Appointments at Mayo Clinic
Alzheimer's treatments: what's on the horizon.
Despite many promising leads, new treatments for Alzheimer's are slow to emerge.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning.
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to progress.
Experts are cautious but hopeful about developing treatments that can stop or delay the progression of Alzheimer's. Experts continue to better understand how the disease changes the brain. This has led to the research of potential Alzheimer's treatments that may affect the disease process.
Future Alzheimer's treatments may include a combination of medicines. This is similar to treatments for many cancers or HIV / AIDS that include more than one medicine.
These are some of the strategies currently being studied.

Taking aim at plaques
Some of the new Alzheimer's treatments target clumps of the protein beta-amyloid, known as plaques, in the brain. Plaques are a characteristic sign of Alzheimer's disease.
Strategies aimed at beta-amyloid include:
Recruiting the immune system. Medicines known as monoclonal antibodies may prevent beta-amyloid from clumping into plaques. They also may remove beta-amyloid plaques that have formed. They do this by helping the body clear them from the brain. These medicines mimic the antibodies your body naturally produces as part of your immune system's response to foreign invaders or vaccines.
In 2023, the U.S. Food and Drug Administration (FDA) approved lecanemab (Leqembi) for people with mild Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease.
A phase 3 clinical trial found that the medicine slowed cognitive decline in people with early Alzheimer's disease. The medicine prevents amyloid plaques in the brain from clumping. The phase 3 trial was the largest so far to study whether clearing clumps of amyloid plaques from the brain can slow the disease.
Lecanemab is given as an IV infusion every two weeks. Your care team likely will watch for side effects and ask you or your caregiver how your body reacts to the drug. Side effects of lecanemab include infusion-related reactions such as fever, flu-like symptoms, nausea, vomiting, dizziness, changes in heart rate and shortness of breath.
Also, people taking lecanemab may have swelling in the brain or may get small bleeds in the brain. Rarely, brain swelling can be serious enough to cause seizures and other symptoms. Also in rare instances, bleeding in the brain can cause death. The FDA recommends getting a brain MRI before starting treatment. It also recommends being monitored with brain MRI s during treatment for symptoms of brain swelling or bleeding.
People who carry a certain form of a gene known as APOE e4 appear to have a higher risk of these serious complications. The FDA recommends being tested for this gene before starting treatment with lecanemab.
If you take a blood thinner or have other risk factors for brain bleeding, talk to your health care professional before taking lecanemab. Blood-thinning medicines may increase the risk of bleeds in the brain.
More research is being done on the potential risks of taking lecanemab. Other research is looking at how effective lecanemab may be for people at risk of Alzheimer's disease, including people who have a first-degree relative, such as a parent or sibling, with the disease.
Another medicine being studied is donanemab. It targets and reduces amyloid plaques and tau proteins. It was found to slow declines in thinking and functioning in people with early Alzheimer's disease.
The monoclonal antibody solanezumab did not show benefits for individuals with preclinical, mild or moderate Alzheimer's disease. Solanezumab did not lower beta-amyloid in the brain, which may be why it wasn't effective.
Preventing destruction. A medicine initially developed as a possible cancer treatment — saracatinib — is now being tested in Alzheimer's disease.
In mice, saracatinib turned off a protein that allowed synapses to start working again. Synapses are the tiny spaces between brain cells through which the cells communicate. The animals in the study experienced a reversal of some memory loss. Human trials for saracatinib as a possible Alzheimer's treatment are now underway.
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes.
Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.
Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
Products and Services
- A Book: Mayo Clinic on Alzheimer's Disease
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's drugs
- Alzheimer's genes
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Lecanemab, the New Alzheimer’s Treatment: 3 Things To Know
BY CARRIE MACMILLAN July 24, 2023
Yale researcher discusses the recent FDA approval of a new Alzheimer's disease treatment.
[Originally published January 19, 2023. Updated: July 24, 2023.]
The Food and Drug Administration (FDA) recently granted full approval to a new Alzheimer’s treatment called lecanemab, which has been shown to moderately slow cognitive and functional decline in early-stage cases of the disease.
Alzheimer’s disease is a progressive disorder that damages and destroys nerve cells in the brain. Over time, the disease leads to a gradual loss of cognitive functions, including the ability to remember, reason, use language, and recognize familiar places. It can also cause a range of behavioral changes.
In January, the FDA gave the medication an accelerated approval based on amyloid plaque clearance. Christopher van Dyck, MD , director of Yale’s Alzheimer’s Disease Research Unit, was the lead author of a study published in the Jan. 5 issue of The New England Journal of Medicine that shared results of a Phase III clinical trial of lecanemab. (Dr. van Dyck is also a paid consultant for the pharmaceutical company Eisai, which funded the trials.)
Sold under the brand name Leqembi™ and made by Eisai in partnership with Biogen Inc., the drug is delivered by an intravenous infusion every two weeks. Lecanemab works by removing a sticky protein from the brain that is believed to cause Alzheimer’s disease to advance.
“It’s very exciting because this is the first treatment in our history that shows an unequivocal slowing of decline in Alzheimer’s disease,” says Dr. van Dyck.
This is the first time in two decades that the FDA has granted full approval to a drug for Alzheimer’s, but there is also a “black box” warning on the medication—the agency’s strongest caution—because of safety concerns.
We talked more with Dr. van Dyck, who answered three questions about the new treatment.
How effective is lecanemab for Alzheimer’s disease?
In a trial that involved 1,795 participants with early-stage, symptomatic Alzheimer’s, lecanemab slowed clinical decline by 27% after 18 months of treatment compared with those who received a placebo.
“The antibody treatment selectively targets the forms of amyloid protein that are thought to be the most toxic to brain cells,” says Dr. van Dyck.
Study participants who received the treatment had a significant reduction in amyloid burden in imaging tests, usually reaching normal levels by the end of the trial. Participants also showed a 26% slowing of decline in a key secondary measure of cognitive function and a 37% slowing of decline in a measure of daily living compared to the placebo group.
“Would I like the numbers to be higher? Of course, but I don’t think this is a small effect,” says Dr. van Dyck. “These results could also indicate a starting point for bigger effects. The data appear encouraging that the longer the treatment period, the better the effect. But we’ll need more studies to determine if that’s true.”
They also beg the question about still-earlier intervention, adds Dr. van Dyck. Lecanemab is already being tested in the global AHEAD study for individuals who are still cognitively normal but at high risk of symptoms due to elevated levels of brain amyloid.
Yale currently has the largest number of participants in the AHEAD study, which is funded by the National Institutes of Health (NIH) and Eisai and is enrolling participants as young as 55. “We may see a larger benefit if we intervene before significant brain damage has occurred,” he says.
Is lecanemab safe?
The most common side effect (26.4% of participants vs. 7.4% in the placebo group) of the treatment is an infusion-related reaction, which may include transient symptoms, such as flushing, chills, fever, rash, and body aches. The majority (96%) of these reactions were mild to moderate, and 75% happened after the first dose.
“We can medicate those individuals in advance if we find they have those side effects repeatedly,” says Dr. van Dyck. “We can use medications such as diphenhydramine or acetaminophen. But this is generally not an issue.”
Another potential side effect associated with lecanemab was amyloid-related imaging abnormalities with edema, or fluid formation on the brain. This occurred in 12.6% of trial participants compared to 1.7% in the placebo group. “It’s usually asymptomatic when it occurs, but we can detect it on MRI scans. We often don’t stop dosing if we see it, unless there are symptoms, in which case we would pause infusions until it fully resolves,” Dr. van Dyck says.
It’s important to note that the studies with lecanemab show substantially lower rates of this side effect than do published trials of other, similar drugs such as aducanumab—they're at about a third of the rate, explains Dr. van Dyck. “So, for drugs in this class, I think lecanemab has a favorable safety profile,” he says.
Lastly, 17.3% of trial participants experienced amyloid-related imaging abnormalities with brain bleeding compared to 9% in the placebo group.
“Most of the time we're really talking about microhemorrhages that are in the order of millimeters,” says Dr. van Dyck. “People with Alzheimer's disease are more prone to these events because of the amyloid deposits in their blood vessels, but a catastrophic bleed is quite rare.”
The medication’s label includes warnings about brain swelling and bleeding and that people with a gene mutation that increases their risk of Alzheimer’s disease are at greater risk of brain swelling on the treatment. The label also cautions against taking blood thinners while on the medication.
When will lecanemab be available for Alzheimer’s disease treatment?
Eisai set the price for Leqembi at $26,500 per year, and it has reportedly been largely unavailable while FDA full approval was pending. That may change now that Medicare has said it will cover 80% of the cost.
More news from Yale Medicine
How to stay connected with a loved one with alzheimer’s disease.

How Anti-Obesity Medications Can Help With Surgery

3 Things to Know About JN.1, the New Coronavirus Strain
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

A molecular ‘warhead’ against disease

Asking the internet about birth control

‘Harvard Thinking’: Facing death with dignity
Start of new era for alzheimer’s treatment.
Alvin Powell
Harvard Staff Writer
Expert discusses recent lecanemab trial, why it appears to offer hope for those with deadly disease
Researchers say we appear to be at the start of a new era for Alzheimer’s treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady, called amyloid-beta, and removing it from the body. Experts say the results offer hope that the slow, inexorable loss of memory and eventual death brought by Alzheimer’s may one day be a thing of the past.
The Gazette spoke with Scott McGinnis , an assistant professor of neurology at Harvard Medical School and Alzheimer’s disease expert at Brigham and Women’s Hospital , about the results and a new clinical trial testing whether the same drug given even earlier can prevent its progression.
Scott McGinnis
GAZETTE: The results of the Clarity AD trial have some saying we’ve entered a new era in Alzheimer’s treatment. Do you agree?
McGINNIS: It’s appropriate to consider it a new era in Alzheimer’s treatment. Until we obtained the results of this study, trials suggested that the only mode of treatment was what we would call a “symptomatic therapeutic.” That might give a modest boost to cognitive performance — to memory and thinking and performance in usual daily activities. But a symptomatic drug does not act on the fundamental pathophysiology, the mechanisms, of the disease. The Clarity AD study was the first that unambiguously suggested a disease-modifying effect with clear clinical benefit. A couple of weeks ago, we also learned a study with a second drug, donanemab, yielded similar results.
GAZETTE: Hasn’t amyloid beta, which forms Alzheimer’s characteristic plaques in the brain and which was the target in this study, been a target in previous trials that have not been effective?
McGINNIS: That’s true. Amyloid beta removal has been the most widely studied mechanism in the field. Over the last 15 to 20 years, we’ve been trying to lower beta amyloid, and we’ve been uncertain about the benefits until this point. Unfavorable results in study after study contributed to a debate in the field about how important beta amyloid is in the disease process. To be fair, this debate is not completely settled, and the results of Clarity AD do not suggest that lecanemab is a cure for the disease. The results do, however, provide enough evidence to support the hypothesis that there is a disease-modifying effect via amyloid removal.
GAZETTE: Do we know how much of the decline in Alzheimer’s is due to beta amyloid?
McGINNIS: There are two proteins that define Alzheimer’s disease. The gold standard for diagnosing Alzheimer’s disease is identifying amyloid beta plaques and tau neurofibrillary tangles. We know that amyloid beta plaques form in the brain early, prior to accumulation of tau and prior to changes in memory and thinking. In fact, the levels and locations of tau accumulation correlate much better with symptoms than the levels and locations of amyloid. But amyloid might directly “fuel the fire” to accelerated changes in tau and other downstream mechanisms, a hypothesis supported by basic science research and the findings in Clarity AD that treatment with lecanemab lowered levels of not just amyloid beta but also levels of tau and neurodegeneration in the blood and cerebrospinal fluid.
GAZETTE: In the Clarity AD trial, what’s the magnitude of the effect they saw?
McGINNIS: The relevant standards in the trial — set by the FDA and others — were to see two clinical benefits for the drug to be considered effective. One was a benefit on tests of memory and thinking, a cognitive benefit. The other was a benefit in terms of the performance in usual daily activities, a functional benefit. Lecanemab met both of these standards by slowing the rate of decline by approximately 25 to 35 percent compared to placebo on measures of cognitive and functional decline over the 18-month studies.
“In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal.”
Steven M. Smith
GAZETTE: What are the key questions that remain?
McGINNIS: An important question relates to the stages at which the interventions were done. The study was done in subjects with mild cognitive impairment and mild Alzheimer dementia. People who have mild cognitive impairment have retained their independence in instrumental activities of daily living — for example, driving, taking medications, managing finances, errands, chores — but have cognitive and memory changes beyond what we would attribute to normal aging. When people transition to mild dementia, they’re a bit further along. The study was for people within that spectrum but there’s some reason to believe that intervening even earlier might be more effective, as is the case with many other medical conditions.
We’re doing a study here called the AHEAD study that is investigating the effects of lecanemab when administered earlier, in cognitively normal individuals who have elevated brain amyloid, to see whether we see a preventative benefit. The hope is that we would at least see a delay to onset of cognitive impairment and a favorable effect not only on amyloid biomarkers, but other biomarkers that might capture progression of the disease.
GAZETTE: Is anybody in that study treatment yet or are you still enrolling?
McGINNIS: There’s a rolling enrollment, so there are people who are in the double-blind phase of treatment, receiving either the drug or the placebo. But the enrollment target hasn’t been reached yet so we’re still accepting new participants.
GAZETTE: Is it likely that we may see drug cocktails that go after tau and amyloid? Is that a future approach?
More like this
Newly identified genetic variant protects against Alzheimer’s
Using AI to target Alzheimer’s

Excessive napping and Alzheimer’s linked in study
McGINNIS: It has not yet been tried, but those of us in the field are very excited at the prospect of these studies. There’s been a lot of work in recent years on developing therapeutics that target tau, and I think we’re on the cusp of some important breakthroughs. This is key, considering evidence that spreading of tau from cell to cell might contribute to progression of the disease. Additionally, for some time, we’ve had a suspicion that we will likely have to target multiple different aspects of the disease process, as is the case with most types of cancer treatment. Many in our field believe that we will obtain the most success when we identify the most pertinent mechanisms for subgroups of people with Alzheimer’s disease and then specifically target those mechanisms. Examples might include metabolic dysfunction, inflammation, and problems with elements of cellular processing, including mitochondrial functioning and processing old or damaged proteins. Multi-drug trials represent a natural next step.
GAZETTE: What about side effects from this drug?
McGINNIS: We’ve known for a long time that drugs in this class, antibodies that harness the power of the immune system to remove amyloid, carry a risk of causing swelling in the brain. In most cases, it’s asymptomatic and just detected by MRI scan. In Clarity AD, while 12 to 13 percent of participants receiving lecanemab had some level of swelling detected by MRI, it was symptomatic in only about 3 percent of participants and mild in most of those cases.
Another potential side effect is bleeding in the brain or on the surface of the brain. When we see bleeding, it’s usually very small, pinpoint areas of bleeding in the brain that are also asymptomatic. A subset of people with Alzheimer’s disease who don’t receive any treatment are going to have these because they have amyloid in their blood vessels, and it’s important that we screen for this with an MRI scan before a person receives treatment. In Clarity AD, we saw a rate of 9 percent in the placebo group and about 17 percent in the treatment group, many of those cases in conjunction with swelling and mostly asymptomatic.
The scenario that everybody worries about is a hemorrhagic stroke, a larger area of bleeding. That was much less common in this study, less than 1 percent of people. Unlike similar studies, this study allowed subjects to be on anticoagulation medications, which thin the blood to prevent or treat clots. The rate of macro hemorrhage — larger bleeds — was between 2 and 3 percent in the anticoagulated participants. There were some highly publicized cases including a patient who had a stroke, presented for treatment, received a medication to dissolve clots, then had a pretty bad hemorrhage. If the drug gets full FDA approval, is covered by insurance, and becomes clinically available, most physicians are probably not going to give it to people who are on anticoagulation. These are questions that we’ll have to work out as we learn more about the drug from ongoing research.
GAZETTE: Has this study, and these recent developments in the field, had an effect on patients?
McGINNIS: It has had a considerable impact. There’s a lot of interest in the possibility of receiving this drug or a similar drug, but our patients and their family members understand that this is not a cure. They understand that we’re talking about slowing down a rate of decline. In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal. So there’s hope. There’s optimism. Our patients, particularly patients who are at earlier stages of the disease, have their lives to live and are really interested in living life fully. Anything that can help them do that for a longer period of time is welcome.
Share this article
You might like.
Approach attacks errant proteins at their roots

Only a fraction of it will come from an expert, researchers say

In podcast episode, a chaplain, a bioethicist, and a doctor talk about end-of-life care
Forget ‘doomers.’ Warming can be stopped, top climate scientist says
Michael Mann points to prehistoric catastrophes, modern environmental victories
Yes, it’s exciting. Just don’t look at the sun.
Lab, telescope specialist details Harvard eclipse-viewing party, offers safety tips
Navigating Harvard with a non-apparent disability
4 students with conditions ranging from diabetes to narcolepsy describe daily challenges that may not be obvious to their classmates and professors
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Cent Nerv Syst Dis
Current and Future Treatments in Alzheimer Disease: An Update
Konstantina g yiannopoulou.
1 Memory Center, Neurological Department, Henry Dunant Hospital Center, Athens, Greece
Sokratis G Papageorgiou
2 Cognitive Disorders/Dementia Unit, 2nd Neurological Department, National and Kapodistrian University of Athens, Attikon General University Hospital, Athens, Greece
Disease-modifying treatment strategies for Alzheimer disease (AD) are still under extensive research. Nowadays, only symptomatic treatments exist for this disease, all trying to counterbalance the neurotransmitter disturbance: 3 cholinesterase inhibitors and memantine. To block the progression of the disease, therapeutic agents are supposed to interfere with the pathogenic steps responsible for the clinical symptoms, classically including the deposition of extracellular amyloid β plaques and intracellular neurofibrillary tangle formation. Other underlying mechanisms are targeted by neuroprotective, anti-inflammatory, growth factor promotive, metabolic efficacious agents and stem cell therapies. Recent therapies have integrated multiple new features such as novel biomarkers, new neuropsychological outcomes, enrollment of earlier populations in the course of the disease, and innovative trial designs. In the near future different specific agents for every patient might be used in a “precision medicine” context, where aberrant biomarkers accompanied with a particular pattern of neuropsychological and neuroimaging findings could determine a specific treatment regimen within a customized therapeutic framework. In this review, we discuss potential disease-modifying therapies that are currently being studied and potential individualized therapeutic frameworks that can be proved beneficial for patients with AD.
Introduction
Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1
Because dementia occurs mostly in people older than 60 years, the growing expansion of lifespan, leading to a rapidly increasing number of patients with dementia, 2 mainly AD, has led to an intensive growth in research focused on the treatment of the disease. However, despite all arduous research efforts, at the moment, there are no effective treatment options for the disease. 3 , 4
The basic pathophysiology and neuropathology of AD that drives the current research suggests that the primary histopathologic lesions of AD are the extracellular amyloid plaques and the intracellular Tau neurofibrillary tangles (NFTs). 5 The amyloid or senile plaques (SPs) are constituted chiefly of highly insoluble and proteolysis-resistant peptide fibrils produced by β-amyloid (Aβ) cleavage. Aβ peptides with Aβ38, Aβ40, and Aβ42 as the most common variants are produced after the sequential cleavage of the large precursor protein amyloid precursor protein (APP) by the 2 enzymes, β-secretase (BACE1) and γ-secretase. Nevertheless, Aβ is not formed if APP is first acted on and cleaved by the enzyme α-secretase instead of β-secretase. 6 According to the “amyloid hypothesis” Aβ production in the brain initiates a cascade of events leading to the clinical syndrome of AD. It is the forming of amyloid oligomers to which neurotoxicity is mainly attributed and initiates the amyloid cascade. The elements of the cascade include local inflammation, oxidation, excitoxicity (excessive glutamate), and tau hyperphosphorylation. 5 Tau protein is a microtubule-associated protein which binds microtubules in cells to facilitate the neuronal transport system. Microtubules also stabilize growing axons necessary for neuronal development and function. Abnormally hyperphosphorylated tau forms insoluble fibrils and folds into intraneuronic tangles. Consequently, it uncouples from microtubules, inhibits transport, and results in microtubule disassembly. 6 Although in the amyloid hypothesis, tau hyperphosphorylation was thought to be a downstream event of Aβ deposition, it is equally probable that tau and Aβ act in parallel pathways causing AD and enhancing each other’s toxic effects. 3 Progressive neuronal destruction leads to shortage and imbalance between various neurotransmitters (eg, acetylcholine, dopamine, serotonin) and to the cognitive deficiencies seen in AD. 5
All of the already established treatments that are used today try to counterbalance the neurotransmitter imbalance of the disease. The acetylocholinesterase inhibitors (AChEIs) which are approved for the treatment of AD are donepezil, galantamine, and rivastigmine. 4 , 5 Their development was based in the cholinergic hypothesis which suggests that the progressive loss of limbic and neocortical cholinergic innervation in AD is critically important for memory, learning, attention, and other higher brain functions decline. Furthermore, neurofibrillary degeneration in the basal forebrain is probably the primary cause for the dysfunction and death of cholinergic neurons in this region, giving rise to a widespread presynaptic cholinergic denervation. The AChEIs increase the availability of acetylcholine at synapses and have been proven clinically useful in delaying the cognitive decline in AD. 7
A further therapeutic agent approved for moderate to severe AD is the low-to-moderate affinity, noncompetitive N -methyl- d -aspartate (NMDA) receptor antagonist memantine. 4 , 5 Memantine binds preferentially to open NMDA receptor–operated calcium channels blocking NMDA-mediated ion flux and ameliorating the dangerous effects of pathologically elevated glutamate levels that lead to neuronal dysfunction. 8
In clinical trials, both Aβ and tau are prime targets for disease-modifying treatments (DMTs) in AD. From this point of view, AD could be prevented or effectively treated by decreasing the production of Aβ and tau; preventing aggregation or misfolding of these proteins; neutralizing or removing the toxic aggregate or misfolded forms of these proteins; or a combination of these modalities. 7
A number of additional pathogenic mechanisms have been described, possibly overlapping with Aβ plaques and NFT formation or induced by them, including inflammation, oxidative damage, iron deregulation, and cholesterol metabolism blood-brain barrier (BBB) dysfunction or α-synuclein toxicity. 9 - 13
This article will review current nonpharmacological and pharmacological management of the cognitive and behavioral symptoms of AD, with a focus on the medications that are currently FDA (Food and Drug Administration)–approved for the treatment of the cognitive and functional deficits of AD. 10 Pharmacological agents under research in phase 1, 2, and 3 clinical trials in AD will be summarized. 11 - 13
Current management of AD
A multifactorial tailored management of AD is attempted nowadays based in the following components:
- Open physician, caregiver, and patient communication: a sincere and successful conveying of information and feelings between them will offer opportune identifying of symptoms, exact evaluation and diagnosis, and suitable guidance.
- - Consistency and simplification of environment 10 ;
- - Established routines 10 ;
- - Communicative strategies such as calm interactions, providing pleasurable activities, using simple language and “saying no” only when safety is concerned 10 ;
- - Timely planning for legal and medical decisions and needs 10 ;
- - Cognitive behavioral therapy 14 , 15 ;
- - Exercise therapy, light therapy, music therapy. 14 , 15
- - Planned short rest periods for the caregiver;
- - Psychoeducation including preparing for effects of dementia on cognition, function and behaviors, expectations, avoiding situations that can worsen the symptoms or increasing the dangers for safety and well-being
- - Encouraging the development of support networks for the caregivers. 10
- Pharmacological interventions.
FDA-approved AD medications
The AChEIs donepezil, galantamine, rivastigmine, and the NMDA antagonist memantine are the only FDA-approved AD medications. 10
AChEIs attempt at reducing the breakdown of acetylcholine levels in the brain of the patients with AD by inhibiting the responsible enzyme acetylcholinesterase in the synaptic cleft. 5 Thus, AChEIs enhance central cholinergic neurotransmission and finally tend to mitigate decline in cognition at least during the first year of treatment. Further decline occurs, but even temporary discontinuation of these drugs results in rapid decline and is associated with greater risk of nursing home placement. 16
Initiation of AChEI treatment as soon as possible after the diagnosis is preferred as patients who started the AChEI 6 months later showed more rapid cognitive decline than those who started the drug immediately. 17 All 3 AChEIs have proved their treatment benefits in delaying decline, stabilizing, or even improving cognition and activities of daily living in randomized placebo-controlled trials up to 52 weeks duration. 10 , 18 Longer term open-label extension studies support also longer term treatment benefits. 10
Significant efficacy differences among the AChEIs have not been reported. Donepezil and rivastigmine have been approved by FDA for mild, moderate, and severe AD, whereas galantamine for mild and moderate AD. 18
The most common adverse effects are triggered by the cholinomimetic action of the AChEIs on the gastrointestinal tract and often include diarrhea, nausea, and vomiting. Rapid eye movement sleep behavior disorder has been also remarked in some individuals. Administration of the drug after a meal in the morning can minimize all of these adverse effects. The transdermal patch of rivastigmine can induce rash at the site of application. Adverse effects affect usually a 5% to 20% of patients but are mostly transient and mild. The AChEIs may also trigger bradycardia and increase the risk of syncope. Thus, AChEIs are contraindicated in conditions including severe cardiac arrhythmias, especially bradycardia or syncope. They are also contraindicated in active peptic ulcer or gastrointestinal bleeding history and uncontrolled seizures. Slow titration over months to years to a maximal tolerated of the indicated dose is important for the safety of the patients. 17 , 18
Pharmacokinetic characteristics differ among AChEIs: the primary route of elimination for donepezil and galantamine is hepatic metabolism, whereas for rivastigmine is liver and intestine metabolism. Donepezil and galantamine inhibit selectively and reversibly the acetylcholinesterase, whereas rivastigmine is a “pseudo-irreversible” inhibitor of acetylcholinesterase and butyrylcholinesterase. Donepezil has a long elimination half-life of 70 hours and galantamine of 6 to 8 hours. The elimination half-life of rivastigmine is very short (1-2 hours for oral and 3-4 hours for transdermal administration), but the duration of action is longer as acetylcholinesterase and butyrylcholinesterase are blocked for around 8.5 and 3.5 hours, respectively. 10 , 17 , 18
Memantine is a noncompetitive low-affinity NMDA-receptor open-channel blocker and affects glutamatergic transmission. 5 Its main elimination route is unchanged via the kidneys with a half-life of 70 hours. It has been approved by FDA for moderate and severe AD either as monotherapy or in combination with an AChEI. 3 Memantine monotherapy has demonstrated short- and long-term benefits for patients with moderate to severe AD as assessed by different scales evaluating activities of daily living, cognition, and behavioral and psychological symptoms of dementia (BPSD). 19
Memantine can be administered in combination with an AChEI, as they have complementary mechanisms of action. Their combination benefits patients with usually additive effects, without any increase in adverse effects. 14 , 15
Duration and persistence of monotherapy or combination treatment with higher doses in moderate or even in advanced dementia are associated with better global function and outcomes. 20
Medications for BPSD
Antipsychotics and antidepressants remain the main medications for BPSD. Selective serotonin reuptake inhibitors are preferred for treating depression and anxiety. Drugs with low anticholinergic effects and an acceptable tolerability, such as sertraline, citalopram, and escitalopram, are more appropriate. Antipsychotics should be administered only when a significant safety risk for the patient or for the caregivers by aggressive behaviors makes them necessary. Controversial and limited evidence cannot adequately support the use of benzodiazepines, anticonvulsants stimulants, or dextromethorphan/quinidine. Pharmacological approaches to managing BPSD are highly individualized and changeable, depending on patient’s comorbidities, stage of the disease, and symptoms’ severity. 21
Removal of superfluous and deleterious medications
Polypharmacy in older patients with dementia is usual (with a prevalence of 25%-98%). 22 Anticholinergics and sedatives are commonly used inappropriate medications. These drugs are prescribed despite strong evidence (Beers Criteria) that they should be avoided in cognitively vulnerable older persons because of their potential adverse cognitive effects. 23 Estrogen is another commonly prescribed potentially inappropriate medication despite evidence that its use is associated with increased cognitive decline in postmenopausal women. 24
Specific examples of usually prescribed potentially harmful medications in the elderly are diphenhydramine, often taken with acetaminophen for insomnia and pain, benzodiazepines for anxiety, anticholinergics (tolderodine, oxybutynin, tamsulosin) for urinary incontinence, biperiden, and pramipexole for extrapyramidal tremor 25 and sedative/hypnotics for sleep disorders. 26
Treating underlying medical conditions
Careful management of vascular risk factors (hyperlipidemia, diabetes, hypertension) is of paramount importance for patients with AD. Hydration, sleep, and nutrition status should also be closely monitored. Disorders in thyroid function or electrolytes, deficiencies in vitamin B 12 , folate, vitamin D, or systemic conditions and diseases that can affect cognition (infections, eg, urinary tract infection, pain, constipation) should be treated. 27
Current Landscape in Treatment Research for AD
No new drug has been approved by FDA for AD since 2003 and there are no approved DMTs for AD, despite many long and expensive trials. 22 , 28 As a matter of fact, more than 200 research projects in the last decade have failed or have been abandoned. 10 Nevertheless, drug pipeline for AD is still full of agents with mechanisms of action (MOA) that target either disease modification or symptoms. 4 , 10 Some of the recent failures of anti-amyloid agents in phase 3 clinical trials in patients with early-stage, mild, or mild-to-moderate stage AD were semagacestat, 29 bapineuzumab, 30 solanezumab 31 and in similar trials of β-secretase inhibitors (BACE) lanabecestat, 32 verubecestat, 33 and atabecestat. 34
The most popular and broadly accepted explanations for the multiple failures of clinical trials of DMT agents for AD include the too late starting of therapies in disease development, the inappropriate drug doses, the wrong main target of the treatment, and mainly an inadequate understanding of the pathophysiology of AD. 35 A novel approach to the problem seems more technical and mathematical than biological and suggests that the selected trials’ clinical endpoint may be extremely premature, and additionally, the variability in diagnostic markers and end points may result in inaccurate diagnosis of patients’ disease state and is finally a definite source of errors. 28 Given the fact that longer trial durations increase the probability of detecting a significant effect but at the same time increase tremendously the costs, the proposed solution seems to be the use of clinical trial simulators. 28 These simulators are constructed with mathematical, computational, and statistical tools and can predict the likelihood that a strategy and clinical end point selection of a given trial are proper or not, before the initiation of the trial. 36 They can also help in the perfecting of the design of the study; hence, they may augment the probability of success of estimated new drugs or save invaluable time and resources, by indicating earlier the forthcoming failure of any inappropriate therapy. 37 Although the use of clinical trial simulators is not frequent in recent research, 38 should this practice be abandoned, especially when potential treatments for diseases with slow progression and long duration, such as AD, are evaluated. 37
At the same time, current research remains focused on the development of therapeutic approaches to slow or stop the disease progression, taking into consideration every new aspect in the biology of the disease, the diagnostic markers, and the precise diagnosis of disease state of every individual and the design of clinical trials. Furthermore, drug development research for AD has become more complicated as preclinical and prodromal AD populations are potentially included in current trials, as well as traditionally included populations of all the clinical stages of AD dementia. 38 Consequently, current guidance provided by the FDA for AD clinical trials further includes use of fluid or neuroradiological biomarkers in disease staging for preclinical and prodromal AD trials and of a single primary outcome in prodromal AD trials. In addition, the use of clinical trial simulators, Bayesian statistics, and modifiable trial designs is strongly suggested. 4
The National Institute on Aging and the Alzheimer’s Association (NIA-AA) proposed a new framework for research, 39 which requires the application of amyloid, tau, and neurodegeneration biomarkers to clinical trials, succeeds in precise classification of patients in AD stages, and can be used to assist clinical trials design.
Tau positron emission tomography (tau PET), neurofilament light, and neurogranin are the new biomarkers that are increasingly used by clinical trials. 40
The above-mentioned biological and statistical advances that are recently integrated in clinical trials may comprise the final assets for succeeding in drug development. The current clinical trials in AD in phases 1, 2, and 3 4 , 11 - 13 are briefly discussed. The tested agents in these trials are classified either as potentially modifying the disease or as symptomatic for the cognitive enhancement, and for the relief of neuropsychiatric symptoms. The new directions in AD clinical trials, such as agents with novel MOA, advanced immunotherapies, the involvement of biomarkers in drug development, and repurposed agents, are highlighted.
A search for phases 1, 2, and 3 “recruiting” or “active but not recruiting” clinical trials for AD in clinicaltrials.gov (accessed August 19, 2019) showed 165 outcomes. The last annual review of the drug development pipeline for AD examined clinicaltrials.gov in February, 2019 (132 agents in 156 trials) and provides information and conclusions available at that time: 28 drugs in 42 clinical trials in phase 3 trials, 74 drugs in 83 phase 2 trials, and 30 drugs in 31 phase 1 trials. 4 The tested agents are classified as DMTs (73%), symptomatic cognitive enhancers (13%), and symptomatic for the treatment of BPSDs (11%). 4 The DMT agents were further separated into small molecules or biologics (monoclonal antibodies [mAbs] and other immunotherapies). The DMT agents were also classified according to their potential MOA as amyloid targeting, as tau-related targeting, and as having other MOA such as anti-inflammatory or metabolic protection, neuroprotection, and growth factor support. 4 The DMTs are suggested to be effective to delay or halt disease progression that would be expressed clinically with long-lasting benefits in cognition over many months to years. Symptomatic agents are supposed to show symptomatic benefits over weeks to many months in cognition improvement or BPSD elimination. 10
In this review, agents currently studied as potential DMTs will be discussed. Furthermore, an approach to a future “precision medicine” multifactorial therapeutic model based on biomarkers profile, genetic analysis, neuropsychologic evaluation, and neuroimaging accomplished with risk factors restriction will be attempted. 2 , 3
Currently studied DMTs for AD
Amyloid-related mechanisms—dmts.
The crucial step in AD pathogenesis is the production of amyloid (Aβ), which forms SPs (insoluble and proteolysis-resistant fibrils). The Aβ derives from a protein overexpressed in AD, APP through sequential proteolysis by β-secretase (BACE1) in the extracellular domain and γ-secretase in the transmembrane region. Full-length APP is first cleaved by α-secretase or β-secretase. The APP cleavage by α-secretase leads to nonamyloidogenic pathway, whereas APP cleavage by β-secretase (BACE1) leads to amyloidogenic pathway. Sequential cleavage of APP by BACE1 in the extracellular and γ-secretase in the transmembrane area results in the Aβ production. Major sites of γ-secretase cleavage usually occur in positions 40 and 42 of Aβ, thus Aβ40 and Aβ42 oligomers are the main products of the sequential APP cleavage, as the amyloidogenic pathway is favored in neurons because of the greater plentifulness of BACE1. On the contrary, the nonamyloidogenic processing is more favored in other cells without BACE1 predominance. 5
“Amyloid hypothesis” suggests that Aβ production in the brain triggers a cascade of pathophysiologic events leading to the clinical expression of AD. Aβ is a protein consisting of 3 main isoforms: Aβ38, Aβ40, and Aβ42. Aβ42 is the most aggregation-prone form and has the tendency to cluster into oligomers. Oligomers can form Aβ-fibrils that will eventually form amyloid plaques. Aβ40 is somewhat aggregation-prone and it is mostly found in the cerebral vasculature as a main component of “cerebral amyloid angiopathy.” Aβ40 usually constitutes more than 50% of total detected Aβ. Aβ38 is soluble, present in the vasculature of patients with sporadic and familial AD. Neurotoxicity is mainly attributed to the forming of amyloid oligomers, which finally initiates the amyloid cascade. 5
Oxidation, inflammation, excessive glutamate, and tau hyperphosphorylation are supposed to be the main pathophysiologic pillars of the cascade. Tau protein binds microtubules in cells to facilitate the neuronal transport system. Microtubules also stabilize growing axons. Hyperphosphorylated tau forms insoluble fibrils and folds into intraneuronic NFTs. Consequently, it inhibits neuronal transport and microtubule function. 2 Although in the initial amyloid hypothesis, tau hyperphosphorylation was thought to be a downstream event of Aβ deposition, it is now equally probable that tau and Aβ act in parallel pathways causing AD and enhancing each other’s toxic effects. 2 The result of massive neuronal destruction is the shortage and imbalance between neurotransmitters, such as acetylcholine, dopamine, serotonin, and to the cognitive and behavioral symptoms of AD. 5
Consequently, anti-amyloid DMTs have focused on 3 major MOAs: (1) reduction of Aβ42 production (γ-secretase inhibitors, β-secretase inhibitors, α-secretase potentiation), (2) reduction of Aβ-plaque burden (aggregation inhibitors, drugs interfering with metals), and (3) promotion of Aβ clearance (active or passive immunotherapy). 10
Reduction of Aβ42 production
γ-secretase inhibitors.
According to the amyloid hypothesis, amyloidogenic pathway is promoted after the sequential cleavage of APP by BACE1 and γ-secretase. Consequently, the inhibition of these enzymes has been considered as a major therapeutic target. Unluckily, concerning γ-secretase, in addition to APP, this particular enzyme acts on many other substances and cleaves different transmembrane proteins. Notch receptor 1, which is essential for control of normal cell differentiation and communication, is among them. 5 This fact is probably responsible for recent failures in clinical trials with γ-secretase inhibitors: semagacestat 29 was associated with worsening of activities in daily leaving and increased rates of infections and skin cancer, avagacestat 41 was associated with higher rate of cognitive decline and adverse dose-limiting effects (skin cancer) and tarenflurbil which showed low brain penetration. 42 Serious safety concerns for γ-secretase inhibitors remove γ-secretase from the role of appropriate target for the treatment of AD 43 until in depth studies on this key enzyme could help to therapeutically target γ-secretase in a safe way. 44 No γ-secretase modulators are currently studied in phase 1-3 clinical trials. 4
BACE inhibitors
Two BACE inhibitors are still elaborated: elenbecestat (E2609) in phase 2 and umibecestat (CNP520) in phase 3. 4 The later agent is studied in asymptomatic individuals at risk of developing AD (APOE4 homozygotes or APOE4 heterozygotes with elevated amyloid, detected by cerebrospinal fluid [CSF] biomarkers or amyloid PET). 45
Fluid and neuroimaging biomarkers indicative of AD pathology or neurodegeneration are integrated in this study.
However, the clinical trials with the BACE inhibitors lanabecestat, 32 verubecestat, 33 and atabecestat 34 have been recently discontinued due to unexpected difficulties. The phase 3 lanabecestat trial was discontinued due to lack of efficacy, whereas verubecestat and atabecestat trials were ceased due to ineffectiveness, as well as safety reasons (rash, falls, liver toxicity, and neuropsychiatric symptoms). 10 , 32 - 34 All agents showed significant and dose-dependent result of reducing CSF Aβ42, but without cognitive or functional benefit while many of them were poorly tolerated and some of them failed in subjects with prodromal AD. These results might support the suggestion that blocking the process of forming of Aβ may be not capable of halting the disease progression. 46
α-secretase modulators
According to the amyloid hypothesis, nonamyloidogenic pathway is promoted after the cleavage of APP by α-secretase. Consequently, the modulation of the enzyme has been considered as a major therapeutic target. However, little is known of the main signaling pathways that could stimulate cleavage of APP by α-secretase. Restricted, nowadays, knowledge assumes that α-secretase activation is promoted through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and may be through γ-aminobutyric acid (GABA) receptor signaling; thus, agents that activate the PI3K/Akt pathway or act as selective GABA receptor modulators are suggested as potential therapeutic drugs for AD. 47 , 48
Etazolate (EHT0202) stimulates the nonamyloidogenic α-secretase pathway acting as a selective modulator of GABA receptors. A previous, phase 2 trial has showed that the agent was safe and well tolerated in patients with mild to moderate AD. However, further evaluation of etazolate in phase 3 trials has not progressed. 48 Etazolate is currently evaluated in animal studies for its preventive effect in post-traumatic stress disorder. 49
Two α-secretase modulators that activate the PI3K/Akt pathway are studied in phase 2 clinical studies: APH-1105 and ID1201. APH-1105 is delivered intranasally and is assessed in mild to moderate AD. 4 ID1201 is a fruit extract of melia toosendan and also induces α-secretase activation. It is evaluated in mild AD. 47
Reduction of Aβ-plaque burden
Aggregation inhibitors (anti-amyloid aggregation agents).
Aggregation inhibitors interact directly with the Aβ peptide to inhibit Aβ42 fiber formation, thus they are considered potential therapeutic for AD.
The last Aβ42 aggregation inhibitor which was tested in humans was the oral agent scyllo-inositol (ELND005). A phase 2 clinical trial in patients with AD did not provide evidence to support a clinical benefit of ELND005 while severe toxicity issues (infections) forced the cessation of the study. Further development of the agent at a lower dose has not progressed in the last 8 years. 50
Nowadays, specific agents in the form of peptidomimetics that inhibit and partially reverse the aggregation of Aβ 42 are tested in transmission electron microscopic studies. KLVFF is a peptide sequence that resembles the hydrophobic central part of the Aβ and gradually replaces natural polypeptides. The KLVFF compound that mainly prevents the aggregation of Aβ 42 and can also dissolve the oligomerics to a limited extend is the final compound 18, which is resilient in proteolytic decomposition. 51
Another newly developed class of peptidomimetics are the “γ-AApeptides.” 52 One of them, compound γ-AA26, seems almost 100-fold as efficient as the compound 18 of the KLVFF in the inhibition of the aggregation of Aβ 42 . 52
In vivo animal studies will be developed to manifest the biological potential of peptidomimetics.
Reduction of Aβ-plaque burden via drugs interfering with metals
Abnormal accumulation or dyshomeostasis of metal ions such as iron, copper, and zinc has been associated with the pathophysiology of AD. 5
Deferiprone is an iron chelating agent which is studied in phase 2 trials in participants with mild and prodromal AD. 4 , 53
A metal protein–attenuating compound, PBT2, has recently progressed in phase 2 AD treatment trials, as it demonstrated promising efficacy data in preclinical studies. 54 In a 3-month phase 2 study, PBT2 succeeded in a 13% reduction of CSF Aβ and an executive function improvement in a dose-related pattern in patients with early AD. 55
Promotion of Aβ clearance (active or passive immunotherapy)
The 2 main immunotherapeutic approaches that intend to promote Aβ clearance and are currently tested in clinical and preclinical studies are active and passive immunization: 56
- Active immunization.
Aβ, phosphorylated tau (ptau) peptides, or specific artificial peptides such as polymerized British amyloidosis (ABri)-related peptide (pBri) 57 are used as immunogens. ABri is a rare hereditary amyloidosis associated with a mutation that results in the production of a highly amyloidogenic protein with a unique carboxyl terminus that has no homology to any other human protein. The pBri peptide corresponds to this terminus and induces an immune response that recognizes Aβ and ptau.
Antigen-presenting cells present the immunogens to B cells. Use of Ab or ptau peptides will produce antibodies to Ab or ptau epitopes, respectively. Use of pBri will produce antibodies to both Aβ and ptau epitopes. 56
- Passive immunization.
Monoclonal Abs to Ab, ptau, or b sheet epitopes are systemically and adequately for BBB penetration infused. As antibodies cross the BBB, they act to clear, degrade, or alternatively disaggregate or neutralize their targets. 56
- Stimulation of innate immunity either by active or passive immunization also ameliorates the pathology of the disease by promoting microglia and macrophage function. 56
Overall, Aβ-targeted strategies seem promising if used very early in the progression of the disease, before the presence of any symptoms; thus, they are developed in current trials in preclinical AD. Strategies that target tau pathology, although promising, bear the risk of toxicity at the moment. Nevertheless, it is hypothesized that, in sporadic late onset AD, ptau and Aβ pathologies could be evolved by separate pathways that can affect each other synergistically. 58 Consequently, it is possible that effective AD immunotherapies must be able to simultaneously target both ptau and Aβ pathologies. 56
Immunotherapeutic approaches have dominated in the past 15 years with negative results until now. However, lessons from these fails have altered the current immunotherapy development research for AD. 56
Active Aβ immunotherapy
Six active immunotherapy agents are currently studied in phase 1, 2, and 3 clinical trials:
CAD106 is an active Aβ immunotherapeutic agent, is studied in preclinical AD under the umbrella of the Alzheimer prevention initiative generation program, which comprises 2 phase 3 studies that evaluate simultaneously the safety and efficacy of CAD106 and umibecestat in asymptomatic individuals at risk of developing AD (60-75 years of age, APOE4 homozygotes, or APOE4 heterozygotes with elevated amyloid in CSF or in amyloid PET). 45
Subjects will be registered in generation study 1 (cohort 1: CAD106 or placebo, cohort 2: umibecestat or placebo) or generation study 2 (umibecestat 50 and 15 mg, or placebo). 45
ABvac40 is evaluated in a phase 2 study, as the first active vaccine against the C-terminal end of Aβ 40 . A phase 1 study was conducted with patients with mild to moderate AD aged 50 to 85 years. Neither incident vasogenic edema nor microhemorrhages were identified. Specific anti-Aβ 40 antibodies were developed in the 92% of the individuals receiving injections of ABvac40. 59
GV1001 peptide (tertomotide) was previously studied as a vaccine against various cancers, whereas now it is evaluated in a phase 2 study for AD. 60
ACC-001 (vanutide cridificar), an Aβ vaccine, was studied in phase 2a extension studies in subjects with mild to moderate AD. It was administered with QS-21 adjuvant. Long-term therapy with this combination was very well tolerated and produced the highest anti-Aβ IgG titers compared with other regimens. 61
UB-311, a synthetic peptide used as Aβ vaccine, has been advanced into an ongoing phase 2 study in patients with mild and moderate AD. In phase 1, it induced a 100% responder rate in patients with AD. The usual adverse effects were swelling in the injection site and agitation. A slower cognitive decline rate was observed in patients with mild AD. 62
Lu AF20513 epitope vaccine is estimated in a phase 1 study in mild AD. 63
The occurrence of encephalitis in previous studies (AN-1792) 64 led to the development of improved anti-Aβ active immunotherapy agents, more specific to Aβ sites less probable to activate T cells, currently studied in clinical trials. 5 , 6
Passive Aβ immunotherapy—via mAbs
Passive Ab immunotherapy via mAbs is the most active and promising class. Cerebral microhemorrhages and vasogenic edema are the main drawbacks in this group of agents. 5 Valuable learning gained from previous failed phase 3 trials of the first agents of this class, bapineuzumab 65 and solanezumab, 66 enlightened the mAbs’ research. Strict inclusion criteria were applied, such as biomarker proof of “amyloid positivity” and enrollment of individuals with preclinical stages of the disease. Furthermore, the design of the studies became more specific and targeted: the characteristics of amyloid-related imaging abnormalities were associated with the dose of antibodies and APOε4 genotyping, higher dosing necessity was recognized, and accurate measures for specific targets, such as reduction of Aβ plaque burden on amyloid PET, were required. 10
Many ongoing mAbs trials are in phase 3, including aducanumab, 67 gantenerumab, 68 and BAN2401 69 in prodromal and very mild AD, and crenezumab, 70 gantenerumab, and solanezumab 71 in studies for preclinical or at-risk populations. First results from aducanumab and BAN2401 trials suggested, at first, a treatment-related result of reducing in cerebral amyloid burden in agreement to deceleration of cognitive decline in patients with prodromal and very mild AD. 71 , 72 On the contrary, the initial trial of gantenerumab in prodromal AD was prematurely stopped for lack of efficacy, but exploratory analyses suggest that higher dosing of gantenerumab may be needed for clinical efficacy and an open-label extension for participating patients with mild AD is continued, simultaneously with a double-blind, placebo-controlled study in patients with mild AD. 4 , 68 Similarly, until now, solanezumab did not delay rates of brain atrophy. 73
Intravenous doses of LY3002813 (donanemab) and LY3372993 are studied in participants with mild cognitive impairment (MCI) and mild to moderate AD in separate phase 1 clinical studies. 4
Passive Aβ immunotherapy—via immunoglobulins
Anti-Aβ antibodies are included in naturally occurring autoantibodies. In contrast to mAbs, blood-derived human anti-Aβ immunoglobulin G (IgG) Abs are polyclonal, with lower avidity for single Aβ molecules, and higher for a broader range of epitopes, especially in Aβ oligomers and fibrils. The natural presence of antibodies against Aβ has been reported in intravenous immunoglobulin (IVIg); thus, IVIg has been considered as a possible AD treatment. Intravenous immunoglobulin is obtained from plasma of healthy donors and is made up of human Abs mainly of the IgG-type. 5 , 74
Nevertheless, the first completed phase 3 trial of IVIg as a treatment for AD demonstrated good tolerability but lack of efficacy of the agent on clinical stability or delay of cognitive or functional decline of participants with mild and moderate AD. 74
Another strategy directed at diminishing the accumulation of Aβ in the brain is based in altering the transportation of Aβ through the BBB. A recent therapeutic method performs plasma exchange (PE) with albumin replacement, inducing the shifting of the existing dynamic equilibrium between plasma and brain Aβ. This therapeutic method is based in the following considerations: (1) high levels of Aβ aggregation in the brain are accompanied by low levels of Aβ in CSF in AD, (2) albumin is the main protein transporter in humans, (3) albumin binds around the 90% of the circulating Aβ, and (4) albumin has proved Aβ-binding ability. Consequently, it is suggested that PE-mediated possession of albumin-bound Aβ would increase the shift of free Aβ from CSF to plasma to correct the imbalance between brain and blood Aβ levels. 75
A phase 3 trial called Alzheimer’s Management by Albumin Replacement (AMBAR) in mild and moderate AD assesses PE with several replacement volumes of albumin, with or without intravenous immunoglobulin. 76
Furthermore, an ongoing phase 2 study evaluates IVIg Octagram 10% in mild and moderate AD. 4
A novel immunotherapeutic strategy that targets simultaneously Aβ and tau is represented by the NPT088 agent. NPT088 is a mixture of the capsid protein of bacteriophage M13 (g3p) and human-IgG 1 -Fc. NPT088 reduced Aβ and ptau aggregates and improved cognition in aged Tg2576 mice. The agent is currently assessed in a phase 1 clinical study. 77
Tau-related mechanisms—DMTs
Anti-phospho-tau approaches consist a major potential treatment strategy, even if there are yet no agents with this specific MOA advanced in phase 3 studies.
Only 1 agent with tau-related mechanism is evaluated in phase 2/3, whereas 10 agents that target tau as one of their mechanisms are evaluated in phase 2, and 5 more agents with tau-related mechanism are assessed in phase 1 studies. 4
Prevention of ptau formation
The hyperphosphorylation of tau is induced by kinases. 78 Thus, kinase inhibitors are examined as potential therapeutic approaches targeting tau. Glycogen synthase kinase 3 (GSK3β) has become prominent as a possible therapeutic target. The most studied GSK3 inhibitor is lithium chloride, a therapeutic agent for affective disorders, which seems to prevent phosphorylation of tau in mouse models. Lithium is currently reassessed within the novel framework for drug research. 79
Another GSK-3 inhibitor, tideglusib, did not meet phase 2 clinical endpoints in patients with mild and moderate AD. 80
ANAVEX 2-73 is evaluated in a phase 2 trial, for eligible subjects AD MCI or mild AD. 81 ANAVEX 2-73 is also a GSK-3b inhibitor but additionally it is a high affinity sigma 1 receptor agonist and a low-affinity muscarinic agonist. 4 Results presented at 2019 Alzheimer’s Association International Conference (AAIC) revealed that patients treated with ANAVEX 2-73 had high levels of 2 gut microbiota families, Ruminococcaceae and Porphyromonadaceae, which were associated with improved activities of daily living. The effect might potentially be reversal of the microbiota imbalances and might have a homeostatic effect on the brain-gut-microbiota axis. 81
Inhibitors of tau aggregation
Methylene blue (MB), a known phenothiazine, is evaluated in AD studies as a potential tau aggregation inhibitor. The problem with this drug is that urine is colored blue, resulting in a lack of blinding. A monotherapy trial with MB on mild and moderate AD ( {"type":"clinical-trial","attrs":{"text":"NCT00515333","term_id":"NCT00515333"}} NCT00515333 ) has demonstrated some clinical benefit in moderate, but not mild AD. 82 However, the methodology of the study, as blinding is impossible, has been highly criticized. 83
Methylene blue’s derivative TRx0237 (LMTX) which was studied in phase 3 failed finally to show efficacy, and based on the analysis of the results, a new phase 2/3 study named LUCIDITY was started 1 year ago in subjects with mild AD with a lower dose of the agent. 84
Microtubule stabilizers
The microtubule-stabilizing agent davunetide was studied in a phase 2 trial but it did not meet the clinical end points. 85
TPI-287 (abeotaxane), a small molecule derived from taxol, is a microtubule protein modulator. It was administered intravenously to patients with mild to moderate AD in a phase 1/2 study ( {"type":"clinical-trial","attrs":{"text":"NCT01966666","term_id":"NCT01966666"}} NCT01966666 ). First results presented report that the agent was not well tolerated by the participants. 84
IONIS MAPTRx (BIIB080), a microtubule-associated protein tau RNA inhibitor, an antisense oligonucleotide, is assessed in a phase 2 clinical study that is still in the recruiting process of patients with mild AD ( {"type":"clinical-trial","attrs":{"text":"NCT02623699","term_id":"NCT02623699"}} NCT02623699 ). 86
Targeting posttranslational modifications of Tau
Another tau modification that promotes aggregation besides phosphorylation is posttranslational modification by lysine acetylation. Thus, the use of inhibitors of tau acetylation is proposed as a possible therapeutic approach for AD.
Nilotinib is a c-Abl tyrosine kinase inhibitor which is used in patients with leukemia. It also appears to trigger intraneuronal autophagy to clear tau. It is now studied in a phase 2 trial in individuals with mild to moderate AD ( {"type":"clinical-trial","attrs":{"text":"NCT02947893","term_id":"NCT02947893"}} NCT02947893 ). 4 , 83
Promotion of Tau clearance—immunotherapy
Recently emerged evidence in various animal models strongly suggests that targeting ptau epitopes is a practical approach to induce antibody responses that are able to promote tau clearance. 81 Hence, a number of active and passive immunotherapy projects have reached clinical trials for AD treatment. 83
Active immunotherapy
AADvac1 contains a synthetic tau peptide and is currently studied in a phase 2 clinical study in mild to moderate AD ( {"type":"clinical-trial","attrs":{"text":"NCT02579252","term_id":"NCT02579252"}} NCT02579252 ). 4 , 10 , 83
Passive immunotherapy
ABBV-8E12 is a humanized anti-tau MAb assessed in a phase 2 clinical study in patients with early AD ( {"type":"clinical-trial","attrs":{"text":"NCT02880956","term_id":"NCT02880956"}} NCT02880956 ). 87
BIIB092 is a humanized IgG4 MAb against tau fragments derived from the stem cells of a patient with familial AD. 84 A phase 2 clinical trial assesses the safety and efficacy of the agent in participants with AD MCI and mild AD. 4
RO7105705 (MTAU9937 A) is an anti-tau MAb which is assessed in a phase 2 study in individuals with prodromal and mild AD ( {"type":"clinical-trial","attrs":{"text":"NCT03289143","term_id":"NCT03289143"}} NCT03289143 ). 83 , 88
Three other anti-tau mAbs (BIIB076, JNJ-63733657, and LY3303560) are currently assessed in phase 1 clinical trials. 4
DMTs with other mechanisms
Neuroprotection.
AGB101 (low-dose extended-release levetiracetam) is an SV2A modulator that is assessed in a phase 3 clinical trial as a repurposed agent (approved for use in another indication, not epilepsy but MCI due to AD). It is supposed to reduce neuronal hyperactivity induced by Aβ ( {"type":"clinical-trial","attrs":{"text":"NCT03486938","term_id":"NCT03486938"}} NCT03486938 ) ( Diagram 1 ). 4

DMTs with other mechanisms. DMTs indicate disease-modifying therapies; hMSCs, human mesenchymal stem cells.
BHV4157 (troriluzole) is a glutamate modulator that reduces synaptic levels of glutamate and is assessed in a phase 3 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT03605667","term_id":"NCT03605667"}} NCT03605667 ). 4
Icosapent ethyl is the eicosapentaenoic acid (EPA) omega-3 fatty acid in a purified form. It is supposed to protect neurons from disease pathology and is assessed in a phase 3 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT02719327","term_id":"NCT02719327"}} NCT02719327 ). 4
There are also 2 biologics and 24 small molecules with neuroprotection as one of their mechanisms 4 assessed in phase 2 clinical studies and 8 small molecules in phase 1 clinical trials. 4
Anti-inflammatory effects
Although neuroinflammation has been proposed as a possible mechanism for the pathogenesis of AD more than 30 years ago, only recently research is spurred into neuroiflammation probably due to 2 enlightening discoveries: first, there is evidence that activated glial cells are involved in the formation of the brain lesions in AD and second, epidemiological studies revealed that patients with rheumatoid arthritis, who are treated with anti-inflammatory drugs for decades, are spared from AD. 89 Further exploration of the inflammatory mechanisms in the disease showed that activation of glial cells, microglia, and astrocytes induces the production of inflammatory cytokines, mainly interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α). More specifically, TNF-α signaling has been proved to exacerbate both Aβ aggregation and tau phosphorylation in vivo, 90 whereas its levels have been found elevated in brain and plasma of patients with AD. 91
According to the previously mentioned neuroinflammatory mechanisms, it is established by multiple biomarker and epidemiological studies of Aβ levels in the CSF and the brain that nonsteroidal anti-inflammatory drugs, complement activation blockers, and other anti-inflammatory agents could postpone the clinical onset of AD if they are timely and for a long time applied, such as in rheumatoid arthritis. 89
Furthermore, the already existing TNF-α inhibitors (TNFIs), which are FDA-approved biologic drugs (mAbs) for the treatment of rheumatoid arthritis, Crohn disease, psoriatic arthritis, and other peripheral inflammatory diseases, are studied as a potential therapeutic strategy for AD. The TNF-α–specific mAbs are the agents infliximab, adalimumab, golimumab, and certolizumab, whereas etanercept is a recombinant fusion protein, which is also a TNFI. 91 The limited BBB penetration of these agents is the main drawback for their development. Peripheral targeting of TNF-α activity is the one proposed method for the tackling of the problem and reengineering of the TNFIs to enable BBB penetration is the other. 91 To sum up, large-scale randomized controlled trials assessing the safety and the effectiveness of TNFIs on patients with AD are warranted.
The following are the anti-inflammatory agents currently assessed in phase 3 clinical trials:
- ALZT-OP1a plus ALZT-OP1b (cromolyn plus ibuprofen) is a combination of a mast cell stabilizer and an anti-inflammatory agent, respectively, assessed in a phase 3 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT02547818","term_id":"NCT02547818"}} NCT02547818 ). 4
- COR388 targets a periodontal pathogen acting as bacterial protease inhibitor that reduces neuroinflammation and consequently hippocampal degeneration and is currently assessed in a phase 3 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT03823404","term_id":"NCT03823404"}} NCT03823404 ). 4
- Masitinib acts on mast cells as a selective tyrosine kinase inhibitor and a modulator of neuroinflammation. It is assessed in a phase 3 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT01872598","term_id":"NCT01872598"}} NCT01872598 ). 4
The following are the anti-inflammatory agents studied in phase 2:
- Elderberry Juice improves the mitochondrial function acting as powerful antioxidant rich in anthocyanins ( {"type":"clinical-trial","attrs":{"text":"NCT02414607","term_id":"NCT02414607"}} NCT02414607 ) and GRF6019, a human plasma protein fraction administered with infusions, based on the hypothesis that brain neuroinflammation can be counteracted by young blood parabiosis ( {"type":"clinical-trial","attrs":{"text":"NCT03520998","term_id":"NCT03520998"}} NCT03520998 , {"type":"clinical-trial","attrs":{"text":"NCT03765762","term_id":"NCT03765762"}} NCT03765762 ). 4
- Anti-inflammatory agents studied in phase 1 are the mAbs AL002, AL003 ( {"type":"clinical-trial","attrs":{"text":"NCT03635047","term_id":"NCT03635047"}} NCT03635047 , {"type":"clinical-trial","attrs":{"text":"NCT03822208","term_id":"NCT03822208"}} NCT03822208 ). 4
Growth factor promotion
NDX-1017 is an hepatocyte growth factor with the role to regenerate neurons, which is studied in a phase 1 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT03298672","term_id":"NCT03298672"}} NCT03298672 ). 4
Metabolic effects
Losartan plus amlodipine plus atorvastatin plus exercise is a combination repurposed agent suggested to succeed significant reduction of the vascular risk capable of preserving cognitive function. It is assessed in a phase 3 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT02913664","term_id":"NCT02913664"}} NCT02913664 ). 4
Candesartan, an angiotensin receptor blocker; formoterol, a β 2 adrenergic receptor agonist; and intranasal insulin glulisine, which rises brain insulin signaling, are currently studied in phase 2 clinical trials ( {"type":"clinical-trial","attrs":{"text":"NCT02646982","term_id":"NCT02646982"}} NCT02646982 , {"type":"clinical-trial","attrs":{"text":"NCT02500784","term_id":"NCT02500784"}} NCT02500784 , {"type":"clinical-trial","attrs":{"text":"NCT02503501","term_id":"NCT02503501"}} NCT02503501 , respectively), whereas intranasal insulin aspart is assessed in a phase 1 clinical study. 4
Stem cell therapies
AstroStem is a stem-cell-based treatment administered 10 times intravenously, which consists of stem cells derived from autologous adipose tissue. AstroStem is currently assessed in a phase 2 study ( {"type":"clinical-trial","attrs":{"text":"NCT03117738","term_id":"NCT03117738"}} NCT03117738 ), whereas hMSCs (human mesenchymal stem cells) treatment is assessed in a phase 1 study ( {"type":"clinical-trial","attrs":{"text":"NCT02600130","term_id":"NCT02600130"}} NCT02600130 ). 4
Symptomatic agents
Symptomatic treatments are agents that target and improve the clinical symptoms of the disease, either cognitive or BPSD, without modifying the pathological steps leading to AD or acting on the evolution of the disease, as DMTs are supposed to do.
Overall, there are 33 symptomatic agents in current trials: 19 agents aim to improve cognition and 14 target BPSD.
Eleven of them are studied in phase 3: 3 cognitive intensifiers and 8 acting on BPSD.
Twenty symptomatic agents are in phase 2: 14 cognitive intensifiers and 6 acting on BPSD.
There are also 2 cognitive intensifiers being studied in phase 1. 4
Arduous research efforts persist to develop effective DMTs for AD, as well as symptomatic therapeutics. A plethora of continuing phase 1, 2, and 3 human studies are focused on various treatment targets in AD. Given the recent experience of a high proportion of lack of success in AD clinical trials on therapeutic agents, more recent trials appear robustly empowered by the integration of developments in biomarkers of AD, of the targeting of a single primary outcome, especially in prodromal AD studies, of the enrollment of earlier populations and the innovative trial designs. 91 - 93
At the same time, innovative research targets the development of more sophisticated diagnostic tools (neuroimaging, fluid, proteomic, and genomic AD biomarkers), whereas prevention studies for the disease are also ongoing. 10
If all these research efforts come to fruition, an effective “precision medicine” context could be applied in every patient with AD in the near future: risk factor elimination, comorbid disease treatment, and personalized advice for lifestyle modification will be provided. An AD biomarkers and neuropsychological evaluation profile will be outlined. Afterward, the patient may start a combination of DMTs tailored to meet his genetic, neuroimaging, biochemical, and neuropsychological requirements. 3 , 94
Furthermore and beyond any DMT perspective, clinicians should always maintain a patient/caregiver-targeted dealing with AD. Establishing a strong therapeutic alliance with the patient and his or her caregivers with a holistic and realistic approach involving psychoeducation, behavioral, and environmental techniques; advanced planning for future care needs; and appropriate pharmaceutical treatment is not only an efficient but also an ethical care in AD.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SGP conceptualized the study, developed the proposal and coordinated the project. KGY completed initial data entry and analysis, and wrote the report. Both authors read and approved the final manuscript.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Dementia articles from across Nature Portfolio
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by neurodegenerative disorders, such as Alzheimer's disease, is usually progressive and can eventually be fatal.
Revealing clinical heterogeneity in a large brain bank cohort
Clinical disease trajectories that describe neuropsychiatric symptoms were identified using natural language processing for 3,042 brain donors diagnosed with various neurodegenerative disorders. Trajectories revealed distinct temporal patterns that result in the identification of new clinical subtypes, and a subset of misdiagnosed donors.
Hearing loss promotes Alzheimer’s disease
Epidemiological studies reveal a correlation between hearing loss and the development and progression of Alzheimer’s disease (AD), but the underlying causal mechanisms remain unclear. A study now provides experimental evidence that hearing loss can promote AD via the growth differentiation factor 1 (GDF1) pathway, which may aid in developing potential AD therapeutic strategies.
- Hong-Bo Zhao
Related Subjects
- Alzheimer's disease
Latest Research and Reviews
Adenosine triphosphate induces amorphous aggregation of amyloid β by increasing Aβ dynamics
- Masahiro Kuramochi
- Momoka Nakamura
- Kazuaki Yoshimune
Multi-modal Neuroimaging Phenotyping of Mnemonic Anosognosia in the Aging Brain
Bueichekú et al. use multimodal in vivo neuroimaging to investigate the brain characteristics of individuals presenting unawareness of memory loss who are at risk of Alzheimer’s disease due to age. They find unawareness of memory decline is an early behavioral sign that a person might develop Alzheimer’s disease.
- Elisenda Bueichekú
- Patrizia Vannini
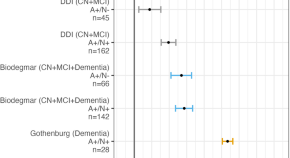
Plasma brain-derived tau is an amyloid-associated neurodegeneration biomarker in Alzheimer’s disease
The authors investigated associations of brain-derived-tau (BD-tau) with Aβ pathology, changes in cognition and MRI signatures. Staging Aβ-pathology according to neurodegeneration, using BD-tau, identifies individuals at risk of near-term cognitive decline and atrophy.
- Fernando Gonzalez-Ortiz
- Bjørn-Eivind Kirsebom
- Kaj Blennow
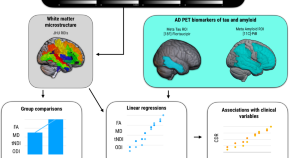
Influences of amyloid-β and tau on white matter neurite alterations in dementia with Lewy bodies
- Robert I. Reid
- Kejal Kantarci
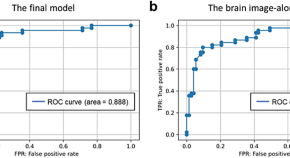
Amyloid-β prediction machine learning model using source-based morphometry across neurocognitive disorders
- Yuki Momota
- Shogyoku Bun
- Masaru Mimura
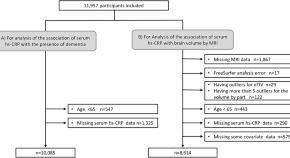
Serum high-sensitivity C-reactive protein and dementia in a community-dwelling Japanese older population (JPSC-AD)
- Ayumi Tachibana
- Jun-ichi Iga
- Yutaka Kiyohara
News and Comment
Marker provides 10-year warning of dementia.
Changes in plasma levels of specific proteins could predict the development of dementia more than 10 years before clinical diagnosis.
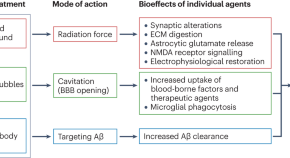
Ultrasound and antibodies — a potentially powerful combination for Alzheimer disease therapy
Success in a trial of low-intensity ultrasound combined with an amyloid-β antibody represents a major stride towards integrating pharmacological and nonpharmacological approaches to reduce the amyloid-β load in patients with mild Alzheimer disease. This trial also highlights the potential of therapeutic ultrasound modalities to combat neurodegenerative diseases.
- Jürgen Götz
- Pranesh Padmanabhan
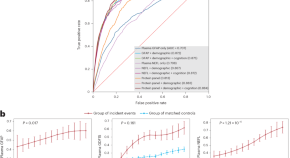
Blood protein markers predict 15-year risk of dementia
Using a data-driven proteomics strategy from a prospective community-based cohort with long-term follow-up, this study reports that plasma levels of glial fibrillary acidic protein (GFAP) can predict the risk of dementia, even 15 years before disease diagnosis. Our findings have important implications for early screening and interventions for dementia.
Clinical staging of behavioral and psychological symptoms of dementia
Clinical staging could be an actionable concept for behavioral and psychological symptoms of dementia (BPSD), providing clinicians with tools to navigate choices of treatment, acceptability of side effects and suitable care settings. This would pave the way for more research into tailored interventions that are much needed in high-stage BPSD.
- Maarten J. A. Van Den Bossche
- Ann T. E. Van Vré
- Mathieu Vandenbulcke
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- RFI Amyloid PET Depletion Following Treatment
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association
As the largest nonprofit funder of Alzheimer's research, the Association is committed to accelerating the global progress of new treatments, preventions and, ultimately, a cure.
Information for Researchers
Research we fund, apply for a grant, find a clinical trial, efforts we lead, the first survivor of alzheimer's is out there, but we won't get there without you., learn how alzheimer’s disease affects the brain..
Take the Brain Tour
Don't just hope for a cure. Help us find one. Volunteer for a clinical trial.
Keep up with alzheimer’s news and events.
As populations age, Alzheimer’s and dementia are becoming more prevalent. A new drug could offer hope

As populations age, the number of cases of dementia rises. Image: Unsplash/centelm
.chakra .wef-1c7l3mo{-webkit-transition:all 0.15s ease-out;transition:all 0.15s ease-out;cursor:pointer;-webkit-text-decoration:none;text-decoration:none;outline:none;color:inherit;}.chakra .wef-1c7l3mo:hover,.chakra .wef-1c7l3mo[data-hover]{-webkit-text-decoration:underline;text-decoration:underline;}.chakra .wef-1c7l3mo:focus,.chakra .wef-1c7l3mo[data-focus]{box-shadow:0 0 0 3px rgba(168,203,251,0.5);} Charlotte Edmond

.chakra .wef-9dduvl{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-9dduvl{font-size:1.125rem;}} Explore and monitor how .chakra .wef-15eoq1r{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;color:#F7DB5E;}@media screen and (min-width:56.5rem){.chakra .wef-15eoq1r{font-size:1.125rem;}} Global Health is affecting economies, industries and global issues

.chakra .wef-1nk5u5d{margin-top:16px;margin-bottom:16px;line-height:1.388;color:#2846F8;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-1nk5u5d{font-size:1.125rem;}} Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:, global health.
Listen to the article
- A new drug, lecanemab, has been shown to reduce the decline in memory and thinking associated with Alzheimer's.
- As populations age, dementia cases are on the rise, with 10 million new people diagnosed each year.
- Dementia is a collective term for a group of diseases or brain injuries that can lead to a change in cognitive functioning as well as other symptoms like lack of emotional control.
It is one of the biggest diseases of our time: 10 million new cases of dementia are diagnosed every year, according to the World Health Organization (WHO). More than 55 million people worldwide live with a form of dementia and it is the seventh leading cause of death among all diseases.
Now a new drug is offering a glimmer of hope after years of searching for a treatment. In clinical trials, lecanemab has been shown to slow the cognitive decline associated with the disease. The drug attacks the protein clumps in the brain that many think are the cause of the disease.
Although dementia patients are currently offered drugs, none of them affect the progression of the disease which is why scientists in the field are so excited about this latest development. Alzheimer's Research UK called the findings "a major step forwards" .
But while this is undoubtedly positive news, the body also points out that the benefits of the drug were small and came with significant side effects. In addition, lecanemab has been proven to work in the early stages of the disease, so would rely on doctors spotting it before it had progressed too far.
With the number of dementia cases expected to rise to 78 million by 2030 and 139 million in 2050, according to the WHO, the race is on for scientific developments and research that will help us understand, treat and possibly prevent the disease.
A global impact
As populations age, the number of cases of dementia rises. While the deterioration of cognitive functioning is not caused by age itself, it does primarily affect the older generation. For many elderly people it also results in disability and loss of independence - which can have psychological, social and economic implications for them and their families, carers and society more broadly.
The estimated global cost of dementia to society was placed at $1.3 trillion in 2019, and is expected to rise to $2.8 trillion by 2030, WHO says.
Alzheimer’s Diesease, a result of rapid ageing that causes dementia, is a growing concern. Dementia, the seventh leading cause of death worldwide, cost the world $1.25 trillion in 2018, and affected about 50 million people in 2019. Without major breakthroughs, the number of people affected will triple by 2050, to 152 million.
To catalyse the fight against Alzheimer's, the World Economic Forum is partnering with the Global CEO Initiative (CEOi) to form a coalition of public and private stakeholders – including pharmaceutical manufacturers, biotech companies, governments, international organizations, foundations and research agencies.
The initiative aims to advance pre-clinical research to advance the understanding of the disease, attract more capital by lowering the risks to investment in biomarkers, develop standing clinical trial platforms, and advance healthcare system readiness in the fields of detection, diagnosis, infrastructure and access.
What is dementia?
Dementia is a collective term for a group of diseases or injuries which primarily or secondarily affect the brain. Alzheimer’s is the most common of these and accounts for around 60-70% of cases. Other types include vascular dementia , dementia with Lewy bodies (abnormal protein clumps) and a group of diseases that contribute to frontotemporal dementia. It can also be triggered by strokes, excessive use of alcohol, repetitive head injuries, nutritional deficiencies, or follow some infections like HIV, the Alzheimer’s Society explains.
The different forms of dementia can often be indistinct and can co-exist.
Different people are affected in different ways, depending on the underlying cause. But the syndrome is usually progressive and can affect a range of functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language and judgement.
Changes in mood and ability to control emotions often accompany these cognitive variations.

Can it be treated?
There is no cure for dementia, although there are numerous treatments being worked on and at clinical trial phase. Dementia care currently focuses on early diagnosis, optimizing health and wellbeing and providing long-term support to carers.
Besides age, there are a number of other risk factors, which if avoided, can decrease the chances of dementia and slow its progression. Preventative steps include being physically active, not smoking, avoiding the harmful use of alcohol, as well as maintaining a healthy diet, weight, blood pressure, cholesterol and blood sugar levels.
Other risk factors associated with dementia include depression, social isolation, low educational attainment, cognitive inactivity and even air pollution .

What is the impact?
People with dementia rely heavily on informal care - i.e., friends and family. These carers spent on average five hours a day looking after people living with dementia in 2019, according to WHO figures. Informal care is thought to cover half of the overall financial burden of dementia.
There is also a disproportionate impact on women. They account for 65% of all dementia-related deaths, and also have a greater number of years affected by the disease. Women also typically provide the majority of informal care - covering over two-thirds of the carer hours for people living with dementia.

What are the latest developments?
The fact that dementia is only diagnosed once symptoms appear means that by the time people take part in clinical trials the disease is often quite well advanced. This can hamper the development of drugs. However, research analyzing data from the UK Biobank has indicated there are a collection of signals that could indicate a problem years before dementia is currently being diagnosed .
Other scientists postulate that, rather than being a disease of the brain, Alzheimer’s is in fact a disorder of the immune system within the brain . They believe research should instead focus on drugs targeting auto-immune pathways .
On a less positive note, researchers found that people who have recently received a dementia diagnosis, or diagnosed with the condition at a younger age, are at an increased risk of suicide . This underlines the importance of a strong support network, particularly among those newly diagnosed.
Have you read?
Here’s how exercise alters our brain chemistry – and could prevent dementia, dementia cases are expected to double in many countries over the next 30 years, these 7 simple habits could halve your risk of dementia, don't miss any update on this topic.
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The agenda .chakra .wef-n7bacu{margin-top:16px;margin-bottom:16px;line-height:1.388;font-weight:400;} weekly.
A weekly update of the most important issues driving the global agenda
.chakra .wef-1dtnjt5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;} More on Global Health .chakra .wef-nr1rr4{display:-webkit-inline-box;display:-webkit-inline-flex;display:-ms-inline-flexbox;display:inline-flex;white-space:normal;vertical-align:middle;text-transform:uppercase;font-size:0.75rem;border-radius:0.25rem;font-weight:700;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;line-height:1.2;-webkit-letter-spacing:1.25px;-moz-letter-spacing:1.25px;-ms-letter-spacing:1.25px;letter-spacing:1.25px;background:none;padding:0px;color:#B3B3B3;-webkit-box-decoration-break:clone;box-decoration-break:clone;-webkit-box-decoration-break:clone;}@media screen and (min-width:37.5rem){.chakra .wef-nr1rr4{font-size:0.875rem;}}@media screen and (min-width:56.5rem){.chakra .wef-nr1rr4{font-size:1rem;}} See all

Could private provision be the key to delivering universal health coverage?
Gijs Walraven
April 7, 2024

A generation adrift: Why young people are less happy and what we can do about it
Andrew Moose and Ruma Bhargava
April 5, 2024

This gene-editing technology lets scientists cut HIV out of cells

Child deaths have reached a historic low – but there's still much more to do
Amira Ghouaibi
April 2, 2024

Call for action on rising cholera cases, and other health stories
Shyam Bishen
March 28, 2024

Here’s how scientists are studying Parkinson’s disease using robots
Here Are the New Drugs and Treatments We Could See in 2024

2023 was a strong year for innovative new drugs, with new medications for Alzheimer’s disease , weight loss , and the first treatment based on the gene-editing technology CRISPR .
But 2024 is also shaping up to be a milestone year for some exciting therapies. Here's what to expect.
Another new Alzheimer's drug
Eli Lilly could debut a new treatment for Alzheimer’s disease that targets amyloid, the protein that builds up in the brains of patients. In studies that the company has submitted to the U.S. Food and Drug Administration (FDA) for approval, people receiving the drug experienced 35% slower cognitive decline according to cognitive tests than those getting placebo, and 40% less decline in their ability to perform daily activities such as driving or holding conversations. That’s a slightly higher efficacy than the existing medications for the neurodegenerative disease, and experts are hoping that if patients start taking it early enough, they might be able to hold off the worst effects of memory loss and cognitive decline for a few years. The FDA is expected to make a decision about the drug in early 2024.
Innovative blood-disorder treatments
After approving the first CRISPR treatment, Casgevy, for sickle cell anemia, the FDA is reviewing the same therapy for another genetic blood disorder called beta thalassemia. In both conditions, people have abnormal blood cells that can’t carry enough oxygen, which leads to painful attacks and frequent blood transfusions and hospitalizations. The gene-editing therapy is a one-time treatment that allows people to make more healthy blood cells, which can reduce the number of painful episodes. U.K. health authorities have granted Casgevy conditional marketing authorization, and the FDA is expected to decide in March whether to approve the treatment for beta thalassemia.
The agency is also considering other gene-based treatments that don’t use CRISPR but rely on more traditional virus-based methods. One is for hemophilia B. Patients with the condition experience moderate-to-severe bleeding episodes because they lack a coagulation factor that the gene therapy provides. In studies by the manufacturer, Pfizer, the therapy lowered the risk of annual bleeding among a few dozen men who tested it by 71% . The FDA is expected to make a decision about the treatment in the second quarter of 2024.
More From TIME
A novel schizophrenia drug.
Later in the year, the FDA will review a new drug treatment for schizophrenia —the first for the psychiatric condition in decades. Karuna Therapeutics has improved upon existing antipsychotics by targeting a different brain chemical than existing medications, which focus on dopamine. In a study of a couple hundred people with schizophrenia, the drug, which works on the muscarinic receptors in the brain involved in regulating positive and negative thoughts, helped to reduce the extremes of symptoms that are typical of the condition. If approved, the drug could help more people with schizophrenia relieve their worst symptoms, since many people stop taking the existing medications because of their side effects.
New year, same old questions of access
As exciting as the possible new medications are, they also raise questions about affordability and accessibility . Innovative drug treatments involving gene therapy and CRISPR, for example, are designed to be one-time treatments that can mitigate the need for repeated and often lifelong medical care. But that means higher upfront costs, and it’s not clear whether insurers will cover such hefty price tags.
As more therapies reach the market, however, they could change the reimbursement structure as insurers will likely feel increasing pressure to cover treatments that could be not just life-changing but also potentially curative—and save millions in long-term health care costs.
More Must-Reads From TIME
- Jane Fonda Champions Climate Action for Every Generation
- Passengers Are Flying up to 30 Hours to See Four Minutes of the Eclipse
- Biden’s Campaign Is In Trouble. Will the Turnaround Plan Work?
- Essay: The Complicated Dread of Early Spring
- Why Walking Isn’t Enough When It Comes to Exercise
- The Financial Influencers Women Actually Want to Listen To
- The Best TV Shows to Watch on Peacock
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]
You May Also Like
MIT Technology Review
- Newsletters
New breakthroughs on Alzheimer’s
MIT scientists have pinpointed the first brain cells to show signs of neurodegeneration in the disorder and identified a peptide that holds potential as a treatment.
- Anne Trafton archive page
Neuronal hyperactivity and the gradual loss of neuron function are key features of Alzheimer’s disease. Now researchers led by Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory, have identified the cells most susceptible to this damage, suggesting a good target for treatment. Even more exciting, Tsai and her colleagues have found a way to reverse neurodegeneration and other symptoms by interfering with an enzyme that is typically overactive in the brains of Alzheimer’s patients.
In one study , the researchers used single-cell RNA sequencing to distinguish two populations of neurons in the mammillary bodies, a pair of structures in the hypothalamus that play a role in memory and are affected early in the disease. Previous work by Tsai’s lab found that they had the highest density of amyloid beta plaques, abnormal clumps of protein that are thought to cause many Alzheimer’s symptoms.
The researchers found that neurons in the lateral mammillary body showed much more hyperactivity and degeneration than those in the larger medial mamillary body. They also found that this damage led to memory impairments in mice and that they could reverse those impairments with a drug used to treat epilepsy.
In the other study , the researchers treated mice with a peptide that blocks a hyperactive version of an enzyme called CDK5, which plays an important role in development of the central nervous system. They found dramatic reductions in neurodegeneration and DNA damage in the brain, and the mice got better at tasks such as learning to navigate a water maze.
CDK5 is activated by a smaller protein known as P35, allowing it to add a phosphate molecule to its targets. However, in Alzheimer’s and other neurodegenerative diseases, P35 breaks down into a smaller protein called P25, which allows CDK5 to phosphorylate other molecules—including the Tau protein, leading to the Tau tangles that are another characteristic of Alzheimer’s.
Pharmaceutical companies have tried to target P25 with small-molecule drugs, but these drugs also interfere with other essential enzymes. The MIT team instead used a peptide—a string of amino acids, in this case a sequence matching that of a CDK5 segment that is critical to binding P25.
In tests on neurons in a lab dish, the researchers found that treatment with the peptide moderately reduced CDK5 activity. But in a mouse model that has hyperactive CDK5, they saw myriad beneficial effects, including reductions in DNA damage, neural inflammation, and neuron loss.
The treatment also produced dramatic improvements in a different mouse model of Alzheimer’s, which has a mutant form of the Tau protein. Tsai hypothesizes that the peptide might confer resilience to cognitive impairment in the brains of people with Tau tangles.
“We found that the effect of this peptide is just remarkable,” she says. “We saw wonderful effects in terms of reducing neurodegeneration and neuroinflammatory responses, and even rescuing behavior deficits.”
The researchers hope the peptide could eventually be used as a treatment not only for Alzheimer’s but for frontotemporal dementia, HIV-induced dementia, diabetes-linked cognitive impairment, and other conditions.
Keep Reading
Most popular, large language models can do jaw-dropping things. but nobody knows exactly why..
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
- Will Douglas Heaven archive page
OpenAI teases an amazing new generative video model called Sora
The firm is sharing Sora with a small group of safety testers but the rest of us will have to wait to learn more.
Google’s Gemini is now in everything. Here’s how you can try it out.
Gmail, Docs, and more will now come with Gemini baked in. But Europeans will have to wait before they can download the app.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
- Casey Crownhart archive page
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.

New 20-minute non-invasive treatment could reverse memory loss, study says
More than 747,000 Canadians currently live with Alzheimer's and other forms of dementia, but a new study suggests age-related memory loss could be reversed with a 20-minute non-invasive treatment.
Researchers from Boston University outlined their findings in a paper published in Nature Neuroscience last week . Their treatment involves a wearable cap equipped with electrodes that sends electrical signals into the brain, and researchers say this could help improve memory function.
"An increasingly older population leads to additional personal, social, healthcare and economic costs. A factor greatly contributing to these costs is the impairment in basic memory systems essential for activities of everyday life, such as making financial decisions or understanding language," lead researcher Robert Reinhart said in a news release published Monday.
In the study, patients received electrical brain stimulation for 20 minutes for four consecutive days. Each day, the patients were given a list of 20 words and were asked to memorize and immediately recite the words.
Two different treatment methods were tested: one targeting short-term memory with low-frequency stimulation and another for improving long-term memory with high-frequency stimulation in the brain's prefrontal cortex. Both methods were tested against a placebo in a randomized double-blind trial.
The researchers found that after three or four days of applying low-frequency electrical signals to the brain, patients exhibited better short-term memory, and the improvements remained a month later.
Sending high-frequency signals into the brain improved long-term memory after Day 2, as well as after a month. The researchers also found that individuals that had lower cognitive function had larger and longer-lasting memory improvement as a result of the treatment.
"Clinically, this is important because there are people with only short-term memory problems and others with only long-term memory problems. So, having tools in hand that can address each of these memory systems is of great value," Reinhart said.
In a 2019 study led by Reinhart's team, the researchers had applied the electrode treatment for one 25-minute session, but patients only exhibited memory benefits for less than an hour before the improvements vanished. However, Reinhart's treatment now applies electrical stimulation for multiple days.
"In this new study, we used multiple, consecutive days of stimulation for 20 minutes to cause long-lasting memory improvements that lasted one month. Previously, the effects lasted only 50 minutes," Reinhart said.
Currently, Reinhart notes that the existing treatment options for age-related impaired cognition produce mixed results and come with several risks and side effects.
"For those reasons, there’s an urgent need to develop innovative therapeutic interventions that can provide rapid and sustainable improvements with minimal side effects," he said.
CTVNews.ca Top Stories

Couple lucky to be alive after piece of Montreal highway crashes into their windshield
A Montreal couple is having a hard time driving without stress and is unhappy with the city's maintenance after a chunk of highway crashed into their windshield while driving on Thursday night.
Will it be cloudy during Monday's solar eclipse? Environment Canada seems to think so
Weather forecasts are predicting that cities along the path of totality during Monday's solar eclipse will have clouds that could obstruct the once-in-a-lifetime celestial event.
A battle for hope: the brewing campaign clash between the Conservatives and the NDP
Conservative Leader Pierre Poilievre's path to power may be by prosecuting Prime Minister Justin Trudeau's past eight years in government, but his road to victory is painted NDP orange.
5 tips for finding the best diet that works for you
With dieting, the conventional wisdom says a person needs to be in calorie-deficit mode to lose weight. If you eat more calories than you burn, you gain weight; if you eat fewer calories, you lose weight.
Iranian official warns Israel that its embassies are not safe after deadly Damascus strike
A top Iranian military adviser on Sunday warned Israel that none of its embassies were safe following a strike in Damascus last week blamed on Israel that killed 12 people, including two elite Iranian generals. Regional tensions have threatened to draw the Middle East into a wider conflict as Israel’s war against Hamas marks six months.
Five things to watch for in the Canadian business world in the coming week
Five things to watch for in the Canadian business world in the coming week:
Solar Eclipse | How to tell if your solar eclipse glasses are fake
As Ontarians prepare for Monday’s solar eclipse, many are discovering that the solar viewing glasses they have purchased may not be safe.
Gunfight at south Florida bar leaves two dead, seven injured
A gunfight at a suburban Miami bar left two people dead and seven injured early Saturday.
'You're the chef': Fast-food chains embrace menu hackers' creative combinations
Some are as simple as coating chicken nuggets in a blend of barbecue and ghost pepper sauces — nicknamed "cowboy caviar" — but others take things to a new level, like dropping pie or mini cinnamon sugar doughnuts into a milkshake or ensconcing a hotdog in onion rings.

School in St. Jerome, Que. forced to toss thousands of counterfeit solar eclipse glasses
A school in Saint-Jerome, Que. was forced to toss out thousands of counterfeit solar eclipse glasses that were purchased on Amazon.
'Huge loss': Fire destroys band office in northern Ontario First Nation
Police are investigating after a fire destroyed a First Nation band office in northern Ontario.
First look at memorial D-Day statue takes place, honouring Royal Regina Rifles
Members of the Royal Regina Rifles along with many dignitaries gathered on the grounds of the legislature Saturday for the first look at a new memorial statue.
'Once-in-a-lifetime event': Next major eclipse in Toronto won't happen for another 120 years
A GTA professor says the upcoming major eclipse is quite literally a “once-in a lifetime event” as the last time it happened in the Toronto region was 1925 and the next one is expected to be in 2144.

International leaders condemn Ecuador after police break into the Mexican Embassy in Quito
International leaders have condemned Ecuador after police in the country's capital broke into the Mexican Embassy to arrest a former vice president who had been granted political asylum.

Biden could face obstacle getting on Ohio’s ballot, secretary of state’s office says
U.S. President Joe Biden may face complications getting on Ohio’s 2024 general election ballot unless Democrats make changes or the state legislature takes action, according to a letter issued by the office of Ohio’s secretary of state, Frank LaRose.
Lebanon's billionaire prime minister denies allegations of money laundering in France
Lebanon's billionaire caretaker prime minister has denied allegations of money laundering after a complaint was filed in France by two anti-corruption groups this week.
Slovak nationalist Peter Pellegrini on course to win country's presidential election
Slovak nationalist left government candidate Peter Pellegrini was on course to win the country's presidential election, results from most voting districts as well as projections showed on Saturday.
Donald Trump is demanding a new judge just days before the start of his hush-money criminal trial
Former U.S. President Donald Trump is demanding a new judge just days before his hush-money criminal trial is set to begin, rehashing longstanding grievances with the current judge in a long-shot, eleventh-hour bid to disrupt and delay the case.
A new declaration in Mexico gives 19 cats roaming the presidential palace food and care fur-ever
They prowl through palace gardens stalking pigeons and make cameos on televised press briefings. Some greet tourists at the doors, while others take a sneaky lick of ice cream from staff.

Federal gov't now doing more than 'fair share' on housing, minister says
Government House Leader Steven MacKinnon says the federal government is now doing "more than our fair share" when it comes to addressing the housing crisis in Canada.
Former RCMP intelligence official Cameron Ortis granted bail pending appeal
Former RCMP intelligence official Cameron Ortis — who was sentenced to 14 years in jail after leaking national secrets — has been granted bail pending appeal, CTV News has confirmed.

P&G recalls 8.2 million bags of Tide, Gain and other laundry detergents
Procter & Gamble is recalling more than eight million bags of Tide, Gain, Ace and Ariel laundry detergent packets sold in the U.S. and Canada due to a defect in the products' child-resistant packaging.
Here are the recalls in Canada this week
Health Canada and the Canadian Food Inspection Agency recalled various items this week, including laundry pods, kids' bike seats, sausages and area rugs.

'You can hear it': Those with low vision can enjoy the eclipse with interactive tools
Eclipses have historically been more difficult to experience for those living with blindness or low vision. This time around, multiple groups hope to change that by using tools and educational materials designed to make the event more accessible.
'A problem for life': Students and staff react to University of Winnipeg cyberattack
Those impacted by the cyberattack that hit the University of Winnipeg last month say they are worried about the possibility of their personal data falling into the wrong hands.
'Plan for the worst': Eclipse viewings may impact cellphone networks
With Monday's solar eclipse expected to draw tens of thousands to regions along the path of totality in Eastern Canada, major cellphone and internet providers say they're ready to handle a surge in wireless traffic in those areas.
Entertainment

Montreal's Sami Zayn is ready for his 'Rocky IV' moment at WrestleMania 40
As he heads to WrestleMania 40 this weekend in Philadelphia, Sami Zayn is not in the main event. But he doesn’t mind taking the scenic route to the top.
Beyonce's new album explores what it means to be country
The top of the country music charts is filled with familiar names: Zach Bryan. Luke Combs. Morgan Wallen. These are country heavyweights — names any country fan would recognize. Then, sitting among them all, face obscured by the tip of a cowboy hat, is Beyonce.
Sacha Baron Cohen and Isla Fisher announce their marriage ended last year
In a joint statement posted to their respective Instagram Stories on Friday, Isla Fisher and Sacha Baron Cohen announced that they filed to end their marriage at some point last year.

32 per cent of Canadians blame grocery stores for rising food prices, more than any other reason: Nanos
Canadians are more likely to blame grocery stores for rising food prices than any other reason, and nearly one-in-five Canadians say they or someone they know has used a food bank in the past year, according to a survey conducted by Nanos Research for CTV News.
Boeing CEO Dave Calhoun was paid US$32.8 million in 2023
Boeing CEO Dave Calhoun was awarded a giant stock bonus on top of his more-than-a-million-dollar salary last year, despite overseeing a company that has been plagued by chronic losses and safety problems.
Oregon Powerball player wins a US$1.3 billion jackpot, ending more than 3 months without a grand prize
A Powerball player in Oregon won a jackpot worth more than US$1.3 billion on Sunday, ending a winless streak that had stretched more than three months.

Canada's Zach Edey and Purdue power their way into NCAA title game, beating N.C. State 63-50
Purdue kept its March Madness dream alive while snuffing out North Carolina State’s, getting 20 points and 12 rebounds from Canada's Zach Edey in a 63-50 victory Saturday that placed the Boilermakers a win from their first NCAA title.
Canadian women rally to defeat Brazil in penalty shootout at SheBelieves Cup
Canada defeated Brazil 4-2 in a penalty shootout at the SheBelieves Cup after the game finished knotted at 1-1 after 90 minutes Saturday.
Saudi Arabia will host the women's tennis WTA Finals for the next three years
Saudi Arabia will host the WTA Finals as part of a three-year deal announced Thursday by the women's professional tennis tour that will increase the prize money for this November's season-ending championship to a record US$15.25 million, a 70 per cent increase from 2023.

Elon Musk announces Tesla will unveil a 'robotaxi' on August 8
Elon Musk has long had an affinity for self-driving vehicles, claiming they will be one of Tesla's most important products. Despite big promises, years have gone by without cars that can, so far, drive on their own.
NEW | What are the chances police can find your stolen car? Canadians believe odds are low
A new poll from Nanos Research for CTV News has found that a majority of Canadians doubt the police are able to recover stolen cars.
Lamborghini written off after 13-year-old takes it for a joyride: West Vancouver police
A 13-year-old is facing several charges after crashing a Lamborghini on a West Vancouver highway, local police say.
Local Spotlight


'Like an underwater puppy': B.C. woman forms lasting friendship with octopus
When Catherine Dobrowolski began doing daily walks by the water, she never expected to make an eight-legged friend.
Ground-breaking Canadian giraffe researcher Anne Innis Dagg dies at 91
Pioneering Canadian giraffe researcher and feminist activist Anne Innis Dagg has died at the age of 91.
Merlin the Macaw leaving Halifax for Ontario due to depression and stress
According to a news release from the Maritime Museum of the Atlantic, Merlin the Macaw, a resident mascot for the facility, is flying off to Safari Niagara in Fort Erie, Ontario.
Ontario family's car stolen from parking lot of Montreal hotel
A family from Ontario says their SUV was stolen from a hotel parking lot in Montreal while the family was on a March break vacation down south.
Ottawa snowbirds embark on epic bicycle journey back to Canada
Two adventurous snowbirds have embarked on an amazing journey back to Canada, and they're inviting the world to join in on their adventure.
'I'm indebted to these guys': First responder, former cop save N.S. man's life in hockey rink
A first responder and a former police officer saved a Nova Scotia man's life as he suffered a heart attack on ice in March.
'Pretty remarkable': Alberta distillery beats out Ireland, Scotland at international whisky competition
A distillery in Parkland County is being internationally recognized for outstanding whisky production – and one bottle in particular is getting all the attention.
This historical tavern in Toronto is closing after nearly 200 years
An historic downtown Toronto bar is closing its doors next week after nearly 200 years in business.
Tipping is off the table at this Toronto restaurant
A Toronto restaurant introduced a surprising new rule that reduced the cost of a meal and raised the salaries of staff.

Vehicle plunges into Fraser River, driver's whereabouts unknown
Crews were able to locate a vehicle that somehow ended up in the Fraser River overnight Saturday, but the fate of its driver is currently unknown.
Langford park closed after person drops off 'explosive material': RCMP
The West Shore RCMP say that Veterans Memorial Park is closed to the public after someone left “explosive material” in the area.
Woman stabs taxi driver with needle, steals cab: Mission RCMP
Mounties in Mission say a woman assaulted a taxi driver and stole his vehicle on Friday night, and are asking for witnesses to come forward.

Impaired driver charged and injured after crashing into tree in Scarborough neighbourhood: police
A person has been sent to hospital and is facing charges after crashing into a tree while driving under the influence in a residential area in Scarborough, police say.
For some who are spiritually inclined, eclipse has added significance
When the Earth, moon and sun align on Monday, Kendra Pape-Green plans to be in a secluded spot in nature.

Oilers keep rolling with 4-2 victory over reeling Flames
Led by a clutch power play and 33 saves from Calvin Pickard, Edmonton inched closer to top spot in the NHL's Pacific Division on Saturday.
Calgary police investigate assault at Market Mall
Police arrested three youths following reports of an assault at Market Mall Saturday afternoon.
More than 300 athletes from across Alberta competing in lifesaving championships in Calgary
Over 300 athletes from 15 different clubs across Alberta are competing this weekend in the 2024 Alberta and Northwest Territories Pool Lifesaving Championships and Junior games at Brookfield Residential YMCA in Calgary.

Here's how it feels in Ottawa, eastern Ontario this Sunday
The countdown for the solar eclipse day continues, but will Ottawa and eastern Ontario have clear skies?
Off-duty Ottawa police officer facing impaired driving charges following 2-vehicle collision
The Ottawa Police Service says one of its officers has been placed on administrative duties after getting involved in a two-vehicle collision while off-duty on March 17.

Man stabbed and dumped at a Montreal hospital, police investigating
Montreal police (SPVM) are investigating after a man with stab wounds was dropped off at a hospital on Sunday morning.
1 dead in 3-vehicle crash near Edmonton International Airport Friday
One person is dead after a multi-vehicle crash Friday night near the Edmonton International Airport.
'It's painful for a parent': Mother protests in support of son with autism who was arrested while playing
The mother of a teen with autism who was arrested by RCMP while playing says she has never received an apology.

Nova Scotia premier joins calls for meeting with Trudeau about carbon pricing
Nova Scotia's Progressive Conservative premier has joined a call from leaders across the country asking for a meeting with Prime Minister Justin Trudeau to discuss carbon pricing.
'Women are getting paid and they're playing their favourite sport': recent women’s sports shattering viewing records
March Madness comes to a close this weekend, and this year on the women’s side, it’s been a ratings blockbuster.
Montreal Canadians superstar Carey Price on his life outside of hockey during visit to Halifax
NHL superstar and goalie for the Montreal Canadians, Carey Price, was recently in Halifax to take part in the Progress Club’s Sports Charity Dinner, which raises money for various charities around the city.

21-year-old facing charges after robbery at Winnipeg outlet mall
A 21-year-old man is facing several charges following a robbery at Outlet Collection Winnipeg on Friday.
Paw-sitive experience: Winnipeg Humane Society’s puppy yoga returns
Puppy yoga classes are back at the Winnipeg Humane Society (WHS), giving participants the chance to get fit with some furry friends.
'Only seemed right': Andrew Harris retiring as Winnipeg Blue Bomber
Hometown hero and four-time Grey Cup Champion Andrew Harris is retiring.

Interested in starting a garden? Here are some tips for beginners
Now that spring is here, some people may be thinking about starting a garden but don’t know where to start.
Here's when street sweeping will begin in Regina
Street sweeping will soon begin in different neighbourhoods in Regina to clear away leaves, as well as remove sand and debris from roads.

Ont. paramedic killed in Swiss avalanche
A Perth County paramedic who died suddenly on vacation in Switzerland is being remembered by her colleagues as a bright, caring person.
Suspects resist arrest, crash into apartment building in Hanover: police
Two people wanted in multiple jurisdictions are facing charges after Hanover police said they tried to resist arrest and crashed a vehicle into an apartment building.
One person killed in Brant county collision
One person has died after a three-vehicle collision on Highway 403 in Brant county.

Saskatoon explores moving Via Rail line to enhance connectivity
Canada's largest passenger train service and a crown corporation, Via Rail, connects the nation's major cities and is at the centre of a new proposal in Saskatoon.
Sask. dancers take stage in Saskatoon
Thousands of dancers from clubs representing all of Saskatchewan gathered at Prairieland Park on Saturday for the annual Maximum Elite Elevation Tour Dance Competition
Blades take series in five
The Saskatoon Blades are on to the second round of the Western Hockey League (WHL) playoffs after a 6-2 win over the Prince Albert Raiders at SaskTel Centre on Friday.
Northern Ontario

EXCLUSIVE | Canadian pilot who exposed Dominican Republic drug trafficking operation suing federal government, Pivot Airlines
A Canadian airline pilot who was detained in the Dominican Republic after he and his crew discovered more than 200 kilos of cocaine on board a flight to Toronto is seeking $16 million from the federal government and his former employer, Pivot Airlines.
Two men fined $2K each for illegal baitfish sales in northern Ont.
An Ontario man and a Quebec resident have each been fined $2,000 for their part in the illegal sale of baitfish in 2021.
Timmins shooting in March leads to April drug bust
Timmins police seized drugs, a handgun, ammo and cash during a raid of a Way Avenue home on Thursday.

London police investigating 'serious' crash between motorcycle, vehicle
London police are investigating following a serious collision between a motorcycle and a vehicle in the city’s east end early Saturday evening.
Still need a pair of glasses for Monday’s solar eclipse? CTV News London has got you covered
With Monday’s total solar eclipse set to bathe the region in darkness during the once-in-a-lifetime spectacle, many people still find themselves scrambling to find a pair of eclipse glasses. But if you need still a pair of glasses, we’ve got you covered.
Auston Matthews scores league-leading 64th goal in 4-2 Maple Leafs win over Canadiens
Auston Matthews scored his league-leading 64th of the season in a four-goal second period for the Maple Leafs, and Toronto defeated the archrival Montreal Canadiens 4-2 on Saturday night at the Bell Centre.

Royal Canadian Navy visits Collingwood for unique training operation
The Royal Canadian Navy is in Collingwood this weekend for a unique training operation.
Two Orillia individuals charged for impaired driving within four hours
Two individuals have been arrested and charged with impaired driving within four hours in Orillia.
Students showcase skills at annual Simcoe County Regional Science and Technology Fair
Students from across the region are showcasing their skills at the Simcoe County Regional Science and Technology Fair this weekend.

2 men suffer head, neck injuries after assault outside bar: Windsor police
Four suspects have been charged and one remains outstanding after two victims were repeatedly struck with beer bottles and kicked outside an Ouelette Avenue bar early Saturday morning.
Free solar eclipse glasses available on Sunday
Solar eclipse glasses have been a hot commodity in Windsor-Essex as the big day approaches.
After a limo collision ended his career 27 years ago, former Detroit Red Wing meets with Canadian fans for 'once-in-a-lifetime' visit
For the first time since a major limousine collision abruptly ended his NHL career, Detroit Red Wings alumni and Stanley Cup champion Vladimir Konstantinov made a visit across the Ambassador Bridge to Canada.
Vancouver Island

How A.I. and underwater microphones are protecting whales in B.C.
On a two-kilometre stretch of Boundary Pass near Saturna Island, underwater microphones known as hydrophones are used to capture whales in action. It’s a practice that’s been in place for years, but newly implemented technology is helping give mariners a heads up when a whale could be in their path.
On-reserve child poverty more than double B.C.'s average, according to data
In late February the First Call Child and Youth Advocacy Society released their annual Poverty Report Card, announcing that in 2021, 14 per cent of children were living in poverty - while on reserves this is more than double the provincial rate.

Evacuation of Kelowna, B.C., apartment near construction site extended for two weeks
More than 80 residents from a low-income apartment building in Kelowna, B.C., have learned they won't be able to return to their homes for at least another two weeks.
2 men injured after 'road rage' incident with Dodge Ram driver, Kelowna RCMP say
Mounties are investigating a reported "road rage" incident in Kelowna, B.C., that left two men injured last week.
Unstable nearby construction site forces evacuation of apartment in Kelowna, B.C.
More than 80 residents of a low-income apartment building in Kelowna, B.C., have been told they need to leave over a 'significant' risk to life and safety.

'Not pleased with the result': Lethbridge Hurricanes looking ahead to next season after first-round exit
The Lethbridge Hurricanes were bounced out of the playoffs earlier this week by the Swift Current Broncos after a double-overtime thriller.
'Hasn't lost its momentum': Green Shirt Day continues to inspire 6 years after Humboldt Broncos crash
This Saturday marks the sixth anniversary of the Humboldt Broncos bus crash and the start of Green Shirt Day, honouring the legacy of Logan Boulet.
Castle Mountain ski season makes a late comeback as resort set to close Sunday
Castle Mountain Resort is making use of the spring snowfall before they close for the season Sunday.
Sault Ste. Marie

Maple Weekend 2024: Ontarians head to the bush for sweet celebrations
It is Maple Weekend in Ontario and events are scheduled across the province to celebrate the region’s favourite pancake topping.
Fires destroy northwestern Ont. First Nation band office, home
Far North Police are investigating fires that destroyed the North Spirit Lake First Nation band office and an abandoned home in the remote northwestern Ontario community.

Lego takes over Newfoundland's biggest museum
Newfoundland's biggest museum has transformed into a giant Lego playground, featuring designs made by creators young and old.
Mysterious Newfoundland shipwreck finally out of the water
It took a few cuts with a chainsaw and the full strength of a 30-ton excavator, but a mysterious Newfoundland shipwreck has finally been pulled out of the water near the small community of Cape Ray.
Premiers making 'political hay' out of carbon pricing increase, Trudeau says
Prime Minister Justin Trudeau says premiers would rather complain and 'make political hay' out of his federal carbon pricing program than present an alternative to reduce greenhouse gas emissions.
Shopping Trends
The Shopping Trends team is independent of the journalists at CTV News. We may earn a commission when you use our links to shop. Read about us.
Editor's Picks
There's a total solar eclipse in canada next week, and here’s how to prepare, 14 of the best interactive dog toys to stimulate your dog's mind, if you're ready to dip your toes into spring cleaning, you'll want to order at least one of these amazon products, if you love cooking, feast your eyes on these 18 aesthetically pleasing kitchen products, 19 amazon canada products that'll solve a bunch of your little problems, our guide to the best office chairs in canada in 2024 (and where to get them), 20 gifts that are so great, you'll want to keep them for yourself, 19 of the best mother's day gifts under $50, 19 foolproof mother's day gifts to order if you want to get your shopping done early, if you hate hate razors and waxing, allow me to introduce you to your new favourite at-home hair removal devices, 16 eye creams that people with mature skin swear by, rosemary oil and 11 other products that reviewers swear by for hair growth, stay connected.

Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Winter 2024 | VOL. 36, NO. 1 CURRENT ISSUE pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Advances in Treatment of Frontotemporal Dementia
- Nahuel Magrath Guimet , M.D. ,
- Lina M. Zapata-Restrepo , M.D. ,
- Bruce L. Miller , M.D.
Search for more papers by this author
In this review, the authors explored the clinical features of frontotemporal dementia (FTD), focusing on treatment. The clinical features of FTD are unique, with disinhibition, apathy, loss of empathy, and compulsions common. Motor changes occur later in the illness. The two major proteins that aggregate in the brain with FTD are tau and TDP-43, whereas a minority of patients aggregate FET proteins, primarily the FUS protein. Genetic causes include mutations in MAPT , GRN , and C9orf72 . There are no medications that can slow FTD progression, although new therapies for the genetic forms of FTD are moving into clinical trials. Once a diagnosis is made, therapies should begin, focusing on the family and the patient. In the setting of FTD, families experience a severe burden associated with caregiving, and the clinician should focus on alleviating this burden. Advice around legal and financial issues is usually helpful. Careful consideration of environmental changes to cope with abnormal behaviors is essential. Most compounds that have been used to treat dementia of the Alzheimer’s disease type are not effective in FTD, and cholinesterase inhibitors and memantine should be avoided. Although the data are scant, there is some evidence that antidepressants and second-generation antipsychotics may help individual patients.
Frontotemporal dementia (FTD) refers to a group of neurodegenerative brain disorders characterized by atrophy of the frontal and anterior temporal lobes ( 1 ) and is one of the most common forms of early-onset dementia ( 2 ). Clinically, there are three main syndromes of FTD that are generally recognized on the basis of their clinical presentations: a behavioral variant FTD (bvFTD) characterized by a progressive deterioration of personality, social comportment and cognition ( 3 ); and two language presentations, classified under primary progressive aphasia (PPA), in which an insidious decline in language skills is the primary feature ( 1 ). These PPAs are divided on the basis of the pattern of language breakdown into a nonfluent variant of aphasia (nfvPPA) and a semantic variant (svPPA). A third form of PPA, the logopenic variant (lvPPA), usually occurs in association with the pathology of Alzheimer’s disease but can also be found in relation to FTD ( 4 ). There are forms of the disease that escape the descriptions of the main syndromes in FTD; an example of this is the right temporal variant, which is often associated with semantic memory impairment, prosopagnosia, and behavioral symptoms often associated with a socioemotional deficit ( 5 ). These syndromes have specific clinical symptoms and neuroimaging and pathological characteristics, although considerable heterogeneity and overlap exist in clinical practice, particularly as the disease progresses ( 1 ). In this article, pharmacological and nonpharmacological treatments for the neuropsychiatric aspects of FTD are reviewed. Promising advances in molecule-based therapies for the genetic forms are highlighted.
NEUROPATHOLOGY
Frontotemporal lobar degeneration (FTLD) is the term used to refer to a group of progressive brain diseases that predominantly affect the frontal and anterior temporal lobes. Thus, FTLDs include the clinical syndromes that are part of FTD: bvFTD, nfvPPA, and svPPA and also include progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and bvFTD with motor neuron disease (FTD-MND). These diseases, although sharing similar anatomy, have diverse etiologic causes at the neuropathological and genetic levels. Pathologically, FTLD is subdivided according to the composition of the abnormal inclusions of misfolded proteins. There are three main subgroups: FTLD-tau, FTLD-TDP, and FTLD-FET. The first two, characterized by the accumulation of tau protein and TDP-43, account for 90%−95% of FTLD cases; the third, a consequence of FET protein accumulation, is related to the remaining 5%−10% of cases ( 6 ).
Tau is a microtubule-associated protein that has been linked to multiple molecular processes, including synaptic plasticity, cell signaling, and regulation of axonal stability ( 7 ). There are six isoforms of tau expressed in the brain from alternative mRNA splicing of a single gene, MAPT . What separates the longer forms of tau from the shorter forms is the inclusion (or exclusion) of the 31 amino acids encoded in exon 10 at the carboxy-terminal end into three isoforms with four repeats (4R forms) and their exclusion into three isoforms with three repeats (3R forms) ( 8 ). The expression of tau is regulated throughout development, and in the adult brain, all six tau isoforms are present, with equal numbers of 3R and 4R forms. In Pick’s disease, we mainly find 3R forms, whereas 4R forms can be related to PSP, CBD, nfvPPA, and other pathologies such as globular glial tauopathy (GGT); mixed 3R-4R forms can be found in Alzheimer’s disease (AD) and chronic traumatic encephalopathy ( 9 ). In addition, the tau protein undergoes posttranslational changes, the most commonly described being phosphorylation, which can modify the affinity of tau for microtubules and lead to self-aggregation. There are 85 known putative phosphorylation sites ( 7 ), and tau posttranslational modifications characterize disease heterogeneity and stage in dementia ( 10 ).
FTLD-TDP is characterized by the accumulation of 43-kDa transactive response DNA binding protein (TDP-43), a multifunctional nucleic-acid-binding protein related to RNA metabolism to which other functions such as neurite outgrowth and axonal repair after injury have been attributed ( 11 ). TDP-43 loss from the nucleus leads to the up- and downregulation of more than 100 different proteins, including stathmin-2 ( 12 ). Upregulation of stathmin-2 is a proposed therapy for FTLD-TDP. In FTLD-TDP, abnormally phosphorylated and ubiquitinated versions of TDP-43 manifest with different morphology and are grouped according to the number of neuronal cytoplasmic inclusions, dystrophic neurites, and glial cytoplasmic inclusions into subtypes A, B, C, and D ( 13 ). Approximately 90% of svPPAs present TDP-43 type C, FTD-MND is almost exclusively related to type B, amyotrophic lateral sclerosis (ALS) is related to types B and D, and bvFTD and nfvPPA are related to types A and B ( 6 , 14 ).
The FUS protein (fused in sarcoma) is one of several FET proteins (also including EWS and TAF15 proteins) associated with FTLD and ALS. FUS, like TDP-43, regulates mRNA and, in addition to being associated with bvFTD and PPA, is also a cause of ALS and neuronal intermediate filament inclusion disease ( 6 ). Functions linked to alternative splicing, transcription, and RNA transport are attributed to FUS. By affecting splicing, FUS dysfunction could affect normal MAPT expression, leading to an increased 4R-Tau–3R-Tau ratio ( 15 ).
FTD is strongly heritable. A positive family history is found in 30%−50% of cases, and in 10%−27%, the inheritance is autosomal dominant ( 2 , 16 – 18 ). In comparison, in less than 1% of AD cases, the inheritance is autosomal dominant ( 19 ). Additionally, genetic causes are found in 1%−10% of sporadic bvFTD cases ( 20 ). Thus, genetics is of fundamental importance in the assessment of patients with FTD ( 21 ). Numerous genes are associated with FTD; however, the most commonly implicated genes are MAPT , GRN , and C9orf72 .
MAPT , located on chromosome 17, is the gene encoding for the tau protein, whose function has been described previously. There are more than 50 known mutations for this gene. These mutations account for 5%−20% of familial FTD cases but are rarely found in sporadic forms of FTD (0%−2%) ( 21 ). Disease onset with these MAPT mutations varies but is often before 60 years of age. These mutations usually cause a bvFTD phenotype, but parkinsonism is often prominent.
Also located on chromosome 17 adjacent to MAPT is the GRN gene encoding for progranulin. This protein functions as a multifunctional growth factor in development, wound repair, neuroinflammation, autophagy, and lysosomal function ( 21 , 22 ). More than 70 known GRN mutations lead to the generation of nonsense mRNA, which is subsequently eliminated by physiological surveillance mechanisms, leading to haploinsufficiency of the progranulin protein, which thus leads to FTLD-TDP pathology by an unclear mechanism ( 23 , 24 ).
In 2011, it was described for the first time that the six-nucleotide noncoding repeat (G4C2) in the first intron of the C9orf72 gene, located on the short arm of chromosome 9, could lead to FTD, ALS, and FTD-MND ( 25 , 26 ). Up to 24 G4C2 repeats have been described in healthy control groups. Although there is no universally established cutoff point, it is suspected that more than 30 expansions increase susceptibility to neurodegeneration ( 26 , 27 ). Although the mechanism of pathogenicity by which this nucleotide expansion leads to the development of FTD is not well defined, multiple hypotheses have been formulated, including haploinsufficiency of the homonymous protein; toxicity from the transcribed, expanded-repeat-containing RNA; up- and downregulation of numerous proteins, including stathmin-2; and toxic dipeptide repeat (DPR) proteins ( 21 ). Regardless of the pathological mechanism by which it produces the disease, the C9orf72 mutation is the leading cause of familial FTD (20%−30% of cases) and the leading known genetic cause of sporadic FTD (6%) ( 21 , 28 – 30 ). In addition, this expansion leads to the accumulation of DPRs that aggregate in the cerebellum and hippocampus ( 31 ) and TDP-43 type A and type B pathology ( 14 ). Clinically, this mutation can present as bvFTD, ALS, or both and is characterized by a shorter disease duration (6.4±4.9 years) relative to other genes such as MAPT or GRN ( 32 ). However, a group of carriers of this mutation may have extremely slow-evolving forms of the disease syndromically indistinguishable from bvFTD, categorized as “FTD phenocopies” ( 33 ). Moreover, between 10% and 50% of patients with this mutation may manifest psychotic symptoms (hallucinations, delusions, or both), which may lead to confusing this disease with psychiatric conditions such as schizophrenia, bipolar disorder, or obsessive-compulsive disorder ( 2 , 27 , 34 ).
There are recommendations for genetic testing for the three main bvFTD-related genes ( MAPT , GRN , and C9orf72 ) in patients with at least one affected first-degree relative. This recommendation extends to FTD or early-onset dementia relatives, but a history of ALS, Parkinson’s disease, or unexplained late-onset psychiatric disorders should also be considered ( 2 ). Also, because of its association with sporadic cases of FTD, one should look for C9orf72 mutations in cases of late-onset behavioral symptoms (even if they do not meet all criteria for bvFTD), and there are no neuroimaging abnormalities, as a diagnostic element ( 2 ). With a significant proportion of apparent sporadic cases that are due to unexpected mutations, several groups are moving toward genetic testing in all FTD cases, even without family history. This approach will become more routine when therapies become available.
Commonly, when mental health professionals explain the diagnosis of FTD to patients and their family members, they are often concerned about the heritability of the disease. Before obtaining genetic testing, the implications for the individual and their family unit should be discussed with a genetic counselor. Family members may also be interested in genetic testing. Genetic counseling has a cost and can have legal and, sometimes, ethical implications. Before testing a family member (or members), the ideal scenario is to have the affected gene identified and search for the mutation in those concerned. This is not always possible, for various reasons. For example, affected relatives may be deceased; the afflicted patient may refuse to be tested, and so forth. When the test for a single gene is negative, another gene may be responsible for the clinical syndrome ( 35 ). Even when the presence of a mutated gene is demonstrated, it is not possible to predict the exact age of onset, severity or type of symptoms, or the course of the disease ( 35 ). Also, there are numerous factors to consider when evaluating whether to perform genetic testing. For example, does the person understand what having the mutation implies, or will the genetic result affect the person’s life decisions? Additionally, it is important to consider what psychological impact this information can have and whether the patient is prepared for this information ( 36 ). These decisions also have legal and economic consequences, including the possibility that the result affects the patient’s health insurance coverage or the possibility that this information can be used by an employer to decide whether to hire the person. In the United States, in 2008, the Genetic Information Nondiscrimination Act, or GINA, was passed at the national level to prohibit information such as this from being used in the context of administrative decisions such as health insurance or employment decisions ( 37 ). However, many countries do not have similar legislation on this type of information, which could potentially expose mutation carriers to stigma and marginalization.
THE DIAGNOSTIC PROBLEM IN FTD
The diagnosis of bvFTD is particularly challenging because of the absence of molecular biomarkers except for imaging and, therefore, depends principally on clinical assessment ( 2 ). In addition, the symptomatic overlap with primary psychiatric disorders (PPDs) including major depressive disorder, bipolar disorder, schizophrenia, obsessive-compulsive disorder, autism spectrum disorders, and even personality disorders ( 38 ) means that PPDs often constitute the main differential diagnosis of bvFTD ( 39 ). Around 50% of patients with bvFTD have received a previous psychiatric diagnosis (most frequently, major depression), and the average diagnostic delay is up to 5–6 years from symptom onset ( 40 , 41 ).
BURDEN OF FTD
The years leading up to a patient’s bvFTD diagnosis are often the most stressful period in the lifetime of a spouse and other family members ( 42 ). Marital strife; financial chaos, or even ruin; and estrangement from friends and family are common and may create resentment toward the patient that persists even after a neurologic diagnosis has been made ( 42 ). Caregiver emotional responses to a bvFTD diagnosis are often mixed ( 42 ). The diagnosis creates enormous grief because of the prognosis: progression to death within 5–7 years ( 43 ). The socioeconomic burden of FTD is high. FTD is associated with substantial direct and indirect costs, diminished quality of life, and increased caregiver burden ( 44 ). The economic burden for FTD in the United States is approximately twice that reported for AD ( 44 ).
LEGAL ASPECTS
Brain disorders have long been considered as a cause of criminal behavior ( 45 ). This seems to be particularly true in the case of FTD, where such behaviors can be found in up to 50% of cases ( 46 , 47 ), up to five times more frequent than in patients with AD ( 48 ). One of the most prominent symptoms in FTD is behavioral disinhibition ( 3 ). These behaviors are often labeled as disinhibited because they break with social norms and frequently transgress legal boundaries ( 49 , 50 ). There are at least two forms of disinhibition ( 51 ): impulsivity, acts involving general rule violations that are related to an impairment of cognitive control mechanisms ( 49 ); and person-based disinhibition, where the behavior is more related to disturbed interpersonal interactions violating social tact and personal boundaries ( 52 ), in which case behaviors may emerge because of the compromise of cognitive systems related to semantic knowledge ( 53 ) or personal salience ( 54 ). This type of behavior has been labeled as “acquired sociopathy” ( 55 ). Because antisocial behavior in patients with FTD arises as a result of compromised functioning of brain structures responsible for directly or indirectly modulating behavior, these cases present a challenge in defining the degree of autonomy in their actions, this being particularly true for the early stages of the disease ( 56 ). Thus, this disease presents a challenge for the criminal justice system.
NEUROPSYCHIATRIC SYMPTOMS AND MANAGEMENT
FTD is a devastating, progressive neurodegenerative disease characterized by changes in personality, behavior, and language. In this sense, bvFTD is the variant that produces the most profound and limiting behavioral changes, compromising social functioning through symptoms such as behavioral disinhibition, apathy, and loss of empathy ( 3 ). In both forms of PPA, language impairment is the predominant feature, leading to significant limitations in social interactions and activities of daily living. Moreover, both svPPA and nfvPPA are not exempt from manifesting, throughout their evolution, behavioral symptoms that are similar to those observed in bvFTD that further compromise the functionality and quality of life of these patients and their caregivers ( 57 ). Additionally, there is evidence that symptoms such as apathy and disinhibition can manifest across the entire FTLD spectrum ( 58 ). This makes the management of neuropsychiatric symptoms a priority for clinicians. No disease-specific treatment interventions for FTD exist, there are only symptomatic treatments that are partially effective. Consequently, treatment largely remains supportive and involves a combination of nonpharmacological and pharmacological measures aimed at reducing the effect of distressing symptoms ( 59 ).
Nonpharmacological Interventions
Behavioral management..
Ikeda et al. ( 60 ) reported that troublesome behavioral symptoms were managed by reintroducing old hobbies and favorite games in six patients with FTD. They also reported that those methods were helpful for reducing social misconduct and disinhibition. They also reported that some patients who were treated with a behavioral therapy called “routinizing therapy”—in which stimulus-bound and stereotypic behaviors are replaced with appropriate behaviors—improved. This therapy was reported to help manage troublesome behaviors and contribute to a stable routine ( 61 ). The antecedent-behavior-consequence (ABC) model is a strategy often used in education for dementia caregivers, although there have been no studies of its effectiveness. The A, or antecedent, is the event or factors that initiate or contribute to the occurrence of the behavior. Antecedents are often called “triggers.” The B, or behavior, is the specific behavioral symptom. The C, or consequences, are all the reactions and responses of others after the behavior ( 62 ). In the case of compulsive activities, substitutions may have positive results. It consists of offering a squeeze ball to hold, instead of touching strangers, or offering a lollipop to diminish repetitive and compulsive vocalizations ( 63 , 64 ). Overall, behavioral management techniques that target disease-specific behaviors and preserved functions seem to be more effective than cognitive training in patients with FTD ( 65 ).
Environmental strategies.
As each patient faces different situations, it is difficult to conduct clinical studies of the environmental strategies. As such, most are mainly based on narrative and clinical experience, and no clinical studies that provide evidence on these environmental strategies exist ( 65 ). Environmental strategies are the least restrictive to the patient and focus on modifying the environment of the patient. Examples may include limiting access to credit cards, changing the family schedule to meet the preferences of the patient, or posting reminder notes and signs ( 62 ). It is recommended that patients keep the same daily routine and that objects or furniture are kept in the same position around them. In addition, to minimize disruptions, caregivers may change their schedule to accommodate a patient’s relatively harmless rituals, or a family may choose restaurants that the patient already knows ( 65 ). Reducing noise and stimulation, lessening clutter, turning off music, or simplifying social situations can help these patients to accurately focus on a designated task or response. Removing access to problematic items (e.g., credit cards, mail) or modifying public outings to reduce the opportunity for inappropriate interactions are examples of FTD-specific environmental manipulations ( 66 ). A supportive environment with normal lighting, moderate sound, a small number of people, and appropriate cueing were more likely to decrease behavioral symptoms in dementia ( 67 ). Exercise has been suggested to reduce behavioral symptoms ( 68 ), and aberrant motor behavior may respond to physical activity ( 69 ). Strategies for psychotic symptoms include environmental modifications such as removing mirrors or increasing lighting, which may reduce the propensity for misinterpretation ( 69 ).
Caregiver intervention.
Caregiver intervention should be the most effective treatment within the context of dementia ( 70 ). Behavioral changes rather than level of disability seem to be correlated with caregiver distress and burden in bvFTD ( 71 ). The ABC strategy has shown a reduction in behavioral symptoms and improved caregiver outcomes for behavior management ( 72 ). The key to reducing caregiver stress seems to lie in increasing their understanding of the symptoms and ways of dealing with challenging behaviors ( 70 ). Programs such as The Savvy Caregiver have shown similar results in promoting caregiver mastery regarding behavior management and reduced caregiver stress ( 73 – 75 ).
Speech therapy.
PPA is a debilitating disorder in which speech and language deteriorate as a result of neurodegenerative disease ( 76 ). Speech therapy is led by a language pathologist, who may offer a variety of interventions and compensatory strategies to patients with PPA ( 77 ). PPA interventions commonly tap strategies training individual word retrieval, trained scripts, and compensatory communication methods ( 78 ). A study by Henry et al. ( 76 ) included ten individuals with mild to moderate nonfluent-agrammatic variant PPA. They examined the immediate and long-term benefits of video-implemented script training for aphasia. They found that this treatment resulted in significant improvement in production of correct, intelligible scripted words for trained topics, a reduction in grammatical errors for trained topics, and an overall increase in intelligibility for trained as well as untrained topics at posttreatment. Follow-up testing revealed maintenance of gains for trained scripts up to 1 year posttreatment on the primary outcome measure. Performance on untrained scripts and standardized tests remained relatively stable during the follow-up period, indicating that treatment helped to stabilize speech and language despite disease progression ( 76 ).
Pharmacological Interventions
Pharmacological interventions should be implemented after a careful analysis of the case, ideally after nonpharmacological approaches have been exhausted, or the magnitude of the symptoms requires these interventions to be implemented promptly. In addition, possible reactions and adverse effects to these medications should be monitored, and the relevance of their indication should be re-evaluated periodically. Table 1 summarizes the pharmacological interventions along with the level of evidence and recommendation for them ( 79 ).
a For further details on the American Hospital Formulary Service Drug Information, see reference 79 . Level of evidence: 1=high strength or quality; 2=moderate strength or quality; 3=low strength or quality; 4=opinion/experience. Grade of recommendation: 1=recommended (accepted); 2=reasonable choice (accepted, with possible conditions); 3=not fully established (unclear risk or benefit, equivocal evidence, inadequate data or experience); 4=not recommended (unaccepted). L-DOPA=levodopa-3,4-dihydroxyphenylalanine.
TABLE 1. Level of evidence and strength of recommendation of frontotemporal dementia pharmacological treatments classified according to the American Hospital Formulary Service Drug Information a
Selective serotonin reuptake inhibitors (SSRIs).
FTD has been shown to have serotonergic network disruption on the basis of autopsy, neuroimaging, and CSF studies ( 80 , 81 ). Some studies suggest that SSRIs are effective in helping with various symptoms of FTD, including disinhibition, impulsivity, repetitive behaviors, and eating disorders ( 82 ), and they also suggest that the behavioral symptoms of FTD may improve after treatment with SSRIs ( 70 , 83 ).
One study concluded that long-term treatment with paroxetine may influence noncognitive aspects of FTD, with improvements in behavior and social conduct as evidenced by a reduction in aggressiveness, agitation, weeping and depressed mood, social conduct, eating problems, and sleep disorders ( 84 ). A small study with paroxetine at doses of 20 mg/day for 14 months showed improvement in scores on the Neuropsychiatric Inventory (NPI) and was well tolerated ( 85 ). However, another study conducted with paroxetine versus placebo with doses increasing up to 40 mg/day reported that there was no significant improvement and that, moreover, it could worsen the cognitive profile of patients ( 86 ). The discrepancies between these studies are attributed to a better response to and tolerability of paroxetine at lower doses (20 mg/day) ( 87 ); however, an alternative hypothesis is that the worse performance, especially in the cognitive domain, with higher doses (40 mg/day) is due to the anticholinergic effect of paroxetine ( 88 ).
A study by Herrmann et al. ( 89 ) reported that citalopram treatment was effective in treating behavioral symptoms, with significant decreases in NPI total score, disinhibition, irritability, and depression scores over 6 weeks. The significant improvement in Frontal Behavior Inventory scores suggested that citalopram was also effective in treating FTD-specific behaviors ( 89 ). A more recent randomized, double-blinded, placebo-controlled study of 12 patients with doses of citalopram at 30 mg/day showed improved disinhibition and related cognitive functions. This effect was attributed to the restoration of impaired serotonergic neurotransmission in bvFTD ( 90 ).
There is limited information on the use of sertraline in FTD; in one study, eight patients showed improvement in stereotyped movements and compulsive behavior with doses of sertraline between 50 and 100 mg over a period of 6 months ( 91 ). A case report of a 53-year-old patient with ALS-FTD and inappropriate sexual behavior reported symptomatic improvement with sertraline at doses of 100 mg/day ( 92 ).
A 12-week open-label study of 16 patients with FTD and behavioral symptoms with fluvoxamine at doses of 50–150 mg/day showed improvement in stereotypic behavior, eating behavior, and roaming behavior ( 93 ). One paper reported two cases of FTD with symptoms of stereotypic behavior and compulsive complaint of abdominal pain where the use of fluvoxamine improved both symptoms ( 94 ).
Trazodone has been effective in treating agitation and aggression in FTD and may also function as a sleep aid, if necessary ( 82 ). In one study, it was also found that trazodone can reduce the behavioral symptoms of FTD that were assessed by the NPI score, especially eating disorder, irritability, agitation, and depressive symptoms ( 95 ).
Second-generation antipsychotics.
Although off-label use of antipsychotics in FTD is common ( 88 ), there are a few studies that support the use of these drugs for the control of behavioral symptoms in this disease. Concern about the association between the use of antipsychotics in older adults and increased risk of stroke led the U.S. Food and Drug Administration (FDA) in 2005 to issue a boxed warning for the use of this medication in dementia-related psychosis. Despite this, guidelines for the management of behavioral symptoms in dementia, such as the American Psychiatric Association guideline ( 96 ) and the International Psychogeriatric Association guideline in 2018, recommend the use of second-generation antipsychotics for symptoms such as agitation and psychosis in dementia. Because of the limited number of studies performed, it is unclear whether it is possible to translate these indications to FTD.
There is evidence that there is a deficit in dopaminergic neurotransmission across the spectrum of FTLD ( 97 ). Mesolimbic and mesocortical dopaminergic pathway involvement may be related to the manifestation of behavioral and cognitive symptoms ( 97 ). However, dopaminergic involvement in the nigrostriatal pathway may be related to the high prevalence of extrapyramidal symptoms observed in the course of the disease, making these patients particularly sensitive to the use of antipsychotics ( 98 – 100 ). Thus, if these drugs are used for the control of behavioral symptoms, antipsychotics with a lower D2 blockade profile (i.e., those with a lower incidence of associated extrapyramidal symptoms) are of choice. Among these drugs, quetiapine could be of particular relevance ( 101 , 102 ). Quetiapine was observed in a case series to demonstrate improvements in agitation in three patients with FTD ( 103 ), but no difference in NPI scores was shown between baseline and quetiapine treatment in a separate double-blind, crossover study with dextroamphetamine and quetiapine involving eight patients with FTD showing behavioral symptoms ( 104 ).
Under the same logic, clozapine is an antipsychotic that could potentially be useful in FTD. Unfortunately, to date, there are no studies on its use in this population. However, a case report of a patient with a diagnosis of FTD, refractory psychosis, and frequent episodes of aggression describes significant clinical improvement after starting clozapine up to a dose of 400 mg/day. Aripiprazole, because of its effect as a partial D2 agonist, could also be presented as a drug with less adverse effects in FTD. However, there are currently only case reports of its clinical use. One report describes symptomatic improvement in a patient with frequent sexual remarks ( 105 ). Similarly, a case of FTD with sexual disinhibition reports symptomatic improvement within 2 weeks with aripiprazole at 18 mg/day ( 106 ). A study with 17 patients related the use of olanzapine to a substantial improvement in symptoms such as delirium, affective lability, wandering, and irritability ( 85 ). However, the use of olanzapine, because of its greater D2 blockade, may increase extrapyramidal symptoms, and it is associated with significant metabolic syndrome and higher mortality than quetiapine ( 88 ). The use of antipsychotics with greater dopaminergic blockade, such as risperidone or first-generation antipsychotics, is not recommended because of the high incidence of motor adverse effects and higher mortality in dementia ( 88 ).
Anticonvulsants.
The use of anticonvulsants as mood stabilizers is common in the treatment of various psychiatric disorders, with bipolar disorder being one of its main indications in psychiatry. However, the use of anticonvulsants in treating patients with FTD is limited. Although there are several case reports where clinical utility is attributed to them, studies demonstrating this are lacking. Three case reports describe improvement in cases of FTD associated with hyperorality with topiramate, two of them in patients with binge eating ( 107 , 108 ) and alcohol misuse ( 109 ). One case report recounts improvement in symptoms of hypersexuality with carbamazepine ( 110 ). Similarly, some case reports related the use of valproate to improvement of symptoms such as hypersexuality and agitation ( 103 , 111 ). However, there is still little evidence to support anticonvulsants as a therapy in FTD.
Carbonate lithium.
As with many anticonvulsants, the use of lithium carbonate as a mood stabilizer in the treatment of patients with bipolar disorder is widespread. However, the evidence for its use in treating patients with FTD is extremely limited. A case series published by Devanand et al. ( 112 ) describes three cases of patients diagnosed as having FTD, previously treated with a combination of antidepressants and antipsychotics, with symptoms such as hallucinations and agitation where the use of low doses of lithium (300–600 mg/day) led to clinical improvement. One of these cases reported lithemia values of 0.4 mmol/L for a dose of 450 mg/day of lithium, whereas another case of the same series recorded lithemia values of 0.8 mmol/L for a dose of 300 mg/day of lithium; similarly, another case recorded symptomatic improvement in agitation with a dose of 600 mg/day but developed tremor and sedation when increasing the dose to 1,200 mg/day. On the other hand, a case reported by Arciniegas ( 113 ) of FTD misdiagnosed as late-onset bipolar disorder reported marked impairment in cognitive, behavioral, and motor functions after reaching lithium levels considered therapeutic; these symptoms improved when lithium was discontinued. A phase 2 study is currently active with low doses of lithium versus placebo for the treatment of behavioral symptoms in patients with FTD ( ClinicalTrials.gov identifier: NCT02862210). However, because of the current limited clinical evidence, the narrow margin of therapeutic safety and the risk of developing lithium toxicity, the use of this molecule should be considered with extreme caution by closely monitoring plasma lithium levels and closely evaluating cognitive, behavioral, or motor changes.
Stimulants.
There is evidence that shows a deficit in dopaminergic transmission in FTD ( 97 ). Dopaminergic dysfunction has been related to the presence of extrapyramidal symptoms but also some behavioral symptoms such as agitation, disinhibition, and apathy ( 114 – 116 ). A small double-blind, placebo-controlled study in patients with bvFTD found that the use of methylphenidate, a dopamine and noradrenaline reuptake inhibitor, at doses of 40 mg was able to improve decision-making behavior of patients ( 117 ). A case report of a 72-year-old patient with FTD and symptoms of apathy, behavioral disinhibition, and irritability treated with 18-mg methylphenidate and 100-mg bupropion showed marked and sustained symptomatic improvement ( 118 ). A study in eight patients with FTD treated with dextroamphetamine versus quetiapine showed that the stimulant was effective in decreasing apathy and disinhibition on the NPI subscales ( 104 ). A limitation of these studies is that they were performed with small samples, so it is difficult to generalize these results. Moreover, the use of psychostimulants carries possible adverse effects that should be considered and monitored in case of their use.
In a study by Jesso et al. ( 119 ), the effects of a single dose of intranasal oxytocin on neuropsychiatric behaviors and emotion processing were evaluated in patients with bvFTD. They designed a randomized, double-blind, placebo-controlled crossover design that included 20 patients with this diagnosis who received a 24-IU dose of intranasal oxytocin or placebo and then completed the emotion recognition tasks. Caregivers completed validated behavior ratings at 8 hours and 1 week after drug administration. There was a significant improvement in NPI scores on the night of oxytocin administration compared with placebo and baseline scores. Oxytocin was also associated with reduced recognition of angry facial expressions by patients with bvFTD. Jesso et al. ( 119 ) concluded that oxytocin is a potentially promising new symptomatic treatment candidate for patients with bvFTD and that further studies were needed. A phase 2 double-blind, randomized, multicenter study is currently active to test the safety, tolerability, and effects of intranasal syntocinon (synthetic oxytocin) versus placebo on behavioral symptoms of FTD ( 120 ).
Pharmacological treatment of motor symptoms.
As described in the section on antipsychotics, it is common for FTD patients to manifest extrapyramidal symptoms during the course of the disease. Up to 70% of patients present symptoms of rigidity, gait dysfunction, or bradykinesia in their evolution ( 98 – 100 , 121 ). The evidence on the use of pharmacological treatment for motor symptoms in FTLD focuses on PSP and CBD. In these cases, the use of L-dopa provides mild and transient improvement ( 122 , 123 ). The use of dopaminergic agonists in FTD is often discouraged because of their incidence of dopaminergic dysregulation syndrome, which may worsen behavioral symptoms ( 88 , 124 ). Within the monoamine oxidase inhibitors, there are data on three case reports that describe improvement in behavioral symptoms ( 125 ).
Memantine is, together with cholinesterase inhibitors, one of the molecules approved by the FDA for the specific treatment of AD. It is postulated that its mechanism of action as a noncompetitive inhibitor of N -methyl- d -aspartate (NMDA) receptors could decrease glutamate-mediated cytotoxicity and thus slow the progression of the disease. Although the neuropathological changes in FTD are different from those in AD, it was believed that its protective effect on neuronal injury could be beneficial in FTD. Likewise, evidence that memantine could improve behavioral symptoms in AD made this molecule a target to explore in FTD. However, two separate double-blind, placebo-controlled studies found that there was no significant improvement between the placebo and memantine groups and that it may even worsen cognition ( 126 , 127 ).
Cholinesterase inhibitors.
Unlike AD, in FTLD, the cholinergic system is relatively preserved ( 97 ). Despite this, approximately 40% of FTD patients receive treatment with cholinesterase inhibitors ( 128 ). Numerous studies have attempted to study the therapeutic efficacy of cholinesterase inhibitors in FTD. Not only have these studies failed to demonstrate a benefit from the use of these drugs, but, on the contrary, there is also evidence that they may actually worsen cognitive and behavioral performance ( 59 , 82 , 87 , 129 ).
CURRENT CLINICAL TRIALS AND BIOMARKERS
Molecule-based therapies are being considered for the genetic forms of FTD and to treat the symptoms of the disease ( Table 2 ). For each of the major genetic subtypes, MAPT , GRN , and C9orf72 , different approaches will be needed. Several advances have made it possible to consider such efforts. First, the discovery of powerful biomarkers such as the neurofilament light-chain protein (NfL) will make it possible to follow progression in a clinical trial, because NfL begins to rise during the transition from asymptomatic to mildly symptomatic FTD ( 130 ). Similarly, structural imaging can detect significant changes in atrophy over 6 months, making it likely that the magnetic resonance imaging (MRI) can also be used as a surrogate marker ( 131 ).
a ALS=amyotrophic lateral sclerosis; bvFTD=behavioral variant FTD; CBD=corticobasal degeneration; FTD=frontotemporal dementia; lvPPA=logopenic variant primary progressive aphasia; nfvPPA=nonfluent variant primary progressive aphasia; PKR=protein kinase R; PSP=progressive supranuclear palsy.
TABLE 2. Active clinical trials in frontotemporal lobar degeneration (FTLD) a
For MAPT carriers, the major emphasis of clinical trials will be to lower tau either by decreasing its production or by increasing its clearance. There is extensive evidence that lowering tau will ameliorate symptoms in animal models of AD and FTD ( 132 , 133 ). Further, in humans, antibodies against tau have been demonstrated to reach the brain and bring tau into the plasma ( 134 ), but clinical trials using antibodies for both AD and FTD have been disappointing. These failures have probably occurred because of relatively low levels of the antibody that cross the blood-brain barrier. Therefore, technologies such as antisense oligonucleotides and CRISPR could lower tau in a highly effective manner. If MAPT carriers treated with effective tau-lowering therapies show slowing or progression or even halting of the disease, these approaches will next be used to treat other tau-related forms of FTD. Other efforts are focused on increasing the degradation of tau in the lysosome or the proteosome ( 135 ).
With GRN , different mechanisms and different approaches are being considered. GRN mutation carriers show markedly reduced brain and blood levels of progranulin, suggesting a haploinsufficiency mechanism with the deficiency of progranulin production on one chromosome sufficient to cause FTD. Many strategies are being considered to increase brain progranulin, with many focused on better ways to deliver progranulin into the brain. Arrant and colleagues found, when using an AAV vector (AAV- Grn ) to deliver progranulin in Grn −/− mice, that lysosomal dysfunction and microglial pathology were both ameliorated ( 136 ). It is likely that, in the coming year, AAV transplantation studies will begin with GRN gene carriers, and others delivery systems are also being considered.
Finally, C9orf72 mutations produce a long hexanucleotide repeat that is already the target for gene carriers with ALS, and therapies for FTD are being considered. As with all these gene-related therapies, multiple questions will need to be answered regarding delivery of the drug to the brain; timing of the therapy (presymptomatic versus symptomatic); the reliability of biomarkers; and, most importantly, efficacy. A new chapter in therapy for FTD and related conditions is beginning. Once the genetic forms of the disease have been effectively treated, new approaches to the sporadic form of the disease are likely to emerge.
CONCLUSIONS
FTD is frequently misdiagnosed, and when the diagnosis occurs, it often comes late in the course of the illness or is missed. Recognition that behavioral changes represent a neurodegenerative condition is difficult, leading clinicians to diagnose a primary psychiatric disorder. Also, diagnostic tools such as blood biomarkers or neuroimaging can be difficult to access, particularly in low- and middle-income communities. Another barrier to its identification is the lack of knowledge and training for health care providers about FTD.
FTD treatment has been limited to the management of neuropsychiatric symptoms, these being the most prominent feature of the disease. Therapeutic strategies have focused on nonpharmacological interventions such as behavioral and environmental manipulation, caregiver interventions, and speech therapy for the language variants of FTD. Also, pharmacological treatment has also been used to treat these symptoms, with variable but sometimes positive results. With the advance of knowledge regarding the pathophysiology of FTD, pharmacological interventions such as the use of SSRIs, trazodone, or second-generation antipsychotics have a solid scientific basis for the treatment of FTD.
In the past 10 years, thanks to new techniques in neuroimaging, genetics, and biomarker analysis, much has been discovered about the phenomena underlying frontotemporal lobar degeneration. This has allowed the design of new molecule-based therapies that are still in the early stages of research but show promising results.
This work was supported by the National Institute on Aging: grant P01-AG019724 to the Frontotemporal Dementia Program of the University of California, San Francisco and grant P30-AG062422 to the Alzheimer’s Disease Research Center.
The authors report no financial relationships with commercial interests.
Dr. Magrath Guimet and Dr. Zapata-Restrepo contributed equally to this study.
The authors thank the Global Brain Health Institute, the Memory and Aging Center, and the Alzheimer Disease Research Center, University of California, San Francisco.
1 Ahmed RM, Hodges JR, Piguet O : Behavioural variant frontotemporal dementia: recent advances in the diagnosis and understanding of the disorder ; in Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, Vol. 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 Crossref , Google Scholar
2 Ducharme S, Dols A, Laforce R, et al. : Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders . Brain 2020 ; 143:1632–1650 Crossref , Medline , Google Scholar
3 Rascovsky K, Hodges JR, Knopman D, et al. : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia . Brain 2011 ; 134:2456–2477 Crossref , Medline , Google Scholar
4 Gorno-Tempini ML, Hillis AE, Weintraub S, et al. : Classification of primary progressive aphasia and its variants . Neurology 2011 ; 76:1006–1014 Crossref , Medline , Google Scholar
5 Chan D, Anderson V, Pijnenburg Y, et al. : The clinical profile of right temporal lobe atrophy . Brain 2009 ; 132:1287–1298 Crossref , Medline , Google Scholar
6 Elahi FM, Miller BL : A clinicopathological approach to the diagnosis of dementia . Nat Rev Neurol 2017 ; 13:457–476 Crossref , Medline , Google Scholar
7 Guo T, Noble W, Hanger DP : Roles of tau protein in health and disease . Acta Neuropathol 2017 ; 133:665–704 Crossref , Medline , Google Scholar
8 Goedert M, Spillantini MG, Falcon B, et al. : Tau protein and frontotemporal dementias ; in Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, Vol. 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 Crossref , Google Scholar
9 Cherry JD, Esnault CD, Baucom ZH, et al. : Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer’s disease . Acta Neuropathol Commun 2021 ; 9:86 Crossref , Medline , Google Scholar
10 Wesseling H, Mair W, Kumar M, et al. : Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease . Cell 2020 ; 183:1699–1713.e13 Crossref , Medline , Google Scholar
11 Klim JR, Pintacuda G, Nash LA, et al. : Connecting TDP-43 pathology with neuropathy . Trends Neurosci 2021 ; 44:424–440 Crossref , Medline , Google Scholar
12 Melamed Z, López-Erauskin J, Baughn MW, et al. : Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration . Nat Neurosci 2019 ; 22:180–190 Crossref , Medline , Google Scholar
13 Mackenzie IRA, Neumann M, Baborie A, et al. : A harmonized classification system for FTLD-TDP pathology . Acta Neuropathol 2011 ; 122:111–113 Crossref , Medline , Google Scholar
14 Neumann M, Lee EB, Mackenzie IR : Frontotemporal lobar degeneration TDP-43-immunoreactive pathological subtypes: clinical and mechanistic significance ; in Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, Vol. 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 Crossref , Google Scholar
15 Ishigaki S, Sobue G : Importance of functional loss of FUS in FTLD/ALS . Front Mol Biosci 2018 ; 5:44 Crossref , Medline , Google Scholar
16 Goldman JS, Farmer JM, Wood EM, et al. : Comparison of family histories in FTLD subtypes and related tauopathies . Neurology 2005 ; 65:1817–1819 Crossref , Medline , Google Scholar
17 Seelaar H, Kamphorst W, Rosso SM, et al. : Distinct genetic forms of frontotemporal dementia . Neurology 2008 ; 71:1220–1226 Crossref , Medline , Google Scholar
18 Rohrer JD, Guerreiro R, Vandrovcova J, et al. : The heritability and genetics of frontotemporal lobar degeneration . Neurology 2009 ; 73:1451–1456 Crossref , Medline , Google Scholar
19 Cacace R, Sleegers K, Van Broeckhoven C : Molecular genetics of early-onset Alzheimer’s disease revisited . Alzheimers Dement 2016 ; 12:733–748 Crossref , Medline , Google Scholar
20 Rademakers R, Neumann M, Mackenzie IR : Advances in understanding the molecular basis of frontotemporal dementia . Nat Rev Neurol 2012 ; 8:423–434 Crossref , Medline , Google Scholar
21 Sirkis DW, Geier EG, Bonham LW, et al. : Recent advances in the genetics of frontotemporal dementia . Curr Genet Med Rep 2019 ; 7:41–52 Crossref , Medline , Google Scholar
22 Kao AW, McKay A, Singh PP, et al. : Progranulin, lysosomal regulation and neurodegenerative disease . Nat Rev Neurosci 2017 ; 18:325–333 Crossref , Medline , Google Scholar
23 Cruts M, Gijselinck I, van der Zee J, et al. : Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21 . Nature 2006 ; 442:920–924 Crossref , Medline , Google Scholar
24 Baker M, Mackenzie IR, Pickering-Brown SM, et al. : Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17 . Nature 2006 ; 442:916–919 Crossref , Medline , Google Scholar
25 Renton AE, Majounie E, Waite A, et al. : A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD . Neuron 2011 ; 72:257–268 Crossref , Medline , Google Scholar
26 DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. : Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS . Neuron 2011 ; 72:245–256 Crossref , Medline , Google Scholar
27 Saracino D, Le Ber I : Clinical update on C9orf72: frontotemporal dementia, amyotrophic lateral sclerosis, and beyond ; in Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, vol. 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 Crossref , Google Scholar
28 Van Mossevelde S, Engelborghs S, van der Zee J, et al. : Genotype-phenotype links in frontotemporal lobar degeneration . Nat Rev Neurol 2018 ; 14:363–378 Crossref , Medline , Google Scholar
29 Deleon J, Miller BL : Frontotemporal dementia . Handb Clin Neurol 2018 ; 148:409–430 Crossref , Google Scholar
30 Pottier C, Ravenscroft TA, Sanchez-Contreras M, et al. : Genetics of FTLD: overview and what else we can expect from genetic studies . J Neurochem 2016 ; 138(Suppl 1):32–53 Crossref , Medline , Google Scholar
31 Mori K, Weng S-M, Arzberger T, et al. : The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS . Science 2013 ; 339:1335–1338 Crossref , Medline , Google Scholar
32 Moore KM, Nicholas J, Grossman M, et al. : Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study . Lancet Neurol 2020 ; 19:145–156 Crossref , Medline , Google Scholar
33 Khan BK, Yokoyama JS, Takada LT, et al. : Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion . J Neurol Neurosurg Psychiatry 2012 ; 83:358–364 Crossref , Medline , Google Scholar
34 Devenney EM, Ahmed RM, Halliday G, et al. : Psychiatric disorders in C9orf72 kindreds: study of 1,414 family members . Neurology 2018 ; 91:e1498–e1507 Crossref , Medline , Google Scholar
35 Roberts JS, Patterson AK, Uhlmann WR : Genetic testing for neurodegenerative diseases: ethical and health communication challenges . Neurobiol Dis 2020 ; 141:104871 Crossref , Medline , Google Scholar
36 Roberts JS, Uhlmann WR : Genetic susceptibility testing for neurodegenerative diseases: ethical and practice issues . Prog Neurobiol 2013 ; 110:89–101 Crossref , Medline , Google Scholar
37 Suter SM : GINA at 10 years: the battle over ‘genetic information’ continues in court . J Law Biosci 2019 ; 5:495–526 Crossref , Medline , Google Scholar
38 Ducharme S, Price BH, Larvie M, et al. : Clinical approach to the differential diagnosis between behavioral variant frontotemporal dementia and primary psychiatric disorders . Am J Psychiatry 2015 ; 172:827–837 Crossref , Medline , Google Scholar
39 Krudop WA, Dols A, Kerssens CJ, et al. : The pitfall of behavioral variant frontotemporal dementia mimics despite multidisciplinary application of the FTDC criteria . J Alzheimers Dis . 2017 ; 60(3):959–975. Crossref , Google Scholar
40 Woolley JD, Khan BK, Murthy NK, et al. : The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease . J Clin Psychiatry 2011 ; 72:126–133 Crossref , Medline , Google Scholar
41 Ducharme S, Bajestan S, Dickerson BC, et al. : Psychiatric presentations of C9orf72 mutation: what are the diagnostic implications for clinicians? J Neuropsychiatry Clin Neurosci 2017 ; 29:195–205 Link , Google Scholar
42 Seeley WW : Behavioral variant frontotemporal dementia . Continuum (Minneap Minn) 2019 ; 25:76–100 Medline , Google Scholar
43 Roberson ED, Hesse JH, Rose KD, et al. : Frontotemporal dementia progresses to death faster than Alzheimer disease . Neurology 2005 ; 65:719–725 Crossref , Medline , Google Scholar
44 Galvin JE, Howard DH, Denny SS, et al. : The social and economic burden of frontotemporal degeneration . Neurology 2017 ; 89:2049–2056 Crossref , Medline , Google Scholar
45 Mendez MF, Shapira JS, Saul RE : The spectrum of sociopathy in dementia . J Neuropsychiatry Clin Neurosci 2011 ; 23:132–140 Link , Google Scholar
46 Diehl-Schmid J, Perneczky R, Koch J, et al. : Guilty by suspicion? Criminal behavior in frontotemporal lobar degeneration . Cogn Behav Neurol 2013 ; 26:73–77 Crossref , Medline , Google Scholar
47 Haußmann R, Krug C, Noppes F, et al. : Criminal behavior in frontotemporal dementia and Alzheimer’s disease [in German]. Der Nervenarzt 2021 . https://link.springer.com/content/pdf/10.1007/s00115-021-01070-8.pdf Google Scholar
48 Shinagawa S, Shigenobu K, Tagai K, et al. : Violation of laws in frontotemporal dementia: a multicenter study in Japan . J Alzheimers Dis 2017 ; 57:1221–1227 Crossref , Medline , Google Scholar
49 Huey ED : A critical review of behavioral and emotional disinhibition . J Nerv Ment Dis 2020 ; 208:344–351 Crossref , Medline , Google Scholar
50 Peet BT, Castro-Suarez S, Miller BL : The neuropsychiatric features of behavioral variant frontotemporal dementia ; in Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, vol 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 Crossref , Google Scholar
51 Magrath Guimet N, Miller BL, Allegri RF, et al. : What do we mean by behavioral disinhibition in frontotemporal dementia? Front Neurol 2021 ; 12:707799 Crossref , Medline , Google Scholar
52 Paholpak P, Carr AR, Barsuglia JP, et al. : Person-based versus generalized impulsivity disinhibition in frontotemporal dementia and Alzheimer disease . J Geriatr Psychiatry Neurol 2016 ; 29:344–351 Crossref , Medline , Google Scholar
53 Benhamou E, Marshall CR, Russell LL, et al. : The neurophysiological architecture of semantic dementia: spectral dynamic causal modelling of a neurodegenerative proteinopathy . Sci Rep 2020 ; 10:16321 Crossref , Medline , Google Scholar
54 Rankin KP : Brain networks supporting social cognition in dementia . Curr Behav Neurosci Rep 2020 ; 7:203–211 Crossref , Google Scholar
55 Mendez MF : What frontotemporal dementia reveals about the neurobiological basis of morality . Med Hypotheses 2006 ; 67:411–418 Crossref , Medline , Google Scholar
56 Dubljević V : The principle of autonomy and behavioural variant frontotemporal dementia . J Bioeth Inq 2020 ; 17:271–282 Crossref , Medline , Google Scholar
57 Karageorgiou E, Miller BL : Frontotemporal lobar degeneration: a clinical approach . Semin Neurol 2014 ; 34:189–201 Crossref , Medline , Google Scholar
58 Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, et al. : Apathy and impulsivity in frontotemporal lobar degeneration syndromes . Brain 2017 ; 140:1792–1807 Crossref , Medline , Google Scholar
59 Boxer AL, Boeve BF : Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies . Alzheimer Dis Assoc Disord 2007 ; 21:S79–S87 Crossref , Medline , Google Scholar
60 Ikeda M, Tanabe H, Horino T, et al. : [Care for patients with Pick’s disease—by using their preserved procedural memory]. Seishin Shinkeigaku Zasshi 1995 ; 97:179–192 Medline , Google Scholar
61 Tanabe H, Ikeda M, Komori K : Behavioral symptomatology and care of patients with frontotemporal lobe degeneration—based on the aspects of the phylogenetic and ontogenetic processes . Dement Geriatr Cogn Disord 1999 ; 10(Suppl 1):50–54 Crossref , Medline , Google Scholar
62 Merrilees J : A model for management of behavioral symptoms in frontotemporal lobar degeneration . Alzheimer Dis Assoc Disord 2007 ; 21:S64–S69 Crossref , Medline , Google Scholar
63 Fick WF, van der Borgh JP, Jansen S, et al. : The effect of a lollipop on vocally disruptive behavior in a patient with frontotemporal dementia: a case-study . Int Psychogeriatr 2014 ; 26:2023–2026 Crossref , Medline , Google Scholar
64 Lavretsky H : Neuropsychiatric symptoms in Alzheimer disease and related disorders: why do treatments work in clinical practice but not in the randomized trials? Am J Geriatr Psychiatry 2008 ; 16:523–527 Crossref , Medline , Google Scholar
65 Shinagawa S, Nakajima S, Plitman E, et al. : Non-pharmacological management for patients with frontotemporal dementia: a systematic review . J Alzheimers Dis 2015 ; 45:283–293 Crossref , Medline , Google Scholar
66 Hodgson NA, Gitlin LN, Winter L, et al. : Undiagnosed illness and neuropsychiatric behaviors in community residing older adults with dementia . Alzheimer Dis Assoc Disord 2011 ; 25:109–115 Crossref , Medline , Google Scholar
67 Trahan MA, Kuo J, Carlson MC, et al. : A systematic review of strategies to foster activity engagement in persons with dementia . Health Educ Behav 2014 ; 41(Suppl):70S–83S Crossref , Medline , Google Scholar
68 Forbes D, Blake CM, Thiessen EJ, et al. : Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia . Cochrane Database Syst Rev 2014 ; 26:CD003946 Google Scholar
69 Livingston G, Johnston K, Katona C, et al. : Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia . Am J Psychiatry 2005 ; 162:1996–2021 Crossref , Medline , Google Scholar
70 Piguet O, Hornberger M, Mioshi E, et al. : Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management . Lancet Neurol 2011 ; 10:162–172 Crossref , Medline , Google Scholar
71 Boutoleau-Bretonnière C, Vercelletto M, Volteau C, et al. : Zarit Burden Inventory and activities of daily living in the behavioral variant of frontotemporal dementia . Dement Geriatr Cogn Disord 2008 ; 25:272–277 Crossref , Medline , Google Scholar
72 Gitlin LN, Winter L, Dennis MP, et al. : A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial . JAMA 2010 ; 304:983–991 Crossref , Medline , Google Scholar
73 Kally Z, Cote SD, Gonzalez J, et al. : The Savvy Caregiver program: impact of an evidence-based intervention on the well-being of ethnically diverse caregivers . J Gerontol Soc Work 2014 ; 57:681–693 Crossref , Medline , Google Scholar
74 Samia LW, Aboueissa A-M, Halloran J, et al. : The Maine Savvy Caregiver Project: translating an evidence-based dementia family caregiver program within the RE-AIM framework . J Gerontol Soc Work 2014 ; 57:640–661 Crossref , Medline , Google Scholar
75 Hepburn K, Lewis M, Tornatore J, et al. : The Savvy Caregiver program: the demonstrated effectiveness of a transportable dementia caregiver psychoeducation program . J Gerontol Nurs 2007 ; 33:30–36 Crossref , Medline , Google Scholar
76 Henry ML, Hubbard HI, Grasso SM, et al. : Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia . Brain 2018 ; 141:1799–1814 Crossref , Medline , Google Scholar
77 Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, Vol 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 Crossref , Google Scholar
78 Volkmer A, Rogalski E, Henry M, et al. : Speech and language therapy approaches to managing primary progressive aphasia . Pract Neurol 2020 ; 20:154–161 Crossref , Medline , Google Scholar
79 AHFS Drug Information 2020 . Bethesda, Md., American Society of Health-System Pharmacists, 2020 Google Scholar
80 Franceschi M, Anchisi D, Pelati O, et al. : Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration . Ann Neurol 2005 ; 57:216–225 Crossref , Medline , Google Scholar
81 Sparks DL, Markesbery WR : Altered serotonergic and cholinergic synaptic markers in Pick’s disease . Arch Neurol 1991 ; 48:796–799 Crossref , Medline , Google Scholar
82 Pressman PS, Miller BL : Diagnosis and management of behavioral variant frontotemporal dementia . Biol Psychiatry 2014 ; 75:574–581 Crossref , Medline , Google Scholar
83 Swartz JR, Miller BL, Lesser IM, et al. : Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors . J Clin Psychiatry 1997 ; 58:212–216 Crossref , Medline , Google Scholar
84 Nardell M, Tampi RR : Pharmacological treatments for frontotemporal dementias: a systematic review of randomized controlled trials . Am J Alzheimers Dis Other Demen 2014 ; 29:123–132 Crossref , Medline , Google Scholar
85 Moretti R, Torre P, Antonello RM, et al. : Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients . Am J Alzheimers Dis Other Demen 2003 ; 18:205–214 Crossref , Medline , Google Scholar
86 Deakin JB, Rahman S, Nestor PJ, et al. : Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial . Psychopharmacology (Berl) 2004 ; 172:400–408 Crossref , Medline , Google Scholar
87 Young JJ, Lavakumar M, Tampi D, et al. : Frontotemporal dementia: latest evidence and clinical implications . Ther Adv Psychopharmacol 2018 ; 8:33–48 Crossref , Medline , Google Scholar
88 Ljubenkov PA, Boxer AL : FTLD treatment: current practice and future possibilities ; in Frontotemporal Dementias: Emerging Milestones of the 21st Century (Advances in Experimental Medicine and Biology, Vol 1281) . Edited by Ghetti B, Buratti E, Boeve B, et al. . Cham, Switzerland, Springer International Publishing, 2021 , pp. 297–310 Crossref , Google Scholar
89 Herrmann N, Black SE, Chow T, et al. : Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia . Am J Geriatr Psychiatry 2012 ; 20:789–797 Crossref , Medline , Google Scholar
90 Hughes LE, Rittman T, Regenthal R, et al. : Improving response inhibition systems in frontotemporal dementia with citalopram . Brain 2015 ; 138:1961–1975 Crossref , Medline , Google Scholar
91 Mendez MF, Shapira JS, Miller BL : Stereotypical movements and frontotemporal dementia . Mov Disord 2005 ; 20:742–745 Crossref , Medline , Google Scholar
92 Anneser JMH, Jox RJ, Borasio GD : Inappropriate sexual behaviour in a case of ALS and FTD: successful treatment with sertraline . Amyotroph Lateral Scler 2007 ; 8:189–190 Crossref , Medline , Google Scholar
93 Ikeda M, Shigenobu K, Fukuhara R, et al. : Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients . Dement Geriatr Cogn Disord 2004 ; 17:117–121 Crossref , Medline , Google Scholar
94 Ishikawa H, Shimomura T, Shimizu T : Stereotyped behaviors and compulsive complaints of pain improved by fluvoxamine in two cases of frontotemporal dementia [in Japanese]. Seishin Shinkeigaku Zasshi 2006 ; 108:1029–1035 Medline , Google Scholar
95 Lebert F, Stekke W, Hasenbroekx C, et al. : Frontotemporal dementia: a randomised, controlled trial with trazodone . Dement Geriatr Cogn Disord 2004 ; 17:355–359 Crossref , Medline , Google Scholar
96 Reus VI, Fochtmann LJ, Eyler AE, et al. : The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia . Am J Psychiatry 2016 ; 173:543–546 Crossref , Medline , Google Scholar
97 Murley AG, Rowe JB : Neurotransmitter deficits from frontotemporal lobar degeneration . Brain 2018 ; 141:1263–1285 Crossref , Medline , Google Scholar
98 Rinne JO, Laine M, Kaasinen V, et al. : Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia . Neurology 2002 ; 58:1489–1493 Crossref , Medline , Google Scholar
99 Padovani A, Agosti C, Premi E, et al. : Extrapyramidal symptoms in frontotemporal dementia: prevalence and clinical correlations . Neurosci Lett 2007 ; 422:39–42 Crossref , Medline , Google Scholar
100 Kertesz A, McMonagle P, Jesso S : Extrapyramidal syndromes in frontotemporal degeneration . J Mol Neurosci 2011 ; 45:336–342 Crossref , Medline , Google Scholar
101 Kurlan R, Cummings J, Raman R, et al. : Quetiapine for agitation or psychosis in patients with dementia and parkinsonism . Neurology 2007 ; 68:1356–1363 Crossref , Medline , Google Scholar
102 Weiden PJ : EPS profiles: the atypical antipsychotics are not all the same . J Psychiatr Pract 2007 ; 13:13–24 Crossref , Medline , Google Scholar
103 Chow TW, Mendez MF : Goals in symptomatic pharmacologic management of frontotemporal lobar degeneration . Am J Alzheimers Dis Other Demen 2002 ; 17:267–272 Crossref , Medline , Google Scholar
104 Huey ED, Garcia C, Wassermann EM, et al. : Stimulant treatment of frontotemporal dementia in 8 patients . J Clin Psychiatry 2008 ; 69:1981–1982 Crossref , Medline , Google Scholar
105 Reeves RR, Perry CL : Aripiprazole for sexually inappropriate vocalizations in frontotemporal dementia . J Clin Psychopharmacol 2013 ; 33:145–146 Crossref , Medline , Google Scholar
106 Nomoto H, Matsubara Y, Ichimiya Y, et al. : A case of frontotemporal dementia with sexual disinhibition controlled by aripiprazole . Psychogeriatrics 2017 ; 17:509–510 Crossref , Medline , Google Scholar
107 Singam C, Walterfang M, Mocellin R, et al. : Topiramate for abnormal eating behaviour in frontotemporal dementia . Behav Neurol 2013 ; 27:285–286 Crossref , Medline , Google Scholar
108 Shinagawa S, Tsuno N, Nakayama K : Managing abnormal eating behaviours in frontotemporal lobar degeneration patients with topiramate . Psychogeriatrics 2013 ; 13:58–61 Crossref , Medline , Google Scholar
109 Cruz M, Marinho V, Fontenelle LF, et al. : Topiramate may modulate alcohol abuse but not other compulsive behaviors in frontotemporal dementia: case report . Cogn Behav Neurol 2008 ; 21:104–106 Crossref , Medline , Google Scholar
110 Poetter CE, Stewart JT : Treatment of indiscriminate, inappropriate sexual behavior in frontotemporal dementia with carbamazepine . J Clin Psychopharmacol 2012 ; 32:137–138 Crossref , Medline , Google Scholar
111 Gálvez-Andres A, Blasco-Fontecilla H, González-Parra S, et al. : Secondary bipolar disorder and Diogenes syndrome in frontotemporal dementia: behavioral improvement with quetiapine and sodium valproate . J Clin Psychopharmacol 2007 ; 27:722–723 Crossref , Medline , Google Scholar
112 Devanand DP, Pelton GH, D’Antonio K, et al. : Low-dose lithium treatment for agitation and psychosis in Alzheimer disease and frontotemporal dementia: a case series . Alzheimer Dis Assoc Disord 2017 ; 31:73–75 Crossref , Medline , Google Scholar
113 Arciniegas DB : New-onset bipolar disorder in late life: a case of mistaken identity . Am J Psychiatry 2006 ; 163:198–203 Crossref , Medline , Google Scholar
114 Engelborghs S, Vloeberghs E, Maertens K, et al. : Evidence for an association between the CSF HVA:5-HIAA ratio and aggressiveness in frontotemporal dementia but not in Alzheimer’s disease . J Neurol Neurosurg Psychiatry 2004 ; 75:1080 Medline , Google Scholar
115 Zamboni G, Huey ED, Krueger F, et al. : Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates . Neurology 2008 ; 71:736–742 Crossref , Medline , Google Scholar
116 Ducharme S, Price BH, Dickerson BC : Apathy: a neurocircuitry model based on frontotemporal dementia . J Neurol Neurosurg Psychiatry 2018 ; 89:389–396 Crossref , Medline , Google Scholar
117 Rahman S, Robbins TW, Hodges JR, et al. : Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia . Neuropsychopharmacology 2006 ; 31:651–658 Crossref , Medline , Google Scholar
118 Goforth HW, Konopka L, Primeau M, et al. : Quantitative electroencephalography in frontotemporal dementia with methylphenidate response: a case study . Clin EEG Neurosci 2004 ; 35:108–111 Crossref , Medline , Google Scholar
119 Jesso S, Morlog D, Ross S, et al. : The effects of oxytocin on social cognition and behaviour in frontotemporal dementia . Brain 2011 ; 134:2493–2501 Crossref , Medline , Google Scholar
120 National Library of Medicine : Intranasal Oxytocin for Frontotemporal Dementia (FOXY) [ClinicalTrials.gov: NCT03260920] . Bethesda, Md, National Library of Medicine, 2021 . https://clinicaltrials.gov/ct2/show/NCT03260920 Google Scholar
121 Gil-Navarro S, Lomeña F, Cot A, et al. : Decreased striatal dopamine transporter uptake in the non-fluent/agrammatic variant of primary progressive aphasia . Eur J Neurol 2013 ; 20:1459–e126 Medline , Google Scholar
122 Litvan I, Grimes DA, Lang AE, et al. : Clinical features differentiating patients with postmortem confirmed progressive supranuclear palsy and corticobasal degeneration . J Neurol 1999 ; 246(Suppl 2):II1–II5 Crossref , Medline , Google Scholar
123 van Balken I, Litvan I : Current and future treatments in progressive supranuclear palsy . Curr Treat Options Neurol 2006 ; 8:211–223 Crossref , Medline , Google Scholar
124 O’Sullivan SS, Evans AH, Lees AJ : Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management . CNS Drugs 2009 ; 23:157–170 Crossref , Medline , Google Scholar
125 Moretti R, Torre P, Antonello RM, et al. : Effects of selegiline on fronto-temporal dementia: a neuropsychological evaluation . Int J Geriatr Psychiatry 2002 ; 17:391–392 Crossref , Medline , Google Scholar
126 Vercelletto M, Boutoleau-Bretonnière C, Volteau C, et al. : Memantine in behavioral variant frontotemporal dementia: negative results . J Alzheimers Dis 2011 ; 23:749–759 Crossref , Medline , Google Scholar
127 Boxer AL, Knopman DS, Kaufer DI, et al. : Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial . Lancet Neurol 2013 ; 12:149–156 Crossref , Medline , Google Scholar
128 Bei Hu, Ross L, Neuhaus J, et al. : Off-label medication use in frontotemporal dementia . Am J Alzheimers Dis Other Demen 2010 ; 25:128–133 Crossref , Medline , Google Scholar
129 Mendez MF, Shapira JS, McMurtray A, et al. : Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia . Am J Geriatr Psychiatry 2007 ; 15:84–87 Crossref , Medline , Google Scholar
130 Rojas JC, Wang P, Staffaroni AM, et al. : Plasma neurofilament light for prediction of disease progression in familial frontotemporal lobar degeneration . Neurology 2021 ; 96:e2296–e2312 Crossref , Medline , Google Scholar
131 Sha SJ, Miller ZA, Min SW, et al. : An 8-week, open-label, dose-finding study of nimodipine for the treatment of progranulin insufficiency from GRN gene mutations . Alzheimers Dement (N Y) 2017 ; 3:507–512 Crossref , Medline , Google Scholar
132 DeVos SL, Miller RL, Schoch KM, et al. : Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy . Sci Transl Med 2017 ; 9:eaag0481 Crossref , Medline , Google Scholar
133 Chang C-W, Shao E, Mucke L : Tau: enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies . Science 2021 ; 371:eabb8255 Crossref , Medline , Google Scholar
134 Yanamandra K, Patel TK, Jiang H, et al. : Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy . Sci Transl Med 2017 ; 9:eaal2029 Crossref , Medline , Google Scholar
135 Scrivo A, Bourdenx M, Pampliega O, et al. : Selective autophagy as a potential therapeutic target for neurodegenerative disorders . Lancet Neurol 2018 ; 17:802–815 Crossref , Medline , Google Scholar
136 Arrant AE, Onyilo VC, Unger DE, et al. : Progranulin Gene Therapy Improves Lysosomal Dysfunction and Microglial Pathology Associated with Frontotemporal Dementia and Neuronal Ceroid Lipofuscinosis . J Neurosci 2018 ; 38:2341–2358 Crossref , Medline , Google Scholar
- Cited by None

- Drug/Psychotherapy Treatment of Neuropsychiatric Disorders

- April 5, 2024 | NASA’s IXPE Triumph: Resurrected To Probe Black Hole Mysteries
- April 4, 2024 | Double Jointed? You May Be at Heightened Risk of Long COVID
- April 4, 2024 | Science Simplified: What Is Autonomous Discovery?
- April 4, 2024 | Scientists Discover Surprising Benefits of Unconsciously Remembering Things
- April 4, 2024 | A New Era of Science on the Lunar Gateway Space Station
Decoding Dementia: How a Brain Protein Could Lead to New Treatments
By Indiana University School of Medicine December 28, 2023
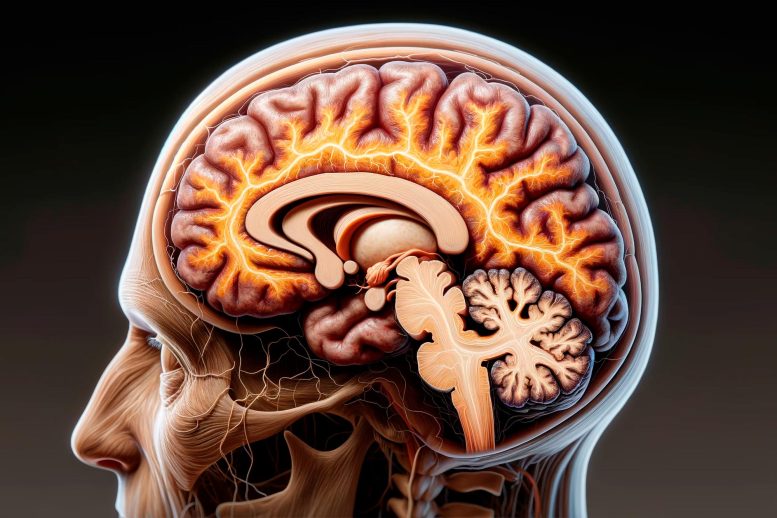
Researchers discovered a crucial protein, TAF15, in frontotemporal dementia (FTD) cases. This finding, offering new treatment avenues, was achieved using advanced neuropathologic and molecular techniques, marking a breakthrough in understanding and potentially treating this form of dementia.
Discovery could lead to new, targeted therapeutics for frontotemporal dementia.
An international team of researchers including experts at the Indiana University School of Medicine has identified a protein found in the brains of people with frontotemporal dementia (FTD), discovering a new target for potential treatments for the disease.
Understanding Frontotemporal Dementia
According to the National Institutes of Health , FTD results from damage to neurons in the frontal and temporal lobes of the brain. People with this type of dementia typically present symptoms, including unusual behaviors, emotional problems, trouble communicating, difficulty with work, or in some cases difficulty with walking, between the ages of 25 and 65.
Research Breakthrough in Neurodegenerative Disorders
Neurodegenerative disorders, including dementias and Amyotrophic Lateral Sclerosis (ALS), occur when specific proteins form amyloid filaments in the nerve cells of the brain and spinal cord. The multidisciplinary team of researchers—including members from the Medical Research Council (MRC) Laboratory of Molecular Biology, the IU School of Medicine and the University College London Queen Square Institute of Neurology—found that in cases of FTD, a protein called TAF15 forms these amyloid filaments in the cells of the brain and the spinal cord. On December 6, they published their findings in the journal Nature .

Cryo-EM structure of TAF15 amyloid filaments as discovered in patients with frontotemporal dementia. Credit: Indiana University
Bernardino Ghetti, MD is a Distinguished Professor at the IU School of Medicine and has been studying neurodegenerative dementias for 50 years. As a lead neuropathologist on the project, Ghetti and his team studied the protein aggregates from brains donated by four people who had frontotemporal dementia and motor weakness. Together with their colleagues in the UK, IU researchers used neuropathologic and molecular techniques and cutting-edge cryo-electron microscopy (cryo-EM) at atomic resolution to discover the presence of the amyloid filaments made of TAF15 protein in multiple brain areas. However, it is important to note that TAF15 amyloid affects also nerve cells of the motor system.
Important Breakthrough
“This discovery represents an important breakthrough that recognizes TAF15 as a potential target for the development of diagnostic and therapeutic strategies toward a lesser-known form of frontotemporal lobar degeneration associated with frontotemporal dementia,” Ghetti said.
Reference: “TAF15 amyloid filaments in frontotemporal lobar degeneration” by Stephan Tetter, Diana Arseni, Alexey G. Murzin, Yazead Buhidma, Sew Y. Peak-Chew, Holly J. Garringer, Kathy L. Newell, Ruben Vidal, Liana G. Apostolova, Tammaryn Lashley, Bernardino Ghetti and Benjamin Ryskeldi-Falcon, 6 December 2023, Nature . DOI: 10.1038/s41586-023-06801-2
Additional authors on the study are the MRC Laboratory of Molecular Biology’s Stephan Tetter, Diana Arseni, Alexey G. Murzin, Sew Y. Peak-Chew and Benjamin Ryskeldi-Falcon; the University College London’s Yazead Buhidma and Tammaryn Lashley; and the IU School of Medicine’s Holly J. Garringer, Kathy L. Newell, Ruben Vidal and Liana G. Apostolova.
The study was in part funded by the NIH’s National Institute on Aging and National Institute of Neurological Disorders and Stroke.
More on SciTechDaily

“Critical Power” Exercise Prescriptions – Greater Improvements and Longer-Lasting Benefits
Restoring Key Brain Rhythm: A Novel Approach to Combat Depression
New RNA Tool Can Illuminate Brain Circuits and Edit Specific Cells
Rutgers Scientists Have Discovered the Origins of the Building Blocks of Life

Research Shows Antarctic Sea Ice Melt Translates to Weather Change in Tropics

New Technology Is Key Step Toward Big Gains in Plastics Recycling

Peering into Cosmic Shadows: BlackGEM Telescopes Join the Hunt for Gravitational Waves

Scientists Regrow Frog’s Lost Leg With a Five-Drug Cocktail
Be the first to comment on "decoding dementia: how a brain protein could lead to new treatments", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- Open access
- Published: 20 December 2023
Statins and cognitive decline in patients with Alzheimer’s and mixed dementia: a longitudinal registry-based cohort study
- Bojana Petek 1 , 2 , 3 ,
- Henrike Häbel 4 ,
- Hong Xu 5 ,
- Marta Villa-Lopez 5 , 6 , 7 ,
- Irena Kalar 1 , 3 , 8 ,
- Minh Tuan Hoang 5 , 9 ,
- Silvia Maioli 1 , 10 ,
- Joana B. Pereira 11 ,
- Shayan Mostafaei 5 , 9 ,
- Bengt Winblad 1 , 12 ,
- Milica Gregoric Kramberger 1 , 3 , 8 ,
- Maria Eriksdotter 5 , 12 &
- Sara Garcia-Ptacek 5 , 12
Alzheimer's Research & Therapy volume 15 , Article number: 220 ( 2023 ) Cite this article
14k Accesses
205 Altmetric
Metrics details
Disturbances in brain cholesterol homeostasis may be involved in the pathogenesis of Alzheimer’s disease (AD). Lipid-lowering medications could interfere with neurodegenerative processes in AD through cholesterol metabolism or other mechanisms.
To explore the association between the use of lipid-lowering medications and cognitive decline over time in a cohort of patients with AD or mixed dementia with indication for lipid-lowering treatment.
A longitudinal cohort study using the Swedish Registry for Cognitive/Dementia Disorders, linked with other Swedish national registries. Cognitive trajectories evaluated with mini-mental state examination (MMSE) were compared between statin users and non-users, individual statin users, groups of statins and non-statin lipid-lowering medications using mixed-effect regression models with inverse probability of drop out weighting. A dose-response analysis included statin users compared to non-users.
Our cohort consisted of 15,586 patients with mean age of 79.5 years at diagnosis and a majority of women (59.2 %). A dose-response effect was demonstrated: taking one defined daily dose of statins on average was associated with 0.63 more MMSE points after 3 years compared to no use of statins (95% CI: 0.33;0.94). Simvastatin users showed 1.01 more MMSE points (95% CI: 0.06;1.97) after 3 years compared to atorvastatin users. Younger (< 79.5 years at index date) simvastatin users had 0.80 more MMSE points compared to younger atorvastatin users (95% CI: 0.05;1.55) after 3 years. Simvastatin users had 1.03 more MMSE points (95% CI: 0.26;1.80) compared to rosuvastatin users after 3 years. No differences regarding statin lipophilicity were observed. The results of sensitivity analysis restricted to incident users were not consistent.
Conclusions
Some patients with AD or mixed dementia with indication for lipid-lowering medication may benefit cognitively from statin treatment; however, further research is needed to clarify the findings of sensitivity analyses.
The brain houses about a quarter of the cholesterol present in the body, making it the richest cholesterol-containing organ [ 1 ]. The essential role of brain cholesterol is reflected in its involvement in numerous physiological processes such as maintaining membrane integrity, neurotransmission and synaptogenesis [ 2 ]. A dysregulation of brain cholesterol homeostasis may be involved in the pathogenesis of Alzheimer’s disease [ 2 ] through interference with the amyloidogenic Aβ pathway [ 3 ], impairment of cerebral blood flow [ 4 ], and other mechanisms [ 5 ]. On the other hand, the association of peripheral hypercholesterolemia and cognition is complex. Peripheral hypercholesterolemia in midlife has been linked to cognitive decline and AD in late-life [ 6 , 7 ] through different mechanisms [ 7 , 8 , 9 , 10 ]. Moreover, genetic polymorphism of brain cholesterol transporter ApoE4 and several additional genetic factors implicated in lipid metabolism could be relevant to AD pathogenesis [ 11 , 12 ]. In contrast, peripheral hyperlipidaemia in late life is a marker of a better general health and cognition [ 13 , 14 ].
The possible cognitive effects of HMG-CoA reductase inhibitors or statins, which are used in cardiovascular disease prevention, have sparked extensive research in the last few decades. Based on their pharmacokinetic characteristics, statins can be divided according to their structure (fungus-derived or synthetical), lipophilicity, metabolism, bioavailability, potency and binding to different proteins and transporters [ 15 ]. The multi-layered effects of statins on cognition are translated through numerous neurodegenerative processes in a cholesterol-dependent as well as independent (´´pleiotropic´´) manner [ 15 , 16 ]. Statins seem to interfere with the amyloidogenic cascade [ 17 ] and phosphorylation of tau [ 18 ], provide beneficial vascular factors through endothelial function and clearance of neurotoxic substances [ 19 ], decrease neuroinflammation and oxidative stress as well as promote neuronal survival and plasticity, synaptogenesis and neurotransmission [ 16 ].
The overall cognitive effects of statins are likely connected to a complex interaction of factors, related to the patient’s characteristics, integrity of blood–brain barrier permeability [ 20 ], characteristics of statins [ 18 ], time of treatment, dosages as well as critical time windows in the pathogenesis of dementia [ 21 , 22 ] (Fig. 1 ).
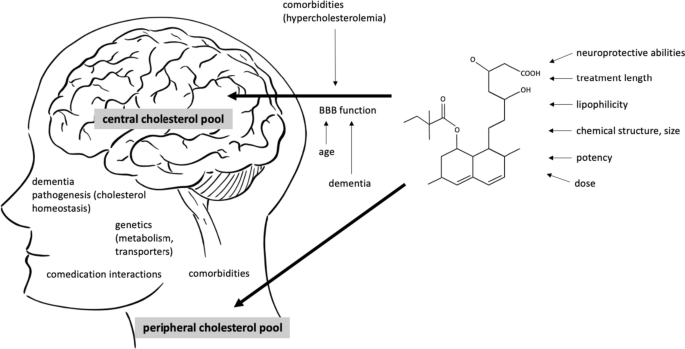
Interaction between the patient’s and medication’s characteristics potentially influence the cognitive effects of statins. Two separate cholesterol pools in the body are thought to be connected to the risk of Alzheimer’s disease (AD), central and peripheral. The brain penetration of statins has been attributed to different factors linked to BBB crossing (lipophilicity of a statin, chemical structure, molecular weight and size of the molecule, different transporters and their genetic polymorphisms). The structure of the barrier itself additionally influences the permeability of statins and is affected by aging, neurodegenerative processes and possibly, peripheral hypercholesterolemia. The overall cognitive effects of statins are likely a result of their central and peripheral actions and are connected to the time of intervention in life and the pathogenesis of AD. Moreover, an interaction of comorbidities and comedication, a sufficient time of treatment and dosages are important. In midlife, protective effect of statins against AD could be achieved through lowering the metabolic risk of hyperlipidaemia. BBB blood–brain barrier, AD Alzheimer’s disease
Despite the extensive number of observational cohort studies and some clinical trials on statins, their ability to prevent dementia or ameliorate cognitive decline after disease onset is still unclear. A number of mild and reversible short-term cognitive adverse effects [ 23 , 24 ] contributed to a warning for the labelling of statins by the US Food and Drug Administration. However, numerous large systematic reviews and meta-analyses have not confirmed these adverse cognitive effects [ 25 , 26 , 27 , 28 , 29 ] and some suggested that the use of statins may lower the risk of AD [ 25 , 30 , 31 , 32 , 33 ]. Clinical trials generally reported a null effect [ 34 , 35 , 36 ] but were commonly underpowered or used less robust cognitive evaluation tools. Comparably less information is available regarding the effect of statins on cognitive decline in patients with established AD [ 37 , 38 , 39 , 40 ]. Epidemiological biases inherent to observational design or a heterogeneous design of studies partly explain these discrepancies [ 41 ].
The aim of our study was to evaluate the association between statin use and cognitive decline over time in a large cohort of patients diagnosed with AD or mixed AD dementia. We hypothesized that statins that cross the BBB would be associated with less cognitive decline evaluated with mini-mental state examination (MMSE) in these patients.
Study design and registries
We performed a longitudinal cohort study of patients with AD or mixed dementia and indication for lipid-lowering treatment, registered in the Swedish registry for dementia (SveDem). SveDem is a nationwide quality-of-care registry, established in 2007 [ 42 ]. All memory clinics and 78 % of primary care centres in Sweden report to SveDem [ 43 ]. From this registry, we obtained demographic information (age, sex, living arrangements), date and care unit of registration, type of dementia diagnosis and cognitive status of the patients (MMSE scores) at baseline and follow-ups. The date of the dementia diagnosis in SveDem was set as the index date; 61% of patients had only one entry, 26% had two, 8% had 3 and 5% had more than 3. In total, 80,004 individual patients with dementia were registered in SveDem between 2007 and 2018. All patients were followed until death, emigration or end of follow-up (16 October 2018).
All patients with a missing MMSE score at index date were excluded from the analyses. Only patients diagnosed with hyperlipidaemia (ICD-10 codes from E78.0 to E78.6 obtained from the the Swedish National Patient Registry (NPR), see below) in the preceding 10 years before the index date or those with a prescription of statins (ICD-10 code C10 obtained from the Swedish Prescribed Drug Registry (PDR), see below) in the preceding 6 months before the index date were included in the analyses. Furthermore, the top 1% of statins users sorted by averaged defined daily doses (DDD) were excluded as well, assuming that their consumption data was falsely high and that these individuals bought medication that they did not consume. Figure 2 shows the patient selection flowchart: 15,586 individuals were included for the main analysis.

Flowchart of study participants selection. Hyperlipid patients with AD or mixed dementia, registered in SveDem from 2007 to 2018 were included in the study. Among these, we compared cognitive trajectories over time, evaluated with MMSE, in different comparison groups: (1) statin users vs non-users of statins, (2) simvastatin users vs atorvastatin users, (3) simvastatin users vs rosuvastatin users, (4) lipophilic statin users vs hydrophilic statin users, (5) fungal statin users vs synthetic statin users and (6) non-statin lipid-lowering medications users vs statin users
The exposure drug use was extracted for every SveDem entry. Drug use was defined from the PDR as either the average DDD during the 6-month period preceding each SveDem entry date or simply as a categorical variable (yes/no) during the same period (time-updated exposure). Time-updated exposure means that presence/absence and dose of statins was examined in each 6-month period leading up to each measurement of MMSE (baseline or follow-up). Individual patients starting their statin treatment after the index date were excluded from the analyses because cognitive decline could have affected prescription. All other patients were included in the analyses as non-users. DDD is defined by the World Health Organization as the assumed average maintenance daily dose of a medication for its primary indication in adults [ 44 ]. One DDD of simvastatin is equivalent to 30 mg of simvastatin or 20 mg of atorvastatin.
Medication use
Medication use with their corresponding ATC codes were collected from the PDR that was established in 2005, which includes all prescription medication dispensed at Swedish pharmacies [ 45 ]. Lipid-lowering medications included simvastatin, pravastatin, fluvastatin, rosuvastatin, pitavastatin, fibrates, bile acid sequestrants, nicotinic acid and derivates and other non-statin lipid lowering medications. Comedications were calculated as time-updated exposures (yes/no) during the 6-month period preceding the index date. Comedications were selected based on known relevance for patients with dementia and included cardiac drugs, vasoprotectives, platelet aggregation inhibitors, anticoagulants, antipsychotics, anxiolytics, hypnotics, antidepressants, cholinesterase inhibitors, memantine and vitamin D ( Appendix ). Assumption on the adherence was made based on the collection of the medication at the pharmacy.
Comorbidities
Comorbidities were obtained with their corresponding ICD-10 codes from the NPR and were coded dichotomously up to 10 years before index date. NPR covers all diagnoses from in-hospital and specialist clinics. Comorbidities were selected based on their known relevance for cognition in patients with dementia and included diabetes mellitus, arrythmia, heart failure, atrial fibrillation, alcohol-related disease, chronic kidney disease, cardiovascular disease, ischemic heart disease, respiratory disease, stroke, anaemia, liver disease, malignancy and obesity ( Appendix ).
Covariates that were considered included age at baseline, sex (male/female), residency (living with another adult/alone/nursing home/missing), type of dementia diagnostic unit (special memory clinic/primary care centre) and calendar year of dementia diagnosis, all at index date. We selected the covariates that are likely associated with cognitive functions or the probability of receiving statins, based on previous research and/or our clinical knowledge.
The linkage of data from the forementioned registries—SveDem, Swedish National Patient Registry, and Swedish Prescribed Drug Registry—was allowed by the personal identification number of each Swedish citizen. Patient identification was pseudonymized and blinded to the researchers.
The main outcome was cognitive decline, evaluated with MMSE points.
Statistical analysis
The data were described in terms of mean and standard deviation (SD) for continuous variables and as positive counts (percentages) for categorical variables.
Linear mixed-effects regression models with random intercept and slope were used to investigate the change in MMSE scores over time and to detect differences between statin users and non-users. The model included statin use and time from index date as continuous variables and an interaction between drug use and time. Following our previous work in SveDem [ 46 ], a linear trend over time was assumed and the model allowed for a random intercept and random slope for each patient. This model is referred to as the crude model. In an adjusted model, comedications, comorbidities and other covariates were included in the model mostly as categorical variables. Only age, MMSE scores and calendar year at index date were treated as continuous variables. Fully adjusted models included clinical and demographic characteristics (MMSE score at baseline and age at diagnosis, year of diagnosis, sex, residency, comorbidities and comedications). Inverse probability weighting was used to account for the potential effects of general attrition from those lost to follow-up due to dropout. For this purpose, a logistic regression model was fitted to the data to estimate the probability of dropping out within the subsequent year. Dropout was defined as the last observed MMSE score without death or study end occurring in the subsequent year. For more details, see Handels et al. [ 47 ].
The analyses were repeated for selected drug groups where drug use was defined as yes/no during the 6-month period before each SveDem entry date and in subgroups defined by gender and age. We split the cohort at the mean age at index date (79.5 years) to create subgroups of younger and older patients. We compared individual statin users. We considered two approaches to divide the statins regarding their functional properties: firstly, lipophilic (simvastatin, atorvastatin, fluvastatin, lovastatin and pitavastatin) or hydrophilic groups (rosuvastatin, pravastatin) [ 48 ]. Moreover, we classified them into fungus-derived (simvastatin, pravastatin, lovastatin) or synthetic statins (atorvastatin, cerivastatin, fluvastatin, rosuvastatin, pitavastatin) [ 15 ]. Finally, we compared statin users to non-statin lipid-lowering medication users. To evaluate the association between the comparison groups after 3 years, we calculated a theoretical linear extrapolation based on the mixed effect models.
Multiple imputations of MMSE scores [ 47 ] and incident users models were two additional models we performed as a sensitivity analysis to check the robustness of our results. The first model deals with bias arising from missing MMSE scores at follow-ups and the second addresses the length of treatment as confounding. Incident users were defined as drug users who did not take out any drug prescription of statins during 12 months before 6-month period preceding each SveDem entry date. Table 1 shows the design of the study.
The cluster robust sandwich estimator was used to estimate standard error of the estimations and two-sided p -values were reported. All analyses were conducted using STATA version 16.1 (StataCorp, College Station, TX).
Characteristics of study population
As shown in Table 2 , our cohort consisted of 15,586 AD or mixed dementia patients with a mean age of 79.5 years (SD = 6.8) at dementia diagnosis. Most patients were women (59.2%). At baseline, all patients scored on average 21 points on MMSE (SD = 5); 10,869 patients (69.7%) used statins in the observation period. The most prescribed statin in the whole cohort was simvastatin (8235, 52.8%) followed by atorvastatin (2210, 14.2%). There were 296 (1.9%) users of a non-statin lipid-lowering medication. Most of the patients resided at home (53.9% with another adult and 40.7% alone) and 5% lived nursing homes. The most common comorbidities in the cohort were hypertension (40.1%), diabetes mellitus (24.4%) and cardiovascular disease (24%).
The average time of follow-up was 0.86 (SD = 1.40) years and average number of MMSE follow-ups for a patient with measures of MMSE was 1.61 (SD = 0.97). The average decline per year of the cohort was −1.20 points MMSE (95% CI: −1.32; −1.09). The average cognitive decline observed among 6113 patients with at least two MMSE measurements was −2.61 points (SD = 4.57).
Statin users were more commonly male (44 % vs 33.5 %, p < 0.001), younger (78.7 vs 80.7 years at baseline, p < 0.001) and had a better cognitive status at baseline (21.3 vs 20.8 MMSE points, p < 0.001), compared to non-users of statins. As expected, there was a higher prevalence of comorbidities in the former compared to the latter group, such as hypertension, cardiovascular disease, liver disease and diabetes mellitus. Statin users were more likely to be prescribed co-medication, such as antithrombotics, antihypertensives, antidiabetics or psycholeptics. More detailed information is presented in Tables 2 and 3 .
The sensitivity analysis revealed that a majority of our cohort had used statins at some point in time. Overall, 844 patients were incident users of statins. Compared to incident simvastatin users ( n = 557), incident atorvastatin users ( n = 267) had a lower baseline MMSE (20.5 vs 21.4 points, p = 0.01) and less commonly received their dementia diagnosis at special memory clinic (60.3 % vs 78.8 %, p < 0.001). (Supplementary table 7 ).
Cognitive decline in different treatment groups
Statin users compared to non- users of statins.
Statin use was associated with a slower cognitive decline over time compared to no use of statins. After taking an average of 1 DDD of statins for a year, statin users had 0.21 more MMSE points (95% CI: 0.12; 0.32) compared to non-users. There was a dose-response effect. After 3 years of taking an average 1 DDD of statins, statin-treated patients had 0.63 points more MMSE points (95% CI: 0.33; 0.94) (Table 4 , Fig. 3 ). These results were consistent in subgroup analysis and when considering imputed missing MMSE values and analysis restricted to incident users (Supplementary table 6 ) and in subgroup analyses (Supplementary table 1 ). We conducted post hoc analyses stratifying by dementia type (Alzheimer or mixed dementia; results not shown) with similar results to those presented for the whole group.
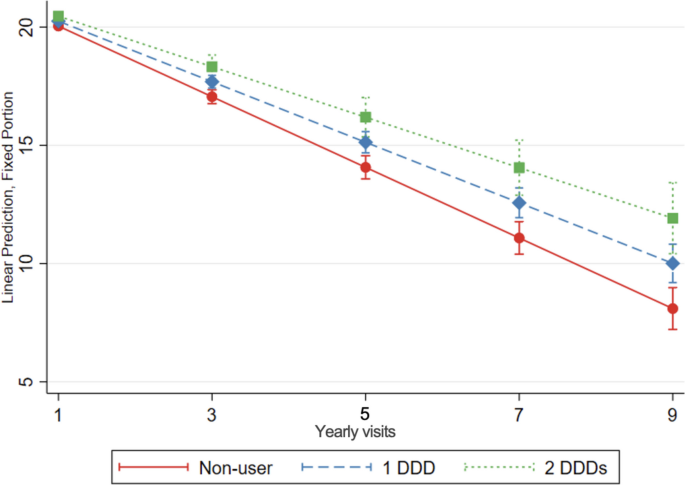
Cognitive decline, evaluated with change in MMSE score over time, in statin users compared to non-users of statins. The graph shows the association between increasing doses of statin treatment and MMSE over time, as predicted from the model. Linear mixed-effects regression model, adjusted for demographic characteristics, comorbidities and comedication, with inverse probability weighting. DDD defined daily dose. DDD is defined by the World Health Organization as the assumed average maintenance daily dose of a medication for its primary indication in adults. Yearly visit 1 represent the first MMSE measurement (baseline)
Simvastatin users compared to atorvastatin users
Simvastatin users exhibited a slower cognitive decline over time, compared to atorvastatin users (0.35 more MMSE points per year of follow-up, 95% CI: 0.03; 0.67 and 1.01 more MMSE points after 3 years, 95% CI: 0.06; 1.97) (Table 4 , Fig. 4 ).
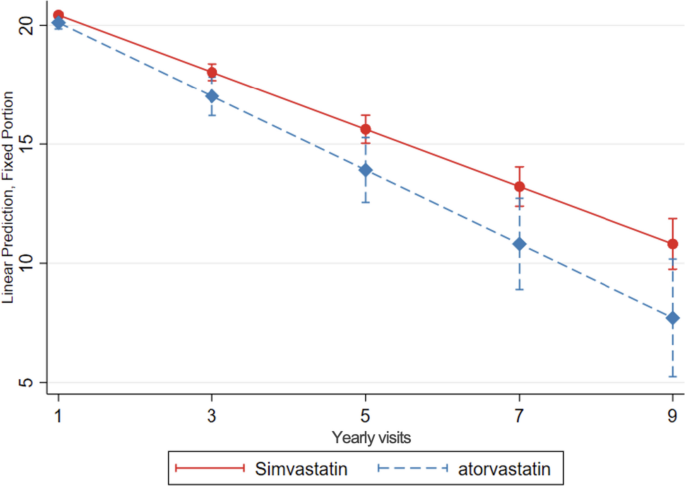
Cognitive decline, evaluated with change in MMSE score over time, in simvastatin compared to atorvastatin users. Linear mixed-effects regression model, adjusted for demographic characteristics, comorbidities and comedication, with inverse probability weighting. Yearly visit 1 represent the first MMSE measurement (baseline)
When stratifying analyses for gender and age, the protective association for cognition of simvastatin compared to atorvastatin, was only statistically significant in the participants aged <79.5 years (which was the mean age of the total sample). Younger users of simvastatin had a slower decline of MMSE (0.28 points more per year, 95% CI: 0.03; 0.54, and 0.80 points more after 3 years, 95% CI: 0.05; 1.55) compared to younger atorvastatin users (Table 5 ).
This protective association was statistically significant in multiple imputations of MMSE model but not in incident users (0.56 less MMSE points per year, 95% CI: -1.13; 0.01) (Supplementary table 6 ). We conducted post hoc analyses stratifying by dementia type (Alzheimer or mixed dementia): in mixed dementia, simvastatin use was associated with 1.57 more MMSE points MMSE at 3 years (95% CI 0.79; 2.34) while results were not significant for the Alzheimer group (0.49 more points MMSE at 3 years; 95% CI -0.76; 1.76).
Simvastatin users compared to rosuvastatin users
Simvastatin users had a slower MMSE decline, compared to rosuvastatin users (0.35 more MMSE points per year of follow-up, 95% CI: 0.09; 0.61, and 1.03 more MMSE points after 3 years, 95% CI: 0.26; 1.80) (Table 4 , Fig. 5 ).

Cognitive decline, evaluated with change in MMSE scores over time, in simvastatin compared to rosuvastatin users. Linear mixed-effects regression model, adjusted for demographic characteristics, comorbidities, and co-medication, with inverse probability weighting. Yearly visit 1 represent the first MMSE measurement (baseline)
In subgroup analysis, these results remained statistically significant in women and younger patients (Supplementary table 2 ).
The associations remained protective when imputing missing MMSE values. However, incident simvastatin users had a faster decline of MMSE compared to incident rosuvastatin users (1.63 less MMSE points per year of follow-up, 95% CI: -3.18; -0.07 and 4.77 less MMSE points after 3 years, 95% CI: -9.46; -0.07) (Supplementary table 6 ).
Lipophilic statin users compared to hydrophilic statin users
We did not find significant differences in MMSE decline in lipophilic statin users (simvastatin, atorvastatin, fluvastatin users) (Table 4 ) or when considering imputed values of missing MMSE, compared to hydrophilic statins users (rosuvastatin, pravastatin users). However, it was faster in incident users of lipophilic statins (1.32 less MMSE points per year, 95% CI: -2.46; -0.18), and 3.84 less points after 3 years, 95% CI: -7.28; -0.41), compared to hydrophilic statins (Supplementary table 6 ). These analyses were not statistically significant in sub-analysis of age groups and sex (Supplementary table 3 ).
Fungal statin users compared to synthetic statin users
Use of fungal statins (simvastatin, pravastatin users) was not associated with a difference in MMSE decline compared to synthetic statin users (atorvastatin, rosuvastatin, fluvastatin users) (Table 4 ). In a subgroup analysis, the MMSE decline was slower in younger fungal statin users (0.26 more points per year, 95% CI: 0.03; 0.49, and 0.73 more points after 3 years, 95% CI: 0.05; 1.42) (Supplementary table 4 ). However, the decline was faster when analysing only incident fungal users, compared to incident synthetic users (0.61 points less per year, 95% CI: -1.19; -0.04, and 1.83 points less after 3 years, 95% CI: -3.55; -0.12) (Supplementary table 6 ).
Non-statin lipid-lowering medication users compared to statin users
We did not observe a significant difference in MMSE decline between non-statin lipid lowering medication users, compared to statin users (Table 4 ).
In some subgroup analyses, users of non-statin lipid lowering medication had a slower MMSE decline (Supplementary table 5 ).
In this longitudinal Swedish registries-based observational study of patients with AD or mixed AD dementia, we discovered a dose-dependent cognitive benefit over time in statin users compared to non-users of statins. Additionally, we discovered a slower MMSE decline over time in patients taking simvastatin, compared to either atorvastatin or rosuvastatin users. Younger users of simvastatin had a slower MMSE decline compared to younger atorvastatin users. We did not observe a difference in MMSE decline depending on lipophilicity. Incident users’ analysis revealed inconsistent findings which could be potentially explained with time-dependant non-linear association between effect of statins on cognitive processes or through differences and selection of these users.
Different statins
Simvastatin was the most used statin in Sweden when our data was collected. Accordingly, this makes comparisons among different statins or groups difficult, often lacking enough power. A beneficial role of simvastatin in early dementia is biologically plausible, when there are high levels of neuroinflammation as this lipophilic statin readily crosses the BBB and could exert various neuroprotective properties, such as protection against tau hyperphosphorylation and mediation of brain cholesterol homeostasis [ 18 ]. Research on animal models of AD further support the beneficial effects of simvastatin on cognition through different mechanisms [ 49 , 50 ]. Clinical trials in patients with mild to moderate AD reported a neutral [ 35 ] or a beneficial effect of simvastatin [ 51 ], using MMSE as a cognitive outcome. Findings were limited by relatively short trial duration and low number of participants and were therefore possibly underpowered. To our best knowledge, our study is the first observational study to compare cognitive decline between different statins in patients with established AD and mixed dementia. A careful adjustment for comedication in general and cholinesterase inhibitors in particular is important, as our group previously discovered a small long-term beneficial effect on cognition in AD and mixed dementia patients treated with cholinesterase inhibitors [ 46 ].
In our study, the analysis including only incident users showed an opposite association. A possible explanation to inconsistent results of incident user design may be related to a temporally dependent biphasic effect of statin therapy on cholesterol metabolites, as shown in a study which included asymptomatic patients at risk of AD [ 52 ]. In this study, statins initially reduced cholesterol metabolites in the cerebrospinal fluid, reaching a nadir at 6–7 months, followed by a return to baseline and an overshoot at two years. Moreover, several differences between incident users compared to all users exist which could partly contribute to the discrepancies. The individual characteristics of incident users, such as baseline MMSE differences, or a possible selection of these smaller groups of patients through individual physicians’ preferences, might have influenced their cognitive trajectories which could not be accounted for in adjusted models.
Lipophilicity and chemical characteristics (fungal or synthetic statins)
Several biochemical characteristics of statins probably influence the functional effects of statins on cholesterol metabolism and cognition. Statins with a higher lipophilicity (e.g., simvastatin, atorvastatin, fluvastatin) may cross the BBB more easily compared to more hydrophilic statins (rosuvastatin, pravastatin) [ 18 ]. Additionally, the size and orientation of a statin molecule may influence the BBB permeability of statins which explained a low ability of a lipophilic atorvastatin to cross the BBB, due to its large size [ 18 ]. In our study, we did not observe a difference in cognitive decline when comparing users of lipophilic to hydrophilic statins in most models and subgroup analyses. However, MMSE decline was faster in incident users of lipophilic statins. Due to forementioned Swedish prescription patterns, the comparisons in our study were driven by simvastatin and atorvastatin users compared to rosuvastatin users. To the best of our knowledge, we are not aware of another observational study which compared cognitive decline between lipophilic and hydrophilic statin users in AD patients.
Fungal statins (simvastatin and pravastatin) differ from the synthetic statins (atorvastatin, rosuvastatin, fluvastatin) in several functional characteristics. Synthetic statins were shown to form more interactions which leads to a stronger inhibition of HMG-CoA reductase and a higher potency [ 53 ]. Moreover, fungus-derived statins were observed to have a high permeability through the blood–brain barrier and cause a reduction of cholesterol levels as well as lower a burden of neurofibrillary tangles in animal models [ 54 ]. In our study, this comparison between fungal and synthetic statins was driven by simvastatin and atorvastatin users, so this classification did not add further information. To the best of our knowledge, no previous studies compared cognitive decline in AD or mixed dementia patients among fungal and synthetic statins. A recent cohort study comparing different incident statin users found a higher risk of AD in fungal statins compared to synthetic, as well as higher risk in lipophilic statins compared to hydrophilic statins; however, the risk was reduced in sensitivity analysis [ 55 ].
Non-statin lipid-lowering medications
The confidence intervals for this comparison were broad and did not reach statistical significance. However, MMSE decline was slower in some subgroups of non-statin lipid-lowering medications (men and younger users). Statins and other hypolipemics, such as gemfibrozil, represent another interesting comparison group since they are both prescribed for hyperlipidaemia, therefore diminishing indication bias, and could exhibit cognitive effects through different metabolic pathways. Gemfibrozil attenuated amyloid burden as well as neuroinflammation and improved the memory in AD mouse models through activation of PPAR-alpha in a recent study [ 56 ], but our study was probably underpowered for this comparison.
Statin dose, potency, treatment length and time window for intervention
The dose, potency or duration of treatment have been recognized as important factors when evaluating the effects of statins on cognition. Most work has been done on evaluating these factors in the prevention of dementia or AD [ 32 , 33 , 57 ]. A dose-response was observed in a large cohort study which included only AD patients [ 58 ]. In our study, a dose-effect was observed when comparing statin users and non-users. The prediction model showed a benefit after 3 years, which is an estimated brain cholesterol turnover rate in adults, but most of the data in our cohort aggregates towards earlier follow-ups. A time window of intervention with statins regarding the neuropathogenesis of dementia, or life course of a patient, might exist as the neurodegenerative pathological changes of AD begin decades prior to clinical symptoms [ 59 ]. The protective effect of statins could be achieved in a long-term amelioration of brain vascular burden, restoration of disturbed central cholesterol homeostasis and neuroprotective effects, possibly in preclinical [ 60 ] or early stages of AD [ 61 , 62 ].
Statin use compared to no use of statins
An extensive evaluation of the possible role of statins in preventing dementia has been performed in the last two decades [ 25 , 26 , 32 , 33 , 41 , 63 , 64 , 65 ] but comparably less studies included patients with already established AD [ 25 , 31 , 38 , 39 , 40 , 41 , 64 ]. Clinical trials of statin use in AD patients did not report a clear benefit to ameliorate cognitive decline [ 38 , 40 , 64 ]. Observational cohort studies of patients with AD and a various follow-up ranging from 10 months to over 10 years, reported a slower [ 61 , 66 , 67 , 68 ] or similar [ 69 ] cognitive decline in statins users compared to non-users. Findings from our analysis comparing statin use to no use which imply a possible dose-dependent beneficial role of statins in patients with AD is in accord with many of these previous studies. However, comparing statin users and no-users introduces several important biases.
Importantly, hyperlipidaemia is an indication for statin use in midlife and represents a risk factor for dementia and AD. On the other hand, low cholesterol level in late life has been recognized as a measure of frailty or prodromal stage of dementia, particularly AD [ 70 ]. These facts can lead an indication bias when comparing users to non-users. Secondly, clinicians might be less likely to prescribe statins to older patients, especially those with pre-existing cognitive decline, frailty, or comorbidities since the risk of possible side effects and diminished life expectancy outweighs the benefit of medication. Furthermore, cognitive impairment could lead to a discontinuation of statins or drop-out from study, which would lead to a false beneficial association [ 41 , 71 ]. Older patients who receive statins for their hypercholesterolemia could naturally possess a lower risk of dementia or reflect a better cognitive trajectory [ 72 ], leading to reverse causation.
Strengths and limitations
Our study has several strengths and limitations. We can report associations but are not able to draw the conclusions on causality. This study was meant as an exploratory analysis which requires confirmation. We considered a variety of comorbidities and comedications in our models; however, a few important covariates were not available for our analysis, such as cholesterol levels, ApoE status and possible genetic polymorphisms specific for different populations [ 73 ]. However, we addressed this issue with a selection of a population with indication for treatment and used multivariate adjusting to balance the differences between the groups as well as performed several sensitivity analyses. Patient adherence to medication was indirectly assumed based on the dispensation of medication at pharmacies. There was a considerable number of drop-out participants and missing values on MMSE. MMSE was the only measure of cognitive decline in our study and is less robust to detect cognitive changes in different cognitive domains. We chose to only include observed MMSE scores in the analyses to limit the risk of creating false data with imputation but there is, of course, a risk of selection bias. We restricted the study population to those individuals with drug use at index date to ensure that we can follow a statin user’s cognitive decline from the beginning. The results from the sensitivity analyses considering multiple imputations of MMSE are in line with our main findings and confirmed our choices. However, the results of analysis on incident users were not consistent. Another important strength of our study lies in carefully selected statistical methods. Use of linear mixed modelling with multiple imputation is currently regarded as a superior method to account for the attrition bias [ 72 ]. We considered a reasonably long follow-up and performed a large, population-based study. We examined the use of statins before dementia to explore the reverse causality of cognition influencing the adherence or use of statin but cannot completely exclude this problem with our current methods.
Our population-based exploratory cohort study of patients with AD or mixed dementia adds to a growing body of evidence that statins are not detrimental for cognition. Moreover, statins might exhibit a long-term cognitive benefit these patients who have indication for lipid-lowering treatment. However, our findings warrant a confirmational study. We believe that our findings should further encourage clinicians to select eligible patients with dementia to benefit from prevention of their cardiovascular and cerebrovascular disease with statins. Further research of the pathogenesis of dementia is warranted. Acknowledging dementia as a complex, multifactorial syndrome where different pathogenic processes and risk factors are at play at different stages of dementia, it would be plausible to examine the combined effects of several medications affecting different metabolic pathways in well-defined subtypes of dementia. Moreover, the role of lipid metabolism dysregulation to the pathogenesis of AD should be further explored, taking the genetic factors into consideration. Further research is needed to decipher the non-consistent results in incident statin users, where time since prescription may be an important factor.
Availability of data and materials
Following the Swedish and EU legislation, the data are not available for public access. In order to obtain the data from Swedish registries, researches must apply to the steering committees of the registries as well as relevant government authorities, after obtaining the ethical approval.
Abbreviations
Alzheimer’s disease
Mini-mental state examination
Swedish Registry for Cognitive/Dementia Disorders
Amyloid beta
Apolipoprotein E4
3-Hydroxy-3-methylglutaryl coenzyme A reductase
Blood–brain barrier
National Patient Registry
Swedish Prescribed Drug Registry
Defined daily dose
International Classification of Diseases, 10th Revision
Standard deviation
Confidence interval
Björkhem I, Meaney S, Fogelman AM. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24(5):806–15.
Article PubMed Google Scholar
Chew H, Solomon VA, Fonteh AN. Involvement of lipids in Alzheimer’s disease pathology and potential therapies. Front Physiol. 2020;9:11.
Google Scholar
Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95(11):6460–4.
Article PubMed PubMed Central CAS Google Scholar
Czuba E, Steliga A, Lietzau G, Kowiański P. Cholesterol as a modifying agent of the neurovascular unit structure and function under physiological and pathological conditions. Metab Brain Dis. 2017;32(4):935–48.
Ismail MAM, Mateos L, Maioli S, Merino-Serrais P, Ali Z, Lodeiro M, et al. 27-Hydroxycholesterol impairs neuronal glucose uptake through an IRAP/GLUT4 system dysregulation. J Exp Med. 2017;214(3):699–717.
Kivipelto M, Solomon A. Cholesterol as a risk factor for Alzheimer’s disease—epidemiological evidence. Acta Neurol Scand. 2006;114(SUPPL. 185):50–7.
Article Google Scholar
Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimer’s Disease. 2017;56:215–28.
Loera-Valencia R, Goikolea J, Parrado-Fernandez C, Merino-Serrais P, Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J Steroid Biochem Mol Biol. 2019;1(190):104–14.
Gamba P, Staurenghi E, Testa G, Giannelli S, Sottero B, Leonarduzzi G. A crosstalk between brain cholesterol oxidation and glucose metabolism in Alzheimer’s disease. Front Neurosci. 2019;13:556.
Article PubMed PubMed Central Google Scholar
Petek B, Villa-Lopez M, Loera-Valencia R, Gerenu G, Winblad B, Kramberger MG, et al. Connecting the brain cholesterol and renin–angiotensin systems: potential role of statins and RAS-modifying medications in dementia. J Intern Med. 2018;284(6):620–42.
Article PubMed CAS Google Scholar
Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54(4):412–36.
de Oliveira FF, Bertolucci PHF, Chen ES, Smith MC. Pharmacogenetic analyses of therapeutic effects of lipophilic statins on cognitive and functional changes in Alzheimer’s disease. J Alzheimers Dis. 2022;87(1):359–72.
Benito-León J, Vega-Quiroga S, Villarejo-Galende A, Bermejo-Pareja F. Hypercholesterolemia in elders is associated with slower cognitive decline: a prospective, population-based study (NEDICES). J Neurol Sci. 2015;350(1–2):69–74.
Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61(5):705–14.
McFarland AJ, Anoopkumar-Dukie S, Arora DS, Grant GD, McDermott CM, Perkins AV, et al. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15(11):20607–37.
Mendoza-Oliva A, Zepeda A, Arias C. The complex actions of statins in brain and their relevance for Alzheimer’s disease treatment: an analytical review. Curr Alzheimer Res. 2014;11(999):1–1.
Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer’s disease: is there a link? Neurology. 2001;57(6):1089–93.
Sierra S, Ramos MC, Molina P, Esteo C, Vázquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimer’s Dis. 2011;23(2):307–18.
Article CAS Google Scholar
Zhou Q, Liao JK. Pleiotropic effects of statins—basic research and clinical perspectives. Circ J. 2010;74(5):818–26.
Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38.
Schultz BG, Patten DK, Berlau DJ. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl Neurodegener. 2018;7(1):5.
Jamshidnejad-Tosaramandani T, Kashanian S, Al-Sabri MH, Kročianová D, Clemensson LE, Gentreau M, et al. Statins and cognition: modifying factors and possible underlying mechanisms. Front Aging Neurosci. 2022;15:14.
Wagstaff LR, Mitton MW, Arvik BML, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–80.
Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29(7):800–11.
Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159(10):688–97.
Samaras K, Brodaty H, Sachdev PS. Does statin use cause memory decline in the elderly? Trends Cardiovasc Med. 2016;26(6):550–65.
Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88(11):1213–21.
Mcguinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;2016(1):CD003160.
PubMed PubMed Central Google Scholar
Adhikari A, Tripathy S, Chuzi S, Peterson J, Stone NJ. Association between statin use and cognitive function: a systematic review of randomized clinical trials and observational studies. J Clin Lipidol. 2021;15(1):22-32.e12.
Chu CS, Tseng PT, Stubbs B, Chen TY, Tang CH, Li DJ, et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2018;8(1):5804.
Zhu X-C, Dai W-Z, Ma T. Overview the effect of statin therapy on dementia risk, cognitive changes and its pathologic change: a systematic review and meta-analysis. Ann Transl Med. 2018;6(22):435–435.
Poly TN, Islam MM, Walther BA, Yang HC, Wu CC, Lin MC, et al. Association between use of statin and risk of dementia: a meta-analysis of observational studies. Neuroepidemiology. 2020;54(3):214–26.
Olmastroni E, Molari G, De Beni N, Colpani O, Galimberti F, Gazzotti M, et al. Statin use and risk of dementia or Alzheimer’s disease: a systematic review and meta-analysis of observational studies. Eur J Prev Cardiol. 2021.
Collins R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22.
Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, Van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77(6):556–63.
Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956–64.
Murphy C, Dyer AH, Lawlor B, Kennelly SP. What is the impact of ongoing statin use on cognitive decline and dementia progression in older adults with mild-moderate Alzheimer disease? PLoS One. 2023;18(5):e0285529.
Liang T, Li R, Cheng O. Statins for treating Alzheimer’s disease: truly ineffective? Eur Neurol. 2015;73(5–6):360–6.
Davis KAS, Bishara D, Perera G, Molokhia M, Rajendran L, Stewart RJ. Benefits and harms of statins in people with dementia: a systematic review and meta-analysis. J Am Geriatr Soc. 2020;68(3):650–8.
Xuan K, Zhao T, Qu G, Liu H, Chen X, Sun Y. The efficacy of statins in the treatment of Alzheimer’s disease: a meta-analysis of randomized controlled trial. Neurol Sci. 2020;41(6):1391–404.
Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia-systematic review and methodological commentary. Nat Rev Neurol. 2015;11(4):220–9.
Religa D, Fereshtehnejad SM, Cermakova P, Edlund AK, Garcia-Ptacek S, Granqvist N, et al. SveDem, the Swedish Dementia Registry - a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS One. 2015;10(2):e0116538.
The Swedish Registry for Cognitive/Dementia Disorders (SveDem) Annual Report 2021. https://www.ucr.uu.se/svedem/om-svedem/arsrapporter/svedem-arsrapport-2021/viewdocument/1063 . Accessed 06 Aug 2023.
Defined Daily Dose. https://www.whocc.no/ddd/definition_and_general_considera/ . Accessed 06 Aug 2023.
Wettermark B, Hammar N, Fored CM, Leimanis A, Olausson PO, Bergman U, et al. The new Swedish Prescribed Drug Register Opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–35.
Xu H, Garcia-Ptacek S, Jönsson L, Wimo A, Nordström P, Eriksdotter M. Long-term effects of cholinesterase inhibitors on cognitive decline and mortality. Neurology. 2021;96(17):e2220-30.
Handels R, Jönsson L, Garcia-Ptacek S, Eriksdotter M, Wimo A. Controlling for selective dropout in longitudinal dementia data: application to the SveDem registry. Alzheimer’s Dement. 2020;16(5):789–96.
Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–25.
Tong XK, Royea J, Hamel E. Simvastatin rescues memory and granule cell maturation through the Wnt/β-catenin signaling pathway in a mouse model of Alzheimer’s disease. Cell Death Dis. 2022;13(4):325.
Hu X, Song C, Fang M, Li C. Simvastatin inhibits the apoptosis of hippocampal cells in a mouse model of Alzheimer’s disease. Exp Ther Med. 2018;15(2):1795–802.
PubMed CAS Google Scholar
Simons M, Schwärzler F, Lütjohann D, Von Bergmann K, Beyreuther K, Dichgans J, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52(3):346–50.
Evans BA, Evans JE, Baker SP, Kane K, Swearer J, Hinerfeld D, et al. Long-term statin therapy and CSF cholesterol levels: implications for Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;27(6):519–24.
Gazzerro P, Proto MC, Gangemi G, Malfitano AM, Ciaglia E, Pisanti S, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64(1):102–46.
Tramontina AC, Wartchow KM, Rodrigues L, Biasibetti R, Quincozes-Santos A, Bobermin L, et al. The neuroprotective effect of two statins: simvastatin and pravastatin on a streptozotocin-induced model of Alzheimer’s disease in rats. J Neural Transm. 2011;118(11):1641–9.
Sinyavskaya L, Gauthier S, Renoux C, Dell’Aniello S, Suissa S, Brassard P. Comparative effect of statins on the risk of incident Alzheimer disease. Neurology. 2018;90(3):e179-87.
Chandra S, Pahan K. Gemfibrozil, a lipid-lowering drug, lowers amyloid plaque pathology and enhances memory in a mouse model of Alzheimer’s disease via peroxisome proliferator-activated receptor α. J Alzheimer’s Dis Reports. 2019;3(1):149–68.
Zhang X, Wen J, Zhang Z. Statins use and risk of dementia: a dose–response meta analysis. Med (United States). 2018;97(30).
Jeong SM, Shin DW, Yoo TG, Cho MH, Jang W, Lee J, et al. Association between statin use and Alzheimer’s disease with dose response relationship. Sci Rep. 2021;11(1):15280.
Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med. 2012;2(5):a006148.
Carlsson CM, Gleason CE, Hess TM, Moreland KA, Blazel HM, Koscik RL, et al. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer’s disease. J Alzheimer’s Dis. 2008;13(2):187–97.
Lin FC, Chuang YS, Hsieh HM, Lee TC, Chiu KF, Liu CK, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Med (United States). 2015;94(47):e2143.
CAS Google Scholar
Sparks DL, Sabbagh M, Connor D, Soares H, Lopez J, Stankovic G, et al. Statin therapy in Alzheimer’s disease. Acta Neurol Scand. 2006;114(SUPPL. 185):78–86.
Wong WB, Lin VW, Boudreau D, Devine EB. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22(4):345–58.
Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30(3):348–58.
Fink HA, Jutkowitz E, McCarten JR, Hemmy LS, Butler M, Davila H, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168(1):39–51.
Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73(9):674–80.
Masse I, Bordet R, Deplanque D, Al Khedr A, Richard F, Libersa C, et al. Lipid lowering agents are associated with a slower cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(12):1624–9.
Rosenberg PB, Mielke MM, Tschanz J, Cook L, Corcoran C, Hayden KM, et al. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(11):883–92.
Kemp EC, Ebner MK, Ramanan S, Godek TA, Pugh EA, Bartlett HH, et al. Statin use and risk of cognitive decline in the ADNI cohort. Am J Geriatr Psychiatry. 2020;28(5):507–17.
Mielke MM, Zandi PP, Sjögren M, Gustafson D, Östling S, Steen B, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64(10):1689–95.
Alsehli AM, Olivo G, Clemensson LE, Williams MJ, Schiöth HB. The Cognitive effects of statins are modified by age. Sci Rep. 2020;10(1).
Samaras K, Makkar SR, Crawford JD, Kochan NA, Slavin MJ, Wen W, et al. Effects of statins on memory, cognition, and brain volume in the elderly. J Am Coll Cardiol. 2019;74(21):2554–68.
Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2017;74(2):225–32.
Download references
Acknowledgements
The authors are grateful to SveDem, www.svedem.se , for providing data for this study. We thank all patients, caregivers, reporting units and coordinators in SveDem as well as SveDem steering committee. SveDem is supported financially by the Swedish Associations of Local Authorities and Regions. Most of the statistical analyses were conducted at the Division of Biostatistics, Institute of Environmental Medicine, Karolinska Institute, 171 77 Solna, Sweden.
Open access funding provided by Karolinska Institute. This study was supported by the regional agreement on medical training and clinical research between the Stockholm Region and the Karolinska Institutet (ALF); the Health, Medicine and Technique grants from the Stockholm Region and Kungliga Tekniska Högskolan-KTH; Swedish medical research council grant (VR) # 2022-01425 (Garcia-Ptacek) and 2020-02014 (Eriksdotter); Stiftelsen Dementia; Margaretha af Ugglas Foundation; Karolinska Institutet Research Foundation; Karolinska Institutet Foundation for Diseases of Aging; Johanniterorden i Sverige/Swedish Order of St John; the Erling Persson foundation; and by the private initiative "Innovative ways to fight Alzheimer´s disease - Leif Lundblad Family and others".
Author information
Authors and affiliations.
Division of Neurogeriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden
Bojana Petek, Irena Kalar, Silvia Maioli, Bengt Winblad & Milica Gregoric Kramberger
Clinical Institute of Genomic Medicine, University Medical Centre Ljubljana, Ljubljana, Slovenia
Bojana Petek
Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Bojana Petek, Irena Kalar & Milica Gregoric Kramberger
Medical Statistics Unit, Department of Learning, Informatics, Management and Ethics, Karolinska Institutet, Stockholm, Sweden
Henrike Häbel
Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden
Hong Xu, Marta Villa-Lopez, Minh Tuan Hoang, Shayan Mostafaei, Maria Eriksdotter & Sara Garcia-Ptacek
Faculty of Medicine, University Complutense of Madrid, Madrid, Spain
Marta Villa-Lopez
Department of Neurology, University of Alberta Hospital, Edmonton, Canada
Department of Neurology, University Medical Centre Ljubljana, Ljubljana, Slovenia
Irena Kalar & Milica Gregoric Kramberger
Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
Minh Tuan Hoang & Shayan Mostafaei
Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Division of Neurogeriatrics, Karolinska Institutet, Stockholm, Sweden
Silvia Maioli
Division of Neuro, Department of Clinical Neurosciences, Karolinska Institutet, Stockholm, Sweden
Joana B. Pereira
Aging and Inflammation Theme, Karolinska University Hospital, Stockholm, Sweden
Bengt Winblad, Maria Eriksdotter & Sara Garcia-Ptacek
You can also search for this author in PubMed Google Scholar
Contributions
BP contributed to study design, interpretation of data, drafted and revised the manuscript. SGP designed the study, contributed to acquisition and analysis of data, drafted, and revised the manuscript, and approved the final version. HH contributed to data preparation, performed the analysis and interpretation of data, revised, and approved the final version of the manuscript. HX contributed to study design, acquisition, and interpretation of data, revised, and approved the final version of manuscript. ME contributed to data acquisition, study design, critically revised the manuscript and approved the final version of the manuscript. MVL, IK, MTH, SM, JBP, SM, BW, MGK critically revised the manuscript and approved the final version of the manuscript
Corresponding authors
Correspondence to Bojana Petek or Sara Garcia-Ptacek .
Ethics declarations
Ethics approval and consent to participate.
This study complies with the Declaration of Helsinki and was approved by the regional human ethics committee in Stockholm (numbers: 2015/743-31/4 and 2017/942-32). All patients and their relatives were informed of inclusion in SveDem at the time of diagnosis and had the right to decline the participation or withdraw the data from the registry at any point. The data were de-identified before analysis.
Consent for publication
Not applicable.
Competing interests
SGP holds stock in AstraZeneca, Bioarctic, Calliditas, Camurus, Dynavax, Moderna, Novo Nordisk, Pfizer, Sanofi, and Vicore. The other authors report no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Supplementary table 1. Cognitive decline in subgroups of statins users vs users of non-users of statins. Supplementary table 2. Cognitive decline in subgroups of simvastatin users vs rosuvastatin users. Supplementary table 3. Cognitive decline in subgroups of lipophilic statins users vs hydrophilic statins users. Supplementary table 4. Cognitive decline in subgroups of fungal statins users vs synthetic statins users. Supplementary table 5. Cognitive decline in subgroups of non-statin lipid-lowering medication users vs statins users. Supplementary table 6. Cognitive decline in different treatment groups, sensitivity analysis. Supplementary table 7. Characteristics of incident users.
ICD-10 codes for comorbidities
Diabetes mellitus/insulin/other antidiabetics (E1[0-4] A10A A10B), arrhythmia (I49), alcohol-related disease (E244 F10 G312 G621 G721 I426 K292 K70 K860 O354 P043 Q860 T51 Y90 Y91 Z502 Z714), chronic kidney disease (N18 N19 I120 I131 N032 N033 N034 N035 N036 N037 N052 N053 N054 N055 N056 N057 N18 N19 N250 T856 T857 Z490 Z491 Z492 Z940 Z992), heart failure (I500, I501, I509), cardiovascular disease (I21[0-4] I219 I220 I221 I228 I229 I25 I63 I739 I70), myocardial infarction (I21 I22 I252), respiratory disease (J4[0-7] J6[0-7] J684 J701 J703), haemorrhagic stroke (I60 I61 I62), other stroke (I63 I64 I69), anaemia (D50 D62), liver failure (K7), ischemic heart disease (I20 I21 I22 I23 I24 I25), malignancy (C[0-9][0-9] D[1-3]D4[0-8]) and obesity (E66).
ATC codes for lipid-lowering medication
Simvastatin (C10AA01 C10BA02 C10BX01 C10BX04), atorvastatin (C10AA05 C10BA05 C10BX03, C10BX06 C10BX08 C10BX11 C10BX12 C10BX15), pravastatin (C10AA03), fluvastatin (C10AA04), rosuvastatin (C10AA07 C10BA06 C10BX05 C10BX07 C10BX09 C10BX10 C10BX13 C10BX14), pitavastatin (C10AA08), fibrates (C10AB), bile acid sequestrants (C10AC), nicotinic acid and derivates (C10AD) and other lipid modifying agents (C10AX).
ATC codes for comedication
Cardiac drugs (C01), vasoprotectives (C05), platelet aggregation inhibitors (B01AC), anticoagulants (B01AA B01AB B01AF B01AE07), antipsychotics (N05A), anxiolytics (N05B), hypnotics (N05C), antidepressants (N06A), anticholinesterases (N06DA), memantine (N06DX01) and vitamin D (A11CC).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Petek, B., Häbel, H., Xu, H. et al. Statins and cognitive decline in patients with Alzheimer’s and mixed dementia: a longitudinal registry-based cohort study. Alz Res Therapy 15 , 220 (2023). https://doi.org/10.1186/s13195-023-01360-0
Download citation
Received : 06 August 2023
Accepted : 28 November 2023
Published : 20 December 2023
DOI : https://doi.org/10.1186/s13195-023-01360-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cohort study
- HMG-CoA reductase inhibitors
- Alzheimer's disease
- Cognitive impairment
- Cholesterol metabolism
Alzheimer's Research & Therapy
ISSN: 1758-9193
- General enquiries: [email protected]
- International edition
- Australia edition
- Europe edition
Hundreds of thousands face being denied revolutionary new dementia drugs in England
Exclusive: Treatments near approval but lack of diagnostic capacity means NHS unprepared for rollout, report says
What are the symptoms of dementia and how do you get a diagnosis?
Hundreds of thousands of dementia patients in England face being denied access to revolutionary new drugs because the diagnostic capacity of the NHS lags behind every other G7 country, according to a damning report.
After decades of research to find a cure for the condition projected to affect 153 million people worldwide by 2050, scientists have successfully developed the first treatments to tackle the underlying causes rather than only relieve the symptoms. Two new drugs could get the green light for use on the NHS within weeks.
However, their effectiveness depends on prompt and early diagnosis of patients . The report, obtained by the Guardian, says the NHS lacks the diagnostic capacity to accurately identify those eligible in time.
The analysis reveals England is unprepared for the rollout of new treatments, with “large gaps in diagnostic capacity” for dementia. It also warns of a £14bn funding black hole that must be plugged if England is to diagnose dementia as quickly as the other G7 countries, the US, Canada, France, Germany, Italy and Japan.
To be eligible for either of the new drugs, lecanemab and donanemab, patients have to be in the early stages of dementia and have had scans to confirm high levels of amyloid in their brain. However, the report says England has the lowest per capita number of PET scanners of any G7 country and the lowest number of MRI scanners. England also has the second-lowest number of dementia specialists needed to diagnose the condition, such as neurologists, old age psychiatrists and geriatricians.
The analysis was produced by experts from organisations including Alzheimer’s Research UK, Alzheimer’s Disease International and the Alzheimer’s Society.
A second study, an NHS briefing paper also seen by the Guardian, estimates that as many as 280,000 patients in England may be eligible for the new treatments if regulators recommend the drugs for use in the health service. However, if the treatments are approved, the NHS would be unprepared for their delivery, the first report says.
“The potential approval of the first of the disease-modifying AD [Alzheimer’s disease] treatments in the UK as early as 2024, and the prospect of subsequent availability in England, shines a light on the stark gap in diagnostic infrastructure needed to provide high-quality dementia care,” it says.
“While future detection and diagnostic technologies might allow for lowering investment levels in later years compared to our projections, the progressive nature of AD means that prolonged wait times would deprive numerous patients of the opportunity to receive a treatment while it will still be effective.”
Dr Susan Mitchell, the head of policy at Alzheimer’s Research UK and a co-author of the report, told the Guardian the NHS had “suffered years of under-investment in diagnostics” and as a result dementia patients were “being left to fall through the cracks”.
“When it comes to diagnosing dementia, timing is everything. Yet right now, too many people are anxiously waiting to get a brain scan or speak to a specialist, and this is stopping them from getting the care and support they deserve. If new Alzheimer’s treatments are approved by regulators, delays to diagnosis could also hurt people’s chances of accessing them, as they are thought to be most effective for those who are in the disease’s earliest stages.”
The most recent NHS figures show fewer than two-thirds of people with dementia (64.5%) have a formal diagnosis – below the government’s target of 67%. People in England wait on average up to two years to receive a diagnosis, and up to four years if under 65.
For the report, the authors compared data and statistics for England with that of the six other countries in the G7. The report found England had the lowest number of PET scanners per 1 million people, at 1.20. Canada has 1.52, Germany has 1.63, France has 2.48, Italy has 3.55, Japan has 4.70, and the US has 5.45.
England also has the lowest number of MRI scanners per 1 million people (6.31), while Canada has 10.06, France has 15.38, Italy has 30.22, Germany has 34.47, the US has 40.44, and Japan has 55.21.
When it comes to dementia specialists, England also fares badly, with 5.04 per 100,000 people, according to the report. Only Canada has a worse record (4.94). France has 6.46, the US has 8.82, Japan has 11.08, and Italy has 15.58. Germany has almost five times as many as England (24.02).
It is estimated that only 2% of people getting a dementia diagnosis in England have access to gold standard tests, the report says.
after newsletter promotion
NHS England officials said dementia diagnosis rates were at their highest for three years. Staff had worked hard to recover services after the Covid-19 crisis when people were less likely to come forward for care, they said.
Mitchell said that without an overhaul of the diagnosis system, people in the earliest stages of dementia could be at risk of missing out on the first-of-a-kind treatments.
“It will be impossible to improve either early or accurate diagnosis without addressing gaps in the workforce and infrastructure – and this research shows just how far England has to go,” she said. “It is shocking to see us sitting at the bottom of the G7 tables for PET and MRI capacity, and only slightly better in terms of staffing. Investment in NHS staff, equipment and facilities is needed to turn the tide.”
The number of people living with dementia in the UK is predicted to rise from 944,000 to 1.6 million by 2050. “This is a crisis that should be at the top of the prime minister’s priority list,” said Mitchell.
The Department of Health and Social Care said it was working to identify and treat more people with dementia, and offer new treatments as they became available.
A spokesperson said: “NHS England is working on improving dementia diagnosis in care homes and taking steps to secure additional diagnostic capacity, including MRI, lumbar puncture and PET-CT scanners.”
- Alzheimer's
- Health policy
- Public services policy
- Mental health

Plans for Royal Mail delivery cuts could risk patient safety, NHS leaders warn

Thousands to be offered blood tests for dementia in UK trial

NHS consultants accept pay offer, ending year-long dispute with government

Mental health trust failings contributed to Norfolk man’s death, coroner finds

Air pollution could be significant cause of dementia – even for those not predisposed
Early blood test to predict dementia is step closer as biological markers identified.

NHS ombudsman warns hospitals are cynically burying evidence of poor care

NHS ombudsman Rob Behrens: ‘There are serious issues of concern’
Blood test could revolutionise diagnosis of Alzheimer’s, experts say
Most viewed.
March 27, 2024
Does Long-Term Benadryl Use Increase Dementia Risk?
Benadryl, which contains diphenhydramine, is a drugstore mainstay and just one medication out of many that could possibly damage brain health
By Hannah Seo

John Kuczala/Getty Images
In the past few months TikTok videos about the over-the-counter antihistamine Benadryl have gone viral because of research suggesting that long-term use of the popular drug is associated with an increased risk of developing dementia. And similar effects on memory and cognition have also been suggested for dozens of other common medications.
Benadryl is a brand-name medication that contains diphenhydramine, the active ingredient in many allergy, cold and anti-itch drugs. It can cause significant drowsiness and is also found in several sleep aids. Diphenhydramine has anticholinergic effects, meaning it blocks the action of a neurotransmitter called acetylcholine. Medical uses of anticholinergics go beyond allergy relief; drugs in this class have long been used as prescription tricyclic antidepressants and incontinence treatments, as well as over-the-counter sleep aids. But experts have been finding evidence that links anticholinergics to increased dementia risks. “That’s now clear, and we have plenty of data to back it up,” says Malaz Boustani, a geriatrician and neuroscientist at the Indiana University School of Medicine. He and other researchers are now trying to determine whether anticholinergics really are contributing to dementia development in any way—and if so, what exactly is happening in the brains of vulnerable adults.
Anticholinergics inhibit the parasympathetic nervous system , which regulates rest and stress responses. The drugs do this by binding to brain cell receptors and blocking acetylcholine, a neurotransmitter that plays a part in many bodily systems, including heart regulation, muscle contractions, urination and digestion. Acetylcholine also affects attention and memory—and a lack of the neurotransmitter in the brain has been linked to Alzheimer’s disease . When bound to receptors, anticholinergics prevent acetylcholine from acting on the parasympathetic nervous system, often causing effects such as dry mouth. These medications can also make people groggy by blocking histamine receptors, which are involved in alertness as well as allergies. Side effects should fade once the drug is out of someone’s system (which in Benadryl’s case would be within a couple of days). But Boustani says less is known about the effects of regular or heavy use.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
A 2015 paper in JAMA Internal Medicine offered some striking insight into possible long-term effects. The hallmark study—which became the basis for the recent viral TikToks—observed adults 65 years or older who had been taking various kinds and amounts of anticholinergic drugs for 10 years. The group reporting the highest amount of such use had a 54 percent increased risk of developing dementia. Since the study, a steady stream of new research has corroborated this link.
The studies show an association between dementia risk and the quantity and frequency of anticholinergic use. One capsule of Benadryl to relieve an allergy every once in a while probably won’t affect someone all that much, but repeated doses of anticholinergic drugs over months or years pose a greater risk, Boustani says. Taking multiple anticholinergics for multiple conditions may also increase your exposure and could raise the risk further, he adds. Another consideration is that some of these drugs have more potent effects than others. Boustani’s team created the Anticholinergic Cognitive Burden (ACB) Scale , a list of drugs with scores based on their potential negative effects on the brain. The scale goes from 1 (possibly an anticholinergic burden) to 3 (definitely a strong anticholinergic burden), Boustani says. For example, someone taking three anticholinergic drugs, all with a score of 1, will have a total anticholinergic cognitive burden score of 3. Boustani says that person may see roughly the same cumulative effects as someone who just takes one anticholinergic with a score of 3. There are currently no protocols or recommendations for doctors and pharmacists to consider a person’s ACB score when prescribing drugs. But the American Geriatrics Society maintains a list of drugs older people should avoid, and it does include many anticholinergic medications.
Researchers are still digging into the specific brain mechanisms that may be involved in elevated dementia risk from these drugs, Boustani says. A leading hypothesis suggests this might be related to how the drugs interact with neurotransmitter receptors. Long-term anticholinergic use might leave acetylcholine receptors perpetually blocked, triggering a series of reactions that either leads to the production of beta-amyloid—a toxic protein thought to be a main cause of dementia—or prevents the brain from clearing this protein out. Researchers have suggested these drugs might affect inflammation and blood flow in the brain, which may result in memory loss and weakened cognition. But so far this research is inconclusive.
Some experts are skeptical that anticholinergics play a role in developing dementia; they suspect the drugs may instead worsen underlying cognitive issues. Ariel Gildengers, a psychiatrist at the University of Pittsburgh, led a 2023 study that did not show a link between anticholinergics and developing dementia. But it did show a link between the drugs and mild cognitive impairment (MCI), a condition in which people have worse cognitive function than expected at their age but do not meet the criteria for dementia. And MCI does have a noticeable chance of turning into dementia. About 10 to 15 percent of people living with MCI develop dementia each year, according to the Alzheimer’s Association . “If you are in the earliest stages of dementia, then anticholinergics may kind of make the dementia more apparent without actually causing the dementia,” Gildengers says.
Research in this area has several major caveats, says Shelly Gray, a pharmacy professor at the University of Washington, who was the first author of the 2015 JAMA paper. “We cannot determine that anticholinergics actually cause dementia” because the 2015 study and others on the relationship have been observational, she says. Clinical trials are needed to confirm causation. Most of these studies are also done in adults aged 65 and older, who are more likely to develop dementia than younger adults and more likely to face medical conditions such as insomnia, which are often treated with anticholinergics. Gray, Boustani and Gildengers say that they keep these possible confounding factors in mind when they design and analyze their studies.
Because research has focused mostly on older adults, there is less evidence on whether younger people who take anticholinergics have increased risk of dementia later in life. Gray says it’s generally a good idea to avoid anticholinergics, however, especially if you’re older. “A big reason why we're seeing anticholinergic medications used by older adults is because they are available over the counter,” Gray says. Many readily available medications have anticholinergic active ingredients, including Benadryl, as well as the antihistamine Dimetapp (which contains the drug brompheniramine), the motion sickness medication Dramamine (dimenhydrinate) and the sleep aid Unisom (doxylamine).
Many anticholinergics can be swapped with other options, Gray says. For example, studies show that cognitive behavioral therapy is often effective for insomnia. "Second-generation antihistamines,” such as those in Claritin and Zyrtec, along with steroid-based nasal sprays such as Flonase, will counteract allergy symptoms without targeting acetylcholine receptors—says Jyothi Tirumalasetty, a clinical assistant professor of medicine at Stanford University, who specializes in allergy and immunology.
The Food and Drug Administration first approved Benadryl as a prescription antihistamine in 1946 and for over-the-counter sales in 1980s. Given the research on potential risks, Tirumalasetty says the FDA might evaluate the drug’s safety differently today and may consider making it available only through prescription. The FDA did not respond to a request for comment by the time of publication . In an e-mail to Scientific American, Kenvue (formerly a division of Johnson & Johnson and Benadryl’s current proprietor) wrote, “We are not aware of any studies that show a causal link between labeled use of diphenhydramine, and an increased risk of developing dementia. Diphenhydramine is an ingredient which is generally recognized as safe and effective by the FDA for [over-the-counter] use.”
Researchers are continuing to investigate anticholinergics and their possible long-term effects on brain health, hoping to help with future clinical and regulation guidance. Boustani says many questions persist, such as how the timing of taking or discontinuing these drugs affects the development of future memory problems.
For now, Boustani is focused on empowering his patients, especially older adults, with the ability to understand their medications and any attendant risks. He says people taking or considering an anticholinergic at any age should ask themselves a few questions: “Are there any alternatives? What symptoms am I addressing, and is alleviating them worth this risk? What is the smallest dose and time period I can take this medication for?”
“The brain is a precious organ,” Boustani says. “There is no health without brain health.”

Thousands of people to trial Alzheimer's blood tests
Memory clinics across the UK are to begin trialling blood tests to see if they can accurately diagnose dementia.
The hope is that more people will be able to access care, support and new drug treatments at an earlier stage.
The research, by University College London and the University of Oxford, will involve around 5,000 volunteers.
The five-year project will study blood tests for Alzheimer's disease and other forms of dementia.
Currently, around a third of patients with dementia never get a formal diagnosis and are left with worry and uncertainty about their condition.
Rogue proteins
Only around 2% of patients have one of the 'gold standard' tests for Alzheimer's - either a specialist PET brain scan or a spinal lumbar puncture.
Both can show the presence of rogue proteins in the brain such as amyloid and tau which start to accumulate up to 20 years before symptoms emerge - but tests are expensive.
The Oxford team will be looking at a range of blood tests, which could be a cheaper and easier way for doctors to spot early signs of the disease.
One blood test will look for traces of these proteins in the blood in order to diagnose Alzheimer's disease, the most common form of dementia. Some tests will also look for potential biomarkers for vascular and frontotemporal dementia, and dementia with Lewy bodies.
The researchers will also look at whether the blood tests can help detect these diseases at various stages.
NHS 'not ready' for new Alzheimer's drugs
NHS exploring Alzheimer's disease blood tests
How common is early Alzheimer's?
Dr Vanessa Raymont, from the University of Oxford, is leading a study which will recruit volunteers from more than 50 UK trial sites, which are all NHS memory clinics.
She told the BBC that although several dementia blood tests had already shown promising results, they had limitations.
"Research has tended to exclude the very elderly, ethnic minorities and those with other medical conditions so we need to understand what the data looks like in the real world, which is why these projects are so important."
The University College London (UCL) team will focus on the most promising biomarker for Alzheimer's disease, called p-tau217, which can indicate levels of amyloid and tau in the brain.
Its trial will see if measuring p-tau217 in the blood can increase the rate of diagnosis for Alzheimer's disease in people with early dementia, but also those with mild but progressive memory problems.
Access to new treatments
Jonathan Schott, professor of neurology at UCL, who is leading the trial, said: "An early, accurate diagnosis of Alzheimer's disease is already important, allowing people to access appropriate care and medications.
"If, as we hope, new treatments that can slow down Alzheimer's disease become available soon, then this will be vital," he said.
"This would pave the way for fair and equitable access to new and potentially life-changing treatments to all who might benefit."
Two treatments have shown in trials that they can slow the progression of early stage Alzheimer's. Doctors say the benefits are modest but they represent the first 'disease-modifying' drugs.
The drugs, lecanemab and donanemab, are currently being considered by the MHRA, the body which approves drugs in the UK.
Even if they are granted licences, they would then need to be given the green light by health assessment bodies which consider their cost-effectiveness for the NHS, before being rolled out to patients.
Dr Sheona Scales, director of research at Alzheimer's Research UK, said: "We've seen the enormous potential that blood tests are showing for improving the diagnostic process for people and their loved ones in other disease areas."
She said it was important to see "the same step-change in dementia", which is the greatest health challenge facing the UK.
The Blood Biomarker Challenge is being funded by Alzheimer's Society, Alzheimer's Research UK, the National Institute for Health and Research and Gates Ventures, including £5m from People's Postcode Lottery.

IMAGES
VIDEO
COMMENTS
By Mayo Clinic Staff. Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning. These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda).
November 08, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
[Originally published January 19, 2023. Updated: July 24, 2023.] The Food and Drug Administration (FDA) recently granted full approval to a new Alzheimer's treatment called lecanemab, which has been shown to moderately slow cognitive and functional decline in early-stage cases of the disease.. Alzheimer's disease is a progressive disorder that damages and destroys nerve cells in the brain.
Researchers say we appear to be at the start of a new era for Alzheimer's treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady ...
Current Landscape in Treatment Research for AD. No new drug has been approved by FDA for AD since 2003 and there are no approved DMTs for AD, despite many long and expensive trials. 22,28 As a matter of fact, more than 200 research projects in the last decade have failed or have been abandoned. 10 Nevertheless, drug pipeline for AD is still ...
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled ...
Trials for new drugs or non-drug-based dementia treatments. Studies on new tests or procedures for diagnosis. Trials that investigate ways to prevent the onset of diseases. Studies exploring ways to improve quality of life for individuals living with a chronic illness, their caregivers and family members. Online studies.
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent.Diagnose. Treat. Care (PDF, 17.5M), a 2022 scientific progress report. This report provides a comprehensive overview of the meaningful progress researchers made from April 2021 through March 2022 to address the enormous challenges of Alzheimer's and related dementia diseases.
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by ...
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. ... the Association is committed to accelerating the global progress of new treatments, preventions and, ultimately, a cure. Our Impact . Information for Researchers . Research We Fund .
Dementia is a collective term for a group of diseases or injuries which primarily or secondarily affect the brain. Alzheimer's is the most common of these and accounts for around 60-70% of cases. Other types include vascular dementia, dementia with Lewy bodies (abnormal protein clumps) and a group of diseases that contribute to frontotemporal ...
By Alice Park. January 4, 2024 2:20 PM EST. 2023 was a strong year for innovative new drugs, with new medications for Alzheimer's disease, weight loss, and the first treatment based on the gene ...
December 20, 2023. PHILADELPHIA - A "chaperone" molecule that slows the formation of certain proteins reversed disease signs, including memory impairment, in a mouse model of Alzheimer's disease, according to a study from researchers at the Perelman School of Medicine at the University of Pennsylvania. In the study, published in Aging ...
Despite this, efforts to develop treatments that break up and remove these tangled proteins have had little success. But a new discovery by University of California, Berkeley, researchers suggests that the accumulation of aggregated proteins isn't what kills brain cells. Rather, it's the body's failure to turn off these cells' stress ...
Anne Trafton. June 27, 2023. A pair of structures in the hypothalamus called the mammillary bodies (highlighted in green) are among the first brain regions to show neurodegeneration in Alzheimer ...
'A defining moment' Kate Lee, Alzheimer's Society CEO said: This is a defining moment for dementia research. But new treatments could mean nothing if we don't fix dementia diagnosis. "We estimate around 720,000 people in the UK could potentially benefit from these emerging new Alzheimer's disease treatments if they're approved for use here.
Published Aug. 29, 2022 2:19 p.m. PDT. Share. More than 747,000 Canadians currently live with Alzheimer's and other forms of dementia, but a new study suggests age-related memory loss could be ...
Read the latest medical research on dementia. Causes, symptoms, lowering the risks, care, medications and new treatments for dementia.
FTD is the No. 1 cause of dementia in patients under 60, with up to 30% of cases attributed to genetics. It has three main variants with symptoms that may overlap. The most common causes dramatic personality shifts, which may manifest as lack of empathy, apathy, impulsivity, compulsive eating, and socially and sexually inappropriate behavior ...
Scientists at the University of Leeds and Lancaster University in the UK have discovered a potential new target for Alzheimer's disease treatment - PDE4B.. Alzheimer's disease is the leading cause of dementia and disability in old age. As the number of people diagnosed with Alzheimer's disease is on the increase, new treatments are urgently needed to improve the quality of life for ...
In this review, the authors explored the clinical features of frontotemporal dementia (FTD), focusing on treatment. The clinical features of FTD are unique, with disinhibition, apathy, loss of empathy, and compulsions common. Motor changes occur later in the illness. The two major proteins that aggregate in the brain with FTD are tau and TDP-43, whereas a minority of patients aggregate FET ...
Discovery could lead to new, targeted therapeutics for frontotemporal dementia. An international team of researchers including experts at the Indiana University School of Medicine has identified a protein found in the brains of people with frontotemporal dementia (FTD), discovering a new target for potential treatments for the disease.
The results of sensitivity analysis restricted to incident users were not consistent. Some patients with AD or mixed dementia with indication for lipid-lowering medication may benefit cognitively from statin treatment; however, further research is needed to clarify the findings of sensitivity analyses.
That may be good news for dementia risk A new study finds brain size has steadily increased for people born after the 1930s ... the center is focused on translating research findings into better tools to diagnose dementia and treatment for patients while focusing on the long-term goal of finding a way to prevent or cure Alzheimer's disease ...
Jonathan Schott, the chief medical officer at Alzheimer's Research UK, will lead a trial on the most promising blood biomarker in tests on 1,100 people across the UK. The second trial will test ...
After decades of research to find a cure for the condition projected to affect 153 million people worldwide by 2050, scientists have successfully developed the first treatments to tackle the ...
Since the study, a steady stream of new research has corroborated this link. The studies show an association between dementia risk and the quantity and frequency of anticholinergic use.
New research suggests factors like diabetes, alcohol consumption, and traffic-related pollution may cause damage to a part of the brain associated with dementia. The brain area of concern is the ...
The long-term hope is that more people will be able to access new treatments at an earlier stage. ... begin trialling blood tests to see if they can accurately diagnose dementia. ... The research ...