
Our research groups
Publications
Study counselling
Administration and service

Medical Cell Biology
Our research areas: Physiology and cell biology with a focus on diabetes, inflammation, cancer, pharmacology and neurobiology.
- Daniel Espes is one of this year's winners of the Göran Gustafsson Prize in Medicine
- Sleep deprivation and circadian rhythm affect public health
- New methods in vascular surgery save lives
- Strengthened cooperation between Japan and Sweden
- The Royal Society of Sciences Thuréus prize awarded to Sebastian Barg
- PhD student
- PhD student in chemokine immunobiology
Recent research papers from MCB
Whole-genome selective sweep analyses identifies the region and candidate gene associated with white earlobe color in Mediterranean chickens
The role of hyaluronan and its CD44 receptor in inflammation and cancer
Surviving COVID-19: patients' experiences of care and path to recovery
Use of cookies
Uppsala University uses cookies to make your website experience as good as possible.
Read more in our cookie policy.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ups J Med Sci
- v.124(1); 2019 Jan

Uppsala Clinical Research Center—development of a platform to promote national and international clinical science
Lars wallentin.
Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden
Bertil Lindahl
Uppsala Clinical Research Center (UCR) is a non-profit organization that provides service for clinical research aiming for development and improvement of health care in Sweden and worldwide. UCR was started in 2001 with the ambition to shift the focus of clinical research from new medications or devices launched by the industry to problem-based research on issues identified in clinical reality, for example through the national quality registries. In order to accomplish these goals, UCR has established services in: 1) clinical trials of new and old methods in health care; 2) quality development of the health care system supported by internet-based national quality registries; 3) biostatistics, epidemiology, and data management; 4) biobanking of biological materials (Uppsala Biobank); 5) high-throughput biochemical analyses (UCR laboratory); and 6) academic leadership by the members of the UCR research faculty. The UCR clinical trials group provides services for investigator-driven projects in all areas of health care, for global mega-trials on new pharmaceutical treatments and devices, for biobanking including biomarker and genetics analyses, and for clinical events adjudication in national as well as global mega-trials. During the last few years, UCR has been a pioneer in establishing the registry-based randomized clinical trial (R-RCT), which today is an international model on how to perform cost-effective pragmatic randomized trials in the real-world environment. In 2002, UCR started the first national competence center for national quality registries, which pioneered the development of the current internet-based technologies for registering, reporting, and supporting continuous systematic improvement of health care. UCR is currently harboring around 20 national quality registries in all areas of health care. Today, UCR is the leading European center for registry-based quality development and evaluation of new medical treatments in cardiovascular care and has started to support other European countries in implementing the UCR registry platform in order to improve quality of care in the European Union.
Introduction
Uppsala Clinical Research Center (UCR) is a non-profit organization that supports research and quality development in health care in Sweden and internationally. The center was founded on 1 July 2001, based on a proposal from the authors of this manuscript. The reasons for the creation of UCR were the needs to support the nationally and internationally successful clinical research groups on cardiovascular, metabolic, cancer, and infectious diseases, and the close collaboration of these research groups with the advanced clinical and basic science laboratories in Uppsala ( 1–4 ). There was also an urgent need to support the national quality registries in cardiovascular care, which at this time pioneered the utilization of internet-based technologies as well as quality development and research in this area ( 5–7 ). At this time, clinical research in Sweden was in a period of crisis because of low status for medical science and medical faculties, few opportunities for systematic training and education, few career positions, and decreasing grant funding (Den kliniska forskningens kris och pris, MFR-rapport 5; 1998 (ISBN:91-85547-12-3)). Clinical research projects were often limited to either being a minor contributor in industry-related international mega-trials or performing local, underpowered, underfunded research projects with small chances to produce results with international impact.
UCR was started with the ambition to shift the focus of clinical research from new medications or devices launched by the industry to problem-based research on issues identified in clinical reality, for example through the national quality registries. The objectives of clinical research needed to be renewed by updating the knowledge on occurrence and natural courses of disease, utilization of old and new treatments, and their effects in real-life health care. Both old and new methods for diagnosis, prognostication, decision support, and treatment should be continuously evaluated by scientific methods. A similar systematic scrutiny should be applied for interventional treatments with surgery, endoscopic, and catheter-based treatments as for new medications. The ambition of UCR was to support the development of a systematic learning process, which should also facilitate a better controlled introduction of new methods and elimination of old ones in the health care system ( Figure 1 ). In addition, a modern scientific approach was also needed to support the inclusion of issues on quality of life, health economy, and prioritization in all clinical research projects ( Table I ).

Evidence-based health care development. UCR’s main objective is to provide services for clinical research aiming for development and improvement of health care based on UCR’s combination of systematic monitoring and continuous evaluation of health care performances and outcomes in registers together with performance of prospective randomized clinical trials of new treatments and finally support of implementation by quality development programs; this has led to the new concept ‘evidence-based health care development’.
Planned services at the foundation of UCR.
From the start, UCR has had the same overriding main objective, which is to provide service for clinical research aiming to develop and improve health care ( Figure 1 ). UCR has had its main activities in six areas ( Figure 2 ). The main reason for the development of UCR was the universally recognized need for improved services in (i) clinical trials of new and old methods in health care. However, the first leaders of UCR also took the, at that time rather unique, initiative to include also (ii) quality development of the health care system supported by internet-based national quality registries as an equally important part of the services. As for all other research services (iii) biostatistics, epidemiology, and data management were also important components of the initial organization. Over the years, the UCR organization has also integrated services for (iv) biobanking of biological materials (Uppsala Biobank) and (v) high-throughput biochemical analyses in plasma samples from large international clinical trials and observational materials (UCR laboratory). Since the start of UCR both the whole organization and most individual projects have been initiated and led by members of the (vi) UCR research faculty.

UCR organization 2013.
As cardiologists started UCR, the three UCR directors as well as most faculty members have been senior researchers in the cardiovascular area. The faculty has gradually been growing to include researchers at all levels from senior professors to research students whose presence at UCR enriches the discussions and acts both as a solid foundation for ongoing projects and as a spearhead for future innovative activities. The UCR faculty group is also the hub for the extensive networking with other national and international researchers and research centers who are often the key contacts for new projects. Over the years, the activities at UCR have gradually expanded and included researchers also in other areas, for example cancer, neurology and care of the elderly, diabetes, nutrition, psychiatry, infection, radiology, and pulmonary, gastrointestinal, and orthopedic diseases.
Clinical trials
In order to perform academic clinical trials, it is today almost a necessity to have support from a clinical research center. Very few clinical trialists can keep the competence needed to perform larger clinical trials within their own organization. Without this support, today’s complicated regulation around clinical trials will form an almost insurmountable obstacle even for the startup of any investigator-initiated trial. In order for a clinical trials center to manage planning, performance, analysis, and reporting of larger multi-center clinical trials, there is a need for a critical mass of personnel and technical resources within several different areas. Therefore, the support for clinical trials concerning new or old methods of diagnosis and treatment is a key part of the development of UCR.
The UCR clinical trials group started with two project managers and three research nurses and has over the years grown to a considerably larger group of around 50 persons. Today, the group provides complete services including study design, protocols, patient information, negotiations, agreements, contracts, applications to authorities, recruitment of countries, centers and investigators, case report form (CRF) and internet-based remote data entry, monitoring and quality assurance, planning and management of biological samples including transportation and storing in the UCR biobank, biochemical and genetic analyses, project coordination, logistics, administration and archiving, consultation, and education. In addition, during the first 10 years UCR was running the UCR clinic, which performed and supported clinical trials in healthy volunteers and outpatients.
The primary purpose for the clinical trials group is to support physicians and other investigators, especially in investigator-initiated and registry-based clinical trials. The same services are also provided for clinical trials in collaboration with external sponsors. The group has been working on investigator-initiated projects within many different areas: cardiovascular disease, internal medicine, obstetrics, rheumatology, renal disease, physiology, odontology, nutrition, cancer, ear–nose–throat diseases, infectious diseases, psychology, and quality development. The projects concern research on pharmaceutical agents, functional foods, medical implants and devices, quality development methods, and national quality registries. They have been, and are, performed in collaboration with governmental departments, Swedish Board of Health and Welfare, Swedish Federation of County Councils, Swedish heart–lung foundations, Swedish Cancer Foundation, Center for Clinical Trials of Foods, and pharmaceutical and medical device companies.
The international breakthrough for the clinical trials group was being one of the leading centers in the development and performance of four global phase III mega-trials in the cardiovascular area, each including 15,000 to 19,000 patients: the RELY ( 8 ), PLATO ( 9 ), ARISTOTLE ( 10 ), and STABILITY ( 11 ) trials which were started between 2005 and 2007. Through these trials, UCR has become an appreciated member of a global network of academic research organizations, VIGOUR, which is continuously taking on new assignments in international clinical trials and has its center at Duke Clinical Research Institute (DCRI) in Durham, NC, USA ( 12 ). A second breakthrough by these trials was the establishment of the UCR laboratory in 2008, which was a necessity as UCR researchers took the initiative and the responsibility for an extensive biomarker and genetic substudy program with analyses of blood samples from all patients in these mega-trials ( 13–18 ). In addition, the large repository of samples from these trials substantially contributed to the third breakthrough, the establishment of Uppsala Biobank in 2008, which was later integrated with the UCR organization. A fourth breakthrough by participation in these mega-trials was the need to set up a center for clinical events adjudication (CEC) ( 19 ). This CEC organization has thereafter rapidly expanded and become one of the world-leading centers for CEC, with assignments for these services in many international trials. Finally, it should be emphasized that a less recognized fifth breakthrough occurred during the first years of UCR clinical trials services, which was the initiation and performance of the very first registry-based randomized clinical trials (R-RCT). These first trials were based on cluster randomization of hospitals to registry-based quality development or usual care and nicely showed the superior results with registry-based care ( 20 , 21 ). During the last few years the concept of R-RCT has been the largest international breakthrough of UCR services which is further discussed in other papers ( 22 , 23 ).
Quality registries
Since its start in 2002, the first national competence center for national quality registries—the Swedish Cardiovascular Registries—constitutes a large part of Uppsala Clinical Research Center. The basis for this activity is the development and maintenance of an advanced internet-based technology for registration, reporting, and support for systematic improvement of health care processes. The focus of the work in the registry group is to maintain and further develop the concept of internet-based interactive and intuitive data entry systems, preferably integrated with electronic patient records, and to further improve the online information with interactive as well as imperative illustrative analyses. The purpose is also to promote all centers to be involved in a continuous quality improvement process supported by internet-based tools for ‘evidenced-based health care development’. The specific tasks are accordingly to develop, maintain, and improve the national quality registries within cardiovascular diseases; to expand the analysis and reporting from these registries; and to provide support to other registries ( 6 ).
The competence center today has the responsibility for around 20 national quality registries, 10 of which are in the cardiovascular area and around 10 in other areas. Today, the UCR registry activities seem to cover all health care areas in life from before birth ( in-vitro fertilization registry) to very old age (Senior Alert). The internet-based registries usually include mainly hospital-based clinics in the around 70 hospitals in Sweden. However, the Senior Alert registry accepts data from as many as 40,000 different care units all over the country. The UCR quality registry group started with as few as three software developers but rapidly grew to 10 system developers in 2006 and further increased to around 40 people in 2018. In addition, the group also contains several data managers and statisticians for the integrated statistical and scientific analysis.
The registry group collaborates closely with a large number of physicians and research nurses, project leaders, and registry coordinators over the whole country and even internationally. The group provides the following services: national and international internet-based databases and registries; electronic computer forms and remote online data entry; imports from and transfer to external data sources; interactive, simple, and advanced statistical analysis; online reports with tabular and graphical presentations; education, support, and monitoring; data security; maintenance and support; quality development and projects supported by consultant and internet-based education; and finally internet portals for information, communication, collaboration, and education. UCR’s IT-based registration technology is also used in international projects and in other countries for evaluation of the effects of new treatments in health care. Therefore, UCR is today established as the leading European center for registry-based quality development and evaluation of new medical treatments in cardiovascular care. In addition, the registry technology has over the last years been developed to integrate tools for registry-based randomized clinical trials (R-RCT). The international interest for taking part in this development has currently placed UCR as one of the world leaders in technologies for cost-effective pragmatic randomized clinical trials within the usual health care system ( 6 , 24 ).
Biostatistics
Biostatistics and epidemiology, supported by data management, are key resources in all medical research. At the beginning, UCR biostatistics group consisted of three statisticians and two data managers. After five years, the group had grown to contain eight biostatisticians, two epidemiologists, and three data managers. Today the group has once more doubled in size and contains several statisticians at the PhD level and specialists in bioinformatics, genetics, and big data analysis. Currently, the group is one of the largest and most experienced in Sweden for management and analysis of quantitative biological data.
The group provides services in planning, recording, management, analysis, and interpretation of data in all areas of clinical research and also develops new statistical models and innovative online tools for interactive statistical analyses. The activities started mainly with services for cardiovascular research but the group now has experience from collaboration in many different areas. The group is now responsible for basic courses of biostatistics in a biomedical program at Uppsala University. Several of the statisticians and epidemiologists are running their own research programs and are acting as tutors for research students. The epidemiology group has recently grown to establish its own organization as an Epidemiological Center within Uppsala University.
Administration
During the early years, UCR had a very limited single-person administration, handling personnel, finance, localities, IT services, and general services. However, over the years also this group has had to grow in order to handle the increasing demands from an expanding organization, including IT solutions and IT security, quality assurance, budget planning, accounting, contracts, etc. In addition, new services have been added including a communications officer and publication services. Finally, after a few years it became apparent that an internal quality system was a necessity to provide a solid framework for the continuous work with standardized operational procedures (SOPs) and all other quality routines.
Although starting on a small and improvised scale in the hands of a few dedicated people, all main activities at UCR became over the years well-established and were shown to work with high quality, able to cope with the increasing service demand. Based on a combination of assignments from research groups, research foundations, hospitals, county councils, National Board of Health and Welfare, and pharmaceutical, device, and diagnostic companies, the UCR finances have gradually been growing and during most years kept in a good balance.
Organization
UCR was thus founded as, and still is, a collaborative project between the Faculty of Medicine at Uppsala University and Uppsala University Hospital within Uppsala County Council. From 2007, UCR has been organized as an independent unit reporting directly to the dean of the Faculty of Medicine and the director of Uppsala University Hospital. The dean of the Faculty of Medicine and the director of Uppsala University Hospital appoint the director of UCR for three years. The board of UCR consists of five voting members (three from Uppsala University, two from Uppsala County Council/Uppsala University Hospital) and the UCR director. A scientific advisory committee has for many years supported the UCR director concerning the strategic planning. The UCR director is further supported by an internal steering group, which has regular meetings concerning planning and performance of the daily operations.
The number of employees at UCR has gradually increased in association with an increasing demand for services. UCR started its activities in 2001 with only around 10–15 employees, in 2006 reaching 40–45 full- or part-time employees, and in 2012 around 100. The UCR finances are managed as a combination of department-based finances within the Uppsala University administration and project-based finances within the Uppsala University Hospital administration. The personnel can thereby be employed either by the University or by the University Hospital. The director of UCR is responsible for the finances of all projects within both administrations. All research groups collaborating with UCR have full responsibility for their own finances. Before the start of collaborative projects, the sharing of costs between the respective group and UCR is established in separate contracts. This organization is ideally suited for its purpose to perform cost-effective clinical research and development by integrating the available resources both in the laboratory-based medical research in the University and in the clinical medicine departments in the hospital environment, with direct access to patients, patient records, registries, and hospital-based resources for clinical investigations and care.
After almost 20 years of development, UCR has now established a stable infrastructure for support of continuous quality development, clinical trials, and translational research in all areas of health care. Currently, UCR is world-leading in the areas of internet-based interactive registry-supported quality development and registry-based randomized clinical trials (R-RCT). UCR has recently been assigned the task to try to implement the SWEDEHEART registry and clinical trials platform in as many European countries as possible. In addition, UCR is one of the world-leading centers for all types of services in conventional randomized clinical trials. In this area, UCR has pioneered international collaboration on biobanking and omics research in order to make personalized and precision medicine an integral part of global collaborative trials. At present, UCR is therefore well positioned to play a gradually expanding role in the national and international development and improvement of health care.
Biographies
Lars Wallentin , senior professor of cardiology, founder and former director of Uppsala Clinical Research Center and the Swedish Cardiovascular Quality Registries; currently senior researcher with focus on prospective clinical trials, registry-based outcome research and biomarker research in international biobanks.
Bertil Lindahl , professor of cardiology, former director of Uppsala Clinical Research Center; currently leading registry-based randomized clinical trials, performing experimental and clinical biomarker studies and outcome research in registries and other cohorts.
Declaration of interest
The authors report no conflicts of interest.
- MedTech / IVD
- Pharma / Biotech
- Marketing Compliance
- Clinical Operations
- Clinical Project Management
- Medical Advice
- Medical Writing
- Data Management
- Biostatistics
- Safety & Pharmacovigilance
- Market Access & RWE
- Quality Assurance
- Pre-clinical Stage & CMC
- Phase I / First in Human
- Phase II-III
- Phase IV & RWE Registries
- Clinical Investigations & Diagnostics
- Post Marketing Services
- IVDD to IVDR Transition
- Decentralized Trials
- Safety Database
- Strategic Advice Group
- Start Up Group
- Outsourcing
- Nordic Benefits
- Therapeutic Experience
- Geographic reach
- Customer Testimonials & Case Studies
Get in touch with us
Please contact us for support on your product development and resourcing needs. One of our experts will get back to you shortly.
Lena Lindeberg
Marketing & Commercial Contact via email
Michael Hakenäs
Business Development Contact via email
Our offices
Uppsala LINK Medical Research AB Slottsgränd 2A SE-753 09 Uppsala
Solna Telegrafgatan 4 SE-169 72 Solna
Malmö Jörgen Kocksgatan 4 SE-211 20 Malmö +46 18 430 31 00
LINK Medical Research AS Drammensveien 288, NO-0283 Oslo, Norway
IRW-Consulting AB, FILIAL I Finland Rajatorpantie 41 C, FI-01640 Vantaa, Finland
LINK Medical ApS Vimmelskaftet 47. 1. Sal 1161 København K +45 31 12 20 18
United Kingdom
LINK Medical Research Ltd 10 John Street London, WC1N 2EB United Kingdom
LINK Medical GmbH Steinmetzstr. 36A 10783 Berlin, Germany +49 30 2461 2025
Don't miss out on the latest updates
Be the first to hear our breaking news at LINK Medical
Get in touch
How can we help you.
Contact us and we will tell you more
Search the site
Search on LINK Medical
Cookie consent
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
- Study Details
- Tabular View
- No Results Posted

Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services

Juniors selected for Texas Medical Center summer research internship
Twelve Augustana College juniors have been selected for the prestigious Texas Medical Center Summer Research Internship Program.
“With placements in medical illustration, health care economics, public health, integrated ethics as well as in more traditional areas of biology, biochemistry, oncology and infectious disease, interns will have the opportunity to explore their chosen areas of interest and build lasting relationships." Dr. Heidi Storl
Philosophy Professor Dr. Heidi Storl said participation in this internship celebrates the achievements of selected students and launches their class-based skills into the world of topnotch labs, clinics, hospitals and related departments.
“With placements in medical illustration, health care economics, public health, integrated ethics as well as in more traditional areas of biology, biochemistry, oncology and infectious disease, interns will have the opportunity to explore their chosen areas of interest and build lasting relationships,” Dr. Storl said.
The following students will take part in the paid, full-time, 10-week experience at the Texas Medical Center in Houston this summer.
- Erik Bergren, an economics and public health double major from Geneseo, Ill.
- Morgan Buchek, a pre-medicine and biochemistry double major from Arlington Heights, Ill.
- Caitlyn Collier, a biology major from Fairview, Ill.
- Clay DeBaillie, a pre-medicine and chemistry double major from Geneso, Ill.
- Yanet Gezu, a public health, business administration-business intelligence, and business analytics triple major from Addis Ababa, Ethiopia
- Christina Harvey, a communication sciences and disorders and psychology double major from Wheaton, Ill.
- Rachel Jocson, a psychology and Spanish double major from Rockford, Ill.
- Abigail Jones, a communication sciences and disorders major from Rolling Meadows, Ill.
- Heather Michalski, an art and biology double major from Batavia, Ill.
- Kira Nelson, a biology and neuroscience double major from Milan, Ill.
- Charlotte Newport, a history major from Baytown, Texas
- Sam Vasich, a psychology and sociology and anthropology double major from Lisle, Ill.
The Texas Medical Center serves 10 million patients per year and performs more than 180,000 surgeries annually. It is home to the world's largest children's hospital and largest cancer hospital, and it is the eighth largest business district in the United States.
Internships are available to Augustana students in all majors. Those selected for the opportunity can establish one-on-one mentoring relationships in a variety of areas, including clinical or research medicine, any area of allied health, psychology, neuroscience or veterinary research. Additional areas of focus include human resources, pastoral care, law, finance, health care policy and clinical ethics.
Previous Augustana students have been placed at the University of Texas MD Anderson Cancer Center, one of the world's leading treatment and research centers; Texas Children's Hospital, an international leader in pediatric medicine; as well as Baylor College of Medicine, Rice University, and the University of Texas Health Science Center's prestigious Center for Laboratory Animal Medicine and Care.
Interns are selected for their curiosity, critical thinking, problem-solving and authentic self-reflection. These traits are fine-tuned in a series of spring, summer and fall seminars on campus, with an emphasis on community building, vocational reflection and ethics.
If you have news, send it to [email protected] ! We love hearing about the achievements of our alumni, students and faculty.

Admission office: +7(910)737-2741 [email protected]
Research Institutes & Labs
MBBS in Russia | MBBS in Moscow | Study in Russia | Study in Moscow | Education in Russia | Education in Moscow | I.M. Sechenov First Moscow State Medical University | FMSMU | MBBS Admission in I.M. Sechenov First Moscow State Medical University | MBBS Fee in I.M. Sechenov First Moscow State Medical University | Direct Admission in I.M. Sechenov First Moscow State Medical University | Apply for MBBS Admission in I.M. Sechenov First Moscow State Medical University | How to Get Direct Admission in MBBS in I.M. Sechenov First Moscow State Medical University | Study in I.M. Sechenov First Moscow State Medical University | About I.M. Sechenov First Moscow State Medical University | Tuition Fee in I.M. Sechenov First Moscow State Medical University | Research Institutes & Labs in I.M. Sechenov First Moscow State Medical University
Research Institutes & Labs
The scientific core of I.M. Sechenov First MSMU is its Research Center (RC) which combines all the scientific departments of the University. RC subdivisions are 7 research institutes (RI), over 30 laboratories and research departments, the Coordinating Research Council, and the Coordinating Department of Young Scientists.
Research Institutes of I.M. Sechenov First MSMU: RI of Medical Parasitology and Tropical Medicine RI of Public Health and Healthcare Management RI of Medical Sociology, Healthcare Economics and Medical Insurance RI of Phthisiopulmonology RI of Molecular Medicine RI of Pharmacy RI of Uronephrology and Reproductive Health
A number of chairs of I.M. Sechenov First MSMU conduct their research at the Research Centers of the Russian Academy of Medical Sciences:
A.N. Bakulev Research Center of Cardiovascular Surgery of the RAMS N.N. Blokhin Russian Research Center of Oncology of the RAMS B.V. Petrovskiy Russian Research Center of Surgery of the RAMS Research Institute of Eye Diseases of the RAMS Research Center of Endocrinology of the RAMS
——————————————————————————————————————————————
RI of Medical Parasitology and Tropical Medicine
RI of Public Health and Healthcare Management
The Scientific-Research Institute of Public Health and Health Management was established in 2002.
The Institute is composed of the managerial board and 8 scientific compounds (4 divisions and 4 independent laboratories). The Institute’s personnel consists of 82 academic staff, including one member from the Academy of Medical Sciences, one correspondent, 16 doctors and 24 candidates of sciences.
Main goals:
- Development of modern technology
- Training of specialists with higher medical and pharmaceutical education, based on the achievements of biomedical research.
Main areas of research are:
- The policy and strategy for the formation of public healthcare
- The development and improvement of technologies for shaping public healthcare
- Strategy for healthcare Management
- Personnel Policy in healthcare
- Economic and resource provision of healthcare
- Standardization in healthcare
- Information technology in healthcare management
- Legal protection of public health and healthcare management
- International healthcare
Departments :
- Department of Medical prevention and health promotion
- Department of Public Health Policy
- Department for problems of public health
- Department of Standardization in Health Care
- Department of Health Management Strategies
- Laboratory of the development, implementation and certification of information technology
- Laboratory of labor efficiency in health care
- Laboratory of coordination of inter-institutional research on public health and health care management
- Laboratory of legislation in health care
- Department of Problems of health information
- Department of socially significant diseases
- Laboratory of clinical and laboratory diagnosis
RI of Medical Sociology, Healthcare Economics and Medical Insurance
Established in 2011 the Institute of Sociology of medicine, health economics and health insurance consists of the managerial board and 6 scientific divisions: 5 departments (sociology of health and illness, sociology of health care management, historical and sociological analysis of medicine, health economics, management, economics and sociology of health insurance) and a laboratory of medical and sociological monitoring.
The Institute is headed by Professor A.V.Reshetnikov academician of the Academy of Medical Sciences, Dr. of Medical Sciences, Dr. of Sociological Sciences, honored worker of health.
Main objectives:
- Coordination of research activities
- Improvement of the quality and quantity of medical and sociological and socio-economic research on fundamentally new directions in the sociology of medicine, health economics and health insurance.
- Training of health managers in areas of: sociology of medicine and health economics.
Main areas of research:
- Generating new knowledge about the basic laws governing the functioning and development of medicine as a social institution,
- The formation of valuable relationships among different segments of the population with healthcare institutions, illnesses and health care, medicine and public health organization,
- Development and implementation of social and economic measures to further improve health and health care organizations.
The research activities of the Institute are based on the principles of: unity and active interaction between the research and educational activities.
—————————————————————————————————————————————–
The RI of Phthisiopulmonology was established in 1918 as the Moscow Institute of Tuberculosis. In 1998 it became a part of I.M. Sechenov First MSMU.
Nowadays the Institute is headed by Sergey V. Smerdin, M.D., honorable physician of the Russian Federation, and comprises board of directors, 7 departments (of epidemiology and TB care management; of TB monitoring; medical; of pediatric and adolescent tuberculosis; of laboratory diagnostics in phthisiology; surgical; of diagnostic study in phthisiology), 17 laboratories, and a vivarium.
Main goal is to stabilize and improve TB epidemiologic situation through developing and implementing new technologies in medical care management, tuberculosis prevention in various risk groups, treatment and rehabilitation of patients with different locations of tuberculosis.
Fundamental research is aimed to – obtain new knowledge in phthisiology; – development new technologies and means of prevention, diagnostics and treatment of tuberculosis; – solve scientific medical issues of public health and healthcare management.
Research activities are based upon – principles of unity and active cooperation between research, education and healthcare; – concentration of efforts and resources on priority directions in phthisiology; – support of the leading scientists and research groups able to provide an outrunning level of research in phthisiology; – translation of results to consumers via publishing and the Internet; – orientation of research groups towards full cycle research and inventions resulting in a product; – integration into the Russian and the international scientific community.
Calendar of events with international participation
RI of Molecular Medicine
The Research Institute of Molecular Medicine was founded in 2000 as a division of the First MGMU in the light of the tremendous scientific and technical progress at the end of the second millennium. The emergence of new technologies in biology took medical science to the level of studying intermolecular interactions. There was a need for specialized academic medical institution which would be engaged in a study of the causes and mechanisms of human diseases, the development of adequate methods of diagnosis, treatment and prevention of diseases carried out at the molecular and cellular levels.
The institute specializes on: clinical laboratory diagnostics, laboratory genetics, management and economics in the field of pharmaceutics and others. Its also engaged in fundamental biomedical research.
- Development of approaches for creating drugs of a new generation,
- Development of methods of early detection and diagnostic tools, and their pre-clinical investigations and referrals for clinical trials on the basis of modern achievements in molecular biology and genetic engineering.
Professionals working in these areas should combine a natural approach and high level of engineering thinking. It is essential that such an institution has links with the international scientific community to ensure the integration of Russian science in the global research process.
The Institute is composed of the managerial board and 9 research units: 2 divisions and 7 laboratories.
The basic principle of the institute in regards to the personnel policy is – continuous improvement of professional skills and increase of the scientific level of personnel. All employees have a degree in either: medicine, biology, pharmacology or chemistry and most are Ph.D. candidates.
Laboratories of the RI of Molecular Medicine:
- Laboratory of biotechnologies
- Laboratory of genetic engineering
- Laboratory of Molecular Biology and Biochemistry
- Laboratory of human molecular genetics
- Laboratory of Chemical Analysis
- Laboratory of biologically active compounds
- Laboratory for the study of reparative processes in skin tissue
Research Institute of Pharmacy
The Research Institute of pharmacy was established in 2001, it carries out its activities in cooperation with the institutions, faculties and departments of the University and its clinics, performs with them collaborative research, and participates in the educational process at the University.
- Development of scientific foundations for organizing the functioning of pharmaceutical companies.
- Development of the basic principles of pharmaceutical research in establishing a manufacture of medicines.
- Research to develop innovative technologies for medicine production.
- Development of requirements for the creation and standardization of medical drugs based on nanotechnology.
- Research on the standardization of general pharmacopoeial analytical methods.
- Research on standardization of dosage forms.
- Research on the standardization of medicinal plants, herbal medicines, including homeopathic ones.
- Research on standardization of excipients.
- Development of scientific and methodological principles of pharmacoeconomic and pharmacoepidemiological studies.
- Pharmacoeconomic studies of drugs used for the treatment of of socially significant diseases.
The results of the research conducted by the staff at the Institute of Pharmacy is published in monographs, textbooks and the following magazines: “Pharmacy”, “New Pharmacy”, “Pharmaceutical Chemistry Journal”, “Bulletin of Voronezh State University,” “Pharmaceutical industry”, “Bulletin of the Scientific Center of Medical Products “,” Pharmacoeconomics “, etc.
The Institute of Pharmacy holds annual scientific conferences and scientific seminars on topical issues of technology, standardization, production and pharmacoeconomics of medicines, with the participation of students, researchers, companies specialized in the manufacture of medicines and the regulatory authorities.
Laboratories of the RI of Pharmacy:
- Laboratory of medicines technologies
- Laboratory of pharmacognosy
- Laboratory of pharmacopoeias
- Laboratory of Pharmaceutical Chemistry
- Laboratory of pharmacoeconomics
RI of Uronephrology and Reproductive Health
The Institute explores the current issues of diagnosis, treatment and prevention of diseases of the urinary system, male fertility disorders, and sexual function.
In it’s clinical departments a wide range of complex medical operations are performed routinely this includes: laparoscopic interventions on the kidney, a wide range of rare and technically complex reconstructive and plastic operations on the organs of the urinary system, percutaneous interventions, minimally invasive treatment of prostate cancer using focused ultrasound, destruction of small renal tumors with radiofrequency ablation and other modern techniques. Promising methods of 3-D modeling of the pathological process of kidney cancer, kidney stones and hydronephrosis were introduced in the institute, significantly improving the effectiveness of surgical treatment for these conditions by creating computer simulations of operations.
Soon the institution will open a well-equipped Laparoscopic Training Division. There are also plans to further develop public educational activity of the institute in the near future.
Admission is open
- Admission process
- Admission requirements
- Application form
- Admission 2020-2021
- Tuition fee
- Apply online
- Visa requirements
- Our representatives
- Russian embassies
- Airport pickup
Admission 2020-2021 is open now. Join to thousands of happy students in First Moscow State Medical University
Admission Office
Admission office for international students.
Adfress: Street, Moscow, Russia
Phone: +7 (000) 000-00-00
Email: [email protected]

July Externship
Twenty two students (twelve medical students, six students in Dental Medicine and four students in Pharmacy) have undergone their summer internship at the training and clinical facilities of Medical University-Varna. For two weeks interns trained at the academic and clinical facilities, under the direct supervision and guidance of leading professors in their respective fields. Summer…

Sechenovsky scientists shared experiences with Chinese counterparts
Our delegation of the Department of Preventive and Emergency Cardiology under the Institute of Vocational Education has participated in three major international events: Second Sino-Russian Conference of Young Scientists on Heart Diseases, Eighth Sino-Russian Conference on Medicine and Pharmacology, Sixth Sino-Russian Conference on Heart Disease in the Cold Climatic Zone. Sechenov First MSMU researchers were…
- Faculties and Chairs
- FMSMU Worldwide
Copyright © 2020 First Moscow State Medical University. All Rights Reserved.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List

Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
5 facts about Hispanic Americans and health care

Hispanic Americans have long faced health care challenges in the United States, including lower health insurance coverage rates and less access to preventative care.
Language and cultural barriers, as well as higher levels of poverty, are among the social and economic factors contributing to disparate health outcomes for Hispanic Americans. These disparities were apparent during the early stages of the COVID-19 pandemic , when Hispanics were far more likely than White Americans to have died from the virus .
Pew Research Center conducted this analysis to highlight Hispanic Americans’ attitudes about and experiences with health care. We surveyed U.S. adults from Nov. 30 to Dec. 12, 2021, including 3,716 Hispanic adults (inclusive of those who identify as any race). A total of 14,497 U.S. adults completed the survey.
The survey was conducted on the Center’s American Trends Panel (ATP) and included an oversample of Black and Hispanic adults from the Ipsos KnowledgePanel. Respondents on both panels are recruited through national, random sampling of residential addresses. This way nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology .
Here are the survey questions used for this analysis , along with responses, and its methodology .
This study was informed by a group of advisers with expertise related to Black and Hispanic Americans’ attitudes and experiences in science, health, STEM education and other areas. Pew Research Center remains solely responsible for all aspects of the research, including any errors associated with its products and findings.
This analysis includes additional information from sources including KFF and the U.S. Census Bureau. Further information about these sources can be found through the links in the text.
Here are five key facts about Hispanic Americans and health care, based on a 2021 Pew Research Center survey of Hispanic adults and other sources:
Hispanic adults are less likely than other Americans to have seen a health care provider recently and to have a primary care provider. Seven-in-ten say they’ve seen a doctor or other health care provider in the past year, compared with 82% among Americans overall. Hispanics are also slightly less likely than Americans overall to say they have a primary care provider (68% vs. 76%).
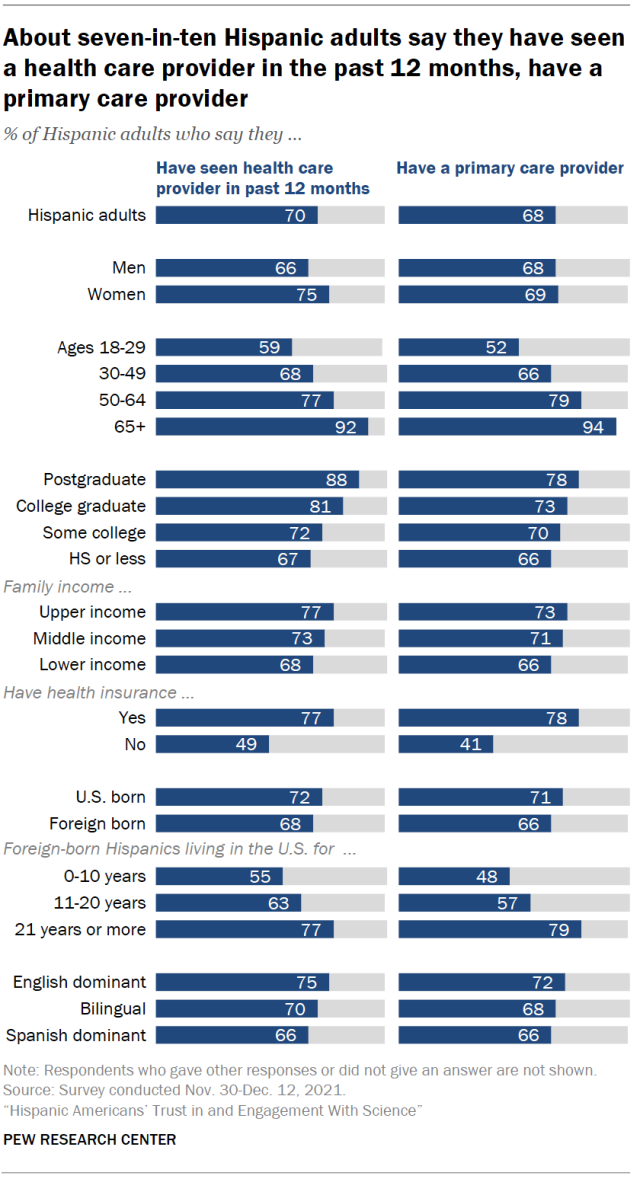
Health care access among Hispanic immigrants differs markedly based on how long they have lived in the U.S. More recent arrivals are less likely than those who have been in the country longer to have seen a doctor recently and to have a primary care provider. For example, 48% of Hispanic immigrants who have been in the U.S. for a decade or less report having a primary care provider, compared with 79% among those who have been in the U.S. for more than two decades.
Recent arrivals make up a declining share of Hispanic immigrants in the U.S. And more broadly, immigrants account for a declining share of the overall U.S. Hispanic population . In 2021, they made up 32% of all Hispanic Americans, down from 37% in 2010.
Hispanic Americans are less likely than people of other racial and ethnic backgrounds to have health insurance. As of 2021, the uninsured rate among Hispanics under age 65 was 19%, according to KFF, formerly known as the Kaiser Family Foundation . That was higher than the share among Black (11%), White (7%) and Asian Americans (6%). (These figures include rates among children as well as adults.)
While comparatively high, the uninsured rate among Hispanic Americans under age 65 in 2021 was down from 33% in 2010, before the implementation of the Affordable Care Act, according to KFF.
Lower rates of health insurance coverage play a major role in Hispanic Americans’ less frequent interactions with health care providers.
The relative youth of the U.S. Hispanic population may be another factor at play. The median age of Hispanic Americans was 30 as of 2020, compared with 41 for non-Hispanic Americans, according to the U.S. Census Bureau . Among both Hispanic and non-Hispanic Americans, younger people are less likely than their elders to have seen a health care provider recently and to have a primary care provider.
Many Hispanic Americans say worse health outcomes for Hispanics are tied to occupational and structural factors. Some 53% of Hispanic adults say a major reason why Hispanic people generally have worse health outcomes is that they’re more likely to work in jobs that put them at risk for health problems. About half (48%) say a major reason is that Hispanic people have less access to quality medical care where they live.
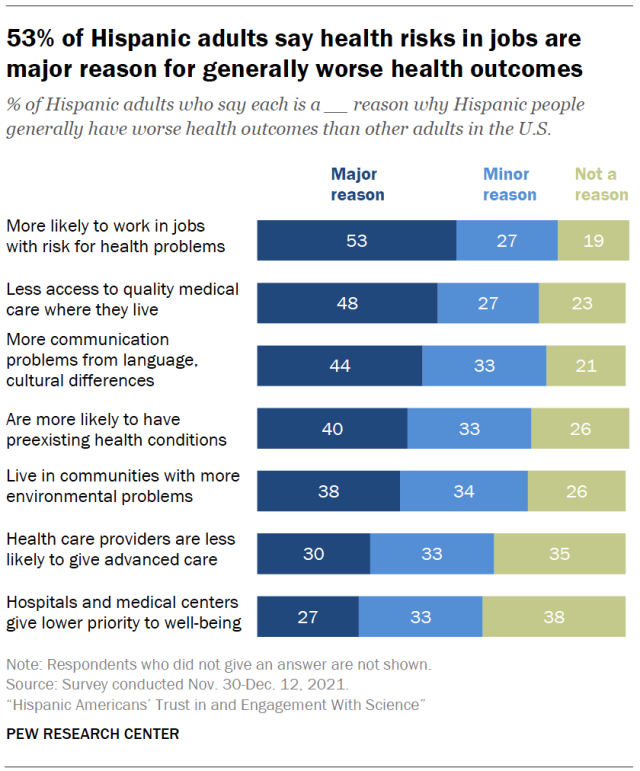
At least four-in-ten Hispanic adults also point to communication problems arising from language or cultural differences (44%) and preexisting health conditions (40%) as major reasons. (Majorities view all of these factors as at least minor reasons for disparate health outcomes among Hispanic adults.)
The coronavirus outbreak took an especially heavy toll on Hispanic Americans when compared with White Americans. Hispanics also face higher rates of certain diseases like diabetes than some other Americans.
When it comes to progress in health outcomes for Hispanic people, 51% of Hispanic adults say health outcomes have gotten a lot or a little better over the past two decades, compared with 13% who say they’ve gotten a lot or a little worse; 34% say they’ve stayed about the same.
About a third of Hispanic Americans – including 58% of Hispanic immigrants – say they prefer to see a Spanish-speaking health care provider. Overall, 35% of Hispanic adults strongly or somewhat prefer seeing a Spanish-speaking doctor or other health care provider for routine care. A larger share (51%) say it makes no difference whether the doctor they see speaks Spanish or not. And 13% say they would rather not see a Spanish-speaking doctor.
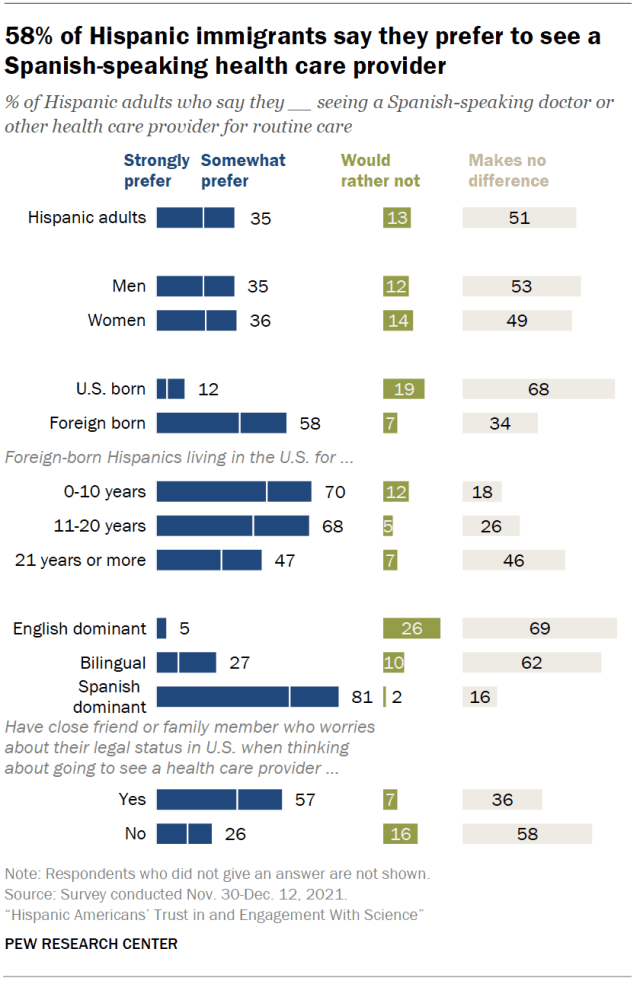
Attitudes are broadly similar when it comes to seeing a Hispanic doctor or health care provider. A third of Hispanic adults say they would prefer to see a Hispanic doctor for routine care, while 59% say it makes no difference and 7% would rather not.
Among Hispanic adults, immigrants are much more likely than those born in the U.S. to prefer seeing a Spanish-speaking doctor (58% vs. 12%) and to prefer seeing a Hispanic doctor (47% vs. 20%). About half of Hispanic immigrants in the U.S. mostly speak and read in Spanish.
Hispanic Americans account for 19% of the U.S. population . But only 9% of the nation’s health care practitioners and technicians are Hispanic, according to a 2021 Pew Research Center analysis of federal government data . And just 7% of all U.S. physicians and surgeons and 7% of registered nurses are Hispanic.
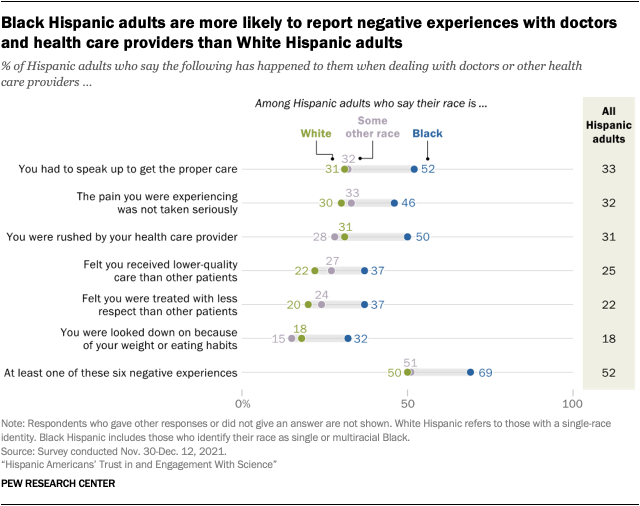
Black Hispanic adults are more likely to report negative health care experiences than other Hispanic adults. Overall, about half of Hispanic adults (52%) say they’ve had at least one of six negative health care experiences asked about in the Center’s 2021 survey, including feeling rushed or having to speak up to get the proper care. This is similar to the share of all U.S. adults who report having at least one of these types of negative experiences.
However, there are notable differences among Hispanics by race. Hispanic Americans who identify as Black are much more likely than White Hispanic adults to have faced negative health care experiences. For instance, 52% of Black Hispanic adults say they’ve had to speak up to get proper care, compared with 31% of White Hispanic adults. And Black Hispanic adults are 15 percentage points more likely than White Hispanic adults to say they’ve received lower-quality care (37% vs. 22%).
While negative health care experiences are fairly common, most Hispanic adults have generally positive opinions about their latest health care interaction. A 56% majority say the quality of care they most recently received from doctors or other health care providers was excellent or very good, while another 28% say it was good. Fewer (14%) say the care they received was only fair or poor. Black and White Hispanic adults are about equally likely to give positive ratings of their most recent health care experience.
Note: Here are the survey questions used for this analysis , along with responses, and its methodology .
- Health Care
- Hispanics/Latinos
- Medicine & Health

9 facts about Americans and marijuana
5 facts about black americans and health care , 8 facts about americans with disabilities, inflation, health costs, partisan cooperation among the nation’s top problems, by more than two-to-one, americans say medication abortion should be legal in their state, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy

Department of Nervous Diseases and Neurosurgery
MBBS in Russia | MBBS in Moscow | Study in Russia | Study in Moscow | Education in Russia | Education in Moscow | I.M. Sechenov First Moscow State Medical University | FMSMU | MBBS Admission in I.M. Sechenov First Moscow State Medical University | MBBS Fee in I.M. Sechenov First Moscow State Medical University | Direct Admission in I.M. Sechenov First Moscow State Medical University | Apply for MBBS Admission in I.M. Sechenov First Moscow State Medical University | How to Get Direct Admission in MBBS in I.M. Sechenov First Moscow State Medical University | Study in I.M. Sechenov First Moscow State Medical University | About I.M. Sechenov First Moscow State Medical University | Tuition Fee in I.M. Sechenov First Moscow State Medical University | About Moscow
The oldest neurological department in Russia is located in the Clinic of Nervous Diseases. AND I. Kozhevnikov Street Rossolimo 11 building 1. The structure of the Clinic includes 3 neurological departments and 1 neurosurgical department, somnological department. Clinic of nervous diseases to them. AND I. Kozhevnikov is equipped with modern diagnostic equipment: MRI (3 Tesla), multispiral CT, functional diagnostics and neurophysiology laboratory. The clinic conducts advisory consultation on all forms of neurological diseases, as well as specialized reception of patients with cognitive impairment (laboratory “memory”), dizziness, headache, back pain, extrapyramidal disorders (Parkinson’s disease and others), neurosurgical reception. The department has leading specialists in our country for the diagnosis, treatment and prevention of cognitive impairment and Alzheimer’s disease, cerebrovascular diseases, primary forms of headache, dizziness, back pain, and neuropathic pain.
The department is trained in nervous diseases, senior students of medical, medical and preventive, pediatric and psychological faculties as well as interns and graduate students. Classes are held in 2 lecture halls and 26 group seminar rooms. Every year more than 1000 students, more than 20 residents and 10 graduate students study at the department. Employees of the department, together with doctors, conduct a weekly round-trip of patients in neurological departments, clinical trials of “difficult” patients. On Tuesday, from 13 to 15 o’clock, conferences are held, during which clinical discussions of “difficult” patients, scientific and methodological meetings of the department staff are held. With the active participation of the department staff, the Neurological Journal (editor-in-chief Academician N.N. Yakhno) and the journal Neurology, Neuropsychiatry and Psychosomatics (editor-in-chief Professor Parfenov V.A.) are recommended. in many international systems, including Scopus. Employees of the department regularly give lectures for doctors and researchers at conferences and congresses organized in the Russian Federation and other countries, and actively participate in multicenter clinical studies.
- Faculties and Departments
- University Leadership
- Clinics of FMSMU
- Mission & Brand Strategy
- Facts & Figures
- Rector's Welcome
- Regulatory Documents
- Preparatory Department
- Undergraduate
- Postgraduate
- e-Learning Courses
- Clinical Facilities
- Non-Degree Programs
- Admission process
- Admission requirements
- Application form
- Admission 2020-2021
- Tuition fee
- Apply online
- Visa requirements
- Our representatives
- Russian embassies
- Airport pickup

+7(910)737-2741
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine-led study identifies novel target for epilepsy treatment
Researchers find that a little-understood part of the brain appears to be involved in starting seizures and keeping them going.
April 17, 2024 - By Kimberlee D'Ardenne
Stanford Medicine researchers and their colleagues found that removing or inhibiting the fasciola cinereum may help epilepsy patients who aren't helped by surgery. Tom - stock.adobe.com
Removing part of the brain’s temporal lobe is the only treatment available to the millions of people with a form of epilepsy that medications often don’t alleviate. But even that approach fails a third of the time.
A new study from Stanford Medicine researchers and their colleagues offers an explanation and suggests a more effective approach to treatment. They found that a previously overlooked region of the hippocampus, the fasciola cinereum, appears to be involved in instigating and propagating seizures. Removing or inhibiting the fasciola cinereum may help those patients who don’t find relief after surgery.
“The hippocampus is the best studied part of the brain by far, but there is shockingly little known about the fasciola cinereum,” said Ivan Soltesz , PhD, the James R. Doty Professor in Neurosurgery and Neurosciences and a senior author on the study. “This relatively small region was consistently involved in seizure activity in mice and in people undergoing pre-surgical electrical recordings. Our findings suggest that all patients with drug-resistant temporal lobe epilepsy should have depth electrodes placed in the fasciola cinereum as part of the surgery planning process.”
The work was published April 17 in Nature Medicine . Soltesz and Vivek Buch , MD, the Christina and Hamid Moghadam Faculty Scholar as well as the surgical director of the Stanford Comprehensive Epilepsy Center , are co-senior authors.
A tale of a tail
Worldwide, 65 million people live with epilepsy. Tens of millions have mesial temporal lobe epilepsy, with seizures originating, in part, from the amygdala, an almond-shaped structure involved in processing emotions, and the hippocampus, a region necessary for forming memories. When people with mesial temporal lobe epilepsy of just one hemisphere do not respond to anti-seizure drug therapies, the standard of care is surgery. In these procedures, the amygdala and most of the hippocampus in one hemisphere are either surgically removed or ablated, a technique that involves using a laser to heat up and destroy tissue. Because of the symmetry of the temporal lobe — both hemispheres of the brain have an amygdala and hippocampus — people who have these surgeries usually have minimal side effects, according to the researchers.

Ivan Soltesz
Before performing the surgery, physicians need to identify the brain tissue responsible for seizure activity. They do this by placing electrodes in areas of the brain suspected of starting or propagating seizures and taking recordings from the electrodes. This process, called stereoelectroencephalography, or sEEG, lets them map where in the brain seizure activity happens.
Though the amygdala and its next-door neighbor the hippocampus are common locations for sEEG recordings, the electrodes are typically placed in only the anterior and middle regions of the hippocampus. The human hippocampus, located deep in each hemisphere of the brain near the level of the ear, looks like a sea horse lying on its side, with its head pointing toward the front of the brain. sEEG electrodes are commonly placed in the anterior and medial regions, corresponding to the head, body and the beginnings of the tail of the sea horse.
The idea to record from the fasciola cinereum — the far tip of the sea horse’s tail — in patients with epilepsy undergoing sEEG for surgical planning first formed about three years ago, when Ryan Jamiolkowski , MD, PhD, co-lead author of the study and a resident in neurosurgery, joined the Soltesz lab.
At the time, Quynh-Anh Nguyen, PhD, co-lead author on the study and former postdoctoral scholar in the Soltesz lab who is now at Vanderbilt University, was screening for the hippocampal neurons that were active during seizures in mice. Unexpectedly, Nguyen discovered that neurons in a posterior region of the hippocampus, the fasciola cinereum, were involved in seizures.
Jamiolkowski and the research team used optogenetic techniques to test whether the fasciola cinereum could be a target for epilepsy interventions. The neurons in the fasciola cinereum were made to contain special proteins capable of shutting down neuronal activity when exposed to blue light. When electrical recordings from the hippocampus showed seizure activity, the researchers shined blue light onto the fasciola cinereum, shortening the duration of seizures in mice.
Recording from the human hippocampus tail
To understand the fasciola cinereum’s role in seizure activity in humans, Jamiolkowski and Buch recorded from the small region in six patients. All were undergoing sEEG to identify the source of their seizures in preparation for future surgeries to cure their epilepsy. The fasciola cinereum contributed recorded seizures in all six patients, including some episodes in which the head and body regions of the hippocampus were quiet.

Ryan Jamiolkowski
One of the patients with mesial temporal lobe epilepsy of the left hemisphere had already undergone laser ablation of the amygdala and anterior and middle regions of the hippocampus. The patient continued having seizures, and follow-up sEEG showed that the only part of the hippocampus that remained, the fasciola cinereum, was involved in all recorded seizures. The patient underwent a second surgical ablation that removed almost all of the fasciola cinereum, and the frequency of the seizures decreased by 83%, from one to two each month to once every three months.
The researchers said that patients whose seizures involve the fasciola cinereum may need to undergo two surgeries because of the shape of the hippocampus.
“The hippocampus curves like a banana, and the optical fiber used for laser ablation is a straight line. Reaching anterior and posterior regions requires different trajectories that are not currently feasible to combine into one procedure. The results of our study do not challenge the importance of ablating the amygdala and anterior hippocampus but suggest considering a second ablation targeting the posterior hippocampal tail for the patients whose seizures recur,” Jamiolkowski said.
Three of the patients had bilateral involvement of the mesial temporal lobe, which means the amygdala and hippocampus in both the right and left hemisphere showed seizure activity. Because new memories cannot be formed without at least one intact hippocampus, these patients instead received responsive neurostimulation from a device that detects and interrupts seizure activity. However, most responsive neurostimulation units can be configured to target only the anterior regions of the hippocampus on both sides of the brain. The findings from this study suggest that a more personalized approach that also allows the device to monitor and interrupt seizure activity in the posterior hippocampal tail region might be more beneficial to patients.
“Because one-third of patients — a high percentage — do not get seizure freedom from surgery, we should be putting sEEG electrodes in the fasciola cinereum in all temporal lobe epilepsy patients; seizure activity in this region could be a reason why these surgeries sometimes fail,” Jamiolkowski added. “Knowing which patients have seizures involving the fasciola cinereum would let us target it with either ablation or neurostimulation and help us treat patients better than a one-size-fits all approach.”
A researcher from Cambridge University contributed to the study.
Funding for this study was provided by the Stanford Maternal and Child Health Research Institute, the Tashia and John Morgridge Endowed Fellowship, the Lennox-Gastaut Syndrome Foundation Cure 365, the Stanford Neuroscience Scholars Program, and the National Institutes of Health (grants R25NS065741, K99NS121399, K99NS126725, NS121106 and P30AG066515).
- Kimberlee D'Ardenne Kimberlee D'Ardenne is a freelance writer.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care

Clinical Neurology
Research activity at neurology is strongly patient-oriented. Scientific questions arise from daily clinical practice and are solved by means of clinical/interventional studies and translational studies using biobank material.
Research fields include common neurological diseases such as epilepsy, movement disorders, acute stroke and multiple sclerosis but also sleep disorders, like narcolepsy, and more rare disorders like neurogenetic disorders, for ex Huntington´s, and low-grade gliomas. Scientific fields like distrubances in the autonomic nervous system have been acknowledged here, and the development of treatments, have lead to successful achivements of our unit.
A common focus is ”Neurotherapy”, a field where our unit for a long time achieved a strong position for developing treatments against neurological disease. Such examples are pharmacotherapy administered vie the gut in Parkinson´s disease, and allogenic stem cell transplantation (HSCT) against MS, further advanced imaging diagnostics in the mapping of brain tumours, and shunt operations in normal pressure hydrocephalus (NPH).

Clinical and interventional studies
- In collaboration with the PET Centre, the value of 11 C-L-DOPA and 18 F-FDG-PET is studied for differential diagnosis of Parkinson’s disease, 11 C-flumazenil as a tracer for focal epilepsy and 11 C-methionine in low-grade gliomas.
- Advanced neuroimaging techniques are evaluated, in collaboration with the Department of Neuroradiology, for diagnosis and follow-up of normal pressure hydrocephalus (NPH), multiple sclerosis (MS), neurogenetic disorders, focal epilepsy, brain tumors, stroke and movement disorders.
- Neurology in Uppsala was first in Sweden to use botulinum toxin, a drug that in addition to focal dystonia is used also for spasticity after stroke and hyperhidrosis, and was first worldwide to use intestinal levodopa/carbidopa gel infusion in advanced Parkinson’s disease. A multicenter trial has been initiated to compare the efficacy of intestinal levodopa/carbidopa gel infusion versus of deep brain stimulation.
- Since 2004, over 100 patients with aggressive MS have beeen treated with haematopoietic stem cell transplantation (HSCT) with a strongly favourable effect on disease activity. A number of prospective trials are ongoing, including a study comparing HSCT with natalizumab in these patients is ongoing.
- Epidemiological studies of narcolepsy, and also on the efficiency, safety, and sociodemographic distribution among recently adopted treatment options in epilepsy are performed.
Clinical translational studies
Translational projects include pathogenetic studies of hereditary neuromuscular disorders and white matter diseases, immunological studies of MS, pharmacokinetic-pharmacodynamic modelling in Parkinson’s disease, identification of prognostic and predictive biomarkers in tumor tissues of adult low-grade gliomas, and in serum and CSF of patients with ALS, NPH and Parkinson´s disease.

Professor at Department of Medical Sciences, Neurology
for Dag Nyholm
Use of cookies
Uppsala University uses cookies to make your website experience as good as possible.
Read more in our cookie policy.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Fatal Traffic Risks With a Total Solar Eclipse in the US
- 1 Department of Medicine, University of Toronto, Toronto, Ontario, Canada
- 2 Evaluative Clinical Science Platform, Sunnybrook Research Institute, Toronto, Ontario, Canada
- 3 Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada
- 4 Division of General Internal Medicine, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
- 5 Center for Leading Injury Prevention Practice Education & Research, Toronto, Ontario, Canada
- 6 Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada
- 7 Centre for Clinical Epidemiology & Evaluation, University of British Columbia, Vancouver, British Columbia, Canada
A total solar eclipse occurs when the moon temporarily obscures the sun and casts a dark shadow across the earth. This astronomical spectacle has been described for more than 3 millennia and can be predicted with high precision. Eclipse-related solar retinopathy (vision loss from staring at the sun) is an established medical complication; however, other medical outcomes have received little attention. 1
Read More About
Redelmeier DA , Staples JA. Fatal Traffic Risks With a Total Solar Eclipse in the US. JAMA Intern Med. Published online March 25, 2024. doi:10.1001/jamainternmed.2023.5234
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Medical Devices
- Medical Devices News and Events
CDRH Issues 2024 Safety and Innovation Reports
Reports highlight CDRH actions to advance medical device safety and innovation and build on these efforts this year.
FOR IMMEDIATE RELEASE April 17, 2024
The following is attributed to Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health (CDRH)
Today, CDRH is issuing two companion reports that detail the Center's commitment to further advance our core pillars of safety and innovation. The CDRH 2024 Safety Report is an update to our 2018 Medical Device Safety Action Plan and features steps we have taken in recent years to assure the safety of medical devices keeps pace with the evolving technology. The CDRH 2024 Innovation Report highlights our work to advance innovation and the progress we have made to make the U.S. market more attractive to top device developers.
As we have long stated, safety and innovation are not polar opposites, but rather two sides of the same coin. Our focus on safety and innovation stems from our vision to protect and promote the public health by assuring that medical devices on the U.S. market are high-quality, safe and effective, and that patients and providers have timely and continued access to these devices.
Since 2009, CDRH has focused our efforts on advancing the development of safer, more effective medical devices that provide a significant benefit to the public health. As such, we enhanced our clinical trial and premarket review programs, including the 510(k) and De Novo pathways, and created new programs like the Breakthrough Devices Program , the Safety and Performance Based Pathway and the Safer Technologies Program to help reduce barriers for innovators. As a result of these actions and other past and ongoing efforts, the number of innovative medical devices authorized annually in the U.S. has increased five-fold since 2009.
In parallel, we took significant actions to improve device safety and enhanced our ability to identify and address new safety signals. We achieved an ambitious set of goals outlined in our 2018 Medical Device Safety Action Plan to help ensure patient safety throughout the Total Product Life Cycle (TPLC) of a medical device. We made improvements and updates to our medical device reporting programs, including updating the Manufacturer and User Facility Device Experience (MAUDE) database, vastly improved our recalls program, and took steps to ensure the timely communication and resolution of new or known safety issues.
And throughout, we partnered with patients and incorporated their voices into our work, including establishing our Patient Science and Engagement Program, because at the end of the day, improving the health and the quality of life of people is at the core of our public health mission.
We are proud of the progress we've made to advance innovation and improve the safety of medical devices, and we continue to build on these efforts, as resources and additional capabilities permit. One of the challenges we face, though, is the sheer volume of products and producers. Today there about 257,000 different types of medical devices on the U.S. market, made by approximately 22,000 manufacturing facilities worldwide, and CDRH authorizes roughly a dozen new or modified devices every business day. Despite that, the number of new or increased known safety issues involve only a small fraction of technologies and many can be addressed without any changes to the device itself. However, the impact to people can be significant, which is why we need to continuously take steps to advance both safety and innovation.
This year, we will take additional actions to help further ensure innovative, high-quality, safe, and effective devices are developed and marketed to U.S. patients. As further detailed in the 2024 Innovation Report, three actions we plan to take this year include: reimagining our premarket review program, expanding our footprint in geographical innovation centers, and launching a new home as a health care hub to extend first-class care into the home. Additionally, as detailed in the 2024 Safety Report, three actions we plan to take this year include: expanding a program to assist companies improve their device quality efforts, strengthening active surveillance, and enhancing the medical device recall process.
Through these new actions and the work detailed in the 2024 Safety and Innovation reports, CDRH remains committed to furthering our mission to protect and promote the public health and ensure our organization is well-positioned to meet the needs of all people and changes in the medical device ecosystem.
Additional Resources:
- 2024 Innovation Report
- 2024 Safety Report
- 2018 Medical Device Safety Action Plan
The request to the URL needs to be verified.
The request to the URL is paused, and must be verified for you to access it. This question is for testing whether you are a human visitor, and to prevent automated spam submission.
What code is in the image submit
Incident ID: 14604593418339799177
For comments and questions: [email protected]

- For the Media
- Open Records
- Division of Marketing & Communications

UGA breaks ground on new medical education and research building

The groundbreaking was a "transformational moment at the University of Georgia"
The University of Georgia broke ground Friday on a new medical education and research building that will significantly expand teaching and research capabilities at the university’s future School of Medicine .
Located on UGA’s Health Sciences campus, preliminary plans for the building include medical simulation suites, standardized patient rooms, clinical skills labs, a gross anatomy lab, and a medical library. The building will also feature student support spaces like conference rooms, study spaces, lounges, and faculty and staff offices dedicated to student support.
In total, the proposed building will measure approximately 92,000 square feet. Roughly 67,000 square feet of the building will be dedicated to medical education while the remaining 25,000 square feet will house biomedical research laboratories.

Gov. Brian Kemp speaks at the groundbreaking ceremony for the Medical School Building on the Health Sciences Campus. (Andrew Davis Tucker/UGA)
The new building will complement existing facilities and provide the UGA School of Medicine with capacity to expand from 60 students per class to 120 in the future.
“Today is an exciting and transformational moment at the University of Georgia,” said UGA President Jere W. Morehead. “As a land-grant university and Georgia’s flagship research institution, the University of Georgia is uniquely positioned to address the health care needs of our state through world-class medical education, research and community outreach.”
Following the recommendation of Governor Brian Kemp, the Georgia General Assembly passed a fiscal year 2024 amended budget that includes $50 million in funding for a new University of Georgia School of Medicine facility.

President Jere W. Morehead speaks along with USG Chancellor Sonny Perdue and Gov. Brian Kemp at the groundbreaking ceremony for the Medical School Building on the Health Sciences Campus. (Andrew Davis Tucker/UGA)
The $50 million in state funding will be matched by private contributions to fund the $100 million medical education and research building.
The University System of Georgia Board of Regents authorized the University of Georgia to establish a new independent School of Medicine in Athens in February.
In March, Dr. Shelley Nuss was named founding dean of the UGA School of Medicine. She previously served as an associate professor of internal medicine and psychiatry in the Augusta University/University of Georgia Medical Partnership. In 2016, she was named campus dean of the Medical Partnership, which has been educating physicians in Athens since 2010.
“The fact is, Georgia needs more doctors, and we need them now,” said Nuss. “The new UGA School of Medicine will increase the number of medical students in the state, translating to more practicing physicians to help address Georgia’s greatest health care challenges.”
The creation of the UGA School of Medicine marks the natural evolution of the longest-serving medical partnership in the United States. Similar programs founded around the same time have already transitioned to independent medical schools.

USG Chancellor Sonny Perdue speaks from the podium along with Gov. Brian Kemp at the groundbreaking ceremony for the Medical School Building on the Health Sciences Campus. (Andrew Davis Tucker/UGA)
UGA will continue to work closely with the Medical College of Georgia to ensure a smooth transition for current medical students as UGA seeks accreditation from the Liaison Committee on Medical Education (LCME).
The development of a new public school of medicine at UGA promises to help address a significant shortage of medical professionals. Georgia’s growing population tops approximately 11 million residents, straining the state’s existing medical infrastructure.
Now the nation’s eighth largest state, Georgia is forecasted to experience further population growth in the coming years, and nearly one-third of the state’s physicians are nearing retirement.
“Georgia is growing,” said Sonny Perdue, chancellor of the University System of Georgia. “We may only be only eighth today, but in just a few short years Georgia could be the fifth largest state. And that means we are going to need more health care, and people are going to get it here and across the state.”

Founding Dean of the School of Medicine Shelley Nuss, middle, is surrounded by medical students at the groundbreaking ceremony for the Medical School Building. (Andrew Davis Tucker/UGA)
Georgia currently ranks No. 40 among U.S. states for the number of active patient care physicians per capita, according to the Association of American Medical Colleges (AAMC), while it ranks No. 41 for the number of primary care physicians and No. 44 for the number of general surgeons per capita. The shortage of medical providers is particularly acute in rural and underserved areas, where access is even more limited.
UGA faculty are already engaged in human health research, and the establishment of a school of medicine will bolster their efforts.
“Our flagship institution, the University of Georgia, is tasked with the vital mission of educating and preparing the next generation of leaders,” said Gov. Brian Kemp. “To that end, one of our top priorities is building a strong health care workforce pipeline. This UGA facility will be an essential part of those efforts.”
Alongside funding from state government, strong private support will fortify efforts to create a School of Medicine at UGA. Donors have demonstrated robust support for UGA initiatives in recent years. In fiscal year 2023, UGA raised over $240 million in gifts and pledges from alumni, friends and foundation and industry partners. The university’s three-year rolling fundraising average is now a record $235 million per year, with annual contributions exceeding $200 million for the past six consecutive years.
You may also like

College of Engineering dean named provost at Ohio…

Undergraduate wins Regents Cup Debate

Lead@3 speakers offer insights on leadership
Uga community has access to ai-powered chatbot, growers hopeful 2024 peach season will rebound from…, innovator, educator inducted into the georgia….

IMAGES
VIDEO
COMMENTS
The programme combines genetic, molecular biology and bioinformatic knowledge with practical research experience and insight into current research, as well as a professional network of contacts. Autumn 2023. Autumn 2024. Uppsala, 100%, On-campus, English. Application and requirements.
At the Department we perform clinical and pre-clinical research in a large number of specialties. Much of the research is conducted in close association with Uppsala University Hospital, and encompasses both basic studies on causative mechanisms of disease and clinical applications such as improved diagnosis and methods of treatment.
The programme syllabus for the Master's Programme in Medical Research was established by the Disciplinary Domain of Medicine and Pharmacy on February 28th, 2013. The general goals for the second-cycle higher education in the Higher Education Act (Chapter 1 Section 9) apply. This advanced level programme is available to students with a BSc in ...
The Master Programme in Medical Research (former Uppsala Graduate School in Biomedical Research, UGSBR) has the overall aim to prepare students that are highly motivated for research for PhD studies. The program is designed to give the students a broad and deep knowledge about present research and development in the biomedical/biotechnological ...
Jonas Oldgren is awarded Region Uppsala's research prize 2022 Uppsala University Hospital and Uppsala University in collaboration, take first place in Diabetesbarometern 2022 ... Department of Medical Sciences, UU Akademiska sjukhuset Entrance 40, floor 5 751 85 Uppsala. See separate visits and delivery addresses for each research group ...
About. The Master's programme in Medical Research at Uppsala University provides you with excellent conditions to specialise in an area of your choice and build a solid ground for future doctoral studies. Uppsala University. Uppsala , Sweden. Top 0.5% worldwide. Studyportals University Meta Ranking. 4.2 Read 117 reviews.
Department of Medical Sciences, UU Akademiska sjukhuset Entrance 40, floor 5 751 85 Uppsala. See separate visits and delivery addresses for each research group
The Department of Medical Cell Biology carries out research and educates PhD students in cell biology and physiology focusing on diabetes, kidney function, inflammation, cancer and neurobiology. ... Universitetet i Uppsala Institutionen för medicinsk cellbiologi Box 571 751 23 Uppsala; Visit us. Find us ; Map ; Visiting address: Husargatan 3 ...
Medical Cell Biology. Our research areas: Physiology and cell biology with a focus on diabetes, inflammation, cancer, pharmacology and neurobiology. ... Universitetet i Uppsala Institutionen för medicinsk cellbiologi Box 571 751 23 Uppsala; Visit us. Find us ; Map ; Visiting address: Husargatan 3 751 23 Uppsala; Shortcuts. Research;
Uppsala Clinical Research center is the leading clinical Academic Research Organization (ARO) in Sweden, providing in-house expertise Our services include management of clinical and observational studies, biostatistics, clinical quality registries, and clinical event adjudication. UCR started in 2001 as a clinical research center within Uppsala ...
Uppsala Clinical Research Center (UCR) is a non-profit organization that supports research and quality development in health care in Sweden and internationally. The center was founded on 1 July 2001, based on a proposal from the authors of this manuscript.
Uppsala LINK Medical Research AB Slottsgränd 2A SE-753 09 Uppsala. Solna Telegrafgatan 4 SE-169 72 Solna. Malmö Jörgen Kocksgatan 4 SE-211 20 Malmö +46 18 430 31 00. Norway. LINK Medical Research AS Drammensveien 288, NO-0283 Oslo, Norway. Finland. IRW-Consulting AB, FILIAL I Finland Rajatorpantie 41 C, FI-01640 Vantaa, Finland. Denmark.
[email protected]. +46 18 471 42 16. For general information or questions about the Master´s Programmes at the Faculty of Medicine, please contact: [email protected]. or. Book an appointment with a study counsellor at the Faculty. For admissions-related or general information, please contact our applicant support team:
To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies. Layout table for eligibility information ... Diseases or medical conditions. Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 ...
Welcome to FMSMU. I.M. Sechenov First Moscow State Medical University is the oldest leading medical university in Russia that has become a cradle of most medical schools and scientific societies of our country. For decades it has been unofficially known as "First Med".University success is based on a blend of glorious traditions and actual ...
April 18, 2024 — With research tackling topics from muscle weakness to cancer, viruses and more, students in the UF College of Medicine's Graduate Program in Biomedical Sciences are aiming to address medical mysteries that can impact the lives of millions of patients globally.. On April 17, eight UF Medicine graduate students presented their thesis research to an audience of peers and ...
Professor at UCR-Uppsala Clinical Research center Email: Bertil.Lindahl [AT-sign] ucr.uu.se Telephone: +4618-6119505 Mobile phone: +46 70 1428580. Professor at Department of Medical Sciences ... Department of Medical Sciences, UU Akademiska sjukhuset Entrance 40, floor 5 751 85 Uppsala. See separate visits and delivery addresses for each ...
April 22, 2024. Twelve Augustana College juniors have been selected for the prestigious Texas Medical Center Summer Research Internship Program. "With placements in medical illustration, health care economics, public health, integrated ethics as well as in more traditional areas of biology, biochemistry, oncology and infectious disease ...
The Innovative Medicine program from Uppsala University is a two-year international Master's programme focusing on translational medical research, modern molecular techniques, innovation and entrepreneurship. Uppsala University Multiple locations. Top 0.5% worldwide. Studyportals University Meta Ranking.
RI of Public Health and Healthcare Management. The Scientific-Research Institute of Public Health and Health Management was established in 2002. The Institute is composed of the managerial board and 8 scientific compounds (4 divisions and 4 independent laboratories). The Institute's personnel consists of 82 academic staff, including one ...
A medical clinic displays signs in Spanish and English in Huntington Park, California, in December 2020. (Dania Maxwell/Los Angeles Times via Getty Images) ... Pew Research Center conducted this analysis to highlight Hispanic Americans' attitudes about and experiences with health care. We surveyed U.S. adults from Nov. 30 to Dec. 12, 2021 ...
The department is trained in nervous diseases, senior students of medical, medical and preventive, pediatric and psychological faculties as well as interns and graduate students. Classes are held in 2 lecture halls and 26 group seminar rooms. Every year more than 1000 students, more than 20 residents and 10 graduate students study at the ...
Students from far and near begin medical studies at Stanford . Explore Education . Learn how we empower tomorrow's leaders . Back. Give . Support Stanford Medicine ... Funding for this study was provided by the Stanford Maternal and Child Health Research Institute, the Tashia and John Morgridge Endowed Fellowship, the Lennox-Gastaut Syndrome ...
Medical science encompasses several subjects that collectively aim to provide knowledge about the structure and function of the human body in health and disease. ... 7.5 credits Introduction to Medical Research, 7.5 credits Research Training in Medical Biochemistry and Microbiology, ... FOLLOW UPPSALA UNIVERSITY ON
First, consider that nearly all medical students pass the Step 1 exam. In 2020, 98% of DO and MD students passed the exam on their first try. But if you don't, the National Board of Medical Examiners allows a maximum of four attempts to pass each different level of the USMLE exam. If you fail, you get a score report that offers feedback on ...
Research activity at neurology is strongly patient-oriented. Scientific questions arise from daily clinical practice and are solved by means of clinical/interventional studies and translational studies using biobank material. Research fields include common neurological diseases such as epilepsy, movement disorders, acute stroke and multiple ...
This astronomical spectacle has been described for more than 3 millennia and can be predicted with high precision. Eclipse-related solar retinopathy (vision loss from staring at the sun) is an established medical complication; however, other medical outcomes have received little attention. 1
April 17, 2024. The following is attributed to Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health (CDRH) Today, CDRH is issuing two companion reports that ...
Outline for Master's Programme in Medical Research. The outline is valid from Autumn 2022. Search. Search. Search suggestions are presented below the search box. Search. ... Strong research environments and research areas. Research projects. Theses and publications. Research ethics. Research infrastructure. PhD studies.
The University of Georgia broke ground Friday on a new medical education and research building that will significantly expand teaching and research capabilities at the university's future School of Medicine.. Located on UGA's Health Sciences campus, preliminary plans for the building include medical simulation suites, standardized patient rooms, clinical skills labs, a gross anatomy lab ...