
- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search

IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breathe (Sheff)
- v.14(2); 2018 Jun

How to obtain informed consent for research
1 University of Messina, “G. Martino” Hospital, Messina, Italy
Amelia Licari
2 University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [1]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [2]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [3].
Short abstract
The process of obtaining informed consent for clinical trials is tightly regulated; complications arise in circumstances when consent may be waived, or when needed from vulnerable populations http://ow.ly/rEMe30j5MVq
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [ 1 ]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [ 2 ]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [ 3 ].
The informed consent form must be written in language easily understood by the subjects, it must minimise the possibility of coercion or undue influence, and the subject must be given sufficient time to consider participation. However, informed consent is not merely a form that is signed, but is a process in which the subject has an understanding of the research and its risks, and it is tightly described in ethical codes and regulations for human subject research [ 2 ].
Educational aims
- To provide a comprehensive overview of issues in obtaining informed consent in clinical research.
- To describe the process of obtaining informed consent in clinical trials.
- To highlight the circumstances under which informed consent can be waived.
- To review the setting of obtaining informed consent from “vulnerable populations”.
The informed consent process
The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [ 4 ]. Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation. Conditions posing practical challenges in obtaining informed consent from the real subject may include situations of medical emergency or obtaining consent from “vulnerable” subjects and/or children [ 5 ].
Research-related information must be presented to enable people to voluntarily decide whether or not to participate as a research subject. For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject’s participation; a detailed description of study treatment or intervention and of any experimental procedures (including, in the case of randomised clinical trials (RCTs), also blinding and randomisation); a statement that participation in research is voluntary; probable risks and benefits associated with research participation; details of the nature of the illness and possible outcome if the condition is left untreated; availability, risks and benefits of alternative treatments; information about procedures adopted for ensuring data protection/confidentiality/privacy, including duration of storage of personal data; details about the handling of any incidental findings of the research; description of any planned genetic tests; details of insurance coverage in case of injury; reference contacts for any further answers to pertinent questions about the research and the subject’s rights and in case of any research-related injury to the subject; and any other information that seems necessary for an informed decision to be taken by the subject. Of particular importance, a statement offering the subject the opportunity to withdraw at any time from the research without consequences must be provided during the information disclosure [ 2 ]. Specific information should be provided in case of research projects involving children, incapacitated adults not able to give informed consent, illiterate populations, etc. (as will be described later in this article).
The information about the research should be given by a physician or by other individuals ( i.e. researchers) with appropriate scientific training and qualifications [ 6 ]. Furthermore, the location where the informed consent is being discussed, and the subject’s physical, emotional and psychological capability, must be taken into consideration when taking consent from a human subject.
Informed consent: when is it not necessary?
After institutional review board (IRB) or independent ethics committee approval is achieved, obtaining informed consent from each human subject prior to his/her participation in clinical trial is mandatory [ 5 ]. However, when specific circumstances occur, the informed consent can be waived, and “research without consent” is possible, which allows enrolment of patients without their consent, under strict regulation [ 7 ]. In order that research without consent is considered justifiable, the following three conditions have to be met: 1) it is impracticable to obtain consent, 2) the research does not infringe the principle of self-determination, and 3) the research provides significant clinical relevance [ 8 ].
The first condition, of “impracticability”, occurs when obtaining informed consent is burdened by high impact in terms of time and economic resources or could compromise the study’s validity [ 8 ]. The second condition means that, although physicians are requested to ensure that the patient has understood the aim of the research and the risks and/or benefits associated with study participation, the researchers are also advised to respect the patient’s decision-making capacity, not interfering with his/her decisions and acting always in the patient’s best interest [ 9 ]. The third condition leads to justification of waiving consent when the clinical relevance and public health importance are potentially high [ 8 ].
The formal literature identifies different types of RCTs and classifies them into three macro-areas: 1) RCTs based on infeasibility of informed consent; 2) RCTs that omit informed consent only for control groups; and 3) RCTs that omit informed consent entirely.
RCTs based on infeasibility of informed consent
Emergency clinical studies, involving critically ill subjects, represent an exception to the requirement of informed consent. The investigated life-saving therapy and the medical intervention may be required immediately, not permitting the researchers to wait and respect all procedures of obtaining informed consent. Within this context, the researchers will be able to proceed with patient recruitment, also without the subject’s consent to treatment, when, prior to the study, the IRB has ascertained the presence of mandatory conditions ( table 1 ) [ 10 ].
Table 1
Conditions to be met in emergency clinical study
Cluster randomised studies include cluster-cluster and individual-cluster research [ 11 ]. In cluster-cluster designs ( e.g. studies on infectious disease prevention), the intervention involves the entire target community, so that single subjects cannot refuse it [ 12 ]. Conversely, in individual-cluster designs ( e.g. studies on primary care), although the intervention involves all the selected community, the right to refuse treatment is allowed. Under this circumstance, the omission of informed consent is justified only when the treatment refusal undermines the validity of the research study and/or procedures [ 13 ].
RCTs that omit informed consent only for control groups
In Zelen’s single-consent model ( e.g. RCTs in infectious or oncological diseases), randomisation occurs prior to any consent, and informed consent is sought only from individuals assigned to experimental treatment [ 14 ]. In the control group, the physicians do not make substantial changes in routine patient care, so informed consent is not required for patient enrolment [ 8 ].
In order to improve study recruitment, Zelen developed the double-consent design. Specifically, informed consent is requested for subjects to be involved in the study but not for the randomisation, preventing psychological distress [ 14 ].
In follow-up studies, the nested consent model ( e.g. for single cohort studies) or cohort multiple RCTs model ( e.g. for multiple cohort studies) is applied. In these variants, patients give their consent for prospective follow-up; however, they remain blinded to any randomised experimental interventions [ 15 ].
In trials using the model of “consent to postponed information”, the informed consent process is carried out after the study is completed [ 16 ].
All these RCT types aim to avoid unnecessary stress in patients who will not receive the new promising experimental treatment. Moreover, these clinical study designs do not affect the standard therapeutic approach or infringe the rights of the patients in the control group; therefore, the clinical trial can proceed without obtaining informed consent [ 8 ].
RCTs that omit informed consent entirely
Based on the fact that patients are assigned to standard care interventions, no informed consent is sought either in low-risk pragmatic RCTs [ 17 ] or in prompted optional randomisation trials [ 18 , 19 ]. However, in a low-risk pragmatic RCT, patients do not have the possibility to choose one of the two standard treatments, whereas in a prompted optional randomisation trial, both the researchers and the enrolled patients can choose one type of treatment over another, despite the randomisation results [ 6 ].
Special needs: vulnerable patients
A “vulnerable population” is defined as a disadvantaged community subgroup unable to make informed choices, protect themselves from inherent or intended risks, or keep their own interests safeguarded [ 20 ]. In the health domain, “vulnerable populations” refers to physical vulnerability ( e.g. pregnant women, fetuses, children, orphans, students, employees, prisoners, the military, and those who are chronically or terminally ill), psychological vulnerability (cognitively and intellectually impaired individuals) and social vulnerability (those who are homeless, from ethnic minorities, are immigrants or refugees) [ 20 ].
Due to a compromised free will and inability to make conscious decisions, several ethical dilemmas (related to communications, privacy and treatment) often arise when research involves these populations. Guaranteeing protection of rights, safety, data privacy and confidentiality of vulnerable subjects are prerogatives of good clinical practice, and law dispositions are regulated and strictly monitored by the applicable authorities [ 21 ].
Physical vulnerability
For a long time, pregnant women were excluded from clinical research because of their “vulnerability”. Although pregnant women are able to make informed and conscious choices, they have been considered “vulnerable” due to the potential risks to the fetus, who is also considered as a “patient” [ 22 ]. More recently, with the consideration of pregnant women as “scientifically complex” rather than “vulnerable” subjects, it has been permitted to involve this category in research trials [ 23 ]. The “scientific complexity” reflects both ethical and physiological complexity. The ethical aspects are secondary to the need to find a balance between interests of the fetus and the mother. The physiological aspects are strictly related to the pregnancy status [ 24 ].
Research studies involving pregnant women and fetuses have to satisfy specific federal regulations ( table 2 ). The following appropriate precautions should be taken in research studies involving pregnant women: no pregnant woman may be involved as a subject in a human clinical research project unless the purpose of the research is to meet the health needs of the mother and the fetus will be placed at risk only to the minimum extent necessary to meet such needs, or the risk to the fetus is minimal [ 25 ].
Table 2
Conditions to be met in research studies involving pregnant women and fetuses
Researchers can enrol pregnant women only when the mother and/or the father are legally competent. In fact, the consent to participate in research may be either self-directed (only the mother’s consent is required) or made with the guidance of the woman’s partner. However, the father’s consent need not be obtained when: 1) the research activity is directed to the health needs of the mother; 2) the father’s identity is doubtful; 3) the father is absent; or 4) a pregnancy from rape has occurred [ 26 ]. The consent signature requirements from the mother and father are summarised in table 3 . Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient’s health (mother and/or fetus) will be in danger.
Table 3
Consent signature requirements for pregnant women and children
# : consent requirements are the same whether the risk is “no more than minimal” or “more than minimal”.
Medical students and employees, who take part in numerous aspects of patient care in primary, secondary and tertiary care settings, are often invited to participate in human studies as volunteers. Frequently, the requesting researcher is their supervisor or instructor, who may push them to participate in the study, which can negatively influence their decision and also violate the consent legitimacy. Therefore, in order to protect these subjects against “coercion” or “undue influence”, when an investigator wishes to recruit medical students or employees, they must first obtain IRB approval for inclusion in the study of these vulnerable subgroups [ 27 ].
Prisoners, defined as any individual involuntarily confined or detained in a penal institution, are considered as “vulnerable” because they may be coerced into study participation, and also, due to both cognitive and psychiatric disorders, they can show an impaired ability to provide voluntary informed consent [ 28 ]. To protect this population, the Office for Human Research Protections has stipulated federal regulations according to which the only studies that may involve prisoners are those with independent and valid reasons for involving them ( table 4 ) [ 25 ].
Table 4
Studies that may involve prisoners
Due to the context of war in which they work, as well as the critical care setting in which they are treated, military subjects often receive medical care and/or participate in biomedical research under an “implied consent” condition. Moreover, the superior–subordinate relationship contributes to favour coercion or undue influence, making this population vulnerable [ 29 ]. To curb this phenomenon and to ensure that participation is truly voluntary, the US Dept of Defense agencies have adopted requirements similar to those that govern medical research that applies to the civilian population. Accordingly, the medical research recruitment session happens in the absence of superiors, and the informed consent is obtained prior to participating in a medical research study. The presence of an ombudsman guarantees and verifies that the participation is voluntary and that the information provided during recruitment is complete, accurate and clear. A payment as an incentive is acceptable but it must not be used to legitimise a coercive interference. Additional protection is provided to students at service academies, especially those aged <18 years. However, when emergency research is conducted or the research study advances the development of a medical product needed by the armed forces, informed consent will not be required [ 29 ].
Psychological vulnerability
Mental disability may compromise the self-determination and decision-making capacities [ 30 ]. Researchers interested in enrolling individuals with cognitive disorders are invited to apply different strategies to promote a better understanding of information-gathering processes. Simplifying the questions and content, adopting supportive technologies, using a more simple language, and spending more time for the information process have been suggested as useful and valid measures. When all these strategies prove to be insufficient, the investigators are required to obtain consent from a legally authorised representative [ 30 ].
Social vulnerability
Similarly to other vulnerable populations, research involving the homeless, ethnic minorities, immigrants and refugees is regulated by laws and specific procedures. Cultural and language differences, “undocumented” migrant status, and the precarious legal positions of these subjects raise several ethical issues, such as whether the participation is truly voluntary, or there are unrealistic expectations, or any benefits for their “status”.
Obtaining informed consent in these groups is extremely complex. A friendly procedure has been identified as the best way to adequately involve these vulnerable groups. A health centre or community building could represent an accessible location. The reimbursement of travel expenses for applicants can be a valid solution to obtain a representative sample for the clinical research. Clear and simple language, emphasising confidentiality, with the help of professional interpreters, can tempt migrants to sign the consent form. Lastly, the possibility of receiving something back in return for their contribution may enable successful enrolment of migrants in research [ 31 ].
Special needs: children
Because of their young age as well as their limited emotional and intellectual abilities, children are considered to be legally incompetent to give valid informed consent; thus, to enrol a child in a research study, the permission by at least one parent or legal representative is mandatory ( table 3 ). For subjects aged <18 years, biological or adoptive parents or legal guardians (persons having both legal capacity and responsibility) can give consent on behalf of their child, exercising free power of choice without any form of coercion. While married mothers and fathers both have parental responsibility, unmarried parents can exert parental responsibility only if they are named individually on the child’s birth certificate. Also, divorced parents maintain parental responsibility, but it is necessary to know to whom the child’s custody has been assigned [ 32 ]. However, on this matter, the European laws and regulations are not harmonised and several discrepancies are present in each country [ 33 ].
Despite potential benefits for the research subjects, the failure of parents to give consent (or their refusal to give consent) is not a rare circumstance [ 34 ]. It can be the case that researchers are dealing with underage parents, so that, although underage parents are responsible for representing their children, as minors themselves they are not considered to be sufficiently mature; therefore, they will be not able to give valid consent. Literacy and socioeconomic levels have been identified as the most common reasons for parental non-response [ 34 ]. Clarity and adequate explanation of research information materials should be part of effective planning to overcome language and social barriers.
In clinical studies in which the adopted methodology constitutes “less than minimal risks” for children, passive parental consent represents a possible way to more easily obtain informed parental consent [ 34 ]. Furthermore, parents can be informed with regard to a possible study involving their children, and, at the time of data collection, only the child’s assent is required. In fact, although the child’s decision-making capacity and understanding of the research project in which he/she will be involved may be limited, the Medical Research Council have shown that, when study details are provided and communicated in a clear and adequate manner, the child can be able to reach a decision and participate consciously in the research [ 35 ]. “Assent” is the term coined to express the child’s willingness to participate in clinical trials despite their young age. The “assent” should include and respect the following key points: 1) helping the child to acquire disease awareness; 2) explaining the potential impact of the experimental treatment; 3) evaluating the child’s ability to understand and adapt to new situations or challenges; and 4) positively influencing the patient’s willingness to participate in clinical trials [ 36 ]. Although the “assent” is not mandatory for research offering a direct benefit for the child, it arises from the need to respect paediatric research subjects [ 37 ]. The evaluation of the capacity to provide the “assent” is based on developmental stage, intellectual abilities and life or disease experience. Usually, the cut-off age of 7 years is used for the beginning of logical thought processes and rational decision making [ 38 ]. However, “assent” for children aged <7 years can be also required once the ability to read and write has been verified [ 32 ]. Figures 1 and and2 2 summarise the parental and assent permission requirements, respectively.

Flow chart of parental permission requirements.

Flow chart of child assent requirements.
When conducting clinical research, the obtaining of informed consent is required. Informed consent is a procedure through which a competent subject, after having received and understood all the research-related information, can voluntarily provide his or her willingness to participate in a clinical trial. However, when it is impracticable to obtain consent, and the research does not infringe the principle of self-determination and also provides significant clinical relevance, the researcher is legally authorised to proceed without informed consent. Furthermore, in order to preserve the self-determination and decision-making rights, specific law dispositions are applied when vulnerable populations are enrolled in clinical trials.
Self-evaluation questions
- a) Diagnosis
- b) Risks and benefits of treatment
- c) Alternatives to treatment
- d) Family’s wishes
- a) When a minor is considered as emancipated
- b) When a patient is found to be incompetent
- c) When immediate treatment is necessary to prevent death or permanent impairment
- d) When the subject is aged >18 years
- a) Minor is married or divorced
- b) Minor on active duty in the US armed forces
- c) Minor is considered self-sufficient by a court
- d) Minor having a son
Suggested answers
- All research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent.
- Voluntary expression of consent and adequate information disclosure about the research are critical and essential elements of the informed consent process.
- When specific circumstances occur, informed consent can be waived: if it is impracticable to obtain consent, if the research does not infringe the principle of self-determination, and if the research provides significant clinical relevance.
- Participation of vulnerable patients in clinical trials is regulated by specific law dispositions.
Conflict of interest: None declared.
Understanding Informed Consent Forms

The informed consent form puts you in control of your health decisions and protects your rights.
Before you join a cancer research study, you’ll receive an informed consent form to review, ask questions about, and sign. The form covers a description of the study’s purpose, procedures and safety measures researchers will follow, and what is expected of study participants.
The form is part of the informed consent process. This process protects your rights. It also gives you control over your choice to take part in research.
Federal law requires that researchers give the informed consent form to potential participants. You will have a chance to ask questions about information you read in the form. Once you understand the study, you can choose if you want to sign the consent form and take part.
Getting comfortable with informed consent forms
Understanding a consent form is an important part of the informed consent process. All informed consent forms are different. But most forms will have the same kinds of information about the study divided into sections.
Overview of study This section tells you:
- what question researchers are hoping to answer with the study
- the main potential risks and benefits of taking part in the study
- your responsibilities as a participant
The overview also describes the choices you have if you do not take part in the study. And it explains the reasons you might leave the study early. This section also lets you know that taking part is voluntary and you may leave the study at any time.
Study design This section describes:
- each study group
- what each group will be asked to do, including tests and procedures you will have and drugs you will take
- how many people will be in each group
- how long the study will last
Risks and benefits This section describes:
- all known potential risks and benefits of taking part in the study
- the most common side effects
- how the study will help doctors learn about your disease
Cost This section explains the costs of taking part in a study. Insurance and the study sponsor should cover some expenses. But you might have others, such as the cost of travel to the trial site. Learn more about who pays for clinical trials .

Safety and Clinical Trials
Explore the many safety measures in place to help keep you safe during a trial or study.
Your rights This section covers your rights:
- if you are injured because of the study or neglect
- to privacy when it comes to sharing your medical information
- to leave the study at any time
How to get more information on the study This section provides ways to:
- learn more about the study
- learn more about your rights as a participant
- reach the study team
Signature As with most legal documents, informed consent forms require a signature. But it’s important to remember that you are not signing away your rights or binding yourself to the study. You may still leave the study at any time. If a participant is under 18 years of age, read about the children’s assent process .
Sample informed consent form
Read a sample consent form to become familiar with the content and sections. Note: this form is for informational purposes only and does not represent a real study. Your form may have other sections, and may present the information in a different way.
Tips for reading informed consent forms
Amy Rose oversees the day-to-day operations of clinical research, including the informed consent process for potential research participants. She is the associate director in Clinical Research Services at University of Pittsburgh Medical Center Hillman Cancer Center.
If you are thinking about taking part in a clinical trial, Amy suggests that you:
- Bring a friend or relative to the appointment when your study team discusses the study and informed consent form.
- Take the form home . Read it in a comfortable place, take notes, and jot down questions for your doctor or study team. “You can highlight things and write down questions right on the consent form,” Amy said.
- Ask a close friend or spouse to read the form so you can discuss it with them. Have them take notes too, and compare your understanding. Reviewing it with your primary care provider can also be helpful, Amy said.
- Don’t be afraid to ask questions . Amy said she and other medical professionals don’t always realize when they aren’t explaining things clearly. They may be in a rush or too steeped in the language of medicine.
- You can decide not to take part before or after you sign the form. “We always tell people their participation is completely voluntary,” Amy said. “You do not have to continue just because you sign this consent today.”
The informed consent process does not end when you sign a form and decide to take part. Your doctor and study team will keep you updated about the study so you can continue to make informed decisions.

Have questions about informed consent?
Connect with our cancer information specialists.
Phone: 1-800-4-CANCER Chat: LiveHelp Email: [email protected]
Available Monday–Friday 9:00 a.m. to 9:00 p.m. ET.
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
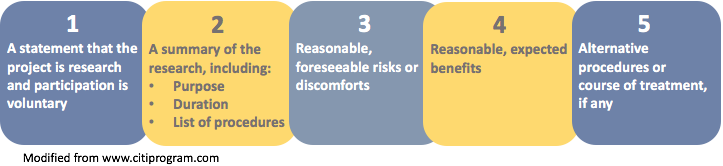
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
Informed consent guidance.
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/10/24
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Brief protocol for exempt research including data management and security questionnaire
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Child assent 12-14
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
Informed Consent for Clinical Trials

On this page you will find information on:
What is Informed Consent
Before enrolling in a clinical trial, the following information must be given to each potential research subject
When Appropriate, one or more of the following elements of information must also be provided in the informed consent document
A potential research subject must have an opportunity to
Informed consent may not include language that
To many, the term informed consent is mistakenly viewed as the same as getting a research participant's signature on the consent form. FDA believes that obtaining a research participant's verbal or written informed consent is only part of the process. Informed consent involves providing a potential participant with:
adequate information to allow for an informed decision about participation in the clinical investigation.
facilitating the potential participant's understanding of the information.
an appropriate amount of time to ask questions and to discuss with family and friends the research protocol and whether you should participate.
obtaining the potential participant's voluntary agreement to participate.
continuing to provide information as the clinical investigation progresses or as the subject or situation requires.
To be effective, the process must provide sufficient opportunity for the participant to consider whether to participate. ( 21 CFR 50.20 .) FDA considers this to include allowing sufficient time for participants to consider the information and providing time and opportunity for the participant to ask questions and have those questions answered. The investigator (or other study staff who are conducting the informed consent interview) and the participant should exchange information and discuss the contents of the informed consent document. This process must occur under circumstances that minimize the possibility of coercion or undue influence. (21 CFR 50.20.)
What is Informed Consent?
As new medical products are being developed, no one knows for sure how well they will work, or what risks they will find. Clinical trials are used to answer questions such as:
Are new medical products safe enough to outweigh the risks related to the underlying condition?,
How should the product be used? (for example, the best dose, frequency, or any special precautions necessary to avoid problems),
How effective is the medical product at relieving symptoms, treating or curing a condition.
The main purpose of clinical trials is to “study” new medical products in people. It is important for people who are considering participation in a clinical trial to understand their role, as a “subject of research” and not as a patient.
While research subjects may get personal treatment benefit from participating in a clinical trial, they must understand that they:
may not benefit from the clinical trial,
may be exposed to unknown risks,
are entering into a study that may be very different from the standard medical practices that they currently know
To make an informed decision about whether to participate or not in a clinical trial, people need to be informed about:
what will be done to them,
how the protocol (plan of research) works,
what risks or discomforts they may experience,
participation being a voluntary decision on their part.
This information is provided to potential participants through the informed consent process. Informed consent means that the purpose of the research is explained to them, including what their role would be and how the trial will work.
A central part of the informed consent process is the informed consent document. The Food and Drug Administration (FDA) does not dictate the specific language required for the informed consent document, but does require certain basic elements of consent be included.

Before enrolling in a clinical trial, the following information must be given to each potential research subject:
A statement explaining that the study involves research.
An explanation of the purposes of the research.
The expected length of time for participation.
A description of all the procedures that will be completed during enrollment on the clinical trial.
Information about all experimental procedures the will be completed during the clinical trial.
A description of any predictable risks.
Any possible discomforts (e.g., injections, frequency of blood test etc.) that could occur as a result of the research.
Any possible benefits that may be expected from the research.
Information about any alternative procedures or treatment (if any) that might benefit the research subject.
A statement describing:
the confidentiality of information collected during the clinical trial,
how records that identify the subject will be kept
the possibility that the FDA may inspect the records.
For research involving more than minimal risk information including
an explanation as to whether any compensation or medical treatments are available if injury occurs,
what they consist of, or
where more information may be found.
questions about the research,
research subjects' rights,
injury related to the clinical trial.
Research subject participation is voluntary,
Research subjects have the right to refuse treatment and will not losing any benefits for which they are entitled,
Research subjects may choose to stop participation in the clinical trial at any time without losing benefits for which they are entitled.
Contact information will be provided for answers to :
A statement that:
When Appropriate, one or more of the following elements of information must also be provided in the informed consent document:
A statement that the research treatment or procedure may involve unexpected risks (to the subject, unborn baby, if the subject is or may become pregnant).
Any reasons why the research subject participation may be ended by the clinical trial investigator (e.g., failing to follow the requirements of the trial or changes in lab values that fall outside of the clinical trial limits).
Added costs to the research subject that may result from participating in the trial.
The consequence of leaving a trial before it is completed (e.g. if the research and procedures require a slow and organized end of participation).
A statement that important findings discovered during the clinical trial will be provided to the research subject.
The approximate number of research subjects that will be enrolled in the study.
A potential research subject must have an opportunity to:
read the consent document,
ask questions about anything they do not understand.
Usually, if one is considering participating in a clinical trial, he or she may take the consent document home to discuss with family, friend or advocate.
An investigator should only get consent from a potential research subject if:
enough time was given to the research subject to consider whether or not to participate
the investigator has not persuaded or influenced the potential research subject.
The information must be in language that is understandable to the research subject.
Informed consent may not include language that:
the research subject is made to ignore or appear to ignore any of the research subject's legal rights,
releases or appears to release the investigator, the sponsor, the institution, or its agents from their liability for negligence.
Participating in clinical trials is voluntary. You have the right not to participate, or to end your participation in the clinical trial at any time. Read the informed consent document carefully. Ask questions about any information you don’t understand or find confusing.
Draft Guidance: Informed Consent Information Sheet Guidance for IRBs, Clinical Investigators, and Sponsors

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Informed Consent FAQs
What is informed consent and when, why, and how must it be obtained.
The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subject’s legally authorized representative, unless (1) the research is exempt under 45 CFR 46.101(b) ; (2) the IRB finds and documents that informed consent can be waived ( 45 CFR 46.116(c) or (d) ); or (3) the IRB finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings. When informed consent is required, it must be sought prospectively, and documented to the extent required under HHS regulations at 45 CFR 46.117 . [Food and Drug Administration (FDA) regulations at 21 CFR part 50 may also apply if the research involves a clinical investigation regulated by FDA.]
The requirement to obtain the legally effective informed consent of individuals before involving them in research is one of the central protections provided for under the HHS regulations at 45 CFR part 46 . This requirement is founded on the principle of respect for persons, one of the three ethical principles governing human subjects research described in the Belmont Report. The principle of respect for persons requires that individuals be treated as autonomous agents and that the rights and welfare of persons with diminished autonomy be appropriately protected. The Belmont Report states that an autonomous agent is “an individual capable of deliberation about personal goals and of acting under the direction of such deliberation.” Respect for persons requires that prospective research subjects “be given the opportunity to choose what shall or shall not happen to them” and thus necessitates adequate standards for informed consent.
The informed consent process involves three key features: (1) disclosing to potential research subjects information needed to make an informed decision; (2) facilitating the understanding of what has been disclosed; and (3) promoting the voluntariness of the decision about whether or not to participate in the research. Informed consent must be legally effective and prospectively obtained. HHS regulations at 45 CFR 46.116 and 45 CFR 46.117 describe the informed consent requirements.
The informed consent process is the critical communication link between the prospective human subject and an investigator, beginning with the initial approach of an investigator to the potential subject (e.g., through a flyer, brochure, or any advertisement regarding the research study) and continuing until the completion of the research study. For the purposes of the HHS regulations at 45 CFR part 46, “investigators” are individuals who conduct human subjects research projects, including individuals directly involved in seeking the voluntary informed consent of potential subjects. Investigators can include physicians, scientists, nurses, administrative staff, teachers, and students, among others.
The informed consent process should be an active process of sharing information between the investigator and the prospective subject. The exchange of information between the investigator and prospective subjects can occur via one or more of the following modes of communication, among others: face-to-face contact; mail; telephone; video; or fax. Prospective subjects should be provided with ample opportunity to ask questions and seek clarification from the investigator. The prospective subjects should be in a position to freely decide whether to initially enroll in the research, or later, to withdraw or continue participating in the research. The informed consent process should ensure that all critical information about a study is completely disclosed, and that prospective subjects or their legally authorized representatives adequately understand the research so that they can make informed choices.
The procedures used in seeking and obtaining informed consent should be designed to communicate with the subject population in terms that they can understand. Information about a research project must be presented in such a way that enables each person to voluntarily decide whether or not to participate as a research subject. Thus, the information must be conveyed in language understandable to those being asked to participate as subjects in the research ( 45 CFR 46.116 ).
For most research, informed consent is documented using a written document that provides key information regarding the research. The consent form is intended, in part, to provide information for the potential subject’s current and future reference and to document the interaction between the subject and the investigator. However, even if a signed consent form is required, it alone does not constitute an adequate consent process. The informed consent process is an ongoing exchange of information between the investigator and the subject and could include, for example, use of question and answer sessions, community meetings, and videotape presentations. In all circumstances, however, individuals should be provided with an opportunity to have their questions and concerns addressed on an individual basis.
The consent process and its documentation should be revised when deficiencies in its accuracy or completeness are noted, when new information about reasonably foreseeable risks and potential benefits becomes available, or when other additional information becomes known that will improve the consent process. Such revisions must be reviewed and approved by an IRB prior to the revised consent being utilized except when necessary to eliminate apparent immediate hazards to subjects ( 45 CFR 46.103(b)(4) ).
Is it possible to obtain legally effective informed consent to research in an urgent or emergency care setting?
Yes, in certain circumstances it is possible to obtain legally effective informed consent in an urgent or emergency care setting. For a particular research study, the answer depends on (1) the expected medical condition of the prospective subject population; (2) the nature of the research; (3) whether there is sufficient time for the potential subjects or their legally authorized representatives to consider participation; and (4) whether the circumstances for obtaining informed consent appropriately minimize the possibility of coercion or undue influence. The Institutional Review Board (IRB) and investigator(s) would have to consider several variables. For example, what is the likely health and emotional condition of the patient population being considered for the proposed research (e.g., conscious but receiving emergency care, undergoing preparation prior to surgery)? What is the likely ability of this population during the consent process to process information, ask questions, and consider the risk involved? What is the timing of the consent process and is it so close to the receipt of care that the patient might blur the distinction between treatment and research?
Because individuals receiving urgent or emergent medical care frequently may be vulnerable to coercion or undue influence, even if temporarily, additional protections may be required to ensure the subject's consent to participate in research is truly voluntary and sought under circumstances that minimize the possibility of coercion or undue influence ( 45 CFR 46.111(b) , ( 45 CFR 46.116 ). In addition, in some cases, it might be possible to obtain consent from a legally authorized representative (e.g., in the case of decisionally incapacitated individuals). In certain emergency circumstances, the Secretarial waiver of informed consent under 45 CFR 46.101(i) may be applicable. It should be noted that if the research is regulated by FDA, the Secretarial waiver permits the research to be conducted under a comparable provision.
See the Office for Human Research Protections' (OHRP) guidance ; and HHS policy (PDF - 22KB) .
What are the basic elements of informed consent?
The basic required elements of informed consent can be found in the HHS regulations at 45 CFR 46.116(a) . Also see OHRP Informed Consent Tips .
The regulations require that the following information must be conveyed to each subject:
- a statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject’s participation, a description of the procedures to be followed, and identification of any procedures which are experimental;
- a description of any reasonably foreseeable risks or discomforts to the subject;
- a description of any benefits to the subject or to others which may reasonably be expected from the research;
- a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject;
- a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained;
- for research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained;
- an explanation of whom to contact for answers to pertinent questions about the research and research subjects’ rights, and whom to contact in the event of a research-related injury to the subject; and
- a statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.
Additional elements are described at 45 CFR 46.116(b)
What additional information might be appropriate to provide during the consent process?
When determined to be appropriate by the Institutional Review Board (IRB), subjects must be provided with one or more of the following additional elements of information during the informed consent process (see 45 CFR 46.116(b) ):
- a statement that the particular treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant) which are currently unforeseeable;
- anticipated circumstances under which the subject’s participation may be terminated by the investigator without regard to the subject’s consent;
- any additional costs to the subject that may result from participation in the research;
- the consequences of a subject’s decision to withdraw from the research and procedures for orderly termination of participation by the subject;
- a statement that significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject; and
- the approximate number of subjects involved in the study.
It is up to the IRB to determine in a particular instance whether some or all of the above additional elements must be included as part of the informed consent process for a particular study. The IRB should make this determination based on the nature of the research and its knowledge of the local research context. If the IRB determines that additional elements are appropriate to the research study, this additional information should be considered just as essential as the eight basic elements of informed consent described in the HHS regulations at 45 CFR 46.116(a) .
Furthermore, an IRB may require that additional information beyond the basic and additional elements be given to subjects during the informed consent process, when in the IRB’s judgment the additional information would meaningfully add to the protection of the rights and welfare of the subjects 45 CFR 46.109(b) .
Can consent or parental permission ever be "passive" or "implied?"
Terms such as “passive” or “implied” consent are not referenced in the HHS regulations. However, OHRP is aware that these terms are sometimes used by investigators or IRBs to describe a process in which consent or parental permission requirements have been altered or waived, or for which the requirement to document consent or parental permission has been waived.
HHS regulations at 45 CFR 46.116 state that no investigator may involve a human being as a subject unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative. However, under conditions specified in the regulations at 45 CFR 46.116(c) or (d) an IRB may approve a consent procedure that does not include, or that alters some or all of the elements of informed consent set forth in 45 CFR 46.116 . In some cases, an IRB also can waive the requirement to obtain consent ( 45 CFR 46.116(c) and (d) ). In addition, under conditions specified in the regulations at 45 CFR 46.117 , an IRB may also waive the requirement for documentation of informed consent. (Note that the regulations at 45 CFR 46.408(c) also permit an IRB to waive parental permission.)
For example, a researcher conducting a survey (that does not qualify for an exemption under 45 CFR 46.101(b) mails a survey questionnaire to a random sample of adults. The survey materials clearly state that by responding to the questions and mailing the survey back, the recipients have agreed to participate in the research. However, the materials accompanying the questionnaire do not include all of the elements of consent listed at 45 CFR 46.116(a) and do not require that the subject sign a consent form. If the IRB has approved this alteration of the consent process and has waived the need for documentation of consent, then such procedures are permissible under the regulations. By sending back a completed survey the recipient has implied that he or she consents to participate but has not signed an informed consent document. Although some might call this “implied informed consent,” OHRP would consider this to be a permissible informed consent process if the IRB has approved the informed consent alteration and waived the requirement for documentation of informed consent.
The term “passive consent” is sometimes used in research with children to describe situations in which the investigator can assume that a parent is permitting a child to participate. For example, researchers collecting survey and behavioral data from children at school provide parents with information regarding the study by mail and ask the parent(s) to return a form if they do not want their child to participate. Sometimes this practice is referred to as an opt out procedure, which is not consistent with the regulatory requirement for seeking and obtaining parental permission. If the IRB determines that the conditions for waiver of parental permission can be met, then the IRB could waive the requirement for parental permission under 45 CFR 46.408(c) or 45 CFR 46.116(c) or (d) . Even though not required by the regulations, an IRB may require that parents be given the opportunity to refuse permission even when the IRB has waived the regulatory requirement to obtain parental permission.
What does it mean to minimize the possibility of coercion or undue influence?
The HHS regulations state that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence” ( 45 CFR 46.116 ). This requirement applies to all nonexempt human subjects research not eligible for a waiver of the consent requirements.
Coercion occurs when an overt or implicit threat of harm is intentionally presented by one person to another in order to obtain compliance. For example, an investigator might tell a prospective subject that he or she will lose access to needed health services if he or she does not participate in the research.
Undue influence , by contrast, often occurs through an offer of an excessive or inappropriate reward or other overture in order to obtain compliance. For example, an investigator might promise psychology students extra credit if they participate in the research. If that is the only way a student can earn extra credit, then the investigator is unduly influencing potential subjects. If, however, she offers comparable non-research alternatives for earning extra credit, the possibility of undue influence is minimized.
In addition to undue influence that can arise with the offering of rewards, undue influence also can be subtle. For example, patients might feel obligated to participate in research if their physician is also the investigator, or students might feel pressure to participate in research if everyone else in the class is doing so. Because influence is contextual, and undue influence is likely to depend on an individual’s situation, it is often difficult for IRBs to draw a bright line delimiting undue influence. It is up to the IRB to use its discretion in determining which circumstances give rise to undue influence. For example, an IRB might consider whether the informed consent process will take place at an appropriate time and in an appropriate setting, and whether the prospective subject may feel pressured into acting quickly or be discouraged from seeking advice from others.
Because of their relative nature and lack of clear-cut standards on the boundaries of inappropriate and appropriate forms of influence, investigators and IRBs must be vigilant about minimizing the possibility for coercion and undue influence. Reasonable assessments can be made to minimize the likelihood of undue influence or coercion occurring. For example, IRBs may restrict levels of financial or nonfinancial incentives for participation and should carefully review the information to be disclosed to potential subjects to ensure that the incentives and how they will be provided are clearly described. Known benefits should be stated accurately but not exaggerated, and potential or uncertain benefits should be stated as such, with clear language indicating how much is known about the uncertainty or likelihood of these potential benefits.
The regulatory requirements for IRB review and approval also specify the need for the IRB -- in order to approve research covered by the HHS regulations -- to ensure that “When some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children , prisoners , pregnant women , mentally disabled persons, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects” ( 45 CFR 46.111(b) ). Thus, inducements that would ordinarily be acceptable in some populations may become undue influences for these vulnerable subject groups.
When does compensating subjects undermine informed consent or parental permission?
The HHS regulations require that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence” ( 45 CFR 46.116 ).
Paying research subjects in exchange for their participation is a common and, in general, acceptable practice. However, difficult questions must be addressed by the IRB. For example, how much money should research subjects receive, and for what should subjects receive payment -- their time, inconvenience, discomfort, or some other consideration -- IRBs must be sensitive to whether any aspect of the proposed remuneration will be an undue influence, thus interfering with the potential subjects’ ability to give voluntary informed consent.
Remuneration for participation in research should be just and fair. However, the specifics of each protocol will influence how those determinations are made. Both researchers and IRBs need to be familiar with the study population and the context of the research in order to make reasonable judgments about how compensation might affect participation. Wherever the remuneration is set, it will influence the decisions of some more than others. In particular, it will be more important to those for whom it will make a significant financial difference. Thus, IRBs should be cautious that payments are not so high that they create an “undue influence” or offer undue inducement that could compromise a prospective subject’s examination and evaluation of the risks or affect the voluntariness of his or her choices.
Information submitted to IRBs should indicate and justify proposed levels and purposes of remuneration, which also should be clearly stated in the accompanying consent forms.
Some institutions have adopted policies regarding the recruitment and payment of volunteers. IRBs and investigators should ensure that the consent process includes a detailed account of the terms of payment, including a description of the conditions under which a subject would receive partial or no payment (e.g., what will happen if he or she withdraws part way through the research or the investigator removes a subject from the study for medical or noncompliance reasons).
Finally, in studies of considerable duration or that involve multiple interactions or interventions, OHRP recommends that payment be prorated for the time of participation in the study rather than delayed until study completion, because the latter could unduly influence a subject’s decision to exercise his or her right to withdraw at any time. For example, if the study is conducted over a period of 6 months, there might be a monthly or bi-monthly payment. Or, if the study involves 12 sessions, there might be payment after every two sessions.
The above principles would apply to remuneration offered to parents whose children are prospective subjects.
[ Note: The previous version of the response to this FAQ included the following sentences. “In no case should remuneration be viewed as a way of offsetting risks; that is, it should not be considered a benefit to be weighed against study risks. The level of remuneration should not be so high as to cause a prospective subject to accept risks that he or she would not accept in the absence of the remuneration.” The first sentence has been struck because this FAQ focuses on potential undue influence in the consent process (45 CFR 46.116) rather than on IRB considerations under 45 CFR 46.111. However, OHRP continues to assert that IRBs should not consider remuneration as a way of offsetting risks. The second sentence has been deleted to clarify that remuneration to subjects may include compensation for risks associated with their participation in research and that compensation may be an acceptable motive for agreeing to participate in research. In addition, the previous version contained the following sentence, which has been struck because it is focused on IRB considerations under 45 CFR 46. 111 rather than informed consent, and was misplaced in this FAQ: “IRBs may need to request of the investigator some plan for monitoring subject recruitment to ensure that such inducements do not result in inequitable subject recruitment (e.g., recruiting only economically disadvantaged individuals).” ]
Can non-financial enrollment incentives constitute undue influence?
Yes, in certain circumstances. Non-monetary incentives (e.g., extra credit for students, access to services or programs) also can create undue influence on a potential subject’s decision about research participation. Informed consent always must be voluntary ( 45 CFR 46.116 ).
IRBs should ensure that non-financial incentives are not so great as to diminish the voluntariness of consent or cloud someone’s appreciation of risks or potential benefits that might be gained from participating in a study ( 45 CFR 46.116 ). Moreover, it must be clear that choosing to not participate will not adversely affect an individual’s relationship with the institution or its staff or the provision of services in any way (e.g., loss of credits or access to programs) ( 45 CFR 46.116(a)(8) ).
Overt coercion (e.g., threatening loss of services or access to programs to which the potential subjects are otherwise entitled) is never appropriate. However, it might be permissible to provide incentives to participate that do not constitute undue influence. Using enrollment incentives to recruit subjects may be ethically permissible as long as the IRB has determined that, although they may be a factor in a subject’s decision to participate, they have not served to unduly influence the subject to participate. To make this determination, IRBs should know who the subject population will be, what incentives are being offered, and the conditions under which the offer will be made.
What constitutes coercion or undue influence when students are involved in research in a college or university setting?
The regulations require that the investigator seek consent only under circumstances that minimize the possibility of coercion or undue influence ( 45 CFR 46.116 ). The Office for Human Research Protections (OHRP) recommends that institutions have policies in place that clarify for students and faculty that any participation of students in research must be voluntary. Reasonable levels of extra credit or rewards may be offered for participating in research. If extra credit or rewards are offered for participation, students must be provided with and informed of non-research alternatives involving comparable time and effort to obtain the extra credit in order for the possibility of undue influence to be minimized. However, if participation in research is a course requirement, students must be informed of non-research alternatives involving comparable time and effort to fulfill those requirements in order for the possibility of undue influence to be minimized. Moreover, students must not be penalized for refusing to participate in research ( 45 CFR 46.116(a)(8) ).
In addition, some research institutions use a so-called “student subject pool” to identify students who might be willing to participate in research, even when the exact nature of the research to be conducted has not yet been determined. Extra credits or other rewards are often offered as an incentive to encourage participation. Students who sign up for such pools have not legally consented to participate in a research study since they have not been provided with sufficient information concerning the exact study in which they would participate. Thus, signing up to be in a subject pool is only a first and preliminary step by which individuals can indicate their willingness to be considered for research participation. The student must also provide informed consent, unless the consent requirement is waived by an IRB once he or she is being considered for a specific study ( 45 CFR 46.116 ). Furthermore, individuals in the pool must be free to decline participation in any available research projects without penalty ( 45 CFR 46.116(a)(8) ).
What constitutes coercion or undue influence when employees are the subjects of research?
The issues involving employees as research subjects are essentially identical to those involving students as research subjects: that is, investigators and IRBs must be cautious about the potential for coercion or undue influence and the need to protect confidentiality.
Employee participation raises questions about the ability of employees to exercise free choice, for example, because of the possibility that a decision to participate could affect performance evaluations or job advancement, even if it is only the employee’s perception that this is the case. In the case of coercion, refusal to participate might result in a loss of benefits (e.g., salary increases, time off). In the case of undue influence, a decision to participate could result in a job promotion. Employees are likely to view their employers as authority figures to whom they must show deference, which could undermine the freedom of their choice.
Should the initial consent or parental permission procedure ever be repeated or supplemented?
Yes, in some circumstances. The HHS regulations require that an investigator obtain legally effective informed consent from subjects or a legally authorized representative before the subjects may be involved in research ( 45 CFR 46.116 ), unless this requirement has been waived by an IRB . Likewise, for research involving children, permission of the potential subjects' parents or guardians must be obtained ( 45 CFR46.408(c) ), unless an IRB has waived this requirement . Ensuring an adequate consent or parental permission process may require repeating or supplementing the initial consent procedure. The regulations also stipulate that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimizes the possibility of coercion or undue influence” ( 45 CFR 46.116 ). This requirement also might necessitate repeating or supplementing the initial consent procedure.
Informed consent and parental permission should be viewed as an ongoing process. The regulations do not explicitly describe all of the circumstances that might require repeating or supplementing the informed consent process. However, they do require that potential subjects be provided, when appropriate, with a “statement that significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject” ( 45 CFR 46.116(b)(5) ). Thus, to ensure that consent remains legally effective -- for example, if the protocol design or risks have changed, or if a substantial period of time has elapsed between the time consent was obtained and the study begins -- it might be necessary to ensure that subjects still want to participate in the research. For example, the prospective subject may no longer be interested in participating, may no longer meet the eligibility criteria, may no longer find the risks acceptable, or may no longer have the time to complete all study-related activities.
The IRB must review and approve any changes in the approved consent procedure, including alterations of the content, as described in the elements listed at 45 CFR 46.116 , or in its timing, and may consider whether there is a need to reiterate the process ( 45 CFR 46.103(b)(4) ). The IRB should take into account whether the changes could potentially affect a subject’s understanding of the nature of the study or potentially affect a subject’s willingness to participate. If so, such changes need to be made in the informed consent document. Even without significant changes to a protocol or informed consent document, periodic reiteration or affirmation of consent is often a good idea, especially if the study takes place over a long period of time or is particularly complex. Minor changes, such as correcting nonsubstantive typographical errors in the consent document, would not generally rise to a level requiring repeating the consent process.
How far in advance of research participation can consent be obtained?
The HHS regulations at 45 CFR part 46 do not specify how far in advance of study entry a subject can provide consent. The amount of time required by a subject to make a decision would presumably depend on the nature of the study, taking into account, among other factors, the degree of risk, potential benefits, alternatives, and desire to consult with family members or others. However, if a prolonged period of time elapses from the date of consent to the date of entry into the study even if there have been no changes in the study design or no new significant findings affecting the study it might be prudent to review the information contained in the consent form with the subject prior to initiating any research procedures with the subject.
Can records or databases be reviewed to identify potential subjects without obtaining informed consent or parental permission?
Yes, under certain circumstances. Although the HHS regulations do not specifically reference this type of activity, sometimes referred to as “preparatory to research,” such an activity must be reviewed and approved by an IRB in accordance with HHS regulations at 45 CFR 46.109(a) when:
- The activity involves human subjects research, as defined by the regulations at 45 CFR 46.102(f) ;
- The research does not meet the criteria for exemption under HHS regulations at 45 CFR 46.101(b) .
In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in accordance with, and to the extent required by, HHS regulations at 45 CFR 46.116 and 45 CFR 46.117 respectively.
However, an IRB may approve a consent or parental permission procedure that does not include, or that alters, some or all of the elements of informed consent, or may waive the requirements to obtain informed consent ( 45 CFR 46.116(c) or (d) ). In order to permit investigators to obtain and record identifiable private information for the purposes of identifying potential subjects, OHRP expects that IRBs routinely will waive the requirement for informed consent for such activities. In assessing the level of risk to determine whether a waiver of informed consent or parental permission is permissible for the identification of potential subjects, the IRB need only consider the risk of investigators accessing the subjects’ identifiable private information, not the risks of the research in toto.
How can the consent and parental permission processes be designed to facilitate understanding?
The procedures used in obtaining informed consent and parental permission should be designed to inform the subject population or the parents of the subject population about the research in terms that they can understand. Therefore, informed consent and parental permission language and its documentation in the accompanying forms (especially explanation of the study’s purpose, duration, experimental procedures, alternatives, risks, and benefits) should be provided in language that is understandable and culturally sensitive to those being asked to participate or provide permission for their child’s participation.
If the prospective subjects include, for example, persons whose primary language is not English, or populations with low literacy levels, the IRB should take special care to ensure that both oral presentations and consent or permission forms are comprehensible to all subjects or the parents of subjects who are children. Subjects who do not speak English should be presented with a consent or permission document written in a language understandable to them. OHRP strongly encourages the use of such a document whenever possible. (See OHRP guidance on this topic at http://www.hhs.gov/ohrp/regulations-and-policy/guidance/obtaining-and-documenting-infomed-consent-non-english-speakers/index.html ; for information about requirements for child assent, see FAQs regarding research with children .)
In general, ordinary language should replace technical terms (e.g., upper extremities are better referred to as arms, venipuncture as taking blood from your arm with a needle, and so forth).
Some IRBs find that their lay members (e.g., community or non-scientist members) are particularly helpful in suggesting necessary modifications to language. Others ask members of the proposed subject population (e.g., clinic patients) to review consent or permission forms and indicate which parts they do not understand.
Can an electronic signature be used to document consent or parental permission?
Yes, under certain circumstances. First, the investigator and the IRB need to be aware of relevant laws pertaining to electronic signatures in the jurisdiction where the research is going to be conducted.
Unless the IRB waives the requirement for the investigator to obtain a signed consent or permission form based on the HHS regulations at 45 CFR 46.117(c) , a written consent or permission form, which may be an electronic version, must be given to and signed by the subjects or the subjects' legally authorized representatives or the parents of subjects who are children. Some form of the consent document must be made available to the subjects or the parents of subjects who are children in a format they can retain. OHRP would allow electronic signature of the document if such signatures are legally valid within the jurisdiction where the research is to be conducted.
OHRP does not mandate a specific method of electronic signature. Rather, OHRP permits IRBs to adopt such technologies for use as long as the IRB has considered applicable issues such as how the electronic signature is being created, if the signature can be shown to be legitimate, and if the consent or permission document can be produced in hard copy for review by the potential subject. One method of allowable electronic signatures in some jurisdictions is the use of a secure system for electronic or digital signature that provides an encrypted identifiable “signature.” If properly obtained, an electronic signature can be considered an “original” for the purposes of recordkeeping.
Is a faxed copy of the signed consent or parental permission form acceptable to document informed consent?
Yes, if it is more convenient for the subjects or parents of children who are subjects to fax a signed copy of the consent or permission form to the investigator, the research subjects or parents may fax the signed form. The subjects or parents need not provide the investigator with the original signed consent or parental permission documents.
Who must sign the informed consent or parental permission document?
When a written consent or parental permission form is used that embodies some or all of the elements of informed consent required by the regulations at 45 CFR 46.116 , the regulations only require that the informed consent or parental permission document be signed by the subjects or the subjects' legally authorized representatives or by the parents of children who are subjects ( 45 CFR 46.117(a) ) and 45 CFR 46.408(d) ). Only in situations where a short form is used, stating that the elements of informed consent required by 45 CFR 46.116 have been presented orally to the subject or the subject’s legally authorized representative or to the parent(s) of a child who is a subject, are there additional requirements for signatures ( 45 CFR 46.117(b)(2) ).
For the consent or parental permission process using the short form, the regulations state that there must be a witness to the oral presentation, who then signs both the short form and a copy of the IRB-approved written summary of what is to be said to the subject or the subject's legally authorized representative or to the parent(s) of a child who is a subject. The subject or the subject’s legally authorized representative or the parent(s) must sign the short form, and the person actually obtaining the consent must sign the copy of the summary ( 45 CFR 46.117(b)(2) ). Thus, three types of persons are involved in this specific consent process -- the subject or legally authorized representative or parent(s) of a child who is a subject, the person obtaining consent, and the witness.
Do signatures on consent forms have to be dated?
Although the HHS regulations at 45 CFR 46.117 do not require the consent form to be dated at the time it is signed, OHRP recommends that it be dated so that the IRB and others can document that informed consent was obtained prior to a subject’s participation in the research.
Who can be a legally authorized representative (LAR) for the purpose of providing consent on behalf of a prospective subject?
Legally authorized representative (LAR) means an individual or judicial or other body authorized under applicable law to consent on behalf of a prospective subject to the subject’s participation in the procedure(s) involved in the research ( 45 CFR 46.102(c) ). The regulations state that “no investigator may involve a human being as a subject in research covered by this policy unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative” ( 45 CFR 46.116 ). The issue as to who can be an LAR is determined by the laws of the jurisdiction in which the research is conducted (e.g., local or state law). Some states have statutes, regulations, or common law that specifically address consent by someone other than the subject for participation in research. Most states have no law specifically addressing the issue of consent in the research context. In these states, law that addresses who is authorized to give consent on behalf of another person to specific medical procedures or generally to medical treatment may be relevant if the research involves those medical procedures or medical treatment.
When the laws of the jurisdiction in which the research is being conducted provide a reasonable basis for authorizing an individual to consent on behalf of a prospective subject to their participation in the research procedure(s), OHRP would consider such an individual to be an LAR as defined by HHS regulations at 45 CFR 46.102(c) . IRBs may wish to consult with legal counsel when deciding who can serve as an LAR for subjects of proposed research.
When may a legally authorized representative provide consent on behalf of an adult with diminished decision-making capacity?
In answering this question, the HHS regulations at 45 CFR part 46 should be consulted in addition to the laws of the jurisdiction in which the research is conducted. As a general matter, if an adult lacks capacity to consent, for example, as a result of trauma, mental retardation, some forms of mental illness, or dementia - whether temporary, progressive, or permanent - only a legally authorized representative for that adult can give consent for participation in the research, unless the requirement to obtain informed consent is waived by the IRB in accordance with the requirements at 45 CFR 46.116(c)(d) , or in accordance with the provisions for emergency waiver, which are permitted under the authority of the HHS Secretary at 45 CFR 46.101(i) .
( See the Federal Register notice of this waiver .) Should the subject regain or develop the capacity to consent, then his or her consent must be obtained for any further research, as the consent of the legally authorized representative is no longer valid.
What should be considered in seeking informed consent from individuals with diminished decision-making capacity?
The HHS regulations are silent on the consent procedures specific to subjects with impaired decision-making capacity, for example, as a result of trauma, mental retardation, some forms of mental illness, or dementia, whether temporary, progressive, or permanent. The regulations do require that the IRB ensure that “additional safeguards have been included in the study to protect the rights and welfare” of all subjects that are “likely to be vulnerable to coercion or undue influence.” The regulations include “mentally disabled persons” in this category ( 45 CFR 46.111(b) ).
In research involving adult subjects with mental illnesses or cognitive impairments, the IRB and investigator(s) must be knowledgeable about the condition and any level of impairment that is likely to be present in the subject population. The regulations do speak to the fact that the IRB must possess “the professional competence necessary to review specific research activities” ( 45 CFR 46.107(a) ). This is achieved either by having members with the appropriate experience and expertise or inviting consultants with competence in the special area to assist in the review of issues that require expertise beyond or in addition to that available on the IRB ( 45 CFR 46.107(a) and (f) ). Ensuring such expertise on the IRB improves its ability to make determinations about subject recruitment, enrollment, and informed consent requirements that best match the needs of the subjects.
In some research, such as longitudinal studies involving progressive disorders or aging populations, enrolled subjects may be competent to consent on their own behalf at the outset, yet may experience effects of progressive or intermittent disorders that lead to decisional impairment during the course of the study. In these situations IRBs and investigators should consider the need to discuss with the prospective subjects whether they should designate someone to serve as a legally authorized representative at the outset of the study, consistent with all applicable laws. Even if a subject has consented on his or her own accord, a designated representative would be ready to step in as the legally authorized representative if the subject’s ability to assess his or her own needs and interests becomes compromised during the study.
What are the requirements for assent and parental permission in research with children?
The IRB must determine, to the extent required by 45 CFR 46.116 , that adequate provisions are made for soliciting the assent of the children -- when in the judgment of the IRB the children are capable of providing assent -- as well as the permission of the parents ( 45 CFR 46.408 ). Permission means the agreement of parent(s) or guardian to the participation of their child or ward in research ( 45 CFR 46.402(c) ).
By regulatory definition, children are “persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted” ( 45 CFR 46.402(a) ). In the United States the legal age of adulthood is a matter of state and local law. This means that who is legally considered a child may vary from state to state; in a large majority of states 18 years of age is the legal age of adulthood, but this is not true in every state, locality, or territory. State law also may address specific circumstances in which a person younger than the age of adulthood is legally authorized to consent to medical procedures: for example, some states allow children younger than the legal age of adulthood to consent to the provision of contraceptive services. Certain states provide a mechanism for the emancipation of minors, through which a child younger than the legal age of adulthood may gain certain civil rights, which might include the legal ability to consent to research participation.
The definition of children also takes into account the particular interventions or interactions involved in the proposed research (e.g., surveys, blood tests). For example, in some places individuals who are 16 years of age may legally consent to certain clinical interventions or interactions. If the involvement of human subjects in a proposed research activity consists of these interventions or interactions, then those individuals may be considered as adults for that purpose. If a proposed activity includes an intervention or interaction for which the subject has not yet reached the legal age of consent, however, that person must be considered a child.
Under 45 CFR 408(b) the IRB may find that the permission of one parent is sufficient for research to be conducted under 45 CFR 46.404 or 45 CFR 46.405 . Where research is conducted under 45 CFR 46.406 or 45 CFR 46.407 , permission must be obtained from both parents unless one parent is deceased, unknown, incompetent, or not reasonably available, or when only one parent has legal responsibility for the care and custody of the child.
Although the regulations state that children are unable to provide legally effective informed consent to participate in research, some might be able to give their assent. Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent ( 45 CFR 46.402(b) ).
If the IRB determines that the capability of some or all of the children is so limited that they cannot reasonably be consulted regarding assent, or that the intervention or procedure involved in the research holds out a prospect of direct benefit that is important to the health or well-being of the children, and is available only in the context of the research, the assent of the children is not a necessary condition for proceeding with the research. Even where the IRB determines that the subjects are capable of assenting, the IRB may still waive the assent requirement under certain circumstances in accord with 45 CFR 46.116 and 45 CFR 46.408(a) .
May the requirement for obtaining informed consent or parental permission be altered or waived?
Waiver or alteration of the requirements for obtaining informed consent from adult subjects can occur under any of the following three provisions:
- the research could not practicably be carried out without the waiver or alteration; and
- public benefit or service programs;
- procedures for obtaining benefits or services under those programs;
- possible changes in or alternatives to those programs or procedures; or
- possible changes in methods or levels of payment for benefits or services under those programs.
- the research involves no more than minimal risk to the subjects;
- the waiver or alteration will not adversely affect the rights and welfare of the subjects;
- the research could not practicably be carried out without the waiver or alteration; and
- whenever appropriate, the subjects will be provided with additional pertinent information after participation.
- Research in emergency settings: an IRB may also waive the requirement for obtaining informed consent if it finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings (PDF) (23KB).
For research involving children, an IRB may waive the requirements for obtaining parental or guardian permission under any of the following four provisions:
- The IRB makes and documents the required findings under 45 CFR 46.116(c) as described above.
- The IRB makes and documents the required findings under 45 CFR 46.116(d) as described above.
- an appropriate mechanism is in place to protect the children, and
- the waiver is not inconsistent with federal, state, or local law ( 45 CFR 46.408(c) ). The choice of an appropriate substitute mechanism (for example, appointing a child advocate or an assent monitor) for protecting children participating in research would depend on the nature and purpose of the activities described in the protocol, the risk and anticipated benefit to the research subjects, and the child’s age, maturity, status, and condition ( 45 CFR 46.408(c) ). Note that an IRB may waive the requirement for obtaining parental or guardian permission under 45 CFR 46.408(c) even if the research involves more than minimal risk to the child subjects.
- The IRB finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings (PDF) (23KB).
What is the definition of guardian in the context of obtaining consent for research involving children?
The term guardian means “an individual who is authorized under applicable State or local law to consent on behalf of a child to general medical care” ( 45 CFR 46.402(e) ) The role of a guardian in the context of research involving a child who is a ward is to provide permission, in lieu of a child’s biological or adoptive parents, for the ward to participate in the research ( 45 CFR 46.402(c) ). For a more extensive discussion see FAQs on Research with Children .
What happens if a child reaches the legal age of consent while enrolled in a study?
The Office for Human Research Protections (OHRP) notes that informed consent should be viewed as an ongoing process throughout the duration of a research project. When a child who was enrolled in research with parental or guardian permission subsequently reaches the legal age of consent to the procedures involved in ongoing research, the subject’s participation in the research is no longer regulated by the requirements of 45 CFR part 46.408 regarding parental or guardian permission and subject assent.
Unless the Institutional Review Board (IRB) determines that the requirements for obtaining informed consent can be waived, the investigators should seek and obtain the legally effective informed consent, as described in 45 CFR 46.116 , for the now-adult subject for any ongoing interactions or interventions with the subjects. This is because the prior parental permission and child assent are not equivalent to legally effective informed consent for the now-adult subject. However, the IRB could approve a waiver of informed consent under 45 CFR 46.116(d) , if the IRB finds and documents that the required conditions are met.
Similarly, if the research does not involve any ongoing interactions or interventions with the subjects, but continues to meet the regulatory definition of “human subjects research” (for example, it involves the continued analysis of specimens or data for which the subject’s identity is readily identifiable to the investigator(s)), then it would be necessary for the investigator(s) to seek and obtain the legally effective informed consent of the now-adult subjects. The IRB may consider, if appropriate, a waiver under 45 CFR 46.116(d) of the requirements for obtaining informed consent in order for the subjects to continue their participation in the research.
What is a waiver or alteration of informed consent or parental permission?
The HHS regulations allow the IRB to waive the requirement for obtaining informed consent or parental permission or to approve a consent procedure that leaves out or alters some or all of the elements of informed consent otherwise required under 45 CFR 46.116(a) and (b) .
Waiving the requirement for obtaining informed consent or parental permission means that the IRB has determined that investigators need not obtain the subjects’ informed consent to participate in research. For example, some research about natural behavior may require that subjects be unaware that the research is taking place. Such research can only be approved by the IRB if the research meets the criteria for a waiver of informed consent under HHS regulations and for approving research according to 45 CFR 46.111 .
An IRB may approve research for which some or all of the elements of informed consent at 45 CFR 46.116 (a) and (b) have been altered, or for which some elements have been left out. For example, some research designs require that subjects be left unaware of the particular purpose of the research, because the subjects’ responses might be biased if they know in advance what the investigators are seeking. Such research designs do not preclude offering potential subjects some information about the research and giving them the opportunity to decide whether to participate. The IRB may approve such research in which investigators will leave out or alter elements of informed consent, so long as the research meets the criteria for approving research in 45 CFR 46.111 , and the research meets the criteria specified in the HHS regulations for leaving out or altering those elements.
What are the regulatory bases for waiving or altering some or all of the required elements of informed consent or parental permission?
The conditions under which an IRB may waive the requirement for obtaining informed consent or parental permission or may approve a consent procedure that leaves out or alters some or all of the elements of informed consent derive from four sources in the HHS regulations.
- At 45 CFR 46.116(c) , the regulations identify when IRBs may waive or approve an alteration of informed consent in some research examining state or local public benefit or service programs , or certain features of those programs.
- At 45 CFR 46.116(d) the regulations identify when IRBs may waive or approve an alteration of informed consent in research that meets four specified criteria .
- At 45 CFR 46.408(c) , the regulations identify when IRBs may approve waiver of parental permission in certain research involving children.
- Under the provisions of 45 CFR 46.101(i) , the Secretary, HHS, has waived the general requirements for obtaining informed consent in a limited class of research in emergency settings .
What are the criteria under 45 CFR 46.116(c) for waiving or altering some or all of the required elements of informed consent or parental permission?
Under 45 CFR 46.116(c) , an IRB may waive the requirement for obtaining informed consent or parental permission or approve a consent or parental permission procedure that leaves out or alters some or all of the elements of informed consent, provided that the IRB finds and documents that the following two criteria are satisfied:
the research or demonstration project is to be conducted by or subject to the approval of state or local government officials and is designed to study, evaluate, or otherwise examine:
- possible changes in methods or levels of payment for benefits or services under those programs; 45 CFR 46.116(c)(1) .
Note that this criterion means that only public benefit or service program research activities that are under state or local authority meet this criterion; similar research conducted under federal authority would not qualify here and is treated elsewhere in the regulations. Research conducted by or subject to the approval of only a private entity also would not qualify.
- the research could not practicably be carried out without the waiver or alteration ( 45 CFR 46.116(c)(2) ).
This criterion means that the practical circumstances of the research are such that the research is not feasible if the informed consent of the subjects must be obtained. For example, a study of identifiable private information about program benefit recipients using 20-year-old records might meet this criterion, if current contact information for those recipients is not available.
What are the criteria under 45 CFR 46.116(d) for waiving or altering some or all of the required elements of informed consent or parental permission?
Under 45 CFR 46.116(d) the IRB may waive the requirement for obtaining informed consent or approve a consent procedure that leaves out or alters some or all of the elements of informed consent, provided that the IRB finds and documents that all of the following four criteria are met:
- the research could not practicably be carried out without the waiver or alteration; and,
Is it possible to waive the informed consent requirement when conducting research in an emergency setting?
In 1996, the HHS Secretary announced, under 45 CFR 46.101(i) , a waiver of the applicability of the regulatory requirement for obtaining and documenting informed consent for a strictly limited class of research, that is, research that may be carried out in human subjects who are in need of emergency therapy and for whom, because of the subjects’ medical condition and the unavailability of legally authorized representatives of the subjects, no legally effective informed consent can be obtained. This waiver applies to research involving adults or children, but does not apply to research involving pregnant women, human fetuses, neonates of uncertain viability, and nonviable neonates, or prisoners.
For more detailed information, see OHRP’s guidance on Emergency Research Consent Waiver . It should be noted that FDA also has a comparable provision for a waiver of informed consent for emergency research at 21 CFR 50.24 .
When may the requirement for documentation of informed consent or parental permission be waived or altered?
When an Institutional Review Board (IRB) has not waived the requirement for seeking prospective informed consent of the subjects or the parental permission of children who are subjects, under the HHS regulations at 45 CFR 46.117(c) , it may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either:
- That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research and the subject’s wishes will govern; or
- That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context (e.g., drawing a blood sample, or asking shoppers in a mall about the ambient lighting or temperature).
Some subjects might refuse a copy of the consent form once signed out of concern that their possession of the form could compromise their privacy. This is fully consistent with the idea behind one of the bases for a waiver of the requirements for documentation of informed consent - that harm would result to the subject if his/her identity were compromised by the documentation itself. The investigator may document that the subject refused a copy of the informed consent document and still include the subject in the study.
In cases in which the documentation requirement is waived, the IRB may require the investigator to provide subjects or the parents of children who are subjects with a written statement regarding the research.
Can parental or guardian permission for research involving children be waived?
Yes, under certain circumstances. An IRB may waive the requirements for obtaining parental or guardian permission if either of the following two conditions is met:
- The IRB makes and documents the required findings under either 45 CFR 46.116(c) or (d) ; or
- An appropriate mechanism is in place to protect the children, and
- The waiver is not inconsistent with federal, state, or local law ( 45 CFR 46.408(c) ).
The choice of an appropriate substitute mechanism (for example, appointing a child advocate or an assent monitor) for protecting children participating in research would depend on the nature and purpose of the activities described in the protocol, the risk and anticipated benefit to the research subjects, and the child’s age, maturity, status, and condition ( 45 CFR 46.408(c) ).
Note that an IRB may waive the requirement for obtaining parental or guardian permission under 45 CFR 46.408(c) even if the research involves more than minimal risk to the child subjects.
Is child assent always required when research involves children?
No, the IRB is responsible for deciding whether child assent is required in proposed research activities. Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent ( 45 CFR 46.402(b) ). Child assent is required, except in the following three circumstances described at 45 CFR 46.408(a) :
- the capability of some or all of the children is so limited that they cannot reasonably be consulted;
- the intervention or procedure involved in the research holds out the prospect of direct benefit to the health or well-being of the children and is available only in the context of the research;
- the research meets the same conditions as those for waiver or alteration of informed consent in research involving adults, as specified in the regulations at either 45 CFR 46.116(c) or 45 CFR 46.116(d) .
How should child assent be documented?
The HHS regulations do not require documentation of assent. The IRB has the discretion to determine the appropriate manner, if any, of documenting child assent. Based on such considerations as the child’s age, maturity, and degree of literacy, the IRB should decide what form of documentation, if any, is most appropriate. If adolescents are involved in research where a consent form would have been used if the subjects were adults, it would generally be appropriate to use a similar form to document an adolescent’s assent.
If young children are involved who are as yet unable to read, documentation should take a form that is appropriate for the purpose of recording that assent took place. The IRB may also decide that documentation of assent is not warranted.
What is the meaning of "legally effective informed consent?"
Informed consent is legally effective if it is both obtained from the subject or the subject’s legally authorized representative and documented in a manner that is consistent with the HHS protection of human subjects regulations and with applicable laws of the jurisdiction in which the research is conducted. In general terms, the regulations stipulate that an investigator should seek consent only under circumstances that provide the prospective subject or the legally authorized representative sufficient opportunity to consider whether to participate and that minimize the possibility of coercion or undue influence. The information provided should be in language that is understandable to the subject or the representative. No informed consent, whether oral or written, may include any exculpatory language .
It is important to note that the informed consent requirements in the regulations are not intended to preempt any applicable federal, state, or local laws that require additional information to be disclosed for consent to be legally effective ( 45 CFR 46.116(e) ).
- Informed consent form
ADOBE ACROBAT
Understand informed consent forms.
Whether you’re running a research study, getting a waiver, or going through an institutional review board, explore the guidelines and processes around clinical research and treatment.
Explore Acrobat

JUMP TO SECTION
What is informed consent
The 3 fundamentals of informed consent
The key elements of rock-solid consent forms
How digital signatures make consent forms easier for everyone
Informed consent basics.
- Informed consent is necessary when a patient is deciding with the help of their doctor about treatment.
- This type of consent is also required for any medical research trial or study using human subjects.
- With electronic signatures, including digital signatures, and forms, patients and research participants can get faster and more accessible treatment.
What is informed consent?
Informed consent is a part of medical ethics and law that states that a patient should have all the necessary information before making a decision about their medical care. This is because navigating healthcare systems can be a complicated and often opaque process for patients, making informed consent one of the pillars of medical ethics.
This consent isn’t limited to medical care in a hospital. If a patient is joining a clinical trial, informed consent is necessary, especially when you’re working with an institutional review board (IRB) who will approve or deny funding. Informed consent can also be referred to as express consent, but that is not the same as implied consent, which refers to consent granted by actions or behaviors within a particular situation.
3 fundamentals of informed consent.
Informed consent has three main principles.
1. Decision capacity and competency
In order for someone to give informed consent, they must have the decision-making capacity, meaning that they must be both conscious and of sound mind to make an informed decision and be able to understand what the informed consent document is asking.
2. Documentation of consent
At the very minimum, informed consent requires the signature of the person consenting to whatever treatment or procedure is on offer. If the person in question is a minor, legally authorized representatives or a parent(s) or guardian must give consent. Often, there is an additional component when children are participating in a trial called child assent, where children are asked to give their consent, but legal requirements vary.
3. Disclosure
For informed consent to occur, the doctor or treatment provider must provide enough information about the procedure so the patient can reasonably consent. While this disclosure doesn’t include every detail, it must have enough so the patient is making an informed decision.
Key elements of consent forms.
When designing an informed consent form, you should always consult with an attorney because you likely need to include many important details and required elements within the form itself. While this article doesn’t include an exhaustive list, below are some steps for building an informed consent form for a research trial with five important components to include.

Statement about the project
The first section describes the overall purpose of the research project, what the goals are, how long it will take, and what the expectations of the participants will be. For example, if this were a drug trial, it would clearly identify itself as such, outline the various effects of the drug in question, and also point out how participants will interact with the trial. Think of this as an information sheet for the participant and keep things easy to read.
Research summary
This section summarizes the research backing the trial, who the principal investigators are (the researchers), and what will be studied within the trial. It includes the purpose of the research, how long it will take, and the procedures that will occur within the scope of the trial.
Get work done. Anywhere.
Adobe Acrobat Pro
View product details

Reasonable risks
In this section, possible side effects and potential risks and discomforts of the trial are specified. This is a critical part of the informed consent form, as this is where the signee gains information to help decide if the benefits outweigh the risks.
Reasonable benefits
This section describes what can be expected to come from the research and the direct benefits that the patient might experience from the drug trial or research.
Alternative procedures or additional treatments
If there are parts of the trial that are reliant upon treatment with other medications or therapies, these are outlined as well, so the patient can consent to them. Finally, the form ends with a signature field, which is where Adobe Acrobat comes in.

How electronic signatures, including digital signatures, make consent forms easier for everyone.
You can configure Adobe Acrobat to support compliance with FDA 21 CFR Part 11, which establishes the Food and Drug Administration’s (FDA) regulations on electronic records and signatures.
Learn how electronic signatures, including digital signatures can jump-start digitizing your paperwork and make life easier.
https://main--dc--adobecom.hlx.page/dc-shared/fragments/seo-articles/acrobat-color-blade

IMAGES
VIDEO
COMMENTS
permission, adult consent, teacher consent, screening consent, etc.). • In this template, "we" refers to the researchers. If there is only one researcher, edit as appropriate. If the PI is a student, always use "we" to include the faculty advisor. • Submit consent documents in MS Word whenever possible. The iMedRIS comparison tool for
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
Click here to view these. Whenever you are proposing research with human participants you must provide a form, known as an Informed Consent Form (ICF), with each proposal to indicate that the research participant has decided to take part in the research of her/his own free will. If the research involves more than one group of individuals, for ...
The informed consent form should be accompanied by an information sheet that describes: 1. General information about the research and the collected research data • Purpose of the research • Type of research intervention, e.g. questionnaire, interview, etc. • Voluntary nature of participation • Benefits and risks of participating
The Research Ethics Board must approve any changes to the consent form before the research begins. Changes to an approved study and its documents are done via an Amendment. Your application will be sent back, and approval delayed, if a complete consent form or consent document is not submitted with your application.
How to Write. Step 1 - Download in PDF, Microsoft Word (.docx), or Open Document Text (.odt). Step 2 - The title of the research study being conducted must be included at the top of the consent form. Step 3 - Enter the following information related to the primary researcher in the fields provided: Step 4 - The purpose of the study, the ...
Instructions: This document provides guidance and language necessary to populate the template informed consent form. The guidance incorporates the regulatory requirements for informed consent. The format may be modified or expanded as indicated to better meet the needs of the research design and/or participant population.
17 ChampollionStreet, El Messalah,Alexandria, Egypt-email; [email protected]. Informed Consent Form Template for Clinical Studies. l C. (This template is for either clinical trials or clinical research) (language used throughout form should be at the level of a local student of class 6th/8th) Alexandria University Faculty of Medicine.
The informed consent process. The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [].Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation.
Understanding Informed Consent Forms. The informed consent form puts you in control of your health decisions and protects your rights. Before you join a cancer research study, you'll receive an informed consent form to review, ask questions about, and sign. The form covers a description of the study's purpose, procedures and safety measures ...
August 14, 2011. Informed consent is a communication process by which researchers reach agreement with people about whether they wish to participate in research. Confusing informed consent with a signed consent form may violate the ethical intent of informed consent, which is to communicate clearly and respectfully, to foster trust ...
The key idea is to go over the information verbally and document the process of gaining consent in field notes so as to leave a written trail. It is still reasonable to leave written material with the participant (e.g., an information letter). Regardless of the way in which consent is sought or documented, the primary focus of ethical concern ...
Informed Consent Guidance. Basic Elements of Informed Consent. PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule.
This resource addresses only consent for the storage and sharing of data and biospecimens collected during a primary research protocol, for the purposes of future secondary research. The approval for use of these data and biospecimens in new research studies that are outside the scope of the primary protocol and consent (i.e., secondary ...
Obtaining genuine informed consent from research participants is best thought of as a process of sharing information and addressing questions and concerns, rather than simply obtaining a signature on a prescribed form. It starts with the researcher developing an awareness of national or regional guidelines, and may involve discussions with, and ...
Consent to participate in a research study should be understood as a process rather than an event. The primary focus of ethical concern should always be on the quality of the consent process. As part of the informed consent process, the consent document as a whole must present information in sufficient detail relating to the research,
When HIPAA applies to the research, the subject must provide HIPAA authorization in addition to informed consent. This can be in the form of either. A combined consent/HIPAA authorization which is a single document that meets the regulatory requirements for research consent and HIPAA; or; A consent form and a stand-alone HIPAA authorization form.
This SOP establishes the procedure for developing an informed consent form (ICF)/ research subject authorization form (RSAF), when applicable, obtaining IRB approval of ICF/RSAF, administering the approved ICF/RSAF, and obtaining informed consent and research subject authorization from subjects, or their legally authorized representative, prior ...
assist research proponents in the design of their informed consent forms (ICF). Researchers are encouraged to use this when creating their informed consent forms to best suit the design of their study. Use of alternative wording or format is allowed. 2. The informed consent form consists of two parts: the information sheet and the consent ...
Informed consent means that the purpose of the research is explained to them, including what their role would be and how the trial will work. A central part of the informed consent process is the ...
This research was approved by an office/committee that oversees the ethics of human subjects research at Brandeis University. If you have any questions about your rights, concerns about the study, or would like to offer input, you may contact them at 781-736-8133 or [email protected] Subject Consent I have read this consent form completely.
If the consent process will be documented in writing with the l ong form of consent documentation: 5.1.1 Obtain the current IRB approved consent form. 5.1.2 Verify that you are using the most current IRB-approved version of the study specific consent form and that the consent form is in language understandable to the subject/representative.
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
5.1.1. Obtain the current IRB-approved consent form. 5.1.2. Verify that you are using the most current IRB-approved version of the study specific consent form and that the consent form is in language understandable to the subject/representative. 5.1.3. Provide a copy of the consent form to the subject/representative. Whenever possible provide the
For most research, informed consent is documented using a written document that provides key information regarding the research. The consent form is intended, in part, to provide information for the potential subject's current and future reference and to document the interaction between the subject and the investigator. ... (PDF) (23KB). For ...
: To provide a checklist for quality control review of informed consent documents to ensure that the consent document complies with all relevant regulations and local IRB requirements, as appropriate. Audience/User . Individuals responsible for reviewing informed consent documents to ensure quality and compliance to relevant requirements . Details
Informed consent has three main principles. 1. Decision capacity and competency. In order for someone to give informed consent, they must have the decision-making capacity, meaning that they must be both conscious and of sound mind to make an informed decision and be able to understand what the informed consent document is asking. 2.