery, theranostics, biosensing

Ramesh Pothuraju, PhD

Joseph Rothwell, PhD
europepmc.org/authors/0000-0002-6927-3360 Research interests: Cancer epidemiology, cancer prevention, cancer risk factors, cohort studies, metabolomics, biomarkers, omics technologies, nutrition

Alessandro Russo, MD, PhD
orcid.org/0000-0002-3365-1972 Research interests: NSCLC; Liquid biopsy; Immunotherapy; SCLC

Mingwang Shen, PhD
orcid.org/0000-0003-4553-800X Research interests: Infectious diseases modelling, health economics, cost-effectiveness analysis, artificial intelligence, genetic screening and gene therapy, cancer screening


Ryota Tamura, MD, PhD
orcid.org/0000-0002-8637-901X Research interests: Malignant glioma; Neurofibromatosis; iPS cells; Neural stem cell; Gene therapy; Immunotherapy; Skull base surgery

Pei Yu, PhD
orcid.org/0000-0002-7485-1751 Research interests: Environmental health, cancer epidemiology

Dake Zhang, MD, PhD
orcid.org/0000-0001-9508-8209 Research interests: Tumor genomics, medical genomics, translational genomics, computational biology, biomarkers, DNA methylation, HBV infection, liver cancer

Wen Zhang, PhD
orcid.org/0000-0003-3150-2751 Research interests: Biotherapy, Immunotherapy, Liquid biopsy, Tumor microenvironment, Biomarkers, Metastasis, Cancer stem cell

Yi Zhang, PhD
orcid.org/0000-0001-5388-1276 Research interests: Bioinformatics, statistics, aging, cancer, preeclampsia, cell-free DNA, in-vitro diagnosis, epigenetics, DNA methylation

Bin Zhao, PhD
orcid.org/0000-0001-7065-2416 Research interests: T cell development/differentiation, B cell development/differentiation, autoimmune diseases, T cell metabolism, B cell metabolism, nutrition, chronic disease prevention and control

Jun Zhong, PhD
orcid.org/0000-0002-3700-9434 Research interests: Cancer genetic epidemiology, computational oncology, cancer multi-omics, driver factors for tumorigenesis, pancreatic cancer, polygenic risk scores (PRS), comprehensive risk prediction, genome/transcriptome-wide association study (GWAS/TWAS), translational genomics, artificial intelligence.

Jian Gou Zhou, MD, PhD
orcid.org/0000-0002-5021-3739 Research interests: EBM, Precision Oncology, Radiation Oncology, Immunotherapy, Biomarkers, bioinformatics Jian-Guo Zhou MD, Dr. rer. biol. hum (Ph.D.) is a oncologist at Department of Oncology, Second Affiliated Hospital of Zunyi Medical University. His research focuses on how to identify the predictive and prognostic biomarkers for cancer patients with ICI and RT. He is an Associate Editor of Technology in Cancer Research & Treatment (TCRT), Frontiers in Oncology and Frontiers in Immunology; Section Editor of Cancer Control; Academic Editor of Pain Research and Management, Disease Markers, Medicine®, and a guest editor of Therapeutic Advances in Medical Oncology. He also served as a reviewer for more than 40 international Journals, e.g. JITC, Oncoimmunology, Gynecologic Oncology, IJC, et al. He got Merit Award in ESMO Asia (2018, 2019), KSMO Young Investigator Award (2020), CSCO 35 under 35 (2019), and received lots of oral presentations in ESMO Asia, EANO. Webpage .
Qihong Deng, PhD

Niina Kolehmainen, PhD
orcid.org/0000-0002-9229-9913 Research interests: child health, complex interventions, behaviour, paediatric, non-pharmacological, mixed methods, population health, applied health, allied health, physiotherapy, occupational therapy, nursing

Primary Care
Bhautesh jani, phd.
orcid.org/0000-0001-7348-514X Research interests: Multimorbidity; Primary care

Getinet Ayano, PhD
orcid.org/0000-0002-9137-4141 Research interests: Mental health, child and adolscent psychiatry, intergenerational transmission of mental health, substance use, comorbid mental and physical disorders, crime, severe mental disorders, mental health interventions, risk and protective factors for mental health, crime and mental health among homeless people.

Ji Chen, PhD

Shanquan Chen, PhD

Runsen Chen, PhD
orcid.org/0000-0003-3398-5750 Research interests: Psychiatry, mental health, suicide, self harm, mood disorder, LGBT

Philipp Homan, MD, PhD

Jianbo Lai, MD
orcid.org/0000-0002-8137-7701 Research interests: Affective disorders, neuroinflammation, brain-gut axis; psychiatric rehabilitation, non-suicidal self-injury, traumatic brain injury, bipolar depression

Ming Li, PhD

Julian Mutz, PhD
orcid.org/0000-0001-5308-1957 Research interests: Epidemiology, Frailty, Ageing, Depression, Bipolar disorder, Brain stimulation, aging, psychiatric genetics, biobank

Heather Palis, PhD
orcid.org/0000-0002-8570-5717 Research interests: Substance use, substance use disorder, opioid agonist treatment, harm reduction, mental illness, incarceration, overdose

Hugo Senra, PhD
orcid.org/0000-0001-8054-6473 Research interests: Adult depression, anxiety, stress, eating disorders, interplay between physical and mental health (long-term medical conditions), vision

Rheumatology
Emre bilgin, md, msc.

Feng Pan, MD, PhD

Xinghao Yu, PhD
orcid.org/0000-0002-0589-0386 Research interests: causal inference, genetic pleiotropy, polygenic risk score, and genome-wide assoication study, Mendelian randomization, bone genetics, amyotrophic lateral sclerosis genetics

- Editorial Team
- Editorial Board
- Call for papers
- Editor’s choice
- Sign up for article alerts and news from this journal
- Manuscript editing services
Annual Journal Metrics
2022 Citation Impact 9.3 - 2-year Impact Factor 10.4 - 5-year Impact Factor 3.011 - SNIP (Source Normalized Impact per Paper) 3.447 - SJR (SCImago Journal Rank)
2023 Speed 6 days submission to first editorial decision for all manuscripts (Median) 145 days submission to accept (Median)
2023 Usage 6,375,113 downloads 24,228 Altmetric mentions
- More about our metrics
Announcements
medRxiv transfers
BMC Medicine is happy to consider manuscripts that have been, or will be, posted on a preprint server. Authors are able to submit their manuscripts directly from medRxiv , without having to re-upload files.
Registered reports
BMC Medicine is accepting Registered Reports. Find out more about this innovative format in our Submission Guidelines .
- Follow us on Twitter
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Call for papers - Data science methodologies
Guest editor.
Imran Ashraf, PhD, Department of Information and Communication Engineering, Yeungnam University, Republic of Korea
Submission Status: Open | Submission Deadline: 17 August 2024

Articles will undergo the journal’s standard peer-review process and are subject to all of the journal’s standard policies . Articles will be added to the Collection as they are published.
The Editors have no competing interests with the submissions which they handle through the peer review process. The peer review of any submissions for which the Editors have competing interests is handled by another Editorial Board Member who has no competing interests.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

BMC Medicine: a decade of open access medical research
Sabina alam.
1 BioMed Central Ltd, 236 Gray’s Inn Road, London WC1X 8HB, UK
Jigisha Patel
On 24 November 2003, BMC Medicine published its first article. Ten years and over 900 articles later we look back at some of the most notable milestones for the journal and discuss advances and innovations in medicine over the last decade. Our editorial board members, Leslie Biesecker, Thomas Powles, Chris Del Mar, Robert Snow and David Moher, also comment on the changes they expect to see in their fields over the coming years.
Just a few months after the Human Genome Project was declared complete [ 1 ] BMC Medicine was launched as an open access [ 2 , 3 ], open peer review journal (i.e. where signed peer review reports are published with the article) [ 4 , 5 ], with the aim of making high impact clinical peer-reviewed research of general interest, accessible to everyone from the basic scientist to the practicing clinician. The journal, initially under the direction of Pritpal Tamber and then Melissa Norton as Editor-in-Chief, was launched amidst a raging debate about the viability of open access publishing [ 6 ]. But open access survived and evolved [ 7 , 8 ], and BMC Medicine now ranks 8 th out of 155 journals in the 2012 general and internal medical journals category of the Journal Citation Reports [ 9 ].
While mainly focused on primary research in its early days, the journal responded to the needs and demands of its readers and contributors by providing, for example, a platform for discussing controversies in medical practice [ 10 - 15 ] and embracing social networking technology to promote open scientific discussion and debate [ 16 - 19 ]. Although proud of its Impact Factor (IF) of 6.68, BMC Medicine recognizes that the IF is a restrictive metric that does not fully reflect the influence of individual articles post-publication. The journal therefore provides informative article metrics which are immediately available on published articles - a feature which has proven to be popular with many of our authors [ 20 ].
Of course, innovations in publishing and technical advances notwithstanding, BMC Medicine owes its success to the scientific contributions made by its authors, reviewers and expert editorial board members. To celebrate its 10 th anniversary, we recently reviewed some of our most successful articles in terms of accesses [ 21 ], citations [ 22 ] and ‘impact’ in news and social media [ 23 ], and also summarized author and reviewer experiences [ 24 ] and explored our author demographics [ 25 ]. As a general medical journal with a very broad scope, it is not possible for us to cover all the main advances in medicine featured in the journal over the last decade, but in this editorial we present a selection of our favorite recent content, together with predictions by our editorial board members on possible future directions for their respective fields of research.
Translational medicine: how far have we come with stem cells, biomarkers and ‘Omics’ research?
Stem cell research and therapy has advanced rapidly in the last decade, and clinical trials for a wide range of diseases are already underway [ 26 , 27 ]. In 2012, the journal published an intriguing study by Zhao and colleagues, who used Stem Cell Educator therapy to safely reverse Type 1 diabetes. The researchers used stem cells from cord blood to ‘re-educate’ T cells in patients with Type 1 diabetes, thereby restoring pancreatic function and reducing the need for insulin [ 28 ]. These compelling results highlight how stem cell therapies may become part of mainstream treatment for many diseases.
Within the last 10 years, major advances have also been made in biomarker research and ‘Omics’ studies in a preclinical setting. Advances in whole genome sequencing have allowed the identification of genes involved in a large number of diseases, and biomarkers that indicate disease severity or susceptibility to treatment are increasingly being characterized [ 29 - 32 ]. As Leslie Biesecker points out (Box 1), clinical exome and genome sequencing are already being used in the clinic for diagnostic and prognostic purposes. However, Biesecker also indicates that these new technologies are not without problems and alludes to the role the journal plays in ensuring the latest research is appropriately validated and disseminated.
Despite the discovery of many biomarkers for cancer in particular, so far very few have been used within the clinical setting [ 33 ], which is partly due to a lack of consistency and clarity in the reporting of prognostic tumor markers. This prompted the development of the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) checklist [ 34 ], which was updated in 2012 by Altman and colleagues [ 35 ] and more recently, the development of criteria to address the lack of scientific rigor when evaluating preclinical evidence to support translation of omics-based predictors to clinical trials [ 36 , 37 ].
The continued identification of new genes and biomarkers specific to disease subtypes and individual patients is essential for translation into personalized medicine, in terms of estimating both disease risk and response to therapy. As highlighted above, the field which has seen the most progress in this area is clinical oncology, and Thomas Powles explains what further changes are required to achieve effective personalized cancer therapies (Box 2).
Leslie G. Biesecker National Human Genome Research Institute, USA
Genomics has provided new modes of discovery in the basic sciences and is now doing the same for clinical research. It has already started to change medical practice with clinical exome and genome sequencing. These two assays are now being used in thousands of patients, for example, to provide a genome-wide diagnostic assay for uncharacterized disorders of birth defects or neurologic disorders and in tumor sequencing to identify targets for cancer therapeutics. BMC Medicine has a critical role to fulfil in this process by providing a forum for critical evaluation of these new technologies. The objective could not be more important - to preserve what works well in medicine and remake what does not. It is hard to imagine a more exciting time for our field.
Thomas Powles St Bartholomew's Hospital, London, UK
The field of medical oncology is moving at a breathtaking speed. A plethora of new agents are now available based on the molecular biology of specific tumors. The next step is to identify subsets of patients who benefit from therapy and move away from ‘one size fits all’ strategies. Therefore, biomarkers predicting response to these therapies are required. The application of whole genome sequencing, novel tracers within the context of functional imaging and circulating biomarkers, such as free circulating tumor DNA will be important pieces in this complex puzzle. There is also a need to develop therapies which focus on inducing longer remission rather than temporary disease controls. A collaborative international approach is required to achieve these goals.
Evidence-based medicine: education, communication and collaboration
There has been increasing international focus on public health initiatives, development of healthcare policies and evidence-based guidelines to improve medical practice [ 38 , 39 ]. This is embedded in effective education strategies, which is evident from a continuing medical education intervention aimed at strengthening links between evidence-based and values-based medicine in healthcare personnel [ 40 ]. Researchers found this intervention led to improved values, such as openness to change, which are essential for improving medical care. Chris Del Mar (Box 3) recommends that going forward a more collaborative approach to decision-making between clinicians, patients and policy makers needs to be developed, and highlights the importance of transparency and communication.
There is also increasing focus on involving researchers based in low-to-middle income countries as principal investigators in local research projects. This is especially important as local knowledge helps to ask the ‘right’ questions in health research, ensures the best available evidence is accumulated and that all ethical aspects have been considered [ 41 - 43 ]. This is vital to guide healthcare policies and identify new tools and strategies; the consequences of not doing so is evident from a recent bibliometric analysis of childhood immunization research output from Africa. Since the onset of the Expanded Program on Immunization in 1974, vaccine research productivity in Africa has skewed toward those funded privately, with minimal research input from African authors, suggesting a need for better communication among all stakeholders [ 44 ]. Robert Snow points out (Box 4), conditions for research are now improving in Africa, and it is important that local researchers and governments work closely to drive the research output from these regions forward.
Chris Del Mar Bond University Gold Coast, Australia
Medicine will enter a new phase of concern about decreasing gains for increasing harms - including not just cost but also over-diagnosis and over-treatment. The medical profession has not been able hitherto to demonstrate an ability to make such cost-benefit decisions sensibly alone, and therefore there will be increasing input from society; increased demand for shared decision-making with the patient; and more directives from government. One important element of quality will soon be considered to be the extent to which the clinician has explicitly, clearly and carefully communicated the evidence in such a way that every patient is in a position to express a preference for the range of management options available. Evidence-based medicine will no longer be some hidden activity that clinicians may (or may not) engage in: it will become the currency expected for patient-clinician communication.
Robert William Snow Kenya Medical Research Institute, Kenya
Since I started work in Africa 30 years ago the landscape of science and research capacity has changed enormously. It is no longer legitimate to make excuses that model-based computing, laboratory science or gene sequencing can only be done in the north. The infrastructure and human capacity now exists in Africa to provide the best possible science for public health problems that face the continent. A fundamental requirement for any form of development is that countries have to take ownership of their problems. The next decade requires an active promotion by governments in Africa, and international partners that support regional development, of the expanding cohorts of African scientists who champion the very highest standards of medical and public health science within the region. Generating new research from within Africa holds untold promise. Unlike external research agendas and funding 30 years ago, this new research will have a much greater and much faster impact on the health of communities in Africa over the next decade.
Enhancing research with reporting guidelines
Without clear guidelines for conducting and analyzing medical research, there is a limit to how far medicine can progress, and the last few years have seen many important improvements in reporting standards. In 2010, BMC Medicine co-published the updated CONSORT (CONsolidated Standards of Reporting Trials) statement by Schulz and colleagues [ 45 ]. This statement guides authors on the reporting of two-parallel design randomized controlled trials by using a checklist and flow diagram based on the latest methodological evidence. More recently, in response to the particular challenges in reporting economic evaluations of health interventions, the Consolidated Health Economic Evaluation Reporting Standards ( CHEERS ) was published [ 46 ]. This statement consolidates existing guidelines with the aim of providing more ‘user-friendly’ guidance for researchers and editors.
As research methods become more sophisticated, so too do the methods via which literature analysis can be conducted. The ‘RAMESES’ (Realist and Meta-review Evidence Synthesis: Evolving Standards) statement [ 47 , 48 ] was published to provide researchers, institutes and journals with guidance on how to conduct these new forms of literature analysis, and adherence to the guidelines will lead to quality assurance and uniform reporting of studies.
Ensuring consistency is a challenging task, and David Moher explains (Box 5) that journals and editors play a key role in providing peer reviewers and authors with the tools and guidance to ensure that medical research is appropriately reported.
We hope you have enjoyed our selection of just some of the most exciting content from BMC Medicine, and hope this has prompted you to seek out favorites of your own.
As an open access general medical journal, we aim to promote better informed clinical decisions and improved therapies. We will continue to publish content that has the potential to improve clinical practice, research and reporting. We especially encourage debate on health related issues not just within the clinical community, but also for the general public who should be, after all, the primary beneficiaries of the research.
David Moher Ottawa Hospital Research Institute, Canada
To reduce the considerable waste of inadequately published research, medical journals will need to develop long-term innovative strategies, such as developing core competencies for editors and peer reviewers, as well as accreditation programs for journals. More immediately, they can help foster greater implementation of reporting guidelines by facilitating the development of applications that can take manuscript content and automatically populate reporting guideline checklists. Such information can provide immediate feedback about the completeness of reporting of manuscripts to authors, editors and peer reviewers.
Competing interests
Both authors are employees of BioMed Central, the publisher of BMC Medicine .
Authors’ information
Sabina Alam is the Editor of BMC Medicine . Jigisha Patel is the Medical Editor at BioMed Central.
Acknowledgements
We thank our academic editorial board and our reviewers for the contribution they have made to the success of BMC Medicine and our authors and readers for their ongoing support.
- Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research: a blueprint for the genomic era. Nature. 2003; 422 :1–13. [ Google Scholar ]
- Tamber PS, Godlee F, Newmark P. Open access to peer-reviewed research: making it happen. Lancet. 2003; 362 :1575–1577. doi: 10.1016/S0140-6736(03)14748-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Suber P. Open Access. Cambridge, MA: MIT Press; 2012. [ Google Scholar ]
- BMC Medicine. Publication and peer review process. http://www.biomedcentral.com/bmcmed/about#publication .
- Godlee F. Making reviewers visible: openness, accountability, and credit. JAMA. 2002; 287 :2762–2765. doi: 10.1001/jama.287.21.2762. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Solomon DJ. Talking past each other: making sense of the debate over electronic publication. First Monday. 2002; 7 http://dx.doi.org/10.5210%2Ffm.v7i8.978 . [ Google Scholar ]
- Björk B-C, Solomon DJ. Open access versus subscription journals - a comparison of scientific impact. BMC Med. 2012; 10 :73. doi: 10.1186/1741-7015-10-73. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laakso M, Björk B-C. Anatomy of open access publishing: a study of longitudinal development and internal structure. BMC Med. 2012; 10 :124. doi: 10.1186/1741-7015-10-124. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Journal Citation Reports® 2013 Release. http://wokinfo.com/products_tools/analytical/jcr/
- Greenhalgh T, Swinglehurst D. Studying technology use as social practice: the untapped potential of ethnography. BMC Med. 2011; 9 :45. doi: 10.1186/1741-7015-9-45. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Autier P, Boniol M. Breast cancer screening: evidence of benefit depends on the method used. BMC Med. 2012; 10 :163. doi: 10.1186/1741-7015-10-163. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Puliti D, Zappa M. Breast cancer screening: are we seeing the benefit? BMC Med. 2012; 10 :106. doi: 10.1186/1741-7015-10-106. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Franco R, Saag MS. When to start antiretroviral therapy: as soon as possible. BMC Med. 2013; 11 :147. doi: 10.1186/1741-7015-11-147. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lundgren JD, Babiker AG, Gordin FM, Borges ÁH, Neaton JD. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med. 2013; 11 :148. doi: 10.1186/1741-7015-11-148. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nemeroff CB, Weinberger D, Rutter M, MacMillan HL, Bryant RA, Wessely S, Stein DJ, Pariante CM, Seemüller F, Berk M, Malhi GS, Preisig M, Brüne M, Lysaker P. DSM-5: a collection of psychiatrist views on the changes, controversies, and future directions. BMC Med. 2013; 11 :202. doi: 10.1186/1741-7015-11-202. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Timimi FK. Medicine, morality and health care social media. BMC Med. 2012; 10 :83. doi: 10.1186/1741-7015-10-83. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Social media in healthcare. http://storify.com/BMCMedicine/draft-storify .
- The growth of open access journals. http://storify.com/BMCMedicine/oaweek2012 .
- Open access, medical research and global health. http://blogs.biomedcentral.com/bmcblog/2013/10/25/open-access-medical-research-and-global-health-a-bmc-medicine-twitter-chat/
- BMC Medicine article metrics FAQ. http://www.biomedcentral.com/bmcmed/about/articlemetrics .
- Barnard C. BMC Medicine’s top 10 most accessed articles. http://blogs.biomedcentral.com/bmcblog/2013/11/25/bmc-medicines-top-10-most-accessed-articles/
- Alam S. BMC Medicine: our ten most highly cited articles. http://blogs.biomedcentral.com/bmcblog/2013/11/26/bmc-medicine-our-10-most-highly-cited-articles/
- Denyer J. Impact of BMC Medicine articles in the news and social media. http://blogs.biomedcentral.com/bmcblog/2013/11/27/impact-of-bmc-medicine-articles-in-the-news-and-social-media/
- D’Souza U. BMC Medicine: authors and reviewers experiences. http://blogs.biomedcentral.com/bmcblog/2013/11/28/bmc-medicine-authors-and-reviewers-experiences/
- Lee L. Demographics over the past 10 years - where are our authors based? http://blogs.biomedcentral.com/bmcblog/2013/11/29/demographics-over-the-past-10-years-where-are-our-authors-based/
- Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011; 9 :52. doi: 10.1186/1741-7015-9-52. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Politis M, Lindvall O. Clinical application of stem cell therapy in Parkinson's disease. BMC Med. 2012; 10 :1. doi: 10.1186/1741-7015-10-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, Li H, Zhang Y, Diao Y, Li Y, Chen Y, Sun X, Fisk MB, Skidgel R, Holterman M, Prabhakar B, Mazzone T. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012; 10 :3. doi: 10.1186/1741-7015-10-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011; 9 :133. doi: 10.1186/1741-7015-9-133. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hsu YC, Chen HY, Yuan S, Yu SL, Lin CH, Wu G, Yang PC, Li KC. Genome-wide analysis of three-way interplay among gene expression, cancer cell invasion and anti-cancer compound sensitivity. BMC Med. 2013; 11 :106. doi: 10.1186/1741-7015-11-106. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brothers JF, Hijazi K, Mascaux C, El-Zein RA, Spitz MR, Spira A. Bridging the clinical gaps: genetic, epigenetic and transcriptomic biomarkers for the early detection of lung cancer in the post-National Lung Screening Trial era. BMC Med. 2013; 11 :168. doi: 10.1186/1741-7015-11-168. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu LY, Yang T, Ji J, Wen Q, Morgan AA, Jin B, Chen G, Lyell DJ, Stevenson DK, Ling XB, Butte AJ. Integrating multiple 'omics' analyses identifies serological protein biomarkers for preeclampsia. BMC Med. 2013; 11 :236. doi: 10.1186/1741-7015-11-236. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Diamandis EP. The failure of protein cancer biomarkers to reach the clinic: why, and what can be done to address the problem? BMC Med. 2012; 10 :87. doi: 10.1186/1741-7015-10-87. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005; 93 :387–391. doi: 10.1038/sj.bjc.6602678. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012; 10 :51. doi: 10.1186/1741-7015-10-51. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McShane LM, Cavenagh MM, Lively TG, Eberhard DA, Bigbee WL, Williams PM, Mesirov JP, Polley MY, Kim KY, Tricoli JV, Taylor JM, Shuman DJ, Simon RM, Doroshow JH, Conley BA. Criteria for the use of omics-based predictors in clinical trials. Nature. 2013; 502 :317–320. doi: 10.1038/nature12564. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McShane LM, Cavenagh MM, Lively TG, Eberhard DA, Bigbee WL, Williams PM, Mesirov JP, Polley MY, Kim KY, Tricoli JV, Taylor JM, Shuman DJ, Simon RM, Doroshow JH, Conley BA. Criteria for the use of omics-based predictors in clinical trials: explanation and elaboration. BMC Med. 2013; 11 :220. doi: 10.1186/1741-7015-11-220. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Birbeck G. Medicine for global health: can "simple interventions" improve the worldwide burden of disease? BMC Med. 2013; 11 :72. doi: 10.1186/1741-7015-11-72. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Birbeck G, Wiysonge CS, Mills EJ, Frenk JJ, Zhou XN, Jha P. Global health: the importance of evidence-based medicine. BMC Med. 2013; 11 :223. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Altamirano-Bustamante MM, Altamirano-Bustamante NF, Lifshitz A, Mora-Magaña I, de Hoyos A, Avila-Osorio MT, Quintana-Vargas S, Aguirre JA, Méndez J, Murata C, Nava-Diosdado R, Martínez-González O, Calleja E, Vargas R, Mejía-Arangure JM, Cortez-Domínguez A, Vedrenne-Gutiérrez F, Sueiras P, Garduño J, Islas-Andrade S, Salamanca F, Kumate-Rodríguez J, Reyes-Fuentes A. Promoting networks between evidence-based medicine and values-based medicine in continuing medical education. BMC Med. 2013; 11 :39. doi: 10.1186/1741-7015-11-39. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jacob ST, Lim M, Banura P, Bhagwanjee S, Bion J, Cheng AC, Cohen H, Farrar J, Gove S, Hopewell P, Moore CC, Roth C, West TE. Integrating sepsis management recommendations into clinical care guidelines for district hospitals in resource-limited settings: the necessity to augment new guidelines with future research. BMC Med. 2013; 11 :107. doi: 10.1186/1741-7015-11-107. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burgess PI, Msukwa G, Beare NA. Diabetic retinopathy in sub-Saharan Africa: meeting the challenges of an emerging epidemic. BMC Med. 2013; 11 :157. doi: 10.1186/1741-7015-11-157. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Giordano J. Ethical considerations in the globalization of medicine - an interview with James Giordano. BMC Med. 2013; 11 :69. doi: 10.1186/1741-7015-11-69. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wiysonge CS, Uthman OA, Ndumbe PM, Hussey GD. A bibliometric analysis of childhood immunization research productivity in Africa since the onset of the Expanded Program on Immunization in 1974. BMC Med. 2013; 11 :66. doi: 10.1186/1741-7015-11-66. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials . BMC Med. 2010; 8 :18. doi: 10.1186/1741-7015-8-18. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement . BMC Med. 2013; 11 :80. doi: 10.1186/1741-7015-11-80. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: meta-narrative reviews. BMC Med. 2013; 11 :20. doi: 10.1186/1741-7015-11-20. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013; 11 :21. doi: 10.1186/1741-7015-11-21. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
404 Not found
BMC Medical Education
Latest collections open to submissions.

Accreditation and program evaluation models in medical education
Guest Edited by Roghayeh Gandomkar and Marta van Zanten

Psychedelic-assisted therapy training
Guest Edited by Terence H. W. Ching and Joseph La Torre
Ultrasound Education
Guest Edited by Florian Recker
- Most accessed
The COVID-19 pandemic and OBGYN residency training: We have a problem and it’s not just masks
Authors: Alexandria C. Kraus, Anthony Bui, Kimberly Malloy, Jessica Morse and Omar M. Young
Unveilling the hidden skillset: exploring non-technical skills in surgical education across spanish medical universities
Authors: Oves-Suarez B, García-Marín JA, Aguayo-Albasini JL and Soria-Aledo V
The importance of creating the right conditions for group intervision sessions among medical residents– a qualitative study
Authors: Anouk Jorissen, Kim van de Kant, Habibe Ikiz, Valerie van den Eertwegh, Walther van Mook and Angelique de Rijk
Creation of a sustainable longitudinal women in Leadership Development (WILD) curriculum focused on graduate medical education trainees
Authors: Colleen A. McGourty, Francine Castillo, Grace Donzelli, Bridget P. Keenan, Margaret Gilbreth and Lekshmi Santhosh
What makes mentors thrive? An exploratory study of their satisfaction in undergraduate medical education
Authors: Elise Pauline Skjevik, Edvin Schei, J. Donald Boudreau, Arne Tjølsen, Unni Ringberg, Abraham Fuks, Monika Kvernenes and Eirik H. Ofstad
Most recent articles RSS
View all articles
Student perspective of classroom and distance learning during COVID-19 pandemic in the undergraduate dental study program Universitas Indonesia
Authors: Lisa R. Amir, Ira Tanti, Diah Ayu Maharani, Yuniardini Septorini Wimardhani, Vera Julia, Benso Sulijaya and Ria Puspitawati
Impact of the COVID-19 pandemic on teaching and learning in health professional education: a mixed methods study protocol
Authors: Arunaz Kumar, Mahbub Sarkar, Elizabeth Davis, Julia Morphet, Stephen Maloney, Dragan Ilic and Claire Palermo
The relationship between learning styles and academic performance in TURKISH physiotherapy students
Authors: Nursen İlçin, Murat Tomruk, Sevgi Sevi Yeşilyaprak, Didem Karadibak and Sema Savcı
Wikis, blogs and podcasts: a new generation of Web-based tools for virtual collaborative clinical practice and education
Authors: Maged N Kamel Boulos, Inocencio Maramba and Steve Wheeler
Determinants of good academic performance among university students in Ethiopia: a cross-sectional study
Authors: Mesfin Tadese, Alex Yeshaneh and Getaneh Baye Mulu
Most accessed articles RSS
Aims and scope
Become an editorial board member.

We are recruiting new Editorial Board Members to join our international editorial board, helping to provide expertise on a wide range of different subject disciplines.
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
BMC Series Blog

2024 BMC Ecology and Evolution and BMC Zoology Image Competition
28 March 2024

Highlights of the BMC Series – February 2024
21 March 2024

BMC Infectious Diseases Scope Expansion: Animal Models
29 February 2024
Important information
Editorial board
For authors
For editorial board members
For reviewers
- Manuscript editing services
Annual Journal Metrics
2022 Citation Impact 3.6 - 2-year Impact Factor 3.9 - 5-year Impact Factor 1.792 - SNIP (Source Normalized Impact per Paper) 0.914 - SJR (SCImago Journal Rank)
2023 Speed 41 days submission to first editorial decision for all manuscripts (Median) 191 days submission to accept (Median)
2023 Usage 6,205,310 downloads 3,103 Altmetric mentions
- More about our metrics
Peer-review Terminology
The following summary describes the peer review process for this journal:
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published: Review reports. Reviewer Identities reviewer opt in. Author/reviewer communication
More information is available here
- Follow us on Twitter
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Volume 24, issue 1, December 2024
82 articles in this issue
Bioequivalence trials for the approval of generic drugs in Saudi Arabia: a descriptive analysis of design aspects
Authors (first, second and last of 16).
- Turki A. Althunian
- Bader R. Alzenaidy
- Abdulmohsen A. Alsaleh
- Content type: Research
- Open Access
- Published: 05 April 2024
- Article: 82
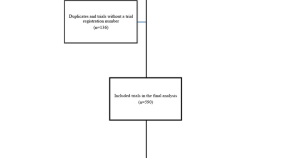
Overcoming denominator problems in refugee settings with fragmented electronic records for health and immigration data: a prediction-based approach
Authors (first, second and last of 4).
- Stella Erdmann
- Kayvan Bozorgmehr
- Published: 01 April 2024
- Article: 81
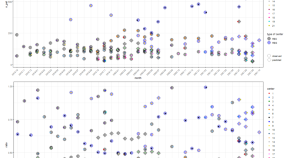
Optimal futility stopping boundaries for binary endpoints
- Michaela Maria Freitag
- Geraldine Rauch
- Published: 28 March 2024
- Article: 80
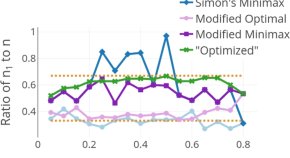
Assessment of the E-value in the presence of bias amplification: a simulation study
- Eric Barrette
- Lucas Higuera
- Kael Wherry
- Article: 79
- Causal Inference and Observational Data ,
- Causal Inference and Observational Data
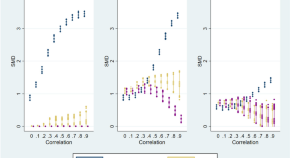
Methodological insights into ChatGPT’s screening performance in systematic reviews
Authors (first, second and last of 9).
- Mahbod Issaiy
- Hossein Ghanaati
- Kavous Firouznia
- Published: 27 March 2024
- Article: 78
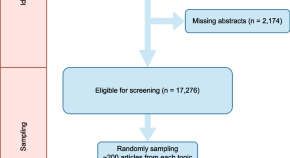
Evaluating methods for risk prediction of Covid-19 mortality in nursing home residents before and after vaccine availability: a retrospective cohort study
- Komal Aryal
- Fabrice I. Mowbray
- Aaron Jones
- Article: 77
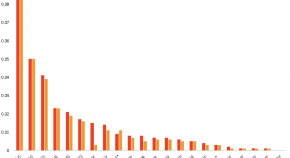
A database for oncological research and quality assurance: implementation and first experiences with the University Clinical Cancer Registry Regensburg
Authors (first, second and last of 11).
- Anna Saibold
- Michael Koller
- Julia Maurer
- Article: 76

Socio-environmental predictors of diabetes incidence disparities in Tanzania mainland: a comparison of regression models for count data
- Sauda Hatibu Mbwambo
- Maurice C. Mbago
- Gadde Srinivasa Rao
- Published: 26 March 2024
- Article: 75
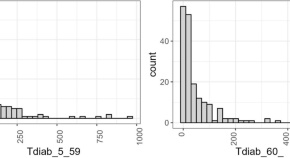
Estimating individualized treatment effects using an individual participant data meta-analysis
Authors (first, second and last of 6).
- Florie Bouvier
- Anna Chaimani
- Raphaël Porcher
- Published: 25 March 2024
- Article: 74
Development of the multivariate administrative data cystectomy model and its impact on misclassification bias
- Luke T. Lavallee
- Carl van Walraven
- Published: 21 March 2024
- Article: 73
An empirical evaluation of approximate and exact regression-based causal mediation approaches for a binary outcome and a continuous or a binary mediator for case-control study designs
- Miguel Caubet
- Kevin L’Espérance
- Geneviève Lefebvre
- Published: 20 March 2024
- Article: 72
Implementation and external validation of the Cambridge Multimorbidity Score in the UK Biobank cohort
- Hannah Harrison
- Samantha Ip
- Antonis C. Antoniou
- Article: 71
Utilization of EHRs for clinical trials: a systematic review
- Leila R. Kalankesh
- Elham Monaghesh
- Published: 18 March 2024
- Article: 70
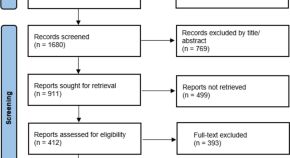
Predicting health outcomes with intensive longitudinal data collected by mobile health devices: a functional principal component regression approach
- Meilin Jiang
- Ryan J. Shaw
- Published: 17 March 2024
- Article: 69
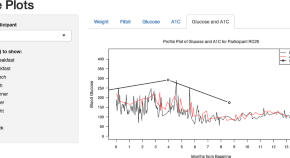
An analysis of published study designs in PubMed prisoner health abstracts from 1963 to 2023: a text mining study
Authors (first, second and last of 7).
- George Karystianis
- Wilson Lukmanjaya
- Tony Butler
- Article: 68
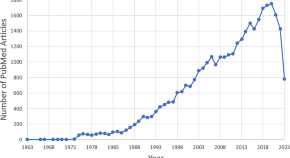
Survival analysis under imperfect record linkage using historic census data
Authors (first, second and last of 5).
- Arielle K. Marks-Anglin
- Frances K. Barg
- Wei-Ting Hwang
- Published: 13 March 2024
- Article: 67
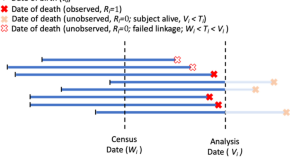
Assessing the properties of patient-specific treatment effect estimates from causal forest algorithms under essential heterogeneity
- John M. Brooks
- Cole G. Chapman
- Neset Hikmet
- Article: 66
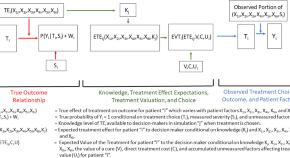
The methodological quality of systematic reviews regarding the Core Outcome Set (COS) development
Authors (first, second and last of 15).
- Jiaxing Zhang
- Published: 11 March 2024
- Article: 65
- Medical reversals in healthcare: factors, assessment, quantification ,
- Medical reversals in healthcare: factors, assessment, quantification
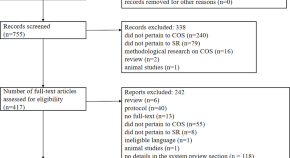
Distributive randomization: a pragmatic fractional factorial design to screen or evaluate multiple simultaneous interventions in a clinical trial
- Skerdi Haviari
- France Mentré
- Article: 64
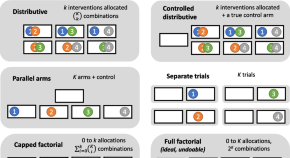
Text analysis framework for identifying mutations among non-small cell lung cancer patients from laboratory data
- Amman Yusuf
- Devon J. Boyne
- Tamer N. Jarada
- Article: 63
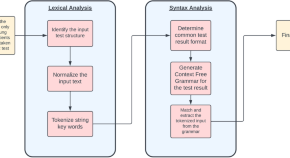
Design and statistical analysis reporting among interrupted time series studies in drug utilization research: a cross-sectional survey
Authors (first, second and last of 10).
- Yuanjin Zhang
- Published: 09 March 2024
- Article: 62
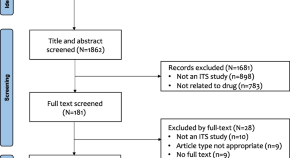
An assessment of the informative value of data sharing statements in clinical trial registries
- Christian Ohmann
- Maria Panagiotopoulou
- Pablo Emilio Verde
- Article: 61
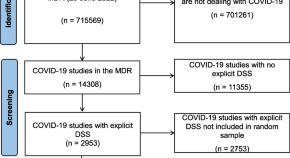
The minimal important difference of patient-reported outcome measures related to female urinary incontinence: a systematic review
- Jordana Barbosa-Silva
- Letícia Bojikian Calixtre
- Susan Armijo-Olivo
- Published: 08 March 2024
- Article: 60

Using the Super Learner algorithm to predict risk of major adverse cardiovascular events after percutaneous coronary intervention in patients with myocardial infarction
- Article: 59

Clarifying the biological and statistical assumptions of cross-sectional biological age predictors: an elaborate illustration using synthetic and real data
- Marije H. Sluiskes
- Jelle J. Goeman
- Mar Rodríguez-Girondo
- Article: 58
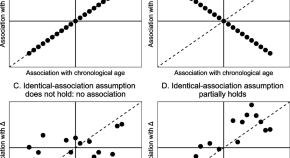
Power calculation for detecting interaction effect in cross-sectional stepped-wedge cluster randomized trials: an important tool for disparity research
- Asem Berkalieva
- Deukwoo Kwon
- Published: 02 March 2024
- Article: 57
Flexible Bayesian semiparametric mixed-effects model for skewed longitudinal data
- Melkamu M. Ferede
- Getachew A. Dagne
- Simon M. Karanja
- Published: 01 March 2024
- Article: 56
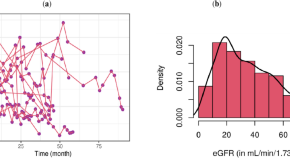
REDCapDM: An R package with a set of data management tools for a REDCap project
- João Carmezim
- Pau Satorra
- Cristian Tebé
- Content type: Software
- Article: 55

A Bayesian Bernoulli-Exponential joint model for binary longitudinal outcomes and informative time with applications to bladder cancer recurrence data
- Michael Safo Oduro
- Article: 54
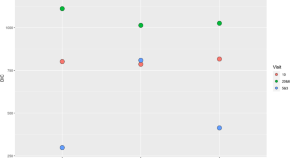
Human biomonitoring without in-person interaction: public health engagements during the COVID-19 pandemic and future implications
- Alyssa J. Mattson
- Susie Y. Dai
- Published: 28 February 2024
- Article: 53
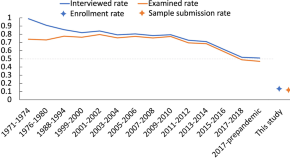
Selecting a randomization method for a multi-center clinical trial with stochastic recruitment considerations
Authors (first, second and last of 8).
- Oleksandr Sverdlov
- Yevgen Ryeznik
- Article: 52
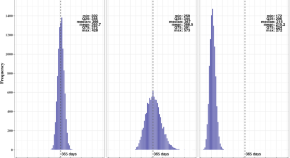
Revising model for end-stage liver disease from calendar-time cross-sections with correction for selection bias
- H. C. de Ferrante
- M. van Rosmalen
- F. C. R. Spieksma
- Article: 51
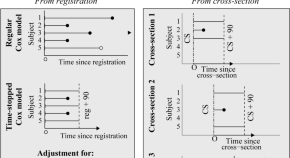
GADNN: a revolutionary hybrid deep learning neural network for age and sex determination utilizing cone beam computed tomography images of maxillary and frontal sinuses
- Omid Hamidi
- Mahlagha Afrasiabi
- Marjan Namaki
- Published: 27 February 2024
- Article: 50
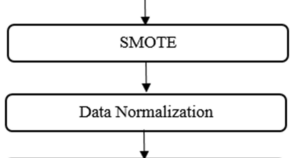
Change analysis for intermediate disease markers in nutritional epidemiology: a causal inference perspective
- Article: 49
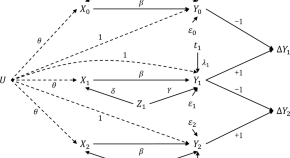
A parametric additive hazard model for time-to-event analysis
- Dina Voeltz
- Annika Hoyer
- Published: 24 February 2024
- Article: 48

Treatment effect estimation using the propensity score in clinical trials with historical control
- Saki Kanamori
- Masahiro Takeuchi
- Published: 22 February 2024
- Article: 47
Barriers and facilitators for recruiting and retaining male participants into longitudinal health research: a systematic review
- Danielle J. Borg
- Melina Haritopoulou-Sinanidou
- Article: 46
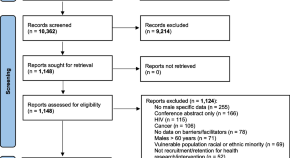
A scoping review on the methodological and reporting quality of scoping reviews in China
- Xintong Tang
- Article: 45
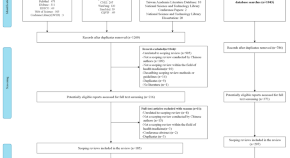
Smoothed quantile residual life regression analysis with application to the Korea HIV/AIDS cohort study
- Soo Min Kim
- Korea HIV/AIDS cohort study
- Published: 17 February 2024
- Article: 44
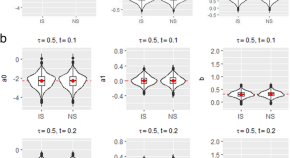
How do quantitative studies involving people with dementia report experiences of standardised data collection? A narrative synthesis of NIHR published studies
- Kate Gridley
- Kate Baxter
- Yvonne Birks
- Published: 16 February 2024
- Article: 43
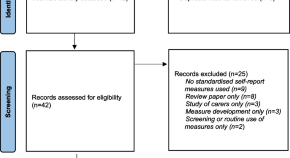
Group sequential designs for pragmatic clinical trials with early outcomes: methods and guidance for planning and implementation
- Nick R. Parsons
- Joydeep Basu
- Nigel Stallard
- Article: 42

Comparison of the effects of imputation methods for missing data in predictive modelling of cohort study datasets
- Article: 41
Extraction frequent patterns in trauma dataset based on automatic generation of minimum support and feature weighting
- Zahra Kohzadi
- Ali Mohammad Nickfarjam
- Mehrdad Mahdian
- Article: 40
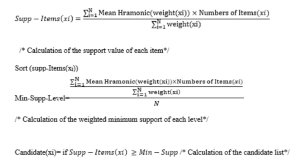
Comparing Bayesian hierarchical meta-regression methods and evaluating the influence of priors for evaluations of surrogate endpoints on heterogeneous collections of clinical trials
- Willem Collier
- Benjamin Haaland
- Article: 39
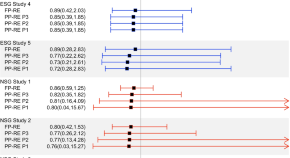
Identifying biologically implausible values in big longitudinal data: an example applied to child growth data from the Brazilian food and nutrition surveillance system
Authors (first, second and last of 14).
- Juliana Freitas de Mello e Silva
- Natanael de Jesus Silva
- Maurício L. Barreto
- Published: 15 February 2024
- Article: 38

Modeling heart rate of individual and team manual handling with one hand using generalized additive mixed models
- Mohammad Hamed Hosseini
- Rashid Heidarimoghaddam
- Leili Tapak
- Article: 37
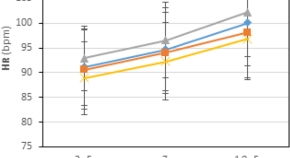
Calibration and XGBoost reweighting to reduce coverage and non-response biases in overlapping panel surveys: application to the Healthcare and Social Survey
- Luis Castro
- María del Mar Rueda
- Andrés Cabrera-León
- Article: 36

Contextual effects: how to, and how not to, quantify them
- Tobias Saueressig
- Hugo Pedder
- Daniel L Belavy
- Content type: Review
- Published: 13 February 2024
- Article: 35
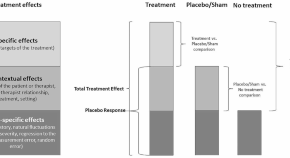
A data-adaptive method for investigating effect heterogeneity with high-dimensional covariates in Mendelian randomization
- Haodong Tian
- Brian D. M. Tom
- Stephen Burgess
- Published: 10 February 2024
- Article: 34
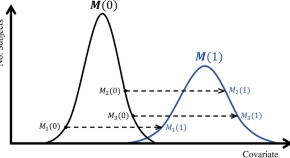
An evaluation of the EASY instrument in a cross-sectional study
- Umesh Ghimire
- Todd Rockwood
- Article: 33
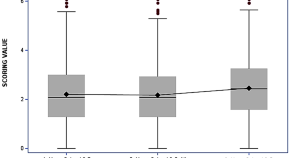
For authors
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 05 April 2024
Bioequivalence trials for the approval of generic drugs in Saudi Arabia: a descriptive analysis of design aspects
- Turki A. Althunian ORCID: orcid.org/0000-0002-8030-7963 1 , 2 ,
- Bader R. Alzenaidy 3 ,
- Raseel A. Alroba 1 ,
- Ohoud A. Almadani 1 ,
- Fahad A. Alqahtani 3 ,
- Albatool A. Binajlan 3 ,
- Amal I. Almousa 3 ,
- Deema K. Alamr 3 ,
- Malak S. Al-Mofada 3 ,
- Nora Y. Alsaqer 3 ,
- Hessa A. Alarfaj 3 ,
- Abdulmohsen A. Bahlewa 3 ,
- Mohammed A. Alharbi 3 ,
- Ali M. Alhomaidan 4 ,
- Abdulaziz A. Alsuwyeh 3 &
- Abdulmohsen A. Alsaleh 3
BMC Medical Research Methodology volume 24 , Article number: 82 ( 2024 ) Cite this article
64 Accesses
1 Altmetric
Metrics details
This retrospective analysis aimed to comprehensively review the design and regulatory aspects of bioequivalence trials submitted to the Saudi Food and Drug Authority (SFDA) since 2017.
This was a retrospective, comprehensive analysis study. The Data extracted from the SFDA bioequivalence assessment reports were analyzed for reviewing the overall design and regulatory aspects of the successful bioequivalence trials, exploring the impact of the coefficient of variation of within-subject variability (CVw) on some design aspects, and providing an in-depth assessment of bioequivalence trial submissions that were deemed insufficient in demonstrating bioequivalence.
A total of 590 bioequivalence trials were included of which 521 demonstrated bioequivalence (440 single active pharmaceutical ingredients [APIs] and 81 fixed combinations). Most of the successful trials were for cardiovascular drugs (84 out of 521 [16.1%]), and the 2 × 2 crossover design was used in 455 (87.3%) trials. The sample size tended to increase with the increase in the CVw in trials of single APIs. Biopharmaceutics Classification System Class II and IV drugs accounted for the majority of highly variable drugs (58 out of 82 [70.7%]) in the study. Most of the 51 rejected trials were rejected due to concerns related to the study center ( n = 21 [41.2%]).
This comprehensive analysis provides valuable insights into the regulatory and design aspects of bioequivalence trials and can inform future research and assist in identifying opportunities for improvement in conducting bioequivalence trials in Saudi Arabia.
Peer Review reports
The development of generic drugs has played a pivotal role in increasing patient access to affordable medications (90% of the prescribed drugs in the United States [U.S.] are dispensed as generics with $2.4 trillion dollars cost savings in the past decade), and has been associated with improved patient adherence and clinical outcomes [ 1 , 2 , 3 , 4 , 5 , 6 ]. These impacts were anticipated given the abbreviated nature of developing and approving generic drugs (i.e. generic drug manufacturers are precluded from replicating pre-clinical and clinical development programs of the corresponding brand-name drugs) [ 7 , 8 , 9 , 10 ]. From a clinical perspective, generic drugs are only anticipated to achieve equivalence to their corresponding brand-name drugs in terms of the rate and extent of absorption (i.e. bioequivalence) [ 7 , 8 , 9 , 10 ]. The in vivo assessment of bioequivalence is performed in the context of bioequivalence trials that are conducted mostly in a small sample of at least 24 healthy volunteers ( n = 12 per group), and designed in a crossover fashion to demonstrate equivalence in several pharmacokinetic parameters using pre-defined bioequivalence margins [ 7 , 8 , 9 , 10 ]. However, alternative design aspects/settings might be acceptable by regulators in certain conditions (e.g. drugs with a long plasma elimination half-life, drugs with highly variable pharmacokinetic parameters, or when recruiting healthy volunteers is considered unethical for safety reasons) [ 7 , 8 , 9 , 10 ]. Notwithstanding the submission of thousands of bioequivalence trials to regulatory agencies, little is known about their designs, the extent of alternative design choices were used, and to what extent bioequivalence was demonstrated as judged by regulators both internationally and in Saudi Arabia.
Most reviews on published bioequivalence trials (or those assessed by regulators) were directed towards re-analyzing their original findings (e.g. pharmacokinetic parameter estimation, bioequivalence testing) [ 11 , 12 , 13 ]. A comprehensive design characterization was only provided by one study which covered bioequivalence trials that were assessed by the Ministry of Food and Drug Safety in South Korea [ 14 ]. However, the latter study excluded trials that did not demonstrate bioequivalence and those conducted for fixed-combination products. Additionally, the study did not provide data on some important bioequivalence design aspects (e.g. country of the trial center, patient ages, body measurements, number of patients who completed the study, details about the nature of replicated trials). None of the published reviews was conducted using data on bioequivalence trials from the Middle East in general and in Saudi Arabia in particular. In Saudi Arabia, there is a lack of knowledge about bioequivalence data of the approved generic drugs in the Saudi health market [ 15 , 16 ]. Studies have shown that medical representatives have been the major source of clinical information about generic drugs for physicians, and only small proportion relies on published bioequivalence trials [ 17 , 18 ]. A panel of Saudi experts also recommended providing more data about bioequivalence trials for the approved generic drugs in the Saudi Arabia [ 15 , 18 ].
Since the establishment of the new electronic recording system of bioequivalence trials at the Saudi Food and Drug Authority (SFDA) in 2017, hundreds of bioequivalence trials have been reviewed by the SFDA’s evaluators for the approval of generic drugs in Saudi Arabia. The review process has been based on the SFDA Guidelines for Bioequivalence and on the SFDA’s Product Specific Bioequivalence Guidance [ 7 , 8 ]. This study was aimed to provide comprehensive review of all bioequivalence trials that were assessed by the SFDA since 2017 from a design and regulatory perspective.
Study design and data source
This was a retrospective, comprehensive analysis study. The SFDA generates assessment reports for bioequivalence trials if they were included in generic drug registration applications (one report for all bioequivalence trials per drug application). In each assessment report, all details per trial about the design, technical, and logistic aspects are added, with feedback on each aspect from the SFDA’s scientific evaluators. Each report ends with a decision on whether bioequivalence was demonstrated, was not demonstrated, or whether a decision could not be reached due to concerns related to the design or conduct of the trial. Data for trials since 2017 were extracted by the study team members using several data collection sheets. Approval from the SFDA Institutional Review Board was granted (the data are confidential and not available in the public domain of the SFDA).
SFDA requirements for bioequivalence testing
Based on the SFDA guidelines [ 7 ], the successful demonstration of bioequivalence is conditional mainly on the following:
Achieving statistical equivalence in the targeted pharmacokinetic parameters : The 90% confidence interval (CI) for the ratio of the test generic drug vs. reference brand-name drug must be contained within 80.0 to 125.0% equivalence margins in the targeted pharmacokinetic parameters, mainly the area under the plasma concentration curve from administration to last observed concentration at time t (AUC 0-t ) and the maximum plasma concentration (C max ). Stricter equivalence margins are required for drugs with a narrow therapeutic index. However, more lenient margins (up to a maximum of 69.84 to 143.19 in C max ) might be acceptable with highly variable drugs, i.e., CVw > 30%.
Choosing the right design aspects : The typical 2-sequence, 2-period, crossover design (i.e., 2 × 2) under fasting conditions is generally requested for immediate-release oral formulations, unless the brand-name drug is administered under fed conditions. Two 2 × 2 bioequivalence trials (one under fasting and one under fed conditions) are required for specific dosage forms (e.g., modified-release dosage forms, microemulsions, and solid dispersions). Alternative designs (e.g., replicate and parallel trial designs) could be considered in highly variable drugs or in drugs with a long elimination half-life (t 1/2 ). Other considerations about alternative design aspects are provided in the SFDA Guidelines for Bioequivalence and by the SFDA Product Specific Guidelines.
Compliance with quality and other regulatory standards : Assessing bioequivalence findings cannot be completed without meeting certain quality and regulatory standards. Examples of these standards are the selection of the production batch, specifications of critical quality attributes, whether the trial was conducted in a center accredited by the SFDA, and the choice of bioanalytical methodology.
Study outcomes and statistical analysis
Our review of bioequivalence trials that were assessed by the SFDA was conducted for three outcomes: reviewing the overall design and regulatory aspects of bioequivalence trials that were deemed successful by the SFDA in demonstrating bioequivalence, exploring the nature of within-subject variability in these trials and its relevance to other design aspects (e.g. the trial sample size), and providing an in-depth assessment of bioequivalence trial submissions that were deemed insufficient in demonstrating bioequivalence.
The overall review of the successful bioequivalence trials covered the following: the biopharmaceutics classification system (BCS), the therapeutic class of the active pharmaceutical ingredient (API) which was determined according to the World Health Organization Anatomical Therapeutic Chemical codes (ATC), the dosage form, the safety index (wide vs. narrow therapeutic index), the trial center, the trial design (2 × 2 crossover, replicate or parallel designs), the study condition (fasting vs. fed), masking study participants (blinding vs. open-label designs), the washout period, the sample size, the choice of equivalence margins, the coefficient of variation of within-subject variability (CVw), age, sex, and body mass index. The review was conducted at the single-API and fixed-combination levels.
The extracted CVws from the accepted trials (for both single APIs and fixed combinations) were assessed in relation to the trial sample size , trial design, and the different BCS classes (the recent Korean review found that highly variable drugs are mostly BCS Class II or IV [i.e. drugs with poor solubility]) [ 14 ]. Reasons for rejecting the submission of bioequivalence trials or for rejecting their results were explored in addition to the success rate of study centers per country. All study outcomes were analyzed descriptively using RStudio Version 2022.12.0.
A total of 590 bioequivalence trials were included in the study (Fig. 1 ). Bioequivalence was demonstrated in 521 trials (440 and 81 trials for single-API and fixed combination drugs; respectively), which were used to evaluate bioequivalence for 184 unique APIs and 36 unique fixed combinations. The remaining 69 of 590 were rejected either due to failed submissions of marketing authorization applications or due to the failure of a trial to demonstrate bioequivalence. Of the included trials, 240 (40.7%) were conducted ≥ 2017 (the trial date was not available for one trial). Almost two-third of 590 trial were conducted using Jordanian and Indian populations (237 and 203 trial centers; respectively), and 98 trials (16.6%) were conducted in European centers (only one study was conducted in Saudi Arabia).
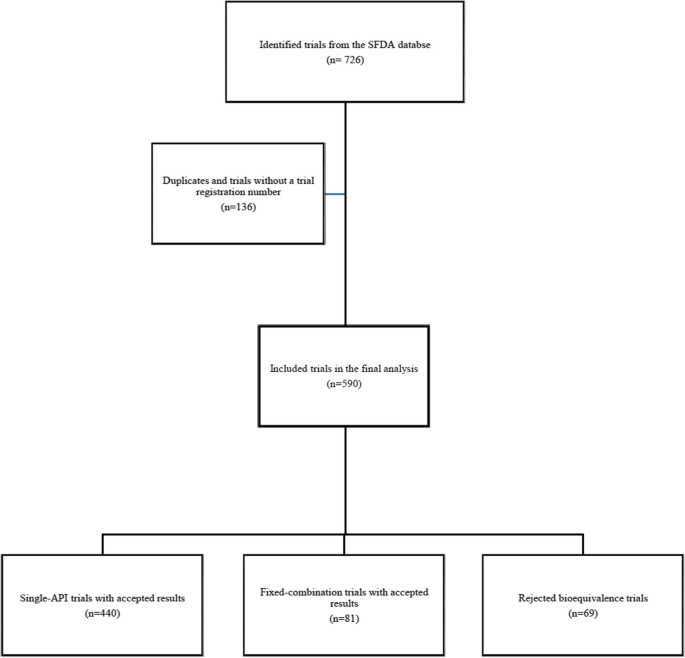
The process of selecting the included bioequivalence trials
The design of the successful bioequivalence trials
Most of the 521 successful trials were conducted for single APIs/fixed-combinations of the cardiovascular system (84 of 521 [16.1%]), antineoplastic and immunomodulating drugs (75 [14.4%]), drugs for alimentary tract and metabolism (73 [14.0%]), and drugs for the nervous system (65 [12.5%]), Fig. 2 . More than half of single-API trials were BCS Class I drugs, and most BCS combinations in the fixed-combinations were BCS Class I and II combination and Class I and III combination (15 combinations each). Almost two-third and all of single-API and fixed-combination trials were conducted for tablet and capsule dosage forms; respectively (Table 1 ). A large proportion (79.0%) was conducted under fasting conditions (85.5% of these trials were conducted for immediate-release dosage oral dosage forms), and all trials. except for six, were conducted for drugs with a wide therapeutic index.
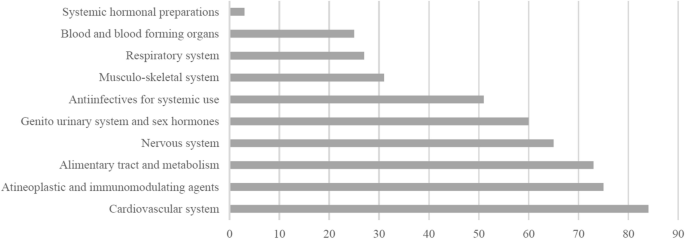
The most common therapeutic classes of successful trials
The typical 2 × 2 crossover design was used in 455 (87.3%) trials. The replicate design was used in 58 trials (wider C max equivalence margins were used in 25 of 58 replicate trials). Nine trials were designed as randomized, parallel design trials (four trials for generic forms of fingolimod hydrochloride, and five for enzalutamide, toremifene, entecavir, and terifunormide). The range of t 1/2 for drugs in these nine parallel trials was 4 to 20 days. The range of the sample size was 12 to 216. The median sample size for the single APIs was 36, and per trial design, the parallel-design trials had the highest median number of study participants ( n = 71), followed by the replicate and 2 × 2 crossover trials (40 and 35; respectively). Similarly, the fixed-combination trials had a median of the sample size ( n = 36), and almost comparable medians per trial design ( n = 39 and 36 for the replicate and 2 × 2 crossover trials).
CVw, sample size, trial design and BCS classification
Figure 3 shows that the sample size tended to increase with the increase in CVw in bioequivalence trials of single APIs. The median sample size in trials of highly variable, single APIs was higher vs. the median in trials of drugs with CVw of ≤30%. This trend was not observed in trials of the fixed-combinations (Fig. 3 ); however, the median sample size in trials of fixed-combinations with a highly variable API was slightly higher vs. those low-CVw APIs (40 vs. 36; respectively).
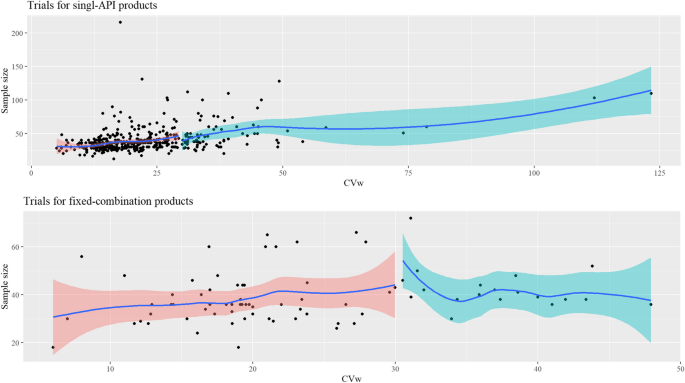
Trials with replicate design had the highest median CVw for both the single APIs (33.5% vs. 18.9% vs. 16.3% compared with the 2 × 2 crossover and parallel trials; respectively) and the fixed combinations (31.1% vs. 19.8% compared with the 2 × 2 crossover trials). BCS Class II and IV drugs accounted for most of the highly variable drugs (58 of 82 [70.7%] of the highly variable drugs in the successful trials were BCS Class II and IV drugs). Figure 4 shows the distribution of CVw at the BCS class level in the single-API trials.
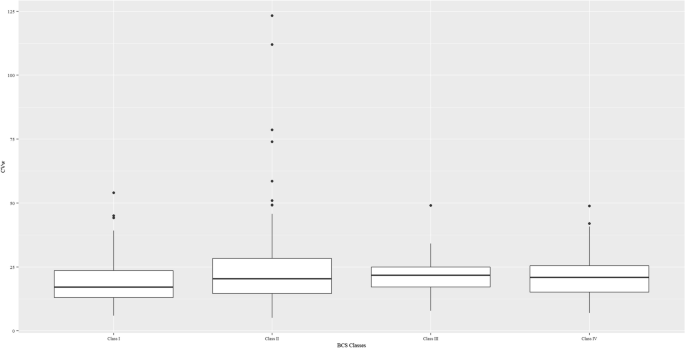
The distribution of CVws per BCS class. Class I: Trials with BCS Class I active pharmaceutical ingredients. Class II: Trials with BCS Class II active pharmaceutical ingredients. Class III: Trials with BCS Class III active pharmaceutical ingredients. Class IV: Trials with Class IV active pharmaceutical ingredients
The rejected bioequivalence trials
Justifications for the SFDA rejection was available for 51 of 69 (73.9%) rejected trials (Table 2 ). Most of these 51 trials ( n = 21 [41.2%]) were rejected due to concerns related to the study centers (e.g. the center was not accredited by the SFDA; the center was suspended by the SFDA or a reference regulatory authority [e.g. US FDA or EMA]). Ten trials (19.6%) failed do demonstrate bioequivalence, and 17 had major regulatory or design concerns or concerns related to the quality of the APIs (Table 2 ).
There were four unsuccessful attempts to obtain waivers for demonstrating bioequivalence through in-vivo testing, commonly known as biowaivers. The first was denied because the request to register a 20 mg generic form of rosuvastatin relied on previous in-vivo bioequivalence data from the unregistered higher strength of 40 mg (i.e., additional strength waiver requests for generic drugs might be acceptable if the maximum strength is approved by the SFDA). Additionally, two other requests for biowaivers were made for generic forms of two drugs, ibuprofen and mirtazapine, which are not classified as BCS Class I (the SFDA only accepts biowaiver requests for BCS Class I drugs). The fourth and final rejected request was for a generic form of dabigatran etexilate mesylate, which failed in the in-vitro comparative dissolution testing—a requirement by the SFDA for biowaiver applications.
Figure 5 illustrates the risk of rejecting a bioequivalence trial per country. There were five countries with a number of conducted bioequivalence trials of higher than 10: Jordan ( n = 237), India (203), Canada ( n = 47), Egypt ( n = 41), and Romania ( n = 15). The highest risk of rejection among these five countries was in Egypt (48.8%), followed by Canada (12.8%), and Jordan (8.4%). The SFDA rejection decision in the Egyptian trials was driven by one of the three trial centers, which led to its suspension by the SFDA.
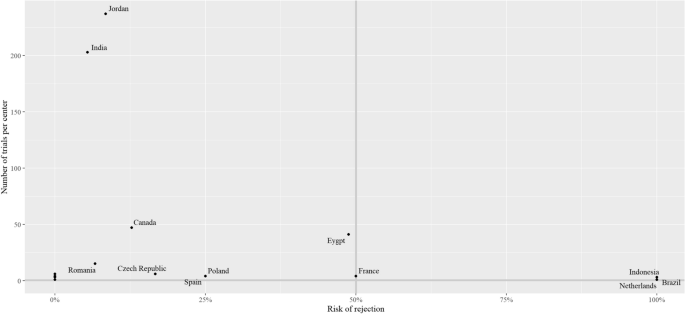
The risk of rejecting bioequivalence trials per country
For the support of the marketing authorization of generic drugs in Saudi Arabia, we found that the majority of submitted bioequivalence trials were accepted by the SFDA. Most were designed in the conventional 2 × 2 crossover format, with a focus on immediate-release dosage forms. These trials were primarily conducted in Jordan and India, with most drugs exhibiting low CVw. Additionally, a significant proportion of the trials involved BCS Class II drugs, and the therapeutic categories most frequently tested were drugs for the cardiovascular system, and antineoplastic agents and immunomodulatory drugs. Studies involving single APIs with high CVw tended to require larger sample sizes, with BCS Class II and IV drugs accounting for the majority of high CVw drugs. Finally, our findings indicate that conducting trials at centers not accredited by the SFDA or suspended by other stringent authorities is likely to result in trial rejection.
Several design aspects of the accepted bioequivalence trials in our study share similarities with those submitted to other regulators [ 13 , 14 , 19 ]. This similarity can be attributed to the common adoption of a 2 × 2 crossover design, immediate-release oral dosage forms, and drugs for the cardiovascular system as the most commonly targeted therapeutic class. Our study identified six parallel trials, which typically had a larger sample size than other designs (median sample sizes were 70, 40, and 35 for parallel, replicate, and crossover trials; respectively). The range of sample sizes in our study was wider compared to those reported in published reviews (12 to 216 versus 12 to 170) [ 13 , 14 ]. Our study also revealed that an increase in sample size was necessary to accommodate an increase in CVw for trials with single APIs. Notably, we observed that the replicate design had the highest median CVw, as expected. Consistent with previous research, which found that CVw was influenced by solubility-limited absorption and/or low permeability, our results indicated that BCS Class II and IV drugs accounted for the highest variability [ 14 , 20 ].
The age range of trial participants in our study was wider (18 to 87 years) than those reported in previous reviews (18 to 60 years) [ 13 , 19 ]. The SFDA might accept an age limit higher than 60 years if adequately justified by the marketing authorization holder. Almost two-third of the trials in our study were conducted under fasting conditions, similar to trials conducted in South Korea [ 19 ]. Only six trials were conducted for narrow-therapeutic index drugs, and no data from published reviews were available for these drugs. Only one study was conducted in Saudi Arabia, with the majority of trials conducted in Jordanian and Indian populations. Further studies are needed to explore opportunities and to identify gaps in conducting bioequivalence in Saudi Arabia, and to determine the extent to which bioequivalence results can be extrapolated directly to the Saudi population as a previous study found differences in fed-between-population pharmacokinetic parameters [ 21 ].
The study findings indicated a potential lack of understanding or insufficient knowledge regarding SFDA requirements and guidelines for the design and conduct of bioequivalence trials. For instance, conducting trials at centers not accredited by the SFDA resulted in a rejection of 23 bioequivalence trials, and two trials were rejected because the approved brand-name drug was not chosen as the reference drug. There might also be insufficient awareness for the conditions of accepting applications for biowaivers. The SFDA published a comprehensive product-specific bioequivalence guideline in 2022 and they have advised marketing authorization holders to seek for an SFDA scientific advice during the design, conduct or submission stages if further clarification is needed [ 8 ]. The number of SFDA bioequivalence scientific advice letters/meetings increased in the first two months of 2024 in comparison with those of 2023 (43 vs. 15; respectively). The SFDA is in the process of updating its guideline for biowaivers to include more details with illustrative examples. These initiatives are expected to facilitate the development of generic drugs and reduce the number of failed or suboptimal applications for demonstrating or waiving bioequivalence [ 22 ].
Our study provides a comprehensive assessment of bioequivalence trials submitted to a regulator for marketing authorizations of generic drugs, making it among the few studies of its kind and the first in the Middle East. We presented a summary of the design, conduct, and regulatory aspects of these trials, investigated the association of CVw with certain design factors, and analyzed trials rejected by the SFDA in an effort to support the implementation of these trials in the region. However, our study had several limitations. Trials submitted for marketing authorization prior to the introduction of the new electronic recording system for bioequivalence trials in 2017 were not included in our analysis. Additionally, we did not evaluate the selection of reference drugs in these trials or explore the submission of marketing authorization applications for biowaivers. Future studies that address these aspects would complement the findings of our study.
Conclusions
In conclusion, this study provides valuable insights into the design, conduct, and regulatory aspects of bioequivalence trials submitted to the SFDA for marketing authorization of generic drugs in Saudi Arabia. The findings of this study highlight the need for further research to explore opportunities and identify gaps in conducting bioequivalence trials in Saudi Arabia.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.
Straka RJ, Keohane DJ, Liu LZ. Potential clinical and economic impact of switching branded medications to generics. Am J Ther. 2017;24(3):e278-289.
Article PubMed Google Scholar
2021 Generic drug and biosimilars access and savings in the U.S. report. Association for Accessible Medicines; 2021. https://accessiblemeds.org/sites/default/files/2021-10/AAM-2021-US-Generic-Biosimilar-Medicines-Savings-Report-web.pdf . Accessed 1 Feb 2022.
Conrad R, Lutter R. Generic competition and drug prices: new evidence linking greater generic competition and lower generic drug prices. U.S. Food and Drug Administration; 2019. https://www.fda.gov/media/133509/download . Accessed 1 Feb 2022.
Dave CH, Hartzema A, Kesselheim AS. Prices of generic drugs associated with numbers of manufacturers 29 citing articles. N Engl J Med. 2017;377:2597–8.
Drug pricing: research on savings from generic drug use. United States Government Accountability Office; 2012. https://www.gao.gov/assets/files.gao.gov/assets/gao-12-371r.pdf . Accessed 1 Feb 2022.
Gupta R, Shah ND, Ross JS. Generic drugs in the United States: policies to address pricing and competition. Clin Pharmacol Ther. 2019;105(2):329–37.
Guidelines for bioequivalence. Saudi Food and Drug Authority; 2021. https://beta.sfda.gov.sa/sites/default/files/2022-08/GCC_Guidelines_Bioequivalence31.pdf . Accessed 1 Feb 2022.
SFDA’s product specific bioequivalence guidance. Saudi Food and Drug Authority; 2022. https://www.sfda.gov.sa/sites/default/files/2022-05/SFDAsProductSpecificBioequivalenceGuidanceV1.pdf . Accessed 1 Jul 2022.
Bioequivalence studies with pharmacokinetic endpoints or drugs submitted under an ANDA: guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2012. https://www.fda.gov/media/87219/download . Accessed 1 Dec 2022.
Committee for Medicinal Products for Human Use (CHMP). Guideline on the investigation of bioequivalence. European Medicines Agency; 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf . Accessed 1 Dec 2022.
Odi R, Franco V, Perucca E, et al. Bioequivalence and switchability of generic antiseizure medications (ASMs): a re-appraisal based on analysis of generic ASM products approved in Europe. Epilepsia. 2021;62(2):285–302.
Tsipotis E, Gupta NR, Raman G, et al. Bioavailability, efficacy and safety of generic immunosuppressive drugs for kidney transplantation: a systematic review and meta-analysis. Am J Nephrol. 2016;44(3):206–18.
Article CAS PubMed Google Scholar
Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583–97.
Huh KY, Kim E, Lee S, et al. Current bioequivalence study designs in South Korea: a comprehensive analysis of bioequivalence study reports between 2013 and 2019. Front Pharmacol. 2021;12:651790.
Article PubMed PubMed Central Google Scholar
Alhawassi TM, Abuelizz HA, Almetwazi M, et al. Advancing pharmaceuticals and patient safety in Saudi Arabia: a 2030 vision initiative. Saudi Pharm J. 2018;26(1):71–4.
Hammami MM, De Padua SJS, Hussein R, et al. Generic-reference and generic-generic bioequivalence of forty-two, randomly-selected, on-market generic products of fourteen immediate-release oral drugs. BMC Pharmacol Toxicol. 2017;18(1):782017.
Article Google Scholar
Alghasham AA. Generic drug prescribing in central Saudi Arabia: perceptions and attitudes of physicians. Ann Saudi Med. 2009;29(1):24–9.
Salhia HO, Ali A, Rezk NL. Perception and attitude of physicians toward local generic medicines in Saudi Arabia: a questionnaire-based study. Saudi Pharm J. 2015;23(4):397–404.
Gurer C, Pehlivanli AC, Demircigil GC. Pooled bioequivalence study database from Turkey: characterization of adverse events and determination of split points based on Gini Index as a promising method. Springerplus. 2016;13(1):709.
Sugihara M, Takeuchi S, Sugita M, et al. Analysis of intra- and intersubject variability in oral drug absorption in human bioequivalence studies of 113 generic products. Mol Pharm. 2015;12:4405–13.
Ozdin D, Varin F, Fuglsang A, et al. Influence of different populations on pharmacokinetic bioequivalence results: can we extrapolate bioequivalence results from one population to another? J Pharm Pharm Sci. 2020;23:357–88.
Article CAS Google Scholar
Sullivan JO, Blake K, Berntgen M, Salmonson T, Welink J, Pharmacokinetics Working Party. Overview of the European Medicines Agency’s development of product-specific bioequivalence guidelines. Clin Pharmacol Ther. 2018;104(3):539–45.
Download references
Acknowledgements
The views expressed in this paper are those of the author(s) and do not necessarily reflect those of the SFDA or its stakeholders. Guaranteeing the accuracy and the validity of the data is a sole responsibility of the research team
No funds, grants, or other support were received.
Author information
Authors and affiliations.
Research Informatics Department, Saudi Food and Drug Authority, Riyadh, Saudi Arabia
Turki A. Althunian, Raseel A. Alroba & Ohoud A. Almadani
College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
Turki A. Althunian
Department of Pharmacokinetics, Saudi Food and Drug Authority, Riyadh, Saudi Arabia
Bader R. Alzenaidy, Fahad A. Alqahtani, Albatool A. Binajlan, Amal I. Almousa, Deema K. Alamr, Malak S. Al-Mofada, Nora Y. Alsaqer, Hessa A. Alarfaj, Abdulmohsen A. Bahlewa, Mohammed A. Alharbi, Abdulaziz A. Alsuwyeh & Abdulmohsen A. Alsaleh
Executive Department of Research and Studies, Saudi Food and Drug Authority, Riyadh, Saudi Arabia
Ali M. Alhomaidan
You can also search for this author in PubMed Google Scholar
Contributions
Turki A. Althunian, Bader R. Alzenaidy, Abdulaziz A. Alsuwyeh, and Abdulmohsen A. Alsaleh designed the study. Turki A. Althunian, Bader R. Alzenaidy, Raseel A. Alroba, Ohoud A. Almadani, Fahad A. Alqahtani, Albatool A. Binajlan, Amal I. Almousa, Deema K. Alamr, Malak S. Al-Mofada, Nora Y. Alsaqer , Hessa A. Alarfaj, Abdulmohsen A. Bahlewa, Mohammed A. Alharbi, Ali M. Alhomaidan, Abdulaziz A. Alsuwyeh, and Abdulmohsen A. Alsaleh involved in the study conduct, data collection and analysis. Turki A. Althunian wrote the manuscript, and the final version of the manuscript was reviewed by all authors and approved by the study supervisors (Turki A. Althunian and Abdulmohsen A. Alsaleh).
Corresponding author
Correspondence to Turki A. Althunian .
Ethics declarations
Ethics approval and consent to participate.
Our study did not involve human participants and consent forms were not required. The study included anonymized human data from anonymized bioequivalence trials. As a result, an assessment by the SFDA’s institutional review board was deemed unnecessary by the board. However, the final draft of the manuscript was approved by the SFDA’s Research Committee for the publication. (approval number: RL-2022-016).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Althunian, T.A., Alzenaidy, B.R., Alroba, R.A. et al. Bioequivalence trials for the approval of generic drugs in Saudi Arabia: a descriptive analysis of design aspects. BMC Med Res Methodol 24 , 82 (2024). https://doi.org/10.1186/s12874-024-02207-4
Download citation
Received : 29 November 2023
Accepted : 22 March 2024
Published : 05 April 2024
DOI : https://doi.org/10.1186/s12874-024-02207-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bioequivalence trials
- Clinical trials
- Generic drugs
- Study design
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
He is currently a Senior Editorial Board member for BMC Medical Research Methodology. Livia Puljak, MD, PhD, Catholic University of Croatia in Zagreb, Croatia. Livia Puljak is a full professor at the Catholic University of Croatia, where she is also the Head of the Center for evidence-based medicine and healthcare.
Editor's Highlights. A data-adaptive method for investigating effect heterogeneity with high-dimensional covariates in Mendelian randomization. Contextual effects: how to, and how not to, quantify them ... BMC Medical Research Methodology does not aim to publish articles describing scientific methods or techniques: ...
If you wish to co-submit a data note describing your data to be published in BMC Research Notes, you can do so by visiting our submission portal. Data notes support open data and help authors to comply with funder policies on data sharing. Co-published data notes will be linked to the research article the data support . Competing interests
BMC Medical Research Methodology does not aim to publish articles describing scientific methods or techniques: these should be directed to the BMC journal covering the relevant biomedical subject area. ... For topics on particular articles, maintain the dialogue through the usual channels with your editor. Developed by: Powered by: Follow us on ...
Find breakthrough research in BMC Medicine, an open access journal with 9.3 Impact Factor and 6 days to first decision. ... health services, clinical trial methodology, meta-analysis, medical education, e-learning, telemedicine, and digital health technologies ... Section Editor of Cancer Control; Academic Editor of Pain Research and Management ...
Analysis of self-report and biochemically verified tobacco abstinence outcomes with missing data: a sensitivity analysis using two-stage imputation. Yiwen Zhang. Xianghua Luo. Janet L. Thomas. Research article. Open Access. Published: 18 December 2018. Article: 170. This is part of 1 collection.
BMC Medical Research Methodology. Volumes and issues. Volume 21, issue 1. Search within journal. Search. Volume 21, issue 1, December 2021. ... Hospital workers mental health during the COVID-19 pandemic: methods of data collection and characteristics of study sample in a university hospital in Milan (Italy) Authors (first, second and last of ...
Volume 1 December 2001. December 2001, issue 1. This is a supplement. December 2001, issue 1. Volumes and issues listings for BMC Medical Research Methodology.
Guest Editor. Imran Ashraf, PhD, Department of Information and Communication Engineering, Yeungnam University, Republic of Korea . Submission Status: Open | Submission Deadline: 17 August 2024. BMC Medical Research Methodology is welcoming submissions for our collection focused on Data science methodologies. Data science has transcended the boundaries of traditional disciplines, creating a ...
Editorial. Just a few months after the Human Genome Project was declared complete [ 1] BMC Medicine was launched as an open access [ 2, 3 ], open peer review journal (i.e. where signed peer review reports are published with the article) [ 4, 5 ], with the aim of making high impact clinical peer-reviewed research of general interest, accessible ...
BMC Medical Research Methodology. Place; About; Article; Application Guidelines ... inference and observing data. Guest Edited by Xiaoyu Liang, Ivan Olier, Victor Volovici, and Yiqiang Zhan. Prediction methods for rare diseases or outcomes ... Clicks here for all journal collections . Editor's Highlights. Data quality considerations for ...
BMC Medical Research Methodology has an h-index of 149.It means 149 articles of this journal have more than 149 number of citations. The h-index is a way of measuring the productivity and citation impact of the publications. The h-index is defined as the maximum value of h such that the given journal/author has published h papers that have each been cited at least h number of times.
BMC Medical Research Methodology. Volumes and issues. Volume 23, issue 1. Search within journal. Search. Volume 23, issue 1, December 2023. 302 articles in this issue. Evaluation of 'implications for research' statements in systematic reviews of interventions in advanced cancer patients - a meta-research study
The ISSN of BMC Medical Research Methodology is 1471-2288 . An ISSN is an 8-digit code used to identify newspapers, journals, magazines and periodicals of all kinds and on all media-print and electronic. BMC Medical Research Methodology Key Factor Analysis
Background Physician bias refers to the unconscious negative perceptions that physicians have of patients or their conditions. Medical schools and residency programs often incorporate training to reduce biases among their trainees. In order to assess trends and organize available literature, we conducted a scoping review with a goal to categorize different biases that are studied within ...
BMC Medical Research Methodology. Volumes and issues. Volume 16, issue 1. Search within journal. Search. Volume 16, issue 1, December 2016. ... Using cognitive pre-testing methods in the development of a new evidenced-based pressure ulcer risk assessment instrument Authors (first, second and last of 9) S. Coleman; J. Nixon;
BMC Medical Research Methodology. Volumes and issues. Volume 22, issue 1. Search within journal. Search. Volume 22, issue 1, December 2022. 339 articles in this issue. Interpretable generalized neural additive models for mortality prediction of COVID-19 hospitalized patients in Hamadan, Iran
Aims and scope. BMC Medical Education is an open access journal publishing original peer-reviewed research articles in relation to the training of healthcare professionals, including undergraduate, postgraduate, and continuing education. The journal has a special focus on curriculum development, evaluations of performance, assessment of ...
BMC Medical Research Methodology. Volumes and issues. Volume 24, issue 1. Search within journal. Search. Volume 24, issue 1, December 2024. 73 articles in this issue. Development of the multivariate administrative data cystectomy model and its impact on misclassification bias Authors (first, second and last of 4)
Background This retrospective analysis aimed to comprehensively review the design and regulatory aspects of bioequivalence trials submitted to the Saudi Food and Drug Authority (SFDA) since 2017. Methods This was a retrospective, comprehensive analysis study. The Data extracted from the SFDA bioequivalence assessment reports were analyzed for reviewing the overall design and regulatory aspects ...