

Welcome to Research Placements & Experiences (formerly Nuffield Research Placements)
The Research Placements & Experience programme (formerly Nuffield Research Placements) provides engaging, hands-on projects, where Year 12 students have the opportunity to make a meaningful contribution towards the work of a host organisation through a well-supervised but independent placement collaboration relating to an area of science, quantitative social science, computing, technology, engineering or maths (or a combination!).
Students can apply via the button below but please make sure you've checked your eligibility requirements
Check eligibility requirements here
Which placement?
Students can participate in Research Placements or Experience Placements. Both offer a rewarding experience for enthusiastic and curious young people
Research Placements
Research Placements are 2-week collaborations with a STEM-related knowledge expert on a live research question or area of development. While producing a scientific or technical report and poster, this opportunity ensures that students contribute meaningfully to the host organisation's current work.
Experience Placements
Experience Placements are 5-day explorations with industry experts to identify essential skills needed for employment in a specific STEM sector of interest. While producing a workbook and reflective report, students gain insight into working in
professional environments as well as knowledge of the challenges for different sectors, in turn preparing them for employment.
Employers who are interested in supporting students through a supervised placement can register below. Alternatively, feel free to contact your Regional Coordinating Team to hear more about the benefits of providing a placement and what's involved. Contact details can be found HERE .
The benefits of a Nuffield Research Placement
Title real-world experience.
Challenge yourself with a meaningful and rewarding supervised research project in a professional working environment.
Title Gain new skills
Learn to write a scientific report and develop your research, critical thinking and time management skills.
Title Valuable insight
Gain workplace skills and take an inside look at the sort of careers available to you.
Title Build your confidence
Take responsibility for your own project that’ll be useful to professional scientists, social scientists, engineers, technologists and/or mathematicians.
Title Exploration
Engage in a unique experience with professionals and become more confident working on novel situations.
Title Networking
Connect with your peers and make new network contacts you can keep in touch with in the future.
Previous student experiences
Title Abrar and Mutahir
Abrar and Mutahir talk about their placements at the University of Manchester.
Title Ife and Ian
Watch what happened on Ife and Ian’s placements at UCL.
Title Asma and Senel
Learn more from Asma and Senel about their placements at the Natural History Museum.
- Our Culture
- Open and FAIR Data
- Research projects
- Publications
- Cellular Genomics
- Decoding Biodiversity
- Delivering Sustainable Wheat
- Earlham Biofoundry
- Transformative Genomics
- Scientific Groups Our groups work at the forefront of life science, technology development, and innovation.
- High-Performance Sequencing Dedicated and efficient high-throughput genomics led by experts in sequencing and bioinformatics.
- Single-cell and Spatial Analysis Platforms to support single- or multi-cell analysis, from cell isolation, to library preparation, sequencing and analysis.
- Earlham Biofoundry Providing expertise in synthetic biology approaches and access to laboratory automation
- Tools and resources Explore our software and datasets which enable the bioscience community to do better science.
- Cloud Computing Infrastructure for Data-intensive Bioscience
- Web Hosting for Sites, Tools and Web Services
- Earlham Enterprises Ltd
- Events Calendar Browse through our upcoming and past events.
- About our training High-quality, specialist training and development for the research community.
- Year in industry Supporting undergraduate students to develop skills and experience for future career development.
- Internships and opportunities Opportunities for the next generation of scientists to develop their skills and knowledge in the life sciences.
- Immersive visitors A bespoke, structured training programme, engaging with the faculty, expertise and facilities at the Earlham Institute.
- News Catch up on our latest news and browse the press archive.
- Articles Explore our science and impact around the world through engaging stories.
- Impact Stories Find out how we are contributing to the major challenges of our time.
- Impact Through Policy Advocacy Engaging across the political spectrum to exchange knowledge and inform public policy.
- Public engagement and outreach Communicating our research to inspire and engage learning.
- Communications at EI We work across digital, multimedia, creative design and public relations to communicate our research.
- Our Vision and Mission
- Inclusivity, diversity, equality and accessibility
- Scientific Advisory Board
- Our Management Team
- Operations Division
- Careers overview
- Postgraduate Studies
- Group leaders
- Fellowships
- Life at Earlham Institute
- Living in Norfolk

Nuffield: getting to grips with science in the real world
Since 2011 we’ve welcomed students from the Nuffield scheme, who have been getting to grips with research projects at the cutting edge of science.
Since 2011 we’ve welcomed students from the Nuffield scheme , who have been getting to grips with research projects at the cutting edge of science. This year, the students got their hands dirty with a metagenomics project looking at the hidden life in soil.
Article by Georgie Lorenzen, Science Communications Trainee
Year 13 is a critical time for students. With the end of their post-16 education in sight, they must make a decision about what to do next. Whilst many will go on to study higher education courses, choosing which ones to apply for can be a daunting decision; over 50,000 undergraduate courses offered at more than 395 providers in the UK alone. That’s before you count the other options: apprenticeships, degree apprenticeships and higher national diplomas.
One way for students to better equip themselves in navigating this minefield of possibilities is by participating in placements, to get real life work experience. The Nuffield research placement scheme gives sixth form students the opportunity to get a taste of what it’s like to work in the scientific sector. Placements run for 4-6 weeks during the summer holidays, with students working alongside scientists to carry out research and develop both their laboratory and computational skills. It is offered by many different providers in the UK, such as universities and research institutes.
We have been offering placements to Nuffield students since 2011 as both a standalone project provider and in partnerships, such as with the John Innes Centre . Previous projects have covered topics such as circadian rhythms in clocks, sequencing European polecats and generating phylogenetic trees of pathogens.

The Nuffield research placement scheme gives sixth form students the opportunity to get a taste of what it’s like to work in the scientific sector.

This year, it was the turn of Thomas Searle and Anne Bongaerts. Their project involved exploring the use of long read sequencing to sequence the genomes of organisms within a population, working under the supervision of Darren Heavens and Samuel Martin , both from the Leggett group .
Darren, who was the student’s primary supervisor, thinks that having the chance to be a mentor is a great way to help inspire the next generation of scientists: “I have worked with two previous Nuffield students and personally get a great deal from them being here. I was very fortunate that in my first job I had two excellent role models who taught me a great deal about working in a laboratory and I see these placements as an opportunity to give something back.”

What is metagenomics?
Commonly referred to as environmental genomics, metagenomics involves studying the genomes of multiple species within a community without the need for obtaining a pure culture.
This could be a sample of the bacteria which make up our gut microbiome, or those found in an environmental sample, such as that from a river. These samples will contain the DNA from a range of species, some of which may have not been sequenced before.
The nature of these samples can make traditional, short-read sequencing difficult. That’s because the genome assembly step is harder when there is DNA from lots of different organisms - particularly if you don’t know what the organisms are!
Developing bioinformatics pipelines is one way that scientists are working to harness the data collected from metagenomic DNA samples. Another method is using long-read sequencing, such as nanopore technologies from Illumina, which read DNA strands in one go as opposed to chopping it up first.
I caught up with Thomas, a student at Thorpe St Andrew sixth form in Norwich, on his last day at EI to find out how the experience had impacted him.
What were you studying?
“We extracted DNA from a soil sample, then used electrophoresis to separate a sample of the DNA into twelve different size fractions. We then investigated if that made a difference to community composition when we sequenced and analysed it.”

We extracted DNA from a soil sample, then used electrophoresis to separate a sample of the DNA into twelve different size fractions.
What have you learned during the placement?
“I'd never done anything to do with DNA sequencing or metagenomics before so everything was brand new! I feel like the project was a really good way of learning about it. I've learned firsthand the importance of good planning and hopefully I've developed my scientific writing a little bit too.”
What was the most interesting part of the placement?
“I thought that using the HPC was really exciting and I definitely feel like I underestimated the importance of computational biology.”

I thought that using the HPC was really exciting and I definitely feel like I underestimated the importance of computational biology.
What did you think of EI?
“EI is incredibly welcoming and everyone here has been really lovely. I think it was a great insight into the working environment. I definitely appreciated the coffee machine and the table football!”
Sowing a seed
The experience has encouraged Thomas to consider the importance of bioinformatics in biological research as he continues his academic studies:
“I was already quite interested in bioinformatics and learning about it at EI has made me want to focus more on being a mathematically and computer literate biologist in the future.”
Not only was the placement a positive experience for the students, Sam told me that he felt it was valuable for the researchers that they worked with, too:
“I haven’t hosted Nuffield students before, but I think it is useful for the institute in terms of outreach, and also for me, personally, to develop teaching and mentoring skills. I was impressed by how quickly the students learned the new skills necessary for the analysis we performed, and how enthusiastic they were to go beyond what I had planned.”
Darren added, “I have really enjoyed seeing them grow as scientists and observe the dynamic of how they worked together. I think that Setpoint do a good job in identifying students with immense potential and this year was no different.”

I was already quite interested in bioinformatics and learning about it at EI has made me want to focus more on being a mathematically and computer literate biologist in the future.
~ Thomas Searle, Nuffield Research Placement Student (2019)

How can you get involved as a student or researcher?
Fancy doing a Nuffield placement in the future? There is a strict application criteria, so you’ll need to speak to your teachers first to check your eligibility. Applications are made via an online application process. They do not come directly to EI, but rather a local administration group. Read more about applying here .
Researchers!
Fancy giving some bright students a great opportunity? If you want to look into hosting a Nuffield placement, the first step is to get in contact with your local Nuffield coordinator. You can find this information here and read more about the scheme here .
Related reading.

How Earlham Institute influenced my career path

How do you know if you’ll hack it in the real world?
Our year in industry: setting the stage for a career in science
Women in Science: Elena Rodriguez - the mind of the gut microbiome

Women in computing: learning the ropes in bioinformatics

My week at Earlham Institute: more than just science

Matthew Dale: Loving science through communication

Where are the bees? Tracking down which flowers they pollinate

UEA Engagement award for the EI ‘Pink Pigeon Trail’

New approaches for metagenome assembly with short reads
Nanopore sequencing for interactive, real-time metagenomics
Metagenomic assembly algorithms
- Scientific Groups
- High-Performance Sequencing
- Single-cell and Spatial Analysis
- Tools and resources
- Events Calendar
- About our training
- Year in industry
- Internships and opportunities
- Immersive visitors
- Impact Stories
- Impact Through Policy Advocacy
- Public engagement and outreach
- Communications at EI
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Delve into a wide range of chemical concepts and processes with this collection of over 200 step-by-step practicals
Developed by the Nuffield Foundation and the Royal Society of Chemistry, each resource contains detailed information for teachers and technicians.
‘Dissolving’ polystyrene in acetone
In association with Nuffield Foundation
Investigate what happens to polystyrene when it is placed in propanone (acetone) in this demonstration. Includes kit list and safety instructions.

‘Magic’ writing with colour changing reactions
Reveal invisible messages or pictures drawn with aqueous solutions by spraying them with suitable reagents in this demonstration. Includes kit list and safety instructions.

A chromate–dichromate equilibrium
Try this class practical to investigate an equilibrium between chromate(VI), dichromate(VI) and hydrogen ions. Includes kit list and safety instructions.
A controlled explosion using hydrogen and air
Show how a hydrogen–air mixture can gain explosive properties using a plastic drink bottle in this demonstration. Includes kit list and safety instructions.

A hydrogen powered rocket
Try this spectacular demonstration to make a rocket using a plastic drink bottle fuelled by hydrogen and air. Includes kit list and safety instructions.

A microscale acid–base titration
Use microscale titration to complete an acid–base neutralisation with sodium hydroxide in this class practical. Includes kit list and safety instructions.
A microscale oxidation of alcohols
Use this practical to investigate the oxidation reactions of various alcohols with acidified potassium dichromate. Includes kit list and safety instructions.
A red–blue oscillating reaction
Use this practical or demonstration to provide a visual illustration of an oscillating reaction and redox equilibria. Includes kit list and safety instructions.

A reversible reaction of hydrated copper(II) sulfate
A class practical which investigates the reversible reaction of hydrated copper(II) sulfate. Includes kit list and safety instructions.
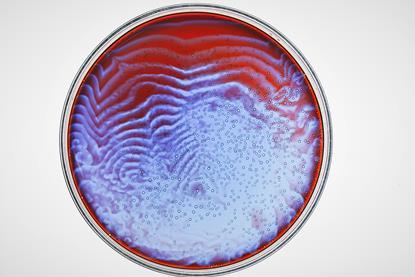
A simple oscillating reaction
Use this demonstration to illustrate an oscillating reaction as bromate ions oxidise malonic acid to carbon dioxide. Includes kit list and safety instructions.

A solid–solid reaction between lead nitrate and potassium iodide
Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide.

A spontaneous exothermic reaction
Illustrate the reaction between glycerol and potassium manganate(VII) to produce flames and steam in this demonstration. Includes kit list and safety instructions.

A test to distinguish between ethanol and methanol
A class practical to distinguish between methanol and ethanol using the iodoform reaction. Includes kit list, safety instructions, procedure and teaching notes.

A thermometric titration
Use this class practical to practise locating end-points in titration by measuring temperature during the reaction. Includes kit list and safety instructions.
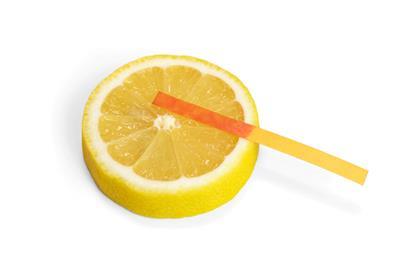
Acid or alkali? Acidic or alkaline? A litmus paper test
Test a variety of substances to see if they are acidic or alkaline, using litmus paper as the indicator. Includes kit list and safety instructions.

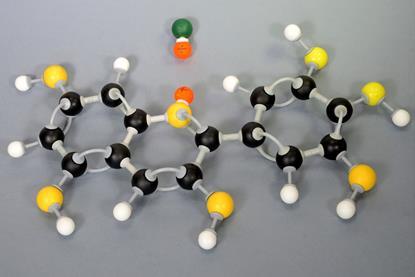
Addition polymerisation with phenylethene
Use this practical or demonstration as an example of addition polymerisation using phenylethene to form polyphenylethene. Includes kit list and safety instructions.

Allotropes of sulfur
Use this practical to explore the changes in the colour and consistency of sulfur as you heat it, melt it and eventually boil it. Includes kit list and safety instructions.

Ammonia fountain demonstration
Try this experiment to make a miniature chemical fountain using only soluble ammonia and atmospheric pressure. Includes kit list and safety instructions.

Ammonium dichromate volcano
Try this demonstration to create a mini volcanic eruption illustrating the decomposition of ammonium dichromate. Includes kit list and safety instructions.
An equilibrium using copper(II) and ammonia
Try this practical to explore an equilibrium involving copper(II) ions, with copper(II) sulfate, ammonia and sulfuric acid. Includes kit list and safety instructions.

Anodising aluminium
Explore an application of electrolysis in this demonstration by anodising aluminium to improve corrosion resistance. Includes kit list and safety instructions.

Burning money: what makes combustion happen?
Surprise your students by soaking a piece of paper (or an old £5 note) in ethanol and water and igniting it. Includes kit list and safety instructions.

Cannon fire
Increase the rate of burning with the inclusion of oxygen, in this loud exothermic practical

Carbon filtration and activated charcoal
Try this practical to remove objectionable tastes and odours from water using carbon in the form of activated charcoal. Includes kit list and safety instructions.

Catalysing the reaction of sodium thiosulfate and hydrogen peroxide
Illustrate the effect of a catalyst as sodium thiosulfate is oxidised by hydrogen peroxide in this demonstration. Includes kit list and safety instructions.

Catalysis of a sodium thiosulfate and iron(III) nitrate reaction
Investigate the effect of transition metal catalysts on the reaction between iron(III) nitrate and sodium thiosulfate. Includes kit list and safety instructions.

Catalysis of the reaction between zinc and sulfuric acid
Compare the rate of reaction between zinc and sulfuric acid with copper as a catalyst in this simple class practical. Includes kit list and safety instructions.

Catalysts for the thermal decomposition of potassium chlorate
Try this demonstration to investigate the effectiveness of various catalysts for the decomposition of potassium chlorate. Includes kit list and safety instructions.

Catalytic oxidation of potassium sodium tartrate
Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. Includes kit list and safety instructions.

Chemiluminescence of luminol: a cold light experiment
Show how the energy of a chemical reaction can be given out as light. Includes kit list and safety instructions.

Chromatography of sweets | 11–14 years
In association with Nuffield Foundation , By Holly Walsh and Sandrine Bouchelkia
Try this class practical to carry out chromatography using dye from different coloured M&M’s®. Includes kit list and safety instructions.

Colourful reactions using ammonia solution
Turn ammonia solution red, white or blue by adding phenolphthalein, lead nitrate or copper(II) sulfate in this demonstration. Includes kit list and safety instructions.

Colourimetric determination of copper ore
Use this practical to introduce students to the determination of copper ore by colourimetry using copper(II) sulfate. Includes kit list and safety instructions.

Combustion of ethanol
Illustrate the large energy changes that take place during the combustion of alcohols with this spectacular demonstration. Includes kit list and safety instructions.

Combustion of hydrogen in air
Try this demonstration or class experiment to investigate how varying amounts of fuel and oxygen affect combustion. Includes kit list and safety instructions.

Comparing heat energy from burning alcohols
Investigate the amounts of heat energy produced by the combustion of different alcohols in this class experiment. Includes kit list and safety instructions.

Comparing light- and heavy-duty detergents
Try this set of experiments to compare the effects of light- and heavy-duty detergents with different pH values. Includes kit list and safety instructions.

Comparing the melting points of solder, tin and lead
Test the melting points of lead, tin and solder to investigate solder as a solid mixture and alloy in this practical. Includes kit list and safety instructions.

Corrosion in different atmospheric conditions
Try this practical to test the corrosion of metals in dry air, moist air and air polluted by acidic sulfur dioxide. Includes kit list and safety instructions.

Cracking hydrocarbons in liquid paraffin with a catalyst
Model the industrial process of cracking larger hydrocarbons to produce smaller alkanes in this demonstration or class practical. Includes kit list and safety instructions.
Cracking hydrocarbons on a microscale
Use this microscale experiment to illustrate hydrocarbon cracking using paraffin, bromine water and aluminium oxide. Includes kit list and safety instructions.
Dehydration of ethanol to form ethene
Use this class practical or demonstration to produce ethene gas as an example of an unsaturated hydrocarbon. Includes kit list and safety instructions.

Detecting starch in food on a microscale
Test different foodstuffs for the presence of starch using iodine in this microscale class practical. Includes kit list and safety instructions.

Detergents, soaps and surface tension
A series of brief experiments on the effects of detergents and soaps on the surface tension of purified and hard water. Includes kit list and safety instructions.

Determining relative molecular mass by weighing gases
Use this demonstration to determine the relative molecular masses of different gases using the ideal gas equation. Includes kit list and safety instructions.

Determining the relative atomic mass of magnesium
Use this practical to determine the relative atomic mass of magnesium using its reaction with hydrochloric acid. Includes kit list and safety instructions.

Determining the relative molecular mass of butane
Use this demonstration to calculate the relative molecular mass of butane using simple apparatus. Includes kit list and safety instructions.

Diffusion in liquids
Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Includes kit list and safety instructions.

Diffusion of gases: ammonia and hydrogen chloride
A demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. Includes kit list and safety instructions.

Displacement reactions between metals and their salts
Students will investigate competition reactions of metals and determine a reactivity series of the four metals used. Includes kit list and safety instructions.

Displacement reactions of non-metals
Investigate a displacement series of non-metals using oxygen and chlorine in this class practical or demonstration. Includes kit list and safety instructions.

Dissolved substances in tap water and seawater
Compare the solids and gases dissolved in tap water and seawater in this class practical and demonstration. Includes kit list and safety instructions.

Distribution of iodine between two immiscible solvents
Use this class experiment or demonstration to create an equilibrium distribution using iodine in two immiscible solvents. Includes kit list and safety instructions.

Dyeing three colours from the same dye bath
Show how dyeing involves chemical interactions between dyes and the molecular nature of different fibres in this demonstration. Includes kit list and safety instructions.

Electrolysis of brine
Use this colourful practical to introduce students to the electrolysis of brine, or sodium chloride solution. Includes kit list and safety instructions.

Electrolysis of copper(II) sulfate solution
Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Includes kit list and safety instructions.

Electrolysis of molten lead(II) bromide
Introduce your students to the study of electrolysis through the production of metallic lead and bromine in this demonstration. Includes kit list and safety instructions.

Electrolysis of molten zinc chloride
Try this demonstration to show how an ionic salt will conduct electricity when molten but not when solid. Includes kit list, video and safety instructions.

Emulsifiers in the kitchen
Test a range of common ingredients to see which ones stabilise an oil and water emulsion in this class practical. Includes kit list and safety instructions.

Endothermic solid–solid reactions
Observe an endothermic reaction between two solids in this demonstration or class experiment. Includes kit list and safety instructions.

Energy content in foods
Try this class experiment to investigate how much energy different foods contain. Includes kit list and safety instructions.

Equilibria involving carbon dioxide in aqueous solution
Use this demonstration or class practical to illustrate changes to equilibria in carbonated soda water. Includes kit list and safety instructions.

Estimating the concentration of bleach
Compare the chlorine content and concentration of sodium hypochlorite in different bleaches in this class practical. Includes kit list and safety instructions.

Exothermic metal displacement reactions
Try this class experiment to explore what happens when different metals are added to a copper(II) sulfate solution. Includes kit list and safety instructions.

Exothermic metal–acid reactions
Use this class practical to explore the temperature changes resulting from adding different metals to hydrochloric acid. Includes kit list and safety instructions.

Exothermic or endothermic? Classifying reactions
Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. Includes kit list and safety instructions.
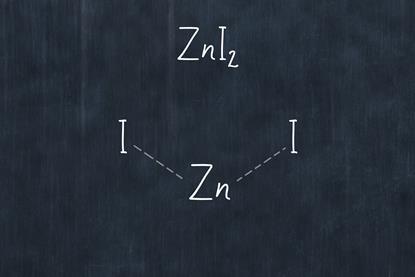
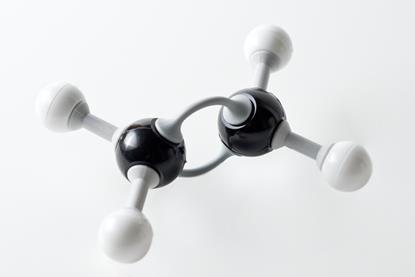
Exothermic redox reaction of zinc with iodine
Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound.

Exploding a tin can using methane
Use this demonstration to illustrate how methane can create an explosive mixture with the oxygen in air. Includes kit list and safety instructions.

Exploding bubbles of hydrogen and oxygen
Create a small explosion in this demonstration by electrolysing water to produce hydrogen and oxygen bubbles. Includes kit list, video and safety instructions.

Extracting copper from copper(II) carbonate
Use this practical to produce copper from copper(II) carbonate, modelling the extraction of copper from malachite. Includes kit list and safety instructions.

Extracting iodine from seaweed
Discover how ribbon seaweed (or kelp) can be used as a source of iodine in this demonstration or class experiment. Includes kit list and safety instructions.

Extracting iron from breakfast cereal
Try this class practical or demonstration to extract food-grade iron from breakfast cereals using neodymium magnets. Includes kit list and safety instructions.

Extracting metals with charcoal
Try this class practical to illustrate the idea of competition reactions between metals and carbon. Includes kit list and safety instructions.

Extraction of iron on a match head
Try this practical as a small scale example of metal extraction, reducing iron(III) oxide with carbon on a match head. Includes kit list and safety instructions.

Fat-pan fires and the conditions for combustion
Use this demonstration to illustrate the conditions required to start combustion, and how to put out a pan fire safely. Includes kit list and safety instructions.

Fermentation of glucose using yeast | 14–16 years
In association with Nuffield Foundation , By Neil Goalby
Use this class practical to investigate the fermentation of glucose by yeast and test for ethanol. Includes kit list, safety instructions, questions and answers

Finding the formula of copper(II) oxide
Use this class practical with your students to deduce the formula of copper(II) oxide from its reduction by methane. Includes kit list and safety instructions.

Finding the formula of hydrated copper(II) sulfate
In this experiment students will measure the mass of hydrated copper(II) sulfate before and after heating and use mole calculations to find the formula.

Flame colours: a demonstration
Explore how different elements rect when exposed to a flame, and discuss how alkali metals, alkaline earth metals, and metal salts change the colour of fire.

Floating and sinking bubbles
Make bubbles of carbon dioxide, hydrogen or methane in this demonstration exploring density, diffusion and solubility. Includes kit list and safety instructions.

Generating, collecting and testing gases
Read our standard guidance on generating, collecting and testing gases during practical experiments, including carbon dioxide, hydrogen, oxygen and chlorine.

Halogen reactions with iron wool
Illustrate an exothermic redox reaction by heating iron wool with chlorine, bromine and iodine with this demonstration. Includes kit list and safety instructions.

Halogens in aqueous solution and their displacement reactions
Explore the chemical properties of halogens using this demonstration or class experiment. Includes kit list and safety instructions.

Handling liquid bromine and preparing bromine water
Find out how to handle liquid bromine and prepare bromine water safely using these health, safety and technical notes.

Heating chocolate and egg
Use this practical to introduce students to physical and chemical changes and the safe use of Bunsen burners. Includes kit list and safety instructions.

Heating copper in air
Explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. Includes kit list and safety instructions.

Heating group 1 metals in air and in chlorine
Use this demonstration to illustrate the reactions of lithium, sodium and potassium in air and in chlorine. Includes kit list, video and safety instructions.

How can hardness in water be removed?
Investigate the effects of three treatments for softening hard water in this class practical and demonstration. Includes kit list and safety instructions.

How much oxygen is used when iron wool rusts?
Try this practical to investigate how much oxygen is used in rusting and calculate the percentage of oxygen in air. Includes kit list and safety instructions.

Hydrogels in plant water storage crystals
Investigate plant water storage crystals as one application of hydrogels in this fun class practical. Includes kit list and safety instructions.

Identifying polymers by density
Investigate and identify a variety of polymers used in everyday materials by testing their density in this practical. Includes kit list and safety instructions.

Identifying the products of combustion
Illustrate the presence of water and carbon dioxide in the products of hydrocarbon combustion in this demonstration. Includes kit list and safety instructions.

Identifying the products of electrolysis
Try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. Includes kit list and safety instructions.

Indicators and dry ice demonstration
Create bubbles, ‘fog’ and a colour change adding dry ice to alkaline ammonia or sodium hydroxide solution in this demonstration. Includes kit list and safety instructions.

Investigating hydrogels in nappies and hair gel
Investigate hydrogels as polymeric smart materials in this series of activities using nappies and hair gel. Includes kit list and safety instructions.

Investigating the role of water in acidity
Try this practical or demonstration to explore the importance of water for acidity using hydrogen chloride and methylbenzene. Includes kit list and safety instructions.

Investigating the solubility of lead halides
Encourage students to make and test predictions about the pattern of solubility among lead halides in this practical. Includes kit list and safety instructions.

Iodine clock reaction demonstration method
Use this iodine clock reaction demonstration to introduce your students to rates of reaction and kinetics. Includes kit list and safety instructions.

Iron and sulfur reaction
This demonstration or class experiment shows the exothermic reaction of iron and sulphur. Includes kit list and safety instructions.

Leaf chromatography
Try this class practical to use paper chromatography to separate and investigate the pigments in a leaf. Includes kit list and safety instructions.

Liquefying chlorine gas
Use this demonstration to produce liquid chlorine and compare it with bromine and iodine in their condensed state. Includes kit list and safety instructions.

Making a crystal garden
Create chemical gardens with your students by growing crystals of coloured silicates in this class practical. Includes kit list and safety instructions.

Making a pH indicator using red cabbage
Try this class practical to make a pH indicator from red cabbage with your students. Includes kit list and safety instructions.

Making a photographic print using silver chloride
Try this practical or demonstration to create a photographic image of an object using light sensitive silver chloride. Includes kit list and safety instructions.

Making and testing ammonia
In this experiment, students make ammonia, investigate its solubility in water and test its alkaline nature. Includes kit list and safety instructions.

Making esters from alcohols and acids
Investigate the reactions between a range of alcohols and acids by producing a variety of esters in this class experiment. Includes kit list and safety instructions.

Making glass
Try this class practical to make samples of glass using lead oxide, zinc oxide and boric acid. Includes kit list and safety instructions.

Making glue from milk
Try this class practical to prepare a polymer glue from milk using the protein casein. Includes kit list and safety instructions.

Making magnesium carbonate: the formation of an insoluble salt in water
Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Includes kit list and safety instructions.

Making plastic from potato starch
Try this class practical to make a plastic using potato starch and investigate the effects of adding a ‘plasticiser’. Includes kit list and safety instructions.

Making rayon
Use this demonstration to produce rayon fibres in the classroom using cotton wool or filter paper. Includes kit list and safety instructions.

Making soaps and detergents using castor oil
Try this class practical to make a soap or detergent using castor oil and either sodium hydroxide or sulfuric acid. Includes kit list and safety instructions.

Making solder as an alloy of tin and lead
Try this practical to make solder by heating together the metals tin and lead before investigating the alloy’s properties. Includes kit list and safety instructions.

Melting and freezing stearic acid
In this class practical students take the temperature of stearic acid at regular intervals as they heat and cool it. Includes kit list and safety instructions.

Microscale extraction of copper
Try this practical to reduce copper(II) oxide to copper using hydrogen, revealing their positions in the reactivity series. Includes kit list and safety instructions.

Microscale reactions of positive ions with sodium hydroxide
Try this microscale practical exploring the reactions of various positive ions with sodium hydroxide. Includes kit list and safety instructions.

Microscale oxidation of cyclohexanol by potassium dichromate(VI)
Use this quick class experiment to observe the oxidation of cyclohexanol to produce cyclohexanone. Includes kit list and safety instructions.

Microscale preparation of ethyl benzoate
Try this class practical to prepare the ester ethyl benzoate on a microscale by warming ethanol and benzoic acid. Includes kit list and safety instructions.

Modelling alloys with plasticine
Try this class activity to explore how alloying can be used to change the properties of a metal. Includes kit list and teaching notes.

Modelling the greenhouse effect
Use this demonstration to illustrate the greenhouse effect and the role of carbon dioxide as a greenhouse gas. Includes kit list and safety instructions.
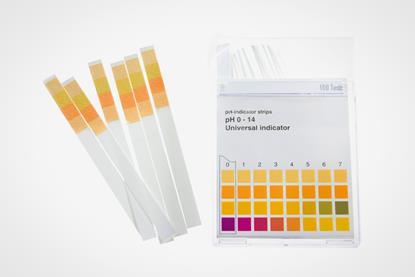
Neutralising an acidic solution
Use this simple practical to illustrate the pH and temperature changes as an acidic solution is neutralised. Includes kit list and safety instructions.

Oxidation of ethanol
In this class practical, ethanol is oxidised by acidified sodium dichromate to form ethanal and then ethanoic acid. Includes kit list and safety instructions.

Phenol-methanal polymerisiation
Make Bakelite in class and investigate its properties using phenol, methanal and ethanoic acid in this demonstration. Includes kit list and safety instructions.

Preferential discharge of cations during electrolysis
Use this practical to show that metal cations are preferentially discharged, in relation to the metal’s position in the reactivity series. Includes kit list and safety instructions.

Preparing a soluble salt by neutralisation
In this practical, students react alkaline ammonia with sulfuric acid to form the soluble salt ammonium sulfate. Includes kit list and safety instructions.

Preparing an insoluble salt in a precipitation reaction
Produce an insoluble salt precipitate by reacting two soluble metal salts together in this class experiment. Includes kit list and safety instructions.

Preparing and using cobalt chloride indicator papers
Make your own cobalt chloride indicator papers, which can be used to test for the presence of water. Includes kit list and safety instructions.

Preparing salts by neutralisation of oxides and carbonates
Try these class experiments to illustrate the production of soluble salts from insoluble metal oxides and carbonates. Includes kit list and safety instructions.

Preventing rust
Try this practical to test methods for preventing rust on iron nails, including painting, greasing and sacrificial protection. Includes kit list and safety instructions.

Properties of alkali metal compounds
Try this class practical to explore the physical and chemical properties of various alkali metal compounds. Includes kit list and safety instructions.

Purifying an impure solid
Purify alum as an example of obtaining a pure chemical from an impure sample in this class practical. Includes kit list and safety instructions.


PVA polymer slime
In this fun class experiment student will make slime by adding borax solution to PVA. Includes kit list and safety instructions.
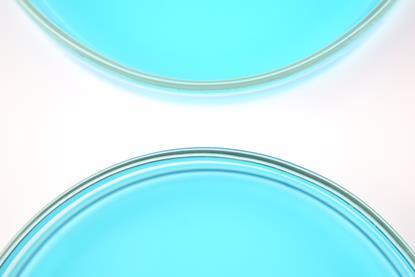
Quantitative electrolysis of aqueous copper(II) sulfate
Use this demonstration to find the value of the Faraday constant from electrolysis of copper(II) sulfate solution. Includes kit list and safety instructions.

Rate of evaporation
Use this class practical to measure and compare the rate of evaporation of propanone under different conditions. Includes kit list and safety instructions.

Rate of reaction of potassium manganate(VII) and oxalic acid
Investigate the effect of surface area or concentration on rate of reaction using oxalic acid in rhubarb and potassium manganate(VII). Includes kit list and safety instructions.

Reacting aluminium and iodine
Illustrate the spectacular reaction between aluminium and iodine with water as a catalyst in this demonstration. Includes kit list and safety instructions.

Reacting copper(II) oxide with sulfuric acid
Illustrate the reaction of an insoluble metal oxide with a dilute acid to produce crystals of a soluble salt in this class practical. Includes kit list and safety instructions.

Reacting magnesium with copper(II) oxide
Illustrate reduction, oxidation and the relative reactivity of magnesium and copper(II) oxide in this demonstration. Includes kit list and safety instructions.

Reacting zinc and copper(II) oxide
Illustrate competition reactions using the exothermic reaction between copper(II) oxide and zinc in this class demonstration. Includes kit list and safety instructions.

Reactions of chlorine with water or halide ions
Generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. Includes kit list and safety instructions.

Reactions of chlorine, bromine and iodine with aluminium
Try this demonstration to produce some spectacular exothermic redox reactions by reacting aluminium with halogens. Includes kit list and safety instructions.

Reactions of metals with acids producing salts
Explore the production of hydrogen gas and salts when metals react with acids in this class experiment. Includes kit list and safety instructions.

Reactivity trends of the alkali metals
Use this experiment to demonstrate the trend in reactivity down group 1 of the Periodic Table, exploring the physical and chemical properties of the alkali metals.

Rechargeable cells: the lead–acid accumulator
Use this practical to demonstrate the chemistry behind rechargeable batteries, using a lead–acid accumulator cell. Includes kit list and safety instructions.

Recovering water from a solution using a condenser
Use this demonstration to show how pure water can be recovered from copper sulfate solution using a condenser. Includes kit list and safety instructions.

Recovering water from copper(II) sulfate solution
Try this practical to introduce students to aqueous solutions by distilling water from copper(II) sulfate solution. Includes kit list and safety instructions.

Separating salts from seawater
Try this simple practical to show that seawater contains a mixture of different salts. Includes kit list and safety instructions.

Separating sand and salt by filtering and evaporation
Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Includes kit list and safety instructions.

Silver and lead halides
Try this practical or demonstration to produce silver and lead halides in a series of precipitation reactions. Includes kit list and safety instructions.

Sodium ethanoate ‘stalagmite’
Quickly grow your own ‘stalagmite’ from a supersaturated solution of sodium ethanoate in this demonstration. Includes kit list and safety instructions.

Solubility patterns of halogen anions
Try this microscale practical to identify and explain patterns in the solubility of fluoride, chloride, bromide and iodide anions. Includes kit list and safety instructions.
Solubility trends of metal halides
Investigate patterns in the solubility of halides of silver and some Group 1 and 2 metals in this microscale practical. Includes kit list and safety instructions.

Sulfuric acid as a dehydrating agent
Try these two demonstrations to illustrate the difference between dehydration and drying using sulfuric acid. Includes kit list and safety instructions.

Supercooling and the energetics of freezing
Explore what happens when a liquid is supercooled using sodium thiosulfate in this class practical. Includes kit list and safety instructions.
Testing for catalase enzymes
Try this class experiment to detect the presence of enzymes as they catalyse the decomposition of hydrogen peroxide. Includes kit list and safety instructions.

Testing the pH of different solutions
Use this practical to reinforce students’ understanding of pH by preparing and testing acidic and alkaline solutions. Includes kit list and safety instructions.

Testing the pH of oxides
Use this class practical to investigate the pH of different metal and non-metal oxides using a universal indicator. Includes kit list and safety instructions.

Testing the hardness of water
Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions.

The ‘blue bottle’ experiment
In this demonstration, the redox indicator Methylene blue can be oxidised many times by shaking. Includes kit list and safety instructions.

The ‘breathalyser’ reaction | 16–18 years
In association with Nuffield Foundation , By Tim Jolliff and Sandrine Bouchelkia
Try this demonstration to recreate an early ‘breathalyser’ test, passing ethanol vapour through potassium dichromate. Includes kit list and safety instructions

The ‘Old Nassau’ or Halloween clock reaction
Illustrate dramatic colour changes as a result of redox and precipitation reactions in this vivid demonstration. Includes kit list and safety instructions.

The ‘whoosh’ bottle demonstration
This exciting demonstration is a combustion reaction where a mixture of alcohol and air in a large bottle is ignited. Includes kit list and safety instructions.

The acidic reactions of ethanoic acid
Explore the properties of ethanoic acid as a weak organic acid in this class experiment. Includes kit list and safety instructions.

The change in mass when magnesium burns
A class practical to measure the change in mass when magnesium burns and to find the formula of magnesium oxide. Includes kit list and safety instructions.

The chemistry of cooking potatoes
Use this class practical to investigate what happens to potatoes and potato cells when they are boiled. Includes kit list and safety instructions.
The combustion of iron wool
Try this quick teacher demonstration to demonstrate the increase in mass as iron wool is heated in air. Includes kit list and safety instructions.

The composition and formula of water
Try this demonstration to determine the formula of water through the reaction of copper(II) oxide with hydrogen. Includes kit list and safety instructions.
The conversion of alcohols to halogenoalkanes
Try this practical or demonstration to produce bromoethane in a substitution reaction between ethanol and phosphorus tribromide. Includes kit list and safety instructions.

The cornflour ‘bomb’
Create a small explosion inside a tin can using cornflour in this demonstration, illustrating energy transformation. Includes kit list and safety instructions.

The density of carbon dioxide
Illustrate the higher density of carbon dioxide relative to air by pouring it over a lighted candle in this demonstration. Includes kit list and safety instructions.

The density of ice
Demonstrate to students what happens as ice cubes floating on oil start to melt and the density of the water changes. Includes kit list and safety instructions.

The effect of concentration and temperature on an equilibrium | Le Chatelier’s principle
Try this demonstration to illustrate how changing chlorine concentration or temperature shifts the position of an equilibrium. Includes kit list and safety instructions.
The effect of concentration on equilibrium | Le Chatelier’s principle
Illustrate the reversible reaction between bismuth(III) oxychloride and bismuth(III) chloride in this demonstration. Includes kit list and safety instructions.

The effect of pressure and temperature on equilibrium | Le Chatelier’s principle
Try this demonstration to explore the effects of pressure and temperature on an equilibrium mixture with your students. Includes kit list and safety instructions.

The equilibrium between two coloured cobalt species
In this demonstration the equilibrium between two different coloured cobalt species is disturbed. Le Chatelier’s principle is used to predict a colour change.

The formation of a sol
Convert a rusty-brown precipitate of iron(III) hydroxide into a cherry-red iron(III) oxide sol in this demonstration. Includes kit list and safety instructions.

The fractional distillation of crude oil
Try this class practical or demonstration to simulate the industrial fractional distillation of crude oil. Includes kit list and safety instructions.

The hydration of alkenes
Try this demonstration and class practical to illustrate the addition reactions of alkenes, with the preparation and purification of an organic liquid.

The migration of ions during electrolysis of potassium manganate(VII)
Try this class practical to investigate the migration of ions during electrolysis as evidence for the ionic model. Includes kit list and safety instructions.

The properties of alcohols
Ethanol and propan-1-ol are tested for pH, reaction with sodium, combustion and oxidation with acidified dichromate(VI) solution. Includes kit list and safety instructions.
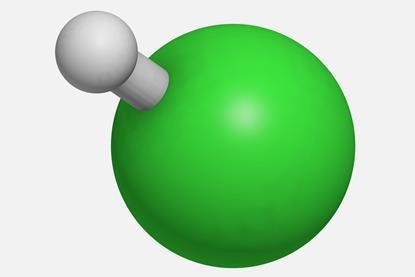
The properties of hydrogen chloride
Use this demonstration and practical to investigate properties of hydrogen chloride, such as its solubility in water. Includes kit list and safety instructions.
The rate of reaction of magnesium with hydrochloric acid
A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Includes kit list and safety instructions.

The reaction of aluminium and copper(II) sulfate
Try this practical or demonstration to illustrate the displacement of copper from copper sulfate using aluminium foil, with kit list and safety instructions.

The reaction of carbon dioxide with water
Form a weak acid from the reaction of carbon dioxide with water in this class practical. Includes kit list and safety instructions.

The reaction of ethyne with chlorine
Try this teacher demonstration with your students to illustrate the spontaneous reaction of ethyne and chlorine. Includes kit list and safety instructions.

The reactivity of iron
Illustrate iron’s position in the reactivity series by heating it with copper and magnesium oxides in this practical. Includes kit list and safety instructions.

The silver mirror test with Tollens’ reagent
Try this practical to explore the mirror-making reaction between silver nitrate (Tollens’ reagent) and glucose. Includes kit list, video and safety instructions.

The structure and properties of chocolate
Investigate how melting chocolate changes its structure and affects properties like taste, texture and melting point. Includes kit list and safety instructions.

The sublimation of air freshener | 11–14 years
In association with Nuffield Foundation , By Dorothy Warren
Use this experiment to demonstrate sublimation, showing how solid air freshener changes directly from a solid to a gas. Includes kit list and safety instructions.

The thermal properties of water
Explore water’s boiling point, specific heat capacity and thermal conductivity in this demonstration. Includes kit list and safety instructions.

The thermite reaction between aluminium and iron(III) oxide
Illustrate a highly exothermic thermite reaction resulting in molten iron in this teacher demonstration. Includes kit list and safety instructions.

Thermal decomposition of calcium carbonate
A class practical on the thermal decomposition of calcium carbonate. Includes kit list and safety instructions.

Thermal decomposition of nitrates: ‘writing with fire’
Make an invisible message ‘glow’ by applying a lighted splint to filter paper treated with sodium nitrate in this demonstration. Includes kit list and safety instructions.

Thermal decomposition of metal carbonates
Use this class practical to compare the thermal stabilities of carbonates of reactive and less reactive metals. Includes kit list and safety instructions.

Titrating sodium hydroxide with hydrochloric acid
Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions.

Turning ‘red wine’ into ‘water’
Use acidified potassium permanganate – or ‘red wine’ – to make ‘water’, ‘milk’ and ‘lemonade’ in this engaging demonstration. Includes kit list and safety instructions.

Turning copper coins into ‘silver’ and ‘gold’
Perform what looks like alchemy with ordinary copper coins in this teacher demonstration. Includes kit list and safety instructions.

Universal indicator ‘rainbow’
Try this demonstration to create a rainbow effect using universal indicator, hydrochloric acid and sodium hydroxide. Includes kit list and safety instructions.

Unsaturation in fats and oils
Use this class practical to investigate the amounts of unsaturated fats and oils in different foods. Includes kit list and safety instructions.

Urea-methanal polymerisation
Explore condensation polymerisation by creating and investigating the properties of a thermosetting polymer in this demonstration. Includes kit list and safety instructions.

Using indigestion tablets to neutralise an acid
Investigate and measure the neutralising effect of indigestion tablets on hydrochloric acid in this class practical. Includes kit list and safety instructions.

Water expands when it freezes
Use this demonstration to show that water expands when it freezes, showing students how it can break a bottle. Includes kit list and safety instructions.

What are the dissolved solids in seawater?
Analyse the salts that crystallise from evaporating seawater, illustrating cation and anion tests in this demonstration. Includes kit list and safety instructions.

What causes iron to rust?
Use this class experiment to help students investigate what conditions are needed for iron to rust. Includes kit list and safety instructions.

What ions cause hardness in water?
Investigate how different cations and anions in dissolved salts affect the formation of a lather in this experiment. Includes kit list and safety instructions.

What makes a substance acidic?
Try these experiments to investigate acidity and learn how the acidic properties of some substances require water. Includes kit list and safety instructions.

Where is carbon in the reactivity series?
Determine the position of carbon in the reactivity series by heating with metal oxides in this practical and demonstration. Includes kit list and safety instructions.

Which substances conduct electricity?
In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. Includes kit list and safety instructions.

Yeast and the expansion of bread dough
Try this class practical to investigate how temperature affects yeast and the expansion of bread dough. Includes kit list and safety instructions.
- Contributors
- Email alerts
Site powered by Webvision Cloud
- STEM Ambassadors
- School trusts
- ITE and governors
- Invest in schools
- STEM careers inspiration
- Benefits and impact
- Our supporters
- Become a STEM Ambassador
- Request a STEM Ambassador
- Employer information
- Training and support
- STEM Ambassadors Partners
- Working with community groups
- Search icon
- Join the STEM Community
Nuffield Science Activities for GCSE
The three packs of Nuffield Science activity sheets were designed to match the National Curriculum Science at Key stage Four from 1996. The ideas were drawn from materials produced by various Nuffield projects over the previous ten years.
Each pack contains activities which exemplify the Nuffield approach to learning science. Many of the activities require apparatus and laboratory facilities. Others lend themselves to group discussion or private study at school or at home.
- Teacher guidance
- Include Physical Resources
Nuffield Biology Activities for GCSE
This pack of Nuffield Biology activity sheets was designed to match the National Curriculum Science: Biology at Key stage Four from 1996. The ideas were drawn from materials produced by various Nuffield projects over the previous ten years. The pack includes student...
Nuffield Chemistry Activities for GCSE
This pack of Nuffield Chemistry activity sheets was designed to match the National Curriculum Science: Chemistry at Key stage Four from 1996. The ideas were drawn from materials produced by various Nuffield projects over the previous ten years. The pack includes student...
Nuffield Physics Activities for GCSE
This pack of Nuffield Physics activity sheets was designed to match the National Curriculum Science: Physics at Key stage Four from 1996. The ideas were drawn from materials produced by various Nuffield projects over the previous ten years. The pack includes student sheets for 97 different activities.
Share this resource
Did you like this resource.
- Work For Us
Summer of science for budding researchers
25th september 2019.
Students from schools and colleges across the North East had the opportunity to work alongside academics on real-life research projects this summer, thanks to a partnership between Northumbria University and the Nuffield Research Placement programme.
The competitive programme, which lasts between four to six weeks, aims to provide talented, motivated students with the opportunity to undertake science, quantitative social science, computing, technology, engineering or maths (STEM) research projects in a professional environment.
Each of the 24 students involved was partnered with an academic or PhD researcher and given the opportunity to assist on a real research project.
Explaining the benefits of the programme, Dr Ian Walshe, a Senior Lecturer within Northumbria’s Department of Sport, Exercise and Rehabilitation, said: “The step between school and higher education can be quite daunting and I feel this is a great stepping stone which can really enhance a student’s CV.
“During their time with us, students are able to experience the working environment of a university, and take part in real research trials.”

“My project has been really interesting – I’m researching land contamination in relation to civil engineering. It’s been a great experience and something which will be great for my CV when I’m applying to university.”

“I’m interested in studying medicine at university and as part of my placement I have been working with older people in the community.
“It’s been great to talk to them about their clinical experiences and hear first-hand from the people using medical services. I’ve also been involved in data analysis and literature reviews so it’s been a great experience.”

“It’s been an amazing experience. The work is challenging but being able to be involved in real research and making new discoveries is something not many people get to experience at our age so I feel really lucky to be part of the placement scheme.”
The Nuffield Research Placement is a widening participation scheme, with a focus on students who are the first in their family to attend University. The majority of students who are offered a placement are eligible for a full bursary of £100 a week, with all student travel expenses covered.
Gill Drinkald, from Northumbria’s Research and Innovation Services team, coordinated the placements this year. Explaining the benefits, she said: “The academics involved provided access to such interesting research topics, as well as guidance and support which enabled the students to produce a scientific report at the end of their placement.
“Recruiting and working with twenty four gifted young Nuffield Research Placement students, has been a real insight. I have witnessed the students grow in confidence and enthusiasm for their research subjects as a result of working with academics and their teams in a real research environment whether that be lab-based, fieldwork etc.
“Northumbria’s involvement in the Nuffield Research Placement programme demonstrates the University’s commitment to widening participation and encouraging students from underrepresented groups to consider higher education, and providing the routes to making that possible.”
Students applying for the Nuffield Research Placement programme must have good GCSE results, be aiming to go on to study at university, and have demonstrated enthusiasm and commitment to working on a research project. The programme is provided by the Nuffield Foundation as part of its mission to improve social well-being and educational opportunity across the UK.
Some of the students taking part in the Northumbria placements have entered their work into the North East heat of the Big Bang Competition – a young scientists and engineers fair which takes place in October. If successful there they will go through to the national final at the Big Bang Fair in March 2020.

Department of Applied Sciences

Department of Mathematics, Physics and Electrical Engineering

Sport Exercise and Rehabilitation Related Courses

News and Features
This is the place to find all the latest news releases, feature articles, expert comment, and video and audio clips from Northumbria University

Northumbria Open Days
Open Days are a great way for you to get a feel of the University, the city of Newcastle upon Tyne and the course(s) you are interested in.

Research at Northumbria
Research is the life blood of a University and at Northumbria University we pride ourselves on research that makes a difference; research that has application and affects people's lives.

Explore NU World
Find out what life here is all about. From studying to socialising, term time to downtime, we’ve got it covered.
Latest News and Features

EXPERT COMMENT: Russia and the Taliban: here’s why Putin wants to get closer to Afghanistan’s current rulers
In this article originally written for The Conversation*, Intigam Mamedov, Postdoctoral research…

Pioneering businesswoman honoured by Northumbria
Roisin Currie, Chief Executive Officer (CEO) of Greggs PLC, has been awarded an honorary degree…

Prestigious nomination for Northumbria cyber security students
Two Northumbria University cyber security students have been shortlisted at the globally recognised…

New exhibition honours the life of a North East icon
As fans prepare for the 2024 World Snooker Championship to get under way this weekend, a new…

EXPERT COMMENT: The rising flood of space junk is a risk to us on Earth – and governments are on the hook
In this article originally written for The Conversation*, Dr Thomas Cheney, Vice Chancellor's…

No link between Instagram use and levels of anxiety, depression and loneliness in adults, new study reveals
Adults who use Instagram are no more likely to suffer from anxiety, depression or loneliness…

“Going to university has transformed my life” – Professor Andy Long talks about being the first in his family to attend university as part of #100Faces campaign
Professor Andy Long, Vice-Chancellor and Chief Executive at Northumbria University, has been…

Cultural-led regeneration to help Gateshead flourish
A Northumbria University academic has received levelling up funding to help creative and cultural…
Upcoming events

Why are so many problems worse in more unequal societies?
Lecture Theatre 002

Virtual Event - Your Wellbeing

Legal Professional Privilege in the 21st Century: Adversarialism, Confidentiality and Justice
Northumbria University

Virtual Event - LLM Space Law & Cyber Law Taster
11am - 12pm
Back to top


Research projects will partner students with DOE national labs to help students develop hands-on research experience
WASHINGTON, D.C . - Today, the U.S. Department of Energy (DOE) announced $16 million in funding for four projects providing classroom training and research opportunities to train the next generation of accelerator scientists and engineers needed to deliver scientific discoveries.
U.S. global competitiveness in discovery science relies on increasingly complex charged particle accelerator systems that require world-leading expertise to develop and operate. These programs will train the next generation of scientists and engineers, providing the expertise needed to lead activities supported by the DOE Office of Science. These programs will develop new curricula and guide a diverse cadre of graduate students working towards a master’s or Ph.D. thesis in accelerator science and engineering.
“Particle accelerator technology enables us to tackle challenges at the frontiers of science and benefits our nation’s high-tech industries, modern medicine, and national security,” said Regina Rameika, DOE Associate Director of Science for High Energy Physics. “The awards announced today will help to develop the workforce to advance the state-of-the-art in accelerator technology while helping deploy these technologies in commercial applications in the health, security, environmental, and industrial sectors. These programs at American universities will help ensure that our nation has a skilled and diverse workforce to develop the accelerator technology needed to meet the scientific challenges of the future.”
Research projects will partner students with DOE national labs to help students develop hands-on research experience. These projects include opportunities for graduate research across a broad range including beam physics at the systems level, technologies of large accelerators, high reliability design and failure analysis, and the fundamentals of project management. Students may also explore the material science, design methodology, fabrication techniques, and operations constraints needed to produce and operate superconducting radiofrequency accelerators. Additional research opportunities in the areas of high-reliability, high-power radiofrequency systems and large-scale cryogenic systems, particularly liquid helium systems, are available through these programs.
The projects were selected by competitive peer review under the DOE Funding Opportunity Announcement for DOE Traineeship in Accelerator Science & Engineering.
Total funding is $16 million for projects lasting up to five years in duration, with $3 million in Fiscal Year 2024 dollars and outyear funding contingent on congressional appropriations. Funding is provided by the High Energy Physics and the Accelerator R&D and Production programs. The list of projects and more information can be found on the High Energy Physics program homepage and the Accelerator R&D and Production program homepage.
Selection for award negotiations is not a commitment by DOE to issue an award or provide funding. Before funding is issued, DOE and the applicants will undergo a negotiation process, and DOE may cancel negotiations and rescind the selection for any reason during that time.
Looking for our teaching and curriculum resources?
Sir Ernest Ryder | Making justice work

Series Changing lives for the better: priorities for the next 20 years
Address by Rt Honourable Sir Ernest Ryder , Nuffield Foundation Trustee, at our Justice conference
The Nuffield Foundation has a purpose which is to advance social well-being.
Our work cuts across education , welfare , and justice . We specifically aim to address the inequalities, disadvantage, discrimination and vulnerabilities people face in justice and consider the ethical implications of systems, processes, technologies and decision-making.
In this, our 80 th anniversary year , our challenge is to ask how we can build and maintain the effective, accountable and trustworthy institutions that we need to support our society and its communities and maintain the legitimacy of the rule of law as the glue that holds us together.
Justice systems are not simply the buildings in which the judiciary sit, or the quality of the judgments they undoubtedly produce. They are much more. At the very least there are, in addition, three aspects that I suggest are of critical importance to their effectiveness:
First, the observational or performative perceptions of trust, respect and confidence in these systems by people and their communities, who will often have different and sometimes conflicting or incommensurable values that need to be respected if not resolved.
Second, the impact of those systems on people and their communities as measured by data on outcomes that are quantifiable so that we are able to understand access to justice and whether the systems are effective in addressing inequalities, disadvantage, discrimination and vulnerability and answer the question whether there is in fact equality before the law.
Third, the quality of our governance of those systems. The leadership of justice is a partnership between the Executive or Government, including the devolved administrations, and the Judiciary. That leadership has a relationship with Parliament that reflects the constitutional principle of accountability while respecting the separation of powers. Without that delicate balance being demonstrated there will be a democratic deficit in the exercise of power.
We have over time developed careful conventions to regulate and re-calibrate our governance: for example:
- Constitutional norms such as sovereignty, accountability, separation of powers, independence of the judiciary and access to justice;
- The principles of the Rule of Law which are well described in international instruments; and
- Ethical standards and codes of conduct, for example concerning independence, impartiality and integrity.
I would suggest, however, that these are not in themselves enough because they do not cause us to measure outcomes, perceptions, in particular trust, and the effect of inadequate governance. The impact of these critical components is most obviously experienced when systems are inadequately resourced so that access to justice is adversely affected with the consequence that the individual is unable to obtain an appropriate, timely and affordable remedy. The consequence of the scarcity of resources will often be insufficient advice and representation, insufficient judges, a poor quality justice environment both as to place and facilities and insufficient investment in training, development, diversity and leadership and management across the justice sector.
Justice that is delayed by backlogs that are a consequence of inadequate funding and inadequately addressed structural questions leads to justice being denied for some of our users. There is an urgent need to go beyond our principles and judgments and examine the effectiveness of our arrangements. There are real concerns about the political leadership of justice and its commitment to the Rule of Law, not I emphasise by individual justice Ministers, although there have been exceptions, but by Government generally, its understanding of constitutional issues, the impact of policies on people and their communities and the effect of inequality and inadequate resources which are questions of legitimate concern.
It is now 20 years since the government of Tony Blair embarked on a new constitutional settlement. Between 2004 and 2008 two Acts of Parliament changed the way that the constitutional partnership between the Executive and the Judiciary was to be led and managed. The Constitutional Reform Act 2005, the Tribunals Courts and Enforcement Act 2007 and the various constitutional instruments that are associated with that legislation: Concordats, Frameworks and Agreements, transferred executive functions to the senior judiciary, in particular from the Lord Chancellor.

The administration of justice and the justice systems that exist had always been a partnership between the Executive and the independent Judiciary, with at least a nod to the constitutional principles of accountability and sovereignty in the way those two limbs of the State engaged with Parliament but the consequence of the changes that took place between 2004 and 2008 remain both poorly understood and researched.
The heads of jurisdiction or chief justices became Presidents of their own courts and tribunals and, as a consequence, executive responsibilities were transferred to them. Statutory duties were imposed on them, and organisational changes were put in place in an attempt to match the strategic and operational functions with capabilities that were intended to help them to discharge those duties.
The changes were so significant that it was necessary to put in place Framework Agreements to regulate the new partnership and reflect the power vested in both the Lord (now Lady) Chief Justice and the Senior President of Tribunals to address Parliament should the arrangements become ineffective. That power has never been used not least because it would bring the partnership to an end but the tensions underlying the same can readily be ascertained from the annual funding and major projects discussions that occur between the senior judiciary and Ministers and their representatives. Let us not forget that on one occasion since the constitutional settlement the arrangements did not work and the questions that arose were not settled until the landmark judgment of the Supreme Court in the UNISON case.
I am not criticising the senior judiciary for the state of justice or the effectiveness of our justice systems. Far from it. The time, energy, strategic and operational skill that has been put into the multiplicity of boards, committees, agency bodies, bilaterals and trilaterals has been notable. It is a major endeavour supported by a skilled Judicial Office. I am equally not criticising His Majesty’s Courts & Tribunals Service or their equivalents in Scotland and Northern Ireland which are the delivery bodies responsible for justice systems. HMCTS in England and Wales has been served by dedicated Chief Executives, senior leadership teams and board members. The problem is systemic.
Justice and the Rule of Law are not regarded as political priorities until something goes wrong. There are few effective environments in which justice systems and their effectiveness are discussed in a transparent and informed way. Justice Councils are notable exceptions, but they do not have the infrastructure of think tanks or the research-intensive bodies we have here at the Nuffield: the Family Justice Observatory , the Nuffield Council on Bioethics and the Ada Lovelace Institute. Bodies that are able to set strategic objectives, commission and help fund research, identify data and its absence, analyse performance and outcomes, provide timely syntheses of good practice and solutions to problems.
There are two very important deficits that have plagued effective delivery for most of the last 20 years: the lack of quality data and the lack of research and analysis of data. In the presentation which follows these remarks, Dr Natalie Byrom will summarise an independent paper that the Nuffield Foundation commissioned from her to highlight these problems with a focus on access to justice. I will not steal her thunder, but the evidence and conclusions are stark.
- Access to legal information and advice is implicit in an individual’s ability to secure access to justice and yet cuts to Government spending and the lack of a coherent plan to provide alternatives to face to face advice have delivered advice deserts.
- Access to formal legal systems is impeded by backlogs, the lack of realistic dispute resolution alternatives and the incomprehensibility of the processes individuals have to navigate.
- Access to a fair and effective hearing is damaged by the lack of representation, and quite insufficient attention to non-adversarial procedures and protections that would help. Justice may be preventative, consensual, facilitative or adjudicative, but it does not always have to be adversarial to be fair.
- Access to a decision according to law is a constitutional right. Where factors presently not analysed because of the lack of data adversely influence that access, for example, ethnicity or post code conceptions of the availability of service delivery, these ought to be surfaced.
- Access to a remedy and enforcement are key to ensuring that justice systems do not make it futile or irrational to bring a claim.
- Read Dr Byrom’s review 1MB | pdf
Neither resource rationing nor political ideology, let alone both together, are an adequate basis for the way we lead and / or deliver justice, in particular in circumstances where austerity or ideology reinforce division rather than respect i.e. increase real inequality and / or the perception of inequality.
The consequence is that political initiatives from the Executive do not always hear the voice of users or the independent judiciary or sometimes even Parliament. Partnership working that is necessary to the way justice is delivered becomes fragmented, we lose sight of the good outcomes that ought to be the hallmark of access to remedies and we end up with inadequate advice, systems that are poorly matched to the needs of the user or victim and very real impacts that are both intended and unintended consequences that can damage the Rule of Law.
Scarcity of resources affects the Rule of Law. The poor decisions of users have consequences that include a wide variety of cases where remedies are vital. These may include, for example, financial debt leading to destitution and improper reliance on unregulated and unethical providers; real health and social care consequences; poor family choices with relationship breakdown, domestic violence and public care outcomes having a direct correlation with the impact of scarcity on decision making and relationships; benefits availability and use and much more.
The consequences in relation to criminal behaviour, civil and family disputes, mental health tribunals, benefits appeals and all manor of jurisdictions that are provided by our justice systems are patent but insufficiently researched. We do not identify or measure justice system outcomes, let alone analyse how scarcity and inequality impact on justice system users. We do not know whether we exacerbate those impacts by the ways in which we work including the lack of advice and support.
In simple terms, it is an obligation on leaders of justice systems, including the judiciary, to have regard to how their leadership of those systems impacts access to justice but no-one can operate the levers of institutions, particularly institutions as complex as justice systems, without tools. The lack of research and funding of research into reforms and also into the previously existing non-digital or analogue methods of making decisions, is marked.
The lack of data to analyse is a matter of serious concern and that all has the consequence that there is insufficient strategic thinking or commentary about justice systems and the outcomes for users.
May I finish where I began.
If we are to address inequalities, disadvantage, discrimination and vulnerabilities we must collect the data that is missing and fund research into outcomes. We must provide an evidence base for strategic thinking so that as a consequence we build and maintain trustworthy institutions.
Justice systems deserve our help.
Read the key takeaways from our Justice conference
news | 22/04/2024
Where has my justice gone? Improving people’s legal experiences
Related news, events and opinion.
news | 27/02/2024
In-flux identities: a child-centric approach to care
news | 21/02/2024
Understanding the identities of young people in care
news | 15/11/2023
New research on financial settlements in divorce
opinion | 01/11/2023
A break-down of new evidence on financial settlements in divorce
opinion | 28/06/2023
Funding call: addressing the growing SEND challenge
news | 31/05/2023
Youth Justice Spotlight: Dr Tim Bateman
news | 25/05/2023
Youth Justice Spotlight: Dr Vicky Kemp
news | 17/05/2023
Youth Justice Spotlight: Dr Maria Adams
news | 11/05/2023
Youth Justice Spotlight: Professor Stephen Case
news | 04/05/2023
Youth Justice Spotlight: Dr Claire Fitzpatrick
news | 20/01/2023
2023 funding call: Research to improve justice in action
news | 26/09/2022
2021 annual report: £28.5 million committed to charitable spending
news | 06/09/2022
Using ‘common sense’ and gender inequalities behind COVID-19 rule breaking
opinion | 09/08/2022
Funding opportunity: cost of living crisis
Explore our projects.
Justice | Welfare | 2024 - 2026
Challenging justice inequalities with children in conflict with the law
Justice | 2024 - 2026
Special guardianship families: experiences and support needs
Breaking networks of youth serious violence.
Justice | 2024 - 2024
Crossing boundaries: Co-designing support for vulnerable young people
Justice | 2023 - 2025
Applicants’ experience of the Criminal Cases Review Commission (CCRC)
Linking household benefits, financial precarity and child welfare, improving safeguarding outcomes after adoption or special guardianship.
Justice | 2023 - 2026
Parental Advocacy in England: a realist evaluation of implementation
Children in police custody: piloting a ‘child first’ approach , physical punishment and child outcomes in the uk.
Welfare | 2023 - 2024
Evidencing the outsourcing of social care provision in England
Access to justice through artificial intelligence.
Justice | 2019 - 2023
Born into care: best practice guidelines
Justice | 2022 - 2025
Exploring racial disparity in diversion from the youth justice system
Justice | 2022 - 2024
Administrative fairness in the digital welfare state
Transparency and judicial review: a study of the duty of candour.
Welfare | 2022 - 2024
Links between cognitive impairment and exploitation in England
Child first: examining children’s collaboration in the youth justice system, the role of communities and connections in social welfare legal advice, voluntary care plans for children in scotland: using section 25 orders, rethinking domestic abuse in child protection: responding differently, understanding local legal needs and supporting early intervention.
Justice | Welfare | 2022 - 2024
Race, religion and representation among care-experienced children
Justice | 2020 - 2023
Discharge of care orders: a national study
Justice | 2021 - 2024
Substituted parenting: what does this mean in the family court?
Justice | 2021 - 2023
Delivering administrative justice after the pandemic
Justice | 2020 - 2021
Guidance to judges on the anonymisation of children judgements
Justice | 2019 - 2021
The production of witness statements by lawyers and litigants in person
Justice | 2020 - 2022
When is a wedding not a marriage? Exploring non-legally binding ceremonies
Justice | 2019 - 2022
The Edinburgh Study: causes and impacts of criminal justice pathways
Justice | Welfare | 2015 - 2018
Bridging the Evidence Gap in Family Proceedings
Justice | 2016 - 2018
The impact of Litigants in Person on the Northern Ireland court system
Justice | 2016 - 2017
Transparency and privacy in family courts
Justice | 2016 - 2019
Implementation of recommendations of the Carlile report
Justice | 2017 - 2020
Enhancing problem-solving practice in youth court
Justice | 2013 - 2019
Timely disclosures mean timely interventions for young offenders and victims
We improve people’s lives by funding research that informs social policy, primarily in Education , Welfare and Justice . We also fund student programmes that give young people skills and confidence in science and research.
We offer our grant-holders the freedom to frame questions and enable new thinking. Our research must stand up to rigorous academic scrutiny, but we understand that to be successful in effecting change, it also needs to be relevant to people’s experience.
Privacy and cookie policy
Privacy overview.
Underground carbon storage top of mind for student team
- Travis Williams
22 Apr 2024
- Share on Facebook
- Share on Twitter
- Copy address link to clipboard

Researching how to successfully store carbon underground is helping a group of Virginia Tech students reach new heights.
“Carbon sequestration is really kind of an elegant solution for global warming,” said Lars Koehn, a third-year Ph.D. candidate in the Department of Geosciences. “Normally, we pull carbon out of the ground in the form of fossil fuels, use it for energy, and then put it into the air. But with carbon sequestration, we’re going to put it straight back into the ground into the same types of rocks we pulled it from. So it’s kind of like a circular economy for carbon.”
Koehn is one of eight Virginia Tech graduate students who recently teamed up to research and create a plan for a carbon sequestration project off the coast of Louisiana as a part of the Society of Exploration Geophysicists’ (SEG) EVOLVE Carbon Solutions Professional Program . After students presented their work at the Carbon Capture, Utilization, and Storage Conference in March, the global not-for-profit organization selected Virginia Tech as the host of the first virtual version of its U.S. regional geoscience trivia contests, officially titled “Challenge Bowl.”
“Some of the SEG EVOLVE teams really require a mentor to provide detailed guidance through the project, but this team has been extremely self-driven,” said Annabella Betancourt, managing director of programs for the society. “They were so diligent and responsible. If they could energize every team with the same spirit and enthusiasm, it would show others how to find the most success within this program.”
The Virginia Tech chapter of the Society of Exploration Geophysicists’ will host the bowl on April 29. The event will feature two-person teams from universities and colleges spanning the country competing for the opportunity to advance to the International Challenge Bowl Finals at IMAGE, the International Meeting for Applied Geoscience and Energy , in Houston this August. Participation in the event is free for qualifying students with the top three teams earning cash prizes.
Hosting the virtual competition marks a milestone for the Virginia Tech SEG EVOLVE team, which consists of four students from the Department of Geosciences and four from the Department of Mining and Minerals Engineering . Armed with seismic and oil drilling data donated by industry partner Fairfield Geotechnologies, the team was tasked with developing a full plan for the implementation of a carbon sequestration site, including the transportation of the carbon, adherence to government regulations, and projections of economic impacts.
“They did a full-blown feasibility study for carbon storage along the Gulf of Mexico, and it was peer-reviewed by their industry mentors,” said Ryan Pollyea, associate professor in the Department of Geosciences and the team’s faculty advisor. “It speaks volumes that this group did such a good job, that SEG is seeking deeper engagement with Virginia Tech.”

Jessica Dostal, a student and early career advisor with the society, said connecting SEG EVOLVE teams with mentors from industry was a critical part of the program’s goal of providing the type of real-life experience that empowers students to “hit the ground running” once they graduate.
“The whole point of the program is to develop those professional practices that help bridge the gap between what you learn in school and industry, and really learn to apply what you’ve learned,” Dostal said.
Providing students with real-world learning opportunities is also in line with the mission of Virginia Tech Advantage.
Koehn said the project did help bridge that classroom-to-workplace gap by posing the team with questions and concerns outside the scope of the traditional classroom or textbook.
“We were addressing things we don’t normally address as students,” he said. “Stuff like, how could this project make money, what are the costs of the project, and how would you get this project approved by the state.”
Along with the opportunities for individual growth, the students said they also see a collective opportunity for the university in this field of research and the SEG U.S. Regional Challenge Bowl is just the tip of the iceberg.
“Virginia Tech can be a major player in carbon sequestration, if we play our cards right,” said Matt Tascione, a Ph.D. student studying geophysics, team member, and president of the Virginia Tech student chapter of the society. “The opportunities are here for us to really latch on to it, we just have to make the most of that. And I think this is a really great way to start doing that.”
540-231-6468
- Climate Action
- College of Engineering
- College of Science
- Geosciences
- Graduate Education
- Graduate Research
- Mining and Minerals Engineering
- Responsible Consumption and Production
Related Content

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

5 NSF projects transforming how researchers understand plastic waste
The U.S. National Science Foundation champions research on how plastic impacts the planet. These five projects are changing how researchers think about plastic and what happens after it is tossed away.
Plastic is everywhere. Humans produce so much plastic that we end up throwing away about 400 million tons of plastic-related trash every year. And researchers are learning that this trash doesn't stay where it is deposited. From land to sea, plastic is found virtually everywhere on the planet.
Earth Day 2024 is highlighting the plastics problem with the theme: "Planet vs. Plastic." "Our reliance on harmful plastics is not sustainable," said Alexandra Isern, NSF assistant director for Geosciences. "We are committed to funding research that will address the plastics challenge to create a safer future for generations to come."
Here are five NSF-driven projects that look at the versatile material in both expected and unusual places and examine its impacts on the planet and the creatures who call it home.

1. In soils
About half of the 400 million tons of plastic that people worldwide discard annually migrates beyond landfills.
Brian Giebel, an assistant research professor at the City University of New York, and Benjamin Bostick, a professor at Columbia University, are studying how these discarded plastics can affect soil health and function . The team is especially interested in plastic's potential to change how soils emit climate-warming gases like carbon dioxide and methane.
How does a piece of plastic eventually end up as a gas? First, it breaks down through chemical and physical processes in soils. When it degrades to less than 5 micrometers in size, slightly bigger than a speck of dust, it can become a tasty lunch for microorganisms, which then release carbon dioxide and methane into the atmosphere.
The team will use a variety of laboratory techniques, like stable isotope measurements and X-ray microscopy, to track plastic's degradation, microbial uptake and eventual transformation to gas.

2. In urban streams
From plastic wrappers to plastic bottles, plastics dominate daily life. Once used, however, plastic can often end up as litter within waterways.
Anne Jefferson, a professor at the University of Vermont, and her team are using time-lapse photography and repeat field surveys to understand how discarded plastic moves through and sometimes stays in streams . "I kept seeing trash everywhere in the urban streams where I was doing research for other projects," Jefferson said. "Since stopping litter from entering streams seemed like a losing battle, I wanted to know more about what happens to the litter once it got into a stream and how it interacted with other elements of the stream channel."
Jefferson's findings will improve litter tracking models that follow plastic from streams to oceans. She wants to learn how much plastic is stored in flood plains or within stream and river channels rather than entering the ocean. Her findings will also help guide litter management, environmental cleanup and ecosystem restoration efforts.

3. On the ocean's surface
Just like humans, plastic is carbon-based. Aron Stubbins, a professor at Northeastern University, is using this fact to better understand whether plastic pollution has fundamentally changed the ocean's surface.
Plastic has been accumulating at the ocean's surface ever since mass production started about 70 years ago. Stubbins and his team are collecting plastic samples from the open ocean and measuring natural organic carbon and plastic-carbon concentrations to determine if the plastic carbon now makes up a significant fraction of the total surface ocean carbon. If that is the case, as the team suspects, then it's very likely that the plastic carbon levels on the ocean surface today are unprecedented.
The team collected samples from the Atlantic Ocean on a research cruise last summer. The anticipated findings will reveal whether ocean scientists need to consider the role of plastic carbon as an active component of the surface ocean carbon cycle.
4. In the Arctic
Bits of plastic smaller than 5 millimeters can come from larger plastic pieces that have broken apart, byproducts of plastic manufacturing or microbeads used in health and beauty products.
These microplastics litter the seas, even reaching the remote Arctic Ocean. Alexandra Jahn, an associate professor at the University of Colorado Boulder, is studying how sea ice moves microplastics in polar regions.
Jahn and her collaborators at the NSF National Center for Atmospheric Research, the University of Washington and the Woods Hole Oceanographic Institute are investigating why observed concentrations of microplastics in sea ice are many times higher than in the underlying ocean and how this affects where microplastics end up. The team is also investigating whether sea ice is more likely to melt when it contains dark microplastics, which increase sunlight absorption.
To help answer these questions, the team is growing sea ice embedded with microplastics in a laboratory and adding microplastics to numerical models of various complexity.
5. In the atmosphere
Manufacturers add certain chemicals to plastic to make it stronger, more flexible and more durable. However, when plastic waste ends up in the ocean, these often toxic additives can leach into the water and accumulate in the sea surface microlayer, where the top of the ocean meets the atmosphere.
Nate Slade, an assistant professor at the University of California San Diego is studying how these chemicals can stick to droplets as they evaporate into the air , travel long distances across the ocean, pollute air quality, and eventually end up in a person's airways.
Slade and his team want to know how long plastic additives can last when stuck to those droplets, known as aerosols, and how other chemicals can affect their transport.
These and related NSF-supported projects will help scientists better understand how plastic impacts the planet and how to use that knowledge to build a resilient planet.
About the Author
Related stories.

NSF 101: The NSF brand identity

2023: A tremendous year for science that sets stage for an exciting 2024 and beyond
Mapping science: How GIS transformed our view of the world
Skip to Content
CU Engineering faculty land prestigious multidisciplinary Department of Defense projects
- Share via Twitter
- Share via Facebook
- Share via LinkedIn
- Share via E-mail
Three faculty members from the CU Boulder College of Engineering and Applied Science are conducting projects awarded through the U.S. Department of Defense’s Multidisciplinary University Research Initiative (MURI) Program .
The highly competitive research program has been enabling major contributions to military capabilities and producing commercial sector applications since 1985.
“Our college emphasizes collaboration across various research disciplines,” said Michael Gooseff, associate dean for research in the College of Engineering and Applied Science. “By prioritizing programs like MURI, we harness the diverse expertise across STEM fields to push the envelope for scientific breakthroughs.”
The three new MURI projects in the college include:
- Mahmoud Hussein , professor in aerospace engineering sciences and in physics, will improve air flow across the wings and bodies of hypersonic aircraft through the use of phononic subsurface materials;
- Francois Barthelat , professor in mechanical engineering, will develop and validate models for the failure of materials and structures under extreme loads; and
- Scott Diddams , professor in electrical, computer and energy engineering and in physics, will examine the fundamental limits in heterodyne detection of thermal radiation with laser light.
Hussein is the main principal investigator and represents CU Boulder as the lead institution for that MURI project. Barthelat and Diddams will be collaborating on projects led by faculty from other peer institutions.
Each project will receive an average award of $7.5 million over the next five years.
2024 Department of Defense CU Engineering MURI Projects

Control of Hypersonic Flows by Phononic Subsurfaces
Mahmoud Hussein - Ann and H.J. Smead Department of Aerospace Engineering Sciences and Department of Physics (courtesy)
This grant will establish a new paradigm for passive manipulation of air flow over hypersonic vehicles using ideas rooted in condensed matter physics. This endeavor will delay laminar-to-turbulent transitions around these vehicles, significantly reducing drag and surface temperature levels — two targets that promise to bring the dream of hypersonic passenger airplanes much closer to reality. A future hypersonic airplane could cut travel time for passengers to destinations across the globe by five times or more.
This research is based on a concept Hussein discovered when he started a collaboration with the late Professor Sedat Biringen in Smead Aerospace more than 15 years ago. The main premise of this concept is to tailor elastodynamic properties of an elastic structure interfaced with a surface exposed to a flow in a manner that creates favorable coherent wave interactions between the solid and the flow.
This research will augment efforts to develop the phononic subsurfaces technology to benefit potentially subsonic aircraft like commercial airliners, significantly improving fuel economy and bringing tangible environmental rewards.

Predicting Performance Outcomes for Heterogeneous Materials Under Complex Loading
Francois Barthelat - Paul M. Rady Department of Mechanical Engineering
The objective of this project is to develop and validate models for the failure of materials and structures under extreme mechanical loads. Engineers can generally predict the failure of materials and structures subjected to relatively moderate loading conditions based on averaged properties. However, in the case of extreme loading conditions or impact, defects and heterogeneities make predictions more challenging, and failure might occur unexpectedly and away from the impact site.
This research project can help predict and prevent the collapse of mines, dams, bridges or buildings; predict earthquakes, which are triggered by mechanical failure along faults in the Earth’s crust; and even predict avalanches — a serious danger in Colorado. An important application could be the prediction and prevention of ruptured aneurysm, which can also be interpreted as a mechanical failure of a blood vessel. In all these examples, these models can incorporate uncertainties in material properties, existing defects, accumulating damage and loading conditions for more accurate predictions and therefore more effective failure prevention.

Fundamental Limits of Passive Heterodyne Photodetection of Incoherent, Broadband Sources
Scott Diddams - Department of Electrical, Computer & Energy Engineering and Department of Physics (courtesy)
The goal of this MURI is to understand the fundamental limits in heterodyne detection of thermal radiation with laser light. This is anticipated to lead to powerful new approaches in imaging and spectroscopy by combining signals from multiple apertures or telescopes to provide images with higher spatial resolution.
These techniques are common in radio astronomy, but the application to high-frequency light fields is largely unexplored. Since light is fundamentally composed of photons, the team will leverage new opportunities in manipulating the quantum properties of the heterodyne process to yield better sensitivity in these measurements. They envision these methods will also be applicable for remote sensing, trace gas detection and atmospheric sciences.
Diddams will be collaborating with Professor Thomas Schibli in the Department of Physics on this project.
Other CU Engineering MURI Projects
Previous MURI projects awarded to College of Engineering and Applied Science faculty include:
- Iain Boyd (2022) in aerospace engineering sciences as main principal investigator for Development of Validated Hypersonic Plasma Kinetics Models including Atomic Excitation;
- Brian Argrow (2017) in aerospace engineering sciences as main principal investigator for Integrated Measurement and Modeling Characterization of Stratospheric Turbulence; and
- Richard Regueiro (2011) in civil engineering as main principal investigator for Soil Blast Modeling and Simulation: Continuation Research Proposal.
Apply Visit Give
Departments
- Ann and H.J. Smead Aerospace Engineering Sciences
- Chemical & Biological Engineering
- Civil, Environmental & Architectural Engineering
- Computer Science
- Electrical, Computer & Energy Engineering
- Paul M. Rady Mechanical Engineering
- Applied Mathematics
- Biomedical Engineering
- Creative Technology & Design
- Engineering Education
- Engineering Management
- Engineering Physics
- Environmental Engineering
- Integrated Design Engineering
- Materials Science & Engineering
Affiliates & Partners
- ATLAS Institute
- BOLD Center
- Colorado Mesa University
- Colorado Space Grant Consortium
- Discovery Learning
- Engineering Honors
- Engineering Leadership
- Entrepreneurship
- Herbst Program for Engineering, Ethics & Society
- Integrated Teaching and Learning
- International Programs
- Mortenson Center for Global Engineering
- National Center for Women & Information Technology
- Western Colorado University

Help Discover Worlds With NASA

The Exoplanet Watch project invites you to use your smartphone or personal telescope to help track worlds outside our solar system.
More than 5,000 planets have been confirmed to exist outside our solar system, featuring a wide array of characteristics like clouds made of glass and twin suns . Scientists estimate there could be millions more exoplanets in our home galaxy alone, which means professional astronomers could use your help tracking and studying them.This is where Exoplanet Watch comes in. Participants in the program can use their own telescopes to detect planets outside our solar system, or they can look for exoplanets in data from other telescopes using a computer or smartphone.
Exoplanet Watch began in 2018 under NASA’s Universe of Learning , one of the agency’s Science Activation programs that enables anyone to experience how science is done and discover the universe for themselves. Until recently there were limits on how many people could help look through the data collected by other telescopes, but now this program is easily available to anyone. By following the site’s instructions , participants can download data to their device or access it via the cloud, and then assess it using a custom data analysis tool.
“With Exoplanet Watch you can learn how to observe exoplanets and do data analysis using software that actual NASA scientists use,” said Rob Zellem, the creator of Exoplanet Watch and an astrophysicist at NASA’s Jet Propulsion Laboratory in Southern California. “We’re excited to show more people how exoplanet science is really done.”
Helping Without a Telescope
Participants without telescopes can help astronomers comb through data that’s already been taken. The project has 10 years of exoplanet observations, collected by a small ground-based telescope south of Tucson, Arizona. This year, the project will start collecting additional data from two other telescopes at the Table Mountain facility in Southern California, which JPL manages.
These telescopes look at nearby stars and search for what scientists call exoplanet transits : regular dips in a star’s brightness caused by a planet passing between the star and Earth. Essentially, a transit is an observation of a planet’s silhouette against the bright glare of its star.
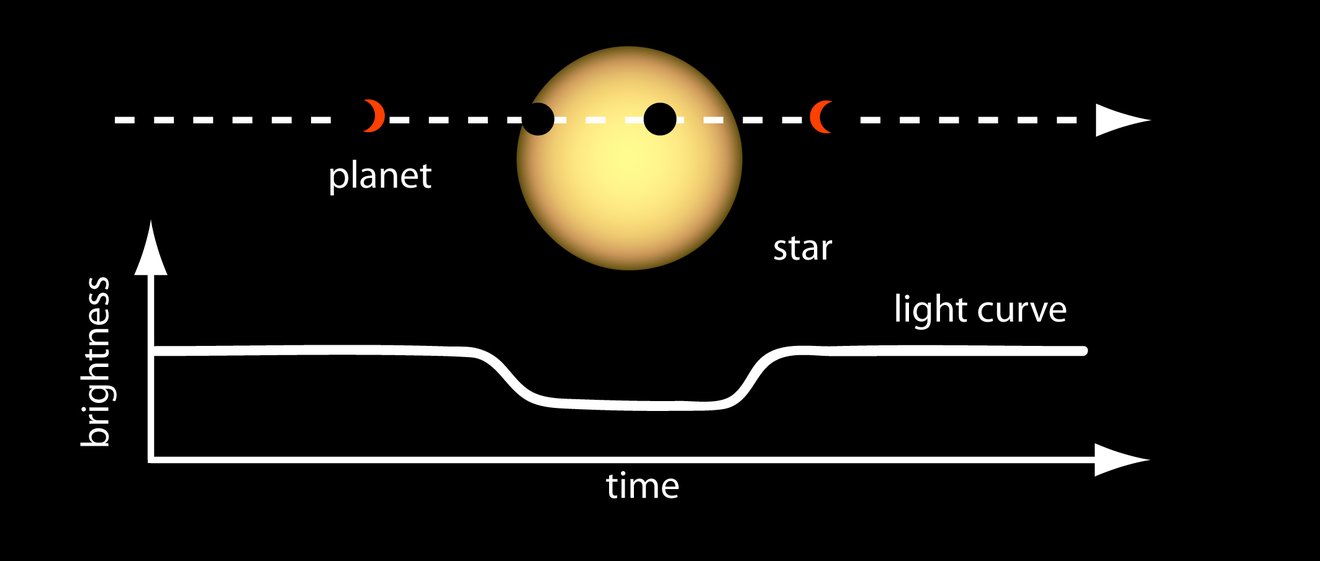
Multiple NASA telescopes look for exoplanet transits as a way to discover new planets, but Exoplanet Watch participants primarily observe transits by planets that have already been discovered to gain more information about their orbits. The time between exoplanet transits reveals how long it takes an exoplanet to orbit its parent star; the more transits that are measured, the more precisely the length of the orbit is known. If the timing of the orbit isn’t measured precisely, scientists who want to study those planets in more detail with large ground-based or space-based telescopes can lose valuable observing time while they wait for the planet to appear. Having volunteers sort through the data will save significant computing and processing time.
Exoplanet Watch participants will also look for variations in the apparent brightness of stars – changes caused by features such as flares (outbursts of light) and star spots (dark spots on a star’s surface). In transit measurements, these changes make a planet appear smaller or larger than it actually is. This work will help scientists anticipate the variability of a particular star before they study its exoplanets with large, sensitive telescopes like NASA’s James Webb Space Telescope .
Helping With Your Own Telescope
Want to take your own data? Although the number of targets you can see increases with the size of the telescope used, there’s no minimum size requirement. For example, Exoplanet Watch can help you detect exoplanet transits for hundreds of nearby stars with just a 6-inch (15-centimeter) telescope.
Exoplanet Watch combines observations of the same target by multiple sky watchers in order to get a higher-fidelity measurement. Combining observations is also useful if the planet’s transit lasts longer than the time a star is visible in the sky for a single observer: Multiple participants at different locations around the globe can collectively watch the duration of a long transit.
That was the case with a planet called HD 80606 b , which Webb will observe this year . A recent study of this planet led by Kyle Pearson, the Exoplanet Watch deputy science lead at JPL, combined observations from more than 20 Exoplanet Watch participants. The volunteer effort on HD 80606 b will free up almost two hours of time on Webb for other observations. And on missions that aim to observe hundreds or thousands of exoplanets, the number of minutes saved by refining planet transit measurements can add up and free a significant amount of observing time, according to Zellem.
One of the program’s policies requires that the first paper to make use of the observations or analysis done by volunteers will list those volunteers as co-authors, which was the case with the study led by Pearson. “I hope this program lowers barriers to science for a lot of people and inspires the next generation of astronomers to join our field,” said Zellem.
Related Terms
- Exoplanet Detection Methods
- Exoplanet Science
- Exoplanet Transits
- Science Activation
- Studying Exoplanets
Explore More
My NASA Data Milestones: Eclipsed by the Eclipse!

Students Present GLOBE Research at INFINITY Science Center with NASA ASTRO CAMP Community Partners Team

That Starry Night Sky? It’s Full of Eclipses
Discover more topics from nasa.
Search for Life

Black Holes


IMAGES
VIDEO
COMMENTS
The Research Placements & Experience programme (formerly Nuffield Research Placements) provides engaging, hands-on projects, where Year 12 students have the opportunity to make a meaningful contribution towards the work of a host organisation through a well-supervised but independent placement collaboration relating to an area of science, quantitative social science, computing, technology ...
Nuffield Research Placements are funded by the Nuffield Foundation and delivered by STEM Learning. They are engaging, hands-on research projects, where students have the opportunity to make a meaningful contribution towards the work of a host organisation. They are a fantastic opportunity for students to apply skills and knowledge learned at ...
Nuffield Research Placements. Programme guide for students . Introduction . Nuffield Research Placements (NRP), formerly known as Nuffield Science Bursaries, has supported approximately 20,000 post-16 students over the last 25 years to engage in real life research working alongside Science, Technology, Engineering and Maths ' (STEM ...
Since 1962, the Foundation has supported major curriculum projects and the creation of a wealth of teaching resources across STEM subjects and beyond. Our work in education includes funding research and supporting its translation into policy and practice. The Foundation has supported some 60 major curriculum projects and countless smaller ones ...
This summer, around 1,200 sixth formers and college students have undertaken Nuffield Research Placements in science (including social science), technology, engineering and maths (STEM) at workplaces across the UK.. Nuffield Research Placements change lives. They give students from more disadvantaged backgrounds, particularly those without a family history of higher education, the opportunity ...
What is a Nuffield Research Placement? When? Summer holidays after Year 12 (in England, Wales and Northern Ireland) or S5 (in Scotland). How long? Four to six weeks. What? A well-supervised but independent research project relating to an area of science, quantitative social science, computing, technology, engineering or maths (or a combination ...
the Nuffield Research Placements (NRP) programme has been adapted so that an entirely virtual ... A well-supervised but independent research project relating to an area of science, quantitative social science, computing, technology, engineering or maths (or a combination!).
Published: Oct 11, 2021 3 min read. [email protected]. STEM Learning are now in their second year of delivering this highly regarded and prestigious programme thanks to ongoing funding from the Nuffield Foundation and support from UK Research and Innovation - and applications are now open for 2022! We hope to encourage Year 12/S5/Year 13 ...
The Nuffield Science Teaching Project was a programme to develop a better approach to teaching science in British secondary schools, under the auspices of the Nuffield Foundation. ... Journal of Research in Science Teaching 7.4 (December 1970) 283-302 (pdf, payment required).
What is a Nuffield Research Placement? Nuffield Research Placements give young people from disadvantaged backgrounds the opportunity to gain skills and confidence in science and research. ... A well-supervised but independent research project suitable for a 16/17 year-old. Projects must have broadly scientific, quantitative or technical content ...
We provide access to outputs from all our research projects and work to strengthen their collective impact. View all projects. 12 of 558 results. Filter. Nuffield Foundation research aims to improve social well-being by funding research and innovation in education, justice and welfare.
The Nuffield Primary Science materials were based on the findings of a research project called the Science Processes and Concept Exploration (SPACE). This was the first set of resources published in the UK for primary science teaching that was explicitly based on a constructivist view of learning. The research aims.
Nuffield Research Placements (NRPs) are 2-week collaborations with a STEM-related knowledge expert on a live research question or area of development. While producing a scientific or technical report and poster, this opportunity ensures that students contribute meaningfully to the host organisation's current work.
The Nuffield Research Placements programme provides engaging, hands-on research projects, where Year 12 students have the opportunity to make a meaningful contribution towards the work of a host organisation through a well-supervised but independent research collaboration. What is a Nuffield Research Placement. When?
The Nuffield project is the brainchild of the Nuffield Foundation set up by founder of Morris Motors, William Morris, Lord Nuffield in 1943; funding research for better social policy in education, welfare and justice. ... Institute, the foundation also seeks to provide opportunities for young people to develop skills and confidence in science ...
Since 2011 we've welcomed students from the Nuffield scheme, who have been getting to grips with research projects at the cutting edge of science. This year, the students got their hands dirty with a metagenomics project looking at the hidden life in soil.
Our goal is to find ways to improve educational outcomes through policy change and interventions that are grounded in robust evidence. We fund research and development projects relating to education across all life stages - from early years through school, to further and higher education and vocational learning. We want to understand young ...
Fermentation of glucose using yeast | 14-16 years. In association with Nuffield Foundation, By Neil Goalby. Use this class practical to investigate the fermentation of glucose by yeast and test for ethanol. Includes kit list, safety instructions, questions and answers.
Quality Assured. Subject: Science Physics. This pack of Nuffield Physics activity sheets was designed to match the National Curriculum Science: Physics at Key stage Four from 1996. The ideas were drawn from materials produced by various Nuffield projects over the previous ten years. The pack includes student sheets for 97 different activities.
25th September 2019. Students from schools and colleges across the North East had the opportunity to work alongside academics on real-life research projects this summer, thanks to a partnership between Northumbria University and the Nuffield Research Placement programme. The competitive programme, which lasts between four to six weeks, aims to ...
Research projects will partner students with DOE national labs to help students develop hands-on research experience. WASHINGTON, D.C.. - Today, the U.S. Department of Energy (DOE) announced $16 million in funding for four projects providing classroom training and research opportunities to train the next generation of accelerator scientists and engineers needed to deliver scientific discoveries.
The Nuffield Foundation has a purpose which is to advance social well-being. Our work cuts across education, welfare, and justice. We specifically aim to address the inequalities, disadvantage, discrimination and vulnerabilities people face in justice and consider the ethical implications of systems, processes, technologies and decision-making.
Eight Virginia Tech graduate students recently researched and created a plan for a carbon sequestration project off the coast of Louisiana as a part of the Society of Exploration Geophysicists' EVOLVE Carbon Solutions Professional Program. Their impressive work led the global not-for-profit organization to select Virginia Tech as the host of its first virtual regional geoscience trivia contest.
The U.S. National Science Foundation champions research on how plastic impacts the planet. These five projects are changing how researchers think about plastic and what happens after it is tossed away. By Elizabeth Jeffers April 22, 2024. Plastic is everywhere. Humans produce so much plastic that we end up throwing away about 400 million tons ...
The Nuffield Science Teaching Project was a programme to develop a better approach to teaching science in British secondary schools, under the auspices of the Nuffield Foundation. Although not intended as a curriculum, it gave rise to alternative national examinations, and its use of discovery learning was influential in the 1960s and 1970s.
May 8, 2024. Have you heard about the initiative at the National Institutes of Health (NIH) to improve the peer review of research project grant and fellowship applications? Join us as NIH describes the steps the agency is taking to simplify its process of assessing the scientific and technical merit of applications, better identify promising ...
Three faculty members from the CU Boulder College of Engineering and Applied Science are conducting projects awarded through the U.S. Department of Defense's Multidisciplinary University Research Initiative (MURI) Program.. The highly competitive research program has been enabling major contributions to military capabilities and producing commercial sector applications since 1985.
Exoplanet Watch began in 2018 under NASA's Universe of Learning, one of the agency's Science Activation programs that enables anyone to experience how science is done and discover the universe for themselves. Until recently there were limits on how many people could help look through the data collected by other telescopes, but now this program is easily available to anyone.