- Library databases
- Library website

Evidence-Based Research: Levels of Evidence Pyramid
Introduction.
One way to organize the different types of evidence involved in evidence-based practice research is the levels of evidence pyramid. The pyramid includes a variety of evidence types and levels.
- systematic reviews
- critically-appraised topics
- critically-appraised individual articles
- randomized controlled trials
- cohort studies
- case-controlled studies, case series, and case reports
- Background information, expert opinion
Levels of evidence pyramid
The levels of evidence pyramid provides a way to visualize both the quality of evidence and the amount of evidence available. For example, systematic reviews are at the top of the pyramid, meaning they are both the highest level of evidence and the least common. As you go down the pyramid, the amount of evidence will increase as the quality of the evidence decreases.

Text alternative for Levels of Evidence Pyramid diagram
EBM Pyramid and EBM Page Generator, copyright 2006 Trustees of Dartmouth College and Yale University. All Rights Reserved. Produced by Jan Glover, David Izzo, Karen Odato and Lei Wang.
Filtered Resources
Filtered resources appraise the quality of studies and often make recommendations for practice. The main types of filtered resources in evidence-based practice are:
Scroll down the page to the Systematic reviews , Critically-appraised topics , and Critically-appraised individual articles sections for links to resources where you can find each of these types of filtered information.
Systematic reviews
Authors of a systematic review ask a specific clinical question, perform a comprehensive literature review, eliminate the poorly done studies, and attempt to make practice recommendations based on the well-done studies. Systematic reviews include only experimental, or quantitative, studies, and often include only randomized controlled trials.
You can find systematic reviews in these filtered databases :
- Cochrane Database of Systematic Reviews Cochrane systematic reviews are considered the gold standard for systematic reviews. This database contains both systematic reviews and review protocols. To find only systematic reviews, select Cochrane Reviews in the Document Type box.
- JBI EBP Database (formerly Joanna Briggs Institute EBP Database) This database includes systematic reviews, evidence summaries, and best practice information sheets. To find only systematic reviews, click on Limits and then select Systematic Reviews in the Publication Types box. To see how to use the limit and find full text, please see our Joanna Briggs Institute Search Help page .
You can also find systematic reviews in this unfiltered database :
To learn more about finding systematic reviews, please see our guide:
- Filtered Resources: Systematic Reviews
Critically-appraised topics
Authors of critically-appraised topics evaluate and synthesize multiple research studies. Critically-appraised topics are like short systematic reviews focused on a particular topic.
You can find critically-appraised topics in these resources:
- Annual Reviews This collection offers comprehensive, timely collections of critical reviews written by leading scientists. To find reviews on your topic, use the search box in the upper-right corner.
- Guideline Central This free database offers quick-reference guideline summaries organized by a new non-profit initiative which will aim to fill the gap left by the sudden closure of AHRQ’s National Guideline Clearinghouse (NGC).
- JBI EBP Database (formerly Joanna Briggs Institute EBP Database) To find critically-appraised topics in JBI, click on Limits and then select Evidence Summaries from the Publication Types box. To see how to use the limit and find full text, please see our Joanna Briggs Institute Search Help page .
- National Institute for Health and Care Excellence (NICE) Evidence-based recommendations for health and care in England.
- Filtered Resources: Critically-Appraised Topics
Critically-appraised individual articles
Authors of critically-appraised individual articles evaluate and synopsize individual research studies.
You can find critically-appraised individual articles in these resources:
- EvidenceAlerts Quality articles from over 120 clinical journals are selected by research staff and then rated for clinical relevance and interest by an international group of physicians. Note: You must create a free account to search EvidenceAlerts.
- ACP Journal Club This journal publishes reviews of research on the care of adults and adolescents. You can either browse this journal or use the Search within this publication feature.
- Evidence-Based Nursing This journal reviews research studies that are relevant to best nursing practice. You can either browse individual issues or use the search box in the upper-right corner.
To learn more about finding critically-appraised individual articles, please see our guide:
- Filtered Resources: Critically-Appraised Individual Articles
Unfiltered resources
You may not always be able to find information on your topic in the filtered literature. When this happens, you'll need to search the primary or unfiltered literature. Keep in mind that with unfiltered resources, you take on the role of reviewing what you find to make sure it is valid and reliable.
Note: You can also find systematic reviews and other filtered resources in these unfiltered databases.
The Levels of Evidence Pyramid includes unfiltered study types in this order of evidence from higher to lower:
You can search for each of these types of evidence in the following databases:
TRIP database
Background information & expert opinion.
Background information and expert opinions are not necessarily backed by research studies. They include point-of-care resources, textbooks, conference proceedings, etc.
- Family Physicians Inquiries Network: Clinical Inquiries Provide the ideal answers to clinical questions using a structured search, critical appraisal, authoritative recommendations, clinical perspective, and rigorous peer review. Clinical Inquiries deliver best evidence for point-of-care use.
- Harrison, T. R., & Fauci, A. S. (2009). Harrison's Manual of Medicine . New York: McGraw-Hill Professional. Contains the clinical portions of Harrison's Principles of Internal Medicine .
- Lippincott manual of nursing practice (8th ed.). (2006). Philadelphia, PA: Lippincott Williams & Wilkins. Provides background information on clinical nursing practice.
- Medscape: Drugs & Diseases An open-access, point-of-care medical reference that includes clinical information from top physicians and pharmacists in the United States and worldwide.
- Virginia Henderson Global Nursing e-Repository An open-access repository that contains works by nurses and is sponsored by Sigma Theta Tau International, the Honor Society of Nursing. Note: This resource contains both expert opinion and evidence-based practice articles.
- Previous Page: Phrasing Research Questions
- Next Page: Evidence Types
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.
- Privacy Policy
Buy Me a Coffee

Home » Evidence – Definition, Types and Example
Evidence – Definition, Types and Example
Table of Contents

Definition:
Evidence is any information or data that supports or refutes a claim, hypothesis, or argument. It is the basis for making decisions, drawing conclusions, and establishing the truth or validity of a statement.
Types of Evidence
Types of Evidence are as follows:
Empirical evidence
This type of evidence comes from direct observation or measurement, and is usually based on data collected through scientific or other systematic methods.
Expert Testimony
This is evidence provided by individuals who have specialized knowledge or expertise in a particular area, and can provide insight into the validity or reliability of a claim.
Personal Experience
This type of evidence comes from firsthand accounts of events or situations, and can be useful in providing context or a sense of perspective.
Statistical Evidence
This type of evidence involves the use of numbers and data to support a claim, and can include things like surveys, polls, and other types of quantitative analysis.
Analogical Evidence
This involves making comparisons between similar situations or cases, and can be used to draw conclusions about the validity or applicability of a claim.
Documentary Evidence
This includes written or recorded materials, such as contracts, emails, or other types of documents, that can provide support for a claim.
Circumstantial Evidence
This type of evidence involves drawing inferences based on indirect or circumstantial evidence, and can be used to support a claim when direct evidence is not available.
Examples of Evidence
Here are some examples of different types of evidence that could be used to support a claim or argument:
- A study conducted on a new drug, showing its effectiveness in treating a particular disease, based on clinical trials and medical data.
- A doctor providing testimony in court about a patient’s medical condition or injuries.
- A patient sharing their personal experience with a particular medical treatment or therapy.
- A study showing that a particular type of cancer is more common in certain demographics or geographic areas.
- Comparing the benefits of a healthy diet and exercise to maintaining a car with regular oil changes and maintenance.
- A contract showing that two parties agreed to a particular set of terms and conditions.
- The presence of a suspect’s DNA at the crime scene can be used as circumstantial evidence to suggest their involvement in the crime.
Applications of Evidence
Here are some applications of evidence:
- Law : In the legal system, evidence is used to establish facts and to prove or disprove a case. Lawyers use different types of evidence, such as witness testimony, physical evidence, and documentary evidence, to present their arguments and persuade judges and juries.
- Science : Evidence is the foundation of scientific inquiry. Scientists use evidence to support or refute hypotheses and theories, and to advance knowledge in their fields. The scientific method relies on evidence-based observations, experiments, and data analysis.
- Medicine : Evidence-based medicine (EBM) is a medical approach that emphasizes the use of scientific evidence to inform clinical decision-making. EBM relies on clinical trials, systematic reviews, and meta-analyses to determine the best treatments for patients.
- Public policy : Evidence is crucial in informing public policy decisions. Policymakers rely on research studies, evaluations, and other forms of evidence to develop and implement policies that are effective, efficient, and equitable.
- Business : Evidence-based decision-making is becoming increasingly important in the business world. Companies use data analytics, market research, and other forms of evidence to make strategic decisions, evaluate performance, and optimize operations.
Purpose of Evidence
The purpose of evidence is to support or prove a claim or argument. Evidence can take many forms, including statistics, examples, anecdotes, expert opinions, and research studies. The use of evidence is important in fields such as science, law, and journalism to ensure that claims are backed up by factual information and to make decisions based on reliable information. Evidence can also be used to challenge or question existing beliefs and assumptions, and to uncover new knowledge and insights. Overall, the purpose of evidence is to provide a foundation for understanding and decision-making that is grounded in empirical facts and data.
Characteristics of Evidence
Some Characteristics of Evidence are as follows:
- Relevance : Evidence must be relevant to the claim or argument it is intended to support. It should directly address the issue at hand and not be tangential or unrelated.
- Reliability : Evidence should come from a trustworthy and reliable source. The credibility of the source should be established, and the information should be accurate and free from bias.
- Sufficiency : Evidence should be sufficient to support the claim or argument. It should provide enough information to make a strong case, but not be overly repetitive or redundant.
- Validity : Evidence should be based on sound reasoning and logic. It should be based on established principles or theories, and should be consistent with other evidence and observations.
- Timeliness : Evidence should be current and up-to-date. It should reflect the most recent developments or research in the field.
- Accessibility : Evidence should be easily accessible to others who may want to review or evaluate it. It should be clear and easy to understand, and should be presented in a way that is appropriate for the intended audience.
Advantages of Evidence
The use of evidence has several advantages, including:
- Supports informed decision-making: Evidence-based decision-making enables individuals or organizations to make informed choices based on reliable information rather than assumptions or opinions.
- Enhances credibility: The use of evidence can enhance the credibility of claims or arguments by providing factual support.
- Promotes transparency: The use of evidence promotes transparency in decision-making processes by providing a clear and objective basis for decisions.
- Facilitates evaluation : Evidence-based decision-making enables the evaluation of the effectiveness of policies, programs, and interventions.
- Provides insights: The use of evidence can provide new insights and perspectives on complex issues, enabling individuals or organizations to approach problems from different angles.
- Enhances problem-solving : Evidence-based decision-making can help individuals or organizations to identify the root causes of problems and develop more effective solutions.
Limitations of Evidence
Some Limitations of Evidence are as follows:
- Limited availability : Evidence may not always be available or accessible, particularly in areas where research is limited or where data collection is difficult.
- Interpretation challenges: Evidence can be open to interpretation, and individuals may interpret the same evidence differently based on their biases, experiences, or values.
- Time-consuming: Gathering and evaluating evidence can be time-consuming and require significant resources, which may not always be feasible in certain contexts.
- May not apply universally : Evidence may be context-specific and may not apply universally to other situations or populations.
- Potential for bias: Even well-designed studies or research can be influenced by biases, such as selection bias, measurement bias, or publication bias.
- Ethical concerns : Evidence may raise ethical concerns, such as the use of personal data or the potential harm to research participants.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

What is Art – Definition, Types, Examples

What is Anthropology – Definition and Overview

What is Literature – Definition, Types, Examples

Economist – Definition, Types, Work Area

Anthropologist – Definition, Types, Work Area

What is History – Definitions, Periods, Methods

- Research Process
Levels of evidence in research
- 5 minute read
Table of Contents
Level of evidence hierarchy
When carrying out a project you might have noticed that while searching for information, there seems to be different levels of credibility given to different types of scientific results. For example, it is not the same to use a systematic review or an expert opinion as a basis for an argument. It’s almost common sense that the first will demonstrate more accurate results than the latter, which ultimately derives from a personal opinion.
In the medical and health care area, for example, it is very important that professionals not only have access to information but also have instruments to determine which evidence is stronger and more trustworthy, building up the confidence to diagnose and treat their patients.
5 levels of evidence
With the increasing need from physicians – as well as scientists of different fields of study-, to know from which kind of research they can expect the best clinical evidence, experts decided to rank this evidence to help them identify the best sources of information to answer their questions. The criteria for ranking evidence is based on the design, methodology, validity and applicability of the different types of studies. The outcome is called “levels of evidence” or “levels of evidence hierarchy”. By organizing a well-defined hierarchy of evidence, academia experts were aiming to help scientists feel confident in using findings from high-ranked evidence in their own work or practice. For Physicians, whose daily activity depends on available clinical evidence to support decision-making, this really helps them to know which evidence to trust the most.
So, by now you know that research can be graded according to the evidential strength determined by different study designs. But how many grades are there? Which evidence should be high-ranked and low-ranked?
There are five levels of evidence in the hierarchy of evidence – being 1 (or in some cases A) for strong and high-quality evidence and 5 (or E) for evidence with effectiveness not established, as you can see in the pyramidal scheme below:
Level 1: (higher quality of evidence) – High-quality randomized trial or prospective study; testing of previously developed diagnostic criteria on consecutive patients; sensible costs and alternatives; values obtained from many studies with multiway sensitivity analyses; systematic review of Level I RCTs and Level I studies.
Level 2: Lesser quality RCT; prospective comparative study; retrospective study; untreated controls from an RCT; lesser quality prospective study; development of diagnostic criteria on consecutive patients; sensible costs and alternatives; values obtained from limited stud- ies; with multiway sensitivity analyses; systematic review of Level II studies or Level I studies with inconsistent results.
Level 3: Case-control study (therapeutic and prognostic studies); retrospective comparative study; study of nonconsecutive patients without consistently applied reference “gold” standard; analyses based on limited alternatives and costs and poor estimates; systematic review of Level III studies.
Level 4: Case series; case-control study (diagnostic studies); poor reference standard; analyses with no sensitivity analyses.
Level 5: (lower quality of evidence) – Expert opinion.
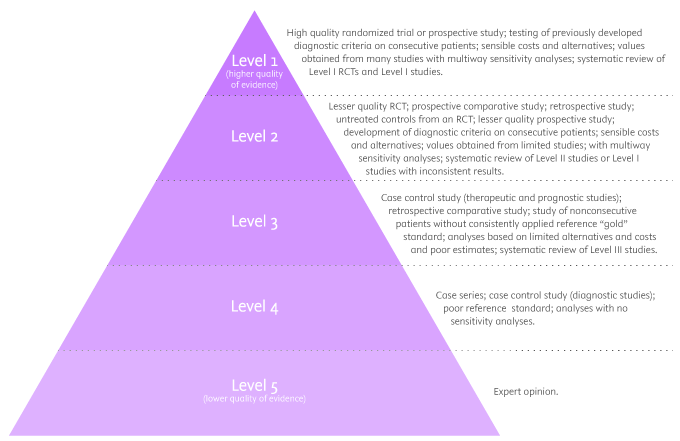
By looking at the pyramid, you can roughly distinguish what type of research gives you the highest quality of evidence and which gives you the lowest. Basically, level 1 and level 2 are filtered information – that means an author has gathered evidence from well-designed studies, with credible results, and has produced findings and conclusions appraised by renowned experts, who consider them valid and strong enough to serve researchers and scientists. Levels 3, 4 and 5 include evidence coming from unfiltered information. Because this evidence hasn’t been appraised by experts, it might be questionable, but not necessarily false or wrong.
Examples of levels of evidence
As you move up the pyramid, you will surely find higher-quality evidence. However, you will notice there is also less research available. So, if there are no resources for you available at the top, you may have to start moving down in order to find the answers you are looking for.
- Systematic Reviews: -Exhaustive summaries of all the existent literature about a certain topic. When drafting a systematic review, authors are expected to deliver a critical assessment and evaluation of all this literature rather than a simple list. Researchers that produce systematic reviews have their own criteria to locate, assemble and evaluate a body of literature.
- Meta-Analysis: Uses quantitative methods to synthesize a combination of results from independent studies. Normally, they function as an overview of clinical trials. Read more: Systematic review vs meta-analysis .
- Critically Appraised Topic: Evaluation of several research studies.
- Critically Appraised Article: Evaluation of individual research studies.
- Randomized Controlled Trial: a clinical trial in which participants or subjects (people that agree to participate in the trial) are randomly divided into groups. Placebo (control) is given to one of the groups whereas the other is treated with medication. This kind of research is key to learning about a treatment’s effectiveness.
- Cohort studies: A longitudinal study design, in which one or more samples called cohorts (individuals sharing a defining characteristic, like a disease) are exposed to an event and monitored prospectively and evaluated in predefined time intervals. They are commonly used to correlate diseases with risk factors and health outcomes.
- Case-Control Study: Selects patients with an outcome of interest (cases) and looks for an exposure factor of interest.
- Background Information/Expert Opinion: Information you can find in encyclopedias, textbooks and handbooks. This kind of evidence just serves as a good foundation for further research – or clinical practice – for it is usually too generalized.
Of course, it is recommended to use level A and/or 1 evidence for more accurate results but that doesn’t mean that all other study designs are unhelpful or useless. It all depends on your research question. Focusing once more on the healthcare and medical field, see how different study designs fit into particular questions, that are not necessarily located at the tip of the pyramid:
- Questions concerning therapy: “Which is the most efficient treatment for my patient?” >> RCT | Cohort studies | Case-Control | Case Studies
- Questions concerning diagnosis: “Which diagnose method should I use?” >> Prospective blind comparison
- Questions concerning prognosis: “How will the patient’s disease will develop over time?” >> Cohort Studies | Case Studies
- Questions concerning etiology: “What are the causes for this disease?” >> RCT | Cohort Studies | Case Studies
- Questions concerning costs: “What is the most cost-effective but safe option for my patient?” >> Economic evaluation
- Questions concerning meaning/quality of life: “What’s the quality of life of my patient going to be like?” >> Qualitative study
Find more about Levels of evidence in research on Pinterest:
17 March 2021 – Elsevier’s Mini Program Launched on WeChat Brings Quality Editing Straight to your Smartphone
- Manuscript Review
Professor Anselmo Paiva: Using Computer Vision to Tackle Medical Issues with a Little Help from Elsevier Author Services
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
Systematic Reviews
- Levels of Evidence
- Evidence Pyramid
- Joanna Briggs Institute
The evidence pyramid is often used to illustrate the development of evidence. At the base of the pyramid is animal research and laboratory studies – this is where ideas are first developed. As you progress up the pyramid the amount of information available decreases in volume, but increases in relevance to the clinical setting.
Meta Analysis – systematic review that uses quantitative methods to synthesize and summarize the results.
Systematic Review – summary of the medical literature that uses explicit methods to perform a comprehensive literature search and critical appraisal of individual studies and that uses appropriate st atistical techniques to combine these valid studies.
Randomized Controlled Trial – Participants are randomly allocated into an experimental group or a control group and followed over time for the variables/outcomes of interest.
Cohort Study – Involves identification of two groups (cohorts) of patients, one which received the exposure of interest, and one which did not, and following these cohorts forward for the outcome of interest.
Case Control Study – study which involves identifying patients who have the outcome of interest (cases) and patients without the same outcome (controls), and looking back to see if they had the exposure of interest.
Case Series – report on a series of patients with an outcome of interest. No control group is involved.
- Levels of Evidence from The Centre for Evidence-Based Medicine
- The JBI Model of Evidence Based Healthcare
- How to Use the Evidence: Assessment and Application of Scientific Evidence From the National Health and Medical Research Council (NHMRC) of Australia. Book must be downloaded; not available to read online.
When searching for evidence to answer clinical questions, aim to identify the highest level of available evidence. Evidence hierarchies can help you strategically identify which resources to use for finding evidence, as well as which search results are most likely to be "best".

Image source: Evidence-Based Practice: Study Design from Duke University Medical Center Library & Archives. This work is licensed under a Creativ e Commons Attribution-ShareAlike 4.0 International License .
The hierarchy of evidence (also known as the evidence-based pyramid) is depicted as a triangular representation of the levels of evidence with the strongest evidence at the top which progresses down through evidence with decreasing strength. At the top of the pyramid are research syntheses, such as Meta-Analyses and Systematic Reviews, the strongest forms of evidence. Below research syntheses are primary research studies progressing from experimental studies, such as Randomized Controlled Trials, to observational studies, such as Cohort Studies, Case-Control Studies, Cross-Sectional Studies, Case Series, and Case Reports. Non-Human Animal Studies and Laboratory Studies occupy the lowest level of evidence at the base of the pyramid.
- Finding Evidence-Based Answers to Clinical Questions – Quickly & Effectively A tip sheet from the health sciences librarians at UC Davis Libraries to help you get started with selecting resources for finding evidence, based on type of question.
- << Previous: What is a Systematic Review?
- Next: Locating Systematic Reviews >>
- Getting Started
- What is a Systematic Review?
- Locating Systematic Reviews
- Searching Systematically
- Developing Answerable Questions
- Identifying Synonyms & Related Terms
- Using Truncation and Wildcards
- Identifying Search Limits/Exclusion Criteria
- Keyword vs. Subject Searching
- Where to Search
- Search Filters
- Sensitivity vs. Precision
- Core Databases
- Other Databases
- Clinical Trial Registries
- Conference Presentations
- Databases Indexing Grey Literature
- Web Searching
- Handsearching
- Citation Indexes
- Documenting the Search Process
- Managing your Review
Research Support
- Last Updated: Apr 8, 2024 3:33 PM
- URL: https://guides.library.ucdavis.edu/systematic-reviews

- What is the best evidence and how to find it
Why is research evidence better than expert opinion alone?
In a broad sense, research evidence can be any systematic observation in order to establish facts and reach conclusions. Anything not fulfilling this definition is typically classified as “expert opinion”, the basis of which includes experience with patients, an understanding of biology, knowledge of pre-clinical research, as well as of the results of studies. Using expert opinion as the only basis to make decisions has proved problematic because in practice doctors often introduce new treatments too quickly before they have been shown to work, or they are too slow to introduce proven treatments.
However, clinical experience is key to interpret and apply research evidence into practice, and to formulate recommendations, for instance in the context of clinical guidelines. In other words, research evidence is necessary but not sufficient to make good health decisions.
Which studies are more reliable?
Not all evidence is equally reliable.
Any study design, qualitative or quantitative, where data is collected from individuals or groups of people is usually called a primary study. There are many types of primary study designs, but for each type of health question there is one that provides more reliable information.
For treatment decisions, there is consensus that the most reliable primary study is the randomised controlled trial (RCT). In this type of study, patients are randomly assigned to have either the treatment being tested or a comparison treatment (sometimes called the control treatment). Random really means random. The decision to put someone into one group or another is made like tossing a coin: heads they go into one group, tails they go into the other.
The control treatment might be a different type of treatment or a dummy treatment that shouldn't have any effect (a placebo). Researchers then compare the effects of the different treatments.
Large randomised trials are expensive and take time. In addition sometimes it may be unethical to undertake a study in which some people were randomly assigned not to have a treatment. For example, it wouldn't be right to give oxygen to some children having an asthma attack and not give it to others. In cases like this, other primary study designs may be the best choice.
Laboratory studies are another type of study. Newspapers often have stories of studies showing how a drug cured cancer in mice. But just because a treatment works for animals in laboratory experiments, this doesn't mean it will work for humans. In fact, most drugs that have been shown to cure cancer in mice do not work for people.
Very rarely we cannot base our health decisions on the results of studies. Sometimes the research hasn't been done because doctors are used to treating a condition in a way that seems to work. This is often true of treatments for broken bones and operations. But just because there's no research for a treatment doesn't mean it doesn't work. It just means that no one can say for sure.
Why we shouldn’t read studies
An enormous amount of effort is required to be able to identify and summarise everything we know with regard to any given health intervention. The amount of data has soared dramatically. A conservative estimation is there are more than 35,000 medical journals and almost 20 million research articles published every year. On the other hand, up to half of existing data might be unpublished.
How can anyone keep up with all this? And how can you tell if the research is good or not? Each primary study is only one piece of a jigsaw that may take years to finish. Rarely does any one piece of research answer either a doctor's, or a patient's questions.
Even though reading large numbers of studies is impractical, high-quality primary studies, especially RCTs, constitute the foundations of what we know, and they are the best way of advancing the knowledge. Any effort to support or promote the conduct of sound, transparent, and independent trials that are fully and clearly published is worth endorsing. A prominent project in this regard is the All trials initiative.
Why we should read systematic reviews
Most of the time a single study doesn't tell us enough. The best answers are found by combining the results of many studies.
A systematic review is a type of research that looks at the results from all of the good-quality studies. It puts together the results of these individual studies into one summary. This gives an estimate of a treatment's risks and benefits. Sometimes these reviews include a statistical analysis, called a meta-analysis , which combines the results of several studies to give a treatment effect.
Systematic reviews are increasingly being used for decision making because they reduce the probability of being misled by looking at one piece of the jigsaw. By being systematic they are also more transparent, and have become the gold standard approach to synthesise the ever-expanding and conflicting biomedical literature.
Systematic reviews are not fool proof. Their findings are only as good as the studies that they include and the methods they employ. But the best reviews clearly state whether the studies they include are good quality or not.
Three reasons why we shouldn’t read (most) systematic reviews
Firstly, systematic reviews have proliferated over time. From 11 per day in 2010, they skyrocketed up to 40 per day or more in 2015.[1][2] Some have described this production as having reached epidemic proportions where the large majority of produced systematic reviews and meta-analyses are unnecessary, misleading, and/or conflicted.[3][4] So, finding more than one systematic review for a question is the rule more than the exception, and it is not unusual to find several dozen for the hottest questions.
Second, most systematic reviews address a narrow question. It is difficult to put them in the context of all of the available alternatives for an individual case. Reading multiple reviews to assess all of the alternatives is impractical, even more if we consider they are typically difficult to read for the average clinician, who will need to solve several questions each day.[5]
Third, systematic reviews do not tell you what to do, or what is advisable for a given patient or situation. Indeed, good systematic reviews explicitly avoid making recommendations.
So, even though systematic reviews play a key role in any evidence-based decision-making process, most of them are low-quality or outdated, and they rarely provide all the information needed to make decisions in the real world.
How to find the best available evidence?
Considering the massive amount of information available, we can quickly discard periodically reviewing our favourite journals as a means of sourcing the best available evidence.
The traditional approach to search for evidence has been using major databases, such as PubMed or EMBASE . These constitute comprehensive sources including millions of relevant, but also irrelevant articles. Even though in the past they were the preferred approach to searching for evidence, information overload has made them impractical, and most clinicians would fail to find the best available evidence in this way, however hard they tried.
Another popular approach is simply searching in Google. Unfortunately, because of its lack of transparency, Google is not a reliable way to filter current best evidence from unsubstantiated or non-scientifically supervised sources.[6]
Three alternatives to access the best evidence
Alternative 1 - Pick the best systematic review Mastering the art of identifying, appraising, and applying high-quality systematic reviews into practice can be very rewarding. It is not easy, but once mastered it gives a view of the bigger picture: of what is known, and what is not known.
The best single source of highest-quality systematic reviews is produced by an international organisation called the Cochrane Collaboration, named after a well-known researcher.[4] They can be accessed at The Cochrane Library .
Unfortunately, Cochrane reviews do not cover all of the existing questions and they are not always up to date. Also, there might be non-Cochrane reviews out-performing Cochrane reviews.
There are many resources that facilitate access to systematic reviews (and other resources), such as Trip database , PubMed Health , ACCESSSS , or Epistemonikos (the Cochrane Collaboration maintains a comprehensive list of these resources).
Epistemonikos database is innovative both in simultaneously searching multiple resources and in indexing and interlinking relevant evidence. For example, Epistemonikos connects systematic reviews and their included studies, and thus allows clustering of systematic reviews based on the primary studies they have in common. Epistemonikos is also unique in offering an appreciable multilingual user interface, multilingual search, and translation of abstracts in more than nine languages.[6] This database includes several tools to compare systematic reviews, including the matrix of evidence, a dynamic table showing all of the systematic reviews, and the primary studies included in those reviews.
Additionally, Epistemonikos partnered with Cochrane, and during 2017 a combined search in both the Cochrane Library and Epistemonikos was released.
Alternative 2 - Read trustworthy guidelines Although systematic reviews can provide a synthesis of the benefits and harms of the interventions, they do not integrate these factors with patients’ values and preferences or resource considerations to provide a suggested course of action. Also, to fully address the questions, clinicians would need to integrate the information of several systematic reviews covering all the relevant alternatives and outcomes. Most clinicians will likely prefer guidance rather than interpreting systematic reviews themselves.
Trustworthy guidelines, especially if developed with high standards, such as the Grading of Recommendations, Assessment, Development, and Evaluation ( GRADE ) approach, offer systematic and transparent guidance in moving from evidence to recommendations.[7]
Many online guideline websites promote themselves as “evidence based”, but few have explicit links to research findings.[8] If they don’t have in-line references to relevant research findings, dismiss them. If they have, you can judge the strength of the commitment to evidence to support inference, checking whether statements are based on high-quality versus low-quality evidence using alternative 1 explained above.
Unfortunately, most guidelines have serious limitations or are outdated.[9][10] The exercise of locating and appraising the best guideline is time consuming. This is particularly challenging for generalists addressing questions from different conditions or diseases.
Alternative 3 - Use point-of-care tools Point-of-care tools, such as BMJ Best Practice, have been developed as a response to the genuine need to summarise the ever-expanding biomedical literature on an ever-increasing number of alternatives in order to make evidence-based decisions. In this competitive market, the more successful products have been those delivering innovative, user-friendly interfaces that improve the retrieval, synthesis, organisation, and application of evidence-based content in many different areas of clinical practice.
However, the same impossibility in catching up with new evidence without compromising quality that affects guidelines also affects point-of-care tools. Clinicians should become familiar with the point-of-care information resource they want or can access, and examine the in-line references to relevant research findings. Clinicians can easily judge the strength of the commitment to evidence checking whether statements are based on high-quality versus low-quality evidence using alternative 1 explained above. Comprehensiveness, use of GRADE approach, and independence are other characteristics to bear in mind when selecting among point-of-care information summaries.
A comprehensive list of these resources can be found in a study by Kwag et al .
Finding the best available evidence is more challenging than it was in the dawn of the evidence-based movement, and the main cause is the exponential growth of evidence-based information, in any of the flavours described above.
However, with a little bit of patience and practice, the busy clinician will discover evidence-based practice is far easier than it was 5 or 10 years ago. We are entering a stage where information is flowing between the different systems, technology is being harnessed for good, and the different players are starting to generate alliances.
The early adopters will surely enjoy the first experiments of living systematic reviews (high-quality, up-to-date online summaries of health research that are updated as new research becomes available), living guidelines, and rapid reviews tied to rapid recommendations, just to mention a few. [13][14][15]
It is unlikely that the picture of countless low-quality studies and reviews will change in the foreseeable future. However, it would not be a surprise if, in 3 to 5 years, separating the wheat from the chaff becomes trivial. Maybe the promise of evidence-based medicine of more effective, safer medical intervention resulting in better health outcomes for patients could be fulfilled.
Author: Gabriel Rada
Competing interests: Gabriel Rada is the co-founder and chairman of Epistemonikos database, part of the team that founded and maintains PDQ-Evidence, and an editor of the Cochrane Collaboration.
Related Blogs
Living Systematic Reviews: towards real-time evidence for health-care decision making
- Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010 Sep 21;7(9):e1000326. doi: 10.1371/journal.pmed.1000326
- Epistemonikos database [filter= systematic review; year=2015]. A Free, Relational, Collaborative, Multilingual Database of Health Evidence. https://www.epistemonikos.org/en/search?&q=*&classification=systematic-review&year_start=2015&year_end=2015&fl=14542 Accessed 5 Jan 2017.
- Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016 Sep;94(3):485-514. doi: 10.1111/1468-0009.12210.
- Page MJ, Shamseer L, Altman DG, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross-sectional study. PLoS Med. 2016;13(5):e1002028.
- Del Fiol G, Workman TE, Gorman PN. Clinical questions raised by clinicians at the point of care: a systematic review. JAMA Intern Med. 2014 May;174(5):710-8. doi: 10.1001/jamainternmed.2014.368.
- Agoritsas T, Vandvik P, Neumann I, Rochwerg B, Jaeschke R, Hayward R, et al. Chapter 5: finding current best evidence. In: Users' guides to the medical literature: a manual for evidence-based clinical practice. Chicago: MacGraw-Hill, 2014.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347
- Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017
- Alonso-Coello P, Irfan A, Solà I, Gich I, Delgado-Noguera M, Rigau D, Tort S, Bonfill X, Burgers J, Schunemann H. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010 Dec;19(6):e58. doi: 10.1136/qshc.2010.042077
- Martínez García L, Sanabria AJ, García Alvarez E, Trujillo-Martín MM, Etxeandia-Ikobaltzeta I, Kotzeva A, Rigau D, Louro-González A, Barajas-Nava L, Díaz Del Campo P, Estrada MD, Solà I, Gracia J, Salcedo-Fernandez F, Lawson J, Haynes RB, Alonso-Coello P; Updating Guidelines Working Group. The validity of recommendations from clinical guidelines: a survival analysis. CMAJ. 2014 Nov 4;186(16):1211-9. doi: 10.1503/cmaj.140547
- Kwag KH, González-Lorenzo M, Banzi R, Bonovas S, Moja L. Providing Doctors With High-Quality Information: An Updated Evaluation of Web-Based Point-of-Care Information Summaries. J Med Internet Res. 2016 Jan 19;18(1):e15. doi: 10.2196/jmir.5234
- Banzi R, Cinquini M, Liberati A, Moschetti I, Pecoraro V, Tagliabue L, Moja L. Speed of updating online evidence based point of care summaries: prospective cohort analysis. BMJ. 2011 Sep 23;343:d5856. doi: 10.1136/bmj.d5856
- Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JP, Mavergames C, Gruen RL. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 2014 Feb 18;11(2):e1001603. doi: 10.1371/journal.pmed.1001603
- Vandvik PO, Brandt L, Alonso-Coello P, Treweek S, Akl EA, Kristiansen A, Fog-Heen A, Agoritsas T, Montori VM, Guyatt G. Creating clinical practice guidelines we can trust, use, and share: a new era is imminent. Chest. 2013 Aug;144(2):381-9. doi: 10.1378/chest.13-0746
- Vandvik PO, Otto CM, Siemieniuk RA, Bagur R, Guyatt GH, Lytvyn L, Whitlock R, Vartdal T, Brieger D, Aertgeerts B, Price S, Foroutan F, Shapiro M, Mertz R, Spencer FA. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ. 2016 Sep 28;354:i5085. doi: 10.1136/bmj.i5085
Discuss EBM
- What does evidence-based actually mean?
- Simply making evidence simple
- Six proposals for EBMs future
- Promoting informed healthcare choices by helping people assess treatment claims
- The blind leading the blind in the land of risk communication
- Transforming the communication of evidence for better health
- Clinical search, big data, and the hunt for meaning
- Living systematic reviews: towards real-time evidence for health-care decision making
- The rise of rapid reviews
- Evidence for the Brave New World on multimorbidity
- Genetics and personalised medicine: where’s the revolution?
- Policy, practice, and politics
- The straw men of integrative health and alternative medicine
- Where’s the evidence for teaching evidence-based medicine?
EBM Toolkit home
Learn, Practise, Discuss, Tools
Evidence-Based Research Series-Paper 1: What Evidence-Based Research is and why is it important?
Affiliations.
- 1 Johns Hopkins Evidence-based Practice Center, Division of General Internal Medicine, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA.
- 2 Digital Content Services, Operations, Elsevier Ltd., 125 London Wall, London, EC2Y 5AS, UK.
- 3 School of Nursing, McMaster University, Health Sciences Centre, Room 2J20, 1280 Main Street West, Hamilton, Ontario, Canada, L8S 4K1; Section for Evidence-Based Practice, Western Norway University of Applied Sciences, Inndalsveien 28, Bergen, P.O.Box 7030 N-5020 Bergen, Norway.
- 4 Department of Sport Science and Clinical Biomechanics, University of Southern Denmark, Campusvej 55, 5230, Odense M, Denmark; Department of Physiotherapy and Occupational Therapy, University Hospital of Copenhagen, Herlev & Gentofte, Kildegaardsvej 28, 2900, Hellerup, Denmark.
- 5 Musculoskeletal Statistics Unit, the Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, Nordre Fasanvej 57, 2000, Copenhagen F, Denmark; Department of Clinical Research, Research Unit of Rheumatology, University of Southern Denmark, Odense University Hospital, Denmark.
- 6 Section for Evidence-Based Practice, Western Norway University of Applied Sciences, Inndalsveien 28, Bergen, P.O.Box 7030 N-5020 Bergen, Norway. Electronic address: [email protected].
- PMID: 32979491
- DOI: 10.1016/j.jclinepi.2020.07.020
Objectives: There is considerable actual and potential waste in research. Evidence-based research ensures worthwhile and valuable research. The aim of this series, which this article introduces, is to describe the evidence-based research approach.
Study design and setting: In this first article of a three-article series, we introduce the evidence-based research approach. Evidence-based research is the use of prior research in a systematic and transparent way to inform a new study so that it is answering questions that matter in a valid, efficient, and accessible manner.
Results: We describe evidence-based research and provide an overview of the approach of systematically and transparently using previous research before starting a new study to justify and design the new study (article #2 in series) and-on study completion-place its results in the context with what is already known (article #3 in series).
Conclusion: This series introduces evidence-based research as an approach to minimize unnecessary and irrelevant clinical health research that is unscientific, wasteful, and unethical.
Keywords: Clinical health research; Clinical trials; Evidence synthesis; Evidence-based research; Medical ethics; Research ethics; Systematic review.
Copyright © 2020 Elsevier Inc. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Biomedical Research* / methods
- Biomedical Research* / organization & administration
- Clinical Trials as Topic / ethics
- Clinical Trials as Topic / methods
- Clinical Trials as Topic / organization & administration
- Ethics, Research
- Evidence-Based Medicine / methods*
- Needs Assessment
- Reproducibility of Results
- Research Design* / standards
- Research Design* / trends
- Systematic Reviews as Topic
- Treatment Outcome
- Archives & Special Collections home
- Art Library home
- Ekstrom Library home
- Kornhauser Health Sciences Library home
- Law Library home
- Music Library home
- University of Louisville Hospital home
- Interlibrary Loan
- Off-Campus Login
- Renew Books
- Cardinal Card
- My Print Center
- Business Ops
- Cards Career Connection
Search Site
Search catalog, evidence-based practice: types of evidence.
- Introduction
- Finding Evidence (PICO)
- Types of Evidence
- Appraising Evidence
- EBP Resources
- Write a Systematic Review
- Evidence-Based Dentistry
- Evidence-Based Nursing
- Evidence-Based SPL and Audiology
- Evidence-Based Public Health
Types of Research
Once you have your focused question, it's time to decide on the type of evidence you need to answer it. Understanding the types of research will help guide you to proper evidence that will support your question.
Evidence Based Pyramid
Hierarchy of evidence and research designs.

As you move up the pyramid, the study designs are more rigorous and are less biased.
What type of study should you use?
Question definitions:.
Intervention/Therapy: Questions addressing the treatment of an illness or disability.
Etiology: Questions addressing the causes or origins of disease (i.e., factors that produce or predispose toward a certain disease or disorder).
Diagnosis: Questions addressing the act or process of identifying or determining the nature and cause of a disease or injury through evaluation.
Prognosis/Prediction: Questions addressing the prediction of the course of a disease.
The type of question you have will often lead you to the type of research that will best answer the question:
Intervention/Prevention: RCT > Cohort Study > Case Control > Case Series
Therapy: RCT > Cohort > Case Control > Case Series
Prognosis/Prediction: Cohort Study > Case Control > Case Series
Diagnosis/Diagnostic: Prospective, blind comparison to Gold Standard
Etiology: RCT > Cohort Study > Case Control > Case Series
Definitions
Cebm study design tree.

The type of study can generally be worked at by looking at three issues:
Q1. What was the aim of the study?
- To simply describe a population (PO questions) descriptive
- To quantify the relationship between factors (PICO questions) analytic.
Q2. If analytic, was the intervention randomly allocated?
- Yes? RCT
- No? Observational study
For observational study the main types will then depend on the timing of the measurement of outcome, so our third question is:
Q3. When were the outcomes determined?
- Some time after the exposure or intervention? cohort study (‘prospective study’)
- At the same time as the exposure or intervention? cross sectional study or survey
- Before the exposure was determined? case-control study (‘retrospective study’ based on recall of the exposure)
from Centre for Evidence-Based Medicine https://www.cebm.net/2014/04/study-designs/
- << Previous: Finding Evidence (PICO)
- Next: Appraising Evidence >>
- Last Updated: Jan 16, 2024 2:02 PM
- Librarian Login

Navigating Scientific Evidence: Types and Definitions
This article provides an insightful exploration into the varied landscape of scientific evidence. It delves into its principles, utility, and its role in hypothesis testing.
With perspectives from philosophers and scientists, it presents a comprehensive understanding of evidence statements and hypotheses.
The article underscores the significance of testing scientific ideas and the influence of special interest groups on research. It also highlights the integral role of the scientific community in the progression of knowledge.
Key Takeaways
- Different background beliefs can lead to different conclusions drawn from the same scientific evidence.
- Scientific evidence plays a central role in Karl R. Popper’s theory of the scientific method.
- Falsifiability is an important concept in determining the utility of scientific evidence.
- Scientists actively seek evidence to test their ideas, even if it requires significant effort.
Understanding the Principles of Scientific Evidence
The principles of scientific evidence, which form the bedrock of empirical research, entail a meticulous understanding of how observations relate with hypotheses and how these relationships are interpreted, often influenced by underlying assumptions or beliefs. This scientific definition of evidence underscores the inherent objectivity and rigorousness of the scientific method.
Evidence serves as a tangible manifestation of abstract theories, often swaying the pendulum of scientific discourse. It is through the judicious application of these principles that scholars can transform raw data into reliable knowledge.
Evidence, then, is not merely an accumulation of facts but a dynamic tool employed to challenge, validate, or refine existing hypotheses. This intricate process underscores the importance of both the acquisition and interpretation of scientific evidence.
Distinguishing Philosophical and Scientific Views on Evidence
In the realm of academia, distinguishing between philosophical and scientific views on evidence presents a fascinating discourse, highlighting the divergences and synergies in interpretation, methodology, and epistemological underpinnings.
Philosophically, evidence is evaluated through abstract, conceptual analysis while scientific evidence is more empirically grounded. The scientific evidence definition rests on the premise that it is concrete, measurable, reproducible, and consistent with theoretical expectations. Philosophy, on the contrary, explores the nuances of evidence, often delving into the subjective realm.
Nevertheless, both views contribute to an all-encompassing understanding of evidence, underlining its multifaceted nature.
Ultimately, the ongoing dialogue between philosophical and scientific perspectives deepens our comprehension of evidence and its pivotal role in advancing knowledge.
Exploring Different Concepts of Evidence
Several concepts of evidence provide a diverse range of perspectives that aid in comprehending the multifaceted nature of scientific inquiry. These concepts, categorised under different types of scientific evidence, play an integral role in the formulation and validation of theories.
For instance, subjective evidence is based on individual perceptions, while veridical evidence is grounded in observable facts. Importantly, potential evidence represents unverified data that may, upon validation, solidify into veridical evidence.
Scientists often seek veridical evidence due to its factual basis but also employ other evidence types to develop comprehensive analyses. The utilisation of these varied concepts contributes to a more robust and nuanced understanding of phenomena, emphasising the complexity and richness of scientific investigation.

The Crucial Role of Hypothesis Testing in Science
Undeniably, hypothesis testing serves as a fundamental pillar in scientific research, facilitating rigorous scrutiny and validation of theories. It enables researchers to draw conclusions from scientific evidence, thus propelling the growth of knowledge. Through hypothesis testing, theories are either supported or rejected based on the strength and credibility of the evidence.
This process underscores the integral role of scientific evidence in research. Without hypothesis testing, scientific research would lack objectivity, reliability, and credibility. Therefore, the importance of hypothesis testing in validating scientific evidence cannot be overstated.
The Relationship Between Evidence and Ideas in Scientific Practice
Remarkably, the relationship between evidence and ideas in scientific practice serves as the cornerstone of any meaningful scientific discovery, with evidence acting as the litmus test for the validity and reliability of ideas. This relationship is critical, as it provides a structured pathway for scientists to formulate, test, and validate their hypotheses.
To illustrate this, consider these examples of scientific evidence:
- Fossils serving as evidence for evolution.
- The Doppler shift in light from distant galaxies as evidence for the Big Bang theory.
- Genetic mutations as evidence for natural selection.
- Climate data as evidence for global warming.
These examples highlight the pivotal role evidence plays in shaping and refining scientific theories, ultimately driving scientific progress.
Sign up for our Publishing Newsletter and start delivering creative, concise content
Frequently asked questions, how has the concept of scientific evidence evolved over the centuries.
The concept of scientific evidence has transformed over centuries, influenced by advancements in technology and methodology. It has evolved from anecdotal observations to rigorous, replicable experiments, quantitative analysis, and sophisticated statistical inference.
What Is the Hierarchy of Scientific Evidence?
The hierarchy of scientific evidence is a system used to rank the reliability of different types of research findings. At the top are systematic reviews and meta-analyses, which synthesise data from multiple studies, followed by randomised controlled trials, cohort studies, case-control studies, case series/reports, and expert opinion. This ranking helps guide decision-making by indicating which evidence is most likely to be accurate.
What Are the Ethical Considerations When Gathering and Interpreting Scientific Evidence?
Ethical considerations in gathering and interpreting scientific evidence include ensuring accuracy, objectivity, and integrity. This involves unbiased data collection, transparent methodologies, thorough data analysis, and honest reporting of findings, while respecting confidentiality and privacy norms.
How Can Non-Scientists Evaluate the Reliability of Scientific Evidence Presented in the Media?
Non-scientists can evaluate the reliability of scientific evidence presented in the media by scrutinising the source, checking for peer reviews, looking at the methodology, and cross-verifying the information with other credible sources.
How Does the Process of Peer Review Contribute to the Validation of Scientific Evidence?
The peer review process validates scientific evidence by ensuring it undergoes rigorous scrutiny by other experts in the field. This process verifies the integrity, accuracy, and reliability of the research before it is published.
How Does Cultural or Societal Context Influence the Interpretation of Scientific Evidence?
Cultural or societal context greatly influences the interpretation of scientific evidence, as it can shape individual perception and understanding. Bias, traditions, and societal norms can impact how scientific data is evaluated and accepted.
How Does Scientific Evidence affect Scientific Content Creation?
Scientific evidence fundamentally shapes scientific content creation by providing the empirical foundation upon which accurate, reliable, and meaningful content is built. In the process of content creation, whether it be for academic journals, educational materials, or public science communication, the inclusion of scientific evidence ensures that information is grounded in verified research and observations. This reliance on evidence allows for the formulation of hypotheses, theories, and models that accurately reflect the natural world.
In conclusion, the interpretation of scientific evidence is guided by principles, philosophical views, and systematic testing of hypotheses.
The role of the scientific community in progressing knowledge is irrefutable.
However, the influence of special interest groups can potentially skew results.
A clear understanding of differing concepts of evidence and the relationship between evidence and ideas is vital in the pursuit and application of scientific knowledge.
Discover the ScioWire research newsfeed: summarised scientific knowledge ready to digest.
- Evidence-Based Medicine
- Finding the Evidence
- eJournals for EBM
Levels of Evidence
- JAMA Users' Guides
- Tutorials (Learning EBM)
- Web Resources
Resources That Rate The Evidence
- ACP Smart Medicine
- Agency for Healthcare Research and Quality
- Clinical Evidence
- Cochrane Library
- Health Services/Technology Assessment Texts (HSTAT)
- PDQ® Cancer Information Summaries from NCI
- Trip Database
Critically Appraised Individual Articles
- Evidence-Based Complementary and Alternative Medicine
- Evidence-Based Dentistry
- Evidence-Based Nursing
- Journal of Evidence-Based Dental Practice
Grades of Recommendation
Critically-appraised individual articles and synopses include:
Filtered evidence:
- Level I: Evidence from a systematic review of all relevant randomized controlled trials.
- Level II: Evidence from a meta-analysis of all relevant randomized controlled trials.
- Level III: Evidence from evidence summaries developed from systematic reviews
- Level IV: Evidence from guidelines developed from systematic reviews
- Level V: Evidence from meta-syntheses of a group of descriptive or qualitative studies
- Level VI: Evidence from evidence summaries of individual studies
- Level VII: Evidence from one properly designed randomized controlled trial
Unfiltered evidence:
- Level VIII: Evidence from nonrandomized controlled clinical trials, nonrandomized clinical trials, cohort studies, case series, case reports, and individual qualitative studies.
- Level IX: Evidence from opinion of authorities and/or reports of expert committee
Two things to remember:
1. Studies in which randomization occurs represent a higher level of evidence than those in which subject selection is not random.
2. Controlled studies carry a higher level of evidence than those in which control groups are not used.
Strength of Recommendation Taxonomy (SORT)
- SORT The American Academy of Family Physicians uses the Strength of Recommendation Taxonomy (SORT) to label key recommendations in clinical review articles. In general, only key recommendations are given a Strength-of-Recommendation grade. Grades are assigned on the basis of the quality and consistency of available evidence.
- << Previous: eJournals for EBM
- Next: JAMA Users' Guides >>
- Last Updated: Jan 25, 2024 4:15 PM
- URL: https://guides.library.stonybrook.edu/evidence-based-medicine
- Request a Class
- Hours & Locations
- Ask a Librarian
- Special Collections
- Library Faculty & Staff
Library Administration: 631.632.7100
- Stony Brook Home
- Campus Maps
- Web Accessibility Information
- Accessibility Barrier Report Form

Comments or Suggestions? | Library Webmaster

Except where otherwise noted, this work by SBU Libraries is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License .
- Chamberlain University Library
- Chamberlain Library Core
Finding Types of Research
- Evidence-Based Research
On This Guide
About this guide, understand evidence-based practice, identify research study types.
- Quantitative Studies
- Qualitative Studies
- Meta-Analysis
- Systematic Reviews
- Randomized Controlled Trials
- Observational Studies
- Literature Reviews
- Finding Research Tools This link opens in a new window
Throughout your schooling, you may need to find different types of evidence and research to support your course work. This guide provides a high-level overview of evidence-based practice as well as the different types of research and study designs. Each page of this guide offers an overview and search tips for finding articles that fit that study design.
Note! If you need help finding a specific type of study, visit the Get Research Help guide to contact the librarians.
What is Evidence-Based Practice?
One of the requirements for your coursework is to find articles that support evidence-based practice. But what exactly is evidence-based practice? Evidence-based practice is a method that uses relevant and current evidence to plan, implement and evaluate patient care. This definition is included in the video below, which explains all the steps of evidence-based practice in greater detail.
- Video - Evidence-based practice: What it is and what it is not. Medcom (Producer), & Cobb, D. (Director). (2017). Evidence-based practice: What it is and what it is not [Streaming Video]. United States of America: Producer. Retrieved from Alexander Street Press Nursing Education Collection
Quantitative and Qualitative Studies
Research is broken down into two different types: quantitative and qualitative. Quantitative studies are all about measurement. They will report statistics of things that can be physically measured like blood pressure, weight and oxygen saturation. Qualitative studies, on the other hand, are about people's experiences and how they feel about something. This type of information cannot be measured using statistics. Both of these types of studies report original research and are considered single studies. Watch the video below for more information.


Study Designs
Some research study types that you will encounter include:
- Case-Control Studies
- Cohort Studies
- Cross-Sectional Studies
Studies that Synthesize Other Studies
Sometimes, a research study will look at the results of many studies and look for trends and draw conclusions. These types of studies include:
- Meta Analyses
Tip! How do you determine the research article's study type or level of evidence? First, look at the article abstract. Most of the time the abstract will have a methodology section, which should tell you what type of study design the researchers are using. If it is not in the abstract, look for the methodology section of the article. It should tell you all about what type of study the researcher is doing and the steps they used to carry out the study.
Read the book below to learn how to read a clinical paper, including the types of study designs you will encounter.
- Search Website
- Library Tech Support
- Services for Colleagues
Chamberlain College of Nursing is owned and operated by Chamberlain University LLC. In certain states, Chamberlain operates as Chamberlain College of Nursing pending state authorization for Chamberlain University.

What this handout is about
This handout will provide a broad overview of gathering and using evidence. It will help you decide what counts as evidence, put evidence to work in your writing, and determine whether you have enough evidence. It will also offer links to additional resources.
Introduction
Many papers that you write in college will require you to make an argument ; this means that you must take a position on the subject you are discussing and support that position with evidence. It’s important that you use the right kind of evidence, that you use it effectively, and that you have an appropriate amount of it. If, for example, your philosophy professor didn’t like it that you used a survey of public opinion as your primary evidence in your ethics paper, you need to find out more about what philosophers count as good evidence. If your instructor has told you that you need more analysis, suggested that you’re “just listing” points or giving a “laundry list,” or asked you how certain points are related to your argument, it may mean that you can do more to fully incorporate your evidence into your argument. Comments like “for example?,” “proof?,” “go deeper,” or “expand” in the margins of your graded paper suggest that you may need more evidence. Let’s take a look at each of these issues—understanding what counts as evidence, using evidence in your argument, and deciding whether you need more evidence.
What counts as evidence?
Before you begin gathering information for possible use as evidence in your argument, you need to be sure that you understand the purpose of your assignment. If you are working on a project for a class, look carefully at the assignment prompt. It may give you clues about what sorts of evidence you will need. Does the instructor mention any particular books you should use in writing your paper or the names of any authors who have written about your topic? How long should your paper be (longer works may require more, or more varied, evidence)? What themes or topics come up in the text of the prompt? Our handout on understanding writing assignments can help you interpret your assignment. It’s also a good idea to think over what has been said about the assignment in class and to talk with your instructor if you need clarification or guidance.
What matters to instructors?
Instructors in different academic fields expect different kinds of arguments and evidence—your chemistry paper might include graphs, charts, statistics, and other quantitative data as evidence, whereas your English paper might include passages from a novel, examples of recurring symbols, or discussions of characterization in the novel. Consider what kinds of sources and evidence you have seen in course readings and lectures. You may wish to see whether the Writing Center has a handout regarding the specific academic field you’re working in—for example, literature , sociology , or history .
What are primary and secondary sources?
A note on terminology: many researchers distinguish between primary and secondary sources of evidence (in this case, “primary” means “first” or “original,” not “most important”). Primary sources include original documents, photographs, interviews, and so forth. Secondary sources present information that has already been processed or interpreted by someone else. For example, if you are writing a paper about the movie “The Matrix,” the movie itself, an interview with the director, and production photos could serve as primary sources of evidence. A movie review from a magazine or a collection of essays about the film would be secondary sources. Depending on the context, the same item could be either a primary or a secondary source: if I am writing about people’s relationships with animals, a collection of stories about animals might be a secondary source; if I am writing about how editors gather diverse stories into collections, the same book might now function as a primary source.
Where can I find evidence?
Here are some examples of sources of information and tips about how to use them in gathering evidence. Ask your instructor if you aren’t sure whether a certain source would be appropriate for your paper.
Print and electronic sources
Books, journals, websites, newspapers, magazines, and documentary films are some of the most common sources of evidence for academic writing. Our handout on evaluating print sources will help you choose your print sources wisely, and the library has a tutorial on evaluating both print sources and websites. A librarian can help you find sources that are appropriate for the type of assignment you are completing. Just visit the reference desk at Davis or the Undergraduate Library or chat with a librarian online (the library’s IM screen name is undergradref).
Observation
Sometimes you can directly observe the thing you are interested in, by watching, listening to, touching, tasting, or smelling it. For example, if you were asked to write about Mozart’s music, you could listen to it; if your topic was how businesses attract traffic, you might go and look at window displays at the mall.
An interview is a good way to collect information that you can’t find through any other type of research. An interview can provide an expert’s opinion, biographical or first-hand experiences, and suggestions for further research.
Surveys allow you to find out some of what a group of people thinks about a topic. Designing an effective survey and interpreting the data you get can be challenging, so it’s a good idea to check with your instructor before creating or administering a survey.
Experiments
Experimental data serve as the primary form of scientific evidence. For scientific experiments, you should follow the specific guidelines of the discipline you are studying. For writing in other fields, more informal experiments might be acceptable as evidence. For example, if you want to prove that food choices in a cafeteria are affected by gender norms, you might ask classmates to undermine those norms on purpose and observe how others react. What would happen if a football player were eating dinner with his teammates and he brought a small salad and diet drink to the table, all the while murmuring about his waistline and wondering how many fat grams the salad dressing contained?
Personal experience
Using your own experiences can be a powerful way to appeal to your readers. You should, however, use personal experience only when it is appropriate to your topic, your writing goals, and your audience. Personal experience should not be your only form of evidence in most papers, and some disciplines frown on using personal experience at all. For example, a story about the microscope you received as a Christmas gift when you were nine years old is probably not applicable to your biology lab report.
Using evidence in an argument
Does evidence speak for itself.
Absolutely not. After you introduce evidence into your writing, you must say why and how this evidence supports your argument. In other words, you have to explain the significance of the evidence and its function in your paper. What turns a fact or piece of information into evidence is the connection it has with a larger claim or argument: evidence is always evidence for or against something, and you have to make that link clear.
As writers, we sometimes assume that our readers already know what we are talking about; we may be wary of elaborating too much because we think the point is obvious. But readers can’t read our minds: although they may be familiar with many of the ideas we are discussing, they don’t know what we are trying to do with those ideas unless we indicate it through explanations, organization, transitions, and so forth. Try to spell out the connections that you were making in your mind when you chose your evidence, decided where to place it in your paper, and drew conclusions based on it. Remember, you can always cut prose from your paper later if you decide that you are stating the obvious.
Here are some questions you can ask yourself about a particular bit of evidence:
- OK, I’ve just stated this point, but so what? Why is it interesting? Why should anyone care?
- What does this information imply?
- What are the consequences of thinking this way or looking at a problem this way?
- I’ve just described what something is like or how I see it, but why is it like that?
- I’ve just said that something happens—so how does it happen? How does it come to be the way it is?
- Why is this information important? Why does it matter?
- How is this idea related to my thesis? What connections exist between them? Does it support my thesis? If so, how does it do that?
- Can I give an example to illustrate this point?
Answering these questions may help you explain how your evidence is related to your overall argument.
How can I incorporate evidence into my paper?
There are many ways to present your evidence. Often, your evidence will be included as text in the body of your paper, as a quotation, paraphrase, or summary. Sometimes you might include graphs, charts, or tables; excerpts from an interview; or photographs or illustrations with accompanying captions.
When you quote, you are reproducing another writer’s words exactly as they appear on the page. Here are some tips to help you decide when to use quotations:
- Quote if you can’t say it any better and the author’s words are particularly brilliant, witty, edgy, distinctive, a good illustration of a point you’re making, or otherwise interesting.
- Quote if you are using a particularly authoritative source and you need the author’s expertise to back up your point.
- Quote if you are analyzing diction, tone, or a writer’s use of a specific word or phrase.
- Quote if you are taking a position that relies on the reader’s understanding exactly what another writer says about the topic.
Be sure to introduce each quotation you use, and always cite your sources. See our handout on quotations for more details on when to quote and how to format quotations.
Like all pieces of evidence, a quotation can’t speak for itself. If you end a paragraph with a quotation, that may be a sign that you have neglected to discuss the importance of the quotation in terms of your argument. It’s important to avoid “plop quotations,” that is, quotations that are just dropped into your paper without any introduction, discussion, or follow-up.
Paraphrasing
When you paraphrase, you take a specific section of a text and put it into your own words. Putting it into your own words doesn’t mean just changing or rearranging a few of the author’s words: to paraphrase well and avoid plagiarism, try setting your source aside and restating the sentence or paragraph you have just read, as though you were describing it to another person. Paraphrasing is different than summary because a paraphrase focuses on a particular, fairly short bit of text (like a phrase, sentence, or paragraph). You’ll need to indicate when you are paraphrasing someone else’s text by citing your source correctly, just as you would with a quotation.
When might you want to paraphrase?
- Paraphrase when you want to introduce a writer’s position, but their original words aren’t special enough to quote.
- Paraphrase when you are supporting a particular point and need to draw on a certain place in a text that supports your point—for example, when one paragraph in a source is especially relevant.
- Paraphrase when you want to present a writer’s view on a topic that differs from your position or that of another writer; you can then refute writer’s specific points in your own words after you paraphrase.
- Paraphrase when you want to comment on a particular example that another writer uses.
- Paraphrase when you need to present information that’s unlikely to be questioned.
When you summarize, you are offering an overview of an entire text, or at least a lengthy section of a text. Summary is useful when you are providing background information, grounding your own argument, or mentioning a source as a counter-argument. A summary is less nuanced than paraphrased material. It can be the most effective way to incorporate a large number of sources when you don’t have a lot of space. When you are summarizing someone else’s argument or ideas, be sure this is clear to the reader and cite your source appropriately.
Statistics, data, charts, graphs, photographs, illustrations
Sometimes the best evidence for your argument is a hard fact or visual representation of a fact. This type of evidence can be a solid backbone for your argument, but you still need to create context for your reader and draw the connections you want them to make. Remember that statistics, data, charts, graph, photographs, and illustrations are all open to interpretation. Guide the reader through the interpretation process. Again, always, cite the origin of your evidence if you didn’t produce the material you are using yourself.
Do I need more evidence?
Let’s say that you’ve identified some appropriate sources, found some evidence, explained to the reader how it fits into your overall argument, incorporated it into your draft effectively, and cited your sources. How do you tell whether you’ve got enough evidence and whether it’s working well in the service of a strong argument or analysis? Here are some techniques you can use to review your draft and assess your use of evidence.
Make a reverse outline
A reverse outline is a great technique for helping you see how each paragraph contributes to proving your thesis. When you make a reverse outline, you record the main ideas in each paragraph in a shorter (outline-like) form so that you can see at a glance what is in your paper. The reverse outline is helpful in at least three ways. First, it lets you see where you have dealt with too many topics in one paragraph (in general, you should have one main idea per paragraph). Second, the reverse outline can help you see where you need more evidence to prove your point or more analysis of that evidence. Third, the reverse outline can help you write your topic sentences: once you have decided what you want each paragraph to be about, you can write topic sentences that explain the topics of the paragraphs and state the relationship of each topic to the overall thesis of the paper.
For tips on making a reverse outline, see our handout on organization .
Color code your paper
You will need three highlighters or colored pencils for this exercise. Use one color to highlight general assertions. These will typically be the topic sentences in your paper. Next, use another color to highlight the specific evidence you provide for each assertion (including quotations, paraphrased or summarized material, statistics, examples, and your own ideas). Lastly, use another color to highlight analysis of your evidence. Which assertions are key to your overall argument? Which ones are especially contestable? How much evidence do you have for each assertion? How much analysis? In general, you should have at least as much analysis as you do evidence, or your paper runs the risk of being more summary than argument. The more controversial an assertion is, the more evidence you may need to provide in order to persuade your reader.
Play devil’s advocate, act like a child, or doubt everything
This technique may be easiest to use with a partner. Ask your friend to take on one of the roles above, then read your paper aloud to them. After each section, pause and let your friend interrogate you. If your friend is playing devil’s advocate, they will always take the opposing viewpoint and force you to keep defending yourself. If your friend is acting like a child, they will question every sentence, even seemingly self-explanatory ones. If your friend is a doubter, they won’t believe anything you say. Justifying your position verbally or explaining yourself will force you to strengthen the evidence in your paper. If you already have enough evidence but haven’t connected it clearly enough to your main argument, explaining to your friend how the evidence is relevant or what it proves may help you to do so.
Common questions and additional resources
- I have a general topic in mind; how can I develop it so I’ll know what evidence I need? And how can I get ideas for more evidence? See our handout on brainstorming .
- Who can help me find evidence on my topic? Check out UNC Libraries .
- I’m writing for a specific purpose; how can I tell what kind of evidence my audience wants? See our handouts on audience , writing for specific disciplines , and particular writing assignments .
- How should I read materials to gather evidence? See our handout on reading to write .
- How can I make a good argument? Check out our handouts on argument and thesis statements .
- How do I tell if my paragraphs and my paper are well-organized? Review our handouts on paragraph development , transitions , and reorganizing drafts .
- How do I quote my sources and incorporate those quotes into my text? Our handouts on quotations and avoiding plagiarism offer useful tips.
- How do I cite my evidence? See the UNC Libraries citation tutorial .
- I think that I’m giving evidence, but my instructor says I’m using too much summary. How can I tell? Check out our handout on using summary wisely.
- I want to use personal experience as evidence, but can I say “I”? We have a handout on when to use “I.”
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
Lunsford, Andrea A., and John J. Ruszkiewicz. 2016. Everything’s an Argument , 7th ed. Boston: Bedford/St Martin’s.
Miller, Richard E., and Kurt Spellmeyer. 2016. The New Humanities Reader , 5th ed. Boston: Cengage.
University of Maryland. 2019. “Research Using Primary Sources.” Research Guides. Last updated October 28, 2019. https://lib.guides.umd.edu/researchusingprimarysources .
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
12.1 Introducing Research and Research Evidence
Learning outcomes.
By the end of this section, you will be able to:
- Articulate how research evidence and sources are key rhetorical concepts in presenting a position or an argument.
- Locate and distinguish between primary and secondary research materials.
- Implement methods and technologies commonly used for research and communication within various fields.
The writing tasks for this chapter and the next two chapters are based on argumentative research. However, not all researched evidence (data) is presented in the same genre. You may need to gather evidence for a poster, a performance, a story, an art exhibit, or even an architectural design. Although the genre may vary, you usually will be required to present a perspective , or viewpoint, about a debatable issue and persuade readers to support the “validity of your viewpoint,” as discussed in Position Argument: Practicing the Art of Rhetoric . Remember, too, that a debatable issue is one that has more than a single perspective and is subject to disagreement.
The Research Process
Although individual research processes are rhetorically situated, they share some common aspects:
- Interest. The researcher has a genuine interest in the topic. It may be difficult to fake curiosity, but it is possible to develop it. Some academic assignments will allow you to pursue issues that are personally important to you; others will require you to dive into the research first and generate interest as you go.
- Questions. The researcher asks questions. At first, these questions are general. However, as researchers gain more knowledge, the questions become more sharply focused. No matter what your research assignment is, begin by articulating questions, find out where the answers lead, and then ask still more questions.
- Answers. The researcher seeks answers from people as well as from print and other media. Research projects profit when you ask knowledgeable people, such as librarians and other professionals, to help you answer questions or point you in directions to find answers. Information about research is covered more extensively in Research Process: Accessing and Recording Information and Annotated Bibliography: Gathering, Evaluating, and Documenting Sources .
- Field research. The researcher conducts field research. Field research allows researchers not only to ask questions of experts but also to observe and experience directly. It allows researchers to generate original data. No matter how much other people tell you, your knowledge increases through personal observations. In some subject areas, field research is as important as library or database research. This information is covered more extensively in Research Process: Accessing and Recording Information .
- Examination of texts. The researcher examines texts. Consulting a broad range of texts—such as magazines, brochures, newspapers, archives, blogs, videos, documentaries, or peer-reviewed journals—is crucial in academic research.
- Evaluation of sources. The researcher evaluates sources. As your research progresses, you will double-check information to find out whether it is confirmed by more than one source. In informal research, researchers evaluate sources to ensure that the final decision is satisfactory. Similarly, in academic research, researchers evaluate sources to ensure that the final product is accurate and convincing. Previewed here, this information is covered more extensively in Research Process: Accessing and Recording Information .
- Writing. The researcher writes. The writing during the research process can take a range of forms: from notes during library, database, or field work; to journal reflections on the research process; to drafts of the final product. In practical research, writing helps researchers find, remember, and explore information. In academic research, writing is even more important because the results must be reported accurately and thoroughly.
- Testing and Experimentation. The researcher tests and experiments. Because opinions vary on debatable topics and because few research topics have correct or incorrect answers, it is important to test and conduct experiments on possible hypotheses or solutions.
- Synthesis. The researcher synthesizes. By combining information from various sources, researchers support claims or arrive at new conclusions. When synthesizing, researchers connect evidence and ideas, both original and borrowed. Accumulating, sorting, and synthesizing information enables researchers to consider what evidence to use in support of a thesis and in what ways.
- Presentation. The researcher presents findings in an interesting, focused, and well-documented product.
Types of Research Evidence
Research evidence usually consists of data, which comes from borrowed information that you use to develop your thesis and support your organizational structure and reasoning. This evidence can take a range of forms, depending on the type of research conducted, the audience, and the genre for reporting the research.
Primary Research Sources
Although precise definitions vary somewhat by discipline, primary data sources are generally defined as firsthand accounts, such as texts or other materials produced by someone drawing from direct experience or observation. Primary source documents include, but are not limited to, personal narratives and diaries; eyewitness accounts; interviews; original documents such as treaties, official certificates, and government documents detailing laws or acts; speeches; newspaper coverage of events at the time they occurred; observations; and experiments. Primary source data is, in other words, original and in some way conducted or collected primarily by the researcher. The Research Process: Where to Look for Existing Sources and Compiling Sources for an Annotated Bibliography contain more information on both primary and secondary sources.
Secondary Research Sources
Secondary sources , on the other hand, are considered at least one step removed from the experience. That is, they rely on sources other than direct observation or firsthand experience. Secondary sources include, but are not limited to, most books, articles online or in databases, and textbooks (which are sometimes classified as tertiary sources because, like encyclopedias and other reference works, their primary purpose might be to summarize or otherwise condense information). Secondary sources regularly cite and build upon primary sources to provide perspective and analysis. Effective use of researched evidence usually includes both primary and secondary sources. Works of history, for example, draw on a large range of primary and secondary sources, citing, analyzing, and synthesizing information to present as many perspectives of a past event in as rich and nuanced a way as possible.
It is important to note that the distinction between primary and secondary sources depends in part on their use: that is, the same document can be both a primary source and a secondary source. For example, if Scholar X wrote a biography about Artist Y, the biography would be a secondary source about the artist and, at the same time, a primary source about the scholar.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/writing-guide/pages/1-unit-introduction
- Authors: Michelle Bachelor Robinson, Maria Jerskey, featuring Toby Fulwiler
- Publisher/website: OpenStax
- Book title: Writing Guide with Handbook
- Publication date: Dec 21, 2021
- Location: Houston, Texas
- Book URL: https://openstax.org/books/writing-guide/pages/1-unit-introduction
- Section URL: https://openstax.org/books/writing-guide/pages/12-1-introducing-research-and-research-evidence
© Dec 19, 2023 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Using Research and Evidence

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
What type of evidence should I use?
There are two types of evidence.
First hand research is research you have conducted yourself such as interviews, experiments, surveys, or personal experience and anecdotes.
Second hand research is research you are getting from various texts that has been supplied and compiled by others such as books, periodicals, and Web sites.
Regardless of what type of sources you use, they must be credible. In other words, your sources must be reliable, accurate, and trustworthy.
How do I know if a source is credible?
You can ask the following questions to determine if a source is credible.
Who is the author? Credible sources are written by authors respected in their fields of study. Responsible, credible authors will cite their sources so that you can check the accuracy of and support for what they've written. (This is also a good way to find more sources for your own research.)
How recent is the source? The choice to seek recent sources depends on your topic. While sources on the American Civil War may be decades old and still contain accurate information, sources on information technologies, or other areas that are experiencing rapid changes, need to be much more current.
What is the author's purpose? When deciding which sources to use, you should take the purpose or point of view of the author into consideration. Is the author presenting a neutral, objective view of a topic? Or is the author advocating one specific view of a topic? Who is funding the research or writing of this source? A source written from a particular point of view may be credible; however, you need to be careful that your sources don't limit your coverage of a topic to one side of a debate.
What type of sources does your audience value? If you are writing for a professional or academic audience, they may value peer-reviewed journals as the most credible sources of information. If you are writing for a group of residents in your hometown, they might be more comfortable with mainstream sources, such as Time or Newsweek . A younger audience may be more accepting of information found on the Internet than an older audience might be.
Be especially careful when evaluating Internet sources! Never use Web sites where an author cannot be determined, unless the site is associated with a reputable institution such as a respected university, a credible media outlet, government program or department, or well-known non-governmental organizations. Beware of using sites like Wikipedia , which are collaboratively developed by users. Because anyone can add or change content, the validity of information on such sites may not meet the standards for academic research.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Course: LSAT > Unit 1
- Getting started with Logical Reasoning
- Introduction to arguments
- Catalog of question types
- Types of conclusions
Types of evidence
- Types of flaws
- Identify the conclusion | Quick guide
- Identify the conclusion | Learn more
- Identify the conclusion | Examples
- Identify an entailment | Quick guide
- Identify an entailment | Learn more
- Strongly supported inferences | Quick guide
- Strongly supported inferences | Learn more
- Disputes | Quick guide
- Disputes | Learn more
- Identify the technique | Quick guide
- Identify the technique | Learn more
- Identify the role | Quick guide
- Identify the role | learn more
- Identify the principle | Quick guide
- Identify the principle | Learn more
- Match structure | Quick guide
- Match structure | Learn more
- Match principles | Quick guide
- Match principles | Learn more
- Identify a flaw | Quick guide
- Identify a flaw | Learn more
- Match a flaw | Quick guide
- Match a flaw | Learn more
- Necessary assumptions | Quick guide
- Necessary assumptions | Learn more
- Sufficient assumptions | Quick guide
- Sufficient assumptions | Learn more
- Strengthen and weaken | Quick guide
- Strengthen and weaken | Learn more
- Helpful to know | Quick guide
- Helpful to know | learn more
- Explain or resolve | Quick guide
- Explain or resolve | Learn more
Types of Evidence
- This can help you avoid getting “lost” in the words; if you’re reading actively and recognizing what type of evidence you’re looking at, then you’re more likely to stay focused.
- Different types of evidence are often associated with specific types of assumptions or flaws, so if a question presents a classic evidence structure, you may be able to find the answer more quickly.
Common Evidence Types
Examples as evidence.
- [Paola is the best athlete in the state.] After all, Paola has won medals in 8 different Olympic sports.
- Paola beat last year's decathlon state champion on Saturday, so [she is the best athlete in the state].
What others say
- [Paola is the best athlete in the state.] We know this because the most highly-acclaimed sports magazine has named her as such.
- Because the population voted Paola the Best Athlete in the state in a landslide, [it would be absurd to claim that anyone else is the best athlete in the state].
Using the past
- [Paola is the best athlete in the state.] She must be, since she won the state championships last year, two years ago, three years ago, and four years ago.
- [Paola is the best athlete in the state], because she won the most athletic awards. Look at Jude, who's currently the Best Chef in the State because he won the most cooking awards.
Generalizing from a Sample
- [Paola is the best athlete in the state], because she won every local tournament in every spring sport.
Common Rebuttal Structures
Counterexamples, alternate possibilities, other types of argument structures, conditional.
- Penguins win → Flyers make big mistake
- Flyers make big mistake → coach tired
- Friday → coach is not tired
Causation based on correlation
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

- SMU Libraries
- Scholarship & Research
- Teaching & Learning
- Bridwell Library
- Business Library
- DeGolyer Library
- Fondren Library
- Hamon Arts Library
- Underwood Law Library
- Fort Burgwin Library
- Exhibits & Digital Collections
- SMU Scholar
- Special Collections & Archives
- Connect With Us
- Research Guides by Subject
- How Do I . . . ? Guides
- Find Your Librarian
- Writing Support
Types of Research Papers: Overview
- Types of Research Questions
A research paper is simply a piece of writing that uses outside sources. There are different types of research papers with varying purposes and expectations for sourcing.
While this guide explains those differences broadly, disciplines and assignments vary. Ask your professor for clarification on the purpose and types of appropriate research questions and sources.
Need More Help?
Related guides.
- Literature Reviews
- Annotated Bibliographies
- Starting Your Research
Research and Writing Lab
Need last minute help but didn't book an appointment? Every week we offer online drop-in labs.
Tuesdays 3:00pm - 4:30pm via Zoom @ https://smu.zoom.us/j/92637892352 and in-person, Fondren Red 1st floor (near elevators)
- Next: Types of Research Questions >>
- Last Updated: Apr 19, 2024 4:29 PM
- URL: https://guides.smu.edu/researchpapertypes
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Major UK non-commercial sponsors’ efforts to reduce research waste: a mixed-methods study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-9346-3319 Till Bruckner 1 , 2 ,
- Aminul Schuster 3 ,
- Belén Chavarría 4 ,
- Carolina Cruz 5 ,
- Fabiola Karely Lizárraga Illán 5 ,
- Ronak Borana 6 ,
- Tungamirai Ishe Bvute 7 ,
- Daniel Sánchez 5
- 1 UiT The Arctic University , Tromso , Norway
- 2 TranspariMED , Bristol , UK
- 3 University of Westminster , London , UK
- 4 National Autonomous University of Nicaragua-Leon , Leon , Nicaragua
- 5 Universidad de Guadalajara , Guadalajara , Mexico
- 6 XLRI , Jamshedpur , Jharkhand , India
- 7 Independent Researcher , Dublin , Ireland
- Correspondence to Dr Till Bruckner, UiT The Arctic University of Norway, Tromso, Troms, Norway; tillbruckner{at}gmail.com
https://doi.org/10.1136/bmjebm-2023-112540
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- Health Services Research
- PUBLIC HEALTH
Worldwide, a significant proportion of clinical trials end up as costly research waste because their results are never made public. 1–3 The resulting gaps in the medical evidence base harm patients and undermine public health. 4 The Declaration of Helsinki and the WHO both call for all clinical trial results to be made public. 5 6
In the wake of a 2018 UK parliamentary enquiry, non-commercial clinical trial sponsors in the UK substantially improved outcome reporting for drug trials (Clinical Trials of Investigative Medicinal Products, CTIMPs) by uploading the summary results of many CTIMPs onto the European Union Clinical Trials Register (EUCTR) which exclusively lists drug trials. However, these efforts typically did not extend to other types of trials, which are listed on other trial registries.
This study assesses the current publication status of 145 clinical trials that are not CTIMPs that were sponsored by ten major UK non-commercial sponsors and were completed or terminated in 2017, allowing a 5-year follow-up period for publication.
The lead researcher (TB) identified the 10 most prolific non-commercial sponsors of clinical trials in the UK by accessing the EU Trials Tracker on 27 October 2022, employing the number of CTIMPs run by each sponsor as a proxy indicator of overall trial activity. 7 The lead researcher then identified other clinical trials run by these sponsors on the two other trial registries commonly used by UK sponsors, ClinicalTrials.gov and ISRCTN.
The final sample consists of 145 interventional clinical trials run by ten major non-commercial UK sponsors that are registered on ClinicalTrials.gov or ISRCTN and that were completed or terminated during 2017. These trials had a combined (actual or planned) enrolment of 34 102 patients.
The study protocol defined trial outcomes published as tabular summary results on clinical trial registries, as articles published in peer-reviewed journals, or as PhD theses as ‘fully reported’. Conference abstracts, posters, presentation slides and other documents containing trial outcomes were classified as ‘grey literature’.
During the initial data extraction, 25/145 trials were identified as having tabular summary results available on ClinicalTrials.gov; these were marked as ‘reported’ prior to the literature search. The study team then used a three-step process for locating publications for the remaining 120/145 trials. The search strategy is detailed in the study protocol ( https://osf.io/rh3m9 ). The lead researcher reviewed all search results.
The lead researcher contacted all sponsors with a list of their trials asking them to verify the publication status. All sponsors responded.
The study protocol was registered on the Open Science Framework (OSF, https://osf.io/rh3m9 ). An ethics waiver was secured. This study was funded by the charity HealthSense UK. The authors have no conflicts of interest to declare. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research. The outcomes of this study are reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for cohort studies. The study protocol, dataset, literature search guide, ethics waiver and sponsors’ responses are available on GitHub ( https://github.com/TillBruckner/UKtrials ).
In total, 116/145 trials (80%) had reported results on a registry, in the academic literature, or in a PhD thesis. The outcomes of 11/145 trials (8%) had been reported in the grey literature. Results for 18/145 trials (12%) remained completely unpublished (see table 1 ).
- View inline
Number and percentage of trials that have not fully reported results
In total, 637 people were enrolled in the 18 clinical trials that remained completely unreported. Conservatively assuming an average cost per trial of £500 000, 8 the total aggregate cost of those 18 trials was £9 million.
Sponsors indicated that they still planned to make public the results of the 14/18 unreported trials. For the remaining 4/18 trials, sponsors indicated that there were no data of value to publish due to early termination of the trial (3/18 trials) or data quality issues (1/18 trials).
This study found a registry or journal publication rate of 80% for this sample of clinical trials after a follow-up period of 5–6 years. This rate is far higher than what comparable studies have found in other countries. 1 2
However, the findings cannot be generalised to all UK university-sponsored non-CTIMPs as the study sample was small and restricted to large sponsors. Also, at least one sponsor rapidly reported several results following our outreach. A key strength is that we succeeded in verifying the publication status of every clinical trial with the responsible sponsor.
The is currently no UK legal requirement to report non-CTIMP results. Nonetheless, all sponsors initiated efforts to publish missing results following our outreach. The entire budget of this project was far less than the cost of a typical clinical trial. Replicating this project on a larger scale could be a highly cost-effective way to reduce research waste.
The UK’s national #MakeItPublic strategy commits the UK Health Research Authority (HRA) to identifying and following up on all future unreported clinical trials (see table 2 ). 9 The HRA has so far failed to deliver on this promise. Full implementation of the strategy could significantly and sustainably reduce future research waste in the UK. Requiring all trial results to be made public on trial registries, as recommended by the WHO, could lower HRA monitoring costs and improve the speed, accuracy and completeness of outcome reporting. 10
Unreported clinical trials and future publication plans
Ethics statements
Patient consent for publication.
Not applicable.
- Nelson JT ,
- Puplampu-Dove Y , et al
- Franzen DL ,
- Carlisle BG ,
- Salholz-Hillel M , et al
- Rodgers F ,
- Pepperrell T ,
- Keestra S , et al
- World Medical Association
- World Health Assembly
- Goldacre B ,
- DeVito NJ ,
- Heneghan C , et al
- Harrison J ,
- Vrijens F , et al
- UK Health Research Authority
- World Health Organization
X @TranspariMED, @aminul_schuster, @BelnChavarria03
Contributors TB conceived and designed the study, wrote the protocol and drafted the manuscript. AS, BC, CC, FKLI, RB, TIB and DS extracted and analysed the data, and critically reviewed the manuscript prior to submission. All authors approved the final manuscript and agreed to be held accountable for its contents.
Funding This study was funded by HealthSense UK (none).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Anaesth
- v.60(9); 2016 Sep
Types of studies and research design
Mukul chandra kapoor.
Department of Anesthesiology, Max Smart Super Specialty Hospital, New Delhi, India
Medical research has evolved, from individual expert described opinions and techniques, to scientifically designed methodology-based studies. Evidence-based medicine (EBM) was established to re-evaluate medical facts and remove various myths in clinical practice. Research methodology is now protocol based with predefined steps. Studies were classified based on the method of collection and evaluation of data. Clinical study methodology now needs to comply to strict ethical, moral, truth, and transparency standards, ensuring that no conflict of interest is involved. A medical research pyramid has been designed to grade the quality of evidence and help physicians determine the value of the research. Randomised controlled trials (RCTs) have become gold standards for quality research. EBM now scales systemic reviews and meta-analyses at a level higher than RCTs to overcome deficiencies in the randomised trials due to errors in methodology and analyses.
INTRODUCTION
Expert opinion, experience, and authoritarian judgement were the norm in clinical medical practice. At scientific meetings, one often heard senior professionals emphatically expressing ‘In my experience,…… what I have said is correct!’ In 1981, articles published by Sackett et al . introduced ‘critical appraisal’ as they felt a need to teach methods of understanding scientific literature and its application at the bedside.[ 1 ] To improve clinical outcomes, clinical expertise must be complemented by the best external evidence.[ 2 ] Conversely, without clinical expertise, good external evidence may be used inappropriately [ Figure 1 ]. Practice gets outdated, if not updated with current evidence, depriving the clientele of the best available therapy.

Triad of evidence-based medicine
EVIDENCE-BASED MEDICINE
In 1971, in his book ‘Effectiveness and Efficiency’, Archibald Cochrane highlighted the lack of reliable evidence behind many accepted health-care interventions.[ 3 ] This triggered re-evaluation of many established ‘supposed’ scientific facts and awakened physicians to the need for evidence in medicine. Evidence-based medicine (EBM) thus evolved, which was defined as ‘the conscientious, explicit and judicious use of the current best evidence in making decisions about the care of individual patients.’[ 2 ]
The goal of EBM was scientific endowment to achieve consistency, efficiency, effectiveness, quality, safety, reduction in dilemma and limitation of idiosyncrasies in clinical practice.[ 4 ] EBM required the physician to diligently assess the therapy, make clinical adjustments using the best available external evidence, ensure awareness of current research and discover clinical pathways to ensure best patient outcomes.[ 5 ]
With widespread internet use, phenomenally large number of publications, training and media resources are available but determining the quality of this literature is difficult for a busy physician. Abstracts are available freely on the internet, but full-text articles require a subscription. To complicate issues, contradictory studies are published making decision-making difficult.[ 6 ] Publication bias, especially against negative studies, makes matters worse.
In 1993, the Cochrane Collaboration was founded by Ian Chalmers and others to create and disseminate up-to-date review of randomised controlled trials (RCTs) to help health-care professionals make informed decisions.[ 7 ] In 1995, the American College of Physicians and the British Medical Journal Publishing Group collaborated to publish the journal ‘Evidence-based medicine’, leading to the evolution of EBM in all spheres of medicine.
MEDICAL RESEARCH
Medical research needs to be conducted to increase knowledge about the human species, its social/natural environment and to combat disease/infirmity in humans. Research should be conducted in a manner conducive to and consistent with dignity and well-being of the participant; in a professional and transparent manner; and ensuring minimal risk.[ 8 ] Research thus must be subjected to careful evaluation at all stages, i.e., research design/experimentation; results and their implications; the objective of the research sought; anticipated benefits/dangers; potential uses/abuses of the experiment and its results; and on ensuring the safety of human life. Table 1 lists the principles any research should follow.[ 8 ]
General principles of medical research

Types of study design
Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. Three main areas in primary research are basic medical research, clinical research and epidemiological research [ Figure 2 ]. Basic research includes fundamental research in fields shown in Figure 2 . In almost all studies, at least one independent variable is varied, whereas the effects on the dependent variables are investigated. Clinical studies include observational studies and interventional studies and are subclassified as in Figure 2 .

Classification of types of medical research
Interventional clinical study is performed with the purpose of studying or demonstrating clinical or pharmacological properties of drugs/devices, their side effects and to establish their efficacy or safety. They also include studies in which surgical, physical or psychotherapeutic procedures are examined.[ 9 ] Studies on drugs/devices are subject to legal and ethical requirements including the Drug Controller General India (DCGI) directives. They require the approval of DCGI recognized Ethics Committee and must be performed in accordance with the rules of ‘Good Clinical Practice’.[ 10 ] Further details are available under ‘Methodology for research II’ section in this issue of IJA. In 2004, the World Health Organization advised registration of all clinical trials in a public registry. In India, the Clinical Trials Registry of India was launched in 2007 ( www.ctri.nic.in ). The International Committee of Medical Journal Editors (ICMJE) mandates its member journals to publish only registered trials.[ 11 ]
Observational clinical study is a study in which knowledge from treatment of persons with drugs is analysed using epidemiological methods. In these studies, the diagnosis, treatment and monitoring are performed exclusively according to medical practice and not according to a specified study protocol.[ 9 ] They are subclassified as per Figure 2 .
Epidemiological studies have two basic approaches, the interventional and observational. Clinicians are more familiar with interventional research, whereas epidemiologists usually perform observational research.
Interventional studies are experimental in character and are subdivided into field and group studies, for example, iodine supplementation of cooking salt to prevent hypothyroidism. Many interventions are unsuitable for RCTs, as the exposure may be harmful to the subjects.
Observational studies can be subdivided into cohort, case–control, cross-sectional and ecological studies.
- Cohort studies are suited to detect connections between exposure and development of disease. They are normally prospective studies of two healthy groups of subjects observed over time, in which one group is exposed to a specific substance, whereas the other is not. The occurrence of the disease can be determined in the two groups. Cohort studies can also be retrospective
- Case–control studies are retrospective analyses performed to establish the prevalence of a disease in two groups exposed to a factor or disease. The incidence rate cannot be calculated, and there is also a risk of selection bias and faulty recall.
Secondary research
Narrative review.
An expert senior author writes about a particular field, condition or treatment, including an overview, and this information is fortified by his experience. The article is in a narrative format. Its limitation is that one cannot tell whether recommendations are based on author's clinical experience, available literature and why some studies were given more emphasis. It can be biased, with selective citation of reports that reinforce the authors' views of a topic.[ 12 ]
Systematic review
Systematic reviews methodically and comprehensively identify studies focused on a specified topic, appraise their methodology, summate the results, identify key findings and reasons for differences across studies, and cite limitations of current knowledge.[ 13 ] They adhere to reproducible methods and recommended guidelines.[ 14 ] The methods used to compile data are explicit and transparent, allowing the reader to gauge the quality of the review and the potential for bias.[ 15 ]
A systematic review can be presented in text or graphic form. In graphic form, data of different trials can be plotted with the point estimate and 95% confidence interval for each study, presented on an individual line. A properly conducted systematic review presents the best available research evidence for a focused clinical question. The review team may obtain information, not available in the original reports, from the primary authors. This ensures that findings are consistent and generalisable across populations, environment, therapies and groups.[ 12 ] A systematic review attempts to reduce bias identification and studies selection for review, using a comprehensive search strategy and specifying inclusion criteria. The strength of a systematic review lies in the transparency of each phase and highlighting the merits of each decision made, while compiling information.
Meta-analysis
A review team compiles aggregate-level data in each primary study, and in some cases, data are solicited from each of the primary studies.[ 16 , 17 ] Although difficult to perform, individual patient meta-analyses offer advantages over aggregate-level analyses.[ 18 ] These mathematically pooled results are referred to as meta-analysis. Combining data from well-conducted primary studies provide a precise estimate of the “true effect.”[ 19 ] Pooling the samples of individual studies increases overall sample size, enhances statistical analysis power, reduces confidence interval and thereby improves statistical value.
The structured process of Cochrane Collaboration systematic reviews has contributed to the improvement of their quality. For the meta-analysis to be definitive, the primary RCTs should have been conducted methodically. When the existing studies have important scientific and methodological limitations, such as smaller sized samples, the systematic review may identify where gaps exist in the available literature.[ 20 ] RCTs and systematic review of several randomised trials are less likely to mislead us, and thereby help judge whether an intervention is better.[ 2 ] Practice guidelines supported by large RCTs and meta-analyses are considered as ‘gold standard’ in EBM. This issue of IJA is accompanied by an editorial on Importance of EBM on research and practice (Guyat and Sriganesh 471_16).[ 21 ] The EBM pyramid grading the value of different types of research studies is shown in Figure 3 .

The evidence-based medicine pyramid
In the last decade, a number of studies and guidelines brought about path-breaking changes in anaesthesiology and critical care. Some guidelines such as the ‘Surviving Sepsis Guidelines-2004’[ 22 ] were later found to be flawed and biased. A number of large RCTs were rejected as their findings were erroneous. Another classic example is that of ENIGMA-I (Evaluation of Nitrous oxide In the Gas Mixture for Anaesthesia)[ 23 ] which implicated nitrous oxide for poor outcomes, but ENIGMA-II[ 24 , 25 ] conducted later, by the same investigators, declared it as safe. The rise and fall of the ‘tight glucose control’ regimen was similar.[ 26 ]
Although RCTs are considered ‘gold standard’ in research, their status is at crossroads today. RCTs have conflicting interests and thus must be evaluated with careful scrutiny. EBM can promote evidence reflected in RCTs and meta-analyses. However, it cannot promulgate evidence not reflected in RCTs. Flawed RCTs and meta-analyses may bring forth erroneous recommendations. EBM thus should not be restricted to RCTs and meta-analyses but must involve tracking down the best external evidence to answer our clinical questions.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- Open access
- Published: 18 April 2024
Dissociation of prepotent response inhibition and interference control in problematic internet use: evidence from the Go/No-Go and Flanker tasks
- Shao-Shuai Zhang 1 , 2 ,
- Yu-qing Zhong 1 , 2 ,
- Xu Li 1 , 2 &
- Ming Peng 1 , 2
BMC Psychology volume 12 , Article number: 216 ( 2024 ) Cite this article
Metrics details
Problematic Internet Use (PIU), characterized by failures to control the overuse of internet, is associated with a range of functional impairments. However, there is limited research on the specific impact of PIU on inhibitory control functions, particularly in terms of differentiating between prepotent response inhibition and interference control. Therefore, the main objective of this study is to investigate these two components of inhibitory control in individuals with PIU.
Thirty participants who met the PIU criteria and 30 control participants were included in the present study. All participants completed the Go/No-Go and Flanker tasks, in which internet-related images and words were used as task stimuli.
In the Go/No-Go task, all participants exhibited poorer performance in inhibiting internet-related stimuli compared to internet-unrelated stimuli, during the No-Go trials. In the Flanker task, results revealed a three-way interaction of Group, Stimulus type and Congruency. Specifically, in the incongruent condition, participants with PIU exhibited slower responses for internet-unrelated targets compared to internet-related targets, whereas no similar effect was observed among individuals with low internet use.
Conclusions
The findings suggest that difficulties in controlling the interference effect of internet-related information represent a key dysfunction in inhibitory control of PIU.
Peer Review reports
The global internet user population has reached a staggering 5.19 billion individuals, representing approximately 64.5% of the total global population (Datareportal; https://datareportal.com/global-digitaloverview ). While this digital expansion has facilitated vast opportunities such as information exchange and social connection, it has also been paralleled with issues related to internet overuse. Problematic Internet Use (PIU) is characterized by excessive and compulsive engagement with the internet, leading individuals to exhibit obsessive preoccupation, difficulty in reducing or discontinuing online activities, and a tendency to isolate themselves from real-life interactions [ 1 ]. Contrasting with Internet Gaming Disorder (IGD), which is specifically focused on excessive gaming online, PIU provides a broader perspective on internet-related disorders [ 2 ]. Importantly, PIU has been recognized as a prominent contributing factor to a range of functional impairments and mental health concerns. For example, PIU has been associated with heightened sleep disturbances, elevated levels of depressive and anxiety symptoms, and increased feelings of loneliness [ 3 ], all of which further exert detrimental effects on individuals’ academic and work performance, as well as their overall daily functioning.
Inhibitory control, a subcomponent of executive functions, encompasses the ability to suppress habitual, dominant, and prepotent responses that are deemed inappropriate within a given context. It also involves the capacity to resist interference caused by distractors [ 4 ]. Deficits in inhibitory control are proposed to underpin cognitive dysfunctions in many mental disorders such as attention deficit hyperactivity disorder, obsessive compulsive disorder and substance abuse. Importantly, previous research has established a connection between PIU and prominent inhibitory control failures, for example, individuals with PIU experience difficulties in controlling their impulsivity to use the internet, struggle to resist cravings for prolonged internet use, and display withdrawal-like symptoms when unable to access the internet. Empirical studies have reported positive correlations between the self-report severity of impulsivity symptoms and PIU [ 5 , 6 ]. Additionally, it has been proposed that the impulsive symptoms observed in individuals with PIU may be indicative of an underlying deficiency in inhibitory control [ 7 ], implicating that disrupted inhibitory control serving as a fundamental cognitive vulnerability factor for PIU.
It is noteworthy that inhibitory control, although often treated as a unitary construct, is increasingly recognized as a multifaceted phenomenon according to research from cognitive and behavioral neuroscience, and the two most commonly recognized components are prepotent response inhibition and interference control [ 8 , 9 ]. Prepotent response inhibition refers to the suppression of dominant responses, involving the inhibition of prepotent but inappropriate responses. On the other hand, interference control refers to the ability to suppress interference from goal-irrelevant information. This distinction is supported by numerous studies in both healthy populations and various clinical samples, such as schizophrenia, autism spectrum disorder, depression, and obsessive-compulsive disorder. The Go/No-Go task and the stop-signal task are consistently classified as measures of prepotent response inhibition [ 10 ], in which participants are required to cancel or withdraw ongoing responses, while the Flanker task, the Simon task are widely used as indices of interference control, in which participants are instructed to exert inhibitory control by suppressing interference from irrelevant Flankers. In addition, the Stroop task, which involves conflict monitoring and interference suppression, has also been used to assess interference control, despite the ongoing controversies surrounding the inhibitory mechanisms that underpin this task and its limited convergent validity when compared to other measures [ 8 , 11 ].
The majority of previous research has explored the relationship between PIU and prepotent response inhibition [ 10 ]. For example, participants with PIU show poor performance than controls in the Go/No-Go task [ 12 , 13 ] and have lower proportion of successful stop in the stop-signal task [ 14 ]. However, some other studies have reported no differences between participants with and without PIU on response inhibition, either in the Go/No-Go or in the stop-signal task [ 2 , 15 ]. The inconsistency might be related to task materials. For example, in the study by Nie et al. [ 16 ], it was found participants with PIU made more errors in stop trials for the internet-related words compared to internet-unrelated words. In the study by Chamberlain et al. [ 15 ], arrows, which were internet-unrelated, were used in the stop-signal task, and no differences on stop-signal reaction times were found between participants with PIU and controls. Studies using digit or letter as task materials in the Go/No-Go task also found no differences regarding accuracy of No-Go trials or response times of Go trials between participants with PIU and controls [ 2 , 17 ]. These results highlight that PIU might be related to specific deficits in inhibiting internet-related information rather than general deficits in prepotent response inhibition.
With regard to interference control function in PIU, very few studies have been conducted. In a study using the Flanker task, it is found that individuals with PIU performed worse than healthy controls, and their response times in error trials were shorter than did controls [ 18 ], suggesting difficulties in inhibiting irrelevant stimuli in PIU. Similarly, another study found that poorer performances on the Flanker task predicted more severe symptoms of PIU in college students [ 19 ]. Furthermore, higher level of social media use was associated with higher levels of self-report distraction in daily life, and participants with excessive social media use were slower in the color-word Stroop task compared to controls [ 3 ]. However, another study did not find a significant effect of social media use on Stroop task performance [ 20 ]. How internet-related information interfered with target processing in PIU individuals remains inadequately investigated.
Taken together, whether and how PIU is associated with deficits in prepotent response inhibition and interference control remains unclear and inconclusive. Moreover, although several studies have separately examined the two inhibitory control components among individuals with PIU, there is a lack of research that simultaneously examines both functions to better understand their respective roles in PIU. In addition, among the limited studies that have investigated interference control function in PIU, none of them utilized internet-related stimuli thus limiting the generalizability of these findings to the processing of internet-related information. To address these gaps and achieve a more comprehensive understanding of inhibitory control in PIU, the current study examined prepotent response inhibition and interference control in a group of participants with PIU using the Go/No-Go and the Flanker tasks, with internet-related and internet-unrelated images and words as task stimuli. We hypothesized that participants with PIU would exhibit impaired performance in both the Go/No-Go and the Flanker tasks than controls, particularly in conditions involving internet-related stimuli.
Participants
A total of 292 undergraduates were invited to complete the Revised Chinese Internet Addiction Scale (CIAS-R) [ 21 ]. The CIAS-R has been commonly used for assessing PIU in Chinese populations, it consists of 26 items on a four-point Likert scale, 1 being ‘does not match my experience’ and 4 indicating ‘definitely matches my experience’, with higher total scores indicating a greater severity of PIU. In line with previous studies [ 16 ], individuals who scored higher than 53 on the CIAS-R were categorized as individuals with PIU, and those who scored lower than 46 were classified as controls. Of our participants who completed the CIAS-R-2 ( n = 292), 37.7% met the inclusion criteria for PIU and 30.1% met the inclusion criteria for controls and were invited to participate in our study. Finally, thirty participants (12 female) who met the PIU criteria and 30 control participants (17 female) were included in the present study. The sample size was determined by a power analysis, with a medium effect size f = 0.25, an alpha level of 0.05 and a statistical power of 0.9. All participants had normal color vision and normal or corrected-to-normal visual acuity.
Experimental design
For the Flanker task, a 2 (Group: PIU vs. Control) × 2 (Stimulus Type: Internet-related words vs. Internet-unrelated words) × 2 (Congruency: Congruent vs. Incongruent) mixed factorial design was used, with Group as a between-subjects variable, and Stimulus type and Congruency as within-subjects variables. For the Go/No-Go task a 2 (Group: PIU vs. Control) × 2 (Stimulus Type: Internet-related vs. Internet-unrelated) mixed factorial design was used, with Group as a between-subjects variable, and Stimulus type as a within-subjects variable. Both tasks were programmed with E-prime 2.0. The stimuli were displayed on a laptop with a 15.6 inch screen, with a screen resolution of 1920 × 1080 and refresh rate of 60 Hz. To control for potential order effects and standardize the testing procedure across participants, the Flanker task was always performed before the Go/No-Go task.
The Flanker task
To study interference controls in PIU, a variant of the Flanker task was employed, with the internet-related and internet-unrelated words as stimuli.
To ensure that the words used in the study were matched on arousal, valence, and familiarity, and also differed on their relevance to internet, a total of 76 two-Chinese character words were firstly collected. There were 38 internet-related words (e.g., download) and 38 internet-unrelated words (e.g., stairs) respectively. A group of 36 participants were recruited to rate these words on four dimensions: arousal (with 1 being extreme calmness and 7 indicating high arousal), emotional valence (with 1 being extreme unpleasantness and 7 indicating high pleasantness), familiarity (with 1 being extremely unfamiliar and 7 indicating extremely familiar), and relevance to the internet (with 1 being extremely irrelevant and 7 indicating highly relevant). Finally, 40 words were selected, with twenty being internet-related and the remaining 20 being internet-unrelated. The selected internet-related and internet-unrelated words were matched in terms of arousal, emotional valence and familiarity ( ps > 0.05). As expected, internet-related words ( M = 6.46, SD = 0.83) were rated as significantly more relevant to the internet than internet-unrelated words ( M = 1.82, SD = 1.03), t = 27.07, p < 0.001.
In the Flanker task, each trial began with a fixation at the center of the screen for 1000 ms, and one blank screen was then displayed for 20ms-200ms, and then a stimulus array was displayed for 1000 ms, followed by a blank screen for 500ms. Three words were presented, participants were instructed to determine the identity of the word shown (either internet-related or internet-unrelated target) in the middle, while disregarding the Flanker words. The central word and the Flanker words were presented in either a congruent condition, where they belonged to the same category, or an incongruent condition, where they belonged to different categories. The Flanker words were positioned at the upper and lower sides of the central word, as depicted in Fig. 1 . Participants were instructed to press “F” for internet-related target and press “J” for internet-unrelated target, as accurately and quickly as possible. After participants provided their response, the stimuli array was immediately removed, and a blank screen was presented for a duration of 500 ms.
Stimulus display in the Flanker task. Note: 书桌 = desk, 流量 = (mobile) data, 下载 = download, 楼梯 = stairs
The Flanker task consisted of 160 trials, and there were 40 trials for each of the four conditions formed by the combination of Congruency (i.e., congruent, incongruent) and Stimulus type of the target word (i.e., internet-related, internet-unrelated). The sequence of congruent and incongruent trials was pseudorandomized within each block to minimize any potential order effects. Participants were given the opportunity to rest after completing every 50 trials. Prior to the formal experiment, participants underwent a practice block comprising eight trials, with two trials for each condition. The formal experiment would not start until participants achieved an accuracy equal to or higher than 80% during the practice session.
The Go/No-Go task
To study response inhibition in PIU, the Go/No-Go task was used, with internet-related and internet-unrelated images as stimuli.
To ensure that the images used in the study were matched on arousal and valence, and also differed on relevance to internet, a total of 40 images were firstly collected from the internet. Out of them, 20 images were internet-related (e.g., the WeChat icon) and 20 were internet-unrelated (e.g., an image of a plate). A group of 30 participants who did not participate the formal experiment were recruited to rate these images from three dimensions: arousal (with 1 being extreme calmness and 9 indicating high arousal), emotional valence (with 1 being extreme unpleasantness and 9 indicating high pleasantness), and relevance to the internet (with 1 being extremely irrelevant and 9 indicating highly relevant). Finally, eight images were selected, four of them were internet-related, and four were internet-unrelated. The selected images were matched in terms of arousal and emotional valence ( ps > 0.05). As expected, internet-related images ( M = 8.28, SD = 0.13) were rated as significantly more relevant to the internet than internet-unrelated images ( M = 2.23, SD = 0.11), t = 68.914, p < 0.001.
In the Go/No-Go task, each trial began with the display of a fixation at the center of the screen for 500 ms, one image was then displayed for 500ms, followed by a blank screen for 1000ms. Each image was standardized to a resolution of 513 × 384 pixels. Participants were instructed to press “H” for Go trials and withhold response for No-Go trials.
The Go/No-Go task consisted of six blocks, with each block comprising 40 trials. Specifically, three blocks involved internet-related images as the Go stimuli and internet-unrelated images as the No-Go stimuli, while the other three blocks had internet-unrelated images as Go stimuli and internet-related images as No-Go stimuli. Each block consisted 32 Go trials and 8 No-Go trials, which were pseudorandomized. Participants first completed a practice block of eight trials (six of them were Go trials), the formal experiment would not start until participants achieved an accuracy equal to or higher than 80% during the practice session.
Statistical analysis
Statistical analyses were performed using SPSS 17.0. For the analysis of RTs, trials with incorrect responses or reaction times (RTs) below 200ms were excluded from the analysis.
Group differences between the PIU and control groups in demographic variables were first examined. To investigate group differences on performance of the Go/No-Go task, repeated measures analysis of variances (ANOVAs) were conducted, with Group (PIU group, control group) as the between-subjects factor, Stimulus type (internet-related, internet-unrelated) as the within-subject factor, and the dependent variables were RT in Go trials and the proportion of successful stops (accuracy) in No-Go trials. For the Flanker task, the Group (PIU group, control group) × Congruency (congruent, incongruent) × Stimulus type (internet-related, internet-unrelated) ANOVAs were conducted, the dependent variables were RTs and response accuracy in each condition.
Demographic and psychometric variables
Descriptive statistics were listed in Table 1 . There was no significant difference on age, years of education, or sex between participants with PIU and controls, p s > 0.05. Participants with PIU scored significantly higher on the CIAS-R than controls, t (58) = 21.082, p < 0.001, d = 5.44.
Performance on the Go/No-Go task
Analyses of group differences in RTs of Go trials revealed a significant main effect for Stimulus type, participants responded faster to internet-unrelated stimuli (463 ± 49 ms) than to internet-related stimuli (477 ± 46 ms), F (1,58) = 23.43, p < 0.001, η 2 = 0.29. Neither the main effect of Group nor the interaction effect between Group and Stimulus type was significant, F (1,58) = 0.46, p = 0.501, η 2 = 0.008; F (1,58) = 2.92, p = 0.093, η 2 = 0.048. Descriptive statistics were presented in Table 2 .
For accuracy in No-Go trials, the analysis revealed a significant main effect of Stimulus type, participants exhibited poorer performance in inhibiting internet-related stimuli (0.83 ± 0.08) compared to internet-unrelated stimuli (0.91 ± 0.06), F (1,58) = 69.55, p < 0.001, η 2 = 0.55. Neither the main effect of Group nor the interaction effect between Group and Stimulus type was significant, F (1,58) = 0.33, p = 0.567, η 2 = 0.006; F (1,58) = 0.52, p = 0.473, η 2 = 0.009. Descriptive statistics were listed in Table 2 .
Performance on the Flanker task
For RTs, the ANOVA revealed a significant main effect of Congruency, longer RTs were observed in incongruent trials compared to congruent trials, F (1,57) = 56.74, p < 0.001, η 2 = 0.50. The three-way interaction of Group × Stimulus type × Congruency was significant, F (1,57) = 4.08, p = 0.048, η 2 = 0.07. Further analysis showed a significant interaction effect between Group and Stimulus type in incongruent condition (Fig. 2 ), F (1,57) = 5.58, p = 0.022, η 2 = 0.09. Specifically, participants with PIU exhibited longer RTs in conditions with internet-unrelated stimulus as targets (634 ± 48 ms) than in conditions with internet-related stimulus as targets (617 ± 40 ms), p = 0.02, that is, internet-related distractors elicited greater interference effect than internet-unrelated distractors in participants with PIU. For the control group, no significant difference on RTs was found between conditions with internet-unrelated targets and conditions with internet-related targets, p = 0.882. The interaction effect between Group and Stimulus type in congruent condition was not significant, neither the main effect of Group nor the main effect of Stimulus type was significant, ps > 0.05.
For accuracy, the main effect of Congruency was significant, with accuracy in congruent trials (0.92 ± 0.06) being significantly higher than that in incongruent trials (0.86 ± 0.07), F (1,57) = 98.76, p < 0.001, η 2 = 0.63. No other significant effects were found, ps > 0.05.
Interaction effect of Group and Stimulus type on RTs in incongruent condition of the Flanker task (error bars represent standard error)
The current study used the Go/No-Go task and the Flanker task to investigate prepotent response inhibition and interference control function among individuals with PIU. Interestingly, the results showed no significant differences between the PIU and control group in the Go/No-Go task, and all participants responded slower to internet-related Go stimuli but had difficulties withholding the prepotent responses to internet-related No-Go stimuli. However, in the Flanker task, the PIU group showed poorer performance in controlling the interference effect of internet-related information compared to controls. These findings emphasize the varying effects of PIU on the two components of inhibitory control, and highlighting the important role of interference control in relation to PIU.
Prepotent response inhibition in PIU
Results from the Go/No-Go task showed that individuals with PIU did not differ significantly in prepotent response inhibition from controls. This finding was in line with previous studies [ 2 , 17 ], which found no difference between participants with PIU and controls on accuracy of No-Go trials. In addition, Chamberlain et al. [ 15 ] also found no difference between participants with PIU and controls in withholding the inappropriate prepotent response in the stop-signal task. Nevertheless, we noticed that these findings were not consistent with studies conducted in individuals with IGD [ 22 , 23 ]. For example, in the study by Yao et al. [ 23 ], it was shown that male participants with IGD committed more errors than healthy controls in No-Go trials. This discrepancy might be related to sampling bias, as their study only included male participants due to the higher prevalence of IGD among males. The impact of sex differences on PIU has been documented. A meta-analytic study, which analyzed 115 independent samples from 34 countries, further corroborated that males tend to exhibit a higher tendency towards PIU compared to females [ 24 ]. In addition, it is noteworthy that PIU is an umbrella term encompassing various forms of internet overuse problems, whereas IGD represents a distinct subset of PIU characterized by more severe issues specifically related to addiction to internet gaming and has been included as a formal disorder in the 11th revision of the International Classification of Diseases (ICD-11) (ICD-11; WHO, 2019) [ 25 ]. Individuals diagnosed with IGD were found to exhibit more pronounced impairments in response inhibition compared to those with overall internet overuse [ 26 ].
Furthermore, internet-related stimuli elicited slower responses than internet-unrelated stimuli in Go trials in all participants. Theslower response times to Internet-related stimuli may represent a compensatorymechanism that allows individuals to reduce inhibitory errors. Using thedot-probe task in combination with eye-tracking, longer gaze duration werefound for social networking sites images than for control images [ 27 ]. Moreover, all participants were found to demonstrate greater difficulties in inhibiting prepotent responses to internet-related stimuli compared to internet-unrelated stimuli. Internet-related stimuli could hold stronger salience, leading to difficulties in inhibiting internet-related information. However, in the current study, internet-related and internet-unrelated stimuli were matched on emotional valence and arousal, thus differences observed in the study were not due to differences in variations in emotional dimensions between the two types of stimuli. Still, it is premature to draw a definitive conclusion that PIU is not related to impairments in response inhibition, as studies utilizing the stop-signal task revealed poorer response inhibition performances in PIU than controls, and both PIU and controls responded faster to internet-related words than tointernet-unrelated words in Go trials [ 16 ]. Future research could benefit from utilizing neurophysiological and neuroimaging techniques to further explore the neural mechanisms involved in the processing of internet-related stimuli among individuals with PIU. Notably, a recent study has reported a larger N2pc for Go trials in individuals with PIU, indicating an early attentional facilitation effect specifically for internet stimuli related to PIU [ 28 ].
Interference control in PIU
In the incongruent condition of the Flanker task, participants with PIU exhibited poorer performance in resisting the distraction from internet-related information than internet-unrelated information, whereas no such effect was found in the control group. This finding is in line with previous study in Flanker task using neutral, internet-unrelated stimuli, which showed that individuals with PIU performed worse than controls [ 18 ]. In addition, research has shown that individuals with IGD have difficulties in inhibiting the interference caused by gaming-related contents [ 29 ]. Therefore, the current study adds to the existing evidence by suggesting that individuals with PIU specifically struggle with resisting internet-related content, rather than experiencing general impairments in interference control. Under the incongruent condition, characterized by the presentation of two conflicting response options, there is an increased need for conflict monitoring and resolution. Consequently, greater efforts in cognitive control are necessary to effectively control interference from the incongruent flankers. For individuals with PIU, the challenge of disengaging from and suppressing internet-related information, especially when it is unrelated to the current task, may relate to dysfunctions in the prefrontal-parietal network, evidenced by enhanced resting functional connectivity density in the right dorsolateral prefrontal cortex in PIU [ 30 ]. Additional research is required to fully understand the causal mechanism linking PIU and the observed changes in brain function.
The divergent performance of individuals with PIU on the Go/No-Go and Flanker tasks adds to the growing body of evidence supporting the dissociation between the two components of inhibitory control. Moreover, neuroimaging studies have provided evidence supporting the unique and distinct nature of prepotent response inhibition and interference control in relation to neural networks. Specifically, prepotent response inhibition relies primarily on the frontal-basal ganglia network, with the right inferior frontal gyrus sending “stop” signals to the primary motor cortex via basal ganglia to inhibit prepotent responses [ 31 ]. On the other hand, increased activation during the Flanker task has been observed in the left middle frontal gyrus and left dorsal anterior cingulate [ 32 ].
The findings have important implications for the design of cognitive training programs for individuals with PIU, and future research could target enhancing the interference control function in this population. Previous studies conducted in healthy individuals and clinical samples have tested the effectiveness of interference control training in improving the ability to inhibit task-irrelevant information [ 33 , 34 ], providing support for its efficacy. Importantly, to maximize the training effect for individuals with PIU, it is crucial to include internet-related distractors in the training program.
The present study has some limitations. First, it is important to note that no clinical diagnoses were conducted in our study to determine if participants met the criteria for IGD. As a result, the generalizability of the findings to individuals with IGD is limited. Additionally, PIU encompasses a broad spectrum of behaviors, including online compulsive buying, excessive use of social media platforms, and online gambling. The extent to which these varied forms of PIU share common mechanisms of inhibitory control dysfunction remains to be fully explored. Third, we did not control for word frequency in the flanker task, although we did control for word familiarity. Given that word frequency and word familiarity are two related but distinct psycholinguistic features of language, the potential confounding effects of word frequency in influencing task performance should be controlled for in future research. Furthermore, our experimental tasks followed a fixed order, with the Flanker task administered before the Go/No-Go task. This design choice may introduce order effects that could influence the results. Future studies may benefit from counterbalancing the order of the tasks to mitigate such effects and provide a more robust estimate of inhibitory control across tasks.
In conclusion, this study examined the prepotent response inhibition and interference control in individuals with PIU. Our findings revealed no significant differences in prepotent response inhibition between individuals with PIU and controls, while they responded slower to Internet-related content in Go trials and made more errors to Internet-related content in No-Go trials. In the Flanker task, compared to controls, individuals with PIU demonstrated significant impairments in controlling the interference effect of Internet-related stimuli. The current study contributes additional evidence supporting the dissociation of the two components of inhibitory control and emphasizes the significant role of interference control in relation to PIU. Further studies are needed to investigate the neural correlates underlying deficits in the control of interference associated with PIU. These findings provide valuable insights for the development of cognitive remediation programs targeting PIU and other forms of addiction.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Problematic internet use
Internet Gaming Disorder
Reaction times
The Revised Chinese Internet Addiction Scale
Kuss DJ, Lopez-Fernandez O. Internet addiction and problematic internet use: a systematic review of clinical research. World J Psychiatry. 2016;6(1):143–76. https://doi.org/10.5498/wjp.v6.i1.143 .
Article PubMed PubMed Central Google Scholar
Vargas T, Maloney J, Gupta T, Damme KSF, Kelley NJ, Mittal VA. Measuring facets of reward sensitivity, inhibition, and impulse control in individuals with problematic internet use. Psychiatry Res. 2019;275:351–8. https://doi.org/10.1016/j.psychres.2019.03.032 .
Carli V, Durkee T, Wasserman D, Hadlaczky G, Despalins R, Kramarz E, Wasserman C, Sarchiapone M, Hoven CW, Brunner R, Kaess M. The association between pathological internet use and comorbid psychopathology: a systematic review. Psychopathology. 2013;46(1):1–13. https://doi.org/10.1159/000337971 .
Article PubMed Google Scholar
Diamond A. Executive functions. Ann Rev Psychol. 2013;64(1):135–68. https://doi.org/10.1146/annurev-psych-113011-143750 .
Article Google Scholar
Chen SK, Lo MT, Lin SSJ. Impulsivity as a precedent factor for problematic internet use: how can we be sure? Int J Psychol. 2017;52(5):389–97. https://doi.org/10.1002/ijop.12231 .
Meerkerk G-J, van den Eijnden RJJM, Franken IHA, Garretsen HFL. Is compulsive internet use related to sensitivity to reward and punishment, and impulsivity? Comput Hum Behav. 2010;26(4):729–35. https://doi.org/10.1016/j.chb.2010.01.009 .
Ding W, Sun J, Sun Y, Chen X, Zhou Y, Zhuang Z, Li L, Zhang Y, Xu J, Du Y. Trait impulsivity and impaired prefrontal impulse inhibition function in adolescents with internet gaming addiction revealed by a Go/No-Go fMRI study. Behav Brain Funct. 2014;10(1):20. https://doi.org/10.1186/1744-9081-10-20 .
Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133(1):101–35. https://doi.org/10.1037/0096-3445.133.1.101 .
Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126(2):220–46. https://doi.org/10.1037/0033-2909.126.2.220 .
Ioannidis K, Hook R, Goudriaan AE, Vlies S, Fineberg NA, Grant JE, Chamberlain SR. Cognitive deficits in problematic internet use: Meta-analysis of 40 studies. Br J Psychiatry. 2019;215(5):639–46. https://doi.org/10.1192/bjp.2019.3 . 2023-07-17.
Paap KR, Anders-Jefferson R, Zimiga B, Mason L, Mikulinsky R. Interference scores have inadequate concurrent and convergent validity: should we stop using the Flanker, Simon, and spatial Stroop tasks? Cogn Research: Principles Implications. 2020;5(1):7. https://doi.org/10.1186/s41235-020-0207-y .
Zhou Z-H, Yuan G-Z, Yao J-J, Li C, Cheng Z-H. An event-related potential investigation of deficient inhibitory control in individuals with pathological internet use. Acta Neuropsychiatrica. 2010;22(5):228–36. https://doi.org/10.1111/j.1601-5215.2010.00444.x .
Zhou Z, Zhou H, Zhu H. Working memory, executive function and impulsivity in internet-addictive disorders: a comparison with pathological gambling. Acta Neuropsychiatrica. 2016;28(2):92–100. https://doi.org/10.1017/neu.2015.54 .
Li Q, Nan W, Taxer J, Dai W, Zheng Y, Liu X. Problematic internet users show impaired inhibitory control and risk taking with losses: evidence from stop signal and mixed gambles tasks. Front Psychol. 2016;7. https://doi.org/10.3389/fpsyg.2016.00370 .
Chamberlain SR, Redden SA, Leppink E, Grant JE. Problematic internet use in gamblers: impact on clinical and cognitive measures. CNS Spectr. 2017;22(6):495–503. https://doi.org/10.1017/S1092852917000037 .
Nie J, Zhang W, Chen J, Li W. Impaired inhibition and working memory in response to internet-related words among adolescents with internet addiction: a comparison with attention-deficit/hyperactivity disorder. Psychiatry Res. 2016;236:28–34. https://doi.org/10.1016/j.psychres.2016.01.004 .
Dong G, Lu Q, Zhou H, Zhao X. Impulse inhibition in people with internet addiction disorder: electrophysiological evidence from a Go/NoGo study. Neurosci Lett. 2010;485(2):138–42. https://doi.org/10.1016/j.neulet.2010.09.002 .
Zhou Z, Li C, Zhu H. (2013). An error-related negativity potential investigation of response monitoring function in individuals with internet addiction disorder. Frontiers in Behavioral Neuroscience , 7:131 . https://doi.org/10.3389/fnbeh.2013.00131 .
Khoo SS, Yang H. Resisting problematic smartphone use: distracter resistance strengthens grit’s protective effect against problematic smartphone use. Pers Indiv Differ. 2022;194:111644. https://doi.org/10.1016/j.paid.2022.111644 .
Lara RS, Bokoch R. Cognitive functioning and social media: has technology changed us? Acta Psychol. 2021;221:103429. https://doi.org/10.1016/j.actpsy.2021.103429 .
Chen W, Su Y-J, Wu H-M, Yang P-F. Development of a Chinese internet addiction scale and its psychometric study. Chin J Psychol. 2003;45(3):279–94.
Google Scholar
Choi S-W, Kim H, Kim G-Y, Jeon Y, Park S, Lee J-Y, Jung H, Sohn B, Choi J-S, Kim D-J. Similarities and differences among internet gaming disorder, gambling disorder and alcohol use disorder: a focus on impulsivity and compulsivity. J Behav Addictions. 2014;3(4):246–53. https://doi.org/10.1556/jba.3.2014.4.6 .
Yao Y-W, Wang L-J, Yip SW, Chen P-R, Li S, Xu J, Zhang J-T, Deng L-Y, Liu Q-X, Fang X-Y. Impaired decision-making under risk is associated with gaming-specific inhibition deficits among college students with internet gaming disorder. Psychiatry Res. 2015;229(1–2):302–9. https://doi.org/10.1016/j.psychres.2015.07.004 .
Su W, Han X, Jin C, Yan Y, Potenza MN. Are males more likely to be addicted to the internet than females? A meta-analysis involving 34 global jurisdictions. Comput Hum Behav. 2019;99:86–100. https://doi.org/10.1016/j.chb.2019.04.021 .
World Health Organization (WHO). (2019). 6C51 Gaming disorder. Retrieved online https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1448597234 .
Wong HY, Mo HY, Potenza MN, Chan MNM, Lau WM, Chui TK, Pakpour AH, Lin C-Y. Relationships between severity of internet gaming disorder, severity of problematic social media use, sleep quality and psychological distress. Int J Environ Res Public Health. 2020;17(6):1879. https://doi.org/10.3390/ijerph17061879 .
Nikolaidou M, Fraser DS, Hinvest N. Attentional bias in internet users with problematic use of social networking sites. J Behav Addictions. 2019;8(4):733–42. https://doi.org/10.1556/2006.8.2019.60 .
Balconi M, Angioletti L. Neurophysiology of gambling behavior and internet use vulnerability: a comparison between behavioral and EEG measures. Clin EEG Neurosci. 2022;53(4):268–77. https://doi.org/10.1177/15500594211038469 .
Dong G, DeVito EE, Du X, Cui Z. Impaired inhibitory control in ‘Internet addiction disorder’: a functional magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 2012;203(2–3):153–8. https://doi.org/10.1016/j.pscychresns.2012.02.001 .
Heuer A, Mennig M, Schubö A, Barke A. Impaired disengagement of attention from computer-related stimuli in internet gaming disorder: behavioral and electrophysiological evidence. J Behav Addictions. 2021;10(1):77–87. https://doi.org/10.1556/2006.2020.00100 .
Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–68. https://doi.org/10.1016/j.biopsych.2010.07.024 .
Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18(1):42–57. https://doi.org/10.1006/nimg.2002.1319 .
Maraver MJ, Bajo MT, Gomez-Ariza CJ. Training on working memory and inhibitory control in young adults. Front Hum Neurosci. 2016;10:588. https://doi.org/10.3389/fnhum.2016.00588 .
Millner AJ, Jaroszewski AC, Chamarthi H, Pizzagalli DA. Behavioral and electrophysiological correlates of training-induced cognitive control improvements. NeuroImage. 2012;63(2):742–53. https://doi.org/10.1016/j.neuroimage.2012.07.032 .
Download references
Acknowledgements
The authors gratefully acknowledge all participants for their contribution in this study.
This work has been supported by the National Natural Science Foundation of China (31700957), the Major Program of the National Social Science Foundation of China (grant No. 22&ZD187), MOE (Ministry of Education in China) Project of Humanities and Social Sciences (17YJC190014), and Key Laboratory of Adolescent Cyberpsychology and Behavior Central China Normal University (CCNU), Ministry of Education (2019B06).
Author information
Authors and affiliations.
Key Laboratory of Adolescent Cyberpsychology and Behavior Central China Normal University (CCNU), Ministry of Education, Wuhan, China
Shao-Shuai Zhang, Yu-qing Zhong, Xu Li & Ming Peng
Hubei Human development and mental health key Laboratory (Central China Normal University), School of Psychology, Central China Normal University, No. 382, XiongChu Road, Hongshan District, 430079, Wuhan, Hubei Province, China
You can also search for this author in PubMed Google Scholar
Contributions
S.S.Z., Y.Q.Z. and X.L. contributed to the design and implementation of the research, to the analysis of theresults and to the writing of the manuscript. M.P. commented on previous versions of the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Correspondence to Xu Li or Ming Peng .
Ethics declarations
Ethics approval and consent to participate.
All procedures were in accordance with the ethical standards of the institutional research committee. Informed consent was obtained from all individual participants included in the study. The study was approved by the ethical committee of Central China Normal University. Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, SS., Zhong, Yq., Li, X. et al. Dissociation of prepotent response inhibition and interference control in problematic internet use: evidence from the Go/No-Go and Flanker tasks. BMC Psychol 12 , 216 (2024). https://doi.org/10.1186/s40359-024-01698-6
Download citation
Received : 25 December 2023
Accepted : 01 April 2024
Published : 18 April 2024
DOI : https://doi.org/10.1186/s40359-024-01698-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Inhibitory control
- Prepotent response inhibition
- Interference control
- Distractor resistance
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
What the data says about abortion in the U.S.
Pew Research Center has conducted many surveys about abortion over the years, providing a lens into Americans’ views on whether the procedure should be legal, among a host of other questions.
In a Center survey conducted nearly a year after the Supreme Court’s June 2022 decision that ended the constitutional right to abortion , 62% of U.S. adults said the practice should be legal in all or most cases, while 36% said it should be illegal in all or most cases. Another survey conducted a few months before the decision showed that relatively few Americans take an absolutist view on the issue .
Find answers to common questions about abortion in America, based on data from the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, which have tracked these patterns for several decades:
How many abortions are there in the U.S. each year?
How has the number of abortions in the u.s. changed over time, what is the abortion rate among women in the u.s. how has it changed over time, what are the most common types of abortion, how many abortion providers are there in the u.s., and how has that number changed, what percentage of abortions are for women who live in a different state from the abortion provider, what are the demographics of women who have had abortions, when during pregnancy do most abortions occur, how often are there medical complications from abortion.
This compilation of data on abortion in the United States draws mainly from two sources: the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, both of which have regularly compiled national abortion data for approximately half a century, and which collect their data in different ways.
The CDC data that is highlighted in this post comes from the agency’s “abortion surveillance” reports, which have been published annually since 1974 (and which have included data from 1969). Its figures from 1973 through 1996 include data from all 50 states, the District of Columbia and New York City – 52 “reporting areas” in all. Since 1997, the CDC’s totals have lacked data from some states (most notably California) for the years that those states did not report data to the agency. The four reporting areas that did not submit data to the CDC in 2021 – California, Maryland, New Hampshire and New Jersey – accounted for approximately 25% of all legal induced abortions in the U.S. in 2020, according to Guttmacher’s data. Most states, though, do have data in the reports, and the figures for the vast majority of them came from each state’s central health agency, while for some states, the figures came from hospitals and other medical facilities.
Discussion of CDC abortion data involving women’s state of residence, marital status, race, ethnicity, age, abortion history and the number of previous live births excludes the low share of abortions where that information was not supplied. Read the methodology for the CDC’s latest abortion surveillance report , which includes data from 2021, for more details. Previous reports can be found at stacks.cdc.gov by entering “abortion surveillance” into the search box.
For the numbers of deaths caused by induced abortions in 1963 and 1965, this analysis looks at reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. In computing those figures, we excluded abortions listed in the report under the categories “spontaneous or unspecified” or as “other.” (“Spontaneous abortion” is another way of referring to miscarriages.)
Guttmacher data in this post comes from national surveys of abortion providers that Guttmacher has conducted 19 times since 1973. Guttmacher compiles its figures after contacting every known provider of abortions – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, and it provides estimates for abortion providers that don’t respond to its inquiries. (In 2020, the last year for which it has released data on the number of abortions in the U.S., it used estimates for 12% of abortions.) For most of the 2000s, Guttmacher has conducted these national surveys every three years, each time getting abortion data for the prior two years. For each interim year, Guttmacher has calculated estimates based on trends from its own figures and from other data.
The latest full summary of Guttmacher data came in the institute’s report titled “Abortion Incidence and Service Availability in the United States, 2020.” It includes figures for 2020 and 2019 and estimates for 2018. The report includes a methods section.
In addition, this post uses data from StatPearls, an online health care resource, on complications from abortion.
An exact answer is hard to come by. The CDC and the Guttmacher Institute have each tried to measure this for around half a century, but they use different methods and publish different figures.
The last year for which the CDC reported a yearly national total for abortions is 2021. It found there were 625,978 abortions in the District of Columbia and the 46 states with available data that year, up from 597,355 in those states and D.C. in 2020. The corresponding figure for 2019 was 607,720.
The last year for which Guttmacher reported a yearly national total was 2020. It said there were 930,160 abortions that year in all 50 states and the District of Columbia, compared with 916,460 in 2019.
- How the CDC gets its data: It compiles figures that are voluntarily reported by states’ central health agencies, including separate figures for New York City and the District of Columbia. Its latest totals do not include figures from California, Maryland, New Hampshire or New Jersey, which did not report data to the CDC. ( Read the methodology from the latest CDC report .)
- How Guttmacher gets its data: It compiles its figures after contacting every known abortion provider – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, then provides estimates for abortion providers that don’t respond. Guttmacher’s figures are higher than the CDC’s in part because they include data (and in some instances, estimates) from all 50 states. ( Read the institute’s latest full report and methodology .)
While the Guttmacher Institute supports abortion rights, its empirical data on abortions in the U.S. has been widely cited by groups and publications across the political spectrum, including by a number of those that disagree with its positions .
These estimates from Guttmacher and the CDC are results of multiyear efforts to collect data on abortion across the U.S. Last year, Guttmacher also began publishing less precise estimates every few months , based on a much smaller sample of providers.
The figures reported by these organizations include only legal induced abortions conducted by clinics, hospitals or physicians’ offices, or those that make use of abortion pills dispensed from certified facilities such as clinics or physicians’ offices. They do not account for the use of abortion pills that were obtained outside of clinical settings .
(Back to top)
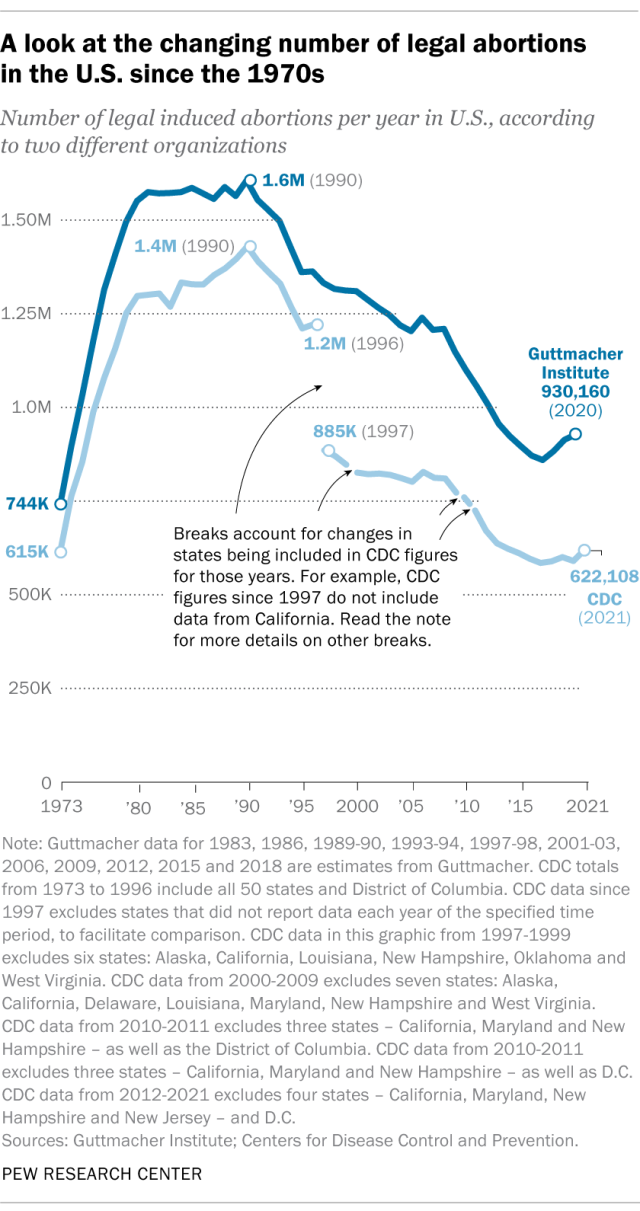
The annual number of U.S. abortions rose for years after Roe v. Wade legalized the procedure in 1973, reaching its highest levels around the late 1980s and early 1990s, according to both the CDC and Guttmacher. Since then, abortions have generally decreased at what a CDC analysis called “a slow yet steady pace.”
Guttmacher says the number of abortions occurring in the U.S. in 2020 was 40% lower than it was in 1991. According to the CDC, the number was 36% lower in 2021 than in 1991, looking just at the District of Columbia and the 46 states that reported both of those years.
(The corresponding line graph shows the long-term trend in the number of legal abortions reported by both organizations. To allow for consistent comparisons over time, the CDC figures in the chart have been adjusted to ensure that the same states are counted from one year to the next. Using that approach, the CDC figure for 2021 is 622,108 legal abortions.)
There have been occasional breaks in this long-term pattern of decline – during the middle of the first decade of the 2000s, and then again in the late 2010s. The CDC reported modest 1% and 2% increases in abortions in 2018 and 2019, and then, after a 2% decrease in 2020, a 5% increase in 2021. Guttmacher reported an 8% increase over the three-year period from 2017 to 2020.
As noted above, these figures do not include abortions that use pills obtained outside of clinical settings.
Guttmacher says that in 2020 there were 14.4 abortions in the U.S. per 1,000 women ages 15 to 44. Its data shows that the rate of abortions among women has generally been declining in the U.S. since 1981, when it reported there were 29.3 abortions per 1,000 women in that age range.
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher’s data, the CDC’s figures also suggest a general decline in the abortion rate over time. In 1980, when the CDC reported on all 50 states and D.C., it said there were 25 abortions per 1,000 women ages 15 to 44.
That said, both Guttmacher and the CDC say there were slight increases in the rate of abortions during the late 2010s and early 2020s. Guttmacher says the abortion rate per 1,000 women ages 15 to 44 rose from 13.5 in 2017 to 14.4 in 2020. The CDC says it rose from 11.2 per 1,000 in 2017 to 11.4 in 2019, before falling back to 11.1 in 2020 and then rising again to 11.6 in 2021. (The CDC’s figures for those years exclude data from California, D.C., Maryland, New Hampshire and New Jersey.)
The CDC broadly divides abortions into two categories: surgical abortions and medication abortions, which involve pills. Since the Food and Drug Administration first approved abortion pills in 2000, their use has increased over time as a share of abortions nationally, according to both the CDC and Guttmacher.
The majority of abortions in the U.S. now involve pills, according to both the CDC and Guttmacher. The CDC says 56% of U.S. abortions in 2021 involved pills, up from 53% in 2020 and 44% in 2019. Its figures for 2021 include the District of Columbia and 44 states that provided this data; its figures for 2020 include D.C. and 44 states (though not all of the same states as in 2021), and its figures for 2019 include D.C. and 45 states.
Guttmacher, which measures this every three years, says 53% of U.S. abortions involved pills in 2020, up from 39% in 2017.
Two pills commonly used together for medication abortions are mifepristone, which, taken first, blocks hormones that support a pregnancy, and misoprostol, which then causes the uterus to empty. According to the FDA, medication abortions are safe until 10 weeks into pregnancy.
Surgical abortions conducted during the first trimester of pregnancy typically use a suction process, while the relatively few surgical abortions that occur during the second trimester of a pregnancy typically use a process called dilation and evacuation, according to the UCLA School of Medicine.
In 2020, there were 1,603 facilities in the U.S. that provided abortions, according to Guttmacher . This included 807 clinics, 530 hospitals and 266 physicians’ offices.
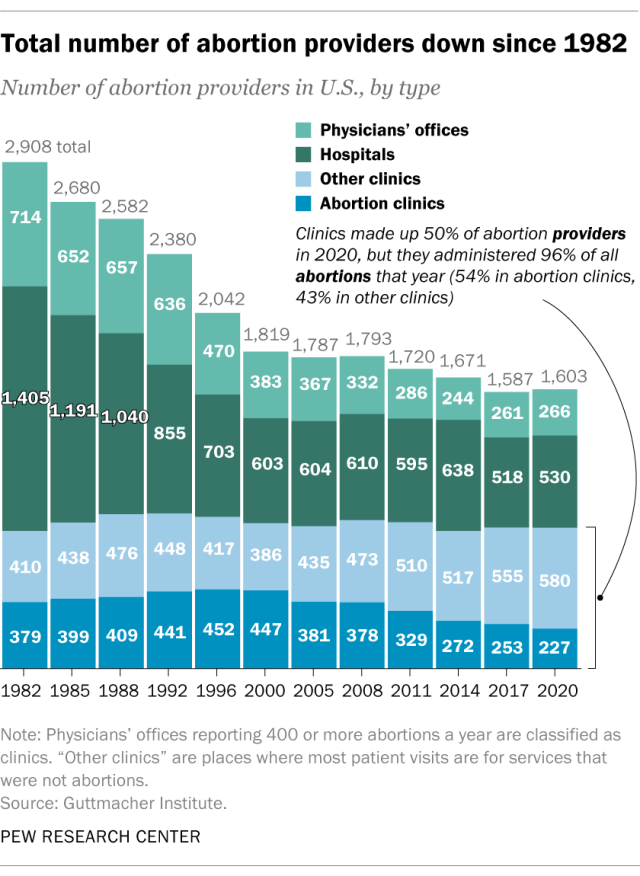
While clinics make up half of the facilities that provide abortions, they are the sites where the vast majority (96%) of abortions are administered, either through procedures or the distribution of pills, according to Guttmacher’s 2020 data. (This includes 54% of abortions that are administered at specialized abortion clinics and 43% at nonspecialized clinics.) Hospitals made up 33% of the facilities that provided abortions in 2020 but accounted for only 3% of abortions that year, while just 1% of abortions were conducted by physicians’ offices.
Looking just at clinics – that is, the total number of specialized abortion clinics and nonspecialized clinics in the U.S. – Guttmacher found the total virtually unchanged between 2017 (808 clinics) and 2020 (807 clinics). However, there were regional differences. In the Midwest, the number of clinics that provide abortions increased by 11% during those years, and in the West by 6%. The number of clinics decreased during those years by 9% in the Northeast and 3% in the South.
The total number of abortion providers has declined dramatically since the 1980s. In 1982, according to Guttmacher, there were 2,908 facilities providing abortions in the U.S., including 789 clinics, 1,405 hospitals and 714 physicians’ offices.
The CDC does not track the number of abortion providers.
In the District of Columbia and the 46 states that provided abortion and residency information to the CDC in 2021, 10.9% of all abortions were performed on women known to live outside the state where the abortion occurred – slightly higher than the percentage in 2020 (9.7%). That year, D.C. and 46 states (though not the same ones as in 2021) reported abortion and residency data. (The total number of abortions used in these calculations included figures for women with both known and unknown residential status.)
The share of reported abortions performed on women outside their state of residence was much higher before the 1973 Roe decision that stopped states from banning abortion. In 1972, 41% of all abortions in D.C. and the 20 states that provided this information to the CDC that year were performed on women outside their state of residence. In 1973, the corresponding figure was 21% in the District of Columbia and the 41 states that provided this information, and in 1974 it was 11% in D.C. and the 43 states that provided data.
In the District of Columbia and the 46 states that reported age data to the CDC in 2021, the majority of women who had abortions (57%) were in their 20s, while about three-in-ten (31%) were in their 30s. Teens ages 13 to 19 accounted for 8% of those who had abortions, while women ages 40 to 44 accounted for about 4%.
The vast majority of women who had abortions in 2021 were unmarried (87%), while married women accounted for 13%, according to the CDC , which had data on this from 37 states.
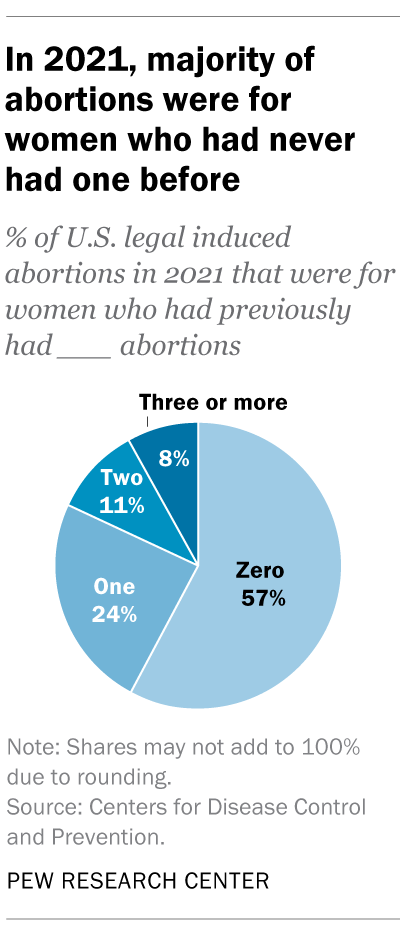
In the District of Columbia, New York City (but not the rest of New York) and the 31 states that reported racial and ethnic data on abortion to the CDC , 42% of all women who had abortions in 2021 were non-Hispanic Black, while 30% were non-Hispanic White, 22% were Hispanic and 6% were of other races.
Looking at abortion rates among those ages 15 to 44, there were 28.6 abortions per 1,000 non-Hispanic Black women in 2021; 12.3 abortions per 1,000 Hispanic women; 6.4 abortions per 1,000 non-Hispanic White women; and 9.2 abortions per 1,000 women of other races, the CDC reported from those same 31 states, D.C. and New York City.
For 57% of U.S. women who had induced abortions in 2021, it was the first time they had ever had one, according to the CDC. For nearly a quarter (24%), it was their second abortion. For 11% of women who had an abortion that year, it was their third, and for 8% it was their fourth or more. These CDC figures include data from 41 states and New York City, but not the rest of New York.
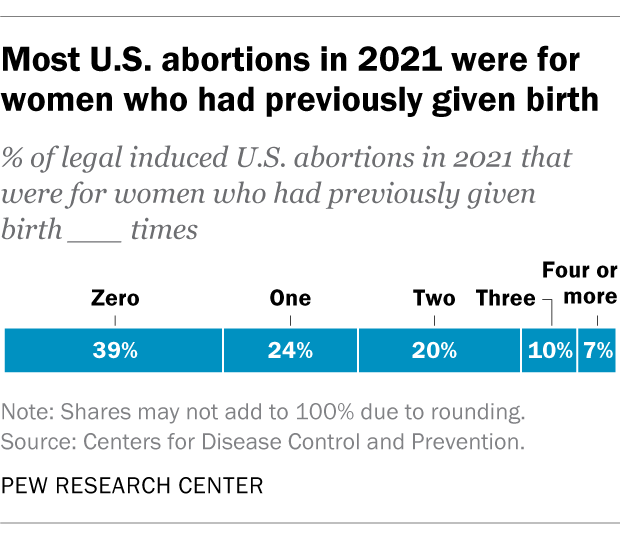
Nearly four-in-ten women who had abortions in 2021 (39%) had no previous live births at the time they had an abortion, according to the CDC . Almost a quarter (24%) of women who had abortions in 2021 had one previous live birth, 20% had two previous live births, 10% had three, and 7% had four or more previous live births. These CDC figures include data from 41 states and New York City, but not the rest of New York.
The vast majority of abortions occur during the first trimester of a pregnancy. In 2021, 93% of abortions occurred during the first trimester – that is, at or before 13 weeks of gestation, according to the CDC . An additional 6% occurred between 14 and 20 weeks of pregnancy, and about 1% were performed at 21 weeks or more of gestation. These CDC figures include data from 40 states and New York City, but not the rest of New York.
About 2% of all abortions in the U.S. involve some type of complication for the woman , according to an article in StatPearls, an online health care resource. “Most complications are considered minor such as pain, bleeding, infection and post-anesthesia complications,” according to the article.
The CDC calculates case-fatality rates for women from induced abortions – that is, how many women die from abortion-related complications, for every 100,000 legal abortions that occur in the U.S . The rate was lowest during the most recent period examined by the agency (2013 to 2020), when there were 0.45 deaths to women per 100,000 legal induced abortions. The case-fatality rate reported by the CDC was highest during the first period examined by the agency (1973 to 1977), when it was 2.09 deaths to women per 100,000 legal induced abortions. During the five-year periods in between, the figure ranged from 0.52 (from 1993 to 1997) to 0.78 (from 1978 to 1982).
The CDC calculates death rates by five-year and seven-year periods because of year-to-year fluctuation in the numbers and due to the relatively low number of women who die from legal induced abortions.
In 2020, the last year for which the CDC has information , six women in the U.S. died due to complications from induced abortions. Four women died in this way in 2019, two in 2018, and three in 2017. (These deaths all followed legal abortions.) Since 1990, the annual number of deaths among women due to legal induced abortion has ranged from two to 12.
The annual number of reported deaths from induced abortions (legal and illegal) tended to be higher in the 1980s, when it ranged from nine to 16, and from 1972 to 1979, when it ranged from 13 to 63. One driver of the decline was the drop in deaths from illegal abortions. There were 39 deaths from illegal abortions in 1972, the last full year before Roe v. Wade. The total fell to 19 in 1973 and to single digits or zero every year after that. (The number of deaths from legal abortions has also declined since then, though with some slight variation over time.)
The number of deaths from induced abortions was considerably higher in the 1960s than afterward. For instance, there were 119 deaths from induced abortions in 1963 and 99 in 1965 , according to reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. The CDC is a division of Health and Human Services.
Note: This is an update of a post originally published May 27, 2022, and first updated June 24, 2022.
Support for legal abortion is widespread in many countries, especially in Europe
Nearly a year after roe’s demise, americans’ views of abortion access increasingly vary by where they live, by more than two-to-one, americans say medication abortion should be legal in their state, most latinos say democrats care about them and work hard for their vote, far fewer say so of gop, positive views of supreme court decline sharply following abortion ruling, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
- Plan for College and Career
- Take the ACT
- School and District Assessment
- Career-Ready Solutions
- Students & Parents
- Open Search Form
- ACT Research
- All Services and Resources
- Growth Modeling Resources
- Data and Visualization
- All Reports
- ACT Research Publications
- Technical Documentation
- Studies and Partnerships
- About ACT Research
- Meet Our Experts
- Get Research Updates
Other ACT Services and Products
ACT Partners with Nexus Capital Management
ACT’s partnership with Nexus Capital Management will enhance and advance our education and work readiness solutions to support the needs of students, educators, and employers. Learn more.
New Featured Apr 16, 2024
Generating Social and Emotional Skill Items: Humans vs. ChatGPT

Large language models (LLMs), such as ChatGPT, are becoming increasingly prominent. Can we use ChatGPT in the field of social and emotional learning assessment development to enhance our productivity?
Size: 309KB | Pages: 8
This action will open a new window. Do you want to proceed?
Welcome to ACT
If you are accessing this site from outside the United States, Puerto Rico, or U.S. Territories, please proceed to the non-U.S. version of our website.

Find Your Local NAMI
Call the NAMI Helpline at
800-950-6264
Or text "HelpLine" to 62640
Depressive disorder, frequently referred to simply as depression, is more than just feeling sad or going through a rough patch. It’s a serious mental health condition that requires understanding and medical care. Left untreated, depression can be devastating for those who have it and their families. Fortunately, with early detection, diagnosis and a treatment plan consisting of medication, psychotherapy and healthy lifestyle choices, many people can and do get better.
Some will only experience one depressive episode in a lifetime, but for most, depressive disorder recurs. Without treatment, episodes may last a few months to several years.
About 21 million U.S. adults— 8.4% of the population—had at least one major depressive episode in 2020. People of all ages and all racial, ethnic and socioeconomic backgrounds experience depression, but it does affect some groups more than others.
Personal Perspectives on Major Depressive Disorder
In this 2-part podcast series, NAMI Chief Medical Officer Dr. Ken Duckworth guides discussions on major depressive disorder that offer insights from individuals, family members and mental health professionals. Read the transcript . Note: Content includes discussions on topics such as suicide attempts and may be triggering.
Depression can present different symptoms, depending on the person. But for most people, depressive disorder changes how they function day-to-day, and typically for more than two weeks. Common symptoms include:
- Changes in sleep
- Changes in appetite
- Lack of concentration
- Loss of energy
- Lack of interest in activities
- Hopelessness or guilty thoughts
- Changes in movement (less activity or agitation)
- Physical aches and pains
- Suicidal thoughts
Depression does not have a single cause. It can be triggered by a life crisis, physical illness or something else—but it can also occur spontaneously. Scientists believe several factors can contribute to depression:
- Trauma . When people experience trauma at an early age, it can cause long-term changes in how their brains respond to fear and stress. These changes may lead to depression.
- Genetics . Mood disorders, such as depression, tend to run in families.
- Life circumstances . Marital status, relationship changes, financial standing and where a person lives influence whether a person develops depression.
- Brain changes . Imaging studies have shown that the frontal lobe of the brain becomes less active when a person is depressed. Depression is also associated with changes in how the pituitary gland and hypothalamus respond to hormone stimulation.
- Other medical conditions . People who have a history of sleep disturbances, medical illness, chronic pain, anxiety and attention-deficit hyperactivity disorder (ADHD) are more likely to develop depression. Some medical syndromes (like hypothyroidism) can mimic depressive disorder. Some medications can also cause symptoms of depression.
- Drug and alcohol misuse . Adults with a substance use disorder are at significantly higher risk for experiencing a major depressive episode. Co-occurring disorders require coordinated treatment for both conditions, as alcohol can worsen depressive symptoms.
To be diagnosed with depressive disorder, a person must have experienced a depressive episode lasting longer than two weeks. The symptoms of a depressive episode include:
- Loss of interest or loss of pleasure in all activities
- Change in appetite or weight
- Sleep disturbances
- Feeling agitated or feeling slowed down
- Feelings of low self-worth, guilt or shortcomings
- Difficulty concentrating or making decisions
- Suicidal thoughts or intentions
Although depressive disorder can be a devastating illness, it often responds to treatment. The key is to get a specific evaluation and treatment plan. Safety planning is important for individuals who have suicidal thoughts. After an assessment rules out medical and other possible causes, a patient-centered treatment plans can include any or a combination of the following:
- Psychotherapy including cognitive behavioral therapy, family-focused therapy and interpersonal therapy.
- Medications including antidepressants, mood stabilizers and antipsychotic medications.
- Exercise can help with prevention and mild-to-moderate symptoms.
- Brain stimulation therapies can be tried if psychotherapy and/or medication are not effective. These include electroconvulsive therapy (ECT) for depressive disorder with psychosis or repetitive transcranial magnetic stimulation (rTMS) for severe depression.
- Light therapy , which uses a light box to expose a person to full spectrum light in an effort to regulate the hormone melatonin.
- Alternative approaches including acupuncture, meditation, faith and nutrition can be part of a comprehensive treatment plan.
Reviewed August 2017

IMAGES
VIDEO
COMMENTS
Not all evidence is the same, and appraising the quality of the evidence is part of evidence-based practice research.The hierarchy of evidence is typically represented as a pyramid shape, with the smaller, weaker and more abundant research studies near the base of the pyramid, and systematic reviews and meta-analyses at the top with higher validity but a more limited range of topics.
Type of evidence; I: High quality prospective cohort study with adequate power or systematic review of these studies: II: ... This allows the reader to know the level of evidence of the research but the designated level of evidence does always guarantee the quality of the research. It is important that readers not assume that level 1 evidence ...
One way to organize the different types of evidence involved in evidence-based practice research is the levels of evidence pyramid. The pyramid includes a variety of evidence types and levels. Filtered resources: pre-evaluated in some way. systematic reviews. critically-appraised topics. critically-appraised individual articles.
Law: In the legal system, evidence is used to establish facts and to prove or disprove a case. Lawyers use different types of evidence, such as witness testimony, physical evidence, and documentary evidence, to present their arguments and persuade judges and juries. Science: Evidence is the foundation of scientific inquiry.
This kind of evidence just serves as a good foundation for further research - or clinical practice - for it is usually too generalized. Of course, it is recommended to use level A and/or 1 evidence for more accurate results but that doesn't mean that all other study designs are unhelpful or useless. It all depends on your research question.
Levels of Evidence. The evidence pyramid is often used to illustrate the development of evidence. At the base of the pyramid is animal research and laboratory studies - this is where ideas are first developed. As you progress up the pyramid the amount of information available decreases in volume, but increases in relevance to the clinical ...
The best answers are found by combining the results of many studies. A systematic review is a type of research that looks at the results from all of the good-quality studies. It puts together the results of these individual studies into one summary. This gives an estimate of a treatment's risks and benefits.
Evidence-based research is the use of prior research in a systematic and transparent way to inform a new study so that it is answering questions that matter in a valid, efficient, and accessible manner. Results: We describe evidence-based research and provide an overview of the approach of systematically and transparently using previous ...
Once you have your focused question, it's time to decide on the type of evidence you need to answer it. Understanding the types of research will help guide you to proper evidence that will support your question. Primary Research. Secondary Research. The data that is obtained during a study that has been conducted.
The hierarchy of scientific evidence is a system used to rank the reliability of different types of research findings. At the top are systematic reviews and meta-analyses, which synthesise data from multiple studies, followed by randomised controlled trials, cohort studies, case-control studies, case series/reports, and expert opinion.
After excluding duplicates, a total of 54 different definitions of 'evidence' were identified. There were 42 intensional definitions and 12 extensional definitions. The top three definiens were 'information', 'fact' and 'research/study'. The definition of 'evidence' differed between health and social sciences.
Level III: Evidence from evidence summaries developed from systematic reviews. Level IV: Evidence from guidelines developed from systematic reviews. Level V: Evidence from meta-syntheses of a group of descriptive or qualitative studies. Level VI: Evidence from evidence summaries of individual studies. Level VII: Evidence from one properly ...
Even when using randomized studies with a high quality of evidence, evaluating the quality of evidence precisely helps determine the strength of recommendations in the meta-analysis. One method of evaluating the quality of evidence in non-randomized studies is the Newcastle-Ottawa Scale, provided by the Ottawa Hospital Research Institute 1 ...
Level 7 Evidence Expert opinion: Recommendations from persons with established expertise in a specific clinical area often based on clinical experience; not considered a research method because systematic (or critical) inquiry is lacking. The level of evidence of systematic reviews and meta-analyses depends on the types of studies reviewed.
Throughout your schooling, you may need to find different types of evidence and research to support your course work. This guide provides a high-level overview of evidence-based practice as well as the different types of research and study designs. Each page of this guide offers an overview and search tips for finding articles that fit that ...
An interview is a good way to collect information that you can't find through any other type of research. An interview can provide an expert's opinion, biographical or first-hand experiences, and suggestions for further research. ... This type of evidence can be a solid backbone for your argument, but you still need to create context for ...
Types of Research Evidence. Research evidence usually consists of data, which comes from borrowed information that you use to develop your thesis and support your organizational structure and reasoning. This evidence can take a range of forms, depending on the type of research conducted, the audience, and the genre for reporting the research. ...
A significant part of evidence-based practice is the levels of evidence or hierarchy of evidence in research. Generally, it applies to any type of research and evaluates the strength of scientific results. While there are specific levels of evidence in various disciplines, the most developed is from medicine and allied health (Hugel, 2013)
The proposed new evidence-based medicine pyramid. (A) The traditional pyramid. (B) Revising the pyramid: (1) lines separating the study designs become wavy (Grading of Recommendations Assessment, Development and Evaluation), (2) systematic reviews are 'chopped off' the pyramid. (C) The revised pyramid: systematic reviews are a lens through ...
There are two types of evidence. First hand research is research you have conducted yourself such as interviews, experiments, surveys, or personal experience and anecdotes. Second hand research is research you are getting from various texts that has been supplied and compiled by others such as books, periodicals, and Web sites. Regardless of ...
Types of Evidence. It can be useful to separate and identify different types of evidence used in an argument to support a conclusion. This can help you avoid getting "lost" in the words; if you're reading actively and recognizing what type of evidence you're looking at, then you're more likely to stay focused.
rigorous research. Common Source of this Evidence Type (follow link for example): marketing testimonials Descriptive: Measures of Outcomes over Time Descriptive evidence summarizes characteristics of program participants and their outcomes over a period of time. This type of evidence is commonly found in marketing materials and news articles.
Ask your professor for clarification on the purpose and types of appropriate research questions and sources. Type. Purpose. Research question. Use of sources. Academic argument essay. To argue for a single claim or thesis through logic with evidence and analysis. Typically answers questions of how or why. Question is focused, answerable through ...
Worldwide, a significant proportion of clinical trials end up as costly research waste because their results are never made public.1-3 The resulting gaps in the medical evidence base harm patients and undermine public health.4 The Declaration of Helsinki and the WHO both call for all clinical trial results to be made public.5 6 In the wake of a 2018 UK parliamentary enquiry, non-commercial ...
Some studies have proposed indirect mechanisms through which IL6 enhances insulin secretion, and some studies have found no evidence supporting a direct role of IL6 in regulating β-cell function. These discrepancies may stem from variations in experimental conditions, research models, cell types, and concentrations of IL6 used in different ...
Types of study design. Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. Three main areas in primary research are basic medical research, clinical research ...
Additional research is required to fully understand the causal mechanism linking PIU and the observed changes in brain function. The divergent performance of individuals with PIU on the Go/No-Go and Flanker tasks adds to the growing body of evidence supporting the dissociation between the two components of inhibitory control.
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher's data, the CDC's figures also suggest a general decline in the abortion rate over time.
ACT Tessera Workforce was a multitrait multimethod assessment that measured six essential skills—work ethic, collaboration, resilience, creativity, leadership, and integrity. It employed three item types—Likert items, situational judgment tests (SJTs), and forced choice items. One important piece of information ACT gathered from the market was the need for a brief assessment—one much ...
Connect by phone 800-950-6264 or text "Helpline". to 62640, or chat. In a crisis call or text 988.*. Over the past several decades, NAMI has helped change the conversation around mental health, but there's more to do to ensure everyone can access the mental health care and support they need. Choose a one-time amount.