Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Guide to Experimental Design | Overview, Steps, & Examples

Guide to Experimental Design | Overview, 5 steps & Examples
Published on December 3, 2019 by Rebecca Bevans . Revised on June 21, 2023.
Experiments are used to study causal relationships . You manipulate one or more independent variables and measure their effect on one or more dependent variables.
Experimental design create a set of procedures to systematically test a hypothesis . A good experimental design requires a strong understanding of the system you are studying.
There are five key steps in designing an experiment:
- Consider your variables and how they are related
- Write a specific, testable hypothesis
- Design experimental treatments to manipulate your independent variable
- Assign subjects to groups, either between-subjects or within-subjects
- Plan how you will measure your dependent variable
For valid conclusions, you also need to select a representative sample and control any extraneous variables that might influence your results. If random assignment of participants to control and treatment groups is impossible, unethical, or highly difficult, consider an observational study instead. This minimizes several types of research bias, particularly sampling bias , survivorship bias , and attrition bias as time passes.
Table of contents
Step 1: define your variables, step 2: write your hypothesis, step 3: design your experimental treatments, step 4: assign your subjects to treatment groups, step 5: measure your dependent variable, other interesting articles, frequently asked questions about experiments.
You should begin with a specific research question . We will work with two research question examples, one from health sciences and one from ecology:
To translate your research question into an experimental hypothesis, you need to define the main variables and make predictions about how they are related.
Start by simply listing the independent and dependent variables .
Then you need to think about possible extraneous and confounding variables and consider how you might control them in your experiment.
Finally, you can put these variables together into a diagram. Use arrows to show the possible relationships between variables and include signs to show the expected direction of the relationships.
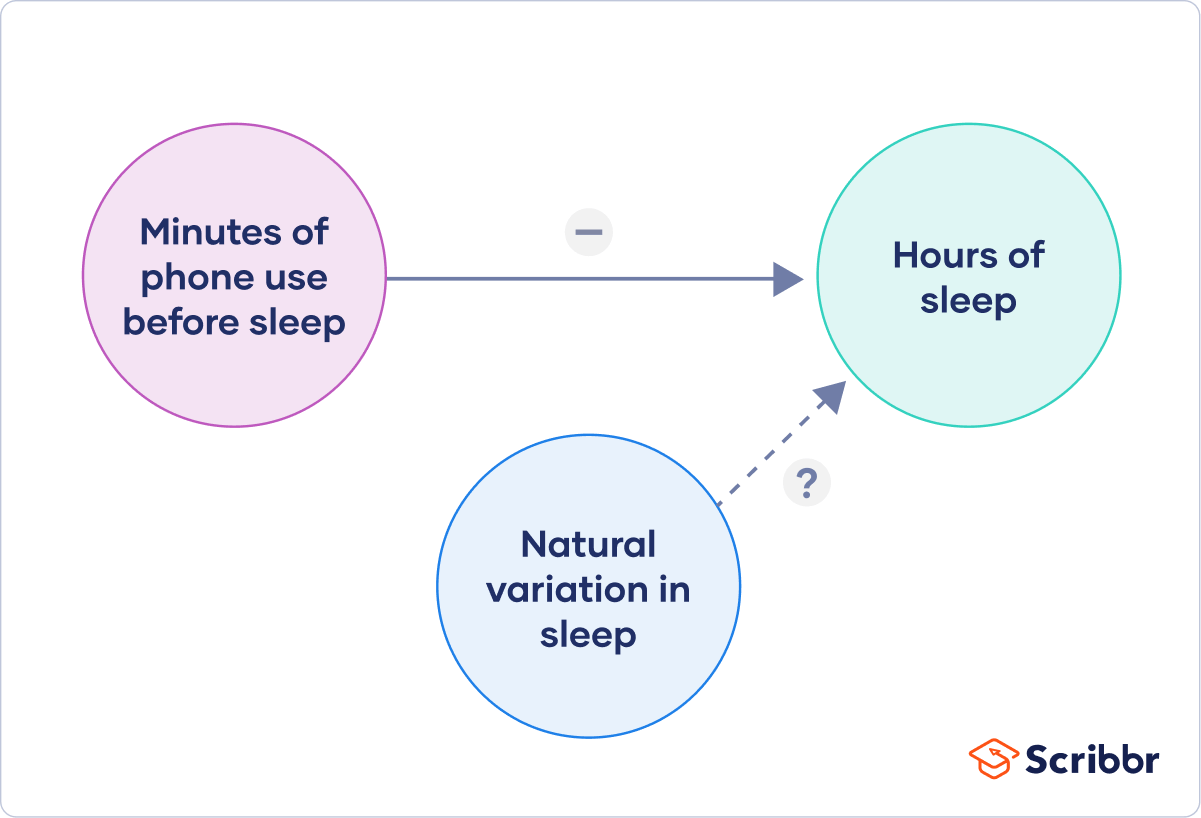
Here we predict that increasing temperature will increase soil respiration and decrease soil moisture, while decreasing soil moisture will lead to decreased soil respiration.
Prevent plagiarism. Run a free check.
Now that you have a strong conceptual understanding of the system you are studying, you should be able to write a specific, testable hypothesis that addresses your research question.
The next steps will describe how to design a controlled experiment . In a controlled experiment, you must be able to:
- Systematically and precisely manipulate the independent variable(s).
- Precisely measure the dependent variable(s).
- Control any potential confounding variables.
If your study system doesn’t match these criteria, there are other types of research you can use to answer your research question.
How you manipulate the independent variable can affect the experiment’s external validity – that is, the extent to which the results can be generalized and applied to the broader world.
First, you may need to decide how widely to vary your independent variable.
- just slightly above the natural range for your study region.
- over a wider range of temperatures to mimic future warming.
- over an extreme range that is beyond any possible natural variation.
Second, you may need to choose how finely to vary your independent variable. Sometimes this choice is made for you by your experimental system, but often you will need to decide, and this will affect how much you can infer from your results.
- a categorical variable : either as binary (yes/no) or as levels of a factor (no phone use, low phone use, high phone use).
- a continuous variable (minutes of phone use measured every night).
How you apply your experimental treatments to your test subjects is crucial for obtaining valid and reliable results.
First, you need to consider the study size : how many individuals will be included in the experiment? In general, the more subjects you include, the greater your experiment’s statistical power , which determines how much confidence you can have in your results.
Then you need to randomly assign your subjects to treatment groups . Each group receives a different level of the treatment (e.g. no phone use, low phone use, high phone use).
You should also include a control group , which receives no treatment. The control group tells us what would have happened to your test subjects without any experimental intervention.
When assigning your subjects to groups, there are two main choices you need to make:
- A completely randomized design vs a randomized block design .
- A between-subjects design vs a within-subjects design .
Randomization
An experiment can be completely randomized or randomized within blocks (aka strata):
- In a completely randomized design , every subject is assigned to a treatment group at random.
- In a randomized block design (aka stratified random design), subjects are first grouped according to a characteristic they share, and then randomly assigned to treatments within those groups.
Sometimes randomization isn’t practical or ethical , so researchers create partially-random or even non-random designs. An experimental design where treatments aren’t randomly assigned is called a quasi-experimental design .
Between-subjects vs. within-subjects
In a between-subjects design (also known as an independent measures design or classic ANOVA design), individuals receive only one of the possible levels of an experimental treatment.
In medical or social research, you might also use matched pairs within your between-subjects design to make sure that each treatment group contains the same variety of test subjects in the same proportions.
In a within-subjects design (also known as a repeated measures design), every individual receives each of the experimental treatments consecutively, and their responses to each treatment are measured.
Within-subjects or repeated measures can also refer to an experimental design where an effect emerges over time, and individual responses are measured over time in order to measure this effect as it emerges.
Counterbalancing (randomizing or reversing the order of treatments among subjects) is often used in within-subjects designs to ensure that the order of treatment application doesn’t influence the results of the experiment.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Finally, you need to decide how you’ll collect data on your dependent variable outcomes. You should aim for reliable and valid measurements that minimize research bias or error.
Some variables, like temperature, can be objectively measured with scientific instruments. Others may need to be operationalized to turn them into measurable observations.
- Ask participants to record what time they go to sleep and get up each day.
- Ask participants to wear a sleep tracker.
How precisely you measure your dependent variable also affects the kinds of statistical analysis you can use on your data.
Experiments are always context-dependent, and a good experimental design will take into account all of the unique considerations of your study system to produce information that is both valid and relevant to your research question.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Likert scale
Research bias
- Implicit bias
- Framing effect
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
Experimental design means planning a set of procedures to investigate a relationship between variables . To design a controlled experiment, you need:
- A testable hypothesis
- At least one independent variable that can be precisely manipulated
- At least one dependent variable that can be precisely measured
When designing the experiment, you decide:
- How you will manipulate the variable(s)
- How you will control for any potential confounding variables
- How many subjects or samples will be included in the study
- How subjects will be assigned to treatment levels
Experimental design is essential to the internal and external validity of your experiment.
The key difference between observational studies and experimental designs is that a well-done observational study does not influence the responses of participants, while experiments do have some sort of treatment condition applied to at least some participants by random assignment .
A confounding variable , also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design , it’s important to identify potential confounding variables and plan how you will reduce their impact.
In a between-subjects design , every participant experiences only one condition, and researchers assess group differences between participants in various conditions.
In a within-subjects design , each participant experiences all conditions, and researchers test the same participants repeatedly for differences between conditions.
The word “between” means that you’re comparing different conditions between groups, while the word “within” means you’re comparing different conditions within the same group.
An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not. They should be identical in all other ways.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bevans, R. (2023, June 21). Guide to Experimental Design | Overview, 5 steps & Examples. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/methodology/experimental-design/
Is this article helpful?
Rebecca Bevans
Other students also liked, random assignment in experiments | introduction & examples, quasi-experimental design | definition, types & examples, how to write a lab report, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”

Writing Center: Experimental Research Papers
- How to Set Up an Appointment Online
- Documentation Styles
- Parts of Speech
- Types of Clauses
- Punctuation
- Spelling & Mechanics
- Usage & Styles
- Resources for ESL Students
- How to Set up an APA Paper
- How to Set up an MLA Paper
- Adapt to Academic Learning
- Audience Awareness
- Learn Touch Typing
- Getting Started
- Thesis Statement
- The First Draft
- Proofreading
- Writing Introductions
- Writing Conclusions
- Chicago / Turabian Style
- CSE / CBE Style
- Avoiding Plagiarism
- Cross-Cultural Understanding
- Writing Resources
- Research Paper - General Guidelines
- Annotated Bibliographies
- History Papers
- Science Papers
- Experimental Research Papers
- Exegetical Papers
- FAQs About Creative Writing
- Tips For Creative Writing
- Exercises To Develop Creative Writing Skills
- Checklist For Creative Writing
- Additional Resources For Creative Writing
- FAQs About Creating PowerPoints
- Tips For Creating PowerPoints
- Exercises to Improve PowerPoint Skills
- Checklist For PowerPoints
- Structure For GRE Essay
- Additional Resources For PowerPoints
- Additional Resources For GRE Essay Writing
- FAQs About Multimodal Assignments
- Tips For Creating Multimodal Assignments
- Checklist For Multimodal Assignments
- Additional Resources For Multimodal Assignments
- GRE Essay Writing FAQ
- Tips for GRE Essay Writing
- Sample GRE Essay Prompts
- Checklist For GRE Essays
- Cover Letter
- Personal Statements
- Resources for Tutors
- Chapter 2: Theoretical Perspectives on Learning a Second Language
- Chapter 4: Reading an ESL Writer's Text
- Chapter 5: Avoiding Appropriation
- Chapter 6: 'Earth Aches by Midnight': Helping ESL Writers Clarify Their Intended Meaning
- Chapter 7: Looking at the Whole Text
- Chapter 8: Meeting in the Middle: Bridging the Construction of Meaning with Generation 1.5 Learners
- Chapter 9: A(n)/The/Ø Article About Articles
- Chapter 10: Editing Line by Line
- Chapter 14: Writing Activities for ESL Writers
- Resources for Faculty
- Writing Center Newsletter
- Writing Center Survey
FAQs About Experimental Research Papers (APA)
What is a research paper?
A researcher uses a research paper to explain how they conducted a research study to answer a question or test a hypothesis. They explain why they conducted the study, the research question or hypothesis they tested, how they conducted the study, the results of their study, and the implications of these results.
What is the purpose of an experimental research paper?
A research paper is intended to inform others about advancement in a particular field of study. The researcher who wrote the paper identified a gap in the research in a field of study and used their research to help fill this gap. The researcher uses their paper to inform others about the knowledge that the results of their study contribute.
What sections are included in an experimental research paper?
A typical research paper contains a Title Page, Abstract, Introduction, Methods, Results, Discussion, and References section. Some also contain a Table and Figures section and Appendix section.
What citation style is used for experimental research papers?
APA (American Psychological Association) style is most commonly used for research papers.
Structure Of Experimental Research Papers (APA)
- Answers the question of “What is this paper about and who wrote it?”
- Located on the first page of the paper
- The author’s note acknowledges any support that the authors received from others
- A student paper also includes the course number and name, instructor’s name, and assignment due date
- Contains a title that summarizes the purpose and content of the research study and engages the audience
- No longer than 250 words
- Summarizes important background information, the research questions and/or hypothesis, methods, key findings, and implications of the findings
- Explains what the topic of the research is and why the topic is worth studying
- Summarizes and discusses prior research conducted on the topic
- Identifies unresolved issues and gaps in past research that the current research will address
- Ends with an overview of the current research study, including how the independent and dependent variables, the research questions or hypotheses, and the objective of the research
- Explains how the research study was conducted
- Typically includes 3 sections: Participants, Materials, and Procedure
- Includes characteristics of the subjects, how the subjects were selected and recruited, how their anonymity was protected, and what feedback was provided to the participants
- Describes any equipment, surveys, tests, questionnaires, informed consent forms, and observational techniques
- Describes the independent and dependent variables, the type of research design, and how the data was collected
- Explains what results were found in the research study
- Describes the data that was collected and the results of statistical tests
- Explains the significance of the results
- Accepts or denies the hypotheses
- Details the implications of these findings
- Addresses the limitations of the study and areas for future research
- Includes all sources that were mentioned in the research study
- Adheres to APA citation styles
- Includes all tables and/or figures that were used in the research study
- Each table and figure is placed on a separate page
- Tables are included before figures
- Begins with a bolded, centered header such as “ Table 1 ”
- Appends all forms, surveys, tests, etc. that were used in the study
- Only includes documents that were referenced in the Methods section
- Each entry is placed on a separate page
- Begins with a bolded, centered header such as “ Appendix A ”
Tips For Experimental Research Papers (APA)
- Initial interest will motivate you to complete your study
- Your entire study will be centered around this question or statement
- Use only verifiable sources that provide accurate information about your topic
- You need to thoroughly understand the field of study your topic is on to help you recognize the gap your research will fill and the significance of your results
- This will help you identify what you should study and what the significance of your study will be
- Create an outline before you begin writing to help organize your thoughts and direct you in your writing
- This will prevent you from losing the source or forgetting to cite the source
- Work on one section at a time, rather than trying to complete multiple sections at once
- This information can be easily referred to as your write your various sections
- When conducting your research, working general to specific will help you narrow your topic and fully understand the field your topic is in
- When writing your literature review, writing from general to specific will help the audience understand your overall topic and the narrow focus of your research
- This will prevent you from losing sources you may need later
- Incorporate correct APA formatting as you write, rather than changing the formatting at the end of the writing process
Checklist For Experimental Research Papers (APA)
- If the paper is a student paper, it contains the title of the project, the author’s name(s), the instructor's name, course number and name, and assignment due date
- If the paper is a professional paper, it includes the title of the paper, the author’s name(s), the institutional affiliation, and the author note
- Begins on the first page of the paper
- The title is typed in upper and lowercase letters, four spaces below the top of the paper, and written in boldface
- Other information is separated by a space from the title
Title (found on title page)
- Informs the audience about the purpose of the paper
- Captures the attention of the audience
- Accurately reflects the purpose and content of the research paper
Abstract
- Labeled as “ Abstract ”
- Begins on the second page
- Provides a short, concise summary of the content of the research paper
- Includes background information necessary to understand the topic
- Background information demonstrates the purpose of the paper
- Contains the hypothesis and/or research questions addressed in the paper
- Has a brief description of the methods used
- Details the key findings and significance of the results
- Illustrates the implications of the research study
- Contains less than 250 words
Introduction
- Starts on the third page
- Includes the title of the paper in bold at the top of the page
- Contains a clear statement of the problem that the paper sets out to address
- Places the research paper within the context of previous research on the topic
- Explains the purpose of the research study and what you hope to find
- Describes the significance of the study
- Details what new insights the research will contribute
- Concludes with a brief description of what information will be mentioned in the literature review
Literature Review
- Labeled as “ Literature Review”
- Presents a general description of the problem area
- Defines any necessary terms
- Discusses and summarizes prior research on the selected topic
- Identifies any unresolved issues or gaps in research that the current research plans to address
- Concludes with a summary of the current research study, including the independent and dependent variables, the research questions or hypotheses, and the objective of the research
- Labeled as “ Methods ”
- Efficiently explains how the research study was conducted
- Appropriately divided into sections
- Describes the characteristics of the participants
- Explains how the participants were selected
- Details how the anonymity of the participants was protected
- Notes what feedback the participants will be provided
- Describes all materials and instruments that were used
- Mentions how the procedure was conducted and data collected
- Notes the independent and dependent variables
- Includes enough information that another researcher could duplicate the research
Results
- Labeled as “ Results ”
- Describes the data was collected
- Explains the results of statistical tests that were performed
- Omits any analysis or discussion of the implications of the study
Discussion
- Labeled as “ Discussion ”
- Describes the significance of the results
- Relates the results to the research questions and/or hypotheses
- States whether the hypotheses should be rejected or accepted
- Addresses limitations of the study, including potential bias, confounds, imprecision of measures, and limits to generalizability
- Explains how the study adds to the knowledge base and expands upon past research
- Labeled as “ References ”
- Correctly cites sources according to APA formatting
- Orders sources alphabetically
- All sources included in the study are cited in the reference section
Table and Figures (optional)
- Each table and each figure is placed on a separate page
- Tables and figures are included after the reference page
- Tables and figures are correctly labeled
- Each table and figure begins with a bolded, centered header such as “ Table 1 ,” “ Table 2 ,”
Appendix (optional)
- Any forms, surveys, tests, etc. are placed in the Appendix
- All appendix entries are mentioned in the Methods section
- Each appendix begins on a new page
- Each appendix begins with a bolded, centered header such as “ Appendix A, ” “ Appendix B ”
Additional Resources For Experimental Research Papers (APA)
- https://www.mcwritingcenterblog.org/single-post/how-to-conduct-research-using-the-library-s-resources
- https://www.mcwritingcenterblog.org/single-post/how-to-read-academic-articles
- https://researchguides.ben.edu/source-evaluation
- https://researchguides.library.brocku.ca/external-analysis/evaluating-sources
- https://writing.wisc.edu/handbook/assignments/planresearchpaper/
- https://nmu.edu/writingcenter/tips-writing-research-paper
- https://writingcenter.gmu.edu/guides/how-to-write-a-research-question
- https://www.unr.edu/writing-speaking-center/student-resources/writing-speaking-resources/guide-to-writing-research-papers
- https://drive.google.com/drive/folders/1F4DFWf85zEH4aZvm10i8Ahm_3xnAekal?usp=sharing
- https://owl.purdue.edu/owl/research_and_citation/apa_style/apa_formatting_and_style_guide/general_format.html
- https://libguides.elmira.edu/research
- https://www.nhcc.edu/academics/library/doing-library-research/basic-steps-research-process
- https://libguides.wustl.edu/research
- << Previous: Science Papers
- Next: Exegetical Papers >>
- Last Updated: Sep 14, 2023 10:30 AM
- URL: https://mc.libguides.com/writingcenter
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- A Quick Guide to Experimental Design | 5 Steps & Examples
A Quick Guide to Experimental Design | 5 Steps & Examples
Published on 11 April 2022 by Rebecca Bevans . Revised on 5 December 2022.
Experiments are used to study causal relationships . You manipulate one or more independent variables and measure their effect on one or more dependent variables.
Experimental design means creating a set of procedures to systematically test a hypothesis . A good experimental design requires a strong understanding of the system you are studying.
There are five key steps in designing an experiment:
- Consider your variables and how they are related
- Write a specific, testable hypothesis
- Design experimental treatments to manipulate your independent variable
- Assign subjects to groups, either between-subjects or within-subjects
- Plan how you will measure your dependent variable
For valid conclusions, you also need to select a representative sample and control any extraneous variables that might influence your results. If if random assignment of participants to control and treatment groups is impossible, unethical, or highly difficult, consider an observational study instead.
Table of contents
Step 1: define your variables, step 2: write your hypothesis, step 3: design your experimental treatments, step 4: assign your subjects to treatment groups, step 5: measure your dependent variable, frequently asked questions about experimental design.
You should begin with a specific research question . We will work with two research question examples, one from health sciences and one from ecology:
To translate your research question into an experimental hypothesis, you need to define the main variables and make predictions about how they are related.
Start by simply listing the independent and dependent variables .
Then you need to think about possible extraneous and confounding variables and consider how you might control them in your experiment.
Finally, you can put these variables together into a diagram. Use arrows to show the possible relationships between variables and include signs to show the expected direction of the relationships.
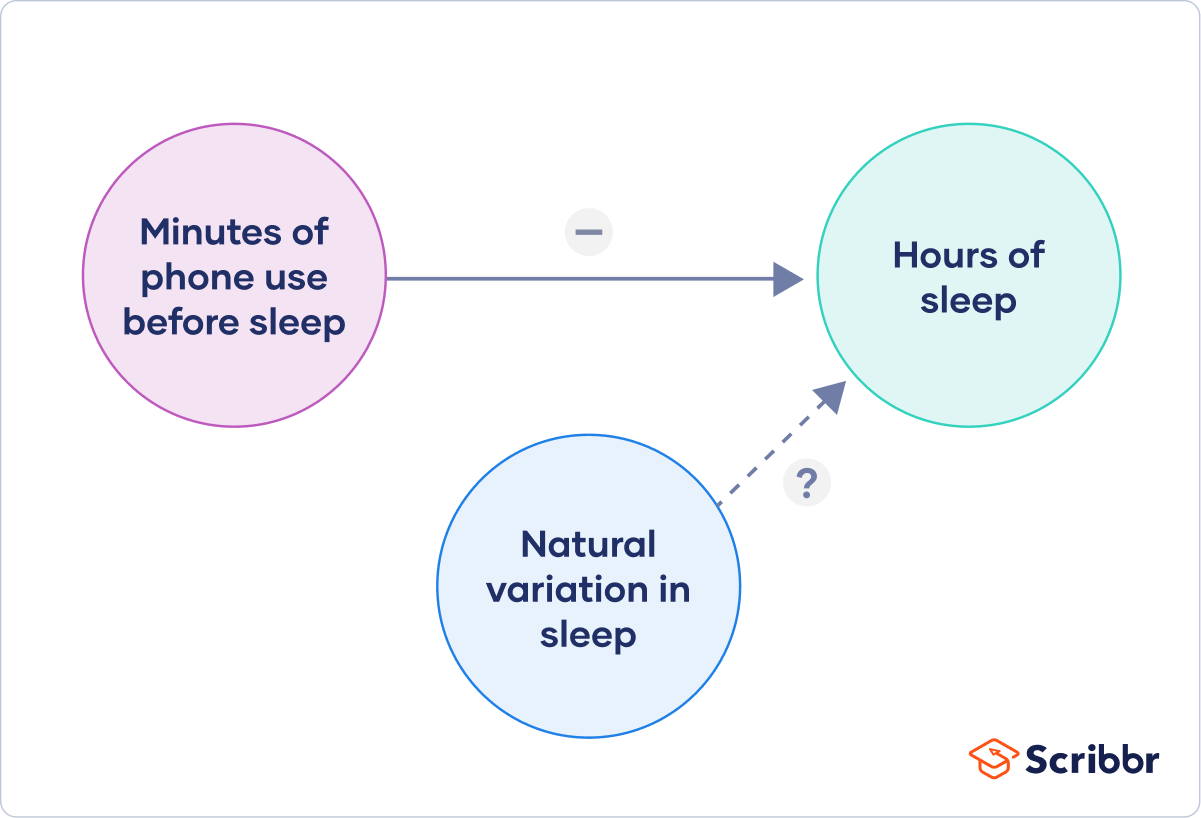
Here we predict that increasing temperature will increase soil respiration and decrease soil moisture, while decreasing soil moisture will lead to decreased soil respiration.
Prevent plagiarism, run a free check.
Now that you have a strong conceptual understanding of the system you are studying, you should be able to write a specific, testable hypothesis that addresses your research question.
The next steps will describe how to design a controlled experiment . In a controlled experiment, you must be able to:
- Systematically and precisely manipulate the independent variable(s).
- Precisely measure the dependent variable(s).
- Control any potential confounding variables.
If your study system doesn’t match these criteria, there are other types of research you can use to answer your research question.
How you manipulate the independent variable can affect the experiment’s external validity – that is, the extent to which the results can be generalised and applied to the broader world.
First, you may need to decide how widely to vary your independent variable.
- just slightly above the natural range for your study region.
- over a wider range of temperatures to mimic future warming.
- over an extreme range that is beyond any possible natural variation.
Second, you may need to choose how finely to vary your independent variable. Sometimes this choice is made for you by your experimental system, but often you will need to decide, and this will affect how much you can infer from your results.
- a categorical variable : either as binary (yes/no) or as levels of a factor (no phone use, low phone use, high phone use).
- a continuous variable (minutes of phone use measured every night).
How you apply your experimental treatments to your test subjects is crucial for obtaining valid and reliable results.
First, you need to consider the study size : how many individuals will be included in the experiment? In general, the more subjects you include, the greater your experiment’s statistical power , which determines how much confidence you can have in your results.
Then you need to randomly assign your subjects to treatment groups . Each group receives a different level of the treatment (e.g. no phone use, low phone use, high phone use).
You should also include a control group , which receives no treatment. The control group tells us what would have happened to your test subjects without any experimental intervention.
When assigning your subjects to groups, there are two main choices you need to make:
- A completely randomised design vs a randomised block design .
- A between-subjects design vs a within-subjects design .
Randomisation
An experiment can be completely randomised or randomised within blocks (aka strata):
- In a completely randomised design , every subject is assigned to a treatment group at random.
- In a randomised block design (aka stratified random design), subjects are first grouped according to a characteristic they share, and then randomly assigned to treatments within those groups.
Sometimes randomisation isn’t practical or ethical , so researchers create partially-random or even non-random designs. An experimental design where treatments aren’t randomly assigned is called a quasi-experimental design .
Between-subjects vs within-subjects
In a between-subjects design (also known as an independent measures design or classic ANOVA design), individuals receive only one of the possible levels of an experimental treatment.
In medical or social research, you might also use matched pairs within your between-subjects design to make sure that each treatment group contains the same variety of test subjects in the same proportions.
In a within-subjects design (also known as a repeated measures design), every individual receives each of the experimental treatments consecutively, and their responses to each treatment are measured.
Within-subjects or repeated measures can also refer to an experimental design where an effect emerges over time, and individual responses are measured over time in order to measure this effect as it emerges.
Counterbalancing (randomising or reversing the order of treatments among subjects) is often used in within-subjects designs to ensure that the order of treatment application doesn’t influence the results of the experiment.
Finally, you need to decide how you’ll collect data on your dependent variable outcomes. You should aim for reliable and valid measurements that minimise bias or error.
Some variables, like temperature, can be objectively measured with scientific instruments. Others may need to be operationalised to turn them into measurable observations.
- Ask participants to record what time they go to sleep and get up each day.
- Ask participants to wear a sleep tracker.
How precisely you measure your dependent variable also affects the kinds of statistical analysis you can use on your data.
Experiments are always context-dependent, and a good experimental design will take into account all of the unique considerations of your study system to produce information that is both valid and relevant to your research question.
Experimental designs are a set of procedures that you plan in order to examine the relationship between variables that interest you.
To design a successful experiment, first identify:
- A testable hypothesis
- One or more independent variables that you will manipulate
- One or more dependent variables that you will measure
When designing the experiment, first decide:
- How your variable(s) will be manipulated
- How you will control for any potential confounding or lurking variables
- How many subjects you will include
- How you will assign treatments to your subjects
The key difference between observational studies and experiments is that, done correctly, an observational study will never influence the responses or behaviours of participants. Experimental designs will have a treatment condition applied to at least a portion of participants.
A confounding variable , also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design , it’s important to identify potential confounding variables and plan how you will reduce their impact.
In a between-subjects design , every participant experiences only one condition, and researchers assess group differences between participants in various conditions.
In a within-subjects design , each participant experiences all conditions, and researchers test the same participants repeatedly for differences between conditions.
The word ‘between’ means that you’re comparing different conditions between groups, while the word ‘within’ means you’re comparing different conditions within the same group.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Bevans, R. (2022, December 05). A Quick Guide to Experimental Design | 5 Steps & Examples. Scribbr. Retrieved 8 April 2024, from https://www.scribbr.co.uk/research-methods/guide-to-experimental-design/
Is this article helpful?
Rebecca Bevans
Research Paper Format
The format described below is one that is used for essentially all research published in physics, astronomy, and engineering journals. This is the format you should use for your muon paper.
The major sections listed below are usually, but not always, included in an experimental research paper.
Title Pick a descriptive title.
Author List List of authors' names.
Date Date of submission of the paper.
Abstract The abstract is a brief summary of the paper. It is typically one paragraph long, and is a concise summary of what was done and the principal results. It may be assumed that the reader has some knowledge of the subject, but the abstract should be intelligible without reference to the paper. Don't cite sections, tables, or figures in the abstract. The title of the paper is part of the abstract, so the opening sentence should be framed without repetition of the title. Write the abstract after you have written the rest of the paper; then you know what the paper claims to do and does.
Introduction This section introduces the topic of your paper to the reader. It usually includes an historical overview with references to previous work The introduction might include any theoretical calculations or results that you will need later in the paper, althougjh many times detailed theoretical calculations are included in a separate theory section or in an appendix. However, the most important purpose of the introduction is to descri e the objectves of your experiment.
Experimental Setup In this section you describe how the experiment was done and summarize the data taken. One typically describes the instruments and detectors used in this section. Describe the procedure followed to collect the data. If the experiment is complex, the procedure mght be described in a separate section.
Data Collection This is where you include the date you took the data. Put the data in tabular form if appropriate. This section and the Experimental Setup sections can be combined for short papers.
Data Analysis In this section you use the theory developed in the introduction to analyze the data.
Results and Discussion This section is the real meat of the paper. This is where you present and interpret your results. You may wish to break this (or any other section) into subsections if it makes the paper clearer. In this section you should interpret your results in light of theory and other information contained in the Introduction section. This is where you would compare your result with theory or other observations. Describe how the result fits or doesn't fit current models. This could be combined with the Data Analysis section for papers where the data analysis is straightforward.
Conclusion No new information is presented here. Briefly summarize your main results and draw conclusions from them. Do your results confirm or deny current models or theories? If appropriate, suggest observations that might resolve issues your observations weren't able to resolve. Often the abstract and conclusion are the only part of the paper that a casual reader will read.
Acknowlegements Acknowledge those who helped your authors.
Appendices Include an appendix if you wish to derie an important result or describe an aspect of the experiment that isn't appropriate to include in the main part of the text.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

A guideline for reporting experimental protocols in life sciences
Olga giraldo.
1 Ontology Engineering Group, Campus de Montegancedo, Boadilla del Monte, Universidad Politécnica de Madrid, Madrid, Spain
Alexander Garcia
2 Technische Universität Graz, Graz, Austria
Oscar Corcho
Associated data.
- Dryad 2017. [7 July 2017]. Dryad homepage. http://datadryad.org/
- Figshare 2017. [7 July 2017]. Figshare. http://figshare.com
- Giraldo O, Garcia A, Corcho O. 2018a. Corpus of protocols [Data set] Zenodo. [ CrossRef ]
- Giraldo O, Garcia A, Corcho O. 2018b. Guidelines for reporting experimental protocols [Data set] Zenodo. [ CrossRef ]
- Giraldo O, Garcia A, Corcho O. 2018c. Survey—reporting an experimental protocol [Data set] Zenodo. [ CrossRef ]
- Gómez FL, Alexander, Giraldo O. 2018. SMARTProtocols/SMARTProtocols.github.io: first release of SMARTProtocols.github.io. Zenodo. [ CrossRef ]
The following information was supplied regarding data availability:
Federico López Gómez, Alexander Garcia & Olga Giraldo. (2018, March 26). SMARTProtocols/SMARTProtocols.github.io: First release of SMARTProtocols.github.io (Version v1.0.0). Zenodo. http://doi.org/10.5281/zenodo.1207846 .
Olga Giraldo. (2018, March 22). oxgiraldo/SMART-Protocols: First release of SMART-Protocols repository (Version v1.0.0). Zenodo. http://doi.org/10.5281/zenodo.1205247 .
Olga Giraldo, Alexander Garcia, & Oscar Corcho. (2018). Survey - reporting an experimental protocol [Data set]. Zenodo. http://doi.org/10.5281/zenodo.1204916 .
Olga Giraldo, Alexander Garcia, & Oscar Corcho. (2018). Guidelines for reporting experimental protocols [Data set]. Zenodo. http://doi.org/10.5281/zenodo.1204887 .
Olga Giraldo, Alexander Garcia, & Oscar Corcho. (2018). Corpus of protocols [Data set]. Zenodo. http://doi.org/10.5281/zenodo.1204838 .
Experimental protocols are key when planning, performing and publishing research in many disciplines, especially in relation to the reporting of materials and methods. However, they vary in their content, structure and associated data elements. This article presents a guideline for describing key content for reporting experimental protocols in the domain of life sciences, together with the methodology followed in order to develop such guideline. As part of our work, we propose a checklist that contains 17 data elements that we consider fundamental to facilitate the execution of the protocol. These data elements are formally described in the SMART Protocols ontology. By providing guidance for the key content to be reported, we aim (1) to make it easier for authors to report experimental protocols with necessary and sufficient information that allow others to reproduce an experiment, (2) to promote consistency across laboratories by delivering an adaptable set of data elements, and (3) to make it easier for reviewers and editors to measure the quality of submitted manuscripts against an established criteria. Our checklist focuses on the content, what should be included. Rather than advocating a specific format for protocols in life sciences, the checklist includes a full description of the key data elements that facilitate the execution of the protocol.

Introduction
Experimental protocols are fundamental information structures that support the description of the processes by means of which results are generated in experimental research ( Giraldo et al., 2017 ; Freedman, Venugopalan & Wisman, 2017 ). Experimental protocols, often as part of “Materials and Methods” in scientific publications, are central for reproducibility; they should include all the necessary information for obtaining consistent results ( Casadevall & Fang, 2010 ; Festing & Altman, 2002 ). Although protocols are an important component when reporting experimental activities, their descriptions are often incomplete and vary across publishers and laboratories. For instance, when reporting reagents and equipment, researchers sometimes include catalog numbers and experimental parameters; they may also refer to these items in a generic manner, e.g., “ Dextran sulfate, Sigma-Aldrich ” ( Karlgren et al., 2009 ). Having this information is important because reagents usually vary in terms of purity, yield, pH, hydration state, grade, and possibly additional biochemical or biophysical features. Similarly, experimental protocols often include ambiguities such as “ Store the samples at room temperature until sample digestion ” ( Brandenburg et al., 2002 ); but, how many Celsius degrees? What is the estimated time for digesting the sample? Having this information available not only saves time and effort, it also makes it easier for researchers to reproduce experimental results; adequate and comprehensive reporting facilitates reproducibility ( Freedman, Venugopalan & Wisman, 2017 ; Baker, 2016 ).
Several efforts focus on building data storage infrastructures, e.g., 3TU. Datacentrum ( 4TU, 2017 ), CSIRO Data Access Portal ( CSIRO, 2017 ), Dryad ( Dryad, 2017 ), figshare ( Figshare, 2017 ), Dataverse ( King, 2007 ) and Zenodo ( Zenodo, 2017 ). These data repositories make it possible to review the data and evaluate whether the analysis and conclusions drawn are accurate. However, they do little to validate the quality and accuracy of the data itself. Evaluating research implies being able to obtain similar, if not identical results. Journals and funders are now asking for datasets to be publicly available for reuse and validation. Fully meeting this goal requires datasets to be endowed with auxiliary data providing contextual information e.g., methods used to derive such data ( Assante et al., 2016 ; Simmhan, Plale & Gannon, 2005 ). If data must be public and available, shouldn’t methods be equally public and available?
Illustrating the problem of adequate reporting, Moher et al. (2015) have pointed out that fewer than 20% of highly-cited publications have adequate descriptions of study design and analytic methods. In a similar vein, Vasilevsky et al. (2013) showed that 54% of biomedical research resources such as model organisms, antibodies, knockdown reagents (morpholinos or RNAi), constructs, and cell lines are not uniquely identifiable in the biomedical literature, regardless of journal Impact Factor. Accurate and comprehensive documentation for experimental activities is critical for patenting, as well as in cases of scientific misconduct. Having data available is important; knowing how the data were produced is just as important. Part of the problem lies in the heterogeneity of reporting structures; these may vary across laboratories in the same domain. Despite this variability, we want to know which data elements are common and uncommon across protocols; we use these elements as the basis for suggesting our guideline for reporting protocols. We have analyzed over 500 published and non-published experimental protocols, as well as guidelines for authors from journals publishing protocols. From this analysis we have derived a practical adaptable checklist for reporting experimental protocols.
Efforts such as the Structured, Transparent, Accessible Reporting (STAR) initiative ( Marcus, 2016 ; Cell Press, 2017 ) address the problem of structure and standardization when reporting methods. In a similar manner, The Minimum Information about a Cellular Assay (MIACA) ( MIACA, 2017 ), The Minimum Information about a Flow Cytometry Experiment (MIFlowCyt) ( Lee et al., 2008 ) and many other “minimal information” efforts deliver minimal data elements describing specific types of experiments. Soldatova et al. (2008) and Soldatova et al. (2014) proposes the EXACT ontology for representing experimental actions in experimental protocols; similarly, Giraldo et al. (2017) proposes the S e MA ntic R epresen T ation of Protocols ontology (henceforth SMART Protocols Ontology) an ontology for reporting experimental protocols and the corresponding workflows. These approaches are not minimal; they aim to be comprehensive in the description of the workflow, parameters, sample, instruments, reagents, hints, troubleshooting, and all the data elements that help to reproduce an experiment and describe experimental actions.
There are also complementary efforts addressing the problem of identifiers for reagents and equipment; for instance, the Resource Identification Initiative (RII) ( Force11, 2017 ), aims to help researchers sufficiently cite the key resources used to produce the scientific findings. In a similar vein, the Global Unique Device Identification Database (GUDID) ( NIH, 2018 ) has key device identification information for medical devices that have Unique Device Identifiers (UDI); the Antibody Registry ( Antibody Registry, 2018 ), gives researchers a way to universally identify antibodies used in their research, and also the Addgene web-application ( Addgene, 2018 ) makes it easy for researchers to identify plasmids. Having identifiers make it possible for researchers to be more accurate in their reporting by unequivocally pointing to the resource used or produced. The Resource Identification Portal ( RIP, 2018 ), makes it easier to navigate through available identifiers, researchers can search across all the sources from a single location.
In this paper, we present a guideline for reporting experimental protocols; we complement our guideline with a machine-processable checklist that helps researchers, reviewers and editors to measure the completeness of a protocol. Each data element in our guideline is represented in the SMART Protocols Ontology. This paper is organized as follows: we start by describing the materials and methods used to derive the resulting guidelines. In the “Results” section, we present examples indicating how to report each data element; a machine readable checklist in the JavaScript Object Notation (JSON) format is also presented in this section. We then discuss our work and present the conclusions.
Materials and Methods
We have analyzed: (i) guidelines for authors from journals publishing protocols ( Giraldo, Garcia & Corcho, 2018b ), (ii) our corpus of protocols ( Giraldo, Garcia & Corcho, 2018a ), (iii) a set of reporting structures proposed by minimal information projects available in the FairSharing catalog ( McQuilton et al., 2016 ), and (iv) relevant biomedical ontologies available in BioPortal ( Whetzel et al., 2011 ) and Ontobee ( Xiang et al., 2011 ). Our analysis was carried out by a domain expert, Olga Giraldo; she is an expert in text mining and biomedical ontologies with over ten years of experience in laboratory techniques. All the documents were read, and then data elements, subject areas, materials (e.g., sample, kits, solutions, reagents, etc.), and workflow information were identified. Resulting from this activity we established a baseline terminology, common and non common data elements, as well as patterns in the description of the workflows (e.g., information describing the steps and the order for the execution of the workflow).
Instructions for authors from analyzed journals
Publishers usually have instructions for prospective authors; these indications tell authors what to include, the information that should be provided, and how it should be reported in the manuscript. In Table 1 we present the list of guidelines that were analyzed.
Corpus of protocols
Our corpus includes 530 published and unpublished protocols. Unpublished protocols (75 in total) were collected from four laboratories located at the International Center for Tropical Agriculture (CIAT) ( CIAT, 2017 ). The published protocols (455 in total) were gathered from the repository “Nature Protocol Exchange” ( NPE, 2017 ) and from 11 journals, namely: BioTechniques, Cold Spring Harbor Protocols, Current Protocols, Genetics and Molecular Research ( GMR, 2017 ), JoVE, Plant Methods ( BioMed Central, 2017 ), Plos One ( PLOS ONE, 2017 ), Springer Protocols, MethodsX, Bio-Protocol and the Journal of Biological Methods. The analyzed protocols comprise areas such as cell biology, molecular biology, immunology, and virology. The number of protocols from each journal is presented in Table 2 .
Minimum information standards and ontologies
We analyzed minimum information standards from the FairSharing catalog, e.g., MIAPPE ( MIAPPE, 2017 ), MIARE ( MIARE, 2017 ) and MIQE ( Bustin et al., 2009 ). See Table 3 for the complete list of minimum information models that we analyzed.
We paid special attention to the recommendations indicating how to describe specimens, reagents, instruments, software and other entities participating in different types of experiments. Ontologies available at Bioportal and Ontobee were also considered; we focused on ontologies modeling domains, e.g., bioassays (BAO), protocols (EXACT), experiments and investigations (OBI). We also focused on those modeling specific entities, e.g., organisms (NCBI Taxon), anatomical parts (UBERON), reagents or chemical compounds (ERO, ChEBI), instruments (OBI, BAO, EFO). The list of analyzed ontologies is presented in Table 4 .
Methods for developing this guideline
Developing the guideline entailed a series of activities; these were organized in the following stages: (i) analysis of guidelines for authors, (ii) analysis of protocols, (iii) analysis of Minimum Information (MI) standards and ontologies, and (iv) evaluation of the data elements from our guideline. For a detailed representation of our workflow, see Fig. 1

Analyzing guidelines for authors
We manually reviewed instructions for authors from nine journals as presented in Table 1 . In this stage (step A in Fig. 1 ), we identified bibliographic data elements classified as “desirable information” in the analyzed guidelines. See Table 5 .
In addition, we identified the rhetorical elements. These have been categorized in the guidelines for authors as: (i) required information (R), must be submitted with the manuscript; (ii) desirable information (D), should be submitted if available; and (iii) optional (O) or extra information. See Table 6 for more details.
Analyzing the protocols
In 2014, we started by manually reviewing 175 published and unpublished protocols; these were from domains such as cell biology, biotechnology, virology, biochemistry and pathology. From this collection, 75 are unpublished protocols and thus not available in the dataset for this paper. These unpublished protocols were collected from four laboratories located at the CIAT. In 2015, our corpus grew to 530; we included 355 published protocols gathered from one repository and eleven journals as listed in Table 2 . Our corpus of published protocols is: (i) identifiable, i.e., each document has a Digital Object Identifier (DOI) and (ii) in disciplines and areas related to the expertise provided by our domain experts, e.g., virology, pathology, biochemistry, biotechnology, plant biotechnology, cell biology, molecular and developmental biology and microbiology. In this stage, step B in Fig. 1 , we analyzed the content of the protocols; theory vs. practice was our main concern. We manually verified if published protocols were following the guidelines; if not, what was missing , what additional information was included? We also reviewed common data elements in unpublished protocols.
Analyzing minimum information standards and ontologies
Biomedical sciences have an extensive body of work related to minimum information standards and reporting structures, e.g., those from the FairSharing initiative. We were interested in determining whether there was any relation to these resources. Our checklist includes the data elements that are common across these resources. We manually analyzed standards such as MIQE, used to describe qPCR assays; we also looked into MIACA, it provides guidelines to report cellular assays; ARRIVE, which provides detailed descriptions of experiments on animal models and MIAPPE, addressing the descriptions of experiments for plant phenotyping. See Table 3 for a complete list of the standards that we analyzed. Metadata, data, and reporting structures in biomedical documents are frequently related to ontological concepts. We also looked into relations between data elements and biomedical ontologies available in BioPortal and Ontobee. We focused on ontologies representing materials that are often found in protocols; for instance, organisms, anatomical parts (e.g., CLO, UBERON, NCBI Taxon), reagents or chemical compounds (e.g., ChEBI, ERO), and equipment (e.g., OBI, BAO, EFO). The complete list of the ontologies that we analyzed is presented in Table 4 .
Generating the first draft
The first draft is the main output from the initial analysis of instructions for authors, experimental protocols, MI standards and ontologies, see (step D in Fig. 1 ). The data elements were organized into four categories: bibliographic data elements such as title, authors; descriptive data elements such as purpose, application; data elements for materials, e.g., sample, reagents, equipment; and data elements for procedures, e.g., critical steps, Troubleshooting. The role of the authors, provenance and properties describing the sample (e.g., organism part, amount of the sample, etc.) were considered in this first draft. In addition properties like “name”, “manufacturer or vendor” and “identifier” were proposed to describe equipment, reagents and kits.
Evaluation of data elements by domain experts
This stage entailed three activities. The first activity was carried out at CIAT with the participation of 19 domain experts in areas such as virology, pathology, biochemistry, and plant biotechnology. The input of this activity was the checklist V. 0.1 (see step E in Fig. 1 ). This evaluation focused on “ What information is necessary and sufficient for reporting an experimental protocol? ”; the discussion also addressed data elements that were not initially part of guidelines for authors -e.g., consumables. The result of this activity was the version 0.2 of the checklist; domain experts suggested to use an online survey for further validation. This survey was designed to enrich and validate the checklist V. 0.2. We used a Google survey that was circulated over mailing lists; participants did not have to disclose their identity (see step F in Fig. 1 ). A final meeting was organized with those who participated in workshops, as well as in the survey (23 in total) to discuss the results of the online poll. The discussion focused on the question: Should the checklist include data elements not considered by the majority of participants? Participants were presented with use cases where infrequent data elements are relevant in their working areas. It was decided to include all infrequent data elements; domain experts concluded that this guideline was a comprehensive checklist a opposed to a minimal information. Also, after discussing infrequent data elements it was concluded that the importance of a data element should not bear a direct relation to its popularity. The analogy used was that of an editorial council; some data elements needed to be included regardless of the popularity as an editorial decision. The output of this activity was the checklist V. 1.0. The survey and its responses are available at ( Giraldo, Garcia & Corcho, 2018c ). This current version includes a new bibliographic element “license of the protocol”, as well as the property “equipment configuration” associated to the datum equipment. The properties: alternative, optional and parallel steps were added to describe the procedure. In addition, the datum “PCR primers” was removed from the checklist, it is specific and therefore should be the product of a community specialization as opposed to part of a generic guideline.
Our results are summarized in Table 7 ; it includes all the data elements resulting from the process illustrated in Fig. 1 . We have also implemented our checklist as an online tool that generates data in the JSON format and presents an indicator of completeness based on the checked data elements; the tool is available at https://smartprotocols.github.io/checklist1.0 ( Gómez, alexander & Giraldo, 2018 ). Below, we present a complete description of the data elements in our checklist. We have organized the data elements in four categories, namely: (i) bibliographic data elements, (ii) discourse data elements, (iii) data elements for materials, and iv) data elements for the procedure. Ours is a comprehensive checklist, the data elements must be reported whenever applicable.
Bibliographic data elements
From the guidelines for authors, the datum “author identifier” was not considered, nor was this data element found in the analyzed protocols. The “provenance” is proposed as “desirable information” in only two of the guidelines (Nature Protocols and Bio-protocols), as well as “updates of the protocol” (Cold Spring Harbor Protocols and Bio-protocols). A total of 72.5% (29) of the protocols available in our Bio-protocols collection and 61.5% (24) of the protocols available in our Nature Protocols Exchange collection reported the provenance ( Fig. 2 ). None of the protocols collected from Cold Spring Harbor Protocols or Bio-protocols had been updated–last checked December 2017.

NC, Not Considered in guidelines; D, Desirable information if this is available.
As a result of the workshops, domain experts exposed the importance of including these three data elements in our checklist. For instance, readers sometimes need to contact the authors to ask about specific information (quantity of the sample used, the storage conditions of a solution prepared in the lab, etc.); occasionally, the correspondent author does not respond because he/she has changed his/her email address, and searching for the full name could retrieve multiple results. By using author IDs, this situation could be resolved. The experts asserted that well-documented provenance helps them to know where the protocol comes from and whether it has changed. For example, domain experts expressed their interest in knowing where a particular protocol was published for the first time, who has reused it, how many research papers have used it, how many people have modified it, etc. In a similar way, domain experts also expressed the need for a version control system that could help them to know and understand how, where and why the protocol has changed. For example, researchers are interested in tracking changes in quantities, reagents, instruments, hints, etc. For a complete description of the bibliographic data elements proposed in our checklist, see below.
Title. The title should be informative, explicit, and concise (50 words or fewer). The use of ambiguous terminology and trivial adjectives or adverbs (e.g., novel, rapid, efficient, inexpensive, or their synonyms) should be avoided. The use of numerical values, abbreviations, acronyms, and trademarked or copyrighted product names is discouraged. This definition was adapted from BioTechniques ( Giraldo, Garcia & Corcho, 2018b ). In Table 8 , we present examples illustrating how to define the title.
Issues in the ambiguous tittle:
Author name and author identifier. The full name(s) of the author(s) is required together with an author ID, e.g., ORCID ( ORCID, 2017 ) or research ID ( ResearcherID, 2017 ). The role of each author is also required; depending on the domain, there may be several roles. It is important to use a simple word that describes who did what. Publishers, laboratories, and authors should enforce the use of an “author contribution section” to identify the role of each author. We have identified two roles that are common across our corpus of documents.
- • Creator of the protocol: This is the person or team responsible for the development or adaptation of a protocol.
- • Laboratory-validation scientist: Protocols should be validated in order to certify that the processes are clearly described; it must be possible for others to follow the described processes. If applicable, statistical validation should also be addressed. The validation may be procedural (related to the process) or statistical (related to the statistics). According to the Food and Drug Administration (FDA) ( FDA, 2017 ), validation is “ establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes ” ( Das, 2011 ).
Updating the protocol. The peer-reviewed and non peer-reviewed repositories of protocols should encourage authors to submit updated versions of their protocols; these may be corrections, retractions, or other revisions. Extensive modifications to existing protocols could be published as adapted versions and should be linked to the original protocol. We recommended to promote the use of a version control system; in this paper we suggest to use the version control guidelines proposed by the National Institute of Health (NIH) ( NIH, 2017 ).
- • Document dates: Suitable for unpublished protocols. The date indicating when the protocol was generated should be in the first page and, whenever possible, incorporated into the header or footer of each page in the document.
- – Draft document version number: Suitable for unpublished protocols. The first draft of a document will be Version 0.1. Subsequent drafts will have an increase of “0.1” in the version number, e.g., 0.2, 0.3, 0.4, ... 0.9, 0.10, 0.11.
- – Final document version number and date: Suitable for unpublished and published protocols. The author (or investigator) will deem a protocol final after all reviewers have provided final comments and these have been addressed. The first final version of a document will be Version 1.0; the date when the document becomes final should also be included. Subsequent final documents will have an increase of “1.0” in the version number (1.0, 2.0, etc.).
- • Documenting substantive changes: Suitable for unpublished and published protocols. A list of changes from the previous drafts or final documents will be kept. The list will be cumulative and identify the changes from the preceding document versions so that the evolution of the document can be seen. The list of changes and consent/assent documents should be kept with the final protocol.
Provenance of the protocol. The provenance is used to indicate whether or not the protocol results from modifying a previous one. The provenance also indicates whether the protocol comes from a repository, e.g., Nature Protocols Exchange, protocols.io ( Teytelman et al., 2016 ), or a journal like JoVE, MethodsX, or Bio-Protocols. The former refers to adaptations of the protocol. The latter indicates where the protocol comes from. See Table 9 .
License of the protocol. The protocols should include a license. Whether as part of a publication or, just as an internal document, researchers share, adapt and reuse protocols. The terms of the license should facilitate and make clear the legal framework for these activities.
Data elements of the discourse
Here, we present the elements considered necessary to understand the suitability of a protocol. They are the “overall objective or purpose”, “applications”, “advantages,” and “limitations”. 100% of the analyzed guidelines for author suggest the inclusion of these four elements in the abstract or introduction section. However, one or more of these four elements were not reported. For example, “limitations” was reported in only 20% of the protocols from Genetic and Molecular Research and PLOS One, and in 40% of the protocols from Springer. See Fig. 3 .

Interestingly, 83% of the respondents considered the “limitations” to be a data element that is necessary when reporting a protocol. In the last meeting, participants considered that “limitations” represents an opportunity to make suggestions for further improvements. Another data element discussed was “advantages”; 43% of the respondents considered the “advantages” as a data element that is necessary to be reported in a protocol. In the last meeting, all participants agreed that “advantages” (where applicable) could help us to compare a protocol with other alternatives commonly used to achieve the same result. For a complete description of the discourse data elements proposed in our checklist, see below.
Overall objective or Purpose. The description of the objective should make it possible for readers to decide on the suitability of the protocol for their experimental problem. See Table 10 .
Application of the protocol. This information should indicate the range of techniques where the protocol could be applied. See Table 10 .
Advantage(s) of the protocol. Here, the advantages of a protocol compared to other alternatives should be discussed. See Table 10 . Where applicable, references should be made to alternative methods that are commonly used to achieve the same result.
Limitation(s) of the protocol. This datum includes a discussion of the limitations of the protocol. This should also indicate the situations in which the protocol could be unreliable or unsuccessful. See Table 10 .
Data elements for materials
From the analyzed guidelines for authors, the datum “sample description” was considered only in the Current Protocols guidelines. The “laboratory consumables or supplies” datum was not included in any of the analyzed guidelines. See Fig. 4 .

NC, Not Considered in guidelines; D, Desirable information if this is available; R, Required information.
Our Current Protocols collection includes documents about toxicology, microbiology, magnetic resonance imaging, cytometry, chemistry, cell biology, human genetics, neuroscience, immunology, pharmacology, protein, and biochemistry; for these protocols the input is a biological or biochemical sample. This collection also includes protocols in bioinformatics with data as the input. 100% of the protocols from our Current Protocols collection includes information about the input of the protocol (biological/biochemical sample or data). In addition, 87% of protocols from this collection include a list of materials or resources (reagents, equipment, consumables, software, etc.).
We also analyzed the protocols from our MethodsX collection. We found that despite the exclusion of the sample description in guidelines for authors, the authors included this information in their protocols. Unfortunately, these protocols do not include a list of materials. Only 29% of the protocols reported a partial list of materials. For example, the protocol published by Vingataramin & Frost (2015) , includes a list of recommended equipment but does not list any of the reagents, consumables, or other resources mentioned in the protocol instructions. See Fig. 5 .

Domain experts considered that the input of the protocol (biological/biochemical sample or data) needs an accurate description; the granularity of the description varies depending on the domain. If such description is not available then the reproducibility could be affected. In addition, domain experts strongly suggested to include consumables in the checklist. It was a general surprise not to find these data elements in the guidelines for authors that we analyzed. Domain experts shared with us bad experiences caused by the lack of information about the type of consumables. Some of the incidents that may arise from the lack of this information include: (i) cross contamination, when no information suggesting the use of filtered pipet tips is available; (ii) misuse of containers, when no information about the use of containers resistant to extreme temperatures and/or impacts is available; (iii) misuse of containers, when a container made of a specific material should be used, e.g., glass vs. plastic vs. metal. This is critical information; researchers need to know if reagents or solutions prepared in the laboratory require some specific type of containers in order to avoid unnecessary reactions altering the result of the assay. Presented below is the set of data elements related to materials or resources used for carrying out the execution of a protocol.
Sample. This is the role played by a biological substance; the sample is an experimental input to a protocol. The information required depends on the type of sample being described and the requirements from different communities. Here, we present the data elements for samples commonly used across the protocols and guidelines that we analyzed.
- Strain, genotype or line: This datum is about subspecies such as ecotype, cultivar, accession, or line. In the case of crosses or breeding results, pedigree information should also be provided.
- – whole organism Typical examples are multicellular animals, plants, and fungi; or unicellular microorganisms such as a protists, bacteria, and archaea.
- – organism part Typical examples of an organism part include a cell line, a tissue, an organ, corporal bodily fluids protoplasts, nucleic acids, proteins, etc.
- – organism/sample identifier This is the unique identifier assigned to an organism. The NCBI taxonomy id, also known as “taxid”, is commonly used to identify an organism; the Taxonomy Database is a curated classification and nomenclature for all organisms in the public sequence databases. Public identification systems, e.g., the Taxonomy Database, should be used when ever possible. Identifiers may be internal; for instance, laboratories often have their own coding system for generating identifiers. When reporting internal identifiers it is important to also state the source and the nature (private or pubic) of the identifier, e.g., A0928873874, barcode (CIAT-DAPA internal identifier) of a specimen or sample.
- Amount of Bio-Source: This datum is about mass (mg fresh weight or mg dry weight), number of cells, or other measurable bulk numbers (e.g., protein content).
- Developmental stage: This datum includes age and gender (if applicable) of the organism.
- Bio-source Supplier: This datum is defined as a person, company, laboratory or entity that offers a variety of biosamples or biospecimens.
- Growth substrates: This datum refers to an hydroponic system (type, supplier, nutrients, concentrations), soil (type, supplier), agar (type, supplier), and cell culture (media, volume, cell number per volume).
- Growth environment: This datum includes, but is not limited to, controlled environments such as greenhouse (details on accuracy of control of light, humidity, and temperature), housing conditions (light/dark cycle), and non-controlled environments such as the location of the field trial.
- Growth time: This datum refers to the growth time of the sample prior to the treatment.
- • Sample pre-treatment or sample preparation: This datum refers to collection, transport, storage, preparation (e.g., drying, sieving, grinding, etc.), and preservation of the sample.
Laboratory equipment. The laboratory equipment includes apparatus and instruments that are used in diagnostic, surgical, therapeutic, and experimental procedures. In this subsection, all necessary equipment should be listed; manufacturer name or vendor (including the homepage), catalog number (or model), and configuration of the equipment should be part of this data element. See Table 11 .
- • Laboratory equipment name: This datum refers to the name of the equipment as it is given by the manufacturer (e.g., FocalCheck fluorescence microscope test slide).
- • Manufacturer name: This datum is defined as a person, company, or entity that produces finished goods (e.g., Life Technologies, Zeiss).
- • Laboratory equipment ID (model or catalog number): This datum refers to an identifier provided by the manufacturer or vendor (e.g., {"type":"entrez-nucleotide","attrs":{"text":"F36909","term_id":"4822535","term_text":"F36909"}} F36909 —catalog number for FocalCheck fluorescence microscope test slide from Life Technologies).
- • Equipment configuration: This datum should explain the configuration of the equipment and the parameters that make it possible to carry out an operation, procedure, or task (e.g., the configuration of an inverted confocal microscope).
Laboratory consumables or supplies. The laboratory consumables include, amongst others, disposable pipettes, beakers, funnels, test tubes for accurate and precise measurement, disposable gloves, and face masks for safety in the laboratory. In this subsection, a list with all the consumables necessary to carry out the protocol should be presented with manufacturer name (including the homepage) and catalog number. See Table 12 .
- • Laboratory consumable name: This datum refers to the name of the laboratory consumable as it is given by the manufacturer e.g., Cryogenic Tube, sterile, 1.2 ml.
- • Manufacturer name: This datum is defined as a person, enterprise, or entity that produces finished goods (e.g., Nalgene, Thermo-scientific, Eppendorf, Falcon)
- • Laboratory consumable ID (catalog number): This datum refers to an identifier provided by the manufacturer or vendor; for instance, 5000-0012 (catalog number for Cryogenic Tube, sterile, 1.2 mL from Nalgene).
Recipe for solutions. A recipe for solutions is a set of instructions for preparing a particular solution, media, buffer, etc. The recipe for solutions should include the list of all necessary ingredients (chemical compounds, substance, etc.), initial and final concentrations, pH, storage conditions, cautions, and hints. Ready-to-use reagents do not need to be listed in this category; all purchased reagents that require modification (e.g., a dilution or addition of β -mercaptoethanol) should be listed. See Table 13 for more information.
- • Solution name: This is the name of the preparation that has at least 2 chemical substances, one of them playing the role of solvent and the other playing the role of solute. If applicable, the name should include the following information: concentration of the solution, final volume and final pH. For instance, Ammonium bicarbonate (NH4HCO3), 50 mM, 10 ml, pH 7.8.
- • Chemical compound name or reagent name: This is the name of a drug, solvent, chemical, etc.; for instance, agarose, dimethyl sulfoxide (DMSO), phenol, sodium hydroxide. If applicable, a measurable property, e.g., concentration, should be included.
- • Initial concentration of a chemical compound: This is the first measured concentration of a compound in a substance.
- • Final concentration of chemical compound: This is the last measured concentration of a compound in a substance.
- • Storage conditions: This datum includes, among others, shelf life (maximum storage time) and storage temperature for the solutions e.g., “Store the solution at room temperature”, “maximum storage time, 6 months”. Specify whether or not the solutions must be prepared fresh.
- • Cautions: Toxic or harmful chemical compounds should be identified by the word ‘CAUTION’ followed by a brief explanation of the hazard and the precautions that should be taken when handling e.g., “CAUTION: NaOH is a very strong base. Can seriously burn skin and eyes. Wear protective clothing when handling. Make in fume hood”.
- • Hints: The “hints” are commentaries or “tips” that help the researcher to correctly prepare the recipe e.g., “Add NaOH to water to avoid splashing”.
Reagents. A reagent is a substance used in a chemical reaction to detect, measure, examine, or produce other substances. List all the reagents used when performing the protocol, the vendor name (including homepage), and catalog number. Reagents that are purchased ready-to-use should be listed in this section. See Table 14 .
- • Reagent name: This datum refers to the name of the reagent or chemical compound. For instance, “Taq DNA Polymerase from Thermus aquaticus with 10X reaction buffer without MgCl2”.
- • Reagent vendor or manufacturer: This is the person, enterprise, or entity that produces chemical reagents e.g., Sigma-Aldrich.
- • Reagent ID (catalog number): This is an identifier provided by the manufacturer or vendor. For instance, D4545-250UN (catalog number for Taq DNA Polymerase from Thermus aquaticus with 10X reaction buffer without MgCl2 from Sigma-Aldrich).
Kits. A kit is a gear consisting of a set of articles or tools for a specific purpose. List all the kits used when carrying out the protocol, the vendor name (including homepage), and catalog number.
- • Kit name: This datum refers to the name of the kit as it is given by the manufacturer e.g., Spectrum Plant Total RNA Kit, sufficient for 50 purifications.
- • Kit vendor or manufacturer: This is the person, enterprise, or entity that produces the kit e.g., Sigma-Aldrich.
- • Kit ID (catalog number): This is an identifier provided by the manufacturer or vendor e.g., STRN50, catalog number for Spectrum ™ Plant Total RNA Kit, sufficient for 50 purifications.
Software. Software is composed of a series of instructions that can be interpreted or directly executed by a processing unit. In this subsection, please list software used in the experiment including the version, as well as where to obtain it.
- • Software name: This datum refers to the name of the software. For instance, “LightCycler 480 Software”.
- • Software version: A software version number is an attribute that represents the version of software e.g., Version 1.5.
- • Software availability: This datum should indicate where the software can be downloaded from. If possible, license information should also be included; for instance, https://github.com/MRCIE-U/ariesmqtl, GPL3.0.
Data elements for the procedure
All the analyzed guidelines include recommendations about how to document the instructions; for example, list the steps in numerical order, use active tense, organize the procedures in major stages, etc. However, information about documentation of alternative, optional, or parallel steps (where applicable) and alert messages such as critical steps, pause point, and execution time was infrequent (available in less than 40% of the guidelines). See Fig. 6 .

NC, Not Considered in guidelines; O, Optional information; D, Desirable information if this is available; R, Required information.
We chose a subset of protocols (12 from our Plant Methods collection, 7 from our Biotechniques collection, and five unpublished protocols from CIAT) to review which data elements about the procedure were documented. 100% of the protocols have steps organized in major stages. 100% of the unpublished protocols list the steps in numerical order, and nearly 60% of the protocols from Plant Methods and Biotechniques followed this recommendation. Alert messages were included in 67% of the Plant Methods protocols and in 14% of the Biotechniques protocols. Neither of the five unpublished protocols included alert messages. Troubleshooting was reported in just a few protocols; this datum was available in 8% of the Plant Methods protocols and in 14% of the Biotechniques protocols. See Fig. 7 .

In this stage, the discussion with domain experts started with the description of steps. In some protocols, the steps are poorly described; for instance, some of them include working temperatures, e.g., cold room, on ice, room temperature; but, what exactly do they mean? Steps involving centrifugation, incubation, washing, etc., should specify conditions, e.g., time, temperature, speed (rpm or g), number of washes, etc. For experts, alert messages and troubleshooting (where applicable) complement the description of steps and facilitate a correct execution. This opinion coincides with the results of the survey, where troubleshooting and alert messages such as critical steps, pause points, and timing were considered relevant by 83%–87% of the respondents. The set of data elements related to the procedure is presented below.
- • Recommendation 1. Whenever possible, list the steps in numerical order; use active tense. For example: “Pipette 20 ml of buffer A into the flask,” as opposed to “20 ml of buffer A are/were pipetted into the flask” ( Nature Protocols, 2012 ).
- • Recommendation 2. Whenever there are two or more alternatives, these should be numbered as sets of consecutive steps Wiley’s Current Protocols (2012) . For example: “Choose procedure A (steps 1–10) or procedure B (steps 11–20); then continue with step 21 . . .”. Optional steps or steps to be executed in parallel should also be included.
- • Recommendation 3. For techniques comprising a number of individual procedures, organize these in the exact order in which they should be executed ( Nature Protocols, 2012 ).
- – Frozen/deep-freeze temperature (−20 °C to −15 °C)
- – Refrigerator, cold room or cold temperature (2 °C to 8 °C)
- – Cool temperature (8 °C to 15 °C)
- – Room/Ambient temperature (15 °C to 25 °C)
- – Warm/Lukewarm temperature (30 °C to 40 °C)
For centrifugation steps, specify time, temperature, and speed (rpm or g). Always state whether to discard/keep the supernatant/pellet. For incubations, specify time, temperature, and type of incubator. For washes, specify conditions e.g., temperature, washing solution and volume, specific number of washes, etc.
Useful auxiliary information should be included in the form of “alert messages”. The goal is to remind or alert the user of a protocol with respect to issues that may arise when executing a step. These messages may cover special tips or hints for performing a step successfully, alternate ways to perform the step, warnings regarding hazardous materials or other safety conditions, time considerations. For instance, pause points, speed at which the step must be performed and storage information (temperature, maximum duration) ( Wiley’s Current Protocols, 2012 ).
- • Pause point: This datum is appropriate after steps in the protocol where the procedure can be stopped. i.e., when the experiment can be stopped and resumed at a later point in time. Any PAUSE POINTS should be indicated with a brief description of the options available. See Table 15 .
- • Timing: This datum is used to include the approximate time of execution of a step or set of steps. Timing could also be indicated at the beginning of the protocol. See Table 15 .
- • Hints: Provide any commentary, note, or hints that will help the researcher to correctly perform the protocol. See Table 15 .
- • Troubleshooting: This datum is used to list common problems, possible causes, and solutions/methods of correction. This can be submitted as a 3-column table or listed in the text. An example is presented in “Table 1.Troubleshooting table”, available at Rohland & Hofreiter (2007) .
Data Elements Represented in the SMART Protocols Ontology
The data elements proposed in our guideline are represented in the SMART Protocols Ontology. This ontology was developed to facilitate the semantic representation of experimental protocols. Our ontology reuses the Basic Formal Ontology (BFO) ( IFOMIS, 2018 ) and the Relation Ontology (RO) ( Smith et al., 2005 ) to characterize concepts. In addition, each term in the SMART Protocols ontology is represented with annotation properties imported from the OBI Minimal metadata. The classes and properties are represented by their respective labels to facilitate the readability; the prefix indicates the provenance for each term. Our ontology is organized in two modules. The document module represents the metadata necessary and sufficient for reporting a protocol. The workflow module represents the executable elements of a protocol to be carried out and maintained by humans. Figure 8 presents the hierarchical organization of data elements into the SMART Protocols Ontology.

In this paper, we have described 17 data elements that can be used to improve the reporting structure of protocols. Our work is based on the analysis of 530 published and non-published protocols, guidelines for authors, and suggested reporting structures. We examined guidelines for authors from journals that specialize in publishing experimental protocols, e.g., Bio-protocols, Cold Spring Harbor Protocols, MethodsX, Nature Protocols, and Plant Methods (Methodology). Although JoVE ( JoVE, 2017 ) is a video methods journal, its guidelines for authors were also considered. Online repositories were also studied; these resources deliver an innovative approach for the publication of protocols by offering platforms tailored for this kind of document. For instance, protocols.io ( protocols.io, 2018 ) structures the protocol by using specific data elements and treats the protocol as a social object, thus facilitating sharing. It also makes it possible to have version control over the document. Protocol Exchange from Nature Protocols is an open repository where users upload, organize, comment, and share their protocols. Our guideline has also benefited from the input from a group of researchers whose primary interest is having reproducible protocols. By analyzing reporting structures and guidelines for authors, we are contributing to the homogenization of data elements that should be reported as part of experimental protocols. Improving the reporting structure of experimental protocols will add the necessary layer of information that should accompany the data that is currently being deposited into data repositories.
Ours was an iterative development process; drafts were reviewed and analyzed, and then improved versions were produced. This made it easier for us to make effective use of the time that domain experts had available. Working with experimental protocols that were known by our group of domain experts helped us to engage them in the iterations. Also, for the domain experts who worked with us during the workshops, there was a pre-existing interest in standardizing their reporting structures. Reporting guidelines are not an accepted norm in biology ( MIBBI, 2017 ); however, experimental protocols are part of the daily activities for most biologists. They are familiar with these documents, the benefits of standardization are easy for them to understand. From our experience at CIAT, once researchers were presented with a standardized format that they could extend and manage with minimal overhead, they adopted it. The early engagement with domain experts in the development process eased the initial adoption; they were familiar with the outcome and aware of the advantages of implementing this practice. However, maintaining the use of the guideline requires more than just availability of the guideline; the long-term use of these instruments requires an institutional policy in data stewardship. Our approach builds upon previous experiences; in our case, the guidelines presented in this paper are a tool that was conceived by researchers as part of their reporting workflow, thus adding a minimal burden on their workload. As domain experts were working with the guideline, they were also gaining familiarity with the Minimum Information for Biological and Biomedical Investigations (MIBBI) ( MIBBI, 2017 ) that were applicable to their experiments. This made it possible for us to also discuss the relation between MIBBIs and the content in the experimental protocols.
The quality of the information reported in experimental protocols and methods is a general cause for concern. Poorly described methods generate poorly reproducible research. In a study conducted by Flórez-Vargas et al. (2014) in Trypanosoma experiments, they report that none of the investigated articles met all the criteria that should be reported in these kinds of experiments. The study reported by Kilkenny et al. (2009) has similar results leading to similar conclusions; key metadata elements are not always reported by researchers. The widespread availability of key metadata elements in ontologies, guidelines, minimal information models, and reporting structures was discussed. These were, from the onset, understood as reusable sources of information. Domain experts understand that they were building on previous experiences; having examples of use is helpful in understanding how to adapt or reuse from existing resources. This helps them to understand the rationale of each data element within the context of their own practice. For us, being able to consult previous experiences was also an advantage. Sharing protocols is a common practice amongst researchers from within the same laboratories or collaborating in the same experiments or projects. However, there are limitations in sharing protocols, not necessarily related to the lack of reporting standards. They are, for instance, related to patenting and intellectual property issues, as well as to giving away competitive advantages implicit in the method.
During our development process, we considered the SMART Protocols ontology ( Giraldo et al., 2017 ); it reuses terminology from OBI, IAO, EXACT, ChEBI, NCBI taxonomy, and other ontologies. Our metadata elements have been mapped to the SMART Protocols ontology; the metadata elements in our guideline could also be mapped to resources on the web such as PubChem ( Kim et al., 2016 ) ( Wang et al., 2017 ) and the Taxonomy database from UniProt ( UniProt, 2017 ). Our implementation of the checklist illustrates how it could be used as an online tool to generate a complement to the metadata that is usually available with published protocols. The content of the protocol does not need to be displayed; key metadata elements are made available together with the standard bibliographic metadata. Laboratories could adapt the online tool to their specific reporting structures. Having a checklist made it easier for the domain experts to validate their protocols. Machine validation is preferable, but such mechanisms require documents to be machine-processable beyond that which our domain experts were able to generate. Domain experts were using the guideline to implement simple Microsoft Word reporting templates. Our checklist does not include aspects inherent to each possible type of experiment such as those available in the MIBBIs; these are based on the minimal common denominator for specific experiments. Both approaches complement each other; where MIBBIs offer specificity, our guideline provides a context that is general enough for facilitating reproducibility and adequate reporting without interfering with records such as those commonly managed by Laboratory Information Management Systems.
In laboratories, experimental protocols are released and periodically undergo revisions until they are released again. These documents follow the publication model put forward by Carole Goble, “ Don’t publish, release ” with strict versioning, changes, and forks ( Goble, 2017 ). Experimental protocols are essentially executable workflows for which identifiers for equipment, reagents, and samples need to be resolved against the Web. The use of unique identifiers can’t be underestimated when supporting adequate reporting; identifiers remove ambiguity for key resources and make it possible for software agents to resolve and enrich these entities. The workflows in protocols are mostly followed by humans, but in the future, robots may be executing experiments ( Yachie, Consortium & Natsume, 2017 ); it makes sense to investigate other publication paradigms for these documents. The workflow nature of these documents is more suitable for a fully machine-processable or -actionable document. The workflows should be intelligible for humans and processable by machines; thus, facilitating the transition to fully automated laboratory paradigms. Entities and executable elements should be declared and characterized from the onset. The document should be “born semantic” and thus inter-operable with the larger web of data. In this way post-publication and linguistic processing activities, such as Named Entity Recognition and annotation, could be more focused.
Currently, when protocols are published, they are treated like any other scientific publication. Little attention is paid to the workflow nature implicit in this kind of document, or to the chain of provenance indicating where it comes from and how it has changed. The protocol is understood as a text-based narrative instead of a self-descriptive Findable Accessible Interoperable and Reusable (FAIR) ( Wilkinson et al., 2016 ) compliant document. There are differences across the examined publications, e.g., JoVE builds the narrative around video, whereas Bio-protocols, MethodsX, Nature Protocols, and Plant Methods primarily rely on a text-based narrative. The protocol is, however, a particular type of publication; it is slightly different from other scientific articles. An experimental protocol is a document that is kept “alive” after it has been published. The protocols are routinely used in laboratory activities, and researchers often improve and adapt them, for instance, by extending the type of samples that can be tested, reducing timing, minimizing the quantity of certain reagents without altering the results, adding new recipes, etc. The issues found in reporting methods probably stem, at least in part, from the current structure of scientific publishing, which is not adequate to effectively communicate complex experimental methods ( Flórez-Vargas et al., 2014 ).
Experimental research should be reproducible whenever possible. Having precise descriptions of the protocols is a step in that direction. Our work addresses the problem of adequate reporting for experimental protocols. It builds upon previous work, as well as over an exhaustive analysis of published and unpublished protocols and guidelines for authors. There is value in guidelines because they indicate how to report; having examples of use facilitate how to adapt them. The guideline we present in this paper can be adapted to address the needs of specific communities. Improving reporting structures requires collective efforts from authors, peer reviewers, editors, and funding bodies. There is no “one size that fits all.” The improvement will be incremental; as guidelines and minimal information models are presented, they will be evaluated, adapted, and re-deployed.
Authors should be aware of the importance of experimental protocols in the research life-cycle. Experimental protocols ought to be reused and modified, and derivative works are to be expected. This should be considered by authors before publishing their protocols; the terms of use and licenses are the choice of the publisher, but where to publish is the choice of the author. Terms of use and licenses forbidding “reuse”, “reproduce”, “modify”, or “make derivative works based upon” should be avoided. Such restrictions are an impediment to the ability of researchers to use the protocols in their most natural way, which is adapting and reusing them for different purposes –not to mention sharing, which is a common practice among researchers. Protocols represent concrete “know-how” in the biomedical domain. Similarly, publishers should adhere to the principle of encouraging authors to make protocols available, for instance, as preprints or in repositories for protocols or journals. Publishers should enforce the use of repository or journal publishing protocols. Publishers require or encourage data to be available; the same principle should be applied to protocols. Experimental protocols are essential when reproducing or replicating an experiment; data is not contextualized unless the protocols used to derive the data are available.
This work is related to the SMART Protocols project. Ultimately we want: (1) to enable authors to report experimental protocols with necessary and sufficient information that allows others to reproduce an experiment, (2) to ensure that every data item is resolvable against resources in the web of data, and (3) to make the protocols available in RDF, JSON, and HTML as web native objects. We are currently working on a publication platform based on linked data for experimental protocols. Our approach is simple, we consider that protocols should be born semantics and FAIR.
Acknowledgments
Special thanks to the research staff at CIAT; in particular, we want to express our gratitude to those who participated in the workshops, survey and discussions. We also want to thank Melissa Carrion for her useful comments and proof-reading. Finally, we would like to thank the editor and reviewers (Leonid Teytelman, Philippe Rocca-Serra and Tom Gillespie) for their valuable comments and suggestions to improve the manuscript.
Funding Statement
This work was supported by the EU project Datos4.0 (No. C161046002). Olga Giraldo has been funded by the I+D+i pre doctoral grant from the UPM, and the Predoctoral grant from the I+D+i program from the Universidad Politécnica de Madrid. Alexander Garcia has been funded by the KOPAR project, H2020-MSCA-IF-2014, Grant Agreement No. 655009. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
The authors declare there are no competing interests.
Olga Giraldo conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Alexander Garcia contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, alexander supervised the research and was a constant springboard for discussion and ideas wrt the checklist and methods.
Oscar Corcho reviewed drafts of the paper, and approved the final draft.

Experimental Study of Multiphase Flow in Porous Media during CO2 Geo-Sequestration Processes pp 51–89 Cite as
Experimental Setup, Material and Procedure
- Ali Saeedi 2
- First Online: 01 January 2012
1675 Accesses
1 Citations
Part of the book series: Springer Theses ((Springer Theses))
A major part of the experimental work related to this research, consisting of a number of different types of core-flooding experiments, was carried out using the state-of-the-art, high pressure-high temperature, three-phase steady-state core-flooding apparatus located within the Department of Petroleum Engineering at Curtin University. To be able to achieve the objectives of this research program, various types of flooding experiments were designed and carried out using the above-mentioned core-flooding rig. In the first part of this chapter a detailed description of the experimental apparatus and its various components is presented.
- Relative Permeability
- Overburden Pressure
- Flooding Experiment
- Composite Core
- Gravity Segregation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
NIST (2010) Thermophysical properties of fluid systems. http://webbook.nist.gov/chemistry/fluid/ , US National Institute of Standards and Technology. Accessed 17 June 2010
Zhenhao Duan Research Group (2010) Interactive online models. http://www.geochem-model.org/models.htm , Institute of Geology and Geophysics, Chinese Academy of Sciences. Accessed 25 June 2010
Leet LD, Judson S (1971) Physical geology. Prentice-Hall, New Jersey
Google Scholar
Sharma S, Cook P, Berly T, Lees M (2009) The CO 2- CRC otway project: overcoming challenges from planning to execution of Australia’s first CCS project. Energy Procedia 1:1965–1972
Article Google Scholar
Knackstedt MA, Dance T, Kumar M, Averdunk H, Paterson L (2010) Enumerating permeability, surface areas, and residual capillary trapping of CO 2 in 3D: digital analysis of CO 2 CRC otway project core, SPE 134625, SPE annual technical conference and exhibition, Society of Petroleum Engineers, Florence, Italy
Spencer L, Xu Q, LaPedalina F, Weir G (2006) Site characterization of the Otway Basin Storage Pilot in Australia. Proceeding of the 8th international conference on greenhouse gas control technologies, Trondheim, Norway
Gluyas J, Swarbrick R (2004) Petroleum geoscience. Blackwell Publishing, Oxford
Byrne M, Patey I (2004) Core sample preparation—an insight into new procedures. International symposium of the society of core analysts, Abu Dhabi, UAE
Chen J, Hirasaki GJ, Flaum M (2006) NMR wettability indices. Effect of OBM on wettability and NMR responses. J Pet Sci Eng 52:161–171
Fleury M, Deflandre F (2003) Quantitative evaluation of porous media wettability using NMR relaxometry. Magn Reson Imaging 21:385–387
Anderson WG (1986) Wettability literature survey–Part 1: rock/oil/brine interactions and the effects of core handling on wettability: SPE 13932. SPE J Pet Technol 38:1125–1144
Wendell DJ, Anderson WG, Meyers JD (1987) Restored-state core analysis for the hutton reservoir: SPE 14298. SPE Form Eval 2:509–517
Mungan N (1966) Certain wettability effects in laboratory waterfloods: SPE 1203. SPE J Pet Technol 18:247–252
Coates GR, Xiao L, Prammer MG (1999) NMR logging-principles and applications. Halliburton Energy Services, Houston
Clennell B, Raven M, Borysenko A, Sedev R, Dewhurst D (2006) Shale petrophysics: electrical, dielectric and nuclear magnetic resonance studies of mudrocks and clays. SPWLA 47th annual logging symposium, Society of Petrophysicists and Well Log Analysts, Veracruz, Mexico
Chen Q, Kinzelbach W, Ye C, Yue Y (2002) Variations of permeability and pore size distribution of porous media with pressure. J Environ Qual 31:500–505
Ennis-King J, Paterson L (2007) Coupling of geochemical reactions and convective mixing in the long-term geological storage of carbon dioxide. Int J Greenh Gas Control 1:86–93
Ennis-King J, Paterson L, Gale J, Kaya Y (2003) Rate of dissolution due to convective mixing in the underground storage of carbon dioxide. Greenhouse gas control technologies—6th international conference, Kyoto, Japan
Ali JK (1997) Developments in measurement and interpretation techniques in coreflood tests to determine relative permeabilities, SPE 39016. Latin American and Caribbean petroleum engineering conference, Society of Petroleum Engineers, Rio de Janeiro, Brazil
Boukadi FH, Bemani AS, Babadagli T (2005) Investigating uncertainties in relative permeability measurements. Energy Sources Part A: Recovery Util Environ Eff 27:719–728
Heaviside J, Black CJJ (1983) Fundamentals of relative permeability: experimental and theoretical considerations, SPE 12173. SPE annual technical conference and exhibition, Society of Petroleum Engineers of AIME, San Francisco, California
Honarpour M, Koederitz L, Harvey AH (1986) Relative permeability of petroleum reservoirs. CRC Press, Boca Raton
Craig FFJ, Sanderlin JL, Moore DW, Geffen TM (1957) A laboratory study of gravity segregation in frontal drives: SPE 676-G. Pet Trans AIME 210:275–282
Guo Y Nilsen V, Hovland F (1991) Gravity effect under steady-state and unsteady-state core flooding and criteria to avoid it. The second society of core analysts european core analysis symposium London, UK
Bed Jr BA, Nunes CS (1984) Velocity and gravity effects in relative permeability measurements. MSc theses, The Department of Petroleum Engineering Stanford University
Kinzel LD, Hill GA (1989) Experimental study of dispersion in a consolidated sandstone. Can J Chem Eng 67:39–44
Buckley SE, Leverett MC (1942) Mechanism of fluid displacement in sands: SPE 942107. Pet Trans AIME 146:107–116
Donnez P (2007) Essentials of reservoir engineering. Editions Technip, Paris
Huang DD, Honarpour MM (1998) Capillary end effects in coreflood calculations. J Pet Sci Eng 19:103–117
Rapoport LA, Leas WJ (1953) Properties of linear waterfloods: SPE 213-G. Pet Trans AIME 198:139–148
Haugen J (1990) Scaling criterion for relative permeability experiments on samples with intermediate wettability. Society of core analysts symposium, London, UK
Peters EJ, Flock DL (1981) The onset of instability during two-phase immiscible displacement in porous media: SPE 8371. SPE J 21:249–258
Chuoke RL, van Meurs P, van der Poel C (1959) The instability of slow, immiscible, viscous liquid-liquid displacements in permeable media: SPE 1141. Pet Trans AIME 216:188–194
Peters EJ, Khataniar S (1987) The effect of instability on relative permeability curves obtained by the dynamic-displacement method: SPE 14713. SPE Form Eval 2:469–474
Huppler JD (1969) Waterflood relative permeabilities in composite cores. J Pet Technol 21:539–540
Langaas K, Ekrann S, Ebeltoft E (1998) A criterion for ordering individuals in a composite core. J Pet Sci Eng 19:21–32
Leverett MC (1941) Capillary behavior in porous solids: SPE 941152. Pet Trans AIME 142:152–169
Abu-Khamsin SA, Ayub M, Al-Marhoun MA, Menouar H (1993) Waterflooding in a tarmat reservoir laboratory model. J Pet Sci Eng 9:251–261
Hinkley RE, Davis LA (1986) Capillary pressure discontinuities and end effects in homogeneous composite cores: effect of flow rate and wettability, SPE 15596. SPE annual technical conference and exhibition, Copyright 1986, Society of Petroleum Engineers, Inc, New Orleans, Louisiana
Øyno L, Uleberg K, Whitson CH (1995) Dry gas injection in fractured chalk reservoirs–An experimental approach. Society of Core Analysts Symposium, San Francisco, CA, USA
Zekri AY, Almehaideb RA (2006) Relative permeability measurements of composite cores: An experimental approach. Pet Sci Technol 24:717–736
Honarpour M, Mahmood SM (1988) Relative-permeability measurements: an overview: SPE 18565. SPE J Pet Technol 40:963–966
Johnson EF, Bossler DP, Naumann VO (1959) Calculation of relative permeability from displacement experiments. Pet Trans AIME 216:370–372
Jones SC, Roszelle WO (1978) Graphical techniques for determining relative permeability from displacement experiments: SPE 6045. SPE J Pet Technol 30:807–817
Archer JS, Wong SW (1973) Use of a reservoir simulator to interpret laboratory waterflood data: SPE 3551. SPE J 13:343–347
Van Spronsen E (1982) Three-phase relative permeability measurements using the centrifuge method, SPE 10688. SPE Enhanced oil recovery symposium, Copyright 1982, Society of Petroleum Engineers of AIME, Tulsa, Oklahoma
Hagoort J (1980) Oil recovery by gravity drainage: SPE 7424. SPE J 20:139–150
Bennion B, Bachu S (2008) Drainage and imbibition relative permeability relationships for supercritical CO 2 /brine and H 2 S/brine systems in intergranular sandstone, carbonate, shale, and anhydrite rocks: SPE 99326. SPE Reserv Eval Eng 11:487–496
Download references
Author information
Authors and affiliations.
Department of Petroleum Engineering, Curtin University, Dick Perry Avenue 26, Kensington, WA, 6151, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ali Saeedi .
Rights and permissions
Reprints and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter.
Saeedi, A. (2012). Experimental Setup, Material and Procedure. In: Experimental Study of Multiphase Flow in Porous Media during CO2 Geo-Sequestration Processes. Springer Theses. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-25041-5_3
Download citation
DOI : https://doi.org/10.1007/978-3-642-25041-5_3
Published : 04 January 2012
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-25040-8
Online ISBN : 978-3-642-25041-5
eBook Packages : Earth and Environmental Science Earth and Environmental Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
How to write an experimental research paper
Affiliation.
- 1 Marmara University Institute of Neurological Sciences, Istanbul, Turkey.
- PMID: 12442630
- DOI: 10.1007/978-3-7091-6743-4_18
The art and practice of academic neurosurgery are mastered by defining and learning the pertinent basic principles and skills. This article aims to present general guidelines to one of the many roles of a neurosurgeon: Writing an experimental research paper. Every research report must use the "IMRAD formula: introduction, methods, results and discussion". After the IMRAD is finished, abstract should be written and the title should be "created". Your abstract should answer these questions: "Why did you start?, what did you do?, what answer did you get?, and what does it mean?". Title of the research paper should be short enough to catch glance and memory of the reader and be long enough to give the essential information of what the paper is about. Writing about the results of the experiment is no easier than the research itself. As surgery, writing a scientific paper is also an improvisation, but general principles should be learned and used in practice. The most effective style of learning basic skills to construct a research paper is the "trial and error" type.
- Guidelines as Topic
- Internship and Residency
- Manuscripts, Medical as Topic
- Neurosurgery* / education
- Research* / education
- Writing* / standards

IMAGES
VIDEO
COMMENTS
Step 1: Define your variables. You should begin with a specific research question. We will work with two research question examples, one from health sciences and one from ecology: Example question 1: Phone use and sleep. You want to know how phone use before bedtime affects sleep patterns.
Abstract. This chapter discusses some of the general principles involved in building experimental setups, such as deliberated appropriateness, simplicity, compactness, and safety. The chapter ...
Abstract. Creating experimental setups is a fundamental step in a physicist's discovery process. This task is particularly challenging for quantum systems, since the behavior of such systems is often unintuitive. In this chapter, we discuss how a special kind of reinforcement learning, called projective simulation, can help to automate the ...
A research paper is intended to inform others about advancement in a particular field of study. The researcher who wrote the paper identified a gap in the research in a field of study and used their research to help fill this gap. The researcher uses their paper to inform others about the knowledge that the results of their study contribute ...
A good experimental design requires a strong understanding of the system you are studying. There are five key steps in designing an experiment: Consider your variables and how they are related. Write a specific, testable hypothesis. Design experimental treatments to manipulate your independent variable.
Research Paper Format. ... Write the abstract after you have written the rest of the paper; then you know what the paper claims to do and does. ... Experimental Setup In this section you describe how the experiment was done and summarize the data taken. One typically describes the instruments and detectors used in this section. Describe the ...
This article aims to present general guidelines to one of the many roles of a neurosurgeon: Writing an experimental research paper. Every research report must use the "IMRAD formula: introduction, methods, results and discussion". After the IMRAD is finished, abstract should be written and the title should be "created".
The standard experiment set up requires a pre-test of the dependent variable (prior to the treatment) of the treatment and the control group. This is only required if you establish a baseline to evaluate change from. ... Writing an experimental research paper follows the principles and structure detailed in Chap. 4. However, there are some ...
After the successful measurement of the 7Be(n,α)α cross section, the 7Be(n,p)7Li reaction was studied in order to provide still missing cross section data of relevance for Big Bang Nucleosynthesis (BBN), in an attempt to find a solution to the cosmological Lithium abundance problem. This paper describes the experimental setup employed in such ...
when writing a paper, the experimental section is often seen as a tedious stage through which an author must suffer on the road to publication. It is true that the experimental section may lack the up-front glory, but these details enable the research community to build upon your results, leading to the longevity of your hard work.
When a research psychologist talks about "writing a paper," he is talking about a lengthy and complicated chain of events that includes a great deal more than just reporting research results. In this chapter I outline these events from start to finish.
From writing research papers, experimental reports, grants, and research proposals to authoring books, scientists encounter several instances where they need to execute profound and convincing writing skills. All forms of technical writing are equally significant, but this article categorically emphasizes the skills and techniques required for ...
In this chapter, we outline these events from start to finish: PLANNING EXPERIMENTAL RESEARCH. Getting an Idea. The basis of any good research is a viable idea that results in a study that contributes to the current state of knowledge. However, getting ideas, and especially getting good ideas, often turns out to be one of the hardest parts of ...
Abstract. Experimental protocols are key when planning, performing and publishing research in many disciplines, especially in relation to the reporting of materials and methods. However, they vary in their content, structure and associated data elements. This article presents a guideline for describing key content for reporting experimental ...
The goal of this project is to design an experimental setup to measure the transfer of moisture between a sheet of paper and airflow through it and also a procedure to extract the mass transfer coefficient. In fig. 1.2 the principle of the inkjet process in a roll-to-roll type printer is shown. The paper is stored in a cylindrical roll inside ...
Designing an experiment means planning exactly how you'll test your hypothesis to reach valid conclusions. This video will walk you through the decisions you...
How to Write an Experimental Research Paper Initiated by the ever-increasing number of papers published each year (over two million in about 20.000 journals), a working group was set up at McMaster University in Canada to improve the quality of the information in abstracts [1]. The idea of "Structured Abstracts" was introduced and supported.
California Institute of Technology
Every research report must use the "IMRAD formula: introduction, methods, results and discussion". After the IMRAD is finished, abstract should be written and the title should be "created ...
Experimental Setup, Material and Procedure 3.1 Introduction A major part of the experimental work related to this research, consisting of a number of different types of core-flooding experiments, was carried out using the state-of-the-art, high pressure-high temperature, three-phase steady-state core-
Title of the research paper should be short enough to catch glance and memory of the reader and be long enough to give the essential information of what the paper is about. Writing about the results of the experiment is no easier than the research itself. As surgery, writing a scientific paper is also an improvisation, but general principles ...
In general the experimental section should give all the information necessary for someone to actually repeat your experiments successfully. The Experimental Section is always written in the third person, past tense. Under the Experimental Section, each experiment is described in a separate paragraph that has a brief, descriptive title in bold.