- Scoping Review
- Open access
- Published: 14 November 2021

Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis
- Qiao Liu 1 na1 ,
- Chenyuan Qin 1 , 2 na1 ,
- Min Liu 1 &
- Jue Liu ORCID: orcid.org/0000-0002-1938-9365 1 , 2
Infectious Diseases of Poverty volume 10 , Article number: 132 ( 2021 ) Cite this article
56k Accesses
204 Citations
372 Altmetric
Metrics details
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
We searched PubMed, Embase and Web of Science from inception to July 22, 2021. Observational studies that examined the effectiveness and safety of SARS-CoV-2 vaccines among people vaccinated were included. Random-effects or fixed-effects models were used to estimate the pooled vaccine effectiveness (VE) and incidence rate of adverse events after vaccination, and their 95% confidence intervals ( CI ).
A total of 58 studies (32 studies for vaccine effectiveness and 26 studies for vaccine safety) were included. A single dose of vaccines was 41% (95% CI : 28–54%) effective at preventing SARS-CoV-2 infections, 52% (31–73%) for symptomatic COVID-19, 66% (50–81%) for hospitalization, 45% (42–49%) for Intensive Care Unit (ICU) admissions, and 53% (15–91%) for COVID-19-related death; and two doses were 85% (81–89%) effective at preventing SARS-CoV-2 infections, 97% (97–98%) for symptomatic COVID-19, 93% (89–96%) for hospitalization, 96% (93–98%) for ICU admissions, and 95% (92–98%) effective for COVID-19-related death, respectively. The pooled VE was 85% (80–91%) for the prevention of Alpha variant of SARS-CoV-2 infections, 75% (71–79%) for the Beta variant, 54% (35–74%) for the Gamma variant, and 74% (62–85%) for the Delta variant. The overall pooled incidence rate was 1.5% (1.4–1.6%) for adverse events, 0.4 (0.2–0.5) per 10 000 for severe adverse events, and 0.1 (0.1–0.2) per 10 000 for death after vaccination.
Conclusions
SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Graphical Abstract
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. As of August 8, 2021, COVID-19 has already killed more than 4.2 million people and more than 203 million people were infected [ 1 ]. Given its alarming-spreading speed and the high cost of completely relying on non-pharmaceutical measures, we urgently need safe and effective vaccines to cover susceptible populations and restore people’s lives into the original [ 2 ].
According to global statistics, as of August 2, 2021, there are 326 candidate vaccines, 103 of which are in clinical trials, and 19 vaccines have been put into normal use, including 8 inactivated vaccines and 5 protein subunit vaccines, 2 RNA vaccines, as well as 4 non-replicating viral vector vaccines [ 3 ]. Our World in Data simultaneously reported that 27.3% of the world population has received at least one dose of a COVID-19 vaccine, and 13.8% is fully vaccinated [ 4 ].
To date, COVID-19 become increasingly fierce due to the emergence of variants [ 5 , 6 , 7 ]. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [ 6 , 8 ]. Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials (RCT) yet [ 9 , 10 , 11 , 12 , 13 ].
In general, RNA vaccines are the most effective, followed by viral vector vaccines and inactivated virus vaccines [ 10 , 11 , 12 , 13 ]. The current safety of COVID-19 vaccines is acceptable for mass vaccination, but long-term monitoring of vaccine safety is needed, especially in older people with underlying conditions [ 9 , 10 , 11 , 12 , 13 ]. Inactivated vaccines had the lowest incidence of adverse events and the safety comparisons between mRNA vaccines and viral vectors were controversial [ 9 , 10 ].
RCTs usually conduct under a very demanding research circumstance, and tend to be highly consistent and limited in terms of population characteristics and experimental conditions. Actually, real-world studies differ significantly from RCTs in terms of study conditions and mass vaccination in real world requires taking into account factors, which are far more complex, such as widely heterogeneous populations, vaccine supply, willingness, medical accessibility, etc. Therefore, the real safety and effectiveness of vaccines turn out to be a major concern of international community. The results of a mass vaccination of CoronaVac in Chile demonstrated a protective effectiveness of 65.9% against the onset of COVID-19 after complete vaccination procedures [ 14 ], while the outcomes of phase 3 trials in Brazil and Turkey were 50.7% and 91.3%, reported on Sinovac’s website [ 14 ]. As for the Delta variant, the British claimed 88% protection after two doses of BNT162b2, compared with 67% for AZD1222 [ 15 ]. What is surprising is that the protection of BNT162b2 against infection in Israel is only 39% [ 16 ]. Several studies reported the effectiveness and safety of the COVID-19 vaccine in the real world recently, but the results remain controversial [ 17 , 18 , 19 , 20 ]. A comprehensive meta-analysis based upon the real-world studies is still in an urgent demand, especially for evaluating the effect of vaccines on variation strains. In the present study, we aimed to systematically evaluate the effectiveness and safety of the COVID-19 vaccine in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
Search strategy and selection criteria
Our methods were described in detail in our published protocol [PROSPERO (Prospective register of systematic reviews) registration, CRD42021267110]. We searched eligible studies published by 22 July 2021, from three databases including PubMed, Embase and Web of Science by the following search terms: (effectiveness OR safety) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination). We used EndNoteX9.0 (Thomson ResearchSoft, Stanford, USA) to manage records, screen and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We included observational studies that examined the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines among people vaccinated with SARS-CoV-2 vaccines. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 vaccination as the exposure; (2) insufficient data to calculate the rate for the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, or adverse events after vaccination; (3) duplicate studies or overlapping participants; (4) RCT studies, reviews, editorials, conference papers, case reports or animal experiments; and (5) studies that did not clarify the identification of COVID-19.
Studies were identified by two investigators (LQ and QCY) independently following the criteria above, while discrepancies reconciled by a third investigator (LJ).
Data extraction and quality assessment
The primary outcome was the effectiveness of SARS-CoV-2 vaccines. The following data were extracted independently by two investigators (LQ and QCY) from the selected studies: (1) basic information of the studies, including first author, publication year and study design; (2) characteristics of the study population, including sample sizes, age groups, setting or locations; (3) kinds of the SARS-CoV-2 vaccines; (4) outcomes for the effectiveness of SARS-CoV-2 vaccines: the number of laboratory-confirmed COVID-19, hospitalization for COVID-19, admission to the ICU for COVID-19, and COVID-19-related death; and (5) outcomes for the safety of SARS-CoV-2 vaccines: the number of adverse events after vaccination.
We evaluated the risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies and case–control studies [ 21 ]. and assess the methodological quality using the checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [ 22 ]. Cohort studies and case–control studies were classified as having low (≥ 7 stars), moderate (5–6 stars), and high risk of bias (≤ 4 stars) with an overall quality score of 9 stars. For cross-sectional studies, we assigned each item of the AHRQ checklist a score of 1 (answered “yes”) or 0 (answered “no” or “unclear”), and summarized scores across items to generate an overall quality score that ranged from 0 to 11. Low, moderate, and high risk of bias were identified as having a score of 8–11, 4–7 and 0–3, respectively.
Two investigators (LQ and QCY) independently assessed study quality, with disagreements resolved by a third investigator (LJ).
Data synthesis and statistical analysis
We performed a meta-analysis to pool data from included studies and assess the effectiveness and safety of SARS-CoV-2 vaccines by clinical outcomes (rates of the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, and adverse events after vaccination). Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity between estimates ( I 2 ). Fixed-effects models were used if I 2 ≤ 50%, which represented low to moderate heterogeneity and random-effects models were used if I 2 > 50%, representing substantial heterogeneity.
We conducted subgroup analyses to investigate the possible sources of heterogeneity by using vaccine kinds, vaccination status, sample size, and study population as grouping variables. We used the Q test to conduct subgroup comparisons and variables were considered significant between subgroups if the subgroup difference P value was less than 0.05. Publication bias was assessed by funnel plot and Egger’s regression test. We analyzed data using Stata version 16.0 (StataCorp, Texas, USA).
A total of 4844 records were searched from the three databases. 2484 duplicates were excluded. After reading titles and abstracts, we excluded 2264 reviews, RCT studies, duplicates and other studies meeting our exclude criteria. Among the 96 studies under full-text review, 41 studies were excluded (Fig. 1 ). Ultimately, with three grey literatures included, this final meta-analysis comprised 58 eligible studies, including 32 studies [ 14 , 15 , 17 , 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] for vaccine effectiveness and 26 studies [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] for vaccine safety. Characteristics of included studies are showed in Additional file 1 : Table S1, Additional file 2 : Table S2. The risk of bias of all studies we included was moderate or low.
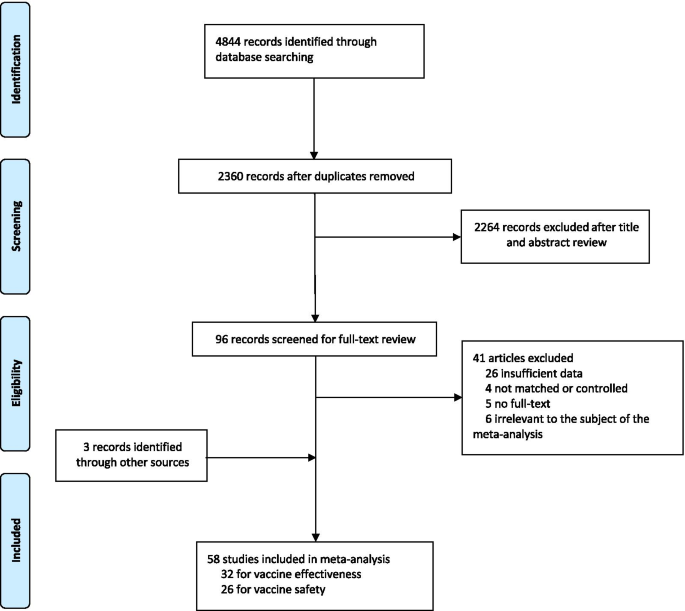
Flowchart of the study selection
Vaccine effectiveness for different clinical outcomes of COVID-19
We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
For the first dose of SARS-CoV-2 vaccines, the pooled VE was 41% (95% CI : 28–54%) for the prevention of SARS-CoV-2 infection, 52% (95% CI : 31–73%) for the prevention of symptomatic COVID-19, 66% (95% CI : 50–81%) for the prevention of hospital admissions, 45% (95% CI : 42–49%) for the prevention of ICU admissions, and 53% (95% CI : 15–91%) for the prevention of COVID-19-related death (Table 1 ). The subgroup, ≥ 21 days after the first dose, was found to have the highest VE in each clinical outcome of COVID-19, regardless of ≥ 1 dose group (Table 1 ).
For the second dose of SARS-CoV-2 vaccines, the pooled VE was 85% (95% CI : 81–89%) for the prevention of SARS-CoV-2 infection, 97% (95% CI : 97–98%) for the prevention of symptomatic COVID-19, 93% (95% CI: 89–96%) for the prevention of hospital admissions, 96% (95% CI : 93–98%) for the prevention of ICU admissions, and 95% (95% CI : 92–98%) for the prevention of COVID-19-related death (Table 1 ). VE was 94% (95% CI : 78–98%) in ≥ 21 days after the second dose for the prevention of SARS-CoV-2 infection, higher than other subgroups, regardless of 2 dose group (Table 1 ). For the prevention of symptomatic COVID-19, VE was also relatively higher in 21 days after the second dose (99%, 95% CI : 94–100%). Subgroups showed no statistically significant differences in the prevention of hospital admissions, ICU admissions and COVID-19-related death (subgroup difference P values were 0.991, 0.414, and 0.851, respectively).
Vaccine effectiveness for different variants of SARS-CoV-2 in fully vaccinated people
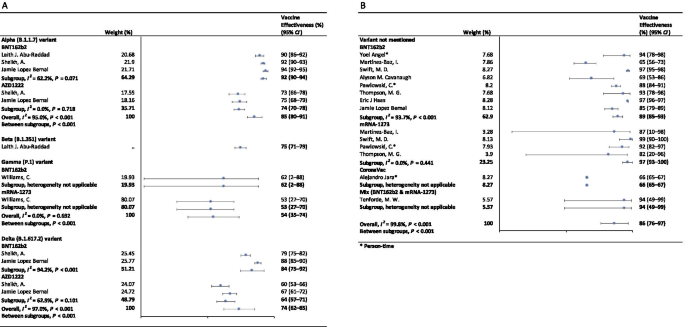
In the fully vaccinated groups (over 14 days after the second dose), the pooled VE was 85% (95% CI: 80–91%) for the prevention of Alpha variant of SARS-CoV-2 infection, 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. There was only one study [ 23 ] focused on the Beta variant, which showed the VE was 75% (95% CI : 71–79%) for the prevention of the Beta variant of SARS-CoV-2 infection. BNT162b2 vaccine had the highest VE in each variant group; 92% (95% CI : 90–94%) for the Alpha variant, 62% (95% CI : 2–88%) for the Gamma variant, and 84% (95% CI : 75–92%) for the Delta variant (Fig. 2 ).
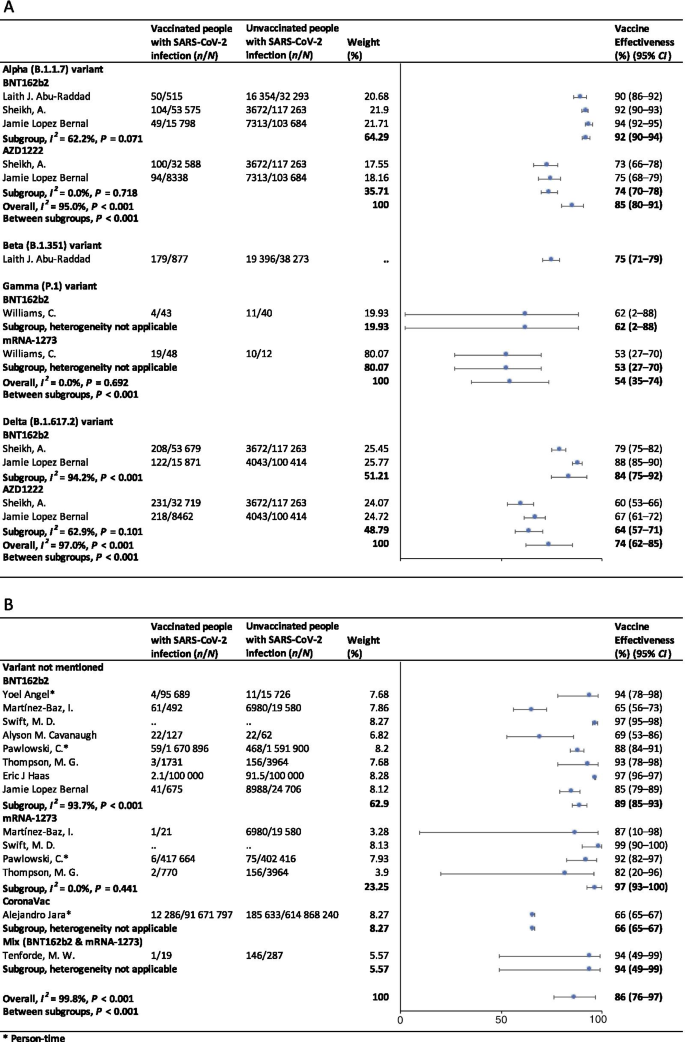
Forest plots for the vaccine effectiveness of SARS-CoV-2 vaccines in fully vaccinated populations. A Vaccine effectiveness against SARS-CoV-2 variants; B Vaccine effectiveness against SARS-CoV-2 with variants not mentioned. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019, CI confidence interval
For studies which had not mentioned the variant of SARS-CoV-2, the pooled VE was 86% (95% CI: 76–97%) for the prevention of SARS-CoV-2 infection in fully vaccinated people. mRNA-1273 vaccine had the highest pooled VE (97%, 95% CI: 93–100%, Fig. 2 ).
Safety of SARS-CoV-2 vaccines
As Table 2 showed, the incidence rate of adverse events varied widely among different studies. We conducted subgroup analysis by study population (general population, patients and healthcare workers), vaccine type (BNT162b2, mRNA-1273, CoronaVac, and et al.), and population size (< 1000, 1000–10 000, 10 000–100 000, and > 100 000). The overall pooled incidence rate was 1.5% (95% CI : 1.4–1.6%) for adverse events, 0.4 (95% CI : 0.2–0.5) per 10 000 for severe adverse events, and 0.1 (95% CI : 0.1–0.2) per 10 000 for death after vaccination. Incidence rate of adverse events was higher in healthcare workers (53.2%, 95% CI : 28.4–77.9%), AZD1222 vaccine group (79.6%, 95% CI : 60.8–98.3%), and < 1000 population size group (57.6%, 95% CI : 47.9–67.4%). Incidence rate of sever adverse events was higher in healthcare workers (127.2, 95% CI : 62.7–191.8, per 10 000), Gam-COVID-Vac vaccine group (175.7, 95% CI : 77.2–274.2, per 10 000), and 1000–10 000 population size group (336.6, 95% CI : 41.4–631.8, per 10 000). Incidence rate of death after vaccination was higher in patients (7.6, 95% CI : 0.0–32.2, per 10 000), BNT162b2 vaccine group (29.8, 95% CI : 0.0–71.2, per 10 000), and < 1000 population size group (29.8, 95% CI : 0.0–71.2, per 10 000). Subgroups of general population, vaccine type not mentioned, and > 100 000 population size had the lowest incidence rate of adverse events, severe adverse events, and death after vaccination.
Sensitivity analysis and publication bias
In the sensitivity analyses, VE for SARS-CoV-2 infections, symptomatic COVID-19 and COVID-19-related death got relatively lower when omitting over a single dose group of Maria et al.’s work [ 33 ]; when omitting ≥ 14 days after the first dose group and ≥ 14 days after the second dose group of Alejandro et al.’s work [ 14 ], VE for SARS-CoV-2 infections, hospitalization, ICU admission and COVID-19-related death got relatively higher; and VE for all clinical status of COVID-19 became lower when omitting ≥ 14 days after the second dose group of Eric et al.’s work [ 34 ]. Incidence rate of adverse events and severe adverse events got relatively higher when omitting China CDC’s data [ 74 ]. P values of Egger’s regression test for all the meta-analysis were more than 0.05, indicating that there might not be publication bias.
To our knowledge, this is a comprehensive systematic review and meta-analysis assessing the effectiveness and safety of SARS-CoV-2 vaccines based on real-world studies, reporting pooled VE for different variants of SARS-CoV-2 and incidence rate of adverse events. This meta-analysis comprised a total of 58 studies, including 32 studies for vaccine effectiveness and 26 studies for vaccine safety. We found that a single dose of SARS-CoV-2 vaccines was about 40–60% effective at preventing any clinical status of COVID-19 and that two doses were 85% or more effective. Although vaccines were not as effective against variants of SARS-CoV-2 as original virus, the vaccine effectiveness was still over 50% for fully vaccinated people. Normal adverse events were common, while the incidence of severe adverse events or even death was very low, providing reassurance to health care providers and to vaccine recipients and promote confidence in the safety of COVID-19 vaccines. Our findings strengthen and augment evidence from previous review [ 75 ], which confirmed the effectiveness of the BNT162b2 mRNA vaccine, and additionally reported the safety of SARS-CoV-2 vaccines, giving insight on the future of SARS-CoV-2 vaccine schedules.
Although most vaccines for the prevention of COVID-19 are two-dose vaccines, we found that the pooled VE of a single dose of SARS-CoV-2 vaccines was about 50%. Recent study showed that the T cell and antibody responses induced by a single dose of the BNT162b2 vaccine were comparable to those naturally infected with SARE-CoV-2 within weeks or months after infection [ 76 ]. Our findings could help to develop vaccination strategies under certain circumstances such as countries having a shortage of vaccines. In some countries, in order to administer the first dose to a larger population, the second dose was delayed for up to 12 weeks [ 77 ]. Some countries such as Canada had even decided to delay the second dose for 16 weeks [ 78 ]. However, due to a suboptimum immune response in those receiving only a single dose of a vaccine, such an approach had a chance to give rise to the emergence of variants of SARS-CoV-2 [ 79 ]. There remains a need for large clinical trials to assess the efficacy of a single-dose administration of two-dose vaccines and the risk of increasing the emergence of variants.
Two doses of SARS-CoV-2 vaccines were highly effective at preventing hospitalization, severe cases and deaths resulting from COVID-19, while the VE of different groups of days from the second vaccine dose showed no statistically significant differences. Our findings emphasized the importance of getting fully vaccinated, for the fact that most breakthrough infections were mild or asymptomatic. A recent study showed that the occurrence of breakthrough infections with SARS-CoV-2 in fully vaccinated populations was predictable with neutralizing antibody titers during the peri-infection period [ 80 ]. We also found getting fully vaccinated was at least 50% effective at preventing SARS-CoV-2 variants infections, despite reduced effectiveness compared with original virus; and BNT162b2 vaccine was found to have the highest VE in each variant group. Studies showed that the highly mutated variants were indicative of a form of rapid, multistage evolutionary jumps, which could preferentially occur in the milieu of partial immune control [ 81 , 82 ]. Therefore, immunocompromised patients should be prioritized for anti-COVID-19 immunization to mitigate persistent SARS-CoV-2 infections, during which multimutational SARS-CoV-2 variants could arise [ 83 ].
Recently, many countries, including Israel, the United States, China and the United Kingdom, have introduced a booster of COVID-19 vaccine, namely the third dose [ 84 , 85 , 86 , 87 ]. A study of Israel showed that among people vaccinated with BNT162b2 vaccine over 60 years, the risk of COVID-19 infection and severe illness in the non-booster group was 11.3 times (95% CI: 10.4–12.3) and 19.5 times (95% CI: 12.9–29.5) than the booster group, respectively [ 84 ]. Some studies have found that the third dose of Moderna, Pfizer-BioNTech, Oxford-AstraZeneca and Sinovac produced a spike in infection-blocking neutralizing antibodies when given a few months after the second dose [ 85 , 87 , 88 ]. In addition, the common adverse events associated with the third dose did not differ significantly from the symptoms of the first two doses, ranging from mild to moderate [ 85 ]. The overall incidence rate of local and systemic adverse events was 69% (57/97) and 20% (19/97) after receiving the third dose of BNT162b2 vaccine, respectively [ 88 ]. Results of a phase 3 clinical trial involving 306 people aged 18–55 years showed that adverse events after receiving a third dose of BNT162b2 vaccine (5–8 months after completion of two doses) were similar to those reported after receiving a second dose [ 85 ]. Based on V-safe, local reactions were more frequently after dose 3 (5323/6283; 84.7%) than dose 2 (5249/6283; 83.5%) among people who received 3 doses of Moderna. Systemic reactions were reported less frequently after dose 3 (4963/6283; 79.0%) than dose 2 (5105/6283; 81.3%) [ 86 ]. On August 4, WHO called for a halt to booster shots until at least the end of September to achieve an even distribution of the vaccine [ 89 ]. At this stage, the most important thing we should be thinking about is how to reach a global cover of people at risk with the first or second dose, rather than focusing on the third dose.
Based on real world studies, our results preliminarily showed that complete inoculation of COVID-19 vaccines was still effective against infection of variants, although the VE was generally diminished compared with the original virus. Particularly, the pooled VE was 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. Since the wide spread of COVID-19, a number of variants have drawn extensive attention of international community, including Alpha variant (B.1.1.7), first identified in the United Kingdom; Beta variant (B.1.351) in South Africa; Gamma variant (P.1), initially appeared in Brazil; and the most infectious one to date, Delta variant (B.1.617.2) [ 90 ]. Israel recently reported a breakthrough infection of SARS-CoV-2, dominated by variant B.1.1.7 in a small number of fully vaccinated health care workers, raising concerns about the effectiveness of the original vaccine against those variants [ 80 ]. According to an observational cohort study in Qatar, VE of the BNT162b2 vaccine against the Alpha (B.1.1.7) and Beta (B.1.351) variants was 87% (95% CI : 81.8–90.7%) and 75.0% (95% CI : 70.5–7.9%), respectively [ 23 ]. Based on the National Immunization Management System of England, results from a recent real-world study of all the general population showed that the AZD1222 and BNT162b2 vaccines protected against symptomatic SARS-CoV-2 infection of Alpha variant with 74.5% (95% CI : 68.4–79.4%) and 93.7% (95% CI : 91.6–95.3%) [ 15 ]. In contrast, the VE against the Delta variant was 67.0% (95% CI : 61.3–71.8%) for two doses of AZD1222 vaccine and 88% (95% CI : 85.3–90.1%) for BNT162b2 vaccine [ 15 ].
In terms of adverse events after vaccination, the pooled incidence rate was very low, only 1.5% (95% CI : 1.4–1.6%). However, the prevalence of adverse events reported in large population (population size > 100 000) was much lower than that in small to medium population size. On the one hand, the vaccination population in the small to medium scale studies we included were mostly composed by health care workers, patients with specific diseases or the elderly. And these people are more concerned about their health and more sensitive to changes of themselves. But it remains to be proved whether patients or the elderly are more likely to have adverse events than the general. Mainstream vaccines currently on the market have maintained robust safety in specific populations such as cancer patients, organ transplant recipients, patients with rheumatic and musculoskeletal diseases, pregnant women and the elderly [ 54 , 91 , 92 , 93 , 94 ]. A prospective study by Tal Goshen-lag suggests that the safety of BNT162b2 vaccine in cancer patients is consistent with those previous reports [ 91 ]. In addition, the incidence rate of adverse events reported in the heart–lung transplant population is even lower than that in general population [ 95 ]. On the other hand, large scale studies at the national level are mostly based on national electronic health records or adverse event reporting systems, and it is likely that most mild or moderate symptoms are actually not reported.
Compared with the usual local adverse events (such as pain at the injection site, redness at the injection site, etc.) and normal systemic reactions (such as fatigue, myalgia, etc.), serious and life-threatening adverse events were rare due to our results. A meta-analysis based on RCTs only showed three cases of anaphylactic shock among 58 889 COVID-19 vaccine recipients and one in the placebo group [ 11 ]. The exact mechanisms underlying most of the adverse events are still unclear, accordingly we cannot establish a causal relation between severe adverse events and vaccination directly based on observational studies. In general, varying degrees of adverse events occur after different types of COVID-19 vaccination. Nevertheless, the benefits far outweigh the risks.
Our results showed the effectiveness and safety of different types of vaccines varied greatly. Regardless of SARS-CoV-2 variants, vaccine effectiveness varied from 66% (CoronaVac [ 14 ]) to 97% (mRNA-1273 [ 18 , 20 , 45 , 46 ]). The incidence rate of adverse events varied widely among different types of vaccines, which, however, could be explained by the sample size and population group of participants. BNT162b2, AZD1222, mRNA-1273 and CoronaVac were all found to have high vaccine efficacy and acceptable adverse-event profile in recent published studies [ 96 , 97 , 98 , 99 ]. A meta-analysis, focusing on the potential vaccine candidate which have reached to the phase 3 of clinical development, also found that although many of the vaccines caused more adverse events than the controls, most were mild, transient and manageable [ 100 ]. However, severe adverse events did occur, and there remains the need to implement a unified global surveillance system to monitor the adverse events of COVID-19 vaccines around the world [ 101 ]. A recent study employed a knowledge-based or rational strategy to perform a prioritization matrix of approved COVID-19 vaccines, and led to a scale with JANSSEN (Ad26.COV2.S) in the first place, and AZD1222, BNT162b2, and Sputnik V in second place, followed by BBIBP-CorV, CoronaVac and mRNA-1273 in third place [ 101 ]. Moreover, when deciding the priority of vaccines, the socioeconomic characteristics of each country should also be considered.
Our meta-analysis still has several limitations. First, we may include limited basic data on specific populations, as vaccination is slowly being promoted in populations under the age of 18 or over 60. Second, due to the limitation of the original real-world study, we did not conduct subgroup analysis based on more population characteristics, such as age. When analyzing the efficacy and safety of COVID-19 vaccine, we may have neglected the discussion on the heterogeneity from these sources. Third, most of the original studies only collected adverse events within 7 days after vaccination, which may limit the duration of follow-up for safety analysis.
Based on the real-world studies, SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
Coronavirus disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
Vaccine effectiveness
Confidence intervals
Intensive care unit
Random clinical trials
Preferred reporting items for systematic reviews and meta-analyses
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. https://coronavirus.jhu.edu/map.html . Accessed 20 Aug 2021.
Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021. https://doi.org/10.3390/healthcare9091163 .
Article Google Scholar
COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ . Accessed 20 Aug 2021.
Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations . Accessed 20 Aug 2021.
Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–1. https://doi.org/10.1016/s2213-2600(21)00005-9 .
Article CAS PubMed PubMed Central Google Scholar
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–1. https://doi.org/10.1038/d41586-021-00121-z .
Article CAS PubMed Google Scholar
Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021. https://doi.org/10.1038/d41586-021-01986-w .
Article PubMed Google Scholar
Li R, Liu J, Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48(2):102–6. https://doi.org/10.1016/j.jgg.2021.03.001 .
Article PubMed PubMed Central Google Scholar
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://doi.org/10.1186/s40249-021-00878-5 .
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021. https://doi.org/10.1002/jmv.27203 .
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467 .
Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, van Teijlingen ER, Kord-Varkaneh H, Mekkodathil AA, Subramanya SH, et al. Development and implementation of a potential coronavirus disease 2019 (COVID-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. 2021;11(1):959–82. https://doi.org/10.3126/nje.v11i1.36163 .
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.11.03.20224998 .
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107715 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2108891 .
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness. 2021. https://www.cnbc.com/2021/07/23/delta-variant-pfizer-covid-vaccine-39percent-effective-in-israel-prevents-severe-illness.html . Accessed 20 Aug 2021.
Zacay G, Shasha D, Bareket R, Kadim I, Hershkowitz Sikron F, Tsamir J, Mossinson D, Heymann AD. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8(6): ofab262. https://doi.org/10.1093/ofid/ofab262 .
Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, Guevara M, Ezpeleta C, Castilla J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.21.2100438 .
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.007 .
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 20 Aug 2021.
Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/ . Accessed 20 Aug 2021
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–9. https://doi.org/10.1056/NEJMc2104974 .
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. https://doi.org/10.1001/jama.2021.7152 .
Azamgarhi T, Hodgkinson M, Shah A, Skinner JA, Hauptmannova I, Briggs TWR, Warren S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021;12(1):3698. https://doi.org/10.1038/s41467-021-23927-x .
Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, Tafuri S, Stefanizzi P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab262 .
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. https://doi.org/10.15585/mmwr.mm7011e3 .
Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, Tobias J, Lunn S, Miller T, Thoroughman D, et al. COVID-19 outbreak associated with a SARS-CoV-2 R1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639–43. https://doi.org/10.15585/mmwr.mm7017e2 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y .
Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6): e2115985. https://doi.org/10.1001/jamanetworkopen.2021.15985 .
Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab438 .
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765 .
Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. VacCInes (Basel). 2021. https://doi.org/10.3390/vaccines9060628 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/s0140-6736(21)00947-8 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x .
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00330-3 .
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2021.05.044 .
Knobel P, Serra C, Grau S, Ibañez R, Diaz P, Ferrández O, Villar R, Lopez AF, Pujolar N, Horcajada JP, et al. COVID-19 mRNA vaccine effectiveness in asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.287 .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373: n1088. https://doi.org/10.1136/bmj.n1088 .
Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53, 2020 to 13 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.24.2100452 .
Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 US Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. https://doi.org/10.15585/mmwr.mm7020e2 .
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/s0140-6736(21)01358-1 .
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00289-9 .
Skowronski DM, Setayeshgar S, Zou M, Prystajecky N, Tyson JR, Galanis E, Naus M, Patrick DM, Sbihi H, El Adam S, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia,Canada. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab616 .
Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab361 .
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107058 .
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. https://doi.org/10.1016/s0140-6736(21)00677-2 .
Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, Gitterman L, Fedsin S, Godoy M, Kozak R, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program—Ontario, April–May 2021. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab617 .
Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060674 .
Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–21. https://doi.org/10.26355/eurrev_202106_26153 .
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054 .
Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, Varghese DR, Krishnan N, Shenoy P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41(8):1441–5. https://doi.org/10.1007/s00296-021-04917-0 .
Chevallier P, Coste-Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, Imbert BM, Drumel T, Le Gouill S, Moreau P, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem. 2021. https://doi.org/10.1002/jha2.242 .
Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220231 .
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220647 .
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3 .
Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese health care workers; a secondary analysis of initial post-approval safety data. J Travel Med. 2021. https://doi.org/10.1093/jtm/taab090 .
Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8: 670370. https://doi.org/10.3389/fmed.2021.670370 .
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, Venkatakrishnan AJ, Anand P, Agarwal V, O’Horo JC, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.006 .
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/s1470-2045(21)00213-8 .
Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, Asprea V, Staneloni MI, Zingoni P, Vidal G, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MediCIna (B Aires). 2021;81(3):408–14.
Google Scholar
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.04.003 .
Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021. https://doi.org/10.1016/j.jhqr.2021.05.002 .
Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.06.024 .
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. https://doi.org/10.1016/j.ejca.2021.06.002 .
Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060673 .
Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac Side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021. https://doi.org/10.3390/jcm10122629 .
Rosman Y, Lavi N, Meir-Shafrir K, Lachover-Roth I, Cohen-Engler A, Mekori YA, Confino-Cohen R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J Allergy Clin Immunol Pract. 2021. https://doi.org/10.1016/j.jaip.2021.06.032 .
Signorelli C, Odone A, Gianfredi V, Capraro M, Kacerik E, Chiecca G, Scardoni A, Minerva M, Mantecca R, Musarò P, et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann Ig. 2021;33(5):499–512. https://doi.org/10.7416/ai.2021.2456 .
Vallée A, Chan-Hew-Wai A, Bonan B, Lesprit P, Parquin F, Catherinot É, Choucair J, Billard D, Amiel-Taieb C, Camps È, et al. Oxford-AstraZeneca COVID-19 vaccine: need of a reasoned and effective vaccine campaign. Public Health. 2021;196:135–7. https://doi.org/10.1016/j.puhe.2021.05.030 .
Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.04.026 .
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1925112 .
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2 .
Prevention CCfDCa. Information analysis of COVID-19 vaccine adverse reaction monitoring in China. 2021-5-28. http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html . Accessed 20 Aug 2021.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90. https://doi.org/10.1007/s10787-021-00839-2 .
Angyal A, Longet S, Moore S, Payne RP, Harding A et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=3820576 or https://doi.org/10.2139/ssrn.3820576 . Accessed 20 Aug 2021.
Pimenta D, Yates C, Pagel C, Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372: n710. https://doi.org/10.1136/bmj.n710 .
Tauh T, Mozel M, Meyler P, Lee SM. An updated look at the 16-week window between doses of vaccines in BC for COVID-19. BC Med J. 2021;63(3):102–3.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for COVID-19 vaccination. N Engl J Med. 2021;384(9): e28. https://doi.org/10.1056/NEJMclde2101987 .
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109072 .
Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, Aguayo-Hiraldo P, Pinsky BA, Pekosz A, Shen L, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. https://doi.org/10.1101/2021.02.27.21252099 .
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–3. https://doi.org/10.1056/NEJMc2031364 .
Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–6. https://doi.org/10.1056/NEJMsb2104756 .
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. https://doi.org/10.1056/NEJMoa2114255 .
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–84. https://doi.org/10.15585/mmwr.mm7039e4 .
Furlow B. Immunocompromised patients in the USA and UK should receive third dose of COVID-19 vaccine. Lancet Rheumatol. 2021. https://doi.org/10.1016/s2665-9913(21)00313-1 .
Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. https://doi.org/10.1016/s0140-6736(21)01699-8 .
Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.08.010 .
WHO. WHO press conference on coronavirus disease (COVID-19)—4 August 2021. 2021. https://www.who.int/multi-media/details/who-press-conference-on-coronavirus-disease-(covid-19)---4-august-2021 . Accessed 20 Aug 2021.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.2675 .
Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. https://doi.org/10.1097/tp.0000000000003780 .
Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021. https://doi.org/10.1002/uog.23729 .
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983 .
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–62. https://doi.org/10.1016/j.healun.2021.04.003 .
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110345 .
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2105290 .
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2113017 .
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. https://doi.org/10.1016/s0140-6736(21)01429-x .
Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12(3):215–21. https://doi.org/10.4103/japtr.JAPTR_229_20 .
Burgos-Salcedo J. A rational strategy to support approved COVID-19 vaccines prioritization. Hum Vaccin Immunother. 2021;17(10):3474–7. https://doi.org/10.1080/21645515.2021.1922060 .
Download references
Acknowledgements
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major infectious disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Author information
Qiao Liu and Chenyuan Qin are joint first authors
Authors and Affiliations
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
Qiao Liu, Chenyuan Qin, Min Liu & Jue Liu
Institute for Global Health and Development, Peking University, Beijing, 100871, China
Chenyuan Qin & Jue Liu
You can also search for this author in PubMed Google Scholar
Contributions
LQ and QCY contributed equally as first authors. LJ and LM contributed equally as correspondence authors. LJ and LM conceived and designed the study; LQ, QCY and LJ carried out the literature searches, extracted the data, and assessed the study quality; LQ and QCY performed the statistical analysis and wrote the manuscript; LJ, LM, LQ and QCY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Min Liu or Jue Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: table s1..
Characteristic of studies included for vaccine effectiveness.
Additional file 2: Table S2.
Characteristic of studies included for vaccine safety.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Q., Qin, C., Liu, M. et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 10 , 132 (2021). https://doi.org/10.1186/s40249-021-00915-3
Download citation
Received : 07 September 2021
Accepted : 01 November 2021
Published : 14 November 2021
DOI : https://doi.org/10.1186/s40249-021-00915-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
- Meta-analysis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults
Affiliations.
- 1 Thibodaux Regional Health System, Thibodaux, LA, USA. Electronic address: [email protected].
- 2 Unit of Innovation and Organization, Navarre Health Service, Spain. Electronic address: [email protected].
- 3 Institute of Evidence-Based Healthcare, Bond University, Gold Coast, QLD, Australia. Electronic address: [email protected].
- 4 Fielding School of Public Health and College of Letters and Science, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 5 Geffen School of Medicine, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected].
- 7 School of Pharmacy, University of Maryland, Baltimore, MD, USA. Electronic address: [email protected].
- PMID: 36055877
- PMCID: PMC9428332
- DOI: 10.1016/j.vaccine.2022.08.036
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
Methods: Secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults ( NCT04368728 and NCT04470427 ), focusing analysis on Brighton Collaboration adverse events of special interest.
Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and 42.2 (95 % CI -0.4 to 20.6 and -3.6 to 33.8), respectively. Combined, the mRNA vaccines were associated with an excess risk of serious adverse events of special interest of 12.5 per 10,000 vaccinated (95 % CI 2.1 to 22.9); risk ratio 1.43 (95 % CI 1.07 to 1.92). The Pfizer trial exhibited a 36 % higher risk of serious adverse events in the vaccine group; risk difference 18.0 per 10,000 vaccinated (95 % CI 1.2 to 34.9); risk ratio 1.36 (95 % CI 1.02 to 1.83). The Moderna trial exhibited a 6 % higher risk of serious adverse events in the vaccine group: risk difference 7.1 per 10,000 (95 % CI -23.2 to 37.4); risk ratio 1.06 (95 % CI 0.84 to 1.33). Combined, there was a 16 % higher risk of serious adverse events in mRNA vaccine recipients: risk difference 13.2 (95 % CI -3.2 to 29.6); risk ratio 1.16 (95 % CI 0.97 to 1.39).
Discussion: The excess risk of serious adverse events found in our study points to the need for formal harm-benefit analyses, particularly those that are stratified according to risk of serious COVID-19 outcomes. These analyses will require public release of participant level datasets.
Keywords: Adverse events of special interest; Brighton Collaboration; COVID-19; COVID-19 vaccines; Coalition for Epidemic Preparedness Innovations; Moderna COVID-19 vaccine mRNA-1273; NCT04368728 ; NCT04470427 ; Pfizer-BioNTech COVID-19 vaccine BNT162b2; SARS-CoV-2; Safety Platform for Emergency vACcines; Serious adverse events; Vaccines; mRNA vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Publication types
- Clinical Trial, Phase III
- Randomized Controlled Trial
- COVID-19 Vaccines / adverse effects
- COVID-19* / prevention & control
- RNA, Messenger
- Randomized Controlled Trials as Topic
- Vaccination / adverse effects
- Vaccines, Synthetic
- mRNA Vaccines
- COVID-19 Vaccines
Associated data
- ClinicalTrials.gov/NCT04368728
- ClinicalTrials.gov/NCT04470427
- Research article
- Open access
- Published: 17 November 2021
The “anti-vax” movement: a quantitative report on vaccine beliefs and knowledge across social media
- Staci L Benoit ORCID: orcid.org/0000-0003-3437-2358 1 &
- Rachel F. Mauldin 1
BMC Public Health volume 21 , Article number: 2106 ( 2021 ) Cite this article
125k Accesses
33 Citations
73 Altmetric
Metrics details
Social media use has become a mainstay of communication and with that comes the exchange of factual and non-factual information. Social media has given many people the opportunity to speak their opinions without repercussions and create coalitionS of like-minded people. This also has led to the development of a community know as anti-vaxxers or vaccine deniers. This research explores the extent to which vaccine knowledge has reached on social media.
This cross sectional research explored the relationship between the spread of information regarding vaccines in relation to social media use. A sample of 2515 people over the age of 18 around the world completed the survey via a link distributed on Twitter, Facebook and Instagram. A series of questions on vaccine knowledge and beliefs were compounded to create an individual’s “knowledge score” and a “belief score”. Knowledge scores were ranked from low knowledge to high knowledge with increasing scores. Belief scores were ranked from belief in myths to disbelief in myths with higher scores. This score was then analysed, using a Welch test and post hoc testing when applicable, across demographics and questions relating to social media use.
Significant relations were found in both the knowledge and belief categories, many of which were similar findings between the two. North Americans had significantly lower knowledge and belief scores compared to all other continents. While the majority of people primarily use Facebook, Twitter users were significantly more knowledgeable. It was also found that higher education was correlated with higher knowledge and belief scores.
Conclusions
Overall, these correlations are important in determining ways to intervene into the anti-vax movement through the use of social media. Cross demographics were not analysed in this study but could be in future studies. To better understand the social media exposures related to vaccine information a follow up structured interview research study would be beneficial. Note that due to the cross sectional nature of this study, causal relationships could not be made.
Peer Review reports
Fear of vaccines dates as far back as vaccines themselves as evident by Edmund Massey's [ 29 ] Sermon titled “A sermon against the dangerous and sinful practice of inoculation.” This appears to be the first objection to any forms of inoculation to prevent disease with Massey stating “Let us not sinfully endeavour to alter the Course of Nature” [ 29 ]. Next in notable vaccine objections was when the smallpox vaccine was introduced, “many skeptics […] found it counterintuitive that deliberately infecting a person with a disease” [ 38 ]. This is when the world began to see a group of people who not only refused vaccination but made an effort to inform others of the “dangers” through propaganda. However this propaganda largely consisted of arguments of infringement of rights and anti-socialism [ 15 ].
Since the infamous 1998 paper by Andrew Wakefield, this was later retracted because it incorrectly related the MMR vaccine to autism, a group of people known as vaccine deniers or more commonly known as anti-vaxxers have been exponentially growing. A vaccine denier or anti-vaxxer will be defined in this study as someone who believes vaccines do not work, are not safe or refuse vaccines for themselves and their children if applicable. Claims about vaccine safety, efficacy, and adverse effects have been evolving and have now spread to almost every vaccine available. Surveys from the American Academy of Pediatrics found that the rate of parents who refused one or more recommended vaccines increased from 9.1% in 2006 to a staggering 16.7% in 2013 [ 30 ]. The problem being faced today is the wealth of information that is not only accessible but easily spread across social media platforms regardless of veracity. It is clear that the internet is now patching a significant time in health literacy and decision making. A survey by Fox [ 18 ] found "(72%) [Of] adult internet users say they have searched online for information about a range of health issues[...] (26%) adult internet users say they have read or watched someone else’s health experience about health or medical issues in the past 12 months. And 16% of adult internet users in the U.S. have gone online in the past 12 months to find others who share the same health concerns. There are an estimated 58 milion followers on anti-vaccination pages across socialmedia ] 1 ]. This study uses Social media propagation, to reach the study population.
The purpose of this study is to evaluate the current knowledge and beliefs about vaccines in people who use social media and the differences between scores and demographics. Past research has found that the strongest influence for positive vaccine views is having factual knowledge [ 11 ]. This study hopes to determine how much factual information is known and what differentiates social media users who have adequate and inadequate knowledge. This cross sectional study hypothesizes that there are correlations between each of the individual demographic questions and the respondent’s knowledge of vaccines. Knowing what differentiates people who have adequate and inadequate knowledge can be beneficial for determining how to reach people in future vaccine campaigns. This information could also be used in an attempt to better educate or correct misinformation by means of social media in those who classify under this study as having insufficient knowledge. Additionally this research will investigate the individual’s beliefs about vaccinations in people who use social media, whether positive or negative. Uncovering individual characteristics in the respondents with their different beliefs can be helpful for deciding how to correctly reach each person group in the future in regards to vaccinations. We can use this information for educational purposes through social media to help those who were found to have negative or lower beliefs toward vaccinations.
Knowledge about vaccines, both true and false, can be easily accessible but also easily confused. The internet has become a huge influence on vaccine knowledge [ 26 ] and the emergence of social media has created a vast community that allows multi-person discussion to happen instantaneously and with little supervision [ 9 ]. “Anti-vaccination activists use [social media] to disseminate messages, facts and beliefs that oppose some or all recommended vaccinations” [ 6 ]. Brunson [ 8 ] found that the most significant factor influencing parents online is the percent of parents within a parents online network that are nonconforming. Because of these factors refusal to vaccinate is becoming a concern of public safety.
Regardless of vaccination rates, legitimate information about vaccine safety is “not reaching parents in an effective or convincing manner” [ 19 ]. This study seeks to assess whether factual knowledge is reaching social media users. This is because it has been found that the strongest predictor of positive attitudes towards vaccines is better knowledge [ 11 ]. In a study of Serbian University students it was found that 47.9% of participants thought that giving multiple vaccines at one time overloads the immune system [ 11 ]. Further research will determine if views such as the one above are perceived across all social media users and not just students.
Investigations of the dissemination of vaccine knowledge across social media have been substantially less studied until recent years, especially with the development of the COVID-19 vaccine. In a study of vaccine attitudes in Twitter users Mitra et al. found that anti-vaccination tweets had a wider reach (seen by more people) than pro-vaccine tweets [ 31 ]. In a study of HPV vaccine tweets it was found that there was “an association between prior exposure to negative tweets about HPV vaccines and the subsequent posting of negative tweets about HPV vaccines” which allowed for the sharing of negative opinions to more susceptible people [ 14 ]. From the information aforementioned it can be predicted that the majority of information about vaccines on social media has a negative connotation. What is still unclear is the ability of the general population to distinguish between fact and fiction, and whether this information has an influence on their knowledge and eventually decisions.
To determine what questions would be used and what is to be expected by demographic results this study looked at Duggan and Brenner's [ 13 ] journal article on social media users. This study found several useful social media user characteristics. Firstly, women are statistically significantly more likely to be social media users compared to men. This study also shows that with age, there is a statistically significant progressive decline in social media use. In terms of education they found that social media use was approximately equal across all levels of education. Equity across races was also seen. This demographic data is important for determining what questions should be asked, but also what can be expected in the results.
It is important to determine what factors influence people to not vaccinate. Yaqub et al. found that “‘distrust of doctors’, ‘distrust of government sources’, and ‘distrust of pharmaceutical companies’ as reason for hesitancy” [ 40 ]. In that same study they found that very few people reported that they did not have access to adequate information. While this is important, it does not elaborate on the quality of information these people are using. A cross sectional study in Australia in 2012 found that although 92% of 452 parents reported that their children were adequately vaccinated, 52% reported concerns including but not limited to vaccine safety and source of vaccine knowledge [ 10 ]. However due to discrepancies in defining anti-vaxxers or people who are vaccine hesitant, this study will focus on what knowledge people have and not ask their vaccine practices. Rather, it will look into how far the anti-vax messages have reached and the characteristics of those participants that have or have not been swayed by these messages.
Since the collection of the data related to this research the world has faced the COVID-19 pandemic. Social media and vaccine hesitancy has become a huge topic of discussion and research relating to combating COVID-19. Several studies worldwide have indicated that older individuals, females, those with higher incomes and those with higher education levels were more likely to accept a vaccine [ 34 ]. Research in this area has exploded due to fast paced development and deployment of the COVID 19 vaccine. Globally there has been varying intention to get the COVID-19 vaccine ranging from 41 and 89% [ 17 ]. A survey conducted in the United States of America showed only 57.6% of respondents intended to be vaccinated but also that “(62%) [of respondents] believed that sociopolitical factors and pressures may lead to a rushed approval for the COVID vaccine without the assurances of safety and efficacy” [ 27 ].
This research was conducted by a cross sectional multiple choice study created via Survey Monkey. Survey Monkey subscription was provided by Saint James School of Medicine and was chosen based on the platforms ability to export data to IBM's SPSS. Data of both qualitative (demographic) and quantitative (Belief and Knowledge Scores as described below) nature were collected. Survey was designed to be completed in 5–10 min. It was designed with no open ended questions, no intended question bias, and no implied judgement. Survey questions were validated by pilot participation and follow up to determine if there was any ambiguity that needed to be addressed. Demographic questions were designed to be all inclusive. Belief and Knowledge questions were based off of key arguments of vaccine deniers as determined by popular social media posts. Research of key argument involved exploring multiple social media platforms and investigating posts/comments regarding vaccine denial.
Inclusion/exclusion
Subjects were included based on completion of all parts of the survey. All demographics (country, race, gender, socioeconomic status, preferred social media platform) were included except for those under 18 years of age. Those participants under 18 were excluded because in most countries the age at which an individual can consent for medical treatment such as vaccination is 18 year old [ 39 ]. Therefore, vaccine decision making if deferred to parents and guardians over 18 years of age. Those participants who no do not consent to their information being used for research purposes (first question of the survey) were excluded from the study. Survey’s that contained any missing data/questions were excluding from analysis as scoring could not be completed. The survey was designed to not allow advancement onto next question without answering the current question. If participants clicked out of browser before completing final question their survey was invalidated and subsequently not extrapolated for analysis. Survey was available for completion from August 15, 2018 till November 1, 2018.
Snowball sampling of social media users was used. Snowball sampling was used to help perpetuate the survey through social media, where social media is the quality of referral. The study population was aimed at being as demographically diverse as possible among people who use social media. Snowball sampling was chosen in particular for its ability to perpetuate hard to reach communities, such as those who identify as vaccine deniers [ 23 ]. Additionally, using Facebook with snowball sampling is effective at diversifying the geographical scope and increasing the overall response rate [ 3 ].
Recruitment
Subjects were recruited through the three largest social media platforms (Facebook, Twitter, Instagram) via a shareable web link and asked to consent before completion of the research survey. Initial survey link was posted publicly on social media platforms outlined above with information on the survey and encouragement to further share the survey once completed. A web survey was chosen due to its ease, speed, cost, and ability to obtain a geographically diverse population [ 20 ].
All questions in the survey were only available in English language and can be found in the supplementary material, document 1 titled Survey. The first half of the survey consisted of demographics and questions pertaining to use of social media and its relation to vaccine information. The latter half of the survey had six questions relating to vaccine knowledge and six questions relating to vaccine myths. The method of scoring was designed by the authors to create a numerical scale for comparative analytics. Lack of knowledge and belief in myths is not a negative feature but more so an area of improvement and discussion. As such, the design only uses positive numerals for scoring each question. This scoring system was created by the authors of this study specifically for this research. Question content was selected by authors through observation of social media posts pertaining to vaccines (both pro vaccine and anti-vaccine content) for common misconceptions and rebuttals.
The six vaccine knowledge questions were scored on a two point scale. Questions were scored by awarding two points for the answer of belief in the vaccine statement, one point for uncertainty, and zero points for the answer of disbelief in the statement. All questions were then totaled for a score on a 12 point scale. Higher values (9–12) suggesting adequate vaccine knowledge, middle range (5–8) suggesting some vaccine knowledge but with uncertainty and lower values (0–4) suggesting inadequate vaccine knowledge. This score could then be appropriately analyzed.
The six vaccine belief questions were scored on a two point scale. Two points were given for the answer choice “disbelief in the vaccine statement”, one point for uncertainty, and zero points for the answer of belief in the statement. All questions were then totaled for a score on a 12-point scale. Similar to knowledge values, higher values were indicative of disbelief in common myths, whereas lower values indicated a belief in common myths. This score could then be appropriately analyzed.
All data analysis was conducted using IBM’s SPSS. Significance testing was performed using the Welch test. This test was chosen based on the negatively skewed data distribution with non-homogeneity of variances and sample sizes [ 16 ]. The Welch test has historically been shown to better control Type 1 error for these parameters compared to other tests [ 35 ]. Post hoc analysis was completed with Games Howell due to its robustness and utility in non-normal distributions [ 21 ]. A standard P value of 0.05 was used for statistical significance but reported up to < 0.001 which is the limit on statistical software. Raw and descriptive data is available from openICPSR.org project ID openicpsr-120,505 [ 5 ].
Of the 2517 respondents, 2417 were included in the analysis based on the inclusion/exclusion criteria. The age of participants showed 446 (18.5%) people aged 18–24, 715 (29.6%) people aged 25–34, 591 (24.5%) people aged 35–44, 394 (16.3%) people aged 45–54, 189 (7.8%) people aged 55–64 and 82 (3.4%) people over the age of 65. Females accounted for 80.1% of the respondents ( n = 1937 people) and males accounted for 18.8% ( n = 454 people). Respondents were predominantly North American with 70.3% ( n = 1700) from the USA and 12.9% ( n = 312) from other North American Countries. The remaining were divided into 7.4% ( n = 180) Australia/Oceanic, 7.3% ( n = 176) European, 0.9% ( n = 22) Asian, 0.6% ( n = 15) African, 0.5% ( n = 11) South American, and 1 respondent from Antarctica. The education levels showed that 0.1% ( n = 2) had no formal schooling, 0.4% ( n = 10) completed elementary school (grade level 1–8), 21.2% ( n = 509) completed high school (grade level 9–12/13), 22.3% ( n = 540) completed an Associates (2 year) degree, 34.5% ( n = 833) completed a Bachelor (4 year) degree, 13.9% ( n = 336) completed a Master’s degree, and 7.7% ( n = 187) completed a Professional degree (PhD, MD, DC, DO, etc.). Individuals identified themselves as Lower class socioeconomic status (SES) comprised 9.8% ( n = 238) of the population, 82.2% ( n = 1987) as Middle class SES, and 7.9% ( n = 192) as Upper class SES.
Facebook is the most commonly used social media type in the population with 69.8% ( n = 1688), followed by Twitter 15.6% ( n = 378), Instagram 12.9% ( n = 311), then other forms of social media 1.7% ( n = 40). Other forms of social media were identified as Snapchat, Tumblr, Reddit, Pinterest, or using all platforms equally. Most people 47.9% ( n = 1158) claimed to only spend 0–2 h on social media daily, followed by 40.9% ( n = 989) using 3–4 h, 8.4% ( n = 204) using 5–6 h, 1.5% ( n = 36) using 7–8 h, and 1.2% ( n = 30) using social media for over 9 h. Most respondents 92.7% ( n = 2240) have seen posts on social media about vaccines and only 7.3% ( n = 177) have not. These posts influence 5.4% ( n = 130) of users to think vaccines are worse than previously thought, 13.6% ( n = 328) to think vaccines are better than previous thought, 76.4% ( n = 1846) claim to not have been influenced by the posts, and 4.7% ( n = 113) had not seen any posts. Lastly, people claimed to trust doctors 89.4% ( n = 2160) the most with their immunization related information/decisions. The remaining people trust the internet 4.1% ( n = 100), family 2.0% ( n = 48), peers and friends 2.3% ( n = 55), social media 0.2% ( n = 5) and the government 2.0% ( n = 49) with their information and decisions.
Table 1, found in the supplementary document 2 labeled “Tables”, depicts the frequency of knowledge scores in the sample population. As described in the methods, knowledge scores are based on a scale from 0 to 12 derived from 6 questions with answers ranked from 0 to 2 points. Scores toward 0 represent negatively skewed knowledge, or lack of correct information. Scores toward 12 represent positively skewed knowledge, or adequate vaccine knowledge. Scores of 6 represent uncertainness.
Analysis of all demographic questions against the respondent’s knowledge score was completed by Welch and then further analyzed by Games Howell. Explanations of why these tests were chosen can be found in Methods. When age was compared with knowledge scores a Welch statistical value of 0.763 and the significance of 0.576 ( p > 0.05). Post hoc was not necessary.
Gender analysis showed a Welch statistic of 1.627 with a significance value of 0.204 ( p > 0.05). Post hoc analysis was not examined because there was no significance.
Geographical Welch testing showed a statistic value of 11.552 with a significance of < 0.001( p < 0.05). Since this value is statically significant post hoc analysis was examined. North Americans (USA) has significantly lower knowledge scores compared to Europe (mean difference − 0.78309, significance < 0.001), and Australia/Oceania (means difference − 0.84316, significance < 0.001). North Americans (Other) also showed significantly lower knowledge scores compared to Europe (means difference − 0.76122, significance 0.001) and Australia/Oceania (means difference − 0.84316, significance 0.001). Values from Asia, Africa, and South American should be looked at with caution because of low responses. Antarctica was excluded from these calculations because there was only one respondent.
Analysis of respondents highest level of education completed showed a Welch statistic of 13.030 and significance of 0.001 ( p < 0.05). Post hoc showed that those who completed a Professional degree had significantly higher scores than Bachelor’s degree (means difference 0.55353, significance of 0.007), Associates degree (means difference 1.21578, significance of < 0.001), and high school (means difference 1.11273, significance < 0.001). Those with Masters Degrees were significantly higher scoring than Associate degrees (means difference of 0.80000, significance < 0.001), and high school (means difference of 0.69695, significance of 0.001). Bachelor’s degree holders had significantly higher scores compared to Associates degree (means difference of 0.66224, significance of < 0.001) and High school (means difference of 0.55920, significance of 0.003). Those values from who have no formal school or only completion of elementary school should be looked at with caution due to low frequencies.
Socioeconomic class compared to knowledge scores yielded a Welch statistic of 0.266 and a significance of 0.767 ( p > 0.05). No further analysis was needed.
The type of social media used compared to knowledge score showed a Welch statistic of 7.175 and significance of < 0.001( p < 0.05). Games Howell determined that Twitter users had significantly higher scores than Facebook (means difference 0.43812, significance of 0.001) and Instagram (means difference 0.69491, significance of 0.001).
Hours spent on social media showed a Welch statistic of 2.531 and significance of 0.044 ( p < 0.05). Post hoc testing showed significantly lower values in those who use social media for 3–4 h compared to 0–2 h (means difference 0.33869, significance of 0.018). No other means from this analysis were significant.
Whether or not a respondent had seen anything on social media about vaccines was not analyzed because there are only 2 categories and therefore the question is noncompliant with the Welch analysis. The influence of vaccine posts on social media had a Welch statistic of 145.202 with a significance of < 0.001 ( p < 0.05). Post hoc testing revealed that those who now perceived their opinion of vaccine of being worse than previously thought had significantly lower scores compared to those who now think vaccines are better (means difference − 6.36712, significance of < 0.001), no influence/change in opinion (means difference − 5.83564, significance of < 0.001) and those who had not seen anything (means difference − 4.70483, significance of < 0.001). Those who think vaccines are better after seeing social media posts had significantly higher scores compared to worsened opinions (as mentioned before), those who were not influenced (means difference 0.53148, significance < 0.001) and those who have not seen anything (means difference 1.66229, significance < 0.001). In addition, those who have not been influenced by posts had significantly higher scores than those who have not seen any posts (means difference 1.13081, significance < 0.001).
Lastly, those trusted for immunization related information and decisions was analyzed and found a Welch statistic of 83.032 with significance of < 0.001 ( p < 0.05). Post hoc analysis showed those who trusted Doctors the most have significantly higher scores than those who trusted the internet (means difference of 5.32139, significance of < 0.001), family (means difference 5.94306, significance < 0.001), and peers (means difference 6.31957, significance of < 0.001). Those who trusted the government the most also had significantly higher scores than internet (means difference 5.13429, significance < 0.001), family (means difference 5.75595, significance of < 0.001) and peers (means difference 6.13247, significance of < 0.001). Trusting of social media should be looked at with caution due to low frequencies.
Depiction of the frequency of belief scores in the sample population can be found in Table 2, found in the supplementary document 2 labeled “Tables”. As noted in the methods, the remaining 6 questions were scored on a two point scale resulting in a belief score from 0 to 12. Scores toward 0 represent negatively skewed beliefs or belief in common myths. Scores toward 12 represent positively skewed beliefs or disbelief in common myths. Scores of 6 represent uncertainness.
The analysis of the demographic questions against the individual’s belief score was completed by Welch and then further analyzed by Games Howell. Explanations of why these tests were chosen can be found in methods. When age was compared with belief score a Welch statistical value of 2.923 and significance of 0.013 ( p < 0.05). Post Hoc revealed 65-year-olds and older had significantly lower scores than 10–24-year-olds (mean difference − 1.37750, significance 0.014) and 24–34 year olds (mean difference − 1.19606, significance 0.047).
Gender analysis showed a Welch statistic of 0.320 with a significance value of 0.728 ( p > 0.05). Post hoc analysis was not examined because there was no significance.
Geographical Welch testing showed a statistic value of 29.212 with a significance of < 0.001( p < 0.05). Due to this value being statically significant, post hoc analysis was examined. North Americans (USA) had significantly lower belief scores compared to Europe (mean difference − 1.47989, significance < 0.001), and Australia/Oceania (means difference − 1.81575, significance < 0.001). North Americans (Other) also showed significantly lower belief scores compared to Europe (means difference − 1.29021, significance < 0.001) and Australia/Oceania (means difference − 1.62607, significance < 0.001). Values from Asia, Africa, and South American should be looked at cautiously because of the low response rate. Antarctica not included in these calculations because there was only one individual who responded.
The analysis of individuals with the highest level of education completed showed a Welch statistic of 17.789 and significance of < 0.001 ( p < 0.05). Post hoc showed that those who completed a professional degree had significantly higher scores than those with master’s degree (mean difference 0.74516, significance of 0.009), bachelor’s degree (means difference 1.10881, significance of < 0.001), associate’s degree (means difference 2.02797, significance of < 0.001), and high school (means difference 1.97009, significance < 0.001). Respondents with master’s degrees were significantly higher scoring than those with associate degrees (means difference of 1.28280, significance < 0.001), and high school (means difference of 1.22493, significance of < 0.001). Those with bachelor’s degrees had significantly higher scores compared to those with associate’s degrees (means difference of 0.91916, significance of < 0.001) and high school (means difference of 0.86128, significance of < 0.001). The values from those individuals that had no formal schooling, or only completion of elementary school, should be looked at cautiously due to low frequencies.
Socioeconomic class, compared to belief scores, resulting in a Welch statistic of 0.028 and a significance of 0.972 ( p > 0.05). No further analysis was needed.
The type of social media users compared to belief score showed a Welch statistic of 8.011 and a significance of < 0.001( p < 0.05). Games Howell determined that Twitter users had significantly higher scores than Facebook (means difference 0.55094, significance of 0.001) and Instagram (means difference 0.98733, significance of < 0.001).
Hours spent on social media showed a Welch statistic of 3.162 and a significance of 0.016 ( p < 0.05). Post hoc testing showed significantly lower values in those who used social media for 3–4 h compared to 0–2 h (means difference 0.39195, significance of 0.034). No other means from this analysis were significant.
Exposure to posts on social media about vaccinations was not analyzed because there were only two categories and therefore, noncompliant with the Welch analysis. The influence of vaccine posts on social media had a Welch statistic of 312.900 with a significance of < 0.001 ( p < 0.05). Post hoc testing revealed that those who now perceived their opinion of vaccines of being worse than previously thought, had significantly lower scores compared to those who now think vaccines are better (means difference − 7.97280, significance of < 0.001), No influence or change in opinion (means difference − 7.27248, significance of < 0.001), and those who had not seen anything (means difference − 4.97992, significance of < 0.001). Those who thought vaccines were better after seeing social media posts had significantly higher scores compared to worsened opinions, as mentioned before, and to those who were not influenced (means difference 0.70031, significance < 0.001), and those who had not seen anything (means difference 2.99288, significance < 0.001). Also, those who had not been influenced by social media posts had significantly higher scores than those who had not seen any posts at all (means difference 2.29256, significance < 0.001).
Finally, those trusted in immunization related information and decisions were analyzed and found a Welch statistic of 150.953 with a significance of < 0.001 ( p < 0.05). Post hoc analysis showed those who trusted doctors the most had significantly higher scores than those who trusted social media (means difference of 6.50713, significance of < 0.001), family (means difference 7.28796, significance < 0.001), and peers (means difference 7.41258, significance of < 0.001). Individuals who trusted the government the most also had significantly higher scores than social media (means difference 6.87265, significance < 0.001), family (means difference 7.65349, significance of < 0.001) and peers (means difference 7.77811, significance of < 0.001). Trusting of social media should be looked at cautiously due to its low frequencies.
The significance found in this study can help us understand who is being influenced by posts about vaccines on social media. It is important to note that the sample in this study was originally started in North America; hence the vast majority of respondents reported residing on this continent. Beyond that, significant values from the statistics should be examined to determine what it means for this research and for further implications.
Mean scores
Overall this study found that respondents were very knowledgeable with a mean knowledge score of 10.4. Very few people had negatively skewed knowledge 0–4 (138 people, 5.7% of the total study population). Further investigation into those people that scored lowest would be able to show greater detail into the minds of those people and where the lack of factual knowledge is coming from or what hurdle needs to be faced.
Respondents had mainly positive beliefs about vaccines with a mean belief score of 9.68, with a standard deviation of 3.14. Few people had negatively skewed beliefs 0–4 (236 people, 9.8% of the total study population). Looking deeper into the individuals that had lower scores would be able to show greater explanation into why their beliefs about vaccines were negative, and how we might be able to change these beliefs.
In a study in 2009, 75.64% of people aged 18–64 were internet users and 74% of users aged 18–24 were social media users (Chou et al). This is important because the high social media use group from that study is now 27–31 years old and could now be making decisions about vaccines for their children.
When looking at ages, we saw that 65 years and older tend to have more negative beliefs than those individuals that are 18–24 and 25–34, with a significant difference of 0.013 ( p < 0.05). Meaning, older people tend to believe the things they see on social media more than someone who is younger. We saw that in the previous research, older people are less likely to use social media [ 4 ], but in this study, those who did use social media scored lower. There were no significant correlations regarding age for knowledge scores.
Gender and socioeconomic status (SES)
A comprehensive research paper by UNICEF in 2013 [ 36 ] titled “Tracking anti-vaccination sentiment in eastern European social media networks” found that females are more likely to discuss developmental disabilities, chemical, toxins and potential side effects whereas males are more likely to discuss conspiracy theories, religion and distrust of the government.
Across all genders and all SES, we saw no significant differences in knowledge or beliefs. This is significant to note because as previous literature and this one found, women are the predominant social media users. Since gender and SES have no impact on vaccine knowledge or beliefs we look deeper into other variables.
For geography, analysis was according to continent lived on and subsequently found statistical significance. North Americans compared to all continents are significantly less knowledgeable about vaccines and had more negatively skewed beliefs. Although this research does not give a reason to why North Americans are less knowledgeable and believe more myths it gives us insight into areas that need to be studied.
There was little data available on Asia, Africa, and South America and none on Antarctica making analysis unreliable and therefore all of which should be disregarded. It is evident that Asia is showing a lower mean knowledge than other areas but without significance due to low frequency. This is paralleled when looking at beliefs. This may be explained by previous research has found that “among people who use the internet, those in developing countries often turn out to be more likely than their counterparts in advanced economies to network via platforms like Facebook and Twitter” [ 33 ]. Further investigation should determine if Asian countries do in fact have lower knowledge of vaccines and why this is. Some things to determine include whether there is a quantifiable difference between continental education, social media, use, cultural perceptions of vaccination, etc.
There is a steady incline in mean knowledge and mean belief score as education level increases. Due to low response frequencies of “no education” and “elementary education” results pertaining to these categories should be disregarded. There are significant differences between all other education levels in an increasing fashion for both knowledge and beliefs. This tells us that those with higher education are in some way or another seeking and finding valid information about vaccines. “Formal schooling adds significant value to innate ability in the form of higher-order cognitive skills crucial to decisions about health” [ 2 ]. This is important in terms of whom to focus on for future vaccine education and propaganda.
Social media platform
A comprehensive research paper titled “Tracking Anti Vaccination Sentiment in Eastern European Social Media Networks” by UNICEF in 2013 [ 36 ] found that vaccine influencers (people or pages that speak publicly about vaccines, both positively and negatively) are most prominent on Facebook and Twitter. This study found that Twitter users are significantly more knowledgeable about vaccines than Facebook or Instagram users and also had more statistically significant positive beliefs about vaccines. Somehow, information is reaching Twitter users but not reaching other forms of social media. Upon further investigation research has showed “information sought from Facebook may be obtained socially (i.e. by asking other users), whereas the information sought on Twitter might be more cognitively based, such as academic or political information that is best gained by reading source materials, for which links are often ‘tweeted’ “[ 22 ].
Time on social media
While the majority of people claimed to only spend 0–2 h on social media daily, those who spent more time, namely 3–4 h, were significantly more knowledgeable about vaccines and had significantly more positive beliefs about vaccines. Although not explored in this study, other studies have found that upwards of 90% of young adults use social media and this number is increasing every year and the majority of this time is spend on smartphones [ 37 ]. Possible reasons for the increased scores may be that those who spend more time on social media could be spending that time reading more deeply into conversations or information. Although it does not appear to be currently studied, there could also be an association between access to a smartphone and the ability to fact check information on the spot.
Vaccine related posts
One of the most interesting takeaways from the data analysis is how vaccine posts have influenced opinions. Those who reported that after seeing vaccine posts they now think vaccines are worse have significantly lower knowledge and belief scores. The opposite is true for those who reported more positive opinions since seeing posts, their scores were significantly higher. The importance of this is that people are able to accurately self-report how these vaccine posts affect them. Those people who see posts about vaccines that make them think vaccines are “bad” have less knowledge or their knowledge has been changed from correct to incorrect with these posts.
A European study found that over 40% of respondents had some degree of “negative feelings about vaccine safety” [ 28 ]. These “fear” posts are typically full of myths, although not inherently known to be myths by the reader, about vaccines and as a result people became more likely to believe the myths. While it cannot be inferred why this is happening it is an interesting statistic. The presence of this significance lets health/vaccine promoters, such as government; know that the vaccine posts with valid knowledge and promotion are working. Those respondents that reported they were not influenced by social media posts, had lower belief scores compared to those who said they were positively influenced by social media posts. Those who reported they were not influenced had similar knowledge scores to those who were positively influenced. This may be due to the ability to weed through information to find facts versus fiction.
The internet is now the easiest and most accessible way to get information. A google search from the United States of America for the term “vaccination” yielded 71% anti-vaccination pages and only 29% pro vaccination pages [ 25 ]. Many of these anti-vaccinations sites claimed that vaccines contain “poisons” and were damaging to the human body [ 25 ]. “In many cases, [those who perceive vaccines as harmful] may become the only individuals who voice their opinions [ 32 ]. This allows for a greater propagation of anti-vax information rather than pro-vaccination and factual knowledge. Googling information about the MMR vaccine and autism only 51% of the search results yielded correct information [ 41 ]. Not only is it important to understand the diversity of search results but also the quality of the websites appearing. Many of “sites masquerading as official scientific sites, some web users may not question the veracity of such material” [ 12 ]. Although this study does not specifically focus on the internet, social media allows for easy sharing of not only opinions but also links to some of these “pseudo-science” websites.
Trusted source
Lastly, the analysis of who people trust most with their vaccine related information and decisions. Literature review revealed a study that found that parents who used the internet to get their information about vaccines were more likely to think children do not benefit from vaccines [ 24 ]. It is comforting to know that the vast majority of respondent in this study do in fact rely on the information they receive from their doctor and government. While many anti-vax campaigns are grounded on the fact that authorities are misconstruing vaccine information, the idea of big pharma, it is evident that that ideal is not being taken up by social media users. Those who trust the government and doctors with their information were significantly more knowledgeable about vaccine and more positive beliefs facts than those who trust another group. While it is known that many anti-vaxxers rely on social media to disseminate their message, very few people trust social media as a source. This is very good news for doctors whose patients seek information from them and government run websites such as health departments and health organizations.
Limitations
Some limitations faced by this research include the cross sectional nature of this study. Cross sectional studies cannot infer causation, only correlations. It also limits this survey to only surface information and not important individual details. In addition, a limitation may be that demographics were not cross analyzed. This means that while it was found that professionals had the highest knowledge score this research does not delve into the gender, location, age, etc. of these people. This same information could be analyzed in this way for further investigation. It is also important to note that this information is limited by some frequencies being too low for confidence. These areas would ideally have to be isolated and looked at again with higher number of participants. These categories were mentioned in the discussion and results stating they should be disregarded.
This study is also limited by the snowball sampling method. This limits the study by which participants decided to share, and where the majority of their friends are located, as seen by the North American predominance. While great caution was taken when constructing this survey to be nonjudgmental, some valuable information is lost in the fact that respondents were not asked their vaccine practices.
As with all online surveys, no interviewer was present. Respondents were not able to clarify answers that they felt needed explanation. Without an interviewer there is no probing into deeper answers. Information this study found could be used in the future for interviewer based research to determine a more in depth understanding of those who lacked vaccine knowledge. Another limitation of being an online survey is that there is no accountability for answers. Respondents could have clicked through the survey to complete all the questions as quickly as possible without regard for what the answer choices were.
The information portrayed in this research by the authors favors scientific fact as described by other journal articles and not personal opinions/bias. Every effort was made not to use incriminating or offensive language and statements in the creation of the survey. This core of this research was completed by the authors when they were medical students. Saint James School of Medicine ethics committee approved this research and all of its modalities. Survey platform was provided by Saint James School of Medicine.
As social media continues to grow exponentially, it can be expected that anti-vaxxers will further spread their messages across these platforms. While this research does not delve into the totality and extent of the anti-vax movement on social media it does provide some insightful information. As mentioned previously, it is possible for governments and doctors to use this information to intervene and correct false information about vaccines and their safety. Given that trust in doctors resulted in significantly higher knowledge and belief scores, it is imperative that physicians create a trusting environment and relationship with their patients and guardians of pediatric patients. This also tells us that the information patients are getting from their doctors and the conversations they are having are influencing vaccine decision making in a positive way. What was not studied, and would be beneficial to investigate going forward, is whether doctors have the ability to change a patient’s perspective on vaccines once they have been exposed to negatively skewed vaccine information. It is also evident that those who trust the internet with their vaccine information are exposed to more misinformation. There may be an avenue for addressing misinformation on the internet through this trusted source and that could be via physician social media influencers. We have started to see the importance of this avenue with the prevalence of COVID-19. Social media has create an easily accessibly way to reach credible sources such as CDC and WHO, who made their presence known during the height of the pandemic through social media campaigns [ 7 ]. This appears to be especially important for direct intervention on Facebook, where the majority of social media use is, and unfortunately lower rates of knowledge and more negatively skewed beliefs. In North America, actions should be taken to combat the misinformation and myths that are reaching its population more than anywhere else in the world.
Further research is recommended to understand why some countries, age groups, social network users, etc. are not getting adequate information on vaccines. Future research would benefit from using a structured interviewer based approach to allow for expansion and clarification of answers. Further evaluation of the data collected here could yield more in-depth understanding of demographics. As mentioned before this research did not cross analyze demographics.
Availability of data and materials
Raw and descriptive data is available from openICPSR.org project ID openicpsr-120505.
Abbreviations
Anti- vaccinator / Vaccine denier
Human Papilloma Virus vaccine
Measles Mumps Rubella vaccine
United Nations International Children’s Emergency Fund
Statistical package for social sciences
Socioeconomic status
Armitage R. Online ‘anti-vax’campaigns and COVID-19: censorship is not the solution. Public Health. 2021;190:e29. https://doi.org/10.1016/j.puhe.2020.12.005 .
Article CAS PubMed Google Scholar
Baker DP, Leon J, Smith Greenaway EG, Collins J, Movit M. The education effect on population health: a reassessment. Popul Dev Rev. 2011;37(2):307–32. https://doi.org/10.1111/j.1728-4457.2011.00412.x .
Article PubMed PubMed Central Google Scholar
Baltar F, Brunet I. Social research 2.0: virtual snowball sampling method using Facebook. Internet research; 2012.
Google Scholar
Bell C, Fausset C, Farmer S, Nguyen J, Harley L, Fain WB. Examining social media use among older adults. In: Proceedings of the 24th ACM conference on hypertext and social media; 2013. p. 158–63.
Chapter Google Scholar
Benoit SL, Mauldin R. The “anti-vax” movement: a quantitative report on vaccine beliefs and knowledge across social media. Ann Arbor: Inter-university Consortium for Political and Social Research [distributor]; 2020. https://doi.org/10.3886/E120505V2
Betsch C, Brewer NT, Brocard P, Davies P, Gaissmaier W, Haase N, et al. Opportunities and challenges of web 2.0 for vaccination decisions. Vaccine. 2012;30(25):3727–33. https://doi.org/10.1016/j.vaccine.2012.02.025 .
Article PubMed Google Scholar
Brindha MD, Jayaseelan R, Kadeswara S. Social media reigned by information or misinformation about COVID-19: a phenomenological study. Alochana Chakra J. 2020;9(5):585–602.
Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397 peds-2012.
Article Google Scholar
Chou WYS, Hunt YM, Beckjord EB, Moser RP, Hesse BW. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11(4):e48. https://doi.org/10.2196/jmir.1249 .
Chow MYK, Danchin M, Willaby HW, Pemberton S, Leask J. Parental attitudes, beliefs, behaviours and concerns towards childhood vaccinations in Australia: a national online survey. Aust Fam Physician. 2017;46(3):145.
Cvjetkovic SJ, Jeremic VL, Tiosavljevic DV. Knowledge and attitudes toward vaccination: a survey of Serbian students. J Infect Public Health. 2017;10(5):649–56. https://doi.org/10.1016/j.jiph.2017.05.008 .
Davies P, Chapman S, Leask J. Antivaccination activists on the world wide web. Arch Dis Child. 2002;87(1):22–5. https://doi.org/10.1136/adc.87.1.22 .
Article CAS PubMed PubMed Central Google Scholar
Duggan M, Brenner J. The demographics of social media users, 2012, vol. 14. Washington, DC: Pew Research Center’s Internet & American Life Project; 2013.
Dunn AG, Leask J, Zhou X, Mandl KD, Coiera E. Associations between exposure to and expression of negative opinions about human papillomavirus vaccines on social media: an observational study. J Med Internet Res. 2015;17(6):e144. https://doi.org/10.2196/jmir.4343 .
Durbach N. Bodily matters: the anti-vaccination movement in England, 1853–1907: Duke University Press; 2005. https://doi.org/10.1215/9780822386506 .
Fagerland MW, Sandvik L. Performance of five two-sample location tests for skewed distributions with unequal variances. Contemp Clin Trials. 2009;30(5):490–6. https://doi.org/10.1016/j.cct.2009.06.007 .
Feleszko, W., Lewulis, P., Czarnecki, A., & Waszkiewicz, P.. Flattening the curve of COVID-19 vaccine rejection—a global overview. 2020. Available at SSRN.
Book Google Scholar
Fox S, Jones S. The social life of health information. Washington, DC: Pew internet & American life project; 2009.
Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125:654 peds-2009.
Fricker RD, Schonlau M. Advantages and disadvantages of internet research surveys: evidence from the literature. Field Methods. 2002;14(4):347–67. https://doi.org/10.1177/152582202237725 .
Hilton A, Armstrong R. Statnote 6: post-hoc ANOVA tests; 2006.
Hughes DJ, Rowe M, Batey M, Lee A. A tale of two sites: twitter vs. Facebook and the personality predictors of social media usage. Comput Hum Behav. 2012;28(2):561–9. https://doi.org/10.1016/j.chb.2011.11.001 .
Johnson, T. P.. Snowball sampling: introduction. 2014. Wiley StatsRef : Statistics Reference Online.
Jones AM, Omer SB, Bednarczyk RA, Halsey NA, Moulton LH, Salmon DA. Parents’ source of vaccine information and impact on vaccine attitudes, beliefs, and nonmedical exemptions. Adv Prev Med. 2012;2012:1–8. https://doi.org/10.1155/2012/932741 .
Kata A. A postmodern Pandora’s box: anti-vaccination misinformation on the internet. Vaccine. 2010;28(7):1709–16. https://doi.org/10.1016/j.vaccine.2009.12.022 .
Kata A. Anti-vaccine activists, web 2.0, and the postmodern paradigm–an overview of tactics and tropes used online by the anti-vaccination movement. Vaccine. 2012;30(25):3778–89. https://doi.org/10.1016/j.vaccine.2011.11.112 .
Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J Community Health. 2021;46(2):270–7. https://doi.org/10.1007/s10900-020-00958-x .
Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. https://doi.org/10.1016/j.ebiom.2016.08.042 .
Massey, E.. A sermon against the dangerous and sinful practice of inoculation . 1722. William Meadows.
McCauley MM, Kennedy A, Basket M, Sheedy K. Exploring the choice to refuse or delay vaccines: a national survey of parents of 6-through 23-month-olds. Acad Pediatr. 2012;12(5):375–83. https://doi.org/10.1016/j.acap.2012.06.007 .
Mitra T, Counts S, Pennebaker JW. Understanding anti-vaccination attitudes in social media. In: ICWSM; 2016. p. 269–78.
Poland GA, Jacobson RM. Understanding those who do not understand: a brief review of the anti-vaccine movement. Vaccine. 2001;19(17–19):2440–5. https://doi.org/10.1016/S0264-410X(00)00469-2 .
Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Res Cent. 2018;22:2–19.
Soares P, Rocha JV, Moniz M, Gama A, Laires PA, Pedro AR, et al. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021;9(3):300. https://doi.org/10.3390/vaccines9030300 .
Tomarken AJ, Serlin RC. Comparison of ANOVA alternatives under variance heterogeneity and specific noncentrality structures. Psychol Bull. 1986;99(1):90–9. https://doi.org/10.1037/0033-2909.99.1.90 .
UNICEF. Tracking anti vaccination sentiment in eastern European social media networks. New York: UNICEF; 2013.
Villanti AC, Johnson AL, Ilakkuvan V, Jacobs MA, Graham AL, Rath JM. Social media use and access to digital technology in US young adults in 2016. J Med Internet Res. 2017;19(6):e196. https://doi.org/10.2196/jmir.7303 .
Weigmann K. An injection of confidence: scientists explore new and old methods to counter anti-vaccine propaganda and overcome vaccine hesitancy so as to increase vaccination rates. EMBO Rep. 2017;18(1):21–4. https://doi.org/10.15252/embr.201643589 .
World Health Organization. Considerations regarding consent in vaccinating children and adolescents between 6 and 17 years old (No. WHO/IVB/14.04): World Health Organization; 2014.
Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: a critical review. Soc Sci Med. 2014;112:1–11. https://doi.org/10.1016/j.socscimed.2014.04.018 .
Zimmerman RK, Wolfe RM, Fox DE, Fox JR, Nowalk MP, Troy JA, et al. Vaccine criticism on the world wide web. J Med Internet Res. 2005;7(2). https://doi.org/10.2196/jmir.7.2.e17 .
Download references
Acknowledgements
Dr. Branka Filipovich from Saint James School of Medicine for research guidance and draft editing. Dr. Zarina Merchant for supervising the survey portion of this research.
There is no funding associated with this project.
Author information
Authors and affiliations.
Saint James School of Medicine, Park Ridge, Illinois, USA
Staci L Benoit & Rachel F. Mauldin
You can also search for this author in PubMed Google Scholar
Contributions
SB; Lead author, designed analytic scoring/method and data analyst. RM; co-author, co-conceived experimental idea, coordination of timeline, data collection. All authors have reviewed and approved the manuscript.
Corresponding author
Correspondence to Staci L Benoit .
Ethics declarations
Ethics approval and consent to participate.
This research was approved by the ethics committee of Saint James School of Medicine. This research included a consent page present at the beginning of the survey which included consent to participate. Participation was limited to those over 18 years of age. Failure to consent resulted in termination of the remainder of the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Survey. Anti- Vax research survey. This file includes the questions and multiple choice options that were used to build the research’s SurveyMonkey Survey.
Additional file 2: Table 1.
Frequency of Knowledge Scores 0-12 with percent of total sample population. Table 2. Frequency of Belief scores 0-12 with percent of total sample population.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Benoit, S.L., Mauldin, R.F. The “anti-vax” movement: a quantitative report on vaccine beliefs and knowledge across social media. BMC Public Health 21 , 2106 (2021). https://doi.org/10.1186/s12889-021-12114-8
Download citation
Received : 03 August 2020
Accepted : 27 October 2021
Published : 17 November 2021
DOI : https://doi.org/10.1186/s12889-021-12114-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Social media
- Vaccine denier
- Social network
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Emerging Infections; Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing The Science and Response Capabilities: Workshop Summary. Washington (DC): National Academies Press (US); 2002.

Biological Threats and Terrorism: Assessing The Science and Response Capabilities: Workshop Summary.
- Hardcopy Version at National Academies Press
3 Vaccines: Research, Development, Production, and Procurement Issues
Vaccines not only afford the best protection against infectious disease but can serve as strong deterrence factors as well. From a bioterrorist perspective, vaccine-resistant agents are more difficult to engineer than drug-resistant agents. But the potential market has been too small and uncertain to encourage the vaccine industry to make large investments in research, development, and manufacturing of new products. This is alarming considering the eight to ten years often needed to develop a new vaccine, compared to only two to three years to develop a new bioweapon.
Even among the four major vaccine manufacturers, there is insufficient production capacity. It was suggested during this session that in order to move animal and clinical testing forward, incentives need to be established to reduce the current challenges of vaccine development; vaccine production priorities need to be set and a central office or leader authorized to declare top priorities; and the role of the major vaccine manufacturers needs to be facilitated by clear directions and active collaboration between industry and government.
The use of vaccines as a civilian biodefense measure presents multiple challenges that are quite different from those of vaccine use by the military. Much of the challenge is due to the fact that the threats are uncertain and risk-benefit information difficult to assess. The very nature of terrorism produces a high level of uncertainty about what to expect and how to prepare. Additionally, DoD has developed vaccines to be used in normal healthy adults between the ages of 18 and 65, not pediatric, geriatric, immunocompromised or other subsets of the civilian population. Currently, there is no policy in place for immunizing the civilian population as a bioweapons defense measure, however several government agencies are working at unprecedented speed to put the correct policies into place.
The threat of a global pandemic makes smallpox one of the top vaccine priorities. An aggressive clinical development plan is currently in place; its goal is to build the stockpile with enough vaccine to protect the entire country within the year. The vaccine immune globulin (VIG) supply also needs to be expanded. Long-term goals include developing a safer vaccine that can be used in immunocompromised or other at-risk individuals.
Anthrax vaccine is another top priority. As of May 2001, over two million doses of the current anthrax vaccine have been administered to over 500,000 individuals, mostly military personnel. But there is an urgent need for more anthrax vaccine for the immunization of high risk civilian populations, as well as for use in medical management of exposed individuals in conjunction with antibiotics. Currently, there is only one manufacturer of licensed anthrax vaccine, but production is limited because of regulatory problems. Several commercial firms have offered to aid in scaled-up production, but the inherent variability of the manufacturing process and the risk of failure when scaling up so rapidly to such a high volume could create problems. Other mid to long-term anthrax vaccine needs include the development of a second-generation vaccine (e.g., a recombinant protective antigen vaccine) as well as better delivery technologies (e.g., plasmid DNA).
Of lesser importance than vaccines against smallpox and anthrax are vaccines against bacterial infections for which antibiotics can be used and other viral agents that, for the present, seem to be a lesser threat.
A recent independent review of DoD's vaccine acquisition program recommended an integrated approach between DoD and industry and the establishment of a dedicated national vaccine production facility that allows for maximal flexibility and expandable manufacturing capability for the production of various types of vaccines. Whether the proposed facility will be government-owned and contractor-operated or contractor-owned and contractor operated is open for discussion.
Ebola virus provides a useful paradigm for how a molecular-level understanding of the pathogenesis of a virus can be used to develop a new vaccine for an infectious agent that would otherwise be difficult to tackle. This type of molecular genetics approach can reveal possible targets for antiviral drugs as well. For example, recent studies have shown that one of the domains of the ebola virus forms a coil-to-coil structure that is similar to structures found in other viruses, including HIV and influenza. This similarity suggests that the approach being used to develop products for antiviral use against HIV may also be useful for targeting the coil-to-coil region of ebola virus. In fact, targeting this coil-to-coil structure may prove to be a useful general antiviral strategy against many different viruses.
Other vaccine issues that were raised during this session include:
- Improving the usefulness of DNA vaccines, which work well in rodents but not primates.
- Consideration of combination vaccines, for example can we use what we have learned from ebola to make a combination vaccine for use against all hemorrhagic fevers?
- Application of genomics to vaccine research could have, for example if we could use the new high throughput technology to identify genomic biomarkers for vaccine efficacy, then we could use these biomarkers in the future to move forward more quickly toward licensure.
- The need for a strong infrastructure to receive the intense flow of resources that would be expected with a rapid deployment of vaccines in response to out-breaks.
- The need for ways to accelerate vaccine FDA licensure without compromising product safety, for example use of the proposed animal efficacy rule for products that are either not feasible or ethical for human efficacy trials.
VACCINES FOR THREATENING AGENTS: ENSURING THE AVAILABILITY OF COUNTERMEASURES FOR BIOTERRORISM *
Affiliations.
Recent events have brought the subject of vaccines as a defense against bioterrorism into very sharp focus. We have been forced to take action in an area that, for the civilian sector, had previously been largely an academic debate and planning exercise with inadequate definitive action. We have changed from a nation of skeptics concerning the threat of bioterrorism to a nation of believers. Several government agencies are working at unprecedented speed to acquire the needed vaccines and put the correct policies into place for utilization.
However, the use of vaccines for defense against bioterrorism presents multiple challenges that are quite different from the traditional public health use of vaccines for protection against endemic or epidemic diseases. The issues are also quite different from those faced by the armed forces. The appropriate use of vaccines as a defense against bioterrorism presents major challenges in public policy development as well as public education. The ongoing public debates in the media highlight the complexity of the issues and reveal the widespread lack of understanding of the limitations of the current vaccines, especially vaccinia vaccine. For example, there is a call for widespread vaccination against smallpox but, in contrast, there is much misinformation and inappropriate fear about the effects of anthrax vaccine.
Some of the challenges involved with developing vaccine policies for defense against bioterrorism lie in the uncertainty of the threats. In contrast, policies for the use of vaccines against naturally-occurring disease threats are based on a wealth of historical and current epidemiologic information about disease burden and potential. Additionally, there is extensive data available on the safety of widely used vaccines that can be used to confidently assess risk benefit and cost effectiveness. In the case of agents of bioterrorism, however, risk assessment is much more difficult. The great difficulty in obtaining timely and reliable intelligence on the threat of biologic terrorism is a major part of the problem. Critical policy decisions—such as which vaccines will be needed, how large the stockpiles should be, and how the vaccine should be used—are greatly influenced by perceptions of threat. The very nature of terrorism produces a high level of uncertainty about what to expect and prepare for, and there is a wide and varying spectrum of perceived threats.
Obtaining the vaccines that are needed to protect our military and civilian populations depends entirely on effective government action. The potential market has been too small, at least up to the present time, to encourage the vaccine industry to make the large investments needed in research, development and manufacturing facilities. This has changed dramatically in the past two months. Nevertheless, the current situation is a result of past misjudgments, which resulted in insufficient government investment in vaccine research and development, and manufacturing capacity. There is an urgent need for rapid progress in R&D, manufacturing, and licensing processes, all of which are painfully slow processes when done by the usual methodologies.
Vaccines have varying usefulness in defense against bioterrorism. At the top of the list is the need for smallpox vaccine to prevent an outbreak from becoming a catastrophic global pandemic. Both smallpox and anthrax vaccines would be very useful in the medical management of exposed individuals, if the vaccines were readily available and placed in geographic proximity to multiple centers for distribution. Less important to the civilian populations are vaccines against bacterial agents that can be managed with antibiotics and viral agents which, at least for the present, seem to be lesser threats. These include plague, tularemia, hemorrhagic fever viruses, alphavirus encephalidities, Rift Valley fever, and others. However, several of these vaccines should be available for both civilian and military use. A government-owned production facility may be the best means for meeting the needs of these lower priority vaccines which will probably, at least initially, be made in much smaller quantities than smallpox and anthrax vaccines.
Smallpox Vaccine
The acquisition of a smallpox vaccine stockpile for civilian use started in 1999, with an Acambis contract for 40 million doses which now has been increased to 54 million doses. The seed virus was developed by cloning a New York City Board of Health strain derived from Wyeth Dry Vax. Animal model studies indicate that this strain appears to be somewhat less neurovirulent than the parent virus. The clinical development plan is aggressive; the phase I clinical trial should occur, as planned, in the latter part of January 2002. A very rapid procurement action has been in progress over the past weeks. The goal is to stockpile enough smallpox vaccine to protect the entire nation within the year. The response from the vaccine industry has been very heartening and has provided excellent options for utilizing existing manufacturing capacity to meet current requirements. Every effort will be made by CDC, FDA and NIH to assure that these contractors succeed to meet goals, time lines, and regulatory requirements. This will require truly unprecedented coordination and responsiveness by both the manufacturers and the various agencies.
Although the first step in building the smallpox vaccine stockpile is to ensure that vaccine manufacturing is underway, there are several other immediate issues that need to be addressed:
- Vaccination policy issues continue to be controversial. The CDC recently sent out a draft smallpox response plan to the states for comment. The plan calls for primary reliance on ring vaccination—the traditional method—to control an outbreak. The CDC has vaccinated 140 staff members who are most likely to be involved in investigating an outbreak, but no further vaccination with potential responders or health care providers is planned at this time. Laboratory personnel working with pox viruses will, of course, continue to be vaccinated.
- There is a need for more vaccine immune globulin (VIG) or VIG substitute to deal with the consequences of vaccination in immunosuppressed or other high risk subsets of the population. An interagency working group is currently exploring options for expanding the VIG supply.
- There is a need to develop a safer vaccine for use in immunosuppressed individuals, pregnant women, and other individuals for which the current vaccine is contraindicated. This will not only be a challenging research and development problem but also a challenging regulatory problem due to the difficulties in proving efficacy.
Anthrax Vaccine
The current licensed U.S. anthrax vaccine is a filtrate of culture media that contains a high level of PA (protective antigen) absorbed to alum; it probably contains small amounts of the other factors as well. An ongoing study at CDC is testing immunization schedules that involve fewer than the currently recommended six doses for this vaccine. Conventional wisdom has it that the live attenuated vaccines used in Russia and China are too reactogenic to be licensed in the United States. Israeli scientists have published reports on animal studies of experimental vaccines engineered to over-express recombinant protective antigen, but no clinical data are available.
The problems that the manufacturer has had with meeting regulatory criteria have limited the U.S. supply. A small amount of anthrax vaccine has been made available to DHHS by DoD, but that amount is far below what will be needed. There is an urgent need for a sufficient supply of anthrax vaccine for vaccinating high risk populations and for use as post-exposure vaccination in conjunction with antibiotics.
There are several immediate issues that need to be addressed. The production method for current licensed vaccine must be scaled up. Several commercial firms have made informal proposals to do this. However, this is a high risk option because of the inherent variability of the manufacturing process and the high risk of failure when scaling up so rapidly to such a high volume. There needs to be more serious consideration of the applications of the various platform technologies—such as plasmid DNA, viral vectors, and a variety of other delivery technologies—that are being developed within the biotech industry.
Finally, we need to accelerate development of a second generation vaccine. The time to availability could be shortened by overlapping large scale production with clinical trials. It has been suggested that we might have a stockpile of IND recombinant protective antigen (PA) vaccine within 18 months. This may be an achievable goal if all involved interests work in an effective, coordinated manner. A recombinant PA vaccine produced in E. coli will likely be the first to enter a phase I trial.
In order to address this issue of a second generation vaccine, the National Institute of Allergy and Infectious Diseases has put together a team with contractor help. Efforts are underway to gather all available information on ongoing or planned development efforts for a second generation anthrax vaccine, and compile the information in a systematic fashion and convene several advisors to review the resulting data, findings, and policy options. This may involve a major research and development contract program similar to what exists for smallpox vaccine and which will hopefully build on the work that has been done by DoD and DoD-DHHS collaboration. It will hopefully involve some new players as well, including the large vaccine manufacturers. Although it is difficult to predict which particular options will receive aggressive support, there is nonetheless a system now in place that will hopefully pave the way for pursuing an effective strategy in a reasonable period of time. The speed at which a second generation anthrax vaccine is developed will depend on both the underlying science and the responsiveness of the vaccine industry to national needs.
THE DEPARTMENT OF DEFENSE AND THE DEVELOPMENT AND PROCUREMENT OF VACCINES AGAINST DANGEROUS PATHOGENS: A ROLE IN THE MILITARY AND CIVILIAN SECTOR? *
Introduction.
In October 2001, the threat of bioterrorism became a reality. In support of this Forum's efforts to identify the obstacles to preparing an optimal response to bioterrorism—particularly as it relates to the complexities of interaction between private industry, research and public health agencies, regulatory agencies, policymakers, academic researchers, and the public—this paper will highlight emerging opportunities for more effective collaboration as well as scientific and programmatic needs for responding to bioterrorism. The focus of this paper is on the potential opportunities and issues related to Department of Defense (DoD) support for the research, development, and production of biological defense vaccines for the military and civilian populations to protect against bioterrorist threats. This paper will address the following topics:
- Current medical biological defense research and development efforts;
- Current biological defense vaccine capabilities;
- Proposed national biological defense vaccine production facility; and,
- Issues related to the use of biological defense vaccines.
In accordance with Congressional direction, DoD established a Joint Service Chemical and Biological Defense Program in 1994. The vision of the program is to ensure U.S. military personnel are the best equipped and best prepared force in the world for operating in future battlespaces that may feature chemical or biological contamination. The capabilities being developed for the military may have applicability to protection of civilians, especially as the military mission may increasingly support homeland security. Vaccines to protect against biological agents provide one critical capability to protect against the threat.
Medical Biological Defense Research and Development Efforts
The primary research program for the development of biological defense vaccines to protect U.S. forces is the Medical Biological Defense Research Program (MBDRP). In developing countermeasures to biological agents, the MBDRP uses a technical approach that focuses on four areas:
- Identify mechanisms involved in disease process;
- Develop and evaluate products (vaccines or drugs) to prevent or counter effects of toxins, bacteria, viruses, and genetically engineered threats;
- Develop methods to measure effectiveness of countermeasures in animal models that predict human response; and,
- Develop diagnostic systems and reagents
Biological defense vaccines are being developed to counter viruses, toxins, bacteria, and genetically engineered biological threat agents. Research activities start with basic research activities and proceed through the following steps, as research demonstrates successful candidates: (1) construction of the infectious clone, (2) identification of attenuating mutations, (3) construction of vaccine candidates, (4) testing in rodent models, (5) testing in non-human primates, (6) final selection, and (7) formulation. The formulated production may then become a candidate for an Investigational New Drug (IND) application for transition to advanced development and clinical trials, then ultimately licensed production.
An example of a product being developed within the MBDRP is the Next Generation Anthrax Vaccine. In cooperation with the National Institutes of Health, the next generation vaccine will provide greater or equal protection, require fewer doses to produce immunity, and have fewer adverse effects than the current anthrax vaccine. The reduced number of doses would provide greater flexibility to military forces by reducing the time constraint for developing immunity, hence accelerating the time for fielding a protected force. The next generation vaccine is based on recombinant protective antigen (rPA), which binds to the lethal factor (LF) and edema factor (EF) of B. anthracis . The recombinant production technology would eliminate need for spore-forming anthrax, and hence the need for a dedicated production facility. Overall, the next generation anthrax vaccine would decrease production cost, allow a greater range of potential vaccine production facilities, and potentially allow for streamlining of the regulatory approval process.
Another example of a product being developed within the MBDRP is Multiagent Vaccines (MAV) for Biological Warfare (BW) Threat Agents. The MAV project is a proof-of-principle effort to construct a vaccine or vaccine delivery approach that could concurrently immunize an individual against a range of BW threats. Bioengineered and recombinant vaccine technologies will be exploited to achieve vaccines that are directed against multiple agents, yet use the same basic construct for all of the agents. The MAV would be analogous to commercial vaccines (e.g., measles-mumps-rubella) but would exploit new approaches—naked DNA vaccines and replicon vaccines. The MAV would result in a reduced number of doses and thus provide greater flexibility to military forces by reducing the time constraint for developing immunity, hence accelerating the time for fielding a protected force. The MAV also could decrease production cost, allow for greater range of potential vaccine production facilities, and potentially allow for streamlining of the regulatory approval process.
Current Biological Defense Vaccine Capabilities
Joint vaccine acquisition program (jvap).
In order to enable the transition of candidate biological defense vaccines developed under the MBDRP or from other sources, a Prime Systems Contract was awarded in November 1997 to DynPort Vaccine Production Corporation, LLC. The JVAP was established for the purpose of developing, testing, and Food and Drug Administration (FDA) licensure of vaccine candidates, and production and storage of vaccine stockpiles. A major objective of the program is to establish a viable industrial base for vaccine production. The next generation anthrax vaccine (rPA) is one of several vaccines being investigated for development by the JVAP. Other vaccines in advanced development include smallpox, pentavalent Botulinum Toxoid, and tularemia. The Prime Systems Contract also provides options for other biological defense vaccines. Currently, all vaccines in the JVAP are in the development phase.
Anthrax Vaccine Adsorbed (AVA) and the Anthrax Vaccine Immunization Program (AVIP)
The only vaccine currently licensed for use in the United States to protect against anthrax is AVA. AVA is cell-free filtrate, produced by an avirulent strain of Bacillus anthracis . It is manufactured by BioPort Corporation in Lansing, Michigan and procured under a separate contract. It was licensed by the FDA in 1970. Six doses of the vaccine are required for full immunity, including doses at 0, 2, and 4 weeks, 6, 12, and 18 months, followed by an annual booster.
On December 15, 1997, the Secretary of Defense approved the decision to vaccinate all of the U.S. armed forces against anthrax, contingent on the successful completion of four conditions, which were met: supplemental testing of the vaccine; tracking of immunizations; approved operational and communications plans; and review of health and medical aspects of the program by an independent expert. Implementation is determined in accordance with DoD Directive 6205.3, “DoD Immunization Program for Biological Warfare Defense,” November 26, 1993, with complete implementation of the plan contingent upon adequate supply of the licensed vaccine
On May 28, 1998, the Secretary of Defense directed vaccination of the total force. Implementation of this directive was administered by the AVIP. As of May 29, 2001, more than two million doses were administered to more than 500,000 military personnel, with at least 70,000 completing the full six-shot regimen. Since then, there has been only a few who have received vaccinations. As outlined in a June 8, 2001 memorandum, the Secretary of the Army ordered a slowdown in immunization to accommodate delays in release of vaccine pending FDA approval. Implementation of the vaccination continues to designated special mission units, to vaccine manufacturing and DoD personnel conducting anthrax research, and others conducting Congressionally mandated anthrax vaccine research. Detailed information on the status of the AVIP is available at www.anthrax.osd.mil .
What Does Producing a Vaccine Mean?
With no vaccines currently in production under the JVAP and AVA as the only currently available FDA licensed vaccine for protection against BW threats, DoD is evaluating other mechanisms to increase and sustain vaccine production. In order to identify the status of vaccines, it is important to understand the major phases of research, development, and production through which they must proceed. Within different phases of vaccine development and production, there will be varying levels of production risk and overall risk. There are three major phases in the development and production of new vaccines—science and technology base, development and licensure, and licensed production. Following is a summary comparing different activities within each phase.
Production Approach
Within the science and technology phase, production is focused on small quantities and relies on bench top methods, which may include many different approaches, including new state-of-the-art experimental approaches. When a candidate product transitions to the next phase, a best approach is selected (or in some cases two or three promising approaches) and tested for scale up for full scale production. Following licensure, production proceeds at full scale and relies on a single, fixed method. Changes in the method typically require further testing and require approval by the FDA.
Vaccine Recipients
Perhaps the most obvious difference among the phases are the numbers and types of vaccine recipients and the purposes for which they receive the vaccine. Within the science and technology phase, recipients are primarily laboratory animals and include hundreds of animals. The primary purpose for using these recipients is to demonstrate the potential effectiveness of a vaccine candidate, that is proof-of-principle testing. During the development and licensure phase, vaccine recipients are humans, who participate in clinical trials. All recipients are volunteers, who participate in clinical trials that comply with FDA regulations. The focus of these investigations is to determine the safety and efficacy of a vaccine as well as to optimize dosing and scheduling. The final phase is production and includes providing a licensed vaccine to all individuals who may be at risk, in accordance with the FDA license and based on quantities available, for the purpose of providing protection against potential threats. The effected populations could be on the order of millions of individuals.
Production Risk
Production risk during the science and technology phase is moderate since only small quantities can be produced yet only small quantities are needed. Risk is minimized since FDA approval of the product is not required. During the development and licensure phase, production risk is usually high because of the risks involved in scaling up pilot lot product to full scale production. Overall risk is also high because of reliance on and surrogate models or biomarkers to determine efficacy, since law prohibits exposure of humans to chemical or biological agents.
Overall Risk
Overall risk for production of biological defense vaccines will vary depending on the type of vaccine being produced and the policy implemented for immunization. For example, use of a live vaccine (e.g., vaccinia live vaccine) poses risk that inoculated individual may be giving off live vaccinia viruses until scarification has occurred (2–5 days), hence potentially exposing unprotected individuals. Another risk is that low rates of adverse effects may become more apparent in a large scale immunization program than had occurred during testing. For example, if 1,000 people are tested in clinical trials and only one had a serious adverse reaction, there may be hundreds of reactions if the total military force is vaccinated.
Biological Defense Vaccine Development and Production Issues
One of the major factors limiting the availability of biological defense vaccines is the limited interest from the pharmaceutical industry in supporting the production of these vaccines. In contrast to vaccines to support public health needs (e.g., childhood diseases, influenza), most vaccine needs are fulfilled by the private sector. However, the private sector has some challenges in fulfilling public health vaccine needs. The vaccine production industrial base is nearly at full capacity to meet public health priorities. This will pose a challenge for the production of biological defense vaccines if production of biological defense vaccines results in the deferral of production of public health vaccines. Biological defense vaccines are considered specialty biologics and interest is primarily centered on a few small to mid-sized companies. Industry interest is limited in part because of requirements to conduct large, complicated clinical studies to demonstrate safety, immunogenicity, and efficacy (where possible).
Another major factor effecting the timely availability of biological defense vaccines are issues related to compliance with Chapter 21 of the Code of Federal Regulations (21 CFR), Food and Drug Administration (FDA). The specific issue relates to the ability to determine the clinical efficacy of biological defense vaccines. 21 CFR requires that for efficacy to be established, vaccines must be tested in informed, volunteer human subject who are exposed to the condition against which the vaccine is intended to protect. However, legal and ethical constraints prohibit exposing human subjects to biological agents. This constraint plus limited availability of human data for most vaccines mean that under current regulations, biological defense vaccine efficacy cannot be established. In order to address this constraint, FDA published a proposed rule on October 5, 1999 entitled, “New Drug and Biological Products; Evidence Needed to Demonstrate Efficacy of New Drugs for Use Against Lethal or Permanently Disabling Toxic Substances When Efficacy Studies in Humans Ethically Cannot Be Conducted; Proposed Rule.” (FDA rules are available at http://www.fda.gov/cber/rules.htm .) The proposed rule is expected to be finalized during 2002. Under this rule, efficacy may be determined based on data from clinical testing on animals (using at least two different species with preference that non-human primates be one of the species.) Animal data would serve as a surrogate for human data, but there would need to be significant data demonstrating that the effects in animals is related to effects in humans. Without the ability to license vaccines based on surrogate test data, biological defense vaccines would remain as investigational new drugs, which would continue to limit availability.
Proposed National Biological Defense Vaccine Production Facility
Following years of research, development, and efforts to produce biological defense vaccines in sufficient quantities to meet DoD needs, a different approach is currently being planned. In July 2001, DoD submitted a report to Congress detailing biological defense vaccine efforts within DoD. Known as the “Top Report”—because it provides the results of an independent expert panel chaired by Franklin Top, M.D.—this report summarized key shortcomings of current biological defense vaccine acquisition efforts. The report made the following findings and recommendations:
- The scope and complexity of the DoD biological warfare defense requirements are too great for either the DoD or the pharmaceutical industry to accomplish alone,
- The panel recommended a combined integrated approach whereupon DoD would work closely with the vaccine industry and national scientific base, and
- The panel recommended the construction of a government-owned, contractor operated (GOCO) vaccine production facility, which would include production capacity for up to eight vaccines over the next 7–12 years and would cost an estimated $2.4–$3.2 billion over that time.
The report recognized that in order for the GOCO to be successful, it would require long-term government commitment, increased resources, innovative DoD business and program management practices, and effective participation by established pharmaceutical industry leaders in vaccine discovery, licensure, and manufacturing.
The design concept for a GOCO biological defense vaccine production facility would accommodate three bulk vaccine production suites, each with different processes: spore-forming bacteria (for which FDA requires separate facilities), microbial fermentation, and tissue culture (viral vaccines). A modular design would allow flexible and expandable manufacturing capacity for production of DoD-cntical vaccines that are intended for force health protection.
The scale of the facility will be determined in part by the quantity of vaccines to be produced. The assumptions for the production capacity requirement are categorized into three tiers. Tier 1 is the baseline requirement and reflects current production requirements, which is the same as current requirements for the JVAP and AVIP. This tier includes sufficient anthrax vaccine for the entire force (approximately 2.4 million doses). It additionally would require 300,000 Troop Equivalent Doses (TEDs) for other biological defense vaccines. (Troop equivalent dose is defined as the number of vaccine administrations to reach full immunity. Boosters are not included.) Tier 2 would require three million TEDs (2.4 million for U.S. forces + 0.6 million for Commanders Reserve) of each vaccine to be produced to allow for total force protection plus sufficient quantities to support annual requirements due to personnel turnover. This requirement was the basis for the initial GOCO cost estimate. Tier 3 would require approximately 300 million TEDs of each vaccine to support civilian protection for the entire U.S. population.
In order to define the requirements for vaccine production and to ensure that it addresses national, and not just DoD needs, an interagency advisory group has been established. Interagency participation has been led by DoD and the Department of Health and Human Services, with participation from several organizations (including the Office of Homeland Security) to ensure a broad perspective. Federal participation is essential since biological defense vaccine needs are not being met by private industry. No individual department has the sufficient, full-spectrum capability and capacity to support vaccine needs. A national vaccine authority may be essential to ensure interagency needs are addressed not only in the planning phase but also in implementation. The details of the national vaccine authority are being developed, though it is not likely to be established as a new agency.
Issues Related to the Use Of Biological Defense Vaccines
Why vaccinate vaccine use risk management decisions.
BW agents pose high risk to military forces and operations, and at least ten countries are pursuing offensive BW programs. Vaccines are the lowest risk, most effective form of protection against BW threats. Vaccines are more effective and have fewer adverse effects than antibiotics or other treatments following exposure. While masks may provide highly effective protection, they may impede performance and must be worn to provide protection. Vaccines enable force protection by providing continuous, long-lasting protection. In addition, there are currently no real-time BW detection systems available. While there are systems that provide the ability to detection respirable aerosols in near real-time, the best available systems today take 15–45 minutes to identify a specific BW agent.
Vaccines are unusual among medical products in that they are given to healthy people to keep them healthy. Table 3-1 shows several of the vaccines commonly given to protect against infectious diseases and contrasts them with the limited number of biological defense vaccines currently available. Biological agents that may be used as weapons may be naturally occurring but have a very low incidence of natural occurrence (at least in the United States.)
Selected infectious diseases vaccines and biological defense vaccines.
The risk assessment for using biological defense vaccines is different from naturally occurring infectious diseases ( Grabenstein and Wilson 1999 ). Because to vaccinate is based on potential risk of disease outbreak rather than actual incidences. Consequently, a proper risk assessment for biological defense vaccines should not be a trade-off assessment between the actual adverse effects of a vaccine vs. the actual adverse effects of the disease, but the actual adverse effects of a vaccine vs. the potential adverse effects of the disease.
The policies on the use of biological defense vaccines will affect biological defense vaccine manufacturing. The two basic options for immunization are stockpiling vaccines in anticipation of a specific contingency or routine use immunization to ensure continued general readiness. If vaccines are stockpiled, manufacturing must address issues related to maintaining the stockpile as a result of the limited shelf life of some vaccines. Additionally, if vaccines are produced in bulk, once the required quantities are produced, manufacturers must ensure that the facilities remain capable of retaining an FDA facility license when production is not ongoing.
The assessment of potential and actual effects may effect product development. For example, as polio has been significantly reduced as a result of extensive vaccination, the Centers for Disease Control have recommended use of the inactivated polio vaccine (IPV) rather than the oral polio vaccine (OPV). While OPV has greater efficacy, it is also linked with rare occurrences of vaccine-associated paralysis. As cases of polio have been virtually eliminated in the United States, the risk of rare occurrences of adverse effects of the vaccine has exceeded the risk of the occurrences and effects of the disease.
If biological defense vaccines are produced and planned for use—especially among civilians populations—vaccine development criteria may place greater emphasis on vaccine safety than on vaccine effectiveness. Risk assessments may be complicated by the fact that the limited industrial base capacity for biological defense vaccine production will likely result in only one vaccine being available for military and civilian use.
There are other key differences between the military and civilian populations that make risk assessment difficult. One factor is that biological defense vaccines made for the military population are intended for use only in healthy adults. By contrast, the general population will also include significant subgroups for which vaccine safety, efficacy, or dosing information may not be fully understood, including pediatrics, geriatrics, pregnant women, and immune-compromised individuals. Currently there is no policy in place to immunize the civilian population absent a naturally occurring threat. If a licensed biological defense vaccine were available for use by the general population, an immunization policy for civilian use would be needed to address several issues before immunization could begin. Some of the issues that would need to be addressed are, for example, who would be vaccinated—the entire population, or a subgroup? Which subgroup(s)? Those living in specific regions? First responders? If symptoms of biological agent do not appear, would that be interpreted as the absence of a threat or the effectiveness of the defense? Paradoxically, would the demand for the vaccine diminish as the apparent threat also diminished? Civilians may also have greater concerns about the long term safety effects as a result of vaccine use. Additionally, there may be concerns regarding the unknown safety of the use of biological defense vaccines when interacting with other medical products. While there is no adequate basis to assess safety, there is no basis for extraordinary concern ( Institute of Medicine, 1996 ).
Conclusions
The Department of Defense may bring valuable assets to bear to counter the use of biological agents by terrorists. Currently, the DoD mission is focused on responding to threats to the military. Because of DoD's experience in defending against biological threats, DoD will continue to play a role in addressing the threat to the civilian population as well. DoD will continue to work with other agencies, including the new Director of Homeland Security, to determine what role it will play in homeland security, which will be defined in The Federal Response Plan, presidential directives, and other sources.
The availability of vaccine to protect against anthrax and other biological agents is based on several factors. One key factor is sustained resources to transition products from the science and technology base to advanced development. Resources include not only adequate funding, but also trained personnel, which is a critical factor since the biotechnology and pharmaceutical industry as a whole is facing shortages of skilled personnel. A second factor limiting the availability of biological defense vaccines is that they are similar to orphan drugs. There is no commercial incentive for manufacturers to produce vaccines. Federal investment may be required to retain the services and capabilities of the biotechnology and pharmaceutical industry.
While the availability of vaccines is critical, the decisions of whether to vaccinate will remain equally important. Vaccination decisions will continue to have greater physiological consequences than non-medical measures to protection against the threat (e.g., whether to wear masks). The decision will need to weigh the risk of actual low rates of adverse effects against the potential for protecting against catastrophic effects. In making these decisions based on risk, communicating the risk decision will be at least as important as risk assessment. Failure to have a coordinated public policy decision on vaccination support for civilians may result in individuals self-prescribing treatments or failing to comply with recommended guidelines.
APPLICATIONS OF MODERN TECHNOLOGY TO EMERGING INFECTIONS AND DISEASE DEVELOPMENT: A CASE STUDY OF EBOLA VIRUS *
In recent years, increasing attention has been focused on the Ebola virus as a potential public health problem, either from natural or deliberate outbreaks. Like the genetically related Marburg virus, Ebola is a filovirus that causes highly lethal hemorrhagic fever in humans and primates. Infection rapidly progresses from flu-like symptoms to hemorrhage, fever, hypotensive shock, and eventually, in about 50–90% of cases, death ( Peters et al., 1996 ; Peters and Khan, 1999 ). The molecular mechanisms underlying the pathogenicity of the Ebola virus are not well understood, in part because it has emerged only relatively recently (for reviews see Balter, 2000 ; Colebunders and Borchert, 2000 ). There was a series of outbreaks in central Africa in the mid-1970s and again in the 1990s (i.e., the Ivory Coast in 1994, Gabon in 1994–1996, Zaire in 1995, Gulu, Uganda in 2000 and presently in Gabon and the Republic of Congo). Ebola virus infection has appeared once in the United States, in Reston, Virginia. The Reston strain is not pathogenic in humans, and the outbreak was fortunately restricted to non-human primates.
One of the reasons that Ebola is highly lethal is that this virus replicates at an overwhelming rate ( Sanchez et al., 1996a ). Thousands of Ebola virus particles per host cell can completely envelop the cell and take over its entire protein synthetic machinery. We have only recently begun to understand the molecular mechanisms underlying this phenomenon. Although we have a descriptive understanding of the cytopathic effects of viral replication, we lack a clear understanding of how these various changes in cell structure and viability occur. Elucidating these details will be critical for developing vaccines and other antiviral therapies.
Aside from the obvious immediate health threat that would be posed if it were introduced into the population, Ebola virus represents a useful paradigm for dissecting the molecular genetics of a virus. Most of what is known about Ebola pathogenesis is derived from genetic studies of the virus. Although Ebola is very similar to the genetically related Marburg virus, it differs in at least one important respect. The gene that encodes the viral glycoprotein in Ebola generates two gene products, whereas in Marburg, this gene encodes a single protein (Sanchez et al., 1996). One of the gene products is secreted as a soluble 50 to 70 kDa glycoprotein, whereas the other is a full-length 120 to 150 kDa glycoprotein that inserts into the viral membrane ( Volchkov et al., 1995 ; Sanchez et al., 1996). The secreted form was originally believed to serve as an immunological decoy for the full-length glycoprotein, allowing the full-length glycoprotein to attach to the target cell. However, more recent evidence now suggests that this hypothesis is unlikely. Instead, the secreted form appears to inhibit early steps in neutrophil activation and thereby inhibit the host inflammatory response to the virus ( Yang et al., 1998 ). The secreted glycoproteins have been shown to bind quite well to neutrophils, but bind poorly to endothelial cells ( Yang et al., 1998 ). In contrast, the full-length glycoprotein interacts with endothelial cells but binds poorly to neutrophils ( Yang et al., 1998 ). This glycoprotein enables the Ebola virus to recognize and introduce its viral contents into the endothelial cell lining of the blood vessels, as well as monocytes/macrophages, thereby resulting in the cellular damage that is associated with the devastating symptoms of Ebola infection.
Antiviral Targets
Detailed analyses of the mechanisms of viral entry, replication, and cell damage have identified the Ebola glycoprotein 2 (GP2) as a potential antiviral target. In particular, there is a region in the GP2 ectodomain of Ebola virus that forms a coiled coil, or hairpin-like structure similar to what exists in the human immunodeficiency virus (HIV), influenza, respiratory syncytial virus, and a variety of other viruses ( Weissenhorn et al., 1998a , 1998b ; Malashkevich et al., 1999 ). This coiled-coil region contributes to membrane fusion by undergoing conformational changes after the glycoprotein binds to the membrane receptor ( Weissenhorn et al., 1998b ; Watanabe et al., 2000 ). The fact that this structure is conserved in a number of different viruses suggests that it may represent a potential target for antiviral therapy. In fact, a peptide product directed at the analogous structure in HIV has potent antiviral effects and is currently being developed for the clinical treatment of AIDS. This or similar peptides could be useful against many other viruses as well, including Ebola.
Not only does the transmembrane glycoprotein direct the Ebola virus into specific cells, but the glycoprotein itself is also highly toxic to cells. For example, when full-length Ebola glycoprotein is overexpressed in cultured renal epithelial cells, it inserts into the membrane and causes morphological changes and detachment from culture dishes ( Yang et al., 2000 ). This finding suggests that there is a genetic determinant in the glycoprotein that mediates its toxicity and, therefore, might represent another potential target for antiviral therapies. Mapping studies identified a serine-threonine-rich, mucin-like core domain region of the glycoprotein that is required for cytotoxicity in human endothelial cells ( Yang et al., 2000 ). When the mucin-like region of the glycoprotein was deleted, its cytotoxicity was abolished, but protein expression and function remained unchanged ( Yang et al., 2000 ). Every possible open reading frame in the Ebola virus genome has been tested for toxicity, except for the polymerase region. To date, only the glycoprotein has been shown to induce toxic cytopathic changes. However, a better understanding of the detailed molecular mechanism of virus assembly may eventually provide insight into other potential antiviral targets as well.
Vaccine Development
Not only does the glycoprotein play an important role in toxicity, increasing evidence suggests that it also plays an important role in the pathogenesis of Ebola infection. Infection of cultured cells with adenoviral vectors encoding the glycoprotein causes considerable cellular damage that correlates with toxicity. However, overexpression of a glycoprotein that is unable to insert into the cell membrane is not cytotoxic. In fact, injecting adenoviral vectors, or DNA forms of these vectors, into mice, rabbits, and primates actually protects the animals from disease by inducing an effective vaccine response. No human vaccine against Ebola is currently available. However, studies in animals suggest that DNA vaccines, together with replication-defective adenoviral vectors, may be particularly promising. In the DNA vaccination platform, a plasmid expression vector is injected into muscle, thereby enabling muscle to synthesize large quantities of proteins that stimulate the immune system to generate an effective immune response. DNA vaccination technology could greatly simplify the vaccination production process that would otherwise rely on very large-scale plants for making these complex and highly purified proteins. However, although current DNA vaccines work well in rodents, they are not as effective in non-human primates and are even less robust in humans. Thus, one of the important challenges for developing an effective DNA vaccination platform technology is to improve immune responses in non-human primates and humans.
The first successful studies of a DNA vaccine for Ebola virus were carried out in guinea pigs (Xu et al., 1999). Animals that were immunized with sufficient levels of Ebola virus glycoprotein to induce a high-titer antibody response survived infection. Guinea pigs with intermediate levels of titers exhibited an intermediate chance of survival. In contrast, none of the control animals, immunized with vector alone, survived Ebola infection (Xu et al., 1999). “Prime-boost” strategies combine DNA immunization and boosting with adenoviral vectors that encode viral proteins to specifically target dendritic cells. Such DNA vector-viral vector combinations can be very potent. Animals are first immunized with a DNA vector, and typically develop titers ranging from 1:1,500 to about 1:3,500. Following the adenovirus boost, antibody titers increase dramatically, ranging from 1:50,000 to 1:100,000. This far exceeds the minimum threshold that is considered to be necessary for an effective immune response in primates. A modified prime-boost strategy was recently used to immunize cynomolgus macaques against several strains of the Ebola glycoprotein ( Sullivan et al., 2000 ). Several months later, animals were boosted with recombinant adenovirus expressing the Ebola (Zaire) glycoprotein. Control animals received empty vectors consisting of plasmid DNA and ADV-DE1 recombinant adenovirus in a parallel injection regimen. When animals were subsequently challenged with a lethal dose of the Zaire subtype of Ebola virus, all control animals (6 out of 6) exhibited rapid increases in their viral antigen levels and succumbed to infection within seven days. In striking contrast, all animals immunized with the combination DNA-adenovirus vaccine survived Ebola virus challenge (4 out of 4). The level of antibody production and the cellular proliferative response were closely correlated with immunoprotection.
It is of interest to note that vaccines are not only clinically useful, but they can also serve an important function as deterrents against bioweapons. It is much more difficult to engineer vaccine resistance than drug resistance in an organism. Having well-defined, publicly known, and effective vaccines is a critical preventive, or deterrent, strategy. Another benefit of a successful vaccine is that it opens the way for developing novel immunotherapies. In the case of Ebola virus, for example, hyperimmune serum from animals that are protected from the disease is currently being examined, to determine if it can be used during the course of infection as a possible post-exposure therapy.
Role of Genomics in Vaccine Development and Biodefense
Genomic approaches hold enormous potential for vaccine development, and these possibilities are only just beginning to be explored. For example:
- Analysis of global gene expression patterns can facilitate the early identification of both environmental and disease-associated pathogens.
- Gene expression patterns can be used to identify specific genetic susceptibility and resistance markers.
- Biomarkers for vaccine efficacy could be incorporated into the experimental design of efficacy trials, which could then accelerate approval and licensure processes.
- High throughput technology can be used to improve vaccine design, by allowing researchers to readily monitor how specific structural changes in the vaccine affect the cellular response to immunization.
It is possible that enough information will eventually be available and implemented within the technology that simply knowing the sequence of a particular open reading frame will be sufficient to understand how to generate an effective vaccine. Such technology would be useful not only as a defense measure against bioterroism, but also for the prevention or treatment of naturally occurring outbreaks, such as influenza. The influenza virus constantly mutates, but if genetic information could be acquired quickly enough, it may become possible to develop more effective countermeasures.
In conclusion, the process of vaccine development must evolve to become more responsive to the changing needs and emerging outbreaks of society today. In other words, more agile vaccines are needed. Agility includes the ability to rapidly deploy vaccines in the event of an outbreak; to accelerate immunization regimens so that such an outbreak could be effectively managed; and, finally, new technology must be applied to develop better vaccines and to accelerate the development process.
MEETING THE REGULATORY AND PRODUCT DEVELOPMENT CHALLENGES FOR VACCINES AND OTHER BIOLOGICS TO ADDRESS TERRORISM *
The FDA plays an important role in multiple stages of the product development process, from initial clinical studies through licensure, manufacturing and post-marketing studies which may be used to further evaluate safety and effectiveness. For these reasons, FDA is committed to working together with the scientific and clinical communities and with industry and the public to fulfill its regulatory and public health role in facilitating the development of biodefense biologics and therapeutics. Recent and ongoing FDA biodefense-related activities include, for example, meeting with sponsors and sister agencies and departments to encourage interest in developing safe and effective new products needed for public health biodefense, performing research that ultimately facilitates the development of these products; and providing intensive and early interactions with product sponsors to speed their availability.
As with any medical product, bioterrorism products need to be regulated to ensure consistent and objective protection of the public safety. While there is currently a sense of emergency and a set of urgent needs to address, the desire for rapid and innovative responses must not be allowed to compromise the objective assessment of safety and effectiveness. Thus we need a regulatory agency that can step back and provide a more objective perspective. If and when things go wrong in the wake of decision(s) made in a time of crisis, few people will remember the crisis and that the decision was in fact made with the best intentions. The public expects safe and effective products, and safety expectations are especially high for vaccines administered to healthy individuals. Maintaining public confidence in vaccines and medical products, in general, is critical to maintaining overall confidence in our nation's public health programs and leadership in matters extending far beyond bioterrorism. For these reasons, even in difficult times, we must continue to make and communicate clearly the best possible scientific and public health decisions about product development, licensure, availability and use.
Furthermore, bioterrorism is a moving target, not a single disease of predictable epidemiology, and all potential product uses may not be anticipated. This complicates many decisions about product use. For example, a vaccine, such as the licensed anthrax vaccine, which may have been originally studied and used in a limited population effectively and without major safety concerns may raise more significant public concerns about uncommon adverse events, whether coincidental or due to the vaccine, if and when it is administered for similar reasons to hundreds of thousands of people or when unanticipatedly used for post-exposure prophylaxis.
There are several factors that account for why we do not have an adequate supply of vaccines for bioterrorism defense:
Financial Disincentives
- Uncertain markets, especially for potentially more limited use products such as a tularemia or plague vaccine.
- Uncertain longevity of the needs, markets and of resources; short attention spans in government budgeting.
- The fact that vaccines are complex biological products that carry a high risk of uncertainty, unpredictability of success, and financial loss.
- The rigorous safety requirements and low public tolerance of risk—in part because they are often administered to healthy people as a preventative measure—and associated costs of developing biologics.
- The fact that preventive measures are generally undervalued, both perceptually and financially. Vaccines are often expected to be sold for very low prices, and the expected profit for the producer is therefore lower than for other products (e.g., drugs for treatment) competing for the same resources. However, while difficult to model when risks are unclear, it would be interesting to conduct more comprehensive and long-term cost-benefit analyses concerning the personal health impacts and the social and economic costs versus potential benefits of vaccine compared to treatment strategies for specific agents of interest.
- The added cost of the large clinical trials needed to address potential wide use including in diverse populations.
- The presence of advocacy groups with various points of view.
- A fair amount of concern about possible adverse effects of vaccines, ranging from specific disease issues to more general anti-vaccine sentiment on the part of a proportion of the public.
- A mistrust of government and industry
- Product liability issues.
Scientific Challenges
- Lack of historical or recent precedents for vaccines against many pathogens, which makes it difficult to establish good surrogates.
- The potential for genetic or other manipulation of antigenic determinants. (Although this is presently more difficult in many cases to engineer than antibacterial resistance.)
- The potential complications of live vaccine administration to increasing immunocompromised populations.
- The intense flow of resources demanded by urgent perceived needs (sometimes referred to as the “disease du jour” phenomenon), in contrast to the more normal lengthy product development cycle.
The FDA Response
There are several regulatory approaches and mechanisms that the FDA has employed in an attempt to safely speed up product availability and licensure:
- Early and frequent consultation between the sponsor producing the product, the potential end users (e.g., health officials and providers in the military and civilian sectors), and the FDA is very resource-intensive but important. This kind of up-front investment can greatly improve the product development process by identifying creative study designs, recognizing factors that are normally not anticipated in developing a product, and reducing misunderstandings and the likelihood of unwelcome surprises. Early dialogue also increases accountability.
- Emergency use under IND (investigational new drug status) allows rapid access to products that have not yet completed requirements for licensure. INDs require acceptable evidence of safety; a reasonable though not necessarily formally proven scientific basis for efficacy; a favorable nsk:benefit ratio; and an intent to license. While allowing availability of potentially lifesavmg products, a disadvantage to emergency use under this rule is that the product is not licensed, which not only reflects the true scientific limitations of the data but also raises important issues about public perception.
- Fast track processes can speed up the review process for products that will provide meaningful therapeutic benefits compared to existing therapies for serious or life-threatening illnesses. Fast track allows the FDA to review information as it becomes available and as the sponsor submits it.
- Accelerated approval through the use of surrogate end points to demonstrate benefit. The use of CD4 cells for assessment of antiviral treatment of HIV was one of the first surrogates to be approved under this rule. For bioterrorist agents, protective antibody levels for a vaccine or immunoglobulin could serve as potential surrogate end points. Clinical end points can also be utilized. There still must be good post-licensure studies to demonstrate the effects on disease outcomes and to collect additional safety information, and the FDA can place restrictions on use and promotion and even withdraw the product if agreements are violated or the product proves unsafe or ineffective. Thus far, this process has worked fairly well although, once a product is licensed, or if a disease is rare, it may be difficult to obtain patients for studies, and sponsors sometimes are unable or unmotivated to fulfill their commitments. But because most accelerated approval products also receive priority review, this process can allow for rapid approval of a product based on more limited and simpler-to-obtain clinical data than may be the case with large, randomized control trials and/or longer-term endpoints.
- Priority review is applied when a product is considered a significant advance or will be used for serious or life-threatening illness.
- Approval under the forthcoming “Animal Rule” has very important biodefense implications. In fact, the rule is specifically oriented to drugs or biologics that reduce or prevent serious or life threatening conditions caused by exposure to lethal or disabling toxic, chemical, biologic, or nuclear threats. The products should be expected to provide a meaningful therapeutic benefit over existing treatments. Human efficacy trials should either be not feasible or unethical, and the use of the animal efficacy data should be scientifically appropriate. In this proposed rule, the end point should be related to the desired benefit in humans, usually a significant outcome such as mortality or major morbidity. Clinical studies in representative populations are still needed, however, both for establishing pharmacokinetics (including, in the case of many vaccines, immunogenicity) and for assessing safety. Such studies are critical because civilian populations often include vulnerable or pharmacokinetically variable subsets. Finally, similar to the fast track and accelerated approvals, the animal rule has post-marketing and labeling commitments and restrictions. It does not apply if the product could be approved based on any other standard in FDA's regulation. It is a rule of last resort, but it certainly would be applicable to many of the situations that have been described in this workshop.
In addition to its regulatory responsibilities, the FDA's Center for Biologics conducts a significant amount of biodefense-related research, supporting approximately sixty ongoing projects that are directly relevant to identified high threat agents. The general goal is to meet otherwise unmet research needs, often with regulatory implications. Examples include how to better determine potency; defining immune and other correlates of protection; how to make safer and purer products (including characterization of the safety of cell substrates and detection of adventitious agents); better assessment of adverse events and efficacy under conditions of use, and studies which allow the agency to make regulations more scientific and less “defensive.” These types of research can benefit not only the public, but also multiple companies across industry, but are often not performed by a given sponsor as they may not provide a direct and/or immediate benefit. Furthermore, through its research and related scientific interactions, the center maintains the type of cutting edge expertise that is increasingly needed for dealing intelligently and proactively with evolving products and their underlying biotechnology. This expertise and confidence fosters the science-based objectivity necessary for anticipating and/or reacting appropriately to the issues raised during the development of a product which, ultimately, accelerates the regulatory and licensure process.
By maintaining its scientific, objective regulatory stance, the FDA can increase confidence in the likely efficacy of products primarily approved based on surrogate/animal data and reduce the likelihood of serious adverse events. The FDA brings several other unique attributes to the product development process as well, including:
- Knowledge of scientific and industrial capabilities, which is very helpful when it is necessary to identify people with specific expertise. This includes knowledge of emerging technologies which are cross-cutting among diverse products that nobody else may have the opportunity to see; knowledge of manufacturing capabilities; and knowledge of potential new uses of both licensed and investigational products, for example anti-sepsis and immune modulators.
- Day-to-day participation in what it takes to develop a product, including clinical trials, quality assurance, adverse event monitoring, timelines, etc.
- A unique ability to match product needs to industrial and academic capabilities. Much of this is informal, but it can be very helpful in getting the job done well.
However, there are several things that the FDA cannot do. FDA cannot
- provide monetary or tax incentives;
- assure that anyone will make a product;
- sponsor or directly assume the burden of product development, since this would be a conflict of interest;
- provide indemnification or compensation for injuries;
Furthermore, while the prelicensure process can provide reasonable assurances about the degree of safety and effectiveness, FDA cannot
- guarantee absolute safety;
- guarantee human efficacy under field conditions based on non-human data such as animal studies or surrogate endpoints (or, for that matter, based on efficacy observed in the controlled setting of a clinical trial).
In addition to expedited regulatory pathways, as well as orphan drug status, there are several potential incentives—both push and pull—which are outside the mission of FDA but that could be evaluated with respect to their potential to stimulate product development. Push incentives, which could be considered where markets are small or uncertain, could include:
- direct financial awards or contracts;
- tax credits;
- enhanced exclusivity;
- partnerships in product development; and
- research and development assistance to reduce the financial sting and risk of product development.
Possible pull incentives, which are probably more valuable, include:
- known markets;
- longer term financial contracts;
- defining prices that more accurately reflect known and potential public health benefit, which will require more economic discussion and modeling; and
- where possible, developing dual or multiple use products/concepts which can used not just for meeting bioterrorism needs but also for enhancing general public health and medical care.
In summary, FDA and CBER are highly committed to working with multiple partners in and outside of government to help in meeting the challenges posed by bioterrorism. Especially in times of threat and crisis, there is a need for a responsive, yet independent and science-based regulatory process. Relevant research and expertise remains critical in meeting the challenge. Existing laws and regulations can help facilitate product development in a timely manner. There are significant financial disincentives which have and may continue to impede the industrial development of some needed products where markets may be small or uncertain. Careful and open communication with the public about what is and is not known about proposed bioterrorism responses using new and existing products is critical not only in responding to specific threats and protecting the public but also in maintaining confidence in and support for the public health system as a whole.
- MOVING THE VACCINE AGENDA FORWARD: OBSTACLES AND OPPORTUNITIES
The vaccine industry is highly concentrated with only four major manufacturers providing more than three quarters of the market. Even within these four mam manufacturers production capacity may be insufficient for unseen circumstances or urgent need, as has been demonstrated by shortages of DT Acellular pertussis vaccine and pneumococcal conjugate vaccine. Following September 11th, all four major manufacturers expressed interest in biodefense/military vaccines. But how long will patriotism sustain this interest? The industry is both high risk (e.g., the rotavirus vaccine which had to be withdrawn from use) and risk adverse (e.g., certain vaccines for pregnant women have yet to be developed). There are several factors that impede vaccine product development:
- The vaccine industry is market-driven, and the major manufacturers are simply not interested if the market is insufficient.
- The industry is highly regulated, and perceived regulatory hurdles can impede product development.
- Intellectual property conflicts can prevent companies from developing products.
- Negative marketing assessment impedes vaccine production. Marketing departments often make unchecked predictions.
- An uncertain or dubious proof of concept creates reluctance to develop a particular new product.
- Biohazard to personnel, especially with regards to biodefense vaccines, can create reluctance.
The production of an adequate biodefense vaccine supply will depend on many factors:
- Development of biodefense vaccines must be in collaboration with DoD, NIH, CDC, and other agencies with national interests. With regard to the development of a new smallpox vaccine, for example, efficacy is an important issue that requires the use of monkey models for which the vaccine industry must turn to DoD or NIH.
- Development of biodefense vaccines requires better adverse reaction surveillance. With the military anthrax vaccine, for example, initially there were no data to support the claim that the vaccine was indeed safe. New vaccines must have built-in surveillance in order to discredit unsubstantiated claims about adverse reactions based on anecdotal data.
- New vaccine production requires that liability indemnification be guaranteed. One possible solution would be to place these vaccines on a list of compensable vaccines. We need to create a no-fault system which indemnifies companies against non-negligent harm but also provides some relief for injured individuals.
- Access to new technology would likely stimulate more interest in vaccine development. For example, a number of companies have pursued DNA vaccines because they are an interesting new technology which could likely be applied to a number of different targets. After all, the mam role of the vaccine industry is not to conduct basic research but apply basic research to the development of new products.
- Finally, vaccine production priorities need to be set and somebody authorized to say “this is the top priority.” For example, Gary Nabel has presented some very elegant work on an Ebola vaccine. But what does intelligence say is the real risk of Ebola? Should a virus that cannot be spread as an aerosol be considered a priority?
In summary, the major vaccine manufacturers will not be able to provide all of the needed biodefense vaccines. However, they should be asked to play a significant role. That role will be facilitated by clear directions, clear priority setting, and a tight collaboration between industry and government in order to move animal and clinical testing forward.
A. Johnson-Winegar
- Grabenstein JD, Wilson JP. Are Vaccines Safe? Risk Communication Applied to Vaccination. Hospital Pharmacy. 1999; 34 (6):713–729.
- Institute of Medicine. Interaction of Drugs, Biologics, and Chemicals in U.S. Military Forces. Washington, D.C.: National Academy Press; 1996. [ PubMed : 25121292 ]
- Use of Anthrax Vaccine in the United States—Recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2000 December 15; 49 (RR15):1–20. [ PubMed : 11145529 ]
- Balter M. Science. 2000; 290 :923–925. [ PubMed : 11184732 ]
- Colebunders R, Borchert M. Journal of Infections. 2000; 40 :16–20. [ PubMed : 10762106 ]
- Malashkevich VN, Schneider BJ, McNally ML, Milhollen MA, Pang JX, Kim PS. Proceeding of the National Academy of Sciences, U S A. 1999; 96 :2662–2667. [ PMC free article : PMC15825 ] [ PubMed : 10077567 ]
- Peters CJ, Sanchez A, Rollin PE, Ksiazek TG, Murphy FA. Fields Virology. Fields BN, Knipe DM, Howley PM, editors. Lippincott-Raven; Philadelphia: 1996. pp. 1161–1176.
- Peters CJ, Khan AS. Current Topics in Microbiology and Immunology. Klenk HD, editor. Vol. 235. Springer-Verlag; Berlin: 1999. pp. 85–95. [ PubMed : 9893380 ]
- Sanchez A, Khan AS, Zaki SR, Nabel GJ, Ksiazek TG, Peters CJ. Fields Virology. Fields BN, Knipe DM, Howley PM, editors. Lippincott-Raven; Philadelphia: 1996. pp. 1279–1304.
- Sanchez A, Trappier SG, Mahy BWJ, Peters CJ, Nichol ST. Proceedings of the National Academy of Sciences, USA. 1996; 93 :3602–3607. [ PMC free article : PMC39657 ] [ PubMed : 8622982 ]
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Nature. 2000; 408 :605–609. [ PubMed : 11117750 ]
- Volchkov VE, Becker S, Volchkova VA, Ternovoj VA, Kotov AN, Netesov SV, Klenk HD. Virology. 1995; 214 :421–430. [ PubMed : 8553543 ]
- Watanabe S, Takada A, Watanabe T, Ito H, Kida H, Kawaoka Y. Journal of Virology. 2000; 74 :10194–10201. [ PMC free article : PMC102058 ] [ PubMed : 11024148 ]
- Weissenhorn W, Calder LJ, Wharton SA, Skehel JJ, Wiley DC. Proceedings of the National Academy of Sciences, USA. 1998; 95 :6032–6036. [ PMC free article : PMC27580 ] [ PubMed : 9600912 ]
- Weissenhorn W, Carfi A, Lee KH, Skehel JJ, Wiley DC. Molecular Cell. 1998; 5 :605–616. [ PubMed : 9844633 ]
- Xu L, Sanchez A, Yang Z, Zaki SR, Nabel EG, Nichol ST, Nabel GJ. Nature Medicine. 1998; 4 :37–42. [ PubMed : 9427604 ]
- Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. Science. 1998; 279 :1034–1037. [ PubMed : 9461435 ]
- Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Nature Medicine. 2000; 6 :886–889. [ PubMed : 10932225 ]
This statement reflects the professional view of the author and should not be construed as an official position of the Department of Health and Human Services.
The information provided in this paper reflects the professional view of the author and not an official position of the U.S. Department of Defense.
This statement reflects the professional view of the author and should not be construed as an official position of the National Institute for Allergy and Infectious Diseases, National Institutes of Health.
This statement reflects the professional view of the author and should not be construed as an official position of the Food and Drug Administration.
- Cite this Page Institute of Medicine (US) Forum on Emerging Infections; Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing The Science and Response Capabilities: Workshop Summary. Washington (DC): National Academies Press (US); 2002. 3, Vaccines: Research, Development, Production, and Procurement Issues.
- PDF version of this title (5.5M)
In this Page
- VACCINES FOR THREATENING AGENTS: ENSURING THE AVAILABILITY OF COUNTERMEASURES FOR BIOTERRORISM
- THE DEPARTMENT OF DEFENSE AND THE DEVELOPMENT AND PROCUREMENT OF VACCINES AGAINST DANGEROUS PATHOGENS: A ROLE IN THE MILITARY AND CIVILIAN SECTOR?
- APPLICATIONS OF MODERN TECHNOLOGY TO EMERGING INFECTIONS AND DISEASE DEVELOPMENT: A CASE STUDY OF EBOLA VIRUS
- MEETING THE REGULATORY AND PRODUCT DEVELOPMENT CHALLENGES FOR VACCINES AND OTHER BIOLOGICS TO ADDRESS TERRORISM
Other titles in this collection
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Vaccines: Research, Development, Production, and Procurement Issues - Biological... Vaccines: Research, Development, Production, and Procurement Issues - Biological Threats and Terrorism
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Vaccines articles from across Nature Portfolio
Vaccines are a clinical product that is composed of live or dead material from an infectious agent – bacterium, virus, fungus or parasite – that elicit protective immunity against the pathogen when administered. These substances are used to prevent the spread of infectious diseases.
Peptide vaccines get an OS update
Peptide vaccines use antigenic peptide fragments to induce an immune response but are problematic because of the short half-life of peptides. A study now reports thioamide substitution in the peptide backbone as a strategy to enhance resistance to proteolysis and promote binding to the MHC I complex for T cell activation.
- Martin Zacharias
- Sebastian Springer
Related Subjects
- Cell vaccines
- Conjugate vaccines
- DNA vaccines
- Inactivated vaccines
- Live attenuated vaccines
- Peptide vaccines
- Protein vaccines
- RNA vaccines
Latest Research and Reviews
A comprehensive synthetic library of poly- N -acetyl glucosamines enabled vaccine against lethal challenges of Staphylococcus aureus
Poly-β-(1–6)- N -acetylglucosamine (PNAG) is an important vaccine target, but the impact of the number and position of free amine vs N -acetylation on its antigenicity is not well understood. Here, the authors report a divergent strategy to synthesize a comprehensive library of PNAG pentasaccharides, enabling the identification of enhanced epitopes for vaccines against Staphylococcus aureus including drug resistant strains.
- Weizhun Yang
- Xuefei Huang
Multi-omics analysis reveals COVID-19 vaccine induced attenuation of inflammatory responses during breakthrough disease
Here, Drury et al study gene, microRNA and protein expression during COVID-19, in a randomised controlled trial of ChAdOx1 nCoV19 vaccine and find that ChAdOx1 nCoV-19 attenuates the inflammatory response, thought to be the basis for severe COVID-19.
- Ruth E. Drury
- Susana Camara
- Daniel O’Connor
An ancestral SARS-CoV-2 vaccine induces anti-Omicron variants antibodies by hypermutation
Repeat vaccination with COVID-19 mRNA vaccines has been shown to increase breadth of the antibody response. Here the authors demonstrate that B cell clones induced by the ancestral COVID-19 vaccine develop into daughter clones with different reactivity to individual SARS-CoV-2 variants through the accumulation of somatic hypermutations.
- Seoryeong Park
- Jaewon Choi
- Junho Chung
Strep A: challenges, opportunities, vaccine-based solutions, and economics
- David E. Bloom
- Jonathan Carapetis
SARS-CoV-2 booster vaccine dose significantly extends humoral immune response half-life beyond the primary series
- Chapin S. Korosec
- David W. Dick
- James Watmough
Diet switch pre-vaccination improves immune response and metabolic status in formerly obese mice
Diet switching from high-fat to standard diet before influenza vaccination affects the metabolic state of T cells, restores their responses and improves vaccine efficacy in mice.
- Rebekah Honce
- Ana Vazquez-Pagan
- Stacey Schultz-Cherry
News and Comment

Harnessing our lived experience for science communication
Adrian Liston, professor of pathology at the University of Cambridge, UK, has published several illustrated children’s books on the topic of vaccination and has developed a computer game called ‘VirusFighter’. Here, he shares his thoughts on how to become an effective science communicator.
- Adrian Liston
The role of correlates of protection in overcoming barriers to vaccine development and demonstrating efficacy
- Deborah F. King
- Helen Groves
- Charlotte Weller
Facilitating broad antibody responses
- Laurie A. Dempsey

Going the extra mile to increase vaccine uptake
Deployment of mobile vaccination teams to remote communities in Sierra Leone substantially increased COVID-19 vaccine uptake, and could potentially be bundled with other health interventions.
- Karen O’Leary
The power of memory T cells minus antibodies
T cell- and antibody-based immunological protection are generally considered to function together, but data now show how T cells conferred by previous SARS-CoV-2 infection or two-dose vaccination can elicit heterologous protection in mice against subsequent SARS-CoV-2 infection, even in the absence of antibodies.
- Thi H. O. Nguyen
- Katherine Kedzierska
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

COMMENTS
A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with more than a 99 ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
The issue of a third vaccine dose in these non-responsive patients is an intriguing one that will be usefully explored in further research. In this context, a large cohort has been opened in France (COV-POPART) to evaluate the immune response in a number of particular populations including patients with cancer, transplant recipients as well as ...
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Metrics. Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine ...
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
The first COVID-19 vaccine outside a clinical trial setting was administered on Dec 8, 2020. To ensure global vaccine equity, vaccine targets were set by the COVID-19 Vaccines Global Access (COVAX) Facility and WHO. However, due to vaccine shortfalls, these targets were not achieved by the end of 2021.
We identified eight phase-3 RCTs that reported primary or preliminary CODIV-19 vaccine efficacy, with contributory data from nine publications 6,7,8,9,10,11,24,25,39.. The search and selection ...
Vaccine effectiveness for different clinical outcomes of COVID-19. We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
2. Vaccination strategies. Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 (Dhama et al., 2020).As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the ...
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
After 5 months, vaccine effectiveness remained high among individuals younger than 55 years. Booster doses restore vaccine effectiveness. Adverse reactions after booster doses were similar to those after the second dose. Homologous booster schedules had fewer reported systemic side-effects than heterologous boosters.
Tetanus vaccines: WHO position paper, February 2017 — recommendations. Vaccine 36, 3573-3575 ... (SAGE) and a National Institute for Health Research (NIHR) Senior Investigator. The views ...
A comprehensive research paper titled "Tracking Anti Vaccination Sentiment in Eastern European Social Media Networks" by UNICEF in 2013 found that vaccine influencers (people or pages that speak publicly about vaccines, both positively and negatively) are most prominent on Facebook and Twitter. This study found that Twitter users are ...
Vaccines alone, the researchers find, accounted for 40 percent of the decline in infant mortality. The paper — authored by a team of researchers led by WHO epidemiologist and vaccine expert Naor ...
WASHINGTON — A new report from the National Academies of Sciences, Engineering, and Medicine reviews evidence for 19 potential harms of the COVID-19 vaccines, and for nine potential shoulder injuries from intramuscular administration of vaccines more broadly. The committee that conducted the review identified sufficient evidence to draw 20 conclusions about whether these vaccines could cause ...
The Pregnancy Research Ethics for Vaccines, Epidemics, and New Technologies (PREVENT) Working Group has published a roadmap to guide the inclusion of the interests of pregnant women in the ...
2020 has been a difficult year for all, but has seen 58 vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) be developed and in clinical trials,1 with some vaccines reportedly having more than 90% efficacy against COVID-19 in clinical trials. This remarkable achievement is much-needed good news as COVID-19 cases are currently at their highest daily levels globally.2 ...
World Health Organization (WHO)
Vaccines not only afford the best protection against infectious disease but can serve as strong deterrence factors as well. From a bioterrorist perspective, vaccine-resistant agents are more difficult to engineer than drug-resistant agents. But the potential market has been too small and uncertain to encourage the vaccine industry to make large investments in research, development, and ...
Scripps Research in La Jolla says it has taken a promising step toward developing a vaccine to fight the effects of xylazine, an animal tranquilizer that's illicitly added to fentanyl and heroin ...
Vaccines are a clinical product that is composed of live or dead material from an infectious agent - bacterium, virus, fungus or parasite - that elicit protective immunity against the pathogen ...