Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
AUTHOR DISCLOSURE
Drs Rajagopalan and Ilboudo have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Anuradha Rajagopalan , Christelle M. Ilboudo; Malaria. Pediatr Rev March 2019; 40 (3): 151–153. https://doi.org/10.1542/pir.2017-0225
Download citation file:
- Ris (Zotero)
- Reference Manager
“With tears and toiling breath, I find thy cunning seeds, O million-murdering Death," wrote Sir Ronald Ross after his discovery in 1897 that the malaria parasite was transmitted by the Anopheles mosquito. Today, more than a century later, malaria continues to cause disease in millions of people worldwide. In 2015 alone, there were approximately 429,000 deaths due to malaria. Most of these deaths occur in sub-Saharan Africa in children younger than 5 years. Malaria is endemic in Africa, Latin America, Asia, parts of the Caribbean, Eastern Europe, and the South Pacific. Although global mortality and morbidity are improving, the number of cases in travelers is increasing. Per GeoSentinel Global Surveillance data, falciparum malaria is the most common serious febrile illness in returning travelers and the main cause of death. There were 1,724 cases of malaria imported to the United States in 2014. Most of these imported cases occur in people visiting friends and relatives in endemic areas of Africa and Asia. Of the 1,715 patients with imported malaria whose ages were known, 265 (15.5%) were children younger than 18 years.
Malaria is caused by protozoa of the genus Plasmodium . The 4 species infecting humans consistently are Plasmodium falciparum , Plasmodium vivax , Plasmodium ovale , and Plasmodium malariae . Human infections from the simian parasite Plasmodium knowlesi are intermittently reported from Southeast Asia. Transmission of all species occurs through the bite of an infective female Anopheles . Other less common modes of transmission are through blood transfusion, organ transplant, needle sharing, and vertical transmission from mother to fetus.
Initial malaria symptoms can be nonspecific, with headaches, abdominal discomfort, fatigue, and myalgia. This is followed by fever, chills, perspiration, anorexia, vomiting, and worsening malaise. Young children can present with lethargy, poor feeding, and cough. The clinical picture in an immunologically naive traveler differs from that of a patient living in a malaria-endemic area, who has partial immunity. Relative immunity to malaria develops in areas of intense malarial transmission with repeated exposure to different strains of malarial antigen. This inhibits parasite multiplication and results in asymptomatic parasitemia (premunition). Immunity wanes over time with lack of exposure to the malarial antigen. People who have previously lived in endemic areas and are visiting friends and relatives often do not take chemoprophylaxis because they consider themselves immune. They are, therefore, at high risk for disease. In nonimmune travelers who have never been exposed to malaria, the incubation period is short, with most travelers developing symptoms of falciparum malaria within a month of leaving the endemic area. Clinical symptoms are severe and can progress rapidly, making it a medical emergency.
Rapid diagnosis and treatment is critical because full recovery is possible. Once progression occurs to severe malaria, most cases are fatal if left untreated. Severe malaria is characterized by 1 or more of the following; coma, metabolic acidosis, severe anemia, hypoglycemia, acute renal failure, or acute pulmonary edema.
The gold standard for laboratory diagnosis is light microscopy. Both a thick and a thin smear are examined for the parasite. The thick smear is a more sensitive tool for detection of the parasite, whereas the thin smear enables species identification and parasite count. The accuracy of detection depends on the skill of the microscopist. A skilled technician can detect parasites at densities of less than 10/μL of blood. A negative smear should be followed by 2 more smears at 6- to 12-hour intervals. A rapid diagnostic test, which detects parasite-specific antigen by immunochromatography, can be considered if a skilled technician is not available. The limitations of this test are that it cannot be used for species identification or parasite count. It cannot be used to distinguish new infections from recently treated ones because the rapid diagnostic test for P falciparum HRP2 remains positive for 1 to 5 weeks after therapy. Polymerase chain reactions are highly sensitive and useful for detecting mixed infections at low parasite densities, but results can take as long as 10 days and are performed only in reference laboratories. They can be used to study drug resistance and are generally reserved for special circumstances, such as treatment failure.
Certain genetic factors, such as the sickle cell trait, provide relative protection against P falciparum . Hence, sickle cell trait is more frequent in people of African ancestry than in other population groups. Similarly, hemoglobin C, the thalassemias, and glucose-6-phosphate dehydrogenase deficiency are more prevalent in malaria-endemic areas. Red blood cells negative for the Duffy blood group are resistant to infection by P vivax . Most Africans are Duffy negative, and, therefore, P vivax is rare in sub-Saharan Africa.
It is important to consider malaria in any traveler with a febrile illness who has returned from a malaria-endemic area in the past year, regardless of chemoprophylaxis history. In most cases surveyed, travelers with imported malaria had not adhered to a chemoprophylaxis regimen that was appropriate for the region of travel per the Centers for Disease Control and Prevention (CDC) recommendations.
Treatment is based on the infecting Plasmodium species, the clinical status of the patient, and the drug susceptibility of the infecting parasite. Children younger than 8 years diagnosed as having uncomplicated chloroquine-resistant P falciparum in the United States are treated with atovaquone-proguanil or artemether-lumefantrine as first-line agents. Quinine sulphate combinations with doxycycline and tetracycline, which are the next option, are not preferred in this age group. Quinine may not be available in capsule form, making dosing difficult. The pediatric dosage should be adjusted by weight and should never exceed the recommended adult dosage.
Children younger than 8 years with chloroquine-resistant P vivax are treated with mefloquine as first-line. In cases of P vivax and P ovale infections, primaquine is necessary for preventing relapses. Glucose-6-phosphate dehydrogenase screening is required before primaquine therapy. Severe malaria is treated in the United States with parenteral quinidine combined with doxycycline, tetracycline, or clindamycin as combination therapy. Intravenous artesunate therapy is preferred. This treatment is provided in an intensive care setting with continuous cardiac monitoring.
It is essential for health care providers to familiarize themselves with prevention strategies because millions of US residents travel each year to malaria-endemic regions. Prevention measures available are mosquito avoidance and chemoprophylaxis. There is no current vaccine for travelers. However, there are phase 3 trials investigating RTS,S/AS01, an injectable 4-dose vaccine series that offers partial protection against P falciparum in children aged 5 to 17 months. It was piloted in 2018 in sub-Saharan Africa.
Addressing malaria prevention in travelers is a balance between ensuring that all people at risk for infection are protected adequately while preventing rare adverse effects associated with these interventions. Chemoprophylaxis is the most effective method of preventing infection. Education about the risk of disease and evaluating for potential adverse effects by starting chemoprophylaxis 3 to 4 weeks before travel can help improve compliance. Malaria, although a deadly disease, is both preventable and curable. Clinicians need to maintain a high index of suspicion for this disease in febrile travelers returning from a malaria-endemic area.
COMMENT: Having worked in Liberia and Uganda, treatment of malaria was a common occurrence and was often done on an outpatient basis. But in my practice in the United States, cases have included children whose families traveled abroad either to their countries of origin or for vacations. It is critical for primary care providers to know how and when to prescribe chemoprophylaxis, and I have found the CDC travel website, https://wwwnc.cdc.gov /travel, incredibly informative. Choices in chemoprophylaxis will vary based on the age of the patient, potential adverse effects, and individual patient choices of frequency of doses and duration of treatment. It is critical to review these issues and find the optimal individualized chemoprophylaxis for families because adherence is key to prevention. As noted in this In Brief, more than 80% of travelers who became infected with malaria did not adhere to their recommended chemoprophylaxis. Appropriate testing for malaria is key to diagnosis, so asking about a travel history in any patient presenting with fever is essential. Once the diagnosis is made or highly suspected, accessing infectious disease consultants is important to ensure implementation of high-quality and best-care practices.

Competing Interests
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- ABP Content Spec Map
- Pediatrics On Call
- Online ISSN 1526-3347
- Print ISSN 0191-9601
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

solar eclipse
25 templates

palm sunday
5 templates

26 templates

14 templates

28 templates
weather report
12 templates
Malaria Disease
Malaria disease presentation, free google slides theme and powerpoint template.
If you want to explain some details about malaria, use this medical presentation. Provide some explanations about its diagnosis, recommendations, pathology, treatments and conclusions. It’s very creative and full of cartoons.
Features of this template
- A orangish design with cartoon style illustrations
- 100% editable and easy to modify
- 29 different slides to impress your audience
- Contains easy-to-edit graphics such as tables, charts, diagrams and maps
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.


S. DAVID SHAHBODAGHI, MD, MPH, AND NICHOLAS A. RATHJEN, DO
Editor's Note: This article has been updated to incorporate the February 2023 guidance update from the Centers for Disease Control and Prevention.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2022;106(3):270-278
Author disclosure: No relevant financial relationships.
Each year, malaria causes an estimated 500,000 deaths worldwide. Most of these deaths occur in Africa and disproportionally affect children younger than five years worldwide. Human malarial disease is caused by protozoan parasites of the genus Plasmodium . The primary means of infection is through the bite of a female Anopheles mosquito. The incidence of malaria in the United States has increased since 2011, in conjunction with the increase in worldwide travel. An estimated 2,000 cases of malaria occur annually in the United States. All travelers to malaria-endemic regions should be prescribed prophylaxis. Malaria has a broad range of clinical presentations. Travelers who have symptoms of malaria should seek medical attention as soon as possible. All febrile travelers who have recently returned from a malarious area should be evaluated for malaria. The accurate, timely, and species-specific diagnosis of malaria is essential for successful treatment. Direct microscopy of Giemsa-stained blood smears is the reference standard for laboratory diagnosis. Rapid testing for malaria has emerged as an important adjunctive diagnostic modality. Malaria treatment is determined by individual patient factors and geography. The World Health Organization recommends treating uncomplicated cases of malaria with artemisinin combination therapy. [corrected] Severe malaria is mainly caused by Plasmodium falciparum . Children, pregnant patients, and people who are not from endemic regions are at highest risk of severe malaria. Intravenous artesunate is the treatment of choice for severe malaria.
Malaria has infected humans since the beginning of recorded history. 1 Some estimates place its total mortality burden at one-half of all people who have ever lived. 2 Each year, the disease continues to cause an estimated 500,000 deaths worldwide. 2 Most of these deaths occur in Africa and disproportionally affect children younger than five years worldwide. 3
Human malarial disease is caused by protozoan parasites of the genus Plasmodium , which has five known species: P. falciparum , P. vivax , P. ovale , P. malariae , and the emerging zoonotic parasite P. knowlesi . Most deaths are caused by P. falciparum . 4 The primary means of human infection is through the bite of a female Anopheles mosquito.
Malaria poses a threat to one-half of the world's population. 5 The incidence of malaria in the United States has continued to increase annually since 2011, in conjunction with the increase in worldwide travel. 6 Malaria, formerly endemic to the United States, was successfully eradicated in the country during the mid-20th century. 7 In the United States today, malaria is almost exclusively found in travelers to and immigrants from endemic regions of the world. 7 However, transmission can rarely occur via other means, such as exposure to infected blood products, congenital transmission, or local mosquito-borne outbreaks. 8 In the United States, an estimated 2,000 cases of malaria occur annually. 7
Before a patient travels internationally, the physician should conduct a personalized risk assessment, including travel location, the season of travel, and the proposed itinerary. The regions with the highest rates of malaria transmission are sub-Saharan Africa, the Indian subcontinent, and Southeast Asia. The risk of contracting malaria varies seasonally, with the highest risk occurring during and just after the rainy season, typically between May and December. 9
The primary method of malaria prevention is avoiding mosquito bites. Anopheles mosquitoes primarily feed at night; most malaria transmission occurs between dusk and dawn. Prevention strategies include personal protective measures such as using insecticide-treated bed nets, wearing clothes that minimize exposed skin, and applying mosquito-repelling chemicals. The most effective insect repellents contain 20% to 30% N , N -diethyl- m -toluamide (DEET) or 20% picaridin. 10 Higher concentrations are not associated with greater protection. Applying permethrin to clothing increases protection against penetrating insect bites. 11
All travelers to malaria-endemic regions should be prescribed prophylaxis. 9 The choice of agent should be based on location and duration of travel, malarial resistance patterns, and the patient's medical history ( Table 1 12 , 13 ) . All prevention regimens involve beginning the medication before departure, taking the medication while in the high-risk area, and continuing the medication for a defined period after travel has ended. The use of antimalarial agents does not negate the need for personal protective measures. The Centers for Disease Control and Prevention (CDC) provides country-specific prophylaxis recommendations at http://www.cdc.gov/malaria/travelers/country_table/a.html .
In 2021, the first malaria vaccine approved for widespread use was recommended by the World Health Organization for the prevention of P. falciparum malaria in children living in endemic areas. The vaccine has been administered to more than 1 million children in Ghana, Malawi, and Kenya. 14 , 15
Clinical Presentation
The clinical presentation of malaria ranges from asymptomatic parasitemia or uncomplicated disease to severe disease or death. The differential diagnosis of malaria is summarized in Table 2 . 16 Symptoms of malaria can develop within six to seven days of exposure, but the presentation may be delayed for several months after leaving an endemic region. 17 Symptomatic malaria is characterized by fevers, chills, headaches, myalgias, and malaise. It may also present as fever without a specific or obvious cause or as gastrointestinal symptoms in children. There are no typical features of malaria. 10 , 17
In the absence of a detailed travel history, malaria is often misdiagnosed as a nonspecific viral illness. 18 Travelers who have symptoms of malaria should seek medical attention as soon as possible, regardless of whether prophylaxis or preventive measures were used. All febrile travelers who have recently returned from a malarious area should be evaluated for malaria. 19 Suspicion of P. falciparum malaria is a medical emergency. Physicians should use only laboratory-based diagnostic methods. 18 Because most patients with malaria have no specific fever pattern, a pattern should not be considered in the diagnosis. 17 Clinical deterioration or death can occur within 24 to 36 hours in a malaria-naive patient. 7
Diagnostic Testing
The accurate, timely, and species-specific diagnosis of malaria is essential for successful treatment. Microscopic examination of Giemsa-stained blood smears is the reference standard for laboratory diagnosis. Thick blood smears are used to detect the presence of malarial parasites, and thin blood smears are used to determine the species and quantify parasitemia. 18 , 20 When malaria is suspected, urgent microscopy should be performed by an individual with expertise in examining blood smears and diagnosing malaria. 17 Multiple blood smears may be needed to produce a positive result. Three negative results, 12 hours apart, are needed to rule out malaria. 21
Rapid testing for malaria has emerged as an important adjunctive diagnostic modality. Rapid diagnostic tests have excellent sensitivity and negative predictive value with results available in five to 20 minutes. 22 , 23 Rapid diagnostic tests for malaria are simple to use, do not require laboratory facilities or diagnostic expertise, and enable prompt diagnosis. 24 However, rapid diagnostic tests can detect only P. falciparum and P. vivax , and they do not provide data regarding parasite density. 17 , 23 , 24 In the United States, rapid diagnostic tests for malaria should be used only in conjunction with thick and thin blood smears. 23 , 24 The usefulness of these rapid tests ends with diagnosis because further testing and monitoring must be completed via microscopy. 23 Binax-NOW is the only rapid diagnostic test approved by the U.S. Food and Drug Administration for malaria, 25 but a variety of other assays are available worldwide.
The CDC-recommended treatment of malaria is based on four variables: the clinical status of the patient (uncomplicated vs. severe disease), the species involved, the patient's history of prophylaxis, and the geographic region where the infection occurred. 25 Under certain circumstances, laboratory testing may not be readily available. If clinical suspicion for malaria is high, empiric treatment should be initiated promptly, especially in the setting of severe disease. Patients who used prophylaxis should be treated with different antimalarial medications than those used for prophylaxis. 13 , 25 , 26
Patients who are immunocompromised, patients with no previous malarial immunity, children, pregnant patients, and patients with signs of severe disease should be hospitalized. Severe disease is defined as the presence of at least one of the following: impaired consciousness (Glasgow Coma Scale score less than 11), convulsions, severe anemia (hemoglobin less than 7 g per dL [70 g per L] in adults or less than 5 g per dL [50 g per L] in children younger than 12 years), acute kidney injury, hypoglycemia, acute respiratory distress syndrome, shock, disseminated intravascular coagulation, acidosis, coma, liver dysfunction, or parasite density greater than 5%. 25
Hospitalized patients should receive standard supportive care, including intravenous fluids, antipyretics, and antiemetics. Outpatient treatment with close clinical follow-up can be considered in patients without an indication for hospitalization. Malaria specialists are available 24 hours a day, seven days a week to aid physicians with diagnosis and treatment ( Table 3 ) .
UNCOMPLICATED MALARIA
The World Health Organization recommends treating uncomplicated cases of malaria with artemisinin combination therapy (ACT), which comprises an artemisinin derivative and a partner drug. However, artemisinin should not be used in the first trimester of pregnancy with the exception of artemether/lumefantrine (Coartem), which is acceptable for all trimesters ( https://www.cdc.gov/malaria/new_info/2023/Coartem.html ). 26 [ corrected] ACTs are well tolerated and highly effective against all Plasmodium species. Patients should be informed that counterfeit and substandard antimalarials are widespread in resource-limited and lower-income countries.
Malaria Caused by Plasmodium falciparum or Unknown Species . If ACT is not available and the infection likely occurred in an area with chloroquine-sensitivity, chloroquine or hydroxychloroquine (Plaquenil) may be used. If ACT is unavailable and the infection occurred in an area with chloroquine resistance, atovaquone/proguanil (Malarone), a combination of quinine (Qualaquin) plus tetracycline, doxycycline, or clindamycin (Cleocin) should be used. Mefloquine is a treatment of last resort. Table 4 summarizes treatment options for acute uncomplicated malaria. 25 , 26
Malaria Caused by Plasmodium ovale or Plasmodium vivax. Initial treatment is the same as for uncomplicated malaria due to P. falciparum or unknown species, as described previously. In addition, patients infected with P. ovale or P. vivax require treatment against hypnozoites (dormant forms), which are responsible for relapsing infections. Patients should be tested for glucose-6-phosphate dehydrogenase (G6PD) deficiency because the drugs of choice, primaquine and tafenoquine, are associated with hemolytic anemia in people with G6PD deficiency. Tafenoquine should not be used in patients younger than 16 years or in patients with neuropsychiatric disorders. Tafenoquine is used only if chloroquine or hydroxychloroquine was used for the acute infection.
For people with G6PD deficiency who cannot tolerate primaquine or tafenoquine, chloroquine prophylaxis should be continued for one year. In those with intermediate G6PD deficiency, primaquine may be considered in close consultation with infectious disease or tropical medicine specialists. Table 5 summarizes antirelapse treatment options. 25 , 26
Malaria Caused by Plasmodium malariae or Plasmodium knowlesi. Although resistance to chloroquines is not widely documented with P. malariae or P. knowlesi , the World Health Organization recommends the use of ACT, regardless of geographic region of infection. 26 P. knowlesi is associated with severe disease, and patients should be hospitalized if this species is isolated. If ACT is not available and the infection is likely from a chloroquine-sensitive area, chloroquine or hydroxychloroquine may be used. 25 , 26
SEVERE MALARIA
P. falciparum and, to a lesser degree, P. knowlesi cause almost all cases of severe malaria. 26 Children, pregnant patients, and people who are not from endemic regions are at highest risk of severe malaria. Intravenous artesunate is the treatment of choice for severe disease and should be initiated as soon as possible ( Table 6 ) . 25 , 26 The dosage for adults and children is 2.4 mg per kg at 0, 12, and 24 hours. Blood smears should be obtained every 12 hours. If the parasite density is less than 1% at least four hours after the third dose, the patient should be transitioned to a full course of an oral medication, ideally ACT. If the parasite density is greater than 1% after the third artesunate dose, artesunate should be continued as a single daily dose until parasitemia is less than 1%, not to exceed seven days. Artesunate is well tolerated, and allergy to artemisinins is the only absolute contraindication. 25 , 26
If artesunate is not immediately available, the preferred oral medication for severe disease is artemether/lumefantrine (Coartem). Other options also include atovaquone/proguanil, quinine, and mefloquine. Tetracyclines and clindamycin should not be used because of their delayed onset of action. Once intravenous artesunate therapy becomes available, the oral medication should be discontinued.
The CDC no longer recommends the use of exchange transfusions as an adjunctive therapy for severe malaria. 25 All patients treated for severe malaria should be evaluated for hemolytic anemia within 30 days after completing treatment.
PREGNANT PATIENTS
Malaria is associated with significant morbidity and mortality in pregnant patients. ACTs may be used in the second and third trimesters except for artemether/lumefantrine, which may be used in the first trimester as well. [corrected] Chloroquine, hydroxychloroquine, and quinine with clindamycin or mefloquine may be used throughout pregnancy. Artemether/lumefantrine may be used in the first trimester if no other options are available. Primaquine should not be used during pregnancy. Tafenoquine should not be used in patients who are pregnant or breastfeeding.
Infants born to mothers who had P. vivax or P. ovale infection during pregnancy should be tested for G6PD deficiency. If results are normal, the mother should be treated with primaquine while breastfeeding. If G6PD deficiency is diagnosed, chloroquine should be used for one year after the initial treatment to prevent relapse. 25 , 26
This article updates previous articles on this topic by Johnson and Kalra , 8 Lo Re and Gluckman , 27 and Juckett . 28
Data Sources: PubMed was searched using the key words prevention, diagnosis, treatment, malaria, surveillance, travel medicine, chemoprophylaxis, and malaria treatment. The search was limited to English-language studies published since 2000. Secondary references from the key articles identified by the search were also used. Search dates: January 2018, October 2021, June 2022.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army at large.
Centers for Disease Control and Prevention. The history of malaria, an ancient disease. Last reviewed November 14, 2018. Accessed October 23, 2021. https://www.cdc.gov/malaria/about/history
Whitfield J. Portrait of a serial killer [published online October 3, 2002]. Nature . Accessed October 23, 2021. https://www.nature.com/articles/news021001-6
World Health Organization. World malaria report 2021. Accessed May 20, 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021
- Foster WA, Walker ED. Mosquitoes ( Culicidae ). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology . 3rd ed. Elsevier; 2019:261–325.
- Dye-Braumuller KC, Kanyangarara M. Malaria in the USA: how vulnerable are we to future outbreaks? Curr Trop Med Rep. 2021;8(1):43-51.
Cullen KA, Mace KE, Arguin PM Centers for Disease Control and Prevention (CDC). Malaria surveillance—United States, 2013. MMWR Surveill Summ. 2016;65(2):1-22.
Centers for Disease Control and Prevention. About malaria. Accessed October 23, 2021. https://www.cdc.gov/malaria/about/index.html
- Johnson BA, Kalra MG. Prevention of malaria in travelers [published correction appears in Am Fam Physician . 2012;86(3):222]. Am Fam Physician. 2012;85(10):973-977.
Briët OJ, Vounatsou P, Gunawardena DM, et al. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008;7:77.
Sanford C, McConnell A, Osborn J. The pretravel consultation. Am Fam Physician. 2016;94(8):620-627.
Banks SD, Murray N, Wilder-Smith A, et al. Insecticide-treated clothes for the control of vector-borne diseases: a review on effectiveness and safety. Med Vet Entomol. 2014;28(suppl 1):14-25.
Bazemore AW, Huntington M. The pretravel consultation. Am Fam Physician. 2009;80(6):583-590.
Centers for Disease Control and Prevention. Choosing a drug to prevent malaria. Last reviewed November 15, 2018. Accessed December 4, 2021. https://www.cdc.gov/malaria/travelers/drugs.html
World Health Organization. Malaria vaccine implementation programme. Accessed March 9, 2022. https://www.who.int/initiatives/malaria-vaccine-implementation-programme
Alonso PL, O'Brien KL. A malaria vaccine for Africa—an important step in a century-long quest. N Engl J Med. 2022;386(11):1005-1007.
Centers for Disease Control and Prevention. CDC Yellow Book 2020: Health Information for International Travel . Oxford University Press; 2019. October 23, 2021. https://wwwnc.cdc.gov/travel/page/yellowbook-home-2020
Lalloo DG, Shingadia D, Bell DJ, et al.; PHE Advisory Committee on Malaria Prevention in UK Travellers. UK malaria treatment guidelines 2016. J Infect. 2016;72(6):635-649.
Amir A, Cheong F-W, De Silva JR, et al. Diagnostic tools in childhood malaria. Parasit Vectors. 2018;11(1):53.
Plewes K, Leopold SJ, Kingston HWF, et al. Malaria: what's new in the management of malaria?. Infect Dis Clin North Am. 2019;33(1):39-60.
Feder HM, Mansilla-Rivera K. Fever in returning travelers: a case-based approach. Am Fam Physician. 2013;88(8):524-530.
Mbakilwa H, Manga C, Kibona S, et al. Quality of malaria microscopy in 12 district hospital laboratories in Tanzania. Pathog Glob Health. 2012;106(6):330-334.
World Health Organization. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 6 (2014–2015). Accessed March 9, 2022. https://apps.who.int/iris/bitstream/handle/10665/204118/9789241510035_eng.pdf
Enane LA, Sullivan KV, Spyridakis E, et al. Clinical impact of malaria rapid diagnostic testing at a US children's hospital. J Pediatric Infect Dis Soc. 2020;9(3):298-304.
Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in travel medicine. Clin Microbiol Infect. 2013;19(5):408-415.
Centers for Disease Control and Prevention. Treatment of malaria: guidelines for clinicians (United States). Last reviewed November 2, 2020. Accessed October 23, 2021. https://www.cdc.gov/malaria/diagnosis_treatment/clinicians1.html
World Health Organization. WHO guidelines for malaria. February 16, 2021. Accessed October 23, 2021. https://reliefweb.int/report/world/who-guidelines-malaria
- Lo Re V III, Gluckman SJ. Prevention of malaria in travelers. Am Fam Physician . 2003;68(3):509–514.
Juckett G. Malaria prevention in travelers. Am Fam Physician. 1999;59(9):2523-2530.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
Issues related to clinical manifestations and diagnosis of malaria will be reviewed here. Technical aspects of laboratory tools for diagnosis of malaria are discussed further separately.
The epidemiology, pathogenesis, diagnosis, and treatment of malaria are discussed separately:
● (See "Malaria: Epidemiology, prevention, and control" .)
● (See "Treatment of uncomplicated falciparum malaria in nonpregnant adults and children" .)
- Correspondence
- Open access
- Published: 07 March 2019
What causes severe malaria and its complications in children? Lessons learned over the past 15 years
- Andrea L. Conroy 1 ,
- Dibyadyuti Datta 1 &
- Chandy C. John 1
BMC Medicine volume 17 , Article number: 52 ( 2019 ) Cite this article
15k Accesses
26 Citations
8 Altmetric
Metrics details
Over the past 15 years, malaria mortality has reduced by approximately 50%. However, malaria still causes more than 400,000 deaths annually, most of which occur in African children under 5 years of age. Significant advances in understanding the pathogenesis of the disease provide a basis for future work to prevent severe malaria and its complications. Herein, we provide an overview of advances in our understanding of severe malaria in African children over the past 15 years, highlighting key complications and identifying priorities to further reduce malaria-associated mortality.
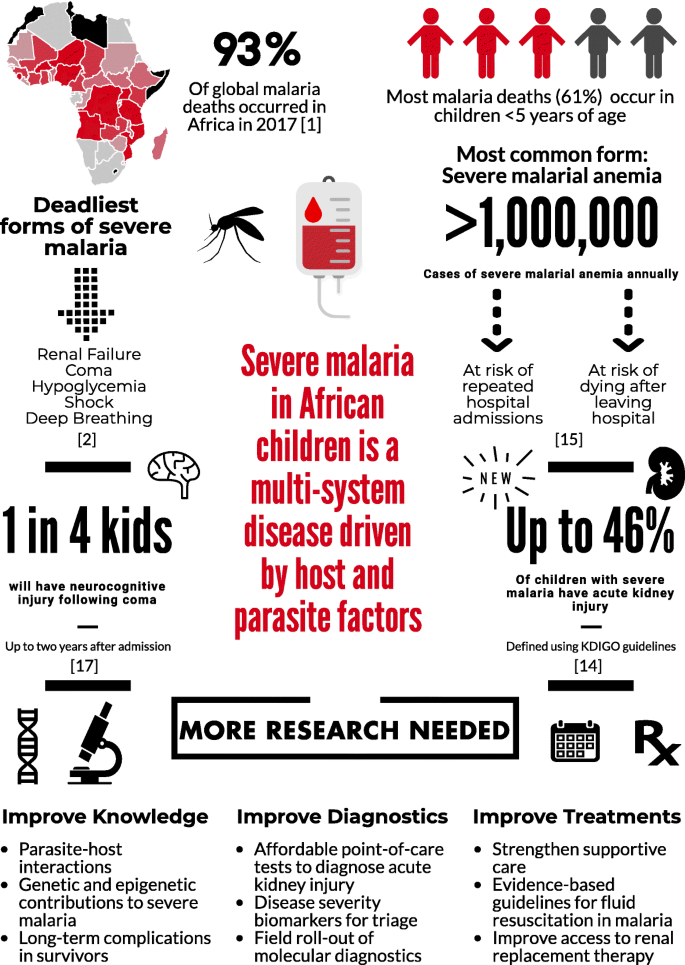
Plasmodium falciparum accounts for the majority of malaria deaths, and is the predominant malaria species in Africa (Fig. 1 ) [ 1 ]. Severe malaria (SM) is defined by the detection of P. falciparum by microscopy or a rapid diagnostic test and at least one criterion for severe disease (impaired consciousness, respiratory distress, multiple convulsions, prostration, shock, pulmonary edema, abnormal bleeding, jaundice, severe anemia, hypoglycemia, acidosis, hyperlactatemia, renal impairment, or hyperparasitemia) [ 2 ]. Further, mortality can exceed 50% when multiple prognostic factors are present [ 3 ].
Severe malaria and its complications
SM is a multi-system disease characterized by a systemic inflammatory response. A central feature in SM is the sequestration of parasitized red blood cells (pRBCs) in vascular beds, leading to impaired tissue perfusion and lactic acidosis. Parasite ligand P. falciparum erythrocyte membrane protein-1 (PfEMP1) is expressed on the pRBC membrane, where it binds to endothelial receptors (e.g., CD36, soluble ICAM-1). Recently, the discovery of endothelial protein C receptor (EPCR) as a novel and conserved host receptor for PfEMP1 binding transformed our understanding of SM pathogenesis (reviewed by Bernabeu and Smith [ 4 ]), providing a link between endothelial activation, inflammation, coagulation, and genetic susceptibility to SM driven by host and parasite genetics.
Endothelial activation is common in SM and is associated with upregulated cellular adhesion molecules on endothelium and their concomitant increase in plasma through ectodomain cleavage [ 5 ]. The angiopoietin-Tie2 signaling pathway has an important association with disease severity and mortality in pediatric SM [ 6 ], and has been implicated in blood–brain barrier breakdown and death in experimental cerebral malaria (CM) [ 7 ].
Diverse forms of SM
SM is a multi-system disease driven by both host and parasite factors. CM is the deadliest form of SM. Children with CM often have malaria retinopathy, presenting with hemorrhages, retinal whitening, and vessel color changes, all of which can be visualized by trained observers using ophthalmoscopy. Interestingly, these changes mirror findings in the brain at autopsy [ 8 ]. Further, the development of radiologic capacity in low-resource settings has led to advances in our understanding of CM, with studies in Malawi showing that cerebral edema predicts mortality in CM [ 9 ] and is associated with EPCR-binding parasites [ 10 ].
Respiratory distress is a common form of SM best characterized by deep acidotic breathing. Like other forms of SM, respiratory distress is multifactorial. Impaired tissue perfusion secondary to pRBC sequestration and reduced oxygen-carrying capacity in severe anemia contribute to acidosis [ 11 ]. It is estimated that lactic acid contributes to half the acid load in SM, with several other organic acids being elevated in SM [ 12 ]. The kidney is important in acid metabolism and excretion, and may be involved in acidosis in SM. Additionally, respiratory distress and acute kidney injury (AKI) are linked by oxidative stress from the destruction of pRBCs and the release of free heme [ 13 ].
Fifteen years ago, renal failure was considered a rare complication in children with SM, yet it is now recognized as one of the strongest predictors of mortality in SM [ 2 ]. The recognition that small changes in kidney function independently predict mortality in critical illness led to the development of new guidelines to define AKI (Kidney Disease: Improving Global Outcomes, KDIGO) [ 14 ]. In a prospective cohort of Ugandan children with SM, AKI was common, occurring in 46% of young children with SM [ 15 ]. Although data suggest AKI is related to reduced kidney perfusion, additional studies are needed to evaluate the spectrum of AKI over hospitalization to define the etiology and pathophysiology of AKI in pediatric SM.
Severe malarial anemia (SMA) is the most common form of SM. The etiology of SMA is complex, involving increased destruction and removal of infected and uninfected RBCs, and reduced RBC production due to bone marrow dyserythropoiesis (recently reviewed by White [ 16 ]). SMA can occur in the absence of other SM complications in children with repeated or inadequately treated infections, and mortality is low with appropriate transfusion [ 11 ]. However, SMA is not benign – it contributes to significant long-term morbidity, including impaired neurocognitive functioning [ 17 ], repeated hospitalizations, and post-discharge mortality [ 16 ].
Long-term complications associated with SM
One in four children prospectively enrolled in studies with CM develop neurocognitive impairments that persist at least 2 years following exposure [ 18 ]. Retrospective studies suggest impairments may last 8 years or longer, and may include behavioral problems, mental health issues, and the development of epilepsy (reviewed by Idro et al. [ 19 ]). Children with SMA have long-term complications related to cognition [ 17 ]; therefore, given the huge burden of SMA, it may be a significant contributor to neurocognitive impairment in African children. Nevertheless, the mechanisms leading to brain injury and subsequent neurocognitive complications due to SM are not well understood. In particular, questions remain about how an intravascular parasite can lead to such a breadth of complications, and why these complications are only observed in a fraction of the population with SM [ 19 ].
Elevated cerebrospinal fluid (CSF) levels of TNF-α are associated with prolonged coma duration, neurologic deficits, and long-term cognitive deficits in children over 5 years [ 20 ]. A strong correlation of the CSF-to-plasma TNF-α ratio and CSF-to-plasma albumin index (an indicator of blood–brain barrier impairment) suggest a degree of permeability across the blood–brain barrier [ 20 ]. Metabolites of the kynurenine pathway are markedly elevated in the CSF of children with CM and have been associated with prolonged coma and impaired attention in children over 5 years [ 19 , 21 ]. Axonal injury marker tau is elevated in CSF of children with CM and is associated with acute neurologic deficits (reviewed by Idro et al. [ 19 ]). Our studies in Ugandan children with CM suggest elevated CSF-tau is associated with prolonged coma duration and long-term cognitive impairment, particularly in children over 5 years, and may be mediated in part by blood–brain barrier impairment. Additional studies are needed to delineate the mechanisms leading to neurocognitive complications in SM, particularly in children without overt clinical signs suggestive of brain injury.
Conclusions
Figure 1 outlines the burden of SM and the ways in which this burden could be lessened in the future. Prevention of SM and its complications will require better implementation of known preventive measures, including primary measures to prevent infection (e.g., insecticide-treated bed-nets) and secondary measures to prevent severe disease such as rapid access to care, use of appropriate malaria diagnostics, and effective treatment of uncomplicated malaria. Increased knowledge of clinical prognostic signs and implementation of point-of-care tools to identify children at risk of clinical deterioration or death could facilitate directed use of intrarectal artesunate in primary health centers prior to referral and transport to tertiary centers. Better knowledge on pRBC and endothelial cell interactions – in particular ICAM-1 and EPCR-binding parasites – may lead to novel vaccine targets. Further, research on the etiology and pathogenesis of AKI in SM, an important complication that remains poorly understood, is critical. Point-of-care tools to facilitate prompt recognition of AKI, development of resource-appropriate kidney care guidelines, and early referral to higher levels of care could reduce the long-term impact of AKI on children’s health. Finally, a better understanding of the pathogenesis of neurodevelopmental complications, as well as the long-term health costs of these complications, may lead to interventions to reduce neurodevelopmental disability in survivors. In the long run, investment in both primary malaria prevention and better management of SM will lead to substantial health benefits for children in sub-Saharan Africa.
World Health Organization. World Malaria Report 2018. Geneva: WHO; 2018.
Book Google Scholar
Sypniewska P, Duda JF, Locatelli I, Althaus CR, Althaus F, Genton B. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med. 2017;15:147.
Article Google Scholar
von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, Nguah SB, Bojang K, Deen JL, Evans J, et al. Predicting the clinical outcome of severe falciparum malaria in african children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8):1080–90.
Bernabeu M, Smith JD. EPCR and malaria severity: the center of a perfect storm. Trends Parasitol. 2017;33(4):295–308.
Turner GD, Ly VC, Nguyen TH, Tran TH, Nguyen HP, Bethell D, Wyllie S, Louwrier K, Fox SB, Gatter KC, et al. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152(6):1477–87.
CAS PubMed PubMed Central Google Scholar
Conroy AL, Glover SJ, Hawkes M, Erdman LK, Seydel KB, Taylor TE, Molyneux ME, Kain KC. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study*. Crit Care Med. 2012;40(3):952–9.
Article CAS Google Scholar
Higgins SJ, Purcell LA, Silver KL, Tran V, Crowley V, Hawkes M, Conroy AL, Opoka RO, Hay JG, Quaggin SE, et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci Transl Med. 2016;8(358):358ra128.
Barrera V, Hiscott PS, Craig AG, White VA, Milner DA, Beare NAV, MacCormick IJC, Kamiza S, Taylor TE, Molyneux ME, et al. Severity of retinopathy parallels the degree of parasite sequestration in the eyes and brains of Malawian children with fatal cerebral malaria. J Infect Dis. 2015;211(12):1977–86.
Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372(12):1126–37.
Kessler A, Dankwa S, Bernabeu M, Harawa V, Danziger SA, Duffy F, Kampondeni SD, Potchen MJ, Dambrauskas N, Vigdorovich V, et al. Linking EPCR-binding PfEMP1 to brain swelling in pediatric cerebral malaria. Cell Host Microbe. 2017;22(5):601–14.e605.
Brand NR, Opoka RO, Hamre KES, John CC. Differing causes of lactic acidosis and deep breathing in cerebral malaria and severe malarial anemia may explain differences in acidosis-related mortality. PLoS One. 2016;11(9):e0163728.
Sriboonvorakul N, Ghose A, Hassan MMU, Hossain MA, Faiz MA, Pukrittayakamee S, Chotivanich K, Sukthana Y, Leopold SJ, Plewes K, et al. Acidosis and acute kidney injury in severe malaria. Malaria J. 2018;17:128.
Elphinstone RE, Conroy AL, Hawkes M, Hermann L, Namasopo S, Warren HS, John CC, Liles WC, Kain KC. Alterations in systemic extracellular heme and hemopexin are associated with adverse clinical outcomes in Ugandan children with severe malaria. J Infect Dis. 2016;214(8):1268–75.
KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(Suppl):1–138.
Google Scholar
Conroy AL, Hawkes M, Elphinstone RE, Morgan C, Hermann L, Barker KR, Namasopo S, Opoka RO, John CC, Liles WC, et al. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis. 2016;3(2):ofw046.
White NJ. Anaemia and malaria. Malaria J. 2018;17:371.
Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, Shapiro E, John CC. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis. 2014;59(3):336–44.
John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92–9.
Idro R, Marsh K, John CC, Newton CRJ. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68(4):267–74.
Shabani E, Ouma BJ, Idro R, Bangirana P, Opoka RO, Park GS, Conroy AL, John CC. Elevated cerebrospinal fluid tumor necrosis factor is associated with acute and long-term neurocognitive impairment in cerebral malaria. Parasite Immunol. 2017;39(7):e12438.
Holmberg D, Franzén-Röhl E, Idro R, Opoka RO, Bangirana P, Sellgren CM, Wickström R, Färnert A, Schwieler L, Engberg G, et al. Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malaria J. 2017;16:303.
Download references
Author information
Authors and affiliations.
Ryan White Center for Pediatric Infectious Diseases and Global Health, Indiana University School of Medicine, 1044 W Walnut St R4 402D, Indianapolis, IN, USA
Andrea L. Conroy, Dibyadyuti Datta & Chandy C. John
You can also search for this author in PubMed Google Scholar
Contributions
AC, DD, and CJ reviewed the literature and contributed to writing the manuscript. AC wrote the first draft of the manuscript and created the figure. AC, DD, and CJ contributed to editing and revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Correspondence to Chandy C. John .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Conroy, A.L., Datta, D. & John, C.C. What causes severe malaria and its complications in children? Lessons learned over the past 15 years. BMC Med 17 , 52 (2019). https://doi.org/10.1186/s12916-019-1291-z
Download citation
Received : 14 February 2019
Accepted : 14 February 2019
Published : 07 March 2019
DOI : https://doi.org/10.1186/s12916-019-1291-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe malaria
- Cerebral malaria
- Severe malarial anemia
- Pathogenesis
- Plasmodium falciparum
- Acute kidney injury
- Neurodevelopmental
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Treatment and prevention of malaria in children
Affiliations.
- 1 Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit, Vientiane, Laos; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 2 Timika Research Facility, Papuan Health and Community Development Foundation, Timika, Indonesia; Department of Child Health, Faculty of Medicine, Public Health and Nursing, Gadjah Mada University, Yogyakarta, Indonesia.
- PMID: 32946831
- DOI: 10.1016/S2352-4642(20)30127-9
Malaria disproportionately affects children younger than 5 years. Falciparum malaria is responsible for more than 200 000 child deaths per year in Africa and vivax malaria is well documented as a cause of severe anaemia and excess mortality in children in Asia and Oceania. For the treatment of malaria in children, paediatric dosing recommendations for several agents, including parenteral artesunate and dihydroartemisinin-piperaquine, have belatedly been shown to be suboptimal. Worsening antimalarial resistance in Plasmodium falciparum in the Greater Mekong Subregion threatens to undermine global efforts to control malaria. Triple antimalarial combination therapies are being evaluated to try to impede this threat. The RTS,S/AS01 vaccine gives partial protection against falciparum malaria and is being evaluated in large, pilot studies in Ghana, Malawi, and Kenya as a complementary tool to other preventive measures. Seasonal malaria chemoprevention in west Africa has resulted in declines in malaria incidence and deaths and there is interest in scaling up efforts by expanding the age range of eligible recipients. Preventing relapse in Plasmodium vivax infection with primaquine is challenging because treating children who have G6PD deficiency with primaquine can cause acute haemolytic anaemia. The safety of escalating dose regimens for primaquine is being studied to mitigate this risk.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Antimalarials / therapeutic use*
- Artemisinins / therapeutic use*
- Child Welfare / statistics & numerical data*
- Dose-Response Relationship, Drug
- Drug Resistance, Multiple
- Malaria / drug therapy*
- Malaria / epidemiology
- Malaria / prevention & control*
- Malaria Vaccines / therapeutic use*
- Vaccination / statistics & numerical data
- Antimalarials
- Artemisinins
- Malaria Vaccines
- artemisinin
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 5 - Leishmaniasis, Visceral
- Section 5 - Onchocerciasis / River Blindness
CDC Yellow Book 2024
Author(s): Kathrine Tan, Francisca Abanyie
Infectious Agent
Transmission, epidemiology, clinical presentation.
INFECTIOUS AGENT: Plasmodium spp.
Multiple countries in Africa, the Americas, and Asia
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Avoid insect bites
Use malaria chemoprophylaxis
DIAGNOSTIC SUPPORT
- 770-488-7788 (M–F 9 a.m.–5 p.m. Eastern)
- 770-488-7100 (after hours)
Parasitological diagnosis: DPDx
Malaria in humans is caused by protozoan parasites of the genus Plasmodium , including Plasmodium falciparum , P. malariae , P. ovale , and P. vivax . In addition, zoonotic forms have been documented as causes of human infections and some deaths, especially P. knowlesi , a parasite of Old World (Eastern Hemisphere) monkeys, in Southeast Asia.
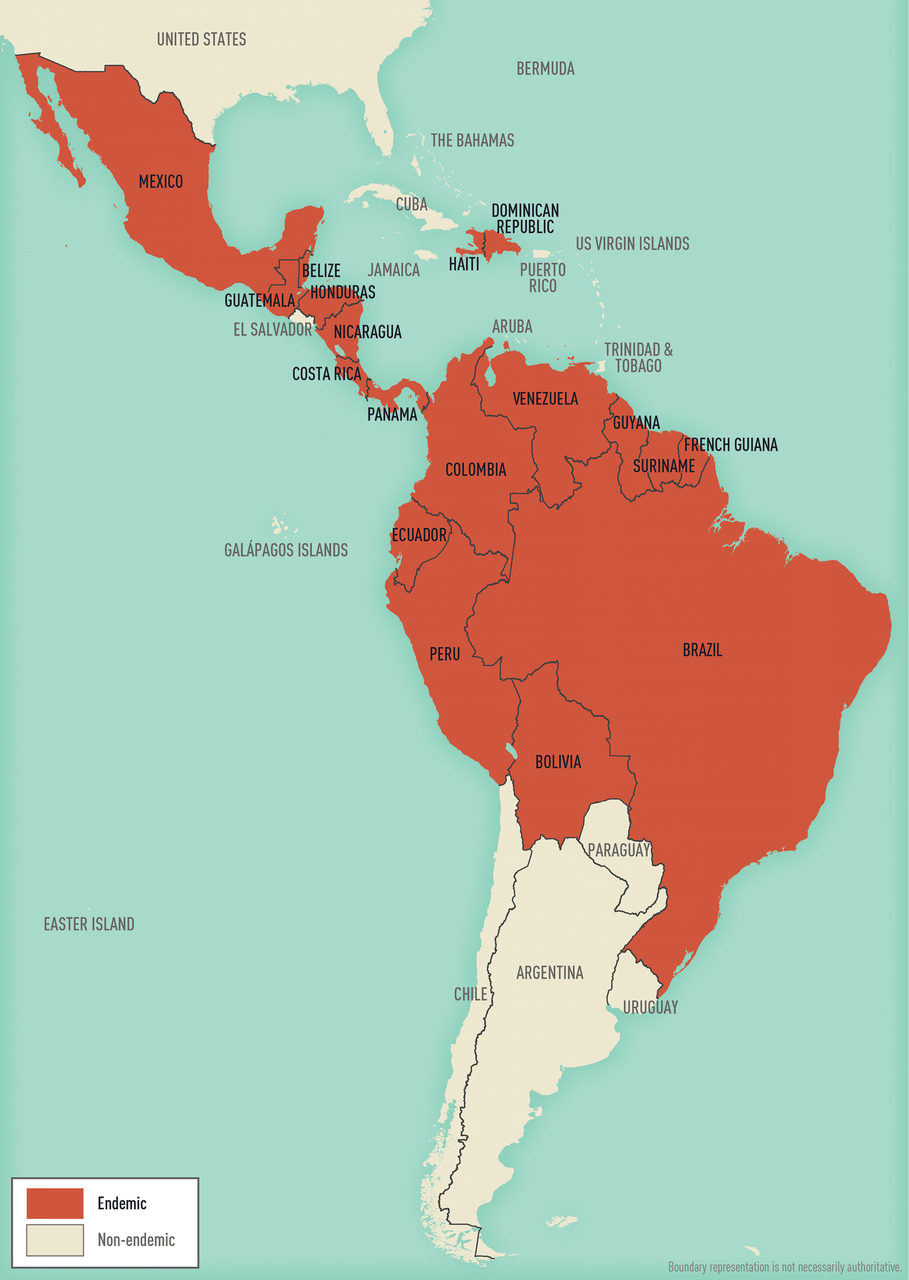
Plasmodium species are transmitted by the bite of an infective female Anopheles mosquito. Occasionally, transmission occurs by blood transfusion, needle sharing, nosocomially, organ transplantation, or vertically from mother to fetus. Malaria transmission occurs in large areas of Africa, Latin America, and parts of the Caribbean, Eastern Europe, the South Pacific, and in Asia including South Asia, Southeast Asia, and the Middle East ( Map 5-12 , Map 5-13 , and Map 5-14 ).
Map 5-12 Malaria-endemic destinations in the Americas & the Caribbean
View Larger Figure
Malaria-endemic destinations are labeled using black font; destinations not endemic for malaria are labeled using gray font. Countries with areas endemic for malaria are shaded completely even if transmission occurs only in a small part of the country. For more specific within-country malaria transmission information, see Section 2, Yellow Fever Vaccine & Malaria Prevention Information, by Country .
Map 5-13 Malaria-endemic destinations in Africa & the Middle East
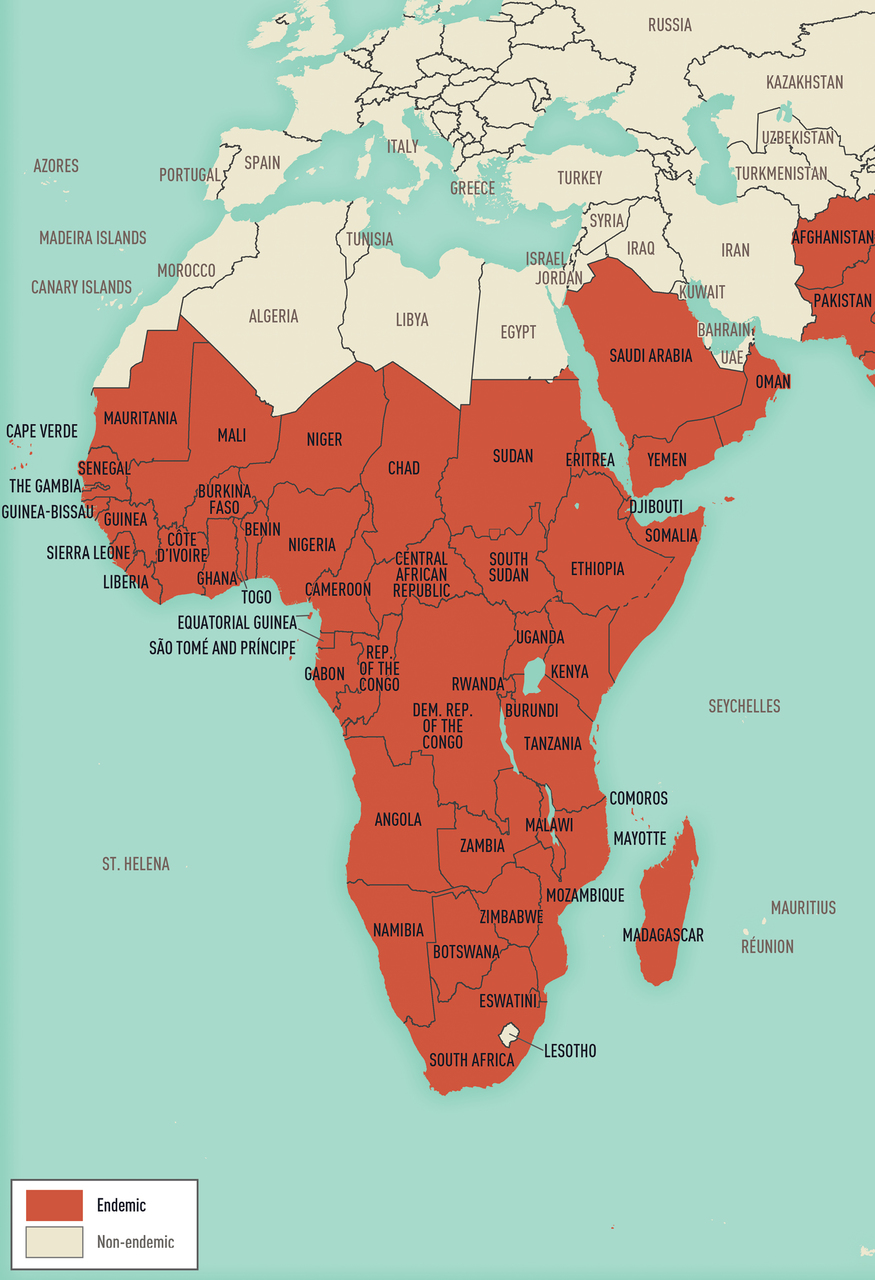
Map 5-14 Malaria-endemic destinations in Asia & Oceania
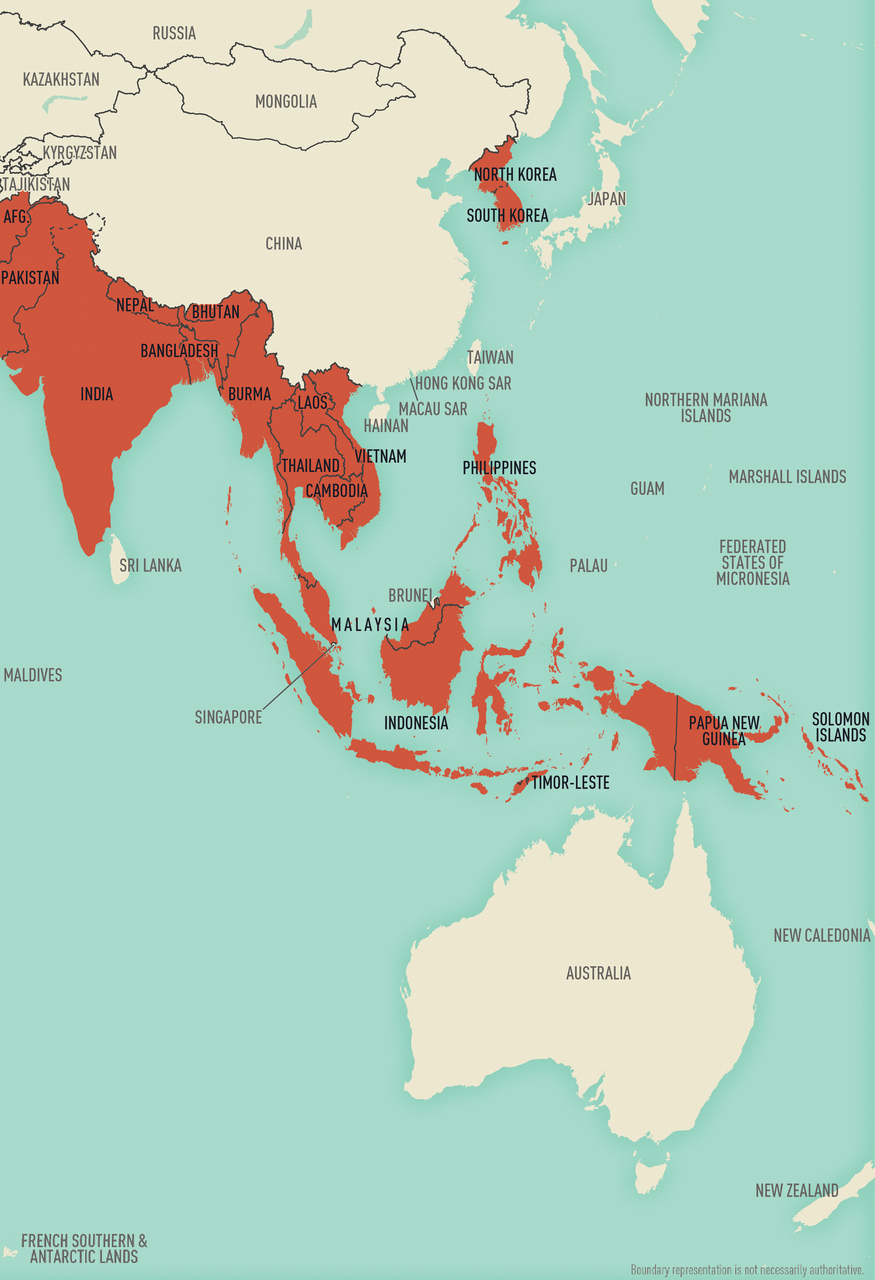
Malaria is a major international public health problem. According to the World Health Organization (WHO) World Malaria Report 2019, >90 countries reported ≈228 million infections and ≈405,000 deaths in 2018. Travelers going to malaria-endemic countries are at risk of contracting the disease, and almost all the ≈2,000 cases of malaria that occur each year in the United States are imported.
The risk of acquiring malaria differs substantially from traveler to traveler and from region to region, even within a single country. This variability is a function of the intensity of transmission within the various regions and the itinerary, duration, season, and type of travel. Risk also varies by travelers’ adherence to mosquito precautions and prophylaxis recommendations. In 2016, 2,078 cases of malaria (including 7 deaths) were diagnosed in the United States and its territories and were reported to the Centers for Disease Control and Prevention (CDC). Of cases for which country of acquisition was known, 85% were acquired in Africa, 9% in Asia, 5% in the Caribbean and the Americas, and 1% in Oceania or the Eastern Mediterranean. Of US residents with malaria who reported a reason for travel, 69% were visiting friends and relatives.
Information about malaria transmission in specific countries is derived from various sources, including WHO (see Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country ). The information presented here was accurate at the time of publication; the risk for malaria can change rapidly and from year to year, however, because of changes in local weather conditions, mosquito vector density, and prevalence of infection. See updated information CDC website.
Malaria is characterized by fever and influenza- like symptoms, including chills, headache, myalgias, and malaise; symptoms can occur intermittently. In severe disease, acute kidney injury, acute respiratory distress syndrome, mental confusion, seizures, coma, and death can occur. Malaria symptoms can develop as early as 7 days after being bitten by an infectious mosquito in a malaria-endemic area and as late as several months or more after exposure. Suspected or confirmed malaria, especially P. falciparum , is a medical emergency requiring urgent intervention, because clinical deterioration can occur rapidly and unpredictably. See Box 5-10 for frequently asked clinical questions.
Box 5-10 Frequently asked clinical questions
How do i address concerns about side effects from prophylaxis.
- Prophylaxis can be started earlier if the traveler has concerns about tolerating a particular medication. For example, mefloquine can be started 3–4 weeks in advance to allow potential adverse events to occur before travel. If unacceptable side effects develop, the clinician has time to change the medication before the traveler’s departure.
- The drugs used for antimalarial prophylaxis are generally well tolerated. Side effects can occur, however. Minor side effects usually do not require stopping the drug. Clinicians should determine if symptoms are related to the medicine and make a medication change if needed.
WHAT SHOULD A TRAVELER DO IF THEY MISS A DOSE OF PROPHYLAXIS?
- Compared with drugs with short half-lives, which are taken daily, drugs with longer half-lives, which are taken weekly, offer the advantage of a wider margin of error if the traveler is late with a dose.
- For a weekly drug, prophylactic blood levels can remain adequate if the dose is only 1–2 days late. If this is the case, the traveler can take a dose as soon as possible, then resume weekly doses on the originally scheduled day. If the traveler is >2 days late, blood levels might not be adequate. The traveler should take a dose as soon as possible. The weekly doses should resume at this new day of the week (the next dose is 1 week later, then weekly thereafter).
- For a daily drug, if the traveler is 1–2 days late, protective blood levels are less likely to be maintained. The traveler should take a dose as soon as possible and resume the daily schedule at the new time of day.
WHAT HAPPENS IF TOO HIGH A DOSE OF PROPHYLAXIS IS TAKEN?
- Overdose of antimalarial drugs, particularly chloroquine, can be fatal. Medications should be stored in childproof containers out of reach of infants and children.
ISN’T MALARIA A TREATABLE DISEASE? WHY NOT CARRY A TREATMENT DOSE OF ANTIMALARIALS INSTEAD OF TAKING MALARIA PROPHYLAXIS?
- Malaria could be fatal even when treated, which is why prevention is always preferable to treating infections after they occur.
WHAT SHOULD BE DONE IF FEVER DEVELOPS WHILE TRAVELING IN A MALARIA-ENDEMIC AREA?
- Malaria and other potentially life-threatening infections acquired during travel could be fatal if treatment is delayed. Travelers should promptly seek medical help and continue to take malaria prophylaxis while in the malaria-endemic area.
WHAT SHOULD BE DONE IF A TRAVELER WHO TOOK MALARIA PROPHYLAXIS DEVELOPS FEVER AFTER RETURNING FROM THEIR TRIP?
- Malaria prophylaxis, while highly effective, is not 100% effective. Travelers should be advised to seek medical care immediately if fever develops, report their travel history, get tested for malaria, and get treated promptly if infection is confirmed.
- Malaria smear or a rapid diagnostic test must be performed, and results obtained immediately (within a few hours). These tests should not be sent out to reference laboratories that take days to weeks to return results. Empiric treatment with antimalarial drugs is not recommended because the malaria smear provides critical information for appropriate treatment. If a patient has an illness suggestive of severe malaria and a compatible travel history in an area where malaria transmission occurs, and malaria testing is not immediately available, start treatment as soon as possible, even before the diagnosis is established. See CDC recommendations for malaria treatment .
Travelers with symptoms of malaria should seek medical evaluation as soon as possible, even if still traveling. Consider malaria in any patient with a febrile illness who has recently returned from a malaria-endemic country. Diagnostic assistance is available from state public health laboratories or CDC. The CDC malaria laboratory can assist in speciating malaria by blood smear microscopy, or confirm species by PCR testing. The CDC laboratory also can assess malaria parasites for mutations that confer resistance to medications. Serologic testing , used in certain situations (e.g., case investigations), can also be done by CDC laboratories.
In the United States, malaria is a notifiable disease. Health care providers must report cases of malaria diagnosed via microscopy or PCR in the United States and its territories to local or state health departments. See more information on reporting malaria .

Blood Smear Microscopy
Blood smear microscopy remains the most important method for malaria diagnosis. Microscopy can provide immediate information about the presence of parasites, allow quantification of the density of the infection, and allow determination of the species of the malaria parasite—all of which are necessary for providing the most appropriate treatment. Tests should be performed immediately when ordered by a health care provider, and microscopy results should be available as soon as possible, ≤24 hours of the patient’s presentation. Assistance with speciation of malaria on smears is available from state health departments or CDC.
In resource-limited settings, and particularly in sub-Saharan Africa, overdiagnosis and the rate of false-positive microscopy for malaria can be high; warn travelers that a local diagnosis of malaria could be incorrect. In such cases, acutely ill travelers should seek the best available medical services and continue their prophylaxis regimen until they have a definitive diagnosis.
Rapid Diagnostic Testing
Rapid diagnostic tests (RDTs) for malaria detect antigens derived from malaria parasites. Malaria RDTs are immunochromatographic tests that most often use a dipstick or cassette format and provide results in 2–15 minutes. RDTs offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not immediately available. Although RDTs can detect malaria antigens within minutes, they have several limitations. RDTs cannot distinguish between all Plasmodium species that affect humans, they might be less sensitive than expert microscopy or PCR for diagnosis, they cannot quantify parasitemia, and an RDT-positive test result might persist for days or weeks after an infection has been treated and cleared. Thus, RDTs are not useful for assessing response to therapy. Furthermore, in some areas, mutations are increasingly being observed in malaria parasites, resulting in an absence of the malaria antigen usually detected by many RDTs, including the only RDT used in the United States. The absence of this parasite antigen in peripheral blood can lead to false-negative RDT test results.
Both positive and negative RDT results must always be confirmed by microscopy. Microscopy confirmation of the RDT result should occur as soon as possible, because the information on the presence, density, and parasite species is critical for optimal management of malaria. The US Food and Drug Administration (FDA) has approved an RDT (the BinaxNOW Malaria test) for hospital and commercial laboratory use; the test is not approved for use by clinicians or patients. Laboratories that do not provide in-house, on-the-spot microscopy services should maintain a stock of malaria RDTs so that they will be able to perform immediate malaria diagnostic testing when needed.
PCR Testing
PCR tests also are available to detect malaria parasites. These tests are more sensitive than routine microscopy, but results are not usually available as quickly as microscopy results, thus limiting the utility of PCR for acute diagnosis and initial clinical management. Use of PCR testing is encouraged to confirm the species of malaria parasite and detect mixed infections.
Malaria can be treated effectively if treatment begins early in the disease; delaying therapy, however, can have serious or even fatal consequences. Specific treatment options depend on the species of malaria, the severity of infection, the likelihood of drug resistance (based on where the infection was acquired), the patient’s age, and whether the patient is pregnant or breastfeeding.
See detailed CDC recommendations for malaria treatment . For assistance with the diagnosis or treatment of malaria, call the CDC Malaria Hotline (770-488-7788 or toll-free at 855-856-4713) from 9 a.m. to 5 p.m. Eastern Time. After hours, on weekends, or on holidays, call the CDC Emergency Operations Center at 770-488-7100 and ask the operator to contact the subject matter expert on call for the Malaria Branch. In addition, consult a clinician specializing in travel or tropical medicine or infectious diseases.
Travelers who decline to take prophylaxis, who choose a suboptimal drug regimen (e.g., chloroquine in an area with chloroquine-resistant P. falciparum ), or who require a less-than-optimal drug regimen for medical reasons are at increased risk for acquiring malaria and then needing prompt treatment while abroad. Medications not used in the United States to treat malaria (e.g., halofantrine, sulfadoxine-pyrimethamine) are widely available abroad. CDC does not recommend halofantrine for treatment because of documented adverse cardiac events, including deaths. These adverse events have occurred in people with and without preexisting cardiac problems, and both in the presence and absence of other antimalarial drugs. Sulfadoxine-pyrimethamine is not recommended because of widespread drug-resistant Plasmodium .
Reliable Supply of Malaria Treatment
Some travelers who take effective prophylaxis but who will be in remote areas might decide, in consultation with their travel health provider, to also carry a reliable supply of a full course of an approved malaria treatment regimen. In the event a traveler carrying a reliable supply is diagnosed with malaria, they will have immediate access to an approved treatment.
CDC recommends that the reliable supply be acquired in the United States, so clinicians can consider the traveler’s other medical conditions or medications when selecting an antimalarial drug and to avoid the possibility of travelers obtaining counterfeit drugs in the local pharmacy or market, or depleting local resources. In rare instances when access to medical care is not available and the traveler develops a febrile illness consistent with malaria, the reliable supply medication can be self-administered presumptively. Advise travelers that self-treatment of a possible malarial infection is only a temporary measure, and that prompt medical evaluation is imperative.
Two malaria treatment regimens available in the United States can be prescribed as a reliable supply for self-treatment: atovaquone-proguanil and artemether-lumefantrine. To treat malaria, CDC recommends against using the same (or related) drug that has been taken for prophylaxis. For example, atovaquone-proguanil can be used as a reliable supply medication by travelers who are not taking atovaquone-proguanil for prophylaxis. See Table 5-26 for dosing recommendations.
Table 5-26 Reliable supply regimens for malaria treatment 1
PEDIATRIC DOSE
ATOVAQUONE-PROGUANIL 2 Adult tablets:
- Atovaquone 250 mg
- Proguanil 100 mg
Pediatric tablets:
- Atovaquone 62.5 mg
- Proguanil 25 mg
4 adult tablets taken orally (as a single daily dose) for 3 consecutive days
Weight-based daily dose taken orally (as a single daily dose) for 3 consecutive days
5–8 kg: 2 pediatric tablets 9–10 kg: 3 pediatric tablets 11–20 kg: 1 adult tablet 21–30 kg: 2 adult tablets 31–40 kg: 3 adult tablets >41 kg: 4 adult tablets
Contraindicated in people with severe renal impairment (creatinine clearance <30 mL/min).
Not recommended for people taking atovaquone-proguanil prophylaxis.
Not recommended for children weighing <5 kg, or people who are pregnant or breastfeeding infants weighing <5 kg.
ARTEMETHER-LUMEFANTRINE 2 One tablet
- Artemether 20 mg
- Lumefantrine 120 mg
ADULT & PEDIATRIC DOSE
Weight-based treatment schedule for both adult and pediatric patients. Patients should take an initial dose, followed by a second dose 8 hours later, then 1 dose twice a day for the next 2 days (total of 6 oral doses over 3 days).
5 kg to <15 kg: 1 tablet per dose 15 kg to <25 kg: 2 tablets per dose 25 kg to <35 kg: 3 tablets per dose ≥35 kg: 4 tablets per dose
Not recommended for people taking mefloquine prophylaxis.
Not recommended for children weighing <5 kg, or people breastfeeding infants weighing <5 kg.
1 A reliable supply is a complete course of an approved malaria treatment regimen obtained in the United States before travel. A reliable supply is not counterfeit or substandard; will not interact adversely with the patient’s other medicines, including malaria chemoprophylaxis; will not deplete local resources in the destination country.
2 If used for presumptive self-treatment, patients should seek medical care as soon as possible.
Malaria prevention consists of a combination of mosquito avoidance measures and chemoprophylaxis. Prevention measures must address all malaria species in the travel area and apply to both short-term and long-term travelers. Although highly efficacious, interventions are not 100% effective, so all febrile persons returning from malaria-endemic areas should be tested for malaria even if they took chemoprophylaxis.
Preventing malaria involves striking a balance between effectiveness and safety: ensuring that all people at risk for infection use the recommended prevention measures, and preventing rare occurrences of adverse effects. Conduct an individual risk assessment for every traveler by collecting a detailed travel itinerary, including countries, specific areas to be visited in those countries (e.g., cities, rural areas, both), types of accommodation, season, and style of travel. Modify the risk assessment depending on traveler characteristics (e.g., pregnancy, underlying health conditions) and malaria characteristics at the destination (e.g., intensity of transmission, local parasite resistance to drugs). Depending on the level of risk, it might be appropriate to recommend no specific interventions, mosquito avoidance measures only, or mosquito avoidance measures plus chemoprophylaxis.
Several factors increase a traveler’s risk for malaria. Travel, even for short periods of time, to areas with intense malaria transmission can result in infection. Malaria transmission is not distributed homogeneously throughout a country, so review the exact itinerary to determine if travel will occur in highly endemic areas. In countries where malaria is seasonal, travel during peak transmission season also increases risk. Travelers going to rural areas or staying in accommodations without screens or air conditioning also will be at greater risk. The greatest risk for malaria is associated with first- and second-generation immigrants living in nonendemic countries who return to their countries of origin to visit friends and relatives (VFRs). VFR travelers might perceive themselves to be at no risk because they grew up in a malaria-endemic country and consider themselves immune to the disease. Tolerance acquired through continuous exposure to malaria is quickly lost, however; consider VFRs to have the same risk as other nonimmune travelers (see Sec. 9, Ch. 9, Visiting Friends & Relatives: VFR Travel ). Also remind travelers that they could become infected even if they had malaria before, and they still need to take preventive measures.
Mosquito Avoidance Measures
Because of the nocturnal feeding habits of Anopheles mosquitoes, malaria transmission occurs primarily between dusk and dawn. Travelers can reduce contact with mosquitoes by remaining in enclosed air-conditioned rooms or well-screened areas, sleeping under mosquito nets (preferably insecticide-treated), using an effective insecticide spray or mosquito coils in living and sleeping areas during evening and nighttime hours, and wearing clothes that cover most of the body.
All travelers should use an effective mosquito repellent, such as those that contain DEET (see Sec. 4, Ch. 6, Mosquitoes, Ticks & Other Arthropods ). Repellents should be applied to exposed parts of the skin. If travelers are also wearing sunscreen, they should apply sunscreen first and insect repellent second. In addition to using a topical insect repellent, a permethrin-containing product can be applied to mosquito nets and clothing for additional protection against mosquitoes. Mosquito repellant–impregnated clothing also is available.
Chemoprophylaxis
Choosing a drug to prevent malaria.
All recommended primary prophylaxis regimens involve taking a medicine before, during, and after travel to an area with malaria. Beginning the drug before travel allows the antimalarial agent to be in the blood before the traveler is exposed to malaria parasites. In choosing a prophylaxis regimen before travel, the traveler and the travel health provider should consider several factors, including the presence of antimalarial drug resistance in the area of travel, length of travel, the patient’s other medical conditions, allergy history, other medications prescribed or already being taken (to assess possible drug interactions), potential side effects, and the cost of the antimalarial. Long-term travelers, defined as people who travel for ≥6 months, have additional considerations (see Box 5-11 ). Table 5-27 lists some of the benefits and limitations of medicines used for malaria prophylaxis; see additional information about choosing a malaria prophylaxis regimen .
Recommendations for drugs to prevent malaria by country of travel can be found in Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country . Recommended drugs for each country are listed in alphabetical order and have comparable efficacy in that country. When >1 drug is recommended, Table 5-27 can help with the decision-making process. No antimalarial drug is 100% protective; therefore, travelers must combine prophylaxis with mosquito avoidance and personal protective measures (e.g., insect repellent, long sleeves, long pants, sleeping in a mosquito-free setting, using an insecticide-treated mosquito net).
Table 5-27 Malaria chemoprophylaxis: prescribing considerations
REASONS TO CONSIDER USING THIS DRUG
REASONS TO CONSIDER AVOIDING THIS DRUG
ATOVAQUONE-PROGUANIL
Good for last-minute travelers because the drug is started 1–2 days before travel.
Some people prefer to take a daily medicine.
Good choice for shorter trips because the traveler takes the medicine for only 7 days after leaving malaria-endemic area, rather than for 4 weeks.
Well tolerated and side effects uncommon.
Pediatric tablets are available and might be more convenient.
Cannot be used by people who are pregnant or who are breastfeeding a child that weighs <5 kg.
Cannot be taken by people with severe renal impairment.
Tends to be more expensive than some of the other options, especially for long trips.
Some people (including children) would rather not take medicine every day.
CHLOROQUINE
Some people would rather take medicine weekly.
Good choice for long trips because it is taken only weekly.
Some people are already taking hydroxychloroquine chronically for rheumatologic conditions; in those instances, they might not have to take an additional medicine.
Can be used in all trimesters of pregnancy.
Cannot be used in areas with chloroquine or mefloquine resistance.
Can exacerbate psoriasis.
ome people would rather not take a weekly medication.
For short trips, some people would rather not take medication for another 4 weeks after leaving malaria-endemic areas.
Not a good choice for last-minute travelers, because drug needs to be started 1–2 weeks before travel.
DOXYCYCLINE
Tends to be the least expensive antimalarial drug.
People already taking doxycycline chronically to prevent acne do not have to take an additional medicine.
Doxycycline also can prevent other infections (e.g., rickettsial infections, leptospirosis); thus, might be preferred by people planning to camp, hike, and swim in fresh water where risk is high
Cannot be used by people who are pregnant or who are breastfeeding a child, or by children aged <8 years.
Some people would rather not take medicine every day.
People prone to getting vaginal yeast infections when taking antibiotics might prefer taking a different medicine.
People might want to avoid the increased risk of sun sensitivity.
Some people are concerned about the potential of getting an upset stomach from doxycycline.
Can be used in all trimesters of pregnancy and during breastfeeding.
Cannot be used in areas with mefloquine-resistant Plasmodium spp.
Cannot be used in patients with certain psychiatric conditions; some travelers without psychiatric conditions would prefer not taking a medication with known neuropsychiatric side effects.
Cannot be used in patients with a seizure disorder.
Not recommended for people with cardiac conduction abnormalities.
Not a good choice for last-minute travelers because drug needs to be started ≥2 weeks before travel.
Some people would rather not take a weekly medication.
One of the most effective drugs for prevention of P. vivax; thus, a good choice for travel to places with >90% P. vivax .
Good choice for shorter trips because the traveler takes the medicine for 7 days after leaving a malaria-endemic area, rather than for 4 weeks.
Cannot be used in patients with G6PD deficiency.
Cannot be used in patients who have not been tested for G6PD deficiency.
Costs and delays associated with getting a quantitative G6PD test might prohibit testing; however, the test only has to be done once. After a normal G6PD level is verified and documented, the test does not have to be repeated the next time primaquine or tafenoquine is considered.
Cannot be used by people who are pregnant.
Cannot be used by people who are breastfeeding unless the infant has also been tested for G6PD deficiency.
Some people are concerned about the potential of getting an upset stomach from primaquine.
TAFENOQUINE
One of the most effective drugs for prevention of P. vivax malaria but also prevents P. falciparum .
Good choice for shorter trips because the traveler takes the medicine once, 1 week after leaving malaria-endemic area, rather than for 4 weeks.
Good for last-minute travelers because the drug is started 3 days before travel.
Cannot be used in people with G6PD deficiency.
Costs and delays associated with getting a quantitative G6PD test might prohibit testing; however, the test only has to be done once. After a normal G6PD level is verified and documented, the test does not have to be repeated the next time tafenoquine or primaquine is considered.
Cannot be used by children.
Not recommended for patients with psychotic disorders.
Abbreviations: G6PD, glucose-6-phosphate-dehydrogenase
Box 5-11 Malaria prevention & prophylaxis considerations for the long-term traveler (travel >6 months)
Considerations.
- Malaria prevention measures are the same for both short- and long-term travelers.
- Longer stays mean longer duration of exposure and increased risk of acquiring malaria.
- Travelers’ attention to mosquito avoidance can wane over time.
- Travelers might not adhere to a lengthy course of malaria prophylaxis due to forgetfulness, fear of side effects, and the possible declining sense of risk and need over time.
- Travelers might move between highly endemic or low endemic areas within a country or region.
- Travelers might have a decreased sense of risk and concern about malaria after engaging in local conversations and lore, particularly regarding malaria immunity over time.
- Travelers who become ill with malaria in countries with limited access and quality of health care might not receive appropriate or effective treatment.
ADDITIONAL ADVICE FOR LONG-TERM TRAVELERS
- Travelers should not count on being able to obtain safe, reliable malaria prophylaxis medication abroad; strongly advise that before leaving the United States they purchase enough medication to last them for the entire duration of their travel to malaria-endemic areas.
- Emphasize continued adherence to and safety of malaria prophylaxis drugs.
- Develop a plan for seeking immediate care when ill with fever, including where to get promptly tested and treated for malaria.
- Advise travelers to purchase travel insurance, including contingencies for medical evacuation.
- Consider having a reliable supply of a treatment dose of antimalarial drugs available in case malaria is diagnosed while traveling.
Medications Used for Prophylaxis
Atovaquone-proguanil.
Atovaquone-proguanil (Malarone) is a fixed combination of the drugs atovaquone and proguanil. Prophylaxis should begin 1–2 days before travel to malaria-endemic areas; the medication should then be taken daily, at the same time each day, while in the malaria-endemic areas, and daily for 7 days after leaving the endemic areas (see Table 5-28 for recommended dosages). Atovaquone-proguanil is well tolerated, and side effects are rare. The most common adverse effects reported in people using atovaquone-proguanil for prophylaxis or treatment are abdominal pain, nausea, vomiting, and headache.
Atovaquone-proguanil is not recommended for prophylaxis in children weighing <5 kg (11 lb), pregnant people, people breastfeeding infants <5 kg, or patients with severe renal impairment (creatinine clearance <30 mL/min). Proguanil can increase the effect of warfarin, so travelers might need international normalized ratio monitoring or adjustment of warfarin dosage. No data are available, however, regarding the clinical impact of taking atovaquone-proguanil and warfarin at the same time.
Table 5-28 Malaria chemoprophylaxis: dosing information
INDICATIONS
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Prophylaxis in all malaria-endemic areas
Adult tablets:
1 adult tablet taken orally, 1×/day
Weight-based daily dosing schedule (taken orally, 1×/day) 5 kg to <8 kg: 1/2 pediatric tablet 8 kg to <10 kg: 3/4 pediatric tablet 10 kg to <20 kg: 1 pediatric tablet 20 kg to <30 kg: 2 pediatric tablets 30 kg to <40 kg: 3 pediatric tablets ≥40 kg: 1 adult tablet
Begin taking 1–2 days before travel to malaria-endemic areas.
Take 1×/day, at the same time each day, while in malaria-endemic areas. Continue taking 1×/day for an additional 7 days after leaving endemic areas.
Take with food or a milky drink.
A pharmacist might need to prepare and dispense partial tablet doses in individual capsules, as described in the text.
Prophylaxis only in areas with chloroquine-sensitive malaria
300 mg base (500 mg salt) taken orally, once a week
5 mg/kg base (8.3 mg/kg salt), up to a maximum dose of 300 mg base (500 mg salt), taken orally, 1×/week
Begin taking 1–2 weeks before travel to malaria-endemic areas.
Take 1×/week, on the same day each week, while in malaria-endemic areas.
Continue taking 1×/week for another 4 weeks after leaving endemic areas.
100 mg taken orally, 1×/day
≥8 years of age: 2.2 mg/kg, up to a maximum dose of 100 mg, taken orally, 1×/day
Take 1×/day, at the same time each day, while in malaria-endemic areas. Continue taking 1×/day for another 4 weeks after leaving endemic areas.
Contraindicated in children aged <8 years and in people who are pregnant.
HYDROXY-CHLOROQUINE
An alternative to chloroquine for prophylaxis only in areas with chloroquine-sensitive malaria
310 mg base (400 mg salt) taken orally, 1×/week
5 mg/kg base (6.5 mg/kg salt), up to a maximum dose of 310 mg base (400 mg salt), taken orally, 1×/week
Prophylaxis in areas with mefloquine-sensitive malaria
228 mg base (250 mg salt) taken orally, 1×/week
Weight-based weekly dosing schedule (taken orally, 1×/week) ≤9 kg: 4.6 mg/kg base (5 mg/kg salt) >9–19 kg: 1/4 tablet >19–30 kg: 1/2 tablet >30–45 kg: 3/4 tablet >45 kg: 1 tablet
Begin taking ≥2 weeks before travel to malaria-endemic areas.
Contraindicated in people allergic to mefloquine or related compounds (quinidine, quinine) and in people with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, other major psychiatric disorders, or seizures.
Use with caution in people with psychiatric disturbances or a previous history of depression.
PRIMAQUINE 1
Prophylaxis for short-duration travel to areas with principally P. vivax . Terminal prophylaxis (presumptive antirelapse therapy) to decrease the risk for relapses of P. vivax and P. ovale .
30 mg base (52.6 mg salt) taken orally, 1×/day. Same dose used for both primary and terminal prophylaxis; duration of therapy differs.
0.5 mg/kg base (0.8 mg/kg salt), up to maximum dose of 30 mg base (52.6 mg salt), taken orally, 1×/day Same dose for used both primary and terminal prophylaxis; duration of therapy differs.
Terminal prophylaxis indicated for people with prolonged exposure to P. ovale , P. vivax , or both. Take daily for 14 days after departure from the malaria-endemic area.
Contraindicated in people with G6PD deficiency.
Also contraindicated during pregnancy and breastfeeding unless the breastfed infant has a documented normal G6PD level.
TAFENOQUINE 1
200 mg orally
Not indicated for use in children
Begin taking 3 days before travel to malaria-endemic areas. Take 1×/week, on the same day each week, while in malaria-endemic areas. Take 1 additional dose 1 week after leaving endemic areas. Contraindicated in people with G6PD deficiency. Also contraindicated during pregnancy and breastfeeding unless the breastfed infant has a documented normal G6PD level.
1 Before prescribing primaquine or tafenoquine to any patient, document a normal G6PD level using a quantitative test.
Chloroquine & Hydroxychloroquine
Chloroquine phosphate or hydroxychloroquine sulfate (Plaquenil) can be used to prevent malaria only in destinations where chloroquine-resistant Plasmodium spp. are not active (see Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country ). Prophylaxis should begin 1–2 weeks before travel to malaria-endemic areas. Travelers should continue taking the drug once a week, on the same day of the week, during travel in malaria-endemic areas, and for 4 weeks after they leave endemic areas (see Table 5-28 for recommended dosages).
Reported side effects of chloroquine and hydroxychloroquine include blurred vision, dizziness, gastrointestinal disturbance, headache, insomnia, and pruritus, but generally, these effects do not require travelers to discontinue the drug. High doses of chloroquine (e.g., those used to treat rheumatoid arthritis) have been associated with retinopathy; this serious side effect appears to be extremely unlikely when chloroquine is used for routine weekly malaria prophylaxis. Chloroquine and related compounds reportedly can exacerbate psoriasis. People who experience uncomfortable side effects after taking chloroquine might tolerate the drug better by taking it with meals. As an alternative, a traveler experiencing side effects might better tolerate the related compound, hydroxychloroquine sulfate.
Doxycycline
Doxycycline prophylaxis should begin 1–2 days before travel to malaria-endemic areas. Doxycycline should then be taken once a day, at the same time each day, during travel in malaria-endemic areas and daily for 4 weeks after the traveler leaves endemic areas. Insufficient data exist on the antimalarial prophylactic efficacy of related compounds (e.g., minocycline, commonly prescribed for the treatment of acne). People on a long-term regimen of minocycline who need malaria prophylaxis should stop taking minocycline 1–2 days before travel and start doxycycline instead. Minocycline can be restarted after the full course of doxycycline is completed (see Table 5-28 for recommended dosages).
Doxycycline can cause photosensitivity, usually manifested as an exaggerated sunburn reaction. The risk for such a reaction can be minimized by avoiding prolonged, direct exposure to the sun and by using sunscreen (see Sec. 4, Ch. 1, Sun Exposure ). In addition, doxycycline use is associated with an increased frequency of vaginal yeast infections.
Gastrointestinal side effects (nausea, vomiting) can be minimized by taking the drug with a meal or by specifically prescribing doxycycline monohydrate or the enteric-coated doxycycline hyclate, rather than the generic doxycycline hyclate, which is often less expensive. To reduce the risk for esophagitis, advise travelers to swallow the medicine with sufficient fluids and to avoid taking doxycycline shortly before going to bed.
Doxycycline is contraindicated in people with an allergy to tetracyclines, in pregnant people, and in infants and children aged <8 years. Vaccination with the oral typhoid vaccine Ty21a should be completed ≥24 hours before taking a dose of doxycycline.
Mefloquine prophylaxis should begin ≥2 weeks before travel to malaria-endemic areas. Travelers should continue taking the drug weekly, on the same day each week, during travel in malaria-endemic areas and for 4 weeks after leaving endemic areas (see Table 5-28 for recommended dosages).
At prophylactic doses, mefloquine has been associated with rare but serious adverse reactions (e.g., psychosis, seizures); these reactions are more frequent with the higher doses used for treatment. Other side effects reported in prophylaxis studies include abnormal dreams, anxiety disorder, depression, dizziness, gastrointestinal disturbance, headache, insomnia, and visual disturbances. Other neuropsychiatric disorders occasionally reported include aggressive behavior, agitation or restlessness, confusion, encephalopathy, forgetfulness, hallucinations, mood changes, panic attacks, paranoia, and sensory and motor neuropathies (e.g., ataxia, paresthesia, tremors). On occasion, psychiatric symptoms have been reported to continue long after mefloquine has been stopped. FDA also includes a boxed warning about rare reports of persistent dizziness after mefloquine use.
Mefloquine is contraindicated for travelers with a known hypersensitivity to the drug or related compounds (e.g., quinidine, quinine) and in people with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia and other major psychiatric disorders, or seizures. Mefloquine should be avoided in people with psychiatric disturbances or a history of depression.
A review of available data suggests that mefloquine can be used safely in people concurrently taking beta-blockers if they have no underlying arrhythmia. Mefloquine is not recommended for people with cardiac conduction abnormalities, however. Any traveler receiving a prescription for mefloquine must also receive a copy of the FDA medication guide [PDF].
Primaquine can cause potentially life-threatening hemolysis in people with glucose-6-phosphate-dehydrogenase (G6PD) deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing primaquine to patients.
Primaquine phosphate has 2 distinct uses for malaria prevention in people with normal G6PD levels: primary prophylaxis in areas with primarily P. vivax , and terminal prophylaxis for travelers who have had prolonged exposure in malaria-endemic areas. Among people with normal G6PD levels taking primaquine, the most common adverse event is gastrointestinal upset; this occurs most commonly if the drug is taken on an empty stomach, and can be minimized or eliminated if it is taken with food.
Primary Prophylaxis
When taken for primary prophylaxis, primaquine should be taken 1–2 days before travel to malaria-endemic areas, daily (at the same time each day) while in the malaria-endemic area, and daily for 7 days after leaving the area (see Table 5-28 for recommended dosages).
Terminal Prophylaxis
In addition to primary prophylaxis, terminal prophylaxis (also known as presumptive antirelapse therapy) generally is indicated for long-term travelers (e.g., military personnel, missionaries, Peace Corps volunteers) with prolonged exposure to P. ovale or P. vivax malaria. Terminal prophylaxis involves taking primaquine toward the end of the exposure period (or immediately thereafter) for the presumptive purpose of eliminating hypnozoites (dormant liver stages) of P. ovale or P. vivax , thereby preventing relapses or delayed-onset clinical presentations of malaria. Because most malaria-endemic areas of the world (except the Caribbean) have ≥1 species of relapsing malaria, travelers to these areas have some risk for acquiring either P. ovale or P. vivax , although the actual risk for an individual traveler is difficult to define.
When indicated, travelers should take primaquine for 14 days after leaving a malaria-endemic area, concurrently with their primary prophylaxis medication. If chloroquine, doxycycline, or mefloquine are used for primary prophylaxis, prescribe primaquine for travelers to take during the last 2 weeks of postexposure prophylaxis. When atovaquone-proguanil is used for primary prophylaxis, travelers can take primaquine during the final 7 days of atovaquone-proguanil, and then for an additional 7 days. If concurrent administration of primary and terminal prophylaxis is not feasible, instruct travelers to take primaquine after completing their primary prophylaxis medication. Primary prophylaxis with primaquine or with tafenoquine (see the following section) obviates the need for terminal prophylaxis.
Tafenoquine
Tafenoquine can cause potentially life-threatening hemolysis in people with G6PD deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing tafenoquine to patients.
Tafenoquine (Arakoda 100 mg tablets) can be used to prevent malaria in adults (see Table 5-28 for recommended dosages). Travelers should take a daily loading dose of tafenoquine for 3 days before leaving for a malaria-endemic area; starting 7 days after the loading dose is complete, they should take a weekly maintenance dose while in the malaria-endemic area; then take a final dose in the week after leaving the malaria-endemic area. Doses should be taken on the same day each week.
Tafenoquine is contraindicated in pregnant people and during breastfeeding. Avoid prescribing tafenoquine for people with a history of psychotic disorder; rare psychiatric adverse events have been observed in people with a history of psychotic disorder using higher doses of tafenoquine. The most common adverse events reported with use of tafenoquine are dizziness, gastrointestinal disturbances, headache, and clinically insignificant decreases in hemoglobin. Tafenoquine should be taken with food.
As of 2020, CDC no longer recommends tafenoquine for terminal prophylaxis of P. ovale or P. vivax malaria.
Prophylaxis for Infants, Children & Adolescents
All children traveling to malaria-endemic areas should use recommended prevention measures, which often include taking an antimalarial drug. In the United States, antimalarial drugs are not available in liquid formulation and can taste bitter. Calculate pediatric doses carefully according to the patient’s body weight, but never exceed the adult dose. Pharmacists can pulverize tablets and prepare gelatin capsules for each measured dose. If a child is unable to swallow capsules or tablets, parents should prepare the child’s medication dose by breaking open the gelatin capsule or crushing the pill and mixing the drug with a small amount of something sweet (e.g., condensed milk, chocolate syrup, chocolate spread) to ensure the entire dose is delivered to the child. Giving the dose on a full stomach can minimize stomach upset and vomiting.
Atovaquone-proguanil can be used as prophylaxis for infants and children weighing ≥5 kg (11 lb); prophylactic dosing for children weighing <11 kg (24 lb) constitutes off-label use in the United States. Chloroquine and mefloquine are options for infants and children of all ages and weights, depending on drug resistance at the destination. Doxycycline can be used for children aged ≥8 years. Primaquine can be used for children who are not G6PD-deficient and who are traveling to areas with principally P. vivax. Pediatric dosing regimens are included in Table 5-28 .
Prophylaxis During Pregnancy
Malaria infection can be more severe in pregnant than in nonpregnant people. Malaria increases the risk for adverse pregnancy outcomes, including premature birth, spontaneous abortion, and stillbirth; thus, because no prophylaxis regimen is completely effective, advise people who are pregnant or likely to become pregnant to avoid travel to areas with malaria transmission if possible (see Sec. 7, Ch. 1, Pregnant Travelers ). If travel to a malaria-endemic area cannot be deferred, an effective prophylaxis regimen and mosquito avoidance measures are essential.
Pregnant people traveling to areas where chloroquine-resistant P. falciparum has not been reported can take chloroquine prophylaxis. Chloroquine has not been found to have harmful effects on the fetus when used in the recommended doses for malaria prophylaxis; therefore, pregnancy is not a contraindication for malaria prophylaxis with chloroquine or hydroxychloroquine.
For travel to areas with known chloroquine-resistant Plasmodium , mefloquine is the only medication recommended for malaria prophylaxis during pregnancy. Studies of mefloquine use during pregnancy have found no indication of adverse effects on the fetus.
Atovaquone-proguanil is not recommended for use during pregnancy because of limited availability of data on its safety, and because other options are available. If other antimalarial drug options are not feasible, however, clinicians and patients should weigh the options, risks, and benefits of using atovaquone-proguanil to make the best decision for the patient. Doxycycline is contraindicated for malaria prophylaxis during pregnancy because of the risk for adverse effects seen with tetracycline, a related drug, on the fetus. These adverse effects include discoloration and dysplasia of the teeth and inhibition of bone growth. Neither primaquine nor tafenoquine should be used during pregnancy; both drugs can be passed transplacentally to a G6PD-deficient fetus and cause hemolytic anemia in utero.
People planning to become pregnant can use the same medications recommended for use during pregnancy (chloroquine or mefloquine, depending on the area of travel). CDC does not make recommendations about delaying pregnancy after the use of malaria prophylaxis medicines. If the traveler or their health care provider wishes to decrease the amount of antimalarial drug in the body before conception, however, Table 5-29 provides information on the half-lives of the recommended malaria prophylaxis medicines. After 2 half-lives, ≈25% of the drug remains in the body, ≈6% remains after 4 half-lives, and ≈2% remains after 6 half-lives.
Table 5-29 Malaria chemoprophylaxis: half-lives
Prophylaxis during breastfeeding.
The quantities of antimalarial drugs excreted in the breast milk of lactating people are insufficient to provide adequate protection to nursing infants. Therefore, infants who require prophylaxis should receive the recommended dosages of antimalarial drugs listed in Table 5-28 . Because chloroquine and mefloquine can be prescribed safely to infants, infants also can be safely exposed to the small amounts excreted in breast milk. Data about the use of doxycycline in lactating people are very limited; most experts, however, consider the theoretical possibility of adverse events to the infant to be remote.
Although no information is available on the amount of primaquine or tafenoquine that enters human breast milk, test both the person breastfeeding and the infant for G6PD deficiency before initiating chemoprophylaxis with either one of these drugs. Because data are not yet available on the safety of atovaquone-proguanil prophylaxis in infants weighing <5 kg (11 lb), CDC does not recommend this drug to prevent malaria in people who are breastfeeding infants weighing <5 kg. Atovaquone-proguanil can, however, be used to treat people who are breastfeeding infants of any weight when the potential benefit outweighs the potential risk to the infant (e.g., treating a breastfeeding person who has acquired P. falciparum malaria in an area of multidrug-resistant strains and who cannot tolerate other treatment options).
Travel to Areas with Chloroquine-Resistant Malaria
Chloroquine-resistant P. falciparum is found in all parts of the world except the Caribbean and countries west of the Panama Canal. Although chloroquine-resistant P. falciparum predominates in Africa, it is found in combination with chloroquine-sensitive P. vivax malaria in South America and Asia. Chloroquine-resistant P. vivax has been confirmed only in Papua New Guinea and Indonesia. For destinations with known chloroquine-resistant Plasmodium spp., in addition to mosquito avoidance measures, prescribe atovaquone-proguanil, doxycycline, mefloquine, or tafenoquine as prophylaxis.
Travel to Areas with Chloroquine-Sensitive Malaria
Areas with chloroquine-sensitive Plasmodium spp. include many Latin American countries where malaria predominantly is caused by P. vivax . Chloroquine-sensitive P. falciparum is present in the Caribbean and Central American countries west of the Panama Canal. For destinations with known chloroquine-sensitive Plasmodium spp., in addition to mosquito avoidance measures, the many effective prophylaxis options include chloroquine, atovaquone-proguanil, doxycycline, mefloquine, and tafenoquine. In countries where P. vivax predominates, primaquine is also an option.
Travel to Areas With Mefloquine-Resistant Malaria
Mefloquine-resistant P. falciparum has been confirmed in Southeast Asia on the borders of Thailand with Burma (Myanmar) and Cambodia, in the western provinces of Cambodia, in the eastern states of Burma on the border between Burma and China, along the borders of Burma and Laos, and in southern Vietnam. For destinations with known mefloquine-resistant Plasmodium spp., in addition to mosquito avoidance measures, prophylaxis options are atovaquone-proguanil, doxycycline, and tafenoquine.
Travel to Areas With Limited Malaria Transmission
For destinations where malaria cases occur sporadically and risk for infection to travelers is considered low, CDC recommends that travelers use mosquito avoidance measures only, and no chemoprophylaxis (see Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country ).
Changing Medications as a Result of Side Effects During Prophylaxis
Medications recommended for malaria prophylaxis have different modes of action that affect the parasites at different stages of the life cycle. Thus, if the medication needs to be changed because of side effects before a full course has been completed, some special considerations exist (see Table 5-30 ).
Table 5-30 Malaria chemoprophylaxis: changing medications due to side effects
Obtaining medications overseas.
Medications recommended for malaria prophylaxis might be available at overseas destinations. Combinations of these medications and additional drugs that are not recommended might be commonly prescribed and used in other countries, however. Strongly discourage travelers from obtaining prophylaxis medications while abroad. The quality of these products is not known; products might be produced under substandard manufacturing practices, be counterfeit, contain contaminants, not be protective, or be dangerous. Additional information on medications obtained while traveling can be found in Sec. 6, Ch. 3, . . . perspectives: Avoiding Poorly Regulated Medicines & Medical Products During Travel , and on the FDA website .
Blood Donation After Travel to Malaria-Endemic Areas
People who have been in an area where malaria transmission occurs should defer donating blood after returning from the malaria-endemic area to prevent transmission of malaria through blood transfusion (see Table 5-31 ).
Risk assessments can differ between travel health providers and blood banks. A travel health provider advising a traveler going to a country with relatively low malaria transmission for a short period of time and engaging in low-risk behaviors might suggest the traveler use only mosquito bite precautions and no prophylaxis. Upon the traveler’s return, however, a blood bank might still choose to defer blood donations from that traveler for 1 year because of travel to an area where transmission occurs.
Table 5-31 US Food and Drug Administration recommendations for deferring blood donation in people returning from malaria-endemic areas
CDC website: Malaria
The following authors contributed to the previous version of this chapter: Kathrine R. Tan, Paul M. Arguin
Bibliography
Andrejko KL, Mayer RC, Kovacs S, Slutsker E, Bartlett E, Tan KR, Gutman JR. The safety of atovaquone-proguanil for the prevention and treatment of malaria in pregnancy: a systematic review. Travel Med Infect Dis. 2019;27:20–6.
Angelo KM, Libman M, Caumes E, Hamer DH, Kain KC, Leder K, et al. Malaria after international travel: a GeoSentinel analysis, 2003–2016. Malar J. 2017;16(1):293.
Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg. 2007;76(2):208–23.
Davlantes EA, Tan KR, Arguin PM. Quantifying malaria risk in travelers: a quixotic pursuit. J Travel Med. 2017;24(6):tax066. Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75(3):402–15.
Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK. Severe morbidity and mortality risk from malaria in the United States, 1985–2011. Open Forum Infect Dis. 2014;1(1):ofu034.
Lupi E, Hatz C, Schlagenhauf P. The efficacy of repellents against Aedes , Anopheles, Culex and Ixodes spp.—a literature review. Travel Med Infect Dis. 2013;11(6):374–411.
Mace KE, Arguin PM, Lucchi NW, Tan KR. Malaria surveillance—United States, 2016. MMWR Surveill Summ 2019;68(SS-5):1–35.
Novitt-Moreno A, Ransom J, Dow, G, Smith B, Read LT, Toovey S. Tafenoquine for malaria prophylaxis in adults: an integrated safety analysis. Travel Med Infect Dis. 2017;17:19–27.
Tan KR, Magill AJ, Parise ME, Arguin PM. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84(4):517–31.
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Choosing a Drug to Prevent Malaria
Considerations when choosing a drug for malaria prophylaxis:
- Recommendations for drugs to prevent malaria differ by country of travel and can be found in Malaria Information by Country. Recommended drugs for each country are listed in alphabetical order and have comparable efficacy in that country.
- No antimalarial drug is 100% protective and must be combined with the use of personal protective measures, (i.e., insect repellent, long sleeves, long pants, sleeping in a mosquito-free setting or using an insecticide-treated bednet).
- For all medicines, also consider the possibility of drug-drug interactions with other medicines that the person might be taking as well as other medical contraindications, such as drug allergies.
- When several different drugs are recommended for an area, the following table might help in the decision process.
- Atovaquone/Proguanil (Malarone)
- Chloroquine
- Doxycycline
- Tafenoquine (ArakodaTM)
To receive email updates about this page, enter your email address:
New! Locally Acquired Cases of Malaria in Florida, Texas, Maryland, and Arkansas
New! Update to Guidance for use of Artemether-Lumefantrine (Coartem®) in Pregnancy for Uncomplicated Malaria New! Discontinuation of CDC’s Distribution of Intravenous Artesunate as Commercial Drug Guidance for Malaria Diagnosis in Patients Suspected of Ebola Infection in the United States -->
See all Malaria Notices
- New! Malaria is a Serious Disease
- New! La malaria (paludismo) es una enfermedad grave
- How to Report a Case of Malaria
- CDC Yellow Book
- Red Pages: Malaria-endemic areas by country
- Drugs for Prevention
- Drugs for Treatment in the U.S.
- Frequently Asked Questions (FAQs)
- Blood Banks
Click here for contact information
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Share full article
Advertisement
Supported by
Kent Campbell, Pivotal Figure in the Fight Against Malaria, Dies at 80
Among his accomplishments in a four-decade career in public health, he helped pioneer programs providing bed nets in Africa.

By Michael S. Rosenwald
Kent Campbell, an instrumental figure in the global battle against malaria — most notably in Africa, where he led an innovative program providing bed nets to protect rural villagers from the mosquitoes carrying the disease — died on Feb. 20 in Oro Valley, Ariz., a suburb of Tucson. He was 80.
His death, in a nursing care facility, was caused by complications of cancer, his children said.
As chief of the malaria branch of the Centers for Disease Control and Prevention from 1981 to 1993, and later as an adviser to UNICEF and the Bill & Melinda Gates Foundation, Dr. Campbell is credited with helping to save lives on multiple continents.
In Zambia, where he began working on a program with the Gates Foundation in 2005 distributing bed nets and newer antimalarial drugs, malaria cases were cut in half within three years. The program was later expanded to more than 40 other countries in Africa.
“His legacy in my country is as one of the people who greatly contributed to the control and prevention of malaria,” Kafula Silumbe, a Zambian public health specialist who worked closely with Dr. Campbell, said in an interview. “It was a collective effort, but he definitely was part of that initial push.”
Tall and lanky, with a Southern drawl that revealed his Tennessee upbringing, Dr. Campbell stumbled on what would become a four-decade-long career in public health.
In 1972, during his pediatric residency in Boston, he joined the C.D.C. as a conscientious objector to the Vietnam War. Not long after, he was sent to Sierra Leone to help investigate an outbreak of Lassa fever , a virulent hemorrhagic virus.
“I had never heard of Lassa fever,” he said in a video history of the C.D.C. “Probably couldn’t even spell it if I’d been asked to.”
He had little to no training in the importance or use of personal protective equipment. For relief from the intense heat, he poked holes in his breathing apparatus, which he later admitted was a bad idea.
Hoping to learn more about Lassa fever, agency officials dispatched him to Ireland to conduct serologic, or antibody-detecting, tests on nuns who had previously worked in Sierra Leone. He traveled there with his wife, Elizabeth (Knight) Campbell, whom he had married in 1966.
A few days later, he nearly collapsed from an intense headache, high fever and an excruciating sore throat.
Dr. Campbell and his wife then traveled to London so that he could be treated at a hospital with expertise in tropical diseases. The episode then took a surreal turn: When U.S. officials sent a military transport plane to retrieve the couple, they shipped inside it a spare Apollo space capsule, which the Campbells rode in as a precautionary measure.
“In retrospect, it’s not clear whether I had Lassa fever,” Dr. Campbell said. “But clearly I didn’t die.”
With a reprieve on life and a newfound appreciation for disease hunting, he stayed on with the C.D.C. He moved to El Salvador in 1973 to take on malaria, which had been essentially orphaned by global public health agencies and aid groups.
“He was indignant about the injustice and unfairness of things,” Laurie Garrett, who wrote about Dr. Campbell in her book “The Coming Plague: Newly Emerging Diseases in a World Out of Balance” (1994), said in an interview. “It just didn’t seem right to him that a scourge like malaria that was killing millions of people every single year wasn’t getting investment and concern and global attention because most of the people dying of it were poor.”
Carlos Clinton Campbell III was born on Jan. 9, 1944, in Knoxville, Tenn. His father was a life insurance salesman, and his mother, Betty Ann (Murphy) Campbell, managed the household. His parents wanted to call him Clint, but his younger sister, Ann, had trouble saying the name, and he wound up as Kent.
He took an early interest in medicine after his sister and mother both died from cancer — Ann when she was 5, their mother when he was in high school.
He studied biology at Haverford College in Pennsylvania, graduating in 1966. He earned his medical degree from Duke University in 1970 and received a master’s in public health at Harvard University after completing his pediatric residency there.
Dr. Campbell bounced around the world, from the corridors of public health to isolated villages, and back.
“He had a deceptive demeanor because of his Southern, laconic exterior,” Ms. Garrett said. “Almost every time you’d go into his office, these gigantic, long legs would go up on the desk, and he’d lean back in his chair. And because he’s so tall, he would automatically fill up, you know, 12 feet of space.”
This made him seem easygoing.
“But then, when he got going, you could feel everything boiling up to the surface,” she added. “He was incredibly impatient, and I think that drove him to ask big questions and to take bold steps to try and help things.”
Following his work at the C.D.C., Dr. Campbell helped create a college of public health at the University of Arizona and consulted for several global health organizations. In 2005, he joined PATH , a health equity nonprofit based in Seattle, as director of the malaria program in Africa funded by the Gates Foundation.
With malaria becoming resistant to the most common drug treatments, he focused on prevention.
“The vector in Africa is basically a single species that is distributed all over the continent called Anopheles gambiae,” he said in an interview with AllAfrica, a Pan-African news organization. “It is like the superstar of transmitters.”
Two years after the bed-net program began in Zambia, the country saw a 29 percent decrease in child mortality, according to PATH.
“To put that in perspective: There’s nothing matching that, which is reflective of how much death malaria caused in Zambia and how powerful bed nets are to decrease transmission,” Dr. Campbell told AllAfrica. “That’s all it really took. It was just remarkable. Clinics emptied out during the transmission season.”
He is survived by his wife; his children, Dr. Kristine Campbell and Dr. Patrick Campbell; his brothers, Robert and John Campbell; his stepsisters, Melissa Hansen and Rebecca Arrants; and four grandchildren.
Dr. Campbell retired from PATH in 2015.
“I hadn’t set out to battle this infection and disease,” he wrote of his professional career. “In reality, it chose me.”
He added, “We chose not to listen to the naysayers.”
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Infect Dis

The Clinical Profile of Severe Pediatric Malaria in an Area Targeted for Routine RTS,S/AS01 Malaria Vaccination in Western Kenya
Samuel akech.
1 Kenya Medical Research Institute/Wellcome Trust Research Programme, Nairobi, Kenya
Mercy Chepkirui
Morris ogero, ambrose agweyu, grace irimu.
2 Department of Paediatrics and Child Health, University of Nairobi, Nairobi, Kenya
Mike English
3 Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, United Kingdom
Robert W Snow
Associated data.
The malaria prevalence has declined in western Kenya, resulting in the risk of neurological phenotypes in older children. This study investigates the clinical profile of pediatric malaria admissions ahead of the introduction of the RTS,S/AS01 vaccine.
Malaria admissions in children aged 1 month to 15 years were identified from routine, standardized, inpatient clinical surveillance data collected between 2015 and 2018 from 4 hospitals in western Kenya. Malaria phenotypes were defined based on available data.
There were 5766 malaria admissions documented. The median age was 36 months (interquartile range, 18–60): 15% were aged between 1–11 months of age, 33% were aged 1–23 months of age, and 70% were aged 1 month to 5 years. At admission, 2340 (40.6%) children had severe malaria: 421/2208 (19.1%) had impaired consciousness, 665/2240 (29.7%) had an inability to drink or breastfeed, 317/2340 (13.6%) had experienced 2 or more convulsions, 1057/2340 (45.2%) had severe anemia, and 441/2239 (19.7%) had severe respiratory distress. Overall, 211 (3.7%) children admitted with malaria died; 163/211 (77% deaths, case fatality rate 7.0%) and 48/211 (23% deaths, case fatality rate 1.4%) met the criteria for severe malaria and nonsevere malaria at admission, respectively. The median age for fatal cases was 33 months (interquartile range, 12–72) and the case fatality rate was highest in those unconscious (44.4%).
Conclusions
Severe malaria in western Kenya is still predominantly seen among the younger pediatric age group and current interventions targeted for those <5 years are appropriate. However, there are increasing numbers of children older than 5 years admitted with malaria, and ongoing hospital surveillance would identify when interventions should target older children.
( See the Editorial Commentary by Castelli on pages 381–2 .)
A decline in the malaria parasite prevalence in western Kenya over the last 25 years has led to indications of a shift of severe malaria to older children, but malaria is still predominantly seen in the younger, pediatric age group.
There has been an unprecedented decline in the intensity of Plasmodium falciparum transmission in Africa since 2000 [ 1 ], resulting in a decline in the malaria burden across most countries [ 2 ]. These reductions have been attributed to a scaling up of vector control and improved case management [ 2 ]. However, these declines have stagnated in recent years [ 1 , 2 ]; therefore, funding, the scope of coverage of existing interventions, and new interventions must increase to accelerate any future declines in transmission and the disease burden. As the landscape of malaria transmission changes, countries are being encouraged to tailor existing and novel sub-national strategic controls to more nuanced local epidemiology [ 3 ].
Over the last 25 years in Kenya, there has been an 88% overall reduction in the prevalence of malaria infection; however, 8 counties surrounding Lake Victoria remain highly endemic, despite a declining transmission since 2000 [ 4 ], and are the current focus of efforts to increase levels of vector control though the distribution of insecticide-treated nets, indoor residual house spraying, and larval source management [ 5 , 6 ]. Following the World Health Organization (WHO) recommendations for the phased, monitored introduction of RTS,S/AS01 in 2016 [ 7 , 8 ], Kenya plans to introduce RTS,S/AS01 into the highly endemic 8 counties in western Kenya in 2019 [ 9 ].
The clinical epidemiology of severe, life-threatening malaria was characterized during the 1990s [ 10 ] and its changing patterns have been described over 25 years along the Kenyan coast [ 11 ]. In western Kenya, during the early 1990s, the community-based malaria infection prevalence around the Siaya District hospital was over 80% [ 4 , 10 ]. Under this level of transmission intensity, 75% of pediatric malaria hospitalizations were below 2 years of age, predominantly due to severe malaria anemia, with a few cases of cerebral malaria [ 10 ]. During the 1990s at Kilifi, on the Kenyan coast, the community prevalence of malaria infection was lower than in Siaya and malaria hospitalizations were among older children, with relatively more presentations with cerebral malaria [ 10 ]. As transmission intensity at Kilifi has declined over the last 25 years to very low levels, the mean age of malaria hospitalization has systematically increased [ 11 , 12 ].
The intensity of transmission has declined in western Kenya since the 1990s [ 4 ]; however, there have been no detailed clinical descriptions of the patient ages, pathogenesis, and outcomes of pediatric malaria in this area since 1997. Here, we analyzed pediatric admission data assembled over 2 complete years since 2015 to provide a current understanding of the clinical epidemiology of malaria in an area poised to launch a scaling of existing and new vector-control strategies and RTS,S/AS01 vaccination among children aged 6 to 24 months.
Study Sites
The present study was undertaken at 4 county referral–level hospitals located in western Kenya ( Figure 1 ). The study is a secondary analysis of prospective data assembled as part of a system established in 2013 as part of a Clinical Information Network (CIN), as described previously [ 13 , 14 ]. The 4 selected hospitals are in high–malaria transmission settings (parasite prevalences in children ≥30% in 2015 [ 4 ]) around Lake Victoria. The catchment areas to these hospitals form part of a wider area of similar malaria transmission intensity [ 4 ] that has been proposed for a pilot implementation of the RTS,S/AS01 malaria vaccine from 2019 through the existing routine immunization program, and are part of a multi-country postregistration study [ 8 ].

Map showing locations of study county hospitals in Western Kenya.
Clinical Surveillance
The CIN surveillance system has been described elsewhere [ 13 , 14 ]. In brief, slides for malaria microscopy and other tests are ordered by clinicians at the outpatient department prior to admission or from the ward. Patients who are admitted are further assessed by pediatric ward clinicians and observations are recorded using a standardized pediatric admission record. The record captures the patient’s history, vital signs, general clinical examinations of the airway, and respiratory, circulatory, and neurological systems. The neurological status is assessed using the alert, response to voice, response to pain, unconscious (AVPU) scale and the ability to drink or breastfeed, as appropriate for age. Anemia is clinically assessed by an examination for palmar pallor (recorded as either absent, mild, or severe). Respiratory distress is assessed by an examination of the chest for indrawing and/or deep, acidotic breathing. Information on laboratory tests ordered at admission and during hospitalization, treatments prescribed at admission, and final discharge information are also collected from medical notes and entered into the database. CIN does not support laboratory testing and any tests or results are based on a hospital’s capacity.
Standard Management for Malaria Admissions
CIN supplies, promotes, and monitors use of Kenyan pediatric guidelines [ 15 ], which are an adapted version of WHO guidelines [ 16 ], in each hospital. These guidelines recommend that children diagnosed with severe malaria are managed with parenteral artesunate at 3 mg/kg of body weight for children weighing 20 kg or less, or 2.4 mg/kg of body weight for children weighing over 20 kg. Parenteral artesunate, which is widely available [ 17 , 18 ], is recommended for at least 24 hours, with administration continued until the child has improved and is able to take a full course of oral, artemisinin-based combination therapy medication. The supportive therapy recommended for severe malaria includes the treatment of hypoglycemia with dextrose when the glucose level is <2.2 mmol/l; a blood transfusion for children with hemoglobin levels less than 4 g/dL or 4–5 g/dL with respiratory distress, although delays in transfusion may occur [ 19 ]; maintenance intravenous fluids for children with circulatory impairment or impaired consciousness (sometimes through nasogastric feeding for impaired consciousness); and oxygen therapy for children with hypoxia (pulse oximeter reading <90%) or severe respiratory distress. However, experience from CIN hospitals has shown poor compliance for glucose testing for severely ill children unable to feed [ 20 ], linked to challenges in the continuous supply of appropriate diagnostics and the variable use of pulse oximetry [ 13 ].
Data Capture and Verification
All medical notes are reviewed by data clerks stationed at the hospital and entered each day into a database designed in Research Electronic Data Capture [ 21 , 22 ] that includes logical range checks, together with local and server-level cleaning scripts [ 21 , 22 ], and any clerical errors are reconciled within 7 days. Approximately 10 records are randomly sampled and reentered on site bimonthly for accuracy at each hospital [ 17 ].
Laboratory Surveillance
Malaria slide results are recorded as positive or negative, with neither species identification nor parasite density. Hematology measurements (hemoglobin) are recorded when requested; however, children may receive blood transfusions based on a clinical diagnosis of severe anemia or a documented severe pallor. Blood glucose, creatinine, lactate, bilirubin, and blood gas tests are rarely performed, and microbiology remains unreliable at all the hospital settings [ 13 , 20 ].
Case Definitions
Malaria was defined based upon a reanalysis of characteristics documented in medical notes at admission, in-patient notes, and information recorded at discharge. A malaria diagnosis was classified as (1) the presence of fever (defined as a history of fever or an axillary temperature ≥ 37.5°C) plus a positive malaria slide and a primary discharge diagnosis of malaria; (2) the presence of fever plus a primary clinical discharge diagnosis of malaria where results of a malaria test were not documented; or (3) if both a history of fever and a temperature were not recorded at admission but the patient had a positive malaria slide and a primary discharge diagnosis of malaria ( Supplementary Figure 1 ).
It was not possible to define the broad classification of severe malaria according to the WHO definition [ 23 , 24 ], which includes hypoglycemia, hyperlactatemia, hemoglobinuria, hyperparasitemia, radiological pulmonary edema, shock, jaundice (hyperbilirubinemia), and renal failure. The focus is, therefore, on common clinical syndromes of severe malaria in African children [ 25 ], for which information was available to define signs denoting cerebral involvement (AVPU), 2 or more convulsions, measures or signs of severe anemia, and respiratory distress ( Table 1 ).
Case Definitions for Severe Malaria Syndromes
Abbreviations: A, alert; AVPU, alert, response to voice, response to pain, unconscious scale; P, responsive to pain; U, unconscious; V, responsive to a voice; WHO, World Health Organization.
Data were analyzed for 2 complete years on either side of a national health worker strike, from December 2015 to November 2016 and from November 2017 to October 2018 [ 26 ]. The analysis was in all pediatric admissions aged between 1 month to 15 years.
Malaria Admissions
A total of 14 999 children aged <15 years were admitted to the 4 hospitals over the 24 months of surveillance between 1 December 2015 and 31 October 2018 ( Supplementary Figure ). In brief, complete data on a measured temperature and/or history of fever was available for 82% of all presentations. Of those with fever documentation, a blood film result for malaria microscopy was available at presentation or during admission among 7919/10 345 (76.5%) patients, of which 4445 (56.1%) were positive for malaria parasites. Among the 2725 patients admitted without a recorded history of fever or axillary temperature, 863 had a blood slide taken, of whom 332 were positive. Among 2423 patients with a recorded fever but without a blood slide result, 989 had a final discharge diagnosis of malaria. In summary, we treated 5766 patients (38.4% of all admissions aged 1 month to 15 years) as having a primary diagnosis of malaria, based predominately (82.8%) on a combination of fever, the presence of malaria parasites, and a final discharge diagnosis of malaria ( Supplementary Figure ). Information on age, discharge diagnosis, and outcome were available for all malaria-defined admissions.
Severe Malaria Classifications
Among 5766 malaria admissions aged <15 years, complete records were available for 5219 (90.5%) on the AVPU score, 5147 (89.3%) on the ability to drink/breastfeed, and 5308 (92.1%) on the history of convulsions. Convulsions were reported in 1650 children and the number of convulsions were recorded in 1292 (78.3% of those with a history of convulsions). Hemoglobin results were available for 2085 (36.2%) of malaria admissions and, where this was not available, information on an assessment for severe pallor (5272, 91%) or whether they received a blood transfusion (5450, 95%) was available to identify those patients with severe anemia. Documented evidence of indrawing and/or deep breathing was available for 5307 (92.0%) malaria admissions. A total of 2340 (40.6%) children admitted with malaria had severe malaria, defined by the presence of any criteria in Table 1 .
Among malaria admissions, the strict definition of cerebral malaria (AVPU score = U, cerebral malaria 1) was present in 81 (1.6%) of children at admission. A wider definition of cerebral involvement (AVPU score = P or U, cerebral malaria 2) was present in 299 (5.7%) children. There were 122 (2.3%) regarded as conscious but with an inappropriate response to voice (AVPU score = V). The inability to drink/breastfeed was present in 665 (12.6%) of children at presentation to the pediatric ward ( Table 2 ). Among all malaria admissions, 1057 (18.3%) had severe anemia, of whom 576 had hemoglobin levels ≤5 g/dL and 481 had severe anemia identified using clinical criteria (severe pallor or blood transfusion). Severe respiratory distress was present in 441 (8.3%) malaria admissions: 92 had deep breathing only, 305 had chest indrawing only, and 44 had both deep breathing and chest indrawing. The distributions of the various clinical phenotypes in severe malaria are summarized in Figure 2A .
Malaria Admissions and the Severe Disease Syndromes
Denomitators less than 2340 show cases with complete data; children may have had more than 1 severity feature.
Abbreviations: AVPU, alert, response to voice, response to pain, unconscious scale; CI, confidence interval; Hb, hemoglobin; IQR, interquartile range; P, responsive to pain; U, unconscious; V, responsive to a voice.

A , Overlap of malaria clinical syndromes in all malaria admissions (n = 5766), n represents total number of admissions with malaria. Square brackets denote percentage case fatality rates. B , Overlap of malaria clinical syndromes in malaria deaths (n = 211), n represents the total number of deaths in children admitted with malaria.
Mortality in Children With Various Severe Malaria Clinical Syndromes
Overall, 211 (3.7%) children admitted with malaria died during hospitalization and 163 deaths occurred in children with severe malaria (case fatality rate 7.0%). The median age for fatal cases was 33 months (interquartile range [IQR] 12–72) and surviving children were of a similar age (median 36 months, IQR 18–60).
Case fatality rates were highest among unconscious children (AVPU score = U; 44.4%); however, fatality rates were also high for children who had an AVPU score of either P or U (20.7%) or of V (7.4%; Table 2 ). The case fatality rate in those with an inability to drink/breastfeed was much lower (3.3%). The case fatality rate for 317 children with 2 or more convulsions at admission was 3.8%. The case fatality rate for the composite definition of severe anemia was 8.2%, with rates of 5.7% in those with hemoglobin levels ≤5 g/dL and 11.2% in cases of clinically defined severe anemia without a hemoglobin measurement. The overall case fatality rate for those with severe respiratory distress was 13.8%, and this was higher in those with deep breathing (20.6%), compared to those with chest indrawing (12.6%; Table 2 ).
The mortality rate in children with severe anemia plus impaired consciousness (AVPU < alert or inability drink/breastfeed) was 15.3% (n = 35); it was 17.0% (n = 19) in those with severe anemia plus severe respiratory distress, 23.9% (n = 39) in those with impaired consciousness plus respiratory distress, and was highest, 25% (n = 14), in those children with a combination of severe anemia, severe respiratory distress, and impaired consciousness ( Figure 2B ). Otherwise, all 3 phenotypes were relatively common among the deaths ( Figure 2B ).
There were 48 deaths in children who did not have any of the severity features shown in Table 2 at admission, representing 23% of all the hospital deaths from malaria, but there was a case fatality rate of only 1.4% among children classified as having nonsevere malaria at admission. These deaths were in children without obvious comorbidities (data not shown) and 90% (43/48) had positive malaria slides, but they were older (median 48 months, IQR 14–98) compared to survivors (median 36 months, IQR 18–66) who had the same clinical status at admission. The mortality rate overall was higher among children aged 1 to 3 months (10.0%, 9/90), despite representing a small group of admissions, compared to the overall mortality rate amongst older children (3.6%). All 9 children aged 1 to 3 months who died had a positive malaria smear.
Age Distribution of Malaria Admissions and Clinical Syndromes
The median age for all malaria admissions was 36 months (IQR 18–60). Of all malaria admissions to the pediatric ward, 15% were aged between 1–11 months of age, 33% were aged 1–23 months of age, and 70% were aged 1 month to 5 years ( Figure 3A ). Only 1.6% (90/5766) of malaria admissions were aged less than 3 months, and 82% had a positive malaria smear. Of the 211 deaths that occurred, 22% were in children aged 1–11 months, 41% were in children aged 1–23 months, and 66% were in children aged between 1 month and 5 years ( Figure 3B ). Admissions and deaths in children aged above 10 years were rare, comprising 489 (10%) of malaria admissions and 21 (10%) of malaria deaths. Children defined as having cerebral malaria based on an AVPU score of U were, on average, older than those with an AVPU score of P or U ( Table 1 ; Figure 3C ). Children who had a composite definition of severe malaria anemia ( Figure 3D ) or respiratory distress ( Figure 3E ) were younger on average than children with cerebral involvement ( Table 1 ).

Percentage age distributions among children aged 1 month to 15 years for ( A ) 5766 malaria admissions, ( B ) 211 malaria deaths; ( C ) 81 cerebral malaria case definition 1 (AVPU score = U; light gray) and 299 cerebral malaria case definition 2 (AVPU score = P or U; dark gray); ( D ) 1057 severe malaria anemia cases; and ( E ) 414 malaria cases with respiratory distress. Abbreviations: AVPU, alert, response to voice, response to pain, unconscious scale; P, responsive to pain; U, unconscious.
Pediatric malaria and severe malaria hospitalizations in western Kenya still predominantly affect younger pediatric age groups ( Figure 3A ); however, the mean age at admission has shifted toward older children, when compared to hospital data from a neighboring hospital from the 1990s [ 10 ]. There are increasing numbers of children presenting at older age groups, including 30% who present above 5 years of age ( Figure 3A ), and an increasing proportion of admissions with cerebral involvement ( Figure 3C ). Comparisons between hospital settings 20 years ago suggested that as the intensity of transmission declined, functional immunity to severe malaria would be acquired later in childhood, resulting in risks among older children with different disease phenotypes [ 10 , 27–31 ]. Subsequent studies of the long-term follow-up hospital surveillance of age and clinical phenotype changes against changing malaria transmission have been few. Where these studies have taken place, confirmation of a shifting age pattern toward older children has been documented when transmission declines [ 11 , 12 , 32 , 33 ].
The case fatality rates for severe malaria remain high, despite the adoption of improved case-management guidelines in these hospitals [ 15 , 17 , 18 , 20 ] ( Table 2 ; Figure 3 ). Of malaria deaths, 48 (23%) did not have any of the prognostic characteristics of severe malaria on admission, and these deaths tended to occur among older children, compared to their surviving counterparts. It is possible that these children had some of the extended features of severe malaria not captured in our surveillance (previously identified as prognostic factors), progressed to have signs of severe malaria during admission, or had another condition unidentified because of limited diagnostics. Consistent with other site-specific studies [ 34 , 35 ] and clinical trials [ 36 ] since the early 1990s [ 25 ], cerebral malaria, other neurological complications, severe anemia, and respiratory distress in this area of Kenya continue to have high case fatality rates, with increasing probabilities of death as symptoms overlap ( Table 2 ; Figure 2 ). In our series, only 36% of malaria admissions had hemoglobin measured. The group we clinically defined as having severe anemia (severe pallor and/or blood transfusion) in the absence of a hemoglobin measurement had a higher mortality rate, compared to those where an admission hemoglobin measurement was used to define severe anemia ( Table 2 ). In previous clinical observations of severe malaria, case fatality rates of severe malaria anemia have been lower than those of other syndromes [ 25 ]. This was true of our group, with a recorded hemoglobin level of <5 g/dl; however, those with a clinical definition may have other complications leading to high fatality rates, as previously described [ 37 ].
Preventing severe disease progression and improving timely access to emergency care must remain a priority across all settings in Africa. Access to hospital care in the area studied here is comparatively better than other areas of Kenya [ 38 ]; however, there remain unmet needs in managing severely ill patients with malaria in Africa [ 39 ]. As disease presentations become increasingly complex, with declining malaria transmission, improvements in supportive and primary treatments for severe malaria are still required [ 34 , 40 , 41 ].
Hospital surveillance of severe malaria and its outcomes provide important insights into the age profiles of life-threatening diseases in the surrounding communities, enabling policy-makers to redesign disease prevention targets. Despite a reduction in malaria transmission in western Kenya and a coincidental increase in the mean ages of patients with severe malaria, hospitalization, and deaths in hospital, malaria continues to be concentrated in children aged less than 36 months (60%; Figure 3A ). The use of intermittent presumptive treatment in infancy [ 42 ] with effective drugs would provide an additional strategy to existing vector-control approaches in this area. Similarly, the RTS,S/AS01 vaccine will provide preerythrocytic immunity, with clinical protection for children up to 5 years of age [ 43 ], and this may have the effect of reducing immunity to blood-stage parasites, resulting in prolonged vulnerability and increased incidences of neurological phenotypes in older children [ 43 , 44 ]. Both intermittent presumptive treatment in infancy and vaccination are strategies delivered through the routine, expanded program on immunization (EPI) services. It is, however, possible that transmission will change further in western Kenya following expanded vector control, and it might be anticipated that the disease burden will shift toward an age group not covered by EPI, in which case additional tools will need consideration. Presently, 30%, 29%, and 33% of the overall malaria hospital disease burden, severe disease burden, and malaria deaths, respectively, occur among children aged ≥5 years.
The surveillance system described here has been developed in partnership with the national and county-level ministries of health [ 13 , 20 ]. The system involves training of staff, simplified electronic data capture tools, and quality assurance and feedback. It has not been designed specifically for malaria surveillance; as such, there are characteristics of severe malaria we were unable to describe, including hypoglycemia, hyperlactatemia, hyperbilirubinemia, and hyperparasitemia. It is possible, therefore, that we misclassified febrile syndromes as caused by malaria in children with incidental parasitemia, especially in settings with limited diagnostic capabilities. With improvement in point-of-care tests [ 45–48 ], future severe malaria surveillance can be improved. As part of the proposed evaluation of the RTS,S/AS01 in Kenya, pediatric surveillance will include modifications to existing tools to improve our ability to compare coma scoring, document hemoglobinuria, provide pathogen diagnoses of meningitis, and provide improved coverage of all clinical and hematological examinations among febrile presentations. This will enable a description of any changes in the clinical epidemiology of malaria and severe disease following the introduction of the vaccine. Hospitals provide unique settings to understanding the changing clinical epidemiology of pediatric infectious diseases and vaccine-preventable disease surveillance [ 49–54 ].
In an area of western Kenya where the RTS,S/AS01 malaria vaccination is proposed as part of routine EPI, malaria admissions, severe malaria, and hospital malaria deaths continue to predominantly affect the younger pediatric age group. Case fatality rates are high among patients with cerebral malaria, which was an infrequent presentation in this part of Kenya 25 years ago. Neurological complications may increase with declining malaria transmission and may involve increasingly older patients outside of the EPI protection range. Hospital surveillance provides a routine, sustainable means to track the severe malaria phenotype, as well as future impacts of community-based controls.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
ciz844_suppl_Supplementary_Figure
Author contributions. S. A. and R. W. S. designed the experiment and concept. M. C., M. O., and S. A. undertook the data cleaning and management and conducted the preliminary analysis. S. A. and R. W. S. reviewed the final data and analysis. S. A., M. E., and R. W. S. drafted the manuscript. All authors contributed to and approved the final version.
Acknowledgments. The authors thank the Ministry of Health, which gave permission for this work to be developed and has supported the implementation of the Clinical Information Network (CIN); the county health executives, hospital management teams, Kenya Paediatric Association, Kenya Ministry of Health, and University of Nairobi for promoting the aims of the CIN; teams based in Vihiga (Victor Juma), Kakamega (Nick Aduro, Boniface Nyumbile, and Roselyne Malangachi), Busia (Emma Sarah Namulala) and Kisumu (Magdalene Kuria); members of the Kenya Medical Research Institute-Wellcome Trust program (David Kyalo, Abraham Lagat, Cynthia Khazenzi, and Basil Okola) for technical data assistance; and Philip Bejon and Kathryn Maitland for comments on an earlier version of the manuscript.
Disclaimer. This work is published with the permission of the Director of the Kenya Medical Research Institute (KEMRI). The KEMRI Scientific and Ethical Review Unit (SERU) approved the CIN study (SERU #2465 and #3459). Data for this report are under the primary jurisdiction of the Ministry of Health in Kenya. Enquiries about using the data can be made to the KEMRI-Wellcome Trust Research Programme Data Governance Committee. The funders had no role in drafting or submitting this manuscript.
Financial support. This work was supported by a Senior Wellcome Fellow award (number 207522 to M. E.) that provided funding to manage the CIN surveillance system and by the Wellcome Trust (grant numbers 092654 and 203077 to the Kenya Major Overseas Programme). S. A. is supported by the Initiative to Develop African Research Leaders Wellcome Trust (award number 107769). M. E. is supported as a Senior Wellcome Fellow (award number 207522). R. W. S. is funded as a Principal Wellcome Fellow (award numbers 103602 and 212176).
Potential conflicts of interest. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.

IMAGES
COMMENTS
Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female mosquitoes. About 3.2 billion people - almost half of the world's population - are at risk of malaria. Young children, pregnant women and non-immune travelers from malaria-free areas are particularly vulnerable to ...
Today, more than a century later, malaria continues to cause disease in millions of people worldwide. In 2015 alone, there were approximately 429,000 deaths due to malaria. Most of these deaths occur in sub-Saharan Africa in children younger than 5 years. Malaria is endemic in Africa, Latin America, Asia, parts of the Caribbean, Eastern Europe ...
Ch 1: Malaria surveillance as a core intervention • Accurate parasitological diagnosis of a malaria case is the foundation of a malaria surveillance system. • All major components of a malaria surveillance system should be integrated into broader health management information systems (HMIS), including, where applicable, systems for
EPIDEMIOLOGY. Although rarely encountered in the United States, malaria causes approximately 45% of the world's population to be at risk of infection. 2 P falciparum and P vivax are the most common causes of human malaria and have distinct geographic distributions, as in Fig. 1. P malariae is found in a similar distribution as P falciparum; P ovale is primarily found in West Africa, but ...
ppt/slides/slide71.xmläVmO#7 þ^©ÿÁÚïaó !º€H€ö$Ê!'»ïÆëM,¼¶±½K¢Sÿ{Ç/› ½iOU¿ìÚ û™™Ç3žùtº(8ª¨6LŠaÒ:h&ˆ "3&fÃäëô²ÑO ±Xd˜KA‡É'šäôä×_>© á ‚Ó ð0™ ...
Malaria in Children Lauren M. Cohee, MD, Miriam K. Laufer, MD, MPH* INTRODUCTION Malaria causes substantial morbidity and mortality in many of the most resource-limited areas of the world. In addition, malaria is a threat to travelers to endemic areas and should be considered in the evaluation of any traveler returning from a malaria-
Malaria disproportionately affects children younger than 5 years. Falciparum malaria is responsible for more than 200 000 child deaths per year in Africa and vivax malaria is well documented as a cause of severe anaemia and excess mortality in children in Asia and Oceania. For the treatment of malaria in children, paediatric dosing recommendations for several agents, including parenteral ...
Free Google Slides theme and PowerPoint template. If you want to explain some details about malaria, use this medical presentation. Provide some explanations about its diagnosis, recommendations, pathology, treatments and conclusions. It's very creative and full of cartoons.
The main clinical presentations of malaria in children are divided into uncomplicated malaria and severe . malaria, which is typically categorised as either cerebral malaria or severe malarial anaemia. Congenital malaria . Key messages • Most malaria-related deaths are in children • Paediatric dosing regimens for several antimalarials have
Background. Plasmodium falciparum accounts for the majority of malaria deaths, and is the predominant malaria species in Africa (Fig. (Fig.1) 1) [].Severe malaria (SM) is defined by the detection of P. falciparum by microscopy or a rapid diagnostic test and at least one criterion for severe disease (impaired consciousness, respiratory distress, multiple convulsions, prostration, shock ...
Malaria has a broad range of clinical presentations. Travelers who have symptoms of malaria should seek medical attention as soon as possible. ... J Pediatric Infect Dis Soc. 2020;9(3):298-304 ...
The clinical manifestations of malaria vary with parasite species, epidemiology, immunity, and age. Issues related to clinical manifestations and diagnosis of malaria will be reviewed here. Technical aspects of laboratory tools for diagnosis of malaria are discussed further separately. The epidemiology, pathogenesis, diagnosis, and treatment of ...
Malaria in Children. Malaria remains widespread throughout the planet and increasing global travel continues to lead to imported cases of malaria in travelers, including children. This article provides an overview of pediatric malaria, including its epidemiology, clinical features, diagnosis, treatment, and prevention in travelers.
Over the past 15 years, malaria mortality has reduced by approximately 50%. However, malaria still causes more than 400,000 deaths annually, most of which occur in African children under 5 years of age. Significant advances in understanding the pathogenesis of the disease provide a basis for future work to prevent severe malaria and its complications. Herein, we provide an overview of advances ...
Malaria Case Surveillance Report Form for instructions on how to report a malaria case. Evaluation and Diagnosis . ... pediatric doses. It is important to note that the base/salt conversions for antimalarials are a recurren t source of confusion and can contribute to treatment errors. In the treatment table, where appropriate, the antimalarial
Pregnancy. In malaria-endemic areas, IPTp is now recommended for all pregnant women, regardless of the number of pregnancies. Previously, it was recommended only during a woman's first and second pregnancies. WHO has updated it recommendations for 3 key malaria prevention strategies targeting children and pregnant women.
Clinical characteristics of locally acquired mosquito-transmitted malaria — U.S., May-July 2023. All 8 individuals were adults and had fever. 7/8 (88%) individuals were hospitalized. All 8 individuals received oral antimalarial treatment. All received treatment to prevent future disease relapse.
Antimalarials. Artemisinins. Malaria Vaccines. artemisinin. Malaria disproportionately affects children younger than 5 years. Falciparum malaria is responsible for more than 200 000 child deaths per year in Africa and vivax malaria is well documented as a cause of severe anaemia and excess mortality in children in Asia and Oceania.
Malaria is a mosquito-borne disease caused by five protozoa: Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and most recently implicated P.knowlesi. Infection with P. falciparum is being accounted for more than 90% of the world's malaria mortality and therefore remains an important threat to public health on a global scale.[1][2] The World Health Organization (WHO) World Malaria ...
Malaria in humans is caused by protozoan parasites of the genus Plasmodium, including Plasmodium falciparum, P. malariae, P. ovale, and P. vivax. In addition, zoonotic forms have been documented as causes of human infections and some deaths, especially P. knowlesi, a parasite of Old World (Eastern Hemisphere) monkeys, in Southeast Asia.
Education and information regarding choosing a drug to prevent malaria, including a list of all available drugs and reasons for taking or not taking a certain drug. ... Children: 5-8 kg: ½ pediatric tablet daily. 8-10 kg: ¾ pediatric tablet daily. 10-20 kg: 1 pediatric tablet daily. 20-30 kg: 2 pediatric tablets daily. 30-40 kg 3 pediatric ...
The incidences of severe pediatric malaria in both hospitals in 2020 were calculated. Inclusion, exclusion, and blood transfusion criteria were identified. Results. We analyzed 236 pediatric cases. The main clinical symptoms among all patients were severe anemia, vomiting, prostration, poor appetite, dysphoria, and dyspnea. Over 50% of patients ...
In 1972, during his pediatric residency in Boston, he joined the C.D.C. as a conscientious objector to the Vietnam War. ... With malaria becoming resistant to the most common drug treatments, he ...
The Clinical Profile of Severe Pediatric Malaria in an Area Targeted for Routine RTS,S/AS01 Malaria Vaccination in Western Kenya. ... As disease presentations become increasingly complex, with declining malaria transmission, improvements in supportive and primary treatments for severe malaria are still required [34, 40, 41].