- Search Menu
- Thematic Series
- High-Impact Research Collection
- Author Guidelines
- Submission Site
- Open Access
- About GigaScience
- Editorial Board
- Reviewer Guidelines
- Editorial Policies
- Authorship Guidelines
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Conclusions, availability of supporting data, additional files, abbreviations, competing interests, author contributions, acknowledgements.
- < Previous
The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation
These authors contributed equally to this work.
- Article contents
- Figures & tables
- Supplementary Data
Conghui Liu, Yan Zhang, Yuwei Ren, Hengchao Wang, Shuqu Li, Fan Jiang, Lijuan Yin, Xi Qiao, Guojie Zhang, Wanqiang Qian, Bo Liu, Wei Fan, The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation, GigaScience , Volume 7, Issue 9, September 2018, giy101, https://doi.org/10.1093/gigascience/giy101
- Permissions Icon Permissions
The golden apple snail ( Pomacea canaliculata ) is a freshwater snail listed among the top 100 worst invasive species worldwide and a noted agricultural and quarantine pest that causes great economic losses. It is characterized by fast growth, strong stress tolerance, a high reproduction rate, and adaptation to a broad range of environments.
Here, we used long-read sequencing to produce a 440-Mb high-quality, chromosome-level assembly of the P. canaliculata genome. In total, 50 Mb (11.4%) repeat sequences and 21,533 gene models were identified in the genome. The major findings of this study include the recent explosion of DNA/hAT-Charlie transposable elements, the expansion of the P450 gene family, and the constitution of the cellular homeostasis system, which contributes to ecological plasticity in stress adaptation. In addition, the high transcriptional levels of perivitelline genes in the ovary and albumen gland promote the function of nutrient supply and defense ability in eggs. Furthermore, the gut metagenome also contains diverse genes for food digestion and xenobiotic degradation.
These findings collectively provide novel insights into the molecular mechanisms of the ecological plasticity and high invasiveness.
The golden apple snail Pomacea canaliculata (family Ampullariidae, order Architaenioglossa) is a freshwater snail listed among the world's top 100 worst invasive species [ 1 ] and is considered an agricultural and quarantine pest worldwide [ 2 ]. Native to tropical and subtropical South America, P. canaliculata gradually spread to nonindigenous regions, such as Southeast and East Asia [ 3 ], Africa [ 4 ], North America [ 5 ], Oceania [ 6 ], and even Europe [ 7 ]. Its successful biological invasion was closely related to its polyphagous feeding habits [ 8 ], voracious appetite [ 9 ], broad environmental adaptability [ 10 ], and rapid growth and high rate of reproduction [ 11 ]. In addition to its ecological impact, P. canaliculata ravages a wide range of crops, including grains, fruits, and vegetables [ 12 ], causing severe economic losses each year as a result of yield loss, replanting cost, and expenditures on control [ 13 ]. More seriously, P. canaliculata has been involved in the transmission of a fatal human disease, eosinophilic meningitis, which first appeared in East Asia where people frequently consume these snails [ 14 ]. During this pathophoresis, P. canaliculata acts as an important intermediate host of the pathogenic parasite Angiostrongylus cantonensis , and the range of infected regions is still expanding, creating a great challenge in terms of human health [ 15, 16 ].
Molluscs are a highly diverse group, second only to arthropods in species number [ 17 ], and their high biodiversity makes them an excellent model to address issues such as biogeography, adaptability, and evolutionary processes [ 18 ]. The worldwide invasive species P. canaliculata provides valuable potential in these fields [ 19 ]. As a primitive circumtropical species, P. canaliculata possesses strong ecological plasticity with many advantages, including low-temperature resistance [ 20 ] and drought tolerance [ 21 ], which has contributed to its competitive success in resource acquisition. Pomacea canaliculata has been reported to establish populations at temperatures ranging from 10°C to 35°C [ 20 , 22 ]. Additionally, P. canaliculata tolerates heavy metal contamination. When living in contaminated water, the gill is enriched with a high concentration of heavy metals, and histopathological changes in the digestive tract are detected; however, an extremely low mortality rate is observed [ 23 ]. The conspicuous coloration and neurotoxic lectin could confer a survival advantage on the eggs, defending the embryos against potential predators [ 24 ]. Moreover, an immune-neuroendocrine system can also be detected in P. canaliculata , as demonstrated by the existence of a specific immune memory after bacterial challenge [ 25, 26 ], broadening the study of invertebrate immunology.
The rich phenotypic and genetic diversity of molluscs makes them an excellent species group for addressing many important issues in evolution, ecology, and function. However, the genomic resources on Mollusca are still insufficient compared with those of other close phyla, such as Arthropoda and Nematoda, and few molluscs can be used as model organisms. Pomacea canaliculata , however, possesses the potential to be a model organism among molluscs because of several inherent characteristics. For example, P. canaliculata is easy to acquire because it has a broad global distribution originating from a primarily circumtropical environment. Moreover, its high adaptability, rapid growth, and efficient reproduction facilitate the cultivation of P. canaliculata in the laboratory.
In recent years, the genomic features of P. canaliculata have been increasingly studied. After the discovery of 14 pachytene bivalents in the karyotype [ 27 ], molecular markers were identified to investigate the genetic diversity of the P. canaliculata population, including 369 amplified fragment length polymorphism loci [ 28 ], 16,717 simple sequence repeats [ 29, 30 ], and 15,412 single-nucleotide polymorphisms [ 31 ]. In addition, multiple transcriptome analyses have been performed to investigate the adaptation, invasion, and immune mechanisms of P. canaliculata . For instance, Sun et al. reported 128,436 unigenes based on a de novo assembly of Illumina reads [ 31 ]; transcriptome changes in response to heat stress and starving incubation were used to characterize its invasive and adaptive abilities [ 32, 33 ]; a transcriptome analysis comparing invasive P. canaliculata and indigenous Cipangopaludina cathayensis provided insights into biological invasion [ 30 ]; and 402 immune-related differentially expressed genes (DEGs) in response to lipopolysaccharide challenge were used to explore the mechanisms of defense against pathogens [ 34 ]. Furthermore, proteomics tools such as isobaric tags for relative and absolute quantitation, and liquid chromatography-tandem mass spectrometry were also applied in the study of protein expression during estivation and oviposition [ 35, 36 ], together providing plentiful omics- data for the functional analysis of P. canaliculata .
However, research at the whole-genome level in P. canaliculata still lags far behind that in other mollusc species due to the lack of a high-quality reference genome. Multiple draft genomes of molluscs have been published, including the genomes of the California sea hare [ 37 ], Pacific oyster [ 38 ], pearl oyster [ 39, 40 ], owl limpet [ 41 ], California two-spot octopus [ 42 ], golden mussel [ 43 ], and Biomphalaria snails [ 44 ], greatly promoting research on mollusc genomics. In this study, we present a chromosome-level genome assembly of P. canaliculata with high-quality gene annotation, transcriptome data from several tissues and under various conditions, and metagenomic data from the intestinal tracts, all of which were then applied to study the species-specific environmental adaptation characteristics, such as the cellular homeostasis system underlying strong stress and the color and nutrient contents of the eggs. Our data will not only strengthen the understanding of the evolutionary mechanisms of molluscs and the molecular basis of biological invasion but also foster the development of approaches to control the invasion of P. canaliculata and provide a basis for interrupting the transmission of pathogenetic nematode parasites.
Complete genome assembly at the chromosome level
We generated 26.6 Gb (60.1 X) of Pacific Biosciences (PacBio) single-molecule real-time (SMRT) raw reads with an average read length of 10.1 kb, and 291 Gb (652.4 X) of Illumina HiSeq paired-end reads with an average read length of 150–250 bp using DNA extracted from a single adult P. canaliculata ( Supplementary Table S1 ). The 24.4 Gb (55.4 X) of clean PacBio SMRT reads that passed quality filtering were assembled using smartdenovo [ 45 ], resulting in an assembly of 1,234 raw contigs with a total length of 473.0 Mb and an N50 length of 1.0 Mb. After filtering of alternatively heterozygous contigs, the 745 resulting contigs with a total length of 440.1 Mb and an N50 length of 1.1 Mb were taken as the final contigs. Previous karyotype research has shown that the haploid P. canaliculata genome consists of 14 chromosomes [ 27 ]. Based on the Hi-C data, 439.5 Mb (99.9%) of final contigs were anchored and oriented into 14 large scaffolds, each corresponding to a natural chromosome (Fig. 1a and 1b ), with the longest 45.4 Mb and the shortest 27.2 Mb. This assembly quality is much better than that of the other molluscan genomes published thus far (Table 1 ). In addition to the length and continuity of the assembled sequences, another important aspect for evaluating genome assembly is the ratio of genome coverage. With an estimated genome size of 446 Mb and genome heterozygosity between 1% and 2% based on the distribution of k -mer frequency [ 46 ] ( Supplementary Fig. S1 ), ∼98.6% of the P. canaliculata genome has been assembled. To further confirm the accuracy and completeness of the assembly, we mapped the Illumina shotgun reads to the assembled reference genome. Significantly, 97% and 95% of the genome-derived and transcriptome-derived reads, respectively, could be aligned to the reference genome, suggesting no obvious bias in sequencing and assembly. Additionally, the mitochondrial genome of P. canaliculata was assembled as a single contig 15,707 bp in length, which has 99.9% sequence identity to the published mitochondrial genome (GenBank: KJ739609.1 ) ( Supplementary Fig. S2 ). This high-quality reference genome provides a good foundation for gene annotation.
![research paper about golden apple snail The genome characteristics of P. canaliculata. (a) Circos plot showing the genomic features. Track 1: 14 linkage groups of the genome; track 2: distribution of transposon elements in chromosomes; track 3: protein-coding genes located on chromosomes; track 4: distribution of Guanine and Cytosine (GC) contents. (b) A genome-wide contact matrix from Hi-C data between each pair of the 14 chromosomes using a 100-kb window size. The color value indicates the base 2 logarithm of the number of valid reads (log2[valid reads]). (c) Distribution of coding DNA sequence length in six closely related species.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/gigascience/7/9/10.1093_gigascience_giy101/1/m_giy101fig1.jpeg?Expires=1717615713&Signature=MOYT1OBKTOBUazNXAwi~IjTCGVz1ZHDG12Tn5ik2XtY~fTl4qU9WMs8aP9KiRtl0aP8Y06C7LFwXEZoZY9ucFwewNQYOE2vltZo1GkPg8SOMjF5wdYhLKS~kmfMwakM5vSI7rV-NeDGU35~GLU1IA5HbKmqihoEReWuyJtvq1VmXkzDVB4Ew9CC7cCXQkeNYsH-v8x9wUlGaaPEmJGa10mCwtKWk75-lTshnfO4g2rSL8CeWnRubBvhbnLmBS7QCGHTx6eQtYEykAN8Z9KC5JWMgqWzPhTrk5k0hPWACPKl39XsavNGCp7LkqDtpfSxxah~NHPBoANOGW0qM9CS9NQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The genome characteristics of P. canaliculata . (a) Circos plot showing the genomic features. Track 1: 14 linkage groups of the genome; track 2: distribution of transposon elements in chromosomes; track 3: protein-coding genes located on chromosomes; track 4: distribution of Guanine and Cytosine (GC) contents. (b) A genome-wide contact matrix from Hi-C data between each pair of the 14 chromosomes using a 100-kb window size. The color value indicates the base 2 logarithm of the number of valid reads (log 2 [valid reads]). (c) Distribution of coding DNA sequence length in six closely related species.
Summary of assembly and annotation of mollusc genomes
The protein-coding genes were predicted on the reference genome by EVM, integrating evidence from de novo prediction, transcriptome, and homology data. In total, 21,533 gene models were predicted as the reference gene set, with coding regions spanning ∼32.2 Mb (7.3%) of the genome (Table 1 and Supplementary Table S2 ). The distribution of coding DNA sequence Coding sequence (CDS) length in P. canaliculata is similar to that in closely related species (Fig. 1c ). Overall, 97.5% of the reference genes were supported by transcriptome data, and 98.0% of eukaryote core genes from OrthoDB [ 47 ] were identified in the reference gene set by Benchmarking Universal Single-Copy Ortholog (BUSCO). These results were comparable to those in other published molluscan genomes (Table 1 ). In functional annotation, 19,815 (91.9%) reference genes were annotated by at least one functional database. Specifically, 15,662 (72.7%), 13,769 (63.4%), 17,081 (79.3%), 18,847 (87.5%), and 17,003 (79.9%) reference genes were annotated with the eggNOG, Kyoto Encyclopedia of Genes and Genomes (KEGG), NR, InterPro, and UniProt databases, respectively ( Supplementary Fig. S3 ).
Signs of adaptive evolution in P. canaliculata genome
To gain insight into the evolutionary perspective of P. canaliculata , a phylogenetic tree was built based on 306 high-confidence single-copy orthologous genes from nine related species ( P. canaliculata, Lottia gigantea, Aplysia californica, Biomphalaria glabrata, Crassostrea gigas, Octopus bimaculoides, Pinctada fucata, Lingula anatina , and Limnoperna fortunei ) by PhyML [ 48 ] and the divergence time was estimated using MCMCTree [ 49 ]. The results show that P. canaliculata diverged from the ancestor of B. glabrata and A. californica 372 million years ago (Mya) and from L. gigantea 491 Mya (Fig. 2a ).
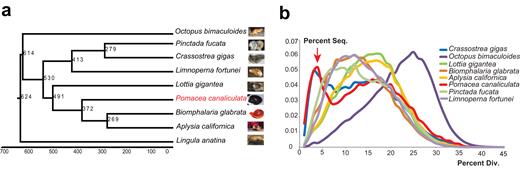
Evolutionary genomic analysis of P. canaliculata . (a) Phylogenetic placement of P. canaliculata within the dated tree of molluscs. The estimated divergence time is shown at each branching point, and P. canaliculata is shown in red. (b) Distribution of divergence rate for the class of DNA transposons in mollusc genomes. The divergence rate was calculated by comparing all transposable element (TE) sequences identified in the genome to the corresponding consensus sequence in each TE subfamily. The red arrow indicates that P. canaliculata and C. gigas had a recent explosion of TEs at a divergence rate of ∼4%.
Then, the molluscan orthologous genes were investigated for adaptive evolution. Utilizing pairwise protein sequence similarities, gene family clustering was conducted using orthoFinder [ 50 ]. A total of 239,541 reference genes from the nine species were clustered into 69,582 orthologous groups, among which 14,766 orthologous groups contained at least two genes each. We identified 66 orthologous groups that underwent common expansion in both P. canaliculata and L. fortunei but not the other seven species. The functions of these orthologous groups are mainly related to signal transduction; replication and repair; translation, glycan biosynthesis, and metabolism; lipid metabolism; and the endocrine, immune, and nervous systems ( Supplementary Fig. S4 ). These relations suggest that the gene families that underwent expansion may play important roles in adaptation to the environment as invasive species.
The high-coverage genome assembly enables a comprehensive analysis of the transposable elements (TEs), which play multiple roles in driving genome evolution in eukaryotes [ 51 ]. We identified 49.6 Mb TE sequences in the assembled P. canaliculata genome (Table 1 ), including 3.4 Mb long terminal repeats, 27.2 Mb long interspersed elements, 17.5 Mb DNA transposons, and 1.5 Mb short interspersed elements. Next, we analyzed the divergence rate of each class of TEs among the available sequenced mollusc genomes. Notably, the TE class of DNA transposons showed a specific peak at a divergence rate of ∼4% divergence rate for P. canaliculata and C. gigas (Fig. 2b ), indicating a recent explosion of DNA transposons in these two species. We analyzed the expression of 709 genes, including DNA elements restricted to the 4% peak inside the gene region, compared with that of the other genes outside the 4% peak ( Supplementary Fig. S5 ). DEGs were defined here by P values smaller than 0.05 for comparison of the treatment (heat, cold, heavy metal, and air exposure) and control data. The percentages of DEGs in the 4% peak were higher than those of genes outside the peak (10.2% higher for heat, 8.6% higher for cold, 8.6% higher for heavy metal, and 7.3% higher for air exposure). Among the DEGs in the 4% peak, approximately half were upregulated, and the other half were downregulated. Moreover, the DEGs in the 4% peak were mainly enriched in cellular metabolic process, response to stimulus, localization, and signaling according to Gene Ontology (GO) annotation. These results indicated that genes in the 4% peak were likely to be more active in the response to stimulus, promoting potential plasticity in stress adaptation. TEs are powerful facilitators of evolution that generate “evolutionary potential” to introduce small adaptive changes within a lineage, and the importance of TEs in stress responses and adaptation has been reported in numerous studies [ 52, 53 ]. The recent explosion of DNA TEs in P. canaliculata could also play an important role in promoting the potential plasticity in stress adaptation.
Investigation of cellular homeostasis system underlying strong stress adaptation
The homeostasis system plays a crucial role in stress adaptability, providing the molecular basis for re-establishing dynamic equilibrium after challenges by various environmental stressors, including temperature, air exposure, anthropogenic pollution, and pathogens [ 54 ]. In this study, we addressed three constituent parts of the cellular homeostasis system that contribute to the successful ecological plasticity of P. canaliculata (Fig. 3 ). The transcriptomes of the hemocytes after different stimuli (cold, heat, heavy metal, and air exposure) were also sequenced and analyzed to address the potential roles of these genes in the cellular homeostasis system.
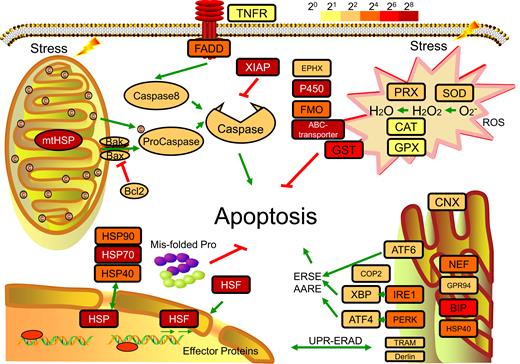
The cellular homeostasis system in P. canaliculata . The unfolded protein response (UPR) system includes HSPs and HSF in the heat shock response and CNX, NEF, GRP94, BIP, HSP40, ATF6, IRE1, PERK, COP2, XBP, ATF4, TRAM, and Derlin in the endoplasmic reticulum unfolded protein response (UPR-ERAD). Apoptotic pathways include XIAPs, Bcl2, caspases, TNFR, and FADD. The antioxidant systems include PRX, SOD, CAT, and GPX. The xenobiotic biotransformation system includes EPHX3, P450, FMO, and ABC transporter. The colors of the boxes for gene families represent the degree of upregulation (FPKM-stimulus/FPKM-control) as an overall result of stress, including heat, cold, heavy metal, and air exposure. Pathways and genes were obtained based on KEGG annotation.
The unfolded protein response (UPR) system is the central component of protein homeostasis [ 55 ]. Heat shock proteins (HSPs) act as molecular chaperones to maintain correct folding, and heat shock transcription factor 1 (HSF1) is responsible for the transcriptional induction of HSPs [ 56 ]. In the P. canaliculata genome, 13 HSP70s, 6 HSP90s, 7 HSP40s, and 11 HSFs were identified ( Supplementary Table S3 ), and the expression of HSP90s and HSFs was highly induced in response to heat, cold, heavy metal, and air exposure ( Supplementary Table S4 and Fig. S6 ). Inositol-requiring enzyme 1 (IRE 1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) are three mediators recruited by the endoplasmic reticulum (ER) to regulate the UPR [ 57 ]. We found putative coding genes of the three core mediators, their respective downstream transcription factors, and the corresponding recognition chaperones in the P. canaliculata genome ( Supplementary Table S3 ).
The xenobiotic biotransformation system helps the molluscs adapt to toxicants, especially pesticides in aquatic environments [ 58 ]. Manual annotation of this genome identified 157 cytochrome P450s (CYP450s), 15 flavin-containing monooxygenases (FMOs), 53 glutathione S-transferases, and 105 ATP binding cassette (ABC) transporters, most of which showed upregulated expression under stress ( Supplementary Tables S3 and S4 ). These proteins have been shown to function in contaminant detection, conjugative modification, and expulsion for xenobiotic detoxification [ 59-61 ].
The massive production of reactive oxygen species (ROS) and reactive oxygen intermediates induced by stress leads to many pathological conditions, and antioxidant systems protect the organism from superoxide [ 62 ]. Four main antioxidant enzyme classes, namely, superoxide dismutase (SOD), catalase (CAT), peroxidase (PRX), and glutathione peroxidase (GPX), were found in P. canaliculata and showed elevated global expression in response to stress ( Supplementary Tables S3 and S4 ).
Apoptosis is a process of cell death when sensing stress, and the regulation of apoptosis contributes to the dynamic homeostasis of the internal environment. In P. canaliculata , we propose the existence of both intrinsic and extrinsic apoptotic signaling pathways, evidenced by the presence of homologous genes involved in both pathways. These two pathways could be activated by cytochrome C and tumor necrosis factor receptor (TNFR), respectively ( Supplementary Table S3 ). Inhibitors of apoptosis, such as XIAP, Bcl2, and Bak, are also detected and show increased expression in response to stress ( Supplementary Table S4 ), which is expected to delay the process of apoptosis and cell death in the stress response.
Expansion of the P450 gene family contributes to stress tolerance
Cytochrome P450 (CYP) enzymes are a monooxygenase family with highly diverse structures and functions that have been widely identified in all kingdoms of life [ 63 ]. P450s catalyze the reductive scission of molecular oxygen and are responsible for the synthesis and metabolism of various molecules, including drugs, hormones, antibiotics, pesticides, carcinogens, and toxins [ 64 ]. The hormones they synthesize, such as glucocorticoids, mineralocorticoids, progestins, and sex hormones, are critical to stress response, growth, and reproduction, and the endogenous and exogenous chemical metabolism participate in combatting toxic compounds [ 65 ].
We found that the P. canaliculata CYP gene family had undergone an expansion compared to that in the other molluscs. We identified 157 genes in the genome of P. canaliculata and 128, 102, 135, 115, 78, 52, and 94 genes in A. californica, B. glabrata, C. gigas, L. fortunei, L. gigantea, O. bimaculoides , and P. fucata , respectively, using the same standard (Fig. 4a ). An expansive trend was also observed in comparison with other model species, such as Homo sapiens (57), Mus musculus (102), Danio rerio (94), and Drosophila melanogaster (94) [ 66 ]. Gene expansion was mainly found in the CYP2U and CYP3A subfamilies, whereas fewer genes were expanded in CYP4F. In mammals, CYP2U participates in the metabolism of fatty acids to generate bioactive eicosanoid derivatives, potentially regulating the development of immune function [ 67 ]. In P. canaliculata , 40 genes formed the CYP2U clade, mainly expressed in the hepatopancreas (Fig. 4b and Supplementary Table S5a, S5b ). CYP3A is a versatile enzyme that metabolizes a wide range of xenobiotics, and its production promotes the growth of various cell types [ 68 ]. The 56 CYP3A genes are comprehensively expressed in the hepatopancreas, gill, and kidney (Fig. 4b and Supplementary Table S5a, S5b ). CYP4F possesses epoxygenase activity, metabolizing fatty acids to epoxides to suppress hypertension, pain perception, and inflammation [ 69 ]. Twenty genes were identified in CYP4F, and Pc06G011748, Pc06G011460, Pc06G011458, Pc06G011459, Pc04G006708, Pc04G006710, and Pc04G006707 exhibited highly induced expression levels under cold, heat, heavy metal, and air exposure stress, indicating their critical roles in the stress tolerance (Fig. 4b and Supplementary Table S5a and S5b ).
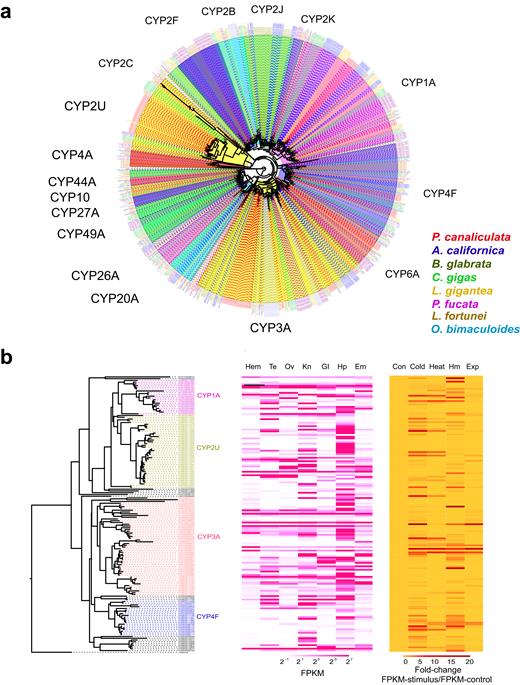
The expansion of the P450 gene family in P. canaliculata . (a) Phylogenetic tree demonstrating orthologous and paralogous relationships of all P450 genes from eight species including P. canaliculata , A. californica , B. glabrata , C. gigas , L. fortunei, L. gigantea , O. bimaculoides , and P. fucata . P450 genes from eight species were obtained based on Pfam annotation (Interpro) with an E-value of 10 −5 . Clades are labeled by P450 subfamily names. The tree was constructed using the maximum likelihood method in MEGA7, and the branch length scale indicates the average number of residue substitutions per site. (b) Phylogenetic tree of P450 genes in P. canaliculata , which is a subset of the phylogenetic tree for the species, and their heat map of expression (FPKM) in tissues (Hem, hemocytes; Te, testis; Ov, ovary and albumen gland; Kn, kidney; Gl, gill; Hp, hepatopancreas; Em, embryo) and heat map of induced expression (FPKM-stimulus/FPKM-control) under stress (Con: control; heat; cold; Hm: heavy metal; Exp: air exposure).
Identification of perivitelline genes and their high transcriptional levels in the ovary and albumen gland
Pomacea canaliculata has eggs characterized by abundant nutrients, reddish or pinkish color, aerial oviposition, and neurotoxicity [ 24 , 70 ] due to the perivitelline fluid (PVF), which fills the space between the eggshell and the embryo and consists of carbohydrates, lipids, and proteins (Fig. 5a ). The PVF proteins in P. canaliculata include three major components, PcOvo, PcPV2, and PcPV3 [ 71 ], collectively named perivitellines, which make up 90% of the total proteins, whereas most of the other dozens of low-abundance components each account for less than 1% of the total proteins [ 36 ]. The perivitellines are not only responsible for the major supply of materials and energy during embryogenesis but also provide warning pigments and deadly toxicants against predators [ 24 , 72, 73 ].
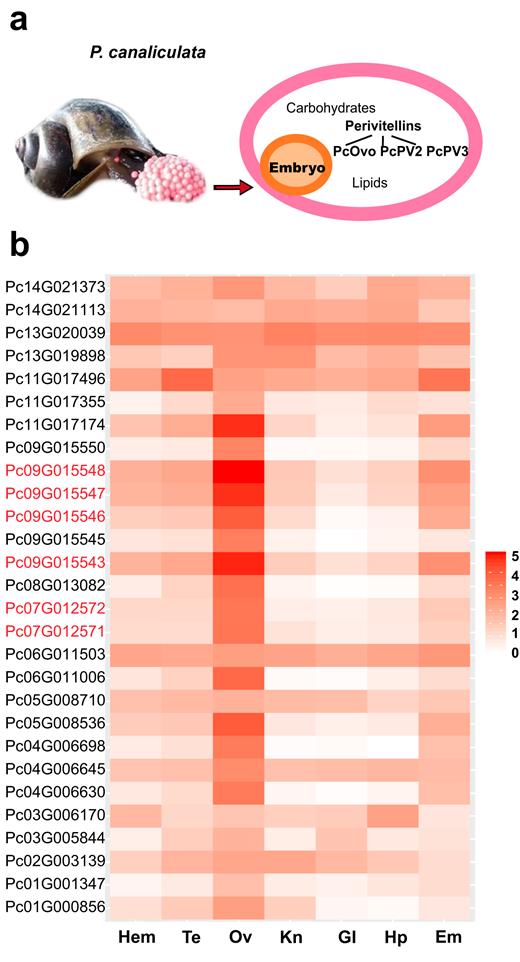
The composition and expression of the P. canaliculata perivitellines in different tissues. (a) Perivitelline fluid (PVF) lies under the eggshell and surrounds the embryo. It contains carbohydrates, lipids, and proteins. The proteins are also known as perivitellines and are classified into three categories, PcOvo, PcPV2, and PcPV3. (b) The displayed expression value of PVF proteins is the base 10 logarithm of FPKM (log 10 FPKM). The genes marked in red encode perivitellines. The tissues examined are abbreviated as follows: Hem, hemocytes; Te, testis; Ov, ovary and albumen gland; Kn, kidney; Gl, gill; Hp, hepatopancreas; Em, embryo.
We identified 28 candidate PVF genes in P. canaliculata by mapping each of the 59 fragmental PVF protein sequences derived from a previous proteomics study by Sun [ 36 ] to its best hit in the reference gene set of P. canaliculata , using Basic Local Alignment Search Tool for proteins (BLASTP) with requirements of over 85% identity and at least 50% alignment length ( Supplementary Table S6 ). Then, the functional annotation of those fragmental proteins was also transferred to our identified PVF genes. The transcriptome data show that 22 (79%) of the 28 candidate PVF genes exhibit their highest expression in the ovary and albumen gland (PVF protein synthesis factory) among all seven tissues (Fig. 5b and Supplementary Table S7 ), confirming that most of them are genuine functional PVF genes. Six of these 28 candidate PVF genes are perivitelline genes, including two PcOvo genes, Pc09G015543 (PcOvo2) and Pc09G015548 (PcOvo3); two PcPV2 genes, Pc07G012572 (PcPV2-31) and Pc07G012571 (PcPV2-67); and two possible PcPV3 genes, Pc09G015546 and Pc09G015547. The expression levels of these six genes in the ovary and albumen gland are much higher than those of the other 22 candidate PVF genes.
By analyzing the orthoFinder gene families that include orthologous and paralogous genes from P. canaliculata and eight other sequenced mollusc species, we found that these 28 candidate PVF genes were classified into 20 multiple-gene families (two or more genes) and seven single-gene families (only one gene) ( Supplementary Table S8 ). Notably, five of the six perivitelline genes were classified into single-gene families, except for Pc07G012571 (PcPV2-67), which not only has homologous genes in other mollusc species but also has three paralogous genes in P. canaliculata itself. However, none of these three PcPV2-67 paralogous genes in P. canaliculata showed higher expression in the ovary and albumen gland than in other tissues, indicating that they are likely not PVF-related genes, i.e., only Pc07G012571 plays a role in PVF. The nearly unique and single-copy nature of the six perivitelline genes in P. canaliculata may be explained by the long evolutionary distance, more than 200 Mya for P. canaliculata and its most closely related species, A. californica , as well as numerous differences in their living characteristics and egg structures. Another possible explanation is that these six major PVF genes may have experienced rapid evolution in their history to adapt to the changing environment.
The gut microbiome plays important roles in stress resistance and food digestion
The gut microbiome is regarded as the “second genome” of the host animal due to the fact that gut microbiota contributes to the food digestion, immune system development, and many other processes important to the host. To investigate the relationship between the gut microbiome and the invasive lifestyle of P. canaliculata , we collected gut digesta samples from 70 P. canaliculata snails and generated 31 Gb of high-quality metagenomic data on the Illumina HiseqX10 platform. To our knowledge, this study is the first in-depth sequencing of the snail gut microbiome. A total of 1,142,095 nonredundant (NR) genes were obtained with an average ORF length of 604 bp ( Supplementary Table S9 ). The taxonomic composition analysis showed that at the phylum level, Proteobacteria was predominant, followed by Verrucomicrobia, Bacteroidetes, Firmicutes, Spirochaetes, and Actinobacteria ( Supplementary Table S10a ). At the genus level, the most abundant genera included Aeromonas , Enterobacter , Desulfovibrio , Citrobacter , Comamonas , Klebsiella , and Pseudomonas ( Supplementary Table S10b ), most of which were also present in Achatina fulica [ 74, 75 ].
Interestingly, some of the most abundant genera, such as Desulfovibrio , Citrobacter , and Pseudomonas , were reported as having strong abilities to remove heavy metals by bioprecipitation and bioabsorption [ 76-78 ]. For example, the sulfur-reducing bacteria Desulfovibrio produce hydrogen sulfide, which precipitates metals and therefore reduces the toxic effects of dissolved metals [ 76 ]. Based on the KEGG pathway database, the complete sulfate reduction metabolism pathway was identified in the P. canaliculata gut microbiome. We suggested that these gut microbes might help P. canaliculata survive the environmental stress of heavy metals in harsh conditions. In addition, a large number of genes in xenobiotic biodegradation and metabolism pathways were annotated, corresponding to 288 KEGG orthologous groups (KOs) and 21 pathways ( Supplementary Table S11 ). As many of the pathways, such as benzoate degradation, toluene degradation, xylene degradation, and steroid degradation, could not be identified in the host genome through KO analysis, we suggested that microbial detoxification abilities may contribute to the ability of P. canaliculata to resist stresses caused by xenobiotics such as pesticides and environmental pollutants.
In digestion, the gut microbes are directly involved in the breakdown of the cellulose portion of the diet, and previous studies have isolated cellulolytic bacteria and evaluated the cellulolytic enzyme activities [ 79 ]. in our work we found a broader range of carbohydrate active enzymes (CAZymes). Of the 208 annotated CAZyme families, 99 were glycoside hydrolase families ( Supplementary Table S12 ). Enzymes that could be classified as cellulases, endohemicelluloses, debranching enzymes, and oligosaccharide-degrading enzymes were all identified. These findings indicate that the gut microbiome provides assistance in digesting a broad range of food sources, enabling P. canaliculata to grow rapidly and adapt to an invasive lifestyle.
Given its environmental invasiveness, broad stress adaptability, and rapid reproduction, the golden apple snail P. canaliculata has received a vast amount of attention worldwide. However, the underlying genetic mechanisms of these properties have not been comprehensively uncovered. The chromosome-level genome of P. canaliculata presented in this study sheds the first light on the genomic basis of its ecological plasticity in response to various stressors. The major findings of this study include the recent explosion of DNA/hAT-Charlie TEs, the expansion of the P450 gene family, and the constitution of the cellular homeostasis system, all of which contribute to the plasticity of the organism in stress adaptation. Although the function of the recently originated TEs could not be confirmed, TEs are considered powerful facilitators in adaptive evolution, suggesting that their increased numbers play an important role in the stress resistance of P. canaliculata . The UPR system, xenobiotic biotransformation system, and ROS system are all major components of the cellular homeostasis system, and the P450s in particular underwent expansion with specific functions. In addition, exclusive perivitelline genes were identified in the P. canaliculata genome, and they are believed to contribute to the high reproductive rate and the expansion of habitats. Furthermore, the gut metagenome contains diverse genes for food digestion and xenobiotic degradation. These findings collectively provide novel insight into the molecular mechanisms of ecological plasticity and high invasiveness.
In this study, we report a fine reference genome of P. canaliculata , the first chromosome-level Mollusca genome published. With widespread distribution, rapid growth, and efficient reproduction, P. canaliculata possesses the potential to be a model organism of Mollusca. As its cellular complexity and conservation of pathways also make P. canaliculata a useful representative of Mollusca, the genome described in this study can be used to advance our understanding of the molecular mechanisms involved in various scientific questions regarding Mollusca.
Sample collection and sequencing
Adult P. canaliculata were collected from a local paddy field in Shenzhen, Guangdong province, China, and maintained in aerated freshwater at 15 ± 2°C for a week before processing. Genomic DNA was extracted from the foot muscles of a single P. canaliculata for constructing polymerase chain reaction-free Illumina 350-bp insert libraries and PacBio 20-kb insert library and sequenced on Illumina HiSeq 2500 and PacBio SMRT platforms, respectively. The Hi-C library was prepared using the muscle tissue of another single P. canaliculata by the following methods: nuclear DNA was cross-linked in situ , extracted, and then digested with a restriction enzyme. The sticky ends of the digested fragments were biotinylated, diluted, and then ligated to each other randomly. Biotinylated DNA fragments were enriched and sheared again for preparing the sequencing library, which was then sequenced on a HiSeq X Ten platform (Illumina).
Seven tissues including embryos (2 days post-fertilization), gill, hemocytes, hepatopancreas, kidney, ovary and albumen gland, and testis from six animals were collected as parallel samples. Next, animals were cultivated at 37°C and 10°C for 24-hour heat and cold tolerance; in Cr 3+ (2 mg/L), Cu 2+ (0.2 mg/L), and Pb 2+ (1 mg/L) for 24-hour heavy metal tolerance, and in a waterless tank for 7 days air exposure. Then, the hemocytes were harvested and stored, with three replicates for each group. Total RNAs were extracted from the stored tissues of P. canaliculata materials. Then, mRNAs were pulled out by beads with poly-T for constructing cDNA libraries (insert 350-bp) and sequenced on an Illumina HiSeq 2500 sequencer.
The intestinal digesta from 70 adult snails of P. canaliculata were collected, pooled into six samples, and stored at −20°C until microbial DNA was extracted. A combination of cell lysis treatments was applied, including five freeze-thaw cycles (alternating between 65°C and liquid nitrogen for 5 minutes), repeated beads-beating in A stool lysis (ASL) buffer (cat. no. 19 082; Qiagen Inc.), and incubated at 95°C for 15 minutes. DNA was isolated following the reported protocol [ 80 ]. Paired-end libraries of metagenomic DNA were prepared with an insert size of 350 bp following the manufacture's protocol (cat. no. E7645L; New England Biolabs). Sequencing was performed on Illumina HiSeq X10.
Genome assembly and annotation
The Illumina raw reads were filtered by trimming the adapter sequence and low-quality regions [ 81 ], resulting in clean and high-quality reads with an average error rate of <0.001. For the PacBio raw data, the short subreads (<2 kb) and low-quality (error rate >0.2) subreads were filtered out, and only one representative subread was retained for each PacBio read. The clean PacBio reads were assembled by the software smartdenovo [ 82 ], after which Illumina reads were aligned to the contigs by BWA-MEM (BWA, RRID:SCR_010910 ), and single base errors in the contigs were corrected by Pilon v1.16 (Pilon, RRID:SCR_014731 ) with the parameters “-fix bases, -nonpf, -minqual 20.” The P. canaliculata genome is highly heterozygous, as illustrated by the double peaks on the distribution curve of k -mer frequency, and the current assembly algorithm tends to collapse homozygous regions and report heterozygous regions in alternative contigs. To obtain a haploid reference contig, we employed a whole-genome alignment strategy with MUMmer v3.23 to recognize and selectively remove alternative heterozygous contigs, which were characterized by shorter length (less than 200 kb) and the ability of most regions (more than 50%) to be aligned to another larger contig with confident identity (higher than 80%). Next, Hi-C sequencing data were aligned to the haploid reference contigs by BWA-MEM, and then these contigs were clustered into chromosomes with LACH-ESIS [ 83 ].
A de novo repeat library for P. canaliculata was constructed by RepeatModeler v. 1.0.4 (RepeatModeler, RRID:SCR_015027 ; [ 84 ]). TEs in the P. canaliculata genome were also identified by RepeatMasker v4.0.6 (RepeatMasker, RRID:SCR_012954 ; [ 85 ]) using both the Repbase library and the de novo library. Tandem repeats in the P. canaliculata genome were predicted using Tandem Repeats Finder v4.07b [ 86 ]. The divergence rates of TEs were calculated between the identified TE elements in the genome and their consensus sequence at the TE family level.
The gene models in the P. canaliculata genome were predicted by EVidence Modeler v1.1.1 [ 87 ], integrating evidence from ab initio predictions, homology-based searches, and RNA sequencing (RNA-seq) alignments. Then, these gene models were annotated by RNA-seq data, UniProt database, and InterProScan software (InterProScan, RRID:SCR_005829 ) [ 88 ]. Finally, the gene models were retained if they had at least one piece of supporting evidence from the UniProt database, InterProScan domain, and RNA-seq data. Gene functional annotation was performed by aligning the protein sequences to the National Center for Biotechnology Information (NCBI) NR, UniProt, COG, and KEGG databases with BLASTp v2.3.0+ under an E-value cutoff of 10 −5 and choosing the best hit. Pathway analysis and functional classification were conducted based on the KEGG database [ 89 ]. InterProScan was used to assign preliminary GO terms, Pfam domains and IPR domains to the gene models.
Evolutionary analysis
Orthologous and paralogous groups were assigned from nine species ( P. canaliculata, Lottia gigantea, Aplysia californica, Biomphalaria glabrata, Crassostrea gigas, Octopus bimaculoides, Pinctada fucata, Limnoperna fortunei , and Lingula anatina ) by OrthoFinder [ 50 ] with default parameters. Orthologous groups that contained only one gene for each species were selected to construct the phylogenetic tree. The protein sequences of each gene family were independently aligned by muscle v3.8.31 [ 90 ] and then concatenated into one super-sequence. The phylogenetic tree was constructed by maximum likelihood (ML) using PhyML v3.0 (PhyML, RRID:SCR_014629 ) [ 48 ] with the best-fit model (LG+I+G) estimated by ProtTest3 [ 91 ]. The Bayesian relaxed molecular clock approach was adopted to estimate the neutral evolutionary rate and species divergence time using the program MCMCTree, implemented in the PAML v4.9 package (PAML, RRID:SCR_014932 ) [ 49 ]. The tree was calibrated with the following time frames to constrain the age of the nodes between the species: minimum = 260 Mya and maximum = 290 Mya for P. fucata and C. gigas [ 92 ]; minimum = 450 Mya and maximum = 480 Mya for A. californica (or B. glabrata ) and L. gigantea [ 93 ]. The calibration time (fossil record time) interval (550–610 Mya) of O. bimaculoides was adopted from previous results [ 94 ]. To identify the common expanded gene families, we compared the P. canaliculata and L. fortunei with the other seven species. The gene numbers of the orthologous group in P. canaliculata and L. fortunei were two or more times that in all of other species, respectively. Additionally, these gene families with P value less than 0.01 were considered as expansion by z-test.
Transcriptome data analysis
Transcriptome reads were trimmed using the same method for genomic reads [ 81 and then mapped to the reference genome of P. canaliculata using TopHat v. 2.1.0 (TopHat, RRID:SCR_013035 ) with default settings. The expression level of each reference gene in terms of FPKM was computed by cufflinks v2.2.1 (cufflinks, RRID:SCR_014597 ). A gene was considered to be expressed if its FPKM was >0. Differential gene expression analysis was conducted using cuffdiff v2.2.1.
Metagenome data analysis
The Illumina raw reads were filtered by trimming the adapter sequence and low-quality regions [ 81 ], resulting in high-quality reads with an average error rate of <0.001. Then, the reads mapped to the following genomes by BWA-MEM were filtered out [ 95 ] [ 96 ] to exclude the contaminated host, food, parasite, and human DNA sequences. The genomes include the P. canaliculata genome, the Brassica rapa genome, the Oryza sativa genome, two Angiostrongylus cantonensis genomes, the Caenorhabditis elegans genome, the Schistosoma mansoni genome, the Clonorchis sinensis genome, the Fasciola hepatica genome, the Danio rerio genome, and the Homo sapiens hg38 genome. Finally, short reads (length < 75 bp) and unpaired reads were excluded to form a set of clean reads.
The clean reads were assembled by metaSPAdes (v3.11.1) [ 97 ] in paired-end mode for each sample. Then, gene prediction was performed on contigs longer than 500 bp by Prodigal v2.6.3 (Prodigal, RRID:SCR_011936 ) [ 98 ] with the parameter “-p meta,” and gene models with cds length less than 102 bp were filtered out. An NR gene set (539,344 genes) was constructed using the gene models predicted from each sample by cd-hit-est (v4.6.6) [ 99 ] with the parameter “-c 0.95 -n 10 -G 0 –a S 0.9,” which adopts a greedy incremental clustering algorithm and the criteria of identity >95% and overlap >90% of the shorter genes. Then, the clean reads were mapped onto this NR gene set by BWA-MEM with the criteria of alignment length ≥50 bp and identity >95%. The unmapped reads from all samples were assembled together, and the genes were predicted again. The newly predicted genes were combined with the previous gene set by cd-hit-est to obtain a new NR gene set (1,147,339 genes). After the taxonomic assignments to the new NR gene set, 5,244 genes classified as Eukaryota but not fungi were removed, and the final NR gene set (1,142,095 genes) was obtained.
The taxonomic assignments of the final NR genes were made on the basis of DIAMOND (DIAMOND, RRID:SCR_016071 ) [ 100 ] protein alignment against the NCBI NR database by CARMA3 [ 101 ]. Functional annotation was performed by aligning all the protein sequences to the KEGG [ 102 ] database (release 79) using DIAMOND and taking the best hit with the criteria of E-value <1e-5. CAZymes were annotated with dbCAN (release 5.0) [ 103 ] using Hmmer v3.0 hmmscan (Hmmer, RRID:SCR_005305 ) [ 104 ] by taking the best hit with an E-value <1e-18 and coverage > .35.
The clean reads from each sample were aligned against the gene catalogue (1,142,095 genes) by BWA-MEM with the criteria of alignment length ≥50 bp and identity >95%. Sequence-based gene abundance profiling was performed as previously described [ 105 ]. The taxonomic profiles of the samples were calculated by summing the gene abundance according to the taxonomic assignment result.
Supplementary Tables S1−S12 and Figs. S1− to S6 are available in the additional file. The raw sequencing data have been deposited in DDBJ/EMBL/GenBank under project accession PRJNA427478 , SRR6425828 for genomic Illumina_PE125 sequencing data, SRR6425829 for genomic Illumina_PE150 sequencing data, SRR6425827 for genomic Pacific Biosciences sequencing data, SRR6429132∼SRR6429164 for transcriptome sequencing data, and SRR6472920∼SRR6472925 for gut microbiome data. Other supporting data, including genome assemblies, annotations, phylogenetic tree files, and Benchmarking Universal Single-Copy Ortholog results, are available via the GigaScience repository GigaDB [ 106 ].
Supplemental_Information-final.doc
ABC, ATP binding cassette; ATF6, activating transcription factor 6; BLASTp: Basic Local Alignment Search Tool for Proteins; CAT, catalase; CAZymes, carbohydrate active enzymes;CDS, coding sequences; CYP450s, cytochrome P450s; DEGs, differentially expressed genes; dRNA-seq: differential RNA sequencing; Em, embryo; ER, endoplasmic reticulum; FMOs, flavin-containing monooxygenases; GI, gill; GO: Gene Ontology; GPX, glutathione peroxidase; GSTs, glutathione S-transferases; Hem, hemocytes; Hp, hepatopancreas; HSF1, heat shock transcription factor 1; HSPs, heat shock proteins; Kn, kidney; KEGG, Kyoto Encyclopedia of Genes and Genomes; KOs, KEGG orthologous groups; LINE, long interspersed elements; Mya, million years ago; NCBI: National Center for Biotechnology Information; NR, nonredundant genes; ORF, open reading frame; Ov, ovary and albumen gland; Ovo, ovorubin; PERK, protein kinase RNA-like ER kinase; Prx, peroxidase; PS: processing site; PVF, pervitelline luid; RNA-seq: RNA sequencing; ROS, reactive oxygen species; SNP: single nucleotide polymorphism; SOD, superoxide dismutase; Te, testis; TEs, transposable elements; TNFR, tumor necrosis factor receptor; TSS, transcriptional start site; UPR, unfolded protein response; UTR, untranslated region: GC rate, guanine and cytocine rate; ASL buffer, a stool lysis buffer.
The authors declare that they have no competing interests.
W.F., W.Q., and C.L. conceived the study and designed the experiments. C.L. performed the genome sequencing and assembly, and B.L. performed annotation and evolutionary analysis. C.L. performed the stress tolerance analysis, Y.R. performed the reproduction analysis, and Y.Z. performed the metagenome analysis. H.W., S.L., F.J., L.Y., and X.Q. provided suggestions and helped checking. C.L., W.F., B.L., Y.R., and Y.Z. wrote the manuscript, and G.Z. helped revise the manuscript. All authors read and approved the final manuscript.
This project is supported by the National Key Research and Development Program of China (2016YFC1200600), the Shenzhen Science and Technology Program (JCYJ20150630165133395), Fund of Key Laboratory of Shenzhen (ZDSYS20141118170111640), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences & Elite Youth Program of Chinese Academy of Agricultural Sciences. We thank Fanghao Wan, Jue Ruan, and Yutao Xiao for providing constructive suggestions to this project.
Lowe S , Browne M , Boudjelas S , et al. 100 of the World's Worst Invasive Alien Species: A Selection from the Global Invasive Species Database . Auckland, New Zealand : World Conservation Union (IUCN) ; 2000 .
Google Scholar
Google Preview
Ranamukhaarachchi SL , Wickramasinghe S . Golden apple snails in the world: introduction, impact, and control measures . Global advances in ecology and management of golden apple snails . 2006 : 133 – 52 .
Naylor R . Invasions in agriculture: assessing the cost of the golden apple snail in Asia . Royal Swedish Academy of Sciences . 1996 ; 25 : 443 – 8 .
Berthold T . Vergleichende Anatomie, Phylogenie und historische Biogeographie der Ampullariidae: (Mollusca, Gastropoda) . 1991 .
Howells RG , Burlakova LE , Karatayev AY et al. , Native and introduced Ampullaridae in North America: history, status, and ecology . Global advances in ecology and management of golden apple snails . 2006 73-112 .
Halwart M , Bartley DM . International Mechanisms for the Control and Responsible Use of Alien Species in Aquatic Ecosystems, with Special Reference to the Golden Apple Snail . Los Baños, Philippines : Philippine Rice Research Institute (PhilRice) ; 2006 .
López MA , Altaba CR , Andree KB , et al. First invasion of the apple snail Pomacea insularum in Europe . Tentacle . 2010 ; 18 : 26 – 8 .
Estebenet AL , Martín PR . Pomacea canaliculata (Gastropoda: Ampullariidae): life-history traits and their plasticity . Biocell . 2002 ; 26 : 83 – 9 .
Lach L . The spread of the introduced freshwater apple snail Pomacea canaliculata (Lamarck) (Gastropoda Ampullariidae) on Oahu, Hawaii . Bishop Museum Occasional Papers . 1999 ; 58 : 66 – 71 .
Yusa Y , Sugiura N , Wada T . Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae), in Southern Japan . Biol Invasions . 2006 ; 8 : 137 – 47 .
Lach L , Britton DK , Rundell RJ et al. Food preference and reproductive plasticity in an invasive freshwater snail . Biol Invasions . 2000 ; 2 : 279 – 88 .
Mochida O . Spread of freshwater Pomacea snails (Pilidae, Mollusca) from Argentina to Asia . Micronesica . 1991 ; 3 : 51 – 62 .
Invasive Speices Compendium for golden apple snail , https://www.cabi.org/isc/datasheet/68490 .
Shan L , Zhang Y , Steinmann P , et al. Emerging angiostrongyliasis in mainland China . Emerg Infect Dis . 2008 ; 14 : 161 – 4 .
Caldeira RL , Mendonca CL , Goveia CO , et al. First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil . Memórias do Instituto Oswaldo Cruz . 2007 ; 102 : 887 – 9 .
McMichael AJ , Beaglehole R . The changing global context of public health . Lancet (London, England) . 2000 ; 356 : 495 – 9 .
Chapman A . Numbers of Living Species in Australia and the World . Australian Biological Resources Study ; 2009 . 1-78.
Lindberg DR , Ponder WF , Haszprunar G . T he Mollusca: Relationships and Patterns from Their First Half-Billion Years . Oxford : Oxford University Press ; 2004 .
Hayes KA , Cowie RH , Thiengo SC . A global phylogeny of apple snails: Gondwanan origin, generic relationships, and the influence of outgroup choice (Caenogastropoda: Ampullariidae) . Biol J Linn Soc Lond . 2009 ; 98 : 61 – 76 .
Matsukura K , Tsumuki H , Izumi Y , et al. Physiological response to low temperature in the freshwater apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae) . J Exp Biol . 2009 ; 212 : 2558 – 63 .
Yusa Y , Wada T , Takahashi S . Effects of dormant duration, body size, self-burial and water condition on the long-term survival of the apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae) . Appl Entomol Zool . 2006 ; 41 : 627 – 32 .
Seuffert ME , Burela S , Martín PR . Influence of water temperature on the activity of the freshwater snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) at its southernmost limit (Southern Pampas, Argentina) . J Therm Biol . 2010 ; 35 : 77 – 84 .
Kruatrachue M , Sumritdee C , Pokethitiyook P , et al. Histopathological effects of contaminated sediments on golden apple snail ( Pomacea canaliculata , Lamarck 1822) . Bull Environ Contam Toxicol . 2011 ; 86 : 610 – 4 .
Dreon MS , Frassa MV , Ceolín M , et al. Novel animal defenses against predation: a snail egg neurotoxin combining lectin and pore-forming chains that resembles plant defense and bacteria attack toxins . PLoS One . 2013 ; 8 : e63782 .
Ottaviani E , Caselgrandi E , Fontanili P et al. Evolution, immune responses and stress: studies on molluscan cells . Acta Biol Hung . 1992 ; 43 : 293 – 8 .
Ottaviani E , Accorsi A , Rigillo G , et al. Epigenetic modification in neurons of the mollusc Pomacea canaliculata after immune challenge . Brain Res . 2013 ; 1537 : 18 – 26 .
Mercado Laczkó AC , Lopretto EC . Estudio cromosómico y cariotípico de p omacea canaliculata (Lamarck, 1801) (Gastropoda, Ampullariidae) . Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” Hidrobiología . 1998 ; 8 : 15 – 20 .
Xu J , Han X , Li N , et al. Analysis of genetic diversity of three geographic populations of Pomacea canaliculata by AFLP . Acta Ecol Sin . 2009 ; 29 : 4119 – 26 .
Chen L , Xu H , Li H et al. Isolation and characterization of sixteen polymorphic microsatellite loci in the golden apple snail Pomacea canaliculata . Int J Mol Sci . 2011 ; 12 : 5993 – 8 .
Mu X , Hou G , Song H , et al. Transcriptome analysis between invasive Pomacea canaliculata and indigenous Cipangopaludina cahayensis reveals genomic divergence and diagnostic microsatellite/SSR markers . BMC Genet . 2015 ; 16 : 12 .
Sun J , Wang M , Wang H et al. De novo assembly of the transcriptome of an invasive snail and its multiple ecological applications . Mol Ecol Resour . 2012 ; 12 : 1133 – 44 .
Mu H , Sun J , Fang L , et al. Genetic basis of differential heat resistance between two species of congeneric freshwater snails: insights from quantitative proteomics and base substitution rate analysis . J Proteome Res . 2015 ; 14 : 4296 – 308 .
Yang L , Cheng TY , Zhao FY . Comparative profiling of hepatopancreas transcriptomes in satiated and starving Pomacea canaliculata . BMC Genet . 2017 ; 18 : 18 .
Xiong YM , Yan ZH , Zhang JE et al. Analysis of albumen gland proteins suggests survival strategies of developing embryos of Pomacea canaliculata . Molluscan Res . 2018 : 38:99 – 114 .
Sun J , Mu H , Zhang H , et al. Understanding the regulation of estivation in a freshwater snail through iTRAQ-based comparative proteomics . J Proteome Res . 2013 ; 12 : 5271 – 80 .
Sun J , Zhang H , Wang H , et al. First proteome of the egg perivitelline fluid of a freshwater gastropod with aerial oviposition . J Proteome Res . 2012 ; 11 : 4240 – 8 .
Aplysia Genome Project . Broad Institute. Vertebrate Biology Group . 2009 . https://www.broadinstitute.org/aplysia/aplysia-genome-project .
Zhang G , Fang X , Guo X et al. The oyster genome reveals stress adaptation and complexity of shell formation . Nature . 2012 ; 490 : 49 – 54 .
Du X , Fan G , Jiao Y , et al. The pearl oyster Pinctada fucata martensii genome and multi-omic analyses provide insights into biomineralization . GigaScience . 2017 ; 6 : 1 – 12 .
Takeuchi T , Kawashima T , Koyanagi R et al. Draft genome of the pearl oyster Pinctada fucata : a platform for understanding bivalve biology . DNA Res . 2012 ; 19 : 117 – 30 .
Simakov O , Marletaz F , Cho SJ , et al. Insights into bilaterian evolution from three spiralian genomes . Nature . 2013 ; 493 : 526 – 31 .
Albertin CB , Simakov O , Mitros T , et al. The octopus genome and the evolution of cephalopod neural and morphological novelties . Nature . 2015 ; 524 : 220 – 4 .
Uliano-Silva M , Dondero F , Dan Otto T , et al. A hybrid-hierarchical genome assembly strategy to sequence the invasive golden mussel Limnoperna fortunei . GigaScience . 2018 ; 7 : gix128 .
Adema CM , Hillier LW , Jones CS et al. Corrigendum: whole genome analysis of a schistosomiasis-transmitting freshwater snail . Nat Commun . 2017 ; 8 : 16153 .
SMARTdenovo on github. https://github.com/ruanjue/smartdenovo
Liu B , Shi Y , Yuan J et al. Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects . Quantitative Biology . 2013 : arXiv : 1308.2012 [q-bio.GN] .
Ortho Database website. http://www.orthodb.org/
Guindon S , Dufayard JF , Lefort V , et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0 . Syst Biol . 2010 ; 59 : 307 – 21 .
Yang Z . PAML 4: phylogenetic analysis by maximum likelihood . Mol Biol Evol . 2007 ; 24 : 1586 – 91 .
Emms DM , Kelly S . OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy . Genome Biol . 2015 ; 16 : 157 .
Feschotte C , Wessler SR . Mariner-like transposases are widespread and diverse in flowering plants . Proc Natl Acad Sci U S A . 2002 ; 99 : 280 – 5 .
Hua-Van A , Le Rouzic A , Boutin TS , et al. The struggle for life of the genome's selfish architects . Biol Direct . 2011 ; 6 : 19 .
Werren JH . Selfish genetic elements, genetic conflict, and evolutionary innovation . Proc Natl Acad Sci U S A . 2011 ; 108 : 10863 – 70 .
Chrousos GP . Stress and disorders of the stress system . Nat Rev Endocrinol . 2009 ; 5 : 374 – 81 .
Vabulas RM , Raychaudhuri S , Hayer-Hartl M . Protein folding in the cytoplasm and the heat shock response . Cold Spring Harbor Perspectives in Biology . 2010 ; 2 : a004390 .
Chen B , Retzlaff M , Roos T et al. Cellular strategies of protein quality control . Cold Spring Harbor Perspectives in Biology . 2011 ; 3 : a004374 .
Korennykh A , Walter P . Structural basis of the unfolded protein response . Annu Rev Cell Dev Biol . 2012 ; 28 : 251 – 77 .
Chambers JE , Yarbrough JD . Xenobiotic biotransformation systems in fishes . Comp Biochem Physiol C . 1976 ; 55 : 77 – 84 .
Mello DF , de Oliveira ES , Vieira RC et al. Cellular and transcriptional responses of Crassostrea gigas hemocytes exposed in vitro to brevetoxin (PbTx-2) . Mar Drugs . 2012 ; 10 : 583 – 97 .
Boutet I , Tanguy A , Moraga D . Characterisation and expression of four mRNA sequences encoding glutathione S-transferases pi, mu, omega and sigma classes in the Pacific oyster Crassostrea gigas exposed to hydrocarbons and pesticides . Mar Biol . 2004 ; 146 : 53 – 64 .
Deeley RG , Westlake C , Cole SP . Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins . Physiol Rev . 2006 ; 86 : 849 – 99 .
Liu C , Zhang T , Wang L , et al. The modulation of extracellular superoxide dismutase in the specifically enhanced cellular immune response against secondary challenge of Vibrio splendidus in Pacific oyster ( Crassostrea gigas ) . Dev Comp Immunol . 2016 ; 63 : 163 – 70 .
Lamb DC , Lei L , Warrilow AG , et al. The first virally encoded cytochrome p450 . J Virol . 2009 ; 83 : 8266 – 9 .
Urlacher VB , Girhard M . Cytochrome P450 monooxygenases: an update on perspectives for synthetic application . Trends Biotechnol . 2012 ; 30 : 26 – 36 .
Sanderson T , van den Berg M . Topic 3.1: interactions of xenobiotics with the steroid hormone biosynthesis pathway . Pure Appl Chem . 2003 ; 75 : 1957 – 71 .
Goldstone JV , McArthur AG , Kubota A , et al. Identification and developmental expression of the full complement of cytochrome P450 genes in zebrafish . BMC Genomics . 2010 ; 11 : 643 .
Chuang SS , Helvig C , Taimi M , et al. CYP2U1, a novel human thymus- and brain-specific cytochrome P450, catalyzes omega- and (omega-1)-hydroxylation of fatty acids . J Biol Chem . 2004 ; 279 : 6305 – 14 .
Fleming I . The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease . Pharmacol Rev . 2014 ; 66 : 1106 – 40 .
Zhang G , Kodani S , Hammock BD . Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer . Prog Lipid Res . 2014 ; 53 : 108 – 23 .
de Jong-Brink M , Boer HH , Joosse J . Mollusca . In: Adiyodi KG Adiyodi RG (Eds.), Reproductive Biology of invertebrates. Oogenesis oviposition and oosorption, vol. 1 . New York : John Wiley & Sons Ltd , 1983 ; pp. 297 – 355 .
Garin CF , Heras H , Pollero RJ . Lipoproteins of the egg perivitelline fluid of Pomacea canaliculata snails (Mollusca: Gastropoda) . J Exp Zool . 1996 ; 276 : 307 – 14 .
Dreon MS , Schinella G , Heras H et al. Antioxidant defense system in the apple snail eggs, the role of ovorubin . Arch Biochem Biophys . 2004 ; 422 : 1 – 8 .
Dreon MS , Ituarte S , Heras H . The role of the proteinase inhibitor ovorubin in apple snail eggs resembles plant embryo defense against predation . PLoS One . 2010 ; 5 : e15059 .
Cardoso AM , Cavalcante JJV , Vieira RP et al. Gut bacterial communities in the giant land snail Achatina fulica and their modification by sugarcane-based diet . PLoS One . 2012 ; 7 : e33440 .
Cardoso AM , Cavalcante JJV , Cantão ME et al. Metagenomic analysis of the microbiota from the crop of an invasive snail reveals a rich reservoir of novel genes . PLoS One . 2012 ; 7 : e48505 .
Cabrera G , Pérez R , Gómez JM , et al. Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp strains . J Hazard Mater . 2006 ; 135 : 40 – 6 .
Finlay JA , Allan VJ , Conner A et al. Phosphate release and heavy metal accumulation by biofilm-immobilized and chemically-coupled cells of a Citrobacter sp. pre-grown in continuous culture . Biotechnol Bioeng . 1999 ; 63 : 87 – 97 .
Valls M , de Lorenzo V , Gonzalez-Duarte R et al. Engineering outer-membrane proteins in Pseudomonas putida for enhanced heavy-metal bioadsorption . J Inorg Biochem . 2000 ; 79 : 219 – 23 .
Pinheiro GL , Correa RF , Cunha RS , et al. Isolation of aerobic cultivable cellulolytic bacteria from different regions of the gastrointestinal tract of giant land snail Achatina fulica . Front Microbiol . 2015 ; 6 : 860 .
Zoetendal EG , Heilig HG , Klaassens ES , et al. Isolation of DNA from bacterial samples of the human gastrointestinal tract . Nat Protoc . 2006 ; 1 ( 2 ): 870 – 3 .
Adaptor cut and low quality base cut software on github. https://github.com/fanagislab/common_use
LACHESIS on github. http://shendurelab.github.io/LACHESIS/
Repeatmodeler website. http://www.repeatmasker.org/RepeatModeler.html
Repeatmasker website. http://www.repeatmasker.org/
Benson G . Tandem repeats finder: a program to analyze DNA sequences . Nucleic Acids Res . 1999 ; 27 : 573 – 80 .
Haas BJ , Salzberg SL , Zhu W et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments . Genome Biol . 2008 ; 9 : R7 .
Quevillon E , Silventoinen V , Pillai S et al. InterProScan: protein domains identifier . Nucleic Acids Res . 2005 ; 33 : W116 – 20 .
Kanehisa M , Goto S , Sato Y , et al. KEGG for integration and interpretation of large-scale molecular data sets . Nucleic Acids Res . 2012 ; 40 : D109 – D14 .
Edgar RC . MUSCLE: multiple sequence alignment with high accuracy and high throughput . Nucleic Acids Res . 2004 ; 32 : 1792 – 7 .
Darriba D , Taboada GL , Doallo R et al. ProtTest 3: fast selection of best-fit models of protein evolution . Bioinformatics . 2011 ; 27 : 1164 – 5 .
Sun J , Zhang Y , Xu T , et al. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes . Nature Ecology & Evolution . 2017 ; 1 : 121 .
Benton MJ , Donoghue PCJ , Asher RJ Hedges SB Kumar S (eds.). The Timetree of Life: Calibrating and Constraining Molecular Clocks . Oxford University Press , 2009 , 35 – 86 .
Zapata F , Wilson NG , Howison M et al. Phylogenomic analyses of deep gastropod relationships reject Orthogastropoda . Proc Biol Sci . 2014 ; 281 ( 1794 ): 20141739 .
Metagenome analysis pipeline github. https://github.com/fanagislab/metagenome_analysis.git
Li H , Durbin R . Fast and accurate short read alignment with Burrows-Wheeler transform . Bioinformatics . 2009 ; 25 : 1754 – 60 .
Nurk S , Meleshko D , Korobeynikov A , et al. metaSPAdes: a new versatile metagenomic assembler . Genome Res . 2017 ; 27 : 824 – 34 .
Hyatt D , LoCascio PF , Hauser LJ , et al. Gene and translation initiation site prediction in metagenomic sequences . Bioinformatics . 2012 ; 28 : 2223 – 30 .
Fu L , Niu B , Zhu Z , et al. CD-HIT: accelerated for clustering the next-generation sequencing data . Bioinformatics . 2012 ; 28 : 3150 – 2 .
Buchfink B , Chao X , Huson DH . Fast and sensitive protein alignment using DIAMOND . Nat Methods . 2015 ; 12 : 59 – 60 .
Gerlach W , Stoye J . Taxonomic classification of metagenomic shotgun sequences with CARMA3 . Nucleic Acids Res . 2011 ; 39 : e91 .
Kanehisa M , Goto S , Kawashima S , et al. The KEGG resource for deciphering the genome . Nucleic Acids Res . 2004 ; 32 : D277 – 80 .
Yin Y , Mao X , Yang J , et al. dbCAN: a web resource for automated carbohydrate-active enzyme annotation . Nucleic Acids Res . 2012 ; 40 : W445 – 51 .
Eddy SR . Accelerated profile HMM searches . Plos Comput Biol . 2011 ; 7 : e1002195 .
Qin JJ , Li YR , Cai ZM et al. A metagenome-wide association study of gut microbiota in type 2 diabetes . Nature . 2012 ; 490 : 55 – 60 .
Liu C , Zhang Y , Ren Y , et al. Supporting data for “The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation.” . GigaScience Database . 2018 . http://dx.doi.org/10.5524/100485
Author notes
Supplementary data.
3/12/2018 Reviewed
5/25/2018 Reviewed
3/17/2018 Reviewed
03/20/2018 Reviewed
05/31/218 Reviewed
Email alerts
Citing articles via.
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 2047-217X
- Copyright © 2024 BGI
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation
Affiliations.
- 1 Agricultural Genomics Institute, Chinese Academy of Agricultural Sciences, Pengfei Road Shenzhen, Guangdong, 518120, China.
- 2 BGI-Shenzhen, Shenzhen, Guangdong, 518083, China.
- PMID: 30107526
- PMCID: PMC6129957
- DOI: 10.1093/gigascience/giy101
Background: The golden apple snail (Pomacea canaliculata) is a freshwater snail listed among the top 100 worst invasive species worldwide and a noted agricultural and quarantine pest that causes great economic losses. It is characterized by fast growth, strong stress tolerance, a high reproduction rate, and adaptation to a broad range of environments.
Results: Here, we used long-read sequencing to produce a 440-Mb high-quality, chromosome-level assembly of the P. canaliculata genome. In total, 50 Mb (11.4%) repeat sequences and 21,533 gene models were identified in the genome. The major findings of this study include the recent explosion of DNA/hAT-Charlie transposable elements, the expansion of the P450 gene family, and the constitution of the cellular homeostasis system, which contributes to ecological plasticity in stress adaptation. In addition, the high transcriptional levels of perivitelline genes in the ovary and albumen gland promote the function of nutrient supply and defense ability in eggs. Furthermore, the gut metagenome also contains diverse genes for food digestion and xenobiotic degradation.
Conclusions: These findings collectively provide novel insights into the molecular mechanisms of the ecological plasticity and high invasiveness.
Publication types
- Research Support, Non-U.S. Gov't
- Acclimatization / genetics*
- Introduced Species*
- Snails / genetics*
- Stress, Physiological / genetics*
- Open access
- Published: 13 September 2016
Effects of dietary supplementation of golden apple snail ( Pomacea canaliculata ) egg on survival, pigmentation and antioxidant activity of Blood parrot
- Song Yang 1 ,
- Qiao Liu 1 ,
- Yue Wang 1 ,
- Liu-lan Zhao 1 ,
- Yan Wang 1 ,
- Shi-yong Yang 1 ,
- Zong-jun Du 1 &
- Jia-en Zhang 2
SpringerPlus volume 5 , Article number: 1556 ( 2016 ) Cite this article
3398 Accesses
2 Citations
1 Altmetric
Metrics details
An Erratum to this article was published on 03 October 2016
This study aims to evaluate the effects of supplementing golden apple snail ( Pomacea canaliculata ) eggs powder (EP) in the diet as a source of natural carotenoids on survival, pigmentation and antioxidant activity of Blood parrot. A total of 90 fish were divided into three treatment groups with three replicates per treatment. Blood parrot were fed with diets containing 0 (control), 5 % (EP 5 %), and 15 % (EP 15 %) dry powder of golden apple snail egg for 60 days, and nine fish per group were sampled at 20, 40, and 60 days. No differences in survival of the fish among treatments were found throughout the experiment. The body coloration of Blood parrot was enhanced in the skin and caudal fin with increasing content of golden apple snail egg powder in the diet. At the end of the experiment, the carotenoid content in the caudal fin and the number of scale chromatophores of the fish fed dietary with EP were higher ( P < 0.05) than those of the control group. The EP 15 % treated fishes showed a significant higher ( P < 0.05) in the activities of SOD after 60 days, but we could not observe significant changes ( P > 0.05) in CAT activities. Results demonstrated that golden apple snail eggs can be used as a colorant to promote the pigmentation efficacy of Blood parrot.
Blood parrot is a hybrid of female Cichlasoma synspilum and male Cichlasoma citrinellum artificially cross-bred in Taiwan as early as 1980s (Yang et al. 2012 ). Currently, Blood parrot is one of the most popular and high-value ornamental fish because of its bright body color, their size is often 10–12 cm. At present, they can be breed and produced with commercial feed in fish farm. Nonetheless, the fish cannot synthesize their own carotenoid color. As the body color is the major factor that affects the market value of ornamental fish, enhancing it by supplementing pigments in diets is therefore necessary (Gouveia et al. 2003 ; Mills and Patterson 2009 ; Olson and Owens 1998 ). Astaxanthin is a pigment widely used in the foodstuff and forage industries as a dietary supplement for developing prospects (Spiller and Dewell 2003 ). In aquaculture, the carotenoids can be produced synthetically and are commonly used for pigmentation of fishes; furthermore, alternative natural carotenoid sources (yeast, algae, higher plants, and crustacean meal) have also been studied (Büyükçapar et al. 2007 ; Chatzifotis et al. 2011 ; Teimouri et al. 2013 ; Kalinowski et al. 2007 ; Lee et al. 2010 ; Pham et al. 2014 ; Wang et al. 2006 ; Whyte and Sherry 2001 ). In addition, carotenoids were reported to be able to change antioxidant activity of fish (Pham et al. 2014 ; Wang et al. 2006 ).
Golden apple snail ( Pomacea canaliculata ) is a freshwater gastropod that has become a serious pest of agriculture and included in the world’s 100 worst invasive alien species (Lowe et al. 2000 ). This species has invaded several European, North American, and Asian countries and damages rice and aquatic organisms (Accorsi et al. 2014 ; Horgan et al. 2014 ; Karraker and Dudgeon 2014 ). Given the adverse effect of this species, physical, chemical, and biological control techniques have been established; such methods include crop rotation (Wada et al. 2004 ), use of molluscicides (Cruz et al. 2000 ; Quijano et al. 2014 ), and use of predators (Su Sin 2006 ; Ip et al. 2014 ; Yusa et al. 2006 ). As an abundant aquatic living resource, utilization of egg of golden apple snails has been rarely reported.
This snail eggs cemented outside water present bright colors and contain 72 nmol carotenoids per gram (Dreon et al. 2004 ). The major carotenoid was astaxanthin in its free (40 %), monoester (24 %), and diester (35 %) forms, mainly esterified with 16:0 fatty acid (Dreon et al. 2004 ). Previous studies reported that natural or synthetic astaxanthin can be used as an additive to enhance fish body coloration and antioxidant activity (Ho et al. 2013 ; Yang et al. 2012 ; Yedier et al. 2014 ). Furthermore, additions dietary of astaxanthin extracted from golden apple snail eggs showed efficiency for improved skin pigmentation in fancy carp ( Cyprinus carpio ) (Boonyapakdee et al. 2014 ). Despite its suitability as an additive, the high production cost of astaxanthin limits its commercial application on the large scale (Yang et al. 2012 ). Thus, the use of golden apple snail eggs would benefit to aquaculture and agriculture industry.
This study was conducted to evaluate the effects of adding golden apple snail eggs as a dietary carotenoid source on the survival, pigmentation and antioxidant activity of Blood parrot.
All experimental procedures and sample collection were approved by the Institutional Animal Care and Use Committee (IACUC) of the College of Animal Science and Technology of Sichuan Agricultural University, Sichuan China, under permit No. DKY-B20121403.
Experimental diets
Apple snail eggs were collected from the trunks or stems of the plants or on the walls above the water in rural paddy fields, lotus ponds, and streams in Ya’an city, Sichuan Province, southwest China, in May 2014. The eggs were dried in an oven at lower than 50 °C and then powdered with a grinder to avoid destructing the components in the eggs. The egg powder was sifted and added to the diet at different proportions. The powder content protein, total lipid, total carbohydrate, ash and moisture are 15.05, 1.96, 0.41, 57.26, 5.55 % respectively.
Three experimental treatments were designed as follows: control diet without golden apple snail egg powder; a diet with 5 % egg powder (EP 5 %), and a diet with 15 % egg powder (EP 15 %). The mineral and vitamin premixes used were supplied by Tong Wei Co., Ltd., Chengdu, China. Ingredient contents and proximate composition are shown in Table 1 . The basal diet was obtained from Tong Wei Co., Ltd. All dietary ingredients were thoroughly mixed, moistened by adding water, and then minced into pellets. The diets were dried overnight under 25 ± 2 °C and then stored at −20 °C until use.
Fish rearing condition
Blood parrot with an average weight of 26.53 ± 4.07 g were obtained from a commercial fish farm in Guangdong Province, China. Prior to the experiment, the fish were fed with the basal diet for 2 weeks to acclimatize them to the laboratory culturing system. Ninety Blood parrots were divided into three treatment groups with three replicates per treatment and cultivated in an aquarium with dimensions of 116 cm × 36 cm × 27 cm under continuous aeration. Manual feeding was continued until apparent satiation, twice daily at 9:00 and 17:00. One-third of the water was changed at a specific time daily from 8:00 to 9:00 am, and the temperature was maintained at 27–29 °C. The feeding experiment lasted for 60 days.
Carotenoid analysis
Nine fish obtained from each treatment group were used for carotenoid analysis. The fish were anesthetized with an dose of MS-222 (about 100 mg/L) before the skin and caudal fin were removed at every 20 days until the end of the experiment. Three other fish were randomly selected for carotenoid analysis as control at the start trial. The removed skin and fin samples were homogenized with a mortar, and 0.05–0.08 g of the samples were obtained for analysis. The samples were then transferred into 10-mL pre-weighed glass tubes and extracted twice. Tissue was weighted and carotenoid extraction done with acetone, until no colour was observed. Partial evaporation of the combined acetone extracts was carried out until approximately 5 mL was attained. Supernate were transfered into another tube. The step was performed again with add approximate 5 mL acetone, afterwards the lower phase (hypophase) was allowed to separate with n-hexane until no colour was observed.
After 24 h, the absorption of the extracts was determined at 480 nm by using a spectrophotometer (Shanghai Metash Instruments CO. LTD, model UV8000A).
Carotenoid content was determined according to the method described by Kalinowski et al. ( 2007 ) and calculated using the formula:
where S is the carotenoid content (mg/kg), A is the absorbance, K is a constant (10 4 ), V is the volume of extracting solution (mL), E is the extinction coefficient (2500), and G is the sample weight (g).
Chromatophore observation
At the end of the experiment, three to four scales were removed with forceps from the dorsal scale area of each fish and were photographed under a microscope (BX51; Olympus). The number of chromatophores was counted under the microscope at 20× magnification (Van der Salm et al. 2005 ).
Antioxidant system assays in liver
The liver of the fishes were homogenized in ice-cold physiological saline to form liver homogenate (1 g tissue in 9 mL physiological saline). The homogenates were then centrifuged at 2500 rpm for 10 min and the supernatants were used for the subsequent tests. The concentration of protein and the activities of SOD and CAT in the live homogenate were assayed using kits which purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).

Statistical analysis
All values were presented as mean ± standard deviation (SD). Data were analyzed using Two-Way ANOVA to examine the main effects of carotenoid content in same tissue, Fisher’s protected least-significant difference (PLSD) test with the SPSS 17.0 system. Differences were considered significant at the 0.05 level.
After 60 days of the experiment, no mortality occurred throughout the experiment.
Coloration effect
At the end of diet experiment, an evident coloration effect was observed and is presented in Fig. 1 . The body coloration of Blood parrot was enhanced in the skin and caudal fin, particularly in the dorsal fin, caudal fin, anal fin, and both of the body lateral lines, with increasing amount of dry powder of golden apple snail eggs added in the diet.

Coloring effects on Blood parrot in each experimental group fed for 20, 40, and 60 days
Carotenoid concentration
After 60 days of the experiment, the carotenoid content was higher in EP (5 %) and EP (15 %) fed with diets supplemented with apple snail egg powder than that in the control group fed without egg powder. The total carotenoid contents in the fish skin and caudal fin are summarized in Table 2 . EP (5 %) and EP (15 %) presented increasing trends, in which the carotenoid content in the skin was directly related to the amount of the added egg powder. Conversely, a decreasing trend was observed in the control group (Fig. 2 ).
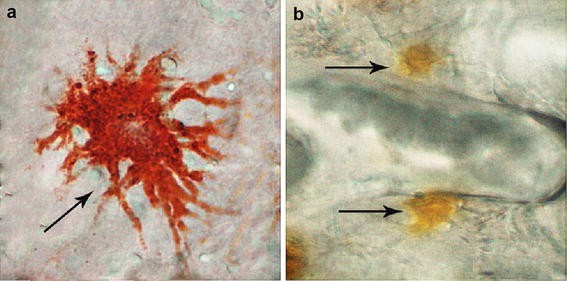
Erythrophores and xanthophores in the scales of blood parrot (40×). a Erythrophores ( arrow ). b Xanthophores ( arrow )
Chromatophores
Erythrophores and xanthophores were detected in the scale of Blood parrot (Fig. 2 ). The total number of erythrophores and xanthophores in the scales was evaluated under a microscope at 20×. After 60 days of experiment, the total amount calculated significantly differed ( P < 0.01) among treatment groups, which was 19.80 ± 1.64 in Control group, 40.75 ± 5.12 in EP 5 % group, and 48.69 ± 5.99 in EP 15 % group (Fig. 3 ).
Total number of xanthophores and erythrophores in the scales under the microscope at ×20 (** P < 0.01) after 60 days
Antioxidant activities
Superoxide dismutase (SOD) and catalase (CAT) activities of the liver of Blood parrot are summarized in Tables 3 and 4 . The EP15 % treated fishes showed level significant higher (P < 0.05) in the activities of SOD after 60 days. An increased trend was found (P > 0.05) in CAT activities, a increase trend were founded, especially in SOD activities (Table 3 ).
Carotenoids are an important class of pigments in fish, and its concentration in tissues determines the color of the fish (Pham et al. 2014 ; Sefc et al. 2014 ). Color is the primary indicator of quality and market price of ornamental fish (Pham et al. 2014 ). Nonetheless, fish cannot completely synthesize their own carotenoid color, which must be included in their diets (Mills and Patterson 2009 ; Olson and Owens 1998 ). Given this limitation, researchers conducted numerous studies on the effects of different carotenoid sources on fish pigmentation. Pham et al. ( 2014 ) reported that dietary inclusion of paprika ( Capsicum annum L.) and Haematococcus pluvialis extract with approximately 100 mg/kg carotenoid can increase the total carotenoid skin pigmentation of olive flounder ( Paralichthys olivaceus ). The addition of red pepper ( Capsicum annum L.) and marigold flower ( Tagetes erecta ) can also increase the pigmentation level of rainbow trout (Büyükçapar et al. 2007 ). The carotenoid concentration in the skin of red porgy ( Pagrus pagrus ) is augmented with increasing amount of shrimp shell meal diet (Kalinowski et al. 2007 ). On the other hand, Boonyapakdee et al. ( 2014 ) reported that dietary concentrations with 50 mg/kg astaxanthin extracted from golden apple snail eggs can improve the skin pigmentation (L* and b* values) of fancy carp, but in this study, the total carotenoid content in fish was undetermined and whether fish coloration could be improved by feeding with diet EP remains ambiguous.
In the present work, EP was added as a source of natural carotenoid in the diet of Blood parrot. Supplementation of this diet increased the carotenoid concentration in the skin and caudal fin of the fish. The high and low levels of the added EP significantly increased the carotenoid content in the caudal fin of the fish on 20 and 40 days, respectively. Moreover, the carotenoid concentration in the skin was significantly higher in the groups supplemented with high levels of EP for 60 days. The results indicate that EP can improve the pigmentation in the caudal fin, but the effect on the skin is evident at high concentration only. In addition, the carotenoid concentration in the skin and caudal fins of Blood parrot decreased during 20–60 days of supplementing diet without EP, this phenomenon is also observed in other species, such as rainbow trout (Torrissen 1985 ) and Amphiprion ocellaris (Ho et al. 2013 ). This decreased concentration can be dilution by the growth of the fish (Torrissen 1985 ).
Fish color is primarily dependent on the presence of chromatophores (melanophores, xanthophores, erythrophores, iridophores, leucophores, and cyanophores) containing pigments, such as melanins, carotenoids (e.g., astaxanthin, canthaxanthin, lutein, and zeaxanthin), pteridines, and purines (Chatzifotis et al. 2011 ). Carotenoid pigments are stored in xanthophores and erythrophores (yellow and red pigment cells, respectively) (Chatzifotis et al. 2011 ; Mills and Patterson 2009 ; Sefc et al. 2014 ). In the present experiment, the number of xanthophores and erythrophores in the scales was significantly affected by the concentration of apple snail egg powder. This result showed that EP can be efficiently utilized for scale deposition and coloration of Blood parrot. Furthermore, the presence of different types of carotenoids in diet will result in different colors of fish. Yedier reported that astaxanthin in the diet increases the red–orange color in the skin of the red zebra cichlid, whereas Spirulina intake increases the orange and yellow tones (Yedier et al. 2014 ). In the present study, Blood parrot showed the red–orange color at the end of the experiment. The appearance of this color could be attributed to the main carotenoid content, namely, esterified and non-esterified astaxanthin, in apple snail eggs.
The antioxidant defense system, especial the enzymatic scavengers SOD, CAT and glutathione peroxidase, provides protection against potentially harmful ROS produced constantly during aerobic cell respiration (Lygren et al. 1999 ). SOD speeds the conversion of superoxide to hydrogen peroxide, whereas CAT and glutathione peroxidase convert hydrogen peroxide to water (Finkel and Holbrook 2000 ). In some fish, the supplemental dietary vitamin E or carotenoid may have an effect on the antioxidant enzyme activities. Pham et al. ( 2014 ) found that SOD activities in liver and plasma of juvenile olive flounder fed diets containing carotenoids were lower than those of the control group. Similar results were observed in juvenile tiger prawn ( Penaeus monodon) (Chien et al. 2003 ) and characins ( Hyphessobrycon callistus) (Wang et al. 2006 ). Lygren et al. ( 1999 ) reported that supplemental diet with high levels of fat-soluble antioxidants (astaxanthin and vitamin E) caused a reduced need for endogenous antioxidant enzymes, such as CAT and total SOD, in protection against H 2 O 2 and ·O 2 − , respectively. However, in this study, SOD and CAT activities of the fish increased after supplementing with 15 % dry powder of golden apple snail egg, which is abundant and rich in carotenoid pigments, for 60 days. The SOD and CAT activities were inconsistent with the reported papers in liver of the fish compared with pure carotenoids.
According to Sun’s reports, more than 59 proteins from the perivitelline fluid (PVF) of P. canaliculata had been identified during its embryonic development (Sun et al. 2010 , 2012 ). Among the proteins, ovorubin is a proteinase inhibitor (PI), whose role is to limit predator’s ability to digest egg nutrients. In fact, the antinutritive or antidigestive defense is exactly the egg defensive strategies (Dreon et al. 2010 , 2014 ). Thus, the complex composition of EP may lead to failure of increased antioxidant activity of this fish.
As one of the world’s 100 worst invasive alien species, P. canaliculata has gained considerable attention worldwide. Currently, P. canaliculata are mainly controlled through physical and biological techniques to benefit the economy, public health, and ecosystems. Utilization of its eggs could destroy the life chains of P. canaliculata and thus prevent its spread. Bright-colored golden apple snails crawl out of the water to lay egg masses on plants, concrete walls, and stones above the water surface. These masses are bright pink to red in color and are easy to find and collect. Fresh apple snail eggs can be utilized to extract astaxanthin, but the process is inconvenient as it requires handling of fresh eggs. In the present study, fresh eggs were dried after collection and this method is preferred for storage and utilization of golden apple snail eggs. This technique also exhibits no negative effects on human beings, non-target organisms, and the ecological environment.
Conclusions
We have found obvious pigmentation effects of supplementing golden apple snail ( Pomacea canaliculata ) eggs powder (EP) in the diet as a source of natural carotenoids on Blood parrot. During a 60-days feeding experiment, no differences in survival of the fish among treatments were found. There were no significant changes in CAT activities ( P > 0.05), but a significantly higher ( P < 0.05) in the activities of SOD were observed at the end of experiment in EP 15 % treated fishes after 60 days. The body coloration of Blood parrot was enhanced in the skin and caudal fin with increasing content of golden apple snail egg powder in the diet. At the end of the experiment, the carotenoid content in the caudal fin and the number of scale chromatophores of the fish fed dietary with EP were higher ( P < 0.05) compared with the control group. Results demonstrated that golden apple snail eggs can be used as a colorant to promote the pigmentation efficacy of Blood parrot.
Accorsi A, Ottaviani E, Malagoli D (2014) Effects of repeated hemolymph withdrawals on the hemocyte populations and hematopoiesis in Pomacea canaliculata . Fish Shellfish Immunol 38:56–64
Article PubMed Google Scholar
Boonyapakdee A, Pootangon Y, Laudadio V, Tufarelli V (2015) Astaxanthin extraction from golden apple snail ( Pomacea canaliculata ) eggs to enhance colours in fancy carp ( Cyprinus carpio ). J Appl Anim Res 43(3):291–294
Article CAS Google Scholar
Büyükçapar HM, Yanar M, Yanar Y (2007) Pigmentation of rainbow trout ( Oncorhynchus mykiss ) with carotenoids from Marigold flower ( Tagetes erecta ) and red pepper ( Capsicum annum ). Turkish J Vet Anim Sci 31:7–12
Google Scholar
Chatzifotis S, Vaz J, Kyriazi P, Divanach P, Pavlidis M (2011) Dietary carotenoids and skin melanin content influence the coloration of farmed red porgy ( Pagrus pagrus ). Aquac Nutr 17:e90–e100
Article Google Scholar
Chien Y, Pan C, Hunter B (2003) The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 216:177–191
Cruz MS, Joshi RC, Martin EC (2000) Potential effects of commercial molluscicides used in controlling golden apple snails on the native snail Vivipara costata (Quoy & Gaimard). Philipp Entomol 14:149–157
Dreon MS, Schinella G, Heras H, Pollero RJ (2004) Antioxidant defense system in the apple snail eggs, the role of ovorubin. Arch Biochem Biophys 422:1–8
Article CAS PubMed Google Scholar
Dreon MS, Ituarte S, Heras H (2010) The role of the proteinase inhibitor ovorubin in apple snail eggs resembles plant embryo defense against predation. PLoS ONE 5:e15059
Article ADS CAS PubMed PubMed Central Google Scholar
Dreon MS, Fernández PE, Gimeno EJ, Heras H (2014) Insights into embryo defenses of the invasive apple snail Pomacea canaliculata : egg mass ingestion affects rat intestine morphology and growth. PLoS Negl Trop Dis 8:e2961
Article PubMed PubMed Central Google Scholar
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Article ADS CAS PubMed Google Scholar
Gouveia L, Rema P, Pereira O, Empis J (2003) Colouring ornamental fish ( Cyprinus carpio and Carassius auratus ) with microalgal biomass. Aquac Nutr 9:123–129
Ho AL, O’Shea SK, Pomeroy HF (2013) Dietary esterified astaxanthin effects on color, carotenoid concentrations, and compositions of clown anemonefish, Amphiprion ocellaris , skin. Aquacult Int 21:361–374
Horgan FG, Felix MI, Portalanza DE, Sánchez L, Moya Rios WM, Farah SE, Wither JA, Andrade CI, Espin EB (2014) Responses by farmers to the apple snail invasion of Ecuador’s rice fields and attitudes toward predatory snail kites. Crop Protect 62:135–143
Ip KK, Liang Y, Lin L, Wu H, Xue J, Qiu J (2014) Biological control of invasive apple snails by two species of carp: effects on non-target species matter. Biol Control 71:16–22
Kalinowski CT, Izquierdo MS, Schuchardt D, Robaina LE (2007) Dietary supplementation time with shrimp shell meal on red porgy ( Pagrus pagrus ) skin colour and carotenoid concentration. Aquaculture 272:451–457
Karraker NE, Dudgeon D (2014) Invasive apple snails ( Pomacea canaliculata ) are predators of amphibians in South China. Biol Invasions 16(9):1785–1789
Lee CR, Pham MA, Lee SM, Lee CR, Pham MA (2010) Effects of dietary paprika and lipid levels on growth and skin pigmentation of pale chub ( Zacco platypus ). Asian Australas J Anim Sci 23:724–732
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species Specialist Group Species Survival Commission, World Conservation Union (IUCN), Auckland
Lygren B, Hamre K, Waagbø R (1999) Effects of dietary pro-and antioxidants on some protective mechanisms and health parameters in Atlantic salmon. J Aquat Anim Health 11:211–221
Mills MG, Patterson LB (2009) Not just black and white: pigment pattern development and evolution in vertebrates. Semin Cell Dev Biol 20:72–81
Olson VA, Owens IP (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Pham MA, Byun H, Kim K, Lee S (2014) Effects of dietary carotenoid source and level on growth, skin pigmentation, antioxidant activity and chemical composition of juvenile olive flounder Paralichthys olivaceus . Aquaculture 431:65–72
Quijano M, Ruíz CR, Barragán A, Miranda M, Orellana T, Manzano P (2014) Molluscicidal activity of the aqueous extracts from Solanum mammosum L ., Sapindus saponaria L . and Jatropha curcas L . against Pomacea canaliculata . Emirates J Food Agric 26(10): 871–877
Sefc KM, Brown AC, Clotfelter ED (2014) Carotenoid-based coloration in cichlid fishes. Comp Biochem Physiol A: Mol Integr Physiol 173:42–51
Spiller GA, Dewell A (2003) Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: a randomized clinical trial. J Med Food 6:51–56
Su Sin T (2006) Evaluation of different species of fish for biological control of golden apple snail Pomacea canaliculata (Lamarck) in rice. Crop Protect 25:1004–1012
Sun J, Zhang Y, Thiyagarajan V, Qian PY, Qiu JW (2010) Protein expression during the embryonic development of a gastropod. Proteomics 10:2701–2711
Sun J, Zhang H, Wang H, Heras H, Dreon MS, Ituarte S, Ravasi T, Qian P, Qiu J (2012) First proteome of the egg perivitelline fluid of a freshwater gastropod with aerial oviposition. J Proteome Res 11:4240–4248
Teimouri M, Amirkolaie AK, Yeganeh S (2013) The effects of dietary supplement of Spirulina platensis on blood carotenoid concentration and fillet color stability in rainbow trout ( Oncorhynchus mykiss ). Aquaculture 414–415:224–228
Torrissen OJ (1985) Pigmentation of salmonids: factors affecting carotenoid deposition in rainbow trout ( Salmo gairdneri ). Aquaculture 46:133–142
Van der Salm AL, Spanings FAT, Gresnigt R, Wendelaar SE, Flik G (2005) Background adaptation and water acidification affect pigmentation and stress physiology of tilapia, Oreochromis mossambicus . Gen Comp Endocrinol 144(1):51–59
Wada T, Ichinose K, Yusa Y, Sugiura N (2004) Decrease in density of the apple snail Pomacea canaliculata (Lamarck) (Gastropoda: Ampullariidae) in paddy fields after crop rotation with soybean, and its population growth during the crop season. Appl Entomol Zool 39:367–372
Wang Y, Chien Y, Pan C (2006) Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus . Aquaculture 261:641–648
Whyte JN, Sherry KL (2001) Pigmentation and composition of flesh of Atlantic salmon fed diets supplemented with the yeast Phaffia rhodozyma . N Am J Aquac 63:52–57
Yang H, Mu X, Luo D, Hu Y, Song H, Liu C, Luo J (2012) Sodium taurocholate, a novel effective feed-additive for promoting absorption and pigmentation of astaxanthin in blood parrot ( Cichlasoma synspilum ♀ × Cichlasoma citrinellum ♂). Aquaculture 350:42–45
Yedier S, Gümüs E, Livengood EJ, Chapman FA (2014) The relationship between carotenoid type and skin color in the ornamental red zebra cichlid Maylandia estherae . AACL Bioflux 7:207–216
Yusa Y, Sugiura N, Wada T (2006) Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae), in southern Japan. Biol Invasions 8:137–147
Download references
Authors’ contributions
SY, QL and LLZ wrote the manuscript. YW, QL and SYY prepared the experiment. SY, LLZ and QL prepared the figures and tables. Specially, ZJD and JEZ prepared the fish in the experiment. All authors read and approved the final manuscript.
Acknowledgements
This research was supported by the “Double Support Project” fund of Sichuan Agricultural University, SICAU (No. 03570202). We are also grateful for the assistance obtained from Jin-wei Yang and Tao Yan of the College of Animal Science and Technology, SCAU, as well as Qiang-qiang Wu of Tongwei Co., Ltd.
Competing interests
The authors declared that they have no competing interests.
Author information
Authors and affiliations.
College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, 611130, Sichuan, China
Song Yang, Qiao Liu, Yue Wang, Liu-lan Zhao, Yan Wang, Shi-yong Yang & Zong-jun Du
Institute of Tropical and Subtropical Ecology, South China Agricultural University, Guangzhou, 510642, Guangdong, China
Jia-en Zhang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Liu-lan Zhao .
Additional information
Song Yang and Qiao Liu have contributed equally to this work
An erratum to this article can be found at http://dx.doi.org/10.1186/s40064-016-3327-6 .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Yang, S., Liu, Q., Wang, Y. et al. Effects of dietary supplementation of golden apple snail ( Pomacea canaliculata ) egg on survival, pigmentation and antioxidant activity of Blood parrot. SpringerPlus 5 , 1556 (2016). https://doi.org/10.1186/s40064-016-3051-2
Download citation
Received : 06 June 2016
Accepted : 11 August 2016
Published : 13 September 2016
DOI : https://doi.org/10.1186/s40064-016-3051-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Golden apple snail egg
- Blood parrot
- Pigmentation
- Antioxidant activity
The Development of Golden Apple Snail Eggs Picker
- Conference paper
- First Online: 02 October 2021
- Cite this conference paper

- Wan Zaiyana Yusof 17 , 18 ,
- Azmir Mamat Nawi 19 &
- Puteri Fadzline Muhamad Tamyez 18
Part of the book series: Lecture Notes in Mechanical Engineering ((LNME))
860 Accesses
Several potential benefits of Golden Apple Snail, or GAS have been reported, including its use as a source of food, use in the trade of aquariums, biological control of plants, as a source of protein for fish, ducks and crocodiles, and as a liquid biofertilizer. However, its physiological adaptability and capacity to travel a long distance within a water system has become an invader of new habitats. Thus, GAS is now a major pest in the cultivation of rice. Numerous research have shown that GAS can be managed at various stages of cultivation by combining chemical, mechanical, and biological steps. Nevertheless, GAS eradication has become a laborious and expensive task. This study aims to design the biological tool or prototype to eradicate this major pest. More particularly, the invention relates to a tool that adapts hand-picking method in controlling and eradicating the eggs of these pests. This study provides a society implication for paddy farmers, in which the creation of this tool is effective and important to drive the farmers to better productivity with the eradication of GAS eggs.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Yusa Y, Wada T (1999) Impact of the introduction of apple snails and their control in Japan. Naga ICLARM Q 22(3):9–13
Google Scholar
Ranamukhaarachchi L, Wickramasinghe S (2006) Golden apple snails in the world: introduction, impact, and control measures. Global advances in ecology and management of golden apple snails. Philippine Rice Research Institute, Nueva Ecija, pp 133–152
Robert HC (2002) Apple snails (Ampullariidae) as agricultural pests: their biology, impacts and management. Barke. Centre for Agriculture and Bioscience International, pp 145–192
Sin TS (2003) Damage potential of the golden apple snail Pomacea canaliculata (Lamarck) in irrigated rice and its control by cultural approaches. Int J Pest Manag 49(1):49–55
Article Google Scholar
San Martin R, Mackenna AV (2006) Recent development in the use of botanical molluscicides against golden apple snails ( Pomacea canaliculata )
Yahaya H, Nordin M, Hisham MNM, Sivapragasam A, Joshi RC, Cowie RH, Sebastian LS (2017) Invasive apple snails in Malaysia. Biology and management of invasive apple snails, 2nd edn. Philippine Rice Research Institute, pp 169–195
Mamat Nawi A (2011) Design through research: handpicking tools case study as facilitator to collaborative product development/Azmir Mamat Nawi and Wan Zaiyana Mohd Yusof. Universiti Teknologi MARA, Kedah
International Rice Research Institute (IRRI) (1990) Low-external input rice production (LIRP) Technology Information Kit, p 292
Nawi AM, Karim ISA, Rahim R (2002) New tool for golden apple snails eradication: case study Kuala Muda Area. In: I-CAST 2012, international conference on arts, social sciences and technology
Download references
Author information
Authors and affiliations.
Faculty of Art and Design, Universiti Teknologi MARA, Shah Alam, Malaysia
Wan Zaiyana Yusof
Faculty of Industrial Management, Universiti Malaysia Pahang, Kuantan, Malaysia
Wan Zaiyana Yusof & Puteri Fadzline Muhamad Tamyez
Art and Design Department, Universiti Teknologi MARA, Merbok, Kedah, Malaysia
Azmir Mamat Nawi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Puteri Fadzline Muhamad Tamyez .
Editor information
Editors and affiliations.
Faculty of Mechanical and Automotive Engineering Technology, Universiti Malaysia Pahang, Pekan, Pahang, Malaysia
Mohd Hasnun Arif Hassan
Human Engineering Research Group (HUMEN), Faculty of Mechanical and Automotive Engineering Technology, Universiti Malaysia Pahang, Pekan, Pahang, Malaysia
Zulkifli Ahmad (a) Manap
Mohamad Zairi Baharom
Nasrul Hadi Johari
Ummu Kulthum Jamaludin
Muhammad Hilmi Jalil
Idris Mat Sahat
Mohd Nadzeri Omar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper.
Yusof, W.Z., Nawi, A.M., Tamyez, P.F.M. (2022). The Development of Golden Apple Snail Eggs Picker. In: Hassan, M.H.A., et al. Human-Centered Technology for a Better Tomorrow. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-4115-2_32
Download citation
DOI : https://doi.org/10.1007/978-981-16-4115-2_32
Published : 02 October 2021
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-4114-5
Online ISBN : 978-981-16-4115-2
eBook Packages : Engineering Engineering (R0)
Share this paper
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Golden Apple Snails
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Nematode Follow Following
- Trematodes Follow Following
- Lymnaeid Snails Follow Following
- Larval Trematodes Follow Following
- Snails in the Philippines Follow Following
- Biopesticides Follow Following
- Ecomorphology Follow Following
- Crop Protection Follow Following
- Natural product research Follow Following
- Malacology Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
VIDEO
COMMENTS
A Review of the Potential of Golden Apple Snail (Pomacea Canaliculata) as a Raw Material for Aquaculture Feed July 2023 International Journal of Scientific Multidisciplinary Research 1(6):655-664
The mammalian parasite A. cantonensis finds the golden apple snail a suitable intermediate host. This parasite, whose diffusion is a cause for concern due to its wide distribution [ 13 ], triggers the formation of recognizable nodules in the infected snails [ 14 ], although the involvement of hemocytes in this process has not been documented.
The golden apple snail (Pomacea canaliculata) is a freshwater snail listed among the top 100 worst invasive species worldwide and a noted agricultural and quarantine pest that causes great economic losses. It is characterized by fast growth, strong stress tolerance, a high reproduction rate, and adaptation to a broad range of environments.
Potential ranges of golden apple snail derived from native and introduced occurrence records. (a) Red represents the potential ranges of native golden apple snail, covering ca. 25,6761 km 2. (b) Red represents the potential ranges of golden apple snail in invaded regions, covering ca. 15,063,498 km 2. Gray indicates no potential range.
Background: The golden apple snail (Pomacea canaliculata) is a freshwater snail listed among the top 100 worst invasive species worldwide and a noted agricultural and quarantine pest that causes great economic losses. It is characterized by fast growth, strong stress tolerance, a high reproduction rate, and adaptation to a broad range of environments.
Ecology is a leading journal publishing original research and synthesis papers on all aspects of ecology, with particular emphasis on cutting-edge research and new concepts. We investigated the effects of an exotic snail, the golden apple snail (Pomacea canaliculata) on biodiversity and ecosystem functioning in tropical wetland ecosystems. ...
Abstract. Golden apple snails are tremendously serious global invasive pests originated from South America. They were introduced into China in 1979 as commercial production. During last 30 years, researches on species identity, spread and origination, as well as the management have been progressed. It has been clarified that there are Pomacea ...
DNA · Golden apple snails · Invasive species · Biomonitoring Introduction Invasive alien species (transported and established non-natives) pose extreme threats to the areas to which they are introduced, by creating an imbalance in the natural ecosystem, carrying disease, and elimi-nating local populations (Tobin, 2018; Shabani et al., 2020).
The golden apple snail Pomacea canaliculata (Lamarck) is a recently introduced rice pest in Asia. Its management is based on preventative and corrective methods emphasizing cultural and mechanical practices, with molluscicide as a measure of last resort. The planting method greatly influences the period of susceptibility to snail damage: wetbed ...
RESEARCH PAPER OPEN ACCESS Landmark-based relative warp analysis of Golden Apple Snail (Pomacea caniculata) from Agusan del Norte and Sur rice ... golden apple snails (P.caniculata) were randomly collected in the rice fields of Bayugan City, Antongalon Butuan City, RTR and Buenavista, Agusan del Norte. To determine shell shape variations among ...
The invasive and widespread golden apple snail (GAS, Pomacea canaliculata ) is a harmful crop pest in many parts of Asia. The heavy use of molluscicides to control GAS could result in soil and water pollution as well as in loss of biodiversity. ... This paper reviews the research progress on the biological characteristics and control strategies ...
This study aims to evaluate the effects of supplementing golden apple snail (Pomacea canaliculata) eggs powder (EP) in the diet as a source of natural carotenoids on survival, pigmentation and antioxidant activity of Blood parrot. A total of 90 fish were divided into three treatment groups with three replicates per treatment. Blood parrot were fed with diets containing 0 (control), 5 % (EP 5 % ...
Thirty adult giant African snails (Lissachatina fulica) and golden apple snails (Pomacea canaliculata) ... filtered through Whatman No. 1 filter paper using vacuum filtration, and stored at 4 °C. ... Our research has shown that the mucus from L. fulica and P. canaculata possess antioxidant and anti-inflammatory properties. We have also studied ...
Semantic Scholar extracted view of "Understanding the golden apple snail (Pomacea canaliculata): biology and early initiatives to control the pest in the Philippines" by C. Adalla et al. ... Search 218,097,719 papers from all fields of science. Search. Sign In Create Free Account. ... AI-powered research tool for scientific literature, based at ...
Corpus ID: 90241388; Nutritional components and utilization values of golden apple snails (Pomacea canaliculata) in different habitats @inproceedings{Du2012NutritionalCA, title={Nutritional components and utilization values of golden apple snails (Pomacea canaliculata) in different habitats}, author={Luo Du and Mu Xidong and Song Hongmei and Gu Dang'en and Yang Yexin and Wang Xuejie and Lu ...
Abstract. The golden apple snail, Pomacea canaliculata (Lamarck, 1822) (Mesogastropoda: Pilidae), has recently been introduced to several Asian countries where it has unexpectedly developed into a pest of rice. Reasons for the introduction as well as the economic and ecological impact of the snail are described. Most farmers have resorted to chemical control, with implications for human health ...
Pomacea canaliculate, also referred to as golden apple snails (GAS), was first detected in Malaysia in 1991, and it took almost 10 years before it became one of the country's main rice pests.These snails reproduce quickly and usually lay eggs on any plants, leaves and other items above the surface of the water at night, with a quantity of 1000-1200 eggs per month.
Mating frequency was related with snail abundance (r s = 0.26; p<0.05) and water temperature (r s = 0.34; p<0.05) and the abundance of egg masses is related with snail abundance (r s = 0.46; p<0.05). In general, we observed that reproductive activity of P. flagellata at Bacalar lagoon is related with the warmer months and with higher rainfall.
Abstract— The purpose of this research is to evaluate Golden Apple Snail (GAS) shells as an alternative source of stabilizing agent for sandy clay loam soils. However, testing revealed that the acquired soil had a higher plasticity index, indicating that it contains a high percentage of clay.