Advertisement

Association between smoking and environmental tobacco smoke with lung cancer risk: a case–control study in the Fujian Chinese population
- Original Article
- Published: 10 May 2021
- Volume 30 , pages 2047–2057, ( 2022 )
Cite this article

- Jinman Zhuang 1 , 2 , 3 na1 ,
- Zhi qiang Liu 1 , 2 , 3 na1 ,
- Rendong Xiao 4 na1 ,
- Qiu ping Xu 5 ,
- Wei min Xiong 1 , 2 , 3 ,
- Lin Cai 1 , 2 , 3 &
- Fei He 1 , 2 , 3
548 Accesses
2 Citations
Explore all metrics
To investigate the association between smoking, environmental tobacco smoke (ETS), and lung cancer risk.
This case–control study included 1622 newly diagnosed cases of lung cancer and 1622 healthy frequency-, age-, and gender-matched control participants. Epidemiological data were collected by in-person interviews using a standard questionnaire.
Smoking was a risk factor for lung cancer in men (odds ratio (OR) = 4.486, 95% confidence interval (95%CI) 3.586–5.611). In addition, decreased starting age, increased number of cigarettes smoked per day, duration of smoking, pack–years, and depth of inhalation were all risk factors that met the dose–response relationship ( P < 0.001). The risk of lung cancer was lower among men who had quit smoking for more than 10 years compared to current smokers. Additionally, male smokers with lung squamous cell carcinoma were at a higher risk of lung cancer than male smokers with lung adenocarcinoma. Workplace ETS increased the risk for lung cancer for male nonsmokers (OR = 2.452, 95%CI 1.534–3.920). In contrast, household ETS increased the risk for lung cancer for female nonsmokers (OR = 2.224, 95%CI 1.644–3.009). Approximately 65.93% cases of lung cancer in men could be attributed to smoking, whereas approximately 31.03% cases of lung cancer among nonsmokers could be attributed to ETS.
Conclusions
Smoking is the main risk factor for lung cancer. Workplace ETS is associated with increased lung cancer risk in male nonsmokers, while household ETS is associated with increased lung cancer risk in nonsmoking women. Thus, smoking and ETS increase the risk of lung cancer and are major public health concerns.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Risk of lung cancer in relation to various metrics of smoking history: a case-control study in Montreal
Risk of lung cancer among women in relation to lifetime history of tobacco smoking: a population-based case-control study in france (the welca study).
Association between secondhand smoke and cancers in adults in the US population
Availability of data and material.
Not applicable.
Code availability
Avino P, Scungio M, Stabile L, Cortellessa G, Buonanno G, Manigrasso M (2018) Second-hand aerosol from tobacco and electronic cigarettes: evaluation of the smoker emission rates and doses and lung cancer risk of passive smokers and vapers. Sci Total Environ 642:137–147. https://doi.org/10.1016/j.scitotenv.2018.06.059
Article CAS PubMed Google Scholar
Becher H, Belau M, Winkler V, Aigner A (2018) Estimating lung cancer mortality attributable to second hand smoke exposure in Germany. Int J Public Health 63:367–375. https://doi.org/10.1007/s00038-017-1022-1
Article PubMed Google Scholar
Behera S, Xian H, Balasubramanian R (2014) Human health risk associated with exposure to toxic elements in mainstream and sidestream cigarette smoke. Sci Total Environ 472:947–956. https://doi.org/10.1016/j.scitotenv.2013.11.063
Cao M, Li H, Sun D, Chen W (2020) Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (London, England) 40:205–210. https://doi.org/10.1002/cac2.12025
Article Google Scholar
Chang CM, Edwards S, Arab A, Del Valle-Pinero A, Yang L, Hatsukami DK (2016) Biomarkers of Tobacco Exposure: Summary of an FDA-sponsored Public Workshop. Cancer Epidemiol Biomarkers Prev 26:291–302 https://doi.org/10.1158/1055-9965.EPI-16-0675
Chen Z et al (2015) Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet (London, England) 386:1447–1456. https://doi.org/10.1016/s0140-6736(15)00340-2
Chen W et al (2016) Cancer statistics in China, 2015 CA. Cancer J Clin 66:115–132. https://doi.org/10.3322/caac.21338
Clark S, Molloy P (2017) Smoke-induced changes to the epigenome provide fertile ground for oncogenic mutation. Cancer Cell 32:278–280. https://doi.org/10.1016/j.ccell.2017.08.016
Cozen et al. (2017) Case-control study of cumulative cigarette tar exposure and lung and upper aerodigestive tract cancers. Int J Cancer 140:2040–2050. https://doi.org/10.1002/ijc.30632
Donny E et al (2015) Randomized trial of reduced-nicotine standards for cigarettes. New England J Med 373:1340–1349. https://doi.org/10.1056/NEJMsa1502403
Article CAS Google Scholar
Du Y et al (2020) Lung cancer occurrence attributable to passive smoking among never smokers in China: a systematic review and meta-analysis. Trans Lung Cancer Res 9:204–217. https://doi.org/10.21037/tlcr.2020.02.11
Feng R, Zong Y, Cao S, Xu R (2019) Current cancer situation in China: good or bad news from the 2018 global Cancer statistics? Cancer Commun 39:22. https://doi.org/10.1186/s40880-019-0368-6
Giovino G et al (2012) Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 380:668–679. https://doi.org/10.1016/s0140-6736(12)61085-x
Higgins S et al (2017) addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiat 74:1056–1064. https://doi.org/10.1001/jamapsychiatry.2017.2355
Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K (2016) Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Japanese J Clin Oncol 46:942–951. https://doi.org/10.1093/jjco/hyw091
Houston K, Henley S, Li J, White M, Richards T (2014) Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer 86:22–28. https://doi.org/10.1016/j.lungcan.2014.08.001
Kim C et al (2014a) Smoky coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung Cancer 84:31–35. https://doi.org/10.1016/j.lungcan.2014.01.004
Kim C et al (2014b) Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the international lung cancer consortium (ILCCO). Int J Cancer 135:1918–1930. https://doi.org/10.1002/ijc.28835
Article CAS PubMed PubMed Central Google Scholar
Kocyigit A, Selek S, Celik H, Dikilitas M (2011) Mononuclear leukocyte DNA damage and oxidative stress: the association with smoking of hand-rolled and filter-cigarettes. Mutation Res 721:136–141. https://doi.org/10.1016/j.mrgentox.2011.01.013
Kubo S, Kobayashi M, Masunaga Y, Ishii H, Hirano Y, Takahashi K, Shimizu Y (2005) Cytokine and chemokine expression in cigarette smoke-induced lung injury in Guinea pigs. European Respiratory J 26:993–1001. https://doi.org/10.1183/09031936.05.00042405
Li M, Liu X, Zhang L (2018) The relationship of indoor coal use and environmental tobacco smoke exposure with lung cancer in China: a meta-analysis. J Cancer Res Therapeutics 14:S7–S13. https://doi.org/10.4103/0973-1482.168965
Lin X, Zhong WL, Lin SG (2008) Epidemiological survey on smoking situation for adults in Fujian. Strait J Prevent Med 14:1–3
CAS Google Scholar
López-Campos J, Ruiz-Ramos M, Fernandez E, Soriano J (2018) Recent lung cancer mortality trends in Europe: effect of national smoke-free legislation strengthening. Eur J Cancer Prev 27:296–302. https://doi.org/10.1097/cej.0000000000000354
Mowls D, McCaffree D, Beebe L (2015) Trends in lung cancer incidence rates, Oklahoma 2005-2010. PLoS One 10:e0119251. https://doi.org/10.1371/journal.pone.0119251
Ngu N, McEvoy M (2017) Environmental tobacco smoke and peripheral arterial disease. A Rev Atherosclerosis 266:113–120. https://doi.org/10.1016/j.atherosclerosis.2017.09.024
Ni X, Xu N, Wang Q (2018) Meta-analysis and systematic review in environmental tobacco smoke risk of female lung cancer by research type. Int J Environ Res Public Health 15:1348. https://doi.org/10.3390/ijerph15071348
Okazaki I, Ishikawa S, Ando W, Sohara Y (2016) Lung adenocarcinoma in never smokers: problems of primary prevention from aspects of susceptible genes and carcinogens. Anticancer Res 36:6207–6224. https://doi.org/10.21873/anticanres.11215
Ramström L (2017) Insufficient knowledge about use of multiple tobacco/nicotine products. Addict Behav 76:384–385. https://doi.org/10.1016/j.addbeh.2017.01.027
Sharp B, Chen H (2019) Neurogenetic determinants and mechanisms of addiction to nicotine and smoked tobacco. Eur J Neurosci 50:2164–2179. https://doi.org/10.1111/ejn.14171
Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. https://doi.org/10.3322/caac.21660
Wang A et al (2015a) Active and passive smoking in relation to lung cancer incidence in the Women’s health initiative observational study prospective cohort. Annals Oncol 26:221–230. https://doi.org/10.1093/annonc/mdu470
Wang G et al (2015b) Tobacco smoke induces production of chemokine CCL20 to promote lung cancer. Cancer Lett 363:60–70. https://doi.org/10.1016/j.canlet.2015.04.005
WHO (2015) Smoke-free policies in china–evidence of effectiveness and implications for action. https://iris.wpro.who.int/handle/10665.1/12047
Xiao J, Zhou Y, Jiang H, Lin Y, Ma J (2015) Analysis of cancer incidence and mortality from cancer registries of Fujian province in 2011 Zhonghua yu fang yi xue za zhi. Chin J Prevent Med 49:738–740
Xu T, Li W, Hu B (2010) Survey of smoking and passive smoking status among chinese adults in 11 provinces. Chin J Prevent Control Chronic Diseases 18:229–230
Google Scholar
Download references
Acknowledgments
We thank all the staff from the Department of Thoracic Surgery, The First Affiliated Hospital of Fujian Medical University. We also would like to express our appreciation to the patients who participated in our study.
This study was supported by the National Natural Science Foundation of China [grant number 81402738].
Author information
Jinman Zhuang, Zhi qiang Liu and Rendong Xiao contributed equally to this work.
Authors and Affiliations
Department of Epidemiology, School of Public Health, Fujian Medical University, Fuzhou, China
Jinman Zhuang, Zhi qiang Liu, Wei min Xiong, Lin Cai & Fei He
Fujian Provincial Key Laboratory of Environment factors and Cancer, School of Public Health, Fujian Medical University, Fuzhou, China
Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China
Department of Thoracic Surgery, The first affiliated hospital of Fujian Medical University, Fuzhou, China
Rendong Xiao & Xu Li
Medical Department, The Affifiliated Hospital of Putian University, Putian, China
Qiu ping Xu
You can also search for this author in PubMed Google Scholar
Contributions
FH and LC conceived and designed the experiments; ZQL, QPX, and WMX did the survey; FH, ZQL, JMZ, and RDX analyzed the data; RDX and XL contributed materials; JMZ and RDX wrote the paper.
Corresponding author
Correspondence to Fei He .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethical Review Committee of Fujian Medical University (Fuzhou, China).
Consent to participate
All of the participants consented to complete the questionnaire.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
(PDF 210 kb)
Rights and permissions
Reprints and permissions
About this article
Zhuang, J., Liu, Z.q., Xiao, R. et al. Association between smoking and environmental tobacco smoke with lung cancer risk: a case–control study in the Fujian Chinese population. J Public Health (Berl.) 30 , 2047–2057 (2022). https://doi.org/10.1007/s10389-021-01573-3
Download citation
Received : 09 September 2020
Accepted : 19 April 2021
Published : 10 May 2021
Issue Date : August 2022
DOI : https://doi.org/10.1007/s10389-021-01573-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Environmental tobacco smoke
- Lung cancer
- Case–control studies
- Find a journal
- Publish with us
- Track your research
Public-Place Smoking Laws and Exposure to Environmental Tobacco Smoke (ETS)
Public-place smoking restrictions are the most important non-price tobacco control measures worldwide, yet surprisingly little is known about their effects on exposure to environmental tobacco smoke (ETS). We study these laws in Canada using data with questions about respondents' ETS exposure in public and private places. In fixed-effects models we find these laws had no effects on smoking but induced large and statistically significant reductions in public-place ETS exposure, especially in bars and restaurants. We do not find significant evidence of ETS displacement to private homes. Our results indicate wide latitude for health improvements from banning smoking in public places.
We thank Marianne Bitler, Claire de Oliveira, Susumu Imai, Ian Irvine, Dean Lillard, Madeline Zavodny, three anonymous referees, and seminar participants at UC Berkeley ARE, UC Irvine, NBER, the 2010 American Society of Health Economists Conference, and the 2009 Canadian Economics Association meetings for useful comments. Some of the results in this paper are based on confidential data accessed at the Queen's RDC which are available in the Canadian Research Data Centres; interested readers can contact Warman for details on gaining access. A previous version of this paper circulated under the title "Public-Place Smoking Laws and Exposure to Environmental Tobacco Smoke (ETS) in Public Places." While the research and analysis are based on data from Statistics Canada, the opinions expressed do not represent the views of Statistics Canada nor do they necessarily reflect the views of the National Bureau of Economic Research. All errors are our own.
MARC RIS BibTeΧ
Download Citation Data
- March 24, 2010
Published Versions
More from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Anniversary
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 13, Issue 1
- Households contaminated by environmental tobacco smoke: sources of infant exposures
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- G E Matt 1 ,
- P J E Quintana 2 ,
- M F Hovell 2 ,
- J T Bernert 3 ,
- N Novianti 2 ,
- T Juarez 2 ,
- J Floro 1 ,
- C Gehrman 4 ,
- M Garcia 1 ,
- 1 Department of Psychology, San Diego State University, San Diego, California, USA
- 2 Graduate School of Public Health, San Diego State University
- 3 US Centers for Disease Control, Atlanta, Georgia, USA
- 4 SDSU/UCSD Joint Doctoral Program in Clinical Psychology, San Diego
- 5 San Diego State University Foundation, WIC Program, San Diego
- Correspondence to: G E Matt PhD, Department of Psychology, San Diego State University, San Diego, CA 92182-4611, USA; gmattsciences.sdsu.edu
Objectives: To examine (1) whether dust and surfaces in households of smokers are contaminated with environmental tobacco smoke (ETS); (2) whether smoking parents can protect their infants by smoking outside and away from the infant; and (3) whether contaminated dust, surfaces, and air contribute to ETS exposure in infants.
Design: Quasi-experiment comparing three types of households with infants: (1) non-smokers who believe they have protected their children from ETS; (2) smokers who believe they have protected their children from ETS; (3) smokers who expose their children to ETS.
Setting: Homes of smokers and non-smokers.
Participants: Smoking and non-smoking mothers and their infants ⩽ 1 year.
Main outcome measures: ETS contamination as measured by nicotine in household dust, indoor air, and household surfaces. ETS exposure as measured by cotinine levels in infant urine.
Results: ETS contamination and ETS exposure were 5–7 times higher in households of smokers trying to protect their infants by smoking outdoors than in households of non-smokers. ETS contamination and exposure were 3–8 times higher in households of smokers who exposed their infants to ETS by smoking indoors than in households of smokers trying to protect their children by smoking outdoors.
Conclusions: Dust and surfaces in homes of smokers are contaminated with ETS. Infants of smokers are at risk of ETS exposure in their homes through dust, surfaces, and air. Smoking outside the home and away from the infant reduces but does not completely protect a smoker’s home from ETS contamination and a smoker’s infant from ETS exposure.
- secondhand smoke contamination
- environmental tobacco smoke
- CDC, US Centers for Disease Control and Prevention
- CTS, California Tobacco Survey
- DEG, direct exposure group
- ETS, environmental tobacco smoke
- IEG, indirect exposure group
- NEG, no exposure group
- PAH, polycyclic aromatic hydrocarbons
- PPM, Pearson product moment
- RSP, respirable suspended particles
- WIC, Women, Infants, and Children Supplemental Food and Nutrition Program
https://doi.org/10.1136/tc.2003.003889
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Environmental tobacco smoke (ETS)—also known as secondhand smoke—is a complex mixture of more than 4000 chemical compounds that are generated during the burning of tobacco products. This mixture contains numerous irritants and toxicants with acute health effects as well as toxicants with carcinogenic effects in humans. ETS is known to increase morbidity and mortality risks in infants, children, and adult non-smokers. 1– 4
Data from the California Tobacco Survey (CTS) indicate that, in 1999, 72.8% of homes in California were smoke-free, leaving approximately one in four homes at risk of contributing to tobacco exposure of non-smokers. 5 In homes with children under 6 years of age where all adults smoked, 56.7% of respondents reported having a complete smoking ban. In homes with children where only some adults smoked, 73.1% were reportedly smoke-free in 1999. A similar picture emerges for the USA at large. Data from the National Health and Nutrition Examination Survey III show that 43% of US children (aged 2 months to 11 years) lived in a home with at least one smoker, and that 37% of adult non-tobacco users lived in a home with a smoker or reported exposure to ETS at work. 6, 7 More recently, the 2000 Behavioral Risk Factor Surveillance System collected data from 20 states about smoking policies at home. The percentage of adults reporting no smoking at home ranged from 61% (Virginia) to 79% (Colorado), 7 suggesting that 20–40% of US homes contribute to tobacco exposure of non-smokers.
The best understood route of exposure to ETS is the inhalation of contaminated indoor air. 1, 2 In addition to gas and vapour phase ETS components, contaminated air also contains ETS particles. Because the ETS particles have a mass median aerodynamic diameter of well below 2.5 μm, 8 they become respirable suspended particles (RSPs) that cannot be easily filtered and removed by the protective mechanisms of nose and throat. The size of these particles allows them to enter the deep lung and to cause damage due to their size alone. 9 To this effect can be added the chemical toxicity of the particles that enter the deep lung. Thus, both the size of ETS particles and the systemic effects of the chemical toxicity of ETS components may contribute to morbidity.
Inhaling ETS while a cigarette is being smoked is the most noticeable, though not the only exposure occasion. From ETS chamber and field studies, it is known that ETS components are rapidly dispersed after emission and undergo further dynamic chemical reactions. 8 Vapour phase components deposit and are adsorbed onto walls, furniture, clothes, toys, and other objects within 10 of minutes to hours after tobacco smoke has been emitted. From there, they are re-emitted into the air over the course of hours to months. ETS particulate matter can deposit on surfaces within hours after smoking occurred, from where it may be re-suspended or react with vapour phase compounds. Through this dynamic behaviour, ETS can contaminate house dust, carpets, walls, furniture, and other household objects for weeks and months after ETS was emitted from a cigarette. Findings from controlled chamber and field studies suggest that residential indoor environments become reservoirs for ETS, turning contaminated dust, carpets, and other household objects into potential sources of ETS exposure long after smoking has stopped. 8, 10
Infants of smoking parents are at a particular risk of secondhand smoke exposure through contaminated house dust and surfaces. During their first year of life, infants spend much time indoors, are in close proximity to contaminated dust and objects (for example, blankets, carpets, floors), and are in close physical contact with their smoking parents. At approximately 0.05–0.25 g/day, the dust ingestion rate in infants is estimated to be more than twice that of adults. 11 Moreover, because of their developmental stage, infants exhibit a much higher frequency of hand-to-mouth and object–to-mouth contacts and ingestion of non-food items (that is, pica behaviour) than older children or adults. 12 In addition to increased inhalation of contaminated dust, infants may also be exposed to ETS through ingesting and touching contaminated objects and surfaces. As infants and young children are highly active near the floor, they may also be exposed to higher levels of re-suspended floor dust than adults.
House dust and contaminated surfaces are known to be a major source of contaminants such as lead, allergens, pesticides, and polycyclic aromatic hydrocarbons (PAH). 11 However, little research is available on ETS contamination of house dust. Hein et al 13 were the first to detect nicotine in house dust from homes of smokers. They compared house dust from homes of 34 smokers and 38 non-smokers and found a strong positive correlation ( r = 0.65) between amount smoked and the nicotine concentration in the house dust. The amount of nicotine inhaled during one hour was estimated for someone in a home with high nicotine concentration in the house dust to be 12 ng, a relatively small amount compared to that inhaled by an active smoker (600–3000 ng/h). However, considering that an infant may spend the entire day indoors, has a higher respiration rate (factor 3–8) and a lower body weight than an adult (factor 10–20), this relatively low dosage of ETS exposure may accumulate over the course of weeks to levels equivalent to several hours of active adult smoking. Thus, long term exposure to contaminated house dust raises the possibility of significant exposure to toxic agents in ETS, which might contribute to disease aetiology or exacerbation of pre-existing illness.
This study explored the potential role of dust and surface contamination as sources of exposure to the contents of ETS for infants. We compared three types of households. The “direct exposure group” (DEG) consisted of households in which parents smoked indoors at home and in the presence of their child. The “indirect exposure group” (IEG) consisted of households in which parents smoked and attempted to protect their infants by smoking outside of the home and in the absence of their child. The control group (“no exposure group”, NEG) consisted of households with parents who have never smoked, in which no smoking took place indoors, and the infant was not knowingly exposed to tobacco smoke elsewhere. Multiple measures of air, dust, and surface contamination and multiple measures of the infants’ exposure to tobacco smoke were examined to address the following questions:
Are house dust and surfaces in households of smokers contaminated with secondhand smoke?
Do smoking parents protect their infants by smoking outside and away from the infant?
Do contaminated household dust and surfaces contribute to the overall exposure of infants to secondhand smoke?
Participants
Participants were recruited through advertisement at WIC (Women, Infants, and Children Supplemental Food and Nutrition Program; 96%) sites in San Diego County and in the local news media (4%). Interested mothers were contacted by phone to determine their eligibility. To qualify, all mothers had to have an infant between 2–12 months old and could not have breast fed their baby within the past 30 days. Though we understand that breast feeding may enhance the health of an infant, we elected to omit families where the mother was breastfeeding the infant because breastfeeding by a smoker (or mother exposed to ETS) may transmit nicotine and confound our cotinine measures of ETS. Subjects were paid up to $100 for participating in the study. Forty nine infants aged 2–13 months and their mothers completed the study.
Table 1 provides sociodemographic information about the household, mothers, and infants in the three exposure groups. The three groups did not differ (all p > 0.15) with respect to household size and income, size of the home, age and sex of the infant, and mother’s age and employment status. Mothers in the non-exposure group tended to be more educated (35% completed college) than mothers in the indirect (6%) and direct (0%) exposure groups (χ 2 (2) = 11.0, p = 0.004) . Moreover, the proportion of white non-Hispanic mothers was lower in the NEG (41%) than the IEG (69%) and DEG (75%) households, although this difference was not significant (χ 2 (2) = 4.5, p = 0.103).
- View inline
Demographic characteristics of participants in different exposure groups
Design and setting
This study relied on a non-equivalent group design, comparing three types of households in which infants were not exposed, indirectly exposed, or directly exposed to tobacco smoke. To qualify for the no exposure control group (NEG), all of the following conditions had to be met at the time of screening: (1) all household residents were non-smokers (that is, consumed no tobacco products) for at least one year; (2) no regular visitors smoked in the residence during the last year; (3) no visitors (regular or occasional) smoked cigarettes in the residence within the past 30 days; (4) there were no visits to a home where someone smoked in the same room with the infant within the past 30 days. In summary, NEG households (n = 17) serve as the baseline for ETS contamination and exposure measures.
To qualify for the indirect exposure group (IEG), all of the following conditions had to be met: (1) the mother had to smoke every day and at least 20 cigarettes/week over the past three months; (2) the mother or other household residents may not have smoked any cigarettes in the same room (or car) with the infant over the past three months; (3) the mother must have smoked at least 10 cigarettes/week at home outside or in a different room from the infant over the past three months. To rule out that smoking indoors at home in a different room contributed to direct ETS exposure, we identified households in which reportedly no indoor smoking took place during the assessment period. Findings are reported separately for all IEG households and those without indoor smoking. In summary, IEG households (n = 17) represent smoking parents who have made serious attempts to protect their children from ETS by not smoking in their presence. This group comes closest to what are commonly referred to as households with smoking bans. 5
To qualify for the direct exposure group (DEG) all of the following conditions had to be met: (1) the mother had to smoke every day and at least 20 cigarettes/week over the past three months; (2) the mother or other household residents must have smoked at least 20 cigarettes per week at home over the past three months; (3) the mother or other household residents had to smoke one or more cigarettes per day (or seven cigarettes per week) at home in the same room with the infant. In summary, DEG households (n = 15) represent smoking parents without a smoking ban at home who do not systematically attempt to protect their children from tobacco smoke.
Measurement schedule
Each residence was visited three times over the course of one week. All visits took place on a Tuesday, Friday, Monday schedule or a Friday, Monday, and Thursday schedule, such that all measures included exposures during a weekend. Each visit consisted of an interview with the mother, the collection of dust and surface wipe samples in the living room and the infant’s bedroom, the collection of a urine sample from the infant, and the collection of a wipe sample from the mother’s index finger of her dominant hand. Air nicotine monitors were placed in the living room and the infant’s bedroom at the first visit and collected for analyses at the third visit. A hair sample was obtained from the infant at the third visit.
Face-to-face interview
At the first visit, a face-to-face interview was conducted with the mother about: (a) the mother’s sociodemographic background; (b) household composition; (c) home characteristics, including type of home, size, pets, and furnishing; (d) cleaning activities over the past 30 days; (e) typical infant activities, schedule, and mouthing behaviours; (f) mother’s smoking history; (g) smoking behaviour of the mother and other household residents over the past three days; (h) infant’s exposure to secondhand smoke over the past three days. Parts (g) and (h) of the interview were repeated at the second and third visit. Based on these interviews, the following reported measures were determined: (1) mother’s average number of cigarettes smoked per day; (2) the average number of cigarettes per day to which the infant was exposed; and (3) the total number of cigarettes to which the infant was exposed (that is, cigarettes smoked in the presence of the infant). All interview measures reflect behaviours over a 10 day period, consisting of seven study days plus the three days preceding the first visit. Matt et al 14, 15 present relevant findings on the reliability and validity of parent reported secondhand smoke exposure.
Behaviour diary
At the first and second visit, the mother was given a diary, and she was instructed to record until the next visit the child’s whereabouts, activities, presence of smokers, exposure to tobacco smoke, and mother’s smoking. Based on these diaries, the following additional reported measures were determined: (4) average number of cigarettes per day smoked indoors by the mother, other household residents, and visitors; (5) mother’s average number of cigarettes smoked per day; (6) total number of cigarettes to which the infant was exposed; and (7) average number of cigarettes per day to which the infant was exposed. All diary based measures reflect behaviours over the seven study days only. The interview (1), (2), and (3) and diary based measures (5), (6), and (7) assessed the same behaviours over slightly different reference periods. Thus, they served as a check for consistency between retrospective reported behaviour and prospectively recorded practices, but they could not be compared directly because they represented slightly different time frames.
Air nicotine in living room and bedroom
Air levels of vapour phase nicotine were measured with passive diffusion monitor badges developed by Hammond et al 16 and used by us previously. 15, 17 The badges were placed in the baby’s home for the duration of the week, placed on the first and removed on the third visit. One badge was placed in the living area and one in the baby’s sleeping area. The height of the monitors was 2 feet from the floor, and badges were placed away from doors and windows. Unmarked non-analysed badges were placed in all other rooms such that all rooms appeared to have air monitors, in keeping with a bogus pipeline procedure. 18 This was employed to prevent smokers moving to a room without a monitoring badge. The number of hours placed in the home was recorded. The badges consisted of a modified 37 mm diffusive sampling cassette with a sodium bisulfate treated Teflon coated glass fibre filter. The badges were stored at −20°C and sent to K Hammond (University of California, Berkeley) for analysis as previously described. 16 Briefly, the nicotine was extracted into hexane and analysed on a gas chromatograph with a nitrogen detector, and results expressed as μg of nicotine/m 3 of air. The level of detection for one full week of exposure is 0.02 μg/m 3 .
Dust nicotine in living room and bedroom
Two area floor dust samples per visit were collected with a high volume, small surface sampler (HVS3, CS-3 Inc, Sandpoint, Idaho, USA), from a 150 cm×150 cm area, if possible. Some homes had a smaller area sampled, with none being less than 100 cm × 100 cm. One sample was obtained from the living room area and the other from the infant’s sleeping area. Areas were carefully measured from reference points in the home to allow collection of dust from the same area each time, without leaving any marks visible to the occupants. Floor dust samples were collected into Teflon bottles, transported cooled, weighed, and sieved with a stainless steel, methanol washed, 150 μm mesh sieve. Sieved dust was weighed and stored in glass bottles at −70°C until analysis. Analysis for nicotine was performed on a gas chromatograph equipped with mass spectrometry (GC-MS, HP 6890) using a method adapted from one developed at the US Centers for Disease Control and Prevention (CDC) 19 for analysis of nicotine in wipe samples. Cotinine and its labelled reference methyl-d3 cotinine were originally included in all analysis, but when approximately half of the samples had been analysed with cotinine detected in only two, cotinine was dropped from further analysis. The limit of detection was 0.03 μg/mg dust (CDC method) to 0.002 μg/mg dust (SDSU method, J Polansky modifications).
Surface nicotine on furniture in living room and bedroom
Two area wipe samples per visit were taken with a pre-screened wipe, covering a 10 × 10 cm area. Wipes were soaked in freshly prepared 0.1% (w/v) ascorbic acid to preserve the nicotine. One wipe was taken from the living room area (typically the coffee table). The other wipe was taken from the baby’s sleeping area (typically the bed frame). The same locations were wiped each visit. Wipes were placed into glass bottles, transported cooled, and stored at −70°C until analysis. Levels were expressed as weight of nicotine per wipe. The limit of detection was 0.06 μg/wipe (CDC) to 0.01 μg/wipe (SDSU method, J Polansky modifications).
Nicotine on mother’s index finger
A wipe sample of the mother’s index finger from the hand used to hold a cigarette was taken at each visit. Wipe was moistened and processed as above. In order to keep costs down while investigating the hypothesis that some nicotine might be present on mother’s hands, only one sample was chosen for analysis from four mothers in the IEG and four mothers in the DEG groups.
Urine cotinine
Urine samples were collected from the infant at each visit using a standard urine collection bag for infants or a cotton roll placed in the diaper. 14, 15 Cotton rolls were placed in a sterile 20 ml syringe and expressed into sterile 5 ml plastic cryovials. Samples were immediately frozen at −20°C before they were packed in dry ice and shipped to the CDC for analysis using high performance liquid chromatography, atmospheric pressure chemical ionisation tandem mass spectrometry (HPLC APCI-MS 20 ). Cotinine levels reported are “total” cotinine, combining bound and unbound quantities of the metabolite. The assay is sensitive to levels as low as 0.05 ng/ml.
Hair nicotine and cotinine
In combination with the urine cotinine measure (1–3 days’ half life), hair cotinine provides a measure of exposure over a longer period of time (1–2 months). 21, 22 Hair samples were obtained at the last visit by cutting 1 cm of 10–15 hair shafts (approximately 10 mg in weight) close to the scalp from the back of the head (posterior vertex, occipital bone) using methanol cleaned scissors. Samples were stored in sterile vials and sent to J Klein (University of Toronto) for analysis as described. 23 The limits of detection were 0.02 ng/mg and 0.05 ng/mg for cotinine and nicotine, respectively.
Statistical analyses
Statistical analyses were conducted using STATA 7.0 24 and SPSS 10.1. 25 All outcome measures were subjected to logarithmic transformation before analyses were conducted to deal with skewed error distribution and to stabilise error variances. Relations between measures of contamination and exposure were examined using rank order and Pearson product moment (PPM) correlations. Because findings do not differ substantially, we only report those for PPM correlations. Significance was set at α = 0.05.
Differences in outcome measures between groups were tested via Tobit regression models, 26 in which an observation was defined as left censored if the value fell below the detection limit of a particular outcome measure. In addition, we used robust estimates of standard errors based on the Huber-White sandwich estimator of variance 27 to protect against the undue influence of outliers on statistical tests in this relatively small sample.
The contribution of house dust and surface contamination to overall exposure was examined using OLS regression models with robust standard errors based on the Huber-White sandwich estimator of variance. 26, 27
Smoking behaviour and smoking policies
Table 2 presents descriptive information regarding smoking behaviour and smoking policies in the three exposure groups. In NEG households, nobody was a smoker and no smokers had reportedly visited during the 30 days before the interview.
Smoking behaviours in different exposure groups
The IEG and DEG households did not differ significantly with respect to the number of smokers and the percentage of visitors smoking outside of the home. Mothers in DEG households smoked more than mothers in IEG households based on interview data (9.34 v 5.38 cigs/day (t(30) = 2.49, p = 0.018) but not based on diary data (6.20 v 5.41 cigs/day; t(31) = 0.44, p = 0.662). Moreover, DEG households were more likely than IEG households to have visitors who smoked indoors (66.7% v 6.3; χ 2 (1) = 10.2, p < 0.01) during the past 30 days. This difference is also reflected in home policies about smoking. Significantly larger proportions of IEG households declared that smokers at home always or almost always smoked outside (88% v 27%; χ 2 (2) = 9.3, p < 0.01) and shut the doors or windows when smoking outside (69% v 13%; χ 2 (2) = 13.6, p < 0.01).
In the IEG households, all mothers were smokers and about two out of three households had one or more additional smokers. Four of the 17 IEG households reported that cigarettes were smoked in the home, for an average of 1.06 cigs/day in these four households. Three of these four households also reported that their infants were in a room or car where cigarettes were smoked at home or away from home. In the three households where this occurred, the infants were directly exposed to an average of 0.38 cigs/day.
To control for the occasional indoor exposure of some infants in the IEG group, we identified a subgroup of IEG households in which reportedly no cigarettes were smoked in the home during the assessment week and infants were not knowingly exposed to tobacco smoke (for example, at home, in a car, at someone else’s home). This was done to investigate whether smoking indoors during the assessment period contributed to ETS contamination at home and the child’s exposure. There were no statistically (all p > 0.20) or practically significant differences on any of the exposure measures between the “no indoor smoking/no direct exposure” IEG subgroup (n = 12) and the “occasional indoor smoking/occasional direct exposure” IEG subgroup (n = 4). Similarly, there were no significant differences between the two IEG subgroups in contamination measures, with the exception of the maximum nicotine loading in living room and bedroom dust (table 3). We also investigated whether excluding the four households with occasional indoor smoking and direct exposure would alter findings concerning group differences between NEG, IEG, and DEG households. However, this was not the case. Therefore, all subsequent statistical analyses rely on the entire group of 17 IEG households to maintain sufficient statistical power. In tables 2, 3, and 4, we report separately findings for all 17 IEG households and the subgroup of 12 households without indoor smoking.
Geometric means and their 95% confidence intervals for measures of nicotine contamination in different exposure groups
Geometric means and their 95% confidence intervals for measures of secondhand smoke exposure in different exposure groups
Contamination of the indoor home environment
Table 3 presents the nicotine levels found in the air, in dust, on surfaces, and on fingers in the three exposure groups. To investigate whether contamination levels differed between exposure groups, NEG households were compared to IEG households (contrast 1, C1) and IEG households were compared to DEG households (contrast 2, C2).
Air nicotine levels
Nicotine was detected in the living room air and the bedroom air in all smoker households and 97% of non-smoker households. Air nicotine concentrations in the living rooms and infant bedrooms of IEG households were approximately three and two times higher, respectively, than those found in the living and bedroom of NEG households (0.32 μg/m 3 v 0.10 μg/m 3 ; 0.22 μg/m 3 v 0.09 μg/m 3 ). Air nicotine levels in living rooms and infant bedrooms of DEG households were eight times and seven times higher than in IEG households (2.57 μg/m 3 v 0.32 μg/m 3 ; 1.50 μg/m 3 v 0.22 μg/m 3 ).
Significant differences were found in air nicotine levels between NEG and IEG (C1) and between IEG and DEG (C2) in the living room (that is, χ 2 (2) = 38.75, p < 0.001; C1: t(46) = 6.19, p < 0.001; C2: t(46) = 9.69, p < 0.001) and bedrooms (that is, χ 2 (2) = 38.75, p < 0.001; C1: t(46) = 4.77, p < 0.001; C 2: t(46) = 7.62, p < 0.001). These findings suggest that while parents in the IEG were able to reduce air nicotine levels compared to DEG households, their children were not protected from exposure to nicotine in the indoor air at home.
Surface nicotine levels
No nicotine was detected on surfaces examined in the living room and infant bedrooms of NEG households. In IEG households, 51% and 53% revealed nicotine levels above the limit of detection on living room and bedroom surfaces, respectively. The average level of the highest nicotine level per household was 19.89 μg/m 2 ; the average of the mean nicotine level per household was 10.68 μg/m 2 .
Nicotine was detected on 88% (49 of 56 samples) of the living room and 88% (35 of 40 samples) of bedroom surfaces in DEG households. Nicotine contamination of surfaces in DEG households was three to five times higher than those found in IEG households. On living room and bedroom surfaces, the average of the highest nicotine levels per household were 73.05 μg/m 2 and 56.26 μg/m 2 , respectively. The average levels of the mean nicotine level per household were 51.33 μg/m 2 and 41.85 μg/m 2 in the living room and bedroom, respectively.
Tobit regression analyses showed significant differences between IEG and DEG households for surface nicotine levels in the living rooms (that is, χ 2 (1) = 9.50, p = 0.002; C2: t(30) = 3.21, p = 0.003) and bedrooms (that is, χ 2 (1) = 16.29, p = < 0.001; C2: t(30) = 4.59, p < 0.001). These findings suggest that IEG households had lower nicotine levels on household surfaces compared to DEG households. However, IEG households showed surface contamination significantly higher than zero (see confidence interval in table 3). Wipe samples collected in NEG households revealed no detectable levels of nicotine and had to be excluded from the analyses.
Nicotine on fingers
No nicotine was detected on the fingers of mothers in the NEG households. However, nicotine was detected on the fingers of 100% and 92% of mothers in the IEG and DEG households, respectively. The average nicotine levels in both groups were 0.63 μg/wipe and 0.65 μg/wipe in the IEG and DEG, respectively. Given the surface area of a typical index finger (< 100 cm 2 ), the average nicotine loading on the fingers of the smoking mothers in the IEG and DEG households is more than twice as high as the nicotine loading on living room surfaces of DEG households. Note that the confidence intervals are noticeably large because of the small sample sizes.
Tobit regression analyses revealed that nicotine levels found on the index fingers of smoking mothers were significantly larger than zero (t(9) = 2.63, p = 0.025). Controlling for smoking frequency, no significant differences were found in finger nicotine between mothers in the IEG and DEG groups (that is, χ 2 (2) = 0.06, p = 0.97; C2: t(10) = 0.13, p = 0.90).
Nicotine in household dust
Approximately equal amounts of dust were found in bedrooms and living rooms of IEG and DEG households. On day 1 of dust collection, 1.50 g (95% CI 0.75 to 2.58 g) and 1.21 g (95% CI 0.61 to 2.04 g) were collected in the living rooms and bedrooms of IEG households, and 1.50 g, (95% CI 0.57 to 2.98) and 1.57 g (95% CI 0.72 to 2.82) in the DEG households. Summed across all three dust collections, 3.83 g (95% CI 1.82 to 7.28 g) and 2.42 g (95% CI 1.07 to 4.67 g) were collected in the living rooms and bedrooms of IEG households and 3.07 g (95% CI 1.34 to 6.09 g) and 2.87 g (95% CI 1.50 to 4.99 g) in the DEG households.
Nicotine was detected in 38% and 52% of dust samples taken from the living rooms and bedrooms of IEG households. The averages of highest nicotine levels found in the living rooms and bedrooms of each household were 4.43 μg/m 2 and 3.22 μg/m 2 , respectively. The averages of the mean nicotine levels per household were 2.22 μg/m 2 and 0.89 μg/m 2 for the living rooms and infant bedrooms, respectively.
Nicotine was detected in 55% and 70% of dust samples taken from the living rooms and bedrooms of DEG households. The averages of highest nicotine levels found in the living rooms and bedrooms of each household were 64.0 μg/m 2 and 15.8 μg/m 2 , respectively. The averages of the mean nicotine levels per household were 6.85 μg/m 2 and 5.37 μg/m 2 for the living rooms and infant bedrooms, respectively.
Tobit regression analyses revealed significant differences between dust nicotine levels in the living rooms (that is, χ 2 (1) = 5.37, p = 0.02; C2: t(27) = 2.17, p = 0.04) and bedrooms of IEG and DEG households (that is, χ 2 (1) = 5.48, p = 0.02; C2: t(27) = 2.29, p = 0.03). These findings suggest that IEG households had lower dust nicotine levels compared to DEG households. Note that dust samples were analysed from IEG and DEG households only, because pilot data revealed no detectable nicotine levels in nonsmoking household.
Infant exposure to tobacco
Mother reported exposure.
Mothers in the NEG households reported that their infants were not exposed tobacco smoke either at home or away from home. In the IEG group, 76% of mothers indicated their child was not exposed to tobacco smoke, and 24% reported exposure to tobacco smoke away outside of the home (for example, car, friend’s home). All mothers in the DEG group reported that their child was exposed to tobacco at home as well as away from home. As indicated by the number of cigarettes smoked in the presence of the child per day, infants in IEG households were directly exposed to 0.03 and 0.06 cigs/day according to the interview and behavioural diary, respectively. Infants in the DEG households were directly exposed to 5.57 and 5.75 cigs/day based on interview and diary reports, respectively.
Tobit regression models indicated that mother reported exposure levels in the IEG group were not significantly larger than zero (t(28) = 1.75, p = 0.091), indicating that mothers noticed little if any ETS exposure. Infant exposure as reported by mothers differed significantly between IEG and DEG households (that is, χ 2 (1) = 14.18, p ⩽ 0.001; C2: t(28) = 3.79, p = 0.001), indicating that smoking in the presence of the child was substantially higher in DEG than in IEG households.
In the NEG households, infant urine cotinine levels averaged 0.33 ng/ml and 0.43 ng/ml based on the mean and the maximum over the three sample days. Urine cotinine levels of infants in the IEG households were approximately eight times higher based on the average (2.47 ng/ml) and the maximum (3.49 ng/ml) over the three sample days. Compared to the IEG households, urine cotinine levels in the DEG households were more than six times higher. The mean levels were 15.47 ng/ml and 20.43 ng/ml based on the average and the maximum across the three sample days, respectively.
Tobit regression analyses showed significant differences in infant urine cotinine levels between NEG and IEG (C1) and between IEG and DEG (C2) (that is, χ 2 (2) = 76.22, p < 0.001; C1: t(45) = 10.85, p < 0.001; C2: t(45) = 12.76, p < 0.001). Moreover, urine cotinine levels in the IEG differed significantly from zero (t(45) = 19.09, p < 0.001). These findings suggest that while infants in the IEG households showed lower exposure levels compared to DEG households, they were not completely protected from secondhand smoke exposure.
We observed a correlation of r = 0.81 (t(34) = 66.9, p < 0.001) between log transformed nicotine and cotinine levels in hair. Hair nicotine and cotinine levels among children in the NEG households were .53 ng/mg and 0.08 ng/mg, respectively. In comparison, hair nicotine and cotinine levels of infants in the IEG households were more than five times higher at 2.65 ng/mg and 0.52 ng/mg, respectively. Infants in the DEG households showed nicotine and cotinine levels approximately twice as high as those in the IEG households at 5.95 ng/mg and 1.05 ng/mg.
Tobit regression analyses revealed significant differences in infant hair cotinine levels between NEG and IEG (C1) and between IEG and DEG (C2) (that is, χ 2 (2) = 21.55, p < 0.001; C1: t(33) = 4.70, p < 0.001; C2: t(33) = 4.48, p < 0.001). The same group differences were found for hair nicotine levels (that is, χ 2 (2) = 25.40, p < 0.001; C1: t(33) = 5.44, p < 0.001; C2: t(33) = 4.77, p < 0.001). These findings indicate again that infants in the IEG households were not protected from secondhand smoke exposure.
Exploring the contribution of air, dust, surface, and finger contamination to overall exposure
Our findings showed that infants in the IEG and DEG groups live in homes with ETS contaminated air, dust, and surfaces. To explore how air, dust, and surface contamination in living rooms and bedrooms may contribute to the overall exposure to ETS, we first examined their bivariate relations. Air and surface nicotine showed consistently positive and medium to large correlations, ranging from 0.85 (living room and bedroom surface nicotine) and 0.84 (living room and bedroom air nicotine) to 0.49 (living room air and living room surface) and 0.51 (living room surface and bedroom air). In contrast, dust nicotine levels showed low to medium correlations (<0.40) with other air and surface nicotine levels.
We examined next the extent to which air, dust, and surface nicotine levels in living rooms and bedrooms predicted average urine cotinine levels. In the subset of 27 households for which measures on all variables were available, living room and bedroom surface nicotine (t(21) = −2.16, p = 0.043; t(21) = 3.12, p = 0.005), living room and bedroom dust nicotine (t(21) = −2.22, p = 0.038; t(21) = 2.07, p = 0.050), and bedroom air nicotine(t(21) = 3.47, p = 0.002) each accounted for a significant proportion of variance for a total R 2 = 0.78 (F(5,21) = 34.98, p < 0.001).
A similar model was fit in the larger subset of 41 households for which data were available on urine cotinine, air and surface nicotine in living rooms and bedrooms. In this sample, living room air nicotine (t(38) = 4.62, p < 0.001; semi-partial r 2 = 0.23) and bedroom surface nicotine (t(38) = 2.38, p = 0.022; semi-partial r 2 = 0.06) accounted for significant proportions of variance for a total R 2 = 0.74 (F(2,38) = 45.57, p < 0.001).
This study investigated air, dust, surfaces, and mother’s index fingers to determine whether they are contaminated with nicotine, the single best marker of ETS and its chemical constituents. Nicotine was detected in the living and bedroom air of infants in the non-smoker and smoker households. Nicotine was also detected in dust and on surfaces of living rooms and bedrooms of infants in IEG and DEG households. Moreover, nicotine was detected on the index fingers of smoking mothers. Although IEG and DEG households had about the same amount of dust, we found three times as much nicotine per square metre in the living rooms of DEG than in IEG households, and we found about six times as much nicotine per square metre in the bedrooms of DEG and IEG households. That is, differences in amount of dust collected in the IEG and DEG households do not account for differences in dust nicotine.
Compared to non-smoker households, average contamination levels in IEG households were 5–7 times higher. Average contamination levels in DEG households were 3–8 times higher than in IEG households. As expected, nicotine contamination of mothers’ index fingers was approximately the same in the DEG and IEG households. This is consistent with the observation that mothers in the IEG and DEG groups had approximately equal smoking rates. Consistent with the different levels of contamination, infants in IEG households showed exposure levels 5–8 times higher than those of infants in NEG households. Exposure levels were 2–6 times higher in infants of DEG households than those in IEG households.
Multiple sources of exposure
Infants of smokers live in homes that are contaminated with ETS and are exposed to ETS. This study showed that ETS contamination is not limited to the indoor air, but includes surfaces and dust in living rooms and bedrooms 8, 13, 28 and on smokers’ skin. This puts infants at risk of exposure to the toxics components of ETS through multiple sources and multiple pathways, including the inhalation of contaminated air, the inhalation and ingestion of dust, ingestion and skin contact with contaminated household surfaces, and the skin of smokers.
This study provided preliminary evidence in support of the multiple exposure risk in infants. Our findings suggest that nicotine contamination of air, dust, and surfaces in living rooms and bedrooms independently account for variance in infants’ urine cotinine levels. Specifically, higher levels of bedroom air, dust, and surface contamination are associated with higher levels of urine cotinine.
Protecting infants from ETS exposure
This study suggests that smokers can reduce household contamination and ETS exposure of their infants by implementing a strict smoking ban in the home and by not smoking in the proximity of the infant outside the home. These findings differ from those reported by Al-Delaimy et al 29 with respect to hair nicotine, who found no significant effect on hair nicotine levels of children (aged 3 months to 10 years) if household members smoked outside or inside the home. The fact that our sample consisted of infants under 12 months (mean 7 months) may partly explain why we found differences in exposure levels between infants in households with and without indoor smoking bans. Because Al-Delaimy et al ’s study 29 did not include measures of ETS contamination, it is unclear whether households with and without indoor smoking bans actually differed in ETS contamination of air, dust, and surfaces. Moreover, it is unclear the extent to which ETS exposure outside the home may have contributed to the overall exposure of children in their study.
Although smoking bans appear to reduce indoor ETS contamination and ETS exposure of infants, smokers will find it difficult—if not impossible—to protect their children from ETS and its toxics components. These findings are consistent with those of Al-Delaimy et al . 29 While parents in the IEG households were successful in reducing dust, surface, and air contamination and exposure levels compared to DEG households, they were unable to reduce ETS contamination and exposure to levels found in non-smoker households. Moreover, skin contamination did not differ between mothers in the DEG and IEG households as is expected because smoking rates were similar in the two groups.
To better understand the challenge to protect children of smokers from secondhand smoke, it is important to consider the parents’ efforts to do so. Almost 90% of parents in the IEG households always or almost always smoked outside, and approximately two thirds always or almost always closed doors and windows when smoking outside. In only four IEG households were any cigarettes reportedly smoked indoors during the study period. The average number of cigarettes reportedly smoked in the proximity of the infants in IEG households (for example, at home, in the car, or outside when child was present) was less than 0.1 per day. It appears that parents tried their best to protect their children from tobacco smoke and had reason to believe that they succeeded in doing so. While parents were able to lower ETS contamination and ETS exposure, these efforts were insufficient to achieve levels of nicotine contamination in the homes and exposure found in infants of non-smoking parents.
Our findings point to some of the sources of ETS exposure that parents cannot easily control through indoor smoking bans. ETS can remain in the home even if smoking took place days, weeks and months earlier 1, 10, 30 through contaminated dust and surfaces, including the frame of an infant’s bed and a smoker’s finger. Additionally, ETS may find its way into the home through windows and doors if cigarettes are smoked outside and through contaminated clothes, skin, and dust carried into the home if cigarettes were smoked elsewhere.
This line of research has many important implications for the comprehensive measurement of ETS contamination and exposure, the study of health risks, the control of secondhand smoke, and public health policies. The comprehensive assessment of secondhand smoke contamination must consider the multiple sources of exposure, including but not limited to, air, dust, surfaces, and skin. 8 Because ETS is not uniformly distributed throughout a home and over time, 28 different household members may be at different risk of exposure to different sources of ETS contamination and different ETS components. For example, if exposure risks in infants are the primary concern, air samples should be taken at lower heights, and objects and surfaces should be sampled with which an infant is more likely to have contact. If smoking takes place irregularly, the duration and frequency of sampling must become an important consideration. If rooms are well ventilated during smoking, highly volatile ETS compounds and ETS particles may contribute less to long term ETS contamination than other compounds. 8
Little is currently known about the differential health risks associated with the inhalation or ingestion of ETS and its toxic components or the health risks associated with ETS exposure within minutes, days, or months after tobacco smoke was emitted. As a first step, research is needed to better understand the validity of nicotine as a marker of ETS in air, dust, and surfaces over the time course of ETS contamination. Next, efforts are necessary to better measure and model the cumulative effects of exposure to ETS through different contamination sources. This and other studies suggest that dose of exposure is a complex function not only of amount of secondhand smoke, timing, and duration but also of different sources and routes of exposure.
Findings of this study suggest that interventions and public policies to reduce secondhand smoke exposure may have to be revised. 31, 32 There are three major concerns. First, smoking outdoors, in different rooms, or when non-smokers are absent does not completely protect non-smokers from tobacco smoke, although it significantly reduces the likely level of exposure. Thus, children of smokers, non-smoking staff cleaning designated smoking areas in hotels and restaurant, and non-smokers renting or buying cars, apartments, and houses of smokers, are at risk of secondhand smoke exposure and the associated health risks. Second, because ETS contaminates surfaces, dust, and skin, serious consideration should be given to efforts necessary to decontaminate homes, cars, furniture, etc, of smokers. Third, because contaminated indoor environments may present significant health risks to unsuspecting non-smokers, public policies may be needed, requiring disclosure of the smoking status of former tenants of apartments and offices and/or owners of cars and homes. To understand and evaluate the health risks associated with ETS exposure, we must take into account the complex physical and chemical properties of ETS, the extent and persistence of ETS contamination of residential environments, the multiple exposure pathways, the cumulative effects of ETS exposure, and the differential vulnerability of risk populations. There is yet much to be learned before we know how to comprehensively assess the risks of ETS exposure and effectively protect non-smokers from ETS.
What this paper adds
To our knowledge, this is the first study to document that surfaces, dust, and air are contaminated in homes of smokers with infants. Infants of smokers are at risk of ETS exposure in their homes through dust, surfaces, and air. Smoking outside the home and away from the infant reduces but does not protect a smoker’s home from ETS contamination and a smoker’s infant from ETS exposure.
Acknowledgments
This study was supported by grant 7IT-0087 from the Tobacco Related Disease Research Program (TRDRP) and by the United States Centers for Disease Control.
- ↵ California Environmental Protection Agency . Health effects of exposure to environmental tobacco smoke: final report . Sacramento, CA: The Office of Environmental Health Hazard Assessment, 1997 .
- ↵ National Research Council . Committee on Passive Smoking. Environmental tobacco smoke: measuring exposures and assessing health effects . Washington DC: National Academy Press, 1986 .
- US Department of Health and Human Services . The health consequences of involuntary smoking. A report of the Surgeon General, 1986 . Rockville, Maryland: Public Health Service, Centers for Disease Control, 1986 . (DHHS Publication No (CDC) 87-8398.).
- ↵ U.S. Environmental Protection Agency . Respiratory health effects of passive smoking: lung cancer and other disorders, Vol 4. Washington, DC: US Department of Health and Human Services, US Environmental Protection Agency, 1993 .
- ↵ Pierce JP , Gilpin EA, Emery SL, et al. Tobacco control in California: who’s winning the war? An Evaluation of the Tobacco Control Program, 1989–1996 . La Jolla, California: University of California, San Diego, 1998 .
- ↵ Pirkle JL , Flegal KM, Bernert JT, et al. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA 1996 ; 275 : 1233 –40. OpenUrl CrossRef PubMed Web of Science
- ↵ US Centers for Disease Control and Prevention . State-specific prevalence of current cigarette smoking among adults, and policies and attitudes about secondhand smoke—United States, 2000. JAMA 2002 ; 287 : 309 –10. OpenUrl CrossRef PubMed
- ↵ Daisey JM . Tracers for assessing exposure to environmental tobacco smoke: what are they tracing? Environ Health Perspect May 1999 ; 107 (suppl 2): 319 –27.
- ↵ Cohen D , Arai SF, Brain JD. Smoking impairs long-term dust clearance from the lung. Science 4 May 1979 ; 204 (4392): 514 –17.
- ↵ Vaughan WM , Hammond SK. Impact of “designated smoking area” policy on nicotine vapor and particle concentrations in a modern office building. J Air Waste Manage Assoc 1990 ; 40 : 1012 –17. OpenUrl PubMed
- ↵ Roberts JW , Dickey P. Exposure of children to pollutants in house dust and indoor air. Rev Environ Contam Toxicol 1995 ; 143 : 59 –78. OpenUrl PubMed
- ↵ Tulve NS , Suggs JC, McCurdy T, et al. Frequency of mouthing behavior in young children. J Expo Anal Environ Epidemiol 2002 ; 12 : 259 –64. OpenUrl CrossRef PubMed Web of Science
- ↵ Hein HO , Suadicani P, Skov P, et al. Indoor dust exposure: an unnoticed aspect of involuntary smoking. Arch Environ Health 1991 ; 46 : 98 –101. OpenUrl PubMed Web of Science
- ↵ Matt GE , Hovell MF, Zakarian JM, et al. Measuring secondhand smoke exposure in babies: the reliability and validity of mother reports in a sample of low-income families. Health Psychol 2000 ; 19 : 232 –41. OpenUrl CrossRef PubMed Web of Science
- ↵ Matt GE , Wahlgren DR, Hovell MF, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tobacco Control 1999 ; 8 : 282 –9. OpenUrl Abstract / FREE Full Text
- ↵ Hammond SK , Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environmental Science & Technology 1987 ; 21 : 494 –7.
- ↵ Hovell MF , Zakarian JM, Matt GE, et al. Effect of counselling mothers on their children’s exposure to environmental tobacco smoke: randomised controlled trial. BMJ 2000 ; 321 : 337 –42. OpenUrl Abstract / FREE Full Text
- ↵ Murray DM , O’Connell CM, Schmid LA, et al. The validity of smoking self-reports by adolescents: a reexamination of the bogus pipeline procedure. Addict Behav 1987 ; 12 : 7 –15. OpenUrl CrossRef PubMed Web of Science
- ↵ Song S , Quintana PJE, Matt GE, et al. GC-MS measurement of nicotine and cotinine in indoor dust. Paper presented at: 47th Annual ASMS Conference on Mass Spectrometry adn Allied Topics. Dallas, Texas, 1999 .
- ↵ Bernert JT Jr , Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and non-smokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 1997 ; 43 : 2281 –91. OpenUrl Abstract / FREE Full Text
- ↵ Al-Delaimy WK . Hair as a biomarker for exposure to tobacco smoke. Tobacco Control Sep 2002 ; 11 : 176 –82. OpenUrl Abstract / FREE Full Text
- ↵ Al-Delaimy WK , Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health 2002 ; 56 : 66 –71. OpenUrl Abstract / FREE Full Text
- ↵ Klein J , Koren G. Hair analysis—a biological marker for passive smoking in pregnancy and childhood. Hum Exp Toxicol Apr 1999 ; 18 : 279 –82. OpenUrl Abstract / FREE Full Text
- ↵ StataCorp . Stata statistical software: Release 7.0 . College Station, Texas: Stata Corporation, 2001 .
- ↵ SPSS Inc . SPSS for Windows. Release 11.0 . Chicago, Illinois: SPSS Inc, 2002 .
- ↵ Johnston J , DiNardo J. Econometric models . New York: McGraw-Hill, 1997 .
- ↵ Hamilton LC . Regression with graphics. A second course in applied statistics . Belmont, California: Duxbury, 1991 .
- ↵ Lofroth G . Environmental tobacco smoke: multicomponent analysis and room-to-room distribution in homes. Tobacco Control 1993 ; 2 : 222 –5. OpenUrl CrossRef
- ↵ Al-Delaimy WK , Crane J, Woodward A. Passive smoking in children: effect of avoidance strategies, at home as measured by hair nicotine levels. Arch Environ Health 2001 ; 56 : 117 –22. OpenUrl PubMed Web of Science
- ↵ Daisey JM , Mahanama KR, Hodgson AT. Toxic volatile organic compounds in simulated environmental tobacco smoke: emission factors for exposure assessment. J Expo Anal Environ Epidemiol 1998 ; 8 : 313 –34. OpenUrl PubMed Web of Science
- ↵ Ashley MJ , Ferrence R. Reducing children’s exposure to environmental tobacco smoke in homes: issues and strategies. Tobacco Control 1998 ; 7 : 61 –5. OpenUrl Abstract / FREE Full Text
- ↵ Hovell MF , Zakarian JM, Wahlgren DR, et al. Reducing children’s exposure to environmental tobacco smoke: the empirical evidence and directions for future research. Tobacco Control 2000 ; 9 (suppl II): ii40 –7. OpenUrl Abstract / FREE Full Text
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2022
Tobacco and nicotine use
- Bernard Le Foll 1 , 2 ,
- Megan E. Piper 3 , 4 ,
- Christie D. Fowler 5 ,
- Serena Tonstad 6 ,
- Laura Bierut 7 ,
- Lin Lu ORCID: orcid.org/0000-0003-0742-9072 8 , 9 ,
- Prabhat Jha 10 &
- Wayne D. Hall 11 , 12
Nature Reviews Disease Primers volume 8 , Article number: 19 ( 2022 ) Cite this article
34k Accesses
69 Citations
96 Altmetric
Metrics details
- Disease genetics
- Experimental models of disease
- Preventive medicine
Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will die prematurely from tobacco-related complications. In addition, people who smoke have a significant reduction in their quality of life. Neurobiological findings have identified the mechanisms by which nicotine in tobacco affects the brain reward system and causes addiction. These brain changes contribute to the maintenance of nicotine or tobacco use despite knowledge of its negative consequences, a hallmark of addiction. Effective approaches to screen, prevent and treat tobacco use can be widely implemented to limit tobacco’s effect on individuals and society. The effectiveness of psychosocial and pharmacological interventions in helping people quit smoking has been demonstrated. As the majority of people who smoke ultimately relapse, it is important to enhance the reach of available interventions and to continue to develop novel interventions. These efforts associated with innovative policy regulations (aimed at reducing nicotine content or eliminating tobacco products) have the potential to reduce the prevalence of tobacco and nicotine use and their enormous adverse impact on population health.
Similar content being viewed by others

Associations between classic psychedelics and nicotine dependence in a nationally representative sample
Use of electronic cigarettes and heated tobacco products during the covid-19 pandemic.
Smoking cessation behaviors and reasons for use of electronic cigarettes and heated tobacco products among Romanian adults
Introduction.
Tobacco is the second most commonly used psychoactive substance worldwide, with more than one billion smokers globally 1 . Although smoking prevalence has reduced in many high-income countries (HICs), tobacco use is still very prevalent in low-income and middle-income countries (LMICs). The majority of smokers are addicted to nicotine delivered by cigarettes (defined as tobacco dependence in the International Classification of Diseases, Tenth Revision (ICD-10) or tobacco use disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)). As a result of the neuro-adaptations and psychological mechanisms caused by repeated exposure to nicotine delivered rapidly by cigarettes, cessation can also lead to a well-characterized withdrawal syndrome, typically manifesting as irritability, anxiety, low mood, difficulty concentrating, increased appetite, insomnia and restlessness, that contributes to the difficulty in quitting tobacco use 2 , 3 , 4 .
Historically, tobacco was used in some cultures as part of traditional ceremonies, but its use was infrequent and not widely disseminated in the population. However, since the early twentieth century, the use of commercial cigarettes has increased dramatically 5 because of automated manufacturing practices that enable large-scale production of inexpensive products that are heavily promoted by media and advertising. Tobacco use became highly prevalent in the past century and was followed by substantial increases in the prevalence of tobacco-induced diseases decades later 5 . It took decades to establish the relationship between tobacco use and associated health effects 6 , 7 and to discover the addictive role of nicotine in maintaining tobacco smoking 8 , 9 , and also to educate people about these effects. It should be noted that the tobacco industry disputed this evidence to allow continuing tobacco sales 10 . The expansion of public health campaigns to reduce smoking has gradually decreased the use of tobacco in HICs, with marked increases in adult cessation, but less progress has been achieved in LMICs 1 .
Nicotine is the addictive compound in tobacco and is responsible for continued use of tobacco despite harms and a desire to quit, but nicotine is not directly responsible for the harmful effects of using tobacco products (Box 1 ). Other components in tobacco may modulate the addictive potential of tobacco (for example, flavours and non-nicotine compounds) 11 . The major harms related to tobacco use, which are well covered elsewhere 5 , are linked to a multitude of compounds present in tobacco smoke (such as carcinogens, toxicants, particulate matter and carbon monoxide). In adults, adverse health outcomes of tobacco use include cancer in virtually all peripheral organs exposed to tobacco smoke and chronic diseases such as eye disease, periodontal disease, cardiovascular diseases, chronic obstructive pulmonary disease, stroke, diabetes mellitus, rheumatoid arthritis and disorders affecting immune function 5 . Moreover, smoking during pregnancy can increase the risk of adverse reproductive effects, such as ectopic pregnancy, low birthweight and preterm birth 5 . Exposure to secondhand cigarette smoke in children has been linked to sudden infant death syndrome, impaired lung function and respiratory illnesses, in addition to cognitive and behavioural impairments 5 . The long-term developmental effects of nicotine are probably due to structural and functional changes in the brain during this early developmental period 12 , 13 .
Nicotine administered alone in various nicotine replacement formulations (such as patches, gum and lozenges) is safe and effective as an evidence-based smoking cessation aid. Novel forms of nicotine delivery systems have also emerged (called electronic nicotine delivery systems (ENDS) or e-cigarettes), which can potentially reduce the harmful effects of tobacco smoking for those who switch completely from combustible to e-cigarettes 14 , 15 .
This Primer focuses on the determinants of nicotine and tobacco use, and reviews the neurobiology of nicotine effects on the brain reward circuitry and the functioning of brain networks in ways that contribute to the difficulty in stopping smoking. This Primer also discusses how to prevent tobacco use, screen for smoking, and offer people who smoke tobacco psychosocial and pharmacological interventions to assist in quitting. Moreover, this Primer presents emerging pharmacological and novel brain interventions that could improve rates of successful smoking cessation, in addition to public health approaches that could be beneficial.
Box 1 Tobacco products
Conventional tobacco products include combustible products that produce inhaled smoke (most commonly cigarettes, bidis (small domestically manufactured cigarettes used in South Asia) or cigars) and those that deliver nicotine without using combustion (chewing or dipping tobacco and snuff). Newer alternative products that do not involve combustion include nicotine-containing e-cigarettes and heat-not-burn tobacco devices. Although non-combustion and alternative products may constitute a lesser risk than burned ones 14 , 15 , 194 , no form of tobacco is entirely risk-free.
Epidemiology
Prevalence and burden of disease.
The Global Burden of Disease Project (GBDP) estimated that around 1.14 billion people smoked in 2019, worldwide, increasing from just under a billion in 1990 (ref. 1 ). Of note, the prevalence of smoking decreased significantly between 1990 and 2019, but increases in the adult population meant that the total number of global smokers increased. One smoking-associated death occurs for approximately every 0.8–1.1 million cigarettes smoked 16 , suggesting that the estimated worldwide consumption of about 7.4 trillion cigarettes in 2019 has led to around 7 million deaths 1 .
In most populations, smoking prevalence is much higher among groups with lower levels of education or income 17 and among those with mental health disorders and other co-addictions 18 , 19 . Smoking is also more frequent among men than women (Figs 1 – 3 ). Sexual and/or gender minority individuals have disproportionately high rates of smoking and other addictions 17 , 20 . In addition, the prevalence of smoking varies substantially between regions and ethnicities; smoking rates are high in some regions of Asia, such as China and India, but are lower in North America and Australia. Of note, the prevalence of mental health disorders and other co-addictions is higher in individuals who smoke compared with non-smokers 18 , 19 , 21 . For example, the odds of smoking in people with any substance use disorder is more than five times higher than the odds in people without a substance use disorder 19 . Similarly, the odds of smoking in people with any psychiatric disorder is more than three times higher than the odds of smoking in those without a psychiatric diagnosis 22 . In a study in the USA, compared with a population of smokers with no psychiatric diagnosis, subjects with anxiety, depression and phobia showed an approximately twofold higher prevalence of smoking, and subjects with agoraphobia, mania or hypomania, psychosis and antisocial personality or conduct disorders showed at least a threefold higher prevalence of smoking 22 . Comorbid disorders are also associated with higher rates of smoking 22 , 23 .
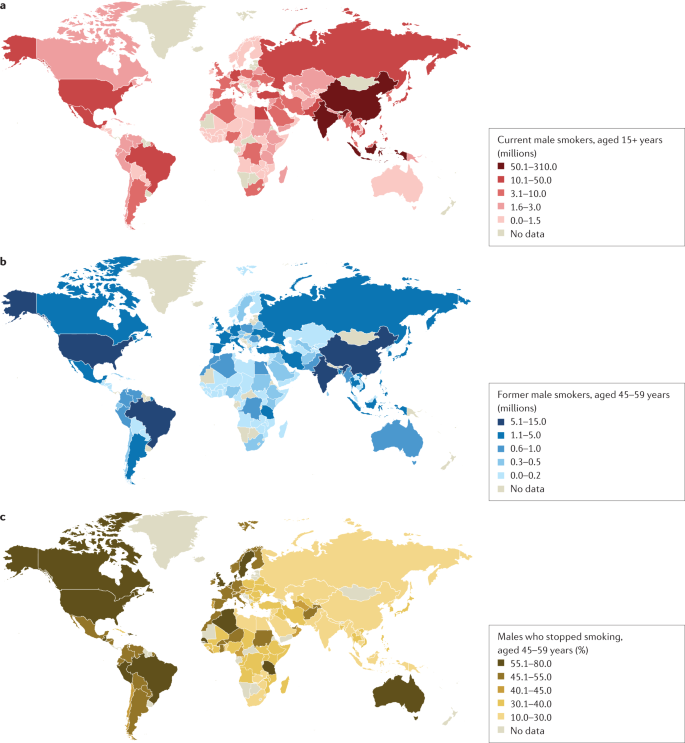
a | Number of current male smokers aged 15 years or older per country expressed in millions. b | Former male smokers aged 45–59 years per country expressed in millions. c | Former male smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for male smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among males is less variable than among females. Data from ref. 1 .
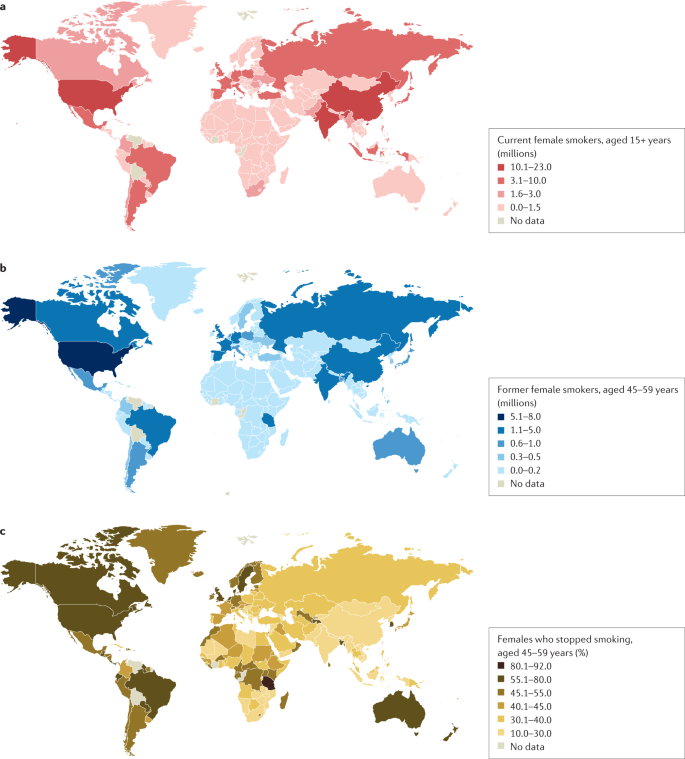
a | Number of current female smokers aged 15 years or older per country expressed in millions. b | Former female smokers aged 45–59 years per country expressed in millions. c | Former female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for female smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among females is much lower in East and South Asia than in Latin America or Eastern Europe. Data from ref. 1 .
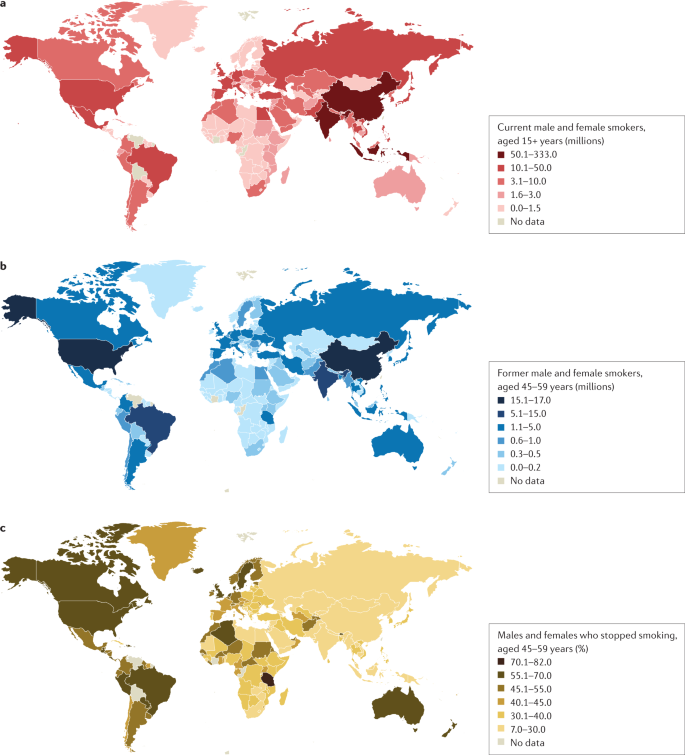
a | Number of current male and female smokers aged 15 years or older per country expressed in millions. b | Former male and female smokers aged 45–59 years per country expressed in millions. c | Former male and female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for the period 2015–2019 from countries with direct smoking surveys. Cessation rates are higher in high-income countries, but also notably high in Brazil. Cessation is far less common in South and East Asia and Russia and other Eastern European countries, and also low in South Africa. Data from ref. 1 .
Age at onset
Most smokers start smoking during adolescence, with almost 90% of smokers beginning between 15 and 25 years of age 24 . The prevalence of tobacco smoking among youths substantially declined in multiple HICs between 1990 and 2019 (ref. 25 ). More recently, the widespread uptake of ENDS in some regions such as Canada and the USA has raised concerns about the long-term effects of prolonged nicotine use among adolescents, including the possible notion that ENDS will increase the use of combustible smoking products 25 , 26 (although some studies have not found much aggregate effect at the population level) 27 .
Smoking that commences in early adolescence or young adulthood and persists throughout life has a more severe effect on health than smoking that starts later in life and/or that is not persistent 16 , 28 , 29 . Over 640 million adults under 30 years of age smoke in 22 jurisdictions alone (including 27 countries in the European Union where central efforts to reduce tobacco dependence might be possible) 30 . In those younger than 30 years of age, at least 320 million smoking-related deaths will occur unless they quit smoking 31 . The actual number of smoking-related deaths might be greater than one in two, and perhaps as high as two in three, long-term smokers 5 , 16 , 29 , 32 , 33 . At least half of these deaths are likely to occur in middle age (30–69 years) 16 , 29 , leading to a loss of two or more decades of life. People who smoke can expect to lose an average of at least a decade of life versus otherwise similar non-smokers 16 , 28 , 29 .
Direct epidemiological studies in several countries paired with model-based estimates have estimated that smoking tobacco accounted for 7.7 million deaths globally in 2020, of which 80% were in men and 87% were current smokers 1 . In HICs, the major causes of tobacco deaths are lung cancer, emphysema, heart attack, stroke, cancer of the upper aerodigestive areas and bladder cancer 28 , 29 . In some lower income countries, tuberculosis is an additional important cause of tobacco-related death 29 , 34 , which could be related to, for example, increased prevalence of infection, more severe tuberculosis/mortality and higher prevalence of treatment-resistant tuberculosis in smokers than in non-smokers in low-income countries 35 , 36 .
Despite substantial reductions in the prevalence of smoking, there were 34 million smokers in the USA, 7 million in the UK and 5 million in Canada in 2017 (ref. 16 ), and cigarette smoking remains the largest cause of premature death before 70 years of age in much of Europe and North America 1 , 16 , 28 , 29 . Smoking-associated diseases accounted for around 41 million deaths in the USA, UK and Canada from 1960 to 2020 (ref. 16 ). Moreover, as smoking-associated diseases are more prevalent among groups with lower levels of education and income, smoking accounts for at least half of the difference in overall mortality between these social groups 37 . Any reduction in smoking prevalence reduces the absolute mortality gap between these groups 38 .
Smoking cessation has become common in HICs with good tobacco control interventions. For example, in France, the number of ex-smokers is four times the number of current smokers among those aged 50 years or more 30 . By contrast, smoking cessation in LMICs remains uncommon before smokers develop tobacco-related diseases 39 . Smoking cessation greatly reduces the risks of smoking-related diseases. Indeed, smokers who quit smoking before 40 years of age avoid nearly all the increased mortality risks 31 , 33 . Moreover, individuals who quit smoking by 50 years of age reduce the risk of death from lung cancer by about two-thirds 40 . More modest hazards persist for deaths from lung cancer and emphysema 16 , 28 ; however, the risks among former smokers are an order of magnitude lower than among those who continue to smoke 33 .
Mechanisms/pathophysiology
Nicotine is the main psychoactive agent in tobacco and e-cigarettes. Nicotine acts as an agonist at nicotinic acetylcholine receptors (nAChRs), which are localized throughout the brain and peripheral nervous system 41 . nAChRs are pentameric ion channels that consist of varying combinations of α 2 –α 7 and β 2 –β 4 subunits, and for which acetylcholine (ACh) is the endogenous ligand 42 , 43 , 44 . When activated by nicotine binding, nAChR undergoes a conformational change that opens the internal pore, allowing an influx of sodium and calcium ions 45 . At postsynaptic membranes, nAChR activation can lead to action potential firing and downstream modulation of gene expression through calcium-mediated second messenger systems 46 . nAChRs are also localized to presynaptic membranes, where they modulate neurotransmitter release 47 . nAChRs become desensitized after activation, during which ligand binding will not open the channel 45 .
nAChRs with varying combinations of α-subunits and β-subunits have differences in nicotine binding affinity, efficacy and desensitization rate, and have differential expression depending on the brain region and cell type 48 , 49 , 50 . For instance, at nicotine concentrations found in human smokers, β 2 -containing nAChRs desensitize relatively quickly after activation, whereas α 7 -containing nAChRs have a slower desensitization profile 48 . Chronic nicotine exposure in experimental animal models or in humans induces an increase in cortical expression of α 4 β 2 -containing nAChRs 51 , 52 , 53 , 54 , 55 , but also increases the expression of β 3 and β 4 nAChR subunits in the medial habenula (MHb)–interpeduncular nucleus (IPN) pathway 56 , 57 . It is clear that both the brain localization and the type of nAChR are critical elements in mediating the various effects of nicotine, but other factors such as rate of nicotine delivery may also modulate addictive effects of nicotine 58 .
Neurocircuitry of nicotine addiction
Nicotine has both rewarding effects (such as a ‘buzz’ or ‘high’) and aversive effects (such as nausea and dizziness), with the net outcome dependent on dose and others factors such as interindividual sensitivity and presence of tolerance 59 . Thus, the addictive properties of nicotine involve integration of contrasting signals from multiple brain regions that process reward and aversion (Fig. 4 ).
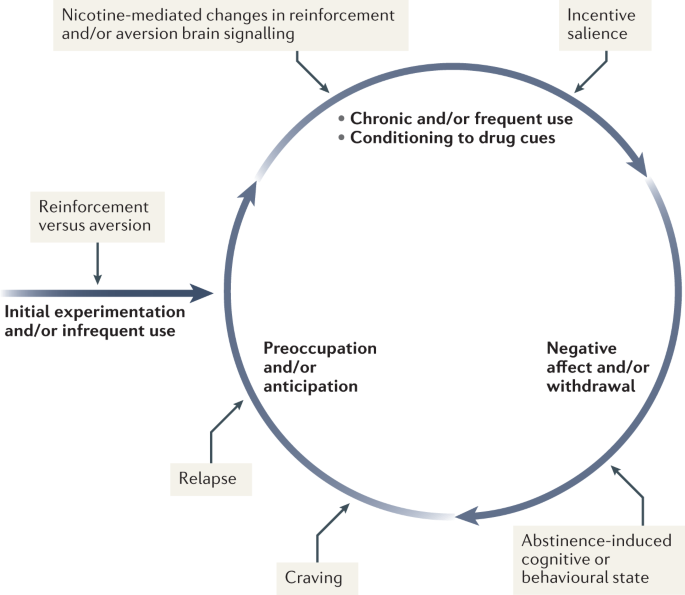
During initial use, nicotine exerts both reinforcing and aversive effects, which together determine the likelihood of continued use. As the individual transitions to more frequent patterns of chronic use, nicotine induces pharmacodynamic changes in brain circuits, which is thought to lead to a reduction in sensitivity to the aversive properties of the drug. Nicotine is also a powerful reinforcer that leads to the conditioning of secondary cues associated with the drug-taking experience (such as cigarette pack, sensory properties of cigarette smoke and feel of the cigarette in the hand or mouth), which serves to enhance the incentive salience of these environmental factors and drive further drug intake. When the individual enters into states of abstinence (such as daily during sleep at night or during quit attempts), withdrawal symptomology is experienced, which may include irritability, restlessness, learning or memory deficits, difficulty concentrating, anxiety and hunger. These negative affective and cognitive symptoms lead to an intensification of the individual’s preoccupation to obtain and use the tobacco/nicotine product, and subsequently such intense craving can lead to relapse.
The rewarding actions of nicotine have largely been attributed to the mesolimbic pathway, which consists of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens and prefrontal cortex 60 , 61 , 62 (Fig. 5 ). VTA integrating circuits and projection regions express several nAChR subtypes on dopaminergic, GABAergic, and glutamatergic neurons 63 , 64 . Ultimately, administration of nicotine increases dopamine levels through increased dopaminergic neuron firing in striatal and extrastriatal areas (such as the ventral pallidum) 65 (Fig. 6 ). This effect is involved in reward and is believed to be primarily mediated by the action of nicotine on α 4 -containing and β 2 -containing nAChRs in the VTA 66 , 67 .
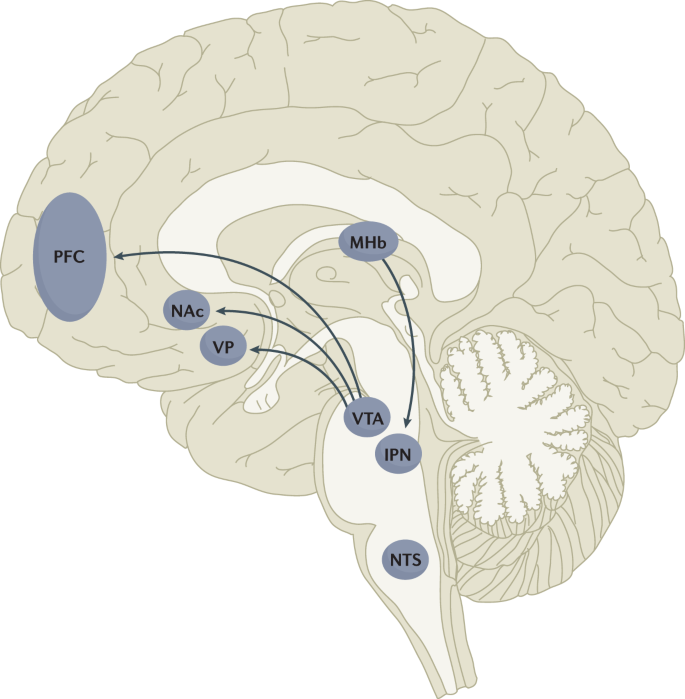
Multiple lines of research have demonstrated that nicotine reinforcement is mainly controlled by two brain pathways, which relay predominantly reward-related or aversion-related signals. The rewarding properties of nicotine that promote drug intake involve the mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). By contrast, the aversive properties of nicotine that limit drug intake and mitigate withdrawal symptoms involve the fasciculus retroflexus projection from the medial habenula (MHb) to the interpeduncular nucleus (IPN). Additional brain regions have also been implicated in various aspects of nicotine dependence, such as the prefrontal cortex (PFC), ventral pallidum (VP), nucleus tractus solitarius (NTS) and insula (not shown here for clarity). All of these brain regions are directly or indirectly interconnected as integrative circuits to drive drug-seeking and drug-taking behaviours.
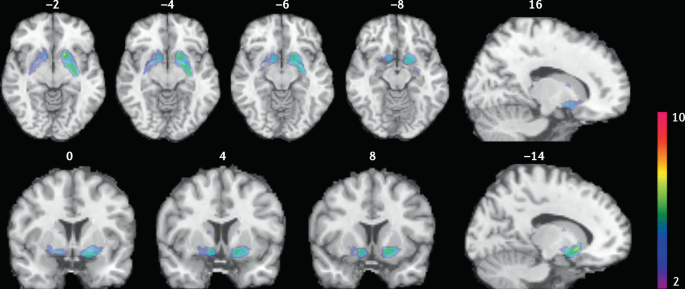
Smokers received brain PET scans with [ 11 C]PHNO, a dopamine D 2/3 PET tracer that has high sensitivity in detecting fluctuations of dopamine. PET scans were performed during abstinence or after smoking a cigarette. Reduced binding potential (BP ND ) was observed after smoking, indicating increased dopamine levels in the ventral striatum and in the area that corresponds to the ventral pallidum. The images show clusters with statistically significant decreases of [ 11 C]PHNO BP ND after smoking a cigarette versus abstinence condition. Those clusters have been superimposed on structural T1 MRI images of the brain. Reprinted from ref. 65 , Springer Nature Limited.
The aversive properties of nicotine are mediated by neurons in the MHb, which project to the IPN. Studies in rodents using genetic knockdown and knockout strategies demonstrated that the α 5 -containing, α 3 -containing and β 4 -containing nAChRs in the MHb–IPN pathway mediate the aversive properties of nicotine that limit drug intake, especially when animals are given the opportunity to consume higher nicotine doses 68 , 69 , 70 , 71 , 72 . In addition to nAChRs, other signalling factors acting on the MHb terminals in the IPN also regulate the actions of nicotine. For instance, under conditions of chronic nicotine exposure or with optogenetic activation of IPN neurons, a subtype of IPN neurons co-expressing Chrna5 (encoding the α 5 nAChR subunit) and Amigo1 (encoding adhesion molecule with immunoglobulin-like domain 1) release nitric oxide from the cell body that retrogradely inhibits MHb axon terminals 70 . In addition, nicotine activates α 5 -containing nAChR-expressing neurons that project from the nucleus tractus solitarius to the IPN, leading to release of glucagon-like peptide-1 that binds to GLP receptors on habenular axon terminals, which subsequently increases IPN neuron activation and decreases nicotine self-administration 73 . Taken together, these findings suggest a dynamic signalling process at MHb axonal terminals in the IPN, which regulates the addictive properties of nicotine and determines the amount of nicotine that is self-administered.
Nicotine withdrawal in animal models can be assessed by examining somatic signs (such as shaking, scratching, head nods and chewing) and affective signs (such as increased anxiety-related behaviours and conditioned place aversion). Interestingly, few nicotine withdrawal somatic signs are found in mice with genetic knockout of the α 2 , α 5 or β 4 nAChR subunits 74 , 75 . By contrast, β 2 nAChR-knockout mice have fewer anxiety-related behaviours during nicotine withdrawal, with no differences in somatic symptoms compared with wild-type mice 74 , 76 .
In addition to the VTA (mediating reward) and the MHb–IPN pathway (mediating aversion), other brain areas are involved in nicotine addiction (Fig. 5 ). In animals, the insular cortex controls nicotine taking and nicotine seeking 77 . Moreover, humans with lesions of the insular cortex can quit smoking easily without relapse 78 . This finding led to the development of a novel therapeutic intervention modulating insula function (see Management, below) 79 , 80 . Various brain areas (shell of nucleus accumbens, basolateral amygdala and prelimbic cortex) expressing cannabinoid CB 1 receptors are also critical in controlling rewarding effects and relapse 81 , 82 . The α 1 -adrenergic receptor expressed in the cortex also control these effects, probably through glutamatergic afferents to the nucleus accumbens 83 .
Individual differences in nicotine addiction risk
Vulnerability to nicotine dependence varies between individuals, and the reasons for these differences are multidimensional. Many social factors (such as education level and income) play a role 84 . Broad psychological and social factors also modulate this risk. For example, peer smoking status, knowledge on effect of tobacco, expectation on social acceptance, exposure to passive smoking modulate the risk of initiating tobacco use 85 , 86 .
Genetic factors have a role in smoking initiation, the development of nicotine addiction and the likelihood of smoking cessation. Indeed, heritability has been estimated to contribute to approximatively half of the variability in nicotine dependence 87 , 88 , 89 , 90 . Important advances in our understanding of such genetic contributions have evolved with large-scale genome-wide association studies of smokers and non-smokers. One of the most striking findings has been that allelic variation in the CHRNA5 – CHRNA3 – CHRNB4 gene cluster, which encodes α 5 , α 3 and β 4 nAChR subunits, correlates with an increased vulnerability for nicotine addiction, indicated by a higher likelihood of becoming dependent on nicotine and smoking a greater number of cigarettes per day 91 , 92 , 93 , 94 , 95 . The most significant effect has been found for a single-nucleotide polymorphism in CHRNA5 (rs16969968), which results in an amino acid change and reduced function of α 5 -containing nAChRs 92 .
Allelic variation in CYP2A6 (encoding the CYP2A6 enzyme, which metabolizes nicotine) has also been associated with differential vulnerability to nicotine dependence 96 , 97 , 98 . CYP2A6 is highly polymorphic, resulting in variable enzymatic activity 96 , 99 , 100 . Individuals with allelic variation that results in slow nicotine metabolism consume less nicotine per day, experience less-severe withdrawal symptoms and are more successful at quitting smoking than individuals with normal or fast metabolism 101 , 102 , 103 , 104 . Moreover, individuals with slow nicotine metabolism have lower dopaminergic receptor expression in the dopamine D2 regions of the associative striatum and sensorimotor striatum in PET studies 105 and take fewer puffs of nicotine-containing cigarettes (compared with de-nicotinized cigarettes) in a forced choice task 106 . Slower nicotine metabolism is thought to increase the duration of action of nicotine, allowing nicotine levels to accumulate over time, therefore enabling lower levels of intake to sustain activation of nAChRs 107 .
Large-scale genetic studies have identified hundreds of other genetic loci that influence smoking initiation, age of smoking initiation, cigarettes smoked per day and successful smoking cessation 108 . The strongest genetic contributions to smoking through the nicotinic receptors and nicotine metabolism are among the strongest genetic contributors to lung cancer 109 . Other genetic variations (such as those related to cannabinoid, dopamine receptors or other neurotransmitters) may affect certain phenotypes related to smoking (such as nicotine preference and cue-reactivity) 110 , 111 , 112 , 113 , 114 , 115 .
Diagnosis, screening and prevention
Screening for cigarette smoking.
Screening for cigarette smoking should happen at every doctor’s visit 116 . In this regard, a simple and direct question about a person’s tobacco use can provide an opportunity to offer information about its potential risks and treatments to assist in quitting. All smokers should be offered assistance in quitting because even low levels of smoking present a significant health risk 33 , 117 , 118 . Smoking status can be assessed by self-categorization or self-reported assessment of smoking behaviour (Table 1 ). In people who smoke, smoking frequency can be assessed 119 and a combined quantity frequency measure such as pack-year history (that is, average number of cigarettes smoked per day multiplied by the number of years, divided by 20), can be used to estimate cumulative risk of adverse health outcomes. The Association for the Treatment of Tobacco Use and Dependence recommends that all electronic health records should document smoking status using the self-report categories listed in Table 1 .
Owing to the advent of e-cigarettes and heat-not-burn products, and the popularity of little cigars in the US that mimic combustible cigarettes, people who use tobacco may use multiple products concurrently 120 , 121 . Thus, screening for other nicotine and tobacco product use is important in clinical practice. The self-categorization approach can also be used to describe the use of these other products.
Traditionally tobacco use has been classified according to whether the smoker meets criteria for nicotine dependence in one of the two main diagnostic classifications: the DSM 122 (tobacco use disorder) and the ICD (tobacco dependence) 123 . The diagnosis of tobacco use disorder according to DSM-5 criteria requires the presence of at least 2 of 11 symptoms that have produced marked clinical impairment or distress within a 12-month period (Box 2 ). Of note, these symptoms are similar for all substance use disorder diagnoses and may not all be relevant to tobacco use disorder (such as failure to complete life roles). In the ICD-10, codes allow the identification of specific tobacco products used (cigarettes, chewing tobacco and other tobacco products).
Dependence can also be assessed as a continuous construct associated with higher levels of use, greater withdrawal and reduced likelihood of quitting. The level of dependence can be assessed with the Fagerström Test for Nicotine Dependence, a short questionnaire comprising six questions 124 (Box 2 ). A score of ≥4 indicates moderate to high dependence. As very limited time may be available in clinical consultations, the Heaviness of Smoking Index (HSI) was developed, which comprises two questions on the number of cigarettes smoked per day and how soon after waking the first cigarette is smoked 125 . The HSI can guide dosing for nicotine replacement therapy (NRT).
Other measures of cigarette dependence have been developed but are not used in the clinical setting, such as the Cigarette Dependence Scale 126 , Hooked on Nicotine Checklist 127 , Nicotine Dependence Syndrome Scale 128 , the Wisconsin Inventory of Smoking Dependence Motives (Brief) 129 and the Penn State Cigarette Dependence Index 130 . However, in practice, these are not often used, as the most important aspect is to screen for smoking and encourage all smokers to quit smoking regardless of their dependence status.
Box 2 DSM-5 criteria for tobacco use disorder and items of the Fagerström Test for nicotine dependence
DSM-5 (ref. 122 )
Taxonomic and diagnostic tool for tobacco use disorder published by the American Psychiatric Association.
A problematic pattern of tobacco use leading to clinically significant impairment or distress as manifested by at least two of the following, occurring within a 12-month period.
Tobacco often used in larger amounts or over a longer period of time than intended
A persistent desire or unsuccessful efforts to reduce or control tobacco use
A great deal of time spent in activities necessary to obtain or use tobacco
Craving, or a strong desire or urge to use tobacco
Recurrent tobacco use resulting in a failure to fulfil major role obligations at work, school or home
Continued tobacco use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of tobacco (for example, arguments with others about tobacco use)
Important social, occupational or recreational activities given up or reduced because of tobacco use
Recurrent tobacco use in hazardous situations (such as smoking in bed)
Tobacco use continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by tobacco use
Tolerance, defined by either of the following.
A need for markedly increased amounts of tobacco to achieve the desired effect
A markedly diminished effect with continued use of the same amount of tobacco
Withdrawal, manifesting as either of the following.
Withdrawal syndrome for tobacco
Tobacco (or a closely related substance, such as nicotine) taken to relieve or avoid withdrawal symptoms
Fagerström Test for Nicotine Dependence 124
A standard instrument for assessing the intensity of physical addiction to nicotine.
How soon after you wake up do you smoke your first cigarette?
Within 5 min (scores 3 points)
5 to 30 min (scores 2 points)
31 to 60 min (scores 1 point)
After 60 min (scores 0 points)
Do you find it difficult not to smoke in places where you should not, such as in church or school, in a movie, at the library, on a bus, in court or in a hospital?
Yes (scores 1 point)
No (scores 0 points)
Which cigarette would you most hate to give up; which cigarette do you treasure the most?
The first one in the morning (scores 1 point)
Any other one (scores 0 points)
How many cigarettes do you smoke each day?
10 or fewer (scores 0 points)
11 to 20 (scores 1 point)
21 to 30 (scores 2 points)
31 or more (scores 3 points)
Do you smoke more during the first few hours after waking up than during the rest of the day?
Do you still smoke if you are so sick that you are in bed most of the day or if you have a cold or the flu and have trouble breathing?
A score of 7–10 points is classified as highly dependent; 4–6 points is classified as moderately dependent; <4 points is classified as minimally dependent.
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Young people who do not start smoking cigarettes between 15 and 25 years of age have a very low risk of ever smoking 24 , 131 , 132 . This age group provides a critical opportunity to prevent cigarette smoking using effective, evidence-based strategies to prevent smoking initiation and reduce escalation from experimentation to regular use 131 , 132 , 133 , 134 , 135 .
Effective prevention of cigarette uptake requires a comprehensive package of cost-effective policies 134 , 136 , 137 to synergistically reduce the population prevalence of cigarette smoking 131 , 135 . These policies include high rates of tobacco taxation 30 , 134 , 137 , 138 , widespread and rigorously enforced smoke-free policies 139 , bans on tobacco advertising and promotions 140 , use of plain packaging and graphic warnings about the health risks of smoking 135 , 141 , mass media and peer-based education programmes to discourage smoking, and enforcement of laws against the sale of cigarettes to young people below the minimum legal purchase age 131 , 135 . These policies make cigarettes less available and affordable to young people. Moreover, these policies make it more difficult for young people to purchase cigarettes and make smoking a much less socially acceptable practice. Of note, these policies are typically mostly enacted in HICs, which may be related to the declining prevalence of smoking in these countries, compared with the prevalence in LMICs.
Pharmacotherapy
Three evidence-based classes of pharmacotherapy are available for smoking cessation: NRT (using nicotine-based patches, gum, lozenges, mini-lozenges, nasal sprays and inhalers), varenicline (a nAChR partial agonist), and bupropion (a noradrenaline/dopamine reuptake inhibitor that also inhibits nAChR function and is also used as an antidepressant). These FDA-approved and EMA-approved pharmacotherapies are cost-effective smoking cessation treatments that double or triple successful abstinence rates compared with no treatment or placebo controls 116 , 142 .
Combinations of pharmacotherapies are also effective for smoking cessation 116 , 142 . For example, combining NRTs (such as the steady-state nicotine patch and as-needed NRT such as gum or mini-lozenge) is more effective than a single form of NRT 116 , 142 , 143 . Combining NRT and varenicline is the most effective smoking cessation pharmacotherapy 116 , 142 , 143 . Combining FDA-approved pharmacotherapy with behavioural counselling further increases the likelihood of successful cessation 142 . Second-line pharmacotherapies (for example, nortriptyline) have some potential for smoking cessation, but their use is limited due to their tolerability profile.
All smokers should receive pharmacotherapy to help them quit smoking, except those in whom pharmacotherapy has insufficient evidence of effectiveness (among adolescents, smokeless tobacco users, pregnant women or light smokers) or those in whom pharmacotherapy is medically contraindicated 144 . Table 2 provides specific information regarding dosing and duration for each FDA-approved pharmacotherapy. Extended use of pharmacotherapy beyond the standard 12-week regimen after cessation is effective and should be considered 116 . Moreover, preloading pharmacotherapy (that is, initiating cessation medication in advance of a quit attempt), especially with the nicotine patch, is a promising treatment, although further studies are required to confirm efficacy.
Cytisine has been used for smoking cessation in Eastern Europe for a long time and is available in some countries (such as Canada) without prescription 145 . Cytisine is a partial agonist of nAChRs and its structure was the precursor for the development of varenicline 145 . Cytisine is at least as effective as some approved pharmacotherapies for smoking cessation, such as NRT 146 , 147 , 148 , and the role of cytisine in smoking cessation is likely to expand in the future, notably owing to its much lower cost than traditional pharmacotherapies. E-cigarettes also have the potential to be useful as smoking cessation devices 149 , 150 . The 2020 US Surgeon General’s Report concluded that there was insufficient evidence to promote cytisine or e-cigarettes as effective smoking cessation treatments, but in the UK its use is recommended for smoking cessation (see ref. 15 for regularly updated review).
Counselling and behavioural treatments
Psychosocial counselling significantly increases the likelihood of successful cessation, especially when combined with pharmacotherapy. Even a counselling session lasting only 3 minutes can help smokers quit 116 , although the 2008 US Public Health Service guidelines and the Preventive Services Task Force 151 each concluded that more intensive counselling (≥20 min per session) is more effective than less intensive counselling (<20 min per session). Higher smoking cessation rates are obtained by using behavioural change techniques that target associative and self-regulatory processes 152 . In addition, behavioural change techniques that will favour commitment, social reward and identity associated with changed behaviour seems associated with higher success rates 152 . Evidence-based counselling focuses on providing social support during treatment, building skills to cope with withdrawal and cessation, and problem-solving in challenging situations 116 , 153 . Effective counselling can be delivered by diverse providers (such as physicians, nurses, pharmacists, social workers, psychologists and certified tobacco treatment specialists) 116 .
Counselling can be delivered in a variety of modalities. In-person individual and group counselling are effective, as is telephone counselling (quit lines) 142 . Internet and text-based intervention seem to be effective in smoking cessation, especially when they are interactive and tailored to a smoker’s specific circumstances 142 . Over the past several years, the number of smoking cessation smartphone apps has increased, but there the evidence that the use of these apps significantly increases smoking cessation rates is not sufficient.
Contingency management (providing financial incentives for abstinence or engagement in treatment) has shown promising results 154 , 155 but its effects are not sustained once the contingencies are removed 155 , 156 . Other treatments such as hypnosis, acupuncture and laser treatment have not been shown to improve smoking cessation rates compared with placebo treatments 116 . Moreover, no solid evidence supports the use of conventional transcranial magnetic stimulation (TMS) for long-term smoking cessation 157 , 158 .
Although a variety of empirically supported smoking cessation interventions are available, more than two-thirds of adult smokers who made quit attempts in the USA during the past year did not use an evidence-based treatment and the rate is likely to be lower in many other countries 142 . This speaks to the need to increase awareness of, and access to, effective cessation aids among all smokers.
Brain stimulation
The insula (part of the frontal cortex) is a critical brain structure involved in cigarette craving and relapse 78 , 79 . The activity of the insula can be modulated using an innovative approach called deep insula/prefrontal cortex TMS (deep TMS), which is effective in helping people quit smoking 80 , 159 . This approach has now been approved by the FDA as an effective smoking cessation intervention 80 . However, although this intervention was developed and is effective for smoking cessation, the number of people with access to it is limited owing to the limited number of sites equipped and with trained personnel, and the cost of this intervention.
Quality of life
Generic instruments (such as the Short-Form (SF-36) Health Survey) can be used to evaluate quality of life (QOL) in smokers. People who smoke rate their QOL lower than people who do not smoke both before and after they become smokers 160 , 161 . QOL improves when smokers quit 162 . Mental health may also improve on quitting smoking 163 . Moreover, QOL is much poorer in smokers with tobacco-related diseases, such as chronic respiratory diseases and cancers, than in individuals without tobacco-related diseases 161 , 164 . The dimensions of QOL that show the largest decrements in people who smoke are those related to physical health, day-to-day activities and mental health such as depression 160 . Smoking also increases the risk of diabetes mellitus 165 , 166 , which is a major determinant of poor QOL for a wide range of conditions.
The high toll of premature death from cigarette smoking can obscure the fact that many of the diseases that cause these deaths also produce substantial disability in the years before death 1 . Indeed, death in smokers is typically preceded by several years of living with the serious disability and impairment of everyday activities caused by chronic respiratory disease, heart disease and cancer 2 . Smokers’ QOL in these years may also be adversely affected by the adverse effects of the medical treatments that they receive for these smoking-related diseases (such as major surgery and radiotherapy).
Expanding cessation worldwide
The major global challenge is to consider individual and population-based strategies that could increase the substantially low rates of adult cessation in most LMICs and indeed strategies to ensure that even in HICs, cessation continues to increase. In general, the most effective tools recommended by WHO to expand cessation are the same tools that can prevent smoking initiation, notably higher tobacco taxes, bans on advertising and promotion, prominent warning labels or plain packaging, bans on public smoking, and mass media and educational efforts 29 , 167 . The effective use of these policies, particularly taxation, lags behind in most LMICs compared with most HICs, with important exceptions such as Brazil 167 . Access to effective pharmacotherapies and counselling as well as support for co-existing mental health conditions would also be required to accelerate cessation in LMICs. This is particularly important as smokers living in LMICs often have no access to the full range of effective treatment options.
Regulating access to e-cigarettes
How e-cigarettes should be used is debated within the tobacco control field. In some countries (for example, the UK), the use of e-cigarettes as a cigarette smoking cessation aid and as a harm reduction strategy is supported, based on the idea that e-cigarette use will lead to much less exposure to toxic compounds than tobacco use, therefore reducing global harm. In other countries (for example, the USA), there is more concern with preventing the increased use of e-cigarettes by youths that may subsequently lead to smoking 25 , 26 . Regulating e-cigarettes in nuanced ways that enable smokers to access those products whilst preventing their uptake among youths is critical.
Regulating nicotine content in tobacco products
Reducing the nicotine content of cigarettes could potentially produce less addictive products that would allow a gradual reduction in the population prevalence of smoking. Some clinical studies have found no compensatory increase in smoking whilst providing access to low nicotine tobacco 168 . Future regulation may be implemented to gradually decrease the nicotine content of combustible tobacco and other nicotine products 169 , 170 , 171 .
Tobacco end games
Some individuals have proposed getting rid of commercial tobacco products this century or using the major economic disruption arising from the COVID-19 pandemic to accelerate the demise of the tobacco industry 172 , 173 . Some tobacco producers have even proposed this strategy as an internal goal, with the idea of switching to nicotine delivery systems that are less harmful ( Philip Morris International ). Some countries are moving towards such an objective; for example, in New Zealand, the goal that fewer than 5% of New Zealanders will be smokers in 2025 has been set (ref. 174 ). The tobacco end-game approach would overall be the best approach to reduce the burden of tobacco use on society, but it would require coordination of multiple countries and strong public and private consensus on the strategy to avoid a major expansion of the existing illicit market in tobacco products in some countries.
Innovative interventions
The COVID-19 pandemic has shown that large-scale investment in research can lead to rapid development of successful therapeutic interventions. By contrast, smoking cessation has been underfunded compared with the contribution that it makes to the global burden of disease. In addition, there is limited coordination between research teams and most studies are small-scale and often underpowered 79 . It is time to fund an ambitious, coordinated programme of research to test the most promising therapies based on an increased understanding of the neurobiological basis of smoking and nicotine addiction (Table 3 ). Many of those ideas have not yet been tested properly and this could be carried out by a coordinated programme of research at the international level.
GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021). This study summarizes the burden of disease induced by tobacco worldwide .
Google Scholar
West, R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32 , 1018–1036 (2017).
PubMed PubMed Central Google Scholar
West, R. The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD 6 , 277–283 (2009).
PubMed Google Scholar
Fagerström, K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob. Res. 14 , 75–78 (2012).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 2 , 739–748 (1950).
CAS PubMed PubMed Central Google Scholar
Royal College of Physicians. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases (Pitman Medical Publishing, 1962).
Henningfield, J. E., Smith, T. T., Kleykamp, B. A., Fant, R. V. & Donny, E. C. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology 233 , 3829–3848 (2016).
Le Foll, B. & Goldberg, S. R. Effects of nicotine in experimental animals and humans: an update on addictive properties. Hand. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_12 (2009).
Article Google Scholar
Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control. 21 , 87–91 (2012).
Hall, B. J. et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol. Biochem. Behav. 120 , 103–108 (2014).
Musso, F. et al. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology 191 , 159–169 (2007).
CAS PubMed Google Scholar
Goriounova, N. A. & Mansvelder, H. D. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2 , a012120 (2012).
Fagerström, K. O. & Bridgman, K. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addictive Behav. 39 , 507–511 (2014).
Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9 , CD010216 (2021).
Jha, P. The hazards of smoking and the benefits of cessation: a critical summation of the epidemiological evidence in high-income countries. eLife https://doi.org/10.7554/eLife.49979 (2020).
Article PubMed PubMed Central Google Scholar
Palipudi, K. M. et al. Social determinants of health and tobacco use in thirteen low and middle income countries: evidence from Global Adult Tobacco Survey. PLoS ONE 7 , e33466 (2012).
Goodwin, R. D., Pagura, J., Spiwak, R., Lemeshow, A. R. & Sareen, J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alcohol. Depend. 118 , 127–133 (2011).
Weinberger, A. H. et al. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002-14: emerging disparities in vulnerable populations. Addiction 113 , 719–728 (2018).
Evans-Polce, R. J., Kcomt, L., Veliz, P. T., Boyd, C. J. & McCabe, S. E. Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates. Am. J. Psychiatry 177 , 1073–1081 (2020).
Hassan, A. N. & Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J. Clin. Psychiatry https://doi.org/10.4088/JCP.20m13637 (2021).
Article PubMed Google Scholar
Smith, P. H., Mazure, C. M. & McKee, S. A. Smoking and mental illness in the U.S. population. Tob. Control. 23 , e147–e153 (2014).
Bourgault, Z., Rubin-Kahana, D. S., Hassan, A. N., Sanches, M. & Le Foll, B. Multiple substance use disorders and self-reported cognitive function in U.S. adults: associations and sex-differences in a nationally representative sample. Front. Psychiatry https://doi.org/10.3389/fpsyt.2021.797578 (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health 6 , e472–e481 (2021).
Warner, K. E. How to think–not feel–about tobacco harm reduction. Nicotine Tob. Res. 21 , 1299–1309 (2019).
Soneji, S. et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171 , 788–797 (2017).
Levy, D. T. et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob. Control. 28 , 629–635 (2019).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Jha, P. & Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 370 , 60–68 (2014). This review covers the impact of tobacco, of quitting smoking and the importance of taxation to impact prevalence of smoking .
Jha, P. & Peto., R. in Tobacco Tax Reform: At the Crossroads of Health and Development . (eds Marquez, P. V. & Moreno-Dodson, B.) 55–72 (World Bank Group, 2017).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368 , 341–350 (2013).
Banks, E. et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 13 , 38 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Jha, P. et al. A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. 358 , 1137–1147 (2008).
Chan, E. D. et al. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis 94 , 544–550 (2014).
Wang, M. G. et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect. Drug Resist. 11 , 873–887 (2018).
Jha, P. et al. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet 368 , 367–370 (2006).
Jha, P., Gelband, H, Irving, H. & Mishra, S. in Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (eds Vaccarella, S et al.) 161–166 (IARC, 2018).
Jha, P. Expanding smoking cessation world-wide. Addiction 113 , 1392–1393 (2018).
Jha, P. Avoidable global cancer deaths and total deaths from smoking. Nat. Rev. Cancer 9 , 655–664 (2009).
Wittenberg, R. E., Wolfman, S. L., De Biasi, M. & Dani, J. A. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177 , 108256 (2020).
Boulter, J. et al. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl Acad. Sci. USA 84 , 7763–7767 (1987).
Couturier, S. et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5 , 847–856 (1990).
Picciotto, M. R., Addy, N. A., Mineur, Y. S. & Brunzell, D. H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 84 , 329–342 (2008).
Changeux, J. P. Structural identification of the nicotinic receptor ion channel. Trends Neurosci. 41 , 67–70 (2018).
McKay, B. E., Placzek, A. N. & Dani, J. A. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 74 , 1120–1133 (2007).
Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20 , 92–98 (1997).
Wooltorton, J. R., Pidoplichko, V. I., Broide, R. S. & Dani, J. A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23 , 3176–3185 (2003).
Gipson, C. D. & Fowler, C. D. Nicotinic receptors underlying nicotine dependence: evidence from transgenic mouse models. Curr. Top. Behav. Neurosci. 45 , 101–121 (2020).
Hamouda, A. K. et al. Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, nicotinic acetylcholine receptors reduces nicotine self-administration and withdrawal symptoms. Neuropharmacology 190 , 108568 (2021).
Lallai, V. et al. Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro https://doi.org/10.1523/ENEURO.0051-19.2019 (2019).
Benwell, M. E., Balfour, D. J. & Anderson, J. M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 50 , 1243–1247 (1988).
Perry, D. C., Davila-Garcia, M. I., Stockmeier, C. A. & Kellar, K. J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 289 , 1545–1552 (1999).
Marks, M. J. et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 12 , 2765–2784 (1992).
Le Foll, B. et al. Impact of short access nicotine self-administration on expression of α4β2* nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 233 , 1829–1835 (2016).
Meyers, E. E., Loetz, E. C. & Marks, M. J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 130 , 1–8 (2015).
Zhao-Shea, R., Liu, L., Pang, X., Gardner, P. D. & Tapper, A. R. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr. Biol. 23 , 2327–2335 (2013).
Jensen, K. P., Valentine, G., Gueorguieva, R. & Sofuoglu, M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 237 , 1359–1369 (2020).
Villanueva, H. F., James, J. R. & Rosecrans, J. A. Evidence of pharmacological tolerance to nicotine. NIDA Res. Monogr. 95 , 349–350 (1989).
Corrigall, W. A., Coen, K. M. & Adamson, K. L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653 , 278–284 (1994).
Nisell, M., Nomikos, G. G., Hertel, P., Panagis, G. & Svensson, T. H. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22 , 369–381 (1996).
Rice, M. E. & Cragg, S. J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7 , 583–584 (2004).
Mameli-Engvall, M. et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50 , 911–921 (2006).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76 , 116–129 (2012).
Le Foll, B. et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology 39 , 415–424 (2014). This brain imaging study identified the brain areas in which smoking elevates dopamine levels .
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436 , 103–107 (2005). This article discusses the implication of the β 2 - containing nAChRs in the VTA in mammalian cognitive function .
Picciotto, M. R. et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 , 173–177 (1998). This article discusses the implication of the β 2 - containing nAChRs in addictive effects of nicotine .
Fowler, C. D., Lu, Q., Johnson, P. M., Marks, M. J. & Kenny, P. J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471 , 597–601 (2011). This article discusses the implication of the α5 nicotinic receptor located in the MHb in a mechanism mediating the aversive effects of nicotine .
Elayouby, K. S. et al. α3* Nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0127-19.2020 (2020).
Ables, J. L. et al. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl Acad. Sci. USA 114 , 13012–13017 (2017).
Frahm, S. et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70 , 522–535 (2011).
Jackson, K. J. et al. Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 334 , 137–146 (2010).
Tuesta, L. M. et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci. 20 , 708–716 (2017).
Salas, R., Pieri, F. & De Biasi, M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J. Neurosci. 24 , 10035–10039 (2004).
Salas, R., Sturm, R., Boulter, J. & De Biasi, M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29 , 3014–3018 (2009).
Jackson, K. J., Martin, B. R., Changeux, J. P. & Damaj, M. I. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325 , 302–312 (2008).
Le Foll, B. et al. Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 52 , 86–93 (2014).
Naqvi, N. H., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315 , 531–534 (2007). This article discusses the implication of the insular cortex in tobacco addiction .
Ibrahim, C. et al. The insula: a brain stimulation target for the treatment of addiction. Front. Pharmacol. 10 , 720 (2019).
Zangen, A. et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20 , 397–404 (2021). This study validated the utility of deep insula/prefrontal cortex rTMS for smoking cessation .
Le Foll, B., Forget, B., Aubin, H. J. & Goldberg, S. R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 13 , 239–252 (2008).
Kodas, E., Cohen, C., Louis, C. & Griebel, G. Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194 , 161–171 (2007).
Forget, B. et al. Noradrenergic α1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35 , 1751–1760 (2010).
Garrett, B. E., Dube, S. R., Babb, S. & McAfee, T. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob. Res. 17 , 892–897 (2015).
Polanska, K., Znyk, M. & Kaleta, D. Susceptibility to tobacco use and associated factors among youth in five central and eastern European countries. BMC Public Health 22 , 72 (2022).
Volkow, N. D. Personalizing the treatment of substance use disorders. Am. J. Psychiatry 177 , 113–116 (2020).
Li, M. D., Cheng, R., Ma, J. Z. & Swan, G. E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98 , 23–31 (2003).
Carmelli, D., Swan, G. E., Robinette, D. & Fabsitz, R. Genetic influence on smoking–a study of male twins. N. Engl. J. Med. 327 , 829–833 (1992).
Broms, U., Silventoinen, K., Madden, P. A. F., Heath, A. C. & Kaprio, J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res. Hum. Genet. 9 , 64–72 (2006).
Kendler, K. S., Thornton, L. M. & Pedersen, N. L. Tobacco consumption in Swedish twins reared apart and reared together. Arch. Gen. Psychiat 57 , 886–892 (2000).
Saccone, N. L. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 69 , 6848–6856 (2009).
Bierut, L. J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165 , 1163–1171 (2008). This study demonstrates that nAChR gene variants are important in nicotine addiction .
Bierut, L. J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16 , 24–35 (2007).
Berrettini, W. et al. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13 , 368–373 (2008).
Sherva, R. et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 103 , 1544–1552 (2008).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42 , 448–453 (2010).
Ray, R., Tyndale, R. F. & Lerman, C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J. Neurogenet. 23 , 252–261 (2009).
Bergen, A. W. et al. Drug metabolizing enzyme and transporter gene variation, nicotine metabolism, prospective abstinence, and cigarette consumption. PLoS ONE 10 , e0126113 (2015).
Mwenifumbo, J. C. et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin. Pharmacol. Ther. 83 , 115–121 (2008).
Raunio, H. & Rahnasto-Rilla, M. CYP2A6: genetics, structure, regulation, and function. Drug Metab. Drug Interact. 27 , 73–88 (2012).
CAS Google Scholar
Patterson, F. et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 84 , 320–325 (2008).
Rodriguez, S. et al. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J. Psychopharmacol. 25 , 915–923 (2011).
Strasser, A. A., Malaiyandi, V., Hoffmann, E., Tyndale, R. F. & Lerman, C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob. Res. 9 , 511–518 (2007).
Liakoni, E. et al. Effects of nicotine metabolic rate on withdrawal symptoms and response to cigarette smoking after abstinence. Clin. Pharmacol. Ther. 105 , 641–651 (2019).
Di Ciano, P. et al. Influence of nicotine metabolism ratio on [11C]-(+)-PHNO PET binding in tobacco smokers. Int. J. Neuropsychopharmacol. 21 , 503–512 (2018).
Butler, K. et al. Impact of Cyp2a6 activity on nicotine reinforcement and cue-reactivity in daily smokers. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntab064 (2021).
Benowitz, N. L., Swan, G. E., Jacob, P. 3rd, Lessov-Schlaggar, C. N. & Tyndale, R. F. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80 , 457–467 (2006).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51 , 237–244 (2019).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49 , 1126–1132 (2017).
Chukwueke, C. C. et al. The CB1R rs2023239 receptor gene variant significantly affects the reinforcing effects of nicotine, but not cue reactivity, in human smokers. Brain Behav. 11 , e01982 (2021).
Ahrens, S. et al. Modulation of nicotine effects on selective attention by DRD2 and CHRNA4 gene polymorphisms. Psychopharmacology 232 , 2323–2331 (2015).
Harrell, P. T. et al. Dopaminergic genetic variation moderates the effect of nicotine on cigarette reward. Psychopharmacology 233 , 351–360 (2016).
Lerman, C. et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 31 , 231–242 (2006).
Le Foll, B., Gallo, A., Le Strat, Y., Lu, L. & Gorwood, P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 20 , 1–17 (2009).
Chukwueke, C. C. et al. Exploring the role of the Ser9Gly (rs6280) dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci. Rep. 10 , 4085 (2020).
The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am. J. Prev. Med. 35 , 158–176 (2008).
Hackshaw, A., Morris, J. K., Boniface, S., Tang, J. L. & Milenković, D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 360 , j5855 (2018).
Qin, W. et al. Light cigarette smoking increases risk of all-cause and cause-specific mortality: findings from the NHIS cohort study. Int. J. Env. Res. Public Health https://doi.org/10.3390/ijerph17145122 (2020).
Rodu, B. & Plurphanswat, N. Mortality among male smokers and smokeless tobacco users in the USA. Harm Reduct. J. 16 , 50 (2019).
Kasza, K. A. et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med. 376 , 342–353 (2017).
Richardson, A., Xiao, H. & Vallone, D. M. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob. Res. 14 , 927–932 (2012).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders 5th edn (American Psychiatric Association, 2013).
World Health Organization. Tobacco fact sheet. WHO https://www.who.int/news-room/fact-sheets/detail/tobacco (2021).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K. O. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86 , 1119–1127 (1991).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., Rickert, W. & Robinson, J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 84 , 791–799 (1989).
Etter, J. F., Le Houezec, J. & Perneger, T. V. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28 , 359–370 (2003).
DiFranza, J. R. et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 156 , 397–403 (2002).
Shiffman, S., Waters, A. & Hickcox, M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 6 , 327–348 (2004).
Smith, S. S. et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob. Res. 12 , 489–499 (2010).
Foulds, J. et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob. Res. 17 , 186–192 (2015).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing tobacco use among youth and young adults: a report of the Surgeon General (Centers for Disease Control and Prevention, 2012).
World Health Organization. Tobacco control to improve child health and development. Thematic brief (WHO, 2021).
Lantz, P. M. et al. Investing in youth tobacco control: a review of smoking prevention and control strategies. Tob. Control. 9 , 47–63 (2000).
Leão, T., Kunst, A. E. & Perelman, J. Cost-effectiveness of tobacco control policies and programmes targeting adolescents: a systematic review. Eur. J. Public Health 28 , 39–43 (2018).
Royal College of Physicians. Smoking and health 2021: a coming of age for tobacco control? (RCP, 2021).
Higashi, H. et al. Cost effectiveness of tobacco control policies in Vietnam: the case of population-level interventions. Appl. Health Econ. Health Policy 9 , 183–196 (2011).
Ranson, M. K., Jha, P., Chaloupka, F. J. & Nguyen, S. N. Global and regional estimates of the effectiveness and cost-effectiveness of price increases and other tobacco control policies. Nicotine Tob. Res. 4 , 311–319 (2002).
International Agency for Research on Cancer. I ARC Handbooks of Cancer Prevention: Tobacco control Vol. 14 (IARC, 2011).
Frazer, K. et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2 , CD005992 (2016).
Hoffman, S. J. & Tan, C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health 15 , 744 (2015).
McNeill, A. et al. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst. Rev. 4 , CD011244 (2017).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General (Department of Health and Human Services, 2020).
Lindson, N. et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 4 , CD013308 (2019).
Krist, A. H. et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 325 , 265–279 (2021).
Tutka, P. & Zatonski, W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol. Rep. 58 , 777–798 (2006).
Courtney, R. J. et al. Effect of cytisine vs varenicline on smoking cessation: a randomized clinical trial. JAMA 326 , 56–64 (2021).
Walker, N. et al. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 371 , 2353–2362 (2014). This study validated the utility of cytisine for smoking cessation .
West, R. et al. Placebo-controlled trial of cytisine for smoking cessation. N. Engl. J. Med. 365 , 1193–1200 (2011).
Hajek, P. et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol. Assess. 23 , 1–82 (2019).
Walker, N. et al. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir. Med. 8 , 54–64 (2020).
Siu, A. L., U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 163 , 622–634 (2015).
Black, N. et al. Behaviour change techniques associated with smoking cessation in intervention and comparator groups of randomized controlled trials: a systematic review and meta-regression. Addiction 115 , 2008–2020 (2020).
Center for Substance Abuse and Treatment. Detoxification and Substance Abuse Treatment (Center for Substance Abuse and Treatment, 2006).
Cahill, K., Hartmann-Boyce, J. & Perera, R. Incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub5 (2015).
Secades-Villa, R., Aonso-Diego, G., García-Pérez, Á. & González-Roz, A. Effectiveness of contingency management for smoking cessation in substance users: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 88 , 951–964 (2020).
Cahill, K. & Perera, R. Competitions and incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub4 (2011).
Trojak, B. et al. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimul. 8 , 1168–1174 (2015).
Wing, V. C. et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 6 , 221–230 (2013).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 , 742–749 (2014).
Goldenberg, M., Danovitch, I. & IsHak, W. W. Quality of life and smoking. Am. J. Addict. 23 , 540–562 (2014).
Heikkinen, H., Jallinoja, P., Saarni, S. I. & Patja, K. The impact of smoking on health-related and overall quality of life: a general population survey in Finland. Nicotine Tob. Res. 10 , 1199–1207 (2008).
Moayeri, F., Hsueh, Y. A., Dunt, D. & Clarke, P. Smoking cessation and quality of life: insights from analysis of longitudinal Australian data, an application for economic evaluations. Value Health 24 , 724–732 (2021).
Taylor, G. M. et al. Smoking cessation for improving mental health. Cochrane Database Syst. Rev. 3 , CD013522 (2021).
López-Nicolás, Á., Trapero-Bertran, M. & Muñoz, C. Smoking, health-related quality of life and economic evaluation. Eur. J. Health Econ. 19 , 747–756 (2018).
Morris, A. Linking nicotine addiction and T2DM. Nat. Rev. Endocrinol. 16 , 6 (2020).
Willi, C., Bodenmann, P., Ghali, W. A., Faris, P. D. & Cornuz, J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 298 , 2654–2664 (2007).
World Health Organization. WHO report on the global tobacco epidemic (WHO, 2019).
Donny, E. C. et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373 , 1340–1349 (2015). This study tested the impact of reducing the quantity of nicotine present in cigarettes on smoking .
Benowitz, N. L. & Henningfield, J. E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 331 , 123–125 (1994).
Benowitz, N. L. & Henningfield, J. E. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 22 , i14–i17 (2013).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377 , 1111–1114 (2017).
Hall, W. & West, R. Thinking about the unthinkable: a de facto prohibition on smoked tobacco products. Addiction 103 , 873–874 (2008).
Ioannidis, J. P. A. & Jha, P. Does the COVID-19 pandemic provide an opportunity to eliminate the tobacco industry? Lancet Glob. Health 9 , e12–e13 (2021).
Smokefree. Smokefree 2025. Smokefree https://www.smokefree.org.nz/smokefree-in-action/smokefree-aotearoa-2025 (2021).
Morgan, C. J., Das, R. K., Joye, A., Curran, H. V. & Kamboj, S. K. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38 , 2433–2436 (2013).
Elsaid, S., Kloiber, S. & Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 167 , 25–75 (2019).
Butler, K. & Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: endocannabinoid modulation. Expert Opin. Drug Discov. 15 , 1065–1080 (2020).
D’Souza, D. C. et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6 , 35–45 (2019).
Robinson, J. D. et al. Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict. Biol. 23 , 291–303 (2018).
Gueye, A. B. et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyw068 (2016).
Yammine, L. et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: a pilot randomized controlled trial. Nicotine Tob. Res. 23 , 1682–1690 (2021).
Eren-Yazicioglu, C. Y., Yigit, A., Dogruoz, R. E. & Yapici-Eser, H. Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 14 , 614884 (2020).
Vanderkam, P. et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta-analysis. Addiction 116 , 1011–1020 (2021).
Sokoloff, P. & Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 45 , 2–19 (2017).
David, S. P., Lancaster, T., Stead, L. F., Evins, A. E. & Prochaska, J. J. Opioid antagonists for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003086.pub3 (2013).
Ray, L. A. et al. Efficacy of combining varenicline and naltrexone for smoking cessation and drinking reduction: a randomized clinical trial. Am. J. Psychiatry 178 , 818–828 (2021).
Mooney, M. E. et al. Bupropion and naltrexone for smoking cessation: a double-blind randomized placebo-controlled clinical trial. Clin. Pharmacol. Ther. 100 , 344–352 (2016).
Justinova, Z., Le Foll, B., Redhi, G. H., Markou, A. & Goldberg, S. R. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology 233 , 1791–1800 (2016).
Le Foll, B., Wertheim, C. E. & Goldberg, S. R. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci. Lett. 443 , 236–240 (2008).
Franklin, T. R. et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol. Depend. 103 , 30–36 (2009).
Lotfy, N., Elsawah, H. & Hassan, M. Topiramate for smoking cessation: systematic review and meta-analysis. Tob. Prev. Cessat. 6 , 14 (2020).
Shanahan, W. R., Rose, J. E., Glicklich, A., Stubbe, S. & Sanchez-Kam, M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob. Res. 19 , 944–951 (2017).
Higgins, G. A., Fletcher, P. J. & Shanahan, W. R. Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205 , 107417 (2020).
Stead, L. F. & Lancaster, T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005231.pub2 (2007).
Download references
Acknowledgements
B.Le F. is supported by a clinician-scientist award from the Department of Family and Community Medicine at the University of Toronto and the Addiction Psychiatry Chair from the University of Toronto. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The authors thank H. Fu (University of Toronto) for assistance with Figs 1–3.
Author information
Authors and affiliations.
Translational Addiction Research Laboratory, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
Bernard Le Foll
Departments of Family and Community Medicine, Psychiatry, Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada
Department of Medicine, University of Wisconsin, Madison, WI, USA
Megan E. Piper
University of Wisconsin Center for Tobacco Research and Intervention, Madison, WI, USA
Department of Neurobiology and Behaviour, University of California Irvine, Irvine, CA, USA
Christie D. Fowler
Section for Preventive Cardiology, Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway
Serena Tonstad
Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA
Laura Bierut
Institute of Mental Health, Peking University Sixth Hospital, Peking University, Beijing, China
National Institute on Drug Dependence, Peking University Health Science Center, Beijing, China
Centre for Global Health Research, Unity Health Toronto, University of Toronto, Toronto, Ontario, Canada
- Prabhat Jha
National Centre for Youth Substance Use Research, The University of Queensland, St Lucia, Queensland, Australia
Wayne D. Hall
Queensland Alliance for Environmental Health Sciences, The University of Queensland, Woolloongabba, Queensland, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (B.Le F.); Epidemiology (P.J. and W.D.H.); Mechanisms/pathophysiology (C.D.F., L.B., L.L. and B.Le F.); Diagnosis, screening and prevention (P.J., M.E.P., S.T. and B.Le F.); Management (M.E.P., S.T., W.D.H., L.L. and B.Le F.); Quality of life (P.J. and W.D.H.); Outlook (all); Conclusions (all). All authors contributed substantially to the review and editing of the manuscript.
Corresponding author
Correspondence to Bernard Le Foll .
Ethics declarations
Competing interests.
B.Le F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. B.Le F. has received some in-kind donations of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.Le F. has obtained industry funding from Canopy (through research grants handled by CAMH or the University of Toronto), Bioprojet, ACS, Indivior and Alkermes. B.Le F. has received in-kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. B.Le F. has been an advisor to Shinoghi. S.T. has received honoraria from Pfizer the manufacturer of varenicline for lectures and advice. All other authors declare no competing interests.
Peer review
Peer reviewer information.
Nature Reviews Disease Primers thanks Jamie Brown, Elisardo Iglesias and Ming Li for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Related links
CTRI website: https://d3futrf33lk36a.cloudfront.net/wp-content/uploads/sites/240/2019/04/Meds-Chart.pdf
Philip Morris International: https://www.pmi.com/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Le Foll, B., Piper, M.E., Fowler, C.D. et al. Tobacco and nicotine use. Nat Rev Dis Primers 8 , 19 (2022). https://doi.org/10.1038/s41572-022-00346-w
Download citation
Accepted : 07 February 2022
Published : 24 March 2022
DOI : https://doi.org/10.1038/s41572-022-00346-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrated mrna- and mirna-sequencing analyses unveil the underlying mechanism of tobacco pollutant-induced developmental toxicity in zebrafish embryos.
- Jiasheng Chen
- Xiaoping Zhong
Journal of Translational Medicine (2024)
Influence of substance use on male reproductive health and offspring outcomes
- Jamie O. Lo
- Jason C. Hedges
- Charles A. Easley
Nature Reviews Urology (2024)
Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries
- Guillermo Paraje
- Mauricio Flores Muñoz
Nature Medicine (2024)
Impact of benzoic acid and 2,2’-methylenebis (6-tert-butyl-4-methylphenol) on the metabolome of flue-cured tobacco and rhizosphere microbial communities: implications for continuous cropping obstacles
- Qiulian Peng
- Jiayan Zhang
Plant and Soil (2024)
Nicotinic regulation of microglia: potential contributions to addiction
- Alexa R. Soares
- Marina R. Picciotto
Journal of Neural Transmission (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 09 November 2023
Environmental tobacco smoke and children’s health: a bibliometric and altmetric analysis of 100 most cited articles
- Sneha S. Patil 1 , 2 ,
- Naveen Puttaswamy 1 ,
- Sachin C. Sarode 3 ,
- Gargi S. Sarode 3 ,
- Smita S. Patil 4 ,
- Andres Cardenas 5 ,
- Rajesh Kumar Gandhirajan 6 &
- Kalpana Balakrishnan 1
BMC Public Health volume 23 , Article number: 2208 ( 2023 ) Cite this article
1114 Accesses
1 Altmetric
Metrics details
Exposure to environmental tobacco smoke (ETS) is arguably the most ubiquitous and hazardous, even at very low levels, starting in early life. The objective of this study was to describe the state of research and future trends on ETS exposure and Children’s Health (CH) topics with bibliometrics and altmetrics.
An electronic search was performed in Scopus database on January 31, 2023. Consensus was arrived on 100 most-cited articles by two reviewers. These papers were then cross matched with citations harvested from Web of Science (WoS) and Google Scholar. Altmetric Attention Score (AAS) and Dimension counts were also collected. Analysis and network visualization of authors, countries, and keywords were generated using VOSviewer software.
Among a total of 1107 articles published on ETS and CH, the 100 top-cited articles appeared in 54 journals, with Pediatrics (n = 12) contributing a maximum number of articles. The time period between 2000 and 2009 accounted for 44% of all publications. With respect to the research design employed across these studies, cross-sectional design took precedence over others accounting for approximately 40%. Predominantly, articles focused on childhood asthma; however, current research trends have shifted towards emerging fields such as children’s oral health and DNA methylation. Twitter, policy documents, and news outlets were the main platforms where outputs were discussed. The AAS was not associated with journal impact factor or access type. Weak correlations were observed between AAS and citation count in Scopus, WoS, and Google Scholar (r = 0.17 to 0.27) while a positive association existed between dimension count and the number of citations across all three databases (r = 0.84 to 0.98).
This study demonstrates the evolution, digital dissemination and research hotspots in the field of ETS and CH, predicting the possible future research directions. High-quality studies with more specific exposure classification are warranted to better understand the relationship between ETS and CH.
Peer Review reports
Exposure to environmental tobacco smoke (ETS) or second-hand smoke (SHS) significantly contributes to children’s morbidity and mortality. In 2004, it is estimated that ETS exposure led to approximately 603,000 premature deaths globally, with children accounting for 28% of these fatalities [ 1 ]. The literature is replete with compelling causality evidence between early life exposure (i.e., pregnancy to eight years) to ETS and numerous health outcomes in children. Parental smoking during pregnancy and exposure to ETS has been linked to impaired fetal growth, sudden infant death syndrome, preterm birth, low-birth weight, otitis media, respiratory illness, cardiovascular problems, neurodevelopmental effects, cancer and socio-behavioral inequities in adolescence and adult life [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ].
Due to the growing burden of this condition, a formidable number of articles have been published. Subsequently, with massive literature it is an arduous task for researchers to narrow their search for a feasible number of high-quality papers. Citation-based indicators have traditionally been employed to assess this impact. Bibliometrics have been carried out on key scientific topics in various fields since 1987. Despite its widespread use, in recent years certain impetus in the foundation has emerged, challenging its position as the leading indicator of research impact. Use of alternative metrics has invoked several studies to address the realm of other indicators [ 13 ]. Altmetrics has recently emanated as a web-based screening tool that evaluates the individual influence of an article through online attention. They can include (but are not limited to) citations on Wikipedia and in public policy documents, patents, discussions on research blog, multi-media sites (YouTube), online reference managers like Mendeley, CiteULike, and social networks (Facebook, Twitter) [ 14 ]. Altmetric computes an Altmetric Attention Score (AAS) formulated on its mention in these platforms, which are ascribed specific weights and amalgamated into a single index [ 15 ].
To date, there has been no bibliometric analysis on ETS and children’s health (CH) despite its growing public health concern, exploding publication records, and mounting scientific evidence. In addition, no comprehensive study has assessed the relationship between traditional and alternative metrics on this topic. Thus, this study aimed to analyze the 100 most cited articles using bibliometric and altmetric methods to provide an overview of the current research on ETS exposure and its impact on children’s health.
A comprehensive search of the Scopus database was performed on January 31, 2023. The search terms used were “environmental tobacco smoke,” “secondhand tobacco smoke,” “passive smoking,” “involuntary smoking” and “child health”. A total of 1,107 articles were retrieved, without restrictions on publication date or language. The first 300 items were exported in a CSV (comma-separated values) file format. The titles and abstracts of studies identified from the search were scanned independently by two authors and if necessary, the full-text articles were analyzed. Every paper was screened in consonance with the inclusion criteria: (a) articles focused on any aspect of ETS and CH (b) original research, case series/reports, and reviews. Papers not related to ETS and CH were excluded. A total of 189 articles were scrutinized and 89 were discarded in accordance with the selection criteria. The 100 included articles were then ranked according to the decreasing number of their citations. When articles had equal citation counts, the paper published recently was graded higher.
Evaluation with other data sources
The selected articles were cross-examined with the citation data from Web of Science (WoS) Core Collection and Google Scholar to compare the number of citations. The Altmetric bookmarklet was added to the Google Chrome browser toolbar. The article under consideration was accessed on PubMed and evaluated using the ‘Altmetric It’ tool, with scores retrieved from the resulting doughnut popup. Further details regarding Altmetric scores were obtained by clicking on a ‘click for more details’ button. Dimensions citation count was also captured through the hyperlink.
Data extraction
Two review authors assessed the selected articles and extracted the citation attributes (title, authors, country, authors affiliations, funding sources, year of publication, citations, the title of the scientific journal, impact factor, quartile scores). Furthermore, the study design, the topic addressed, and article access (i.e., subscription for access vs. free access) was discerned.
Bibliometric network
VOSviewer (version 1.6.19, Leiden University, Netherlands) was used to create co-authorship, countries, and keyword co-occurrence networks. For the co-occurrence analysis of ‘all keywords,’ items that appeared in singular and plural form, like ‘risk factor’ and ‘risk factors’ and differed by a hyphen, such as ‘preschool’ and ‘pre-school’ were selected from the CSV file and combined. Irrelevant keywords were excluded from the analysis.
Data analysis
The Journal Impact Factor (JIF) in 2022 was accessed in the WoS’s Incites Journal Citation Reports. Articles were also tabulated using the recent edition of the International Classification of Diseases (ICD-11). Descriptive statistics were used to describe the primary data set. Pearson correlation coefficient (r) was used to evaluate the relationship between citation counts for individual papers, AAS, and Dimensions count. A p-value </=0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences version 29.0.0 (IBM Corporation, Armonk, NY, USA).
Citation counts
Additional file 1 shows the ranking of the 100 most-cited publications. The top-cited articles accrued a total of 10,463 (Scopus), 6305 (WoS), and 17,149 (Google Scholar) citations. DiFranza et al.’s article “Prenatal and Postnatal Environmental Tobacco Smoke Exposure and Children’s Health” published in the Pediatrics journal (2004) was the most cited paper, with 641 (Scopus), 549 (Web of Science), and 984 (Google Scholar) citations and a mean citation density of 90.4. The article “Prenatal Tobacco Smoke Exposure affects global and Gene-specific DNA methylation” ranked second, with 484 (Scopus), 435 (Web of Science), and 675 (Google Scholar) citations, and a mean citation density of 86.93. Thirty-seven articles were not detected by WoS.
Publication characteristics
Journal characteristics and year of publication.
The articles recognized in the search came from 54 journals, amid them, 42 were positioned in the first quartile, nine and three in the second and third quartile respectively (Additional file 2). Twenty journals were edited in the United Kingdom and 15 in the United States. A maximum number of publications were contributed by the Pediatrics journal (n = 12), followed by the American Journal of Epidemiology and the International Journal of Epidemiology (n = seven each). Thirty-nine journals provided only a single paper. The JIF ranged from 0 to 93.333 (mean 8.48 ± 13.18). Free full-text was accessible (Open Access) for 22 articles, while 78 papers required a subscription. The distribution of citation counts significantly differed between freely available studies (median − 99.5, SD 50.38, total number of citations − 2350) and restricted access papers (median − 71.5, SD 97.64, total number of citations − 8113). The years 2009 and 2013 had the highest number of articles, eight and seven respectively. Our findings revealed that the number of papers published on ETS and CH reached its peak between 2000 and 2009 (44%). Twenty-three articles were published between 1985 and 1999, while 33 papers between 2011 and 2019. There was a positive association between the mean citation density and age of publication, however, it was weak (r = 0.08; p = 0.04) as shown in Fig. 1 .
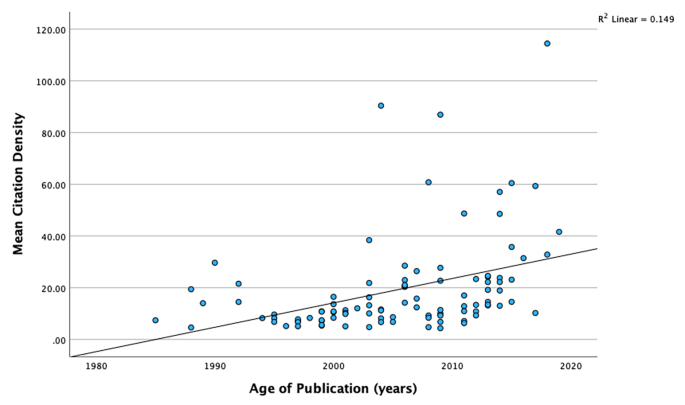
Association between age of publication and mean citation density
Authors, country, institution, and funding source distribution
One hundred and sixty researchers contributed to the top-cited articles. The articles were published by Frank D. Gilliland (six articles; 1029 citations), Martin Weitzman (five articles; 1413 citations), Kiros Berhane, Jouni J.K. Jaakkola, and Lam Tai Hing (four articles each; 497, 322, 272 citations respectively). Sixty-six publications were authored by one to six researchers, whereas 34 studies had seven to forty authors. A co-authorship relation was also developed (Fig. 2 ). Fifteen of the 51 most productive authors were integrated into the confederation network. This was led by Frank D. Gilliland and Kiros Berhane involving five authors each. The influential articles emerged from 38 diverse nations. The United States contributed to majority of the publications (46 articles; 5420 citations), followed by the United Kingdom (17 articles; 1537 citations), Sweden (9 articles; 682 citations), Italy (8 articles; 1064 citations), and China (7 articles; 493 citations). Figure 3 displays the collaboration network of countries that were drawn to meet the threshold of a minimum of three publications. The United States, the United Kingdom, and Sweden had a substantial number of international collaborations. The countries were categorized into clusters, with each cluster depicted by color. The node size is an indicator of the number of papers published by each nation. The publications co-authored are represented by the joining lines, with thicker lines signifying a stronger link between the two countries. The University of California, Berkeley allied the most papers (n = 8), followed by seven articles from the Harvard TH Chan School of Public Health, and six articles from Keck School of Medicine, University of Southern California. The National Institute of Environmental Health Sciences was the topmost organization to fund 15 studies, whereas the National Heart, Lung, and Blood Institute and the National Cancer Institute sponsored six and five studies respectively.
Coauthor contribution in the top-cited papers
Visualisation network of international collaboration for ETS and CH publications
Research design, health outcome addressed and keyword co-occurrence network analysis
Cross-sectional studies were observed to be the most prevalent study design accounting for 40% (3623 citations). Narrative reviews, cohort studies, systematic reviews, and case-control studies constituted about 32% (3945 citations),15% (1333 citations), 5% (602 citations), and 5% (806 citations) respectively (Fig. 4 a). The assessment of health outcomes addressed based on the International Classification of Diseases (ICD-11) disclosed that respiratory diseases (n = 159) were the most frequently cited. This was followed by conditions originating in the perinatal period (n = 59), neurobehavioural disorders (n = 32), endocrine and metabolic diseases (n = 11), childhood cancer, and cardiovascular effects (n = 10 each) (Fig. 4 b). Figure 4 c demonstrates the disease distribution by study design. A total of 1442 keywords that formed five clusters were detected in the present analysis. Figure 5 demonstrates the keyword co-occurrence relation. The most prominent node was “environmental exposure” which emerged 61 times. This was followed by “passive smoking” (60), “child health” (59), and “tobacco smoke pollution” (58). Surprisingly, the keyword “environmental tobacco smoke” and “secondhand smoke” appeared only a mere 13 and three times respectively. Cluster 1 in red mainly included “child health,” “environmental exposure,” “maternal exposure,” “biomarkers,” “birth weight,” “allergy,” “pneumonia,” and “DNA damage.” It reflected the researchers’ focus on how ETS exposure affects children’s health. Cluster 2 in green constituted “air pollution,” “atmospheric pollution,” “ambient air,” “smoke,” “home environment,” and “questionnaire.” It investigated the components and discussed the conditions in which ETS may be harmful to public health. Cluster 3 in blue primarily covered “adverse outcome,” “low birth weight,” “cognitive defect,” “childhood obesity,” “childhood cancer,” “respiratory tract infections,” and “middle ear disease.” Cluster 3 indicated they were interested in a range of child health problems, a sub-theme of Custer 1. Cluster 4 in yellow contained “maternal smoking,” “maternal age,” “educational status,” “pregnancy,” “hypertension,” and “father.” This emphasized the role of parental influence on ETS. While cluster 5 which discussed the potential mechanism of ETS comprised “pathophysiology,” “risk assessment,” and “respiratory function test.” From 2010 to 2019, “nicotiana tabacum,” “rhinitis,” “lower respiratory tract infections,” “birth weight,” “DNA,” “child behavior disorders,” and “fetal development” have started to draw attention.
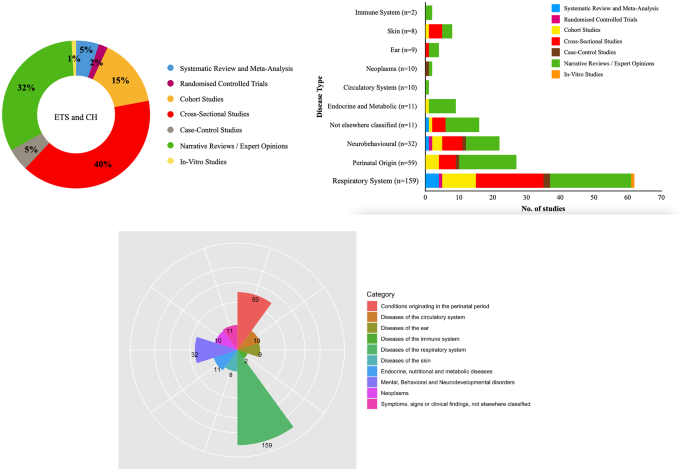
Distribution of ( a ) articles by study design ( b ) disease type by study design ( c ) disease type based on ICD-11
Keyword co-occurrence map of the most-cited articles
Altmetric indicators
The total AAS for the papers was 937 (median = 3) with individual values ranging from 0 to 149. Thirty-two percent of the top-cited articles had no AAS. The outputs were mostly discussed on Twitter (median = 2; range from 0 to 25), policy documents (median = 1; range from 0 to 6), and news outlets (median = 2; range from 0 to 19). Uploaded videos and patents were much less significant. Strzelak’s ‘Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma, and other lung diseases: A mechanistic review’ was the most popular online article (AAS = 149). The breakdown of the AAS revealed this research was cited in 19 new outlets, seven tweets, one blog post, one Facebook page, one Wikipedia page, one video uploader and referenced 431 times in Mendeley (Additional file 3). The AAS was not significantly associated with the JIF (r = -0.01, p > 0.05). The AAS was also not significantly correlated between articles published in Q1 journals compared to those published in Q2 and Q3 journals. Similarly, no significant difference was noted in AAS between articles with unrestricted access and those that require a subscription (p > 0.05). There was a weak correlation between the AAS and citation counts in Scopus (r = 0.17, p = 0.16), WoS (r = 0.27, p = 0.02), and Google Scholar (r = 0.17, p = 0.16). Conversely, a positive association was found between the dimensions citation count and the number of citations in Scopus (r = 0.98, p < 0.000), WoS (r = 0.84, p < 0.000), Google Scholar (r = 0.94, p < 0.000). It should be emphasized that a Pearson’s correlation analysis was carried out on only those papers with a greater than one Altmetric score and Dimension count (Additional file 4).
Analysis of the top 100 cited articles on exposure to ETS and its impact on children’s health provides a varied yet persuasive read. This study links conventional indicators of bibliometrics with the modern digital dissemination measures for studies relating to ETS and CH. Currently, they appear to have discrete but reciprocal parts in assessing the broadcasting and influence of these publications.
One of the most striking features of the list is papers that appeared in journals with a low IF garnered substantial citations, whereas articles emerging in high-IF journals received limited references. The Pediatrics journal had the maximum number of articles (n = 12, JIF 9.703), whilst the British Medical Journal with maximal IF 93.333, presented only four studies. This suggests that citations are more dependent on the content and scientific ‘popularity’ of the research topic among researchers than the JIF. This study observed 33 articles with 100 or greater citation counts, thus making them citation classics [ 16 ]. They were cited between 100 and 641 times when the evaluation was employed with Scopus. A comparison across multiple data sources revealed variations in citation numbers; citations varied between ranges of 41–641 (Scopus), 35–549 (WoS), and 38–984 (Google Scholar). This difference underscores the purport of selecting a relevant scientometric database. Scopus provides a wide breadth of journals (n = 12,850) than WoS (n = 8,700) and quicker citation analysis. WoS and Google Scholar were not used as benchmark data sources for numerous grounds. In WoS, missing and incorrect references are major issues. Google Scholar includes citation data from books, preprints, theses, and dissertations which may influence the evaluation of the top publications [ 17 ]. Interestingly, two highly cited papers by Weitzman M on “Maternal Smoking and Childhood Asthma” and “Maternal Smoking and Behavior Problems of Children” were only found in Scopus and Google Scholar but not in WoS. It is worth noting that while citation counts do not delineate the study quality, it imitates its acclaim within the research community and impact on shaping discussions, controversies, practice guidelines or further investigations [ 18 ].
Although older literature is likely to be more frequently referenced, we observed a significant inclination towards recently published articles, with 33 papers that were released within the last decade. This can be attributed in part to the increasingly prominent role of digital platforms in evidence-based medicine, enabling manuscripts to explore novel concepts and guide future research trajectories. Interestingly, over the years the number of co-authors has risen substantially, with a preponderance of publications having more than three authors. A possible explanation could be increased awareness and interest among researchers of numerous institutes and countries about the potential benefits that studies in the purview of ETS could provide in children’s health. The average number of researchers per publication was 6.19. Frank D. Gilliland, a leading investigator in air pollution research, respiratory health, and gene-environment interactions, was on top of the list with six articles and a mean citation density of 29.31. In this analysis, it was observed that authors tend to collaborate quite frequently with authors affiliated with the same university or country. Frank D. Gilliland and Kiros Berhane had maximum collaborations with researchers. More coalition amongst investigators can be expected in the future.
As evidenced by the present study and in concordance with other bibliometric studies in varied fields, the majority of studies stemmed from academic institutes in the United States. Countries with a stronger economic background are inclined towards biomedical research, perhaps due to better scientific resources and funding. Despite the high prevalence and fatalities associated with exposure to ETS among children in low- and middle-income nations, there were limited population-based investigations performed within these regions. This study recognized a trend towards collation between the United States and several other nations, including the United Kingdom, Sweden, Italy, Netherlands, Poland, Germany, Switzerland, Greece, Denmark, Spain, China, Canada, Australia. Notably, among the top 100 cited articles, there were only two randomized controlled trials and five systematic reviews, while narrative reviews dominated with a count of 32. It is important to acknowledge the challenges of conducting randomised controlled trials for hazardous exposures like ETS even when trying to implement beneficial interventions. Furthermore, with the colossal size of publications, researchers may incline to consolidate and synthesize the existing information on a topic in the form of a literature review. Though Cochrane reviews have been internationally acclaimed as the highest level of the evidence base, they could ensure only one position in this study. A plausible explication of the lower citation counts could be that they are yet to attain a substantial age of publication. Fifty-five percent of the research papers were observational (cohort or cross-sectional). This finding could be attributed to the relatively lower resource requirements and costs associated with these study designs. Fundamental explorations in the etiopathogenesis of ETS have emanated from this study design. As the evidence-based philosophy is being propagated globally, it is essential to prioritize meticulously planned high-quality clinical studies on ETS and CH. Urgent attention must be directed towards conducting large longitudinal studies that span from preconception until childhood to gain a better understanding of how exposure to ETS impacts subclinical childhood health outcomes, such as neuropsychologic impairments. Additionally, large-scale case-control studies are required to investigate gene-environment interactions for relatively uncommon diseases like childhood malignancies. However, there are two challenges present within this field: exposure misclassification and statistical methodologies required for dealing with intricate interactions comprising multiple dimensions. Future research efforts could immensely benefit from using archives of exposure biomarkers which hold crucial information on prenatal and childhood determinants of adult diseases. While the primary target organ for ETS exposure is the lungs it comes as no surprise that a considerable number of studies (n = 31) focussed on respiratory outcomes such as asthma, wheezing, pneumonia, acute respiratory infections, and lower respiratory infections. There exists a substantial amount of evidence to support the causal relationship between exposure to ETS and respiratory ailments as compared to other conditions. There was a scarcity of studies assessing the association between ETS and atopic eczema (n = 9) or otitis media (n = 8). Similarly, the number of articles about ETS and snoring, and obesity were also low. The relationship between ETS and childhood dental caries is an area of research that is expanding. Furthermore, the expeditious growth of DNA methylation has aided the ranking of epigenetic papers, a part of Precision Environmental Health, to gain notable traction in the past ten years. It is paramount to take cognizance of the detrimental effects of ETS on childhood illnesses that could potentially influence their health trajectory throughout adult life. A collaborative effort between communities, healthcare professionals and government bodies at all levels must be pursued to explore novel solutions within the realm of children’s environmental health. Thereby, successfully translating and communicating research findings into actionable interventions. Finally, the process of triangulation of evidence by means of reviews and pointing sources of bias in different study designs can help strengthen the degree of causality from multiple study designs [ 19 ].
The evaluation also focused on both the authors’ chosen keywords and those indexed in the papers. The commonly used term “human” was frequently observed, along with gender-specific words such as “male” and “female.“ Thus, when searching for papers related to ETS and CH, employing generic keywords may result in a more compendious search.
The conventional citation-based indicators do not assess the social media realm. As highlighted in additional file 3 the highest altmetirc score was displayed by a mechanistic review of tobacco smoke altering the immune responses in the lung triggering inflammation by Strzelak et al. (2018). This article was broadcasted through various news outlets and tweets; nineteen and seven times, respectively. On the contrary, the second article ‘Housing Characteristics and Children’s Respiratory Health in the Russian Federation’ published in 2004, was broadcasted by seven agencies but received low Twitter dissemination. From this study, we see the growth in Twitter and news outlets’ distribution of research cognates by a regress in blogs, CiteULike, and Facebook’s use to exchange scientific literature. Conjectures can be derived if these configurations demonstrate an alteration in the overall repute or if more distinct role changes amid social network types have led to this makeshift; however, further investigation is warranted. The percentage of papers with the maximal AAS suggests a huge diversity among the journals with 8% published in the Pediatrics journal followed by 5% each in the International Journal of Epidemiology and Environmental Research journal.
The relationship between the citations in WoS, Scopus, Google Scholar, and the observed AAS was poor. The lack of relation between the number of citations and AAS can be elucidated either by the varied nature of the items which have been taken for estimation or the distinct responses of a scholar/populace to a publication. A strong correlation was noted between Dimensions count and Scopus, WOS, and Google Scholar citation count. Dimensions count may be paramount since it can partially overcome the bias of Altmetric owing to the inconsistent features of social networks [ 20 ]. The AAS of environmental tobacco smoke and child health articles was not significantly correlated with the quartile of the journals. Similar results have been stated by other studies [ 21 , 22 ]. Altmetric outcomes need to be conferred with prudence since the articles published before the burgeoning of the social media landscape may be under-represented [ 23 ]. Altmetrics evaluates the immediate influence of an article, in contrast to the traditional metrics where papers may take more than a decade to attain maximal citations [ 22 ]. Our findings displayed social media mentions reached a peak in the first five years after publication, this is in accordance with similar studies [ 21 , 24 ].
Besides the aforementioned time delay in citations, the results of the study should be expounded with caution. Bibliometric and altmetric analyses have numerous inherent limitations. Firstly, landmark studies, over time, achieve fewer citations as their findings are absorbed into current knowledge without the necessity for referencing. This is referred to as “obliteration by incorporation” [ 16 ]. To mitigate this, we discerned articles by citation density. Second, self-citations can have an impact on citation counts. In this analysis, however, a major variance between the total number of citation counts and citations was not reported after excluding self-citations. Third, only articles published since July 2011 are picked up by Twitter. Also, the Bookmarklet works only on PubMed, arXiv, or Google Scholar pages containing a DOI [ 14 ]. Hence, the probability of influential articles not being cited by social media scientometrics cannot be ruled out. Fourth, altmetrics recognize the level of online activity of research without distinguishing between the publicity or the research output quality [ 15 ]. Fifth, altmetrics weight allocation in the generation of scores is related to the developer’s inference about their anticipated goal for every media platform [ 24 ]. Thus, there may be an imbalance in the contribution of diverse sources to AAS. Sixth, researchers can “game” the system by generating added mentions for their projects on a social forum [ 25 ]. This type of manipulation bias was improbable in the present study as Altmetric Explorer was used as a search engine.
Alternative metrics are in their early stages, and there is meager data about the elements of social platforms to certainly elucidate a definite association amidst novel metrics and bibliometrics. It is ambiguous if media presence leads to higher citations or if aspects that steer greater citation counts lead to increased social networking activity. Although the social web may have some cogency on the distribution of an article, alternative metrics ought to be employed alongside traditional bibliometric measures for assessing research impact comprehensively. Future investigations should explore methods to construct a comprehensive stratagem that integrates both citation-based and social media-based indicators for evaluating research outcomes.
This article provides scientometric and digital dissemination of ETS and CH research between 1985 and 2019. Numerous publications providing strong evidence of causality linking ETS exposure to several pediatric illnesses were noted. However, additional long-term studies of ETS exposure and CH are needed particularly in low- and middle-income countries to provide more precise estimates of these effects. A poor association between the citations in Scopus, WoS, Google Scholar, and the AAS existed, whilst the Dimensions score had a strong relationship with the data sources. To enhance the social influence of research on ETS and CH, sharing research outputs through social media platforms should be encouraged by editors and publishers to reach wider audiences including researchers, academicians, and policy analysts.
Data Availability
All data are contained within the manuscript and its additional files.
Abbreviations
Environmental Tobacco Smoke
Children’s Health
Web of Science
Altmetric Attention Score
Journal Impact Factor
Öberg M, Woodward A, Jaakkola MS, Peruga A, Prüss-Üstün A. Global estimate of the burden of disease from second-hand smoke. World Health Organization; 2010.
Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA. 1995;273(10):795–8.
Article CAS PubMed Google Scholar
Adair-Bischoff CE, Sauve RS. Environmental tobacco smoke and middle ear disease in preschool-age children. Arch Pediatr Adolesc Med. 1998;152:127–33.
Eskenazi B, Castorina R. Association of prenatal maternal or postnatal child environmental tobacco smokes exposure and neurodevelopmental and behavioral problems in children. Environ Health Perspect. 1999;107(12):991–1000.
Article CAS PubMed PubMed Central Google Scholar
Stein RT, Holberg CJ, Sherrill D. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s respiratory study. Am J Epidemiol. 1999;149(11):1030–7.
U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2006.
Google Scholar
Lee C-C, Middaugh NA, Howie SRC, Ezzati M. Association of secondhand smoke exposure with pediatric invasive bacterial disease and bacterial carriage: a systematic review and meta-analysis. PLoS Med. 2010;7:e1000374.
Article PubMed PubMed Central Google Scholar
Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics. 2011;127(4):734–41.
Article PubMed Google Scholar
Nicoletti D, Appel LD, Siedersberger Neto P, Guimarães GW, Zhang L. Maternal smoking during pregnancy and birth defects in children: a systematic review with meta-analysis. Cad Saude Publica. 2014;30:2491–529.
Raghuveer G, White DA, Hayman LL, Woo JG, Villafane J, Celermejer D, Faber T, Kumar A, Mackenbach JP, Millett C, Basu S, Sheikh A et al. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. Lancet Public Heal 2017;2:e420–37.
Faber T, Kumar A, Mackenbach JP, Millett C, Basu S, Sheikh A et al. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. Lancet Public Heal 2017;2:e420–37.
Cao Y, Lu J, Lu J. Paternal smoking before conception and during pregnancy is associated with an increased risk of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2020;42:32–40.
Kwok R. Research impact: altmetrics make their mark. Nature. 2013;500(7463):491–3.
Priem J, Taraborelli D, Groth P, Neylon C. Altmetrics: a manifesto. Available at: URL- http://altmetrics.org/manifesto/ . Accessed on February 5, 2023.
Williams C. The altmetric score is now the altmetric attention score. Available at: URL- https://www.altmetric.com/blog/the-altmetric-score-is-now-the-altmetric-attention-score/ . Accessed on February 5, 2023.
Garfield E. 100 citation classics from the journal of the American Medical Association. JAMA. 1987;257:52–9.
Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2008;22(2):338–42.
Horton NJ, Switzer SS. Statistical methods in the journal. N Engl J Med. 2005;353:1977–9.
Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–86.
PubMed Google Scholar
Thelwall M. Dimension: a competitor to Scopus and web of science? J Informetr. 2018;12:430–5.
Article Google Scholar
Hassona Y, Qutachi T, Dardas L, Alrashdan MS, Sawair F. The online attention to oral cancer research: an altmetric analysis. Oral Dis. 2019;25(6):1502–10.
Costas R, Zahedi Z, Wouters P. Do “altmetrics” correlate with citations? Extensive comparison of altmetric indicators with citations from a multidisciplinary perspective. J Assoc Inf Sci Technol. 2015;66:2003–19.
Delli K, Livas C, Spijkervet FKL, Vissink A. Measuring the social impact of dental research: an insight into the most influential articles on the web. Oral Dis. 2017;23(8):1155–61.
Garcovich D, Adobes Martin M. Measuring the social impact of research in Paediatric Dentistry: an altmetric study. Int J Paediatr Dent. 2019;00:1–9.
Eysenbach G. Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
Download references
Acknowledgements
Not applicable.
This work was supported by grants from the US National Institutes of Health Fogarty International Center, National Institute of Dental and Craniofacial Research (D43TW010540), and Sri Ramachandra Institute of Higher Education and Research - Founder-Chancellor Shri N.P.V Ramasamy Udayar Research Fellowship (U02D200668).
Author information
Authors and affiliations.
Department of Environmental Health Engineering, Faculty of Public Health, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India
Sneha S. Patil, Naveen Puttaswamy & Kalpana Balakrishnan
Department of Pediatric and Preventive Dentistry, Dr. D.Y. Patil Dental College and Hospital, Dr. D.Y. Patil Vidyapeeth, Sant-Tukaram Nagar, Pimpri, Pune, India
Sneha S. Patil
Department of Oral Pathology and Microbiology, Dr. D.Y. Patil Vidyapeeth, Sant-Tukaram Nagar, Pimpri, Pune, India
Sachin C. Sarode & Gargi S. Sarode
Private Practitioner, Pune, India
Smita S. Patil
Department of Epidemiology and Population Health, Stanford School of Medicine, Stanford, CA, USA
Andres Cardenas
Department of Human Genetics, Faculty of Biomedical Sciences, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India
Rajesh Kumar Gandhirajan
You can also search for this author in PubMed Google Scholar
Contributions
All authors have made substantial contribution to the conceptualisation and design of the study. SP, NP, and SS were involved in data collection and data analysis. SP, NP, SS, GS, SP, AC, RG and KB were involved in data interpretation, drafting the manuscript and revising it critically.
Corresponding author
Correspondence to Sneha S. Patil .
Ethics declarations
Ethics approval.
Institutional Ethic Committee (IEC-NI22/JUL/83/82), Sri Ramachandra Institute of Higher Education and Research.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, supplementary material 4, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Patil, S.S., Puttaswamy, N., Sarode, S.C. et al. Environmental tobacco smoke and children’s health: a bibliometric and altmetric analysis of 100 most cited articles. BMC Public Health 23 , 2208 (2023). https://doi.org/10.1186/s12889-023-16242-1
Download citation
Received : 28 March 2023
Accepted : 04 July 2023
Published : 09 November 2023
DOI : https://doi.org/10.1186/s12889-023-16242-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bibliometric analysis
- Environmental tobacco smoke
- Child health
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Tobacco, Nicotine, and E-Cigarettes Research Report What are the effects of secondhand and thirdhand tobacco smoke?
Secondhand smoke is a significant public health concern and driver of smoke-free policies. Also called passive or secondary smoke, secondhand smoke increases the risk for many diseases. 55 Exposure to environmental tobacco smoke among nonsmokers increases lung cancer risk by about 20 percent. 48 Secondhand smoke is estimated to cause approximately 53,800 deaths annually in the United States. 55 Exposure to tobacco smoke in the home is also a risk factor for asthma in children. 56
Smoking also leaves chemical residue on surfaces where smoking has occurred, which can persist long after the smoke itself has been cleared from the environment. This phenomenon, known as "thirdhand smoke," is increasingly recognized as a potential danger, especially to children, who not only inhale fumes released by these residues but also ingest residues that get on their hands after crawling on floors or touching walls and furniture. More research is needed on the risks posed to humans by thirdhand smoke, but a study in mice showed that thirdhand smoke exposure has several behavioral and physical health impacts, including hyperactivity and adverse effects on the liver and lungs. 57
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
Protocol for the ‘Su p po r t i ng Young Cancer S urvivors who S m oke’ study (PRISM): Informing the development of a smoking cessation intervention for childhood, adolescent and young adult cancer survivors in England
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Population Health Sciences Institute, Newcastle University, Newcastle upon Tyne, United Kingdom, Newcastle University Centre for Cancer, Newcastle University, Newcastle upon Tyne, United Kingdom
Roles Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing
Affiliation Centre for Preventive Medicine and Digital Health (CPD), Division for Prevention of Cardiovascular and Metabolic Disease, Medical Faculty Mannheim, Heidelberg University, Heidelberg, Germany
Affiliations Newcastle University Centre for Cancer, Newcastle University, Newcastle upon Tyne, United Kingdom, Department of Paediatric and Adolescent Haematology and Oncology, Great North Children’s Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Roles Funding acquisition, Methodology, Visualization, Writing – review & editing
Affiliation Department of Behavioural Science and Health, University College London, London, United Kingdom
Roles Funding acquisition, Methodology, Writing – review & editing
Affiliations Department of Paediatric Oncology, Leeds Children’s Hospital, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, Leeds Institute of Medical Research, University of Leeds, Leeds, United Kingdom
Affiliation Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
Affiliations Division of Cancer Sciences, The University of Manchester, Manchester, United Kingdom, The Christie NHS Foundation Trust, Manchester, United Kingdom
Roles Funding acquisition, Visualization, Writing – review & editing
Affiliation Patient and Public Representatives for the Study, United Kingdom
- Morven C. Brown,
- Vera Araújo-Soares,
- Roderick Skinner,
- Jamie Brown,
- Adam W. Glaser,
- Helena Hanratty,
- Martin G. McCabe,
- Ana-Ecaterina Amariutei,
- Sabrina Mauri,
- Linda Sharp

- Published: May 15, 2024
- https://doi.org/10.1371/journal.pone.0299321
- Peer Review
- Reader Comments
Childhood, adolescent and young adult (CAYA) cancer survivors are vulnerable to adverse late-effects. For CAYA cancer survivors, tobacco smoking is the most important preventable cause of ill-health and early death. Yet, effective strategies to support smoking cessation in this group are lacking. The PRISM study aims to undertake multi-method formative research to explore the need for, and if appropriate, inform the future development of an evidence-based and theory-informed tobacco smoking cessation intervention for CAYA cancer survivors.
Materials and methods
PRISM involves three phases of: 1) an environmental scan using multiple strategies to identify and examine a) smoking cessation interventions for CAYA cancer survivors that are published in the international literature and b) current smoking cessation services in England that may be available to, or tailorable to, CAYA cancer survivors; 2) a qualitative study involving semi-structured interviews with CAYA cancer survivors (aged 16–29 years and who are current or recent ex-smokers and/or current vapers) to explore their views and experiences of smoking, smoking cessation and vaping; and 3) stakeholder workshops with survivors, healthcare professionals and other stakeholders to consider the potential for a smoking cessation intervention for CAYA cancer survivors and what such an intervention would need to target and change. Findings will be disseminated to patient groups, healthcare professionals and researchers, through conference presentations, journal papers, plain English summaries and social media.
PRISM will explore current delivery of, perceived need for, and barriers and facilitators to, smoking cessation advice and support to CAYA cancer survivors from the perspective of both survivors and healthcare professionals. A key strength of PRISM is the user involvement throughout the study and the additional exploration of survivors’ views on vaping, a behaviour which often co-occurs with smoking. PRISM is the first step in the development of a person-centred, evidence- and theory-based smoking cessation intervention for CAYA cancer survivors who smoke, which if effective, will reduce morbidity and mortality in the CAYA cancer survivor population.
Citation: Brown MC, Araújo-Soares V, Skinner R, Brown J, Glaser AW, Hanratty H, et al. (2024) Protocol for the ‘Su p po r t i ng Young Cancer S urvivors who S m oke’ study (PRISM): Informing the development of a smoking cessation intervention for childhood, adolescent and young adult cancer survivors in England. PLoS ONE 19(5): e0299321. https://doi.org/10.1371/journal.pone.0299321
Editor: Jesse T. Kaye, University of Wisconsin-Madison, UNITED STATES
Received: October 13, 2023; Accepted: February 7, 2024; Published: May 15, 2024
Copyright: © 2024 Brown et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: This a protocol paper and there is no associated data. For future publications resulting from this study, data will be assigned a persistent identifier (DOI) that will allow the data to be discoverable but not openly accessible. The DOI will be included in any data access statement in publications. The corresponding author can be contacted for the data availability of future publications.
Funding: This article presents independent research funded by the National Institute for Health Research (NIHR) under the Research for Patient Benefit programme [NIHR202768]. The project was subjected to an external peer review as part of the application process. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Childhood, adolescent and young adult (CAYA) cancer survivors are a growing population. Approximately 4000 young people aged 0–24 years are diagnosed with cancer in the United Kingdom (UK) annually [ 1 , 2 ]. Due to treatment advances, over 80% of those diagnosed will achieve long-term cure [ 1 , 2 ]. However, late-effects of some cancer treatments—in particular, pulmonary and cardiac toxicities—can leave survivors vulnerable to chronic health conditions.
For CAYA cancer survivors, as with the general population, smoking tobacco is the most important health behaviour associated with ill-health and early death [ 3 ]. Smoking is particularly risky for this group because their health is already compromised by the cancer and its treatment. Smoking exacerbates survivors’ risks of cardiovascular and respiratory diseases, and increases risk of a subsequent cancer, making these the most common causes of morbidity and mortality in this population [ 4 , 5 ].
Children diagnosed with cancer have 3.6 to 6.4-fold increased risk of developing another cancer in later life compared to those in the general population [ 6 ]. Using both non-cancer controls and population data, those diagnosed in adolescence or young adulthood have been found to have a 2 to 3-fold increased risk of developing smoking-related cancers (e.g., lung) [ 6 – 8 ]; this rises to a 5-fold excess risk among some subgroups (e.g., survivors of Hodgkin lymphoma) [ 7 ]. British CAYA survivors diagnosed up to 19 years of age have 3–4 times excess risk of cardiac mortality than the general population [ 9 , 10 ]. In addition, by the age of 40, half of UK survivors have been admitted to hospital for a respiratory condition [ 11 ], and the risk of respiratory mortality is raised 2-fold in those diagnosed with cancer in adolescence or young adulthood, and 7-fold in those diagnosed as children compared to what would be expected in the general population [ 12 ]. Despite these risks, a substantial proportion of CAYA survivors in the UK and United States (US) smoke tobacco: surveys of self-reported smoking cite figures of 14–35% [ 13 – 16 ]. However, it is worth nothing that these may be underestimates as in clinical groups, where smoking is especially stigmatized (e.g., pregnant women, patients with cardiac disease, cancer patients), patients may be particularly reticent about disclosing their tobacco use [ 17 , 18 ].
Clinical practice guidelines for the follow-up care of CAYA cancer survivors from the UK and US, as well as recent harmonized international guidelines [ 19 – 22 ], state that survivors should be advised on both tobacco smoking and smoking cessation. However, a large proportion of CAYA survivors (including those who smoke) report not receiving such advice [ 23 ]. A UK survey of 95 healthcare professionals (HCPs) caring for these survivors found that only 50% of physicians and 36% of nurses reported providing smoking advice to most patients, with many stating they felt they were not the right person to do so [ 24 ]. This echoes findings from adult oncology in the UK, which shows that HCPs can feel uneasy discussing smoking with survivors, and lack awareness and knowledge of smoking cessation services [ 25 ]. Moreover, it is not known what—and how—smoking cessation support should be provided to CAYA cancer survivors to promote successful quitting, and whether existing services could support this (either in their current format, or if appropriately adapted). However, it also needs to be highlighted that although there is much evidence to support effective strategies for smoking cessation in adults, and the provision of cessation services, there is still limited evidence for how to effectively help young people in general to quit smoking.
There is limited empirical research on the views and attitudes of CAYA cancer survivors regarding smoking [ 23 , 26 ]. Although cancer diagnosis, treatment and survivorship are often thought to offer ‘teachable moments’ for a lifestyle change such as quitting smoking [ 27 ], evidence suggests that survivors of adult cancers experience barriers to smoking cessation that are specific to their illness experience (e.g., smoking to help with cancer-related stress and to maintain personal control after their diagnosis [ 25 , 27 ]), and which may affect the uptake, and effectiveness, of current smoking cessation services and support [ 28 ]. Whether this also holds in survivors of CAYA cancer is unclear and fundamental evidence is lacking on why CAYA cancer survivors’ smoke, what affects whether they want to quit and what helps and hinders successful quitting.
In exploring CAYA survivors’ views of smoking, it would be pertinent to also explore their views of e-cigarette use (also known as vaping). In England, around a quarter of young people aged 16–24 use e-cigarettes, whilst 14–20% smoke [ 29 , 30 ]; evidence suggests that around half of those who vape, also smoke tobacco (i.e., are dual users) [ 29 ]. Dual users may find it harder to quit smoking and may not view themselves as ‘smokers’ [ 31 ], thus they may not perceive a study, or indeed an intervention, for smoking cessation to be relevant to them.
There is an urgent need to develop effective strategies to support smoking cessation among CAYA cancer survivors [ 3 ]. However, few interventions exist and of those that have been published [ 16 , 32 – 34 ], none appear to have involved: (i) a systematic framework of intervention development; (ii) a thorough understanding of smoking behaviours in CAYA survivors; (iii) user involvement; or (iv) application of appropriate theory. These are prerequisites for developing successful interventions [ 35 – 38 ], suggesting considerable groundwork is needed to inform the development of effective smoking cessation interventions for CAYA cancer survivors.
The PRISM study will begin to address tobacco smoking in CAYA cancer survivors by undertaking formative research in order to provide a foundation for the future development of a smoking cessation intervention targeted to this population, with the ultimate goal of reducing smoking-related morbidity and mortality.
PRISM aims to inform the future development of a person-centred, evidence-based and theoretically-informed tobacco smoking cessation intervention which can be tailored, as required, to the needs of CAYA cancer survivors.
- a) To identify and describe the features of tobacco smoking cessation interventions for CAYA cancer survivors which have been published internationally; b) To identify and describe the features of tobacco smoking cessation services which currently exist for i) adolescents and/or young people with a medical diagnosis (cancer or another diagnosis) or ii) people of any age with cancer/cancer survivors in England;
- To identify and explore perceived influences on tobacco smoking and vaping behaviour among CAYA cancer survivors;
- To identify and explore perceived barriers to, and facilitators for, tobacco smoking cessation among CAYA cancer survivors;
- To explore CAYA cancer survivors’ views and experiences of tobacco smoking cessation advice and services, and what may help or hinder them to engage with such advice/services;
- To engage with survivors, healthcare professionals and other key stakeholders to develop a preliminary logic model of the problem and identify areas for future development of a tobacco smoking cessation intervention for CAYA cancer survivors.
Overall study design
PRISM will address shortcomings of previous research by following established guidance to generate evidence to inform intervention development [ 35 – 38 ]. It focuses on the planning domain of intervention development—that is, it will seek to understand the problem being addressed, agree the aims and goals of the future intervention, identify possible ways of addressing the problem, and consider real-world issues which may affect future implementation [ 39 ].
Phase 1 will involve an environmental scan where we will identify smoking cessation interventions for CAYA cancer survivors that have been published internationally. We will also identify existing smoking cessation services in England that may be relevant for CAYA cancer survivors (objective 1). Phase 2 will involve semi-structured interviews with 25–30 CAYA cancer survivors who are current or recent ex-smokers and/or current vapers (objectives 2–4). Evidence gathered from these phases will feed into Phase 3 (objective 5), which will involve workshops with key stakeholders and development of a preliminary logic model of the problem which will begin to define what an intervention will need to target and change, and its expected outcomes [ 37 , 40 ].
The methods for each phase are presented below.
Phase 1: Environmental scan.
Environmental scans seek, gather and interpret data from a wide range of sources, enabling assessment of the current state of healthcare services [ 41 ]. We will utilise multiple strategies to identify and characterise existing smoking interventions and cessation services which are either currently available to, or which could be adapted to the needs (retrofitted), of CAYA cancer survivors in England. These strategies will encompass: 1) a scoping search of published literature; 2) a comprehensive search of grey literature and 3) consultation with key informants and stakeholders.
Scoping search of published literature . We will search bibliographic databases (MEDLINE, Embase, PsycINFO, CINAHL, Scopus) to identify smoking cessation interventions with CAYA cancer survivors in the published literature. Search strategies will be informed by PICO search strategy tool [ 42 ]. Search terms will relate to CAYA cancer survivors, interventions and smoking cessation and a combination of subject headings and key words and will be adapted for each database. Searches will be limited to the English language.
Comprehensive search of grey literature . To rigorously identify grey information, we will search: 1) clinical trial registries (ISRCTN registry, ClinicalTrials.gov) and a grey literature database (OpenGrey); 2) results from a popular Internet search engine ( Google.co.uk ); 3) web-sites of relevant organisations (e.g., National Health Service (NHS); and 4) App stores (Google Play and Apple App Store) [ 43 , 44 ].
A Medical Sciences Librarian will advise on a customised search strategy for each source. Search strategies will be based on two components–the intervention/services of interest (e.g., smoking cessation) and population of interest (e.g., young people, cancer patients/survivors).
1 . Grey literature and trial database .
In trial databases, advanced search functions will be utilised to identify smoking cessation trials. Separate searches will be run with restrictions for participant age (child) and condition/disease (cancer). Searches will include all trial statuses (e.g., ongoing, completed, suspended) and will be limited by country (England/UK, as database allows). The OpenGrey database will also be searched.
2 . Google .
Multiple combinations of terms for the two search components will be run using Google Advanced Search. Searches will be restricted to the UK and language (English) and to the first 100 results of each search. Results will be archived by copying results into a Microsoft Word document, retaining the page titles, site links and brief description of the page to enable review for eligibility.
3 . Websites .
Using Google Advanced Search, searches will use a combination of key terms and will be restricted by domain name in order to search the content of specific websites (e.g., cancerresearchuk.org ) and language (English). The first 50 results of each search will be archived for review.
4 . App stores .
Google Play (via play.google.com ) and the Apple App store (via an Iphone13) will be searched using lay language keywords for smoking cessation (e.g., stop smoking, quit smoking), limiting to the first 100 results. App stores do not enable results to be exported, therefore eligibility will be determined by one researcher based on the app’s marketing description. Any apps deemed potentially relevant will be downloaded.
Consultation with key informants and stakeholders . Consultation with key informants . To identify services we will consult with key informants (e.g., smoking cessation services, Cancer Alliances, relevant HCPs in primary and secondary/tertiary care, local public health departments, smoking cessation researchers). We will use a snowball approach by asking contacts if they are aware of other relevant organisations or individuals.
Healthcare professional survey.
We will disseminate a brief online survey via professional associations for HCPs involved in the care of CAYA cancer survivors (Children’s Cancer and Leukaemia Group; Teenagers and Young Adults with Cancer; Teenage Cancer Trust) and social media. The survey will seek information on which (if any) smoking cessation services HCPs refer patients/survivors to. Additional questions will investigate current practices of HCPs with regards to offering advice and support about smoking/smoking cessation to survivors, and the perceived influences on these behaviours. These questions will be informed by the Theoretical Domains Framework (TDF) [ 45 , 46 ], a widely used theoretical approach for identifying determinants of behaviour.
Identifying relevant interventions and services . Eligible studies identified via the scoping search of published literature must report on a smoking cessation intervention for CAYA cancer survivors in any geographical location. To be eligible for inclusion in the grey literature scan, services, interventions and apps must state that they are targeted towards either 1) adolescents and/or young people with a medical diagnosis (cancer or another diagnosis) or 2) people of any age with cancer/cancer survivors. These interventions and services must be available to individuals residing in England, and any apps must be in English. We envisage that few, if any, services will be aimed specifically to CAYA cancer survivors; conducting a wider search will therefore identify services which may provide a basis for a future smoking cessation intervention for CAYA survivors.
Data extraction and analysis . Information will be extracted on the name of service/intervention; target population (who is eligible to access the service); any tailoring; how it is accessed; location and/or type or format; aim and description of what it entails; who provides or delivers it; how long individuals are enrolled in the service and how frequently they are asked to attend (if applicable); how many individuals access it annually; cost (if applicable); and whether it has been evaluated [ 47 ]. Excel will be used to build a database of extracted information to enable synthesis. Narrative synthesis will be undertaken [ 48 ].
Phase 2: Qualitative study.
Study setting and participants . Phase 2 will involve semi-structured interviews with CAYA cancer survivors who are current or recent tobacco smokers and/or current vapers ( Table 1 shows eligibility/exclusion criteria). Survivors will be primarily recruited via four clinical sites (Newcastle upon Tyne Hospitals NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Sheffield Teaching Hospitals NHS Foundation Trust; and The Christie NHS Foundation Trust) which provide care for CAYA cancer survivors. To reach a wider range of survivors we will also promote the study via the social media pages of the research team and, with permission, the pages of local charities (e.g., Children’s Cancer North). Phase 2 received approval from West Midlands–South Birmingham Research Ethics Committee (REC reference: 22/WM/0102).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0299321.t001
Recruitment . Identification and screening of CAYA cancer survivors began in January 2023, with recruitment expected to end 01 July 2024. Young people’s disclosure of tobacco smoking can be influenced by the setting they are in, the perceived disapproval of others and anonymity [ 49 ], and indeed whether they view themselves as a smoker or not. Therefore, we will adopt a range of approaches for recruitment. Maximum variation purposive sampling (with strata including age, gender, ethnicity, socio-economic status, diagnosis, treatment) will be used to ensure elicitation of varied views and experiences.
The primary recruitment route will be via CAYA cancer survivor follow-up services in the Trusts. HCPs will identify potentially eligible survivors through medical records and at clinic, and inform them about the study by letter, telephone or verbally face-to-face. All those approached will receive a participant information sheet. Those interested in participating will either contact the study’s researcher directly or give permission for their details to be provided to the researcher. Where possible, the researcher will attend clinics to be able to speak to eligible patients about the study. We have successfully used these approaches to recruit CAYA survivors to several studies [ 50 , 51 ]. In describing the study to survivors (both verbally and via the participant information sheet) neutral, non-judgmental language will be used. It will be made clear that it is not assumed that they wish to stop smoking or vaping, nor does the study involve efforts to persuade them to do so; we simply want to hear their views on smoking and vaping.
These targeted methods rely on the HCPs identifying CAYA survivors whom they know, or suspect, to smoke tobacco or vape. If required, this approach will be supplemented by Trusts distributing a brief screening questionnaire to survivors who fulfil inclusion criteria 1–4 ( Table 1 ). Those who declare themselves to be current smokers, recent ex-smokers and/or current vapers will be invited to provide their contact details if they are interested in receiving information about the study. Survivors will return the questionnaire in a sealed envelope to the researcher to ensure confidentiality.
For recruitment via social media, posts promoting the study will ask interested survivors to contact the researcher who will screen them against the inclusion criteria and send them a participant information sheet.
Procedures/data collection and analysis . CAYA cancer survivors wishing to take part will be able to choose their preferred mode of interview—telephone, online (e.g., Zoom) or face-to-face at a location of their choice. Offering multiple options can increase research participation of more marginalised groups, or those traditionally less likely to participate [ 52 ]. Prior to interview, participants will provide informed consent. Written consent will be obtained from those interviewed in person. For interviews conducted remotely, verbal consent will be audio-recorded. The researcher will read out each point of the consent form and will ask the participant to indicate their agreement or otherwise. The researcher will then complete a form on the participant’s behalf. Interviews will be guided by a topic guide, which will be used flexibly (available in S1 File ). Interviews will adopt a non-judgemental attitude and will explore: cancer beliefs; awareness of late-effects; smoking and vaping-related health beliefs/risk perceptions; views and experiences of smoking cessation advice/resources/services; quit attempts; perceived influences on their smoking and/or vaping behaviour.
Elements of the interview will be informed by the TDF to: help support comprehensive assessment of behavioural determinants; enable subsequent identification of the most relevant theory; and identify what needs to be targeted by strategies to bring about behaviour change [ 45 ]. As recommended [ 53 ], the TDF will be used flexibly to support identification of key determinants of (i) tobacco smoking and vaping and (ii) successful smoking cessation. Interviews are expected to last approximately 60–90 minutes. All interviewees will be offered a £20 shopping voucher to thank them for their time, as well as reimbursement of any travel expenses.
Interviews will be audio-recorded and transcribed verbatim. Inductive reflexive analysis will occur concurrently with data collection to ensure any new issues raised are explored in subsequent interviews [ 54 ]. Two team members will code preliminary interviews, discuss and agree codes and themes. These codes will be applied to the remaining interviews, incorporating any new codes and themes as they are identified. In a second, deductive, phase of analysis, codes which relate to the influences on smoking/smoking cessation (and vaping) will be mapped onto the TDF, distinguishing between those factors which can, and cannot, be modified. Coding and analysis will be facilitated by QSR International’s NVivo software (Release 1.6, 2022).
Sample size and data saturation . Recruitment will continue until reasonable data saturation has been achieved [ 55 ]. Determination of data saturation will be primarily based on theoretical sufficiency (conceptual depth) regarding survivors’ perceived influences on smoking behaviour. In addition, we will ensure no new themes have been identified in the last three interviews as regards other objectives [ 56 ]. Based on our past research [ 50 , 51 ], and recommendations for sample size for semi-structured interviews [ 57 ], we anticipate 25–30 interviews will be required.
Phase 3: Stakeholder workshops.
Study setting and participants . Phase 3 will involve working with CAYA cancer survivors, HCPs (e.g., oncologists, nurses, general practitioners) and other stakeholders (e.g., public health, smoking cessation service providers) to begin to identify what a smoking cessation intervention for CAYA cancer survivors will need to target.
We will hold two (parallel) workshops, one for survivors, and the other for HCPs (oncologists, nurses, general practitioners) and other stakeholders (e.g., public health, service providers). If required, and if time permits, additional workshops may be held.
Recruitment t o survivor workshop .
CAYA survivors who participate in Phase 2 will be asked to register their interest in workshop attendance. Clinical co-applicants/collaborators will advertise this Patient and Public Involvement (PPI) opportunity via their service and appropriate links at their Trusts (e.g., support groups). We will also invite members of our network of CAYA cancer survivors interested in PPI in Northern England to participate. For greater reach, the opportunity will be advertised via cancer charities and organisations (e.g., Children’s Cancer North), support groups and social media.
Recruitment t o professional workshop .
Stakeholders (e.g., public health, smoking cessation service providers) will be identified via Phase 1. HCPs (e.g., oncologists, nurses, general practitioners) who care for CAYA cancer survivors will be identified via the contacts of the clinical co-applicants and advertisement via professional associations (e.g., Children’s Cancer and Leukaemia Group) and social media.
Procedures/data collection and analysis . If the workloads of HCPs permit, workshops will take place face-to-face at an easily accessible location; if this is not possible, remote workshops will be held using a video conferencing platform that is accessible to workshop participants (e.g., Zoom for survivors, MS Teams for HCPs). These workshops will draw on co-design approaches [ 58 ], in that the research team and stakeholders will work together during them to co-create an understanding of the problem and the potential for a solution.
At each workshop, the Phase 1 and 2 findings will be presented (in the form of evidence statements) [ 58 ], and initial ideas for what would be useful, and what is needed, to promote smoking cessation among CAYA cancer survivors will be explored. The survivor workshop will explore issues around the acceptability of any intervention to support smoking cessation (e.g., perceived need for intervention, potential stigma of using intervention), factors which may encourage or prevent CAYA survivors engaging in an intervention (e.g., referral by HCP, low readiness to quit), and initial views on intervention delivery (e.g., where, how and by whom). Attendees at the survivor workshop will be offered a £75 honorarium [ 59 ]. The workshop with HCPs and wider stakeholders will begin to consider issues around the feasibility of health service/HCP involvement in any intervention and potential contextual factors that might affect an intervention. Based on these workshops, the logic model of the problem will be refined and potential areas for future intervention development identified.
Dissemination of findings.
To reach academic and clinical audiences, dissemination will be via journal publications and conferences. We will work with our PPI co-applicants on dissemination methods to reach CAYA cancer survivors. We will produce a plain English summary of the project results for dissemination through HCP and survivor networks and organisations (e.g., Children’s Cancer and Leukaemia Group).
Patient and public involvement.
PPI input shaped the study. A brief survey about smoking/smoking cessation was posted in two Facebook groups for CAYA cancer survivors. Discussions were held with members of Perspectives , an adult cancer PPI group. Members of the Young Person’s Advisory Group North-East provided input on the content of participant information sheets, recruitment methods and the appropriateness and conduct of interviews. PPI co-applicants (AA and SM) will advise and assist with strategies and patient-facing materials for recruitment to Phase 2 and 3. They will help to shape project direction and decision making, and be invited to contribute to the interpretation and dissemination of study findings. We will use the GRIPP2 short form checklist to report PPI involvement in our research in publications [ 60 ].
The increased risk of smoking-related health conditions among CAYA cancer survivors indicates the need for smoking cessation interventions, but high-quality, evidence-based and theoretically-informed interventions are currently lacking. There is also a lack of research exploring CAYA survivors’ perceived needs for smoking cessation advice and support, and perceived barriers and facilitators to smoking cessation. To our knowledge, there are also no studies which explore the views of CAYA cancer survivors who vape, a behaviour which may have important implications for smoking cessation efforts. PRISM will generate this information to underpin future development of tobacco smoking cessation interventions for this group.
Evidence suggests that cancer professionals lack awareness of what smoking cessation services are available [ 25 ]. By mapping the smoking cessation landscape in England, PRISM will both identify any interventions/services which could potentially be retrofitted to CAYA cancer survivors (thus enabling agile intervention development) [ 35 ], and provide information that may help professionals in the short-term to implement the guidelines advising CAYA survivors on smoking cessation [ 19 – 21 ].
Tobacco smoking often co-occurs with other behaviours such as vaping e-cigarettes and smoking cannabis [ 61 , 62 ]. These are related but distinct behaviours, all of which have very different drivers [ 61 – 64 ]. In the UK, e-cigarettes are tightly regulated and recommended as a smoking cessation aid for those aged 18 and above and while 16% of young people report using cannabis [ 65 ], it remains to be illegal. PRISM focuses specifically on smoking tobacco as CAYA cancer survivors have increased risk of tobacco-related diseases but due to the complexities that may be caused by dual use of e-cigarettes and tobacco cigarettes (e.g., dual users not identifying as smokers), we also include CAYA survivors who vape in this study. Contrary to the public health recommendations of other countries (e.g., Australia, the US), vaping is promoted as a much safer alternative to smoking in the UK. However, little is known about the possible long-term health risks of vaping, and emerging evidence suggests links to increased risk of respiratory and cardiovascular diseases [ 66 ], which should be of particular concern to CAYA survivors. CAYA cancer survivors who smoke cannabis will also be eligible for Phase 2 provided they also smoke tobacco separately (and/or vape), thus the study may also provide some (albeit limited) information about cannabis smoking in this population.
Adolescence is a critical period for smoking initiation, therefore, addressing tobacco use in this patient group will likely benefit from efforts to both prevent uptake in survivors and also support smoking cessation for those who already smoke. By speaking to survivors who smoke and vape we hope to be able to explore their views on the initiation of these behaviours (e.g., when and why they started), by doing so, this may provide information useful to the consideration of smoking prevention in this group.
PRISM is the first step in the development of a person-centred, evidence- and theory-based smoking cessation intervention aimed at CAYA cancer survivors who smoke tobacco. This intervention will seek to support smoking cessation, in order to reduce long-term morbidity and mortality. Therefore, in the long-term the project has considerable potential to yield significant benefits for the CAYA cancer survivor population. In addition, if, in the long-term, it results in an effective tobacco smoking cessation intervention for CAYA cancer survivors, it could yield significant cost savings for the NHS, by preventing admissions of respiratory and cardiac conditions and second cancers.
Supporting information
https://doi.org/10.1371/journal.pone.0299321.s001
- View Article
- Google Scholar
- PubMed/NCBI
- 37. Bartholomew Eldredge LK, Markham C. M., Ruiter R. A. C., Fernandez M. E., Kok G, & Parcel G. S. Planning health promotion programs: An intervention mapping approach 4th ed. San Fransisco, CA:: Jossey-Bass.; 2016.
- 40. Green L, Kreuter M. Health program planning: An educational and ecological approach. 4th Edition ed. New York: McGraw Hill; 2005.
- 54. Braun V, Clarke V. Successful qualitative research: a practical guide for beginners. London: SAGE; 2013.
- Share full article
For more audio journalism and storytelling, download New York Times Audio , a new iOS app available for news subscribers.
Stormy Daniels Takes the Stand
The porn star testified for eight hours at donald trump’s hush-money trial. this is how it went..
This transcript was created using speech recognition software. While it has been reviewed by human transcribers, it may contain errors. Please review the episode audio before quoting from this transcript and email [email protected] with any questions.
It’s 6:41 AM. I’m feeling a little stressed because I’m running late. It’s the fourth week of Donald J. Trump’s criminal trial. It’s a white collar trial. Most of the witnesses we’ve heard from have been, I think, typical white collar witnesses in terms of their professions.
We’ve got a former publisher, a lawyer, accountants. The witness today, a little less typical, Stormy Daniels, porn star in a New York criminal courtroom in front of a jury more accustomed to the types of witnesses they’ve already seen. There’s a lot that could go wrong.
From “The New York Times,” I’m Michael Barbaro. This is “The Daily.”
Today, what happened when Stormy Daniels took the stand for eight hours in the first criminal trial of Donald J. Trump. As before, my colleague Jonah Bromwich was inside the courtroom.
[MUSIC PLAYING]
It’s Friday, May 10th.
So it’s now day 14 of this trial. And I think it’s worth having you briefly, and in broad strokes, catch listeners up on the biggest developments that have occurred since you were last on, which was the day that opening arguments were made by both the defense and the prosecution. So just give us that brief recap.
Sure. It’s all been the prosecution’s case so far. And prosecutors have a saying, which is that the evidence is coming in great. And I think for this prosecution, which is trying to show that Trump falsified business records to cover up a sex scandal, to ease his way into the White House in 2016, the evidence has been coming in pretty well. It’s come in well through David Pecker, former publisher of The National Enquirer, who testified that he entered into a secret plot with Trump and Michael Cohen, his fixer at the time, to suppress negative stories about Trump, the candidate.
It came in pretty well through Keith Davidson, who was a lawyer to Stormy Daniels in 2016 and negotiated the hush money payment. And we’ve seen all these little bits and pieces of evidence that tell the story that prosecutors want to tell. And the case makes sense so far. We can’t tell what the jury is thinking, as we always say.
But we can tell that there’s a narrative that’s coherent and that matches up with the prosecution’s opening statement. Then we come to Tuesday. And that day really marks the first time that the prosecution’s strategy seems a little bit risky because that’s the day that Stormy Daniels gets called to the witness stand.
OK, well, just explain why the prosecution putting Stormy Daniels on the stand would be so risky. And I guess it makes sense to answer that in the context of why the prosecution is calling her as a witness at all.
Well, you can see why it makes sense to have her. The hush money payment was to her. The cover-up of the hush money payment, in some ways, concerns her. And so she’s this character who’s very much at the center of this story. But according to prosecutors, she’s not at the center of the crime. The prosecution is telling a story, and they hope a compelling one. And arguably, that story starts with Stormy Daniels. It starts in 2006, when Stormy Daniels says that she and Trump had sex, which is something that Trump has always denied.
So if prosecutors were to not call Stormy Daniels to the stand, you would have this big hole in the case. It would be like, effect, effect, effect. But where is the cause? Where is the person who set off this chain reaction? But Stormy Daniels is a porn star. She’s there to testify about sex. Sex and pornography are things that the jurors were not asked about during jury selection. And those are subjects that bring up all kinds of different complex reactions in people.
And so, when the prosecutors bring Stormy Daniels to the courtroom, it’s very difficult to know how the jurors will take it, particularly given that she’s about to describe a sexual episode that she says she had with the former president. Will the jurors think that makes sense, as they sit here and try to decide a falsifying business records case, or will they ask themselves, why are we hearing this?
So the reason why this is the first time that the prosecution’s strategy is, for journalists like you, a little bit confusing, is because it’s the first time that the prosecution seems to be taking a genuine risk in what they’re putting before these jurors. Everything else has been kind of cut and dry and a little bit more mechanical. This is just a wild card.
This is like live ammunition, to some extent. Everything else is settled and controlled. And they know what’s going to happen. With Stormy Daniels, that’s not the case.
OK, so walk us through the testimony. When the prosecution brings her to the stand, what actually happens?
It starts, as every witness does, with what’s called direct examination, which is a fancy word for saying prosecutors question Stormy Daniels. And they have her tell her story. First, they have her tell the jury about her education and where she grew up and her professional experience. And because of Stormy Daniels’s biography, that quickly goes into stripping, and then goes into making adult films.
And I thought the prosecutor who questioned her, Susan Hoffinger, had this nice touch in talking about that, because not only did she ask Daniels about acting in adult films. But she asked her about writing and directing them, too, emphasizing the more professional aspects of that work and giving a little more credit to the witness, as if to say, well, you may think this or you may think that. But this is a person with dignity who took what she did seriously. Got it.
What’s your first impression of Daniels as a witness?
It’s very clear that she’s nervous. She’s speaking fast. She’s laughing to herself and making small jokes. But the tension in the room is so serious from the beginning, from the moment she enters, that those jokes aren’t landing. So it just feels, like, really heavy and still and almost oppressive in there. So Daniels talking quickly, seeming nervous, giving more answers than are being asked of her by the prosecution, even before we get to the sexual encounter that she’s about to describe, all of that presents a really discomfiting impression, I would say.
And how does this move towards the encounter that Daniels ultimately has?
It starts at a golf tournament in 2006, in Lake Tahoe, Nevada. Daniels meets Trump there. There are other celebrities there, too. They chatted very briefly. And then she received a dinner invitation from him. She thought it over, she says. And she goes to have dinner with Trump, not at a restaurant, by the way. But she’s invited to join him in the hotel suite.
So she gets to the hotel suite. And his bodyguard is there. And the hotel door is cracked open. And the bodyguard greets her and says she looks nice, this and that. And she goes in. And there’s Donald Trump, just as expected. But what’s not expected, she says, is that he’s not wearing what you would wear to a dinner with a stranger, but instead, she says, silk or satin pajamas. She asked him to change, she says. And he obliges.
He goes, and he puts on a dress shirt and dress pants. And they sit down at the hotel suite’s dining room table. And they have a kind of bizarre dinner. Trump is asking her very personal questions about pornography and safe sex. And she testifies that she teased him about vain and pompous he is. And then at some point, she goes to the bathroom. And she sees that he has got his toiletries in there, his Old Spice, his gold tweezers.
Very specific details.
Yeah, we’re getting a ton of detail in this scene. And the reason we’re getting those is because prosecutors are trying to elicit those details to establish that this is a credible person, that this thing did happen, despite what Donald Trump and his lawyers say. And the reason you can know it happened, prosecutors seem to be saying, is because, look at all these details she can still summon up.
She comes out of the bathroom. And she says that Donald Trump is on the hotel bed. And what stands out to me there is what she describes as a very intense physical reaction. She says that she blacked out. And she quickly clarifies, she doesn’t mean from drugs or alcohol. She means that, she says, that the intensity of this experience was such that, suddenly, she can’t remember every detail. The prosecution asks a question that cuts directly to the sex. Essentially, did you start having sex with him? And Daniels says that she did. And she continues to provide more details than even, I think, the prosecution wanted.
And I think we don’t want to go chapter and verse through this claimed sexual encounter. But I wonder what details stand out and which details feel important, given the prosecution’s strategy here.
All the details stand out because it’s a story about having had sex with a former president. And the more salacious and more private the details feel, the more you’re going to remember them. So we’ll remember that Stormy Daniels said what position they had sex in. We’ll remember that she said he didn’t use a condom. Whether that’s important to the prosecution’s case, now, that’s a much harder question to answer, as we’ve been saying.
But what I can tell you is, as she’s describing having had sex with Donald Trump, and Donald Trump is sitting right there, and Eric Trump, his son, is sitting behind him, seeming to turn a different color as he hears this embarrassment of his father being described to a courtroom full of reporters at this trial, it’s hard to even describe the energy in that room. It was like nothing I had ever experienced. And it was just Daniels’s testimony and, seemingly, the former President’s emotions. And you almost felt like you were trapped in there with both of them as this description was happening.
Well, I think it’s important to try to understand why the prosecution is getting these details, these salacious, carnal, pick your word, graphic details about sex with Donald Trump. What is the value, if other details are clearly making the point that she’s recollecting something?
Well, I think, at this point, we can only speculate. But one thing we can say is, this was uncomfortable. This felt bad. And remember, prosecutor’s story is not about the sex. It’s about trying to hide the sex. So if you’re trying to show a jury why it might be worthwhile to hide a story, it might be worth —
Providing lots of salacious details that a person would want to hide.
— exposing them to how bad that story feels and reminding them that if they had been voters and they had heard that story, and, in fact, they asked Daniels this very question, if you hadn’t accepted hush money, if you hadn’t signed that NDA, is this the story you would have told? And she said, yes. And so where I think they’re going with this, but we can’t really be sure yet, is that they’re going to tell the jurors, hey, that story, you can see why he wanted to cover that up, can’t you?
You mentioned the hush money payments. What testimony does Daniels offer about that? And how does it advance the prosecution’s case of business fraud related to the hush money payments?
So little evidence that it’s almost laughable. She says that she received the hush money. But we actually already heard another witness, her lawyer at the time, Keith Davidson, testify that he had received the hush money payment on her behalf. And she testified about feeling as if she had to sell this story because the election was fast approaching, almost as if her leverage was slipping away because she knew this would be bad for Trump.
That feels important. But just help me understand why it’s important.
Well, what the prosecution has been arguing is that Trump covered up this hush money payment in order to conceal a different crime. And that crime, they say, was to promote his election to the presidency by illegal means.
Right, we’ve talked about this in the past.
So when Daniels ties her side of the payment into the election, it just reminds the jurors maybe, oh, right, this is what they’re arguing.
So how does the prosecution end this very dramatic, and from everything you’re saying, very tense questioning of Stormy Daniels about this encounter?
Well, before they can even end, the defense lawyers go and they consult among themselves. And then, with the jury out of the room, one of them stands up. And he says that the defense is moving for a mistrial.
On what terms?
He says that the testimony offered by Daniels that morning is so prejudicial, so damning to Trump in the eyes of the jury, that the trial can no longer be fair. Like, how could these jurors have heard these details and still be fair when they render their verdict? And he says a memorable expression. He says, you can’t un-ring that bell, meaning they heard it. They can’t un-hear it. It’s over. Throw out this trial. It should be done.
Wow. And what is the response from the judge?
So the judge, Juan Merchan, he hears them out. And he really hears them out. But at the end of their arguments, he says, I do think she went a little too far. He says that. He said, there were things that were better left unsaid.
By Stormy Daniels?
By Stormy Daniels. And he acknowledges that she is a difficult witness. But, he says, the remedy for that is not a mistrial, is not stopping the whole thing right now. The remedy for that is cross-examination. If the defense feels that there are issues with her story, issues with her credibility, they can ask her whatever they want. They can try to win the jury back over. If they think this jury has been poisoned by this witness, well, this is their time to provide the antidote. The antidote is cross-examination. And soon enough, cross-examination starts. And it is exactly as intense and combative as we expected.
We’ll be right back.
So, Jonah, how would you characterize the defense’s overall strategy in this intense cross-examination of Stormy Daniels?
People know the word impeach from presidential impeachments. But it has a meaning in law, too. You impeach a witness, and, specifically, their credibility. And that’s what the defense is going for here. They are going to try to make Stormy Daniels look like a liar, a fraud, an extortionist, a money-grubbing opportunist who wanted to take advantage of Trump and sought to do so by any means necessary.
And what did that impeachment strategy look like in the courtroom?
The defense lawyer who questions Stormy Daniels is a woman named Susan Necheles. She’s defended Trump before. And she’s a bit of a cross-examination specialist. We even saw her during jury selection bring up these past details to confront jurors who had said nasty things about Trump on social media with. And she wants to do the same thing with Daniels. She wants to bring up old interviews and old tweets and things that Daniels has said in the past that don’t match what Daniels is saying from the stand.
What’s a specific example? And do they land?
Some of them land. And some of them don’t. One specific example is that Necheles confronts Daniels with this old tweet, where Daniels says that she’s going to dance down the street if Trump goes to jail. And what she’s trying to show there is that Daniels is out for revenge, that she hates Trump, and that she wants to see him go to jail. And that’s why she’s testifying against him.
And Daniels is very interesting during the cross-examination. It’s almost as if she’s a different person. She kind of squares her shoulders. And she sits up a little straighter. And she leans forward. Daniels is ready to fight. But it doesn’t quite land. The tweet actually says, I’ll dance down the street when he’s selected to go to jail.
And Daniels goes off on this digression about how she knows that people don’t get selected to go to jail. That’s not how it works. But she can’t really unseat this argument, that she’s a political enemy of Donald Trump. So that one kind of sticks, I would say. But there are other moves that Necheles tries to pull that don’t stick.
So unlike the prosecution, which typically used words like adult, adult film, Necheles seems to be taking every chance she can get to say porn, or pornography, or porn star, to make it sound base or dirty. And so when she starts to ask Daniels about actually being in pornography, writing, acting, and directing sex films, she tries to land a punch line, Necheles does. She says, so you have a lot of experience making phony stories about sex appear to be real, right?
As if to say, perhaps this story you have told about entering Trump’s suite in Lake Tahoe and having sex with him was made up.
Just another one of your fictional stories about sex. But Daniels comes back and says, the sex in the films, it’s very much real, just like what happened to me in that room. And so, when you have this kind of combat of a lawyer cross-examining very aggressively and the witness fighting back, you can feel the energy in the room shift as one lands a blow or the other does. But here, Daniels lands one back. And the other issue that I think Susan Necheles runs into is, she tries to draw out disparities from interviews that Daniels gave, particularly to N-TOUCH, very early on once the story was out.
It’s kind of like a tabloid magazine?
But some of the disparities don’t seem to be landing quite like Necheles would want. So she tries to do this complicated thing about where the bodyguard was in the room when Daniels walked into the room, as described in an interview in a magazine. But in that magazine interview, as it turns out, Daniels mentioned that Trump was wearing pajamas. And so, if I’m a juror, I don’t care where the bodyguard is. I’m thinking about, oh, yeah, I remember that Stormy Daniels said now in 2024 that Trump was wearing pajamas.
I’m curious if, as somebody in the room, you felt that the defense was effective in undermining Stormy Daniels’s credibility? Because what I took from the earlier part of our conversation was that Stormy Daniels is in this courtroom on behalf of the prosecution to tell a story that’s uncomfortable and has the kind of details that Donald Trump would be motivated to try to hide. And therefore, this defense strategy is to say, those details about what Trump might want to hide, you can’t trust them. So does this back and forth effectively hurt Stormy Daniels’s credibility, in your estimation?
I don’t think that Stormy Daniels came off as perfectly credible about everything she testified about. There are incidents that were unclear or confusing. There were things she talked about that I found hard to believe, when she, for instance, denied that she had attacked Trump in a tweet or talked about her motivations. But about what prosecutors need, that central story, the story of having had sex with him, we can’t know whether it happened.
But there weren’t that many disparities in these accounts over the years. In terms of things that would make me doubt the story that Daniels was telling, details that don’t add up, those weren’t present. And you don’t have to take my word for that, nor should you. But the judge is in the room. And he says something very, very similar.
What does he say? And why does he say it?
Well, he does it when the defense, again, at the end of the day on Thursday, calls for a mistrial.
With a similar argument as before?
Not only with a similar argument as before, but, like, almost the exact same argument. And I would say that I was astonished to see them do this. But I wasn’t because I’ve covered other trials where Trump is the client. And in those trials, the lawyers, again and again, called for a mistrial.
And what does Judge Marchan say in response to this second effort to seek a mistrial?
Let me say, to this one, he seems a little less patient. He says that after the first mistrial ruling, two days before, he went into his chambers. And he read every decision he had made about the case. He took this moment to reflect on the first decision. And he found that he had, in his own estimation, which is all he has, been fair and not allowed evidence that was prejudicial to Trump into this trial. It could continue. And so he said that again. And then he really almost turned on the defense. And he said that the things that the defense was objecting to were things that the defense had made happen.
He says that in their opening statement, the defense could have taken issue with many elements of the case, about whether there were falsified business records, about any of the other things that prosecutors are saying happened. But instead, he says, they focused their energy on denying that Trump ever had sex with Daniels.
And so that was essentially an invitation to the prosecution to call Stormy Daniels as a witness and have her say from the stand, yes, I had this sexual encounter. The upshot of it is that the judge not only takes the defense to task. But he also just says that he finds Stormy Daniels’s narrative credible. He doesn’t see it as having changed so much from year to year.
Interesting. So in thinking back to our original question here, Jonah, about the idea that putting Stormy Daniels on the stand was risky, I wonder if, by the end of this entire journey, you’re reevaluating that idea because it doesn’t sound like it ended up being super risky. It sounded like it ended up working reasonably well for the prosecution.
Well, let me just assert that it doesn’t really matter what I think. The jury is going to decide this. There’s 12 people. And we can’t know what they’re thinking. But my impression was that, while she was being questioned by the prosecution for the prosecution’s case, Stormy Daniels was a real liability. She was a difficult witness for them.
And the judge said as much. But when the defense cross-examined her, Stormy Daniels became a better witness, in part because their struggles to discredit her may have actually ended up making her story look more credible and stronger. And the reason that matters is because, remember, we said that prosecutors are trying to fill this hole in their case. Well, now, they have. The jury has met Stormy Daniels. They’ve heard her account. They’ve made of it what they will. And now, the sequence of events that prosecutors are trying to line up as they seek prison time for the former President really makes a lot of sense.
It starts with what Stormy Daniels says with sex in a hotel suite in 2006. It picks up years later, as Donald Trump is trying to win an election and, prosecutors say, suppressing negative stories, including Stormy Daniels’s very negative story. And the story that prosecutors are telling ends with Donald Trump orchestrating the falsification of business records to keep that story concealed.
Well, Jonah, thank you very much. We appreciate it.
Of course, thanks for having me.
The prosecution’s next major witness will be Michael Cohen, the former Trump fixer who arranged for the hush money payment to Stormy Daniels. Cohen is expected to take the stand on Monday.
Here’s what else you need to know today. On Thursday, Israeli Prime Minister Benjamin Netanyahu issued a defiant response to warnings from the United States that it would stop supplying weapons to Israel if Israel invades the Southern Gaza City of Rafah. So far, Israel has carried out a limited incursion into the city where a million civilians are sheltering, but has threatened a full invasion. In a statement, Netanyahu said, quote, “if we need to stand alone, we will stand alone.”
Meanwhile, high level ceasefire negotiations between Israel and Hamas have been put on hold in part because of anger over Israel’s incursion into Rafah.
A reminder, tomorrow, we’ll be sharing the latest episode of our colleague’s new show, “The Interview” This week on “The Interview,” Lulu Garcia-Navarro talks with radio host Charlamagne Tha God about his frustrations with how Americans talk about politics.
If me as a Black man, if I criticize Democrats, then I’m supporting MAGA. But if I criticize, you know, Donald Trump and Republicans, then I’m a Democratic shill. Why can’t I just be a person who deals in nuance?
Today’s episode was produced by Olivia Natt and Michael Simon Johnson. It was edited by Lexie Diao, with help from Paige Cowett, contains original music by Will Reid and Marion Lozano, and was engineered by Alyssa Moxley. Our theme music is by Jim Brunberg and Ben Landsverk of Wonderly.
That’s it for “The Daily.” I’m Michael Barbaro. See you on Monday.

- May 15, 2024 • 25:48 The Possible Collapse of the U.S. Home Insurance System
- May 14, 2024 • 35:20 Voters Want Change. In Our Poll, They See It in Trump.
- May 13, 2024 • 27:46 How Biden Adopted Trump’s Trade War With China
- May 10, 2024 • 27:42 Stormy Daniels Takes the Stand
- May 9, 2024 • 34:42 One Strongman, One Billion Voters, and the Future of India
- May 8, 2024 • 28:28 A Plan to Remake the Middle East
- May 7, 2024 • 27:43 How Changing Ocean Temperatures Could Upend Life on Earth
- May 6, 2024 • 29:23 R.F.K. Jr.’s Battle to Get on the Ballot
- May 3, 2024 • 25:33 The Protesters and the President
- May 2, 2024 • 29:13 Biden Loosens Up on Weed
- May 1, 2024 • 35:16 The New Abortion Fight Before the Supreme Court
- April 30, 2024 • 27:40 The Secret Push That Could Ban TikTok
Hosted by Michael Barbaro
Featuring Jonah E. Bromwich
Produced by Olivia Natt and Michael Simon Johnson
Edited by Lexie Diao
With Paige Cowett
Original music by Will Reid and Marion Lozano
Engineered by Alyssa Moxley
Listen and follow The Daily Apple Podcasts | Spotify | Amazon Music | YouTube
This episode contains descriptions of an alleged sexual liaison.
What happened when Stormy Daniels took the stand for eight hours in the first criminal trial of former President Donald J. Trump?
Jonah Bromwich, one of the lead reporters covering the trial for The Times, was in the room.
On today’s episode

Jonah E. Bromwich , who covers criminal justice in New York for The New York Times.

Background reading
In a second day of cross-examination, Stormy Daniels resisted the implication she had tried to shake down Donald J. Trump by selling her story of a sexual liaison.
Here are six takeaways from Ms. Daniels’s earlier testimony.
There are a lot of ways to listen to The Daily. Here’s how.
We aim to make transcripts available the next workday after an episode’s publication. You can find them at the top of the page.
The Daily is made by Rachel Quester, Lynsea Garrison, Clare Toeniskoetter, Paige Cowett, Michael Simon Johnson, Brad Fisher, Chris Wood, Jessica Cheung, Stella Tan, Alexandra Leigh Young, Lisa Chow, Eric Krupke, Marc Georges, Luke Vander Ploeg, M.J. Davis Lin, Dan Powell, Sydney Harper, Mike Benoist, Liz O. Baylen, Asthaa Chaturvedi, Rachelle Bonja, Diana Nguyen, Marion Lozano, Corey Schreppel, Rob Szypko, Elisheba Ittoop, Mooj Zadie, Patricia Willens, Rowan Niemisto, Jody Becker, Rikki Novetsky, John Ketchum, Nina Feldman, Will Reid, Carlos Prieto, Ben Calhoun, Susan Lee, Lexie Diao, Mary Wilson, Alex Stern, Dan Farrell, Sophia Lanman, Shannon Lin, Diane Wong, Devon Taylor, Alyssa Moxley, Summer Thomad, Olivia Natt, Daniel Ramirez and Brendan Klinkenberg.
Our theme music is by Jim Brunberg and Ben Landsverk of Wonderly. Special thanks to Sam Dolnick, Paula Szuchman, Lisa Tobin, Larissa Anderson, Julia Simon, Sofia Milan, Mahima Chablani, Elizabeth Davis-Moorer, Jeffrey Miranda, Renan Borelli, Maddy Masiello, Isabella Anderson and Nina Lassam.
Jonah E. Bromwich covers criminal justice in New York, with a focus on the Manhattan district attorney’s office and state criminal courts in Manhattan. More about Jonah E. Bromwich
Advertisement
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options
Ifeanyichukwu o. onor.
1 Xavier University of Louisiana College of Pharmacy, 1 Drexel Drive, New Orleans, LA 70125, USA; moc.liamg@21irosa (D.L.S.); ude.alux@ailliwrs (S.R.W.); moc.oohay@sttebnnad (D.B.); ude.alux@lohgroba (A.B.); ude.alux@1sirrahm (M.B.H.); moc.liamg@sneradbt (T.B.D.); moc.liamtoh@yalcedrahs (S.D.C.)
2 Department of Medicine, School of Medicine, Louisiana State University Health Sciences Center New Orleans, 1542 Tulane Avenue, New Orleans, LA 70112, USA
Daniel L. Stirling
Shandrika r. williams, daniel bediako, amne borghol, martha b. harris, tiernisha b. darensburg, sharde d. clay, samuel c. okpechi.
3 Stanley S. Scott Cancer Center, School of Medicine, Louisiana State University Health Sciences Center New Orleans, 1700 Tulane Avenue, New Orleans, LA 70112, USA; moc.liamg@ihcepkoleumas
Daniel F. Sarpong
4 Center for Minority Health and Health Disparities Research and Education, Xavier University of Louisiana College of Pharmacy, 1 Drexel Drive, New Orleans, LA 70125, USA; ude.alux@gnoprasd
Cigarette smoking—a crucial modifiable risk factor for organ system diseases and cancer—remains prevalent in the United States and globally. In this literature review, we aim to summarize the epidemiology of cigarette smoking and tobacco use in the United States, pharmacology of nicotine—the active constituent of tobacco, and health consequence of cigarette smoking. This article also reviews behavioral and pharmacologic interventions for cigarette smokers and provides cost estimates for approved pharmacologic interventions in the United States. A literature search was conducted on Google Scholar, EBSCOhost, ClinicalKey, and PubMed databases using the following headings in combination or separately: cigarette smoking, tobacco smoking, epidemiology in the United States, health consequences of cigarette smoking, pharmacologic therapy for cigarette smoking, and non-pharmacologic therapy for cigarette smoking. This review found that efficacious non-pharmacologic interventions and pharmacologic therapy are available for cessation of cigarette smoking. Given the availability of efficacious interventions for cigarette smoking cessation, concerted efforts should be made by healthcare providers and public health professionals to promote smoking cessation as a valuable approach for reducing non-smokers’ exposure to environmental tobacco smoke.
1. Introduction
Tobacco use, in any form, can be described as a behavioral process which elicits psychological and physiologic addictive mood among users. Nicotine, the active ingredient in tobacco, is highly addictive, resulting in sustained tobacco use. Tobacco use is divided into combustible and noncombustible tobacco products. Combustible tobacco products include: cigarettes, cigars, cigarillos, small cigars, water pipes (hookah), and pipes. Noncombustible tobacco products include electronic cigarettes and tobacco formulations developed for chewing, dipping, or snuffing.
According to the 2013–2014 National Adult Tobacco Survey (NATS), the United States’ national prevalence for current tobacco product use was 21.3% in adults aged ≥18 years [ 1 ]. Distribution of tobacco product use include: 17% for cigarettes, 1.8% for cigars/cigarillos/filtered little cigars, 0.3% for pipes, 0.6% for water pipes/hookah, 3.3% for electronic cigarettes, and 2.5% for smokeless tobacco [ 1 ]. These trends sharply contrast with tobacco use in the 1800s—a period which saw predominant use of chewing tobacco and pipe tobacco because there was no mass manufacturing of cigarettes. These less popular methods of tobacco use, while still unhealthy, theoretically were associated with fewer cancers and tobacco related deaths. Now, with its unique design and accessibility, cigarette smoking has become the choice of tobacco use among many youth and adults globally. Cigarettes are designed to allow deep inhalation of smoke into the lungs, delivering high levels of nicotine to the brain within 10–20 s of inhalation [ 2 ]. This rapid rise in nicotine levels makes cigarette smoking the most reinforcing and dependence-producing form of tobacco use [ 2 ]. The epidemiologic impact and adverse health effects of cigarette smoking are significant. Reducing the prevalence of cigarette smoking and the resultant smoking-induced disease is imperative.
This article reviews the epidemiology of cigarette smoking in the United States, pharmacology of nicotine, and health impact of cigarette smoking alongside behavioral and pharmacological interventions available for smoking cessation in the United States. We performed a literature search on Google Scholar, EBSCOhost, ClinicalKey, and PubMed databases using the following keywords in combination or separately: cigarette smoking, tobacco smoking, epidemiology in the United States, health consequences of cigarette smoking, pharmacologic therapy for cigarette smoking, and non-pharmacologic therapy for cigarette smoking. We reviewed and included literature that provided the most relevant and up-to-date information on our search terms. Excluding the epidemiology data, which focused on the United States, our literature search was inclusive of literature without any geographic constraint. The aim of this review is to provide accessible information on the clinical effects of cigarette smoking, interventions available for cigarette smoking cessation, and the cost estimates for U.S. Food and Drug Administration (FDA)—approved pharmacotherapy options for cigarette smoking.
2. Epidemiology of Cigarette (Tobacco) Smoking in the United States
Although cigarette smoking is the most commonly used form of tobacco in the U.S., the prevalence of cigarette smoking amongst adults has been declining in recent years. According to the 2015 National Health Interview Survey (NHIS), the percentage of adults aged ≥18 years who smoked cigarettes was 15.1% in 2015, a decrease from 20.9% in 2005 [ 3 ]. This general trend of decline in tobacco smoking in the United States has also been observed globally [ 4 ]. The World Health Organization (WHO) reports that among adults over 15 years, the global rate of smoking declined from 23.5% in 2007 to 20.7 in 2015, reflecting a 2.8% smoking rate reduction [ 4 ]. Although there has been a decline in the prevalence of smoking globally, the number of people smoking worldwide has remained at 1.1 billion from 2007 to 2015 because of population growth [ 4 ]. Several factors linked to declines in the prevalence of smoking include population-based interventions such as raising tobacco taxes, tobacco price increases, anti-tobacco mass media campaigns, comprehensive smoke-free laws, enhanced access to help quitting tobacco use, and implementation of governmental regulations of tobacco products [ 1 , 4 , 5 ]. Of all these factors, WHO reports that raising tobacco taxes has been the single most effective way to reduce tobacco use [ 4 , 5 ].
The result of the NHIS also highlights several disparities in the prevalence of cigarette smoking [ 3 ]. Cigarette (tobacco) smoking is more prevalent among adult males than adult females [ 3 ]. The prevalence of cigarette smoking in 2015 was 16.7% among adult males and 13.6% among adult females [ 3 ]. Prevalence was highest among adults aged 25–44 years (14.8%) and lowest among persons aged ≥65 years [ 3 ]. Racial and ethnic differences also exist. The prevalence was highest amongst American Indian/Alaska Natives (21.9%), and lowest among Asians (7.0%) [ 3 ]. When examining education level, prevalence was variable. It was highest among those with a General Education Development Certificate (GED) (34.1%) and lowest among those with a graduate degree (3.6%) [ 3 ]. When examining socioeconomic status, prevalence was highest among persons living below poverty level (26.1%) and lowest among persons living at or above poverty level (13.9%) [ 3 ].
A history of substance abuse disorders and mental illness increases cigarette smoking [ 6 , 7 ]. Cooperman et al. reported a high prevalence of cigarette smoking (80%) among opiate dependent smokers on methadone treatment and Santhosh et al. disclosed a 2013 report which showed that although patients with mental illness and substance abuse disorders made up 24.8% of adults in the United States, they consumed nearly 40% of all cigarettes [ 6 , 7 ]. Additional data from the National Surveys on Drug Use and Health corroborate the strong association among cigarette use, mental illness, and substance abuse across gender and age [ 7 ].
Cigarette (tobacco) smoking is not only common among adults, but is also common among youth. With the current trends of monetary investment into the tobacco industry, smoking poses a bigger threat to the younger population in American society. According to the Executive Summary of the U.S. Surgeon General Office report in 2012, everyday 3800 youth under the age of 18 start smoking [ 8 ]. Most adult smokers, 88%, smoked their first cigarette before the age of 18 [ 8 ]. According to the National Survey on Drug Use and Health 2012, the mean age of smoking initiation was 15.3 years and less than 1.5% of cigarette smokers began smoking in adulthood (after 26 years of age) [ 9 ]. Although cigarette smoking most often begins during youth and young adulthood, the use of cigarettes among this population has been declining in recent years. Among high school students, 9.3% reported current cigarette smoking in 2015, a decrease from 15.8% in 2011 [ 10 ]. Among middle school students, 2.3% reported current cigarette smoking in 2015, a decrease from 4.3% in 2011 [ 10 ]. While the use of cigarettes among youth has declined, the use of electronic cigarettes in this population is increasing. Electronic cigarettes are currently the most commonly used form of tobacco among middle and high school students. In 2015, 16% of high school students reported current electronic cigarette use, an increase from 1.5% in 2011 [ 10 ]. Among middle school students, 5.3% reported current electronic cigarette use, an increase from 0.6% in 2011 [ 10 ]. These trends in cigarette and electronic cigarette use highlight the importance of targeting smoking prevention efforts at youth and young adults.
Electronic cigarettes (e-cigarettes) are rapidly increasing in popularity [ 11 ]. There was an increase in e-cigarette use from 1.9% in 2012–2013 to 3.3% in 2013–2014 according to the National Adult Tobacco Survey [ 1 ]. Young adults between 18–24 years account for the highest prevalence of use of newly emerging products, including e-cigarettes and water pipes/hookahs [ 1 ]. E-cigarettes use a battery-powered heating device to deliver nicotine via a vapor that is drawn into the mouth, upper airways and possibly lungs [ 11 ]. The device uses a battery-powered heating element activated by suction or manually to heat a nicotine solution and transform it into vapor [ 11 ]. In a study by D’Ruiz et al., they observed that e-cigarettes had blood plasma nicotine levels lower than that of conventional tobacco cigarettes, yet the reduction in craving was comparable between e-cigarettes and conventional tobacco cigarettes [ 12 ]. E-cigarettes usually contain nicotine dissolved in a solution made up of propylene glycol and/or glycerin, and flavorings [ 12 , 13 , 14 ]. Other toxic substances such as formaldehyde and acrolein may be present in very low levels in e-cigarettes compared to conventional cigarettes [ 14 ]. Although the use of e-cigarette is soaring, several review articles evaluating studies of e-cigarettes have concluded that the short- and long-term effects of e-cigarettes are limited or lacking [ 13 , 14 ]. Even with limited data on the health effects of e-cigarettes, in 2016, the U.S. Food and Drug Administration (FDA)—under authority granted to it by the Congress under the Family Smoking Prevention and Tobacco Control Act of 2009—took a historic step to protect America’s youth from the harmful effects of using e-cigarettes by extending its regulatory authority over the manufacturing, distribution, and marketing of e-cigarettes [ 15 ].
Although current gaps exist in scientific evidence on the spectrum of health effects of e-cigarettes, we know that compared with older adults, brain of youth and young adults is more vulnerable to the negative consequences of nicotine exposure [ 15 ]. These effects include addiction, priming for use of other addictive substances, reduced impulse control, deficits in attention and cognition, and mood disorders [ 15 ]. The U.S. Surgeon General in his report on “E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General” raised awareness on the exponential growth of youth and young adults who are using e-cigarettes and encourages concerted societal effort to prevent and reduce the use of e-cigarettes by youth and young adults in order to prevent the well documented harmful effects of nicotine use-which is more pronounced in the development of adolescent brain [ 15 ].
The CDC also discusses the potential for harm and benefit associated with e-cigarette use [ 16 ]. E-cigarettes can cause harm to the public, which is more notable if used by defined populations (youth, young adults, pregnant women). Some of the harms include increased risk for using nicotine and/or other tobacco products, leading former smokers to relapse to nicotine and/or tobacco product use, delay smoking cessation among current smokers, exposure to second-hand aerosol, and nicotine poisoning [ 16 ]. E-cigarette use also contributes to environmental tobacco smoke and may mimic the effects of passive (second-hand) smoking seen with use of conventional cigarettes [ 17 ]. Potential benefit of e-cigarette is that it can help us transition our society to little or no combustible tobacco use [ 16 ]. There is also emerging data suggesting that e-cigarettes may facilitate smoking cessation but further research is needed to compare the effectiveness and safety of e-cigarettes compared to other nicotine replacement therapies [ 13 , 14 ]. Consistent with the U.S. FDA regulatory oversight and the U.S. Surgeon General report, it may be prudent to investigate further the health effects of e-cigarettes prior to widespread advocacy favoring its use as a replacement for combustible tobacco use, given that the public health effects of e-cigarettes are yet to be fully understood.
3. Pharmacology of Nicotine
Nicotine (C 10 H 14 N 2 )—see Figure 1 —is a plant alkaloid found in the tobacco plant and is the principal constituent of tobacco responsible for its addictive character [ 18 , 19 ]. Nicotine acts as a ganglionic nicotinic cholinergic agonist in the autonomic ganglia, brain, spinal cord, neuromuscular junctions and adrenal medulla [ 18 , 20 , 21 ]. Nicotine has dose-dependent pharmacological effects and has both stimulant and depressant action [ 20 , 22 ].

The chemical structure of nicotine [ 23 ].
The effects of nicotine on the central nervous system (CNS) and its peripheral stimulating effects are mediated through the release of several neurotransmitters, including acetylcholine, beta-endorphin, dopamine, norepinephrine, serotonin, and adrenocorticotropic hormone (ACTH) [ 18 ]. Notable stimulant effects of nicotine stimulant activities include peripheral vasoconstriction, elevated blood pressure, tachycardia, increased cardiac output, and enhanced mental alertness and cognitive function [ 18 , 20 , 22 ]. Depressant effects of nicotine include muscle relaxation and anxiety reduction [ 20 , 22 ]. At higher doses, nicotine stimulates the “reward” center in the limbic system of the brain [ 20 ].
Nicotine use produces a feeling of pleasure and relaxation [ 20 ]. In dependent smokers, the urge to smoke cigarettes correlates with a low blood nicotine level, as though smoking were a means to achieve certain nicotine level, reap the rewarding feeling associated with nicotine and avoid withdrawals [ 22 ]. Repetitive exposure to nicotine leads to neuroadaptation and building of tolerance to nicotine’s initial effects [ 20 ]. Accumulation of nicotine in the body leads to a more substantial withdrawal reaction if cessation is attempted [ 20 ]. Common withdrawal symptoms include anxiety, difficulty concentrating, irritability, and strong cravings for tobacco [ 20 ]. Onset of these withdrawal symptoms occurs within 24 h and can last for days, weeks, or longer [ 20 ]. Nicotine replacement therapies neither achieve the peak levels seen with cigarettes nor produce the same magnitude of subjective effects of cigarette smoking, they do, however, suppress the symptoms of nicotine withdrawal [ 22 ].
Nicotine from cigarette is carried on inhaled tar particles into the lungs where a large alveolar surface area allows rapid absorption into the pulmonary circulation [ 21 ]. Nicotine is well distributed with a volume of distribution of about 2.6 L/kg [ 21 ]. It undergoes primarily hepatic (80–90%) metabolism—with the remainder of the metabolism taking place in the lungs and kidney—to inactive metabolite: cotinine. Nicotine has a half-life of 1–4 h and about 2–35% is excreted unchanged in the urine [ 21 ].
4. Health Effects of Cigarette (Tobacco) Smoking
Annually, more than 400,000 individuals die prematurely in the United States from cigarette use; this represents almost one of every five deaths in the United States [ 9 ]. Approximately 40% of cigarette smokers will die prematurely due to cigarette smoking unless they are able to quit [ 9 ]. Between 1965 and 2014, over 20 million Americans died either from chronic conditions caused by smoking or exposure to secondhand smoke, complications caused by smoking during pregnancy, or smoking related fires in residential buildings [ 9 ]. Table 1 outlines the common causes of smoking-related deaths between 1965 and 2014 [ 9 ].
Premature deaths caused by smoking and exposure to secondhand smoke, 1965–2014 [ 9 ].
Cigarette smoking affects the human body in myriad ways, causing the development of chronic diseases and cancers. Figure 2 categorizes common health effects of tobacco smoking. The health effects are seen not only in smokers, but also individuals exposed to secondhand smoke. The impact of cigarette smoking on health depends on the duration of smoking over years and the exposure to cigarette (tobacco) smoke. The mechanism by which cigarette (tobacco) smoke causes adverse health outcomes involves multiple complex steps resulting from the exposure to free radicals from the components of tobacco smoke, leading to increased oxidative stress, inflammation, and DNA damage [ 9 ]. The chemical toxins in tobacco smoke are transferred from the lungs to the blood stream, where it is transported to nearly every part of the human body.

Effects of tobacco smoking [ 25 ]. (AA) Aortic aneurysm; (CHD) Coronary heart disease; (PVD) Peripheral Vascular Disease; (COPD) Chronic obstruction pulmonary disease.
4.1. Cancer
Smoking is currently the largest preventable cause of cancer-related deaths, accounting for approximately 30% of cancer related deaths [ 24 ]. Carcinogens in cigarette smoke bind to human DNA, resulting in DNA damage and gene mutations. These genetic changes lead to uncontrolled cell growth and inhibit normal mechanisms that restrain cell growth and spread, resulting in cancer. A causal relationship has been established between cigarette (tobacco) smoking and lung cancer, the leading cause of cancer-related deaths in the U.S. There is also a causal relationship between cigarette smoking and cancers of the head, neck, liver, bladder, cervix, esophagus, colon, and rectum [ 9 ]. The evidence is insufficient to conclude that there is a causal relationship between smoking and cancers of the breast and prostate, however there is an increased risk of dying from cancer in smokers with breast, prostate, and other cancers [ 9 ].
4.2. Cardiovascular Diseases
There is a causal relationship between cigarette smoking and cardiovascular events. Major mechanisms underlying smoking-induced cardiovascular disease include endothelial dysfunction, prothrombotic effects, inflammation, altered lipid metabolism, increased demand for myocardial oxygen and blood, decreased supply of myocardial blood and oxygen, and insulin resistance [ 9 ]. Cigarette smoking and exposure to second hand smoke are major causes of coronary heart disease, stroke, aortic aneurysm, and peripheral arterial disease [ 25 ]. Cigarette smoking and secondhand smoking are also a major cause of death due to CVD. Annually, 194,000 deaths from cardiovascular disease in the U.S. are smoking-related [ 9 ].
4.3. Respiratory Diseases
Cigarette (tobacco) smoking is also associated with the development of chronic pulmonary diseases. In fact, cigarette smoking is the primary cause of COPD in the U.S. [ 26 , 27 ]. Some of the mechanisms involved are loss of cilia in the lungs, mucus gland hyperplasia, and overall inflammation resulting in the abnormal functioning of the lungs as well as injury. Cigarette smoking may exacerbate asthma in adults. Underlying mechanisms may include chronic airway inflammation, impaired mucociliary clearance, increased bronchial hyperresponsiveness, increased development of T helper cell 2 (Th2) pathways relative to Th1 pathways, increased production of IgE, and greater allergic sensitization [ 9 , 25 ]. Smoking also increases the risk of developing tuberculosis and dying from tuberculosis [ 9 ].
4.4. Reproductive Effects
Maternal cigarette (tobacco) smoking causes several reproductive abnormalities. Carbon monoxide in cigarette smoke binds to hemoglobin, depriving the fetus of oxygen, ultimately resulting in low birth weight [ 25 ]. Other toxins in tobacco smoke including nicotine, cadmium, lead, mercury, and polycyclic aromatic hydrocarbons, have been found to cause sudden infant death syndrome, premature births, and decreased fertility in women [ 9 , 24 ]. More recent evidence indicates a causal relationship between maternal cigarette smoking and orofacial clefts and ectopic pregnancies [ 9 ]. A causal relationship between smoking and erectile dysfunction in men has also been established [ 9 ].
4.5. Additional Effects
Smoking impairs immune function, resulting in an increased risk of pulmonary infections and rheumatoid arthritis [ 9 ]. It also affects the gastrointestinal tract, increasing the risk of peptic ulcer disease. There is also increased risk of hip fractures and low bone mineral density in postmenopausal women who smoke. Additionally, smokers with diabetes have a higher risk of developing complications, including nephropathy, blindness, peripheral neuropathy, and amputations [ 25 ]. Recent evidence indicates that the risk of developing type 2 diabetes is 30–40% higher in smokers that nonsmokers [ 9 ]. Passive (second-hand) smoking has also been linked with negative health consequences such as low-birth rate in offspring of mothers exposed to second-hand smoke, sudden infant death syndrome, and type 2 diabetes mellitus [ 28 ].
5. Non-Pharmacologic Treatment of Cigarette (Tobacco) Smoking
About 70% of cigarette smokers visit a physician each year [ 29 ]. Even more smokers visit pharmacists, dentists, nurses, and other healthcare professionals. Clinicians are, therefore, in an excellent position to identify smokers. It is recommended that tobacco use of every patient treated in a healthcare setting be assessed and documented at every visit [ 29 ]. Identifying smokers in the healthcare setting offers a good opportunity for clinicians to recognize and guide effective interventions for smoking cessation [ 30 ]. Patients who begin any major behavioral or lifestyle change go through successive stages of change. To plan an effective intervention, it is important to understand these major stages of change [ 26 ]. Intervention strategies should target the individual’s current stage of change, with an initial objective of moving the individual to the next stage and an overall goal of moving the individual to the maintenance stage. Table 2 below reviews the stages of change.
Stages of behavior change [ 26 ].
A simple five-step algorithm called the 5 A’s (Ask, Advise, Assess, Assist, Arrange) can be used by clinicians to offer a brief counseling intervention in the primary care setting [ 29 ]. The 5 A’s are concisely described in Table 3 . Some of the myriad reasons that patients may be unwilling to quit are as follows. They may be unaware of the harmful effects of tobacco or do not understand the benefits of quitting. They may not have the financial resources to facilitate the smoking cessation process. Also, they may have fears or concerns about quitting, or may be demoralized because of failed quit attempts. Patients in this category or others who are unwilling to quit may respond to brief motivational interventions that are based on principles of Motivational Interviewing (MI) [ 29 ]. The 5 R’s of smoking cessation summarize the areas that should be addressed in Motivational Interviewing (MI). The 5 R’s are described in Table 4 .
The “5A’s” model for treating tobacco use and dependence [ 29 ].
Enhancing motivation to quit tobacco—The “5 R’s” [ 29 ].
Specific non-pharmacologic interventions for smoking cessation can be categorized into three approaches: clinical approaches, public health approaches, and alternative approaches. Clinical approaches to smoking cessation include self-help programs, telephone counseling, cognitive-behavioral approaches such as individual and group counseling, healthcare provider interventions, and exercise programs. Public health approaches include community-level interventions, workplace interventions, multimedia interventions, and public policy changes [ 31 ]. Alternative approaches include acupuncture, aversive therapy, and hypnosis. These various interventions are briefly discussed in Table 5 . It is also important to understand barriers to smoking cessation and effectively address these barriers using motivational intervention technique. A systematic review by Twyman et al. reported common barriers to smoking cessation [ 32 ]. The review found that smoking for stress management, lack of support from health and other service providers, and the high prevalence and acceptability of smoking in vulnerable communities were three consistent barriers to smoking cessation common to six-select vulnerable groups (low socioeconomic status, indigenous, mental illness and substance abuse, homeless; prisoners; and at-risk youth) [ 32 ]. Knowledge of the barriers to smoking cessation and implementation of methods to address these barriers are imperative in helping patients quit smoking.
Non-pharmacologic interventions for smoking cessation [ 31 , 33 , 34 , 35 ].
6. Pharmacologic Treatment of Cigarette (Tobacco) Smoking
All patients who are trying to quit smoking should be offered pharmacologic intervention except when these medications are contraindicated or in certain populations where there is insufficient evidence of effectiveness (e.g., pregnancy, adolescence, light smokers) [ 29 ]. Pharmacologic therapy should be used in addition to behavioral support for smoking cessation [ 36 ]. There are seven FDA approved medications for smoking cessation: transdermal nicotine patch, nicotine gum, nicotine lozenge, nicotine inhaler, nicotine nasal spray, bupropion sustained-release (SR), and varenicline. These medications should be considered first line therapy according to the U.S. Public Health Service guidelines [ 29 ]. First line agents are summarized in Table 6 . Patients who do not respond to any first line medications or who have contraindications to first line agents may be prescribed second line agents. Second line agents include clonidine and nortriptyline. Second line agents are not FDA approved for smoking cessation but have demonstrated some effectiveness in treating tobacco use [ 37 ]. Combination therapy of pharmacologic agents is often used in patients who have failed to achieve cessation with monotherapy. Combination therapy involves adding short acting nicotine replacement therapy (nicotine gum, lozenge, inhaler, or nasal spray) to longer acting agents, such as the nicotine patch or bupropion SR [ 37 ]. Table 7 includes the wholesale acquisition cost of the FDA approved smoking cessation therapy for consideration by providers and patients. Clinicians tasked with selecting appropriate pharmacologic therapy for smoking cessation should consider using the first line agents prior to considering the second line therapy, except when there are contraindications to first line agents or when patients did not respond to first line therapy. Clinicians should also consider other factors such as cost, adverse effect profile, and route of medication delivery. The goal of therapy should be to administer an affordable agent with proven efficacy and good tolerability profile. Selecting a medication formulation that helps patients to achieve medication adherence is also desirable.
Pharmacologic agents for smoking cessation [ 29 , 30 , 37 , 38 ].
Smoking cessation medications and cost [ 54 ].
a Dosage reduction may be needed for hepatic or renal impairment. b Appropriate WAC for 30 days’ treatment at the maximum usual maintenance dosage. WAC = wholesaler acquisition cost, or manufacturer’s published price to wholesalers. WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: Red Book Online ® System (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com/ (cited: 10/10/2016). c Same price for all dosages. d See specific label for instructions for dose titration. e Cost for 28 transdermal patches. f One spray per nostril. Maximum of 40 doses/day should not be used for >3 months. g Cost of four 10-mL bottles. h Cost of 168 10-mg cartridges; each cartridge delivers 4 mg of nicotine. i Not FDA-approved for this indication. j Only the generic 150 mg SR tablets are FDA-approved for this indication. k Initial dosage is 150 mg once/day for 3 days. l Initial dosage is 0.5 mg once/day for 3 days, then bid for 4–7 days.
6.1. Nicotine Replacement Therapy (NRT)
Five nicotine replacement therapy (NRT) products are approved by the U.S. Food and Drug Administration for tobacco dependence treatment: nicotine gum, nicotine lozenge, nicotine nasal spray, nicotine inhaler, and the transdermal nicotine patch [ 38 ]. The nicotine inhaler and nasal spray are prescription drugs in the U.S., whereas the nicotine gum, lozenge and patch are available over the counter. NRTs work to reduce severity and duration of withdrawal symptoms by partially replacing nicotine obtained by tobacco use. A 2008 meta-analysis of 69 clinical trials found that all five nicotine replacement products are superior to placebo, approximately doubling abstinence rates [ 39 ]. A Cochrane Review of 150 trials also found that all forms of nicotine replacement therapy (inhaler, oral tablets/lozenges, gum, patch, and nasal spray) increased rates of quitting smoking by 50–70% [ 40 ]. A study enrolling 504 patients found that all forms of NRT evaluated (gum, patch, nasal spray, and inhaler) produced similar quit rates and were equally effective at reducing the frequency, duration, and severity of urges to smoke [ 41 ]. NRT is generally well tolerated with mild adverse effects. The three most commonly reported adverse effects of NRT in observational studies were headache, nausea and vomiting, and other gastrointestinal symptoms [ 38 , 42 ]. Adverse effects of NRT are generally formulation specific, depending on the delivery system used [ 38 , 42 ]. NRT must be used with caution in patients with known cardiovascular conditions, but have generally found to be safe in patients with these conditions [ 38 , 42 ]. All products are pregnancy category D with the exception of the nicotine gum (category C), although the benefit of replacement therapy may outweigh the risks [ 37 , 43 ].
6.2. Bupropion Sustained-Release (SR)
Bupropion is the first non-nicotine agent to demonstrate efficacy in the treatment of tobacco dependence [ 37 ]. Bupropion sustained-release is FDA approved for smoking cessation and is regarded as a first line therapy by the U.S. Public Health Service guideline [ 29 ]. Bupropion is an inhibitor of dopamine and norepinephrine reuptake, but its mechanism of action in smoking cessation is not well understood [ 37 ]. A systematic review of 44 clinical trials, published in 2014, found that sole therapy with bupropion significantly increased long-term (≥6 months) smoking abstinence (RR = 1.62; 95% CI, 1.49–1.76) [ 44 ]. The most common adverse effects with bupropion, when used for smoking cessation, are insomnia, which occurs in about 30–40% of patients, and dry mouth, which occurs in 10% of patients [ 37 ]. A more serious side effect is seizure, which can occur because bupropion reduces the seizure threshold. Two large studies reported seizure incidence of 0.1% [ 37 ]. Bupropion has a boxed warning for development of neuropsychiatric symptoms ranging from agitation to suicidal ideation and behavior in patients using this medication [ 45 ]. In 2009, the FDA issued an alert to healthcare professionals reporting that cases of neuropsychiatric symptoms have occurred in patients without pre-existing psychiatric illness and have worsened in patients with pre-existing psychiatric illness [ 45 ]. The FDA recommends close monitoring of neuropsychiatric symptoms in patients receiving Bupropion and to stop Bupropion therapy when necessary and to monitor patient closely until neuropsychiatric symptoms resolve [ 45 ]. Bupropion is pregnancy category C and has been shown to be safe and effective in patients with known cardiovascular conditions [ 46 , 47 ].
6.3. Varenicline
This is a first line agent for smoking cessation. Varenicline is a partial agonist specific for the neuronal nicotinic acetylcholine receptor subtype α 4 β 2 . As a partial agonist, it binds to and produces partial stimulation of the nicotinic receptor, thereby reducing the symptoms of nicotine withdrawal [ 37 ]. Varenicline also stimulates dopamine turnover, which provides relief from nicotine cravings and withdrawal symptoms that can occur when a patient is trying to quit [ 37 ]. A 2008 meta-analysis found that varenicline increased the odds of quitting three times than that of placebo (OR 3.1, 95% CI 2.5–3.8) and produced a quit rate of 33 percent at six month follow-up [ 29 ]. In a systematic review of 39 clinical trials comparing nicotine partial agonists, varenicline significantly increased smoking abstinence at 6 months or longer compared to placebo (RR = 2.24; 95% CI, 2.06–2.34) or bupropion (RR = 1.39; 95% CI, 1.25–1.54). [ 48 ] A 2008 meta-analysis also found varenicline to be superior to placebo (OR 2.55; 95% CI, 1.99–3.24) and bupropion (OR 2.18, 95% CI, 1.09–4.08) [ 39 ]. Varenicline is generally well tolerated, with the most common adverse events being nausea, insomnia, and headache [ 37 ]. Varenicline can be used in patients who have concurrent CVD, but with caution. It should be noted that in 2011 the FDA published a warning, based on data from a clinical trial of smokers with CVD which stated that “cardiovascular adverse events were infrequent overall, however, certain events, including heart attack, were reported more frequently in patients treated with Chantix ® (Varenicline) than in patients treated with placebo” [ 49 , 50 ]. Varenicline is pregnancy category C and, like bupropion, carries a black box warning for increased risk of behavior change, agitation, depressed mood, and suicidal ideation and behavior [ 49 , 50 , 51 ].
6.4. Clonidine
Clonidine should be used as a second line agent when primary therapies are found to be ineffective. Clonidine is only FDA approved for hypertension but has shown to be efficacious in smoking cessation. Clonidine is a α 2 -adrenergic agonist, whose effect in smoking is thought to be based on its ability to counteract CNS features of nicotine withdrawal, including craving and anxiety. Results from a Cochrane Review article found that clonidine approximately doubled the rate of abstinence compared to placebo (OR, 1.89; 95% CI, 1.30–2.74) [ 52 ]. Clonidine is limited by its adverse effect profile, which includes postural hypotension, extreme drowsiness, fatigue, and dry mouth [ 37 ].
6.5. Nortriptyline
Nortriptyline should be used as a second line agent when primary therapies are found to be ineffective. Nortriptyline is a tricyclic antidepressant, whose effects in smoking cessation are not well understood. A meta-analysis review of 6 randomized clinical trials indicated that nortriptyline treatment doubles the odds of smoking cessation, with an OR for abstinence of 2.1 (95% CI, 1.5–3.1) [ 53 ]. The most common side effects of nortriptyline are related to its anticholinergic effects, including dry mouth, constipation, and sedation [ 37 ].
7. Conclusions
Although its prevalence has declined in recent years, cigarette smoking remains the most common method of tobacco use. The adverse health effects associated with cigarette smoking are numerous; thus, continual efforts to reduce the prevalence of cigarette smoking are imperative. Current trends on cigarette smoking highlight the importance of smoking prevention and smoking cessation initiatives that target youth. Promotion of smoking cessation can be a strong public health approach for reducing non-smokers’ environmental exposure to environmental tobacco smoke. Treating tobacco dependence should include both behavioral and pharmacologic interventions. First line agents for smoking cessation include bupropion SR, varenicline, and nicotine replacement therapies.
One of the goals of Healthy People 2020 is to “reduce the illness, disability, and death related to tobacco use and secondhand smoke exposure” [ 55 ]. Twenty-one national objectives, related to tobacco use, are outlined to achieve this goal. Recommended strategies for achieving this goal include: increasing the cost of tobacco products; fully funding tobacco control programs; banning smoking in public places; anti-tobacco media campaigns, particularly those targeted towards youth; community, school, and college anti-tobacco programs; encouraging and assisting tobacco users to quit; expanding insurance coverage of smoking cessation agents; and expanding state quit line capacity [ 55 , 56 ].
Acknowledgments
This manuscript was partially supported by the Grant from National Institutes of Health (NIH), Department of Health and Human Services (DHHS); 5 S21 MD 000100-12 from the National Institute on Minority Health and Health Disparities (NIMHD).
Author Contributions
IfeanyiChukwu O. Onor and Daniel L. Stirling conceived the project. IfeanyiChukwu O. Onor, Daniel L. Stirling, Shandrika R. Williams, Daniel Bediako, Amne Borghol, Martha B. Harris, Tiernisha B. Darensburg, and Sharde D. Clay performed literature search and drafted sections of the manuscript. Samuel C. Okpechi and Daniel F. Sarpong contributed to the manuscript draft and provided critical revision of the manuscript. All authors have approved the submitted version.
Conflicts of Interest
The authors declare no conflict of interest.

IMAGES
VIDEO
COMMENTS
While the health effects of cigarette smoking are well recognized and documented, the environmental impacts of tobacco are less appreciated and often overlooked. Here, we evaluate tobacco's global footprint across its entire supply chain, looking at resource needs, waste, and emissions of the full cradle-to-grave life cycle of cigarettes. The cultivation of 32.4 Mt of green tobacco used for ...
Decades after its ill effects on human health were first documented, tobacco smoking remains one of the major global drivers of premature death and disability. In 2017, smoking was responsible for ...
Background and objectives: Despite reductions in prevalence in recent years, tobacco smoking remains one of the main preventable causes of ill-health and premature death worldwide.This paper reviews the extent and nature of harms caused by smoking, the benefits of stopping, patterns of smoking, psychological, pharmacological and social factors that contribute to uptake and maintenance of ...
Abstract. This paper examines the immediate and long-term effects of public smoking bans on smoking prevalence, smoking regularity, smoking intensity, and secondhand tobacco smoke exposure. We supplement the extensive literature on the effects of various types of tobacco control legislation on smoking behavior in developed countries by studying ...
Exposure to environmental tobacco smoke (ETS) or second-hand smoke (SHS) significantly contributes to children's morbidity and mortality. In 2004, ... Fifty-five percent of the research papers were observational (cohort or cross-sectional). This finding could be attributed to the relatively lower resource requirements and costs associated ...
3.1 Tobacco smoke 20 3.2 Third-hand smoke pollution 22 4 Post-consumer waste 24 4.1 Reducing harm caused by tobacco product waste 24 ... The overview highlights the current lack of scientific research into the environmental impact of tobacco, including the health and economic consequences that result from the cultivation, production ...
Aim To investigate the association between smoking, environmental tobacco smoke (ETS), and lung cancer risk. Methods This case-control study included 1622 newly diagnosed cases of lung cancer and 1622 healthy frequency-, age-, and gender-matched control participants. Epidemiological data were collected by in-person interviews using a standard questionnaire. Results Smoking was a risk factor ...
Environmental tobacco smoke (ETS) and its sequelae are among the largest economic and healthcare burdens in the United States and worldwide. The relationship between active smoking and ...
female smoking rates are 68% and 3.2%, respectively, while thegeneralsmoking-quittingrateis<10%(Chenetal.2015); therefore, the smoking control status does not appear promis-ing.In particular, the indoor smoking rate in China is approx-imately 70%. Nearly 740,000,000 people are exposed to en-vironmental tobacco smoke (ETS), which is one of the ...
Environmental tobacco smoke (ETS) and its sequelae are among the largest economic and healthcare burdens in the United States and worldwide. The relationship between active smoking and atherosclerosis is well-described in the literature. However, the specific mechanisms by which ETS influences atherosclerosis are incompletely understood. In this paper, we highlight the definition and chemical ...
We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer ...
Revision Date December 2011. Public-place smoking restrictions are the most important non-price tobacco control measures worldwide, yet surprisingly little is known about their effects on exposure to environmental tobacco smoke (ETS). We study these laws in Canada using data with questions about respondents' ETS exposure in public and private ...
Abstract. Objectives: To examine (1) whether dust and surfaces in households of smokers are contaminated with environmental tobacco smoke (ETS); (2) whether smoking parents can protect their infants by smoking outside and away from the infant; and (3) whether contaminated dust, surfaces, and air contribute to ETS exposure in infants. Design: Quasi-experiment comparing three types of households ...
Abstract. Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will ...
From the first issue of Indoor and Built Environment in 1992 through to February, 2004, 484 papers were published (table).Of these, 66 (14%) had either a minor or principal focus on environmental tobacco smoke. Of these, 17 (26%) concluded that this smoke is hazardous in some way; 32 (48%) concluded that environmental tobacco smoke is not a significant hazard; eight (12%) concluded that more ...
Involuntary exposure to environmental tobacco smoke (ETS), or "passive smoking," has been extensively investigated with respect to its potential health effects, particularly on respiratory health. There is a significant body of research on its potential effects regarding the incidence, prevalence, and exacerbation of established asthma. While attention has focused upon possible ...
Background Exposure to environmental tobacco smoke (ETS) is arguably the most ubiquitous and hazardous, even at very low levels, starting in early life. The objective of this study was to describe the state of research and future trends on ETS exposure and Children's Health (CH) topics with bibliometrics and altmetrics. Methods An electronic search was performed in Scopus database on January ...
Secondhand smoke is a significant public health concern and driver of smoke-free policies. Also called passive or secondary smoke, secondhand smoke increases the risk for many diseases. 55 Exposure to environmental tobacco smoke among nonsmokers increases lung cancer risk by about 20 percent. 48 Secondhand smoke is estimated to cause approximately 53,800 deaths annually in the United States ...
DOI: 10.1164/ajrccm-conference.2024.209.1_meetingabstracts.a2887 Corpus ID: 269534875; Cotinine Correlates With Sleep Parameters in Children Exposed to Environmental Tobacco Smoke @article{Arwas2024CotinineCW, title={Cotinine Correlates With Sleep Parameters in Children Exposed to Environmental Tobacco Smoke}, author={Noga Arwas and I. Etzion and S. Greenberg-Dotan and A. Tarasiuk and A ...
environmental tobacco smoke described it as one among other indoor air contaminants, with at least a brief discussion on it. Papers with a principal focus on environmental tobacco smoke examined the issue in detail as a primary objective of the review or study. We excluded papers with no focus on environmental tobacco smoke from our review.
Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer. ... and to help identify the mechanisms through which environmental agents, such as cigarette ...
We conducted a meta-analysis of over 30 genome wide association studies (GWAS) in over 1.2 million participants with European ancestry on nicotine and substance use. Specifically, we targeted different stages and kinds of substance use from initiation (smoking initiation and age of regular smoking initiation) to regular use (drinks per week and cigarettes per day) to cessation (smoking cessation).
Background Childhood, adolescent and young adult (CAYA) cancer survivors are vulnerable to adverse late-effects. For CAYA cancer survivors, tobacco smoking is the most important preventable cause of ill-health and early death. Yet, effective strategies to support smoking cessation in this group are lacking. The PRISM study aims to undertake multi-method formative research to explore the need ...
In the late 1980s, the international tobacco industry assisted in the establishment of the International Society of the Built Environment, which published the journal Indoor and Built Environment. Using evidence from tobacco industry documents, we examine the industry associations of the Society's executive, the journal's editor and board, and the extent to which the journal publishes papers ...
Environmental tobacco smoke (ETS) occurs in homes, at workplaces, and in public places. The acute irritating and noxious effects of involuntary exposure to ETS, or ''passive smoking,'' are well established. Based in part on these irritating properties of ETS, a recent report of the NRC recommended a ban on smoking in the small enclosed spaces of airliner cabins (National Research Council, 1986 ...
Jonah E. Bromwich, who covers criminal justice in New York for The New York Times. Stormy Daniels leaving court on Thursday, after a second day of cross-examination in the Manhattan criminal trial ...
E-cigarette use also contributes to environmental tobacco smoke and may mimic the effects of passive (second-hand) smoking seen with use of ... There is also emerging data suggesting that e-cigarettes may facilitate smoking cessation but further research is needed to compare the effectiveness and safety of e-cigarettes compared to other ...