47 case interview examples (from McKinsey, BCG, Bain, etc.)

One of the best ways to prepare for case interviews at firms like McKinsey, BCG, or Bain, is by studying case interview examples.
There are a lot of free sample cases out there, but it's really hard to know where to start. So in this article, we have listed all the best free case examples available, in one place.
The below list of resources includes interactive case interview samples provided by consulting firms, video case interview demonstrations, case books, and materials developed by the team here at IGotAnOffer. Let's continue to the list.
- McKinsey examples
- BCG examples
- Bain examples
- Deloitte examples
- Other firms' examples
- Case books from consulting clubs
- Case interview preparation

Click here to practise 1-on-1 with MBB ex-interviewers
1. mckinsey case interview examples.
- Beautify case interview (McKinsey website)
- Diconsa case interview (McKinsey website)
- Electro-light case interview (McKinsey website)
- GlobaPharm case interview (McKinsey website)
- National Education case interview (McKinsey website)
- Talbot Trucks case interview (McKinsey website)
- Shops Corporation case interview (McKinsey website)
- Conservation Forever case interview (McKinsey website)
- McKinsey case interview guide (by IGotAnOffer)
- McKinsey live case interview extract (by IGotAnOffer) - See below
2. BCG case interview examples
- Foods Inc and GenCo case samples (BCG website)
- Chateau Boomerang written case interview (BCG website)
- BCG case interview guide (by IGotAnOffer)
- Written cases guide (by IGotAnOffer)
- BCG live case interview with notes (by IGotAnOffer)
- BCG mock case interview with ex-BCG associate director - Public sector case (by IGotAnOffer)
- BCG mock case interview: Revenue problem case (by IGotAnOffer) - See below
3. Bain case interview examples
- CoffeeCo practice case (Bain website)
- FashionCo practice case (Bain website)
- Associate Consultant mock interview video (Bain website)
- Consultant mock interview video (Bain website)
- Written case interview tips (Bain website)
- Bain case interview guide (by IGotAnOffer)
- Bain case mock interview with ex-Bain manager (below)
4. Deloitte case interview examples
- Engagement Strategy practice case (Deloitte website)
- Recreation Unlimited practice case (Deloitte website)
- Strategic Vision practice case (Deloitte website)
- Retail Strategy practice case (Deloitte website)
- Finance Strategy practice case (Deloitte website)
- Talent Management practice case (Deloitte website)
- Enterprise Resource Management practice case (Deloitte website)
- Footloose written case (by Deloitte)
- Deloitte case interview guide (by IGotAnOffer)
5. Accenture case interview examples
- Case interview workbook (by Accenture)
- Accenture case interview guide (by IGotAnOffer)
6. OC&C case interview examples
- Leisure Club case example (by OC&C)
- Imported Spirits case example (by OC&C)
7. Oliver Wyman case interview examples
- Wumbleworld case sample (Oliver Wyman website)
- Aqualine case sample (Oliver Wyman website)
- Oliver Wyman case interview guide (by IGotAnOffer)
8. A.T. Kearney case interview examples
- Promotion planning case question (A.T. Kearney website)
- Consulting case book and examples (by A.T. Kearney)
- AT Kearney case interview guide (by IGotAnOffer)
9. Strategy& / PWC case interview examples
- Presentation overview with sample questions (by Strategy& / PWC)
- Strategy& / PWC case interview guide (by IGotAnOffer)
10. L.E.K. Consulting case interview examples
- Case interview example video walkthrough (L.E.K. website)
- Market sizing case example video walkthrough (L.E.K. website)
11. Roland Berger case interview examples
- Transit oriented development case webinar part 1 (Roland Berger website)
- Transit oriented development case webinar part 2 (Roland Berger website)
- 3D printed hip implants case webinar part 1 (Roland Berger website)
- 3D printed hip implants case webinar part 2 (Roland Berger website)
- Roland Berger case interview guide (by IGotAnOffer)
12. Capital One case interview examples
- Case interview example video walkthrough (Capital One website)
- Capital One case interview guide (by IGotAnOffer)
13. Consulting clubs case interview examples
- Berkeley case book (2006)
- Columbia case book (2006)
- Darden case book (2012)
- Darden case book (2018)
- Duke case book (2010)
- Duke case book (2014)
- ESADE case book (2011)
- Goizueta case book (2006)
- Illinois case book (2015)
- LBS case book (2006)
- MIT case book (2001)
- Notre Dame case book (2017)
- Ross case book (2010)
- Wharton case book (2010)
Practice with experts
Using case interview examples is a key part of your interview preparation, but it isn’t enough.
At some point you’ll want to practise with friends or family who can give some useful feedback. However, if you really want the best possible preparation for your case interview, you'll also want to work with ex-consultants who have experience running interviews at McKinsey, Bain, BCG, etc.
If you know anyone who fits that description, fantastic! But for most of us, it's tough to find the right connections to make this happen. And it might also be difficult to practice multiple hours with that person unless you know them really well.
Here's the good news. We've already made the connections for you. We’ve created a coaching service where you can do mock case interviews 1-on-1 with ex-interviewers from MBB firms . Start scheduling sessions today!
The IGotAnOffer team
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Microbiol
BCG as a Case Study for Precision Vaccine Development: Lessons From Vaccine Heterogeneity, Trained Immunity, and Immune Ontogeny
Asimenia angelidou.
1 Division of Newborn Medicine, Boston Children’s Hospital and Beth Israel Deaconess Medical Center, Boston, MA, United States
2 Precision Vaccines Program, Boston Children’s Hospital, Boston, MA, United States
3 Harvard Medical School, Boston, MA, United States
Joann Diray-Arce
4 Division of Infectious Diseases, Boston Children’s Hospital, Boston, MA, United States
Maria Giulia Conti
5 Department of Maternal and Child Health, Sapienza University of Rome, Rome, Italy
Kinga K. Smolen
Simon daniël van haren, david j. dowling, robert n. husson.
Vaccines have been traditionally developed with the presumption that they exert identical immunogenicity regardless of target population and that they provide protection solely against their target pathogen. However, it is increasingly appreciated that vaccines can have off-target effects and that vaccine immunogenicity can vary substantially with demographic factors such as age and sex. Bacille Calmette-Guérin (BCG), the live attenuated Mycobacterium bovis vaccine against tuberculosis (TB), represents a key example of these concepts. BCG vaccines are manufactured under different conditions across the globe generating divergent formulations. Epidemiologic studies have linked early life immunization with certain BCG formulations to an unanticipated reduction (∼50%) in all-cause mortality, especially in low birthweight males, greatly exceeding that attributable to TB prevention. This mortality benefit has been related to prevention of sepsis and respiratory infections suggesting that BCG induces “heterologous” protection against unrelated pathogens. Proposed mechanisms for heterologous protection include vaccine-induced immunometabolic shifts, epigenetic reprogramming of innate cell populations, and modulation of hematopoietic stem cell progenitors resulting in altered responses to subsequent stimuli, a phenomenon termed “trained immunity.” In addition to genetic differences, licensed BCG formulations differ markedly in content of viable mycobacteria key for innate immune activation, potentially contributing to differences in the ability of these diverse formulations to induce TB-specific and heterologous protection. BCG immunomodulatory properties have also sparked interest in its potential use to prevent or alleviate autoimmune and inflammatory diseases, including type 1 diabetes mellitus and multiple sclerosis. BCG can also serve as a model: nanoparticle vaccine formulations incorporating Toll-like receptor 8 agonists can mimic some of BCG’s innate immune activation, suggesting that aspects of BCG’s effects can be induced with non-replicating stimuli. Overall, BCG represents a paradigm for precision vaccinology, lessons from which will help inform next generation vaccines.
The BCG Vaccine
BCG, the live attenuated vaccine against tuberculosis (TB), is one of the world’s most widely used vaccines ( Andersen and Doherty, 2005 ; Aaby et al., 2010 ) and continues to be the only vaccine used to prevent TB. It contains an attenuated strain of the bovine tubercle bacillus Mycobacterium bovis and was first introduced in humans in 1921. BCG is used to induce immunity against TB and is part of the World Health Organization’s (WHO’s) Expanded Program on Immunization (EPI) with more than 100 million children vaccinated with BCG every year ( World Health and Organization, 2004 ). Universal vaccination at birth with a single dose of BCG is recommended in developing countries where TB is highly endemic or where there is high risk of exposure to TB. Because of the declining incidence of TB in Europe and the United States, BCG immunization is mostly recommended for high-risk groups in these regions. A database of global BCG vaccination policies and practices can be found online 1 ( Zwerling et al., 2011 ).
BCG has an excellent and long-standing record of safety ( Saroha et al., 2015 ) and tolerability with the most common adverse effect being regional suppurative lymphadenitis, which is a rare occurrence. The most serious complication of BCG vaccination is disseminated BCG infection (rate of 0.06–1.56 cases per million doses of vaccine administered), occurring primarily in immunocompromised individuals, including neonates with undiagnosed primary immunodeficiency ( Marciano et al., 2014 ). Possible factors affecting the rate of adverse reactions include the BCG dose, vaccine strain, and method of vaccine administration ( Lotte et al., 1984 ).
TB-Specific Protection Conferred by BCG Vaccine
Multiple aspects of BCG remain incompletely characterized, including its overall efficacy, duration of protective immunity, and how age at vaccination affects protection. The variability of BCG protective efficacy has been systematically studied ( Mangtani et al., 2014 ). In children, BCG confers 58% protection against progression of TB infection to disease ( Roy et al., 2014 ) and ∼80% protection against severe or disseminated forms of TB, such as meningitis and miliary disease ( Rodrigues et al., 1993 ; Trunz et al., 2006 ). Decreasing BCG coverage in European countries was followed by an increased incidence of TB ( Romanus et al., 1992 ; Kelly et al., 1997 ) and other mycobacterial diseases ( Romanus et al., 1995 ; Dowling et al., 2017 ). In adults, BCG reduces the risk of pulmonary TB by ∼50% but has variable efficacy in different populations ( Colditz et al., 1994 ; Brewer, 2000 ). In summary, across many studies BCG efficacy is variable, with some studies showing minimal benefit, while in others it appears to provide limited protection against infection and progression to TB disease. BCG vaccination has no sizeable impact on TB transmission dynamics as its effectiveness has been mainly demonstrated in childhood, when TB is rarely contagious ( Loeffler, 2003 ).
BCG is considered a ‘self-adjuvanted’ vaccine, as components of the formulation capable of engaging multiple Pattern Recognition Receptors (PRRs), including Toll-like receptor (TLR)2 and TLR4 ( Heldwein et al., 2003 ), TLR8 ( Dowling et al., 2017 ), as well as the C-type lectin receptors Dectin-1 and Mincle ( Yadav and Schorey, 2006 ; Matsunaga and Moody, 2009 ; Schoenen et al., 2010 ) are thought to enhance vaccine-induced immunity. Unlike hepatitis B vaccine which requires multiple doses to achieve lymphoproliferation, BCG induces single shot lymphoproliferation ( Sanchez-Schmitz et al., 2018 ). Most recently, in an Indian adult human cohort, a hypermorphic gain of function single nucleotide polymorphism in TLR8, a PRR that is activated by microbial single stranded RNA, was associated with improved BCG vaccine-mediated protection against pulmonary TB ( Ugolini et al., 2018 ).
BCG-induced protection against TB is, at least in part, attributed to a T-helper (Th)1 response. BCG elicits a Th1 cell response in adults, and overcomes the Th2 immune bias present in infants, by inducing adult-like IFNγ responses ( Marchant et al., 1999 ). IFNγ production to many stimuli is muted in newborn T cells, however IFNγ can be produced in vitro by neonatal NK cells in response to live microbial stimuli such as BCG after priming with recombinant IFNγ, at least for certain geographic populations ( van den Biggelaar et al., 2009 ). In BCG-vaccinated infants, unconventional gamma-delta (γδ) T cells are also increasingly recognized as a source of IFNγ production ( Zufferey et al., 2013 ), in addition to their bridging role between innate and adaptive immunity against TB infection ( Meraviglia et al., 2011 ). Although protective immunity against TB requires IFNγ responses, a direct association between the concentrations of vaccine-induced IFNγ responses and degree of immune protection has not been seen ( Hoft et al., 2002 ). Further, recent evidence suggests that IFNγ-independent immune responses, including generation of highly avid antibodies and CD40L+/CD154+ T cells, are associated with absence of TB disease in highly exposed contacts of persons with highly infectious TB, though the role of these responses in protection is not clear ( Lu et al., 2019 ). After boost vaccination with a candidate TB vaccine, MVA85A, BCG-induced protection against TB was not enhanced in infants despite more durable T cell responses ( Tameris et al., 2013 ). However, weak immunogenicity was also noted in this trial. Furthermore, dysregulated or excessive CD4 + T cell activation can enhance host susceptibility to Mycobacterium tuberculosis (Mtb) infection; as such, effector T cell responses must be tightly regulated for host survival to TB ( Tzelepis et al., 2018 ). Although anti-BCG T cell-mediated immunity alone is not adequate to confer protection from TB infection and disease, it can serve as an immune correlate of TB infection and disease risk ( Kagina et al., 2010 ; Fletcher et al., 2016 ). Parameters such as presence and size of BCG scar and delayed-type hypersensitivity do not predict protective efficacy in humans ( Ota et al., 2006 ; Kagina et al., 2010 ; Fletcher et al., 2016 ).
Mycobacterium bovis BCG infection induces macrophage production of GM-CSF that may contribute to the host response against mycobacterial infection by favoring macrophage M1 polarization ( Benmerzoug et al., 2018 ). GM-CSF and IFNγ may have an additive effect in promoting macrophage control of intracellular bacterial replication ( Rothchild et al., 2017 ). GM-CSF is produced by a variety of cells, including macrophages and parenchyma cells. It stimulates differentiation of myeloid progenitors into macrophages and neutrophils, regulates hematopoietic cell proliferation and differentiation, and modulates the function of mature hematopoietic cells ( Martinez and Gordon, 2014 ). Clinical observations linking the presence of anti-GM-CSF autoantibodies with susceptibility to cryptococcal meningitis and pulmonary TB support an important role for GM-CSF for host defense against infection ( Rosen et al., 2013 ), and Mtb infection in particular. In addition to activating macrophages to limit the intracellular growth of Mtb in vitro ( Denis and Ghadirian, 1990 ), proposed antimicrobial mechanisms of GM-CSF include preserving the integrity of alveolar epithelial cells, regulating cellular lipid metabolism in alveolar macrophages ( Rothchild et al., 2017 ) and facilitating containment of virulent mycobacteria in pulmonary granulomas ( Szeliga et al., 2008 ). Interestingly, GM-CSF along with IL-3 priming of CD14+ human monocytes enhanced TNF production and monocyte renewal (as evaluated by the degree of cell confluency and increased cell number by fluorescence and time-lapse microscopy) upon subsequent LPS stimulation, indicating a potential mechanism of trained immunity ( Borriello et al., 2016 ). As detailed in the following sections, trained immunity refers to the ability of innate immune cells to mount an enhanced subsequent response to diverse microbes, a phenomenon whose underlying mechanisms are under intense investigation.
IL-17 is associated with a protective role against infection with clinically virulent Mtb isolates ( Gopal et al., 2014 ) and enhanced protection in mouse models ( Aguilo et al., 2016 ). However, BCG delivered systemically is not a strong inducer of Th17, one potential explanation being that BCG strains lack the region of difference 1 (RD1) region ( Dockrell and Smith, 2017 ), resulting in loss of the protein secretion system ESAT-6 that governs phagosomal rupture and host cell lysis. In fact, when complemented with the ESAT-6 containing RD1 region, BCG shows improved protective efficacy and enhanced Th17 responses in mice ( Chatterjee et al., 2011 ). More recently, local pulmonary BCG administration via endobroncheal instillation in a rhesus macaque Mtb challenge model induced mucosal protective immunity mediated by Th17 polyfunctional cells and IgA production ( Dijkman et al., 2019 ).
In contrast to cell-mediated immunity, the human humoral response against Mtb has been conventionally thought to exert little immune control over the course of Mtb infection or in response to BCG vaccination ( Jacobs et al., 2016 ), due to the paradigm that humoral immunity plays little role in the protection against intracellular pathogens. However, the contribution of BCG vaccination specific Abs to specific and non-specific protection is a revived area of interest (see Box 1 ).
Box 1. Vaccine-induced antibody-mediated immunity against mycobacteria.
The ‘central dogma’ of anti-mycobacterial immunity outlines that T cell production of IFNγ activates macrophages to kill intracellular Mtb. Accordingly, measurement of IFNγ produced by T cells is the most widely used method for detecting immune responses following infection or vaccination with BCG ( Nunes-Alves et al., 2014 ). Current strategies to develop next generation BCG vaccines are generally focused on the enhancement of IFNγ production by CD4+ T cells (i.e., Th1 cell-mediated immunity) ( Achkar and Casadevall, 2013 ). Recent attention has been focused toward understanding the role, if any, of vaccine-induced antibodies (Abs) to prevent infection ( Izzo, 2017 ). Various reported mechanisms of Ab-mediated protection against Mtb include direct antimycobacterial activity, opsonization, activation of complement, clearance of immunomodulatory mycobacterial antigens, increase of macrophage Ca2+ signaling, release of oxidants enhancing intracellular killing and other mechanisms of enhancing cell-mediated immunity ( Achkar et al., 2014 ). How and whether BCG vaccination specific Abs may contribute to protective mechanisms remains unclear ( Lu et al., 2016 ). Indeed, maternal infection with Mtb, and subsequently maternal Abs, do not seem to play a role in protecting neonates and young infants against mycobacterial infection, although maternal Abs inhibited purified protein derivative (PPD)-specific T cell responses in BCG vaccinated infants ( Mawa et al., 2015 ). However, recent studies in mice ( Ai et al., 2013 ; Alvarez et al., 2013 ) and humans ( Zimmermann et al., 2016 ) have indicated a potential role for IgA Abs. Currently, the most compelling evidence for human IgG Ab-mediated immunity against mycobacteria may come from studies investigating IFN-independent markers of mycobacterial exposure. When compared to subjects with classic latent Mtb infection, Mtb ‘resisters’ display enhanced Ab avidity and distinct Mtb-specific IgM and IgG Fc profiles ( Lu et al., 2019 ). BCG may also enhance Ab responses and, in some cases, T cell responses to other early life vaccines, such as hepatitis B, pertussis, and pneumococcal vaccines ( Ota et al., 2002 ; Ritz et al., 2013 ; Scheid et al., 2018 ). Overall, understanding formulation-specific BCG-induced responses may necessitate complimentary investigation of functional Ab responses to vaccination, which may prove to be as important as inducing T cell production of IFNγ and/or heterologous responses. Such studies will shed fresh light on the mechanisms of BCG-induced protection and may inform development of next generation TB vaccines.
Route of BCG Administration
The route of BCG administration can affect immune responses. Intradermal injection is the most common method of BCG vaccination and the route currently recommended by the WHO. Percutaneous administration is the only route licensed for use of BCG (Tice strain) as a TB vaccine in the United States. Given the more unpredictable nature of percutaneous administration, percutaneously administered formulations are manufactured to contain more colony forming units (CFU) compared to those meant for intradermal administration. A human adult randomized trial comparing the two methods showed that percutaneous BCG Tice vaccination was associated with lower reactogenicity, immunogenicity (as measured by lymphoproliferative responses) and delayed hypersensitivity responses (assessed using the mean size of PPD response) compared to intradermal vaccination ( Kemp et al., 1996 ). Of note, the CFU dose for intradermal use of BCG Tice was adjusted in this study by diluting BCG, to match the WHO’s standard recommended dose. A later study comparing intradermal vs. percutaneous BCG Japan administration found significantly greater Thl cytokine and lymphoproliferative responses with percutaneous BCG ( Davids et al., 2006 ). This study involved an infant cohort and CFUs were not adjusted for route of administration. Divergent results across studies may be related to the strain used (Tice vs. Japan) and also raise concerns about the administration routes of the vaccine, both of which have their challenges: percutaneous administration results in variable delivery of CFU subject to skin penetration, while intradermal delivery requires training and skill for optimal execution. Interestingly, a randomized trial in South African infants vaccinated at birth with intradermal vs. percutaneous BCG Japan found an equivalent incidence of TB over 2 years, questioning the relevance of administration route to clinical efficacy, though heterologous effects were not specifically assessed in this study ( Hawkridge et al., 2008 ). A recent study in non-human primates demonstrated that intravenous administration of BCG provided 90% protection against TB as compared to the conventional intradermal route. Further studies of route of BCG administration are needed to inform optimal administration to humans ( Darrah et al., 2020 ).
Overview of Different BCG Vaccine Strains
The evolution of bcg strains.
BCG is not a single vaccine, but rather a family of historically evolving and divergent vaccine formulations, further complicating the crucial task of defining mechanisms of action for these vaccines and their correlates of protection. The basis of BCG attenuation was the deletion of the genomic region RD1, which is absent from all M. bovis BCG strains, resulting in loss of the protein secretion system ESAT-6 that governs phagosomal rupture and host cell lysis ( Mahairas et al., 1996 ; Brosch et al., 2007 ). Since its introduction in 1921 ( Calmette, 1931 ), BCG seed lots were distributed globally for vaccine production at multiple sites. Based on historical records and phylogeny derived through molecular typing, a genealogy of BCG strains has been established, demonstrating the temporal relationship of their production and their dichotomy into “early” strains (e.g., Japan, Russia, Moreau, Sweden) and “late” strains (e.g., Pasteur, Tice, Denmark, Glaxo) ( Behr and Small, 1999 ; Brosch et al., 2007 ; Abdallah et al., 2015 ). Before freeze-dried seed lots were derived from a single spreading colony in the 1960s, BCG strains were sub-cultured in different laboratories, yielding minority subpopulations that can impact virulence ( Kroger et al., 1994 ), immunogenicity ( Davids et al., 2006 ; Aguirre-Blanco et al., 2007 ), viability ( Gheorghiu and Lagrange, 1983 ), colony size/counts and heterologous effects ( Shann, 2015 ). BCG has continued to change with in vitro passage, resulting in further genetic diversity among strains ( Figure 1 ). Comparative genome and transcriptome analysis of representative early and late BCG daughter strains, such as BCG Japan and BCG Pasteur respectively, has shown amplification of polymorphisms such as the tandem duplication DU2 in the later strains with implications for the expression level of known surface proteins and immunodominant prominent antigens ( Brosch et al., 2007 ). The potential influence of these differences on the protective efficacy, immunogenicity, safety and heterologous effects of BCG immunization has generated considerable challenges for international TB immunization initiatives and highlights the importance of future studies comparing the different licensed BCG formulations ( Wu et al., 2007 ; Ritz et al., 2008 , 2012 ; Hayashi et al., 2009 ; Biering-Sorensen et al., 2015 ; Shann, 2015 ).

Licensed BCG formulations are derived from a parent strain developed in Paris, France. Multiple sub-strains have been generated using diverse culture methods, classified by genomic sequencing, resulting in a genealogy/timeline of BCG vaccine strains. Such BCG sub-strains differ in colony morphology, growth characteristics, biochemistry, immunogenicity, and virulence. The French (Pasteur) strain 1173 P2, Denmark (Statens Serum Institute) strain 1331, Glaxo strain 1077, Japan/Tokyo strain 172-1, Russian strain BCG-I, and Moreau RDJ, account for >90% of the BCG vaccines in use worldwide. The scheme depicts the distribution of vaccine formulations into four main groups (circles) based on their tandem duplication 2 (DU2) variant, which distinguishes the early (DU group I) from the late (DU group II-IV) vaccines. The lines indicate the chronology of derivation for each group. Modified from Brosch et al. (2007) .
Challenges in BCG Propagation in vitro
BCG strains supplied for clinical use vary depending on the original seed strain. Different culture or manufacturing conditions likely result in different genotypes within the same strain ( Behr and Small, 1999 ) as well as epigenetic changes, even within a single genotype ( Biering-Sorensen et al., 2015 ). Further variation may have occurred over time after a lab acquired the source and before freeze-drying, resulting in batch effects ( Biering-Sorensen et al., 2015 ). Issues of batch or vaccine strain variability have proven very challenging to study at scale, as the EPI program has historically employed different vaccine strains as well as different batches of the same vaccine strain within the same region. Due to strain divergence and subsequent evolution, it has been difficult to assess the various bacterial strains using a single, consistent approach.
Over 14 different licensed BCG vaccine formulations comprised of distinct daughter strains of attenuated M. bovis are used globally with UNICEF being the largest supplier ( UNICEF, 2015 ). Most countries import BCG from one of the international WHO prequalified manufacturers, while a few produce their own. However, there is no standardized culture methodology or one single culture medium recommended for the culture of BCG. This was demonstrated during the international collaborative study to evaluate and establish WHO reference reagents for BCG vaccine, where each of the 11 participating labs used their preferred culture medium to evaluate BCG candidate vaccines by culturable viable counts (e.g., Löwenstein-Jensen, Middlebrook 7H11 or 7H10, Ogawa, and Dubos) ( Markey et al., 2009 ). Results between labs were highly variable, though reportedly within expected ranges, and may be partially attributable to challenges in standardizing colony counting due to variable colony sizes and the clump-forming nature of M. bovis .
Minor differences in production techniques can have profound effects on BCG growth ( Shann, 2015 ). For example, inconsistent production methods may result in both type-by-type (e.g., BCG Denmark vs. BCG Russia) and lot-to-lot variability that can affect clinical efficacy. Growth and phenotypes of M. bovis BCG can be significantly influenced by the choice of media and the duration of culture incubation. For example, shorter time to detection of colonies was observed for M. bovis isolated from bovine tissues grown on 7H11 versus egg-based media ( Corner et al., 2012 ). A study compared the immunogenicity of BCG vaccine grown in 7H9 medium, the most commonly used medium in laboratory studies, against that grown in Sauton medium, which is used for growing BCG by some manufacturers. This study showed clear differences in the efficacy of BCG grown in these different culture media, including variation in persistence within macrophages in vitro , apoptosis of infected cells, as well as cellular and humoral immune responses in mice in vivo ( Venkataswamy et al., 2012 ). However, this study was largely limited to the BCG Pasteur strain, which might have behaved differently than other formulations, and did not examine specific BCG growth characteristics across culture media. Variable components between commercially available Oleic Albumin Dextrose Catalase (OADC) enrichment supplements can stimulate or inhibit the growth of mycobacteria and influence performance of Middlebrook 7H11 medium ( Butler et al., 1990 ). Discrepancies in culture growth may alternatively indicate differences in viability after lyophilization or reconstitution. Slower growth has been associated with inocula that contain fewer viable bacilli ( Corner et al., 2012 ). The number of live bacilli in the vaccine product decreases with time ( Messina et al., 2018 ), as does survival after freeze-drying. Lastly, divergence in growth between BCG formulations may indicate unique nutrient needs, as BCG strains vary in their ability to catabolize amino acids, which act as the nitrogen source for BCG growth ( Chen et al., 2003 ).
The presence and selection of minority populations within strains has been demonstrated by serial subculture under experimental conditions ( Osborn, 1983 ) and is partially attributed to maintenance procedures of BCG lines. Specifically, BCG Tokyo 172 (the mother strain of BCG Japan, derived from the Pasteur strain in 1925), and Denmark 1331 (derived from the Pasteur strain in 1931), have a minority population of non-spreading colonies, as did BCG Pasteur before the seed lot system was introduced in 1961 ( Osborn, 1983 ). Non-spreading colonies are characterized by opacity and lack of orientation. In contrast, in spreading colonies organisms have the tendency to adhere to one another in the direction of their long axis, and appear as a dense and opaque center surrounded by a halo, which consists of serpentine strands folded close together. BCG Japan substrains differ in cell wall lipid composition and antigenicity ( Naka et al., 2011 ), with phenolic glycolipid and phthiocerol dimycocerosate found only in the substrains forming smooth colonies but not in those forming rough colonies. BCG Russia, the first documented daughter strain distributed by Institut Pasteur to Russia in 1924, is a natural recA mutant, preventing its genomic evolution ( Keller et al., 2008 ) with unclear effects on BCG culture growth and the vaccine’s protective efficacy. BCG Russia is associated with lower effectiveness against tuberculosis, and lower frequency of BCG scars than BCG Denmark and BCG Japan. Of note, the genome of the BCG Russia strain features variably sized deletions of the polyketide synthase 12 ( pks12) gene, necessary for β-phosphomycoketide production and the CD1c-mediated T cell response ( Abdallah et al., 2015 ), potentially directly affecting immunogenicity of the daughter strains ( Matsunaga et al., 2004 ).
Different BCG Formulations Can Induce Distinct but Broad Ranges of Immunologic Responses in Humans
It is not currently known which BCG strain/formulation offers the best protection from TB disease, as immune correlates of protection are lacking. This limits inferences from in vitro studies. However, in vitro immunological and microbiological studies could provide critical insight in divergence of essential properties of the different strains, such as viability and host immune activating potential ( Angelidou et al., 2020 ). Even in the absence of a defined correlate of protection these outcomes are probably critical to protection against TB.
Human data on cytokine induction after BCG administration are inconclusive as existing studies have low numbers of study participants, heterogeneous study designs, and variable formulations are tested with incomplete information on which formulation was used. As outlined in Table 1 , comparative studies were largely incomplete in terms of comparing all available strains or formulations. Even though some patterns emerge such as BCG Denmark and BCG Japan perhaps being more immunogenic, generalizability of conclusions is difficult due to heterogeneous study designs, variable formulations, study populations, assays performed and endpoints studied. In a Mexican neonatal cohort vaccinated with BCG Denmark, Brazil (derived from BCG Moreau) or Japan, Mtb-specific recall immune responses after 1 year were examined ( Wu et al., 2007 ). Upon activation of peripheral blood mononuclear cells (PBMCs) with Mtb proteins, BCG Denmark- or Brazil-immunized newborns demonstrated mRNA expression of cytokines important to adaptive immunity (IL-12, IL-27, IFNγ), while BCG Japan preferentially induced cytokines associated with acute inflammatory responses (IL-1α/β, IL-6, IL-24) ( Wu et al., 2007 ). A randomized controlled trial in Australia showed that BCG Denmark and BCG Japan given at birth induced higher proportions of mycobacterial-specific polyfunctional [IFNγ(+)TNF(+)IL-2(+)] CD4 T cells than BCG Russia ( Ritz et al., 2012 ). The impact of different BCG strains on the ontogeny of vaccine-specific and heterologous vaccine immunogenicity in the first 9 months of life was also examined in two African birth cohorts ( Kiravu et al., 2019 ), where BCG Denmark vaccinated infants mounted significantly higher frequencies of polyfunctional CD4+ T cells, compared with infants vaccinated with BCG Bulgaria and BCG Russia. BCG-naïve adult volunteers immunized with BCG Denmark showed divergent whole blood pro-inflammatory and regulatory T cell responses, with significant induction of polyfunctional [IFNγ(+)TNF(+)IL-2(+)] CD4 T cells and IFNγ production confined to individuals with strong local skin inflammation, compared to regulatory-like CD8 T cell induction in individuals with mild skin inflammation ( Boer et al., 2015 ). Polyfunctional CD4 cells have been associated with enhanced Th1 cytokine production and implicated as memory cells responsible for antigen-specific long-term protection ( Darrah et al., 2007 ). However, whether their presence correlates with protective immunity remains highly controversial. In the limited studies done, there is no direct evidence that genetic variation of the vaccine strains accounts for the variability in efficacy and/or protection against TB based on the year a particular vaccine strain was given ( Mangtani et al., 2014 ). Overall, these observations indicate that further clinical studies directly comparing different licensed BCG formulations/strains currently in use are needed to address these questions.
Summary of human infant studies of BCG-induced innate, heterologous and mycobacteria-specific immunity.
BCG-Induced Heterologous Effects
Human newborns are highly susceptible to infection due to functionally distinct innate ( Kollmann et al., 2012 ) and adaptive immune responses ( Kollmann et al., 2017 ) compared to other age groups. Epidemiologic studies have linked early life BCG immunization to an unanticipated reduction (∼50%) in all-cause mortality, which greatly exceeds a reduction in mortality attributable to TB ( Higgins et al., 2014 ; Jensen et al., 2015 ). These observations suggest BCG induces heterologous protection against antigenically diverse, unrelated pathogens. One of the suggested mechanisms for heterologous protection against infection in the context of BCG vaccination is innate immune memory, also known as “trained immunity” ( Netea et al., 2011 ).
The Concept of Trained Immunity
Trained immunity is the ability of innate immune cells to mount an altered response against infection following a previous unrelated infection or vaccination ( Figure 2 ). Innate immune memory is well described in plant immunology and invertebrates which lack adaptive immune response mechanisms ( Kurtz, 2005 ). In contrast to adaptive memory mediated by B- and T-cells, innate memory primarily involves mononuclear phagocytes. Mammalian studies suggest that the innate host defense of vertebrates possesses similar properties. Vaccination of mice with BCG protects against secondary infections with Candida albicans or Schistosoma mansoni through activation of tissue macrophages ( van ’t Wout et al., 1992 ). Injection of attenuated strains of Candida in athymic mice induced protection toward virulent Candida strains but also toward Staphylococcus aureus , through macrophage activation and proinflammatory cytokine production ( Bistoni et al., 1986 ). Human innate immunity also exhibits immunological memory mediated by epigenetic and metabolic reprogramming of innate immune cells and their bone marrow precursors ( Netea et al., 2019 ).

Influence of “trained” immunity on the magnitude of immune responses later in life. Certain forms and combinations of early life immune-stimulation, including BCG, can induce epigenetic changes in innate immune cells that can enhance or inhibit innate immune responses following future exposure to diverse antigenically unrelated pathogens ( Netea and van der Meer, 2017 ).
Examples of Trained Immunity in Human Cohorts and Mechanistic Insights
Several observations in human epidemiologic studies support the notion that trained immunity occurs in the human neonate. One observation that supports a role for trained immunity in early life is the association of bloodstream infections in critically ill preterm newborns with enhanced pathogen-specific mononuclear cell PRR expression in the setting of subsequent Gram-positive or Gram-negative bacteremia ( Zhang et al., 2010 ). This finding suggests that the neonatal innate immune system can remember previous activation such that responses to subsequent microbial challenges are altered. Similarly, histologic chorioamnionitis affecting preterm infants is associated with a significantly reduced risk of late onset sepsis, both with coagulase-negative Staphylococcus (most common) and other bacteria ( Strunk et al., 2012 ), implying that perinatal inflammation may enhance functional maturation of the preterm immune system.
Immunization of human newborns may also trigger trained immunity. In observational studies in Guinea-Bissau, BCG vaccine had beneficial effects on overall mortality compared to no/delayed BCG vaccination ( Kristensen et al., 2000 ), especially during the first 2 months of life (unadjusted MRR 0.74, adjusted MRR 0.55). Near halving of neonatal mortality in low-birth weight children vaccinated with BCG at birth was replicated in two subsequent randomized-controlled trials ( Aaby et al., 2011 ; Biering-Sorensen et al., 2012 ). The reduction in neonatal mortality was associated with fewer cases of neonatal sepsis, respiratory infections and fever ( Aaby et al., 2011 ). In another randomized-controlled trial between 2008 and 2013 including 2,320 low birth weight children, BCG given early (at birth) vs. late (>2.5 kg or when infant was 2 months old per the established practice) conferred a rapid survival benefit as early as 1 month of age (MRR 0.55), which was sustained up to 1 year of age (MRR 0.83) ( Jensen et al., 2015 ). In the same trial, early BCG immunization led to increased production of Th1 polarizing and monocyte-derived pro-inflammatory cytokines, particularly IL-1β, IL-6, TNF and IFNγ, upon heterologous challenge of the infants’ whole blood in vitro with TLR-2, -4 or -7/8 agonists, or PPD, demonstrating a potentiating effect on innate cytokine responses ( Jensen et al., 2015 ).
In addition to reduced mortality, heterologous beneficial BCG effects include decreases in infectious morbidities. Case control studies in Guinea-Bissau suggest that BCG vaccination and the presence of a scar among BCG-immunized infants was associated with a reduced risk of acute lower respiratory infection (ALRI) compared to unimmunized controls, with the association being stronger for females ( Stensballe et al., 2005 ). In fact, children with ALRI were ∼3-fold more likely to have not received BCG vaccine compared to children without ALRI. Similar results were found in an exploratory analysis of national health survey data from 33 low- and middle-income countries between 2000 and 2010, where 0–5 year-old BCG vaccinated children had 17–37% lower risk of suspected ALRI compared to unvaccinated controls ( Hollm-Delgado et al., 2014 ). A retrospective epidemiologic study in Spain used data from the Official Spanish Registry of Hospitalizations to identify differences in hospitalization rates in BCG-vaccinated children (Basque Country, where universal neonatal BCG vaccination is practiced) as compared to non-BCG-vaccinated children (rest of Spain, where BCG is not routinely used) ( de Castro et al., 2015 ). Analysis of 464,611 hospitalization episodes over a 15-year period showed that neonatal BCG immunization was associated with fewer hospitalizations for respiratory infections (the preventive fraction, defined as the attributable proportion of disease cases prevented by BCG exposure, was 40% and statistically significant among all age groups) and sepsis (preventive fraction 36%, statistically significant among the infant group) ( de Castro et al., 2015 ). Differences diluted with age suggesting a time-limited protective effect of BCG vaccination vs. lower rates of hospitalization for respiratory infection in older children. No significant differences in the already low mortality rates were observed.
BCG scarring has been correlated with heterologous protective effects. A recent prospective study in rural Guinea-Bissau showed that children vaccinated with the BCG Moscow strain (also known as BCG Russia) who developed a scar had 26% lower mortality compared to children who did not develop a scar, mainly attributable to prevention of deaths from respiratory infections (mortality rate ratio [MRR] 0.2) ( Storgaard et al., 2015 ). This correlation of BCG scarring and improved survival has been replicated over different time periods and with different BCG strains; however, scarification rates differ by BCG formulation. For example, BCG Russia is less likely to produce a scar compared to BCG Japan and Denmark ( Frankel et al., 2016 ; Funch et al., 2018 ). BCG-induced scarring in Ugandan newborns was associated with higher IFNγ responses to heterologous stimuli (tetanus toxoid, phytohaemagglutinin) at 1 year, and differed across strains (93% with BCG Denmark vs. 64% with BCG Bulgaria vs. 52% with BCG Russia) ( Anderson et al., 2012 ). An RCT in Guinea-Bissau showed increased scarring induced by BCG Denmark and Japan compared to BCG Russia, but no significant differences in morbidity and mortality, at least by 6 weeks of age ( Schaltz-Buchholzer et al., 2019 ), possibly because BCG Russia also induced relatively high scarification rates in this cohort compared to others. Even though development of scarring also depends on additional factors such as vaccination technique, preservation of the cold chain, nutritional status of the recipient, age at time of vaccination and prior exposure to non-tuberculous mycobacteria, variable scarification rates may still predict variable heterologous protection in populations vaccinated with different BCG formulations.
In adults, BCG immunization induces specific epigenetic markers associated with the acquisition of a trained or tolerant phenotype after BCG vaccination ( Saeed et al., 2014 ). In healthy volunteers BCG induces trained immunity and heterologous protection from infections through epigenetic reprogramming of monocytes ( Kleinnijenhuis et al., 2012 ), specifically trimethylation of histone H3 at lysine 4 (H3K4me3) at the level of cytokine and TLR4 promoters. To further characterize BCG-induced innate immune regulation, adult PBMCs were cultured with BCG in vitro . Following heterologous stimulation with TLR ligands and bacteria, there was increased production of TNF, an effect mediated through the Nucleotide-Binding Oligomerization Domain Containing 2 pathway ( Kleinnijenhuis et al., 2012 ). In a randomized placebo-controlled adult study, yellow fever virus vaccine recipients who had been BCG vaccinated with the Denmark strain 1 month prior, had significantly lower yellow fever viremia compared to subjects who had received placebo vaccination ( Arts et al., 2018 ). BCG vaccination conferred protection against yellow fever experimental infection by inducing genome-wide epigenetic reprogramming of monocytes involving genes related to signal transduction molecules, epidermal growth factor receptor, fibroblast growth factor, and vascular endothelial growth factor signaling pathways, as well as genes such as AKT1, MAPKs, and PI3K-related that have been shown to be important in β-glucan-induced trained immunity, the prototypical trained immunity-inducing agonist in vitro . This effect correlated with induction of cytokine responses indicative of trained immunity: higher pro-inflammatory cytokine production (TNF, IL-1β, IL-6) from BCG-vaccinated volunteers, compared to placebo-treated individuals, with a crucial role for IL-1β production and release. These observations suggest potential mechanisms for heterologous protection that could also apply to infants, as epidemiological studies have shown that BCG vaccination results in lower all-cause mortality in infants ( Roth et al., 2006 ).
More recently, immune-gene priming long non-coding RNAs (lncRNAs), positioned at the nexus of RNA, DNA, and protein interactions, have emerged as key regulators of gene transcription in trained immunity by positioning themselves at the nexus of RNA, DNA, and protein interactions. Taking advantage of the three-dimensional nuclear architecture and the close proximity of functionally related immune genes in topologically associated domains (TADs), lncRNAs contribute to accumulation of H3K4me3 at the promoters of trained immune genes in human monocytes ( Fanucchi et al., 2019 ).
Growing evidence that innate immune engagement by BCG enhances responses to other pathogens raises the possibility that some, or conceivably even most, of its clinical benefit is due to heterologous effects. However, the extent, mechanism and ontogeny of trained immunity in early life remain incompletely defined. Understanding how BCG-induced innate immune engagement, including BCG-induced enhancement of Th-polarizing cytokine production by antigen-presenting cells, varies by BCG strain and age is of basic and translational importance ( Arts et al., 2015 ; Storgaard et al., 2015 ).
The Role of Immunometabolism in BCG-Induced Trained Immunity
Intracellular metabolism plays key roles in regulating innate immune memory. In particular, different training programs induce metabolites that function as cofactors for epigenetic enzymes, which in turn induce chromatin and DNA modifications and modulate gene transcription upon re-challenge with a second stimulus ( Netea et al., 2016 ). The Warburg Effect , first described in neoplastic cells, is a metabolic pathway important to trained immunity ( Vander Heiden et al., 2009 ). Under normoxia, in resting cells, there is a low level of glycolysis and preferential pyruvate oxidation in the mitochondrion (oxidative phosphorylation), which confers slow but very efficient ATP production. In activated and proliferating cells, there is a metabolic switch from a state of oxidative phosphorylation to a state of glycolysis, crucial for the induction of the histone modifications and functional changes underlying BCG-induced trained immunity ( Arts et al., 2016 ).
Epigenetic and metabolic reprogramming of hematopoietic progenitors may account for the long-term maintenance of trained immunity ( Mitroulis et al., 2018 ). Trained immunity affects myeloid cells as well as precursor cells of the innate immune system in the bone marrow ( Mitroulis et al., 2018 ). Administration of β-glucan in mice induced selective expansion of myeloid stem and progenitor cells accompanied by a global increase in energy metabolism in bone marrow progenitors, particularly enhancement of cholesterol biosynthesis and glycolysis. Cytokine analysis in the bone marrow extracellular fluid revealed elevated IL-1β levels, important in shaping immunometabolism within the bone marrow. In a randomized placebo-controlled human BCG immunization study with subsequent yellow fever vaccine challenge, reduction of viremia was highly correlated with the upregulation of IL-1β, a cytokine associated with the induction of trained immunity, but not with the specific IFNγ response ( Arts et al., 2018 ), supporting a key role for IL-1β as a mediator of trained immunity responses ( Moorlag et al., 2018 ). In mice, access of BCG to the bone marrow reshaped the transcriptional landscape of hematopoietic stem cells resulting in preferential myelopoiesis vs. lymphopoiesis and generation of macrophages that provided improved protection against TB ( Kaufmann et al., 2018 ).
Changes in glucose, glutamine and cholesterol metabolism enable maintenance and longevity of trained immunity via accumulation of immunologically active intermediate metabolites ( Fok et al., 2018 ). Examples include: (a) cholesterol, which participates in cell membrane remodeling and increased sensitivity to subsequent stimuli, (b) succinate and fumarate, which antagonize histone demethylation and suppress anti-inflammatory genes, (c) acetyl-CoA, an essential substrate for acetylating processes, and (d) NAD + which is important for epigenetic changes resulting in a switch from glucose to fatty acid oxidation during LPS-induced tolerance and sepsis-induced immune paralysis ( Conti et al., 2019 ). Immunometabolic changes may be different between newborns and adults, reflecting the differential nutritional and metabolic needs of the two groups, as well as their distinct immune response to pathogens ( Kan et al., 2018 ; Dreschers et al., 2019 ). Indeed the ontogeny of immunometabolism is an emerging and promising area of research ( Conti et al., 2019 ) (see Box 2 ).
Box 2. Applying systems biology to systems vaccinology.
Systems vaccinology, the application of global molecular techniques such as metabolomics, proteomics, or transcriptomics, can provide unique insights into vaccine-induced immune responses by identifying molecular signatures that may predict and give insight into vaccine-induced immunogenicity and protection ( Pulendran, 2014 ). The systems biology can provide valuable insights into host–pathogen interaction with Mtb as well as generate tools for early and proper diagnosis of TB, identification of BCG protective efficacy, and accelerated development of better TB vaccines. The metabolome, the inventory of all metabolites present in a given sample, reflecting both genetic and epigenetic influences, shifts upon immune activation and can in turn shape immune responses ( O’Neill et al., 2016 ). Metabolic phenotype influences vaccine immunogenicity and together with orthogonal datasets can identify correlates of vaccine immunity ( Li et al., 2017 ). Lipid metabolism is pivotal in the regulation of inflammatory signaling hence making lipidomics, an in-depth profiling of lipid metabolites, a valuable modality as well. Lipid metabolism regulates immune cells via cell membrane synthesis ( Lochner et al., 2015 ) and is important to epigenetic reprogramming of immune cells ( Kleinnijenhuis et al., 2014 , 2015 ). Mass-spectrometry-based metabolomics, together with computational tools, can identify and correlate metabolic pathways between samples, providing a powerful approach for clinical diagnostics ( Johnson et al., 2016 ). More studies are warranted to build the area of biomarker identification while addressing the challenges of identifying correlates of protection against TB.
BCG-Mediated Immune Modulation of Autoimmune and Inflammatory Diseases
BCG has been recognized as a potent immunomodulator for decades with extensive use for cancer and particularly bladder cancer treatment ( Rosenthal, 1988 ; Ravaud et al., 1990 ). In the past decade, there has been revived interest in BCG vaccine for potential new therapeutic uses in type 1 diabetes mellitus and treatment of other forms of autoimmunity. When administered to young NOD (autoimmune-prone) mice, BCG could not only stop new-onset diabetes but also reverse end-stage diabetes, owing to induction of suppressive regulatory T cell (Treg) expansion ( Ryu et al., 2001 ; Kodama et al., 2003 ), thereby preventing the immune system from attacking the body’s own tissue. In a clinical trial involving humans with longstanding type 1 diabetes mellitus, repeat BCG administration (2 doses) led to transient restoration of pancreatic cell islet function in vivo (for 4–6 weeks after vaccination) ( Faustman et al., 2012 ). The suspected mechanism was BCG-induced proliferation of Tregs and selective elimination/suppression of auto-reactive cytotoxic T cells, possibly via TNF induction/TNF receptor 2 agonism ( Faustman, 2018 ). Long-lasting improvements in glycemic control as evidenced by sustained decreases in hemoglobin A1c were achieved via accelerated glucose utilization induced by a systemic shift from oxidative phosphorylation to aerobic glycolysis ( Kuhtreiber et al., 2018 ).
In a double-blind, placebo-controlled trial conducted in Italy involving subjects with early symptoms consistent with multiple sclerosis (MS), participants were randomly assigned to receive BCG or placebo and monitored monthly with brain Magnetic Resonance Imaging (MRI) (6 scans) ( Ristori et al., 2014 ). By the end of the study, 58% of those vaccinated had not developed MS, compared with 30% of those who received placebo ( Ristori et al., 2014 ). Overall clinical benefits after BCG administration in new onset MS were durable and even enhanced at 5 years. In another trial, BCG vaccination was found to decrease MS disease activity and prevent progression of brain lesions in patients with relapsing-remitting MS ( Paolillo et al., 2003 ). A phase III clinical trial of BCG to reverse progression of MS is now underway.
BCG vaccination has also been associated with a reduced risk of atopic disorders as noted in a Japanese cohort ( Shirakawa et al., 1997 ), as well as in African children, where the reduction in atopy associated with BCG was greater the earlier the age at vaccination, with the largest reduction seen in children vaccinated in the first week of life ( Aaby et al., 2000 ). This observation is consistent with BCG being a powerful inducer of a Th1 phenotype in infants ( Marchant et al., 1999 ) and shifting their immune response away from the Th2-type that is typically favored in early life. Importantly, these immune polarizing effects of BCG may be yet another result of trained immunity, which may contribute to host survival in early life and affect the risks of infection, allergic and chronic inflammation later in life ( Levy and Netea, 2014 ). A randomized controlled trial to determine if BCG immunization at birth reduces allergy and infection in infants is currently underway in Australia (Melbourne Infant Study, {"type":"clinical-trial","attrs":{"text":"NCT01906853","term_id":"NCT01906853"}} NCT01906853 ).
The Role of Immune Ontogeny in Shaping BCG-Induced Tb-Specific and Heterologous Immunity
Few studies have investigated the influence of age at and timing of immunization on BCG-induced immunogenicity and protection against TB. BCG-specific effector CD4 T cell responses demonstrate increased antigen-specific CD4 T cell proliferative capacity in infants compared to older children ( Whittaker et al., 2018b ). Vaccination at birth induces a broad Th1/Th2/IL-17/Treg anti-mycobacterial response but the Th1/Th17 response is reduced when delaying the vaccine from birth to 4 1/2 months of age ( Burl et al., 2010 ). In a randomized trial of low birth weight newborns, BCG significantly increased in vitro whole blood cytokine responses to heterologous TLR agonists and to PPD in infants 4 weeks post-vaccination, particularly cytokines IL1β, IL-6, TNF, and IFNγ ( Jensen et al., 2015 ), potentially contributing to broad protection against infections. These studies illustrate that timing of BCG administration can be crucial for its immunogenicity with distinct effects depending on which outcomes are studied (mycobacterial-specific vs. heterologous). Mechanistic studies are needed to provide a basis for understanding the impact of immune ontogeny on BCG immunogenicity.
Comparable CD4 and CD8 T cell anti-mycobacterial responses and whole blood cytokine production were noted in Australian infants who received BCG Denmark at birth (early BCG) compared to 2 months after birth (late BCG) ( Ritz et al., 2016 ). However, in TB-endemic regions such as Cape Town and South Africa, delaying immunization with BCG Denmark 10 weeks post-birth led to increased frequencies of memory CD4 T cells at 1 year of age ( Kagina et al., 2009 ). These two seemingly contradictory studies emphasize the importance of immune ontogeny, as well as genetic and epigenetic host factors, including prior and ongoing host exposure to non-tuberculous mycobacteria, to the immunogenicity of live vaccines ( Plotkin, 2013 ).
BCG vaccination in children ( Jensen et al., 2015 ) results in different cytokine induction patterns compared to adults ( Kleinnijenhuis et al., 2012 ). Vaccine efficacy rates were indeed higher in studies conducted in populations vaccinated during childhood compared with populations vaccinated at older ages ( Colditz et al., 1995 ). The longevity of BCG clinical effects remains largely unknown and may in part depend on age of immunization. In the largest community-based controlled trial of BCG vaccination conducted in southern India in the 1960s, vaccine recipients were reevaluated 15 years after BCG vaccination ( Tuberculosis Research Centre, 2013 ): protective efficacy in persons who had been vaccinated as children was found to be 17%, while no protective effect was seen in people who had been vaccinated as adolescents or adults ( Tuberculosis Research Centre, 2013 ).
WHO currently recommends BCG at birth for countries where TB is endemic since birth is the first point of contact for the newborn with the healthcare system. In practice, however, many healthcare systems continue to institute policies such that BCG is not administered unless a certain number of infants are present to receive immunization from the multi-dose BCG vial resulting in missed opportunities to administer it at the earliest possible age per WHO recommendations ( Schaltz-Buchholzer et al., 2017 ). However, based on the above, a “one size fits all” policy on optimal BCG timing may not be realistic and immunization should be tailored to different global populations with different risk factors in different settings. Further investigations involving the ontogenetic aspects of BCG-induced immunogenicity and protection against TB are needed. Highly standardized comparison studies should account for the environmental (local and regional) exposure, genetic and epigenetic factors, biological age, and immunological status of vaccinated participants. Such studies would further inform the variation of heterologous effects seen as a result of BCG vaccination.
BCG as a Model to Build Next Generation Vaccines
The ability of live vaccines such as BCG to induce heterologous immunity raises the possibility of leveraging such broadly protective effects in the development of novel vaccine formulations ( Whittaker et al., 2018a ), in the form of “trained immunity-based” vaccines ( Sanchez-Ramon et al., 2018 ). Firstly, increased awareness of innate memory may be employed to define new classes of vaccine adjuvants ( Topfer et al., 2015 ), crucial tools to optimize current vaccines and develop new ones ( Dowling and Levy, 2015 ). Adjuvants enhance responses to vaccine antigens by a variety of mechanisms ( Coffman et al., 2010 ), but like BCG, many are capable of acting via PRR signaling (e.g., TLRA), which possibly could hold the potential of inducing innate memory and could thereby mediate long-term changes in host defense. Also, recent advances in adjuvant discovery and delivery have opened up a new toolbox on how vaccinologists can employ adjuvants, including synthetic small molecule PRR agonists ( Dowling and Levy, 2015 ). Thus, to confer protective immunity a strategy might be the combination of adjuvants, with potential of inducing beneficial non-specific trained immunity responses, formulated along with the specific selected antigen epitopes. An important aspect to take into account is that it is not yet known whether or not all PRR stimuli produce trained immunity-like responses. As different adjuvants may trigger different cell activation pathways and have age-specific activity, it is likely that more than one trained immunity pathway could be targeted for perturbation. In addition, putative target cell populations for innate training may vary, including progenitor cells, tissue resident or circulating monocytes, which may be optimally targeted via specific routes of administration or by rationally selected adjuvant formulations ( Dowling and Levy, 2015 ; Nanishi et al., 2020 ).
Secondly, characterizing mechanisms by which BCG enhances neonatal immunity may inform rational design of scalable, synthetic subunit vaccine formulations for newborns. Initially, TLR7/8a imidazoquinolines were shown to induce trained immunity in newborn mice ( Wynn et al., 2008 ), raising the possibility that such an approach could generate a vaccine that may also induce “BCG-like” trained immunity. However, since free un-formulated molecules may have off target effects, another approach is to build “BCG-like” synthetic “non-live” particulate vaccines that may mimic BCG’s immune-enhancing effects. Inclusion of an imidazoquinoline small molecule TLR8 agonist in a polymersome nanoparticle (∼150 nm diameter) induced robust Th1 polarizing responses from human newborn monocyte-derived dendritic cells in vitro that at least matched and for some biomarkers such as IL-12p70 exceeded those induced by BCG Denmark ( Dowling et al., 2017 ). Of note, when co-loaded with the M. tuberculosis antigen 85B peptide 25, the TLR8-agonist containing polymersome nanoparticles were comparable to BCG in inducing antigen-specific T cell responses in human TLR8-expressing neonatal mice in vivo ( Dowling et al., 2017 ). This is promising, since BCG reduces the risk of disseminated early life TB safely, elicits Th1-type neonatal immune responses and requires only a single dose at or shortly after the time of birth. The key role of TLR8 agonists for protection against Mtb challenge was recently verified by others with humanized TLR8 mice ( Tang et al., 2017 ) and in human studies, wherein humans with hypermorphic alleles of TLR8 demonstrated enhanced BCG-induced protection against TB ( Ugolini et al., 2018 ).
Thirdly, the robust safety and immunogenicity profile of BCG has rendered it an attractive vector for vaccine development against other infectious diseases ( Hernandez-Pando et al., 2007 ; Bastos et al., 2009 ; Nieuwenhuizen and Kaufmann, 2018 ). Recombinant BCG technology has been studied in the context of vaccination against HIV ( Aldovini and Young, 1991 ), Lyme disease ( Stover et al., 1993 ), malaria ( Matsumoto et al., 1998 ), measles ( Zhu et al., 1997 ), and HCV ( Uno-Furuta et al., 2003 ). When administered in early life, BCG can act as an adjuvant enhancing antibody responses to recombinant hepatitis B surface antigen (rHBsAg) both in mice and in human infants ( Ota et al., 2002 ; Zimmermann et al., 2019 ). In another approach, a recombinant strain of M. bovis BCG that secretes high levels of functional murine monocyte chemotactic protein 3 (BCG MCP– 3 ) attenuated vaccine virulence in immunodeficient mice, while maintaining protective efficacy against Mtb in mice by enhancing antigen-specific IFNγ T cell responses, as compared to a control BCG (Pasteur strain 1173P2) ( Ryan et al., 2007 ). A recombinant BCG strain expressing listeriolysin O to enhance cytosolic entry of BCG antigens for MHC I presentation, named VPM1002, induced both CD4 and CD8 responses and demonstrated safety and immunogenicity in a phase 2 clinical study in South African newborns ( Loxton et al., 2017 ). Overall, insights into BCG vaccine-induced heterologous and specific immunity may provide insights into the development of a broad spectrum of anti-infective vaccine formulations.
Despite nearly a century of use, policies and practices around BCG immunization vary widely across the world. Much remains to be learned regarding the relative protective efficacy of different licensed BCG formulations and it is important to ensure that BCG vaccines selected for use in large-scale immunization schemes maintain the stability of their characteristics. Our growing understanding of the distinct neonatal immune response and of innate immune memory in early life will increasingly inform optimal immunization in this age group. Epidemiologic studies suggest that the benefit of BCG vaccination may vary by BCG formulation and age of administration with optimal timing in early life to maximize both specific and heterologous beneficial effects. Future studies should directly compare licensed BCG formulations, including their optimal timing of administration, and measure both heterologous and specific protection in high mortality populations. Characterizing activation of age-specific immune responses by BCG strains and defining potential correlates of BCG-induced protection via correlation with known relative heterologous clinical benefit, can inform optimization of BCG’s use. This may involve potential BCG (re)introduction in national immunization schedules, BCG utilization in prime-boost schedules, use of BCG as a vector for other vaccinal antigens, as well as design of new vaccines that mimic BCG to harness innate immune memory for clinical benefit ( Dowling et al., 2017 ).

Author Contributions
AA conceived the manuscript. AA, JD-A, DD, and MC wrote the manuscript. DD created the manuscript figures. KS, SvH, DD, RH, and OL revised it critically for important intellectual content. All authors read and approved the submitted version.
Conflict of Interest
OL and DD are named inventors on several vaccine adjuvant formulation patent applications. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Precision Vaccines Program Coordinators, Ms. Diana Vo and Bianca Dy, for important administrative support.
Abbreviations
1 http://www.bcgatlas.org
MC was sponsored by Sapienza University of Rome and was a recipient of the Admeto Pettinari e Paolo Andreini graduate scholarship for specialization courses in Italy and abroad. OL is supported by U.S. National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID) awards, including Human Immunology Project Consortium (HIPC) 1U19AI118608-01A1, Molecular Mechanisms of Combination Adjuvants (1U01AI124284-01), Adjuvant Discovery (HHSN272201400052C and 75N93019C00044) and Development (HHSN272201800047C) Program Contracts and the Precision Vaccines Program at Boston Children’s Hospital. DD was supported by NIH/NIAID grant 1R21AI137932-01A1 and Adjuvant Discovery Program (75N93019C00044).
- Aaby P., Martins C. L., Garly M. L., Bale C., Andersen A., Rodrigues A., et al. (2010). Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ 341 : c6495 . 10.1136/bmj.c6495 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aaby P., Roth A., Ravn H., Napirna B. M., Rodrigues A., Lisse I. M., et al. (2011). Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 204 245–252. 10.1093/infdis/jir240 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aaby P., Shaheen S. O., Heyes C. B., Goudiaby A., Hall A. J., Shiell A. W., et al. (2000). Early BCG vaccination and reduction in atopy in Guinea-Bissau. Clin. Exp. Allergy 30 644–650. 10.1046/j.1365-2222.2000.00803.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Abdallah A. M., Hill-Cawthorne G. A., Otto T. D., Coll F., Guerra-Assuncao J. A., Gao G., et al. (2015). Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci. Rep. 5 : 15443 . 10.1038/srep15443 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Achkar J. M., Casadevall A. (2013). Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 13 250–262. 10.1016/j.chom.2013.02.009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Achkar J. M., Chan J., Casadevall A. (2014). Role of B cells and antibodies in acquired immunity against Mycobacterium tuberculosis. Cold Spring Harb. Perspect. Med. 5 : a018432 . 10.1101/cshperspect.a018432 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aguilo N., Alvarez-Arguedas S., Uranga S., Marinova D., Monzon M., Badiola J., et al. (2016). Pulmonary but Not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17-dependent mechanism. J. Infect. Dis. 213 831–839. 10.1093/infdis/jiv503 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aguirre-Blanco A. M., Lukey P. T., Cliff J. M., Dockrell H. M. (2007). Strain-dependent variation in Mycobacterium bovis BCG-induced human T-cell activation and gamma interferon production in vitro. Infect. Immun. 75 3197–3201. 10.1128/iai.01611-06 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ai W., Yue Y., Xiong S., Xu W. (2013). Enhanced protection against pulmonary mycobacterial challenge by chitosan-formulated polyepitope gene vaccine is associated with increased pulmonary secretory IgA and gamma-interferon(+) T cell responses. Microbiol. Immunol. 57 224–235. 10.1111/1348-0421.12027 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aldovini A., Young R. A. (1991). Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351 479–482. 10.1038/351479a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alvarez N., Otero O., Camacho F., Borrero R., Tirado Y., Puig A., et al. (2013). Passive administration of purified secretory IgA from human colostrum induces protection against Mycobacterium tuberculosis in a murine model of progressive pulmonary infection. BMC Immunol. 14 ( Suppl. 1):S3 . 10.1186/1471-2172-14-S1-S3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andersen P., Doherty T. M. (2005). The success and failure of BCG – implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3 656–662. 10.1038/nrmicro1211 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson E. J., Webb E. L., Mawa P. A., Kizza M., Lyadda N., Nampijja M., et al. (2012). The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine 30 2083–2089. 10.1016/j.vaccine.2012.01.053 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Angelidou A., Conti M. G., Diray-Arce J., Benn C. S., Frank S., Netea M., et al. (2020). Licensed bacille calmette-guerin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine 38 229–2240. 10.1016/j.vaccine.2019.11.060 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arts R. J., Blok B. A., Aaby P., Joosten L. A., de Jong D., van der Meer J. W., et al. (2015). Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity. J Leukoc Biol. 98 995–1001. 10.1189/jlb.4MA0215-059R [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arts R. J. W., Carvalho A., La Rocca C., Palma C., Rodrigues F., Silvestre R., et al. (2016). Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 17 2562–2571. 10.1016/j.celrep.2016.11.011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arts R. J. W., Moorlag S., Novakovic B., Li Y., Wang S. Y., Oosting M., et al. (2018). BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23 89–100.e5. 10.1016/j.chom.2017.12.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bastos R. G., Borsuk S., Seixas F. K., Dellagostin O. A. (2009). Recombinant Mycobacterium bovis BCG. Vaccine 27 6495–6503. 10.1016/j.vaccine.2009.08.044 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Behr M. A., Small P. M. (1999). A historical and molecular phylogeny of BCG strains. Vaccine 17 915–922. 10.1016/s0264-410x(98)00277-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benmerzoug S., Marinho F. V., Rose S., Mackowiak C., Gosset D., Sedda D., et al. (2018). GM-CSF targeted immunomodulation affects host response to M. tuberculosis infection. Sci. Rep. 8 : 8652 . 10.1038/s41598-018-26984-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Biering-Sorensen S., Aaby P., Napirna B. M., Roth A., Ravn H., Rodrigues A., et al. (2012). Small randomized trial among low-birth-weight children receiving bacillus calmette-guerin vaccination at first health center contact. Pediatr. Infect. Dis. J. 31 306–308. 10.1097/INF.0b013e3182458289 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Biering-Sorensen S., Jensen K. J., Aamand S. H., Blok B., Andersen A., Monteiro I., et al. (2015). Variation of growth in the production of the BCG vaccine and the association with the immune response. An observational study within a randomised trial. Vaccine 33 2056–2065. 10.1016/j.vaccine.2015.02.056 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. (1986). Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 51 668–674. 10.1128/iai.51.2.668-674.1986 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boer M. C., Prins C., van Meijgaarden K. E., van Dissel J. T., Ottenhoff T. H., Joosten S. A. (2015). Mycobacterium bovis BCG vaccination induces divergent proinflammatory or regulatory T cell responses in adults. Clin. Vaccine Immunol. 22 778–788. 10.1128/CVI.00162-15 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Borriello F., Iannone R., Di Somma S., Loffredo S., Scamardella E., Galdiero M. R., et al. (2016). GM-CSF and IL-3 modulate human monocyte TNF-alpha production and renewal in in vitro models of trained immunity. Front. Immunol. 7 : 680 . 10.3389/fimmu.2016.00680 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brewer T. F. (2000). Preventing tuberculosis with bacillus calmette-guerin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31 ( Suppl. 3 ), S64–S67. 10.1086/314072 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brosch R., Gordon S. V., Garnier T., Eiglmeier K., Frigui W., Valenti P., et al. (2007). Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. U.S.A. 104 5596–5601. 10.1073/pnas.0700869104 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burl S., Adetifa U. J., Cox M., Touray E., Ota M. O., Marchant A., et al. (2010). Delaying bacillus calmette-guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J. Immunol. 185 2620–2628. 10.4049/jimmunol.1000552 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Butler W. R., Warren N. G., Kubica G. P., Kilburn J. O. (1990). Modified method for testing the quality of albumin-containing enrichments used in growth media for mycobacteria. J. Clin. Microbiol. 28 1068–1070. 10.1128/jcm.28.5.1068-1070.1990 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Calmette A. (1931). Preventive vaccination against tuberculosis with BCG. Proc. R. Soc. Med. 24 1481–1490. 10.1177/003591573102401109 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chatterjee S., Dwivedi V. P., Singh Y., Siddiqui I., Sharma P., Van Kaer L., et al. (2011). Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 7 : e1002378 . 10.1371/journal.ppat.1002378 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen J. M., Alexander D. C., Behr M. A., Liu J. (2003). Mycobacterium bovis BCG vaccines exhibit defects in alanine and serine catabolism. Infect. Immun. 71 708–716. 10.1128/iai.71.2.708-716.2003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coffman R. L., Sher A., Seder R. A. (2010). Vaccine adjuvants: putting innate immunity to work. Immunity 33 492–503. 10.1016/j.immuni.2010.10.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Colditz G. A., Berkey C. S., Mosteller F., Brewer T. F., Wilson M. E., Burdick E., et al. (1995). The efficacy of bacillus calmette-guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96 (1 Pt 1), 29–35. [ PubMed ] [ Google Scholar ]
- Colditz G. A., Brewer T. F., Berkey C. S., Wilson M. E., Burdick E., Fineberg H. V., et al. (1994). Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271 698–702. 10.1001/jama.271.9.698 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Conti M. G., Angelidou A., Diray-Arce J., Smolen K. K., Lasky-Su J., De Curtis M., et al. (2019). Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr. Res. 87 399–405. 10.1038/s41390-019-0647-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Corner L. A., Gormley E., Pfeiffer D. U. (2012). Primary isolation of Mycobacterium bovis from bovine tissues: conditions for maximising the number of positive cultures. Vet. Microbiol. 156 162–171. 10.1016/j.vetmic.2011.10.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Darrah P. A., Patel D. T., De Luca P. M., Lindsay R. W., Davey D. F., Flynn B. J., et al. (2007). Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13 843–850. 10.1038/nm1592 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Darrah P. A., Zeppa J. J., Maiello P., Hackney J. A., Wadsworth M. H., II, Hughes T. K., et al. (2020). Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577 95–102. 10.1038/s41586-019-1817-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davids V., Hanekom W. A., Mansoor N., Gamieldien H., Gelderbloem S. J., Hawkridge A., et al. (2006). The effect of bacille calmette-guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J. Infect Dis. 193 531–536. 10.1086/499825 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- de Castro M. J., Pardo-Seco J., Martinon-Torres F. (2015). Nonspecific (Heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin. Infect. Dis. 60 1611–1619. 10.1093/cid/civ144 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Denis M., Ghadirian E. (1990). Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol. Lett. 24 203–206. 10.1016/0165-2478(90)90049-v [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dijkman K., Sombroek C. C., Vervenne R. A. W., Hofman S. O., Boot C., Remarque E. J., et al. (2019). Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 25 255–262. 10.1038/s41591-018-0319-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Djuardi Y., Sartono E., Wibowo H., Supali T., Yazdanbakhsh M. (2010). A longitudinal study of BCG vaccination in early childhood: the development of innate and adaptive immune responses. PLoS One 5 : e14066 . 10.1371/journal.pone.0014066 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dockrell H. M., Smith S. G. (2017). What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 8 : 1134 . 10.3389/fimmu.2017.01134 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dowling D. J., Levy O. (2015). Pediatric vaccine adjuvants: components of the modern vaccinologist’s toolbox. Pediatr. Infect. Dis. J. 34 1395–1398. 10.1097/INF.0000000000000893 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dowling D. J., Scott E. A., Scheid A., Bergelson I., Joshi S., Pietrasanta C., et al. (2017). Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 140 1339–1350. 10.1016/j.jaci.2016.12.985 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dreschers S., Ohl K., Lehrke M., Mollmann J., Denecke B., Costa I., et al. (2019). Impaired cellular energy metabolism in cord blood macrophages contributes to abortive response toward inflammatory threats. Nat. Commun. 10 : 1685 . 10.1038/s41467-019-09359-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fanucchi S., Fok E. T., Dalla E., Shibayama Y., Borner K., Chang E. Y., et al. (2019). Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 51 138–150. 10.1038/s41588-018-0298-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Faustman D. L. (2018). TNF, TNF inducers, and TNFR2 agonists: a new path to type 1 diabetes treatment. Diabetes Metab. Res. Rev. 34 . 10.1002/dmrr.2941 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Faustman D. L., Wang L., Okubo Y., Burger D., Ban L., Man G., et al. (2012). Proof-of-concept, randomized, controlled clinical trial of bacillus-calmette-guerin for treatment of long-term type 1 diabetes. PLoS One 7 : e41756 . 10.1371/journal.pone.0041756 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fletcher H. A., Snowden M. A., Landry B., Rida W., Satti I., Harris S. A., et al. (2016). T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat. Commun. 7 : 11290 . 10.1038/ncomms11290 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fok E. T., Davignon L., Fanucchi S., Mhlanga M. M. (2018). The lncRNA connection between cellular metabolism and epigenetics in trained immunity. Front. Immunol. 9 : 3184 . 10.3389/fimmu.2018.03184 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frankel H., Byberg S., Bjerregaard-Andersen M., Martins C. L., Aaby P., Benn C. S., et al. (2016). Different effects of BCG strains – A natural experiment evaluating the impact of the Danish and the russian BCG strains on morbidity and scar formation in guinea-bissau. Vaccine 34 4586–4593. 10.1016/j.vaccine.2016.07.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Funch K. M., Thysen S. M., Rodrigues A., Martins C. L., Aaby P., Benn C. S., et al. (2018). Determinants of BCG scarification among children in rural guinea-bissau: a prospective cohort study. Hum. Vaccin Immunother. 14 2434–2442. 10.1080/21645515.2017.1421879 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gheorghiu M., Lagrange P. H. (1983). Viability, heat stability and immunogenicity of four BCG vaccines prepared from four different BCG strains. Ann. Immunol. (Paris) 134C 125–147. 10.1016/s0769-2625(83)80157-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gopal R., Monin L., Slight S., Uche U., Blanchard E., Fallert Junecko B. A., et al. (2014). Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 10 : e1004099 . 10.1371/journal.ppat.1004099 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hawkridge A., Hatherill M., Little F., Goetz M. A., Barker L., Mahomed H., et al. (2008). Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ 337 : a2052 . 10.1136/bmj.a2052 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hayashi D., Takii T., Fujiwara N., Fujita Y., Yano I., Yamamoto S., et al. (2009). Comparable studies of immunostimulating activities in vitro among Mycobacterium bovis bacillus calmette-guerin (BCG) substrains. FEMS Immunol. Med. Microbiol. 56 116–128. 10.1111/j.1574-695X.2009.00559.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heldwein K. A., Liang M. D., Andresen T. K., Thomas K. E., Marty A. M., Cuesta N., et al. (2003). TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J. Leukoc. Biol. 74 277–286. 10.1189/jlb.0103026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hernandez-Pando R., Castanon M., Espitia C., Lopez-Vidal Y. (2007). Recombinant BCG vaccine candidates. Curr. Mol. Med. 7 365–372. 10.2174/156652407780831610 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Higgins JPT, Soares-Weiser K., Reingold A. (2014). Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. Available at: http://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf (accessed February 28, 2020). [ Google Scholar ]
- Hoft D. F., Worku S., Kampmann B., Whalen C. C., Ellner J. J., Hirsch C. S., et al. (2002). Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J. Infect. Dis. 186 1448–1457. 10.1086/344359 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hollm-Delgado M. G., Stuart E. A., Black R. E. (2014). Acute lower respiratory infection among bacille calmette-guerin (BCG)-vaccinated children. Pediatrics 133 e73–e81. 10.1542/peds.2013-2218 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Izzo A. A. (2017). Tuberculosis vaccines – perspectives from the NIH/NIAID Mycobacteria vaccine testing program. Curr. Opin. Immunol. 47 78–84. 10.1016/j.coi.2017.07.008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jacobs A. J., Mongkolsapaya J., Screaton G. R., McShane H., Wilkinson R. J. (2016). Antibodies and tuberculosis. Tuberculosis (Edinb) 101 102–113. 10.1016/j.tube.2016.08.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jayaraman K., Adhisivam B., Nallasivan S., Krishnan R. G., Kamalarathnam C., Bharathi M., et al. (2018). Two randomized trials of the effect of BCG-russia alone or with oral polio vaccine on neonatal mortality in infants weighing <2000 G in India. Pediatr. Infect. Dis. J. 198 198–202. 10.1097/INF.0000000000002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jensen K. J., Larsen N., Biering-Sorensen S., Andersen A., Eriksen H. B., Monteiro I., et al. (2015). Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J. Infect. Dis. 211 956–967. 10.1093/infdis/jiu508 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnson C. H., Ivanisevic J., Siuzdak G. (2016). Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17 451–459. 10.1038/nrm.2016.25 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kagina B. M., Abel B., Bowmaker M., Scriba T. J., Gelderbloem S., Smit E., et al. (2009). Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine 27 5488–5495. 10.1016/j.vaccine.2009.06.103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kagina B. M., Abel B., Scriba T. J., Hughes E. J., Keyser A., Soares A., et al. (2010). Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus calmette-guerin vaccination of newborns. Am. J. Respir Crit. Care Med. 182 1073–1079. 10.1164/rccm.201003-0334OC [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kan B., Michalski C., Fu H., Au H. H. T., Lee K., Marchant E. A., et al. (2018). Cellular metabolism constrains innate immune responses in early human ontogeny. Nat. Commun. 9 : 4822 . 10.1038/s41467-018-07215-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaufmann E., Sanz J., Dunn J. L., Khan N., Mendonca L. E., Pacis A., et al. (2018). BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172 176–190.e119. 10.1016/j.cell.2017.12.031 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Keller P. M., Bottger E. C., Sander P. (2008). Tuberculosis vaccine strain Mycobacterium bovis BCG Russia is a natural recA mutant. BMC Microbiol. 8 : 120 . 10.1186/1471-2180-8-120 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kelly P., McKeown D., Clancy L. (1997). Neonatal BCG vaccination in Ireland: evidence of its efficacy in the prevention of childhood tuberculosis. Eur. Respir. J. 10 619–623. [ PubMed ] [ Google Scholar ]
- Kemp E. B., Belshe R. B., Hoft D. F. (1996). Immune responses stimulated by percutaneous and intradermal bacille calmette-guerin. J. Infect. Dis. 174 113–119. 10.1093/infdis/174.1.113 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kiravu A., Osawe S., Happel A. U., Nundalall T., Wendoh J., Beer S., et al. (2019). Bacille calmette-guerin vaccine strain modulates the ontogeny of both mycobacterial-specific and heterologous T cell immunity to vaccination in infants. Front. Immunol. 10 : 2307 . 10.3389/fimmu.2019.02307 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L. A., Ifrim D. C., Saeed S., et al. (2012). Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A. 109 17537–17542. 10.1073/pnas.1202870109 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L. A., Jacobs C., Xavier R. J., et al. (2014). BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155 213–219. 10.1016/j.clim.2014.10.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kleinnijenhuis J., van Crevel R., Netea M. G. (2015). Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans. R. Soc. Trop. Med. Hyg. 109 29–35. 10.1093/trstmh/tru168 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kodama S., Kuhtreiber W., Fujimura S., Dale E. A., Faustman D. L. (2003). Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 302 1223–1227. 10.1126/science.1088949 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kollmann T. R., Kampmann B., Mazmanian S. K., Marchant A., Levy O. (2017). Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46 350–363. 10.1016/j.immuni.2017.03.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kollmann T. R., Levy O., Montgomery R. R., Goriely S. (2012). Innate immune function by toll-like receptors: distinct responses in newborns and the elderly. Immunity 37 771–783. 10.1016/j.immuni.2012.10.014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kristensen I., Aaby P., Jensen H. (2000). Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 321 1435–1438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kroger L., Brander E., Korppi M., Wasz-Hockert O., Backman A., Kroger H., et al. (1994). Osteitis after newborn vaccination with three different bacillus calmette-guerin vaccines: twenty-nine years of experience. Pediatr. Infect. Dis. J. 13 113–116. [ PubMed ] [ Google Scholar ]
- Kuhtreiber W. M., Tran L., Kim T., Dybala M., Nguyen B., Plager S., et al. (2018). Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 3 : 23 . 10.1038/s41541-018-0062-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kurtz J. (2005). Specific memory within innate immune systems. Trends Immunol. 26 186–192. 10.1016/j.it.2005.02.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levy O., Netea M. G. (2014). Innate immune memory: implications for development of pediatric immunomodulatory agents and adjuvanted vaccines. Pediatr. Res. 75 184–188. 10.1038/pr.2013.214pr2013214 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li S., Sullivan N. L., Rouphael N., Yu T., Banton S., Maddur M. S., et al. (2017). Metabolic phenotypes of response to vaccination in humans. Cell 169 862–877.e817. 10.1016/j.cell.2017.04.026 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lochner M., Berod L., Sparwasser T. (2015). Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36 81–91. 10.1016/j.it.2014.12.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Loeffler A. M. (2003). Pediatric tuberculosis. Semin. Respir. Infect. 18 272–291. [ PubMed ] [ Google Scholar ]
- Lotte A., Wasz-Hockert O., Poisson N., Dumitrescu N., Verron M., Couvet E. (1984). A bibliography of the complications of BCG vaccination. A comprehensive list of the world literature since the introduction of BCG up to July 1982, supplemented by over 100 personal communications. Adv. Tuberc Res. 21 194–245. [ PubMed ] [ Google Scholar ]
- Loxton A. G., Knaul J. K., Grode L., Gutschmidt A., Meller C., Eisele B., et al. (2017). Safety and Immunogenicity of the recombinant Mycobacterium bovis BCG Vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin. Vaccine Immunol. 24 : e00439-16 . 10.1128/CVI.00439-16 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lu L. L., Chung A. W., Rosebrock T. R., Ghebremichael M., Yu W. H., Grace P. S., et al. (2016). A functional role for antibodies in tuberculosis. Cell 167 433–443.e414. 10.1016/j.cell.2016.08.072 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lu L. L., Smith M. T., Yu K. K. Q., Luedemann C., Suscovich T. J., Grace P. S., et al. (2019). IFN-gamma-independent immune markers of Mycobacterium tuberculosis exposure. Nat. Med. 25 977–987. 10.1038/s41591-019-0441-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mahairas G. G., Sabo P. J., Hickey M. J., Singh D. C., Stover C. K. (1996). Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis . J. Bacteriol. 178 1274–1282. 10.1128/jb.178.5.1274-1282.1996 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P. E., et al. (2014). Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 58 470–480. 10.1093/cid/cit790 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marchant A., Goetghebuer T., Ota M. O., Wolfe I., Ceesay S. J., De Groote D., et al. (1999). Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus calmette-guerin vaccination. J. Immunol. 163 2249–2255. [ PubMed ] [ Google Scholar ]
- Marciano B. E., Huang C. Y., Joshi G., Rezaei N., Carvalho B. C., Allwood Z., et al. (2014). BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J. Allergy Clin. Immunol. 133 1134–1141. 10.1016/j.jaci.2014.02.028 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Markey K., Ho M. M., Rigsby P., Hockley J., Corbel M. J. (2009). International Collaborative Study to Evaluate and Establish WHO Reference Reagents for BCG Vaccine of Three Different Substrains. Geneva: WHO Press. [ PubMed ] [ Google Scholar ]
- Martinez F. O., Gordon S. (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6 : 13 . 10.12703/P6-13 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matsumoto S., Yukitake H., Kanbara H., Yamada T. (1998). Recombinant Mycobacterium bovis bacillus calmette-guerin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J. Exp. Med. 188 845–854. 10.1084/jem.188.5.845 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matsunaga I., Bhatt A., Young D. C., Cheng T. Y., Eyles S. J., Besra G. S., et al. (2004). Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J. Exp. Med. 200 1559–1569. 10.1084/jem.20041429 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matsunaga I., Moody D. B. (2009). Mincle is a long sought receptor for mycobacterial cord factor. J. Exp. Med. 206 2865–2868. 10.1084/jem.20092533 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mawa P. A., Nkurunungi G., Egesa M., Webb E. L., Smith S. G., Kizindo R., et al. (2015). The impact of maternal infection with Mycobacterium tuberculosis on the infant response to bacille calmette-guerin immunization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370 : 20140137 . 10.1098/rstb.2014.0137 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meraviglia S., El Daker S., Dieli F., Martini F., Martino A. (2011). gammadelta T cells cross-link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011 : 587315 . 10.1155/2011/587315 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Messina N. L., Germano S., Bonnici R., Lee L. Y., Daley A. J., Bustamante A., et al. (2018). Can colony-forming unit testing be used to extend the shelf life of BCG vaccines? Tuberculosis (Edinb) 111 188–192. 10.1016/j.tube.2018.06.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mitroulis I., Ruppova K., Wang B., Chen L. S., Grzybek M., Grinenko T., et al. (2018). Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172 147–161.e12. 10.1016/j.cell.2017.11.034 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moorlag S., Roring R. J., Joosten L. A. B., Netea M. G. (2018). The role of the interleukin-1 family in trained immunity. Immunol. Rev. 281 28–39. 10.1111/imr.12617 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Naka T., Maeda S., Niki M., Ohara N., Yamamoto S., Yano I., et al. (2011). Lipid phenotype of two distinct subpopulations of Mycobacterium bovis bacillus calmette-guerin tokyo 172 substrain. J. Biol. Chem. 286 44153–44161. 10.1074/jbc.M111.310037 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nanishi E., Dowling D. J., Levy O. (2020). Toward precision adjuvants: optimizing science and safety. Curr. Opin. Pediatr. 32 125–138. 10.1097/MOP.0000000000000868 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netea M. G., Joosten L. A., Latz E., Mills K. H., Natoli G., Stunnenberg H. G., et al. (2016). Trained immunity: a program of innate immune memory in health and disease. Science 352 : aaf1098 . 10.1126/science.aaf1098 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netea M. G., Quintin J., van der Meer J. W. (2011). Trained immunity: a memory for innate host defense. Cell Host Microbe 9 355–361. 10.1016/j.chom.2011.04.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netea M. G., Schlitzer A., Placek K., Joosten L. A. B., Schultze J. L. (2019). Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25 13–26. 10.1016/j.chom.2018.12.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netea M. G., van der Meer J. W. (2017). Trained immunity: an ancient way of remembering. Cell Host Microbe 21 297–300. 10.1016/j.chom.2017.02.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nieuwenhuizen N. E., Kaufmann S. H. E. (2018). Next-generation vaccines based on bacille calmette-guerin. Front. Immunol. 9 : 121 . 10.3389/fimmu.2018.00121 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nunes-Alves C., Booty M. G., Carpenter S. M., Jayaraman P., Rothchild A. C., Behar S. M. (2014). In search of a new paradigm for protective immunity to TB. Nat. Rev. Microbiol. 12 289–299. 10.1038/nrmicro3230 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Neill L. A., Kishton R. J., Rathmell J. (2016). A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16 553–565. 10.1038/nri.2016.70 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Osborn T. W. (1983). Changes in BCG strains. Tubercle 64 1–13. 10.1016/0041-3879(83)90044-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ota M. O., Goetghebuer T., Vekemans J., Okoko B. J., Newport M. J., McAdam K. P., et al. (2006). Dissociation between tuberculin skin test and in vitro IFN-gamma responses following neonatal BCG vaccination. J. Trop Pediatr. 52 136–140. 10.1093/tropej/fmi087 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ota M. O., Vekemans J., Schlegel-Haueter S. E., Fielding K., Sanneh M., Kidd M., et al. (2002). Influence of Mycobacterium bovis bacillus calmette-guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168 919–925. 10.4049/jimmunol.168.2.919 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paolillo A., Buzzi M. G., Giugni E., Sabatini U., Bastianello S., Pozzilli C., et al. (2003). The effect of bacille calmette-guerin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS. J. Neurol. 250 247–248. 10.1007/s00415-003-0967-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Plotkin S. A. (2013). Complex correlates of protection after vaccination. Clin. Infect. Dis. 56 1458–1465. 10.1093/cid/cit048 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pulendran B. (2014). Systems vaccinology: probing humanity’s diverse immune systems with vaccines. Proc. Natl. Acad. Sci. U.S.A. 111 12300–12306. 10.1073/pnas.1400476111 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ravaud A., Eghbali H., Trojani M., Hoerni-Simon G., Soubeyran P., Hoerni B. (1990). Adjuvant bacillus calmette-guerin therapy in non-Hodgkin’s malignant lymphomas: long-term results of a randomized trial in a single institution. J. Clin. Oncol. 8 608–614. 10.1200/JCO.1990.8.4.608 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ristori G., Romano S., Cannoni S., Visconti A., Tinelli E., Mendozzi L., et al. (2014). Effects of bacille calmette-guerin after the first demyelinating event in the CNS. Neurology 82 41–48. 10.1212/01.wnl.0000438216.93319.ab [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ritz N., Casalaz D., Donath S., Tebruegge M., Dutta B., Connell T. G., et al. (2016). Comparable CD4 and CD8 T cell responses and cytokine release after at-birth and delayed BCG immunisation in infants born in Australia. Vaccine 34 4132–4139. 10.1016/j.vaccine.2016.06.077 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ritz N., Dutta B., Donath S., Casalaz D., Connell T. G., Tebruegge M., et al. (2012). The influence of bacille calmette-guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am. J. Respir. Crit. Care Med. 185 213–222. 10.1164/rccm.201104-0714OC [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ritz N., Hanekom W. A., Robins-Browne R., Britton W. J., Curtis N. (2008). Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 32 821–841. 10.1111/j.1574-6976.2008.00118.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ritz N., Mui M., Balloch A., Curtis N. (2013). Non-specific effect of bacille calmette-guerin vaccine on the immune response to routine immunisations. Vaccine 31 3098–3103. 10.1016/j.vaccine.2013.03.059 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rodrigues L. C., Diwan V. K., Wheeler J. G. (1993). Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22 1154–1158. 10.1093/ije/22.6.1154 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Romanus V., Hallander H. O., Wahlen P., Olinder-Nielsen A. M., Magnusson P. H., Juhlin I. (1995). Atypical mycobacteria in extrapulmonary disease among children. Incidence in Sweden from 1969 to 1990, related to changing BCG-vaccination coverage. Tuber Lung Dis. 76 300–310. 10.1016/s0962-8479(05)80028-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Romanus V., Svensson A., Hallander H. O. (1992). The impact of changing BCG coverage on tuberculosis incidence in Swedish-born children between 1969 and 1989. Tuber Lung Dis. 73 150–161. 10.1016/0962-8479(92)90149-E [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosen L. B., Freeman A. F., Yang L. M., Jutivorakool K., Olivier K. N., Angkasekwinai N., et al. (2013). Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J. Immunol. 190 3959–3966. 10.4049/jimmunol.1202526 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosenthal S. R. (1988). Surgery, recall antigens, immunity, and bacillus calmette-guerin vaccination. Am. J. Surg. 156 1–3. 10.1016/s0002-9610(88)80158-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roth A., Garly M. L., Jensen H., Nielsen J., Aaby P. (2006). Bacillus calmette-guerin vaccination and infant mortality. Expert Rev. Vaccines 5 277–293. 10.1586/14760584.5.2.277 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rothchild A. C., Stowell B., Goyal G., Nunes-Alves C., Yang Q., Papavinasasundaram K., et al. (2017). Role of granulocyte-macrophage colony-stimulating factor production by T cells during Mycobacterium tuberculosis infection. MBio 8 e1514–e1517. 10.1128/mBio.01514-17 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roy A., Eisenhut M., Harris R. J., Rodrigues L. C., Sridhar S., Habermann S., et al. (2014). Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 349 : g4643 . 10.1136/bmj.g4643 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ryan A. A., Spratt J. M., Britton W. J., Triccas J. A. (2007). Secretion of functional monocyte chemotactic protein 3 by recombinant Mycobacterium bovis BCG attenuates vaccine virulence and maintains protective efficacy against M. tuberculosis infection. Infect. Immun. 75 523–526. 10.1128/iai.00897-06 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ryu S., Kodama S., Ryu K., Schoenfeld D. A., Faustman D. L. (2001). Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J. Clin. Invest. 108 63–72. 10.1172/JCI12335 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saeed S., Quintin J., Kerstens H. H., Rao N. A., Aghajanirefah A., Matarese F., et al. (2014). Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345 : 1251086 . 10.1126/science.1251086 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sanchez-Ramon S., Conejero L., Netea M. G., Sancho D., Palomares O., Subiza J. L. (2018). Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front. Immunol. 9 : 2936 . 10.3389/fimmu.2018.02936 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sanchez-Schmitz G., Stevens C. R., Bettencourt I. A., Flynn P. J., Schmitz-Abe K., Metser G., et al. (2018). Microphysiologic human tissue constructs reproduce autologous age-specific BCG and HBV primary immunization in vitro. Front. Immunol. 9 : 2634 . 10.3389/fimmu.2018.02634 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saroha M., Faridi M. M., Batra P., Kaur I., Dewan D. K. (2015). Immunogenicity and safety of early vs delayed BCG vaccination in moderately preterm (31-33 weeks) infants. Hum. Vaccin Immunother. 11 2864–2871. 10.1080/21645515.2015.1074361 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schaltz-Buchholzer F., Bjerregaard-Andersen M., Oland C. B., Golding C., Stjernholm E. B., Monteiro I., et al. (2019). Early vaccination with BCG-denmark or BCG-Japan versus BCG-russia to healthy newborns in guinea-bissau: a randomized controlled trial. Clin. Infect. Dis. [Epub ahead of print]. [ PubMed ] [ Google Scholar ]
- Schaltz-Buchholzer F., Frankel H. N., Benn C. S. (2017). The real-life number of neonatal doses of bacille calmette-guerin vaccine in a 20-dose vial. Glob Health Action 10 1–4. 10.1080/16549716.2017.1267964 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scheid A., Borriello F., Pietrasanta C., Christou H., Diray-Arce J., Pettengill M. A., et al. (2018). Adjuvant effect of bacille calmette-guerin on hepatitis B vaccine immunogenicity in the preterm and term newborn. Front. Immunol. 9 : 29 . 10.3389/fimmu.2018.00029 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schoenen H., Bodendorfer B., Hitchens K., Manzanero S., Werninghaus K., Nimmerjahn F., et al. (2010). Cutting edge: mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 184 2756–2760. 10.4049/jimmunol.0904013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shann F. (2015). Editorial commentary: different strains of bacillus calmette-guerin vaccine have very different effects on tuberculosis and on unrelated infections. Clin. Infect. Dis. 61 960–962. 10.1093/cid/civ454 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shey M. S., Nemes E., Whatney W., de Kock M., Africa H., Barnard C., et al. (2014). Maturation of innate responses to mycobacteria over the first nine months of life. J. Immunol. 192 4833–4843. 10.4049/jimmunol.1400062 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shirakawa T., Enomoto T., Shimazu S., Hopkin J. M. (1997). The inverse association between tuberculin responses and atopic disorder. Science 275 77–79. 10.1126/science.275.5296.77 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stensballe L. G., Nante E., Jensen I. P., Kofoed P. E., Poulsen A., Jensen H., et al. (2005). Acute lower respiratory tract infections and respiratory syncytial virus in infants in guinea-bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine 23 1251–1257. 10.1016/j.vaccine.2004.09.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Storgaard L., Rodrigues A., Martins C., Nielsen B. U., Ravn H., Benn C. S., et al. (2015). Development of BCG Scar and subsequent morbidity and mortality in rural guinea-bissau. Clin. Infect. Dis. 61 950–959. 10.1093/cid/civ452 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stover C. K., Bansal G. P., Hanson M. S., Burlein J. E., Palaszynski S. R., Young J. F., et al. (1993). Protective immunity elicited by recombinant bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J. Exp. Med. 178 197–209. 10.1084/jem.178.1.197 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Strunk T., Doherty D., Jacques A., Simmer K., Richmond P., Kohan R., et al. (2012). Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 129 e134–e141. 10.1542/peds.2010-3493 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Szeliga J., Daniel D. S., Yang C. H., Sever-Chroneos Z., Jagannath C., Chroneos Z. C. (2008). Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinb) 88 7–20. 10.1016/j.tube.2007.08.009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tameris M. D., Hatherill M., Landry B. S., Scriba T. J., Snowden M. A., Lockhart S., et al. (2013). Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381 1021–1028. 10.1016/S0140-6736(13)60177-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tang J., Sun M., Shi G., Xu Y., Han Y., Li X., et al. (2017). Toll-like receptor 8 agonist strengthens the protective efficacy of ESAT-6 immunization to Mycobacterium tuberculosis infection. Front. Immunol. 8 : 1972 . 10.3389/fimmu.2017.01972 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Topfer E., Boraschi D., Italiani P. (2015). Innate immune memory: the latest frontier of adjuvanticity. J. Immunol. Res. 2015 : 478408 . 10.1155/2015/478408 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Trunz B. B., Fine P., Dye C. (2006). Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367 1173–1180. 10.1016/S0140-6736(06)68507-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tuberculosis Research Centre. (2013). Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Tuberculosis research centre. Indian. J. Med. Res. 110 56–69. [ PubMed ] [ Google Scholar ]
- Tzelepis F., Blagih J., Khan N., Gillard J., Mendonca L., Roy D. G., et al. (2018). Mitochondrial cyclophilin D regulates T cell metabolic responses and disease tolerance to tuberculosis. Sci. Immunol. 3 : eaar4135 10.1126/sciimmunol.aar4135 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ugolini M., Gerhard J., Burkert S., Jensen K. J., Georg P., Ebner F., et al. (2018). Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat. Immunol. 19 386–396. 10.1038/s41590-018-0068-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- UNICEF. (2015). BCG Vaccine: Current Supply & Demand Outlook. New York, NY: UNICEF. [ Google Scholar ]
- Uno-Furuta S., Matsuo K., Tamaki S., Takamura S., Kamei A., Kuromatsu I., et al. (2003). Immunization with recombinant calmette-guerin bacillus (BCG)-hepatitis C virus (HCV) elicits HCV-specific cytotoxic T lymphocytes in mice. Vaccine 21 3149–3156. 10.1016/s0264-410x(03)00256-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van den Biggelaar A. H., Prescott S. L., Roponen M., Nadal-Sims M. A., Devitt C. J., Phuanukoonnon S., et al. (2009). Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. J. Allergy Clin. Immunol. 124 544–550, 550.e1-2. 10.1016/j.jaci.2009.03.040 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van ’t Wout J. W., Poell R., van Furth R. (1992). The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 36 713–719. [ PubMed ] [ Google Scholar ]
- Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324 1029–1033. 10.1126/science.1160809 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vekemans J., Ota M. O., Sillah J., Fielding K., Alderson M. R., Skeiky Y. A., et al. (2004). Immune responses to mycobacterial antigens in the Gambian population: implications for vaccines and immunodiagnostic test design. Infect. Immun. 72 381–388. 10.1128/iai.72.1.381-388.2004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venkataswamy M. M., Goldberg M. F., Baena A., Chan J., Jacobs W. R., Jr., et al. (2012). In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine 30 1038–1049. 10.1016/j.vaccine.2011.12.044 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Whittaker E., Goldblatt D., McIntyre P., Levy O. (2018a). Neonatal immunization: rationale, current state, and future prospects. Front. Immunol. 9 : 532 . 10.3389/fimmu.2018.00532 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Whittaker E., Nicol M. P., Zar H. J., Tena-Coki N. G., Kampmann B. (2018b). Age-related waning of immune responses to BCG in healthy children supports the need for a booster dose of BCG in TB endemic countries. Sci. Rep. 8 : 15309 . 10.1038/s41598-018-33499-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health, and Organization (2004). BCG vaccine. WHO position paper. Wkly Epidemiol. Rec. 79 27–38. [ PubMed ] [ Google Scholar ]
- Wu B., Huang C., Garcia L., Ponce, de Leon A., Osornio J. S., et al. (2007). Unique gene expression profiles in infants vaccinated with different strains of Mycobacterium bovis bacille Calmette-Guerin. Infect. Immun. 75 3658–3664. 10.1128/iai.00244-07 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn J. L., Scumpia P. O., Winfield R. D., Delano M. J., Kelly-Scumpia K., Barker T., et al. (2008). Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112 1750–1758. 10.1182/blood-2008-01-130500 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yadav M., Schorey J. S. (2006). The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108 3168–3175. 10.1182/blood-2006-05-024406 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang J. P., Yang Y., Levy O., Chen C. (2010). Human neonatal peripheral blood leukocytes demonstrate pathogen-specific coordinate expression of TLR2, TLR4/MD2, and MyD88 during bacterial infection in vivo. Pediatr. Res. 68 479–483. 10.1203/PDR.0b013e3181f90810 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhu Y. D., Fennelly G., Miller C., Tarara R., Saxe I., Bloom B., et al. (1997). Recombinant bacille Calmette-Guerin expressing the measles virus nucleoprotein protects infant rhesus macaques from measles virus pneumonia. J. Infect. Dis. 176 1445–1453. 10.1086/514140 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zimmermann N., Thormann V., Hu B., Kohler A. B., Imai-Matsushima A., Locht C., et al. (2016). Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis . EMBO Mol. Med. 8 1325–1339. 10.15252/emmm.201606330 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zimmermann P., Donath S., Perrett K. P., Messina N. L., Ritz N., Netea M. G., et al. (2019). The influence of neonatal bacille calmette-guerin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine 37 3735–3744. 10.1016/j.vaccine.2019.03.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zufferey C., Germano S., Dutta B., Ritz N., Curtis N. (2013). The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNgamma response in Bacille Calmette-Guerin (BCG)-immunized infants. PLoS One 8 : e77334 . 10.1371/journal.pone.0077334 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zwerling A., Behr M. A., Verma A., Brewer T. F., Menzies D., Pai M. (2011). The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 8 : e1001012 . 10.1371/journal.pmed.1001012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Partner Sites

Inspiring and informing your business school journey
Case study interview | tips from bain, bcg & mckinsey.

Bain, BCG, or McKinsey, the case study interview is a key part of the consulting interview process ©Philip McMaster
If you want to work in consulting, you’ll have to sit a case study interview. Here’s everything you need to know about the case study interview with tips from experts at Bain, BCG, and McKinsey

Mon Oct 11 2021
The consulting case study interview might just be one part of the application process, but it’s where you can stand out to your prospective employer and show you’ve got what it takes to crack cases day in day out.
Whether you have your eyes set on Bain, BCG, or McKinsey, or a smaller boutique consulting firm , here’s everything you need to know about the case study interview:
What is the consulting case study interview?
The consulting case study interview requires you to solve a simulated problem for a client. There are two types of case interview: The interviewer-led approach and the candidate-led approach.
“We use a client-like problem such as how to reduce a carbon footprint, how to make a workforce more diverse, how to leverage technology, or how to grow a customer base, and ask you three questions that simulate the kind of problem-solving our teams do in a client engagement,” says Amy Ross ( pictured) , senior expert in McKinsey’s global assessment team based in New York.
The three questions aren’t in a set order, but you can expect to focus on identifying the issues, then doing analysis based on information the team collects (that McKinsey provide), then coming up with insights and developing a conclusion. The McKinsey case study interview is consistent across global offices.
The interviewer-led approach of the McKinsey case interview means you’ll be guided through the process by your interviewer. However, there’s a caveat, explains Eugene Goh, the cofounder of HR tech startup, HireQuotient, who worked as a principal for BCG for more than eight years.
“They’re expecting a lot more detail and depth,” says Eugene, who’s recently cowritten From the Interviewer’s Seat: The Insider’s Guide for Aspiring Consultants , a book on the consulting interview.
Candidate-led approach
The candidate-led BCG case study interview will similarly present you with a real BCG case from previous client work. You’ll then be presented with the client’s challenges and have 45 minutes to walk your interviewer through your solution, rather than being led question by question. The BCG case study requires you to build your solution step by step.
“They’re looking for people who can structure their approach from end to end,” Eugene explains.
How can you stand out in your case study interview?
The case interview is supposed to simulate the problem-solving approach of the firm you’re interviewing with, which at McKinsey involves a lot of back-and-forth between team members, says Amy. You'll need to tackle case study interview questions that mimic a real consulting case.
“Candidates should listen to the client context and think about what it means, rather than repeat back everything the interviewer says.
“If the case covers an industry that is unfamiliar, candidates might consider whether there is an analogous industry they are familiar with and see if that helps them think of good ideas.
You should ask questions to clarify your understanding of the data and the issues at hand. Amy advises that you take a moment before speaking to collect your thoughts.
“Listen carefully, making sure you consider the information provided and the meaning behind the specific questions. You’ll stand out by putting the client front-and-center and by sharing interesting insights.”
The Bain case study, like the BCG case study, is often candidate-led. To stand out, your interviewers are looking for your analytical skills; the ability to break down challenging problems into parts you can tackle in a sequence; strong communication skills; the ability to simplify complex concepts; teamwork; and the ability to work successfully among others. That's according to Keith Bevans, head of global consultant recruitment at Bain & Company.
He explains that tackling a Bain case study is akin to playing in a jazz quartet. There’s no script, so you’re going to play a bit, and improvise. Improvisation is a key thing interviewers are looking for when you're solving a Bain case study.
“I think some students want to be perfect and play classical music and don’t want to share their insight or preliminary analysis until it’s right," says Keith. "The truth is I need them to share because what they share may not be perfect, but it’ll spark something in somebody else. I need students who are comfortable in that sort of environment.”
That's why MBA and business master's graduates are so well placed to enter consulting. They develop the skills that the Big Three consulting firms are looking for through live consulting projects, and constant group work that tests their ability to manage and lead diverse teams of peers to solve complex business problems.
Skills that will help you stand out
How to prepare for your case study interview?
The best thing you can do ahead of your consulting case study interview is prepare. Make sure you work with real case study interview examples.
Your business school will likely have a consulting club that offers consulting case study prep sessions, which will give you ample opportunity to work through mock case study interview questions. You may also have on hand a network of business school alumni who likely work in consulting, as well as professors—use them.
Amy from McKinsey recommends going to the website of the firm you’re applying to—McKinsey have case interview examples you can use to brush up on your casing. That way, you’ll know what to expect when you face your case study interview questions in real time.
She adds that there are many coaches and preparation services available to candidates, but the firm doesn’t expect you to use them.
“Frankly, we are worried there’s a lot of misleading advice out there so, again, we advise to consult our website, and feel free to ask your recruiter to arrange for you to meet one of our consultants who can be your interview coach,” she asserts.
Approach the case study interview like a McKinsey consultant
→ Make sure you understand the information provided.
→ Ask questions.
→ Collect yourself before diving into your answer.
→ Alongside logical ideas, challenge yourself to provide a few that are more ‘out there’, things you and a client team would want to test first. Sometimes, those bolder ideas are the best ones and often they really show McKinsey how you think.
Beware though, as you can see too many case study interview examples and overprepare. Angela Michalik, MBA recruiter at BCG, says that to avoid being overprepared focus on the quality not quantity of your prep.
Do one case, then get feedback, she says. At the end you should know where you were weak, and then in your next case go hard on the areas in which you’re weakest.
“I feel by doing that, students really improve,” she says. “You have to reflect on what you need to work on and practice.”
If you notice that you’re going straight into applying a framework to a case before you take the time to think, you might be overpreparing.
“Each client problem is different and deserves an initial approach that meets their needs. We realize interviews are filled with uncertainty, but the link between preparation and success in our process is not so strong,” Amy from McKinsey notes.
“Practice enough so you know what to expect, stay current with what’s new in the business world, and then bring curiosity and an open mind to your case interview.”
READ: Bain, BCG, McKinsey: How To Get Hired By The Big Three Consulting Firms
Granted, you need to be ready for ambiguity, something that can be hard to prepare for. But there’s a step-by-step approach Eugene says can help break away from the fixed framework approach:
- Define the objectives. What are you trying to do? Understand a bit about the client’s constraints. What’s the timeframe for the project, their budget, for example.
- Dive into the diagnosis. Why does the client have a problem?
- Option generation. What approaches could the client take?
- Option evaluation before coming to a decision
“It sounds generic but almost every problem, even in the real world, requires you to roughly go through those steps,” Eugene ( pictured ) says.
“That is a more helpful approach as it applies to all problems, therefore all cases.”
Case study interview prep is a key component of your overall application. But don’t stress. Focus on the quality of your preparation and lean on your business school network of MBA alumni in the consulting sector, as well as the resources available from your school’s consulting club.
Run through case interview examples from the firm’s you’re interested in and breathe before you approach a problem. That way, you’ll be best placed to ace your interview and launch your post-business school career as a consultant.
Where Do McKinsey, Bain, BCG Hire The Most MBAs?
Main image in this article is credited to Philip McMaster and used under this license
- MBB Consulting
You might like:

Colombia to Dubai: How This Asia School of Business Graduate Landed A Job At Kraft

Careers In Finance: How I Used My MBA To Land A Role At S&P Global

Sustainability in Business: How MBA Students Are Benefiting From China's Green Revolution
Bentley University strives to make web content accessible to all users. If you are having difficulty accessing information and need this content in an alternate format, please contact The Pulsifer Undergraduate Career Development Center at 781-891-2165 or [email protected] .

- What is a Career Community?
- Analytics/Actuarial
- Healthcare/Biotech/Pharma
- MarComm / Media
- Non-Profit/Public Policy
- Professional Sales
- Real Estate
- Sustainability
- African American/Black/Brown Students
- Asian Students
- First Generation Students
- First-Year & Sophomore Community
- Hispanic/Latinx Students
- International Students
- Students with Disabilities
- Alumni on Linkedin
- LinkedIn Online Course
- LinkedIn Learning
- Credit Internship Information
- Featured Jobs
- Micro-Internships (Pangea)
- Micro-Internships (Parker Dewey)
- Hire Our Students
- Career Closet
- Academic Resources
- Big Interview Mock Interview System
- Booking an Interview Room
- Career Planning
- Dress for Success Guide
- General Resources
- Graduate and Law School Information
- Interstride
- Job Postings & Career Planning
- Professional Associations and Organizations
- Retrieve Your Unofficial Transcript/Academic Record
- Student Organizations
- Using AI in Career Development
- Vault Guides
- Vmock Smart Editor Guide
- Your Major by the Numbers – 2023 Placement Data
- How HIRE Education Works
- Career Design Seminars (CDI 101, 201, 301)
- The Board of HIRE Education
- 2023 Student Outcomes
- Accountancy Majors
- Actuarial Science Majors
- Business Economics Majors
- Computer Information Systems Majors
- Corporate Finance & Accounting Majors
- Data Analytics Majors
- Economics-Finance Majors
- Finance Majors
- Management Majors
- Marketing, Communications, & Media Majors
- Our Services
- Employment Statistics
- Student Colleagues
- Contact Us!
Students Should Utilize BCG’s Interactive Case Library to Practice Case Interviews!
- Share This: Share Students Should Utilize BCG’s Interactive Case Library to Practice Case Interviews! on Facebook Share Students Should Utilize BCG’s Interactive Case Library to Practice Case Interviews! on LinkedIn Share Students Should Utilize BCG’s Interactive Case Library to Practice Case Interviews! on X
The Boston Consulting Group website has an amazing Interactive Case Library that students can use to practice case interviews. This tool allows you to select a sample case (I tested an Airline Case) and then a fictional interviewer starts the mock interview with you. You first receive background information about the client and then will work through multiple screens where you are asked a question (Ex. What factors should we explore for their impact on our client’s profitability?) and are provided with anywhere from two to five options to select as your response. At some points, you are asked to complete calculations or analyze graphs and it does a really nice job of mimicking a real case interview.
When you finish the case, you will see a short recap and a summary of your results across 4 areas (Rigor, Structuring, Business Judgment and Synthesis). In addition, you will see how you compare to the Average and the 90th Percentile. Enjoy!

ul]:list-disc [&>ul]:pl-10">Digital Strategy Case Study
Want to know what to expect from a digital strategy case challenge when applying to Boston Consulting Group? Try this example, and incorporate the tips presented into your approach.
Note: BCG is not tracking or scoring candidate responses.

ul]:list-disc [&>ul]:pl-10">We use cookies (and other similar technologies) to collect data to improve your experience on our site. You’re agreeing to the collection of data as described in our terms and conditions by using our website. ul]:list-disc [&>ul]:pl-10">I accept
ul]:list-disc [&>ul]:pl-10">Change your preferences
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
By WIRED Brand Lab
A Leader in AI: Boston Consulting Group

For nearly 60 years, Boston Consulting Group (BCG) has been recognized as one of the world’s most prestigious strategy and technology consulting firms—and with good reason. Driven by its core purpose—unlocking the potential of those who advance the world—and fueled by some of the brightest minds in the industry, BCG has helped countless businesses realize their digital ambitions by harnessing the combined power of new technologies like artificial intelligence and human capabilities to drive growth, innovation, transformation and change. But sometimes, even the transformation consultants need to transform a little themselves.
That’s why BCG is partnering with the team at WIRED, one of the nation’s foremost technology publications, to help tell the story of how artificial intelligence is not only affecting the world right now, but how it promises to shape our lives for years to come.
“The work that we do—helping businesses strike the right partnership between human and tech to unlock their full potential—affects how people, processes, culture, and strategy come together for a company but also society,” said Vladimir Lukic, Managing Director and Partner at BCG. “There’s a broader context to what we do and that’s the story we want to tell.”
Together, WIRED and BCG are proud to present a new content database —devoted entirely to artificial intelligence—that provides that context. Built for business leaders, AI enthusiasts, and the curious minded, this database is designed to be a key resource in how both businesses and the public alike understand the real-world implications of AI—and how to plan for its future.
The new, searchable database blends the best of WIRED’s AI editorial content with BCG’s wide variety of use cases, sourced from years of experience in building and implementing practical AI solutions for organizations of all shapes and sizes. Behind a team of more than 1,300 data scientists and AI experts, BCG has built a deep history of these impactful case studies because that caliber of work has been its primary focus. BCG isn’t just an AI thought leader—it’s an AI impact leader .
The WIRED team has long focused on the intersection of technology and humanity—how our species crafts new and innovative technologies, and in turn how those technologies shape the lives we live. BCG shares this ambition, focusing on the importance of “bionic” companies: organizations that seek to combine the strengths of people and technology to create and scale extraordinary opportunities. The story of this partnership is fundamentally a human one—because humans are a fundamental part of AI’s future.
Now is the right time to explore this story with you. AI’s capabilities are scaling exponentially, but more importantly, the COVID-19 pandemic has forced a new kind of global digital transformation for businesses and individuals alike. All of us are interfacing with AI solutions more than ever before—and that means we must recognize their impact accordingly.
BCG understands commercial AI solutions better than anyone. WIRED understands the technology enthusiast better than anyone. Together, we’re ready to share not just our stories, but your stories—humankind’s stories. BCG has been an integral part of AI’s past and present.
Now we’ll all be a central part of its future.

Megan Farokhmanesh
Will Knight

Amanda Hoover

Reece Rogers

Steven Levy


Bewerbung und Interviews bei BCG: How to join the Group.
Der bewerbungs- und interviewprozess bei bcg, deine bewerbung: a wie anschreiben bis z wie zeugnisse.
- Stell dich kurz vor und sag uns, was deine Motivation ist, dich bei BCG zu bewerben.
- Sei authentisch und erzähl uns von deinen beruflichen Vorstellungen und Wünschen.
- Verzichte auf Standardfloskeln – wir wünschen uns eine persönliche Bewerbung von dir
- Fasse dich kurz (max. eine Seite) und achte auf eine klare Struktur.
- Mach uns neugierig: Was suchst du? Worauf dürfen wir uns freuen? Warum die Group?
- Erwähne gerne, wenn wir uns schon mal bei einem Event oder Vortrag kennengelernt haben.
Dein Vorstellungsgespräch bei BCG
First round, second round, du bist bereit für deinen job bei bcg , tipps für dein case interview, dein kontakt zu unserem recruiting team.
- Around 90% of participants improved their performance when using GenAI for creative ideation. People did best when they did not attempt to edit GPT-4’s output.
- When working on business problem solving, a task outside the tool’s current competence, many participants took GPT-4's misleading output at face value. Their performance was 23% worse than those who didn’t use the tool at all.
- Adopting generative AI is a massive change management effort. The job of the leader is to help people use the new technology in the right way, for the right tasks and to continually adjust and adapt in the face of GenAI’s ever-expanding frontier.
Subscribe to our Artificial Intelligence E-Alert.

Generative AI
/ article, how people can create—and destroy—value with generative ai.
By François Candelon , Lisa Krayer , Saran Rajendran , and David Zuluaga Martínez
Key Takeaways
Generative AI will be a powerful enabler of competitive advantage for companies that crack the code of adoption. In a first-of-its-kind scientific experiment, we found that when GenAI is used in the right way, and for the right tasks, its capabilities are such that people’s efforts to improve the quality of its output can backfire. But it isn’t obvious when the new technology is (or is not) a good fit, and the persuasive abilities of the tool make it hard to spot a mismatch. This can have serious consequences: When it is used in the wrong way, for the wrong tasks, generative AI can cause significant value destruction.
We conducted our experiment with the support of a group of scholars from Harvard Business School, MIT Sloan School of Management, the Wharton School at the University of Pennsylvania, and the University of Warwick. 1 1 We designed the study with input from Professor Karim R. Lakhani, Dr. Fabrizio Dell’Acqua, and Professor Edward McFowland III of Harvard Business School; Professor Ethan R. Mollick of the Wharton School at the University of Pennsylvania; Professor Hila Lifshitz-Assaf at the University of Warwick; and Professor Katherine C. Kellogg at the MIT Sloan School of Management. Our academic colleagues analyzed our data. Please see our scholarly paper for more details. Notes: 1 We designed the study with input from Professor Karim R. Lakhani, Dr. Fabrizio Dell’Acqua, and Professor Edward McFowland III of Harvard Business School; Professor Ethan R. Mollick of the Wharton School at the University of Pennsylvania; Professor Hila Lifshitz-Assaf at the University of Warwick; and Professor Katherine C. Kellogg at the MIT Sloan School of Management. Our academic colleagues analyzed our data. Please see our scholarly paper for more details. With more than 750 BCG consultants worldwide as subjects, it is the first study to test the use of generative AI in a professional-services setting—through tasks that reflect what employees do every day. The findings have critical implications across industries.
The opportunity to boost performance is astonishing: When using generative AI (in our experiment, OpenAI’s GPT-4) for creative product innovation, a task involving ideation and content creation, around 90% of our participants improved their performance. What’s more, they converged on a level of performance that was 40% higher than that of those working on the same task without GPT-4. People best captured this upside when they did not attempt to improve the output that the technology generated.
Creative ideation sits firmly within GenAI’s current frontier of competence. When our participants used the technology for business problem solving, a capability outside this frontier, they performed 23% worse than those doing the task without GPT-4. And even participants who were warned about the possibility of wrong answers from the tool did not challenge its output.
Our findings describe a paradox: People seem to mistrust the technology in areas where it can contribute massive value and to trust it too much in areas where the technology isn’t competent. This is concerning on its own. But we also found that even if organizations change these behaviors, leaders must watch for other potential pitfalls: Our study shows that the technology’s relatively uniform output can reduce a group’s diversity of thought by 41%.
The precise magnitude of the effects we uncovered will be different in other settings. But our findings point to a crucial decision-making moment for leaders across industries. They need to think critically about the work their organization does and which tasks can benefit from or be damaged by generative AI. They need to approach its adoption as a change management effort spanning data infrastructure, rigorous testing and experimentation, and an overhaul of existing talent strategies. Perhaps most important, leaders need to continually revisit their decisions as the frontier of GenAI’s competence advances.
The Value at Stake
Our findings make clear that generative AI adoption is a double-edged sword. In our experiment, participants using GPT-4 for creative product innovation outperformed the control group (those who completed the task without using GPT-4) by 40%. But for business problem solving, using GPT-4 resulted in performance that was 23% lower than that of the control group. (See Exhibit 1.)
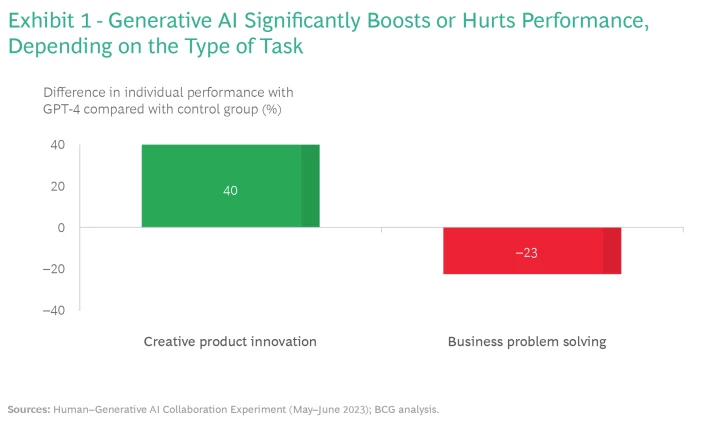
The creative product innovation task asked participants to come up with ideas for new products and go-to-market plans. The business problem-solving task asked participants to identify the root cause of a company’s challenges based on performance data and interviews with executives. (See “Our Experiment Design and Methodology.”) Perhaps somewhat counterintuitively, current GenAI models tend to do better on the first type of task; it is easier for LLMs to come up with creative, novel, or useful ideas based on the vast amounts of data on which they have been trained. Where there’s more room for error is when LLMs are asked to weigh nuanced qualitative and quantitative data to answer a complex question. Given this shortcoming, we as researchers knew that GPT-4 was likely to mislead participants if they relied completely on the tool, and not also on their own judgment, to arrive at the solution to the business problem-solving task (this task had a “right” answer).
Our Experiment Design and Methodology
Task design.
- You are working for a footwear company in the unit developing new products. Generate ideas for a new shoe aimed at a specific market or sport that is underserved. Be creative and give at least ten ideas.
- Come up with a list of steps needed to launch the product. Be concise but comprehensive.
- Use your best knowledge to segment the footwear market by users. Develop a marketing slogan for each segment you are targeting.
- Suggest three ways of testing whether your marketing slogan works well with the customers you have identified.
- Write marketing copy for a press release of the product.
- Using this information, if the CEO must pick one brand to focus on and invest in to drive revenue growth in the company, what brand should that be? What is the rationale for this choice? Please support your views with data and/or interview quotations.
Measuring Baseline Proficiency
Grading rubric, experimental treatment design.
- Group A: Those who used GPT-4 to solve the task after a 30-minute training on best practices on GPT-4 use (see the sidebar on training).
- Group B: Those who used GPT-4 to solve the task without any training.
- Group C: Those who did not use GPT-4 to solve the task (control group).
Incentive Structure
Grading methodologies.
We also knew that participants were capable of finding the answer to the business problem-solving task on their own: 85% of participants in the control group did so. Yet many participants who used GPT-4 for this task accepted the tool’s erroneous output at face value. It’s likely that GPT-4’s ability to generate persuasive content contributed to this result. In our informal conversations with participants, many confirmed that they found the rationale GPT-4 offered for its output very convincing (even though as an LLM, it came up with the rationale after the recommendation, rather than creating the recommendation on the basis of the rationale).
The double-edged-sword effect holds across all levels of baseline proficiency. (At the start of the experiment, participants completed a baseline task without using GPT-4 that we then graded and ranked; see the sidebar on our design and methodology). This has an important caveat: The lower the individual’s baseline proficiency, the more significant the effect tended to be; for the creative product innovation task, these individuals boosted performance by 43%. Still, the effect was material even for the top-ranked baseline performers, among whom the upside and downside of using GPT-4 on the two tasks were 17% and -17%, respectively. (See Exhibit 2.) (Throughout, our discussion of participants’ performance is not indicative of their absolute levels of competence and talents with respect to these or other tasks.)

The strong connection between performance and the context in which generative AI is used raises an important question about training: Can the risk of value destruction be mitigated by helping people understand how well-suited the technology is for a given task? It would be rational to assume that if participants knew the limitations of GPT-4, they would know not to use it, or would use it differently, in those situations.
Our findings suggest that it may not be that simple. The negative effects of GPT-4 on the business problem-solving task did not disappear when subjects were given an overview of how to prompt GPT-4 and of the technology’s limitations. (See “Our Use of Training in the Experiment.”)
Our Use of Training in the Experiment
Even more puzzling, they did considerably worse on average than those who were not offered this simple training before using GPT-4 for the same task. (See Exhibit 3.) This result does not imply that all training is ineffective. But it has led us to consider whether this effect was the result of participants’ overconfidence in their own abilities to use GPT-4—precisely because they’d been trained.

New Opportunities for Human Talent
Effects at the group level, like the ones discussed above, aren’t necessarily indicative of how generative AI impacts individuals. When we look behind the averages, we find that the use of GPT-4 has two distinct effects on individual performance distribution. (See Exhibit 4.) First, the entire distribution shifts to the right, toward higher levels of performance. This underscores the fact that the 40% performance boost discussed above is not a function of “positive” outliers. Nearly all participants (around 90%), irrespective of their baseline proficiency, produced higher-quality results when using GPT-4 for the creative product innovation task. Second, the variance in performance is dramatically reduced: A much higher share of our participants performed at or very close to the average level.

In other words, participants with lower baseline proficiency, when given access to generative AI, ended up nearly matching those with higher baseline proficiency. Being more proficient without the aid of technology doesn’t give one much of an edge when everyone can use GPT-4 to perform a creative product innovation task. (See Exhibit 5.) The fact that we observed this effect among our well-educated, high-achieving sample suggests that it may turn out to be even more pronounced in contexts that are more heterogenous, with a wider spread in proficiency.

Digging deeper, we find that because GPT-4 reaches such a high level of performance on the creative product innovation task, it seems that the average person is not able to improve the technology’s output. In fact, human efforts to enhance GPT-4 outputs decrease quality. (See the sidebar on our design and methodology for a description of how we measured quality.) We found that “copy-pasting” GPT-4 output strongly correlated with performance: The more a participant’s final submission in the creative product innovation task departed from GPT-4’s draft, the more likely it was to lag in quality. (See Exhibit 6.) For every 10% increase in divergence from GPT-4’s draft, participants on average dropped in the quality ranking by around 17 percentile points.

It appears that the primary locus of human-driven value creation lies not in enhancing generative AI where it is already great, but in focusing on tasks beyond the frontier of the technology’s core competencies.
Interestingly, we found that most of our participants seemed to grasp this point intuitively. In general, they did not feel threatened by generative AI; rather, they were excited by this change in their roles and embraced the idea of taking on tasks that only humans can do. As one participant observed, “I think there is a lot of value add in what we can do as humans. You need a human to adapt an answer to a business’s context; that process cannot be replaced by AI.” Another noted, “I think it’s an opportunity to do things more efficiently, to stop wasting time on things that are very repetitive and actually focus on what’s important, which is more strategic.”
However, it is worth keeping in mind the population of this study: highly skilled young knowledge workers who are more likely to be able to make this transition easily. Other professionals may feel greater fear or experience more difficulty adapting their role to the new technology.
The Creativity Trap
Even if you use GenAI in the right way, and for the right tasks, our research suggests that there are risks to creativity.

The first risk is a tradeoff between individual performance gains and collective creativity loss. Because GPT-4 provides responses with very similar meaning time and again to the same sorts of prompts, the output provided by participants who used the technology was individually better but collectively repetitive. The diversity of ideas among participants who used GPT-4 for the creative product innovation task was 41% lower compared with the group that did not use the technology. (See Exhibit 7.) People didn’t appreciably add to the diversity of ideas even when they edited GPT-4’s output.

The second risk is drawn from a sample of our interviews with participants. Roughly 70% believe that extensive use of GPT-4 may stifle their creative abilities over time. (See Exhibit 8.) As one participant explained, “Like any technology, people can rely on it too much. GPS helped navigation immensely when it was first released, but today people can’t even drive without a GPS. As people rely on a technology too much, they lose abilities they once had.” Another participant noted, “This [phenomenon] is definitely a concern for me. If I become too reliant on GPT, it will weaken my creativity muscles. This already happened to me during the experiment.” Businesses will need to be mindful of their employees’ perceptions of and attitudes about generative AI , and how those might affect their ability to drive innovation and add value.
We don’t yet have data to confirm our participants’ perceptions; this is a topic for further study. But if employees’ concerns bear out, it could compound the group-level risk. Specifically, the loss of collective diversity of ideas may be exacerbated if employees experience some atrophy of individual creativity.
The Generative AI Change Imperative
Inspired by the findings from our research, we envision a series of questions, challenges, and options that can help business leaders make generative AI adoption a source of differentiation—and, as such, an enabler of sustained competitive advantage.
Data Strategy. Any company that incorporates GenAI can realize significant efficiency gains in areas where the technology is competent. But if multiple firms apply the technology across similar sets of tasks, it can produce a leveling effect among organizations analogous to the pattern observed among participants in our experiment. As a result, one of the keys to differentiation will be the ability to fine-tune generative AI models with large volumes of high-quality, firm-specific data.
This is easier said than done. In our experience, not all companies have the advanced data infrastructure capabilities needed to process their proprietary data. Developing these capabilities has been a key focus of AI transformations, but with the arrival of generative AI, it becomes all the more important: As we have argued elsewhere , the power of GenAI often lies in the identification of unexpected—even counterintuitive—patterns and correlations. To reap these benefits, companies need a comprehensive data pipeline, combined with a renewed focus on developing internal data engineering capabilities.
Roles and Workflows. For tasks that generative AI systems have mastered—which, of course, is an ever-expanding list—people need to radically revise their mindset and their approach to work. Instead of the default assumption that technology creates a helpful first draft that requires revision, people should regard the output as a plausible final draft that they should check against firm-established guardrails but otherwise largely leave as is.
The value at stake lies not only in the promise of greater efficiency but also in the possibility for people to redirect time, energy, and effort away from tasks that generative AI will take over. Employees will be able to double down on the tasks that remain beyond the frontier of this technology, reaching higher levels of proficiency.
Turning the lens on ourselves, we can already envision our employees spending less time manually summarizing research or polishing slides and instead investing even more effort in driving complex change management initiatives. The impact of generative AI’s disruption will of course vary dramatically across job categories. But at least some workers—including the majority of our participants—are confronting this prospect with optimism.
Strategic Workforce Planning. To get the AI–human dynamics right in complex organizations, leaders must grapple with four questions that have no easy answers:
- Which capabilities will you need? As with any other technology, it will take people to define what and how generative AI will be used. But it isn’t obvious which human capabilities are best suited to maximizing the tool’s value or how often these capabilities will change. We’re seeing this uncertainty play out in real time with respect to LLMs: The role of “prompt engineer” didn’t exist a year ago, but demand for this role during Q2 2023 was nearly seven times higher than it was in Q1. 2 2 BCG analysis based on global job postings in the Lightcast (formerly BurningGlass) platform through 8/24/2023. Notes: 2 BCG analysis based on global job postings in the Lightcast (formerly BurningGlass) platform through 8/24/2023. (GPT-4 was launched toward the end of Q1, on March 14, 2023.) And yet, prompt engineers may no longer be needed once generative AI itself has mastered the task of breaking down complex problems into optimal prompts (as it appears it soon will with autonomous agents). Even the selection of optimal LLMs for specific business applications, which is largely done by humans at present, may in the future be outsourced to AI systems themselves.
- What is your hiring strategy? Because generative AI is a great leveler of proficiency on certain tasks, raw talent may not be a good predictor of high performance in a world of widespread GenAI use. For example, some people may have lower baseline proficiency for a type of task while being quite capable of partnering with generative AI to outperform peers. Finding these individuals will be an important goal for future talent strategies, but the underlying traits are not yet clearly identified.
How will you train people effectively? As our findings indicate, straightforward training won’t be sufficient. Effective training will likely need to explicitly address any cognitive biases that may lead people to over-rely on generative AI in situations where the technology has not yet reached the right level of competence.
We also see a potentially deeper issue: Even as certain tasks are fully handed over to GenAI, some degree of human oversight will be necessary. How can employees effectively manage the technology for tasks that they themselves have not learned how to do on their own?
- How will you cultivate diversity of thought? Our results suggest that GenAI detracts from collective creativity by limiting the range of perspectives that individuals bring to the table. This loss in diversity of thought may have ripple effects beyond what we can currently envision. One plausible risk is that it could shrink the long-term innovation capacity of organizations—for example, by making ideation more homogenous. It’s a slippery slope, as a decline in innovation capabilities means less differentiation from competitors, which could impede growth potential. The good news is that the ideas that humans generate on their own and the ideas that they generate when assisted by generative AI are vastly different. Setting aside the degree of diversity in each group, when we compared the output of the control and experimental groups, the overlap (semantic similarity) was less than 10%. The key for leaders will be to use both approaches to ideation—which ultimately will create an even wider circle of ideas.
Experimentation and Testing. Generative AI systems continue to develop at a stunning rate: In just the few months between the releases of OpenAI’s GPT-3.5 and GPT-4, the model made huge performance leaps across a wide range of tasks. Tasks for which generative AI is ill-suited today will likely fall within its frontier of competence soon—perhaps in the very near future. This is likely to happen as LLMs become multi-modal (going beyond text to include other formats of data), or as models grow larger, both of which increase the likelihood of unpredictable capabilities.
Given this lack of predictability, the only way to understand how generative AI will impact your business is to develop experimentation capabilities—to establish a “generative AI lab” of sorts that will enable you to keep pace with an expanding frontier. And as the technology changes, the collaboration model between humans and generative AI will have to change as well. Experimentation may yield some counterintuitive or even uncomfortable findings about your business, but it will also enable you to gain invaluable insights about how the technology can and should be used. We put our feet to the fire with this experiment—and we believe all business leaders should do the same.
Generative AI will likely change much of what we do and how we do it, and it will do so in ways that no one can anticipate. Success in the age of AI will largely depend on an organization’s ability to learn and change faster than it ever has before.
In addition to the collaborators from the academic team listed above, the authors would like to thank Clément Dumas, Gaurav Jha, Leonid Zhukov, Max Männig, and Maxime Courtaux for their helpful comments and suggestions. The authors would also like to thank Lebo Nthoiwa, Patrick Healy, Saud Almutairi, and Steven Randazzo for their efforts interviewing the experiment participants. The authors also thank all their BCG colleagues who volunteered to participate in this experiment.

The BCG Henderson Institute is Boston Consulting Group’s strategy think tank, dedicated to exploring and developing valuable new insights from business, technology, and science by embracing the powerful technology of ideas. The Institute engages leaders in provocative discussion and experimentation to expand the boundaries of business theory and practice and to translate innovative ideas from within and beyond business. For more ideas and inspiration from the Institute, please visit our website and follow us on LinkedIn and X (formerly Twitter) .

Managing Director & Senior Partner; Global Director, BCG Henderson Institute

Project Leader
Washington, DC

Project Leader, BCG Henderson Institute Ambassador
San Francisco - Bay Area

Partner, BCG Henderson Institute Ambassador
ABOUT BOSTON CONSULTING GROUP
Boston Consulting Group partners with leaders in business and society to tackle their most important challenges and capture their greatest opportunities. BCG was the pioneer in business strategy when it was founded in 1963. Today, we work closely with clients to embrace a transformational approach aimed at benefiting all stakeholders—empowering organizations to grow, build sustainable competitive advantage, and drive positive societal impact.
Our diverse, global teams bring deep industry and functional expertise and a range of perspectives that question the status quo and spark change. BCG delivers solutions through leading-edge management consulting, technology and design, and corporate and digital ventures. We work in a uniquely collaborative model across the firm and throughout all levels of the client organization, fueled by the goal of helping our clients thrive and enabling them to make the world a better place.
© Boston Consulting Group 2024. All rights reserved.
For information or permission to reprint, please contact BCG at [email protected] . To find the latest BCG content and register to receive e-alerts on this topic or others, please visit bcg.com . Follow Boston Consulting Group on Facebook and X (formerly Twitter) .
Related Content
What’s Next
Read more insights from BCG’s teams of experts.

Artificial Intelligence
Scaling artificial intelligence can create a massive competitive advantage. Learn how our AI-driven initiatives have helped clients extract value.

Generative artificial intelligence is a form of AI that uses deep learning and GANs for content creation. Learn how it can disrupt or benefit businesses.

Why AI Regulation Is an Opportunity for CEOs
Companies can ensure compliance with new legislation while engaging with regulators to establish effective safeguards that leave room for innovation.

How AI Startups Can Become Trusted Business Transformers
For AI startups, success runs through incumbents, but overcoming incumbent reluctance to partner with startups requires more than the best tech.

Engaging Consumers in a Generative AI World
Large language model-powered virtual assistants are about to get between traditional companies and their customers, forcing executives to make tough choices sooner than expected.

What’s Missing from Your AI Transformation Is a Transformer
Writing in Fortune, BCG’s François Candelon, Rémi Lanne, and Clément Dumas explain why some businesses are turning to AI transformers—they provide access to custom technology and support for talent, training, and change management. But many companies remain hesitant about working with AI startups. “For industry incumbents to get more out of AI, they need to change their mindset and their behaviors to allow for meaningful collaboration with transformers,” the authors write.

IMAGES
VIDEO
COMMENTS
An important step in the interview process for client-facing roles, case interviews are designed to simulate real-world problems faced by client teams, so you'll be able to experience the type of work we do, show off your ability to problem-solve, and demonstrate any technical or specialized skills related to the role for which you're applying.
The remaining slides are intended to familiarize you with the format of the online case. • There are 4 questions which should take you no more than 8 minutes to complete. • In an actual online case, you would have more than 20 questions and 45 minutes. • In an actual online case, as in this example, the screen will be divided into 2 parts.
Case interview examples and sample questions from the leading consulting firms, including McKinsey, BCG, Bain, Deloitte, PWC, Accenture, etc. ... BCG, or Bain, is by studying case interview examples. There are a lot of free sample cases out there, but it's really hard to know where to start. So in this article, we have listed all the best free ...
to a "case study." You will be confronted with a real-life business case. We advise you to prepare by familiarizing yourself with case frameworks and the kind of analysis you will be asked to do in the interview. You can find case examples in this booklet and on our website. SECOND ROUND When you have successfully completed your first-round ...
Five Case Studies of Transformation Excellence. November 03, 2014 By Lars Fæste , Jim Hemerling , Perry Keenan, and Martin Reeves. In a business environment characterized by greater volatility and more frequent disruptions, companies face a clear imperative: they must transform or fall behind. Yet most transformation efforts are highly complex ...
Case control studies in Guinea-Bissau suggest that BCG vaccination and the presence of a scar among BCG-immunized infants was associated with a reduced risk of acute lower respiratory infection (ALRI) compared to unimmunized controls, with the association being stronger for females (Stensballe et al., 2005). In fact, children with ALRI were ∼ ...
m cases Average price per liter 30 40 3 4 10 20 1 2 0 0 F93 F00F94 F95 F97F96 F98 F01F99 F02 F03E The average price per liter has grown at a CAGR of 6.1% p.a. over the last decade. However, this has slowed markedly over the last few years. Average price per liter grew just 0.7% in F02. Avg export price per litre Aust. wine export shipment (m cases)
The McKinsey case study interview is consistent across global offices. The interviewer-led approach of the McKinsey case interview means you'll be guided through the process by your interviewer. However, there's a caveat, explains Eugene Goh, the cofounder of HR tech startup, HireQuotient, who worked as a principal for BCG for more than ...
Share Students Should Utilize BCG's Interactive Case Library to Practice Case Interviews! on Facebook Share Students Should Utilize BCG's Interactive Case Library to Practice Case Interviews! on LinkedIn Share Students Should Utilize BCG's ... 1st and 2nd year student Drop-Ins are available in-person on Monday - Friday from 1:00pm - 3 ...
1 / 10. The Power of Social Business. The Power of Social Business: Lessons from Corporate Engagements with Grameen, a report published by The Boston Consulting Group and Yunus Social Businesses, is based on an analysis of ten social businesses that operate in Bangladesh today and the lessons they offer to social and commercial businesses alike.
To understand the scale and scope of the problem, BCG created a food loss and waste model. (See "Quantifying the Food Waste Challenge.") That work reveals a disturbing upward trend line: BCG projects the volume of food loss and waste will rise 1.9% annually from 2015 to 2030 while the dollar value will rise 1.8%. 3 3 In 2015 dollars.
Climate Case Challenge. Want to know what to expect from a case challenge when applying to Boston Consulting Group? Try this example, and incorporate the tips presented into your approach. Note: BCG is not tracking or scoring candidate responses. You're welcome to go through the case study multiple times. Let's begin.
Digital Strategy Case Study. Want to know what to expect from a digital strategy case challenge when applying to Boston Consulting Group? Try this example, and incorporate the tips presented into your approach. Note: BCG is not tracking or scoring candidate responses. Start quiz. Want to know what to expect from a digital strategy case ...
MBB 101. We're a team of McKinsey, Bain and BCG consultants sharing our knowledge and experiences to boost your chances of landing an offer from all three firms, or any other consulting firm. Our students have a 25% acceptance rate vs the 1% industry average! We teach you everything you need to know about nailing case interviews, from ...
Behind a team of more than 1,300 data scientists and AI experts, BCG has built a deep history of these impactful case studies because that caliber of work has been its primary focus. BCG isn't ...
Improving the Customer and Employee Experience. BCG helped a global telecom company create a digital transformation program that has generated two times the amount of e-commerce traffic and a gain of over $250 million in revenue in one year alone. Learn more about our technology, media, and telecommunications services.
Der Bewerbungs- und Interviewprozess bei BCG. Der Einstellungsprozess ist vor allem eine Sache der Einstellung. Und wir in der Group sind der Überzeugung, dass wir dir weder den Prozess machen noch starren Abläufen folgen wollen, sondern mit dir einen Dialog auf Augenhöhe führen möchten. Wie das genau abläuft, erfährst du hier.
The Business Case for Carbon Capture. September 24, 2019 By Alex Dewar and Bas Sudmeijer. The widespread adoption of carbon capture technology is crucial for meeting the Paris Agreement's goal of limiting the rise in the global temperature to well below 2°C. According to international energy and climate change agencies, the technology offers ...
BCG consultants using AI completed 12.2% more tasks while doing it 25.1% faster. But there is a big catch. ... Moreover, the Harvard and BCG study underscores the importance of a collaborative ...
The BCG matrix on Pitchspot. Plotting growth rates against market share relative to competitors yields the four quadrants of the Growth Share Matrix: Stars, Question Marks, Cash Cows, and Dogs.
The latest insights, ideas, and perspectives from BCG. Explore a cross-section of up-to-date content on the trends shaping the future of business and society. ... We partner with effective organizations and educators to improve student outcomes and learning models—from K-12 through higher education—locally, nationally, and globally ...
A first-of-its-kind scientific experiment finds that people mistrust generative AI in areas where it can contribute tremendous value and trust it too much where the technology isn't competent. Around 90% of participants improved their performance when using GenAI for creative ideation. People did best when they did not attempt to edit GPT-4 ...