Resume Worded | Career Strategy
14 regulatory affairs specialist cover letters.
Approved by real hiring managers, these Regulatory Affairs Specialist cover letters have been proven to get people hired in 2024. A hiring manager explains why.


Table of contents
- Regulatory Affairs Specialist
- Senior Regulatory Affairs Specialist
- Regulatory Affairs Manager
- Regulatory Affairs Associate
- Alternative introductions for your cover letter
- Regulatory Affairs Specialist resume examples
Regulatory Affairs Specialist Cover Letter Example
Why this cover letter works in 2024, highlighting quantifiable achievements.
In this cover letter, the job seeker showcases their accomplishments by providing specific numbers of regulatory applications managed. This demonstrates their ability to handle workload and achieve success in the role.
Process Improvement Example
By mentioning the development of a streamlined process, the candidate shows initiative and problem-solving skills, which are highly valuable in the regulatory affairs field.
Showing Genuine Interest
This sentence effectively conveys the candidate's excitement about the role and their passion for contributing to the company's mission, making them stand out from other applicants who may have a more generic approach.
Establishing personal connection
By explaining your personal connection to the company's mission, you're indicating to the employer that you're not just interested in this role for a paycheck. Your passion and drive come from a personal place which can mean more motivation and dedication to the role. This can really resonate with recruiters and make you a more memorable candidate.
Demonstrating impact through metrics
Providing concrete examples of your success, especially when they're quantified, can really underline your capabilities. This shows you're results-oriented and understand what it takes to achieve those results - traits any employer would appreciate.
Tailoring your skills to the company's needs
Linking your skills to the specific needs or goals of the company you're applying to can be a great way to show your awareness and understanding of their business. This shows you're already thinking about how you can contribute to their success.
Conveying genuine excitement for the job
Expressing genuine excitement for the role can be quite powerful. It shows that you're enthusiastic about the work and eager to make a positive impact. This can really resonate with employers and help you stand out.
Highlight Impactful Achievements
The fact that you led a critical compliance project and managed to speed up the FDA approval process by 20% is a big deal. It shows you can handle responsibility and create impactful results. This is a surefire way to catch the attention of hiring managers.
Show Alignment with Company's Mission
Speaking to Pfizer's mission in the context of what excites you about the job shows that you understand and align with their values. Employers want to know you're enthusiastic about the same things they are.
Express Motivation
Highlighting the chance to be part of a team that is at the forefront of innovative solutions is an effective way to convey your motivation. It clearly shows that you are driven not just by personal success but by being part of something bigger.
Express Eagerness to Discuss Further
Looking forward to discussing the opportunity further is a nice, straightforward way to express your interest and initiative. It also leaves the door open for dialogue, which is always a good thing.
Show your passion for the role and company
Talking about your excitement and drawing a personal connection to the company’s mission makes your cover letter memorable. It shows you're not just looking for any job, you're looking for this job.
Highlight your expertise in regulatory affairs
Mentioning your experience in navigating complex regulatory landscapes demonstrates your capability to handle the challenges of the role. It reassures the hiring manager of your proficiency.
Demonstrate impact with concrete results
By showing how you improved efficiency or solved a problem in your previous role, you appeal directly to what hiring managers look for: someone who can deliver results and make a significant contribution.
Emphasize your commitment to making a difference
Expressing a desire to impact patient lives connects your personal values with the company’s goals. It suggests you will be a motivated and passionate contributor.
Show eagerness to discuss your fit
Ending your cover letter with an invitation to discuss your application further demonstrates confidence in your qualifications and a proactive attitude towards securing the role.
Does writing cover letters feel pointless? Use our AI
Dear Job Seeker, Writing a great cover letter is tough and time-consuming. But every employer asks for one. And if you don't submit one, you'll look like you didn't put enough effort into your application. But here's the good news: our new AI tool can generate a winning cover letter for you in seconds, tailored to each job you apply for. No more staring at a blank page, wondering what to write. Imagine being able to apply to dozens of jobs in the time it used to take you to write one cover letter. With our tool, that's a reality. And more applications mean more chances of landing your dream job. Write me a cover letter It's helped thousands of people speed up their job search. The best part? It's free to try - your first cover letter is on us. Sincerely, The Resume Worded Team
Want to see how the cover letter generator works? See this 30 second video.
Connect with the company's mission
Sharing your initial attraction to the company's groundbreaking work immediately establishes a personal connection and shows that your values align with theirs.
Highlight regulatory landscape expertise
Demonstrating your understanding of the regulatory field and your success in navigating its challenges proves your capability and readiness for the role.
Demonstrate impact through project management
Your ability to lead successful projects and improve processes showcases your leadership skills and potential for positive impact within the company.
Show inspiration drawn from the company's work
Expressing how the company's commitment to innovation and patient care inspires you underscores your personal commitment to these values in your professional work.
Thank the reader and express eagerness for dialogue
A polite thank you and expression of hope for a discussion about your alignment with the role reflects professionalism and genuine interest in the opportunity.
Connect personal experience with company mission
When you share a personal story that links to the company's goals, it not only shows your genuine interest but also your understanding of the company's impact on real lives.
Demonstrate regulatory affairs success
Telling us about a specific achievement, like speeding up FDA clearance, proves you can handle key responsibilities and deliver results, which is exactly what we look for in a regulatory affairs specialist.
Highlight expertise in regulatory landscape
Expressing eagerness to apply your skills at a new company shows confidence in your ability to navigate complex regulatory environments, signaling that you're ready to tackle industry-specific challenges.
Share initiatives for team improvement
Initiating programs that lead to better performance and fewer errors tells us you're not just a team player but a proactive one who seeks continuous improvement in regulatory affairs practices.
State your contribution to the company's mission
Reiterating your excitement to contribute signifies a strong alignment with the company's mission, making you a more compelling candidate for the regulatory affairs team.
Senior Regulatory Affairs Specialist Cover Letter Example
Aligning personal and company mission.
When you align your professional mission with the company’s mission, you show that your values are in sync with the company’s. This sends a strong message to the employer that you’re not just there for a job, but you believe in what the company does. It shows you're committed and invested in their cause.
Highlighting successes and improvements
By mentioning your achievement, like managing regulatory strategies with a 100% approval rate, you're showing the employer that you're reliable and can deliver tangible results. Accomplishments like these, especially if they include improvements or innovations you've made, can be a major selling point in your cover letter.
Matching experience with company culture
When you discuss your experience and skills in the context of the company's environment and culture, you're showing the employer that you'd be a good fit not just for the role, but for the team and company as a whole.
Expressing alignment of ambition and role responsibilities
When you express that the role aligns with your personal ambitions, you're telling the employer that you're not just there for the job, but are genuinely interested in the work and its impact. This can make you seem more dedicated and committed to the role, which is always a plus in the eyes of employers.
Express enthusiasm for the company’s mission
Starting your cover letter by acknowledging the company’s contributions to the industry sets a positive tone and aligns your interests with theirs from the beginning.
Share a standout achievement
Discussing a significant accomplishment, like leading a successful drug approval, showcases your leadership skills and ability to drive critical projects to completion.
Highlight your proactive approach to challenges
Illustrating your ability to foresee and manage potential issues reassures the hiring manager that you are a forward-thinking problem solver.
Detail your meticulousness and proactive strategies
Conveying your attention to detail and your strategy for maintaining compliance emphasizes your thoroughness and reliability, qualities highly valued in regulatory roles.
Connect your aspirations with the company’s goals
Expressing admiration for the company’s commitment and your desire to contribute to its mission shows that your professional values are in harmony with theirs, making you a culturally compatible candidate.
Show your passion for the industry
When you express genuine interest in the biotech field and acknowledge the company's impact, it sets a positive tone. This approach shows that you're not just looking for any job, but you're eager to contribute to specific advancements and innovations.
Highlight your successful project management
Talking about your direct involvement in developing and executing regulatory plans not only showcases your capability but also demonstrates your hands-on experience with critical projects. This makes you a more compelling candidate because it proves you can handle the responsibilities of a senior role.
Demonstrate your collaborative skills
By mentioning your ability to maintain relationships and navigate regulatory pathways, you're highlighting important soft skills. These are critical in roles that require cross-functional teamwork and dealing with external agencies, showing that you can be a linchpin in complex processes.
Aligning your motivations with the company's goals, especially in terms of patient-focused drug development, suggests that your values and work ethic match the company's culture. This can make you a more attractive candidate to employers who value mission-driven employees.
Express gratitude and eagerness to contribute
Closing with a thank you and a forward-looking statement indicates professionalism and a positive attitude. It wraps up your cover letter on a courteous note, leaving a lasting impression of your eagerness to join their team.
Show admiration for the company's work
Expressing long-term admiration for a company's innovation demonstrates both familiarity with their work and alignment with their mission, key for a senior regulatory affairs specialist.
Illustrate impact through strategic leadership
Describing how you led a strategy that significantly reduced time-to-market highlights your leadership skills and ability to make impactful decisions in regulatory affairs.
Emphasize capabilities in overcoming regulatory challenges
Showing eagerness to bring your experience in navigating regulatory hurdles to a new setting underlines your problem-solving skills and readiness for complex tasks.
Focus on talent development and retention
Creating a training program that improved retention rates proves you value team growth and can contribute to a positive and sustainable work environment in regulatory affairs.
Communicate enthusiasm for contributing to healthcare innovation
Conveying excitement to join and contribute to a company's mission showcases your motivation and eagerness to be part of their life-changing work, making you an attractive candidate for the senior regulatory affairs specialist role.
Regulatory Affairs Manager Cover Letter Example
Show how you overcame challenges.
Leading a team through the intricate process of obtaining CE marking for a novel medical device and expanding your market reach is a great story. It showcases your leadership, problem-solving skills, and your ability to overcome regulatory challenges.
Link Your Skills to Company's Mission
Linking your expertise in global regulatory strategies to Johnson & Johnson's mission demonstrates that you understand how your skills can contribute to the company's larger goals. This makes you come across as someone who thinks strategically and can contribute at a high level.
Value Company's Focus
Expressing excitement about the prospect of working in an environment that values regulatory excellence shows you appreciate and value the company's focus. It reassures the employer that you understand the importance of their work and are ready to uphold their standards.
Offer Unique Skills
By highlighting your strategic approach to regulatory affairs, your experience with market expansion, and your passion for healthcare innovation, you are effectively showcasing your unique blend of skills and experiences. This helps the hiring team picture how you could fit into their organization.
Express Desire to Contribute
Expressing that you look forward to the chance to contribute to the team's success shows your eagerness to make a positive impact. It's an excellent way to end your cover letter on an enthusiastic note.
Regulatory Affairs Associate Cover Letter Example
Show your passion for regulatory affairs work.
A strong interest in the specific mission of a company, like transforming lives through rare disease therapies, shows you're not just looking for any job but a place where you can fulfill your passions.
Value of attention to detail in regulatory documents
Highlighting your ability to handle complex regulatory guidelines and ensure document compliance demonstrates your readiness for the meticulous nature of regulatory affairs work.
Convey excitement for the role
Expressing eagerness to contribute to life-changing therapies showcases your motivation and potential to be a value-adding team member.
Express gratitude and enthusiasm to contribute
Ending your cover letter on a note of thanks coupled with a restatement of your desire to make a meaningful impact leaves a positive and memorable impression.
Offer to discuss alignment with company goals
Inviting further discussion about how your skills and passion meet the company's mission highlights your proactive approach and eagerness to contribute to their success.
Share your initial attraction to the company
Starting with why you were drawn to the company highlights your enthusiasm and shows that you've done your homework. This approach makes your application more personal and suggests you're a candidate who will be committed to the company's mission.
Illustrate your attention to detail
By detailing your experience with regulatory filings and emphasizing your focus on accuracy, you're assuring the employer of your capability to manage essential tasks. It speaks volumes about your professionalism and potential to contribute to the company's regulatory compliance efforts.
Showcase your efficiency and adaptability
Mentioning how you improved a document management process not only proves your initiative but also your capacity to enhance efficiency. Employers value such qualities in a fast-paced environment, marking you as a resourceful and adaptable candidate.
Express your career development goals
Stating your excitement for professional growth at the company signifies your long-term interest and commitment. It indicates that you're not just looking for a job but an opportunity to evolve and contribute significantly.
Conclude with gratitude and readiness
Ending your cover letter by thanking the employer and showing eagerness to discuss your role further demonstrates good manners and genuine interest. It's a positive and professional way to conclude, suggesting you're ready and keen to take on the challenges of the position.
Show your admiration for the company's mission
Talking about your respect for the company's work helps me see your genuine interest. It's good to know you understand what we do and want to be part of it.
Highlight your relevant experience
When you share a specific success story, it gives me a clear picture of what you can do. It's helpful to see you've done similar work before and succeeded.
Connect your skills to our needs
Explaining how your abilities can help with our projects makes your application stronger. It tells me you're thinking about what you can bring to the team.
Emphasize ongoing learning in regulatory affairs
Showing you're actively learning about regulatory changes demonstrates dedication. It's important in this field to stay updated, and your effort to do so is a big plus.
Express excitement about the role
Your enthusiasm about joining the company is infectious. It makes me more interested in meeting you and seeing how your passion and skills could benefit our team.
Alternative Introductions
If you're struggling to start your cover letter, here are 6 different variations that have worked for others, along with why they worked. Use them as inspiration for your introductory paragraph.
Cover Letters For Jobs Similar To Regulatory Affairs Specialist Roles
- Regulatory Affairs Associate (Entry Level) Cover Letter Guide
- Regulatory Affairs Manager Cover Letter Guide
- Regulatory Affairs Specialist Cover Letter Guide
Other Legal Cover Letters
- Attorney Cover Letter Guide
- Contract Specialist Cover Letter Guide
- Lawyer Cover Letter Guide
- Underwriter Cover Letter Guide

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.


Regulatory Affairs Associate Cover Letter Examples & Writing Tips
Use these Regulatory Affairs Associate cover letter examples and writing tips to help you write a powerful cover letter that will separate you from the competition.

Table Of Contents
- Regulatory Affairs Associate Example 1
- Regulatory Affairs Associate Example 2
- Regulatory Affairs Associate Example 3
- Cover Letter Writing Tips
Regulatory affairs associates are responsible for ensuring that their company’s products meet all applicable regulatory requirements. They work with a variety of departments, including marketing, R&D, and manufacturing, to make sure that products are safe and compliant with regulations.
To get a job in regulatory affairs, you need to have a strong cover letter. Use these examples and tips to write a cover letter that will help you stand out from the competition.
Regulatory Affairs Associate Cover Letter Example 1
I am excited to be applying for the Regulatory Affairs Associate position at Topdown Pharmaceuticals. I have a degree in biology and three years of experience working in the pharmaceutical industry. I am confident that I have the skills and qualifications that you are looking for in a Regulatory Affairs Associate.
I have experience working in a regulatory affairs department where I was responsible for compiling and submitting regulatory submissions to the FDA. I have also worked in quality assurance, where I was responsible for reviewing and approving product labeling, advertising, and promotional materials. I have a strong understanding of the regulatory process and the requirements of the FDA.
I am a motivated and detail-oriented individual who is able to work independently and under pressure. I am confident that I would be a valuable asset to your team. I look forward to discussing the Regulatory Affairs Associate position with you in more detail. Thank you for your time and consideration.
Regulatory Affairs Associate Cover Letter Example 2
I am writing to apply for the Regulatory Affairs Associate position that was recently posted on your website. I am confident that I have the skills and qualifications that you are looking for, and I am eager to put my experience to work for your company.
I have more than three years of experience working in the regulatory affairs field. In this time, I have gained a deep understanding of the regulatory process and the necessary skills to navigate it. I am well-versed in the regulations of both the FDA and the EPA, and I have experience working with a variety of stakeholders, including regulatory agencies, manufacturers, and distributors.
I am a strategic thinker with a proven track record of success. I have a keen eye for detail, and I am able to think outside the box to come up with creative solutions to problems. I am also a strong team player, and I have a track record of working well with others to achieve common goals.
I am confident that I have the skills and qualifications to be a valuable asset to your team. I would be grateful for the opportunity to discuss my qualifications further with you in an interview. Thank you for your time and consideration.
Regulatory Affairs Associate Cover Letter Example 3
I am writing to express my interest in the Regulatory Affairs Associate position that you have posted. I believe that my experience and education make me a strong candidate for this position.
I have been working in the pharmaceutical industry for the past five years, most recently as a Regulatory Affairs Specialist at Pfizer. In this role, I was responsible for managing all aspects of regulatory affairs for a portfolio of products including biologics, vaccines, and small molecules. My responsibilities included developing and maintaining regulatory strategies, preparing submissions to the FDA and other regulatory agencies, and providing regulatory support to clinical trial sites.
In addition to my work experience, I have also completed an internship with the Food and Drug Administration (FDA) where I gained valuable insight into how the agency works. I believe that my background makes me a strong candidate for your position.
I would appreciate the opportunity to discuss my qualifications with you in person. Thank you for your time and consideration.
Regulatory Affairs Associate Cover Letter Writing Tips
1. showcase your experience.
When applying for a regulatory affairs associate position, it’s important to showcase your experience in the field. This can be done by providing specific examples of how you’ve helped companies meet regulatory requirements in the past.
You can also highlight your knowledge of the regulatory process by describing how you stay up-to-date with the latest changes. For example, you might mention reading industry journals or attending regulatory affairs events.
2. Customize your cover letter
Since regulatory affairs associates are responsible for ensuring that companies comply with regulations, it’s important that your cover letter reflects your understanding of the company’s specific needs.
To do this, you can customize your cover letter by referencing the company’s website or job description. For example, if the company is looking for someone with experience in a specific area of regulatory affairs, be sure to mention any relevant experience you have.
3. Demonstrate your problem-solving skills
As a regulatory affairs associate, you’ll be responsible for solving problems and addressing any compliance issues. To demonstrate that you have the skills needed for the job, explain how you’ve tackled difficult problems in the past.
For example, you might talk about how you developed a new process to streamline regulatory submissions or how you created a training program to help employees understand new regulations.
4. Proofread your cover letter
Child care assistant cover letter examples & writing tips, market researcher cover letter examples & writing tips, you may also be interested in..., insurance analyst cover letter examples & writing tips, middle school teacher cover letter examples & writing tips, commercial manager cover letter examples, health and wellness coordinator cover letter examples & writing tips.

Regulatory Affairs Specialist Cover Letter Examples (Template & 20+ Tips)
Create a standout regulatory affairs specialist cover letter with our online platform. browse professional templates for all levels and specialties. land your dream role today.

Are you looking to break into the field of Regulatory Affairs? Our comprehensive guide to writing a Regulatory Affairs Specialist Cover Letter will provide you with all the resources you need to craft an effective and engaging letter. We'll show you how to grab the attention of potential employers and demonstrate your knowledge of regulatory compliance. Get ready to take the first step in your career!
We will cover:
- How to write a cover letter, no matter your industry or job title.
- What to put on a cover letter to stand out.
- The top skills employers from every industry want to see.
- How to build a cover letter fast with our professional Cover Letter Builder .
- What a cover letter template is, and why you should use it.
Related Cover Letter Examples
- Trial Attorney Cover Letter Sample
- Litigation Paralegal Cover Letter Sample
- Intellectual Property Attorney Cover Letter Sample
- Real Estate Attorney Cover Letter Sample
- Legal Assistant Cover Letter Sample
- Trust Officer Cover Letter Sample
- Claims Investigator Cover Letter Sample
- Regulatory Affairs Manager Cover Letter Sample
- Government Contractor Cover Letter Sample
- Legal Secretary Cover Letter Sample
- Advocate Cover Letter Sample
- Contract Negotiator Cover Letter Sample
- Bankruptcy Paralegal Cover Letter Sample
- Funeral Director Cover Letter Sample
- Litigation Legal Assistant Cover Letter Sample
- Contract Attorney Cover Letter Sample
- Priest Cover Letter Sample
- Trademark Paralegal Cover Letter Sample
- Defense Attorney Cover Letter Sample
- Claims Examiner Cover Letter Sample
Regulatory Affairs Specialist Cover Letter Sample
Dear Hiring Manager,
I am writing to express my interest in the Regulatory Affairs Specialist position at XYZ Corporation. My extensive experience with regulatory affairs and compliance management make me an ideal candidate for this role.
I have over five years of experience in regulatory affairs. I have an in-depth knowledge of the various rules and regulations related to the development, manufacturing, distribution, and sale of medical devices. I am well-versed in preparing and submitting regulatory applications, such as 510(k)s, PMAs, and IDE applications. I am also experienced in developing and implementing compliance programs, such as ISO and FDA requirements.
In my current role as Regulatory Affairs Specialist at ABC Corporation, I am responsible for leading the regulatory affairs and compliance team. I have successfully managed the development, review, and submission of a variety of regulatory applications, and I have developed and implemented compliance programs that ensure that our products meet all regulatory requirements. My work has enabled us to quickly bring our products to market and maintain compliance with all applicable regulations.
I am an organized and detail-oriented professional who is able to work independently and as part of a team. I am confident that my experience and skills make me an ideal candidate for this role. I would welcome the opportunity to discuss my qualifications in more detail.
Thank you for your consideration.
Sincerely, John Doe
Why Do you Need a Regulatory Affairs Specialist Cover Letter?
- A Regulatory Affairs Specialist cover letter is essential for anyone looking to make a career in the field of regulatory affairs.
- It is important to demonstrate your understanding of regulatory affairs principles and practices, as well as your ability to work in a team environment and to meet deadlines.
- A Regulatory Affairs Specialist cover letter will also allow you to highlight any relevant qualifications, such as certifications, or other experience that may set you apart from other applicants.
- In addition, a Regulatory Affairs Specialist cover letter can help to showcase your communication skills, problem-solving ability, and knowledge of regulatory processes.
- Overall, a Regulatory Affairs Specialist cover letter is an important tool to help you stand out among other applicants and show potential employers that you are the right candidate for the job.
A Few Important Rules To Keep In Mind
- Address the letter to the hiring manager or recruiter.
- Include a brief introduction that explains why you are writing.
- Outline your relevant qualifications and expertise.
- Provide examples of your accomplishments in regulatory affairs.
- Explain why you are interested in the position.
- Highlight your communication and organizational skills.
- Close the letter with a call to action.
- Proofread the letter for any mistakes or typos.
What's The Best Structure For Regulatory Affairs Specialist Cover Letters?
After creating an impressive Regulatory Affairs Specialist resume , the next step is crafting a compelling cover letter to accompany your job applications. It's essential to remember that your cover letter should maintain a formal tone and follow a recommended structure. But what exactly does this structure entail, and what key elements should be included in a Regulatory Affairs Specialist cover letter? Let's explore the guidelines and components that will make your cover letter stand out.
Key Components For Regulatory Affairs Specialist Cover Letters:
- Your contact information, including the date of writing
- The recipient's details, such as the company's name and the name of the addressee
- A professional greeting or salutation, like "Dear Mr. Levi,"
- An attention-grabbing opening statement to captivate the reader's interest
- A concise paragraph explaining why you are an excellent fit for the role
- Another paragraph highlighting why the position aligns with your career goals and aspirations
- A closing statement that reinforces your enthusiasm and suitability for the role
- A complimentary closing, such as "Regards" or "Sincerely," followed by your name
- An optional postscript (P.S.) to add a brief, impactful note or mention any additional relevant information.
Cover Letter Header
A header in a cover letter should typically include the following information:
- Your Full Name: Begin with your first and last name, written in a clear and legible format.
- Contact Information: Include your phone number, email address, and optionally, your mailing address. Providing multiple methods of contact ensures that the hiring manager can reach you easily.
- Date: Add the date on which you are writing the cover letter. This helps establish the timeline of your application.
It's important to place the header at the top of the cover letter, aligning it to the left or center of the page. This ensures that the reader can quickly identify your contact details and know when the cover letter was written.
Cover Letter Greeting / Salutation
A greeting in a cover letter should contain the following elements:
- Personalized Salutation: Address the hiring manager or the specific recipient of the cover letter by their name. If the name is not mentioned in the job posting or you are unsure about the recipient's name, it's acceptable to use a general salutation such as "Dear Hiring Manager" or "Dear [Company Name] Recruiting Team."
- Professional Tone: Maintain a formal and respectful tone throughout the greeting. Avoid using overly casual language or informal expressions.
- Correct Spelling and Title: Double-check the spelling of the recipient's name and ensure that you use the appropriate title (e.g., Mr., Ms., Dr., or Professor) if applicable. This shows attention to detail and professionalism.
For example, a suitable greeting could be "Dear Ms. Johnson," or "Dear Hiring Manager," depending on the information available. It's important to tailor the greeting to the specific recipient to create a personalized and professional tone for your cover letter.
Cover Letter Introduction
An introduction for a cover letter should capture the reader's attention and provide a brief overview of your background and interest in the position. Here's how an effective introduction should look:
- Opening Statement: Start with a strong opening sentence that immediately grabs the reader's attention. Consider mentioning your enthusiasm for the job opportunity or any specific aspect of the company or organization that sparked your interest.
- Brief Introduction: Provide a concise introduction of yourself and mention the specific position you are applying for. Include any relevant background information, such as your current role, educational background, or notable achievements that are directly related to the position.
- Connection to the Company: Demonstrate your knowledge of the company or organization and establish a connection between your skills and experiences with their mission, values, or industry. Showcasing your understanding and alignment with their goals helps to emphasize your fit for the role.
- Engaging Hook: Consider including a compelling sentence or two that highlights your unique selling points or key qualifications that make you stand out from other candidates. This can be a specific accomplishment, a relevant skill, or an experience that demonstrates your value as a potential employee.
- Transition to the Body: Conclude the introduction by smoothly transitioning to the main body of the cover letter, where you will provide more detailed information about your qualifications, experiences, and how they align with the requirements of the position.
By following these guidelines, your cover letter introduction will make a strong first impression and set the stage for the rest of your application.
Cover Letter Body
As an experienced Regulatory Affairs Specialist, I am confident in my ability to make a positive contribution to your organization. I understand the importance of staying up to date on regulations and laws and am experienced in developing and implementing compliance strategies. I have a successful track record of helping organizations maintain compliance with applicable laws and regulations, while also providing guidance and support on related issues.
My qualifications include:
- Knowledge of Regulatory Processes and Compliance: I have a comprehensive knowledge of regulatory processes, including those associated with product development, registration, and compliance. I am experienced in developing compliance strategies and implementing plans that are effective in meeting regulatory requirements.
- Project Management: I am highly organized and have a proven ability to handle multiple projects simultaneously while meeting deadlines. I am comfortable working in fast-paced environments and thrive when presented with challenging projects.
- Communication: I have excellent oral and written communication skills and am comfortable presenting information to colleagues and stakeholders. I am a confident negotiator and have a track record of building strong relationships with internal and external stakeholders.
- Problem-Solving: I am experienced in identifying issues and developing effective solutions. I have a proven ability to think strategically and analyze complex problems. I am confident in my ability to develop strategies and plans that effectively address issues and ensure compliance.
I am confident that I have the qualifications and experience your organization needs and I look forward to the opportunity to discuss my qualifications in person. Thank you for your time and consideration.
Complimentary Close
The conclusion and signature of a cover letter provide a final opportunity to leave a positive impression and invite further action. Here's how the conclusion and signature of a cover letter should look:
- Summary of Interest: In the conclusion paragraph, summarize your interest in the position and reiterate your enthusiasm for the opportunity to contribute to the organization or school. Emphasize the value you can bring to the role and briefly mention your key qualifications or unique selling points.
- Appreciation and Gratitude: Express appreciation for the reader's time and consideration in reviewing your application. Thank them for the opportunity to be considered for the position and acknowledge any additional materials or documents you have included, such as references or a portfolio.
- Call to Action: Conclude the cover letter with a clear call to action. Indicate your availability for an interview or express your interest in discussing the opportunity further. Encourage the reader to contact you to schedule a meeting or provide any additional information they may require.
- Complimentary Closing: Choose a professional and appropriate complimentary closing to end your cover letter, such as "Sincerely," "Best Regards," or "Thank you." Ensure the closing reflects the overall tone and formality of the letter.
- Signature: Below the complimentary closing, leave space for your handwritten signature. Sign your name in ink using a legible and professional style. If you are submitting a digital or typed cover letter, you can simply type your full name.
- Typed Name: Beneath your signature, type your full name in a clear and readable font. This allows for easy identification and ensures clarity in case the handwritten signature is not clear.
Common Mistakes to Avoid When Writing a Regulatory Affairs Specialist Cover Letter
When crafting a cover letter, it's essential to present yourself in the best possible light to potential employers. However, there are common mistakes that can hinder your chances of making a strong impression. By being aware of these pitfalls and avoiding them, you can ensure that your cover letter effectively highlights your qualifications and stands out from the competition. In this article, we will explore some of the most common mistakes to avoid when writing a cover letter, providing you with valuable insights and practical tips to help you create a compelling and impactful introduction that captures the attention of hiring managers. Whether you're a seasoned professional or just starting your career journey, understanding these mistakes will greatly enhance your chances of success in the job application process. So, let's dive in and discover how to steer clear of these common missteps and create a standout cover letter that gets you noticed by potential employers.
- Not customizing the cover letter to the specific role
- Using incorrect company name, job title, or other details
- Using a generic opening statement
- Failing to highlight relevant qualifications
- Including too many details
- Using informal language or slang
- Using an unprofessional email address
- Making spelling or grammar mistakes
- Using generic closing statements
- Not including a call to action
Key Takeaways For a Regulatory Affairs Specialist Cover Letter
- Demonstrate knowledge of FDA regulations and laws related to the medical device and pharmaceuticals industries.
- Highlight experience in regulatory affairs, such as filing submissions, preparing regulatory documents, and conducting research and analysis.
- Mention any relevant certifications, such as Certified Regulatory Affairs Professional (CRA).
- Showcase attention to detail and ability to work under tight deadlines.
- Illustrate strong communication and organizational skills.
- Emphasize commitment to accuracy, compliance, and quality assurance.
- Express enthusiasm for the position and company.


Regulatory Affairs Associate Cover Letter Example
A Regulatory Affairs Associate is responsible for ensuring that an organization’s products comply with all applicable regulations and laws. They work closely with legal and regulatory personnel to ensure that company products are safe and effective, as well as in line with national and international regulations.
You can’t get a job until you have a compelling cover letter, and this means have to do justice when you write your Regulatory Affairs Associate Cover Letter . There is no use if you submit a mediocre cover letter, as this will not impress your hiring manager. But, if you take sufficient time and write a quality cover letter application, then your cover letter would be taken seriously. Typical Cover Letters will not work always, and writing a high-quality one is not that easy unless you research and play with sentences. Here is a Cover Letter Sample that masters these two concepts simultaneously.

- Cover Letters
A Regulatory Affairs Associate is responsible for providing support to regulatory operations, in compliance with national and international regulations. This includes managing regulatory submissions, responding to regulatory authorities, keeping up to date on regulatory changes and trends, and working with cross-functional teams in the organization. The ideal Regulatory Affairs Associate should have strong knowledge of the pharmaceutical regulatory environment, excellent communication and organizational skills, and experience in a regulatory filing.
What to Include in a Regulatory Affairs Associate Cover Letter?
Roles and responsibilities.
- Researching and staying informed of applicable laws and regulations.
- Developing and maintaining a thorough understanding of regulatory requirements.
- Preparing and submitting applications for product registration and licensure.
- Assisting in the development and implementation of regulatory strategies .
- Coordinating with other departments to ensure compliance with regulations.
- Supporting and managing internal regulatory audits.
- Communicating with regulatory agencies on behalf of the company.
- Maintaining regulatory files, policies, and procedures.
- Providing guidance and training to other departments on regulatory requirements.
Education & Skills
Regulatory affairs associate skills:.
- Knowledge of FDA regulations, as well as other international regulatory systems.
- Familiarity with clinical development processes.
- Ability to interpret and apply regulatory requirements.
- Excellent writing and communication skills.
- Ability to work in a fast-paced environment.
- Proficiency in Microsoft Office.
- Project management experience.
- Ability to multi-task and prioritize tasks.
- Ability to work well with cross-functional teams.
- Attention to detail and accuracy.
Regulatory Affairs Associate Education Requirements:
- Bachelor’s degree in a related field such as biology, chemistry, pharmacy, or a related field.
- Master’s degree in a related field such as regulatory affairs.
- Knowledge of relevant regulations, laws, and guidelines.
Regulatory Affairs Associate Cover Letter Example (Text Version)
Dear Mr./Ms.
I am writing to express my interest in the Regulatory Affairs Associate position at [Company Name]. With more than seven years of experience in the regulatory affairs field, I believe I can be a valuable addition to your team.
I have extensive experience in working with the FDA, EMA, and other regulatory bodies, to ensure that all regulatory requirements are met. I have a strong understanding of the regulatory processes and the challenges this field can present. I am also skilled at developing strategies to ensure compliance and staying up to date with changing regulations.
My qualifications include:
- 7+ years of experience in the medical device industry, including experience as a Regulatory Affairs Associate and Regulatory Affairs Manager.
- Comprehensive knowledge of FDA requirements and other applicable regulations.
- Proven ability to develop, implement, and maintain regulatory strategies.
- Expertise in preparing and submitting documentation to regulatory agencies.
- Ability to analyze, interpret, and revise regulatory documents and policies.
- Proficiency in researching to identify and resolve regulatory issues.
- Achieved successful completion of a variety of FDA submissions and clearance requests.
- Adept at coordinating with internal teams and external organizations to ensure compliance.
I have experience in conducting gap analyses, preparing regulatory documents and applications, and monitoring and tracking submissions. I have a strong ability to interpret and apply regulations to ensure compliance. Additionally, I have experience in liaising with cross-functional teams to ensure successful product launches.
I am confident that I would be an asset to your organization. I have a proven track record of success in regulatory affairs and I am eager to bring my experience and knowledge to your team. I am available for an interview at any time and I look forward to hearing from you.
Sincerely, [Your Name]
When writing a cover letter for a Regulatory Affairs Associate role, focus on demonstrating your knowledge of regulatory principles, your attention to detail, and your ability to work effectively with stakeholders. Highlight your research, communication, and organizational skills, and provide specific examples of your success in relevant roles. Finally, emphasize your enthusiasm for the position and your commitment to the organization.
Need some tips for writing your resume, refer to our Regulatory Affairs Associate Resume Samples !

Customize Regulatory Affairs Associate Cover Letter
Get hired faster with our free cover letter template designed to land you the perfect position.
Related Legal Cover Letters

- Resume Builder
- Resume Templates
- Resume Formats
- Resume Examples
- Cover Letter Builder
- Cover Letter Templates
- Cover Letter Formats
- Cover Letter Examples
- Career Advice
- Interview Questions
- Resume Skills
- Resume Objectives
- Job Description
- Job Responsibilities
- FAQ’s
Regulatory Affairs Specialist Cover Letter Example
Writing a cover letter can be an intimidating task. Crafting a persuasive and compelling letter that stands out from the competition requires careful consideration of the employer’s needs and an understanding of the skills and knowledge you have to offer. This guide provides a comprehensive overview of how to write a regulatory affairs specialist cover letter, from introduction to conclusion. It also includes a sample cover letter as an example to help you better understand the structure and content of an effective letter. Hopefully, this guide will give you the confidence and guidance to write a compelling cover letter that will increase your chances of securing an interview.
If you didn’t find what you were looking for, be sure to check out our complete library of cover letter examples .

Download the Cover Letter Sample in Word Document – Click Below

Start building your dream career today!
Create your professional cover letter in just 5 minutes with our easy-to-use cover letter builder!
Regulatory Affairs Specialist Cover Letter Sample
Dear [Hiring Manager],
I am writing to apply for the position of Regulatory Affairs Specialist that I recently discovered on your website. With more than 5 years of experience in the regulatory affairs field, I am confident that I have the qualifications, knowledge and experience to make a valuable contribution to your organization.
Throughout my career, I have developed extensive experience in regulatory compliance, product registration, regulatory filing, and product labeling. I have worked extensively with the FDA, ensuring compliance with all regulatory standards and guidelines. I have also developed strong skills in data analysis and interpretation, as well as problem- solving and strategic planning.
In addition to my regulatory affairs experience, I have a Master’s degree in Public Health and am currently completing an Advanced Regulatory Affairs Certification program. I have excellent organizational and communication skills, both written and oral. I am also proficient in various regulatory software programs and have experience working with FDA databases.
I am excited by the prospect of joining your team and am confident that I can bring my knowledge, skills and experience to help your organization achieve its objectives. I look forward to discussing my qualifications in further detail and I am available for an interview at your convenience.
[Your Name]
Create My Cover Letter
Build a profession cover letter in just minutes for free.
Looking to improve your resume? Our resume examples with writing guide and tips offers extensive assistance.
What should a Regulatory Affairs Specialist cover letter include?
A Regulatory Affairs Specialist cover letter should provide an engaging summary of your professional qualifications and experience. It should demonstrate your ability to analyze and interpret regulatory requirements and enforce compliance.
The cover letter should offer a snapshot of your experience, skills and qualifications and demonstrate why you are the ideal candidate for the role. It should also include a description of your experience in regulatory affairs and any relevant certifications you may have.
In addition, your cover letter should include information about your ability to work effectively and efficiently with regulatory agencies such as the FDA, ensuring that all compliance standards are met. You should also demonstrate your ability to assess and analyze products for regulatory compliance and develop strategies for regulatory approval.
Finally, your cover letter should highlight your excellent team- working and communication skills, along with any other related expertise that will benefit the employer. This will help to make you stand out from the crowd and give the employer an insight into your personality and enthusiasm.
Regulatory Affairs Specialist Cover Letter Writing Tips
Are you looking to land a job as a Regulatory Affairs Specialist? Writing a compelling cover letter is an essential step in the job search process. With the right strategies and techniques, your cover letter can be just as effective as your resume in helping you stand out from other qualified candidates. Here are some tips to help you write a strong cover letter for a Regulatory Affairs Specialist position:
- Identify the target position: Make sure to personalize your cover letter for each job you apply for, highlighting how your skills and experience make you the best fit for the role.
- Highlight your qualifications: Showcase the qualifications that make you an ideal candidate for the Regulatory Affairs Specialist role. Focus on the skills, credentials, and experience that are most relevant to the position.
- Show enthusiasm: Demonstrate your enthusiasm for the position and company in your cover letter. Let the hiring manager know that you are excited to be considered for the job.
- Keep it concise: Aim to keep your cover letter to one page in length. Write in a clear and concise manner, using language that is easy to understand.
- Proofread: Before you submit your cover letter, make sure to review it for any typos or errors. Have someone else read it over to make sure it’s error- free.
By following these cover letter writing tips, you can create a compelling cover letter that will showcase your qualifications and help you stand out from the competition. Good luck in your job search!
Common mistakes to avoid when writing Regulatory Affairs Specialist Cover letter
A cover letter is a critical part of a successful job search and a great way to introduce yourself to potential employers. As a Regulatory Affairs Specialist, it’s important to make sure your cover letter is spot on so you stand out from the competition. Here are some common mistakes to avoid when writing your Regulatory Affairs Specialist cover letter:
- Not tailoring your letter to the position: It’s important to customize your cover letter to the specific job you’re applying for. Include information that is directly related to the job description and the company.
- Not including a professional summary: A professional summary helps to quickly introduce yourself and explain why you would be a good fit for the role.
- Not addressing the letter to the right person: Make sure that you’re addressing your letter to the right person. If you’re not sure, it’s best to call the company to find out.
- Overusing technical terms and jargon: Although it’s important to demonstrate your expertise and knowledge, don’t use too much technical language when writing your cover letter.
- Not proofreading: It’s essential to proofread your cover letter for spelling and grammar mistakes.
- Not ending with a call to action: Make sure that you end your cover letter with a call to action to encourage the hiring manager to contact you.
By avoiding these common mistakes and taking the time to create a well- written cover letter, you’ll be well on your way to impressing your potential employer and landing the job.
Key takeaways
Writing an effective cover letter for a Regulatory Affairs Specialist role is essential to securing an interview. Your cover letter should showcase your experience and highlight how you meet the qualifications of the position. Here are some key takeaways to remember when crafting your cover letter:
- Start with a strong introduction that identifies your qualifications and why you are the perfect candidate for the role.
- Make sure to incorporate keywords from the job description to demonstrate that you are an ideal fit for the position.
- Be specific about your experience with regulatory affairs and how it can benefit the organization you’re applying to.
- Showcase your professional accomplishments and how you’ve helped organizations succeed in the past.
- Demonstrate your ability to work independently and collaboratively.
- Stress how you are a self- starter and can take initiative to complete projects successfully.
- Close by expressing your enthusiasm for the position and reiterating your interest in being considered.
By using these key takeaways, you can craft an impressive cover letter that will help you stand out from other candidates and give you a competitive edge.
Frequently Asked Questions
1. how do i write a cover letter for an regulatory affairs specialist job with no experience.
When writing a cover letter for an Regulatory Affairs Specialist job with no experience, you should emphasize your educational background and any relevant certifications or licenses. You should also highlight any transferable skills you possess that could be beneficial for the position. Additionally, you should include any volunteer or internship experience that is specific to the role you are applying for. Be sure to mention any other experiences that could demonstrate your commitment to the Regulatory Affairs Specialist field such as taking courses or attending industry conferences.
2. How do I write a cover letter for an Regulatory Affairs Specialist job experience?
When writing a cover letter for an Regulatory Affairs Specialist job with experience, you should focus on your past successes and accomplishments. Be sure to provide examples of how you applied your skills and knowledge to achieve positive results. Highlight any projects you may have worked on that are relevant to the position. Additionally, showcase your ability to work with cross- functional teams to achieve better outcomes.
3. How can I highlight my accomplishments in Regulatory Affairs Specialist cover letter?
In your Regulatory Affairs Specialist cover letter, you should highlight your accomplishments in a way that demonstrates your qualifications for the position. For example, you can discuss any successful projects you may have completed and how you were able to use your skills and knowledge to achieve better outcomes. Additionally, you can include any professional certifications or licenses you may have that are related to the role. Additionally, you can mention any awards or recognitions you have received for your work.
4. What is a good cover letter for an Regulatory Affairs Specialist job?
A good cover letter for an Regulatory Affairs Specialist job should include information on your educational background, any relevant certifications or licenses you possess, and any volunteer or internship experience you may have in the field. Additionally, you should include any professional accomplishments you have achieved that would be beneficial for the job. Be sure to showcase your ability to work with cross- functional teams to achieve better outcomes. Finally, you should also highlight any awards or recognitions you have received for your work.
In addition to this, be sure to check out our cover letter templates , cover letter formats , cover letter examples , job description , and career advice pages for more helpful tips and advice.
Let us help you build your Cover Letter!
Make your cover letter more organized and attractive with our Cover Letter Builder

Regulatory Affairs Associate Resume Guide
Regulatory Affairs Associates are responsible for ensuring that a company’s products and services comply with relevant regulatory requirements. They collaborate closely with other departments to ensure compliance, prepare documents and submissions for required approvals, monitor changes in regulations, develop strategies to address compliance issues, and provide advice on policies related to the regulation of their organization’s activities.
Your knowledge in regulatory affairs and compliance is unparalleled, but employers don’t know who you are. To make them aware of your qualifications and expertise, you must write an impressive resume that stands out from the competition.
This guide will walk you through the entire process of creating a top-notch resume. We first show you a complete example and then break down what each resume section should look like.

Table of Contents
The guide is divided into sections for your convenience. You can read it from beginning to end or use the table of contents below to jump to a specific part.
Regulatory Affairs Associate Resume Sample
Sydnie Krajcik Regulatory Affairs Associate
[email protected] 752-818-9237 linkedin.com/in/sydnie-krajcik
Skilled regulatory affairs associate with 8+ years of experience in the medical device industry. Adept at developing regulatory strategies and executing projects to achieve compliance for products within a global scope. Proven track record of success in obtaining timely approval from both domestic and international agencies such as FDA, ISO, CE Mark, etc. Seeking to join ABC Pharma’s Regulatory Affairs team to continue driving successful product launches worldwide.
Regulatory Affairs Associate, Employer A Grand Rapids, Jan 2018 – Present
- Successfully complied with FDA, EPA and OSHA regulations to ensure the highest quality of medical products were produced in accordance with industry standards; reduced non-compliance incidents by 40%.
- Spearheaded a project to update all product labeling information across 10+ countries, ensuring that language was consistent and compliant per each country’s regulatory guidelines within 3 months.
- Streamlined internal processes for document submission and review when registering new products across international markets, resulting in an 800% increase in efficiency during the onboarding process.
- Utilized best practices from previous experience to proactively identify potential compliance issues before they arose, preventing costly delays or penalties due to incorrect procedures or misinterpretations of policy/law changes.
- Assisted senior management team on various legal initiatives related to manufacturing agreements & patent applications for company’s product portfolio; achieved positive outcomes on 95% of cases handled independently over 2 year period.
Regulatory Affairs Associate, Employer B Palmdale, Mar 2012 – Dec 2017
- Represented a large pharmaceutical company in over 70 regulatory submissions to the FDA, ensuring that all guidelines and regulations were met; reduced review time by 25%.
- Structured an efficient filing system for documentation related to registration, certification, product labeling and safety reports; facilitated faster recall processes during emergency situations.
- Investigated potential customer complaints about products or services with regard to compliance issues within 6 hours of receipt; prevented costly litigation costs due to non-compliance issues in 90% of cases.
- Revised existing standards & procedures associated with local & international regulations concerning medical device manufacturing operations; improved overall efficiency of company’s quality control system by 30%.
- Effectively communicated changes in global healthcare laws & industry trends regarding clinical trials, drug safety data requirements and other areas relevant to the business on a weekly basis.
- Regulatory Affairs
- Pharmaceutical Industry
- Regulatory Submissions
- Biotechnology
- Medical Devices
Bachelor’s Degree in Regulatory Affairs Educational Institution XYZ Nov 2011
Certifications
Regulatory Affairs Certification (RAC) Regulatory Affairs Professionals May 2017
Related Resume Examples
- Regulatory Affairs Specialist
- Regulatory Affairs Coordinator
- Quality Assurance Associate
- Pharmacy Technician
- Quality Assurance Specialist
- Quality Control Analyst
- Quality Assurance Officer
1. Summary / Objective
A resume summary/objective for a Regulatory Affairs Associate should provide the hiring manager with an overview of your experience and qualifications. In this section, you can highlight key skills such as knowledge of FDA regulations and guidelines, expertise in preparing documents for regulatory submissions, and success in obtaining product approvals from government agencies. You could also mention any relevant certifications or awards that demonstrate your commitment to excellence in the field.
Below are some resume summary examples:
Talented Regulatory Affairs Associate with 7+ years of experience in drug development, clinical trials, and regulatory compliance. Proven success in developing strategies for obtaining FDA approval for new drugs and ensuring ongoing adherence to federal regulations. Adept at providing guidance on labeling requirements, marketing authorization applications (MAA), international registrations (CTD), IND submissions/amendments, etc.
Proficient regulatory affairs associate with 5+ years of experience in the medical device industry. Adept at monitoring regulatory changes and ensuring product compliance for both domestic and international markets. At XYZ, led a team of 7 regulatory professionals in developing strategies to ensure compliance with FDA regulations. Developed training materials that improved overall quality assurance processes by 25%.
Seasoned Regulatory Affairs Associate with 7+ years of experience in the healthcare sector, including 4 years at ABC Pharmaceuticals. Skilled in managing regulatory affairs activities to ensure compliance with global and local regulations for drug development programs across multiple therapeutic areas. Successfully developed comprehensive strategies for obtaining approval from health authorities worldwide.
Hard-working Regulatory Affairs Associate with 5+ years of experience in researching, preparing and submitting regulatory documents for pharmaceutical products. Professional background includes a Regulatory Compliance Certification from ABC University and proven success in obtaining FDA approval for seven products. Skilled at developing strategic plans to ensure compliance with all applicable regulations.
Driven Regulatory Affairs Associate with 5+ years of experience managing compliance and regulatory processes for a prominent pharmaceutical company. Proven success in developing, implementing, and maintaining comprehensive policies to ensure adherence to industry guidelines. Seeking an opportunity at ABC Pharma to use my expertise in global regulations and standards while growing within the organization.
Reliable and detail-oriented regulatory affairs associate with 7+ years of experience in the pharmaceutical industry. Skilled at developing and submitting high quality documentation to meet compliance requirements for various countries worldwide. Seeking a role at ABC Pharma, where I can use my knowledge and expertise to ensure that products are compliant with all applicable regulations.
Well-rounded Regulatory Affairs Associate with 5+ years of experience in the medical device industry. Adept at developing regulatory strategies, preparing documentation and reports for submission to relevant agencies, and leading product registration processes globally. Experienced in working closely with Quality Assurance teams to ensure that products meet international standards.
Enthusiastic Regulatory Affairs Associate with 5+ years of experience in coordinating and managing the regulatory process for pharmaceutical products. Skilled in assessing product registration requirements, developing strategies to ensure compliance, and preparing high quality submissions. Aiming to leverage my knowledge of global regulations as part of ABC’s Regulatory team.
2. Experience / Employment
The employment (or experience) section is where you provide details on your work history. This should be written in reverse chronological order, meaning the most recent job is listed first.
You want to stick with bullet points when writing this section; doing so makes it easier for the reader to take in all of the information that you are providing. When stating what you did, make sure to include quantifiable results and detail about how you achieved them.
For example, instead of saying “Managed regulatory documents,” say something like: “Maintained an up-to-date database of over 500 regulatory documents from both domestic and international sources which enabled compliance with FDA requirements.”
To write effective bullet points, begin with a strong verb or adverb. Industry specific verbs to use are:
- Interpreted
- Implemented
- Coordinated
- Investigated
Other general verbs you can use are:
- Demonstrated
- Facilitated
- Participated
- Reorganized
- Represented
- Spearheaded
- Streamlined
Below are some example bullet points:
- Reorganized regulatory filing system to streamline document submission process, reducing regulatory approval times by 25%.
- Documented and maintained records of all government regulations related to product development and manufacturing processes; identified non-compliance issues quickly due to proactive research efforts.
- Reduced the risk of FDA or other agency compliance violations by 50% through careful formulation reviews and testing protocols implementation that met company standards for product safety & efficacy.
- Presented detailed reports on new legislation impacting the medical device industry at executive meetings, keeping upper management informed about current trends in their field of expertise.
- Diligently followed up with internal teams regarding pending submissions or inquiries from applicable governmental agencies; kept track of project milestones and timelines without fail or delay.
- Monitored and assessed regulatory trends and developments in the pharmaceutical industry to ensure compliance with FDA regulations, resulting in a 100% satisfactory rating from company auditors.
- Achieved cost savings of $5K annually by streamlining internal processes related to submission documents preparation and management.
- Prepared and submitted new product applications for over 20 products on time while meeting all applicable laws; successfully launched 15+ products within specified timelines after approval.
- Submitted reports, changes control forms & other documentation including clinical trial protocols as part of drug registration activities; achieved successful registrations in 10 countries across Europe within 6 months period.
- Resourcefully identified potential risks associated with post-marketing surveillance activities & proposed corrective action plans to mitigate them before they could cause any harm or financial losses for the organization.
- Introduced regulatory compliance strategies and updates to over 500 staff members, resulting in a 40% reduction of non-compliance incidents.
- Optimized the regulatory affairs workflow process by streamlining 20+ time-consuming tasks, saving 30 hours of work each week.
- Meticulously monitored safety standards & industry regulations related to medical device products; identified 9 potential risks areas that were resolved before being reported externally.
- Assessed product labeling requirements for accuracy across 15 countries and ensured they complied with local laws; reduced errors by 17%.
- Participated in various internal meetings with cross-functional teams regarding changes or amendments to regulatory policies and procedures; proposed 10 new ideas which were accepted into practice within 6 months.
- Compiled and submitted regulatory documents for more than 50 medical device applications, ensuring 100% compliance with FDA regulations and successfully obtaining approval from the agency in all cases.
- Thoroughly researched applicable local and international laws regarding product safety standards to ensure that projects met relevant requirements; identified 15 potential issues which were addressed beforehand, leading to a 10% reduction in costs associated with non-compliance penalties.
- Researched industry trends related to clinical trials for medical devices, creating an up-to-date resource library of best practices used by leading pharmaceutical companies; improved overall efficiency of premarketing efforts by 30%.
- Improved existing internal processes through the implementation of new procedures and systems designed to streamline operations while simultaneously maintaining quality control; reduced paperwork processing time by 50 hours per week on average without compromising accuracy or precision levels.
- Mentored three junior associates on regulatory affairs topics such as documentation preparation & filing requirements, risk management strategies and good manufacturing practices (GMP); provided guidance on numerous projects resulting in successful outcomes within established deadlines each time.
- Advised a team of 8 regulatory affairs specialists in preparing, submitting and managing over 150 applications for new product registration with a 95% success rate.
- Updated corporate compliance policies to reflect changes in local government regulations; organized training sessions and workshops for 60+ employees to ensure adherence to these guidelines.
- Implemented risk management strategies across the organization that reduced potential fines due to non-compliance by $20,000 per year on average.
- Actively monitored market dynamics and industry trends impacting regulatory decisions; identified key opportunities that resulted in 5 additional approvals from governmental agencies within 6 months of analysis period end date.
- Analyzed complex clinical data sets with statistical software packages such as SAS & SPSS, providing vital information used by senior executives when making strategic decisions related to products’ labelling & marketing campaigns.
- Facilitated regulatory compliance for 40+ clinical trials, ensuring timely submission and approval of associated documents to governing agencies.
- Coordinated with cross-functional teams to develop regulatory strategies that met global standards; reduced the time needed for product registration in target markets by 20%.
- Expedited review processes through efficient tracking of all regulatory records while liaising with external vendors on documentation requirements; decreased turnaround times by 30% on average.
- Competently evaluated potential risks related to new products and proposed mitigation plans based on relevant local regulations, leading to successful project completion within budgeted timelines and resources allocated.
- Formulated detailed reports outlining past developments in international medical device regulation laws as well as current trends influencing the industry landscape; contributed towards highly rated submissions during FDA inspection visits twice a year.
- Interpreted and implemented regulatory policies and procedures to ensure compliance with FDA and other international regulations, resulting in a 25% decrease in non-compliance incidents.
- Efficiently managed the coordination of multiple activities related to drug development process such as preclinical testing, clinical trial applications, post-marketing studies etc., thereby reducing timeline for product approval by 35%.
- Reviewed documentation for accuracy prior to submission and identified any potential regulatory risks; successfully submitted over 80 new products into foreign markets within six months of joining the firm.
- Developed strategies to improve processes from idea through launch that resulted in an increase of 20% operational efficiency while complying with all applicable laws & regulations across all countries/regions where company operates business operations.
- Demonstrated expertise in pharmaceuticals assessment & interpretation; provided training sessions on key industry standards & government requirements which led to improved quality control metrics across departments by 15%.
Two organizations that have advertised for a position with the same title may be searching for individuals whose skills are quite different. For instance, one may be looking for someone with experience in the medical device industry and another for a candidate who is familiar with pharmaceuticals.
It is therefore important to tailor the skills section of your resume to each job that you apply for, as this will help ensure that it passes through any applicant tracking systems used by employers.
You should also use other sections of your resume (such as summary or experience) to further elaborate on specific skills, such as regulatory compliance knowledge or project management expertise.
Below is a list of common skills & terms:
- Analytical Chemistry
- Biochemistry
- Cell Culture
- Change Control
- Clinical Research
- Clinical Trials
- Communication
- Data Analysis
- Laboratory Skills
- Life Sciences
- Lifesciences
- Molecular Biology
- Pharmaceutics
- Pharmacology
- Pharmacovigilance
- Product Development
- Quality Assurance
- Quality Control
- Quality System
- Regulatory Requirements
- Standard Operating Procedure
- Team Leadership
- Time Management
- U.S. Food and Drug Administration
4. Education
Including an education section on your resume will depend on how far along you are in your career. If you have just graduated and have no prior work experience, mention your education below the resume objective. On the other hand, if you already have a few years of regulatory affairs associate experience under your belt, an education section may not be necessary.
If including an educational background is relevant to the position you’re applying for, try to include courses and subjects related to regulatory affairs that demonstrate knowledge about this field.
5. Certifications
Certifications demonstrate to employers that you have the necessary skills and knowledge for a particular job. It also shows them that you are committed to staying up-to-date with industry trends and developments.
Including certifications on your resume can give hiring managers an indication of how well qualified you are for the position, so make sure to list any relevant ones in this section.
6. Contact Info
Your name should be the first thing a reader sees when viewing your resume, so ensure its positioning is prominent. Your phone number should be written in the most commonly used format in your country/city/state, and your email address should be professional.
You can also choose to include a link to your LinkedIn profile, personal website, or other online platforms relevant to your industry.
Finally, name your resume file appropriately to help hiring managers; for Sydnie Krajcik, this would be Sydnie-Krajcik-resume.pdf or Sydnie-Krajcik-resume.docx.
7. Cover Letter
Including a cover letter with your job application is a great way to introduce yourself and demonstrate why you are the perfect fit for the role. It should typically be made up of 2 to 4 paragraphs, in which you can provide more detail about your skills and experience that may not have been covered in your resume.
Although cover letters aren’t always required for most jobs, it’s highly recommended that you write one as it gives recruiters an insight into who you are and how passionate you feel about applying for this particular position.
Below is an example cover letter:
Dear London,
I am writing to apply for the Regulatory Affairs Associate position at [company name]. I have a Bachelor’s degree in Biology and a Master’s degree in Regulatory Affairs, and I am passionate about using my skills to help bring new products to market. In my previous role as a Regulatory Affairs Specialist, I was responsible for preparing and submitting regulatory filings to the FDA, managing clinical trials, and interacting with clients. I am confident that I can use my experience and knowledge to support your team in meeting all regulatory requirements.
I am knowledgeable of all relevant regulations governing the development and commercialization of pharmaceuticals, biologics, medical devices, and cosmetics. I have experience preparing IND/IDE applications, clinical trial protocols/reports, 510(k) submissions, annual reports, safety reports, etc. In addition to being detail-oriented and organized, I possess excellent written and verbal communication skills which will be valuable when interfacing with clients or other members of your team.
I believe that my qualifications make me an ideal candidate for this position on your team. My attached resume provides additional details regarding my education & training as well as professional experience. Please do not hesitate to contact me if you have any questions or would like more information about myself; I look forward to hearing from you soon! Thank you for your consideration!
Regulatory Affairs Associate Resume Templates
Regulatory Affairs Specialist Cover Letter Examples
A great regulatory affairs specialist cover letter can help you stand out from the competition when applying for a job. Be sure to tailor your letter to the specific requirements listed in the job description, and highlight your most relevant or exceptional qualifications. The following regulatory affairs specialist cover letter example can give you some ideas on how to write your own letter.

or download as PDF
Cover Letter Example (Text)
Shawniece Tarnowski
(639) 990-8310
Dear Danyella Mikha,
I am writing to express my interest in the Regulatory Affairs Specialist position at Pfizer Inc., as advertised. With a robust background in regulatory affairs complemented by five years of experience at Johnson & Johnson, I am excited about the opportunity to bring my expertise to your esteemed team.
At Johnson & Johnson, I honed my skills in ensuring compliance with all applicable global regulations and standards across a range of healthcare products. My responsibilities included preparing regulatory submissions, developing strategies for regulatory approval, and maintaining up-to-date knowledge of regulatory legislation. I have a proven track record of successfully managing multiple projects simultaneously while meeting tight deadlines and maintaining meticulous attention to detail.
I am particularly proud of my involvement in the expedited approval process of several high-profile products, which required close collaboration with cross-functional teams and regulatory agencies. My ability to navigate complex regulatory environments and my commitment to continuous learning have been pivotal in achieving favorable outcomes.
I am drawn to Pfizer Inc. because of your commitment to innovation and patient care, values that I share and have been integral to my professional journey. I believe that my experience in managing regulatory processes, coupled with my proactive approach and problem-solving abilities, will make a significant contribution to your company.
I am eager to further discuss how my background, skills, and enthusiasms align with the goals of Pfizer Inc. Thank you for considering my application. I look forward to the possibility of contributing to your team and the exciting challenges ahead.
Warm regards,
Related Cover Letter Examples
- Regulatory Affairs Associate
- Regulatory Affairs Manager
- Public Affairs Specialist
- Regulatory Compliance Specialist
- Regulatory Specialist
- Regulatory Analyst
- ResumeBuild
- Regulatory Affairs
5 Amazing regulatory affairs Resume Examples (Updated 2023) + Skills & Job Descriptions
Build your resume in 15 minutes, regulatory affairs: resume samples & writing guide, norman bailey, employment history.
- Provide guidance and support on regulatory matters to other departments
- Represent the organization in legal proceedings, such as arbitrations and litigation
- Advise on regulatory compliance issues related to products and services
- Maintain and update knowledge of relevant laws and regulations
- Identify and assess legal risks associated with business activities
- Develop and implement strategies to ensure ongoing compliance
- Develop and maintain regulatory compliance policies and procedures
Do you already have a resume? Use our PDF converter and edit your resume.
Professional Summary
- Monitor and analyze changes to applicable laws and regulations
- Draft, review, and negotiate contracts and other legal documents
- Provide advice and counsel to internal stakeholders on legal matters
- Create and maintain regulatory databases
- Manage relationships with regulatory bodies and other external stakeholders
Pauline Patterson
Norman wilson.
- Train internal stakeholders on regulatory compliance requirements
- Prepare and submit regulatory filings, such as license applications and notifications
Not in love with this template? Browse our full library of resume templates

Table of Content
- Introduction
- Resume Samples & Writing Guide
- Resume Example 1
- Resume Example 2
- Resume Example 3
- Resume Example 4
- Resume Example 5
- Jobs Description
- Jobs Skills
- Technical Skills
- Soft Skills
- How to Improve Your Resume
- How to Optimize Your Resume
- Cover Letter Example
regulatory affairs Job Descriptions; Explained
If you're applying for an regulatory affairs position, it's important to tailor your resume to the specific job requirements in order to differentiate yourself from other candidates. Including accurate and relevant information that directly aligns with the job description can greatly increase your chances of securing an interview with potential employers. When crafting your resume, be sure to use action verbs and a clear, concise format to highlight your relevant skills and experience. Remember, the job description is your first opportunity to make an impression on recruiters, so pay close attention to the details and make sure you're presenting yourself in the best possible light.
regulatory affairs
- Country Leader for Regulatory Affairs (Total Categories: Foods, Refreshments, Home Care & Personal Care) for Unilever Malaysia and Singapore, covering Brunei market too.
regulatory affairs Job Skills
For an regulatory affairs position, your job skills are a key factor in demonstrating your value to the company and showing recruiters that you're the ight fit for the role. It's important to be specific when highlighting your skills and ensure that they are directly aligned with the job requirements, as this can greatly improve your chances of being hired. By showcasing your relevant skills and experience, you can make a compelling case for why you're the best candidate for the job.
How to include technical skills in your resume:
Technical skills are a set of specialized abilities and knowledge required to perform a particular job effectively. Some examples of technical skills are data analysis, project management, software proficiency, and programming languages, to name a few. Add the technical skills that will get hired in your career field with our simple-to-use resume builder. Select your desired resume template, once you reach the skills section of the builder, manually write in the skill or simply click on "Add more skills". This will automatically generate the best skills for your career field, choose your skill level, and hit "Save & Next."
- Data Analysis
- Regulatory Compliance
- Contract Negotiation
- Legislative Knowledge
- Legal Writing
- Regulatory Requirements
- Regulatory Submissions
- Legal Research
- Regulatory Strategies
- Risk Assessment
- Litigation Support
- Statutory Interpretation
- Regulatory Interpretation
- Case Management
- Regulatory Processes
- Regulatory Monitoring
- Legal Analysis
- Corporate Law
- International Law
- Administrative Law
How to include soft skills in your resume:
Soft skills are non-technical skills that relate to how you work and that can be used in any job. Including soft skills such as time management, creative thinking, teamwork, and conflict resolution demonstrate your problem-solving abilities and show that you navigate challenges and changes in the workplace efficiently. Add competitive soft skills to make your resume stand-out to recruiters! Simply select your preferred resume template in the skills section, enter the skills manually or use the "Add more skills" option. Our resume builder will generate the most relevant soft skills for your career path. Choose your proficiency level for each skill, and then click "Save & Next" to proceed to the next section.
- Communication
- Interpersonal
- Time Management
- Problem Solving
- Decision Making
- Critical Thinking
- Adaptability
- Organization
- Public Speaking
- Negotiation
- Conflict Resolution
- Attention to Detail
- Self-Motivation
- Stress Management
- Collaboration
- Strategic Thinking
- Emotional Intelligence
- Flexibility
- Reliability
- Professionalism
- Computer Literacy
- Project Management
- Customer Service
- Presentation
- Written Communication
- Social Media
- Troubleshooting
- Quality Assurance
- Supervisory
- Risk Management
- Database Management
- Documentation
- Financial Management
- Visualization
- Business Acumen
- Process Improvement
- Relationship Management.
How to Improve Your regulatory affairs Resume
Navigating resume pitfalls can mean the difference between landing an interview or not. Missing job descriptions or unexplained work history gaps can cause recruiters to hesitate. Let's not even talk about the impact of bad grammar, and forgetting your contact info could leave your potential employer hanging. Aim to be comprehensive, concise, and accurate.
Floyd Franklin
Include your contact information and job descriptions, missing job descriptions lessens your chances of getting hired..
- Employers want to know what you've accomplished, so make sure to include descriptions for all of your previous jobs.
- Keep job descriptions short but don't just list your jobs.
- Never copy-paste a job description to post on your resume. Get inspired and use tools to help you write customized descriptions.
How to Optimize Your regulatory affairs Resume
Keep an eye out for these resume traps. Neglecting to detail your job roles or explain gaps in your career can lead to unnecessary doubts. Grammar blunders can reflect negatively on you, and without contact information, how can employers reach you? Be meticulous and complete.
Ursula Hawkins
- Identifynd assess legal risks assosiated wit business activities.
- Devellop and maintane regulatry complience policys and proceduers.
- Develp and implement strateges too ensure on-going complience.
- Mointor and anaylze changes too applicable laws and regulatiosn.
- Conducts internall compliances audits an reviews.
- Advices on regulatory compliances issues relateds to products and services.
- Povide guidence an suport on regulatiry mattters too othr departments.
- Maintainnd updat knowledge of relevant laws and regulatons.
- Preparend submit regulatory filings, such as licens applications and notifications.
Correct Grammar and Address Gap Years in Your Resume
Don't leave unexplained gaps in your work history..
- When explaining gaps in your employment section, start by being honest.
- Elaborate on the gap and show that you never stopped learning.
- Explain and elaborate any gap in your work history by highlighting new skills.
regulatory affairs Cover Letter Example
A cover letter can be a valuable addition to your job application when applying for an regulatory affairs position. Cover letters provide a concise summary of your qualifications, skills, and experience, also it also gives you an opportunity to explain why you're the best fit for the job. Crafting a cover letter that showcases your relevant experience and enthusiasm for the Accounts Payable role can significantly improve your chances of securing an interview.
To the respected Hogan Lovells Hiring Team
I am a results-driven Regulatory Affairs with 5 years of experience in the Legal field. I am excited to submit my application for the Lead Regulatory Affairs role at Hogan Lovells, where I believe I can make a valuable contribution to your team.
My diverse life experiences have taught me the importance of adaptability, creativity, and resilience. Whether it was on the job, or simply on my day to day, I have learned to navigate challenges and find innovative solutions. I am confident that I possess the skills and expertise necessary to excel in the position at Hogan Lovells and I am excited about the opportunity to grow with a team that values these qualities and contribute to your organization's growth and success.
I appreciate the time and consideration you have given my application. I am confident that if we work together we could achieve great things and so I look forward to the opportunity to join your team.
Showcase your most significant accomplishments and qualifications with this cover letter. Personalize this cover letter in just few minutes with our user-friendly tool!
Related Resumes & Cover Letters

Contemporary

Professional

Looking to explore other career options within the Legal field?
Check out our other resume of resume examples.
- Counsel Resume
- Attorney Resume
- Regulatory Affairs Resume
- Lawyer Resume
- Paralegal Resume
- Legal Assistant Resume
FIND EVERYTHING YOU NEED HERE.
IF YOU HAVE QUESTIONS, WE HAVE ANSWERS.

4 Ways a Career Test Can Jump-Start Your Future (and Help Your Resume)
If you’re looking for a fresh path or a new passion, a career test could help you find it. You can take these tests online, in the comfort of your...

Avoid These 3 Resume Mistakes at All Costs
Your resume is your first impression for a prospective employer. The way you present yourself in that little document can make or break you – it can clinch you an...

Resume Design Tips and Tricks
Creating a resume that stands out from the rest doesn’t have to be rocket science. With just a few tips and tricks, you can make your professional resume a shining...
Build your Resume in 15 minutes
Regulatory Affairs resume example
A career in regulatory affairs offers plenty of room for progression, as well as the opportunity to do important work and make a real difference.
This can make it a very attractive industry to work in, but first, you’ve got to prove you have the skills and qualifications to excel.
If you’re hoping to land your next role in regulatory affairs, check out our step-by-step resume writing guide below, along with our regulatory affairs resume example.
Resume templates
Regulatory Affairs Resume Example

This example Regulatory Affairs Director resume is well-structured to provide a pleasant reading experience for recruiters, and contains all of the crucial information they want to see.
The rest of the guide will show you exactly how you can achieve this in your own resume.

Regulatory Affairs resume layout and formatting
Formatting is often overlooked when writing resume, but it’s a crucial element of it”s success.
Creating a document that not only looks good, but is easily comprehended, is the key to gaining and holding the attention of busy hiring managers.
Use these formatting tips for best results.
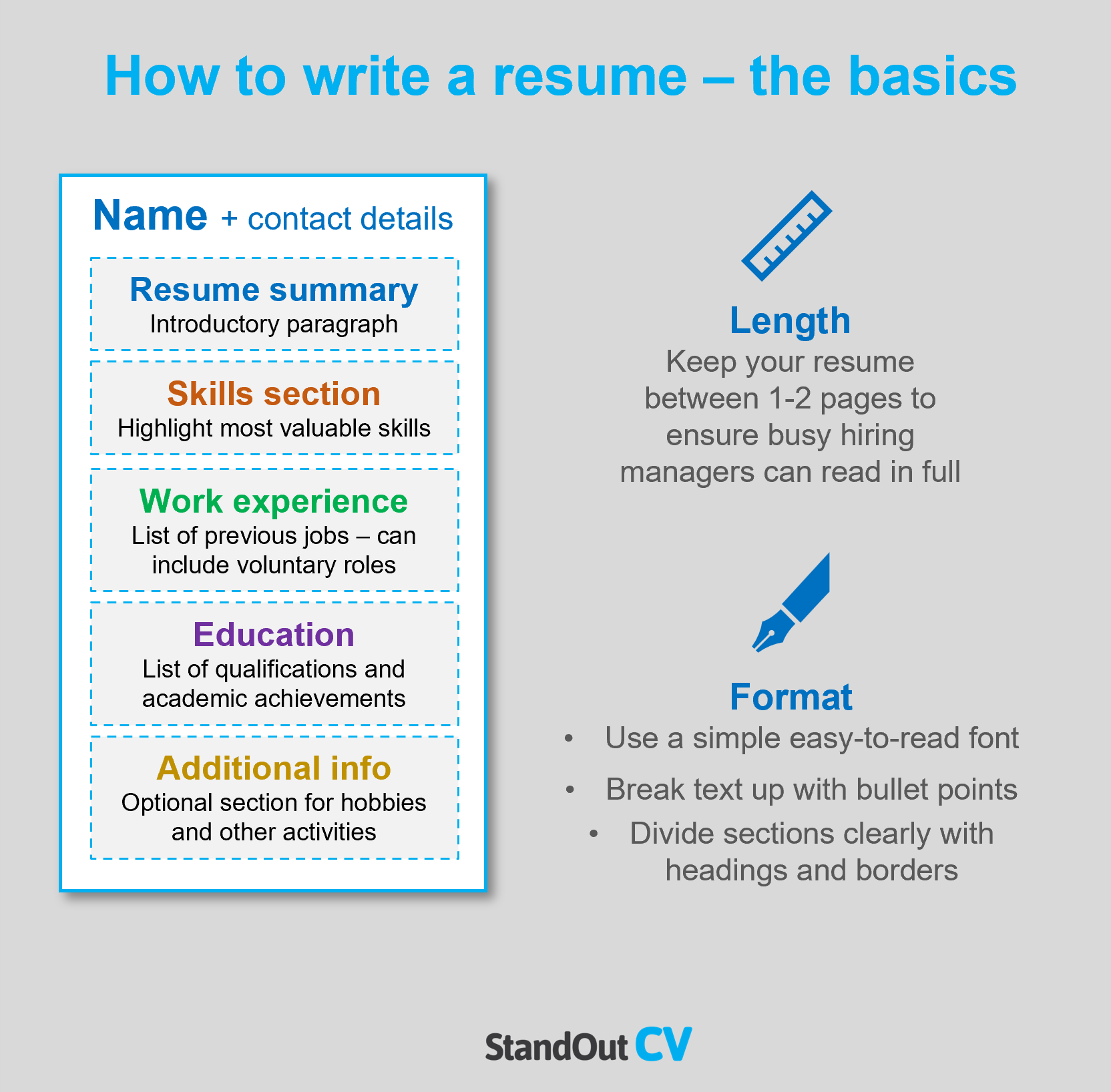
Formatting your resume
- Length: To ensure that recruiters will read all of your resume, limit its length to 2 pages – as they someteimes read hundreds of resumes daily.
- Font & readability : The key to an effective resume is its readability, so it’s best to use a clear and simple font and format it with bullet points and short paragraphs to make it easy for recruiters to read through quickly.
- Layout & Structure: Go with a resume design that looks good, but also allows for easy reading and navigation for employers. Ensure the page is clearly split up into sections by adding large font headings and dividing borders. Keep the color scheme simple and don’t overcrowd the page.
- Photos: While adding a photo to your resume is not mandatory in the USA, it can be beneficial if you are applying to organizations in creative industries.
Quick tip: Achieving a professional look for your resume can be difficult and time-consuming. If you want to create an attractive resume quickly, try our quick-and-easy Resume Builder and use one of their eye-catching resume templates.

Resume layout
Organize the document into these sections when you write your resume .
- Name and contact details – Employers need to know how to get in touch with you – so list your email and cell phone number here.
- Resume summary – An intro paragraph at the top of the resume which summarizes your suitability for target jobs.
- Skills section – A bullet-pointed list of your most relevant skills and knowledge.
- Work experience – A list of your previous jobs (or at least the most relevant and recent ones)
- Education – Add academic and professional qualifications that prove you can carry out the job
- Additional info – If they are relevant to the jobs you are applying for, you can add an extra section for things like hobbies and interests.
Here’s what to include in each part of your resume.
Contact Details

Make it easy for hiring managers to contact you by adding your contact details to the top of your resume.
Keep this section small to save space and include the following.
- Name and profession title
- Telephone number – Ideally your cell phone so you can answer quickly.
- Location – Add your general location such as LA or New York
- Email address – Use a professional looking one with no nicknames.
You can add a link to your LinkedIn profile if you have one – you do not need to include personal details like date of birth or marital status.
Regulatory Affairs Resume Summary
Create a strong opening for your resume by adding a compelling summary to the top that highlights your most valuable skills and experience.
This short but important paragraph is designed convince recruiters that you’re the perfect candidate for the job and entice them to read more of your resume.

How to create a resume summary that will excite recruiters:
- Keep it short: To capture a recruiter’s attention and keep them interested in your resume, limit your summary to 4-7 lines as you only have a few seconds to make an impression
- Tailor to target jobs: Optimize your summary to match the requirements of your target jobs, by mirroring the key words from the job description as closely as possible.
- Avoid using cliches: Recruiters always see cringey cliches like “ hardworking guru who works well in a team or individually ” – they don’t mean much to anyone, so focus your summary on tangible skills and experience.
Regulatory Affairs resume summary example
What to include in your regulatory affairs resume summary.
- Summary of your experience: Summarize the type of work you have done in the past and the benefits you have delivered for the organizations you worked at.
- Relevant skills: Instantly showcase your suitability for Regulatory Affairs jobs by including your skills that are highly relevant to them.
- Qualifications: Showcase your level of education with a quick mention of any qualifications that are essential for the Regulatory Affairs roles you are applying to.
Quick tip: Choose from hundreds of pre-written summaries across all industries, and add one to your resume with one-click in our quick-and-easy Resume Builder . All written by recruitment experts and easily tailored to suit your unique skillset and style.
Core skills section
Your core skills section, positioned just below your resume summary, provides recruiters with a quick glance at 4-10 of your most in-demand skills.
For Regulatory Affairs jobs, where hiring managers may receive hundreds of applications, this section can help you stand out and immediately grab their attention.
To be effective, this section should consist of 2-3 columns of bullet points that highlight attributes that are highly relevant to the jobs you are targeting.
Best skills for your Regulatory Affairs resume
Knowledge of regulations – Maintaining a thorough understanding of regulations related to the industry, such as FDA, EU MDR, and ISO standards.
Compliance – Ensuring that products and processes comply with applicable laws, regulations, and guidelines.
Regulatory strategy – Developing regulatory strategies to support product development, launch, and post-market surveillance.
Technical writing – Utilizing excellent technical writing skills to prepare regulatory documents, such as technical files, reports, and submissions.
Product evaluation – Reviewing and evaluating product design and development documents to ensure compliance with regulations.
Stakeholder communication – Communicating regulatory requirements and changes to stakeholders, such as R&D, manufacturing, and quality teams.
Auditing – Utilizing knowledge of auditing principles and experience in preparing for and participating in regulatory audits.
Quality management – Utilizing knowledge of quality management principles and their application in a regulatory affairs context.
Project management – Managing regulatory projects, including timelines, budgets, and resources, to ensure regulatory requirements are met.
Risk management – Utilizing knowledge of risk management principles and their application in a regulatory affairs context, including risk assessments and risk mitigation strategies.
Quick tip: Our quick-and-easy Resume Builder contains thousands of in-demand skills for every profession that can be added to your resume in seconds – saving you time and greatly improving your chances of landing job interviews and getting hired.
Work experience
Now that you’ve reeled recruiters in with your awesome summary, it’s time to delve into your work experience.
Here you’ll list your previous jobs (starting with your most recent and working backward) and showcase how you apply your skills in the workplace.
Provide lots of detail in recent jobs, and less in older roles.
If you have no relevant paid experience, you can include voluntary work and placements – but if you have lots of experience, you can leave out some of the really old jobs.

Structuring your jobs
Without a good structure, your job description can look messy and overwhelming to anyone reading them.
Make it easy for recruiters to read your work experience by structuring your roles like this.

Job outline
Starting each job with a brief summary of the organization, your position within it, and the primary goal of your role can help recruiters quickly understand the context of your work.
Key responsibilities
The bulk of the role description should be comprised of bullet points that explain all of your duties in the job.
Keep the sentences short and simple to make them easy for recruiters to digest.
Key achievements
Show employers the value you can bring to them by adding a few achievements to your jobs.
Whether you’ve saved the company money or improved an internal process, let recruiters know
Add some numbers to give readers a real scale of the impact, e.g. “reduced call wait time by 10%”
Example job for Regulatory Affairs resume
Provide strategic and operational regulatory leadership in the development, commercialization, and life cycle management of oncology products, for an organization that transforms the future of healthcare by unlocking the power of what science can do for people and society.
Key Responsibilities
- Develop and execute global regulatory strategies for product development, approval, competitive labeling, and registration for rare cancer and neurological disease programs.
- Conduct frequent regulatory intelligence concerning assigned programs and disseminate relevant information to cross-functional personnel.
- Craft health authority engagement and interaction plans and drive the formulation of briefing documents focused on scientific content.
- Guide project teams whose recommendations fall in-line with company goals and international laws, inclusive of Phase 3 drug development and commercial applications.
Quick tip: Create impressive job descriptions easily in our quick-and-easy Resume Builder by adding pre-written job phrases for every industry and career stage.
Education section
Towards the bottom of your resume, add your education section.
Here you should list your professional qualifications and academic record, such as high school diplomas or college degrees.
If you have lots of work experience, you can keep this section brief (because recruiters will be more interested in your career. If you have little/no experience then you should bulk this section up with plenty of detail.
Additional information
The bottom of your resume is a place to add any “additional info”
Any other info that didn’t fall into any of the previous sections can be added here.
If you have hobbies that are related to your profession or any awards or publications – add them here.
Writing your own winning Regulatory Affairs resume
Crafting a strong Regulatory Affairs resume can be a daunting task, but implementing the steps outlined above will significantly increase your chances of securing multiple interview opportunities.
Good luck with your job search!
- • Led a team in composing and submitting 12 premarket notifications (510(k)s) with a 100% acceptance rate, speeding up the device certification process by 20%
- • Managed project timelines and documentation for 3 major CE Mark applications, resulting in successful certification and market entry into European countries
- • developed a comprehensive understanding of APAC, LATAM, Middle East, and African medical device regulations, which facilitated the international registration of 5 new IVD products
- • Coordinated with interdisciplinary teams to review and update 15+ product technical files, ensuring compliance with evolving global directives and regulations
- • Spearheaded process improvement initiatives using RPA, reducing document processing time by 35%, and increasing team efficiency
- • Acted as a regulatory liaison on 4 product development teams, providing strategic guidance and ensuring regulatory requirements were seamlessly integrated
- • Co-authored four successful IDE submissions, facilitating essential clinical trials for groundbreaking IVD devices
- • Initiated and maintained product registration for over 20 medical devices in multiple high-profile demographic regions
- • Played a key role in the interdepartmental review of engineering changes, promotional materials, and product documentation for over 30 products
- • Implemented an internal audit of regulatory procedures, identifying areas for improvement that increased overall departmental compliance by 10%
- • Assisted with the preparation and submission of annual establishment registration and device listings for the company
- • Supported the submission of two PMA applications for novel IVD devices, resulting in FDA approval and market launch
- • Collaborated with Quality Assurance to ensure proper maintenance of technical files for 10+ existing products
- • Conducted industry research and analysis to keep the company abreast of regulatory trends and changes across various global markets
- • Assisted with the review of labeling and promotional materials for compliance with applicable regulatory requirements
5 Regulatory Affairs Resume Examples & Guide for 2024
Ensure your regulatory affairs resume prominently features your comprehensive understanding of regulatory guidelines and submission procedures. Highlight your experience with FDA, EMA, or other relevant regulatory body interactions. Demonstrate your track record in successfully navigating the product approval process within tight deadlines. Articulate your ability to coordinate cross-functional teams to ensure regulatory compliance.
All resume examples in this guide

Resume Guide
Resume Format Tips
Resume Experience
Skills on Resume
Education & Certifications
Resume Summary Tips
Additional Resume Sections
Key Takeaways

Crafting a resume for regulatory affairs positions can be daunting due to the need to balance technical expertise with an understanding of ever-changing compliance protocols. Our guide offers clear strategies and examples to help you articulate your qualifications and stay ahead in the competitive landscape of regulatory affairs.
- Incorporate regulatory affairs job advert keywords into key sections of your resume, such as the summary, header, and experience sections;
- Quantify your experience using achievements, certificates, and more in various regulatory affairs resume sections;
- Apply practical insights from real-life regulatory affairs resume examples to enhance your own profile;
- Choose the most effective regulatory affairs resume format to succeed in any evaluation process.
- Government Resume Example
- Policy Analyst Resume Example
- Military Resume Example
- Canvasser Resume Example
- Federal Resume Example
- Grant Writer Resume Example
The importance of format and layout in your regulatory affairs resume
Achieve this balance by:
- Listing your experience, beginning with the most recent and relevant , in reverse chronological order;
- Ensuring your header contains essential information, such as contact details , a headline, and a portfolio link. Include a professional photo in the regulatory affairs resume header if you have one;
- Including only the most important and relevant resume sections to showcase your expertise and stand out from other candidates;
- Editing your regulatory affairs resume to be no longer than two pages if you have extensive relevant experience. Use your limited resume space judiciously.
Also, remember that your regulatory affairs resume might initially be scanned by an Applicant Tracker System (ATS).
When it comes to ATS:
- Opt for simple and legible fonts like Raleway, Rubik, Lato, etc., making your experience easy for the ATS to scan;
- Use serif and sans-serif fonts, both of which are ATS-friendly;
- Avoid overused options like Arial and Times New Roman, which, while suitable, may lack personality.
Contrary to a common myth, our recent study shows that the ATS can effectively process both one-column and two-column resumes. Learn more about this in the ATS myths guide .
Finally, when submitting your regulatory affairs resume, always export it as a PDF to ensure all information remains intact, making the document easier to print, read, and scan.
Upload & Check Your Resume
Drop your resume here or choose a file . PDF & DOCX only. Max 2MB file size.
The more time and effort you've put into obtaining the relevant certificate, the closer to the top it should be listed. This is especially important for more senior roles and if the company you're applying for is more forward-facing.
Essential sections that should make up your regulatory affairs resume include:
- The header - with your contact details (e.g. email and telephone number), link to your portfolio, and headline
- The summary (or objective) - to spotlight the peaks of your professional career, so far
- The experience section - with up to six bullets per role to detail specific outcomes
- The skills list - to provide a healthy mix between your personal and professional talents
- The education and certification - showing your most relevant degrees and certificates to the regulatory affairs role
What recruiters want to see on your resume:
- Experience with regulatory submissions (e.g., NDAs, ANDAs, BLAs, MAAs) and knowledge of relevant guidelines (e.g., FDA, EMA, ICH)
- Demonstrated understanding of regulatory strategy and lifecycle management for pharmaceuticals or medical devices
- Familiarity with Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP) regulations
- Strong communication and negotiation skills for interactions with regulatory authorities and cross-functional teams
- Evidence of continued professional development in regulatory affairs, such as RAC certification or related advanced degrees
Writing your regulatory affairs resume experience
Within the body of your regulatory affairs resume is perhaps one of the most important sections - the resume experience one. Here are five quick tips on how to curate your regulatory affairs professional experience:
- Include your expertise that aligns to the job requirements;
- Always ensure that you qualify your achievements by including a skill, what you did, and the results your responsibility led to;
- When writing each experience bullet, ensure you're using active language;
- If you can include a personal skill you've grown, thanks to your experience, this would help you stand out;
- Be specific about your professional experience - it's not enough that you can "communicate", but rather what's your communication track record?
Wondering how other professionals in the industry are presenting their job-winning regulatory affairs resumes? Check out how these regulatory affairs professionals put some of our best practices into action:
- Developed and implemented regulatory strategies for new oncology drugs, leading to successful FDA approvals for three novel therapies.
- Oversaw a cross-functional team that prepared and submitted over 50 INDs and associated amendments, contributing to a streamlined development process.
- Handled regulatory submissions for international markets, achieving a 30% reduction in time-to-market for key products in the European Union.
- Liaised with regulatory agencies to negotiate and define the scope of regulatory submissions, ensuring compliance with local and international standards.
- Led the preparation of an exhaustive Environmental Risk Assessment for a new agricultural biotechnology product, facilitating its clearance.
- Managed complex regulatory projects for medical devices, including the compilation and review of technical documentation, resulting in a 20% increase in approval efficiency.
- Authored comprehensive regulatory dossiers for biologics and was instrumental in obtaining market authorization for two blockbuster immunotherapies.
- Pioneered an initiative to digitize submission processes, greatly enhancing the accessibility and tracking of regulatory documents.
- Played a key role in the cross-departmental task force that addressed compliance issues, significantly reducing potential regulatory infractions.
- Executed due diligence assessments for potential acquisitions, identifying regulatory risks and opportunities that influenced business decisions.
- Contributed to the development of a proprietary regulatory intelligence database, streamlining data retrieval and analysis efforts for the whole department.
- Spearheaded the initiative to train junior regulatory affairs staff, enhancing team knowledge and productivity by 25%.
- Directed regulatory strategy for advanced gene therapy products, playing a pivotal role in the attainment of first-ever FDA approval for a gene therapy treatment in a specific genetic disorder.
- Cultivated productive relationships with key stakeholders at the FDA, EMA, and other health authorities, facilitating smoother communications and negotiations.
- Introduced an automated system for tracking regulatory commitments, which bolstered compliance rates by 35% across multiple product lines.
- Crafted regulatory submission plans that aligned with corporate timelines, optimizing resources and reducing go-to-market time by an average of 4 months.
- Fostered a culture of continuous improvement by instituting regular regulatory process audits, enhancing overall efficiency by 15%.
- Served as the primary regulatory representative in multidisciplinary project teams, ensuring all regulatory requirements were understood and met for each new product launch.
- Managed the submission and maintenance of regulatory filings for a portfolio of over 20 pharmaceutical products, securing prolonged market presence and compliance.
- Strategized and executed a successful pathway for orphan drug designation, leading to tax credits and grant opportunities for the company.
- Orchestrated the creation and delivery of training programs for global regulatory requirements, equipping the team with the knowledge to tackle diverse international markets.
- Engaged actively in the reformulation of clinical development plans in light of changing regulatory guidelines, ensuring no disruptions to ongoing clinical trials.
- Provided expert guidance on the regulatory landscape to influence product development and lifecycle management from a regulatory perspective.
- Conducted rigorous analysis and reporting on post-marketing surveillance data, enhancing patient safety and maintaining compliance with post-marketing commitments.
Quantifying impact on your resume
- Include the number of regulatory submissions managed and their success rate to demonstrate project management and effectiveness.
- List the specific number of countries in which you’ve secured product approvals to show international regulatory expertise.
- Mention the percentage of compliance issues resolved within deadlines to highlight problem-solving skills and efficiency.
- Quantify the amount of training sessions or people you’ve trained on regulatory affairs to establish leadership and knowledge-sharing capabilities.
- Specify the number and type of audits conducted, along with any improvements implemented, to illustrate due diligence and continuous improvement.
- Display the reduction in approval times achieved under your guidance to emphasize process optimization.
- Detail the volume of documentation prepared or reviewed to show thoroughness and attention to detail.
- Report the financial impact of regulatory strategies you’ve implemented, such as cost savings or avoidance of fines.
Action verbs for your regulatory affairs resume
What can candidates do about their resume, if they have no experience
Job requirements can sometimes be answered by other elements you could make more prominent in your regulatory affairs resume.
Thus, you'd be substituting your lack of experience with your relevant:
- Education with details of skills you've obtained that align with the job
- Internships and short-term jobs that are once more dedicated to putting your expertise in the spotlight
- Skills section answering basic and - potentially - more specific job qualifications
- Strengths or accomplishments to show the unique value you present, even as a candidate with less or no professional experience in the industry.
Recommended reads:
- How to List a Major & Minor on Your Resume (with Examples)
- Should You Include Eagle Scout On Your Resume?
If you happen to have some basic certificates, don't invest too much of your regulatory affairs resume real estate in them. Instead, list them within the skills section or as part of your relevant experience. This way you'd ensure you meet all job requirements while dedicating your certificates to only the most in-demand certification across the industry.
Regulatory Affairs resume skills: the essential hard skills and soft skills checklist
Ultimately, your Regulatory Affairs resume should hint to recruiters that you possess an array of talents that are indispensable to the role.
For example, listing the technologies and software you're apt at using (or your hard skills) and how you apply them in your day-to-day responsibilities would ensure you meet the technical requirements of the role.
But is this enough to ensure that you make a good impression on recruiters?
Go a step further by detailing the soft skills or personality traits you've attained thanks to your work and life experience.
The best way to balance hard skills and soft skills on your Regulatory Affairs resume is by:
- Highlighting up to three of your most noteworthy career accomplishments in a separate section.
- Listing at least one hard skill and one soft skill you've used to solve a particular challenge or problem.
- Feature niche skills and technologies that would help you stand out amongst candidates.
- Think back on the social impact your efforts have had towards improving the work environment - were you able to always maintain a professional ethic, while enhancing the team culture? Write about your contribution to the role, department, or organization itself as a metric of success.
The skills section of your resume provides you with plenty of opportunities to detail your technical and personal traits.
All you have to do is select the talents that best fit your application and expertise. Make note of some of the most prominent hard and soft skills across the industry from our list:
Top skills for your regulatory affairs resume:
Regulatory Compliance
Regulatory Submission Management
Data Analysis
Legal Research
Risk Management
Product Lifecycle Management
Quality Assurance
Good Clinical Practice (GCP)
Knowledge of International Regulations
Regulatory Strategy Development
Attention to Detail
Problem-Solving
Communication
Project Management
Time Management
Adaptability
Critical Thinking
Negotiation
Organizational Skills
Listing your relevant degrees or certificates on your regulatory affairs resume is a win-win situation. Not only does it hint at your technical capabilities in the industry, but an array of soft skills, like perseverance, adaptability, and motivation.
Showcase academic background with education and certifications' sections
Listing your education and certifications should be a rudimentary part of your resume writing.
Including your relevant academic background - in the form of your higher education degree and niche-specific certificates - will prove knowledge of the industry.
For your education section:
- Start by including your degree, followed by start and graduation dates, as well as the institution;
- You could include relevant coursework, major/minor , or GPA, only if your've just graduated from college or if this information would further support your application;
- If you have an "ongoing" degree, you can still list it in case you think your diploma can impress recruiters or it's required;
Follow a similar logic for your certifications section by listing the institution, alongside dates you've obtained the certificate. For some of the most recent and relevant industry certificates , check out the next part of our guide:
The top 5 certifications for your regulatory affairs resume:
- Regulatory Affairs Certification (RAC) - Regulatory Affairs Professionals Society (RAPS)
- Certified in Healthcare Compliance (CHC) - Compliance Certification Board (CCB)
- Certified Regulatory Compliance Manager (CRCM) - American Bankers Association (ABA)
- Medical Device Regulatory Affairs Certification (MDRAC) - Association of International Certified Professional Accountants
- Pharmaceutical Regulatory Affairs Certification (PRAC) - Association of International Certified Professional Accountants
If you happen to have plenty of certificates, select the ones that are most applicable and sought-after across the industry. Organize them by relevance to the role you're applying for.
- How To List Certifications On A Resume (Examples Included)
- How To Include Your Relevant Coursework On A Resume
Which one to use: a resume summary or a resume objective?
The regulatory affairs resume summary or objective serves as a good introduction to your experience for recruiters.
Have you ever wondered which one (the summary or objective) will be more appropriate for your regulatory affairs resume?
- If you are a less experienced professional, write a resume objective statement. The objective is about three sentences long and provides recruiters with information about your career goals, strengths, and achievements . It should basically denote how you see yourself in this particular role, and what is your relevant experience and/or know-how;
- If you happen to have plenty of relevant experience, select your most impressive achievements for your resume summary. The summary is no longer than five sentences and serves as a storytelling instrument - highlighting your greatest career wins . Don't forget to align your summary with the job requirements to ensure your resume stays relevant to the role.
Read on for more information and examples of resume summaries and objectives from real world professionals.
Resume summaries for a regulatory affairs job
- Accomplished regulatory affairs professional with over a decade of experience in leading the successful submission of multiple drug applications, instrumental in steering the pivotal phase III trials for a groundbreaking oncology therapy. Adept in FDA and EMA guidelines, committed to ensuring stringent compliance within a fast-paced biotech environment.
- Dedicated pharmacist transitioning into regulatory affairs, leveraging an 8-year background in community and hospital pharmacy settings, extensive knowledge of compounding and distribution regulations, and a keen interest in applying these skills to the regulatory domain to facilitate the strategic development of compliant healthcare products.
- Seasoned engineer with 12 years in aerospace project management, seeking to bring a robust analytical skillset and a history of cross-functional team leadership to the regulatory sector. Proven track record in achieving ISO certifications and implementing rigorous quality control systems that exceed industry standards.
- Eager recent graduate with a Master's degree in Biochemistry and internships at top-tier research laboratories, ready to contribute a fresh perspective and scientific acumen to the field of regulatory affairs. Demonstrated aptitude in data analysis and a passion for regulatory science, aiming to ensure public access to safe and effective medical innovations.
- With a fervent interest in the intersection of law and science, I am determined to leverage my legal studies background and in-depth understanding of healthcare legislation to facilitate the successful navigation of regulatory pathways. Proactive and analytical, I aim to commence my career in regulatory affairs by significantly contributing to the advancement of public health initiatives.
- As a dynamic professional with a strong foundation in environmental policy and a recent certification in regulatory affairs, I aspire to integrate my skills in sustainability with the robust framework of pharmaceutical compliance. Enthusiastic about championing environmental health and safety regulations in a new capacity, I am set to embark on a dedicated path in regulatory affairs.
Miscellaneous regulatory affairs resume sections for a more personalized approach
Your regulatory affairs resume can reflect even more upon your personality and best qualities - that is if you decide on including a couple of additional resume sections to support your application.
Some of the best-accepted industry-wide choices include the:
- Resume projects - getting into the outcomes of your most important work, so far;
- Languages on your resume - detailing your proficiency level;
- Special recognitions - dedicated to your most prominent industry awards;
- Hobbies and interests - defining how you spend your free time.
Key takeaways
- All aspects of your resume should be selected to support your bid for being the perfect candidate for the role;
- Be intentional about listing your skill set to be balanced with both technical and people capabilities, while aligning with the job;
- Include any experience items that are relevant to the role and ensure you feature the outcomes of your responsibilities;
- Use the summary or objective as a screenshot of your best experience highlights;
- Curate various resume sections to showcase personal, transferable skills.

Looking to build your own Regulatory Affairs resume?

- Resume Examples
How to List GED on Your Resume
Hard skills on resume: top hard skills by industry sector, how to craft & tell a resume story behind your work success, how to include study abroad on a resume, how to get a green industry job – essential skills and 20+ green careers (with salaries), should you have hobbies & interests on your resume.
- Create Resume
- Terms of Service
- Privacy Policy
- Cookie Preferences
- Resume Templates
- AI Resume Builder
- Resume Summary Generator
- Resume Formats
- Resume Checker
- Resume Skills
- How to Write a Resume
- Modern Resume Templates
- Simple Resume Templates
- Cover Letter Builder
- Cover Letter Examples
- Cover Letter Templates
- Cover Letter Formats
- How to Write a Cover Letter
- Resume Guides
- Cover Letter Guides
- Job Interview Guides
- Job Interview Questions
- Career Resources
- Meet our customers
- Career resources
- English (UK)
- French (FR)
- German (DE)
- Spanish (ES)
- Swedish (SE)
© 2024 . All rights reserved.
Made with love by people who care.

IMAGES
VIDEO
COMMENTS
11. Regulatory Affairs Specialist. Cover Letters. Approved by real hiring managers, these Regulatory Affairs Specialist cover letters have been proven to get people hired in 2024. A hiring manager explains why. Compiled by: Kimberley Tyler-Smith. Senior Hiring Manager. 20+ Years of Experience. Jump to a Cover Letter.
Breanna Wallace. City, State, Zip Code. Home : 000-000-0000 Cell: 000-000-0000. [email protected]. Dear Ms. Schwartz, I came across your notice on Indeed.com for a Regulatory Affairs Associate with MacProductions Foods Unlimited. I am submitting my resume. I strongly feel my experiences and education puts me high on the list of potential applicants.
Cover Letter Body. Dear Hiring Manager, I am writing to express my interest in the Regulatory Affairs Associate position at your company. With a strong background in regulatory compliance, I am confident in my ability to contribute to your team and support your company's objectives.
Regulatory Affairs Manager Cover Letter Writing Tips. 1. Showcase your experience. When applying for a regulatory affairs manager position, it's important to showcase your experience in the field. This can be done by providing specific examples of how you've helped companies achieve compliance with regulations in the past.
This shows attention to detail and professionalism. For example, a suitable greeting could be "Dear Ms. Johnson," or "Dear Hiring Manager," depending on the information available. It's important to tailor the greeting to the specific recipient to create a personalized and professional tone for your cover letter.
Here is the Knowledgeable Regulatory Affairs Associate Cover Letter Example: Dear Ms. Bertha Manhart, I have recently seen your notice regarding an opening for a regulatory affairs associate at your company Fougera Pharmaceuticals Inc. and would like to be considered for the position. I currently hold a master's degree in biology and have two ...
Regulatory Affairs Associate Cover Letter Writing Tips. 1. Showcase your experience. When applying for a regulatory affairs associate position, it's important to showcase your experience in the field. This can be done by providing specific examples of how you've helped companies meet regulatory requirements in the past.
Cover Letter Example (Text) Ota Tonsor. (620) 923-5714. [email protected]. Dear Ms. Genslinger, I am writing to express my keen interest in the Regulatory Affairs Manager position at Pfizer Inc., as advertised. With a solid foundation of five years' experience at Merck & Co., Inc., I have honed my skills in navigating the complex regulatory ...
Cover Letter Header. A header in a cover letter should typically include the following information: Your Full Name: Begin with your first and last name, written in a clear and legible format. Contact Information: Include your phone number, email address, and optionally, your mailing address.
Here is a Cover Letter Sample that masters these two concepts simultaneously. A Regulatory Affairs Associate is responsible for providing support to regulatory operations, in compliance with national and international regulations. This includes managing regulatory submissions, responding to regulatory authorities, keeping up to date on ...
A Regulatory Affairs Specialist cover letter should provide an engaging summary of your professional qualifications and experience. It should demonstrate your ability to analyze and interpret regulatory requirements and enforce compliance. The cover letter should offer a snapshot of your experience, skills and qualifications and demonstrate why ...
Regulatory Affairs Associate resume sample including summary/objective, experience, skills, education, certifications, templates & cover letter. ... Below is an example cover letter: Dear London, I am writing to apply for the Regulatory Affairs Associate position at [company name]. I have a Bachelor's degree in Biology and a Master's degree ...
The following regulatory affairs specialist cover letter example can give you some ideas on how to write your own letter.Regulatory Affairs Specialist Cover Letter Example Use this template. or download as PDF. Cover Letter Example (Text) Shawniece Tarnowski (639) 990-8310. [email protected]. Dear Danyella Mikha, I am writing to ...
regulatory affairs Cover Letter Example. A cover letter can be a valuable addition to your job application when applying for an regulatory affairs position. Cover letters provide a concise summary of your qualifications, skills, and experience, also it also gives you an opportunity to explain why you're the best fit for the job. ...
Regulatory affairs resume example Consider using this resume example to help you create your own: Stu Gomez Chicago, Illinois [email protected] 555-555-5555 Professional summary Detail-oriented regulatory affairs specialist with three years of experience in medical and pharmaceutical device and drug review. Currently in the process of obtaining ...
Example job for Regulatory Affairs resume. Outline. Provide strategic and operational regulatory leadership in the development, commercialization, and life cycle management of oncology products, for an organization that transforms the future of healthcare by unlocking the power of what science can do for people and society. Key Responsibilities.
Before delving into our step-by-step guide, we have selected some relevant regulatory affairs resume examples that might be beneficial for you. Government Resume Example. Policy Analyst Resume Example. Military Resume Example. Canvasser Resume Example. Federal Resume Example.