An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Korean Med Sci
- v.38(25); 2023 Jun 26
- PMC10293659


Ethics Committees: Structure, Roles, and Issues
Pankti mehta.
1 Department of Clinical Immunology and Rheumatology, King George’s Medical University, Lucknow, India.
Olena Zimba
2 Department of Clinical Rheumatology and Immunology, University Hospital in Krakow, Krakow, Poland.
3 National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland.
4 Department of Internal Medicine N2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine.
Armen Yuri Gasparyan
5 Departments of Rheumatology and Research and Development, Dudley Group NHS Foundation Trust (Teaching Trust of the University of Birmingham, UK), Russells Hall Hospital, Dudley, UK.
Birzhan Seiil
6 Department of Biology and Biochemistry, South Kazakhstan Medical Academy, Shymkent, Kazakhstan.
Marlen Yessirkepov
An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty. MEDLINE, Scopus, and Directory of Open Access Journals were searched for studies relevant to this topic. This review is focused on the types of research articles that need EC approval, the submission process, and exemptions. It further highlights the constitution of ECs, their duties, the review process, and the assessment of the risk-benefit of the proposed research including privacy issues. It’s pertinent for academicians and researchers to abide by the rules and regulations put forth by ECs for upholding of human rights and protecting research subjects primarily, as well as avoiding other issues like retraction of publications. Despite various issues of cost, backlogs, lack of expertise, lesser representation of laypersons, need for multiple approvals for multisite projects, conflicts of interest, and monitoring of ongoing research for the continued safety of participants, the ECs form the central force in regulating research and participant safety. Data safety and monitoring boards complement the ECs for carrying out continuous monitoring for better protection of research subjects. The establishment of ECs has ensured safe study designs, the safety of human subjects along with the protection of researchers from before the initiation until the completion of a study.
Graphical Abstract

INTRODUCTION
The journey of the role of ethics in biomedical research began with “The Doctor’s Trial” post-World War II in which 23 doctors and administrators were tried for war crimes, crimes against humanity, and conducting research without informed consent. This judgment, known as the “Nuremberg Code” was one of the first international ethical standards which gave a ten-point rule with respect to the protection of human research participants. The core principle was the requirement of voluntary consent of human subjects and respecting human autonomy. 1 , 2
However, some researchers continued to ignore the code and violations like the Willow Brook Hepatitis Study (1956), Jewish Chronic Disease Study (1963), and 22 others were highlighted by Beecher in 1966. 3 , 4 This led to the composition of the Declaration of Helsinki by the World Medical Association in Finland in 1964 with revisions at regular intervals. 5 This affirmed the principles highlighted in the Nuremberg Code stating that research should be conducted upholding the interests and rights of the human subjects. It proposed for the first time, the submission of a research protocol to an ethics committee (EC) before the initiation of the study ( Fig. 1 ). 6

EC refers to “Committees established by professional societies, health facilities, or other institutions to consider decisions that have bioethical implications. The role of these committees may include consultation, education, mediation, and/or review of policies and practices.” Committees that consider the ethical dimensions of patient care are Clinical ECs whereas committees established to protect the welfare of research subjects are Research ECs. 7 In this review, we will be using the terms ECs and Research ECs interchangeably.
It was in the 1960s that most nations developed guidelines regarding the formation of ECs with the main task of protection of human subjects. 8 EC is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects. ECs can be of two types—Institutional Review Boards (IRBs) or Institutional ECs (IECs) (referred to IRB or IEC by different countries) that are formally constituted by an institution to review research projects for that institute. An independent EC is an autonomous EC that is not part of any institute and performs the same functions independently. It is helpful for institutes that don’t have an IRB.
Despite these regulations, the unethical standards of the Tuskegee Syphilis study emerged in 1972 in which treatment was denied to the participants in order to study the natural course of the disease. This was followed by the conversion of the National Research Act in the USA into law (1974) and the setting up of the national commission of ‘International Ethical Guidelines for Biomedical Research Involving Human Subjects’ that submitted the Belmont report in 1979. The Belmont report described the role of assessment of risk-benefit of research involving human subjects, appropriate guidelines for selection of human subjects, and definition of informed consent. It was based on the three pillars of ethics- respect, beneficence, and justice. 9 , 10 It stressed the need for the approval of studies by an EC in accordance with the 1975 revision of the World Medical Association in Tokyo. Subsequently, countries like China, India, and South Korea adopted and legalized the need for submission of protocols to ECs from the 1980s onwards. 11 , 12 , 13 , 14
ECs function on six basic principles 15 :
- 1. Autonomy: respect the patient’s right to act on his/her own value and choice.
- 2. Justice: fair treatment of the research subjects.
- 3. Beneficence: work for the benefit of the patient.
- 4. Nonmaleficence: primum non-nocere or first do no harm to the patient.
- 5. Confidentiality: privacy protection.
- 6. Honesty: truthfulness in terms of the study.
Ethics approval is required for most research studies to uphold the above-mentioned principles, and protect the participants as well as the researcher. 16
In this narrative review, we aim to study the structure and function of ECs or IRBs with a focus on the composition, role, violations, and development perspectives of ECs.
Searches through MEDLINE (PubMed) and Scopus were performed in line with previously published recommendations. 17
Articles published till March 15, 2023 were reviewed using the following keywords: ("Ethics Committees, Clinical/classification"[Mesh] OR "Ethics Committees, Clinical/economics"[Mesh] OR "Ethics Committees, Clinical/ethics"[Mesh] OR "Ethics Committees, Clinical/history"[Mesh] OR "Ethics Committees, Clinical/legislation and jurisprudence"[Mesh] OR "Ethics Committees, Clinical/organization AND administration"[Mesh] OR "Ethics Committees, Clinical/standards"[Mesh] OR "Ethics Committees, Clinical/statistics and numerical data"[Mesh] OR "Ethics Committees, Clinical/trends"[Mesh]). Additional searches about subtopics were also carried out (“Data Safety Monitoring Boards” OR “Independent Data Review Committees”, “Institutional Review Boards” OR “Ethics Committees” and “Problems” OR “Issues”).
Articles in languages other than English, and reviews, conference proceedings, and editorials were excluded. Relevant articles searchable at the Directory of Open Access Journals and references of included articles were also processed for eligibility and inclusion for this narrative review. 18 , 19 , 20 , 21
RESEARCH, SUBMISSION PROCESS, AND EXEMPTIONS
IRB approval is required for most research to protect human rights and assess the scientific soundness of the research. For this, we first need to understand what research is. Research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge” ( Table 1 ). 8
IRB = Institutional Review Board.
An EC approval is required for studies with more than minimal risk to the subjects where the intention is to publish findings or contribute to the scientific knowledge, studies involving the compilation or analysis of data containing patient identifying information, studies with any risk of physical or mental discomfort to participants or their families, and studies on vulnerable groups. 22 Minimal risk refers to the probability of discomfort posed by the research is not greater than that ordinarily encountered in routine daily life activities of an average healthy individual. 5 , 8 , 22
Thus, even surveys and archived data that contain patient identifying information (name, age, address) and sensitive information (illicit drug use, comorbidities, communicable diseases, e.g., HIV AIDS) need ethical approval to uphold the privacy and anonymity of the participants as well as protection the possibility of psychological discomfort to them. 10 , 23 , 24 , 25
Some studies may be exempted from ethical approval including most educational research, case reports on one to three patients (without any hypothesis testing), those that pose no risk to the participants, involve information freely available in the open domain for the community, analysis of open-source datasets or anonymized datasets obtained from other researchers with due informed consent taken at the time of primary data collection, research evaluating the public health programs or government public schemes. 26 , 27 However, a formal exemption is to be decided by the IRB and not the investigator. 8 , 28
For projects requiring an EC approval, the type of reviews includes expedited and a full board review. Expedited review is for research involving no more than minimal risk to the subjects, minor revisions of an already approved study, and is usually conducted by an experienced person or the chair of the IRB. A full board review on the other hand is for research with greater than minimal risk to the subjects or those involving vulnerable populations. This is reviewed extensively by a full IRB meeting.
The documents usually required for a full ethics review include the name of the applicant with designation, approval of the head of the department, research/trial protocol, ethical issues if any, and plans to address them, written informed consent form (and assent forms) in the language the participant understands, data collection tools, patient information sheet, regulatory clearances (e.g., Drug Controller General of India in India for drug trials), finance and funding details, Insurance, statement of conflicts of interest, information about payment or compensation to the subjects, scientific or departmental review board permission, Curriculum Vitae of the investigators, declaration of interests and any other relevant information. 29 , 30 Waivers of consent may be provided for no more than minimal risk to the subjects when the waiver will not endanger the rights and welfare of the subjects like retrospective studies, secondary analysis of data wherein consent had been taken previously, use of open access databases with anonymized data, and emergency research as seen fit by the EC. 31 , 32 In emergencies like the coronavirus disease 2019 pandemic, waivers may be provided if the patient is incapacitated or in life-threatening situations where there is no time for informed consent. Pandemics like these may even call for common documents for risk disclosure and audio/video/electronic consent. 33 , 34
EC PERSONNEL, THEIR EXPERIENCE, AND DUTIES
ECs have the primary responsibility of reviewing research and its alignment with the Good Clinical Practice (GCP) guidelines. 35 The research design must be scientifically sound and conducted in an ethical way to include human subjects with voluntary informed consent.
The composition of ECs varies depending on the country, center, volume, and nature of the research reviewed. However, there are some basic recommendations laid down by national authorities and GCP. 30
- a) Most countries in Europe, the USA, and South Korea have a requirement of at least five members whereas recommendations in China and India need a minimum of seven members and a maximum of 12–15 members. 14 , 36 , 37 , 38 , 39
- b) At least one member who is autonomous, independent of the institution or trial site. It is mandatory that the chairperson of the EC is not part of the institution where the research is to be conducted.

Others include a member secretary from within the institution and members from the scientific field. The composition should have an adequate gender and age representation with a blend of basic scientists, clinician scientists, one legal expert, one social scientist, one philosopher, and one layperson. Review of research involving vulnerable populations like children, pregnant women, handicapped, prisoners, etc., must involve one member with expertise in dealing with that population. 40 , 41 It is also desirable to have a member or expert advisor for special areas of research who has proficiency in that field.
Responsibilities
The chairperson has the primary responsibility of independent and smooth functioning of the EC, ensuring the participation of all members, seeking Conflict of Interests from all members, and handling complaints against the researchers and EC members. 39 It’s the responsibility of the member secretary to schedule EC meetings, handle documentation, organize an effective review of proposals, define and maintain adherence to standard operating procedures (SOPs), train EC members, and assess the need for expedited reviews/exemption from review. 39 The members of the scientific community have the primary responsibility of reviewing the research protocols and their scientific soundness. The non-scientist member is crucial to safeguard the human subjects and practical issues of the research. 40 , 41 However, studies have shown lesser participation by laypersons as compared to scientific members. A study conducted across 10 academic centers across the USA with 20 IRB meetings recorded noted that 29 community members were present in 17 of those meetings. They were primary reviewers in only two of the 93 submitted protocols due to refusal on grounds of lack of knowledge regarding medical research. Even as secondary and tertiary reviewers, they were less active and were more likely to focus on issues related to confidentiality. However, they played a greater role when they were not designated reviewers. 42
The EC or IRBs function to review and approve research protocols, monitor ongoing research involving human subjects with the aims of continual protection of human volunteers, advancement of research, and protecting the institute from litigation. Its main role is the protection of the human rights, autonomy, confidentiality, and welfare of the research subjects especially vulnerable populations. The GCP recommends the following for duties of the IRB ( Table 2 , Fig. 3 ) 35 :
e.g., An immunosuppressive drug “X” being evaluated for patients with Lupus Nephritis.
IRB = Institutional Review Board, SOC = standard of care, GCP = Good Clinical Practice, MMF = mycophenolate mofetil, CYC = cyclophosphamide, ICU = intensive care unit, SOP = standard operating procedure, RCT = randomized controlled trial.

- • The IRB should obtain and review all the necessary documents for the research/trial within a reasonable time and document its views following standardized operating procedures with clear identification of the dates for approval, modifications, disapproval, or termination of an ongoing trial that was initially approved in writing.
- • Qualification of the investigators should be considered for the proposed research.
- • Reviewing of ongoing research as appropriate to the risks involved (at least once a year).
- • Protocols indicating exemption of prior consent of the subject or their legally acceptable representative (e.g., emergency situations) should be assessed in detail for all the regulatory needs.
- • Review the sum and method of compensatory payment to subjects if required.
- • Functions should be performed as per written SOPs which should comply with the GCP guideline.
Most IRBs conduct meetings regularly (one–two per month depending on the number of protocols) and SOPs are followed as per the national governing authority.
An EC review is a continuous process and is needed before the initiation of research, before the extension of the approval period, prior to modifications to an already approved study, for monitoring of any adverse events, and until all the data collection and analysis is complete. 8 An oversight to the monitoring of trials (usually single center, early phase, less risky) is provided by the IRBs through annual reviews, adverse event monitoring, and reporting of undue events by the principal investigator (PI). However, complex clinical trials and/or multicenter, randomized controlled trials, interventional studies with pre-existing concerns about safety, or study participants who might need additional protection through an additional committee referred to as the Data Safety Monitoring Board (DSMB). 43 , 44
RISKS, BENEFITS, CONFIDENTIALITY, AND PRIVACY ISSUES IN RESEARCH PROTOCOLS
The role of the EC is not only to provide direct protection to human subjects from physical or mental harm but also to weigh the risks and benefits involved in the research. It must be assessed if the study is designed to add to the current scientific knowledge base and help society. 8
The research protocol is the document that includes the research question, aims and objectives, a critical literature review, methodology, and statistical plan. It is pertinent that the IRB reviews the protocol with respect to the clarity and focus of the research question; and whether the study design is suitable to answer the same. This is decided by the chair or a special departmental committee ( Table 2 , Fig. 3 ).
Privacy and confidentiality are a part and parcel of every physician-patient relationship. Needless to say, this must be maintained in a researcher-human subject relationship as well. It helps build trust, curbs participant anxiety, maintains their dignity, and above all their autonomy. 10 The International Committee of Medical Journal Editors recommends that authors must ensure that nonessential information like names, initials hospital record numbers, etc., are omitted during data collection, storage, and publication whenever possible. 45 However, there’s an extent to which this confidentiality can be maintained. Information required for scientific purposes (e.g., clinical photographs) or those with mandated legal reporting may breach participant privacy. This needs to be explained to the participant and recorded in written informed consent ( Table 2 ).
The role of the IRB with respect to privacy and confidentiality is to:
- • Review the consent document and assess the sensitivity of the information, the duration for which it will be held, the usefulness of the information, and the ability to protect it.
- • For multicenter projects, review the measures taken by the research team to maintain the privacy of the research subjects including the number of personnel with access to the information, data storage, and transfer.
- • An ongoing review of the research must include monitoring of confidentiality issues to check for maintenance of the same and the need for a revised privacy protection plan.
- • Educate researchers and IRB members regarding the data privacy and protection process. 46
Review of informed consent by IRBs is especially important in low-middle-income countries. There are various issues related to the lack of understanding of the information provided, maintaining privacy due to interference by family members, and the inability to assess risk and benefit by the research participant. IRBs have an additional responsibility to ensure that studies have minimal/no risk to the participant, the consent forms are clear and simple to understand and ensure the proper process of obtaining informed consent is being followed without undue pressure or coercion to participate in the study. 47
VIOLATIONS OF ETHICS APPROVAL RULES AND REGULATIONS
Violations of IRB approval rules like lack of approval, lack of approval of modifications to the protocol, and lack of informed consent can result in dire aftermaths for the authors. It can result in the withdrawal of the article if it’s still in press, retraction if it’s already published, and even removal if it has legal consequences. The number of papers retracted as searched on the retraction database 48 is steadily increasing by the decade from 474 in the 1990s to 6120 in the 2010s. The most common reason for retraction is plagiarism whereas violation of IRB rules accounts for 4–5% of all retractions. 49 , 50 When consultations for ethical inquiries to the Korean Association of Medical Journal Editor were analyzed, the most common reason was duplicate publications (12 of 80) with issues with IRB approval (5 of 80) and informed consent (6 of 80). 51 Some of the examples of types of studies and their reasons for retractions have been summarized in Table 3 .
Violations can be assessed before the studies are published for those with IRB approval. It is the responsibility of the IRBs to monitor whether ongoing studies are abiding by the ethical regulations and whether the approved protocol is being followed. A study conducted in India by an IRB at a tertiary care hospital in Mumbai monitored 12 clinical trials from 2011–2017. The most common violations were related to informed consent, followed by a lack of understanding of protocol and protocol deviations. This was corrected by re-taking of the informed consent and retraining in GCP by the IRB. 52 A similar study in Uganda done from 2007–2010 with monitoring of 40 research projects also found a similar frequency and reasons for violations. 53
Journal editors routinely check if a statement mentioning whether ethics approval was sought has been mentioned in the manuscript. Depending on the journal and type of article, further details of the EC approval can be sought by the journal editorial board. 54
ISSUES AND ONGOING DEVELOPMENTS
ECs were developed to provide ethical oversight to clinical research. But here are various issues associated with the functioning of IRBs.
- • Composition: Most studies indicate a skewed gender representation in the structure IRBs. Further, the participation of laypersons on the board is minimal. 14 , 42 , 55 , 56 , 57
- • Overburdened IRBs, delays, and operational costs: The IRB reviews have been associated with delays from over 4 to 7 months on average from surveys conducted across the USA. 58 , 59 A delay in biomedical research can translate into more than monetary loss as biomedical research saves lives and a delay in the approvals can result in greater loss of life. 60 An older survey conducted across 63 institutions (with 20 being low volume, 24 intermediate volume, and 19 being high volume centers) in the USA in 2005 reported the median amount spent by academic medical centers on IRB was $750,000/year with an average of $559 per review. The main costs are divided across staff salary, board salary, space, outsourcing of the reviews, travel, supplies, and equipment. 61 Over the years, there is a definite increase in the number of ongoing research projects thus increasing these costs further. Furthermore, documentation of Food and Drug Administration (FDA) warning letters to IRBs was predominantly related to paperwork stressing on documentation of reviews and meetings rather than ethical issues. 62 Increasing paperwork further results in delays and added costs. These deficiencies are more marked in developing nations like India and China dealing with issues like lack of regulation, informal ethics reviews, lack of supervision, and insufficient ethics review capacity. 63 , 64
- • Multi-site projects: With multicenter projects on the rise, a single protocol is often reviewed by multiple IRBs. In a review of 17 articles reported from UK, USA and Europe, which underwent multiple IRB reviews of the same protocol there were discrepancies in the judgment. Five of 26 reported rejection at some and acceptance by some IRBs. However, there were great differences in the protocol revisions, consent, patient information sheets, risk-benefit assessment, and compensation arrangements. 65 Keeping these issues in mind, the Common Rule in the USA was revised in 2017 with IRB approval required only from one center for multisite projects. 66 This may be extrapolated to other nations or consideration of an expedited review at other sites when fully reviewed at one IRB can be considered.
- • Independent EC and IEC: Independent ECs have inherent tissues of limitation of knowledge about the local community and use of these may promote IRB shopping. Whereas, local IRBs can have conflicts of interest as colleagues of investigators may be on the review board. Thus, a central IRB can alleviate some of these concerns by avoiding repetitive reviews, minimizing conflicts, and establishing a centralized adverse event reporting system. 67 , 68 , 69 A central IRB can be formed by experts on a particular subject or by a group of institutes like the National Cancer Institute’s Central IRB and the Biomedical Research Alliance of New York respectively. 70 , 71
- • Scientific expertise of the IRB reviewers: The IRB reviewers may lack the scientific expertise to review sophisticated research projects that may affect the quality of the research. 11 , 14 , 57 , 72 Regular training in research ethics and GCP along with adequate consultations with external experts is needed. This can be done at a national, regional, and international level. First, by identifying core issues and then solutions for them by focused training. 73 Training of EC members is conducted across Central Asia and Eastern Europe under the framework of Forum for Ethics Committees in the Confederation of Independent States and Strategic Initiative for Developing Capacity in Ethical Review program that train members regarding GCP, bioethics, the establishment of an EC, review processes and SOPs, choosing independent consultants, and confidentiality agreements. 74
- • Review of studies involving complementary and integrative medicine (CIM) is a challenge due to the lack of quality evidence to support the basis for their use. Moreover, most international regulatory bodies and research regulations do not address CIM, thus leaving the review process and decision-making to the IRBs. However, it is to be emphasized here studies irrespective of the type (modern or CIM) must be reviewed using the same principles of respect, beneficence, and justice. Well-designed studies on CIM are essential to ascertain the health and safety of patients. 75
DSMB is defined by the FDA, USA as “a group of individuals with pertinent scientific expertise that review research data of an ongoing trial on a regular basis, advises the sponsor/or researcher regarding the continuing safety of research subjects and those yet to be recruited into the research trial, and advises as to the continuing validity and scientific merit of the trial.” 76 It’s an autonomous entity independent of the researchers, sponsors, and the IRB so as to control data sharing and protect the authenticity of the clinical trial from unfavorable impact. 35 It was first developed in the USA in the 1960s as the NIH began sponsoring multicenter trials, the first trial was the Coronary Drug Project which used a DSMB for monitoring. 77 Over time, it became a common practice for the sponsors to have experienced scientific personnel serving on these committees. Although the FDA does not mandate DSMB for all trials, DSMBs are generally recommended for large, multi-site studies evaluating treatments that intend to reduce mortality and morbidity.
DSMBs are usually constituted for:
- • The study outcome is such that a highly encouraging or detrimental result is a possibility in an interim analysis that may require an early termination of the study on ethical grounds.
- • When the safety concerns are high, e.g., invasive therapy is administered.
- • Previous data suggesting serious toxicity with the study treatment.
- • Studies involving vulnerable populations.
- • Studies including subjects at an increased risk of death or serious outcomes.
- • Large, multisite, long-duration studies.
In India, it is recommended by the Indian GCP guidelines that the sponsor may establish a DSMB to assess the progress of the trial, and in 2006 Indian Council of Medical Research (ICMR) mandated a DSMB to review data emerging from research on interventions in the emergency setting. 39 These were updated in 2012 by the ICMR to include all stem cell research involving human subjects. The SOPs for the constitution and responsibilities of the DSMB are laid down by the World Health Organization and are similar across USA, Europe, and South Korea. 78 , 79
DSMBs are constituted by scientific members and are appointed by the funding agency, before the recruitment of the first subject in the trial. It can consist of as few as three members and is typically constituted of clinicians and at least one biostatistician. Others that may be included are medical ethicists, other scientists, etc. The most important requisite is that the members should be independent of the sponsors, investigators IRBs, regulatory authorities, and site or study staff. They should have no conflicts of interest with the sponsors, researchers, or study staff.
The functions of the DSMB are:
- • To uphold participant safety.
- • Ensure credibility and integrity of the trial for future subjects.
- • Ensure the timely conclusion of the study so that the results can be disseminated.
- • Identify protocol violations if any.
- • Identify unexpectedly high dropouts and evaluate for the same.
- • Ensure the validity of the results.
The above functions are carried out by an initial organizational meeting to understand the protocol and safety monitoring plan followed by an early safety review meeting to review early safety information. Continuing periodic reviews to assess safety, efficacy, and the progress of the trial are then carried out with reporting of serious adverse events. 44 A final meeting is to be held at the termination of a study. DSMBs function independently of the IRBS but the PIs must submit DSMB reports or minutes to the IRB.
Dramatic instances in which trials have been stopped prematurely on the recommendation of the DSMB include the withdrawal of rofecoxib and celecoxib in two trials on the prevention of colonic polyps due to increased cardiovascular events. 80 , 81
We have come a long way from the horrific ethical compromises in clinical studies in history to establishing adequate safety for the human subjects participating in clinical research today. The establishment of the IRB or EC has ensured safe study designs and the safety of human subjects right from before the study initiation until its completion. This is further supplanted by additional boards like DSMBs. However, we still need studies assessing the outcomes of the ECs on a global basis and addressing various issues that are still pertinent to the working of the ECs. 82
Disclosures: The authors have no potential conflicts of interest to disclose.
Author Contributions:
- Conceptualization: Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M.
- Data curation: Mehta P.
- Writing - original draft: Mehta P.
- Writing - review & editing: Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M.

- Ethics of Neurotechnology
- Recommendation on ethics of artificial intelligence
- International Declaration on Human Genetic Data
- Universal Declaration on Bioethics and Human Rights
- International Bioethics Committee
- Intergovernmental Bioethics Committee
- Assisting Bioethics Committees
- Ethics Education Programme
- UN Inter-Agency Committee on Bioethics
International Bioethics Committee (IBC)

Created in 1993, the IBC provides the only global forum for reflection in bioethics.
IBC Sessions
The Director-General of UNESCO convenes the IBC at least once a year. Through its sessions and working groups, the Committee produces advice and recommendations on specific issues that are adopted by consensus and are widely disseminated and submitted to the Director-General for transmission to the Member States, the Executive Board and the General Conference.
- 13th Session of the IGBC / 30 th (Ordinary) Session of the IBC / 13 th (Ordinary) Session of COMEST , UNESCO Paris, France, 18-22 September 2023
- 29th (Ordinary) Session of the IBC / Joint Session of the IBC and the IGBC / 12th (Extraordinary) Session of COMEST , UNESCO Paris, France, 19-23 September 2022
- Public meetings of the 28th (Ordinary) Session of the IBC and 12th Ordinary Session of COMEST , Online, 2-3 December 2021
- Extraordinary Session of the IBC / Joint Session of the IBC and the IGBC / Eleventh (Extraordinary) Session of COMEST , Online, 22-26 February 2021
- Twenty-seventh (Ordinary) Session of the IBC , Online, 17 December 2020
IBC Sessions 1993 - 2019
- Twenty-sixth (Ordinary) Session of the IBC - Eleventh (Ordinary) Session of the World Commission on the Ethics of Scientific Knowledge and Technology (COMEST) , Bangkok, Thailand, 5-6 July 2019
- Twenty-fifth (Ordinary) Session of the IBC / Joint Session of the IBC and the IGBC / Tenth Extraordinary Session of COMEST , UNESCO Paris, France, 11-12 September 2018
- Twenty-fourth (Ordinary) Session of the IBC and Tenth (Ordinary) Session of the World Commission on the Ethics of Scientific Knowledge and Technology (COMEST) , UNESCO Paris, France, 12-13 September 2017
- Twenty-third (Ordinary) Session of the IBC / Joint Session of the IBC and the Intergovernmental Bioethics Committee (IGBC) / Extraordinary Session of the IGBC / Ninth Extraordinary Session of the World Commission on the Ethics of Scientific Knowledge and Technology (COMEST) , UNESCO Paris, France, 12-16 September 2016
- Twenty-second Session of the IBC and Ninth Session of the World Commission on the Ethics of Scientific Knowledge and Technology (COMEST) , UNESCO Paris, France, 29 September - 1 October 2015
- Twenty-first Session and Joint Session of the IBC and the Intergovernmental Bioethics Committee (IGBC) , Paris, France, 8-12 September 2014
- Twentieth Session , Seoul, Republic of Korea, 19-21 June 2013
- Joint Session of the IBC and the Intergovernmental Bioethics Committee (IGBC) , Paris, France, 13-14 September 2012
- Nineteenth Session , Paris, France, 11-12 September 2012
- Eighteenth Session , Baku, Azerbaijan, 31 May - 2 June 2011
- Joint Session of the IBC and the Intergovernmental Bioethics Committee (IGBC) , Paris, France, 28-29 October 2010
- Seventeenth Session , Paris, France, 26-27 October 2010
- Sixteenth session , Mexico City, Mexico, 23-25 November 2009
- Joint session of the IBC and the Intergovernmental Bioethics Committee (IGBC) , Paris, France, 30–31 October 2008
- Fifteenth Session , Paris, France, 28–29 October 2008
- Fourteenth Session , Nairobi, Kenya, 17-19 May 2007
- Thirteenth Session , Paris, France, 20-22 November 2006
- Twelfth Session , Tokyo, Japan, 15-17 December 2005
- Extraordinary Session , Paris, France, 28 January 2005
- Joint Session of the IBC and the Intergovernmental Bioethics Committee (IGBC) , Paris, France, 26-27 January 2005
- Eleventh Session , Paris, France, 23-24 August 2004
- Extraordinary Session on "Towards a Declaration on Universal Norms on Bioethics" , Paris, France, 27-29 April 2004
- Tenth Session , Paris, France, 12-14 May 2003
- Ninth Session , Montreal, Canada, 26-28 November 2002
- Eighth Session , Paris, France, 12-14 September 2001
- Seventh Session , Quito, Ecuador, 7-9 November 2000
- Sixth Session , Rabat, Morocco, 7-13 October 1999
- Fifth Session , Noordwijk, The Netherlands, 2-4 December 1998
- Fourth Session , Paris, France, 3-4 October 1996
- Third Session , Paris, France, 27-29 September 1995
- Second Session , Paris, France, 20-22 September 1994
- First Session , Paris, France, 15-16 September 1993
Who can participate in or attend IBC sessions?
- Member States, Associate Members of UNESCO may take part as official observers in the meetings of the IBC, while non-Member States that have set up a permanent observer mission may do so at the invitation of the Director-General.
- The United Nations and the other organizations of the United Nations system that have an agreement with UNESCO for reciprocal representation may take also part as observers in the meetings of the IBC.
- International governmental or non-governmental organizations with similar objectives to those of the IBC may be invited to take part as observers in the meetings of the IBC.
- Specialists or other relevant persons or groups may be consulted on matters within the competence of the IBC.
- Any individual or representative of an institution who wishes to attend a public session of the IBC should contact the Secretariat of the IBC to receive an invitation.
How does the IBC work?
Since 1998, the IBC has had Statutes defining its mandate, composition, etc.
- Statutes
- Rules of Procedure
Committee tasks
- To promote reflection on the ethical and legal issues raised by research in the life sciences and their applications.
- To encourage the exchange of ideas and information.
- To encourage action to heighten awareness among the general public, specialized groups and public and private decision-makers involved in bioethics.
- To co-operate with the international governmental and non-governmental organizations concerned by the issues raised in the field of bioethics, as well as with the national and regional bioethics committees and similar bodies.
- To contribute to the dissemination of the principles set out in the UNESCO Declarations in the field of bioethics, and to the further examination of issues raised by their applications and by the evolution of the technologies in question.
How are the IBC members chosen?
The Director-General appoints the IBC's 36 members to serve in their personal capacities for four-year terms. The selection is made taking into account cultural diversity, balanced geographical representation and nominations from States of qualified specialists in the life sciences and in the social and human sciences, including law, human rights, philosophy, education and communication.
List of members
AARONS Dr (Mr) Derrick (Jamaica) Medical Doctor and Bioethicist Ethics Consultant, The Caribbean Public Health Agency (CARPHA) Consultant and Faculty, Center for Global Bioethics, St. George's University, Grenada, West Indies Member, the Editorial Board, The Developing World Bioethics Journal Member, The Executive Council, UNESCO Latin American and Caribbean Bioethics Network (REDBIOETICA-UNESCO) Former Head of the Health Professions Authority in the Turks and Caicos Islands, West Indies Former Member of the International Advisory Board for Bioethics of the Pan American Health Organization (PAHO) Former President and Founder, The Bioethics Society of the English-speaking Caribbean (BSEC) Former Bioethicist and Deputy Chairman for the National Bioethics Committee of Jamaica Former Bioethicist, The Ethics Committee, Faculty of Medical Sciences, University of the West Indies Former Chairman, The Advisory Panel on Ethics and Medico-Legal Affairs, Ministry of Health, Jamaica
ABAKAR Dr (Mr) Mahamat Fayiz (Chad) Member of research group to enhance health services in Chad Country coordinator of the Zoonotic Disease Integrated Action (experience in zoonotic diseases in public health and veterinary institution) Council member and former Chair, African biological Safety Association (AfBSA) Member of the scientific Committee on COVID-19 at the Public Health Ministry of Chad Member of the national Coordination of health Response
ACOSTA SARIEGO Prof. (Mr) José Ramón (Cuba) Medical Doctor, Postgraduate in Public Health, Master Degree in Bioethics, PhD Full Professor and Full Researcher, Medical Sciences University of Havana Academic Coordinator, Master Degree in Bioethics, University of Havana Vice-Chair, UNESCO Latin American and Caribbean Bioethics Network (REDBIOÉTICA) (2018-2021), actually Member of the Directive Council Founder and Member, Bioethics Chair, Institute of Basic and Preclinical Sciences “Victoria de Girón”, University of Medical Sciences of Havana Member, Félix Varela Centre of Ethics Founder and Member, Applied Ethics Chair, University of Havana Founder and Honorary Member, Bioethics Committee, University of Havana Honorary Member, Bioethics Chair, Latin American School of Medicine President, Martian Club of Bioethics, Cultural Society “José Martí” (2007-present). President, Institutional Ethics of Research Committee, Institute of Basic and Preclinical Sciences “Victoria de Girón”, University of Medical Sciences of Havana (2017-present) President, Neuroethics Chapter, Cuban Neeurosciences Society (2019-present) Member, National Cuban Bioethics Committee (1997-2002 and 2019-present)
AL-ATIYYAT Prof. (Ms) Nijmeh (Jordan) Associate Professor of Oncology Nursing and Mentor of Oncology Nursing Master Programme, Faculty of Nursing, Hashemite University Chair holder, UNESCO Chair on Bioethics (2019), Hashemite University (1484) Member, Jordan National Committee for Ethics of Science and Technology Member, National Committee of Palliative Care Member, National Committee of Cancer Control Founder and Organizer, Scientific day Cancer Control Innovation Vice-Chairperson of the IBC (2022-2023) Co-Editor of IBC/UNESCO Newsletter
ANDANDA Prof. (Ms) Pamela (Kenya) Professor of Law, University of the Witwatersrand, South Africa Member, Ethics Advisory Council, International COVID-19 Data Research Alliance (ICODA) Member, Wellcome Trust Discovery Award Interview Panel Member, Academy of Science of South Africa (ASSAf) Standing Committee on Biosafety and Biosecurity Member, the Social Sciences and Humanities standing panel, South African National Research Foundation (2019-2023) Advocate, High Court of Kenya Member, Strathmore University’s institutional ethics review committee Member, Data and Biospecimen Access Committee (DBAC) of the Human Heredity and Health in Africa (H3Africa) (2016-2023) Member, University of the Witwatersrand, Johannesburg Research Committee Chair, University of the Witwatersrand’s Advisory Committee on Ethics
BETZLER Prof. (Ms) Monika (Switzerland) Chair for Practical Philosophy and Ethics, Philosophy Department, Ludwig Maximilan University Munich (LMU), Germany Director of the Munich Graduate School for Practical Ethics, LMU Elected Member, Swiss National Committee for Bioethics Member of the Scientific Board, Fritz Thyssen Foundation Spokeswoman and Founding Member of the Center for Ethics and Philosophy in Practice, LMU Director of the Executive Master Program “Philosophy – Politics – Economics (PPW), LMU Member of the Expert Team, Doc-CH Evaluation Committee of the Swiss National Science Foundation Elected Member of the Review Board “Philosophy”, German Science Foundation (DFG) Member of the Scientific Advisory Board for the Faculty for Philosophy and Education, University of Vienna, Austria
CEKANAUSKAITE Dr. (Ms) Asta (Lithuania) Director of Lithuanian Bioethics Committee Lecturer (bioethics, medical ethics), Vilnius University Expert for ethics assessments, European Commission
CHOI Prof. (Mr) Kyungsuk (Republic of Korea) Professor of Bioethics, School of Law, Ewha Womans University Director, Ewha Institute for Biomedical Law & Ethics, Ewha Womans University Chair, Bioethics Policy Studies, Graduate School, Ewha Womans University Chair, Expert Advisory Committee for Human Material, National Bioethics Committee Executive Member of Editorial Board, Korean Society for Ethics Chief of Editorial Board, Korean Society of Medical Ethics Former Vice President, Korean Bioethics Association Former Member, Internal Review Board (IRB), Samsung Seoul Hospital
CROSS Prof. (Ms) Emily (United Kingdom/USA) Professor of Human Neuroscience, Department of Cognitive Science, Macquarie University, Australia Professor of Social Robotics, Institute of Neuroscience and Psychology, University of Glasgow, Scotland Honorary Professor of Cognitive Neuroscience, School of Psychology, Bangor University, Wales Member, Young Academy of Europe Member, Royal Society of Edinburgh’s Young Academy of Scotland Philip Leverhulme Prize for early career contributions to Psychology (2018) British Science Association Jacob Bronowski Award for early contributions to science and the arts (2017)
DARWISH Prof. (Mr) Bahaa (Egypt) Professor of Philosophy, College of Arts, Minia University, Egypt Former Vice Dean for Postgraduate Studies and Scientific Research and Ex-vice Dean for Students Affairs, College of Arts, Minia University Founding and Board Member, International Association of Education in Ethics, Pittsburgh, USA Board Member, Egyptian Society of Philosophy Member, Ethics of Scientific Research Council, Academy of Scientific Research and Technology, Egypt Member, Philosophy and Sociology Committee, Supreme Council of Culture, Egypt
DESCHENES (Ms) Mylène (Canada) Lawyer Director, Ethics and Legal Affairs, Office of the Québec Chief Scientist, Fonds de recherche du Québec Vice-Chair, Commission on Research Integrity of Luxembourg Member, Research ethics boards (between 1998 and 2012), including the National Research Council of Canada, the Montreal Heart Institute and Héma Québec Responsible for the drafting and implementation of the FRQ policy (of provincial application) on scientific integrity in research "Policy on Responsible Conduct in Research" 2014, updated in 2022 Former Executive Director, P3G Consortium “Public Population Genomics Project” (2005-2012) Former Senior Ethics Policy Advisor, Ethics Office, Canadian Institutes of Health Research (CIHR) (2003-2005)
DHAI Prof. (Ms) Ames (South Africa) Specialist Ethicist, Office of the President and CEO of the South African Medical Research Council (SAMRC) Professor Bioethics and Health Law, School of Clinical Medicine, Wits University Member Academy of Sciences of South Africa Member Working Group on Recommended Guidelines on the Principles of Good Governance for Research Institutions, Council of International Organizations of Medical Sciences CIOMS) Former Member, Working Group on Clinical Research in Resource Limited Settings, Council of International Organizations of Medical Sciences CIOMS) Former Member, Working Groups on the Declarations of Helsinki and Taipei, World Medical Association Editor, South African Journal of Bioethics and Law Chairperson of the IBC (2022-2023)
FERNANDO Prof. (Ms) Anoja Indrakanthi (Sri Lanka) Professor Emeritus, Pharmacology, and Former Dean, Faculty of Medicine, University of Ruhuna Chairperson, Ethics Committee, National Science Foundation of Sri Lanka (from 2021) Member, National Health Research Council, Ministry of Health Former Chair, National Bioethics Committee of Sri Lanka Former President, Asian Bioethics Association Former President, Sri Lanka Medical Association
FORUS Dr (Ms) Anne (Norway) Senior Adviser, Department of Health Legislation and Biotechnology, Norwegian Directorate of Health Member (2003-), Bureau Member (2011-2012) and Former Chairperson (2013-2014), Council of Europe Steering Committee for Human Rights in the fields of Biomedicine and Health Deputy Member, Norwegian Privacy Appeals Board (2013-2017) Member (2008-2012), Executive board for stem cell research, Norwegian Research Council Member (2003-2007), Executive board for ELSA research (Ethical, legal and societal aspects of biotechnology) Vice-Chairperson of the IBC (2022-2023)
FUJITA Prof. (Ms) Misao (Japan) Head and Professor, Uehiro Research Division for iPS Cell Ethics, Kyoto University Professor, Institute for the Advanced Study of Human Biology (WPI-ASHBi), KUIAS Kyoto University Member, Expert Panel on Bioethics, Cabinet Office’s Council for Science, Technology and Innovation Member, Ethics Committee, International Society for Stem Cell Research Clinical Psychologist
GRECO Prof. (Mr) Dirceu Bartolomeo (Brazil) Medical Doctor Professor Emeritus of Bioethics and Infectious Diseases, Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil Chair, Brazilian Bioethics Society (BBS) -(2019-2021) Member, Brazilian National Health Council –CNS (2019-2024) Member, WHO COVID-19 Ethics & Governance Working Group (2022-) Former Member, Brazilian National Commission on AIDS, Brazilian Ministry of Health Former Member, National Commission on Anti-HIV Vaccines, Brazilian Ministry of Health Former Member, Brazilian National Commission for Research Ethics – CONEP (2007-2010) Former Director, Department of IST, AIDS and Viral Hepatitis, Brazilian Ministry of Health (2010-2013) Vice-Chairperson of the IBC (2020-2023)
IOAN Prof. (Ms) Beatrice Gabriela (Romania) Professor of Forensic Medicine and Bioethics and Coordinator of the Forensic Medicine Department, Grigore T. Popa University of Medicine and Pharmacy, Iasi, Romania Forensic Physician, Institute of Forensic Medicine, Iasi, Romania Former Chairperson (2016-2018) and Member, Committee on Bioethics of the Council of Europe (DH-BIO) Chairperson, Bioethics Committee, Romanian College of Physicians
KHIATI Prof. (Mr) Mostéfa (Algeria) Professor at the University of Algiers Founding President, National Foundation for the Promotion of Health and Research Development (FOREM) Member, National Council of Ethics and Deontology of Algeria Member, Ethics Committee, National Center for Biotechnology Research (CRBT) Member, Ethics Committee, Thematic Agency of Research in Health Sciences (ATRSS) Former Chair, National Council for Research Evaluation (2015-2019) Former Member, National Commission for the Promotion and Protection of Human Rights of Algeria (2001-2016) Former Professor of General Medicine and Pediatric, University of Algeria Signatory on behalf of Algeria of the Arab Charter on Ethics in Higher Education initiated by Unesco and the Arab League (2018)
KILLIAN Prof. (Ms) Bernadeta (Tanzania) Deputy Vice Chancellor-Research at University of Dar es Salaam Associate Professor, Dept. of Political Science, University of Dar es Salaam Former Principal, Dar es Salaam University College of Education (DUCE) (2018-2022) Former Principal, Mkwawa University College of Education (MKWAWA) (2013-2017) Former Board Chairperson, Tanzania Institute of Education (TIE), (2017- 2021) Former Chairperson, Award Committee, Tanzania Education Authority (TEA) (2016- 2019) Former Chairperson, Academic Committee, Tanzania Public Service College (2017-2020)
LEE PROF. (Mr) Eng Hin (Singapore) Emeritus Professor of Orthopaedic Surgery, Yong Loo Lin School of Medicine, National University of Singapore (NUS) Emeritus consultant, Division of Paediatric Orthopaedics, National University hospital Chairman, Bioethics Advisory Committee of Singapore (BAC) Former Chair, Human Embryo and Chimera Research Work Group, BAC (2007-2008) Member, Ethics Committee, Singapore Medical Council Member, Working Committee, Ethical Code and Ethical Guidelines & Handbook on Medical Ethics, Singapore Medical Council (2016) Chair, Advisory Committee for Restricted Human Biomedical Research, Ministry of Health of Singapore Member, Healthcare Ethics Capability Committee, Ministry of Health, Singapore (2019-2022) Vice-Chairperson of the IBC (2022-2023)
LEZAUN Prof. (Mr) Javier (Spain) Associate Professor in the School of Anthropology and Museum Ethnography Director of the Institute for Science, Innovation and Society (InSIS), Oxford University Principal Investigator, Diseased landscapes, a British Academy- funded research project Co-Investigator, Oxford Martin Programme on Transboundary Resource Management research programme
LIKINDA BOFONDA Prof. (M.) Evariste (Democratic Republic of Congo) Vice Dean responsible for education, Faculty of Medicine, University of Mbandaka Professor of Neurosurgery, Medical Ethics and Bioethics, University of Mbandaka Neurosurgeon and Head, Department of Surgery, University Hospital of Mbandaka President, National Bioethics Committee of the Democratic Republic of Congo Expert, Program of Hospital Reforms and Services, Directorate General for the Organization and Management of Health Care, Ministry of Public Health, Hygiene and Prevention
MARTINHO DA SILVA Dr (Ms) Paula (Portugal) Lawyer Visiting Professor, Bioethics Institute, Portuguese Catholic University Member, Ethics Committee, Champalimaud Foundation Member, Ethics Committee, Centro Hospitaler de Lisboa Central Former Chair, Ethics Council of José de Mello Saúde Former Member, European group of Ethics in Science and New Technologies of the European Commission (EGE) Former Chair, National Council of Ethics for the Life Sciences
MEZINSKA Dr (Ms) Signe (Latvia) Associate Professor, Faculty of Medicine, University of Latvia Senior Researcher, Institute of Clinical and Preventive Medicine, University of Latvia Chairperson, Research Ethics Committee for Life Sciences and Medicine, University of Latvia Member, Central Medical Ethics Committee of Latvia ELSI expert for BBMRI-ERIC Former Vice-Chairperson, Medical and Biomedical Research Ethics Committee, Riga East Clinical University Hospital Support Foundation Former Board Member, Health Action International (HAI) Europe Rapporteur of the IBC (2022-2023)
MORALES ORDÓÑEZ Prof. (Mr) Juan Cristobal (Ecuador) Professor and director of the Ethics Programme, University of Azuay, Cuenca - Ecuador Member of the National Bioethics Committee of the Ministry of Public Health of Ecuador. Coordinator of the Academy of Philosophy of Law (Cuenca – Ecuador) Judge of the Supreme Court of Justice of Ecuador (2005 to 2011) Coordinator of the Red University and Citizen Network of Ethics and Bioethics (RUCEB), Ecuador Special Advisor to the International Group of Brazilian Universities
ORER Prof. (Mr) Hakan S. (Türkiye) Professor, Department of Medical Pharmacology, School of Medicine, Koç University Chair, Allied Ethics Committees on Clinical Trials, Biomedical Research, and Social Sciences and Humanities, Koç University Chair, Bioethics Steering Committee, National Commission for UNESCO Elected Member, Science Academy (Türkiye)
PALAZZANI Prof. (Mrs) Laura (Italy) Professor of Philosophy of Law and Biolaw, Department of Law, Lumsa University Member, European Group on Ethics in Science and New Technologies, European Commission Delegate of Italy, Committee on Bioethics (DH-BIO) of the Council of Europe
PINSART Prof. (Ms) Marie-Geneviève (Belgium) Professor, Department of Philosophy and Ethics, Free University of Brussels Présidente du Pôle de recherches en éthique appliquée (Université Libre de Bruxelles) Member and former Chairperson, Consultative Committee on Bioethics of Belgium Former President of the Ethics Committee of St-Jean Hospital (Brussels) Member, Ethics Advisory Committee for Partnership Research (French National Research Institute for Development) Member, INRA-CIRAD-IFREMER-IRD Joint Consultative Ethics Committee (France)
ROZYNSKA Dr (Ms) Joanna (Poland) Assistant Professor, Faculty of Philosophy, Center for Bioethics & Biolaw, University of Warsaw Director, Master Programme in Bioethics, University of Warsaw Chairperson, Committee for Bioethics, Presidium of the Polish Academy of Sciences Head, Polish Unit (Warsaw) of the International Network in Bioethics (WMA Cooperation Centre) Member, Compassionate Use Advisory Committee (CompAC), NYU School of Medicine Member, Ethics Commission, National Transplantation Council Member, Research Ethics Committee, University of Warsaw
RUEDA BARRERA Prof. (Mr) Eduardo Alfonso (Colombia) President, Latin American and Caribbean Network for Bioethics Education (REDLACEB) Director, Network doe Ethics and Citizenship Education (REDLACEB) Former Director and Professor, Institute of Bioethics, Pontificia Universidad Javeriana (2012-2019) Former Head, Latin American Working Group on Political Philosophy, Latin American Social Sciences Council (CLACSO) (2012-2019) Board Member, UNESCO Latin American and Caribbean Bioethics Network (REDBIOÉTICA) Board Member, International Network on Biolaw Director of the inter-institutional Chair on Citizenship and Integrity, and Professor on Ethics and Bioethics, Universidad Nacional de Colombia Member, Research Ethics Committee, Colombian National Department of Statistics
SCHÖNE-SEIFERT Prof. (Ms) Bettina (Germany) Director of the Institute for Ethics, History and Theory of Medicine, University of Muenster (until 2023) Full Professor for Medical Ethics, University of Muenster (until 2023) Member, Academia Europaea Member, German Academy of Scientists Leopoldina Fellow, the Hastings Center Former Member, German National Ethics Council
SHAMSI GOOSHKI Prof. (Mr) Ehsan (Islamic Republic of Iran) Associate Professor of Biomedical Ethics, School of Medicine, Tehran University of Medical Sciences International Deputy, Medical Ethics and History of Medicine Research Center, Tehran University of Medical Sciences (UNESCO Chair for Studies in Bioethics) Member of Tehran University of Medical Sciences Research Ethics Committee Clinical Ethics Consultant, Shariati University Hospital, Tehran, Iran Member of World Health Organization Research Ethics Committee (ERC) Member of Eastern Mediterranean Regional office of World Health Organization Research Ethics Committee Member, Biomedical Ethics and Philosophy of Medicine Council, Iran Academy of Medical Science Member, National Bioethics Committee, Iranian National Commission for UNESCO
SIVERINO BAVIO Prof. (Ms) Paula (Argentina) Doctor in Law Former Coordinator of the National Program of Restitution of Rights (Trafficking, Refugees and International Restitution), National Secretariat of Childhood Adolescence and Family Member, Bioethics Counsel, International Institute of Human Rights (Chapter for the Americas) Former Professor of Civil Law and Bioethics, Pontificia Universidad Católica del Perú
SULEMAN Dr (Ms) Mehrunisha (United Kingdom) Director of Medical Ethics and Law Education, Oxford University Member, Nuffield Council on Bioethics Trustee, Arthur Rank Hospice, Cambridge, UK Expert, UNESCO’s Ethics Teacher Training Programme
TOMB Prof. (Mr) Roland R. (Lebanon) Dean, Faculty of Medicine, Saint Joseph University Chairperson and Professor, Department of Bioethics, Saint Joseph University Former Member, Lebanese National Consultative Committee of Bioethics Former Rapporteur, Intergovernmental Bioethics Committee of UNESCO (IGBC) Vice-President of IBC (2018-2021)
YONGYUTH Prof. (Mr) Yuthavong (Thailand) Advisor to the President, National Science and Technology Development Agency (NSTDA) Senior Research Fellow, National Centre for Genetic Engineering and Biotechnology, NSTDA Former Minister, Ministry of Science and Technology (2006-2008) Former Deputy Prime Minister (2014-2015) Former Chairperson, Intergovernmental Bioethics Committee of UNESCO (IGBC) Former President, Thai Academy of Science and Technology (1999-2003)
Download in PDF format:
- IBC Composition for 2022-2023
- IBC Composition for 2020-2021
- IBC Composition for 2018-2019
- IBC Composition for 2016-2017
- IBC Composition for 2014-2015
- Previous Compositions of the IBC
During its 30th (Ordinary) Session, the IBC proceeded with the election of its Bureau: a Chairperson, four Vice-Chairpersons and a Rapporteur, who will remain in office until the end of the 32nd (Ordinary) Session in 2025, provided that they remain members of the Committee.
The following members were elected to the Bureau:
Chairperson
- Ms Mylène DESCHENES (Canada)
- Ms Paula SIVERINO BAVIO (Argentina)
Vice-Chairpersons
- Ms Joanna ROZYNSKA (Poland)
- Mr Ehsan SHAMSI GOOSHKI (Islamic Republic of Iran)
- Ms Bernadeta KILLIAN (Tanzania)
- Mr Bahaa DARWISH (Egypt)
Work Programme for 2024-2025
Based on the discussions during the 30th (Ordinary) Session of the International Bioethics Committee (Paris, September 2023) and subsequent online meetings, the Bureau of the IBC defined the work programme of the Committee for 2024-2025 as follows:
- The Committee will address the topic of the Ethics of Synthetic Biology.
- The Committee will also address the topic of Mental Health.
The Committee remains open to address other emerging challenges related to bioethics during the 2024-2025 biennium.
Reports and Advices
- Draft report of the IBC on the Covid-19 Pandemic: lessons learnt and recommendations for future directions (2023)
- Draft report of the IBC on the principle of solidarity and cooperation in global health challenges (2023)
- Report of the IBC on ethical issues of neurotechnology (2021)
- Report of the IBC on the principle of protecting future generations (2021)
- Report of the IBC on assisted reproductive technologies (ART) and parenthood (2019)
- Report of the IBC on the Principle of Individual Responsibility as related to Health (2019)
- Report of the IBC on Big Data and Health (2017)
- Report of the IBC on the Bioethical Response to the Situation of Refugees (2017)
- Report of the IBC on the Principle of the Sharing of Benefits (2015)
- Report of the IBC on Updating Its Reflection on the Human Genome and Human Rights (2015)
- Report of the IBC on the Principle of Non-Discrimination and Non-Stigmatization (2014)
- Report of the IBC on the Principle of Respect for Human Vulnerability and Personal Integrity (2013)
- Report of the IBC on social responsibility and health (2010)
- Report of IBC on Human Cloning and International Governance (2009)
- Report of IBC on Consent (2008)
- Report of the IBC on the Possibility of Elaborating a Universal Instrument on Bioethics (2003) Rapporteurs: Giovanni Berlinguer and Leonardo De Castro
- Report of the IBC on Pre-implantation Genetic Diagnosis and Germ-line Intervention (2003) Rapporteur: Hans Galjaard
- Human Genetic Data: Preliminary Study by the IBC on its Collection, Processing, Storage and Use (2002) Rapporteurs: Sylvia Rumball and Alexander McCall Smith
- Report of the IBC on Ethics, Intellectual Property and Genomics (2002) Rapporteur: Judge Michael Kirby
- Report of the IBC on Solidarity and International Co-operation between Developed and Developing Countries concerning the Human Genome (2001) [in French] Rapporteur: Mehmet Öztürk
- The Use of Embryonic Stem Cells in Therapeutic Research (2001) Rapporteurs: Alexander McCall Smith and Michel Revel
- Report on Confidentiality and Genetic Data (2000) Report of the Working Group of the IBC
- Ethical Considerations Regarding Access to Experimental Treatment and Experimentation on Human Subjects (1996) Rapporteurs: Harold Edgar and Ricardo Cruz-Coke
- Food, Plant Biotechnology and Ethics (1995) Rapporteur: Darryl Macer
- Bioethics and Human Population Genetics Research (1995) By Chee Heng Leng, Laila El-Hamamsy, John Fleming, Norio Fujiki, Genoveva Keyeux, Bartha Maria Knoppers and Darryl Macer
- Genetic Counselling (1995) Rapporteur: Michel Revel
- Ethics and Neurosciences (1995) Rapporteur: Mr Jean-Didier Vincent
- Report on Human Gene Therapy (1994) Rapporteurs: Mr Harold Edgar and Mr Thomas Tursz
- Report on Genetic Screening and Testing (1994) Rapporteur: Mr David Shapiro
- Advice of the IBC on the Patentability of the Human Genome
Statements on ethical issues related to the COVID-19 pandemic
Since the beginning of the COVID-19 pandemic, the International Bioethics Committee (IBC) together with the World Commission on the Ethics of Scientific Knowledge and Technology (COMEST) , has spared no effort to provide the international community with reflections and recommendations on the ethical issues raised by the health crisis, through several statements, as follows:
UNESCO’s Ethics Commissions call for the temporary waiving of patents related to vaccines as an exceptional measure (September 2021) This statement calls for equal access for all to vaccines and therapeutics developed to confront COVID-19.
- Read the full statement
UNESCO’s Ethics Commissions call to address ethical issues of COVID-19 certificates to leave no one behind (June 2021) This statement calls for policy-makers to address ethical issues involved in COVID-19 certificates and vaccine passports.
- Read the news
UNESCO calls for COVID-19 vaccines to be considered a global public good (February 2021) In this joint statement, the IBC and COMEST call for a change of course in current COVID-19 vaccination strategies, urging for global vaccines equity and solidarity.
- Read the press release
Statement on COVID-19 : Ethical Considerations from a Global Perspective (April 2020) In this joint statement, the IBC and COMEST guide policy-makers and inform the public about essential ethical considerations that need to be addressed during the global fight against the COVID-19 pandemic.
COVID-19 Vaccines as global public goods , N° 2, July 202
Ethics as the global compass , N° 1, July 2020
Related items
- Social and human sciences
- Artificial intelligence
- Guidelines and tools
- Norms & Standards
- Sharing knowledge
- Intergovernmental Committee
- Life sciences
- Ethics of science
- Ethics of technology
- Environmental ethics
- Ethics of artificial intelligence
- Medical ethics
- Philosophy and ethics
- See more add
Advertisement
Reassessing the Role of the Biomedical Research Ethics Committee
- Published: 07 October 2012
- Volume 10 , pages 335–352, ( 2012 )
Cite this article

- Merryn Ekberg 1
524 Accesses
7 Citations
Explore all metrics
The role of the Research Ethics Committee (REC) in the design, conduct and dissemination of scientific research is still evolving and many important questions remain unanswered. Hence, the aim of this paper is to address some of the uncertainty that exists around the role and responsibilities of RECs and to discuss some of the controversy that exists over the criteria that RECs should follow when evaluating a research proposal. The discussion is organised around five of the major roles currently performed by RECs when assessing proposals in the biomedical sciences. It will be shown that these five roles need to be critically evaluated and reassessed. The five roles addressed are: assessing the legitimacy and validity of the informed consent process, second, conducting a comprehensive risk/benefit analysis, third, assessing the validity of a research proposal, fourth, ensuring that researchers observe the social norms, values, customs, traditions and laws that prevail in the community or jurisdiction in which the research will be conducted and finally, monitoring the research project as it unfolds and providing an ongoing advisory and consultancy service to both new and experienced researchers. In reassessing the role of the REC, this paper concludes with a set of general recommendations for RECs. These provide some guidance on the minimum criteria that should be followed when RECs evaluate proposals. These guidelines will be beneficial for new and experienced members of REC, and will help to make the process a more objective, efficient and standardised process. The guidelines will also be beneficial for researchers in the biomedical sciences who are preparing proposals for ethical review.
This is a preview of subscription content, log in via an institution to check access.

Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

A charter for biomedical research ethics in a progressive, caring society
Research ethics board (reb) members’ preparation for, and perceived knowledge of research ethics, what do international ethics guidelines say in terms of the scope of medical research ethics.
For an interesting case study that challenged the principle of absolute confidentiality see Lowman and Palys 2000 .
Allen, G. (2008). Getting beyond form filling: the role of institutional governance in human research ethics. Journal of Academic Ethics, 6 , 105–116.
Article Google Scholar
Andanda, P. (2005). Module two: informed consent. Developing World Bioethics, 5 (1), 14–25.
Appelbaum, P. (2002). Clarifying the ethics of clinical research: a path towards avoiding the therapeutic misconception. American Journal of Bioethics, 2 , 22–23.
Google Scholar
Beauchamp, T. (2011). Informed consent: its history, meaning and present challenges. Cambridge Quarterly of Healthcare Ethics, 20 , 515–523.
Beauchamp, T., & Childress. (2009). Principles of biomedical ethics . Oxford: Oxford University Press.
Beck, U. (1992). Risk society: Towards a new modernity . London: Sage.
Bentley, J., & Thacker, P. (2004). The influence of risk and monetary payment on the research participation decision making process. Journal of Medical Ethics, 30 , 293–298.
Cave, E., & Nichols, C. (2007). Clinical audit and reform of the UK research ethics review system. Theoretical Medicine and Bioethics, 28 , 181–203.
Council for International Organizations of Medical Sciences (CIOMS). (1991). International guidelines for ethical review of epidemiological studies. In Z. Bankowski, J. Bryant, & J. Last (Eds.), Ethics and epidemiology: International guidelines . Geneva: CIOMS.
Council for International Organizations of Medical Sciences (CIOMS). (1993). International ethical guidelines for biomedical research involving human subjects. In Z. Bankowski & R. Levine (Eds.), Ethics and research of human subjects: International guidelines . Geneva: CIOMS.
Cummins, D. (2002). The professional status of bioethics consultation. Theoretical Medicine, 23 , 19–43.
Curran, W. (1973). The Tuskegee syphilis study. New England Journal of Medicine, 287 , 730–731.
Department of Health (DH). (2001). Governance arrangements for NHS Research Ethics Committees . London: HMSO. ( http://www.dh.gov.uk ).
Department of Health (DH). (2005). Research Governance Framework . London: HMSO.
Edwards, S. (2005). Research participation and the right to withdraw. Bioethics, 19 (2), 112–130.
Edwards, S., Ashcroft, R., & Kirchin, S. (2004a). Research ethics committees: differences and moral judgement. Bioethics, 18 (5), 408–427.
Edwards, S., Kirchin, S., & Huxtable, R. (2004b). Research ethics committees and paternalism. Journal of Medical Ethics, 30 , 88–91.
European Forum of Good Clinical Practice. (2001) European Guidelines for Auditing Ethics Committees. ( http://www.efgcp.org/index.asp ).
Feyerabend, P. (1975). Against method: Outline of an anarchistic theory of knowledge . Atlantic Highlands: Humanities Press.
HFEA. (1990; 2008). Human Fertilisation and Embryology Act. ( http://www.hfea.gov.uk )
Hirtle, M., Lemmens, T., & Sprumont, D. (2000). A comparative analysis of research ethics review mechanisms and the ICH Good clinical practice guideline. European Journal of Health Law, 7 , 265–292.
International Committee of Medical Journal Editors (ICMJE). (2010). Uniform requirements for manuscripts submitted to biomedical journals. ( http://www.icmje.org/urm_full.pdf ).
Jones, M., & Slater, B. (2003). The governance of human genetics: policy discourse and constructions of public trust. New Genetics and Society, 22 (1), 21–41.
Karunaratne, A., Myles, P., Ago, M., & Komesaroff, P. (2006). Communication deficiencies in research and monitoring by ethics committees. Internal Medicine Journal, 36 , 86–91.
Loff, B., & Black, J. (2004). Research ethics committees: what is their contribution? Medical Journal of Australia, 181 (8), 440–441.
Lowman, J., & Palys, T. (2000). Ethics and institutional conflicts of interest: the research controversy at Simon Fraser University. Sociological Practice: A Journal of Clinical and Applied Sociology, 2 (4), 245–264.
Macpherson, C. (1999). Research ethics committees: a regional approach. Theoretical Medicine and Bioethics, 20 , 161–179.
McDonald, M., & Cox, S. (2009). Moving towards evidence-based human participant protection. Journal of Academic Ethics, 7 (1), 1–16.
Montgomery, K., & Oliver, A. (2009). Shifts in guidelines for ethical scientific conduct: how public and private organisations create and change norms of research integrity. Social Studies of Science, 39 (1), 137–155.
Nuffield Council on Bioethics (NCB). (1999). The ethics of clinical research in developing countries: a discussion paper . London.
Nuffield Council on Bioethics (NCB). (2002). The ethics of research related to healthcare in developing countries. London.
Pence, G. (2004). Classic cases in medical ethics (3rd ed.). New York: McGraw-Hill.
Petryna, A. (2007). Clinical trials offshored: on private sector science and public health. Biosocieties, 2 , 21–40.
Rothman, D., & Rothman, S. (1984). The Willowbrook Wars . New York: Harper & Row.
Salter, B., & Salter, C. (2007). Bioethics in the global moral economy. The cultural politics of human embryonic stem cell science. Science, Technology and Human Values, 32 , 554–581.
Shaul, R. (2002). Reviewing the reviewers: the vague accountability of research ethics committees. Critical Care, 6 (2), 121–122.
Sutrop, M. (2011). Changing ethical frameworks: from individual rights to the common good? Cambridge Quarterly of Healthcare Ethics, 20 , 533–545.
Tinker, A., & Coomber, V. (2004). University research ethics committees: Their role, remit and conduct . London: King’s College London.
UNESCO. (1997). Universal Declaration on the Human Genome and Human Rights. ( www.unesco.org ).
UNESCO. (2005). Bioethics Committees at work: Policies and Procedures. UNESCO. ( www.unesco.org ).
van den Hoonaard, W. (2006). New angles and tangles in the ethics review of research. Journal of Academic Ethics, 4 , 261–274.
Wendler, D., & Grady, C. (2008). What should research participants understand to understand they are participants in research? Bioethics, 22 (4), 203–208.
Whittaker, E. (2005). Adjudicating entitlements: the emerging discourses of research ethics boards. Health, 9 (4), 513–535.
World Medical Association. (WMA). (1964; 1975; 1983; 1989; 1996; 2000). Declaration of Helsinki. ( http://www.wma.net/en/30publications/10policies/b3/index.html ).
Download references
Author information
Authors and affiliations.
University of Northampton, Boughton Green Road, Northampton, UK
Merryn Ekberg
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Merryn Ekberg .
Rights and permissions
Reprints and permissions
About this article
Ekberg, M. Reassessing the Role of the Biomedical Research Ethics Committee. J Acad Ethics 10 , 335–352 (2012). https://doi.org/10.1007/s10805-012-9171-6
Download citation
Published : 07 October 2012
Issue Date : December 2012
DOI : https://doi.org/10.1007/s10805-012-9171-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research ethics
- Research ethics committee
- Informed consent
- Quality assurance
- Social norms
- Find a journal
- Publish with us
- Track your research
- For Clinicians
- For Medical Students
- For Scientists
- Our Victories
- Internships
- Annual & Financial Reports
- Barnard Medical Center
- May 13, 2024
Ethics—Both Human and Animal—Demand We Move Away From Animal Use in Biomedical Research Urgently and Focus Entirely on Human Biology Instead
- Share on Facebook
- Share on Twitter
- Share via Email
New paper by Jarrod Bailey, PhD, Director of Medical Research at the Physicians Committee, and co-author Professor Michael Balls, summarizes the case for a paradigm shift in medical research that will help animals and humans.
In their invited review in Anesthesiology Clinics , the authors discuss ethical issues and considerations concerning the use of animals in science, and how these can be used by some to justify animal experimentation. They also make the case that human ethics must take a much more prominent role. If biomedical science is not sufficiently human relevant, then it is letting down billions of people relying on science to increase the understanding of human diseases, and to provide new drugs to ease human suffering—and perpetuating harm to tens of millions of animals in laboratories every year.
One of the reasons for the continuation of large-scale animal research and testing is that there is, and has been, far too little reflection and critical questioning of animal models of human diseases and drug efficacy and safety. This widespread failure to follow the scientific method—in which critical evaluation of what is being done, what models are being used to generate data and test hypotheses—is often absent, in the face of unprecedented and substantial evidence against it.
The paper makes the case that animal ‘models’ of human biology and diseases are poor, due to intractable species differences that cannot be overcome or sufficiently taken into account. These differences are amplified by considerable variability within species: Individual animals and humans often differ significantly from one another, making the relevance of experimental data even to other members of the same species problematic. Such differences between individual human beings must be factored into research, and this can only be achieved through human-specific research approaches. This includes so-called New Approach Methodologies (NAMs)—advanced methods of growing and maintaining human cells, tissues, and miniature organs, often derived from patient skin and blood samples, which reflect the variable biology of specific, different patients. Huge strides in our understanding of human diseases, and in what we can do to develop new, safe, and effective therapies for them, are being made in this way, including for diseases which have been poorly served by the use of animals.
The article concludes that a rapid phase-out of animal use in science is essential and must be replaced by a focus on human biology at all stages—for the sake of animals and humans.
Dr Bailey’s new paper can be found here, free, until June 22:
https://authors.elsevier.com/a/1j19V6tLd7CNeS
More on Ethical Science
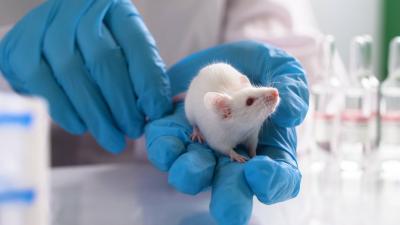
Good Science Digest

Join the Kickstart
Prevention starts today. Join the 21-Day Vegan Kickstart.
21-Day Kickstart
Get Healthy With Good Nutrition
Food for Life classes teach you how to improve your health with a plant-based diet.
Find a Class

COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Director of Operations
- Columbia University Medical Center
- Opening on: May 15 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $125,000 - $140,000
Position Summary
- The Division of Pediatric Hematology, Oncology and Stem Cell Transplantation at Columbia University Irving Medical Center is recruiting a Director of Operations for the International Initiative for Pediatrics and Nutrition (IIPAN). The individual would support the Program for advancing nutritional health in children located in low and middle-income countries. The candidate would advance IIPAN’s offers of international as well as domestic educational opportunities in academic medical institutions abroad and work closely with IIPAN’s collaborators within the World Health Organization and Ministries of Health. Current active programs are located in Central and South America, Africa, and several countries in South Asia. The director of operations will be responsible for directing the operations of IIPAN, overseeing and expanding site implementation (monitoring and evaluation), develop and oversee expansion of IIPAN’s technological imprint in the professional and lay populations, and work alongside development to grow IIPAN’s programmatic objectives. The candidate may also serve as a mentor to graduate students at CUIMC who may be interested in global health activities. The candidate will direct operations projects with the Columbia Global Centers to advance regional nutrition operations within the region.
At CUIMC, we are leaders in teaching, research, and patient care and are proud of the service and support we provide to our community. We apply the same rigor in our commitment to fostering an inclusive, thriving community and caring for our employees and their loved ones. We offer immediate eligibility and invest in our employees’ families through comprehensive Health and Welfare , Employee Assistance , Tuition Programs , and Retirement Benefits .
“Subject to business needs, we may support flexible and hybrid work arrangements. Options will be discussed during the interview process”
Responsibilities
- Direct all aspects of operations, encompassing development, marketing, events, and public relations, alongside collaboration with respective departments at CUIMC.
- Assess current operational structures and devise innovative processes for implementation.
- Conceptualize and refine new workflows for implementation, while actively incorporating team member perspectives into the formulation of proposals.
- Develop and direct cross-departmental collaboration to optimize operations and policies.
- Develop and direct online and automated processes and new technology to enhance efficiency of operations.
- Provide strategic direction and informed recommendations on organizational policies, resource allocation, budgetary considerations, and daily decision-making processes.
- Spearhead comprehensive strategic planning initiatives across programs, shaping the trajectory of service delivery and operational strategies.
- Supply budgeting, financial and staffing input to the Director for the development of proposals.
- Direct executive management meetings and deliver comprehensive progress reports on project progress.
- Direct and advise staff and local team members on operational issues including those relating to grants and compliance, technology, and facility needs.
- Pioneer and manage initiatives that will enhance and advance commitment to diversity, equity, and inclusion.
- Participate in Board meetings (and Committee meetings as needed) and review and provide feedback on reports to be delivered at meetings.
- Abide by all applicable professional standards of ethics and practice adhering to CUIMC policies.
- Performs related duties & responsibilities as assigned/requested.
Minimum Qualifications
- Bachelor’s degree or equivalent in education and experience required; plus, five years of related experience.
Preferred Qualifications
- 8-10+ years of experience progressively responsible for designing, implementing, and directing nonprofit operation workflows.
- A passion for the organization's mission with a demonstrated history supporting international humanitarian organizations.
- Excellent oral and written communication skills with the ability to communicate with the executive leadership team, community members, and donors.
- Experience with Microsoft Office programs, social media platforms, ticket purchasing systems, and project management software.
- Ability to work collaboratively in a team setting listening to ideas from all parties.
- Ability and willingness to travel to program sites and international meetings.
- Demonstrated competence in working with diverse clients, client communities and organizations. Strong sense of customer service and the ability to follow through on projects and deliverables.
- Strong coaching, teaching, training, organizational and time management skills.
- Proven ability and willingness to work under pressure, meet tight deadlines and handle multiple tasks simultaneously while maintaining attention to detail.
- Excellent analytical and problem-solving skills.
- Demonstrated resourcefulness and ability to take initiative in development and completion of projects.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Non-Student Short-Term Casual
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .
Take the Quiz: Find the Best State for You »
What's the best state for you », russia says us 'playing with fire' in 'indirect war' with moscow.
Russia Says US 'Playing With Fire' in 'Indirect War' With Moscow

FILE PHOTO: Russian Deputy Foreign Minister Sergei Ryabkov attends a meeting chaired by Russian President Vladimir Putin on operational issues, including the course of Russia-Ukraine conflict and the continuing conflict between Israel and Hamas, at the Novo-Ogaryovo state residence outside Moscow, Russia October 16, 2023. Sputnik/Gavriil Grigorov/Pool via REUTERS/File Photo
MOSCOW (Reuters) - A top Russian diplomat said on Friday the United States had long since entered into a state of indirect war with Moscow and was playing with fire over Ukraine by behaving in such a way that the situation could spin out of control.
The comments by deputy foreign minister Sergei Ryabkov in an interview with state TASS news agency reflect growing Russian concern over what Moscow casts as dangerous Western escalation in Ukraine as Russian forces advance in several places.
"We warn that they are playing with fire. They have long been in a state of indirect war with the Russian Federation," Ryabkov told TASS, referring to the United States.
"They somehow fail to realise that, in order to satisfy their own geopolitical ideas, they are approaching a phase in which it will be very difficult to control what is happening and to prevent a dramatic crisis."
Ukraine and the West accuse Russia of waging an unprovoked war of aggression in Ukraine aimed at seizing land. Moscow says what it calls its "special military operation", launched in February 2022, is defensive and aimed at bolstering Russian security against a hostile West.
Russia has interpreted recent comments from Western diplomats as an aggressive shift in position and the Russian defence ministry said this month that President Vladimir Putin had ordered drills to rehearse using tactical nuclear weapons in what officials said was a response to Western rhetoric.
British Foreign Secretary David Cameron, during a visit to Kyiv on May 3, said Ukraine had a right to use the weapons provided by Britain to strike targets inside Russia, and that it was up to Kyiv whether or not to do so.
U.S. Secretary of State Antony Blinken made similar comments about U.S.-supplied weapons during a visit on Wednesday to Kyiv, where he accused Putin of "ramping up yet another offensive against Ukraine" in the east.
The Latest Photos From Ukraine

Blinken said Washington had "not encouraged or enabled strikes outside of Ukraine, but ultimately Ukraine has to make decisions for itself about how it’s going to conduct this war."
Ukraine has targeted military and energy targets across Russia, which is bombarding targets inside Ukraine in attacks that have killed thousands of civilians, to try to degrade Moscow's military capacities.
Steps by the U.S. and the European Union to look at the possibility of using frozen Russian assets or the profits on them to help Ukraine have also angered Moscow. Russia accuses Washington of trying to bully European countries into taking more radical steps to thwart it in Ukraine.
LIVING 'IN A BOX'
Ryabkov told TASS that Washington appeared oblivious to the grave risks attached to its behaviour.
"This rhetoric, this drumming, this constant baiting of their allies to help Ukraine even more, to expand their support, shows only one thing: people are living, as they themselves say, 'in a box'," he said.
"This is the great risk of the current situation, because it is impossible to get through to them (the Americans).
Ryabkov complained that the West had adopted a stance of strategic uncertainty and ambiguity towards Russia, trying to make it difficult for Moscow to predict how NATO will react in various situations, including with nuclear weapons.
"Russia will put the topic of 'red lines' aside and will respond to the West in a mirror manner," Ryabkov said.
Russia's diplomacy with the West was now in crisis management mode and was focused on trying to ensure that tensions do not spill over into a large-scale conflict, he added.
(Reporting by Reuters; Writing by Andrew Osborn; Editing by Tim Heritage)
Copyright 2024 Thomson Reuters .
Photos You Should See - May 2024

Join the Conversation
Tags: Ukraine , Russia , United States , Asia , European Union , Belarus , Europe
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Flag Display Rattles SCOTUS Experts
Lauren Camera May 17, 2024

Will Trump Testify in His Own Trial?
Laura Mannweiler May 17, 2024

Viral House Spat Shows Chaotic Congress
Aneeta Mathur-Ashton May 17, 2024

QUOTES: Trump on Gun Control Policy
Cecelia Smith-Schoenwalder May 17, 2024

Leading Indicators: Economy Is Softening
Tim Smart May 17, 2024

Key Moments From Cohen Cross-Examination
Laura Mannweiler May 16, 2024


COMMENTS
Abstract. An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty.
GUIDELINE 23: REQUIREMENTS FOR ESTABLISHING RESEARCH ETHICS COMMITTEES AND FOR THEIR REVIEW OF PROTOCOLS ... CIOMS, in association with WHO, undertook its work on ethics in biomedical research in the late 1970s. Accordingly, CIOMS set out, in cooperation with WHO, to prepare guidelines. The aim of the
UKZN Research Office: Biomedical Research Ethics. Research Division. A place to access UKZN Research Policies, Grant Application Forms and Guidelines, Research-related news, and innovative research ideas. UKZN Research Office @ westville University Road. A relaxed environment where the academic research community can meet with colleagues and ...
Because addressing issues in medical ethics often requires multidisciplinary expertise — in philosophy, biomedical research, clinical practice, law, policy, and communication, among other fields ...
Former Vice-Chairperson, Medical and Biomedical Research Ethics Committee, Riga East Clinical University Hospital Support Foundation Former Board Member, Health Action International (HAI) Europe Rapporteur of the IBC (2022-2023) MORALES ORDÓÑEZ Prof. (Mr) Juan Cristobal (Ecuador)
The role of the Research Ethics Committee (REC) in the design, conduct and dissemination of scientific research is still evolving and many important questions remain unanswered. Hence, the aim of this paper is to address some of the uncertainty that exists around the role and responsibilities of RECs and to discuss some of the controversy that exists over the criteria that RECs should follow ...
67 Operational guidelines for ethics committees that review biomedical research 68 Registration of an institutional review board (IRB) or independent ethics committee (IEC) 69 International guidelines on bioethics (informal listing of selected international codes, declarations, guidelines etc. on medical ethics/bioethics/health care ethics ...
Biomedical Research Ethics Committees in Sub-Saharan Africa: A Collective Review of Their Structure, Functioning, and Outcomes Blessing Silaigwana1 and Douglas Wassenaar1 Abstract Research Ethics Committees (RECs) are mandated to protect human participants by conducting ethical reviews of biomedical research. To date, there is a dearth of ...
Research Ethics Committees (RECs)—or Institutional Review Boards (IRBs), as they are known in the US—were created about 50 years ago to independently assess the ethical acceptability of research projects involving human subjects, their fundamental role being the protection of the dignity and rights of research participants.
In this paper, we describe how ethics expertise can contribute to biomedical research through real‐time engagement and some of the challenges associated with such efforts. To do so, we offer our experiences with two particular examples: organoid technology and umbilical cord blood (UCB) banking and transplantation.
This document has been developed for individuals and organizations involved in health-related research with human participants, including biomedical, behavioural, social science, and epidemiological research (throughout this document, the term "research" is meant to include, and refers to, all of these domains).
Guide for research ethics commitee members. This Guide is intended to be used as a tool for research ethics committee (REC) members. The text has been elaborated by the Group of Specialists on Biomedical Research (CDBI-CO-GT2) working under the authority of the Steering Committee on Bioethics (CDBI) of the Council of Europe.
Ethics Committees That Review Biomedical Research World Health Organization Geneva 2000. This document is not issued to the general public, and all rights are ... tribute to the highest attainable quality in the science and ethics of biomedical research. States should promote, as appropriate, the es-tablishment of ECs at the national ...
This document is intended to provide guidance to research ethics committees on which organizations rely to review and oversee the ethical aspects of research, as well as to the researchers who design and carry out health research studies. Other languages. Arabic pdf, 1.3Mb; Spanish pdf, 1.56Mb
A Guide for the Ethics Committee Editors Johan PE Karlberg and Marjorie A Speers ... 1.2 Clinical Trials in the Context of Biomedical Research _____ 19 Clinical Trials on Medicinal Products _____ 19 ... research nurses, research support staff, ethics committee . 6 administrators, contract and budget development administrative staff, monitors ...
Role of the Ethics Committee: Brief Summary Safeguarding the dignity, rights, safety, and well-being of all actual or potential research participants Providing independent, competent, and timely ethical review of the proposed study Initial review Continuing review Products of an Ethics Committee -1- Decision/opinion on the research protocol
This review discusses three pieces of work, that is, a conference panel and two books, that deal with the role of research ethics committees (RECs) in regulating biomedical research and medical anthropological research. We summarise the papers and conversations of a panel we convened on this topic during the 2020 European Association for Social Anthropologists (EASA) conference.
Biomedical Research The Additional Protocol to the Convention on Human Rights and Biomedicine on Biomedical Research is intended to build on the principles embodied in the Convention, with a view to protecting human rights and dignity in the specific field of biomedical research. ... independent examination of research by an ethics committee ...
In their invited review in Anesthesiology Clinics, the authors discuss ethical issues and considerations concerning the use of animals in science, and how these can be used by some to justify animal experimentation.They also make the case that human ethics must take a much more prominent role. If biomedical science is not sufficiently human relevant, then it is letting down billions of people ...
According to Chapter IV of the 'New Drugs and Clinical Trials Rules 2019', notified by The Ministry of Health and Family Welfare (Department of Health and Family Welfare), any organization conducting Biomedical and Health Research involving human participants, shall be required to have an ethics committee to review and oversee and conduct of such research as detailed in the 'National ...
Nonanimal biomedical research methods have advanced rapidly over the last decade making them the first-choice model for ... . 5,20-23 It also has serious implications for the progress of researchers' careers and for animal research ethics ... Advisory committee to the director working group on catalyzing the development and use of novel ...
The Protocol is to cover the full range of biomedical research activities involving interventions on human beings. ... scientific quality, independent examination of research by an ethics committee, information to be submitted to the ethics committee, information for research participants, confidentiality and the right to information, dependent ...
Indeed, NIH is the largest public funder of biomedical research in the world. Nearly 83 percent of the NIH budget is used to support extramural research through competitive grants and contracts. In FY 2023 alone, NIH supported almost 59,000 awards and issued grants to 2,743 academic institutions, research institutes, hospitals, small businesses ...
Advisory Committee Charter Renewals AGENCY: Department of Veterans Affairs. ACTION: ... may address research questions within the general area of biomedical and behavioral research or clinical science research. The Board does ... An ethics review is conducted for each selected nominee. Dated: May 14, 2024.
The director of operations will be responsible for directing the operations of IIPAN, overseeing and expanding site implementation (monitoring and evaluation), develop and oversee expansion of IIPAN's technological imprint in the professional and lay populations, and work alongside development to grow IIPAN's programmatic objectives.
Justice Samuel Alito's ethics kerfuffle is the latest to hit the high court, and comes as justices consider a major decision related to Trump's attempts to interfere with the election. Lauren ...