- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
News releases.
News Release
Wednesday, July 19, 2023
New atlas of human kidney cells to help unlock kidney disease research
NIH-funded effort provides interactive resource for global research community.
In a major breakthrough toward understanding and treating kidney disease, a nationwide research team funded by the National Institutes of Health has created the most comprehensive atlas of the human kidney. Data from the Kidney Tissue Atlas will allow the comparison of healthy kidney cells to those injured by kidney disease, helping investigators understand the factors that contribute to the progression of kidney disease and kidney failure or recovery from injury. The atlas, part of the Kidney Precision Medicine Project (KPMP), was supported by NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), as published in Nature .
Due to the complexity of the kidney, scientists have struggled to develop kidney models that accurately represent human kidney structures and function. The lack of human kidney models has limited the ability to develop new drugs to treat or prevent kidney disease.
The Kidney Tissue Atlas comprises maps of 51 main kidney cell types that include rare and novel cell populations, 28 kidney cellular states that represent injury or disease, a repository of raw gene data, and interactive 3D models of cells and microenvironment relationships created from 45 healthy donor kidneys and 48 kidney disease biopsies. The atlas thus establishes a critical foundation for KPMP’s overall goal to help discover new treatments for chronic kidney disease (CKD) and acute kidney injury (AKI), medical conditions that present a significant global health burden. The publicly available data created by KPMP, including all 3D renderings and analytical tools, can be accessed at atlas.kpmp.org .
“KPMP’s new atlas represents open, public science at its best,” said Dr. Eric Brunskill, KPMP program director in NIDDK’s Division of Kidney, Urologic, and Hematologic Diseases. “With the atlas, we’ve created an interactive, hypothesis-generating resource for kidney disease investigators and clinicians around the world.”
While CKD and AKI have historically been described as single, uniform diseases, KPMP builds on growing consensus that kidney disease can have several different root causes and disease pathways leading to subgroups of CKD and AKI. Instead of a “one size fits all” approach to treating kidney disease, precision medicine explores more personalized treatments. KPMP’s kidney atlas is intended to help identify disease subgroups within CKD and AKI, leading to the discovery of new, and possibly individualized, ways to treat CKD and AKI.
The study also received support from the Human Cell Atlas initiative, an international research effort to gather information on at least 10 billion human cells, and NIH’s Human BioMolecular Atlas Program (HuBMAP). HuBMAP’s goal is to develop an open and global platform to map healthy cells in the human body; the KPMP and HuBMAP teams worked closely to align the outputs of this molecular atlas as an example of cross-consortia collaborations.
“KPMP brings together the best of new technology, patient engagement, and partnership, and represents an evolution in the way we think about kidney disease,” said NIDDK Director Dr. Griffin P. Rodgers. “We’re confident the Kidney Tissue Atlas will help us discover new ways to get the right kidney disease treatment to the right patient at the right time.”
Data related to this research are available for request at the NIDDK Central Repository .
Research reported in this study was funded by NIDDK (grants U2C DK114886, UH3 DK114861, UH3 DK114866, UH3 DK114870, UH3 DK114908, UH3 DK114915, UH3 DK114926, UH3 DK114907, UH3 DK114923 and UH3 DK114933). The research was also supported by National Institute of Health (S10 OD026929), National Cancer Institute (P30 CA91842), and National Center for Advancing Translational Sciences (UL1 TR002345). HuBMAP is supported by NIH (OT2 D033760), National Heart, Lung, and Blood Institute (U54 HL145608), and NIDDK (U54 DK134301). Additional NIH support was provided by NIDDK (K08 DK107864, R01 DK111651, P01 DK056788, U2C DK114886, U54 DK083912, P30 DK081943, K23 DK125529, and U54 DK083912), National Institute of Mental Health (U01 MH114828), and National Cancer Institute (UH3 CA246632).
About the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): The NIDDK, a component of the National Institutes of Health (NIH), conducts and supports research on diabetes and other endocrine and metabolic diseases; digestive diseases, nutrition and obesity; and kidney, urologic and hematologic diseases. Spanning the full spectrum of medicine and afflicting people of all ages and ethnic groups, these diseases encompass some of the most common, severe, and disabling conditions affecting Americans. For more information about the NIDDK and its programs, see www.niddk.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Lake BB, et al. An atlas of healthy and injured cell states and niches in the human kidney . Nature. 2023.
Connect with Us
- More Social Media from NIH
Clinical Trials
Chronic kidney disease.
Displaying 38 studies
By comparing people who have kidney stones to people who do not have kidney stones, we hope to learn what causes kidney stones to form and determine if kidney stones lead to loss of kidney function or kidney disease.
The purpose of this study is to determine the clearance rate of biotin from the serum in patients with impaired renal function following ingestion of over-the-counter biotin supplements.
The purpose of this study is to determine non-inferiority in safety and efficacy when Quanta SC+ is used in the self-care home environment compared to a hemodialysis facility.
To compare the effect of senolytic drugs on cellular senescence, physical ability or frailty, and adipose tissue-derived MSC functionality in patients with chronic kidney disease. Primary Objectives: To assess the efficacy of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on clearing senescent adipose-derived MSC in patients with CKD. To assess the efficacy of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on improving adipose-derived MSC functionality in patients with CKD. Secondary Objective: To assess the short-term effect of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on ...
The primary purpose of this study is to investigate the effect of empagliflozin on kidney disease progression or cardiovascular death versus placebo on top of standard of care in patients with pre-existing chronic kidney disease.
The purpose of this multi-center event-driven study in non-dialysis (ND) participants with anemia associated with chronic kidney disease (CKD) is to evaluate the safety and efficacy of daprodustat compared to darbepoetin alfa.
The purpose of this study is to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease
This study aims to determine the potential barriers that contribute to fewer patients with Chronic Kidney Disease (CKD) being placed on the waiting list for a kidney transplant and that accomplished through evaluating:
The purpose of this multi-center study is to evaluate the efficacy and safety of daprodustat in subjects with anemia associated with CKD.
As the global epidemic of obesity and diabetes mellitus spreads, an exponential rise in incident chronic kidney disease (CKD) complicated by end stage renal disease (ESRD) is predicted, leaving healthcare systems overwhelmed worldwide. Hence, there is urgent need for novel therapies to slow the progression of DKD and optimize the health of this patient population. The purpose of this study is to examine the effect of a supplement on mesenchymal stem cells, physical body function (or frailty), kidney function, and total clearance of senescent cells in individuals with CKD. At present, we are enrolling participants with CKD, with a subset ...
The purpose of this study is to assess the safety, tolerability, optimal dosing, effectiveness signals reflecting kidney repair, and markers of mesenchymal stem cells (MSC) function that relate to response to allogenenic adipose tissue-derived MSC in patients with Chronic Kidney Disease (CKD).
The purpose of this study is to assess the safety and tolerability of intravenously delivered mesenchymal steml cells (MSC) in one of two fixed dosing regimens at two time points in patients with chronic kidney disease.
To determine the effect of 12 weeks of chronic PDEV inhibition with Tadalafil versus placebo on basal cardiorenal and humoral function and on the integrated cardiorenal and humoral response to acute sodium loading in subjects with preclinical systolic dysfunction (PSD) and renal (kidney) dysfunction.
The purpose of this study is to investigate the central hypothesis that autologous, adipose-derived MSC preconditioned by hypoxia will have superior kidney angiogenic function compared to untreated MSC from humans with HN and CKD.
This trial will compare two effective therapies, allopurinol and febuxostat, to lower serum uric acid and therefore prevent further gout attaches. These therapies have never been compared at appropriate doses. Further, they will be study in patients with kidney disease for the first time.
The purpose of this multi-center event-driven study in participants with anemia associated with chronic kidney disease (CKD) to evaluate the safety and efficacy of daprodustat.
The purpose of this study is to evaluate the pharmacokinetics, pharmacodynamics, safety and tolerability of multiple doses of lixivaptan in Autosomal Dominant Polycystic Kidney Disease subjects with chronic kidney disease in stages CKD1, CKD2 or CKD3.
This parallel-group, randomized, placebo-controlled study will examine the incidence and severity of acute kidney injury (AKI) in patients with chronic kidney disease (CKD) stage III/IV following an i.v. injection of iso-osmolar iodinated contrast material iodixanol (Visipaque™ Injection 320 mgI/mL), as compared with patients who received saline and underwent a non-enhanced CT (NECT) and duplex ultrasound (US) during their scheduled post-EVAR surveillance imaging.
The purpose of this study is to define the effects of decreasing the furosemide (lasix) dose on heart, kidney and biochemical balance, in people with compensated heart failure and kidney dysfunction and also in people with compensated heart failure without kidney dysfunction.
The aims of this study are to establish a registry and biorepository of patients with PH-CKD, and to identify clinical risk factors and biomarkers associated with PH-CKD and different hemodynamic phenotypes.
The purpose of this study is to develop educational materials that will help patients and clinicians talk about treatment options for patients with advanced kidney disease.
The purpose of the ISCHEMIA-CKD trial is to determine the best management strategy for patients with stable ischemic heart disease (SIHD), at least moderate ischemia and advanced chronic kidney disease (CKD; estimated glomerular filtration rate [eGFR] <30 or on dialysis). This is a multicenter randomized controlled trial with a target randomization of ~1000 patients with advanced CKD and at least moderate ischemia on stress testing. Participants will be assigned at random to a routine invasive strategy (INV) with cardiac catheterization (cath) followed by revascularization plus optimal medical therapy (OMT) or to a conservative strategy (CON) of OMT, with cath and ...
The purpose of this study is to evaluate the impact of palliative care consultations on quality of life and advance care planning in end-stage renal disease patients on hemodialysis, evaluate hemodialysis patients’ and caregivers’ satisfaction with palliative care consultations, and to compare quality of life and advance directive completion in dialysis patients who have and have not received palliative care consultations.
The purpose of this study of obinutuzumab administered as intravenous (IV) infusion in adults with end stage renal disease is to assess the safety and tolerability of the regimen at week 24 of the desensitization phase and at week 28 post kidney transplantation. All participants will be monitored for a minimum of 12 months following the last obinutuzumab infusion.
Systolic Pressure Intervention Trial (SPRINT) is a large scale randomized trial of ~ 9250 adults aged 50 years or older with high cardiovascular risk sponsored by NIH. The study is designed to recruit 45% of the study population with Chronic Kidney Disease (CKD). The trial will test the effects of low systolic blood pressure (SBP) goal of < 120 mm Hg versus the standard goal of < 140 mm Hg on the primary composite of cardiovascular events and death. One of the pre-specified secondary outcome is the progression of kidney disease. In this ancillary named SPRINT - Factors affecting Atherosclerosis ...
The purpose of this study is to determine the effectiveness of Basis™ (Nicotinamide Riboside and Pterostilbene) in preventing acute kidney injury (AKI) among patients undergoing complex aortic aneurysm repair and aortic arch reconstruction.
The purpose of this study is to assess the safety and tolerability of intra-arterially delivered mesenchymal stem/stromal cells (MSC) to a single kidney in one of two fixed doses at two time points in patients with progressive diabetic kidney disease.
Diabetic kidney disease, also known as diabetic nephropathy, is the most common cause of chronic kidney disease and end-stage kidney failure requiring dialysis or kidney transplantation. Regenerative, cell-based therapy applying MSCs holds promise to delay the progression of kidney disease in individuals with diabetes mellitus. Our clinical trial will use MSCs processed from each study participant to test the ...
The purpose of this study is to evaluate whether or not semaglutide can slow down the growth and worsening of chronic kidney disease in people with type 2 diabetes. Participants will receive semaglutide (active medicine) or placebo ('dummy medicine'). This is known as participants' study medicine - which treatment participants get is decided by chance. Semaglutide is a medicine, doctors can prescribe in some countries for the treatment of type 2 diabetes. Participants will get the study medicine in a pen. Participants will use the pen to inject the medicine in a skin fold once a week. The study will close when ...
The purpose of this study is to determine the natural history of the hereditary forms of nephrolithiasis and chronic kidney disease (CKD), primary hyperoxaluria (PH), cystinuria, Dent disease and adenine phosphoribosyltransferase deficiency (APRTd) and acquired enteric hyperoxaluria (EH). The investigator will measure blood and urinary markers of inflammation and determine relationship to the disease course. Cross-comparisons among the disorders will allow us to better evaluate mechanisms of renal dysfunction in these disorders.
The purpose of this research is to gather information on the effectiveness of an innovative device called the “DialySafe” intended to help reduce the chance of needle infiltrations during hemodialysis procedures. The DialySafe concept is a light-based device intended to make the AV fistula and graft easily visible for needle insertion. Light is generated from a red LED embedded in the surface of the device. The device will use transillumination technology to visualize the access, which consists of shining light directly into the skin where blood absorbs some of the light, resulting in a true representation of the ...
The purpose of this study is to evaluate the Renasight™ test, a next generation sequencing (NGS) gene mutation assay, for patients with chronic kidney disease (CKD) which utilizes genomic DNA from patient blood or buccal swab samples to analyze over 300 genes that are associated with autosomal dominant, autosomal recessive and X-linked disorders. Patients undergoing Renasight™ testing are offered optional genetic information sessions in addition to their test results.
The purpose of this study is to evaluate the Renasight™ test, a next generation sequencing (NGS) gene mutation assay, for patients with chronic kidney disease (CKD) which utilizes genomic DNA from patient blood or buccal swab samples to analyze over 300 genes that are associated with autosomal dominant, autosomal recessive and X-linked disorders. Patients undergoing Renasight™ testing are offered optional genetic information sessions in addition to their test results.
Patients with previously treated multiple myeloma and kidney dysfunction will be treated with lenalidomide and low-dose dexamethasone. Phase I will study the side effects and best dose of lenalidomide when given together with low-dose dexamethasone therapy. After the maximum safe and tolerated dose is found in Phase I, the study will proceed to Phase II. Phase II will study how well the the treatment works in patients with previously treated (relapsed or refractory) multiple myeloma and kidney dysfunction.
Biological therapies, such as lenalidomide, may stimulate the immune system in different ways and stop cancer cells from growing. Drugs used ...
The purpose of this study is to evaluate how advancing stages of chronic kidney disease (CKD) may impact the hypothalamic-pituitary-gonadal axis,and how alterations in sex hormones and menstrual cycles correlate with changes in endothelial health and sexual function before and after transplant.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
GRAIL is using deep sequencing of circulating cell-free nucleic acids (cfNAs) to develop assays to detect cancer early in blood. The purpose of this study is to collect biological samples from donors with a new diagnosis of cancer (blood and tumor tissue) and from donors who do not have a diagnosis of cancer (blood) in order to characterize the population heterogeneity in cancer and non-cancer subjects and to develop models for distinguishing cancer from non-cancer.
Falls are common and catastrophic in cancer patients. Cancer patients are vulnerable to falls due to muscle loss. In prescribing exercise in a data driven manner to cancer patients, our hypothesis is this "prescription" for exercise will eventually be demonstrated to reduce the occurrence of injurious falls.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Advances in the...
Advances in the management of chronic kidney disease
- Related content
- Peer review
- Teresa K Chen , assistant professor 1 ,
- Melanie P Hoenig , associate professor 2 ,
- Dorothea Nitsch , professor 3 ,
- Morgan E Grams , professor 4
- 1 Kidney Health Research Collaborative and Division of Nephrology, Department of Medicine, University of California San Francisco; and San Francisco VA Health Care System, San Francisco, CA, USA
- 2 Division of Nephrology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
- 3 Department of Non-Communicable Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, UK
- 4 Department of Medicine, New York University Langone School of Medicine, New York, NY, USA
- Correspondence to: M E Grams Morgan.Grams{at}nyulangone.org
Chronic kidney disease (CKD) represents a global public health crisis, but awareness by patients and providers is poor. Defined as persistent abnormalities in kidney structure or function for more than three months, manifested as either low glomerular filtration rate or presence of a marker of kidney damage such as albuminuria, CKD can be identified through readily available blood and urine tests. Early recognition of CKD is crucial for harnessing major advances in staging, prognosis, and treatment. This review discusses the evidence behind the general principles of CKD management, such as blood pressure and glucose control, renin-angiotensin-aldosterone system blockade, statin therapy, and dietary management. It additionally describes individualized approaches to treatment based on risk of kidney failure and cause of CKD. Finally, it reviews novel classes of kidney protective agents including sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, non-steroidal selective mineralocorticoid receptor antagonists, and endothelin receptor antagonists. Appropriate, widespread implementation of these highly effective therapies should improve the lives of people with CKD and decrease the worldwide incidence of kidney failure.
Introduction
Chronic kidney disease (CKD) affects approximately 10% of the world’s population and is associated with substantial morbidity and mortality. 1 Risks of kidney failure, acute kidney injury, heart failure, cardiovascular disease, and hospital admissions are all heightened in people with CKD. 2 The Global Burden of Disease Consortium projects that CKD will be in the top five conditions contributing to years of life lost by 2040. 3 However, CKD remains under-recognized by both patients and providers. 1 A diverse entity, CKD is most commonly attributed to diabetes or high blood pressure, but many other causes exist, from genetic causes to adverse effects of drugs to autoimmune processes. 2 In this review, we summarize the evidence for current paradigms of disease identification and classification, discuss new equations developed for estimating glomerular filtration rate (GFR) and harmonizing different measures of albuminuria, report major progress in individualized risk estimation of kidney failure and other adverse outcomes both for CKD in general and within specific disease entities, and describe longstanding and novel treatment strategies. Notable advances have been made in both general and cause specific therapies, including sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, non-steroidal selective mineralocorticoid receptor antagonists (MRA), and endothelin receptor antagonists. Finally, we describe major guidelines in CKD and highlight common themes as well as differences in their recommendations.
Sources and selection criteria
We searched PubMed for peer reviewed articles in the English language from 1 January 2010 to 14 July 2023 using the keywords listed in the web appendix. We additionally reviewed reference lists of selected articles, prioritizing randomized controlled trials, systematic reviews, and meta-analyses when possible but also including observational studies and reviews that were of high quality. We included older articles if we deemed them to be of high importance. Finally, we reviewed guidelines from websites of professional societies and advisory committees (for example, the National Institute for Health and Care Excellence (NICE), Kidney Disease: Improving Global Outcomes (KDIGO), US Centers for Disease Control and Prevention, US Department of Health and Human Services, and International Society of Hypertension).
Epidemiology
CKD is a global public health crisis. Recent estimates suggest that more than 700 million people have CKD, with greater burdens in low income and middle income countries. 1 4 Determining the global, regional, and national burden of disease is challenging owing to inconsistent use of estimating equations for GFR, laboratory assay standardization, and albuminuria testing. Despite this, some important observations can still be made. The prevalence of CKD increases with age and is greatest in people over 70 years. 2 In the US, compared with White people, Black people have substantially higher rates of kidney failure, followed by Native Americans, people of Hispanic ethnicity, and people of Asian descent. 5
The most commonly reported risk factors for CKD are diabetes mellitus and hypertension. 6 7 Social determinants of health are also important and likely contribute to racial disparities in kidney disease. Specific genetic variants increase risk of CKD, including variants in the APOL1 and HBB genes that are present in far greater proportions among people of African ancestry. 8 9 10 11 In Central America, Sri Lanka, Egypt, and Central India, defined geographic areas exist where many cases of CKD of unknown cause have been identified. 12 Some experts postulate that heat stress or pesticides may contribute.
Whereas the incidence of CKD is difficult to estimate, reliant as it is on testing for GFR and albuminuria, the incidence of kidney failure with the receipt of replacement therapy (KFRT) is more readily captured. Many countries have developed national registries of patients with kidney failure, allowing comparison of incidence across ages and countries. 13 For example, the countries with the highest incidence of treated kidney failure in 2020 were Taiwan, the US, and Singapore, whereas the countries with the highest prevalence were Taiwan, the Republic of Korea, and Japan. 5
Definition and classification of CKD: cause, GFR, and albuminuria staging
CKD is defined as persistent abnormalities in kidney structure or function for more than three months, manifest as either low GFR or presence of a marker of kidney damage. 2 Specifically, diagnosis requires one or more of the following: albuminuria, defined as an albumin-to-creatinine ratio (ACR) ≥30 mg per gram of creatinine (approximately ≥3 mg/mmol) or albumin excretion of ≥30 mg/day; GFR <60 mL/min/1.73 m 2 ; abnormalities on urine sediment, histology, or imaging; electrolyte or other abnormalities attributed to tubular disorders; or history of kidney transplantation. The KDIGO heat map helps with understanding of overall risk (low, moderately increased, high, and very high) of patients according to level of albuminuria (A category), level of GFR (G category), and cause of disease ( fig 1 ), such that people with normal estimated GFR but higher albuminuria have a similar risk to people with moderately reduced estimated GFR and no albuminuria.

Kidney Disease: Improving Global Outcomes heat map with guidance on monitoring. 2 Numbers in boxes indicate recommended frequency of monitoring (number of times per year). Colors denote risk as follows: green (low risk), yellow (moderately increased risk), orange (high risk), and red (very high risk). CKD=chronic kidney disease; GFR=glomerular filtration rate
- Download figure
- Open in new tab
- Download powerpoint
Clinical manifestations of CKD
Albuminuria.
Albuminuria is often the first sign of kidney damage, and its detection drives many treatment decisions. 2 The prevalence of albuminuria in people with diabetes or hypertension is estimated to be 32% and 22%, respectively. 14 However, only a minority of patients receive urine screening tests. 14 15 For example, the mean albuminuria screening rates across health systems in the US were 35% among adults with diabetes and 4% among adults with hypertension. 14
The gold standard for assessing albuminuria is either a sample collected mid-stream from an early morning urine void or a 24 hour urine collection; however, in situations where this is not possible, a spot collection is reasonable. 2 Quantification of albumin is preferred over that of total protein. 2 16 This preference is because the sensitivity of the total protein assay to different protein components can vary by laboratory, as well as the fact that proteinuria assessments do not easily discriminate A1 and A2 categories. Both urine albumin and urine protein are typically indexed to urine creatinine to account for differences in dilution, as urine ACR or urine protein-to-creatinine ratio (PCR). Dipstick protein assessment is generally more economical than both methods; however, like PCR, dipstick assessment can be insensitive in A1 and A2 categories. Although conversion calculators exist to aid in the harmonization of ACR and PCR measures; they do not work well at lower ranges of albuminuria. 17 18
The second axis for CKD classification focuses on GFR. 2 The gold standard for assessing GFR is direct measurement from clearance of an exogenous filtration marker such as iohexol or iothalamate; however, this is relatively cumbersome and rarely done in clinical practice. Instead, GFR is usually estimated by using plasma or serum concentrations of endogenous filtration markers, such as creatinine and cystatin C, and demographic variables. Early equations for adults, such as Modification of Diet in Renal Disease (MDRD) and CKD Epidemiology Collaboration (CKD-EPI) 2009 equations, used filtration markers along with age, sex, and race (Black versus non-Black) to estimate GFR. 19 20 21 The newer European Kidney Function Consortium equation, which allows for seamless GFR evaluation from infancy to old age, uses a population specific divisor to adjust creatinine values (for example, separate values for Black European and White European populations). 22 However, the use of race in GFR estimation has faced strong criticism and, in 2021, the US based American Society of Nephrology-National Kidney Foundation Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease recommended immediate adoption of the race-free CKD-EPI 2021 estimating equations, which exist for creatinine alone (eGFRcr) as well as for creatinine and cystatin C (eGFRcr-cys). 23 24 25 Cystatin C has distinct confounders (non-GFR determinants) of its relation with GFR compared with creatinine ( fig 2 ). 2 26 Thus, eGFRcr-cys is a more accurate estimate of GFR than eGFRcr alone, irrespective of equation used, in most scenarios, including those in which large differences exist between eGFRcr and that estimated solely using cystatin C (eGFRcys). 25 27 28 However, the newest GFR estimating equations have not been tested extensively in Asian populations. 29 30

Common non-glomerular filtration rate (GFR) determinants of blood concentrations of creatinine and cystatin C. 2 26 eGFR=estimated glomerular filtration rate
The third axis for classification is cause of CKD, which is generally ascertained through imaging, assessment of extrarenal manifestations and biomarkers, or kidney biopsy. 2 Classification of cause typically hinges on the presence or absence of systemic disease (for example, obesity, diabetes, hypertension, systemic autoimmune disease) and the specific location of the kidney pathology (for example, glomeruli, tubulointerstitium, vasculature, or cystic/congenital abnormality). Unfortunately, the cause of CKD is often unknown, limiting its utility. Molecular phenotyping and genetic testing are increasingly being used to assign cause of disease. Targeted gene panels offered commercially may have high diagnostic yields in select populations, such as patients with glomerular disease, nephrotic syndrome, or congenital anomalies of the kidney and urinary tract. 31 One study suggested that for appropriately selected patients, 34% had disease either reclassified or assigned on the basis of genetic testing, thus changing clinical management. 32 The European Renal Association and the European Rare Kidney Disease Reference Network have issued a joint statement providing recommendations for how to provide genetic testing, including specific settings in which it may be considered ( box 1 ). 33
European Renal Association and European Rare Kidney Disease Reference Network recommendations for settings in which genetic testing might be considered 33
Most tubulopathies
Glomerulopathies:
Congenital nephrotic syndrome
Nephrotic syndrome refractory to standard steroid therapy
Multi-organ phenotypes suggestive of syndromic steroid resistant nephrotic syndrome
Complement disorders:
Immune complex mediated membranoproliferative glomerulonephritis
C3 glomerulopathy
Atypical hemolytic uremic syndrome
Renal ciliopathies
Congenital anomalies of the kidney and urinary tract
Patients aged <50 years with severe CKD of unknown cause
Patients aged >50 years with adult onset CKD and family history of CKD
CKD=chronic kidney disease
Individualized prognosis and treatment
Identifying the cause of CKD is critical as different causes of CKD carry different prognoses and can have distinct treatments. 2 For example, autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic cause of CKD and is typically associated with faster progression than other disease entities. 32 34 Individualized prognosis is often determined by using disease specific risk classification or calculators (for example, the Mayo classification or the ADPKD Prognostic Tool), and screening and treatment recommendations such as increased fluid intake and tolvaptan are unique to this entity. 35 36 37 38 IgA nephropathy, the most common type of glomerulonephritis worldwide, particularly in East Asian and Pacific Asian countries, 39 has its own prognostic aids, such as the International IgA Nephropathy Prediction Tool, 40 41 and treatments specific to IgA nephropathy are in various stages of development. 42 The APOL1 high risk genotypes confer about twofold higher risk of kidney failure in the general population and are common in people of African ancestry. 8 43 44 45 A recently published phase 2A study of targeted therapy for APOL1 related disease showed promising reductions in albuminuria; the phase 3 study is ongoing. 46 Other disease specific therapies are increasingly available, such as belimumab in lupus nephritis and lumasiran for primary hyperoxaluria type 1. 47 48
Individualized risk prediction is also available for more general populations of patients with CKD. The most widely known and validated is the kidney failure risk equation (KFRE), which is used in patients with GFR <60 mL/min/1.73 m 2 . 49 Tested in more than 30 countries and 700 000 people, the tool provides probabilities of kidney failure at two years and five years based on age, sex, and estimated GFR and albuminuria levels. 50 Like all risk equations, the KFRE may perform better with recalibration to absolute risk levels of local populations, but the discriminatory ability (that is, distinguishing people at high risk from those at low risk) has been extremely consistent across all studies. The KFRE has also been validated in recipients of kidney transplants. 51 52 Although the KFRE does not explicitly take into account the competing risk of death, estimates are quite accurate except among the members of the oldest segments of the population at the highest risk. 53 One study suggested that the KFRE provides more accurate prediction of kidney failure than both patients and providers. 54 Even within categories of GFR and urine ACR, the KFRE provides a wide estimate of risk prediction, which can be helpful in the counseling and referral of patients ( fig 3 ). Some centers will refer patients with a two year risk of kidney failure greater than 20-40% for vascular access and kidney transplantation evaluation, on the basis that tools that incorporate albuminuria provide more accurate and unbiased time to kidney failure than does estimated GFR alone. 55 Studies suggest that the KFRE is robust to different GFR equations (specifically, CKD-EPI 2009 and CKD-EPI 2021) and that many patients value being counseled using this information. 53 56

Range of predicted risk of kidney failure using the kidney failure risk equation (KFRE) within G and A categories of chronic kidney disease (CKD). The KFRE ( ckdpcrisk.org/kidneyfailurerisk ) was used to estimate two year risk of kidney failure in 350 232 patients with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m 2 from the Optum Laboratories Data Warehouse (OLDW). OLDW is a longitudinal, real world data asset with deidentified administrative claims and electronic health record data. Patients with eGFR and albuminuria (urine albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio, or dipstick protein) within a two year window were included in this analysis. Different measures of albuminuria were harmonized to ACR levels for A categories ( ckdpcrisk.org/pcr2acr )
Other risk equations exist to predict the risk of cardiovascular disease and death in CKD; some of these do consider the competing risk of death ( www.ckdpcrisk.org ). For example, the advanced CKD risk tool provides simultaneous estimates of kidney failure, cardiovascular disease, and death for patients with estimated GFR <30 mL/min/1.73 m 2 , which can inform decisions on access placement and reinforce the importance of cardiovascular risk reduction. 57 Estimating risks of cardiovascular disease is particularly relevant given that many more patients with CKD have cardiovascular disease events than need KFRT. 58 Other efforts incorporate estimated GFR and albuminuria into existing tools, such as SCORE2 and the pooled cohort equation for the prediction of cardiovascular disease. 59 60
Patient specific prognostic clues may stem from discrepant estimated GFR values between eGFRcr and eGFRcys. 61 62 63 When eGFRcys is substantially lower than eGFRcr, the risk for kidney related laboratory abnormalities (for example, anemia, hyperuricemia, and hyperphosphatemia) and subsequent adverse outcomes (for example, kidney failure, heart failure, and mortality) is higher. 61 64 65 By contrast, having a lower eGFRcr than eGFRcys is associated with lower risk of adverse outcomes. 66 Risk factors for having a discrepancy between eGFRcr and eGFRcys include older age, female sex, higher body mass index, recent weight loss, and smoking.
General principles of management
The mainstays of therapy for patients with CKD include treating the underlying cause if known, and correcting risk factors (for example, albuminuria) for CKD progression and other CKD related complications ( fig 4 ). 2

Comprehensive care of patients with chronic kidney disease (CKD), irrespective of cause
Blood pressure targets
The three major studies for evaluating the optimal blood pressure target in CKD were the Modification of Diet in Renal Disease Study (MDRD), African American Study of Kidney Disease and Hypertension (AASK), and Systolic Blood Pressure Intervention Trial (SPRINT). 67 68 69 In both MDRD and AASK, intensive blood pressure control did not slow GFR decline overall. 67 68 However, in MDRD, participants with baseline proteinuria of ≥3 g/day seemed to benefit from intensive blood pressure control, with slower mean rates of GFR decline compared with their counterparts in the usual blood pressure control group. 67 Among SPRINT participants with baseline CKD (n=2646), aiming for a systolic blood pressure goal of <120 mm Hg versus <140 mm Hg did not significantly reduce the risk for a composite kidney outcome that included a ≥50% reduction in estimated GFR, long term dialysis, or kidney transplant. 69 70 However, benefits of intensive blood pressure control were seen with respect to prevention of the composite cardiovascular outcome (defined as myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes—hazard ratio 0.75, 95% confidence interval 0.64 to 0.89) and all cause mortality (hazard ratio 0.73, 0.60 to 0.90), regardless of CKD status. 69 Blood pressure control can also reduce albuminuria, as shown in the Chlorthalidone in Chronic Kidney Disease (CLICK) trial of chlorthalidone in advanced CKD. 71
Glycemic targets
Among patients with diabetes and CKD, glycemic control is an important component of comprehensive care. 72 The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) was the largest trial of intensive glucose control to enroll patients with CKD. 73 Among the 11 140 trial participants, 19% had an estimated GFR <60 mL/min/1.73 m 2 and 31% had albuminuria at baseline. 74 Compared with standard glucose control, intensive glucose control was associated with 9% (hazard ratio 0.91, 0.85 to 0.98), 30% (0.70, 0.57 to 0.85), and 65% (0.35, 0.15 to 0.83) lower risks of developing new onset ACR 30-300 mg/g, ACR >300 mg/g, and end stage kidney disease (ESKD), respectively.
Specific classes of therapy
Angiotensin converting enzyme inhibitors and angiotensin receptor blockers.
When choosing antihypertensive agents, those that act by inhibiting the renin-angiotensin-aldosterone system (RAAS) have particular relevance in CKD. A 2001 meta-analysis of 11 studies suggested that, for non-diabetic CKD, the use of angiotensin converting enzyme (ACE) inhibitors resulted in a 30% reduction in risk of KFRT or doubling of serum creatinine. 75 Clinical trials in populations with CKD and diabetes (for example, IDNT, RENAAL) have also shown benefit of angiotensin receptor blockers (ARB) in preventing CKD progression ( table 1 ). 77 78 RAAS inhibition also plays a role in prevention of cardiovascular disease. The Heart Outcomes Prevention Evaluation (HOPE) study showed that ACE inhibitors reduced the risks of myocardial infarction, stroke, and cardiovascular death in populations at high risk for cardiovascular disease, including those with diabetes and albuminuria. 80 The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) showed that ACE inhibitors and ARB were generally equivalent in the prevention of cardiovascular events. 81 Because of the increased risk of hyperkalemia and acute kidney injury, dual therapy with both an ACE inhibitor and an ARB is typically avoided. 82
Landmark randomized clinical trials on angiotensin converting enzyme inhibitors or angiotensin receptor blockers in chronic kidney disease
- View inline
When GFR declines, providers often grapple with whether RAAS inhibitors should be continued. The Benazepril in Advanced CKD study showed that benazepril reduced the risk of the primary composite kidney endpoint by 43% compared with placebo, thus suggesting that RAAS inhibitors are beneficial even in advanced CKD (baseline serum creatinine 3.1-5.0 mg/dL). 79 Three recent reports further explored this question, also examining the benefits in prevention of death and cardiovascular events associated with continuation of RAAS inhibitors. 83 84 85 A retrospective, propensity score matched study of patients with estimated GFR <30 mL/min/1.73 m 2 showed higher risk of all cause mortality and major adverse cardiovascular events in those who stopped RAAS inhibitors compared with those who continued them, 83 as did a Swedish trial emulation study. 84 The risk of kidney replacement therapy associated with cessation of RAAS inhibitors was not statistically significant in the first study and lower in the second study. 83 84 In an open label randomized trial, cessation of RAAS inhibitors did not show significant between group differences in long term decline in estimated GFR or initiation of kidney replacement therapy, providing reassurance that RAAS inhibitors can be safely continued as GFR declines. 85
SGLT-2 inhibitors
One of the biggest advancements in CKD management over the past decade was the discovery that SGLT-2 inhibitors have robust protective effects on the heart and kidneys in patients with and without diabetes. Recent trials showed an approximate 30% reduction in risk for diverse kidney outcomes among patients with baseline estimated GFR values as low as 20 mL/min/1.73 m 2 ( table 2 ). 86 88 89 91 Importantly, the three trials designed with primary kidney outcomes (Canagliflozin and Renal Events in Diabetes and Established Nephropathy Clinical Evaluation (CREDENCE), Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD), and Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY)) were terminated early because pre-specified efficacy criteria were met, with median follow-up times ranging from 2.0 to 2.6 years. 88 89 91 The overwhelming majority of trial participants were taking an ACE inhibitor or ARB before randomization, showing that the benefits of SGLT-2 inhibitors on slowing CKD progression are additive to those of RAAS inhibitors. One simulation study estimated that a 50 year old adult with non-diabetic albuminuric CKD would have seven extra years free from doubling of serum creatinine, kidney failure, or all cause mortality if treated with an SGLT-2 inhibitor and RAAS inhibitor. 92
Landmark randomized clinical trials on sodium-glucose co-transporter 2 inhibitors in chronic kidney disease (CKD)
Subgroup analyses of the DAPA-CKD and EMPA-KIDNEY trials have provided additional insights on the wide range of patients who are likely to benefit from SGLT-2 inhibitors. 89 91 In DAPA-CKD, dapagliflozin was favored over placebo in all pre-specified subgroups by baseline age, sex, race, diabetes status, systolic blood pressure, estimated GFR (<45 v ≥45 mL/min/1.73 m 2 ), and ACR (≤1000 v >1000 mg/g or ≤113 v >113 mg/mmol). 89 Similarly, in EMPA-KIDNEY, empagliflozin was associated with lower risk of the primary composite outcome compared with placebo regardless of baseline diabetes status or estimated GFR (<30 v ≥30 mL/min/1.73 m 2 to <45 v ≥45 mL/min/1.73 m 2 ). 91 The risk of the primary outcome was not lower among patients with ACR ≤300 mg/g (approximately ≤30 mg/mmol). In exploratory analyses, however, empagliflozin was associated with slower annual rates of decline in estimated GFR compared with placebo among participants with ACR between 30 and 300 mg/g (approximately 3-30 mg/mmol) and slower chronic slope (from two months to the final follow-up visit) among all ACR subgroups.
The DAPA-CKD trial also showed that the kidney protective effects of SGLT-2 inhibitors extend to patients with IgA nephropathy and perhaps also those with focal segmental glomerulosclerosis (FSGS). 93 94 Among 270 participants with IgA nephropathy (mean estimated GFR 44 mL/min/1.73 m 2 ; median ACR 900 mg/g (102 mg/mmol)), dapagliflozin was associated with a 71% lower risk of developing the primary outcome and a 70% lower risk of ESKD compared with placebo. 93 Among the 104 participants with FSGS (mean estimated GFR 42 mL/min/1.73 m 2 ; median ACR 1248 mg/g (141 mg/mmol)), dapagliflozin was not associated with a lower risk of the primary composite outcome, although this analysis was limited in power (only 11 events). 94 In exploratory analyses, dapagliflozin was associated with slower chronic decline in estimated GFR in the FSGS population. Investigations on the use of SGLT-2 inhibitors in other patient populations, such as those with polycystic kidney disease and kidney transplant recipients, are ongoing (clinicaltrials.gov).
SGLT-2 inhibitors, which act at the level of the proximal tubule to block the reabsorption of glucose and sodium, 95 are generally safe to use in patients with CKD. Early signals of heightened risks of volume depletion, serious genital infections, bone fractures, and need for limb amputation in the Canagliflozin Cardiovascular Assessment Study (CANVAS) were not observed in subsequent studies—CREDENCE, DAPA-CKD, and EMPA-KIDNEY—thus assuaging these concerns ( table 3 ). 86 88 89 91 A pooled analysis of 15 081 participants with type 2 diabetes and CKD G3-4 showed similar rates of serious adverse events for empagliflozin versus placebo, with a higher rate only of mild genital infections with the SGLT-2 inhibitor. 96 A real world study of patients receiving SGLT-2 inhibitors compared with dipeptidyl peptidase-4 (DPP-4) inhibitors found no increased risk of outpatient urinary tract infections or severe urinary tract infection events requiring hospital admission. 97
Adverse effects of SGLT-2 inhibitors * in CANVAS, CREDENCE, DAPA-CKD, and EMPA-KIDNEY trials
GLP-1 receptor agonists
GLP-1 receptor agonists have also been shown to improve kidney outcomes among patients with type 2 diabetes, albeit in trials that were designed for primary cardiac outcomes ( table 4 ). 98 99 100 101 102 103 104 105 106 107 108 109 The reduction in risk of kidney outcomes, which included albuminuria, ranged from 15% to 36%. A large meta-analysis of approximately 44 000 participants from the six trials in table 4 reported that use of GLP-1 receptor agonists was associated with a 21% lower risk of developing the composite kidney outcome, defined as new onset albuminuria >300 mg/g, doubling of serum creatinine, ≥40% decline in estimated GFR, kidney replacement therapy, or death due to kidney causes, compared with placebo. 100 This risk reduction seemed to be driven by the reduction in incident albuminuria >300 mg/g; associations between GLP-1 receptor agonists and CKD progression and kidney failure were not statistically significant. However, results were more promising in A Study Comparing Dulaglutide with Insulin Glargine on Glycemic Control in Participants with Type 2 Diabetes and Moderate or Severe Chronic Kidney Disease (AWARD-7), a clinical trial designed to evaluate change in glycated hemoglobin. 110 Among 577 adults with type 2 diabetes and CKD G3-4 randomized to open label dulaglutide 1.5 mg once weekly, dulaglutide 0.75 mg once weekly, or insulin glargine daily, both dulaglutide groups had slower estimated GFR declines compared with the insulin glargine group; among participants with baseline albuminuria >300 mg/g, dulaglutide was associated with greater ACR reductions in a dose dependent manner over the one year follow-up.
Landmark randomized clinical trials on associations of glucagon-like peptide-1 (GLP-1) receptor agonists with secondary kidney outcomes among patients with type 2 diabetes mellitus
Exact mechanisms by which the GLP-1 receptor agonists slow decline in estimated GFR and/or reduce albuminuria are not entirely clear, but proposed mechanisms include improved glycemic control, weight loss, increased natriuresis, and reduced inflammation and oxidative stress. 111 112 113 Adverse effects observed with this class of drugs have included diarrhea, nausea, and vomiting. 103 104 107 109 110
Mineralocorticoid receptor antagonists
Several MRAs are available and can be useful adjuncts to RAAS inhibitors, particularly among populations with albuminuria and/or diabetes. Two common steroidal non-selective MRAs, spironolactone and eplerenone, both lower albuminuria. 72 In a meta-analysis of 372 participants from seven trials, combination therapy with a non-selective MRA and an ACE inhibitor and/or ARB was associated with a significant reduction in proteinuria, albeit with a higher risk of hyperkalemia. 114 Finerenone, a non-steroidal selective MRA, was also recently approved. 115 Compared with the steroidal non-selective MRAs, finerenone has a stronger selectivity for the mineralocorticoid receptor, a shorter half life, less of a blood pressure lowering effect, and a more favorable side effect profile, as well as potentially greater anti-inflammatory and antifibrotic effects. 115 116 117 The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial and the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial were two complementary phase 3 clinical trials designed to investigate the kidney and cardiovascular benefits of finerenone, respectively, in people with albuminuria levels ≥30 mg/g and type 2 diabetes ( table 5 ). 116 118 Both trials included patients taking maximally tolerated ACE inhibitor or ARB, with participants in FIDELIO-DKD generally having more severe baseline CKD. In a pooled analysis of the two trials, finerenone was associated with a 15-23% lower risk of developing the kidney composite outcomes and a 32% lower mean change in ACR from baseline to four months. 119 Hyperkalemia was more frequent among patients randomized to finerenone (14%) compared with placebo (7%). In pre-specified analyses, baseline SGLT-2 inhibitor use (n=877) or GLP-1 receptor agonist use (n=944) did not modify the beneficial effect of finerenone on the kidney composite outcome, thus suggesting a potential role for dual therapy (for example, finerenone plus SGLT-2 inhibitor or GLP-1 receptor agonist) among patients with type 2 diabetes and CKD.
Landmark randomized clinical trials on finerenone in chronic kidney disease
Endothelin receptor antagonists
Endothelin receptor antagonists have emerged as novel treatments for a variety of kidney diseases. The Study of Diabetic Nephropathy with Atrasentan (SONAR) evaluated the effect of atrasentan on a composite kidney outcome (defined as a doubling of serum creatinine or ESKD) among adults with type 2 diabetes, estimated GFR 25-75 mL/min/1.73 m 2 , and urine ACR 300-5000 mg/g taking a stable dose of ACE inhibitor or ARB. 120 After a six week enrichment period during which all participants received atrasentan 0.75 mg daily (n=5517), those who responded (defined as a ≥30% reduction in urine ACR without the development of substantial fluid retention or increase in serum creatinine by >0.5 mg/dL and 20% from baseline; n=2648) were randomized to receive atrasentan or placebo. Over a median follow-up of 2.2 years, the atrasentan group had a 35% lower risk of developing the composite kidney outcome compared with the placebo group, although fluid retention and anemia were more frequent in the former. Of note, the frequency of hyperkalemia was low (1%) in both treatment groups. Sparsentan, a dual endothelin and angiotensin II receptor antagonist, is also being investigated as a treatment for FSGS and IgA nephropathy. 121 122 In a phase 2, randomized, double blind, active control trial, 109 adults with biopsy proven FSGS (estimated GFR >30 mL/min/1.73 m 2 and urine PCR ≥1 g/g) received varying doses of sparsentan (200, 400, or 800 mg daily) or irbesartan 300 mg daily. 121 At eight weeks, participants receiving sparsentan had greater reductions in urine PCR compared with those receiving irbesartan. In an interim analysis of the PROTECT phase 3 trial, adults with biopsy proven IgA nephropathy (urine PCR ≥1 g/day) randomized to sparsentan 400 mg daily had a 41% greater reduction in urine PCR over 36 weeks and threefold higher odds of achieving complete remission of proteinuria at any point compared with their counterparts who were randomized to irbesartan 300 mg daily. 122 Based in part on the results of this study, the US Food and Drug Administration (FDA) granted accelerated approval for the use of this drug in adults with primary IgA nephropathy considered to be at risk of rapid disease progression. 123
Endothelin 1 has been implicated in the pathogenesis of kidney disease via various mechanisms including vasoconstriction, vascular hypertrophy, endothelial and podocyte injury, inflammation, cell proliferation, extracellular matrix accumulation, and fibrosis. 124 Systemic and local kidney production of endothelin 1 is augmented in CKD.
Other nephroprotective and cardiovascular risk reduction strategies
A bidirectional association exists between CKD and cardiovascular disease: cardiovascular disease is both a risk factor for CKD and a common outcome in patients with CKD. 125 126 Thus, patients with CKD are likely to benefit from efforts at cardiovascular risk reduction including administration of a statin as well as the gamut of lifestyle changes. 2 127
Lipid management
The Study of Heart and Renal Protection (SHARP) trial evaluated the efficacy of ezetimibe and simvastatin combination therapy in patients with moderate to severe CKD (33% on dialysis; 67% not on dialysis with mean estimated GFR of 27 mL/min/1.73 m 2 ). 128 Treatment with these low density lipoprotein (LDL) cholesterol lowering agents led to a 17% risk reduction for development of a first major atherosclerotic event compared with placebo, although this benefit was seen only in the patients not requiring maintenance dialysis. Those at very high risk (for example, with previous major atherosclerotic cardiovascular disease events) may benefit from additional therapies to lower LDL cholesterol, including evolocumab. 129 Evolocumab is a monoclonal antibody for proprotein convertase subtilisin/kexin type 9, which increases LDL cholesterol receptors and hence clearance of LDL; this novel therapy also seems to be safe and efficacious in patients with CKD. 129 130
Physical activity
Exercise has been shown to benefit patients with CKD. Several small, randomized trials have reported that exercise training programs in patients with moderate to severe CKD are safe, feasible, and effective in improving physical activity levels, cardiorespiratory fitness, and quality of life. 131 132 133 134 135 Whether these interventions also slow CKD progression remains to be determined, as many of these studies were underpowered for this outcome.
For patients with obesity, weight loss may reduce the risk of CKD progression, whether it comes from intensive lifestyle intervention such as in the Look AHEAD (Action for Health in Diabetes) trial or, as in observational studies, from bariatric surgery. 136 137 138 Micronutrient and macronutrient composition of diets may also matter. 139
Traditional recommendations about diet in the setting of CKD have focused on limiting protein and dietary acid intake. Experimental evidence suggests that protein intake can increase intraglomerular pressure and cause glomerular hyperfiltration. 140 141 142 Observational data from large cohort studies suggest that the type of protein may be important; a diet high in animal protein may increase risk, whereas protein from plant sources may be better tolerated. 143 144 For example, an observational study in Singapore found a strong correlation between red meat intake and risk of ESKD. 145 Little clinical trial evidence for protein restriction exists. The MDRD study randomized patients to different levels of protein restriction but found no statistically significant difference in the rate of GFR decline. 67
A second line of investigation has been into the benefits of increasing nutritional alkali intake, with a body of open label trials suggesting benefits on kidney function and prevention of starting dialysis. 146 A phase 3 double blinded, placebo controlled trial reported that veverimer (a potent acid binder that acts in the intestine) was effective in raising or normalizing serum bicarbonate among patients with CKD and chronic metabolic acidosis. 147 Other double blinded studies using veverimer suggested that treating acidosis in CKD improves quality of life and overall physical function. 148 However, a recent trial evaluating veverimer in slowing progression of CKD was negative. 149
Although patients with CKD are prone to hyperkalemia, potassium intake has a beneficial effect on blood pressure, cardiovascular disease, and death independent of and opposite to that of sodium intake. 150 151 152 153 One large randomized controlled trial suggested that substituting 25% of sodium chloride intake with potassium chloride reduced the risk of major adverse cardiovascular events by 13% in the general population. 154 Similarly, small studies suggest that diets rich in potassium may be beneficial in CKD. A feeding trial in people with CKD G3 observed that 100 mmol compared with 40 mmol of dietary potassium per day increased serum potassium by 0.21 mmol/L, 155 similar to the increase seen with finerenone. 156 Many dietary studies have evaluated patterns of diet rather than potassium alone: for example, plant based diets tend to be rich in not only potassium but also alkali and fiber. Observational data from prospective cohorts suggest that plant based diets are associated with less CKD progression. 143 157 158 Evidence is also emerging to suggest that increasing fiber intake benefits the gut microbiome, decreases inflammation, and possibly slows CKD progression. 159
Appropriate drug dosing and nephrotoxin avoidance
An important component of care for patients with CKD is avoidance of additional insults. Many drugs are cleared by glomerular filtration or tubular secretion by the kidney, and reduced GFR can lead to accumulation of the drug or its metabolites resulting in adverse effects. 160 Careful estimation of GFR is generally a first step in determining dosage for renally excreted drugs. 161 The US FDA guidance to industry suggests that estimated GFR based on serum creatinine may be used in pharmacokinetic studies. 162 If drugs are dosed on the basis of estimated GFR (rather than estimated creatinine clearance from the Cockcroft-Gault equation, an equation that is known to be flawed), estimated GFR must be “de-indexed” by multiplying the standardized estimated GFR by the individual’s calculated body surface area and dividing by 1.73 m 2 . 163 164 165 This is because drug clearance is thought to be proportional to a person’s GFR and not the GFR standardized to body surface area. Antibiotics and antiviral agents, direct oral anticoagulants, drugs for diabetes mellitus, and chemotherapeutic agents are the most common drugs that require attention to dosing in CKD. 2 160 164
Some drugs should be avoided or minimized in CKD because of their potential to worsen kidney function. For example, non-steroidal anti-inflammatory drugs (NSAIDs) can exacerbate hypertension, cause fluid retention, and contribute to the risk of acute kidney injury. 166 Particularly when used with RAAS inhibitors and diuretics, NSAIDs are ideally avoided. 167 In select patients with CKD, however, some clinicians will prescribe an abbreviated course of NSAIDs given that the most common alternative, opioids, also have significant adverse effects. 168 Proton pump inhibitors can lead to acute or chronic interstitial nephritis and have been associated with incident CKD, progression of CKD, and ESKD. 169 170 Although the mechanism by which proton pump inhibitors contribute to CKD remains unclear, most experts agree that these agents should be used judiciously.
Emerging treatments
Many phase 3-4 clinical trials are ongoing to evaluate emerging treatments for kidney disease (clinicaltrials.gov). These include, but are not limited to, investigations on the use of dapagliflozin in advanced CKD (for example, estimated GFR <25 mL/min/1.73 m 2 , on maintenance dialysis with residual daily urine output of >500 mL, and kidney transplant recipients with estimated GFR ≤45 mL/min/1.73 m 2 ; NCT05374291 ); finerenone in non-diabetic CKD ( NCT05047263 ); and monteluklast ( NCT05362474 ) and pentoxyifylline ( NCT03625648 ) in diabetic CKD. Several therapies are also being tested for rarer causes of kidney disease: obinutuzumab ( NCT04629248 ), zanubrutinib ( NCT05707377 ), and SNP-ACTH (1-39) gel ( NCT05696613 ) in membranous nephropathy; voclosporin ( NCT05288855 ), atacicept ( NCT05609812 ), anifrolumab ( NCT05138133 ), inanalumab ( NCT05126277 ), secukinumab ( NCT04181762 ), obinutuzumab ( NCT04221477 ), and ACTHar gel ( NCT02226341 ) in lupus nephritis; VX-147 in APOL1 related kidney disease ( NCT05312879 ); imlifidase in antiglomerular basement membrane disease ( NCT05679401 ); sparsentan in focal segmental glomerulosclerosis ( NCT03493685 ); and pegcetacoplan ( NCT05067127 ) in immune complex glomerulonephritis. IgA nephropathy, in particular, is an area of high interest, as recent work suggests that disease activity may be driven by the overproduction of galactose deficient IgA antibodies that are recognized as autoantigens, triggering glomerular deposition of immune complexes. 171 Monoclonal antibodies to signaling molecules that enhance IgA production are in phase 3 trials, as are immunosuppressive and non-immunosuppressive agents (for example, those acting on the endothelin-1 and angiotensin II pathways): budesonide ( NCT03643965 ), sparsentan ( NCT03762850 ), atrasentan ( NCT04573478 ), LNP023 ( NCT04578834 ), RO7434656 ( NCT05797610 ), atacicept ( NCT04716231 ), and sibeprenlimab ( NCT05248646 ; NCT05248659 ).
Major guidelines in CKD are issued by the international KDIGO group ( https://kdigo.org/ ), and locally in the UK by NICE ( www.nice.org.uk/guidance/ng28/chapter/Recommendations#chronic-kidney-disease ), with the most recent issuances primarily from 2023 (currently in public review) and 2021, respectively. KDIGO publishes guidelines on the evaluation and management of patients with CKD in general, as well as myriad other aspects (for example, diabetes, blood pressure, lipids, anemia, mineral and bone disease, hepatitis C, ADPKD, glomerular diseases). With the expansion of therapeutic options, both organizations are updating recommendations frequently. Other guideline producing organizations such as the American College of Cardiology, the American Heart Association, the European Society of Cardiology, the European Society of Hypertension, the International Society of Hypertension, and the American Diabetes Association (ADA) provide more limited statements of recommendation for the specific aspects of the management of patients with CKD. 172 173 174 175
Annual screening for CKD (including testing for albuminuria) is widely recommended in people with diabetes. 72 174 175 176 177 Guidelines in hypertension are less clear. 178 The 2020 Global Hypertension Practice Guideline from the International Society of Hypertension is a notable exception and now recommends routine assessment of albuminuria in addition to estimated GFR in people with hypertension. 173 KDIGO and NICE also recommend testing anyone who is at risk for CKD, which includes those with hypertension, cardiovascular disease, diabetes, and previous acute kidney injury, along with multiple other, less common conditions. 179 For CKD, the KDIGO guidelines recommend at least annual albuminuria testing with greater frequency in higher risk categories ( fig 1 ). 2 The NICE guidelines, on the other hand, recommend annual ACR testing with individualization based on clinical characteristics, risk of progression, and whether a change in ACR would lead to a change in management. 16
KDIGO guidelines and those from NICE differ slightly on staging CKD. KDIGO recommends using a validated equation for GFR estimation and suggests that using “race as a distinct variable in the computation of GFR” is not appropriate. 179 NICE recommends using the CKD-EPI 2009 equation, which did include race, but using the computed value for non-Black people for everyone, a position that is also endorsed by other European groups. 16 180 181 The KDIGO guidelines recommend staging CKD by eGFRcr-cys when cystatin C is available, as well as when precise estimates of GFR are needed for clinical decision making. 2 179 The NICE guidelines recommend direct measurement of GFR rather than the use of cystatin C in clinical situations requiring additional precision. 16
Both KDIGO and NICE emphasize the importance of risk assessment in patients with CKD. The NICE guidelines suggest that primary care providers should counsel patients using the KFRE five year risk estimate, with referral to a specialist if risk is greater than 5%. 16 KDIGO 2023 additionally suggests that the two year risk estimate can drive referral for multidisciplinary care (>10%) and preparation for kidney replacement therapy, including vascular access planning and referral for transplantation (>40%). 179 The KDIGO 2023 guidelines also emphasize the importance of cardiovascular risk assessment using equations developed in people with CKD or that encompasses estimated GFR and albuminuria and the use of disease specific tools in IgA nephropathy and ADPKD. 179
Multiple guidelines comment on target blood pressures in the setting of CKD. The NICE guidelines recommend a target of <140/90 mm Hg, or <130/80 mm Hg if ACR is ≥70 mg/mmol (approximately 700 mg/g). 16 Guidelines from the American College of Cardiology, American Heart Association, European Society of Cardiology, and European Society of Hypertension recommend a systolic blood pressure target of <130 mm Hg as a best practice target, with the European Society of Cardiology and European Society of Hypertension specifically advising against lower targets. 172 The KDIGO guidelines on hypertension in CKD advocate for a systolic blood pressure goal of <120 mm Hg, as assessed using standardized office measurements. 182 This recommendation is based largely on data from SPRINT and the observed benefits in cardiovascular endpoints and survival rather than benefits in kidney endpoints. 70
Of note, disparate guideline recommendations may reflect different emphasis on standardized blood pressure measurement techniques, which can result in measured blood pressure that is substantially lower than measurement in an uncontrolled setting. 183 Joint statements from several international groups including KDIGO stress the importance of proper technique when assessing blood pressure. 184 Both NICE and KDIGO recommend RAAS inhibitors (either ACE inhibitor or ARB) as first line antihypertensive treatment for people without diabetes but with albuminuria (NICE: urine ACR >70 mg/mmol; KDIGO: A3) as well as those with diabetes and CKD G1-G4, A2-A3. 16 182 KDIGO 2023 suggests continuation of RAAS inhibitors even when estimated GFR is <30 mL/min/1.73 m 2 . 179
For patients with diabetes and CKD not treated with dialysis, KDIGO recommends a hemoglobin A 1c target ranging from <6.5% to <8%. 72 NICE does not provide specific recommendations for people with CKD, instead emphasizing shared decision making but a general goal of hemoglobin A 1c <7% for people with diabetes treated with drugs associated with hypoglycemia and <6.5% for people with diabetes managed by lifestyle or a single drug not associated with hypoglycemia. 185
KDIGO and ADA guidelines recommend SGLT-2 inhibitors as first line drug therapy for all people with type 2 diabetes, CKD, and an estimated GFR ≥20 mL/min/1.73 m 2 ( fig 5 ). 72 174 175 179 The NICE guidelines recommend that an SGLT-2 inhibitor should be offered when ACR is >30 mg/mmol (approximately >300 mg/g) and considered when ACR is between 3 and 30 mg/mmol (approximately 30 to 300 mg/g) in patients with type 2 diabetes and CKD who are already taking an ACE inhibitor or ARB and meet estimated GFR thresholds. 185 The NICE guidelines further specify that dapagliflozin should also be considered in people with estimated GFR 25-75 mL/min/1.73 m 2 and ACR ≥22.6 mg/mmol (approximately 200 mg/g) regardless of diabetes status 186 ; KDIGO is broader and recommends SGLT-2 inhibitors in general in people with ACR ≥200 mg/g and estimated GFR ≥20 mL/min/1.73 m 2 , as well as in those with CKD and heart failure. 179 KDIGO further specifies that once started, a SGLT-2 inhibitor can be continued even if the estimated GFR drops below 20 mL/min/1.73 m 2 , as long as it is tolerated and kidney replacement therapy has not yet been started. 72 179 The KDIGO and ADA guidelines recommend the use of GLP-1 receptor agonists in patients with type 2 diabetes and CKD who are unable to tolerate metformin or an SGLT-2 inhibitor or do not meet their individualized glycemic target with these drugs. 72 174 175 179

Kidney Disease: Improving Global Outcomes/American Diabetes Association recommendations on the management of diabetes in populations with chronic kidney disease. 72 174 ACR=albumin-to-creatinine ratio; ASCVD=atherosclerotic cardiovascular disease; BP=blood pressure; CCB=calcium channel blocker; CVD=cardiovascular disease; eGFR=estimated glomerular filtration rate; GLP-1 RA=glucagon-like peptide-1 receptor agonist; HTN=hypertension; MRA=mineralocorticoid receptor antagonist; PCSK9i=proprotein convertase subtilisin/kexin type 9 inhibitor; RAS=renin-angiotensin system; SGLT2i=sodium-glucose cotransporter-2 inhibitor
In patients with diabetes and CKD, the KDIGO and ADA guidelines recommend that finerenone should be used as add-on therapy to maximally tolerated ACE inhibitor or ARB if ACR is ≥30 mg/g (approximately ≥3 mg/mmol) and potassium is within normal limits (that is, ≤4.8 mmol/L based on trial and ≤5.0 mmol/L as per FDA). 72 174 175 179 More specifically, the starting dose should be 10 mg daily when estimated GFR is 25-59 mL/min/1.73 m 2 and 20 mg daily when it is ≥60 mL/min/1.73 m 2 . The guidelines also recommend that potassium concentration should be checked at four weeks after starting treatment, with each dose change, and routinely during treatment. If potassium is >5.5 mmol/L, the drug should be stopped and restarted at the lower dose of 10 mg daily when potassium is ≤5.0 mmol/L. Additionally, finerenone need not be stopped when estimated GFR falls below 25 mL/min/1.73 m 2 as long as the patient is normokalemic. 174 175
With respect to cardiovascular risk reduction, the KDIGO guidelines suggest that all patients aged over 50 with CKD G3-G5 but not treated with chronic dialysis or kidney transplantation should be treated with a statin, irrespective of cholesterol concentrations or a statin/ezetimide combination. 179 187 The NICE recommendation is broader, recommending starting atorvastatin 20 mg for all people with CKD. 188 KDIGO recommends regular physical activity for people with CKD, for at least 150 minutes a week of moderate intensity exercise. 179 NICE simply suggests providing lifestyle advice, including encouragement of exercise, maintenance of healthy weight, and smoking cessation, and specifically recommends against offering low protein diets (defined as dietary protein intake <0.8 g/kg/day). 16 KDIGO recommends maintaining sodium intake <2 g/day and a protein intake of 0.8 g/kg/day but no higher than 1.3 g/kg/day. 179
People with CKD face high risks of many adverse outcomes, including requirement for kidney replacement therapy, cardiovascular events, and death. Fortunately, major advances have been made in the field of CKD over the past decade. Estimating equations for GFR and ACR have evolved for more precise classification of disease. Individualized risk prediction tools exist to assist in the counseling, referral, and treatment of patients. Novel therapies build on the fundamentals—a healthy lifestyle, blood pressure and glucose control, and statin therapy and RAAS blockade—to provide effective preventive strategies for CKD progression and cardiovascular events.
Glossary of abbreviations
ACE—angiotensin converting enzyme
ACR—albumin-to-creatinine ratio
ADA—American Diabetes Association
ADPKD—autosomal dominant polycystic kidney disease
ARB—angiotensin receptor blockers
CKD—chronic kidney disease
CKD-EPI—CKD Epidemiology Collaboration
DPP-4—dipeptidyl peptidase-4
eGFRcr—estimated glomerular filtration rate using creatinine
eGFRcr-cys—estimated glomerular filtration rate using creatinine and cystatin C
eGFRcys—estimated glomerular filtration rate using cystatin C
ESKD—end stage kidney disease
FDA—Food and Drug Administration
FSGS—focal segmental glomerulosclerosis
GFR—glomerular filtration rate
GLP-1—glucagon-like peptide-1
KDIGO—Kidney Disease: Improving Global Outcomes
KFRE—kidney failure risk equation
KFRT—kidney failure with replacement therapy
LDL—low density lipoprotein
MDRD—Modification of Diet in Renal Disease
MRA—mineralocorticoid receptor antagonists
NICE—National Institute for Health and Care Excellence
NSAID—non-steroidal anti-inflammatory drug
PCR—protein-to-creatinine ratio
RAAS—renin-angiotensin-aldosterone system
SGLT-2—sodium-glucose cotransporter-2
Questions for future research
How do the race-free estimating equations perform in global populations?
Where can genetic testing add value in patient care?
Can cause of chronic kidney disease be incorporated into risk prediction tools?
How can medical therapy be best tailored for the individual patient with chronic kidney disease?
Patient perspective
Increasing awareness of chronic kidney disease is key to empowering patients to make lifestyle changes and seek treatments to improve their health outcomes. We are pleased to offer our perspective as husband and wife, and as physicians, who have been affected by kidney disease. Roberta M Falke is a patient with autosomal dominant polycystic kidney disease (ADPKD), a kidney transplant recipient, and a retired hematologist-oncologist. Andrew S Levey is a kidney donor and a nephrologist. Our knowledge of Roberta’s family history enabled early diagnosis and treatment. 189 Although we have benefited from our training and positions in the healthcare system, all patients can benefit from early diagnosis.
RMF —My ADPKD was diagnosed when I developed pyelonephritis at age 22 years. Thereafter, I had prophylaxis and prompt treatment of recurrent urinary tract infections and, as the disease progressed, complications of kidney and liver cysts, hypertension, hyperparathyroidism, vitamin D deficiency, acidosis, hyperkalemia, and ultimately kidney failure, with fatigue, dietary restrictions, and a long list of medications to take every day. I had always known that living donor kidney transplantation would be the best treatment for my kidney failure. Over time, family members without ADPKD donated to others, and when I was ready at age 60 years no family members were available. Fortunately, Andy stepped up. I felt better immediately after the transplant, and in the 13 years since then I have continued to take medications daily but have had few complications. I am grateful to all those who have cared for me for many years and enabled me to make the best choices I could to help myself, and I’m especially grateful to Andy who gave me the gift of life.
ASL —I knew that Roberta would develop kidney failure and hoped that a living kidney donor would be available for her. I wanted to donate, but our blood group incompatibility was an obstacle, so it was exciting when paired donor exchange was conceived and implemented in our region. I believe that kidney donors benefit from donation, not only by fulfilling their spirit of altruism but by improving their own lives. In my case, donating has been life changing. Roberta and I have been able to have an active, fulfilling life for more than a decade after the transplant, without the demands and complications of kidney failure or dialysis. I hope that we will have many more years together. I am also grateful to all those who enabled me to achieve my goal and to Roberta, who always takes full responsibility for caring for her kidney disease.
Acknowledgments
We thank Andrew S Levey and Roberta M Falke for providing both their perspective as patients affected by kidney disease and their input on the manuscript itself. We also acknowledge Alix Rosenberg and Yingying Sang for their help with the boxes and figures.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: All authors were involved in the conception, writing, and revision of the manuscript. MEG is the guarantor.
Funding: TKC is supported by NIH/NIDDK K08DK117068; MEG is supported by NIH/NIDDK R01DK108803, R01DK100446, R01DK115534, R01DK124399, and NIH/NHLBI K24HL155861.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: TKC and MEG received an honorarium from the American Society of Nephrology (nephSAP).
Patient involvement: We invited a husband and wife, Andrew S Levey and Roberta M Falke, who are affected by chronic kidney disease, to write a patient perspective together. They also reviewed and provided input on the penultimate draft of the paper.
Provenance and peer review: Commissioned; externally peer reviewed.
- GBD Chronic Kidney Disease Collaboration
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group
- Foreman KJ ,
- Marquez N ,
- Dolgert A ,
- Kovesdy C ,
- Langham R ,
- Rosenberg M ,
- ↵ United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. 2022. https://adr.usrds.org/2022 .
- Garcia-Garcia G ,
- Friedman DJ ,
- Derebail VK ,
- Genovese G ,
- Johnson RJ ,
- Wesseling C ,
- Johnson DW ,
- O’Shaughnessy MM ,
- CKD Prognosis Consortium
- Shlipak MG ,
- ↵ National Institute for Health and Care Excellence. NICE guideline [NG203]: Chronic kidney disease: assessment and management. 2021. www.nice.org.uk/guidance/ng203 .
- Nadkarni GN ,
- Chronic Kidney Disease Prognosis Consortium
- Weaver RG ,
- Modification of Diet in Renal Disease Study Group
- Chronic Kidney Disease Epidemiology Collaboration
- Stevens LA ,
- Schmid CH ,
- CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration)
- Delgado C ,
- Eneanya ND ,
- ↵ Chen DC, Potok OA, Rifkin D, Estrella MM. Advantages, Limitations, and Clinical Considerations in Using Cystatin C to Estimate GFR. Kidney360 2022;3:1807-1814.
- Khandpur S ,
- Awasthi A ,
- Behera MR ,
- Nestor JG ,
- Groopman EE ,
- Cameron-Christie S ,
- Antignac C ,
- Bergmann C ,
- Sexton DJ ,
- Collins AJ ,
- Torres VE ,
- Chapman AB ,
- Devuyst O ,
- TEMPO 3:4 Trial Investigators
- Müller RU ,
- Messchendorp AL ,
- Irazabal MV ,
- Rangel LJ ,
- Bergstralh EJ ,
- CRISP Investigators
- ↵ QxMD. Total Kidney Volume (height-adjusted) Calculator & ADPKD Prognostic Tool using Kidney Dimensions calculator. https://qxmd.com/calculate/calculator_490/total-kidney-volume-height-adjusted-calculator-adpkd-prognostic-tool-using-kidney-dimensions .
- Rodrigues JC ,
- ↵ QxMD. International IgAN Prediction Tool at biopsy - adults. https://qxmd.com/calculate/calculator_499/international-igan-prediction-tool-at-biopsy-adults .
- Barbour SJ ,
- International IgA Nephropathy Network
- Selvaskandan H ,
- Gonzalez-Martin G ,
- Barratt J ,
- Foster MC ,
- Fornage M ,
- AASK Study Investigators ,
- CRIC Study Investigators
- Zimmerman B ,
- VX19-147-101 Study Group
- Houssiau F ,
- Garrelfs SF ,
- Frishberg Y ,
- Hulton SA ,
- ILLUMINATE-A Collaborators
- Griffith J ,
- Ferguson TW ,
- Fallahzadeh MK ,
- McCulloch CE ,
- Brunskill NJ ,
- Ballew SH ,
- Nguyen HA ,
- Abdelmalek JA ,
- Woodell TB ,
- Greene TH ,
- Sparkes D ,
- Harasemiw O ,
- Thorsteinsdottir B ,
- Matsushita K ,
- Kaptoge S ,
- Hageman SHJ ,
- Jassal SK ,
- Scherzer R ,
- Farrington DK ,
- Surapaneni A ,
- Seegmiller JC ,
- Carrero JJ ,
- Wright JT Jr . ,
- African American Study of Kidney Disease and Hypertension Study Group
- Williamson JD ,
- Whelton PK ,
- SPRINT Research Group
- Agarwal R ,
- Cramer AE ,
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group
- MacMahon S ,
- Chalmers J ,
- ADVANCE Collaborative Group
- Perkovic V ,
- Heerspink HL ,
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia)
- Brenner BM ,
- Cooper ME ,
- de Zeeuw D ,
- RENAAL Study Investigators
- Hunsicker LG ,
- Clarke WR ,
- Collaborative Study Group
- Sleight P ,
- Dagenais G ,
- Heart Outcomes Prevention Evaluation Study Investigators
- ONTARGET Investigators
- Emanuele N ,
- VA NEPHRON-D Investigators
- Bhandari S ,
- STOP ACEi Trial Investigators
- Mahaffey KW ,
- CANVAS Program Collaborative Group
- Jardine MJ ,
- CREDENCE Trial Investigators
- Heerspink HJL ,
- Stefánsson BV ,
- Correa-Rotter R ,
- DAPA-CKD Trial Committees and Investigators
- Wheeler DC ,
- Stefansson BV ,
- Batiushin M ,
- Herrington WG ,
- Staplin N ,
- The EMPA-KIDNEY Collaborative Group
- Vaduganathan M ,
- DeFronzo RA ,
- Reeves WB ,
- Tuttle KR ,
- Nangaku M ,
- Schneeweiss S ,
- Fralick M ,
- Muskiet MHA ,
- Tonneijck L ,
- Pfeffer MA ,
- Claggett B ,
- ELIXA Investigators
- Kristensen SL ,
- Bentley-Lewis R ,
- Aguilar D ,
- Riddle MC ,
- Ørsted DD ,
- Brown-Frandsen K ,
- LEADER Steering Committee and Investigators
- Daniels GH ,
- LEADER Steering Committee ,
- LEADER Trial Investigators
- Consoli A ,
- SUSTAIN-6 Investigators
- Holman RR ,
- Bethel MA ,
- EXSCEL Study Group
- Merrill P ,
- Gerstein HC ,
- Colhoun HM ,
- Dagenais GR ,
- REWIND Investigators
- REWIND Trial Investigators
- Rosenstock J ,
- AMPLITUDE-O Trial Investigators
- Lakshmanan MC ,
- Dieter BP ,
- Alicic RZ ,
- O’Neil PM ,
- Birkenfeld AL ,
- McGowan B ,
- Pratley R ,
- PIONEER 4 investigators
- Navaneethan SD ,
- Nigwekar SU ,
- Sehgal AR ,
- Strippoli GF
- Epstein M ,
- Kovesdy CP ,
- Pecoits-Filho R
- Bakris GL ,
- FIDELIO-DKD Investigators
- Barrera-Chimal J ,
- Lima-Posada I ,
- Filippatos G ,
- FIGARO-DKD Investigators
- FIDELIO-DKD and FIGARO-DKD investigators
- Parving HH ,
- Andress DL ,
- SONAR Committees and Investigators
- Trachtman H ,
- DUET Study Group
- Radhakrishnan J ,
- Alpers CE ,
- PROTECT Investigators
- ↵ US Food and Drug Administration. Highlights of prescribing information: Filspari. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216403s000lbl.pdf .
- Kalyesubula R ,
- Schaeffner E ,
- Ishigami J ,
- Trevisan M ,
- Grundy SM ,
- Bailey AL ,
- Baigent C ,
- Landray MJ ,
- SHARP Investigators
- Lloyd-Jones DM ,
- Morris PB ,
- Ballantyne CM ,
- Writing Committee
- Charytan DM ,
- Sabatine MS ,
- Pedersen TR ,
- FOURIER Steering Committee and Investigators
- Weiner DE ,
- Uchiyama K ,
- Muraoka K ,
- Beetham KS ,
- Krishnasamy R ,
- Stanton T ,
- Howden EJ ,
- Coombes JS ,
- Douglas B ,
- Campbell KL ,
- Ikizler TA ,
- Robinson-Cohen C ,
- Look AHEAD Research Group
- Sadeghirad B ,
- Hostetter TH
- Caulfield LE ,
- Garcia-Larsen V ,
- Bushinsky DA
- Wesson DE ,
- Mathur VS ,
- ↵ Tangri N, Mathur VS, Bushinsky DA, Inker LA, et al. VALOR-CKD: A Multicenter, Randomized, Double-Blind Placebo-Controlled Trial Evaluating Veverimer in Slowing Progression of CKD in Patients With Metabolic Acidosis. 2022, Florida. https://www.asn-online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3801835 .
- Aburto NJ ,
- Gutierrez H ,
- Elliott P ,
- Cappuccio FP
- Narasaki Y ,
- O’Donnell M ,
- Rangarajan S ,
- PURE Investigators
- Juraschek SP ,
- Miller ER 3rd . ,
- Fioretto P ,
- Anderson CAM ,
- Whittaker CF ,
- Miklich MA ,
- Matzke GR ,
- Aronoff GR ,
- Atkinson AJ Jr . ,
- ↵ US Food and Drug Administration, Center for Drug Evaluation and Research. Pharmacokinetics in patients with impaired renal function - study design, data analysis, and impact on dosing. 2020. https://www.fda.gov/media/78573/download .
- Vondracek SF ,
- Teitelbaum I ,
- Hudson JQ ,
- Perazella MA
- Azoulay L ,
- Nessim SJ ,
- Doerfler RM ,
- Lazarus B ,
- Wilson FP ,
- Balasubramanian S ,
- Pattrapornpisut P ,
- Avila-Casado C ,
- Charchar F ,
- de Boer IH ,
- Sadusky T ,
- Tummalapalli SL ,
- Boulware LE ,
- Conference Participants
- American Diabetes Association Professional Practice Committee
- ↵ Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2023 Clinical Practice Guideline For the Evaluation and Management of Chronic Kidney Disease - Public Review Draft July 2023. 2023. https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2023-CKD-Guideline-Public-Review-Draft_5-July-2023.pdf .
- Gansevoort RT ,
- Anders HJ ,
- Cozzolino M ,
- Delanaye P ,
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group
- Muntner P ,
- Einhorn PT ,
- Cushman WC ,
- 2017 National Heart, Lung, and Blood Institute Working Group
- Cheung AK ,
- ↵ National Institute for Health and Care Excellence. NICE guideline [NG28]: Type 2 diabetes in adults: management. 2022. https://www.nice.org.uk/guidance/ng28/chapter/Recommendations#chronic-kidney-disease .
- ↵ National Institute for Health and Care Excellence. Technology appraisal guidance [TA775]: Dapagliflozin for treating chronic kidney disease. 2022. https://www.nice.org.uk/guidance/ta775/chapter/1-Recommendations .
- Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group
- ↵ National Institute for Health and Care Excellence. NICE guideline [CG181]: Cardiovascular disease: risk assessment and reduction, including lipid modification. 2023. https://www.nice.org.uk/guidance/cg181/chapter/Recommendations#lipid-modification-therapy-for-the-primary-and-secondary-prevention-of-cardiovascular-disease .
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Systemic immune-inflammatory indicators and bone mineral density in chronic kidney disease patients: A cross-sectional research from NHANES 2011 to 2018
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft
Affiliation Department of Nephrology, Jinshan Hospital, Fudan University, Shanghai, China
Roles Methodology, Project administration, Validation, Writing – review & editing
* E-mail: [email protected]
- Yuying Jiang,
- Xiaorong Bao

- Published: April 25, 2024
- https://doi.org/10.1371/journal.pone.0302073
- Peer Review
- Reader Comments
The purpose of this study was to look at the relationship between the Systemic Immune Inflammatory Index (SII) and bone mineral density (BMD) in the pelvis, left upper and lower limbs, lumbar spine, thoracic spine, and trunk in a chronic kidney disease (CKD) population in the United States.
The National Health and Nutrition Examination Survey (2011–2016) yielded 2302 people with CKD aged >18 years. CKD was defined as eGFR less than 90 ml/min/1.73 m 2 or eGFR greater than 90 ml/min/1.73 m 2 with urine ACR greater than 30 mg/L.SII was calculated as PC * (NC / LC) from platelet count (PC), neutrophil count (NC), and lymphocyte count (LC). Multiple logistic regression was used to examine the relationship between BMD and SII at different sites in CKD patients, smoothed curve-fitting and generalized weighting models were used to investigate non-linear relationships, and a two-tailed linear regression model was used to find potential inflection points in the model.
We discovered a negative correlation between SII and pelvic BMD among 2302 participants after controlling for gender, age, and race [β = -0.008; 95% confidence value -0.008; 95% confidence interval (CI) -0.014, -0.002]. Lower PEBMD was related to increasing SII (trend p = 0.01125). After additional correction, only pelvic BMD remained adversely linked with SII [value -0.006; 95% CI -0.012, -0.000, p = 0.03368]. Smoothed curve fitting revealed a consistent inverse relationship between SII and pelvic BMD. Further stratified analyses revealed a substantial positive negative connection between SII and pelvic BMD in individuals who did not have hypertension, diabetes, a BMI of more than 30 kg/m 2 , or stage 2 CKD. The connection between SII and PEBMD in people without diabetes revealed a strong inverted U-shaped curve.
In individuals with CKD in the United States, there was a negative connection between the systemic immunoinflammatory index (SII) and pelvic BMD. The SII might be a low-cost and simple test for CKD-related BMD loss.
Citation: Jiang Y, Bao X (2024) Systemic immune-inflammatory indicators and bone mineral density in chronic kidney disease patients: A cross-sectional research from NHANES 2011 to 2018. PLoS ONE 19(4): e0302073. https://doi.org/10.1371/journal.pone.0302073
Editor: Ewa Tomaszewska, University of Life Sciences in Lublin, POLAND
Received: January 29, 2024; Accepted: March 27, 2024; Published: April 25, 2024
Copyright: © 2024 Jiang, Bao. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Publicly available datasets were analyzed for this study from the NHANES database ( www.cdc.gov/nchs/nhanes/ ).
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
More than 10% of the world’s population and more than 80 billion people are affected by chronic kidney disease (CKD), one of the world’s leading non-communicable causes of death [ 1 ]. By 2024, it is expected to rank as the fifth leading cause of life expectancy loss globally [ 2 ]. Patients with CKD frequently develop mineral bone disorders, such as osteoporosis and renal osteodystrophy, which worsen with deteriorating renal function, which is extremely common and harmful [ 3 ]. The 2017 KDIGO recommendations state that when evaluating the diagnostic and treatment requirements for osteoporosis in individuals with CKD, testing BMD may be preferred to doing a bone biopsy [ 4 ]. Furthermore, a 2022 meta-analysis showed that lower BMD values were associated with an increased risk of all-cause mortality in patients with CKD [ 5 ]. In patients with CKD, the clinical utility of BMD measurements for assessing bone loss and other related health conditions remains worth investigating due to the presence of various hormonal and metabolic alterations [ 6 ].
In fact, ongoing low-grade inflammation and immunological dysfunction are now recognized as distinguishing characteristics of CKD and are linked to patient death [ 7 ]. Numerous investigations have demonstrated that inflammation worsens renal function. White blood cell count, interleukin-6, his-CRP, and tumor necrosis factor-alpha receptor were found to have a favorable correlation with the outcome of CKD in a cross-sectional investigation by Shankar et al. [ 8 ]. Inflammation may be a sign of a poor prognosis in CKD patients, according to cohort research by Amdur RL et al. [ 9 ].
Due to their shared developmental ecological niche, the immune system and bone currently function as a tightly coupled functional unit (the bone immune system), with numerous anatomical and vascular sites of ongoing interaction between the two [ 10 ]. The role of the immune system in various skeletal pathologies is currently well established. Through direct or indirect influences on the physiological functions of bone cells, immune cells can eventually alter bone density [ 11 , 12 ]. Some indices reflecting systemic immune and inflammatory status, such as the sex granulocyte-lymphocyte ratio [ 13 ], derived from immune cell counts, have also been correlated with BMD changes [ 14 ]. To better monitor the health of CKD patients, researchers are looking for novel indices based on immune cell counts to evaluate the risk of bone loss in patients.
A novel index that uses platelet, neutrophil, and lymphocyte counts called the Systemic Immune Inflammation Index (SII) can be used to measure the level of systemic inflammation [ 15 , 16 ]. Growing data suggests that it gives insight into the body’s overall immunological and inflammatory status and may be used to forecast risk and evaluate prognosis in conditions such as tumors, coronary artery disease, and bone loss [ 17 – 20 ]. Additionally, Qin et al. noted that higher SII was linked to a higher incidence of albuminuria in adults [ 21 ]. The association between BMD and SII in CKD patients is still unclear due to the small number of studies, and further research is required to determine the function of SII in the problems of bone loss in CKD patients.
The purpose of this study was to evaluate the association between SII and BMD in CKD patients and to assess the correlation between SII and the risk of bone loss/osteoporosis in CKD patients based on the theoretical backdrop discussed above. We hypothesized that increasing SII is linked to a higher risk of osteoporosis and that SII is inversely correlated with bone mineral density (BMD).
Materials and methods
Study subjects.
The National Health and Nutrition Examination Survey (NHANES), which is based on a cross-sectional cohort study and intended to evaluate the nutritional and health status of the general population in the United States (US), served as the source for all subject data. Every two years, NHANES is updated and is associated with the US Centers for Disease Control and Prevention. Data from the NHANES 2011–2016 (2011–2012, 2013–2014, and 2015–2016) were retrieved. The authors do not have access to information that could identify individual participants during data collection.
Out of the 29,903 participants, we eliminated 5,324 people because their lymphocyte, neutrophil, and platelet counts were missing; 13,366 people because their blood creatinine, sex, race, and BMD measurements were missing; 8,541 people because they did not have CKD; 370 people because they were under 18; and 2,302 people because there were no pregnant people among the sample. The inclusion and exclusion details of the study population in this study are shown in Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0302073.g001
Ethics statement
The NHANES procedure was approved by the National Center for Health Statistics Research Ethics Review Board, and written informed permission was acquired. The NHANES data were made publicly accessible after being anonymized. It allowed scholars to transform the data into a format that could be learned. To make sure that data were only utilized for statistical analyses and that all experiments were carried out in compliance with the relevant standards and laws, we agreed to abide by the study’s data usage rules.
SII calculations
Using automated hematology analytical tools, the lymphocyte, neutrophil, and platelet counts (expressed as 103 cells/L) were calculated. On the NHANES website, laboratory procedures for full blood count testing are described. Based on recent research [ 15 ], SII was calculated as PC * (NC / LC) for the platelet count (PC), neutrophil count (NC), and lymphocyte count (LC).
Diagnosis of CKD
eGFR 90 ml/min/1.73 m 2 or eGFR >90 ml/min/1.73 m 2 and urine ACR >30 mg/L were both considered signs of CKD [ 22 ]. Both urinary ACR and serum creatinine were measured. Serum creatinine, ethnicity, and gender were used to compute the estimated glomerular filtration rate (eGFR) using the CKD-EPI equation [ 23 ]. The Kidney Disease Guidelines for Improving Global Outcomes [ 24 ] were used to identify the stage of CKD.
Acquisition of bone density
In actuality, no one location or method can completely satisfy the therapeutic demands for bone mineral assessments. The evaluation of osteoporosis can be impacted by biological variations in bone composition that exist between populations and locations, as well as by technological inaccuracies in the accuracy of various measures [ 25 ]. The most used bone densitometry technology, DXA, is used to measure the mineral content of bone at several places across the skeleton, particularly those that are most likely to fracture [ 26 ]. Hip fractures are a serious consequence of osteoporosis in clinical terms [ 27 ]. The World Health Organization defines osteoporosis as having a femoral neck DXA score that is below normal [ 25 ]. The majority of bone loss in CKD patients comes from the cortical bone [ 28 ], and hip fracture risk is higher in ESRD and milder stages of CKD [ 29 ]. The frequency of poor BMD, however, varied from 50 to 80 percent in the radius, 16 to 47 percent in the femoral neck, and 13 to 29 percent in the lumbar spine in many investigations on hemodialysis patients [ 30 ]. In order to better link BMD with inflammation at various places, we included BMD of the pelvis, left upper and lower limbs, and lumbar spine in the current investigation. We also included BMD of the thoracic spine and trunk in the study.
A qualified radiographer used a Hologic QDR-4500A fan-beam densitometer (Hologic; Bedford, MA, USA) to perform dual-energy X-ray absorptiometry (DXA) exams on all participants, including those who were included in the final analyses. Using Hologic APEX software (version 4.0), all DXA examination data were analyzed. The NHANES website offers further details.
This study additionally included covariates in the analysis to account for the possible impact of other variables on bone metabolism. Age, race, body mass index (BMI), smoking, hypertension, diabetes mellitus, blood alkaline phosphatase, serum uric acid, blood calcium, blood phosphorus, blood vitamin D, and blood triglycerides were eventually added as covariates based on other research [ 31 , 32 ]. In this instance, the final blood pressure reading was calculated as the average of the body measures, and hypertension was defined as a mean systolic blood pressure of greater than 140 mmHg and/or a mean diastolic blood pressure of greater than 90 mmHg. Diabetes mellitus was deemed to exist when the hemoglobin A1c (HbA1c) level reached 6.5%. How these variables were estimated is fully described on the NHANES website ( https://www.cdc.gov/nchs/nhanes/ ).
Analyses of statistics
With statistical significance set at P 0.05, we conducted all statistical analyses using R ( http://www.r-project.org ) and EmpowerStats ( http://www.empowerstats.com ). Model 1 included no covariate adjustments, Model 2 had ethnicity, age, and gender adjustments, and Model 3 included all of the covariate adjustments from Table 1 . Additionally, subgroup analyses were performed. To deal with nonlinearities, the Generalised Additive Model (GAM) and smooth curve fitting were employed.
https://doi.org/10.1371/journal.pone.0302073.t001
Baseline characteristics of the SII-strategized population
2,302 people were examined in this study. Depending on their SII levels, the research sample was evenly split into Qs (Q1-Q4). Table 1 displays weighted demographic and medical information. The study had 2,302 adult participants in total. The Qs were statistically different (p < 0.05) for urinary albumin, blood alkaline phosphatase, blood phosphorus, blood uric acid, blood creatinine, blood cholesterol, blood urea nitrogen, glomerular filtration rate, bone density in the left arm, bone density in the left leg, bone density in the thoracic vertebrae, sex, ethnicity, whether or not they were smokers, BMI, and diabetes mellitus, whereas the age of There were no statistically significant differences in comparisons of total calcium, blood vitamin D, urinary creatinine, urinary albumin-creatinine ratio, lumbar spine bone density, pelvic bone density, trunk bone density, and hypertension. Additionally, we noticed that patients in the first quartile of SII had greater blood cholesterol and glomerular filtration rates compared to other subgroups, but lower blood creatinine and blood urea nitrogen.
The GFR values for the CKD patients in this study were computed using the CKD-EPI algorithm, and the staging of the participating patients was done in accordance with the KDIGO recommendations: In terms of lumbar spine bone mineral density and current smoking status across CKD subgroups, there was no statistically significant difference between individuals with different CKD stages (p> 0.05). The sample was mostly focused on CKD stage 2, and SII tended to rise as CKD advanced, despite statistically significant differences between the remaining variables. The result is shown in Table 2 .
https://doi.org/10.1371/journal.pone.0302073.t002
Relationship between SII and BMD in CKD patients
Table 3 displays the outcomes of the multivariate regression analysis. We initially created an unadjusted model to investigate the relationship between SII and BMD at various locations. We discovered that the SII percentile was significantly linked with BMD in the left arm alone (0.006, 95% CI: 0.002, 0.009, p = 0.00370). However, after controlling for sex, age, and race in adjusted model I, SII was associated with pelvic BMD, suggesting that a higher SII percentile was associated with lower odds of pelvic BMD. This association also suggested that the higher SII percentile was associated with a trend towards decreasing pelvic BMD as the SII percentile increased (trend p = 0.01125). Age, sex, race, blood alkaline phosphatase, blood urea nitrogen, blood calcium, blood triglycerides, blood phosphorus, blood creatinine, blood uric acid, total active vitamin D, 2,5-hydroxyvitamin D2, presence of hypertension, diabetes mellitus, BMI, and calculated GFR were all taken into account in the adjusted Model II. Further analyses for pelvic BMD and SII were then carried out, and only pelvic BMD remained substantially linked with SII.
https://doi.org/10.1371/journal.pone.0302073.t003
Fig 2 displays the smoothed curve fits and scatter plots. Model III was used to fit a smoothed curve to represent the nonlinear connection between SII and PEBMD. There were no statistically significant optimum breakpoints identified using a two-stage linear regression model. The detailed results of the threshold effect analysis are presented in Table 4 . Overall, SII and PEBMD have a negative correlation, as can be observed.
(A) Each black dot represents a sample. (B) The solid red line represents a smooth curve fit between the variables. Blue bands indicate 95% confidence intervals of the fit. SII, systemic immunoinflammatory index; PEBMD, pelvic bone mineral density.
https://doi.org/10.1371/journal.pone.0302073.g002
https://doi.org/10.1371/journal.pone.0302073.t004
SII and pelvic bone density in CKD patients: Stratified analysis and investigation of the threshold effect
In order to analyze changes in pelvic BMD caused by SII in patients with CKD, the sample was stratified using the previously reported adjusted model. Because there weren’t enough stage 5 patients with CKD, stage 4 and stage 5 patients were analyzed together:
Our findings indicated that the negative connection between SII and pelvic BMD was independently and significantly positive in men [0.0008 (-0.023, -0.006)] but not statistically significant in the female models when subgroup analyses stratified by sex were performed. Independent statistical significance for different racial groups was absent. Patients without diabetes, hypertension, or a BMI of more than 30 kg/m 2 demonstrated a substantial positive connection between SII and pelvic BMD rather than the expected negative correlation. Patients with CKD stage 2 showed a negative connection between SII and pelvic BMD when we grouped the patients based on CKD stage [0.0222 (-0.015, -0.001)]. The results are shown in Table 5 .
https://doi.org/10.1371/journal.pone.0302073.t005
Fig 3 displays the outcomes of further stratified analyses based on patient gender, the presence of diabetes, hypertension, and a BMI greater than 30 kg/m 2 , as well as smoothed curve fitting using model 3.
https://doi.org/10.1371/journal.pone.0302073.g003
The relationship between SII and PEBMD showed a significant inverted U-shaped curve with a fold point of 0.969 ((1,000 cells/l)) after stratifying the sample for patients who did not have diabetes mellitus, according to a threshold effect analysis using a two-stage linear regression model. The connection between SII and PEBMD likewise displayed an inverted U-shaped curve in individuals without and with hypertension, with fold points of 0.938 (1,000 cells/l) and 2.947 (1,000 cells/l), respectively. SII and PEBMD had a more complicated non-linear association in individuals with BMI more than 30 kg/m 2 , with no significant fold points. Tables 6 and 7 show the findings of the threshold effect investigation.
https://doi.org/10.1371/journal.pone.0302073.t006
https://doi.org/10.1371/journal.pone.0302073.t007
Some hints are given by the variations in the association between SII and PEBMD in the various subgroups, but more research is required to validate these correlations and dive further into the underlying processes.
Long recognized as a prognostic factor in a variety of illnesses, the immune system. According to recent findings from cross-sectional studies of the NHANES-III 2011–2016 database, SII levels in individuals with CKD correlate with pelvic BMD, which declines with rising SII.
To our knowledge, this is the first community-based, nationally representative cohort of people from the United States to evaluate the relationship between SII, a less expensive clinical measure, and reduced bone density, a typical consequence of CKD.
We discovered that SII levels were negatively correlated with pelvic BMD in patients with CKD by comparing regression models after unadjusted modeling, the addition of demographic variables, and the remaining pertinent variables. This finding corroborated the persistent finding that hip BMD can be used to predict fractures, as mentioned in the KDIGO guidelines [ 33 ]. Additionally, when examining the relationship between SII and PEBMD, the findings of stratified analyses revealed that men who were obese, male, and free of diabetes or hypertension had a stronger negative association between SII and PEBMD. These might serve as some benchmarks for further clinical choices.
According to a 2014 study by Hu [ 15 ] et al., SII is a thorough and unique inflammatory biomarker. Prior research has routinely employed SII as a predictor of the development of kidney transplant rejection as well as the incidence of acute or chronic renal injury [ 34 – 38 ]. Numerous studies connected to SII have been conducted in recent years, and they have thoroughly examined its clinical importance. Lai et al. [ 34 ] in a cohort study showed that elevated levels of SII prior to CAG were an important and independent risk factor for postoperative AKI, and Halpern et al. [ 39 ] in a single-center cohort study showed that elevated levels of SII were independently associated with increased survival only in post-transplant patients. For instance, Xie et al. [ 40 ] in 2022 found that elevated levels of SII were associated with hepatic steatosis but not with hepatic fibrosis. A cohort study was conducted by Halpern et al. in one center. Regarding prognosis, prospective cohort research by Shi [ 31 ] et al. showed that in CKD patients with ACS, higher SII was linked to poor cardiovascular outcomes. In a similar vein, Xie et al. [ 41 ] discovered a favorable correlation between SII and abdominal aortic calcification, a frequent CKD consequence. In a cohort investigation of critically ill AKI patients, Lan et al. [ 42 ] discovered a J-shaped relationship between SII and all-cause death in these patients.
Studying the onset of CKD’s consequences, however, can be more successful in enhancing the quality of patients’ survival because CKD is a long-term chronic disease. Long-term, chronic inflammation can also exacerbate renal anemia and renal function [ 43 ], as well as promote malnutrition, in the kidney, the site of the majority of renal disorders [ 44 ]. To preserve the kidneys, the body suppresses inflammation through autophagy [ 45 ]. The very diverse etiology of bone disease, as well as the restrictions and particular adverse effects of available treatments, make it difficult to diagnose and treat osteoporosis in individuals with severe CKD [ 46 ]. Therefore, it is crucial to identify osteoporosis early and take steps to avoid it. We opted to investigate inflammatory markers, a mechanism frequently present in BMD decrease and nephropathy, and ultimately found specific outcomes.
Our research has a few drawbacks. Firstly, since this research is cross-sectional, temporality cannot be established. Additionally, even after controlling for a number of pertinent confounders, we were unable to completely exclude the impact of other confounders, therefore it is important to proceed with care when interpreting our results. Third, while patients with CKD typically use oral drugs like hormones depending on the underlying condition, our findings could not accurately reflect the true situation because the NHANES database’s constraints prevented us from including individuals’ medication use as a covariate. Fourth, although the CKD-EPI equation is the most accurate GFR estimation equation that has been tested in a wide range of populations and is appropriate for general clinical use [ 47 ], its accuracy is still not comparable to filtered marker measurements. The degree of renal impairment in this study was extrapolated from the CKD-EPI equation.
Despite these drawbacks, our study provides a number of advantages. Our study is typical of the multiracial and gender-diverse adult population of the United States since we employed a nationally representative sample. Furthermore, the size of the sample used in our study allowed us to do a subgroup analysis.
In our study, pelvic bone density in CKD patients was associated with SII levels, and pelvic bone density reduced as SII increased. The change in BMD with SII in CKD patients without diabetes may be at a turning point. SII and pelvic bone density were substantially associated with individuals with stage 2 CKD. This may offer recommendations for the prevention and management of problems from osteoporosis in CKD patients. To support our findings, further comprehensive prospective cohort studies are still required.
- View Article
- PubMed/NCBI
- Google Scholar
Why Is Chronic Kidney Disease on the Rise? 6 Things to Know
BY KATHY KATELLA April 24, 2024
Chronic kidney disease (CKD) is a condition that affects an estimated 37 million American adults—or one in seven. And yet, many people don’t even know they have it.
That’s because CKD is a silent disease, progressing without symptoms as the kidneys gradually—and permanently—lose function over months or years. When people finally start to experience symptoms, such as itchy skin, an impaired ability to urinate, and unexplained weight loss, among others, it means the disease has reached an irreversible stage. At that point, they may have lost so much kidney function that they will need dialysis or a kidney transplant.
CKD is also on the rise. "Diabetes is the number one cause of kidney disease; so, because more patients have diabetes, we're seeing more kidney disease,” says Randy Luciano, MD , a Yale Medicine nephrologist. High blood pressure is the second leading cause, and that’s on the rise as well. (There are other causes, including obesity and, less commonly, polycystic kidney disease, a genetic condition marked by multiple cysts in the kidneys.)
Once you have CKD, it and the accompanying kidney damage cannot be reversed. However, diagnosing and treating CKD early may help stop it from advancing. There are new treatments in the past few years that have been described as “game-changing” for their ability to slow the progression of CKD for years and possibly even decades, Dr. Luciano says.
Below, Dr. Luciano answers common questions about chronic kidney disease.
1. What, exactly, is chronic kidney disease?
Chronic kidney disease is the medical term used to describe the gradual loss of kidney function over a period of at least three months. Because of this, excess fluid and waste from the blood remain in the body and may cause other health problems, such as heart disease and stroke , in addition to kidney failure.
CKD can occur for a variety of reasons, but diabetes and high blood pressure are the two most common ones. If someone has diabetes, high blood sugar can clog and narrow the kidney’s tiny blood vessels, leading to kidney damage. In people with high blood pressure, there is an increase in the force of blood pushing against vessel walls throughout the body, including in the kidneys, affecting their ability to eliminate waste and extra fluid from the body—key kidney functions. Other issues that can lead to CKD include glomerulonephritis, an inflammation of the tiny filters in the kidneys; lupus nephritis , an autoimmune disease; polycystic kidney disease ; and kidney cancer .
2. How do you know if you’re at risk for CKD?
While anyone can develop CKD, some people are at higher risk.
Risk increases with age, with people over 65 most likely to be diagnosed with CKD. In addition to having diabetes (type 1 or 2) and high blood pressure , people are also at higher risk if they have heart disease, obesity, or past damage to the kidneys from an infection or surgery.
Family history matters, too. "If you have a family history of CKD, kidney failure, or inherited kidney disorders, you should talk to your provider about kidney health when you’re in your 20s,” Dr. Luciano says. “People with first-degree relatives affected by CKD are at higher risk, and those with other relatives who have the condition have an elevated risk as well. A young patient may not have CKD that will impact their life for the next 10 or 15 years, but we can take steps to lower their risk as they age.”
Race, ethnicity, and socioeconomic issues may play a role as well. As many as 20% of non-Hispanic Black adults in the U.S. are estimated to have CKD compared to 11.7% for non-Hispanic white adults, according to the CDC. “This is probably multifactorial,” says Dr. Luciano, adding that access to health care and affordable medications can be a factor in some cases. And minority populations are more likely to have diabetes, heart disease, high blood pressure, and obesity, all conditions that raise the risk for CKD, he says.
“We also know that some Black patients have certain genetic risks that may increase their chances of developing CKD,” Dr. Luciano says. A nephrologist can arrange genetic testing, which involves a tissue swab of the cheek or a blood draw the patient can perform at home. If a patient tests positive for a genetic mutation linked to CKD, “the goal is to be preemptive and manage diabetes or blood pressure using the latest medicines aggressively to get that disease under control,” he says.
3. How is CKD diagnosed?
CKD can be detected (and monitored) using two simple tests: a blood test and a urine test. The first is often done as part of a regular physical examination with routine bloodwork that includes a test called glomerular filtration rate (GFR)—the GFR score is based on the level of creatinine in a person’s blood, combined with their age and sex. (Creatinine is a waste product, resulting from the normal breakdown of muscle tissue and digestion of protein from food; too much of it in the blood is a sign that the kidneys are not cleaning out waste efficiently.)
The second test is a urinalysis to evaluate the urine for albumin, the main protein found in blood. Albumin in the urine is called albuminuria, and its presence is a sign that the kidneys are damaged.
There are five stages of kidney disease, which are determined by GFR scores that remain consistent for at least three months:
- Stage 1. GFR: 90 or higher. Stage 1 CKD means you have a normal GFR, but there is protein in your urine. The presence of protein alone means you are in Stage 1 CKD, even if there is no kidney damage.
- Stage 2. GFR: 60-89. This suggests mild kidney damage, but the kidneys still work well. Because most people don’t notice symptoms until Stage 3, this stage of CKD often goes unnoticed; however, if caught, it may still be possible to slow down the loss of kidney function with lifestyle changes.
- Stage 3. GFR: 30-59. This stage is divided into two subcategories: 3a, which is mild-to-moderate damage, with a GFR between 45 and 59, and 3b, which is moderate-to-severe damage, with a GFR between 30 and 44. At this stage, CKD can cause complications, such as anemia and bone disease. Although some people still may not have symptoms, many experience one or more of a range of symptoms, including feeling weak and tired, lower back pain, dry or itchy skin, urinating more or less than usual, or having foamy or darker-colored urine. Although at this point the kidneys are irreversibly damaged and don’t work as well as they should, with treatment and healthy life changes, many people in this stage do not move to Stage 4 or Stage 5.
- Stage 4. GFR: 15-29. This is the last stage before kidney failure, when damage is severe. The kidneys have a difficult time filtering out waste, which then builds up in the body where it can cause a variety of health problems, including heart disease and stroke. Patients and their providers should begin to plan for future dialysis or a kidney transplant.
- Stage 5. GFR: Less than 15. The kidneys are close to failure or have already failed, the latter of which is called end-stage CKD (ESKD). Symptoms of kidney failure may include some of the issues that some people start to experience in Stage 3, along with some new ones, such as rashes; foamy, frothy, or bubbly urine; feeling less hungry than normal; and feeling sick in the stomach and throwing up. This stage calls for dialysis or a kidney transplant to keep patients alive.
4. How can you stop CKD from progressing?
Treatment usually depends on the underlying cause of kidney disease. “Not all chronic kidney disease progresses in the same way,” Dr. Luciano says. For instance, “if we know a patient’s kidney disease is due to diabetes, we focus on treating that,” he says, because improving diabetes will slow further damage. Likewise, patients with high blood pressure must get their blood pressure under control, whether that includes making lifestyle changes or taking medication.
In the past few years, several relatively new CKD medications have become available. Some of these are also used to treat diabetes and other CKD-related conditions, including obesity and heart disease. These drugs include:
- SGLT-2 inhibitors (also called flozins). These include several medicines, available as pills, that are used in diabetes treatment. Several, including empagliflozin (Jardiance®), are FDA-approved for CKD. One way these medicines work is by preventing the kidneys from reabsorbing blood sugar; the glucose instead goes to the urine and is eliminated from the body, thereby protecting the kidneys.
- Finerenone (Kerendia®) . This is an FDA-approved pill aimed at reducing the risk of kidney function decline and failure, as well as serious heart conditions and events associated with type 2 diabetes . It works by blocking proteins called MRs that can become overactivated in type 2 diabetes, leading to kidney inflammation and scarring.
- GLP-1 receptor agonists (glucagon-like peptide-1 receptor agonists). These include drugs that are FDA-approved for type 2 diabetes and may help people lose weight. One is semaglutide (Ozempic®), which is approved for weight loss under the brand name Wegovy®. These drugs have shown promise in reducing the risk of kidney failure, although they are not FDA-approved for that purpose at this time.
High blood pressure can be both a cause and a result of CKD, so blood pressure medications are often prescribed—and were used to treat CKD for decades before the newer drugs became available. These include ACE inhibitors (angiotensin receptor blockers) and ARBs (angiotensin-converting enzymes). They help blood vessels relax, so the blood can flow smoothly, preserving kidney function.
A series of other medications may also help. For instance, because poor kidney function can weaken bones, calcium and vitamin D supplements may be prescribed to keep them strong. Diuretics, which can help kidneys eliminate salt and water and facilitate urination, may be used to reduce swelling. And iron supplements can help address anemia.
It may also be important to stop taking certain medicines that can worsen kidney damage, such as NSAIDs (nonsteroidal anti-inflammatory drugs) and some arthritis medicines.
5. If you have CKD, will you need dialysis or a kidney transplant?
It depends. Having CKD doesn’t always mean the disease will progress to ESKD and require dialysis or a kidney transplant . “As kidney specialists, we try to establish the cause of the disease, treat it, and determine if a patient will require dialysis in the future,” Dr. Luciano says. “Sometimes, the last part is difficult because we don't know how they're going to respond to treatment, how their kidney disease is going to progress over time, and what other circumstances can develop in their life that can impact the disease.”
If, however, you progress to ESKD and your kidneys fail, you will need either dialysis or a kidney transplant to replace the work of the kidneys.
Out of almost 808,000 people in the U.S. who have ESKD, 69% are on dialysis, which was once done only in a health care setting. Now, many people choose types of dialysis that can be performed at home.
There are two types of dialysis treatment, both of which involve several treatments a week.
- Peritoneal dialysis: In a surgical procedure, a catheter (or plastic tube) is placed in the patient's belly. The patient hooks up a plastic bag of cleansing fluid to the tube, which transports the fluid to the abdomen, and is then used to drain it. Treatment can be continuous, as the person goes about their normal activities, or automated, in which the cleansing fluid is delivered and drained while the person sleeps.
- Hemodialysis: Blood is pumped out of the body through a tube and into an artificial kidney machine, which removes the waste and extra fluid before it’s sent back to the body. This is done three or four times a week, often in a health care setting, but it can sometimes be done at home.
“For those who can do it, we often recommend home dialysis,” Dr. Luciano says. “It tends to be easier for people, gives them more flexibility with their schedule, and is gentler on the body.”
Still, a kidney transplant is often the best solution—preferably before a patient reaches the point of requiring dialysis, Dr. Luciano says. That’s because while dialysis can take over the function of cleaning waste and excess water from the body, treatments can be time-consuming and cause such side effects as skin infections, low blood pressure, muscle cramps, weakness, and fatigue. It can also have a major impact on a person’s life, both emotionally and physically.
“The hope is that some of the newer medications will allow patients to stay on the waiting list longer without needing dialysis,” Dr. Luciano says.
In 2020, the remaining 31% of the almost 808,000 people in the U.S. with ESKD had kidney transplants, according to the CDC. People wait three to five years, more or less, for an organ from a deceased donor, depending on such factors as geographic location and blood type. “But if you have a living donor—either someone you know or a stranger willing to donate a kidney—and everything works out, you may get a kidney within three to six months,” Dr. Luciano says.
There are also kidney registries that can help match people with a living donor who is not a relative.
6. How can you avoid CKD?
The best thing to protect your kidneys is to regularly see your primary care provider and, if relevant, share any family history of kidney disease or dialysis, Dr. Luciano says.
“If you have a yearly physical and bloodwork every two or three years, you’ll be much less likely to miss CKD,” he says. If there is an abnormality, such as an elevated level of creatinine, he recommends repeating the bloodwork once a year. “Your doctor may notice over time that your creatinine is a little off, which will trigger the follow-up appointment with a kidney specialist who can look into it more carefully.”
For anyone with diabetes, high blood pressure, or heart disease, and/or who is 65 or older, it’s especially important to talk to a doctor about CKD risk. People who are young and healthy but have a first-degree relative with a history of kidney disease or dialysis should consider being screened, Dr. Luciano says. “Chances are they're not going to have a serious case of CKD at an early age. But it's always good to make sure that we're not missing anything that would put them at risk as they age.”
As with many diseases, preventing a serious condition from developing also comes down to good general health, he says. This includes eating a diet rich in fruits and vegetables and low in salt, fat, and sugar; drinking enough water; being active for at least 30 minutes most days; sleeping seven to eight hours each night; avoiding smoking; and minimizing alcohol intake.
“There are some people who are going to develop kidney disease no matter what they do, just as there are people who will develop heart disease,” Dr. Luciano says. “But good general care can mean early diagnosis and treatment, which can keep people healthy.”
More news from Yale Medicine

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts

Long-term health outcomes associated with hydration status
- Natalia I. Dmitrieva
- Manfred Boehm
- Sofia Enhörning
Current issue
Organ trafficking — a continuing challenge.
- Thomas F. Mueller
- Sanjay Nagral
Innovating dialysis through computational modelling of hollow-fibre haemodialysers
- Ruhit Sinha
- Michael V. Rocco
- Anne E. Staples
Switching off SOX9 for epithelial recovery after AKI
- Monica Wang
The multifaceted links between hearing loss and chronic kidney disease
- Dina Greenberg
- Norman D. Rosenblum
- Marcello Tonelli
Sirtuins in kidney health and disease
- Luca Perico
- Giuseppe Remuzzi
- Ariela Benigni

Announcements

WEBINAR ON Digital Determinants of Health
Watch the recording of our webinar on "Digital Determinants of Health: AI and the need for equity-focused innovation in digital health" with Leo Celi and Kianoush Kashani.
SERIES ON Tools & Technologies
A series of articles that aim to explore the tools and techniques that are improving our understanding of renal development, physiology and disease mechanisms as well as contributing to advances in the screening, diagnosis and management of kidney diseases.
Focus on the Sustainable Development Goals
This Focus issue examines how tackling the three dimensions of sustainable development — social, economic and environmental — is essential to improving global kidney health.

FOCUS on regulated necrosis in the kidney
This Focus issue explores the potential contribution of regulated necrosis pathways — ferroptosis, necroptosis and pyroptosis – to kidney injury.
Advertisement
Latest Reviews & Analysis
Not every organ ticks the same
A new study describes the development of proteomics-based ageing clocks that calculate the biological age of specific organs and define features of extreme ageing associated with age-related diseases. Their findings support the notion that plasma proteins can be used to monitor the ageing rates of specific organs and disease progression.
- Khaoula Talbi
- Anette Melk
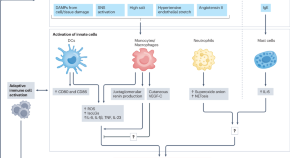
Immune mechanisms in the pathophysiology of hypertension
This Review outlines the roles of innate and adaptive immune cells in hypertension. The authors discuss the mechanisms and important properties of immune cells that contribute to hypertension pathogenesis, such as memory and plasticity.
- Bianca A. Nguyen
- Matthew R. Alexander
- David G. Harrison
Calcium signalling and transport in the kidney
Calcium reabsorption along the nephron is essential for calcium homeostasis and whole-body electrolyte balance. Here, Staruschenko et al. highlight signalling pathways and molecules involved in renal calcium handling in health and disease, and discuss progress in the integration of systems-level and molecular understanding of calcium transport and regulation.
- Alexander Staruschenko
- R. Todd Alexander
- Daria V. Ilatovskaya
Epithelial cell states associated with kidney and allograft injury
This Review describes parallels in the injury mechanisms that underlie acute kidney injury, chronic kidney disease and allograft injury, and explains how our understanding of the molecular changes that occur in epithelia in the context of kidney disease may contribute to the therapeutic targeting of specific epithelial cell phenotypes for the treatment of transplantation complications.
- Christian Hinze
- Svjetlana Lovric
- Kai M. Schmidt-Ott
A new era in the science and care of kidney diseases
Despite notable progress in basic, clinical and translational nephrology research in the past 50 years, many challenges remain. In this Review, the authors provide an overview of the current status and future directions in nephrology research and patient care.
- Carmine Zoccali
- Francesca Mallamaci
- Raymond Vanholder
Combination therapy for kidney disease in people with diabetes mellitus
- Daniël H. van Raalte
- Petter Bjornstad
- Hiddo J. L. Heerspink
Chronic kidney disease and the global public health agenda: an international consensus
- Anna Francis
- Meera N. Harhay
- Vivekanand Jha
Drug stewardship in chronic kidney disease to achieve effective and safe medication use
- Rasheeda K. Hall
- Rümeyza Kazancıoğlu
- Juan J. Carrero
Role of biophysics and mechanobiology in podocyte physiology
- Jonathan Haydak
- Evren U. Azeloglu
News & Comment
Considerations of sex as a binary variable in clinical algorithms.
Clinical algorithms that are used to guide medical decision-making often include sex as a variable. However, binary considerations of sex and/or gender might introduce bias due to potentially inaccurate assumptions about sex and gender-specific physiology, hormones and exposures. An equity-focused approach to sex and gender is essential when using clinical algorithms to ensure health equity across populations.
- Dinushika Mohottige
- Samira Farouk
- Selma Feldman Witchel
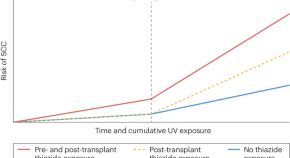
Thiazides in kidney transplant recipients: skin in the game
Clinical trial data suggest that thiazides and thiazide-like drugs could be beneficial for blood-pressure lowering in patients with severe chronic kidney disease. However, prolonged exposure to these photosensitizing drugs could translate into increased risk of squamous cell carcinoma and post-transplant diabetes in the already extremely vulnerable kidney transplant population.
- Steven Van Laecke
Kidney disease: a global health priority
The prevalence of kidney disease and its associated morbidity and mortality continue to rise. This crisis cannot be tackled unless kidney disease is made a global public health priority.

The global landscape of kidney registries: immense challenges and unique opportunities
Kidney registries are essential to understanding the burden of kidney disease and facilitating the development of sustainable and effective programs for kidney disease prevention and care. Key barriers to implementation of registries at a global scale include funding and data quality. These issues warrant the attention of the global nephrology community.
- Christopher H. Grant
- Fergus J. Caskey
- Samira Bell

Sex differences in kidney metabolism and DKD
- Ellen F. Carney
Clonal haematopoiesis and AKI
- Susan J. Allison
Trending - Altmetric
Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup
Role of necroptosis in kidney health and disease

The pathophysiology of distal renal tubular acidosis
The role of lysosomes in metabolic and autoimmune diseases
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Endocr Connect
- v.10(5); 2021 May 1
Chronic kidney disease in patients with diabetes mellitus
Espen nordheim.
1 Department of Transplantation Medicine, Section of Nephrology, Oslo University Hospital, Rikshospitalet, Oslo, Norway
2 Faculty of Medicine, University of Oslo, Oslo, Norway
Trond Geir Jenssen
3 Metabolic and Renal Research Group, Faculty of Health Sciences, UiT- The Arctic University of Norway, Tromsø

Chronic kidney disease is a common complication and concomitant condition of diabetes mellitus. The treatment of patients with diabetes and chronic kidney disease, including intensive control of blood sugar and blood pressure, has been very similar for type 1 and type 2 diabetes patients. New therapeutic targets have shown promising results and may lead to more specific treatment options for patients with type 1 and type 2 diabetes.
Introduction
Diabetes mellitus is among the leading causes of chronic kidney disease and end-stage kidney disease in the western world. It was the most common diagnosis for the initiation of renal replacement therapy in the United States in 2018, accounting for 47% of the cases ( 1 ).
Type 1 and type 2 diabetes mellitus share many clinical characteristics and long-term complications, but they are in fact two different diseases with diverging pathophysiology ( 2 ). While type 1 diabetes results from autoimmune destruction of the insulin-producing beta-cells within the pancreatic islets of Langerhans, type 2 diabetes is most often characterized by insulin resistance together with insufficient insulin response, at least in White people.
Type 1 and type 2 diabetes mellitus can both cause long-term microvascular and macrovascular complications, contributing to the increased morbidity and mortality among these patients. Kidney disease in patients with diabetes can be a result of microvascular complications from diabetes, a concomitant kidney disease of other origin or a combination of the two. In type 1 diabetes patients, microvascular disease secondary to diabetes is the most common etiology to chronic kidney disease, while a spectrum of etiologies can cause kidney disease in type 2 diabetes patients.
The established treatment principals to prevent and halt progression of chronic kidney disease have been very similar in the two diabetic phenotypes. This review article intends to highlight the established and upcoming treatment for patients with type 1 and type 2 diabetes and chronic kidney disease. We have reviewed landmark papers published up till the fall of 2020 and publications dealing with new potential therapeutic targets. This review is based on literature search in Pubmed with the following combinations of keywords: 'diabetic nephropathy', 'diabetic kidney disease', 'diabetic nephropathy treatment' and 'diabetic kidney disease treatment' to complete our own bibliography. The search yielded a total of 79.367 search results and was closed on October 26, 2020. The relevance of the articles was initially assessed on the basis of title and abstract before relevant articles in English language were read in full text. Reference lists of all articles read were analyzed in order to identify reference articles for the review.
Kidney disease in patients with type 1 diabetes mellitus
Type 1 diabetes usually affects young and middle-aged patients and among these patients, chronic kidney disease is most often caused by diabetes-related microvascular disease ( 3 ), a condition which has been referred to as diabetic nephropathy or 'diabetic kidney disease' in the literature.
Chronic kidney disease in type 1 diabetes patients typically can cause a progressive decline in renal function. Hyperglycaemia starts the pathophysiologic mechanisms with a subsequent interplay of altered haemodynamics, metabolic and inflammatory pathways ( 4 ). When glomerular foot processes merge and the integrity of the glomerular basement membrane is compromised by pathological processes, albumin and subsequently, larger proteins can leak into the urine. Urine albumin/creatinine ratio (UACR) spot analysis of first morning collection is preferred for proteinuria quantification, above assessment of albumin in 24 h urine collection. UACR are staged into moderate albuminuria, previously called microalbuminuria (30–300 mg/g) and overt albuminuria, previously called proteinuria (≥300 mg/g).
Chronic kidney disease in type 1 diabetes patients is initially characterized by hyperfiltration due to increased glomerular filtration pressure ( 5 ). Cherney et al . postulated hyperglycaemia-dependent hyperfiltration to be mediated through upregulated back-transportation of sodium and glucose from the renal tubular system ( 6 ). Sodium-glucose-co-transporter-2 (SGLT2) contributes to 90% of this transportation reducing distal tubular flux of glucose and sodium. Due to reduced sodium flux in the loop of Henle, macula densa signals dilatation of the afferent arteriolar tone through a tubuloglomerular feedback mechanism which increases tubular sodium flux at the expense of increase of intraglomerular pressure and hyperfiltration at the nephron level. Hyperfiltration is in the clinic seen as an increase in glomerular filtration rate (GFR) ( 5 , 6 ). Albuminuria and hypertension subsequently occur as the kidney disease develops. After the initial hyperfiltration phase, nephrons are lost resulting in a steady GFR decline ranging 3–6 mL/min/year ( 4 ). Renal failure requiring replacement therapy may eventually occur within 20–25 years. During this process, the remaining nephrons compensate by hyperfiltration not only due to hyperglycemia but now also due to reduced total filtration surface. This represents a vicious circle with progressive loss of nephrons.
In his first model of the disease, Mogensen suggested that the pathological processes in chronic kidney disease in type 1 diabetes patients were irreversible and that the disease gradually progressed ( 5 ). Later, this model has been challenged by reports describing that renal function may be reduced without concomitant proteinuria and that proteinuria may cease spontaneously in the course of the disease ( 7 ). These reports used estimated GFR based on cystatin c measurements, and they have, thus, been challenged since these estimates of GFR may differ substantially from measured GFR ( 8 ).
Kidney disease in patients with type 2 diabetes mellitus
While chronic kidney disease in type 1 diabetes most often is secondary to diabetes microvascular disease, there is a whole spectrum of chronic kidney disease etiologies in type 2 diabetes. Type 2 diabetes patients are often older at the time of diagnosis and kidney disease due to other causes than diabetes is likely to occur. Several studies have verified that kidney disease in type 2 diabetes may be a more compounded entity than what is seen in type 1 diabetes ( 9 ). One study from the United States which examined kidney biopsies in patients with type 2 diabetes and kidney disease found that typical diabetic microvascular disease were present in 37% of the cases, non-diabetic kidney disease in 36% of the cases, such as nephrosclerosis or immunological kidney disease, while mixed forms of diabetic and non-diabetic kidney disease were found in 27% of the cases ( 10 ). Interestingly, one study has found different insulin resistance phenotypes in diabetes to be associated with different risks for chronic kidney disease ( 11 ), but this needs to be studied further.
Regardless of kidney disease etiology, strict blood glucose control is on a group level the single-most important intervention to prevent kidney disease to develop in patients with type 1 and type 2 diabetes ( 12 , 13 , 14 ). Normalization of blood glucose might act renoprotective through different mechanisms: reduced hyperfiltration on the nephron level ( 15 , 6 ), reduced generation of toxic intermediates such as reactive oxygen species (ROS) ( 16 ) and reduced activity in pathogenetic signalling pathways including the polyol, hexasamine, protein kinase C and advanced glycation end-product pathways ( 17 ).
Still as the chronic kidney disease progresses, GFR is reduced through nephron loss and hyperfiltration in remaining nephrons drives the process further.
How to diagnose kidney disease in patients with diabetes?
Typically, patients with established diabetes are diagnosed with kidney disease when albuminuria is identified as elevated in two out of three spot urine analyzes, corresponding to urine albumin/creatinine ratio (UACR) at or above 30 mg/g and/or persistently impaired renal function, defined as estimated glomerular filtration rate (eGFR) below 60 mL/1.73 m 2 .
Our clinical impression is that, in most cases, patients with diabetes are not referred for diagnostic kidney biopsy when kidney disease is verified, especially if the clinical characteristics do not differ from what can be expected from chronic kidney disease in diabetes (i.e. increased albuminuria, hypertension and slow decrease in estimated GFR). However, other diseases must be suspected when no other microvascular complications of diabetes are present and also in the presence of microscopic haematuria ( 9 ). The pathological classification system developed for chronic kidney disease in type 1 diabetes also includes type 2 diabetes ( 3 ). It has been argued that a common classification system is challenging due to the differences in renal lesions among the two diseases ( 18 ). While type 1 diabetes patients typically have glomerular lesions characterized by thickening of the glomerular basement membrane, mesangial expansion and glomerulosclerosis, only 30% of type 2 diabetes patients with microalbuminuria and 50% with proteinuria demonstrate such lesions ( 19 ).
Established treatment of chronic kidney disease for type 1 and type 2 diabetes
Intensive blood sugar control.
Long-term hyperglycaemia causes glomerular hyperfiltration together with glycation of cell proteins, especially intranuclar proteins and nucleic acids. Intensified blood sugar control reduces the risk for proteinuria to develop and also to progress ( 12 , 13 , 14 ). Prevention of overt proteinuria is beneficial for maintaining renal function over time ( 12 , 13 , 14 ). The effect is well-documented in patients with type 1 diabetes ( 12 , 13 ) and has also been demonstrated at a group level for patients with type 2 diabetes ( 14 ). Most guidelines recommend glucose control corresponding to HbA1c around 53 mmol/mol (7%), provided good quality of life is maintained without causing repeated or even severe episodes of hypoglycaemia ( 20 , 21 ).
The risk for severe hypoglycaemic episodes increases when GFR falls below 45 mL/min. Hypoglycaemic episodes may develop due to reduced gluconeogenesis and counter-regulation in the kidneys ( 22 ) and also the half-life of blood glucose-lowering drugs can be prolonged when renal function is impaired, which may necessitate dose-lowering or cessation of drugs such as insulin or sulfonylureas. The increased risk for metformin-related lactacidosis must also been taken into consideration when renal function is impaired ( 23 ).
Regulation of blood pressure and albuminuria is beneficial for chronic kidney disease in diabetes
Carl Erik Mogensen reported in 1976 on how blood pressure must be lowered in order to prevent decline in renal function in patients with type 1 diabetes ( 24 ). The treatment target for this patient group is blood pressure below 130/80 mmHg ( 25 , 26 ). Blocking of the renin-angiotensin-aldosterone (RAAS) system is particularly effective in order to reduce proteinuria and preserve renal function, regardless of the blood pressure, first demonstrated for captopril in patients with type 1 diabetes and nephropathy ( 27 ) and later for losartan and irbesartan in type 2 diabetes ( 28 , 29 ). Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARBs) reduce intraglomerular filtration pressure by dilating the efferent glomerular arteriole, and the clinical effects of these two classes of drugs are considered as equivalent ( 30 ). If proteins are present in the urine, it is crucial to reduce the level of proteinuria in order to reduce the risk for kidney disease progression ( 31 ). To achieve a reduction of proteinuria, RAAS-blocking drugs are often needed in higher doses to reduce proteinuria than for reaching the blood pressure target alone ( 32 ). To be able to monitor and evaluate treatment effect, the amount of proteinuria should be quantified and followed with ACR in urine, which can be easily analyzed in spot samples of morning urine ( 33 ).
Other interventions beneficial for chronic kidney disease in diabetes
A comprehensive care is recommended for the management of diabetes patients in the recent KDIGO clinical practice guidelines ( 21 ), including statins to all patients with diabetes and kidney disease regardless of cholesterol levels to prevent cardiovascular disease. Moderate physical activity for at least 150 min per week is recommended in addition to smoking cessation, weight loss in the case of obesity, salt restriction and protein-reduced diet.
Recent studies on glucose-lowering treatment with clinical impact for patients with type 2 diabetes
Patients with type 1 diabetes need treatment with exogenous insulin to obtain blood glucose control, while type 2 diabetes patients might reach treatment targets with life-style modifications, with or without addition of oral glucose lowering agents. The last decade has presented a large selection of blood-glucose lowering drugs for type 2 diabetes patients, and it has been demonstrated that especially two classes of drugs have particular beneficial effects in patients with kidney disease beyond the blood-glucose lowering effect: receptor agonists of the glucagon-like protein 1 receptor (GLP-1 RA) and inhibitors of sodium-glucose-co-transporter-2 (SGLT2i). In secondary analyses of the SAVOR-TIMI 53 and CARMELINA studies, the dipeptidyl peptidase-4 inhibitors (DPP4i) – saxagliptin and linagliptin were associated with less development of macroalbuminuria ( 34 , 35 ) but with no effect on major cardiovascular endpoints ( 36 , 35 ). However, the antiproteinuric effect is more pronounced with GLP-1 RAs. Renal outcomes were included in six out of the major GLP-1 RAs studies on cardiovascular outcome (CVOT): lixisenatide (ELIXA), liraglutide (LEADER), semaglutide (SUSTAIN-6), exenatide (EXSCEL), dulaglutide (REWIND and AWARD-7) ( 37 , 38 , 39 , 40 , 41 , 42 ). Major adverse cardiovascular events and death were the primary outcomes of all these studies, while composite renal endpoints were among the secondary outcomes. The renal endpoints were not standardized, but all included proteinuria and change in estimated GFR. Lixisenatide, liraglutide, semaglutide, exenatide and dulaglutide all seemed to demonstrate a protective effect on composite renal outcomes. This effect was apparently driven by reduced development of macroalbuminuria and not by stabilization of GFR ( 37 , 38 , 40 ). However, dulaglutide seemed to preserve renal function better compared to insulin glargine after 1 year of treatment in patients with type 2 diabetes in AWARD-7 ( 42 ).
How GLP-1 RAs provide beneficial effects in the kidneys, outside lowering of blood glucose, is not fully understood. It has been suggested that GLP-1 agonism mediates reduced inflammation and oxidative stress. GLP-1 RA increases natriuresis, probably by inhibiting sodium/hydrogen isoform 3 (NHE3) in the renal tubules ( 43 ), but the renal haemodynamic effects seem neutral as the drug also causes glomerular vasodilatation ( 44 ). Weight loss, another known effect of GLP-1 RA leading to reduced hyperfiltration and improved proteinuria, might also have a role.
Promising results were found with the SGLT2-inhibitors early in the cardiovascular safety studies, for example, with empagliflozin (EMPA-REG), canagliflozin (CANVAS) and dapagliflozin (DECLARE- TIMI 58) ( 45 , 46 , 47 , 48 ). Composite renal outcomes were studied as secondary endpoints also in these studies, and it was observed that SGLT2i not only seemed to reduce proteinuria but also to delay deterioration of renal function (eGFR).
How can we understand the SGLT2i effects in chronic kidney disease?
SGLT2-mediated retention of glucose in the kidneys is probably a calorie saving mechanism which enabled survival in times when access to exogenous energy resources were more restricted than today. In a normal diet, the kidneys reabsorb 180 g glucose (720 kilocalories) daily. During diabetes and hyperglycaemia, the reabsorption of glucose and sodium is increased even more and less sodium is presented to macular densa distal to loop of Henle. This in turn initiates a tubuloglomerular feedback mechanism which dilates the afferent arteriole leading to an increase in GFR. Thus, tubular sodium flux is reestablished at the expense of hyperfiltration which is detrimental for the glomerular tuft in the long run.
The excretion of sodium into the urine increases due to the co-inhibitory effect of SGLT2i, which again normalizes the afferent arteriole tone through its effect on macula densa ( 6 ). Increased afferent arteriole tone translates into a reduced filtration pressure in the glomeruli and, thus, reduced load on the glomerular tuft. Additional potential beneficial mechanisms of SGLT2i may be increased oxygenation of tubular cells, possible reduction of toxic effects on renal tubules secondary to reduced albuminuria, preservation of intravascular volume and reduced volume overload due to loop-diuretic sparing effect in addition to improved metabolic parameters (i.e. body weight and HbA1c) ( 49 ). One study found post-glomerular vasodilatation of the SGLT2 inhibitor dapagliflozin rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes ( 50 ).
The CREDENCE study was the first study that primarily included type 2 diabetes patients with kidney disease ( 51 ). This was a randomized placebo-controlled trial of more than 4000 type 2 diabetes patients with estimated glomerular filtration rate (eGFR) between 30 and 90 mL/min/1.73 m 2 and UACR ranging 300–5000 mg/g. The pre-specified efficacy criteria for the study were achieved earlier than expected and led to an early termination of the study ( https://www.jnj.com/phase-3-credence-renal-outcomes-trial-of-invokana-canagliflozin-is-being-stopped-early-for-positive-efficacy-findings ; accessed October 26, 2020). After a median follow-up of 2.6 years, the treatment group had a significant reduction in the combined renal-specific endpoint (development of terminal kidney failure, doubling of serum creatinine or death from renal-specific cause). These results were valid also for the patients with the most reduced renal function in the study. In fact, in a subset of patients with a GFR drop to less than 30 mL/min/1.73 m 2 just prior to randomization, safety and efficacy of the drug was in line with the whole study population ( 52 ). Renoprotective effects occur at GFR thresholds where glucose lowering is no longer observed. The ability for SGLT2i to lower blood glucose decreases sharply when estimated GFR falls below 60 mL/min/1.73 m 2 ( 53 ), while the CREDENCE study documented kidney protective effects in the patient group with estimated GFR ranging from 30 to 45 mL/min/1.73 m 2 and even lower.
The DAPA-CKD study studied whether the SGLT2 inhibitor dapagliflozin could have beneficial renal effects also in non-diabetic patients with chronic kidney disease (CKD). Due to superior effects on the renal endpoints, the study was halted before scheduled time ( 54 ). The study involved 4.304 patients with CKD out of which only 68% had type 2 diabetes, and the primary end-point was: decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Patients with eGFR down to 25 mL/min/1.73 m 2 were included. The study found significant reduction in the primary endpoint and the effect was found independently of presence of diabetes. The number of patients needed to be treated to prevent one incident was as low as 19 during follow-up (median 2.4 years). The EMPEROR-Reduce study, published simultaneously, studied the effect of empagliflozin in heart failure patients already on RAAS blockage and found that empagliflozin was superior to placebo in preventing cardiovascular death or hospitalization for heart failure ( 55 ). Rate of eGFR decline was a secondary outcome of the study. The annual decline in eGFR was slower in the empagliflozin group compared to the placebo group (0.55 mL/min/1.73 m 2 vs 2.28 mL/min/1.73 m 2 ). The DAPA-HF trial studied the effect of dapagliflozin in heart failure patients and found a lower risk of heart failure worsening or death from cardiovascular causes in the treatment group compared to placebo, regardless of the presence of absence of diabetes ( 56 ). The incidence of the prespecified renal composite outcome did not differ between the treatment groups. The number of patients with kidney disease in the DAPA-HF trial was low which may have reduced the power to detect statistically significant differences.
The VERTIS study could not demonstrate a significant effect of ertugliflozin on the primary major adverse cardiovascular events endpoint, but the results showed numerically beneficial outcome for preservation of GFR compared to placebo ( 57 ). Table 1 summarizes the recent SGLT2i studies with demonstrated effects on renal endpoint.
Summary of recent SGLT2i studies with effects on primary and secondary renal endpoints.
The ongoing EMPA-KIDNEY study also addresses the renal outcome of using the SGLT2 inhibitor empagliflozin in persons with CKD. In this study also patients with type 1 diabetes are included, in addition to patients with type 2 diabetes or no diabetes (ClinicalTrials.gov. {"type":"clinical-trial","attrs":{"text":"NCT03594110","term_id":"NCT03594110"}} NCT03594110 ). The study plans to recruit in total 5000 patients including type 1 diabetes, type 2 diabetes and non-diabetic patients with impaired renal function (eGFR ≥ 20 to <45 mL/min/1.73 m 2 or eGFR ≥ 45 to <90 mL/min/1.73 m 2 and UACR ≥ 200 mg/g) and the results are estimated to be published by summer 2022.
In the 2020 KDIGO guidelines for diabetes management in chronic kidney disease, the combination of metformin and SGLT2i is now recommended as first line treatment for all type 2 diabetes patients with chronic kidney disease if eGFR is above 30 mL/min per 1.73 m 2 regardless of glucose control, even in patients with HbA1c within the target range. If the patient does not achieve the individualized glycaemic target, the addition of a GLP-1 RA is recommended ( 21 ). Previous and upcoming studies on SGLT2-inhibitors studies are all performed in patients already treated with RAAS blockage or inhibition. Thus, one might expect that current treatment with ACEi or ARBs will still be a basic therapy in the future.
Other therapeutic targets recently tested
Atrasentan, an endothelin A-receptor antagonist, was investigated in the SONAR study ( 58 ). Atrasentan is thought to have beneficial effects in several pathophysiological processes involved in chronic kidney disease in patients with diabetes, altered haemodynamics, inflammation and fibrosis. The study included type 2 diabetes patients with eGFR 25–75 mL/min/1.73 m 2 and UACR 300–5000 mg/g and demonstrated a lower reduction in eGFR and reduced proteinuria when compared to placebo. Another and smaller clinical study on pirfenidion, an inhibitor of TGF-β (transforming grown factor beta), included patients with both type 1 and type 2 diabetes with kidney failure and proteinuria and reported improvement in eGFR after 1 year treatment compared to placebo ( 59 ). Sulodexide, a glycosaminoglycan described as being able to prevent structural changes in the glomerular basement membrane, has shown positive effects on proteinuria in two small studies ( 60 , 61 ). Bardoxolone mathyl is demonstrated to improve inulin GFR in a Japanese phase 2 study, the phase 3 study results are expected early in 2022 ( 62 ). Lowering of the serum urate level with allopurinol was ,however, not shown to slow the decrease in the glomerular filtration rate (GFR) in persons with type 1 diabetes and early-to-moderate diabetic nephropathy ( 63 ). FIDELIO-DKD found promising results with finerenone, a selective mineralocorticoid, on diabetic nephropathy and cardiovascular endpoints ( 64 ).
Studies in progress
In addition to the EMPA-KIDNEY study which examines SGLT2i in type 1 diabetes patients, other studies are of potential interest. The FLOW trial (Semaglutide on the progression of renal impairment in subjects with type 2 diabetes and chronic kidney disease) is designed to show whether the GLP-1 RA semaglutide can slow the decline in eGFR among type 1 diabetes patients with chronic kidney disease. The trial is still recruiting patients and will evaluate when a sufficient amount of end-points has been obtained, alternatively after 5 years. The primary endpoint is persistent eGFR decline of >50%, reaching end-stage renal disease, death from kidney disease or death from cardiovascular disease (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03819153","term_id":"NCT03819153"}} NCT03819153 ).
FIGARO-DKD will give us more answers to the role of finerenone in the treatment of chronic kidney disease in type 1 diabetes patients and cardiovascular endpoints (ClinicalTrials.gov: {"type":"clinical-trial","attrs":{"text":"NCT02545049","term_id":"NCT02545049"}} NCT02545049 ). Another interesting study investigates whether reduced oxidative stress by inhibiting NADPH oxidase can reduce proteinuria and preserve renal function in patients with type 1diabetes and moderate renal failure ( 65 ).
The established treatment of diabetes patients with chronic kidney disease has up till now been very similar for type 1 or type 2 diabetes patients. Recent studies have, however, demonstrated that type 2 diabetes patients with impaired renal function have beneficial renal effects beyond the glucose- lowering effects of especially SGLT2i but also GLP-1 RA. Future studies will clarify if new drugs will arrive in the treatment of type 1 diabetes patients with chronic kidney disease.
Declaration of interest
EN has no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. TGJ has received lecture fees from AstraZeneca, Mundipharma, Boehringer Ingelheim, and Novo Nordisk.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
- Patient Care & Health Information
- Diseases & Conditions
- Chronic kidney disease
- What is kidney disease? An expert explains
Learn more from kidney doctor Andrew Bentall, M.D.
I'm Dr. Andrew Bentall, a kidney doctor at Mayo Clinic. I look after patients with kidney disease, either in the early stages, or with more advanced kidney disease considering dialysis and transplantation as treatment options. In this video, we'll cover the basics of chronic kidney disease. What is it? Who gets it? The symptoms, diagnosis and treatment. Whether you are looking for answers for yourself or for someone you love, we're here to give you the best information available.
Chronic kidney disease is a disease characterized by progressive damage and loss of function in the kidneys. It's estimated that chronic kidney disease affects about one in seven American adults. And most of those don't know they have it. Before we get into the disease itself, let's talk a little bit about the kidneys and what they do. Our kidneys play many important roles keeping our bodies in balance. They remove waste and toxins, excess water from the bloodstream, which is carried out of the body in urine. They helped to make hormones to produce red blood cells, and they turn vitamin D into its active form, so it's usable in the body.
There are quite a few things that can cause or put you at higher risk for chronic kidney disease. Some of them are not things that can be avoided. Your risk is simply higher if you have a family history of certain genetic conditions like polycystic kidney disease or some autoimmune diseases like lupus or IgA nephropathy. Defects in the kidney structure can also cause your kidneys to fail, and you have an increased risk as you get older. Sometimes, other common medical conditions can increase your risk. Diabetes is the most common cause of kidney disease. Both type 1 and type 2 diabetes. But also heart disease and obesity can contribute to the damage that causes kidneys to fail. Urinary tract issues and inflammation in different parts of the kidney can also lead to long-term functional decline. There are things that are more under our control: Heavy or long-term use of certain medications, even those that are common over-the-counter. Smoking can also be a contributing factor to chronic kidney disease.
Often there are no outward signs in the earlier stages of chronic kidney disease, which is grouped into stages 1 through 5. Generally, earlier stages are known as 1 to 3. And as kidney disease progresses, you may notice the following symptoms. Nausea and vomiting, muscle cramps, loss of appetite, swelling via feet and ankles, dry, itchy skin, shortness of breath, trouble sleeping, urinating either too much or too little. However, these are usually in the later stages, but they can also happen in other disorders. So don't automatically interpret this as having kidney disease. But if you're experiencing anything that concerns you, you should make an appointment with your doctor.
Even before any symptoms appear, routine blood work can indicate that you might be in the early stages of chronic kidney disease. And the earlier it's detected, the easier it is to treat. This is why regular checkups with your doctor are important. If your doctor suspects the onset of chronic kidney disease, they may schedule a variety of other tests. They may also refer you to a kidney specialist, a nephrologist like myself. Urine tests can reveal abnormalities and give clues to the underlying cause of the chronic kidney disease. And this can also help to determine the underlying issues. Various imaging tests like ultrasounds or CT scans can be done to help your doctor assess the size, the structure, as well as evaluate the visible damage, inflammation or stones of your kidneys. And in some cases, a kidney biopsy may be necessary. And a small amount of tissue is taken with a needle and sent to the pathologist for further analysis.
Treatment is determined by what is causing your kidneys to not function normally. Treating the cause is key, leading to reduced complications and slowing progression of kidney disease. For example, getting better blood pressure control, improved sugar control and diabetes, and reducing weight are often key interventions. However, existing damage is not usually reversible. In some conditions, treatment can reverse the cause of the disease. So seeking medical review is really important. Individual complications vary, but treatment might include high blood pressure medication, diuretics to reduce fluid and swelling, supplements to relieve anemia, statins to lower cholesterol, or medications to protect your bones and prevent blood vessel calcification. A lower-protein diet may also be recommended. It reduces the amount of waste your kidneys need to filter from your blood. These can not only slow the damage of kidney disease, but make you feel better as well. When the damage has progressed to the point that 85 to 90 percent of your kidney function is gone, and they no longer work well enough to keep you alive, it's called end-stage kidney failure. But there are still options. There's dialysis, which uses a machine to filter the toxins and remove water from your body as your kidneys are no longer able to do this. Where possible, the preferred therapy is a kidney transplant. While an organ transplant can sound daunting, it's actually often the better alternative, and the closest thing to a cure, if you qualify for a kidney transplant.
If you have kidney disease, there are lifestyle choices. Namely quit smoking. Consuming alcohol in moderation. If you're overweight or obese, then try to lose weight. Staying active and getting exercise can help not only with your weight, but fatigue and stress. If your condition allows, keep up with your routine, whether that's working, hobbies, social activities, or other things you enjoy. It can be helpful to talk to someone you trust, a friend or relative who's good at listening. Or your doctor could also refer you to a therapist or social worker. It can also be helpful to find a support group and connect with people going through the same thing. Learning you have chronic kidney disease and learning how to live with it can be a challenge. But there are lots of ways to help you to be more comfortable for longer before more drastic measures are needed. And even then, there is plenty of hope. If you'd like to learn even more about chronic kidney disease, watch our other related videos or visit mayoclinic.org. We wish you well.
Chronic kidney disease, also called chronic kidney failure, involves a gradual loss of kidney function. Your kidneys filter wastes and excess fluids from your blood, which are then removed in your urine. Advanced chronic kidney disease can cause dangerous levels of fluid, electrolytes and wastes to build up in your body.
In the early stages of chronic kidney disease, you might have few signs or symptoms. You might not realize that you have kidney disease until the condition is advanced.
Treatment for chronic kidney disease focuses on slowing the progression of kidney damage, usually by controlling the cause. But, even controlling the cause might not keep kidney damage from progressing. Chronic kidney disease can progress to end-stage kidney failure, which is fatal without artificial filtering (dialysis) or a kidney transplant.
- How kidneys work
One of the important jobs of the kidneys is to clean the blood. As blood moves through the body, it picks up extra fluid, chemicals and waste. The kidneys separate this material from the blood. It's carried out of the body in urine. If the kidneys are unable to do this and the condition is untreated, serious health problems result, with eventual loss of life.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- A Book: The Body's Keepers
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of chronic kidney disease develop over time if kidney damage progresses slowly. Loss of kidney function can cause a buildup of fluid or body waste or electrolyte problems. Depending on how severe it is, loss of kidney function can cause:
- Loss of appetite
- Fatigue and weakness
- Sleep problems
- Urinating more or less
- Decreased mental sharpness
- Muscle cramps
- Swelling of feet and ankles
- Dry, itchy skin
- High blood pressure (hypertension) that's difficult to control
- Shortness of breath, if fluid builds up in the lungs
- Chest pain, if fluid builds up around the lining of the heart
Signs and symptoms of kidney disease are often nonspecific. This means they can also be caused by other illnesses. Because your kidneys are able to make up for lost function, you might not develop signs and symptoms until irreversible damage has occurred.
When to see a doctor
Make an appointment with your doctor if you have signs or symptoms of kidney disease. Early detection might help prevent kidney disease from progressing to kidney failure.
If you have a medical condition that increases your risk of kidney disease, your doctor may monitor your blood pressure and kidney function with urine and blood tests during office visits. Ask your doctor whether these tests are necessary for you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
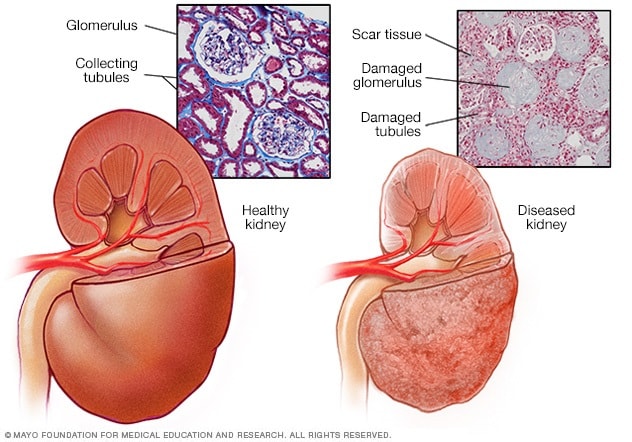
- Healthy kidney vs. diseased kidney
A typical kidney has about 1 million filtering units. Each unit, called a glomerulus, joins a tubule. The tubule collects urine. Conditions such as high blood pressure and diabetes harm kidney function by damaging these filtering units and tubules. The damage causes scarring.
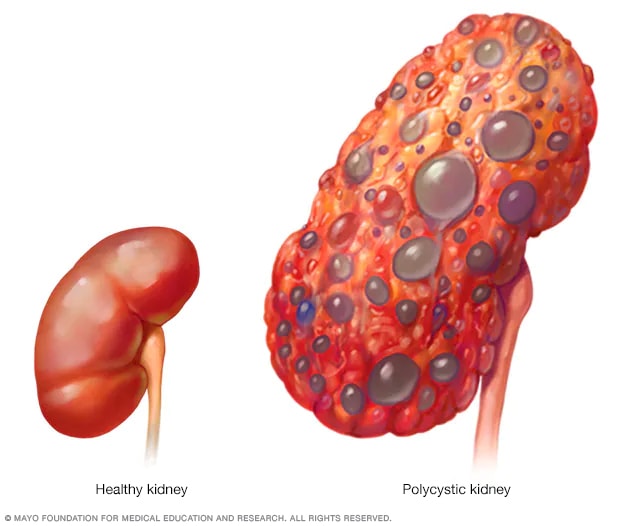
- Polycystic kidney
A healthy kidney (left) eliminates waste from the blood and maintains the body's chemical balance. With polycystic kidney disease (right), fluid-filled sacs called cysts develop in the kidneys. The kidneys grow larger and gradually lose the ability to function as they should.
Chronic kidney disease occurs when a disease or condition impairs kidney function, causing kidney damage to worsen over several months or years.
Diseases and conditions that cause chronic kidney disease include:
- Type 1 or type 2 diabetes
- High blood pressure
- Glomerulonephritis (gloe-mer-u-low-nuh-FRY-tis), an inflammation of the kidney's filtering units (glomeruli)
- Interstitial nephritis (in-tur-STISH-ul nuh-FRY-tis), an inflammation of the kidney's tubules and surrounding structures
- Polycystic kidney disease or other inherited kidney diseases
- Prolonged obstruction of the urinary tract, from conditions such as enlarged prostate, kidney stones and some cancers
- Vesicoureteral (ves-ih-koe-yoo-REE-tur-ul) reflux, a condition that causes urine to back up into your kidneys
- Recurrent kidney infection, also called pyelonephritis (pie-uh-low-nuh-FRY-tis)
Risk factors
Factors that can increase your risk of chronic kidney disease include:
- Heart (cardiovascular) disease
- Being Black, Native American or Asian American
- Family history of kidney disease
- Abnormal kidney structure
- Frequent use of medications that can damage the kidneys
Complications
Chronic kidney disease can affect almost every part of your body. Potential complications include:
- Fluid retention, which could lead to swelling in your arms and legs, high blood pressure, or fluid in your lungs (pulmonary edema)
- A sudden rise in potassium levels in your blood (hyperkalemia), which could impair your heart's function and can be life-threatening
- Heart disease
- Weak bones and an increased risk of bone fractures
- Decreased sex drive, erectile dysfunction or reduced fertility
- Damage to your central nervous system, which can cause difficulty concentrating, personality changes or seizures
- Decreased immune response, which makes you more vulnerable to infection
- Pericarditis, an inflammation of the saclike membrane that envelops your heart (pericardium)
- Pregnancy complications that carry risks for the mother and the developing fetus
- Irreversible damage to your kidneys (end-stage kidney disease), eventually requiring either dialysis or a kidney transplant for survival
To reduce your risk of developing kidney disease:
- Follow instructions on over-the-counter medications. When using nonprescription pain relievers, such as aspirin, ibuprofen (Advil, Motrin IB, others) and acetaminophen (Tylenol, others), follow the instructions on the package. Taking too many pain relievers for a long time could lead to kidney damage.
- Maintain a healthy weight. If you're at a healthy weight, maintain it by being physically active most days of the week. If you need to lose weight, talk with your doctor about strategies for healthy weight loss.
- Don't smoke. Cigarette smoking can damage your kidneys and make existing kidney damage worse. If you're a smoker, talk to your doctor about strategies for quitting. Support groups, counseling and medications can all help you to stop.
- Manage your medical conditions with your doctor's help. If you have diseases or conditions that increase your risk of kidney disease, work with your doctor to control them. Ask your doctor about tests to look for signs of kidney damage.
Chronic kidney disease care at Mayo Clinic
Living with chronic kidney disease?
Connect with others like you for support and answers to your questions in the Transplants support group on Mayo Clinic Connect, a patient community.
Transplants Discussions

22 Replies Wed, Apr 24, 2024

35 Replies Wed, Apr 24, 2024

16 Replies Tue, Apr 23, 2024
- Goldman L, et al., eds. Chronic kidney disease. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. http://www.clinicalkey.com. Accessed April 27, 2021.
- Chronic kidney disease (CKD). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd#:~:text=Chronic kidney disease (CKD) means,family history of kidney failure. Accessed April 26, 2021.
- Rosenberg M. Overview of the management of chronic kidney disease in adults. https://www.uptodate.com/contents/search. Accessed April 26, 2021.
- Chronic kidney disease (CKD) symptoms and causes. National Kidney Foundation. https://www.kidney.org/atoz/content/about-chronic-kidney-disease. Accessed April 26, 2021.
- Chronic kidney disease. Merck Manual Professional Version. https://www.merckmanuals.com/professional/genitourinary-disorders/chronic-kidney-disease/chronic-kidney-disease?query=Chronic kidney disease. Accessed April 26, 2021.
- Ammirati AL. Chronic kidney disease. Revista da Associação Médica Brasileira. 2020; doi:10.1590/1806-9282.66.S1.3.
- Chronic kidney disease basics. Centers for Disease Control and Prevention. https://www.cdc.gov/kidneydisease/basics.html. Accessed April 26, 2021.
- Warner KJ. Allscripts EPSi. Mayo Clinic; April 21, 2021.
- Office of Patient Education. Chronic kidney disease treatment options. Mayo Clinic; 2020.
- Chronic kidney disease: Is a clinical trial right for me?
- Eating right for chronic kidney disease
- Effectively managing chronic kidney disease
- Kidney biopsy
- Kidney disease FAQs
- Low-phosphorus diet: Helpful for kidney disease?
- MRI: Is gadolinium safe for people with kidney problems?
- Renal diet for vegetarians
Associated Procedures
- Deceased-donor kidney transplant
- Hemodialysis
- Kidney transplant
- Living-donor kidney transplant
- Nondirected living-donor transplant
- Peritoneal dialysis
- Preemptive kidney transplant
News from Mayo Clinic
- Mayo Clinic Minute: Why Black Americans are at higher risk of chronic kidney disease March 05, 2024, 05:00 p.m. CDT
- Mayo Clinic Minute: Can extra salt hurt your kidneys? Feb. 16, 2024, 04:00 p.m. CDT
- Mayo Clinic Minute: Using AI to predict kidney failure in patients with polycystic kidney disease April 06, 2023, 04:00 p.m. CDT
- Mayo Clinic Q and A: Understanding chronic kidney disease March 23, 2023, 12:35 p.m. CDT
- Mayo Clinic Minute: Game-changing treatment for chronic kidney disease could slow down progression of the disease March 06, 2023, 04:01 p.m. CDT
- Science Saturday: Seeking a cellular therapy for chronic kidney disease Nov. 12, 2022, 12:00 p.m. CDT
- Science Saturday: Mayo Clinic researchers integrate genomics into kidney disease diagnosis, care Sept. 17, 2022, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
Kidney Disease
- Español
The kidneys are two bean-shaped organs. Each kidney is about the size of a fist. Your kidneys filter extra water and wastes out of your blood and make urine. Kidney disease means your kidneys are damaged and can’t filter blood the way they should.
You are at greater risk for kidney disease if you have diabetes or high blood pressure. If you experience kidney failure, treatments include kidney transplant or dialysis. Other kidney problems include acute kidney injury, kidney cysts, kidney stones, and kidney infections.

Featured Topics
- Chronic Kidney Disease (CKD) Overview
- Preventing Chronic Kidney Disease
- Eating Right for Chronic Kidney Disease
- Quick Reference on UACR & GFR
- Kidney Failure
- Diabetic Kidney Disease
- IgA Nephropathy
- Polycystic Kidney Disease (PKD)
- Simple Kidney Cysts
- Kidney Infection (Pyelonephritis)
- Kidney Stones
Understanding Your Kidneys & CKD
Common questions and brief answers about the kidneys, chronic kidney disease, and how to reduce your risk.
Children & Youth
- Kidney Disease in Children
- Growth Failure in Children with Chronic Kidney Disease
- Helping Your Child Adapt to Life with Chronic Kidney Disease
- Nutrition for Chronic Kidney Disease in Children
- Kidney Stones in Children
- Hemolytic Uremic Syndrome in Children
- Multicystic Dysplastic Kidney
- Nephrotic Syndrome in Children
- IgA Vasculitis
Kidney Disease Topics
Chronic kidney disease.
- Anemia in CKD
- Causes of CKD
- CKD Overview
- CKD Tests & Diagnosis
- Diet & Nutrition for Adults with Advanced Chronic Kidney Disease
- Eating Right for CKD
- High Blood Pressure & Kidney Disease
- Managing CKD
- Mineral & Bone Disorder in CKD
- Preventing CKD
- Eating & Nutrition for Hemodialysis
- Financial Help for Treatment of Kidney Failure
- Hemodialysis
- Kidney Transplant
- Peritoneal Dialysis
Other Kidney Topics
- Acquired Cystic Kidney Disease
- Amyloidosis & Kidney Disease
- Diabetes Insipidus
- Ectopic Kidney
- Glomerular Disease
- Anti-GBM (Goodpasture's) Disease
- Lupus Nephritis
- Medullary Sponge Kidney
- Nephrotic Syndrome in Adults
- Pain Medicine & Kidney Damage
- Renal Artery Stenosis
- Renal Tubular Acidosis
- Solitary or Single-functioning Kidney
- Your Kidneys & How They Work
- Your Urinary Tract & How It Works

Statistics for Kidney Disease in the United States
Community Health & Outreach

Kidney Disease for Health Professionals
- Identify & Manage Patients
- Laboratory Evaluation
- Talking With Your Patients About Kidney Disease
Healthy Moments Radio
Listen to health tips from Dr. Rodgers in his weekly 1-minute episodes.
- Get Involved in Kidney Research
- Keeping Your Kidneys Healthy
- What is Kidney Disease?
Clinical Trials

Research Discoveries & News
- Scientists discover potential treatment approaches for polycystic kidney disease
- New atlas of human kidney cells to help unlock kidney disease research
- Being hospitalized with acute kidney injury may increase risk for rehospitalization and death
- Resolving key details of polycystic kidney disease genetics
The browser you are using is no longer supported and for that reason you will not get the best experience when using our website.
You currently have JavaScript disabled in your web browser, please enable JavaScript to view our website as intended.
New £10.4 million research centre will unlock new tests, treatments and cures for people living with rare kidney diseases
Professor Jonathan Barratt
Thousands of people living with rare kidney disease will have access to improved diagnostics, treatments and potentially cures, thanks to the creation of a new research centre, involving experts from the University of Leicester.
It follows a major report from Kidney Research UK which showed that kidney failure could overwhelm the health care system within ten years.
The LifeArc-Kidney Research UK Centre for Rare Kidney Diseases will provide urgent focus and resource, uniting researchers, patients and healthcare professionals and building on strong established resources, including the national registry of rare kidney diseases (RaDaR), the national renal sample biobank (NURTuRE) and care guidelines.
It signals the start of a transformation in all 13 of the UK’s children’s kidney centres to embed a culture of research by connecting the systems to accelerate discoveries and advance the treatment of rare kidney diseases.
Medical research charity, LifeArc, has invested £9.4 million into the new translational centre, with a further £1 million contribution from Kidney Research UK to support work over the next five years.
It will be led by Dr Louise Oni, Senior Lecturer in Paediatric Nephrology at the University of Liverpool and honorary consultant paediatric nephrologist at Alder Hey Children’s Hospital.
Professor Jonathan Barratt leads the Renal Research Group within the University of Leicester. His research is focused on a bench to bedside approach to improving understanding of the pathogenesis of IgA nephropathy – a common global cause of kidney failure. As such he will be heavily involved in the new centre and said: “It will offer a transformative approach to the study and care of children with rare kidney diseases.
“Having worked with Louise to help develop the LifeArc proposal it is clear to me that this programme of work has the potential to transform how adult and paediatric nephrologists work together to find cures for rare kidney diseases.
“LifeArc will leverage the 25 years plus expertise we have in Leicester in the study of IgAN and IgA vasculitis, two rare causes of kidney disease that affect both adults and children, and will utilise the cutting-edge technologies we have available in The Mayer IgA Nephropathy Laboratories to study changes at the molecular level in the kidneys of children with these diseases.”
Dr Louise Oni said: “This UK wide project aims to create a culture of constant learning to bring rapid advances to patients of all ages living with kidney diseases. It will start by focusing on children with rare kidney diseases to attempt to halt the journey to kidney failure and then upscale into adult patients. Through collaboration, the templates presented by these rare disease centres will support mass transformation to benefit many other patients.”
Related stories
Leicester and leicestershire can breathe easy as university unveils pollen monitor, potential new worlds rescued by citizens of earth, prestigious award for mathematician exploring climate by numbers, student nurses shortlisted for prestigious awards, new network launched to advance research into huntington’s disease, nhs staff retention to be investigated in new study.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 22, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Leukocyte glucose index, arteriovenous fistula failure linked in ESKD
by Elana Gotkine
For patients with end-stage kidney disease (ESKD), a high preoperative leukocyte glucose index (LGI) is associated with arteriovenous fistula (AVF) failure, according to a study published online April 1 in the Journal of Clinical Medicine .
Adrian Vasile Muresan, Ph.D., from the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures in Romania, and colleagues examined the impact of LGI on long-term primary patency of AVF following initiation of dialysis in 158 patients with ESKD. AVF failure, defined as the impossibility of performing chronic dialysis due to severe restenosis or AVF thrombosis, was examined as the primary end point.
The researchers found that the prevalence rates of atrial fibrillation and diabetes were higher in patients with AVF failure, and they had a higher LGI value. In a receiver operating characteristic analysis, the strongest association with the outcome was seen for LGI, with an area under the curve of 0.729 and an optimal cutoff of 0.95 (sensitivity and specificity of 72.4 and 68 percent, respectively). Patients in the highest versus the lowest tertile of LGI had a significantly higher incidence of AVF failure in Kaplan-Meier survival analyses. The risk for AVF failure during follow-up was significantly higher for patients with higher baseline LGI values (hazard ratio, 1.48); the association was independent of age and sex, cardiovascular risk factors , and preoperative vascular mapping determinations (hazard ratios, 1.65, 1.63, and 3.49, respectively).
"LGI can be used as a potential biomarker to identify patients from risk groups that require more careful monitoring of AVF in order to improve the management and care of patients with ESKD," the authors write.
Copyright © 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Living at higher altitudes in India linked to increased risk of childhood stunting
2 hours ago

Study confirms effectiveness of bivalent COVID-19 vaccine
4 hours ago

How does aging start? Scientists explain how IgG antibodies are a driving factor

Study reveals emotional turmoil experienced after dog-theft is like that of a caregiver losing a child

Targeting specific protein regions offers a new treatment approach in medulloblastoma
5 hours ago

Study recommends exposing deaf children to sign language before and after cochlear implantation

Study reveals racial disparities in COVID-19 testing delays among health care workers

Study reports new compound that halts replication of COVID by targeting 'Mac-1' protein in cell models

Shoulder surgeons should rethink a common practice, new study suggests

Study finds vitamin D alters mouse gut bacteria to give better cancer immunity
6 hours ago
Related Stories
Paclitaxel-coated balloon effective for coronary in-stent restenosis: Study
Mar 11, 2024

Smoking tied to earlier death in dialysis patients
Oct 24, 2022
In LVSD, diabetes tied to higher risk of heart failure
Apr 20, 2018
Ablation cuts risk of recurrent stroke in patients with A-fib
Nov 28, 2017

Blacks, Hispanics on dialysis get more staph infections than whites: CDC
Feb 6, 2023
Study shows postoperative atrial fibrillation tied to worse outcomes after valve surgery
Dec 18, 2023
Recommended for you

Food in sight? The liver is ready in minutes: Study shows how adapting sugar metabolism starts in the brain

Creatine found to improve cognitive performance during sleep deprivation
9 hours ago

Understanding the cellular mechanisms of obesity-induced inflammation and metabolic dysfunction
11 hours ago

Link between depression and cardiovascular disease explained: They partly develop from same gene module
20 hours ago

Circadian rhythms can influence drugs' effectiveness
Apr 24, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
A woman with failing kidneys receives genetically modified pig organs

Dr. Jeffrey Stern, assistant professor in the Department of Surgery at NYU Grossman School of Medicine, and Dr. Robert Montgomery, director of the NYU Langone Transplant Institute, prepare the gene-edited pig kidney with thymus for transplantation. Joe Carrotta for NYU Langone Health hide caption
Dr. Jeffrey Stern, assistant professor in the Department of Surgery at NYU Grossman School of Medicine, and Dr. Robert Montgomery, director of the NYU Langone Transplant Institute, prepare the gene-edited pig kidney with thymus for transplantation.
NEW YORK — Lisa Pisano was lying in a hospital bed at NYU Langone Health, hooked up to beeping monitors and an array of tubes. Her surgical wounds were still healing, and she looked tired. But the 54-year-old New Jersey woman said she hasn't felt this good in years.
"I'm feeling better and better and better every day," said Pisano, 54, of Cookstown, N.J. "I got somewhat of me back. Not there yet. But I'm getting there."
Ten days earlier, Pisano became the second living person in the world to get a kidney from a genetically modified pig transplanted into her body to replace her own failing organs, her doctors announced Wednesday. A Massachusetts man was the first to get a pig kidney last month.
Pisano also got a thymus gland from the same genetically engineered pig to help prevent her body from rejecting the kidney, as well as a pump to shore up her failing heart.
"I'm amazed," said Pisano during a bedside interview two days before her kidney transplant was announced publicly. "I'm absolutely amazed that it's an option for me. Because I didn't think I even had that option."
Progress in human transplant of animal organs
Pisano's transplant is the latest development in the fast-moving effort to use genetically modified pigs to solve the persistent shortage of organs for transplants. More than 103,000 people are currently on the waiting list for organs. About 17 die every day because they can't get one.
"We're in a new universe in transplantation," said Dr. Robert Montgomery , who runs the NYU Langone Transplant Institute where the operation was performed. "This would be a sustainable, unlimited source of organs. This would be transformative."

Shots - Health News
How genetically modified pigs could end the shortage of organs for transplants.
Many transplant specialists are excited by the research. But the effort is also raising some concerns. Some doctors worry pig organs could spread viruses to people. Some critics are uncomfortable with the prospect of breeding thousands of genetically modified animals to be slaughtered for their organs. Some also are concerned about using vulnerable patients for experimentation.
"This is a real landmark procedure," said Karen Maschke , a bioethicist at The Hastings Center, a bioethics think tank in Garrison, N.Y. "But there are lot of issues that need to be discussed."
When Pisano arrived at the hospital, she was within weeks — maybe even days — of dying, Montgomery said. Years of diabetes had taken a terrible toll. She had suffered multiple heart attacks and was on dialysis to compensate for her failing kidneys.
"I didn't really have a life," she said. "I didn't do anything. I just sat around. I couldn't get up and do anything. I couldn't even cook dinner. I couldn't vacuum. I couldn't play with my grandkids because I couldn't bend down to get them. I just couldn't do anything with them. And that is the most horrible feeling in the world. That was really, really tearing me apart. I was almost at the point of giving up. It was terrible."
Pisano wasn't eligible to get a human organ transplant because she had too many other health problems, especially serious heart problems. So she jumped at the chance to get a pig kidney.
"My first thought was: 'Wow, I can't even believe that was even possible.' So when it was brought to my attention I was like, 'You know what? I'm going to try it.' I said, 'You know what? I'm going to do it. I have to do it — for myself and for the rest of my family.' "
A transplant for research — and to buy time
The hope is the pig kidney will give Pisano at least a little more time, and provide researchers with important information they could use to improve the outcome in future transplants. Pisano also needs to take anti-rejection medication.
"When we brought her into the hospital, she was in really bad shape," said Montgomery. "None of us could have imagined that it would have gone this smoothly."
"Her kidney is working better than yours or mine. So we're optimistic that she'll be able to go home and spend time with her children and grandchildren and live a comfortable life," Montgomery told NPR in an interview before the announcement.
He stressed, however, that she will probably need several months to recover in the hospital before she can go home. He also said he couldn't predict how much more time Pisano may gain from the procedure.
Beyond Pisano's case, much more research is needed before organs from genetically modified pigs could become commonly used. "It's still early," Montgomery said. "These are early days. There's still a lot we need to learn and perfect."

First human transplant of a genetically modified pig kidney performed
Surgeons had previously transplanted kidneys and livers from genetically modified cloned pigs into baboons and a handful of people whose brains had stopped functioning. Surgeons at the University of Maryland even tested hearts in two men who had run out of other options. They lived for several weeks after the procedures. The Massachusetts man who got the pig kidney left the hospital within weeks and is still doing well, according to Massachusetts General Hospital.
Pig organs are genetically modified for human compatibility
The organs come from pigs that have been genetically modified to minimize the risk they will be rejected by the human body, spread pig viruses to people or cause other complications.
NPR recently got exclusive access to one research farm breeding pigs for Revivicor Inc. of Blacksburg, Va., the biotech company that produced the kidney and thymus Pisano received. The kidney transplanted in Boston came from a pig created by eGenesis of Cambridge, Mass.
The companies hope that someday an ample supply of genetically modified pigs will save thousands of lives.
The modified organs haven't been reviewed or approved for widespread use.
The transplant of the pig hearts and kidneys were made possible by the Food and Drug Administration as part of a " compassionate use " program aimed at helping desperate patients.
"I think there are worries about conducting these experiments in this way, where we are finding the most desperate patients who have no other options," said L. Syd Johnson , a bioethicist at SUNY Upstate Medical University in Syracuse, N.Y.
"Maybe those patients will benefit. Maybe they believe they will benefit and that the risks are worthwhile for them. But I do worry about whether or not we are taking advantage of particularly vulnerable and desperate patients in conducting these experiments," Johnson said.
The doctors performing the transplants, and some independent observers, say the volunteers are fully informed of the risks and potential benefits.
But some would prefer the FDA approve a formal study to fully evaluate the approach instead of granting individual patient's approval.
"It's a remarkable development. It is incredible how quickly this is progressing," said Michael Gusmano , a bioethicist at Lehigh University. "But I remain concerned about the use of the expanded access protocol in lieu of moving forward with a trial."
The companies hope the FDA will authorize a formal study soon.
Pisano just hopes for some more time with her grandchildren.
"Any time on this Earth is better than none," she said. "So if I get two years, that's two years that I didn't have before."
- xenotransplantation
- kidney transplants
- organ transplants
Honoring Three NKF Volunteers with The Arthur P. Pasquarella Leadership in Action Award
April 25, 2024, 8:10am EDT

At the National Kidney Foundation, we are committed to educating people about their risk of kidney disease while dismantling structural inequities in kidney care, dialysis, and transplantation.
We've made tremendous strides over the years, including the adoption of a race-free eGFR equation and passing the Securing the U.S. Organ Procurement and Transplantation Network Act.
None of this would have been possible without the countless volunteers and Voices for Kidney Health advocates who work alongside us.
Today, we honor three volunteers who've gone above and beyond in their kidney advocacy.
Kelli Strother
"Anything we can do to make a patients time on dialysis better and to make them feel supported and cared for is great. Anything we can do to increase living donations and get people off dialysis is even better." says living donor, Kelli Strother. https://t.co/c2qC37Bq78 — National Kidney Foundation (@nkf) October 25, 2022
Kelli Strother first visited the Colorado NKF office in 2018. She went on behalf of her employer to brainstorm mental health support for those with kidney disease. After discussing mental health, Kelli shared that her father was on dialysis. Touched by her story, the NKF team invited her to future events.
"I showed up and saw all these kidney donors and recipients that were participating. One man wore a shirt that said 'rock one kidney'. The attitude towards it was fun," said Kelli. "I got to ask questions and talk one-on-one with donors. One donor was an ultra-distance runner who had done 100-mile runs with one kidney."
Inspired, Kelli participated in a paired kidney donor exchange , which resulted in life-saving transplants for her father and another recipient.
Her involvement didn't end there. Kelli continued to advocate with NKF and was pivotal in passing legislation protecting living kidney donors in Colorado. Kelli continues to attend NKF events and lobby for improved kidney disease policies on a national level.
Read Kelli Strother's kidney donation story .
David Sultzer
The Kidney Walk was great for Jean's Team. #Heartyourkidneys pic.twitter.com/P3GI1GUR5r — Dave Sultzer (@StatsPM) October 13, 2020
Dave Sultzer, a retired military officer, has dedicated over a decade to volunteering with NKF. His journey began after he donated a kidney to his mother. He saw an NKF kidney disease screening event ad and felt called to volunteer.
Since then, Dave has consistently attended nearly every NKF event, from KEEP Healthy Kidney screenings to the Kidney Walk and NKF Golf Classic . Though his mother has passed away, Dave honors her legacy through his advocacy efforts.
Driven by the desire to encourage early kidney function testing and make living donation more obtainable, Dave passionately advocates for legislative change. He believes that kidney disease does not discriminate based on age, race, or demographics and emphasizes its impact on patients and their caregivers.
“Young people, old people, Black, white, Asian, Hispanic, it doesn't matter," Dave told Spectrum News New York 1. "Your population, your race, your demographic, your age–this disease can affect you and change not only your but the life of your caregivers."
Dave’s advocacy continues. He holds leadership positions in NKF's Kidney Advocacy Committee and the Wizards Organ Donation Awareness Tournament. He also serves on the NKF/NCA Board of Advisors and the Medical Advisory Board.
Learn more about NKF's Kidney Advocacy Committee .
Sheila Gordon
"It has been an honor to serve as Chair of the Authors Luncheon for many years. …The event has raised awareness of kidney disease and raised millions of dollars in support of the work of the NKF.” – Sheila Gordon, Event Chair. pic.twitter.com/SXZ4ldw9ES — NatlKidneyFoundPNW (@NKFPNW) October 10, 2023
Sheila Gordon has been involved in the NKF Authors Luncheon for over three decades–first as an NKF employee and then as a volunteer and chair of the Authors Luncheon Planning Committee.
Each year, she generously shares her time, talents, and expertise to ensure that the Authors Luncheon planning and execution are financially and experientially successful.
"It's an honor to serve as Chair of this incredible event and reach this significant milestone of 35 years celebrating literature while increasing awareness of kidney disease and raising millions of dollars to support NKF's work," said Sheila Gordon. "In the decades I have been doing this work, kidney care has undergone remarkable transformations–living donor transplants and chain donations are common, xenotransplantation is on the horizon and could alleviate wait times for transplants and home dialysis is now possible for millions of patients."
Sheila has helped raise hundreds of thousands of dollars fo r NKF. Her wealth of knowledge, experience, and close relationships with longtime supporters are invaluable. She's contributed greatly to the event's growth and funds raised over the years!
Read about NKF's 35th Annual Authors Luncheon .
Get involved
Do Kelli, David, and Sheila inspire you? Get involved with NKF . Whether you want to give, volunteer, or advocate, we need your help to win the fight against kidney disease.
Related content
Meet five inspiring Voices for Kidney Health advocates at the forefront of the battle for kidney equity. They faced kidney disease head-on, and now they're helping lea...
It's time to celebrate all the achievements Voices for Kidney Health advocates drove forward this past year. We passed the U.S. Organ Procurement and Transplantation N...

IMAGES
VIDEO
COMMENTS
Kidney diseases are hereditary and nonhereditary disorders that affect the kidney. Diabetes mellitus and high blood pressure are important risk factors for kidney disease. Obstructive nephropathy ...
A policy call to address rare kidney disease in health care plans. Clin. J. Am. Soc. ... Hocher, B. & Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev.
In a major breakthrough toward understanding and treating kidney disease, a nationwide research team funded by the National Institutes of Health has created the most comprehensive atlas of the human kidney. ... clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases ...
Loss of kidney function is an important health challenge whether it occurs suddenly or over a long period of time. The NIDDK supports basic and clinical research on kidney development; the causes of kidney disease; improving kidney health equity and reducing kidney health disparities; the underlying mechanisms leading to progression of kidney ...
Introduction. Current international guidelines define chronic kidney disease (CKD) as an abnormality of kidney function or structure that is present for at least 3 months, regardless of underlying causes, with implications for health. [ 1] The prevalence of CKD varies worldwide due to differences in socioeconomic conditions and ethnicity.
About AJKD. First published in 1981, the American Journal of Kidney Diseases (AJKD) is the official journal of the National Kidney Foundation Opens in new window , AJKD is recognized worldwide as a leading source of information devoted to clinical nephrology research and practice. Learn more.
Here are nine of the biggest headlines from the nephrology research field from 2023. Breakthrough #1: New atlas of human kidney cells to help unlock kidney disease research | National Institutes of Health (NIH) In July, the National Institutes of Health (NIH) announced it had created a "comprehensive atlas of the human kidney."
Kidney tissues taken for study undergo state-of-the-art processing, including genetic sequencing to identify meaningful cellular pathways and proteins linked to disease patterns, Parikh said, toward the goal of more personalized therapies for patients. "People didn't know that so many cell types exist in the kidney," Parikh says. "It ...
The study is designed to recruit 45% of the study population with Chronic Kidney Disease (CKD). The trial will test the effects of low systolic blood pressure (SBP) goal of < 120 mm Hg versus the standard goal of < 140 mm Hg on the primary composite of cardiovascular events and death.
October 7, 2021, New York, NY — The National Kidney Foundation (NKF) released today a research roadmap that, if funded by Congress, would quickly accelerate innovations in treatment and increase understanding of kidney disease. Kidney disease is growing in the United States so much so that now 1 in 3 adults are at risk, yet the pace of ...
Chronic kidney disease (CKD) has emerged as one of the most prominent causes of death and suffering in the 21 st century. Due in part to the rise in risk factors, such as obesity and diabetes mellitus, the number of patients affected by CKD has also been increasing, affecting an estimated 843.6 million individuals worldwide in 2017. 1 Although mortality has declined in patients with end-stage ...
Chronic kidney disease (CKD) represents a global public health crisis, but awareness by patients and providers is poor. Defined as persistent abnormalities in kidney structure or function for more than three months, manifested as either low glomerular filtration rate or presence of a marker of kidney damage such as albuminuria, CKD can be identified through readily available blood and urine tests.
Kidneys are remarkable organs. Both blood filters and endocrine organs, kidneys remove waste, regulate electrolytes and acid-base homoeostasis, control fluid balance and blood pressure, and regulate bone metabolism and red blood cell production. They are intimately connected with the functioning of other organs, such as the heart and liver, and can be devastated by diseases as varied as ...
In a preclinical model of human diabetic kidney disease, turning off this process by administering an antibody that binds to IL-11 led to proliferation of the kidney tubule cells and reversal of ...
Rare kidney diseases comprise more than 150 conditions, most of which are inherited.1 Rare kidney diseases have a prevalence of approximately 60-80 cases per 100 000 people in Europe and the USA.2 The diagnosis of a rare kidney disease is often delayed,3 and a substantial number of patients will progress to kidney failure and require kidney replacement therapy. However, there is little ...
Background The purpose of this study was to look at the relationship between the Systemic Immune Inflammatory Index (SII) and bone mineral density (BMD) in the pelvis, left upper and lower limbs, lumbar spine, thoracic spine, and trunk in a chronic kidney disease (CKD) population in the United States. Methods The National Health and Nutrition Examination Survey (2011-2016) yielded 2302 ...
Chronic kidney disease is the medical term used to describe the gradual loss of kidney function over a period of at least three months. Because of this, excess fluid and waste from the blood remain in the body and may cause other health problems, such as heart disease and stroke, in addition to kidney failure.. CKD can occur for a variety of reasons, but diabetes and high blood pressure are ...
A new era in the science and care of kidney diseases. Despite notable progress in basic, clinical and translational nephrology research in the past 50 years, many challenges remain. In this Review ...
Introduction. Diabetes mellitus is among the leading causes of chronic kidney disease and end-stage kidney disease in the western world. It was the most common diagnosis for the initiation of renal replacement therapy in the United States in 2018, accounting for 47% of the cases ().Type 1 and type 2 diabetes mellitus share many clinical characteristics and long-term complications, but they are ...
Dry, itchy skin. High blood pressure (hypertension) that's difficult to control. Shortness of breath, if fluid builds up in the lungs. Chest pain, if fluid builds up around the lining of the heart. Signs and symptoms of kidney disease are often nonspecific. This means they can also be caused by other illnesses.
The kidneys are two bean-shaped organs. Each kidney is about the size of a fist. Your kidneys filter extra water and wastes out of your blood and make urine. Kidney disease means your kidneys are damaged and can't filter blood the way they should. You are at greater risk for kidney disease if you have diabetes or high blood pressure.
Applying a person-centred lens has implications for several aspects of risk prediction research. Incorporating the patient voice may involve partnering with patients as researchers to identify the target outcome for the tool and/or determine priorities for outcomes related to the kidney disease domain of interest.
About kidney failure. Kidney failure means your kidneys are no longer able to work well enough to keep you alive. With kidney failure, 85-90% of your kidney function is gone. People with kidney failure have stage 5 CKD (also known as end-stage kidney disease or ESKD). People with kidney failure will need dialysis or a kidney transplant to survive.
It signals the start of a transformation in all 13 of the UK's children's kidney centres to embed a culture of research by connecting the systems to accelerate discoveries and advance the treatment of rare kidney diseases. Medical research charity, LifeArc, has invested £9.4 million into the new translational centre, with a further £1 ...
by Elana Gotkine. For patients with end-stage kidney disease (ESKD), a high preoperative leukocyte glucose index (LGI) is associated with arteriovenous fistula (AVF) failure, according to a study ...
NEW YORK — Lisa Pisano was lying in a hospital bed at NYU Langone Health, hooked up to beeping monitors and an array of tubes. Her surgical wounds were still healing, and she looked tired. But ...
Amino acids are essential for normal pregnancy and fetal development. Disruptions in maternal amino acid metabolism have been associated with various adult diseases later in life, a phenomenon referred to as the developmental origins of health and disease (DOHaD). In this review, we examine the recent evidence highlighting the significant impact of amino acids on fetal programming, their ...
Honoring Three NKF Volunteers with The Arthur P. Pasquarella Leadership in Action Award. April 25, 2024, 8:10am EDT. At the National Kidney Foundation, we are committed to educating people about their risk of kidney disease while dismantling structural inequities in kidney care, dialysis, and transplantation. We've made tremendous strides over ...