Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.

What’s New in Breast Cancer
This section gives an overview of new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, and prevent, detect, diagnose and treat breast cancer. Advances in breast cancer care are evaluated through a rigorous process that includes clinical trials and regulatory approvals before being considered standards of care and included in breast cancer care guidelines. Komen’s research team monitors the rapidly evolving breast cancer landscape, and here we will highlight new breast cancer treatment breakthroughs, innovations in technology or key advances that may be added or are new to guidelines. We will share these research advancements to empower patients with knowledge to help them make informed decisions with their doctors.
Use these links to jump to the topics below.
- Emerging Areas in Metastatic Breast Cancer Treatment
- Clinical Trials
Treatments and Drugs
For patients, new treatments can mean more options and more hope. Researchers are working to develop new breast cancer treatment breakthroughs, such as more effective drugs that will specifically target breast cancer cells, minimize side effects and prevent breast cancer cells from coming back. While some treatments increase the effectiveness of existing drugs, others may offer new, innovative strategies for attacking tumor cells.
As of August 2023, the following new treatments and drugs are currently in clinical trials and have not yet received FDA approval:
- A new antibody-drug conjugate called datopotamab deruxtecan (Dato-DXd) is currently being evaluated in three Phase 3 clinical trials for advanced estrogen receptor-positive (ER+) [2] breast cancer, metastatic triple negative [ 3 ] breast cancer and early triple negative [ 4 ] breast cancer (TNBC). Dato-DXd specifically targets a protein called TROP2, a biomarker that can be used to target cancer cells instead of healthy cells. Another TROP2-targeting therapy called sacituzumab govitecan has already been approved for TNBC and estrogen-receptor-positive breast cancer. Dato-DXd uses a different chemotherapy drug and delivery system compared to sacituzumab govitecan.
- HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results for a Phase 2 clinical trial studying HER3-DXd, a new HER3-targeting antibody-drug conjugate for people with metastatic breast cancer . [ 1 ]. While the study found that 35% of patients responded positively to HER3-DXd, researchers will continue to evaluate which patients could benefit most from this drug through future Phase 3 clinical trials.
- CDK4/6 inhibitors are commonly used to treat estrogen receptor-positive breast cancer, but a new CDK4/6 inhibitor called trilaciclib is being tested to treat TNBC. Results from a Phase 2 clinical trial showed that trilaciclib improved outcomes for people with advanced TNBC, and the drug is currently being evaluated in the Phase 3 PRESERVE 2 clinical trial [ 5 ]. Researchers believe that unlike currently available CDK4/6 inhibitors, trilaciclib may improve response to immunotherapy and mitigate some of the side effects of chemotherapy . If this clinical trial is successful, this would be the first CDK4/6 inhibitor approved for people with TNBC.
New and improved technologies may be able to increase the speed and accuracy of detecting, diagnosing or monitoring breast cancer for progression and response to treatment.
- Doctors may use PET scans, or positron emission tomography, to scan for evidence that breast cancer has spread or metastasized. Once breast cancer has spread, the metastases may have evolved to a different type of breast cancer than the original tumor. These differences mean the metastases and the original tumor may not respond to the same treatments. A diagnostic imaging agent called Cerianna (fluoroestradiol F-18 or FES PET) allows doctors to use PET scans to learn if estrogen receptors are present in metastatic lesions. If a person has metastatic lesions that are estrogen receptor-positive, they may respond well to hormone therapy. This agent was recently incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [ 6 ] as an option for some people with metastatic or recurrent estrogen receptor-positive breast cancer to consider [ 7 ].
- Ovarian suppression increases the effectiveness of hormone therapy in some premenopausal women but comes with additional side effects that can affect quality of life. A study presented at the 2022 San Antonio Breast Cancer Symposium [ 8 ] suggests that the Breast Cancer Index , a tumor profiling test that looks at genes to predict how likely a cancer is to metastasize, may be able to identify premenopausal women that would benefit most from ovarian suppression. This test would give doctors a new tool to personalize treatment for premenopausal women with estrogen receptor-positive breast cancer. More data are needed to confirm these results.
- Doctors are getting closer to identifying which patients with early HER2-positive breast cancer can safely avoid chemotherapy by using the HER2DX genomic test. HER2DX is the first test specifically designed to identify HER2-positive patients at high and low risk for recurrence . For some people, being able to avoid chemotherapy without comprising long-term outcomes will lead to a better quality of life.

Research can take decades to reach the bedside, but what discoveries are just around the corner for patients? Susan G. Komen shares all of this and more through Breast Cancer Breakthroughs, a virtual education series focusing on the new science and technology advancements that are poised to make a difference for patients in the near future. Sign up for Breast Cancer Breakthroughs to never miss an episode.

Kimberly’s Story: Finding Joy in the Midst of a Metastatic Breast Cancer Diagnosis
After Kimberly Reinika’s mother passed away in 2019 from ovarian cancer, she worried that it would ultimately take her life, too. “That was the cancer I was checking for,” she said.
Approaches to Care
With knowledge gained from clinical trials, researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining certain drugs, finding which patients can skip certain elements of treatment or changing the order of their treatments to maximize effectiveness or minimize side effects.
- Patients with early estrogen receptor-positive breast cancer generally have a good prognosis, but some people have a higher risk of recurrence for as long as 20 years. Researchers are seeking new strategies to reduce this risk of recurrence. CDK4/6 inhibitors are used to treat advanced breast cancer, but the Phase 3 NATALEE clinical trial, presented at the 2023 American Society of Clinical Oncology Annual Meeting [ 9 ], found that using the CDK4/6 inhibitor ribociclib for two years in the adjuvant setting reduced the risk of recurrence for people with estrogen receptor-positive breast cancer.
- Inflammatory breast cancer is difficult to diagnose because its symptoms often mimic infections. Additionally, because some medical professionals don’t see it often, they may lack experience in recognizing and treating inflammatory breast cancer. In partnership with the Inflammatory Breast Cancer Research Foundation and the Milburn Foundation, Susan G. Komen launched a first-of-its kind diagnostic tool for inflammatory breast cancer. Through this scoring system, the tool considers the defining features of inflammatory breast cancer and provides data that can help providers accurately determine whether a person has inflammatory breast cancer. The goal of this tool is to increase the accuracy of diagnosing inflammatory breast cancer so that people will receive the appropriate care they need to treat this aggressive disease.
- Immunotherapy targets the immune system to help the body fight off tumors. Immunotherapy is currently only available for some patients with triple negative breast cancer, but researchers are aiming to bring this cutting edge therapy to more people. In a recent announcement [ 10 ], positive results were announced for a clinical trial that evaluated the immunotherapy drug pembrolizumab in patients with early estrogen receptor-positive breast cancer. Komen will be closely monitoring the results of this study at upcoming scientific conferences and hopes to see more promising data suggesting that a new treatment option may soon be available for patients with early estrogen receptor-positive breast cancer.
- Clinical trials are often designed using the maximum tolerated dose of a drug. However, many drugs may give the same effect with a smaller dose that results in fewer side effects for the patient. The X-7/7 clinical trial, which was presented at the 2023 ASCO Annual Meeting, tested the impact of a new treatment schedule for the chemotherapy drug capecitabine to treat metastatic breast cancer. Researchers found that people who took a higher dose of capecitabine over fewer days had fewer side effects and were able to remain on their treatment longer compared to the standard regimen. This new approach can improve the quality of life for those living with metastatic breast cancer without compromising the effectiveness of their treatments.
Komen will be closely monitoring the results of these studies and more at upcoming scientific conferences and hopes to see more promising data regarding new ways to prevent, detect, diagnose and treat breast cancer.

It Looks Promising: Uncovering New Possibilities in Breast Cancer Prevention
Is breast cancer prevention possible? Komen Scientific Advisory Board Member Dr. Kornelia Polyak is exploring a new strategy to identify and eliminate cell precursors from which tumors can grow.

Help discover cures to breast cancer, faster. New treatment breakthroughs for breast cancer come from researchers learning from people who have breast cancer, but our current data sources only represent a small portion of the breast cancer community. Help us discover the cures to breast cancer, faster, by joining ShareForCures.
What’s New in Breast Cancer References
- Hamilton, E. P., et al. (2023). “A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC).” Journal of Clinical Oncology 41(16_suppl): 1004-1004. https://meetings.asco.org/abstracts-presentations/219699
- https://classic.clinicaltrials.gov/ct2/show/NCT05104866
- https://clinicaltrials.gov/study/NCT05374512
- https://classic.clinicaltrials.gov/ct2/show/NCT05629585
- https://classic.clinicaltrials.gov/ct2/show/NCT04799249
- https://www.gehealthcare.com/about/newsroom/press-releases/ge-healthcare-announces-fes-pet-imaging-recommendation-in-nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines
- https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf (page 16)
- https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
- Stroyakovskiy, D., et al. (2023). “Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial.” Journal of Clinical Oncology 41(17_suppl): LBA500-LBA500.
- https://www.merck.com/news/merck-announces-phase-3-keynote-756-trial-met-primary-endpoint-of-pathological-complete-response-pcr-rate-in-patients-with-high-risk-early-stage-er-her2-breast-cancer/
TOOLS & RESOURCES
NEED HELP OR MORE INFORMATION?
1-877 GO KOMEN (1-877-465-6636)
Educational Resources
Komen Financial Assistance Program
Breast Cancer Research Results and Study Updates
See Advances in Breast Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
Some people with no evidence of cancer in nearby lymph nodes after presurgical chemotherapy can skip radiation to that area without increasing the risk of the cancer returning, a clinical trial found. But some experts caution that more details are needed.
For women in their 70s and older, the risk of overdiagnosis with routine screening mammography is substantial, a new study suggests. The findings highlight the need for conversations between older women and their health care providers about the potential benefits and harms of continuing screening mammography.
Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
For younger women with advanced breast cancer, the combination of ribociclib (Kisqali) and hormone therapy was much better at shrinking metastatic tumors than standard chemotherapy treatments, results from an NCI-funded clinical trial show.
In a large clinical trial, a condensed course of radiation therapy was as effective and safe as a longer standard course for those with higher-risk early-stage breast cancer who had a lumpectomy. This shorter radiation course makes treatment less of a burden for patients.
Adding the immunotherapy drug pembrolizumab (Keytruda) to chemotherapy can help some patients with advanced triple-negative breast cancer live longer. In the KEYNOTE-355 trial, overall survival improved among patients whose tumors had high levels of the PD-L1 protein.
People with metastatic breast cancer whose tumors had low levels of HER2 protein lived longer after treatment with trastuzumab deruxtecan (Enhertu) than those treated with standard chemotherapy, results of the DESTINY-Breast04 clinical trial show.
NCI researchers have shown that an experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer who have exhausted all other treatment options.
Most breast cancer risk tools were developed with data mainly from White women and don’t work as well for Black women. A new tool that estimates risk for Black women may help identify those who might benefit from earlier screening, enabling earlier diagnosis and treatment.
In people with metastatic HER2-positive breast cancer, the targeted drug trastuzumab deruxtecan (Enhertu) markedly lengthened progression-free survival compared with trastuzumab emtansine (Kadcycla), new study results show.
In a large clinical trial, women with HR-positive, HER2-negative metastatic breast cancer treated with ribociclib (Kisqali) and letrozole (Femara) as their initial treatment lived approximately 1 year longer than women treated with letrozole only.
Women with early-stage breast cancer who had one or both breasts surgically removed (a unilateral or bilateral mastectomy) had lower scores on a quality-of-life survey than women who had breast-conserving surgery, a new study has found.
For women undergoing chemotherapy for breast cancer, meeting the national physical activity guidelines may help alleviate cognitive issues, a new study suggests. The benefits may be even greater for patients who were physically active before treatment.
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC). The update follows last year’s accelerated approval of the drug for people with TNBC.
For some people with ER-positive breast cancer, a new imaging test may help guide decisions about receiving hormone therapy, according to a new study. The test can show whether estrogen receptors in tumors are active and responsive to estrogen.
The test, which helps guide treatment decisions, was not as good at predicting the risk of death from breast cancer for Black patients as for White patients, a new study has found. The findings highlight the need for greater racial diversity in research studies.
The drug abemaciclib (Verzenio) may be a new treatment option for people with the most common type of breast cancer, with new study findings suggesting that it can reduce the risk of the cancer returning.
Fertility preservation for young women with breast cancer doesn’t increase their risk of dying in the ensuing decades, a new study affirmed. Experts said the findings support routinely offering fertility preservation to patients who want it.
Some postmenopausal women with HR-positive, HER2-negative breast cancer may not benefit from chemotherapy and can safely forgo the treatment, according to clinical trial results presented at the San Antonio Breast Cancer Symposium.
A heart-related event, like a heart attack, may make breast cancer grow faster, a new study suggests. In mice, heart attacks accelerated breast tumor growth and human studies linked cardiac events with breast cancer recurrence, researchers reported.
FDA has approved sacituzumab govitecan (Trodelvy) for the treatment of triple-negative breast cancer that has spread to other parts of the body. Under the approval, patients must have already undergone at least two prior treatment regimens.
Women with high-risk breast cancer who engaged in regular exercise before their cancer diagnosis and after treatment were less likely to have their cancer return or to die compared with women who were inactive, a recent study found.
Researchers have developed a “microscaled” approach to analyze the proteins and genetic changes (proteogenomics) of a tumor that uses tissue from a core needle biopsy. The analyses can provide important information that may help guide treatment.
Tucatinib improved survival for women in the HER2CLIMB trial, including some whose cancer had spread to the brain. Trastuzumab deruxtecan improved survival and shrank many tumors in the DESTINY-Breast01 trial, which led to its accelerated approval.
A TAILORx analysis shows women with early-stage breast cancer and high recurrence scores on the Oncotype DX who received chemotherapy with hormone therapy had better long-term outcomes than what would be expected from hormone therapy alone.
Men with breast cancer may be more likely to die of the disease than women, particularly during the first 5 years after diagnosis, a new study suggests. The higher likelihood of death was linked in part to undertreatment and later diagnosis.
In a survey of nearly 600 breast cancer survivors, researchers found that the cost of care factored into the decisions the women made about what type of surgery to get. Many women also reported never discussing costs with their physicians.
FDA has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla), also called T-DM1, to include adjuvant treatment in some women with early-stage HER2-positive breast cancer.
Many women diagnosed with ovarian and breast cancer are not undergoing tests for inherited genetic mutations that can provide important information to help guide decisions about treatment and longer-term cancer screening, a new study has found.
FDA has approved atezolizumab (Tecentriq) in combination with chemotherapy for the treatment of some women with advanced triple-negative breast cancer. This is the first FDA-approved regimen for breast cancer to include immunotherapy.
The build-up of connective tissue around some types of cancer can act as a barrier to immunotherapy. A new study uses a bone marrow transplant drug, plerixafor, to break down this barrier and improve the efficacy of immune checkpoint inhibitors in animal models of breast cancer.
A new study in mice shows that disrupting the relationship between breast cancer cells that spread to bone and normal cells surrounding them makes the cancer cells sensitive to treatment.
In women with early-stage breast cancer, two clinical trials have shown that both whole- and partial-breast radiation therapy are effective at preventing the cancer from returning after breast-conserving surgery.
Researchers are testing a topical-gel form of the drug tamoxifen to see if it can help prevent breast cancer as effectively as the oral form of the drug but with fewer side effects.
Findings from a clinical study and a mouse study may shed light on genetic risk factors for developing cancer-related cognitive problems in older breast cancer survivors. The results suggest a gene associated with Alzheimer’s disease may play a role.
Arsenic trioxide and retinoic acid work together to target the master regulator protein Pin1, a new study shows. In cancer cell lines and mice, the drug combination slowed the growth of triple-negative breast cancer tumors.
FDA has expanded the approved uses of ribociclib (Kisqali) for women with advanced breast cancer, including new uses in pre- and postmenopausal women. It’s the first approval under a new FDA program to speed the review of cancer drugs.
Using a liquid biopsy to test for tumor cells circulating in blood, researchers found that, in women with breast cancer, the presence of these cells could identify women at risk of their cancer returning years later.
Findings from the TAILORx clinical trial show chemotherapy does not benefit most women with early breast cancer. The new data, released at the 2018 ASCO annual meeting, will help inform treatment decisions for many women with early-stage breast cancer.
Do cancer study participants want to receive their genetic test results? A recent study involving women with a history of breast cancer tested an approach for returning genetic research results and evaluated the impact those results had on the women.
Researchers compared the risk of death for women with breast cancer who had low skeletal muscle mass, or sarcopenia, at the time of their cancer diagnosis and women who had adequate muscle mass.
Some people who have been treated for breast cancer or lymphoma have a higher risk of developing congestive heart failure than people who haven’t had cancer, results from a new study show.
FDA has approved the CDK4/6 inhibitor abemaciclib (Verzenio) as a first-line treatment in some women with advanced or metastatic breast cancer. Under the approval, the drug must be used in combination with an aromatase inhibitor.
A new study in mice raises the possibility that using microscopic, oxygen-carrying bubbles may improve the effectiveness of radiation therapy in the treatment of breast cancer.
The drug olaparib (Lynparza®) is the first treatment approved by the Food and Drug Administration for patients with metastatic breast cancer who have inherited mutations in the BRCA1 or BRCA2 genes.
Joint pain caused by aromatase inhibitors in postmenopausal women with breast cancer can cause some women to stop taking the drugs. Reducing their symptoms may translate into better adherence to therapy.
A new study suggests that the cells in treatment-resistant tumors in women with metastatic breast cancer share important characteristics that could potentially make tumors vulnerable to therapies that otherwise might not have been considered.
A large nationwide clinical trial called TMIST has been launched to compare two techniques used for mammograms: tomosynthesis, often called 3D mammography, and standard 2D digital mammography.
FDA approved abemaciclib (Verzenio™) for the treatment of some people with advanced or metastatic HR-positive, HER2-negative breast cancer whose disease has progressed after treatment with hormone therapy.
Long-term results from a large clinical trial confirm that, for some women with early-stage breast cancer who have lumpectomy as their surgical treatment, a less extensive lymph node biopsy approach is sufficient.
When given at the same time, two immune checkpoint inhibitors were ineffective against breast cancer growth in mice, a new study found. The combination was more effective and safer if the two inhibitors were given in a specific sequence.
FDA has expanded its approval of fulvestrant (Faslodex®) as a standalone treatment for postmenopausal women with advanced HR-positive, HER2-negative breast cancer who have not previously undergone endocrine therapy.
Many women who receive taxane-based chemotherapy to treat breast cancer experience long-term nerve damage, or peripheral neuropathy, data from a large clinical trial show.
Researchers recognized the potential of endoxifen as a treatment for breast cancer and, with NCI support, developed the compound into a drug now being tested in clinical trials.
Researchers have used modified stem cells to deliver a cancer drug selectively to metastatic breast cancer tumors in mice. The stem cells target metastatic tumors by homing in on the stiff environment that typically surrounds them.
FDA has approved neratinib for patients with early-stage HER2-positive breast cancer who have finished at least 1 year of adjuvant therapy with trastuzumab.
Many survivors of early-stage breast cancer prefer that their oncologist handle aspects of routine medical care usually overseen by primary care practitioners, leading to concerns about gaps in care.
Results from the first large prospective study of breast and ovarian cancer risk in women with inherited mutations in the BRCA 1 or BRCA2 genes confirm the high risks estimated from earlier, retrospective studies.
Two clinical trials show that trastuzumab emtansine (T-DM1) improves survival compared with other standard treatments for patients with HER2-positive metastatic breast cancer that has progressed after treatment with other HER2-targeted drugs.
Using one of the largest collections of tumor samples from African Americans with breast cancer, researchers tried to assess the extent to which the molecular characteristics on these tumors might help to explain breast cancer disparities.
A new study shows that the number of women in the United States living with distant metastatic breast cancer (MBC), the most severe form of the disease, is growing. This is likely due to the aging of the U.S. population and improvements in treatment.
In a randomized trial, low-income women who role-played talking with their doctor about their survivorship care plan in a counseling session reported receiving more of their recommended care than women who did not get counseling.
The FDA has approved a new targeted therapy, ribociclib, and expanded its earlier approval of another targeted therapy, palbociclib, for some women with metastatic breast cancer.
Researchers have found that duloxetine (Cymbalta®), a drug most commonly used to treat depression, may also reduce joint pain caused by aromatase inhibitors in some women being treated for early-stage breast cancer.

Search form
- Find Stories
- For Journalists
Researchers uncover on/off switch for breast cancer metastasis
New research from Stanford and the Arc Institute could lead to a new and more effective immunotherapy and help clinicians better predict patient response to existing medicines.

Songnan Wang (left) and Lingyin Li (right) found that a protein called ENPP1 acts as an on/off switch for breast cancer metastases. High protein levels lead to a high chance of metastasis (as seen by cells growing in the dish on the left), while low levels lead to no metastasis (as seen by no cells growing in the dish on the right). (Image credit: Lingyin Li and Songnan Wang)
Despite their promise, immunotherapies fail to treat many cancers, including over 80% of some of the most advanced breast cancers. And many of those patients who do respond still experience metastases eventually. New research from Stanford University and the Arc Institute has revealed a better way to predict and improve patient responses.
A team led by Lingyin Li , associate professor of biochemistry at Stanford and Arc Core Investigator, found that a protein called ENPP1 acts as an on/off switch that controls breast cancer’s ability to both resist immunotherapy and metastasize. The study, published on Dec. 20 in the Proceedings of the National Academy of Sciences , showed that ENPP1 is produced by cancer cells and by healthy cells in and around the tumor, and that high patient ENPP1 levels are linked to immunotherapy resistance and subsequent metastases. The research could lead to new, more effective immunotherapies and help clinicians better predict patient response to existing medicines.
“Our study should offer hope for everyone,” said Li, who is also an institute scholar at Sarafan ChEM-H .
Thawing cold tumors
Immunotherapies, like pembrolizumab (Keytruda), work by blocking an immune-dampening interaction between a cancer cell and a T cell, a kind of immune cell. For this to be effective, though, T cells need to permeate the tumor. So-called “hot” tumors, like those in melanoma and a subset of lung cancer, are treatable through immunotherapies, but many others, like breast and pancreatic cancers, are “cold,” devoid of T cell infiltration.
In her quest to turn cold tumors hot, Li started with cGAMP, a molecule that cells produce when their DNA is damaged, which happens when a cell becomes cancerous. If left intact, cGAMP activates an immune response through what is known as the STING pathway, which can help make a tumor hot. Li previously discovered that cGAMP is exported outside the cells but often, before it can trigger a response, a protein called ENPP1 chews up these molecular “danger” signals. ENPP1, she proposed, helped keep cold tumors cold.
High levels of ENPP1 correlate with poor prognosis in many cancers, but the protein can perform many actions in the body, so Li set out to determine if its cGAMP-chewing ability is behind its clinical significance.
An on/off switch
Li began collaborating with two professors at the University of California, San Francisco: Hani Goodarzi, also an incoming Arc Institute Core Investigator, and Laura Van’t Veer, a clinician who leads the I-SPY 2 Trial, a groundbreaking breast cancer trial. ENPP1 levels naturally vary across individuals, so the team looked at data from patients in the I-SPY 2 Trial to see how responses to pembrolizumab varied with ENPP1 levels at the time of diagnosis.
The results were astounding. Patients with high ENPP1 levels had low response to pembrolizumab and high chance of metastases. Those with low ENPP1 levels had a high response to pembrolizumab and no metastases. ENPP1 predicted both response to immunotherapy and likelihood of relapse.
Two things were suddenly clear: that ENPP1 was critical in metastases, not just in primary tumors; and that they should be looking at ENPP1 in healthy cells, not only in cancer cells.
“Using the finest molecular scalpels developed in our lab, I was excited to dig deeper and figure out exactly how ENPP1 has such a dramatic influence on clinical outcomes,“ said Songnan Wang , an MD-PhD student in biochemistry, Arc researcher, and first author on the paper.
In a series of mouse studies, Wang proved that removing ENPP1 entirely or eliminating only its cGAMP-chewing ability in normal and cancer cells yielded exactly the same result: decreased tumor growth and decreased metastases. And the team proved that it resulted directly from suppressing the STING pathway. They found an on/off switch.
On top of the waterfall
Immune pathways are often described as “cascades” with a series of signals that trigger downstream actions that eventually lead to a response.
“For cancers to stop the immune system from detecting them, they need to build dams that block the signal from flowing,” said Li. “We have shown that ENPP1 acts like a big dam at the top of the waterfall.”
This means that clinicians can use ENPP1 levels to better determine appropriate treatment for breast cancer patients. It also means that drugs that destroy the ENPP1 dam could make existing therapies more effective – and several ENPP1 inhibitors are already in clinical development.
While this work focused on breast cancer, Li believes that ENPP1 plays a critical role in other kinds of “cold” tumors.
“I hope to inspire clinicians who treat cancers – including lung cancer, glioblastoma, and pancreatic cancer – to investigate ENPP1’s role in patient outcomes,” said Li.
Li is also a member of Stanford Bio-X and the Stanford Cancer Institute . Other Stanford co-authors include Alby Joseph and Valentino Sudyaryo (of Stanford and Arc); Volker Böhnert, Gemini Skariah, and Xuchao Lyu (of Stanford). Additional co-authors are from the University of California, San Francisco, and Arc.
This work was supported by the Arc Institute, the Stanford Chi-Li Pao Foundation Alpha Omega Alpha Student Research Fellowship, the Stanford Medical Scholars Research Program, a Stanford Graduate Fellowship, the Chemistry/Biology Interface (CBI) Predoctoral Training Program at Sarafan ChEM-H, the NSF Graduate Research Fellowship Program, an Era of Hope Scholar Award, an NIH New Innovator Award, a Pew-Steward Scholars for Cancer Research award, and the National Institutes of Health.
Lingyin Li and Volker Böhnert have filed two patents on ENPP1 inhibitors (PCT/US2020/015968 and PCT/US2018/050018) that are licensed to Angarus Therapeutics. Li is a co-founder of Angarus Therapeutics.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

How old is too old to run?

America’s graying. We need to change the way we think about age.

Can we talk?
Estrogen a more powerful breast cancer culprit than we realized.
Getty Images
Ekaterina Pesheva
HMS Communications
Potential path to better testing in findings that identify hormone as ‘a catalyst and a cause’ in disease
In what may turn out to be a long-missing piece in the puzzle of breast cancer, Harvard Medical School researchers have identified the molecular sparkplug that ignites cases of the disease currently unexplained by the classical model of breast-cancer development.
A report on the team’s work is published May 17 in Nature.
“We have identified what we believe is the original molecular trigger that initiates a cascade culminating in breast tumor development in a subset of breast cancers that are driven by estrogen,” said study senior investigator Peter Park , professor of Biomedical Informatics in the Blavatnik Institute at HMS.
The researchers said as many as one-third of breast cancer cases may arise through the newly identified mechanism.
The study also shows that the sex hormone estrogen is the culprit behind this molecular dysfunction because it directly alters a cell’s DNA.
Most, though not all, breast cancers are fueled by hormonal fluctuations . The prevailing view of estrogen’s role in breast cancer is that it acts as a catalyst for cancer growth because it stimulates the division and proliferation of breast tissue, a process that carries the risk for cancer-causing mutations. The new work, however, shows that estrogen causes mischief in a far more direct manner.
“Our work demonstrates that estrogen can directly induce genomic rearrangements that lead to cancer, so its role in breast cancer development is both that of a catalyst and a cause,” said study first author Jake Lee , a former research fellow in the Park lab who is now a medical oncology fellow at Memorial Sloan Kettering Cancer Center.
Although the work has no immediate implications for therapy, it could inform the design of tests that can track treatment response and could help doctors detect the return of tumors in patients with a history of certain breast cancers.
Birth of a cancer cell
The human body is made up of hundreds of trillions of cells. Most of these cells are constantly dividing and replicating, a process that sustains the function of organs day after day, over a lifetime.
With each division, a cell makes a copy of its chromosomes — bundles of tightly compressed DNA — into a new cell. But this process sometimes goes awry, and DNA can break. In most cases, these DNA breaks get swiftly mended by the molecular machinery that guards the integrity of the genome. However, every now and then, the repair of broken DNA gets botched, causing chromosomes to get misplaced or scrambled inside a cell.
Many human cancers arise in this manner during cell division, when chromosomes get rearranged and awaken dormant cancer genes that can trigger tumor growth.
One such chromosomal scramble can occur when a chromosome breaks, and a second copy of the broken chromosome is made before the break gets fixed.
Then, in what ends up being a botched repair attempt, the broken end of one chromosome is fused to the broken end of its sister copy rather than to its original partner. The resulting new structure is a misshapen, malfunctioning chromosome.
During the next cell division, the misshapen chromosome is stretched between the two emerging daughter cells and the chromosome “bridge” breaks, leaving behind shattered fragments that contain cancer genes to multiply and get activated.
“Our work demonstrates that estrogen can directly induce genomic rearrangements that lead to cancer, so its role in breast cancer development is both that of a catalyst and a cause.” Jake Lee, medical oncology fellow at Memorial Sloan Kettering Cancer Center
Certain human cancers, including some breast cancers, arise when a cell’s chromosomes get rearranged in this way. This malfunction was first described in the 1930s by Barbara McClintock , who went on to win the Nobel Prize in physiology or medicine in 1983.
Cancer experts can often identify this particular aberration in tumor samples by using genomic sequencing. Yet, a portion of breast cancer cases do not harbor this mutational pattern, raising the question: What is causing these tumors?
These were the “cold” cases that intrigued study authors Park and Lee. Looking for answers, they analyzed the genomes of 780 breast cancers obtained from patients diagnosed with the disease. They expected to find the classical chromosomal disarray in most of the tumor samples, but many of the tumor cells bore no trace of this classic molecular pattern.
Instead of the classic misshapen and improperly patched-up single chromosome, they saw that two chromosomes had fused, suspiciously near “hot spots” where cancer genes are located.
Just as in McClintock’s model, these rearranged chromosomes had formed bridges, except in this case, the bridge contained two different chromosomes. This distinctive pattern was present in one-third (244) of the tumors in their analysis.
Lee and Park realized they had stumbled upon a new mechanism by which a “disfigured” chromosome is generated and then fractured to fuel the mysterious breast cancer cases.
A new role for estrogen in breast cancer?
When the researchers zoomed onto the hot spots of cancer-gene activation, they noticed that these areas were curiously close to estrogen-binding areas on the DNA.
Estrogen receptors are known to bind to certain regions of the genome when a cell is stimulated by estrogen. The researchers found that these estrogen-binding sites were frequently next to the zones where the early DNA breaks took place.
This offered a strong clue that estrogen might be somehow involved in the genomic reshuffling that gave rise to cancer-gene activation.
Lee and Park followed up on that clue by conducting experiments with breast cancer cells in a dish. They exposed the cells to estrogen and then used CRISPR gene editing to make cuts to the cells’ DNA.
As the cells mended their broken DNA, they initiated a repair chain that resulted in the same genomic rearrangement Lee and Park had discovered in their genomic analyses.
Estrogen is already known to fuel breast cancer growth by promoting the proliferation of breast cells. However, the new observations cast this hormone in a different light.
They show estrogen is a more central character in cancer genesis because it directly alters how cells repair their DNA.
The findings suggest that estrogen-suppressing drugs such as tamoxifen — often given to patients with breast cancer to prevent disease recurrence — work in a more direct manner than simply reducing breast cell proliferation.
“In light of our results, we propose that these drugs may also prevent estrogen from initiating cancer-causing genomic rearrangements in the cells, in addition to suppressing mammary cell proliferation,” Lee said.
The study could lead to improved breast cancer testing. For instance, detecting the genomic fingerprint of the chromosome rearrangement could alert oncologists that a patient’s disease is coming back, Lee said.
A similar approach to track disease relapse and treatment response is already widely used in cancers that harbor critical chromosomal translocations, including certain types of leukemias.
More broadly, the work underscores the value of DNA sequencing and careful data analysis in deepening the biology of cancer development, the researchers said.
“It all started with a single observation. We noticed that the complex pattern of mutations that we see in genome sequencing data cannot be explained by the textbook model,” Park said. “But now that we’ve put the jigsaw puzzle together, the patterns all make sense in light of the new model. This is immensely gratifying.” Additional authors included Youngsook Lucy Jung, Taek-Chin Cheong, Jose Espejo Valle-Inclan, Chong Chu, Doga C. Gulhan,Viktor Ljungstrom, Hu Jin, Vinayak Viswanadham, Emma Watson, Isidro Cortes-Ciriano, Stephen Elledge, Roberto Chiarle, and David Pellman.
This work was funded by grants from Ludwig Center at Harvard, Cancer Grand Challenges, Cancer Research UK, and the Mark Foundation for Cancer Research, National Institutes of Health grant 1R01-CA222598, and with additional support from the Office of Faculty Development/CTREC/BTREC Career Development Fellowship.
Share this article
You might like.
No such thing, specialist says — but when your body is trying to tell you something, listen

Experts say instead of disability, focus needs to shift to ability, health, with greater participation, economically and socially

Study finds that conversation – even online – could be an effective strategy to help prevent cognitive decline and dementia
When math is the dream
Dora Woodruff was drawn to beauty of numbers as child. Next up: Ph.D. at MIT.
Seem like Lyme disease risk is getting worse? It is.
The risk of Lyme disease has increased due to climate change and warmer temperature. A rheumatologist offers advice on how to best avoid ticks while going outdoors.
Three will receive 2024 Harvard Medal
In recognition of their extraordinary service
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
siRNA treatment targeting integrin α11 overexpressed via EZH2-driven axis inhibits drug-resistant breast cancer progression
Authors: Prakash Chaudhary, Kiran Yadav, Ho Jin Lee, Keon Wook Kang, Jongseo Mo and Jung-Ae Kim
Quantitative characterization of breast lesions and normal fibroglandular tissue using compartmentalized diffusion-weighted model: comparison of intravoxel incoherent motion and restriction spectrum imaging
Authors: Litong He, Yanjin Qin, Qilan Hu, Zhiqiang Liu, Yunfei Zhang and Tao Ai
AMD1 promotes breast cancer aggressiveness via a spermidine-eIF5A hypusination-TCF4 axis
Authors: Ruocen Liao, Xingyu Chen, Qianhua Cao, Longchang Bai, Chenglong Ma, Zhijun Dai and Chenfang Dong
NSABP FB-10: a phase Ib/II trial evaluating ado-trastuzumab emtansine (T-DM1) with neratinib in women with metastatic HER2-positive breast cancer
Authors: Samuel A. Jacobs, Ying Wang, Jame Abraham, Huichen Feng, Alberto J. Montero, Corey Lipchik, Melanie Finnigan, Rachel C. Jankowitz, Mohamad A. Salkeni, Sai K. Maley, Shannon L. Puhalla, Fanny Piette, Katie Quinn, Kyle Chang, Rebecca J. Nagy, Carmen J. Allegra…
Screening mammography performance according to breast density: a comparison between radiologists versus standalone intelligence detection
Authors: Mi-ri Kwon, Yoosoo Chang, Soo-Youn Ham, Yoosun Cho, Eun Young Kim, Jeonggyu Kang, Eun Kyung Park, Ki Hwan Kim, Minjeong Kim, Tae Soo Kim, Hyeonsoo Lee, Ria Kwon, Ga-Young Lim, Hye Rin Choi, JunHyeok Choi, Shin Ho Kook…
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .
Breast cancer rates rising among Canadian women in their 20s, 30s and 40s
Researchers highlights need for immediate shift in public health policy as early detection is key to reducing breast cancer death and complications.
Rates of breast cancer in women under the age of 50 are rising in Canada according to a study which showed an increase in breast cancer diagnoses among females in their Twenties, Thirties, and Forties.
Led by Dr. Jean Seely, this study published in the Canadian Association of Radiologists Journal reviewed breast cancer cases over 35 years to shed light on trends in breast cancer detection in Canada.
"Breast cancer in younger women tends to be diagnosed at later stages and is often more aggressive," said Dr. Seely, Head of Breast Imaging at The Ottawa Hospital and Professor in the Department of Radiology at the University of Ottawa. "It's alarming to see rising rates among women in their Twenties and Thirties because they are not regularly screened for breast cancer."
Risk increases with age
Using data from the National Cancer Incidence Reporting System (1984-1991) and the Canadian Cancer Registry (1992-2019) at Statistics Canada, the research team, which included Larry Ellison from Statistics Canada and Dr. Anna Wilkinson, an Associate Professor in the Faculty of Medicine, looked at all women aged 20 to 54 who were diagnosed with breast cancer.
Their findings included:
- For women in their Twenties, there were 3.9 cases per 100,000 people between 1984 and 1988, compared to 5.7 cases per 100,000 between 2015 and 2019 for a 45.5% increase.
- For women in their Thirties, there were 37.7 cases per 100,000 people between 1984 and 1988, compared to 42.4 cases per 100,000 between 2015 and 2019 for a 12.5% increase.
- For women in their Forties, there were 127.8 cases per 100,000 people between 1984 and 1988, compared to 139.4 cases per 100,000 between 2015 and 2019 for a 9.1% increase.
The study's results show the importance of targeting younger women in breast cancer awareness campaigns and screening programs. Most public health efforts focus on women over 50, but these findings suggest that younger women are increasingly at risk and may benefit from earlier and more frequent screenings.
Personal experience
Chelsea Bland is one of those women.
Hearing about a death from breast cancer at age 33 led Chelsea -- then 28 -- to conduct her own self-examination, where she discovered a lump. This led to screenings which ultimately led to a breast cancer diagnosis and subsequent treatment. While she is two years cancer free, she remains on hormone therapy today. The entire experience led Chelsea to help establish a local group that provides peer support for younger women -- the average ages range between 28 to 40.
"I hope that by bringing awareness to this study it makes people think twice about saying that being in your twenties, thirties and forties is too young to have breast cancer. In my support group, I have heard the same story over and over again," Chelsea says. "Young women are not being taken seriously after they find a lump because they are told they are too young for breast cancer. This has ultimately led to delays in being diagnosed and being diagnosed at a more advanced stage. We are not too young for this and this is happening to women who do not have any high-risk genetic markers for breast cancer, myself included."
Improving awareness
The investigators say more research is needed to understand the root cause of rising breast cancer rates among younger women, information that could be used to develop targeted intervention strategies.
"We're calling for increased awareness among health-care professionals and the public regarding the rising incidence of breast cancer in younger women," said Dr. Seely, who alongside Dr. Wilkinson have long documented the benefits of early detection with screening for women in their forties. "We need to adapt our strategies and policies to reflect these changing trends, ensuring that all women, regardless of age, have access to the information and resources they need to detect and combat this disease."
- Breast Cancer
- Women's Health
- Breastfeeding
- Colon Cancer
- Diseases and Conditions
- Lung Cancer
- Breast cancer
- Mammography
- Breast reconstruction
- Breast implant
- Cervical cancer
- Ovarian cancer
- Colorectal cancer
Story Source:
Materials provided by University of Ottawa . Original written by Paul Logothetis. Note: Content may be edited for style and length.
Journal Reference :
- Jean M. Seely, Larry F. Ellison, Jean-Michel Billette, Shary X. Zhang, Anna N. Wilkinson. Incidence of Breast Cancer in Younger Women: A Canadian Trend Analysis . Canadian Association of Radiologists Journal , 2024; DOI: 10.1177/08465371241246422
Cite This Page :
Explore More
- Mice Given Mouse-Rat Brains Can Smell Again
- New Circuit Boards Can Be Repeatedly Recycled
- Collisions of Neutron Stars and Black Holes
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Breast Cancer Treatments: Updates and New Challenges
Anna burguin.
1 Department of Molecular Medicine, Faculty of Medicine, Université Laval, Quebec City, QC G1T 1C2, Canada; [email protected]
2 Cancer Research Center, CHU de Québec-Université Laval, Quebec City, QC G1V 4G2, Canada; [email protected]
Caroline Diorio
3 Department of Preventive and Social Medicine, Faculty of Medicine, Université Laval, Quebec City, QC G1T 1C2, Canada
Francine Durocher
Associated data.
The study did not report any data.
Breast cancer (BC) is the most frequent cancer diagnosed in women worldwide. This heterogeneous disease can be classified into four molecular subtypes (luminal A, luminal B, HER2 and triple-negative breast cancer (TNBC)) according to the expression of the estrogen receptor (ER) and the progesterone receptor (PR), and the overexpression of the human epidermal growth factor receptor 2 (HER2). Current BC treatments target these receptors (endocrine and anti-HER2 therapies) as a personalized treatment. Along with chemotherapy and radiotherapy, these therapies can have severe adverse effects and patients can develop resistance to these agents. Moreover, TNBC do not have standardized treatments. Hence, a deeper understanding of the development of new treatments that are more specific and effective in treating each BC subgroup is key. New approaches have recently emerged such as immunotherapy, conjugated antibodies, and targeting other metabolic pathways. This review summarizes current BC treatments and explores the new treatment strategies from a personalized therapy perspective and the resulting challenges.
1. Introduction
Breast cancer (BC) is the most frequent cancer and the second cause of death by cancer in women worldwide. According to Cancer Statistics 2020, BC represents 30% of female cancers with 276,480 estimated new cases and more than 42,000 estimated deaths in 2020 [ 1 ].
Invasive BC can be divided into four principal molecular subtypes by immunohistological technique based on the expression of the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) [ 2 ]. Luminal A BC (ER+ and/or PR+, and HER2-) represents around 60% of BC and is associated with a good prognosis [ 3 ]. Luminal B BC (ER+ and/or PR+, and HER2+) represents 30% of BC and is associated with high ki67 (>14%), a proliferation marker, and a poor prognosis [ 4 ]. HER2 BC (ER-, PR-, and HER2+) represents 10% of BC and is also associated with a poor prognosis [ 5 ]. Lastly, triple-negative BC (TNBC) (ER-, PR-, and HER2-) represents 15–20% of BC and is associated with more aggressivity and worse prognosis compared to other BC molecular subtypes and often occurs in younger women [ 6 ]. Characteristics of BC by molecular subtypes are described in Figure 1 .

Characteristics of breast cancer molecular subtypes. ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer. a . Frequency derived from Al-thoubaity et al. [ 12 ] and Hergueta-Redondo et al. [ 13 ]. b . Grade derived from Engstrom et al. [ 14 ]. c . Prognosis derived from Hennigs et al. [ 15 ] and Fragomeni et al. [ 16 ]. d . The 5–year survival rate derived from the latest survival statistics of SEER [ 7 ].
The 5-year relative BC-specific survival rate of BC is encouraging with 90.3% for all subtypes and stages. However, for metastatic BC the 5-year relative cancer-specific survival rate is still low: 29% regardless of subtype and can drop to 12% for metastatic TNBC [ 7 ]. This clearly indicates that strategies of treatment for metastatic BC patients are not effective enough to ensure a good survival rate. Thus, it is crucial to find new solutions for the treatment of metastatic BC and especially TNBC.
Treatment choice is based on the grade, stage, and BC molecular subtype to have the most personalized, safe, and efficient therapy. The grade describes the appearance of tumor cells compared to normal cells. It includes tubule differentiation, nuclear pleomorphism, and the mitotic count [ 8 ]. The stage is used to classify the extent of cancer in the body and is defined using the TNM system comprising tumor size, lymph node status, and the presence of metastases [ 9 ]. For non-metastatic BC, the strategic therapy involves removing the tumor by complete or breast-conserving surgery with preoperative (neoadjuvant) or postoperative (adjuvant) radiotherapy and systemic therapy including chemotherapy, and targeted therapy. Targeted therapy comprises endocrine therapy for hormone receptor-positive (HR+) BC and anti-HER2 therapy for HER2+ BC. Unfortunately, there is no available targeted therapy for the TNBC subtype. For metastatic BC the priority is to contain tumor spread as this type of BC remains incurable. The same systemic therapies are used to treat metastatic BC [ 10 ].
Challenges in the treatment of BC including dealing with treatment resistance and recurrence. Indeed, 30% of early-stage BC have recurrent disease, mostly metastases [ 11 ]. Thus, it is crucial to develop new strategic therapies to treat each BC subgroup effectively.
This review will summarize current treatments for invasive BC, the underlying resistance mechanisms and explore new treatment strategies focusing on personalized therapy and the resulting challenges.
2. Common Treatments for All Breast Cancer Subtypes
In addition to surgery, radiotherapy and chemotherapy are used routinely to treat all BC subtypes [ 17 ].
2.1. Surgery
The most standard breast surgery approaches are either total excision of the breast (mastectomy), usually followed by breast reconstruction, or breast-conserving surgery (lumpectomy). Lumpectomy entails the excision of the breast tumor with a margin of surrounding normal tissue. The recommended margins status is defined as “no ink on tumor”, meaning no remaining tumor cells at the tissue edge [ 18 ]. Studies show that total mastectomy and lumpectomy plus irradiation are equivalent regarding relapse-free and overall survival (OS) [ 19 ]. Contraindications for breast-conserving surgery include the presence of diffuse microcalcifications (suspicious or malignant-appearing), disease that cannot be incorporated by local excision with satisfactory cosmetic result, and ATM (ataxia-telangiesctasia mutated) mutation (biallelic inactivation) [ 18 ].
The surgery to remove axillary lymph nodes is useful to determine cancerous cell spread and for therapeutic purposes. For instance, axillary lymph node dissection (ALND) can improve survival rated by removing remaining tumor cells. ALND used to be the goal standard for removing positive lymph nodes. However, clinical trials showed that sentinel lymph node biopsy (SLNB) had the same effect as ALND regarding disease-free survival (DFS) and OS [ 20 ]. Other clinical trials demonstrated that ALND was not necessary for all patients with positive lymph nodes. Moreover, most patients who receive radiation and systemic treatment after SLNB have negative lymph nodes as these treatments are sufficient in eliminating residual tumor cells [ 21 ].
2.2. Radiotherapy
Radiation therapy has been used to treat cancer since Röngten discovered the X-ray in 1895 [ 22 ]. High-energy radiations are applied to the whole breast or a portion of the breast (after breast-conservative surgery), chest wall (after mastectomy), and regional lymph nodes [ 23 ]. A meta-analysis showed that radiation following conservative surgery offered more benefits to patients with higher-risk BC while patients with small, low-grade tumors could forego radiation therapy [ 24 ]. Postmastectomy radiation to the chest wall in patients with positive lymph nodes is associated with decreased recurrence risk and BC mortality compared to patients with negative lymph nodes [ 25 ]. A radiation boost to the regional node radiation treatment can be incorporated after mastectomy for patients at higher risk for recurrence [ 26 ]. This additional radiation boost to regional nodes following mastectomy is associated with improved (DFS) but is also associated with an increase in radiation toxicities such as pneumonitis and lymphedema [ 27 ]. Radiotherapy can be administered concurrently with personalized therapy (anti-HER2 therapy or endocrine therapy).
As one of the major side effects of radiotherapy is cardiotoxicity, it is critical to minimize exposure to the heart and lungs [ 28 ]. Additional techniques can be used to reduce the radiation exposure to the heart, lungs, and normal tissue such as prone positioning, respiratory control, or intensity-modulated radiotherapy [ 29 ].
Advanced invasive BC can exhibit radiation therapy resistance [ 30 ]. The hypoxic tumor microenvironment, which lacks oxygen, leads to increased cell proliferation, apoptosis resistance, and radiotherapy resistance [ 31 ]. The major player of this resistance is the HIF-1α (hypoxia-inducible factor 1 alpha) protein [ 32 ]. Indeed, HIF-1α overexpression is caused by low oxygen levels within the microenvironment and promotes the maintenance of hypoxia by allowing tumoral cells to survive in a hypoxic microenvironment [ 33 , 34 , 35 ]. Cancer stem cells (CSC) could also have a role in radiation therapy resistance [ 36 ]. CSC can self-renew and initiate subpopulations of differential progeny, and a hypoxic microenvironment is ideal for CSC survival and proliferation [ 37 , 38 ].
Radiation therapy is used to treat all BC subtypes, but its implication is more important for TNBC, as there is no personalized therapy for this subtype. It has been shown that radiotherapy benefits TNBC patients both after conserving surgery and mastectomy [ 39 ].
2.3. Chemotherapy
BC chemotherapy comprises several families of cytotoxic drugs, including alkylating agents, antimetabolites and tubulin inhibitors [ 40 ]. Cyclophosphamide is a nitrogen mustard alkylating agent causing breakage of the DNA strands [ 41 ]. The mechanism of action for anthracyclines (doxorubicin, daunorubicin, epirubicin, and idarubicin) includes DNA intercalation, thereby inhibiting macromolecular biosynthesis [ 42 ]. Taxanes, including docetaxel and paclitaxel, bind to microtubules and prevent their disassembly, leading to cell cycle arrest and apoptosis [ 43 ].
Chemotherapy can be administered in the neoadjuvant or adjuvant setting and for metastatic BC treatment.
2.3.1. Neoadjuvant Chemotherapy (NAC)
Neoadjuvant chemotherapy was initially administered for non-metastatic but inoperable BC, defined as unreachable tumors [ 44 ]. Then, chemotherapy was used before the surgery for operable tumors to facilitate breast conservation [ 45 ].
Studies demonstrated that chemotherapy administered before surgery is as effective as administered after surgery [ 46 , 47 , 48 ]. The NSABP-B-18 trial compared the effects of doxorubicin and cyclophosphamide administered either postoperatively or preoperatively. This trial showed that NAC reduces the rate of axillary metastases in node-negative BC patients [ 48 ].
Some patients fail to achieve pathologic complete response after a full course of NAC. Unfortunately, there is no consensus regarding the treatment strategy to follow for patients with residual disease after surgery [ 49 , 50 ]. The BC subtype plays an important role in the response to NAC. Indeed, TNBC and HER2+ BC are more likely to be sensitive to chemotherapy. Hence, NAC is a good strategy to maximize pathologic complete response in these BC subtypes [ 45 ].
2.3.2. Adjuvant Chemotherapy
Adjuvant chemotherapy is administered to BC patients with lymph nodes metastases or a high risk of recurrence [ 51 ]. The standard chemotherapy treatment comprises an anthracycline and a taxane. The two most common regimens are cyclophosphamide and doxorubicin for four cycles followed by paclitaxel for four cycles. Then patients are given the previous combination of therapies followed by either weekly paclitaxel for 12 weeks, or docetaxel every 3 weeks for four cycles [ 52 , 53 ].
Like neoadjuvant therapy, patients with HR-negative BC receive more benefits from adjuvant therapy (i.e., reduction of BC recurrence and mortality) than HR+ BC patients [ 54 ]. However, for patients with HR+, node-negative BC associated with a high Oncotype recurrence score (≥31), calculated from the expression of 16 BC-related genes and 5 reference genes, adjuvant chemotherapy reduces the risk of recurrence [ 55 ]. The TAILORx clinical trial showed that HR+ BC patients with a low Oncotype recurrence score do not benefit from chemotherapy alone [ 56 ].
According to the molecular BC subtype, chemotherapy can be administered with targeted therapies. Patients with HR+ BC should receive endocrine therapy after chemotherapy is completed, and HER2+ BC patients should receive trastuzumab combined with chemotherapy [ 57 ]. For TNBC patients, front-line therapy includes a combination of taxane and anthracycline [ 58 ].
One of the major drawbacks of chemotherapy is its side effects. The early side effects (0–6 months of treatment) involve fatigue, alopecia, cytopenia (reduction in the number of normal blood cells), muscle pain, neurocognitive dysfunction, and chemo-induced peripheral neuropathy. The chronic or late side effects (after 6 months of treatment) include cardiomyopathy, second cancers, early menopause, sterility, and psychosocial impacts [ 59 ].
As mentioned previously in this review, chemotherapy is composed of taxanes, anthracyclines and cyclophosphamide. Each of these molecules can lead to resistance in BC patients [ 60 ].
One mechanism of resistance is by overexpressing p-glycoprotein, an ATP-binding cassette (ABC) family member, which confers resistance to anthracycline and taxanes [ 61 ]. Breast cancer resistance protein (BCRP), another ABC family member, induces resistance to anthracycline but not taxanes when overexpressed [ 62 ]. Microtubule alterations can also lead to taxane resistance. The overexpression of β-tubulin III induces paclitaxel resistance [ 63 ]. Moreover, mutations in microtubule-associated proteins (MAPs) affect microtubule dynamics and improve taxane resistance [ 64 ]. Multiple enzymes are known to be involved in the cyclophosphamide detoxification, leading to its resistance. For example, aldehyde dehydrogenase upregulation detoxifies aldophosphamide a type of cyclophosphamide, and mutations in glutathione S-transferases, enzymes involved in drug-metabolizing conjugation reactions, can also affect cyclophosphamide detoxification [ 65 , 66 ].
Surgery, radiotherapy, and chemotherapy are complementary strategies in the treatment of BC patients. However, they are not sufficient to effectively treat all BC molecular subtypes, as they do not have the same response to radiotherapy or chemotherapy. Thus, personalized therapies are essential in the process for BC treatment.
3. Current Personalized Treatments for Breast Cancer: Strengths and Weaknesses
The current strategies of treatment are principally based on the tumor progression and BC molecular subtypes in order to offer the most personalized treatment for BC patients. The algorithm of BC treatment is represented in Figure 2 .

Breast cancer treatment flow diagram. ( A ). Early-stage breast cancer. ( B ). Metastatic/advanced breast cancer. a Neoadjuvant chemotherapy for HR+ BC patients is not systematic. It is mainly administered to luminal B BC patients and/or elder BC patients. HR+: hormone receptors positive; HER2+: human epidermal growth factor receptor 2 positive; TNBC: triple-negative breast cancer; AIs: aromatase inhibitors; T-DM1: trastuzumab-emtansine.
3.1. Endocrine Therapy
Endocrine therapy is the main strategy to treat HR positive invasive BC. The purpose of this therapy is to target the ER directly (selective estrogen receptors modulators and degraders) or the estrogen synthesis (aromatase inhibitors) [ 67 ]. The most common types of endocrine therapy are selective estrogen receptor modulators (SERMs), selective modulators estrogen receptor degraders (SERDs), and aromatase inhibitors (AIs) [ 68 ]. Endocrine therapy mechanism of action and resistance are described in Figure 3 .

Endocrine therapy mechanisms of action and resistance. The left part of the figure shows the mechanism of endocrine therapy through aromatase inhibitors, tamoxifen, and fulvestrant. The right part of the figure describes the mechanisms of resistance to endocrine therapy through the epigenetic modifications, the increase of coactivators and cell cycle actors, and the activation of other signaling pathways. Estrogens can go through the plasma membrane by a. diffusion as they are small non-polar lipid soluble molecules; b. binding to membrane ER initiating the activation of Ras/Raf/MAPK and PI3K/Akt signaling pathways which are blocked by tamoxifen. 1: inhibition of ER dimerization; 2: blockage of nucleus access; 3: ER degradation. ER: estrogen receptor; AIB1: amplified in breast cancer 1; IGF-1R: insulin growth factor receptor 1; IGF: insulin growth factor; HER: human epidermal receptors; EGF: epidermal growth factor; HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor alpha; MEK/MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3-kinase; mTOR: mammalian target of rapamycin; Me: methylation; Ac: acetylation.
3.1.1. Selective Estrogen Receptor Modulators (SERMs)
SERMs, such as tamoxifen, toremifene, bazedoxifene, and raloxifene, are antiestrogens that compete with estrogen by binding to the ER. This binding changes the conformation of the ER ligand-binding domain, and once ER is translocated to the nucleus, it blocks co-factor recruitment and subsequent genes transcription involved in cell cycle progression (cyclin D1), cell proliferation (like IGF-1), or cell migration (collagenase) [ 69 , 70 ].
The most used SERMs is tamoxifen, approved by the US Food and Drugs Administration (FDA) in 1977. It is an adjuvant therapy orally administered for 5 to 10 years according to tumor aggressivity. Tamoxifen adjuvant treatment reduces recurrence risk by 50% for the first 5 years and 30% for the next 5 years [ 71 ]. Tamoxifen is given to either premenopausal or postmenopausal patients. However, for high-risk premenopausal patients, adding ovarian suppression is more effective than tamoxifen alone [ 72 ]. Tamoxifen can also be administered as neoadjuvant treatment, especially for elderly BC patients [ 73 ]. However, studies have demonstrated no difference in OS for ER+ BC patients when neoadjuvant tamoxifen is compared to surgery [ 74 , 75 ].
Other SERMs have since been developed, such as toremifene approved by the FDA in 1997 [ 76 ]. Studies comparing the effect of toremifene and tamoxifen in premenopausal patients with ER+ advanced BC have shown that toremifene efficacy and safety are similar to tamoxifen [ 77 , 78 ]. Bazedoxifene and raloxifene are administered as prevention treatment to postmenopausal patients at high risk of developing invasive BC and for preventing osteoporosis [ 79 , 80 , 81 ].
The most frequent adverse events of SERMs are hot flushes, nausea, vomiting, vaginal bleeding/discharges, and increased risk of thromboembolic events [ 82 ]. Of note, about 40% of HR+ BC patients will develop resistance to SERMs [ 83 ]. SERMs resistance can occur by the loss of ER expression or functions. Epigenetic modifications such as hypermethylation of CpG islands or histone deacetylation can lead to transcriptional repression of ER [ 84 ]. Another potential mechanism for ER expression loss is the overpopulation of ER-negative cells in heterogenous ER+ tumors [ 85 ]. Mutations in the ligand-binding domain of ER gene ( ESR1 ) inhibit the binding of estrogen to the ER leading to the abolition of downstream signaling. Moreover, abnormal splicing can lead to truncated, nonfunctional ER protein [ 86 , 87 ]. Another explanation for SERMs resistance is the abnormal expression of ER coregulators [ 88 ]. Coregulators are very important in the ER pathway as they can increase or decrease ER activity depending on incoming signals [ 89 ]. The most studied coregulator involved in SERMs resistance is the AIB1 (Amplified in breast cancer 1) coactivator protein, often overexpressed in resistant breast tumors [ 90 ]. In particular, in ER+ cells that overexpress HER2, there is a crosstalk between HER2 and AIB1. HER2 induces phosphorylation of AIB1 leading to evasion and subsequent activation of the ER signaling pathway even though it is inhibited by SERMs [ 91 ]
3.1.2. Selective Estrogen Receptor Degraders (SERDs)
To counteract the large proportion of tamoxifen-resistant tumors, a new type of therapeutic agents with a different mechanism of action has been developed: SERDs. In contrast to SERMs, SERDs completely block the ER signaling pathway.
Fulvestrant is the main SERD administered. It was discovered by Wakeling and collaborators in 1987 and demonstrated pure anti-estrogen activity [ 92 ]. Fulvestrant binds to ER with a higher affinity than tamoxifen. Once it binds to the ER, it inhibits receptor dimerization and then blocks ER translocation to the nucleus leading to its degradation [ 93 , 94 , 95 ].
Fulvestrant is administered by intramuscular injections, and common adverse effects are nausea, pain, and headaches [ 96 ]. Fulvestrant is approved to treat postmenopausal and premenopausal patients with ovarian function suppression, with ER+ advanced or metastatic BC on prior endocrine therapy [ 97 ]. More recently (in 2017), fulvestrant was approved as first-line monotherapy for advanced ER+ breast cancer [ 98 ]. According to the 2021 NCCN guidelines, fulvestrant combined with endocrine therapy or CDK4/6 inhibitors is one of the preferred regimens for second-line therapy in ER+ advanced or metastatic BC [ 99 ]. The combination of fulvestrant with other endocrine therapies has not shown any advantages over fulvestrant used in monotherapy [ 100 , 101 ]. Clinical studies have shown benefits from fulvestrant when administered in higher doses to patients with ESR1 -mutated advanced BC [ 102 , 103 ]. Indeed, ESR1 mutations occur in nearly 20% of cases of ER+ BC [ 86 ].
However, fulvestrant can lead to resistance by different mechanisms. For example, by upregulating the PI3K (phosphatidylinositol 3-kinase), mTOR (mammalian target of rapamycin) and Ras-ERK (extracellular signal-regulated kinase) signaling pathways. PI3K/Akt/mTOR is a downstream signaling pathway of ER activation and plays an important role in antiestrogen therapy resistance [ 104 ]. PI3K pathway activation can occur independently of ER by binding to the epidermal growth factor (EGF) [ 105 ]. Moreover, it has been shown that Akt overexpression leads to fulvestrant resistance [ 106 ]. IGF-1R activation (insulin-like growth factor 1 receptor) may be another mechanism of resistance to fulvestrant. IGF-1R expression is involved in cell survival and promotes metastatic cell proliferation. The interaction between IGF-1R and ER initiates the activation of IGF-1R/MAPK (mitogen-activated protein kinase) and IGF-1R/PI3K signaling leading to antiestrogen resistance [ 107 ].
3.1.3. Aromatase Inhibitors (AIs)
Aromatase is a cytochrome P50 enzyme involved in the synthesis of androgens and estrogens [ 108 ]. Aromatase is found in the breast, uterus, and other estrogen-sensitive tissues in specific levels depending on menopausal status [ 109 , 110 ]. Aromatase expression is increased in breast tumors and associated with high estrogen levels. Therefore, high expression of aromatase promotes ER+ tumor proliferation [ 111 ].
Aromatase inhibitors (AIs) block aromatase enzyme activity, leading to the inhibition of estrogen synthesis. Current AIs can be classified into two categories: steroidal AIs and non-steroidal AIs [ 112 ]. Exemestane, a steroidal AI, has a steroid-like structure similar to androstenedione, which is the aromatase substrate. Exemestane irreversibly binds to the aromatase substrate-binding site leading to its inactivation [ 113 ]. Non-steroidal AIs include letrozole and anastrozole. They both bind non-covalently and competitively to the aromatase substrate-binding site and prevent the binding of androgens by saturating the binding site [ 112 ].
AIs are an oral treatment administered only to postmenopausal women (including patients that become postmenopausal following ovarian suppression). It is administered alone or in combination with tamoxifen as adjuvant therapy for HR+ BC patients [ 114 , 115 , 116 , 117 ]. AIs can be administered for 5 years or 2–3 years if followed by tamoxifen and up to 5 years after previous tamoxifen or AI treatment. For advanced or metastatic HR+ BC, AIs can be delivered as first-line and second-line therapy. Patients who become postmenopausal after or during the 5 years of tamoxifen treatment can receive AIs, such as letrozole, as an extended treatment strategy [ 118 , 119 ].
Estrogens have protective effects on the cardiovascular system by regulating serum lipids concentrations and increasing vasodilatation [ 120 ]. Hence, AIs might increase the risk of developing cardiovascular diseases by reducing estrogen levels in the blood [ 121 ]. Other adverse effects of AIs include hot flushes, vaginal dryness, fatigue, and osteoporosis [ 122 ]. ER+ tumors can acquire AI resistance. Some mechanisms of AI resistance are similar to those conferring SERM or SERD resistance, such as ESR1 mutations, epigenetic modifications, and PI3K pathway upregulation [ 123 ]. However, other mechanisms of action are involved in AI resistance. For example, the upregulation of cyclin-dependent kinase 4 (CDK4) or cyclin-dependent kinase 6-retinoblastoma (CDK6-RB) pathways can lead to an estrogen-dependent cell progression [ 124 ]. Clinical studies have shown better benefits from CDK4-CDK6 inhibitors in combination with AIs compared to AIs alone [ 125 , 126 ].
Endocrine therapy is a well-established treatment strategy for HR+ tumors. Over the last decades, SERMs, SERDs and AIs have been proven as safe and effective personalized therapy for HR+ BC patients, and these therapeutic strategies have shown continued improvements. However, the main drawback of endocrine therapy is acquired or de novo resistance [ 127 ]. Hence, it is essential to develop new therapeutic agents that use different modes of action to treat HR+ BC more efficiently.
3.2. Anti-HER2 Therapy
The overexpression of HER2 is associated with worse survival outcome compared to HR-positive/HER2-negative BC [ 128 , 129 ]. Hence, therapies targeting HER2 are essential to treat HER2-positive BC. The current anti-HER2 therapies comprise antibodies that target specific HER2 epitopes, tyrosine kinase inhibitors (TKIs) and, more recently, antibody-drug conjugates (ADCs) [ 130 ]. Anti-HER2 mechanisms of action and resistance are described in Figure 4 .

Anti-HER2 therapy mechanisms of action and resistance. The left part of the figure describes the mechanism of action of anti-HER2 therapy through anti-HER2 antibody (trastuzumab and pertuzumab), tyrosine kinase inhibitors (lapatinib and nerotinib), and trastuzumab-emtansine (T-DM1). The right part of the figure describes the mechanism of resistance to anti-HER2 therapy through constitutive active p95 HER2 fragment, activation of other signaling pathways, and rapid recycling of HER2-T-DM1. ADCC: antibody-dependent cellular cytotoxicity; HER2: human epidermal growth factor receptor 2; EGF: epidermal growth factor, HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor alpha; T-DM1: trastuzumab-emtansine; IGF-1R: insulin growth factor receptor 1; IGF: insulin growth factor; HGF: hepatocyte growth factor; MEK/MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3-kinase; mTOR: mammalian target of rapamycin; PTEN: phosphatase and tensin homolog.
3.2.1. Antibodies Targeting HER2
The first developed HER2-targeted antibody, trastuzumab (Herceptin), was approved by the FDA in 1998 [ 131 , 132 ]. Trastuzumab targets subdomain IV of the HER2 extracellular domain. However, the mechanism underlying trastuzumab’s therapeutic effect is not well understood. Multiple studies have reported hypotheses to explain trastuzumab’s mechanism of action. For instance, trastuzumab may inhibit the formation of the HER2-HER3 heterodimer, known to be the most oncogenic pair in the HER family [ 133 ]. It could also inhibit the formation of the active p95 HER2 fragment by preventing cleavage of the HER2 extracellular domain [ 134 ]. An indirect antitumor effect could be activating antibody-dependent cellular cytotoxicity (ADCC) by engaging with Fc receptors on immune effector cells [ 135 ].
Initially, trastuzumab was approved for administration in metastatic HER2+ BC, increasing the clinical benefits of first-line chemotherapy [ 132 ]. Trastuzumab has also demonstrated its efficacy and safety in early-stage HER2+ BC. It is given as neoadjuvant or adjuvant therapy in combination with other anti-HER2 treatments and/or with chemotherapy [ 136 , 137 , 138 ]. The recommended dose for intravenous trastuzumab is 4 mg/kg followed by 2 mg/kg weekly for 1 year in the adjuvant setting for early-stage HER2+ BC and until disease-free progression for metastatic HER2+ BC [ 139 ].
Pertuzumab (Perjeta) is another antibody that targets the HER2 extracellular domain but binds to subdomain II. Once it binds to HER2, pertuzumab prevents HER2 heterodimerization with other HER family members, leading to inhibition of downstream signaling pathways [ 140 ]. Like trastuzumab, one of pertuzumab’s indirect antitumor effects is activating the ADCC pathway [ 141 ]. Multiple clinical trials have shown that pertuzumab, combined with trastuzumab and chemotherapy, improved OS in metastatic HER2+ BC patients compared to trastuzumab and chemotherapy alone [ 142 , 143 , 144 , 145 ]. The benefits of pertuzumab have also been shown in early-stage HER2+ BC, as pertuzumab can be used in the neoadjuvant or adjuvant setting combined with trastuzumab and chemotherapy [ 146 , 147 , 148 , 149 ]. Pertuzumab is administered in fixed doses of 840 mg followed by 420 mg every three weeks [ 150 ].
Despite the major positive impacts of trastuzumab and pertuzumab in HER2+ BC treatment, only one-third of BC patients with HER2+ tumors benefit from anti-HER2 antibodies [ 151 ]. One of the hypotheses explaining this resistance concerns structural modifications of HER2, which hinder antibody binding. Alternative splicing can lead to a truncated isoform lacking the extracellular domain, thus forming a constitutive active p95 HER2 fragment [ 152 ]. The overexpression of other tyrosine kinases can bypass the signaling pathways mediated by HER2. It has been shown that cells overexpressing IGF-1R overcome cell cycle arrest by increasing CDK2 kinase activity [ 153 ]. Moreover, the overexpression of c-Met (a hepatic growth factor receptor) synergizes with HER2 signaling to confer resistance to anti-HER2 antibodies. Indeed, c-Met physically interacts with HER2, and c-Met depletion renders cells more sensitive to trastuzumab [ 154 , 155 ]. Another hypothesis for anti-HER2 antibody resistance is intracellular alterations in HER2 downstream signaling pathways. HER2 activates PI3K/Akt signaling, and PTEN (phosphatase and tensin homolog) is a well-known inhibitor of this pathway [ 156 ]. Tumors with a loss of PTEN function and/or constitutive activation of PI3K due to alteration mutations achieve worse therapeutic outcomes with trastuzumab [ 157 , 158 ].
3.2.2. Tyrosine Kinase Inhibitors (TKIs)
Since tumors may be resistant to anti-HER2 antibodies, new approaches have been developed. TKIs such as lapatinib, neratinib, or pyrotinib are small molecules that compete with ATP at the catalytic domain of the receptor to prevent tyrosine phosphorylation and HER2 downstream signaling [ 159 ].
Lapatinib is a dual EGFR/HER2 TKI blocking both HER1 and HER2 activation [ 160 ]. In metastatic BC, clinical trials have shown that lapatinib offers more benefits than chemotherapy alone [ 161 , 162 , 163 ]. The effects of lapatinib in the neoadjuvant/adjuvant setting have also been evaluated. As a neoadjuvant treatment, lapatinib plus trastuzumab combined with chemotherapy were more efficient than chemotherapy combined with lapatinib or trastuzumab alone [ 164 ]. Lapatinib as adjuvant treatment showed modest antitumor efficacy compared to placebo in a randomized, controlled, and multicenter phase III trial (TEACH) [ 165 ]. For luminal B (ER/PR+; HER2+) advanced or metastatic BC, lapatinib can be administered in combination with AIs.
Neratinib is an irreversible TKI targeting HER1, HER2, and HER4 [ 166 ]. The FDA approved Neratinib in 2017 as an extended adjuvant treatment for patients with HER2+ early-stage BC and combination with trastuzumab in the adjuvant setting [ 167 , 168 ]. Neratinib can be delivered in combination with capecitabine as a third-line and beyond therapy for HER2+ advanced or metastatic BC.
More recently, pyrotinib, a new generation TKI targeting HER1, HER2 and HER4, has been developed [ 169 ]. Pyrotinib is still under clinical trials to prove its efficacy and safety [ 170 ]. However, in 2018, the Chinese State Drug Administration approved pyrotinib in combination with or after chemotherapy treatment for patients with HER2+ advanced or metastatic BC [ 171 ].
Despite the recent development of TKI treatments, patients can still exhibit intrinsic or acquired resistance to these agents. Three mechanisms of action have been hypothesized: (1) activation of compensatory pathways, (2) HER2 tyrosine kinase domain mutation, and (3) other gene amplification [ 172 ]. For instance, activation of the PI3K/Akt pathway and FOXO3A (Forkhead transcription factor) by the upregulation of HER3 can lead to lapatinib resistance [ 173 ]. Other tyrosine kinases can be involved, such as c-Met, also known to be implicated in trastuzumab resistance. C-Met induces the activation of PI3K/Akt signaling in lapatinib-resistant BC [ 174 ]. Mutations in the HER2 tyrosine kinase domain lead to the constitutive activation of HER2 by substituting individual amino acids [ 175 ]. Lastly, it has been shown that the amplification of the NIBP (TRAPPC9, Trafficking Protein Particle Complex 9) gene occurs in HER2+ lapatinib-resistant tumors. The inhibition of NIBP makes resistant cells sensitive to lapatinib [ 176 ].
3.2.3. Trastuzumab-Emtansine (T-DM1)
Trastuzumab-emtansine (T-DM1) is an antibody-drug conjugate (ADC), which is a conjugate of trastuzumab and a cytotoxic molecule, DM1, a derivative of maytansine [ 177 ]. T-DM1 binds to HER2 with the trastuzumab part. The formed complex is then internalized for degradation, releasing DM1 metabolites into the cytoplasm. DM1 then inhibits microtubule assembly causing cell death [ 178 , 179 ]. Thus, T-DM1 consists of the antitumor effects of trastuzumab and those associated with DM1 metabolites [ 180 ].
Three phase III clinical trials have evaluated the safety and efficacy of T-DM1 for HER2+ metastatic BC [ 181 , 182 , 183 ]. They have shown that T-DM1 improves OS and DFS of HER2+ metastatic BC patients compared to lapatinib in combination with trastuzumab or chemotherapy [ 181 , 182 , 183 ]. T-DM1 as neoadjuvant treatment has less efficacy compared with trastuzumab or pertuzumab with chemotherapy [ 146 ]. This suggests that T-DM1 should not be administered as a neoadjuvant treatment but as a first-line or second-line therapy for HER2+ metastatic BC. The 2021 NCCN guidelines recommend using T-DM1 as second-line therapy for HER2+ advanced or metastatic BC [ 99 ].
The mechanism of action of T-DM1 involves those related to trastuzumab and DM1, so the observed resistance to T-DM1 could come from interference in one or both constituents [ 184 ]. The mechanism of T-DM1 resistance has been hypothesized to involve (1) the loss of trastuzumab mediated activity, (2) the dysfunctional intracellular trafficking of T-DM1, and (3) the impairment of DM1 mediated cytotoxicity [ 185 ].
As previously described in this review, the reduction of trastuzumab effects can occur by reduced HER2 expression, dysregulation of PI3K signaling, or the activation of alternative tyrosine kinase receptors [ 153 , 154 , 156 , 186 ]. The alteration of HER2-T-DM1 complex internalization can go through a rapid recycling of HER2 to the plasma membrane leading to the inhibition of DM1 metabolism released into the cytoplasm [ 187 ]. The internalization of the HER2-T-DM1 complex occurs through the formation of lysosomes. These vesicles enclose lysosomal enzymes involved in HER2-T-DM1 complex degradation. In T-DM1-resistant tumors, the level of lysosomal enzymes is inhibited [ 188 , 189 ]. T-DM1 also disrupts microtubule assembly causing incomplete spindle formation resulting in mitotic catastrophe and apoptosis [ 190 ]. Cells resistant to T-DM1 can avoid this process by reducing the induction of Cyclin-B1, an enzyme essential for cell cycle progression [ 191 ].
HER2+ BC are aggressive and associated with poor prognosis and metastasis, and recurrences. Anti-HER2 therapy has greatly improved the management of HER2+ BC. However, 25% of early-stage HER2+ BC patients will have a recurrence after the initial anti-HER2 treatment [ 192 ]. The emergence of new therapeutic agents specific for HER2+ BC provides new hope to treat this particularly aggressive BC subtype.
3.3. PARP Inhibitors
The prevalence of BRCA (Breast Cancer genes) mutations in TNBC patients is approximately 20% [ 193 ]. BRCA1 and BRCA2 are proteins involved in the DNA damage response to repair DNA lesions [ 194 ]. Mutations in BRCA 1/2 genes are associated with an increased risk of breast and ovarian cancers [ 195 ].
PARP (poly-(ADP-ribose) polymerase protein) proteins are also involved in the DNA damage response as they recruit DNA repair proteins, such as BRCA1 and BRCA2, to the damage site [ 196 ]. PARP inhibitors (PARPi) were developed to inhibit DNA repair in BRCA-mutated BC since cells defective in BRCA functions cannot repair DNA damage when PARP is inhibited [ 197 ]. The principal PARPis currently in clinical development are olaparib, talazoparib, veliparib, and rucaparib [ 198 ]. PARP inhibitors mechanisms of action and resistance are described in Figure 5 .

PARP inhibitors mechanisms of action and resistance. The left part of the figure describes the mechanism of PARP inhibitors in the context of BRCA mutated breast cancer. The right part of the figure describes the mechanism of resistance to PARP inhibitors through secondary intragenic mutations restoring BRCA proteins functions and the decrease of the recruitment of nucleases (MUS81 or MRE11) to protect the replication fork. PARP: poly-(ADP-ribose) polymerase protein; PARPi: PARP inhibitors; BRCA: breast cancer protein; MUS81: methyl methanesulfonate ultraviolet sensitive gene clone 81; MRE11: meiotic recombination 11.
3.3.1. Olaparib
Olaparib is the first FDA-approved PARPi for the treatment of BRCA -mutated BC [ 199 ]. Phase I and phase II trials evaluating the effects of olaparib monotherapy in germline BRCA-mutated (gBRCAm) BC proved its clinical benefits by improving progression-free survival (PFS) [ 200 , 201 , 202 , 203 ]. The phase III, randomized, open-label, OlympiAD trial compared olaparib monotherapy vs. standard chemotherapy in patients with BRCA mutated HER2-negative BC. This trial showed that olaparib has better efficacy and tolerability than standard chemotherapy for this group of patients [ 204 ]. Olaparib has also been tested in combination with chemotherapy. A phase I study evaluated the effects of olaparib in combination with paclitaxel in unselected TNBC patients [ 205 ]. The overall response rate (ORR) for these patients was 37%. Two phase I studies evaluating the combination of olaparib with cisplatin or carboplatin in gBRCAm BC patients showed improved ORR [ 206 , 207 ].
3.3.2. Talazoparib
Talazoparib has the highest PARP-DNA trapping efficiency among the PARPis [ 208 ]. A phase II trial testing the effects of talazoparib on gBRCAm early-stage BC showed decreased tumor size in all patients included [ 209 ]. Other phase I and II trials with gBRCAm BC patients receiving talazoparib confirmed the efficiency of this PARPi [ 210 , 211 ]. The EMBRACA study, an open-label phase III trial, compared talazoparib monotherapy to chemotherapy in gBRCAm, HER2-negative BC patients [ 212 ]. PFS and ORR were improved with talazoparib compared to chemotherapy alone.
3.3.3. Veliparib
Veliparib has been mostly evaluated in combination with chemotherapy. For example, the phase II multicenter I-SPY2 trial tested the combination of veliparib and neoadjuvant chemotherapy in unselected TNBC patients [ 213 ]. The predicted complete response rate (pCR) was 51% with veliparib and chemotherapy vs. 26% in the control arm (chemotherapy alone). The phase II BROCADE study evaluated the combination of veliparib with carboplatin and paclitaxel in gBRCAm BC patients [ 214 ]. The ORR was improved with the combination of veliparib and chemotherapy compared to chemotherapy alone. Lastly, the phase III BRIGHTNESS study evaluated the addition of veliparib to carboplatin in the standard neoadjuvant chemotherapy setting [ 211 ]. The addition of veliparib showed no further benefit to chemotherapy.
3.3.4. Rucaparib
Rucaparib is the second PARPi that has been FDA approved for gBRCAm BC patients [ 215 ]. Intravenous rucaparib was tested in a phase II trial of gBRCAm BC patients [ 216 ]. Stable disease, meaning no tumor development, was reported in 44% of patients. Rucaparib was also tested in combination with chemotherapy in unselected TNBC patients [ 217 ]. This phase I study showed that rucaparib could be safely used in combination with chemotherapy. The phase II, a randomized BRE09-146 trial, evaluated rucaparib in combination with cisplatin vs. cisplatin alone in gBRCAm patients with residual disease following neoadjuvant therapy [ 218 ]. DFS was similar in the two arms, as low-dose rucaparib did not affect cisplatin toxicity. However, the rucaparib dose may not have been sufficient to inhibit PARP activity.
Tumor cells can become resistant to PARPi by different mechanisms [ 219 ].
First, secondary intragenic mutations that restore BRCA proteins functions can lead to PARPi resistance [ 220 ]. These genetic events can lead to the expression of nearly full-length proteins or full-length wild-type proteins with complete restored functions [ 221 ]. This has been reported mostly in ovarian cancer patients, and it has also been demonstrated in BC cell line models [ 222 ]. Tumor cells with missense mutations conserving the N-terminal and C-terminal domains of BRCA proteins also lead to poor PARPi response [ 223 ]. Another mechanism of action leading to PARPi resistance is decreased expression of PARP enzymes. Indeed, tumor cells with low PARP1 expression acquire resistance to veliparib [ 224 ].
In addition, tumor cells can find alternative mechanisms to protect the replication fork. It has been shown that PARPi-resistant cells can reduce the recruitment of the MRE11 (meiotic recombination 11) nuclease to the damage site, leading to the protection of the fork by blocking its access [ 225 ]. Another study has shown that BRCA2 -mutated tumors acquired PARPi resistance by reducing the recruitment of the MUS81 (methyl methanesulfonate ultraviolet sensitive gene clone 81) nuclease to protect the replication fork [ 226 ].
Chemotherapy has been the pioneer treatment strategy for TNBC for decades. The development of PARPis has been a major improvement in the treatment of TNBC and, more specifically, gBRCAm TNBC, as they have shown more benefits over chemotherapy [ 227 ]. However, TNBC is a heterogenous BC subtype, and PARPis cannot treat all TNBCs as it is administered only for gBRCAm TNBC [ 228 ]. Therefore, it is necessary to develop specific targeted therapies to treat each TNBC subtype.
4. New Strategies and Challenges for Breast Cancer Treatment
4.1. emerging therapies for hr-positive breast cancer.
As mentioned in Section 3.1 , the major mechanisms of action of current endocrine therapy resistance occur via (1) the mTOR/PI3K/Akt signaling pathway and (2) the actors of the cell cycle progression CDK4/6. Therefore, emerging therapies for HR+ BC mainly target these pathways to bypass estrogen-independent cell survival [ 229 ]. The most recent completed clinical trials on emerging therapies for HR+ BC are presented in Table 1 .
Most recent completed clinical trial on emerging therapies for HR-positive breast cancer.
HR+: hormone receptors positive; HER2-: human epidermal growth factor receptor 2 negative; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; pCR: pathologic complete response; HR: hazard ratio.
4.1.1. mTOR/PI3K/AKT Pathway Inhibitors
The mTOR/PI3K/Akt pathway inhibitors can be divided into different categories according to the target in the pathway. Specific inhibitors have been developed to target all or specific isoforms of PI3K, mTORC1 and Akt [ 251 ].
Pan-Pi3K Inhibitors
Pan-PI3K inhibitors target all PI3K isoforms resulting in significant off-target effects. The main pan-PI3K inhibitors are buparlisib and pictilisib [ 252 ]. Multiple clinical trials have tested the effects of pan-PI3K inhibitors in luminal BC.
The phase III randomized double-blinded BELLE-2 trial compared buparlisib combined with fulvestrant, to fulvestrant monotherapy in luminal A advanced or metastatic BC patients [ 230 ]. The results of this trial showed a modest improvement in PFS when buparlisib was added to fulvestrant. Another phase III clinical trial (BELLE-3) studied the effects of buparlisib plus fulvestrant in luminal A advanced or metastatic BC patients with no benefits from endocrine therapy [ 231 ]. Though PFS was significantly improved with buparlisib, there were severe adverse effects such as hyperglycemia, dyspnea, or pleural effusion. Lastly, the phase II/III BELLE-4 clinical trial evaluated buparlisib plus paclitaxel in HER2-negative locally advanced or metastatic BC patients [ 232 ]. The addition of buparlisib to paclitaxel did not improve PFS in these patients. Thus, further studies on buparlisib in HR+ BC were not conducted. The phase II randomized, double-blinded FERGI clinical trial analyzed the effects of pictilisib plus fulvestrant in luminal A BC patients resistant to AI [ 233 ]. The addition of pictilisib to fulvestrant did not improve PFS. Moreover, severe adverse effects occurred when the dose of pictilisib was increased. These results were confirmed for pictilisib plus paclitaxel, as the phase II PEGGY study showed no benefit from pictilisib in PI3K-mutated HER2-negative BC patients [ 234 ].
Hence, pan-PI3K inhibitors are not optimal to treat HR+ BC due to their toxicity and lack of efficacy.
Isoform-Specific PI3K Inhibitors
To sort out issues related to off-target effects and toxicities with pan-PI3K inhibitors, isoform-specific PI3K inhibitors have been developed. These isoform-specific PI3K inhibitors can specifically target the PI3K p110α, p110β, p110δ, and p110γ isoforms [ 252 ]. Multiple clinical trials have tested the effects of isoform-specific PI3K inhibitors.
PI3K p110α is the most commonly mutated isoform in BC [ 253 ]. Alpelisib is the first FDA-approved PI3K p110α isoform inhibitor. A phase Ib clinical trial tested the effects of alpelisib and letrozole in patients with ER+ metastatic BC refractory to endocrine therapy [ 235 ]. The clinical benefit of the alpselisib and letrozole combination was higher for patients with PI3K-mutated BC, but clinical activity was still observed in patients with non-mutated tumors. The phase III randomized SOLAR-1 clinical trial compared the effects of alpelisib plus fulvestrant to fulvestrant alone in luminal A advanced BC patients who received no benefits from prior endocrine therapy [ 236 ]. The addition of alpelisib improved PFS for patients with PI3K-mutated BC.
Taselisib targets the PI3K p110α, p110γ and p110δ isoforms [ 254 ]. Taselisib was tested in the SANDPIPER study, a phase III randomized clinical trial, in combination with fulvestrant in patients with ER+ metastatic BC resistant to AIs [ 238 ]. Although the addition of taselisib slightly improved PFS, further clinical trials with taselisib were interrupted since high rates of severe adverse events were detected.
mTORC1 Inhibitors
mTORC1 inhibitors, such as everolimus, block the mTORC1 dependent phosphorylation of s6k1 [ 255 ]. The BOLERO-2 phase III randomized clinical trial investigated the effects of exemestane with or without everolimus in AI-resistant ER+ metastatic BC patients [ 240 ]. The combination of everolimus and exemestane improved PFS. The TAMRAD phase II randomized open-label study compared the effects of tamoxifen with or without everolimus in AI-resistant luminal A BC patients [ 241 ]. This study showed an improvement in overall survival (OS) when everolimus was given in combination with tamoxifen. The findings of these two clinical trials led to FDA approval of everolimus. More recently, the PrE0102 phase II randomized clinical trial showed that the addition of everolimus to fulvestrant improved PFS of patients with AI-resistant ER+ BC compared to fulvestrant alone [ 242 ].
Akt Inhibitors
Akt inhibitors target all Akt isoforms as Akt 1, 2, and 3 isoforms share very similar structures [ 256 ]. Capivasertib is the principal Akt inhibitor under investigation in different clinical trials. The FAKTION phase II multi-centered randomized clinical trial compared the effects of capivasertib plus fulvestrant to fulvestrant plus placebo in AI-resistant luminal A advanced BC patients [ 243 ]. PFS was significantly improved with the combination of capivasertib and fulvestrant in comparison with the placebo arm.
The AKT1 E17K activating mutation is the most common in Akt and occurs in approximately 7% of ER+ metastatic BC. This mutation in the Akt lipid-binding pocket leads to constitutive Akt activation by modifying its localization to the membrane [ 257 ]. A phase I study analyzed the effects of capivasertib alone or in combination with fulvestrant in a cohort of patients with AKT1 E17K mutation ER+ metastatic BC [ 244 ]. Capivasertib, in combination with fulvestrant, demonstrated clinically meaningful activity and better tolerability compared to capivasertib alone.
4.1.2. CDK4/6 Inhibitors
There are currently three CDK4/6 inhibitors approved to treat HR+/HER2- metastatic BC: palbociclib, ribociclib, and abemaciclib. They can be administered as first-line treatment combined with AIs or as second-line treatment combined with fulvestrant [ 258 ].
First-Line Treatment
Palbociclib, a highly selective CDK4/6 inhibitor, is the first FDA-approved CDK4/6 inhibitor as first-line treatment combined with AIs for metastatic or advanced HR+ BC patients [ 259 ].
PALOMA-1 is an open-label, randomized phase II study that evaluated the effects of palbociclib in combination with letrozole vs. letrozole alone as first-line treatment for HR+ advanced BC patients [ 126 ]. The addition of palbociclib to letrozole significantly improved PFS in HR+ BC patients. A phase III study was performed (PALOMA-2) to confirm these findings and expand the efficacy and safety of palbociclib, [ 245 ]. This double-blinded clinical trial tested the combination of palbociclib and letrozole in postmenopausal BC patients without prior systemic therapy for advanced BC. The addition of palbociclib to letrozole significantly improved PFS and ORR.
Ribociclib is the second FDA-approved CDK4/6 inhibitor for first-line treatment in postmenopausal advanced BC patients in combination with AIs [ 260 ]. The phase III MONALEESA-2 clinical trial results showed improved PFS and ORR with the combination of ribociclib and letrozole in HR+ metastatic BC patients. The clinical benefits and manageable tolerability observed with ribociclib and letrozole are maintained with longer follow-up compared to letrozole alone [ 247 ].
Abemaciclib has been tested in the phase III randomized double-blinded MONARCH-3 study [ 250 ]. HR+ advanced BC patients with no prior systemic therapy received abemaciclib plus anastrozole or letrozole or AIs plus placebo in the control arm. PFS and ORR were significantly improved with the combination of abemaciclib and AIs.
Second-Line Treatment
As second-line treatment, palbociclib can be given in combination with fulvestrant in advanced or metastatic BC patients with disease progression after endocrine therapy [ 261 ]. This was confirmed in the phase III multi-centered randomized double-blinded PALOMA-3 trial [ 246 ]. BC patients who received palbociclib plus fulvestrant had significantly longer PFS compared to fulvestrant plus placebo.
The phase III MONALEESA-3 study tested the effects of ribociclib plus fulvestrant in patients with HR+ advanced BC who received prior endocrine therapy in the advanced setting [ 248 ]. The PFS and ORR were significantly improved when ribociclib was added to fulvestrant. Thus, ribociclib plus fulvestrant can be considered as second-line treatment for these BC patients.
Abemaciclib has been recently approved in combination with fulvestrant for HR+ advanced or metastatic BC patients with disease progression after endocrine therapy. This was based on the results of the phase III, double-blinded MONARCH 2 study [ 249 ]. The combination of abemaciclib and fulvestrant demonstrated a significant improvement of PFS and ORR compared to fulvestrant plus placebo in HR+ metastatic BC patients who experienced relapse or progression after prior endocrine therapy.
mTOR/PI3K/Akt inhibitors and CDK4/6 inhibitors show great promise for advanced HR+ BC resistant to endocrine therapy. To leverage the potential of these two types of therapies, some preclinical studies have evaluated a triple therapy combination including PI3K inhibitors, CDK4/6 inhibitors, and endocrine therapy (see the summarized table at the end of the manuscript) [ 262 ].
4.2. New Strategic Therapies for HER2-Positive Breast Cancer
As mentioned in Section 3.2 , HER2+ BC is currently treated with specific HER2 targeting antibodies or tyrosine kinase inhibitors (TKIs), and more recently, with TDM-1, an antibody-drug conjugate. These treatments have greatly improved HER2+ BC survival. However, 25% of HER2+ BC patients will still develop resistance to anti-HER2 treatment. Hence, new therapeutic strategies are emerging, such as new antibodies targeting HER2, new TKIs, vaccines, and PI3K/mTOR and CDK4/6 inhibitors [ 263 ]. The most recent completed clinical trials on new strategies for HER2+ BC treatment are gathered in Table 2 .
Most recent completed clinical trials on emerging therapies for HER2+ breast cancer.
HER2+: human epidermal growth factor receptor 2 positive; ER+: estrogen receptor positive; HLA2/3: human leucocyte antigen 2/3; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival OS: overall survival GM-CSF: granulocyte macrophage colony-stimulated factor; HR: hazard ratio.
4.2.1. New Antibodies
Novel types of antibodies have been developed to target HER2+ BC more efficiently. They can be divided into three categories: antibody-drug conjugates (ADC), modified antibodies, and bispecific antibodies.
Antibody-Drug Conjugates (ADC)
ADCs are the combination of a specific monoclonal antibody and a cytotoxic drug that is released in the antigen-expressing cells [ 280 ]. The most common ADC is TDM-1, and the promising results with TDM-1 have led to the development of new ADCs.
Trastuzumab-deruxtecan (DS-8201a) is a HER2-targeting antibody (trastuzumab) linked to a DNA topoisomerase I inhibitor (deruxtecan) [ 281 ]. A phase I study demonstrated that DS-8201a had antitumor activity even with HER2 low-expressing tumors [ 282 ]. These results led to phase II and phase III clinical trials. The DESTINY-Breast01 clinical trial is an open-labeled, single-group, multicentered phase II study [ 264 ] was evaluated in HER2+ metastatic BC patients who received prior TDM-1 treatment. DS-8201a showed durable antitumor activity for these patients. Two phase III clinical trials are currently evaluating DS-8201a. DESTINY-Breast02 (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03523585","term_id":"NCT03523585"}} NCT03523585 ) is comparing DS-8201a to standard treatment (lapatinib or trastuzumab) in HER2+ metastatic BC patients previously treated with TDM-1. The DESTINY-Breast03 (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03529110","term_id":"NCT03529110"}} NCT03529110 ) trial is evaluating the effects of DS-8201a vs. TDM-1 in HER2+ metastatic BC patients with prior trastuzumab and taxane treatment.
Trastuzumab-duocarmycin (SYD985) is a HER-2 targeting antibody (trastuzumab) conjugate with a cleavable linker-duocarmycin payload that causes irreversible alkylation of the DNA in tumor cells leading to cell death [ 283 ]. A dose-escalation phase I study evaluated the effects of SYD85 in BC patients with variable HER2 status and refractory to standard cancer treatment [ 284 ]. Trastuzumab-duocarmycin showed clinical activity in heavily pretreated HER2+ metastatic BC patients, including TDM-1 resistant and HER2-low BC patients. After these promising results, a phase I expansion cohort study was performed on the same type of patients (heavily pretreated HER2+ or HER2-low BC patients) [ 265 ]. This study confirmed previous results on the efficacy of STD985. A phase III clinical trial (TULIP-ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03262935","term_id":"NCT03262935"}} NCT03262935 ) is ongoing to compare SYD985 to the treatment chosen by the physician in HER2+ metastatic BC patients. Other ADCs are under clinical trials to test their safety and activity for HER2+ advanced BC patients. RC48 is an anti-HER2 antibody conjugated with monomethyl auristatin E that demonstrated promising efficacy and a manageable safety profile in an open-labeled, multicentered phase II study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02881138","term_id":"NCT02881138"}} NCT02881138 ) [ 248 ]. PF06804103 conjugates an anti-HER2 monoclonal antibody and the cytotoxic agent, Aur0101. In a phase I study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03284723","term_id":"NCT03284723"}} NCT03284723 ), PF06804103 showed manageable toxicity and promising antitumor activity [ 249 ]. XMT1522 showed encouraging results in a dose-escalation phase I study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02952729","term_id":"NCT02952729"}} NCT02952729 ) [ 250 ]. MEDI4276, which targets two different HER2 epitopes and is linked to a microtubule inhibitor, showed promising clinical activity in a phase I study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02576548","term_id":"NCT02576548"}} NCT02576548 ) [ 254 ] (see the summarized table at the end of the manuscript).
Chimeric Antibody
Margetuxumab (MGAH22) is a human/mouse chimeric IgG1 targeting HER2 monoclonal antibody. It is based on trastuzumab as it binds to the same epitope (subdomain IV or HER2 extracellular domain) but with an enhanced Fcγ domain. The substitution of five amino acids into the IgG1 Fc domain increases CD16A affinity, a receptor found on macrophages and natural-killer cells, and decreases CD32B affinity, leading to increased antibody-dependent cell-mediated cytotoxicity (ADCC) [ 285 ]. A phase I study evaluated margetuximab toxicity and tumor activity on HER2+ BC patients for whom no standard treatment was available [ 266 ]. This study showed promising single-agent activity of margetuximab as well as good tolerability. The phase III randomized open-labeled SOPHIA clinical trial (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT02492711","term_id":"NCT02492711"}} NCT02492711 ) compared margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in pretreated HER2+ advanced BC patients [ 286 ]. The combination of margetuximab and chemotherapy significantly improved the PFS of patients compared to trastuzumab plus chemotherapy. This study is still under investigation to collect data on OS (see the summarized table at the end of the manuscript).
Bispecific Antibodies
Bispecific antibodies (BsAbs) can target two different epitopes in the same or different receptors by combining the functionality of two monoclonal antibodies [ 287 ]. MCLA-128 targets both HER2 and HER3 and have an enhanced ADCC activity [ 288 ]. A phase I/II study evaluated the safety, tolerability, and antitumor activity of MCLA-128 in patients with pretreated HER2+ metastatic BC.
Preliminary results showed encouraging clinical benefits of MCLA-128. An open-labeled, multicentered phase II study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03321981","term_id":"NCT03321981"}} NCT03321981 ) is ongoing to evaluate the effects of MCLA-128 in combination with trastuzumab and chemotherapy in HER2+ metastatic BC patients.
ZW25 is a BsAb biparatopic that binds the IV and II subdomains of the HER2 extracellular domain, the binding epitopes of trastuzumab and pertuzumab, respectively [ 289 ]. The efficacy of ZW25 was evaluated in a phase I study given alone or in combination with chemotherapy in patients with advanced or metastatic HER2+ BC. The results of this study showed promising antitumor activity, and no-dose limiting was observed.
T-cell bispecific antibodies (TCBs) are another type of BsAbs recently developed. TCBs target the CD3-chain of the T-cell receptor and tumor-specific antigens, resulting in lymphocyte activation and tumor cell death [ 290 ].
GBR1302 targets both HER2 and CD3 receptors and directs T-cells to HER2+ tumor cells. A phase II study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03983395","term_id":"NCT03983395"}} NCT03983395 ) is ongoing to determine the safety profile of the GBR1302 single agent in previously treated HER2+ metastatic BC. PRS-343 targets HER2 and the immune receptor CD137, a member of the tumor necrosis factor receptor family. Two clinical trials are investigating the effects of PRS-343 monotherapy (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03330561","term_id":"NCT03330561"}} NCT03330561 ) or in combination with other treatments (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03650348","term_id":"NCT03650348"}} NCT03650348 ) (see the summarized table at the end of the manuscript).
4.2.2. HER2-Derived Peptide Vaccines
One of the strategies of immunotherapy is activating the patient’s immune system to kill cancer cells. Vaccination is an emerging approach to induce a tumor-specific immune response by targeting tumor-associated antigens, such as HER2 [ 291 ]. HER2-derived peptide vaccines comprise different parts of the HER2 molecule, such as E75 (extracellular domain), GP2 (transmembrane domain), and AE37 (intracellular domain) [ 292 ].
E75 (HER2/neu 369–377: KIFGSLAFL) has high affinity for HLA2 and HLA3 (human leucocyte antigen) that can stimulate T-cells against HER2 overexpressing tumor cells [ 293 ]. The efficacy of the E75 vaccine to prevent BC recurrence has been evaluated in a phase I/II study, in which high-risk HER2+ HLA2/3+ BC patients received the E75 vaccine [ 269 ]. The results demonstrated the safety and clinical efficacy of the vaccine as PFS was improved in the E75-vaccinated group compared to the unvaccinated group. Other clinical trials are currently investigating the efficacy of the E75 vaccine on HER2+ BC (see he summarized table at the end of the manuscript).
GP2 (654-662: IISAVVGIL) is a subdominant epitope with poor affinity for HLA2 [ 294 ]. A phase I trial evaluating the effects of a GP2 vaccine in disease-free BC patients showed that it was safe and tolerable with HER2-specific immune response [ 295 ]. The GP2 vaccine has been tested in a randomized, open-labeled phase II study to prevent BC recurrence. The patients that received the GP2 vaccine had HER2+ and HLA2+ BC and were disease-free with a high risk of recurrence at the time of the study [ 270 ]. The results demonstrated that the GP2 vaccine was safe and clinically beneficial for patients with HER2+ BC who received the full vaccine series.
AE37 (Ii-key hybrid of MHC II peptide AE36 (HER2/neu 776–790)) can stimulate CD8+ and CD4+ cells. A randomized, single-blinded phase II study evaluated the effects of an AE37 vaccine to prevent BC recurrence. Patients with a high risk of recurrence and HER2+ BC received the AE37 vaccine [ 271 ]. The vaccination demonstrated no benefit in the overall intention-to-treat analysis, a method that considers the randomized treatment to avoid bias happening after the randomization [ 296 ]. However, the study showed that the AE37 vaccine was safe, and results suggested that it could be effective for HER2-low BC, such as TNBC.
4.2.3. New Tyrosine Kinase Inhibitors (TKIs)
As previously described in this review (see Section 3.2.2 Tyrosine kinase inhibitors (TKIs)), TKIs are small molecules targeting the HER2 intracellular catalytic domain [ 159 ]. New TKIs have been developed with better efficacy and less toxicity in the treatment of HER2+ metastatic BC, such as tucatinib and poziotinib.
Tucatinib is a TKI with high selectivity for HER2, leading to less EGFR-related toxicities, common with other HER TKIs [ 297 ]. A phase I dose-escalation trial evaluated the combination of tucatinib and trastuzumab in BC patients with progressive HER2+ brain metastases [ 298 ]. This study showed preliminary evidence of tucatinib efficacy and tolerability in these patients. Tucatinib was also tested in combination with TDM-1 in a phase Ib trial in HER2+ metastatic BC patients with heavy pre-treatment [ 299 ]. The combination of tucatinib and TDM-1 showed acceptable toxicity and antitumor activity in these patients. Tucatinib was FDA approved in combination with trastuzumab and capecitabine for patients with advanced or metastatic HER2+ BC who received prior anti-HER2 in the metastatic setting [ 300 ]. This was based on the results of the phase II HER2CLIMB clinical trial, where HER2+ metastatic BC patients received tucatinib or placebo in combination with trastuzumab and capecitabine [ 267 ]. The addition of tucatinib to trastuzumab and capecitabine improved PFS and OS of heavily pretreated HER2+ metastatic BC patients.
Poziotinib is a pan-HER kinase inhibitor that irreversibly inhibits the HER family members’ kinase activity [ 301 ]. A phase I study evaluated the efficacy and tolerability of poziotinib in advanced solid tumors. The results showed encouraging antitumor activity against different types of HER2+ cancers as poziotinib was safe and well-tolerated by the patients [ 302 ]. The phase II NOV120101-203 study evaluated the safety and efficacy of poziotinib monotherapy in heavily pretreated HER2+ metastatic BC patients [ 268 ]. Poziotinib showed meaningful activity in these patients with no severe toxicities.
4.2.4. mTOR/PI3K Inhibitors and CDK4/6 Inhibitors
As mentioned in the previous Section 4.1 , mTOR/PI3K inhibitors and CDK4/6 inhibitors have been evaluated as potential new strategic therapies for HR+ BC resistant to endocrine therapy. The mTOR/PI3K signaling pathway and CDK4/6 also play a role in the mechanisms leading to treatment resistance in HER2+ BC [ 303 ]. Thus, targeting them with mTOR/PI3K and CDK4/6 inhibitors is also being investigated in HER2+ BC subtype.
mTOR/PI3K Inhibitors
Alpelisib and taselisib are PI3K isoform-specific inhibitors that were also evaluated in HR+ BC [ 235 , 236 , 238 , 253 , 254 ]. A phase I study evaluated alpelisib in combination with trastuzumab and LJM716 (a HER3-targeted antibody) in patients with PI3KCA mutant HER2+ metastatic BC [ 272 ]. Unfortunately, the results of this study were limited by high gastrointestinal toxicity. Another phase I study tested alpelisib in combination with TDM-1 in HER2+ metastatic BC patients pretreated with trastuzumab [ 273 ]. The combination of alpelisib and TDM-1 demonstrated tolerability and antitumor activity in trastuzumab-resistant HER2+ metastatic BC patients. Taselisib is being tested in an ongoing phase Ib dose-escalation trial in combination with anti-HER2 therapies (trastuzumab, pertuzumab and TDM-1) in HER2+ advanced BC patients (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02390427","term_id":"NCT02390427"}} NCT02390427 ).
Copanlisib is a highly selective and potent pan-class I PI3K inhibitor [ 304 ]. A phase Ib (PantHER) study evaluated the tolerability and activity of copanlisib in combination with trastuzumab in heavily pretreated HER2+ metastatic BC patients [ 274 ]. The combination of copanlisib and trastuzumab was safe and tolerable. Preliminary evidence of tumor stability was observed in these patients.
Everolimus is a mTORC1 inhibitor also tested in HR+ BC [ 240 , 241 , 242 ]. Everolimus was tested in phase III clinical trials, in combination with trastuzumab and docetaxel (BOLERO-1), or in combination with trastuzumab and vinorelbine (BOLERO-3) in trastuzumab-resistant advanced HER2+ BC [ 275 , 276 ]. Unfortunately, results showed an increase of adverse effects with everolimus. Moreover, the BOLERO-1 clinical trial showed no improvement in PFS with the combination of trastuzumab and everolimus. By contrast, PFS was significantly longer when everolimus was added to vinorelbine in BOLERO-3. A study analyzing the molecular alterations found in patients in the BOLERO-1 and BOLERO-3 clinical trials demonstrated that HER2+ BC patients could derive more benefit from everolimus if the tumors had PI3KCA mutations, PTEN loss or a hyperactive PI3K pathway [ 305 ].
CDK4/6 Inhibitors
Palbociclib, ribociclib and abemaciclib are CDK4/6 inhibitors that have been FDA approved to treat HR+ BC as first-line treatments [ 247 , 250 , 259 ]. They have also been evaluated in multiple clinical trials for advanced HER2+ BC. Palbociclib has been tested in combination with trastuzumab in the phase II SOLTI-1303 PATRICIA clinical trial in heavily pretreated advanced HER2+ BC patients [ 277 ]. Palbociclib combined with trastuzumab demonstrated safety and encouraging survival outcomes in these patients. Palbociclib has also been evaluated in combination with TDM-1 in HER2+ advanced BC patients pretreated with trastuzumab and taxane therapy [ 306 ]. The results of this phase I/Ib study showed safety, tolerability, and antitumor activity in these patients.
Ribociclib was evaluated in a phase Ib/II trial in combination with trastuzumab to treat advanced HER2+ BC patients previously treated with multiple anti-HER2 therapies [ 278 ]. The combination of ribociclib and trastuzumab was safe, but there was limited activity in heavily pretreated patients. The conclusions of this study suggest that CDK4/6 inhibitor/anti-HER2 combination should be administered in patients with few previous therapies.
Abemaciclib has been tested in the phase II randomized open-labeled MonarcHER trial in combination with trastuzumab with or without fulvestrant vs. trastuzumab with standard chemotherapy in HR+/HER2+ BC patients [ 279 ]. The combination of abemaciclib, trastuzumab, and fulvestrant significantly improved PFS in these patients, with a tolerable safety profile.
There are multiple ongoing clinical trials for advanced HER2+ BC testing the combination of palbociclib, trastuzumab, pertuzumab, and anastrozole (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03304080","term_id":"NCT03304080"}} NCT03304080 ); or palbociclib and trastuzumab plus letrozole (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03054363","term_id":"NCT03054363"}} NCT03054363 ). Preliminary results are expected around July 2021 and March 2022, respectively (see he summarized table at the end of the manuscript).
A great proportion of HER2+ BC patients develop resistance to traditional anti-HER2 therapies, and 40–50% of patients with advanced HER2+ BC develop brain metastases [ 307 ]. Thus, developing new therapies to overcome resistance is essential. The therapeutic strategies that have been described in this section provide new hope for HER2+ BC patients, especially for advanced or metastatic HER2+ BC patients.
4.3. Emerging Therapies for Triple Negative Breast Cancer (TNBC)
TNBC is the most aggressive BC subtype. The fact that TNBC lacks ER and PR expression and does not overexpress HER2, combined with its high heterogeneity, has contributed to the difficulties in developing efficient therapies [ 308 ]. Thus, multiple strategic therapies have been developed to treat all TNBC subtypes. These include conjugated antibodies, targeted therapy, and immunotherapy. An overview of the most recent and completed clinical trials on emerging therapies for TNBC is presented in Table 3 .
Most recent completed clinical trials on emerging therapies for TNBC.
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor; HR: hormonal receptor; MBC: metastatic breast cancer; BC: breast cancer; AR: androgen receptor; PPV: personalized peptide vaccine; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; IDFS: invasive disease-free survival; OS: overall survival; TTP: time to progression; pCR: pathologic complete response; HR: hazard ratio.

4.3.1. Antibodies-Drug Conjugates (ADC)
Antibody drug conjugates (ADCs) deliver a cytotoxic drug into the tumor cell by the specific binding of an antibody to a surface molecule [ 280 ]. Multiple ADCs have been investigated in TNBC such as sacituzumab govitecan, ladiratuzumab vedotin, or trastuzumab deruxtecan.
Sacituzumab govitecan combines an antibody targeting trophoblast antigen 2 (Trop-2) and a topoisomerase I inhibitor SN-38 [ 334 ]. Trop-2, a CA 2+ signal transducer, is expressed in 90% of TNBCs and is associated with poor prognosis [ 335 , 336 ]. A single-arm, multicentered phase I/II study evaluated sacituzumab govitecan in heavily pretreated metastatic TNBC patients [ 336 , 337 ]. The efficacy and safety of scituzumab govitecan was shown in these patients, as it was associated with durable objective response. Based on these results, a randomized phase III trial (ASCENT) tested sacituzumab govitecan compared to single-agent chemotherapy chosen by the physician in patients with relapsed or refractory metastatic TNBC [ 309 ]. Sacituzumab govitecan significantly improved PFS and OS of metastatic TNBC patients compared to chemotherapy.
Ladiratuzumab vedotin is composed of a monoclonal antibody targeting the zinc transporter LIV-1 and a potent microtubule disrupting agent, monoethyl auristatin E (MMAE) [ 338 ]. LIV-1 is a transmembrane protein with potent zinc transporter and metalloproteinase activity, expressed in more than 70% of metastatic TNBC tumors [ 339 ]. All clinical trials investigating ladiratuzumab vedotin are still ongoing. A dose-escalation phase I study is evaluating the safety and efficacy of ladiratuzumab vedotin in heavily pretreated metastatic TNBC patients (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT01969643","term_id":"NCT01969643"}} NCT01969643 ). Preliminary results showed encouraging antitumor activity and tolerability of ladiratuzumab vedotin with an objective response rate of 32% [ 340 ]. The estimated study completion date is June 2023. Two phase Ib/II trials are testing ladiratuzumab vedotin in combination with immunotherapy agents in metastatic TNBC patients, such as pembrolizumab (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03310957","term_id":"NCT03310957"}} NCT03310957 ) with expected preliminary results in February 2022, or in combination with multiple immunotherapy-based treatments (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03424005","term_id":"NCT03424005"}} NCT03424005 ) with expected preliminary results in January 2023.
Trastuzumab deruxtecan is an ADC developed as a treatment for metastatic HER2+ BC patients. Its mechanism of action is described in Section 3.2 . Even though trastuzumab deruxtecan was developed to treat HER2+ BC, it showed antitumor activity in HER2-low tumors in a phase I study [ 282 ]. Based on these results, an ongoing open-labeled, multicentered phase III study (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03734029","term_id":"NCT03734029"}} NCT03734029 ) is recruiting patients with HER2-low metastatic BC to test trastuzumab deruxtecan vs. standard treatment chosen by the physician. Preliminary results are expected in January 2023 (see Table 4 ).
Ongoing clinical trials on emerging therapies for BC treatment for all BC molecular subtypes.
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor 2; ER: estrogen receptor; MBC: metastatic breast cancer; BC: breast cancer; HR: hormonal receptor; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival; OS: overall survival; TTP: time to progression. pCR: pathologic complete response; GM-CSF: granulocyte macrophage colony-stimulated factor; DLT: dose-limiting toxicities; MTD: maximum tolerated dose; TTF: time to treatment failure; TTR: time to treatment response; iDFS: invasive disease-free survival; RFS: recurrence free survival; DDFS: distant disease-free survival; iEFS: invasive events-free survival; CR: clinical response; DoCB: duration of clinical benefit; SD: stable disease; DoR: duration of response; IAEs: incidence of adverse events; TDR: treatment discontinuation rate; PR: partial response; DCR: disease control rate; HR: hazard ratio.
4.3.2. Targeted Therapies
Targeted therapy is the current standard of care to treat HR+ and HER2+ BC, but it cannot be administered to patients with TNBC as these tumors lack the expression of these biomarkers. Hence, the next logical step is to identify biomarkers associated with TNBC to develop specific targeted therapies. Several emerging targeted therapies are being clinically trialed with limited or mixed results.
VEGF and EGFR Inhibitors
Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are overexpressed in most TNBC patients [ 341 , 342 ]. Bevacizumab and cetuximab are antibodies developed to specifically target VEGF and EGFR, respectively. Unfortunately, clinical trials studying the effects of these antibodies in TNBC patients demonstrated limited results. The phase III, randomized BEATRICE study evaluating adjuvant bevacizumab-continuing therapy in TNBC demonstrated no significant benefit in OS [ 310 ]. A phase II trial evaluating the impact of adding bevacizumab or cisplatin to neoadjuvant chemotherapy to stage II to III TNBC concluded that further investigation of bevacizumab in this setting was unlikely [ 311 ].
The phase II randomized TBCRC 001 trial testing the combination of cetuximab and carboplatin in stage IV TNBC showed a response in fewer than 20% of patients [ 312 ]. Another randomized phase II study compared the effects of cetuximab plus cisplatin to cisplatin alone in metastatic TNBC patients. Adding cetuximab to cisplatin prolonged PFS and OS, warranting further investigation of cetuximab in TNBC [ 313 ]. Based on these results, bevacizumab is not recommended for the treatment of TNBC.
mTOR/PI3K/AKT Inhibitors
mTOR/PI3K/Akt signaling pathway is an important target involving all BC subtypes. Inhibitors of mTOR, PI3K, and Akt have been tested in HR+ and HER2+ BC patients and have also been tested in TNBC patients. The mTOR inhibitor everolimus has been tested in a randomized phase II trial in combination with chemotherapy vs. chemotherapy alone in stage II/III TNBC patients [ 314 ]. Unfortunately, the addition of everolimus was associated with more adverse effects, without improving pCR or clinical response. A phase I study testing the combination of everolimus and eribulin in metastatic TNBC patients showed that this combination was safe, but the efficacy was modest [ 343 ].
The Akt inhibitor ipatasertib has been tested in combination with paclitaxel (vs. placebo) for metastatic TNBC patients in the phase II multicentered double-blinded randomized LOTUS trial [ 315 ]. The results showed improved PFS when patients received ipatasertib. Another phase II double-blinded randomized trial, FAIRLANE, testing neoadjuvant ipatasertib plus paclitaxel for early TNBC, showed no clinically or statistically significant improvement in the pCR rate, but ipatasertib’s antitumor effect was more pronounced in patients with PI3K/AKT1/PTEN-altered tumors [ 316 ]. Capivasertib, another Akt inhibitor, has been tested in combination with paclitaxel (vs. placebo), first-line therapy for metastatic TNBC patients in the phase II double-blinded randomized PAKT trial [ 317 ]. The addition of capivasertib to paclitaxel significantly improved PFS and OS, with better benefits for patients with PI3K/AKT1/PTEN-altered tumors.
Androgen Receptor Inhibitors
The androgen receptor (AR) is a steroidal hormonal receptor that belongs to the nuclear receptor family and is expressed in 10% to 50% of TNBC tumors [ 344 ]. Tumors expressing AR have better prognosis but are less responsive to chemotherapy [ 345 ]. Multiple clinical trials have tested AR inhibitors in TNBC [ 318 , 319 , 320 ].
Bicalutamide, an AR agonist, was tested in a phase II study in patients with AR+, HR- metastatic BC [ 318 ]. The results showed promising efficacy and safety for these patients.
Enzalutamide, a nonsteroidal antiandrogen, has been tested in a phase II study in patients with locally advanced or metastatic AR+ TNBC [ 319 ]. Enzalutamide demonstrated significant clinical activity and tolerability, warranting further investigation.
Abiraterone, a selective inhibitor of CYP17, has been evaluated in combination with prednisone in AR+ locally advanced or metastatic TNBC patients [ 320 ]. This combination was beneficial for 20% of the patients.
Several clinical trials are currently testing AR inhibitors alone or combined with other treatments for TNBC patients; expecting results between 2022 and 2027 (see Table 4 ).
4.3.3. Immunotherapy
Targeted antibodies.
The immune system plays a crucial role in BC development and progression. Tumor cells can escape the immune system by regulating T-cell activity leading to the inhibition of immune response [ 346 , 347 ]. Two principal biomarkers found in TNBC are associated with this bypass: the programmed cell death protein receptor (PD-1) and its ligand PDL-1, and the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) [ 348 ].
PD-1 is an immune checkpoint receptor expressed on the surface of activated T-cells. PDL-1, its ligand, is expressed on the surface of dendritic cells or macrophages. The interaction of PD-1 and PDL-1 inhibits T-cell response [ 349 ]. CTLA-4 is expressed on T-cells and inhibits T-cell activation by binding to CD80/CD86, leading to decreased immune response [ 350 ].
Atezolizumab, an anti-PDL-1 antibody, has demonstrated safety and efficacy in a phase I study for metastatic TNBC patients [ 351 ]. Based on these results, atezolizumab was tested in combination with nab-paclitaxel for unresectable locally advanced or metastatic TNBC in the phase III double-blinded placebo-controlled randomized Impassion130 study [ 321 ]. Atezolizumab plus nab-paclitaxel prolonged PFS and OS in both the intention-to-treat population and PDL1+ subgroup. Another double-blinded, randomized phase III study (Impassion031) compared atezolizumab in combination with nab-paclitaxel and anthracycline-based chemotherapy vs. placebo for early-stage TNBC [ 322 ]. This combination significantly improved pCR with an acceptable safety profile.
Durvalumab, another anti-PDL-1 antibody, has been tested in combination with an anthracycline taxane-based neoadjuvant therapy for early TNBC in the randomized phase II GeparNuevo study [ 323 ]. This combination increased pCR rate, particularly in patients pretreated with durvalumab monotherapy before chemotherapy. Another randomized phase II study, SAFIRO BREAST-IMMUNO, compared durvalumab to maintenance chemotherapy in a cohort including TNBC patients [ 324 ]. Results showed that durvalumab, as a single agent therapy, could improve outcomes in TNBC patients. A phase I study tested durvalumab in combination with multiple TNBC therapies: PARP inhibitor olaparib and VEGFR1-3 inhibitor cediranib for patients with recurrent cancers including TNBC [ 325 ]. This combination was well tolerated and showed preliminary antitumor activity in all of these patients.
The safety and efficacy of avelumab, another anti-PDL-1 antibody, was evaluated in the phase Ib JAVELIN study in patients with locally advanced or metastatic BC, including TNBC [ 326 ]. Avelumab showed an acceptable safety profile and clinical activity, particularly in tumors expressing PDL-1.
Pembrolizumab is an anti-PD-1 antibody that has been tested in multiple clinical trials. The phase Ib KEYNOTE-012 study demonstrated the safety and efficacy of pembrolizumab on advanced TNBC patients [ 352 ]. Based on these results, the phase II KEYNOTE-086 study evaluated pembrolizumab monotherapy for pretreated or non-pretreated metastatic TNBC patients [ 327 , 353 ]. Pembrolizumab monotherapy showed a manageable safety profile and durable antitumor activity for both pretreated and non-pretreated subgroups. The randomized open-labeled phase III KEYNOTE-119 trial compared pembrolizumab monotherapy to standard chemotherapy in metastatic TNBC [ 354 ]. Pembrolizumab monotherapy did not significantly improve OS compared to chemotherapy in these patients. These findings suggest that pembrolizumab should be investigated in a combinational approach rather than in monotherapy. Based on these results, pembrolizumab was tested in combination with chemotherapy (vs. placebo) for pretreated locally recurrent or metastatic TNBC patients in the phase III double-blinded randomized KEYNOTE-355 trial [ 328 ]. The combination of pembrolizumab plus chemotherapy significantly and clinically improved PFS compared to chemotherapy plus placebo. Pembrolizumab has also been evaluated for early TNBC as neoadjuvant therapy in combination with chemotherapy (vs. placebo) in the phase III KEYNOTE-522 trial [ 329 ]. The combination of pembrolizumab plus chemotherapy significantly improved pCR rate in these patients compared to placebo plus chemotherapy.
Tremelimumab is an anti-CTLA-4 antibody. A dose-escalation phase I study evaluating the safety and efficacy of tremelimumab in patients with metastatic BC showed good tolerability [ 330 ].
Vaccination is an emerging approach to prevent recurrence in high-risk BC patients. As mentioned earlier, TNBC is the most aggressive BC subtype with a higher risk of distant recurrence [ 331 ]. Thus, developing vaccines to prevent recurrence in TNBC patients is of great interest.
Takahashi et al. have developed a novel regimen of personalized peptide vaccination (PPV) based on the patient’s immune system to select vaccine antigens from a pool of peptide candidates [ 332 ]. They performed a phase II study where metastatic recurrent BC patients with prior chemotherapy and/or hormonal therapies received a series of personalized vaccines. This vaccination demonstrated safety, possible clinical benefit, and immune response, especially for TNBC patients [ 332 ]. A multicentered, randomized, double-blinded phase III study analyzed the effects of sialyl-TN keyhole limpet hemocyanin (STn-KLH) on metastatic BC patients [ 333 ]. STn-KLH consists of a synthetic STn, an epitope expressed in BC and associated with aggressive and metastatic tumors, and a high molecular weight protein carrier KLH [ 355 ]. Stn-KLH demonstrated good tolerability, but no benefits in time to progression (TTP) or survival were found. Thus, this vaccination is not recommended for metastatic BC patients [ 333 ].
PVX-410 is a multiple peptide vaccine that activates T-cell to target tumor cells and was developed to treat myeloma. A phase Ib/II study demonstrated the safety and immunogenicity in myeloma patients [ 356 ]. Based on these results, a PVX-410 vaccine is currently being tested to treat TNBC in multiple clinical trials (see Table 4 ).
Finding new treatments for TNBC is an ongoing challenge. The therapeutic strategies that have been described in this section offer great hope to treat TNBC patients. However, because TNBC is highly heterogeneous, it is difficult to find a single treatment efficient for all TNBC subtypes [ 228 ].
5. Conclusions
This review clearly demonstrates that the treatment of BC is complex and is constantly evolving with a large number of ongoing clinical trials on emerging therapies. Indeed, the BC molecular subtype will determine the personalized therapeutic approach, such as targeted treatments like endocrine therapy for HR+ BC or anti-HER2 therapy for HER2+ BC. These therapies have demonstrated their safety and efficacy in treating BC over the years. However, it is essential to go beyond these conventional treatments as BC is a complex disease and not all patients can benefit from personalized treatment. One of the major challenges in BC treatment is finding effective therapies to treat TNBC patients since conventional targeted therapies cannot be administered for this specific BC subtype, which has the worst survival outcomes.
Another important issue in BC treatment is the acquisition of treatment resistance. This is a common phenomenon for either endocrine therapy, anti-HER2 therapy, and chemotherapy.
Hence, understanding the mechanisms underlying drug resistance is a good strategy to develop novel treatments for BC. For example, the mTOR/PI3K/Akt pathway is involved in the mechanism of resistance in all BC molecular subtypes, and thus developing specific inhibitors targeting this pathway is a promising BC treatment approach.
Acknowledgments
The authors would also like to thank team members from the C.D. and F.D. research groups for their valuable assistance.
Abbreviation
Author contributions.
A.B. conceptualized and drafted the manuscript. F.D. and C.D. supervised the project. All authors did critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
This work was supported by the “Fond de recherche du Québec–Santé (FRQS)” associated with the Canadian Tumor Repository Network (CTRNet). Caroline Diorio is a senior Research Scholar from the FRSQ. Anna Burguin holds a Bourse d’excellence en recherche sur le cancer du sein—Faculté de médecine-Université Laval.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- IT’S ADVANCING OUR UNDERSTANDING OF BREAST CANCER
- IT’S SAVING LIVES, IMPROVING OUTCOMES
- IT’S LEADING TO PREVENTION & A CURE
- RESEARCH IS THE REASON STORIES
- Our Approach
- The Ground We’ve Gained
- Areas of Focus
- Meet Our Researchers
- Collaborative Initiatives
- Start Your Fundraiser
- Make a planned gift
- Game for BCRF
- Other Ways to Give
- Become a Partner
- Find an event
- Our History
- Board of Directors
- Scientific Advisors
- Corporate Partners
- Affiliate Organizations
- Major Donors
- Blog: The Progress Report
- Podcasts: Investigating Breast Cancer
- Video Series: Behind the Breakthroughs
- Stories: Research is the reason
- BCRF Publications
- Research is the reason
Research Moves Us Forward
Your gift fuels the research that gives people who have breast cancer longer, healthier lives.
The Breast Cancer Research Foundation is dedicated to ending breast cancer by advancing the world’s most promising research.
This year, BCRF is the largest private funder of breast cancer research—and metastatic breast cancer research—worldwide and is the highest-rated breast cancer research organization in the country.

THIS YEAR'S INVESTMENT
One-third of our research program is dedicated to metastasis.
METASTATIC BREAST CANCER
BCRF explains this form and highlights our MBC investment.
BREAST CANCER RACIAL DISPARTIES
Explore how BCRF researchers are working to end disparities.

Research Is the Reason
Nasreen is grateful to research for the fact that her metastatic breast cancer has responded well to treatment.
From Our Blog

The Breast Cancer Research Foundation Announces $10 Million Donation from Karen and Rob Hale

Broadway Stars Align to Support Metastatic Breast Cancer Research

Support Research This May With BCRF’s Virtual Fitness Challenge

BCRF-Supported Trial Shows Lifestyle Interventions May Improve Breast Cancer Outcomes

Breast Cancer Risk Assessment Explained

Improving Treatments for Invasive Lobular Carcinoma with Dr. Adrian Lee
Meet our researchers.
Learn more about the over 250 investigators BCRF funds.
FUNDRAISE FOR BCRF
We make it easy to make a difference. Get started today.
SHOP PINK, SAVE LIVES
Support brands and products that fuel BCRF’s research.
MAKE A PLANNED GIFT
Use our FreeWill tool to build your legacy: a world without breast cancer.
Get The Latest
Connect with us.
Please remember BCRF in your will planning. Learn More
Breast Cancer Research Foundation 28 West 44th Street, Suite 609, New York, NY 10036
General Office: 646-497-2600 | Toll Free: 1-866-346-3228 [email protected] | BCRF is a 501 (c)(3) | EIN: 13-3727250
- Privacy Policy
Breast Cancer Survivors Face Higher Odds for Second Cancer
By Ernie Mundell HealthDay Reporter

THURSDAY, April 25, 2024 (HealthDay News) -- People lucky enough to survive a breast cancer may still face heightened risks for other cancers later, a new study shows.
The researchers stressed that the absolute risk of a secondary cancer to any one survivor is still low. However, relative to folks who've never had breast cancer , the risk is raised.
"This is the largest study to date to look at the risk in breast cancer survivors of developing a second cancer," noted study senior author Antonis Antoniou , of the department of public health and primary care at the University of Cambridge in Britain.
His team looked at data on over 580,000 female and more than 3,500 male breast cancer survivors enrolled in the U.K.'s National Cancer Registration Dataset. They had all received their initial breast cancer diagnosis between 1995 and 2019.
U.S. Cities With the Most Homelessness

First off, the team looked at the odds that a breast cancer survivor would go on to to be diagnosed with cancer in the previously unaffected breast.
Female breast cancer survivors had double the odds of a tumor arising in the other breast compared to women who'd never had breast cancer before, the team reported April 24 in the journal Lancet Regional Health – Europe .
For male survivors (in whom breast cancer is a much more rare disease), the odds of developing cancer in the other breast rose 55-fold, the study found.
As for other tumor types, female breast cancer survivors were found to have an 87% higher odds of developing endometrial cancer, a 58% higher risk of myeloid leukemia and a 25% upped odds for ovarian cancer, compared to women without any history of breast cancer.
A patient's age at first diagnosis of breast cancer also mattered: Women who had been diagnosed before the age of 50 faced an 86% higher odds for a second cancer later, whereas women first diagnosed after the age of 50 were only 17% more prone to secondary tumors, the data showed.
Why did age matter? It's possible that women who develop a breast cancer when young are more likely to carry cancer-linked genes than women whose cancers emerge later in life, Antoniou's team said.
Economics may also play a role in the risk of second cancers, with breast cancer survivors from poorer backgrounds having a 35% higher odds for a secondary tumor compared to women from the most affluent areas.
“This is further evidence of the health inequalities that people from more deprived backgrounds experience," said study lead author Isaac Allen , a PhD student at Cambridge.
"We need to fully understand why they are at greater risk of second cancers so that we can intervene and reduce this risk," he said in a Cambridge news release.
More information
Find out more about the care of breast cancer survivors at the American Cancer Society.
SOURCE: University of Cambridge, news release, April 24, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: breast cancer
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

RFK Jr.: By the Numbers
Laura Mannweiler April 26, 2024

Biden’s Student Loan Chief to Step Down
Lauren Camera April 26, 2024

What to Know: Bird Flu Virus in Milk
Cecelia Smith-Schoenwalder April 26, 2024

Inflation a Stubborn Foe for the Fed
Tim Smart April 26, 2024

The Curse of the Modern Vice President

‘A Rule for the Ages’
Lauren Camera April 25, 2024

Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Charity News
Cancer vaccines could be game changing, but they’re not a one-shot solution to beating cancer

26 April 2024
The science that helped bring the COVID-19 pandemic under control is continuing to show promise in treating other diseases.
I recently returned from a large conference of cancer experts in the US where there were several presentations on promising early trials of cancer vaccines. Things are really moving in the right direction to bring this type of treatment to more patients sooner.
Today’s announcement about Moderna and MSD’s melanoma cancer vaccine moving to Phase 3 clinical trials means the vaccine will now be tested in a larger number of patients with melanoma post-surgery, to ensure it is an effective treatment for this type of cancer.
If the trial is successful, the same technology could be potentially used to target other cancers in combination with existing treatments such as chemotherapy, radiotherapy and immunotherapy.
It’s not just Moderna’s COVID-19 vaccine technology being repurposed: Cancer Research UK and the CRIS Cancer Foundation recently announced up to £1.7million funding to investigate whether Oxford-AstraZeneca’s COVID-19 vaccine platform can be used to make LungVax, the world’s first vaccine to prevent lung cancer.
We also shouldn’t forget that the HPV vaccine has reduced cases of cervical cancer by nearly 90% in women in their 20s who received the vaccine at age 12 to 13. It’s possible that we could eliminate this cancer type as a public health problem.
As there are over 200 types of cancer, vaccines are unlikely to be a solution to beating all cancer types. We desperately need a systemic approach to addressing the complex challenges associated with cancer.
Research must be sufficiently funded to make more breakthroughs possible. We face a R&D funding gap of more than £1billion by the end of the next decade in cancer research. We want to work with all major political parties, industry and academia to ensure we can plug this gap and ensure the innovations of tomorrow reach patients.
We need long-term strategies for cancer care to help diagnose more cases earlier, reduce inequalities in accessing treatment, and reduce waiting times. We also need to make sure that groundbreaking therapies, should they become available, are accessible to all.
Cancer vaccines progressing through clinical trials are exciting news. These breakthroughs should give us much cause to be optimistic, but we can’t lose sight of the complex challenges ahead, tackling them head on will benefit all of us in the long run.
Iain Foulkes
About the author
Iain Foulkes is Executive Director of Research and Innovation at Cancer Research UK .
This article was originally published in iNews
Tell us what you think
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Read our comment policy .
Highlighted content
Are ultra-processed foods linked to cancer, making cancer screening work for you, clearing the smoke on age of sale: the hidden tactics of the tobacco industry, that cancer conversation podcast – longer, better lives: ep.2 why did a doctor have to wait for her cancer treatment, what's it like to be diagnosed with cancer as a teenager, can vaping cause changes in our cells, flame of hope awards 2024: spotlight on our inspiring volunteers, related topics.
- Preventing cancer
- Expert Opinion
- Research and trials
- Vaccination
- International edition
- Australia edition
- Europe edition

Deprivation linked to higher second cancer risk among England breast cancer survivors
Cambridge study finds those from poorest areas have 35% higher risk of second non-breast cancer
Female survivors of breast cancer living in the most deprived areas have a 35% higher risk of developing second, unrelated cancers, compared with those from the most affluent areas, research shows.
Breast cancer is the most commonly diagnosed cancer in the UK, with about 56,000 people being told they have it each year. Improved diagnosis and treatments mean that five-year survival rates are now 86% in England.
People who survive breast cancer have a greater likelihood of second primary (unrelated) cancer, but until now the exact risk has not been clear.
A team of researchers led by the University of Cambridge analysed NHS data from almost 600,000 patients in England and found, compared with the general female population, women who had survived breast cancer had an increased risk of developing 12 other primary cancers.
They had double the risk of developing cancer in the unaffected (contralateral) breast, an 87% higher risk of endometrial cancer, a 58% increased likelihood of myeloid leukaemia and a 25% higher risk of ovarian cancer.
The study, published in Lancet Regional Health – Europe, found that the risk of second primary cancers was higher in people living in areas of greater socioeconomic deprivation.
Compared with the most affluent, the least well-off female survivors of breast cancer had a 166% greater chance of developing lung cancer, a 78% higher risk of stomach cancer, more than 50% increased risk of bladder and oesophagus cancers, 48% higher risk of head and neck cancer and 43% increased risk of kidney cancer.
Overall, those from the most deprived areas had a 35% higher risk of a second non-breast cancer.
This may be because risk factors such as smoking, obesity and alcohol consumption are more common among more deprived groups. A 2023 study found that deprivation causes 33,000 extra cancer cases in the UK each year .
The first author, Isaac Allen, from the department of public health and primary care at the University of Cambridge, said: “This is the biggest study ever to examine second cancers after breast cancer and the first to show that women diagnosed with breast cancer in deprived regions are more likely to get second cancers. Many cancers are caused by deprivation, but more research is clearly needed to identify the specific factors driving the higher risks and how best to reduce these inequalities.”
In addition to data from more than 580,000 women, the authors examined the risk of second primary cancers for more than 3,500 male breast cancer survivors diagnosed between 1995 and 2019 using the national cancer registration dataset.
Male breast cancer survivors were 55 times more likely than the general male population to develop contralateral breast cancer, 58% more likely than the general male population to develop prostate cancer and had four times the risk of thyroid cancer, although the actual numbers of these cancers were low.
Responding to the findings, Prof Pat Price, a leading oncologist and co-founder of the Catch Up With Cancer campaign , said: “This highlights yet another instance of alarming inequalities within cancer, underscoring the urgent need for a dedicated cancer plan. Where one comes from or their socioeconomic status should not determine the chances of developing or surviving cancer.”
Dr Simon Vincent, the director of research, support and influencing at Breast Cancer Now, said while the higher risk of secondary cancer may occur due to genetic factors or the effects of initial breast cancer treatment, more research was needed into the causes of second primary cancers and how to follow up patients completing primary breast cancer treatment.
- Breast cancer
- Women's health
- Social exclusion
Medics design AI tool to predict side-effects in breast cancer patients

Labour MP Foy says she has breast cancer and urges others to get checked

Cost of breast cancer to UK economy could reach £3.6bn by 2034, study shows

Consumer genetic test results ‘causing unnecessary breast cancer alarm’
Most early-stage breast cancer patients will be long-term survivors – study
Breast cancer drug cuts risk of most common form returning by 25%
Should I worry about the cancer risk from hormonal contraceptives?
Advising older patients against breast cancer surgery is ‘age bias’, UK study finds
Drug may help more women survive hereditary breast cancer
Most viewed.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 May 2023
Global trends and forecasts of breast cancer incidence and deaths
- Yuyan Xu 1 na1 ,
- Maoyuan Gong 1 na1 ,
- Yue Wang 2 ,
- Yang Yang 1 ,
- Shu Liu 2 &
- Qibing Zeng ORCID: orcid.org/0000-0002-6694-1503 1
Scientific Data volume 10 , Article number: 334 ( 2023 ) Cite this article
10k Accesses
26 Citations
10 Altmetric
Metrics details
- Cancer epidemiology
- Risk factors
Breast cancer (BC) is one of the major public health challenges worldwide. Studies that address the new evidence on trends of BC are of great importance for preventing and controlling the occurrence and development of diseases and improving health. The aim of this study was to analyze the outcomes for the global burden of disease (GBD), incidence, deaths, and risk factors for BC from 1990 to 2019, and predict the GBD of BC until 2050 to inform global BC control planning efforts. In this study, the results show that the regions with low levels of socio-demographic index (SDI) will have the largest disease burden of BC in the future. The leading global risk factor for death attributable to BC in 2019 was metabolic risks, followed by behavioral risks. This study supports the worldwide urgent need for comprehensive cancer prevention and control strategies to reduce exposure, early screening, and improve treatment to effectively reduce the GBD of BC.
Similar content being viewed by others
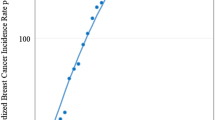
Forecast of a future leveling of the incidence trends of female breast cancer in Taiwan: an age-period-cohort analysis

Trends in female breast cancer incidence, mortality, and survival in Austria, with focus on age, stage, and birth cohorts (1983–2017)

A multilevel assessment of the social determinants associated with the late stage diagnosis of breast cancer
Introduction.
Breast cancer (BC) is a most common malignant tumor, and its global burden of disease (GBD) has become one of the important factors that endanger the health of the world population, especially the health of women 1 . The global BC statistics report shows that in 2020, there will be 2.261 million new cases and 685,000 deaths worldwide, and BC has become the number one malignant tumor in the world 2 . Although some cancer cases cannot be prevented, governments can develop a range of health interventions to minimize exposure to known cancer risk factors, such as environmental factors, lifestyle behaviors, dietary habits, metabolic factors, etc 3 . Therefore, understanding the relative contributions of modifiable risk factors to the GBD of BC and their long-term trends is critical to inform local and global cancer control efforts.
Global Health Data Exchange provides two important research tools (GBD Comparison Tool and GBD Results Tool) that have been open sourced to quantify GBD, assessing GBD by age group, sex and time (1990 to 2019) in countries around the world), attributed to a wide range of modifiable risk factors 4 . GBD 2019 is the latest iteration of the GBD study, which provides an opportunity to assess the global cancer burden attributable to risk factors. Previous studies assessed the global, regional, and national burden of breast cancer until 2017 5 , 6 , 7 , 8 , 9 . A recent study 10 evaluated the GBD of female BC from 1990 to 2019 and predicted the GBD of female BC in 2035. However, these studies know little about the global cancer burden attributable to metabolic, behavioral, diet, physical activity factors and its more longer-term future forecasts to 2050.
In this study, we report for the first time the GBD for BC attributable to a comprehensive inventory of metabolism, behavior, diet, and physical activity from 1990 to 2019, using breast cancer incidence, deaths, and risk factor results. Furthermore, this study provides a new perspective on the attributable cancer burden by estimating the risk-attributable cancer burden at global levels using incidence and deaths.
Global burden of disease and temporal trends of breast cancer
To assess the global GBD and changing trends of BC, the incident cases, death cases and ASR of BC in 1990 and 2019 were calculated, and the estimated annual percentage change (EAPC) was used to demonstrate the temporal trends from 1990 to 2019. The global GBD and temporal trends of BC are presented in Supplementary Tables 1 and 2 . Globally, the incident cases of BC increased from 876,990 in 1990 to 2,002,350 in 2019, and the EAPC for incidence increased by an average 0.33% per year. Although the death cases of BC in 2019 is higher than in 1990 worldwide, the EAPC for deaths decreased by an average 0.56% per year. In terms of gender, the number of cases and ASR of women are higher than that of men, regardless of morbidity and death. However, it is worth noting that the EAPC for incidence in men increased by an average 0.91% per year, which is higher than the woman with 0.36%. And the EAPC for deaths in different gender population both gradually decreased. Compared with other SDI regions, the incident cases, death cases and ASR of BC in high SDI regions were at a higher level. However, it is exciting to note that the EAPC for incidence began to decline in high SDI regions, and the EAPC for deaths also decreased the most in this group. In the other hand, we also observed a fast increase in the EAPC for incidence in the middle SDI regions and the EAPC for deaths in the low SDI regions. Further observation of the GBD and temporal trends of 21 GBD regions found that the highest incident cases and ASR for incidence of BC is in East Asia region, and the largest decline for EAPC is in Central Asia region. Moreover, Western Sub-Saharan Africa is the only region where EAPC for incidence continues to grow. Western Europe, Oceania and High-income North America are the region with higher breast cancer deaths in 1990, but by 2019, only the Oceania region was found to still be at relatively high levels. The EAPC for deaths in the Western Sub-Saharan Africa region increased fast, but High-income North America, Australasia and Western Europe regions decreased more obviously.
Figures 1 and 2 show the GBD of BC incidence and mortality for 204 countries and territories. As shown, the countries with the highest incidence and deaths of ASR in 1990 were concentrated in high-income countries (Figs. 1a and 2a ). However, the top 2 countries with the highest incidence and deaths of ASR in 2019 are not high-income countries, such as Lebanon and Solomon Islands with the highest incidence (Fig. 1b ) and Pakistan and Solomon Islands with the highest death (Fig. 2b ). Subsequently, we further analyzed global changes in cancer case (Figs. 1c and 2c ) and EAPC (Figs. 1d and 2d ) to better indicate temporal trends in GBD. From the perspective of changes in cancer cases, only 2 countries have seen a decline in incidence, while 72 countries have seen a decline in deaths. Among them, 52 countries with an increase for incidence of more than 300%, but only 3 countries had an increase for deaths of more than 300%, and the largest increase was both in the Solomon Islands.
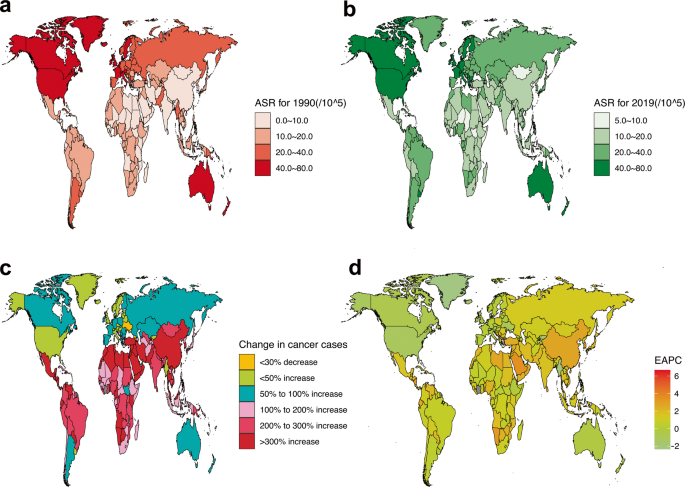
Global GBD and temporal trends of BC incidence in 204 countries or territories. ASR: age standardized rate; BC: breast cancer; EAPC: estimated annual percentage change; GBD: global burden of disease. ( a ) The ASR per 100,000 people in 1990; ( b ) The ASR per 100,000 people in 2019; ( c ) The change in cancer cases; ( d ) EAPC in different countries or territories.
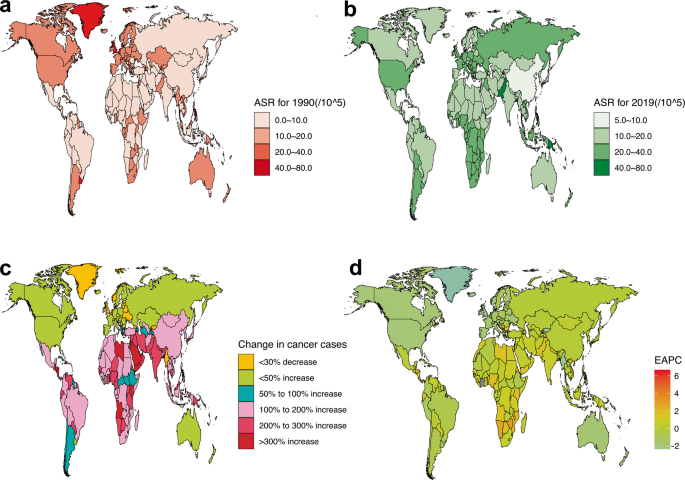
Global GBD and temporal trends of BC deaths in 204 countries or territories. ASR: age standardized rate; BC: breast cancer; EAPC: estimated annual percentage change; GBD: global burden of disease. ( a ) The ASR per 100,000 people in 1990; ( b ) The ASR per 100,000 people in 2019; ( c ) The change in cancer cases; ( d ) EAPC in different countries or territories.
Supplementary Fig. 1 combines EAPC for incidence and deaths data in a hierarchical cluster analysis to identify countries with similar annual growth rates in incidence and deaths. As shown in the multimedia appendices, 35 countries (or territories) were cluster into the significant increase group, including the Northern Mariana Islands, Taiwan (Province of China), Netherlands, Germany, Viet Nam, Gambia, etc . A total of 39 countries (or territories) were categorized into the minor increase group, including United States of America, United Kingdom, Pakistan, Canada, etc . Another 120 countries (or territories) were grouped into the remained stable or minor decrease group, including China, Japan, France, Mexico, and Solomon Islands. The remaining 10 countries (or territories) were categorized into the significant decrease group, including Turkmenistan, Uzbekistan, Puerto Rico, Kazakhstan, Bahrain, Colombia, Singapore, Maldives, Chile.
Global burden of disease of breast cancer attributable to risk factors
The results of GBD of BC attributable to the risk factors were shown in Fig. 3 and Supplementary Figs. 2 and 3 . As revealed in the Figure, the leading risk factor in terms of attributable BC deaths was metabolic risks worldwide, which accounted for 31.98% in 1990, and has a gradual increasing trend in 2019, accounting for 46.87%. Alcohol use, tobacco, dietary risks, and low physical activity were the next greatest risk factors. The percentage of BC deaths due to metabolic risks was significantly heterogeneous all over the world, with the highest percentage observed in Oceania region (55.48% in 1990 and 63.76% in 2019), followed by Southeast Asia region (47.34% in 1990 and 63.69% in 2019). The largest increase in the percentage for BC deaths due to the metabolic risks from 1990 to 2019 are Southern Sub-Saharan Africa (17.66%), South Asia (17.29%), Andean Latin America (16.59%), Southeast Asia (16.35%) regions. At the same time, we also observed a gradual decrease in the percentage of BC deaths due to behavioral risks such as such as alcohol use and tobacco. Dietary risks and low physical activity have remained relatively stable over the past 20 years. When we assessed the time trends of attributable risk factors at the SDI level, we found that the most increase in the percentage for BC deaths due to the metabolic risks from 1990 to 2019 are in the middle (14.42%), low-middle (14.41%) and middle-high (13.29%) SDI areas. Multimedia Appendix 2 shows the two metabolic risks attributable to breast cancer death. As shown in the figure, the global and low, middle-low and high SDI regions accounted for half and half percentage of BC deaths due to high fasting plasma glucose and high body mass index, but the fasting plasma glucose in the middle, middle-high SDI region was very high, accounting for 70.10%~ 84.62%. Furthermore, the proportion of low, low-middle, and middle SDI areas attributed to the high body mass index is increasing, especially in the low-middle SDI areas, from 42.65% in 1990 to 54.70% in 2019.
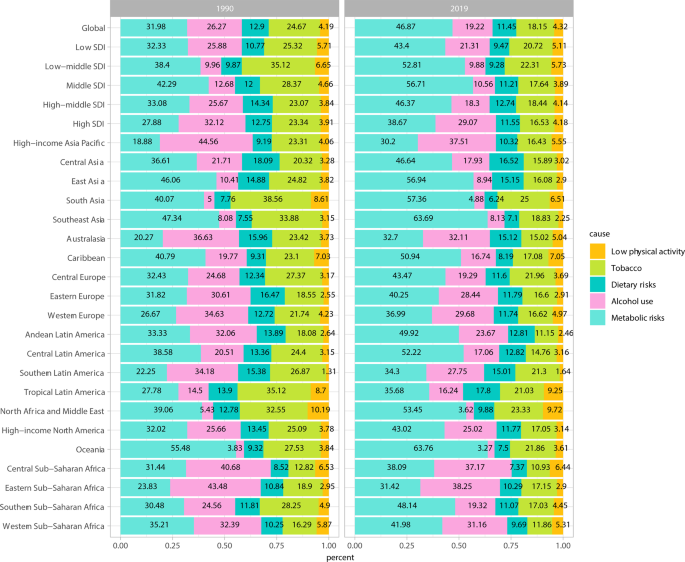
GBD of BC attributable to risk factors in 1990 and 2019. BC: breast cancer; GBD: global burden of disease; SDI, socio-demographic index.
Factors influencing the estimated annual percentage change in the global burden of disease
To better explain GBD in BC, we analyzed influencing factors that may affect EAPC, including ASIR, ASDR, and HDI (which can be used as an indicator of the level and availability of medical care in each country) (Fig. 4 ). As illustrated in the Figs. 4a,b , a significant negative correlation was found between EAPC and ASIR, ASDR in 1990 ( r = −0.607, −0.583; P all < 0.001). In contrast, this negative correlation in 2019 gradually weakened or disappeared. EAPC had a weak negative correlation with ASIR ( r = −0.152; P = 0.030) in 2019, but a positive correlation with ASDR ( r = 0.315; P < 0.001). Figure 4c,d show the correlation between the EAPC and HDI. As revealed in the figure, whether in 1990 or 2019, the relationship between EAPC and HDI is not a simple linear correlation, on the contrary it is more like a “parabola”. when the HDI was limited to below 0.50 in 1990 or 0.55 in 2019, a significant positive correlation was found between EAPC for incidence and deaths and HDI. In contrast, for a HDI above 0.50 in 1990 or 0.55 in 2019, the positive association gradually disappeared, and EAPC for incidence and deaths has a significant negative correlation with HDI in 1990 ( r = −0.312, −0.548; P all < 0.001) and 2019 ( r = −0.300, −0.582; P all < 0.001).
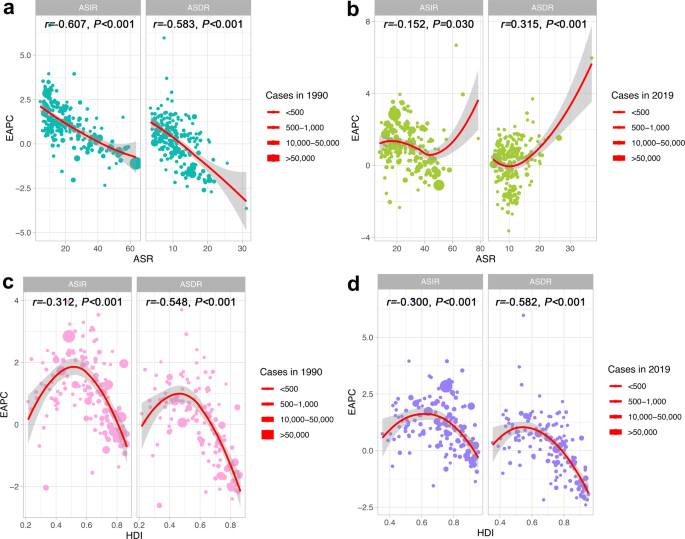
Factors Influencing EAPC in the GBD. ASR: age standardized rate; ASIR: age standardized incidence rate; ASDR: age standardized death rate; BC: breast cancer; EAPC: estimated annual percentage change; GBD: global burden of disease; HDI: human development index. ( a ) The correlation between EAPC and ASR in 1990. ( b ) The correlation between EAPC and ASR in 2019. ( c ) The correlation between EAPC and HDI in 1990. ( d ) The correlation between EAPC and HDI in 2019.
Future forecasts of global burden of disease in breast cancer
Figure 5 show the future forecasts of GBD in BC. As illustrated in the Fig. 5a,b , the ASR of BC incidence in the world will gradually increase. It is estimated that by 2050, the ASR of BC incidence in female will be 59.63 per 100,000, an increase of 32.13% compared with 2019; the ASR of BC incidence in male will be 0.65 per 100,000, an increase of 1.74% compared with 2019. Subsequently, we also estimated the ASR of global BC deaths from 2020 to 2050 (Fig. 5c,d ). Over time, the ASR of BC death in female increased slightly, but the ASR of BC deaths in male gradually decreased. It is estimated that by 2050, the ASR of female BC deaths will be 16.42/100,000, an increase of 4.69% compared with 2019; the ASR of BC deaths in male will be 0.26 cases per 100,000, a decrease of 19.84% compared with 2019. According to the United Nations world population forecast data, there will be 4,781,849 incident cases (4,714,393 women and 67,456 men) and 1,503,694 death cases (1,481,463 women and 22,231 men) of BC in the world in 2050.
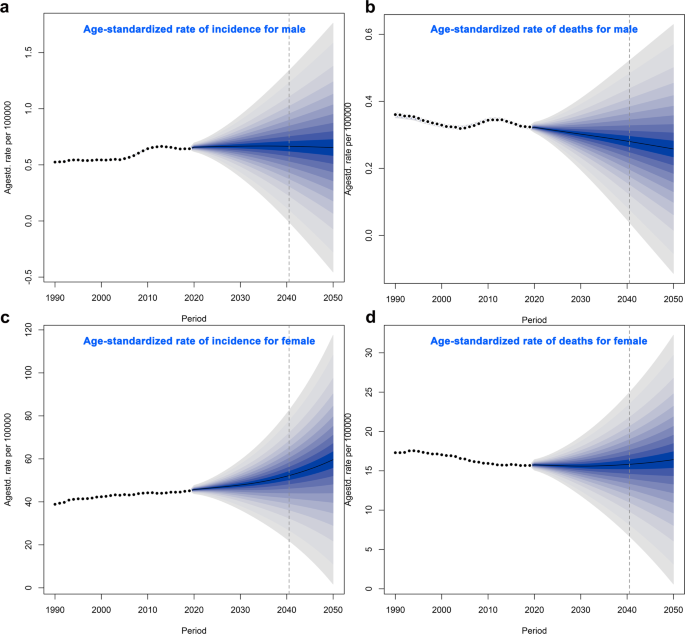
Future Forecasts of GBD in BC. ASIR: age standardized incidence rate; ASDR: age standardized death rate; BC: breast cancer. ( a ) The ASIR per 100,000 for male; ( b ) The ASDR per 100,000 for male; ( c ) The ASIR per 100,000 for female; ( d ) The ASDR per 100,000 for female.
Breast cancer incidence burden
Our analysis found that the global incident cases of BC increased from 876,990 in 1990 to 2,002,350 in 2019, a total increase of 1.28 times. This is an increase of another 5 percentage points compared to the 2017 GBD data for BC 11 , which showed that the global incident cases of BC increased by 123% between 1990 and 2017. Although we have seen a sharp increase in the BC incident cases worldwide in the past 20 years, the ASR has not shown a trend of rapid growth. And this slow growth trend is also confirmed by our EAPC results and other study 11 , which also found that from 1990 to 2017, the incidence of breast cancer worldwide increased by 123%, but the change in ASR was not obvious. Previous study 12 has found that the changes in the number of BC cases are largely attributable to population growth and aging. This seems to explain the findings in this study well, since our study only found a significant increase in the incident cases of BC, not ASR. It suggests that reducing the global population may be one of the key factors in reducing BC incidence.
For the gender, we saw an absolute predominance of women, which is logical, but it is worth noting that the EAPC for incidence in men is significantly higher than that in women and continues to increase at an average rate of 0.91% per year. These results suggest that we should not ignore men in the health monitoring of BC in the future, especially for those who have bad behavior factors, such as smoking, alcohol use. The latest study 1 found that tobacco is the main risk factor of cancer for male, followed by alcohol use, dietary risks and air pollution.
SDI is a composite index calculated based on the total fertility rate of women under the age of 25, the per capita lagged distribution income and the average education level of individuals aged 15 and above 13 . Our results showed that the higher the SDI level, the higher incident cases and ASR of BC, but the EAPC did not appear consistent. On the contrary, in the meddle-high and high SDI regions, the EAPC of BC incidence was significantly reduced, especially in the high SDI region even showed negative growth. These findings were also confirmed by further association analysis, and it was found that EAPC showed a significant negative correlation with ASIR. One possible explanation is that the ASR of BC incidence in these regions was higher in the past, with limited room for increase. Furthermore, due to the general increase in the education level of the population in these areas, people’s awareness of health has been continuously strengthened, which has limited the growth of BC to a certain extent. However, it is worth noting that in low, low-middle, and middle SDI regions, changes in the ASR and EAPC due to population growth and due to the global rise in SDI levels, they are re expected to impose increasing burdens on individuals and societies.
From the analysis of countries or regions, the countries with high ASR of BC incidence 20 years ago were mainly concentrated in high-income countries (such as the United States, New Zealand and the Netherlands), but in 2019, some low-income countries (such as Solomon Islands, Lebanon) rapidly occupy the position of high ASR incidence. These results support our previous hypothesis that low, low-meddle, and middle SDI regions are projected to impose increasing burdens on individuals and society. Furthermore, East Asia is the region with the highest ASR of BC incidence, sub-Saharan Africa is the only region where the EAPC of BC continues to grow, and Solomon Islands is the only country where EAPC has increased by more than 6%. These countries and regions should be the focus of future BC disease burden monitoring.
Further GBD future forecasts for BC demonstrated that that from 2020 to 2050, the global BC incidence and total incidence will increase year by year. Therefore, how to control modifiable risk factors and reduce the incidence of BC becomes the key to alleviating GBD of BC.
Breast cancer deaths burden
ASR for deaths, as one of the commonly used indicators in disease burden research, can measure the level of risk to the population from the perspective of life 9 . Our study demonstrated that the global death cases of BC in 2019 (700,660 cases) was higher than in 1990 (380,910 cases). There will be 1,503,694 death cases (1,481,463 women and 22,231 men) of BC in the world in 2050. These results suggest that the GBD from breast cancer deaths will remain severe for some time to come. The incidence of BC is dominant in females, so the relatively higher death rate in females may be related to the higher incidence of BC in females than in males. Previous studies 9 , 11 have shown that high-income countries such as North America and Western Europe have a higher GBD of BC due to death. Our GBD study based on 1990 also came to a similar conclusion. But what is exciting is that by 2019, the ASR for deaths in high-income countries such as North America and Western Europe has gradually declined the most obvious in all countries. These findings were also confirmed by the results of death cases and death ASR in different SDI index countries and the significant negative association between HDI and EPAC. A logical explanation is that the application in widespread mammography for early-stage BC diagnosis in high-income and high-SDI countries 7 , 14 and improved treatment facilities in terms of chemotherapy, radiation therapy, and targeted approaches may be the underlying reasons for the decline in BC mortality in these countries. In addition, our study also found that the growth of EAPC for deaths was particularly rapid in low and low-medium SDI regions and in sub-Saharan Africa. Possible solutions behind this growing trend are access to widespread mammograms, improved BC awareness, increased exercise and greater access to healthcare, among others. It is worth noting that in low-income countries, individuals suffering from severe illnesses may opt to discontinue their treatment because of the considerable financial burden it places on their families. This decision can lead to a rapid deterioration of their condition and ultimately hasten their demise, a phenomenon known as “near-suicide.” Research indicates that as the severity of the illness increases, patients are more likely to forego treatment due to familial responsibilities 15 . These findings underscore the importance of providing accessible and affordable healthcare for individuals dealing with serious illnesses.
Risk factors attributable to breast cancer burden
The latest research evidence 1 shown that 44.4% of global cancer deaths and 42.0% of global cancer disability-adjusted life years can be attributed to GBD 2019 estimated risk factors. Our study demonstrates that the major risk factor globally attributable to BC deaths is metabolic risks. High body mass index and high fasting glucose have also been identified as potential risk factors attributable to BC deaths 11 , and our study found that the proportion of contribution of the two risk factors was quite different in various SDI regions of the world. In most countries with high SDI, the growth rate of national wealth is also the fastest, and the growth rate of national wealth is often proportional to the increase in body weight 16 . Our study did not observe a significant increase in the proportion of BC death risk attributable to high body mass index in high-income countries, which may be related to the traditional low-calorie diet 17 and high physical activity in the part of countries, such as walking 18 . Conversely, the proportion of BC deaths attributable to high body mass index is increasing in low, low-middle SDI regions. It suggests that the body weight control is the key to reduce the risk of BC disease in the future, in these regions, especially in low-middle SDI region. However, glycemic control may be more important for middle and middle-high SDI regions, where a very high proportion of BC deaths are attributable to high fasting glucose.
Alcohol use is one of the important risk factors for BC death 11 , and a pioneering study 19 has revealed a possible dose-response relationship between alcohol consumption and BC. Our study found that the proportion of disease burden of BC deaths attributable to alcohol use gradually decreased in meddle-high and high SDI countries, which may be very much related to the significant decline in the prevalence of daily alcohol consumption globally 20 . These results may also better explain why the incidence and deaths of BC in high-income countries such as North America and Europe have gradually decreased, despite high-calorie, high-metabolic diets.
The GBD 2019 Study shows that smoking remains the leading cause of cancer death and health loss worldwide 1 . Our study found that the proportion of BC disease burden attributable to smoking decreased gradually over time in low, low- middle, and middle SDI regions, which may be related to the decline in smoking prevalence in these regions. Because previous study 21 has shown that smoking rates decline with the lower SDI. In addition, our study indicates that the dietary risk and low physical activity also play a role in the burden of BC disease. Therefore, reducing the global burden of breast cancer requires a comprehensive cancer prevention and control strategy. On the one hand, reduce the incidence and death of breast cancer by controlling adverse metabolic risks and behavioral risks (such as alcohol use, tobacco, dietary risk, low physical activity); on the other hand, by promoting mammography for early diagnosis of breast cancer, and improvements in effective treatments to effectively reduce the global burden of disease.
Strengths and limitations
To our knowledge, this GBD-based study is the largest effort to date to reveal global BC incidence and deaths, determine the global cancer burden attributable to the most relevant risk factors, and predict the future burden of BC. The study will help to enrich the research evidence of global BC risk and attributable disease burden 11 , 22 , 23 , 24 , 25 , which is of great important to prevent and control the occurrence and development of BC and improve health. However, our study also has limitations. First, some countries (or territories) do not have population-based cancer registries, leaving an important source of data for estimating cancer burden missing. Second, the GBD2019 only provides some behavioral risks (such as tobacco, alcohol use, dietary risks and low physical activity) and metabolic risks (including high fasting glucose and high body mass index) that can be used for further research 26 , are important for a comprehensive assessment of the burden of breast cancer attributable to risk factors. Finally, the disability-adjusted life years, as an index that can simultaneously consider premature death from disease and health loss from disability, has received increasing attention in the field of international cancer disease burden evaluation 27 , and this study focuses on diseases caused by BC incidence and deaths burden.
Moreover, data sharing, a practice that enhances research integrity and transparency, facilitating peer validation and enabling further exploration of the study’s findings, offers valuable resources for scientific research and evidence-based policymaking, particularly relevant to developing countries 28 , 29 . In this sense, our research has significant meaning, because all data available free of charge. Our data provide a rigorous and comparable measure of the global disease burden of breast cancer, all freely downloadable, and can be used by policymakers in the future to generate the evidence they need on how to allocate resources to best improve the population Health makes informed decisions. Nonetheless, it is worth noting that our findings may be delayed as they reflect past disease burden. While our analysis provides valuable insights into the historical trends of breast cancer, predicting future trends necessitates confirmation by more recent data.
Overall, our study provides some evidence that regions with low levels of SDI will have the largest disease burden of breast cancer in the future. Metabolic risk factors increased the most from 1990 to 2019, compared with the behavioral factors. The findings of this study may be of great value for preventing and controlling the incidence and deaths of BC, as well as for improving the health of population. Furthermore, our study results may aid decision makers in formulating more reasonable and effective preventive health policies, and solutions for BC, and related health inequalities.
Study design
In this study covering data of GBD on incidence, deaths, and their temporal trends in 204 countries or territories and 21 regions from 1990 to 2019, different changing trends of BC burden were observed, with significant differences by sex, region, country, and sociodemographic index. The logical flowchart of this study is shown in Supplementary Fig. 4 .
Data sources
Annual incident cases, age standardized incidences and deaths of BC from 1990 to 2019, by sex, region, country, and risk factors (metabolic risks, dietary risks, tobacco, alcohol use and low physical activity) were obtained from the GBD 2019 through the Global Health Data Exchange (GHDx) query tool ( https://ghdx.healthdata.org/gbd-2019 ).
To create the source dataset, we follow a procedure. First, we access the data acquisition interface of the database and click on the “query tool” hyperlink located under the “GBD Results Tool” menu. This leads us to the data retrieval interface where we have the option to select different GBD evaluation options from the “GBD Estimate” drop-down menu. By default, cause of death or injury is selected. Next, we can choose from a variety of disease evaluation indicators such as morbidity, prevalence, mortality, and disease burden, including disability-adjusted life years, from the “Measure” drop-down menu. We can also select different measurement indicators like number, percent, and rate from the “Metric” drop-down menu. The “Cause” drop-down menu provides an extensive list of common causes such as tumor, high blood pressure, and diabetes, among others. Similarly, the “Location” drop-down menu displays all the countries and regions in the world which are categorized in great detail, including China (national level) and East Asia (regional level). For some countries like the United States and the United Kingdom, intra-country state or provincial level data is also available although this feature is not currently available for China. Moreover, we can filter data by age and sex using the “Age and Sex” drop-down menu, while the “Year” drop-down menu allows us to choose a time range between 1990–2019. GBD 2019 is the most up to date and ongoing global collaboration, and all epidemiological data are available as open source. Simply enter your desired query in the search box above and click “Search” to retrieve the relevant information. Alternatively, you may choose to directly download the CSV file by clicking on the “Download CSV” button.
Data records
A total of 204 countries or territories and 21 regions were selected in this study. The human development index (HDI) data at the national level were collected from the United Nations Development Programme ( https://hdr.undp.org/data-center/human-development-index#/indicies/HDI ). Rates in this study are reported per 100,000 people, and age-standardized rates are calculated based on GBD world population standards 30 . Some of the results were provided by the sociodemographic index (SDI) to describe differences in GBD of BC. The quintiles of the SDI index are used to define low (~20), low-middle (~40), middle (~60), middle-high (~80) and high (~100) SDI countries in 2019 27 . The global population forecast data for 2017–2100 were obtained from the Institute for Health Metrics and Evaluation ( https://ghdx.healthdata.org/record/ihme-data/global-population-forecasts-2017-2100 ). The data supports this finding have recorded in the Figshare 31 ( https://doi.org/10.6084/m9.figshare.22787405 ). The document “GBD for BC.xlsx” comprises six main worksheets. The first worksheet, named “BC_nation,” is primarily utilized to analyze the country’s morbidity and mortality related to BC, enabling quantification of this data. The following worksheets - “BC_region,” “BC_region_SDI,” and “BC_region_SEX” - are used to examine the morbidity and mortality of BC quantified by region, SDI, and sex. To assess trends in BC incidence and mortality, an estimation of the change in cancer cases from 1990 to 2019, along with the EAPC and its 95% confidence interval, are used. Finally, the “BC_percent” worksheet focuses on estimating the cancer burden attributable to risk factors.
Data analysis
Referring to previous literature report 32 , the age-standardized ratio (ASR) and its 95% uncertainty interval was used to quantify the incidence and deaths of BC by time, sex, region, country and SDI. Then, the changes in cancer cases, the estimated annual percentage change (EAPC) and its 95% confidence interval from 1990 to 2019 was used to assess the incidences and deaths trend of BC. Finally, we combined EAPC data for incidences and deaths to perform hierarchical cluster analysis to identify countries with similar annual increases in incidences and deaths. All countries were divided into 4 groups, including minor increase, remained stable or minor decrease, significant decrease, and significant increase.
GBD 2019 includes three categories of attributable risks, such as environment or occupation risks, behavior risks and metabolism risks. We first identified the BC risk factors with convincing or likely causal evidence based on World Cancer Research Fund criteria. Then, the proportion of cancer-specific burden attributable to each risk factor was calculated in different year, region, country and SDI. Finally, temporal trends of attributable risk factors were assessed at the SDI level.
We selected two-time nodes, 1990 and 2019, and calculated the age-standardized incidence rates (ASIR) and age-standardized deaths rates (ASDR) at the country level. Then, HDI, ASIR, ASDR were selected as the candidate indicators to determine the influencing factors of EAPC by correlation analysis.
Considering that the incidence and mortality rates of different sexes are different, in this study we separately predicted the incidence and deaths rates of men and women from 2020 to 2050 to assess the future GBD of BC. This GBD forecasts is primarily based on the Global Population Forecasts 2017–2100 data and age-standardized BC incidence and deaths data from 1990 to 2019.
All statistical analysis of data were performed using the R Project for Statistical Computing (version 4.2.2; R Core Team). We used the ASR and EAPC to quantify the BC incidence and deaths trends. Constituent ratios were used to evaluate the cancer burden attributable to risk factors. Pearson correlation analysis was used to determine the association of HDI, ASIR, ASDR with EAPC. For the future forecasts of GBD in BC, we used the BACP package. A threshold of P value less than 0.05 was set to determine the significant differences.
Usage Notes
Our data and code are freely available as open source. The analysis codes presented in the article were written using the R language. To conduct your own analysis, you will need to first install the necessary environment for R, including packages such as dplyr, ggplot2, ggsci, factoextra, ggmap, rgdal, maps, devtools, and others. Moving forward, these data can be utilized to examine the disease burden of breast cancer and its changing trends, categorized by time, sex, region, country, and SDI. Additionally, if you intend to analyze other disease burdens apart from breast cancer, our open-source R language code is also well-suited for your needs.
Data availability
The data supports this finding can be accessed from the Figshare 31 ( https://doi.org/10.6084/m9.figshare.22787405 ). The “Date.xlsx” file contains separate sheets that provide pertinent metadata for assessing the incidence and mortality rates of breast cancer based on various factors such as time, gender, region, country, and socio-demographic index (SDI). In addition to this information, the document also includes data on the World population age standard, the HDI of different countries in 1990, and Global Population Forecasts spanning from 2017 to 2100.
Code availability
All R code supporting the conclusions of this study can be accessed and downloaded via Github 33 ( https://doi.org/10.5281/zenodo.7915783 ). The main computational tools used in this study are R language based. The scripts used in this study include “GBD_Incidence_region.R,” which calculates incident cases, deaths, ASR, and EAPC for BC worldwide during 1990 and 2019. Another script, “GBD_Incidence_map.R” is employed to generate visualizations of BC incidence and mortality using GBD data from 204 countries and regions around the world. Additionally, “GBD_cluster.R” was used to perform hierarchical cluster analysis to identify countries with similar annual increases in BC incidence and mortality. To calculate the percentage of major risk factors globally attributable to BC mortality, “GBD_Percent.R” was utilized. The correlation between EAPC and ASIR, ASDR, and HDI was analyzed using “GBD_COR.R”. Finally, “Global_BAPC_prediction.R” was implemented to predict the future burden of BC using GBD data.
Collaborators, G. B. D. C. R. F. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400 , 563–591, https://doi.org/10.1016/S0140-6736(22)01438-6 (2022).
Article Google Scholar
Sung, H. et al . Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71 , 209–249, https://doi.org/10.3322/caac.21660 (2021).
Article PubMed Google Scholar
Winn, A. N., Ekwueme, D. U., Guy, G. P. Jr. & Neumann, P. J. Cost-Utility Analysis of Cancer Prevention, Treatment, and Control: A Systematic Review. Am J Prev Med 50 , 241–248, https://doi.org/10.1016/j.amepre.2015.08.009 (2016).
Global Burden of Disease Cancer, C. et al . Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 5 , 1749–1768, https://doi.org/10.1001/jamaoncol.2019.2996 (2019).
Safiri, S. et al . Burden of female breast cancer in the Middle East and North Africa region, 1990-2019. Arch Public Health 80 , 168, https://doi.org/10.1186/s13690-022-00918-y (2022).
Article PubMed PubMed Central Google Scholar
Mubarik, S. et al . Evaluation of lifestyle risk factor differences in global patterns of breast cancer mortality and DALYs during 1990-2017 using hierarchical age-period-cohort analysis. Environ Sci Pollut Res Int 28 , 49864–49876, https://doi.org/10.1007/s11356-021-14165-1 (2021).
Mubarik, S. et al . A Hierarchical Age-Period-Cohort Analysis of Breast Cancer Mortality and Disability Adjusted Life Years (1990-2015) Attributable to Modified Risk Factors among Chinese Women. Int J Environ Res Public Health 17 , https://doi.org/10.3390/ijerph17041367 (2020).
Mubarik, S., Hu, Y. & Yu, C. A multi-country comparison of stochastic models of breast cancer mortality with P-splines smoothing approach. BMC Med Res Methodol 20 , 299, https://doi.org/10.1186/s12874-020-01187-5 (2020).
Mubarik, S. et al . Epidemiological and sociodemographic transitions of female breast cancer incidence, death, case fatality and DALYs in 21 world regions and globally, from 1990 to 2017: An Age-Period-Cohort Analysis. J Adv Res 37 , 185–196, https://doi.org/10.1016/j.jare.2021.07.012 (2022).
Li, Y. et al . Global Burden of Female Breast Cancer: Age-Period-Cohort Analysis of Incidence Trends From 1990 to 2019 and Forecasts for 2035. Front Oncol 12 , 891824, https://doi.org/10.3389/fonc.2022.891824 (2022).
Li, N. et al . Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol 12 , 140, https://doi.org/10.1186/s13045-019-0828-0 (2019).
Global Burden of Disease Cancer, C. et al . Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 3 , 524–548, https://doi.org/10.1001/jamaoncol.2016.5688 (2017).
Deng, Y. et al . Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw Open 3 , e208759, https://doi.org/10.1001/jamanetworkopen.2020.8759 (2020).
Arshi, A. et al . Expression Analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in Breast Cancer Tissues from Young Women and Women over 45 Years of Age. Mol Ther Nucleic Acids 12 , 751–757, https://doi.org/10.1016/j.omtn.2018.07.014 (2018).
Article CAS PubMed PubMed Central Google Scholar
Vuong, Q. H. et al . Near-Suicide Phenomenon: An Investigation into the Psychology of Patients with Serious Illnesses Withdrawing from Treatment. Int J Environ Res Public Health 20 , https://doi.org/10.3390/ijerph20065173 (2023).
Masood, M. & Reidpath, D. D. Effect of national wealth on BMI: An analysis of 206,266 individuals in 70 low-, middle- and high-income countries. PLoS One 12 , e0178928, https://doi.org/10.1371/journal.pone.0178928 (2017).
Lee, M. J., Popkin, B. M. & Kim, S. The unique aspects of the nutrition transition in South Korea: the retention of healthful elements in their traditional diet. Public Health Nutr 5 , 197–203, https://doi.org/10.1079/PHN2001294 (2002).
Article CAS PubMed Google Scholar
Mori, N., Armada, F. & Willcox, D. C. Walking to school in Japan and childhood obesity prevention: new lessons from an old policy. Am J Public Health 102 , 2068–2073, https://doi.org/10.2105/AJPH.2012.300913 (2012).
Seitz, H. K., Pelucchi, C., Bagnardi, V. & La Vecchia, C. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol Alcohol 47 , 204–212, https://doi.org/10.1093/alcalc/ags011 (2012).
Zakhari, S. & Hoek, J. B. Alcohol and breast cancer: reconciling epidemiological and molecular data. Adv Exp Med Biol 815 , 7–39, https://doi.org/10.1007/978-3-319-09614-8_2 (2015).
Collaborators, G. B. D. T. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389 , 1885–1906, https://doi.org/10.1016/S0140-6736(17)30819-X (2017).
Guerra, M. R. et al . Inequalities in the burden of female breast cancer in Brazil, 1990-2017. Popul Health Metr 18 , 8, https://doi.org/10.1186/s12963-020-00212-5 (2020).
Liu, W. et al . [Disease burden of breast cancer in women in China, 1990-2017]. Zhonghua Liu Xing Bing Xue Za Zhi 42 , 1225–1230, https://doi.org/10.3760/cma.j.cn112338-20200908-01139 (2021).
Yan, X. X. et al . [DALYs for breast cancer in China, 2000-2050: trend analysis and prediction based on GBD 2019]. Zhonghua Liu Xing Bing Xue Za Zhi 42 , 2156–2163, https://doi.org/10.3760/cma.j.cn112338-20210506-00373 (2021).
Azamjah, N., Soltan-Zadeh, Y. & Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac J Cancer Prev 20 , 2015–2020, https://doi.org/10.31557/APJCP.2019.20.7.2015 (2019).
Britt, K. L., Cuzick, J. & Phillips, K. A. Key steps for effective breast cancer prevention. Nat Rev Cancer 20 , 417–436, https://doi.org/10.1038/s41568-020-0266-x (2020).
Diseases, G. B. D. & Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222, https://doi.org/10.1016/S0140-6736(20)30925-9 (2020).
Vuong, Q.-H. The (ir)rational consideration of the cost of science in transition economies. Nature Human Behaviour 2 , 5, https://doi.org/10.1038/s41562-017-0281-4 (2018).
Vuong, Q.-H. Reform retractions to make them more transparent. Nature 582 , 149, https://doi.org/10.1038/d41586-020-01694-x (2020).
Article ADS CAS Google Scholar
Collaborators, G. B. D. D. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1160–1203, https://doi.org/10.1016/S0140-6736(20)30977-6 (2020).
Zeng, Q. GBD for Breast Cancer, Figshare , https://doi.org/10.6084/m9.figshare.22787405 (2023).
Liu, Z. et al . The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol 70 , 674–683, https://doi.org/10.1016/j.jhep.2018.12.001 (2019).
Zeng, Q. zengqibing/GBD-for-Breast-Cancer: Code of GBD for Breast Cancer (Code-GBD-for-Breast-Cancer), Zenodo , https://doi.org/10.5281/zenodo.7915783 (2023).
Download references
Acknowledgements
We highly appreciate the works by the Global Burden of Disease Study 2019 collaborators. This work was supported by the National Key Research and Development Program “Precision Medicine Initiative” of China (Grant 2017YFC0907301).
Author information
These authors contributed equally: Yuyan Xu, Maoyuan Gong.
Authors and Affiliations
Guizhou Medical University, the Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education & Guizhou Provincial Engineering Research Center of Ecological Food Innovation & School of Public Health, Guiyang, 550025, China
Yuyan Xu, Maoyuan Gong, Yang Yang & Qibing Zeng
The Affiliated Hospital of Guizhou Medical University, Department of Breast Surgery, Guiyang, 550004, China
Yue Wang & Shu Liu
You can also search for this author in PubMed Google Scholar
Contributions
Y.X. and M.G. performed data collection and analysis, results visualization, and wrote the manuscript. Y.W. and Y.Y. made contributions to data acquisition and analysis. S.L. and Q.Z. made significant contributions to the conceptualization, methodology, data analysis, supervision, validation, and writing– review & editing of the study. Final manuscript read and approved by all authors. Correspondence to Shu Liu and Qibing Zeng.
Corresponding authors
Correspondence to Shu Liu or Qibing Zeng .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Xu, Y., Gong, M., Wang, Y. et al. Global trends and forecasts of breast cancer incidence and deaths. Sci Data 10 , 334 (2023). https://doi.org/10.1038/s41597-023-02253-5
Download citation
Received : 02 March 2023
Accepted : 18 May 2023
Published : 27 May 2023
DOI : https://doi.org/10.1038/s41597-023-02253-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Prospective study to evaluate efficacy of single versus double drains in breast cancer patients undergoing surgery.
- Shubhajeet Roy
- Shikhar S. Gupta
- Anand Kumar Misra
Indian Journal of Surgical Oncology (2024)
MDM2 Gene rs2279744 Polymorphism and Breast Cancer Risk: Evidence from Meta-Analysis and Meta-Regression Analysis
- Mohammad Masoud Eslami
- Payam Mohammadi
- Amirhossein Sahebkar
Indian Journal of Gynecologic Oncology (2024)
Restoring microRNA-34a overcomes acquired drug resistance and disease progression in human breast cancer cell lines via suppressing the ABCC1 gene
- Shaymaa M. M. Yahya
- Heba K. Nabih
- Sohair M. Salem
Breast Cancer Research and Treatment (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

Three UWI academics chosen for international programme on breast cancer research
24 April 2024
From Left: Dr. Heather Harewood, Dr. Natalie Greaves and Dr. Simone Badal
Return to all news
The University of the West Indies Cave Hill Barbados, W.I.
Tel: (246) 417-4000
Information About
- Admissions & Aid
- Administration
- Academic Calendar
- Campus Life
Information For
- New Students
- Current Students
- Undergraduates
- Postgraduates
- Faculty & Staff
- Media Centre
Emergency Contacts | Campus Contacts | Campus Map | CITS Helpdesk

IMAGES
COMMENTS
An experimental form of immunotherapy that uses an individual's own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute's (NCI) Center for Cancer Research, part of the National Institutes of Health.
HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results ...
Possible environmental causes of breast cancer have also received more attention in recent years. While much of the science on this topic is still in its earliest stages, this is an area of active research. Breast cancer prevention. Researchers are looking for ways to help reduce breast cancer risk, especially for women who are at high risk.
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for breast cancer prevention, screening, and treatment. Breast Cancer Research Results. The following are some of our latest news articles on breast cancer research and study updates:
Patients with new metastatic triple-negative breast cancer at initial diagnosis were also eligible for the trial. Patients who received taxane, gemcitabine, or platinum agents as neoadjuvant or ...
The long-sought discovery of HER2 as an actionable and highly sensitive therapeutic target was a major breakthrough for the treatment of highly aggressive HER2-positive breast cancer, leading to ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
December 20, 2023 Researchers uncover on/off switch for breast cancer metastasis. New research from Stanford and the Arc Institute could lead to a new and more effective immunotherapy and help ...
In this international, open-label, randomized, phase 3 trial, we randomly assigned patients with HR-positive, HER2-negative early breast cancer in a 1:1 ratio to receive ribociclib (at a dose of ...
Latest Research and Reviews Impact of dose reductions on adjuvant abemaciclib efficacy for patients with high-risk early breast cancer: analyses from the monarchE study Matthew P. Goetz
Estrogen is already known to fuel breast cancer growth by promoting the proliferation of breast cells. However, the new observations cast this hormone in a different light. They show estrogen is a more central character in cancer genesis because it directly alters how cells repair their DNA. The findings suggest that estrogen-suppressing drugs ...
Panels A-C show the number of clinical trials in breast cancer since early 2000, by immunotherapeutic approach (A), by trial setting (B), and by trial phase (C).Panel D shows the major immune ...
Ribociclib is a targeted therapy called a small molecule inhibitor. It works by targeting proteins in breast cancer cells called CDK4 and CDK6, which modulate cell growth, including the growth of ...
Ribociclib plus Endocrine Therapy in Early Breast Cancer. D. Slamon and OthersN Engl J Med 2024;390:1080-1091. In patients with stage II or III early breast cancer, the addition of ribociclib to ...
Breast cancer is the second most common cancer among American women. Breast cancer death rates have been falling over the past 30 years. But nearly 13% of women are still diagnosed in their lifetime. Men can get breast cancer too, although it's rare. Cancer is caused by changes to genes that control the way our cells function.
Sep. 10, 2019 — Researchers have discovered that a cell adhesion protein, E-cadherin, allows breast cancer cells to survive as they travel through the body and form new tumors, a process termed ...
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic, biochemical, and cellular basis of breast cancer.
July 19, 2023 — Women diagnosed and treated for breast cancer have increased biological aging compared to women who remain free of breast cancer, according to a new study. Among women diagnosed ...
Glucocorticoids promote breast cancer metastasis. In patient-derived xenograft models of breast cancer in mice, an increase in stress hormones during progression or treatment with their synthetic ...
1. Introduction. Breast cancer (BC) is the most frequent cancer and the second cause of death by cancer in women worldwide. According to Cancer Statistics 2020, BC represents 30% of female cancers with 276,480 estimated new cases and more than 42,000 estimated deaths in 2020 [].Invasive BC can be divided into four principal molecular subtypes by immunohistological technique based on the ...
A new way to boost breast cancer treatment. In early 2023, our researchers found a way to potentially improve treatment effectiveness, by targeting a different form of a common protein. Dr Ahmet Ucar and this team from the University of Manchester found that a protein called RAC1B can help the disease become resistant to treatment, spread and ...
The mission of the Breast Cancer Research Foundation is to prevent and cure breast cancer by advancing the world's most promising breast cancer research. Why Research . ... 28 West 44th Street, Suite 609, New York, NY 10036. General Office: 646-497-2600 | Toll Free: 1-866-346-3228
THURSDAY, April 25, 2024 (HealthDay News) -- People lucky enough to survive a breast cancer may still face heightened risks for other cancers later, a new study shows. The researchers stressed ...
Iain Foulkes reflects on today's news that Moderna and MSD's melanoma cancer vaccine is moving to Phase 3 clinical trials. ... Breast cancer; All cancer types; By cancer topic; New treatments; Cancer biology; Cancer drugs; ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103 ...
Breast cancer cases in patients under 40 are on the rise. New research sheds light on the comparatively rare cancer, for both oncologists and patients. In 2016, 21-year-old Jessica Florence found ...
Women who survive breast cancer before the age of 50 are almost twice as likely as their peers to develop second cancers, research shows.. Across all ages they have an 87 per cent increased risk ...
Female survivors of breast cancer living in the most deprived areas have a 35% higher risk of developing second, unrelated cancers, compared with those from the most affluent areas, research shows ...
Risk factors attributable to breast cancer burden. The latest research evidence 1 shown that 44.4% of global cancer deaths and 42.0% of global cancer disability-adjusted life years can be ...
The UWI Regional Headquarters, Jamaica W.I. Wednesday, April 24, 2024—Three academics from The University of the West Indies (The UWI) have been selected as part of the first cohort of the Rising Scholars: Breast Cancer Programme, an international breast cancer research initiative for Early Career Researchers (ECRs).The UWI's Dr. Simone Badal, Dr. Heather Harewood, and Dr. Natalie Greaves ...