Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 30 March 2022

Closing in on a cure for hepatitis B
- Elie Dolgin 0
Elie Dolgin is a science journalist in Somerville, Massachusetts.
You can also search for this author in PubMed Google Scholar
Illustration: Chiara Zarmati
For Thomas Tu, eliminating hepatitis B is a deeply personal goal.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 603 , S46-S48 (2022)
doi: https://doi.org/10.1038/d41586-022-00812-1
This article is part of Nature Outlook: Hepatitis B , an editorially independent supplement produced with the financial support of third parties. About this content .
The Polaris Observatory Collaborators. Lancet Gastroenterol. Hepatol. 3 , 383–403 (2018).
Article PubMed Google Scholar
Yan, H. et al. eLife 13 , e00049 (2012).
Article Google Scholar
Revill, P., Testoni, B., Locarnini, S. & Zoulim, F. Nature Rev. Gastroenterol. Hepatol. 13 , 239–248 (2016).
Revill, P. A. et al. Lancet Gastroenterol. Hepatol. 4 , 545–558 (2019).
Yang, Y.-C. & Yang, H.-C. Viruses 14 , 4 (2022).
Yuen, M.-F. et al. Nature Med. 27 , 1725–1734 (2021).
Tu, T., Block, J. M., Wang, S. Cohen, C. & Douglas, M. W. Viruses 12 , 515 (2020).
Download references
Related Articles

Sponsor feature: Charting a new frontier in chronic hepatitis B research to improve lives worldwide
Sponsor feature: Addressing the unmet need for a functional cure for chronic hepatitis B
- Drug discovery
- Therapeutics
- Medical research
‘Mini liver’ will grow in person’s own lymph node in bold new trial
News 02 APR 24

Green space near home has an antidepressant effect
Research Highlight 01 APR 24

The future of at-home molecular testing
Outlook 21 MAR 24
‘A landmark moment’: scientists use AI to design antibodies from scratch
News 19 MAR 24

A spotlight on the stark imbalances of global health research
Nature Index 13 MAR 24

Can non-profits beat antibiotic resistance and soaring drug costs?
News Feature 28 FEB 24
Anti-ageing antibodies revive the immune system
News & Views 27 MAR 24

How AI is being used to accelerate clinical trials

Yaws could soon be eradicated — 70 years behind schedule
Outlook 11 JAN 24
Postdoctoral Associate- Cell Biology
Houston, Texas (US)
Baylor College of Medicine (BCM)
Head of ClinicalTrials.gov
National Institutes of Health (NIH) National Library of Medicine (NLM) National Center for Biotechnology Information (NCBI) Information Engineering...
Washington D.C. (US)
National Library of Medicine, National Center for Biotechnology Information
POSTDOCTORAL FELLOWSHIP IN SYSTEMS BIOLOGY: PRECISION VACCINE PROGRAM (PVP) - BOSTON CHILDREN'
The Data Management and Analysis Core (DMAC) within the Precision Vaccine Program (PVP) at Boston Children’s Hospital and Harvard Medical School (H...
Boston, Massachusetts
Boston Children's Hospital - Department of Pediatrics
2024 Recruitment notice Shenzhen Institute of Synthetic Biology: Shenzhen, China
The wide-ranging expertise drawing from technical, engineering or science professions...
Shenzhen,China
Shenzhen Institute of Synthetic Biology
Global Talent Recruitment (Scientist Positions)
Global Talent Gathering for Innovation, Changping Laboratory Recruiting Overseas High-Level Talents.
Beijing, China
Changping Laboratory
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
Media Advisory
Tuesday, December 10, 2019
NIH strategic plan details pathway to achieving Hepatitis B cure

A highly effective vaccine to prevent hepatitis B virus (HBV) infection has been available for nearly 40 years, yet millions of people worldwide continue to become infected with the liver-attacking virus and more than 600,000 die from its complications each year, according to the U.S. Centers for Disease Control and Prevention. A new strategic plan from the National Institutes of Health outlines efforts to intensify ongoing HBV research with the goals of developing a cure and improving scientific understanding of the virus while creating improved strategies for screening and treating patients.
HBV is transmitted through sex, contact with infected blood or bodily fluids, or from an infected mother to her baby, and can result in either an acute infection that resolves or a chronic infection. Chronic HBV infection can lead to serious health issues including cirrhosis (scarring of the liver), liver failure or liver cancer. Approximately 80% to 90% of infants infected during the first year of life will develop chronic infection, while only 5% of people infected as adults will become chronically infected. Between 850,000 and 2 million people in the United States have chronic hepatitis B; globally, that number is 257 million, according to the World Health Organization.
Although treatments are available to control HBV infection, they must be taken for years if not for life. Additionally, high medication costs, the need for continuous disease monitoring and adhering to treatment regimens present significant burdens for people with chronic hepatitis B. Even among those treated for chronic hepatitis B, the risk of developing cirrhosis and liver cancer remains elevated.
Scientific discoveries within the past decade suggest that a hepatitis B cure is possible. The new NIH plan defines a hepatitis B “cure” as a sustained loss of a specific protein on the surface of HBV called hepatitis B virus surface antigen — preferably with antibodies against the antigen and undetectable viral DNA after completion of a finite course of treatment. Ideally, a hepatitis B cure would also reduce a person’s risk of liver failure and liver cancer. To effectively address the global public health challenge posed by HBV, a curative treatment will need to complement better approaches to screening, follow-up care, and vaccination coverage.
The Strategic Plan for Trans-NIH Research to Cure Hepatitis B focuses on three key research areas. The first priority calls for a better understanding of hepatitis B biology, including the viral and host factors that lead to disease, immunity, reactivation and transmission, as well as the impact of co-infections with other hepatitis viruses and HIV. An improved understanding of these factors and the HBV lifecycle is essential to developing a cure.
The second priority emphasizes the development and sharing of tools and resources to support fundamental research and product development. This includes standardizing and sharing reagents and assays; improving and creating new animal models to study the progression of human liver disease and mother-to-child transmission; and establishing biomarkers for disease progression and response to therapy.
The third priority calls for the creation of strategies to cure and prevent hepatitis B infection. A cure could include approaches to blocking viral replication, stimulating the anti-HBV immune responses or eliminating HBV-infected cells. However, achieving a cure requires strengthening existing public health efforts for promoting hepatitis screening, ensuring that high-risk and underserved populations have access to vaccination, and prioritizing follow-up to care and adherence to treatment, according to the plan.
The strategic plan builds on NIH’s ongoing hepatitis B research portfolio and the U.S. National Viral Hepatitis Action Plan . NIH sought input from academia, patient advocacy organizations, private and nonprofit companies, government organizations, and clinical trial networks funded by the NIH in the development of this strategic plan.
The Strategic Plan for Trans-NIH Research to Cure Hepatitis B
Anthony S. Fauci, M.D., director of the National Institute of Allergy and Infectious Diseases, is available to comment on the strategic plan.
To schedule interviews, please contact NIAID Office of Communications, (301) 402-1663, [email protected] .
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Understanding If There’s a Cure for Hepatitis B
- Is There a Cure?
- Acute vs. Chronic
- Treatment Options
- Risk Factors
Frequently Asked Questions
When news broke in 2014 that hepatitis C , a viral disease affecting the liver, could be cured thanks to a new class of direct-acting antiviral drugs , many began to wonder how soon it would be before the same occurred with its cousin, hepatitis B .
Scientists have yet to find a cure for this potentially severe form of viral hepatitis , which affects anywhere from 2.4 million to 4.7 million people in the United States.
This article takes a look at hepatitis B and ongoing cure research, including the development of direct-acting antivirals similar to those used to treat hepatitis C. It also explains how hepatitis B is currently treated and prevented with medications and vaccines.
FatCamera / Getty Images
Is There a Cure for Hepatitis B?
The long and short answer is that there is not yet a cure for hepatitis B. Understanding why requires insight into the virus itself and the challenges cure researchers face.
Hepatitis B is an infectious disease caused by the hepatitis B virus (HBV). While most people exposed to hepatitis B will spontaneously clear the virus (eliminating it from the body) soon after infection, a proportion will go on to develop a chronic (persistent) infection.
Of these, around one in four will develop severe liver complications, including cirrhosis (extensive scarring of the liver) and liver cancer , typically years after the initial infection.
Efforts to find a cure for hepatitis B have been underway since the virus was first identified by scientists at the National Institutes of Health in 1966. It soon became clear, however, that numerous hurdles would need to be overcome before an actual cure could be achieved. Chief among these are:
- Poor innate immunity : For reasons that are not entirely clear, HBV is not readily recognized by the immune system during early-stage infection and is poorly eliminated by the body's frontline innate immune response .
- Poor adaptive immunity : Over time, the body's disease-specific adaptive immune response also weakens due to a phenomenon known as T-cell exhaustion. When this occurs, the immune system is less able to recognize and launch an immune assault against the virus.
- Viral reservoirs : In chronically infected people, HBV will embed itself within tissues inside and outside the liver, called viral reservoirs. Within these reservoirs, the virus is largely shielded from immune detection and is difficult to reach with antiviral drugs.
- cccDNA : What differentiates hepatitis B from hepatitis C is the unique structure of its viral DNA, called covalently closed circular DNA (cccDNA). Antiviral drugs have limited effectiveness against this seemingly indestructible "mini-chromosome" that continues to pump out new viruses from infected liver cells.
Overcoming the Hurdles
Despite the challenges in finding a cure, scientists have a greater understanding of how HBV infects, replicates, and persists. By targeting and blocking these mechanisms with either one or a combination of therapies, scientists hope to one day render the virus harmless or eliminate it.
Among some of the leading drug candidates are:
- Bepirovirsen : An experimental direct-acting antiviral that may block cccDNA from delivering the genetic code used to build new viruses
- HBsAg monoclonal antibody : An experimental form of immunotherapy used to boost the immune system's ability to recognize and launch a targeted immune attack against HBV
- JNJ-64300535 : An experimental therapeutic vaccine that may help activate the adaptive immune response in people with chronic hepatitis B infection
- REP 2139/2165 : An experimental antiviral direct-acting antiviral that the limits the production of HBV subviral particles, which are thought to lessen the effects of the immune system
- RO7049389 : An experimental direct-acting antiviral that blocks the assembly of new viruses
Clinical Trials
Today, there are at least 50 different HBV therapies—including more than 25 experimental direct-acting antivirals—undergoing clinical trials, with more expected to follow.
Difference Between Acute and Chronic Hepatitis B
Acute hepatitis B is the stage of infection immediately following exposure to the virus. Many of these infections are asymptomatic , meaning without symptoms.
Of those who do develop symptoms, some of the more common include:
- Persistent fatigue
- Loss of appetite
- Nausea or vomiting
- Abdominal pain
- Clay-colored stools
- Jaundice (yellowing of the skin and the whites of the eyes)
Clearing Acute Hepatitis B
Some studies suggest that up to 95% of adults with acute HBV infection will spontaneously clear the virus, usually within six months, with no lasting repercussions.
Chronic hepatitis B occurs when the immune system does not clear the virus. Around one of every 20 people acutely infected with HBV will progress to this persistent stage of infection.
Chronic hepatitis B is a slowly progressive disease in which ongoing inflammation causes the gradual scarring of the liver. This can lead to cirrhosis (the loss of liver function due to scarring) and hepatocellular carcinoma (the most common form of liver cancer).
However, the course of chronic HBV infection is not set. Some people may progress faster than others, while others may never develop overt symptoms.
Statistically speaking:
- The risk of cirrhosis in people with chronic hepatitis B is approximately 10% to 20% over 20 years, increasing to 40% after 30 years.
- The risk of hepatocellular carcinoma increases by 2% and 3% per year in people with HBV and cirrhosis. People without cirrhosis can also get it, but the annual risk drops to around 0.02%.
Clearing Chronic Hepatitis B
The vast majority of people with chronic hepatitis B will have it for a lifetime. Even so, around 0.5% of those with non-progressing chronic hepatitis B spontaneously clear the virus every year.
How Hepatitis B Is Treated
Hepatitis B cannot be cured, but newer, less toxic drug therapies have effectively slowed the progression of the disease in chronically infected people. Even those with advanced liver disease have longer survival and better quality of life thanks to newer drug therapies.
Acute Hepatitis B
There is no specific treatment for acute hepatitis B infection. If you experience acute symptoms of hepatitis B and test positive for the virus , the treatment would be focused on managing symptoms and providing nutritional support.
An exception is in people with fulminant hepatitis , an uncommon but severe form of liver failure that typically occurs within eight weeks of the appearance of hepatitis symptoms.
Fulminant hepatitis is treated with the antiviral drug Epivir (lamivudine) to reduce the risk of liver damage and the need for a liver transplant . Epivir may also be considered in people with acute hepatitis B who experience severe symptoms.
There are no drugs able to clear an HBV infection after it occurs.
With that said, many people with acute hepatitis will spontaneously clear the virus and, in turn, be afforded lifelong immunity to HBV.
Chronic Hepatitis B
Chronic hepatitis B is definitively diagnosed when blood tests are able to detect a protein called hepatitis B surface antigen ( HBsAg ). lt can take up to six months to accurately detect HBsAg after an infection occurs.
Most people with chronic hepatitis B require treatment for a lifetime to slow the progression of the disease. This may involve:
- Antiviral drugs : These medications are taken by mouth every day and work in different ways to block the replication of HBV. The six options approved for use in the United States are Baraclude (entecavir) , Epivir (lamivudine), Hepsera (adefovir) , Tyzeka (telbivudine), Vemlidy (tenofovir AF), and Viread (tenofovir DF) .
- Pegasys (pegylated interferon alfa-2A) : This drug is injected subcutaneously (under the skin) that interferes with the replication of HBV. It also enhances the immune response to the virus. It is typically used as part of combination antiviral therapy.
- Liver transplantation : This is an option if you experience liver failure or liver cancer. The organ usually comes from a deceased donor. Less commonly, a portion of a living donor's liver can be transplanted.
Is Hepatitis B Preventable?
Chronic hepatitis B infection affects an estimated 290 million people worldwide, causing over 820,000 deaths annually. It is also a major cause of liver cancer, which causes over 25,000 deaths in the U.S. every year. The CDC now recommends that all adults get screened for hepatitis B at least once, including those who are not at greater risk of exposure.
Unlike hepatitis C, hepatitis B can be prevented with vaccines. If you are accidentally exposed to the virus, there are also drug therapies you can take—called postexposure prophylaxis—to avert the infection.
Hepatitis B Vaccine
The four hepatitis B vaccines approved for use by the Food and Drug Administration (FDA) are:
- Recombivax HB
The vaccines are given by injection into a large muscle in either two or three doses over six months. The dosage varies by the person's age, immune status , and choice of vaccine.
Who Should Get the Hepatitis B Vaccine?
The Advisory Committee on Immunization Practices (ACIP) recommends that the following groups receive the hepatitis B vaccine series:
- All infants
- Unvaccinated children under the age of 19
- Adults age 19–59
- Adults age 60 and older with risk factors for hepatitis B
Adults age 60 and up without known risk factors may also opt for vaccination given that the benefits of HBV vaccination generally outweigh the risks.
Postexposure Prophylaxis
Postexposure prophylaxis (PEP) is a treatment designed to prevent an infection after a recent exposure. For hepatitis B, PEP may involve:
- Hepatitis B vaccination (or revaccination) : A three-dose vaccine series typically is advised.
- Hepatitis B immunoglobulins (HBIG) : This is a purified solution of hepatitis antibodies derived from donated blood. It is delivered by injection to bolster the body's natural immune defenses.
Hepatitis B vaccination is considered the mainstay of PEP. In cases in which the source of the exposure is known to have hepatitis B, both hepatitis B vaccination and HBIG would be used.
Hepatitis B PEP should ideally be started within 24 hours of the suspected exposure, although it may still have benefits up to seven days after the exposure.
How Do You Get Hepatitis B?
The hepatitis B virus is found mainly in the blood but also in semen and vaginal secretions.
The virus is passed when body fluids from someone with hepatitis B enter the body of someone without hepatitis B. This can happen when sharing needles or syringes, engaging in vaginal or anal sex, or during childbirth, when the virus can be passed from mother to baby.
Unlikely Sources of Infection
Trace levels of HBV can also be found in saliva, tears, urine, and feces but in amounts that are highly unlikely to cause infection.
While vaccination remains the cornerstone of HBV prevention, there are ways to further reduce the risk of transmission , especially if you or someone in your household has hepatitis B:
- Wash your hands with soap and water if exposed to blood.
- Avoid sharing razors or toothbrushes.
- Use condoms during sex.
- Cover all cuts carefully.
- Discard tampons and sanitary napkins into individual plastic bags.
- Avoid sharing needles, syringes, or other drug paraphernalia.
- Only used licensed tattoo or body piercing studios.
- Be sure that new, sterile needles are used for acupuncture.
Hepatitis B can be treated and prevented, but it cannot be cured. Research is underway to investigate different drugs and drug combinations that may one day offer cure rates similar to those seen with hepatitis C.
Until then, it is important to seek treatment if you are diagnosed with chronic hepatitis B. Doing so can slow the progression of the disease and reduce the risk of cirrhosis, liver failure, or liver cancer.
Hepatitis B vaccination is recommended for children and all people at risk of getting hepatitis B.
A Word From Verywell
Until scientists find a safe and effective cure for hepatitis B, you need to focus on protecting yourself and others from this potentially serious viral infection. Hepatitis B vaccination is central to this, offering protection of between 98% and 100%.
If you are unsure whether you've ever been vaccinated against hepatitis B, speak with your healthcare provider. If you're still unsure, consider undergoing the two- to three-dose series just to be safe, especially if you are at risk of infection.
The four approved hepatitis B vaccines are regarded as safe and effective. Side effects tend to be mild and may include headache, fever, and injection site soreness or redness.
One of the main reasons that there is a cure for hepatitis C but not hepatitis B is due to the structure of their DNA. Hepatitis B has a unique DNA structure, called covalently closed circular DNA (cccDNA) that is seemingly indestructible and able to generate new viruses even when exposed to antiviral therapy.
The course of chronic hepatitis B can vary from one person to the next. Some older studies suggest that an asymptomatic carrier has a near-normal life expectancy of around 72 years. As a group, however, chronic hepatitis B is associated with an average loss of 14 years compared to the general population.
In most cases it does. Studies suggest that up to 95% of people who are infected with hepatitis B will clear the virus spontaneously, usually within six months of exposure to it. Many will have no idea they were even affected.
It can be in some cases, but not always. Studies suggest between 10% and 20% of people with chronic hepatitis B will develop cirrhosis after 20 years, increasing to 40% after 30 years. A small percentage of these people will go on to develop liver cancer.
On the one hand, hepatitis B is more common and accounts for more cancer diagnoses and liver-related deaths worldwide than hepatitis C. On the other, hepatitis C is more likely to turn into a chronic infection, increasing the likelihood of cirrhosis and liver cancer from an individual perspective.
Ghany MG, Gara N. The quest for a cure for hepatitis C - the end is in sight . Lancet. 2014;384(9941):381–3. doi:10.1016/S0140-6736(14)60807-2
Department of Health and Human Services. Viral hepatitis in the United States: data and trends .
World Health Organization. Hepatitis B: key facts .
Alter HJ, Blumberg BS. Further studies on a "new" human isoprecipitin system (Australia antigen) . Blood. 1966;27(3):297-309. doi:10.1182/blood.V27.3.297.297 copy editor: retain, it is appropriate as the hallmark paper on the topic.
Tang J, Wu ZY, Dai RJ, Ma J, Gong GZ. Hepatitis B virus-persistent infection and innate immunity defect: cell-related or virus-related? World J Clin Cases. 2018;6(9):233–41. doi:10.12998/wjcc.v6.i9.233
Dushieko G. Towards the elimination and eradication of hepatitis B . J Virus Erad. 2015;1(1):4–12.
Xia H, Guo H. Hepatitis B virus cccDNA: formation, regulation and therapeutic potential . Antiviral Res . 2020;180:104824. doi:10.1016/j.antiviral.2020.104824
Yuen MF, Heo J, Jang JW, et al. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: a phase 2 randomized controlled trial . Nat Med. 2021:27:1725-34. doi:10.1038/s41591-021-01513-4
Beretta M, Mouquet H. Advances in human monoclonal antibody therapy for HBV infection . Curr Opinion Virol. 2022;53:10125. doi:10.1016/j.coviro.2022.101205
Revill PA, Penicaud C, Brechot C, Zoulim F. Meeting the challenge of eliminating chronic hepatitis B infection . Genes (Basel). 2019;10(4):260. doi:10.3390/genes10040260
Bazinet M, Pantea V, Placinta G, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naïve to nucleos(t)ide therapy . Gastroenterology . 2020;158(8):2180-94. doi:10.1053/j.gastro.2020.02.058
Feng SH, Gane E, Schwabe C, et al. A five-in-one first-in-human study to assess safety, tolerability, and pharmacokinetics of RO7049389, an inhibitor of hepatitis B virus capsid assembly, after single and multiple ascending doses in healthy participants . Antimicrob Agents Chemother . 2020;64(11):e01323-20. doi:10.1128/AAC.01323-20
Martinez MG, Villeret F, Testoni B, Zoulim F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int . 2020 Feb;40 Suppl 1:27-34. doi:10.1111/liv.14364
Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics . Cold Spring Harb Perspect Med. 2014;4(12):a024935. doi:10.1101/cshperspect.a024935
Chou HH, Chien WH, Wu LL, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota . Proc Natl Acad Sci U S A. 2015;112(7):2175–80. doi:10.1073/pnas.1424775112
Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer . Philos Trans R Soc Lond B Biol Sci . 2017;372(1732):20160274. doi:10.1098/rstb.2016.0274
Zamor PJ, deLemos AS, Russo MW. Viral hepatitis and hepatocellular carcinoma: etiology and management . J Gastrointest Oncol . 2017;8(2):229-42. doi:10.21037/jgo.2017.03.14
Wilkins T, Sams R, Carpenter M. Hepatitis B: screening, prevention, diagnosis, and treatment . Am Fam Physician. 2019;99(5):314-23.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance . Hepatology . 2018;67(4):1560-99. doi:10.1002/hep.29800
Wendon J, Cordoba J, Dhawan A, et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure . J Hepatol . 2017;66(5):1047-81. doi:10.1016/j.jhep.2016.12.003
Centers for Disease Control and Prevention. Liver Cancer .
Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations - United States, 2023 . MMWR Recomm Rep . 2023;72(1):1-25. Published 2023 Mar 10. doi:10.15585/mmwr.rr7201a1
Centers for Disease Control and Prevention. Hepatitis B questions and answers for health professionals .
Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices . MMWR Recomm Rep. 2018;67(No. RR-1):1–31. doi:10.15585/mmwr.rr6701a1
Minnesota State Department of Health. Recommended postexposure prophylaxis for percutaneous exposure to hepatitis B virus.
Canadian Centre for Occupational Health and Safety. Hepatitis B .
Department of Health and Human Services. Hepatitis B basic information .
Centers for Disease Control and Prevention. Hepatitis B vaccine: safety information .
Wang T. Model of life expectancy of chronic hepatitis B carriers in an endemic region . J Epidemiol. 2009;19(6):311–8. doi:10.2188/jea.JE20090039
Bixler D, Zhong Y, Ly KN, et al. Mortality Among Patients With Chronic Hepatitis B Infection: The Chronic Hepatitis Cohort Study (CHeCS) . Clin Infect Dis . 2019;68(6):956-963. doi:10.1093/cid/ciy598
Hepatitis B Foundation. What’s the difference: hepatitis B vs hepatitis C?
Saeed U, Waheed Y, Ashraf M. Hepatitis B and hepatitis C viruses: a review of viral genomes, viral induced host immune responses, genotypic distributions and worldwide epidemiology . Asian Pac J Trop Dis. 2014 Apr;4(2):88–96. doi:10.1016/S2222-1808(14)60322-4
By James Myhre & Dennis Sifris, MD Dennis Sifris, MD, is an HIV specialist and Medical Director of LifeSense Disease Management. James Myhre is an American journalist and HIV educator.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Call for Papers
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 1, Issue 2
- Opportunities and challenges for hepatitis B cure
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Armando Andres Roca Suarez 1 , 2 , 3 and
- http://orcid.org/0000-0002-2245-0083 Fabien Zoulim 1 , 2 , 3 , 4
- 1 INSERM U1052, CNRS UMR-5286, Cancer Research Center of Lyon (CRCL) , Lyon , France
- 2 University of Lyon, Université Claude-Bernard (UCBL) , Lyon , France
- 3 Hepatology Institute of Lyon , Lyon , France
- 4 Department of Hepatology, Croix Rousse hospital, Hospices Civils de Lyon , Lyon , France
- Correspondence to Professor Fabien Zoulim; fabien.zoulim{at}inserm.fr
In spite of the fact that safe and effective vaccines have been available for over 40 years, hepatitis B virus (HBV) remains a major public health problem, as there are 296 million chronically HBV-infected individuals worldwide and 820 000 HBV-related deaths taking place every year. Achieving the goal of HBV cure remains a challenge due to the particularities of the HBV cycle underlying viral persistence. The new understanding of HBV biology and antiviral immune responses has allowed to identify novel drug targets. This has led to a renewed interest in developing new curative strategies and combinations for HBV. In the present review, we aim to summarise the biological and clinical challenges associated with chronic HBV infection. Moreover, we consider the lessons that have been learnt in the past years regarding the preclinical and clinical evaluation of compounds against HBV and how this is driving the field to explore new directions.
- Hepatitis, Viral, Human
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/egastro-2023-100021
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
The development of antiviral treatments for hepatitis C virus (HCV) infection is one of the most remarkable stories in translational medicine. Since the discovery of HCV in 1989, 1 the scientific community was able to develop the necessary research and diagnostic tools that led to the design of antiviral molecules, nowadays allowing HCV elimination in more than 98% of cases. 2 Although HCV remains a global health burden, 3 the success of these therapies has shown that it is possible to surmount the challenges associated with virally induced liver diseases. In particular, there has been a renewed interest in developing curative therapies for hepatitis B virus (HBV), a pathogen discovered decades before HCV and for which safe and effective vaccines have been available for over 40 years. In spite of this head start, it is estimated that there are 296 million chronically HBV-infected individuals worldwide and 820 000 HBV-related deaths taking place every year. 4 To address the challenges of HBV cure, an important question is to identify the aspects that make HBV elimination such a unique and complex endeavour.
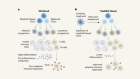
In the present review, we aim to summarise the clinical challenges that stem from the particular biology of HBV. Moreover, we consider the lessons that have been learnt in the past years regarding the preclinical and clinical evaluation of compounds against HBV and how this is driving the field to explore new directions ( figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Opportunities and challenges for hepatitis B cure. Particularities of the HBV cycle that favour the development of chronic infection (centre), the clinical challenges stemming from these biological characteristics (top) and the opportunities currently under development to achieve the goal of HBV cure (bottom). cccDNA, covalently closed circular DNA; 3D, three-dimensional; DCs, dendritic cells; FNAs, fine-needle aspirates; HBe, hepatitis B e; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; MPs, macrophages; NUCs, nucleos(t)ide analogues; PCLS, precision-cut liver slices; pegIFN-α, pegylated interferon alpha; PHH, primary human hepatocytes; WHV, woodchuck hepatitis virus.
Challenges for hepatitis B cure
The determinants of viral persistence, particularities of the hbv cycle.
One the most relevant characteristics of the HBV viral cycle in regard to its ability to favour chronic infection is the formation of covalently closed circular DNA (cccDNA), which serves as a viral reservoir and template for viral replication. 5 The HBV cccDNA is associated with cellular histones and non-histone proteins and organised into a chromatin-like structure, which regulates its transcription via epigenetic modifications. 5 6 The intrahepatic pool of this highly stable mini-chromosome is maintained via new rounds of infection and intracellular recycling. The dual source of cccDNA, in combination with its long half-life, results in highly stable concentrations that are minimally affected even after long-term antiviral treatment. 7 8 In addition, HBV DNA can persist in the form of integrated sequences within the host cell genome. Although integrated HBV sequences cannot sustain viral replication, they can generate hepatitis B surface antigen (HBsAg) and the transcriptional regulator HBx. 9 10 These integration events occur early during infection, with their number representing a driver for the development of hepatocellular carcinoma (HCC). 11
Genetic variability of HBV
HBV can be classified into 10 genotypes (A–J) based on an intergroup divergence of ≥8% in their nucleotide sequence. 12 HBV genotypes present particular geographical distributions and have been described to influence patient outcomes, such as hepatitis B e antigen (HBeAg) seroconversion, mutational patterns and response to therapy. 13 Moreover, the lack of polymerase activity during reverse transcription of the HBV pregenomic RNA (pgRNA) leads to genetic mutations that ultimately result in the rise of viral quasi-species in infected patients. These viral populations evolve according to their interplay with the host immune responses or antiviral treatment, potentially driving the emergence of HBV mutants able to escape them. 14 15
Impairment of immune responses during HBV infection
Considering the particularities of the liver immune microenvironment in which HBV infection is established and spread in the liver is equally relevant to understand its persistence. Indeed, the liver presents a wide variety of regulatory mechanisms that induce a bias towards immune unresponsiveness. 16 As immune cells from the blood slowly transit through the liver, the presence of fenestrations in the hepatic sinusoid allows the interaction between lymphocytes and liver resident cells. 17 It is believed that in context of HBV infection, this contact mediates the intrahepatic priming of T lymphocytes by non-professional antigen-presenting cells, such as hepatocytes. 18 The hepatic priming of HBV-specific CD8 + T cells, for example, results in their proliferation and activation, but these lymphocytes fail to differentiate into effector cells, thus contributing to the establishment of a persistent HBV infection. 19 In addition, the persistent exposure of T cells to HBV antigens leads to the establishment of a functionally exhausted phenotype, which is characterised by high expression levels of inhibitory molecules such as programmed cell death 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4. 20–22 As discussed in later sections, the functional restoration of exhausted T cell populations via targeting of these inhibitory checkpoints is an active research field.
The important role of B cell responses in controlling HBV can be exemplified by the use of B cell-targeting therapies (eg, rituximab), which results in HBV reactivation in patients with chronic hepatitis B (CHB) or in those with resolved infection. 23 In this regard, it has been shown that most patients with CHB do not present detectable levels of anti-HBsAg antibodies, with recent evidence suggesting that this stems from an impaired function of HBsAg-specific B cells and not a decrease in their number. 24 25 Hepatitis B core antigen (HBcAg)-specific B cells are present at much higher frequencies than HBsAg-specific B cells and are associated with elevated liver inflammation and viral replication. 26 27 A possible mechanism explaining these observations could be related to the high circulating levels of HBsAg as compared with HBcAg, resulting in the sequestration of available antibodies as immune complexes. 19
Barriers in the preclinical evaluation of novel therapies against HBV
Tumour-derived cell lines and primary human hepatocytes (PHHs) are some of the most common in vitro models to study HBV infection. In this context, cell lines overexpressing the HBV receptor sodium taurocholate cotransporting polypeptide (NTCP) represent a flexible system that is able to support the whole HBV cycle. 28 Nonetheless, these cells (eg, HepG2-NTCP) lack multiple cellular components implicated in the immune response, which hinders the evaluation of compounds targeting these signalling pathways. 29 PHHs present a non-transformed phenotype, in addition to several practical advantages that include a relatively simple isolation procedure and the possibility for cryopreservation. 30 However, PHHs cannot be expanded and progressively undergo dedifferentiation when in culture. 31 Moreover, PHHs as a model lack the polarity, zonation and presence of additional cell types that characterise the hepatic microenvironment. The latter point is of particular relevance for the preclinical evaluation of novel therapies against HBV, as an ideal model should allow characterisation of the HBV cycle and the interplay with immune cells, in order to evaluate direct-acting antivirals (DAAs) and host-targeting agents (HTAs).
Immunomodulatory agents can be evaluated in vivo using the woodchuck model in context of woodchuck hepatitis virus (WHV) infection. 32 However, this model differs from HBV infection in several aspects, including genomic divergence, particularities in the mechanisms regulating viral transcription, the course of liver disease with specific integration events and rapid HCC development, and the particular expression pattern of immune components between both woodchucks and humans. 33 Chimpanzees are the only non-human primate model for HBV infection and their use has been fundamental to study the host response against the virus, as well as the development and testing of prophylactic vaccines. 34 However, the HBV field has moved away from this model due to ethical concerns and its ban in many countries. 35 Therefore, current efforts are focused in the development of small animal models for HBV infection. For instance, human liver chimeric mice are based on the engraftment of human hepatocytes in immunodeficient animals. 36 Although this model is inadequate for the evaluation of HTAs, it recapitulates the HBV cycle in its entirety and is therefore useful for the evaluation of DAAs.
Clinical challenges associated with HBV infection
Current therapeutic agents for chb.
Although current therapies based on pegylated interferon alpha (pegIFN-α) and nucleos(t)ide analogues (NUCs) can suppress HBV replication and decrease the risk of complications such as cirrhosis and HCC, HBV is never fully eliminated. Thus, these regimens require indefinite treatment to prevent the virological relapse that usually occurs after treatment discontinuation. 37 Moreover, it is unrealistic to expect all patients to adhere to lifelong non-curative regimens, with a strong patient preference for finite therapy. This is of particular relevance in the case of IFN treatment, as its use is limited by an unfavourable tolerability profile. 38 Finally, the economic burden of long-term treatment and monitoring of these patients is an important issue to consider in highly endemic areas.
Phases of chronic HBV infection and patient heterogeneity
CHB is a highly heterogeneous disease with different clinical phases, stemming from the complex balance between viral replication and immune responses against it. This has led to a classification of chronic HBV infection that takes into account these factors and divides it into four phases: HBeAg-positive chronic HBV infection (HBeAg+, high HBV DNA and normal alanine aminotransferase (ALT)), HBeAg-positive chronic hepatitis B (HBeAg+, high HBV DNA and high ALT), HBeAg-negative chronic HBV infection (HBeAg−, low HBV DNA and normal ALT) and HBeAg-negative CHB (HBeAg− and fluctuating levels of HBV DNA and ALT). 13 These phases do not always progress in a linear manner and patients can go from one phase to another. Classification of patients according to HBV phase is an important predictor of long‐term outcome and a valuable mean to define treatment initiation and monitor treatment response. 39
Monitoring viral and immune parameters in the liver microenvironment
Taking into account the particularities of intrahepatic immune responses and the observation that serum markers do not seem to reflect cccDNA levels at certain disease phases (eg, HBeAg-negative chronic infection), 40 it is considered that intrahepatic cccDNA quantification will be essential in longitudinal studies aiming to evaluate new curative strategies. 41 In this context, core liver biopsy has remained the gold standard for liver histology analysis and CHB staging, as it allows the assessment of host and viral parameters associated with HBV infection. However, as both patients and clinicians favour less-invasive assessments whenever possible, developing relevant less-invasive or non-invasive methods to assess the liver reservoir of HBV is a high priority. Such procedures that could be repeated at short intervals, as in the case of longitudinal studies, would represent an asset to assist the clinical development of novel antiviral strategies.
Design of clinical trials
Considering the above-mentioned biological and clinical characteristics of HBV, the design of clinical trials is crucial in order to evaluate novel therapies for CHB. This is a challenging matter, as there needs to be a balance between selecting the best responders (eg, patients with viral suppression and low HBsAg levels) to provide proof of principle regarding efficacy, against the evaluation of potential therapies in more heterogeneous groups that represent the diversity of CHB in the real world. Moreover, multiple factors need to be considered, including HBV genotype, ethnic background and stage of liver disease.
As described in the subsequent sections, it is considered that achieving HBV cure with finite treatment regimens will require not only the development of novel agents, but also their use as combination therapies. The concept behind the combination of molecules with different mechanisms of action is to induce: suppression of HBV replication, decrease in viral antigen expression (eg, HBsAg) and activation of the immune response. 41 Defining the need and timing of immunomodulatory therapy is particularly difficult. Indeed, emerging virological and immunological markers to predict patients’ response and guide interventions are still at an exploratory stage.
Regarding clinical trial endpoints, the goal of new compounds against HBV would be to eliminate all traces of the virus. However, achieving a sterilising cure, with elimination of cccDNA and integrated DNA, seems to be a scenario beyond what can be attained with existing treatments in development. 42 Therefore, functional cure was suggested as a new goal and was defined as a sustained (>6 months) HBsAg loss with or without seroconversion to anti-HBsAg antibodies and undetectable HBeAg and HBV DNA after therapy. Unfortunately, very few of the drugs under evaluation have resulted in HBsAg loss at the end of therapy and even fewer have achieved a sustained response. Therefore, there is a need to define alternative clinical trial endpoints that could be useful for the evaluation of novel therapies and combinations against HBV.
Opportunities for hepatitis B cure
Novel therapeutic targets and combinations against hbv, inhibition of hbv entry.
Because de novo infection is a central step in the maintenance of the cccDNA pool and the persistence of HBV infection, targeting viral entry would be a sensible approach to halt progression of the viral cycle. Bulevirtide, a synthetic peptide containing 47 amino acids of the HBsAg pre-S1 domain, was developed to compete with NTCP and prevent virion uptake by hepatocytes. 43 Although the clinical evaluation of bulevirtide monotherapy was unsatisfactory regarding its effect to decrease HBsAg levels, 44 entry inhibition may still represent a useful approach in combination with agents targeting other steps of the HBV cycle. Indeed, blocking new rounds of infection could be an adequate strategy to favour clearance of HBV-infected hepatocytes harbouring cccDNA as a consequence of cell turnover. Bulevirtide is currently developed to treat coinfections with hepatitis D virus (HDV), a situation in which the underlying high turnover of viral infection has supported the clinical development of this antiviral agent. 44 45
Monoclonal antibodies against HBsAg are a potential strategy to neutralise viral particles and prevent HBV entry. Moreover, this approach could present the advantage to decrease circulating HBsAg levels, resulting in a reinvigoration of immune responses and enhanced viral clearance. VIR-3434 is a neutralising antibody against HBsAg, which has been Fc-engineered in order to extend its serum half-life and increase binding to activating Fc gamma receptors supporting a potential vaccinal effect. VIR-3434 has been reported to neutralise HBV infection in vitro and to decrease circulating HBsAg levels and HBV spread in vivo. 46 Regarding its clinical evaluation, preliminary results have reported that VIR-3434 is well tolerated, with the majority of patients presenting at least a 1 log IU/mL drop in HBsAg in the first week after treatment. 47 Early reports from a subsequent phase II study showed that combination of VIR-3434 with the small interfering RNA (siRNA) VIR-2218 achieved reductions in HBsAg of >2.5 log IU/mL. 48 Triple combination of VIR-3434, VIR-2218 and pegIFN-α is currently under phase II evaluation ( NCT04856085 ).
Capsid assembly modulators
Capsid assembly modulators (CAMs) are a class of molecules that interfere with the HBV cycle by favouring spontaneous capsid nucleation or accelerating their formation, which leads to the production of aberrant or empty capsids devoid of pgRNA. 49 Moreover, it has been proposed that this could have an effect on the formation of nascent cccDNA in de novo infected cells by preventing newly formed capsids from cycling back to the nucleus. While there was initial excitement for the use of CAMs, 50–52 discouraging results from later trials have dampened the enthusiasm for these agents. Preliminary results from an attempt to stop therapy in patients with no detectable serum markers of HBV replication after a year of treatment with an NUC/CAM (ie, vebicorvir) combination showed that this led to an immediate relapse. 53 Consistently, a large phase II trial showed limited effect on HBsAg and HBeAg levels. 54 This suggests that with the use of the currently available CAMs, the reservoir of transcriptionally active cccDNA had not been eliminated from the liver. In addition, liver toxicity with the use of some CAMs and reports of an apparent antagonism with siRNAs have left the future of CAMs uncertain. 55–57
It would be important to determine if new generation of CAMs can exert a direct effect on the cccDNA pool and if their use would require longer duration of treatment in order to achieve HBsAg loss.
Targeting HBV RNA
The compact nature of the HBV genome and its overlapping open reading frames offers the opportunity to target multiple HBV transcripts with individual siRNAs or antisense oligonucleotides (ASOs). These strategies could interfere not only with HBV replication (eg, targeting pgRNA), but also decrease HBsAg production in order to reinvigorate immune responses against HBV. Some of the compounds under evaluation include the siRNAs JNJ-3989, VIR-2218, AB-729 and RG6346, and the ASO bepirovirsen.
Although siRNA monotherapy or their combination with NUCs is associated with on-treatment HBsAg responses, HBsAg loss is rarely achieved. However, strategies combining pegIFN-α with siRNAs seem to have an additive effect on HBsAg levels. In this context, preliminary results from the combination of VIR-2218, an N-acetylgalactosamine (GalNAc)-conjugated siRNA, with pegIFN-α have shown that this led to HBsAg loss in 30.8% of patients with CHB under NUC therapy. Although the long-term durability of this approach still needs to be confirmed, combinations using these agents could be an important strategy for HBV cure. 58 59
In a phase II clinical trial, the ASO bepirovirsen has shown promising results as monotherapy or in combination with NUCs, reporting HBsAg loss in ~10% of patients by the end of 24-week follow-up. 60 It is worth noting that bepirovirsen is not GalNAc-conjugated, thus potentially internalised not only by hepatocytes, but also by non-parenchymal cells in the liver such as macrophages. Indeed, preliminary results suggesting the activation of Toll-like receptor 8 (TLR8) by bepirovirsen have been shown in transgenic mice expressing the human version of this receptor. 61
Inhibition of antigen secretion
Nucleic acid polymers (NAPs) are a class of compounds that block the release of subviral particles from HBV-infected hepatocytes. As in the case of agents targeting HBV expression, NAPs could present the advantage of decreasing circulating levels of HBsAg and thus potentially favour clearance of the virus by the immune system. Combination of NAPs (ie, REP 2139 or REP 2165) with pegIFN-α and tenofovir has been reported to achieve high rates of HBsAg seroconversion (50%) and HBsAg loss (35%) after 1 year of treatment-free follow-up. Interestingly, a significant number of patients presented ALT flares at the time of HBsAg decline. 62 Clinical evaluation of REP 2139 in context of HBV/HDV coinfection showed that its combination with pegIFN-α was well tolerated and associated with significant HBsAg declines and HDV RNA clearance, which was sustained up to 3 years off-treatment. 63 Although the results look promising, further mechanistic and clinical studies in larger patient cohorts will be required to assess the role of NAPs in future therapeutic combinations.

Innate immunity activators
Considering that natural HBsAg clearance is based on immune mechanisms, there has been a major focus on developing immunomodulatory approaches to achieve a cure of HBV. Besides the long use of IFN-α for the treatment of CHB, the clinical evaluation of agents targeting innate immune responses has included agonists of retinoic acid-inducible gene I (RIG-I), TLR7 and TLR8. The RIG-I agonist inarigivir was reported to inhibit HBV replication via induction of IFN-α in vitro. Despite an initial assessment concluding that inarigivir was well tolerated following 12 weeks of treatment, 64 a second longer clinical trial reported severe toxicity in several patients and the development of multiorgan failure and death in one individual. 65
Similarly, initial reports of the TLR7 agonist GS-9620 in the WHV and chimpanzee models were promising. 66–68 However, its clinical evaluation was disappointing, as no significant decreases in HBsAg were observed despite target engagement. 69 More recently, clinical evaluation of the TLR8 agonist selgantolimod in NUC-suppressed patients reported HBsAg loss in 5% of participants and HBeAg loss in 16% of them, with a mean HBsAg reduction of <1 log IU/mL. 70
Checkpoint inhibitors
Checkpoint inhibitors reinvigorate pre-existing antiviral immunity by preventing the action of cell components and pathways that limit immune responses. Considering that these factors are upregulated in HBV-specific T cells, checkpoint inhibitors could represent a sensible strategy to restore T cell responsiveness. 71 Preliminary results from the phase II clinical evaluation of ASC22 (envafolimab), a humanised anti-programmed death-ligand 1 antibody, in virally suppressed patients reported a mean HBsAg decrease of 0.38 log IU/mL. Moreover, 42.9% of patients with baseline HBsAg ≤100 IU/mL obtained a sustained HBsAg loss. 72
Therapeutic vaccines
Unlike checkpoint inhibitors, therapeutic vaccines prime new antiviral responses. This strategy mainly relies on the induction of effective CD4 + and CD8 + T cells and to a lesser extent on B cells and the production of antibodies. Although the use of therapeutic vaccines has shown disappointing results, 73 74 new approaches in their development and combination have sparked interest in this strategy once again.
The therapeutic vaccine VTP-300 was evaluated in NUC-suppressed patients with or without the PD-1 inhibitor nivolumab. Preliminary results from this evaluation showed that only 16.6% of patients treated with VTP-300 alone achieved HBsAg declines of 0.7–1.4 log IU/mL. Patients who received VTP-300 and low-dose nivolumab achieved HBsAg declines of 1.15 log IU/mL, which persisted 8 months after the final dose. 75
Encouraging preliminary results have been reported from the clinical evaluation of CHB targeted immunotherapy (CHB-TI), a strategy consisting of the administration of viral vectors in a heterologous prime boost regimen combined with adjuvanted recombinant HBc and HBs proteins. CHB-TI treatment in patients with CHB was associated with an increase of HBV-specific CD8 + T cells. 76 Similarly, an alternating immunisation strategy based on the combination of the arenavirus vectors GS-2829 and GS-6779 has shown promising preclinical results. Indeed, GS-2829/GS-6779 administration in cynomolgus macaques induced a strong polyfunctional CD8 T cell immunity, as well as an anti-HBsAg antibody response. 77
Finally, an interesting concept that has been explored in preclinical models consists of the combination of agents that reduce HBsAg expression (eg, siRNAs) with therapeutic vaccines. 78 By decreasing antigen levels before vaccination, T cells may be better able to respond to vaccine antigens. It is currently unclear for how long and to which extent HBV antigens would need to be reduced before T cells respond to a therapeutic vaccine. In addition, it remains to be determined if additional strategies will be necessary to revive the activity of exhausted T/B cells. In this context, CHB-TI is currently being evaluated in combination with bepirovirsen for patients with CHB under NUC treatment ( NCT05276297 ), similarly to the combination of VTP-300 with AB-729 (ACTRN12622000317796).
Directly targeting cccDNA
Although currently at the preclinical stages, strategies aimed to deplete the intrahepatic cccDNA pool remain a high priority, as this could drastically change the perspective for HBV cure. 79 Indeed, approaches to directly target cccDNA using clustered regularly interspaced short palindromic repeat/Cas9, base and new base editors that do not induce double-stranded DNA breaks have shown promising results in vitro and in animal models. 80–82 Further characterisation of their effect and safety profiles, as well as improvements in their delivery, could make these strategies a valuable tool for future combination therapies. More recently, it has been reported the discovery of a small molecule (ccc_R08) that is able to decrease the HBV cccDNA reservoir in multiple models. Although the exact mode of action of this compound remains to be determined, the analyses presented in this work suggest it to be most likely mediated by the modulation of host regulatory networks. 83 84
New preclinical models for the characterisation of therapies against CHB
Aimed to circumvent the limitations of classic in vitro approaches and more closely recapitulate the cellular context observed in vivo, advanced three-dimensional (3D) culture models have emerged as a viable alternative to study the liver. In this regard, 3D microfluidic PHH cultures have been reported to better recapitulate the liver microenvironment, as they present functional bile canaliculi and a complete cell polarisation. Moreover, this type of system allows HBV infection and the co-culture of PHHs with additional cell types, such as Kupffer cells (KCs). 85 In this model, activation of KCs with lipopolysaccharides was able to reduced HBsAg levels, suggesting that 3D microfluidic cultures could be a useful tool to characterise novel compounds targeting innate immune responses against HBV. Other in vitro models, such as liver organoids, have been reported to support the entire HBV cycle, which can be experimentally halted by the use of tenofovir or bulevirtide. 86 Considering that organoids can be expanded and biobanked, this could represent a practical approach for the screening of novel molecules against HBV. 87 Moreover, it has recently been shown that liver organoids can be adapted to liver-on-chip systems, exhibiting enhanced in vivo-like functions and potential utility for drug-induced liver injury (DILI) risk assessment. Using this model, tenofovir/inarigivir-associated hepatotoxicity was observed and correlated with the clinical manifestation of DILI reported in patients. 88 Finally, ex vivo models such as precision-cut liver slices (PCLS) have the advantage of retaining the complex multicellular architecture of the hepatic environment, while offering the practical aspects of an in vitro model. PCLS have been described to allow HBV infection, having the potential to be a valuable tool for the preclinical characterisation of novel HTAs against HBV. 89
Although there are ethical concerns associated with the use of animal models, the in vivo characterisation of therapeutic compounds against HBV is an approach that presents several advantages. Indeed, these models provide the ability not only to examine the hepatic microenvironment, but also the interorgan relations the liver shares. Moreover, there is the possibility to establish mice that present a humanised immune system and chimeric liver. These mice have been reported to support the HBV cycle and to develop an immune response against it, with NUC therapy decreasing HBV loads and restoring a naïve-immune phenotype. 90 91
Improvements in the design of clinical trials
Regardless of the approach employed, it is fundamental for future clinical trials to have a better understanding of the viral and immune changes taking place within the intrahepatic compartment. 92 In this context, fine-needle aspirates (FNAs) represent a promising alternative to liver biopsies that could allow sequential liver sampling during the natural history of the disease or antiviral therapy, while minimising risk and discomfort to patients. Indeed, characterisation of liver FNAs by single-cell technologies has already provided a great opportunity to dissect intrahepatic immune responses. 93 Moreover, quantification of cccDNA and 3.5 kb RNA in FNAs by droplet digital PCR has proved as reliable as in liver biopsies. 94 The use of FNAs has started to be applied in clinical trials, as in the B-Fine Study ( NCT04544956 ) or the IP-cure-B Study ( NCT05045261 ), which will include repeated FNA sampling in order to characterise the immune changes associated with bepirovirsen treatment or selgantolimod followed by NUC cessation, respectively. Moreover, efforts have been made to standardise cccDNA quantification in research laboratory assays. 95 These new data will support studies to better stratify patients and guide the use of therapeutic agents until new reliable biomarkers and non-invasive methods are available. 96 Nonetheless, promising candidates have emerged in recent years, including the quantification of hepatitis B core-related antigen and circulating HBV RNA to assess the cccDNA reservoir in a non-invasive manner. 97–100
Regarding clinical trial endpoints, it is important to consider that the best predictor of HBsAg loss is low HBsAg levels before treatment. Therefore, future trials should stratify patients according to baseline HBsAg levels. Patients with low HBsAg levels could also take part in clinical trials designed to evaluate monotherapy or short treatment using combination approaches. Finally, the use of stringent definitions of success during clinical evaluation (eg, functional cure) could have a negative impact in the development of new therapies, potentially leading to an early halt of molecules able to improve the clinical management of HBV infection. Therefore, alternative endpoints have been suggested such as partial HBV cure , defined as HBsAg positive at low levels, HBeAg negative and undetectable serum HBV DNA after discontinuation of a finite course of treatment. 42 Considering that partial HBV cure is rarely achieved following NUC discontinuation, this alternative endpoint could represent a useful milestone in the path towards functional cure.
Perspectives
The landscape of therapeutic options against HBV has expanded considerably in the past years as drug discovery efforts are progressing. Now, it will be necessary to refine our understanding of which combinations are more often associated with favourable outcomes, the appropriate timing for their use and the patient populations that could benefit the most from these interventions. In this context, therapeutic strategies targeting HBV transcripts (eg, siRNAs, ASOs) or the secretion of viral antigens (eg, NAPs) have reported the highest rates of HBsAg decline and HBsAg loss. Although the long-term durability of this effect remains to be established, the use of these molecules as a therapeutic backbone could be an important approach for HBV cure. Moreover, their combination with immunomodulatory agents could help to restore HBV-specific immune responses. Therefore, longitudinal studies aimed to characterise viral and immunological responses in the intrahepatic compartment could be useful not only to monitor patients under treatment, but also to gain highly valuable mechanistic insights and the identification of novel CHB biomarkers. Altogether these investigations should provide new insight in the path towards the best combination strategies that could include potent inhibition of viral replication and blockade of cccDNA turnover, decrease of viral antigen expression to modify the T and B cell exhausting environment, immune invigoration through innate immunity boosting or immune checkpoint inhibition, and specific stimulation (or replacement) of adaptive immunity. 9 41 Currently, the most promising approach under investigation is the use of ASO (ie, bepirovirsen) in combination with NUCs which has just entered phase III clinical trials ( NCT05630807 ). For instance, it will be interesting to see in bepirovirsen-treated patients if add-on therapies with other modes of actions or treatment stopping strategies will enhance the rate of HBsAg loss.
Although the path towards HBV cure has not been straightforward, the multiple molecules under clinical evaluation, the development of novel preclinical models and sampling techniques are important milestones that on the long term will contribute to address this unmet medical need.
- Weiner AJ , et al
- Terrault NA ,
- World Health Organization
- Martinez MG ,
- Combe E , et al
- Werle-Lapostolle B ,
- Locarnini S , et al
- Fung J , et al
- Fanning GC ,
- Hou J , et al
- Wooddell CI ,
- Chan H-Y , et al
- Imbeaud S ,
- La Bella T , et al
- Goldmann N ,
- Lauber C , et al
- European Association for the Study of the Liver. Electronic address: [email protected], European Association for the Study of the Liver
- Rajoriya N ,
- Zoulim F , et al
- Bhardwaj N ,
- Hedskog C , et al
- Vollmar B ,
- Iannacone M ,
- Guidotti LG
- Fisicaro P ,
- Valdatta C , et al
- Schurich A ,
- Lopes AR , et al
- Bertoletti A ,
- Liu Y , et al
- Salimzadeh L ,
- Le Bert N ,
- Dutertre C-A , et al
- Gill US , et al
- Vanwolleghem T ,
- Groothuismink ZMA ,
- Kreefft K , et al
- Xu G , et al
- Hengstler JG ,
- Steinberg P , et al
- Heslop JA ,
- Walsh J , et al
- Tennant BC ,
- Michalak TI
- Allweiss L ,
- Strick-Marchand H
- Anderson RT ,
- Mishra P , et al
- Dusheiko G , et al
- Meier M-A ,
- Ketterer S , et al
- Baumert TF ,
- Boni C , et al
- Cornberg M ,
- Terrault NA , et al
- Schulze A ,
- Schieck A ,
- Ni Y , et al
- Wedemeyer H ,
- Schöneweis K ,
- Bogomolov P , et al
- Degasperi E ,
- Anolli MP ,
- Uceda Renteria SC , et al
- Cameroni E , et al
- Agarwal K ,
- Wedemeyer H , et al
- Dobryanska M , et al
- Zlotnick A ,
- Buchholz S , et al
- Vandenbossche JJ , et al
- Gane EJ , et al
- Nguyen T , et al
- Sulkowski M ,
- Ma X , et al
- Janssen HLA ,
- Asselah T , et al
- Berliba E ,
- Sukeepaisarnjaroen W , et al
- Asselah T ,
- Jacobson IM , et al
- van Bömmel F , et al
- Kim JB , et al
- Plesniak R , et al
- Delahaye J ,
- Ermler M , et al
- Bazinet M ,
- Placinta G , et al
- Cebotarescu V , et al
- Liu C-J , et al
- Coffin C , et al
- Lanford RE ,
- Chavez D , et al
- Liu KH , et al
- Daffis S , et al
- Elkhashab M , et al
- Dunbar PR ,
- Brooks AE , et al
- Verdon DJ ,
- Xie Y , et al
- Gehring AJ ,
- Lang-Meli J ,
- Neumann-Haefelin C ,
- Swinnen K ,
- Romero-Gomez M , et al
- Schmidt S ,
- Mengistu M ,
- Michler T ,
- Kosinska AD ,
- Festag J , et al
- Smekalova E ,
- Kaldine H ,
- Cathomen T , et al
- Xu D , et al
- Steer CJ , et al
- Zhang JD , et al
- Ortega-Prieto AM ,
- Skelton JK ,
- Wai SN , et al
- De Crignis E ,
- Hossain T ,
- Romal S , et al
- O’Meara MJ , et al
- Doornebal EJ ,
- Billerbeck E ,
- Mommersteeg MC ,
- Shlomai A , et al
- Dusséaux M ,
- Masse-Ranson G ,
- Darche S , et al
- Boettler T ,
- Allweiss L , et al
- Nkongolo S ,
- Mahamed D ,
- Kuipery A , et al
- Testoni B ,
- Roca Suarez AA ,
- Battisti A , et al
- Yu M , et al
- Kramvis A ,
- Chang K-M ,
- Dandri M , et al
- Lebossé F ,
- Scholtes C , et al
- Scholtès C ,
- Hamilton AT ,
- Plissonnier M-L , et al
- Lisker-Melman M , et al
- Scholtes C ,
- Plissonnier ML
Twitter @andresroca
Contributors Both authors wrote the manuscript.
Funding This work is supported by the French National Research Agency Investissements d’Avenir Programme (CirB-RNA project–ANR-17-RHUS-0003) and by the European Union’s Horizon 2020 research and innovation programme under grant agreement number 847939 (IP-cure-B Project) to FZ and by the Agence Nationale pour la Recherche sur le SIDA et les hepatites virales (ANRS) to AARS (ECTZ206376) and FZ.
Competing interests FZ received grants from Assembly, Beam Therapeutics and Janssen; and had consulting activities with Assembly, Blue Jay, Gilead and GSK. FZ is an Associate Editor for eGastroenterology and was not involved in the peer-review process.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- News Archive
Optimizing treatment regimens for adults with chronic hepatitis B
In adults with chronic hepatitis B participating in NIDDK-funded Hepatitis B Research Network (HBRN) studies across North America, investigators tested whether a combination treatment regimen could increase long-term clearance of the virus. Chronic hepatitis B, a form of viral hepatitis, is a global problem that disproportionately affects people living in or originating from certain geographic areas, such as Asia and sub-Saharan Africa. If not appropriately treated, the disease can lead to cirrhosis, liver failure, and liver cancer. Effective treatments for chronic hepatitis B include interferon-based therapy, which targets immune cell function, and a class of drugs called nucleoside analogues that inhibit viral enzyme activity. However, these drugs’ effectiveness varies across individuals, in terms of reliably clearing the virus and ultimately preventing development of severe liver disease. In addition, these drugs often must be taken lifelong to prevent recurrence of disease; therefore, better treatments that clear the virus long-term are needed.
The NIDDK-funded HBRN conducted clinical trials of treatment approaches for chronic hepatitis B in a study population that was primarily men, women, and children of Asian descent. Though the Network studies concluded in 2022, data analysis and publication of results has continued, with study samples available for additional research through the NIDDK Central Repository. One HBRN clinical trial in adults, results of which were recently published, assessed the safety and efficacy of combining two treatments—a long-lasting form of interferon called peginterferon and the nucleoside analogue tenofovir—to increase the currently low or variable rates of viral clearance. Two hundred people with hepatitis B were treated, all of whom had active disease with high levels of viral DNA and elevations in serum liver enzymes, which indicate liver inflammation or disease. Half of the study participants’ samples contained the hepatitis B e antigen (HBeAg), a protein produced by the hepatitis B virus that signals an active infection. All study participants were treated with tenofovir for approximately 4 years; half also received peginterferon, but only for the first 6 months. After 4 years, those individuals who had received combination therapy had a higher rate of clearing the viral proteins and viral DNA, though nearly all study participants had an excellent clinical and biochemical response. The overall complete response with clearance of all hepatitis B proteins, however, was uncommon. Furthermore, almost all responses occurred in people with HBeAg and a single type of hepatitis B virus called genotype A2, found mostly in White and Black populations and rarely among those of Asian ancestry. At the 4-year point, study participants were eligible to continue or to stop tenofovir therapy, based on withdrawal of therapy being one approach to increasing the rate of complete viral clearance. One year after withdrawal of tenofovir therapy, slightly more of the participants who stopped treatment had complete clearance than those who continued therapy. Furthermore, a proportion of the study participants who elected to withdraw from further tenofovir therapy had a severe flare of hepatitis and had to be restarted on treatment.
These results indicate that the addition of peginterferon to tenofovir therapy for hepatitis B leads to an increased rate of response, but only in people with the viral protein HBeAg. Withdrawal of therapy after 4 years did not seem to increase the rate of complete response and could be followed by worsening of the hepatitis requiring restarting of therapy. Future studies will continue to build on these findings to develop more effective, individualized approaches to treating people with hepatitis B.
Terrault NA, Lok AS, Wahed AS,…Janssen HLA for the Hepatitis B Research Network. Randomized trial of tenofovir with or without peginterferon alfa followed by protocolized treatment withdrawal in adults with chronic hepatitis B . Am J Gastroenterol doi: 10.14309, 2022.
Our team assists with new WHO guidelines for treatment of hepatitis B -- read more here

News & Events
- In the news: Coverage of our programs and people
- Journal Articles on Hepatitis B Research, Vaccination and Public Education
- Emerging Scholars Scientific and Medical Advisors
- Journal articles recommended by our Emerging Scholars Scientific and Medical Advisors
- WHO announces new guidelines for hepatitis B treatment
- People living with hepatitis B should have a voice in new treatment guidelines, advocates say
- Hepatitis B Foundation launches training website with user-friendly courses for everyone interested in hepatitis B and D
- Pennsylvania couple to receive Hepatitis B Foundation’s Community Leadership Award
- Hepatitis B Foundation, Blumberg Institute and PABC congratulates John Crowley of Amicus on being named BIO President and CEO
- Hepatitis B Foundation releases report on first-ever Externally Led Patient-Focused Drug Development meeting for hepatitis B
- Hepatitis B Foundation extends its engagement in Africa
- Sen. Steven J. Santarsiero honored at the Pennsylvania Biotechnology Center (PABC)
- NIH scientist conducting influential hepatitis research to receive 2024 Baruch S. Blumberg Prize
- Hepatitis B Foundation invites providers and the public to participate in The Liver Meeting, Nov. 10-14, in Boston and online
- Hepatitis B Foundation coordinates 2023 International HBV Meeting, a scientific conference held Sept. 19-23 in Kobe, Japan
- Hep B United Commemorates World Hepatitis Day, July 28
- Gala puts the spotlight on creating family and support for those living with hepatitis B
- Global leader, physician treating people living with hepatitis B takes new role with Hepatitis B Foundation
- Hepatitis B Foundation releases white paper calling health care providers into action following new hepatitis B screening and vaccination recommendations
- New CDC Universal Screening Recommendations will save lives, Hepatitis B Foundation president says
- Hepatitis B Foundation invites everyone to participate in the online silent auction fundraiser.
- Hepatitis B Foundation president responds to Janssen decision on the company’s hepatitis B drug development program
- Globally prominent advocate and physician chosen for Hepatitis B Foundation’s 2023 Community Commitment Award
- Dr. Yasmin Ibrahim appointed to national Patient Engagement Collaborative
- Comprehensive hepatitis B program in Vietnam provides an excellent model for other countries
- Hepatitis B Foundation Announces third series of continuing education program on hepatitis B for health care providers and public health professionals
- Hepatitis B Foundation hails decision by U.S. Public Health Service Corps to accept future applicants living with chronic hepatitis B infection and HIV
- German scientist, inventor of new, first-in-class treatment for hepatitis D, to receive the 2023 Baruch S. Blumberg Prize
- Researchers and people living with hepatitis B meet in Paris at the third Hepatitis B Community Forum
- Hepatitis B Foundation mourns Bill Mason, an accomplished scientist whose discovery led to current treatments for hepatitis B
- Hepatitis B Foundation raises alarm about findings from new federal viral hepatitis surveillance report
- Hepatitis B Foundation strongly supports Congressional letters urging Biden administration to end discriminatory military policy
- Longtime Board Chair honored by his colleagues
- Hepatitis B Foundation creates two Global Community Advisory Boards
- Pa DOH Highlights the Importance of Viral Hepatitis Awareness, Need for Expansion of Syringe Services
- Hepatitis B Foundation, StoryCenter release new #justB stories from people with lived experience
- Pediatric Hepatitis Outbreaks
- Philadelphia City Council recognizes May as Hepatitis Awareness Month
- Hepatitis B Foundation hosts Princeton Workshop on Liver Cancer
- Annual Gala raises a record amount for the local Hepatitis B Foundation
- Hepatitis D Roundtable to Address Unmet Needs of Patients
- DiRx teams up with Hepatitis B Foundation to offer low-cost medications
- Many more U.S. adults to get vaccinated against hepatitis B following move by U.S. Centers for Disease Control and Prevention (CDC)
- Hepatitis B Foundation receives Congressional funding for a Center of Public Health Excellence
- CTC Foundation of Princeton donating $100,000 to Hepatitis B Foundation
- Hepatitis B Foundation senior vice president and board member speak on popular podcast
- Anonymous donor provides record gift for hepatitis B research
- Two hepatitis B medications available free through Hepatitis B Foundation and Rx Outreach partnership
- Canadian scientist chosen for the 2022 Hepatitis B Foundation’s Blumberg Prize
- Landmark vote by CDC’s Advisory Committee on Immunization Practices (ACIP) to recommend universal hepatitis B vaccination
- Hepatitis B Foundation strongly endorses the Liver Illness Visibility, Education and Research (LIVER) Act of 2021
- Dr. Chari A. Cohen becomes President of the Hepatitis B Foundation
- New president announced for the Pennsylvania Biotechnology Center (PABC)
- Major successes on Capitol Hill
- CDC awards a $1.375 million, five-year grant to the Hepatitis B Foundation for expansion of Hep B United, a nationwide coalition
- Hepatitis B Foundation Members Selected to Participate as Consumer Reviewers in the Congressionally Directed Medical Research Program’s Peer Reviewed Medical Research Program for the U.S. Department of Defense
- Hepatitis B Foundation supports launch of new global online forum dedicated to supporting people with hepatitis B, connecting with health experts
- Hepatitis B Foundation launches the first global registry of discrimination against people living with hepatitis B
- Hepatitis B Foundation to hold its annual Crystal Ball Gala on April 30
- Hepatitis B Foundation mourns the passing of John C. Martin, pharmaceutical industry leader
- Chronic hepatitis B is far more prevalent among U.S. residents than previously reported
- All of Us research program
- Hepatitis B Foundation Mourns Loss of Co-Founder Paul Witte, Longtime New Hope Resident
- Hepatitis B Foundation launches continuing education series on hepatitis B for health care providers and public health professionals
- HBF applauds President Biden’s Memorandum denouncing racism, xenophobia, and intolerance against Asian Americans and Pacific Islanders
- Hepatitis B Foundation applauds release of National Hepatitis Strategic Plan
- Bristol Myers Squibb awards grant to Hepatitis B Foundation
- Federal Task Force Recommendation for Hepatitis B Screening Fails to Close Gaps in Diagnosis Rates
- Hepatitis B community leaders convene to address eliminating hepatitis B during COVID-19 pandemic
- Pennsylvania Biotechnology Center’s Kassa named one of The 10 Best COOs of 2020
- Hepatitis B Foundation announces recipient of 2021 Baruch S. Blumberg Prize
- Hepatitis B Foundation co-founders chosen for major new award from the American Association for the Study of Liver Diseases
- Hepatitis B Foundation applauds HHS letter on discrimination against people living with hepatitis B who are pursuing careers in health care
- Hepatitis B Foundation launches new tool to assist people living with hepatitis B in making decisions on health insurance
- Nobel Prize to Hepatitis B Foundation and Blumberg Institute Advisors
- Work begins on a $19 million expansion of the Pennsylvania Biotechnology Center (PABC)
- Hepatitis B Foundation launches B the Voice Story Bank
- Hepatitis B Foundation expresses appreciation for the work of Dr. Ding-Shinn Chen with the announcement of his death
- Hepatitis B Foundation and Hep B United Statement on the Federal Government's Rollback of Critical Health Care Protections
- Hepatitis B Foundation stands in solidarity with black communities, calls for action against institutional racism
- Two Powerful Editorials Published by the Hepatitis B Foundation
- Hepatitis B Foundation Expands Hepatitis B Prevention Policy Initiatives
- HBV Vaccinations Save Lives, Reduce New Infections: National Adult Hepatitis B Vaccination Awareness Day
- Hepatitis B Foundation Hepatitis B Foundation Says “Thank You!” to its 100- Volunteers
- Hepatitis B Foundation Announces Annual Fundraising Event to Go Virtual on April 24
- Hepatitis B Foundation, Baruch S. Blumberg Institute and Pennsylvania Biotechnology Center Announce New Director of Communications and Marketing
- Message from Dr. Timothy Block, Hepatitis B Foundation President
- Hepatitis B Foundation Commends New Rx Outreach Program to Provide Access to Affordable Hepatitis B Medication
- Hepatitis B Foundation Endorses the Liver Illness Visibility, Education, and Research (LIVER) Act of 2019
- Hepatitis B Foundation Announces 2020 Baruch S. Blumberg Prize Winner
- U.S. Falls Short in Reaching 2020 Goals for Hepatitis B
- Hepatitis B Foundation Calls for Increased Resources for Hepatitis B Prevention in Response to CDC 2017 Surveillance Data Report
- Our Voices Made a Difference: CVS Caremark to Cover Vemlidy Prescriptions
- Hepatitis B Foundation Announces 2019 Baruch S. Blumberg Prize Winner
- Hep B United Applauds Bipartisan Legislation to Combat the Opioid Crisis and Opioid Related Infectious Diseases
- Hepatitis B Foundation Endorses the Liver Illness Visibility, Education, and Research (LIVER) Act of 2018
- Hepatitis B Foundation Calls for Universal Screening for Hepatitis B
- Hepatitis B Leaders Call for the Elimination of Hepatitis B
- Hepatitis B Foundation Joins Forces with Grace Meng
- Hepatitis B Foundation Releases New #justB Stories
- Hepatitis B Foundation Strongly Supports the Strategic Plan for Trans‐NIH Research to Cure Hepatitis B
- Hepatitis B Foundation Crystal Ball Gala
- New Two-Dose HBV Vaccine Recommended by ACIP
- Timothy M. Block, PhD, President of Hepatitis B Foundation and its Baruch S. Blumberg Institute, named a 2017 National Academy of Inventors Fellow
- Hepatitis B Foundation Applauds FDA Approval of New Hepatitis B Vaccine
- CDC National Progress Report on Hepatitis Elimination Reveals Rise in Acute HBV Infections and Low Birth Dose Vaccination Rates in the U.S.
- Hepatitis B Foundation Announces Promotion of Chari Cohen, DrPH, MPH, to Vice President, Public Health and Programs
- Hepatitis B Foundation Mourns the Loss of Pioneering Hepatitis B Physician-Scientist Dr. W. Thomas London
- Hepatitis B Foundation's #justB Campaign Gives Voice to Personal Stories During May Hepatitis Awareness Month
- Executive Director Retires After 25 Years of Service
- Hepatitis B Foundation Bets on a Cure at the 2017 Crystal Ball
- Targets to Eliminate Hepatitis B in U.S.
- Fred Beans Family of Dealerships Donates $30,000..
- International Leaders to Its Scientific and Medical Advisory Board
- Hepatitis B Foundation Opposes the American Health Care Act
- Appoints Global Expert Dr. Nat Brown
- Hepatitis B Foundation Launches #justB Storytelling Campaign
- Dr. Richard G. Pestell Joins the Baruch S. Blumberg Institute
- Dr. Bud Tennant Leaves Behind a Distinguished Scientific Legacy
- Be About It
- Nationwide Hepatitis Delta Virus (HDV) Campaign
- Current and Past "B Informed" Newsletters
Hepatitis B: Is a Cure Possible?
- Calendar of Events
- Witte Lecture
- International HBV Meeting
- Externally Led Patient-Focused Drug Development Meeting
- A statement regarding COVID-19 from the Hepatitis B Foundation Scientific and Medical Advisory Board (SMAB) to the hepatitis B community
- Why World Hepatitis Day is July 28
- Princeton Workshop 2022
- Commentary on the Cure
- Pajama Gala April 24, 2020
- NYC Marathon
With the momentum growing around hepatitis B drug discovery research, we are closer than ever to a cure.
From the Spring 2016 B Informed Newsletter
With the momentum growing around hepatitis B drug discovery research, how far are we from a cure?
Closer than ever, according to Timothy Block, PhD, president and co-founder of the Hepatitis B Foundation and its research arm, the Baruch S. Blumberg Institute. He points out that hepatitis C, initially thought to be incurable, can now be cured with new combination treatments.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceuticals (Basel)

Recent Advances in Hepatitis B Treatment
Georgia-myrto prifti.
1 Department of Pharmacy, Division of Pharmaceutical Chemistry, School of Health Sciences, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece; moc.liamg@itfirpotrym (G.-M.P.); moc.liamg@mijsonaiom (D.M.); rg.aou.mrahp@naigve (E.G.); rg.aou.mrahp@drapikilisav (V.P.)
Dimitrios Moianos
Erofili giannakopoulou, vasiliki pardali, john e. tavis.
2 Molecular Microbiology and Immunology, Saint Louis University, Saint Louis, MO 63104, USA; [email protected]
Grigoris Zoidis
Associated data.
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Hepatitis B virus infection affects over 250 million chronic carriers, causing more than 800,000 deaths annually, although a safe and effective vaccine is available. Currently used antiviral agents, pegylated interferon and nucleos(t)ide analogues, have major drawbacks and fail to completely eradicate the virus from infected cells. Thus, achieving a “functional cure” of the infection remains a real challenge. Recent findings concerning the viral replication cycle have led to development of novel therapeutic approaches including viral entry inhibitors, epigenetic control of cccDNA, immune modulators, RNA interference techniques, ribonuclease H inhibitors, and capsid assembly modulators. Promising preclinical results have been obtained, and the leading molecules under development have entered clinical evaluation. This review summarizes the key steps of the HBV life cycle, examines the currently approved anti-HBV drugs, and analyzes novel HBV treatment regimens.
1. Introduction
Hepatitis B is a liver disease caused by the Hepatitis B Virus (HBV). HBV belongs to the Hepadnaviridae family and is classified into ten genotypes (A to J) [ 1 ]. It is transmitted by exposure to infectious blood or other body fluids (e.g., semen, vaginal secretions—sexual intercourse) as well as perinatally from infected mothers to infants [ 2 ]. The acute phase of the infection can be either symptomatic or asymptomatic. Acute infections can either spontaneously resolve or proceed to chronic infections. Chronic HBV infection is among the leading causes of hepatic cirrhosis and is the single largest cause of hepatocellular carcinoma (HCC). According to the World Health Organization (WHO), over 250 million people are chronically infected, and HBV caused 887,000 deaths in 2015 [ 3 ]. The highest epidemic prevalence is present in SE Asian, African, and Western Pacific countries [ 4 ].
The hepatitis B surface antigen (HBsAg), originally known as “Australia antigen” (AusAg), was firstly identified in the serum of indigenous Australians by Baruch Samuel Blumberg in 1965 [ 5 ]. This antigen was later related with viral hepatitis [ 6 ].
The goal of the current therapeutic development is a “functional cure” defined as sustained undetectable levels of HBsAg and HBV DNA in serum, with or without seroconversion to hepatitis B surface antibodies (anti-HBs) after the end of the treatment [ 7 ]. This reduction has been associated with an improved clinical condition and significantly decreased the chance of infection rebound. Other important HBV biomarkers include serum HBV DNA, hepatitis B core antigen (HBcAg), and its antibody anti-HBc, hepatitis B e antigen (HBeAg), and anti-HBe antibody [ 8 , 9 , 10 ]. HBeAg is a secreted variant of HBcAg, and viral infections are classified either as HbeAg-positive or HbeAg-negative, with HBeAg-positive patients having higher viral titers and a more frequent and rapid disease progression [ 11 ]. These biomarkers are used to guide treatment decisions following guidelines established by the major hepatology medical societies [ 12 , 13 , 14 ].
Despite the existence of a safe and effective vaccine, no therapeutic regimen that routinely induces a “functional cure” for chronic HBV has been identified yet. This review summarizes the HBV replication cycle, the existing treatment options and their significant disadvantages, and novel therapeutic approaches that are currently the subject of extensive scientific research, with the ultimate goal of achieving a “functional cure” of the disease.
2. HBV Replication Cycle
2.1. virion structure and genome.
HBV particles, also known as Dane particles ( Figure 1 A), were firstly identified by Dane and colleagues in 1970 [ 15 ]. Their shape is spherical, with a diameter of ∼42 nm. They consist of an outer envelope, which is a host-derived lipid bilayer containing three different-sized HBV surface antigens (HBsAg or HBs)—large (L-HBs), middle (M-HBs) and small (S-HBs)—surrounding the viral nucleocapsid. The nucleocapsid (∼27 nm diameter) is icosahedral and comprises the HBV core protein (HBcAg), as well as the viral DNA genome and the viral DNA polymerase (P) [ 16 , 17 ]. The virus also secretes a wide range of defective particles ( Figure 1 B), including enveloped nucleocapsids that are empty or contain defective immature genomes and subviral lipid particles containing the viral surface antigens. The subviral particles are secreted along with the infectious virions at levels that are thousands of times higher, and they play an important role in suppressing antibody responses to the virus [ 18 ].

Hepatitis B Virus particles. ( A ) Infectious HBV virion (Dane particle). The lipid envelope, bearing three types of surface proteins—small (S-HBs), middle (M-HBs) and large (L-HBs)—surrounds the nucleocapsid, consisting of HBV relaxed circular DNA (rcDNA), the viral DNA polymerase (P), and the core protein (HBcAg). ( B ) Non-infectious HBV particles; enveloped nucleocapsids containing immature or defective DNA/RNA, subviral particles, and naked nucleocapsids.
The HBV genome is a 3.2 kb circular, partially double-stranded DNA (relaxed circular DNA; rcDNA). The negative-sense, non-coding (−) DNA strand is complete and complementary to the mRNA transcripts, whereas the positive (+) DNA strand is incomplete and has a fixed 5′-end and a variable-size 3′-end [ 19 , 20 , 21 ]. The former contains four overlapping open reading frames (ORFs)—C, P, S, and X ( Figure 2 ). These are transcribed into five RNA transcripts of varying lengths and are subsequently translated into seven functional proteins. HBcAg is produced from ORF-C, HBeAg is produced from ORF preC + C, DNA polymerase from ORF P, and HBV X protein (HBx) from ORF X. The ORF S, because of its multiple in-frame start codons, encodes the L-HBs, M-HBs, and S-HBs envelope proteins (pre-S1 + pre-S2 + S, pre-S2 + S, or S, respectively) [ 17 , 19 ]. This compact nature of the HBV genome results in approximately two thirds of nucleotides encoding more than one functional element [ 22 , 23 ]. The overlap of more than 1000 nucleotides between the P and S genes is the largest gene overlap of any known animal virus [ 24 ].

Hepatitis B Virus genome. Partially double-stranded, relaxed circular DNA (rcDNA) with four overlapping open reading frames (ORFs).
2.2. Viral Entry
The HBV virion binds to the heparan sulfate proteoglycans (HSPGs) cell-surface receptors, via low-affinity and non-specific interactions. Afterwards, the Na(+)-taurocholate co-transporting polypeptide (NTCP) functions as a high affinity receptor for the recognition and attachment of the pre-S1 domain of L-HBsAg. NTCP is a liver-specific peptide that mediates the uptake of bile salts into hepatocytes, and it is also an entry receptor for Hepatitis D virus (HDV) [ 17 , 25 ]. Interactions between HBV and NTCP are responsible for the viral endocytosis ( Figure 3 ). According to recent studies, a complex formed between NTCP and the epidermal growth factor receptor (EGFR) contributes to the HBV entry [ 26 ]. Due to its complicated structure, NTCP can be oligomerized, and this process seems to affect the viral internalization into the cell. Following entry, the nucleocapsid is released into the cytoplasm, followed by uncoating, and then the rcDNA is transported to the nucleus via the nuclear pore complexes [ 17 , 19 , 27 ].

Main features of the hepatitis B virus replication cycle and potential therapeutic targets. (1) HBV entry inhibitors. Lipopeptides mimicking the pre-S1 region of HBV, monoclonal antibodies, and other small molecules under evaluation. (2) Targeting cccDNA. Damage and destruction of cccDNA via sequence-specific nucleases. Direct targeting of the HBx protein. (3) RNA interference. Small interfering RNAs (siRNAs), antisense oligonucleotides (ASOs). (4) HBV polymerase inhibitors. Reverse transcriptase inhibitors (nucleos(t)ide analogues) are part of the current treatment. RNaseH inhibitors are in preclinical evaluation. (5) Nucleocapsid assembly inhibitors or modulators can affect HBV capsid formation, reverse transcription, and pgRNA encapsidation. NTCP; Na(+) taurocholate co-transporting polypeptide, HSPG; heparan sulfate proteoglycan, rc-DNA; relaxed circular DNA, PF-rcDNA; protein-free rcDNA, cccDNA; covalently closed circular DNA, pgRNA; pregenomic RNA, preC; precore, mRNA; messenger RNA, P; polymerase, L-HBs; large hepatitis B surface protein, M-HBs; middle hepatitis B surface protein, S-HBs; small hepatitis B surface protein, HBx; hepatitis B X protein, HBsAg; hepatitis B surface antigen, HBeAg; hepatitis B e antigen, dslDNA; double-stranded linear DNA.
2.3. cccDNA Formation/Maintenance
Multiple cellular factors repair the HBV rcDNA to form the episomal covalently closed circular DNA (cccDNA) that is located in the nucleus ( Figure 3 ). Both the viral P protein which is bound to the 5′-end of the minus-polarity DNA strand [ 28 ] and the RNA primer attached to the 5′-end of the plus DNA strand are removed to leave a protein-free rcDNA (PF-rcDNA). The gaps in both strands are filled and circularized to form the cccDNA. Cellular factors believed to be involved in this process include the DNA repair enzyme tyrosyl-DNA phosphodiesterase 2 (TDP2) by presumably breaking the phosphodiesterase bond between the HBV P and rcDNA [ 29 , 30 ]. Another enzyme that breaks down the RNA primer at the 5′-end of the minus strand is the flap endonuclease 1 (FEN1) [ 31 ]. After removal of the proteins, the fill-in of the strands, DNA ligation, and DNA repair are conducted by other host enzymes such as DNA polymerases (κ and α), DNA ligases (LIG1 and LIG3), and topoisomerases I and II (TOP1 and TOP2) [ 17 ]. However, there is some functional redundancy among these factors, and it is not fully clear which of them function in an infected liver. The cccDNA is the template for transcription of viral RNAs. The stability of cccDNA is regulated by several cellular factors, such as the APOBEC3 protein family, that triggers cccDNA degradation [ 32 , 33 ]. The cccDNA is rather stable during antiviral therapy, declining by only ~1 log 10 after more than a year of nucleos(t)ide analogue therapy [ 34 ]. However, the half-life of cccDNA has been measured at only approximately 40 days in HepG2 cells [ 17 , 35 ], and studies of cccDNA replacement during the reversion of nucleoside analogue resistance following the cessation of the therapy indicate that the cccDNA half-life in the liver is 16–28 weeks [ 36 ].
2.4. Transcription-Translation-Reverse Transcription-Nucleocapsid Assembly
Using the cccDNA as a template, the host RNA polymerase II transcribes five RNAs of different lengths: three subgenomic mRNAs of 0.7, 2.1, and 2.4 kb and two longer than genomic mRNAs of 3.5 kb ( Figure 3 ). All of them are heterogenous, positively orientated, and have a 5′-cap and a 3′-polyadenylated tail [ 21 ]. The 3.5 kb pregenomic RNA (pgRNA) has two functions: it is the template for reverse transcription to generate the minus DNA strand and also the mRNA for the translation of the core protein and HBV P. The preC mRNA is slightly longer than the pgRNA. It contains an open reading frame that starts about 90 nucleotides upstream of the HBc ORF on the 3.5 kb pre-C mRNA and encodes the precore protein, which is converted to HBeAg upon post-translational processing in the endoplasmic reticulum (ER). The 2.4 kb mRNA encodes the L-HBs, whilst both M-HBs and S-HBs are encoded by the 2.1 kb transcript. The shortest transcript encodes the HBx protein [ 37 , 38 , 39 ]. The transcription process is regulated by four promoters (precore/core, pre-S1, pre-S2, and X) and two enhancers (Enh1 and Enh2), as well as several cis-acting negative regulatory elements [ 19 , 40 , 41 , 42 , 43 ].
HBx is the only purely regulatory protein encoded by HBV and has a multifunctional role. HBx promotes the degradation of the Smc5/6 complex (structural maintenance of chromosomes) host factor, thus enhancing the transcription of cccDNA [ 44 , 45 , 46 , 47 ]. Moreover, HBx represses development of the immune response to HBV infection, protecting the infected hepatocytes from immune-mediated apoptosis, and interferes with the host gene expression, facilitating the development of HCC [ 48 , 49 , 50 ].
HBV P contains four domains: the terminal protein (TP), the spacer, the reverse transcriptase (RT) domain, and the ribonuclease H (RNaseH) domain [ 51 , 52 ]. The pgRNA binds to P via the ε -stem loop located close to its 5′-end with specific motifs in the TP, spacer, RT, and RNaseH domains to form a pgRNA-P ribonucleoprotein (RNP) complex [ 53 , 54 , 55 , 56 , 57 ]. This interaction is of great importance, as it is essential for the RNA packaging into nucleocapsids and initiation of reverse transcription [ 58 , 59 ]. Specifically, the RNP complex is packaged within HBcAg to form immature nucleocapsids, where reverse transcription occurs, producing either rcDNA, or less often, double-stranded linear DNA (dslDNA) forms ( Figure 3 ).
HBV replicates by reverse transcription. The RT activity of the P protein primes DNA synthesis using a tyrosine in the TP domain, covalently linking the enzyme to the product DNA. P then catalyzes the synthesis of the (−) DNA strand, which is the pattern for the (+) DNA strand synthesis, to form double-stranded rcDNA and mature DNA nucleocapsids [ 19 , 60 ]. During the (−) DNA strand synthesis, the RNaseH domain degrades the pgRNA template inside the capsids after it is copied into the minus-polarity DNA [ 61 , 62 ]. Either the newly formed mature nucleocapsids are surrounded by HBsAg and secreted non-cytolytically as virions that can infect new hepatocytes or they can re-enter the nucleus to maintain the cccDNA reservoir ( Figure 3 ). This intracellular cccDNA recycling is likely one factor that makes the complete elimination of the HBV infection in a patient so difficult. Smaller, non-infectious subviral particles (∼22 nm in diameter) are also released from the hepatocytes in vast excess over infectious virions [ 17 ]. These include empty envelopes of HBsAg (subviral particles), virions containing RNA, or a defective DNA genome, as well as naked nucleocapsids (lacking envelope) ( Figure 1 ) [ 63 ]. These defective particles do not participate in viral replication, although the subviral HBsAg particles help suppress antiviral immunity [ 18 ].
The dslDNA is an aberrant reverse transcription product that is able to integrate into the cellular genome early after the initial HBV infection, and it has been associated with promoting the development of HCC. The integrated HBV DNA does not replicate, but it contributes to HBsAg expression, which contributes to HBV pathogenesis and modulates the immune response [ 64 ].
3. Current Therapies
Two types of treatment are currently available against hepatitis B viral infection, interferon α derivatives (IFNs), and nucleos(t)ide analogues (NAs).
Interferon α (IFN-α) was first approved for the treatment of HBV infection in 1991 [ 65 ]. However, the addition of a polyethylene glycol chain to IFN-α led to significantly improved pharmacological properties. Thus, IFN-α was replaced by its pegylated counterpart, PEG-IFN-α, in 2005. There are two forms of PEG-IFN-α available today, PEG-IFN-α2a (Pegasys © , Roche) and PEG-IFN-α2b (Pegintron © , Merck). They have improved pharmacokinetics and allowed for a longer half-life, enabling a weekly administration [ 66 ]. PEG-IFN-α is administered subcutaneously and has direct antiviral as well as immunomodulatory activity [ 67 , 68 , 69 , 70 ]. One year of PEG-IFN-α treatment in HbeAg-positive patients led to HbeAg seroconversion in 29–32% of the patients and sustainable reduced HbsAg levels in 3–7% of the patients, 24 weeks after the end of the treatment, highlighting the effectiveness of PEG-IFN-α against HBV [ 2 , 71 ]. Nevertheless, the PEG-IFN-α treatment causes adverse reactions including flu-like symptoms, bone marrow suppression, fatigue, and depression, and is contraindicated for patients suffering from hepatic failure or cirrhosis [ 2 , 72 ]. Patient compliance is also low due to the subcutaneous administration.
Nucleoside analogues ( Figure 4 ) inhibit the HBV reverse transcriptase activity and therefore block HBV DNA replication. The active form of most of these drugs is the triphosphate that results from their phosphorylation by hepatocyte kinases. Nucleoside triphosphate analogues are substrates for the RT. During reverse transcription, they act as immediate or delayed transcriptional terminators and prevent the synthesis of both (−) and (+) HBV DNA strands. They are administered per os , having acceptable pharmacokinetics and limited drug-drug interactions. NAs suppress viremia at clinically undetectable levels in up to 76% of HBeAg (+) and 93% of HBeAg (−) patients after one year of treatment. Efficacy can vary in patients with different HBV genotypes [ 73 , 74 ]. Although some HBeAg (−) patients can discontinue treatment with NAs, their use is essentially life-long for the large majority of patients. However, virological relapse almost always occurs. Eight NAs have been approved against the HBV, of which the current recommended ones are entecavir and the two tenofovir prodrugs, disoproxil and alafenamide [ 73 ].

Nucleos(t)ide Analogues (NAs) approved for the treatment of hepatitis B [ 28 , 73 ].
The first approved NA which was effective against HBV was lamivudine (3TC, LMV, Epivir © , Zeffix © , Heptodin © , Hepitec © ). It was approved in the United States of America in 1998 [ 73 ], and it is administered once daily, with few side effects. It is no longer widely used because it is less potent than newer drugs and most patients develop resistance within one to five years [ 65 ]. Data from a randomized controlled trial showed that treatment with LMV for a median duration of approximately 32 months reduced the frequency of HCC occurrence [ 75 ]. As shown in another study, receiving LMV reduced the risk of HCC even in patients with liver cirrhosis [ 76 ]. The long-term use of LMV is limited by the development of resistance associated with mutations in the YMDD (tyrosine-methionine-aspartic acid-aspartic acid) motif in the viral RT active site. A study carried out by Kwon et al. in 2013 [ 77 ] suggests that treatment in patients without mutations in this region may be continued for more than five years until the complete loss of HBsAg is achieved [ 77 ]. However, the sustained viral response obtained with LMV for more than five years showed no further decrease in the incidence of HCC [ 75 ]. In the opposite direction, Eun et al. [ 78 ] found that long-term LMV administration and subsequent prolonged viral suppression had a beneficial impact on the risk of HCC [ 75 ]. Lamivudine therapy has been confirmed to reduce liver-related mortality in patients with HBV and even in patients with co-infection with human immunodeficiency virus (HIV), especially along with other NA as combination therapy [ 75 , 79 ].
The next approved ΝΑ was adefovir dipivoxil (bis(POM) PMEA, ADV, Hepsera © or Preveon © ) in 2002 [ 73 ]. Given once daily, it has shown only few side effects; however, the renal function should be monitored to avoid the development of renal impairment. It is considered a second-line treatment option, except for the case of LMV resistance, where it is used as the drug of choice [ 65 ]. Although ADV monotherapy is effective in HBV patients and its long-term use reduced the rate of liver fibrosis, resistant mutations conferred decreased susceptibility to ADV [ 75 ].
Entecavir (BMS-200475-01, ETV, Baraclude © ) was approved in 2005 [ 73 ]. It is taken once daily and causes few side effects. It is a first-line treatment with exceptional resistance profile [ 65 ], and it has been proved that it reduces the incidence of HCC [ 80 ]. The monitoring of serum alanine aminotransferase (ALT), an enzyme released by dead hepatocytes, is recommended at 6 and 12 months of treatment with ETV, since normal ALT levels are related to a reduced risk of developing HCC. Furthermore, the follow-up monitoring of serum alpha-fetoprotein as a biomarker for HCC is suggested [ 75 ].
Telbivudine (LdT, TBV, Tyzeka © or Sebivo © ) was approved in 2006 as a second-line treatment option [ 65 ]. Randomized clinical trials revealed that TBV is superior to lamivudine and adefovir in the treatment of patients with chronic HBV, regardless of the HBeAg detection [ 81 , 82 , 83 ]. In 2013, Tsai et al. [ 84 ] found that the cumulative incidence of HCC in patients who had received telbivudine was 2.5% and 4.1% at two and three years, respectively, rates similar to that of entecavir administration (3.1% and 7.5% in two and three years, respectively). TBV is associated with few side effects, including muscle toxicity and peripheral neuropathy [ 85 , 86 ]. Renal function should be considered when choosing between NAs, and it is worth noting that TBV can prevent nephrotoxicity [ 75 ]. At the same year, clevudine (L-FMAU, CLV, Levovir © or Revovir © ) was approved in South Korea and the Philippines. It was soon recalled due to the induction of skeletal myopathy caused by mitochondrial dysfunction [ 73 ].
Tenofovir Disoproxil Fumarate (bis(POC) PMPA Fumarate, TDF, Viread © ) was first released in 2008. It is taken once daily and has few serious adverse effects, including dose-limiting renal toxicity. Although being a first-line treatment, TDF is also effective as a second-line rescue treatment after therapy with other nucleos(t)ide analogues has failed due to resistance evolution [ 65 , 75 ]. To a great extent, TDF is not susceptible to resistance development, and thus its use provides sufficient virological suppression [ 87 ]. Some studies demonstrate that patients receiving TDF have a lower incidence of HCC compared with patients receiving entecavir [ 75 , 88 , 89 , 90 ]. Contrarily, other studies indicate that both tenofovir and entecavir monotherapies display a comparable risk for HCC [ 91 , 92 ]. Tenofovir alafenamide fumarate (GS-7340-03, TAF, Vemlidy © ) was first released in 2016, and it was developed to tackle the dose-limiting renal toxicity of TDF. The primary purpose of another analogue, tenofovir exalidex (a prodrug which is in early clinical development) is to improve the safety compared to formulations of TDF [ 93 ]. In 2017 another analogue was discovered, besifovir dipivoxil maleate (ANA-380/LB80380 maleate, BSV dipivoxil maleate, Besivo © ), showing significantly reduced bone and kidney toxicity, compared to tenofovir [ 73 , 93 ].
Overall, NAs which are administered per os, require long-term duration of therapy, achieve better control of HBV replication, and show many fewer side effects compared to PEG-IFN-α. Treatment with PEG-IFN-α is shorter in duration and can lead to stable, off-treatment multi-log10 reductions in viral titers in about 30% of patients [ 2 ], but it is not well tolerated in many patients because of severe side effects [ 7 , 94 ].
4. Novel Therapeutic Strategies
Major scientific breakthroughs, such as the identification of the NTCP cell surface receptor, detailed knowledge gained about cccDNA formation, regulation and its epigenetic control, the mechanism of cccDNA and pgRNA degradation, and the determination of the HBx protein’s role in viral transcription, have enabled an in-depth understanding of the HBV life cycle. In addition, innovative cell and animal models have improved the in vitro and in vivo assessment of the antiviral activity and potential toxicity of novel compounds. All of the above have paved the way for investigating multiple new therapeutic targets that will lead to substantial progress toward achieving a functional HBV cure [ 7 , 95 ].
4.1. HBV Entry Inhibitors
The discovery of NTCP as the entry receptor for HBV provided key knowledge on the viral entry mechanism, thus facilitating the identification of a variety of compounds that block the viral entry into the host hepatocytes [ 96 ]. As mentioned in Section 2.2 , interactions between the pre-S1 domain of L-HBsAg and NTCP are the key process for viral entry [ 97 ].
Various strategies have been proposed for the inhibition of HBV entry into hepatocytes. Small, acetylated peptides derived from the pre-S1 domain of L-HBs can effectively inhibit viral entry, exhibiting promising results both in vitro and in vivo [ 98 , 99 , 100 ]. In a recent report, novel cyclic peptides led to a significant HBsAg loss in vivo, with IC 50 values between 0.66 and 2.54 μΜ, without affecting the physiological function of the NTCP receptor [ 101 ]. Another study revealed that peptide 4B10 was able to inhibit HBV infection in a human hepatocyte culture, with IC 50 values in the nM range and no observed cytotoxicity [ 102 ]. The most important compound of this category is Myrcludex B (also known as Bulevirtide). Myrcludex B is a synthetic myristoylated lipopeptide consisting of 47 amino acids of the pre-S1 region. It strongly inhibits the HBV entry in the cell culture (IC 50 = 80 pM) and in a uPA/SCID humanized mouse model of HBV infection [ 103 , 104 , 105 ]. Moreover, Myrcludex B inhibits bile salt uptake only at much higher concentrations (IC 50 = 52.5 nM) [ 106 ]. The safety and efficacy results from clinical trials IIb are also excellent [ 107 , 108 ]. A liposomal formulation of Myrcludex B is under development for per os administration with an excellent pharmacological drug-drug interaction profile [ 109 , 110 ].
Several FDA-approved compounds have recently been identified as efficient inhibitors of the NTCP-L-HBs interaction. Those include the immunosuppressant cyclosporin A and its derivatives [ 106 , 111 , 112 ], the antihyperlipidemic ezetimibe [ 113 ], the angiotensin II receptor antagonist irbesartan [ 114 ], and the immunosuppressant rapamycin [ 115 ], among many drugs already in clinical use [ 116 , 117 , 118 ]. Another study identified that the green tea flavonoid epigallocatechin-3-gallate can efficiently block the NTCP-mediated viral entry [ 119 ]. Other recently identified HBV entry inhibitors include zafirlukast, vanitaracin A, proanthocyanidin, and its analogues, betulin derivatives, and novel synthetic compounds like B7 ( Table 1 ) [ 117 , 120 , 121 , 122 , 123 , 124 ].
Compounds that inhibit HBV entry in hepatocytes and their in vitro IC 50 values for HBV infection, measured in HepG2 cell cultures.
1 Inhibitor concentration required for 50% inhibition; 2 EC 50 value (half-maximal effective concentration).
Monoclonal antibodies are also efficient HBV entry inhibitors [ 125 , 126 , 127 , 128 ]. Studies have proven that monoclonal antibodies not only inhibit viral entry but also block the secretion of new infectious virions from hepatocytes [ 129 ]. The combination of two monoclonal antibodies, HBV-Ab17 and HBV-Ab19, has been evaluated in phase I clinical trials, demonstrating great safety and efficacy against HBV infection [ 130 ].
4.2. Directly Targeting cccDNA
The ability to eliminate or inactivate HBV cccDNA has been considered as the “holy grail” of HBV treatments [ 93 ] because achieving a “functional cure” for chronic HBV infections requires the permanent inactivation or degradation of the cccDNA [ 131 ]. Steps toward disrupting cccDNA have been enabled by understanding the roles of host enzymes such as TDP2, FEN1, and Pol-K in cccDNA formation. Similarly, the identification of host cell nuclear histones and chromatin-modifying enzymes that are essential for viral minichromosome formation and function has also enabled novel drug discovery avenues. Thus, targeting the cccDNA formation has led to many novel candidates in the pipeline of HBV chemotherapy [ 132 , 133 ]. As referred in Section 2.3 , APOBEC3 proteins can trigger cccDNA degradation. Several studies have proven that IFN-α administration induces APOBEC3 expression, resulting in the elimination of cccDNA in infected hepatocytes [ 134 ].
A potentially useful tool for the complete inactivation of cccDNA is the CRISPR-Cas9 endonuclease system to perform RNA-guided disruption and mutagenesis of cccDNA. The CRISPR-Cas9 endonuclease is complexed with a synthetic guide RNA (gRNA) that perfectly matches with the target sequence of cccDNA, resulting in the cleavage of the selected region. Therefore, to inactivate cccDNA, several gRNAs, targeting multiple different sites in the HBV genome, are required [ 133 , 135 , 136 ]. Integrated HBV DNA is also sensitive in CRISPR-Cas9-mediated inactivation, which could cause alterations in the host genome and subsequent gene malfunction [ 132 ]. Before using this genome editing approach in clinical practice, a number of serious potential issues need to be addressed. These include the incomplete cccDNA degradation, the need of a delivery system that will transfer the CRISPR-Cas9 system to all infected hepatic and extrahepatic cells, potential off-target effects, an immune response induced against the bacterial enzyme that could provoke serious toxicity, and the unpredictable effects of editing the integrated chromosomal HBV DNA [ 133 , 137 ].
Zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) are other methods that can destroy HBV cccDNA [ 133 , 138 ]. This treatment could slow down the growth of resistant HBV strains and increase the probability of a prolonged viral response [ 139 , 140 , 141 ], yet off-target activity, limited efficacy, effects on integrated HBV DNAs, and the potential induction of immune responses remain serious obstacles [ 140 ]. TALENs are newly developed nucleases that cleave selected DNA sequences, thus leading to gene disruptions. Several types of TALENs have been developed that target conserved regions of viral DNA among different HBV genotypes. Overall, TALENs can target and inactivate the HBV genome with a higher specificity than ZFNs and ameliorate the antiviral activity in synergy with IFN-α. Thus, a potential therapeutic strategy for the treatment of chronic hepatitis B infection is provided [ 140 , 141 , 142 ].
Another method that could contribute to the transcriptional control of cccDNA is direct targeting of the HBV X protein. HBx induces the proteasomal degradation of the Smc5/6 complex, that normally suppresses cccDNA transcription. Consequently, HBx protein inhibition will prevent the expression of all HBV transcripts from existing cccDNA molecules and suppress the formation of new cccDNA molecules [ 132 , 143 ]. cccDNA formation can also be directly targeted with substituted sulfonamides that interfere with the conversion of rcDNA to cccDNA ( Figure 5 ) [ 144 , 145 ].

Disubstituted Sulfonamides (DSS) [ 144 ].
4.3. Immune Therapy
4.3.1. targeting innate immunity.
The innate immune responses constitute the first line of defense against pathogens. These systems include membrane and cytoplasmic pattern recognition receptors (PRRs). PRRs interact with specific components, essential for pathogens’ survival, called pathogen-associated molecular patterns (PAMPs), and trigger the production of pro-inflammatory factors, like cytokines from immune cells [ 146 , 147 , 148 , 149 ]. Thus, TLR, and RIG-I agonists can stimulate the immune response against HBV infection and contribute significantly to its “functional cure”. Several TLR7, TLR8, and TLR9 agonists are being evaluated in clinical trials [ 150 , 151 , 152 , 153 ]. Phase I clinical trials for TLR7 agonists RO7020531, RG7795 (ANA773), and RG7854 (Roche © ) are currently underway. TLR7 agonist JNJ-64794964 (Janssen © ) demonstrated an excellent safety and tolerability profile in healthy adults during a double-blinded, randomized phase I trial [ 154 ]. Phase II clinical trial results for TLR7 agonist GS-9620 (also known as vesatolimod) revealed that it is safe and well-tolerated in chronic hepatitis B patients receiving NAs, although no significant HBsAg loss was observed after 24 weeks of treatment [ 155 ]. The same compound also caused no significant HBsAg decline in combination with tenofovir in treatment-naïve patients [ 156 ]. Pyrimidine analogues were recently identified as potent dual TLR7/8 modulators ( Figure 6 ii) [ 157 ]. Structural modifications led to novel 2,4-diaminoquinazoline dual TLR7/8 agonists with increased potency and proved that changing the stereochemistry in one single stereocenter leads to TLR8 selectivity ( Figure 6 iii) [ 158 ]. TLR8 agonist GS-9688 (also known as selgantolimod) is under phase II clinical trial evaluation [ 150 , 159 ]. Finally, RIG-I agonist SB-9200 (also known as Inarigrivir) showed promising results in a woodchuck model of HBV infection [ 160 , 161 ], and phase II clinical trials demonstrated the increased benefit of combining classic antiviral treatment with immune therapy [ 162 ].

Innate immunity modulators; ( i ) Selective TLR7 agonist [ 163 ] ( ii ) TLR7/8 dual agonist [ 157 ] ( iii ) Dual TLR7/8 agonist. (R) isomer results in selective TLR8 agonist [ 158 ] ( iv ) Selective TLR8 agonist [ 159 ].
4.3.2. Targeting Adaptive Immunity
The PD-1 (programmed death-1) receptor is expressed on HBV-specific T cells, and compounds that block the interactions with its physiological ligand, PD-L1, can increase the number and response of HBV-specific T cells, resulting in increased cytotoxic T cell activity against HBV-infected cells’ anti-HBV-antigen production by B cells [ 164 , 165 , 166 ]. Ex vivo studies have shown that blocking PD-1/PD-L1 interactions in chronically infected patients can partially restore the HBV-specific T and B cells’ function [ 167 , 168 , 169 ]. PD-1 antagonists have been associated with a high risk of hepatic failure [ 170 ]. On the other hand, the anti-PD-1:PD-L1 monoclonal antibody nivolumab has already been evaluated in phase I and II clinical trials in over 100 patients with advanced HCC and no hepatotoxicity incidents were observed [ 171 , 172 ].
4.4. RNA Interference—Post-Transcriptional Control
The inhibition of HBV replication by targeting mRNA production and stability is an innovative method for the therapy of chronic hepatitis B, whilst several inhibitors have made it into phase II clinical trials [ 7 , 28 , 93 , 173 ]. Inhibitors should bind to HBV mRNA with high specificity and therefore disrupt HBV protein expression by suppressing mRNA translation or inducing mRNA degradation. Such compounds are either small RNA interference (RNAi) molecules, antisense oligonucleotides (ASOs), or possibly even specific ribonucleic acid enzymes (riboenzymes) [ 133 ]. RNA interference is mediated by a sequence of 20–30 nucleotides, known as small interfering RNAs (siRNAs) [ 174 ]. An advantage stemming from HBV’s transcriptional profile is that multiple mRNA copies of HBV can be targeted selectively at the same time by selecting siRNAs that bind within the overlapping coding regions [ 133 ]. To date, three types of siRNAs with different modes of administration are under preclinical evaluation and/or in early-phase clinical trials [ 7 ].
An early RNAi drug against HBV, tested in human clinical trials, is ARC-520. The injection consists of two cholesterol-conjugated siRNAs, along with N -acetylgalactosamine (NAG) to achieve hepatocyte-specific delivery via the asialoglycoprotein receptor [ 39 , 93 ]. Potential use limitations are the intravenous administration, the contingent hepatotoxicity, and the off-target binding, as well as the risk of immune activation by PRRs [ 7 ]. Despite the barriers mentioned, ARC-520 seems to be very efficient in reducing HBV DNA, HBeAg, and HBsAg levels after experiments on chimpanzees [ 39 , 93 ]. Having passed through phase I with only few hypersensitivity reactions, it proceeded to phase II trials [ 28 ]. The co-administration of antihistamines is also recommended [ 28 ]. The next step in siRNA evolution came with JNJ-3989 (formerly ARO-HBV, phase II clinical trials), designed to target different HBV genome sites. It is administered subcutaneously, affecting transcripts from both cccDNA and integrated HBV DNA, is safe, and has not shown serious drug-drug interactions [ 39 , 93 , 175 ].
The HBV inhibitor RG7834 ( Figure 7 ), has been studied for its complicity in hepatitis B and has been confirmed not to act as an RNAi molecule, but as an HBV transcription suppressor, in a specific, unknown manner [ 28 , 176 ]. Furthermore, the combination of RG7834, entecavir, and PEG-IFN-α significantly decreases HBV DNA and HBsAg levels [ 133 ]. AB-729 is also an siRNA molecule which is administered subcutaneously conjugated with NAG. This compound showed an important suppression of HBsAg in mice models infected by HBV [ 93 ].

HBV transcription inhibitor [ 176 ].
Antisense oligonucleotides are short, single-stranded fragments of nucleic acids, either DNA or RNA, that bind to the complementary sequence of viral mRNAs through base pairing. As a result, when binding to RNA, they form hybrids of DNA:RNA (antisense DNA) and duplexes of RNA:RNA (antisense RNA), respectively. The subsequent degradation of the transcribed RNA and the silencing of protein expression occur via a host RNase H-dependent mechanism [ 7 , 177 , 178 ].
4.5. Ribonuclease H Inhibitors
Ribonucleases H are endonuclease enzymes that catalyze cleavage of RNA sequences in DNA:RNA hybrids [ 179 , 180 ]. The HBV RNaseH degrades the viral pgRNA during minus-polarity DNA strand synthesis by reverse transcriptase within immature nucleocapsids [ 181 ]. Inhibiting HBV RNaseH activity results in the accumulation of long DNA:RNA hybrids and halts the reverse transcription process [ 62 ]. Consequently, newly synthesized virions are non-infectious since they contain a defective genome [ 182 ]. Thus, compounds that inhibit RNaseH are promising antiviral candidates against HBV infection.
The RNaseH catalytic site includes a ‘DEDD’ (aspartic acid-glutamic acid-aspartic acid-aspartic acid) motif that coordinates two Mg 2+ ions. Both Mg 2+ are essential during the RNA hydrolysis process [ 183 , 184 ]. All known HBV RNaseH inhibitors contain three electron donors (O or N) that chelate these two cations [ 185 ]. RNaseH inhibitors primarily belong to two chemical classes: α-hydroxytropolones (α-HTs) and N -hydroxyimides. The latter include N -hydroxyisoquinolinediones (HIDs), N -hydroxynapthyrydinones (HNOs), N -hydroxypyridinediones (HPDs), and N -hydroxypyrimidinediones [ 185 , 186 , 187 , 188 ].
One of the first identified HBV RNaseH inhibitors was β -thujaplicinol (compound 46, Table 2 ), an α-HT isolated from the heartwood of western red cedar. β -thujaplicinol blocks the RNaseH of HBV genotypes D and H with EC 50 values of 5.9 and 2.3 μΜ, respectively [ 189 ]. This finding led to the design and synthesis of several novel hydroxylated tropolone analogues that suppress HBV replication in EC 50 values as low as 0.34 μM (compound 110) [ 185 , 190 , 191 ], and with CC 50 values up to 100 μM. Therapeutic index values were up to 200 [ 191 ]. Compound 110 has also been found to inhibit the HBV RNaseH activity, in a molecular beacon assay, and to suppress viremia in animal models [ 192 ]. Further structure-activity relationship studies on the hydroxylated tropolone ring revealed that the α-OH substitution is essential for the HBV RNaseH inhibition. Bulky substitution in positions R 1 , R 2 and R 3 leads to a decreased inhibitory activity, indicating that sulfonyl- or lactone substituents can increase the efficacy [ 190 , 191 , 193 ]. These findings have been validated by recent studies, which also highlighted the increased efficacy of amide-substituted α-HTs [ 194 , 195 , 196 ].
HBV Ribonuclease inhibitors. The highlighted atoms chelate the two Mg 2+ ions in the enzyme’s catalytic site. HID; N -hydroxyisoquinolinedione, HNO; N -hydroxynaphthyridinone, HPD; N -hydroxypyridinedione, EC 50 ; half-maximal effective concentration, CC 50 ; 50% cytotoxic concentration, TI; therapeutic index (TI = CC 50 /EC 50 ).
All four mentioned classes of N -hydroxyimides contain N or O atoms in suitable positions in order to chelate the two Mg 2+ ions and inhibit RNaseH in the same way as the α-HTs [ 185 ]. Several analogues have been synthesized and assessed pharmacologically for their HBV RNaseH inhibitory activity, exhibiting low EC 50 values (as low as 110 nM), limited cytotoxicity (most CC 50 values between 25 and 100 μΜ), and TI values >300 [ 187 , 188 , 197 , 198 ]. One compound from this class has also been evaluated in vivo, and the results verified N -hydroxyimides as being effective HBV RNaseH inhibitors [ 192 ]. Finally, RNaseH inhibition is unlikely to be affected by HBV’s large genetic diversity, and RNaseH inhibitors have demonstrated great synergistic activity with antiviral compounds with a different mechanism of action, indicating that they can potentially be used in effective, combination therapeutic schemes against HBV infection [ 199 , 200 ]. The structures of several HBV RNaseH inhibitors identified up to date are shown in Table 2 .
4.6. Nucleocapsid Assembly Inhibitors or Modulators
The HBV core particle is actively involved in the HBV replication cycle. It is required for the transfer of the viral genome to and from the nucleus of the infected hepatocyte, as well as for a successful reverse transcription [ 39 ]. Thus, it is a promising target for antiviral drugs [ 11 ]. New regulators or inhibitors of nucleocapsid assembly can affect various stages of the HBV replication cycle, including capsid formation, reverse transcription, and pgRNA encapsidation [ 28 ]. Based on the three-dimensional structure of capsids when they interact with a ligand, two categories of analogues have been developed [ 28 ].
The first category is the Class I core protein allosteric modulators (CpAMs), represented by heteroaryldihydropyrimidines (HAPs) such as GLS4, RO7049389, and Bay41-4109 [ 28 , 201 ]. Class I CpAMs induce the formation of deformed nucleocapsids [ 11 , 39 , 133 , 145 ]. The other category is that of Class II CpAMs, such as phenylpropenamides (PP) or sulfamoylbenzamides (SBA), its main representatives being: AT-130, NVR-3778, JNJ6379, JNJ0440, JNJ-632, JNJ56136379, AB-423. These compounds accelerate the assembly of morphologically normal HBV capsids that lack the viral genome [ 28 , 133 , 201 , 202 ]. NVR 3-778 is the first SBA derivative developed in the USA, administered per os , and is under phase Ia clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT02112799","term_id":"NCT02112799"}} NCT02112799 , {"type":"clinical-trial","attrs":{"text":"NCT02401737","term_id":"NCT02401737"}} NCT02401737 ), exhibiting synergistic effects in combination with PEG-IFN-α, after evaluation in HBV-infected mice with a humanized liver [ 7 , 28 , 39 , 175 ]. Recent in vitro studies in primary human hepatocytes have shown that JNJ-632 (SBA) and Bay41-4109 (HAP) inhibit cccDNA formation and decrease both intracellular HBV RNA and HBeAg and HBsAg levels [ 28 ]. Further in vitro studies have proved that phenylpropenamide derivatives demonstrate improved antiviral activity when combined with NAs [ 145 ]. JNJ-6379 binds to the HBV core protein and disrupts the encapsidation of pgRNA. It also blocks the cccDNA formation. This drug seems to have a very long half-life, of approximately 120–140 h [ 175 , 203 , 204 ]. ABI-H0731 marked the beginning of a new category of compounds. It is a dibenzo-thiazepine-2-carboxamide derivative, and it has been shown to cause a significant reduction in HBV DNA and RNA levels in phase I clinical trials, as a core protein modulator [ 39 , 205 ].
Both classes of CpAMs inhibit the release of viral particles. Thus, the amount of HBV DNA and RNA leaving the hepatocyte is reduced. They also prevent de novo cccDNA formation due to blocking the formation of functional capsids, and hence viral replication [ 133 ]. The structures of the abovementioned compounds are shown in Figure 8 .

Nucleocapsid assembly modulators or inhibitors [ 201 , 205 , 206 , 207 , 208 ].
5. Perspectives
Hepatitis B vaccines are very effective in preventing infection, and antiviral drugs are partially effective in reducing disease progression and death from the infection. However, access to both the vaccine and the drugs remains a challenge for a large percentage of the world’s population because the majority of chronically infected patients live in developing countries, most often in sub-Saharan Africa or southeast Asia [ 209 ], with varying degrees of access to medical care. Therefore, developing an affordable and readily deliverable cure for chronic hepatitis B is urgent. It is widely believed that achieving a broadly applicable “functional cure” for chronic HBV infection will require a combination therapy using agents that target multiple different viral targets plus immune modulators that harness the power of the patients’ defenses against the virus [ 132 , 210 ]. Drugs to improve control and eliminate HBV will have to tackle the unique features of this infection, particularly the durability of the cccDNA during current therapies, which is the reason why the existing drugs so rarely induce a “functional cure”. Fortunately, there is a very wide range of drugs in preclinical and clinical development, so chances are high that combinations of these strategies may be found that substantially improve treatment for HBV patients.
Author Contributions
G.-M.P. writing—original draft, D.M. writing—original draft, E.G. writing—review & editing and preparing the original manuscript figures, V.P. writing—review & editing, J.E.T. review & editing G.Z. conceptualization, methodology, review & editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
The APC was funded by the Special Account for Research Grants and the National and Kapodistrian University of Athens.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Safety and efficacy of vebicorvir administered with entecavir in treatment-naïve patients with chronic hepatitis B virus infection
Affiliations.
- 1 Johns Hopkins University School of Medicine, Baltimore, MD, USA. Electronic address: [email protected].
- 2 Institute of Liver Studies, King's College Hospital, London, UK.
- 3 Office of Xiaoli Ma, Philadelphia, PA, USA.
- 4 T Nguyen Research and Education, Inc., San Diego, CA, USA.
- 5 Schiff Center for Liver Diseases, University of Miami School of Medicine, Miami, FL, USA.
- 6 Department of Medicine, Division of Gastroenterology and Hepatology, Thomas Jefferson University Hospital, Philadelphia, PA, USA.
- 7 Department of Medicine, Division of Liver Diseases, Icahn School of Medicine, Mount Sinai Hospital, New York, NY, USA.
- 8 ID Care, Hillsborough, NJ, USA.
- 9 NYU Langone Health, New York, NY, USA.
- 10 Sing Chan, MD, New York, NY, USA.
- 11 Pfleger Liver Institute, University of California, Los Angeles, CA, USA.
- 12 New Zealand Clinical Studies, Auckland, New Zealand.
- 13 Medical Associates Research Group, San Diego, CA, USA.
- 14 Assembly Biosciences, South San Francisco, CA, USA.
- 15 Quest Clinical Research, San Francisco, CA, USA.
- 16 Cedars-Sinai Medical Center, Los Angeles, CA, USA.
- 17 Waikato Hospital, Hamilton, New Zealand.
- 18 Gastrohealth, Catonsville, MD, USA.
- 19 Gastrointestinal Research Institute, Vancouver, BC, Canada.
- 20 Stanford University Medical Center, Stanford, CA, USA.
- 21 Toronto Liver Centre, Toronto, ON, Canada.
- 22 Southern California Research Center, Coronado, CA, USA.
- 23 Asian Pacific Liver Center, Los Angeles, CA, USA.
- 24 University of Toronto, Toronto, ON, Canada.
- 25 Department of Medicine and State Key Laboratory of Liver Research, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
- PMID: 35697332
- DOI: 10.1016/j.jhep.2022.05.027
Background & aims: Nucleos(t)ide reverse transcriptase inhibitors do not completely suppress HBV DNA in chronic HBV infection (cHBV). Vebicorvir (VBR) is an investigational core inhibitor that interferes with multiple aspects of HBV replication. This phase II trial evaluated the safety and efficacy of VBR in combination with entecavir (ETV) in treatment-naïve patients with cHBV.
Methods: HBeAg-positive, treatment-naïve patients without cirrhosis were randomised 1:1 in a double-blind manner to once-daily VBR 300 mg+ETV 0.5 mg or placebo (PBO)+ETV 0.5 mg for 24 weeks. The primary endpoint was change in mean log 10 HBV DNA from Baseline to Week 12 and 24.
Results: All patients in both treatment groups (PBO+ETV: 12/12; VBR+ETV: 13/13) completed the study. At Week 12, VBR+ETV led to a greater mean (SD) reduction from Baseline in log 10 IU/ml HBV DNA (-4.45 [1.03]) vs. PBO+ETV (-3.30 [1.18]; p = 0.0077). At Week 24, VBR+ETV led to a greater reduction from Baseline in log 10 IU/ml HBV DNA (-5.33 [1.59]) vs. PBO+ETV (-4.20 [0.98]; p = 0.0084). Greater mean reductions in pregenomic RNA were observed at Week 12 and 24 in patients receiving VBR+ETV vs. PBO+ETV (p <0.0001 and p <0.0001). Changes in viral antigens were similar in both groups. No drug interaction between VBR and ETV was observed. Two patients experienced HBV DNA rebound during treatment, with no resistance breakthrough detected. The safety of VBR+ETV was similar to PBO+ETV. All treatment-emergent adverse events and laboratory abnormalities were Grade 1/2. There were no deaths, serious adverse events, or evidence of drug-induced liver injury.
Conclusions: In this 24-week study, VBR+ETV provided additive antiviral activity over PBO+ETV in treatment-naïve patients with cHBV, with a favourable safety and tolerability profile.
Clinical trial number: NCT03577171 LAY SUMMARY: Hepatitis B is a long-lasting viral infection of the liver. Current treatments can suppress hepatitis B virus but do not offer the opportunity of cure, hence, new treatment approaches are required. Herein, we show that the combination of the novel core inhibitor vebicorvir with an existing antiviral (entecavir) in treatment-naïve patients chronically infected with hepatitis B virus demonstrated greater antiviral activity than entecavir alone. Additionally, vebicorvir was safe and well tolerated. Thus, further studies evaluating its potential role in the treatment of chronic hepatitis B are warranted.
Keywords: ABI-H0731; Vebicorvir; chronic hepatitis B virus; core inhibitor; entecavir; nucleos(t)ide reverse transcriptase inhibitor; phase 2; treatment-naïve.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Publication types
- Clinical Trial, Phase II
- Research Support, Non-U.S. Gov't
- Antiviral Agents* / adverse effects
- Double-Blind Method
- Drug Therapy, Combination / adverse effects
- Guanine / analogs & derivatives
- Hepatitis B e Antigens
- Hepatitis B virus
- Hepatitis B, Chronic* / drug therapy
- Reverse Transcriptase Inhibitors / therapeutic use
- Treatment Outcome
- Antiviral Agents
- Reverse Transcriptase Inhibitors
Associated data
- ClinicalTrials.gov/NCT03577171

Characteristics and antiviral treatment eligibility of newly diagnosed hepatitis B patients at a teaching hospital in Ghana: Implications for prevention and management
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Joseph Daniels
- For correspondence: [email protected]
- ORCID record for Yvonne A Nartey
- ORCID record for Francis Djankpa
- Info/History
- Preview PDF
Hepatitis B virus (HBV) infection poses a considerable public health challenge in limited-resource settings especially in the sub-Saharan African region. Even though HBV infection is incurable, timely treatment is effective in preventing disease progression to liver cirrhosis or hepatocellular carcinoma. However, not all infected patients require treatment. The aim of this study was to determine the clinical, immunological, and virological profiles of newly diagnosed adult HBV patients at a tertiary healthcare center in Ghana and to determine the antiviral treatment eligibility rate based on current guidelines of the World Health Organization (WHO). A hospital-based cross-sectional study involving total sampling of 220 treatment naïve HBV surface antigen positive clients was carried out. A structured questionnaire was used to collect data and detailed clinical and laboratory assessment (serological, biochemical and virological) was carried out. Data were entered and analyzed with STATA version 16. The median age at diagnosis was 34 years (IQR 26.0 – 41.5) with a male to female ratio of 1:1.5. A total of 138 participants (62.7%) were diagnosed with HBV infection following voluntary testing. There was a median delay of 8.5 months (IQR 3.0 – 22.5) between initial diagnosis and patients’ presentation for medical care. In all, 24 patients (10.9%) had abnormal clinical examination findings, 172 patients (78.2%) had HBV DNA levels ≤ 2000 IU/ml while 8 (3.6%) were seropositive for HBeAg. A small proportion of patients had concomitant human immunodeficiency virus (2.7%) and hepatitis C virus (1.4%) infections. Treatment eligibility rate was very low among newly diagnosed HBV infected patients seeking medical care (n=14, 6.4%) following the WHO guidelines for treatment eligibility. Thus, increasing screening rate among the general population, early linkage to clinical care of screen positives and vaccination of screen negatives will help reduce HBV related clinical conditions in resource limited countries.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
The author(s) received no specific funding for this work.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Written informed consent was obtained from all participants of the study. The study was approved by the ethical review committee of the Cape Coast Teaching Hospital (Reference number: CCTHERC/EC/2019/084).
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Infectious Diseases (except HIV/AIDS)
- Addiction Medicine (315)
- Allergy and Immunology (617)
- Anesthesia (159)
- Cardiovascular Medicine (2269)
- Dentistry and Oral Medicine (279)
- Dermatology (201)
- Emergency Medicine (369)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (798)
- Epidemiology (11559)
- Forensic Medicine (10)
- Gastroenterology (676)
- Genetic and Genomic Medicine (3560)
- Geriatric Medicine (336)
- Health Economics (615)
- Health Informatics (2297)
- Health Policy (912)
- Health Systems and Quality Improvement (860)
- Hematology (334)
- HIV/AIDS (748)
- Infectious Diseases (except HIV/AIDS) (13133)
- Intensive Care and Critical Care Medicine (755)
- Medical Education (358)
- Medical Ethics (100)
- Nephrology (388)
- Neurology (3341)
- Nursing (191)
- Nutrition (506)
- Obstetrics and Gynecology (650)
- Occupational and Environmental Health (643)
- Oncology (1753)
- Ophthalmology (524)
- Orthopedics (208)
- Otolaryngology (284)
- Pain Medicine (223)
- Palliative Medicine (66)
- Pathology (437)
- Pediatrics (999)
- Pharmacology and Therapeutics (419)
- Primary Care Research (402)
- Psychiatry and Clinical Psychology (3050)
- Public and Global Health (5980)
- Radiology and Imaging (1217)
- Rehabilitation Medicine and Physical Therapy (712)
- Respiratory Medicine (809)
- Rheumatology (367)
- Sexual and Reproductive Health (348)
- Sports Medicine (315)
- Surgery (386)
- Toxicology (50)
- Transplantation (170)
- Urology (142)
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Epidemiology and treatment outcomes of tuberculosis with chronic hepatitis b infection—california, 2016–2020.
- Article contents
- Figures & tables
- Supplementary Data
J Bradford Bertumen, Lisa Pascopella, Emily Han, Rosie Glenn-Finer, Robert J Wong, Amit Chitnis, Devan Jaganath, Mirna Jewell, Prabhu Gounder, Sara McElroy, Lauren Stockman, Pennan Barry, Epidemiology and Treatment Outcomes of Tuberculosis with Chronic Hepatitis B Infection—California, 2016–2020, Clinical Infectious Diseases , 2024;, ciae169, https://doi.org/10.1093/cid/ciae169
- Permissions Icon Permissions
Improved epidemiologic and treatment data for active tuberculosis (TB) with chronic hepatitis B virus (cHBV) infection might inform and encourage screening and vaccination programs focused on persons at risk of having both conditions.
We matched the California Department of Public Health TB registry during 2016–2020 to the cHBV registry using probabilistic matching algorithms. We used chi-square analysis to compare the characteristics of persons with TB and cHBV with those with TB only. We compared TB treatment outcomes between these groups using modified Poisson regression models. We calculated the time between reporting of TB and cHBV diagnoses for those with both conditions.
We identified 8,435 persons with TB, including 316 (3.7%) with cHBV. Among persons with TB and cHBV, 256 (81.0%) were non-U.S.-born Asian vs 4,186 (51.6%) with TB only ( P <0.0001). End-stage renal disease (26 [8.2%] vs 322 [4.0%]; P <0.001) and HIV (21 [6.7%] vs 247 [3.0%]; P value = 0.02) were more frequent among those with TB and cHBV compared with those with TB only. Among those with both conditions, 35 (11.1%) had TB diagnosed >60 days before cHBV (median 363 days) and 220 (69.6%) had TB diagnosed >60 days after cHBV (median 3,411 days).
Persons with TB and cHBV were found more frequently in certain groups compared with TB only, and infrequently had their conditions diagnosed together. This highlights an opportunity to improve screening and treatment of TB and cHBV in those at high risk for coinfection.
- epidemiology
- hepatitis b, chronic
- tuberculosis
- treatment outcome
Supplementary data
Email alerts, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

COMMENTS
For Thomas Tu, eliminating hepatitis B is a deeply personal goal. Tu, a molecular virologist at the Westmead Institute for Medical Research in Sydney, Australia, learnt he had chronic hepatitis B ...
The National Institutes of Health has updated its Strategic Plan for NIH Research to Cure Hepatitis B, a roadmap for ending the hepatitis B epidemic, focused on developing a cure as well as improved strategies for vaccination, screening and follow-up care. The revised plan incorporates lessons from the COVID-19 pandemic and recent advances in technology.
DOI: 10.1056/NEJMra2211764. VOL. 388 NO. 1. Chronic hepatitis B is caused by the hepatitis B virus (HBV), a hepatotropic DNA virus that can replicate at high levels and cause minimal disease or ...
Chronic hepatitis B virus (HBV) infection impacts an estimated 257-291 million people globally. The current approach to treatment for chronic HBV infection is complex, reflecting a risk:benefit approach driven by the lack of an effective curative regimen. This complexity and the lack of a durable tr …
Hepatitis B virus (HBV) infection is a leading cause of chronic liver disease worldwide. Antiviral agents, such as interferon and nucleos (t)ide analogues (NAs), have been used to treat patients with chronic hepatitis B (CHB). Antiviral treatment strongly suppresses HBV replication and slows progression to cirrhosis and hepatocellular carcinoma ...
B is built on three priority areas that are vital to developing a cure: 1. Understanding hepatitis B biology, public health challenges posed by HBV infection, a. 2. Developing tools and resources to advance HBV curative treatment will need to go hand in hand research, and 3.
The Strategic Plan for Trans-NIH Research to Cure Hepatitis B focuses on three key research areas. The first priority calls for a better understanding of hepatitis B biology, including the viral and host factors that lead to disease, immunity, reactivation and transmission, as well as the impact of co-infections with other hepatitis viruses and ...
The International Coalition to Eliminate HBV (ICE-HBV) is an international, independent, research-based forum created in 2016 to accelerate HBV cure research. ICE-HBV aims to coordinate, promote, and foster collaborative partnerships working towards an HBV cure and the alleviation of suffering for all individuals living with chronic hepatitis B ...
Core Tip: Given the hepatitis B viral life cycle's unique characteristics, a true cure is lacking.Most recent guidelines from multiple societies including the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, Asia Pacific Association for the Study of the Liver, and Taiwan Association for the Study of the Liver, primarily include data ...
This article takes a look at hepatitis B and ongoing cure research, including the development of direct-acting antivirals similar to those used to treat hepatitis C. It also explains how hepatitis B is currently treated and prevented with medications and vaccines.
In spite of the fact that safe and effective vaccines have been available for over 40 years, hepatitis B virus (HBV) remains a major public health problem, as there are 296 million chronically HBV-infected individuals worldwide and 820 000 HBV-related deaths taking place every year. Achieving the goal of HBV cure remains a challenge due to the particularities of the HBV cycle underlying viral ...
Figure 2: Trans-NIH Strategic Plan to Cure Hepatitis B proposes to maximize the use of resources by leveraging clinical cohorts and biorepositories, sharing resources, and drawing on multidisciplinary biomedical collaborations. The plan's strategic priorities are: 1. Understand hepatitis B biology, 2.
The NIDDK-funded HBRN conducted clinical trials of treatment approaches for chronic hepatitis B in a study population that was primarily men, women, and children of Asian descent. Though the Network studies concluded in 2022, data analysis and publication of results has continued, with study samples available for additional research through the ...
Background: The hepatitis B virus (HBV) affects an estimated 290 million individuals worldwide and is responsible for approximately 900 000 deaths annually, mostly from complications of cirrhosis and hepatocellular carcinoma. Although current treatment is effective at preventing complications of chronic hepatitis B, it is not curative, and often must be administered long term.
With the recent advances in hepatitis B research, scientists are optimistic that another big leap in the search for a cure is possible if new complementary drugs are identified. The Baruch S. Blumberg Institute of the Hepatitis B Foundation is at the forefront of research efforts to discover such new drugs.
Professor Wong commented, "With almost 300 million persons living with chronic hepatitis B in the world, there is a high - and critical - unmet medical need for a functional cure that is not only effective, but also safe. These first clinical data suggest that VRON-0200 is safe and well tolerated, after a single injection into the arm muscle.
With the recent advances in hepatitis B research, scientists are optimistic that another big leap in the search for a cure is possible if other complementary drugs can be found. The Baruch S. Blumberg Institute of the Hepatitis B Foundation is at the forefront of research efforts to discover such new drugs. Blumberg Institute at the forefront
Abstract. Hepatitis B virus infection affects over 250 million chronic carriers, causing more than 800,000 deaths annually, although a safe and effective vaccine is available. Currently used antiviral agents, pegylated interferon and nucleos (t)ide analogues, have major drawbacks and fail to completely eradicate the virus from infected cells.
WHO has released new Guidelines on prevention, diagnosis and treatment of chronic hepatitis B (HBV) infection at the 2024 Asian Pacific Conference for the Study of Liver Disease (APASL) in Kyoto, Japan. These guidelines provide a substantial simplification and expansion of eligibility for treatment to overcome barriers in access to HBV testing and treatment.
New treatment strategies and hepatitis B cure agenda Research is ongoing to develop and test new agents that can "cure" hepatitis B by eliminating all replicative forms, including cccDNA and thus reduce the need for lifelong treatment. The goal is to achieve "functional" cure, defined as a sustained loss of HBsAg (undetectable <0.05
Overview . Hepatitis B (HBV) infection is a major public health problem and cause of chronic liver disease. The 2024 HBV guidelines provide updated evidence-informed recommendations on key priority topics. These include expanded and simplified treatment criteria for adults but now also for adolescents; expanded eligibility for antiviral prophylaxis for pregnant women to prevent mother-to-child ...
NCT03577171 LAY SUMMARY: Hepatitis B is a long-lasting viral infection of the liver. Current treatments can suppress hepatitis B virus but do not offer the opportunity of cure, hence, new treatment approaches are required. Herein, we show that the combination of the novel core inhibitor vebicorvir w …
Future research is needed to perfect some antiviral therapy schemes that shorten the treatment period but also decrease the rate of progression towards decompensated cirrhosis and liver adenocarcinoma. ... M.S.; Xiong, S. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B ...
Hepatitis B virus (HBV) infection poses a considerable public health challenge in limited-resource settings especially in the sub-Saharan African region. Even though HBV infection is incurable, timely treatment is effective in preventing disease progression to liver cirrhosis or hepatocellular carcinoma. However, not all infected patients require treatment. The aim of this study was to ...
NIAID seeks applications through the notice of funding opportunity (NOFO) Multidisciplinary Research to Accelerate Hepatitis B Cure in Persons Living with HIV and HBV (U19, Clinical Trial Not Allowed).The NOFO's purpose is to support research on the impact of host and viral heterogeneity on pathogenesis of disease, viral persistence, and immunopathology of Hepatitis B virus (HBV) and inform ...
Persons with tuberculosis and chronic hepatitis B are more likely to be non-US born Asian people and have chronic medical conditions. ... We compared TB treatment outcomes between these groups using modified Poisson regression models. ... It furthers the University's objective of excellence in research, scholarship, and education by publishing ...