Cornell Chronicle
- Architecture & Design
- Arts & Humanities
- Business, Economics & Entrepreneurship
- Computing & Information Sciences
- Energy, Environment & Sustainability
- Food & Agriculture
- Global Reach
- Health, Nutrition & Medicine
- Law, Government & Public Policy
- Life Sciences & Veterinary Medicine
- Physical Sciences & Engineering
- Social & Behavioral Sciences
- Coronavirus
- News & Events
- Public Engagement
- New York City
- Photos of the Week
- Big Red Sports
- Freedom of Expression
- Student Life
- University Statements
- Around Cornell
- All Stories
- In the News
- Expert Quotes
- Cornellians

Large-scale study reveals new genetic details of diabetes
By wynne parry weill cornell medicine.
In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research furthers efforts to better understand and ultimately treat this common metabolic disease.
Previous studies have generally examined the influence of individual genes. In research described Oct. 18 in Cell Metabolism, senior co-author Shuibing Chen , the Kilts Family Professor of Surgery at Weill Cornell Medicine, working alongside senior co-author Dr. Francis Collins , a senior investigator at the Center for Precision Health Research within the National Human Genome Research Institute of the U.S. National Institutes of Health, took a more comprehensive approach. Together, they looked at the contribution of 20 genes in a single effort.
“It’s very difficult to believe all these diabetes-related genes act independently of each other,” Chen said. By using a combination of technologies, the team examined the effects of shutting each down. By comparing the consequences for cell behavior and genetics, she said, “we found some common themes.”
As with other types of diabetes, Type 2 diabetes occurs when sugar levels in the blood are too high. In Type 2 diabetes, this happens in part because specialized cells in the pancreas, known as β-cells, don’t produce enough insulin, a hormone that tells cells to take sugar out of the blood for use as an energy source. Over time, high levels of blood sugar damage tissues and cause other problems, such as heart and kidney disease. According to the United States Centers for Disease Control and Prevention, nearly 9% of adults in the United States have been diagnosed with Type 2 diabetes.
Both genetic and environmental factors, such as obesity and chronic stress, can increase risk for it. Yet evaluating the role of the genetic contributors alone is a massive project. So far, researchers have identified more than 290 locations within the genome where changes to DNA can raise the likelihood of developing the disease. Some of these locations fall within known genes, but most are found in regions that regulate the expression of nearby genes.
For the new research, the team focused on 20 genes clearly identified as contributors. They began their investigation by using the gene editing system CRISPR-Cas9 to shut down these genes, one at a time, within 20 sets of identical stem cells.
These stem cells had the potential to generate any kind of mature cell, but the researchers coaxed them into becoming insulin-producing β-cells. They then examined the effects of losing each gene on five traits related to insulin production and the health of β-cells. They also documented the accompanying changes in gene expression and the accessibility of DNA for expression.
To make sense of the massive amount of data they collected, the team developed their own computational models to analyze it, leading to several discoveries: By comparing the effects of all 20 mutations on β-cells, they identified four additional genes, each representing a newly discovered pathway that contributes to insulin production. They also found that, of the original 20 genes, only one, called HNF4A, contributed to all five traits, apparently by acting as a master controller that regulates the activity of other genes. In one specific example, they explained how a small variation, located in a space between genes, contributes to the risk of diabetes by interfering with HNF4A’s ability to regulate nearby genes.
Ultimately, this study and others like it hold the promise of benefiting patients, Collins said. “We need to understand all the genetic and environmental factors involved so we can do a better job of preventing diabetes, and to develop new ideas about how to effectively treat it.”
Collins and Chen note that their approach may have relevance beyond diabetes, to other common diseases, such as Alzheimer’s, Parkinson’s and Crohn’s disease, that involve many genetic factors.
The work reported in this newsroom story was supported in part by the United States’ National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and the American Diabetes Association.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see the profile for Shuibing Chen .
Wynne Parry is a freelance writer for Weill Cornell Medicine.
Media Contact
Krystle lopez.
Get Cornell news delivered right to your inbox.
You might also like

Gallery Heading
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 19, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
Review summarizes the latest knowledge on type 2 diabetes
by Cactus Communications
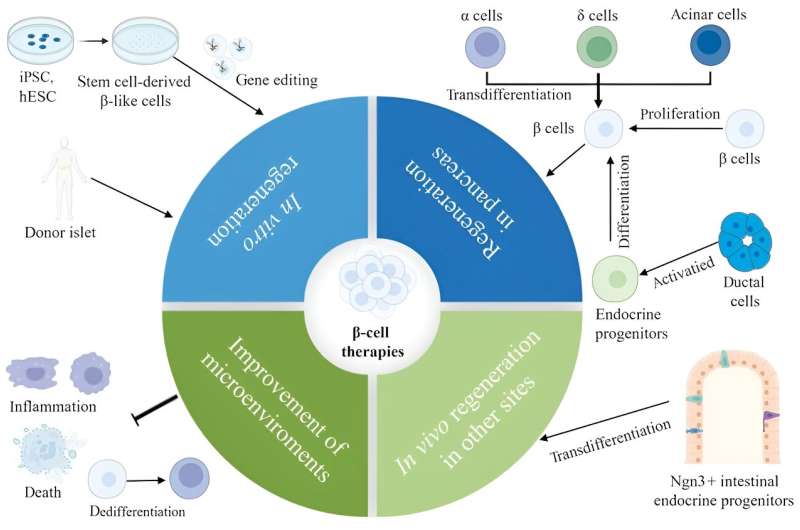
Diabetes is a widespread metabolic disorder affecting over 500 million adults worldwide. The most common form is type-2 diabetes (T2D), characterized by peripheral insulin resistance.
This implies that in patients with T2D, their peripheral tissues, such as muscle and fat cells, do not respond well to insulin, ultimately resulting in issues during the regulation of blood sugar levels. Another key feature of T2D is the progressive loss of function of b-cells in the pancreas, which are responsible for producing, storing, and releasing insulin.
T2D is generally manageable, with some patients relying on a strictly controlled diet, whereas others monitor their blood glucose levels and administer insulin as needed. Yet, poor management of the disease can lead to life-threatening consequences. To add to it, a definitive cure for T2D remains elusive.
Against this backdrop, a research team led by Professor Zhiguang Su from Sichuan University, China, recently decided to conduct an extensive literature review to summarize some of the latest knowledge pertaining to T2D. In their review, they touched upon the mechanisms that contribute to the failure of b-cells and potential therapeutic strategies.
The article was published in the Chinese Medical Journal .
The researchers first review the current understanding of the physiology of B-cells under normal conditions. They delve into the finer details of insulin production and secretion in these cells, exploring the regulated functions. Worth noting is the observation that insulin secretion is not a straightforward process regulated simply by sensing blood glucose levels ; it rather resembles a complex chemical orchestration, influenced by various factors such as adenosine triphosphate (ATP), GABA neurotransmitters, and signaling between different pancreatic cell types.
The article then delves into the latest scientific progress aimed at understanding the origin and development of T2D, technically known as ' T2D pathogenesis.' As mentioned previously, insulin resistance is one of the hallmarks of T2D. It renders cells throughout the body unable to respond normally to insulin and coordinate a blood glucose-lowering response.
Interestingly, available evidence suggests that long-term insulin resistance cascades into the failure of b-cells. Explaining further, Prof. Su says, "Hyperglycemia, accompanied by obesity, particularly visceral adiposity, causes insulin resistance, and more insulin is needed to override the ineffectiveness of insulin. Pancreatic b-cells detect this requirement and adaptively augment insulin synthesis and secretion through compensatory expansion of their mass to restore glucose homeostasis."
Adding further, he says, "Ultimately, with increasing time, the number of b-cells as well as their secretory function progressively decline, and glucose homeostasis is impaired, eventually causing diabetes." The failure of b-cells is complex and is a result of a combination of mechanisms, including aging, genetics, oxidative stress, inflammation, and even the transformation of b-cells into other cell types.
Finally, the review explores diverse therapeutic strategies aimed at replenishing and regenerating b-cells. As the review suggests, an optimal solution could involve stimulating the proliferation of one's b-cells through chemical signaling. Scientists have reported some success in using molecules produced by organs besides the pancreas and small-molecule drugs to induce B-cell proliferation. However, it is essential to note that these studies were conducted in mice, and thorough testing is required to determine the efficacy of these methods in humans.
Discussing these observations, an optimistic Professor Su says, "With the increasing appreciation of the mechanisms involved in promoting human b-cell proliferation and the development of high-throughput screening tools, it is anticipated that more small molecules and drugs expanding functional b-cell mass will be identified."
An alternative approach to address T2D is through pancreas transplants, either complete or partial. While promising a T2D cure, transplanted tissues face the challenge of immune system targeting, requiring recipients to take immunosuppressants, which can lead to a battery of new problems.
Another potential treatment for T2D could be the generation of b-cells using stem cells , presenting an immune system-friendly option. However, some important challenges will still remain to be addressed. For one, it appears not all b-cells are equal, and their proper functioning in the pancreas depends on having an appropriate balance of subpopulations. Moreover, so far, stem cell-derived B-cells do not perform as well as regular B-cells do.
In short, the review article presents a comprehensive summary of the recent advances in this field of research and will hopefully inspire researchers toward further research. With any luck, a cure for this common disorder might be within reach.
As Prof. Su remarks, "Although many challenges remain unanswered, we believe that b-cell regenerative therapies will represent a viable cure for diabetes in the not-too-distant future with the recognition of mechanisms responsible for b-cell development and mature endocrine cell plasticity, remarkable advances in improved protocols to generate b-cells from stem cells and single-cell studies."
Explore further
Feedback to editors

Can animals count? Neuroscientists identify a sense of numeracy among rodents
2 hours ago

Pressure to lose weight in adolescence linked to how people value themselves almost two decades later
4 hours ago

Many people with breast cancer 'systematically left behind' due to inaction on inequities and hidden suffering
5 hours ago

How trauma gets 'under the skin': Research investigates impaired muscle function caused by childhood trauma
8 hours ago

New mechanism uncovered in early stages of Alzheimer's disease

Study reveals AI enhances physician-patient communication

Newly found rare cells could be a missing link in color perception

New vaccine strategy may mean the end of the line for endless boosters
9 hours ago

Research explores why we remember what we remember

Epilepsy drug prevents brain tumors in mice with neurofibromatosis type 1
Related stories.
Research reveals that stimulating nerves connected to the pancreas can regenerate insulin-producing cells
Dec 4, 2023
Novel molecular mechanisms in the early development of diabetes mellitus
Nov 21, 2023
Closing in on the ultimate quest to regenerate insulin in pancreatic stem cells
Jan 2, 2024

Boosting function and survival of stem cell-derived pancreatic cells by genetic engineering
Mar 3, 2022
Study shows reactivation of beta-like cells in the pancreas to produce insulin
Jun 16, 2023
Protein coding gene coordinates cell division, protein synthesis, and mitochondrial activity in β-cells
Sep 7, 2023
Recommended for you

Study suggests light physical activity as a child is key to reducing risk of type 2 diabetes
Apr 9, 2024

Electronic sock detects unhealthy walking style linked to cardiovascular and diabetic complications
Apr 8, 2024

New insights into adult-onset type 1 diabetes
Apr 1, 2024
Researchers report clear shift in arterial diseases in diabetes
Mar 28, 2024

Risk prediction using genes and gut bacteria can improve early detection of diseases like type 2 diabetes
Mar 26, 2024

Insulin copayment caps not associated with an increase in insulin use among commercially insured populations
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
The use of potentially interacting supplement-drug pairs in adults with type 2 diabetes: A large population-based cohort study in the UK Biobank
Affiliations.
- 1 School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China.
- 2 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China.
- 3 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China; Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China.
- 4 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China; Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China; Phase 1 Clinical Trial Centre, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China.
- 5 School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong Special Administrative Regions of China. Electronic address: [email protected].
- PMID: 38583779
- DOI: 10.1016/j.diabres.2024.111658
Aims: To examine the patterns of use of potentially interacting supplement-drug pairs in adults with type 2 diabetes (T2D) in real-world settings, and to explore the impact of potentially interacting supplement-drug pairs on downstream outcomes.
Methods: Potentially interacting supplement-drug pairs were identified from four tertiary databases. We categorized the potential pharmacodynamic interactions into different clinical types according to their related outcomes and explored their associations with incident outcomes using Cox models.
Results: 26,394 participants with T2D in the UK Biobank were included. Half (48.5 %) were supplement users, of whom 85.0 % were taking potentially interacting supplement-drug pairs. The potential pharmacodynamic interactions were related to various clinical outcomes, including reducing the effects of glucose-lowering drugs (50.7 %), hypotension (49.8 %), bleeding (50.4 %) and hepatotoxicity (34.8 %). Exploratory analyses found that the use of potentially interacting supplement-drug pairs was associated with incident hepatic diseases (hazard ratio = 1.26, 95 % confidence interval 1.10-1.44, P < 0.001).
Conclusions: Real-world data suggests that most adults with T2D who concurrently used supplements and drugs were on potentially interacting supplement-drug combinations, with the potential of causing adverse outcomes such as incident hepatic diseases. Clinicians should communicate with patients and assess the potential risk of supplement-drug interactions in clinical settings.
Keywords: Dietary supplement; Supplement–drug interactions; Type 2 diabetes.
Copyright © 2024 Elsevier B.V. All rights reserved.
This outdated diabetes drug still has something to offer
By learning how an old diabetes drug works, researchers are discovering new, safer treatment options.
Thiazolidinediones (TZDs) are a class of drug that can be used to treat type 2 diabetes by reversing insulin resistance, one of the main hallmarks of the disease. While TZDs were extremely popular in the 1990's and early 2000's, they have fallen out of use among physicians in recent decades because they were discovered to cause unwanted side effects, including weight gain and excess fluid accumulation in body tissues.
Now, researchers at University of California San Diego School of Medicine are exploring how to isolate the positive effects of these drugs, which could help yield new treatments that don't come with the old side effects. In a new study published in Nature Metabolism , the researchers discovered how one of the most well-known TZD drugs works at the molecular level and were able to replicate its positive effects in mice without giving them the drug itself.
"For decades, TZDs have been the only drugs we have that can reverse insulin resistance, but we seldom use them anymore because of their side effects profile," said Jerrold Olefsky, M.D., a professor of medicine and assistant vice chancellor for integrative research at UC San Diego Health Sciences. "Impaired insulin sensitivity is the root cause of type 2 diabetes, so any treatment we can develop to safely restore this would be a major step forward for patients."
The main driver of insulin resistance in type 2 diabetes is obesity, which currently affects more than 40 percent of Americans and in 2021 bore an annual medical cost of nearly $173 billion. In addition to causing adipose tissue (fat) to expand, obesity also causes low levels of inflammation. This inflammation causes immune cells, called macrophages, to accumulate in adipose tissue, where they can comprise up to 40 percent of the total number of cells in the tissue.
When adipose tissue is inflamed, these macrophages release tiny nanoparticles containing instructions for surrounding cells in the form of microRNAs, small fragments of genetic material that help regulate gene expression. These microRNA-containing capsules, called exosomes, are released into the circulation and can travel through the bloodstream to be absorbed by other tissues, such as the liver and muscles. This can then lead to the varied metabolic changes associated with obesity, including insulin resistance. For the current study, the researchers wanted to understand how TZD drugs, which restore insulin resistance, affect this exosome system.
The researchers treated a group of obese mice with rosiglitazone, a type of TZD drug. Those mice became more sensitive to insulin, but they also gained weight and retained excess fluid, known side effects of rosiglitazone. However, by isolating exosomes from the adipose tissue macrophages of the mice who had received the drug and injecting them into another group of obese mice that had not received it, the researchers were able to deliver the positive effects of rosiglitazone without transferring the negative effects.
"The exosomes were just as effective in reversing insulin resistance as the drug itself but without the same side effects," said Olefsky. "This indicates that exosomes can ultimately link obesity-related inflammation and insulin resistance to diabetes. It also tells us that we may be able to leverage this system to boost insulin sensitivity."
The researchers were also able to identify the specific microRNA within the exosomes that was responsible for the beneficial metabolic effects of rosiglitazone. This molecule, called miR-690, could eventually be leveraged into new therapies for type 2 diabetes.
"It's likely not practical to develop exosomes themselves as a treatment because it would be difficult to produce and administer them, but learning what drives the beneficial effects of exosomes at the molecular level makes it possible to develop drugs that can mimic these effects," said Olefsky. "There's also plenty of precedent for using microRNAs themselves as drugs, so that's the possibility we're most excited about exploring for miR-690 going forward."
- Personalized Medicine
- Colon Cancer
- Pharmacology
- Diet and Weight Loss
- HIV and AIDS
- Diseases and Conditions
- Diabetes mellitus type 1
- Drug discovery
- Pharmaceutical company
- Diabetes mellitus type 2
- Nocebo - Placebo
- Erectile dysfunction
Story Source:
Materials provided by University of California - San Diego . Original written by Miles Martin. Note: Content may be edited for style and length.
Journal Reference :
- Theresa V. Rohm, Felipe Castellani Gomes Dos Reis, Roi Isaac, Cairo Murphy, Karina Cunha e Rocha, Gautam Bandyopadhyay, Hong Gao, Avraham M. Libster, Rizaldy C. Zapata, Yun Sok Lee, Wei Ying, Charlene Miciano, Allen Wang, Jerrold M. Olefsky. Adipose tissue macrophages secrete small extracellular vesicles that mediate rosiglitazone-induced insulin sensitization . Nature Metabolism , 2024; DOI: 10.1038/s42255-024-01023-w
Cite This Page :
Explore More
- No Two Worms Are Alike
- Quantum Effects in Electron Waves
- Star Trek's Holodeck Recreated Using ChatGPT
- Cloud Engineering to Mitigate Global Warming
- Detecting Delayed Concussion Recovery
- Bonobos: More Aggressive Than Thought
- Brightest Gamma-Ray Burst
- Stellar Winds of Three Sun-Like Stars Detected
- Fences Causing Genetic Problems for Mammals
- Ozone Impact On Fly Species
Trending Topics
Strange & offbeat.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Research Programs & Contacts
Clinical Research in Type 2 Diabetes
Studies in humans aimed at the prevention, treatment, and diagnosis of Type 2 Diabetes and the mechanistic aspects of its etiology.
The Clinical Research in Type 2 Diabetes (T2D) program supports human studies across the lifespan aimed at understanding, preventing and treating T2D. This program includes clinical trials that test pharmacologic, behavioral, surgical or practice-level approaches to the treatment and/or prevention of T2D, including promoting the preservation of beta cell function. Studies may also advance the development of new surrogate markers for use in clinical trials. Studies can be designed to understand the pathophysiology of T2D, including the role of gestational diabetes and metabolic imprinting on the development of T2D, as well as factors influencing the response to treatment. The program also encompasses epidemiologic studies that improve our understanding of the natural history and pathogenesis of T2D, and the development of diagnostic criteria to distinguish type 1 and type 2 diabetes, especially in the pediatric population. The program also supports research to understand and test approaches to accelerate the translation of efficacious interventions into real-world practice and adoption; and to address health equity by reducing health disparities in the incidence and/or clinical outcomes of T2D.
NIDDK Program Staff
- Shavon Artis Dickerson, Dr.P.H., M.P.H. Health Equity and Implementation Science
- Henry B. Burch, M.D. Clinical studies utilizing existing digital health technology for the prevention and treatment of type 2 diabetes, clinical and basic science studies involving non-neoplastic disorders of the thyroid, clinical studies involving medical and novel dietary treatment of type 2 diabetes.
- Maureen Monaghan Center, Ph.D., CDCES Health Psychology, Behavioral Science, Clinical Management of Diabetes
- Jean M. Lawrence, Sc.D., M.P.H., M.S.S.A. Type 2 diabetes risk and prevention after gestational diabetes; Studies of adults with diabetes/pre-diabetes using secondary data and observational designs, and natural experiments
- Hanyu Liang, M.D., Ph.D. Hepatic Metabolism; Insulin Resistance; Type 2 Diabetes; Obesity; Bariatric Surgery
- Barbara Linder, M.D., Ph.D. Type 2 diabetes in children and youth; human studies of metabolic imprinting
- Saul Malozowski, M.D., Ph.D., M.B.A. Neuroendocrinology of hypothalamic-pituitary axis, neuropeptide signaling and receptors; hormonal regulation of bone and mineral metabolism; HIV/AIDS-associated metabolic and endocrine dysfunction
- Pamela L. Thornton, Ph.D. Health Equity and Translational Research; Centers for Diabetes Translation Research (P30) Program
- Theresa Teslovich Woo, Ph.D. Human behavior, developmental cognitive neuroscience, and brain-based mechanisms involved in obesity and diabetes
Recent Funding Opportunities
Mentored patient-oriented research career development award (parent k23 independent clinical trial required), mentored patient-oriented research career development award (parent k23 independent clinical trial not allowed), rare diseases clinical research consortia (rdcrc) for the rare diseases clinical research network (rdcrn) (u54 clinical trial optional), adaptation of diabetes control technologies for older adults with t1d (r01 clinical trial optional), diabetes research centers (p30 clinical trial optional), related links.
View related clinical trials from ClinicalTrials.gov.
Study sections conduct initial peer review of applications in a designated scientific area. Visit the NIH’s Center for Scientific Review website to search for study sections.
Research Resources
NIDDK makes publicly supported resources, data sets, and studies available to researchers to accelerate the rate and lower the cost of new discoveries.
- Ancillary Studies to Major Ongoing Clinical Studies to extend our knowledge of the diseases being studied by the parent study investigators under a defined protocol or to study diseases and conditions not within the original scope of the parent study but within the mission of the NIDDK.
- NIDDK Central Repository for access to clinical resources including data and biospecimens from NIDDK-funded studies.
- NIDDK Information Network (dkNET) for simultaneous search of digital resources, including multiple datasets and biomedical resources relevant to the mission of the NIDDK.
Additional Research Programs
Research training.
NIDDK supports the training and career development of medical and graduate students, postdoctoral fellows, and physician scientists through institutional and individual grants.
Diversity Programs
The NIDDK offers and participates in a variety of opportunities for trainees and researchers from communities underrepresented in the biomedical research enterprise. These opportunities include travel and scholarship awards, research supplements, small clinical grants, high school and undergraduate programs, and a network of minority health research investigators.
Small Business
Small business programs.
NIDDK participates in the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs. These programs support innovative research conducted by small businesses that has the potential for commercialization.
Human Subjects Research
NIDDK provides funding for pivotal clinical research, from preliminary clinical feasibility to large multi-center studies.
Translational Research
NIDDK provides funding opportunities and resources to encourage translation of basic discoveries into novel therapeutics.
Meetings & Workshops

Supports researchers with tools to enhance scientific rigor, reproducibility, and transparency, and provides a big data knowledge base for genomic and pathway hypothesis generation.

Providing education and training for the next generation of biomedical and behavioral scientist

Stay informed about the latest events, or connect through social media.

Learn about current projects and view funding opportunities sponsored by the NIH Common Fund .
Registration is required at eRA Commons and grants.gov and can take 4 weeks.
Research Summaries
Keep up with the latest diabetes and diabetes-related studies with these brief overviews. Each summary provides main points, methods, and findings and includes a link to the article.
Diabetes Management and Education
Reaching treatment goals could help people living with type 2 diabetes increase their life expectancy by 3 years or in some cases by as much as 10 years. Read the summary .
Adults who receive diabetes education are more likely to follow recommended preventive care practices that lead to better diabetes management. Read the summary .
In 2017, the total cost of diabetes complications was over $37 billion among Medicare beneficiaries 65 or older with type 2 diabetes. Read the summary .
Kids and teens can get both type 1 and type 2 diabetes. New research shows how diabetes rates in young people may rise by 2060. Read the summary .
New USPSTF and ADA guidelines lower the age for prediabetes and type 2 diabetes screening to 35. This study examined if testing practices aligned with guidelines and which populations were less likely to receive testing. Read the summary .
The SEARCH for Diabetes in Youth study reports trends in young people who are being diagnosed with type 1 and type 2 diabetes. Read the summary .
Recent guidelines recommend newer types of diabetes medications, and most Americans living with type 2 diabetes are eligible. Read the summary .
Chronic Kidney Disease
End-stage kidney disease—kidney failure that requires dialysis or a kidney transplant—can lead to disability and early death, is expensive to treat, and cases are on the rise. Read the summary .
- Reports and Publications
- Research Projects
- US Diabetes Surveillance System
To receive updates about diabetes topics, enter your email address:
- Diabetes Home
- State, Local, and National Partner Diabetes Programs
- National Diabetes Prevention Program
- Native Diabetes Wellness Program
- Chronic Kidney Disease
- Vision Health Initiative
- Heart Disease and Stroke
- Overweight & Obesity
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Talk to us about diabetes
0345 123 2399
customer support
The biggest diabetes research stories of 2022
2022 has been a big year for diabetes research, filled with historic moments and world-firsts. Here we take a look back at some of the incredible progress Diabetes UK-funded scientists and diabetes researchers across the globe have been behind this year.

First, we’ll take a look at what’s happened in the world of type 1 diabetes research.

Type 1 diabetes
A game-changing partnership to lead the race towards a type 1 cure .
In April we announced a new partnership with JDRF and the Steve Morgan Foundation , following the Foundation’s incredible £50 million donation.
The single largest ever gift to type 1 diabetes research in the UK is funding the Type 1 Diabetes Grand Challenge . This will see us bring together science dream teams to work on bigger and bolder ideas in three areas that hold the greatest potential to transform type 1 treatments and lives.
- Treatments to replace or rescue insulin-making beta cells in the pancreas.
- Treatments to stop the immune system’s attack that destroys insulin-making beta cells.
- Next generation insulins, such as those that respond to changing blood sugar levels.
In 2023, the first Grand Challenge projects will be getting underway, and you can stay up to date with all the news on the newly launched Type 1 Diabetes Grand Challenge website .
UK’s first ever type 1 screening programme
On World Diabetes Day, we announced the launch of the UK’s first ever trial screening programme to identify children who have a high risk of developing type 1 diabetes in their lifetime.
The ELSA study will screen children for signals that can be detected in the blood that indicate the immune system has started to prepare an attack on the pancreas. The signals can appear years, or sometimes decades, before people begin to experience any symptoms and receive a type 1 diagnosis.
ELSA will help our researchers figure out how a widespread screening programme for type 1 diabetes would best be rolled out in the UK. Screening has the potential to revolutionise the way we identify and treat type 1 diabetes.
If you have a child aged 3-13 years, find out more about taking part.
US approves first drug to delay type 1 diabetes
Just days after we launched ELSA, the first-ever immunotherapy drug was approved for use in the U.S, to hold off the development of type 1 diabetes in people at high risk of the condition.
The drug called teplizumab , or Tzield, works to weaken the advancing immune attack and help insulin-making beta cells to survive for longer, delaying the start of type 1 diabetes.
The approval of teplizumab in the US was perfectly in sync with the launch of our ELSA study. Without screening programmes, it would be impossible to identify and treat those at risk of type 1 who could benefit from immunotherapies.
Teplizumab is currently being reviewed for use in the UK, and together with screening programmes, could open the door to a new era in type 1 diabetes treatment.
New findings on FLASH
Our researchers at the University of Manchester led a clinical trial to investigate the impact of second-generation Flash (FreeStyle Libre 2 ) on blood sugar levels and quality of life for people living with type 1 diabetes. They found that Flash not only helped people to reduce their average blood sugar levels and spend time in range, but it also helped to reduce some of the day-in day-out burden of living with type 1 diabetes.
The findings highlight how crucial it is for everyone who could benefit from this technology to have access to it.
AI helps to diagnose type 1 sooner
With our funding, Dr Julia Townson and her team used artificial intelligence and electronic health records from over 1 million children to develop a predictive tool that could detect patterns that flag cases of potential undiagnosed type 1 diabetes in children.
They found the tool successfully identified 75% of children who would go on to develop type 1 in the following 90 days, which could help them to get a diagnosis, and started on life-saving insulin, sooner.
It’s a promising step forward, which could lead to the widespread use of this tool in GPs in the future. This could help more children get an accurate and rapid diagnosis and have the best possible start to life with type 1 diabetes.
Type 2 diabetes
It has also been a jam-packed year for progress in the world of type 2 diabetes research.
Unlocking the genes for fat storage
At the beginning of the year, our researchers at the Universities of Brunel and Exeter discovered that the genes which control where our body fat is stored play a direct role in causing type 2 diabetes.
The team studied information from around 500,000 people and found that some people have genes that mean they store higher levels of fat everywhere, including under the skin, liver and pancreas. This is linked to a higher risk of conditions such as type 2 diabetes. While others have genes that mean they have higher fat under the skin but lower liver fat, and therefore, a lower risk of conditions like type 2 diabetes.
This work is an exciting step forward in helping us to understand more about the underlying biology of type 2 diabetes and why the risk of developing type 2 can vary so much between people of similar bodyweights.
Remission possible for more people
Earlier this year, Professor Roy Taylor announced new findings from his Diabetes UK-funded project, called ReTUNE , a world-first study into type 2 diabetes remission in people with lower body weights.
After losing weight following a low-calorie diet programme, 70% of participants went into remission.
The findings showed for the first time that people with type 2 diabetes and lower body weights can be supported to put their type 2 into remission through a structured low-calorie diet programme. And that the key to this is losing harmful fat from the liver and pancreas.
This work could help to make sure many more people have the chance to put their type 2 diabetes into remission.
A NewDAWN for remission
This year we joined forces with the National Institute for Health and Care Research (NIHR) to fund NewDAWN , a £2.2 million project to help give more people the chance of going into remission.
The project is aiming to create a brand-new, national NHS support service for people newly diagnosed with type 2 diabetes and living with overweight or obesity. The service will allow people to try out different weight loss programmes to find the one that’s right for them, giving everyone the best chance of remission.
Insomnia found to play a role in type 2
Earlier this year, our researchers at the University of Bristol, and supported by the universities of Manchester, Exeter and Harvard revealed that treating insomnia could help to prevent or treat type 2 diabetes, by helping to combat higher blood sugars.
The team studied the sleep behaviours and blood sugar levels of 337,000 people and found that people who frequently find it hard to get to sleep or stay asleep had higher blood sugar levels than people without these difficulties.
These findings are helping us to understand how sleep problems can affect the development of type 2 diabetes, but we need to know more about what’s going on inside the body before this work could open up new ways to treat or manage the condition.
New drugs to treat and prevent type 2 getting closer
Innovative new types of drugs, called GLP-1 receptor agonists, were approved in the US this year to help people living with type 2 diabetes to manage their blood sugar levels.
The medications work by mimicking hormones that tell our bodies to release insulin and tell our brains when we are full.
In 2022, we also heard promising results from clinical trials showing that these medications also worked to help people living with obesity to lose weight. The medications aren’t yet approved to treat obesity. But we know that living obesity or overweight is an important risk factor for type 2 diabetes, so with further research they could offer new and improved ways to both treat and prevent type 2 diabetes.
It’s thanks to your support and generosity throughout 2022 that this amazing progress has been possible. Our scientists simply couldn’t make breakthroughs without you.
Share this Page
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

Diabetes test ‘may be inaccurate for thousands of South Asian people in UK’
A common type 2 diabetes test may be inaccurate for tens of thousands of South Asian people in the UK, new research suggests.
The research identified a genetic variant among people of South Asian heritage that affects the results of the HbA1c test.
The findings suggest that the test, which measures average blood sugar levels, may give falsely lower results in South Asian people who carry this genetic variant, leading to delayed diagnosis.
This evidence showing that the accuracy of a common test to diagnose and monitor type 2 diabetes is linked to a person's ethnicity should be urgently investigated further
Dr Elizabeth Robertson
Data suggests that there are more than 420,000 people from a South Asian background living with diabetes in England , and more than 230,000 have a diagnosis of prediabetes and are therefore at high risk of developing type 2 diabetes.
With around 7.6% of South Asians carrying this variant, this suggests the HbA1c test is underestimating blood sugar levels in around 32,000 South Asian people with diabetes and 17,500 with prediabetes in England alone.
The test is crucial for diagnosing type 2 diabetes, monitoring prediabetes, and guiding diabetes treatment.
Dr Miriam Samuel at Queen Mary University London and colleagues in the Genes and Health Research Team identified the genetic variant that is found in 7.6% of people of South Asian heritage but is ultra rare in other ethnicities.
Dr Samuel said: “Many genetic variants linked to red blood cell conditions are ultra-rare amongst the Northern Europeans who have historically dominated genetic studies.

Conservationists and celebrities urge Government to back Bill to ban peat sales

Rise in voter registrations as deadline arrives for May 2 elections

Bid to boost youth vote as polling suggests widespread disillusionment

Make your next trip to the West End a celebration of women in culture
“We demonstrate one such variant that is carried by 7.6% of South Asians which could affect the accuracy of HbA1c and cause delays in diabetes diagnosis.
Every individual at risk or with diabetes, regardless of their background, deserves equal access to effective diabetes care to live a healthy life and mitigate long-term diabetes complications
Dr Miriam Samuel
“Studies such as Genes and Health, focusing on populations who are underserved in genetic research, are vital to understand the different pathways that may contribute to diabetes inequalities in these communities.”
Dr Elizabeth Robertson, director of research at Diabetes UK , said: “This evidence showing that the accuracy of a common test to diagnose and monitor type 2 diabetes is linked to a person’s ethnicity should be urgently investigated further.
“It’s incredibly important that healthcare professionals are armed with a precise way to evaluate average blood sugar levels over extended periods.
“Without this, they are navigating in the dark and potentially at risk of overlooking cases of type 2 diabetes.
“Every individual at risk or with diabetes, regardless of their background, deserves equal access to effective diabetes care to live a healthy life and mitigate long-term diabetes complications.”
The researchers looked at genetic and health data from the Genes and Health study – which included more than 60,000 individuals of Bangladeshi or Pakistani ethnicity living in England – and the UK Biobank.
They found that people with this gene were found to have falsely lower HbA1c levels and differences in their full blood count.
HbA1c tests estimate average blood sugar levels by measuring how much sugar is attached to haemoglobin in red blood cells.
The research suggests that the genetic variant is linked to changes in red blood cells, and that this affects HbA1c test results.
According to the findings, people who had one copy of the genetic variant were diagnosed with type 2 diabetes on average one year later.
While those with two copies were diagnosed on average two years later, than those without the genetic variant.
Inaccurate results might also mean people do not receive timely and appropriate treatments needed to manage blood sugar levels and reduce risk of long-term complications, which could include heart attacks, strokes, amputations, and sight loss, the experts suggest.
The findings indicate additional blood sugar testing, such as fasting glucose and oral glucose tolerance tests, and alternative monitoring methods might be needed in South Asian people who carry the variant.
The findings will be presented at the Diabetes UK Professional Conference 2024.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Epidemiol Glob Health
- v.10(1); 2020 Mar

Epidemiology of Type 2 Diabetes – Global Burden of Disease and Forecasted Trends
Moien abdul basith khan.
1 Department of Family Medicine, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates
Muhammad Jawad Hashim
Jeffrey kwan king, romona devi govender, halla mustafa, juma al kaabi.
2 Department of Internal Medicine, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates
The rising burden of type 2 diabetes is a major concern in healthcare worldwide. This research aimed to analyze the global epidemiology of type 2 diabetes. We analyzed the incidence, prevalence, and burden of suffering of diabetes mellitus based on epidemiological data from the Global Burden of Disease (GBD) current dataset from the Institute of Health Metrics, Seattle. Global and regional trends from 1990 to 2017 of type 2 diabetes for all ages were compiled. Forecast estimates were obtained using the SPSS Time Series Modeler. In 2017, approximately 462 million individuals were affected by type 2 diabetes corresponding to 6.28% of the world’s population (4.4% of those aged 15–49 years, 15% of those aged 50–69, and 22% of those aged 70+), or a prevalence rate of 6059 cases per 100,000. Over 1 million deaths per year can be attributed to diabetes alone, making it the ninth leading cause of mortality. The burden of diabetes mellitus is rising globally, and at a much faster rate in developed regions, such as Western Europe. The gender distribution is equal, and the incidence peaks at around 55 years of age. Global prevalence of type 2 diabetes is projected to increase to 7079 individuals per 100,000 by 2030, reflecting a continued rise across all regions of the world. There are concerning trends of rising prevalence in lower-income countries. Urgent public health and clinical preventive measures are warranted.
1. INTRODUCTION
Type 2 diabetes is recognized as a serious public health concern with a considerable impact on human life and health expenditures. Rapid economic development and urbanization have led to a rising burden of diabetes in many parts of the world [ 1 ]. Diabetes affects individuals’ functional capacities and quality of life, leading to significant morbidity and premature mortality [ 2 ]. Recently, concerns have been raised that more than one-third of the diabetes-related deaths occur in people under the age of 60 [ 3 ]. Increased consumption of unhealthy diets and sedentary lifestyles, resulting in elevated Body Mass Index (BMI) and fasting plasma glucose, have been blamed for these trends [ 4 ]. In particular, persons with higher BMI are more likely to have type 2 diabetes [ 5 ]. The aging of the human population is another contributor, as diabetes tends to affect older individuals [ 6 ]. The cost of diabetes care is at least 3.2 times greater than the average per capita healthcare expenditure, rising to 9.4 times in presence of complications [ 7 ]. Control of blood glucose, blood pressure, and other targets remains suboptimal for many patients [ 8 ]. This has been partly attributed to the lack of awareness and health promotion needed for diabetes control [ 9 ].
Unfortunately, the global epidemiology of diabetes has not been re-evaluated since the availability of recent high-quality data [ 10 ]. We found no studies providing global forecasts for the intermediate future, which would be a critical piece of information for health policymakers.
This research project examines the latest dataset of the Global Burden of Disease (GBD) to assess the burden of type 2 diabetes worldwide. The aim is to study the current global epidemiology of diabetes and highlight the current distribution of disease and emerging epidemiologic trends.
2. MATERIALS AND METHODS
We analyzed descriptive epidemiological data from the GBD dataset managed by the Institute of Health Metrics and Evaluation at the University of Washington, Seattle [ 11 ]. The GBD dataset is actively maintained and updated based on research data, epidemiology studies, and governmental publications from more than 100,000 sources. As a systematic public health project, it carefully builds models and statistical estimates for health loss due to illness, injury, and risk factors based on empirical data. GBD produces annual estimates of disease measures, such as prevalence, incidence, deaths, and Disability-Adjusted Life Years (DALYs). DALYs combine years of life lost due to premature death and years lived with disability, and are a more accurate reflection of human suffering resulting from a disease than prevalence or mortality alone.
We used the latest data refresh from GBD (the 2017 update). This dataset includes annual figures from 1990 to 2017 for type 2 diabetes in all countries and regions. We selected four world regions (Asia, Europe, America, and Africa) instead of other classification schemes based on economic development. All data were directly retrieved from GBD without any adjustments. Estimates were not age adjusted for differences in underlying population age distributions. Thus, the rates for different countries represent the actual burden on their respective health systems.
2.1. Statistical Data Analysis
Forecasting was conducted using IBM SPSS version 25 (IBM Corp., Armonk, NY, USA). The Time Series Modeler was used to develop a forecast model using the Expert Modeler option without any events. None of the observed values were marked as outliers.
Globally, an estimated 462 million individuals are affected by type 2 diabetes, corresponding to 6.28% of the world’s population ( Table 1 ). More than 1 million deaths were attributed to this condition in 2017 alone, ranking it as the ninth leading cause of mortality. This is an alarming rise when compared with 1990, when type 2 diabetes was ranked as the eighteenth leading cause of deaths. In terms of human suffering (DALYs), diabetes ranks as the seventh leading disease.
Disease burden of type 2 diabetes, 2017
The prevalence of type 2 diabetes shows a distribution pattern that matches socio-economic development ( Figure 1 ). Developed regions, such as Western Europe, show considerably higher prevalence rates that continue to rise despite public health measures ( Figure 2 ). The rate of increase does not appear to be slowing down.

Global distribution of diabetes mellitus type 2 prevalence. Note: Colors indicate prevalence rates per 100,000 population in 2017.

Trends in the prevalence of type 2 diabetes. Note: Forecast estimates using SPSS Time Series Modeler (Ljung Box Q, p = 0.16). Dotted lines indicate upper and lower confidence limits.
Remarkably, certain regions, such as Pacific Ocean island nations, are sustaining the highest prevalence of disease. These countries include Fiji (20,277 per 100,000), Mauritius (18,545), American Samoa (18,312), and Kiribati (17,432). Southeast Asian countries, such as Indonesia, Malaysia, Thailand, and Vietnam, have moved up the ranks in the last two decades. Owing to their large population sizes, China (88.5 million individuals with type 2 diabetes), India (65.9 million), and the US (28.9 million) retain the top spots as the countries with the greatest total number of individuals with this condition.
Males show a slightly higher prevalence than females (6219 compared with 5898 cases per 100,000), although this difference is within the margin of uncertainty. The age of onset of new diagnosis is also somewhat earlier among males and shows expected patterns of rising prevalence with increasing age, whereas the incidence peaks at 55–59 years ( Figure 3 ). There appears to be no major shift in the age distribution from 1990 to 2017.

Age distribution of diabetes mellitus type 2, worldwide. (A) Incidence vs. prevalence (both 2017). (B) Incidence in 1990 vs. 2017. p < 0.0001, chi-square test.
Even though it afflicts individuals later in life, type 2 diabetes ranks seventh among the leading causes of disability and years of life lost (DALYs). It has jumped ranks from nineteenth position in 1990, indicating a global transition in disease patterns toward noncommunicable diseases.
Statistical forecasting using a model based on the 1990–2017 data showed that global diabetes prevalence could increase to 7079 per 100,000 by 2030 and 7862 by 2040. This estimate for 2040 is flanked by an upper confidence limit of 9904 and a lower limit of 5821 per 100,000.
4. DISCUSSION
This study reports on the current trends in the global burden of diabetes with emphasis on the burden of human suffering. The high prevalence of type 2 diabetes worldwide continues to rise, and there are no signs of it stabilizing. A concerning finding is the rapidly rising burden in lower-income countries. These findings have implications for health policy planners, physicians, healthcare professionals, and the public.
The burden of suffering due to diabetes, as measured by DALYs, is increasing despite significant investment in clinical care and pharmaceutical research. This increase is in excess of population growth and aging. Notably, Western Europe has a rate of increase greater than that of global and Asian averages. Even with the high levels of clinical and public health expenditure, this region is losing the battle against diabetes. One explanation might be non-modifiable risk factors, such as age and family history [ 12 ]. However, factors like a highly processed, calorie-dense western diet and a sedentary lifestyle may also be contributing. Developed countries like Italy and the US endure the highest burdens of human suffering (DALYs) due to diabetes. Advanced economies in Asia, such as South Korea and Taiwan, are joining the ranks of these countries, based on GBD data. Thus, our findings support the correlation between diabetes and economic development [ 13 ]. We speculate that our current approach to diabetes management, which focuses on expensive oral medications and insulin, is not working. Lowering blood glucose levels is perhaps not sufficient by itself nor effective in reducing all-cause mortality among these patients.
Prevention of new cases of diabetes appears to be not working as well based on our findings from global data. Although research is ongoing to reduce the progression from metabolic syndrome and prediabetes to diabetes, most interventions being tried seem to be unsuccessful in affecting the incidence. According to our data, there is no evidence of a decrease in incidence. Alarmingly high incidence rates recorded in island nations in the Pacific region are an indication of the interaction between genetic predisposition and the effect of rapid nutritional change on these indigenous populations. Meanwhile, the sheer number of individuals with diabetes is testing health systems in China, India, and the US to the limit. Rapid urbanization and its effects on diet and lifestyle has been implicated [ 14 ]. These findings have direct implications for health systems planning and resource allocation. Clearly, hospital-based management and subspecialist care are not sustainable strategies. Resource allocation in healthcare budgets for prevention of diabetes needs to be comparable to expenditures on treatment. Strengthening of primary care and community restructuring for active lifestyles and healthy nutrition are perhaps more likely to be cost effective [ 15 ]. Sadly, the rising tide of type 2 diabetes is out pacing preventive efforts by a wide margin [ 16 ].
The rising incidence of type 2 diabetes at earlier ages warrants closer attention. Previous clinic-based studies have reported a high number of young adults being diagnosed with type 2 diabetes, most of whom are obese [ 17 ]. There appears to be an age gradient with early-onset type 2 diabetes patients (those younger than 45) showing more obesity, dyslipidemia, smoking, sedentary lifestyles, and low-grade inflammation [ 18 ]. In our study, although the incidence of diabetes in young adults has increased over the past decades, the rise is across all ages. Thus, there appears to be no clear indication that the age of onset of type 2 diabetes has shifted to younger age groups. In any case, rising life expectancy in many countries will lead to a substantially greater burden of diabetes in the elderly.
The main limitations of our study include reliance on secondary data, which in turn is affected by the accuracy of measurement, changes in case definition, and heterogeneity in study designs. Yet as GBD evolves and matures, its estimation techniques have become more accurate and reliable. These statistical estimates provide a more complete and continuous picture of disease epidemiology than relying on raw data from isolated studies [ 11 ]. Ultimately, the goal is to guide decision making in clinical care and public health policy.
5. CONCLUSION
Type 2 diabetes continues to increase in prevalence, incidence, and as a leading cause of human suffering and deaths. Despite significant investments in clinical care, research, and public health interventions, there appears to be no sign of reduction in the rate of increase. Certain regions of the world, such as Western Europe and island states in the Pacific, are experiencing a disproportionately high burden. This epidemic will require an urgent and unwavering commitment to aggressive solutions at national levels with public policies, public health funding, and economic incentives for local communities to start diabetes prevention programs. Healthy eating options need to be subsidized, and unhealthy foods need to be taxed or otherwise disincentivized. Healthcare organizations and individual healthcare providers from multiple disciplines (doctors, nurses, pharmacists, dieticians, and diabetes educators) must be given time and resources to collaborate as they educate and care for individual and groups of patients. Unless urgent measures are instituted to reduce unhealthy eating, sedentary lifestyles, rapid urbanization, and other factors related to economic development, the burden of diabetes is expected to continue rising.
ACKNOWLEDGMENT
We would like to thank the Institute of Health Metrics, Seattle for compiling global epidemiological statistics and allowing access to data.
Data availability statement: The data that support the findings of this study are openly available in Global Health Data Exchange by the Institute of Health Metrics at http://ghdx.healthdata.org/gbd-results-tool .
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
MK contributed to writing the manuscript including the literature review. MJH designed the study/basic concept, wrote sections of the manuscript, analyzed the data, and provided overall supervision of the study. JK wrote parts of the manuscripts, proofread, and provided insights into the interpretation. RDG revised the manuscript and provided additional interpretation of results. HM compiled data and wrote the table. JAK revised and proofread the manuscript and provided additional interpretation of results.
This study did not receive any external grants from government, private or commercial sources.
What to eat to prevent spikes in your blood sugar
Over the past three decades, the prevalence of Type 2 diabetes has risen dramatically worldwide.
According to the International Federation of Diabetes, by 2045 one in eight adults will be living with diabetes, an increase of 46 per cent.
A recent study of dietary and health data from 184 countries found that poor carbohydrate quality – eating too many refined grains and too few whole grains – was the leading dietary driver of Type 2 diabetes cases.
Now, a global study conducted in 20 countries adds to existing evidence that carbohydrate quality matters when it comes to staving off the disease.
The findings strongly suggest that eating foods with a low glycemic index – ones that don’t spike blood glucose and insulin after eating – is protective.
Here’s a breakdown of the latest research, plus what to eat to prevent blood sugar spikes.
Glycemic index and glycemic load defined
The glycemic index (GI), developed by University of Toronto researchers Dr. David Jenkins and Dr. Thomas Wolever in 1981, assigns carbohydrate-containing foods a score of 0 to 100 based on how rapidly they raise blood glucose compared to pure glucose.
A surge in blood glucose triggers an outpouring of the hormone insulin; over time these events can lead to glucose intolerance and Type 2 diabetes.
Foods with a high GI (70 or more) cause a sharp increase in blood glucose that declines rapidly. Examples include white bread, whole wheat bread, soda crackers, rice cakes, jasmine rice, instant rice, baked russet potato, instant oats, refined breakfast cereals, croissants, doughnuts, cakes and raisins.
Foods with a low GI (55 or less) lead to a slower and lower rise in blood glucose that declines gradually. Low GI foods include dense multigrain breads, sourdough bread, 100-per-cent bran cereals, steel-cut and rolled oats, barley, quinoa, brown rice, al dente pasta, beans and lentils, sweet potato, winter squash, most fruit and yogurt.
The glycemic load (GL) gives a more accurate picture of how foods affect your blood glucose. It considers not only the food’s glycemic index but also how much carbohydrate it contains per serving.
For example, if you eat a high glycemic food that contains only a small amount of carbohydrate, it won’t have much impact on blood glucose and its GL will be low.
The new research findings
The study, published April 5 in the journal Lancet Diabetes & Endocrinology, included 127,954 adults ages 35-70, enrolled in the PURE study (Prospective Urban and Rural Epidemiology).
Participants were from 20 low-income, middle-income and high-income countries and, at the study’s outset, did not have Type 2 diabetes.
Diet information was collected and used to calculate dietary glycemic index and glycemic load.
After 12 years, 7,326 participants had developed Type 2 diabetes.
Compared to those whose diets had the lowest GI and GL, those with the highest scores had a significantly greater risk of Type 2 diabetes. The increased risk was more pronounced in people with a high body mass index.
The researchers accounted for other factors that could influence diabetes risk such as family history, smoking, physical activity and intake of calories, fibre and whole grains.
The study’s strengths are its long duration of follow-up, large sample size and the inclusion of participants from low- to high-income countries.
Limitations include the fact that diet was measured only at the beginning of the study; dietary habits could have changed over time. Dietary information was also self-reported which is prone to error.
The study was observational; it doesn’t prove that a low glycemic diet prevents Type 2 diabetes.
How a high glycemic diet can harm metabolic health
This isn’t the first study to link high GI diets to an increased risk of Type 2 diabetes.
A review of large studies published earlier this year turned up similar findings.
High GI diets have been tied to reduced insulin sensitivity, impaired insulin secretion and poor blood glucose control.
Large spikes in blood glucose after eating have been shown to increase inflammation and oxidative stress, factors thought to promote the development of Type 2 diabetes.
Diet strategies to balance blood sugar
To lower the glycemic load of meals, choose unprocessed or minimally processed carbohydrates (e.g., whole grains, sweet potato, winter squash, beans and lentils, whole fruit) over refined carbohydrates.
These foods deliver fibre, which delays the rate that carbohydrates are digested and absorbed into the bloodstream.
Balance meals with protein and healthy fats, macronutrients that also slow down carbohydrate digestion.
Adding vinegar to meals (e.g., vinaigrette dressing) can also blunt the rise in postmeal glucose by slowing digestion and increasing glucose uptake by cells.
Consider food order too. Studies have found that eating vegetables, protein or fat first and eating refined carbohydrates last (e.g., white rice, pasta or bread) helps minimize blood sugar spikes.
Leslie Beck, a Toronto-based private practice dietitian, is director of food and nutrition at Medcan. Follow her on Twitter @LeslieBeckRD
Report an editorial error
Report a technical issue
Editorial code of conduct
Follow related authors and topics

Authors and topics you follow will be added to your personal news feed in Following .
Interact with The Globe

- Latest News
- North & East
- Environment
- International
- Social Love
- Horse Racing
- World Champs
- Commonwealth Games
- FIFA World Cup 2022
- Art & Culture
- Tuesday Style
- Food Awards
- JOL Takes Style Out
- Design Week JA
- Black Friday
- Relationships
- Motor Vehicles
- Place an Ad
- Jobs & Careers
- Study Centre
- Jnr Study Centre
- Supplements
- Entertainment
- Career & Education
- Classifieds
- Design Week
New study into Type 2 diabetes treatment yields promising results
KINGSTON, Jamaica – New research has yielded promising results for more effective treatment and management options for patients suffering from Type 2 diabetes.
The study, titled ‘Combined Supplementation of S-Nitro glutathione and Glutathione Improves Glycaemic Control in Type 2 Diabetic Rats’, was led by a master’s graduate from the Faculty of Medical Sciences at the University of the West Indies (UWI) Mona campus, Amarley Wright.
It focuses on the use of the antioxidant Glutathione, combined with another substance known as Nitric oxide, to significantly lower blood sugar levels in diabetic rats.
“So, these are some promising results, and it highlights the possible role that this combination treatment could play in improving the lives of diabetic patients,” Wright said.
Diabetes is a disorder in which an individual develops an abnormally high blood sugar level due to inadequate or lack of insulin production by the pancreas or the inability of the body to respond properly to the hormone.
Insulin is needed to control the amount of glucose (sugar) in the blood.
Wright said Type 2 diabetes represents between 90 and 95 per cent of all diabetes cases globally, with 11.6 per cent of Jamaicans currently living with the condition.
“More than likely, each one of us knows somebody with diabetes. This is the reason why my research is of major importance. There are millions of people worldwide living with diabetes, and in Jamaica, the Economic and Social Survey showed that diabetes was one of the three main causes of death for both men and women in 2021,” he noted.
Common symptoms associated with diabetes include excessive thirst, extreme hunger, frequent urination, fatigue, and blindness.
Wright stated that the major test used to diagnose Type 2 diabetes is the Oral Glucose Tolerance Test, also known as the OGTT.
“This test involves an overnight fast. Thereafter, blood is taken from the patient and a fasting blood sugar level is measured. Then, they’re given fluids to drink, which contain glucose, and their blood sugar level is measured one hour and two hours afterwards. Normally a reading that is greater than or equal to 200 milligrams per decilitre indicates diabetes,” he explained.
In patients with diabetes, there is the development of a phenomenon called ‘oxidative stress’ where bad compounds in the body, such as free radicals become present. Good compounds known as antioxidants help fight against these bad compounds.
For Wright’s research, a major antioxidant, Glutathione, is combined with another substance known as Nitric oxide to form S-Nitro glutathione (GSNO).
“These two compounds are the focus of my work. So, I administered these compounds in Type 2 diabetic rats,” he said.
Wright’s research revealed that, among other things, Glutathione on its own was effective in significantly reducing the blood-sugar levels of the diabetic rats, which were administered the compound.
His research findings also showed an increase in the insulin concentration for the rats, which were treated as part of the study when compared to those that were left untreated.
Wright added that further work needs to be done “in terms of evaluating the toxicity as well as other biomedical parameters that can be measured so that we can know more about the mechanism that these compounds work by”.
While clinical trials on the use of the combination of compounds as treatment for diabetes in humans have begun in other jurisdictions such as India, Jamaica currently has no such programme.
Wright said his research has the potential to change that reality.
“We could engage the clinical transitional research unit from the Faculty of Medical Sciences at the University of the West Indies, Mona, to see how this could be done. We could also seek further information from them about what other drug tests we need to do to see if we can push this forward to clinical trials.
“Overall, we want to improve the quality of life for diabetic patients. We see that diabetes is not only a topical issue it is a growing one. There is expected to be an increase in the prevalence of diabetes as well,” Wright pointed out.
He added that the healthcare expenditure associated with treating diabetes is also an area of concern, in terms of insulin and other medications associated with the condition that afflicted patients have to purchase.
“So, we’re talking about effective treatment, and we see that these compounds, administering them together, could lead to a possible pharmaceutical option for treating Type 2 diabetes,” he said.
For his research, Wright received the award for ‘Best Student Oral Presentation’ at the 14th Annual National Health Research Conference held in November 2023.
HOUSE RULES
- We welcome reader comments on the top stories of the day. Some comments may be republished on the website or in the newspaper; email addresses will not be published.
- Please understand that comments are moderated and it is not always possible to publish all that have been submitted. We will, however, try to publish comments that are representative of all received.
- We ask that comments are civil and free of libellous or hateful material. Also please stick to the topic under discussion.
- Please do not write in block capitals since this makes your comment hard to read.
- Please don't use the comments to advertise. However, our advertising department can be more than accommodating if emailed: [email protected] .
- If readers wish to report offensive comments, suggest a correction or share a story then please email: [email protected] .
- Lastly, read our Terms and Conditions and Privacy Policy
- Privacy Policy
- Editorial Code of Conduct

This May Be the Best Way to Exercise if You Have Type 2 Diabetes
- A new study found that individuals with type 2 diabetes may benefit from curating their exercise routines, particularly by strategizing the time of day they work out and the type of workouts they do.
- Working out in the evening, after a meal, and incorporating cardio, strength, and flexibility exercises into your weekly routine can provide helpful results.
- Experts emphasize the benefits of prioritizing exercise for type 2 diabetes care, in tandem with a healthy diet and any necessary, prescribed medication.
Exercising at certain times of the day and implementing certain types of activity can help people with type 2 diabetes get the most out of a workout, a new study finds.
About 10% of adults in the United States have type 2 diabetes , and about one-third have prediabetes. Unlike type 1 diabetes , type 2 diabetes can sometimes be reversed with healthy lifestyle interventions.
Exercise is usually prescribed as a treatment alongside diet and medication, but on its own, exercise can induce short-term glycemic (blood sugar) control.
“Exercise is nature’s insulin sensitizer, meaning it makes your body more sensitive to the effects of insulin,” Joanne Dushay, MD , an endocrinologist at Beth Israel Deaconess Medical Center who was not involved with the new research, told Health .
Muscle and fat cells act like sponges, absorbing glucose from the bloodstream and transporting it into cells, which lowers blood sugar levels. Exercise helps this process along, especially in people with type 2 diabetes, where muscles may be unable to absorb glucose like they normally should.
“Regular exercise is better than pretty much any medication for improving insulin resistance,” Dushay told Health .
Related: What Is Insulin Resistance?
Using Exercise to Regulate Type 2 Diabetes
To understand how exercise helps regulate type 2 diabetes, people must first understand what goes wrong in the body to cause the disease in the first place, Steven Malin, PhD, an associate professor of kinesiology and health at Rutgers University, told Health .
First, over time, the body becomes less responsive to the hormone insulin. As the body becomes less responsive to insulin, muscles don’t take up glucose as they normally would. The body tries to compensate by producing more insulin.
This overexertion causes the pancreas—the organ that secretes insulin—to become exhausted. As a result, it no longer makes enough insulin.
Malin, who led the study, emphasized the benefits of using exercise to counteract insulin resistance.
Not only does physical activity help regulate blood sugar and insulin, but it can also ward off some common complications of diabetes, including heart disease and nerve damage.
To determine how people with type 2 diabetes can best optimize exercise, Malin and his team analyzed existing studies that looked at how different types of exercises—such as high-intensity interval training (HIIT) and strength training—impact glycemic control in people with type 2 diabetes, as well as whether or not it matters when the exercise is being done.
“Either type of exercise, aerobic or strength, and exercise at any time of day is thought to be beneficial for helping the body become more insulin sensitive,” Malin told Health. So the first focus should be on getting any type of exercise whenever you can, he added.
The next step up can be optimizing those workouts to get the most benefits—but being physically active in any way remains the most important thing, Malin said.
Post-Meal and Evening Workouts Are Best
Several studies have found that exercising after eating a meal is the best way to optimize the benefits of a workout when it comes to glycemic control.
After eating, a person’s blood sugar spikes. But a post-meal workout can help quell these spikes by prompting the muscles to take up more glucose from the blood. Since dinner is typically the largest meal of the day in the U.S., exercising in the afternoon or evening is slightly better for managing glucose than exercising in the morning.
If you exercise in the evening, those benefits last until the next morning, Malin said.
“If you’re in a spot where you have to make a decision and you have barriers in your way, pick whichever option, before or after a meal, that is easier and get it done,” he said. “But if you have more flexibility, the evidence is pointing towards exercising after the meal.”
Mixing Up Kinds of Exercise Activities Can Help
According to Dushay, a combination of cardio , strength, and flexibility exercises is best, especially as people get older.
“Each has unique and important benefits,” she said. “Cardio helps with cardiovascular health; strength helps maintain muscle mass as we age; flexibility helps with balance and to prevent falls.”
If you can only pick two, stick with cardio and strength training. “If you’re able to do both strength and aerobic exercise you’re going to get the best benefits for glucose control,” Malin said.
Mixing it up can also keep exercise interesting and allow you to do most workouts at the time of day you prefer, even if it isn’t post-meal or in the evening.
“Don’t get caught up in doing the same thing every day, you can mix it up," he said. "If you prefer morning you can do it once or twice a week in the evening but primarily in the morning."
Using Exercise to Break Insulin Barriers
Studies have also shown that one of the big barriers to exercise is time, Malin said. The key to eliminating that barrier may be to break down exercise into smaller increments.
“People will say 'I don’t have 45 minutes to get out and do the exercise,' but you can break that apart and do 15 minutes after each meal,” said Malin. “Studies have shown that breaking up the exercise over the course of the day is just as good if not even a little better than one burst of 45 minutes.”
A 2021 study found that even opting to take the stairs decreased a person’s risk of developing metabolic syndrome, which includes type 2 diabetes.
Dushay said marching in place, climbing stairs, or strength training for just 5 or 10 minutes several times a day can significantly improve blood sugar among people with type 2 diabetes.
“I can't emphasize this enough, exercise is hands down the best medicine for diabetes,” she said, adding that it should still be used in tandem with a healthy diet and, if needed, medication.
Related: Timing of Meals for Diabetes
For more Health news, make sure to sign up for our newsletter!
Read the original article on Health .

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 11 September 2023
Physiology and Biochemistry
Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions
- Mohammad Azam Ansari ORCID: orcid.org/0000-0002-6122-5479 1 ,
- Waseem Chauhan 2 ,
- Shoaib Shoaib 3 ,
- Sami A. Alyahya 4 ,
- Mubashshir Ali 5 ,
- Hamid Ashraf 6 ,
- Mohammad N. Alomary 7 &
- Ebtesam A. Al-Suhaimi 8
International Journal of Obesity volume 47 , pages 1179–1199 ( 2023 ) Cite this article
1915 Accesses
3 Citations
Metrics details
- Gastrointestinal hormones
Diabetes is a serious health issue that causes a progressive dysregulation of carbohydrate metabolism due to insufficient insulin hormone, leading to consistently high blood glucose levels. According to the epidemiological data, the prevalence of diabetes has been increasing globally, affecting millions of individuals. It is a long-term condition that increases the risk of various diseases caused by damage to small and large blood vessels. There are two main subtypes of diabetes: type 1 and type 2, with type 2 being the most prevalent. Genetic and molecular studies have identified several genetic variants and metabolic pathways that contribute to the development and progression of diabetes. Current treatments include gene therapy, stem cell therapy, statin therapy, and other drugs. Moreover, recent advancements in therapeutics have also focused on developing novel drugs targeting these pathways, including incretin mimetics, SGLT2 inhibitors, and GLP-1 receptor agonists, which have shown promising results in improving glycemic control and reducing the risk of complications. However, these treatments are often expensive, inaccessible to patients in underdeveloped countries, and can have severe side effects. Peptides, such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), are being explored as a potential therapy for diabetes. These peptides are postprandial glucose-dependent pancreatic beta-cell insulin secretagogues and have received much attention as a possible treatment option. Despite these advances, diabetes remains a major health challenge, and further research is needed to develop effective treatments and prevent its complications. This review covers various aspects of diabetes, including epidemiology, genetic and molecular basis, and recent advancements in therapeutics including herbal and synthetic peptides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
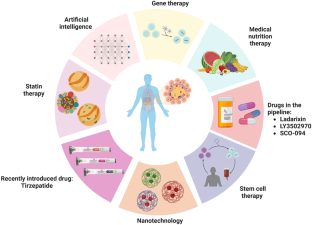
Similar content being viewed by others
Novel therapies with precision mechanisms for type 2 diabetes mellitus
Leigh Perreault, Jay S. Skyler & Julio Rosenstock

Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1
Rola Hammoud & Daniel J. Drucker
Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action
John R. Ussher & Daniel J. Drucker
Data availability
The datasets utilized and/or analyzed during this study are included in the manuscript.
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–7.
Fareed M, Chauhan W, Fatma R, Din I, Afzal M, Ahmed Z. Next-generation sequencing technologies in diabetes research. Diabetes Epidemiol Manag. 2022;7:100097.
Google Scholar
Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, et al. Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care. 2010;33:104–8.
CAS PubMed Google Scholar
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H. et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21:6275.
PubMed PubMed Central Google Scholar
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342.
Skoczek D, Dulak J, Kachamakova-Trojanowska N. Maturity onset diabetes of the young-new approaches for disease modelling. Int J Mol Sci. 2021;14:7553.
Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–90.
Ikle JM, Gloyn AL. 100 YEARS OF INSULIN: a brief history of diabetes genetics: insights for pancreatic beta-cell development and function. J Endocrinol. 2021;250:R23–R35.
CAS PubMed PubMed Central Google Scholar
Redondo M, Yu L, Hawa M, Mackenzie T, Pyke D, Eisenbarth G, et al. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354–62.
Mychaleckyj JC, Noble JA, Moonsamy PV, Carlson JA, Varney MD, Post J, et al. HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clinical Trials. 2010;7:S75–S87.
PubMed Google Scholar
Ounissi-Benkalha H, Polychronakos C. The molecular genetics of type 1 diabetes: new genes and emerging mechanisms. Trends Mol Med. 2008;14:268–75.
Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11:533–42.
Sanchez-Mazas A, Meyer D. The relevance of HLA sequencing in population genetics studies. J Immunol Res. 2014;2014.
Buhler S, Sanchez-Mazas A. HLA DNA sequence variation among human populations: molecular signatures of demographic and selective events. PloS One. 2011;6:e14643.
Eisenbarth GSJD. Banting Lecture 2009: An unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes. 2010;59:759.
Wyatt RC, Lanzoni G, Russell MA, Gerling I, Richardson SJ. What the HLA-I!-Classical and non-classical HLA Class I and their potential roles in type 1 diabetes. Curr Diab Rep. 2019;19:159.
Redondo MJ, Steck AK. Pugliese AJPd. Genetics of type 1. Diabetes. 2018;19:346–53.
Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007732.
Laine AP, Valta M, Toppari J, et al. Non-HLA Gene Polymorphisms in the Pathogenesis of Type 1 Diabetes: Phase and Endotype Specific Effects. Front Immunol. 2022;13:909020.
Pociot F, Akolkar B, Concannon P, et al. Genetics of type 1 diabetes: what’s next?. Diabetes. 2010;59:1561–71.
Pérez de Nanclares G, Bilbao JR, Calvo B, Vitoria JC, Vázquez F, Castaño L. 5′‐Insulin gene VNTR polymorphism is specific for type 1 diabetes: no association with celiac or Addison’s disease. Ann N Y Acad Sci. 2003;1005:319–23.
Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–7.
Bennett S, Lucassen A, Gough S, Powell E, Undlien D, Pritchard L, et al. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet. 1995;9:284–92.
Wang J, Liu L, Ma J, Sun F, Zhao Z, Gu M. Common variants on cytotoxic T lymphocyte antigen-4 polymorphisms contributes to type 1 diabetes susceptibility: evidence based on 58 studies. PloS One. 2014;9:e85982.
Kavvoura FK, Ioannidis JP. CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol. 2005;162:3–16.
Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8.
Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–7.
Lempainen J, Laine AP, Hammais A, et al. Non-HLA gene effects on the disease process of type 1 diabetes: From HLA susceptibility to overt disease. J Autoimmun. 2015;61:45–53.
Steck AK, Dong F, Wong R, Fouts A, Liu E, Romanos J, et al. Improving prediction of type 1 diabetes by testing non‐HLA genetic variants in addition to HLA markers. Pediatr Diabetes. 2014;15:355–62.
Lempainen J, Hermann R, Veijola R, Simell O, Knip M, Ilonen J. Effect of the PTPN22 and INS risk genotypes on the progression to clinical type 1 diabetes after the initiation of β-cell autoimmunity. Diabetes. 2012;61:963–966.
Bugawan TL, Mirel DB, Valdes AM, Panelo A, Pozzilli P, Erlich HA. Association and interaction of the IL4R, IL4, and IL13 loci with type 1 diabetes among Filipinos. Am J Hum Genet. 2003;72:1505–14.
Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun. 2015;64:101–12.
Shastry A, Sedimbi S, Rajalingam R, Nikitina‐Zake L, Rumba I, Wigzell H, et al. Combination of KIR 2DL2 and HLA‐C1 (Asn80) confers susceptibility to type 1 diabetes in Latvians. Int J of Immunogenet. 2008;35:439–46.
CAS Google Scholar
Sabater L, Ferrer-Francesch X, Sospedra M, Caro P, Juan M, Pujol-Borrell R. Insulin alleles and autoimmune regulator (AIRE) gene expression both influence insulin expression in the thymus. J Autoimmun. 2005;25:312–8.
Visperas A, Vignali DA. Are regulatory T cells defective in type 1 diabetes and can we fix them? J Immunol. 2016;197:3762–70.
Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–4.
Udler MS, Kim J, von Grotthuss M, Bonàs-Guarch S, Cole JB, Chiou J, et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 2018;15:e1002654.
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–13.
Udler MS. Type 2 diabetes: multiple genes, multiple diseases. Curr Diab Rep. 2019;19:1–9.
Ge Y, Onengut-Gumuscu S, Quinlan AR, Mackey AJ, Wright JA, Buckner JH, et al. Targeted deep sequencing in multiple-affected sibships of European ancestry identifies rare deleterious variants in PTPN22 that confer risk for type 1 diabetes. Diabetes. 2016;65:794–802.
Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878–84.
Chen J, Sun M, Adeyemo A, Pirie F, Carstensen T, Pomilla C, et al. Genome-wide association study of type 2 diabetes in Africa. Diabetologia. 2019;62:1204–11.
Qi Q, Stilp AM, Sofer T, Moon J-Y, Hidalgo B, Szpiro AA, et al. Genetics of type 2 diabetes in US Hispanic/Latino individuals: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes. 2017;66:1419–25.
Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240–5.
Mahajan A, Wessel J, Willems SM, Zhao W, Robertson NR, Chu AY, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. 2018;50:559–71.
Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46:234–44.
Mahajan A, Sim X, Zhang W, Below JE, Kitajima H, Speidel L, et al. 303-OR: ADA presidents’ select abstract: transethnic association study of type 2 diabetes in more than a million individuals. Diabetes. 2019;68.
Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, Huffman JE, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52:680–91.
Langenberg C, Lotta LA. Genomic insights into the causes of type 2 diabetes. Lancet. 2018;391:2463–74.
Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019;570:71–6.
Dwivedi OP, Lehtovirta M, Hastoy B, Chandra V, Krentz NA, Kleiner S, et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat Genet. 2019;51:1596–606.
Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536:41–7.
Jambaljav B, Tanaka D, Nagashima K, Harashima SI, Harada N, Harada T, et al. Whole-exome sequencing in a Japanese family with highly aggregated diabetes identifies a candidate susceptibility mutation in ADAMTSL3. Diabetes Res Clin Pract. 2018;135:143–9.
Johansson S, Irgens H, Chudasama KK, Molnes J, Aerts J, Roque FS, et al. Exome sequencing and genetic testing for MODY. PLoS One. 2012;7:e38050.
Yki-Jarvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315:224–30.
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia. 2013;56:1462–70.
Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–90.
Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005.
Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Magi R, Reschen ME, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47:1415–25.
Portha B, Chavey A, Movassat J. Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res. 2011;2011:105076.
Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2018;10:23–31.
Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, et al. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65:719–31.
Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500.
Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–55.
Christensen AA, Gannon M. The beta cell in type 2 diabetes. Curr Diab Rep. 2019;19:81.
Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–8.
Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, et al. Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic beta cell. J Biol Chem. 2019;294:168–81.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34.
Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol Behav. 2017;176:139–48.
Venkatasamy VV, Pericherla S, Manthuruthil S, Mishra S, Hanno R. Effect of physical activity on insulin resistance, inflammation and oxidative stress in diabetes mellitus. J Clin Diagn Res. 2013;7:1764–6.
Sircana A, Framarin L, Leone N, Berrutti M, Castellino F, Parente R, et al. Altered gut microbiota in type 2 diabetes: just a coincidence? Curr Diab Rep. 2018;18:98.
Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18:4–17.
Crittenden S, Goepp M, Pollock J, Robb CT, Smyth DJ, Zhou Y, et al. Prostaglandin E(2) promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci Adv. 2021;7.
Han Q, Wang J, Li W, Chen ZJ, Du Y. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome. 2021;9:101.
Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. 2022;12:834485.
Takagi T, Naito Y, Kashiwagi S, Uchiyama K, Mizushima K, Kamada K, et al. Changes in the gut microbiota are associated with hypertension, hyperlipidemia, and type 2 diabetes mellitus in Japanese subjects. Nutrients. 2020;12.
Wang TY, Zhang XQ, Chen AL, Zhang J, Lv BH, Ma MH, et al. A comparative study of microbial community and functions of type 2 diabetes mellitus patients with obesity and healthy people. Appl Microbiol Biotechnol. 2020;104:7143–53.
Yang K, Niu J, Zuo T, Sun Y, Xu Z, Tang W, et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology. 2021;161:1257–69.e13.
Al Bataineh MT, Dash NR, Bel Lassen P, Banimfreg BH, Nada AM, Belda E, et al. Revealing links between gut microbiome and its fungal community in Type 2 Diabetes Mellitus among Emirati subjects: a pilot study. Sci Rep. 2020;10:9624.
Federation I IDF Diabetes Atlas Eighth edition 2019. International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels. Belgium: International Diabetes Federation 2019.
Forouhi NG, Wareham N. Epidemiology of diabetes. Medicine. 2019;47:22–7.
Fitzmaurice C, Abate D, Abbasi N. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systemic analysis for the Global Burden of Disease Study. Jama Oncol. 2020;6:444.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
Herman WH, Ye W, Griffin SJ, Simmons RK, Davies MJ, Khunti K, et al. Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION-Europe). Diabetes Care. 2015;38:1449–55.
Chong S, Ding D, Byun R, Comino E, Bauman A, Jalaludin B. Lifestyle changes after a diagnosis of type 2 diabetes. diabetes spectr. Diabetes Spectr. 2017;30:43–50.
Cotter AP, Durant N, Agne AA, Cherrington A. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J Diabetes Complications. 2014;28:243–51.
Contreras I, Vehi J. Artificial intelligence for diabetes management and decision support: literature review. J Med Internet Res. 2018;20:e10775.
Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133:895–900.
Buch V, Varughese G, Maruthappu M. Artificial intelligence in diabetes care. Diabetic Med. 2018;35:495–7.
Food U Drug Administration (FDA). FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. 2018.
Association AD. 11. Microvascular complications and foot care: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S135–S51.
Ellahham S, Ellahham N. Use of artificial intelligence for improving patient flow and healthcare delivery. J Comp Sci Syst Biol. 2019;12:2.
Bellemo V, Lim G, Rim TH, Tan GS, Cheung CY, Sadda S, et al. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr Diab Rep. 2019;19:1–12.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359:eaan4672.
Tsokos GC, Nepom GT. Gene therapy in the treatment of autoimmune diseases. J Clin Invest. 2000;106:181–3.
Chellappan DK, Yap WS, Na BAS, Gupta G, Dua K. Current therapies and targets for type 2 diabetes mellitus. Panminerva Med. 2018;60:117–31.
Bakay M, Pandey R, Hakonarson H. Genes involved in type 1 diabetes: an update. Genes. 2013;4:499–521.
Chan L, Fujimiya M, Kojima H. In vivo gene therapy for diabetes mellitus. Trends Mol Med. 2003;9:430–5.
Zalzman M, Gupta S, Giri RK, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci USA. 2003;100:7253–8.
Kwak SH, Park KS. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp Mol Med. 2016;48:e220.
Abderrazak A, El Hadri K, Bosc E, Blondeau B, Slimane M-N, Büchele B, et al. Inhibition of the inflammasome NLRP3 by arglabin attenuates inflammation, protects pancreatic β-cells from apoptosis, and prevents type 2 diabetes mellitus development in ApoE2Ki mice on a chronic high-fat diet. J Pharmacol Exp Ther. 2016;357:487–94.
O'Doherty RM, Jensen PB, Anderson P, et al. Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J Clin Invest. 2000;105:479–88.
Newgard CB, Brady MJ, O'Doherty RM, Saltiel AR. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes. 2000;49:1967–77.
Dong H, SLJTiE Woo. Hepatic insulin production for type 1 diabetes. Trends Endocrinol Metab. 2001;12:441–6.
Auricchio A, Gao GP, Yu QC, et al. Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Ther. 2002;9:963–71.
Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603.
Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–13.
Tiwari P. Recent trends in therapeutic approaches for diabetes management: a comprehensive update. J Diabetes Res. 2015;2015.
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90.
Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37:S120–S43.
Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78.
DiSanto RM, Subramanian V, Gu Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:548–64.
Aloke C, Egwu CO, Aja PM, Obasi NA, Chukwu J, Akumadu BO, et al. Current advances in the management of diabetes mellitus. Biomedicines. 2022;10:2436.
Tamborlane W, Beck R, Bode B, Buckingham B, Chase H, Clemons R. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med.2008;359:1464–76.
Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265–74.
Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57.
Scognamiglio V. Nanotechnology in glucose monitoring: advances and challenges in the last 10 years. Biosens Bioelectron. 2013;47:12–25.
Grunberger G. The need for better insulin therapy. Diabetes Obes Metab. 2013;15:1–5.
Lagopati N, Pavlatou E. Nanotechnology in diabetes management. Interv Obes Diabetes. 2021;5:419–24.
Lemmerman LR, Das D, Higuita-Castro N, Mirmira RG, Gallego-Perez D. Nanomedicine-based strategies for diabetes: diagnostics, monitoring, and treatment. Trends Endocrinol Metab. 2020;31:448–58.
McCall MD, Toso C, Baetge EE, Shapiro AJ. Are stem cells a cure for diabetes? Clin Sci. 2010;118:87–97.
Abdulazeez SS. Diabetes treatment: a rapid review of the current and future scope of stem cell research. Saudi Pharm J. 2015;23:333–40.
Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993;42:1715–20.
Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song K-H, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci. 2000;97:7999–8004.
Gao R, Ustinov J, Pulkkinen M-A, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–15.
Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–50.
Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow–derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–70.
Shah RV, Goldfine AB. Statins and risk of new-onset diabetes mellitus. Circulation. 2012;126:e282–e4.
Chen Y-H, Feng B, Chen Z-W. Statins for primary prevention of cardiovascular and cerebrovascular events in diabetic patients without established cardiovascular diseases: a meta-analysis. Exp Clin Endocrinol Diabetes. 2012;120:116–20.
Buse J. Statin treatment in diabetes mellitus. Clin Diabetes. 2003;21:168–72.
Drummond RS, Lyall M, McKnight J. Statins should be routinely prescribed in all adults with diabetes. Pract Diabetes Int. 2010;27:404–6a.
Food U, Administration D FDA approves novel, dual-targeted treatment for type 2 diabetes. FDA News Release. 2022.
Bertsch T. An introduction to tirzepatide. Clin Diabetes. 2022;40:371–2.
Bailey CJ, Flatt PR, Conlon JM. An update on peptide-based therapies for type 2 diabetes and obesity. Peptides. 2023;161:170939.
Nauck MA, Mirna AEA, Quast DR. Meta-analysis of head-to-head clinical trials comparing incretin-based glucose-lowering medications and basal insulin: An update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide. Diabetes Obes Metab. 2023;25:1361–71.
Madsbad S, Holst JJ. Cardiovascular effects of incretins: focus on glucagon-like peptide-1 receptor agonists. Cardiovasc Res. 2023;119:886–904.
Piemonti L, Keymeulen B, Gillard P, Linn T, Bosi E, Rose L, et al. Ladarixin, an inhibitor of the interleukin‐8 receptors CXCR1 and CXCR2, in new‐onset type 1 diabetes: a multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2022;24:1840.
Kawai T, Sun B, Yoshino H, Feng D, Suzuki Y, Fukazawa M, et al. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc Natl Acad Sci. 2020;117:29959–67.
Fesenko I, Azarkina R, Kirov I, Kniazev A, Filippova A, Grafskaia E, et al. Phytohormone treatment induces generation of cryptic peptides with antimicrobial activity in the Moss Physcomitrella patens. BMC Plant Biol. 2019;19:9.
Karami Z, Akbari-Adergani B. Bioactive food derived peptides: a review on correlation between structure of bioactive peptides and their functional properties. J Food Sci Technol. 2019;56:535–47.
Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive peptides: synthesis, sources, applications, and proposed mechanisms of action. Int J Mol Sci. 2022;23.
Qiao Q, Chen L, Li X, Lu X, Xu Q. Roles of dietary bioactive peptides in redox balance and metabolic disorders. Oxid Med Cell Longev. 2021;2021:5582245.
Antony P, Vijayan R. Bioactive peptides as potential nutraceuticals for diabetes therapy: a comprehensive review. Int J Mol Sci. 2021;22.
Muttenthaler M, King GF, Adams DJ, Alewood PF. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021;20:309–25.
Yan J, Zhao J, Yang R, Zhao W. Bioactive peptides with antidiabetic properties: a review. Int J Food Sci Technol. 2019;54:1909–19.
Singh AK. Dipeptidyl peptidase-4 inhibitors: novel mechanism of actions. Indian J Endocrinol Metab. 2014;18:753–9.
Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, et al. Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther. 2022;7:48.
Zaky AA, Simal-Gandara J, Eun JB, Shim JH, Abd El-Aty AM. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: a review. Front Nutr. 2021;8:815640.
Furman BL. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon. 2012;59:464–71.
Wajcberg E, Amarah A. Liraglutide in the management of type 2 diabetes. Drug Des Devel Ther. 2010;4:279–90.
Wang Z, Zhang X. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. J Sci Food Agric. 2017;97:918–22.
Zambrowicz A, Eckert E, Pokora M, Bobak Ł, Dąbrowska A, Szołtysik M, et al. Antioxidant and antidiabetic activities of peptides isolated from a hydrolysate of an egg-yolk protein by-product prepared with a proteinase from Asian pumpkin (Cucurbita ficifolia). RSC Adv. 2015;5:10460–7.
Wang R, Zhao H, Pan X, Orfila C, Lu W, Ma Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of alpha-glucosidase inhibitory peptides from soy protein. Food Sci Nutr. 2019;7:1848–56.
Karimi A, Azizi MH, Ahmadi Gavlighi H. Frationation of hydrolysate from corn germ protein by ultrafiltration: In vitro antidiabetic and antioxidant activity. Food Sci Nutr. 2020;8:2395–405.
Ktari N, Salem RBS-B, Bkhairia I, Slima SB, Nasri R, Salah RB, et al. Functional properties and biological activities of peptides from zebra blenny protein hydrolysates fractionated using ultrafiltration. Food Biosci. 2020;34:100539.
Dale HF, Jensen C, Hausken T, et al. Effect of a cod protein hydrolysate on postprandial glucose metabolism in healthy subjects: a double-blind cross-over trial [published correction appears in J Nutr Sci. 2019 Jan 18;8:e1]. J Nutr Sci. 2018;7:e33.
Wang T, Zheng L, Zhao T, Zhang Q, Liu Z, Liu X, et al. Anti-diabetic effects of sea cucumber (Holothuria nobilis) hydrolysates in streptozotocin and high-fat-diet induced diabetic rats via activating the PI3K/Akt pathway. J Funct Foods. 2020;75.
Godinho R, Mega C, Teixeira-de-Lemos E, Carvalho E, Teixeira F, Fernandes R, et al. The place of dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapeutics: a “me too” or “the special one” antidiabetic class? J Diabetes Res. 2015;2015:806979.
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–68.
Jin R, Teng X, Shang J, Wang D, Liu N. Identification of novel DPP-IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res Int. 2020;133:109161.
Gao J, Gong H, Mao X. Dipeptidyl peptidase-IV inhibitory activity and related molecular mechanism of bovine alpha-lactalbumin-derived peptides. Molecules. 2020;25.
Gu H, Gao J, Shen Q, Gao D, Wang Q, Tangyu M, et al. Dipeptidyl peptidase-IV inhibitory activity of millet protein peptides and the related mechanisms revealed by molecular docking. LWT. 2021;138:110587.
Wang J, Wu T, Fang L, Liu C, Liu X, Li H, et al. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J Funct Foods. 2020;69.
Depta J, Małkowska P, Wysokińska M, Todorska K, Sierawska O, Hrynkiewicz R, et al. Therapeutic role of antimicrobial peptides in diabetes mellitus. Biologics. 2022;2:92–106.
Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, Franco A, et al. Antimicrobial peptides: a new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol. 2021;11:668632.
Hirsch JG. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956;103:589–611.
Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel). 2013;6:1543–75.
Miller A, Matera-Witkiewicz A, Mikolajczyk A, Wieczorek R, Rowinska-Zyrek M. Chemical “butterfly effect” explaining the coordination chemistry and antimicrobial properties of clavanin complexes. Inorg Chem. 2021;60:12730–4.
Zhang QY, Yan ZB, Meng YM, Hong XY, Shao G, Ma JJ, et al. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res. 2021;8:48.
van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP. Cathelicidins: immunomodulatory antimicrobials. Vaccines (Basel). 2018;6.
Nagaoka I, Tamura H, Reich J. Therapeutic potential of cathelicidin peptide LL-37, an Antimicrobial agent, in a murine sepsis model. Int J Mol Sci. 2020;21.
Contreras G, Shirdel I, Braun MS, Wink M. Defensins: transcriptional regulation and function beyond antimicrobial activity. Dev Comp Immunol. 2020;104:103556.
Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511.
Miani M, Le Naour J, Waeckel-Enee E, Verma SC, Straube M, Emond P, et al. Gut microbiota-stimulated innate lymphoid cells support beta-defensin 14expression in pancreatic endocrine cells, preventing autoimmune diabetes. Cell Metab. 2018;28:557–72.e6.
Tsai YW, Dong JL, Jian YJ, Fu SH, Chien MW, Liu YW, et al. Gut Microbiota-modulated metabolomic profiling shapes the etiology and pathogenesis of autoimmune diseases. Microorganisms. 2021;9.
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–53.
Zainab AJAA, Ashish N, Ragnath V. Salivary levels of antimicrobial peptides in chronic periodontitis patients with type 2 diabetes. J Int Acad Periodontol. 2019;21:36–44.
Soltaninejad H, Zare-Zardini H, Ordooei M, Ghelmani Y, Ghadiri-Anari A, Mojahedi S, et al. Antimicrobial peptides from amphibian innate immune system as potent antidiabetic agents: a literature review and bioinformatics analysis. J Diabetes Res. 2021;2021:2894722.
Musale V, Moffett RC, Owolabi B, Conlon JM, Flatt PR, Abdel-Wahab YHA. Mechanisms of action of the antidiabetic peptide [S4K]CPF-AM1 in db/db mice. J Mol Endocrinol. 2021;66:115–28.
Ramadhan AH, Nawas T, Zhang X, Pembe WM, Xia W, Xu Y. Purification and identification of a novel antidiabetic peptide from Chinese giant salamander (Andrias davidianus) protein hydrolysate against α-amylase and α-glucosidase. Int J Food Properties. 2017;20:S3360–S72.
Nishikawa T, Araki E. Investigation of a novel mechanism of diabetic complications: impacts of mitochondrial reactive oxygen species. Rinsho Byori. 2008;56:712–9.
Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78.
Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–8.
Erbguth F. Diabetes and the central nervous system. Nervenarzt. 2017;88:675–90.
Zarse K, Ristow M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 2015;21:355–6.
Onyango AN. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid Med Cell Longev. 2018;2018:4321714.
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192.
Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–7.
Kwon C, Sun JL, Jeong JH, Jung TW. Humanin attenuates palmitate-induced hepatic lipid accumulation and insulin resistance via AMPK-mediated suppression of the mTOR pathway. Biochem Biophys Res Commun. 2020;526:539–45.
Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the beta cell. FASEB J. 2013;27:4890–8.
Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY). 2016;8:796–809.
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–54.
Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl). 2019;97:473–85.
Zhai D, Ye Z, Jiang Y, Xu C, Ruan B, Yang Y, et al. MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA. Mol Immunol. 2017;92:151–60.
Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care. 2003;26:1619–23.
Simsek S. Angiotensin I-converting enzyme, dipeptidyl peptidase-IV, and α-glucosidase inhibitory potential of hazelnut meal protein hydrolysates. J Food Measurement Charact. 2021;15:4490–6.
Yu Z, Yin Y, Zhao W, Liu J, Chen F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012;135:2078–85.
Mudgil P, Kilari BP, Kamal H, Olalere OA, FitzGerald RJ, Gan C-Y, et al. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J Cereal Sci. 2020;96:103130.
Vilcacundo R, Martínez-Villaluenga C, Hernández-Ledesma B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J Funct Foods. 2017;35:531–9.
Fuentes LR, Richard C, Chen L. Sequential alcalase and flavourzyme treatment for preparation of α-amylase, α-glucosidase, and dipeptidyl peptidase (DPP)-IV inhibitory peptides from oat protein. J Funct Foods. 2021;87:104829.
Pathan S, Siddiqui RA. Nutritional composition and bioactive components in Quinoa (Chenopodium quinoa Willd.) greens: a review. Nutrients. 2022;14.
Salami M, Sadeghian Motahar SF, Ariaeenejad S, Sheykh Abdollahzadeh Mamaghani A, Kavousi K, Moosavi-Movahedi AA, et al. The novel homologue of the human alpha-glucosidase inhibited by the non-germinated and germinated quinoa protein hydrolysates after in vitro gastrointestinal digestion. J Food Biochem. 2022;46:e14030.
Luan F, Wang Z, Yang Y, Ji Y, Lv H, Han K, et al. Juglans mandshurica Maxim.: a review of its traditional usages, phytochemical constituents, and pharmacological properties. Front Pharmacol. 2020;11:569800.
Ghafoor K, Özcan MM, AL-Juhaımı F, Babıker EE, Sarker ZI, Ahmed IAM, et al. Nutritional composition, extraction, and utilization of wheat germ oil: a review. Eur J Lipid Sci Technol. 2017;119:1600160.
Liu W, Li H, Wen Y, Liu Y, Wang J, Sun B. Molecular mechanism for the alpha-glucosidase inhibitory effect of wheat germ peptides. J Agric Food Chem. 2021;69:15231–9.
Rasane P, Jha A, Sabikhi L, Kumar A, Unnikrishnan VS. Nutritional advantages of oats and opportunities for its processing as value added foods - a review. J Food Sci Technol. 2015;52:662–75.
Grundy MM, Fardet A, Tosh SM, Rich GT, Wilde PJ. Processing of oat: the impact on oat’s cholesterol lowering effect. Food Funct. 2018;9:1328–43.
Zhang Y, Wu F, He Z, Fang X, Liu X. Optimization and molecular mechanism of novel alpha-glucosidase inhibitory peptides derived from camellia seed cake through enzymatic hydrolysis. Foods. 2023;12.
Feng J, Ma YL, Sun P, Thakur K, Wang S, Zhang JG, et al. Purification and characterisation of α‐glucosidase inhibitory peptides from defatted camellia seed cake. Int J Food Sci Technol. 2021;56:138–47.
Brown R, Ware L, Tey SL. Effects of hazelnut consumption on cardiometabolic risk factors and acceptance: a systematic review. Int J Environ Res Public Health. 2022;19.
Gul K, Yousuf B, Singh AK, Singh P, Wani AA. Rice bran: nutritional values and its emerging potential for development of functional food—a review. Bioact Carbohydr Diet Fibre. 2015;6:24–30.
Uraipong C, Zhao J. In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on alpha-glucosidase and angiotensin I converting enzyme. J Sci Food Agric. 2018;98:758–66.
Tan SP, Kha TC, Parks SE, Roach PD. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: a review. Food Rev Int. 2016;32:181–202.
Poovitha S, Parani M. In vitro and in vivo alpha-amylase and alpha-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement Altern Med. 2016;16:185.
Kumar P, Mahato DK, Kamle M, Borah R, Sharma B, Pandhi S, et al. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: an overview. Phytother Res. 2021;35:6010–29.
Ren Y, Liang K, Jin Y, Zhang M, Chen Y, Wu H, et al. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J Funct Foods. 2016;26:439–50.
Telapolu S, Kalachavedu M, Punnoose AM, Bilikere DMD-1. a poly herbal formulation indicated in diabetes mellitus ameliorates glucose uptake and inhibits adipogenesis - an in vitro study. BMC Complement Altern Med. 2018;18:113.
Butala MA, Kukkupuni SK, Venkatasubramanian P, Vishnuprasad CN. An ayurvedic anti-diabetic formulation made from Curcuma longa L. and Emblica officinalis L. inhibits α-amylase, α-glucosidase, and starch digestion, in vitro. Starch Stärke. 2018;70:1700182.
Panda V, Deshmukh A, Singh S, Shah T, Hingorani L. An ayurvedic formulation of Emblica officinalis and Curcuma longa alleviates insulin resistance in diabetic rats: involvement of curcuminoids and polyphenolics. J Ayurveda Integr Med. 2021;12:506–13.
Mehrzadi S, Tavakolifar B, Huseini HF, Mosavat SH, Heydari M. The effects of Boswellia serrata gum resin on the blood glucose and lipid profile of diabetic patients: a double-blind randomized placebo-controlled clinical trial. J Evid Based Integr Med. 2018;23:2515690X18772728.
Ahmed A, Zeng G, Azhar M, Lin H, Zhang M, Wang F, et al. Jiawei Shengmai San herbal formula ameliorates diabetic associate cognitive decline by modulating AKT and CREB in rats. Phytother Res. 2020;34:3249–61.
Yella SST, Kumar RN, Ayyanna C, Varghese AM, Amaravathi P, Vangoori Y. The combined effect of Trigonella foenum seeds and Coriandrum sativum leaf extracts in alloxan-induced diabetes mellitus wistar albino rats. Bioinformation. 2019;15:716–22.
Mehrzadi S, Mirzaei R, Heydari M, Sasani M, Yaqoobvand B, Huseini HF. Efficacy and safety of a traditional herbal combination in patients with type II diabetes mellitus: a randomized controlled trial. J Diet. 2021;18:31–43.
Wasana KGP, Attanayake AP, Weerarathna TP, Jayatilaka K. Efficacy and safety of a herbal drug of Coccinia grandis (Linn.) Voigt in patients with type 2 diabetes mellitus: a double blind randomized placebo controlled clinical trial. Phytomedicine. 2021;81:153431.
Wang H, Tan H, Zhan W, Song L, Zhang D, Chen X, et al. Molecular mechanism of Fufang Zhenzhu Tiaozhi capsule in the treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease based on network pharmacology and validation in minipigs. J Ethnopharmacol. 2021;274:114056.
Perez Gutierrez RM, Martinez Jeronimo FF, Contreras Soto JG, Muniz Ramirez A, Estrella, Mendoza MF. Optimization of ultrasonic-assisted extraction of polyphenols from the polyherbal formulation of Cinnamomum verum, Origanum majorana, and Origanum vulgare and their anti-diabetic capacity in zebrafish (Danio rerio). Heliyon. 2022;8:e08682.
Naik A, Adeyemi SB, Vyas B, Krishnamurthy R. Effect of co-administration of metformin and extracts of Costus pictus D.Don leaves on alloxan-induced diabetes in rats. J Tradit Complement Med. 2022;12:269–80.
Kumar A, Negi AS, Chauhan A, Semwal R, Kumar R, Semwal RB, et al. Formulation and evaluation of SGLT2 inhibitory effect of a polyherbal mixture inspired from Ayurvedic system of medicine. J Tradit Complement Med. 2022;12:477–87.
Majd FS, Talebi SS, Ahmad Abadi AN, Poorolajal J, Dastan D. Efficacy of a standardized herbal product from Pistacia atlantica subsp. Kurdica in type 2 diabetic patients with hyperlipidemia: a triple-blind randomized clinical trial. Complement Ther Clin Pract. 2022;48:101613.
Nguyen TT, Le QT, Hoang DT, et al. Massively parallel sequencing uncovered disease-associated variant spectra of glucose-6-phosphate dehydrogenase deficiency, phenylketonuria and galactosemia in Vietnamese pregnant women. Mol Genet Genomic Med. 2022;10:e1959.
Klak M, Gomółka M, Kowalska P, et al. Type 1 diabetes: genes associated with disease development. Cent Eur J Immunol. 2020;45:439–53.
Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57:241–54.
Rafique I, Mir A, Saqib MAN, Naeem M, Marchand L, Polychronakos C. Causal variants in Maturity Onset Diabetes of the Young (MODY) - A systematic review. BMC Endocr Disord. 2021;21:223.
Huang SL, Jao CL, Ho KP, Hsu KC. Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides. 2012;35:114–21.
Nongonierma AB, FitzGerald RJ. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J Funct Foods. 2013;5:1909–17.
Harnedy PA, Parthsarathy V, McLaughlin CM, et al. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res Int. 2018;106:598–606.
Zhang Y, Chen R, Chen X, Zeng Z, Ma H, Chen S. Dipeptidyl peptidase iv-inhibitory peptides derived from silver carp (hypophthalmichthys molitrix val.) proteins. J Agric Food Chem. 2016;64:831–9.
Valencia-Mejía E, Batista KA, Fernández JJA, Fernandes KF. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res Int. 2019;121:238–46.
Download references
Acknowledgements
WC and SS are thankful to Indian Council of Medical Research (ICMR), New Delhi and Council of Scientific and Industrial Research (CSIR), New Delhi for providing senior research fellowship, respectively.
Author information
Authors and affiliations.
Department of Epidemic Disease Research, Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam, 31441, Saudi Arabia
Mohammad Azam Ansari
Department of Hematology, Duke University, Durham, NC, 27710, USA
Waseem Chauhan
Department of Biochemistry, Faculty of Medicine, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
Shoaib Shoaib
Wellness and Preventive Medicine Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, 11442, Saudi Arabia
Sami A. Alyahya
USF Health Byrd Alzheimer’s Center and Neuroscience Institute, Department of Molecular Medicine, Tampa, FL, USA
Mubashshir Ali
Rajiv Gandhi Center for Diabetes and Endocrinology, Faculty of Medicine, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
Hamid Ashraf
Advanced Diagnostic and Therapeutic Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, 11442, Saudi Arabia
Mohammad N. Alomary
King Abdulaziz & his Companions Foundation for Giftedness & Creativity, Riyadh, Saudi Arabia
Ebtesam A. Al-Suhaimi
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: MAA, SS, and WC; writing—original draft: SS, WC, and MAA; writing—review and editing: SAA, MA, HA, MNA, and EAA; All authors were involved in drafting, reviewing and revising the final paper.
Corresponding authors
Correspondence to Mohammad Azam Ansari , Mohammad N. Alomary or Ebtesam A. Al-Suhaimi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Ansari, M.A., Chauhan, W., Shoaib, S. et al. Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions. Int J Obes 47 , 1179–1199 (2023). https://doi.org/10.1038/s41366-023-01369-3
Download citation
Received : 09 April 2023
Revised : 04 July 2023
Accepted : 17 August 2023
Published : 11 September 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s41366-023-01369-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
New cause of diabetes discovered, offering potential target for new classes of drugs to treat the disease. ScienceDaily . Retrieved April 13, 2024 from www.sciencedaily.com / releases / 2023 / 12 ...
St. Petersburg, Fla. - September 12, 2023 - Scientists at Johns Hopkins All Children's Hospital, along with an international team of researchers, are shedding new light on the causes of Type 2 diabetes. The new research, published in the journal Nature Communications, offers a potential strategy for developing new therapies that could restore dysfunctional pancreatic beta-cells or ...
Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia). It arises from a resistance to and relative deficiency ...
This was an impressive piece of work that makes important new insights into type 2 diabetes biology and hints at causes of ancestral differences in the disease. ... The T2D-genetics research ...
Large-scale study reveals new genetic details of diabetes. In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research ...
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Read the latest Research articles in Type 2 diabetes from Nature Reviews Endocrinology ... This Review highlights new insights into mechanisms of glucocorticoid-induced diabetes mellitus and ...
Introduction. Insulin resistance and β-cell dysfunction are the 2 major hallmarks of type 2 diabetes mellitus (T2DM) that appear as the result of disturbed homeostasis [].Failure of β-cells (∼80% of their β-cell function) and insulin resistance in muscles and the liver is a vicious triumvirate responsible for the core physiological defects.
There are many known risk factors for type 2 diabetes, including family members having it, being of African or Asian ancestry, high blood pressure, obesitym and polycystic ovary syndrome (PCOS ...
However, new research methodologies are needed to obtain more accurate and useful insights into the biological and clinical processes involved in diabetic complication development. During the last few years, new approaches for clinical research have incorporated digital tools to analyze the complex physiopathological background of type 2 diabetes.
The most common form is type-2 diabetes (T2D), characterized by peripheral insulin resistance. ... New insights into adult-onset type 1 diabetes. Apr 1, 2024 ... Daily science news on research ...
This new molecule is stable over long periods of time and can be easily integrated into miniaturized systems. Now, Dr. Wang is in the process of patenting his invention and intends to continue research on this new molecule so that it can eventually benefit people living with diabetes. Wang, B ... Determining the role of BPA in type 2 diabetes risk.
This research will provide insights into the role of the brain in the control of blood sugar levels and has potential to facilitate the development of novel approaches to diabetes treatment." The problem: Type 2 diabetes (T2D) is among the most pressing and costly medical challenges confronting modern society. Even with currently available ...
A new study shows that tiny biodegradable particles helped improve insulin sensitivity in the liver cells of humans and in diabetic mice. By using these nanoscavengers, researchers were able to ...
Aims: To examine the patterns of use of potentially interacting supplement-drug pairs in adults with type 2 diabetes (T2D) in real-world settings, and to explore the impact of potentially interacting supplement-drug pairs on downstream outcomes. Methods: Potentially interacting supplement-drug pairs were identified from four tertiary databases.
Your source for the latest research news. Follow: Facebook X/Twitter Subscribe: RSS Feeds. ... This molecule, called miR-690, could eventually be leveraged into new therapies for type 2 diabetes.
Dr Elizabeth Robertson, our Director of Research, said: "Diabetes UK is proud to have funded over a decade of research that has forged new frontiers for people with type 2 diabetes and put remission on the map. "Remission from type 2 diabetes lifts the burden of 24/7 diabetes management and can transform health and wellbeing.
Two decades ago, if you asked a diabetes specialist to explain the cause of type 2 diabetes, they would say insulin resistance. Insulin is a hormone made by the pancreas that helps the glucose in ...
The Clinical Research in Type 2 Diabetes (T2D) program supports human studies across the lifespan aimed at understanding, preventing and treating T2D. This program includes clinical trials that test pharmacologic, behavioral, surgical or practice-level approaches to the treatment and/or prevention of T2D, including promoting the preservation of ...
Type 1 diabetes; Type 2 diabetes; Latest Research and Reviews. ... The largest genome-wide association study for type 2 diabetes so far, which included several ancestry groups, led to the ...
An Additional 12 Million US Adults Become Eligible for Diabetes Screening. New USPSTF and ADA guidelines lower the age for prediabetes and type 2 diabetes screening to 35. This study examined if testing practices aligned with guidelines and which populations were less likely to receive testing. Read the summary.
Earlier this year, Professor Roy Taylor announced new findings from his Diabetes UK-funded project, called ReTUNE, a world-first study into type 2 diabetes remission in people with lower body weights. After losing weight following a low-calorie diet programme, 70% of participants went into remission. The findings showed for the first time that ...
A total of 3260 patients were assigned to receive empagliflozin and 3262 to receive placebo. During a median follow-up of 17.9 months, a first hospitalization for heart failure or death from any ...
Dr Elizabeth Robertson, director of research at Diabetes UK, said: "This evidence showing that the accuracy of a common test to diagnose and monitor type 2 diabetes is linked to a person's ...
Global and regional trends from 1990 to 2017 of type 2 diabetes for all ages were compiled. Forecast estimates were obtained using the SPSS Time Series Modeler. In 2017, approximately 462 million individuals were affected by type 2 diabetes corresponding to 6.28% of the world's population (4.4% of those aged 15-49 years, 15% of those aged ...
Over the past three decades, the prevalence of Type 2 diabetes has risen dramatically worldwide. According to the International Federation of Diabetes, by 2045 one in eight adults will be living ...
HOUSE RULES. KINGSTON, Jamaica - New research has yielded promising results for more effective treatment and management options for patients suffering from Type 2 diabetes. The study, titled ...
Fact checked by Sarah Scott A new study found that individuals with type 2 diabetes may benefit from curating their exercise routines, particularly by strategizing the time of day they work out ...
It is crucial to take HLA context into account when analyzing genetic association data from non-HLA loci. ... Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among ...