Five-Kingdom Classification and the Origin and Evolution of Cells
Cite this chapter.

- Lynn Margulis 4
420 Accesses
32 Citations
6 Altmetric
This chapter will argue that modern biologists, in spite of social pressures and historical precedents, need to replace the traditional two-kingdom animal-plant distinction, which has outlived its usefulness, with a multikingdom classification of living organisms. For reasons discussed below, based on recent discoveries from a variety of disciplines, it seems that Whittaker’s five-kingdom system (Whittaker, 1969) is the most logical and consistent yet devised. Whittaker’s system is expanded below the phylum level and slightly modified on the basis of cell evolutionary considerations; suggestions for its adoption by zoologists, botanists, and microbiologists are made.*
- Fossil Record
- Green Plant
- Cellular Slime Mold
- Microbial Form
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
This is a preview of subscription content, log in via an institution to check access.

Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Alexopoulos, C. J., 1962, Introductory Mycology , 2nd ed., Wiley, New York.
Google Scholar
Altman, P. L., and Dittmer, D. S. (eds.), 1972, Biology Data Book , Federation of American Societies for Experimental Biology, Bethesda, Md.
Arnold, C. A., 1947, Introduction to Paleobotany , McGraw-Hill, New York.
Banks, H. P., 1970a, Major evolutionary events and the geological record of plants, Biol. Rev. 45 :451–454.
Article Google Scholar
Banks, H. P., 1970b, Evolution and Plants of the Past , 170 pp., Wadsworth, Belmont, Calif.
Banks, H. P., 1972, The stratigraphic occurrence of early land plants, Paleontology 15 :365–397.
Bold, H. C., 1967, Morphology of Plants , 2nd ed., Harper and Row, New York and Evanston.
Breed, R. S., Murray, E. G. D., and Smith, N. R., 1957, Bergey’s Manual of DeterminativeBacteria , 7th ed., Balliere, Trudall and Cox, London.
Brock, T. D., 1970, Biology of Microorganisms , Prentice-Hall, Englewood Cliffs, N.J.
Campbell, L. L., and Postgate, J. R., 1965, Classification of the spore forming sulfate reducing bacteria, Bacteriol. Rev. 29 :359–363.
PubMed CAS Google Scholar
Cohen, S. S., 1970, Are/were mitochondria and chloroplasts microorganisms? Am. Scientist 58 :281–289.
Copeland, H. F., 1956, Classification of the Lower Organisms , Pacific Books, Palo Alto, Calif.
Cowan, S. T., 1962, The microbial species—A macromyth? in: Microbial Classification (12th Symposium of the Society for General Microbiology) (G. C. Ainsworth and P. H. A. Sneath, eds.), pp. 433–455, Cambridge University Press, London.
Cronquist, A., 1968, Evolution and Classification of Flowering Plants , Houghton-Mifflin, Boston.
Cronquist, A., 1971, Introductory Botany . 2nd ed., pp. 365–374. Harper & Row, Publ., New York.
Curtis, H., 1968, Biology , Worth, New York.
Dayhoff, M. O., 1972, Atlas of Protein Sequence and Structure , National Biomedical Research Organization, Bethesda, Md.
Dibble, C. E., and Anderson, A. J. O., 1963, Florentine Codex, Earthly Things, 11th BookWhich Telleth of the Different Animals, the Birds, the Fishes: and the Trees and theHerbs; the Metals Resting in the Earth—Tin, Lead, and Still Others; and the DifferentStones , Published by School of American Research and the University of Utah, Santa Fe, N.M.
Dodson, E. O., 1971, The kingdoms of organisms, Syst. Zool. 20 :265–281.
DeLey, J., 1968, Molecular biology and bacterial phylogeny, Evol. Biol. 2 :104–154.
Echlin, P., and Morris, L, 1965, The relationship between blue-green algae and bacteria, Biol.Rev. 40 :143.
Article PubMed CAS Google Scholar
Eglinton, G., and Murphy, M. T., 1969, Organic Geochemistry , Springer-Verlag, New York.
Fritsch, F. E., 1935, The Structure and Reproduction of the Algae , Vol. 1, Cambridge University Press, London.
Glaessner, M. F., 1968, Biological events and the Precambrian time scale, Canad. J. EarthSci. 5 :585–590.
Golubic, S., 1973, The relationship between blue-green algae and carbonate deposition, in: TheBiology of Blue-Green Algae (N. G. Carr and B. A. Whitton, Eds.) University of California Press, p. 439–472.
Grant, V., 1971, Plant Speciation , Columbia University Press, New York.
Greenwood, P. H., Rosen, D. E., Weitzman, S. H., and Myers, G. S., 1966, Phyletic studies of teleostean fishes, with a provisional classification of living forms, Bull. Am. Mus. Nat.Hist. 131 :339–456.
Hale, M. E., Jr., 1967, The Biology of Lichens , Edward Arnold, London.
Honigberg, B. M., Balamuth, W., Bovee, E. C., Corliss, J. O., Godjics, M., Hall, R. D., Kudo, R. R., Levine, N. D., Leoblich, A. R., Jr., Weiser, J., and Wenrich, D. H., 1964, A revised classification of the phylum Protozoa, J. Protozool. 11 :7–20.
Hutchinson, G. E., 1967, Treatise on Limnology , Vol. 2, Wiley, New York.
Hutchinson, J., 1959, The Families oj Flowering Plants , 2nd ed., Vol’. 1: Dicotyledons , Clarendon Press, Oxford.
Hutchinson, J., 1969, Evolution and Phytogeny of Flowering Plants. Dictoyledons: Facts andTheory , Academic Press, London and New York.
International Code of Botanical Nomenclature (F. A. Stafleu, ed.), 1969, Eleventh International Botanical Congress, Seattle.
International Code of Zoological Nomenclature , (N. R. Stoll, R. P. Dollfus, J. Forest, N. D. Riley, C. W. Sabrosky, C. W. Wright, and R. V. Melville, eds.), 1964, XV International Congress of Zoology, London.
Leedale, G., 1974, How many are the kingdoms of organisms? Taxon 23 :37–47.
Lerner, I. M., 1963, Heredity, Evolution and Society , Freeman, San Francisco.
Luykx, P., 1970, Cellular Mechanisms of Chromosome Distribution , Academic Press, New York.
Lwoff, A., and Tournier, M., 1966, Classification of viruses, Ann. Rev. Microbiol. 20 :45–74.
Article CAS Google Scholar
Keeton, W., 1972, Biological Science , 2nd ed., 888 pp., Norton, New York.
Klein, R. M., and Cronquist, A., 1967, A consideration of the evolutionary and taxonomic significance of some biochemical, micromorphological and physiological characters in the Thallophyta, Quart. Rev. Biol. 42 :105–296.
Mandel, M., 1969, New approaches of bacterial taxonomy: Perspective and prospects, Ann.Rev. Microbiol. 23 :239–274.
Margulis, L., 1968, Evolutionary criteria in thallophytes: A radical alternative, Science 161 :1020–1022.
Margulis, L., 1970, Origin of Eukaryotic Cells , Yale University Press, New Haven.
Margulis, L., 1971 a , Whittaker’s five kingdoms: Minor modifications based on considerations of the origins of mitosis, Evolution 25 :242–245.
Margulis, L., 1971 b , Early cell evolution, in: Exobiology (C. Ponnamperuma, ed.), pp. 342–368, North-Holland, Amsterdam.
Margulis, L. 1974 a , The classification of prokaryotes and eukaryotes, in: Handbook ofGenetics (R. C. King, ed.), Chap. 1, Plenum Press, New York.
Margulis, L. 1974 b , On the origin and possible mechanism of colchicine-sensitive mitotic movements, Bio Systems 6 :16–36.
Margulis, L., 1974c, Origin and evolution of the eukaryotic cell, Taxon 23 :225–226.
Mayr, E., 1970, Populations, Species and Evolution , Harvard University Press, Cambridge, Mass.
McLaughlin, P., and Dayhoff, M. O., 1973, Eukaryote evolution: A view based on cytochrome c sequence data, J. Mol. Evol. 2 :99–116.
Morowitz, H. J., 1967, Biological self-replicating systems, Progr. Theoret. Biol. 1 :35–58.
Olive, L. S., 1970, The Mycetozoa: A revised classification, Bot. Rev. 36 :59–89.
Pickett-Heaps, J., 1974, Evolution of mitosis and the eukaryote condition, BioSystems , 6 :37–45.
Romer, A. S., 1968, The Procession of Life (1972 Anchor Books edition), 384 pp., World, Cleveland.
Romer, A. S., 1970, The Vertebrate Body , 4th ed., 452 pp., Saunders, Philadelphia.
Schopf, J. W., 1972, Precambrian Paleobiology, in: Exobiology (C. Ponnamperuma and R. Buvet, eds.), pp. 16–61, North-Holland, Amsterdam.
Schopf, J. W., and Blacic, J. M., 1971, New microorganisms from the Bitter Springs Formation (Late Precambrian) of the north-central Amadeus Basin, Australia, J. Paleontol. 45 :925–961.
Schulthorpe, C. D., 1967, The Biology of Aquatic Vascular Plants , Edward Arnold, London.
Simpson, G. G., 1954, The Meaning of Evolution , Harper and Row, New York.
Simpson, G. G., 1960, The history of life, in: Evolution After Darwin (S. Tax, ed.), pp. 117–180, University of Chicago Press, Chicago.
Simpson, G. G., 1961, Principles of Animal Taxonomy , 247 pp., Columbia University Press, New York.
Simpson, G. G., 1963, Major Features of Evolution , Columbia University Press, New York.
Stafleu et al. (see International Code).
Stanier, R., Douderoff, M., and Adelberg, E., 1970, The Microbial World , 3rd ed., Prentice-Hall, Englewood Cliffs, N.J.
Starr, M. P., and Seidler, R. J., 1971, The Bdellovibrios, Ann. Rev. Microbiol. 25:649–678.
Stoll et al. (see International Code).
Sylvester-Bradley, P., 1971, Carbonaceous chondrites and the prebiological origin of food, in: Molecular Evolution (L. Buvet and C. Ponnamperuma, eds.), pp. 499–504, North-Holland, Amsterdam.
Taylor, F. J. R., 1974, Implications and extensions of the serial endosymbiosis theory of the origin of eukaryotes, Taxon 23 :229–258.
Thomas, C. A., Jr., 1971, The genetic organization of chromosomes, Ann. Rev. Genet. 5 :237–256.
Worcel, A., and Burgi, E., 1972, On the structure of the folded chromosome of E. coli, J. Mol. Biol. 71 :127–138.
CAS Google Scholar
Whitehouse, H. K. L., 1969, Towards an Understanding of the Mechanism of Heredity , 2nd ed., St. Martin’s Press, New York.
Whittaker, E. H., 1969, New concepts of the kingdoms of organisms, Science 163 :150–160.
Younger, K. B., Banerjee, S., Kelleher, J. K., Winston, M., and Margulis, L., 1972, Evidence that the synchronized production of new basal bodies is not associated with DNA synthesis in Stentor coeruleus, J. Cell Sci. 11 :621–637.
Download references
Author information
Authors and affiliations.
Department of Biology, Boston University, Boston, Massachusetts, USA
Lynn Margulis
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Genetics, University of California, Davis, California, USA
Theodosius Dobzhansky
Queens College, Flushing, New York, USA
Max K. Hecht
New York Botanical Garden, Bronx, New York, USA
William C. Steere
Rights and permissions
Reprints and permissions
Copyright information
© 1974 Plenum Press, New York
About this chapter
Margulis, L. (1974). Five-Kingdom Classification and the Origin and Evolution of Cells. In: Dobzhansky, T., Hecht, M.K., Steere, W.C. (eds) Evolutionary Biology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-6944-2_2
Download citation
DOI : https://doi.org/10.1007/978-1-4615-6944-2_2
Publisher Name : Springer, Boston, MA
Print ISBN : 978-1-4615-6946-6
Online ISBN : 978-1-4615-6944-2
eBook Packages : Springer Book Archive
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Search Menu
- Advance articles
- Editor's Choice
- Special Collections
- Author Guidelines
- Submission Site
- Open Access
- Reasons to submit
- About BioScience
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Potentially Offensive Content
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Whittaker's classification of communities and kingdoms, biology during the cold war, the development of whittaker's five-kingdom system, the five-kingdom system and cold war educational reforms, domains and kingdoms, acknowledgments, references cited.
- < Previous
Five Kingdoms, More or Less: Robert Whittaker and the Broad Classification of Organisms
Joel B. Hagen ( [email protected] ) is affiliated with the Biology Department at Radford University, in Radford, Virginia.
- Article contents
- Figures & tables
- Supplementary Data
Joel B. Hagen, Five Kingdoms, More or Less: Robert Whittaker and the Broad Classification of Organisms, BioScience , Volume 62, Issue 1, January 2012, Pages 67–74, https://doi.org/10.1525/bio.2012.62.1.11
- Permissions Icon Permissions
Robert Whittaker's five-kingdom system was a standard feature of biology textbooks during the last two decades of the twentieth century. Even as its popularity began to wane at the end of the century, vestiges of Whittaker's thinking continued to be found in most textbook accounts of biodiversity. Whittaker's early thinking about kingdoms was strongly shaped by his ecological research, but later versions were also heavily influenced by concepts in cell biology. This historical episode provides insights into important intellectual, institutional, and social changes in biology after World War II. Consideration of the history of Whittaker's contributions to the classification of kingdoms also sheds light on the impact of Cold War politics on science education and educational reforms that continue to shape the presentation of biological topics in introductory textbooks today.
During the late twentieth century, Robert Whittaker's five-kingdom system was a standard feature of biology textbooks, serving as an important organizing scheme for discussing biodiversity. Even as its popularity waned at the end of the century, vestiges of Whittaker's thinking continued to be found in textbooks. Beginning with the germ of an idea in 1957, Whittaker significantly revised his concept in a series of articles published during the subsequent decade. He started with a three-kingdom system that challenged the traditional plant–animal dichotomy, quickly proposed an alternative four-kingdom system, and arrived at his well-known five-kingdom system only after a decade of critical reflection. At last, Whittaker had crafted a system that biologists and educators found attractive because it seemed to capture fundamental properties of living organisms. At its roots, the five-kingdom system was an ecological idea, but Whittaker increasingly relied on cell biology—particularly, the distinction between prokaryotes and eukaryotes—as a central organizing principle for later versions of his system. Thus, the five-kingdom system reflected important intellectual developments in biology during the post–World War II era. Equally important, the success of Whittaker's system owed much to changes in the institutional structure of biology and in science education during the Cold War. Although some of Whittaker's ideas eventually fell victim to molecular systematics, cladistics, and other recent biological developments, the persistence of his system testifies to its broad appeal.
Robert Whittaker (1920–1980) was one of the most influential modern ecologists and made important contributions to a wide range of fields ( Westman and Peet 1985 ). Although the five-kingdom system was only a minor part of his work, it reflected two of Whittaker's fundamental interests. The first was the structure and function of communities and ecosystems. Whittaker's early research on biogeochemical cycles was focused on trophic levels, which provided the initial idea for his kingdom system. The second interest was what Whittaker referred to as “broad classification”—classifying communities and kingdoms in a rigorous way ( Whittaker 1948 , 1959 , 1962 , 1972 , 1978 ).
Early in his career, Whittaker became known as one of the critics responsible for overthrowing Frederic Clements' idea that plant communities are highly organized systems comparable to organisms ( Westman and Peet 1985 , Nicolson and McIntosh 2002 , Kohler 2008 ). Clements' organismic idea implied that the boundaries between communities were quite sharp and well defined, but Whittaker's dissertation on the vegetation of the Smoky Mountains demonstrated that populations and communities were independently scattered along environmental gradients ( Whittaker 1948 , 1956 ). Ecotones between communities were usually gradual and ill defined. In his dissertation, Whittaker struggled with his research's implications for classifying communities. The philosophical position that he took was a form of nominalism. Although he believed that populations and species were real, Whittaker argued that communities had only a “low degree of reality” (pp. 168–170); indeed, they were simply names applied by ecologists to areas with similar vegetation ( Whittaker 1948 ). In the field, the ecologist was faced by a multitude of plant populations with broadly overlapping distributions. The task for the ecologist was to analyze these distributions and then impose subdivisions on what was, in fact, a continuum ( Whittaker 1948 ).
The tension between the belief that species are distributed independently and the necessity of classifying vegetation into a coherent system provided a creative spark that drove much of Whittaker's later research ( Whittaker 1962 , 1972 , 1978 ). Although he acknowledged that classifying vegetation always involved a large degree of subjectivity, he hoped that the methods employed by ecologists could be rigorously objective. Achieving this goal led Whittaker to develop mathematically sophisticated methods of ordination ( Whittaker 1978 ) but also to develop simple graphical approaches illustrating how the broad pattern of plant communities could be explained in terms of a few climatic variables ( figure 1 ). Although he was quick to point out that numerous exceptions occurred and that community boundaries could never be precisely predicted by temperature or rainfall, Whittaker claimed that his mosaic diagrams captured the “broad relations of natural communities” ( Whittaker 1970 , pp. 64–65; also see Whittaker 1948 , 1956 ). Modified versions of these graphical representations became standard features in biology and ecology textbooks. Whittaker employed similar diagrams to represent the relationships among kingdoms. Using the two axes of mode of nutrition and cellular organization, Whittaker was able to present a conceptual map of the broad contours of the living world ( figure 2 ). The important point that needs to be stressed is that although Whittaker was drawn into taxonomic controversies over kingdom classifications, his early and enduring ideas about classification were strongly shaped by his experiences studying plant communities as a graduate student.
![5 kingdoms hypothesis Diagram depicting major types of plant communities in relation to temperature and precipitation (in centimeters [cm]). The dotted lines enclose environments where several different community types might exist, depending on variables other than temperature and precipitation. Source: Figure illustrated by John Norton, adapted from Whittaker (1970).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bioscience/62/1/10.1525_bio.2012.62.1.11/1/m_bio.2012.62.1.11-f01.jpeg?Expires=1714511576&Signature=pKRyWdSCV5xIJJYaDsgpoOAqFaW8SZVeb65xmd3OS3IJVgdaosBgytCK2ixccH6tIYhsZEC80JMReMYI44fSgt1wYXsW38OUTHvKEKsULt11usGNuBafylvGN6sHhG0nTA0Yz94x7gGDX8ASQHBi~IkFHAMRj8RaiN0vs-76KgDM6gOWkwkHekbDtYHQnuc-Qs76zoUqnYSNPyv1g5FzDK2Lg2wicpxAMTBUNmo57uIGlE6xRdPDTqqAemIc-3sF4~EpEz-BR-jQUzsUbJys~Xh9pZGf-EdccfouSTLruo5DfezHZM7SVtdBwIeWB51V5eBQINtPTbS8S47zenCiEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Diagram depicting major types of plant communities in relation to temperature and precipitation (in centimeters [cm]). The dotted lines enclose environments where several different community types might exist, depending on variables other than temperature and precipitation. Source: Figure illustrated by John Norton, adapted from Whittaker (1970) .

Whittaker's early four-kingdom system, based on three modes of nutrition and the distinction between unicellular and multicellular body plans. The dotted lines represent groups that include both unicellular and multicellular forms. Source: Reprinted from Robert Whittaker, “On the Broad Classification of Organisms,” Quarterly Review of Biology 34 (1959): 210–226, with permission from University of Chicago Press.
By coincidence, Whittaker (1957) published his first article on kingdoms just a few months before the launch of Sputnik 1, but the success of the five-kingdom system owed much to the Cold War context within which it was created. Biologists eagerly turned to large-scale funding from the National Science Foundation, the Atomic Energy Commission, and other post–World War II federal agencies to support new areas of research. Partly as a result, traditional taxonomy and natural history became marginalized. E. O. Wilson later noted that during the 1960s, “biology spun through a ninety-degree turn in its approaches to life” (p. 225) as many biologists turned away from studying whole organisms and biodiversity in order to focus on cells and molecules ( Wilson 2006 ). This trend away from traditional botany and zoology was evident not only in the rise of molecular biology but also in ecology. In his influential ecology textbook, Eugene Odum (1959) described biology as a layer cake: The slices represented taxonomic divisions such as entomology or ornithology, but the “more basic” and fundamental layers represented disciplines such as genetics, cell biology, and ecology. These broad intellectual changes were reflected institutionally, by traditional botany and zoology departments' increasing consolidation into biology departments or replacement by specialized departments along disciplinary lines that cut across taxonomic divisions. Whittaker's own career tracked these changes. Although much of his research fell within traditional plant ecology, he began his professional career in a research position in which he studied the biogeochemical cycling of radionuclides at the Hanford National Laboratory in central Washington. This ecosystem-level research—conducted within the immediate Cold War context of US nuclear weapons production—strongly focused Whittaker's sights on trophic levels ( Whittaker 1961 ), which provided the intellectual foundation for his initial foray into the classification of kingdoms.
Shortly after leaving Hanford, Whittaker published a brief note in Ecology , arguing that the traditional dichotomy between plants and animals was artificial ( Whittaker 1957 ). According to Whittaker, a better classification would recognize three broad kingdoms based on ecological trophic levels: producers (plants), consumers (animals), and decomposers (fungi and bacteria). He acknowledged that these kingdoms did not correspond very closely with taxonomic groupings in the traditional two kingdoms or with alternative three- and four-kingdom revisions that had been proposed earlier by some taxonomists. Instead, he appealed to a combined ecological and evolutionary justification: All ecological communities, past and present, included producers, consumers, and decomposers. Although these groups were heterogeneous, the three nutritional modes that characterized the trophic levels were conceptually clear cut and represented three “major directions of evolution.” Whittaker argued that recognizing kingdoms by ecological function provided an intellectual coherence that was lacking in systems based on morphological characteristics or speculative phylogenetic relationships.
Not only did Whittaker intend to replace the traditional two-kingdom system, but he also intended to forestall several competing three- and four-kingdom alternatives. In particular, Whittaker took aim at a four-kingdom proposal published a year earlier by Herbert Copeland (1956) . Copeland also criticized the traditional plant–animal dichotomy, but both his approach and his solution were strikingly different from Whittaker's. Primarily interested in “lower organisms,” Copeland proposed a new kingdom, Mychota, to include all prokaryotic organisms and another kingdom, Protoctista, for all eukaryotic organisms that were not plants or animals. The appeal of this system rested on making the plant and animal kingdoms monophyletic and solving the problem of intermediates like Euglena that had been claimed by both botanists and zoologists. Combining the fungi, various algae, protozoans, slime molds, and other organisms that lacked true tissues made the kingdom Protoctista very heterogeneous. Nonetheless, Copeland claimed that this new kingdom was monophyletic because in the distant past, all of its diverse members shared a common ancestor. Copeland placed bacteria, which had traditionally also been included in the plant kingdom, into the kingdom Mychota on the basis of their unique prokaryotic cell structure.
In his book The Classification of Lower Organisms , Copeland (1956) provided a detailed taxonomic system subdividing his new kingdoms into phyla, classes, and orders. He paid considerable attention to important taxonomic issues of nomenclature, priority, stability, and phylogenetic relationships. For example, he provided a long historical account of various taxonomic revisions and group names that led to his new kingdom, Protoctista. Whittaker had little use for these technical taxonomic considerations and argued that kingdoms should correspond primarily to clear-cut ecological distinctions and should serve only secondarily as taxonomic units. Whittaker was particularly critical of Copeland's decision to include the fungi, red and brown algae, and numerous groups of microscopic eukaryotes in kingdom Protoctista. According to Whittaker, “The kingdom Protoctista seems more a product of taxonomic definitions than a grouping of organisms with coherent meaning or common evolutionary theme” ( Whittaker 1957 , p. 536). From Whittaker's perspective, fungi and algae were two very different types of organisms, and it made no sense—ecologically or evolutionarily—to place them in the same kingdom. Imposing order on a complex and chaotic nature required conceptual clarity. From Whittaker's perspective, Copeland's kingdom Protoctista badly failed this criterion.
It would be tempting to portray Copeland as Whittaker's unsuccessful competitor, and, indeed, Whittaker effectively used the taxonomist as a target for criticism. At a time when taxonomy was being marginalized in American biology, Copeland's elaborate taxonomic scheme provided an easy foil for Whittaker's conceptually simpler approach. However, Copeland played at least two important positive roles in the subsequent development of Whittaker's approach to kingdoms. In the late 1950s, Whittaker was relatively unfamiliar with microorganisms, and Copeland's detailed system acted as an important spur to developing Whittaker's later ideas about unicellular life. Second, Copeland's distinction between prokaryotic and eukaryotic cells eventually became a critical part of Whittaker's final five-kingdom system, even though he resisted it for over a decade.
The clash of ideas between Whittaker and Copeland was evident in a long review article published two years later ( Whittaker 1959 ). Analyzing the history of kingdom classification, Whittaker presented a clear overview of several alternative systems. At the same time, he argued forcefully for his own ideas. This didactic approach was one that Whittaker also effectively used in his writing about community classification ( Whittaker 1962 , Westman and Peet 1985 ). In both cases, Whittaker argued that there were no absolute criteria for choosing among competing classification systems but only relative criteria, which included important practical, theoretical, and aesthetic considerations. A successful system needed to be useful and biologically coherent but also needed to provide the “subjective satisfaction” of a well-organized set of categories ( Whittaker 1962 ). Weighing the advantages and disadvantages of alternative systems, Whittaker argued cogently for a new four-kingdom system that he had devised, which included plants, animals, fungi, and a new kingdom that Whittaker called Protista ( figure 2 ).
Throughout the 1959 article, Whittaker contrasted his ecological approach with the taxonomic approach used by Copeland and other biologists who classified kingdoms. First, he continued to argue for “functional” kingdoms that were primarily ecological and only secondarily taxonomic groupings. The idea that kingdoms should be defined in terms of ecological function was the origin of Whittaker's approach to the subject, and in his 1959 article, he tied this idea more explicitly to the ecosystem theory that had rapidly developed during the 1950s ( Hagen 1992 , Golley 1993 , Kingsland 2005 ). Second, Whittaker argued for a classification system that was broadly evolutionary, although not necessarily phylogenetic. Phylogenetic relationships were important but, according to Whittaker, monophyletic grouping needed to be balanced with other important criteria, such as ecological function and cellular organization. In addition, Whittaker resisted a purely phylogenetic basis for classification because he considered many of the phylogenetic claims made by Copeland and other taxonomists to be highly speculative. Before the widespread acceptance of cladistics, which did not occur until the 1970s, Whittaker's views on phylogeny were held by many biologists.
The differences between Whittaker's ecological approach and Copeland's traditional taxonomic approach are evident in the way they treated several important groups of organisms. Both Whittaker and Copeland argued that the fungi should be removed from the plant kingdom, but for very different reasons. Copeland chose to group the fungi with other “lower” eukaryotic organisms that lacked tissues in his kingdom Protoctista. Although Copeland claimed that the kingdom was monophyletic, Whittaker challenged this view and also argued that the Protoctista were biologically “incoherent” because the kingdom was a hodgepodge of unicellular and multicellular organisms with very different modes of nutrition. Whittaker created a separate kingdom for the fungi, not because the group was monophyletic, but because the fungi were united by their ecological role as multicellular decomposers in ecosystems. This emphasis on decomposition as an ecological process worthy of defining a kingdom reflected Whittaker's own work with biogeochemical cycling and also the growing prominence of biogeochemistry in ecosystem ecology ( Hagen 1992 ). To further support a separate kingdom for the fungi, Whittaker pointed to recent research that cast doubt on the belief that modern fungi had descended from photosynthetic ancestors similar to filamentous algae. According to Whittaker, the evidence against this earlier claim undercut both the traditional grouping of fungi within the plant kingdom and Copeland's decision to combine the fungi with various algae in his kingdom Protoctista.
In place of the Protoctista, Whittaker (1959) now proposed a purely unicellular kingdom, Protista—an idea that he attributed to Ernst Haeckel. Although he acknowledged that many groups such as Chlorophyta had both unicellular and multicellular members, Whittaker argued that the distinction between unicellular and multicellular body plans was conceptually clear cut and biologically meaningful. Furthermore, Whittaker pointed to the symmetry between the multicellular kingdoms of animals, plants, and fungi and the various subgroups within the Protista. In both cases, one could find the three major directions of evolution and ecological functions: producers, consumers, and decomposers. Therefore, by using two fundamental characteristics—mode of nutrition and cellular organization—Whittaker created a system of classification that was both simple and conceptually coherent ( figure 2 ).
The addition of kingdom Protista to Whittaker's original three-kingdom scheme highlighted another major difference between his ecological approach and Copeland's taxonomic approach. Copeland restricted his plant kingdom to a monophyletic group of vascular plants and their close relatives. Whittaker originally wanted to include all producers in the plant kingdom. He was now willing to relegate unicellular algae and cyanobacteria to his new kingdom Protista, but Whittaker continued to place all multicellular producers in kingdom Plantae. Whittaker's functional plant kingdom was an admittedly polyphyletic group of land plants, brown algae, and red algae. He justified this grouping on the grounds of both ecological function and cellular structure. The brown and red algae included large, complex, multicellular organisms that played the same ecological role in marine ecosystems that plants played in terrestrial ecosystems: They were, indeed, “functional plants.”
Whittaker's delineation of the kingdoms Plantae and Protista was later rejected even by some of his strongest supporters ( Margulis 1971 , 1974 ), but it highlights the distinction between his functional kingdoms and traditional taxonomic kingdoms. It also illustrates the philosophical underpinnings of Whittaker's approach to classifying both kingdoms and communities. Just as one could not always use environmental variables to precisely determine whether an area would be forest or grassland, so one could not neatly place groups such as the Chlorophyta into one or another kingdom on the basis of cellularity ( figures 1 and 2 ). Despite the ambiguity, Whittaker (1959) argued that his system provided the better alternative because it was conceptually more coherent than Copeland's system. Interestingly, later biologists tended to define kingdom Protista using a combination of criteria borrowed from both the Whittaker and the Copeland systems.
A decade later, Whittaker published his definitive five-kingdom system in the high-profile journal Science , ensuring that his ideas would reach a broad audience ( Whittaker 1969 ). Although the article repeated much of the line of reasoning that Whittaker employed in 1959, there were several substantive differences in both content and style. Most importantly, Whittaker now accepted Copeland's earlier decision to place all prokaryotic organisms into their own kingdom. Although he had considered this possibility in 1959, Whittaker made the more conservative decision to include the bacteria as a subkingdom of the Protista. The prokaryotic kingdom Monera now joined kingdoms Protista, Fungi, Plantae, and Animalia in the final version of Whittaker's system.
Whittaker justified adding the new kingdom Monera to his system for several reasons. By the end of the 1960s, the prokaryote–eukaryote distinction was a mainstream idea accepted by leading microbiologists ( Sapp 2005 , 2006 , 2009 ). Citing the still-controversial endosymbiotic theory being championed by Lynn Margulis as an attractive explanation for the evolution of eukaryotic cells, Whittaker now claimed that the prokaryote–eukaryote boundary represented the most fundamental division in the living world. Finally, Whittaker argued that the absorptive nutritional mode that characterized most Monerans was the original method of gaining energy. Photosynthesis had evolved in a few Monerans, but the three nutritional modes became well established only after the first eukaryotic protists evolved through endosymbiosis. Therefore, organisms could be placed into one of three structural grades: prokaryotes, unicellular eukaryotes, and multicellular eukaryotes. Within the two higher grades, various lineages of producers, consumers, and decomposers could be clearly identified, although only producers and decomposers were found at the prokaryotic grade.
Stylistically, Whittaker departed from the broad review of competing systems that he had used in 1959 and presented classification as a choice between two alternatives: Copeland's four-kingdom system and Whittaker's new five-kingdom system. Both the importance of the choice and the rationale for making it were also new. Whittaker now emphasized the pedagogical importance of revising the traditional two-kingdom system with one that better represented the broad contours of the living world. Noting that several introductory biology textbooks questioned the plant–animal dichotomy, Whittaker had an obvious motivation for highlighting the differences between the two alternative replacements. Compared with Copeland's elaborate taxonomic system, Whittaker claimed that his functional kingdoms rested on two criteria that biologists considered important and that students could easily understand.
The Soviet launch of Sputnik 1 in 1957 served as a potent catalyst for educational change ( Grobman 1969 , Sundberg et al. 1992 , Rudolph 2002 ). Exploiting fears that the United States was falling behind the Soviet Union in science, educational reformers pushed for revamping the nation's outdated approach to biology. Critics complained that existing textbooks were little more than dry surveys of plant and animal phyla, emphasizing anatomical description rather than unifying principles ( Grobman 1969 , Rudolph 2002 ). Drawing on expanded federal funding, new organizations such as the Biological Sciences Curriculum Study (BSCS) and the Commission on Undergraduate Education in the Biological Sciences (CUEBS) designed innovative curricula, textbooks, and laboratory exercises ( Sundberg et al. 1992 , Engleman 2001 ). Highlighting how difficult this was, BSCS published three different high school textbooks because of disagreements over fundamental biological principles. Two of these textbooks (the “blue” and “green” versions) departed radically from earlier textbooks by emphasizing evolution, the process of science, and unifying principles of cell and molecular biology (blue version) and ecology (green version). Students were exposed to a variety of organisms but in the context of discussing these broader biological concepts, rather than as a taxonomic survey.
CUEBS never produced comparable products at the college level, but its recommendations influenced the writing of new college textbooks that were profoundly different from their predecessors ( Sundberg et al. 1992 ). Popular pre-Sputnik textbooks were based on the pedagogical assumption that understanding topics such as genetics or ecology required a thorough familiarity with plant and animal taxa ( Johnson et al. 1956 ). Therefore, chapters on heredity and ecology were tucked at the end of the book, where critics complained they were rarely read ( Rudolph 2002 ). Conscious of the educational reforms proposed by CUEBS, later editions of these established textbooks added more chapters on cell biology, genetics, and ecology ( Johnson et al. 1966 ) but retained the pedagogical premise that familiarity with biodiversity was a prerequisite for understanding the unity of life. By contrast, a new generation of post-Sputnik textbooks emphatically rejected this traditional pedagogical approach. Rather than detailed taxonomic and anatomical surveys, these books shifted much greater attention to cell biology, genetics, development, animal behavior, and ecology ( figure 3 ). These topics were organized around three overarching themes: evolution, the molecular and cellular basis of life, and energetics.

A comparison of coverage of topics in pre- and post-Sputnik introductory biology textbooks.
The new design adopted by the authors of post-Sputnik textbooks posed serious challenges for discussing biodiversity. The emphasis on unifying principles, combined with a much-reduced taxonomic survey, demanded a more compelling way to describe the broad classification of organisms than the traditional plant–animal dichotomy. By emphasizing the importance of both ecological trophic levels and cellular structure, Whittaker's five-kingdom system organized biological diversity using the very themes that new biology textbooks stressed so heavily. Still, the two most popular post-Sputnik textbooks did not immediately adopt Whittaker's system but only gradually came to embrace it in later editions. Examining this transition sheds light on the difficulties of presenting biodiversity in the context of a new biology that deemphasized traditional taxonomy and the study of organisms.
William Keeton was an invertebrate taxonomist, but he also turned a boyhood interest in training homing pigeons into a successful research career in avian orientation and navigation at Cornell University ( Emlen 1981 ). When the life sciences were reorganized at Cornell, Keeton moved from the Department of Entomology to a newly established program in Neurobiology and Behavior. During this period, he designed and taught an extremely popular introductory biology course and spent five years writing his highly successful textbook ( Keeton 1967 , Emlen 1981 ).
In some ways, Keeton's (1967) , Biological Science was a major departure from older textbooks, but it initially retained the traditional focus on plants and animals. Indeed, Keeton's teaching innovation was combining botany and zoology into a single course ( Emlen 1981 ), and this was reflected in the textbook that he wrote. Although he briefly discussed the kingdom Monera, Keeton stuck closely to the traditional taxonomic system of plants and animals. He acknowledged the weaknesses of the plant–animal dichotomy but justified his choice in two ways: First, organisms familiar to students tended to be either plants or animals, so the traditional two-kingdom system provided a common-sense way to organize biodiversity. Most of the examples used by Keeton to illustrate unifying biological principles were drawn from multicellular plants and animals. Second, Keeton argued that phylogenetic relationships among protists and fungi were highly speculative and provided little support for newer classification systems. Although he briefly presented several alternative systems in a table, it was not until the third edition, in 1979, that Keeton adopted Whittaker's five-kingdom system. By this time, Whittaker, who was Keeton's colleague at Cornell, was acting as a consultant on the textbook. Not only did Keeton now use Whittaker's system to reorganize the five chapters on biodiversity, but he also devoted a page of the introductory chapter to discussing the logic of Whittaker's system in relation to the other major themes of the textbook. Thus, the five-kingdom system joined natural selection, energetics, and cell theory as broad explanatory principles that provided the foundation for discussing all of the other topics in the book. This approach was widely copied by later biology textbooks.
Helena Curtis's (1968) , Biology was an even greater departure from traditional textbooks, because less than 25% of the book was devoted to organisms ( figure 3 ). Curtis was a highly successful science writer, who made up for a lack of professional training in biology by enlisting a lineup of distinguished scientists as consultants. The result of this collaboration was a textbook widely acclaimed for its engaging style ( Luria 1969 , Villager 2005 ). Curtis initially dismissed the choice of kingdom classification as a technical matter of interest only to professional taxonomists ( Curtis 1968 ). Like Keeton, she emphasized that phylogenetic relationships—particularly among the protists—were highly speculative. Because there was little compelling support for any of the competing systems, Curtis was ambivalent about her choice of adding a third kingdom of microorganisms to the traditional plant and animal kingdoms. Despite her initial reluctance to strongly endorse any system of kingdom classification, Curtis's approach to introducing biological concepts harmonized well with the logic of Whittaker's approach. Like Keeton, Curtis emphasized energetics at both the cellular and ecological levels, and she presented the distinction between autotrophs and heterotrophs as fundamental. Similarly, her emphasis on cellular evolution (including endosymbiosis) and the prokaryote–eukaryote dichotomy for understanding cell structure provided another rationale for eventually accepting the five-kingdom system.
Curtis significantly reorganized the chapters of her textbook for the third edition, published in 1979, using two broad thematic divisions: the unity of life and the diversity of life. Despite misgivings about Whittaker's kingdom Protista, Curtis now endorsed the five-kingdom system as the best alternative for understanding the general contours of biodiversity. Just as Darwinian evolution, cell theory, and energetics served as fundamental principles for understanding the unity of life, Curtis now used the five-kingdom system as a basic principle underlying the section of her book devoted to the diversity of life. Both the “unity and diversity of life” themes and the use of Whittaker's system for organizing diversity were widely copied by later textbooks that tried to compete with the textbooks of Curtis and Keeton during the final two decades of the twentieth century.
Why Keeton and Curtis did not more quickly adopt Whittaker's five-kingdom system is an intriguing historical question. Whittaker suggested that the continued use of the two-kingdom system by biologists was largely attributable to intellectual conservatism and that acceptance of the five-kingdom system required a kind of cultural evolution in biological thinking ( Whittaker 1969 , Whittaker and Margulis 1978 ). The two-kingdom system had long been criticized, and several alternatives had been suggested, beginning in the late nineteenth century. In the first two editions of his textbook, Keeton acknowledged these alternatives without strongly endorsing any of them. His continued use of the plant–animal dichotomy for organizing biodiversity until the late 1970s was a conservative element in an otherwise highly innovative textbook. When Keeton and Curtis finally adopted the five-kingdom system in the third editions of their textbooks, both of them justified the switch on the basis of a gradual shift among biologists toward supporting Whittaker's system. Several reasons can be suggested for the gradualness of this change. The decline of traditional botany and zoology—as disciplines, departments, and introductory courses—made the plant–animal dichotomy less attractive, but this shift occurred in a piecemeal way during the Cold War era. Conversely, the increasing prominence of ecology in the biology curriculum—partly in response to popular environmental movements—reached a peak during the 1970s. This, in addition to new developments in cell biology, contributed importantly to the success of Whittaker's system. Perhaps most significantly, an alliance between Whittaker and Lynn Margulis closely linked the five-kingdom system with the controversial but increasingly influential theory of endosymbiosis ( Margulis 1970 , 1971 , 1974 , Whittaker and Margulis 1978 ). Margulis quickly embraced the five-kingdom system, focused considerable scientific attention on unicellular organisms, and played a major role in refining Whittaker's problematic kingdom Protista. The growing linkage between endosymbiosis and the five-kingdom system appears to have been important for both Curtis and Keeton, who each placed the topics back to back in the third editions of their textbooks. All of these changes took time, but a decade after Whittaker introduced his system, the tide had turned decisively toward the acceptance of five kingdoms. Suffice it to say that during the final two decades of the twentieth century, all major biology textbooks followed Keeton and Curtis in using some version of Whittaker's five-kingdom system to organize discussions of biodiversity.
Ironically, as the five-kingdom system became a prominent and well-established feature of introductory textbooks, the rationale for Whittaker's approach was being undermined in a number of important ways. Molecular systematists rejected the earlier belief that phylogenetic relationships among protists and bacteria were inherently speculative and perhaps unknowable ( Sapp 2009 ). As molecular sequences rapidly accumulated, along with advanced computational techniques to analyze them, confidence grew among biologists that monophyletic classification of formerly problematic groups was within reach. This undercut the logic of Whittaker's system, which was broadly evolutionary but not phylogenetic. Whittaker's belief that phylogeny was only one of several equally valid criteria for classification had also been widely shared when he began writing about kingdoms, but with the rapid rise of cladistics during the 1970s, biologists increasingly rejected this view. The seemingly fundamental distinction between prokaryotes and eukaryotes was also challenged by the discovery of the archaea (initially referred to as archaebacteria ) and Carl Woese's claim that all living organisms belonged to one of three broad domains: archaea, bacteria, and eukarya ( Woese et al. 1990 , Sapp 2009 ). Woese was highly critical of the prokaryote–eukaryote dichotomy, both as a basis for classification and as a supposedly useful distinction between types of cells ( Sapp 2006 , 2009 ). Woese claimed that the dichotomy was based on a false distinction that was phylogenetically misleading; he opposed defining the kingdom Monera negatively, on the basis of the lack of a structure (i.e., the nucleus); and he argued that the dichotomy was incompatible with the three-domain system that he championed. In short, he wanted to eliminate the terms prokaryote and eukaryote from the biological vocabulary ( Sapp 2006 ).
Textbooks quickly adopted Woese's idea of three domains, but his critique of the prokaryote–eukaryote dichotomy was ignored. Therefore, Woese's three domains and the remnants of Whittaker's five kingdoms rest somewhat uncomfortably in modern textbook discussions of biodiversity. Many textbooks recognize a new kingdom for the archaea, but both the archaea and bacteria are typically discussed in the chapter devoted to prokaryotic life. Similarly, although most textbook authors have abandoned the polyphyletic kingdom Protista, they continue to devote a chapter to “protists.” The persistence of Whittaker's ideas about kingdoms cannot be explained entirely by intellectual inertia but rather by genuine ambiguities in the broad classification of organisms. This ambiguity is reflected in the spirited debate over the implications of recognizing Woese's three domains and the controversy over Woese's critique of the prokaryote– eukaryote dichotomy ( Mayr 1998 , Woese 1998 , Sapp 2006 , 2009 ). Despite the popularity of Woese's domains, most educators find the distinction between prokaryotes and eukaryotes to be useful, and textbooks continue to highlight the significance of the two cell types. The strong support that some prominent biologists continue to voice for the five- (or six-) kingdom system—albeit in modified form—is another reason that textbooks have not completely abandoned Whittaker's approach. For example, Margulis and Chapman (2009) criticized Woese's domains for being based exclusively on molecular data and ignoring other important biological characteristics of organisms. As a result, Margulis and Chapman continued to argue for maintaining a prokaryotic superkingdom that includes both bacteria and archaea. Margulis and Chapman also pointed out that a completely monophyletic classification would have so many kingdoms that it would lose any pedagogical value for students' understanding of biodiversity. This pedagogical point highlights the tension between basing a kingdom system strictly on phylogeny while still “providing a synoptic view of the living world” ( Whittaker and Margulis 1978 , p. 11). The need for this “synoptic view” reinforces the major strengths of Whittaker's system: its simplicity and close ties to easily understandable ecological and cellular principles. Whittaker's grouping of organisms according to cellular structure and ecological function constituted a manageable and conceptually pleasing scheme—one that seems difficult to completely abandon, despite its acknowledged shortcomings.
I thank Christine Small, Fred Singer, and three anonymous reviewers for their comments on an earlier draft of this article. I also thank John Norton for preparing the figures.
Copeland HF . 1956 . The Classification of Lower Organisms . Pacific Books .
Google Scholar
Google Preview
Curtis H . 1968 . Biology . Worth .
Emlen ST . 1981 . In memoriam: William T. Keeton . Auk 98 : 167 – 172 .
Engleman L , ed. 2001 . The BSCS Story: A History of the Biological Sciences Curriculum Study . BSCS .
Golley FB . 1993 . A History of the Ecosystem Concept in Ecology: More than the Sum of the Parts . Yale University Press .
Grobman AB . 1969 . The Changing Classroom: The Role of the Biological Sciences Curriculum Study . Doubleday .
Hagen JB . 1992 . An Entangled Bank: The Origins of Ecosystem Ecology . Rutgers University Press .
Johnson WH Laubengayer RA DeLanney LE . 1956 . General Biology . Holt .
Johnson WH Laubengayer RA DeLanney LE . 1966 . General Biology , 2nd ed. Holt .
Keeton WT . 1967 . Biological Science . Norton .
Kingsland SE . 2005 . The Evolution of American Ecology, 1890–2000 . Johns Hopkins University Press .
Kohler RE . 2008 . Plants and Pigeonholes: Classification as a practice in American ecology . Historical Studies in the Natural Sciences 38 : 77 – 108 .
Luria SE . 1969 . On teaching biology in a biological revolution . Scientific American 220 : 131 – 134 .
Margulis L . 1970 . Origin of Eukaryotic Cells . Yale University Press .
Margulis L . 1971 . Whittaker's five kingdoms of organisms: Minor revisions suggested by considerations of the origin of mitosis . Evolution 25 : 242 – 245 .
Margulis L . 1974 . Five-kingdom classification and the origin and evolution of cells . Evolutionary Biology 7 : 45 – 78 .
Margulis L Chapman MJ . 2009 . Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth , 4th ed. Academic Press .
Mayr E . 1998 . Two empires or three? Proceedings of the National Academy of Sciences 95 : 9720 – 9723 .
Nicolson M McIntosh RP . 2002 . H. A. Gleason and the individualistic hypothesis revisited . Bulletin of the Ecological Society of America 83 : 133 – 142 .
Odum EP . 1959 . Fundamentals of Ecology , 2nd ed. Saunders .
Rudolph JL . 2002 . Scientists in the Classroom: The Cold War Reconstruction of American Science Education . Palgrave .
Sapp J . 2005 . The prokaryote–eukaryote dichotomy: Meanings and mythology . Microbiology and Molecular Biology Reviews 69 : 292 – 305 .
Sapp J . 2006 . Two faces of the prokaryote concept . International Microbiology 9 : 163 – 172 .
Sapp J . 2009 . The New Foundations of Evolution: On the Tree of Life . Oxford University Press .
Sundberg MD Kormondy EJ Carter JL Moore JA Postlethwait SN Thornton JW . 1992 . Reassessing the commission on undergraduate education in the biological sciences . BioScience 42 : 442 – 447 .
Villager . 2005 . Obituary: Helena Curtis, 81, wrote 'elegant' science textbooks . The Villager 74 . February 23–March 1. (5 July 2011; www.thevillager.com/vil_95/helenacurtis81.html ) .
Westman WE Peet RK . 1985 . Robert Whittaker (1920–1980): The man and his work . Pages 6 – 30 in Peet RK , ed. Plant Community Ecology: Papers in Honor of Robert H.Whittaker . Junk .
Whittaker RH . 1948 . A Vegetation Analysis of the Great Smoky Mountains . PhD dissertation . University of Illinois, Urbana .
Whittaker RH . 1956 . Vegetation of the Great Smoky Mountains . Ecological Monographs 26 : 1 – 80 . 11 .
Whittaker RH . 1957 . The kingdoms of the living world . Ecology 38 : 536 – 538 .
Whittaker RH . 1959 . On the broad classification of organisms . Quarterly Review of Biology 34 : 210 – 226 .
Whittaker RH . 1961 . Experiments with radiophosphorus tracer in aquarium microcosms . Ecological Monographs 31 : 157 – 188 .
Whittaker RH . 1962 . Classification of natural communities . Botanical Review 28 : 1 – 239 .
Whittaker RH . 1969 . New concepts of kingdoms of organisms . Science 163 : 150 – 160 .
Whittaker RH . 1970 . Communities and Ecosystems . Macmillan .
Whittaker RH . 1972 . Introduction . Pages 1 – 6 in Whittaker RH , ed. Ordination and Classification of Communities . Junk .
Whittaker RH . 1978 . Approaches to classifying vegetation . Pages 1 – 33 in Whittaker RH , ed. Classification of Plant Communities . Junk .
Whittaker RH Margulis L . 1978 . Protist classification and the kingdoms of organisms . BioSystems 10 : 3 – 18 .
Wilson EO . 2006 . Naturalist , 2nd ed. Island Press .
Woese CR . 1998 . Default taxonomy: Ernst Mayr's view of the microbial world . Proceedings of the National Academy of Sciences 95 : 11043 – 11046 .
Woese CR Kandler O Wheelis ML . 1990 . Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya . Proceedings of the National Academy of Sciences 87 : 4576 – 4579 .
Author notes
Email alerts, citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1525-3244
- Copyright © 2024 American Institute of Biological Sciences
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
LYNN MARGULIS
Our unruly earth mother, and a contrarian to the end., five kingdoms of life.
When Lynn Margulis was a young woman, all life was divided into two great kingdoms, known as plants and animals. But Margulis and others saw that this division did not accurately reflect the diversity of life: many organisms are neither. To combat this, Carl Woese in 1990 introduced the three-domain system, meant to expand on the previously narrow categories of life. In 1969, life on earth was classified into five kingdoms (Plants, Animals, Fungi, Protists and Monera, as introduced by Robert Whittaker.
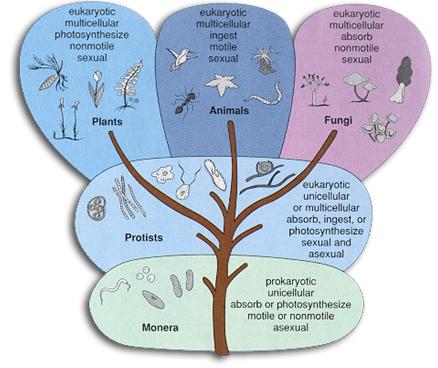
Illustration depicting Whittaker’s 5 kingdom classification
Margulis became the most important supporter and defender of this proposed theory, as s he rejected the three-domain system which gained wide acceptance, believing that there were more domains that were neglected by this system.
However, while in support of parts of the new classification as it was superior to the three domain system, Margulis was also a big critic of Whittaker’s newly introduced 5 kingdom classification and she was the first to recognise the limitations of Whittaker’s classification of microbes. She introduced a modified classification by which all life forms, including the newly discovered, could be integrated into the classical five kingdoms. According to her the main problem, archaea, falls under the kingdom Prokaryotae alongside bacteria (in contrast to the three-domain system, which treats archaea as a higher taxon than kingdom, or the six-kingdom system, which holds that it is a separate kingdom).
Her concept is given in detail in her book, “Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth”, written with Karlene V. Schwartz. Stephen Jay Gould, in the Foreword, called the book the “rarest of intellectual treasures” and drew upon its contents in pointing out that the greatest division among living beings was not “between plants and animals, but within the once- ignored microorganisms—the prokaryotic Bacteria and the eukaryotic Protoctista.” The famous hand symbol used to illustrate the 5 classifications, where each part of the hand represents one of the tenets of the proposed classification, is now globally recognised.

Margulis’ book, Five Kingdoms, written with Karlene V. Schwartz
It is believed that it is mainly because of Margulis and her propagation of this theory that the five-kingdom system survived. But later discoveries of new organisms, such as archaea, and emergence of molecular taxonomy challenged the concept. By the mid-2000s, most scientists began to agree that there are more than five kingdoms.
- Biology Article
- Five Kingdoms Classification
Five Kingdom Classification
The system of assembling organisms into groups or sets on the basis of likenesses and variances is called classification. It simplifies the study of a wide variety of organisms in a very systematic manner.

R.H. Whittaker proposed the five-kingdom classification in 1969. This classification was based upon certain characters like mode of nutrition, thallus organization, cell structure, phylogenetic relationships and reproduction. This form of kingdom classification includes five kingdoms Monera, Protista, Fungi, Plantae and Animalia.
Also Read: Taxonomy
The five-kingdom classification that we see today was not the initial result of the classification of living organisms. Carolus Linnaeus first came up with a two-kingdom classification, which included only kingdom Plantae and kingdom Animalia.
The two-kingdom classification lasted for a very long time but did not last forever because it did not take into account many major parameters while classifying. There was no differentiation of the eukaryotes and prokaryotes; neither unicellular and multicellular; nor photosynthetic and the non-photosynthetic.
Putting all the organisms in either plant or animal kingdom was insufficient because there were a lot of organisms which could not be classified as either plants or animals.
All this confusion led to a new mode of classification which had to take into account cell structure, the presence of cell wall, mode of reproduction and mode of nutrition. As a result, R H Whittaker came up with the concept of the five-kingdom classification.
The five-kingdom classification of living organisms included the following kingdoms:
Kingdom Monera
Bacteria are categorized underneath the Kingdom Monera.
Features of Monerans
They possess the following important features:
- Bacteria occur everywhere and they are microscopic in nature.
- They possess a cell wall and are prokaryotic.
- The cell wall is formed of amino acids and polysaccharides.
- Bacteria can be heterotrophic and autotrophic.
- The heterotrophic bacteria can be parasitic or saprophytic. The autotrophic bacteria can be chemosynthetic or photosynthetic.
Types of Monerans
Bacteria can be classified into four types based on their shape:
- Coccus (pl.: cocci) – These bacteria are spherical in shape
- Bacillus (pl.: bacilli) – These bacteria are rod-shaped
- Vibrium (pl.: vibrio) – These bacteria are comma-shaped bacteria
- Spirillum (pl.: spirilla) – These bacteria are spiral-shaped bacteria
Monera has since been divided into Archaebacteria and Eubacteria.
Kingdom Protista
Features of protista.
Protista has the following important features:
- They are unicellular and eukaryotic organisms.
- Some of them have cilia or flagella for mobility.
- Sexual reproduction is by a process of cell fusion and zygote formation.
Sub-groups of Protista
Kingdom Protista is categorized into subsequent groups:
- Chrysophytes : The golden algae (desmids) and diatoms fall under this group. They are found in marine and freshwater habitats.
- Dinoflagellates : They are usually photosynthetic and marine. The colour they appear is dependent on the key pigments in their cells; they appear red, blue, brown, green or yellow.
- Euglenoids : Most of them live in freshwater habitation in motionless water. The cell wall is absent in them, instead, there is a protein-rich layer called a pellicle.
- Slime Moulds : These are saprophytic. The body moves along putrefying leaves and twigs and nourishes itself on organic material. Under favourable surroundings, they form an accumulation and were called Plasmodial slime moulds.
- Protozoans : They are heterotrophs and survive either as parasites or predators.
Kingdom Fungi
The kingdom fungi include moulds, mushroom, yeast etc. They show a variety of applications in domestic as well as commercial purposes.
Features of Kingdom Fungi
- The fungi are filamentous, excluding yeast (single-celled).
- Their figure comprises slender, long thread-like constructions called hyphae. The web of hyphae is called mycelium.
- Some of the hyphae are unbroken tubes which are jam-packed with multinucleated cytoplasm. Such hyphae are labelled Coenocytic hyphae.
- The other type of hyphae has cross-walls or septae.
- The cell wall of fungi is composed of polysaccharides and chitin.
- Most of the fungi are saprophytes and are heterotrophic.
- Some of the fungi also survive as symbionts. Some are parasites. Some of the symbiont fungi live in association with algae, like lichens. Some symbiont fungi live in association with roots of higher plants, as mycorrhiza.
Kingdom Plantae
Features of kingdom plantae.
- The kingdom Plantae is filled with all eukaryotes which have chloroplast.
- Most of them are autotrophic in nature, but some are heterotrophic as well.
- The Cell wall mainly comprises cellulose.
- Plants have two distinct phases in their lifecycle. These phases alternate with each other. The diploid saprophytic and the haploid gametophytic phase. The lengths of the diploid and haploid phases vary among dissimilar groups of plants. Alternation of Generation is what this phenomenon is called.
Kingdom Animalia
Features of kingdom animalia.
- All multicellular eukaryotes which are heterotrophs and lack cell wall are set aside under this kingdom.
- The animals are directly or indirectly dependent on plants for food. Their mode of nutrition is holozoic. Holozoic nutrition encompasses ingestion of food and then the use of an internal cavity for digestion of food.
- Many of the animals are adept for locomotion.
- They reproduce by sexual mode of reproduction.
Also Read: Kingdom Monera, Protista and Fungi
The five-kingdom classification of living organisms took a lot into consideration and is till now the most efficient system.
The older system of classification was based only on one single characteristic according to which two highly varied organisms were grouped together. For example, the fungi and plants were placed in the same group based on the presence of the cell wall. In the same way, unicellular and multicellular organisms were also grouped together.
Therefore, all the organisms were classified again into the five kingdoms known as the five-kingdom classification, starting with Monera, where all the prokaryotic unicellular organisms were placed together.
Following that, all the eukaryotic unicellular organisms were placed under the kingdom Protista.
The organisms were then classified based on the presence and absence of a cell wall. The ones without the cell wall were classified under kingdom Animalia and the ones with cell wall were classified under kingdom Plantae.
The organisms under kingdom Plantae were further classified into photosynthetic and non-photosynthetic, which included Plantae and fungi respectively.
This system of classification of living organisms is better than following the older classification of plants and animals because it eradicated the confusion of putting one species in two different kingdoms.
Also Read: Basis of Biological Classification
For more information on the five-kingdom classification or any other kingdom classification of organisms, keep visiting BYJU’S website or download BYJU’S app, for further reference.
Frequently Asked Questions
What is classification.
Classification is the arrangement of plants and animals in taxonomic groups according to the similarities and differences observed.
What is kingdom classification?
Kingdom classification is the highest classification into which the organisms are grouped in the taxonomy. It is ranked above the phylum.
What is the two kingdom classification?
The two-kingdom classification was proposed by Carolus Linnaeus. He classified the living organisms on the basis of nutrition and mobility. The living organisms were classified into Kingdom Plantae and Kingdom Animalia.
On what basis are the living organisms divided in the five-kingdom classification?
The living organisms are divided into five different kingdoms – Protista, Fungi, Plantae, Animalia, and Monera on the basis of their characteristics such as cell structure, mode of nutrition, mode of reproduction and body organization.
What is the basic unit of classification?
Species are the basic unit of classification. The organisms that have the same characteristics and can breed with each other to produce fertile offspring are known to belong to the same species.
What was the drawback of two-kingdom classification?
In the two-kingdom classification, the plants included photosynthetic and non-photosynthetic species. Fungi, which feed on dead organic matter, were placed under photosynthetic plants. Therefore, there arose a need for another system of classification where the organisms with the same characteristics were clubbed into one kingdom.
What are the different levels of classification?
The organisms are classified according to the following different levels- Kingdom, Phylum, Class, Order, Family, Genus and Species.
In which kingdom are the prokaryotes classified?
The prokaryotes are classified into kingdom Monera. There are two other kingdoms, including prokaryotes- Eubacteria and Archaea.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Thanks for this am really impressed and happy for knowing more about the 5 kingdoms and their characteristics (features)
Thanks for the good work done I am already on cloud nine for I have learnt from this.
very helpful
This helped me a lot. Thank you so much😊😊
This help me very much thanks so much
Thank you, this helps me very much
In the diagram, in the Kingdom bacteria, is it Euglena? If yes, could you explain how?
Thank you, these people help very much
this is great thank you so much
It’s really awesome and very very helpful thank you Byju’s teachers
This helped me a lot thank you.
Grateful to you
This is very easy to learn 5kingdom classification It is very helpful for me thanks a lot
Thank you so much!
it’s very easy to understand thank you sooo much.
Thank you so much! This helped me a lot!
This helped me a lot in my work, Thank u BYJU’S
it is very good and i often use such byjus free material because they provide almost all info.
Thnx alot!♥️♥️
Thnx alot!!!♥️♥️
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


Biology Notes Web
An Overview On The Five Kingdom Classification System
In the five kingdom classification system organisms are divided into Monera, Protista, Fungi, Plantae & Animalia based on cells, nutrition, and evolution.
Classification organizes organisms into groups, or sets based on their similarities and differences. The study of organisms becomes simpler with this systematic approach..
A Brief History of Biological Classification
Humans have long sought to organize the living world systematically. Aristotle divided all organisms into two groups: plants and animals.
The system remained simple for centuries until advances in microscopy and evolution theory allowed for more complex groupings.
In 1866, Ernst Haeckel proposed adding a third kingdom Protista to include single celled organisms like protozoans and algae.
Later, Robert Whittaker built upon Haeckel’s system by identifying differences between fungi and other plants, creating the Fungi kingdom.
But it was not until 1969 that Whittaker outlined his seminal five-kingdom classification scheme, which biologists still employ today.
By relying on evolutionary relationships and important cellular distinctions, Whittaker categorized life into five kingdoms: Monera, Protista, Fungi, Plantae, and Animalia, setting his system apart..
The Five Kingdom Classification System
Whittaker’s five kingdoms represent the broad categorical levels of the taxonomic hierarchy applied to all living organisms on Earth. We can define each kingdom based on distinguishing characteristics:
Kingdom Monera
The Kingdom Monera contains all prokaryotic single-celled microorganisms that lack a nucleus or other membrane-bound organelles in their cells.
Within Moneran cells, the genetic material comprises a single loop of DNA floating freely in the cytoplasm rather than enclosed within a nuclear membrane. The cell walls of Monera comprise polysaccharides and proteins rather than cellulose.
Monerans use diverse metabolic strategies. Some species are autotrophs, like cyanobacteria, that can make their food through photosynthesis, while others are heterotrophs that use organic compounds for nutrition.
Many Monerans have simple spherical shapes, like cocci bacteria, while others have more complex morphologies.
Rod-shaped bacilli and spiral-shaped spirilla bacteria exemplify the morphological diversity within the kingdom. Mycoplasma bacteria represent an unusual Monerans group that entirely lacks a cell wall.
Within the Kingdom Monera, someone currently divides organisms into two major categories based on fundamental genetic and biochemical differences: Bacteria and Archaea.
Eubacteria and cyanobacteria fall under the traditional bacterial domain, while extremophile organisms classified as Archaea inhabit harsh environments like salt lakes, hot springs, and Antarctic ice.
Kingdom Monera contains many organisms of tremendous ecological and medical importance. Cyanobacteria produce oxygen through photosynthesis, while other Monerans cycle through vital elements like nitrogen, sulfur, and carbon.
Major Taxa Within Kingdom Monera
- Archaebacteria –Single-celled prokaryotes capable of living in extreme environments.
- Eubacteria –The more typical bacterial prokaryotes like Escherichia coli . Responsible for cycles of nutrient transformation.
- Cyanobacteria –Photosynthetic bacteria that produce oxygen. Crucial in shaping Earth’s atmosphere.
- Mycoplasmas –Bacteria lacking cell walls. Many are important pathogens.
Species within the genus Clostridium include pathogens that cause diseases like tetanus and botulism. Still, other Monerans live symbiotically with plants and animals, providing essential functions for their hosts.
Monerans use diverse metabolisms and inhabit every environment on Earth.
Kingdom Protista
The Kingdom Protista encompasses all eukaryotic unicellular life forms not classified within the kingdoms’ Plantae, Fungi, or Animalia.
These organisms are extremely diverse but share basic eukaryotic cell structures containing membrane-bound nuclei and organelles. However, Protists lack the complex multicellular differentiation and tissue organization found in plants, fungi, and animals.
Protists display a wide variety of forms and functions. Some Protists are autotrophic, like photosynthetic algae that can produce their own food.
Other Protist species are heterotrophs that consume organic compounds as food and include protozoans, unicellular slime molds, and kelp.
Many Protists have flagella or cilia for locomotion. Pseudopodia allows amoeboid Protists to engulf food through phagocytosis.
Still, other groups like the chlorophyte algae Volvox form loose colonies or simple multicellular structures. This shows the beginnings of multicellularity within the kingdom.
Protists inhabit aquatic and damp environments, with many species living as plankton in aquatic food webs. Others live in or on other organisms as parasites or symbiotic forms. Protist diversity includes:
- Algae–Aquatic, photosynthetic protists like seaweed. Provide nourishment and oxygen to ocean ecosystems.
- Amoebas – Blob-like protists that engulf food through phagocytosis. Some cause disease.
- Paramecium–Complex single-celled organisms with specialized organelles. Move via cilia.
- Plasmodium–Parasitic protists transmitted by mosquitos that cause malaria.
- Protozoans such as amoebas, paramecia, and plasmodium cause human diseases
- Unicellular slime molds and water molds that feed on microbes
- Kelp that forms large multicellular bodies from eukaryotic cells
- Phytoplankton like Emiliania huxleyi form the base of marine food chains.
Major Taxa Within Kingdom Protista
- Algae – Aquatic, photosynthetic protists like seaweed. Provide nourishment and oxygen to ocean ecosystems.
- Amoebas – Blob-like protists that engulf food through phagocytosis. Some cause disease.
- Paramecium – Complex single-celled organisms with specialized organelles. Move via cilia.
- Plasmodium – Parasitic protists transmitted by mosquitos that cause malaria.
Kingdom Protista has unicellular eukaryotes important for ecosystems, pathogen transmission, and aquatic food webs.
Kingdom Fungi
Kingdom Fungi contain multicellular eukaryotic organisms that cannot photosynthesize food, unlike plants and animals. Fungi are heterotrophs, feeding by absorption after releasing digestive enzymes onto food sources.
This absorptive nutrition strategy distinguishes fungi from ingestive animals. The main body of a fungus comprises thin filament-like structures called hyphae.
A web of hyphae collectively makes up the vegetative structure called mycelium. Fungi can be unicellular, like yeasts, or develop complex multicellular bodies, as seen in mushrooms.
A defining trait of fungi is chitin on their cell walls. Chitin provides structural support and contributes to edible mushrooms’ distinctive texture. Most fungi reproduce via spores, which allow both survival and dispersal.
Fungi fill diverse ecological roles as decomposers, parasites, and mutualistic symbionts:
- Cherry oyster mushrooms and other decomposer fungi break down dead material and release important nutrients..
- Parasitic fungi, including tinea, cause diseases in plants and animals by invading living tissue.
- Mutualistic fungi form important symbioses like mycorrhizal associations with plant roots that enhance growth.
Humans cultivate and harvest edible fungi like portobello mushrooms and truffles for food.. Other fungi produce antibiotics like penicillin or psychedelic compounds.

Major Taxa Within Kingdom Fungi
- Mushrooms – Fleshy fungi with reproductive spore-producing gills. Biologically closest group to animals.
- Molds – Fungi is composed of filamentous hyphae. Help decompose organic matter.
- Yeasts – Unicellular fungi like Saccharomyces used to ferment wine and beer.
- Lichens – Mutualistic symbioses between fungi and algae or cyanobacteria.
The Kingdom Fungi contains indispensable organisms recycling nutrients in ecosystems, causing plant and animal diseases, and generating valuable medicines and foods for humanity.
Kingdom Plantae
Kingdom Plantae covers all photosynthetic eukaryotic life forms ranging from microscopic unicellular algae to giant multicellular trees. Possessing chloroplasts, plants synthesize chemical energy from sunlight via photosynthesis. This phototrophic capacity distinguishes the Plantae from heterotrophic fungi and animals.
The rigid cell walls of plants contain the carbohydrate cellulose. This cellulosic cell wall and vacuoles that maintain water pressure provide structural support for plants. Plants lack mobility but can disperse through seeds and spores.
Multicellular plants display proper tissues organized into functional structures like roots for nutrient uptake, stems for support, and leaves for photosynthesis. Vascular plants have specialized conductive tissues that transport water and nutrients internally.
Major Taxa Within Kingdom Plantae
- Bryophytes –Small plants like mosses and liverworts that need water for reproduction. No proper vascular tissues.
- Gymnosperms –Seed plants with “naked seeds” not enclosed in fruits. Conifers like pines are a key example.
- Angiosperms– Flowering plants that produce seeds within protective fruits. Most plant species fall into this category.
In addition to providing the foundation for terrestrial food webs, human life relies on plants for food, fuel, fiber, medicine, and oxygen generation. Photosynthesis by the plant kingdom also helps regulate global carbon dioxide levels.
kingdom Animalia – Five kingdom classification
The final eukaryotic kingdom, Animalia, contains all multicellular heterotrophic organisms that ingest food. Animals exhibit mobility at some stage in their life cycles thanks to specialized tissues, including muscle and nervous systems.
Without cell walls, animal cells can form flexible tissues optimized for functions like digestion, nutrient transport, sensation, and locomotion. Diploid animal bodies develop from zygotes with haploid gametes produced via sexual reproduction.
Like plants, animals display true multicellularity, with differentiated cells forming tissues and organs. But only animals show complex embryological development guided by genetic programs.
The Kingdom Animalia contains a vast diversity of body plans and ecologies.
- Invertebrates like sponges, jellyfish, worms, mollusks, and arthropods lack backbones and comprise 95% of known animal species.
- Vertebrates, including fish, amphibians, reptiles, birds, and mammals, possess internal skeletal support from backbones and skulls.
- Aquatic animals span vertebrate fish and jawless hagfish to marine invertebrates like octopuses, sea stars, and sponges.
- Terrestrial animals range from annelid worms and arthropods to reptiles, birds, and mammals inhabiting land environments.
- Parasitic roundworms, leeches, tapeworms, and other animals consume nutrients from living host organisms.
- Predators like lions, sharks, hawks, and spiders hunt other animals as food sources.
Major Taxa Within Kingdom Animalia
- Porifera – Sponges with permeable bodies optimized for filter feeding.
- Cnidaria – Simple aquatic animals like jellyfish and coral armed with stinging cells.
- Arthropoda – Invertebrates with exoskeletons and jointed bodies. Includes insects, spiders, and crabs.
- Chordata – Vertebrates characterized by a notochord supporting their nerve cord. Consists of fish, amphibians, reptiles, birds, and mammals.
The incredible innovations of multicellular heterotrophic eukaryotes enabled the success and dominance of the Animalia on Earth today, in the seas, skies, and across terrestrial habitats.
Applications and Limitations of the Five kingdom classification
The five-kingdom classification system helps biologists compare and contrast broad categories of life. It provides a biological context on scales from molecular genetics to global ecology. Practical applications include:
- Developing effective antibiotics based on distinguishing bacteria from human cells.
- Tracing the evolutionary origins of diverse diseases that afflict plants, animals, and fungi.
- Understanding principles of vaccine development by studying natural immune responses across kingdoms.
- Managing ecosystems holistically through knowledge of interrelationships between kingdoms.
- Investigating potential cancer cures being produced by various fungi, plants, and marine invertebrates.
However, the five-kingdom model has limitations . Examples include:
- Ambiguous Boundaries – Some organisms blur the lines between kingdoms. Viruses possess characteristics of living and nonliving entities.
- Oversimplification – Placing all bacteria in one kingdom obscures tremendous diversity within prokaryotes. Analogous issues arise in other kingdoms.
- Hierarchy Implications – Ranking kingdoms inaccurately convey evolutionary relationships between organisms like fungi and animals.
- Technological Advances – Genomic analysis reveals that traditional organismal traits reflect only a fragment of evolutionary history. Broader phylogenetic relationships exist.
Thus, while still indispensable in education and research, the five kingdoms represent one of many biological classification frameworks. Scientists continue developing systems that better reflect the latest knowledge and technologies.
Kingdoms Provide the Highest Ranks in Biological Taxonomy.
Kingdoms represent just the first tier in the hierarchical organization of life. Each kingdom contains many nested levels below it, moving from broad to increasingly specific:
Kingdom > Phylum > Class > Order > Family > Genus > Species
For instance, Animalia lives in the phylum Chordata within the kingdom, which includes the class Mammalia. This class holds the order Carnivora, composed of families like Felidae. The faily family Felidae contains genera including Felis , holding having species like the domestic cat, and Felis cactus .
This taxonomic structure allows precisely categorizing all organisms using a standardized binomial naming system. The first part of a species’ scientific name denotes its genus, while the second shows the specific species.
While kingdoms provide a universal starting point, lower taxonomic ranks capture the richness of life’s diversity. Let’s explore some key subgroups within each kingdom.
The Significance of Classifying Earth’s Diversity
Biological classification systems empower us to perceive order in the natural world and facilitate scientific inquiry. Key benefits of taxonomy include:
- Providing a universal language to communicate unambiguously about any species.
- Revealing meaningful relationships between organisms based on morphological, genetic, or evolutionary associations.
- Simplifying memorization of vast biodiversity by imposing hierarchy and structure.
- Enabling organization of knowledge for easy retrieval and expansion.
- Focusing research programs within bounded domains of life.
- Illuminating patterns and trends that spur questions driving research.
- Identifying unknown organisms and determining appropriate study methods.
From Aristotle’s divisions to Whittaker’s kingdoms, our grasp of biological diversity grows ever deeper thanks to developing classification frameworks.
These systems empower us to investigate life’s complexity, harness nature’s gifts, preserve disappearing species, and appreciate our shared evolutionary heritage.
While odysseys of discovery always lie ahead, life’s diversity becomes more wondrous and comprehensible when viewed through biological taxonomy–the essential science of classifying Earth’s endlessly surprising species.
Frequently Asked Questions About Five kingdom classification.
Taxonomy and classification may seem esoteric, but this foundational biological understanding has profound everyday affects. Here are answers to some common questions:
Traditionally studied by biochemists rather than taxonomists, I discovered viruses after the proposal of the five-kingdom system. Their acellular nature also precludes easy classification within established kingdoms.
The five-kingdom classification system proposed by Robert Whittaker is based on differences in cell structure, body organization, mode of nutrition, and evolutionary relationships between organisms. The primary criteria used to separate organisms into five kingdoms are cell type (prokaryotic vs eukaryotic), the complexity of multicellularity, the method of acquiring nutrients, and inferred evolutionary distance.
They classified organisms into five kingdoms – Monera, Protista, Fungi, Plantae, and Animalia – to categorize life into broad groups based on significant genetic, physiological, and morphological differences. This allows organisms with common characteristics to be studied while distinguishing between the unique attributes of prokaryotes, plants, animals, fungi, and single-celled eukaryotes.
Biologists favor the five-kingdom classification system because it represents major evolutionary divergences in the life on Earth. The system differentiates prokaryotic and eukaryotic cells, photosynthetic vs heterotrophic nutrition, unicellular vs multicellular complexity, and degrees of organismal specialization. This provides a more nuanced organizational structure than the previous two or three kingdom systems.
Advantages include recognizing prokaryotic/eukaryotic differences, separating non-photosynthetic fungi from plants, and grouping organisms according to inferred phylogenetic relationships and significant trait differences. Disadvantages are that boundaries between kingdoms are still fuzzy, vast diversity exists within each kingdom, and evolutionary relatedness doesn’t fully align with the taxonomic rankings used. No classification scheme perfectly captures the complexity of life.
References and Sources
- https://www.toppr.com/guides/biology/diversity-in-living-organisms/five-kingdom-classification/
- https://kids.britannica.com/students/article/biological-classification/611149
- https://biologyteach.com/five-kingdom-classification-system/
- https://www.brainkart.com/article/Whittaker—s-System-of-Classification_35254/
- https://biologydictionary.net/fungi/
- https://organismalbio.biosci.gatech.edu/biodiversity/eukaryotes-and-their-origins/
- https://quizlet.com/286045995/biology-the-kingdoms-flash-cards/
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.

The Diversity of Life
The fact that biology, as a science, has such a broad scope has to do with the tremendous diversity of life on earth. The source of this diversity is evolution, the process of gradual change during which new species arise from older species. Evolutionary biologists study the evolution of living things in everything from the microscopic world to ecosystems.
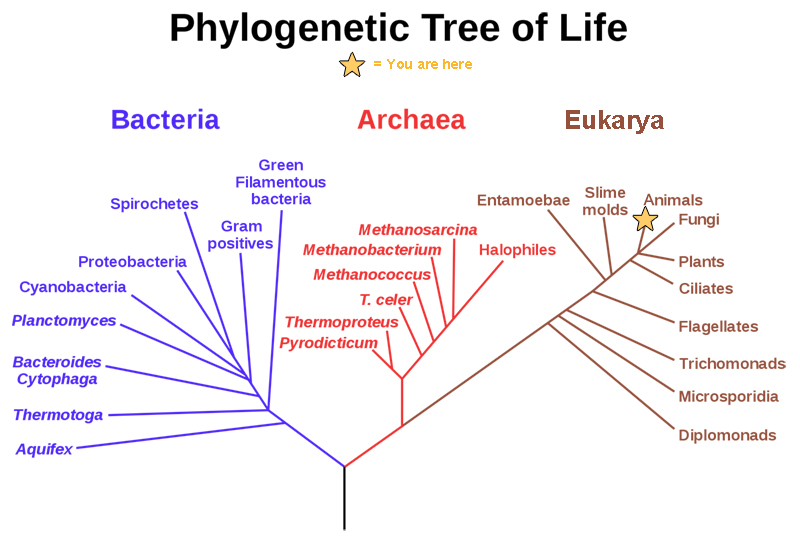
The evolution of various life forms on Earth can be summarized in a phylogenetic tree (Figure 1). A phylogenetic tree is a diagram showing the evolutionary relationships among biological species based on similarities and differences in genetic or physical traits or both. A phylogenetic tree is composed of branches (the lines) and nodes (places where two lines diverge). The internal nodes represent ancestors and are points in evolution when, based on scientific evidence, an ancestor is thought to have diverged to form two new species. The length of each branch is proportional to the time elapsed since the split.
While this is the most common way that is used to group organisms, other divisions have been proposed.
- Some scientists believe that organisms should be divided into two groups: Prokaryota (or Monera) and Eukaryota. In this method, Archae is typically included in Prokaryota. This view has become less popular due to scientific advancements, specifically genetic analysis of various organisms.
- Another two-group division groups Archae with Eukaryotes. This is often called the “Eocyte hypothesis”. This hypothesis has become more popular as the genomes of more Archaeic organisms are sequenced.
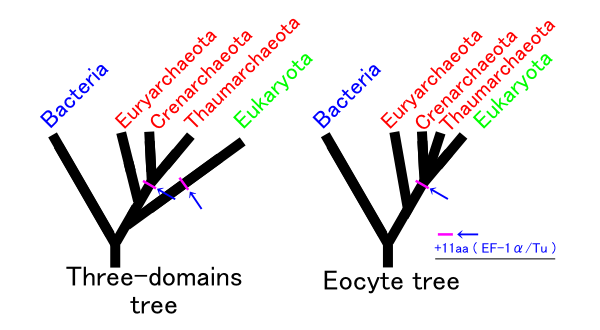
None of the three systems currently include non-cellular life. As of 2011 there is talk about Nucleocytoplasmic large DNA viruses possibly being a fourth branch domain of life, a view supported by researchers in 2012.
Stefan Luketa in 2012 proposed a five-domain system, adding Prionobiota (acellular and without nucleic acid) and Virusobiota (acellular but with nucleic acid) to the traditional three domains.
Evolution Connection
Carl woese and the phylogenetic tree.
In the past, biologists grouped living organisms into five kingdoms: animals, plants, fungi, protists, and bacteria. The organizational scheme was based mainly on physical features, as opposed to physiology, biochemistry, or molecular biology, all of which are used by modern systematics. The pioneering work of American microbiologist Carl Woese in the early 1970s has shown, however, that life on Earth has evolved along three lineages, now called domains—Bacteria, Archaea, and Eukarya. The first two are prokaryotic cells with microbes that lack membrane-enclosed nuclei and organelles. The third domain contains the eukaryotes and includes unicellular microorganisms together with the four original kingdoms (excluding bacteria). Woese defined Archaea as a new domain, and this resulted in a new taxonomic tree (Figure 1). Many organisms belonging to the Archaea domain live under extreme conditions and are called extremophiles. To construct his tree, Woese used genetic relationships rather than similarities based on morphology (shape).
Woese’s tree was constructed from comparative sequencing of the genes that are universally distributed, present in every organism, and conserved (meaning that these genes have remained essentially unchanged throughout evolution). Woese’s approach was revolutionary because comparisons of physical features are insufficient to differentiate between the prokaryotes that appear fairly similar in spite of their tremendous biochemical diversity and genetic variability (Figure 3). The comparison of homologous DNA and RNA sequences provided Woese with a sensitive device that revealed the extensive variability of prokaryotes, and which justified the separation of the prokaryotes into two domains: bacteria and archaea.

Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax .
Text adapted from:
OpenStax , Concepts of Biology. OpenStax CNX. May 25, 2017 https://cnx.org/contents/[email protected]:gNLp76vu@13/Themes-and-Concepts-of-Biology
Eocyte Hypothesis, Wikipedia. May 25, 2017. https://en.wikipedia.org/wiki/Eocyte_hypothesis
Domain (biology), Wikipedia. May 25, 2017. https://en.wikipedia.org/wiki/Domain_(biology)
Principles of Biology Copyright © 2017 by Lisa Bartee, Walter Shriner, and Catherine Creech is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
Modern Classification Systems
Article objectives.
- To identify the four new kingdoms that were added to the original Linnaean taxonomy.
- To describe the three domains of the three-domain system of classification.
- To explain why the three-domain system may need revision in the future.
Linnaeus established two kingdoms of organisms in his classification system: Plantae (the plant kingdom) and Animalia (the animal kingdom). Since then, scientists have repeatedly revised the Linnaean system. They have added several new kingdoms and other taxa. These changes were necessary as scientists learned more about life on Earth.
New Kingdoms
Between 1866 and 1977, a total of four new kingdoms were added to the original plant and animal kingdoms identified by Linnaeus. The new kingdoms include Protista (protists), Fungi, Monera (eubacteria), and Archaea (archaebacteria). Table 1 identifies the scientists who introduced the kingdoms and the dates the kingdoms were introduced. The table starts with the two-kingdom system introduced by Linnaeus in 1735.
Table 1: Kingdoms in the Classification of Organisms
The Protist Kingdom
When Linnaeus created his taxonomy, microorganisms were almost unknown. As scientists began studying single-celled organisms under the microscope, they generally classified them as either plants and or animals. For example, bacteria are single-celled organisms, some of which make their own food. They were classified as plants, which also make their own food. Protozoa are single-celled organisms that can move on their own. They were classified as animals, which are organisms that have independent movement.
As more single-celled organisms were identified, many didn’t seem to fit in either the plant or the animal kingdom. As a result, scientists could not agree on how to classify them. To address this problem, in 1866, biologist Ernst Haeckel created a third kingdom for all single-celled organisms. He called this kingdom Protista. Figure 1 shows drawings that Haeckel made of several different types of protists as they looked under a microscope. The drawings show some of the diversity of microorganisms.
Figure 1: Diversity of Protists . Biologist Ernst Haeckel made these drawings of various types of single-celled organisms as viewed under a microscope. Based on his extensive knowledge of the diversity of microorganisms, Haeckel introduced a new kingdom just for single-celled life forms, called the protist kingdom. This was the first major change in the original Linnaean taxonomy.
The Bacteria Kingdom Haeckel’s protist kingdom represented all known single-celled organisms, including both bacteria and protozoa. In the early 1900s, scientists discovered that bacterial cells are very different not only from plant and animal cells but also from the cells of protists, such as protozoa. Figure 2 shows a bacterial cell, a protozoan cell, and an animal cell. When you compare the three cells, what differences do you see? The major difference is that, unlike the protozoan and animal cells, the bacterial cell does not contain a nucleus surrounded by a nuclear membrane. Instead, its DNA is found in the cytoplasm of the cell. Organelles in the bacterial cell also lack surrounding membranes.
Figure 2: Prokaryote and Eukaryote Cells. Prokaryote and eukaryote cells differ significantly in their structure. Unlike prokaryote cells (upper figure), eukaryote cells (middle figure, protist cell; lower figure, animal cell) have a nucleus, which is separated by membranes from the cytoplasm of the cell. Their organelles also have membranes. Herbert Copeland thought that these and other differences were significant enough to place prokaryote and eukaryote organisms in different superkingdoms.
In the 1920s, microbiologist Edouard Chatton gave bacteria the name prokaryotes. He defined prokaryote as an organism whose cells lack nuclei. He gave the name eukaryotes to all other organisms. He defined eukaryote as an organism whose cells have nuclei. Chatton proposed placing prokaryotes and eukaryotes in a new taxon above the kingdom, called the superkingdom. However, this idea did not catch on, and most biologists continued to place bacteria in the protist kingdom.
Over the next several decades, scientists learned more about the tremendous number and diversity of bacteria. They started to see a need for a separate bacteria kingdom. By 1956, biologist Herbert Copeland proposed placing bacteria in a new kingdom called Monera. With the addition of the Monera kingdom, Linnaean taxonomy became a four-kingdom system (See Table 1).
Bacteria are the most numerous organisms on Earth. In a single gram of soil, there are typically 40 million bacterial cells. The human body also contains 10 times as many bacterial cells as human cells. Most of these bacteria are on the skin or in the digestive tract.
The Fungi Kingdom
In the late 1960s, ecologist Robert Whittaker proposed adding a fifth kingdom to Linnaean taxonomy to represent fungi. Fungi are eukaryote organisms such as mushrooms and molds. Up until then, fungi had been classified in the plant kingdom. Whittaker separated fungi from plants on the basis of differences in metabolism. Plants make their own food in the process of photosynthesis, whereas fungi obtain nutrients by breaking down dead organisms. Separating fungi from plants resulted in five kingdoms, which are illustrated in Figure 3. The five-kingdom system soon became widely accepted.
Figure 3: This five-kingdom system of classification was proposed by ecologist Robert Whittaker in the late 1960s. Whittaker added the Fungi kingdom to the earlier four-kingdom classification system.
Two Bacterial Kingdoms
By the 1970s, scientists had started to classify organisms in ways that reflected evolutionary relationships. They had also started using nucleic acid base sequences to identify these relationships. Nucleic acid sequence data are especially useful for studying bacteria. These organisms are so small that they have few physical traits.
Studies have bacterial nucleic acid sequences have yielded some surprising results. For example, in their research on ribosomal RNA base sequences, microbiologist Carl Woese and his colleagues discovered that bacteria actually include two very different groups of organisms. They called the two groups Eubacteria and Archaebacteria . Examples of organisms from each group are shown in Figure 4. Although the two types of organisms are similar in appearance, their ribosomal RNA sequences are very different. In 1977, Woese and his colleagues suggested that the original bacteria kingdom should be divided into two new kingdoms, called Eubacteria and Archaebacteria. This resulted in a six-kingdom taxonomy that has been widely accepted for many years.
Figure 4: Left, Eubacteria (now called Bacteria), Right, Archaebacteria (now called Archaea). Appearances can be deceiving! These two microorganisms are very different from one another, despite their outward similarities. Both organisms used to be classified in the bacteria kingdom. Woese suggested placing them in different kingdoms, called the eubacteria and archaebacteria kingdoms.
Woese wasn’t completely happy with the six-kingdom system. It didn’t show that all four eukaryote kingdoms are more closely related to each other than to the two bacteria kingdoms. It also didn’t show that the two bacteria kingdoms are as different from each other as they are from the eukaryote kingdoms. To show these similarities and differences, Woese introduced a new taxon called the domain . He defined domain as a taxon higher than the kingdom.
The Three-Domain System
In 1990, Woese and his colleagues proposed a new classification system containing three domains: Bacteria, Archaea, and Eukarya. As shown in Figure 5, the Bacteria domain was formerly the Eubacteria kingdom, and the Archaea domain was formerly the Archaebacteria kingdom. The Eukarya domain includes all four eukaryote kingdoms: plants, animals, protists, and fungi. The three-domain system emphasizes the similarities among eukaryotes and the differences among eukaryotes, bacteria, and archaea. By using domains, Woese was able to show these relationships without replacing the popular six-kingdom system.
Figure 5: This diagram shows how the three-domain system of classification is related to the six-kingdom system. Both Eubacteria and Archaebacteria kingdoms are raised to the level of domains (Bacteria and Archaea domains, respectively) in the three-domain system. The other four kingdoms make up the third domain (Eukarya domain).
Archaea were first found in extreme environments. For example, they were found in the hot water geysers in Yellowstone National park. Archaea have since been found in all of Earth’s habitats. They are now known to be present everywhere in high numbers. They may contribute as much as 20 percent to Earth’s total biomass.
Woese’s three-domain system was quickly adopted by many other biologists. There were some critics, however, who argued that the system put too much emphasis on the uniqueness of Archaea. Later studies confirmed how different Archaea are from other organisms. For example, organisms belogning to Archaea were found to differ from both Eukarya and Bacteria in the composition of their cell membranes and the system they use for DNA replication. These differences convinced most critics that the three-domain system was justified. After its introduction in 1990, the three-domain system became increasingly popular. Within a decade of its introduction, it had largely replaced earlier classifications.
How Are the Three Domains Related?
Comparing ribosomal RNA base sequences, Woese and his colleagues also showed that organisms belonging to Eukarya are more similar to Archaea than they are to Bacteria. Figure 6 is a phylogenetic tree based on their analysis. This tree places Archaea and Eukarya in the same clade. It represents the hypothesis that Archaea and Eukarya shared a more recent common ancestor with each other than with Bacteria.
The results of a study published in 2007 seem to conflict with this hypothesis. Comparing DNA base sequences, the 2007 study suggested that the domain Archaea may be older than either Bacteria or Eukarya. That would make Archaea the most ancient group of organisms on Earth. It is not yet known, which, if either, hypothesis is correct. Scientists need to learn more about Archaea and their relationships with other organisms to resolve these questions.
Figure 6: This phylogenetic tree is based on comparisons of ribosomal RNA base sequences among living organisms. The tree divides all organisms into three domains: Bacteria, Archaea, and Eukarya. Humans and other animals belong to the Eukarya domain. From this tree, organisms that make up the domain Eukarya appear to have shared a more recent common ancestor with Archaea than Bacteria.
The Future of Classification
The three-domain system is unlikely to be the final word on classification. The system is based on the current state of knowledge. As knowledge increases, the three-domain system may need revision. For example, the number of domains may change as scientists learn more about those life forms we currently know least about.
A recent discovery illustrates this point. In 2003, scientists identified a new virus called mimivirus. It resembles bacteria in size and number of genes. However, the virus cannot respond to stimuli or grow by cell division, both of which are traits of bacteria and other living organisms. Mimivirus’ unique combination of traits seems to place it at the boundary between living and nonliving things. Some scientists think mimivirus might represent a new domain of life.
Table and Images courtesy of:
Table Source: http://en.wikipedia.org/wiki/Kingdom_%28biology%29, License: GNU Free Documentation
http://en.wikipedia.org/wiki/Image:Protist_collage.jpg. Public Domain.
http://water.me.vccs.edu/courses/ENV108/lesson6b.htm http://en.wikipedia.org/wiki/Image:Animal_cell_structure.svg. Public Domain, CC-BY-SA, Public Domain.
http://upload.wikimedia.org/wikipedia/en/a/a4/5kingdoms.png. Commons.
http://en.wikipedia.org/wiki/Image:Halobacteria.jpg. Public Domain, Public Domain.
http://en.wikipedia.org/wiki/Image:Phylogenetic_tree.svg. Commons.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.9.1: Kingdom Plantae - Evolution and Phylogeny
- Last updated
- Save as PDF
- Page ID 105417

- Tara Jo Holmberg
- Northwestern Connecticut Community College
Kingdom Plantae - Evolution and Phylogeny
- Please read and watch the following Learning Resources
- Reading the material for understanding, and taking notes during videos, will take approximately 90 minutes.
- Optional Activities are embedded.
- Bolded terms are located at the end of the unit in the Glossary. There is also a Unit Summary at the end of the Unit.
- If on a mobile device, use the Contents menu at the top of the page OR the links at the bottom of the page.
Learning Objectives
- Identify the challenges to life on land for plants
- Describe the timeline of plant evolution and the impact of land plants on other living things
- Identify the main characteristics of bryophytes
- Identify the new traits that first appear in pterophytes
- Describe the type of seeds produced by gymnosperms, as well as other characteristics of gymnosperms
- State which period saw the first appearance of gymnosperms and explain when they were the dominant plant life
- List the four groups of modern-day gymnosperms and provide examples of each
- Explain why angiosperms are the dominant form of plant life in most terrestrial ecosystems
- Describe the two main groups of flowering plants
Introduction
The kingdom Plantae constitutes large and varied groups of organisms. All are eukaryotic, multicellular with differentiated tissues, and photosynthetic. There are more than 300,000 species of cataloged plants. Of these, more than 260,000 are seed plants. Mosses, ferns, conifers, and flowering plants are all members of the plant kingdom.
Evolutionary History of Plants
The major event to mark the Ordovician, more than 500 Ma, was the colonization of land by the ancestors of modern land plants. Fossilized cells, cuticles, and spores of early land plants have been dated as far back as the Ordovician period in the early Paleozoic era. The oldest-known vascular plants have been identified in deposits from the Devonian. One of the richest sources of information is the Rhynie chert, a sedimentary rock deposit found in Rhynie, Scotland, where embedded fossils of some of the earliest vascular plants have been identified (Figure \(\PageIndex{1}\)).

Current evolutionary thought holds that green algae and land-dwelling plants are monophyletic. The evolutionary transition from water to land imposed severe constraints on plants. They had to develop strategies to avoid dehydration, disperse reproductive cells, support themselves without water, and capture and filter sunlight. These adaptations were required for the colonization of land begun by the bryophytes (seedless, non-vascular plants) and their ancestors. While seed plants developed adaptations that allowed them to populate even arid habitats, full independence from water did not happen in all plants. Most seedless plants still require a moist environment.
An incredible variety of seedless plants populates the terrestrial landscape (Figure \(\PageIndex{2}\)). Mosses may grow on a tree trunk, and horsetails may display their jointed stems and spindly leaves across the forest floor. Ferns dominate some forest floors. Today, seedless plants represent only a small fraction of the plants in our environment; yet, three hundred million years ago, seedless plants dominated the landscape and grew in the enormous swampy forests of the Carboniferous period. Their decomposition created large deposits of coal that we mine today.

Seeds and pollen, two critical adaptations, distinguish the seed plants from other (seedless) vascular plants. Seed plants, such as conifers and flowering plants, do not rely on water for reproduction like the seedless plants do (Figure \(\PageIndex{3}\)). They play an integral role in all aspects of life on the planet, shaping the physical terrain, influencing the climate, and maintaining life as we know it.
Fossils place the earliest distinct seed plants at about 350 Ma. The first reliable record of gymnosperms (the cone-bearing plants) dates their appearance to the Pennsylvanian period, about 319 Ma. Gymnosperms dominated the landscape in the early (Triassic) and middle (Jurassic) Mesozoic era. Angiosperms (the flowering plants) surpassed gymnosperms by the middle of the Cretaceous (about 100 Ma) in the late Mesozoic era, and today are the most abundant plant group in most terrestrial biomes.

The Major Divisions (i.e. Phyla) of Land Plants
The green algae and land plants are grouped together into a subphylum called the Streptophytina, and thus are called Streptophytes. In a further division, land plants are classified into two major groups (knowns as Divisions rather than phyla) according to the absence or presence of vascular tissue, as detailed in Figures \(\PageIndex{4}\).
Plants that lack vascular tissue (the bryophytes), which is formed of specialized cells for the transport of water and nutrients, are referred to as non-vascular plants . Liverworts, mosses, and hornworts are seedless, non-vascular plants that likely appeared early in land plant evolution. Vascular plants developed a network of cells that conduct water and solutes.
The first vascular plants (the tracheophytes ) appeared in the late Ordovician and were probably similar to lycophytes , which include club mosses (not to be confused with the mosses) and the pterophytes (ferns, horsetails, and whisk ferns). Lycophytes and pterophytes are referred to as seedless vascular plants , because they do not produce seeds.
The seed plants, or spermatophytes , form the largest group of all existing plants, and hence dominate the landscape. Seed plants include gymnosperms, most notably conifers, which produce “naked seeds,” and the most successful of all plants, the angiosperms, which produce flowers. Angiosperms protect their seeds inside chambers at the center of a flower; the walls of the chamber later develop into a fruit.
The evolutionary history of the development of each of these groups is detailed in the cladogram in Figure \(\PageIndex{5}\). Each taxa is described in more detail below.
Optional Activity \(\PageIndex{1}\)
Figure \(\PageIndex{4}\): Which of the following statements about plant divisions is false?
- Lycophytes and pterophytes are seedless vascular plants.
- All vascular plants produce seeds.
- All nonvascular embryophytes are bryophytes.
- Seed plants include angiosperms and gymnosperms.
Evolution Connection: Algae and Evolutionary Paths to Photosynthesis
Some scientists consider all algae to be plants, while others assert that only the Charophytes belong in the kingdom Plantae. These divergent opinions are related to the different evolutionary paths to photosynthesis selected for in different types of algae. While all algae are photosynthetic—that is, they contain some form of a chloroplast—they did not all become photosynthetic via the same path.
The ancestors to the green algae became photosynthetic by engulfing, but not metabolising, a green, photosynthetic bacterium about 1.65 billion years ago. That algal line evolved into the Charophytes, and eventually into the modern mosses, ferns, gymnosperms, and angiosperms. Their evolutionary trajectory was relatively straight and monophyletic. In contrast, the other algae—red, brown, golden, stramenopiles, and so on—all became photosynthetic by secondary, or even tertiary, endosymbiotic events; that is, they endosymbiosed cells that had already endosymbiosed a cyanobacterium. These latecomers to photosynthesis are parallels to the Charophytes in terms of autotrophy, but they did not expand to the same extent as the Charophytes, nor did they colonize the land.
The different views on whether all algae are Plantae arise from how these evolutionary paths are viewed. Scientists who solely track evolutionary straight lines, consider only the Charophytes as plants. To biologists who cast a broad net over living things that share a common characteristic (in this case, photosynthetic eukaryotes), all algae are plants.
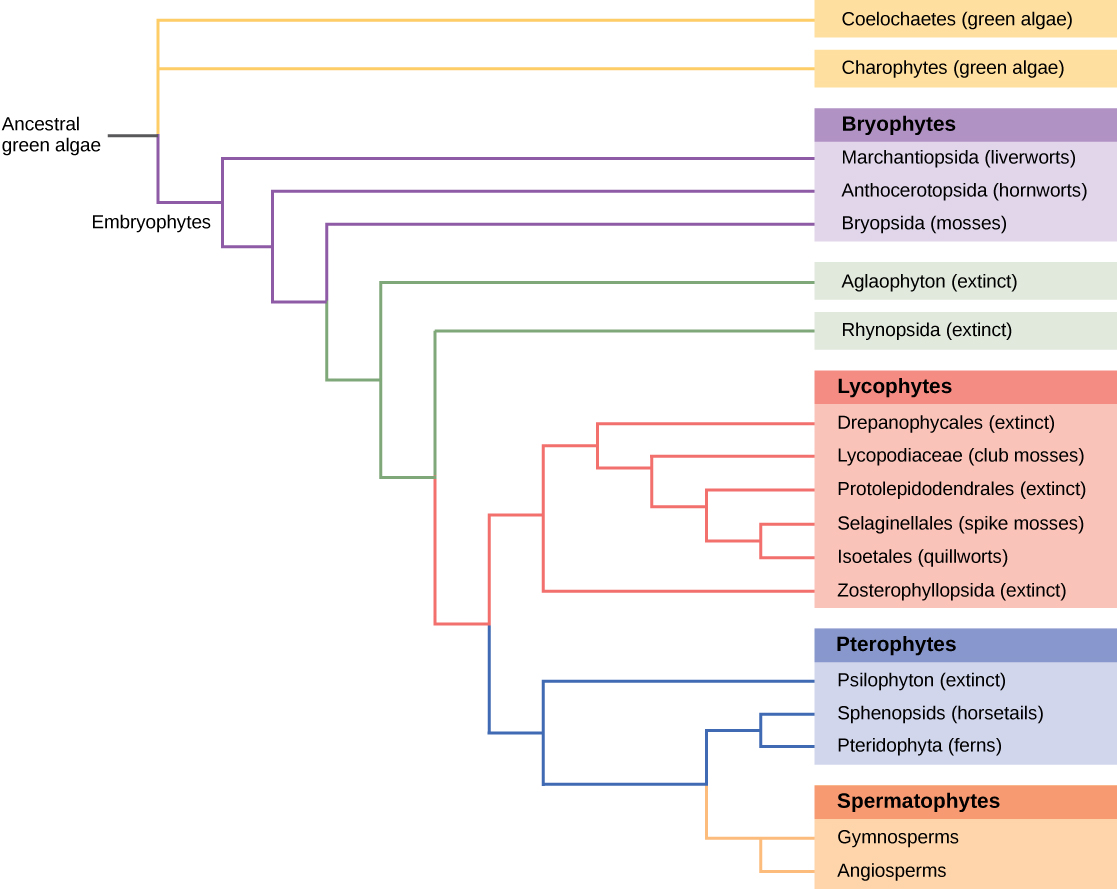
Optional Activity \(\PageIndex{2}\)
What evolved first: eukaryotic cells or cells capable of photosynthesis?
Bacteria capable of photosynthesis (cyanobacteria) evolved first and were engulfed by larger bacterial cells without being degraded. These photosynthetic cells evolved a mutualistic relationship with their hosts and are now known as chloroplasts within some eukaryotic cells.
The Seedless Plants
Bryophytes (non-vascular, seedless plants).
Bryophytes are the group of plants that are the closest extant relatives of early terrestrial plants. The first bryophytes (liverworts) most likely appeared in the Ordovician period, about 450 million years ago. Because of the lack of lignin and other resistant structures in these plants, the likelihood of bryophytes forming fossils is rather small. By the Silurian period, however, vascular plants had spread through the continents. This compelling fact is used as evidence that non-vascular plants must have preceded the Silurian period.
Today, more than 25,000 species of bryophytes thrive in mostly damp habitats, although some live in deserts. They constitute the major flora of inhospitable environments like the tundra, where their small size and tolerance to desiccation offer distinct advantages. They generally lack lignin and do not have tracheids (xylem cells specialized for forming conduits inside the plant to conduct water). Rather, water and nutrients circulate inside specialized conducting cells. Although the term non-tracheophyte is more accurate, bryophytes are commonly called nonvascular plants.
In a bryophyte, all the conspicuous vegetative organs—including the photosynthetic leaf-like structures, the thallus, stem, and the rhizoid that anchors the plant to its substrate—belong to the haploid organism or gametophyte . The sporophyte (the diploid organism) is barely noticeable. The gametes formed by bryophytes swim with a flagellum, as do gametes in a few of the tracheophytes. The sporangium , the multicellular sexual reproductive structure, is present in bryophytes and absent in the majority of algae. The bryophyte embryo also remains attached to the parent plant, which protects and nourishes it. This is a characteristic of land plants.
Bryophytes may have been successful at the transition from an aquatic habitat to land, but they are still dependent on water for reproduction and absorb moisture and nutrients through the gametophyte surface. The lack of roots for absorbing water and minerals from the soil, as well as a lack of reinforced conducting cells, limits bryophytes to small sizes. Although they may survive in reasonably dry conditions, they cannot reproduce and expand their habitat range in the absence of water.
The bryophytes are divided into three phyla: the liverworts ( Marchantiophyta ), the hornworts ( Anthocerotophyta ), and the mosses ( Bryophyta ).
Learn more about Bryophytes in this 8-minute video. Questions after watching: How would you recognize a bryophyte next time you go on a hike in the forest? What are their distinctive features? If they have no root system, how do they take in water? What about other nutrients?
Hank introduces us to nonvascular plants - liverworts, hornworts & mosses - which have bizarre features, kooky habits, and unique sex lives. Nonvascular plants inherited their reproductive cycle from algae, but have perfected it to the point where it is now used by all plants in one way or another.
The Seedless Vascular Plants
Hank introduces us to one of the most diverse and important families in the tree of life - the vascular plants. These plants have found tremendous success and their secret is also their defining trait: conductive tissues that can take food and water from one part of a plant to another part.
The vascular plants, or tracheophytes , are the dominant and most conspicuous group of land plants. More than 260,000 species of tracheophytes represent more than 90 percent of Earth’s vegetation. Several evolutionary innovations explain their success and their ability to spread to all habitats. Vascular plants can achieve enormous heights, thus competing successfully for light. Photosynthetic organs become leaves. Pipe-like cells or vascular tissues transport water, minerals, and macromolecules (especially carbohydrates) throughout the organism. By the late Devonian period, plants had evolved vascular tissue, well-defined leaves, and root systems. With these advantages, plants increased in height and size. During the Carboniferous period, swamp forests of club mosses ( Lycopodiophyta ) and ferns, horsetails, and whisk ferns ( Pteridophyta ), with some specimens reaching heights of more than 30 m, covered most of the land. These forests gave rise to the extensive coal deposits that gave the Carboniferous its name.
Water is still required for the fertilization of seedless vascular plants , and most favor a moist environment as the sperm must swim on a layer of moisture to reach the egg. This step in reproduction explains why ferns and their relatives are more abundant in damp environments. Modern-day seedless tracheophytes include club mosses, horsetails, ferns, and whisk ferns. In seedless vascular plants, the diploid sporophyte is the dominant phase of the lifecycle. The gametophyte is now an inconspicuous, but still independent, organism. Throughout plant evolution, there is an evident reversal of roles in the dominant phase of the lifecycle.
Learn more about lycophytes in this 5.5-minute video. Questions after watching: What's unique about the "plumbing system" of lycophytes? What is the most significant impact that lycophytes have had on our society?
In this 7-minute video, you will learn more about monilophytes (a smaller clade of the Pteridophyta). Question after watching: How are monilophytes different to the lycophytes you just heard about in the last video?
Gymnosperms
The fossil plant Elkinsia polymorpha , a "seed fern" from the Devonian period, about 400 Ma, is considered the earliest seed plant currently known. Seed ferns produced their seeds along their branches without specialized structures (Figure \(\PageIndex{6}\)). They are the first true seed plants due to structures called cupules that enclose and protect the ovule , the female gametophyte, and associated tissues, which then developed into a seed upon fertilization. Seed plants resembling modern tree ferns became more numerous and diverse in the swamps of the Carboniferous period.
Following the wet Mississippian and Pennsylvanian periods, which were dominated by giant fern trees, the Permian period was dry. This gave a reproductive edge to seed plants, which are better adapted to survive dry spells. The Ginkgoales, a group of gymnosperms with only one surviving species, Gingko biloba, were the first gymnosperms to appear during the lower Jurassic. Gymnosperms expanded in the Mesozoic era (about 240 Ma), supplanting ferns in the landscape, and reaching their greatest diversity during this time. The Jurassic period was as much the age of the cycads (palm-tree-like gymnosperms) as the age of the dinosaurs. Gingkoales and the more familiar conifers also dotted the landscape.
Gymnosperms , meaning “naked seeds,” are a diverse group of seed plants and are paraphyletic. Their characteristics include naked seeds, separate female and male gametes, pollination by wind, and tracheids (which transport water and solutes in the vascular system). Gymnosperm seeds are not enclosed in an ovary ; rather, they are exposed on cones or modified leaves. Sporophylls are specialized leaves that produce sporangia. The term strobilus describes a tight arrangement of sporophylls around a central stalk, as seen in cones. Some seeds are enveloped by sporophyte tissues upon maturation.
Modern gymnosperms are classified into four taxa: Coniferophyta (the conifers), Cycadophyta (the cycads), and Ginkgophyta (the ginko) are similar in their production of secondary cambium (cells that generate the vascular system of the trunk or stem and are partially specialized for water transportation) and their pattern of seed development. However, the three phyla are not closely related phylogenetically to each other. Gnetophyta (a group of 70 species of three genera) are considered the closest group to angiosperms because they produce true xylem tissue with vessel elements.

Although angiosperms (flowering plants) are the major form of plant life in most biomes, gymnosperms still dominate some ecosystems, such as the taiga (boreal forests - see Unit 4.4.3 ) and the alpine forests at higher mountain elevations because of their adaptation to cold temperatures and dry growth conditions (Figure \(\PageIndex{7}\)).

Figure \(\PageIndex{7}\): This boreal forest (taiga) has low-lying plants and conifer trees. Government of Canada: Boreal Forest
Angiosperms (The Flowering Plants)
Undisputed fossil records place the massive appearance and diversification of angiosperms in the middle to late Mesozoic era. Angiosperms (“seed in a vessel”) produce a flower containing male and/or female reproductive structures. Fossil evidence indicates that flowering plants first appeared in the Lower Cretaceous, about 125 million years ago, and were rapidly diversifying by the Middle Cretaceous, about 100 Ma (Figure \(\PageIndex{8}\)). Earlier traces of angiosperms are scarce but fossilized pollen recovered from Jurassic geological material has been attributed to possible angiosperms. A few early Cretaceous rocks show clear imprints of leaves resembling angiosperm leaves. By the mid-Cretaceous, a staggering number of diverse flowering plants crowd the fossil record. The same geological period is also marked by the appearance of many modern groups of insects, including pollinating insects that likely played a key role in ecology and the evolution of flowering plants.
Although several hypotheses have been offered to explain this sudden profusion and variety of flowering plants, none have garnered the consensus of paleobotanists (scientists who study ancient plants). New data in comparative genomics and paleobotany have, however, shed some light on the evolution of angiosperms. Rather than being derived from gymnosperms, angiosperms formed a sister clade that developed in parallel with the gymnosperms. The two innovative structures of flowers and fruit represent an improved reproductive strategy that served to protect the embryo, while increasing genetic variability and range. Paleobotanists debate whether angiosperms evolved from small woody bushes, or were basal angiosperms related to tropical grasses. Both views draw support from cladistics studies, and the so-called woody magnoliid hypothesis, which proposes that the early ancestors of angiosperms were shrubs, also offers molecular biological evidence.

From their humble and still obscure beginning during the early Jurassic period, the angiosperms—or flowering plants—have evolved to dominate most terrestrial ecosystems. With more than 250,000 species, the angiosperm phylum (Anthophyta) is second only to insects in terms of diversification. The success of angiosperms is due to two novel reproductive structures: flowers and fruit (Figure \(\PageIndex{9}\)). The function of the flower is to facilitate pollination , which helps plants mix up their genes with that of other plants to produce offspring. Flowers also provide protection for the ovule and developing embryo inside a receptacle. The function of the fruit is seed dispersal. They also protect the developing seed and assist with germination. Different fruit structures or tissues on fruit—such as sweet flesh, wings, parachutes, or spines that grab—reflect the dispersal strategies that help spread seeds.

Angiosperms are classified in a single phylum: the Anthophyta . Modern angiosperms appear to be a monophyletic group, which means that they originate from a single ancestor. Flowering plants are divided into two major groups, according to the structure of the cotyledons (the first leaves formed after germination), veins (the vascular tissue of xylem and phloem), pollen grains (haploid sperm cells), and other structures such as flower petal numbers (Table \(\PageIndex{1}\)). Monocots include grasses and lilies, and eudicots or dicots form a polyphyletic group. Basal angiosperms are a group of plants that are believed to have branched off before the separation into monocots and eudicots because they exhibit traits from both groups. They are categorized separately in many classification schemes. The Magnoliidae (magnolia trees, laurels, and water lilies) and the Piperaceae (peppers) belong to the basal angiosperm group.
Marlene Simon interviews Ernesto Sandoval to review the basic features and diversity of Angiosperms.
Highlight: Sweetgrass (Angiosperm)
"Sweetgrass is a fragrant grass with long, satiny leaves. Also known as vanillagrass, mannagrass, and holy grass, it is well known to [some] Indigenous people in Canada and the United States as a material for baskets, as well as a scent, medicine and smudge. Two closely related species are native to Canada: common sweetgrass ( Hierochloë hirta subspecies arctica ) and alpine sweetgrass ( H. alpina ). As a widely used and revered sacred plant, sweetgrass is still harvested today, and continues to play an important role in Indigenous cultures.
Sweetgrass is a perennial grass, in the family Poaceae. Usually placed in the genus Hierochloë , it is sometimes included within the genus Anthoxanthum . It has often been treated as one species, Hierochloë odorata , but currently taxonomists recognize up to four species, two of which grow in Canada: common sweetgrass ( Hierochloë hirta subspecies arctica ) and alpine sweetgrass ( H. alpina ).
The flat, bright green leaves can grow [more than] half a meter long, and have a distinctively reddish colour at their base. The flowers are small and greenish, and grow in branching clusters at the top of slender, upright stalks. The seeds are small and often infertile, and rarely germinate to create new plants. Sweetgrass’s most common means of reproduction comes from its creeping, underground stems. These stems, known as rhizomes, grow horizontally under the earth, creating dense patches of grass that can be manually divided up to produce new plants.
Sweetgrass thrives in diverse habitats and is circumpolar in distribution. Members of the genus are native to northern Eurasia, Greenland, Iceland, Canada and the United States (except in the south central and southeastern regions). In Canada, common sweetgrass grows near rivers, lake edges, and wet meadows, often forming dense patches, from sea level to subalpine zones. Alpine sweetgrass grows near sea level in the Arctic, and in meadows and on rocky slopes in subalpine and alpine areas in other parts of Canada, especially in the Pacific Coastal and Rocky Mountains."
Adapted from: Turner, N. (2018). Sweetgrass. In The Canadian Encyclopedia . Retrieved from https://www.thecanadianencyclopedia.ca/en/article/sweetgrass

"Sweetgrass" (Matt Lavin; CC BY-SA 2.0)
Thumbnail: Fern plants. (CC BY-SA 3.0; Sanjay ach via Wikimedia Commons).

- Scholarly Community Encyclopedia
- Log in/Sign up

Video Upload Options
- MDPI and ACS Style
- Chicago Style
In biology, a kingdom (Latin: regnum, plural regna) is the second highest taxonomic rank, just below domain. Kingdoms are divided into smaller groups called phyla. Traditionally, some textbooks from the United States and Canada used a system of six kingdoms (Animalia, Plantae, Fungi, Protista, Archaea/Archaebacteria, and Bacteria/Eubacteria) while textbooks in Great Britain, India, Greece, Brazil and other countries use five kingdoms only (Animalia, Plantae, Fungi, Protista and Monera). Some recent classifications based on modern cladistics have explicitly abandoned the term kingdom, noting that some traditional kingdoms are not monophyletic, meaning that they do not consist of all the descendants of a common ancestor. The terms flora (for plants), fauna (for animals), and, in the 21st century, funga (for fungi) are also used for life present in a particular region or time.
1. Definition and Associated Terms
When Carl Linnaeus introduced the rank-based system of nomenclature into biology in 1735, the highest rank was given the name "kingdom" and was followed by four other main or principal ranks: class, order, genus and species. [ 1 ] Later two further main ranks were introduced, making the sequence kingdom, phylum or division, class, order, family, genus and species. [ 2 ] In 1990, the rank of domain was introduced above kingdom. [ 3 ]
Prefixes can be added so subkingdom ( subregnum ) and infrakingdom (also known as infraregnum ) are the two ranks immediately below kingdom. Superkingdom may be considered as an equivalent of domain or empire or as an independent rank between kingdom and domain or subdomain. In some classification systems the additional rank branch (Latin: ramus ) can be inserted between subkingdom and infrakingdom, e.g., Protostomia and Deuterostomia in the classification of Cavalier-Smith. [ 4 ]
2.1. Two Kingdoms of Life
The classification of living things into animals and plants is an ancient one. Aristotle (384–322 BC) classified animal species in his History of Animals , while his pupil Theophrastus ( c. 371– c. 287 BC) wrote a parallel work, the Historia Plantarum , on plants. [ 5 ]
Carl Linnaeus (1707–1778) laid the foundations for modern biological nomenclature, now regulated by the Nomenclature Codes, in 1735. He distinguished two kingdoms of living things: Regnum Animale ('animal kingdom') and Regnum Vegetabile ('vegetable kingdom', for plants). Linnaeus also included minerals in his classification system, placing them in a third kingdom, Regnum Lapideum .

2.2. Three Kingdoms of Life
In 1674, Antonie van Leeuwenhoek, often called the "father of microscopy", sent the Royal Society of London a copy of his first observations of microscopic single-celled organisms. Until then, the existence of such microscopic organisms was entirely unknown. Despite this, Linnaeus did not include any microscopic creatures in his original taxonomy.
At first, microscopic organisms were classified within the animal and plant kingdoms. However, by the mid–19th century, it had become clear to many that "the existing dichotomy of the plant and animal kingdoms [had become] rapidly blurred at its boundaries and outmoded". [ 6 ]
In 1860 John Hogg proposed the Protoctista , a third kingdom of life composed of "all the lower creatures, or the primary organic beings"; he retained Regnum Lapideum as a fourth kingdom of minerals. [ 6 ] In 1866, Ernst Haeckel also proposed a third kingdom of life, the Protista , for "neutral organisms" or "the kingdom of primitive forms", which were neither animal nor plant; he did not include the Regnum Lapideum in his scheme. [ 6 ] Haeckel revised the content of this kingdom a number of times before settling on a division based on whether organisms were unicellular (Protista) or multicellular (animals and plants). [ 6 ]
2.3. Four Kingdoms
The development of microscopy revealed important distinctions between those organisms whose cells do not have a distinct nucleus (prokaryotes) and organisms whose cells do have a distinct nucleus (eukaryotes). In 1937 Édouard Chatton introduced the terms "prokaryote" and "eukaryote" to differentiate these organisms. [ 7 ]
In 1938, Herbert F. Copeland proposed a four-kingdom classification by creating the novel Kingdom Monera of prokaryotic organisms; as a revised phylum Monera of the Protista, it included organisms now classified as Bacteria and Archaea. Ernst Haeckel, in his 1904 book The Wonders of Life , had placed the blue-green algae (or Phycochromacea) in Monera; this would gradually gain acceptance, and the blue-green algae would become classified as bacteria in the phylum Cyanobacteria. [ 6 ] [ 7 ]
In the 1960s, Roger Stanier and C. B. van Niel promoted and popularized Édouard Chatton's earlier work, particularly in their paper of 1962, "The Concept of a Bacterium"; this created, for the first time, a rank above kingdom—a superkingdom or empire —with the two-empire system of prokaryotes and eukaryotes. [ 7 ] The two-empire system would later be expanded to the three-domain system of Archaea, Bacteria, and Eukaryota. [ 8 ]
2.4. Five Kingdoms
The differences between fungi and other organisms regarded as plants had long been recognised by some; Haeckel had moved the fungi out of Plantae into Protista after his original classification, [ 6 ] but was largely ignored in this separation by scientists of his time. Robert Whittaker recognized an additional kingdom for the Fungi. The resulting five-kingdom system, proposed in 1969 by Whittaker, has become a popular standard and with some refinement is still used in many works and forms the basis for new multi-kingdom systems. It is based mainly upon differences in nutrition; his Plantae were mostly multicellular autotrophs, his Animalia multicellular heterotrophs, and his Fungi multicellular saprotrophs.
The remaining two kingdoms, Protista and Monera, included unicellular and simple cellular colonies. [ 9 ] The five kingdom system may be combined with the two empire system. In the Whittaker system, Plantae included some algae. In other systems, such as Lynn Margulis's system of five kingdoms, the plants included just the land plants (Embryophyta), and Protoctista has a broader definition. [ 10 ]
Following publication of Whittaker's system, the five-kingdom model began to be commonly used in high school biology textbooks. [ 11 ] But despite the development from two kingdoms to five among most scientists, some authors as late as 1975 continued to employ a traditional two-kingdom system of animals and plants, dividing the plant kingdom into subkingdoms Prokaryota (bacteria and cyanobacteria), Mycota (fungi and supposed relatives), and Chlorota (algae and land plants). [ 12 ]
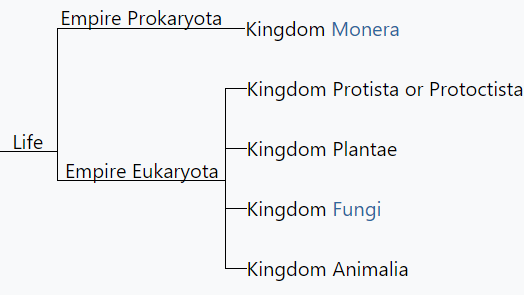
2.5. Six Kingdoms
In 1977, Carl Woese and colleagues proposed the fundamental subdivision of the prokaryotes into the Eubacteria (later called the Bacteria) and Archaebacteria (later called the Archaea), based on ribosomal RNA structure; [ 13 ] this would later lead to the proposal of three "domains" of life, of Bacteria, Archaea, and Eukaryota. [ 3 ] Combined with the five-kingdom model, this created a six-kingdom model, where the kingdom Monera is replaced by the kingdoms Bacteria and Archaea. [ 14 ] This six-kingdom model is commonly used in recent US high school biology textbooks, but has received criticism for compromising the current scientific consensus. [ 11 ] But the division of prokaryotes into two kingdoms remains in use with the recent seven kingdoms scheme of Thomas Cavalier-Smith, although it primarily differs in that Protista is replaced by Protozoa and Chromista. [ 15 ]
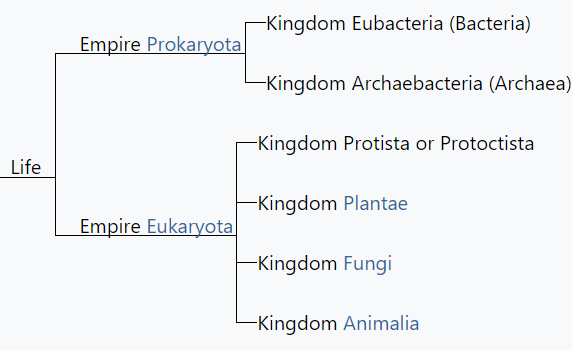
2.6. Eight Kingdoms
Thomas Cavalier-Smith supported the consensus at that time, that the difference between Eubacteria and Archaebacteria was so great (particularly considering the genetic distance of ribosomal genes) that the prokaryotes needed to be separated into two different kingdoms. He then divided Eubacteria into two subkingdoms: Negibacteria (Gram negative bacteria) and Posibacteria (Gram positive bacteria). Technological advances in electron microscopy allowed the separation of the Chromista from the Plantae kingdom. Indeed, the chloroplast of the chromists is located in the lumen of the endoplasmic reticulum instead of in the cytosol. Moreover, only chromists contain chlorophyll c. Since then, many non-photosynthetic phyla of protists, thought to have secondarily lost their chloroplasts, were integrated into the kingdom Chromista.
Finally, some protists lacking mitochondria were discovered. [ 16 ] As mitochondria were known to be the result of the endosymbiosis of a proteobacterium, it was thought that these amitochondriate eukaryotes were primitively so, marking an important step in eukaryogenesis. As a result, these amitochondriate protists were separated from the protist kingdom, giving rise to the, at the same time, superkingdom and kingdom Archezoa. This superkingdom was opposed to the Metakaryota superkingdom, grouping together the five other eukaryotic kingdoms (Animalia, Protozoa, Fungi, Plantae and Chromista). This was known as the Archezoa hypothesis, which has since been abandoned; [ 17 ] later schemes did not include the Archezoa–Metakaryota divide. [ 4 ] [ 15 ]
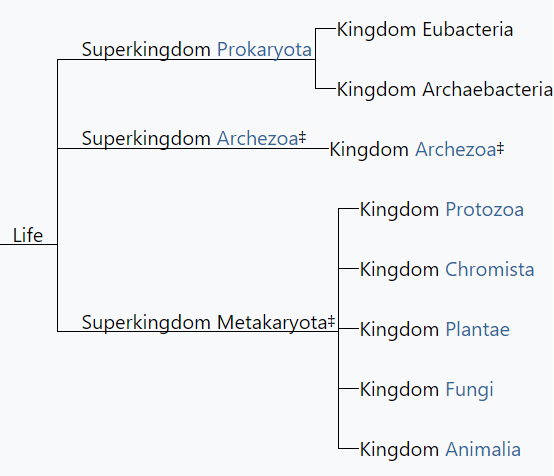
‡ No longer recognized by taxonomists.
2.7. Six Kingdoms (1998)
In 1998, Cavalier-Smith published a six-kingdom model, [ 4 ] which has been revised in subsequent papers. The version published in 2009 is shown below. [ 18 ] [ 19 ] [ 20 ] Cavalier-Smith no longer accepted the importance of the fundamental Eubacteria–Archaebacteria divide put forward by Woese and others and supported by recent research. [ 21 ] The kingdom Bacteria (sole kingdom of empire Prokaryota) was subdivided into two sub-kingdoms according to their membrane topologies: Unibacteria and Negibacteria. Unibacteria was divided into phyla Archaebacteria and Posibacteria; the bimembranous-unimembranous transition was thought to be far more fundamental than the long branch of genetic distance of Archaebacteria, viewed as having no particular biological significance.
Cavalier-Smith does not accept the requirement for taxa to be monophyletic ("holophyletic" in his terminology) to be valid. He defines Prokaryota, Bacteria, Negibacteria, Unibacteria, and Posibacteria as valid paraphyla (therefore "monophyletic" in the sense he uses this term) taxa, marking important innovations of biological significance (in regard of the concept of biological niche).
In the same way, his paraphyletic kingdom Protozoa includes the ancestors of Animalia, Fungi, Plantae, and Chromista. The advances of phylogenetic studies allowed Cavalier-Smith to realize that all the phyla thought to be archezoans (i.e. primitively amitochondriate eukaryotes) had in fact secondarily lost their mitochondria, typically by transforming them into new organelles: Hydrogenosomes. This means that all living eukaryotes are in fact metakaryotes, according to the significance of the term given by Cavalier-Smith. Some of the members of the defunct kingdom Archezoa, like the phylum Microsporidia, were reclassified into kingdom Fungi. Others were reclassified in kingdom Protozoa, like Metamonada which is now part of infrakingdom Excavata.
Because Cavalier-Smith allows paraphyly, the diagram below is an 'organization chart', not an 'ancestor chart', and does not represent an evolutionary tree.

2.8. Seven Kingdoms
Cavalier-Smith and his collaborators revised their classification in 2015. In this scheme they introduced two superkingdoms of Prokaryota and Eukaryota and seven kingdoms. Prokaryota have two kingdoms: Bacteria and Archaea. (This was based on the consensus in the Taxonomic Outline of Bacteria and Archaea, and the Catalogue of Life). The Eukaryota have five kingdoms: Protozoa, Chromista, Plantae, Fungi, and Animalia. In this classification a protist is any of the eukaryotic unicellular organisms. [ 15 ]

2.9. Summary
The kingdom-level classification of life is still widely employed as a useful way of grouping organisms, notwithstanding some problems with this approach:
- Kingdoms such as Protozoa represent grades rather than clades, and so are rejected by phylogenetic classification systems.
- The most recent research does not support the classification of the eukaryotes into any of the standard systems. (As of April 2010) , no set of kingdoms is sufficiently supported by research to attain widespread acceptance. In 2009, Andrew Roger and Alastair Simpson emphasized the need for diligence in analyzing new discoveries: "With the current pace of change in our understanding of the eukaryote tree of life, we should proceed with caution." [ 22 ]
3. Beyond Traditional Kingdoms
While the concept of kingdoms continues to be used by some taxonomists, there has been a movement away from traditional kingdoms, as they are no longer seen as providing a cladistic classification, where there is emphasis in arranging organisms into natural groups. [ 23 ]
3.1. Three Domains of Life
From around the mid-1970s onwards, there was an increasing emphasis on comparisons of genes at the molecular level (initially ribosomal RNA genes) as the primary factor in classification; genetic similarity was stressed over outward appearances and behavior. Taxonomic ranks, including kingdoms, were to be groups of organisms with a common ancestor, whether monophyletic ( all descendants of a common ancestor) or paraphyletic ( only some descendants of a common ancestor).
Based on such RNA studies, Carl Woese thought life could be divided into three large divisions and referred to them as the "three primary kingdom" model or "urkingdom" model. [ 13 ] In 1990, the name "domain" was proposed for the highest rank. [ 3 ] This term represents a synonym for the category of dominion (lat. dominium), introduced by Moore in 1974. [ 24 ] Unlike Moore, Woese et al. (1990) did not suggest a Latin term for this category, which represents a further argument supporting the accurately introduced term dominion. [ 25 ] Woese divided the prokaryotes (previously classified as the Kingdom Monera) into two groups, called Eubacteria and Archaebacteria, stressing that there was as much genetic difference between these two groups as between either of them and all eukaryotes.
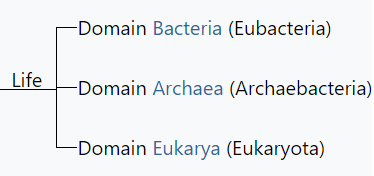
According to genetic data, although eukaryote groups such as plants, fungi, and animals may look different, they are more closely related to each other than they are to either the Eubacteria or Archaea. It was also found that the eukaryotes are more closely related to the Archaea than they are to the Eubacteria. Although the primacy of the Eubacteria-Archaea divide has been questioned, it has been upheld by subsequent research. [ 21 ] There is no consensus on how many kingdoms exist in the classification scheme proposed by Woese.
3.2. Eukaryotic Supergroups
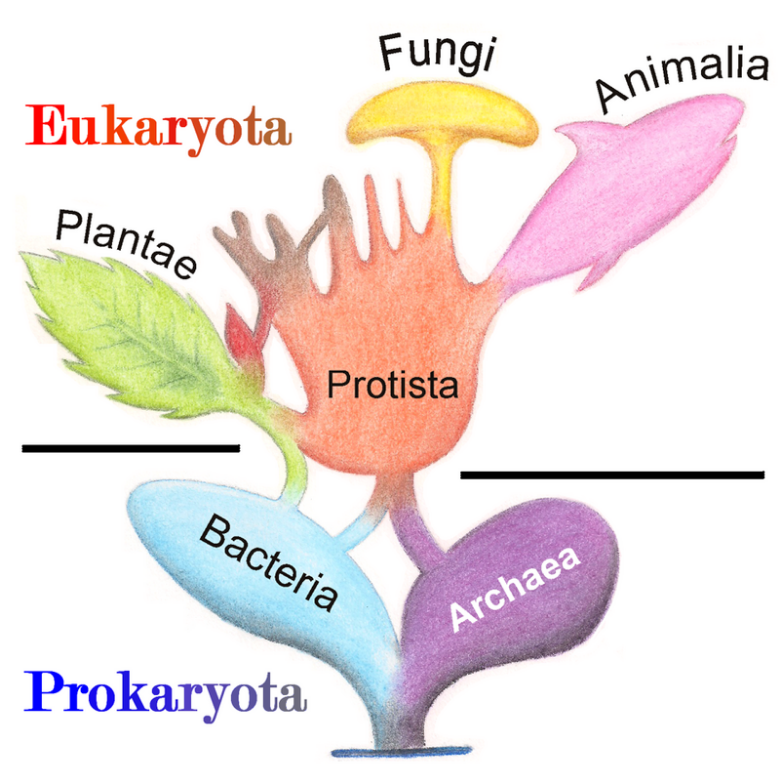
In 2004, a review article by Simpson and Roger noted that the Protista were "a grab-bag for all eukaryotes that are not animals, plants or fungi". They held that only monophyletic groups should be accepted as formal ranks in a classification and that – while this approach had been impractical previously (necessitating "literally dozens of eukaryotic 'kingdoms'") – it had now become possible to divide the eukaryotes into "just a few major groups that are probably all monophyletic". [ 23 ]
On this basis, the diagram opposite (redrawn from their article) showed the real "kingdoms" (their quotation marks) of the eukaryotes. [ 23 ] A classification which followed this approach was produced in 2005 for the International Society of Protistologists, by a committee which "worked in collaboration with specialists from many societies". It divided the eukaryotes into the same six "supergroups". [ 26 ] The published classification deliberately did not use formal taxonomic ranks, including that of "kingdom".
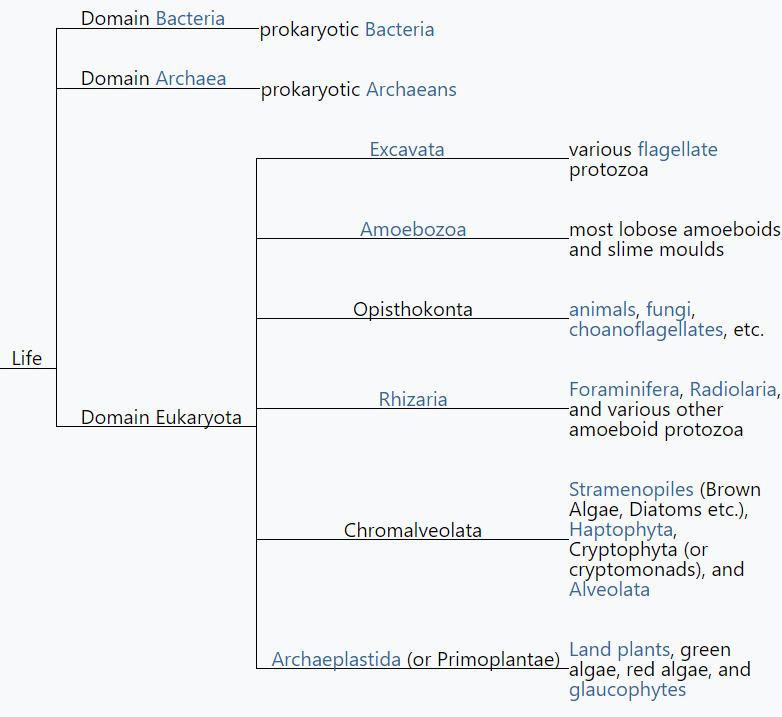
In this system the multicellular animals (Metazoa) are descended from the same ancestor as both the unicellular choanoflagellates and the fungi which form the Opisthokonta. [ 26 ] Plants are thought to be more distantly related to animals and fungi.
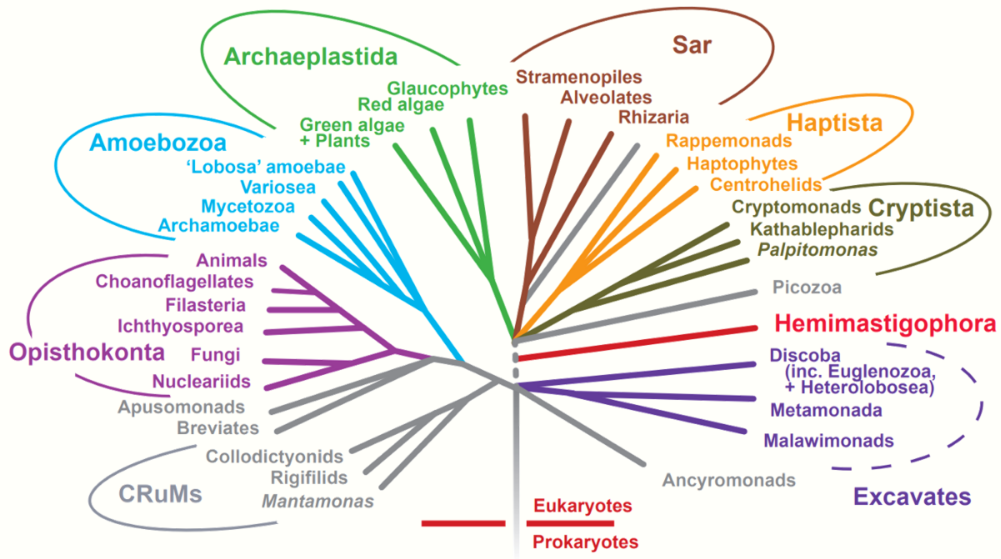
However, in the same year as the International Society of Protistologists' classification was published (2005), doubts were being expressed as to whether some of these supergroups were monophyletic, particularly the Chromalveolata, [ 27 ] and a review in 2006 noted the lack of evidence for several of the six proposed supergroups. [ 28 ]
(As of 2010) , there is widespread agreement that the Rhizaria belong with the Stramenopiles and the Alveolata, in a clade dubbed the SAR supergroup, [ 29 ] so that Rhizaria is not one of the main eukaryote groups. [ 18 ] [ 30 ] [ 31 ] [ 32 ] [ 33 ] Beyond this, there does not appear to be a consensus. Rogozin et al. in 2009 noted that "The deep phylogeny of eukaryotes is an extremely difficult and controversial problem." [ 34 ] (As of December 2010) , there appears to be a consensus that the six supergroup model proposed in 2005 does not reflect the true phylogeny of the eukaryotes and hence how they should be classified, although there is no agreement as to the model which should replace it. [ 30 ] [ 31 ] [ 35 ]
3.3. Comparison of Top Level Classification
Some authors have added non-cellular life to their classifications. This can create a "superdomain" called "Acytota", also called "Aphanobionta", of non-cellular life; with the other superdomain being "cytota" or cellular life. [ 36 ] [ 37 ] The eocyte hypothesis proposes that the eukaryotes emerged from a phylum within the archaea called the Thermoproteota (formerly known as eocytes or Crenarchaeota). [ 38 ] [ 39 ]
The International Committee on Taxonomy of Viruses uses the taxonomic rank "kingdom" for the classification of viruses (with the suffix -virae ); but this is beneath the top level classifications of realm and subrealm. [ 40 ]
There is ongoing debate as to whether viruses can be included in the tree of life. The arguments against include the fact that they are obligate intracellular parasites that lack metabolism and are not capable of replication outside of a host cell. [ 41 ] [ 42 ] Another argument is that their placement in the tree would be problematic, since it is suspected that viruses have arisen multiple times, and they have a penchant for harvesting nucleotide sequences from their hosts.
On the other hand, arguments favor their inclusion. [ 43 ] One comes from the discovery of unusually large and complex viruses, such as Mimivirus, that possess typical cellular genes. [ 44 ]
- Linnaeus, C. (1735). Systemae Naturae, sive regna tria naturae, systematics proposita per classes, ordines, genera & species.
- See e.g. McNeill, J., ed (2006). "International Code of Botanical Nomenclature (Vienna Code) adopted by the Seventeenth International Botanical Congress, Vienna, Austria, July 2005" (electronic ed.). Vienna: International Association for Plant Taxonomy. http://ibot.sav.sk/icbn/main.htm. Retrieved 2011-02-20. ,"article 3.1". http://ibot.sav.sk/icbn/no%20frames/0007Ch1Art003.htm.
- Woese, C.R.; Kandler, O.; Wheelis, M.L. (1990). "Towards a natural systs: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America 87 (12): 4576–9. doi:10.1073/pnas.87.12.4576. PMID 2112744. Bibcode: 1990PNAS...87.4576W. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=54159
- Cavalier-Smith, T. (1998). "A revised six-kingdom system of life". Biological Reviews 73 (3): 203–66. doi:10.1111/j.1469-185X.1998.tb00030.x. PMID 9809012. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=685.
- Singer, Charles J. (1931). A short history of biology, a general introduction to the study of living things. Oxford: Clarendon Press. OCLC 1197036. http://www.worldcat.org/oclc/1197036
- Scamardella, Joseph M. (1999). "Not plants or animals: a brief history of the origin of Kingdoms Protozoa, Protista and Protoctista". International Microbiology 2 (4): 207–16. PMID 10943416. http://www.ncbi.nlm.nih.gov/pubmed/10943416
- Sapp, J. (2005). "The Prokaryote-Eukaryote Dichotomy: Meanings and Mythology". Microbiology and Molecular Biology Reviews 69 (2): 292–305. doi:10.1128/MMBR.69.2.292-305.2005. PMID 15944457. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1197417
- Stanier, R.Y.; Van Neil, C.B. (1962). "The concept of a bacterium". Archiv für Mikrobiologie 42 (1): 17–35. doi:10.1007/BF00425185. PMID 13916221. https://dx.doi.org/10.1007%2FBF00425185
- Whittaker, R.H. (January 1969). "New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms". Science 163 (3863): 150–60. doi:10.1126/science.163.3863.150. PMID 5762760. Bibcode: 1969Sci...163..150W. https://dx.doi.org/10.1126%2Fscience.163.3863.150
- Margulis, Lynn; Chapman, Michael J. (2009-03-19). Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Academic Press. ISBN 9780080920146. https://books.google.com/books?id=9IWaqAOGyt4C.
- Case, Emily (2008-10-01). "Teaching Taxonomy: How Many Kingdoms?" (in en). American Biology Teacher 70 (8): 472–477. doi:10.2307/30163328. https://eric.ed.gov/?id=EJ813862. Retrieved 2020-07-28.
- Palmer, E. Laurence; Fowler, Seymour H (January 1975). Fieldbook of Natural History (2nd ed.). McGraw-Hill. ISBN 978-0-070-48425-2. https://archive.org/details/fieldbookofnatur00palm.
- Balch, W.E.; Magrum, L.J.; Fox, G.E.; Wolfe, C.R.; Woese, C.R. (August 1977). "An ancient divergence among the bacteria". J. Mol. Evol. 9 (4): 305–311. doi:10.1007/BF01796092. PMID 408502. Bibcode: 1977JMolE...9..305B. https://dx.doi.org/10.1007%2FBF01796092
- "The Six Kingdoms". Rhode Island College. http://www.ric.edu/faculty/ptiskus/six_kingdoms/index.htm.
- Ruggiero, Michael A.; Gordon, Dennis P.; Orrell, Thomas M.; Bailly, Nicolas; Bourgoin, Thierry; Brusca, Richard C.; Cavalier-Smith, Thomas; Guiry, Michael D. et al. (2015). "A higher level classification of all living organisms". PLOS ONE 10 (4): e0119248. doi:10.1371/journal.pone.0119248. PMID 25923521. Bibcode: 2015PLoSO..1019248R. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4418965
- Cavalier-Smith, Thomas (March 26, 1987). "Eucaryotes with no mitochondria". Nature 326 (6111): 332–333. doi:10.1038/326332a0. PMID 3561476. Bibcode: 1987Natur.326..332C. https://dx.doi.org/10.1038%2F326332a0
- Poole, Anthony; Penny, David (21 June 2007). "Engulfed by speculation". Nature 447 (7147): 913. doi:10.1038/447913a. PMID 17581566. http://www.cecm.usp.br/~cewinter/aulas/artigos/2007/eukarya_orig.pdf. Retrieved 15 March 2011.
- Cavalier-Smith, Thomas (2009). "Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree". Biology Letters 6 (3): 342–345. doi:10.1098/rsbl.2009.0948. PMID 20031978. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2880060
- Compared to the version Cavalier-Smith published in 2004, the alveolates and the rhizarians have been moved from Kingdom Protozoa to Kingdom Chromista.
- Cavalier-Smith, T. (2004). "Only six kingdoms of life". Proceedings of the Royal Society of London B 271 (1545): 1251–1262. doi:10.1098/rspb.2004.2705. PMID 15306349. PMC 1691724. http://www.cladocera.de/protozoa/cavalier-smith_2004_prs.pdf. Retrieved 2010-04-29.
- Dagan, T.; Roettger, M.; Bryant; Martin, W. (2010). "Genome Networks Root the Tree of Life between Prokaryotic Domains". Genome Biology and Evolution 2: 379–92. doi:10.1093/gbe/evq025. PMID 20624742. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2997548
- Roger, A.J.; Simpson, A.G.B. (2009). "Evolution: Revisiting the Root of the Eukaryote Tree". Current Biology 19 (4): R165–7. doi:10.1016/j.cub.2008.12.032. PMID 19243692. https://dx.doi.org/10.1016%2Fj.cub.2008.12.032
- Simpson, Alastair G.B.; Roger, Andrew J. (2004). "The real 'kingdoms' of eukaryotes". Current Biology 14 (17): R693–R696. doi:10.1016/j.cub.2004.08.038. PMID 15341755. https://dx.doi.org/10.1016%2Fj.cub.2004.08.038
- Moore, R.T. (1974). "Proposal for the recognition of super ranks". Taxon 23 (4): 650–652. doi:10.2307/1218807. http://www.iapt-taxon.org/historic/Congress/IBC_1975/Prop034bis-037.pdf.
- Luketa, S. (2012). "New views on the megaclassification of life". Protistology 7 (4): 218–237. http://protistology.ifmo.ru/num7_4/luketa_protistology_7-4.pdf.
- "The new higher-level classification of eukaryotes with emphasis on the taxonomy of protists". Journal of Eukaryotic Microbiology 52 (5): 399–451. 2005. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873. https://dx.doi.org/10.1111%2Fj.1550-7408.2005.00053.x
- Harper, J. T.; Waanders, E.; Keeling, P. J. (2005). "On the monophyly of chromalveolates using a six-protein phylogeny of eukaryotes". International Journal of Systematic and Evolutionary Microbiology 55 (Pt 1): 487–496. doi:10.1099/ijs.0.63216-0. PMID 15653923. https://dx.doi.org/10.1099%2Fijs.0.63216-0
- Parfrey, Laura W.; Barbero, Erika; Lasser, Elyse; Dunthorn, Micah; Bhattacharya, Debashish; Patterson, David J.; Katz, Laura A. (2006). "Evaluating support for the current classification of eukaryotic diversity". PLOS Genetics 2 (12): e220. doi:10.1371/journal.pgen.0020220. PMID 17194223. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1713255
- Burki et al. 2007, p. 4
- Burki, Fabien; Shalchian-Tabrizi, Kamran; Minge, Marianne; Skjæveland, Åsmund; Nikolaev, Sergey I.; Jakobsen, Kjetill S.; Pawlowski, Jan (2007). Butler, Geraldine. ed. "Phylogenomics reshuffles the eukaryotic supergroups". PLOS ONE 2 (8): e790. doi:10.1371/journal.pone.0000790. PMID 17726520. Bibcode: 2007PLoSO...2..790B. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1949142
- Burki, Fabien; Shalchian-Tabrizi, Kamran; Pawlowski, Jan (2008). "Phylogenomics reveals a new 'megagroup' including most photosynthetic eukaryotes". Biology Letters 4 (4): 366–369. doi:10.1098/rsbl.2008.0224. PMID 18522922. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2610160
- Burki, F.; Inagaki, Y.; Brate, J.; Archibald, J. M.; Keeling, P. J.; Cavalier-Smith, T.; Sakaguchi, M.; Hashimoto, T. et al. (2009). "Large-scale phylogenomic analyses reveal that two enigmatic protist lineages, Telonemia and Centroheliozoa, are related to photosynthetic Chromalveolates". Genome Biology and Evolution 1: 231–238. doi:10.1093/gbe/evp022. PMID 20333193. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2817417
- Hackett, J.D.; Yoon, H.S.; Li, S.; Reyes-Prieto, A.; Rummele, S.E.; Bhattacharya, D. (2007). "Phylogenomic analysis supports the monophyly of cryptophytes and haptophytes and the association of Rhizaria with chromalveolates". Mol. Biol. Evol. 24 (8): 1702–1713. doi:10.1093/molbev/msm089. PMID 17488740. https://dx.doi.org/10.1093%2Fmolbev%2Fmsm089
- Rogozin, I.B.; Basu, M.K.; Csürös, M.; Koonin, E.V. (2009). "Analysis of rare genomic changes does not support the unikont–bikont phylogeny, and suggests cyanobacterial symbiosis as the point of primary radiation of eukaryotes". Genome Biology and Evolution 1: 99–113. doi:10.1093/gbe/evp011. PMID 20333181. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2817406
- Kim, E.; Graham, L. E.; Redfield, Rosemary Jeanne (2008). Redfield, Rosemary Jeanne. ed. "EEF2 analysis challenges the monophyly of Archaeplastida and Chromalveolata". PLOS ONE 3 (7): e2621. doi:10.1371/journal.pone.0002621. PMID 18612431. Bibcode: 2008PLoSO...3.2621K. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2440802
- Minelli, Alessandro (1993). Biological systematics: The state of the art. London. ISBN 0-412-36440-9. OCLC 27895507. http://www.worldcat.org/oclc/27895507
- Archibald, John M. (23 December 2008). "The eocyte hypothesis and the origin of eukaryotic cells". PNAS 105 (51): 20049–20050. doi:10.1073/pnas.0811118106. PMID 19091952. Bibcode: 2008PNAS..10520049A. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2629348
- Lake, James A.; Henderson, Eric; Oakes, Melanie; Clark, Michael W. (June 1984). "Eocytes: A new ribosome structure indicates a kingdom with a close relationship to eukaryotes". PNAS 81 (12): 3786–3790. doi:10.1073/pnas.81.12.3786. PMID 6587394. Bibcode: 1984PNAS...81.3786L. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=345305
- "ICTV Code". International Committee on Taxonomy of Viruses. https://talk.ictvonline.org/information/w/ictv-information/383/ictv-code.
- Moreira, David; Purificación López-García (2009). "Ten reasons to exclude viruses from the tree of life". Nature Reviews Microbiology 7 (4): 306–311. doi:10.1038/nrmicro2108. PMID 19270719. https://dx.doi.org/10.1038%2Fnrmicro2108
- Luketa, Stefan (2012). "New views on the megaclassification of life". Protistology 7 (4): 218–237. http://protistology.ifmo.ru/num7_4/luketa_protistology_7-4.pdf.
- Hegde, Nagendra; Maddur, Mohan S.; Kaveri, Srini V.; Bayry, Jagadeesh (2009). "Reasons to include viruses in the tree of life". Nature Reviews Microbiology 7 (8): 615. doi:10.1038/nrmicro2108-c1. PMID 19561628. https://dx.doi.org/10.1038%2Fnrmicro2108-c1
- Raoult, Didier; Audic, Stéphane; Robert, Catherine; Abergel, Chantal; Renesto, Patricia; Ogata, Hiroyuki; La Scola, Bernard; Suzan, Marie et al. (2004). "The 1.2 megabase genome sequence of Mimivirus". Science 306 (5700): 1344–1350. doi:10.1126/science.1101485. PMID 15486256. Bibcode: 2004Sci...306.1344R. https://dx.doi.org/10.1126%2Fscience.1101485

- Terms and Conditions
- Privacy Policy
- Advisory Board


IMAGES
VIDEO
COMMENTS
Lynn Margulis (born Lynn Petra Alexander; March 5, 1938 - December 22, 2011) was an American evolutionary biologist, and was the primary modern proponent for the significance of symbiosis in evolution.Historian Jan Sapp has said that "Lynn Margulis's name is as synonymous with symbiosis as Charles Darwin's is with evolution." In particular, Margulis transformed and fundamentally framed ...
The three morphologically complex eukaryotic kingdoms (plants, animals, and fungi) will be treated as consistently as possible, optimizing both tradition and logic. The approval of specialists is sacrificed for comprehension by geneticists, molecular biologists, ... Five-Kingdom Classification and the Origin of Cells 51
Biology during the Cold War. By coincidence, Whittaker (1957) published his first article on kingdoms just a few months before the launch of Sputnik 1, but the success of the five-kingdom system owed much to the Cold War context within which it was created. Biologists eagerly turned to large-scale funding from the National Science Foundation, the Atomic Energy Commission, and other post ...
Five kingdoms of life. When Lynn Margulis was a young woman, all life was divided into two great kingdoms, known as plants and animals. But Margulis and others saw that this division did not accurately reflect the diversity of life: many organisms are neither. To combat this, Carl Woese in 1990 introduced the three-domain system, meant to ...
The hierarchy of biological classification's eight major taxonomic ranks.A domain contains one or more kingdoms. Intermediate minor rankings are not shown. In biology, a kingdom is the second highest taxonomic rank, just below domain.Kingdoms are divided into smaller groups called phyla.. Traditionally, some textbooks from the United States and Canada used a system of six kingdoms (Animalia ...
17,100. R.H. Whittaker proposed the five-kingdom classification in 1969. This classification was based upon certain characters like mode of nutrition, thallus organization, cell structure, phylogenetic relationships and reproduction. This form of kingdom classification includes five kingdoms Monera, Protista, Fungi, Plantae and Animalia.
According to Whittaker's classification, protists are one of five kingdoms, besides monera, animals, plants and fungi (Hagen 2012). They enclose diverse microorganisms that may be assigned to ...
According to Lynn Margulis and Karlene V. Schwartz (1998), each of the five kingdoms "can be uniquely defined using all the features of the whole organism — molecular, morphological, and developmental.". Every fossil or living organism can be classified into one of the five kingdoms.
Whittaker's (1969) new system of classification into five kingdoms (Monera, Protista, Animalia, Plantae and Fungi) has made a fundamental contribution to the clarification of relationships among … Expand. 36. PDF. Save. New concepts of kingdoms of organisms. R. Whittaker. Biology. 1969; TLDR.
July 22, 2023 by Ramzan Asghar. In the five kingdom classification system organisms are divided into Monera, Protista, Fungi, Plantae & Animalia based on cells, nutrition, and evolution. The Five Kingdom Classification System. Classification organizes organisms into groups, or sets based on their similarities and differences.
Name the four kingdoms of the Domain Eukarya and recognize a description of each. ... It has been estimated that the total number of microbial cells on Earth on the order of 2.5 X 10 30 cells, ... Some of the evidence behind this hypothesis is based on a "superphylum" of bacteria called PVC, members of which share some characteristics with both ...
The 5 Kingdoms of life are: Kingdom Animalia- Eg. Polar Bears. Kingdom Plantae- Eg. Coconut trees. Kingdom Fungi- Eg. Button Mushrooms. Kingdom Monera- Eg. Lactobacillus bacteria.
A phylogenetic tree based on rRNA data, emphasizing the separation of bacteria, archaea, and eukarya as proposed by Carl Woese et al. in 1990, with the hypothetical last universal common ancestor. The three-domain system is a taxonomic classification system that groups all cellular life into three domains, namely Archaea, Bacteria and Eukarya, introduced by Carl Woese, Otto Kandler and Mark ...
Five-Kingdom System of Classification was proposed by American taxonomist R.H.Whittaker in 1969. It excludes viruses from living beings. ... It is a mode of classification in which living beings have been distributed into five kingdoms. ... Concept of Atomic Hypothesis. Preparation of Lyophobic Sols. Nuclear Fission and Liquid Drop Model.
The fact that biology, as a science, has such a broad scope has to do with the tremendous diversity of life on earth. The source of this diversity is evolution, the process of gradual change during which new species arise from older species. Evolutionary biologists study the evolution of living things in everything from the microscopic world to ...
The new kingdoms include Protista (protists), Fungi, Monera (eubacteria), and Archaea (archaebacteria). Table 1 identifies the scientists who introduced the kingdoms and the dates the kingdoms were introduced. The table starts with the two-kingdom system introduced by Linnaeus in 1735. Table 1: Kingdoms in the Classification of Organisms.
Five Kingdoms Lab #1: CLASSIFYING CRITTERS - instructions Material: copy of lab sheets (3 pages), pencil scissors colored pencils (optional) ... Go to lab page 2 and complete the hypothesis. Pair up the creatures as best you can and write your guesses down on the sheet. 3. Lo and behold the "Classification Key to Blobonian Life" arrives in ...
The following points highlight the top six concepts of the kingdom system of organisms classification. The concepts are: 1. Two Kingdom Systems 2. Three Kingdom System 3. Four Kingdom Systems 4. Five Kingdom Systems 5. Six Kingdom System 6. Eight Kingdom System.
As in my 1983 system Bacteria are treated as a single kingdom, and eukaryotes are divided into only five kingdoms: Protozoa, Animalia, Fungi, Plantae and Chromista. Intermediate high level categories (superkingdom, subkingdom, branch, infrakingdom, superphylum, subphylum and infraphylum) are extensively used to avoid splitting organisms into an ...
Angiosperms (the flowering plants) surpassed gymnosperms by the middle of the Cretaceous (about 100 Ma) in the late Mesozoic era, and today are the most abundant plant group in most terrestrial biomes. Figure 5.9.1.3 5.9.1. 3: Seed plants dominate the landscape and play an integral role in human societies.
Biology during the Cold War. By coincidence, Whittaker published his first article on kingdoms just a few months before the launch of Sputnik 1, but the success of the five-kingdom system owed much to the Cold War context within which it was created.Biologists eagerly turned to large-scale funding from the National Science Foundation, the Atomic Energy Commission, and other post—World War II ...
Scientists divide living things into categories based on their common features. One system uses five main groups: monerans, protists, fungi, plants, and animals. These groups are called kingdoms.
The Eukaryota have five kingdoms: Protozoa, Chromista, Plantae, Fungi, and Animalia. In this classification a protist is any of the eukaryotic unicellular organisms. ... The eocyte hypothesis proposes that the eukaryotes emerged from a phylum within the archaea called the Thermoproteota (formerly known as eocytes or Crenarchaeota).