Get Our Latest News!
- Join Our Community


FDA Approves New First-line Treatment Option for Metastatic Pancreatic Cancer: What You Need to Know
by Erin Post — Feb 13, 2024
For the first time in more than a decade, the FDA has approved a new first-line treatment for patients with metastatic pancreatic cancer . After a clinical trial showed a positive survival benefit, the combination chemotherapy called NALIRIFOX is now approved for patients who have not received any previous treatment. For a disease with limited treatment options, today’s FDA announcement is exciting news.
“We are pleased that the U.S. Food and Drug Administration has issued this new approval of the NALIRIFOX regimen. With each new approved treatment, there is more hope for those who will be diagnosed in the future and people currently living with pancreatic cancer may have more time with their loved ones,” said PanCAN President and CEO Julie Fleshman, JD, MBA, in a press release from the pharmaceutical company Ipsen announcing the approval. “We are thankful to the patients who participated in this clinical trial as they play a crucial role in advancing treatments for pancreatic cancer.”
Here, PanCAN answers questions related to this new treatment option.
What is NALIRIFOX?
NALIRIFOX is a chemotherapy treatment. It is a combination of three previously approved pancreatic cancer drugs, liposomal irinotecan (Nal-IRI or Onivyde®), made by the pharmaceutical company Ipsen, plus 5 fluorouracil (5-FU)/leucovorin and oxaliplatin. NALIRIFOX will be delivered intravenously (IV, through a vein under the skin).
What does this FDA approval mean?
NALIRIFOX has been approved by the FDA as a new first-line treatment for metastatic pancreatic cancer. This means patients whose cancer has spread and who have not had treatment yet can now receive this drug combination.
Are these new drugs?
No. All of the drugs in NALIRIFOX have already been approved for other purposes; what is new is the combination of these drugs together as a first-line treatment.
Liposomal irinotecan, in combination with 5-FU/leucovorin, is already approved for people with metastatic pancreatic cancer that has continued to grow after being treated with another chemotherapy called gemcitabine (Gemzar®). Oxaliplatin has also been approved and used to treat other cancers for a long time.
NALIRIFOX is a combination of liposomal irinotecan, 5-FU/leucovorin and oxaliplatin. This combination has now been approved for a new group of patients, those with metastatic pancreatic cancer who have not had any other treatment. This is the first approval for a first-line treatment for metastatic pancreatic cancer in over ten years.
What is the survival benefit?
In a clinical trial, the NALIRIFOX regimen was compared to gemcitabine (Gemzar) plus nab-paclitaxel (Abraxane®), which is one of the current standard-of-care treatments for patients with metastatic pancreatic cancer. The results, published in October 2023 , showed that patients treated with NALIRIFOX had an overall survival of 11.1 months, which was a statistically significant improvement over the 9.2-month overall survival with gemcitabine and nab-paclitaxel.
What are the side effects?
In the clinical trial, patients took NALIRIFOX for a median of six weeks longer than those receiving gemcitabine and nab-paclitaxel, showing that NALIRIFOX was relatively well tolerated. The most frequent side effects reported in the NALIRIFOX group included neutropenia (low levels of a type of immune cell called neutrophils) and hypokalemia (low potassium level), and gastrointestinal disorders like diarrhea and nausea.
Is NALIRIFOX the same as FOLFIRINOX?
The combination chemotherapy FOLFIRINOX is composed of 5-FU, leucovorin, irinotecan and oxaliplatin. In 2010, a clinical trial showed that FOLFIRINOX was effective for the treatment of metastatic pancreatic cancer in people who hadn’t received prior treatment.
The drug liposomal irinotecan replaces irinotecan to make NALIRIFOX. Liposomal irinotecan is a modified form of irinotecan, designed to stay in the body longer before it gets broken down.
Does insurance cover this treatment?
FDA approval means this drug combination is safe and effective, and although the FDA does not decide what is covered by insurance, when a drug gets FDA approval Medicare and Medicaid will usually cover it. Coverage for chemotherapy drugs will vary based on the specific plan and insurance company a person uses.
Contact PanCAN Patient Services for more information on financial assistance programs for those experiencing or anticipating cost-related barriers to care.
I am a patient with pancreatic cancer interested in NALIRIFOX. What should I do?
People diagnosed with pancreatic cancer should talk to their healthcare team about this treatment option. Since this approval is for first-line treatment (the first or initial treatment a person receives after diagnosis), it will impact people who have not received treatment for their pancreatic cancer yet.
For people who have already received treatment with a drug called gemcitabine, a similar chemotherapy containing one of the drugs in NALIRIFOX, liposomal irinotecan, is also approved.
Contact PanCAN Patient Services for additional information and support, including information on what questions to ask and how to seek out a second opinion. Our Case Managers can help you understand your options and connect you with resources to learn more.
- chemotherapy
- clinical trials
- NALIRIFOX
- Patient Services
- research
- treatment
- Most popular
- Latest News
More Than Volunteers, A Family
University of Nebraska Medical Center and Nebraska Medicine, PanCAN’s First City Sponsor, Highlights Commitment to Patients
Celebrities Rally for PanCAN PurpleStride 2024
Case Manager Karina Gutierrez Receives the 4th Annual Carol Kulok Award for Compassion
PanCAN's Legislative Priorities Need Your Support
No one should face pancreatic cancer alone. Learn how we can support you.
(877) 272-6226
Mon – Fri, 7 a.m. – 5 p.m. PST You can also contact us using the below form.
Follow us on social media.
Get the latest news and updates from the Pancreatic Cancer Action Network.

1500 Rosecrans Avenue, Suite 200 Manhattan Beach, CA 90266 - Map
Phone: (310) 725-0025
Toll Free: (877) 573-9971
Fax: (310) 725-0029
Email: [email protected]
Gear, apparel, accessories and more to show off your purple pride.
- What is the Pancreas?
- What is Pancreatic Cancer?
- Living with Pancreatic Cancer
- Patient & Caregiver Services
- Stories of Hope
- PanCAN + Research
- Funding Research
- Latest Research
- Resources for Researchers
- Resources for HCPs
- Donate & Fundraise
- Be an Advocate
- Local Events
- News & Media
- Reports & Financials
©2024 Pancreatic Cancer Action Network. All rights reserved. Terms of Use | Privacy Policy
Pancreatic Cancer Action Network®, PanCAN®, PurpleStride®, Wage Hope®, Know Your Tumor®, Powerful Knowledge. Personal Treatment.®, Precision Promise℠ and Demand Better For Patients. For Survival.℠ are the trademarks of the Pancreatic Cancer Action Network, Inc.
The Pancreatic Cancer Action Network is registered as a 501©3 nonprofit organization. Contributions to the Pancreatic Cancer Action Network are tax-deductible to the extent permitted by law. The Pancreatic Cancer Action Network’s tax identification number is #33-0841281.

Advances in Pancreatic Cancer Research
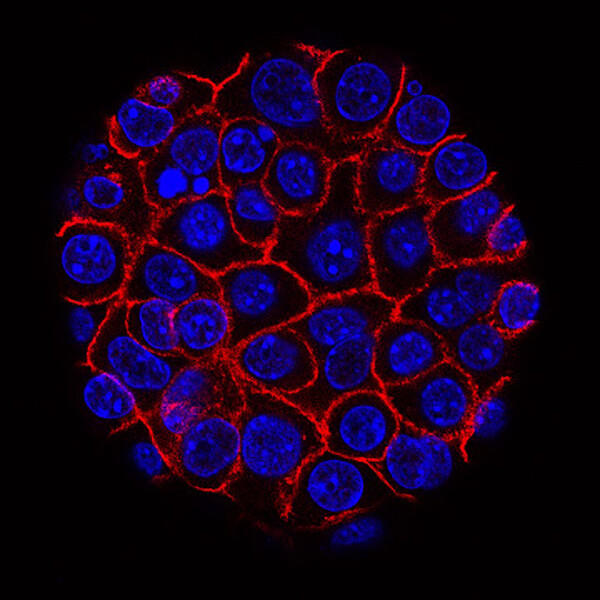
Pancreatic cancer cells (blue) growing as a sphere encased in membranes (red).
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat pancreatic cancer, which includes pancreatic ductal adenocarcinoma (PDAC) and pancreatic neuroendocrine tumors (PNET). PNET is much less common than PDAC and has a better prognosis .
This page highlights some of the latest research in pancreatic adenocarcinoma, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Early Detection of Pancreatic Cancer
Currently, no screening tests exist that can catch pancreatic cancer early, before symptoms develop. NCI is now funding several large research projects that are working to develop such an early-detection tool.
One known risk factor for developing pancreatic cancer is a new diagnosis of diabetes, sometimes called new-onset diabetes . About 1 in 100 people with new onset diabetes are diagnosed with pancreatic cancer within 3 years after learning they have diabetes. And 1 in 4 people who get pancreatic cancer had already been diagnosed with diabetes.
The NCI-funded New Onset Diabetes (NOD) Study , which is scheduled to run through 2025, is currently enrolling 10,000 people with new-onset diabetes or hyperglycemia (also known as prediabetes). The NOD researchers hope to develop a blood test that can identify the few individuals with a new diabetes diagnosis who may need further testing for pancreatic cancer.
Other NCI-funded teams, coordinated through the Pancreatic Cancer Detection Consortium (PCDC) , are trying to create a blood test that could pick up early pancreatic cancer in the general population. PCDC researchers are also working to improve imaging of the pancreas, by developing methods that may be able to pick up tiny deposits of tumor cells.
Pancreatic Cancer Treatment
Pancreatic cancer can be hard to treat surgically due to the location of the organ, and because the disease has often spread in the body by the time it is diagnosed.
Standard treatment for pancreatic cancer usually consists of surgery , chemotherapy , radiation , or combinations of each, depending on the cancer’s stage. Beyond these standard treatments, NCI scientists continue to look for ways to treat pancreatic cancer more effectively. Researchers are looking at the potential of new drugs, ways to combine standard drugs, and new modalities (such as immunotherapy ) to give to patients.
Patients with pancreatic cancer are generally recommended to have both biomarker testing and testing for inherited genetic changes. Both types of testing can help suggest possible treatments and can indicate with a patient’s family members might have an increased risk for pancreatic cancer or other types of cancer.
Testing treatments for early-stage pancreatic cancer
Therapies for early-stage disease that are being tested in clinical trials right now include
- new adjuvant chemotherapy drug combinations Some of these postsurgical drug combinations are already known to extend the lives of patients with metastatic disease, but it's not clear if they are better at killing cancer cells left behind after surgery than standard treatments.
- neoadjuvant chemotherapy This form of chemotherapy is given before surgery, with the goal of improving outcomes by shrinking the tumor before it’s removed. Pre-surgical chemotherapy also may help by killing cancer cells that have escaped from the tumor that would continue to grow as the patient recovers from surgery.
Testing treatments for advanced pancreatic cancer
New treatments for metastatic pancreatic cancer that are being investigated in clinical trials include immunotherapy and targeted therapy . Immunotherapy uses substances to stimulate or suppress the immune system to help the body fight cancer. Targeted therapy uses drugs or other substances to target specific molecules that cancer cells need to survive and spread.
Drug Targets Common Mutation in Pancreatic Cancer
In mice, experimental drug MRTX1133 shrank pancreatic tumors with KRAS G12D mutations.
- Ras-directed therapies . The RAS gene s makes proteins that take part in signaling pathways that control cell growth. Altered forms of these genes are found in more than 90% of pancreatic cancers. Drugs that target mutant forms of RAS are now being tested. One example is a drug that targets a form of RAS that has a mutation called G12C and another drug that targets a more common mutation, G12D.
- olaparib . Olaparib (Lynparza) is used as maintenance therapy in adults with metastatic cancer that has not progressed after platinum chemotherapy and has certain mutations in the BRCA1 or BRCA2 gene.
- pembrolizumab . In rare cases, people with pancreatic cancer have mutations in their tumor that cause high microsatellite instability (MSI) . Pembrolizumab (Keytruda) is an immune checkpoint inhibitor approved for patients with pancreatic cancer that has high MSI.
- novel immune checkpoint inhibitors and combinations . Using one drug for immunotherapy treatment has not been effective for most people with pancreatic cancer. Therefore, researchers are combining several immunotherapies that can act on different parts of the immune system.
- combinations of immunotherapy drugs with other treatments . These include radiation therapy, stromal modifying agents, and other targeted drugs.
- cell therapies . Researchers are exploring the use of cell-based therapies for pancreatic cancer. These therapies use immune cells such as T cells and natural killer cells that are altered in the lab to kill cancer cells.
- the stroma is the fibrous tissue around a tumor that does not contain cancer cells. It is mostly made up of connective tissue , blood vessels , lymphatic vessels , and nerves . Some of these components can help to support cancer cells and/ or prevent the immune system from recognizing cancer cells.
- pancreatic cancers have much denser stroma than most tumors. Agents that help break down or remodel this stroma may help more chemotherapy drugs reach cancer cells. Or they may help reduce cancer cell resistance to killing by other agents.
For a list of specific drugs, see Drugs Approved for Pancreatic Cancer .
Clinical Trials
Because of the complex nature of pancreatic cancer, many experts believe it’s important for all patients to join a clinical trial , even if they have early-stage disease. NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for pancreatic cancer treatment .
NCI-Supported Research Programs
Many NCI-funded researchers at the NIH campus, and across the United States and world, are seeking ways to address pancreatic cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate this basic information into improved patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in pancreatic cancer.
- The Pancreatic Cancer Cohort Consortium consists of more than a dozen prospective epidemiologic cohort studies that investigate the causes and natural history of pancreatic cancer. This includes the launching of a genome-wide association study (GWAS) known as PanScan .
- The Pancreatic Cancer Detection Consortium (PCDC) develops and tests biomarkers to detect early stage pancreatic cancer and identify individuals at high risk for the disease.
- The Pancreatic Ductal Adenocarcinoma (PDAC) Stromal Reprogramming Consortium (PSRC) is a multidisciplinary community of PDAC researchers that will bridge biological research with preclinical/translational research. The goal is to identify and evaluate elements in the tumor microenvironment that drive PDAC progression and response to therapy.
- The Pancreatic Specialized Programs of Research Excellence (Pancreatic SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Pancreatic SPORE grants support new and diverse approaches to the prevention, early detection, diagnosis, and treatment of pancreatic cancer. Two of NCI's Gastrointestinal (GI) SPOREs also conduct research in pancreatic cancer.
- The RAS Initiative looks to understand mutations in RAS genes to ultimately create effective, new therapies for RAS -related cancers. More than 90% of pancreatic cancers are caused by mutations in the RAS family of genes.
Pancreatic Cancer Research Results
The following are some of our latest news articles on pancreatic cancer research:
- No Glucose? Pancreatic Cancer May Have a Ready Energy Alternative
- In Mouse Study, KRAS-Targeted Drug Shows Potential against Pancreatic Cancer
- Abnormal Collagen May Be Weak Spot for Pancreatic Cancer
- Belzutifan Approved to Treat Tumors Linked to Inherited Disorder VHL
- Could A Diabetes Diagnosis Help Detect Pancreatic Cancer Early?
- Boosting Dendritic Cells Helps the Immune System Find Pancreatic Cancer
View the full list of Pancreatic Cancer Research Results and Study Updates .
- Share full article
Advertisement
Supported by
Pancreatic Cancer Vaccine Shows Promise in Small Trial
Using mRNA tailored to each patient’s tumor, the vaccine may have staved off the return of one of the deadliest forms of cancer in half of those who received it.

By Benjamin Mueller
Five years ago, a small group of cancer scientists meeting at a restaurant in a deconsecrated church hospital in Mainz, Germany, drew up an audacious plan: They would test their novel cancer vaccine against one of the most virulent forms of the disease, a cancer notorious for roaring back even in patients whose tumors had been removed.
The vaccine might not stop those relapses, some of the scientists figured. But patients were desperate. And the speed with which the disease, pancreatic cancer, often recurred could work to the scientists’ advantage: For better or worse, they would find out soon whether the vaccine helped.
On Wednesday, the scientists reported results that defied the long odds. The vaccine provoked an immune response in half of the patients treated, and those people showed no relapse of their cancer during the course of the study, a finding that outside experts described as extremely promising.
The study, published in Nature, was a landmark in the yearslong movement to make cancer vaccines tailored to the tumors of individual patients.
Researchers at Memorial Sloan Kettering Cancer Center in New York, led by Dr. Vinod Balachandran, extracted patients’ tumors and shipped samples of them to Germany. There, scientists at BioNTech, the company that made a highly successful Covid vaccine with Pfizer, analyzed the genetic makeup of certain proteins on the surface of the cancer cells.
Using that genetic data, BioNTech scientists then produced personalized vaccines designed to teach each patient’s immune system to attack the tumors. Like BioNTech’s Covid shots, the cancer vaccines relied on messenger RNA. In this case, the vaccines instructed patients’ cells to make some of the same proteins found on their excised tumors, potentially provoking an immune response that would come in handy against actual cancer cells.
“This is the first demonstrable success — and I will call it a success, despite the preliminary nature of the study — of an mRNA vaccine in pancreatic cancer,” said Dr. Anirban Maitra, a specialist in the disease at the University of Texas MD Anderson Cancer Center, who was not involved in the study. “By that standard, it’s a milestone.”
The study was small: Only 16 patients, all of them white, were given the vaccine, part of a treatment regimen that also included chemotherapy and a drug intended to keep tumors from evading people’s immune responses. And the study could not entirely rule out factors other than the vaccine having contributed to better outcomes in some patients.
“It’s relatively early days,” said Dr. Patrick Ott of the Dana-Farber Cancer Institute.
Beyond that, “cost is a major barrier for these types of vaccines to be more broadly utilized,” said Dr. Neeha Zaidi, a pancreatic cancer specialist at the Johns Hopkins University School of Medicine. That could potentially create disparities in access.
But the simple fact that scientists could create, quality-check and deliver personalized cancer vaccines so quickly — patients began receiving the vaccines intravenously roughly nine weeks after having their tumors removed — was a promising sign, experts said.
Since the beginning of the study, in December 2019, BioNTech has shortened the process to under six weeks, said Dr. Ugur Sahin, a co-founder of the company, who worked on the study. Eventually, the company intends to be able to make cancer vaccines in four weeks.
And since it first began testing the vaccines about a decade ago, BioNTech has lowered the cost from roughly $350,000 per dose to less than $100,000 by automating parts of production, Dr. Sahin said.
A personalized mRNA cancer vaccine developed by Moderna and Merck reduced the risk of relapse in patients who had surgery for melanoma, a type of skin cancer, the companies announced last month. But the latest study set the bar higher by targeting pancreatic cancer, which is thought to have fewer of the genetic changes that would make it ripe for vaccine treatments.
In patients who did not appear to respond to the vaccine, the cancer tended to return around 13 months after surgery. Patients who did respond, though, showed no signs of relapse during the roughly 18 months they were tracked.
Intriguingly, one patient showed evidence of a vaccine-activated immune response in the liver after an unusual growth developed there. The growth later disappeared in imaging tests.
“It’s anecdotal, but it’s nice confirmatory data that the vaccine can get into these other tumor regions,” said Dr. Nina Bhardwaj, who studies cancer vaccines at the Icahn School of Medicine at Mount Sinai.
Scientists have struggled for decades to create cancer vaccines, in part because they trained the immune system on proteins found on tumors and normal cells alike.
Tailoring vaccines to mutated proteins found only on cancer cells, though, potentially helped provoke stronger immune responses and opened new avenues for treating any cancer patient, said Ira Mellman, vice president of cancer immunology at Genentech, which developed the pancreatic cancer vaccine with BioNTech.
“Just establishing the proof of concept that vaccines in cancer can actually do something after, I don’t know, thirty years of failure is probably not a bad thing,” Dr. Mellman said. “We’ll start with that.”
Benjamin Mueller is a health and science reporter. Previously, he covered the coronavirus pandemic as a correspondent in London and the police in New York. More about Benjamin Mueller
The Fight Against Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention. Here’s how to avoid one of the deadliest forms of skin cancer.
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why .
Should alcoholic beverages have cancer warning labels? Ireland will require them starting in 2026, and there are nascent efforts elsewhere .
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot .
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Year in Review
- Published: 16 December 2022
Pancreatic cancer in 2022
The war on pancreatic cancer: progress and promise
- Christine A. Iacobuzio-Donahue ORCID: orcid.org/0000-0002-4672-3023 1 , 2
Nature Reviews Gastroenterology & Hepatology volume 20 , pages 75–76 ( 2023 ) Cite this article
1776 Accesses
2 Citations
17 Altmetric
Metrics details
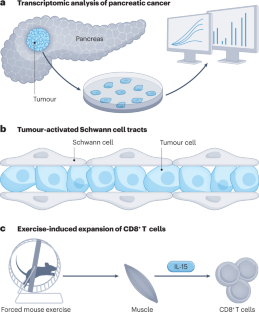
The year 2022 was notable for substantial research progress related to pancreatic ductal adenocarcinoma (PDAC). The first single-cell and spatial transcriptomic atlases of PDAC were reported, a mechanism for how Schwann cells promote perineural invasion was explored, and, finally, the role of exercise in abrogating immunosuppression was shown.
Key advances
Spatial transcriptomics and other single-cell technologies reveal distinct transitional populations linking acinar-to-ductal metaplasia to pancreatic intraepithelial neoplasia and enrichment of metallothionein-expressing inflammatory cancer-associated fibroblasts in chemoresistant pancreatic cancer 3 .
Schwann cells within the tumour microenvironment organize into tumour-activated Schwann cell tracts that promote migration along nerves via activation of JUN 6 .
Aerobic exercise restrains pancreatic cancer growth in mice through IL-15–IL-15RA-mediated activation of CD8 + T cells, and evidence for this relationship was found in humans 7 .
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Park, W., Chawla, A. & O’Reilly, E. M. Pancreatic cancer: a review. JAMA 326 , 851–862 (2021).
Article CAS Google Scholar
Kindler, H. L. et al. Overall survival results from the POLO trial: a phase III study of active maintenance olaparib versus placebo for germline BRCA-mutated metastatic pancreatic cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.21.01604 (2022).
Article Google Scholar
Cui Zhou, D. et al. Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat. Genet. 54 , 1390–1405 (2022).
Grippo, P. J. & Tuveson, D. A. Deploying mouse models of pancreatic cancer for chemoprevention studies. Cancer Prev. Res. 3 , 1382–1387 (2010).
Schlesinger, Y. et al. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat. Commun. 11 , 4516 (2020).
Deborde, S. et al. Reprogrammed Schwann cells organize into dynamic tracks that promote pancreatic cancer invasion. Cancer Discov. 12 , 2454–2473 (2022).
Kurz, E. et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 40 , 720–737.e5 (2022).
Liu, J. et al. Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ. Health Perspect. 115 , 1101–1106 (2007).
Li, G. et al. Recent advances in c-Jun N-terminal kinase (JNK) inhibitors. Curr. Med. Chem. 28 , 607–627 (2021).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52 , 17–35 (2020).
Download references
Author information
Authors and affiliations.
Department of Pathology, David. M. Rubenstein Center for Pancreatic Cancer Research, Human Oncology and Pathogenesis Program, New York, NY, USA
Christine A. Iacobuzio-Donahue
Memorial Sloan Kettering Cancer Center, New York, NY, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christine A. Iacobuzio-Donahue .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Iacobuzio-Donahue, C.A. The war on pancreatic cancer: progress and promise. Nat Rev Gastroenterol Hepatol 20 , 75–76 (2023). https://doi.org/10.1038/s41575-022-00728-1
Download citation
Published : 16 December 2022
Issue Date : February 2023
DOI : https://doi.org/10.1038/s41575-022-00728-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

People with pancreatic cancer are living longer, thanks to improved approaches
Share this:.
By Jessica Saenz
A diagnosis of pancreatic cancer is almost synonymous with hopelessness. As the least survivable type of cancer, the perception is understandable. "As soon as patients were diagnosed, they were often told by their physician to start making arrangements," says Mark Truty, M.D. , a surgical oncologist at Mayo Clinic who specializes in pancreatic surgery.
But the tides are turning, thanks to new and improved treatment methods that are helping people with pancreatic cancer live longer. Dr. Truty and Robert McWilliams, M.D. , a medical oncologist at Mayo Clinic, talk about Mayo Clinic's approach to pancreatic cancer care , and how it's leading to improved survival and quality of life.
Capturing the full picture from the time of diagnosis and beyond
Before moving forward with treatment, Dr. Truty says it's critical to understand as much about each person's cancer as possible. "When a patient is first diagnosed, they need really good imaging and molecular testing to see, not just where the tumor is, but if there's any evidence of spread. We do a lot of tests at the beginning and throughout to make sure that the cancer is truly localized and has not spread."
In most instances, a CT scan or MRI scan is used to identify the location of the cancer and possible spread, but Dr. Truty says standard scans are just one piece of the puzzle. "Historically, patients have gotten a scan where the tumor appears to be localized, and then they underwent surgery. But that paradigm has not resulted in the outcomes we wanted."
This is where PET scans and additional molecular testing play an important role.
Dr. Truty says that PET scans and newer genetic testing are key to staging the cancer and assessing its behavior accurately. They can help determine if treatment is working effectively to shrink the tumor, whereas traditional CT scans have distinct limitations in assessing response in pancreatic primary tumors. "If we see a response we’re anticipating on the PET scan, those are the patients that do very well. If we're not seeing a response, then we have to pivot and switch their therapy to see if we can achieve a better outcome," he says. "We've also been using novel genetic testing developed at Mayo Clinic to test the blood of patients, as well as the fluid of the abdomen through laparoscopy , to see if we can pick up some cancer DNA."
This method is helping cancer experts at Mayo Clinic determine who might be at risk for pancreatic cancer recurrence and individualize their treatment to reduce the risk of the cancer returning. "We're the first center to do this routinely for every single patient we see," Dr. Truty says.
Tailoring testing and treatment for each person
Initial testing and staging of pancreatic cancer can help uncover weaknesses or potential threats for each unique pancreatic cancer case. "As we've learned more about the genetics of pancreatic cancer — and how to find patients who can benefit — we've been able to tailor therapies according to the patient's genetics and their DNA, or the DNA changes that are specific to the cancer itself," says Dr. McWilliams.
In a study led by Mayo Clinic Center for Individualized Medicine , researchers found that nearly 1 in 6 people diagnosed with pancreatic cancer had an inherited cancer-related gene mutation that may have predisposed them to pancreatic cancer. The most common genetic mutation in those patients was the BRCA2 gene, which is linked to breast cancer.
Niloy Jewel Samadder, M.D. , a Mayo Clinic gastroenterologist and hepatologist, and the study's senior author, said that patients with mutations had a 50% longer survival. Data from this study and others have led to recent changes in guidelines that advocate for genetic testing for all pancreatic cancer patients, regardless of their cancer stage or family history of cancer.
Though the majority of people with pancreatic cancer do not have a germline mutation, Dr. McWilliams says it's important to use all the tools available for each patient. While it may not achieve a cure, it can help select therapies to improve quality of life so patients can live longer and more comfortably.
"There's a national trial, called the POLO Trial , which showed that patients on chemotherapy with BRCA1 or BRCA2 mutations are eligible for a maintenance therapy with just a pill, rather than IV chemotherapy, which is really good from a side effects standpoint," says Dr. McWilliams.
Redefining what is considered inoperable
Dr. Truty says patients who are able to have surgery to remove their pancreatic cancer can live significantly longer, but in cases where the tumor has grown outside of the pancreas to encase critical blood vessels, pancreatic cancer has been considered inoperable.
About one-third of pancreatic cancer tumors grow to surround blood vessels outside the pancreas. "Those patients have historically not been considered for surgery," he says. "Theoretically, 50% of patients with diagnosed pancreatic cancer have the potential to undergo an operation. The question is: How do we get them to surgery? And how do we optimize their outcomes to make sure that they live as long as they possibly can?"
Drs. Truty, McWilliams and pancreatic cancer experts at Mayo Clinic use an approach called neoadjuvant therapy, which delivers chemotherapy — or a combination of chemotherapy and radiation — to destroy microscopic cancer cells in the body before surgery. By combining this method with personalized surgery for each patient's anatomy, they can remove tumors entirely and reconstruct blood vessels as needed. This has resulted in the ability to operate on patients who previously did not have that option, leading to better results than ever before.
"We're creating custom surgeries for each patient that aren't being done anywhere else on the planet. That's why so many people come to us after they've been told their tumors are inoperable," says Dr. Truty.
Though surgery can lead to the best outcomes in many cases, Dr. Truty emphasizes that the goal of pancreatic cancer treatment is not surgery. "The goal for anyone with cancer is to extend their life and maintain a reasonable quality of life. Sometimes an operation is necessary to achieve this, and sometimes it will decrease the likelihood of one or the other, or both. That's why before we even consider an operation, we have to make sure that operation has the highest probability that we'll achieve both of those goals."
Pancreatic cancer continues to have the highest mortality rate, but Dr. McWilliams says there's plenty of reason for patients to be hopeful. "It's a very serious cancer. It's something that is life-threatening for a lot of people, but it's not necessarily a death sentence," he says. "It's something that we have treatments for, and our treatments are only getting better."
And this progress, he says, is driven by clinical trials. "Clinical trials are how we advance the science. For patients who are looking for the latest and greatest, and want to help advance the options for their cancer, participation in clinical trials is crucial."
Dr. Truty says he hopes more people with pancreatic cancer seek out second opinions from cancer centers who are leveraging new approaches and providing patients more options. "Historically, it's been such a nihilistic disease, but things have really changed. We have not settled for the standard of care — this results in standard outcomes which have not been good. We have to treat patients differently — starting from the beginning," he says. "And if you can do that all the way through treatment, then those patients really do have exceptional outcomes."
Learn more about panc r eatic cancer and find a pancreatic cancer clinical trial at Mayo Clinic.
Read these articles:
- " 5 things to know about pancreatic cancer "
- " PET/MRI biomarkers guide personalized treatment for people with pancreatic cancer, study finds "
- " Identifying inherited gene mutations in pancreatic cancer can lead to targeted therapies, better survival "
- " Aggressive Approach to Pancreatic Cancer Yields Outstanding Outcome "
Also watch this video: " Mayo Clinic Minute: Advances in pancreatic cancer treatment extending lives
Related Posts

They identified a cell-signaling protein that drives pancreatic cancer cell growth that could be a potential therapeutic target.

Researchers built a highly accurate artificial intelligence model for fully automated cancer detection, including small and otherwise difficult-to-detect tumors.

Rita Krueger shares the lessons she has learned from her experience with pancreatic cancer diagnosis, treatment and survivorship.
New MD Anderson Research Uncovers Drug Combo That Could Eliminate Pancreatic Cancer Tumors
Researchers at the University of Texas MD Anderson Cancer Center published two studies this week on a new approach that could improve treatment for patients with pancreatic cancer. The preclinical studies showed that combining immunotherapy with a KRAS inhibitor can lead to long-lasting tumor elimination.
- Copy Link Copy Link
Researchers at the University of Texas MD Anderson Cancer Center published two studies this week on a new approach that could improve treatment for patients with pancreatic cancer — a disease that an estimated 64,050 U.S. adults will be diagnosed with in 2023.
The preclinical studies showed that combining immunotherapy with a KRAS inhibitor can lead to long-lasting tumor elimination in pancreatic cancer.

Health Benefit Consultants, Share Your Expert Insights in Our Survey
The research explored the functional role of KRAS mutations in pancreatic cancer. KRAS belongs to a family of genes that encode proteins that participate in cell signaling, activating or deactivating to regulate the growth of cells. When KRAS are mutated, they cause the uncontrolled cell growth that occurs in cancer. The oncology community has known “for a while now” that KRAS mutations drive pancreatic cancer, but it has had a hard time figuring out a way to effectively drug these mutated genes, explained Dr. Raghu Kalluri, an author for both studies.
In the study published in Developmental Cell , the research team tested the functional role of KRAS by generating mouse models with a variety of genetic alterations known to go along with KRAS mutations. By thoroughly examining KRAS’ functional role, the research team gained key insights about how to prepare the tumor microenvironment in advanced pancreatic cancer, Dr. Kalluri pointed out.
The research team then genetically suppressed KRAS in the mice, which led to cancer cell death. In some cases, the number of myeloid cells in the tumor decreased significantly, and in others, the tumor was completely eradicated, Dr. Kalluri said.
In his view, prior models didn’t do a great job of replicating the constantly changing tumor microenvironment found in advanced pancreatic cancer. However, the models generated by his team more accurately reflected the tumor microenvironment present in patients with metastatic pancreatic cancer, and this helped them identify immune activation as a vital element for sustained tumor suppression and elimination, he declared.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
In the study published in Cancer Cell , the researchers tested the effects of a KRAS G12D inhibitor known as MRTX1133 in 16 different lab models. They found that the drug reversed both early- and late-stage tumor growth — but not for good. The tumors grew back after some time, letting the research team know that KRAS G12D inhibition will only be successful in the long term if immune cells are activated.
In other words, KRAS inhibitors do a good job of suppressing pancreatic tumors, but these drugs cannot sustain those effects over a long period of time unless they are combined with various immune checkpoint inhibitors, Dr. Kalluri explained.
These preclinical studies have already led to a Phase I clinical trial at MD Anderson, which is testing the use of MRTX1133 in combination with immune checkpoint inhibitors in patients with pancreatic cancer.
Photo: The National Cancer Institute
More From MedCity News

Hospital Discharge Backlogs Impacting Patient Outcomes

Delivering the Right Approach for Virtual Primary Care: 3 Key Insights
Which Medtech Startups Will Take Part in the MedCity INVEST Pitch Perfect Contest?

Transforming Denials Management: Insights and Best Practices for Your Organization
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New hope for early pancreatic cancer intervention via AI-based risk prediction
Press contact :.

Previous image Next image
The first documented case of pancreatic cancer dates back to the 18th century. Since then, researchers have undertaken a protracted and challenging odyssey to understand the elusive and deadly disease. To date, there is no better cancer treatment than early intervention. Unfortunately, the pancreas, nestled deep within the abdomen, is particularly elusive for early detection.
MIT Computer Science and Artificial Intelligence Laboratory (CSAIL) scientists, alongside Limor Appelbaum, a staff scientist in the Department of Radiation Oncology at Beth Israel Deaconess Medical Center (BIDMC), were eager to better identify potential high-risk patients. They set out to develop two machine-learning models for early detection of pancreatic ductal adenocarcinoma (PDAC), the most common form of the cancer. To access a broad and diverse database, the team synced up with a federated network company, using electronic health record data from various institutions across the United States. This vast pool of data helped ensure the models' reliability and generalizability, making them applicable across a wide range of populations, geographical locations, and demographic groups.
The two models — the “PRISM” neural network, and the logistic regression model (a statistical technique for probability), outperformed current methods. The team’s comparison showed that while standard screening criteria identify about 10 percent of PDAC cases using a five-times higher relative risk threshold, Prism can detect 35 percent of PDAC cases at this same threshold.
Using AI to detect cancer risk is not a new phenomena — algorithms analyze mammograms, CT scans for lung cancer, and assist in the analysis of Pap smear tests and HPV testing, to name a few applications. “The PRISM models stand out for their development and validation on an extensive database of over 5 million patients, surpassing the scale of most prior research in the field,” says Kai Jia, an MIT PhD student in electrical engineering and computer science (EECS), MIT CSAIL affiliate, and first author on an open-access paper in eBioMedicine outlining the new work . “The model uses routine clinical and lab data to make its predictions, and the diversity of the U.S. population is a significant advancement over other PDAC models, which are usually confined to specific geographic regions, like a few health-care centers in the U.S. Additionally, using a unique regularization technique in the training process enhanced the models' generalizability and interpretability.”
“This report outlines a powerful approach to use big data and artificial intelligence algorithms to refine our approach to identifying risk profiles for cancer,” says David Avigan, a Harvard Medical School professor and the cancer center director and chief of hematology and hematologic malignancies at BIDMC, who was not involved in the study. “This approach may lead to novel strategies to identify patients with high risk for malignancy that may benefit from focused screening with the potential for early intervention.”
Prismatic perspectives
The journey toward the development of PRISM began over six years ago, fueled by firsthand experiences with the limitations of current diagnostic practices. “Approximately 80-85 percent of pancreatic cancer patients are diagnosed at advanced stages, where cure is no longer an option,” says senior author Appelbaum, who is also a Harvard Medical School instructor as well as radiation oncologist. “This clinical frustration sparked the idea to delve into the wealth of data available in electronic health records (EHRs).” The CSAIL group’s close collaboration with Appelbaum made it possible to understand the combined medical and machine learning aspects of the problem better, eventually leading to a much more accurate and transparent model. “The hypothesis was that these records contained hidden clues — subtle signs and symptoms that could act as early warning signals of pancreatic cancer,” she adds. “This guided our use of federated EHR networks in developing these models, for a scalable approach for deploying risk prediction tools in health care.” Both PrismNN and PrismLR models analyze EHR data, including patient demographics, diagnoses, medications, and lab results, to assess PDAC risk. PrismNN uses artificial neural networks to detect intricate patterns in data features like age, medical history, and lab results, yielding a risk score for PDAC likelihood. PrismLR uses logistic regression for a simpler analysis, generating a probability score of PDAC based on these features. Together, the models offer a thorough evaluation of different approaches in predicting PDAC risk from the same EHR data.
One paramount point for gaining the trust of physicians, the team notes, is better understanding how the models work, known in the field as interpretability. The scientists pointed out that while logistic regression models are inherently easier to interpret, recent advancements have made deep neural networks somewhat more transparent. This helped the team to refine the thousands of potentially predictive features derived from EHR of a single patient to approximately 85 critical indicators. These indicators, which include patient age, diabetes diagnosis, and an increased frequency of visits to physicians, are automatically discovered by the model but match physicians' understanding of risk factors associated with pancreatic cancer.
The path forward
Despite the promise of the PRISM models, as with all research, some parts are still a work in progress. U.S. data alone are the current diet for the models, necessitating testing and adaptation for global use. The path forward, the team notes, includes expanding the model's applicability to international datasets and integrating additional biomarkers for more refined risk assessment.
“A subsequent aim for us is to facilitate the models' implementation in routine health care settings. The vision is to have these models function seamlessly in the background of health care systems, automatically analyzing patient data and alerting physicians to high-risk cases without adding to their workload,” says Jia. “A machine-learning model integrated with the EHR system could empower physicians with early alerts for high-risk patients, potentially enabling interventions well before symptoms manifest. We are eager to deploy our techniques in the real world to help all individuals enjoy longer, healthier lives.”
Jia wrote the paper alongside Applebaum and MIT EECS Professor and CSAIL Principal Investigator Martin Rinard, who are both senior authors of the paper. Researchers on the paper were supported during their time at MIT CSAIL, in part, by the Defense Advanced Research Projects Agency, Boeing, the National Science Foundation, and Aarno Labs. TriNetX provided resources for the project, and the Prevent Cancer Foundation also supported the team.
Share this news article on:
Press mentions, the boston globe.
Researchers from MIT and elsewhere have developed an AI model that is capable of identifying 3 ½ times more people who are at high-risk for developing pancreatic cancer than current standards, reports Felice J. Freyer for The Boston Globe . “This work has the potential to enlarge the group of pancreatic cancer patients who can benefit from screening from 10 percent to 35 percent,” explains Freyer. “The group hopes its model will eventually help detect risk of other hard-to-find cancers, like ovarian.”
Previous item Next item
Related Links
- Martin Rinard
- Computer Science and Artificial Intelligence Laboratory (CSAIL)
- Department of Electrical Engineering and Computer Science
Related Topics
- Diagnostics
- Artificial intelligence
- Health sciences and technology
- Computer science and technology
- Technology and society
- Electrical Engineering & Computer Science (eecs)
- National Science Foundation (NSF)
- Defense Advanced Research Projects Agency (DARPA)
Related Articles
AI model can help determine where a patient’s cancer arose

Seeing into the future: Personalized cancer screening with artificial intelligence

Toward a smarter electronic health record
More mit news.
Researchers detect a new molecule in space
Read full story →

Erin Bahm, Steven Parks named 2024–25 UPS Fellows

Twenty-three MIT faculty honored as "Committed to Caring" for 2023-25

Featured video: Moooving the needle on methane
The many-body dynamics of cold atoms and cross-country running

Heather Paxson named associate dean for faculty of the School of Humanities, Arts, and Social Sciences
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Pancreatic Cancer News
Top headlines, latest headlines.
- Pancreatic and Colorectal Cancers
- Removing Benign Pancreatic Tumors
- Pancreatic Cancer Hijacks Brain-Building Protein
- Unintentional Weight Loss? Get a Check-Up
- Halting Cancer Cell Growth
- Deregulation of RBFOX2: Pancreatic Cancer
- Potential New Pancreatic Cancer Treatment
- Effective Screening of Anticancer Drugs
- Treating 'Scar Tissue' of Pancreatic Cancer
- Insulin-Producing Cells Regenerated in Mice
Earlier Headlines
Monday, december 18, 2023.
- Promising Anticancer Drug Targeting KRAS Protein
Monday, December 4, 2023
- Macrophages 'eat' Insulin-Producing Cells to Regulate Insulin After Mice Have Given Birth
Wednesday, November 29, 2023
- Can We Crack This Cancer's Immune Response?
Tuesday, November 21, 2023
- Novel Molecular Mechanisms in the Early Development of Diabetes Mellitus
Thursday, November 16, 2023
- Pancreatic Cancer Discovery Opens the Door for New Clinical Trial
Wednesday, November 15, 2023
- Nanoparticles for Optimized Cancer Therapy
Thursday, November 9, 2023
- Drug Screen Points Toward Novel Diabetes Treatments
Monday, November 6, 2023
- A Step Closer to Injection-Free Diabetes Care: Innovation in Insulin-Producing Cells
Tuesday, October 31, 2023
- High Insulin Levels Directly Linked to Pancreatic Cancer
Wednesday, October 18, 2023
- New Study Suggests Promising Approach for Treating Pancreatic Cancer
Monday, October 16, 2023
- A Powerful New Tool in the Fight Against One of the Deadliest Cancers
Tuesday, October 10, 2023
- Can Immunity from Routine Vaccines Be Used to Fight Cancer?
Monday, October 9, 2023
- Newfound Mechanism Suggests Drug Combination Could Starve Pancreatic Cancer
Wednesday, September 6, 2023
- 'Super-Enhancer' Super-Charges Pancreatic Tumor Growth
Tuesday, August 29, 2023
- Drug That Targets Scar-Like Tissue in Tumors Shows Promise for Aggressive Pancreatic Cancer
Thursday, August 3, 2023
- Enhanced Tumor Modeling Using Laponite Bioinks for 3D Bioprinting
- New Research Casts Doubt on Role of Fungus in Driving Pancreatic Cancer
Monday, July 17, 2023
- Pancreatic Cancer Vaccine Plus Immunotherapy and Antibody Spark Immune System Response in Pancreatic Cancers
Wednesday, June 28, 2023
- One-Two Punch: Novel Drug Pairing Could Beat Pancreatic Cancer
Thursday, May 18, 2023
- Cancer Cells Use a New Fuel in Absence of Sugar
Thursday, April 13, 2023
- Implantable Device Shrinks Pancreatic Tumors
Friday, March 17, 2023
- Biomarkers Show Promise for Identifying Early Risk of Pancreatic Cancer
Thursday, January 19, 2023
- How Pancreatic Cancer Defies Treatment
Monday, January 16, 2023
- Stress-Tolerant Cells Drive Tumor Initiation in Pancreatic Cancer
Friday, December 30, 2022
- Study Discovers Triple Immunotherapy Combination as Possible Treatment for Pancreatic Cancer
Thursday, December 8, 2022
- Adjuvant Chemotherapy Improves Overall Survival for Pancreatic Cancer Patients
Tuesday, December 6, 2022
- Study Shows Promise of New Anti-KRAS Drug for Pancreatic Cancer
Wednesday, November 30, 2022
- New Genetic Culprit Suspected in the Onset of Pancreatic Cancer
Monday, November 7, 2022
- New Insights Into the Mechanisms Causing Diabetes
Thursday, October 27, 2022
- Cancer Deaths Continue Downward Trend in U.S.; Modest Improvements in Survival for Pancreatic Cancer
Thursday, October 20, 2022
- Gel-Like, Radioactive Tumor Implant Obliterates Pancreatic Cancer in Mice
Wednesday, October 19, 2022
- Two Drugs Reverse Key Pancreatic Cancer Step in the Lab
Wednesday, October 12, 2022
- Scientists Identify Link Between Mitochondria and Pancreatic Cancer Risk
Monday, October 10, 2022
- Researchers Find Tumor Microbiome Interactions May Identify New Approaches for Pancreatic Cancer Treatment
Thursday, October 6, 2022
- Researchers Find Link Between Immune Cells' Closest Neighbors and Survival Time in Patients With Pancreatic Cancer
Wednesday, October 5, 2022
- Genetic Test for Pancreatic Cancer Outperforms Current Guidelines
- Biological Pathways Provide Evidence for How to Overcome Barriers Limiting Cancer Immunotherapies
Tuesday, September 13, 2022
- AI Helps Detect Pancreatic Cancer
Wednesday, August 31, 2022
- Mechanisms at Work in Progression of Pancreatic Cysts to Pancreatic Cancer
Monday, August 22, 2022
- Study Offers Insights Into How Pancreatic Cancer Develops

Friday, July 22, 2022
- Potential Target for Type 1 Diabetes Treatment
Wednesday, July 13, 2022
- Regular Screening of People at High Risk for Pancreatic Cancer Pays Off
Wednesday, June 29, 2022
- Scientists Discover Mechanism Controlling Spread of Pancreatic Cancer
Tuesday, June 14, 2022
- Researchers Develop Pancreatic Beta-Cell Restoring Therapy for Treating Type 1 Diabetes
Thursday, June 9, 2022
- Existing Cancer Therapy in Narrow Use Shows Significant Activity Against Other Cancers
Wednesday, June 1, 2022
- High Fat Diet, Unregulated Athletic Exercise Endurance Enhancers Linked to Risk of Pancreatic Cancer
Thursday, May 5, 2022
- Breaking the Shield That Protects Pancreatic Cancer from Immunotherapy
- Research Pushes Closer to New Therapy for Pancreatic Cancer
- Stem Cell Therapy Protects Against the Side Effects of Cancer Drugs
Tuesday, April 26, 2022
- AI May Detect Earliest Signs of Pancreatic Cancer
Friday, April 22, 2022
- Establishment of a Pancreatic Cancer Animal Model Using the Pancreas-Targeted Hydrodynamic Gene Delivery Method
Thursday, April 21, 2022
- The Protein That Keeps the Pancreas from Digesting Itself
Tuesday, April 19, 2022
- The Origins and ID of Pancreatic Endocrine Cells
Tuesday, April 5, 2022
- Artificial Intelligence May Improve Diabetes Diagnosis, Study Shows
Tuesday, March 29, 2022
- Distinct Classes of Fibroblasts in Tumors Play Opposing Roles, Promoting or Restraining Pancreatic Cancer Growth
Wednesday, March 23, 2022
- Novel Therapeutic Strategy Shows Promise Against Pancreatic Cancer
Tuesday, March 22, 2022
- Could Diet Modification Make Chemotherapy Drugs More Effective for Patients With Pancreatic Cancer?
Monday, March 7, 2022
- Novel Treatment Makes Pancreatic Cancer Susceptible to Immunotherapy, Mouse Study Shows
Thursday, March 3, 2022
- Researchers Produce Fully Functional Pancreatic Beta Cells from Stem Cells
Thursday, February 24, 2022
- Pancreatic Cancer: Cellular Process Suggests Path to New Treatment Options
Wednesday, February 16, 2022
- Pushing Past Pancreatic Tumors’ Defenses
Tuesday, February 8, 2022
- Gut Bacteria Linked to Immune Suppression in Pancreatic Cancer
Thursday, January 27, 2022
- Pancreatic Cancer Cells Feed Off Hyaluronic Acid
Tuesday, January 25, 2022
- Faulty BRCA Genes Linked to Prostate and Pancreatic Cancers
Wednesday, November 24, 2021
- New Findings on Bacteria That Increase Risk of Pancreatic Cancer
Thursday, November 18, 2021
- Strategy to Overcome Tumors’ Resistance to Immunotherapy Generates Promising Clinical Trial Results
Monday, November 8, 2021
- New Symptoms Identified That Could Help Doctors Diagnose Pancreatic Cancer
Thursday, November 4, 2021
- RAS Inhibitors for Use in Fighting More Cancers
- Experimental Drug Boosts Immunotherapy Effectiveness in Pancreatic Cancer in Mice
Friday, October 29, 2021
- Uncovering How Injury to the Pancreas Impacts Cancer Formation
Wednesday, October 6, 2021
- Cancer Costs US More Than $156 Billion Annually, With Drugs a Leading Expense
Thursday, September 30, 2021
- Scientists Reverse Pancreatic Cancer Progression in ‘time Machine’ Made of Human Cells
Tuesday, September 28, 2021
- Antidepressants Inhibit Cancer Growth in Mice
Friday, September 24, 2021
- Lab Grown Tumor Models Could Improve Treatment for Pancreatic Cancer
Thursday, September 16, 2021
- Link Between Inflammation and Pancreatic Cancer Development Uncovered
Monday, September 13, 2021
- New Method Enables 3D Microscopy of Human Organs
- Engineers Grow Pancreatic 'organoids' That Mimic the Real Thing
Tuesday, August 17, 2021
- Pancreatic Cancer Trials Are No More Diverse Now Than Over a Decade Ago
Wednesday, August 11, 2021
- Pumping Iron: Inhibition of Key Pathway Promotes Iron-Dependent Cell Death in Pancreatic Cancer Cells
Friday, August 6, 2021
- New Drug Combo Shows Early Potential for Treating Pancreatic Cancer
Tuesday, July 27, 2021
- New Approach for Cell Therapy Shows Potential Against Solid Tumors With KRAS Mutations
Monday, July 19, 2021
- Why Identical Mutations Cause Different Types of Cancer
Tuesday, June 29, 2021
- Biomarker Could Help to Diagnose Pancreatic Cancer
Tuesday, June 22, 2021
- How Pancreatic Cancer Cells Dodge Drug Treatments
Monday, June 14, 2021
- Targeted Drug Found Effective in Thwarting Pancreatic Tumors
Tuesday, June 1, 2021
- 'Electronic Nose' Accurately Sniffs out Hard-to-Detect Cancers
Thursday, May 13, 2021
- Scientists Show How to Attack the 'fortress' Surrounding Pancreatic Cancer Tumors
Monday, May 10, 2021
- PARP Inhibitor Shrinks Tumors in Pancreatic Cancer Patients With Mutations
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- This Alloy Is Kinky
- Giant Galactic Explosion: Galaxy Pollution
- Flare Erupting Around a Black Hole
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
- Octopus Inspires New Suction Mechanism for ...
- Cities Sinking: Urban Populations at Risk
- Puzzle Solved About Ancient Galaxy
- How 3D Printers Can Give Robots a Soft Touch
- Combo of Multiple Health Stressors Harming Bees
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- World J Gastroenterol
- v.27(27); 2021 Jul 21
Pancreatic cancer: A review of epidemiology, trend, and risk factors
Jian-xiong hu.
Intensive Care Unit (ICU), Affiliated Hospital of Putian University, Putian 351100, Fujian Province, China
Cheng-Fei Zhao
School of Pharmacy and Medical Technology, Putian University, Putian 351100, Fujian Province, China
Key Laboratory of Pharmaceutical Analysis and Laboratory Medicine in University of Fujian Province, Putian University, Putian 351100, Fujian Province, China. moc.361@902iefgnehcoahz
Wen-Biao Chen
Department of Basic Medicine, Quanzhou Medical College, Quanzhou 362011, Fujian Province, China
Department of Reproductive Medicine Centre, First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian Province, China
Department of Priority Laboratory for Zoonoses Research, Fujian Center for Disease Control and Prevention, Fuzhou 350001, Fujian Province, China
Department of Pathology, First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian Province, China
Supported by Fujian Province Medical Health Young and Middle-aged Talents Training Project, No. 2020GGA079 ; National Natural Science Foundation of China, No. 81572442 ; and Natural Science Foundation of Fujian Province, No. 2018J01195 .
Corresponding author: Cheng-Fei Zhao, MD, Associate Professor, School of Pharmacy and Medical Technology, Putian University, No. 1133 Xueyuan Road, Chengxiang District, Putian 351100, Fujian Province, China. moc.361@902iefgnehcoahz
Despite rapid advances in modern medical technology and significant improvements in survival rates of many cancers, pancreatic cancer is still a highly lethal gastrointestinal cancer with a low 5-year survival rate and difficulty in early detection. At present, the incidence and mortality of pancreatic cancer are increasing year by year worldwide, no matter in the United States, Europe, Japan, or China. Globally, the incidence of pancreatic cancer is projected to increase to 18.6 per 100000 in 2050, with the average annual growth of 1.1%, meaning that pancreatic cancer will pose a significant public health burden. Due to the special anatomical location of the pancreas, the development of pancreatic cancer is usually diagnosed at a late stage with obvious clinical symptoms. Therefore, a comprehensive understanding of the risk factors for pancreatic cancer is of great clinical significance for effective prevention of pancreatic cancer. In this paper, the epidemiological characteristics, developmental trends, and risk factors of pancreatic cancer are reviewed and analyzed in detail.
Core Tip: Pancreatic cancer is still a highly lethal gastrointestinal cancer with a low 5-year survival rate and difficulty in early detection. A comprehensive understanding of the risk factors for pancreatic cancer is of great clinical significance for effective prevention of pancreatic cancer. In this review, the latest epidemiology, future trends, and various risk factors of pancreatic cancer are analyzed and summarized, which will provide more guidance and suggestions for the prevention and control of this malignancy.
INTRODUCTION
The pancreas is an about 15-cm-long, spongy, tube-shaped organ located in the upper abdomen between the stomach and spine[ 1 ]. A normal healthy pancreas consists of acinar cells secreting digestive enzyme, ductal cells secreting bicarbonate, centro-acinar cells that are the transitional region between acinar and ductal cells, endocrine islets secreting hormone, and relatively inactive stellate cells[ 2 ]. Pancreatic cancer occurs when abnormal DNA mutations in the pancreas cause pancreatic cells to uncontrollably grow and divide, forming tumors[ 3 ]. Pancreatic cancer is characterized as a fatal disease and one of the most aggressive and lethal malignancies[ 4 , 5 ]. By the time of diagnosis, pancreatic cancer often presents at an advanced stage, and has often spread to other parts of the body. Clinically, pancreatic cancer is the general term for malignant tumor formed in the epithelial cells of glandular structures in the pancreatic ductal cells, referred to as adenocarcinoma[ 6 ], and pancreatic ductal adenocarcinoma (PDAC) accounts for more than 90% of pancreatic cancers[ 7 ]. Due to the poor survival outcomes, PDAC is the seventh leading cause of global cancer death despite being the 10th most common cancer[ 8 ]. Other less common exocrine pancreatic cancers include adenosquamous carcinoma, squamous cell carcinoma, giant cell carcinoma, acinar cell carcinoma, and small cell carcinoma. At present, pancreatic cancer remains a devastating disease whose prognosis has remained largely unchanged over the last two decades[ 9 ]. Improvement in patient outcomes will depend on clear knowledge of epidemiology, reasonable prevention, and scientific regulation of early detection[ 4 ]. Therefore, it is necessary to understand the epidemiological characteristics, development trends, and risk factors of pancreatic cancer in detail, which will eventually establish rational prevention approaches for clinical benefit.
EPIDEMIOLOGY OF PANCREATIC CANCER
Assessing the latest epidemiologic trends in pancreatic cancer is necessary because it is of great importance for preventive measures and clinical care[ 10 ]. Therefore, we present a review of the latest epidemiology of pancreatic cancer.
Pancreatic cancer ranks consistently last among all cancers in terms of prognostic outcomes for patients and is predicted to become the second leading cause of cancer death in some regions[ 11 ]. A study including 84275 patients with at least 5 years of follow-up showed that actual 5-year survival rate in patients rose from 0.9% in 1975 to 4.2% in 2011 for all stages of pancreatic cancer, while in surgically resected patients, it increased from 1.5% to 17.4%[ 12 ]. In non-resected patients, the actual 5-year survival rate was 0.8% in 1975 and 0.9% in 2011, meaning that it remained roughly the same between 1975 and 2011[ 12 ]. The 5-year relative survival rate of pancreatic cancer was 7.2% in China and the lowest level in all cancers[ 13 ]. Cancer Stat Facts showed that the 5-year survival rate at the time of diagnosis is approximately 10% in the United States based on data from Surveillance, Epidemiology, and End Results Program 18 between 2010 and 2016[ 14 ]. Pancreatic cancer has a poor 5-year survival rate, ranging from 2% to 9%, with little difference between high-income countries and low-income and middle-income countries[ 11 , 15 ]. Therefore, the 5-year survival rate of pancreatic cancer varies globally in different regions and countries, but does not exceed 10%. And it is predicted that patients with nonoperative pancreatic cancer have a lower 5-year survival rate.
According to Cancer Statistics 2021, the American Cancer Society reported approximately 60430 new cases and 48220 deaths for pancreatic cancer in the United States, ranking third after lung and bronchus cancer and colorectal cancer[ 16 ]. In the 28 countries of the European Union (EU), it was estimated that approximately 111500 people (55000 in males and 56500 in females) will die from pancreatic cancer by 2025, and the number of recorded deaths from the cancer in 2010 will increase by almost 50% (45% in men and 49% in women), and it has been projected that pancreatic cancer may become the third leading cause of cancer death in the EU after lung and colorectal cancers[ 17 ]. Global Cancer Statistics 2018 showed that the incidence and mortality of pancreatic cancer were 458918 and 432242 in 2018 in the world, respectively, and deaths account for about 94.2% of new cases[ 18 ]. Pancreatic cancer remains the seventh leading cause of cancer death globally, and Global Cancer Statistics 2020 showed that, globally, a total of 495773 new cases and 466003 related deaths were reported for pancreatic cancer in 2020, with almost as many mortality as incidence[ 19 ]. The systematic analysis for the 2017 Global Burden of Disease Study showed that the number of incident cases and deaths from pancreatic cancer in both genders increased 2.3-fold from 195000 incident cases and 196000 deaths in 1990 to 448000 incident cases and 441000 deaths in 2017 globally[ 15 ]. These reports indicate a gradual increase in the number of incident cases and deaths from pancreatic cancer.
Average age-standardized rates (ASRs) of pancreatic cancer incidence and mortality vary widely across regions of the world[ 19 ]. The ASR of the incidence was highest in Eastern Europe, with 9.9 per 100000, followed by Western Europe (9.8), Northern America (9.3), Southern Europe (8.4), Northern Europe (8.3), Australia/New Zealand (7.9), Micronesia/Polynesia (7.7), and Western and Eastern Asia (7.0)[ 19 ]. The ASR of the mortality was highest in Western Europe, with 7.4 per 100000, followed by Northern America (6.9), Northern Europe (6.7), Australia/New Zealand (6.7), Southern Europe (8.4), Eastern Europe (5.6), Eastern Asia (4.8), and Western Asia (4.4)[ 19 ]. The human development index (HDI) is a composite index that measures three dimensions: Life expectancy, education period, and access to essential sources for a suitable and reasonable life[ 20 ]. The ASRs of pancreatic cancer incidence and mortality in regions with a very high HDI were significantly higher than medium or low HDI regions[ 19 ]. The low ASRs of the incidence and mortality were found mainly in South-Central Asia (1.5 per 100000, 0.9 per 100000), Eastern Africa (2.0, 1.7), Middle Africa (2.0, 1.2), Western Africa (2.2, 1.8), Melanesia (2.9, 1.7), and South-Eastern Asia (2.9, 1.8), all of which are medium or low HDI regions[ 19 ]. The top six countries for pancreatic cancer incidence were Hungary (ASR, 11.2), Uruguay (ASR, 10.7), Japan (ASR, 9.9), Slovakia (ASR, 9.6), Czechia (ASR, 9.5), and Austria (ASR, 9.0), with 9.0 and greater per 100000, and a total of 21 countries, including the United States (ASR, 8.2), had an ASR of the incidence between 8.1 and 8.9 per 100000, as shown in Figure Figure1A 1A [ 21 ]. The ASR of pancreatic cancer mortality was highest in Hungary and Uruguay, both at 10.2 per 100000, and a total of 26 countries, not including the United States (ASR, 6.6), had an ASR of the incidence between 7.2 and 8.6 per 100000, as shown in Figure Figure1B 1B [ 21 ]. The proportion of estimated new cases for pancreatic cancer in China was relatively high in East China (9.4 per 100000), Northeast (9.4), Northwest (6.8), and North China (5.3), and was comparatively low in Central China (5.2), Southwest (4.3), and South China (3.6), having obvious regional characteristics[ 13 ]. Age-standardized rates of pancreatic cancer were 3-fold to 4-fold higher in higher HDI countries, compared with lower HDI countries[ 18 ]. The higher incidence and mortality rates of pancreatic cancer were reported in countries and regions with higher levels of HDI and Gross Domestic Product (GDP) per capita, and the coefficients of determination (R 2 ) of HDI and GPD per capita were high for the incidence and mortality[ 22 ]. The higher incidence and mortality rates of pancreatic cancer in countries with higher HDI indicates the importance that paying more attention and implementing appropriate programme to reduce risk factors acts as an effective measure to control the incidence and mortality of the cancer[ 23 ].

Maps showing estimated age-standardized rates of incidence and mortality for pancreatic cancer worldwide in 2020, including both sexes and all ages. A: Incidence; B: Mortality. Citation: Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. [cited 20 Jan 2021]. In: International Agency for Research on Cancer [Internet]. Available from: https://gco.iarc.fr/today . Copyright ©International Agency for Research on Cancer 2021. Published by World Health Organization[ 21 ].
TRENDS OF PANCREATIC CANCER
Over the two decades from 2001 to 2020, the estimated new cases and deaths of pancreatic cancer have been increasing year by year in the United States, and the same trend has been observed among men and women, as shown in Figure Figure2. 2 . Using statistical models for analysis, in the United States, age-adjusted rates of new cases for pancreatic cancer remained stable from 2008 to 2017, and age-adjusted rates of death increased by an average of 0.3% each year from 2009 to 2018[ 14 ]. Prediction of pancreatic cancer incidence burden from the 28 member states of the EU and other selected countries around the world showed that in 2025, 2030, 2035, and 2040, the incidence will be 557688, 639030, 726740, and 815276, respectively, with growth rates of 21.5%, 39.2%, 58.4%, and 77.7%[ 24 ]. The incidence and mortality of pancreatic cancer in Africa will increase by 18327 and 17744 in 2040, respectively, with growth rates of 114.1% and 114.8%, the rates of which will be highest in the world, followed by Latin America and the Caribbean (incidence: + 99.3%; mortality: 101.0%)[ 25 ]. However, in 2040, the growth rates of the incidence and mortality in Europe will be lowest at 29.3% and 31.6%, respectively[ 25 ]. Based on China and India, both countries in Asia with more than one billion population, the incidence and mortality in Asia will increase by 190532 and 182127 in 2040, respectively, which will be the largest increase in terms of number[ 25 ]. In addition, standardized mortality rate of pancreatic cancer increased from 1.30 per 100000 to 3.32 per 100000 over 1991-2014 and might reach the peak in the ensuing 5 years in China, and the mortality rate was higher among elderly people and in urban and northeast/eastern regions than among young people and in rural and middle/western regions[ 26 ]. The incidence of pancreatic cancer was 12.1 per 100000 in 2010 and is predicted to increase to 15.1 and 18.6 per 100000 in 2030 and 2050, respectively, with an average annual growth of 1.1%[ 27 ]. In the age-stratified analysis, the over 65 years group will have the highest projected incidence (31.9 per 100000) in 2050, and the incidence is projected to increase gradually in the sex-stratified analysis, with an average annual growth of 1.3% in males and 0.9% in females[ 27 ].

Estimated new cases and deaths from 2001 to 2020 in the United States. The data is from Cancer Statistics that the American Cancer Society estimates the numbers of new cancer cases and deaths in the United States from 2001 to 2020[ 16 , 28 - 47 ].
The number of years of life lost (YLL) is a measure of premature mortality, taking into account simultaneously the number of deaths and life expectancy at age of death, and projection of YLL due to premature mortality ( i.e. , time-based approach) provides a comprehensive outlook of the fatal burden at a population level[ 27 ]. Due to premature death in individuals with pancreatic cancer, the total YLL was 5604 years in 2010 and is projected to increase to 9784 in 2030 and 14247 in 2050, with an average annual growth of 2.1%[ 27 ]. In the age-stratified analysis, the 40-64 years group will have the highest projected YLL (7588 years) in 2050, and the YLL is projected to increase gradually in the sex-stratified analysis, with an average annual growth of 2.1% in males and 2.2% in females[ 27 ].
In conclusion, pancreatic cancer, like other cancers such as lung, liver, and stomach cancer, will cause a huge economic burden to all countries and related populations in the next 20 years, especially China having a huge population, which is still a developing country. In order to reverse these trends and improve the prognosis of patients with pancreatic cancer, the most simple, direct, and effective way is to understand the risk factors affecting the occurrence and development of pancreatic cancer in detail, which provides comprehensive and reasonable guidance and suggestions for the prevention of pancreatic cancer, and offers reliable and feasible ideas for the early screening of pancreatic cancer.
CAUSES AND RISK FACTORS OF PANCREATIC CANCER
Pancreatic intraepithelial neoplasias (PanINs) are noninvasive epithelial proliferations in smaller pancreatic ducts, which progress from PanIN-1 (low-grade) to PanIN-2 (intermediate-grade) to PanIN-3 (high-grade)[ 48 ]. The differentiation of normal epithelium into PanIN-1/PanIN-2 and then into PanIN-3/invasive pancreatic cancer requires a considerable period of development, while the development process before high-grade PanIN-3 and invasive pancreatic cancer is the golden stage of preventing pancreatic cancer through effective interventions. Therefore, a thorough and comprehensive understanding of pancreatic cancer risk factors is of great practical significance for the prevention of pancreatic cancer. The exact cause of pancreatic cancer is unknown, but many non-modifiable and modifiable risk factors are associated with development of pancreatic cancer. Non-modifiable risk factors include age, gender, ethnicity, ABO blood group, microbiota, diabetes mellitus (DM), and family history and genetic susceptibility, while modifiable risk factors include smoking, alcohol drinking, dietary factors, pancreatitis, obesity, infection, and socioeconomic status and insurance. The influence of these factors on the occurrence, progression, and invasion of pancreatic cancer is analyzed and summarized as follows.
NON-MODIFIABLE RISK FACTORS
Both 89.4% of new cases of pancreatic cancer and 92.6% of deaths occur in patients over 55 years of age in the United States, the new cases are most frequently diagnosed among people 65-74 years of age with a median age at diagnosis of 70 years, and the percent of deaths is also highest among people of the same age group with a median age at death of 72 years[ 14 ]. The proportions at 40-64 years and over 65 years of age were 47.9% and 48.6% in diagnosed patients with pancreatic cancer in China[ 49 ]. The mortality rates of patients aged under 30, 30-44, 45-59, 60-74, and 75 and above among males are 0.1, 1.4, 10.1, 19.3, and 14.6 per 100000 in China, respectively[ 50 ], meaning that male pancreatic cancer population over 60 years of age has a higher mortality rate. The reference does not provide the mortality rates of pancreatic cancer in the five broad age groups in women and both sexes[ 50 ]. Worldwide, it is extremely rare for pancreatic cancer to be diagnosed before the age of 30, so it is typically a disease of the elderly. The risk factor also determines the need for screening and early detection of pancreatic cancer among the population over a certain age.
In the United States, the new cases of pancreatic cancer is 31950 among males and 28480 among females in 2020, and the deaths is 25270 among males and 22950 among females[ 16 ]. In China, the age-standardized respective incidence and mortality rate are 52.2 and 45.6 per 100000 among men in 2015, and 37.9 and 33.8 per 100000 among women[ 50 ]. On a global scale, the new cases are 243033 among men and 215885 among women in 2018, and the deaths are 226910 among men and 205332 among women[ 18 ]. The global respective incidence and mortality rates are 5.5 and 5.1 per 100000 among men in 2018, and 4.0 and 3.8 per 100000 among women[ 18 ]. Globally, the respective cumulative risk of developing pancreatic cancer and dying from it from birth to 74 years is 0.65% and 0.59% among males in 2018, and 0.45% and 0.41% among females[ 18 ]. The ratio of male to female for estimated new cases and deaths increased in the United States from 2001 to 2020, as shown in Figure Figure3. 3 . Thus, the worldwide incidence and mortality of pancreatic cancer are higher among males than females.

Ratio of male to female for estimated new cases and deaths from 2001 to 2020 in the United States. The data is from Cancer Statistics that the American Cancer Society estimates the numbers of new cancer cases and deaths in the United States from 2001 to 2020[ 16 , 28 - 47 ].
ABO blood group
The ABO antigens were first described by Landsteiner as erythrocyte antigens in 1900[ 51 ]. The antigens of ABO blood group are glycoproteins that are expressed on red blood cells and various epithelial cells, including the urothelium and gastrointestinal mucosa[ 52 ]. The phenotypic A and B antigens are terminal carbohydrates synthesized by the addition of monosaccharides catalyzed by a series of specific glycosyltransferases, and the phenotype O is characterized by deficiency of A and B glycosyltransferases[ 52 ]. There is growing evidence that ABO blood group may also be associated with carcinogenesis or progression of pancreatic cancer. A consortia-based evaluation and replication study showed that non-O blood group was associated with an increased risk of pancreatic cancer compared with blood group O[ 53 ]. A register-based cohort study showed that blood group A was associated with an increased risk of pancreatic cancer[ 54 ]. There was a significantly higher risk for developing pancreatic cancer in Chinese patients with the A or AB blood types than for those with type O[ 55 ]. El Jellas et al [ 56 ] reported that the prevalence of blood type A and subtype A1 was highest among the unresected cases, the unresected cases had the lowest frequency of blood group O, and patients with blood group O survived longer than non-O patients in the group of unresected cases. A study at Shanghai Pancreatic Cancer Institute showed that Chinese Han population with blood type A were more likely to develop pancreatic cancer, but people with blood type B were less likely to develop pancreatic neuroendocrine tumors and other types of pancreatic masses, compared with those with blood type O[ 57 ]. Hofmann et al [ 51 ] reported that patients with blood type O had more often well-differentiated PDAC compared with blood type non-O, and they elucidated the novel interaction between blood type immunoglobulin M isoagglutinins and PDAC O-GalNAc glycoproteins, which may contribute to the pathogenesis and progression of pancreatic cancer. Accordingly, the risk for people with blood type O to develop pancreatic cancer is lower than those with other blood types. In addition, the ABO allele that determines blood type A has two major subtypes, namely, A1 and A2, and the association of A1 but not A2 with pancreatic cancer could therefore suggest that the activity of blood type A glycosyltransferase plays a role in carcinogenesis[ 56 ]. The study showed that the A2 subtype has a single base deletion near the carboxyl terminal, and introducing the single base deletion into the expression construct of A1 transferase cDNA significantly reduced the activity of A transferase in DNA-transfected HeLa cells[ 58 ]. Therefore, clarifying the etiological mechanism between the risk of pancreatic cancer and ABO blood type may provide a new perspective for the treatment of this disease.
The burden of exocrine pancreatic disease, including pancreatic cancer, pancreatitis, and pancreatic cyst, differs among various ethnicities, and African-Americans and certain indigenous populations are at the greatest risk of developing these diseases[ 59 ]. Huang et al [ 60 ] observed that African-Americans, Native Americans, and Japanese-Americans had higher rates of developing pancreatic cancer, but no difference between Latino- and European-Americans, and found that African-Americans had a 20% greater risk of pancreatic cancer than European-Americans even after adjusting for known risk factors. In the studies including few minority patients, the neutrophil-to-lymphocyte ratio (NLR) is associated with a reduced overall survival in pancreatic cancer patients, and NLR > 5 was significantly associated with a worse overall survival compared with NLR ≤ 5[ 61 ]. Patients with an NLR ≤ 5 were also more likely to develop locally advanced disease than metastatic cancers and primary tumor located in the head or neck of the pancreas, while patients with an NLR > 5 were more likely to have liver metastases and albumin < 3.4 g/dL, suggesting that elevated NLR is an independent marker for poor prognosis and a potentially valuable factor[ 61 ]. Patients with an NLR ≤ 5 were more likely to be non-Hispanic Black, while patients with an NLR > 5 were more likely to be non-Hispanic White or Hispanic[ 61 ], suggesting that there are different predispositions and outcomes for pancreatic cancer between non-Hispanic Black and non-Hispanic White or Hispanic. Gad et al [ 62 ] found that the incidence of pancreatic cancer among Asian-Americans, especially malignancies of the body and tail of the pancreas, as well as the mortality based on the incidence, was overall on the rise in an epidemiological study, without respect to age, sex, or stage subgroup. Amaral et al [ 63 ] also emphasized the importance of the influence of ethnicity on somatic mutations in Brazilian patients with PDAC. To elucidate the reasons for the racial differences in the incidence of pancreatic cancer may help us improve the understanding and prevention of this disease.
Oral microbiota: Several epidemiological studies have found the direct relationship between oral bacteria and pancreatic cancer[ 64 ]. Farrell et al [ 65 ] reported that the levels of two bacteria biomarkers ( Neisseria elongate and Streptococcus mitis ) were lower in patients with pancreatic cancer than in healthy controls, and found that the combination of the two bacteria biomarkers distinguished pancreatic cancer patients from healthy subjects with an area under the curve value of 0.90, sensitivity of 96.4%, and specificity of 82.1%. Torres et al [ 66 ] found that the ratio of Leptotrichia to Porphyromonas in the saliva of pancreatic cancer patients was significantly higher than that in healthy individuals or those with other disease. Fan et al [ 67 ] found that Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans were associated with a higher risk of pancreatic cancer, and phylum Fusobacteria and its genus Leptotrichia were associated with a decreased risk of this cancer. Olson et al [ 68 ] reported that the mean relative proportions of Firmicutes and related taxa were higher in patients with pancreatic cancer, while the mean relative proportions of Proteobacteria and related taxa were higher in controls.
Gut microbiota: Studies have confirmed that gut microbiota is associated with recognized risk factors for pancreatic cancer, such as obesity and type II diabetes, suggesting the relationship between gut bacteria and pancreatic cancer[ 64 ]. In recent decades, multiple and highly complex effects of gut microbiota on pancreatic cancer have been identified as potential risk factors for the development and progression of this tumor[ 69 ]. A prospective study, for the first time, analyzed gut microbial profile in Chinese pancreatic cancer cohorts by MiSeq sequencing, revealing a significant decline in gut microbial diversity and a unique microbial profile in pancreatic cancer, due in part to the decline in alpha diversity[ 70 ]. Additionally, the microbial profile changed in pancreatic cancer, with an increase in certain pathogens and lipopolysaccharides (LPS)-producing bacteria and a decrease in probiotics and butyrate-producing bacteria[ 70 ]. LPS might play a pro-inflammatory pro-tumor role by activating the nuclear factor-κappa B (NF-κB) pathway, producing proinflammatory cytokines [tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1] and leading to liver inflammatory and oxidative damage[ 71 ]. After LPS treatment, Ras activity in cells prepared from acinar-Ras mice was greatly elevated and maintained at a high level for a long time, and severe chronic pancreatitis and PanIN lesions were induced in acinar-Ras mice, accompanied by sustained elevated Ras activity, whereas there was no observed effects in control mice, suggesting that LPS treatments led to fibrosis and PanIN formation in the presence of oncogenic Ras[ 72 ]. Therefore, LPS may have a greater pathological impact on patients carrying cells expressing oncogenic RAS, which may explain individual differences in response to infection, suggesting the association between chronic bacterial infectious diseases and colon and pancreatic cancers[ 72 ]. As a member of the RAS family of GTP-binding proteins, KRAS mediates a wide variety of cellular functions including proliferation, differentiation, and survival[ 73 ], and the prevalence of oncogenic KRAS mutation in PDAC ranges from 88% to 100%[ 74 ]. Consistent with a central pathogenic role of the KRAS G12D mutation, mice engineered with pancreas-specific expression of this activated KRAS allele sustain classical PanIN lesions that can progress to PDAC in the appropriate tumor suppressor background[ 73 ]. Thomas et al [ 75 ] found that the proportion of poorly differentiated PDAC in the microbiota-intact mice was higher than that in microbiota-depleted mice (89.75 vs 34.8%, respectively), demonstrated that the intestinal microbiota accelerated pancreatic carcinogenesis in the KRAS G12D /PTEN lox/+ mice model of pancreatic cancer, and considered that the intestinal microbiota had a long-distance role on PDAC progression. In addition, based on crucial genera associated with pancreatic cancer, gut microbial markers might achieve an excellent classification capacity between pancreatic cancer and healthy controls, suggesting that the specific alterations of gut microbiota might become non-invasive biomarkers for pancreatic cancer diagnosis[ 70 ].
Pancreatic microbiota: A number of different bacterial taxa are found in pancreatic tissue contents, including those known to inhabit the oral cavity, which suggest that the pancreas is not a sterile organ[ 76 ]. The relative abundance of bacterial taxa at the genus level in the pancreas has a substantial between-person variability, and bacterial composition of the pancreatic duct, head, and tail as well as the duodenum was highly similar in the same individuals[ 76 ]. The study showed that the community composition of the microbiota in the human pancreas failed to discriminate between normal and disease states, and that the acquisition of pancreatic bacteria is not a physiologic process, even under conditions of intestinal inflammation[ 75 ]. Del Castillo et al [ 76 ] found that the presence and relative abundance of Lactobacillus were lower in pancreatic tissue of cancer subjects and the relative abundance of periodontal-related pathogens was higher in cancer subjects, when compared with noncancer subjects. Pushalkar et al [ 77 ] found that the bacteria ( Enterococcus faecalis and Escherichia coli ) could migrate into the pancreas, confirmed that the abundance of intrapancreatic bacteria in both mice and patients with PDAC was markedly greater compared with normal pancreas by 16S rRNA fluorescence in situ hybridization and qPCR analysis, and consider that the bacteria promote the progression of pancreatic oncogenesis in both preinvasive and invasive models[ 77 ]. Maekawa et al [ 78 ] mainly detected Enterococcus and Enterobacter species in bile, demonstrating that Enterococcus and Enterobacter can survive in pancreatic juice and/or bile, and found that 29 of 36 pancreatic juice samples were positive for bacterial DNA[ 78 ]. Enterococcus faecalis was also found in pancreatic tissue from patients with chronic pancreatitis and pancreatic cancer, and serum antibodies to capsular polysaccharide of Enterococcus faecalis were elevated in patients with chronic pancreatitis[ 78 ]. They demonstrated that Enterococcus faecalis is involved in the progression of chronic pancreatitis using the model mice with caerulein-induced chronic pancreatitis, which may ultimately result in the development of pancreatic cancer[ 78 ].
In addition, Pushalkar et al [ 77 ] also found that the microbiome regulates immunogenicity in PDAC and programs tumor-associated macrophages via Toll-like receptor signaling to induce immune tolerance, and bacterial communities are distinct between early and advanced PDAC. Geller et al [ 79 ] demonstrated that bacteria are a component of the PDAC tumor microenvironment, and estimated that bacteria colonized PDAC samples had an average of one bacterium per 146 human cells. Their results indicated that PDACs contain bacteria that can potentially modulate tumor sensitivity to gemcitabine, and they considered that the bacteria play a critical role in mediating resistance to chemotherapy[ 79 ]. By studying colon cancer models, they found that bacteria can metabolize the chemotherapeutic drug gemcitabine (2′,2′-difluorodeoxycytidine) into its inactive form (2′,2′-difluorodeoxyuridine), which depends on the expression of a long isoform of bacterial cytidine deaminase, seen primarily in γ-proteobacteria[ 79 ].
The above various microorganisms have different influences on the occurrence, development, and invasion of pancreatic cancer in different ways. Therefore, the study on the influence of microorganisms on pancreatic cancer will provide more new insights to reveal its etiology. And studying the effects of microorganisms on pancreatic cancer has the potential to be used as a target for the regulation of disease progression and treatment.
Family history and genetic susceptibility
Hereditary pancreatic cancer includes inherited cancer syndromes with a recognized known germline mutation associated with an increased risk of pancreatic cancer and familial pancreatic cancer with two or more cases of pancreatic cancer in their families[ 80 ]. Pancreatic cancer associated with hereditary syndromes or familial pancreatic cancer accounts for about 10% of cases[ 81 ]. Family history of pancreatic cancer was associated with an increased pancreatic cancer risk when compared with cancer-free family history, with the risk being greater when ≥ 2 first-degree relatives suffered pancreatic cancer and among current smokers[ 82 ]. Members of familial pancreatic cancer kindreds having at least one first-degree relative affected by pancreatic cancer had a 9-fold increased risk of developing pancreatic cancer, whereas members of sporadic pancreatic cancer kindreds having a first-degree relative with pancreatic cancer did not have an increased risk[ 83 ]. Risk was higher among members of familial pancreatic cancer kindreds with a young-onset patient (< 50 years) in the kindred than those without a young-onset case in the kindred[ 84 ].
Genetic mutations associated with an increased risk of pancreatic cancer include STK11/LKB1 , CDKN2A (p16) , BRCA1/2 , PRSS1/SPINK1/CFTR , mismatch repair genes ( MLH1/MSH6/MSH2/PMS2 ), ATM , and PALB2 (a new pancreatic cancer susceptibility gene)[ 85 ]. And pancreatic cancer is also found to be associated with these familial cancer syndromes for which genetic mutations correspond, such as Peutz-Jeghers syndrome ( STK11/LKB1 ), familial atypical multiple mole melanoma ( CDKN2A ), hereditary breast cancer ovarian cancer syndrome ( BRCA1/2 ), and hereditary non-polyposis colorectal carcinoma syndrome ( MLH1/MSH6/MSH2/PMS2 )[ 25 , 85 ].
A study from Mayo Clinic showed that the aggregate prevalence was 36/302 (11.9%) for all cases with any positive PDAC family history[ 86 ]. Seven PDAC-associated genes ( ATM , BRCA1 , BRCA2 , CDKN2A , MSH2 , PALB2 , and PMS2 ) and four genes with no known PDAC association ( BARD1 , CHEK2 , MUTYH/MUY , and NBN ) were identified as pathogenic variants in the study[ 86 ]. Kindreds with at least one pair of first-degree relatives who were affected by PDAC were considered FPC, and kindreds with at least two affected blood relatives that did not meet the FPC definition were considered “familial non-FPC”[ 86 ]. Thirty-six (12%) patients carried at least one pathogenic variant in one of 11 genes, and the probabilities of carriers with pathogenic variant among FPC patients and familial non-FPC patients were 14% and 9%, respectively[ 86 ]. Pathogenic variants (n) identified in PDAC patients were BRCA2 (11), ATM (8), CDKN2A (4), CHEK2 (4), MUTYH/MYH (3 heterozygotes, not biallelic), BRCA1 (2), and 1 each in BARD1 , MSH2 , NBN , PALB2 , and PMS2 [ 86 ]. Regardless of FPC status, multiple susceptibility gene testing may be necessary in PDAC patients with a family history of pancreatic cancer, which will provide genetic risk counseling for families[ 86 ]. Therefore, the study of the family characteristics and genetic features of pancreatic cancer is of great clinical significance in identifying the susceptible population of pancreatic cancer, screening the high-risk individuals of pancreatic cancer, and early diagnosis of pancreatic cancer.
Diabetes mellitus
In comparison with patients without diabetes, those who were recently diagnosed with diabetes had an nearly 7-fold increase in risk of developing pancreatic cancer[ 87 ]. Either hyperglycaemia or diabetes is found among as many as 80% of patients, both of which can be detected in the presymptomatic phase, and on the contrary, older patients with new-onset diabetes have about an 8-fold higher risk of developing pancreatic cancer than the general population, suggesting a “dual causality” between diabetes and PDAC[ 88 ], in that both long-standing type 2 DM (T2DM) is a risk factor of developing PDAC and PDAC is assumed to be a cause of diabetes in many cases[ 89 ]. A multiethnic cohort study also showed that recent-onset diabetes is a manifestation of pancreatic cancer and long-standing diabetes is a risk factor of developing this cancer[ 90 ]. At present, the prevalence of diabetes in China is on the rise and has the largest diabetes epidemic worldwide, and in 2013, the estimated total prevalence was 10.9% for diabetes and 35.7% for prediabetes among adults in China, indicating the importance of diabetes as a public health problem in China[ 91 ]. In 2011-2012, the prevalence of diabetes was estimated from 12% to 14% among US adults, and participants who were non-Hispanic black, non-Hispanic Asian, and Hispanic had a higher prevalence[ 92 ]. The global spread of this enormous medical burden further highlights the necessity to better understand the pathophysiological relationship between T2DM and pancreatic cancer.
A growing body of epidemiological and experimental evidence shows that chronic hyperinsulinaemia increases the risk of cancers of the colon and endometrium, and probably other tumours (such as pancreas and kidney)[ 93 ]. Hyperinsulinemia, especially intrapancreatic, due to obesity and insulin resistance in patients with prediabetes or early T2DM may believably conduce to the observed increased risk of developing PDAC[ 89 ]. The high level of islet hormones in blood directly reaches groups of acinar and ductal cells and acts on insulin-like growth factor-1 (IGF-1) receptors to promote survival and proliferation of acinar and ductal cells[ 89 ]. The characteristics of T2DM patients and the overwhelming majority of obese individuals are insulin resistance with ensuing hyperinsulinemia and high levels of IGF-1, which can act as potent growth-promoting factors[ 94 ]. In addition, Butler et al [ 95 ] reported that replication of pancreatic duct cells in lean subjects with T2DM had a 4-fold increase compared with lean non-diabetic controls, suggesting that the increased risk of pancreatitis and pancreatic cancer in T2DM is driven by replication of chronically increased pancreatic duct cell replication[ 95 ].
The desmoplastic response attribute to production and proliferation of extracellular matrix proteins in tumor-associated fibroblasts, activating pancreatic stellate cells (PaSC)[ 94 ]. Yang et al [ 96 ] reported that progression of high-fat diet-induced PDAC in mice is associated with hyperglycemia, hyperinsulinemia, and PaSC activation, and found that the pancreas from patients with T2DM showed substantial collagen deposition and activated PaSC in islet and peri-islet exocrine pancreas compared with normal control[ 96 ]. Both quiescent and activated PaSC coexpress insulin and IGF-1 receptors, the expression of which was modulated by both insulin and glucose[ 96 ]. Insulin induces rapid tyrosine autophosphorylation of insulin/IGF-1 receptors at specific kinase domain activation loop sites, activates Akt/mTOR/p70S6K signaling, and inactivates FoxO1, a transcription factor suppressing cell growth[ 96 ]. In activated PaSC, insulin promotes cell proliferation and production of extracellular matrix proteins, and specific inhibition of mTORC1 and mTORC2 can abolish the above effects, suggesting that increased local glucose and insulin concentrations are associated with obesity and T2DM promotes PaSC growth and fibrosing responses[ 96 ]. In premalignant H6c7-kras cells, hyperglycemia increases secretion and signaling of transforming growth factor beta1 (TGF-β1) and induces properties of cancer stem cells depending on TGF-β1-signaling, suggesting that hyperglycemia promotes pancreatic ductal epithelial cells to acquire the properties of mesenchymal and cancer-stem cells by activating TGF-β signaling[ 97 ]. Li et al found that patients with A blood type who also had DM had a greater odds of having pancreatic cancer, and further research is needed to confirm the results and to identify the mechanisms by which A blood type and DM jointly contribute to the risk of pancreatic cancer progression and development[ 55 ].
There are many mechanisms that explain the effect of DM on pancreatic cancer and the relationship between the two. However, in order to reveal the real relationship between diabetes and pancreatic cancer, it still needs to be further studied to provide new strategies for the prevention of pancreatic cancer.
MODIFIABLE RISK FACTORS
Epidemiological studies have shown that many causative factors are associated with pancreatic cancer, and cigarette smoking has the strongest positive association with the risk of developing the cancer[ 98 ]. Due to smoking, the estimated prevalence was about 30% in many parts of the world and the risk of pancreatic cancer was doubled in smokers, whereas the population-attributable risk caused by smoking is about 25% for pancreatic cancer, meaning that the overall burden of this cancer would be reduced if smoking was completely eliminated[ 99 ]. Patients with pancreatic cancer who smoked prior to diagnosis had an about 40% increased hazard for death compared with those who never smoked[ 100 ]. And long-term smoking portended worse outcomes for current smokers, but former smokers experienced outcomes similar to those who had never smoked, suggesting that quitting smoking can have potential beneficial effects[ 100 ]. A large European case-control study confirmed that current smokers had a 72% increased risk of developing pancreatic cancer compared with never-smokers, and the study also endorsed that around 16% of all pancreatic cancer diagnoses could be avoided through tobacco preventive measures in terms of attributable risk[ 101 ]. And analysis of dose-response relationships confirmed that higher smoking intensity, longer smoking duration, and increased cumulative dose levels were associated with a further increased risk of pancreatic cancer, whereas smoking cessation led to a gradual decline in the risk of pancreatic cancer[ 101 ]. Smoking also notably increases the risk of developing pancreatic cancer in individuals with a family history of this cancer[ 82 ]. These studies suggest that smoking cessation has a potential benefit to improve survival for patients with pancreatic cancer and helpfully prevent pancreatic cancer in those at risk.
As an avoidable risk factor, smoking is of particular concern, and elucidating the mechanisms involved would significantly reduce the number of PDAC cases diagnosed each year[ 102 ]. Smoking-induced inflammation was accompanied by enhanced activation of PaSC and elevated levels of serum retinoic acid-binding protein 4, suggesting increased bioavailability of retinoic acid that is conducive to differentiation of myeloid-derived suppressor cells to tumor-associated macrophages and dendritic cells[ 103 ]. And smoking exposure also leads to partial suppression of the immune system in the early progression of pancreatic cancer[ 103 ]. In xenografts of patient-derived pancreatic cancer, nicotine intervention promoted growth and metastasis of tumor, and it was confirmed that nicotine reduced survival by enhancing paracrine HGF-MET signaling in the pancreatic cancer microenvironment[ 104 ]. In addition, nicotine induced dedifferentiation of acinar cells by activating AKT-ERK-MYC signaling, thereby inhibiting the activity of Gata6 promoter and losing GATA6 protein, and subsequently causing loss of acinar differentiation and over-activation of oncogenic K-Ras[ 105 ]. And metformin could inhibit nicotine-induced carcinogenesis of the pancreas and tumor growth by up-regulating GATA6 expression and promoting programmed differentiation of acinar cell[ 105 ]. Benzo(a)pyrenes, polycyclic aromatic hydrocarbons, and tobacco-specific nitrosamines are several carcinogens identified in tobacco smoke, most of which play a genotoxic role by formation of DNA adducts and generation of reactive oxygen species, leading to mutations in vital genes such as K-Ras and p53 [ 106 ]. Nicotine and other carcinogenic components in tobacco smoke can directly promote growth of tumor cells, change cross-talk between tumor and stromal cells within the tumor microenvironment, and enhance infiltration of myeloid-derived suppressor cells[ 100 ]. Therefore, the study and elucidation of carcinogenic mechanism of carcinogens in tobacco smoke will contribute to the treatment and prevention of pancreatic cancer caused by related factors.
Alcohol drinking
East Asians have a high proportion in inefficient metabolism of acetaldehyde, so alcohol drinking may play a more important role in the developing pancreatic cancer among East Asians[ 107 ]. According to many studies, there is no doubt that the risk of pancreatic cancer is associated with high alcohol consumption (more than three drinks per day), but no association was found with low-to-moderate alcohol consumption[ 25 ]. A population-based study demonstrated that heavy alcohol consumption and binge drinking increased estimated risk of developing pancreatic cancer among males but not among females[ 108 ]. It may also be the reason why pancreatic cancer has a higher incidence and mortality in men than in women. And the study also suggested that either binge or consistent heavy alcohol consumption persistently increased the risk of developing pancreatic cancer regardless of the temporal proximity between alcohol consumption and diagnosis of pancreatic cancer[ 108 ]. A large prospective study suggested that baseline and lifetime alcohol consumption was positively associated with the risk of developing pancreatic cancer, and the estimated risk for beer and spirits/liquors was more apparent than wine[ 109 ]. Alcohol plays an independent role in promoting PDAC associated with fibrosis formed by a stellate cell-independent mechanism and further boosts formation of PanIN lesion and induction of M2 macrophages in the context of chronic pancreatitis[ 110 ]. This is an important finding, namely, M2 macrophages suppress the directed immune mechanisms of cancer and block the recruitment of T cells into the tumor, further promoting cancer progression[ 111 ]. Mice that expressed mutant K-ras gene developed early and advanced forms of the most common pancreatic cancer in humans[ 112 ]. Specific mutations in the K-ras oncogene may be more commonly found in alcohol consumers with pancreatic cancer, and may be initiators or terminators of pancreas cancer associated with heavy alcohol consumption[ 108 ]. Additionally, alcohol might promote the development of cancer by inducing oxidative stress and lipid peroxidation, and alcohol abuse may also accelerate the progression of tumor by boosting pancreatic inflammation[ 112 ].
Two NAD-dependent enzymes, namely, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), are mainly involved in alcohol metabolism in human[ 113 ]. In the body, alcohol is first converted into acetaldehyde by ADH oxidation, and acetaldehyde is then converted into non-toxic acetate by ALDH oxidation for excretion[ 113 ]. In the human liver, there are two kind of ALDHs, including ALDH1 (cytosolic) and ALDH2 (mitochondrial), and only mitochondrial ALDH2 oxidizes acetaldehyde to acetate at physiological concentrations[ 114 ]. The variant ALDH2*2 allele can significantly decrease ALDH2 enzyme activity and affect alcohol response, which is a striking genetic polymorphism[ 113 ].
In many East Asian countries, the ALDH2*2 allele associated with reduced enzyme activity is found in 30%-50% of the population, resulting in the inefficient metabolism of the carcinogenic acetaldehyde generated from alcohol metabolism[ 107 ]. Thus, acetaldehyde accumulates in ALDH2*2 carriers, even after a moderate intake of ethanol (0.5 g/kg)[ 115 ], also leading to a higher risk of developing alcohol-related cancers in individuals carrying the ALDH2*2 allele. Kanda et al [ 116 ] found that the impact of alcohol on pancreatic cancer risk was associated with rapid production or high accumulation of acetaldehyde, indicating that acetaldehyde may play a substantial role in the potential mechanism of pancreatic cancer[ 116 ]. This can also provide guidance and help to prevent pancreatic cancer for people of different groups and races.
Dietary factors
In general, diets high in fruits, vegetables, and other plant-based foods can reduce the risk of developing pancreatic cancer, while dietary patterns rich in meat and animal products can increase the risk of the cancer[ 107 ]. Intake of meat, especially red meat, cooked meat at high temperature, and meat-associated heterocyclic amines, and overall mutagenic activity may induce the development of exocrine pancreatic cancer[ 117 ]. Foods are often heat processed and may contain advanced glycation end products (AGEs) that can be formed by a nonenzymatic reaction between reducing sugars and free amino groups on proteins, peptides, and amino acids[ 118 ]. When the carbonyl group of reducing sugar like glucose or its oxidation or lipid peroxidation products react with the ε-amino group of lysine or the guanidino group of arginine, AGEs can be formed, including imidazolones, pentosidine, pyrraline, and N ε -(carboxymethyl)lysine (CML)[ 118 ]. AGEs accumulate in sera and tissues during the ageing process because of glycolytic and oxidative reactions, reduced activity of the detoxification systems, cigarette smoking, and consumption of high-temperature-processed foods[ 8 ]. Excessively high concentrations of AGEs in human tissue and circulation accelerate oxidative stress and inflammation, which may play a pathogenic role[ 118 ]. Exogenous AGEs, in particular those derived from the diet, have been claimed to contribute to several disease processes, including cancer in general and, specifically PDAC[ 8 ]. CML is frequently used as a marker for AGEs in general[ 119 ]. Jiao et al [ 120 ] proposed a novel mechanism that consumption of heat-treated red meat can cause chronic inflammation and subsequently lead to pancreatic cancer, and considered that consumption of dietary CML-AGE was associated with a modest increase in risk of pancreatic cancer for men, which might partly explain the positive correlation between red meat and pancreatic cancer. A model experiment showed that AGEs markedly accelerated the development of pancreatic cancer and inhibition of AGE prevented the tumor-promoting effect of diabetes[ 8 ]. Therefore, exogenous AGEs from processed/ grilled/baked foods may be involved in the genesis and development of pancreatic cancer.
Many nutrients and phytochemicals in fruits and vegetables have antioxidant, anti-mutagenic, and anti-carcinogenic properties, especially for water-soluble vitamins[ 121 ]. Additionally, isothiocyanates found in cruciferous vegetables powerfully induce the detoxification enzymes, and assist in the removal of potential carcinogens[ 121 ]. Higher intake of fruit and vegetables is associated with a reduced risk of pancreatic cancer[ 122 ], and cruciferous vegetable intake might be inversely associated with pancreatic cancer risk[ 123 ]. A meta-analysis of epidemiological studies showed that high intake of dietary fiber was associated with a risk reduction of pancreatic cancer[ 124 ]. A European Prospective Investigation Into Cancer-Norfolk study showed that high-fiber diet altered a positive correlation between red/processed meats and the PDAC development but not those with lower fiber intake, and fiber intake made few alteration for the PDAC risk in past and current smokers[ 125 ]. This study showed that fiber intake may be beneficial for those with high meat intake, but the findings do not suggest that high fiber intake can protect against the PDAC development[ 125 ]. Therefore, further prospective cohort studies are needed to investigate the effect of fiber intake on the development and progression of pancreatic cancer. A low-fat dietary intervention was associated with a reduced incidence of pancreatic cancer in overweight or obese women in the Women’s Health Initiative Dietary Modification trial[ 126 ]. Therefore, it is of great significance for the prevention of pancreatic cancer by adjusting the diet of pancreatic cancer susceptible population. And it is worth further investigating how both diet and lifestyle may work together to promote or inhibit pancreatic cancer.
Pancreatitis
The majority of burden in exocrine pancreatic disease arises from acute pancreatitis, chronic pancreatitis, and pancreatic cancer[ 127 ]. Acute pancreatitis, an inflammatory disease of exocrine pancreas, is associated with injury and necrosis of tissue[ 128 ]. It has been reported that acute pancreatitis may be an early symptom of pancreatic cancer[ 129 ]. A nationwide matched-cohort study in Denmark showed that patients hospitalized with acute pancreatitis had an increased risk of developing pancreatic cancer compared with age- and gender-matched controls in the general population[ 130 ]. Rijkers et al [ 131 ] found that patients who suffered a first incident of acute pancreatitis and had no further progression to chronic pancreatitis had a 0.4% risk of developing pancreatic cancer, but the risk of pancreatic cancer increased 9-fold for those who progressed to chronic pancreatitis, suggesting that screening for pancreatic cancer after a first incident of acute pancreatitis, especially in patients who had further progression to chronic pancreatitis, could potentially result in more curable resections and improved survival[ 131 ]. The risk of pancreatic cancer increased markedly after an initial diagnosis of acute pancreatitis, regardless of its type, and gradually decreases with passage of time[ 132 ]. An increase in the number of recurrent episodes of acute pancreatitis was associated with an increased risk of developing pancreatic cancer[ 132 ].
Patients who have an episode of acute pancreatitis are 20%-30% more likely to have one or more relapse, and approximately 10% of the relapsing cases progress to chronic pancreatitis[ 133 ]. Chronic pancreatitis is a progressive inflammatory disease, and causes pancreatic parenchyma to be replaced by fibrous tissue, resulting in a loss of acinar and islet cells[ 134 ]. Chronic pancreatitis may cause mild or asymptomatic debilitating pain, attack(s) of acute pancreatitis, endocrine and/or exocrine deficiency, local and/or systemic complications, and pancreatic cancer[ 135 ]. In recent decades, there is accumulating evidence that longstanding pre-existing chronic pancreatitis is a strong risk factor of developing pancreatic cancer[ 25 ]. Low body mass index and pancreatic exocrine insufficiency in patients with chronic pancreatitis define a high-risk population with latent PDAC[ 136 ]. In comparison with the general population, patients with chronic pancreatitis had a significantly increased risk of pancreatic cancer, especially those with an older age at onset and a > 60 pack-year smoking history[ 137 ]. Five years after diagnosis, the risk of pancreatic cancer increased nearly 8-fold in patients with chronic pancreatitis, but the association diminishes with long-term follow-up[ 138 ]. The same risk trend was observed in patients with recurrent acute pancreatitis and chronic pancreatitis, suggesting the need for close follow-up in the first few years after diagnosis of these two types of pancreatitis to avoid neglect of pancreatic cancer. Patients who need surgery to treat chronic pancreatitis have a very high risk of developing pancreatic cancer, and early surgical intervention can play a role in preventing the progression of chronic pancreatitis to pancreatic cancer[ 139 ]. Chronic pancreatitis patients with de novo postoperative diabetes have a high suspicion index of developing pancreatic cancer after surgery[ 139 ].
Patients with early-onset pancreatitis caused by genetic factors appear to have a higher risk of developing pancreatic cancer[ 140 ]. Mutations of susceptibility genes in chronic pancreatitis can determine hereditary pancreatitis, idiopathic chronic pancreatitis, and cystic fibrosis, and Cazacu et al [ 140 ] found that mutations of cystic fibrosis transmembrane conductance regulator ( CFTR ) genes modestly increase the risk of pancreatic cancer in a meta-analysis. A total of 50080 patients were diagnosed with pancreatic cancer, of which 14.8% (7420 cases) were diagnosed with idiopathic pancreatitis prior to the diagnosis of cancer[ 141 ]. After pancreatitis diagnosis, six risk factors significantly associated with pancreatic cancer diagnosis included age between 40 and 90 years, African-American race, male sex, smoking, obesity, and DM, suggesting that it may be warranted to screen patients older than 40 years with unclear etiology of pancreatitis, especially for African-Americans and male population[ 141 ].
The study of the promoting effect of pancreatitis on the development of pancreatic cancer is beneficial to the early detection of pancreatic cancer, and can provide more guidance for the prevention of pancreatic cancer. However, in order to better understand the promoting role of pancreatitis on pancreatic cancer, more studies are needed to clarify the mechanism of pancreatitis in the development of pancreatic cancer.
Obesity has been more and more recognized as a strong but modifiable risk factor for pancreatic cancer[ 142 ]. Relevant studies have confirmed that obesity is associated with an increased incidence of pancreatic cancer and potentially worse outcomes of this cancer[ 142 ]. A cohort study with pooled analysis found that central obesity was associated with increased mortality of pancreatic cancer, independent of body mass index, and also suggested that being overweight or obese during early adulthood may have a significant impact on the mortality risk of pancreatic cancer later in life[ 143 ]. A nationwide study including 1.79 million Israeli adolescents showed that obesity (≥ 95 th percentile) was associated with an increased risk of pancreatic cancer later in life among both men and women compared with normal weight (5 th to- < 85 th percentile)[ 144 ].
There have been two biological mechanisms that were proposed to explain the underlying association between obesity and risk of pancreatic cancer, including inflammation and hormonal misbalance[ 142 ]. Many human cancers result directly from chronic inflammation, and inflammation has emerged to be a key mediator of pancreatic cancer development[ 145 ]. Changes in the fibro-inflammatory microenvironment are the major feature of obesity-associated pancreatic tumors[ 146 ]. Obesity is a pro-inflammatory condition, and both hypertrophied adipocytes and immune cells (primarily lymphocytes and macrophages) residing in adipose tissue contribute to increased circulating levels of pro-inflammatory cytokines like TNF-α, IL-6, leptin, and adiponectin[ 147 ]. The imbalance between these finely regulated pro-inflammatory and anti-inflammatory bioactive molecules leads to changes in tissue microenvironment, which further have influence on cell proliferation, apoptosis, cell invasion, and angiogenesis[ 148 ]. For example, Hertzer et al [ 149 ] reported that conditional KRAS G12D mice with a high fat, high calorie diet exhibited significantly increased inflammation in the peri-pancreatic fat accompanied by elevated levels of several inflammatory cytokines, such as IL6, IL13, and IFN-γ, suggesting that obesity-associated inflammation in peri-pancreatic fat may accelerate pancreatic neoplasia in the model mice[ 149 ].
Obesity is also often associated with insulin resistance and T2DM, along with raised levels of insulin and IGF-1[ 142 ]. Insulin resistance is a hallmark of T2DM, in which insulin fails to trigger adequate glucose uptake, resulting in accumulation of glucose in bloodstream and raised levels of insulin[ 150 ]. Hyperglycemia can enhance the availability of nutrients to cancer cells which metabolize glucose through the Warburg effect[ 151 ]. Islet adaptation enhances hormone production, processing, and secretion in the setting of obesity[ 146 ]. Even moderate overall and abdominal obesity and weight gain during adulthood were independently associated with an increased risk of developing hyperinsulinemia in non-diabetic middle-aged men[ 152 ]. Hyperinsulinemia causes a rise of IGF-1 which activates PI3K/MAPK/mTOR pathways after binding with its receptor, or the IGF receptor[ 148 ]. Overactivation of these pathways can activate the Ras/ERK pathway, leading to an increase in cell division, and IGF-1 activating PI3K/AKT/mTOR pathways promotes proliferation and inhibits apoptosis[ 142 ].
The mutation of oncogenic KRAS is the major event in pancreatic cancer and permanently activates KRAS protein, and then the protein serves on a molecular switch to activate various signaling pathways and transcription factors in cells, inducing cell proliferation, migration, transformation, and survival[ 153 ]. In comparison with lean KC mice, the pancreas of obese KC mice showed an increase in activation of KRAS downstream pathways, including MAPK and PI3K/AKT/ mTORC1[ 154 ]. Chung et al [ 146 ] found that β-cell aberrantly expressed peptide hormone cholecystokinin in response to obesity and showed that islet cholecystokinin promoted oncogenic KRAS-driven tumorigenesis in pancreatic duct.
Helicobacter pylori ( H. pylori ) found mainly in the stomach is a Gram-negative microaerophilic pathogen that chronically infects as much as half the world's population[ 155 ]. H. pylori infection is associated with a variety of malignancies, such as gastric cancer, premalignant lesions of the stomach (atrophic gastritis and intestinal metaplasia), gastric lymphoma, pancreatic cancer, colorectal cancer, and laryngeal cancer[ 156 ]. With an estimated prevalence between 25% and 50% in Westernized countries, H. pylori could result in 4% to 25% of all cases with pancreatic cancer in these countries[ 157 ]. H. pylori infection is closely, albeit weakly, associated with the development of pancreatic cancer, and the association is prominent in Europe and East Asia, but less so in North America[ 158 ]. Cytotoxin-associated antigen A (CagA), a 120-145-kDa protein, was for the first time described as a virulence factor of H. pylori related to peptic ulcers[ 159 ]. A risk of pancreatic cancer increased in individuals with seropositivity for CagA-negative H. pylori , whereas the risk decreased in individuals with seropositivity for CagA-positive H. pylori [ 160 ]. CagA-negative strains of H. pylori might be a causative factor of pancreatic cancer[ 161 ]. Xiao et al [ 158 ] reported that CagA-positive H. pylori strains appear not to be associated with pancreatic cancer.
The net effects of H. pylori colonization in the gastric antrum are paracrine disinhibition of antral G-cell function, hypergastrinemia, and hyperacidity[ 162 ]. The risk of pancreatic cancer is increased by long-term conditions of excess gastric/ duodenal acidity[ 162 ]. Gastric acid drives pancreatic bicarbonate secretion and, a consequence of hyperchlorhydria and suppressed somatostatin increases bicarbonate output from the pancreas in H. pylori carriers[ 162 ]. Low-level, prolonged generating of secretin or pancreatic bicarbonate increases the activity and turnover rate of ductular epithelial cell to sufficiently enhance the carcinogenic effect of environmental or endogenous N-nitroso carcinogens[ 162 ]. Asymptomatic H. pylori colonization, non-ulcer dyspepsia, or duodenal ulcers, and exposure to N-nitroso carcinogens via dietary or other routes in individuals would increase the risk of developing pancreatic cancer by increasing basal secretors or pancreatic bicarbonate[ 162 ]. CagA injected into gastric parietal cells through the interaction of H. pylori with integrin results in the activation of extracellular regulated protein kinases (ERK1/2) to further mobilize NF-κB p50 homodimers into the nucleus, leading to the inhibition of gastric H,K-adenosine triphosphatase (H,K-ATPase) α subunit transcription and the repression of gastric acid secretion[ 163 ]. Seropositivity of CagA-positive H. pylori was shown to protect against pancreatic cancer when compared to CagA-negative H. pylori , suggesting that differential modification of CagA-negative vs CagA-positive strains of H. pylori on chronic gastric acidity may be involved in modulating the risk of pancreatic cancer[ 160 ].
Socioeconomic status and insurance
In contrast with the traditional biomedical model, the bio-psycho-social-medical model highlights the significant role of socioeconomic status in health care services, including insurance status, marital status, and poverty level[ 164 ]. A study suggested that African-Americans, and in some cases Hispanics, had lower rates of surgery, less accessed to aggressive stage specific treatment, and underwent surgery at low volume hospitals and/or by lower volume surgeons, which might contribute to the differences in outcomes[ 165 ]. In addition, underinsured or uninsured patients also tended to receive less aggressive treatment[ 165 ]. A study of a total of 83902 patients with pancreatic cancer showed that patients with lower socioeconomic status were less likely to undergo surgical resection among patients with localized/regional pancreatic cancer[ 166 ]. Among patients with localized/regional pancreatic cancer who underwent surgical resection, patients with higher socioeconomic status have better overall survival, and patients with lower socioeconomic status have worse pancreatic cancer-specific survival compared with patients with higher socioeconomic status[ 166 ]. These findings suggest that racial differences in treatment and outcomes might be attributable to socioeconomic, insurance, and geographic factors[ 165 ]. The study of pancreatic cancer cases from the National Cancer Database from 2004 to 2015 showed that private insurance was associated with more treatment and better survival, higher education was associated with earlier treatment, and treatment was less and delayed among African-Americans despite later diagnosis[ 167 ]. After adjusting for socioeconomic status, African-Americans had about the same rate of survival overall at integrated facilities and the survival was improved, suggesting that higher socioeconomic status was associated with better treatment and survival[ 167 ]. A pan-cancer analysis showed that socioeconomic status was strongly associated with 1-mo postoperative mortality in primary solid tumors, and the risk of dying was high within 1-mo after surgery in socioeconomically disadvantaged people[ 164 ]. And underserved populations around the world often face similar barriers to cancer treatment, largely reflecting inequalities in social factors[ 165 ]. Therefore, socioeconomic status plays an extremely important role in the prevention and prognosis of pancreatic cancer, and the formulation of policies targeting low socioeconomic status patients may improve the low 5-year survival rate of pancreatic cancer.
Over the next 10 years to 20 years, an increase in pancreatic cancer is inevitable. At the same time, in the face of the characteristics of high mortality and difficult early diagnosis of pancreatic cancer, early prevention of pancreatic cancer through understanding the risk factors for pancreatic cancer is an economical and effective means, which is to prevent pancreatic cancer in advance. In view of the non-modifiable factors affecting pancreatic cancer, we may screen the susceptible population to pancreatic cancer, and provide reliable screening strategies and reasonable diagnostic ideas for the early diagnosis of pancreatic cancer. By studying the modifiable risk factors that affect pancreatic cancer, we may provide earlier interventions to prevent pancreatic cancer so that it can be possibly blocked in its early stages of canceration, thus significantly reducing the incidence of pancreatic cancer. Globally, a comprehensive prevention and control strategy for pancreatic cancer should include effective tobacco-control policy, recommendations for healthier lifestyles, and enlarging coverage of screening, education and vaccination programmes to better improve public awareness of the need to take precautions.
ACKNOWLEDGEMENTS
We sincerely thank International Agency for Research on Cancer for granting us permission to use Figure Figure1 1 in this paper.
Conflict-of-interest statement: All the authors of this paper declare that there is no conflict of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: February 8, 2021
First decision: March 6, 2021
Article in press: June 15, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sperti C, Takemura N S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Jian-Xiong Hu, Intensive Care Unit (ICU), Affiliated Hospital of Putian University, Putian 351100, Fujian Province, China.
Cheng-Fei Zhao, School of Pharmacy and Medical Technology, Putian University, Putian 351100, Fujian Province, China. Key Laboratory of Pharmaceutical Analysis and Laboratory Medicine in University of Fujian Province, Putian University, Putian 351100, Fujian Province, China. moc.361@902iefgnehcoahz .
Wen-Biao Chen, Department of Basic Medicine, Quanzhou Medical College, Quanzhou 362011, Fujian Province, China.
Qi-Cai Liu, Department of Reproductive Medicine Centre, First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian Province, China.
Qu-Wen Li, Department of Priority Laboratory for Zoonoses Research, Fujian Center for Disease Control and Prevention, Fuzhou 350001, Fujian Province, China.
Yan-Ya Lin, Intensive Care Unit (ICU), Affiliated Hospital of Putian University, Putian 351100, Fujian Province, China.
Feng Gao, Department of Pathology, First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian Province, China.

- Facebook Icon
- Twitter Icon
- LinkedIn Icon
Cellular Crosstalk Induces Cachexia
Interplay between macrophages and pancreatic cancer cells seems to promote cachexia, researchers reported ( Cancer Cell 2024 Apr 11 [Epub ahead of print] ). The crosstalk between the cell types prompts the tumor cells to secrete TNF-like weak inducer of apoptosis, or TWEAK, which binds to receptors on muscle cells, causing inflammation and muscle wasting. However, the researchers noted that “depletion of macrophages reverses muscle degradation induced by tumor cells.” The finding provides potential therapeutic targets to reduce incidence and severity of muscle atrophy in patients with pancreatic cancer.
Advertisement
- Noted This Week Archive (2011-2023)
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account

- Latest News
View Press Releases
- Post a Press Release
- Reprint Articles
- Digital Advertising
- Lead Generation
- Editorial Calendar
- Whitepapers
- Inside eBooks
- Industry Events
- IT Conferences
- Bio-IT World Expo
- Bio-IT World Expo Europe
- Editorial Profile
- Editorial Calender
- Submit a story
- Innovative Practices Awards
- Linking Policy
- Bio-IT World Boston
- Bio-IT World Europe
Alfa Cytology Launches Full-Scale Research Services for Pancreatic Cancer to Achieve Preclinical Safety Assessment
Alfa Cytology, a premier tumor microenvironment research outsourcing service provider, has recently upgraded pancreatic cancer research services with robust platforms, powerful analytics, and cross-platform collaborations.
Pancreatic cancer (PC) is a severe disease that is difficult to diagnose in the early stages due to its complex and hidden nature. A recent study finds that the incidence rate of PC has been increasing each year, especially in people over 45 years old. Even young patients suffering from PC are showing a significant increase compared to a decade ago, and the malignancy is also higher than before, resulting in a worse prognosis.
To realize preclinical prediction and diagnosis of PC, Alfa Cytology provides a variety of services (molecular expertise of PC, detection & diagnosis of PC, and treatment strategy of PC in full tracking support). The range of PC basic research services includes PC pathology research, PC cell research, PC stem cell research, PC tumor model, and more. Additionally, Alfa Cytology is committed to exploring drug development services for pancreatic cancer .
“The new drug development services focus on the hotspot of PC tumor. We are pleased to develop small molecule drugs and peptide drugs for PC because they are the most promising and potential anti-pancreatic cancer drugs,” said the operation manager of Alfa Cytology. “Furthermore, we are working on a multidimensional study in anticancer. Through our neoantigen research service , Alfa Cytology identifies unique cancer-carrying genes by sequencing tumor cell genes to predict immune presentation affinity. Then we screen out characteristic neoantigens encoded, hoping to make a huge contribution to tumor immunotherapy.”
Scientists of Alfa Cytology work on tailored projects by diagnosing PC and identifying its biomarkers. The ultimate aim of the company is to develop new drugs that are more cost-effective, less toxic, and have higher efficacy and safety to meet the market’s needs. Alfa Cytology possesses a comprehensive understanding of the tumor immunological profile of PC, including intra-tumor heterogeneity, intersection with the tumor-microenvironment, and microenvironmental instability. Hence, it can help clients achieve precise and feasible development applications of biotech to enhance the safety and efficacy of such pharmaceutical innovation for PC.
About Alfa Cytology
Alfa Cytology is a contract research organization (CRO) that specializes in early discovery and preclinical studies. It was founded by experts and scientists and has grown from a small team to a larger one capable of developing new therapies for cancer treatment. Alfa Cytology provides comprehensive research services globally, helping individuals and organizations cost-effectively achieve success.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 22, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New findings on pancreatic anatomy may affect diabetes research and treatment
by Claes Björnberg, Umea University
Researchers at Umeå University have succeeded in imaging an entire human organ, a pancreas, in microscopic resolution. By staining different cell-types with antibodies and then using optical 3D imaging techniques to study the entire organ, their data provides a partially new picture of the pancreas.
The results may be of great importance for diabetes research , especially when developing various new forms of treatment. The study is published in Nature Communications .
The pancreas is a key organ for the development of diabetes, a disease that today affects over half a billion people. It contains millions of small cell clusters, the so-called islets of Langerhans, which function to regulate blood sugar levels in the body.
The islets chiefly contain beta- and alpha-cells that produce the hormones insulin and glucagon, respectively. Insulin is secreted into the bloodstream and acts much like a key to unlock the body's cells so that they can take up sugar (glucose) after a meal, the main form of energy used by the body. Glucagon in turn releases glucose stores when we need a supply of energy. These two cell-types also communicate directly with each other to optimize the correct glucose level in the body.
"Both insulin and glucagon cells were discovered over a hundred years ago, and it has long been believed that the islets should contain both cell types to form a fully functioning unit," says Ulf Ahlgren, professor at the Department of Medical and Translational Biology.
Since the islets of Langerhans make up only a small percentage of the pancreas, even though they occur in such large numbers, they have historically been very difficult to study directly within the pancreas. In most cases, researchers have had to study tissue sections that only provide a 2D image of a very small part of the organ. Now, Umeå researchers have used optical 3D techniques in which different cell-types can be marked with fluorescently colored antibodies.
Entire organ at microscopic resolution
"By dividing the entire organ into smaller parts, we enable the antibodies to get where they need to go. Since we know where each piece comes from, we can then, after scanning the different parts individually, 'reassemble' the entire pancreas again using computer software. This allows us to perform a plethora of calculations and study which cell-types are present, as well as where they are located in 3D space, as we know the 3D coordinates, their volume, shape and other parameters for each and every stained object in the entire organ," says Ahlgren.
In addition to new data on how insulin-producing cells are distributed in the pancreas, the researchers now show that glucagon-producing cells are not present in as many as 50% of the islets of Langerhans that do contain insulin cells. This is contrary to what was previously thought, where islets were believed to contain both insulin- and glucagon-expressing cell-types with the same islet.
"This was a surprise to us, and I believe that these results may be of great importance for diabetes research. First, it shows that the islets have a much more uneven composition, or cellularity, than previously thought. This could mean that islets of different composition might be specifically specialized to respond to different signals and/or operate in different metabolic environments. Of course, we really want to find this out," says Ahlgren.
"Second, a great deal of research in the diabetes field is carried out on isolated islets of Langerhans from deceased donors. Since we also show that this uneven composition is largely linked to islet size, it means that results from such experiments may not fully reflect how the islets are structured and function in the living pancreas. This could potentially be important for everything from islet transplants in type 1 diabetes to studies trying to produce islets of Langerhans from stem cells."
Basis for future studies
The research team will now continue to work to see if their methods can be used to determine whether other cell types in the pancreas also contribute to the formation of the islets in a way that has not previously been known. In addition, they will study whether it looks similar in mouse models, which could affect the use of mice for preclinical diabetes research.
"The methods and data we are now publishing will be able to form an important basis for future studies of human material in order to better understand what happens in the pancreas in the development of type 1 and type 2 diabetes, but also for diseases such as pancreatic cancer," says Ahlgren.
Explore further
Feedback to editors

Study connects enjoyment of nature to lower inflammation levels
21 minutes ago

Bacteria in the intestine that change in response to inflammation could have an impact on our immune system
54 minutes ago

Researchers develop deep-learning model capable of predicting cardiac arrhythmia 30 minutes before it happens

Improving cancer immunotherapy by prolonging T-cell survival

Eye-opener: Pupils enlarge when people focus on tasks
2 hours ago

Study finds COVID-19 pandemic led to some, but not many, developmental milestone delays in infants and young children

Common antibiotic may be helpful in fighting respiratory viral infections

In psychedelic therapy, clinician-patient bond may matter most

Despite AI advancements, human oversight remains essential: Study

Spanish scientists identify the key cell type for strategies to prevent atherosclerosis in progeria syndrome
Related stories.

Pancreas cells in people who have died show significant signs of stress
Apr 2, 2024
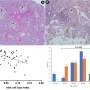
Loss of cells in pancreas in the elderly may cause age-related diabetes
Jan 15, 2024
New DNA methylation-based method for precise assessment of pancreas cell composition
Feb 6, 2024
Understanding the differences between healthy and type 2 diabetes-affected pancreatic islets
Nov 28, 2022
Beta cells under fire
Jun 7, 2017

New method enables 3D microscopy of human organs
Sep 13, 2021
Recommended for you

Advancing high-resolution ultrasound imaging with deep learning
3 hours ago

Siblings with unique genetic mutation help scientists progress drug search for type 1 diabetes
Apr 18, 2024

New study focuses on the placenta for clues to the development of gestational diabetes
Apr 16, 2024

GPT-4 matches radiologists in detecting errors in radiology reports

World-first microscopic stiffness probe could advance early cancer diagnosis
Apr 15, 2024

New AI method captures uncertainty in medical images
Apr 11, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Pancreatic Cancer Action Network to hold annual walk on April 27
by LIZ BONIS & MEGAN BURGASSER, WKRC

CINCINNATI (WKRC) - The public is invited to help raise awareness and money for research to fight pancreatic cancer. It’s helping to fund promising new research.
The Pancreatic Cancer Action Network invites everyone to come together this week. The organization is hosting its annual PurpleStride event. People will take steps together at locations all over the country to end pancreatic cancer.
Those fighting to find a cure for this disease have said the research is a bit behind.
“One thing that we aren’t too great at yet is detecting pancreatic cancer early, and unfortunately, most patients with pancreatic cancer show up in the clinic when it’s already spread to the other organs and lymph nodes,” said Dr. Andrew Waters, communications chair of the PanCAN Cincinnati affiliate.
Barb Thornton is recovering from pancreatic cancer. Her team in the walk is “Barb’s Believers”. She said the warning signs for pancreatic cancer can be subtle. She had back pain.
“I was diagnosed in late summer of '21, stage two pancreatic cancer. Last summer, I progressed to stage four metastatic. This summer, I’m looking forward to being clear. My bloodwork is in the normal range.”
She and Dr. Waters will join thousands of others across the country for the PurpleStride walk on Saturday, April 27. Every PanCAN chapter in the U.S. walks on the same day for this fundraiser.
The public is invited to join walkers to raise money for research, which is showing promise. Dr. Waters said PurpleStride has helped fund drugs now in development. The drugs help target certain gene mutations that appear to help the body fight pancreatic cancer.
“Patients are experiencing deep regressions with these drugs, and these are patients that have failed other chemotherapy treatments. On average, they had failed three lines of treatment. And most people are responding really well. There's one complete response, which is essentially unheard of for pancreatic cancer. So, over the next five years, I think that five-year survival rate, instead of these incremental increases, we're going to see more of a steep incline,” Dr. Waters said.
Again, the PanCAN Purple Stride Walk for Cincinnati is Saturday, April 27. It’s at East Fork State Park. The event village opens at 8 in the morning. Click here for information.
Watch CBS News
Making strides toward a cure with Lustgarten's Walk for Pancreatic Cancer Research
WA experts call for more research funding into deadly pancreatic cancer
When Lisa Melrose first found out she had pancreatic cancer she was given a grim prognosis.
Now, more than a year on, she is still fighting in the hopes of benefiting from growing research into a successful treatment.
Subscribers with digital access can view this article.
Already a subscriber?
Enjoy exclusive member discounts, giveaways and competitions with our subscriber rewards program.

IMAGES
VIDEO
COMMENTS
The new drug candidate permanently modifies a wily cancer-causing mutation, called K-Ras G12D, that is responsible for nearly half of all pancreatic cancer cases and appears in some forms of lung, breast and colon cancer. Pancreatic cancer is less common than these other cancers, but the lack of treatment options makes it more deadly, and it ...
For the first time in more than a decade, the FDA has approved a new first-line treatment for patients with metastatic pancreatic cancer.After a clinical trial showed a positive survival benefit, the combination chemotherapy called NALIRIFOX is now approved for patients who have not received any previous treatment.
One known risk factor for developing pancreatic cancer is a new diagnosis of diabetes, sometimes called new-onset diabetes. About 1 in 100 people with new onset diabetes are diagnosed with pancreatic cancer within 3 years after learning they have diabetes. And 1 in 4 people who get pancreatic cancer had already been diagnosed with diabetes.
By Alison Lee Satake. How cancer spreads or metastasizes is a big question for cancer researchers and patients. Mayo Clinic researchers studying pancreatic cancer — the third deadliest form of cancer in the U.S. — recently made a discovery that advances knowledge of how metastasis unfolds. They identified a cell-signaling protein that drives pancreatic cancer cell growth that could be a ...
A new scientific finding is the foundation for the first clinical trial in the U.S. to study an experimental drug's ability to fight pancreatic cancer with metastasis to the liver.
A team of MIT researchers has developed an immunotherapy strategy that can eliminate pancreatic tumors in mice. The new therapy, a combination of three drugs that boost the body's immune defenses against tumors, is expected to enter clinical trials later this year. ... Working with the Lustgarten Foundation for Pancreatic Cancer Research ...
May 10, 2023. Five years ago, a small group of cancer scientists meeting at a restaurant in a deconsecrated church hospital in Mainz, Germany, drew up an audacious plan: They would test their ...
Pancreatic ductal adenocarcinoma (PDAC) is projected to become the second-leading cause of cancer-related deaths by 2030 in the United States 1.To date, treatment of PDAC with the best available ...
Pancreatic cancer is a disease in which malignant cells originate in the pancreatic tissue. Cancer of the exocrine component of the pancreas (adenocarcinomas) represents the majority of pancreatic ...
The year 2022 was notable for substantial research progress related to pancreatic ductal adenocarcinoma (PDAC). The first single-cell and spatial transcriptomic atlases of PDAC were reported, a ...
Understanding if a blood test can be developed to test people with new-onset diabetes for possible pancreatic cancer ; Treatment. A lot of research is focused on finding better treatments for pancreatic cancer. Improving surgery and radiation therapy are major goals, as is determining the best combination of treatments for people with certain ...
In a study led by Mayo Clinic Center for Individualized Medicine, researchers found that nearly 1 in 6 people diagnosed with pancreatic cancer had an inherited cancer-related gene mutation that may have predisposed them to pancreatic cancer. The most common genetic mutation in those patients was the BRCA2 gene, which is linked to breast cancer.
An experimental approach to treating pancreatic cancer with the messenger RNA (mRNA)-based therapeutic cancer vaccine candidate autogene cevumeran continues to show potential to stimulate an immune response that may reduce the risk of the disease returning after surgery.. New results from a phase 1 clinical trial show that the cancer vaccine candidate activated immune cells that persisted in ...
Currently the third leading cause of death from cancer, pancreatic cancer kills about 50,000 people annually in the United States alone. Despite decades of research, the disease continues to ...
Researchers at the University of Texas MD Anderson Cancer Center published two studies this week on a new approach that could improve treatment for patients with pancreatic cancer — a disease ...
Pancreatic cancer discovery opens the door for new clinical trial. by University of Rochester Medical Center. Graphical abstract. Credit: Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113369 ...
Researchers from MIT and elsewhere have developed an AI model that is capable of identifying 3 ½ times more people who are at high-risk for developing pancreatic cancer than current standards, reports Felice J. Freyer for The Boston Globe.. "This work has the potential to enlarge the group of pancreatic cancer patients who can benefit from screening from 10 percent to 35 percent ...
Preclinical research published in the Journal for ImmunoTherapy of Cancer points to a promising new treatment option for people with pancreatic cancer. Researchers from VCU Massey Comprehensive ...
New Research Casts Doubt on Role of Fungus in Driving Pancreatic Cancer Aug. 3, 2023 — Four years ago, a report that a common species of fungus might fuel pancreatic cancer offered a promising ...
New research in the April 2024 issue of JNCCN-;Journal of the National Comprehensive Cancer Network showcases the feasibility of improving early detection and prevention for pancreatic cancer.
New Blood Test Promises Hope for Pancreatic Cancer. April 9, 2024 - Researchers are getting closer to being able to reliably screen for pancreatic cancer by conducting tests on a person's ...
Both 89.4% of new cases of pancreatic cancer and 92.6% of deaths occur in patients over 55 years ... Li et al found that patients with A blood type who also had DM had a greater odds of having pancreatic cancer, and further research is needed to confirm the results and to identify the mechanisms by which A blood type and DM jointly ...
A 2022 survey by the Pancreatic Cancer Action Network found that 83 per cent of adults are unaware of the signs of the disease. Smoking and type 2 diabetes are major risk factors.
Through our cutting-edge pancreatic cancer research, MD Anderson has: Initiated the first pancreatic cancer high-risk clinic, where family members and those at higher risk for pancreatic cancer (such as adults with new onset diabetes) can be screened and advised. Pioneered the advance of using chemotherapy before surgery (so-called ...
Interplay between macrophages and pancreatic cancer cells seems to promote cachexia, researchers reported (Cancer Cell 2024 Apr 11 [Epub ahead of print]). The crosstalk between the cell types prompts the tumor cells to secrete TNF-like weak inducer of apoptosis, or TWEAK, which binds to receptors on muscle cells, causing inflammation and muscle wasting. However, the researchers noted that ...
Alfa Cytology, a premier tumor microenvironment research outsourcing service provider, has recently upgraded pancreatic cancer research services with robust platforms, powerful analytics, and cross-platform collaborations. Pancreatic cancer (PC) is a severe disease that is difficult to diagnose in the early stages due to its complex and hidden nature.
New findings on pancreatic anatomy may affect diabetes research and treatment. Generation of volumetric and 3D-spatial data sets of the complete β-cell distribution across the human pancreas ...
CINCINNATI (WKRC) - The public is invited to help raise awareness and money for research to fight pancreatic cancer. It's helping to fund promising new research. The Pancreatic Cancer Action ...
Watch CBS News. Making strides toward a cure with Lustgarten's Walk for Pancreatic Cancer Research. The Lustgarten Foundation held its 14th annual New York City walk over the weekend, and our own ...
Claire Sadler The West Australian. Sat, 20 April 2024 11:00AM. Comments. When Lisa Melrose first found out she had pancreatic cancer she was given a grim prognosis. Now, more than a year on, she is still fighting in the hopes of benefiting from growing research into a successful treatment. Subscribers with digital access can view this article.