An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Pediatr

Childhood and Adolescent Obesity: A Review
Alvina r. kansra.
1 Division of Endocrinology, Diabetes and Metabolism, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
Sinduja Lakkunarajah
2 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin Affiliated Hospitals, Milwaukee, WI, United States
M. Susan Jay
3 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure. Adiposity rebound (AR) in early childhood is a risk factor for obesity in adolescence and adulthood. The increasing prevalence of childhood and adolescent obesity is associated with a rise in comorbidities previously identified in the adult population, such as Type 2 Diabetes Mellitus, Hypertension, Non-alcoholic Fatty Liver disease (NAFLD), Obstructive Sleep Apnea (OSA), and Dyslipidemia. Due to the lack of a single treatment option to address obesity, clinicians have generally relied on counseling dietary changes and exercise. Due to psychosocial issues that may accompany adolescence regarding body habitus, this approach can have negative results. Teens can develop unhealthy eating habits that result in Bulimia Nervosa (BN), Binge- Eating Disorder (BED), or Night eating syndrome (NES). Others can develop Anorexia Nervosa (AN) as they attempt to restrict their diet and overshoot their goal of “being healthy.” To date, lifestyle interventions have shown only modest effects on weight loss. Emerging findings from basic science as well as interventional drug trials utilizing GLP-1 agonists have demonstrated success in effective weight loss in obese adults, adolescents, and pediatric patients. However, there is limited data on the efficacy and safety of other weight-loss medications in children and adolescents. Nearly 6% of adolescents in the United States are severely obese and bariatric surgery as a treatment consideration will be discussed. In summary, this paper will overview the pathophysiology, clinical, and psychological implications, and treatment options available for obese pediatric and adolescent patients.
Introduction
Obesity is a complex issue that affects children across all age groups ( 1 – 3 ). One-third of children and adolescents in the United States are classified as either overweight or obese. There is no single element causing this epidemic, but obesity is due to complex interactions between biological, developmental, behavioral, genetic, and environmental factors ( 4 ). The role of epigenetics and the gut microbiome, as well as intrauterine and intergenerational effects, have recently emerged as contributing factors to the obesity epidemic ( 5 , 6 ). Other factors including small for gestational age (SGA) status at birth, formula rather than breast feeding in infancy, and early introduction of protein in infant's dietary intake have been reportedly associated with weight gain that can persist later in life ( 6 – 8 ). The rising prevalence of childhood obesity poses a significant public health challenge by increasing the burden of chronic non-communicable diseases ( 1 , 9 ).
Obesity increases the risk of developing early puberty in children ( 10 ), menstrual irregularities in adolescent girls ( 1 , 11 ), sleep disorders such as obstructive sleep apnea (OSA) ( 1 , 12 ), cardiovascular risk factors that include Prediabetes, Type 2 Diabetes, High Cholesterol levels, Hypertension, NAFLD, and Metabolic syndrome ( 1 , 2 ). Additionally, obese children and adolescents can suffer from psychological issues such as depression, anxiety, poor self-esteem, body image and peer relationships, and eating disorders ( 13 , 14 ).
So far, interventions for overweight/obesity prevention have mainly focused on behavioral changes in an individual such as increasing daily physical exercise or improving quality of diet with restricting excess calorie intake ( 1 , 15 , 16 ). However, these efforts have had limited results. In addition to behavioral and dietary recommendations, changes in the community-based environment such as promotion of healthy food choices by taxing unhealthy foods ( 17 ), improving lunch food quality and increasing daily physical activity at school and childcare centers, are extra measures that are needed ( 16 ). These interventions may include a ban on unhealthy food advertisements aimed at children as well as access to playgrounds and green spaces where families can feel their children can safely recreate. Also, this will limit screen time for adolescents as well as younger children.
However, even with the above changes, pharmacotherapy and/or bariatric surgery will likely remain a necessary option for those youth with morbid obesity ( 1 ). This review summarizes our current understanding of the factors associated with obesity, the physiological and psychological effects of obesity on children and adolescents, and intervention strategies that may prevent future concomitant issues.
Definition of Childhood Obesity
Body mass index (BMI) is an inexpensive method to assess body fat and is derived from a formula derived from height and weight in children over 2 years of age ( 1 , 18 , 19 ). Although more sophisticated methods exist that can determine body fat directly, they are costly and not readily available. These methods include measuring skinfold thickness with a caliper, Bioelectrical impedance, Hydro densitometry, Dual-energy X-ray Absorptiometry (DEXA), and Air Displacement Plethysmography ( 2 ).
BMI provides a reasonable estimate of body fat indirectly in the healthy pediatric population and studies have shown that BMI correlates with body fat and future health risks ( 18 ). Unlike in adults, Z-scores or percentiles are used to represent BMI in children and vary with the age and sex of the child. BMI Z-score cut off points of >1.0, >2.0, and >3.0 are recommended by the World Health Organization (WHO) to define at risk of overweight, overweight and obesity, respectively ( 19 ). However, in terms of percentiles, overweight is applied when BMI is >85th percentile <95th percentile, whereas obesity is BMI > 95th percentile ( 20 – 22 ). Although BMI Z-scores can be converted to BMI percentiles, the percentiles need to be rounded and can misclassify some normal-weight children in the under or overweight category ( 19 ). Therefore, to prevent these inaccuracies and for easier understanding, it is recommended that the BMI Z-scores in children should be used in research whereas BMI percentiles are best used in the clinical settings ( 20 ).
As BMI does not directly measure body fat, it is an excellent screening method, but should not be used solely for diagnostic purposes ( 23 ). Using 85th percentile as a cut off point for healthy weight may miss an opportunity to obtain crucial information on diet, physical activity, and family history. Once this information is obtained, it may allow the provider an opportunity to offer appropriate anticipatory guidance to the families.
Pathophysiology of Obesity
The pathophysiology of obesity is complex that results from a combination of individual and societal factors. At the individual level, biological, and physiological factors in the presence of ones' own genetic risk influence eating behaviors and tendency to gain weight ( 1 ). Societal factors include influence of the family, community and socio-economic resources that further shape these behaviors ( Figure 1 ) ( 3 , 24 ).

Multidimensional factors contributing to child and adolescent obesity.
Biological Factors
There is a complex architecture of neural and hormonal regulatory control, the Gut-Brain axis, which plays a significant role in hunger and satiety ( Figure 2 ). Sensory stimulation (smell, sight, and taste), gastrointestinal signals (peptides, neural signals), and circulating hormones further contribute to food intake ( 25 – 27 ).

Pictorial representation of the Hunger-Satiety pathway a and the various hormones b involved in the pathway. a, Y1/Y5R and MC3/4 are second order neuro receptors which are responsible in either the hunger or satiety pathway. Neurons in the ARC include: NPY, Neuropeptide Y; AgRP, Agouti-Related Peptide; POMC, Pro-Opiomelanocortin; CART, Cocaine-and Amphetamine-regulated Transcript; α-MSH, α-Melanocyte Stimulating Hormone. b, PYY, Peptide YY; PP, Pancreatic Polypeptide; GLP-1, Glucagon-Like Peptide- I; OMX, Oxyntomodulin.
The hypothalamus is the crucial region in the brain that regulates appetite and is controlled by key hormones. Ghrelin, a hunger-stimulating (orexigenic) hormone, is mainly released from the stomach. On the other hand, leptin is primarily secreted from adipose tissue and serves as a signal for the brain regarding the body's energy stores and functions as an appetite -suppressing (anorexigenic) hormone. Several other appetite-suppressing (anorexigenic) hormones are released from the pancreas and gut in response to food intake and reach the hypothalamus through the brain-blood barrier (BBB) ( 28 – 32 ). These anorexigenic and orexigenic hormones regulate energy balance by stimulating hunger and satiety by expression of various signaling pathways in the arcuate nucleus (ARC) of the hypothalamus ( Figure 2 ) ( 28 , 33 ). Dysregulation of appetite due to blunted suppression or loss of caloric sensing signals can result in obesity and its morbidities ( 34 ).
Emotional dysfunction due to psychiatric disorders can cause stress and an abnormal sleep-wake cycles. These modifications in biological rhythms can result in increased appetite, mainly due to ghrelin, and can contribute to emotional eating ( 35 ).
Recently, the role of changes in the gut microbiome with increased weight gain through several pathways has been described in literature ( 36 , 37 ). The human gut serves as a host to trillions of microorganisms, referred to as gut microbiota. The dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes representing 90% of human gut microbiota ( 5 , 38 ). The microbes in the gut have a symbiotic relationship within their human host and provide a nutrient-rich environment. Gut microbiota can be affected by various factors that include gestational age at birth, mode of infant delivery, type of neonatal and infant feeding, introduction of solid food, feeding practices and external factors like antibiotic use ( 5 , 38 ). Also, the maturation of the bacterial phyla that occurs from birth to adulthood ( 39 ), is influenced by genetics, environment, diet, lifestyle, and gut physiology and stabilizes in adulthood ( 5 , 39 , 40 ). Gut microbiota is unique to each individual and plays a specific role in maintaining structural integrity, and the mucosal barrier of the gut, nutrient metabolism, immune response, and protection against pathogens ( 5 , 37 , 38 ). In addition, the microbiota ferments the indigestible food and synthesizes other essential micronutrients as well as short chain fatty acids (SCFAs') ( 40 , 41 ). Dysbiosis or imbalance of the gut microbiota, in particularly the role of SCFA has been linked with the patho-physiology of obesity ( 36 , 38 , 41 , 42 ). SCFAs' are produced by anaerobic fermentation of dietary fiber and indigestible starch and play a role in mammalian energy metabolism by influencing gut-brain communication axis. Emerging evidence has shown that increased ratio of Firmicutes to Bacteroidetes causes increased energy extraction of calories from diets and is evidenced by increased production of short chain fatty acids (SCFAs') ( 43 – 45 ). However, this relationship is not affirmed yet, as a negative relationship between SCFA levels and obesity has also been reported ( 46 ). Due to the conflicting data, additional randomized control trials are needed to clarify the role of SCFA's in obese and non-obese individuals.
The gut microbiota also has a bidirectional interaction with the liver, and various additional factors such as diet, genetics, and the environment play a key role in this relationship. The Gut- Liver Axis is interconnected at various levels that include the mucus barrier, epithelial barrier, and gut microbiome and are essential to maintain normal homeostasis ( 47 ). Increased intestinal mucosal permeability can disrupt the gut-liver axis, which releases various inflammatory markers, activates an innate immune response in the liver, and results in a spectrum of liver diseases that include hepatic steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) ( 48 , 49 ).
Other medical conditions, including type 2 Diabetes Mellitus, Metabolic Syndrome, eating disorders as well as psychological conditions such as anxiety and depression are associated with the gut microbiome ( 50 – 53 ).
Genetic Factors
Genetic causes of obesity can either be monogenic or polygenic types. Monogenic obesity is rare, mainly due to mutations in genes within the leptin/melanocortin pathway in the hypothalamus that is essential for the regulation of food intake/satiety, body weight, and energy metabolism ( 54 ). Leptin regulates eating behaviors, the onset of puberty, and T-cell immunity ( 55 ). About 3% of obese children have mutations in the leptin ( LEP ) gene and the leptin receptor (LEPR) and can also present with delayed puberty and immune dysfunction ( 55 , 56 ). Obesity caused by other genetic mutations in the leptin-melanocortin pathway include proopiomelanocortin (POMC) and melanocortin receptor 4 (MC4R), brain-derived neurotrophic factor (BDNF), and the tyrosine kinase receptor B (NTRK2) genes ( 57 , 58 ). Patients with monogenic forms generally present during early childhood (by 2 years old) with severe obesity and abnormal feeding behaviors ( 59 ). Other genetic causes of severe obesity are Prader Willi Syndrome (PWS), Alström syndrome, Bardet Biedl syndrome. Patients with these syndromes present with additional characteristics, including cognitive impairment, dysmorphic features, and organ-specific developmental abnormalities ( 60 ). Individuals who present with obesity, developmental delay, dysmorphic features, and organ dysfunction should receive a genetics referral for further evaluation.
Polygenic obesity is the more common form of obesity, caused by the combined effect of multiple genetic variants. It is the result of the interplay between genetic susceptibility and the environment, also known as the Gene-Environment Interaction (GEI) ( 61 – 64 ). Genome-wide association studies (GWAS) have identified gene variants [single nucleotide polymorphism (SNPs)] for body mass index (BMI) that likely act synergistically to affect body weight ( 65 ). Studies have identified genetic variants in several genes that may contribute to excessive weight gain by increasing hunger and food intake ( 66 – 68 ). When the genotype of an individual confers risk for obesity, exposure to an obesogenic environment may promote a state of energy imbalance due to behaviors that contribute to conserving rather than expending energy ( 69 , 70 ). Research studies have shown that obese individuals have a genetic variation that can influence their actions, such as increased food intake, lack of physical activity, a decreased metabolism, as well as an increased tendency to store body fat ( 63 , 66 , 67 , 69 , 70 ).
Recently the role of epigenetic factors in the development of obesity has emerged ( 71 ). The epigenetic phenomenon may alter gene expression without changing the underlying DNA sequence. In effect, epigenetic changes may result in the addition of chemical tags known as methyl groups, to the individual's chromosomes. This alteration can result in a phenomenon where critical genes are primed to on and off regulate. Complex physiological and psychological adjustment occur during infancy and can thereafter set the stage for health vs. disease. Developmental origins of health and disease (DOHaD) shows that early life environment can impact the risk of chronic diseases later in life due to fetal programming secondary to epigenetic changes ( 72 ). Maternal nutrition during the prenatal or early postnatal period may trigger these epigenetic changes and increase the risk for chronic conditions such as obesity, metabolic and cardiovascular disease due to epigenetic modifications that may persist and cause intergenerational effect on the health children and adults ( 58 , 73 , 74 ). Similarly, adverse childhood experiences (ACE) have been linked to a broad range of negative outcomes through epigenetic mechanisms ( 75 ) and promote unhealthy eating behaviors ( 76 , 77 ). Other factors such as diet, physical activity, environmental and psychosocial stressors can cause epigenetic changes and place an individual at risk for weight gain ( 78 ).
Developmental Factors
Eating behaviors evolve over the first few years of life. Young children learn to eat through their direct experience with food and observing others eating around them ( 79 ). During infancy, feeding defines the relationship of security and trust between a child and the parent. Early childhood eating behaviors shift to more self-directed control due to rapid physical, cognitive, communicative, and social development ( 80 ). Parents or caregivers determine the type of food that is made available to the infant and young child. However, due to economic limitations and parents having decreased time to prepare nutritious meals, consumption of processed and cheaper energy-dense foods have occurred in Western countries. Additionally, feeding practices often include providing large or super-sized portions of palatable foods and encouraging children to finish the complete meal (clean their plate even if they do not choose to), as seen across many cultures ( 81 , 82 ). Also, a segment of parents are overly concerned with dietary intake and may pressurize their child to eat what they perceive as a healthy diet, which can lead to unintended consequences ( 83 ). Parents' excessive restriction of food choices may result in poor self-regulation of energy intake by their child or adolescent. This action may inadvertently promote overconsumption of highly palatable restricted foods when available to the child or adolescent outside of parental control with resultant excessive weight gain ( 84 , 85 ).
During middle childhood, children start achieving greater independence, experience broader social networks, and expand their ability to develop more control over their food choices. Changes that occur in the setting of a new environment such as daycare or school allow exposure to different food options, limited physical activity, and often increased sedentary behaviors associated with school schedules ( 24 ). As the transition to adolescence occurs, physical and psychosocial development significantly affect food choices and eating patterns ( 25 ). During the teenage years, more independence and interaction with peers can impact the selection of fast foods that are calorically dense. Moreover, during the adolescent years, more sedentary behaviors such as video and computer use can limit physical exercise. Adolescence is also a period in development with an enhanced focus on appearance, body weight, and other psychological concerns ( 86 , 87 ).
Environmental Factors
Environmental changes within the past few decades, particularly easy access to high-calorie fast foods, increased consumption of sugary beverages, and sedentary lifestyles, are linked with rising obesity ( 88 ). The easy availability of high caloric fast foods, and super-sized portions, are increasingly common choices as individuals prefer these highly palatable and often less expensive foods over fruits and vegetables ( 89 ). The quality of lunches and snacks served in schools and childcare centers has been an area of debate and concern. Children and adolescents consume one-third to one-half of meals in the above settings. Despite policies in place at schools, encouraging foods, beverages, and snacks that are deemed healthier options, the effectiveness of these policies in improving children's dietary habits or change in obesity rate has not yet been seen ( 90 ). This is likely due to the fact that such policies primarily focus on improving dietary quality but not quantity which can impact the overweight or obese youth ( 91 ). Policies to implement taxes on sugary beverages are in effect in a few states in the US ( 92 ) as sugar and sugary beverages are associated with increased weight gain ( 2 , 3 ). This has resulted in reduction in sales of sugary drinks in these states, but the sales of these types of drinks has risen in neighboring states that did not implement the tax ( 93 ). Due to advancements in technology, children are spending increased time on electronic devices, limiting exercise options. Technology advancement is also disrupting the sleep-wake cycle, causing poor sleeping habits, and altered eating patterns ( 94 ). A study published on Canadian children showed that the access to and night-time use of electronic devices causes decreased sleep duration, resulting in excess body weight, inferior diet quality, and lower physical activity levels ( 95 ).
Infant nutrition has gained significant popularity in relation to causing overweight/obesity and other diseases later in life. Breast feeding is frequently discussed as providing protection against developing overweight/obesity in children ( 8 ). Considerable heterogeneity has been observed in studies and conducting randomized clinical trials between breast feeding vs. formula feeding is not feasible ( 8 ). Children fed with a low protein formula like breast milk are shown to have normal weight gain in early childhood as compared to those that are fed formulas with a high protein load ( 96 ). A recent Canadian childbirth cohort study showed that breast feeding within first year of life was inversely associated with weight gain and increased BMI ( 97 ). The effect was stronger if the child was exclusively breast fed directly vs. expressed breast milk or addition of formula or solid food ( 97 ). Also, due to the concern of poor growth in preterm or SGA infants, additional calories are often given for nutritional support in the form of macronutrient supplements. Most of these infants demonstrate “catch up growth.” In fact, there have been reports that in some children the extra nutritional support can increase the risk for overweight/obesity later in life. The association, however, is inconsistent. Recently a systemic review done on randomized controlled trials comparing the studies done in preterm and SGA infants with feeds with and without macronutrient supplements showed that macronutrient supplements may increase weight and length in toddlers but did not show a significant increase in the BMI during childhood ( 98 ). Increased growth velocity due to early introduction of formula milk and protein in infants' diet, may influence the obesity pathways, and can impact fetal programming for metabolic disease later in life ( 99 ).
General pediatricians caring for children with overweight/obesity, generally recommend endocrine testing as parents often believe that there may be an underlying cause for this condition and urge their primary providers to check for conditions such as thyroid abnormalities. Endocrine etiologies for obesity are rarely identified and patients with underlying endocrine disorders causing excessive weight gain usually are accompanied by attenuated growth patterns, such that a patient continues to gain weight with a decline in linear height ( 100 ). Various endocrine etiologies that one could consider in a patient with excessive weight gain in the setting of slow linear growth: severe hypothyroidism, growth hormone deficiency, and Cushing's disease/syndrome ( 58 , 100 ).
Clinical-Physiology of Pediatric Obesity
It is a well-known fact that early AR(increased BMI) before the age of 5 years is a risk factor for adult obesity, obesity-related comorbidities, and metabolic syndrome ( 101 – 103 ). Typically, body mass index (BMI) declines to a minimum in children before it starts increasing again into adulthood, also known as AR. Usually, AR happens between 5 and 7 years of age, but if it occurs before the age of 5 years is considered early AR. Early AR is a marker for higher risk for obesity-related comorbidities. These obesity-related health comorbidities include cardiovascular risk factors (hypertension, dyslipidemia, prediabetes, and type 2 diabetes), hormonal issues, orthopedic problems, sleep apnea, asthma, and fatty liver disease ( Figure 3 ) ( 9 ).

Obesity related co-morbidities a in children and adolescents. a, NAFLD, Non-Alcoholic Fatty Liver Disease; SCFE, Slipped Capital Femoral Epiphysis; PCOS, Polycystic Ovary Syndrome; OSA, Obstructive Sleep Apnea.
Clinical Comorbidities of Obesity in Children
Growth and puberty.
Excess weight gain in children can influence growth and pubertal development ( 10 ). Childhood obesity can cause prepubertal acceleration of linear growth velocity and advanced bone age in boys and girls ( 104 ). Hyperinsulinemia is a normal physiological state during puberty, but children with obesity can have abnormally high insulin levels ( 105 ). Leptin resistance also occurs in obese individuals who have higher leptin levels produced by their adipose tissue ( 55 , 106 ). The insulin and leptin levels can act on receptors that impact the growth plates with a resultant bone age advancement ( 55 ).
Adequate nutrition is essential for the typical timing and tempo of pubertal onset. Excessive weight gain can initiate early puberty, due to altered hormonal parameters ( 10 ). Obese children may present with premature adrenarche, thelarche, or precocious puberty (PP) ( 107 ). The association of early pubertal changes with obesity is consistent in girls, and is well-reported; however, data is sparse in boys ( 108 ). One US study conducted in racially diverse boys showed obese boys had delayed puberty, whereas overweight boys had early puberty as compared to normal-weight boys ( 109 ). Obese girls with PP have high leptin levels ( 110 , 111 ). Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) is a cross-sectional study and suggested an indirect relationship between elevated leptin levels, early puberty, and cardiometabolic and inflammatory markers in obese girls ( 112 ). Additionally, obese girls with premature adrenarche carry a higher risk for developing polycystic ovary syndrome (PCOS) in the future ( 113 , 114 ).
Sleep Disorders
Obesity is an independent risk factor for obstructive sleep apnea (OSA) in children and adolescents ( 12 , 115 ). Children with OSA have less deleterious consequences in terms of cardiovascular stress of metabolic syndrome when compared to adolescents and adults ( 116 , 117 ). In children, abnormal behaviors and neurocognitive dysfunction are the most critical and frequent end-organ morbidities associated with OSA ( 12 ). However, in adolescents, obesity and OSA can independently cause oxidative systemic stress and inflammation ( 118 , 119 ), and when this occurs concurrently, it can result in more severe metabolic dysfunction and cardiovascular outcomes later in life ( 120 ).
Other Comorbidities
Obesity is related to a clinical spectrum of liver abnormalities such as NAFLD ( 121 ); the most important cause of liver disease in children ( 122 – 124 ). NAFLD includes steatosis (increased liver fat without inflammation) and NASH (increased liver fat with inflammation and hepatic injury). While in some adults NAFLD can progress to an end-stage liver disease requiring liver transplant ( 125 , 126 ), the risk of progression during childhood is less well-defined ( 127 ). NAFLD is closely associated with metabolic syndrome including central obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension ( 128 ).
Obese children are also at risk for slipped capital femoral epiphysis (SCFE) ( 129 ), and sedentary lifestyle behaviors may have a negative influence on the brain structure and executive functioning, although the direction of causality is not clear ( 130 , 131 ).
Clinical Comorbidities of Obesity in Adolescents
Menstrual irregularities and pcos.
At the onset of puberty, physiologically, sex steroids can cause appropriate weight gain and body composition changes that should not affect normal menstruation ( 132 , 133 ). However, excessive weight gain in adolescent girls can result in irregular menstrual cycles and puts them at risk for PCOS due to increased androgen levels. Additionally, they can have excessive body hair (hirsutism), polycystic ovaries, and can suffer from distorted body images ( 134 , 135 ). Adolescent girls with PCOS also have an inherent risk for insulin resistance irrespective of their weight. However, weight gain further exacerbates their existing state of insulin resistance and increases the risk for obesity-related comorbidities such as metabolic syndrome, and type 2 diabetes. Although the diagnosis of PCOS can be challenging at this age due to an overlap with predictable pubertal changes, early intervention (appropriate weight loss and use of hormonal methods) can help restore menstrual cyclicity and future concerns related to childbearing ( 11 ).
Metabolic Syndrome and Sleep Disorders
Metabolic syndrome (MS) is a group of cardiovascular risk factors characterized by acanthosis nigricans, prediabetes, hypertension, dyslipidemia, and non-alcoholic steatohepatitis (NASH), that occurs from insulin resistance caused by obesity ( 136 ). Diagnosis of MS in adults requires at least three out of the five risk factors: increased central adiposity, hypertension, hyperglycemia, hypertriglyceridemia, or low HDL level. Definitions to diagnose MS are controversial in younger age groups, and many definitions have been proposed ( 136 ). This is due to the complex physiology of growth and development during puberty, which causes significant overlap between MS and features of normal growth. However, childhood obesity is associated with an inflammatory state even before puberty ( 137 ). In obese children and adolescents, hyperinsulinemia during puberty ( 138 , 139 ) and unhealthy sleep behaviors increase MS's risk and severity ( 140 ). Even though there is no consensus on diagnosis regarding MS in this age group, when dealing with obese children and adolescents, clinicians should screen them for MS risk factors and sleep behaviors and provide recommendations for weight management.
Social Psychology of Pediatric Obesity in Children and Adolescents
Obese children and adolescents may experience psychosocial sequelae, including depression, bullying, social isolation, diminished self-esteem, behavioral problems, dissatisfaction with body image, and reduced quality of life ( 13 , 141 ). Compared with normal-weight counterparts, overweight/obesity is one of the most common reasons children and adolescents are bullied at school ( 142 ). The consequence of stigma, bullying, and teasing related to childhood obesity are pervasive and can have severe implications for emotional and physical health and performance that can persist later in life ( 13 ).
In adolescents, psychological outcomes associated with obesity are multifactorial and have a bidirectional relationship ( Figure 4 ). Obese adolescents due to their physique may have a higher likelihood of psychosocial health issues, including depression, body image/dissatisfaction, lower self-esteem, peer victimization/bullying, and interpersonal relationship difficulties. They may also demonstrate reduced resilience to challenging situations compared to their non-obese/overweight counterparts ( 9 , 143 – 146 ). Body image dissatisfaction has been associated with further weight gain but can also be related to the development of a mental health disorder or an eating disorder (ED) or disorder eating habits (DEH). Mental health disorders such as depression are associated with poor eating habits, a sedentary lifestyle, and altered sleep patterns. ED or DEH that include anorexia nervosa (AN), bulimia nervosa (BN), binge-eating disorder (BED) or night eating syndrome (NES) may be related to an individual's overvaluation of their body shape and weight or can result during the treatment for obesity ( 147 – 150 ). The management of obesity can place a patient at risk of AN if there is a rigid focus on caloric intake or if a patient overcorrects and initiates obsessive self-directed dieting. Healthcare providers who primarily care for obese patients, usually give the advice to diet to lose weight and then maintain it. However, strict dieting (hypocaloric diet), which some patients may later engage in can lead to an eating disorder such as anorexia nervosa ( 151 ). This behavior leads to a poor relationship with food, and therefore, adolescents perseverate on their weight and numbers ( 152 ).

Bidirectional relationship of different psychological outcomes of obesity.
Providers may not recognize DEHs when a morbidly obese patient loses the same weight as a healthy weight individual ( 149 ). It may appear as a positive result with families and others praising the individual without realizing that this youth may be engaging in destructive behaviors related to weight control. Therefore, it is essential to screen regarding the process of how weight loss was achieved ( 144 , 150 ).
Support and attention to underlying psychological concerns can positively affect treatment, overall well-being, and reduce the risk of adult obesity ( 150 ). The diagram above represents the complexity of the different psychological issues which can impact the clinical care of the obese adolescent.
Eating family meals together can improve overall dietary intake due to enhanced food choices mirrored by parents. It has also may serve as a support to individuals with DEHs if there is less attention to weight and a greater focus on appropriate, sustainable eating habits ( 148 ).
Prevention and Anticipatory Guidance
It is essential to recognize and provide preventive measures for obesity during early childhood and adolescence ( 100 , 153 , 154 ). It is well-established that early AR is a risk factor for adult obesity ( 66 – 68 ). Therefore, health care providers caring for the pediatric population need to focus on measures such as BMI but provide anticipatory guidance regarding nutritional counseling without stigmatizing or judging parents for their children's overweight/obesity ( 155 ). Although health care providers continue to pursue effective strategies to address the obesity epidemic; ironically, they frequently exhibit weight bias and stigmatizing behaviors. Research has demonstrated that the language that health care providers use when discussing a patient's body weight can reinforce stigma, reduce motivation for weight loss, and potentially cause avoidance of routine preventive care ( 155 ). In adolescents, rather than motivating positive changes, stigmatizing language regarding weight may negatively impact a teen and result in binge eating, decreased physical activity, social isolation, avoidance of health care services, and increased weight gain ( 156 , 157 ). Effective provider-patient communication using motivational interviewing techniques are useful to encourage positive behavior changes ( 155 , 158 ).
Anticipatory guidance includes educating the families on healthy eating habits and identifying unhealthy eating practices, encouraging increased activity, limiting sedentary activities such as screen time. Lifestyle behaviors in children and adolescents are influenced by many sectors of our society, including the family ( Figure 1 ) ( 3 , 24 ). Therefore, rather than treating obesity in isolation as an individual problem, it is crucial to approach this problem by focusing on the family unit. Family-based multi-component weight loss behavioral treatment is the gold standard for treating childhood obesity, and it is having been found useful in those between 2 and 6 years old ( 150 , 159 ). Additionally, empowering the parents to play an equal role in developing and implementing an intervention for weight management has shown promising results in improving the rate of obesity by decreasing screen time, promoting healthy eating, and increasing support for children's physical activity ( 160 , 161 ).
When dietary/lifestyle modifications have failed, the next option is a structured weight -management program with a multidisciplinary approach ( 15 ). The best outcomes are associated with an interdisciplinary team comprising a physician, dietician, and psychologist generally 1–2 times a week ( 15 , 162 ). However, this treatment approach is not effective in patients with severe obesity ( 122 ). Although healthier lifestyle recommendations for weight loss are the current cornerstone for obesity management, they often fail. As clinicians can attest, these behavioral and dietary changes are hard to achieve, and all too often is not effective in patients with severe obesity. Failure to maintain substantial weight loss over the long term is due to poor adherence to the prescribed lifestyle changes as well as physiological responses that resist weight loss ( 163 ). American TV hosts a reality show called “The Biggest Loser” that centers on overweight and obese contestants attempting to lose weight for a cash prize. Contestants from “The Biggest Loser” competition, had metabolic adaptation (MA) after drastic weight loss, regained more than they lost weight after 6 years due to a significant slow resting metabolic rate ( 164 ). MA is a physiological response which is a reduced basal metabolic rate seen in individuals who are losing or have lost weight. In MA, the body alters how efficient it is at turning the food eaten into energy; it is a natural defense mechanism against starvation and is a response to caloric restriction. Plasma leptin levels decrease substantially during caloric restriction, suggesting a role of this hormone in the drop of energy expenditure ( 165 ).
Pharmacological Management
The role of pharmacological therapy in the treatment of obesity in children and adolescents is limited.
Orlistat is the only FDA approved medication for weight loss in 12-18-year-olds but has unpleasant side effects ( 166 ). Another medicine, Metformin, has been used in children with signs of insulin resistance, may have some impact on weight, but is not FDA approved ( 167 ). The combination of phentermine/topiramate (Qsymia) has been FDA approved for weight loss in obese individuals 18 years and older. In studies, there has been about 9–10% weight loss over 2 years. However, caution must be taken in females as it can lead to congenital disabilities, especially with use in the first trimester of pregnancy ( 167 ).
GLP-1 agonists have demonstrated great success in effective weight loss and are approved by the FDA for adult obesity ( 168 – 170 ). A randomized control clinical trial recently published showed a significant weight loss in those using liraglutide (3.0 mg)/day plus lifestyle therapy group compared to placebo plus lifestyle therapy in children between the ages of 12–18 years ( 171 ).
Recently during the EASL conference, academic researchers and industry partners presented novel interventions targeting different gut- liver axis levels that include intestinal content, intestinal microbiome, intestinal mucosa, and peritoneal cavity ( 47 ). The focus for these therapeutic interventions within the gut-liver axis was broad and ranged anywhere from newer drugs protecting the intestinal mucus lining, restoring the intestinal barriers and improvement in the gut microbiome. One of the treatment options was Hydrogel technology which was shown to be effective toward weight loss in patients with metabolic syndrome. Hydrogel technology include fibers and high viscosity polysaccharides that absorb water in the stomach and increasing the volume, thereby improving satiety ( 47 ). Also, a clinical trial done in obese pregnant mothers using Docosahexaenoic acid (DHA) showed that the mothers' who got DHA had children with lower adiposity at 2 and 4 years of age ( 172 ). Recently the role of probiotics in combating obesity has emerged. Probiotics are shown to alter the gut microbiome that improves intestinal digestive and absorptive functions of the nutrients. Intervention including probiotics may be a possible solution to manage pediatric obesity ( 173 , 174 ). Additionally, the role of Vitamin E for treating the comorbidities of obesity such as diabetes, hyperlipidemia, NASH, and cardiovascular risk, has been recently described ( 175 , 176 ). Vitamin E is a lipid- soluble compound and contains both tocopherols and tocotrienols. Tocopherols have lipid-soluble antioxidants properties that interact with cellular lipids and protects them from oxidation damage ( 177 ). In metabolic disease, certain crucial pathways are influenced by Vitamin E and some studies have summarized the role of Vitamin E regarding the treatment of obesity, metabolic, and cardiovascular disease ( 178 ). Hence, adequate supplementation of Vitamin E as an appropriate strategy to help in the treatment of the prevention of obesity and its associated comorbidities has been suggested. Nonetheless, some clinical trials have shown contradictory results with Vitamin E supplementation ( 177 ). Although Vitamin E has been recognized as an antioxidant that protects from oxidative damage, however, a full understanding of its mechanism of action is still lacking.
Bariatric Surgery
Bariatric surgery has gained popularity since the early 2000s in the management of severe obesity. If performed earlier, there are better outcomes for reducing weight and resolving obesity-related comorbidities in adults ( 179 – 182 ). Currently, the indication for bariatric in adolescents; those who have a BMI >35 with at least one severe comorbidity (Type 2 Diabetes, severe OSA, pseudotumor cerebri or severe steatohepatitis); or BMI of 40 or more with other comorbidities (hypertension, hyperlipidemia, mild OSA, insulin resistance or glucose intolerance or impaired quality of life due to weight). Before considering bariatric surgery, these patients must have completed most of their linear growth and participated in a structured weight-loss program for 6 months ( 159 , 181 , 183 ). The American Society for Metabolic and Bariatric Surgery (AMBS) outlines the multidisciplinary approach that must be taken before a patient undergoing bariatric surgery. In addition to a qualified bariatric surgeon, the patient must have a pediatrician or provider specialized in adolescent medicine, endocrinology, gastroenterology and nutrition, registered dietician, mental health provider, and exercise specialist ( 181 ). A mental health provider is essential as those with depression due to obesity or vice versa may have persistent mental health needs even after weight loss surgery ( 184 ).
Roux-en-Y Gastric Bypass (RYGB), laparoscopic Sleeve Gastrectomy (LSG), and Gastric Banding are the options available. RYGB and LSG currently approved for children under 18 years of age ( 166 , 181 , 185 ). At present, gastric banding is not an FDA recommended procedure in the US for those under 18y/o. One study showed some improvements in BMI and severity of comorbidities but had multiple repeat surgeries and did not believe a suitable option for obese adolescents ( 186 ).
Compared to LSG, RYGB has better outcomes for excess weight loss and resolution of obesity-related comorbidities as shown in studies and clinical trials ( 183 , 184 , 187 ). Overall, LSG is a safer choice and may be advocated for more often ( 179 – 181 ). The effect on the Gut-Brain axis after Bariatric surgery is still inconclusive, especially in adolescents, as the number of procedures performed is lower than in adults. Those who underwent RYGB had increased fasting and post-prandial PYY and GLP-1, which could have contributed to the rapid weight loss ( 185 ); this effect was seen less often in patients with gastric banding ( 185 ). Another study in adult patients showed higher bile acid (BA) subtype levels and suggested a possible BA's role in the surgical weight loss response after LSG ( 188 ). Adolescents have lower surgical complication rates than their adult counterparts, hence considering bariatric surgery earlier rather than waiting until adulthood has been entertained ( 180 ). Complications after surgery include nutritional imbalance in iron, calcium, Vitamin D, and B12 and should be monitored closely ( 180 , 181 , 185 ). Although 5-year data for gastric bypass in very obese teens is promising, lifetime outcome is still unknown, and the psychosocial factors associated with adolescent adherence post-surgery are also challenging and uncertain.
Obesity in childhood and adolescence is not amenable to a single easily modified factor. Biological, cultural, and environmental factors such as readily available high-density food choices impact youth eating behaviors. Media devices and associated screen time make physical activity a less optimal choice for children and adolescents. This review serves as a reminder that the time for action is now. The need for interventions to change the obesogenic environment by instituting policies around the food industry and in the schools needs to be clarified. In clinical trials GLP-1 agonists are shown to be effective in weight loss in children but are not yet FDA approved. Discovery of therapies to modify the gut microbiota as treatment for overweigh/obesity through use of probiotics or fecal transplantation would be revolutionary. For the present, ongoing clinical research efforts in concert with pharmacotherapeutic and multidisciplinary lifestyle programs hold promise.
Author Contributions
AK, SL, and MJ contributed to the conception and design of the study. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 18 May 2023
Child and adolescent obesity
- Natalie B. Lister ORCID: orcid.org/0000-0002-9148-8632 1 , 2 ,
- Louise A. Baur ORCID: orcid.org/0000-0002-4521-9482 1 , 3 , 4 ,
- Janine F. Felix 5 , 6 ,
- Andrew J. Hill ORCID: orcid.org/0000-0003-3192-0427 7 ,
- Claude Marcus ORCID: orcid.org/0000-0003-0890-2650 8 ,
- Thomas Reinehr ORCID: orcid.org/0000-0002-4351-1834 9 ,
- Carolyn Summerbell 10 &
- Martin Wabitsch ORCID: orcid.org/0000-0001-6795-8430 11
Nature Reviews Disease Primers volume 9 , Article number: 24 ( 2023 ) Cite this article
33k Accesses
31 Citations
250 Altmetric
Metrics details
- Paediatric research
The prevalence of child and adolescent obesity has plateaued at high levels in most high-income countries and is increasing in many low-income and middle-income countries. Obesity arises when a mix of genetic and epigenetic factors, behavioural risk patterns and broader environmental and sociocultural influences affect the two body weight regulation systems: energy homeostasis, including leptin and gastrointestinal tract signals, operating predominantly at an unconscious level, and cognitive–emotional control that is regulated by higher brain centres, operating at a conscious level. Health-related quality of life is reduced in those with obesity. Comorbidities of obesity, including type 2 diabetes mellitus, fatty liver disease and depression, are more likely in adolescents and in those with severe obesity. Treatment incorporates a respectful, stigma-free and family-based approach involving multiple components, and addresses dietary, physical activity, sedentary and sleep behaviours. In adolescents in particular, adjunctive therapies can be valuable, such as more intensive dietary therapies, pharmacotherapy and bariatric surgery. Prevention of obesity requires a whole-system approach and joined-up policy initiatives across government departments. Development and implementation of interventions to prevent paediatric obesity in children should focus on interventions that are feasible, effective and likely to reduce gaps in health inequalities.
Similar content being viewed by others

Unraveling Complexity about Childhood Obesity and Nutritional Interventions: Modeling Interactions Among Psychological Factors
Keith Feldman, Gisela M. B. Solymos, … Nitesh V. Chawla

Pediatric weight management interventions improve prevalence of overeating behaviors
Stephanie G. Harshman, Ines Castro, … Lauren Fiechtner
Parent and child characteristics associated with treatment non-response to a short- versus long-term lifestyle intervention in pediatric obesity
Sarah Woo, Hong Ji Song, … Kyung Hee Park
Introduction
The prevalence of child and adolescent obesity remains high and continues to rise in low-income and middle-income countries (LMICs) at a time when these regions are also contending with under-nutrition in its various forms 1 , 2 . In addition, during the COVID-19 pandemic, children and adolescents with obesity have been more likely to have severe COVID-19 requiring hospitalization and mechanical ventilation 3 . At the same time, the pandemic was associated with rising levels of childhood obesity in many countries. These developments are concerning, considering that recognition is also growing that paediatric obesity is associated with a range of immediate and long-term negative health outcomes, a decreased quality of life 4 , 5 , an increased presentation to health services 6 and increased economic costs to individuals and society 7 .
Body weight is regulated by a range of energy homeostatic and cognitive–emotional processes and a multifactorial interplay of complex regulatory circuits 8 . Paediatric obesity arises when multiple environmental factors — covering preconception and prenatal exposures, as well as broader changes in the food and physical activity environments — disturb these regulatory processes; these influences are now widespread in most countries 9 .
The treatment of obesity includes management of obesity-associated complications, a developmentally sensitive approach, family engagement, and support for long-term behaviour changes in diet, physical activity, sedentary behaviours and sleep 10 . New evidence highlights the role, in adolescents with more severe obesity, of bariatric surgery 11 and pharmacotherapy, particularly the potential for glucagon-like peptide 1 (GLP1) receptor agonists 12 .
Obesity prevention requires a whole-system approach, with policies across all government and community sectors systematically taking health into account, avoiding harmful health impacts and decreasing inequity. Programmatic prevention interventions operating ‘downstream’ at the level of the child and family, as well as ‘upstream’ interventions at the level of the community and broader society, are required if a step change in tackling childhood obesity is to be realized 13 , 14 .
In this Primer, we provide an overview of the epidemiology, causes, pathophysiology and consequences of child and adolescent obesity. We discuss diagnostic considerations, as well as approaches to its prevention and management. Furthermore, we summarize effects of paediatric obesity on quality of life, and open research questions.
Epidemiology
Definition and prevalence.
The World Health Organization (WHO) defines obesity as “abnormal or excessive fat accumulation that presents a risk to health” 15 . Paediatric obesity is defined epidemiologically using BMI, which is adjusted for age and sex because of the physiological changes in BMI during growth 16 . Global prevalence of paediatric obesity has risen markedly over the past four decades, initially in high-income countries (HICs), but now also in many LMICs 1 .
Despite attempts to standardize the epidemiological classification, several definitions of paediatric obesity are in use; hence, care is needed when comparing prevalence rates. The 2006 WHO Child Growth Standard, for children aged 0 to 5 years, is based on longitudinal observations of multiethnic populations of children with optimal infant feeding and child-rearing conditions 17 . The 2007 WHO Growth Reference is used for the age group 5–19 years 18 , and the 2000 US Centers for Disease Control and Prevention (CDC) Growth Charts for the age group 2–20 years 19 . The WHO and CDC definitions based on BMI-for-age charts are widely used, including in clinical practice. By contrast, the International Obesity Task Force (IOTF) definition, developed from nationally representative BMI data for the age group 2–18 years from six countries, is used exclusively for epidemiological studies 20 .
For the age group 5–19 years, between 1975 and 2016, the global prevalence of obesity (BMI >2 standard deviations (SD) above the median of the WHO growth reference) increased around eightfold to 5.6% in girls and 7.8% in boys 1 . Rates have plateaued at high levels in many HICs but have accelerated in other regions, particularly in parts of Asia. For the age group 2–4 years, between 1980 and 2015, obesity prevalence (IOTF definition, equivalent to an adult BMI of ≥30 kg/m 2 ) increased from 3.9% to 7.2% in boys and from 3.7% to 6.4% in girls 21 . Obesity prevalence is highest in Polynesia and Micronesia, the Middle East and North Africa, the Caribbean and the USA (Fig. 1 ). Variations in prevalence probably reflect different background levels of obesogenic environments, or the sum total of the physical, economic, policy, social and cultural factors that promote obesity 22 . Obesogenic environments include those with decreased active transport options, a ubiquity of food marketing directed towards children, and reduced costs and increased availability of nutrient-poor, energy-dense foods. Particularly in LMICs, the growth of urbanization, new forms of technology and global trade have led to reduced physical activity at work and leisure, a shift towards Western diets, and the expansion of transnational food and beverage companies to shape local food systems 23 .
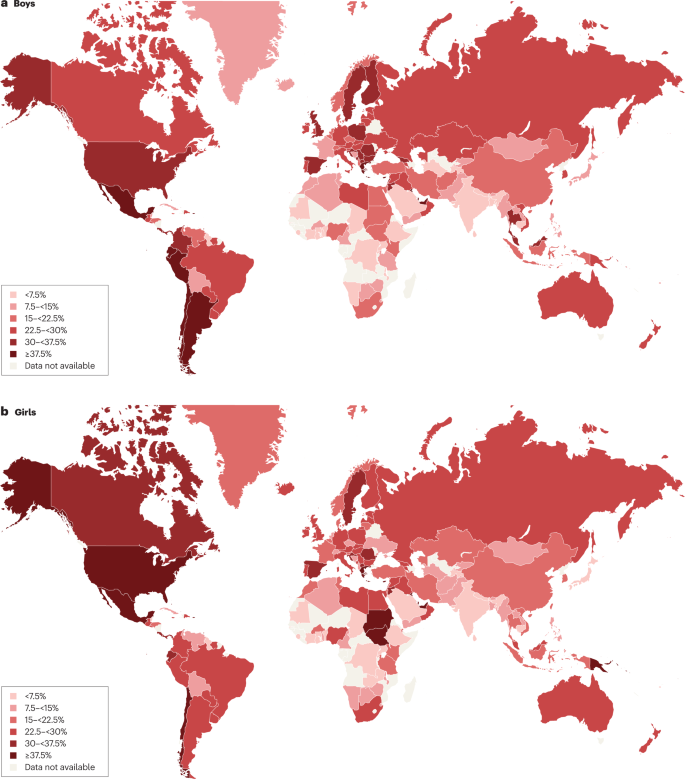
Maps showing the proportions of children and adolescents living with overweight or obesity (part a , boys; part b , girls) according to latest available data from the Global Obesity Observatory . Data might not be comparable between countries owing to differences in survey methodology.
The reasons for varying sex differences in prevalence in different countries are unclear but may relate to cultural variations in parental feeding practices for boys and girls and societal ideals of body size 24 . In 2016, obesity in the age group 5–19 years was more prevalent in girls than in boys in sub-Saharan Africa, Oceania and some middle-income countries in other regions, whereas it was more prevalent in boys than in girls in all HICs, and in East and South-East Asia 21 . Ethnic and racial differences in obesity prevalence within countries are often assumed to mirror variations in social deprivation and other social determinants of obesity. However, an independent effect of ethnicity even after adjustment for socioeconomic status has been documented in the UK, with Black and Asian boys in primary school having higher prevalence of obesity than white boys 25 .
Among individuals with obesity, very high BMI values have become more common in the past 15 years. The prevalence of severe obesity (BMI ≥120% of the 95th percentile (CDC definition), or ≥35 kg/m 2 at any age 26 , 27 ) has increased in many HICs, accounting for one-quarter to one-third of those with obesity 28 , 29 . Future health risks of paediatric obesity in adulthood are well documented. For example, in a data linkage prospective study in Israel with 2.3 million participants who had BMI measured at age 17 years, those with obesity (≥95th percentile BMI for age) had a much higher risk of death from coronary heart disease (HR 4.9, 95% CI 3.9–6.1), stroke (HR 2.6, 95% CI 1.7–4.1) and sudden death (HR 2.1, 95% CI 1.5–2.9) compared with those whose BMI fell between the 5th and 24th percentiles 30 .
Causes and risk factors
Early life is a critical period for childhood obesity development 9 , 31 , 32 , 33 . According to the Developmental Origins of Health and Disease framework, the early life environment may affect organ structure and function and influence health in later life 34 , 35 . Meta-analyses have shown that preconception and prenatal environmental exposures, including high maternal pre-pregnancy BMI and, to a lesser extent, gestational weight gain, as well as gestational diabetes and maternal smoking, are associated with childhood obesity, potentially through effects on the in utero environment 33 , 36 , 37 , 38 . Paternal obesity is also associated with childhood obesity 33 . Birthweight, reflecting fetal growth, is a proxy for in utero exposures. Both low and high birthweights are associated with later adiposity, with high birthweight linked to increased BMI and low birthweight to central obesity 33 , 39 .
Growth trajectories in early life are important determinants of later adiposity. Rapid weight gain in early childhood is associated with obesity in adolescence 32 . Also, later age and higher BMI at adiposity peak (the usual peak in BMI around 9 months of age), as well as earlier age at adiposity rebound (the lowest BMI reached between 4 and 7 years of age), are associated with increased adolescent and adult BMI 40 , 41 . Specific early life nutritional factors, including a lower protein content in formula food, are consistently associated with a lower risk of childhood obesity 42 , 43 . These also include longer breastfeeding duration, which is generally associated with a lower risk of childhood obesity 42 . However, some controversy exists, as these effects are affected by multiple sociodemographic confounding factors and their underlying mechanisms remain uncertain 44 . Some studies comparing higher and lower infant formula protein content have reported that the higher protein group have a greater risk of subsequent obesity, especially in early childhood 41 , 42 ; however, one study with a follow-up period until age 11 years found no significant difference in the risk of obesity, but an increased risk of overweight in the high protein group was still observed 42 , 43 , 45 . A high intake of sugar-sweetened beverages is associated with childhood obesity 33 , 46 .
Many other behavioural factors are associated with an increased risk of childhood obesity, including increased screen time, short sleep duration and poor sleep quality 33 , 47 , reductions in physical activity 48 and increased intake of energy-dense micronutrient-poor foods 49 . These have been influenced by multiple changes in the past few decades in the broader social, economic, political and physical environments, including the widespread marketing of food and beverages to children, the loss of walkable green spaces in many urban environments, the rise in motorized transport, rapid changes in the use of technology, and the move away from traditional foods to ultraprocessed foods.
Obesity prevalence is inextricably linked to relative social inequality, with data suggesting a shift in prevalence over time towards those living with socioeconomic disadvantage, and thus contributes to social inequalities. In HICs, being in lower social strata is associated with a higher risk of obesity, even in infants and young children 50 , whereas the opposite relationship occurs in middle-income countries 51 . In low-income countries, the relationship is variable, and the obesity burden seems to be across socioeconomic groups 52 , 53 .
Overall, many environmental, lifestyle, behavioural and social factors in early life are associated with childhood obesity. These factors cannot be seen in isolation but are part of a complex interplay of exposures that jointly contribute to increased obesity risk. In addition to multiple prenatal and postnatal environmental factors, genetic variants also have a role in the development of childhood obesity (see section Mechanisms/pathophysiology).
Comorbidities and complications
Childhood obesity is associated with a wide range of short-term comorbidities (Fig. 2 ). In addition, childhood obesity tracks into adolescence and adulthood and is associated with complications across the life course 32 , 41 , 54 , 55 .
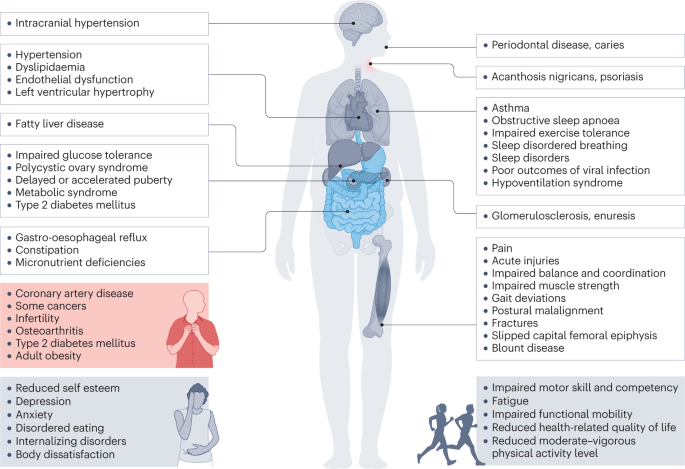
Obesity in children and adolescents can be accompanied by various other pathologies. In addition, childhood obesity is associated with complications and disorders that manifest in adulthood (red box).
Increased BMI, especially in adolescence, is linked to a higher risk of many health outcomes, including metabolic disorders, such as raised fasting glucose, impaired glucose tolerance, type 2 diabetes mellitus (T2DM), metabolic syndrome and fatty liver disease 56 , 57 , 58 , 59 . Other well-recognized obesity-associated complications include coronary heart disease, asthma, obstructive sleep apnoea syndrome (itself associated with metabolic dysfunction and inflammation) 60 , orthopaedic complications and a range of mental health outcomes including depression and low self-esteem 27 , 55 , 57 , 61 , 62 , 63 .
A 2019 systematic review showed that children and adolescents with obesity are 1.4 times more likely to have prediabetes, 1.7 times more likely to have asthma, 4.4 times more likely to have high blood pressure and 26.1 times more likely to have fatty liver disease than those with a healthy weight 64 . In 2016, it was estimated that, at a global level by 2025, childhood obesity would lead to 12 million children aged 5–17 years with glucose intolerance, 4 million with T2DM, 27 million with hypertension and 38 million with fatty liver disease 65 . These high prevalence rates have implications for both paediatric and adult health services.
Mechanisms/pathophysiology
Body weight regulation.
Body weight is regulated within narrow limits by homeostatic and cognitive–emotional processes and a multifactorial interplay of hormones and messenger substances in complex regulatory circuits (Fig. 3 ). When these regulatory circuits are disturbed, an imbalance between energy intake and expenditure leads to obesity or to poor weight gain. As weight loss is much harder to achieve than weight gain in the long term due to the regulation circuits discussed below, the development of obesity is encouraged by modern living conditions, which enable underlying predispositions for obesity to become manifest 8 , 66 .
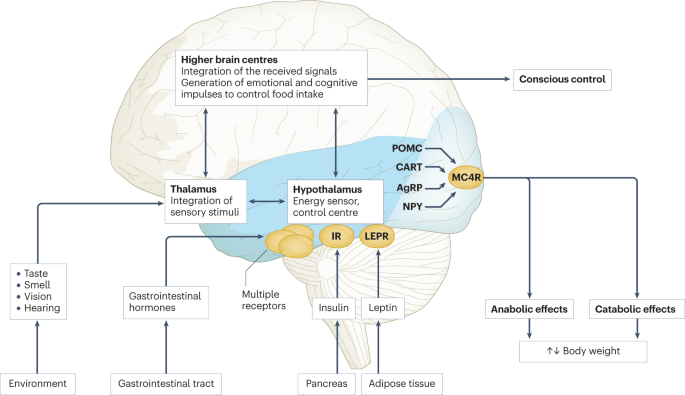
Body weight is predominantly regulated by two systems: energy homeostasis and cognitive–emotional control. Both homeostatic and non-homeostatic signals are processed in the brain, involving multiple hormone and receptor cascades 217 , 218 , 219 . This overview depicts the best-known regulatory pathways. The homeostatic system, which is mainly regulated by brain centres in the hypothalamus and brainstem, operates on an unconscious level. Both long-term signals from the energy store in adipose tissue (for example, leptin) and short-term hunger and satiety signals from the gastrointestinal tract signal the current nutrient status. During gastric distension or after the release of gastrointestinal hormones (multiple receptors are involved) and insulin, a temporary feeling of fullness is induced. The non-homeostatic or hedonic system is regulated by higher-level brain centres and operates at the conscious level. After integration in the thalamus, homeostatic signals are combined with stimuli from the environment, experiences and emotions; emotional and cognitive impulses are then induced to control food intake. Regulation of energy homeostasis in the hypothalamus involves two neuron types of the arcuate nucleus: neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and neurons producing pro-opiomelanocortin (POMC). Leptin stimulates these neurons via specific leptin receptors (LEPR) inducing anabolic effects in case of decreasing leptin levels and catabolic effects in case of increasing leptin levels. Leptin inhibits the production of NPY and AgRP, whereas low leptin levels stimulate AgRP and NPY production resulting in the feeling of hunger. Leptin directly stimulates POMC production in POMC neurons. POMC is cleaved into different hormone polypeptides including α-melanocyte-stimulating hormone which in turn activates melanocortin 4 receptors (MC4R) of cells in the nucleus paraventricularis of the hypothalamus, leading to the feeling of satiety. CART, cocaine and amphetamine responsive transcript; IR, insulin receptor.
In principle, there are two main systems in the brain which regulate body weight 8 , 66 (Fig. 3 ): energy homeostasis and cognitive–emotional control. Energy homeostasis is predominantly regulated by brain centres in the hypothalamus and brainstem and operates at an unconscious level. Both long-term signals from the adipose tissue energy stores and short-term hunger and satiety signals from the gastrointestinal tract signal the current nutrient status 8 , 66 . For example, negative energy balance leading to reduced fat mass results in reduced leptin levels, a permanently reduced urge to exercise and an increased feeling of hunger. During gastric distension or after the release of gastrointestinal hormones and insulin, a temporary feeling of fullness is induced 8 , 66 . Cognitive–emotional control is regulated by higher brain centres and operates at a conscious level. Here, the homeostatic signals are combined with stimuli from the environment (sight, smell and taste of food), experiences and emotions 8 , 66 . Disorders at the level of cognitive–emotional control mechanisms include emotional eating as well as eating disorders. For example, the reward areas in the brain of people with overweight are more strongly activated by high-calorie foods than those in the brain of people with normal weight 67 . Both systems interact with each other, and the cognitive–emotional system is strongly influenced by the homeostatic control circuits.
Disturbances in the regulatory circuits of energy homeostasis can be genetically determined, can result from disease or injury to the regulatory centres involved, or can be caused by prenatal programming 8 , 66 . If the target value of body weight has been shifted, the organism tries by all means (hunger, drive) to reach the desired higher weight. These disturbed signals of the homeostatic system can have an imperative, irresistible character, so that a conscious influence on food intake is no longer effectively possible 8 , 66 . The most important disturbances of energy homeostasis are listed in Table 1 .
The leptin pathway
The peptide hormone leptin is primarily produced by fat cells. Its production depends on the amount of adipose tissue and the energy balance. A negative energy balance during fasting results in a reduction of circulating leptin levels by 50% after 24 h (ref. 68 ). In a state of weight loss, leptin production is reduced 69 . In the brain, leptin stimulates two neuron types of the arcuate nucleus in the hypothalamus via specific leptin receptors: neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and neurons producing pro-opiomelanocortin (POMC). High leptin levels inhibit the production of NPY and AgRP, whereas low leptin levels stimulate AgRP and NPY production. By contrast, leptin directly stimulates POMC production in POMC neurons (Fig. 3 ). POMC is a hormone precursor that is cleaved into different hormone polypeptides by specific enzymes, such as prohormone convertase 1 (PCSK1). This releases α-melanocyte-stimulating hormone (α-MSH) which in turn activates melanocortin 4 receptors (MC4R) of cells in the nucleus paraventricularis of the hypothalamus, leading to the feeling of satiety. Rare, functionally relevant mutations in the genes for leptin and leptin receptor, POMC , PCSK1/3 or MC4R lead to extreme obesity in early childhood. These forms of obesity are potential indications for specific pharmacological treatments, for example setmelanotide 70 , 71 . MC4R mutations are the most common cause of monogenic obesity, as heterozygous mutations can be symptomatic depending on the functional impairment and with variable penetrance and expression. Other genes have been identified, in which rare heterozygous pathological variants are also associated with early onset obesity (Table 1 ).
Pathological changes in adipose tissue
Adipose tissue can be classified into two types, white and brown adipose tissue. White adipose tissue comprises unilocular fat cells and brown adipose tissue contains multilocular fat cells, which are rich in mitochondria 72 . A third type of adipocyte, beige adipocytes, within the white adipose tissue are induced by prolonged exposure to cold or adrenergic signalling, and show a brown adipocyte-like morphology 72 . White adipose tissue has a large potential to change its volume to store energy and meet the metabolic demands of the body. The storage capacity and metabolic function of adipose tissue depend on the anatomical location of the adipose tissue depot. Predominant enlargement of white adipose tissue in the visceral, intra-abdominal area (central obesity) is associated with insulin resistance and an increased risk of metabolic disease development before puberty. Accumulation of adipose tissue in the hips and flanks has no adverse effect and may be protective against metabolic syndrome. In those with obesity, adipose tissue is characterized by an increased number of adipocytes (hyperplasia), which originate from tissue-resident mesenchymal stem cells, and by enlarged adipocytes (hypertrophy) 73 . Adipocytes with a very large diameter reach the limit of the maximal oxygen diffusion distance, resulting in hypoxia, the development of an inflammatory expression profile (characterized by, for example, leptin, TNF and IL-6) and adipocyte necrosis, triggering the recruitment of leukocytes. Resident macrophages switch from the anti-inflammatory M2 phenotype to a pro-inflammatory M1 phenotype, which is associated with insulin resistance, further promoting local sterile inflammation and the development of fibrotic adipose tissue. This process limits the expandability of the adipose tissue for further storage of triglycerides. In the patient, the increase in fat mass in obesity is associated with insulin resistance and systemic low-grade inflammation characterized by elevated serum levels of C-reactive protein and pro-inflammatory cytokines. The limitation of adipose tissue expandability results in storage of triglycerides in other organs, such as the liver, muscle and pancreas 74 .
Genetics and epigenetics in the general population
Twin studies have found heritability estimates for BMI of up to 70% 75 , 76 . In contrast to rare monogenic forms of obesity, which are often caused by a single genetic defect with a large effect, the genetic background of childhood obesity in the general population is shaped by the joint effects of many common genetic variants, each of which individually makes a small contribution to the phenotype. For adult BMI, genome-wide association studies, which examine associations of millions of such variants across the genome at the same time, have identified around 1,000 genetic loci 77 . The largest genome-wide association studies in children, which include much smaller sample sizes of up to 60,000 children, have identified 25 genetic loci for childhood BMI and 18 for childhood obesity, the majority of which overlap 78 , 79 . There is also a clear overlap with genetic loci identified in adults, for example for FTO , MC4R and TMEM18 , but this overlap is not complete, some loci are specific to early life BMI, or have a relatively larger contribution in childhood 78 , 79 , 80 . These findings suggest that biological mechanisms underlying obesity in childhood are mostly similar to those in adulthood, but the relative influence of these mechanisms may differ at different phases of life.
The role of epigenetic processes in childhood and adolescent obesity has gained increasing attention. In children, several studies found associations between DNA methylation and BMI 81 , 82 , 83 , 84 , but a meta-analysis including data from >4,000 children identified only minimal associations 85 . Most studies support the hypothesis that DNA methylation changes are predominantly a consequence rather than a cause of obesity, which may explain the lower number of identified (up to 12) associations in children, in whom duration of exposure to a higher BMI is shorter than in adults, in whom associations with DNA methylation at hundreds of sites have been identified 85 , 86 , 87 . In addition to DNA methylation, some specific circulating microRNAs have been found to be associated with obesity in childhood 84 .
The field of epigenetic studies in childhood obesity is relatively young and evolving quickly. Future studies will need to focus on defining robust associations in blood as well as other tissues and on identifying cause-and-effect relationships. In addition, other omics, such as metabolomics and proteomics, are promising areas that may contribute to an improved aetiological understanding or may provide biological signatures that can be used as predictive or prognostic markers of childhood obesity and its comorbidities.
Parental obesity and childhood obesity
There is an established link between increased parental BMI and increased childhood BMI 88 , 89 . This link may be due to shared genetics, shared environment, a direct intrauterine effect of maternal BMI or a combination of these factors. In the case of shared genetics, the child inherits BMI-increasing genetic variants from one or both parents. Shared environmental factors, such as diet or lifestyle, may also contribute to an increased BMI in both parents and child. In addition, maternal obesity might create an intrauterine environment that programmes metabolic processes in the fetus, which increases the risk of childhood obesity. Some studies show larger effects of maternal than paternal BMI, indicating a potential causal intrauterine mechanism of maternal obesity, but evidence showing similar maternal and paternal effects is increasing. The data may indicate that there is only a limited direct intrauterine effect of maternal obesity on childhood obesity; rather, genetic effects inherited from the mother or father, or both, and/or shared environmental factors may contribute to childhood obesity risk 90 , 91 , 92 , 93 , 94 , 95 .
Diagnosis, screening and prevention
Diagnostic work-up.
The extent of overweight in clinical practice is estimated using BMI based on national charts 96 , 97 , 98 , 99 , 100 . Of note, the clinical classification of overweight or obesity differ depending on the BMI charts used and national recommendations; hence, local guidelines should be referred to. For example, the US CDC Growth Charts and several others use the 85th and 95th centile cut-points to denote overweight and obesity, respectively 19 . The WHO Growth Reference for children aged 5–19 years defines cut-points for overweight and obesity as a BMI-for-age greater than +1 and +2 SDs for BMI for age, respectively 18 . For children <5 years of age, overweight and obesity are defined as weight-for-height greater than +2 and +3 SDs, respectively, above the WHO Child Growth Standards median 17 . The IOTF and many countries in Europe use cut-points of 85th, 90th and 97th to define overweight, obesity and extreme obesity 26 .
BMI as an indirect measurement of body fat has some limitations; for example, pronounced muscle tissue leads to an increase in BMI, and BMI is not independent of height. In addition, people of different ethnicities may have different cut-points for obesity risk; for example, cardiometabolic risk occurs at lower BMI values in individuals with south Asian than in those with European ancestry 101 . Thus, BMI is best seen as a convenient screening tool that is supplemented by clinical assessment and investigations.
Other measures of body fat may help differentiate between fat mass and other tissues. Some of these tools are prone to low reliability, such as body impedance analyses (high day-to-day variation and dependent on level of fluid consumption) or skinfold thickness (high inter-observer variation), or are more expensive or invasive, such as MRI, CT or dual-energy X-ray absorptiometry, than simpler measures of body composition or BMI assessment.
Primary diseases rarely cause obesity in children and adolescents (<2%) 102 . However, treatable diseases should be excluded in those with obesity. A suggested diagnostic work-up is summarized in Fig. 4 . Routine measurement of thyroid-stimulating hormone (TSH) is not recommended 96 . Moderately elevated TSH levels (usually <10 IU/l) are frequently observed in obesity and are a consequence, and not a cause, of obesity 103 . In a growing child with normal height velocity, a normal BMI at the age of 2 years and normal cognitive development, no further diagnostic steps are necessary to exclude primary diseases 96 , 104 .
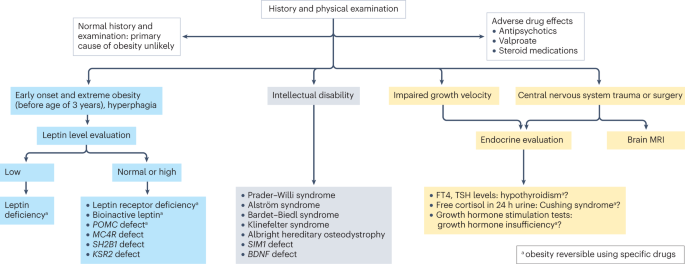
Concerning findings from a detailed medical history and physical examination will lead to further examinations. In individuals with early onset, extreme obesity (before age 3 years) and signs of hyperphagia, serum leptin level should be measured to rule out the extremely rare condition of congenital leptin deficiency. In individuals with normal or high leptin levels, genetic testing is indicated to search for monogenetic obesity. In individuals with intellectual disability, a syndromic disease may be present. Signs of impaired growth velocity or the history of central nervous system trauma or surgery will result in deeper endocrine evaluation and/or brain MRI. BDNF , brain-derived neurotropic factor; FT4, free thyroxin; KSR2 , kinase suppressor of ras 2; MC4R , melanocortin 4 receptor; POMC , pro-opiomelanocortin; SH2B1 , Src-homology 2 (SH2) B adapter protein 1; SIM1 , single-minded homologue 1; TSH, thyroid-stimulating hormone.
Clinical findings which need no further examination include pseudogynaecomastia (adipose tissue mimicking breast development; differentiated from breast tissue by ultrasonography), striae (caused by rapid weight increase) and a hidden penis in suprapubic adipose tissue (differentiated from micropenis by measurement of stretched penis length while pressing down on the suprapubic adipose tissue) 96 , 105 . Girls with obesity tend to have an earlier puberty onset (usually at around 8–9 years of age) and boys with severe obesity may have a delayed puberty onset (usually at around 13–14 years of age) 106 . Thus, if pubertal onset is slightly premature in girls or slightly delayed in boys, no further endocrine assessment is necessary.
Assessment of obesity-associated comorbidities
A waist to height ratio of >0.5 is a simple tool to identify central obesity 107 , 108 . Screening for cardiometabolic risk factors and fatty liver disease is recommended, especially in adolescents, and in those with more severe obesity or central adiposity, a strong family history of T2DM or premature heart disease, or relevant clinical symptoms, such as high blood pressure or acanthosis nigricans 96 , 97 , 98 , 99 , 109 . Investigations generally include fasting glucose levels, lipid profile, liver function and glycated haemoglobin, and might include an oral glucose tolerance test, polysomnography, and additional endocrine tests for polycystic ovary syndrome 96 , 97 , 98 , 99 .
T2DM in children and adolescents often occurs in the presence of a strong family history and may not be related to obesity severity 110 . T2DM onset usually occurs during puberty, a physiological state associated with increased insulin resistance 111 and, therefore, screening for T2DM should be considered in children and adolescents with obesity and at least one risk factor (family history of T2DM or features of metabolic syndrome) starting at pubertal onset 112 . As maturity-onset diabetes of the young (MODY) type II and type III are more frequent than T2DM in children and adolescents in many ethnicities, genetic screening for MODY may be appropriate 112 . Furthermore, type 1 diabetes mellitus (T1DM) should be excluded by measurement of autoantibodies in any individual with suspected diabetes with obesity. The differentiation of T2DM from MODY and T1DM is important as the diabetes treatment approaches differ 112 .
Several comorbidities of obesity should be considered if specific symptoms occur 96 , 109 . For polycystic ovary syndrome in hirsute adolescent girls with oligomenorrhoea or amenorrhoea, moderately increased testosterone levels and decreased sex hormone binding globulin levels are typical laboratory findings 113 . Obstructive sleep apnoea can occur in those with more severe obesity and who snore, have daytime somnolence or witnessed apnoeas. Diagnosis is made by polysomnography 114 . Minor orthopaedic disorders, such as flat feet and genu valgum, are frequent in children and adolescents with obesity and may cause pain. Major orthopaedic complications include slipped capital femoral epiphyses (acute and chronic), which manifest with hip and knee pain in young adolescents and are characterized by reduced range of hip rotation and waddling gait; and Blount disease (tibia vara), typically occurring in children aged 2–5 years 105 , 115 . In addition, children and adolescents with extreme obesity frequently have increased dyspnoea and decreased exercise capacity. A heightened demand for ventilation, elevated work of breathing, respiratory muscle inefficiency and diminished respiratory compliance are caused by increased truncal fat mass. This may result in a decreased functional residual capacity and expiratory reserve volume, ventilation to perfusion ratio abnormalities and hypoxaemia, especially when supine. However, conventional respiratory function tests are only mildly affected by obesity except in extreme cases 116 . Furthermore, gallstones should be suspected in the context of abdominal pain after rapid weight loss, which can be readily diagnosed via abdominal ultrasonography 105 . Finally, pseudotumor cerebri may present with chronic headache, and depression may present with flat affect, chronic fatigue and sleep problems 105 .
Obesity in adolescents can also be associated with disordered eating, eating disorders and other psychological disorders 117 , 118 . If suspected, assessment by a mental health professional is recommended.
A comprehensive approach
The 2016 report of the WHO Commission on Ending Childhood Obesity stated that progress in tackling childhood obesity has been slow and inconsistent, with obesity prevention requiring a whole-of-government approach in which policies across all sectors systematically take health into account, avoiding harmful health impacts and, therefore, improving population health and health equity 13 , 119 . The focus in developing and implementing interventions to prevent obesity in children should be on interventions that are feasible, effective and likely to reduce health inequalities 14 . Importantly, the voices of children and adolescents living with social disadvantage and those from minority groups must be heard if such interventions are to be effective and reduce inequalities 120 .
Figure 5 presents a system for the prevention of childhood obesity within different domains of the socioecological model 121 and highlights opportunities for interventions. These domains can be described on a continuum, from (most downstream) individual and interpersonal (including parents, peers and wider family) through to organizational (including health care and schools), community (including food, activity and environment), society (including media and finally cultural norms) and (most upstream) public policy (from local to national level). Interventions to prevent childhood obesity can be classified on the Nuffield intervention ladder 122 . This framework was proposed by the Nuffield Council on Bioethics in 2007 (ref. 122 ) and distributes interventions on the ladder steps depending on the degree of agency required by the individual to make the behavioural changes that are the aim of the intervention. The bottom step of the ladder includes interventions that provide information, which requires the highest agency and relies on a child, adolescent and/or family choosing (and their ability to choose) to act on that information and change behaviour. The next steps of the ladder are interventions that enable choice, guide choice through changing the default policy, guide choice through incentives, guide choice through disincentives, or restrict choice. On the top-most step of the ladder (lowest agency required) are interventions that eliminate choice.
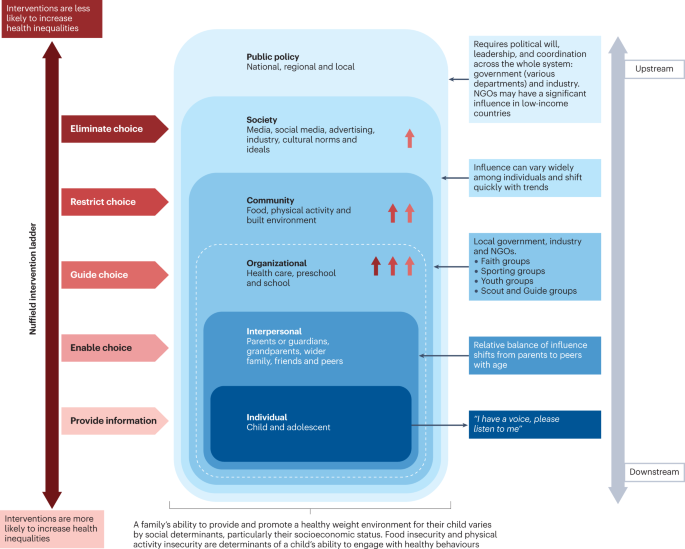
This schematic integrates interventions that were included in a Cochrane review 127 of 153 randomized controlled trials of interventions to prevent obesity in children and are high on the Nuffield intervention ladder 122 . The Nuffield intervention ladder distributes interventions depending on the degree of agency required for the behavioural changes that are the aim of the intervention. The socioecological model 121 comprises different domains (or levels) from the individual up to public policy. Interventions targeting the individual and interpersonal domains can be described as downstream interventions, and interventions within public policy can be described as the highest level of upstream interventions. Within each of these domains, arrow symbols with colours corresponding to the Nuffield intervention ladder category are used to show interventions that were both included in the Cochrane review 127 and that guide, restrict or eliminate choice as defined by the Nuffield intervention ladder 122 . Upstream interventions, and interventions on the top steps of the Nuffield ladder, are more likely to reduce inequalities. NGO, non-governmental organization.
Downstream and high-agency interventions (on the bottom steps of the Nuffield ladder) are more likely to result in intervention-generated inequalities 123 . This has been elegantly described and evidenced, with examples from the obesity prevention literature 124 , 125 . A particularly strong example is a systematic review of 38 interventions to promote healthy eating that showed that food price (an upstream and low-agency intervention) seemed to decrease inequalities, all interventions that combined taxes and subsidies consistently decreased inequalities, and downstream high-agency interventions, especially dietary counselling, seemed to increase inequalities 126 .
Effectiveness of prevention interventions
A 2019 Cochrane review of interventions to prevent obesity in children 127 included 153 randomized controlled trials (RCTs), mainly in HICs (12% were from middle-income countries). Of these RCTs, 56% tested interventions in children aged 6–12 years, 24% in children aged 0–5 years, and 20% in adolescents aged 13–18 years. The review showed that diet-only interventions to prevent obesity in children were generally ineffective across all ages. Interventions combining diet and physical activity resulted in modest benefits in children aged 0–12 years but not in adolescents. However, physical activity-only interventions to prevent obesity were effective in school-age children (aged 5–18 years). Whether the interventions were likely to work equitably in all children was investigated in 13 RCTs. These RCTs did not indicate that the strategies increased inequalities, although most of the 13 RCTs included relatively homogeneous groups of children from disadvantaged backgrounds.
The potential for negative unintended consequences of obesity prevention interventions has received much attention 128 . The Cochrane review 127 investigated whether children were harmed by any of the strategies; for example, by having injuries, losing too much weight or developing damaging views about themselves and their weight. Of the few RCTs that did monitor these outcomes, none found any harms in participants.
Intervention levels
Most interventions (58%) of RCTs in the Cochrane review aimed to change individual lifestyle factors via education-based approaches (that is, simply provide information) 129 . In relation to the socioecological model, only 11 RCTs were set in the food and physical activity environment domain, and child care, preschools and schools were the most common targets for interventions. Of note, no RCTs were conducted in a faith-based setting 130 . Table 2 highlights examples of upstream interventions that involve more than simply providing information and their classification on the Nuffield intervention ladder.
Different settings for interventions to prevent childhood obesity, including preschools and schools, primary health care, community settings and national policy, offer different opportunities for reach and effectiveness, and a reduction in inequalities.
Preschools and schools are key settings for public policy interventions for childhood obesity prevention, and mandatory and voluntary food standards and guidance on physical education are in place in many countries. Individual schools are tasked with translating and implementing these standards and guidance for their local context. Successful implementation of a whole-school approach, such as that used in the WHO Nutrition-Friendly Schools Initiative 131 , is a key factor in the effectiveness of interventions. Careful consideration should be given to how school culture can, and needs to, be shifted by working with schools to tailor the approach and manage possible staff capacity issues, and by building relationships within and outside the school gates to enhance sustainability 132 , 133 .
Primary health care offers opportunities for guidance for obesity prevention, especially from early childhood to puberty. Parent-targeted interventions conducted by clinicians in health-care or community settings have the strongest level of evidence for their effectiveness in reducing BMI z -score at age 2 years 134 . These interventions include group programmes, clinic nurse consultations, mobile phone text support or nurse home visiting, and focusing on healthy infant feeding, healthy childhood feeding behaviours and screen time.
A prospective individual participant data meta-analysis of four RCTs involving 2,196 mother–baby dyads, and involving nurse home visiting or group programmes, resulted in a small but significant reduction in BMI in infants in the intervention groups compared with control infants at age 18–24 months 134 . Improvements were also seen in television viewing time, breastfeeding duration and feeding practices. Interventions were more effective in settings with limited provision of maternal and child health services in the community. However, effectiveness diminished by age 5 years without further intervention, highlighting the need for ongoing interventions at each life stage 135 . Evidence exists that short-duration interventions targeting sleep in very early childhood may be more effective than nutrition-targeted interventions in influencing child BMI at age 5 years 136 .
Primary care clinicians can provide anticipatory guidance, as a form of primary prevention, to older children, adolescents and their families, aiming to support healthy weight and weight-related behaviours. Clinical guidelines recommend that clinicians monitor growth regularly, and provide guidance on healthy eating patterns, physical activity, sedentary behaviours and sleep patterns 97 , 100 . Very few paediatric trials have investigated whether this opportunistic screening and advice is effective in obesity prevention 100 . A 2021 review of registered RCTs for the prevention of obesity in infancy found 29 trials 137 , of which most were delivered, or were planned to be delivered, in community health-care settings, such as nurse-led clinics. At the time of publication, 11 trials had reported child weight-related outcomes, two of which showed a small but significant beneficial effect on BMI at age 2 years, and one found significant improvements in the prevalence of obesity but not BMI. Many of the trials showed improvements in practices, such as breastfeeding and screen time.
At the community level, local public policy should be mindful of the geography of the area (such as urban or rural) and population demographics. Adolescents usually have more freedom in food and beverage choices made outside the home than younger children. In addition, physical activity levels usually decline and sedentary behaviours rise during adolescence, particularly in girls 138 , 139 . These behavioural changes offer both opportunities and barriers for those developing community interventions. On a national societal level, public policies for interventions to prevent obesity in children include the control of advertising of foods and beverages high in fat, sugar and/or salt in some countries. Industry and the media, including social media, can have a considerable influence on the food and physical activity behaviours of children 13 , 119 .
Public policy may target interventions at all domains from the individual to the societal level. The main focus of interventions in most national public policies relies on the ability of individuals to make the behavioural changes that are the aim of the intervention (high-agency interventions) at the individual level (downstream interventions). An equal focus on low-agency and upstream interventions is required if a step change in tackling childhood obesity is to be realized 140 , 141 .
COVID-19 and obesity
Early indications in several countries show rising levels of childhood obesity, and an increase in inequalities in childhood obesity during the COVID-19 pandemic 142 . The substantial disruptions in nutrition and lifestyle habits of children during and since the pandemic include social isolation and addiction to screens 143 . Under-nutrition is expected to worsen in poor countries, but obesity rates could increase in middle-income countries and HICs, especially among vulnerable groups, widening the gap in health and social inequalities 143 . Public health approaches at national, regional and local levels should include strategies that not only prevent obesity and under-nutrition, but also reduce health inequalities.
In summary, although most trials of obesity prevention have occurred at the level of the individual, the immediate family, school or community, effective prevention of obesity will require greater investment in upstream, low-agency interventions.
Treatment goals
Treatment should be centred on the individual and stigma-free (Box 1 ) and may aim for a reduction in overweight and improvement in associated comorbidities and health behaviours. Clinical considerations when determining a treatment approach should include age, severity of overweight and the presence of associated complications 144 , 145 .
Box 1 Strategies for minimizing weight stigma in health care 220 , 221 , 222
Minimizing weight bias in the education of health-care professionals
Improved education of health professionals:
pay attention to the implicit and explicit communication of social norms
include coverage of the broader determinants of obesity
include discussion of harms caused by social and cultural norms and messages concerning body weight
provide opportunities to practise non-stigmatizing care throughout education
Provide causal information focusing on the genetic and/or socioenvironmental determinants of weight.
Provide empathy-invoking interventions, emphasizing size acceptance, respect and human dignity.
Provide a weight-inclusive approach, by emphasizing that all individuals, regardless of size, have the right to equal health care.
Addressing health facility infrastructure and processes
Provide appropriately sized chairs, blood pressure cuffs, weight scales, beds, toilets, showers and gowns.
Use non-stigmatizing language in signage, descriptions of clinical services and other documentation.
Providing clinical leadership and using appropriate language within health-care settings
Senior clinicians and managers should role-model supportive and non-biased behaviours towards people with obesity and indicate that they do not tolerate weight-based discrimination in any form.
Staff should identify the language that individuals prefer in referring to obesity.
Use person-first language, for example a ‘person with obesity’ rather than ‘an obese person’.
Treatment guidelines
Clinical guidelines advise that first-line management incorporates a family-based multicomponent approach that addresses dietary, physical activity, sedentary and sleep behaviours 97 , 99 , 109 , 146 . This approach is foundational, with adjunctive therapies, especially pharmacotherapy and bariatric surgery, indicated under specific circumstances, usually in adolescents with more severe obesity 144 , 145 . Guideline recommendations vary greatly among countries and are influenced by current evidence, and functionality and resourcing of local health systems. Hence, availability and feasibility of therapies differs internationally. In usual clinical practice, interventions may have poorer outcomes than is observed in original studies or anticipated in evidence-based guidelines 147 because implementation of guidelines is more challenging in resource-constrained environments 148 . In addition, clinical trials are less likely to include patients with specialized needs, such as children from culturally diverse populations, those living with social disadvantage, children with complex health problems, and those with severe obesity 149 , 150 .
Behavioural interventions
There are marked differences in individual responses to behavioural interventions, and overall weight change outcomes are often modest. In children aged 6–11 years, a 2017 Cochrane review 150 found that mean BMI z -scores were reduced in those involved in behaviour-changing interventions compared with those receiving usual care or no treatment by only 0.06 units (37 trials; 4,019 participants; low-quality evidence) at the latest follow-up (median 10 months after the end of active intervention). In adolescents aged 12–17 years, another 2017 Cochrane review 149 found that multicomponent behavioural interventions resulted in a mean reduction in weight of 3.67 kg (20 trials; 1,993 participants) and reduction in BMI of 1.18 kg/m 2 (28 trials; 2,774 participants). These effects were maintained at the 24-month follow-up. A 2012 systematic review found significant improvements in LDL cholesterol triglycerides and blood pressure up to 1 year from baseline following lifestyle interventions in children and adolescents 151 .
Family-based behavioural interventions are recommended in national level clinical practice guidelines 97 , 100 , 146 , 152 . They are an important element of intensive health behaviour and lifestyle treatments (IHBLTs) 109 . Family-based approaches use behavioural techniques, such as goal setting, parental monitoring or modelling, taught in family sessions or in individual sessions separately to children and care givers, depending on the child’s developmental level. The priority is to encourage the whole family to engage in healthier behaviours that result in dietary improvement, greater physical activity, and less sedentariness. This includes making changes to the family food environment and requires parental monitoring.
Family-based interventions differ in philosophy and implementation from those based on family systems theory and therapy 153 . All are intensive interventions that require multiple contact hours (26 or more) with trained specialists delivered over an extended period of time (6–12 months) 10 . Changing family lifestyle habits is challenging and expensive, and the therapeutic expertise is not widely available. Moving interventions to primary care settings, delivered by trained health coaches, and supplemented by remote contact (for example by phone), will improve access and equity 154 .
Very few interventions use single psychological approaches. Most effective IHBLTs are multicomponent and intensive (many sessions), and include face-to-face contact. There has been interest in motivational interviewing as an approach to delivery 155 . As client-centred counselling, this places the young person at the centre of their behaviour change. Fundamental to motivational interviewing is the practitioner partnership that helps the young person and/or parents to explore ambivalence to change, consolidate commitment to change, and develop a plan based on their own insights and expertise. Evidence reviews generally support the view that motivational interviewing reduces BMI. Longer interventions (>4 months), those that assess and report on intervention fidelity, and those that target both diet and physical activity are most effective 155 , 156 .
More intensive dietary interventions
Some individuals benefit from more intensive interventions 98 , 144 , 157 , 158 , which include very low-energy diets, very low-carbohydrate diets and intermittent energy restriction 159 . These interventions usually aim for weight loss and are only recommended for adolescents who have reached their final height. These diets are not recommended for long periods of time due to challenges in achieving nutritional adequacy 158 , 160 , and lack of long-term safety data 158 , 161 . However, intensive dietary interventions may be considered when conventional treatment is unsuccessful, or when adolescents with comorbidities or severe obesity require rapid or substantial weight loss 98 . A 2019 systematic review of very low-energy diets in children and adolescents found a mean reduction in body weight of −5.3 kg (seven studies) at the latest follow‐up, ranging from 5 to 14.5 months from baseline 161 .
Pharmacological treatment
Until the early 2020s the only drug approved in many jurisdictions for the treatment of obesity in adolescents was orlistat, a gastrointestinal lipase inhibitor resulting in reduced uptake of lipids and, thereby, a reduced total energy intake 162 . However, the modest effect on weight in combination with gastrointestinal adverse effects limit its usefulness overall 163 .
A new generation of drugs has been developed for the treatment of both T2DM and obesity. These drugs are based on gastrointestinal peptides with effects both locally and in the central nervous system. GLP1 is an incretin that reduces appetite and slows gastric motility. The GLP1 receptor agonist liraglutide is approved for the treatment of obesity in those aged 12 years and older both in the USA and Europe 164 , 165 . Liraglutide, delivered subcutaneously daily at a higher dose than used for T2DM resulted in a 5% better BMI reduction than placebo after 12 months 166 . A 2022 trial of semaglutide, another GLP1 receptor agonist, delivered subcutaneously weekly in adolescents demonstrated 16% weight loss after 68 weeks of treatment, with modest adverse events and a low drop-out rate 12 . Tirzepatide, an agonist of both GLP1 and glucose-dependent insulinotropic polypeptide (GIP), is approved by the FDA for the treatment of T2DM in adults 167 . Subcutaneous tirzepatide weekly in adults with obesity resulted in ~20% weight loss over 72 weeks 168 . Of note, GIP alone increases appetite, but the complex receptor–agonist interaction results in downregulation of the GIP receptors 169 , illustrating why slightly modified agonists exert different effects. A study of the use of tirzepatide in adolescents with T2DM has been initiated but results are not expected before 2027 (ref. 170 ). No trials of tirzepatide are currently underway in adolescents with obesity but without T2DM.
Hypothalamic obesity is difficult to treat. Setmelanotide is a MC4R agonist that reduces weight and improves quality of life in most people with LEPR and POMC mutations 71 . In trials of setmelanotide, 8 of 10 participants with POMC deficiency and 5 of 11 with LEPR deficiency had weight loss of at least 10% at ~1 year. The mean percentage change in most hunger score from baseline was −27.1% and −43.7% in those with POMC deficiency and leptin receptor deficiency, respectively 71 .
In the near future, effective new drugs with, hopefully, an acceptable safety profile will be available that will change the way we treat and set goals for paediatric obesity treatment 171 .
Bariatric surgery
Bariatric surgery is the most potent treatment for obesity in adolescents with severe obesity. The types of surgery most frequently used are sleeve gastrectomy and gastric bypass, both of which reduce appetite 172 . Mechanisms of action are complex, involving changes in gastrointestinal hormones, neural signalling, bile acid metabolism and gut microbiota 173 . Sleeve gastrectomy is a more straightforward procedure and the need for vitamin supplementation is lower than with gastric bypass. However, long-term weight loss may be greater after gastric bypass surgery 174 .
Prospective long-term studies demonstrate beneficial effects of both sleeve gastrectomy and gastric bypass on weight loss and comorbidities in adolescents with severe obesity 175 , 176 . In a 5-year follow-up period, in 161 participants in the US TEEN-LABS study who underwent gastric bypass, mean BMI declined from 50 to 37 kg/m 2 (ref. 11 ). In a Swedish prospective study in 81 adolescents who underwent gastric bypass, the mean decrease in BMI at 5 years was 13.1 kg/m 2 (baseline BMI 45.5 kg/m 2 ) compared with a BMI increase of 3.1 kg/m 2 in the control group 176 . Both studies showed marked inter-individual variations. Negative adverse effects, including gastrointestinal problems, vitamin deficits and reduction in lean body mass, are similar in adults and adolescents. Most surgical complications following bariatric surgery in the paediatric population are minor, occurring in the early postoperative time frame, but 8% of patients may have major perioperative complications 177 . Up to one-quarter of patients may require subsequent related procedures within 5 years 109 . However, many adolescents with severe obesity also have social and psychological problems, highlighting the need for routine and long-term monitoring 109 , 178 .
Recommendations for bariatric surgery in adolescents differ considerably among countries, with information on long-term outcomes emerging rapidly. In many countries, bariatric surgery is recommended only from Tanner pubertal stage 3–4 and beyond, and only in children with severe obesity and cardiometabolic comorbidities 177 . The 2023 American Academy of Pediatrics clinical practice guidelines recommend that bariatric surgery be considered in adolescents ≥13 years of age with a BMI of ≥35 kg/m 2 or 120% of the 95th percentile for age and sex, whichever is lower, as well as clinically significant disease, such as T2DM, non-alcoholic fatty liver disease, major orthopaedic complications, obstructive sleep apnoea, the presence of cardiometabolic risk, or depressed quality of life 109 . For those with a BMI of ≥40 kg/m 2 or 140% of the 95th percentile for age and sex, bariatric surgery is indicated regardless of the presence of comorbidities. Potential contraindications to surgery include correctable causes of obesity, pregnancy and ongoing substance use disorder. The guidelines comment that further evaluation, undertaken by multidisciplinary centres that offer bariatric surgery for adolescents, should determine the capacity of the patient and family to understand the risks and benefits of surgery and to adhere to the required lifestyle changes before and after surgery.
Long-term weight outcomes
Few paediatric studies have investigated long-term weight maintenance after the initial, more intensive, weight loss phase. A 2018 systematic review of 11 studies in children and adolescents showed that a diverse range of maintenance interventions, including support via face-to-face psychobehavioural therapies, individual physician consultations, or adjunctive therapeutic contact via newsletters, mobile phone text or e-mail, led to stabilization of BMI z -score compared with control participants, who had increases in BMI z -score 179 . Interventions that are web-based or use mobile devices may be particularly useful in young people 180 .
One concern is weight regain which occurs after bariatric surgery in general 181 but may be more prevalent in adolescents 176 . For example, in a Swedish prospective study, after 5 years, 25–30% of participants fulfilled the definitions of low surgical treatment effectiveness, which was associated with poorer metabolic outcomes 176 . As with adults, prevention of weight regain for most at-risk individuals might be possible with the combination of lifestyle support and pharmacological treatment 182 . Further weight maintenance strategies and long-term outcomes are discussed in the 2023 American Academy of Pediatrics clinical practice guidelines 109 . The appropriate role and timing of other therapies for long-term weight loss maintenance, such as anti-obesity medications, more intensive dietary interventions and bariatric surgery, are areas for future research.
In summary, management of obesity in childhood and adolescence requires intensive interventions. Emerging pharmacological therapies demonstrate greater short-term effectiveness than behavioural interventions; however, long-term outcomes at ≥2 years remain an important area for future research.
Quality of life
Weight bias describes the negative attitudes to, beliefs about and behaviour towards people with obesity 183 . It can lead to stigma causing exclusion, and discrimination in work, school and health care, and contributes to the inequities common in people with obesity 184 . Weight bias also affects social engagement and psychological well-being of children.
Children and adolescents with obesity score lower overall on health-related quality of life (HRQoL) 4 , 5 . In measures that assess domains of functioning, most score lower in physical functioning, physical/general health and psychosocial areas, such as appearance, and social acceptance and functioning. HRQoL is lowest in treatment-seeking children and in those with more extreme obesity 185 . Weight loss interventions generally increase HRQoL independent of the extent of weight loss 186 , especially in the domains most affected. However, changes in weight and HRQoL are often not strongly correlated. This may reflect a lag in the physical and/or psychosocial benefit from weight change, or the extent of change that is needed to drive change in a child’s self-perception.
Similar observations apply to the literature on self-esteem. Global self-worth is reduced in children and adolescents with obesity, as is satisfaction with physical appearance, athletic competence and social acceptance 187 . Data from intensive interventions suggest the psychological benefit of weight loss may be as dependent on some feature of the treatment environment or supportive social network as the weight loss itself 188 . This may include the daily company of others with obesity, making new friendships, and experienced improvements in newly prioritized competences.
There is a bidirectional relationship between HRQoL and obesity 189 , something also accepted in the relationship with mood disorder. Obesity increases the risk of depression and vice versa, albeit over a longer period of time and which may only become apparent in adulthood 190 . Obesity also presents an increased risk of anxiety 191 .
Structured and professionally delivered weight management interventions ameliorate mood disorder symptoms 192 and improve self-esteem 193 . Regular and extended support are important components beyond losing weight. Such interventions do not increase the risk of eating disorders 194 . This is despite a recognition that binge eating disorder is present in up to 5% of adolescents with overweight or obesity 195 . They are five times more likely to have binge eating symptoms than those with average weight. Importantly, adolescents who do not have access to professionally delivered weight management may be more likely to engage in self-directed dieting, which is implicated in eating disorder development 196 .
The literature linking childhood obesity with either attention deficit hyperactivity disorder or autism spectrum disorder is complex and the relationship is uncertain. The association seems to be clearer in adults but the mechanisms and their causal directions remain unclear 109 , 197 . Young children with obesity, especially boys, are more likely to be parent-rated as having behavioural problems 198 . This may be a response to the behaviour of others rather than reflect clinical diagnoses such as attention deficit hyperactivity disorder or autism spectrum disorder. Conduct and peer relationship problems co-occur in children, regardless of their weight.
Children with obesity experience more social rejection. They receive fewer friendship nominations and more peer rejections, most pronounced in those with severe obesity 199 . This continues through adolescence and beyond. Children with obesity are more likely to report being victimized 200 . Younger children may respond by being perpetrators themselves. While it is assumed that children are victimized because of their weight, very few studies have looked at the nature or reason behind victimization. A substantial proportion of children with obesity fail to identify themselves as being fat-teased 187 . Although the stigma associated with obesity should be anticipated in children, especially in those most overweight, it would be inappropriate to see all as victims. A better understanding of children’s resilience is needed.
Many gaps remain in basic, translational and clinical research in child and adolescent obesity. The mechanisms (genetic, epigenetic, environmental and social) behind the overwhelming association between parental obesity and child and adolescent obesity are still unclear given the paradoxically weak association in BMI between adopted children and their parents in combination with the modest effect size of known genetic loci associated with obesity 201 .
Early manifestation of extreme obesity in childhood suggests a strong biological basis for disturbances of homeostatic weight regulation. Deep genotyping (including next-generation sequencing) and epigenetic analyses in these patients will reveal new genetic causes and causal pathways as a basis for the development of mechanism-based treatments. Future work aiming to understand the mechanisms underlying the development of childhood obesity should consider the complex biopsychosocial interactions and take a systems approach to understanding causal pathways leading to childhood obesity to contribute to evidence-based prevention and treatment strategies.
Long-term outcome data to better determine the risks of eating disorders are required. Although symptoms improve during obesity treatment in most adolescents, screening and monitoring for disordered eating is recommended in those presenting for treatment 202 and effective tools for use in clinical practice are required. A limited number of tools are validated to identify binge eating disorder in youth with obesity 203 but further research is needed to screen appropriately for the full spectrum of eating disorder diagnoses in obesity treatment seeking youth 203 . Recent reviews provide additional detail regarding eating disorder risk in child and adolescent obesity 117 , 202 , 204 .
Most studies of paediatric obesity treatment have been undertaken in HICs and predominantly middle-class populations. However, research is needed to determine which strategies are best suited for those in LMICs and low-resource settings, for priority population groups including indigenous peoples, migrant populations and those living with social disadvantage, and for children with neurobehavioural and psychiatric disorders. We currently have a limited understanding of how best to target treatment pathways for different levels of genetic risk, age, developmental level, obesity severity, and cardiometabolic and psychological risk. Current outcomes for behavioural interventions are relatively modest and improved treatment outcomes are needed to address the potentially severe long-term health outcomes of paediatric obesity. Studies also need to include longer follow-up periods after an intervention, record all adverse events, incorporate cost-effectiveness analyses and have improved process evaluation.
Other areas in need of research include the role of new anti-obesity medications especially in adolescents, long-term outcomes following bariatric surgery and implementation of digital support systems to optimize outcomes and reduce costs of behavioural change interventions 205 . We must also better understand and tackle the barriers to implementation of treatment in real-life clinical settings, including the role of training of health professionals. Importantly, treatment studies of all kinds must engage people with lived experience — adolescents, parents and families — to understand what outcomes and elements of treatment are most valued.
Obesity prevention is challenging because it requires a multilevel, multisectoral approach that addresses inequity, involves many stakeholders and addresses both the upstream and the downstream factors influencing obesity risk. Some evidence exists of effectiveness of prevention interventions operating at the level of the child, family and school, but the very poor progress overall in modifying obesity prevalence globally highlights many areas in need of research and evidence implementation. Studies are needed especially in LMICs, particularly in the context of the nutrition transition and the double burden of malnutrition. A focus on intergenerational research, rather than the age-based focus of current work, is also needed. Systems research approaches should be used, addressing the broader food and physical activity environments, and links to climate change 206 . In all studies, strategies are needed that enable co-production with relevant communities, long-term follow-up, process evaluation and cost-effectiveness analyses. In the next few years, research and practice priorities must include a focus on intervention strategies in the earliest phases of life, including during pregnancy. The effects of COVID-19 and cost of living crises in many countries are leading to widening health inequalities 207 and this will further challenge obesity prevention interventions. Available resourcing for prevention interventions may become further constrained, requiring innovative solutions across agendas, with clear identification of co-benefits. For example, public health interventions for other diseases, such as dental caries or depression, or other societal concerns, such as urban congestion or climate change, may also act as obesity prevention strategies. Ultimately, to implement obesity prevention, societal changes are needed in terms of urban planning, social structures and health-care access.
Future high-quality paediatric obesity research can be enabled through strategies that support data sharing, which avoids research waste and bias, and enables new research questions to be addressed. Such approaches require leadership, careful engagement of multiple research teams, and resourcing. Four national or regional level paediatric weight registries exist 208 , 209 , 210 , 211 , which are all based in North America or Europe. Such registries should be established in other countries, especially in low-resource settings, even if challenging 208 . Another data-sharing approach is through individual participant data meta-analyses of intervention trials, which can include prospectively collected data 212 and are quite distinct from systematic reviews of aggregate data. Two recent examples are the Transforming Obesity Prevention in Childhood (TOPCHILD) Collaboration, which includes early interventions to prevent obesity in the first 2 years of life 213 , and the Eating Disorders in Weight-Related Therapy (EDIT) Collaboration, which aims to identify characteristics of individuals or trials that increase or protect against eating disorder risk following obesity treatment 214 . Formal data linkage studies, especially those joining up routine administrative datasets, enable longer-term and broader outcome measures to be assessed than is possible with standard clinical or public health intervention studies.
Collaborative research will also be enhanced through the use of agreed core outcome sets, supporting data harmonization. The Edmonton Obesity Staging System – Paediatric 215 is one option for paediatric obesity treatment. A core outcome set for early intervention trials to prevent obesity in childhood (COS-EPOCH) has been recently established 216 . These efforts incorporate a balance between wanting and needing to share data and adhering to privacy protection regulations. Objective end points are ideal, including directly measured physical activity and body composition.
Collaborative efforts and a systems approach are paramount to understand, prevent and manage child and adolescent obesity. Research funding and health policies should focus on feasible, effective and equitable interventions.
NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet https://doi.org/10.1016/S0140-6736(17)32129-3 (2017).
Article Google Scholar
Popkin, B. M., Corvalan, C. & Grummer-Strawn, L. M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395 , 65–74 (2020).
Article PubMed Google Scholar
Kompaniyets, L. et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw. Open 4 , e2111182 (2021).
Article PubMed PubMed Central Google Scholar
Griffiths, L. J., Parsons, T. J. & Hill, A. J. Self‐esteem and quality of life in obese children and adolescents: a systematic review. Int. J. Pediatr. Obes. 5 , 282–304 (2010).
Buttitta, M., Iliescu, C., Rousseau, A. & Guerrien, A. Quality of life in overweight and obese children and adolescents: a literature review. Qual. Life Res. 23 , 1117–1139 (2014).
Hayes, A. et al. Early childhood obesity: association with healthcare expenditure in Australia. Obesity 24 , 1752–1758 (2016).
Marcus, C., Danielsson, P. & Hagman, E. Pediatric obesity – long-term consequences and effect of weight loss. J. Intern. Med. 292 , 870–891 (2022).
Berthoud, H. R., Morrison, C. D. & Münzberg, H. The obesity epidemic in the face of homeostatic body weight regulation: what went wrong and how can it be fixed? Physiol. Behav. 222 , 112959 (2020).
Article CAS PubMed PubMed Central Google Scholar
World Health Organization. Report of the commission on ending childhood obesity. WHO https://www.who.int/publications/i/item/9789241510066 (2016). This report from the WHO on approaches to childhood and adolescent obesity has six main recommendations for governments, covering food and physical activity, age-based settings and provision of weight management for those with obesity.
O’Connor, E. A. et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA 317 , 2427–2444 (2017).
Inge, T. H. et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med. 380 , 2136–2145 (2019).
Weghuber, D. et al. Once-weekly semaglutide in adolescents with obesity. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2208601 (2022). To our knowledge, the first RCT of semaglutide 2.4 mg, administered weekly by subcutaneous injection, in adolescents with obesity.
World Health Organization. Report of the Commission on Ending Childhood Obesity: Implementation Plan: Executive Summary (WHO, 2017).
Hillier-Brown, F. C. et al. A systematic review of the effectiveness of individual, community and societal level interventions at reducing socioeconomic inequalities in obesity amongst children. BMC Public Health 14 , 834 (2014).
World Health Organization. Obesity. WHO https://www.who.int/health-topics/obesity#tab=tab_1 (2023).
Mei, Z. et al. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am. J. Clin. Nutr. 75 , 978–985 (2002).
Article CAS PubMed Google Scholar
World Health Organization. Child growth standards. WHO https://www.who.int/tools/child-growth-standards/standards (2006).
World Health Organization. Growth reference data for 5–19 years. WHO https://www.who.int/tools/growth-reference-data-for-5to19-years (2007).
National Center for Health Statistics. CDC growth charts. Centers for Disease Control and Prevention http://www.cdc.gov/growthcharts/ (2022).
Cole, T. J., Bellizzi, M. C., Flegal, K. M. & Dietz, W. H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320 , 1240 (2000).
Di Cesare, M. et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 17 , 212 (2019).
Swinburn, B., Egger, G. & Raza, F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev. Med. 29 , 563–570 (1999).
Ford, N. D., Patel, S. A. & Narayan, K. V. Obesity in low-and middle-income countries: burden, drivers, and emerging challenges. Annu. Rev. Public Health 38 , 145–164 (2017).
Shah, B., Tombeau Cost, K., Fuller, A., Birken, C. S. & Anderson, L. N. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr. Prev. Health 3 , 387–390 (2020).
Public Health England. Research and analysis: differences in child obesity by ethnic group. GOV.UK https://www.gov.uk/government/publications/differences-in-child-obesity-by-ethnic-group/differences-in-child-obesity-by-ethnic-group#data (2019).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr. Obes. 7 , 284–294 (2012).
Kelly, A. S. et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 128 , 1689–1712 (2013).
Garnett, S. P., Baur, L. A., Jones, A. M. & Hardy, L. L. Trends in the prevalence of morbid and severe obesity in Australian children aged 7–15 years, 1985-2012. PLoS ONE 11 , e0154879 (2016).
Spinelli, A. et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes. Facts 12 , 244–258 (2019).
Twig, G. et al. Body-Mass Index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 374 , 2430–2440 (2016).
González-Muniesa, P. et al. Obesity. Nat. Rev. Dis. Primers 3 , 17034 (2017).
Geserick, M. et al. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 379 , 1303–1312 (2018).
Larqué, E. et al. From conception to infancy – early risk factors for childhood obesity. Nat. Rev. Endocrinol. 15 , 456–478 (2019).
Barker, D. J. Fetal origins of coronary heart disease. Br. Med. J. 311 , 171–174 (1995).
Article CAS Google Scholar
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359 , 61–73 (2008).
Philips, E. M. et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: an individual participant data meta-analysis of 229,000 singleton births. PLoS Med. 17 , e1003182 (2020).
Voerman, E. et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 16 , e1002744 (2019). Individual participant data meta-analysis of >160,000 mothers and their children on the associations of maternal BMI and gestational weight gain and childhood overweight or obesity.
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 5 , 47 (2019).
Oken, E. & Gillman, M. W. Fetal origins of obesity. Obes. Res. 11 , 496–506 (2003).
Hughes, A. R., Sherriff, A., Ness, A. R. & Reilly, J. J. Timing of adiposity rebound and adiposity in adolescence. Pediatrics 134 , e1354–e1361 (2014).
Rolland-Cachera, M. F. et al. Tracking the development of adiposity from one month of age to adulthood. Ann. Hum. Biol. 14 , 219–229 (1987).
Koletzko, B. et al. Prevention of childhood obesity: a position paper of the Global Federation of International Societies of Paediatric Gastroenterology, Hepatology and Nutrition (FISPGHAN). J. Pediatr. Gastroenterol. Nutr. 70 , 702–710 (2020).
Weber, M. et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am. J. Clin. Nutr. 99 , 1041–1051 (2014).
Cope, M. B. & Allison, D. B. Critical review of the World Health Organization’s (WHO) 2007 report on ‘evidence of the long‐term effects of breastfeeding: systematic reviews and meta‐analysis’ with respect to obesity. Obes. Rev. 9 , 594–605 (2008).
Totzauer, M. et al. Different protein intake in the first year and its effects on adiposity rebound and obesity throughout childhood: 11 years follow‐up of a randomized controlled trial. Pediatr. Obes. 17 , e12961 (2022).
Deren, K. et al. Consumption of sugar-sweetened beverages in paediatric age: a position paper of the European academy of paediatrics and the European Childhood Obesity Group. Ann. Nutr. Metab. 74 , 296–302 (2019).
Felső, R., Lohner, S., Hollódy, K., Erhardt, É. & Molnár, D. Relationship between sleep duration and childhood obesity: systematic review including the potential underlying mechanisms. Nutr. Metab. Cardiovasc. Dis. 27 , 751–761 (2017).
Farooq, A. et al. Longitudinal changes in moderate‐to‐vigorous‐intensity physical activity in children and adolescents: a systematic review and meta‐analysis. Obes. Rev. 21 , e12953 (2020).
Mahumud, R. A. et al. Association of dietary intake, physical activity, and sedentary behaviours with overweight and obesity among 282,213 adolescents in 89 low and middle income to high-income countries. Int. J. Obes. 45 , 2404–2418 (2021).
Ballon, M. et al. Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. Int. J. Obes. 42 , 1671–1679 (2018).
Buoncristiano, M. et al. Socioeconomic inequalities in overweight and obesity among 6- to 9-year-old children in 24 countries from the World Health Organization European region. Obes. Rev. 22 , e13213 (2021).
Jiwani, S. S. et al. The shift of obesity burden by socioeconomic status between 1998 and 2017 in Latin America and the Caribbean: a cross-sectional series study. Lancet Glob. Health 7 , e1644–e1654 (2019).
Monteiro, C. A., Conde, W. L., Lu, B. & Popkin, B. M. Obesity and inequities in health in the developing world. Int. J. Obes. 28 , 1181–1186 (2004).
Guo, S. S. & Chumlea, W. C. Tracking of body mass index in children in relation to overweight in adulthood. Am. J. Clin. Nutr. 70 , 145S–148S (1999).
Aarestrup, J. et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int. J. Obes. 44 , 1546–1560 (2020).
Eslam, M. et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol. Hepatol. 6 , 864–873 (2021).
Daniels, S. R. et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 111 , 1999–2012 (2005).
Cioana, M. et al. The prevalence of obesity among children with type 2 diabetes: a systematic review and meta-analysis. JAMA Netw. Open 5 , e2247186 (2022).
Gepstein, V. & Weiss, R. Obesity as the main risk factor for metabolic syndrome in children. Front. Endocrinol. 10 , 568 (2019).
Kuvat, N., Tanriverdi, H. & Armutcu, F. The relationship between obstructive sleep apnea syndrome and obesity: a new perspective on the pathogenesis in terms of organ crosstalk. Clin. Respir. J. 14 , 595–604 (2020).
Baker, J. L., Olsen, L. W. & Sorensen, T. I. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 357 , 2329–2337 (2007).
Bjerregaard, L. G. et al. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N. Engl. J. Med. 378 , 1302–1312 (2018).
Kelsey, M. M., Zaepfel, A., Bjornstad, P. & Nadeau, K. J. Age-related consequences of childhood obesity. Gerontology 60 , 222–228 (2014).
Sharma, V. et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes. Rev. 20 , 1341–1349 (2019).
Lobstein, T. & Jackson-Leach, R. Planning for the worst: estimates of obesity and comorbidities in school-age children in 2025. Pediatr. Obes. 11 , 321–325 (2016).
Berthoud, H. R., Münzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152 , 1728–1738 (2017).
Devoto, F. et al. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 94 , 271–285 (2018).
Blum, W. F., Englaro, P., Attanasio, A. M., Kiess, W. & Rascher, W. Human and clinical perspectives on leptin. Proc. Nutr. Soc. 57 , 477–485 (1998).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1 , 754–764 (2019).
Kühnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375 , 240–246 (2016).
Clément, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8 , 960–970 (2020).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156 , 20–44 (2014).
Fischer-Posovszky, P., Roos, J., Zoller, V. & Wabitsch, M. in Pediatric Obesity: Etiology, Pathogenesis and Treatment (ed. Freemark, M. S.) 81–93 (Springer, 2018).
Hammarstedt, A., Gogg, S., Hedjazifar, S., Nerstedt, A. & Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 98 , 1911–1941 (2018).
Silventoinen, K. et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the collaborative project of development of anthropometrical measures in twins (CODATwins) study. Am. J. Clin. Nutr. 104 , 371–379 (2016).
Silventoinen, K. et al. Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: an individual-based pooled analysis of 40 twin cohorts. Am. J. Clin. Nutr. 106 , 457–466 (2017).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet. 27 , 3641–3649 (2018).
Vogelezang, S. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16 , e1008718 (2020).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28 , 3327–3338 (2019). To our knowledge, currently the largest genome-wide association study meta-analysis on childhood obesity in >13,000 individuals with obesity and >15,500 controls.
Couto Alves, A. et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 5 , eaaw3095 (2019).
Ding, X. et al. Genome-wide screen of DNA methylation identifies novel markers in childhood obesity. Gene 566 , 74–83 (2015).
Huang, R. C. et al. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics 10 , 995–1005 (2015).
Rzehak, P. et al. DNA-methylation and body composition in preschool children: epigenome-wide-analysis in the European Childhood Obesity Project (CHOP)-Study. Sci. Rep. 7 , 14349 (2017).
Alfano, R. et al. Perspectives and challenges of epigenetic determinants of childhood obesity: a systematic review. Obes. Rev. 23 , e13389 (2022).
Vehmeijer, F. O. L. et al. DNA methylation and body mass index from birth to adolescence: meta-analyses of epigenome-wide association studies. Genome Med. 12 , 105 (2020). Meta-analysis of epigenome-wide association studies of childhood BMI in >4,000 children.
Richmond, R. C. et al. DNA methylation and BMI: investigating identified methylation sites at HIF3A in a causal framework. Diabetes 65 , 1231–1244 (2016).
Wahl, S. et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541 , 81–86 (2017).
Kivimäki, M. et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am. J. Clin. Nutr. 86 , 1509–1514 (2007).
Whitaker, R. C., Wright, J. A., Pepe, M. S., Seidel, K. D. & Dietz, W. H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337 , 869–873 (1997).
Davey Smith, G., Steer, C., Leary, S. & Ness, A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch. Dis. Child. 92 , 876–880 (2007).
Fleten, C. et al. Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am. J. Epidemiol. 176 , 83–92 (2012).
Gaillard, R. et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 63 , 683–691 (2014).
Lawlor, D. A. et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 5 , e33 (2008).
Patro, B. et al. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann. Nutr. Metab. 63 , 32–41 (2013).
Sorensen, T. et al. Comparison of associations of maternal peri-pregnancy and paternal anthropometrics with child anthropometrics from birth through age 7 y assessed in the Danish National Birth Cohort. Am. J. Clin. Nutr. 104 , 389–396 (2016).
Styne, D. M. et al. Pediatric obesity – assessment, treatment, and prevention: an Endocrine Society Clinical Practice guideline. J. Clin. Endocrinol. Metab. 102 , 709–757 (2017).
National Institute for Health and Care Excellence. Obesity: identification, assessment and managenent: clinical guideline [CG189]. NICE https://www.nice.org.uk/guidance/cg189 (2022). A high quality clinical practice guideline for obesity management.
Barlow, S. E. Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120 (Suppl. 4), S164–S192 (2007).
Canadian Task Force on Preventive Health Care. Recommendations for growth monitoring, and prevention and management of overweight and obesity in children and youth in primary care. Can. Med. Assoc. J. 187 , 411–421 (2015).
US Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 317 , 2417–2426 (2017).
McConnell-Nzunga, J. et al. Classification of obesity varies between body mass index and direct measures of body fat in boys and girls of Asian and European ancestry. Meas. Phys. Educ. Exerc. Sci. 22 , 154–166 (2018).
Reinehr, T. et al. Definable somatic disorders in overweight children and adolescents. J. Pediatr. 150 , 618–622.e5 (2007).
Reinehr, T. Thyroid function in the nutritionally obese child and adolescent. Curr. Opin. Pediatr. 23 , 415–420 (2011).
Kohlsdorf, K. et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int. J. Obes. 42 , 1602–1609 (2018).
Armstrong, S. et al. Physical examination findings among children and adolescents with obesity: an evidence-based review. Pediatrics 137 , e20151766 (2016).
Reinehr, T. & Roth, C. L. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc. Health 3 , 44–54 (2019).
Garnett, S. P., Baur, L. A. & Cowell, C. T. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int. J. Obes. 32 , 1028–1030 (2008).
Maffeis, C., Banzato, C., Talamini, G. & Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 152 , 207–213.e2 (2008).
Hampl, S. E. et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics 151 , e2022060640 (2023). A new, comprehensive clinical practice guideline outlining current recommendations on assessment and treatment of children and adolescents with obesity.
Reinehr, T. et al. Comparison of cardiovascular risk factors between children and adolescents with classes III and IV obesity: findings from the APV cohort. Int. J. Obes. 45 , 1061–1073 (2021).
Reinehr, T. Metabolic syndrome in children and adolescents: a critical approach considering the interaction between pubertal stage and insulin resistance. Curr. Diabetes Rep. 16 , 8 (2016).
Zeitler, P. et al. ISPAD Clinical Practice Consensus Guidelines 2018: type 2 diabetes mellitus in youth. Pediatr. Diabetes 19 , 28–46 (2018).
Ibáñez, L. et al. An International Consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 88 , 371–395 (2017).
Brockmann, P. E., Schaefer, C., Poets, A., Poets, C. F. & Urschitz, M. S. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep Med. Rev. 17 , 331–340 (2013).
Taylor, E. D. et al. Orthopedic complications of overweight in children and adolescents. Pediatrics 117 , 2167–2174 (2006).
Winck, A. D. et al. Effects of obesity on lung volume and capacity in children and adolescents: a systematic review. Rev. Paul. Pediatr. 34 , 510–517 (2016).
PubMed PubMed Central Google Scholar
Jebeile, H., Lister, N., Baur, L., Garnett, S. & Paxton, S. J. Eating disorder risk in adolescents with obesity. Obes. Rev. 22 , e13173 (2021).
Quek, Y. H., Tam, W. W. S., Zhang, M. W. B. & Ho, R. C. M. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes. Rev. 18 , 742–754 (2017).
World Health Organization. Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity . Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity (WHO, 2016).
Pickett, K. et al. The Child of the North: building a fairer future after COVID-19. The Northern Health Science Alliance and N8 Research Partnership https://www.thenhsa.co.uk/app/uploads/2022/01/Child-of-the-North-Report-FINAL-1.pdf (2021).
Bronfenbrenner, U. Toward an experimental ecology of human development. Am. Psychol. 32 , 513–531 (1977).
Nuffield Council on Bioethics. Public Health: Ethical Issues (Nuffield Council on Bioethics, 2007).
Lorenc, T., Petticrew, M., Welch, V. & Tugwell, P. What types of interventions generate inequalities? Evidence from systematic reviews. J. Epidemiol. Community Health 67 , 190–193 (2013).
Adams, J., Mytton, O., White, M. & Monsivais, P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 13 , e1001990 (2016).
Backholer, K. et al. A framework for evaluating the impact of obesity prevention strategies on socioeconomic inequalities in weight. Am. J. Public Health 104 , e43–e50 (2014).
McGill, R. et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health 15 , 457 (2015).
Brown, T. et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 7 , Cd001871 (2019). A Cochrane review involving 153 RCTs of diet and/or physical activity interventions to prevent obesity in children and adolescents, highlighting varying effectiveness of interventions in different age groups.
PubMed Google Scholar
Le, L. K.-D. et al. Prevention of high body mass index and eating disorders: a systematic review and meta-analysis. Eat. Weight Disord. 27 , 2989–3003 (2022).
Nobles, J., Summerbell, C., Brown, T., Jago, R. & Moore, T. A secondary analysis of the childhood obesity prevention Cochrane Review through a wider determinants of health lens: implications for research funders, researchers, policymakers and practitioners. Int. J. Behav. Nutr. Phys. Act. 18 , 22 (2021).
Rai, K. K., Dogra, S. A., Barber, S., Adab, P. & Summerbell, C. A scoping review and systematic mapping of health promotion interventions associated with obesity in Islamic religious settings in the UK. Obes. Rev. 20 , 1231–1261 (2019).
World Health Organization. Nutrition Action in Schools: A Review of Evidence Related to the Nutrition-Friendly Schools Initiative (WHO, 2021).
Daly-Smith, A. et al. Using a multi-stakeholder experience-based design process to co-develop the Creating Active Schools Framework. Int. J. Behav. Nutr. Phys. Act. 17 , 13 (2020).
Tibbitts, B. et al. Considerations for individual-level versus whole-school physical activity interventions: stakeholder perspectives. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph18147628 (2021).
Askie, L. M. et al. Interventions commenced by early infancy to prevent childhood obesity-The EPOCH Collaboration: an individual participant data prospective meta-analysis of four randomized controlled trials. Pediatr. Obes. 15 , e12618 (2020). To our knowledge, the first prospective individual participant data meta-analysis showing that interventions commencing in late pregnancy or very early childhood are associated with healthier BMI z -score at age 18–24 months.
Seidler, A. L. et al. Examining the sustainability of effects of early childhood obesity prevention interventions: follow-up of the EPOCH individual participant data prospective meta-analysis. Pediatr. Obes. 17 , e12919 (2022).
Taylor, R. W. et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the prevention of overweight in infancy (POI) randomized controlled trial at ages 3.5 and 5 y. Am. J. Clin. Nutr. 108 , 228–236 (2018).
Mihrshahi, S. et al. A review of registered randomized controlled trials for the prevention of obesity in infancy. Int. J. Environ. Res. Public Health 18 , 2444 (2021).
Farooq, M. A. et al. Timing of the decline in physical activity in childhood and adolescence: Gateshead Millennium Cohort Study. Br. J. Sports Med. 52 , 1002–1006 (2018).
van Sluijs, E. M. F. et al. Physical activity behaviours in adolescence: current evidence and opportunities for intervention. Lancet 398 , 429–442 (2021).
Griffin, N. et al. A critique of the English national policy from a social determinants of health perspective using a realist and problem representation approach: the ‘Childhood Obesity: a plan for action’ (2016, 2018, 2019). BMC Public Health 21 , 2284 (2021).
Knai, C., Lobstein, T., Petticrew, M., Rutter, H. & Savona, N. England’s childhood obesity action plan II. Br. Med. J. 362 , k3098 (2018).
World Health Organization. WHO European Regional Obesity Report 2022 (WHO, 2022).
Zemrani, B., Gehri, M., Masserey, E., Knob, C. & Pellaton, R. A hidden side of the COVID-19 pandemic in children: the double burden of undernutrition and overnutrition. Int. J. Equity Health 20 , 44 (2021).
Alman, K. L. et al. Dietetic management of obesity and severe obesity in children and adolescents: a scoping review of guidelines. Obes. Rev. https://doi.org/10.1111/obr.13132 (2020).
Pfeiffle, S. et al. Current recommendations for nutritional management of overweight and obesity in children and adolescents: a structured framework. Nutrients https://doi.org/10.3390/nu11020362 (2019).
Scottish Intercollegiate Guidelines Network. Management of obesity. A National Clinical Guideline . SIGN 115 (SIGN, 2010).
Reinehr, T. et al. Two-year follow-up in 21,784 overweight children and adolescents with lifestyle intervention. Obesity 17 , 1196–1199 (2009).
Ells, L. J. et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int. J. Obes. 42 , 1823–1833 (2018).
Al‐Khudairy, L. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 6 , CD012691 (2017). One of three Cochrane reviews looking at lifestyle treatment of paediatric obesity, in this case in adolescents, which identified 44 completed trials, finding low quality evidence of improvements in BMI and moderate quality evidence of improvements in weight.
Mead, E. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev. 6 , CD012651 (2017). A Cochrane Review, involving 70 RCTs, showing that multicomponent behavioural interventions can lead to small, short-term reductions in BMI and related measures in children aged 6–11 years with obesity.
Ho, M. et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics 130 , e1647–e1671 (2012). To our knowledge, the first systematic review of lifestyle interventions in children and adolescents with obesity to show improvements in cardiometabolic outcomes (LDL cholesterol, triglycerides, fasting insulin and blood pressure), as well as weight.
Clinical Practice Guideline Panel. Clinical practice guideline for multicomponent behavioral treatment of obesity and overweight in children and adolescents: current state of the evidence and research needs. American Psychological Association https://www.apa.org/obesity-guideline/clinical-practice-guideline.pdf (2018).
Nowicka, P. & Flodmark, C. E. Family therapy as a model for treating childhood obesity: useful tools for clinicians. Clin. Child. Psychol. Psychiatry 16 , 129–145 (2011).
Wilfley, D. E. et al. Improving access and systems of care for evidence-based childhood obesity treatment: conference key findings and next steps. Obesity 25 , 16–29 (2017).
Amiri, P. et al. Does motivational interviewing improve the weight management process in adolescents? A systematic review and meta-analysis. Int. J. Behav. Med. 29 , 78–103 (2022).
Kao, T. A., Ling, J., Hawn, R. & Vu, C. The effects of motivational interviewing on children’s body mass index and fat distributions: a systematic review and meta-analysis. Obes. Rev. 22 , e13308 (2021).
Hassapidou, M. et al. European Association for the Study of Obesity (EASO) position statement on medical nutrition therapy for the management of overweight and obesity in children and adolescents developed in collaboration with the European Federation of the Associations of Dietitians (EFAD). Obes. Facts https://doi.org/10.1159/000527540 (2022).
Hoelscher, D. M., Kirk, S., Ritchie, L. & Cunningham-Sabo, L. Position of the Academy of Nutrition and Dietetics: interventions for the prevention and treatment of pediatric overweight and obesity. J. Acad. Nutr. Diet. 113 , 1375–1394 (2013).
Hoare, J. K., Jebeile, H., Garnett, S. P. & Lister, N. B. Novel dietary interventions for adolescents with obesity: a narrative review. Pediatr. Obes. 16 , e12798 (2021).
Lister, N. et al. Nutritional adequacy of diets for adolescents with overweight and obesity: considerations for dietetic practice. Eur. J. Clin. Nutr. 71 , 646–651 (2017).
Andela, S. et al. Efficacy of very low-energy diet programs for weight loss: a systematic review with meta-analysis of intervention studies in children and adolescents with obesity. Obes. Rev. 20 , 871–882 (2019).
Srivastava, G. & Apovian, C. M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 14 , 12–24 (2018).
Apperley, L. J. et al. Childhood obesity: a review of current and future management options. Clin. Endocrinol. 96 , 288–301 (2022).
European Medicines Agency. Saxenda. European Medicines Agency https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda (2022).
US Food and Drug Administration. FDA approves weight management drug for patients aged 12 and older. FDA https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older (2021).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382 , 2117–2128 (2020). To our knowledge, the first RCT of liraglutide, administered daily via subcutaneous injection, in adolescents with obesity.
US Food and Drug Administration. FDA approves novel, dual-targeted treatment for type 2 iabetes. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes (2022).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387 , 205–216 (2022).
Holst, J. J. & Rosenkilde, M. M. GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaa327 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT05260021 (2023).
Müller, T. D., Blüher, M., Tschöp, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21 , 201–223 (2022).
Chalklin, C. G., Ryan Harper, E. G. & Beamish, A. J. Metabolic and bariatric surgery in adolescents. Curr. Obes. Rep. 10 , 61–69 (2021).
Albaugh, V. L. et al. Regulation of body weight: lessons learned from bariatric surgery. Mol. Metab. https://doi.org/10.1016/j.molmet.2022.101517 (2022).
Uhe, I. et al. Roux-en-Y gastric bypass, sleeve gastrectomy, or one-anastomosis gastric bypass? A systematic review and meta-analysis of randomized-controlled trials. Obesity 30 , 614–627 (2022).
Inge, T. H. et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 5 , 165–173 (2017).
Olbers, T. et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(16)30424-7 (2017).
Pratt, J. S. et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis. 14 , 882–901 (2018).
Jarvholm, K. et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child Adolesc. Health 4 , 210–219 (2020).
Van Der Heijden, L., Feskens, E. & Janse, A. Maintenance interventions for overweight or obesity in children: a systematic review and meta‐analysis. Obes. Rev. 19 , 798–809 (2018).
Park, J., Park, M.-J. & Seo, Y.-G. Effectiveness of information and communication technology on obesity in childhood and adolescence: systematic review and meta-analysis. J. Med. Internet Res. 23 , e29003 (2021).
Brissman, M., Beamish, A. J., Olbers, T. & Marcus, C. Prevalence of insufficient weight loss 5 years after Roux-en-Y gastric bypass: metabolic consequences and prediction estimates: a prospective registry study. BMJ Open 11 , e046407 (2021).
El Ansari, W. & Elhag, W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps – a scoping review. Obes. Surg. 31 , 1755–1766 (2021).
World Health Organization Regional Office for Europe. Weight Bias and Obesity Stigma: Considerations for the WHO European Region (WHO, 2017).
Puhl, R. M. & Latner, J. D. Stigma, obesity, and the health of the nation’s children. Psychol. Bull. 133 , 557–580 (2007).
Black, W. R. et al. Health-related quality of life across recent pediatric obesity classification recommendations. Children 8 , 303 (2021).
Finne, E., Reinehr, T., Schaefer, A., Winkel, K. & Kolip, P. Changes in self-reported and parent-reported health-related quality of life in overweight children and adolescents participating in an outpatient training: findings from a 12-month follow-up study. Health Qual. Life Outcomes 11 , 1 (2013).
Hill, A. J. Obesity in children and the ‘myth of psychological maladjustment’: self-esteem in the spotlight. Curr. Obes. Rep. 6 , 63–70 (2017).
McGregor, S., McKenna, J., Gately, P. & Hill, A. J. Self‐esteem outcomes over a summer camp for obese youth. Pediatr. Obes. 11 , 500–505 (2016).
Jansen, P., Mensah, F., Clifford, S., Nicholson, J. & Wake, M. Bidirectional associations between overweight and health-related quality of life from 4–11 years: longitudinal study of Australian children. Int. J. Obes. 37 , 1307–1313 (2013).
Mannan, M., Mamun, A., Doi, S. & Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatr. 21 , 51–66 (2016).
Lindberg, L., Hagman, E., Danielsson, P., Marcus, C. & Persson, M. Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med. 18 , 30 (2020).
Jebeile, H. et al. Association of pediatric obesity treatment, including a dietary component, with change in depression and anxiety: a systematic review and meta-analysis. JAMA Pediatr. 173 , e192841 (2019).
Gow, M. L. et al. Pediatric obesity treatment, self‐esteem, and body image: a systematic review with meta‐analysis. Pediatr. Obes. 15 , e12600 (2020).
Jebeile, H. et al. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: a systematic review with meta-analysis. Obes. Rev. 20 , 1287–1298 (2019). To our knowledge, the first systematic review to show that structured and professionally led weight management interventions in children and adolescents with obesity are associated with reductions in eating disorder risk and symptoms.
Kjeldbjerg, M. L. & Clausen, L. Prevalence of binge-eating disorder among children and adolescents: a systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry https://doi.org/10.1007/s00787-021-01850-2 (2021).
Patton, G. C., Selzer, R., Coffey, C., Carlin, J. B. & Wolfe, R. Onset of adolescent eating disorders: population based cohort study over 3 years. BMJ 318 , 765–768 (1999).
Cortese, S. The association between ADHD and obesity: intriguing, progressively more investigated, but still puzzling. Brain Sci. 9 , 256 (2019).
Griffiths, L. J., Dezateux, C. & Hill, A. Is obesity associated with emotional and behavioural problems in children? Findings from the Millennium Cohort Study. Int. J. Pediatr. Obes. 6 , e423–e432 (2011).
Harrist, A. W. et al. The social and emotional lives of overweight, obese, and severely obese children. Child. Dev. 87 , 1564–1580 (2016).
Van Geel, M., Vedder, P. & Tanilon, J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the relation between weight status and bullying. Int. J. Obes. 38 , 1263–1267 (2014).
Albuquerque, D., Nóbrega, C., Manco, L. & Padez, C. The contribution of genetics and environment to obesity. Br. Med. Bull. 123 , 159–173 (2017).
Rancourt, D. & McCullough, M. B. Overlap in eating disorders and obesity in adolescence. Curr. Diabetes Rep. 15 , 78 (2015).
House, E. T. et al. Identifying eating disorders in adolescents and adults with overweight or obesity: a systematic review of screening questionnaires. Int. J. Eat. Disord. 55 , 1171–1193 (2022).
Lister, N. B., Baur, L. A., Paxton, S. J. & Jebeile, H. Contextualising eating disorder concerns for paediatric obesity treatment. Curr. Obes. Rep. 10 , 322–331 (2021).
Hagman, E. et al. Effect of an interactive mobile health support system and daily weight measurements for pediatric obesity treatment, a 1-year pragmatical clinical trial. Int. J. Obes. 46 , 1527–1533 (2022).
Swinburn, B. A. et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet commission report. Lancet 393 , 791–846 (2019).
Whitehead, M., Taylor-Robinson, D. & Barr, B. Poverty, health, and covid-19. Br. Med. J. 372 , n376 (2021).
Morrison, K. M. et al. The CANadian Pediatric Weight Management Registry (CANPWR): lessons learned from developing and initiating a national, multi-centre study embedded in pediatric clinical practice. BMC Pediatr. 18 , 237 (2018).
Kirk, S. et al. Establishment of the pediatric obesity weight evaluation registry: a national research collaborative for identifying the optimal assessment and treatment of pediatric obesity. Child. Obes. 13 , 9–17 (2017).
Hagman, E., Danielsson, P., Lindberg, L. & Marcus, C., BORIS Steering Committee. Paediatric obesity treatment during 14 years in Sweden: lessons from the Swedish Childhood Obesity Treatment Register – BORIS. Pediatr. Obes. 15 , e12626 (2020).
Bohn, B. et al. Changing characteristics of obese children and adolescents entering pediatric lifestyle intervention programs in Germany over the last 11 years: an adiposity patients registry multicenter analysis of 65,453 children and adolescents. Obes. Facts 10 , 517–530 (2017).
Seidler, A. L. et al. A guide to prospective meta-analysis. BMJ 367 , l5342 (2019).
Hunter, K. E. et al. Transforming obesity prevention for children (TOPCHILD) collaboration: protocol for a systematic review with individual participant data meta-analysis of behavioural interventions for the prevention of early childhood obesity. BMJ Open 12 , e048166 (2022).
Lister, N. B. et al. Eating disorders in weight-related therapy (EDIT) collaboration: rationale and study design. Nutr. Res. Rev. https://doi.org/10.1017/S0954422423000045 (2023).
Hadjiyannakis, S. et al. Obesity class versus the Edmonton Obesity Staging System for Pediatrics to define health risk in childhood obesity: results from the CANPWR cross-sectional study. Lancet Child Adolesc. Health 3 , 398–407 (2019).
Brown, V. et al. Core outcome set for early intervention trials to prevent obesity in childhood (COS-EPOCH): agreement on “what” to measure. Int. J. Obes. 46 , 1867–1874 (2022). A stakeholder-informed study that identified the minimum outcomes recommended for collecting and reporting in obesity prevention trials in early childhood.
Han, J. C., Lawlor, D. A. & Kimm, S. Y. Childhood obesity. Lancet 375 , 1737–1748 (2010).
Shin, A. C., Zheng, H. & Berthoud, H. R. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol. Behav. 97 , 572–580 (2009).
Lennerz, B., Wabitsch, M. & Eser, K. Ätiologie und genese [German]. Berufl. Rehabil. 1 , 14 (2014).
Google Scholar
Pont, S. J., Puhl, R., Cook, S. R. & Slusser, W. Stigma experienced by children and adolescents with obesity. Pediatrics 140 , e20173034 (2017).
Talumaa, B., Brown, A., Batterham, R. L. & Kalea, A. Z. Effective strategies in ending weight stigma in healthcare. Obes. Rev. 23 , e13494 (2022).
Rubino, F. et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 26 , 485–497 (2020).
Download references
Author information
Authors and affiliations.
Children’s Hospital Westmead Clinical School, Sydney Medical School, The University of Sydney, Sydney, New South Wales, Australia
Natalie B. Lister & Louise A. Baur
Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead, Sydney, New South Wales, Australia
Natalie B. Lister
Sydney School of Public Health, The University of Sydney, Sydney, New South Wales, Australia
Louise A. Baur
Weight Management Services, The Children’s Hospital at Westmead, Sydney, New South Wales, Australia
The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Janine F. Felix
Department of Paediatrics, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Institute of Health Sciences, School of Medicine, University of Leeds, Leeds, UK
Andrew J. Hill
Division of Paediatrics, Department of Clinical Science Intervention and Technology, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden
Claude Marcus
Vestische Hospital for Children and Adolescents Datteln, University of Witten/Herdecke, Datteln, Germany
Thomas Reinehr
Department of Sport and Exercise Sciences, Durham University, Durham, UK
Carolyn Summerbell
Division of Paediatric Endocrinology and Diabetes, Department of Paediatrics and Adolescent Medicine, Ulm University Medical Centre, Ulm, Germany
Martin Wabitsch
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (L.A.B., J.F.F. and N.B.L.); Epidemiology (L.A.B. and J.F.F.); Mechanisms/pathophysiology (L.A.B., J.F.F., T.R. and M.W.); Diagnosis, screening and prevention (L.A.B., N.B.L., T.R., C.S. and M.W.); Management (L.A.B., N.B.L., A.J.H., C.M. and T.R.); Quality of life (L.A.B., N.B.L. and A.J.H.); Outlook (L.A.B., N.B.L., J.F.F., A.J.H., C.M., T.R., C.S. and M.W.); Overview of the Primer (L.A.B. and N.B.L.).
Corresponding author
Correspondence to Louise A. Baur .
Ethics declarations
Competing interests.
A.J.H. reports receiving payment for consultancy advice for Slimming World (UK). L.A.B. reports receiving honoraria for speaking in forums organized by Novo Nordisk in relation to management of adolescent obesity and the ACTION-Teens study, which is sponsored by Novo Nordisk. L.A.B. is the Australian lead of the study. T.R. received funding from the German Federal Ministry of Education and Research (BMBF; 01GI1120A/B) as part of the German Competence Network Obesity (Consortium ‘Youth with Extreme Obesity’). T.R. receives payment for consultancy advice related to pharmacological treatment of obesity from Novo Nordisk and Lilly, as well as honoraria for lectures in symposia organized by Novo Nordisk, Novartis and Merck. C.M. receives payments for consultancy advice and advisory board participation from Novo Nordisk, Oriflame Wellness, DeFaire AB and Itrim AB. C.M. also receives honoraria for speaking at meetings organized by Novo Nordisk and Astra Zeneca. C.M. is a shareholder and founder of Evira AB, a company that develops and sells systems for digital support for weight loss, and receives grants from Novo Nordisk for epidemiological studies of the effects of weight loss on future heath. M.W. received funding from the German Federal Ministry of Education and Research (BMBF; 01GI1120A/B) as part of the German Competence Network Obesity (Consortium ‘Youth with Extreme Obesity’). M.W. receives payment for consultancy advice related to pharmacological treatment of obesity from Novo Nordisk, Regeneron, Boehringer Ingelheim and LG Chem, as well as honoraria for speaking in symposia organized by Novo Nordisk, Rhythm Pharmaceuticals and Infectopharm. M.W. is principal investigator in phase II and phase III studies of setmelanotide sponsored by Rhythm Pharmaceuticals. N.B.L., J.F.F. and C.S. declare no competing interests.
Peer review
Peer review information.
Nature Reviews Disease Primers thanks C. Maffeis, L. Moreno, R, Weiss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Lister, N.B., Baur, L.A., Felix, J.F. et al. Child and adolescent obesity. Nat Rev Dis Primers 9 , 24 (2023). https://doi.org/10.1038/s41572-023-00435-4
Download citation
Accepted : 12 April 2023
Published : 18 May 2023
DOI : https://doi.org/10.1038/s41572-023-00435-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Methods and Literature Search
Food insecurity and obesity, food insecurity and bmi, food insecurity and bmi z-score, results synthesis, limitations, conclusions, acknowledgments, food insecurity and childhood obesity: a systematic review.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Christine St. Pierre , Michele Ver Ploeg , William H. Dietz , Sydney Pryor , Chioniso S. Jakazi , Elizabeth Layman , Deborah Noymer , Tessa Coughtrey-Davenport , Jennifer M. Sacheck; Food Insecurity and Childhood Obesity: A Systematic Review. Pediatrics July 2022; 150 (1): e2021055571. 10.1542/peds.2021-055571
Download citation file:
- Ris (Zotero)
- Reference Manager
Video Abstract
Addressing food insecurity while promoting healthy body weights among children is a major public health challenge. Our objective is to examine longitudinal associations between food insecurity and obesity in US children aged 1 to 19 years.
Sources for this research include PubMed, CINAHL, and Scopus databases (January 2000 to February 2022). We included English language studies that examined food insecurity as a predictor of obesity or increased weight gain. We excluded studies outside the United States and those that only considered the unadjusted relationship between food security and obesity. Characteristics extracted included study design, demographics, methods of food security assessment, and anthropometric outcomes.
Literature searches identified 2272 articles; 13 met our inclusion criteria. Five studies investigated the relationship between food insecurity and obesity directly, whereas 12 examined its relationship with body mass index or body mass index z-score. Three studies assessed multiple outcomes. Overall, evidence of associations between food insecurity and obesity was mixed. There is evidence for possible associations between food insecurity and obesity or greater weight gain in early childhood, for girls, and for children experiencing food insecurity at multiple time points. Heterogeneity in study methods limited comparison across studies.
Evidence is stronger for associations between food insecurity and obesity among specific subgroups than for children overall. Deeper understanding of the nuances of this relationship is critically needed to effectively intervene against childhood obesity.
The United States faces 2 important public health challenges in reducing childhood obesity while ensuring that children and their families have enough nutritious food for an active, healthy life. From 2017 to 2018, ∼20% of US children aged 2 to 19 were estimated to have obesity, a prevalence level that has increased by nearly 40% over the past 20 years. 1 In 2020, the first year of the COVID-19 pandemic, food insecurity among US households with children was 14.8%, an increase over the 2019 level of 13.6% and a reversal of the declining trend observed over the previous decade. 2
Childhood obesity and food insecurity are more prevalent in lower-income households, 2 , 3 suggesting a potentially simultaneous occurrence of both under- and over-nutrition. Despite almost 3 decades since these dual problems were first observed, 4 no consensus exists about the underlying mechanisms of their relationship. The increases in food insecurity 2 , 5 and accelerated weight gain 6 – 8 observed among children during the COVID-19 pandemic indicate that greater understanding of how these 2 issues interact is of great importance for child health, particularly in terms of associations between food insecurity episodes and weight status over the long term.
In this systematic review, we examined longitudinal associations between food insecurity and obesity in US children aged 1 to 19 years. The review summarizes the overall evidence, then discusses differences in the evidence according to relevant demographics and the experience of multiple food insecurity episodes.
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis. Searches were performed in December 2020 in PubMed, CINHAL, and Scopus databases and restricted to English language studies published between January 1, 2000, and November 30, 2020. Studies published before 2000 were excluded to maximize homogeneity in food security and child weight status assessment tools. Results were uploaded to the Covidence systematic review tool (Covidence, Melbourne, Australia) for screening.
Studies eligible for inclusion were those with participants who were infants or children in the United States from 1 to 19 years of age; that assessed food security or insufficiency at the household or child level; compared outcomes by food security status; and examined obesity or body mass index (BMI) as the primary outcome of interest (see full search strategy Supplemental Table 3 ). Included studies were limited to those conducted in the United States to reduce heterogeneity in the food security assessment tools employed. Studies were excluded if they only reported unadjusted relationships between food security and obesity, or if the study population was limited to only youth with overweight or obesity at baseline. Studies with less than 30 participants, the traditional minimum in statistics for a reliable confidence interval, were also excluded. Title and abstract screening, full-text screening and data extraction were performed by 2 independent reviewers; conflicts were resolved by consultation between researchers. Database searches were re-run in September 2021 and February 2022, and results were hand-searched to add relevant studies published between December 2020 and February 2022.
Study quality was assessed using the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies (NIH, Bethesda, Maryland). Although quality assessment tools for clinical trials are well established, there is no consensus on the best methods to assess the quality of observational nutrition studies. 9 We selected the NIH tool because it could be applied consistently to all included studies. Two researchers applied the assessment tool independently. Disagreements were resolved via discussion among the research team.
The database search yielded 2272 studies after duplicates were removed. Following title and abstract review, 91 papers were retained for full-text screening. Forty-two were excluded for 1 of the following: methods (no adjusted estimate of the relationship between food security and anthropometrics); outcome (did not assess likelihood of obesity or a continuous BMI-related outcome); population (participants over 19 years old); or location (outside the United States). A total of 41 studies met the inclusion criteria after the initial screening, 4 studies were subsequently added following the search and screening process in September 2021, and no additional studies meeting the inclusion criteria were identified in the February 2022 search ( Fig 1 ).

Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram detailing review search process. PRISMA statement distributed under the terms of the Creative Commons Attribution License. Original source: Mohr D, Liberati A, Tetzlaff J, Altmann DC, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. PLoS Med . 2009;6(7);e1000098. 37
A total of 45 papers were originally included for data extraction. Data on sample size, demographic characteristics of study participants, nutrition assistance program participation (when available), food security assessment methods, outcomes measured, method of analysis, covariates included in analysis, adjusted results, and tests for interaction with any subsequent stratified results, were extracted using a piloted, standardized extraction spreadsheet. Although both longitudinal and cross-sectional studies were initially included, only the longitudinal studies ( n = 16) were ultimately retained for evidence analysis because of their overall higher study quality and ability to provide insight into potential relationships between food insecurity and obesity over time.
In the quality analysis, all longitudinal studies were rated “good” or “fair” ( Supplemental Table 4 ). Thirteen studies 10 – 22 used either the “gold standard” USDA 18-item Household Food Security Survey Module or a shorter subset derived directly from the full module to assess food security, whereas 3 studies used single-item measures. These 3 studies were excluded from the overall results synthesis because they lacked a standardized food security assessment instrument.
In the final 13 studies, variation in outcomes measured, food security categorization (eg, binary vs multilevel and categorical versus continuous), and analysis methods prevented us from conducting a meta-analysis. We instead present the results according to the 3 different outcomes analyzed in the included studies. Our primary interest was examining the relationship between food insecurity and obesity. We focused on obesity rather than both overweight and obesity because obesity has a greater sensitivity and specificity for identifying excess body fat and carries a higher risk for adverse health outcomes. 23 To examine potential differences in trajectories of BMI growth, we also synthesized the evidence for associations between food insecurity and changes in BMI and BMI z-score. Both variables present interpretation challenges as longitudinal outcomes. BMI changes are not indexed to age and sex-specific references in studies and may not account for the normative dip in BMI that occurs in early childhood. 24 BMI z-score changes are smaller at higher levels of adiposity and do not adequately reflect large changes in weight and adiposity. 25 Despite these limitations, the growth trajectories identified in these analyses can illuminate associations between food insecurity and excess weight gain in children. When included studies measured multiple outcomes, we included the findings in each of the applicable syntheses. Table 1 summarizes the characteristics of all included studies.
Characteristics of Included Studies
Only analyses meeting our inclusion criteria are presented in the table; studies may have analyzed other outcomes that did not fit our criteria
Five studies examined the association between food insecurity and obesity. 12 , 14 , 19 – 21 Among a cohort of participants in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) in Massachusetts, an association was found between low food security (worried about food but no disruption to eating patterns, as opposed to very low food security, which also includes disruption of eating patterns) when present in both infancy and preschool and greater odds of obesity in preschool. 20 This relationship was also moderated by maternal prepregnancy weight status, with greater odds of obesity for children in households with low food security at both time points and whose mothers were either overweight, had obesity, or were underweight prepregnancy. An analysis of the Early Childhood Longitudinal Study (ECLS) birth cohort using structural equation modeling found no direct association between food insecurity and obesity, but in mediation analysis, food insecurity affected parenting and infant feeding behaviors, which ultimately affected weight. 12 The 3 remaining studies found no significant associations between food insecurity and obesity; 2 used data from the ECLS-K 21 and ECLS-K: 2011 19 cohorts, and the third used findings from a cohort of Hispanic mothers and their children in California (CHAMACOS). 14
Four studies analyzed the relationship between food insecurity and changes in BMI over time. Of the 2 studies using ECLS-K data, 1 found a greater increase in BMI among children whose households were food insecure at 2 time points, 17 and both found higher BMI increases for girls from food insecure households but not for boys. 13 , 17 In a third study using ECLS-K data, the authors reported no significant associations but did not test for interactions by sex or consider food insecurity at multiple time points. 11 The CHAMACOS study found lower BMI gains among children whose households changed from highly food secure to marginally food secure or food insecure across 2 time points. 14
Nine studies investigated associations between food insecurity and changes in BMI z-score. The ECLS-K: 2011 study found an association between food insecurity in first grade and an increased BMI z-score in third grade, but no association between kindergarten food insecurity and third grade BMI z-score. 19 Likewise, a fourth ECLS-K study found an association between fifth grade food insecurity and a higher eighth grade BMI z-score but no significant associations when food insecurity occurred in younger grades. 22 In a Head Start preschool cohort, an association was found between food insecurity and increased BMI z-score for girls, but participants were only followed for an average of 6 months. 16 A birth cohort following infants through 12 months found an association between very low food security and a higher BMI z-score, 10 whereas the WIC cohort found no association in the main analysis but an association between food insecurity in infancy and an increased BMI z-score in early childhood if the mother was overweight or had obesity prepregnancy. 20 In the CHAMACOS cohort, food insecurity at age 9 was associated with a decrease in BMI z-score from ages 9 to 10.5, and food insecurity across 2 time points or changing from food secure to food insecure were also associated with decreased BMI z-score. 14 The remaining 3 studies found no significant associations. 11 , 15 , 18
The studies are categorized by outcome and findings in Table 2 . An association between food insecurity and obesity was found only in early childhood, 20 whereas 6 additional studies found evidence of associations between food insecurity and increases in BMI or BMI z-score in limited age groups or sex-specific analyses. 10 , 13 , 16 , 17 , 19 , 22 One study of an exclusively Hispanic population found evidence of an association between food insecurity and decreases in BMI z-score or BMI, limited to a specific age group or changes in food security status. 14 Although all studies assessed food security based on standardized US Department of Agriculture assessment tools, comparison is challenging because of differences in food security categorization. Most studies categorized participants as either food secure (high or marginal food security according to survey responses) or food insecure (low or very low food security). However, 2 studies combined marginal food security with low and very low food security, 14 , 17 3 studies used more than 2 categories for food security status, 10 , 12 , 20 and 1 used a continuous variable. 11 Studies also differed in the covariates used in their analyses. Child age, sex, race or ethnicity, household income, and parent or maternal education were consistently included as control variables, but other predictors of obesity, such as physical activity level, child birth weight, and maternal BMI, were included in no more than half of the studies. The variations in both the food security variable and covariates may help explain the mixed results observed across studies.
Findings by Outcome
The number of studies does not sum to 13 because some studies investigated multiple outcomes.
Our findings corroborate the previously published literature, indicating that potential relationships between food insecurity and childhood obesity and child weight changes are complex. Although the evidence did not allow us to draw broad conclusions about the relationship between food insecurity and obesity in children, we were nevertheless able to gain deeper insight and identify directions for further research by synthesizing results according to age, sex, and multiple experiences of food insecurity.
We observed associations between food insecurity and increases in BMI or BMI z-score among infants, 10 preschoolers, 16 , 20 elementary students, 17 , 19 and middle school students. 13 , 22 Although 5 of the studies with evidence of higher BMIs among food insecure youth were highly powered cohorts with large samples, 13 , 17 , 19 , 20 , 22 findings were limited to specific age ranges or subgroups within the sample, with the exception of the preschool study. 20 In the localized CHAMACOS cohort, food insecurity was associated with decreased BMI z-scores during mid- to late elementary years. 14 Thus, the mixed evidence is in agreement with the 2015 Dietary Guidelines Advisory Committee (DGAC) conclusion that limited evidence supports an association between food insecurity and higher anthropometric measurements in early childhood. 26 It is also particularly noteworthy that none of the studies followed children beyond eighth grade. Eight of the 13 studies in our review were published after the 2015 DGAC identified the need for additional study of food insecurity and weight changes into the adolescent years, 26 but none provided evidence of potential associations beyond middle school.
Three of the studies in the review presented results stratified by child sex, and all found an association between food insecurity and increased BMI or BMI z-score for girls but not for boys. 13 , 16 , 17 Three additional studies tested for interaction by sex but found no associations. 14 , 20 , 22 Associations between food insecurity and higher BMI for preschool girls have also been found cross-sectionally. 27 , 28 Potential explanations for this association in girls but not in boys could include differential parent feeding practices by gender, 29 or different responses to stress, including the experience of food insecurity. 17 Lack of testing for interaction by sex in many studies could also be masking associations in 1 of the groups, even if a relationship is not found in the overall population. 27 Following youth into adolescence and early adulthood could also help clarify differences in the interactions between food insecurity and weight by sex, particularly given recent evidence among adults that food insecurity was more prevalent among women with greater adiposity. 30
Several of the longitudinal studies in the review categorized food security across multiple time points to examine how changes in food security status or multiple episodes of food insecurity were related to obesity and BMI. 13 , 14 , 16 , 17 , 20 In 3 large studies, food insecurity at multiple time points was associated with obesity or greater BMI growth relative to food security at all time points, 13 , 17 , 20 and 1 preschool study found that for girls, higher BMI z-scores were associated with the household changing from food secure to food insecure over the course of 1 school year. 16 One smaller study found an association with decreased BMI z-scores when food insecurity occurred at multiple time points or when households transitioned from food security to food insecurity, 14 but more evidence points to a potential cumulative positive effect of multiple experiences of food insecurity on weight gain, an effect also observed by the 2015 DGAC. 26 The effects of the duration and episodic nature of food insecurity may be of particular relevance to the increase in childhood obesity that has occurred during the COVID-19 pandemic. 6 , 7
Potential differences by age range, sex, and the unknown effects of fluctuations in food security status over time indicate that a systems or structural modeling approach may provide better insight into how food insecurity and child weight status are related to one another through indirect channels. Household stress may play an important mediating role in the relationship between food insecurity and weight outcomes. One longitudinal study with a small sample size found an association between food insecurity and increased BMI when high stress was present at the child level, 31 and multiple studies have examined how interactions between maternal stress and food insecurity may affect child weight status. 32 – 35 Two of the studies included in this review included structural models that accounted for parental feeding practices, 12 , 18 and another structural model includes child dietary intake and both child and parent physical activity levels. 36 Further research can build on such models to better understand the complex mechanisms that affect the relationship between food insecurity and child weight status. Irrespective of any future conclusive evidence on the relationship mechanisms between food insecurity and childhood obesity, however, effective interventions against child food insecurity should be a public health priority to promote the physical, emotional, and cognitive wellbeing of children and parents.
Although limiting our analysis to longitudinal studies strengthens the evidence relative to cross-sectional findings, following low-income populations over long periods is a challenging endeavor. The ECLS-K studies did not remain nationally representative over the follow-up periods, and 1 of them specifically noted that participants excluded because of missing data were more likely to be of lower socioeconomic status. 19 Recent evidence indicates that racial or ethnic disparities in childhood obesity have increased since the COVID-19 pandemic, 6 but our ability to explore potential differences by race or ethnicity in the food insecurity-childhood obesity relationship was limited by lack of testing for interaction by race or ethnicity. Two of the 3 studies that followed youth into puberty omitted any discussion of pubertal status, despite connections between puberty and anthropometric measurements that could have affected study findings. Differences in covariates, most notably omission of control variables for physical activity in most studies and for dietary quality in all studies, may contribute to the inconsistent findings. Finally, we were limited in our ability to assess the relationship between food insecurity and obesity by the diverse outcomes measured in the included studies. A greater proportion of studies used continuous BMI outcomes relative to weight categories. Although these studies showed changes in BMI trajectories, it was not apparent whether these changes indicated movement across weight categories.
We observed mixed evidence of associations between food insecurity and childhood obesity, but the mechanisms of their relationship remain difficult to ascertain. This review highlights the importance of understanding the many nuances of how food insecurity and childhood obesity interact with one another, which is even more critical as we have observed increased child food insecurity and widening disparities in the prevalence of obesity amid the COVID-19 pandemic. Ongoing and future studies need to consider interactions between food insecurity and salient demographics and the broader context of the household environment to enable us to meet the dual challenges of reducing childhood obesity and ensuring food security for all families.
We thank the anonymous reviewers for their thoughtful and insightful feedback on this paper.
Ms St. Pierre and Dr Ver Ploeg conceptualized and designed the study, coordinated and supervised data collection, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Dietz and Sacheck conceptualized and designed the study and critically reviewed the manuscript for important intellectual content; Ms Pryor, Ms Jakazi, Ms Layman, Ms Noymer, and Ms Coughtrey-Davenport collected data, conducted the initial analyses, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: This research was supported by Healthy Eating Research, a national program of the Robert Wood Johnson Foundation.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no conflicts of interest to disclose.
body mass index
Dietary Guidelines Advisory Committee
Early Childhood Longitudinal Study
National Institutes of Health
Supplemental Nutrition Assistance Program
Special Supplemental Nutrition Program for Women, Infants, and Children
Supplementary data
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Socioeconomic Status and Childhood Obesity: a Review of Literature from the Past Decade to Inform Intervention Research
- Etiology of Obesity (M Rosenbaum, Section Editor)
- Published: 12 August 2020
- Volume 9 , pages 562–570, ( 2020 )
Cite this article
- Christian E. Vazquez ORCID: orcid.org/0000-0002-3792-9150 1 &
- Catherine Cubbin 1
7513 Accesses
78 Citations
18 Altmetric
Explore all metrics
Purpose of Review
This is a review of the patterns, conceptualization, and suggested mechanisms underlying the relationship of socioeconomic status (SES) to obesity in childhood and the implications of these data for interventions going forward.
Recent Findings
Adiposity and SES are negatively associated in high-income countries and positively associated in medium to low-income countries. Several mechanisms, such as early introduction of solid food and parental behaviors, which may explain the association of SES and adiposity, have been identified. Parental education and adiposity and early pediatric nutrition appear to be particularly salient SES-related effectors on adiposity.
There is a clear association of SES and adiposity which is affected by population affluence. Evaluation of the relationship of SES and obesity in children are complicated by the complexity of SES and lack of common definition. A number of SES-related interventional targets have been identified. Intervention research should ensure they are addressing SES-associated issues in the study population.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Does Household Income Affect children’s Outcomes? A Systematic Review of the Evidence
Kerris Cooper & Kitty Stewart
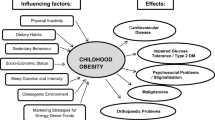
Risk Factors and Implications of Childhood Obesity
Susann Weihrauch-Blüher & Susanna Wiegand

Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem
Ellen P. Williams, Marie Mesidor, … Sharon B. Wyatt
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pratt CA, Loria CM, Arteaga SS, Nicastro HL, Lopez-Class M, de Jesus JM, et al. A systematic review of obesity disparities research. Am J Prev Med. 2017;53(1):113–22.
PubMed Google Scholar
Trust for America’s Health. The state of obesity: better policies for a healthier America. [Internet]. 2019. Available from: https://www.tfah.org/wp-content/uploads/2019/09/2019ObesityReportFINAL-1.pdf
UNICEF. The state of the world’s children 2019. Children, food and nutrition: growing well in a changing world. [Internet]. New York: UNICEF; 2019. Available from: https://www.unicef.org/reports/state-of-worlds-children-2019
Google Scholar
Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–30.
Finkelstein EA, Graham WKC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatr. 2014;133(5):854–62.
Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systemic review. Obes Rev. 2018;19(4):452–63.
CAS PubMed Google Scholar
Sonntag D, Ali S, De Bock F. Lifetime indirect costs of childhood overweight and obesity: a decision analytic model. Obesity. 2016;24(1):200–6.
Gebremariam M, Lien N, Nianogo R, Arah O. Review of mediators of socioeconomic differences in adiposity among youth: a systematic review. Obes Rev. 2017;18(8):880–98.
Vallgarda S. Childhood obesity policies - mighty concerns, meek reactions. Obes Rev. 2018;19(3):295–301.
The World Bank. World bank country and lending groups. [Internet]. 2019. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Safron M, Cislak A, Gaspar T, Luszczynska A. Effects of school-based interventions targeting obesity-related behaviors and body weight change: a systematic umbrella review. J Behav Med. 2011;37(1):15–25.
Cislak A, Safron M, Pratt M, Gaspar T, Luszczynska A. Family-related predictors of body weight and weight-related behaviours among children and adolescents: a systematic umbrella review. Child Care Health Dev. 2012;38(3):321–31.
Rajjo T, Mohammed K, Alsawas M, Ahmed AT, Farah W, Asi N, et al. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab. 2017;102(3):763–75.
Kobes A, Kretschmer T, Timmerman G, Schreuder P. Interventions aimed at preventing and reducing overweight/obesity among children and adolescents: a meta-synthesis. Obes Rev. 2018;19(8):1065–79.
Ells LJ, Rees K, Brown T, Mead E, Al-Khudairy L, Azevedo L, et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int J Obes. 2018;42(11):1823–33.
Jang M, Chao A, Whittemore R. Evaluating intervention programs targeting parents to manage childhood overweight and obesity: a systematic review using the re-aim framework. J Pediatr Nurs. 2015;30(6):877–87.
Ash T, Agaronov A, Young T, Aftosmes-Tobio A, Davison KK. Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis. Int J Behav Nutr Phys Act. 2017;14(1):113.
PubMed PubMed Central Google Scholar
Ling J, Robbins L, Wen F, Zhang N. Lifestyle interventions in preschool children: a meta-analysis of effectiveness. Am J Prev Med. 2017;53(1):102–12.
•• Brown T, Moore T, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2019;7:CD001871 Most recent (at time of publication) systematic review of interventions that reports SES differences.
Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry. 2012;24(3):176–88.
Schroeder K, Kulage KM, Lucero R. Beyond positivism: understanding and addressing childhood obesity disparities through a critical theory perspective. J Spec Pediatr Nurs. 2015;20(4):259–70.
Grant-Guimaraes J, Feinstein R, Laber E, Kosoy J. Childhood overweight and obesity. Gastroenterol Clin N Am. 2016;45(4):715–28.
Sobal J, Stunkard A. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105:260–75.
Wu S, Ding Y, Wu F, Li R, Hu Y, Hou J, et al. Socio-economic position as an intervention against overweight and obesity in children: a systematic review and meta-analysis. Sci Rep. 2015;5(1):11354.
CAS PubMed PubMed Central Google Scholar
Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990-2005. Obesity. 2008;16(2):275–84.
El-Sayed AM, Scarborough P, Galea S. Socioeconomic inequalities in childhood obesity in the United Kingdom: a systematic review of the literature. Obes Facts. 2012;5:671–92.
Barriuso L, Miqueleiz E, Albaladejo R, Villanueva R, Santos JM, Regidor E. Socioeconomic position and childhood-adolescent weight status in rich countries: a systematic review, 1990-2013. BMC Pediatr. 2015;15.
Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes. 2010;34(1):41–7.
CAS Google Scholar
Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011;6(5–6):342–60.
Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proc Natl Acad Sci U S A. 2014;111(4):1338–42.
Robert Wood Johnson Foundation. Declining childhood obesity rates - where are we seeing signs of progress? [Internet]. Health Policy Snapshot - Childhood Obesity. 2013; Available from: https://www.issuelab.org/resources/15536/15536.pdf .
Ogden CL, Carroll MD, Lawman HG, Fryar C, Kruszon-Moran D, Kit B, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–9.
Chung A, Backholer K, Wong E, Palermo C, Keating C, Peeters A. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: a systematic review. Obes Rev. 2016;17(3):276–95.
Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev. 2012;13(11):1067–79.
Byrne M, Schwartz O, Simmons J, Sheeber L, Whittle S, Allen N. Duration of breastfeeding and subsequent adolescent obesity: effects of maternal behavior and socioeconomic status. J Adolesc Health. 2018;62(4):471–9.
Mech P, Hooley M, Skouteris H, Williams J. Review of parent-related mechanisms underlying the social gradient of childhood overweight and obesity: a systematic review. Child Care Health Dev. 2016;42(5):603–24.
• Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research one size does not fit all. JAMA. 2005;294(22):2879–88 Critical analysis of the problems with measuring socioeconomic status and suggested improvements that are reiterated in the current review .
Hillier-Brown FC, Bambra CL, Cairns J, Kasim A, Moore HJ, Summerbell CD. A systematic review of the effectiveness of individual, community and societal level interventions at reducing socioeconomic inequalities in obesity amongst children. BMC Public Health. 2014;14(1):834.
Silventoinen K, Huppertz C, van Beijsterveldt C, Bartels M, Willemsen G, Boomsma D. The genetic architecture of body mass index from infancy to adulthood modified by parental education. Obesity. 2016;24(9):2004–11.
Shackleton N. Is there a link between low parental income and childhood obesity? J Early Child Res. 2017;15(3):238–55.
Powell LM, Wada R, Krauss RC, Wang Y. Ethnic disparities in adolescent body mass index in the United States: the role of parental socioeconomic status and economic contextual factors. Soc Sci Med. 2012;75(3):469–76.
Laws R, Campbell KJ, van der Pligt P, Russell G, Ball K, Lynch J, et al. The impact of interventions to prevent obesity or improve obesity related behaviours in children (0-5 years) from socioeconomically disadvantaged and/or indigenous families: a systematic review. BMC Public Health. 2014;14(1):779.
Morgan EH, Schoonees A, Sriram U, Faure M, Seguin-Fowler RA. Caregiver involvement in interventions for improving children’s dietary intake and physical activity behaviors. Cochrane Database of Syst Rev. 2020;1:CD012547.
Gibbs B, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9(2):135–46.
Chatham RE, Mixer SJ. Cultural influences on childhood obesity in ethnic minorities: a qualitative systematic review. J Transcult Nurs. 2019;31(1):87–99.
Yan J, Liu L, Zhu Y, Huang G, Wang P. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14(1):1267.
Kaur J, Lamb M, Ogden C. The association between food insecurity and obesity in children - the National Health and Nutrition Examination Survey. J Acad Nutr Diet 2015:115(5):751–758.
Jones KM, Power ML, Queenan JT, Schulkin J. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10(4):186–96.
Tremblay MS, Gray CE, Akinroye K, Harrington DM, Katzmarzyk PT, Lambert EV, et al. Physical activity of children: a global matrix of grades comparing 15 countries. J Phys Act Health. 2014;11(suppl 1):S113–25.
Brown T, Smith S, Bhopal R, Kasim A, Summerbell C, Brown T. Diet and physical activity interventions to prevent or treat obesity in South Asian children and adults: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12(1):566–94.
Andrea S, Hooker E, Messer L, Tandy T, Boone-Heinonen J. Does the association between early life growth and later obesity differ by race/ethnicity or socioeconomic status? A systematic review. Ann Epidemiol. 2017;27(9):583–92.
Whitaker R, Wright J, Pepe M, Seidel K, Dietz W. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–73.
Reinehr T, Kleber M, Lass N, Toschke A. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91(5):1165–71.
Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–88.
Fakhouri T, Hughes J, Brody D, Kit B, Ogden C. Physical activity and screen-time viewing among elementary school-aged children in the United States from 2009 to 2010. JAMA Pediatr. 2013;167(3):1–7.
Mcgoron L, Hvizdos E, Bocknek E, Montgomery E, Ondersma S. Feasibility of internet-based parent training for low-income parents of young children. Child Youth Serv Rev. 2018;84:198–205.
de Niet J, Timman R, Jongejan M, Passchier J, van den Akker E. Predictors of participant dropout at various stages of a pediatric lifestyle program. Pediatrics. 2011;127(1):e164–70.
Cui Z, Seburg E, Sherwood N, Faith M, Ward D. Recruitment and retention in obesity prevention and treatment trials targeting minority or low-income children: a review of the clinical trials registration database. Trials. 2015;16(1):564.
Shabbir S, Kwan D, Wang MC, Shih M, Simon PA. Asians and Pacific Islanders and the growing childhood obesity epidemic. Ethn Dis. 2010;20(2):129–35.
Bullock A, Sheff K, Moore K, Manson S. Obesity and overweight in American Indian and Alaska Native children, 2006-2015. Am J Public Health. 2017;107(9):1502–7.
Ligthart KAM, Buitendijk L, Koes BW, van Middelkoop M. The association between ethnicity, socioeconomic status and compliance to pediatric weight-management interventions - a systematic review. Obes Res Clin Pract. 2017;11(5 Suppl 1):1–51.
Barkin SL, Gesell SB, Po’e EK, Escarfuller J, Tempesti T. Culturally tailored, family-centered, behavioral obesity intervention for Latino-American preschool-aged children. Pediatrics. 2012;130(3):445–56.
Perez LG, Arredondo EM, Elder JP, Barquera S, Nagle B, Holub CK. Evidence-based obesity treatment interventions for Latino adults in the US: a systematic review. Am J Prev Med. 2013;44(5):550–60.
Gassman-Pines A, Skinner AT. Psychological acculturation and parenting behaviors in Mexican-immigrant families. J Fam Issues. 2018;39(5):1139–64.
Peeters A, Backholer K. Prioritising and tackling socio-economic inequalities in obesity. BMC Obesity. 2014;1.
Lin B, Smith T, Lee J, Hall K. Measuring weight outcomes for obesity intervention strategies: the case of a sugar-sweetened beverage tax. Econ Hum Biol. 2011;9(4):329–41.
Briggs A, Mytton O, Kehlbacher A, Tiffin R, Rayner M, Scarborough P. Overall and income specific effect on prevalence of overweight and obesity of 20% sugar sweetened drink tax in UK: econometric and comparative risk assessment modelling study. BMJ. 2013;347.
Ruff RR, Zhen C. Estimating the effects of a calorie-based sugar-sweetened beverage tax on weight and obesity in New York city adults using dynamic loss models. Ann Epidemiol. 2015;25(5):350–7.
• Nakhimovsky SS, Feigl AB, Avila C, O’Sullivan G, Macgregor-Skinner E, Spranca M. Taxes on sugar-sweetened beverages to reduce overweight and obesity in middle-income countries: a systematic review. PLoS ONE. 2016;11(9):e0163358 This recent systematic review, based on mostly estimates, suggests taxing SSBs will reduce net energy intake by enough to prevent further growth in obesity prevalence, but not to reduce population weight permanently .
Nakamura R, Mirelman A, Cuadrado C, Silva-Illanes N, Dunstan J, Suhrcke M. Evaluating the 2014 sugar-sweetened beverage tax in Chile: an observational study in urban areas. PLoS Med. 2018;15(7):e1002596.
Gortmaker S, Wang Y, Long M, Giles C, Ward Z, Barrett J, et al. Three interventions that reduce childhood obesity are projected to save more than they cost to implement. Health Aff. 2015;34(11):1932–9.
• Jones A. Race, socioeconomic status, and health during childhood: a longitudinal examination of racial/ethnic differences in parental socioeconomic timing and child obesity risk. Int J Environ Res Public Health. 2018;15(4) Strong recent study suggesting SES, particularly mother’s employment and father’s education, is linked to weight gain differently for White, Black, and Hispanic children at specific ages .
CONSORT. Transparent Reporting of Trials. [Internet]. 2020. Available from: http://www.consort-statement.org/
Hoffmann T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Download references
Author information
Authors and affiliations.
Steve Hicks School of Social Work, The University of Texas at Austin, 1925 San Jacinto Blvd, Austin, TX, 78712, USA
Christian E. Vazquez & Catherine Cubbin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christian E. Vazquez .
Ethics declarations
Conflict of interest.
No conflicts of interest are declared for study authors.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Etiology of Obesity
Rights and permissions
Reprints and permissions
About this article
Vazquez, C.E., Cubbin, C. Socioeconomic Status and Childhood Obesity: a Review of Literature from the Past Decade to Inform Intervention Research. Curr Obes Rep 9 , 562–570 (2020). https://doi.org/10.1007/s13679-020-00400-2
Download citation
Published : 12 August 2020
Issue Date : December 2020
DOI : https://doi.org/10.1007/s13679-020-00400-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Socioeconomic status
- Health disparities
- Interventions
Advertisement
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Advance articles
- Editor's Choice
- Supplements
- Author Guidelines
- Submission Site
- Open Access
- About Journal of Public Health
- About the Faculty of Public Health of the Royal Colleges of Physicians of the United Kingdom
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Advertising and Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, results and review of papers.
- < Previous
Preventing obesity in pre-school children: a literature review
- Article contents
- Figures & tables
- Supplementary Data
Karen L. Saunders, Preventing obesity in pre-school children: a literature review, Journal of Public Health , Volume 29, Issue 4, December 2007, Pages 368–375, https://doi.org/10.1093/pubmed/fdm061
- Permissions Icon Permissions
Obesity in children is increasing worldwide, impacting on both long- and short-term health. Obesity prevention is an important contemporary public health priority and is firmly on the Government's agenda in the UK. Prevention involves addressing the main risk factors of diet and physical inactivity and also involves a wide range of environmental factors including access to sport and leisure, family life, diet, education and information.
A literature review undertaken on preventing obesity in children aged <5.
The review confirms that there is a limited and immature evidence and lack of comprehensive evidence on effective strategies to prevent obesity in younger children. The overall quality of studies is poor.
The need remains for structured, focused and systematic research on child obesity prevention. Well-designed studies examining a range of interventions remain a priority. The findings in this review support the recommendations in the National Institute for Health and Clinical Excellence (NICE) guidelines on obesity.
Child obesity will continue to be a problem without improved understanding of key factors operative during early childhood and identification of effective interventions. 1 The UK Government has responded to rising childhood obesity with a Public Service Agreement target to ‘halt the year on year rise in obesity among children aged under 11 by 2010’. 2
Primary Care Trusts (PCTs) now measure primary school children in the reception year (age, 4–5 years) and year 6 (age, 10–11 years). 3 Many Local Authorities (LAs) have incorporated targets to reduce childhood obesity into Local Area Agreements. 4
Strategic Health Authorities (SHAs), PCTs and LAs are expected to use the best available evidence in establishing plans to tackle child obesity. 5 The Wanless Report identified that the evidence base was particularly weak on interventions to reduce health inequalities due to obesity. 6 The recent NICE guidance reinforces that ‘ for children and young people, it is accepted that the evidence base is far from complete’. 7
At regional and local level, further information is needed on effective interventions. This review of the prevention of obesity in pre-school children has been conducted to inform such policy.
A broad scoping search was undertaken to identify key terms, to assess the breadth and depth of the literature and to establish a broad structure for the review. The search strategy was refined and candidate studies identified by searching PubMed (restricted to reviews), Cochrane and the Department of Health (DH) library catalogue. Inclusion criteria were obesity defined by body mass index (BMI), weight-for-height index and/or skinfold thickness, <5 years, some form of intervention and some assessment of effectiveness. Drug therapy was excluded and searches were restricted to English language. Titles and abstracts were assessed for relevance and where abstracts were unavailable and/or the relevance of the paper uncertain from the title, the full paper was obtained. Further candidate articles were identified from citations and review articles that specifically addressed obesity prevention in pre-school children. Assessment of studies was undertaken with an academic colleague from the University of Birmingham with experience in systematic reviewing.
Given the relatively underdeveloped field of work, with a small crop of peer reviewed papers, Internet searches using the ‘Google’ search engine were undertaken using the term ‘Interventions Preventing Obesity in Preschool Children’. There were 35 100 hits. Following discussions with West Midlands Health Technology Assessment Collaboration, a pragmatic approach was adopted and the first 200 results reviewed.
A data extraction form was developed by the author and was applied to all included papers. Data extracted included the objective and type of study, the setting, sample, intervention undertaken, measures used to evaluate impact, results and the author's comments on the studies. The types of studies ranged from simple observational methods to higher order studies—one randomized control trial (RCT) and two cohort studies.
The literature search identified 832 papers. Six papers met the inclusion criteria (Fig. 1 ). Excluded papers after full review and reasons for rejection are available from the author.
Schematic of literature survey.
The interventions identified in included papers were grouped around themes developed from the scoping search viz breastfeeding, 8 physical activity, 9 , 10 family-based interventions 11 , 12 and professional support. 13 Characteristics of the included studies are given in Table 1 . The quality of each study was assessed in terms of study design including subject numbers, randomization, control for confounding and minimization of bias.
Characteristics of included studies
Armstrong and Reilly 8 tested the hypothesis that breastfeeding is associated with a reduced risk of child obesity in a large, well-conducted cohort study in Scotland, using a population-based sample of 32 000 children. The authors examined the health records of children born in 1995 and 1996 who had undergone routine health screenings as part of the ‘Child Health Surveillance Programme’. During a screening at 6–8 weeks, the health worker asked the mother whether the baby was breastfed only, formula-fed only or fed both breast milk and formula. During a similar screening at 39–42 months, the health worker measured the child's height and weight and calculated the BMI. The prevalence of obesity was significantly lower among breastfed children compared with formula-fed children. This association persisted after adjustment for deprivation, birth weight and sex. The adjusted odds ratio (OR) for obesity was 0.70 (95% CI, 0.61–0.80). The findings suggest that breastfeeding is associated with a modest, but significant, reduction in childhood obesity risk. The authors also suggest that the reduction in risk is present in early childhood. There are limitations to the study given lack of information on other risk factors for obesity, including diet (once children began eating food), parental weight and physical activity.
Mo-suwan et al. 9 evaluated the effect of a school-based aerobic exercise programme on the obesity indexes of pre-school children in Thailand in a RCT. A total of 292 second-year elementary school pupils from two nursery schools were included: 147 (34 from school 1 and 113 from school 2) in the exercise group and 145 (45 from school 1 and 100 from school 2) in the control group. The mean age of the children was 4.5 years. Trained staff encouraged children in the exercise group to take part in a specially designed 30-week exercise programme. One school provided extra swimming for 1 h a week. Weight, height and triceps skinfold (TSF) thickness were measured four times throughout the study. Prevalence of obesity in both the exercise and control groups decreased. The exercise group decreased from 12.2% at baseline to 8.8% (Wilcoxon signed-rank test, P = 0.058), whereas the control group decreased from 11.7 to 9.7% (Wilcoxon signed-rank test, P = 0.179). The reduction in obesity in the exercise group was not significant, but was greater than the control group. A gender difference in the response of BMI to exercise was observed. Girls in the exercise group had a significantly lower likelihood of having an increasing BMI slope than the control girls (OR, 0.32; 95% CI, 0.18–0.56). The effect in boys had the opposite direction of study intention.
Daily dietary intake was not recorded and control of dietary intake may have added benefit. Parental guidance may also have reinforced activity. The study was relatively short and may reflect short-term change. The process of randomization was unclear, as was the sample size calculation and blinding was impossible with intervention and control conducted in each setting leading to the possibility of ‘contamination’. In school 1, there was an extra swimming class and it is not clear how this was controlled for. Other confounders could have been explored including ethnicity and parents' BMI. There are potential biases including children being recruited on teacher's advice, some family reported baseline data and measurement bias (adapted measures used and TSF measurement may not be accurate). There were 104 non-participating children, and it would be interesting to see whether their characteristics differed from those included.
Moore et al. 10 examined the effect of pre-school physical activity on the change in body fatness from pre-school to first grade in the USA in a longitudinal study. This study was part of the ‘Framingham Children's Study’ looking at childhood cardiovascular risk behaviours and began in 1987 with 106 children aged 3–5 years (mean, 4 years) and their parents. The authors analysed 97 healthy children with complete data from study entry into first grade. Physical activity of children and parents was assessed twice per year for five consecutive years using an electronic motion sensor. Each child also had yearly measurements of TSF thickness. Active girls i.e. those with above-median activity levels, had a better outcome and gained 1.0 mm in their TSF thickness from baseline to first grade, whereas inactive girls gained 1.75 mm TSF thickness. Active boys lost an average of 0.75 mm in their TSF thickness, whereas inactive boys gained 0.25 mm in their TSF thickness. Inactive pre-schoolers were almost four times as likely to have larger triceps during follow-up. Inactive preschoolers who were initially fatter were nearly six times as likely to have larger triceps during follow-up.
When age, television viewing, energy intake, baseline triceps and parents' BMI were controlled for, inactive pre-schoolers were 3.8 (95% CI, 1.4–10.6) times as likely as active pre-schoolers to have an increasing triceps slope during follow-up, rather than a stable or decreasing slope. This relative risk estimate was slightly higher for children with more body fat at baseline.
The study suggests that physical activity can affect obesity early in life and found a strong effect of low levels of physical activity on body fatness. Limitations include the small number of subjects and possible measurement bias.
Drucker et al. 11 examined the relationship between maternal parenting style, maternal eating cues and a child's eating behaviour during mealtime in the USA in an observational study using data collected as part of the ‘Stanford Infant Growth Study’, an ongoing longitudinal study. Seventy-seven children (mean age, 3.5 years) were included and visited the laboratory with their mothers for a videotaped lunch. Videotapes were coded for the child's eating rate and maternal parenting style, measured as the level of maternal control and support and the number and type of eating prompts given during a meal. The number and rate of verbal and physical encouragements and discouragements were significantly related to measures of general maternal parenting style and meal duration.
The rates of food offers, food presentations and total prompts were significantly positively related to the child's rate of calorie intake. A mother's level of support or control was not related to the child's eating behaviour. Although general maternal parenting style did not predict the child's eating behaviour, these behaviours were related to the frequency of maternal prompts, which, in turn, were significantly related to the number of calories eaten and the time spent eating. Children who ate fast had mothers who delivered eating prompts more frequently. The authors suggest that children's BMI was significantly and negatively correlated with total discouragement per minute ( r = 0.23, P ≤ 0.05) but not with other maternal prompts. Limitations include the representativeness of the study sample with participants being mainly white, older, working and well-educated mothers. The mother's prompts may also be in response to the child's behaviour rather than encouraging or discouraging certain eating behaviours. The total number of calories consumed was imputed from one meal in a laboratory setting and may not be representative of everyday eating behaviour. The setting may also have influenced behaviours.
In a large observational study, Baughcum et al. 12 developed and analysed two new instruments to assess maternal feeding practices and beliefs. The ‘Infant Feeding Questionnaire’ (IFQ) assessed feeding during the first year of life and was administered to 453 mothers of children aged 11–23 months. The ‘Pre-Schooler Feeding Questionnaire’ (PFQ) assessed feeding of young children between ages of 2–5 years (mean age of children, 39.5 months). Scores were calculated and linked with the children's measured and mothers' self-reported weight and height. Scores from the IFQ and PFQ were compared between obese and non-obese mothers, between those who did and did not have an overweight child and between those who had low and high incomes. There was no significant difference between boys and girls in the prevalence of overweight (20 versus 22%, P = 0.53). Within the low-income group mothers, the prevalence of maternal obesity was higher (27 versus 11%, P < 0.001), and their children were more often overweight (26 versus 13%, P = 0.001). However, overall obese mothers were no more likely than non-obese mothers to have overweight children (26 versus 20%, P = 0.19), and this was true when high and low income groups were examined separately.
Mothers who breast-fed for longer than 3 months were no less likely to have an overweight children than other breastfeeding mothers (14 versus 18%, P = not significant, data not shown). Low-income group mothers introduced solids earlier, but there was no evidence that early introduction of solids or the practice of adding cereal to the bottle was associated with overweight beyond infancy. After controlling for family income, there was no evidence that obese mothers had a different feeding style and the study did not suggest that there is a particular feeding style associated with overweight in young children. The only suggested difference in feeding style for obese mothers was the tendency to give children less control over feeding.
Harvey-Berino and Rourke conducted a low-powered observational study to determine whether maternal participation in a home-visiting obesity prevention plus parenting support (OPPS) intervention would reduce the prevalence of obesity in high-risk Native-American children compared with a parenting support (PS) only intervention. 13 Forty-three mother/child pairs were recruited. Mothers were 26.5 ± 5 years old with a mean BMI of 29.9 ± 3. Children (23 males) were 22 ± 8 months old with mean weight-for-height z- scores (WHZ) of 0.73 ± 1.4. Mothers were randomly assigned to a 16-week OPPS intervention or PS alone. The only difference was the focus of the lessons. The intervention was delivered one-on-one in homes by an indigenous peer educator.
Baseline and 16 week assessments included weight and height dietary intake, physical activity, parental feeding style and maternal outcome expectations, self-efficacy and intention to change diet and exercise behaviours. Children in the OPPS group gained less weight over 4 months than those in PS, but differences were not significant. WHZ scores decreased in the PS condition and increased among the OPPS group (−0.27 ± 1.1 versus 0.31 ± 1.1, P = 0.06), although this is not significant. Children in the OPPS condition significantly decreased energy intake (−316 ± 835 versus 197 ± 608 kcal/day, P < 0.05). Scores on the Child Feeding Questionnaire decreased significantly in the OPPS condition (−0.22 ± 0.42 versus 0.08 ± 0.63, P < 0.05), indicating that mothers in the OPPS group were engaging in less restrictive child-feeding practices over time. The authors considered a home-visiting programme focused on changing lifestyle behaviours and improving parenting skills, which showed promise for obesity prevention in high-risk children. Limitations include small sample size, short duration and the representativeness of the sample, including maternal age, education, employment, breast-feeding rates and childcare.
What is already known on this topic
NICE guidance reinforces that the pre-school years are a key time for shaping attitudes and behaviours; that opportunities for children to be active and to develop healthy eating habits are important as well as the need to involve parents and carers. 7
While there is a need for policy and practice to be evidence based, the review has considered some interventions that are good public health practice per se and should be encouraged in any case, for example, breast feeding and physical activity. As Wanless stated ‘the need for action is too pressing for the lack of a comprehensive evidence-base to be used as an excuse for inertia. Instead, current public health policy and practice, which includes a multitude of promising initiatives, should be evaluated as a series of natural experiments’. 14
What this study adds
The review confirms that there is a limited and immature evidence and a lack of comprehensive evidence on effective strategies to prevent obesity in younger children. There are some interesting individual studies that enhance and support recent NICE guidance around activity, family-based interventions and breast-feeding. The study reinforces that prevention of child obesity requires comprehensive, sustained and evidence-based action. Improvements in the evidence base are needed looking at points of intervention, such as those identified in this review, along with evaluation of those interventions. The evidence that childhood obesity persists into adulthood may justify shorter term monitoring at this age.
Limitations of this study
The review shows that the overall quality of studies is poor, there is no consistent research theme, inconsistent results across studies and compared with clinical decision-making where the evidence base is dominated by RCTs with high internal validity, the evidence base for child obesity prevention is poor. 15 The better quality studies tend to show small, but significantly beneficial, effects particularly for physical activity and breast-feeding suggesting that research should be focused in these areas. The lower order RCT by Mo-suwan 9 shows promise and results suggesting swimming may be effective in preventing obesity are worthy of follow-up. The longitudinal study by Armstrong et al. 8 indicates that breast-feeding is potentially useful for population-based strategies aimed at obesity prevention in children aged <5 years. However, caution is required in translating this research into local practice, given the different settings of the studies and the challenges in applying research including RCTs back into a community setting.
With family-based interventions the need exists for good quality longitudinal studies that carefully assess child growth as well as parental control over infant feeding practices and activity levels. The preliminary results of an unpublished RCT on the effectiveness of a multi-component family-based intervention suggest significant improvements in moderately obese older children (P.M. Sacher, unpublished results).
The findings from some studies suggest there are implications for the development of obesity in children and a correlation is evident between certain parental–child interactions and the relative weight and activity levels of the children. Future research should investigate the types of food being encouraged or discouraged and the intensity of children's activity levels. If findings are replicated in different settings it may, for example, explain the equivocal literature on the influence of children's physical activity on weight. Overweight children may in fact engage in equal frequency of activity, but less intensely.
Child obesity will continue to be a problem without improved understanding of key factors likely to be operative during very early childhood and without identification of those where intervention would have the greatest effect. 1
Greater effort is still required to establish an evidence-based approach to issues surrounding obesity in children.
Google Scholar
- childhood obesity
- overweight and obesity prevention
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1741-3850
- Print ISSN 1741-3842
- Copyright © 2024 Faculty of Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Dietary Interventions to Prevent Childhood Obesity: A Literature Review
Affiliations.
- 1 Faculty of Health Sciences (Nutrition Sciences), University Fernando Pessoa, Rua Carlos da Maia 296, 4200-150 Porto, Portugal.
- 2 EPIUnit-Instituto de Saúde Pública da Universidade do Porto (Institute of Public Health of the University of Porto), Rua das Taipas 135, 4050-600 Porto, Portugal.
- 3 Laboratory for Integrative and Translational Research in Population Health (ITR), Rua das Taipas 135, 4050-600 Porto, Portugal.
- 4 Department of Public Health and Forensic Sciences, and Medical Education, Faculty of Medicine, University of Porto, Alameda Professor Hernâni Monteiro, 4200-319 Porto, Portugal.
- PMID: 34684448
- PMCID: PMC8537925
- DOI: 10.3390/nu13103447
Several dietary interventions have been conducted to prevent/reduce childhood obesity, but most of them are known to have failed in tackling the obesity epidemic. This study aimed to review the existing literature on dietary interventions for the prevention of childhood obesity and their effectiveness. A literature search was conducted using PubMed Central ® . Only articles published between 2009 and 2021, written in English, conducted in humans, and including children and/or adolescents (<18 years old) were considered. The majority of studies were school-based interventions, with some addressing the whole community, and including some interventions in the food sector (e.g., taxation of high fat/sugar foods, front-of-pack labelling) and through mass media (e.g., restrictions on food advertising for children) that directly or indirectly could help to manage childhood obesity. Most of the programs/interventions conducted focus mainly on person-based educational approaches, such as nutrition/diet education sessions, allied to the promotion of physical activity and lifestyles to students, parents, and school staff, and less on environmental changes to offer healthier food choices. Only a few trials have focused on capacity building and macro-policy changes, such as the adaptation of the built environment of the school, serving smaller portion sizes, and increasing the availability and accessibility of healthy foods and water in schools, and restricting the access to vending machines, for example. Overall, most of the intervention studies showed no consistent effects on changing the body mass index of children; they have only reported small weight reductions, clinically irrelevant, or no effects at all. Little is known about the sustainability of interventions over time.
Keywords: children; diet; dietary interventions; pediatric obesity; prevention.
Publication types
- Meta-Analysis
- Child, Preschool
- Diet, Healthy
- Early Intervention, Educational
- Health Behavior
- Pediatric Obesity / etiology
- Pediatric Obesity / prevention & control*
- Pediatric Obesity / therapy
- Residence Characteristics
Grants and funding
- POCI-01-0145-FEDER-030334; PTDC/SAU-EPI/30334/2017/Fundação para a Ciência e a Tecnologia
- IF/01350/2015/Fundação para a Ciência e a Tecnologia
- Open access
- Published: 02 April 2024
Family systems approaches in pediatric obesity management: a scoping review
- Natasha Wills-Ibarra 1 ,
- Keryn Chemtob 1 ,
- Heather Hart 1 ,
- Francesca Frati 1 ,
- Keeley J Pratt 2 , 3 ,
- Geoff DC Ball 4 &
- Andraea Van Hulst 1
BMC Pediatrics volume 24 , Article number: 235 ( 2024 ) Cite this article
75 Accesses
Metrics details
Family-based obesity management interventions targeting child, adolescent and parental lifestyle behaviour modifications have shown promising results. Further intervening on the family system may lead to greater improvements in obesity management outcomes due to the broader focus on family patterns and dynamics that shape behaviours and health. This review aimed to summarize the scope of pediatric obesity management interventions informed by family systems theory (FST). Medline, Embase, CINAHL and PsycInfo were searched for articles where FST was used to inform pediatric obesity management interventions published from January 1980 to October 2023. After removal of duplicates, 6053 records were screened to determine eligibility. Data were extracted from 50 articles which met inclusion criteria; these described 27 unique FST-informed interventions. Most interventions targeted adolescents (44%), were delivered in outpatient hospital settings (37%), and were delivered in person (81%) using group session modalities (44%). Professionals most often involved were dieticians and nutritionists (48%). We identified 11 FST-related concepts that guided intervention components, including parenting skills, family communication, and social/family support. Among included studies, 33 reported intervention effects on at least one outcome, including body mass index (BMI) ( n = 24), lifestyle behaviours (physical activity, diet, and sedentary behaviours) ( n = 18), mental health ( n = 12), FST-related outcomes ( n = 10), and other outcomes (e.g., adiposity, cardiometabolic health) ( n = 18). BMI generally improved following interventions, however studies relied on a variety of comparison groups to evaluate intervention effects. This scoping review synthesises the characteristics and breadth of existing FST-informed pediatric obesity management interventions and provides considerations for future practice and research.
Peer Review reports
Obesity is a major public health concern affecting all age groups [ 1 ]. The high global prevalence of childhood overweight and obesity is concerning given known impacts on several body systems, including the cardiovascular, pulmonary, endocrine, gastrointestinal and musculoskeletal systems [ 2 ]. Obesity persists from childhood into adulthood [ 3 ] resulting in increased risk of morbidity and mortality [ 4 , 5 ]. In addition to its bearings on physical health, childhood overweight and obesity are associated with poor psychosocial outcomes [ 2 , 6 ]. Given its multiple immediate and long-term consequences, managing overweight and obesity in children and adolescents through effective interventions is a priority.
Most pediatric obesity management interventions fall within the umbrella of family-based approaches, targeting specific lifestyle behaviours (e.g., diet, physical activity) for obesity management and including at least one family member (e.g., a parent) in addition to the target child. Family-based behavioural interventions have shown improvements in lifestyle behaviours and in obesity-related outcomes [ 7 , 8 , 9 , 10 ]. However, these interventions may have limited effects if they fail to address the family patterns and dynamics that shape lifestyle behaviours [ 11 ].
Family Systems Theory (FST) has gained attention in pediatric obesity management [ 12 ]. Derived from general systems theory, FST focuses on understanding the interrelationships between elements within a system (e.g., the dynamics of a family unit, communication, and problem-solving). It views families as complex systems in which events or changes in one family member influence other interrelated parts of the system [ 11 ]. FST explicitly recognizes the key roles of family-level influences on children’s lifestyle behaviours and changes therein, with the goal of promoting health and managing obesity [ 13 ]. The integration of a family systems approach in pediatric obesity management interventions may increase their efficacy and sustainability by targeting core family dynamics that challenge lifestyle modifications required for obesity management [ 12 ]. A preliminary search of published systematic reviews on family-based obesity management interventions revealed a limited focus on family systems approaches with few reviews identifying specific intervention components consistent with FST [ 10 , 14 , 15 , 16 , 17 , 18 ]. Family systems concepts (e.g., interpersonal dynamics, family functioning, family problem-solving) were infrequently mentioned or only discussed narrowly [ 12 ]. Moreover, despite the potential benefits of using FST, clinicians have reported a lack of clarity regarding how to apply FST in the context of pediatric obesity management [ 13 ].
This scoping review addresses the following overarching question: How has FST been used in the context of pediatric obesity management interventions? Specifically, this review identifies 1) who is targeted by existing FST-informed interventions; 2) settings where they have been implemented (primary, specialty/tertiary, community); 3) delivery format (e.g., group vs. individual, parents-only vs. child-only vs. family) and professionals involved in the implementation of these interventions; 4) FST-related concepts that are integrated into interventions and tools used to measure these concepts; and 5) effects of FST-informed approaches on obesity outcomes and on FST-related concepts.
A scoping review of the literature was conducted following the Joanna Briggs Institute (JBI) methodology [ 19 ], and the PRISMA-ScR and PRISMA-S guidelines for searches [ 20 , 21 ].
Search strategy
A comprehensive search strategy was used. An academic health sciences librarian (FF) conducted a preliminary search that allowed us to analyse titles, abstracts, and index terms of isolated papers in order to refine our scoping review questions and define the final search strategy. Although we initially wanted to use a broad approach to the definition of FST, for feasibility reasons, we narrowed our review to articles that explicitly mention the use of FST to inform the development of obesity management interventions [ 12 ]. Similarly, although we initially wanted to include both prevention and management interventions, we narrowed our review to interventions focusing on obesity management (i.e., children and adolescents with overweight or obesity). Following these refinements, a final search strategy was developed by FF and a peer review of the search strategy was conducted by a second academic health sciences librarian using the PRESS (Peer Review of Electronic Search Strategies) guideline [ 22 ]. After minor revisions, the final search was run in Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Embase, and PsycInfo on April 4, 2020. Duplicates across databases were removed in EndNote using a simplified method described by Bramer et al. [ 23 ] and additional duplicates were identified in Rayyan [ 24 ]. Our search was based on three main concepts, namely family systems, pediatric obesity, and interventions. The full search strategies for all four databases are presented in Supplemental Table 1 . We also examined reference lists and citations of included studies for further pertinent studies that were not captured through our database searches. This overall search strategy was implemented for studies published between January 1980 and April 2020. No additional limits or search filters were used. In October 2023, we updated our review by conducting the same search in Medline to identify publications indexed between April 4, 2020 and October 27, 2023, the date of this search. We also searched for articles published in the last 3 years that cited previously identified research protocol articles of FST-informed obesity management interventions. This scoping review thus includes articles published between January 1980 and October 2023; this date range was selected to capture early family systems interventions following the increased recognition by the early 1990’s of the role of families in childhood obesity [ 25 ].
Inclusion and exclusion criteria
Details regarding inclusion and exclusion criteria are presented in Table 1 . Articles that used FST to inform the design of a pediatric obesity management intervention or program were included. Specifically, we included publications describing obesity management interventions that focus on children aged 2 to 18 years, with overweight or obesity, the direct involvement of at least one adult family member, and the explicit statement of a family systems-related theory, model, and/or framework [ 12 ]. Review papers, case studies, texts, opinion papers, letters and gray literature were excluded.
Study selection
EndNote (Thomson Reuters, New York, USA) was used to manage records identified from the literature search. Search results from all databases were combined, and duplicates were removed. Records were then imported into Rayyan [ 26 ] to manage decisions on inclusion/exclusion. For the updated search covering the period of April 2020 to October 2023, we used Covidence, a web-based collaboration software platform to manage the flow of records in review studies. Titles and abstracts were screened for inclusion by two out of four independent reviewers (NWI, KC and 2 research assistants), followed by screening of full-text by two of the same reviewers. Discordances at both stages were settled by the senior author (AVH).
Data extraction, analysis and synthesis
Data extraction, analysis and synthesis were conducted by two reviewers (NWI, KC) and verified by the senior author. An adaptation of the JBI data extraction instrument was used to import data into a table with the following fields based on the research questions: country and name of intervention; sample size (if applicable); study design; target population (e.g., age/sex of child, family members targeted, racial/ethnic groups, etc.); type of care setting (e.g., community, hospital); description and duration of the intervention; delivery format of the intervention (e.g., group vs. individual, parents-only vs. child/teen-only vs. family); professionals involved in the intervention; Family Systems related theory or framework and other theories used to inform the intervention; specific Family Systems concepts used (e.g., family dynamics, family functioning, parenting styles, etc.); and measurment of family concepts. The results of articles that reported intervention effects on outcomes were summarized in a separate table, including intervention effects on family systems concepts, mental health, lifestyle behaviours, body mass index (BMI) and other outcomes examined. The type of control group was classified as not applicable (no control group), waitlist control, usual care, or intervention control group, with descriptors provided when available. Intervention effects were summarised based on whether an improvement, a deterioration, or the absence of changes on outcomes were reported. No standardised metrics for outcomes were sought given the diversity of included studies.
All data extracted from articles were compiled using counts and proportions to answer our research questions. A conventional inductive content analysis was completed [ 27 ] in order to identify and summarize the FST-related concepts that were intervened upon in included studies. To do so, keywords and descriptive texts were extracted from the studies’ intervention descriptions and grouped into categories with similar content; once complete, these categories were individually labelled to represent different FST-related concepts.
Database and citation searches allowed us to identify 6053 records after the removal of duplicates, with a total of 50 articles that met inclusion criteria (Fig. 1 ). The most common reasons for exclusion were the absence of FST-related theory in the development of the intervention, and interventions not focusing specifically on children/adolescents with overweight/obesity. Among the included studies, all were published in English, 14 were descriptive articles (e.g., study protocols), 33 reported on at least one measured intervention outcome, 3 used qualitative post-intervention exploratory designs, and one included baseline data only. Supplemental Table 2 provides a summary of the 50 studies included in this review. Among included studies, we identified 27 unique FST-informed interventions which are presented in Table 2 .
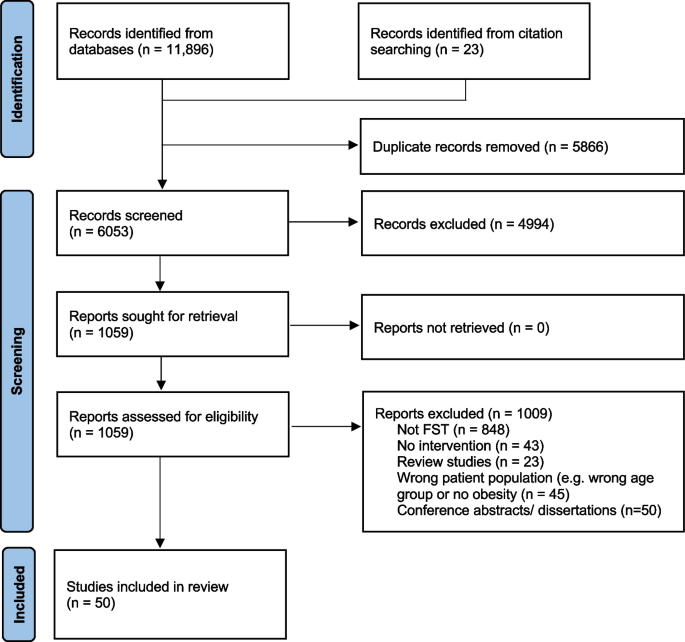
PRISMA flow diagram
Who is targeted by existing FST-informed interventions?
Of the 27 unique interventions, 3 (11%) targeted preschool children exclusively, 7 (26%) targeted school-aged children exclusively, and 12 (44%) targeted adolescents exclusively. In addition, one intervention (4%) targeted both preschool and school-aged children, while 4 (15%) targeted both school-aged children and adolescents. Twenty-three interventions (85%) targeted the child/adolescent and at least one parent/guardian, and the remaining 4 interventions (15%) targeted a parent/guardian without the index child/adolescent. Five interventions (19%) were designed for families with low incomes or living in underserved areas. Some interventions targeted specific ethnic or population sub-groups, including 4 interventions (15%) for African American families, 3 (11%) for Latin American families, one for Hispanic and Black families, and one for female adolescents only.
In which settings are FST-informed approaches implemented?
All studies were conducted in Western countries, including the USA, Europe, and Canada. Four different intervention settings were identified: outpatient hospital (37%, n = 10), community-based (26%, n = 7), pediatric obesity management center (7%, n = 2), and home-based (7%, n = 2). An additional 6 interventions (22%) relied on a combination of settings, 4 of which included a home-based component (15%).
How are FST-informed interventions delivered, and which professionals are involved?
Intervention duration ranged from 1.5 to 24 months (median of 6 months). Most interventions were delivered entirely in person (81%, n = 22). Three interventions (11%) used a combination of in-person and virtual/online sessions, one intervention combined in-person and telephone delivery, and one intervention was delivered entirely over the phone. Twelve interventions (44%) were group-based, 6 (22%) were delivered individually, and 9 (33%) used a combination of group and individual sessions.
In terms of in-session participation, 12 interventions (44%) comprised sessions that included the child/adolescent together with at least one adult family member at all times, whereas another 11 (41%) had a mix of parent-only, child/adolescent-only, and parent–child/adolescent sessions. The remaining 4 interventions (15%) included only parents in their intervention, without the child/adolescent.
Interventions were delivered by a wide range of health professionals, and commonly involved two or more professionals. These included dieticians/nutritionists (48%, n = 13), licensed counsellors/therapists (30%, n = 8), psychologists (30%, n = 8), sports trainers and exercise specialists (30%, n = 8), students in different health-related fields (22%, n = 6), nurses (19%, n = 5), pediatricians (15%, n = 4), occupational therapists (7%, n = 2), physiotherapists (7%, n = 2), social workers (7%, n = 2), health educators (4%, n = 1), and behaviouralists (4%, n = 1). Moreover, 7 of the interventions (26%) included other non-health-related professionals (e.g., local parks and recreation staff, prevention managers, and trained facilitators with unspecified backgrounds), or did not specify the professionals involved.
Which FST-related concepts are included in interventions and how are these concepts measured?
A detailed description of the 11 FST-related concepts identified across interventions, including definitions and examples of how they were integrated within interventions, is presented in Table 3 . The most common concepts related to parenting skills (59%, n = 16), family communication (52%, n = 14), and social/family support (48%, n = 13). Other concepts included family functioning (37%, n = 10), parental role modelling (30%, n = 8), autonomy support (22%, n = 6), shared decision-making (19%, n = 5), home environment (22%, n = 6), empowerment (11%, n = 3), family goal setting (26%, n = 7), and family problem solving (22%, n = 6). Some studies reported in-depth descriptions of how FST-related concepts were integrated while others did not. Few studies included pre- or post-intervention measurements of FST-related concepts as shown in Table 3 .
What are the effects of FST-informed interventions?
Of the 50 articles reviewed, 33 reported on at least one intervention outcome, including BMI or BMI z-scores ( n = 24), lifestyle behaviours (physical activity, diet, and sedentary behaviours) ( n = 18), mental health ( n = 12), FST-related outcomes ( n = 10), and other outcomes (e.g., waist circumference, heart rate, blood pressure, cardiovascular fitness) ( n = 18) (Table 4 ).
As shown in Table 4 , among studies that reported on BMI outcomes, virtually all studies with comparisons to baseline values or to waitlist control groups found post-intervention improvements in BMI. For studies that compared BMI to usual care or control interventions, 6 reported improvements, 4 reported no differences, and 1 reported worse outcomes in the FST intervention compared to the control group. For studies examining changes in physical activity, 4 out of 5 studies that used baseline or waitlist control groups reported improvements, whereas only 6 out of 11 studies with usual care or control intervention comparisons reported improvements in physical activity, and other studies reported no differences. For sedentary behaviour outcomes, 3 out of 4 studies using baseline or waitlist controls reported improvements, whereas no differences were found in the 2 studies with usual care or control interventions. Among studies that examined dietary outcomes, most found no difference, except for 2 studies with usual care or control intervention comparisons, and one relying on baseline comparisons. Most studies that reported improvements in mental health outcomes used baseline and waitlist control comparisons, with mixed findings for intervention effects compared to usual care and control interventions. Lastly, of the 10 studies that measured FST concepts (e.g., family communication, family functioning, family support), 5 reported improvements of which 3 were compared to usual care or control interventions, while the other studies reported no differences or mixed findings.
This scoping review sought to describe the use of FST in pediatric obesity management interventions over the past four decades to map current knowledge and identify research gaps and practice implications. Our review reveals that school-aged children and adolescents are more frequently targeted compared to preschoolers and that few interventions specifically target population sub-groups who are at increased risk of obesity and its complications due to systemic barriers to health (e.g., low socioeconomic status, racial/ethnic minority groups). Interventions were most commonly delivered in outpatient hospital settings by multidisciplinary teams using a variety of delivery modalities, and all studies were conducted in Western countries. We identified 11 FST-related concepts that informed intervention components, with parenting skills, family communication, and social/family support being the most common. However, many interventions did not elaborate on how FST was translated into specific intervention components, and few included measurements of FST-related concepts as part of the baseline and post-intervention assessments. Among studies reporting intervention outcomes, BMI was most frequently reported and generally improved following the intervention; however, there were a variety of comparison groups noted ranging from usual care obesity management to psychoeducation and other control interventions. This variety in comparison groups should be considered in the interpretation of intervention effects given differences between studies in intensity and dosage.
Preschool-aged children were infrequently included in the obesity management interventions we reviewed with inconsistent results for BMI, lifestyle behaviours, and/or family systems-related outcomes [ 51 , 53 , 54 , 55 , 58 , 59 ]. Considering their young age, it is possible that FST-informed obesity interventions targeting preschool-aged children are more likely to be preventative in nature. Inclusion in this review required children to have overweight/obesity at intervention baseline; exploring the use of FST in the prevention of obesity may shed light on the nature and overall usefulness of FST in preventing obesity among children under 5 years of age.
Moreover, given the higher rates of obesity in some ethnic minority groups [ 78 ], culturally adapted FST-informed interventions continue to be a priority. FST concepts integrated in interventions targeting ethnic minority groups did not differ from other interventions, but authors mentioned how cultural considerations and strategies were used to guide implementation. For example, the Supporting Health Interactively through Nutrition (SHINE) study enhanced intervention relevance for African American families through the recruitment of African American providers and community leaders, the usage of photos of African American families in intervention material, and the presentation of data related to African American youth specifically [ 70 ]. Other studies used qualitative methods to explore sociocultural values and barriers that could be integrated in the intervention’s final curriculum [ 35 ]. Of the 8 interventions that focused on ethnic minorities, 5 included measurements of pre- and post-intervention outcomes (e.g., BMI and lifestyle behaviours), and 4 of these resulted in improvements, lending support to the usefulness of culturally adapted FST-informed interventions.
Almost all studies included in this review reported the involvement of professionals from two or more disciplines. This is in line with the multidisciplinary approach recommended for pediatric obesity management [ 79 ]. However, few articles mentioned whether those delivering the interventions were trained in family systems approaches which is essential to ensure appropriate embodiment by involved professionals of core FST intervention components [ 80 , 81 ]. Interestingly, some interventions included staff outside of the traditional health fields (e.g., parks and recreation staff) which may provide a broader perspective of the different multi-sectoral and multi-systemic factors implicated in pediatric obesity and its solutions [ 79 , 82 ].
Although most interventions were group-based and were delivered entirely in person, others were either partially or fully delivered virtually using web-based or telephone modalities. Virtual intervention delivery may facilitate reaching more family members, an important consideration from a family systems perspective. Moreover, overall attendance and retention may be improved for interventions delivered virtually [ 83 ]. Similarly, the use of home visits was reported in 2 interventions of which one (Multisystemic Therapy) reported effects on outcomes. The latter is one of the few interventions that reported improvements across all measured outcomes, including FST-related concepts, BMI, diet, and adiposity in comparison to a control intervention group [ 61 , 62 ]. Home visits may be an important modality to consider for the delivery of FST-informed interventions in pediatric obesity management. It has been shown that families support the use of home visits in the context of obesity management and perceive these as having benefits, namely in terms of convenience, tailored care, and family involvement [ 84 ]. While previous reviews have highlighted the importance of engaging multiple family members in pediatric obesity management [ 12 ], it has been noted that potentially influential family members, such as the other parent (often fathers), siblings, or grandparents, are often neglected in family-based pediatric obesity management interventions [ 85 ]. Home-based approaches may facilitate the involvement and engagement of multiple members within a family unit.
BMI outcomes were the most consistently measured to evaluate FST-informed interventions; they also showed the most consistent improvements, notably in comparison to baseline and waitlist control groups but also in comparison to usual care and to non-FST control interventions. These results are in line with previous reviews of family-based interventions that have reported weight-related improvements [ 10 , 14 , 86 ], and lend support to the use of FST-informed interventions in pediatric obesity management. Findings were generally similar with regard to improvements in physical activity but were largely inconsistent for other outcomes. This review highlights the need for more evidence on the benefits of FST-informed interventions in comparison to usual care and standard family-based obesity management interventions not based on FST. There is also a need for evidence on which families and children may benefit the most from FST-informed interventions in comparison to standard obesity management interventions.
Intervention effects on family systems measures (e.g., parenting skills, family communication, etc.) were either not reported or mixed in the few studies that evaluated these outcomes. This is an important knowledge gap given that one of the goals of FST-informed interventions is to improve dynamics and organisation within the family so as to create family environments and conditions that are supportive of improvements in health and lifestyle behaviour changes [ 11 , 12 , 87 ]. Inconsistency in results may be due to the relatively low number of studies that measured FST-related variables. Some studies used qualitative methods to assess participants’ perspectives on changes in the family system following the intervention, both of which reported perceived improvements [ 36 , 57 ]. Qualitative exploration may allow for a deeper understanding of family beliefs associated with family system concepts at baseline and how these evolve following an intervention. Exploring these perspectives can allow for a more tailored approach to obesity management and can provide a richer understanding of intervention effectiveness related to the family system.
This review highlights the importance of evaluating the family system before and after intervention delivery given its potential role as mediator of intervention effects. Intervening at the family systems level may lead to greater and more sustained changes due to improvements in underlying family dynamics that may hinder or challenge lifestyle modification [ 12 ]. In addition, the health of the family system may predict the response to FST-informed obesity management. For example, although Kitzmann et al. did not see improvements in examined family systems concepts following their intervention, baseline parental support for healthy eating habits and positive parenting styles were associated with greater reductions in BMI over the 6-week study [ 56 ]. Similarly, Spence et al. found that a healthier family system pre-intervention was associated with improved retention in their program [ 65 ].
In order to be included in this review study, studies had to explicitly mention how FST or related theories were used to guide the intervention development. Most studies used FST in combination with other health-related theories to inform certain components of their intervention, but fewer studies used FST as a broader lens through which to approach pediatric obesity at the family system level. Many studies briefly mentioned the use of FST or related theories but lacked a clear embodiment of FST and did not elaborate on the specifics of how these theories were integrated in their intervention delivery. One exception to this was the Families Improving Together (FIT) intervention which was described as deeply rooted in FST [ 35 ]. This intervention targeted a number of different FST-related concepts (e.g., parenting skills, family communication) and was centered on creating a positive social climate and promoting warm and supportive family interactions throughout all intervention sessions [ 35 ]. It further targeted positive parenting skills through parenting style, parental monitoring, shared decision making, and communication, while promoting family bonding and family support in weekly goal setting [ 35 ]. Other interventions that were more explicit on their family systems approaches were the Multisystemic Therapy, which included baseline assessment of the family’s strengths and weaknesses to target individual family needs related to FST concepts [ 62 , 63 , 88 ], the SHINE intervention, which provided detailed and specific descriptions of FST integration in their design [ 70 , 71 ], and ENTREN-F, which focused on behavioural parenting strategies, parental educational styles, feeding practices, communication skills and adaptive dynamics in the home environment [ 30 ].
Previous reviews have also pointed out that existing pediatric obesity interventions based on FST do not fully embody a family systems approach. In their literature review published in 2011, Kitzmann and Beech observed that the majority of pediatric obesity management interventions reviewed had a narrow family focus (e.g., parents were asked to modify health behaviours) while fewer were more broadly family-focused [ 86 ]. Additionally, as noted by Skelton et al. in their review of family theories in pediatric obesity management, FST was often used as a theme to discuss pediatric obesity but was rarely used to guide obesity management interventions [ 12 ]. Family perspectives and beliefs surrounding the family system were infrequently explored in the studies we reviewed. Exploring these beliefs would allow for a more tailored approach to intervention delivery and would promote an individualized, strengths-based design that builds on a family’s existing values and unique strengths to improve intervention outcomes [ 89 ].
Findings from this review provide insight for health care providers seeking to integrate FST into obesity management interventions. FST-informed approaches can be used across the pediatric age groups. Including a combination of in-person and virtual or home-based sessions can facilitate intervening with the family as a whole, and adaptations to increase relevance to specific sociodemographic backgrounds (e.g., socioeconomic status, ethnocultural backgrounds) are key. Training the intervention delivery team in FST and including the assessment of family systems concepts (e.g., baseline and follow-up measures of family communication and family functioning) are essential moving forward.
This review was conducted by a multidisciplinary research team that included health professionals and researchers with expertise in FST and pediatric obesity management as well as a health sciences librarian. We used a broad search strategy to ensure all FST-informed interventions were captured. We included a variety of types of articles such as protocols, intervention descriptions, qualitative studies, randomized controlled trials and quasi-experimental studies. A rigorous approach was used to determine article inclusion/exclusion and to extract data from included studies. For example, a preliminary search guided our final inclusion/exclusion criteria, notably the explicit use of a family systems-related theory in the development of intervention and the focus on obesity management, which allowed us to synthesise evidence from more comparable interventions. In terms of limitations, our review does not include preventive interventions which may have excluded studies targeting preschool-aged children. Additionally, we did not assess the quality of included studies. Although this is not mandatory in scoping reviews, doing so strengthens the synthesised evidence. Lastly, we did not register or publish a protocol for this scoping review.
Conclusions
This review provides some support for FST as a useful theory to inform the development of pediatric obesity management intervention strategies targeting improvements in obesity-related outcomes, lifestyle behaviours (namely physical activity), and mental health. However, it remains unclear whether improvements at the family system level mediate favourable outcomes. This review further highlights the need for additional evidence on the benefits of FST-informed interventions in comparison to standard family-based obesity management interventions not based on FST. Future research should explore family perspectives and beliefs surrounding FST in pediatric obesity management. Assessing the family system prior to intervening, focusing on the family’s strengths, and exploring beliefs related to the family system may optimize the tailoring of pediatric obesity management interventions to the unique needs and context of each family.
Availability of data and materials
The dataset(s) supporting the conclusions of this article are available in the Medline, CINAHL, Embase, and PsycInfo repositories.
Abbreviations
Body Mass Index
Cognitive Behavioral Therapy
Control Intervention
Cumulative Index to Nursing and Allied Health Literature
Diastolic Blood Pressure
The Dyadic Communication Scale
The Family Adaptability and Cohesion Evaluation Scales IV
Multidisciplinary family-based behavioural therapy for obesity
Family Experiences Related to Food Questionnaire
Families Improving Together
Families Improving Together-Telehealth
Fit Kids / Fit Families
Families on the Move
Family Questionnaire
Family Systems Theory
United Families for Health and Wellness
Home Environment Survey-Physical Activity
Joanna Briggs Institute
Lighter Living program
Lund Overweight and Obesity Preschool Study
Motivational + Family Weight Loss Intervention
Mind, Exercise, Nutrition, Do it!
Physical Activity
Parents as Agents of Change
The Parenting Dimensions Inventory
The Parenting Strategies for Eating and Activity Scale
Peer Review of Electronic Search Strategies
Quality of Life
Systolic Blood Pressure
Standard Behavioural Treatment + Enhanced Parenting
Self-Report Family Inventory
Supporting Health Interactively through Nutrition and Exercise
Telephone-based Adiposity prevention for Families
United Kingdom
United States of America
The Weight Control Strategies Scale
Wait list control
The Youth Quality of Life Inventory
Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128· 9 million children, adolescents, and adults. The Lancet. 2017;390(10113):2627–42.
Article Google Scholar
Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251–65.
Article PubMed Google Scholar
Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17(2):95–107.
Article CAS PubMed Google Scholar
Reinehr T. Long-term effects of adolescent obesity: time to act. Nat Rev Endocrinol. 2018;14(3):183–8.
Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev. 2016;17(1):56–67.
Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5(4):282–304.
Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health psychol. 1994;13(5):373.
Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264(19):2519–23.
O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–44.
Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: a systematic review of randomized controlled trials. Obes Rev. 2013;14(4):265–78.
Kaplan SG, Arnold EM, Irby MB, Boles KA, Skelton JA. Family systems theory and obesity treatment: applications for clinicians. Infant Child Adolesc Nutr. 2014;6(1):24–9.
Skelton JA, Buehler C, Irby MB, Grzywacz JG. Where are family theories in family-based obesity treatment?: conceptualizing the study of families in pediatric weight management. Int J Obes (Lond). 2012;36(7):891–900.
Pratt KJ, Skelton JA. Family functioning and childhood obesity treatment: a family systems theory-informed approach. Acad Pediatr. 2018.
Berge JM, Everts JC. Family-based interventions targeting childhood obesity: a meta-analysis. Childhood obesity (Print). 2011;7(2):110–21.
Berry D, Sheehan R, Heschel R, Knafl K, Melkus G, Grey M. Family-based interventions for childhood obesity: a review. J Fam Nurs. 2004;10(4):429–49.
Nowicka P, Flodmark CE. Family in pediatric obesity management: a literature review. Int J Pediatr Obes. 2008;3:44–50.
Ash T, Agaronov A, Young T, Aftosmes-Tobio A, Davison KK. Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis. Int J Behav Nutr Phys Act. 2017;14(1):113.
Article PubMed PubMed Central Google Scholar
Skouteris H, McCabe M, Swinburn B, Newgreen V, Sacher P, Chadwick P. Parental influence and obesity prevention in pre-schoolers: a systematic review of interventions. Obes Rev. 2011;12(5):315–28.
Joanna Briggs Institute. Joanna Briggs Institute Australia: The University of Adelaide; 2018. Available from: http://joannabriggs.org/ .
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73.
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev. 2021;10(1):39.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6.
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–3.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Doherty WJ, Harkaway JE. Obesity and Family Systems: A family FIRO approach to assessment and treatment planning. J Marital Fam Ther. 1990;16(3):287–98.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10.
Bengtsson M. How to plan and perform a qualitative study using content analysis. NursingPlus Open. 2016;2:8–14.
Appelhans BM, French SA, Bradley LE, Lui K, Janssen I, Richardson D. CHECK: A randomized trial evaluating the efficacy and cost-effectiveness of home visitation in pediatric weight loss treatment. Contemp Clin Trials. 2020;88:105891.
Dilley JR, Singletary CR, Ard JD, Giles S, Skelton JA, Heboyan V, et al. Protocol for a randomized controlled feasibility study of a coordinated parent/child weight loss intervention: Dyad Plus. Transl J Am Coll Sports Med. 2020;5(12):e000136.
PubMed PubMed Central Google Scholar
Rojo M, Lacruz T, Solano S, Vivar M, Del Río A, Martínez J, et al. ENTREN-F family-system based intervention for managing childhood obesity: Study protocol for a randomized controlled trial at primary care. Obes Res Clin Pract. 2022;16(4):319–29.
Rojo M, Lacruz T, Solano S, Gutiérrez A, Beltrán-Garrayo L, Veiga OL, et al. Family-reported barriers and predictors of short-term attendance in a multidisciplinary intervention for managing childhood obesity: A psycho-family-system based randomised controlled trial (ENTREN-F). Eur Eat Disord Rev. 2022;30(6):746–59.
Christison AL, Evans TA, Bleess BB, Wang H, Aldag JC, Binns HJ. Exergaming for health: A randomized study of community-based exergaming curriculum in pediatric weight management. Games Health J. 2016;5(6):413–21.
Prado G, Fernandez A, St George SM, Lee TK, Lebron C, Tapia MI, et al. Results of a family-based intervention promoting healthy weight strategies in overweight hispanic adolescents and parents: An RCT. Am J Prev Med. 2020;59(5):658–68.
Perrino T, Brincks AM, Estrada Y, Messiah SE, Prado G. Reducing screen-based sedentary behavior among overweight and obese hispanic adolescents through a family-based intervention. J Phys Act Health. 2022;19(7):509–17.
Alia KA, Wilson DK, McDaniel T, George SMS, Kitzman-Ulrich H, Smith K, et al. Development of an innovative process evaluation approach for the Families Improving Together (FIT) for weight loss trial in African American adolescents. Eval Program Plann. 2015;49:106–16.
Sweeney AM, Wilson DK, Loncar H, Brown A. Secondary benefits of the families improving together (FIT) for weight loss trial on cognitive and social factors in African American adolescents. Int J Behav Nutr Phys Act. 2019;16(1):1–10.
Wilson DK, Kitzman-Ulrich H, Resnicow K, Van Horn ML, George SMS, Siceloff ER, et al. An overview of the Families Improving Together (FIT) for weight loss randomized controlled trial in African American families. Contemp Clin Trials. 2015;42:145–57.
Wilson DK, Sweeney AM, Van Horn ML, Kitzman H, Law LH, Loncar H, et al. The Results of the Families Improving Together (FIT) for weight loss randomized trial in overweight African American adolescents. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2022;56(10):1042–55.
Wilson DK, Sweeney AM, Quattlebaum M, Loncar H, Kipp C, Brown A. The Moderating Effects of the Families Improving Together (FIT) for weight loss intervention and parenting factors on family mealtime in overweight and obese African American adolescents. Nutrients. 2021;13(6):1745.
Wilson DK, Sweeney AM, Law LH, Kitzman-Ulrich H, Resnicow K. Web-based program exposure and retention in the families improving together for weight loss trial. Ann Behav Med. 2018;53(4):399–404.
Article PubMed Central Google Scholar
Quattlebaum M, Wilson DK, Sweeney AM, Zarrett N. Moderating effects of parental feeding practices and emotional eating on dietary intake among overweight African American adolescents. Nutrients. 2021;13(6):1920.
Biggs BK, Rodgers KV, Nayman SJ, Hofschulte DR, Loncar H, Kumar S, et al. Translation of a family-based behavioral intervention for adolescent obesity using the RE-AIM framework and common steps from adaptation frameworks. Translational behavioral medicine. 2023;13(9):700–9.
James KS, Connelly CD, Rutkowski E, McPherson D, Gracia L, Mareno N, et al. Family-based weight management with latino mothers and children. J Spec Pediatr Nurs. 2008;13(4):249–62.
Estabrooks PA, Shoup JA, Gattshall M, Dandamudi P, Shetterly S, Xu S. Automated telephone counseling for parents of overweight children: a randomized controlled trial. Am J Prev Med. 2009;36(1):35–42. e2.
Zoellner JM, You W, Hill JL, Brock DJP, Yuhas M, Alexander RC, et al. A comparative effectiveness trial of two family-based childhood obesity treatment programs in a medically underserved region: Rationale, design & methods. Contemp Clin Trials. 2019;84:105801.
Zoellner JM, You W, Hill JL, Brock DJP, Yuhas M, Price B, et al. Comparing two different family-based childhood obesity treatment programmes in a medically underserved region: Effectiveness, engagement and implementation outcomes from a randomized controlled trial. Pediatric obesity. 2022;17(1):e12840.
Nowicka P, Höglund P, Pietrobelli A, Lissau I, Flodmark C-E. Family Weight School treatment: 1-year results in obese adolescents. Int J Pediatr Obes. 2008;3(3):141–7.
Joosse L, Stearns M, Anderson H, Hartlaub P, Euclide J. Fit Kids/Fit Families: a report on a countywide effort to promote healthy behaviors. Wis Med J. 2008;107(5):231.
Google Scholar
Soltero EG, Konopken YP, Olson ML, Keller CS, Castro FG, Williams AN, et al. Preventing diabetes in obese Latino youth with prediabetes: a study protocol for a randomized controlled trial. BMC Public Health. 2017;17(1):1–12.
Peña A, Olson ML, Hooker E, Ayers SL, Castro FG, Patrick DL, et al. Effects of a diabetes prevention program on type 2 diabetes risk factors and quality of life among Latino youths with prediabetes: a randomized clinical trial. JAMA Netw Open. 2022;5(9):e2231196.
Orban K, Edberg A-K, Thorngren-Jerneck K, Önnerfält J, Erlandsson L-K. Changes in parents’ time use and its relationship to child obesity. Phys Occup Ther Pediatr. 2014;34(1):44–61.
Önnerfält J, Erlandsson L-K, Orban K, Broberg M, Helgason C, Thorngren-Jerneck K. A family-based intervention targeting parents of preschool children with overweight and obesity: conceptual framework and study design of LOOPS-Lund overweight and obesity preschool study. BMC Public Health. 2012;12(1):1–9.
Wilson TA, Liu Y, Adolph AL, Sacher PM, Barlow SE, Pont S, et al. Behavior modification of diet and parent feeding practices in a community-Vs primary care–centered intervention for childhood obesity. JNEB. 2019;51(2):150–61. e1.
Law C, Cole T, Cummins S, Fagg J, Morris S, Roberts H. A pragmatic evaluation of a family-based intervention for childhood overweight and obesity. Public Health Res. 2014;2(5).
Sacher PM, Kolotourou M, Chadwick PM, Cole TJ, Lawson MS, Lucas A, et al. Randomized controlled trial of the MEND program: a family-based community intervention for childhood obesity. Obesity. 2010;18(S1):S62–8.
Kitzman-Ulrich H, Wilson DK, St. George SM, Segal M, Schneider E, Kugler K. A preliminary test of a motivational and parenting weight loss program targeting low-income and minority adolescents. Child Obes. 2011;7(5):379–84.
Chamay Weber C, Camparini N, Lanza L, Narring F. Parents’ integration in the treatment of adolescents with obesity: A qualitative study. Fam Syst Health. 2016;34(4):396.
Bocca G, Kuitert M, Sauer P, Stolk R, Flapper B, Corpeleijn E. A multidisciplinary intervention programme has positive effects on quality of life in overweight and obese preschool children. Acta Paediatr. 2014;103(9):962–7.
Bocca G, Kuitert MW, Sauer PJ, Corpeleijn E. Effect of a multidisciplinary treatment program on eating behavior in overweight and obese preschool children. J Pediatr Endocrinol Metab. 2018;31(5):507–13.
Kitzman-Ulrich H, Hampson R, Wilson DK, Presnell K, Brown A, O’Boyle M. An adolescent weight-loss program integrating family variables reduces energy intake. J Am Diet Assoc. 2009;109(3):491–6.
Ellis DA, Janisse H, Naar-King S, Kolmodin K, Jen KLC, Cunningham P, et al. The effects of multisystemic therapy on family support for weight loss among obese African-American adolescents: findings from a randomized controlled trial. J Dev Behav Pediatr. 2010;31(6):461–8.
Naar-King S, Ellis D, Kolmodin K, Cunningham P, Jen KLC, Saelens B, et al. A randomized pilot study of multisystemic therapy targeting obesity in African-American adolescents. J Adolesc Health. 2009;45(4):417–9.
Idalski Carcone A, MacDonell KE, Naar-King S, Ellis DA, Cunningham PB, Kaljee L. Treatment engagement in a weight loss intervention for African American adolescents and their families. Child Health Care. 2011;40(3):232–52.
Flodmark C-E, Ohlsson T, Rydén O, Sveger T. Prevention of progression to severe obesity in a group of obese schoolchildren treated with family therapy. Pediatrics. 1993;91(5):880–4.
Spence ND, Newton AS, Keaschuk RA, Ambler KA, Jetha MM, Holt NL, et al. Predictors of short-and long-term attrition from the parents as agents of change randomized controlled trial for managing pediatric obesity. J Pediatr Health Care. 2017;31(3):293–301.
Ball GD, Mushquash AR, Keaschuk RA, Ambler KA, Newton AS. Using intervention mapping to develop the Parents as Agents of Change (PAC©) intervention for managing pediatric obesity. BMC Res Notes. 2017;10(1):1–11.
Ball GD, Ambler KA, Keaschuk RA, Rosychuk RJ, Holt NL, Spence JC, et al. Parents as Agents of Change (PAC) in pediatric weight management: The protocol for the PAC randomized clinical trial. BMC Pediatr. 2012;12(1):1–13.
Spence ND, Newton AS, Keaschuk RA, Ambler KA, Holt NL, Jetha MM, et al. Parents as agents of change in managing pediatric obesity: a randomized controlled trial comparing cognitive behavioral therapy versus psychoeducation interventions. Childhood obesity (Print). 2023;19(2):71–87.
Steele RG, Aylward BS, Jensen CD, Cushing CC, Davis AM, Bovaird JA. Comparison of a family-based group intervention for youths with obesity to a brief individual family intervention: A practical clinical trial of positively fit. J Pediatr Psychol. 2011;37(1):53–63.
St. George SM, Wilson DK, Van Horn ML. Project SHINE: Effects of a randomized family-based health promotion program on the physical activity of African American parents. J Behav Med. 2018;41(4):537–49.
St. George SM, Wilson DK, Schneider EM, Alia KA. Project SHINE: Effects of parent–adolescent communication on sedentary behavior in African American adolescents. J Pediatr Psychol. 2013;38(9):997–1009.
Nowicka P, Pietrobelli A, Flodmark C-E. Low-intensity family therapy intervention is useful in a clinical setting to treat obese and extremely obese children. Int J Pediatr Obes. 2007;2(4):211–7.
Hadley W, McCullough MB, Rancourt D, Barker D, Jelalian E. Shaking up the system: the role of change in maternal-adolescent communication quality and adolescent weight loss. J Pediatr Psychol. 2015;40(1):121–31.
Jelalian E, Hadley W, Sato A, Kuhl E, Rancourt D, Oster D, et al. Adolescent weight control: An intervention targeting parent communication and modeling compared with minimal parental involvement. J Pediatr Psychol. 2015;40(2):203–13.
Herget S, Markert J, Petroff D, Gausche R, Grimm A, Hilbert A, et al. Psychosocial well-being of adolescents before and after a 1-year telephone-based adiposity prevention study for families. J Adolesc Health. 2015;57(3):351–4.
Markert J, Herget S, Petroff D, Gausche R, Grimm A, Kiess W, et al. Telephone-based adiposity prevention for families with overweight children (TAFF-Study): one year outcome of a randomized, controlled trial. IJERPH. 2014;11(10):10327–44.
Markert J, Alff F, Zschaler S, Gausche R, Kiess W, Blüher S. Prevention of childhood obesity: Recruiting strategies via local paediatricians and study protocol for a telephone-based counselling programme. Obes Res Clin Pract. 2013;7(6):e476–86.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–90.
Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ. 2007;176(8):S1–13.
Sveinbjarnardottir EK, Svavarsdottir EK, Saveman BI. Nurses attitudes towards the importance of families in psychiatric care following an educational and training intervention program. J Psychiatr Ment Health Nurs. 2011;18(10):895–903.
Larivaara P, Väisänen E, Väisänen L, Kiuttu J. Training general practitioners in family systems medicine. Nord J Psychiatry. 1995;49(3):191–7.
Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clinical nutr. 2017;36(4):917–38.
Moorman EL, Koskela-Staples NC, Mathai BB, Fedele DA, Janicke DM. Pediatric obesity treatment via telehealth: current evidence and future directions. Curr Obes Rep. 2021:1–14.
Gehring ND, Ball GD, Perez A, Holt NL, Neuman D, Spence N, et al. Families’ perceived benefits of home visits for managing paediatric obesity outweigh the potential costs and barriers. Acta Paediatr. 2018;107(2):315–21.
May C, Chai LK, Burrows T. Parent, partner, co-parent or partnership? The need for clarity as family systems thinking takes hold in the quest to motivate behavioural change. Children. 2017;4(4):29.
Kitzmann KM, Beech BM. Family-based interventions for pediatric obesity: methodological and conceptual challenges from family psychology. Couple Family Psychol. 2011;1(S):45–62.
Kitzman-Ulrich H, Wilson DK, George SMS, Lawman H, Segal M, Fairchild A. The integration of a family systems approach for understanding youth obesity, physical activity, and dietary programs. Clin Child Fam Psychol Rev. 2010;13(3):231–53.
Ellis DA, Janisse H, Naar-King S, Kolmodin K, Jen KL, Cunningham P, et al. The effects of multisystemic therapy on family support for weight loss among obese African-American adolescents: findings from a randomized controlled trial. Journal of developmental and behavioral pediatrics : JDBP. 2010;31(6):461–8.
Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117(6):2047–54.
Download references
Acknowledgements
Authors wish to thank Rosa-Elena Ponce Alcala and Rebecca Fox for their support towards this work.
Funding for this scoping review was obtained from the Cardiometabolic Health, Diabetes and Obesity (CMDO) Research Network and from the Fonds de la recherche du Québec – Santé (FRQ-S). N Wills-Ibarra and K Chemtob were supported by McGill University Ingram School of Nursing Summer Research Support Awards. A Van Hulst holds a Junior 1 research award from the FRQ-S.
Author information
Authors and affiliations.
Ingram School of Nursing, Faculty of Medicine and Health Sciences, McGill University, 680 Sherbrooke West Suite 1800, Montreal, QC, Canada
Natasha Wills-Ibarra, Keryn Chemtob, Heather Hart, Francesca Frati & Andraea Van Hulst
Department of Human Sciences, Human Development and Family Science Program, Couple and Family Therapy Specialization, College of Education and Human Ecology, The Ohio State University, Columbus, OH, USA
Keeley J Pratt
Department of Surgery, The Ohio State University Wexner Medical Centre, Columbus, OH, USA
Department of Pediatrics, Faculty of Medicine & Dentistry, University of Alberta, Edmonton, AB, Canada
Geoff DC Ball
You can also search for this author in PubMed Google Scholar
Contributions
NWI and KC identified studies that met inclusion criteria and extracted data from included studies. NWI also drafted the initial version of the manuscript. FF contributed to the development and implementation of the search strategy and provided expertise on the knowledge synthesis review. HH, KJP and GDB provided expertise on family systems theory and childhood obesity in all steps of the knowledge synthesis and contributed to the interpretation of the results. AVH conceptualised the study, provided direct supervision to student authors, contributed to the interpretation of the results, and completed the writing of the manuscript. All authors critically reviewed the manuscript for important intellectual content and provided final approval for the work.
Corresponding author
Correspondence to Andraea Van Hulst .
Ethics declarations
Ethics approval and consent to particiate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wills-Ibarra, N., Chemtob, K., Hart, H. et al. Family systems approaches in pediatric obesity management: a scoping review. BMC Pediatr 24 , 235 (2024). https://doi.org/10.1186/s12887-024-04646-w
Download citation
Received : 03 August 2023
Accepted : 14 February 2024
Published : 02 April 2024
DOI : https://doi.org/10.1186/s12887-024-04646-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Children and adolescents
- Childhood obesity
- Family systems
- Lifestyle behaviours
- Obesity management
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
COMMENTS
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure.
The prevalence of childhood obesity continues to rise despite decades of clinical and public health efforts. Early identification of children at risk of developing obesity is essential using newer electronic health systems, which move beyond traditional growth charts to provide a wealth of information about body mass index and other relevant parameters such as social determinants of health and ...
The present study conducted a systematic literature review to examine obesity research and machine learning techniques for the prevention and treatment of obesity from 2010 to 2020. ... Computerized decision support and machine learning applications for the prevention and treatment of childhood obesity: a systematic review of the literature ...
Introduction. The prevalence of child and adolescent obesity remains high and continues to rise in low-income and middle-income countries (LMICs) at a time when these regions are also contending ...
10.1542/6305605698112Video AbstractPEDS-VA_2021-0555716305605698112BACKGROUND AND OBJECTIVES. Addressing food insecurity while promoting healthy body weights among children is a major public health challenge. Our objective is to examine longitudinal associations between food insecurity and obesity in US children aged 1 to 19 years.METHODS. Sources for this research include PubMed, CINAHL, and ...
The aim of this review is to evaluate both current and future potential management options for childhood obesity, with the main focus on pharmacological interventions. 2 MATERIALS AND METHODS. The search engine PubMed was used to identify literature for this review.
This Review describes current knowledge on the epidemiology and causes of child and adolescent obesity, considerations for assessment, and current management approaches. Before the COVID-19 pandemic, obesity prevalence in children and adolescents had plateaued in many high-income countries despite levels of severe obesity having increased. However, in low-income and middle-income countries ...
This systematic review synthesizes the empirical evidence on the built environment as determinant of childhood obesity. We focused on environmental factors including traffic noise and air pollution, as well as physical factors potentially driving obesity-related behaviors, including neighborhood walkability, and availability and accessibility ...
Early childhood overweight and obesity is a major health concern affecting nearly a quarter of children in the United States, with similar rates in Europe, Canada and Australia (Morris et al., 2015), with Shakleton et al. (2018) reporting that New Zealand has among the highest rates in the world.Rudolf et al. (2019) has reported that as many as 10% of children in the United Kingdom are obese ...
Purpose of Review This is a review of the patterns, conceptualization, and suggested mechanisms underlying the relationship of socioeconomic status (SES) to obesity in childhood and the implications of these data for interventions going forward. Recent Findings Adiposity and SES are negatively associated in high-income countries and positively associated in medium to low-income countries ...
This literature review showed that the caregiver's (CG) and the child's attachment quality, along with controlling or permissive feeding practices, and few family routines are mostly mediated by appetite dysregulation and emotional regulation strategies with the development of child obesity.
Child obesity will continue to be a problem without improved understanding of key factors operative during early childhood and identification of effective interventions. 1 The UK Government has responded to rising childhood obesity with a Public Service Agreement target to ... Results and review of papers. The literature search identified 832 ...
This study aimed to review the existing literature on dietary interventions for the prevention of childhood obesity and their effectiveness. A literature search was conducted using PubMed Central ®. Only articles published between 2009 and 2021, written in English, conducted in humans, and including children and/or adolescents (<18 years old ...
Family-based obesity management interventions targeting child, adolescent and parental lifestyle behaviour modifications have shown promising results. Further intervening on the family system may lead to greater improvements in obesity management outcomes due to the broader focus on family patterns and dynamics that shape behaviours and health. This review aimed to summarize the scope of ...
Several dietary interventions have been conducted to prevent/reduce childhood obesity, but most of them are known to have failed in tackling the obesity epidemic. This study aimed to review the existing literature on dietary interventions for the prevention of childhood obesity and their effectiveness. A literature search was conducted using PubMed Central®. Only articles published between ...
Introduction: Adverse childhood experiences (ACEs) and social determinants of health (SDoH) are associated with increased incidence of pediatric obesity. Recent literature highlights an imperative need to assess ACEs and SDoH among youth and families with obesity to identify those individuals requiring targeted interventions. The primary objective of the present study was to examine the ...
Now obesity is a worldwide health problem that has nearly tripled since 1975. Additionally In 2016, more than 1.9 billion cases of adults have been found to be overweight, and of these cases over 650 million people were found to be obese. On the contrary, 38.9 million children under 5 were found to be diagnostically overweight or obese in 2020.