Purdue Online Writing Lab Purdue OWL® College of Liberal Arts

Tables and Figures

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Note: This page reflects the latest version of the APA Publication Manual (i.e., APA 7), which released in October 2019. The equivalent resources for the older APA 6 style can be found at this page as well as at this page (our old resources covered the material on this page on two separate pages).
The purpose of tables and figures in documents is to enhance your readers' understanding of the information in the document; usually, large amounts of information can be communicated more efficiently in tables or figures. Tables are any graphic that uses a row and column structure to organize information, whereas figures include any illustration or image other than a table.
General guidelines
Visual material such as tables and figures can be used quickly and efficiently to present a large amount of information to an audience, but visuals must be used to assist communication, not to use up space, or disguise marginally significant results behind a screen of complicated statistics. Ask yourself this question first: Is the table or figure necessary? For example, it is better to present simple descriptive statistics in the text, not in a table.
Relation of Tables or Figures and Text
Because tables and figures supplement the text, refer in the text to all tables and figures used and explain what the reader should look for when using the table or figure. Focus only on the important point the reader should draw from them, and leave the details for the reader to examine on their own.
Documentation
If you are using figures, tables and/or data from other sources, be sure to gather all the information you will need to properly document your sources.
Integrity and Independence
Each table and figure must be intelligible without reference to the text, so be sure to include an explanation of every abbreviation (except the standard statistical symbols and abbreviations).
Organization, Consistency, and Coherence
Number all tables sequentially as you refer to them in the text (Table 1, Table 2, etc.), likewise for figures (Figure 1, Figure 2, etc.). Abbreviations, terminology, and probability level values must be consistent across tables and figures in the same article. Likewise, formats, titles, and headings must be consistent. Do not repeat the same data in different tables.
Data in a table that would require only two or fewer columns and rows should be presented in the text. More complex data is better presented in tabular format. In order for quantitative data to be presented clearly and efficiently, it must be arranged logically, e.g. data to be compared must be presented next to one another (before/after, young/old, male/female, etc.), and statistical information (means, standard deviations, N values) must be presented in separate parts of the table. If possible, use canonical forms (such as ANOVA, regression, or correlation) to communicate your data effectively.

A generic example of a table with multiple notes formatted in APA 7 style.
Elements of Tables
Number all tables with Arabic numerals sequentially. Do not use suffix letters (e.g. Table 3a, 3b, 3c); instead, combine the related tables. If the manuscript includes an appendix with tables, identify them with capital letters and Arabic numerals (e.g. Table A1, Table B2).
Like the title of the paper itself, each table must have a clear and concise title. Titles should be written in italicized title case below the table number, with a blank line between the number and the title. When appropriate, you may use the title to explain an abbreviation parenthetically.
Comparison of Median Income of Adopted Children (AC) v. Foster Children (FC)
Keep headings clear and brief. The heading should not be much wider than the widest entry in the column. Use of standard abbreviations can aid in achieving that goal. There are several types of headings:
- Stub headings describe the lefthand column, or stub column , which usually lists major independent variables.
- Column headings describe entries below them, applying to just one column.
- Column spanners are headings that describe entries below them, applying to two or more columns which each have their own column heading. Column spanners are often stacked on top of column headings and together are called decked heads .
- Table Spanners cover the entire width of the table, allowing for more divisions or combining tables with identical column headings. They are the only type of heading that may be plural.
All columns must have headings, written in sentence case and using singular language (Item rather than Items) unless referring to a group (Men, Women). Each column’s items should be parallel (i.e., every item in a column labeled “%” should be a percentage and does not require the % symbol, since it’s already indicated in the heading). Subsections within the stub column can be shown by indenting headings rather than creating new columns:
Chemical Bonds
Ionic
Covalent
Metallic
The body is the main part of the table, which includes all the reported information organized in cells (intersections of rows and columns). Entries should be center aligned unless left aligning them would make them easier to read (longer entries, usually). Word entries in the body should use sentence case. Leave cells blank if the element is not applicable or if data were not obtained; use a dash in cells and a general note if it is necessary to explain why cells are blank. In reporting the data, consistency is key: Numerals should be expressed to a consistent number of decimal places that is determined by the precision of measurement. Never change the unit of measurement or the number of decimal places in the same column.
There are three types of notes for tables: general, specific, and probability notes. All of them must be placed below the table in that order.
General notes explain, qualify or provide information about the table as a whole. Put explanations of abbreviations, symbols, etc. here.
Example: Note . The racial categories used by the US Census (African-American, Asian American, Latinos/-as, Native-American, and Pacific Islander) have been collapsed into the category “non-White.” E = excludes respondents who self-identified as “White” and at least one other “non-White” race.
Specific notes explain, qualify or provide information about a particular column, row, or individual entry. To indicate specific notes, use superscript lowercase letters (e.g. a , b , c ), and order the superscripts from left to right, top to bottom. Each table’s first footnote must be the superscript a .
a n = 823. b One participant in this group was diagnosed with schizophrenia during the survey.
Probability notes provide the reader with the results of the tests for statistical significance. Asterisks indicate the values for which the null hypothesis is rejected, with the probability ( p value) specified in the probability note. Such notes are required only when relevant to the data in the table. Consistently use the same number of asterisks for a given alpha level throughout your paper.
* p < .05. ** p < .01. *** p < .001
If you need to distinguish between two-tailed and one-tailed tests in the same table, use asterisks for two-tailed p values and an alternate symbol (such as daggers) for one-tailed p values.
* p < .05, two-tailed. ** p < .01, two-tailed. † p <.05, one-tailed. †† p < .01, one-tailed.
Borders
Tables should only include borders and lines that are needed for clarity (i.e., between elements of a decked head, above column spanners, separating total rows, etc.). Do not use vertical borders, and do not use borders around each cell. Spacing and strict alignment is typically enough to clarify relationships between elements.

Example of a table in the text of an APA 7 paper. Note the lack of vertical borders.
Tables from Other Sources
If using tables from an external source, copy the structure of the original exactly, and cite the source in accordance with APA style .
Table Checklist
(Taken from the Publication Manual of the American Psychological Association , 7th ed., Section 7.20)
- Is the table necessary?
- Does it belong in the print and electronic versions of the article, or can it go in an online supplemental file?
- Are all comparable tables presented consistently?
- Are all tables numbered with Arabic numerals in the order they are mentioned in the text? Is the table number bold and left-aligned?
- Are all tables referred to in the text?
- Is the title brief but explanatory? Is it presented in italicized title case and left-aligned?
- Does every column have a column heading? Are column headings centered?
- Are all abbreviations; special use of italics, parentheses, and dashes; and special symbols explained?
- Are the notes organized according to the convention of general, specific, probability?
- Are table borders correctly used (top and bottom of table, beneath column headings, above table spanners)?
- Does the table use correct line spacing (double for the table number, title, and notes; single, one and a half, or double for the body)?
- Are entries in the left column left-aligned beneath the centered stub heading? Are all other column headings and cell entries centered?
- Are confidence intervals reported for all major point estimates?
- Are all probability level values correctly identified, and are asterisks attached to the appropriate table entries? Is a probability level assigned the same number of asterisks in all the tables in the same document?
- If the table or its data are from another source, is the source properly cited? Is permission necessary to reproduce the table?
Figures include all graphical displays of information that are not tables. Common types include graphs, charts, drawings, maps, plots, and photos. Just like tables, figures should supplement the text and should be both understandable on their own and referenced fully in the text. This section details elements of formatting writers must use when including a figure in an APA document, gives an example of a figure formatted in APA style, and includes a checklist for formatting figures.
Preparing Figures
In preparing figures, communication and readability must be the ultimate criteria. Avoid the temptation to use the special effects available in most advanced software packages. While three-dimensional effects, shading, and layered text may look interesting to the author, overuse, inconsistent use, and misuse may distort the data, and distract or even annoy readers. Design properly done is inconspicuous, almost invisible, because it supports communication. Design improperly, or amateurishly, done draws the reader’s attention from the data, and makes him or her question the author’s credibility. Line drawings are usually a good option for readability and simplicity; for photographs, high contrast between background and focal point is important, as well as cropping out extraneous detail to help the reader focus on the important aspects of the photo.
Parts of a Figure
All figures that are part of the main text require a number using Arabic numerals (Figure 1, Figure 2, etc.). Numbers are assigned based on the order in which figures appear in the text and are bolded and left aligned.
Under the number, write the title of the figure in italicized title case. The title should be brief, clear, and explanatory, and both the title and number should be double spaced.
The image of the figure is the body, and it is positioned underneath the number and title. The image should be legible in both size and resolution; fonts should be sans serif, consistently sized, and between 8-14 pt. Title case should be used for axis labels and other headings; descriptions within figures should be in sentence case. Shading and color should be limited for clarity; use patterns along with color and check contrast between colors with free online checkers to ensure all users (people with color vision deficiencies or readers printing in grayscale, for instance) can access the content. Gridlines and 3-D effects should be avoided unless they are necessary for clarity or essential content information.
Legends, or keys, explain symbols, styles, patterns, shading, or colors in the image. Words in the legend should be in title case; legends should go within or underneath the image rather than to the side. Not all figures will require a legend.
Notes clarify the content of the figure; like tables, notes can be general, specific, or probability. General notes explain units of measurement, symbols, and abbreviations, or provide citation information. Specific notes identify specific elements using superscripts; probability notes explain statistical significance of certain values.

A generic example of a figure formatted in APA 7 style.
Figure Checklist
(Taken from the Publication Manual of the American Psychological Association , 7 th ed., Section 7.35)
- Is the figure necessary?
- Does the figure belong in the print and electronic versions of the article, or is it supplemental?
- Is the figure simple, clean, and free of extraneous detail?
- Is the figure title descriptive of the content of the figure? Is it written in italic title case and left aligned?
- Are all elements of the figure clearly labeled?
- Are the magnitude, scale, and direction of grid elements clearly labeled?
- Are parallel figures or equally important figures prepared according to the same scale?
- Are the figures numbered consecutively with Arabic numerals? Is the figure number bold and left aligned?
- Has the figure been formatted properly? Is the font sans serif in the image portion of the figure and between sizes 8 and 14?
- Are all abbreviations and special symbols explained?
- If the figure has a legend, does it appear within or below the image? Are the legend’s words written in title case?
- Are the figure notes in general, specific, and probability order? Are they double-spaced, left aligned, and in the same font as the paper?
- Are all figures mentioned in the text?
- Has written permission for print and electronic reuse been obtained? Is proper credit given in the figure caption?
- Have all substantive modifications to photographic images been disclosed?
- Are the figures being submitted in a file format acceptable to the publisher?
- Have the files been produced at a sufficiently high resolution to allow for accurate reproduction?
University of Vermont
Tim plante, md mhs, part 5: baseline characteristics in a table 1 for a prospective observational study, what’s the deal with table 1.
Tables describing the baseline characteristics of your analytical sample are ubiquitous in observational epidemiology manuscripts. They are critical to help the reader understand the study population and potential limitations of your analysis. A table characterizing baseline characteristics is so important that it’s typically the first table that appears in any observational epidemiology (or clinical trial) manuscript, so it’s commonly referred to as a “ Table 1 “. Table 1s are critically important because they help the readers understand internal validity of your study. If your study has poor internal validity, then your results and findings aren’t useful.
The details here are specific to prospective observational studies (e.g., cohort studies), but are generalizable to other sorts of studies (e.g., RCTs, case-control studies).
If you are a Stata user, you might be interested into my primer of using Table1_mc to generate a Table 1 .
Guts of a Table 1
There are several variations of the Table 1, here’s how I do it.
COLUMNS : This is your exposure of interest (i.e., dependent variable). This is not the outcome of interest . There’s a few way to divvy up these columns, depending on what sort of data you have:
- Continuous exposure (e.g., baseline LDL-cholesterol level): Cut this up into quantiles. I commonly use tertiles (3 groups) or quartiles (4 groups). People have very, very strong opinions about whether you use tertiles or quartiles. I don’t see much of a fuss in using either. Of note, there usually is no need to transform your data prior to splitting into quantiles. (And, log transforming continuous data that includes values of zero will replace those zeros with missing data!)
- Dichotomous/binary exposure (e.g., prevalent diabetes status as no/0 or yes/1): This is easy, column headers should be 0 or 1. Make sure to use a descriptive column header like “No prevalent diabetes” and “Prevalent diabetes” instead of numbers 0 and 1.
- Ordinal exposure, not too many groups (e.g., never smoker/0, former smoker/1, current smoker/2): This is also easy, column headers should be 0, 1, or 2. Make sure to use descriptive column headers.
- Ordinal exposure, a bunch of groups (e.g., extended Likert scale ranging from super unsatisfied/1 to super satisfied/7): This is a bit tricker. On one hand, there isn’t any real limitation on how wide a table can be in a software package so you could have columns 1, 2, 3, 4, 5 ,6 and 7. This is a bit unwieldy for the reader, however. I personally think it’s better to collapse really wide groupings into a few groups. Here, you could collapse all of the negative responses (1, 2 and 3), leave the neutral response as its own category (4), and collapse all of the positive responses (5, 6, and 7). Also use descriptive column headers, but also be sure to describe how you collapsed groups in the footer of the table.
- Nominal exposure, not too many groups (e.g., US Census regions of Northeast, Midwest, South, and West): This is easy, just use the groups. Be thoughtful about using a consistent order of these groups throughout your manuscript.
- Nominal exposure, a bunch of groups (e.g., favorite movie): As with ‘Ordinal data, a bunch of groups’ above, I would collapse these into groups that relate to each other, such as genre of movie.
- (Optional) Additional first column showing “Total” summary statistics. This presents summary statistics for the entire study population as a whole, instead of by quantile or discrete groupings. I don’t see much value in these and typically don’t include them.
- Note: Table1_mc for Stata cannot generate a “missingness” row .
- (Optional, but suggest to avoid) Following P-value column that shows comparisons across rows. These have fallen out of favor for clinical trial Table 1s . I see little value of them for prospective observational studies and also avoid them.
ROWS: These include the N for each column, the range of values for continuous exposures, and baseline values. Note that the data here are from baseline.
- N for each group. Make sure that these Ns add up to the expected N in your analytical population at the bottom of your inclusion flow diagram. If it doesn’t match, you’ve done something wrong.
- (For continuous exposures) Range of values for your quantiles and yes I mean minimum and maximum for each quantile, not IQRs.
- Sociodemographics (age, sex, race, ± income, ± region, ± education level, etc.)
- Anthropometrics (height, weight, waist circumference, BMI, etc.)
- Medical problems as relevant to your study (eg, proportion with hypertension, diabetes, etc.)
- Medical data as relevant to your study (eg, laboratory assays, details with radiological imaging, details from cardiology reports)
- Suggest avoiding the outcome(s) of interest as additional rows. I think that presenting the outcomes in this table is inadequate. I prefer to have a separate table or figure dedicated to the outcome of interest that goes much more in-depth than a Table 1 does. Plus, the outcome isn’t ascertained at baseline in a prospective observational study, and describing the population at baseline is the general purpose of Table 1.
- And for the love of Pete, please make sure that all covariates in your final model appear as rows. If you have a model that adjusts for Epworth Sleepiness Score, for example, make sure that fits in somewhere above.
The first column of your Table 1 will describe each row. The appearance of this row will vary based upon the type of data you have.
- N row – I suggest simply using “N”, though some folks use N (upper case) to designate the entire population and n (lower case) to designate subpopulations, so perhaps you might opt to put “n”.
- Continuous variables (including the row for range) – I suggest a descriptive name and the units. Eg, “Height, cm”
- Dichotomous/binary values – In this example, sex is dichotomous (male vs. female) since that’s how it has historically been collected in NIH studies. For dichotomous variables, you can include either (1) a row for ‘Male’ and a row for ‘Female’, or (2) simply a row for one of the two sexes (eg, just ‘Female’) since the other row will be the other sex.
- Other discrete variables (eg, ordinal or nominal) – In this example, we will consider the nominal variable of Race. I suggest having a leading row that provides description of the following rows (eg, “Race group”) then add two spaces before each following race group so the nominal values for the race groups seem nested under the heading.
- (Optional) Headings for groupings of rows – I like including bold/italicized headings for groupings of data to help keep things organized.
Here’s an example of how I think a blank table should appear:
Table 1 – Here is a descriptive title of your Table 1 followed by an asterix that leads to the footer. I suggest something like “Sociodemographics, anthropometrics, medical problems, and medical data ascertained baseline among [#] participants in [NAME OF STUDY] with [BRIEF INCLUSION CRITERIA] and without [BRIEF EXCLUSION CRITERIA] by [DESCRIPTION OF EXPOSURE LIKE ‘TERTILE OF CRP’ OR ‘PREVALENT DIABETES STATUS’]*”
*Footer of your Table 1. I suggest describing the appearance of the cells, eg “Range is minimum and maximum of the exposure for each quantile. Presented as mean (SD) for normally distributed and median (IQR) for skewed continuous variables. Discrete data are presented as column percents.”
Cell contents
The cell contents varies by type of variable and your goal in this table:
- Normally distributed continuous variables : Mean (SD)
- Non-normally distributed continuous variables : Median (IQR)
- Discrete variables : Present column percentages . Not row percentages. For example we’ll consider “income >$75k” by tertile of CRP. A column percentage would show the % of participants in that specific quantile have an income >$75k. A row percentage would show the percentage of participants with income >$75K who were in that specific tertile.
- Note: Table1_mc in Stata cannot report an ‘n’ with continuous variables.
- Dichotomous variables : Present column percentage plus ‘n’. Example for female sex: “45%, n=244”.
A word on rounding: I think there is little value on including numbers after the decimal place. I suggest aggressively rounding at the decimal for most things. For example, for BMI, I suggest showing “27 (6)” and not “26.7 (7.2)”. For things that are obtained at the decimal place, I strongly recommend reporting at the decimal. For example, BP is always measured as a whole number, so reporting out a tenth place for BP isn’t of much value. For example, systolic BP is measured as 142, 112, and 138 — not 141.8, 111.8 and 138.4. For discrete variables, I always round the proportion/percentage at the decimal, but clarify very small proportions to be “<1%" if there are any in that group, but it would round to zero or "0%" if there are none in that group.
The one exception to my aggressive “round at the decimal place” strategy is variables that are commonly reported past the decimal place, such as many laboratory values. Serum creatinine is commonly reported to the hundredths place (e.g., “0.88”), so report the summary statistic for that value to the hundredths place, like 0.78 (0.30).
- About WordPress
- Get Involved
- WordPress.org
- Documentation
- Learn WordPress
- UVM Blogs Home
- Site Directory
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
How to Read a Research Table
The tables in this section present the research findings that drive many recommendations and standards of practice related to breast cancer.
Research tables are useful for presenting data. They show a lot of information in a simple format, but they can be hard to understand if you don’t work with them every day.
Here, we describe some basic concepts that may help you read and understand research tables. The sample table below gives examples.
The numbered table items are described below. You will see many of these items in all of the tables.
Sample table – Alcohol and breast cancer risk
Selection criteria.
Studies vary in how well they help answer scientific questions. When reviewing the research on a topic, it’s important to recognize “good” studies. Good studies are well-designed.
Most scientific reviews set standards for the studies they include. These standards are called “selection criteria” and are listed for each table in this section. These selection criteria help make sure well-designed studies are included in the table.
Types of studies
The types of studies (for example, randomized controlled trial, prospective cohort, case-control) included in each table are listed in the selection criteria.
Learn about the strengths and weaknesses of different types of research studies .
Selection criteria for most tables include the minimum number of cases of breast cancer or participants for the studies in the table.
Large studies have more statistical power than small studies. This means the results from large studies are less likely to be due to chance than results from small studies.
The power of large numbers
You can see the power of large numbers if you think about flipping a coin. Say you are trying to figure out whether a coin is fixed so that it lands on “heads” more than “tails.” A fair coin would land on heads half the time. So, you want to test whether the coin lands on heads more than half of the time.
If you flip the coin twice and get 2 heads, you don’t have a lot of evidence. It wouldn’t be surprising to flip a fair coin and get 2 heads in a row. With 2 coin flips, you can’t be sure whether you have a fair coin or not. Even 3 or 4 heads in a row wouldn’t be surprising for a fair coin.
If, however, you flipped the coin 20 times and got mostly heads, you would start to think the coin might be fixed.
With an increasing number of observations, you have more evidence on which to base your conclusions. So, you have more confidence in your conclusions. It’s a similar idea in research.
Example of study size in breast cancer research
Say you’re interested in finding out whether or not alcohol use increases the risk of breast cancer.
If there are only a few cases of breast cancer among the alcohol drinkers and the non-drinkers, you won’t have much confidence drawing conclusions.
If, however, there are hundreds of breast cancer cases, it’s easier to draw firm conclusions about a link between alcohol and breast cancer. With more evidence, you have more confidence in your findings.
The importance of study design and study quality
Study design (the type of research study) and study quality are also important. For example, a small, well-designed study may be better than a large, poorly-designed study. However, when all else is equal, a larger number of people in a study means the study is better able to answer research questions.
Learn about different types of research studies .
The studies
The first column (from the left) lists either the name of the study or the name of the first author of the published article.
Below each table, there’s a reference list so you can find the original published articles.
Sometimes, a table will report the results of only one analysis. This can occur for a few reasons. Either there’s only one study that meets the selection criteria or there’s a report that combines data from many studies into one large analysis.
Study population
The second column describes the people in each study.
- For randomized controlled trials, the study population is the total number of people who were randomized at the start of the study to either the treatment (or intervention) group or the control group.
- For prospective cohort studies, the study population is the number of people at the start of the study (baseline cohort).
- For case-control studies, the study population is the number of cases and the number of controls.
In some tables, more details on the people in the study are included.
Length of follow-up
Randomized controlled trials and prospective cohort studies follow people forward in time to see who will have the outcome of interest (such as breast cancer).
For these studies, one column shows the length of follow-up time. This is the number or months or years people in the study were followed.
Because case-control studies don’t follow people forward in time, there are no data on follow-up time for these studies.
Tables that focus on cumulative risk may also show the length of follow-up. These tables give the length of time, or age range, used to compute cumulative risk (for example, the cumulative risk of breast cancer up to age 70).
Learn more about cumulative risk .
Other information
Some tables have columns with other information on the study population or the topic being studied. For example, the table Exercise and Breast Cancer Risk has a column with the comparisons of exercise used in the studies.
This extra information gives more details about the studies and shows how the studies are similar to (and different from) each other.
Studies on the same topic can differ in important ways. They may define “high” and “low” levels of a risk factor differently. Studies may look at outcomes among women of different ages or menopausal status.
These differences are important to keep in mind when you review the findings in a table. They may help explain differences in study findings.
Understanding the numbers
All of the information in the tables is important, but the main purpose of the tables is to present the numbers that show the risk, survival or other measures for each topic. These numbers are shown in the remaining columns of the tables.
The headings of the columns tell you what the numbers represent. For example:
- What is the outcome of interest? Is it breast cancer? Is it 5-year survival? Is it breast cancer recurrence?
- Are groups being compared to each other? If so, what groups are being compared?
Relative risks
Most often, findings are reported as relative risks. A relative risk shows how much higher, how much lower or whether there’s no difference in risk for people with a certain risk factor compared to the risk in people without the factor.
A relative risk compares 2 absolute risks.
- The numerator (the top number in a fraction) is the absolute risk among people with the risk factor.
- The denominator (the bottom number) is the absolute risk among those without the risk factor.
The absolute risk of those with the factor divided by the absolute risk of those without the factor gives the relative risk.
The confidence interval around a relative risk helps show whether or not the relative risk is statistically significant (whether or not the finding is likely due to chance).
Learn more about confidence intervals .
Example of relative risk
Say a study shows women who don’t exercise (inactive women) have a 25 percent increase in breast cancer risk compared to women who do exercise (active women).
This statistic is a relative risk (the relative risk is 1.25). It means the inactive women were 25 percent more likely to develop breast cancer than women who exercised.
Learn more about relative risk .
Confidence intervals
A 95 percent confidence interval (95% CI) around a risk measure means there’s a 95 percent chance the “true” measure falls within the interval.
Because there’s random error in studies, and study populations are only samples of much larger populations, a single study doesn’t give the “one” correct answer. There’s always a range of likely answers. A single study gives a “best estimate” along with a 95 % CI of a likely range.
Most scientific studies report risk measures, such as relative risks, odds ratios and averages, with 95% CI.
Confidence intervals and statistical significance
For relative risks and odds ratios, a 95% CI that includes the number 1.0 means there’s no link between an exposure (such as a risk factor or a treatment) and an outcome (such as breast cancer or survival).
When this happens, the results are not statistically significant. This means any link between the exposure and outcome is likely due to chance.
If a 95% CI does not include 1.0, the results are statistically significant. This means there’s likely a true link between an exposure and an outcome.
Examples of confidence intervals
A few examples from the sample table above may help explain statistical significance.
The EPIC study found a relative risk of breast cancer of 1.07, with a 95% CI of 0.96 to 1.19. In the table, you will see 1.07 (0.96-1.19).
Women in the EPIC study who drank 1-2 drinks per day had a 7 percent higher risk of breast cancer than women who did not drink alcohol. The 95% CI of 0.96 to 1.19 includes 1.0. This means these results are not statistically significant and the increased risk of breast cancer is likely due to chance.
The Million Women’s Study found a relative risk of breast cancer of 1.13 with a 95% CI of 1.10 to 1.16. This is shown as 1.13 (1.10-1.16) in the table.
Women in the Million Women’s Study who drank 1-2 drinks per day had a 13 percent higher risk of breast cancer than women who did not drink alcohol. In this case, the 95% CI of 1.10 to 1.16 does not include 1.0. So, these results are statistically significant and suggest there’s likely a true link between alcohol and breast cancer.
For any topic, it’s important to look at the findings as a whole. In the sample table above, most studies show a statistically significant increase in risk among women who drink alcohol compared to women who don’t drink alcohol. Thus, the findings as a whole suggest alcohol increases the risk of breast cancer.
Summary relative risks
Summary relative risks from meta-analyses.
A meta-analysis takes relative risks reported in different studies and “averages” them to come up with a single, summary measure. Findings from a meta-analysis can give stronger conclusions than findings from a single study.
Summary relative risks from pooled analyses
A pooled analysis uses data from multiple studies to give a summary measure. It combines the data from each person in each of the studies into one large data set and analyses the data as if it were one big study. A pooled analysis is almost always better than a meta-analysis.
In a meta-analysis, researchers combine the results from different studies. In a pooled analysis, researchers combine the individual data from the different studies. This usually gives more statistical power than a meta-analyses. More statistical power means it’s more likely the results are not simply due to chance.
Cumulative risk
Sometimes, study findings are presented as a cumulative risk (risk up to a certain age). This risk is often shown as a percentage.
A cumulative risk may show the risk of breast cancer for a certain group of people up to a certain age. Say the cumulative risk up to age 70 for women with a risk factor is 20 percent. This means by age 70, 20 percent of the women (or 1 in 5) with the risk factor will get breast cancer.
Lifetime risk is a cumulative risk. It shows the risk of getting breast cancer during your lifetime (or up to a certain age). Women in the U.S. have a 13 percent lifetime risk of getting breast cancer. This means 1 in 8 women in the U.S. will get breast cancer during their lifetime.
Learn more about lifetime risk .
Sensitivity and specificity
Some tables show study findings on the sensitivity and specificity of screening tests. These measures describe the quality of a breast cancer screening test.
- Sensitivity shows how well the screening test shows who truly has breast cancer. A sensitivity of 90 percent means 90 percent of people tested who truly have breast cancer are correctly identified as having cancer.
- Specificity shows how well the screening test shows who truly does not have breast cancer. A specificity of 90 percent means 90 percent of the people who do not have breast cancer are correctly identified as not having cancer.
The goals of any screening test are:
- To correctly identify everyone who has a certain disease (100 percent sensitivity)
- To correctly identify everyone who does not have the disease (100 percent specificity)
A perfect test would correctly identify everyone with no mistakes. There would be no:
- False negatives (when people who have the disease are missed by the test)
- False positives (when healthy people are incorrectly shown to have the disease)
No screening test has perfect (100 percent) sensitivity and perfect (100 percent) specificity. There’s always a trade-off between the two. When a test gains sensitivity, it loses some specificity.
Learn more about sensitivity and specificity .
Finding studies
You may want more detail about a study than is given in the summary table. To help you find this information, the references for all the studies in a table are listed below the table.
Each reference includes the:
- Authors of the study article
- Title of the article
- Year the article was published
- Title and specific issue of the medical journal where the article appeared
PubMed , the National Library of Medicine’s search engine, is a good source for finding summaries of science and medical journal articles (called abstracts).
For some abstracts, PubMed also has links to the full text articles. Most medical journals have websites and offer their articles either for free or for a fee.
If you live near a university with a medical school or public health school, you may be able to go to the school’s medical library to get a copy of an article. Local public libraries may not carry medical journals, but they may be able to find a copy of an article from another source.
More information on research studies
If you’re interested in learning more about health research, a basic epidemiology textbook may be a good place to start. The National Cancer Institute also has information on epidemiology studies and randomized controlled trials.
Updated 07/25/22
TOOLS & RESOURCES
In Your Own Words
How has having breast cancer changed your outlook?
Share Your Story or Read Others
An Introduction to R for Research
5.5 table 1.
In almost every published article that includes quantitative data, there will be a “Table 1” displaying the descriptive statistics for the study sample. There are many ways to organize this information, but the following are commonly followed principles:
- Different variables are in different rows.
- Different statistics are in different columns.
- Categorical variables are typically summarized by displaying the number (N) and proportion (%) of cases at each level. Sometimes the number of missing values is indicated, as well.
- Continuous variables are typically summarized by displaying the mean and SD (or median and IQR). Sometimes the number of missing values is indicated, as well.
- If the descriptive statistics are to be presented by some other variable, levels of that variable should be in separate columns.
- The units for each variable should be included next to the variable name (e.g., Cholesterol (mg/dL)).
- The reader should be able to understand all the contents of the table, within reason, without reading the text. Clarifying information should be included in the title, headings, and footnotes.
For details on creating a “Table 1”, see Section 3.3 in Introduction to Regression Methods for Public Health . Here, we just present the relevant code for the dataset used in this chapter ( Sjoberg et al. 2023 , 2021 ) .

R news and tutorials contributed by hundreds of R bloggers
Table 1 and the characteristics of study population.
Posted on February 14, 2016 by Klodian Dhana in R bloggers | 0 Comments
[social4i size="small" align="align-left"] --> [This article was first published on DataScience+ , and kindly contributed to R-bloggers ]. (You can report issue about the content on this page here ) Want to share your content on R-bloggers? click here if you have a blog, or here if you don't.
In research, especially in medical research, we describe characteristics of our study populations through Table 1. The Table 1 contain information about the mean for continue/scale variable, and proportion for categorical variable. For example: we say that the mean of systolic blood pressure in our study population is 145 mmHg, or 30% of participants are smokers. Since is called Table 1, means that is the first table in the manuscript.
To create the Table 1 sometimes it can be very time consuming. Imagine if we have 10 variables (e.g. age, gender.. etc) for 3 groups, and for each variable we compute mean (standard deviation) and number of participants (proportion); in the end we have to fill 60 numbers in the table. Moreover, we usually export the table from R to Microsoft Word, and we can be prone to making mistakes if we’re copy/pasting. Therefore, I did a search to find a simple and comprehensive way to make Table 1 with R. I found two very interesting packages named “Tableone” and “ReporteRs”. The TableOne package is created by Kazuki Yoshida and Justin Bohn and is used to create the Table 1 in R. The ReporteRs package is created by David Gohel and in this post I use for exporting Table from R to Microsoft Word.
Create Table 1
I simulated a dataset by using functions rnorm() and sample() . You can download this simulated data set on you desktop to replicate this post. To learn how to upload your dataset into R read this post .
Now we will use the package TableOne to create our Table 1. First we will load the package and then will create the list of variables which we want to place on Table 1. Secondly, we will define the categorical variables.
My first interest is to make the Table 1 for total population.
But, often I am interested to create Table 1 for men and women and to compare their means and proportions. To compute this we run the code below.
You can do a lot more with the TableOne package. For example: you can compute median and inter-quartile range for non normally distributing variables, and run different tests for comparison of the groups.
Export Table 1 from R to Microsoft Word
Now that we have Table 1 ready we want to transfer Table 1 to Microsoft Word document. For this purpose we will use the function FlexTable() from the package "ReporteRs". I found a very good script in StackOverflow to achieve this task. I am sharing the code below. (Credits to the author in StackOverflow ).

If you have any comment or feedback feel free to post a comment below.
To leave a comment for the author, please follow the link and comment on their blog: DataScience+ . R-bloggers.com offers daily e-mail updates about R news and tutorials about learning R and many other topics. Click here if you're looking to post or find an R/data-science job . Want to share your content on R-bloggers? click here if you have a blog, or here if you don't.
Copyright © 2022 | MH Corporate basic by MH Themes
Never miss an update! Subscribe to R-bloggers to receive e-mails with the latest R posts. (You will not see this message again.)
- Privacy Policy
Buy Me a Coffee

Home » Tables in Research Paper – Types, Creating Guide and Examples
Tables in Research Paper – Types, Creating Guide and Examples
Table of Contents

Tables in Research Paper
Definition:
In Research Papers , Tables are a way of presenting data and information in a structured format. Tables can be used to summarize large amounts of data or to highlight important findings. They are often used in scientific or technical papers to display experimental results, statistical analyses, or other quantitative information.
Importance of Tables in Research Paper
Tables are an important component of a research paper as they provide a clear and concise presentation of data, statistics, and other information that support the research findings . Here are some reasons why tables are important in a research paper:
- Visual Representation : Tables provide a visual representation of data that is easy to understand and interpret. They help readers to quickly grasp the main points of the research findings and draw their own conclusions.
- Organize Data : Tables help to organize large amounts of data in a systematic and structured manner. This makes it easier for readers to identify patterns and trends in the data.
- Clarity and Accuracy : Tables allow researchers to present data in a clear and accurate manner. They can include precise numbers, percentages, and other information that may be difficult to convey in written form.
- Comparison: Tables allow for easy comparison between different data sets or groups. This makes it easier to identify similarities and differences, and to draw meaningful conclusions from the data.
- Efficiency: Tables allow for a more efficient use of space in the research paper. They can convey a large amount of information in a compact and concise format, which saves space and makes the research paper more readable.
Types of Tables in Research Paper
Most common Types of Tables in Research Paper are as follows:
- Descriptive tables : These tables provide a summary of the data collected in the study. They are usually used to present basic descriptive statistics such as means, medians, standard deviations, and frequencies.
- Comparative tables : These tables are used to compare the results of different groups or variables. They may be used to show the differences between two or more groups or to compare the results of different variables.
- Correlation tables: These tables are used to show the relationships between variables. They may show the correlation coefficients between variables, or they may show the results of regression analyses.
- Longitudinal tables : These tables are used to show changes in variables over time. They may show the results of repeated measures analyses or longitudinal regression analyses.
- Qualitative tables: These tables are used to summarize qualitative data such as interview transcripts or open-ended survey responses. They may present themes or categories that emerged from the data.
How to Create Tables in Research Paper
Here are the steps to create tables in a research paper:
- Plan your table: Determine the purpose of the table and the type of information you want to include. Consider the layout and format that will best convey your information.
- Choose a table format : Decide on the type of table you want to create. Common table formats include basic tables, summary tables, comparison tables, and correlation tables.
- Choose a software program : Use a spreadsheet program like Microsoft Excel or Google Sheets to create your table. These programs allow you to easily enter and manipulate data, format the table, and export it for use in your research paper.
- Input data: Enter your data into the spreadsheet program. Make sure to label each row and column clearly.
- Format the table : Apply formatting options such as font, font size, font color, cell borders, and shading to make your table more visually appealing and easier to read.
- Insert the table into your paper: Copy and paste the table into your research paper. Make sure to place the table in the appropriate location and refer to it in the text of your paper.
- Label the table: Give the table a descriptive title that clearly and accurately summarizes the contents of the table. Also, include a number and a caption that explains the table in more detail.
- Check for accuracy: Review the table for accuracy and make any necessary changes before submitting your research paper.
Examples of Tables in Research Paper
Examples of Tables in the Research Paper are as follows:
Table 1: Demographic Characteristics of Study Participants
This table shows the demographic characteristics of 200 participants in a research study. The table includes information about age, gender, and education level. The mean age of the participants was 35.2 years with a standard deviation of 8.6 years, and the age range was between 21 and 57 years. The table also shows that 46% of the participants were male and 54% were female. In terms of education, 10% of the participants had less than a high school education, 30% were high school graduates, 35% had some college education, and 25% had a bachelor’s degree or higher.
Table 2: Summary of Key Findings
This table summarizes the key findings of a study comparing three different groups on a particular variable. The table shows the mean score, standard deviation, t-value, and p-value for each group. The asterisk next to the t-value for Group 1 indicates that the difference between Group 1 and the other groups was statistically significant at p < 0.01, while the differences between Group 2 and Group 3 were not statistically significant.
Purpose of Tables in Research Paper
The primary purposes of including tables in a research paper are:
- To present data: Tables are an effective way to present large amounts of data in a clear and organized manner. Researchers can use tables to present numerical data, survey results, or other types of data that are difficult to represent in text.
- To summarize data: Tables can be used to summarize large amounts of data into a concise and easy-to-read format. Researchers can use tables to summarize the key findings of their research, such as descriptive statistics or the results of regression analyses.
- To compare data : Tables can be used to compare data across different variables or groups. Researchers can use tables to compare the characteristics of different study populations or to compare the results of different studies on the same topic.
- To enhance the readability of the paper: Tables can help to break up long sections of text and make the paper more visually appealing. By presenting data in a table, researchers can help readers to quickly identify the most important information and understand the key findings of the study.
Advantages of Tables in Research Paper
Some of the advantages of using tables in research papers include:
- Clarity : Tables can present data in a way that is easy to read and understand. They can help readers to quickly and easily identify patterns, trends, and relationships in the data.
- Efficiency: Tables can save space and reduce the need for lengthy explanations or descriptions of the data in the main body of the paper. This can make the paper more concise and easier to read.
- Organization: Tables can help to organize large amounts of data in a logical and meaningful way. This can help to reduce confusion and make it easier for readers to navigate the data.
- Comparison : Tables can be useful for comparing data across different groups, variables, or time periods. This can help to highlight similarities, differences, and changes over time.
- Visualization : Tables can also be used to visually represent data, making it easier for readers to see patterns and trends. This can be particularly useful when the data is complex or difficult to understand.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
- Methodology
- Open access
- Published: 20 March 2017
Getting started with tables
- Hazel Inskip ORCID: orcid.org/0000-0001-8897-1749 1 ,
- Georgia Ntani 1 ,
- Leo Westbury 1 ,
- Chiara Di Gravio 1 ,
- Stefania D’Angelo 1 ,
- Camille Parsons 1 &
- Janis Baird 1
Archives of Public Health volume 75 , Article number: 14 ( 2017 ) Cite this article
8040 Accesses
1 Citations
3 Altmetric
Metrics details
Tables are often overlooked by many readers of papers who tend to focus on the text. Good tables tell much of the story of a paper and give a richer insight into the details of the study participants and the main research findings. Being confident in reading tables and constructing clear tables are important skills for researchers to master.
Common forms of tables were considered, along with the standard statistics used in them. Papers in the Archives of Public Health published during 2015 and 2016 were hand-searched for examples to illustrate the points being made. Presentation of graphs and figures were not considered as they are outside the scope of the paper.
Basic statistical concepts are outlined to aid understanding of each of the tables presented. The first table in many papers gives an overview of the study population and its characteristics, usually giving numbers and percentages of the study population in different categories (e.g. by sex, educational attainment, smoking status) and summaries of measured characteristics (continuous variables) of the participants (e.g. age, height, body mass index). Tables giving the results of the analyses follow; these often include summaries of characteristics in different groups of participants, as well as relationships between the outcome under study and the exposure of interest. For continuous outcome data, results are often expressed as differences between means, or regression or correlation coefficients. Ratio/relative measures (e.g. relative risks, odds ratios) are usually used for binary outcome measures that take one of two values for each study participants (e.g. dead versus alive, obese versus non-obese). Tables come in many forms, but various standard types are described here.
Clear tables provide much of the important detail in a paper and researchers are encouraged to read and construct them with care.
Peer Review reports
Tables are an important component of any research paper. Yet, anecdotally, many people say that they find tables difficult to understand so focus only on the text when reading a paper. However, tables provide a much richer sense of a study population and the results than can be described in the text. The tables and text complement each other in that the text outlines the main findings, while the detail is contained in the tables; the text should refer to each table at the appropriate place(s) in the paper. We aim to give some insights into reading tables for those who find them challenging, and to assist those preparing tables in deciding what they need to put into them. Producing clear, informative tables increases the likelihood of papers being published and read. Good graphs and figures can often provide a more accessible presentation of study findings than tables. They can add to the understanding of the findings considerably, but they can rarely contain as much detail as a table. Choosing when to present a graph or figure and when to present a table needs careful consideration but this article focuses only on the presentation of tables.
We provide a general description of tables and statistics commonly used when presenting data, followed by specific examples. No two papers will present the tables in the same way, so we can only give some general insights. The statistical approaches are described briefly but cannot be explained fully; the reader is referred to various books on the topic [ 1 – 6 ].
Presentation of tables
The title (or legend) of a table should enable the reader to understand its content, so a clear, concise description of the contents of the table is required. The specific details needed for the title will vary according to the type of table. For example, titles for tables of characteristics should give details of the study population being summarised and indicate whether separate columns are presented for particular characteristics, such as sex. For tables of main findings, the title should include the details of the type of statistics presented or the analytical method. Ideally the table title should enable the table to be examined and understood without reference to the rest of the article, and so information on study, time and place needs to be included. Footnotes may be required to amplify particular points, but should be kept to a minimum. Often they will be used to explain abbreviations or symbols used in the table or to list confounding factors for which adjustment has been made in the analysis.
Clear headings for rows and columns are also required and the format of the table needs careful consideration, not least in regard to the appropriateness and number of rows and columns included within the table. Generally it is better to present tables with more rows than columns; it is usually easier to read down a table than across it, and page sizes currently in use are longer than they are wide. Very large tables can be hard to absorb and make the reader’s work more onerous, but can be useful for those who require extra detail. Getting the balance right needs care.
Types of tables
Many research articles present a summary of the characteristics of the study population in the first table. The purpose of these tables is to provide information on the key characteristics of the study participants, and allow the reader to assess the generalisability of the findings. Typically, age and sex will be presented along with various characteristics pertinent to the study in question, for example smoking prevalence, socio-economic position, educational attainment, height, and body mass index. A single summary column may be presented or perhaps more than one column split according to major characteristics such as sex (i.e. separate columns for males and females) or, for trials, the intervention and control groups.
Subsequent tables generally present details of the associations identified in the main analyses. Sometimes these include results that are unadjusted or ‘crude’ (i.e. don’t take account of other variables that might influence the association) often followed by results from adjusted models taking account of other factors.
Other types of tables occur in some papers. For example, systematic review papers contain tables giving the inclusion and exclusion criteria for the review as well as tables that summarise the characteristics and results of each study included in the review; such tables can be extremely large if the review covers many studies. Qualitative studies often provide tables describing the characteristics of the study participants in a more narrative format than is used for quantitative studies. This paper however, focuses on tables that present numerical data.
Statistics commonly presented in tables
The main summary statistics provided within a table depend on the type of outcome under investigation in the study. If the variable is continuous (i.e. can take any numerical value, between a minimum and a maximum, such as blood pressure, height, birth weight), then means and standard deviations (SD) tend to be given when the distribution is symmetrical, and particularly when it follows the classical bell shaped curve known as a Normal or Gaussian distribution (see Fig. 1a ). The mean is the usual arithmetic average and the SD is an indication of the spread of the values. Roughly speaking, the SD is about a quarter of the difference between the largest and the smallest value excluding 5% of values at the extreme ends. So, if the mean is 100 and the SD is 20 we would expect 95% of the values in our data to be between about 60 (i.e. 100–2×20) and 140 (100 + 2×40).
Distribution of heights and weights of young women from the Southampton Women’s Survey [ 7 ]. a Shows the height distribution, which is symmetrical and generally follows a standard normal distribution, while b shows weight, which is skewed to the right
The median and inter-quartile range (IQR) are usually provided when the data are not symmetrical as in Fig. 1b , which gives an example of data that are skewed, such that if the values are plotted in a histogram there are many values at one end of the distribution but fewer at the other end [ 7 ]. If all the values of the variable were listed in order, the median would be the middle value and the IQR would be the values a quarter and three-quarters of the way through the list. Sometimes the lower value of the IQR is labelled Q1 (quartile 1), the median is Q2, and the upper value is Q3. For categorical variables, frequencies and percentages are used.
Common statistics for associations between continuous outcomes include differences in means, regression coefficients and correlation coefficients. For these statistics, values of zero indicate no association between the exposure and outcome of interest. A correlation coefficient of 0 indicates no association, while a value of 1 or −1 would indicate perfect positive or negative correlation; values outside the range −1 to 1 are not possible. Regression coefficients can take any positive or negative value depending on the units of measurement of the exposure and outcome.
For binary outcome measures that only take two possible values (e.g. diseased versus not, dead versus alive, obese versus not obese) the results are commonly presented in the form of relative measures. These include any measure with the word ‘relative’ or ‘ratio’ in their name, such as odds ratios, relative risks, prevalence ratios, incidence rate ratios and hazard ratios. All are interpreted in much the same way: values above 1 indicate an elevated risk of the outcome associated with the exposure under study, whereas below 1 implies a protective effect. No association between the outcome and exposure is apparent if the ratio is 1.
Typically in results tables, 95% confidence intervals (95% CIs) and/or p -values will be presented. A 95% CI around a result indicates that, in the absence of bias, there is a 95% probability that the interval includes the true value of the result in the wider population from which the study participants were drawn. It also gives an indication of how precisely the study team has been able to estimate the result (whether it is a regression coefficient, a ratio/relative measure or any of the summary measures mentioned above). The wider the 95% CI, the less precise is our estimate of the result. Wide 95% CIs tend to arise from small studies and hence the drive for larger studies to give greater precision and certainty about the findings.
If a 95% CI around a result for a continuous variable (difference in means, regression or correlation coefficient) includes 0 then it is unlikely that there is a real association between exposure and outcome whereas, for a binary outcome, a real association is unlikely if the 95% CI around a relative measure, such as a hazard or odds ratio, includes 1.
The p -value is the probability that the finding we have observed could have occurred by chance, and therefore there is no identifiable association between the exposure of interest and the outcome measure in the wider population. If the p -value is very small, then we are more convinced that we have found an association that is not explained by chance (though it may be due to bias or confounding in our study). Traditionally a p -value of less than 0.05 (sometimes expressed as 5%) has been considered as ‘statistically significant’ but this is an arbitrary value and the smaller the p -value the less likely the result is simply due to chance [ 8 ].
Frequently, data within tables are presented with 95% CIs but without p -values or vice versa. If the 95% CI includes 0 (for a continuous outcome measure) or 1 (for a binary outcome), then generally the p -value will be greater than 0.05, whereas if it does not include 0 or 1 respectively, then the p -value will be less than 0.05 [ 9 ]. Generally, 95% CIs are more informative than p -values; providing both may affect the readability of a table and so preference should generally be given to 95% CIs. Sometimes, rather than giving exact p-values, they are indicated by symbols that are explained in a footnote; commonly one star (*) indicates p < 0.05, two stars (**) indicates p < 0.01.
Results in tables can only be interpreted if the units of measurement are clearly given. For example, mean or median age could be in days, weeks, months or years if infants and children are being considered, and 365, 52, 12 or 1 for a mean age of 1 year could all be presented, as long the unit of measurement is provided. Standard deviations should be quoted in the same units as the mean to which they refer. Relative measures, such as odds ratios, and correlation coefficients do not have units of measurement, but for regression coefficients the unit of measurement of the outcome variable is required, and also of the exposure variable if it is continuous.
The examples are all drawn from recent articles in Archives of Public Health. They were chosen to represent a variety of types of tables seen in research publications.
Tables of characteristics
The table of characteristics in Table 1 is from a study assessing knowledge and practice in relation to tuberculosis control among in Ethiopian health workers [ 10 ]. The authors have presented the characteristics of the health workers who participated in the study. Summary statistics are based on categories of the characteristics, so numbers (frequencies) in each category and the percentages of the total study population within each category are presented for each characteristic. From this, the reader can see that:
the study population is quite young, as only around 10% are more than 40 years old;
the majority are female;
more than half are nurses;
about half were educated to degree level or above.
The table of characteristics in Table 2 is from a study of the relationship between distorted body image and lifestyle in adolescents in Japan [ 11 ]. Here the presentation is split into separate columns for boys and girls. The first four characteristics are continuous variables, not split into categories but, instead, presented as means, with the SDs given in brackets. The three characteristics in the lower part of the table are categorical variables and, similar to Table 1 , the frequency/numbers and percentages in each category are presented. The p -values indicate that boys and girls differ on some of the characteristics, notably height, self-perceived weight status and body image perception.
In Table 3 , considerable detail is given for continuous variables in the table. This comes from an article describing the relationship between mid-upper-arm circumference (MUAC) and weight changes in young children admitted to hospital with severe acute malnutrition from three countries [ 12 ]. For each country, the categorical characteristic of sex is presented as in the previous two examples, but more detail is given for the continuous variables of age, MUAC and height. The mean is provided as in Table 2 , though without a standard deviation, but we are also given the minimum value, the 25th percentile (labelled Q1 – for quartile 1), the median (the middle value), the 75th percentile (labelled Q2, here though correctly it should be Q3 – see above) and the maximum value. The table shows:
Ethiopian children in this study were older and taller than those from the other two countries but their MUAC measurements tended to be smaller;
in Bangladesh, disproportionally more females than males were admitted for treatment compared with the other two countries.
It is unusual to present as much detail on continuous characteristics as is given in Table 3 . Usually, for each characteristic, either (a) mean and SD or (b) median and IQR would be given, but not both.
Tables of results – summary findings
Many results tables are simple summaries and look similar to tables presenting characteristics, as described above. Sometimes the initial table of characteristics includes some basic comparisons that indicate the main results of the study. Table 4 shows part of a large table of characteristics for a study of risk factors for acute lower respiratory infections (ALRI) among young children in Rwanda [ 13 ]. In addition to presenting the numbers of children in each category of a variety of characteristics, it also shows the percentage in each category among those who suffered ALRI in the previous two weeks, and provides p- values for the differences between the categories among those who did and did not suffer from ALRI. Thus only 2.9% of older children (24–59 months) within the study suffered from ALRI, compared with about 5% in the two youngest categories. The p -value of 0.001, well below 0.05, indicates that this difference is statistically significant. The other finding of some interest is that children who took vitamin A supplements appeared to be less likely to suffer from ALRI than those who did not, but the p -value of 0.04 is close to 0.05 so not as remarkable a finding as for the difference between the age groups.
Table 5 shows a summary table of average life expectancy in British Columbia by socioeconomic status [ 14 ]. The average life expectancy at birth and the associated 95% CIs are given according to level of socio-economic status for the total population (column 1), followed by males and females separately. The study is large so the 95% CIs are quite narrow, and the table indicates that there are considerable differences in life expectancy between the three socioeconomic groups, with the lowest category having the poorest life expectancy. The gap in life expectancy between the lowest and highest category is more than three years, as shown in the final row.
Tables of results – continuous outcomes
Continuous outcome measures can be analysed in a variety of ways, depending on the purpose of the study and whether the measure of the exposure is continuous, categorical or binary.
Table 6 shows an example of correlation coefficients indicating the degree of association between the exposure of interest (cognitive test scores) and the outcome measure (academic performance) [ 15 ]. No confidence intervals are presented, but the results show that almost all the particular cognitive test scores are statistically significantly associated ( p -value < 0.05) with the two measures of academic performance. Note that this table is an example of where a footnote is used to give information about the p-values. Not surprisingly, all the correlations are positive; one would expect that as cognitive score increase so too would academic performance. The numbers labelled “N” give the number of children who contributed data to each correlation coefficient.
Table 7 is quite a complex table, but one that bears examination. It presents regression coefficients from an analysis of pregnancy exposure to nitrogen dioxide (NO 2 ) and birth weight of the baby in a large study of four areas in Norway; more than 17,000 women-baby pairs contributed to the complete crude analysis [ 16 ]. Regression coefficients are presented and labelled “Beta”, the usual name for such coefficients, though the Greek letter β, B or b are sometimes used. They are interpreted as follows: for one unit increase in the exposure variable then the outcome measure increases by the amount of the regression coefficient. Regression coefficients of zero indicate no association. In this table, the Beta in the top left of the table indicates that as NO 2 exposure of the mother increases by 1 unit (a ‘unit’ in this analysis is 10 μg/m 3 , see the footnote in the table, which gives the units of measurement used for the regression coefficients: grams per 10 μg/m 3 NO 2 ) then the birth weight of her baby decreases (because the Beta is negative) by 37.9 g. The 95% CI does not include zero and the p -value is small (<0.001) implying that the association is not due solely to chance.
However, reading across the columns of the table gives a different story. The successive sets of columns include adjustment for increasing numbers of factors that might affect the association. While model 1 still indicates a negative association between NO 2 and birth weight that is highly significant ( p < 0.001), models 2 and 3 do not. Inclusion of adjustment for parity or area and maternal weight has reduced the association such that the Betas have shrunk in magnitude to be closer to 0, with 95% CIs including 0 and p -values >0.05.
The table has multiple rows, with each one providing information on a different subset of the data, so the numbers in the analyses are all smaller than in the first row. The second row restricts the analysis to women who did not move address during pregnancy, an important consideration in estimating NO 2 exposure from home addresses. The third row restricts the analysis to those whose gestational age was based on the last menstrual period. These second two rows present ‘sensitivity analyses’, performed to check that the results were not due to potential biases resulting from women moving house or having uncertain gestational ages. The remaining rows in the table present stratified analyses, with results given for each category of various variables of interest, namely geographical area, maternal smoking, parity, baby’s sex, mother’s educational level and season of birth. Only one row of this table has a statistically significant result for models 2 and 3, namely babies born in spring, but this finding is not discussed in the paper. Note the gap in the table in the model 2 column as it is not possible to adjust for area (one of the adjustment factors in model 2) when the analysis is being presented for each area separately.
Tables of results – binary outcomes
Table 8 presents results from a study assessing whether children’s eating styles are associated with having a waist-hip ratio greater or equal to 0.5 (the latter being the outcome variable expressed in binary form – ≥0.5 versus <0.5) [ 17 ]. Results for boys and girls are presented separately, along with the number of children in each of the eating style categories. The main results are presented as crude and adjusted odds ratios (ORs). The adjusted ORs take account of age, exercise, skipping breakfast and having a snack after dinner, all of these being variables thought to affect the association between eating style and waist-hip ratio. Looking at the crude OR column, the value of 2.04 in the first row indicates that, among boys, those who report eating quickly have around twice the odds of having a high waist-hip ratio than those who do not eat quickly (not eating quickly is the baseline category, with an odds ratio given as 1.00). The 95% CI for the crude OR for eating quickly is 1.31 – 3.18. This interval does not include 1, indicating that the elevated OR for eating quickly is unlikely to be a chance finding and that there is a 95% probability that the range of 1.31 – 3.18 includes the true OR. The p -value is 0.002, considerably smaller than 0.05, indicating that this finding is ‘statistically significant’. The other ORs can be considered in the same way, but note that, for both boys and girls, the ORs for eating until full are greater than 1 but their 95% CIs include 1 and the p- values are considerably greater than 0.05, so not ‘statistically significant’, indicating chance findings.
The final columns present the ORs after adjustment for various additional factors, along with their 95% CIs and p -values. The ORs given here differ little from the crude ORs in the table, indicating that the adjustment has not had much effect, so the conclusions from examining the crude ORs are unaltered. It thus appears that eating quickly is strongly associated with a greater waist-hip ratio, but that eating until full is not.
Summary tables of characteristics describe the study population and set the study in context. The main findings can be presented in different ways and choice of presentation is determined by the nature of the variables under study. Scrutiny of tables allows the reader to acquire much more information about the study and a richer insight than if the text only is examined. Constructing clear tables that communicate the nature of the study population and the key results is important in the preparation of papers; good tables can assist the reader enormously as well as increasing the chance of the paper being published.
Abbreviations
Acute lower respiratory infections
Confidence interval
Mid-upper-arm circumference
- Inter-quartile range
Nitrogen dioxide
Quartile 1 (25th percentile)
Quartile 2 (50th percentile = median)
Quartile 3 (75th percentile)
- Standard deviation
Peacock J, Peacock P. Oxford Handbook of Medical Statistics. Oxford: Oxford University Press; 2010.
Book Google Scholar
Bland M. An Introduction to Medical Statistics. 4th ed. Oxford: Oxford University Press; 2015.
Google Scholar
Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed. Oxford: Wiley; 2003.
Altman DG. Practical statistics for medical research. London: Chapman & Hall; 1991.
Everitt BS, Palmer C. Encyclopaedic Companion to Medical Statistics. 2nd ed. Chichester: Wiley; 2010.
Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4th ed. Oxford: Wiley; 2001.
Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. SWS Study Group: Cohort profile: The Southampton Women's Survey. Int J Epidemiol. 2006;35:42–8.
Article PubMed Google Scholar
Sterne JA, Davey Smith G. Sifting the evidence-what's wrong with significance tests? BMJ. 2001;322:226–31.
Article CAS PubMed PubMed Central Google Scholar
Davies HTO, Crombie IK. What are confidence intervals and p-values? http://www.bandolier.org.uk/painres/download/whatis/What_are_Conf_Inter.pdf. or https://grunigen.lib.uci.edu/sites/all/docs/gml/what_are_conf_inter.pdf . Accessed 27 Jan 2017.
Demissie Gizaw G, Aderaw Alemu Z, Kibret KT. Assessment of knowledge and practice of health workers towards tuberculosis infection control and associated factors in public health facilities of Addis Ababa, Ethiopia: A cross-sectional study. Arch Public Health. 2015;73:1–9.
Article Google Scholar
Shirasawa T, Ochiai H, Nanri H, Nishimura R, Ohtsu T, Hoshino H, Tajima N, Kokaze A. The relationship between distorted body image and lifestyle among Japanese adolescents: a population-based study. Arch Public Health. 2015;73:1–7.
Binns P, Dale N, Hoq M, Banda C, Myatt M. Relationship between mid upper arm circumference and weight changes in children aged 6–59 months. Arch Public Health. 2015;73:1–10.
Harerimana J-M, Nyirazinyoye L, Thomson DR, Ntaganira J. Social, economic and environmental risk factors for acute lower respiratory infections among children under five years of age in Rwanda. Arch Public Health. 2016;74:1–7.
Zhang LR, Rasali D. Life expectancy ranking of Canadians among the populations in selected OECD countries and its disparities among British Columbians. Arch Public Health. 2015;73:1–10.
Haile D, Nigatu D, Gashaw K, Demelash H. Height for age z score and cognitive function are associated with Academic performance among school children aged 8–11 years old. Arch Public Health. 2016;74:1–7.
Panasevich S, Håberg SE, Aamodt G, London SJ, Stigum H, Nystad W, Nafstad P. Association between pregnancy exposure to air pollution and birth weight in selected areas of Norway. Arch Public Health. 2016;74:1–9.
Ochiai H, Shirasawa T, Nanri H, Nishimura R, Matoba M, Hoshino H, Kokaze A. Eating quickly is associated with waist-to-height ratio among Japanese adolescents: a cross-sectional survey. Arch Public Health. 2016;74:1–7.
Download references
Acknowledgement
Not applicable.
The work was funded by the UK Medical Research Council which funds the work of the MRC Lifecourse Epidemiology Unit where the authors work. The funding body had no role in the design and conduct of the work, or in the writing the manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Authors’ contributions
HI conceived the idea for the paper in discussion with JB. HI wrote the first draft and all other authors commented on successive versions and contributed ideas to improve content, clarity and flow of the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Ethics approval and consent to participate, author information, authors and affiliations.
MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, SO16 6YD, UK
Hazel Inskip, Georgia Ntani, Leo Westbury, Chiara Di Gravio, Stefania D’Angelo, Camille Parsons & Janis Baird
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hazel Inskip .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Inskip, H., Ntani, G., Westbury, L. et al. Getting started with tables. Arch Public Health 75 , 14 (2017). https://doi.org/10.1186/s13690-017-0180-1
Download citation
Received : 04 December 2016
Accepted : 20 January 2017
Published : 20 March 2017
DOI : https://doi.org/10.1186/s13690-017-0180-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Characteristics
- Regression coefficients
- Correlation coefficients
- Relative measures
Archives of Public Health
ISSN: 2049-3258
- General enquiries: [email protected]
Who is in this study, anyway? Guidelines for a useful Table 1
Affiliations.
- 1 Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY, USA. Electronic address: [email protected].
- 2 Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY, USA.
- 3 Harborview Injury Prevention & Research Center, University of Washington, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
- 4 Department of Epidemiology and Biostatistics, Drexel University Dornsife School of Public Health, Philadelphia, PA, USA.
- PMID: 31229583
- PMCID: PMC6773463
- DOI: 10.1016/j.jclinepi.2019.06.011
Objective: Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this "Table 1" can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses. We aimed to summarize and extend the literature related to reporting descriptive statistics.
Study design and setting: In consultation with existing guidelines, we synthesized and developed reporting recommendations driven by study design and focused on transparency related to potential threats to internal and external validity.
Results: We describe a basic structure for Table 1 and discuss simple modifications in terms of columns, rows, and cells to enhance a reader's ability to judge both internal and external validity. We further highlight several analytic complexities common in epidemiologic research (missing data, sample weights, clustered data, and interaction) and describe possible variations to Table 1 to maintain and add clarity about study validity in light of these issues. We discuss considerations and tradeoffs in Table 1 related to breadth and comprehensiveness vs. parsimony and reader-friendliness.
Conclusion: We anticipate that our work will guide authors considering layouts for Table 1, with attention to the reader's perspective.
Keywords: Clinical research; Descriptive statistics; Epidemiologic methods; External validity; Generalizability; Internal validity; Tables.
Copyright © 2019 Elsevier Inc. All rights reserved.
Publication types
- Research Support, N.I.H., Extramural
- Data Analysis*
- Documentation / methods
- Documentation / standards*
- Epidemiologic Research Design
- Guidelines as Topic*
- Publishing / standards*
- Reproducibility of Results
- Research Design
Grants and funding
- T32 AI114398/AI/NIAID NIH HHS/United States
- T32 HD057822/HD/NICHD NIH HHS/United States
- R01 AG049970/AG/NIA NIH HHS/United States
- T32 ES023772/ES/NIEHS NIH HHS/United States
- K99 LM012868/LM/NLM NIH HHS/United States
- T32 MH013043/MH/NIMH NIH HHS/United States

Effective Use of Tables and Figures in Research Papers
Research papers are often based on copious amounts of data that can be summarized and easily read through tables and graphs. When writing a research paper , it is important for data to be presented to the reader in a visually appealing way. The data in figures and tables, however, should not be a repetition of the data found in the text. There are many ways of presenting data in tables and figures, governed by a few simple rules. An APA research paper and MLA research paper both require tables and figures, but the rules around them are different. When writing a research paper, the importance of tables and figures cannot be underestimated. How do you know if you need a table or figure? The rule of thumb is that if you cannot present your data in one or two sentences, then you need a table .
Using Tables
Tables are easily created using programs such as Excel. Tables and figures in scientific papers are wonderful ways of presenting data. Effective data presentation in research papers requires understanding your reader and the elements that comprise a table. Tables have several elements, including the legend, column titles, and body. As with academic writing, it is also just as important to structure tables so that readers can easily understand them. Tables that are disorganized or otherwise confusing will make the reader lose interest in your work.
- Title: Tables should have a clear, descriptive title, which functions as the “topic sentence” of the table. The titles can be lengthy or short, depending on the discipline.
- Column Titles: The goal of these title headings is to simplify the table. The reader’s attention moves from the title to the column title sequentially. A good set of column titles will allow the reader to quickly grasp what the table is about.
- Table Body: This is the main area of the table where numerical or textual data is located. Construct your table so that elements read from up to down, and not across.
Related: Done organizing your research data effectively in tables? Check out this post on tips for citing tables in your manuscript now!
The placement of figures and tables should be at the center of the page. It should be properly referenced and ordered in the number that it appears in the text. In addition, tables should be set apart from the text. Text wrapping should not be used. Sometimes, tables and figures are presented after the references in selected journals.
Using Figures
Figures can take many forms, such as bar graphs, frequency histograms, scatterplots, drawings, maps, etc. When using figures in a research paper, always think of your reader. What is the easiest figure for your reader to understand? How can you present the data in the simplest and most effective way? For instance, a photograph may be the best choice if you want your reader to understand spatial relationships.
- Figure Captions: Figures should be numbered and have descriptive titles or captions. The captions should be succinct enough to understand at the first glance. Captions are placed under the figure and are left justified.
- Image: Choose an image that is simple and easily understandable. Consider the size, resolution, and the image’s overall visual attractiveness.
- Additional Information: Illustrations in manuscripts are numbered separately from tables. Include any information that the reader needs to understand your figure, such as legends.
Common Errors in Research Papers
Effective data presentation in research papers requires understanding the common errors that make data presentation ineffective. These common mistakes include using the wrong type of figure for the data. For instance, using a scatterplot instead of a bar graph for showing levels of hydration is a mistake. Another common mistake is that some authors tend to italicize the table number. Remember, only the table title should be italicized . Another common mistake is failing to attribute the table. If the table/figure is from another source, simply put “ Note. Adapted from…” underneath the table. This should help avoid any issues with plagiarism.
Using tables and figures in research papers is essential for the paper’s readability. The reader is given a chance to understand data through visual content. When writing a research paper, these elements should be considered as part of good research writing. APA research papers, MLA research papers, and other manuscripts require visual content if the data is too complex or voluminous. The importance of tables and graphs is underscored by the main purpose of writing, and that is to be understood.
Frequently Asked Questions
"Consider the following points when creating figures for research papers: Determine purpose: Clarify the message or information to be conveyed. Choose figure type: Select the appropriate type for data representation. Prepare and organize data: Collect and arrange accurate and relevant data. Select software: Use suitable software for figure creation and editing. Design figure: Focus on clarity, labeling, and visual elements. Create the figure: Plot data or generate the figure using the chosen software. Label and annotate: Clearly identify and explain all elements in the figure. Review and revise: Verify accuracy, coherence, and alignment with the paper. Format and export: Adjust format to meet publication guidelines and export as suitable file."
"To create tables for a research paper, follow these steps: 1) Determine the purpose and information to be conveyed. 2) Plan the layout, including rows, columns, and headings. 3) Use spreadsheet software like Excel to design and format the table. 4) Input accurate data into cells, aligning it logically. 5) Include column and row headers for context. 6) Format the table for readability using consistent styles. 7) Add a descriptive title and caption to summarize and provide context. 8) Number and reference the table in the paper. 9) Review and revise for accuracy and clarity before finalizing."
"Including figures in a research paper enhances clarity and visual appeal. Follow these steps: Determine the need for figures based on data trends or to explain complex processes. Choose the right type of figure, such as graphs, charts, or images, to convey your message effectively. Create or obtain the figure, properly citing the source if needed. Number and caption each figure, providing concise and informative descriptions. Place figures logically in the paper and reference them in the text. Format and label figures clearly for better understanding. Provide detailed figure captions to aid comprehension. Cite the source for non-original figures or images. Review and revise figures for accuracy and consistency."
"Research papers use various types of tables to present data: Descriptive tables: Summarize main data characteristics, often presenting demographic information. Frequency tables: Display distribution of categorical variables, showing counts or percentages in different categories. Cross-tabulation tables: Explore relationships between categorical variables by presenting joint frequencies or percentages. Summary statistics tables: Present key statistics (mean, standard deviation, etc.) for numerical variables. Comparative tables: Compare different groups or conditions, displaying key statistics side by side. Correlation or regression tables: Display results of statistical analyses, such as coefficients and p-values. Longitudinal or time-series tables: Show data collected over multiple time points with columns for periods and rows for variables/subjects. Data matrix tables: Present raw data or matrices, common in experimental psychology or biology. Label tables clearly, include titles, and use footnotes or captions for explanations."
Enago is a very useful site. It covers nearly all topics of research writing and publishing in a simple, clear, attractive way. Though I’m a journal editor having much knowledge and training in these issues, I always find something new in this site. Thank you
“Thank You, your contents really help me :)”
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- Reporting Research
Explanatory & Response Variable in Statistics — A quick guide for early career researchers!
Often researchers have a difficult time choosing the parameters and variables (like explanatory and response…

- Manuscript Preparation
- Publishing Research
How to Use Creative Data Visualization Techniques for Easy Comprehension of Qualitative Research
“A picture is worth a thousand words!”—an adage used so often stands true even whilst…

- Figures & Tables
Effective Use of Statistics in Research – Methods and Tools for Data Analysis
Remember that impending feeling you get when you are asked to analyze your data! Now…
- Old Webinars
- Webinar Mobile App
SCI中稿技巧: 提升研究数据的说服力
如何寻找原创研究课题 快速定位目标文献的有效搜索策略 如何根据期刊指南准备手稿的对应部分 论文手稿语言润色实用技巧分享,快速提高论文质量

Distill: A Journal With Interactive Images for Machine Learning Research
Research is a wide and extensive field of study. This field has welcomed a plethora…
Explanatory & Response Variable in Statistics — A quick guide for early career…
How to Create and Use Gantt Charts

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

What should universities' stance be on AI tools in research and academic writing?
- Writing Tips
Creating tables in scientific papers: row and column titles, units, error values and sample sizes
This is the second post in our series about creating and editing scientific tables. In the first post , we saw how basic table formatting and effective table titles could be used to improve an example of a poorly constructed table.
This post will deal with table row and column titles, units, error values and sample sizes. Let’s continue with the example table that we began to improve in the first post .
Fig. 1: Improved table after placing values in individual cells, formatting and double spacing, and adding an informative title.
Rule 4. Use short, descriptive row and column titles
The title of Table 1 (above) indicates the data in the table is about wheat plants exposed to salinity. Unfortunately, the row titles do not provide any useful information, except to show there were two groups in the experiment (control and test).
If this table was in a scientific paper, you could read the materials and methods section to find out how the control group and test groups were treated. However, every table should be understandable on its own, without having to look at other parts of the paper.
Therefore, the row titles in Table 1 should be the concentration of salt used in each group, perhaps Control (0 mM NaCl) and 50 mM NaCl (instead of control and test).
The column titles (light, 5 days and 10 days) in Table 1 are quite obvious: the researcher probably exposed the wheat plants to different periods of light each day, and then measured plant height after 5 and 10 days.
However, it is important to remember that simple titles such as “light” may be easily misunderstood by someone who is not familiar with your research.
Table 1 could be improved if the row titles provided a little more information, perhaps “Light exposure per day (hours)” or “Light/day (h)” instead of “light”. Similarly, “5 days exposure” and 10 days exposure” would be better than “5 days” and “10 days”.
If you need to use long or complicated titles that don’t easily fit in the column or row titles (for example, non-small cell lung carcinoma) then it is fine to use abbreviations (NSCLC), as long as you remember to define them in the table footnote.
Again, this makes the table easier to read and prevents your reader from having to look through the paper for the definitions for each abbreviation.
Rule 5. Always include the units, error values and number of samples
Although we have improved the content of Table 1 by changing the row and column titles, some very important information is still missing.
You could probably guess that the height of wheat plants is measured in centimeters, and the light exposure per day was measured in hours.
However, you may not be able to guess the correct units in every table (and your reader should never have to guess!!), so the units should be included in every table.
Additionally, it is not known what the numbers placed after the “±” represent in Table 1, as they could be the standard deviation or standard error of the mean. Therefore, every table should include the units (for example cm and hours) and define the error values (for example mean ± S.E.M.).
It is also important to show how many samples (or patients, cultivars, replicates) were in each group, especially if the sample sizes varied. You can choose where to include the units, error values and sample sizes, depending on the layout and information in your table.
For example, the units can be placed after every value, placed in a new row at the top of the table along with the type measurement as shown in Fig. 2 below or placed in a footnote, for example: “Values are mean centimeters ± SEM; ( n = 5 per group).”
Fig. 2: Examples of different ways to include the units, error values and sample size information in a scientific table.
The table is improved by including more information in the row and column titles (rule 4), and defining units, error values and sample sizes (rule 5).
However, there is still some information missing and a few minor mistakes. Can you see any?
In the final post of this series, I will discuss the final pieces of information that should be included in every scientific table.

At Science Editing Experts, we help scientists like you to submit well-written journal papers with confidence and complete your thesis without headaches, so you can focus on your research and career.
Andrea Devlin PhD
Chief editor and owner of Science Editing Experts
Wish scientific writing was less stressful?
Grab my free checklist to significantly improve your paper or thesis!

A small favour: If you liked this post, please share it?
I built this blog page by page and will continue to do so.
Thank you for your support, dear reader!
A share from you will really help this blog to grow and won't take more than 10 seconds of your time.
Simply share this post to your favourite social media profile.

GDPR/Privacy
Website Terms
Course/Coaching Terms
2 Drumgrannon Heights, Moy, Northern Ireland, BT71 7TW, UK.
© Science EditingExperts 2022-2024. All Rights Reserved.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
The instructions provided here pertain to tables included in the main article. The more closely your tables adhere to these requirements at submission, the fewer times you will need to revise your manuscript to meet them. Your tables will therefore publish more accurately and will be less likely to slow down publication of your accepted manuscript.
Formatting Rules and Constraints
Tables must be editable, cell-based objects. We cannot typeset tables that are graphic objects. Use the table tool in your text editing software to create tables. If necessary, create tables in Excel and insert them into the manuscript. Do not insert text boxes or graphics within your tables.
- Text justification. Justification of the text within cells is honored at typesetting. If you have a preference, indicate justification of content within cells by using your text editor’s alignment features (for example, centered or left justified).
- Merged cells. You can use merging to indicate cells that span multiple columns and rows.
- Multi-section tables. Tables with multiple sections must have a consistent number of columns throughout all sub-sections. If necessary, you may divide and re-number the sections as separate tables. Do not insert tables within tables or cells within cells.
- Text color and formatting. Text color is limited to black. Bolded, italicized, underlined, superscript, subscript, and strikethrough text is OK. Meaning can also be expressed with symbols that are explained in the footnotes. Text in header rows will be automatically formatted in bold type.
- Text Font. Use a standard font size and any standard font, except for the font named “Symbol”. To add symbols to the manuscript, use the Insert → Symbol function in your word processor or paste in the appropriate Unicode character.
- Cell shading. Background color can be applied to cells to convey meaningful information. Read the instructions for applying cell shading .
- Size. Tables do not have strict width and height requirements. Do not split your table or otherwise try to make the table appear within the manuscript margins if it does not fit on one page. In Word, tables that run off of the manuscript page can be seen using Draft View. In the PDF version of the published article, very wide tables may be printed sideways, and long tables may span more than one page.
Arrangement of cell content
Use the following rules to arrange content within individual cells and to match alignment across multiple rows and columns.
To view the marks for returns, spaces, and indents in Microsoft Word, click the ¶ (paragraph) button in the toolbar. These paragraph marks are normally hidden from the document, unless you turn on the tools to make them visible. Use the key to understand the paragraph marks shown in the examples on this page.
Arranging content within a cell
- Use only separate cells, ordered lists, unordered lists, or returns to separate content onto individual lines.
- Use a single tab to indent individual pieces of content.
- Do not use spaces to create a new line, indent, or justify content.
Aligning content across rows and columns
- Enter content in separate cells to match the text alignment used in other rows and columns.
- Do not use returns, spaces, or tabs to align content across the table. Alignment set in this manner may not be preserved in the published version of the article.
Cell shading
Color can only be applied to the cell background.
- You may use any shading color, but keep legibility in mind. The Web Content Accessibility Guidelines (WCAG) advise a contrast ratio of 4.5:1 between the text and the background color. PLOS requires the text color to be black.
- Use only solid colors, with no patterns or gradients.
- Lighter shades are recommended in order to clearly contrast against the black text.
- If using more than one shading color, avoid combinations that could make color differentiation difficult for people with colorblindness.
Footnotes referencing background color should describe it using words, not images or colored text. Example: “Comparisons involving any renin-angiotensin receptor blocker are shaded orange.”
Heavy gridlines
You can apply a heavy gridline to individual cells or to entire rows or columns. The line will appear 3 times heavier than a normal gridline.
To apply a heavy gridline, use the Borders and Shading options in your text editor to apply a line weight of 3 pt (point) to the appropriate border.
Note: It is OK if the surrounding normal-weight gridlines appear as dotted lines once this action is performed (as in the example below). The normal-weight gridlines will still be applied to those cells during typesetting.
How to Submit
Please refer to our downloadable sample manuscript (PDF) to ensure that your table captions, citations, and organization in the manuscript meet our formatting requirements.
Correctly formatted table with caption
If you have questions about how to format and submit your tables, contact [email protected] .
Generate accurate APA citations for free
- Knowledge Base
- APA Style 7th edition
- How to format tables and figures in APA Style
APA Format for Tables and Figures | Annotated Examples
Published on November 5, 2020 by Jack Caulfield . Revised on January 17, 2024.
A table concisely presents information (often numbers) in rows and columns. A figure is any other image or illustration you include in your text—anything from a bar chart to a photograph.
Tables and figures differ in terms of how they convey information, but APA Style presents them in a similar format—preceded by a number and title, and followed by explanatory notes (if necessary).
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
Apa table format, apa figure format, numbering and titling tables and figures, formatting table and figure notes, where to place tables and figures, referring to tables and figures in the text, frequently asked questions about apa tables and figures.
Tables will vary in size and structure depending on the data you’re presenting, but APA gives some general guidelines for their design. To correctly format an APA table, follow these rules:
- Table number in bold above the table.
- Brief title, in italics and title case, below the table number.
- No vertical lines.
- Horizontal lines only where necessary for clarity.
- Clear, concise labels for column and row headings.
- Numbers consistently formatted (e.g. with the same number of decimal places).
- Any relevant notes below the table.
An example of a table formatted according to APA guidelines is shown below.
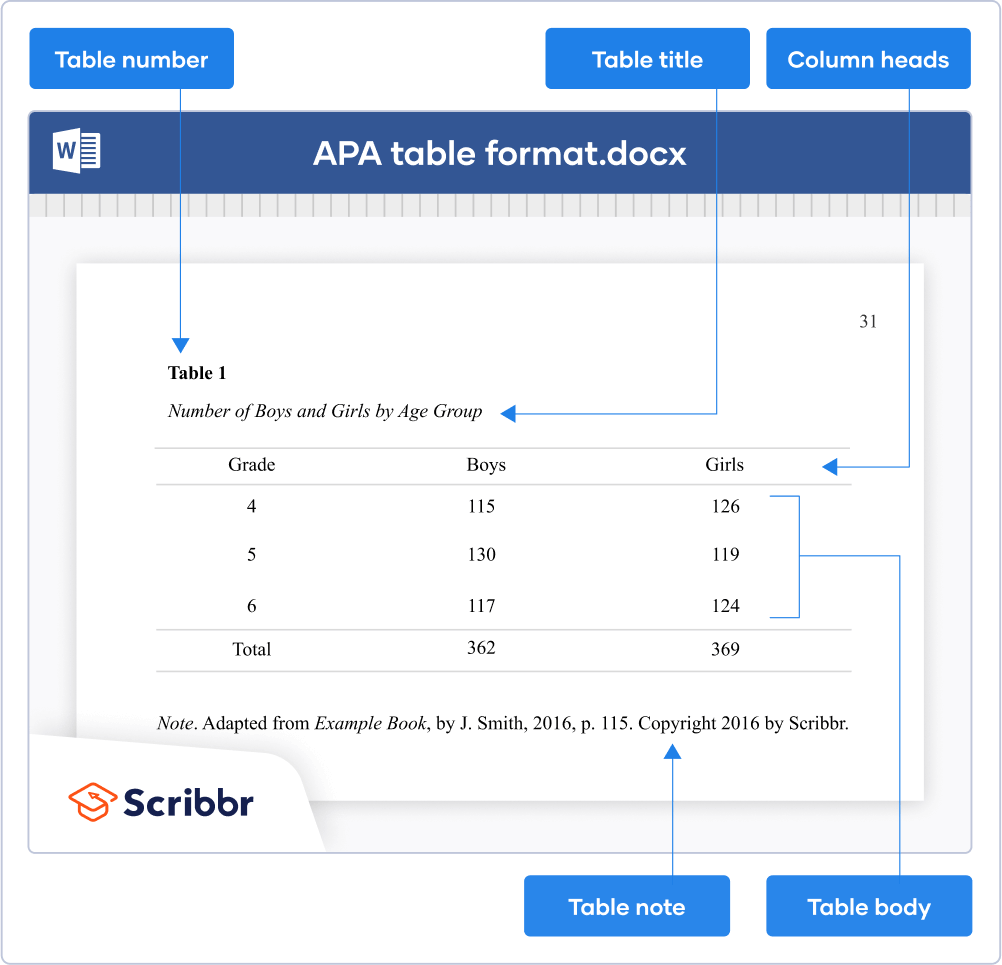
The table above uses only four lines: Those at the top and bottom, and those separating the main data from the column heads and the totals.
Create your tables using the tools built into your word processor. In Word, you can use the “ Insert table ” tool.
Prevent plagiarism. Run a free check.
Any images used within your text are called figures. Figures include data visualization graphics—e.g. graphs, diagrams, flowcharts—as well as things like photographs and artworks.
To correctly format an APA figure, follow these rules:
- Figure number in bold above the figure.
- Brief title, in italics and title case, under the figure number.
- If necessary, clear labels and legends integrated into the image.
- Any relevant notes below the figure.
An example of a figure formatted according to APA guidelines is shown below.
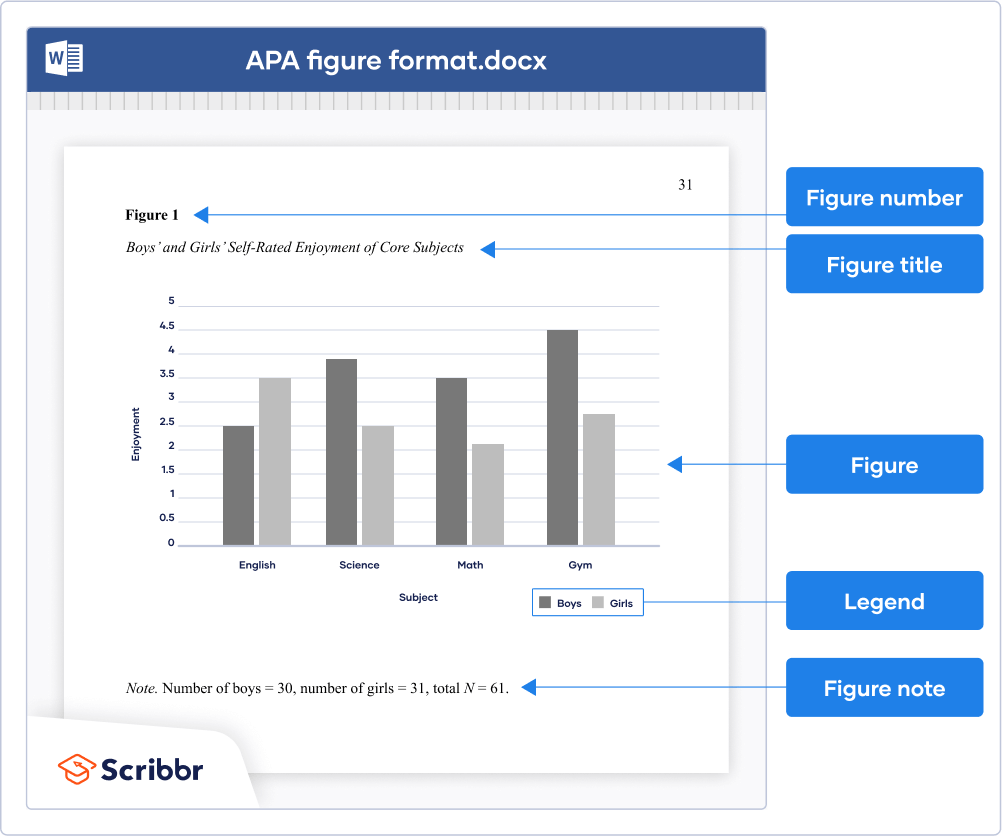
Keep the design of figures as simple as possible. Use colors only where necessary, not just to make the image look more appealing.
For text within the image itself, APA recommends using a sans serif font (e.g. Arial) with a size between 8 and 14 points.
For other figures, such as photographs, you won’t need a legend; the figure consists simply of the image itself, reproduced at an appropriate size and resolution.
Each table or figure is preceded by a number and title.
Tables and figures are each numbered separately, in the order they are referred to in your text. For example, the first table you refer to is Table 1; the fourth figure you refer to is Figure 4.
The title should clearly and straightforwardly describe the content of the table or figure. Omit articles to keep it concise.
The table or figure number appears on its own line, in bold, followed by the title on the following line, in italics and title case.
Where a table or figure needs further explanation, notes should be included immediately after it. These are not your analysis of the data presented; save that for the main text.
There are three kinds of notes: general , specific , and probability . Each type of note appears in a new paragraph, but multiple notes of the same kind all appear in one paragraph.
Only include the notes that are needed to understand the table or figure. It may be that it is clear in itself, and has no notes, or only probability notes; be as concise as possible.
General notes
General notes come first. They are preceded by the word “ Note ” in italics, followed by a period. They include any explanations that apply to the table or figure as a whole and a citation if it was adapted from another source, and they end with definitions of any abbreviations used.

Specific notes
Specific notes refer to specific points in the table or figure. Superscript letters (a, b, c …) appear at the relevant points in the table or figure and at the start of each note to indicate what they refer to. They are used when it’s necessary to comment on a specific data point or term.
Probability notes
Probability notes give p -values for the data in the table or figure. They correspond to asterisks (and/or other symbols) in the table or figure.
You have two options for the placement of tables and figures in APA Style:
- Option 1: Place tables and figures throughout your text, shortly after the parts of the text that refer to them.
- Option 2: Place them all together at the end of your text (after the reference list) to avoid breaking up the text.
If you place them throughout the text, note that each table or figure should only appear once. If you refer to the same table or figure more than once, don’t reproduce it each time—just place it after the paragraph in which it’s first discussed.
Align the table or figure with the text along the left margin. Leave a line break before and after the table or figure to clearly distinguish it from the main text, and place it on a new page if necessary to avoid splitting it across multiple pages.
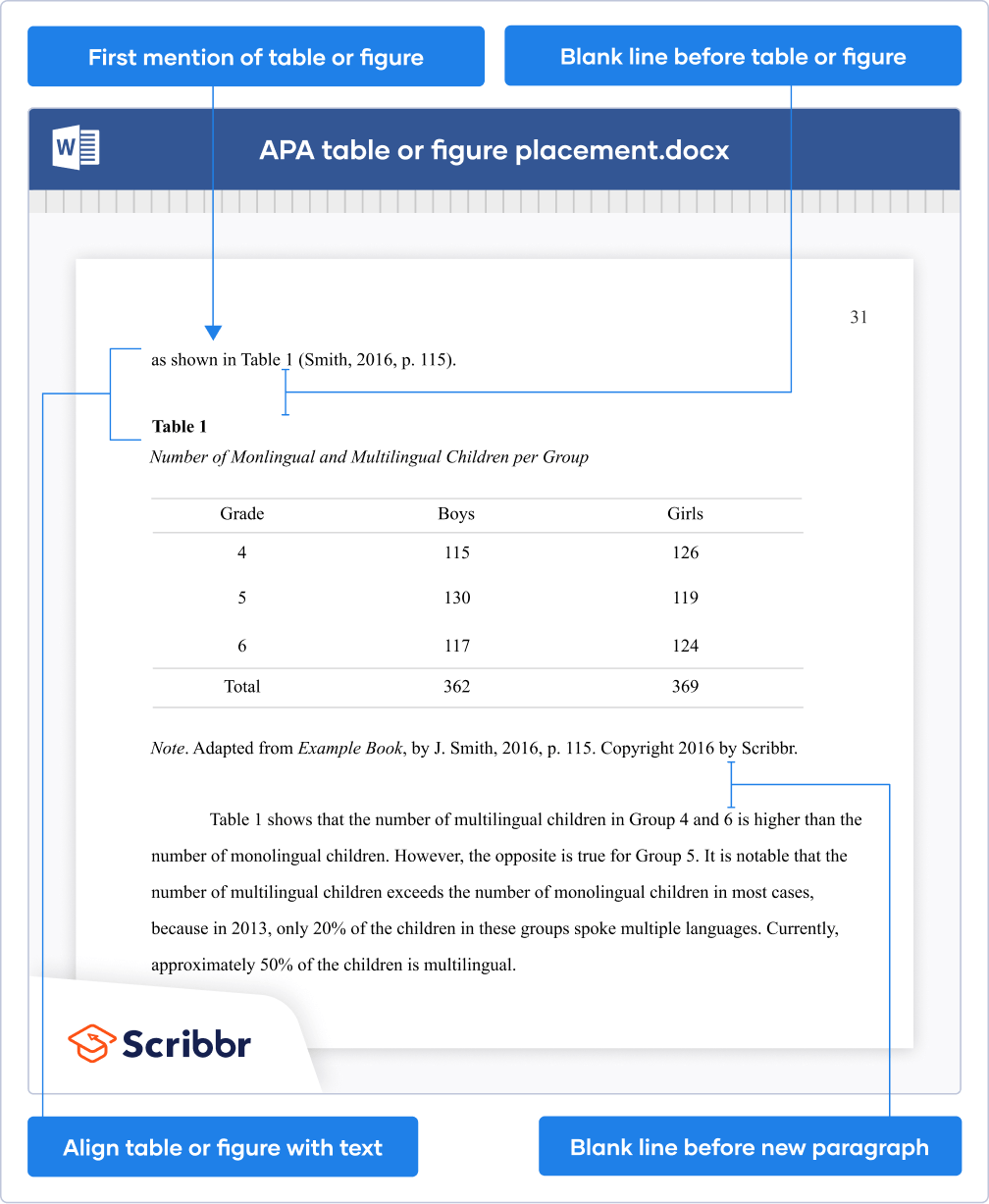
If you place all your tables and figures at the end, you should have one table or figure on each page. Begin with all your tables, then place all your figures afterwards.
Avoid making redundant statements about your tables and figures in your text. When you write about data from tables and figures, it should be to highlight or analyze a particular data point or trend, not simply to restate what is already clearly shown in the table or figure:
- As Table 1 shows, there are 115 boys in Grade 4, 130 in Grade 5, and 117 in Grade 6 …
- Table 1 indicates a notable preponderance of boys in Grade 5. It is important to take this into account because …
Additionally, even if you have embedded your tables and figures in your text, refer to them by their numbers, not by their position relative to the text or by description:
- The table below shows…
- Table 1 shows…
- As can be seen in the image on page 4…
- As can be seen in Figure 3…
- The photograph of a bald eagle is an example of…
- Figure 1 is an example of…
In an APA Style paper , use a table or figure when it’s a clearer way to present important data than describing it in your main text. This is often the case when you need to communicate a large amount of information.
Before including a table or figure in your text, always reflect on whether it’s useful to your readers’ understanding:
- Could this information be quickly summarized in the text instead?
- Is it important to your arguments?
- Does the table or figure require too much explanation to be efficient?
If the data you need to present only contains a few relevant numbers, try summarizing it in the text (potentially including full data in an appendix ). If describing the data makes your text overly long and difficult to read, a table or figure may be the best option.
APA doesn’t require you to include a list of tables or a list of figures . However, it is advisable to do so if your text is long enough to feature a table of contents and it includes a lot of tables and/or figures.
A list of tables and list of figures appear (in that order) after your table of contents , and are presented in a similar way.
If you adapt or reproduce a table or figure from another source, you should include that source in your APA reference list . You should also acknowledge the original source in the note or caption for the table or figure.
Tables and figures you created yourself, based on your own data, are not included in the reference list.
In most styles, the title page is used purely to provide information and doesn’t include any images. Ask your supervisor if you are allowed to include an image on the title page before doing so. If you do decide to include one, make sure to check whether you need permission from the creator of the image.
Include a note directly beneath the image acknowledging where it comes from, beginning with the word “ Note .” (italicized and followed by a period). Include a citation and copyright attribution . Don’t title, number, or label the image as a figure , since it doesn’t appear in your main text.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2024, January 17). APA Format for Tables and Figures | Annotated Examples. Scribbr. Retrieved April 15, 2024, from https://www.scribbr.com/apa-style/tables-and-figures/
Is this article helpful?

Jack Caulfield
Other students also liked, citing tables and figures from other sources in apa style, how to cite an image in apa style, how to write an apa results section, unlimited academic ai-proofreading.
✔ Document error-free in 5minutes ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Korean Med Sci
- v.37(16); 2022 Apr 25

A Practical Guide to Writing Quantitative and Qualitative Research Questions and Hypotheses in Scholarly Articles
Edward barroga.
1 Department of General Education, Graduate School of Nursing Science, St. Luke’s International University, Tokyo, Japan.
Glafera Janet Matanguihan
2 Department of Biological Sciences, Messiah University, Mechanicsburg, PA, USA.
The development of research questions and the subsequent hypotheses are prerequisites to defining the main research purpose and specific objectives of a study. Consequently, these objectives determine the study design and research outcome. The development of research questions is a process based on knowledge of current trends, cutting-edge studies, and technological advances in the research field. Excellent research questions are focused and require a comprehensive literature search and in-depth understanding of the problem being investigated. Initially, research questions may be written as descriptive questions which could be developed into inferential questions. These questions must be specific and concise to provide a clear foundation for developing hypotheses. Hypotheses are more formal predictions about the research outcomes. These specify the possible results that may or may not be expected regarding the relationship between groups. Thus, research questions and hypotheses clarify the main purpose and specific objectives of the study, which in turn dictate the design of the study, its direction, and outcome. Studies developed from good research questions and hypotheses will have trustworthy outcomes with wide-ranging social and health implications.
INTRODUCTION
Scientific research is usually initiated by posing evidenced-based research questions which are then explicitly restated as hypotheses. 1 , 2 The hypotheses provide directions to guide the study, solutions, explanations, and expected results. 3 , 4 Both research questions and hypotheses are essentially formulated based on conventional theories and real-world processes, which allow the inception of novel studies and the ethical testing of ideas. 5 , 6
It is crucial to have knowledge of both quantitative and qualitative research 2 as both types of research involve writing research questions and hypotheses. 7 However, these crucial elements of research are sometimes overlooked; if not overlooked, then framed without the forethought and meticulous attention it needs. Planning and careful consideration are needed when developing quantitative or qualitative research, particularly when conceptualizing research questions and hypotheses. 4
There is a continuing need to support researchers in the creation of innovative research questions and hypotheses, as well as for journal articles that carefully review these elements. 1 When research questions and hypotheses are not carefully thought of, unethical studies and poor outcomes usually ensue. Carefully formulated research questions and hypotheses define well-founded objectives, which in turn determine the appropriate design, course, and outcome of the study. This article then aims to discuss in detail the various aspects of crafting research questions and hypotheses, with the goal of guiding researchers as they develop their own. Examples from the authors and peer-reviewed scientific articles in the healthcare field are provided to illustrate key points.
DEFINITIONS AND RELATIONSHIP OF RESEARCH QUESTIONS AND HYPOTHESES
A research question is what a study aims to answer after data analysis and interpretation. The answer is written in length in the discussion section of the paper. Thus, the research question gives a preview of the different parts and variables of the study meant to address the problem posed in the research question. 1 An excellent research question clarifies the research writing while facilitating understanding of the research topic, objective, scope, and limitations of the study. 5
On the other hand, a research hypothesis is an educated statement of an expected outcome. This statement is based on background research and current knowledge. 8 , 9 The research hypothesis makes a specific prediction about a new phenomenon 10 or a formal statement on the expected relationship between an independent variable and a dependent variable. 3 , 11 It provides a tentative answer to the research question to be tested or explored. 4
Hypotheses employ reasoning to predict a theory-based outcome. 10 These can also be developed from theories by focusing on components of theories that have not yet been observed. 10 The validity of hypotheses is often based on the testability of the prediction made in a reproducible experiment. 8
Conversely, hypotheses can also be rephrased as research questions. Several hypotheses based on existing theories and knowledge may be needed to answer a research question. Developing ethical research questions and hypotheses creates a research design that has logical relationships among variables. These relationships serve as a solid foundation for the conduct of the study. 4 , 11 Haphazardly constructed research questions can result in poorly formulated hypotheses and improper study designs, leading to unreliable results. Thus, the formulations of relevant research questions and verifiable hypotheses are crucial when beginning research. 12
CHARACTERISTICS OF GOOD RESEARCH QUESTIONS AND HYPOTHESES
Excellent research questions are specific and focused. These integrate collective data and observations to confirm or refute the subsequent hypotheses. Well-constructed hypotheses are based on previous reports and verify the research context. These are realistic, in-depth, sufficiently complex, and reproducible. More importantly, these hypotheses can be addressed and tested. 13
There are several characteristics of well-developed hypotheses. Good hypotheses are 1) empirically testable 7 , 10 , 11 , 13 ; 2) backed by preliminary evidence 9 ; 3) testable by ethical research 7 , 9 ; 4) based on original ideas 9 ; 5) have evidenced-based logical reasoning 10 ; and 6) can be predicted. 11 Good hypotheses can infer ethical and positive implications, indicating the presence of a relationship or effect relevant to the research theme. 7 , 11 These are initially developed from a general theory and branch into specific hypotheses by deductive reasoning. In the absence of a theory to base the hypotheses, inductive reasoning based on specific observations or findings form more general hypotheses. 10
TYPES OF RESEARCH QUESTIONS AND HYPOTHESES
Research questions and hypotheses are developed according to the type of research, which can be broadly classified into quantitative and qualitative research. We provide a summary of the types of research questions and hypotheses under quantitative and qualitative research categories in Table 1 .
Research questions in quantitative research
In quantitative research, research questions inquire about the relationships among variables being investigated and are usually framed at the start of the study. These are precise and typically linked to the subject population, dependent and independent variables, and research design. 1 Research questions may also attempt to describe the behavior of a population in relation to one or more variables, or describe the characteristics of variables to be measured ( descriptive research questions ). 1 , 5 , 14 These questions may also aim to discover differences between groups within the context of an outcome variable ( comparative research questions ), 1 , 5 , 14 or elucidate trends and interactions among variables ( relationship research questions ). 1 , 5 We provide examples of descriptive, comparative, and relationship research questions in quantitative research in Table 2 .
Hypotheses in quantitative research
In quantitative research, hypotheses predict the expected relationships among variables. 15 Relationships among variables that can be predicted include 1) between a single dependent variable and a single independent variable ( simple hypothesis ) or 2) between two or more independent and dependent variables ( complex hypothesis ). 4 , 11 Hypotheses may also specify the expected direction to be followed and imply an intellectual commitment to a particular outcome ( directional hypothesis ) 4 . On the other hand, hypotheses may not predict the exact direction and are used in the absence of a theory, or when findings contradict previous studies ( non-directional hypothesis ). 4 In addition, hypotheses can 1) define interdependency between variables ( associative hypothesis ), 4 2) propose an effect on the dependent variable from manipulation of the independent variable ( causal hypothesis ), 4 3) state a negative relationship between two variables ( null hypothesis ), 4 , 11 , 15 4) replace the working hypothesis if rejected ( alternative hypothesis ), 15 explain the relationship of phenomena to possibly generate a theory ( working hypothesis ), 11 5) involve quantifiable variables that can be tested statistically ( statistical hypothesis ), 11 6) or express a relationship whose interlinks can be verified logically ( logical hypothesis ). 11 We provide examples of simple, complex, directional, non-directional, associative, causal, null, alternative, working, statistical, and logical hypotheses in quantitative research, as well as the definition of quantitative hypothesis-testing research in Table 3 .
Research questions in qualitative research
Unlike research questions in quantitative research, research questions in qualitative research are usually continuously reviewed and reformulated. The central question and associated subquestions are stated more than the hypotheses. 15 The central question broadly explores a complex set of factors surrounding the central phenomenon, aiming to present the varied perspectives of participants. 15
There are varied goals for which qualitative research questions are developed. These questions can function in several ways, such as to 1) identify and describe existing conditions ( contextual research question s); 2) describe a phenomenon ( descriptive research questions ); 3) assess the effectiveness of existing methods, protocols, theories, or procedures ( evaluation research questions ); 4) examine a phenomenon or analyze the reasons or relationships between subjects or phenomena ( explanatory research questions ); or 5) focus on unknown aspects of a particular topic ( exploratory research questions ). 5 In addition, some qualitative research questions provide new ideas for the development of theories and actions ( generative research questions ) or advance specific ideologies of a position ( ideological research questions ). 1 Other qualitative research questions may build on a body of existing literature and become working guidelines ( ethnographic research questions ). Research questions may also be broadly stated without specific reference to the existing literature or a typology of questions ( phenomenological research questions ), may be directed towards generating a theory of some process ( grounded theory questions ), or may address a description of the case and the emerging themes ( qualitative case study questions ). 15 We provide examples of contextual, descriptive, evaluation, explanatory, exploratory, generative, ideological, ethnographic, phenomenological, grounded theory, and qualitative case study research questions in qualitative research in Table 4 , and the definition of qualitative hypothesis-generating research in Table 5 .
Qualitative studies usually pose at least one central research question and several subquestions starting with How or What . These research questions use exploratory verbs such as explore or describe . These also focus on one central phenomenon of interest, and may mention the participants and research site. 15
Hypotheses in qualitative research
Hypotheses in qualitative research are stated in the form of a clear statement concerning the problem to be investigated. Unlike in quantitative research where hypotheses are usually developed to be tested, qualitative research can lead to both hypothesis-testing and hypothesis-generating outcomes. 2 When studies require both quantitative and qualitative research questions, this suggests an integrative process between both research methods wherein a single mixed-methods research question can be developed. 1
FRAMEWORKS FOR DEVELOPING RESEARCH QUESTIONS AND HYPOTHESES
Research questions followed by hypotheses should be developed before the start of the study. 1 , 12 , 14 It is crucial to develop feasible research questions on a topic that is interesting to both the researcher and the scientific community. This can be achieved by a meticulous review of previous and current studies to establish a novel topic. Specific areas are subsequently focused on to generate ethical research questions. The relevance of the research questions is evaluated in terms of clarity of the resulting data, specificity of the methodology, objectivity of the outcome, depth of the research, and impact of the study. 1 , 5 These aspects constitute the FINER criteria (i.e., Feasible, Interesting, Novel, Ethical, and Relevant). 1 Clarity and effectiveness are achieved if research questions meet the FINER criteria. In addition to the FINER criteria, Ratan et al. described focus, complexity, novelty, feasibility, and measurability for evaluating the effectiveness of research questions. 14
The PICOT and PEO frameworks are also used when developing research questions. 1 The following elements are addressed in these frameworks, PICOT: P-population/patients/problem, I-intervention or indicator being studied, C-comparison group, O-outcome of interest, and T-timeframe of the study; PEO: P-population being studied, E-exposure to preexisting conditions, and O-outcome of interest. 1 Research questions are also considered good if these meet the “FINERMAPS” framework: Feasible, Interesting, Novel, Ethical, Relevant, Manageable, Appropriate, Potential value/publishable, and Systematic. 14
As we indicated earlier, research questions and hypotheses that are not carefully formulated result in unethical studies or poor outcomes. To illustrate this, we provide some examples of ambiguous research question and hypotheses that result in unclear and weak research objectives in quantitative research ( Table 6 ) 16 and qualitative research ( Table 7 ) 17 , and how to transform these ambiguous research question(s) and hypothesis(es) into clear and good statements.
a These statements were composed for comparison and illustrative purposes only.
b These statements are direct quotes from Higashihara and Horiuchi. 16
a This statement is a direct quote from Shimoda et al. 17
The other statements were composed for comparison and illustrative purposes only.
CONSTRUCTING RESEARCH QUESTIONS AND HYPOTHESES
To construct effective research questions and hypotheses, it is very important to 1) clarify the background and 2) identify the research problem at the outset of the research, within a specific timeframe. 9 Then, 3) review or conduct preliminary research to collect all available knowledge about the possible research questions by studying theories and previous studies. 18 Afterwards, 4) construct research questions to investigate the research problem. Identify variables to be accessed from the research questions 4 and make operational definitions of constructs from the research problem and questions. Thereafter, 5) construct specific deductive or inductive predictions in the form of hypotheses. 4 Finally, 6) state the study aims . This general flow for constructing effective research questions and hypotheses prior to conducting research is shown in Fig. 1 .

Research questions are used more frequently in qualitative research than objectives or hypotheses. 3 These questions seek to discover, understand, explore or describe experiences by asking “What” or “How.” The questions are open-ended to elicit a description rather than to relate variables or compare groups. The questions are continually reviewed, reformulated, and changed during the qualitative study. 3 Research questions are also used more frequently in survey projects than hypotheses in experiments in quantitative research to compare variables and their relationships.
Hypotheses are constructed based on the variables identified and as an if-then statement, following the template, ‘If a specific action is taken, then a certain outcome is expected.’ At this stage, some ideas regarding expectations from the research to be conducted must be drawn. 18 Then, the variables to be manipulated (independent) and influenced (dependent) are defined. 4 Thereafter, the hypothesis is stated and refined, and reproducible data tailored to the hypothesis are identified, collected, and analyzed. 4 The hypotheses must be testable and specific, 18 and should describe the variables and their relationships, the specific group being studied, and the predicted research outcome. 18 Hypotheses construction involves a testable proposition to be deduced from theory, and independent and dependent variables to be separated and measured separately. 3 Therefore, good hypotheses must be based on good research questions constructed at the start of a study or trial. 12
In summary, research questions are constructed after establishing the background of the study. Hypotheses are then developed based on the research questions. Thus, it is crucial to have excellent research questions to generate superior hypotheses. In turn, these would determine the research objectives and the design of the study, and ultimately, the outcome of the research. 12 Algorithms for building research questions and hypotheses are shown in Fig. 2 for quantitative research and in Fig. 3 for qualitative research.

EXAMPLES OF RESEARCH QUESTIONS FROM PUBLISHED ARTICLES
- EXAMPLE 1. Descriptive research question (quantitative research)
- - Presents research variables to be assessed (distinct phenotypes and subphenotypes)
- “BACKGROUND: Since COVID-19 was identified, its clinical and biological heterogeneity has been recognized. Identifying COVID-19 phenotypes might help guide basic, clinical, and translational research efforts.
- RESEARCH QUESTION: Does the clinical spectrum of patients with COVID-19 contain distinct phenotypes and subphenotypes? ” 19
- EXAMPLE 2. Relationship research question (quantitative research)
- - Shows interactions between dependent variable (static postural control) and independent variable (peripheral visual field loss)
- “Background: Integration of visual, vestibular, and proprioceptive sensations contributes to postural control. People with peripheral visual field loss have serious postural instability. However, the directional specificity of postural stability and sensory reweighting caused by gradual peripheral visual field loss remain unclear.
- Research question: What are the effects of peripheral visual field loss on static postural control ?” 20
- EXAMPLE 3. Comparative research question (quantitative research)
- - Clarifies the difference among groups with an outcome variable (patients enrolled in COMPERA with moderate PH or severe PH in COPD) and another group without the outcome variable (patients with idiopathic pulmonary arterial hypertension (IPAH))
- “BACKGROUND: Pulmonary hypertension (PH) in COPD is a poorly investigated clinical condition.
- RESEARCH QUESTION: Which factors determine the outcome of PH in COPD?
- STUDY DESIGN AND METHODS: We analyzed the characteristics and outcome of patients enrolled in the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) with moderate or severe PH in COPD as defined during the 6th PH World Symposium who received medical therapy for PH and compared them with patients with idiopathic pulmonary arterial hypertension (IPAH) .” 21
- EXAMPLE 4. Exploratory research question (qualitative research)
- - Explores areas that have not been fully investigated (perspectives of families and children who receive care in clinic-based child obesity treatment) to have a deeper understanding of the research problem
- “Problem: Interventions for children with obesity lead to only modest improvements in BMI and long-term outcomes, and data are limited on the perspectives of families of children with obesity in clinic-based treatment. This scoping review seeks to answer the question: What is known about the perspectives of families and children who receive care in clinic-based child obesity treatment? This review aims to explore the scope of perspectives reported by families of children with obesity who have received individualized outpatient clinic-based obesity treatment.” 22
- EXAMPLE 5. Relationship research question (quantitative research)
- - Defines interactions between dependent variable (use of ankle strategies) and independent variable (changes in muscle tone)
- “Background: To maintain an upright standing posture against external disturbances, the human body mainly employs two types of postural control strategies: “ankle strategy” and “hip strategy.” While it has been reported that the magnitude of the disturbance alters the use of postural control strategies, it has not been elucidated how the level of muscle tone, one of the crucial parameters of bodily function, determines the use of each strategy. We have previously confirmed using forward dynamics simulations of human musculoskeletal models that an increased muscle tone promotes the use of ankle strategies. The objective of the present study was to experimentally evaluate a hypothesis: an increased muscle tone promotes the use of ankle strategies. Research question: Do changes in the muscle tone affect the use of ankle strategies ?” 23
EXAMPLES OF HYPOTHESES IN PUBLISHED ARTICLES
- EXAMPLE 1. Working hypothesis (quantitative research)
- - A hypothesis that is initially accepted for further research to produce a feasible theory
- “As fever may have benefit in shortening the duration of viral illness, it is plausible to hypothesize that the antipyretic efficacy of ibuprofen may be hindering the benefits of a fever response when taken during the early stages of COVID-19 illness .” 24
- “In conclusion, it is plausible to hypothesize that the antipyretic efficacy of ibuprofen may be hindering the benefits of a fever response . The difference in perceived safety of these agents in COVID-19 illness could be related to the more potent efficacy to reduce fever with ibuprofen compared to acetaminophen. Compelling data on the benefit of fever warrant further research and review to determine when to treat or withhold ibuprofen for early stage fever for COVID-19 and other related viral illnesses .” 24
- EXAMPLE 2. Exploratory hypothesis (qualitative research)
- - Explores particular areas deeper to clarify subjective experience and develop a formal hypothesis potentially testable in a future quantitative approach
- “We hypothesized that when thinking about a past experience of help-seeking, a self distancing prompt would cause increased help-seeking intentions and more favorable help-seeking outcome expectations .” 25
- “Conclusion
- Although a priori hypotheses were not supported, further research is warranted as results indicate the potential for using self-distancing approaches to increasing help-seeking among some people with depressive symptomatology.” 25
- EXAMPLE 3. Hypothesis-generating research to establish a framework for hypothesis testing (qualitative research)
- “We hypothesize that compassionate care is beneficial for patients (better outcomes), healthcare systems and payers (lower costs), and healthcare providers (lower burnout). ” 26
- Compassionomics is the branch of knowledge and scientific study of the effects of compassionate healthcare. Our main hypotheses are that compassionate healthcare is beneficial for (1) patients, by improving clinical outcomes, (2) healthcare systems and payers, by supporting financial sustainability, and (3) HCPs, by lowering burnout and promoting resilience and well-being. The purpose of this paper is to establish a scientific framework for testing the hypotheses above . If these hypotheses are confirmed through rigorous research, compassionomics will belong in the science of evidence-based medicine, with major implications for all healthcare domains.” 26
- EXAMPLE 4. Statistical hypothesis (quantitative research)
- - An assumption is made about the relationship among several population characteristics ( gender differences in sociodemographic and clinical characteristics of adults with ADHD ). Validity is tested by statistical experiment or analysis ( chi-square test, Students t-test, and logistic regression analysis)
- “Our research investigated gender differences in sociodemographic and clinical characteristics of adults with ADHD in a Japanese clinical sample. Due to unique Japanese cultural ideals and expectations of women's behavior that are in opposition to ADHD symptoms, we hypothesized that women with ADHD experience more difficulties and present more dysfunctions than men . We tested the following hypotheses: first, women with ADHD have more comorbidities than men with ADHD; second, women with ADHD experience more social hardships than men, such as having less full-time employment and being more likely to be divorced.” 27
- “Statistical Analysis
- ( text omitted ) Between-gender comparisons were made using the chi-squared test for categorical variables and Students t-test for continuous variables…( text omitted ). A logistic regression analysis was performed for employment status, marital status, and comorbidity to evaluate the independent effects of gender on these dependent variables.” 27
EXAMPLES OF HYPOTHESIS AS WRITTEN IN PUBLISHED ARTICLES IN RELATION TO OTHER PARTS
- EXAMPLE 1. Background, hypotheses, and aims are provided
- “Pregnant women need skilled care during pregnancy and childbirth, but that skilled care is often delayed in some countries …( text omitted ). The focused antenatal care (FANC) model of WHO recommends that nurses provide information or counseling to all pregnant women …( text omitted ). Job aids are visual support materials that provide the right kind of information using graphics and words in a simple and yet effective manner. When nurses are not highly trained or have many work details to attend to, these job aids can serve as a content reminder for the nurses and can be used for educating their patients (Jennings, Yebadokpo, Affo, & Agbogbe, 2010) ( text omitted ). Importantly, additional evidence is needed to confirm how job aids can further improve the quality of ANC counseling by health workers in maternal care …( text omitted )” 28
- “ This has led us to hypothesize that the quality of ANC counseling would be better if supported by job aids. Consequently, a better quality of ANC counseling is expected to produce higher levels of awareness concerning the danger signs of pregnancy and a more favorable impression of the caring behavior of nurses .” 28
- “This study aimed to examine the differences in the responses of pregnant women to a job aid-supported intervention during ANC visit in terms of 1) their understanding of the danger signs of pregnancy and 2) their impression of the caring behaviors of nurses to pregnant women in rural Tanzania.” 28
- EXAMPLE 2. Background, hypotheses, and aims are provided
- “We conducted a two-arm randomized controlled trial (RCT) to evaluate and compare changes in salivary cortisol and oxytocin levels of first-time pregnant women between experimental and control groups. The women in the experimental group touched and held an infant for 30 min (experimental intervention protocol), whereas those in the control group watched a DVD movie of an infant (control intervention protocol). The primary outcome was salivary cortisol level and the secondary outcome was salivary oxytocin level.” 29
- “ We hypothesize that at 30 min after touching and holding an infant, the salivary cortisol level will significantly decrease and the salivary oxytocin level will increase in the experimental group compared with the control group .” 29
- EXAMPLE 3. Background, aim, and hypothesis are provided
- “In countries where the maternal mortality ratio remains high, antenatal education to increase Birth Preparedness and Complication Readiness (BPCR) is considered one of the top priorities [1]. BPCR includes birth plans during the antenatal period, such as the birthplace, birth attendant, transportation, health facility for complications, expenses, and birth materials, as well as family coordination to achieve such birth plans. In Tanzania, although increasing, only about half of all pregnant women attend an antenatal clinic more than four times [4]. Moreover, the information provided during antenatal care (ANC) is insufficient. In the resource-poor settings, antenatal group education is a potential approach because of the limited time for individual counseling at antenatal clinics.” 30
- “This study aimed to evaluate an antenatal group education program among pregnant women and their families with respect to birth-preparedness and maternal and infant outcomes in rural villages of Tanzania.” 30
- “ The study hypothesis was if Tanzanian pregnant women and their families received a family-oriented antenatal group education, they would (1) have a higher level of BPCR, (2) attend antenatal clinic four or more times, (3) give birth in a health facility, (4) have less complications of women at birth, and (5) have less complications and deaths of infants than those who did not receive the education .” 30
Research questions and hypotheses are crucial components to any type of research, whether quantitative or qualitative. These questions should be developed at the very beginning of the study. Excellent research questions lead to superior hypotheses, which, like a compass, set the direction of research, and can often determine the successful conduct of the study. Many research studies have floundered because the development of research questions and subsequent hypotheses was not given the thought and meticulous attention needed. The development of research questions and hypotheses is an iterative process based on extensive knowledge of the literature and insightful grasp of the knowledge gap. Focused, concise, and specific research questions provide a strong foundation for constructing hypotheses which serve as formal predictions about the research outcomes. Research questions and hypotheses are crucial elements of research that should not be overlooked. They should be carefully thought of and constructed when planning research. This avoids unethical studies and poor outcomes by defining well-founded objectives that determine the design, course, and outcome of the study.
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions:
- Conceptualization: Barroga E, Matanguihan GJ.
- Methodology: Barroga E, Matanguihan GJ.
- Writing - original draft: Barroga E, Matanguihan GJ.
- Writing - review & editing: Barroga E, Matanguihan GJ.
- Open access
- Published: 10 April 2024
Development of an index system for the scientific literacy of medical staff: a modified Delphi study in China
- Shuyu Liang 2 na1 ,
- Ziyan Zhai 2 na1 ,
- Xingmiao Feng 2 ,
- Xiaozhi Sun 1 ,
- Jingxuan Jiao 1 ,
- Yuan Gao 1 na2 &
- Kai Meng ORCID: orcid.org/0000-0003-1467-7904 2 , 3 na2
BMC Medical Education volume 24 , Article number: 397 ( 2024 ) Cite this article
128 Accesses
Metrics details
Scientific research activity in hospitals is important for promoting the development of clinical medicine, and the scientific literacy of medical staff plays an important role in improving the quality and competitiveness of hospital research. To date, no index system applicable to the scientific literacy of medical staff in China has been developed that can effectively evaluate and guide scientific literacy. This study aimed to establish an index system for the scientific literacy of medical staff in China and provide a reference for improving the evaluation of this system.
In this study, a preliminary indicator pool for the scientific literacy of medical staff was constructed through the nominal group technique ( n = 16) with medical staff. Then, two rounds of Delphi expert consultation surveys ( n = 20) were conducted with clinicians, and the indicators were screened, revised and supplemented using the boundary value method and expert opinions. Next, the hierarchical analysis method was utilized to determine the weights of the indicators and ultimately establish a scientific literacy indicator system for medical staff.
Following expert opinion, the index system for the scientific literacy of medical staff featuring 2 first-level indicators, 9 second-level indicators, and 38 third-level indicators was ultimately established, and the weights of the indicators were calculated. The two first-level indicators were research literacy and research ability, and the second-level indicators were research attitude (0.375), ability to identify problems (0.2038), basic literacy (0.1250), ability to implement projects (0.0843), research output capacity (0.0747), professional capacity (0.0735), data-processing capacity (0.0239), thesis-writing skills (0.0217), and ability to use literature (0.0181).
Conclusions
This study constructed a comprehensive scientific literacy index system that can assess medical staff's scientific literacy and serve as a reference for evaluating and improving their scientific literacy.
Peer Review reports
Due to the accelerated aging of the population and the growing global demand for healthcare in the wake of epidemics, there is an urgent need for medicine to provide greater support and protection. Medical scientific research is a critical element in promoting medical science and technological innovation, as well as improving clinical diagnosis and treatment techniques. It is the main driving force for the development of healthcare [ 1 ].
Medical personnel are highly compatible with clinical research. Due to their close interaction with patients, medical staff are better equipped to identify pertinent clinical research issues and actually implement clinical research projects [ 2 ]. Countries have created favorable conditions for the research and development of medical personnel by providing financial support, developing policies, and offering training courses [ 3 , 4 ]. However, some clinical studies have shown that the ability of most medical staff does not match current health needs and cannot meet the challenges posed by the twenty-first century [ 5 ]. It is clear that highly skilled professionals with scientific literacy are essential for national and social development [ 6 ]. Given the importance of scientific research in countries and hospitals, it is crucial to determine the level of scientific research literacy that medical personnel should possess and how to train them to acquire the necessary scientific research skills. These issues have significant practical implications.
Scientific literacy refers to an individual's ability to engage in science-related activities [ 7 ]. Some scholars suggest that the scientific literacy of medical personnel encompasses the fundamental qualities required for scientific research work, encompassing three facets: academic moral accomplishment, scientific research theory accomplishment, and scientific research ability accomplishment [ 8 ]. The existing research has focused primarily on the research capabilities of medical staff. According to Rillero, problem-solving skills, critical thinking, communication skills, and the ability to interpret data are the four core components of scientific literacy [ 9 ]. The ability to perform scientific research in nursing encompasses a range of abilities, including identifying problems, conducting literature reviews, designing and conducting scientific research, practicing scientific research, processing data, and writing papers [ 10 ]. Moule and Goodman proposed a framework of skills that research-literate nurses should possess, such as critical thinking capacity, analytical skills, searching skills, research critique skills, the ability to read and critically appraise research, and an awareness of ethical issues [ 11 ]. Several researchers have developed self-evaluation questionnaires to assess young researchers' scientific research and innovative abilities in the context of university-affiliated hospitals (UHAs) [ 12 ]. The relevant indicators include sensitivity to problems, sensitivity to cutting-edge knowledge, critical thinking, and other aspects. While these indicators cover many factors, they do not consider the issue of scientific research integrity in the medical field. The lack of detailed and targeted indicators, such as clinical resource collection ability and interdisciplinary cooperation ability, hinders the effective measurement of the current status of scientific literacy among medical staff [ 12 ]. In conclusion, the current research on the evaluation indicators of scientific literacy among medical personnel is incomplete, overlooking crucial humanistic characteristics, attitudes, and other moral literacy factors. Therefore, there is an urgent need to establish a comprehensive and systematic evaluation index to effectively assess the scientific literacy of medical staff.
Therefore, this study utilized a literature search and nominal group technique to screen the initial evaluation index and subsequently constructed an evaluation index system for medical staff's scientific research literacy utilizing the Delphi method. This index system would serve as a valuable tool for hospital managers, aiding them in the selection, evaluation, and training of scientific research talent. Additionally, this approach would enable medical personnel to identify their own areas of weakness and implement targeted improvement strategies.
Patient and public involvement
Patients and the public were not involved in this research.
Study design and participants
In this study, an initial evaluation index system was developed through a literature review and nominal group technique. Subsequently, a more comprehensive and scientific index system was constructed by combining qualitative and quantitative analysis utilizing the Delphi method to consult with experts. Finally, the hierarchical analysis method and the percentage weight method were employed to empower the index system.
The program used for this study is shown in Fig. 1 .
Study design. AHP, analytic hierarchy process
Establishing the preliminary indicator pool
Search process.
A literature search was performed in the China National Knowledge Infrastructure (CNKI), WanFang, PubMed, Web of Science and Scopus databases to collect the initial evaluation indicators. The time span ranged from the establishment of the database to July 2022. We used a combination of several MeSH terms in our searches:(("Medical Staff"[Mesh] OR "Nurses"[Mesh] OR "Physicians"[Mesh])) AND (("Literacy"[Mesh]) OR "Aptitude"[Mesh]). We also used several Title/Abstract searches, including keywords such as: Evaluation, scientific literacy, research ability.
The inclusion criteria were as follows: (1)The subjects were nurses, medicial staff and other personnel engaged in the medical industry; (2) Explore topics related to scientific literacy, such as research ability, and literature that can clarify the structure or dependency between indicators of scientific literacy; (3) Select articles published in countries such as China, the United States, the United Kingdom, Australia and Canada; (4) Research published in English or Chinese is considered to be eligible. The exclusion criteria are as follows: (1) indicators not applicable to medical staff; (2) Conference abstracts, case reports or review papers; (3) Articles with repeated descriptions; (4) There are no full-text articles or grey literature. A total of 78 articles were retrieved and 60 were retained after screening according to inclusion and exclusion criteria.
The research was conducted by two graduate students and two undergraduate students who participated in the literature search and screening. The entire research process was supervised and guided by one professor. All five members were from the fields of social medicine and health management. The professor was engaged in hospital management and health policy research for many years.
Nominal group technique
The nominal group technique was introduced at Hospital H in Beijing in July 2022. This hospital, with over 2,500 beds and 3,000 doctors, is a leading comprehensive medical center also known for its educational and research achievements, including numerous national research projects and awards.
The interview questions were based on the research question: What research literacy should medical staff have? 16 clinicians and nurses from Hospital H were divided into 2 equal groups and asked to provide their opinions on important aspects of research literacy based on their positions and experiences. Once all participants had shared their thoughts, similar responses were merged and polished. If anyone had further inputs after this, a second round of interviews was held until no new inputs were given. The entire meeting, including both rounds, was documented by researchers with audio recordings on a tape recorder.
Scientific literacy dimensions
Based on the search process, the research group extracted 58 tertiary indicators. To ensure the practicality and comprehensiveness of the indicators, the Nominal group technique was used on the basis of the literature search. Panelists summarized the entries shown in the interviews and merged similar content to obtain 32 third-level indicators. The indicators obtained from the literature search were compared. Several indicators with similar meanings, such as capture information ability, language expression ability, communication ability, and scientific research integrity, were merged. Additionally, the indicators obtained from the literature search, such as scientific research ethics, database use ability, feasibility and analysis ability, were added to the 15 indicators. A total of 47 third-level indicators were identified.
Fengling Dai and colleagues developed an innovation ability index system with six dimensions covering problem discovery, information retrieval, research design, practice, data analysis, and report writing, which represents the whole of innovative activity. Additionally, the system includes an innovation spirit index focusing on motivation, thinking, emotion, and will, reflecting the core of the innovation process in terms of competence [ 13 ]. Liao et al. evaluated the following five dimensions in their study on scientific research competence: literature processing, experimental manipulation, statistical analysis, manuscript production, and innovative project design [ 14 ]. Mohan claimed that scientific literacy consists of four core components: problem solving, critical thinking, communication skills, and the ability to interpret data [ 15 ].
This study structured scientific literacy into 2 primary indicators (research literacy and research competence) and 9 secondary indicators (basic qualifications, research ethics, research attitude, problem identification, literature use, professional capacity, subject implementation, data processing, thesis writing, and research output).
Using the Delphi method to develop an index system
Expert selection.
This study used the Delphi method to distribute expert consultation questionnaires online, allowing experts to exchange opinions anonymously to ensure that the findings were more desirable and scientific. No fixed number of experts is required for a Delphi study, but the more experts involved, the more stable the results will be [ 16 ]; this method generally includes 15 to 50 experts [ 17 ]. We selected clinicians from several tertiary hospitals in the Beijing area to serve as Delphi study consultants based on the following inclusion criteria: (1) they had a title of senior associate or above; (2) they had more than 10 years of work experience in the field of clinical scientific research, and (3) they were presiding over national scientific research projects. The exclusion criteria were as follows: (1) full-time scientific researchers, and (2) personnel in hospitals who were engaged only in management. To ensure that the selected experts were representative, this study selected 20 experts from 14 tertiary hospitals affiliated with Capital Medical University, Peking University, the Chinese Academy of Medical Sciences and the China Academy of Traditional Chinese Medicine according to the inclusion criteria; the hospitals featured an average of 1,231 beds each, and 9 hospitals were included among the 77 hospitals in the domestic comprehensive hospital ranking (Fudan Hospital Management Institute ranking). The experts represented various specialties and roles from different hospitals, including cardiology, neurosurgery, neurology, ear and throat surgery, head and neck surgery, radiology, imaging, infection, vascular interventional oncology, pediatrics, general practice, hematology, stomatology, nephrology, urology, and other related fields. This diverse group included physicians, nurses, managers, and vice presidents. The selected experts had extensive clinical experience, achieved numerous scientific research accomplishments and possessed profound knowledge and experience in clinical scientific research. This ensured the reliability of the consultation outcomes.
Design of the expert consultation questionnaire
The Delphi survey for experts included sections on their background, familiarity with the indicator system, system evaluation, and opinions. Experts rated indicators on importance, feasibility, and sensitivity using a 1–10 scale and their own familiarity with the indicators on a 1–5 scale. They also scored their judgment basis and impact on a 1–3 scale, considering theoretical analysis, work experience, peer understanding, and intuition. Two rounds of Delphi surveys were carried out via email with 20 experts to evaluate and suggest changes to the indicators. Statistical coefficients were calculated to validate the Delphi process. Feedback from the first round led to modifications and the inclusion of an AHP questionnaire for the second round. After the second round, indicators deemed less important were removed, and expert discussion finalized the indicator weights based on their relative importance scores. This resulted in the development of an index system for medical staff scientific literacy. The questionnaire is included in Additional file 1 (first round) and Additional file 2 (second round).
Using the boundary value method to screen the indicators
In this study, the boundary value method was utilized to screen the indicators of medical staff's scientific literacy, and the importance, feasibility, and sensitivity of each indicator were measured using the frequency of perfect scores, the arithmetic mean, and the coefficient of variation, respectively. When calculating the frequency of perfect scores and arithmetic means, the boundary value was set as "mean-SD," and indicators with scores higher than this value were retained. When calculating the coefficient of variation, the cutoff value was set to "mean + SD," and indicators with values below this threshold were retained.
The principles of indicator screening are as follows:
To evaluate the importance of the indicators, if none of the boundary values of the three statistics met the requirements, the indicators were deleted.
If an indicator has two aspects, importance, feasibility, or sensitivity, and each aspect has two or more boundary values that do not meet the requirements, then the indicator is deleted.
If all three boundary values for an indicator meet the requirements, the research group discusses the modification feedback from the experts and determines whether the indicator should be used.
The results of the two rounds of boundary values are shown in Table 1 .
Using the AHP to assign weights
After the second round of Delphi expert consultations, the analytic hierarchy process (AHP) was used to determine the weights of the two first-level indicators and the nine second-level indicators. The weights of the 37 third-level indicators were subsequently calculated via the percentage weight method. The AHP, developed by Saaty in the 1980s, is used to determine the priority and importance of elements constituting the decision-making hierarchy. It is based on multicriteria decision-making (MCDM) and determines the importance of decision-makers' judgments based on weights derived from pairwise comparisons between elements. In the AHP, pairwise comparisons are based on a comparative evaluation in which each element's weight in the lower tier is compared with that of other lower elements based on the element in the upper tier [ 18 ].
AHP analysis involves the following steps:
Step 1: Establish a final goal and list related elements to construct a hierarchy based on interrelated criteria.
Step 2: Perform a pairwise comparison for each layer to compare the weights of each element. Using a score from 1 to 9, which is the basic scale of the AHP, each pair is compared according to the expert’s judgment, and the importance is judged [ 19 , 20 ].
Yaahp software was employed to analyze data by creating a judgment matrix based on the experts' scores and hierarchical model. The index system weights were obtained by combining the experts' scores. The percentage weight method used experts' importance ratings from the second round to calculate weights, ranking indicators by importance, calculating their scores based on frequency of ranking, and determining weighting coefficients by dividing these scores by the total of all third-level indicators' scores. The third-level indicator weighting coefficients were then calculated by multiplying the coefficients [ 21 ].
Data analysis
Expert positivity coefficient.
The expert positivity coefficient is indicated by the effective recovery rate of the expert consultation questionnaire, which represents the level of expert positivity toward this consultation and determines the credibility and scientific validity of the questionnaire results. Generally, a questionnaire with an effective recovery rate of 70% is considered very good [ 22 ].
In this study, 20 questionnaires were distributed in both rounds of Delphi expert counseling, and all 20 were effectively recovered, resulting in a 100% effective recovery rate. Consequently, the experts provided positive feedback on the Delphi counseling.
Expert authority coefficient (CR)
The expert authority coefficient (Cr) is the arithmetic mean of the judgment coefficient (Ca) and the familiarity coefficient (Cs), namely, Cr = \(\frac{({\text{Ca}}+{\text{Cs}})}{2}\) . The higher the degree of expert authority is, the greater the predictive accuracy of the indicator. A Cr ≥ 0.70 was considered to indicate an acceptable level of confidence [ 23 ]. Ca represents the basis on which the expert makes a judgment about the scenario in question, while Cs represents the expert's familiarity with the relevant problem [ 24 ].
Ca is calculated on the basis of experts' judgments of each indicator and the magnitude of its influence. In this study, experts used "practical experience (0.4), "theoretical analysis (0.3), "domestic and foreign peers (0.2)" and "intuition (0.1)" as the basis for judgment and assigned points according to the influence of each basis for judgment on the experts' judgment. Ca = 1 when the basis for judgment has a large influence on the experts, and Ca = 0.5 when the influence of the experts' judgment is at a medium level. When no influence on expert judgment was evident, Ca = 0 [ 25 ] (Table 2 ).
Cs refers to the degree to which the expert was familiar with the question. This study used the Likert scale method to score experts’ familiarity with the question on a scale ranging from 0 to 1 (1 = very familiar, 0.75 = more familiar, 0.5 = moderately familiar, 0.25 = less familiar, 0 = unfamiliar). The familiarity coefficient for each expert (the average familiarity for each indicator) was calculated. The average familiarity coefficient was subsequently computed [ 26 ].
The Cr value of the primary indicator in this study was 0.83, and the Cr value of the secondary indicator was 0.82 (> 0.7); hence, the results of the expert consultation were credible and accurate, as shown in Table 3 .
The degree of expert coordination is an important indicator used to judge the consistency among various experts regarding indicator scores. This study used the Kendall W coordination coefficient test to determine the degree of expert coordination. A higher Kendall W coefficient indicates a greater degree of expert coordination and greater consistency in expert opinion, and P < 0.05 indicates that the difference is significant [ 26 ]. The results of the three-dimensional harmonization coefficient test for each indicator in the two rounds of the expert consultation questionnaire were valid ( p < 0.01 ), emphasizing the consistency of the experts' scores. The values of the Kendall W coordination coefficients for both rounds are shown in Table 4 .
Basic information regarding the participants
The 20 Delphi experts who participated in this study were predominantly male (80.0%) rather than female (20.0%). In addition, the participants’ ages were mainly concentrated in the range of 41–50 years old (60.0%). The majority of the experts were doctors by profession (85.0%), and their education and titles were mainly doctoral degree (90.0%) and full senior level (17.0%). The experts also exhibited high academic achievement in their respective fields and had many years of working experience, with the majority having between 21 and 25 years of experience (40.0%) (Table 5 ).
Index screening
The boundary value method was applied to eliminate indicators, leading to the removal of 6 third-level indicators in the first round. One of these, the ability to use statistical software, was associated with a more significant second-level indicator involving data processing, which was kept after expert review. Six indicators were merged into three indicators due to duplication, and 5 third-level indicators were added, resulting in 2 primary indicators, 10 secondary indicators, and 43 third-level indicators.
In the second round of Delphi expert consultation, 5 third-level indicators were deleted, as shown in Additional file 3 , and only one third-level indicator, "Scientific spirit", remained under the secondary indicator "research attitude". The secondary indicator "Research attitude" was combined with "Research ethics" and the third-level indicator "Scientific spirit" was also considered part of "Research ethics". After expert discussion, these were merged into a new secondary indicator "Research attitude" with three third-level indicators: "Research ethics", "Research integrity", and "Scientific spirit". The final index system included two primary indicators, nine secondary indicators, and thirty-eight third-level indicators, as shown in Additional File 3 .
Final index system with weights
The weights of the two primary indexes, research literacy and research ability, were equal. This was determined using the hierarchical analysis method and the percentage weight method based on the results of the second round of Delphi expert consultation (Table 6 ). The primary indicator of research literacy encompasses the fundamental qualities and attitudes medical staff develop over time, including basic qualifications and approach to research. The primary indicator of research ability refers to medical professionals' capacity to conduct scientific research in new areas using suitable methods, as well as their skills needed for successful research using scientific methods.
In this study, the Delphi method was employed, and after two rounds of expert consultation, in accordance with the characteristics and scientific research requirements of medical staff in China, an index system for the scientific literacy of medical staff in China was constructed. The index system for medical staff's scientific literacy in this study consists of 2 first-level indicators, 9 second-level indicators, and 38 third-level indicators. Medical institutions at all levels can use this index system to scientifically assess medical staff's scientific literacy.
In 2014, the Joint Task Force for Clinical Trial Competency (JTF) published its Core Competency Framework [ 27 ]. The Framework focuses more on the capacity to conduct clinical research. These include principles such as clinical research and quality practices for drug clinical trials. However, this framework does not apply to the current evaluation of scientific literacy in hospitals. Because these indicators do not apply to all staff members, there is a lack of practical scientific research, such as information about the final paper output. Therefore, the experts who constructed the index system in this study came from different specialties, and the indicators can be better applied to scientific researchers in all fields. This approach not only addresses clinical researchers but also addresses the concerns of hospital managers, and the indicators are more applicable.
The weighted analysis showed that the primary indicators "research literacy" and "research ability" had the same weight (0.50) and were two important components of scientific literacy. Research ability is a direct reflection of scientific literacy and includes the ability to identify problems, the ability to use literature, professional capacity, subject implementation capacity, data-processing capacity, thesis-writing skills, and research output capacity. Only by mastering these skills can medical staff carry out scientific research activities more efficiently and smoothly. The ability to identify problems refers to the ability of medical staff to obtain insights into the frontiers of their discipline and to identify and ask insightful questions. Ratten claimed that only with keen insight and sufficient sensitivity to major scientific issues can we exploit the opportunities for innovation that may lead to breakthroughs [ 28 ]. Therefore, it is suggested that in the process of cultivating the scientific literacy of medical staff, the ability to identify problems, including divergent thinking, innovative sensitivity, and the ability to produce various solutions, should be improved. Furthermore, this study included three subentries of the secondary indicator "research attitude", namely, research ethics, research integrity, and scientific spirit. This is likely because improper scientific research behavior is still prevalent. A study conducted in the United States and Europe showed that the rate of scientific research misconduct was 2% [ 13 ]. A small survey conducted in Indian medical schools and hospitals revealed that 57% of the respondents knew that someone had modified or fabricated data for publication [ 28 ]. The weight of this index ranked first in the secondary indicators, indicating that scientific attitude is an important condition for improving research quality, relevance, and reliability. Countries and hospitals should develop, implement, and optimize policies and disciplinary measures to combat academic misconduct.
In addition, the third-level indicator "scheduling ability" under the second-level indicator "basic qualification" has a high weight, indicating that medical staff attach importance to management and distribution ability in the context of scientific research. Currently, hospitals face several problems, such as a shortage of medical personnel, excessive workload, and an increase in the number of management-related documents [ 29 , 30 ]. These factors result in time conflicts between daily responsibilities and scientific research tasks, thereby presenting significant obstacles to the allocation of sufficient time for scientific inquiry [ 31 ]. Effectively arranging clinical work and scientific research time is crucial to improving the overall efficiency of scientific research. In the earlier expert interviews, most medical staff believed that scientific research work must be combined with clinical work rather than focused only on scientific research. Having the ability to make overall arrangements is essential to solving these problems. The high weight given to the second-level index of 'subject implementation capacity', along with its associated third-level indicators, highlights the challenges faced by young medical staff in obtaining research subjects. Before implementing a project, researchers must thoroughly investigate, analyze, and compare various aspects of the research project, including its technical, economic, and engineering aspects. Moreover, potential financial and economic benefits, as well as social impacts, need to be predicted to determine the feasibility of the project and develop a research plan [ 32 ]. However, for most young medical staff in medical institutions, executing such a project can be challenging due to their limited scientific research experience [ 33 ]. A researcher who possesses these skills can truly carry out independent scientific research.
The weights of the second-level index "research output capacity" cannot be ignored. In Chinese hospitals, the ability to produce scientific research output plays a certain role in employees’ ability to obtain rewards such as high pay, and this ability is also used as a reference for performance appraisals [ 34 ]. The general scientific research performance evaluation includes the number of projects, scientific papers and monographs, scientific and technological achievements, and patents. In particular, the publication of papers is viewed as an indispensable aspect of performance appraisal by Chinese hospitals [ 35 ]. Specifically, scientific research papers are the carriers of scientific research achievements and academic research and thus constitute an important symbol of the level of medical development exhibited by medical research institutions; they are thus used as recognized and important indicators of scientific research output [ 36 ]. This situation is consistent with the weight evaluation results revealed by this study.
The results of this study are important for the training and management of the scientific research ability of medical personnel. First, the index system focuses not only on external characteristics such as scientific knowledge and skills but also on internal characteristics such as individual traits, motivation, and attitudes. Therefore, when building a research team and selecting and employing researchers, hospital managers can use the index system to comprehensively and systematically evaluate the situation of researchers, which is helpful for optimizing the allocation of a research team, learning from each other's strengths, and strengthening the strength of the research team. Second, this study integrates the content of existing research to obtain useful information through in-depth interviews with medical staff and constructs an evaluation index system based on Delphi expert consultation science, which comprehensively includes the evaluation of the whole process of scientific research activities. These findings can provide a basis for medical institutions to formulate scientific research training programs, help medical personnel master and improve scientific research knowledge and skills, and improve their working ability and quality. Moreover, the effectiveness of the training can also be evaluated according to the system.
In China, with the emergence of STEM rankings, hospitals pay more and more attention to the scientific research performance of medical personnel. Scientific literacy not only covers the abilities of medical personnel engaged in scientific research, but also reflects their professional quality in this field. Having high quality medical personnel often means that they have excellent scientific research ability, and their scientific research performance will naturally rise. In view of this,,medical institutions can define the meaning of third-level indicators and create Likert scales to survey medical staff. Based on the weights assigned to each indicator, comprehensive scores can be calculated to evaluate the level of scientific literacy among medical staff. Through detailed data analysis, they can not only reveal their shortcomings in scientific research ability and quality, but also provide a strong basis for subsequent training and promotion. Through targeted inspection, we can not only promote the comprehensive improvement of the ability of medical staff, but also promote the steady improvement of their scientific research performance, and inject new vitality into the scientific research cause of hospitals.
Limitations
This study has several limitations that need to be considered. First, the participants were only recruited from Beijing (a city in China), potentially lacking geographical diversity. We plan to select more outstanding experts from across the country to participate. Second, the index system may be more suitable for countries with medical systems similar to those of China. When applying this system in other countries, some modifications may be necessary based on the local context. Last, While this study has employed scientific methods to establish the indicator system, the index system has yet to be implemented on a large sample of medical staff. Therefore, the reliability and validity of the index system must be confirmed through further research. In conclusion, it is crucial to conduct further detailed exploration of the effectiveness and practical application of the index system in the future.
This study developed an evaluation index system using the Delphi method to assess the scientific literacy of medical staff in China. The system comprises two primary indicators, nine secondary indicators, and thirty-eight third-level indicators, with each index assigned a specific weight. The index system emphasizes the importance of both attitudes and abilities in the scientific research process for medical staff and incorporates more comprehensive evaluation indicators. In the current era of medical innovation, enhancing the scientific literacy of medical staff is crucial for enhancing the competitiveness of individuals, hospitals, and overall medical services in society. This evaluation index system is universally applicable and beneficial for countries with healthcare systems similar to those of China. This study can serve as a valuable reference for cultivating highly qualified and capable research personnel and enhancing the competitiveness of medical research.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Coloma J, Harris E. From construction workers to architects: developing scientific research capacity in low-income countries. PLoS Biol. 2009;7(7):e1000156. https://doi.org/10.1371/journal.pbio.1000156 .
Article Google Scholar
Brauer SG, Haines TP, Bew PG. Fostering clinician-led research. Aust J Physiother. 2007;53(3):143–4. https://doi.org/10.1016/s0004-9514(07)70020-x .
The L. China’s research renaissance. Lancet. 2019;393(10179):1385. https://doi.org/10.1016/S0140-6736(19)30797-4 .
Hannay DR. Evaluation of a primary care research network in rural Scotland. Prim Health Care ResDevelop. 2006;7(3):194–200. https://doi.org/10.1191/1463423606pc296oa .
Frenk J, Chen L, Bhutta ZA, Cohen J, Crisp N, Evans T, et al. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet. 2010;376:1923–58.
Xie Y, Wang J, Li S, Zheng Y. Research on the Influence Path of Metacognitive Reading Strategies on Scientific Literacy. J Intell. 2023;11(5):78. https://doi.org/10.3390/jintelligence11050078 . PMID: 37233327; PMCID: PMC10218841.
Pang YH, Cheng JL. Revise of scientific research ability self-evaluation rating scales of nursing staff. Chin Nurs Res. 2011;13:1205–8. https://doi.org/10.3969/j.issn.1009-6493.2011.13.040 .
Zhang J, Jianshan MAO, Gu Y. On the cultivation of scientific research literacy of medical graduate students. Continu Med Educ China. 2023;15(3):179–82. https://doi.org/10.3969/j.issn.1674-9308.2023.03.043 .
Rillero P. Process skills and content knowledge. Sci Act. 1998;3:3–4.
Google Scholar
Liu RS. Study on reliability and validity of self rating scale for scientific research ability of nursing staff. Chinese J Pract Nurs. 2004;9:8–10. https://doi.org/10.3760/cma.j.issn.1672-7088.2004.09.005 .
Moule P, Goodman M. Nursing research: An introduction. London, UK: Sage; 2013.
Xue J, Chen X, Zhang Z, et al. Survey on status quo and development needs of research and innovation capabilities of young researchers at university-affiliated hospitals in China: a cross-sectional survey. Ann Transl Med. 2022;10(18):964. https://doi.org/10.21037/atm-22-3692 .
Fanelli D, Costas R, Fang FC, et al. Testing hypotheses on risk factors for scientific misconduct via matched-control analysis of papers containing problematic image duplications. Sci Eng Ethics. 2019;25(3):771–89. https://doi.org/10.1007/s11948-018-0023-7 .
Liao Y, Zhou H, Wang F, et al. The Impact of Undergraduate Tutor System in Chinese 8-Year Medical Students in Scientific Research. Front Med (Lausanne). 2022;9:854132. https://doi.org/10.3389/fmed.2022.854132 .
Mohan L, Singh Y, Kathrotia R, et al. Scientific literacy and the medical student: A cross-sectional study. Natl Med J India. 2020;33(1):35–7. https://doi.org/10.4103/0970-258X.308242 .
Jorm AF. Using the Delphi expert consensus method in mental health research. Aust N Z J Psychiatry. 2015;49(10):887–97. https://doi.org/10.1177/0004867415600891 .
Xinran S, Heping W, Yule H, et al. Defining the scope and weights of services of a family doctor service project for the functional community using Delphi technique and analytic hierarchy process. Chinese Gen Pract. 2021;24(34):4386–91.
Park S, Kim HK, Lee M. An analytic hierarchy process analysis for reinforcing doctor-patient communication. BMC Prim Care. 2023;24(1):24. https://doi.org/10.1186/s12875-023-01972-3 . Published 2023 Jan 21.
Zhou MLY, Yin H, et al. New screening tool for neonatal nutritional risk in China: a validation study. BMJ Open. 2021;11(4):e042467. https://doi.org/10.1136/bmjopen-2020-042467 .
Wang K, Wang Z, Deng J, et al. Study on the evaluation of emergency management capacity of resilient communities by the AHP-TOPSIS method. Int J Environ Res Public Health. 2022;19(23):16201. https://doi.org/10.3390/ijerph192316201 .
Yuwei Z, Chuanhui Y, Junlong Z, et al. Application of analytic Hierarchy Process and percentage weight method to determine the weight of traditional Chinese medicine appropriate technology evaluation index system. Chin J Tradit Chinese Med. 2017;32(07):3054–6.
Babbie E. The practice of social research. 10th Chinese language edition. Huaxia Publisher, 2005: 253–4.
Liu W, Hu M, Chen W. Identifying the Service Capability of Long-Term Care Facilities in China: an e-Delphi study. Front Public Health. 2022;10:884514. https://doi.org/10.3389/fpubh.2022.884514 .
Zeng G. Modern epidemiological methods and application. Pecking Union Medical College Union Press, 1996.
Geng Y, Zhao L, Wang Y, et al. Competency model for dentists in China: Results of a Delphi study. PLoS One. 2018;13(3):e0194411. https://doi.org/10.1371/journal.pone.0194411 .
Cong C, Liu Y, Wang R. Kendall coordination coefficient W test and its SPSS implementation. Journal of Taishan Medical College. 2010;31(7):487–490. https://doi.org/10.3969/j.issn.1004-7115.2010.07.002 .
Sonstein S, Seltzer J, Li R, et al. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clin Res. 2014;28(3):17–23.
Madan C, Kruger E, Tennant M. 30 Years of dental research in Australia and India: a comparative analysis of published peer review literature. Indian J Dent Res. 2012;23(2):293–4. https://doi.org/10.4103/0970-9290.100447 .
Siemens DR, Punnen S, Wong J, Kanji N. A survey on the attitudes towards research in medical school. BMC Med Educ. 2010;10:4. https://doi.org/10.1186/1472-6920-10-4 .
Solomon SS, Tom SC, Pichert J, Wasserman D, Powers AC. Impact of medical student research in the development of physician-scientists. J Investig Med. 2003;51(3):149–56. https://doi.org/10.1136/jim-51-03-17 .
Misztal-Okonska P, Goniewicz K, Hertelendy AJ, et al. How Medical Studies in Poland Prepare Future Healthcare Managers for Crises and Disasters: Results of a Pilot Study. Healthcare (Basel). 2020;8(3):202. https://doi.org/10.3390/healthcare8030202 .
Xu G. On the declaration of educational scientific research topics. Journal of Henan Radio & TV University. 2013;26(01):98–101.
Ju Y, Zhao X. Top three hospitals clinical nurse scientific research ability present situation and influence factors analysis. J Health Vocational Educ. 2022;40(17):125–8.
Zhu Q, Li T, Li X, et al. Industry gain public hospital medical staff performance distribution mode of integration, exploring. J Health Econ Res. 2022;33(11):6-82–6.
Sun YLL. Analysis of hospital papers published based on performance appraisal. China Contemp Med. 2015;22(31):161–3.
Jian Y, Wu J, Liu Y. Citation analysis of seven tertiary hospitals in Yunnan province from 2008 to 2012. Yunnan Medicine. 2014;(6):700–704.
Download references
Acknowledgements
The authors thank all who participated in the nominal group technique and two rounds of the Delphi study.
This study was supported by the National Natural Science Foundation of China (72074160) and the Natural Science Foundation Project of Beijing (9222004).
Author information
Shuyu Liang and Ziyan Zhai contributed equally to this work and joint first authors.
Kai Meng and Yuan Gao contributed equally to this work and share corresponding author.
Authors and Affiliations
Aerospace Center Hospital, No. 15 Yuquan Road, Haidian District, Beijing, 100049, China
Xiaozhi Sun, Jingxuan Jiao & Yuan Gao
School of Public Health, Capital Medical University, No.10 Xitoutiao, Youanmenwai Street, Fengtai District, Beijing, 100069, China
Shuyu Liang, Ziyan Zhai, Xingmiao Feng & Kai Meng
Beijing Tiantan Hospital, Capital Medical University, No. 119 South Fourth Ring West Road, Fengtai District, Beijing, 100070, China
You can also search for this author in PubMed Google Scholar
Contributions
S.L. and Z.Z. contributed equally to this paper. S.L. took charge of the nominal group technique, data analysis, writing the first draft and revising the manuscript; Z.Z. was responsible for the Delphi survey, data analysis, and writing of the first draft of the manuscript; XF was responsible for the rigorous revision of Delphi methods; X.S. and J.J. were responsible for the questionnaire survey and data collection; Y.G. contributed to the questionnaire survey, organization of the nominal group interview, supervision, project administration and resources; and K.M. contributed to conceptualization, methodology, writing—review; editing, supervision, and project administration. All the authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yuan Gao or Kai Meng .
Ethics declarations
Ethics approval and consent to participate.
This study involved human participants and was approved by the Ethical Review Committee of the Capital Medical University (No. Z2022SY089). Participation in the survey was completely voluntary, and written informed consent was obtained from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liang, S., Zhai, Z., Feng, X. et al. Development of an index system for the scientific literacy of medical staff: a modified Delphi study in China. BMC Med Educ 24 , 397 (2024). https://doi.org/10.1186/s12909-024-05350-0
Download citation
Received : 25 October 2023
Accepted : 26 March 2024
Published : 10 April 2024
DOI : https://doi.org/10.1186/s12909-024-05350-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical staff
- Scientific literacy
- Evaluation indicators
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 5—May 2024
Epidemiologic survey of crimean-congo hemorrhagic fever virus in suids, spain.
Main Article
Distribution and seroprevalence of Crimean-Congo hemorrhagic fever in wild boars and extensively raised Iberian pigs, Spain
*NA, not applicable.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Review article
- Open access
- Published: 09 November 2023
Male contraception: narrative review of ongoing research
- Eli J. Louwagie ORCID: orcid.org/0000-0001-8741-2240 1 ,
- Garrett F.L. Quinn 1 ,
- Kristi L. Pond 1 &
- Keith A. Hansen 2
Basic and Clinical Andrology volume 33 , Article number: 30 ( 2023 ) Cite this article
1931 Accesses
Metrics details
Since the release of the combined oral contraceptive pill in 1960, women have shouldered the burden of contraception and family planning. Over 60 years later, this is still the case as the only practical, effective contraceptive options available to men are condoms and vasectomy. However, there are now a variety of promising hormonal and non-hormonal male contraceptive options being studied. The purpose of this narrative review is to provide clinicians and laypeople with focused, up-to-date descriptions of novel strategies and targets for male contraception. We include a cautiously optimistic discussion of benefits and potential drawbacks, highlighting several methods in preclinical and clinical stages of development.
As of June 2023, two hormonal male contraceptive methods are undergoing phase II clinical trials for safety and efficacy. A large-scale, international phase IIb trial investigating efficacy of transdermal segesterone acetate (Nestorone) plus testosterone gel has enrolled over 460 couples with completion estimated for late 2024. A second hormonal method, dimethandrolone undecanoate, is in two clinical trials focusing on safety, pharmacodynamics, suppression of spermatogenesis and hormones; the first of these two is estimated for completion in December 2024. There are also several non-hormonal methods with strong potential in preclinical stages of development.
Conclusions
There exist several hurdles to novel male contraception. Therapeutic development takes decades of time, meticulous work, and financial investment, but with so many strong candidates it is our hope that there will soon be several safe, effective, and reversible contraceptive options available to male patients.
Depuis la sortie de la pilule contraceptive orale combinée en 1960, les femmes ont assumé le fardeau de la contraception et de la planification familiale. Plus de 60 ans plus tard, c’est toujours le cas, car les seules options contraceptives pratiques et efficaces disponibles pour les hommes sont les préservatifs et la vasectomie. Cependant, il existe maintenant une variété d’options contraceptives masculines hormonales et non hormonales prometteuses qui sont à l’étude. Le but de cette revue narrative est de fournir aux cliniciens et aux profanes des descriptions ciblées et à jour de nouvelles stratégies et cibles pour la contraception masculine. Nous incluons une discussion prudemment optimiste sur les avantages et les inconvénients potentiels, en soulignant plusieurs méthodes aux stades précliniques et cliniques du développement.
En juin 2023, deux méthodes contraceptives masculines hormonales faisaient l’objet d’essais cliniques de phase II pour leur innocuité et leur efficacité. Un essai international de phase IIb à grande échelle, portant sur l’efficacité de l’acétate de ségestérone transdermique (Nestorone) et du gel de testostérone, a recruté plus de 460 couples et devrait être achevé pour la fin de 2024. Une seconde méthode hormonale, l’undécanoate de diméthandrolone, fait l’objet de deux essais cliniques axés sur l’innocuité, la pharmacodynamique, la suppression de la spermatogenèse et des hormones; le premier de ces deux essais devrait être achevé en décembre 2024. Il existe également plusieurs méthodes non hormonales à fort potentiel aux stades précliniques de développement.
Il existe plusieurs obstacles à la nouvelle contraception masculine. Le développement thérapeutique nécessite des décennies de temps, un travail méticuleux et un investissement financier ; mais avec autant de candidats solides, nous espérons qu’il y aura bientôt plusieurs options contraceptives sûres, efficaces et réversibles, disponibles pour les hommes.
Introduction
In the wake of the Dobbs v. Jackson Women’s Health Organization 2022 decision, the resultant “trigger laws” in 13 U.S. states, and the lingering retraction of reproductive rights in many more [ 1 , 2 ], the need for novel contraceptive options has gained urgency across the United States. Unfortunately, due to a complex combination of medical challenges and societal beliefs [ 3 , 4 , 5 , 6 ], the burden of contraception has fallen almost entirely on women, and the only practical effective options available to males are condoms and vasectomy. Even with ‘perfect use’, the failure rate of condoms is still over 10% [ 7 ], and vasectomy is largely irreversible. Further, many of the contraceptive options currently available have high discontinuation rates [ 8 ], contributing to high rates of unintended pregnancy in the United States [ 9 , 10 ]. With that in mind, there is a growing demand for safe, effective, and reversible male contraception that would allow men to share the burden of family planning [ 11 , 12 ].
Male fertility is dependent on production of an adequate number of viable, motile sperm capable of moving through the female reproductive tract and fertilizing oocytes. Fertile males generally have seminal sperm concentrations greater than 15 million sperm/mL [ 13 ], and adequate sperm suppression for contraception requires sperm levels ≤ 1 million/mL [ 14 ]. The process of sperm production is termed spermatogenesis and is controlled by the hypothalamic-pituitary-testicular (HPT) axis (Fig. 1 ) [ 15 ]. Briefly, the hypothalamus produces gonadotropin-releasing hormone (GnRH) in a pulsatile fashion, which stimulates the anterior pituitary to secrete the gonadotrophic hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH stimulates androgen production by testicular Leydig cells, and FSH, along with high levels of intratesticular T, enables spermatogenesis within the seminiferous tubules [ 16 ]. T exerts negative feedback on GnRH release and therefore suppresses LH and FSH secretion; the same effect is seen with exogenous androgens. Similarly, natural and synthetic progesterone, the latter termed progestins, exert negative feedback on the HPT axis to suppress LH and FSH release [ 16 ]. These concepts underlie the mechanisms of hormonal contraceptives discussed in this review, which generally target spermatogenesis, sperm motility, or transport through the vas deferens (Fig. 1 ).
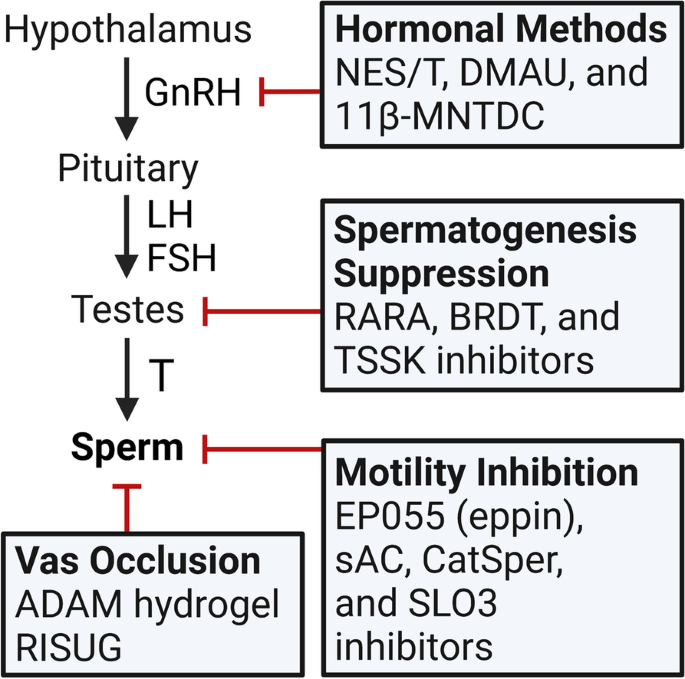
Overview of the hypothalamic-pituitary-testicular (HPT) axis and targets of male contraception. The HPT axis consists of the hypothalamus, pituitary gland, and testes. The hypothalamus releases gonadotropin-releasing hormone (GnRH) in a pulsatile fashion which signals for release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary. LH and FSH drive testosterone (T) production and spermatogenesis in the testes. T and the hormonal contraceptives exert negative feedback on the hypothalamus to inhibit GnRH, LH, and FSH release, therefore suppressing spermatogenesis. Non-hormonal methods focus on distinct targets to inhibit spermatogenesis, sperm motility, or transit through the vas deferens. Pointed arrows indicate activation; red broad-tipped arrows indicate inhibition. NES/T, Nestorone/testosterone; DMAU, dimethandrolone undecanoate; 11β-MNTDC, 11β-methyl-19-nortestosterone dodecylcarbonate; RARA, retinoic acid receptor alpha; BRDT, bromodomain testis-specific protein; TSSK, testis-specific serine/threonine kinase; sAC, soluble adenylyl cyclase; CatSper, cation channel of sperm; SLO3, slowpoke homolog 3; RISUG, reversible inhibition of sperm under guidance. Figure created by EJL using BioRender.com
There are several promising male contraceptive options in development, and they can be broadly categorized as either hormonal or non-hormonal. The purpose of this review is to provide an overview of the most promising male contraceptive methods under study, including how they work, their current state in research and development, and potential side effects or barriers to marketability. We will also briefly discuss some methods in preclinical stages of development to demonstrate that men may soon have access to a variety of safe, effective, and reversible contraceptive options.
Materials and methods
For this narrative review, authors searched the online databases MEDLINE (via PubMed.gov), Cochrane Reviews, CENTRAL (via CochraneLibrary.com), ClinicalTrials.gov, and the World Health Organization’s International Clinical Trials Registry Platform for publications and ongoing clinical trials through 20 June 2023. Search terms included “contraception”, “male contraception”, “hormonal contraception”, “spermatogenesis inhibition”, “vas deferens occlusion”, and terms related to methods discussed below. Authors considered all identified ongoing studies related to male contraception, but we excluded from discussion those evaluating 7α-Methyl-19-nortestosterone (MENT) [ 17 , 18 , 19 ] or T combined with GnRH antagonists [ 20 , 21 ], estradiol [ 22 ], or progestins (medroxyprogesterone acetate [ 23 , 24 ] or norethisterone enanthate [ 25 , 26 , 27 , 28 ]) as these treatments ultimately failed to progress in clinical trials. Several past trials evaluating T injection alone [ 29 , 30 , 31 , 32 , 33 , 34 , 35 ] were also excluded as ongoing trials use T as a supplemental rather than primary compound. Authors EJL, GFLQ, and KLP completed literature search and assessed methodology of ongoing trials with particular focus on sample size ( n ), primary and secondary outcomes, and inclusion and exclusion criteria; none of the studies were excluded due to grossly unsound methodology.
Hormonal methods
Three hormonal methods show great promise in male contraception: segesterone acetate (Nestorone; NES), dimethandrolone undecanoate (DMAU), and 11β-methyl-19-nortestosterone dodecylcarbonate (11β-MNTDC). NES and DMAU are currently in phase II clinical trials, and 11β-MNTDC has completed one phase II trial. Each method will be discussed separately below, and the clinical trials investigating these three compounds are summarized in Table 1 .
Segesterone acetate + testosterone (NES/T)
Segesterone acetate, most often identified by its trade name Nestorone (NES), is a potent progestin with virtually no affinity for androgen receptors (AR) or estrogen receptors (ER) and minimal glucocorticoid activity [ 49 , 50 , 51 ]. NES shows low bioavailability when taken orally but is readily absorbed by transdermal application [ 52 ]; it has been available with ethinyl estradiol in the ANNOVERA vaginal ring (Mayne Pharma, Raleigh, NC) since 2018 and is a well-tolerated female contraceptive with > 97% efficacy [ 53 , 54 , 55 ]. NES is now compounded with T in a transdermal gel (NES/T) in a phase II clinical trial evaluating efficacy [ 36 , 37 ]. T is added to improve suppression of spermatogenesis and minimize potential symptoms of androgen deficiency [ 56 ].
Phase I trials of NES/T daily gel (approximately 8.3 mg/62.5 mg) have demonstrated gonadotropin suppression adequate to suppress spermatogenesis in nearly 90% of participants [ 38 , 39 ], suggesting that NES/T will be an effective form of male birth control [ 57 ]. Importantly, in these same studies there were no severe side effects with treatment. The main adverse effects were similar to the combined estrogen-progestin contraceptive pills used by women [ 58 ] and included minor mood symptoms, acne, and likely transient gastrointestinal symptoms [ 38 , 39 , 40 ]. From the most recent Phase I trial and a survey on attitudes towards NES/T, the majority of participants (79% and 56%, respectively) were satisfied or very satisfied with the treatments, and 50–51% reported that they would use NES/T daily gel as a sole form of contraception [ 38 , 59 ].
A phase IIb trial investigating NES/T efficacy is currently underway at 17 medical centers across 8 U.S. states and 7 other countries [ 36 , 37 ]; it is sponsored by the Population Council and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Participants are self-administering the transdermal gel with one of two T doses (both compounded as NES/T; 8 mg/62 mg or 8 mg/74 mg), and participants showing low serum T with symptoms of hypogonadism will be offered additional T [ 37 ]. The trial is broken down into phases. There is an initial screening phase, after which participants begin daily NES/T. Within 20 weeks of beginning treatment, participants must show sperm suppression to levels ≤ 1 million sperm/mL before entering the 52-week efficacy phase. The recovery phase is intended to assess sperm production after ceasing NES/T application and continue symptom surveillance of both males and their female partners [ 37 ]. Enrollment was completed in November 2022 with 462 couples having started treatment. Completion of the primary endpoint, contraceptive efficacy, is estimated for late 2024, with full study results likely available in early 2025 [ 36 , 37 ].
Dimethandrolone undecanoate (DMAU)
DMAU is a testosterone-derived pro-drug, metabolized to active form dimethandrolone (DMA), with high affinity for AR and, to a lesser degree, progesterone receptors (PR) [ 60 ]. DMAU and DMA are not aromatized and therefore lack estrogenic effects [ 61 ], but DMAU is highly lipophilic and experiences first-pass metabolism by the liver [ 62 ], requiring study of a variety of formulations to determine the optimal delivery method. In preclinical animal studies including non-human primates, DMAU was shown to effectively, reversibly suppress gonadotropins and spermatogenesis while maintaining physiologic androgenic effects without serious side effects [ 63 , 64 , 65 ]; importantly, there were no signs of liver toxicity, a well-characterized side effect of many exogenous androgens [ 66 ]. DMAU has been studied in several clinical trials for safety, pharmacodynamics, and gonadotropin suppression to evaluate its potential in male contraception, and phase I and phase II clinical trials are currently underway.
Early human trials of DMAU evaluated safety and absorption with doses up to 800 mg. In 2014, the first clinical trial orally dosed DMAU in a powder formulation from 25 to 800 mg, fasting or following high-fat meal (50% calories as fat). With a high-fat meal, authors found considerable, dose-escalating absorption of DMAU and suppression of gonadotropins (12 h later) from 200 mg upwards [ 45 ]. In a follow-up 2017 study, authors evaluated daily DMAU absorption at doses up to 400 mg daily and effects on estrogen and T levels [ 44 ]. Similarly, they found improved absorption with high-fat meals and suppression of estrogen and T in the absence of any serious side effects [ 44 ].
In a placebo-controlled, double-blinded, randomized phase I trial, Thirumalai et al. 2019 [ 43 ] investigated safety, tolerability, and adverse events associated with oral DMAU over 28 days of treatment, as well as pharmacokinetics, pharmacodynamics, hormonal changes, and sperm counts. The study found suppression of T at even the lowest dose of DMAU and dose-dependent suppression of LH and FSH, theoretically sufficient to suppress spermatogenesis with treatment for 10 weeks [ 57 ]. No serious side effects were observed; several participants reported decreased libido or erectile dysfunction, particularly at the highest tested dose, but participants did not report this affecting their sexual or erectile satisfaction [ 43 ]. Of note, DMAU was taken after a meal containing 25–30 g of fat, reflecting a typical Western diet but approximately half the fat content of the Ayoub et al. 2017 study [ 44 ]. In a secondary analysis of this trial’s samples and data, Thirumalai et al. 2021 found dose-dependent suppression of T and estrogen as well as an increase in a marker for bone formation over 28 days [ 67 ]. In another secondary analysis comparing metabolic effects of DMAU and 11β-MNTDC (discussed below), Yuen et al. 2021 found that DMAU caused a mean weight gain of 1.2 or 2.0 kg with 200 or 400 mg daily dosing, respectively, and mild lipid changes, but there were no serious adverse effects or signs of overt insulin resistance [ 46 ]. Collectively, these analyses indicate that orally dosed DMAU is well-tolerated and shows promise as a male contraceptive.
Today, there are two ongoing trials with DMAU, run by Drs. Christina Wang, MD, out of the University of California Los Angeles and Stephanie Page, MD, PhD, out of the University of Washington [ 41 , 42 ]. Per ClinicalTrials.gov, both are reportedly still recruiting. The first is a phase I trial comparing a single injection of intramuscular (80-800 mg) vs. subcutaneous (50-200 mg) DMAU and is primarily assessing safety, pharmacodynamics, and hormonal suppression in healthy males [ 41 ]. Completion is estimated for December 2024. The second is a phase II trial primarily investigating the ability of orally dosed DMAU with or without a low dose of levonorgestrel (a progestin) to suppress spermatogenesis after 12 weeks treatment; secondary outcomes include hormonal suppression, serious adverse events, systemic symptoms, and tolerability [ 42 ]. Ideally, these ongoing studies will shed further light on the optimal route and dose of DMAU administration to guide efficacy trials.
11β-methyl-19-nortestosterone dodecylcarbonate (11β-MNTDC)
11β-MNTDC is a testosterone derivative active at both AR and PR; it does not undergo aromatization and therefore lacks estrogenic effects [ 48 , 61 , 65 ]. Like DMAU, 11β-MNTDC is a pro-drug and is converted to 11β-methyl-19-nortestosterone (11β-MNT), which is structurally similar to DMA [ 68 ]. However, 11β-MNT’s affinity for AR and PR is more balanced than that of DMA (which favors AR), so side effect profiles may vary [ 48 ]. In preclinical animal studies, 11β-MNTDC was shown to effectively suppress serum gonadotropins [ 65 ] and exert even less liver toxicity than other androgens, including DMAU [ 63 ].
Several clinical trials have investigated 11β-MNTDC. The first major human trial was directed by Drs. Wang and Page and published in 2019 [ 48 ]. Twelve healthy adult males were given a single oral dose of 100-800 mg 11β-MNTDC with a high-fat meal or fasting, then assessed for pharmacokinetics, adverse effects, serum gonadotropins, and T levels. Like DMAU, 11β-MNTDC absorption was improved with high-fat meal, treatment was overall well-tolerated, and T was suppressed in a dose-dependent manner from 200 mg upwards [ 48 ]. Gonadotropin levels were not significantly reduced with a single dose, but this was addressed in a follow-up study published in 2020 [ 47 ]. This randomized, placebo-controlled phase II trial was again directed by Drs. Wang and Page, and participants received a daily oral dose of 200 or 400 mg 11β-MNTDC for 28 consecutive days. 11β-MNTDC was taken after a meal containing 25–30 g of fat [ 47 ], a more typical fat content per meal than in the previous trial [ 48 ]. Ultimately, 11β-MNTDC was well-tolerated; participants reported no serious adverse events, no one discontinued the trial due to side effects, and all reported side effects were mild or moderate. The most common sides effects were headache, acne, and decreased libido in 16% of participants [ 47 ]. Mood symptoms were reported, but they were comparable to those seen with currently available female estrogen-containing contraceptives [ 69 , 70 , 71 ]. 11β-MNTDC caused dose-dependent suppression of LH and FSH, and more participants in the 400 mg group had suppression to LH and FSH levels < 1.0 IU/L, the threshold at which spermatogenesis will be suppressed in nearly 90% of participants [ 57 ].
Efficacy trials are still needed for 11β-MNTDC, but between the two clinical trials and a secondary analysis comparing metabolic effects of DMAU and 11β-MNTDC (DMAU discussed above), 11β-MNTDC demonstrated acceptable safety profiles. Levels of T, estradiol, and sex hormone binding globulin (SHBG) were all suppressed, but these changes did not correlate with side effects or changes in serum chemistries [ 46 , 47 , 48 ]. 11β-MNTDC slightly increased participant weight and serum low-density lipoprotein (LDL) cholesterol levels, but there were no serious adverse events or signs of overt insulin resistance [ 46 ]. Results-to-date warrant clinical trials evaluating efficacy and safety using a larger number of participants.
Non-hormonal methods
Several non-hormonal methods show promise in the field of male contraception, and two are either near human study or recently began human trials. In theory, these methods lack hormonal side effects, such as acne or mood symptoms, as well as the societal stigmas and false beliefs associated with hormonal contraception in the United States [ 6 , 72 ]. The non-hormonal methods showing the most potential or closest to market, particularly those that inhibit spermatogenesis, motility, or vas deferens passage, will be discussed in greatest depth.
- Spermatogenesis
All-trans retinoic acid (RA), also known as tretinoin, is derived from vitamin A and plays global roles in cell growth and development. RA plays essential roles in spermatogenesis and acts through binding the retinoic acid receptor alpha (RARA) located in the testes [ 73 , 74 ]. The first human trial targeting RARA was conducted over 60 years ago with the non-selective RA biosynthesis inhibitor WIN 18,446 [ 75 ]. Sixty men were treated for one year, and spermatogenesis was suppressed in all participants throughout. However, off-target effects including inhibition of aldehyde dehydrogenase 2 in the liver unfortunately lead to a severe disulfiram-like reaction, effectively making the drug unmarketable [ 75 ]. Since then, the pharmaceutical company Bristol-Myers Squibb (BMS) designed and, with other labs, demonstrated effective, reversible suppression of spermatogenesis in mice with the pan-antagonist BMS-189,453 [ 76 , 77 , 78 ]. Theoretically, reversible alpha-selective agents would effectively and safely suppress sperm production without the systemic side effects of pan-antagonists. In other words, this would be an ideal method of contraception. Early attempts, most notably BMS-189,532 and BMS-189,614, lacked the efficacy of the pan-antagonist (WIN 18,446) by oral, intravenous, or intraperitoneal routes [ 79 ], but RARA remains a strong potential target for male contraception.
Bromodomains are amino acid segments in proteins that facilitate specific protein-protein interactions and a wide variety of cellular functions [ 80 , 81 ]. One of these bromodomains, bromodomain testis-specific protein (BRDT), is required for spermatogenesis, and males with BRDT gene mutations are infertile with abnormal sperm morphology and impaired motility [ 82 , 83 ]. Like RARA inhibition, specific inhibition of BRDT would theoretically suppress sperm production without the systemic effects of pan-inhibitors or hormonal methods. Indeed, inhibition of BRDT has been shown to effectively suppress spermatogenesis in male rodents using the small molecule JQ1 [ 84 ]. In this study, JQ1 was safe, reversible, and lacked obvious transgenerational effects, but authors noted potential off-target binding that could be reduced or prevented through design of more specific molecular inhibitors [ 84 ]. Progress has been made in the search for more specific BRDT inhibitors [ 85 , 86 , 87 , 88 ], but the compounds have yet to be tested in vivo and are therefore far from human trials.
Males express distinct testis-specific serine/threonine kinases (TSSK) that play spermatogenic roles in spermatids [ 89 ]. Mice with TSSK1 and TSSK2, TSSK3, or TSSK 6 deletions and human males with TSSK2 mutations are infertile, suggesting potential non-hormonal targets for contraception [ 90 , 91 , 92 , 93 ]. Of these, research into TSSK2 has shown the most progress. Since generation of enzymatically active, isolated TSSK2 [ 94 ], several inhibitors have demonstrated potent in vitro inhibition of TSSK2 [ 95 ]. To our knowledge, these inhibitors have yet to undergo in vivo study.
In order to reach and fertilize oocytes, sperm must travel through the female reproductive tract. This quality is termed motility , and immotile sperm are a major contributor to male-factor infertility [ 96 ]. Theoretically, by targeting enzymes or receptors that play essential roles in motility and are present only in sperm, one may reversibly immobilize sperm without systemic side effects. Eppin is an enzyme made in the testes that binds to the surface of sperm to play essential roles in motility [ 97 ]. Both immunization against eppin and molecular inhibition using the inhibitor EP055 has been shown to significantly, transiently reduce sperm motility [ 98 , 99 ]. Although these studies were both done with small sample sizes and much work is needed before eppin inhibition may see clinical trials, no severe side effects were noted in these animal studies, suggesting that eppin may hold promise as a non-hormonal target [ 100 ].
In a similar vein as eppin, soluble adenylyl cyclase (sAC) is an intracellular signaling molecule needed for sperm capacitation, motility, and acrosome formation [ 101 , 102 , 103 ]. Several compounds have been tested in preclinical in vitro studies and shown to effectively inhibit sAC in mouse and human sperm [ 101 , 104 ]. Indeed, sAC inhibition stands as a strong candidate for male contraception, and two recent studies have been conducted by Drs. Lonny Levin, PhD, and Jochen Buck, MD, PhD, out of Weill Cornell Medicine.
The first study by the Levin-Buck lab intricately compared capacitation and motility of sperm from sAC null mice and from healthy, wild type mice [ 105 ]. In vitro, they demonstrated that sAC plays essential roles in capacitation. In vivo, sAC null mice mated similarly to wild type mice, but their sperm were unable to migrate through the female reproductive tract. Essentially, these sperm were immotile [ 105 ]. Improving on the inhibitors mentioned above [ 102 ], a recent, well-designed study by the Levin-Buck lab investigated the new compound TDI-11,861; they demonstrated that a single oral or intraperitoneal dose of TDI-11,861 acutely inhibits sAC in mice, impairing capacitation and motility [ 103 ]. Importantly, the mice in this study had no changes in behavior, no obvious toxicity, and no pregnancies when treated within 2.5 h of mating [ 103 ]. With completion of this proof-of-concept study, authors anticipate additional safety and transgenerational studies to follow.
Calcium plays several signaling roles in sperm, including modulation of motility through activating sAC [ 96 ]. Extracellular calcium enters sperm flagella, the organelle that propels sperm, primarily through the cell type-specific cation channel of sperm (CatSper) [ 106 ]. Studies nearly 15 years ago demonstrated that immunologic inhibition of CatSper significantly suppresses sperm motility [ 107 ]; since, several compounds (RU1968 and HC-056456) have demonstrated effective inhibition of CatSper in vitro [ 108 , 109 ] and preliminarily in vivo [ 110 ]. Several new compounds have been identified, synthesized, and tested on human sperm in vitro with excellent efficacy and safety profiles, at least on a cellular level [ 111 ]. Additional in vivo animal studies are anticipated.
Slowpoke homolog 3 (SLO3) is the main potassium channel in sperm and has functions directly related to calcium signaling and the CatSper channel [ 112 , 113 ]. Like CatSper, SLO3 is specific to sperm and has functions essential for male fertility, making it an ideal target for male contraception [ 114 , 115 , 116 ]. A highly specific inhibitor of SLO3, VU0546110, has been identified and shown in vitro to inhibit sperm motility and acrosome reactions [ 117 ]. Better yet, at least one compound (termed “7 a” by Carlson et al. 2022) has been identified that blocks both SLO3 and CatSper, indicating potential for synergistic inhibition of sperm motility [ 111 ].
Vas deferens occlusion
The final target we wish readers to know about is physical obstruction of the vas deferens, termed ‘vas occlusion’, via gel injection to physically disrupt sperm during passage through the vas deferens. The benefits of this approach include fast installation (i.e. a quick injection at an outpatient visit) and relatively fast onset of action. A major barrier has been reversibility, but once overcome this approach may hold strong potential in male contraception. Several distinct polymers have been studied, including two styrene compounds termed “reversible inhibition of sperm under guidance” (RISUG) in India [ 118 , 119 , 120 ] and Valsalgel in the United States [ 121 , 122 , 123 ], and silicone and polyurethane compounds in the People’s Republic of China [ 124 , 125 ]. The most recent trial of RISUG showed high contraceptive efficacy and a favorable safety profile [ 120 ], but human trials demonstrating reversibility of RISUG are needed. Despite these setbacks, one newer compound is being investigated in an ongoing clinical trial [ 126 ]. This new compound is a proprietary hydrogel, named ADAM by its founding company, Contraline Inc. of Charlottesville, Virginia. The trial started enrolling in late 2022 with a planned 25 total male participants through June 2025; ADAM injections will be done at the Epworth Freemasons Hospital in Melbourne, Australia. The primary outcome is adverse events, and secondary outcomes include percentage of participants achieving azoospermia and any serious adverse events [ 126 ].
Limitations of the study
This review is subject to several limitations. The clinical trials discussed above are ongoing, and results have yet to be peer-reviewed and published. This does not yet allow for data-driven conclusions. Although this narrative review focuses on the most recent and ongoing studies of male contraception, authors recognize that it is not comprehensive. As mentioned above in Materials and Methods, several compounds were excluded because they failed to progress to human trials, failed after reaching human trials, or are in early preclinical stages. For these, we advise readers to explore several well-written reviews by Thirumalai and Amory [ 127 ], Long et al. [ 128 ], or the University of California San Diego urology department [ 129 ] that include many of these discontinued approaches.
It is long overdue that male partners share the burden of family planning, and it is the authors’ hope that this will soon be a possibility. Ultimately, we feel that two of the methods discussed above—NES/T and DMAU—show the greatest potential for male contraception in the next decade. However, as clinical trials range from early planning stages to data collection stages, it may be several years before we see the efficacy and safety data needed to apply for FDA approval. In particular, the ongoing phase IIb NES/T trial results will not be published before 2025, and this is the method farthest along ‘the pipeline.’
Despite their many theoretical advantages to hormonal contraception, the non-hormonal targets are further from practical application. Authors recognize that there are many obstacles to reaching human studies, let alone late-stage clinical trials. Clinical trials require years of time, meticulous study, and financial support, and many compounds that perform well in pre-clinical animal studies fall short in human trials. The tools needed to efficiently design and study these non-hormonal targets are relatively young. However, they are already being employed to design and test strong drug candidates. As a society we now possess not only the scientific knowledge, technology, and clinical infrastructure needed to overcome these challenges, but also the social drive. With so many strong candidates, it is our hope that there will soon be several safe, effective, and reversible contraceptive options available to male patients.
Availability of data and materials
Not applicable.
Abbreviations
11β-methyl-19-nortestosterone
11β-methyl-19-nortestosterone dodecylcarbonate
Androgen receptor
Bristol-Myers Squibb
Bromodomain testis-specific protein
Cation channel of sperm
Dimethandrolone
Dimethandrolone undecanoate
Follicle-stimulating hormone
Gonadotropin-releasing hormone
Hypothalamic-pituitary-testicular
Intramuscular
Low-density lipoprotein
Luteinizing hormone
Levonorgestrel
7α-Methyl-19-nortestosterone
Nestorone/testosterone transdermal gel
Eunice Kennedy Shriver National Institute of Child Health and Human Development
Progesterone receptor
All-trans retinoic acid
Retinoic acid receptor alpha
Randomized controlled trial
Soluble adenylyl cyclase
Subcutaneous
Sex hormone binding globulin
Slowpoke homolog 3
Testosterone
Testis-specific serine/threonine kinase
Myers C, Jones R, Upadhyay U. Predicted changes in abortion access and incidence in a post-roe world. Contraception. 2019;100(5):367–73. https://doi.org/10.1016/j.contraception.2019.07.139 .
Article PubMed Google Scholar
Kimport K, Rasidjan MP. Exploring the emotional costs of abortion travel in the United States due to legal restriction. Contraception. 2023;120:109956. https://doi.org/10.1016/j.contraception.2023.109956 .
Page ST, Blithe D, Wang C. Hormonal male contraception: getting to market. Front Endocrinol (Lausanne). 2022;13:891589. https://doi.org/10.3389/fendo.2022.891589 .
Kimport K. More than a physical burden: women’s mental and emotional work in preventing pregnancy. J Sex Res. 2018;55(9):1096–105. https://doi.org/10.1080/00224499.2017.1311834 .
Kimport K. Talking about male body-based contraceptives: the counseling visit and the feminization of contraception. Soc Sci Med. 2018;201:44–50. https://doi.org/10.1016/j.socscimed.2018.01.040 .
Article PubMed PubMed Central Google Scholar
Alspaugh A, Barroso J, Reibel M, Phillips S. Women’s contraceptive perceptions, beliefs, and attitudes: an integrative review of qualitative research. J Midwifery Womens Health. 2020;65(1):64–84. https://doi.org/10.1111/jmwh.12992 .
Sundaram A, Vaughan B, Kost K, Bankole A, Finer L, Singh S, et al. Contraceptive failure in the United States: estimates from the 2006–2010 national survey of family growth. Perspect Sex Reprod Health. 2017;49(1):7–16. https://doi.org/10.1363/psrh.12017 .
Vaughan B, Trussell J, Kost K, Singh S, Jones R. Discontinuation and resumption of contraceptive use: results from the 2002 national survey of family growth. Contraception. 2008;78(4):271–83. https://doi.org/10.1016/j.contraception.2008.05.007 .
Bearak J, Popinchalk A, Ganatra B, Moller AB, Tunçalp Ö, Beavin C, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990–2019. Lancet Glob Health. 2020;8(9):e1152–e61. https://doi.org/10.1016/s2214-109x(20)30315-6 .
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(Suppl 1):43–8. https://doi.org/10.2105/ajph.2013.301416 .
Article Google Scholar
Heinemann K, Saad F, Wiesemes M, White S, Heinemann L. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod. 2005;20(2):549–56. https://doi.org/10.1093/humrep/deh574 .
Eberhardt J, van Wersch A, Meikle N. Attitudes towards the male contraceptive pill in men and women in casual and stable sexual relationships. J Fam Plann Reprod Health Care. 2009;35(3):161–5. https://doi.org/10.1783/147118909788707986 .
Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25(2):271–85. https://doi.org/10.1016/j.beem.2010.08.006 .
Nieschlag E. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. October 22–23, 2006. Contraception. 2007;75(3):166-7. doi: https://doi.org/10.1016/j.contraception.2006.12.001 .
Klein CE. The hypothalamic-pituitary-gonadal Axis. In: Kufe DWPR, Weichselbaum RR, et al. editors. Holland-Frei Cancer Medicine. Online: BC Decker Inc; 2003. https://www.ncbi.nlm.nih.gov/books/NBK13386/ .
Google Scholar
Litwack G. Polypeptide Hormones. In: Human Biochemistry. 2nd ed. Online: Andre Gerhard Wolff, Elsevier Inc. 2022. p. 475–516. doi: https://doi.org/10.1016/B978-0-323-85718-5.00013-3 .
von Eckardstein S, Noe G, Brache V, Nieschlag E, Croxatto H, Alvarez F, et al. A clinical trial of 7 alpha-methyl-19-nortestosterone implants for possible use as a long-acting contraceptive for men. J Clin Endocrinol Metab. 2003;88(11):5232–9. https://doi.org/10.1210/jc.2002-022043 .
Article CAS Google Scholar
Walton MJ, Kumar N, Baird DT, Ludlow H, Anderson RA. 7alpha-methyl-19-nortestosterone (MENT) vs testosterone in combination with etonogestrel implants for spermatogenic suppression in healthy men. J Androl. 2007;28(5):679–88. https://doi.org/10.2164/jandrol.107.002683 .
Article PubMed CAS Google Scholar
García-Becerra R, Ordaz-Rosado D, Noé G, Chávez B, Cooney AJ, Larrea F. Comparison of 7α-methyl-19-nortestosterone effectiveness alone or combined with progestins on androgen receptor mediated-transactivation. Reproduction. 2012;143(2):211–9. https://doi.org/10.1530/rep-11-0171 .
Pavlou SN, Brewer K, Farley MG, Lindner J, Bastias MC, Rogers BJ, et al. Combined administration of a gonadotropin-releasing hormone antagonist and testosterone in men induces reversible azoospermia without loss of libido. J Clin Endocrinol Metab. 1991;73(6):1360–9. https://doi.org/10.1210/jcem-73-6-1360 .
Bagatell CJ, Matsumoto AM, Christensen RB, Rivier JE, Bremner WJ. Comparison of a gonadotropin releasing-hormone antagonist plus testosterone (T) versus T alone as potential male contraceptive regimens. J Clin Endocrinol Metab. 1993;77(2):427–32. https://doi.org/10.1210/jcem.77.2.8345047 .
Handelsman DJ, Wishart S, Conway AJ. Oestradiol enhances testosterone-induced suppression of human spermatogenesis. Hum Reprod. 2000;15(3):672–9. https://doi.org/10.1093/humrep/15.3.672 .
Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88(10):4659–67. https://doi.org/10.1210/jc.2003-030107 .
Handelsman DJ, Conway AJ, Howe CJ, Turner L, Mackey MA. Establishing the minimum effective dose and additive effects of depot progestin in suppression of human spermatogenesis by a testosterone depot. J Clin Endocrinol Metab. 1996;81(11):4113–21. https://doi.org/10.1210/jcem.81.11.8923869 .
Kamischke A, Diebäcker J, Nieschlag E. Potential of norethisterone enanthate for male contraception: pharmacokinetics and suppression of pituitary and gonadal function. Clin Endocrinol (Oxf). 2000;53(3):351–8. https://doi.org/10.1046/j.1365-2265.2000.01097.x .
Kamischke A, Heuermann T, Krüger K, von Eckardstein S, Schellschmidt I, Rübig A, et al. An effective hormonal male contraceptive using testosterone undecanoate with oral or injectable norethisterone preparations. J Clin Endocrinol Metab. 2002;87(2):530–9. https://doi.org/10.1210/jcem.87.2.8218 .
Meriggiola MC, Costantino A, Saad F, D’Emidio L, Morselli Labate AM, Bertaccini A, et al. Norethisterone enanthate plus testosterone undecanoate for male contraception: effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab. 2005;90(4):2005–14. https://doi.org/10.1210/jc.2004-1852 .
Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI, et al. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab. 2016;101(12):4779–88. https://doi.org/10.1210/jc.2016-2141 .
WHO Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336(8721):955–9. https://doi.org/10.1016/0140-6736(90)92416-F .
WHO Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65(6):821. https://doi.org/10.1016/S0015-0282(16)58221-1 .
McLachlan RI, McDonald J, Rushford D, Robertson DM, Garrett C, Baker HWG. Efficacy and acceptability of testosterone implants, alone or in combination with a 5α-reductase inhibitor, for male hormonal contraception. Contraception. 2000;62(2):73–8. https://doi.org/10.1016/S0010-7824(00)00139-6 .
Gu Y-Q, Wang X-H, Xu D, Peng L, Cheng L-F, Huang M-K, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy chinese men. J Clin Endocrinol Metab. 2003;88(2):562–8. https://doi.org/10.1210/jc.2002-020447 .
Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in chinese men. J Clin Endocrinol Metab. 2009;94(6):1910–5. https://doi.org/10.1210/jc.2008-1846 .
Handelsman DJ, Conway AJ, Boylan LM. Suppression of human spermatogenesis by testosterone implants. J Clin Endocrinol Metab. 1992;75(5):1326–32. https://doi.org/10.1210/jcem.75.5.1430094 .
Nieschlag E, Hoogen H, Bölk M, Schuster H, Wickings EJ. Clinical trial with testosterone undecanoate for male fertility control. Contraception. 1978;18(6):607–14. https://doi.org/10.1016/0010-7824(78)90045-8 .
Blithe D, Myer K. Study of daily application of Nestorone® (NES) and testosterone (T) combination gel for male contraception. ClinicalTrials.gov: 2023 https://ClinicalTrials.gov/show/NCT03452111 .
Amory JK, Blithe DL, Sitruk-Ware R, Swerdloff RS, Bremner WJ, Dart C, et al. Design of an international male contraceptive efficacy trial using a self-administered daily transdermal gel containing testosterone and segesterone acetate (Nestorone). Contraception. 2023:110064. https://doi.org/10.1016/j.contraception.2023.110064 .
Anawalt BD, Roth MY, Ceponis J, Surampudi V, Amory JK, Swerdloff RS, et al. Combined nestorone-testosterone gel suppresses serum gonadotropins to concentrations associated with effective hormonal contraception in men. Andrology. 2019;7(6):878–87. https://doi.org/10.1111/andr.12603 .
Article PubMed PubMed Central CAS Google Scholar
Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97(10):3476–86. https://doi.org/10.1210/jc.2012-1384 .
Mahabadi V, Amory JK, Swerdloff RS, Bremner WJ, Page ST, Sitruk-Ware R, et al. Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J Clin Endocrinol Metab. 2009;94(7):2313–20. https://doi.org/10.1210/jc.2008-2604 .
Wang C, Page S. Injectable DMAU for male contraception in healthy male volunteers (CCN015); 2023. https://ClinicalTrials.gov/show/NCT02927210 .
Wang C, Page S. Study of spermatogenesis suppression with DMAU alone or with LNG versus placebo alone in normal men. https://ClinicalTrials.gov/show/NCT03455075 ; 2020.
Thirumalai A, Ceponis J, Amory JK, Swerdloff R, Surampudi V, Liu PY, et al. Effects of 28 days of oral dimethandrolone undecanoate in healthy men: a prototype male pill. J Clin Endocrinol Metab. 2019;104(2):423–32. https://doi.org/10.1210/jc.2018-01452 .
Ayoub R, Page ST, Swerdloff RS, Liu PY, Amory JK, Leung A, et al. Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive. Andrology. 2017;5(2):278–85. https://doi.org/10.1111/andr.12303 .
Surampudi P, Page ST, Swerdloff RS, Nya-Ngatchou JJ, Liu PY, Amory JK, et al. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: a prototype oral male hormonal contraceptive. Andrology. 2014;2(4):579–87. https://doi.org/10.1111/j.2047-2927.2014.00216.x .
Yuen F, Thirumalai A, Fernando FA, Swerdloff RS, Liu PY, Pak Y, et al. Comparison of metabolic effects of the progestational androgens dimethandrolone undecanoate and 11β-MNTDC in healthy men. Andrology. 2021;9(5):1526–39. https://doi.org/10.1111/andr.13025 .
Yuen F, Thirumalai A, Pham C, Swerdloff RS, Anawalt BD, Liu PY, et al. Daily oral administration of the novel androgen 11β-MNTDC markedly suppresses serum gonadotropins in healthy men. J Clin Endocrinol Metab. 2020;105(3):e835–47. https://doi.org/10.1210/clinem/dgaa032 .
Wu S, Yuen F, Swerdloff RS, Pak Y, Thirumalai A, Liu PY, et al. Safety and pharmacokinetics of single-dose novel oral androgen 11β-methyl-19-nortestosterone-17β-dodecylcarbonate in men. J Clin Endocrinol Metab. 2019;104(3):629–38. https://doi.org/10.1210/jc.2018-01528 .
Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65(10–11):629–36. https://doi.org/10.1016/s0039-128x(00)00119-7 .
Kumar N, Fagart J, Liere P, Mitchell SJ, Knibb AR, Petit-Topin I, et al. Nestorone® as a novel progestin for nonoral contraception: structure-activity relationships and brain metabolism studies. Endocrinology. 2017;158(1):170–82. https://doi.org/10.1210/en.2016-1426 .
Sitruk-Ware R, Nath A. The use of newer progestins for contraception. Contraception. 2010;82(5):410–7. https://doi.org/10.1016/j.contraception.2010.04.004 .
Fraser IS, Weisberg E, Kumar N, Kumar S, Humberstone AJ, McCrossin L, et al. An initial pharmacokinetic study with a Metered Dose Transdermal System for delivery of the progestogen nestorone as a possible future contraceptive. Contraception. 2007;76(6):432–8. https://doi.org/10.1016/j.contraception.2007.08.006 .
FDA News Release. : FDA approves new vaginal ring for one year of birth control. https://www.fda.gov/news-events/press-announcements/fda-approves-new-vaginal-ring-one-year-birth-control (2018). Accessed 2023.
Archer DF, Merkatz RB, Bahamondes L, Westhoff CL, Darney P, Apter D, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7(8):e1054–e64. https://doi.org/10.1016/s2214-109x(19)30265-7 .
Virro JJ, Besinque K, Carney CE, Gross D, Bernick B, Mirkin S. Long-lasting, patient-controlled, procedure-free contraception: a review of Annovera with a pharmacist perspective. Pharm (Basel). 2020;8(3). https://doi.org/10.3390/pharmacy8030156 .
Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, et al. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab. 2008;93(5):1774–83. https://doi.org/10.1210/jc.2007-2768 .
Roth MY, Ilani N, Wang C, Page ST, Bremner WJ, Swerdloff RS, et al. Characteristics associated with suppression of spermatogenesis in a male hormonal contraceptive trial using testosterone and nestorone(®) gels. Andrology. 2013;1(6):899–905. https://doi.org/10.1111/j.2047-2927.2013.00135.x .
Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: Side effects and health concerns. In: Crowley WF, Schreiber CA, editors.Online: UpToDate. 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns . Accessed 20 April 2023.
Roth MY, Shih G, Ilani N, Wang C, Page ST, Bremner WJ, et al. Acceptability of a transdermal gel-based male hormonal contraceptive in a randomized controlled trial. Contraception. 2014;90(4):407–12. https://doi.org/10.1016/j.contraception.2014.05.013 .
Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology. 2006;147(6):3016–26. https://doi.org/10.1210/en.2005-1524 .
Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR. Dimethandrolone (7alpha,11beta-dimethyl-19-nortestosterone) and 11beta-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase. J Steroid Biochem Mol Biol. 2008;110(3–5):214–22. https://doi.org/10.1016/j.jsbmb.2007.11.009 .
Sharma S, Ahire D, Basit A, Lajoie M, Wang C, Lee MS, et al. Dimethandrolone, a potential male contraceptive pill, is primarily metabolized by the highly polymorphic UDP-glucuronosyltransferase 2B17 enzyme in human intestine and liver. Drug Metab Dispos. 2022;50(12):1493–500. https://doi.org/10.1124/dmd.122.001041 .
Hild SA, Attardi BJ, Koduri S, Till BA, Reel JR. Effects of synthetic androgens on liver function using the rabbit as a model. J Androl. 2010;31(5):472–81. https://doi.org/10.2164/jandrol.109.009365 .
Hild SA, Marshall GR, Attardi BJ, Hess RA, Schlatt S, Simorangkir DR, et al. Development of l-CDB-4022 as a nonsteroidal male oral contraceptive: induction and recovery from severe oligospermia in the adult male cynomolgus monkey (Macaca fascicularis). Endocrinology. 2007;148(4):1784–96. https://doi.org/10.1210/en.2006-1487 .
Attardi BJ, Marck BT, Matsumoto AM, Koduri S, Hild SA. Long-term effects of dimethandrolone 17β-undecanoate and 11β-methyl-19-nortestosterone 17β-dodecylcarbonate on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrated male rats. J Androl. 2011;32(2):183–92. https://doi.org/10.2164/jandrol.110.010371 .
NIDDK. Androgenic Steroids. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. 2020. https://www.ncbi.nlm.nih.gov/books/NBK548931/ .
Thirumalai A, Yuen F, Amory JK, Hoofnagle AN, Swerdloff RS, Liu PY, et al. Dimethandrolone undecanoate, a novel, nonaromatizable androgen, increases P1NP in healthy men over 28 days. J Clin Endocrinol Metab. 2021;106(1):e171–e81. https://doi.org/10.1210/clinem/dgaa761 .
Attardi BJ, Hild SA, Koduri S, Pham T, Pessaint L, Engbring J, et al. The potent synthetic androgens, dimethandrolone (7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone, do not require 5α-reduction to exert their maximal androgenic effects. J Steroid Biochem Mol Biol. 2010;122(4):212–8. https://doi.org/10.1016/j.jsbmb.2010.06.009 .
Mu E, Kulkarni J. Hormonal contraception and mood disorders. Aust Prescr. 2022;45(3):75–9. https://doi.org/10.18773/austprescr.2022.025 .
de Wit AE, Booij SH, Giltay EJ, Joffe H, Schoevers RA, Oldehinkel AJ. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry. 2020;77(1):52–9. https://doi.org/10.1001/jamapsychiatry.2019.2838 .
Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73(11):1154–62. https://doi.org/10.1001/jamapsychiatry.2016.2387 .
Frost JJ, Lindberg LD, Finer LB. Young adults’ contraceptive knowledge, norms and attitudes: associations with risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44(2):107–16. https://doi.org/10.1363/4410712 .
Noman MAA, Kyzer JL, Chung SSW, Wolgemuth DJ, Georg GI. Retinoic acid receptor antagonists for male contraception: current status†. Biol Reprod. 2020;103(2):390–9. https://doi.org/10.1093/biolre/ioaa122 .
Schleif MC, Havel SL, Griswold MD. Function of retinoic acid in development of male and female gametes. Nutrients. 2022;14(6). https://doi.org/10.3390/nu14061293 .
Heller CG, Moore DJ, Paulsen CA. Suppression of spermatogenesis and chronic toxicity in men by a new series of bis(dichloroacetyl) diamines. Toxicol Appl Pharmacol. 1961;3:1–11. https://doi.org/10.1016/0041-008x(61)90002-3 .
Chung SS, Wang X, Roberts SS, Griffey SM, Reczek PR, Wolgemuth DJ. Oral administration of a retinoic acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology. 2011;152(6):2492–502. https://doi.org/10.1210/en.2010-0941 .
Chung SS, Wang X, Wolgemuth DJ. Prolonged oral administration of a pan-retinoic acid receptor antagonist inhibits spermatogenesis in mice with a rapid recovery and changes in the expression of influx and efflux transporters. Endocrinology. 2016;157(4):1601–12. https://doi.org/10.1210/en.2015-1675 .
Amory JK, Muller CH, Shimshoni JA, Isoherranen N, Paik J, Moreb JS, et al. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl. 2011;32(1):111–9. https://doi.org/10.2164/jandrol.110.010751 .
Chung SS, Cuellar RA, Wang X, Reczek PR, Georg GI, Wolgemuth DJ. Pharmacological activity of retinoic acid receptor alpha-selective antagonists in vitro and in vivo. ACS Med Chem Lett. 2013;4(5):446–50. https://doi.org/10.1021/ml300365k .
Cochran AG, Conery AR, Sims RJ 3. Bromodomains: a new target class for drug development. Nat Rev Drug Discov. 2019;18(8):609–28. https://doi.org/10.1038/s41573-019-0030-7 .
Fujisawa T, Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat Rev Mol Cell Biol. 2017;18(4):246–62. https://doi.org/10.1038/nrm.2016.143 .
Wisniewski A, Georg GI. BET proteins: investigating BRDT as a potential target for male contraception. Bioorg Med Chem Lett. 2020;30(6):126958. https://doi.org/10.1016/j.bmcl.2020.126958 .
Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development. 2007;134(19):3507–15. https://doi.org/10.1242/dev.004481 .
Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno JE, et al. Small-molecule inhibition of BRDT for male contraception. Cell. 2012;150(4):673–84. https://doi.org/10.1016/j.cell.2012.06.045 .
Ayoub AM, Hawk LML, Herzig RJ, Jiang J, Wisniewski AJ, Gee CT, et al. BET bromodomain inhibitors with one-step synthesis discovered from virtual screen. J Med Chem. 2017;60(12):4805–17. https://doi.org/10.1021/acs.jmedchem.6b01336 .
Law RP, Atkinson SJ, Bamborough P, Chung CW, Demont EH, Gordon LJ, et al. Discovery of tetrahydroquinoxalines as bromodomain and extra-terminal domain (BET) inhibitors with selectivity for the second bromodomain. J Med Chem. 2018;61(10):4317–34. https://doi.org/10.1021/acs.jmedchem.7b01666 .
Yu Z, Ku AF, Anglin JL, Sharma R, Ucisik MN, Faver JC, et al. Discovery and characterization of bromodomain 2-specific inhibitors of BRDT. Proc Natl Acad Sci U S A. 2021;118(9). https://doi.org/10.1073/pnas.2021102118 .
Guan X, Cheryala N, Karim RM, Chan A, Berndt N, Qi J, et al. Bivalent BET bromodomain inhibitors Confer increased potency and selectivity for BRDT via protein conformational plasticity. J Med Chem. 2022;65(15):10441–58. https://doi.org/10.1021/acs.jmedchem.2c00453 .
Salicioni AM, Gervasi MG, Sosnik J, Tourzani DA, Nayyab S, Caraballo DA, et al. Testis-specific serine kinase protein family in male fertility and as targets for non-hormonal male contraception†. Biol Reprod. 2020;103(2):264–74. https://doi.org/10.1093/biolre/ioaa064 .
Xu B, Hao Z, Jha KN, Zhang Z, Urekar C, Digilio L, et al. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev Biol. 2008;319(2):211–22. https://doi.org/10.1016/j.ydbio.2008.03.047 .
Nayyab S, Gervasi MG, Tourzani DA, Caraballo DA, Jha KN, Teves ME, et al. TSSK3, a novel target for male contraception, is required for spermiogenesis. Mol Reprod Dev. 2021;88(11):718–30. https://doi.org/10.1002/mrd.23539 .
Spiridonov NA, Wong L, Zerfas PM, Starost MF, Pack SD, Paweletz CP, et al. Identification and characterization of SSTK, a serine/threonine protein kinase essential for male fertility. Mol Cell Biol. 2005;25(10):4250–61. https://doi.org/10.1128/mcb.25.10.4250-4261.2005 .
Zhang H, Su D, Yang Y, Zhang W, Liu Y, Bai G, et al. Some single-nucleotide polymorphisms of the TSSK2 gene may be associated with human spermatogenesis impairment. J Androl. 2010;31(4):388–92. https://doi.org/10.2164/jandrol.109.008466 .
Shetty J, Sinville R, Shumilin IA, Minor W, Zhang J, Hawkinson JE, et al. Recombinant production of enzymatically active male contraceptive drug target hTSSK2 - localization of the TSKS domain phosphorylated by TSSK2. Protein Expr Purif. 2016;121:88–96. https://doi.org/10.1016/j.pep.2016.01.009 .
Hawkinson JE, Sinville R, Mudaliar D, Shetty J, Ward T, Herr JC, et al. Potent pyrimidine and pyrrolopyrimidine inhibitors of testis-specific serine/threonine kinase†|2 (TSSK2). ChemMedChem. 2017;12(22):1857–65. https://doi.org/10.1002/cmdc.201700503 .
Dcunha R, Hussein RS, Ananda H, Kumari S, Adiga SK, Kannan N, et al. Current insights and latest updates in sperm motility and associated applications in assisted reproduction. Reprod Sci. 2022;29(1):7–25. https://doi.org/10.1007/s43032-020-00408-y .
O’Rand MG, Widgren EE, Hamil KG, Silva EJ, Richardson RT. Functional studies of eppin. Biochem Soc Trans. 2011;39(5):1447–9. https://doi.org/10.1042/bst0391447 .
O’Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, et al. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306(5699):1189–90. https://doi.org/10.1126/science.1099743 .
O’Rand MG, Hamil KG, Adevai T, Zelinski M. Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PLoS ONE. 2018;13(4):e0195953. https://doi.org/10.1371/journal.pone.0195953 .
Silva AAS, Raimundo TRF, Mariani NAP, Kushima H, Avellar MCW, Buffone MG, et al. Dissecting EPPIN protease inhibitor domains in sperm motility and fertilizing ability: repercussions for male contraceptive development. Mol Hum Reprod. 2021;27(12). https://doi.org/10.1093/molehr/gaab066 .
Balbach M, Ghanem L, Rossetti T, Kaur N, Ritagliati C, Ferreira J, et al. Soluble adenylyl cyclase inhibition prevents human sperm functions essential for fertilization. Mol Hum Reprod. 2021;27(9). https://doi.org/10.1093/molehr/gaab054 .
Miller M, Rossetti T, Ferreira J, Ghanem L, Balbach M, Kaur N, et al. Design, synthesis, and pharmacological evaluation of second-generation soluble adenylyl cyclase (sAC, ADCY10) inhibitors with slow dissociation rates. J Med Chem. 2022;65(22):15208–26. https://doi.org/10.1021/acs.jmedchem.2c01133 .
Balbach M, Rossetti T, Ferreira J, Ghanem L, Ritagliati C, Myers RW, et al. On-demand male contraception via acute inhibition of soluble adenylyl cyclase. Nat Commun. 2023;14(1):637. https://doi.org/10.1038/s41467-023-36119-6 .
Ramos-Espiritu L, Kleinboelting S, Navarrete FA, Alvau A, Visconti PE, Valsecchi F, et al. Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase. Nat Chem Biol. 2016;12(10):838–44. https://doi.org/10.1038/nchembio.2151 .
Ritagliati C, Ayoub S, Balbach M, Buck J, Levin LR. In vivo characterization of sAC null sperm. Front Cell Dev Biol. 2023;11:1134051. https://doi.org/10.3389/fcell.2023.1134051 .
Rahban R, Nef S, CatSper. The complex main gate of calcium entry in mammalian spermatozoa. Mol Cell Endocrinol. 2020;518:110951. https://doi.org/10.1016/j.mce.2020.110951 .
Li H, Ding X, Guan H, Xiong C. Inhibition of human sperm function and mouse fertilization in vitro by an antibody against cation channel of sperm 1: the contraceptive potential of its transmembrane domains and pore region. Fertil Steril. 2009;92(3):1141–6. https://doi.org/10.1016/j.fertnstert.2008.07.1751 .
Rennhack A, Schiffer C, Brenker C, Fridman D, Nitao ET, Cheng YM, et al. A novel cross-species inhibitor to study the function of CatSper ca(2+) channels in sperm. Br J Pharmacol. 2018;175(15):3144–61. https://doi.org/10.1111/bph.14355 .
Carlson AE, Burnett LA, del Camino D, Quill TA, Hille B, Chong JA, et al. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PLoS ONE. 2009;4(8):e6844. https://doi.org/10.1371/journal.pone.0006844 .
Curci L, Carvajal G, Sulzyk V, Gonzalez SN, Cuasnicú PS. Pharmacological inactivation of CatSper blocks sperm fertilizing ability independently of the capacitation status of the cells: implications for non-hormonal contraception. Front Cell Dev Biol. 2021;9:686461. https://doi.org/10.3389/fcell.2021.686461 .
Carlson EJ, Francis R, Liu Y, Li P, Lyon M, Santi CM, et al. Discovery and characterization of multiple classes of human CatSper blockers. ChemMedChem. 2022;17(15):e202000499. https://doi.org/10.1002/cmdc.202000499 .
Chávez JC, Ferreira JJ, Butler A, De La Vega Beltrán JL, Treviño CL, Darszon A, et al. SLO3 K + channels control calcium entry through CATSPER channels in sperm. J Biol Chem. 2014;289(46):32266–75. https://doi.org/10.1074/jbc.M114.607556 .
Tan Z, Garcia TX. SLO3 in the fast lane: the latest male contraceptive target with a promising small-molecule inhibitor. Proc Natl Acad Sci U S A. 2023;120(8):e2221758120. https://doi.org/10.1073/pnas.2221758120 .
Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K + channel from mammalian spermatocytes. J Biol Chem. 1998;273(6):3509–16. https://doi.org/10.1074/jbc.273.6.3509 .
Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584(5):1041–6. https://doi.org/10.1016/j.febslet.2010.02.005 .
Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K + current in mouse spermatozoa. Proc Natl Acad Sci U S A. 2011;108(14):5879–84. https://doi.org/10.1073/pnas.1100240108 .
Lyon M, Li P, Ferreira JJ, Lazarenko RM, Kharade SV, Kramer M, et al. A selective inhibitor of the sperm-specific potassium channel SLO3 impairs human sperm function. Proc Natl Acad Sci U S A. 2023;120(4):e2212338120. https://doi.org/10.1073/pnas.2212338120 .
Guha SK, Singh G, Ansari S, Kumar S, Srivastava A, Koul V, et al. Phase II clinical trial of a vas deferens injectable contraceptive for the male. Contraception. 1997;56(4):245–50. https://doi.org/10.1016/s0010-7824(97)00142-x .
Sharma RS, Mathur AK, Singh R, Das HC, Singh GJ, Toor DPS, et al. Safety & efficacy of an intravasal, one-time injectable & non-hormonal male contraceptive (RISUG): a clinical experience. Indian J Med Res. 2019;150(1):81–6. https://doi.org/10.4103/ijmr.IJMR_635_18 .
Lohiya NK, Ansari AS, Sadasukhi TC, Pachera S, Khilwani B, Dhaked RK. RISUG® offers early contraception: an experience during phase III clinical trials. J Reprod Healthc Med. 2022;3:11. https://doi.org/10.25259/JRHM_8_2022 .
Waller D, Bolick D, Lissner E, Premanandan C, Gamerman G. Azoospermia in rabbits following an intravas injection of Vasalgel ™. Basic Clin Androl. 2016;26:6. https://doi.org/10.1186/s12610-016-0033-8 .
Waller D, Bolick D, Lissner E, Premanandan C, Gamerman G. Reversibility of Vasalgel™ male contraceptive in a rabbit model. Basic Clin Androl. 2017;27:8. https://doi.org/10.1186/s12610-017-0051-1 .
Colagross-Schouten A, Lemoy MJ, Keesler RI, Lissner E, VandeVoort CA. The contraceptive efficacy of intravas injection of Vasalgel™ for adult male rhesus monkeys. Basic Clin Androl. 2017;27:4. https://doi.org/10.1186/s12610-017-0048-9 .
Zhao SC, Zhang SP, Yu RC. Intravasal injection of formed-in-place silicone rubber as a method of vas occlusion. Int J Androl. 1992;15(6):460–4. https://doi.org/10.1111/j.1365-2605.1992.tb01138.x .
Zhao SC, Lian YH, Yu RC, Zhang SP. Recovery of fertility after removal of polyurethane plugs from the human vas deferens occluded for up to 5 years. Int J Androl. 1992;15(6):465–7. https://doi.org/10.1111/j.1365-2605.1992.tb01139.x .
Eisenfrats K, Lawrentschuk N, Contraline. Safety evaluation of the ADAM system. https://www.clinicaltrials.gov/ct2/show/NCT05134428 ; 2023.
Thirumalai A, Amory JK. Emerging approaches to male contraception. Fertil Steril. 2021;115(6):1369–76. https://doi.org/10.1016/j.fertnstert.2021.03.047 .
Long JE, Lee MS, Blithe DL. Update on novel hormonal and nonhormonal male contraceptive development. J Clin Endocrinol Metab. 2021;106(6):e2381–e92. https://doi.org/10.1210/clinem/dgab034 .
Service CA, Puri D, Hsieh TC, Patel DP. Emerging concepts in male contraception: a narrative review of novel, hormonal and non-hormonal options. Ther Adv Reprod Health. 2023;17:26334941221138323. https://doi.org/10.1177/26334941221138323 .
Download references
Acknowledgements
This manuscript was supported by the University of South Dakota Sanford School of Medicine Department of Obstetrics and Gynecology.
Author information
Authors and affiliations.
University of South Dakota Sanford School of Medicine, 1400 W 22nd St, Sioux Falls, SD, 57105, USA
Eli J. Louwagie, Garrett F.L. Quinn & Kristi L. Pond
Chair and Professor, Dept. of Obstetrics and Gynecology, University of South Dakota Sanford School of Medicine; Reproductive Endocrinologist, Sanford Fertility and Reproductive Medicine, 1500 W 22nd St Suite 102, Sioux Falls, SD, 57105, USA
Keith A. Hansen
You can also search for this author in PubMed Google Scholar
Contributions
First author EJL and senior author KAH conceived and wrote this review paper. EJL created Fig. 1 using BioRender.com. GFLQ and KLP assisted with literature search and interpretation. All authors approved the final version of this manuscript.
Corresponding author
Correspondence to Eli J. Louwagie .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Louwagie, E.J., Quinn, G.F., Pond, K.L. et al. Male contraception: narrative review of ongoing research. Basic Clin. Androl. 33 , 30 (2023). https://doi.org/10.1186/s12610-023-00204-z
Download citation
Received : 05 May 2023
Accepted : 26 July 2023
Published : 09 November 2023
DOI : https://doi.org/10.1186/s12610-023-00204-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Male contraception
- Reproductive health
- Endocrinology
- Sperm motility
- Vas deferens
- Contraception masculine
- Santé reproductive
- Endocrinologie
- Progestatif
- Spermatogenèse
- Mobilité des Spermatozoïdes
- Canal déférent
Basic and Clinical Andrology
ISSN: 2051-4190
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 12 April 2024
Immersed in a reservoir of potential: amniotic fluid-derived extracellular vesicles
- Ishara Atukorala ORCID: orcid.org/0000-0003-0194-5877 1 , 2 ,
- Natalie Hannan 1 , 2 &
- Lisa Hui 1 , 2 , 3 , 4
Journal of Translational Medicine volume 22 , Article number: 348 ( 2024 ) Cite this article
177 Accesses
5 Altmetric
Metrics details
This review aims to encapsulate the current knowledge in extracellular vesicles extracted from amniotic fluid and amniotic fluid derived stem/stromal cells. Amniotic fluid (AF) bathes the developing fetus, providing nutrients and protection from biological and mechanical dangers. In addition to containing a myriad of proteins, immunoglobulins and growth factors, AF is a rich source of extracellular vesicles (EVs). These vesicles originate from cells in the fetoplacental unit. They are biological messengers carrying an active cargo enveloped within the lipid bilayer. EVs in reproduction are known to play key roles in all stages of pregnancy, starting from fertilisation through to parturition. The intriguing biology of AF-derived EVs (AF-EVs) in pregnancy and their untapped potential as biomarkers is currently gaining attention. EV studies in numerous animal and human disease models have raised expectations of their utility as therapeutics. Amniotic fluid stem cell and mesenchymal stromal cell-derived EVs (AFSC-EVs) provide an established supply of laboratory-made EVs. This cell-free mode of therapy is popular as an alternative to stem cell therapy, revealing similar, if not better therapeutic outcomes. Research has demonstrated the successful application of AF-EVs and AFSC-EVs in therapy, harnessing their anti-inflammatory, angiogenic and regenerative properties. This review provides an overview of such studies and discusses concerns in this emerging field of research.
Introduction and background
Composition of amniotic fluid.
Amniotic fluid (AF) is a unique conditioning medium for the developing fetus throughout gestation until birth [ 1 ]. The composition and volume of AF changes across gestation and aligns with key gestational stages [ 2 ]. The AF volume increases linearly from first trimester until about 33 weeks gestation and then reduces towards full-term [ 3 ]. It starts as a by-product of maternal serum consisting of water and electrolytes and gradually changes to fetal products by the late second trimester [ 1 , 4 , 5 , 6 ]. In the early weeks of gestation, the fetal skin is a simple epithelium layer, as such AF freely diffuses across [ 5 ]. However, after keratinization completes, around week 25, fetal urination becomes the main source of increasing AF volume, while fetal lung secretions also contribute significantly [ 3 ]. Fetal “respiration” and swallowing remain the principal routes for AF resorption [ 3 , 7 ]. At term, the human fetus produces 800–1200 ml of urine per day, which can replace the entire AF volume within 12–24 h [ 8 , 9 ].
AF is rich in numerous nutrients and growth factors supporting fetal development [ 10 ], while antibodies and antibacterial agents present within the fluid help to protect the fetus from infections [ 11 ]. Apart from playing an integral part in fetal health, AF has been a useful prenatal diagnostic sample, since amniocentesis was first performed in the late 1960s for fetal karyotyping [ 1 ].
What are extracellular vesicles?
Extracellular vesicles (EVs) are lipid-bilayer membrane-enclosed vesicles that are secreted by virtually all cells [ 12 ]. Their diameter can range from small EVs of 30–150 nm to oncosomes of 10 µm [ 13 ]. Since the first description of EVs in the 1980s [ 14 , 15 ], EVs have been extensively researched in health and disease. There are many classes of EVs, including exosomes, oncosomes, shedding microvesicles, migrasomes and apoptotic bodies. The categorisation is based on their biogenesis and secretion mechanisms, size, and function [ 16 , 17 , 18 ]. EVs secreted by the host cells can mediate both proximal and distal signalling events in organisms [ 19 , 20 , 21 ]. Their biological cargo is transported intact, avoiding degradation through the protection of the lipid bilayer membrane [ 22 ]. Their unrestrictive crossing of the blood–brain barrier makes them an appealing delivery mode for central nervous system therapeutics [ 23 , 24 ].
EVs as a method of studying human reproduction
EVs have been a valuable source of information about human reproduction. Examples include uterine luminal fluid EVs in fertilisation, maintaining the sperm viability in the oviduct and continuity of pregnancy by keeping Ca 2+ homeostasis [ 25 ]. The potential influence can be attributed to their selectively packaged cargo [ 26 ]. They appear to play a critical role in embryo implantation, establishing the first communication between the mother and the conceptus [ 27 , 28 ]. Placental EVs are known to influence uterine spiral arterial remodelling under physiological conditions, but might be compromised under pathological conditions [ 29 ].The role of AF-EVs in parturition [ 30 , 31 ] is discussed later in detail.
It is evident that the molecular signature of AF-EV cargo changes according to feto-maternal pathologies, creating opportunities for many clinical applications. Pregnancy complications such as pre-eclampsia [ 32 ] and preterm labour [ 30 , 33 ], fetal complications such as congenital hydronephrosis [ 34 ] and fetal alcohol syndrome [ 35 ] have been studied using AF-EV borne molecules, which are discussed later in detail. While these studies are beneficial in biomarker discovery and knowledge gain, they are yet to achieve clinical translation.
Amniotic fluid EVs and amniotic fluid stem/stromal cell EVs in therapy
Therapeutic applications of EVs have been investigated by researchers, mostly as drug delivery vehicles [ 23 , 24 , 36 ]. However, AF-EVs and AFSC-EVs are more than a transport mode for exogenous therapeutics. They are loaded with endogenous molecules with therapeutic potential, that can influence tissue regeneration, anti-inflammation, paracrine signalling, and immunomodulation [ 37 , 38 ]. Unmodified EVs isolated from term AF have been tested in pre-clinical models to treat conditions such as bronchopulmonary dysplasia [ 39 ] and azoospermia [ 40 ]. They have also been used in human trials to treat severely ill COVID-19 patients. Case studies performed in the USA demonstrated the safe clinical use of AF-EVs in humans, successfully improving lung function of intubated COVID-19 patients [ 41 , 42 ].
EVs derived from amniotic fluid stem cells/stromal cells (AFSC-EVs) are a popular choice for therapeutic experimentation in pre-clinical models, owing to the easy access to the source material and successful laboratory production. The studies included in this review used several distinct terms to identify the cell populations—stem cells, mesenchymal stem cells and mesenchymal stromal cells . The field of stem cell research acknowledges the potential ambiguity in cell nomenclature by various research groups [ 43 , 44 , 45 ]. Therefore, for the purpose of this review, we have used AFSC-EVs to identify EVs derived from the conditioned media of all three different cell types mentioned.
EVs from AF stem cell cultures appear to have a more consistent paracrine profile than stem cells, thus avoiding the unpredictability that is tied with stem cell therapy [ 38 ]. AFSC-EVs have produced positive responses in preclinical studies of various pathologies, including premature ovarian failure [ 46 ], cardiac injury [ 47 , 48 ], neuroinflammation [ 49 , 50 ] and necrotising enterocolitis [ 51 , 52 ].
The aim of this narrative review is to summarise the current knowledge of AF-EVs and AFSC-EVs, including their isolation and characterisation, physiological and pathological implications, and potential clinical applications. Due to the variability in methods used to isolate EVs, studies discussed in this review include a wide range of EV sizes and categories with varying molecular properties, including microparticles, microvesicles, exosomes and nanovesicles (Table 1 ).
Selection of studies
PubMed Central was searched on the 13th of June 2023, using the keyword combination (exosomes OR extracellular vesicles) AND amniotic fluid, using the advanced search option. A total of 148 search results published from 2000 to June 2023 was retrieved. Articles were included if they were full manuscripts published in English reporting original research on EVs directly isolated from AF or from AF stem cell cultures.
A list of 74 articles was selected for full-text review after screening of titles, abstracts, and keywords, of which 7 irrelevant studies were excluded. Two articles were retrieved after a manual search of reference lists of included articles. A total of 69 full-text articles were included (Additional file 1 . List of included studies) (Fig. 1 ). Forty-four (64%) studies were published since 2020. We performed a narrative overview and content synthesis of the final included articles.
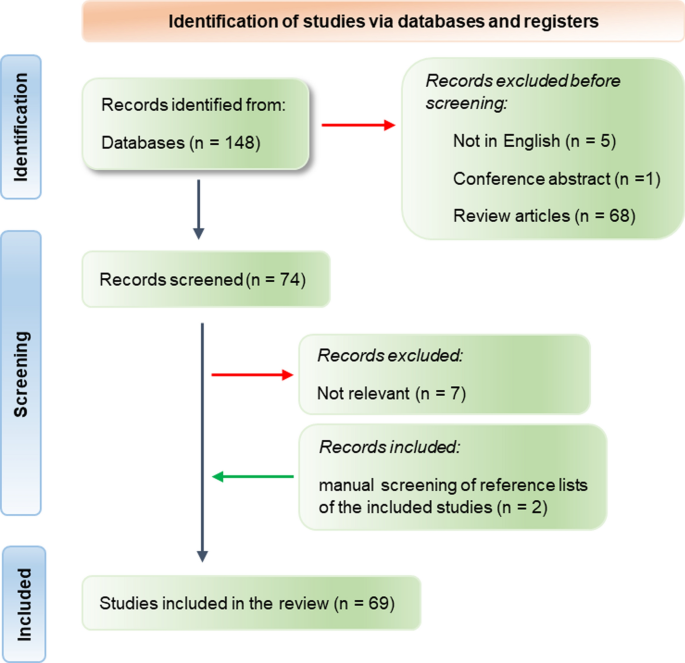
PRISMA flow chart of the study selection criteria for the review. A thorough literature search via NCBI Pubmed resulted in 148 articles, of which 69 were included in this review, after excluding irrelevant studies
AF-EV isolation
The source of af.
The majority of studies derived human AF samples from clinically-indicated amniocentesis (18), term labour or Caesarean section (13). Three studies did not state the source of AF. Two other groups studied murine and ovine AF (Table 2 ).
Lack of standardization in AF-EVs isolation methods
The most common method to isolate small AF-EVs was differential centrifugation coupled with ultracentrifugation. The majority of studies performed centrifugation at 300 g for 15 min to remove cells, followed by 2000 g for 20 min to eliminate cellular debris. This step was most commonly followed by centrifugation at 10,000 g for 30 min and filtration to remove larger vesicles. Ultracentrifugation at 100,000–120,000 g for varying time periods pelleted down small EVs.
Various methods were reported for further purification of EVs following ultracentrifugation. While some researchers opted for density gradient centrifugation or ion exchange chromatography, others used commercially available kits for EV isolation (Table 2 ). Researchers preferred amniocentesis for sample collection over Caesarean section and differential centrifugation for EV isolation as indicated in Table 3 (a summary of Table 2 ).
Ebert and Rai developed an unconventional three-step centrifugation protocol to isolate AF-EVs, that involved addition of dithiothreitol (DTT) to the EV pellet to denature external protein aggregates [ 53 ]. This method may not be suitable for studies focusing on EV membrane proteins as DTT can denature the ectodomains of proteins. Others used a centrifugation-based method in combination with filtration and commercially available chromatography columns for EVs isolation from small volumes (down to 250 µL) of AF [ 54 ]. A comparison of methods study stated that ultracentrifugation resulted in better EV yield from human AF than commercial exosome isolation reagents [ 55 ].
The variability in methods may partly be due to the variability in samples. For example, term AF contains vernix caseosa (white wax-like substance covering the fetal skin) compared to second trimester AF, requiring strenuous sample cleaning steps. While AF can be a challenging sample, one would expect to have largely consistent methods for EV isolation from conditioned media derived from cell cultures.
Amniotic fluid stem/stromal cell EV isolation
Amniotic fluid stem/stromal cell cultures are used as a reliable supply of evs.
Many researchers have isolated AF stem or stromal cells and cultured them to provide a convenient and continuous in vitro source of EVs. These studies used human/murine primary or cryopreserved cells obtained from second-trimester amniocentesis, elective Caesarean sections or both. Five research groups obtained mouse AF stem cells (Table 4 ), presumably to maintain the consistency with experimental animal models. Table 5 summarises this information, providing a count of studies that used different sample sources and EV isolation methods.
Stem cells were most commonly isolated from AF by fluorescence activated cell sorting for c-Kit expression [ 47 , 48 , 52 , 56 , 57 , 58 ] or for CD44/CD105 expression [ 59 ]. Other researchers cultured cells from AF and separated the colonies based on the fibroblast morphology of the cells [ 60 , 61 ]. Whether these different methods impact EV biogenesis and secretion pathways differently in stem cells is yet to be understood.
Majority (79%) of the AFSC-EV studies included in this review referred to their cell populations as stem cells while 2 studies mentioned the isolation of mesenchymal stromal cells. Five other studies mentioned the use of mesenchymal stem cells. Table 4 describes different culture conditions used by research groups to grow the isolated cells.
A variety of isolation methods for AF stem/stromal cell EVs
There is a variety of methods of EV isolation from AF stem cell-conditioned media, but most employed some form of differential centrifugation with many variations in the centrifugation steps. Studies published in the past 2–3 years commonly used the classic approach of differential centrifugation steps to remove live and dead cells (500 g ), cell debris (2000 g ), large vesicles (10,000–15,000 g ) and a final ultracentrifugation collecting small EVs (100,000–120,000 g ) (Table 4 ). A recent study comparing ultracentrifugation and a novel polyethylene glycol (PEG)-based EV precipitation method demonstrated that PEG-based isolation produced approximately five times more EV yield and EV proteins, but one third the EV-RNA content compared to ultracentrifugation [ 62 ]. The choice of isolation method may consequently influence the properties of EVs [ 62 ].
Isolation methods depend on the differential density, solubility factors and size of the target EVs [ 63 ]. Efforts to standardize EV research by the International Society for Extracellular Vesicles is reflected in the studies published since 2020, with a degree of consistency in methods compared to earlier studies. However, all methods result in some degree of variation in size range, purity and protein content of each EV preparation. Some research groups have attempted to standardize their laboratory protocols by adhering to good manufacturing practices (GMP) guidelines [ 41 , 42 , 64 ], or used GMP-grade AF stem cells for culture [ 65 ]. This is an essential step in ensuring that the findings from basic research can eventually be translated into clinical applications and scaled up into commercial products.
Characterisation of EVs should adhere to internationally accepted guidelines
The established guideline for characterising EVs and confirming their successful isolation is the Minimal Information for Studies of Extracellular Vesicles (MISEV2018) statement approved by the International Society for Extracellular Vesicles [ 66 ]. This characterization involves three main steps: (i) nanoparticle tracking analysis to confirm the size range and concentration of the isolated vesicles, (ii) transmission electron microscopy to visualise their morphology, and (iii) screening for standard EV enriched markers such as Alix, TSG-101 and tetraspanins CD63, CD81 and CD9 (Fig. 2 ). Only 23 (36%) of the included studies employed all three characterisation methods.
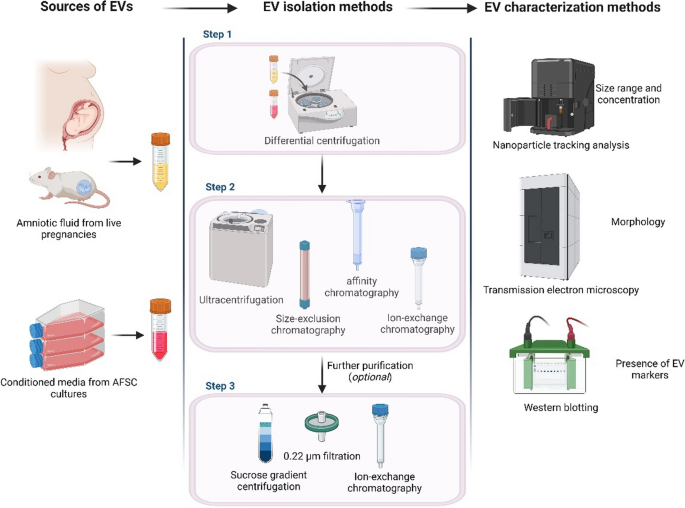
Commonly employed EV isolation and characterisation methods. Human/animal AF or conditioned media of AF stem cell/MSC cultures are first subjected to differential centrifugation to remove cellular debris. The supernatant is subjected to ultracentrifugation/size-exclusion chromatography/affinity chromatography or a combination of these methods. An optional further purification of the isolated EV population is achieved using density gradient centrifugation, filtration, or ion-exchange chromatography. Isolated EVs are characterised using nanoparticle tracking analysis for EV concentration and size range, transmission electron microscopy for EV morphology and Western blotting to analyse EV protein markers. Figure created with BioRender.com
Amniotic fluid EVs are abundant and immunologically active
Human AF appears to be a more concentrated source of EVs compared to other bio-fluids, with AF-EVs concentrations up to 41-times higher than maternal plasma [ 67 ]. AF-derived exosomes are also reportedly smaller (~ 100 nm) than EVs of other sources and contain standard EV markers [ 54 ]. The predominant fetal renal origin of these vesicles has been suggested by the presence of tetraspanin CD24, kidney marker aquaporin-2 [ 68 ] and CD133 [ 32 ]. Other identified proteins in AF-EVs include an obscure, lower molecular weight CA125 species [ 69 ], tubulin and heat shock proteins Hsp72 and Hsc73 [ 70 ]. These extracellularly released heat shock-related proteins are known as alarmins and are expressed under hypoxic, immune or inflammatory stress conditions [ 71 ].
AF-EVs are known for their immunomodulatory properties, which can suppress T-cell activation and pro-inflammatory cytokine release in-vitro [ 72 ]. AF-EVs may act as both pro- and anti-inflammasome activating agents, potentially priming the fetal immunity owing to the presence of bacterial DNA in these vesicles [ 73 ]. Moreover, AF-EVs triggered epithelial-to-mesenchymal transition and myofibroblast activation in stem cells [ 74 ]. These studies have revealed important biological properties of AF-EVs, suggesting their many roles and potential uses.
AF stem/stromal cell-derived EVs are bioactive and have distinct ‘omic profiles
The AFSC-EV therapeutics is a rapidly growing field of research. One of the first studies exploring AFSC-EVs reported on their active immunoregulatory properties [ 75 ]. A recent comparative study confirmed a 25% higher EV yield from AF stem cells compared to human bone marrow-derived stem cells, making them preferable for clinical applications [ 76 ]. They contain a significant amount of the biologically active molecules of the secretome of AF stem cells. AFSC-EVs contain miRNA, but not mRNA, suggesting their role in directly or indirectly regulating existing signalling pathways of recipient cells rather than enforcing new ones [ 47 ].
Researchers have suggested that AFSC-EVs are metabolically independent entities [ 77 ]. Equivalently, EVs isolated from semen of multiple species (human, canine, equine, and bovine origin) produced ATP intrinsically through the glycolytic pathway [ 78 , 79 ]. Presence of active metabolic enzymes, particularly glyoxalases and MG-H1, in AFSC-EVs cargo [ 61 ] adds up to this concept.
AF-EVs contain anti-inflammatory, immunomodulatory, and free radical scavenging properties [ 39 ]. These functions are manifested by stabilizing telomere lengths [ 80 ], increasing cell adhesion and migration, and regulating cytokine production under inflammatory conditions [ 81 ] in recipient cells. These findings indicate that AF-EVs may indirectly modulate the maternal immune system, potentially preventing fetal rejection by the mother’s body.
Selecting the appropriate source of AF stem cells based on desired therapeutic outcome is essential as neonatal and perinatal AFSC-EVs possess distinct proteomic and transcriptomic profiles [ 82 ]. Second trimester amniocentesis-derived immature AFSC-EVs displayed pro-vasculogenic, pro-regenerative, and anti-aging properties, while term pregnancy-derived AFSC-EVs exhibited pronounced immune-modulatory and anti-inflammatory characteristics. However, both types of AFSC-EVs had a rich microRNA signature containing regenerative paracrine factors [ 82 ].
Amniotic fluid derived EVs as potential biomarkers
Exosomal shuttle rna and fetal development.
The RNA cargo in exosomes is known as exosomal shuttle RNA (esRNA) [ 83 ]. esRNA within AF-EVs is protected by the lipid membrane from digestion by nucleases, making transcripts readily available for diagnostic or prognostic purposes [ 22 ]. A number of biomarker discovery studies basing AF-EV esRNA have been published for fetal conditions such as congenital hydronephrosis [ 34 ], congenital diaphragmatic hernia [ 84 ], fetal alcohol exposure, osteogenic differentiation [ 35 ], congenital heart defects [ 85 ] and ureteropelvic junction obstruction [ 86 ]. However, these studies are yet to be translated into clinically useful predictors of perinatal outcomes.
AF-EVs and parturition
Labour is an inflammation driven process. Resident and infiltrating immune cells in reproductive tissue [ 87 , 88 ] and free cytokines in AF are associated with labour, both term and preterm [ 89 , 90 , 91 ]. Preterm labour, intra-amniotic inflammation and infection, all result in differential packaging of cytokines in AF-EVs [ 33 ]. Placental alkaline phosphatase (PLAP)/CD63 ratio in AF-EVs has been suggested as a marker for preterm birth and preterm premature rupture of membranes [ 30 ]. Others have postulated that fetal lung-derived EVs in AF may have a role in parturition, as they induced senescence-associated secretory phenotype and proinflammatory molecules in human amniotic epithelial cells in term pregnancies [ 31 ]. Moreover, transcription regulator HIF1α contained in AF-EVs impacts comparatively shorter interval between amniocentesis and parturition [ 92 ].
AF-EVs in obstetric complications
AF-EVs have been studied in a limited number of obstetric complications. Elevated CD105 (endoglin) in AF-EVs resembled augmented angiogenesis in preeclampsia [ 32 ]. Others studied AF-derived microparticles in disseminated intravascular coagulation and hypotension in amniotic fluid embolism [ 67 ]. These fetal-origin EVs [ 93 ] were predominantly from apoptotic events of epithelial and leukocytic cells [ 94 ]. Their cargo included procoagulant molecules such as phosphatidylserine and tissue factor [ 95 ], and extrinsic tenase complexes [ 96 ].
Congenital cytomegalovirus infection is a common infection worldwide and may result in a range of undesirable outcomes including fetal death [ 97 ]. Identification of the association between the fetal infection and the EV-borne pro-inflammatory cytokine profile [ 98 ], may be a step towards predictive biomarkers for severity of fetal infection.
While these studies have revealed potential AF-EV-borne biomarkers for obstetric complications, they are primarily discovery-phase reports that require to be clinically validated.
Therapeutic applications of AF-EVs and AFSC-EVs
AF and AF cell-derived EVs gained substantial interest as a therapeutic in regenerative medicine. Biological activity of these EVs is dependent on the treatment dose, rather than the specific size or purity of the isolated EV populations [ 99 ]. As a cell-free product loaded with bioactive molecules, they contain many desirable properties. EVs have been shown to modulate inflammation [ 58 , 100 , 101 , 102 ], curb oxidative stress [ 103 ] and augment wound healing [ 104 , 105 ], ultimately leading to tissue regeneration. Moreover, as a natural cell-derived product, EVs present advantages such as biocompatibility and minimal toxicity for recipients. A summary of the preclinical and clinical therapeutic studies retrieved from our literature search is presented in Table 6 .
AF is an accessible human fetal sample with significant biological value. However, until recently, it has been under-explored in reproductive medicine compared to other sources such as maternal plasma and placental tissue. Keller and colleagues first reported the detection of EVs in human and murine AF in 2007 [ 68 ], but the field remained quiescent until the past 4 years. There is an increased interest in AF derived biologics since 2020, making up for 64% of studies in this review.
Researchers have debated the optimal methods for EV isolation and their purity assessment for the last decade [ 63 ]. The community achieved consensus with the publication of the Minimal Information for Studies of Extracellular Vesicles guidelines [ 66 ] regarding basic isolation and characterization of EVs. However, EVs are a heterogenous group and cannot be separated by biogenesis using existing methods [ 18 ]. Therefore, nomenclature of the vesicles is challenging and will remain a discussion for the foreseeable future. At present, large EVs or small EVs seem to be the appropriate terms to describe an EV population, based on the employed isolation methods. Our review shows the inconsistent terminology (Table 1 ) used in reproductive EV research.
Researchers seem to prefer ultracentrifugation over other methods for AF-EVs and AFSC-EVs isolation (Tables 3 and 5 ). However, specific details such as durations of spins and speed were lacking in several studies. Ultracentrifugation is considered the “gold standard” method for EV isolation due to its reliability and optimal yield [ 106 , 107 ]. However, EV samples isolated using ultracentrifugation require further purification methods to achieve homogeneity. The use of other methods such as commercially available chromatography columns and polymeric precipitation were observed when sample sizes were too small for centrifugation. Many factors such as the source material and its volume, EV size range of interest and the downstream use of the isolated EVs can influence the isolation methods. Nonetheless, the choice of isolation method largely appeared to be at the discretion of individual research groups. A clear and globally accepted, robust set of guidelines for the methodologies for AF derived EVs would benefit this emerging research field.
The laborious nature of the differential centrifugation and ultracentrifugation procedures limits the scalability for EV production for clinical use [ 108 ]. Commercial products are attractive solutions but have not gained widespread acceptance as only 17% of studies in this review have utilized them. Methodological studies have compared the commercial EV isolation kits versus ultracentrifugation [ 55 ], and the use of both methods together in the same protocol [ 54 ] resulting in varying inferences. Regardless of these time and labour effective new commercial products, ultracentrifugation remains the preferred method for most researchers. Studies have presented EV concentrations using a range of units such as particles per gram of EV proteins, vesicles per millilitre of fluid (it is unclear if the fluid refers to AF or the EV suspension buffer) and EV proteins (µg) per millilitre. Adoption of a standard unit such as vesicle number per millilitre/gram of starting material (body fluid/tissue) or per million cells would help advance the field by allowing more direct comparisons of results and facilitating replication of studies.
EV isolation from conditioned media requires specific conditions. Use of serum-free culture media or EV-depleted FBS in the media is widely accepted, to avoid introducing exogenous EVs. Other components such as antibiotics, growth factors and supplements can also affect EV biogenesis and their cargo [ 66 ]. Confluence of cells, culture temperature, percentage CO 2 , O 2 and incubation time before EV isolation may all alter EV yield, quality and their biomolecule content [ 109 , 110 ].
Therefore, it is important all information is reported accurately in publications and lack thereof may result in lack of reproducibility. Many groups studied RNA cargo in EVs to develop predictive disease biomarkers. However, the effect of different EV and evRNA purification methods for downstream sequencing and profiling is not known [ 18 ]. Standardization of methodologies and terminology for publications is of central importance going forward. The compliance of experimental protocols with good manufacturing practice guidelines is highly commendable, which improves the quality of research and reproducibility across laboratories, facilitating smooth clinical translation.
Only one clinical application for AF-EVs has progressed to human clinical trials, no doubt accelerated by the urgency to develop novel therapies during the COVID-19 pandemic. Zofin, a human AF derivative enriched for EVs, is being evaluated in COVID-19 patients with severe acute respiratory syndrome in three separate studies, by the same group (NCT05228899, NCT04657406, NCT04384445). These clinical trials are still in progress, but pilot studies have proved safe use of AF-EVs with improved clinical outcomes.
The appeal of AF-EVs for COVID-19 treatment lies in their anti-inflammatory properties and their potential to curb the ‘cytokine storm’ of severe disease. Another clinical trial in Israel (NCT04747574) administered CD24-loaded EVs derived from HEK293 cells to COVID-19 patients, with encouraging outcomes [ 111 ]. Several other groups have also manifested the safety and feasibility of using acellular AF (not enriched for EVs) to treat COVID-19 patients in the clinic [ 112 , 113 ]. Treatments for other inflammatory diseases also have shown the capacity of both AF-EVs and AFSC-EVs to reduce inflammation, restoring tissues or cells to their homeostatic state.
The number of clinical trials using AF-EVs or AFSC-EVs is currently minimal. However, clinical trials have used processed or unprocessed AF to treat chronic wounds (NCT04438174), osteoarthritis (NCT03074526, NCT02768155, NCT04886960), stenosing tenosynovitis (NCT03583151) and venous stasis ulcer (NCT04647240) among many others. The need for expertise, purpose-built instrument and laborious nature of isolating EVs may have delayed AF derived EV research reaching clinical translation.
Regenerative properties of AF-EVs and AFSC-EVs were used to treat necrotizing enterocolitis, premature ovarian failure and wound healing [ 99 , 114 ]. Most studies demonstrated the desirable outcomes of these EV treatments in in-vitro and in-vivo models and some studies deciphered the underlying molecular mechanisms. In-depth understanding of the mechanisms will be beneficial in translating the findings to clinical applications. For example, AFSC-EVs treatment of cystinosis may have revealed a prospective targeted therapy for this rare disease, as the EVs were naturally loaded with cystinosin and reprogrammed the recipient mutant cells [ 115 ].
Stem cell-EV therapy has emerged as an attractive alternative to stem cell therapy, as it omits the challenges of unpredictable host rejection and poor efficacy. The shift in interest was promoted by research studies increasingly implying that the therapeutic effect of stem cells is mediated by the extracellular paracrine factors exerted via EVs [ 38 ]. Many research studies have demonstrated the successful utility of AFSC-EVs in pre-clinical models to treat different pathologies including necrotizing enterocolitis [ 51 , 52 , 100 , 101 ], hypoplastic neonatal lungs [ 65 , 116 , 117 ] and wound healing [ 104 , 105 ]. AF composition is dynamic and often represents the gestation-dependent development of fetal organs [ 118 , 119 ]. Accordingly, the careful choice of gestation for AF collection according to the intended purpose of EVs was observed in these studies (Fig. 3 ). For example, for lung function-related therapies, AF obtained from elective Caesarean sections at term was used for EV or stem cell isolation, as fetal lungs rapidly develop close to parturition [ 120 ]. For other conditions, such as treating wound healing and necrotising enterocolitis, researchers used samples from second-trimester amniocentesis, where the AF is rich with factors implicated in tissue regeneration.
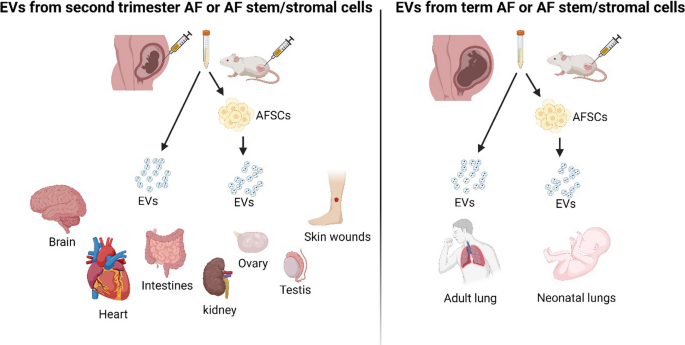
Gestation of amniotic fluid is matched with intended therapeutic use. The gestation at which the AF was collected was often matched to the therapeutic purpose of the research studies/clinical trials. For example, second trimester AF derived EVs were used when the regenerative properties of EVs were desired whereas third trimester AF derived EVs were preferred for lung function therapies. Researchers obtained second trimester AF from amniocentesis and third trimester AF from labour/Caesarean section at term. Figure created with BioRender.com
Our understanding of the biological difference between AF-EVs and AFSC-EVs is narrow and therefore there is currently no definitive evidence to propose biological superiority of one over the other. They conceivably are not bioequivalent and cannot be used inter-changeably. This is a grey area that has not been looked at yet. Researchers seem to be interested in EVs from both sources alike. Thirty-four (49%) articles included in this review used AF-EVs while 35 (51%) used AFSC-EVs. Since AFSC-EVs originate from one cell type, presumably they have minimal batch variations and more predictable biological properties compared to AF-EVs—both beneficial properties for clinical use. Therefore, a comprehensive comparison between AF-EVs and AFSC-EVs can benefit their applications.
If these EVs clear the hurdles to become therapeutics, AF collection and processing mechanisms will need to be increased and standardised. Additional research is needed to assess the inherent variation in AF samples from different donors and the suitability of singular or pooled samples for clinical applications. Despite the great excitement, there is a real risk that many studies of EVs as prognostic markers or therapies may be lost in the ‘valley of death’ between preclinical studies and clinical trials [ 121 ]. Therefore, further research, together with standardisation, may immensely progress the translation of these findings into clinical applications.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341–8.
Article PubMed Google Scholar
Wintour EM, Shandley L. Effects of fetal fluid balance on amniotic fluid volume. Semin Perinatol. 1993;17(3):158–72.
CAS PubMed Google Scholar
Moore TR. Amniotic fluid dynamics reflect fetal and maternal health and disease. Obstet Gynecol. 2010;116(3):759–65.
Sherer DM. A review of amniotic fluid dynamics and the enigma of isolated oligohydramnios. Am J Perinatol. 2002;19(5):253–66.
Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG. Amniotic fluid water dynamics. Placenta. 2007;28(8–9):816–23.
Article CAS PubMed Google Scholar
Shamsnajafabadi H, Soheili ZS. Amniotic fluid characteristics and its application in stem cell therapy: a review. Int J Reprod Biomed. 2022;20(8):627–43.
CAS PubMed PubMed Central Google Scholar
Ross MG, Nijland MJM. Development of ingestive behavior. Am J Physiol Regul Integr Comp Physiol. 1998;274(4):R879–93.
Article CAS Google Scholar
Rabinowitz R, Peters MT, Vyas S, Campbell S, Nicolaides KH. Measurement of fetal urine production in normal pregnancy by real-time ultrasonography. Am J Obstet Gynecol. 1989;161(5):1264–6.
Pierce J, Jacobson P, Benedetti E, Peterson E, Phibbs J, Preslar A, et al. Collection and characterization of amniotic fluid from scheduled C-section deliveries. Cell Tissue Bank. 2016;17(3):413–25.
Tong XL, Wang L, Gao TB, Qin YG, Qi YQ, Xu YP. Potential function of amniotic fluid in fetal development–-novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J Chin Med Assoc. 2009;72(7):368–73.
Mao Y, Pierce J, Singh-Varma A, Boyer M, Kohn J, Reems J-A. Processed human amniotic fluid retains its antibacterial activity. J Transl Med. 2019;17(1):68.
Article PubMed PubMed Central Google Scholar
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–89.
Meehan B, Rak J, Di Vizio D. Oncosomes—large and small: what are they, where they came from? J Extracell Vesicles. 2016;5:33109.
Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–8.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–39.
Kang T, Atukorala I, Mathivanan S. Biogenesis of extracellular vesicles. In: Mathivanan S, Fonseka P, Nedeva C, Atukorala I, editors. New frontiers: extracellular vesicles. Cham: Springer International Publishing; 2021. p. 19–43.
Chapter Google Scholar
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12.
Article CAS PubMed PubMed Central Google Scholar
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360.
Article Google Scholar
Barry OP, Praticò D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102(1):136–44.
Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, et al. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56(2):193–204.
Kalluri R. The biology and function of exosomes in cancer. J Clin Investig. 2016;126(4):1208–15.
Keller S, Ridinger J, Rupp A-K, Janssen JWG, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9(1):86.
Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol Ther. 2017;25(6):1269–78.
Du R, Wang C, Zhu L, Yang Y. Extracellular vesicles as delivery vehicles for therapeutic nucleic acids in cancer gene therapy: progress and challenges. Pharmaceutics. 2022;14(10):2236.
Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS ONE. 2013;8(11): e80181.
Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE. 2014;9(3): e90913.
O’Neil EV, Burns GW, Spencer TE. Extracellular vesicles: novel regulators of conceptus-uterine interactions? Theriogenology. 2020;150:106–12.
Nakamura K, Kusama K, Suda Y, Fujiwara H, Hori M, Imakawa K. Emerging role of extracellular vesicles in embryo-maternal communication throughout implantation processes. Int J Mol Sci. 2020;21(15):5523.
Salomon C, Yee SW, Mitchell MD, Rice GE. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed Res Int. 2014;2014: 693157.
Dixon CL, Sheller-Miller S, Saade GR, Fortunato SJ, Lai A, Palma C, et al. Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor. Endocrinology. 2018;159(5):2229–40.
Wan S, Chen P, Gu M, Liu J, Zhou Q, Zhang F, et al. Fetal lung-derived exosomes in term labor amniotic fluid induce amniotic membrane senescence. Front Cell Dev Biol. 2022;10: 889861.
Gebara N, Scheel J, Skovronova R, Grange C, Marozio L, Gupta S, et al. Single extracellular vesicle analysis in human amniotic fluid shows evidence of phenotype alterations in preeclampsia. J Extracell Vesicles. 2022;11(5): e12217.
Bhatti G, Romero R, Rice GE, Fitzgerald W, Pacora P, Gomez-Lopez N, et al. Compartmentalized profiling of amniotic fluid cytokines in women with preterm labor. PLoS ONE. 2020;15(1): e0227881.
Xie J, Zhou Y, Gao W, Li Z, Xu Z, Zhou L. The relationship between amniotic fluid miRNAs and congenital obstructive nephropathy. Am J Transl Res. 2017;9(4):1754–63.
Tavanasefat H, Li F, Koyano K, Gourtani BK, Marty V, Mulpuri Y, et al. Molecular consequences of fetal alcohol exposure on amniotic exosomal miRNAs with functional implications for stem cell potency and differentiation. PLoS ONE. 2020;15(11): e0242276.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Costa A, Quarto R, Bollini S. Small extracellular vesicles from human amniotic fluid samples as promising theranostics. Int J Mol Sci. 2022;23(2):590.
Johnson J, Shojaee M, Mitchell Crow J, Khanabdali R. From mesenchymal stromal cells to engineered extracellular vesicles: a new therapeutic paradigm. Front Cell Dev Biol. 2021;9: 705676.
Bellio MA, Young KC, Milberg J, Santos I, Abdullah Z, Stewart D, et al. Amniotic fluid-derived extracellular vesicles: characterization and therapeutic efficacy in an experimental model of bronchopulmonary dysplasia. Cytotherapy. 2021;23(12):1097–107.
Mobarak H, Heidarpour M, Rahbarghazi R, Nouri M, Mahdipour M. Amniotic fluid-derived exosomes improved spermatogenesis in a rat model of azoospermia. Life Sci. 2021;274: 119336.
Mitrani MI, Bellio MA, Meglin A, Khan A, Xu X, Haskell G, et al. Treatment of a COVID-19 long hauler with an amniotic fluid-derived extracellular vesicle biologic. Respir Med Case Rep. 2021;34: 101502.
PubMed PubMed Central Google Scholar
Mitrani MI, Bellio MA, Sagel A, Saylor M, Kapp W, VanOsdol K, et al. Case report: administration of amniotic fluid-derived nanoparticles in three severely ill COVID-19 patients. Front Med (Lausanne). 2021;8: 583842.
Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–24.
Robey P. “Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res. 2017;6:F1000.
Lindner U, Kramer J, Rohwedel J, Schlenke P. Mesenchymal stem or stromal cells: toward a better understanding of their biology? Transfus Med Hemother. 2010;37(2):75–83.
Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120.
Mellows B, Mitchell R, Antonioli M, Kretz O, Chambers D, Zeuner MT, et al. Protein and molecular characterization of a clinically compliant amniotic fluid stem cell-derived extracellular vesicle fraction capable of accelerating muscle regeneration through enhancement of angiogenesis. Stem Cells Dev. 2017;26(18):1316–33.
Balbi C, Lodder K, Costa A, Moimas S, Moccia F, van Herwaarden T, et al. Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting using the human amniotic fluid stem cell secretome. Int J Cardiol. 2019;287:87–95.
Castelli V, Antonucci I, d’Angelo M, Tessitore A, Zelli V, Benedetti E, et al. Neuroprotective effects of human amniotic fluid stem cells-derived secretome in an ischemia/reperfusion model. Stem Cells Transl Med. 2021;10(2):251–66.
Gatti M, Zavatti M, Beretti F, Giuliani D, Vandini E, Ottani A, et al. Oxidative stress in Alzheimer’s disease: in vitro therapeutic effect of amniotic fluid stem cells extracellular vesicles. Oxid Med Cell Longev. 2020;2020:2785343.
McCulloh CJ, Olson JK, Wang Y, Zhou Y, Tengberg NH, Deshpande S, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. 2018;53(6):1215–20.
Li B, Lee C, O’Connell JS, Antounians L, Ganji N, Alganabi M, et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. 2020;11(9):750.
Ebert B, Rai AJ. Isolation and characterization of amniotic fluid-derived extracellular vesicles for biomarker discovery. In: Levy B, editor. Prenatal diagnosis. New York: Springer, New York; 2019. p. 287–94.
Sheller-Miller S, Menon R. Chapter ten—isolation and characterization of human amniotic fluid-derived exosomes. In: Spada S, Galluzzi L, editors. Methods in enzymology, vol. 645. Academic Press; 2020. p. 181–94.
Google Scholar
Seyfizadeh N, Seyfizadeh N, Rahbarghazi R, Nourazarian A, Borzouisileh S, Palideh A, et al. Isolation and characterization of human amniotic fluid and SH-SY5Y/BE(2)-M17 cell derived exosomes. Acta Neurobiol Exp (Wars). 2019;79(3):261–9.
Zavatti M, Beretti F, Casciaro F, Bertucci E, Maraldi T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors. 2020;46(1):106–17.
Gatti M, Beretti F, Zavatti M, Bertucci E, Ribeiro Luz S, Palumbo C, et al. Amniotic fluid stem cell-derived extracellular vesicles counteract steroid-induced osteoporosis in vitro. Int J Mol Sci. 2020;22(1):38.
Zavatti M, Gatti M, Beretti F, Palumbo C, Maraldi T. Exosomes derived from human amniotic fluid mesenchymal stem cells preserve microglia and neuron cells from Aβ. Int J Mol Sci. 2022;23(9):4967.
Geng Z, Chen H, Zou G, Yuan L, Liu P, Li B, et al. Human amniotic fluid mesenchymal stem cell-derived exosomes inhibit apoptosis in ovarian granulosa cell via miR-369-3p/YAF2/PDCD5/p53 pathway. Oxid Med Cell Longev. 2022;2022:3695848.
Takov K, He Z, Johnston HE, Timms JF, Guillot PV, Yellon DM, et al. Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic Res Cardiol. 2020;115(3):26.
Romani R, Talesa VN, Antognelli C. The glyoxalase system is a novel cargo of amniotic fluid stem-cell-derived extracellular vesicles. Antioxidants (Basel). 2022;11(8):1524.
Jia L, Li B, Fang C, Liang X, Xie Y, Sun X, et al. Extracellular vesicles of mesenchymal stem cells are more effectively accessed through polyethylene glycol-based precipitation than by ultracentrifugation. Stem Cells Int. 2022;2022:3577015.
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347.
Costa A, Balbi C, Garbati P, Palamà MEF, Reverberi D, De Palma A, et al. Investigating the paracrine role of perinatal derivatives: human amniotic fluid stem cell-extracellular vesicles show promising transient potential for cardiomyocyte renewal. Front Bioeng Biotechnol. 2022;10:902038.
Khalaj K, Antounians L, Figueira RL, Post M, Zani A. Autophagy is impaired in fetal hypoplastic lungs and rescued by administration of amniotic fluid stem cell extracellular vesicles. Am J Respir Crit Care Med. 2022;206(4):476–87.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Uszyński W, Zekanowska E, Uszyński M, Zyliński A, Kuczyński J. New observations on procoagulant properties of amniotic fluid: microparticles (MPs) and tissue factor-bearing MPs (MPs-TF), comparison with maternal blood plasma. Thromb Res. 2013;132(6):757–60.
Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72(9):1095–102.
Mitić N, Kosanović M, Milutinović B, Goč S, Mladenović D, Grubiša I, et al. Nano-sized CA125 antigen glycocamouflage: mucin—extracellular vesicles alliance to watch? Arch Biochem Biophys. 2018;653:113–20.
Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, et al. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol. 2008;79(1):12–7.
Taha EA, Ono K, Eguchi T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int J Mol Sci. 2019;20(18):4588.
del Rivero T, Milberg J, Bennett C, Mitrani MI, Bellio MA. Human amniotic fluid derived extracellular vesicles attenuate T cell immune response. Front Immunol. 2022;13:977809.
Nunzi E, Mezzasoma L, Bellezza I, Zelante T, Orvietani P, Coata G, et al. Microbiota-associated HAF-EVs regulate monocytes by triggering or inhibiting inflammasome activation. Int J Mol Sci. 2023;24(3):2527.
Liu N, Bowen CM, Shoja MM, Castro de Pereira KL, Dongur LP, Saad A, et al. Comparative analysis of co-cultured amniotic cell-conditioned media with cell-free amniotic fluid reveals differential effects on epithelial-mesenchymal transition and myofibroblast activation. Biomedicines. 2022;10(9):2189.
Romani R, Pirisinu I, Calvitti M, Pallotta MT, Gargaro M, Bistoni G, et al. Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1. J Cell Mol Med. 2015;19(7):1593–605.
Tracy SA, Ahmed A, Tigges JC, Ericsson M, Pal AK, Zurakowski D, et al. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: bone marrow and amniotic fluid. J Pediatr Surg. 2019;54(1):86–90.
Mezzasoma L, Bellezza I, Orvietani P, Manni G, Gargaro M, Sagini K, et al. Amniotic fluid stem cell-derived extracellular vesicles are independent metabolic units capable of modulating inflammasome activation in THP-1 cells. FASEB J. 2022;36(4): e22218.
Ronquist KG, Ek B, Morrell J, Stavreus-Evers A, Ström Holst B, Humblot P, et al. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP). Biochimica et Biophysica Acta (BBA) General Subjects. 2013;1830(10):4604–10.
Ronquist KG, Sanchez C, Dubois L, Chioureas D, Fonseca P, Larsson A, et al. Energy-requiring uptake of prostasomes and PC3 cell-derived exosomes into non-malignant and malignant cells. J Extracell Vesicles. 2016;5:29877.
Radeghieri A, Savio G, Zendrini A, Di Noto G, Salvi A, Bergese P, et al. Cultured human amniocytes express hTERT, which is distributed between nucleus and cytoplasm and is secreted in extracellular vesicles. Biochem Biophys Res Commun. 2017;483(1):706–11.
Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288(51):36691–702.
Costa A, Ceresa D, De Palma A, Rossi R, Turturo S, Santamaria S, et al. Comprehensive profiling of secretome formulations from fetal- and perinatal human amniotic fluid stem cells. Int J Mol Sci. 2021;22(7):3713.
Li J, Tian T, Zhou X. The role of exosomal shuttle RNA (esRNA) in lymphoma. Crit Rev Oncol Hematol. 2019;137:27–34.
Fabietti I, Nardi T, Favero C, Dioni L, Cantone L, Pergoli L, et al. Extracellular vesicles and their miRNA content in amniotic and tracheal fluids of fetuses with severe congenital diaphragmatic hernia undergoing fetal intervention. Cells. 2021;10(6):1493.
Yang H, Yang S, Shen H, Wu S, Ruan J, Lyu G. Construction of the amniotic fluid-derived exosomal ceRNA network associated with ventricular septal defect. Genomics. 2021;113(6):4293–302.
Liu R, Zhang W, Luo M, Qin X, Yang F, Wei Q. iTRAQ-based proteomics and in vitro experiments reveals essential roles of ACE and AP-N in the renin–angiotensin system-mediated congenital ureteropelvic junction obstruction. Exp Cell Res. 2020;393(1): 112086.
Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11(6):571–81.
Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(1):104.e1-e11.
Klebanoff M, Searle K. The role of inflammation in preterm birth–focus on periodontitis. BJOG. 2006;113(Suppl 3):43–5.
Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166(11):1312–9.
Halgunset J, Johnsen H, Kjøllesdal AM, Qvigstad E, Espevik T, Austgulen R. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur J Obstet Gynecol Reprod Biol. 1994;56(3):153–60.
Song JE, Park SJ, Lee KY, Lee WJ. Amniotic fluid HIF1α and exosomal HIF1α in cervical insufficiency patients with physical examination-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32(14):2287–94.
Butov KR, Karetnikova NA, Pershin DY, Trofimov DY, Panteleev MA. Procoagulant activity in amniotic fluid is associated with fetal-derived extracellular vesicles. Curr Issues Mol Biol. 2022;44(6):2710–6.
Liu S, Wei L, Zhang Y, Xu M, Wang C, Zhou J. Procoagulant activity and cellular origin of microparticles in human amniotic fluid. Thromb Res. 2014;133(4):645–51.
Hell L, Wisgrill L, Ay C, Spittler A, Schwameis M, Jilma B, et al. Procoagulant extracellular vesicles in amniotic fluid. Transl Res. 2017;184:12-20.e1.
Hu Y, Repa A, Lisman T, Yerlikaya-Schatten G, Hau C, Pabinger I, et al. Extracellular vesicles from amniotic fluid, milk, saliva, and urine expose complexes of tissue factor and activated factor VII. J Thromb Haemost. 2022;20(10):2306–12.
Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76.
Bourgon N, Fitzgerald W, Aschard H, Magny J-F, Guilleminot T, Stirnemann J, et al. Cytokine profiling of amniotic fluid from congenital cytomegalovirus infection. Viruses. 2022;14(10):2145.
Antounians L, Tzanetakis A, Pellerito O, Catania VD, Sulistyo A, Montalva L, et al. The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci Rep. 2019;9(1):1837.
O’Connell JS, Lee C, Farhat N, Antounians L, Zani A, Li B, et al. Administration of extracellular vesicles derived from human amniotic fluid stem cells: a new treatment for necrotizing enterocolitis. Pediatr Surg Int. 2021;37(3):301–9.
Hu X, Zhang R, Liang H, An J, Yang Y, Huo J, et al. Comparison and investigation of exosomes from human amniotic fluid stem cells and human breast milk in alleviating neonatal necrotizing enterocolitis. Stem Cell Rev Rep. 2023;19(3):754–66.
Katifelis H, Filidou E, Psaraki A, Yakoub F, Roubelakis MG, Tarapatzi G, et al. Amniotic fluid-derived mesenchymal stem/stromal cell-derived secretome and exosomes improve inflammation in human intestinal subepithelial myofibroblasts. Biomedicines. 2022;10(10):2357.
Gatti M, Dittlau KS, Beretti F, Yedigaryan L, Zavatti M, Cortelli P, et al. Human neuromuscular junction on a chip: impact of amniotic fluid stem cell extracellular vesicles on muscle atrophy and NMJ integrity. Int J Mol Sci. 2023;24(5):4944.
Zhang Y, Yan J, Liu Y, Chen Z, Li X, Tang L, et al. Human amniotic fluid stem cell-derived exosomes as a novel cell-free therapy for cutaneous regeneration. Front Cell Dev Biol. 2021;9: 685873.
Wgealla M, Liang H, Chen R, Xie Y, Li F, Qin M, et al. Amniotic fluid derived stem cells promote skin regeneration and alleviate scar formation through exosomal miRNA-146a-5p via targeting CXCR4. J Cosmet Dermatol. 2022;21(10):5026–36.
Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3–22.
Momen-Heravi F, Balaj L, Alian S, Mantel PY, Halleck AE, Trachtenberg AJ, et al. Current methods for the isolation of extracellular vesicles. Biol Chem. 2013;394(10):1253–62.
Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing Exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24(3):242–56.
Palviainen M, Saari H, Kärkkäinen O, Pekkinen J, Auriola S, Yliperttula M, et al. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J Extracell Vesicles. 2019;8(1):1596669.
Gudbergsson JM, Johnsen KB, Skov MN, Duroux M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology. 2016;68(4):579–92.
Shapira S, Ben Shimon M, Hay-Levi M, Shenberg G, Choshen G, Bannon L, et al. A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond. EMBO Mol Med. 2022;14(9): e15997.
Joseph ET, Jan P, Nathan H, Giavonni L, John DP, Alyssa M, et al. Safety and feasibility of using acellular sterile filtered amniotic fluid as a treatment for patients with COVID-19: protocol for a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open. 2021;11(2): e045162.
Selzman CH, Tonna JE, Pierce J, Vargas C, Skidmore C, Lewis G, et al. A pilot trial of human amniotic fluid for the treatment of COVID-19. BMC Res Notes. 2021;14(1):32.
Beretti F, Zavatti M, Casciaro F, Comitini G, Franchi F, Barbieri V, et al. Amniotic fluid stem cell exosomes: therapeutic perspective. BioFactors. 2018;44(2):158–67.
Iglesias DM, El-Kares R, Taranta A, Bellomo F, Emma F, Besouw M, et al. Stem cell microvesicles transfer cystinosin to human cystinotic cells and reduce cystine accumulation in vitro. PLoS ONE. 2012;7(8): e42840.
Khalaj K, Figueira RL, Antounians L, Gandhi S, Wales M, Montalva L, et al. Treatment with amniotic fluid stem cell extracellular vesicles promotes fetal lung branching and cell differentiation at canalicular and saccular stages in experimental pulmonary hypoplasia secondary to congenital diaphragmatic hernia. Stem Cells Transl Med. 2022;11(10):1089–102.
Antounians L, Catania VD, Montalva L, Liu BD, Hou H, Chan C, et al. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med. 2021;13(590):eaax5941.
Hui L, Slonim DK, Wick HC, Johnson KL, Bianchi DW. The amniotic fluid transcriptome: a source of novel information about human fetal development. Obstet Gynecol. 2012;119(1):111–8.
Tarca AL, Romero R, Pique-Regi R, Pacora P, Done B, Kacerovsky M, et al. Amniotic fluid cell-free transcriptome: a glimpse into fetal development and placental cellular dynamics during normal pregnancy. BMC Med Genomics. 2020;13(1):25.
Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2017;170:19–27.
Fernandez-Moure JS. Lost in translation: the gap in scientific advancements and clinical application. Front Bioeng Biotechnol. 2016;4:43.
Bellio MA, Bennett C, Arango A, Khan A, Xu X, Barrera C, et al. Proof-of-concept trial of an amniotic fluid-derived extracellular vesicle biologic for treating high risk patients with mild-to-moderate acute COVID-19 infection. Biomater Biosyst. 2021;4: 100031.
Kosanović M, Milutinović B, Goč S, Mitić N, Janković M. Ion-exchange chromatography purification of extracellular vesicles. Biotechniques. 2017;63(2):65–71.
Li P, Lu X, Hu J, Dai M, Yan J, Tan H, et al. Human amniotic fluid derived-exosomes alleviate hypoxic encephalopathy by enhancing angiogenesis in neonatal mice after hypoxia. Neurosci Lett. 2022;768: 136361.
Shahlaei M, Saeidifar M, Zamanian A. Sustained release of sulforaphane by bioactive extracellular vesicles for neuroprotective effect on chick model. J Biomed Mater Res B Appl Biomater. 2022;110(12):2636–48.
Balbi C, Piccoli M, Barile L, Papait A, Armirotti A, Principi E, et al. First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential. Stem Cells Transl Med. 2017;6(5):1340–55.
Sedrakyan S, Villani V, Da Sacco S, Tripuraneni N, Porta S, Achena A, et al. Amniotic fluid stem cell-derived vesicles protect from VEGF-induced endothelial damage. Sci Rep. 2017;7(1):16875.
Thabet E, Yusuf A, Abdelmonsif DA, Nabil I, Mourad G, Mehanna RA. Extracellular vesicles miRNA-21: a potential therapeutic tool in premature ovarian dysfunction. Mol Hum Reprod. 2020;26(12):906–19.
Hu J, Chen X, Li P, Lu X, Yan J, Tan H, et al. Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovasc Diagn Ther. 2021;11(2):348–61.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Obstetrics, Gynaecology & Newborn Health, Melbourne Medical School, The University of Melbourne, Mercy Hospital for Women, 163 Studley Road, Heidelberg, VIC, 3084, Australia
Ishara Atukorala, Natalie Hannan & Lisa Hui
Department of Obstetrics, Gynaecology & Newborn Health, The Northern Centre for Health Education and Research, Northern Health, Epping, VIC, Australia
Department of Perinatal Medicine, Mercy Hospital for Women, Mercy Health, Heidelberg, VIC, Australia
Reproductive Epidemiology Group, Murdoch Children’s Research Institute, Parkville, VIC, Australia
You can also search for this author in PubMed Google Scholar
Contributions
IA and LH conceptualized and designed the manuscript. IA performed the literature review and content synthesis. IA drafted the manuscript while LH edited it. NH provided resources for schematic diagrams and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ishara Atukorala .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
List of included research studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Atukorala, I., Hannan, N. & Hui, L. Immersed in a reservoir of potential: amniotic fluid-derived extracellular vesicles. J Transl Med 22 , 348 (2024). https://doi.org/10.1186/s12967-024-05154-2
Download citation
Received : 08 November 2023
Accepted : 02 April 2024
Published : 12 April 2024
DOI : https://doi.org/10.1186/s12967-024-05154-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Extracellular vesicles
- Amniotic fluid
- Amniotic fluid stem cells
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
Political typology quiz.
Notice: Beginning April 18th community groups will be temporarily unavailable for extended maintenance. Thank you for your understanding and cooperation.
Where do you fit in the political typology?
Are you a faith and flag conservative progressive left or somewhere in between.

Take our quiz to find out which one of our nine political typology groups is your best match, compared with a nationally representative survey of more than 10,000 U.S. adults by Pew Research Center. You may find some of these questions are difficult to answer. That’s OK. In those cases, pick the answer that comes closest to your view, even if it isn’t exactly right.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
SSI Monthly Statistics, March 2024
All federally administered payments.

IMAGES
VIDEO
COMMENTS
Abstract. Objective: Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this "Table 1" can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses.
Number all tables sequentially as you refer to them in the text (Table 1, Table 2, etc.), likewise for figures (Figure 1, Figure 2, etc.). Abbreviations, terminology, and probability level values must be consistent across tables and figures in the same article. Likewise, formats, titles, and headings must be consistent.
Objective: Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this ''Table 1'' can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses.
The simplest Table 1 is a single column of descriptive statistics for the total study sample, with rows containing key study variables (minimally, all variables included in the final main analysis). Inside each cell, descriptive statistics are typically given as n (%) for categorical variables and mean (standard deviation) or median (25th ...
3.3.4 Adding p-values to Table 1. Often, in a published research article, a Table 1 that displays descriptive statistics by the outcome or an exposure includes p-values that test, for each variable, the null hypothesis that the variable has the same mean (or median or proportion) across all groups in the population.
A table characterizing baseline characteristics is so important that it's typically the first table that appears in any observational epidemiology (or clinical trial) manuscript, so it's commonly referred to as a "Table 1". Table 1s are critically important because they help the readers understand internal validity of your study. If ...
The same information from table 1 may be presented as a bar or a pie chart, which can be prepared considering the absolute or relative frequency of the categories. Figures 2 and and3 3 illustrate the same information shown in table 1, but present it as a bar chart and a pie chart, respectively. It can be observed that, regardless of the form of ...
The 95% CI of 0.96 to 1.19 includes 1.0. This means these results are not statistically significant and the increased risk of breast cancer is likely due to chance. The Million Women's Study found a relative risk of breast cancer of 1.13 with a 95% CI of 1.10 to 1.16. This is shown as 1.13 (1.10-1.16) in the table.
5.5. Table 1. In almost every published article that includes quantitative data, there will be a "Table 1" displaying the descriptive statistics for the study sample. There are many ways to organize this information, but the following are commonly followed principles: Different variables are in different rows.
In research, especially in medical research, we describe characteristics of our study populations through Table 1. The Table 1 contain information about the mean for continue/scale variable, and proportion for categorical variable. For example: we say that the mean of systolic blood pressure in our study population is 145 mmHg, or 30% of participants are […]
How to Create Tables in Research Paper. Here are the steps to create tables in a research paper: Plan your table: Determine the purpose of the table and the type of information you want to include. Consider the layout and format that will best convey your information. Choose a table format: Decide on the type of table you want to create.
Background Tables are often overlooked by many readers of papers who tend to focus on the text. Good tables tell much of the story of a paper and give a richer insight into the details of the study participants and the main research findings. Being confident in reading tables and constructing clear tables are important skills for researchers to master. Method Common forms of tables were ...
1 Answer to this question. Answer: Generally speaking, references to specific figures, tables, chapters, sections, etc. within the text are captalized (Section 4, Table 1, Chapter 3, etc.). Most style guides recommend this usage. However, it is always a good idea to check the author guidelines of your target journal to see if they have ...
Abstract. Objective: Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this "Table 1" can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses.
1) Determine the purpose and information to be conveyed. 2) Plan the layout, including rows, columns, and headings. 3) Use spreadsheet software like Excel to design and format the table. 4) Input accurate data into cells, aligning it logically. 5) Include column and row headers for context.
Fig. 1: Improved table after placing values in individual cells, formatting and double spacing, and adding an informative title. Rule 4. Use short, descriptive row and column titles. The title of Table 1 (above) indicates the data in the table is about wheat plants exposed to salinity.
This paper will take the reader through the process of creating a summary table from raw data and presenting the results in RTF format. THE DATA. For this tutorial a data set was generated to simulate what an analyst in the field of public health might encounter. The code for this data set is included in the appendix.
To view the marks for returns, spaces, and indents in Microsoft Word, click the ¶ (paragraph) button in the toolbar. These paragraph marks are normally hidden from the document, unless you turn on the tools to make them visible. Use the key to understand the paragraph marks shown in the examples on this page.
Where to place tables and figures. You have two options for the placement of tables and figures in APA Style: Option 1: Place tables and figures throughout your text, shortly after the parts of the text that refer to them. Option 2: Place them all together at the end of your text (after the reference list) to avoid breaking up the text. If you place them throughout the text, note that each ...
INTRODUCTION. Scientific research is usually initiated by posing evidenced-based research questions which are then explicitly restated as hypotheses.1,2 The hypotheses provide directions to guide the study, solutions, explanations, and expected results.3,4 Both research questions and hypotheses are essentially formulated based on conventional theories and real-world processes, which allow the ...
Sample results of several t tests table. Sample correlation table. Sample analysis of variance (ANOVA) table. Sample factor analysis table. Sample regression table. Sample qualitative table with variable descriptions. Sample mixed methods table. These sample tables are also available as a downloadable Word file (DOCX, 37KB).
Scientific research activity in hospitals is important for promoting the development of clinical medicine, and the scientific literacy of medical staff plays an important role in improving the quality and competitiveness of hospital research. ... The results of the two rounds of boundary values are shown in Table 1. Table 1 Results of the two ...
Research Epidemiologic Survey of Crimean-Congo Hemorrhagic Fever Virus in Suids, Spain Mario Frías, Kerstin Fischer, Sabrina Castro-Scholten, Caroline Bost, David Cano-Terriza, Maria Ángeles Risalde, Pelayo Acevedo, Saúl Jiménez-Ruiz, Balal Sadeghi, Martin H. Groschup, Javier Caballero-Gómez , and Ignacio García-Bocanegra
In the wake of the Dobbs v.Jackson Women's Health Organization 2022 decision, the resultant "trigger laws" in 13 U.S. states, and the lingering retraction of reproductive rights in many more [1, 2], the need for novel contraceptive options has gained urgency across the United States.Unfortunately, due to a complex combination of medical challenges and societal beliefs [3,4,5,6], the ...
Therefore, nomenclature of the vesicles is challenging and will remain a discussion for the foreseeable future. At present, large EVs or small EVs seem to be the appropriate terms to describe an EV population, based on the employed isolation methods. Our review shows the inconsistent terminology (Table 1) used in reproductive EV research.
Take our quiz to find out which one of our nine political typology groups is your best match, compared with a nationally representative survey of more than 10,000 U.S. adults by Pew Research Center. You may find some of these questions are difficult to answer. That's OK. In those cases, pick the answer that comes closest to your view, even if ...
Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this "Table 1" can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses. We aimed to summarize and extend the literature related to reporting ...
Objective. Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this "Table 1" can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses.
Methods: We conducted a repeated cross-sectional analysis of accidental opioid-related deaths between Jan. 1, 2019, and Dec. 31, 2021, across 9 Canadian provinces and territories using aggregated national data. Our primary measure was the burden of premature opioid-related death, measured by potential years of life lost. Our secondary measure was the proportion of all deaths attributable to ...
All Federally Administered Payments. Table 1. Number of recipients, total payments, average monthly payment, and number of awards, by type of payment, eligibility category, and age, March 2024. SOURCE: Social Security Administration, Supplemental Security Record, 100 percent data.