- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board

Cancer Treatments and Research
Learn more about the progress made in improving cancer survival rates
Cancer Treatment Development
Radiotherapy, immunotherapy, targeted therapy.
- Combination Therapies
Diagnostics
Considerable progress has been made in reducing cancer rates and improving cancer survival in the United States since the 1990s. A greater understanding of the immune system , genetics , and cancer pathology has opened the doors to an ever-increasing range of new cancer treatments and diagnostic tools.
Advances in cancer care have been highly specific in terms of the diagnostic and treatment modalities that are recommended for each type of cancer. This article will describe these key treatments as well as the process of cancer treatment development.
sanjeri / Getty Images
Throughout the years, there have been discoveries of drugs and treatment methods that prove to be more successful or reliable than previous ones. These treatment methods are discovered in different ways.
Some are found in nature through the testing and studying of plants, fungi, and animals. Others are found through the study of cancer cells and existing drugs or procedures. But before any type of treatment method is used on patients, there is an important process that ensures its safety and effectiveness.
New cancer drugs typically go through stages of clinical research. These stages are:
- Preclinical research : Preclinical research aims to ensure a form of treatment is safe for human use. Laboratory studies that include animal research and in vitro studies , or experiments usually done in test tubes and Petri dishes, are common in this research stage.
- Clinical research : After preclinical research is successful, clinical research focuses on testing the form of therapy on humans. This clinical research stage can be lengthy (up to 10 years or more) as the discovered treatment goes through phases of clinical trials .
- Post-clinical research : Post-clinical research involves studying a therapy that has gone through the clinical research phase and received approval for human use. This involves collecting data on effectiveness and safety in real-world use.
Advances in and refinement of cancer surgery—including the use of targeted drugs and other medications before and after surgery—that can improve outcomes for cancer patients continue to emerge.
Studies comparing the outcomes of different surgical methods have helped guide doctors in selecting the technique that is most likely to result in a better long-term prognosis.
Video-Assisted Thoracoscopic Surgery (VATS) Lobectomy for Lung Cancer
During a lobectomy , a portion of a lobe of a lung that is affected by cancer is removed.
The minimally invasive technique known as VATS lobectomy, done with general anesthesia , often involves a shorter recovery time than open surgery for lung cancer . The American College of Chest Physicians identifies VATS lobectomy as the preferred method for treating early-stage lung cancer.
During the procedure, a thoracoscope, which is a small tube with a light and camera attached to the end, is inserted between the ribs through a small incision. The affected lung tissue is then removed using special tools.
Open Surgery for Cervical Cancer
In a clinical trial between 2008 and 2013, 631 women were enrolled to compare the efficacy of open surgery with that of minimally invasive surgery for the treatment of cervical cancer .
Postoperative quality of life for both groups was similar. But open surgery resulted in lower rates of cancer recurrence and higher disease-free survival.
Another study found that patients with early-stage cervical cancer who had minimally invasive surgery experienced higher recurrence rates than those who had open surgery, making open surgery a better option for some patients.
Radiation therapy is used as an adjunct to cancer treatment. More effective and targeted radiotherapies are being used to treat early and advanced cancers.
Stereotactic Ablative Radiotherapy (SABR) for Metastatic Cancer
A study demonstrated that patients receiving SABR in addition to standard of care showed improved survival compared with patients receiving palliative standard of care.
SABR for Inoperable Early-Stage Lung Cancer
For patients who are not surgical candidates, SABR offers an alternative. This approach was shown to have excellent local control and well tolerated in a cohort of 273 patients.
Immunotherapy uses the body's immune system to fight cancer. Immunotherapy can boost or change how the immune system works so it can find and attack cancer cells.
Molecular testing, which can help select patients most suitable for immunotherapy, has opened the door to this newer form of treatment. Some of the early and commonly used immunotherapy agents are vaccines, including the first FDA-approved cancer vaccine, sipuleucel-T, for prostate cancer .
Below are some breakthrough agents grouped by category:
- Monoclonal antibodies , such as Trodelvy for metastatic triple-negative breast cancer
- Oncolytic virus therapy , including Imlygic for inoperable melanoma
- CAR T-cell therapy , such as CD22 for acute lymphoblastic leukemia relapse
- Cancer vaccines , such as Provenge for prostate cancer
Targeted therapy is when drugs are directed at specific proteins or genes that promote cancer cell growth. It is designed to attack cancer cells directly.
Some of the targeted drugs commonly used to treat cancer are Tagrisso (osimertinib), Tarceva (erlotinib), and Iressa (gefitinib) for lung cancer, and Kadcyla (ado-trastuzumab), Tykerb (lapatinib), and Afinitor (everolimus) for breast cancer.
Kinase Inhibitors
Dysregulation of protein kinases is involved in many types of cancer, and this protein is the target of several cancer drugs.
Drugs like Rozlytrek (entrectinib) and Tabrecta (capmatinib) are used to treat metastatic non-small cell lung cancer .
- Rozlytrek (entrectinib) is used to treat non-small cell lung cancer that is positive for ROS1 and the neurotrophic receptor tyrosine kinases (NTRK) fusion-positive solid tumors. It inhibits cell-proliferation while targeting ROS1, a receptor tyrosine kinase.
- Tabrecta (capmatinib) is a tyrosine kinase inhibitor that can help to shrink tumors involving a MET mutation. The MET gene produces a receptor tyrosine kinase, which is involved in cell proliferation and cell survival.
Kinase Inhibitor
Our bodies contain enzymes called kinases, which help to regulate functional processes such as cell signaling and cell division. A kinase inhibitor blocks the action of kinases.
PARP Inhibitors
Drugs, such as Zejula, are used to treat ovarian cancer . The drug inhibits the enzymatic activity of enzyme poly (ADP-ribose) polymerase (PARP). In a study of 533 patients who had recurring ovarian cancer, Zejula increased the time experienced without symptoms compared with standard therapy.
Combination Therapies
Combination therapy means using two forms of cancer therapy in conjunction. Newer classes of drugs are being combined with traditional chemotherapy to improve outcomes. This approach becoming the standard of care for treating some types of cancer.
One recent example is the combination of Tecentriq and Avastin in the treatment of liver cancer.
It is an ongoing area of critical research to develop better and more accurate diagnostic and screening techniques. Below are some next-generation technologies that are being developed. However, keep in mind these techniques (aside from ctDNA) have yet to be approved by the FDA.
Artificial Intelligence Mammograms
In a study that involved 28,296 independent interpretations, AI performance was comparable to radiologists' diagnostic ability for detecting breast cancer.
Liquid Biopsy for Breast Cancer
A liquid biopsy can detect circulating levels of cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA).
In a meta-analysis that included 69 published research studies. with 5,736 breast cancer patients, researchers determined that the status of ctDNA mutation predicts disease recurrence and adverse survival results. They also found that the levels of cfDNA can predict metastasis of the axillary lymph node.
Monarch Robotic Endoscopy for Lung Cancer
This may be advantageous for patients with external lung lesions that need biopsy prior to surgery, radiation, targeted therapies, or immunotherapy.
Genomic Cancer Screening in Embryos
A polygenic risk score used by genomic prediction accurately distinguished which person in a set of siblings will inherit a medical condition. The accuracy was cited between 70% and 90%, depending upon the condition.
At-Home Urine Test for Prostate Cancer
A convenient, at-home urine test can be used to detect extracellular vesicle-derived RNA to provide prognostic information for men under active surveillance for prostate cancer.
A Word From Verywell
Cancer research that is investigating better treatments and diagnostic tools is ongoing. Even if you have advanced metastatic cancer, it may be comforting to know that newer treatments are being studied and approved every year. As treatments become better and better, your chances of survival and remission will also improve. If you have been diagnosed with cancer, it may also help to seek a cancer support group to boost your mental well-being and resilience.
American Society of Clinical Oncology: Cancer.Net. How are cancer drugs discovered and developed .
Cancer.net Improvements in Surgery for Cancer: The 2020 Advance of the Year.
Berfield KS, Farjah F, Mulligan MS. Video-assisted thoracoscopic lobectomy for lung cancer . Ann Thorac Surg. 2019 Feb;107(2):603-609. doi: 10.1016/j.athoracsur.2018.07.088
Frumovitz M, Obermair A, Coleman RL, Pareja R, Lopez A, Ribero R. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (Lacc): a secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial . Lancet Oncol . 2020 Jun;21(6):851-860. doi: 10.1016/S1470-2045(20)30081-4
Kim SI, Cho JH, Seol A, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer . Gynecol Oncol . 2019;153(1):3-12. doi:10.1016/j.ygyno.2019.01.008
Palma DA, Olson R, Harrow S, Gaede S, Louie A, Haasbeek C. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (Sabr-comet): a randomised, phase 2, open-label trial. Lancet. 2019 May 18;393(10185):2051-2058. doi: 10.1016/S0140-6736(18)32487-5
Murray L, Ramasamy S, Lilley J, et al. Stereotactic Ablative Radiotherapy (SABR) in Patients with Medically Inoperable Peripheral Early Stage Lung Cancer: Outcomes for the First UK SABR Cohort . Clin Oncol (R Coll Radiol) . 2016;28(1):4-12. doi:10.1016/j.clon.2015.09.007
American Cancer Society. Immunotherapy .
Sastre J, Sastre-Ibañez M. Molecular diagnosis and immunotherapy . Curr Opin Allergy Clin Immunol . 2016 Dec;16(6):565-570. doi: 10.1097/ACI.0000000000000318
Vansteenkiste JF, Van De Kerkhove C, Wauters E, Van Mol P. Capmatinib for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther . 2019;19(8):659-671. doi:10.1080/14737140.2019.1643239
Matulonis UA, Walder L, Nøttrup TJ, et al. Niraparib Maintenance Treatment Improves Time Without Symptoms or Toxicity (TWiST) Versus Routine Surveillance in Recurrent Ovarian Cancer: A TWiST Analysis of the ENGOT-OV16/NOVA Trial . J Clin Oncol . 2019;37(34):3183-3191. doi:10.1200/JCO.19.00917
Breast Cancer Research Foundation. How Combination Therapies Are Changing the Landscape of Breast Cancer Care .
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma . N Engl J Med . 2020;382(20):1894-1905. doi:10.1056/NEJMoa1915745
Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-Alone Artificial Intelligence for Breast Cancer Detection in Mammography: Comparison With 101 Radiologists . J Natl Cancer Inst . 2019;111(9):916-922. doi:10.1093/jnci/djy222
Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: A comprehensive review . Clin Genet . 2019 Jun;95(6):643-660. doi: 10.1111/cge.13514
Murgu SD. Robotic assisted-bronchoscopy: technical tips and lessons learned from the initial experience with sampling peripheral lung lesions. BMC Pulm Med. 2019 May 9;19(1):89. doi: 10.1186/s12890-019-0857-z
Lello L, Raben TG, Hsu SDH. Sibling validation of polygenic risk scores and complex trait prediction. Sci Rep 10 , 13190 (2020). doi.org/10.1038/s41598-020-69927-7
Connell SP, Hanna M, McCarthy F, et al. A Four-Group Urine Risk Classifier for Predicting Outcome in Prostate Cancer Patients [published online ahead of print, 2019 May 20]. BJU Int . 2019;124(4):609-620. doi:10.1111/bju.14811
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New cancer treatment may reawaken the immune system
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
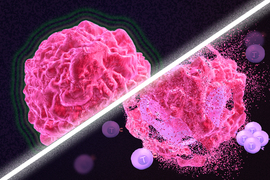
Immunotherapy is a promising strategy to treat cancer by stimulating the body’s own immune system to destroy tumor cells, but it only works for a handful of cancers. MIT researchers have now discovered a new way to jump-start the immune system to attack tumors, which they hope could allow immunotherapy to be used against more types of cancer.
Their novel approach involves removing tumor cells from the body, treating them with chemotherapy drugs, and then placing them back in the tumor. When delivered along with drugs that activate T cells, these injured cancer cells appear to act as a distress signal that spurs the T cells into action.
“When you create cells that have DNA damage but are not killed, under certain conditions those live, injured cells can send a signal that awakens the immune system,” says Michael Yaffe, who is a David H. Koch Professor of Science, the director of the MIT Center for Precision Cancer Medicine, and a member of MIT’s Koch Institute for Integrative Cancer Research.
In mouse studies, the researchers found that this treatment could completely eliminate tumors in nearly half of the mice.
Yaffe and Darrell Irvine, who is the Underwood-Prescott Professor with appointments in MIT’s departments of Biological Engineering and Materials Science and Engineering, and an associate director of the Koch Institute, are the senior authors of the study, which appears today in Science Signaling . MIT postdoc Ganapathy Sriram and Lauren Milling PhD ’21 are the lead authors of the paper.
T cell activation
One class of drugs currently used for cancer immunotherapy is checkpoint blockade inhibitors, which take the brakes off of T cells that have become “exhausted” and unable to attack tumors. These drugs have shown success in treating a few types of cancer but do not work against many others.
Yaffe and his colleagues set out to try to improve the performance of these drugs by combining them with cytotoxic chemotherapy drugs, in hopes that the chemotherapy could help stimulate the immune system to kill tumor cells. This approach is based on a phenomenon known as immunogenic cell death, in which dead or dying tumor cells send signals that attract the immune system’s attention.
Several clinical trials combining chemotherapy and immunotherapy drugs are underway, but little is known so far about the best way to combine these two types of treatment.
The MIT team began by treating cancer cells with several different chemotherapy drugs, at different doses. Twenty-four hours after the treatment, the researchers added dendritic cells to each dish, followed 24 hours later by T cells. Then, they measured how well the T cells were able to kill the cancer cells. To their surprise, they found that most of the chemotherapy drugs didn’t help very much. And those that did help appeared to work best at low doses that didn’t kill many cells.
The researchers later realized why this was so: It wasn’t dead tumor cells that were stimulating the immune system; instead, the critical factor was cells that were injured by chemotherapy but still alive.
“This describes a new concept of immunogenic cell injury rather than immunogenic cell death for cancer treatment,” Yaffe says. “We showed that if you treated tumor cells in a dish, when you injected them back directly into the tumor and gave checkpoint blockade inhibitors, the live, injured cells were the ones that reawaken the immune system.”
The drugs that appear to work best with this approach are drugs that cause DNA damage. The researchers found that when DNA damage occurs in tumor cells, it activates cellular pathways that respond to stress. These pathways send out distress signals that provoke T cells to leap into action and destroy not only those injured cells but any tumor cells nearby.
“Our findings fit perfectly with the concept that ‘danger signals’ within cells can talk to the immune system, a theory pioneered by Polly Matzinger at NIH in the 1990s, though still not universally accepted,” Yaffe says.
Tumor elimination
In studies of mice with melanoma and breast tumors, the researchers showed that this treatment eliminated tumors completely in 40 percent of the mice. Furthermore, when the researchers injected cancer cells into these same mice several months later, their T cells recognized them and destroyed them before they could form new tumors.
The researchers also tried injecting DNA-damaging drugs directly into the tumors, instead of treating cells outside the body, but they found this was not effective because the chemotherapy drugs also harmed T cells and other immune cells near the tumor. Also, injecting the injured cells without checkpoint blockade inhibitors had little effect.
“You have to present something that can act as an immunostimulant, but then you also have to release the preexisting block on the immune cells,” Yaffe says.
Yaffe hopes to test this approach in patients whose tumors have not responded to immunotherapy, but more study is needed first to determine which drugs, and at which doses, would be most beneficial for different types of tumors. The researchers are also further investigating the details of exactly how the injured tumor cells stimulate such a strong T cell response.
The research was funded, in part, by the National Institutes of Health, the Mazumdar-Shaw International Oncology Fellowship, the MIT Center for Precision Cancer Medicine, and the Charles and Marjorie Holloway Foundation.
Share this news article on:
Related links.
- Department of Biology
- Department of Biological Engineering
- Department of Materials Science and Engineering
- Koch Institute
- Ragon Institute
Related Topics
- Biological engineering
Related Articles
Cancer biologists identify new drug combo
A boost for cancer immunotherapy
Fighting cancer with the power of immunity
Previous item Next item
More MIT News
3 Questions: A shared vocabulary for how infectious diseases spread
Read full story →

Seven from MIT elected to American Academy of Arts and Sciences for 2024
Two MIT teams selected for NSF sustainable materials grants

Study demonstrates efficacy of MIT-led Brave Behind Bars program

Bringing an investigator’s eye to complex social challenges

MIT announces 2024 Bose Grants
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 25 March 2020
How cancer genomics is transforming diagnosis and treatment
- Bianca Nogrady 0
Bianca Nogrady is a freelance science writer in Sydney, Australia.
You can also search for this author in PubMed Google Scholar
DNA sequencing allows oncologists to characterize tumours on the basis of genetic mutations. Credit: KTSDESIGN/SPL
When cancer was first described by the ancient Greek physician Hippocrates, he identified just two forms: the non-ulcer-forming carcinos and the ulcer-forming carcinoma. In the late nineteenth century, physicians found, with the help of the microscope, that cancer had multiple cellular forms.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 579 , S10-S11 (2020)
doi: https://doi.org/10.1038/d41586-020-00845-4
This article is part of Nature Outlook: Cancer diagnosis , an editorially independent supplement produced with the financial support of third parties. About this content .
Schmitz, R. et al. N. Engl. J. Med. 378 , 1396–1407 (2018).
Article PubMed Google Scholar
van der Velden, D. L. et al. Nature 574 , 127–131 (2019).
Berland, L. et al. J. Thorac. Dis. 11 , S71–S80 (2019).
Download references
Related Articles
- Health care
Bioengineered ‘mini-colons’ shed light on cancer progression
News & Views 24 APR 24
Tumours form without genetic mutations
Discovery of WRN inhibitor HRO761 with synthetic lethality in MSI cancers
Article 24 APR 24

Ecologists: don’t lose touch with the joy of fieldwork
World View 24 APR 24

Emx2 underlies the development and evolution of marsupial gliding membranes

Ancient DNA traces family lines and political shifts in the Avar empire
Targeting RNA opens therapeutic avenues for Timothy syndrome

WHO redefines airborne transmission: what does that mean for future pandemics?
News 24 APR 24
More work is needed to take on the rural wastewater challenge
Correspondence 23 APR 24
Postdoctoral Associate- Computational Spatial Biology
Houston, Texas (US)
Baylor College of Medicine (BCM)
Staff Scientist - Genetics and Genomics
Technician - senior technician in cell and molecular biology.
APPLICATION CLOSING DATE: 24.05.2024 Human Technopole (HT) is a distinguished life science research institute founded and supported by the Italian ...
Human Technopole
Postdoctoral Fellow
The Dubal Laboratory of Neuroscience and Aging at the University of California, San Francisco (UCSF) seeks postdoctoral fellows to investigate the ...
San Francisco, California
University of California, San Francsico
Postdoctoral Associate
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancer Control
- v.28; Jan-Dec 2021
Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective
Patricia piña-sánchez.
1 Oncology Research Unit, Oncology Hospital, Mexican Institute of Social Security, Mexico
Antonieta Chávez-González
Martha ruiz-tachiquín, eduardo vadillo, alberto monroy-garcía, juan josé montesinos, rocío grajales.
2 Department of Medical Oncology, Oncology Hospital, Mexican Institute of Social Security, Mexico
Marcos Gutiérrez de la Barrera
3 Clinical Research Division, Oncology Hospital, Mexican Institute of Social Security, Mexico
Hector Mayani
Since the second half of the 20th century, our knowledge about the biology of cancer has made extraordinary progress. Today, we understand cancer at the genomic and epigenomic levels, and we have identified the cell that starts neoplastic transformation and characterized the mechanisms for the invasion of other tissues. This knowledge has allowed novel drugs to be designed that act on specific molecular targets, the immune system to be trained and manipulated to increase its efficiency, and ever more effective therapeutic strategies to be developed. Nevertheless, we are still far from winning the war against cancer, and thus biomedical research in oncology must continue to be a global priority. Likewise, there is a need to reduce unequal access to medical services and improve prevention programs, especially in countries with a low human development index.
Introduction
During the last one hundred years, our understanding of the biology of cancer increased in an extraordinary way. 1 - 4 Such a progress has been particularly prompted during the last few decades because of technological and conceptual progress in a variety of fields, including massive next-generation sequencing, inclusion of “omic” sciences, high-resolution microscopy, molecular immunology, flow cytometry, analysis and sequencing of individual cells, new cell culture techniques, and the development of animal models, among others. Nevertheless, there are many questions yet to be answered and many problems to be solved regarding this disease. As a consequence, oncological research must be considered imperative.
Currently, cancer is one of the illnesses that causes more deaths worldwide. 5 According to data reported in 2020 by the World Health Organization (WHO), cancer is the second cause of death throughout the world, with 10 million deaths. 6 Clearly, cancer is still a leading problem worldwide. With this in mind, the objective of this article is to present a multidisciplinary and comprehensive overview of the disease. We will begin by analyzing cancer as a process, focusing on the current state of our knowledge on 4 specific aspects of its biology. Then, we will look at cancer as a global health problem, considering some epidemiological aspects, and discussing treatment, with a special focus on novel therapies. Finally, we present our vision on some of the challenges and perspectives of cancer in the 21 st century.
The Biology of Cancer
Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and epigenomic alterations that lead to cell transformation, the cells where these changes occur, and the processes of invasion and metastasis that, to an important degree, determine tumor aggressiveness.
Cancer Genomics
The genomics of cancer can be defined as the study of the complete sequence of DNA and its expression in tumor cells. Evidently, this study only becomes meaningful when compared to normal cells. The sequencing of the human genome, completed in 2003, was not only groundbreaking with respect to the knowledge of our gene pool, but also changed the way we study cancer. In the post-genomic era, various worldwide endeavors, such as the Human Cancer Genome Project , the Cancer Genome ATLAS (TCGA), the International Cancer Genome Consortium, and the Pan-Cancer Analysis Working Group (PCAWG), have contributed to the characterization of thousands of primary tumors from different neoplasias, generating more than 2.5 petabytes (10 15 ) of genomic, epigenomic, and proteomic information. This has led to the building of databases and analytical tools that are available for the study of cancer from an “omic” perspective, 7 , 8 and it has helped to modify classification and treatment of various neoplasms.
Studies in the past decade, including the work by the PCAWG, have shown that cancer generally begins with a small number of driving mutations (4 or 5 mutations) in particular genes, including oncogenes and tumor-suppressor genes. Mutations in TP53, a tumor-suppressor gene, for example, are found in more than half of all cancer types as an early event, and they are a hallmark of precancerous lesions. 9 - 12 From that point on, the evolution of tumors may take decades, throughout which the mutational spectrum of tumor cells changes significantly. Mutational analysis of more than 19 000 exomes revealed a collection of genomic signatures, some associated with defects in the mechanism of DNA repair. These studies also revealed the importance of alterations in non-coding regions of DNA. Thus, for example, it has been observed that various pathways of cell proliferation and chromatin remodeling are altered by mutations in coding regions, while pathways, such as WNT and NOTCH, can be disrupted by coding and non-coding mutations. To the present date, 19 955 genes that codify for proteins and 25 511 genes for non-coding RNAs have been identified ( https://www.gencodegenes.org/human/stats.html ). Based on this genomic catalogue, the COSMIC (Catalogue Of Somatic Mutations In Cancer) repository, the most robust database to date, has registered 37 288 077 coding mutations, 19 396 fusions, 1 207 190 copy number variants, and 15 642 672 non-coding variants reported up to August 2020 (v92) ( https://cosmic-blog.sanger.ac.uk/cosmic-release-v92/ ).
The genomic approach has accelerated the development of new cancer drugs. Indeed, two of the most relevant initiatives in recent years are ATOM (Accelerating Therapeutics for Opportunities in Medicine), which groups industry, government and academia, with the objective of accelerating the identification of drugs, 13 and the Connectivity Map (CMAP), a collection of transcriptional data obtained from cell lines treated with drugs for the discovery of functional connections between genes, diseases, and drugs. The CMAP 1.0 covered 1300 small molecules and more than 6000 signatures; meanwhile, the CMAP 2.0 with L1000 assay profiled more than 1.3 million samples and approximately 400 000 signatures. 14
The genomic study of tumors has had 2 fundamental contributions. On the one hand, it has allowed the confirmation and expansion of the concept of intratumor heterogeneity 15 , 16 ; and on the other, it has given rise to new classification systems for cancer. Based on the molecular classification developed by expression profiles, together with mutational and epigenomic profiles, a variety of molecular signatures have been identified, leading to the production of various commercial multigene panels. In breast cancer, for example, different panels have been developed, such as Pam50/Prosigna , Blue Print , OncotypeDX , MammaPrint , Prosigna , Endopredict , Breast Cancer Index , Mammostrat, and IHC4 . 17
Currently, the genomic/molecular study of cancer is more closely integrated with clinical practice, from the classification of neoplasms, as in tumors of the nervous system, 18 to its use in prediction, as in breast cancer. 17 Improvement in molecular methods and techniques has allowed the use of smaller amounts of biological material, as well as paraffin-embedded samples for genomic studies, both of which provide a wealth of information. 19 In addition, non-invasive methods, such as liquid biopsies, represent a great opportunity not only for the diagnosis of cancer, but also for follow-up, especially for unresectable tumors. 20
Research for the production of genomic information on cancer is presently dominated by several consortia, which has allowed the generation of a great quantity of data. However, most of these consortia and studies are performed in countries with a high human development index (HDI), and countries with a low HDI are not well represented in these large genomic studies. This is why initiatives such as Human Heredity and Health in Africa (H3Africa) for genomic research in Africa are essential. 21 Generation of new information and technological developments, such as third-generation sequencing, will undoubtedly continue to move forward in a multidisciplinary and complex systems context. However, the existing disparities in access to genomic tools for diagnosis, prognosis, and treatment of cancer will continue to be a pressing challenge at regional and social levels.
Cancer Epigenetics
Epigenetics studies the molecular mechanisms that produce hereditable changes in gene expression, without causing alterations in the DNA sequence. Epigenetic events are of 3 types: methylation of DNA and RNA, histone modification (acetylation, methylation, and phosphorylation), and the expression of non-coding RNA. Epigenetic aberrations can drive carcinogenesis when they alter chromosome conformation and the access to transcriptional machinery and to various regulatory elements (promoters, enhancers, and anchors for interaction with chromatin, for example). These changes may activate oncogenesis and silence tumor-suppressor mechanisms when they modulate coding and non-coding sequences (such as micro-RNAs and long-RNAs). This can then lead to uncontrolled growth, as well as the invasion and metastasis of cancer cells.
While genetic mutations are stable and irreversible, epigenetic alterations are dynamic and reversible; that is, there are several epigenomes, determined by space and time, which cause heterogeneity of the “epigenetic status” of tumors during their development and make them susceptible to environmental stimuli or chemotherapeutic treatment. 22 Epigenomic variability creates differences between cells, and this creates the need to analyze cells at the individual level. In the past, epigenetic analyses measured “average states” of cell populations. These studies revealed general mechanisms, such as the role of epigenetic marks on active or repressed transcriptional states, and established maps of epigenetic composition in a variety of cell types in normal and cancerous tissue. However, these approaches are difficult to use to examine events occurring in heterogeneous cell populations or in uncommon cell types. This has led to the development of new techniques that permit marking of a sequence on the epigenome and improvement in the recovery yield of epigenetic material from individual cells. This has helped to determine changes in DNA, RNA, and histones, chromatin accessibility, and chromosome conformation in a variety of neoplasms. 23 , 24
In cancer, DNA hypomethylation occurs on a global scale, while hypermethylation occurs in specific genomic loci, associated with abnormal nucleosome positioning and chromatin modifications. This information has allowed epigenomic profiles to be established in different types of neoplasms. In turn, these profiles have served as the basis to identify new neoplasm subgroups. For example, in triple negative breast cancer (TNBC), 25 and in hepatocellular carcinoma, 26 DNA methylation profiles have helped to the identification of distinct subgroups with clinical relevance. Epigenetic approaches have also helped to the development of prognostic tests to assess the sensitivity of cancer cells to specific drugs. 27
Epigenetic traits could be used to characterize intratumoral heterogeneity and determine the relevance of such a heterogeneity in clonal evolution and sensitivity to drugs. However, it is clear that heterogeneity is not only determined by genetic and epigenetic diversity resulting from clonal evolution of tumor cells, but also by the various cell populations that form the tumor microenvironment (TME). 28 Consequently, the epigenome of cancer cells is continually remodeled throughout tumorigenesis, during resistance to the activity of drugs, and in metastasis. 29 This makes therapeutic action based on epigenomic profiles difficult, although significant advances in this area have been reported. 30
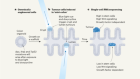
During carcinogenesis and tumor progression, epigenetic modifications are categorized by their mechanisms of regulation ( Figure 1A ) and the various levels of structural complexity ( Figure 1B ). In addition, the epigenome can be modified by environmental stimuli, stochastic events, and genetic variations that impact the phenotype ( Figure 1C ). 31 , 32 The molecules that take part in these mechanisms/events/variations are therapeutic targets of interest with potential impact on clinical practice. There are studies on a wide variety of epidrugs, either alone or in combination, which improve antitumor efficacy. 33 However, the problems with these drugs must not be underestimated. For a considerable number of epigenetic compounds still being under study, the main challenge is to translate in vitro efficacy of nanomolar (nM) concentrations into well-tolerated and efficient clinical use. 34 The mechanisms of action of epidrugs may not be sufficiently controlled and could lead to diversion of the therapeutic target. 35 It is known that certain epidrugs, such as valproic acid, produce unwanted epigenetic changes 36 ; thus the need for a well-established safety profile before these drugs can be used in clinical therapy. Finally, resistance to certain epidrugs is another relevant problem. 37 , 38

Epigenetics of cancer. (A) Molecular mechanisms. (B) Structural hierarchy of epigenomics. (C) Factors affecting the epigenome. Modified from Refs. 31 and 32 .
As we learn about the epigenome of specific cell populations in cancer patients, a door opens to the evaluation of sensitivity tests and the search for new molecular markers for detection, prognosis, follow-up, and/or response to treatment at various levels of molecular regulation. Likewise, the horizon expands for therapeutic alternatives in oncology with the use of epidrugs, such as pharmacoepigenomic modulators for genes and key pathways, including methylation of promoters and regulation of micro-RNAs involved in chemoresponse and immune response in cancer. 39 There is no doubt that integrated approaches identifying stable pharmagenomic and epigenomic patterns and their relation with expression profiles and genetic functions will be more and more valuable in our fight against cancer.
Cancer Stem Cells
Tumors consist of different populations of neoplastic cells and a variety of elements that form part of the TME, including stromal cells and molecules of the extracellular matrix. 40 Such intratumoral heterogeneity becomes even more complex during clonal variation of transformed cells, as well as influence the elements of the TME have on these cells throughout specific times and places. 41 To explain the origin of cancer cell heterogeneity, 2 models have been put forward. The first proposes that mutations occur at random during development of the tumor in individual neoplastic cells, and this promotes the production of various tumor populations, which acquire specific growth and survival traits that lead them to evolve according to intratumor mechanisms of natural selection. 42 The second model proposes that each tumor begins as a single cell that possess 2 functional properties: it can self-renew and it can produce several types of terminal cells. As these 2 properties are characteristics of somatic stem cells, 43 the cells have been called cancer stem cells (CSCs). 44 According to this model, tumors must have a hierarchical organization, where self-renewing stem cells produce highly proliferating progenitor cells, unable to self-renew but with a high proliferation potential. The latter, in turn, give rise to terminal cells. 45 Current evidence indicates that both models may coexist in tumor progression. In agreement with this idea, new subclones could be produced as a result of a lack of genetic stability and mutational changes, in addition to the heterogeneity derived from the initial CSC and its descendants. Thus, in each tumor, a set of neoplastic cells with different genetic and epigenetic traits may be found, which would provide different phenotypic properties. 46
The CSC concept was originally presented in a model of acute myeloid leukemia. 47 The presence of CSCs was later proved in chronic myeloid leukemia, breast cancer, tumors of the central nervous system, lung cancer, colon cancer, liver cancer, prostate cancer, pancreatic cancer, melanoma, and cancer of the head and neck, amongst others. In all of these cases, detection of CSCs was based on separation of several cell populations according to expression of specific surface markers, such as CD133, CD44, CD24, CD117, and CD15. 48 It is noteworthy that in some solid tumors, and even in some hematopoietic ones, a combination of specific markers that allow the isolation of CSCs has not been found. Interestingly, in such tumors, a high percentage of cells with the capacity to start secondary tumors has been observed; thus, the terms Tumor Initiating Cells (TIC) or Leukemia Initiating Cells (LIC) have been adopted. 46
A relevant aspect of the biology of CSCs is that, just like normal stem cells, they can self-renew. Such self-renewal guarantees the maintenance or expansion of the tumor stem cell population. Another trait CSCs share with normal stem cells is their quiescence, first described in chronic myeloid leukemia. 49 The persistence of quiescent CSCs in solid tumors has been recently described in colorectal cancer, where quiescent clones can become dominant after therapy with oxaliplatin. 50 In non-hierarchical tumors, such as melanoma, the existence of slow-cycling cells that are resistant to antimitogenic agents has also been proved. 51 Such experimental evidence supports the idea that quiescent CSCs or TICs are responsible for both tumor resistance to antineoplastic drugs and clinical relapse after initial therapeutic success.
In addition to quiescence, CSCs use other mechanisms to resist the action of chemotherapeutic drugs. One of these is their increased numbers: upon diagnosis, a high number of CSCs are observed in most analyzed tumors, making treatment unable to destroy all of them. On the other hand, CSCs have a high number of molecular pumps that expulse drugs, as well as high numbers of antiapoptotic molecules. In addition, they have very efficient mechanisms to repair DNA damage. In general, these cells show changes in a variety of signaling pathways involved in proliferation, survival, differentiation, and self-renewal. It is worth highlighting that in recent years, many of these pathways have become potential therapeutic targets in the elimination of CSCs. 52 Another aspect that is highly relevant in understanding the biological behavior of CSCs is that they require a specific site for their development within the tissue where they are found that can provide whatever is needed for their survival and growth. These sites, known as niches, are made of various cells, both tumor and non-tumor, as well as a variety of non-cellular elements (extracellular matrix [ECM], soluble cytokines, ion concentration gradients, etc.), capable of regulating the physiology of CSCs in order to promote their expansion, the invasion of adjacent tissues, and metastasis. 53
It is important to consider that although a large number of surface markers have been identified that allow us to enrich and prospectively follow tumor stem cell populations, to this day there is no combination of markers that allows us to find these populations in all tumors, and it is yet unclear if all tumors present them. In this regard, it is necessary to develop new purification strategies based on the gene expression profiles of these cells, so that tumor heterogeneity is taken into account, as it is evident that a tumor can include multiple clones of CSCs that, in spite of being functional, are genetically different, and that these clones can vary throughout space (occupying different microenvironments and niches) and time (during the progression of a range of tumor stages). Such strategies, in addition to new in vitro and in vivo assays, will allow the development of new and improved CSC elimination strategies. This will certainly have an impact on the development of more efficient therapeutic alternatives.
Invasion and Metastasis
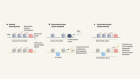
Nearly 90% of the mortality associated with cancer is related to metastasis. 54 This consists of a cascade of events ( Figure 2 ) that begins with the local invasion of a tumor into surrounding tissues, followed by intravasation of tumor cells into the blood stream or lymphatic circulation. Extravasation of neoplastic cells in areas distant from the primary tumor then leads to the formation of one or more micrometastatic lesions which subsequently proliferate to form clinically detectable lesions. 4 The cells that are able to produce metastasis must acquire migratory characteristics, which occur by a process known as epithelial–mesenchymal transition (EMT), that is, the partial loss of epithelial characteristics and the acquirement of mesenchymal traits. 55

Invasion and metastasis cascade. Invasion and metastasis can occur early or late during tumor progression. In either case, invasion to adjacent tissues is driven by stem-like cells (cancer stem cells) that acquire the epithelial–mesenchymal transition (EMT) (1). Once they reach sites adjacent to blood vessels, tumor cells (individually or in clusters) enter the blood (2). Tumor cells in circulation can adhere to endothelium and extravasation takes place (3). Other mechanisms alternative to extravasation can exist, such as angiopelosis, in which clusters of tumor cells are internalized by the endothelium. Furthermore, at certain sites, tumor cells can obstruct microvasculature and initiate a metastatic lesion right there. Sometimes, a tumor cells that has just exit circulation goes into an MET in order to become quiescent (4). Inflammatory signals can activate quiescent metastatic cells that will proliferate and generate a clinically detectable lesion (5).
Although several of the factors involved in this process are currently known, many issues are still unsolved. For instance, it has not yet been possible to monitor in vivo the specific moment when it occurs 54 ; the microenvironmental factors of the primary tumor that promote such a transition are not known with precision; and the exact moment during tumor evolution in which one cell or a cluster of cells begin to migrate to distant areas, is also unknown. The wide range of possibilities offered by intra- and inter-tumoral heterogeneity 56 stands in the way of suggesting a generalized strategy that could resolve this complication.
It was previously believed that metastasis was only produced in late stages of tumor progression; however, recent studies indicate that EMT and metastasis can occur during the early course of the disease. In pancreatic cancer, for example, cells going through EMT are able to colonize and form metastatic lesions in the liver in the first stages of the disease. 52 , 57 Metastatic cell clusters circulating in peripheral blood (PB) are prone to generate a metastatic site, compared to individual tumor cells. 58 , 59 In this regard, novel strategies, such as the use of micro-RNAs, are being assessed in order to diminish induction of EMT. 60 It must be mentioned, however, that the metastatic process seems to be even more complex, with alternative pathways that do not involve EMT. 61 , 62
A crucial stage in the process of metastasis is the intravasation of tumor cells (alone or in clusters) towards the blood stream and/or lymphatic circulation. 63 These mechanisms are also under intensive research because blocking them could allow the control of spreading of the primary tumor. In PB or lymphatic circulation, tumor cells travel to distant parts for the potential formation of a metastatic lesion. During their journey, these cells must stand the pressure of blood flow and escape interaction with natural killer (NK) cells . 64 To avoid them, tumor cells often cover themselves with thrombocytes and also produce factors such as VEGF, angiopoietin-2, angiopoietin-4, and CCL2 that are involved in the induction of vascular permeability. 54 , 65 Neutrophils also contribute to lung metastasis in the bloodstream by secreting IL-1β and metalloproteases to facilitate extravasation of tumor cells. 64
The next step in the process of metastasis is extravasation, for which tumor cells, alone or in clusters, can use various mechanisms, including a recently described process known as angiopellosis that involves restructuring the endothelial barrier to internalize one or several cells into a tissue. 66 The study of leukocyte extravasation has contributed to a more detailed knowledge of this process, in such a way that some of the proposed strategies to avoid extravasation include the use of integrin inhibitors, molecules that are vital for rolling, adhesion, and extravasation of tumor cells. 67 , 68 Another strategy that has therapeutic potential is the use of antibodies that strengthen vascular integrity to obstruct transendothelial migration of tumor cells and aid in their destruction in PB. 69
Following extravasation, tumor cells can return to an epithelial phenotype, a process known as mesenchymal–epithelial transition and may remain inactive for several years. They do this by competing for specialized niches, like those in the bone marrow, brain, and intestinal mucosa, which provide signals through the Notch and Wnt pathways. 70 Through the action of the Wnt pathway, tumor cells enter a slow state of the cell cycle and induce the expression of molecules that inhibit the cytotoxic function of NK cells. 71 The extravasated tumor cell that is in a quiescent state must comply with 2 traits typical of stem cells: they must have the capacity to self-renew and to generate all of the cells that form the secondary tumor.
There are still several questions regarding the metastatic process. One of the persisting debates at present is if EMT is essential for metastasis or if it plays a more important role in chemoresistance. 61 , 62 It is equally important to know if there is a pattern in each tumor for the production of cells with the capacity to carry out EMT. In order to control metastasis, it is fundamental to know what triggers acquisition of the migratory phenotype and the intrinsic factors determining this transition. Furthermore, it is essential to know if mutations associated with the primary tumor or the variety of epigenetic changes are involved in this process. 55 It is clear that metastatic cells have affinity for certain tissues, depending on the nature of the primary tumor (seed and soil hypothesis). This may be caused by factors such as the location and the direction of the bloodstream or lymphatic fluid, but also by conditioning of premetastatic niches at a distance (due to the large number of soluble factors secreted by the tumor and the recruitment of cells of the immune system to those sites). 72 We have yet to identify and characterize all of the elements that participate in this process. Deciphering them will be of upmost importance from a therapeutic point of view.
Epidemiology of Cancer
Cancer is the second cause of death worldwide; today one of every 6 deaths is due to a type of cancer. According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors—including environmental pollutants and processed food—play as cancer inducers and promoters. 74 The types of cancer that produce the greatest numbers of cases and deaths worldwide are indicated in Table 1 . 6
Total Numbers of Cancer Cases and Deaths Worldwide in 2020 by Cancer Type (According to the Global Cancer Observatory, IARC).
Data presented on this table were obtained from Ref. 6.
As shown in Figure 3 , lung, breast, prostate, and colorectal cancer are the most common throughout the world, and they are mostly concentrated in countries of high to very high human development index (HDI). Although breast, prostate, and colorectal cancer have a high incidence, the number of deaths they cause is proportionally low, mostly reflecting the great progress made in their control. However, these data also reveal the types of cancer that require further effort in prevention, precise early detection avoiding overdiagnosis, and efficient treatment. This is the case of liver, lung, esophageal, and pancreatic cancer, where the difference between the number of cases and deaths is smaller ( Figure 3B ). Social and economic transition in several countries has had an impact on reducing the incidence of neoplasms associated with infection and simultaneously produced an increase in the types related to reproductive, dietary, and hormonal factors. 75

Incidence and mortality for some types of cancer in the world. (A) Estimated number of cases and deaths in 2020 for the most frequent cancer types worldwide. (B) Incidence and mortality rates, normalized according to age, for the most frequent cancer types in countries with very high/& high (VH&H; blue) and/low and middle (L&M; red) Human Development Index (HDI). Data include both genders and all ages. Data according to https://gco.iarc.fr/today , as of June 10, 2021.
In the past 3 decades, cancer mortality rates have fallen in high HDI countries, with the exception of pancreatic cancer, and lung cancer in women. Nevertheless, changes in the incidence of cancer do not show the same consistency, possibly due to variables such as the possibility of early detection, exposure to risk factors, or genetic predisposition. 76 , 77 Countries such as Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the United Kingdom have reported a reduction in incidence and mortality in cancer of the stomach, colon, lung, and ovary, as well as an increase in survival. 78 Changes in modifiable risk factors, such as the use of tobacco, have played an important role in prevention. In this respect, it has been estimated that decline in tobacco use can explain between 35% and 45% of the reduction in cancer mortality rates, 79 while the fall in incidence and mortality due to stomach cancer can be attributed partly to the control of Helicobacter pylori infection. 80 Another key factor in the fall of mortality rates in developed countries has been an increase in early detection as a result of screening programs, as in breast and prostate cancer, which have had their mortality rates decreased dramatically in spite of an increase in their incidence. 76
Another important improvement observed in recent decades is the increase in survival rates, particularly in high HDI countries. In the USA, for example, survival rates for patients with prostate cancer at 5 years after initial diagnosis was 28% during 1947–1951; 69% during 1975–1977, and 100% during 2003–2009. Something similar occurred with breast cancer, with a 5-year survival rate of 54% in 1947–1951, 75% in 1975–1977, and 90% in 2003–2009. 81 In the CONCORD 3 version, age-standardize 5-year survival for patients with breast cancer in the USA during 2010–2014 was 90%, and 97% for prostate cancer patients. 82 Importantly, even among high HDI countries, significant differences have been identified in survival rates, being stage of disease at diagnosis, time for access to effective treatment, and comorbidities, the main factors influencing survival in these nations. 78 Unfortunately, survival rates in low HDI countries are significantly lower due to several factors, including lack of information, deficient screening and early detection programs, limited access to treatment, and suboptimal cancer registration. 82 It should be noted that in countries with low to middle HDI, neoplasms with the greatest incidence are those affecting women (breast and cervical cancer), which reflects not only a problem with access to health services, but also a serious inequality issue that involves social, cultural, and even religious obstacles. 83
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western Africa was 2.2, whereas in Polynesia and Eastern Asia was 37.3 and 34.4, respectively. 6 In contrast, the global burden of cancer associated with infection was 15.4%, but in Sub-Saharan Africa it was 30%. 85 Likewise, the incidence of cervical cancer in Eastern Africa was 40.1, in contrast with the USA and Canada that have a rate of 6.2. This makes it clear that one of the challenges we face is the reduction of the risk factors that are potentially modifiable and associated with specific types of cancer.
Improvement of survival rates and its disparities worldwide are also important challenges. Five-year survival for breast cancer—diagnosed during 2010-2014— in the USA, for example, was 90%, whereas in countries like South Africa it was 40%. 82 Childhood leukemia in the USA and several European countries shows a 5-year survival of 90%, while in Latin-American countries it is 50–76%. 86 Interestingly, there are neoplasms, such as pancreatic cancer, for which there has been no significant increase in survival, which remains low (5–15%) both in developed and developing countries. 82
Although data reported on global incidence and mortality gives a general overview on the epidemiology of cancer, it is important to note that there are great differences in coverage of cancer registries worldwide. To date, only 1 out of every 3 countries reports high quality data on the incidence of cancer. 87 For the past 50 years, the IARC has supported population-based cancer registries; however, more than one-third of the countries belonging to the WHO, mainly countries of low and middle income (LMIC), have no data on more than half of the 18 indicators of sustainable development goals. 88 High quality cancer registries only cover 4% of the population in Africa, 8% in Asia, and 7% in Latin America, contrasting with 83% in the USA and Canada, and 33% in Europe. 89 In response to this situation, the Global Initiative for Cancer Registry Development was created in 2012 to generate improved infrastructure to permit greater coverage and better quality registries, especially in countries with low and middle HDI. 88 It is expected that initiatives of this sort in the coming years will allow more and better information to guide strategies for the control of cancer worldwide, especially in developing regions. This will enable survival to be measured over longer periods of time (10, 15, or 20 years), as an effective measure in the control of cancer. The WHO has established as a target for 2025 to reduce deaths by cancer and other non-transmissible diseases by 25% in the population between the ages of 30–69; such an effort requires not only effective prevention measures to reduce incidence, but also more efficient health systems to diminish mortality and increase survival. At the moment, it is an even greater challenge because of the effects of the COVID-19 pandemic which has negatively impacted cancer prevention and health services. 90
Oncologic Treatments
A general perspective.
At the beginning of the 20th century, cancer treatment, specifically treatment of solid tumors, was based fundamentally on surgical resection of tumors, which together with other methods for local control, such as cauterization, had been used since ancient times. 91 At that time, there was an ongoing burst of clinical observations along with interventions sustained on fundamental knowledge about physics, chemistry, and biology. In the final years of the 19 th century and the first half of the 20th, these technological developments gave rise to radiotherapy, hormone therapy, and chemotherapy. 92 - 94 Simultaneously, immunotherapy was also developed, although usually on a smaller scale, in light of the overwhelming progress of chemotherapy and radiotherapy. 95
Thus began the development and expansion of disciplines based on these approaches (surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy), with their application evolving ever more rapidly up to their current uses. Today, there is a wide range of therapeutic tools for the care of cancer patients. These include elements that emerged empirically, arising from observations of their effects in various medical fields, as well as drugs that were designed to block processes and pathways that form part of the physiopathology of one or more neoplasms according to knowledge of specific molecular alterations. A classic example of the first sort of tool is mustard gas, originally used as a weapon in war, 96 but when applied for medical purposes, marked the beginning of the use of chemicals in the treatment of malignant neoplasms, that is, chemotherapy. 94 A clear example of the second case is imatinib, designed specifically to selectively inhibit a molecular alteration in chronic myeloid leukemia: the Bcr-Abl oncoprotein. 97
It is on this foundation that today the 5 areas mentioned previously coexist and complement one another. The general framework that motivates this amalgam and guides its development is precision medicine, founded on the interaction of basic and clinical science. In the forecasts for development in each of these fields, surgery is expected to continue to be the fundamental approach for primary tumors in the foreseeable future, as well as when neoplastic disease in the patient is limited, or can be limited by applying systemic or regional elements, before and/or after surgical resection, and it can be reasonably anticipated for the patient to have a significant period free from disease or even to be cured. With regards to technology, intensive exploration of robotic surgery is contemplated. 98
The technological possibilities for radiotherapy have progressed in such a way that it is now possible to radiate neoplastic tissue with an extraordinary level of precision, and therefore avoid damage to healthy tissue. 99 This allows administration of large doses of ionizing radiation in one or a few fractions, what is known as “radiosurgery.” The greatest challenges to the efficacy of this approach are related to radio-resistance in certain neoplasms. Most efforts regarding research in this field are concentrated on understanding the underlying biological mechanisms of the phenomenon and their potential control through radiosensitizers. 100
“Traditional” chemotherapy, based on the use of compounds obtained from plants and other natural products, acting in a non-specific manner on both neoplastic and healthy tissues with a high proliferation rate, continues to prevail. 101 The family of chemotherapeutic drugs currently includes alkylating agents, antimetabolites, anti-topoisomerase agents, and anti-microtubules. Within the pharmacologic perspective, the objective is to attain a high concentration or activity of such molecules in specific tissues while avoiding their accumulation in others, in order to achieve an increase in effectiveness and a reduction in toxicity. This has been possible with the use of viral vectors, for example, that are able to limit their replication in neoplastic tissues, and activate prodrugs of normally nonspecific agents, like cyclophosphamide, exclusively in those specific areas. 102 More broadly, chemotherapy also includes a subgroup of substances, known as molecular targeted therapy, that affect processes in a more direct and specific manner, which will be mentioned later.
There is no doubt that immunotherapy—to be explored next—is one of the therapeutic fields where development has been greatest in recent decades and one that has produced enormous expectation in cancer treatment. 103 Likewise, cell therapy, based on the use of immune cells or stem cells, has come to complement the oncologic therapeutic arsenal. 43 Each and every one of the therapeutic fields that have arisen in oncology to this day continue to prevail and evolve. Interestingly, the foreseeable future for the development of cancer treatment contemplates these approaches in a joint and complementary manner, within the general framework of precision medicine, 104 and sustained by knowledge of the biological mechanisms involved in the appearance and progression of neoplasms. 105 , 106
Immunotherapy
Stimulating the immune system to treat cancer patients has been a historical objective in the field of oncology. Since the early work of William Coley 107 to the achievements reached at the end of the 20 th century, scientific findings and technological developments paved the way to searching for new immunotherapeutic strategies. Recombinant DNA technology allowed the synthesis of cytokines, such as interferon-alpha (IFN-α) and interleukin 2 (IL-2), which were authorized by the US Food and Drug Administration (FDA) for the treatment of hairy cell leukemia in 1986, 108 as well as kidney cancer and metastatic melanoma in 1992 and 1998, respectively. 109
The first therapeutic vaccine against cancer, based on the use of autologous dendritic cells (DCs), was approved by the FDA against prostate cancer in 2010. However, progress in the field of immunotherapy against cancer was stalled in the first decade of the present century, mostly due to failure of several vaccines in clinical trials. In many cases, application of these vaccines was detained by the complexity and cost involved in their production. Nevertheless, with the coming of the concept of immune checkpoint control, and the demonstration of the relevance of molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed cell death molecule-1 (PD-1), immunotherapy against cancer recovered its global relevance. In 2011, the monoclonal antibody (mAb) ipilimumab, specific to the CTLA-4 molecule, was the first checkpoint inhibitor (CPI) approved for the treatment of advanced melanoma. 110 Later, inhibitory mAbs for PD-1, or for the PD-1 ligand (PD-L1), 111 as well as the production of T cells with chimeric receptors for antigen recognition (CAR-T), 112 which have been approved to treat various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma, among others, have changed the paradigm of cancer treatment.
In spite of the current use of anti-CTLA-4 and anti-PD-L1 mAbs, only a subgroup of patients has responded favorably to these CPIs, and the number of patients achieving clinical benefit is still small. It has been estimated that more than 70% of patients with solid tumors do not respond to CPI immunotherapy because either they show primary resistance, or after responding favorably, develop resistance to treatment. 113 In this regard, it is important to mention that in recent years very important steps have been taken to identify the intrinsic and extrinsic mechanisms that mediate resistance to CPI immunotherapy. 114 Intrinsic mechanisms include changes in the antitumor immune response pathways, such as faulty processing and presentation of antigens by APCs, activation of T cells for tumor cell destruction, and changes in tumor cells that lead to an immunosuppressive TME. Extrinsic factors include the presence of immunosuppressive cells in the local TME, such as regulatory T cells, myeloid-derived suppressor cells (MDSC), mesenchymal stem/stromal cells (MSCs), and type 2 macrophages (M2), in addition to immunosuppressive cytokines.
On the other hand, classification of solid tumors as “hot,” “cold,” or “excluded,” depending on T cell infiltrates and the contact of such infiltrates with tumor cells, as well as those that present high tumor mutation burden (TMB), have redirected immunotherapy towards 3 main strategies 115 ( Table 2 ): (1) Making T-cell antitumor response more effective, using checkpoint inhibitors complementary to anti-CTLA-4 and anti-PD-L1, such as LAG3, Tim-3, and TIGT, as well as using CAR-T cells against tumor antigens. (2) Activating tumor-associated myeloid cells including monocytes, granulocytes, macrophages, and DC lineages, found at several frequencies within human solid tumors. (3) Regulating the biochemical pathways in TME that produce high concentrations of immunosuppressive molecules, such as kynurenine, a product of tryptophan metabolism, through the activity of indoleamine 2,3 dioxygenase; or adenosine, a product of ATP hydrolysis by the activity of the enzyme 5’nucleotidase (CD73). 116
Current Strategies to Stimulate the Immune Response for Antitumor Immunotherapy.
Abbreviations: TME, tumor microenvironment; IL, interleukin; TNF, Tumor Necrosis Factor; TNFR, TNF-receptor; CD137, receptor–co-stimulator of the TNFR family; OX40, member number 4 of the TNFR superfamily; CD27/CD70, member of the TNFR superfamily; CD40/CD40L, antigen-presenting cells (APC) co-stimulator and its ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; STING, IFN genes-stimulator; RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation-associated protein 5; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high mobility group B1 protein; TLR, Toll-like receptor; HVEM, Herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T lymphocyte antigen 4; PD-L1, programmed death ligand-1; TIGIT, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibition motives; CSF1/CSF1R, colony-stimulating factor-1 and its receptor; CCR2, Type 2 chemokine receptor; PI3Kγ, Phosphoinositide 3-Kinase γ; CXCL/CCL, chemokine ligands; LFA1, lymphocyte function-associated antigen 1; ICAM1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indolamine 2,3-dioxigenase; TGF, transforming growth factor; LAG-3, lymphocyte-activation gene 3 protein; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CD73, 5´nucleotidase; ARs, adenosine receptors; Selectins, cell adhesion molecules; CAR-T, chimeric antigen receptor T cell; TCR-T, T-cell receptor engineered T cell.
Apart from the problems associated with its efficacy (only a small group of patients respond to it), immunotherapy faces several challenges related to its safety. In other words, immunotherapy can induce adverse events in patients, such as autoimmunity, where healthy tissues are attacked, or cytokine release syndrome and vascular leak syndrome, as observed with the use of IL-2, both of which lead to serious hypotension, fever, renal failure, and other adverse events that are potentially lethal. The main challenges to be faced by immunotherapy in the future will require the combined efforts of basic and clinical scientists, with the objective of accelerating the understanding of the complex interactions between cancer and the immune system, and improve treatment options for patients. Better comprehension of immune phenotypes in tumors, beyond the state of PD-L1 and TME, will be relevant to increase immunotherapy efficacy. In this context, the identification of precise tumor antigenicity biomarkers by means of new technologies, such as complete genome sequencing, single cell sequencing, and epigenetic analysis to identify sites or subclones typical in drug resistance, as well as activation, traffic and infiltration of effector cells of the immune response, and regulation of TME mechanisms, may help define patient populations that are good candidates for specific therapies and therapeutic combinations. 117 , 118 Likewise, the use of agents that can induce specific activation and modulation of the response of T cells in tumor tissue, will help improve efficacy and safety profiles that can lead to better clinical results.
Molecular Targeted Therapy
For over 30 years, and based on the progress in our knowledge of tumor biology and its mechanisms, there has been a search for therapeutic alternatives that would allow spread and growth of tumors to be slowed down by blocking specific molecules. This approach is known as molecular targeted therapy. 119 Among the elements generally used as molecular targets there are transcription factors, cytokines, membrane receptors, molecules involved in a variety of signaling pathways, apoptosis modulators, promoters of angiogenesis, and cell cycle regulators. 120
Imatinib, a tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, became the first targeted therapy in the final years of the 1990s. 97 From then on, new drugs have been developed by design, and today more than 60 targeted therapies have been approved by the FDA for the treatment of a variety of cancers ( Table 3 ). 121 This has had a significant impact on progression-free survival and global survival in neoplasms such as non-small cell lung cancer, breast cancer, renal cancer, and melanoma.
FDA Approved Molecular Targeted Therapies for the Treatment of Solid Tumors.
Abbreviations: mAb, monoclonal antibody; ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic lymphocyte antigen-4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stroma tumor; mTOR, target of rapamycine in mammal cells; NSCLC, non-small cell lung carcinoma; PARP, poli (ADP-ribose) polimerase; PD-1, programmed death protein-1; PDGFR, platelet-derived growth factor receptor; PD-L1, programmed death ligand-1; ER, estrogen receptor; PR, progesterone receptor; TKR, tyrosine kinase receptors; SERM, selective estrogen receptor modulator; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Modified from Ref. [ 127 ].
Most drugs classified as targeted therapies form part of 2 large groups: small molecules and mAbs. The former are defined as compounds of low molecular weight (<900 Daltons) that act upon entering the cell. 120 Targets of these compounds are cell cycle regulatory proteins, proapoptotic proteins, or DNA repair proteins. These drugs are indicated based on histological diagnosis, as well as molecular tests. In this group there are multi-kinase inhibitors (RTKs) and tyrosine kinase inhibitors (TKIs), like sunitinib, sorafenib, and imatinib; cyclin-dependent kinase (CDK) inhibitors, such as palbociclib, ribociclib and abemaciclib; poli (ADP-ribose) polimerase inhibitors (PARPs), like olaparib and talazoparib; and selective small-molecule inhibitors, like ALK and ROS1. 122
As for mAbs, they are protein molecules that act on membrane receptors or extracellular proteins by interrupting the interaction between ligands and receptors, in such a way that they reduce cell replication and induce cytostasis. Among the most widely used mAbs in oncology we have: trastuzumab, a drug directed against the receptor for human epidermal growth factor-2 (HER2), which is overexpressed in a subgroup of patients with breast and gastric cancer; and bevacizumab, that blocks vascular endothelial growth factor and is used in patients with colorectal cancer, cervical cancer, and ovarian cancer. Other mAbs approved by the FDA include pembolizumab, atezolizumab, nivolumab, avelumab, ipilimumab, durvalumab, and cemiplimab. These drugs require expression of response biomarkers, such as PD-1 and PD-L1, and must also have several resistance biomarkers, such as the expression of EGFR, the loss of PTEN, and alterations in beta-catenin. 123
Because cancer is such a diverse disease, it is fundamental to have precise diagnostic methods that allow us to identify the most adequate therapy. Currently, basic immunohistochemistry is complemented with neoplastic molecular profiles to determine a more accurate diagnosis, and it is probable that in the near future cancer treatments will be based exclusively on molecular profiles. In this regard, it is worth mentioning that the use of targeted therapy depends on the existence of specific biomarkers that indicate if the patient will be susceptible to the effects of the drug or not. Thus, the importance of underlining that not all patients are susceptible to receive targeted therapy. In certain neoplasms, therapeutic targets are expressed in less than 5% of the diagnosed population, hindering a more extended use of certain drugs.
The identification of biomarkers and the use of new generation sequencing on tumor cells has shown predictive and prognostic relevance. Likewise, mutation analysis has allowed monitoring of tumor clone evolution, providing information on changes in canonic gene sequences, such as TP53, GATA3, PIK3CA, AKT1, and ERBB2; infrequent somatic mutations developed after primary treatments, like SWI-SNF and JAK2-STAT3; or acquired drug resistance mutations such as ESR1. 124 The study of mutations is vital; in fact, many of them already have specific therapeutic indications, which have helped select adequate treatments. 125
There is no doubt that molecular targeted therapy is one of the main pillars of precision medicine. However, it faces significant problems that often hinder obtaining better results. Among these, there is intratumor heterogeneity and differences between the primary tumor and metastatic sites, as well as intrinsic and acquired resistance to these therapies, the mechanisms of which include the presence of heterogeneous subclones, DNA hypermethylation, histone acetylation, and interruption of mRNA degradation and translation processes. 126 Nonetheless, beyond the obstacles facing molecular targeted therapy from a biological and methodological point of view, in the real world, access to genomic testing and specific drugs continues to be an enormous limitation, in such a way that strategies must be designed in the future for precision medicine to be possible on a global scale.
Cell Therapy
Another improvement in cancer treatment is the use of cell therapy, that is, the use of specific cells as therapeutic agents. This clinical procedure has 2 modalities: the first consists of replacing and regenerating functional cells in a specific tissue by means of stem/progenitor cells of a certain kind, 43 while the second uses immune cells as effectors to eliminate malignant cells. 127
Regarding the first type, we must emphasize the development of cell therapy based on hematopoietic stem and progenitor cells. 128 For over 50 years, hematopoietic cell transplants have been used to treat a variety of hematologic neoplasms (different forms of leukemia and lymphoma). Today, it is one of the most successful examples of cell therapy, including innovative modalities, such as haploidentical transplants, 129 as well as application of stem cells expanded ex vivo . 130 There are also therapies that have used immature cells that form part of the TME, such as MSCs. The replication potential and cytokine secretion capacity of these cells make them an excellent option for this type of treatment. 131 Neural stem cells can also be manipulated to produce and secrete apoptotic factors, and when these cells are incorporated into primary neural tumors, they cause a certain degree of regression. They can even be transfected with genes that encode for oncolytic enzymes capable of inducing regression of glioblastomas. 132
With respect to cell therapy using immune cells, several research groups have manipulated cells associated with tumors to make them effector cells and thus improve the efficacy and specificity of the antitumor treatment. PB leckocytes cultured in the presence of IL-2 to obtain activated lymphocytes, in combination with IL-2 administration, have been used in antitumor clinical protocols. Similarly, infiltrating lymphocytes from tumors with antitumor activity have been used and can be expanded ex vivo with IL-2. These lymphocyte populations have been used in immunomodulatory therapies in melanoma, and pancreatic and kidney tumors, producing a favorable response in treated patients. 133 NK cells and macrophages have also been used in immunotherapy, although with limited results. 134 , 135
One of the cell therapies with better projection today is the use of CAR-T cells. This strategy combines 2 forms of advanced therapy: cell therapy and gene therapy. It involves the extraction of T cells from the cancer patient, which are genetically modified in vitro to express cell surface receptors that will recognize antigens on the surface of tumor cells. The modified T cells are then reintroduced in the patient to aid in an exacerbated immune response that leads to eradication of the tumor cells ( Figure 4 ). Therapy with CAR-T cells has been used successfully in the treatment of some types of leukemia, lymphoma, and myeloma, producing complete responses in patients. 136

CAR-T cell therapy. (A) T lymphocytes obtained from cancer patients are genetically manipulated to produce CAR-T cells that recognize tumor cells in a very specific manner. (B) Interaction between CAR molecule and tumor antigen. CAR molecule is a receptor that results from the fusion between single-chain variable fragments (scFv) from a monoclonal antibody and one or more intracellular signaling domains from the T-cell receptor. CD3ζ, CD28 and 4-1BB correspond to signaling domains on the CAR molecule.
Undoubtedly, CAR-T cell therapy has been truly efficient in the treatment of various types of neoplasms. However, this therapeutic strategy can also have serious side effects, such as release of cytokines into the bloodstream, which can cause different symptoms, from high fever to multiorgan failure, and even neurotoxicity, leading to cerebral edema in many cases. 137 Adequate control of these side effects is an important medical challenge. Several research groups are trying to improve CAR-T cell therapy through various approaches, including production of CAR-T cells directed against a wider variety of tumor cell-specific antigens that are able to attack different types of tumors, and the identification of more efficient types of T lymphocytes. Furthermore, producing CAR-T cells from a single donor that may be used in the treatment of several patients would reduce the cost of this sort of personalized cell therapy. 136
Achieving wider use of cell therapy in oncologic diseases is an important challenge that requires solving various issues. 138 One is intratumor cell heterogeneity, including malignant subclones and the various components of the TME, which results in a wide profile of membrane protein expression that complicates finding an ideal tumor antigen that allows specific identification (and elimination) of malignant cells. Likewise, structural organization of the TME challenges the use of cell therapy, as administration of cell vehicles capable of recognizing malignant cells might not be able to infiltrate the tumor. This results from low expression of chemokines in tumors and the presence of a dense fibrotic matrix that compacts the inner tumor mass and avoids antitumor cells from infiltrating and finding malignant target cells.
Further Challenges in the 21st Century
Beyond the challenges regarding oncologic biomedical research, the 21 st century is facing important issues that must be solved as soon as possible if we truly wish to gain significant ground in our fight against cancer. Three of the most important have to do with prevention, early diagnosis, and access to oncologic medication and treatment.
Prevention and Early Diagnosis
Prevention is the most cost-effective strategy in the long term, both in low and high HDI nations. Data from countries like the USA indicate that between 40-50% of all types of cancer are preventable through potentially modifiable factors (primary prevention), such as use of tobacco and alcohol, diet, physical activity, exposure to ionizing radiation, as well as prevention of infection through access to vaccination, and by reducing exposure to environmental pollutants, such as pesticides, diesel exhaust particles, solvents, etc. 74 , 84 Screening, on the other hand, has shown great effectiveness as secondary prevention. Once population-based screening programs are implemented, there is generally an initial increase in incidence; however, in the long term, a significant reduction occurs not only in incidence rates, but also in mortality rates due to detection of early lesions and timely and adequate treatment.
A good example is colon cancer. There are several options for colon cancer screening, such as detection of fecal occult blood, fecal immunohistochemistry, flexible sigmoidoscopy, and colonoscopy, 139 , 140 which identify precursor lesions (polyp adenomas) and allow their removal. Such screening has allowed us to observe 3 patterns of incidence and mortality for colon cancer between the years 2000 and 2010: on one hand, an increase in incidence and mortality in countries with low to middle HDI, mainly countries in Asia, South America, and Eastern Europe; on the other hand, an increase in incidence and a fall in mortality in countries with very high HDI, such as Canada, the United Kingdom, Denmark, and Singapore; and finally a fall in incidence and mortality in countries like the USA, Japan, and France. The situation in South America and Asia seems to reflect limitations in medical infrastructure and a lack of access to early detection, 141 while the patterns observed in developed countries reveal the success, even if it may be partial, of that which can be achieved by well-structured prevention programs.
Another example of success, but also of strong contrast, is cervical cancer. The discovery of the human papilloma virus (HPV) as the causal agent of cervical cancer brought about the development of vaccines and tests to detect oncogenic genotypes, which modified screening recommendations and guidelines, and allowed several developed countries to include the HPV vaccine in their national vaccination programs. Nevertheless, the outlook is quite different in other areas of the world. Eighty percent of the deaths by cervical cancer reported in 2018 occurred in low-income nations. This reveals the urgency of guaranteeing access to primary and secondary prevention (vaccination and screening, respectively) in these countries, or else it will continue to be a serious public health problem in spite of its preventability.
Screening programs for other neoplasms, such as breast, prostate, lung, and thyroid cancer have shown outlooks that differ from those just described, because, among other reasons, these neoplasms are highly diverse both biologically and clinically. Another relevant issue is the overdiagnosis of these neoplasms, that is, the diagnosis of disease that would not cause symptoms or death in the patient. 142 It has been calculated that 25% of breast cancer (determined by mammogram), 50–60% of prostate cancer (determined by PSA), and 13–25% of lung cancer (determined by CT) are overdiagnosed. 142 Thus, it is necessary to improve the sensitivity and specificity of screening tests. In this respect, knowledge provided by the biology of cancer and “omic” sciences offers a great opportunity to improve screening and prevention strategies. All of the above shows that prevention and early diagnosis are the foundations in the fight against cancer, and it is essential to continue to implement broader screening programs and better detection methods.
Global Equity in Oncologic Treatment
Progress in cancer treatment has considerably increased the number of cancer survivors. Nevertheless, this tendency is evident only in countries with a very solid economy. Indeed, during the past 30 years, cancer mortality rates have increased 30% worldwide. 143 Global studies indicate that close to 70% of cancer deaths in the world occur in nations of low to middle income. But even in high-income countries, there are sectors of society that are more vulnerable and have less access to cancer treatments. 144 Cancer continues to be a disease of great social inequality.
In Europe, the differences in access to cancer treatment are highly marked. These treatments are more accessible in Western Europe than in its Eastern counterpart. 145 Furthermore, highly noticeable differences between high-income countries have been detected in the cost of cancer drugs. 146 It is interesting to note that in many of these cases, treatment is too costly and the clinical benefit only marginal. Thus, the importance of these problems being approached by competent national, regional, and global authorities, because if these new drugs and therapeutic programs are not accessible to the majority, progress in biomedical, clinical and epidemiological research will have a limited impact in our fight against cancer. We must not forget that health is a universal right, from which low HDI countries must not be excluded, nor vulnerable populations in nations with high HDI. The participation of a well-informed society will also be fundamental to achieve a global impact, as today we must fight not only against the disease, but also against movements and ideas (such as the anti-vaccine movement and the so-called miracle therapies) that can block the medical battle against cancer.
Final Comments
From the second half of the 20th century to the present day, progress in our knowledge about the origin and development of cancer has been extraordinary. We now understand cancer in detail in genomic, molecular, cellular, and physiological terms, and this knowledge has had a significant impact in the clinic. There is no doubt that a patient who is diagnosed today with a type of cancer has a better prospect than a patient diagnosed 20 or 50 years ago. However, we are still far from winning the war against cancer. The challenges are still numerous. For this reason, oncologic biomedical research must be a worldwide priority. Likewise, one of the fundamental challenges for the coming decades must be to reduce unequal access to health services in areas of low- to middle income, and in populations that are especially vulnerable, as well as continue improving prevention programs, including public health programs to reduce exposure to environmental chemicals and improve diet and physical activity in the general population. 74 , 84 Fostering research and incorporation of new technological resources, particularly in less privileged nations, will play a key role in our global fight against cancer.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Hector Mayani https://orcid.org/0000-0002-2483-3782
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Home > About the AACR > Newsroom > News Release > AACR Cancer Progress Report Details Exciting Advances in Cancer Research and Treatment
- News Releases
- Media Advisories
- Media Contacts
- AACR Fast Facts
- Apply for Embargoed Access
- Media Coverage
- AACR Cancer Progress Report 2023
- AACR Annual Meeting 2023
- AACR Cancer Disparities Progress Report 2022
- AACR Report on COVID-19 and Cancer 2022
- The AACR June L. Biedler Prize for Cancer Journalism
- San Antonio Breast Cancer Symposium 2023
- Press and PIO Registration
- AACR Annual Meeting
- Press Program 2024
- Peer-reviewed Journals
- National Cancer Research Month
- Resources for Cancer Center PIOs and Communications Staff
- AACR Stories Index
- AACR Blog: Cancer Research Catalyst
- Cancer Today Magazine
AACR Cancer Progress Report Details Exciting Advances in Cancer Research and Treatment
Report includes call to action outlining steps congress must take to maintain momentum against cancer for all patients.
PHILADELPHIA – Today, the American Association for Cancer Research (AACR) released the 13th edition of its annual Cancer Progress Report , which chronicles how basic, translational, and clinical cancer research and cancer-related population sciences—primarily supported by federal investments in the National Institutes of Health (NIH) and the National Cancer Institute (NCI)—remain vitally important to improving health and saving lives.
In addition to providing the latest statistics on cancer incidence, mortality, and survivorship, the AACR Cancer Progress Report 2023 offers detailed updates and important context regarding the latest research in cancer etiology, early detection, diagnosis, treatment, prevention, and survivorship. Throughout the report, the personal stories of patients who have benefited from innovative, recently approved anticancer therapeutics highlight the real-world impact of cancer research.
This comprehensive report also features a spotlight on cancer immunotherapy and addresses persistent challenges in cancer research, including cancer disparities, slow progress against certain types of cancer, and the physical, psychosocial, and financial hardships faced by cancer patients, survivors, and their caregivers. A closing call to action outlines steps Congress and other stakeholders must take to ensure that the U.S. maintains its momentum against cancer for the benefit of all patients.
“The advances in cancer research, particularly in the last two decades, have been breathtaking,” said AACR President Philip Greenberg, MD, FAACR, faculty member at Fred Hutchinson Cancer Center. “We are in an era of unparalleled opportunity to make even more breakthroughs for patients. For the cancer research community to achieve these breakthroughs, however, our representatives in Congress must continue to prioritize funding for biomedical research, from basic research to clinical trials. Through the AACR Cancer Progress Report 2023 , we are sharing with the public and policy makers the progress that has been made, how that progress has been delivered to patients, how it’s changed people’s lives, and the unparalleled opportunities that now exist from scientific and technologic advances, so they understand how crucial it is that we maintain this momentum through continued support of NIH and NCI.”
PROMISING TRENDS AND ADVANCES IN CANCER CARE
The medical research community—including researchers in academia and industry, physician-scientists, patient advocates, regulators, and many other stakeholders—has maintained impressive momentum against cancer in recent years. As outlined in the AACR Cancer Progress Report 2023 :
- A new gene therapy-based immunotherapeutic for certain patients with bladder cancer
- A first-in-class antibody drug conjugate for patients with ovarian cancer
- Four new T-cell engaging bispecific antibodies for a range of hematologic malignancies
- The first approval of an immune checkpoint inhibitor for pediatric and adult patients with a rare form of sarcoma
- Due in large part to advances in prevention, early detection, and treatment, the age-adjusted overall cancer death rate in the U.S. fell by 33% between 1991 and 2020— an estimated 3.8 million cancer deaths averted .
- Breast cancer mortality declined by 43% between 1989 and 2020 , leading to an estimated 460,000 fewer breast cancer deaths.
- The decrease in lung cancer mortality has accelerated from 0.9% a year between 1995 and 2005 to nearly 5% a year between 2014 and 2020. This rapid decline is the result of a steep reduction in the U.S. smoking rate as well as the development of numerous highly effective molecularly targeted therapeutics and immunotherapeutics.
- More and better treatment options have led to notable progress against many pediatric cancers as well. Among children (14 and younger) and adolescents (15-19), overall cancer death rates declined by 70% and 64%, respectively, between 1970 and 2020.

THE IMMUNOTHERAPY REVOLUTION
Immunotherapy has revolutionized cancer care. Breakthroughs in this field have contributed to much of the progress noted above, such as declines in the death rates for previously intractable cancers like advanced lung cancer and melanoma. The AACR Cancer Progress Report 2023 contains a spotlight on the history of cancer immunotherapy, the current state of this treatment modality, and the immense promise of the next generation of immunotherapeutics. Highlights include:
- Since 2011, the FDA has approved 11 immune checkpoint inhibitors , which release “brakes” on the surface of certain immune cells—called T cells—so that the T cells are able to destroy cancer cells. Many of these drugs are approved for more than one type of cancer, making immune checkpoint inhibitors a treatment option for 20 cancer types and any tumor with certain specific molecular characteristics.
- Since 2017, the FDA has approved six CAR T-cell therapies to treat a range of hematologic malignancies. CAR T-cell therapy is a type of adoptive cell therapy, which is designed to dramatically increase the number of cancer-killing immune cells a patient has.
- The field is expanding in exciting ways, with researchers combining the power of other cells in the immune system with recent advances in gene editing to develop more personalized and effective versions of adoptive cell therapy for treatment of solid tumors; developing mRNA-based vaccines and therapeutics to treat cancer; and targeting the gut microbiome to increase the efficacy of cancer immunotherapy, among many other innovative approaches.
DESPITE PROGRESS, CHALLENGES PERSIST
Despite the extraordinary scientific progress against cancer in recent years, this complex disease remains a significant threat to human health around the world. In the U.S., it is estimated that nearly 2 million new cases of cancer will be diagnosed and more than 609,000 people will die from the disease in 2023.
Indeed, cancer research and patient care face numerous challenges, as outlined in the AACR Cancer Progress Report 2023 :
- Cancer disparities are a pervasive public health problem, with racial and ethnic minorities and other medically underserved U.S. populations shouldering a disproportionally higher burden of cancer. While advances have been made in identifying, understanding, and addressing some of these disparities, more research and policy solutions are urgently needed to ensure equitable progress against cancer.
- There has been uneven progress against different cancer types. Few treatment options exist for patients diagnosed with pancreatic cancer or glioblastoma, for example, and 5-year relative survival rates for these cancers are extremely low.
- Incidence rates for some cancers are increasing, including for early-onset colorectal cancer, pancreatic cancer, and uterine cancer, in part due to the rising rate of obesity.
- Financial toxicity is widespread, exacerbated by the rising cost of cancer care. In 2019, U.S. cancer patients paid an estimated $16.2 billion in out-of-pocket cancer care costs and lost an additional $5 billion in “time costs.”
FEDERAL FUNDING ESSENTIAL FOR CONTINUED PROGRESS
To confront these and other challenges, the AACR Cancer Progress Report 2023 calls on Congress to support robust, sustained, and predictable annual funding growth for NIH and NCI by providing increases of at least $3.465 billion and $2.6 billion, respectively, in their fiscal year 2024 base budgets. This funding is crucial to continued progress for patients. From 2010 to 2019, NIH funding contributed to the development of 354 out of 356 new drugs, including many cancer drugs, approved by the FDA.
The AACR also urges Congress to:
- Provide $1.7 billion in dedicated funding for Cancer Moonshot activities in FY 2024 across NCI, FDA, and Centers for Disease Control and Prevention (CDC) with the assurance that Moonshot funding will supplement rather than supplant NIH funding in FY 2024.
- Appropriate at least $472.4 million in FY 2024 appropriations for the CDC Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $50 million in funding for the Oncology Center of Excellence at FDA in FY 2024 to allow regulators with the capable staff and necessary tools to conduct expedited review of cancer-related medical products.
“We are proud to release the 13 th annual AACR Cancer Progress Report ,” said AACR CEO Margaret Foti, PhD, MD (hc). “It is our hope that this comprehensive resource will help to increase knowledge about the myriad diseases we call cancer as well as the innovative research that is improving and extending lives. The findings in this report, along with the personal stories of the featured patients, underscore the enormous impact that robust, sustained, and predictable funding for cancer research has had on Americans’ health, and why that support must continue.”
- A Message from the AACR
- Molecular Biology in Clinical Oncology Workshop
10 new breakthroughs in the fight against cancer

Medical advances are continuing to help the world fight cancer. Image: Unsplash/National Cancer Institute
.chakra .wef-1c7l3mo{-webkit-transition:all 0.15s ease-out;transition:all 0.15s ease-out;cursor:pointer;-webkit-text-decoration:none;text-decoration:none;outline:none;color:inherit;}.chakra .wef-1c7l3mo:hover,.chakra .wef-1c7l3mo[data-hover]{-webkit-text-decoration:underline;text-decoration:underline;}.chakra .wef-1c7l3mo:focus,.chakra .wef-1c7l3mo[data-focus]{box-shadow:0 0 0 3px rgba(168,203,251,0.5);} Victoria Masterson
Madeleine north.

.chakra .wef-9dduvl{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-9dduvl{font-size:1.125rem;}} Explore and monitor how .chakra .wef-15eoq1r{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;color:#F7DB5E;}@media screen and (min-width:56.5rem){.chakra .wef-15eoq1r{font-size:1.125rem;}} Global Health is affecting economies, industries and global issues

.chakra .wef-1nk5u5d{margin-top:16px;margin-bottom:16px;line-height:1.388;color:#2846F8;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-1nk5u5d{font-size:1.125rem;}} Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:, global health.
Listen to the article
This article was originally published in May 2022, and most recently updated in January 2024 .
- Cancer is one of the world’s biggest killers, with around 10 million deaths per year due to the disease.
- Scientists are using artificial intelligence, DNA sequencing, precision oncology and other technologies to improve treatment and diagnosis.
- The Centre for the Fourth Industrial Revolution India, a collaboration with the World Economic Forum, hopes to accelerate 18 cancer interventions.
Cancer kills around 10 million people a year and is a leading cause of death globally, according to the World Health Organization.
Breast, lung and colon cancer are among the most common. Death rates from cancer were falling before the pandemic . But COVID-19 caused a big backlog in diagnosis and treatment .
There is some good news, however. Medical advances are accelerating the battle against cancer. Here are 10 recent developments.
Test to identify 18 early-stage cancers
Researchers in the US have developed a test they say can identify 18 early-stage cancers. Instead of the usual invasive and costly methods, Novelna's test works by analyzing a patient's blood protein. In a screening of 440 people already diagnosed with cancer, the test correctly identified 93% of stage 1 cancers in men and 84% in women. The researchers believe the findings "pave the way for a cost-effective, highly accurate, multi-cancer screening test that can be implemented on a population-wide scale". It's early days, however. With such a small sample screening and a lack of information on co-existing conditions, the test is currently more of "a starting point for developing a new generation of screening tests for the early detection of cancer".
The seven-minute cancer treatment jab
England's National Health Service (NHS) is to be the first in the world to make use of a cancer treatment injection , which takes just seven minutes to administer, rather than the current time of up to an hour to have the same drug via intravenous infusion. This will not only speed up the treatment process for patients, but also free up time for medical professionals. The drug, Atezolizumab or Tecentriq, treats cancers including lung and breast, and it's expected most of the 3,600 NHS patients in England currently receiving it intravenously will now switch to the jab.
Precision oncology
Precision oncology is the “ best new weapon to defeat cancer ”, the chief executive of Genetron Health, Sizhen Wang, says in a blog for the World Economic Forum. This involves studying the genetic makeup and molecular characteristics of cancer tumours in individual patients. The precision oncology approach identifies changes in cells that might be causing the cancer to grow and spread. Personalized treatments can then be developed. The 100,000 Genomes Project, a National Health Service initiative, studied more than 13,000 tumour samples from UK cancer patients , successfully integrating genomic data to more accurately pin-point effective treatment. Because precision oncology treatments are targeted – as opposed to general treatments like chemotherapy – it can mean less harm to healthy cells and fewer side effects as a result.
Artificial intelligence fights cancer
In India, World Economic Forum partners are using emerging technologies like artificial intelligence (AI) and machine learning to transform cancer care. For example, AI-based risk profiling can help screen for common cancers like breast cancer, leading to early diagnosis. AI technology can also be used to analyze X-rays to identify cancers in places where imaging experts might not be available. These are two of 18 cancer interventions that The Centre for the Fourth Industrial Revolution India, a collaboration with the Forum , hopes to accelerate.

Greater prediction capabilities
Lung cancer kills more people in the US yearly than the next three deadliest cancers combined. It's notoriously hard to detect the early stages of the disease with X-rays and scans alone. However, MIT scientists have developed an AI learning model to predict a person's likelihood of developing lung cancer up to six years in advance via a low-dose CT scan. Trained using complex imaging data, 'Sybil' can forecast both short- and long-term lung cancer risk, according to a recent study. "We found that while we as humans couldn't quite see where the cancer was, the model could still have some predictive power as to which lung would eventually develop cancer," said co-author Jeremy Wohlwend.
Clues in the DNA of cancer
At Cambridge University Hospitals in England, the DNA of cancer tumours from 12,000 patients is revealing new clues about the causes of cancer, scientists say. By analyzing genomic data, oncologists are identifying different mutations that have contributed to each person’s cancer. For example, exposure to smoking or UV light, or internal malfunctions in cells. These are like “fingerprints in a crime scene”, the scientists say – and more of them are being found. “We uncovered 58 new mutational signatures and broadened our knowledge of cancer,” says study author Dr Andrea Degasperi, from Cambridge’s Department of Oncology.
Liquid and synthetic biopsies
Biopsies are the main way doctors diagnose cancer – but the process is invasive and involves removing a section of tissue from the body, sometimes surgically, so it can be examined in a laboratory. Liquid biopsies are an easier and less invasive solution where blood samples can be tested for signs of cancer. Synthetic biopsies are another innovation that can force cancer cells to reveal themselves during the earliest stages of the disease.
The application of “precision medicine” to save and improve lives relies on good-quality, easily-accessible data on everything from our DNA to lifestyle and environmental factors. The opposite to a one-size-fits-all healthcare system, it has vast, untapped potential to transform the treatment and prediction of rare diseases—and disease in general.
But there is no global governance framework for such data and no common data portal. This is a problem that contributes to the premature deaths of hundreds of millions of rare-disease patients worldwide.
The World Economic Forum’s Breaking Barriers to Health Data Governance initiative is focused on creating, testing and growing a framework to support effective and responsible access – across borders – to sensitive health data for the treatment and diagnosis of rare diseases.
The data will be shared via a “federated data system”: a decentralized approach that allows different institutions to access each other’s data without that data ever leaving the organization it originated from. This is done via an application programming interface and strikes a balance between simply pooling data (posing security concerns) and limiting access completely.
The project is a collaboration between entities in the UK (Genomics England), Australia (Australian Genomics Health Alliance), Canada (Genomics4RD), and the US (Intermountain Healthcare).
CAR-T-cell therapy
A treatment that makes immune cells hunt down and kill cancer cells was recently declared a success for leukaemia patients. The treatment, called CAR-T-cell therapy, involves removing and genetically altering immune cells, called T cells, from cancer patients. The altered cells then produce proteins called chimeric antigen receptors (CARs). These recognize and can destroy cancer cells. In the journal Nature, scientists at the University of Pennsylvania announced that two of the first people treated with CAR-T-cell therapy were still in remission 12 years on.
Fighting pancreatic cancer
Pancreatic cancer is one of the deadliest cancers. It is rarely diagnosed before it starts to spread and has a survival rate of less than 5% over five years. At the University of California San Diego School of Medicine, scientists developed a test that identified 95% of early pancreatic cancers in a study. The research, published in Nature Communications Medicine , explains how biomarkers in extracellular vesicles – particles that regulate communication between cells – were used to detect pancreatic, ovarian and bladder cancer at stages I and II.
Have you read?
Cancer: how to stop the next global health crisis, how to improve access to cancer medicines in low and middle-income countries, why is cancer becoming more common among millennials, a tablet to cut breast cancer risk.
A drug that could halve the chance of women developing breast cancer is being tested out by England's National Health Service (NHS). It will be made available to almost 300,000 women seen as being at most risk of developing breast cancer, which is the most common type of cancer in the UK . The drug, named anastrozole, cuts the level of oestrogen women produce by blocking the enzyme aromatase . It has already been used for many years as a breast cancer treatment but has now been repurposed as a preventive medicine. “This is the first drug to be repurposed through a world-leading new programme to help us realize the full potential of existing medicines in new uses to save and improve more lives on the NHS," says NHS Chief Executive Amanda Pritchard.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
The Agenda .chakra .wef-n7bacu{margin-top:16px;margin-bottom:16px;line-height:1.388;font-weight:400;} Weekly
A weekly update of the most important issues driving the global agenda
.chakra .wef-1dtnjt5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;} More on Health and Healthcare Systems .chakra .wef-nr1rr4{display:-webkit-inline-box;display:-webkit-inline-flex;display:-ms-inline-flexbox;display:inline-flex;white-space:normal;vertical-align:middle;text-transform:uppercase;font-size:0.75rem;border-radius:0.25rem;font-weight:700;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;line-height:1.2;-webkit-letter-spacing:1.25px;-moz-letter-spacing:1.25px;-ms-letter-spacing:1.25px;letter-spacing:1.25px;background:none;padding:0px;color:#B3B3B3;-webkit-box-decoration-break:clone;box-decoration-break:clone;-webkit-box-decoration-break:clone;}@media screen and (min-width:37.5rem){.chakra .wef-nr1rr4{font-size:0.875rem;}}@media screen and (min-width:56.5rem){.chakra .wef-nr1rr4{font-size:1rem;}} See all

Equitable healthcare is the industry's north star. Here's how AI can get us there
Vincenzo Ventricelli
April 25, 2024

Bird flu spread a ‘great concern’, plus other top health stories
Shyam Bishen
April 24, 2024

This Earth Day we consider the impact of climate change on human health
Shyam Bishen and Annika Green
April 22, 2024

Scientists have invented a method to break down 'forever chemicals' in our drinking water. Here’s how
Johnny Wood
April 17, 2024

Young people are becoming unhappier, a new report finds

Promoting healthy habit formation is key to improving public health. Here's why
Adrian Gore
April 15, 2024

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Cancer research highlights from 2023
Share this:.
By Mayo Clinic staff
Researchers at Mayo Clinic Comprehensive Cancer Center spent 2023 studying the biology of cancer and new ways to predict, prevent, diagnose and treat the disease. Their discoveries are creating hope and transforming the quality of life for people with cancer today and in the future. Here are some highlights from their research over the past year:
Mayo Clinic researchers link ovarian cancer to bacteria colonization in the microbiome.
A specific colonization of microbes in the reproductive tract is commonly found in people with ovarian cancer, according to a study from the Mayo Clinic Center for Individualized Medicine . Published in Scientific Reports and led by Marina Walther-Antonio, Ph.D. , a Mayo Clinic researcher, and Abigail Asangba, Ph.D., the discovery strengthens the evidence that the bacterial component of the microbiome — a community of microorganisms that also consists of viruses, yeasts and fungi — is an important indicator for early detection, diagnosis and prognosis of ovarian cancer . The study also suggests that a higher accumulation of pathogenic microbes plays a role in treatment outcomes and could be a potential indicator for predicting a patient's prognosis and response to therapy. Read more .
Artificial intelligence is forging a new future for colorectal cancer and other digestive system diseases.
Colonoscopy remains the gold standard in detecting and preventing colorectal cancer , but the procedure has limitations. Some studies suggest that more than half of post-colonoscopy colon cancer cases arise from lesions missed at patients' previous colonoscopies. In 2022, Michael Wallace, M.D. , a Mayo Clinic gastroenterologist, published the results of an international, multicenter study testing the impact of adding artificial intelligence (AI) to routine colonoscopies. His team, including James East, M.D. , a Mayo Clinic gastroenterologist, and other researchers from the U.S., the U.K., Italy, Germany and Ireland, found that incorporating AI into colonoscopies reduced the risk of missing polyps by 50%. Read more .
A big step forward: Bringing DNA sequencing data to routine patient care.
The Tapestry study , an extensive genomic sequencing clinical research study, aims to complete exome sequencing (sequencing the protein-coding regions of a genome) for 100,000 Mayo Clinic patients. The results will be integrated into patients’ electronic health records for three hereditary conditions, and the amassed data will contribute to a research dataset stored within the Mayo Clinic Cloud on the Omics Data Platform. The overall hope of Tapestry is to accelerate discoveries in individualized medicine to tailor prevention, diagnosis and treatment to a patient's unique genetic makeup. It is poised to advance evidence that exome sequencing, when applied to a diverse and comprehensive general population, can proficiently identify carriers of genetic variants that put them at higher risk for a disease, allowing them to take preventive measures. Read more .
Patients with multiple tumors in one breast may not need a mastectomy.
Patients who have multiple tumors in one breast may be able to avoid a mastectomy if surgeons can remove the tumors while leaving enough breast tissue, according to research led by the Alliance in Clinical Trials in Oncology and Mayo Clinic Comprehensive Cancer Center . Patients would receive breast-conserving therapy — a lumpectomy followed by whole-breast radiation therapy — rather than mastectomy . The study is published in the Journal of Clinical Oncology . Historically, women with multiple tumors in one breast have been advised to have a mastectomy. Now, patients can be offered a less invasive option with faster recovery, resulting in better patient satisfaction and cosmetic outcomes, says Judy Boughey, M.D. , lead author, Mayo Clinic breast surgical oncologist and the W.H. Odell Professor of Individualized Medicine. Read more .
Staging pancreatic cancer early with minimally invasive surgery shows positive results in patient prognosis.
A study published in the Journal of the American College of Surgeons reveals that performing a minor surgical procedure on patients newly diagnosed with pancreatic cancer helps to identify cancer spread early and determine the stage of cancer. The researchers add that the surgery ideally should be performed before the patient begins chemotherapy. "This is an important study because it supports that staging laparoscopy may help determine a patient's prognosis and better inform treatment so that patients avoid unhelpful or potentially harmful surgical therapy," says Mark Truty, M.D. , a Mayo Clinic surgical oncologist who led the research. Read more .
Mayo Clinic study reveals proton beam therapy may shorten breast cancer treatment.
In a trial published in The Lancet Oncology , Mayo Clinic Comprehensive Cancer Center researchers uncovered evidence supporting a shorter treatment time for people with breast cancer . The study compared two separate dosing schedules of pencil-beam scanning proton therapy , known for its precision in targeting cancer cells while preserving healthy tissue to reduce the risk of side effects. The investigators found that both 25-day and 15-day proton therapy schedules resulted in excellent cancer control while sparing surrounding non-cancerous tissue. Further, complication rates were comparable between the two study groups. "We can now consider the option of 15 days of therapy for patients based on the similar treatment outcomes observed," says Robert Mutter, M.D. , a Mayo Clinic radiation oncologist and physician-scientist. Read more .
Harnessing the immune system to fight ovarian cancer.
Mayo Clinic research is biomanufacturing an experimental, cell-based ovarian cancer vaccine and combining it with immunotherapy to study a "one-two punch" approach to halting ovarian cancer progression. This research begins with a blood draw from people with advanced ovarian cancer whose tumors have returned after standard surgery and chemotherapy. White blood cells are extracted from the blood, biomanufactured to become dendritic cells and returned to the patient. Dendritic cells act as crusaders that march through the body, triggering the immune system to recognize and fight cancer. "We're building on an earlier phase 1 clinical trial that showed promising results in terms of survival after the dendritic cell-based vaccine," says Matthew Block, M.D., Ph.D. , co-principal investigator and Mayo Clinic medical oncologist. "Of the 18 evaluable patients in the phase 1 study, 11 had cancer return, but seven of them — 40% — have been cancer-free for almost 10 years. We typically expect 90% of patients in this condition to have the cancer return." Read more .
New gene markers detect Lynch syndrome-associated colorectal cancer.
Researchers from Mayo Clinic Comprehensive Cancer Center and Mayo Clinic Center for Individualized Medicine have discovered new genetic markers to identify Lynch syndrome-associated colorectal cancer with high accuracy. Studies are underway to determine if these genetic markers are in stool samples and, if so, how this could lead to a non-invasive screening option for people with Lynch syndrome . The research was published in Cancer Prevention Research , a journal of the American Association for Cancer Research. "This is an exciting finding that brings us closer to the reality that clinicians may soon be able to offer a non-invasive cancer screening option to patients with the highest risk of getting cancer," says Jewel Samadder, M.D. , co-lead author of the paper and a Mayo Clinic gastroenterologist. Read more .
Mayo Clinic prepares to biomanufacture a new CAR-T cell therapy for B-cell blood cancers.
Mayo Clinic research has developed a new type of chimeric antigen receptor-T cell therapy (CAR-T cell therapy) aimed at killing B-cell blood cancers that have returned and are no longer responding to treatment. This pioneering technology, designed and developed in the lab of Hong Qin, M.D., Ph.D. , a Mayo Clinic cancer researcher, killed B-cell tumors grown in the laboratory and tumors implanted in mouse models. The preclinical findings are published in Cancer Immunology, Immunotherapy . "This study shows our experimental CAR-T cell therapy targets several blood cancers, specifically chronic lymphocytic leukemia," says Dr. Qin. "Currently, there are six different CAR-T cell therapies approved for treatment of relapsed blood cancers. While the results are impressive, not everyone responds to this treatment. Our goal is to provide novel cell therapies shaped to each patient's individual need." Read more .
Related Posts

People with early-stage triple-negative breast cancer and high levels of immune cells in their tumors may have a lower risk of recurrence and better survival rates.

Richard Vile, Ph.D., is leading research into genetically engineered viruses called oncolytic viruses for cancer treatment at Mayo Clinic.

After receiving CAR-T cell therapy at Mayo Clinic, Welsh-born John Cadwallader achieved remission and found new hope. He now receives care in the U.K. and is monitored by Mayo in the U.S. and London.

Search form
Asco family of sites.
- ASCO Connection
- ASCO Daily News
- ASCO Journals
- ASCO Practice Central
- ASCO Education
- Cancer.Net - For Patients
- Conquer Cancer
- TAPUR Study
- The ASCO Post

ASCOconnection.org features blogs from members, the online version of the membership magazine, a discussion area, working groups, and links to the Membership Directory, Career Center, and Volunteer Portal.

ASCO Daily News is the official conference reporter for ASCO meetings and symposia, providing high-quality, unbiased research summaries and oncology news to members and oncology health care providers.

ASCO’s growing roster of cutting-edge journals serves readers as the most credible, authoritative, peer-reviewed resources for significant clinical oncology research and research that informs the delivery of efficient, high-quality cancer care across the globe.

ASCO Practice Central helps oncology professionals navigate a complicated and ever-changing practice environment—while providing high-quality patient care.

Stay updated on the latest oncology has to offer with ASCO Education—your online, on-demand resource for timely information, real-world application, and practice-changing care.

A cutting-edge health information technology platform, CancerLinQ® enables practitioners to learn from individual patients. By assembling vast amounts of usable, searchable, real-world data, CancerLinQ seeks to improve the quality and value of cancer care.

Cancer.Net brings the expertise and resources of ASCO to people living with cancer and those who care for and about them to help patients and families make informed health care decisions.

Conquer Cancer, the ASCO Foundation, raises funds to support the world's leading researchers who are improving treatments and discovering cures for every cancer, every patient, everywhere.

ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) Study is a non-randomized clinical trial aiming to describe the performance of commercially available, targeted anticancer drugs prescribed for treatment of patients with advanced cancer with a potentially actionable genomic variant.

The ASCO Post, in partnership with the American Society of Clinical Oncology, communicates news of the highest quality multidisciplinary cancer care to a broad audience of oncology professionals and members.
News Releases
New cancer research impacting treatment, prevention, early diagnosis, and quality of life to be presented at the 2024 asco annual meeting in chicago.
ALEXANDRIA, Va. – The global oncology community will gather in Chicago to share and discuss the latest clinical cancer research impacting patient care at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. Treatment advances involving targeted therapies, immunotherapy, and new uses of technology, as well as research on improving patient quality of life and outcomes are among the topics that will be highlighted in the meeting’s official Press Program.
The 2024 ASCO Annual Meeting will take place May 31-June 4 in Chicago and online. The theme of this year’s conference is The Art and Science of Cancer Care: From Comfort to Cure.
This year, ASCO received a record number of abstract submissions and as a result more than 5,000 abstracts will be presented or published as part of the 2024 ASCO Annual Meeting. ASCO will publish the regular abstracts on Thursday, May 23, at 5:00 PM (ET) on asco.org/abstracts . Late-Breaking Abstracts (LBAs), including Plenary abstracts, will be released at 7:00 AM (CT)/8:00 AM (ET) on their day of scientific presentation at the Meeting. The complete embargo schedule is available online .
EMBARGOED PRE-MEETING PRESS BRIEFING (VIRTUAL EVENT) – Thursday, May 23, 12:00-1:30 PM (ET)
In advance of the release of regular abstracts on May 23, an embargoed, virtual press briefing for credentialed media will be held earlier the same day and will highlight four abstracts. The embargo will lift on May 23 at 5:00 PM (ET) for the following studies:
- Using artificial intelligence to re-engage communities of color who have missed colonoscopy appointments (Abstract 100)
- A prospective study examining long-term fertility outcomes among younger breast cancer survivors. (Abstract 1518)
- Lessons from a prospective trial examining treatment-induced neuropathy in Black women with breast cancer (Abstract 503)
- Impact of HPV vaccination on cancer rates beyond cervical cancer, among both men and women (Abstract 10507)
EMBARGOED PRE-MEETING PRESS BRIEFING (VIRTUAL EVENT) – Wednesday, May 29, 8:30-10:00 AM (ET)
Several Late-Breaking Abstracts (LBAs) being presented on Friday, May 31 and Saturday, June 1 at the meeting, will be discussed in an embargoed, virtual press briefing for credentialed media on May 29:
- Five-year survival and safety outcomes from the phase III CROWN study comparing lorlatinib to crizotinib in treatment-naïve patients with advanced ALK + non-small cell lung cancer. (Abstract LBA8503) Embargo lifts Friday, May 31 at 7:00 AM (CT)/8:00 AM (ET)
- The primary results from the ASC4FIRST study, a randomized phase III trial looking at asciminib compared to investigator-selected tyrosine kinase inhibitors in newly diagnosed patients with chronic myeloid leukemia. (Abstract LBA6500) Embargo lifts Friday, May 31 at 7:00 AM (CT)/8:00 AM (ET)
- Results from the phase III GHSG HD21 study, examining tolerability and efficacy of BrECADD compared to BEACOPP in advanced stage classical Hodgkin lymphoma. (Abstract LBA7000) Embargo lifts Saturday, June 1 at 7:00 AM (CT)/8:00 AM (ET)
RESEARCH TO BE RELEASED DURING THE ANNUAL MEETING
During the Annual Meeting, two press briefings will be held on Saturday, June 1: one from 8:00-9:15 AM (CT) highlighting research from ASCO’s Plenary Session and one from 12:30-1:30 PM (CT) highlighting additional LBAs that will be presented during the meeting. The press briefings will be held in person and livestreamed for credentialed reporters covering the meeting remotely.
LBAs that will be featured in these press briefings include:
- The prospective phase III ESOPEC trial comparing perioperative chemotherapy to neoadjuvant chemoradiation in patients with esophageal cancer. (Plenary, Abstract LBA1) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
- The phase III NADINA trial studying neoadjuvant nivolumab plus ipilimumab compared to adjuvant nivolumab in melanoma. (Plenary, Abstract LBA2) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
- Trial evaluating the effectiveness of early palliative care delivered via telehealth compared to in-person for patients with advanced lung cancer. (Plenary, Abstract LBA3) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
- Primary results of the phase 3 LAURA study examining osimertinib after chemoradiotherapy in patients with unresectable stage III epidermal growth factor receptor-mutated non-small cell lung cancer. (Plenary, Abstract LBA4) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
- The phase III ADRIATIC study evaluating durvalumab with or without tremelimumab as in patients with limited-stage small-cell lung cancer. (Plenary, LBA5) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
- The randomized phase III DREAMM-8 study of belantamab mafodotin plus pomalidomide and dexamethasone compared to pomalidomide plus bortezomib and dexamethasone in relapsed or refractory multiple myeloma. (Abstract LBA105) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
- The CARACO phase III randomized trial, studying the omission of lymphadenectomy in patients with advanced ovarian cancer treated with cytoreductive surgery after neoadjuvant chemotherapy. (Abstract LBA5505) Embargo lifts Sunday, June 2 at 7:00 AM (CT)/8:00 AM (ET)
All press briefings will also be recorded and made available to credentialed reporters in ASCO’s Media Headquarters.
ADDITIONAL STUDIES OF NOTE
The following studies will be included in the official ASCO Tip Sheet, a brief summary of additional noteworthy research. The Tip Sheet will be sent to credentialed reporters ahead of the meeting and will include additional comments from the lead authors and ASCO experts.
The embargo will lift Thursday, May 23 at 5:00 PM (ET) for the following studies:
- Addition of the MUC-1 vaccine tecemotide to neoadjuvant systemic therapy for patients with early breast cancer: Survival results from the prospective randomized ABCSG 34 trial. (Abstract 587)
- Trends in human papilloma virus vaccination uptake by sex and race/ethnicity among US adolescents and young adults, 2011-2020. (Abstract 10519)
- Phase 3 study results of isatuximab, bortezomib, lenalidomide, and dexamethasone (Isa-VRd) versus VRd for transplant-ineligible patients with newly diagnosed multiple myeloma (IMROZ). (Abstract 7500)
- Chemotherapy and liver transplantation versus chemotherapy alone in patients with definitively unresectable colorectal liver metastases: A prospective multicentric randomized trial (TRANSMET). (Abstract 3500)
- A phase 1 study of JNJ-69086420 (JNJ-6420), an actinium-225 (225Ac) -labeled antibody targeting human kallikrein 2 (hK2), for metastatic castration-resistant prostate cancer (mCRPC). (Abstract 5010).
- Evolution of tisagenlecleucel use for the treatment of pediatric and young adult relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL): Center for International Blood & Marrow Transplant Research (CIBMTR) registry results. (Abstract 10016).
- Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: Overall survival and updated 5-year results from the randomized DYNAMIC trial. (Abstract 108).
- The potential role of serial circulating tumor DNA (ctDNA) testing after upfront surgery to guide adjuvant chemotherapy for early stage pancreatic cancer: The AGITG DYNAMIC-Pancreas trial. (Abstract 107).
- Sacituzumab tirumotecan (SKB264/MK-2870) in patients (pts) with previously treated locally recurrent or metastatic triple-negative breast cancer (TNBC): Results from the phase III OptiTROP-Breast01 study. (Abstract 104)
- Survival outcomes associated with first-line PCV or temozolomide in combination with radiotherapy in IDH-mutant 1p/19q-codeleted grade 3 oligodendroglioma. (Abstract 2004)
- First-line inavolisib/placebo + palbociclib + fulvestrant (Inavo/Pbo+Palbo+Fulv) in patients (pts) with PIK3CA-mutated, hormone receptor-positive, HER2‑negative locally advanced/metastatic breast cancer who relapsed during/within 12 months (mo) of adjuvant endocrine therapy completion: INAVO120 Phase III randomized trial additional analyses. (Abstract 1003).
- Circulating kidney injury molecule-1 (KIM-1) biomarker analysis in IMmotion010: A randomized phase 3 study of adjuvant (adj) atezolizumab (atezo) vs placebo (pbo) in patients (pts) with renal cell carcinoma (RCC) at increased risk of recurrence after resection. (Abstract 4506)
- Multi-site randomized trial of stepped palliative care (PC) for patients with advanced lung cancer. ( Abstract 12000)
- A randomized phase II study of gemcitabine and nab-paclitaxel compared with 5-fluorouracil, leucovorin, and liposomal irinotecan in older patients with treatment-naïve metastatic pancreatic cancer (GIANT): ECOG-ACRIN EA2186. (Abstract 4003)
The embargo will lift Friday, May 31 at 7:00 AM (CT)/8:00 AM (ET) for the following study :
- Durable complete responses to PD-1 blockade alone in mismatch repair deficient locally advanced rectal cancer. (Abstract LBA3512)
The embargo will lift Saturday, June 1 at 7:00 AM (CT)/8:00 AM (ET) for the following studies:
- KRYSTAL-12: Phase 3 study of adagrasib versus docetaxel in patients with previously treated advanced/metastatic non-small cell lung cancer (NSCLC) harboring a KRAS G12C mutation. (LBA8509)
- Abemaciclib plus fulvestrant vs fulvestrant alone for HR+, HER2- advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: Primary outcome of the phase 3 postMONARCH trial (LBA1001)
The embargo will lift Monday, June 3 at 7:00 AM (CT)/8:00 AM (ET) for the following studies :
- Subcutaneous amivantamab vs intravenous amivantamab, both in combination with lazertinib, in refractory EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results, including overall survival (OS), from the global, phase 3, randomized controlled PALOMA-3 trial. (Abstract LBA8505)
- IMpower010: Final disease-free survival (DFS) and second overall survival (OS) interim results after ≥5 years of follow up of a phase III study of adjuvant atezolizumab vs best supportive care in resected stage IB-IIIA non-small cell lung cancer (NSCLC). (LBA8035)
MEDIA RESOURCES
Media registration : To participate in the Annual Meeting press briefings and/or register to attend the Meeting in person in Chicago, please visit ASCO’s Media Headquarters at https://profile.asco.org/media/workflow . Step-by-step instructions are available online .
Requests for media credentials must be submitted in Media Headquarters no later than Wednesday, May 15, to participate in the pre-meeting press briefing on May 23. Pre-registration is required for on-site attendance and must also be requested by this date.
Annual Meeting Media Resource Center : Visit asco.org/AMMRC for press releases, the press briefing schedule, embargo policies, high-resolution photos, and the Virtual Press Room (an online repository of corporate and institutional press materials from third-party organizations).
About ASCO :
Founded in 1964, the American Society of Clinical Oncology, Inc. (ASCO®) is committed to the principle that knowledge conquers cancer. Together with the Association for Clinical Oncology, ASCO represents nearly 50,000 oncology professionals who care for people living with cancer. Through research, education, and promotion of high quality, equitable patient care, ASCO works to conquer cancer and create a world where cancer is prevented or cured, and every survivor is healthy. Conquer Cancer, the ASCO Foundation, supports ASCO by funding groundbreaking research and education across cancer’s full continuum. Learn more at www.ASCO.org , explore patient education resources at www.Cancer.Net , and follow us on Facebook , Twitter , LinkedIn , Instagram , and YouTube .
Related News Releases
Related tags.
- Meetings News
- Events Calendar /calendar Events Calendar
- Research & Data ASCO Journals TAPUR Study CDK4/6 Inhibitor Dosing Knowledge (CDK) Study Clinical Trials ASCO Data Library Reports & Studies Research Survey Pool Interactive Map of Oncology /research-data Research & Data
- Guidelines /practice-patients/guidelines Guidelines
- Grants & Awards /career-development/grants-awards Grants & Awards
- Cancer Progress Timeline /news-initiatives/cancer-progress-timeline Cancer Progress Timeline
- Volunteering & Committees Apply to Volunteer FASCO Distinction International Cancer Corps Policies for Volunteers & Committee Members Regional Councils /get-involved/volunteering-committees/volunteer-asco Volunteering & Committees
- Discussion Groups https://myconnection.asco.org Discussion Groups
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
After 40 years of smoking, she survived lung cancer thanks to new treatments

Yuki Noguchi

Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission. Denise Lee hide caption
Denise Lee on her last day of chemo. In addition to chemo and surgery, she was treated with immunotherapy. She's currently in remission.
Denise Lee grew up in Detroit in the mid-1970s and went to an all-girls Catholic high school. She smoked her first cigarette at age 14 at school, where cigarettes were a popular way of trying to lose weight.
Instead, her nicotine addiction lasted four decades until she quit in her mid-50s.
"At some point it got up as high as 2.5 packs a day," Lee, 62, recalls.
Yet she didn't think about lung cancer risk — until she saw a billboard urging former smokers to get screened. Lee, a retired lawyer living in Fremont, Calif., used to drive past it on her way to work.
"The thing that caught my attention was the fact that it was an African American female on the front," she recalls.

Shots - Health News
The american cancer society says more people should get screened for lung cancer.
She eventually got the low-dose CT scan recommended for current and former smokers. When doctors found an early, but dangerous, tumor, Lee cried and panicked. Her mother had cared for her father, who'd died of prostate cancer. "My biggest concern was telling my mom," she says.
But that was six years ago, and Lee is cancer free today. Surgery removed the 2-inch tumor in her lung, then new treatments also boosted her immune system, fighting off any recurrence.
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year – more than breast, prostate and colon cancer combined – which is why many people still think of a diagnosis as synonymous with a death sentence. But with new treatments and technology, the survival rates from lung cancer are dramatically improving, allowing some patients with relatively late-stage cancers to live for years longer.
"If you're gonna have lung cancer, now is a good time," Lee says of the advances that saved her.

Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective. Denise Lee hide caption
Denise Lee has been cancer-free for six years. She says she's grateful she got screened and caught her lung cancer early enough that treatment has been effective.
The key breakthrough, says Robert Winn, a lung cancer specialist at Virginia Commonwealth University, is the ability to better pinpoint the mutations of a patient's particular form of cancer. In the past, treatments were blunt tools that caused lots of collateral damage to healthy parts of the body while treating cancer.
"We've gone from that to molecular characterization of your lung cancer, and it has been a game changer," Winn says. "This is where science and innovation has an impact."
One of those game-changing treatments is called targeted therapy . Scientists identify genetic biomarkers in the mutated cancer cells to target and then deliver drugs that attack those targets, shrinking tumors.

CRISPR gene-editing may boost cancer immunotherapy, new study finds
Another is immunotherapy, usually taken as a pill, which stimulates the body's own defense system to identify foreign cells, then uses the immune system's own power to fight the cancer as if it were a virus.
As scientists identify new cancer genes, they're creating an ever-broader array of these drugs.
Combined, these treatments have helped increase national survival rates by 22% in the past five years – a rapid improvement over a relatively short time, despite the fact that screening rates are very slow to increase. Winn says as these treatments get cheaper and readily available, the benefits are even reaching rural and Black populations with historic challenges accessing health care.
The most remarkable thing about the drugs is their ability to, in some cases, reverse late-stage cancers. Chi-Fu Jeffrey Yang, a thoracic surgeon at Massachusetts General Hospital and faculty at Harvard Medical School, recalls seeing scans where large dark shadows of tumor would disappear: "It was remarkable to see the lung cancer completely melting away."
To Yang, such progress feels personal. He lost his beloved grandfather to the disease when Yang was in college. If he were diagnosed today, he might still be alive.
"Helping to take care of him was a big reason why I wanted to be a doctor," Yang says.
But the work of combating lung cancer is far from over; further progress in lung cancer survival hinges largely on getting more people screened.
Low-dose CT scans are recommended annually for those over 50 who smoked the equivalent of a pack a day for 20 years. But nationally, only 4.5% of those eligible get those scans , compared to rates of more than 75% for mammograms.
Andrea McKee, a radiation oncologist and spokesperson for the American Lung Association, says part of the problem is that lung cancer is associated with the stigma of smoking. Patients often blame themselves for the disease, saying: "'I know I did this to myself. And so I don't I don't think I deserve to get screened.'"
McKee says that's a challenge unique to lung cancer. "And it just boggles my mind when I hear that, because, of course, nobody deserves to die of lung cancer."
Denise Lee acknowledges that fear. "I was afraid of what they would find," she admits. But she urges friends and family to get yearly scans, anyway.
"I'm just so grateful that my diagnosis was early because then I had options," she says. "I could have surgery, I could have chemotherapy, I could be a part of a clinical trial."
And all of that saved her life.
- lung cancer screening
- immunotherapy
- lung cancer
New study offers hope for a rare and devastating eye cancer

After more than a decade studying a rare eye cancer that produces some of the hardest-to-fight tumors, researchers from University of Pittsburgh Medical Center have found a treatment that works on some patients and, more importantly, a tool that can predict when it is likely to succeed.
The work, published in Nature Communications, is being validated in a clinical trial involving at least 30 patients. It could pave the way for similar methods designed to overcome one of the enduring frustrations of cancer care.
Because tumors differ, not only between patients but even inside the same patient, a treatment that works on one mass may fail on another, even when both are of the same cancer type.
The researchers in Pittsburgh tackled this problem in uveal melanoma, an eye cancer that afflicts only 5 people in a million, but that half the time spreads to other parts of the body, often the liver. The median survival once uveal melanoma has spread has been less than seven months, according to a 2018 study in the journal JAMA Ophthalmology.
“We chose this because it was one of the only cancers that 10 years ago when we started, there was nothing approved for it,” said Udai Kammula, who led the study and directs the Solid Tumor Cell Therapy Program at UPMC Hillman Cancer Center in Pittsburgh.
Scientists had long speculated that the reason uveal melanoma is so tough to fight is that something helps the tumor keep out T cells, a key part of the body’s immune system that develops in bone marrow. However, previous studies by Kammula and his colleagues showed that uveal melanoma tumors actually have T cells inside, and they are turned on.
The problem? The cells lie dormant instead of multiplying and reaching numbers large enough to overwhelm the tumor.
The culprit appears to reside somewhere inside the tumor’s ecosystem of cells, molecules and blood vessels, known formally as the tumor’s “microenvironment.” Kammula compares this ecosystem to the infrastructure that supports a city. Something in that infrastructure helps protect uveal melanoma tumors by preventing the critical T cells from multiplying.
“Ultimately, if we’re going to get rid of cancer, we have to get rid of this infrastructure,” Kammula said.
A tool for predicting success
He and his colleagues have had some success using a treatment known as adoptive cell therapy, which was developed in the 1980s by Steven Rosenberg at the National Institutes of Health.
The treatment involves removing the T cells from the tumor, where they have been unable to proliferate. Scientists then take those T cells and grow them outside the body in a lab dish. They treat patients with chemotherapy to kill off the last of their old immune systems. Finally, they reinfuse the lab-grown T cells into the patient’s blood stream and the cells, now in much greater numbers, go on to attack the tumor.
In this treatment, the T cells are often referred to as tumor-infiltrating leukocytes, or TILs.
Kammula said his team has found that tumors shrink partially or completely in about 35 percent of patients who receive the treatment. But they wanted to know why it doesn’t work in the majority of cases, and whether there might be some way to predict beforehand when it will succeed.
To find out, the researchers analyzed samples from 100 different uveal melanoma tumors that had spread to different parts of the body in 84 patients, seeking to examine all of the tumors’ genetic material.
“We basically put the tumor biopsy in a blender that had the stroma [supportive tissue], the blood vessels, the immune cells, the tumor cells. It had everything,” Kammula said, explaining that they then analyzed all of the tumor’s genetic material.
They found 2,394 genes that could have helped make the tumor susceptible to treatment, some of them genes that experts would regard as “the usual suspects” and others that were unexpected. Using this long list of genes, the scientists searched for characteristics that they shared.
The genes were predominantly involved in helping the body defend itself against viruses, bacteria and other foreign invaders by removing the invaders and helping tissue heal. Kammula and the study’s lead author, Shravan Leonard-Murali, a postdoctoral fellow in the lab, used the different activity levels of these genes to develop a clinical tool.
The tool, known as a biomarker, assigns a score to a uveal melanoma tumor based on the likelihood that it will respond well to the treatment ― removing T cells, growing them outside the body, then reinfusing them.
So far, Kammula said, the biomarker has been “extremely good,” in predicting when the treatment will be effective, though he added, “these findings will need confirmation in the current ongoing clinical trial.”
“I thought it was somewhat of a tour de force, honestly,” said Eric Tran, an associate member of the Earle A. Chiles Research Institute, a division of Providence Cancer Institute in Portland, Ore. Tran did not participate in the study.
He said that while it will be important to validate these results, “I was certainly encouraged by their studies. And from my perspective, I wonder if that sort of strategy can be deployed in other cancers.”
Ryan J. Sullivan, an oncologist at Massachusetts General Hospital and associate professor at Harvard Medical School who was not involved in the study, called the team’s work “timely” and said “it is even more significant that they appear to have a [tool] that appears to predict which patients will benefit.”
The team at UPMC is already investigating possible wider application of both the treatment and the biomarker in a second clinical trial that involves a dozen different cancers.


Researchers develop a new way to safely boost immune cells to fight cancer
Researchers in Virginia Tech’s College of Engineering have developed a new cancer immunotherapy to localize cancer-killing cytokines in tumors to improve treatment effectiveness.
- Hailey Wade
19 Apr 2024
- Share on Facebook
- Share on Twitter
- Copy address link to clipboard

Cancer is the monster of our society. Last year alone, more than 600,000 people in the United States died from cancer, according to the American Cancer Society . The relentless pursuit of understanding this complex disease has shaped medical progress on developing treatment procedures that are less invasive while still highly effective.
Immunotherapy is on the rise as a possible solution. Immunotherapy involves harnessing the power of the body’s immune system to fight against cancer cells. Researchers in the College of Engineering have found a way to revamp a treatment procedure into a groundbreaking practice.
Rong Tong , associate professor in chemical engineering , has teamed up with Wenjun "Rebecca" Cai , associate professor in materials science and engineering , to explore a cancer immunotherapy treatment that has long been of interest to researchers. In their newly published article in the journal Science Advances , Tong and Cai detailed their approach, which involves activating the immune cells in the body and reprogramming them to attack and destroy the cancer cells. This therapeutic method is frequently implemented with the protein cytokine. Cytokines are small protein molecules that act as intercellular biochemical messengers and are released by the body's immune cells to coordinate their response.
“Cytokines are potent and highly effective at stimulating the immune cells to eliminate cancer cells,” Tong said. “The problem is they’re so potent that if they roam freely throughout the body, they’ll activate every immune cell they encounter, which can cause an overactive immune response and potentially fatal side effects.”
Tong and Cai, in collaboration with chemical engineering and materials science and engineering graduate students, have developed an innovative approach to employ cytokine proteins as a potential immunotherapy treatment. Unlike previous methods, their technique ensures that the immune cell stimulating cytokines effectively localize within the tumors for weeks while preserving the cytokine’s structure and reactivity levels.
Combining forces to take down cancer cells
Current cancer treatments, such as chemotherapy, cannot distinguish between healthy cells and cancer cells. When someone with cancer is treated with chemotherapy, the treatment attacks all of the cells in their body, which can lead to side effects such as hair loss and fatigue. Stimulating the body’s immune system to attack tumors is a promising alternative to treat cancer. The delivery of cytokines can jump-start immune cells in the tumor, but overstimulating healthy cells can cause severe side effects.
“Scientists determined a while ago that cytokines can be used to activate and fight against tumors, but they didn’t know how to localize them inside the tumor while not exposing toxicity to the rest of the body,” said Tong. “Chemical engineers can look at this from an engineering approach and use their knowledge to help refine and elevate the effectiveness of the cytokines so they can work inside the body effectively.”
The research team’s goal is to find a balance between killing cancer cells in the body while sparing healthy cells.
To accomplish this goal, Tong and his students used their expertise to create specialized particles with distinctive sizes that help determine where the drug is going. These microparticles are designed to stay within the tumor environment after being injected into the body. Cai and her students worked on measuring these particles’ surface properties.
“In the field of materials science and engineering, we study the surface chemistry and mechanical behavior of materials, such as the specialized particle created for this project,” Cai said. “Surface engineering and characterization, along with particle size, play important roles in controlled drug delivery, ensuring prolonged drug presence and sustained therapeutic effectiveness.”
To ensure successful drug delivery, Tong and his chemical engineering students designed a novel strategy that:
- Anchors cytokines to these new microparticles, limiting the harm of cytokines to healthy cells
- Allows the newly particle-anchored cytokines to jump-start immune systems and recruit immune cells to attack cancer cells
“Our strategy not only minimizes cytokine-induced harm to healthy cells, but also prolongs cytokine retention within the tumor,” Tong said. “This helps facilitate the recruitment of immune cells for targeted tumor attack.”
The next step in the process involves combining the new, localized cytokine therapy method with commercially available, Food and Drug Administration (FDA)-approved checkpoint blockade antibodies, which reactivate the tumor immune cells that have been silenced so they can fight back the cancer cells.
“When there is a tumor inside the body, the body’s immune cells are being deactivated by the cancer cells,” Tong explained. “The FDA-approved checkpoint blocking antibody helps “take off the brakes” that tumors put on immune cells, while the cytokine molecules “step on the gas” to jump-start the immune system and get an immune cell army to fight cancer cells. These two approaches work together to activate immune cells.”
Combining the checkpoint antibodies with the particle-anchored cytokine proved to successfully eliminate many tumors in their study.

Engineering an impact on cancer treatment
Team members hope their impact on immunotherapy treatment is part of a greater movement toward cancer treatment approaches that are harmless to healthy cells. The new approach of attaching cytokines to particles also could be used in the future to deliver other types of immunostimulatory drugs, according to the team.
“Researchers are still looking for safer and more effective cancer treatments,” said Tong. “This motivation is what drives us to develop new technologies in the field. The whole class of drugs that are employed to jump-start the immune system to fight cancer cells has largely not yet succeeded. Our goal is to create novel solutions that allow researchers to test these drugs with existing FDA-approved therapeutics, ensuring both safety and enhanced efficacy.”
Cai said the nature of cancer treatment research requires expertise across engineering disciplines.
“I view this project as a perfect marriage between chemical engineering and materials science,” Cai said. “The former focuses on the synthesis and drug delivery part, the latter on applying advanced materials characterization. This collaboration not only accelerates immunotherapy research, but also has the ability to transform cancer treatment.”
Chelsea Seeber
540-231-2108
- Cancer Research
- Chemical Engineering
- College of Engineering
- Good Health and Well-Being
- Materials Science and Engineering
- One Health Frontier
- Virginia Tech Global Distinction
Related Content

For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news

Living well after cancer treatment
Cancer can have a long lasting impact on every aspect of your life. Your medical team at the hospital and your GP work together to care for your needs and worries as they come up. This helps you to live well after cancer treatment.
Healthcare teams use a number of interventions to support people living with cancer. You may hear this being called personalised care and support planning (PCSP) or cancer recovery package.
How does the PCSP work?
The PCSP aims to support people living with cancer to feel more in control and improve their quality of life. It has 4 parts:
holistic needs assessment (HNA)
end of treatment summary
primary care cancer review
health and wellbeing events
PCSP is available throughout England. There are similar programmes in Northern Ireland, Scotland and Wales.
Holistic Needs Assessment (HNA)
The HNA is a simple questionnaire that you complete online or on paper. It looks at the following needs:
Your team uses the questionnaire to discuss your needs and concerns with you. They then create a care and support plan. This can include advice and information on:
self management
local support
a referral to a specialist service
You have the HNA at diagnosis, the end of treatment, or whenever your needs change. You can also ask to have an HNA when you feel you need it.
HNAs are not available in all hospitals. Speak to your specialist nurse if you would like one.
End of treatment summary
The end of treatment summary tells your GP and other health professionals in the community:
what treatment you’ve had
what your needs might be
how they can help you
who to contact at the hospital if they have any questions or concerns
You will also get a copy of your end of treatment summary. You can share it with other health professionals if necessary.
Your end of treatment summary also helps your GP to do your primary care cancer review.
Primary care cancer review
The primary care cancer review is a discussion between you and your GP or practice nurse. You usually have a primary care review within 3 and 12 months of your diagnosis.
During this review, you can discuss different topics with your GP. For example:
what information and support is available in your local area
the financial impact of cancer
free prescriptions for people with cancer
possible side effects of cancer and its treatment
Health and wellbeing events
Health and wellbeing events are sessions where you and your loved ones can learn about topics related to cancer. The events cover topics such as:
self management to help you feel more in control
a healthy lifestyle with information on diet, physical activity, sleep, fatigue and mental health
benefits and financial advice
coping with side effects of treatments
support groups
These events are usually run by your local hospital. Speak with your specialist nurse if you would like to take part in a health and wellbeing event.
- Read about how preparing for treatment (prehabilitation) can help with life after treatment
Related links
What is prehabilitation.
Prehabilitation means getting ready for cancer treatment in whatever time you have before it starts. It is a programme of support and advice that some NHS hospitals are using.
What should I eat to get ready for cancer treatment?
You can help yourself to get ready for cancer treatment by trying to eat a varied diet. This means eating a wide range of foods in the right proportions.
Being physically active, exercise and cancer
You can help yourself to get ready for cancer treatment by increasing your physical activity. You can improve your fitness in as little as two weeks before the treatment starts.
Looking after your mental wellbeing when you have cancer
You can help yourself to get ready for cancer treatment by looking after your mental wellbeing. This might mean reaching out for support if you can’t cope.
Coping with cancer can be difficult. There is help and support available. Find out about the emotional, physical and practical effects of cancer and how to manage them.
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Find a clinical trial
Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
How CRISPR Is Changing Cancer Research and Treatment
July 27, 2020 , by NCI Staff
CRISPR is a highly precise gene editing tool that is changing cancer research and treatment.
Ever since scientists realized that changes in DNA cause cancer , they have been searching for an easy way to correct those changes by manipulating DNA . Although several methods of gene editing have been developed over the years, none has really fit the bill for a quick, easy, and cheap technology.
But a game-changer occurred in 2013, when several researchers showed that a gene-editing tool called CRISPR could alter the DNA of human cells like a very precise and easy-to-use pair of scissors.
The new tool has taken the research world by storm, markedly shifting the line between possible and impossible. As soon as CRISPR made its way onto the shelves and freezers of labs around the world, cancer researchers jumped at the chance to use it.
“CRISPR is becoming a mainstream methodology used in many cancer biology studies because of the convenience of the technique,” said Jerry Li, M.D., Ph.D., of NCI’s Division of Cancer Biology .
Now CRISPR is moving out of lab dishes and into trials of people with cancer. In a small study, for example, researchers tested a cancer treatment involving immune cells that were CRISPR-edited to better hunt down and attack cancer.
Despite all the excitement, scientists have been proceeding cautiously, feeling out the tool’s strengths and pitfalls, setting best practices, and debating the social and ethical consequences of gene editing in humans.
How Does CRISPR Work?
Like many other advances in science and medicine, CRISPR was inspired by nature. In this case, the idea was borrowed from a simple defense mechanism found in some microbes, such as bacteria.
To protect themselves against invaders like viruses, these microbes capture snippets of the intruder’s DNA and store them away as segments called CRISPRs, or clustered regularly interspersed short palindromic repeats. If the same germ tries to attack again, those DNA segments (turned into short pieces of RNA ) help an enzyme called Cas find and slice up the invader’s DNA.
After this defense system was discovered, scientists realized that it had the makings of a versatile gene-editing tool. Within a handful of years, multiple groups had successfully adapted the system to edit virtually any section of DNA, first in the cells of other microbes, and then eventually in human cells.
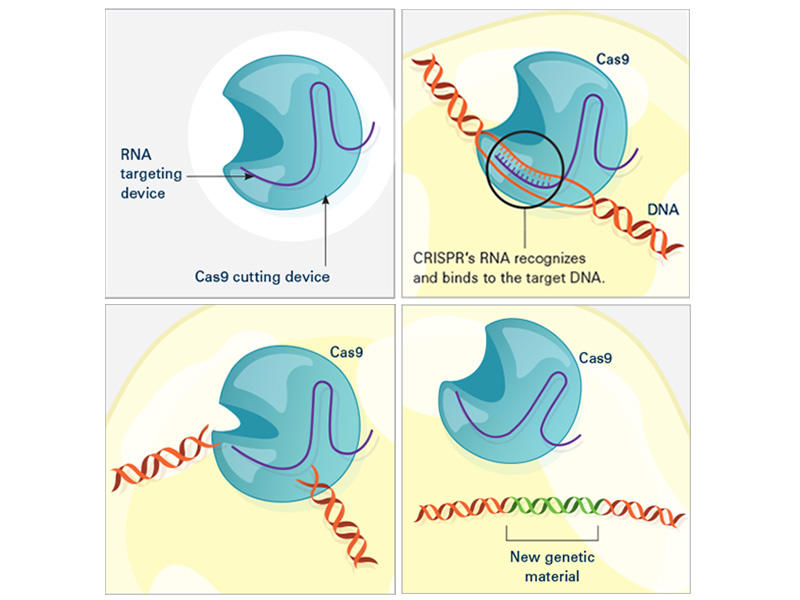
CRISPR consists of a guide RNA (RNA-targeting device, purple) and the Cas enzyme (blue). When the guide RNA matches up with the target DNA (orange), Cas cuts the DNA. A new segment of DNA (green) can then be added.
In the laboratory, the CRISPR tool consists of two main actors: a guide RNA and a DNA-cutting enzyme, most commonly one called Cas9. Scientists design the guide RNA to mirror the DNA of the gene to be edited (called the target). The guide RNA partners with Cas and—true to its name—leads Cas to the target. When the guide RNA matches up with the target gene's DNA, Cas cuts the DNA.
What happens next depends on the type of CRISPR tool that’s being used. In some cases, the target gene's DNA is scrambled while it's repaired, and the gene is inactivated . With other versions of CRISPR, scientists can manipulate genes in more precise ways such as adding a new segment of DNA or editing single DNA letters .
Scientists have also used CRISPR to detect specific targets, such as DNA from cancer-causing viruses and RNA from cancer cells . Most recently, CRISPR has been put to use as an experimental test to detect the novel coronavirus .
Why Is CRISPR a Big Deal?
Scientists consider CRISPR to be a game-changer for a number of reasons. Perhaps the biggest is that CRISPR is easy to use, especially compared with older gene-editing tools.
“Before, only a handful of labs in the world could make the proper tools [for gene editing]. Now, even a high school student can make a change in a complex genome ” using CRISPR, said Alejandro Chavez, M.D., Ph.D., an assistant professor at Columbia University who has developed several novel CRISPR tools.
CRISPR is also completely customizable. It can edit virtually any segment of DNA within the 3 billion letters of the human genome, and it’s more precise than other DNA-editing tools.
And gene editing with CRISPR is a lot faster. With older methods, “it usually [took] a year or two to generate a genetically engineered mouse model , if you’re lucky,” said Dr. Li. But now with CRISPR, a scientist can create a complex mouse model within a few months, he said.
Another plus is that CRISPR can be easily scaled up. Researchers can use hundreds of guide RNAs to manipulate and evaluate hundreds or thousands of genes at a time. Cancer researchers often use this type of experiment to pick out genes that might make good drug targets .
And as an added bonus, “it’s certainly cheaper than previous methods,” Dr. Chavez noted.
What Are CRISPR’s Limitations?
With all of its advantages over other gene-editing tools, CRISPR has become a go-to for scientists studying cancer. There’s also hope that it will have a place in treating cancer, too. But CRISPR isn’t perfect, and its downsides have made many scientists cautious about its use in people.
A major pitfall is that CRISPR sometimes cuts DNA outside of the target gene—what’s known as “off-target” editing. Scientists are worried that such unintended edits could be harmful and could even turn cells cancerous , as occurred in a 2002 study of a gene therapy .
“If [CRISPR] starts breaking random parts of the genome, the cell can start stitching things together in really weird ways, and there’s some concern about that becoming cancer,” Dr. Chavez explained. But by tweaking the structures of Cas and the guide RNA, scientists have improved CRISPR’s ability to cut only the intended target, he added.
Another potential roadblock is getting CRISPR components into cells. The most common way to do this is to co-opt a virus to do the job. Instead of ferrying genes that cause disease, the virus is modified to carry genes for the guide RNA and Cas.
Slipping CRISPR into lab-grown cells is one thing; but getting it into cells in a person's body is another story. Some viruses used to carry CRISPR can infect multiple types of cells, so, for instance, they may end up editing muscle cells when the goal was to edit liver cells.
Researchers are exploring different ways to fine-tune the delivery of CRISPR to specific organs or cells in the human body. Some are testing viruses that infect only one organ, like the liver or brain. Others have created tiny structures called nanocapsules that are designed to deliver CRISPR components to specific cells.
Because CRISPR is just beginning to be tested in humans, there are also concerns about how the body—in particular, the immune system —will react to viruses carrying CRISPR or to the CRISPR components themselves.
Some wonder whether the immune system could attack Cas (a bacterial enzyme that is foreign to human bodies) and destroy CRISPR-edited cells. Twenty years ago, a patient died after his immune system launched a massive attack against the viruses carrying a gene therapy he had received. However, newer CRISPR-based approaches rely on viruses that appear to be safer than those used for older gene therapies.
Another major concern is that editing cells inside the body could accidentally make changes to sperm or egg cells that can be passed on to future generations. But for almost all ongoing human studies involving CRISPR, patients’ cells are removed and edited outside of their bodies. This “ ex vivo ” approach is considered safer because it is more controlled than trying to edit cells inside the body, Dr. Chavez said.
However, one ongoing study is testing CRISPR gene editing directly in the eyes of people with a genetic disease that causes blindness, called Leber congenital amaurosis.
The First Clinical Trial of CRISPR for Cancer
The first trial in the United States to test a CRISPR-made cancer therapy was launched in 2019 at the University of Pennsylvania. The study, funded in part by NCI, is testing a type of immunotherapy in which patients’ own immune cells are genetically modified to better “see” and kill their cancer.
The therapy involves making four genetic modifications to T cells , immune cells that can kill cancer. First, the addition of a synthetic gene gives the T cells a claw-like protein (called a receptor ) that “sees” NY-ESO-1, a molecule on some cancer cells.
Then CRISPR is used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities. The finished product, dubbed NYCE T cells, were grown in large numbers and then infused into patients.
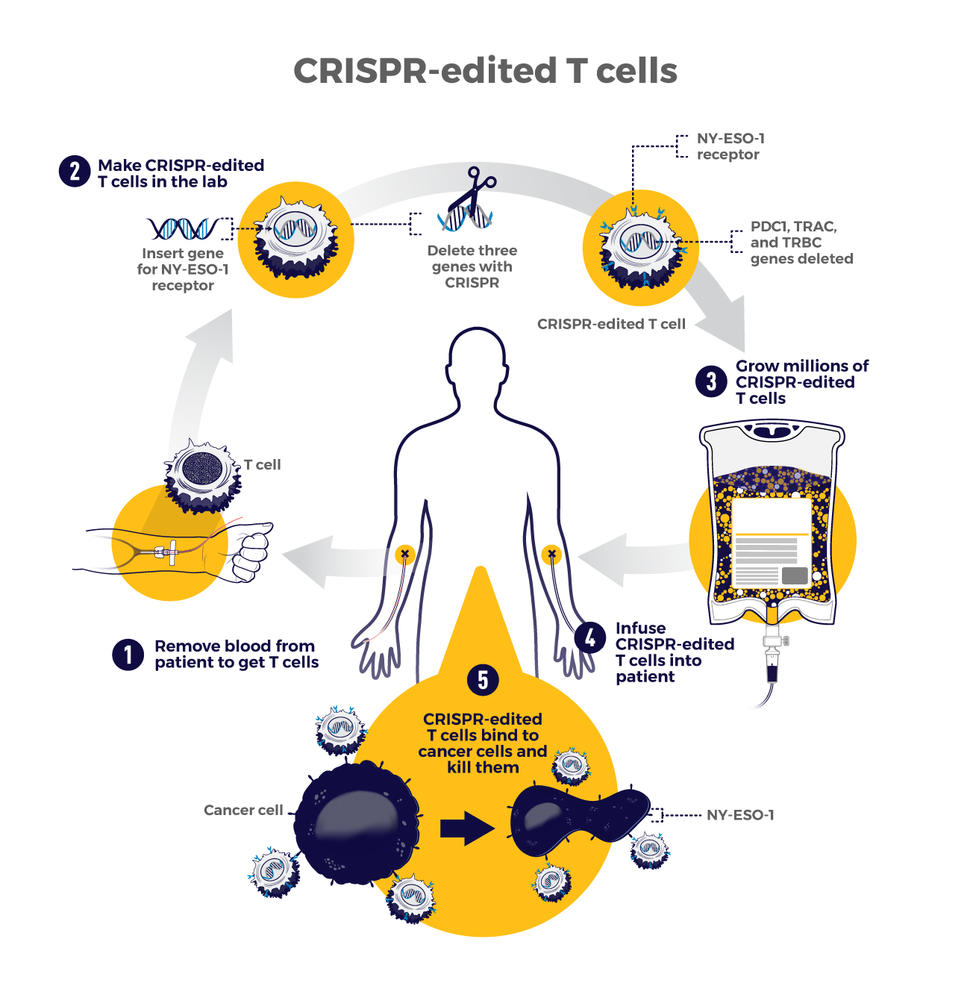
The first trial of CRISPR for patients with cancer tested T cells that were modified to better "see" and kill cancer. CRISPR was used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities.
“We had done a prior study of NY-ESO-1–directed T cells and saw some evidence of improved response and low toxicity ,” said the trial’s leader, Edward Stadtmauer, M.D., of the University of Pennsylvania. He and his colleagues wanted to see if removing the three genes with CRISPR would make the T cells work even better, he said.
The goal of this study was to first find out if the CRISPR-made treatment was safe. It was tested in two patients with advanced multiple myeloma and one with metastatic sarcoma . All three had tumors that contained NY-ESO-1, the target of the T-cell therapy.
Initial findings suggest that the treatment is safe . Some side effects did occur, but they were likely caused by the chemotherapy patients received before the infusion of NYCE cells, the researchers reported. There was no evidence of an immune reaction to the CRISPR-edited cells.
Only about 10% of the T cells used for the therapy had all four of the desired genetic edits. And off-target edits were found in the modified cells of all three patients. However, none of the cells with off-target edits grew in a way that suggested they had become cancer, Dr. Stadtmauer noted.
The treatment had a small effect on the patients’ cancers. The tumors of two patients (one with multiple myeloma and one with sarcoma) stopped growing for a while but resumed growing later. The treatment didn't work at all for the third patient.
It's exciting that the treatment initially worked for the sarcoma patient because “ solid tumors have been a much more difficult nut to crack with cellular therapy," Dr. Stadtmauer said. "Perhaps [CRISPR] techniques will enhance our ability to treat solid tumors with cell therapies.”
Although the trial shows that CRISPR-edited cell therapy is possible, the long-term effects still need to be monitored, Dr. Stadtmauer continued. The NYCE cells are “safe for as long as we’ve been watching [the study participants]. Our plan is to keep monitoring them for years, if not decades,” he said.
More Studies of CRISPR Treatments to Come
While the study of NYCE T cells marked the first trial of a CRISPR-based cancer treatment, there are likely more to come.
“This [trial] was really a proof-of-principle, feasibility, and safety thing that now opens up the whole world of CRISPR editing and other techniques of [gene] editing to hopefully make the next generation of therapies,” Dr. Stadtmauer said.
Other clinical studies of CRISPR-made cancer treatments are already underway. A few trials are testing CRISPR-engineered CAR T-cell therapies , another type of immunotherapy. For example, one company is testing CRISPR-engineered CAR T cells in people with B cell cancers and people with multiple myeloma .
There are still a lot of questions about all the ways that CRISPR might be put to use in cancer research and treatment. But one thing is for certain: The field is moving incredibly fast and new applications of the technology are constantly popping up.
“People are still improving CRISPR methods,” Dr. Li said. “It’s quite an active area of research and development. I’m sure that CRISPR will have even broader applications in the future.”
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)

IMAGES
COMMENTS
People with cancer who take immunotherapy drugs often develop skin side effects, including itching and painful rashes. New research in mice suggests these side effects may be caused by the immune system attacking new bacterial colonies on the skin. Implanted "Drug Factories" Deliver Cancer Treatment Directly to Tumors.
The earliest evidence of cancer treatment can be traced back to an ancient Egyptian medical text, ... These advances reflect the focus placed on cancer research and oncology by governments ...
Selected NCI Activities in Cancer Treatment Research. For more than 50 years, NCI has played an active role in cancer drug development—from conducting preclinical studies in the laboratory to testing potential therapies in humans. NCI researchers conduct clinical trials to test cancer treatments at the National Institutes of Health in ...
Immunotherapy. Immunotherapy uses the body's immune system to fight cancer. Immunotherapy can boost or change how the immune system works so it can find and attack cancer cells. Molecular testing, which can help select patients most suitable for immunotherapy, has opened the door to this newer form of treatment.
Cancer is a global health problem responsible for one in six deaths worldwide. Treating cancer has been a highly complex process. Conventional treatment approaches, such as surgery, chemotherapy, and radiotherapy, have been in use, while significant advances are being made in recent times, including stem cell therapy, targeted therapy, ablation therapy, nanoparticles, natural antioxidants ...
Nevertheless, after years of painstaking research, CAR T-cell therapies have entered the mainstream of cancer treatment, said Steven Rosenberg, M.D., Ph.D., chief of the Surgery Branch in NCI's Center for Cancer Research (CCR), an immunotherapy and CAR T-cell therapy pioneer. " [CAR T cells] are now widely available in the United States and ...
The MIT team began by treating cancer cells with several different chemotherapy drugs, at different doses. Twenty-four hours after the treatment, the researchers added dendritic cells to each dish, followed 24 hours later by T cells. Then, they measured how well the T cells were able to kill the cancer cells.
Cancer therapy describes the treatment of cancer in a patient, often with surgery, chemotherapy and/or radiotherapy. Targeted therapies are also available for some cancer types. A cancer patient ...
But each cancer-causing or cancer-influencing genetic mutation that is discovered is a potential target for drug development, including for cancers for which there are currently few treatment choices.
The current scenario for cancer research is wide, offering many possibilities for the constant improvement of treatment, considering not only patient recovery but also caring for their well-being during therapy. As summarised in Table 1, these new approaches offer many advantages compared to conventional therapies. However, some disadvantages ...
According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors ...
In addition to providing the latest statistics on cancer incidence, mortality, and survivorship, the AACR Cancer Progress Report 2023 offers detailed updates and important context regarding the latest research in cancer etiology, early detection, diagnosis, treatment, prevention, and survivorship. Throughout the report, the personal stories of ...
Medical advances are accelerating the battle against cancer. Here are 10 recent developments. Test to identify 18 early-stage cancers. Researchers in the US have developed a test they say can identify 18 early-stage cancers. Instead of the usual invasive and costly methods, Novelna's test works by analyzing a patient's blood protein.
December 27, 2023. Estimated reading time: 6 minutes. By Mayo Clinic staff. Researchers at Mayo Clinic Comprehensive Cancer Center spent 2023 studying the biology of cancer and new ways to predict, prevent, diagnose and treat the disease. Their discoveries are creating hope and transforming the quality of life for people with cancer today and ...
Applicability of Spatial Technology in Cancer Research. Sangjeong Ahn, Hye Seung Lee. Cancer Res Treat. 2024;56(2):343-356. Published online January 30, ... Editors of Cancer Research and Treatment. Cancer Res Treat. 2024;56(2):697-697. Published online February 20, ...
Some people with cancer will have only one treatment. But most people have a combination of treatments, such as surgery with chemotherapy and/or radiation therapy. You may also have immunotherapy, targeted therapy, or hormone therapy. Clinical trials might also be an option for you. Clinical trials are research studies that involve people.
The global oncology community will gather in Chicago to share and discuss the latest clinical cancer research impacting patient care at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. Treatment advances involving targeted therapies, immunotherapy, and new uses of technology, as well as research on improving patient quality of life and outcomes are among the topics that ...
Lung cancer remains the most lethal form of the disease, killing about 135,000 Americans a year - more than breast, prostate and colon cancer combined - which is why many people still think of ...
Types of Cancer Treatment. Many procedures and drugs are available to treat cancer, with many more being studied. Some are "local" treatments like surgery and radiation therapy, which are used to treat a specific tumor or area of the body. Drug treatments (such as chemotherapy, immunotherapy, or targeted therapy) are often called "systemic" treatments because they can affect the entire body.
Leading cancer researchers from UC San Francisco presented talks about advances in targeted therapy, cancer genomics, eliminating treatment disparities and other cancer research topics at this year's annual meeting of the American Association for Cancer Research (AACR) conference, which was held April 5-10 in San Diego.
After more than a decade studying a rare eye cancer that produces some of the hardest-to-fight tumors, researchers from University of Pittsburgh Medical Center have found a treatment that works on ...
Prediction of Cancer Incidence and Mortality in Korea, 2024. Purpose This study aimed to report the projected cancer incidence and mortality for the year 2024 to estimate Korea's current cancer burden. Materials and Methods Cancer incidence data from 1999 to 2021 were obtained from the Korea National Cancer Incidence Database, and cance...
Treatment for cancer. Your treatment depends on where your cancer is, how big it is, whether it has spread, and your general health. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for breast cancer prevention, screening, and treatment. Breast Cancer Research Results. The following are some of our latest news articles on breast cancer research and study updates:
Researchers in the College of Engineering explore a cancer immunotherapy treatment that involves activating the immune cells in the body and reprogramming them to attack and destroy cancer cells. This therapeutic method frequently uses cytokines, small protein molecules that act as intercellular biochemical messengers and are released by the body's immune cells to coordinate their response.
The research team will use data on 1,000 Veterans with OPC and information from the Computer Vision and Machine Learning in Precision Oncology hub to build and train an AI algorithm to assess chemo-radiation treatment response. The new platform will be called the AI-based Risk Stratification for Oropharyngeal Carcinomas (AIROC).
Immunotherapy is a type of cancer treatment that helps your immune system fight cancer. The immune system helps your body fight infections and other diseases. It is made up of white blood cells and organs and tissues of the lymph system.. Immunotherapy is a type of biological therapy.Biological therapy is a type of treatment that uses substances made from living organisms to treat cancer.
Eventually, Dr Grotz hopes to do the procedure even sooner after diagnosis to prevent the spread of stomach cancer to the peritoneum. The research was published in the Annals of Surgical Oncology ...
Living well after cancer treatment. Cancer can have a long lasting impact on every aspect of your life. Your medical team at the hospital and your GP work together to care for your needs and worries as they come up. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and ...
CRISPR is a highly precise gene editing tool that is changing cancer research and treatment. Ever since scientists realized that changes in DNA cause cancer, they have been searching for an easy way to correct those changes by manipulating DNA. Although several methods of gene editing have been developed over the years, none has really fit the ...